Abstract
Background
Souvenaid is a dietary supplement with a patented composition (Fortasyn Connect™)which is intended to be used by people with Alzheimer's disease (AD). It has been designed to support the formation and function of synapses in the brain, which are thought to be strongly correlated with cognitive function. If effective, it might improve symptoms of Alzheimer's disease and also prevent the progression from prodromal Alzheimer's disease to dementia. We sought in this review to examine the evidence for this proposition.
Objectives
To assess the effects of Souvenaid on incidence of dementia, cognition, functional performance, and safety in people with Alzheimer's disease.
Search methods
We searched ALOIS, i.e. the specialised register of the Cochrane Dementia and Cognitive Improvement Group, MEDLINE (Ovid SP), Embase (Ovid SP), PsycINFO (Ovid SP), Web of Science (ISI Web of Science), Cinahl (EBSCOhost), Lilacs (BIREME), and clinical trials registries up to 24 June 2020. We also reviewed citations of reference lists of landmark papers, reviews, and included studies for additional studies and assessed their suitability for inclusion in the review.
Selection criteria
We included randomised, placebo‐controlled trials which evaluated Souvenaid in people diagnosed with mild cognitive impairment (MCI) due to AD (also termed prodromal AD) or with dementia due to AD, and with a treatment duration of at least 16 weeks.
Data collection and analysis
Our primary outcome measures were incidence of dementia, global and specific cognitive function, functional performance, combined cognitive‐functional outcomes and adverse events. We selected studies, extracted data, assessed the quality of trials and intended to conduct meta‐analyses according to the Cochrane Handbook for Systematic Reviews of Interventions. We rated the quality of the evidence using the GRADE approach. We present all outcomes grouped by stage of AD.
Main results
We included three randomised, placebo‐controlled trials investigating Souvenaid in 1097 community‐dwelling participants with Alzheimer's disease. One study each included participants with prodromal AD, mild AD dementia and mild‐to‐moderate AD dementia. We rated the risks of bias of all trials as low. One study (in prodromal AD) was funded by European grants. The other two studies were funded by the manufacturer of Souvenaid.
One trial investigated the incidence of dementia in people with prodromal AD at baseline, and found little to no difference between the Souvenaid group and the placebo group after 24 months (RR 1.09, 95% CI 0.82 to 1.43; 1 trial, 311 participants; moderate quality of evidence).
In prodromal AD, and in mild and mild‐to‐moderate Alzheimer's disease dementia, Souvenaid probably results in little or no difference in global or specific cognitive functions (moderate quality of evidence). Everyday function, or the ability to perform activities of daily living, were measured in mild and mild‐to‐moderate AD dementia. Neither study found evidence of a difference between the groups after 24 weeks of treatment (moderate quality of evidence). Two studies investigated combined cognitive‐functional outcomes with the Clinical Dementia Rating Sum of Boxes and observed conflicting results. Souvenaid probably results in slight improvement, which is below estimates of meaningful change, in participants with prodromal Alzheimer's disease after 24 months (moderate quality of evidence), but probably has little to no effect in mild‐to‐moderate Alzheimer's disease dementia after 24 weeks (moderate quality of evidence).
Adverse effects observed were low in all trials, and the available data were insufficient to determine any connection with Souvenaid.
Authors' conclusions
Two years of treatment with Souvenaid probably does not reduce the risk of progression to dementia in people with prodromal AD. There is no convincing evidence that Souvenaid affects other outcomes important to people with AD in the prodromal stage or mild‐to‐moderate stages of dementia. Conflicting evidence on combined cognitive‐functional outcomes in prodromal AD and mild AD dementia warrants further investigation. Adverse effects of Souvenaid seem to be uncommon, but the evidence synthesised in this review does not permit us to make a definitive statement on the long‐term tolerability of Souvenaid. The effects of Souvenaid in more severe AD dementia or in people with AD at risk of nutritional deficiencies remain unclear.
Plain language summary
The dietary supplement Souvenaid for preventing dementia or delaying cognitive decline in people with Alzheimer's disease
Review question
We investigated whether people with mild cognitive impairment (MCI) due to Alzheimer's disease (AD) can reduce their risk of developing dementia with a patented dietary supplement called Souvenaid. We also investigated the effect of Souvenaid on memory or other thinking skills, ability to carry out daily activities, and side effects in people with MCI or any stage of dementia due to AD.
Background
Alzheimer's disease is a brain disease. It is the commonest cause of dementia among older people. A person is said to have dementia when there has been a decline in their memory and thinking skills which is severe enough to stop them being fully independent in all their daily activities. Because AD develops slowly, it is also possible to pick up symptoms before dementia is fully developed. This pre‐dementia stage, when people with AD have a detectable decline in memory and thinking skills but are still able to manage their usual activities independently, is known as mild cognitive impairment due to AD, or 'prodromal' AD.
Souvenaid is a patented mix of vitamins and minerals (Fortasyn Connect™) which was designed to improve brain function in AD. It is a drink which is to be taken once a day. It is intended to be consumed under medical supervision, in addition to the usual diet.
Search for evidence
We systematically searched for randomised controlled trials (RCTs) which were published up to June 2020 and which compared treatment with Souvenaid for at least 16 weeks with treatment with a dummy supplement (a placebo). For the comparison to be fair, it had to be decided randomly whether each participant was given Souvenaid or the placebo.
Key results
We found three RCTs with a total of 1097 participants to include in the review. Two of the trials investigated Souvenaid in people with dementia over a treatment period of 24 weeks. One of these included 527 participants with mild‐to‐moderate dementia due to AD and the other included 259 participants with mild dementia due to AD. The third trial investigated the use of Souvenaid for two years in 311 people with prodromal AD.
We considered all of the trials to be well‐designed, but because of differences between them in the severity of the participants' symptoms and in the way the researchers measured their results, we were not able to combine the data numerically from the single trials. All the results we report are therefore based on single trials, which leads us to have only moderate confidence in the findings of this review. This means that results could be changed by further research.
We found that people with prodromal AD who took Souvenaid daily for two years were probably no more or less likely than those taking placebo to develop dementia.
Souvenaid probably had little or no effect on measures of memory or other thinking skills in people with prodromal AD (after two years of treatment) or with mild or mild‐to‐moderate dementia due to AD (after 24 weeks of treatment). It also probably had little or no effect on the ability of people with mild or mild‐to‐moderate dementia due to AD to manage everyday activities (again after 24 weeks).
Two studies used an outcome scale which combined memory and thinking skills with practical skills (described as a combined cognitive‐functional outcome). There was probably a small benefit of Souvenaid on this outcome among people with prodromal AD who took Souvenaid for two years. However, there was probably little or no effect of Souvenaid on this outcome among people with mild‐to‐moderate AD dementia who took it for 24 weeks.
There were only a few adverse events reported in the trials, and it was not possible to know whether any of them were side effects of Souvenaid.
Study funding sources
Two studies were funded by the manufacturer of Souvenaid. The third study (in prodromal AD) was funded by European grants.
Summary of findings
Background
Description of the condition
The world’s population is ageing (United Nations 2017). Improvements in health care in the past century have contributed to people living longer and healthier lives. However, since age is the strongest risk factor for dementia, this has resulted in an increase in the number of people with dementia (WHO 2012, WHO 2019). It is estimated that over 46 million people are currently affected worldwide, resulting in high costs and considerable burden to individuals and societies (Wimo 2015).
The term ‘dementia’ refers to a syndrome occurring in a group of diseases of typically chronic or progressive nature. It involves disturbances of multiple higher cortical functions, such as memory, thinking, orientation, perception and behaviour, and it affects the ability to perform everyday activities. Deterioration in emotional control, social behaviour, or motivation often precedes or accompanies cognitive decline. The most common form of dementia is due to Alzheimer’s disease (AD), which is involved in 60% to 70% of cases. Vascular dementia is also very common. Dementia with Lewy bodies and frontotemporal dementia are less frequent forms. However, mixed forms are frequent and subtypes are indistinct (Prince 2009).
Alzheimer's disease is characterised by the accumulation of misfolded proteins, neuronal dysfunction and cell death in the brain. The deposition of amyloid β peptides in the brain as the central event in Alzheimer's disease pathology has been predominantly discussed over the last decades, but the causative mechanisms of these alterations are still being debated (Andrade‐Moraes 2013; Bloom 2014; Querfurth 2010). However, the concomitant dysfunction of synapses, which mediate information transmission, is strongly correlated with cognitive decline and memory dysfunction in Alzheimer's disease (Querfurth 2010). There is also evidence and consensus that this pathophysiological process begins in very early stages of cognitive decline or even before memory symptoms occur (Dubois 2016; Kryscio 2014; Monsell 2014; Scheff 2007). In a hypothetical model of the Alzheimer's disease continuum, this pathophysiological process is also defined as the 'preclinical stage of Alzheimer's disease' which precedes the first signs of mild cognitive impairment (Sperling 2011). Current diagnostic criteria (i.e. Dubois 2014) therefore distinguish between pathophysiological processes and clinically observable syndromes (Jack 2011).
Mild cognitive impairment (MCI) generally describes a condition of intermediate symptomatology between normal cognitive changes of ageing and fully developed symptoms of dementia of all types (Petersen 1999). The term refers to a pre‐dementia syndrome where individuals themselves, people who know them well, or clinicians observe a progressive cognitive decline, and there is objective evidence of lower‐than‐expected performance in one or more cognitive domains. MCI is distinguished from dementia in that the cognitive impairment does not interfere with the ability to function independently at work or in usual daily activities (McKhann 2011). In order to be considered 'MCI due to Alzheimer's disease', the aetiology of MCI should be consistent with the pathophysiological process of Alzheimer's disease, while other causes for cognitive impairment should be ruled out. These core criteria for the diagnosis of MCI due to Alzheimer's disease may be further supported by genetics or biomarkers (Albert 2011). Although MCI is a risk factor for Alzheimer's disease, we are still not able to predict if and when an individual with MCI might develop Alzheimer's disease dementia (Ritchie 2014; Sperling 2011; Williams 2010).
The term 'prodromal AD', which is used in the diagnostic criteria of the International Working Group (IWG) (Dubois 2007; Dubois 2014), also refers to the pre‐dementia stage of AD but is based on objective measures of memory impairment. While biomarker abnormalities may support the diagnosis of MCI due to AD (Albert 2011), they are required for the diagnosis of prodromal AD (Dubois 2014). Similar to MCI, prodromal AD is distinguished from dementia in that the cognitive deficits do not interfere with the usual performance of "accustomed instrumental activities of daily living (IADL)" (EMA 2018). The European Medicines Agency (EMA 2018) stated in recent guidelines that "It is recognized that the clinical characteristics of patients with prodromal AD/MCI due to AD may overlap with those at the milder end of the AD dementia spectrum and that, despite all efforts for criteria harmonization, operationally defined stages of disease are not clearly demarcated". They further suggest that these populations may be studied together (EMA 2018).
The clinical course of dementia due to Alzheimer's disease is often described in progressive stages, although the stages are continuous and there is much variation between individuals in the way the disease presents. The early stages of the disease are typically characterised by forgetfulness, communication problems and difficulties in carrying out complex activities (e.g. finances). In the middle stage, symptoms become increasingly obvious. Memory loss and confusion progress and individuals gradually lose the ability to care for themselves without considerable support. In the late or severe stages individuals are dependent on others for all care (WHO 2019), and psychiatric and behavioural symptoms are very common (WHO 2012).
Currently, medical treatment options for dementia due to Alzheimer's disease are limited to acetylcholinesterase (AChE) inhibitors and memantine, while non‐medical options to influence early symptoms or the course of the disease are limited to lifestyle modifications which address modifiable risk factors (WHO 2012; WHO 2019). Recent data indicate a falling incidence of dementia which supports the theory that modifying individual risk may be possible (Larson 2013). One option to alter risk is dietary modification, an option that has increasingly gained importance in research into the primary and secondary prevention of dementia (Prince 2014).
Description of the intervention
The dietary supplement, Souvenaid, is marketed as a medical food product for individuals in the early stages of Alzheimer's disease. It is intended to be consumed under medical supervision, in addition to the usual diet. The recommended dose is one bottle (125 ml) daily. The preparation is available in several flavours and contains natural food ingredients in a special patented composition (Fortasyn Connect™) (Nutricia 2014).
According to the manufacturer, the composition is designed to promote synaptic formation, which in turn is assumed to be strongly correlated with cognitive function. The drink contains a combination of docosahexaenoic acid, eicosapentaenoic acid, uridine monophosphate, choline and folate, combined with vitamins, minerals and trace elements (see Table 4). The manufacturer reports no safety concerns. Due to its ingredients, the preparation is not recommended for individuals with galactosaemia (Nutricia 2014).
1. Souvenaid: nutritional composition as provided by manufacturer (Nutricia 2014).
| Contents | per 100 ml |
| Energy | 420 kJ / 100 kcal |
| Protein (12 En%) | 3.0 g |
| Carbohydrate (36 En%): | 13.2 g |
| Sugars | 6.4 g |
| Lactose | <0.025 g |
| Fat (36 En%): | 3.9 g |
| Saturates | 1.3 g |
| Monounsaturates | 0.7 g |
| Polyunsaturates | 1.5 g |
| Eicosapentaenoic acid (EPA) | 240 mg |
| Docosapentaenoic acid (DHA) | 960 mg |
| Dietary fibre (0 En%) | 0 g |
| Minerals and trace elements: | |
| Sodium | 100 (4.3) mg (mmol) |
| Potassium | 150 (3.8) mg (mmol) |
| Chloride | 125 (3.5) mg (mmol) |
| Calcium | 80 (2.0) mg (mmol) |
| Phosphorus | 70 (2.3) mg (mmol) |
| Phosphate | 217 (2.3) mg (mmol) |
| Magnesium | 20 (0.8) mg (mmol) |
| Iron | 1.6 mg |
| Zinc | 1.2 mg |
| Copper | 180 µg |
| Manganese | 0.33 mg |
| Molybdenum | 10 µg |
| Selenium | 48 µg |
| Chromium | 6.7 µg |
| Iodine | 13 µg |
| Vitamins: | |
| Vitamin A | 160 µg‐RE |
| Vitamin D3 | 0.7 µg |
| Vitamin E | 32 mg α‐TE |
| Vitamin K | 5,3 µg |
| Thiamin (B1) | 0.15 mg |
| Riboflavin (B2) | 0.16 mg |
| Niacin (B3) | 1.8 mg‐NE |
| Pantothenic acid (B5) | 0.53 mg |
| Vitamin B6 | 0.80 mg |
| Folic acid | 320 µg |
| Vitamin B12 | 2.4 µg |
| Biotin | 4.0 µg |
| Vitamin C | 64 mg |
| Others: | |
| Uridine‐5'‐monophosphate (UMP) | 500 mg |
| Choline | 320 mg |
| Osmolarity | 490 mOsml/l |
α‐TE: alpha‐tocopherol equivalent; En: energy; NE: niacin equivalent; RE: retinol equivalent
How the intervention might work
The manufacturer of Souvenaid claims that the formulation was primarily designed to support the formation and function of synapses in the brains of people with Alzheimer's disease.
Synapses undergo constant change and remodelling, a phenomenon described as synaptic plasticity. Synaptic loss and dysfunction, one of the hallmarks of Alzheimer's disease, is thought to be closely linked to cognitive decline (Alix 2011; Scheff 2007). Maintaining or remodelling synapses could maintain better functioning of brain networks, resulting in improved cognitive functions. This might be supported directly by the supply of nutrients important in brain cell metabolism. Phosphatide subunits are a major component of synaptic membranes. It has been proposed that their biosynthesis can be supported by the consumption of specific nutrients known to be precursors for membrane phosphatides (Cansev 2008).
Research findings indicate lower plasma levels of several nutrients in individuals with Alzheimer's disease compared with cognitively healthy elderly individuals (Doecke 2012; Lopes 2013). It has been hypothesised that individuals with Alzheimer's disease might have increased requirements for certain nutrients due to alterations in diet, metabolism, uptake or use of nutrients (Mi 2013). Supplementing the diet with these nutrients could improve nutritional status, which in turn could have a favourable effect on brain cell metabolism and hence on memory and cognition.
This theory gains some support from preclinical studies. The main components of Souvenaid (Fortasyn Connect™) are omega‐3 long‐chain poly‐unsaturated fatty acids (omega 3 PUFAs), uridine monophosphate and choline. Several animal studies indicate that additional administration of these nutrients can increase levels of brain phosphatides, synaptic proteins, or the number of dendritic spines on hippocampal neurons (Cansev 2008). Souvenaid also contains several vitamins, minerals and trace elements which are claimed to enhance the bioactivity of the precursor components (Nutricia 2014).
Why it is important to do this review
Souvenaid is claimed to improve the cognitive function of individuals with Alzheimer's disease and to have no significant side effects. Considering the enormous impact of dementia on quality of life, a safe and effective dietary intervention would be of great interest to people with Alzheimer's disease. We believe it is important to systematically review the evidence on the efficacy and safety of Souvenaid in order to help people with Alzheimer's disease make decisions about its use.
Objectives
To assess the effects of Souvenaid on incidence of dementia, cognition, functional performance, and safety in people with Alzheimer's disease.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and cluster‐randomised trials, published or unpublished, reported in any language. Since Alzheimer's disease is a progressive disease, we planned to include only data from the first period of cross‐over randomised trials.
Types of participants
We included individuals with mild cognitive impairment (MCI) due to AD, or prodromal AD, or any severity of dementia due to AD.
A diagnosis of MCI should have been made in accordance with published clinical core criteria for MCI due to Alzheimer's disease (Albert 2011) as follows.
Concern about a change in cognition.
Impairment in one or more cognitive domains.
Preservation of independence in functional abilities.
Not demented (no significant impairment in social or occupational functioning).
We also accepted earlier published criteria for MCI (e.g. Petersen 1999). We included diagnostic criteria incorporating biomarkers if they had been assessed in addition to common MCI criteria, e.g. in the IWG criteria for prodromal AD (Dubois 2007).
Diagnosis of Alzheimer's disease dementia should have been made in accordance with internationally‐accepted guidelines such as Diagnostic and Statistical Manual of Mental Disorders (DSM), International Classification of Diseases (ICD) or the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS‐ADRDA) criteria (DSM III‐R; DSM IV; McKhann 2011; WHO 1992).
Since Alzheimer's disease is the most common cause of dementia (WHO 2012; WHO 2019), we planned in our protocol for this review (Burckhardt 2015) to include RCTs even when MCI or dementia subtype at baseline had not been specified, as long as data from people with dementia in general could be examined separately. We excluded studies specifically investigating other dementia types, or MCI due to other causes (e.g. vascular disease). If data from individuals with dementia or MCI were not presented separately from those of others (e.g. healthy volunteers) we aimed to obtain these data from the trial authors.
We included participants at any stage of dementia who were able to ingest Souvenaid (Fortasyn Connect™) orally or by tube feeding. There were no restrictions based on setting, gender, ethnicity or other characteristics.
Types of interventions
The intervention of interest was Souvenaid (Fortasyn Connect™). We considered any dosage and frequency of administration. The European Medicines Agency (EMA) suggested at the time of the protocol a follow‐up of at least six months, to demonstrate short‐term effects on outcomes related to cognition (EMA 2008). Meanwhile, a minimum trial duration of 18 months has been assumed to be sufficient for disease‐modifying treatments (EMA 2018). However, Souvenaid is not currently subject to the same regulatory requirements as drugs. We therefore did not expect to find many long‐term studies. Following the approach of the German Institute of Quality and Efficiency in Health Care (IQWIG 2008), we decided to compromise and accept studies with a treatment duration of at least 16 weeks.
We investigated the following treatment comparison.
Souvenaid (Fortasyn Connect™) compared with a placebo.
We considered a placebo as appropriate if it was not expected or known to influence cognitive performance.
Types of outcome measures
Primary outcomes
We assessed the effect of Souvenaid on the following.
Incidence of Alzheimer's disease dementia in individuals with MCI/prodromal AD at baseline
-
Changes in global cognitive function and specific cognitive functions (e.g. memory) measured by validated tools such as:
Alzheimer's Disease Assessment Scale‐Cognitive subscale (ADAS‐cog) (Rosen 1984);
Mini‐Mental State Exam (MMSE) (Folstein 1975);
Rey Auditory Verbal Learning Test (RAVLT) (Schmidt 1996);
Wechsler Memory Scale (Wechsler 2010);
Neuropsychological Test Battery (NTB) (Harrison 2007).
-
Changes in functional outcomes (e.g. activities of daily living) measured by validated tools such as:
Alzheimer’s Disease Activities of Daily Living International Scale (ADCS‐ADL) (Galasko 1997);
Gottries‐Bråne‐Steen‐Skala, Activities of Daily Living (ADL) subscale (GBS‐ADL) (Bråne 2001).
-
Changes in combined cognitive‐functional outcomes measured by validated tools such as:
Clinical dementia rating scale ‐ Sum of Boxes (CDR‐SOB) (O'Bryant 2008);
Alzheimer's Disease Co‐operative Study‐Clinical Global Impression of Change (CIBIC‐Plus) (Schneider 1997).
Adverse events and adverse effects
We use the term 'adverse events and adverse effects' in line with the terminology in the PRISMA harms checklist (Zorzela 2016) and the Cochrane Handbook (Peryer 2020), in which adverse event means "An unfavourable outcome that occurs during or after the use of a drug or other intervention but is not necessarily caused by it" and adverse effect means "An unfavourable outcome that occurs during or after the use of a drug or other intervention and the causal relation between the intervention and the event is at least a reasonable possibility" (Zorzela 2016; Peryer 2020). To report the adverse events, we have used an exploratory approach in order to capture all mentioned adverse events without prespecification (Peryer 2020).
Secondary outcomes
The secondary outcomes are:
concordance with intervention;
quality of life;
non‐cognitive symptoms associated with dementia (e.g. changes in mood, alterations in circadian rhythm);
entry to institutional care;
hospital admissions; and
mortality.
We concentrated on outcomes relevant for patients and included no biomarker outcomes.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois), the Cochrane Dementia and Cognitive Improvement Group’s Specialised Register, using the search terms: Souvenaid OR Fortasyn. The most recent search was performed on 24 June 2020.
ALOIS is maintained by the Information Specialist of the Cochrane Dementia and Cognitive Improvement Group and contains dementia and cognitive improvement studies identified from the following.
Monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL, PsycINFO and Lilacs.
Monthly searches of a number of trial registers: metaRegister of Controlled Trials; Umin Japan Trial Register; WHO portal (which covers ClinicalTrials.gov; ISRCTN; Chinese Clinical trials Register; German Clinical trials register; Iranian Registry of Clinical trials and the Netherlands National Trials Regsiter, plus others).
Quarterly search of the Cochrane Library’s Central Register of Controlled trials (CENTRAL).
Six‐monthly searches of a number of grey literature sources: ISI Web of knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses.
To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS website.
We ran additional separate searches in many of the above sources to ensure that we retrieved the most up‐to‐date results. The sources searched and the search strategies used can be seen in Appendix 1.
Searching other resources
We contacted the study authors of the included trials and the sponsor Nutricia for overlooked, unpublished and ongoing trials. We also reviewed reference lists from all included studies and relevant reviews. We screened the reference lists of landmark papers, reviews, and included studies for additional studies, and assessed their suitability for inclusion in the review.
Data collection and analysis
Selection of studies
We managed all references retrieved by the searches using EndNote (X9) (Endnote 2011). We removed duplications of the same references.
Two review authors (MB, AF) independently examined titles and abstracts to identify eligible studies. If the relevance of a study was not clear, we made the decision based on the full text. We resolved differing opinions on the eligibility of studies by discussion, and if necessary by involving a third review author. For all full texts of studies eligible for inclusion, we acquired all errata and supplementary data as well. It was not necessary to employ translation services. We linked multiple reports and conference abstracts of the same study together.
Two review authors (MB, AF) evaluated full texts of relevant articles independently according to the eligibility criteria. They were not blinded to study data.
We resolved disagreements by discussion, and, if necessary, by involving a third review author. We listed final decisions for the exclusion of studies and of articles which were retrieved in full text (see Characteristics of excluded studies). The selection process is documented according to the PRISMA statement (Liberati 2009) in Figure 1.
Data extraction and management
Two review authors (MB, AF) independently read and extracted the data from each included study. If any discrepancies occurred, we involved a third review author to resolve the matter. Depending on the topic, the third review author was a methodologist or a content area expert. In case of language ambiguity, we planned to involve methodologists or healthcare professionals familiar with the language in question, but this was not necessary.
We used an electronic data extraction form, including source, eligibility, methods, participants, interventions, comparators, outcomes, results and miscellaneous notes, according to the Cochrane Handbook for Systematic Reviews of Interventions (Page 2020). We also assessed details of funding source, declarations of interest of the primary investigators, and methods used to control possible conflicts of interests. The form was pre‐tested and used in a comparable review from our review group (Burckhardt 2016).
We extracted the latest available data reported by the study.
For continuous data, we extracted the mean or, if this was not available, the mean change from baseline, standard deviation (SD) and the number of participants used to measure the outcome for each group.
For dichotomous outcomes we extracted the number of participants in each outcome group. If the data provided were insufficient, we attempted to obtain the missing information from the authors and the sponsors of the trials (see the section Dealing with missing data).
If a paper reported only an estimate of an effect size (e.g. mean difference between groups for continuous data, or odds ratio or risk ratio for dichotomous data, along with corresponding standard errors or equivalent measures of uncertainty), then we extracted these data instead.
Whenever possible, we extracted intention‐to‐treat data, i.e. analysing all participants according to the group randomisation. We also extracted information on the amount of missing participant data, the statistical approach used to deal with missing data, and the level of significance as reported. Whenever possible, we also extracted and reported data from available‐case analyses or data from 'per protocol’ analyses. We contacted the main author or the sponsor if we were unable to obtain the necessary data from the trial report.
For adverse events and adverse effects, we recorded under general safety the number of participants who had at least one (serious) adverse event. We also sought data on the number of adverse events and on frequent combinations of adverse events.
One review author (MB) entered the data into Review Manager 5 (RevMan 2019). Another author (from AF and AW) checked the data for accuracy.
We also extracted information on study name, methods, participants, interventions, outcomes, starting date, contact information and other notes on ongoing but apparently eligible trials and trials which we were unable to classify for any reason.
Assessment of risk of bias in included studies
Two review authors (MB, AF) independently assessed the risks of bias for each study, using the Cochrane 'Risk of bias' tool (Higgins 2011a). We resolved any disagreements by discussion, involving the other review authors if necessary. We describe the risks of bias of all included studies in the Characteristics of included studies tables and in narrative form in the main text. We also provide an overall judgement of the included studies with a 'Risk of bias' summary (see Figure 1).
In our protocol we explicitly considered potential methods used to prevent undue industry influence during the clinical trial process. We therefore assessed additional criteria which are presented in detail in Table 5. We intended to use this information to consider whether specific aspects of methodology might have influenced the results of meta‐analyses (i.e. to explore sources of heterogeneity, as advised in the Cochrane Handbook (Higgins 2011a)).
2. Methods used to control bias resulting from conflict of interest.
| Study | Prespecified primary outcomes presented? | Planning phase and funding: role of industry | Conducting phase: role of industry | Analysing process: role of industry | Reporting process: role of industry |
| S‐Connect study 2013 | Yes | "Study design and planning were carried out in conjunction with the sponsor, Nutricia Research [...]". " The sponsor also provided the study products and funding for the research, data collection and analysis." | 2 authors (1 was on the clinical advisory panel for Nutricia Inc, 1 disclosed no COI related to the study) " had full access to the entire dataset and performed an independent, blinded analysis of the dataset." Several authors (including 4 employees of Nutricia Reserach) have supported interpretation and statistical analyses of the data |
The corresponding author had final responsibility for the decision to submit for publication. 4 authors were employees of Nutricia Inc |
|
| Souvenir II study 2012 | Yes | “Study design and planning were carried out in conjunction with the sponsor, Danone Research BV, on behalf of Nutricia Advanced Medical Nutrition, Danone’s specialized healthcare unit. The sponsor also provided the study products and funding for the research and data collection. The Souvenir II study was further supported by the NL Food & Nutrition Delta project, FND N◦10003”. P.234 |
"The sponsor also provided the study products and funding for the research and data collection." P. 234 |
"Data analysis was conducted
by staff of Danone Research and an outside statistician (JWR Twisk) independently and again by
staff at Rush Alzheimer’s Disease Center (S Leurgans, RC Shah, DA Bennett, W Fan) who received the whole data set and preformed a statistical analysis blinded to study treatment on the primary outcome
measure." P. 234 COI unclear: Rush Alzheimer's Disease Center statisticians are not named as authors; no conflict of interest statements provided. It is not mentioned if the results corresponded |
"All authors had full access to the study data. The corresponding author had final responsibility for the decision to submit for publication." P. 234 Published COI of authors: Consulting fees from Danone, Nutricia: N = 7 from (including corresponding author); lecture fees from Danone, Nutricia: n = 4; employees: N = 4 (www.j-alz.com/node/30463?id=1376) |
| LipiDiDiet study 2017 | Yes study protocol was changed before data base was locked |
"The research leading to these results was mainly funded by the European Commission under the 7th framework programme of the European Union (grant agreement number 211696). Additional funding was provided by the EU Joint Programme ‐ Neurodegenerative Disease Research (MIND‐AD grant); Kuopio University Hospital, Finland (EVO/VTR grant); and Academy of Finland (grant 287490)."P. 974. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors "had full access to all the data in the study. The corresponding author had final responsibility for the decision to submit for publication." P. 969 Published COI of authors: No obvious association to Danone/Nutricia. "Nutricia Research, Nutricia Advanced Medical Nutrition" is named as collaborator to the clinical study group. Suppl. P. 2 |
|||
AD: Alzheimer's disease; COI: Conflicts of Interests
Measures of treatment effect
We planned to use mean differences or standardised mean differences with 95% confidence intervals for continuous outcomes, and risk ratio with 95% confidence intervals for dichotomous outcomes.
Commonly‐used scales in dementia trials are often coded ordinally. We treated data measured with scales comprising more than 10 categories as continuous variables, assuming a normal distribution.
Unit of analysis issues
The unit of analysis was the person with dementia.
Dealing with missing data
For all included studies, we sought additional data or clarifications from corresponding authors or the study sponsor.
We considered both published and unpublished data obtained from the study authors. We took the amount and distribution of missing data into account when we considered the risk of bias due to missing data.
We intended to report intention‐to‐treat analyses and, if this was not possible, per protocol analyses along with sensitivity analyses to assess the robustness of their results to imputations assuming poor outcomes.
None of the included trials were able to assess outcomes from all randomised participants. All trials used logistic regression models to predict data from missing participants over time. These models are based on the assumption that data are missing at random. The impact of missing data under the 'missing not at random' assumption was investigated in one trial (LipiDiDiet study 2017).
We intended to report the results of per protocol analyses alongside the results from the advanced models reported, but these were only published for the LipiDiDiet study 2017. For the other trials, we were unable to obtain these analyses from the sponsor Nutricia.
The following terms and definitions were used within the trials. We use them for the presentation of the results unless otherwise stated.
intention‐to‐treat‐population (ITT). This includes all participants randomised (Souvenir II study 2012; S‐Connect study 2013)
All‐subjects‐treated population (AST). This includes all randomised participants who received at least one dose of the study product (Souvenir II study 2012; S-Connect study 2013). All three trials used this population for the safety analysis.
Modified intention‐to‐treat population (mITT). This included all randomised participants "excluding visit data after the start of rescue medication" (LipiDiDiet study 2017).
Per protocol population."The per‐protocol population consists of all participants from the modified intention‐to‐treat population, excluding participants or distinct visits of participants with major protocol deviations" (LipiDiDiet study 2017). Participants were excluded from PP if they did not comply with major eligibility criteria, did not consume at least one study product or lived in the same household with another study participant randomised to the other intervention arm (LipiDiDiet study 2017).
Two trials (Souvenir II study 2012; S‐Connect study 2013) presented descriptive statistics which were stated to be for the ITT population. However, the numbers of the participants on which the means and SDs are based differ from the numbers of participants randomised. We regarded the populations described by these statistics as 'available cases' and use the term 'available‐case analysis' for our analyses accordingly. This term is often used for analyses "in which data are analysed for every participant for whom the outcome was obtained" Higgins 2011b. We use this term also for analyses based on "observed mean changes from baseline", which was presented for the mITT population in the LipiDiDiet study 2017.
Assessment of heterogeneity
To assess clinical heterogeneity, we examined the data extraction tables and considered between‐study variability with respect to participants, interventions, and outcome measurements.
We had intended to evaluate statistical heterogeneity by using Chi2 and I2 statistics.
Assessment of reporting biases
We tried to minimise reporting bias by including both published and unpublished trials. We compared conference abstracts and trial registry entries with published data.
We did not identify enough trials to justify the use of a funnel plot or Egger’s test for asymmetry (Egger 1997) to explore reporting biases or other small‐study effects.
Data synthesis
We intended to perform meta‐analyses for all primary and secondary outcomes using Review Manager 5 (RevMan 2019) where there were sufficient data from included studies to estimate an overall treatment effect. As specified in our protocol (Burckhardt 2015), we intended to use comparable time points (± 1 week) for all meta‐analyses and to investigate the impact of baseline cognition in subgroups.
We examined participants, interventions, and outcomes in the included trials in order to decide whether we could reasonably pool them. The included trials differed in participants, outcome scales, follow‐up times and analysis methods. However, we considered that the LipiDiDiet study 2017 and the Souvenir II study 2012 were sufficiently similar for meta‐analyses to be informative. To perform meta‐analyses, more data would have been needed (means, measures of variance). Since we were unable to obtain the necessary data from the responsible project co‐ordinator of the LipiDiDiet study 2017 or from the sponsor Nutricia (responsible for the data of the Souvenir II study 2012 and S‐Connect study 2013), we provide a narrative account of all results as reported by the authors of the studies.
Subgroup analysis and investigation of heterogeneity
To explore possible sources of heterogeneity, we had prespecified the following subgroup analyses:
Baseline Mini‐Mental State Exam (MMSE) score ≥ 24 versus < 24;
Baseline nutritional status (e.g. higher versus lower plasma triglycerides or higher versus lower Subjective Global Assessment scores).
Given the available data, it was not possible to investigate these subgroups.
Sensitivity analysis
We had prespecified sensitivity analyses to investigate the impact of missing data but were unable to do this because the data were unsuitable for meta‐analysis.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach (Guyatt 2011) to interpret the findings and present them in 'Summary of findings' tables, as recommended by Cochrane (Schünemann 2020). We considered all primary outcomes to be important, and present them in the 'Summary of findings' tables. With this approach, we deviated from our plan to rate all outcomes with a consumer group (see also Differences between protocol and review).
GRADE distinguishes quality in four possible ratings: high, moderate, low and very low. The quality‐of‐ evidence ratings in GRADE describe the degree of confidence which we can place in the estimates of treatment benefits and harms (see explanations below the 'Summary of findings' tables). Results of RCTs without any limitations are generally rated as high quality. Several factors (imprecision of effect estimates, risk of bias in included studies, inconsistency of results, indirectness of evidence and publication bias ) can lead to the downgrading of the evidence (Guyatt 2011). These factors are described below the 'Summary of findings' tables.
Results
Description of studies
Results of the search
The electronic searches from the five searches run for this review (February 2016, January 2017, March 2018, May 2019 and June 2020) by Anna Noel‐Storr and Candida Fenton, Information Specialists for the Cochrane Dementia and Cognitive Improvement Group, retrieved a total of 283 references, which were supplemented by two further references (285 references in total). After deduplication, 229 references were left. We identified no further reference by scanning the reference lists of landmark papers and included studies. We received no information about further published or unpublished studies from experts or from Nutricia. Two review authors (MB, AF) independently assessed the 229 references and discarded 189 which were not relevant. Two review authors (MB, AF) independently assessed the remaining 40 articles and related conference abstracts for eligibility. Two trials did not meet our inclusion criteria (see Characteristics of excluded studies). We included 19 articles covering to three trials (LipiDiDiet study 2017; S‐Connect study 2013; Souvenir II study 2012). One trial registered in the Netherlands Trial Register (NL-ENIGMA) matched our selection criteria for its secondary outcomes. In spring 2019 we contacted the manufacturer and the contact person named in the trial registry. So far, the secondary endpoints relevant for this review have not been published. The selection process is presented in accord with the PRISMA statement (Liberati 2009) (see Figure 1). Because of the small number of studies, we were unable to use the funnel‐plot method to look for any indication of unpublished studies.
1.
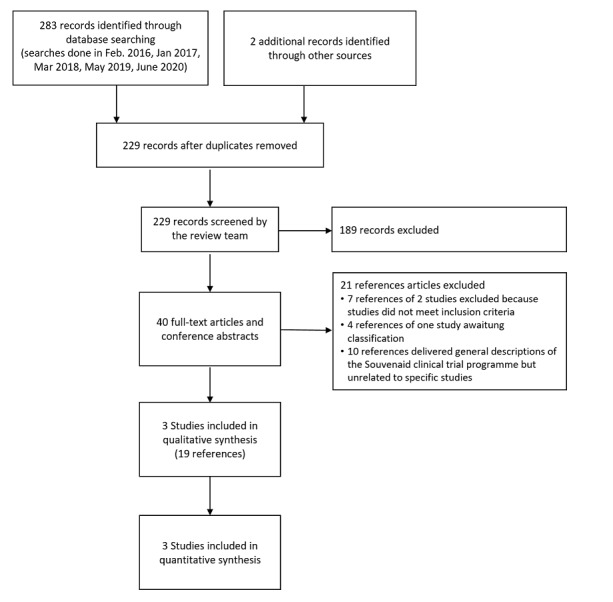
Study flow diagram
Included studies
All three included studies were published, randomised, double‐blind, placebo‐controlled trials with a parallel‐group design (LipiDiDiet study 2017; S‐Connect study 2013; Souvenir II study 2012). Participants in all trials received once daily either 125 ml Souvenaid or an iso‐caloric control drink which was identical in taste, consistency and appearance but lacked Fortasyn Connect™. Two trials (S‐Connect study 2013; Souvenir II study 2012) included participants with diagnosed dementia due to Alzheimer's disease, whereas one (LipiDiDiet study 2017) included participants with prodromal Alzheimer's disease. Two studies (S‐Connect study 2013; Souvenir II study 2012) received funding from the manufacturer of Souvenaid, Nutricia Advanced Medical Nutrition; the third study (LipiDiDiet study 2017) appeared to have no industry funding, although Nutricia Advanced Medical Nutrition is named as a collaborator with the clinical study group. There are some further differences between the studies, which mainly concern the stage of Alzheimer's disease, outcome measurements and study duration, and which are described below. Table 6 provides an overview of the main characteristics.
3. Main characteristics of included studies.
| Study | Number randomised | Alzheimer’s disease severity/ Mean MMSE (SD) | Mean age (SD) | Mean BMI (SD) | Use of AD medication | Treatment duration | Oucomes relevant to this review |
|
Souvenir II Europe |
Total 259 IG 130 CG 129 |
very mild IG 24.9 (2.9) CG 25.0 (2.8) |
IG 74.4 (6.9) CG 73.2 (8.4) |
IG 26.1 (4.1) CG 26.7 (4.2) |
No | 24 weeks | NTB memory function NTB executive function Modified NTB composite score DAD Tolerance and safety |
|
S‐connect USA |
Total 527 IG 265 CG 262 |
mild‐to‐moderate IG 19.5 (3.2) CG 19.4 (3.0) |
IG 76.6 (8.2) CG 76.9 (8.2) |
IG 26.2 (4.2) CG 26.6 (4.6) |
AChEI: 34% Memantine: 6% Combined: 60% |
24 weeks | ADAS‐cog Cognitive test battery CDR SoB MMSE ADCS‐ADL Tolerance and safety |
|
LIPIDIDIET Europe |
Total 311 IG 153 CG 158 |
prodromal IG 26.4 (2.1) CG 26.9 (1.9) |
IG 71.3 (7.0) CG 70.7 (6.2) |
not reported | No | 24 months | NTB Cognitive function (modified version) NTB memory function NTB executive function Progression to dementia CDR SoB Tolerance and safety |
AChEI: acetylcholinesterase inhibitors; AD: Alzheimer's disease; ADAS‐Cog: Alzheimer's Disease Assessment Scale ‐ Cognitive subscale; ADCS‐ADL: Alzheimer's Disease Cooperative Study ‐ Activities of Daily Living; BMI: body mass index; CDR‐SOB: Clinical Dementia Rating ‐ Sum of Boxes; CG: control group; DAD: Disability Assessment for Dementia; IG: intervention group; MMSE: Mini‐Mental State Examination; NTB: neuropsychological test battery; PP: per protocol; SD: standard deviation.
The largest trial (S‐Connect study 2013) was conducted in the USA. It investigated Souvenaid in 527 participants with mild‐to‐moderate dementia due to AD over a treatment duration of 24 weeks. All participants received US Food and Drug Administration (FDA)‐approved AD medication at a stable dose for at least four months prior to baseline (an inclusion criterion) and during the study period. Participants were recruited from the community or from specialised dementia clinics but were not institutionalised in nursing homes. Further inclusion criteria were: age 50 years or more, diagnosis of probable AD (NINCDS‐ADRA) with MMSE score between 14 and 24, and availability of a responsible study partner. People were excluded if they had neurological or psychiatric diseases other than AD significantly contributing to cognitive difficulties, drug abuse, depression score above 4 (on a 15‐item scale), recent use of defined nutritional supplements (e.g. omega‐3 PUFAs or high‐energy drinks) or medication (e.g. anticholinergic), or were living in a nursing home. The study population had a mean MMSE score of 19.45 (SD 2.0) at the beginning of the trial and had a mean age of 76.75 years (SD 8.19).
The Souvenir II study 2012 also investigated Souvenaid over a treatment duration of 24 weeks. The primary focus of the trial was on memory performance. It included 259 participants with mild dementia due to Alzheimer's disease, who were receiving no FDA‐approved medication for AD. The trial was conducted in Europe and the participants were recruited in specialised AD centres in Spain, Italy, Germany, The Netherlands, Belgium and France. Important entry criteria were diagnosis of probable AD according to the NINCDS‐ADRA criteria, no other cause of dementia shown by medical imaging, MMSE score of 20 or more, and a responsible caregiver. Among other reasons, participants were excluded if they had received approved medication for AD within three months prior to baseline, lived in a nursing home, had a depression score above 6 (on a 15‐item scale) or consumed relevant nutritional supplements (e.g. omega‐3 PUFAs) or medication. The groups did not differ by demographic characteristics at baseline. The study population had very mild dementia, with a mean MMSE score of 24.94 (SD 2.85) and a mean age of 73.80 (SD 7.69).
The LipiDiDiet study 2017 lasted 24 months and was a multicentre trial conducted in Finland, Germany, the Netherlands and Sweden. The 11 study sites investigated Souvenaid in 311 participants with prodromal Alzheimer's disease, defined as a disorder of episodic memory and evidence of AD disease pathology (according to Dubois 2007). Major exclusion criteria were medication for AD, depressive disorders (DSM‐IV) and regular high intakes of B, C or E vitamins and fish oils. Progression to Alzheimer's dementia was used as a secondary endpoint. The study population had a mean MMSE score of 26.65 (SD 2.01) at the beginning of the trial and had a mean age of 71.00 years (SD 6.60).
We contacted the sponsor Nutricia, responsible for the data of two of the studies (S‐Connect study 2013; Souvenir II study 2012), and the project co‐ordinator of the LipiDiDiet study 2017. We asked for six‐month data to perform meta‐analyses, for statistical clarifications, and for information on other aspects of the studies. We report the outcomes of these requests in the relevant sections and tables.
Outcomes
A variety of outcome measures were used in the trials. Table 6 summarises their use in the included studies. To aid interpretation, we present related estimates of clinically important changes as identified in the literature. Appropriate methods for defining valid estimates of minimum clinically important differences (MCIDs) are not yet fully developed and for scales covering multiple constructs (e.g. global severity, or combined cognitive‐functional scales) are almost impossible to determine (Molnar 2009). Furthermore, what is estimated to be a clinically important difference depends on the population (e.g. severity of dementia) and contextual characteristics (e.g. balance between side effects and efficacy) and might vary from different points of view (e.g. researcher or patient) (Revicki 2008) . It would therefore have been ideal if participants taking Souvenaid had estimated their own features of a MCID from their point of view. However, none of the trials used this approach. The estimates we present of clinically important changes have been developed with varying methods and address different circumstances and disease severities. They should therefore be considered with caution.
Incidence of Alzheimer's dementia in individuals with MCI at baseline
Incidence of Alzheimer's dementia was measured in LipiDiDiet study 2017 according to DSM IV diagnostic criteria (McKhann 2011).
Global and specific cognitive function measures
The Neuropsychological Test Battery (NTB) (Harrison 2007) is a combination of validated cognitive tests measuring memory, praxis, language, working memory, attention, and psychomotor speed. Higher scores indicate better cognitive function. The memory domain of the test comprises the Wechsler Memory Scale Verbal Paired Associates test (immediate and delayed) and the Rey Auditory Verbal Learning Test (immediate and delayed). The executive domain includes Wechsler Memory Digit Span, Controlled Oral Word Association Test, Category Fluency, Trail‐Making Test parts A and B. The data can be analysed at the level of the individual tests or can be standardised into z‐scores, which in turn can be averaged to obtain z‐scores for memory or executive sub‐domains. It is also possible to get a composite (global) score of all test results. The NTB was developed for measuring cognitive change in Alzheimer's disease clinical trials in participants with relatively high Mini‐Mental State Examination (MMSE) scores. Since its development the test battery has been modified in several trials (Harrison 2011). Contemporary versions of the NTB now commonly include measures of working memory, attention, and psychomotor speed in addition to the more traditionally‐measured functions such as memory, praxis, and language. The two included trials (LipiDiDiet study 2017; Souvenir II study 2012) which used the NTB both refer to the same publication (Harrison 2007), although the NTB subdomains and the NTB total composite scores used in the two trials were composed differently. The components of the respective Neuropsychological Test Battery and the composition of the individual subscores used in these studies are shown in Table 7. We found no estimates in the literature of a meaningful change. Z‐scores can be interpreted in accordance with Cohen (Cohen 1988), where an effect of 0.2 corresponds to a small effect, 0.5 to a moderate effect and 0.8 to a large effect.
Another composite cognitive test battery was used in one study (S‐Connect study 2013) to measure attention, concentration, executive function, processing speed and semantic memory. The test comprised the Digit Span from the Wechsler Memory Scale – Third Edition, the Concept Shifting Test, the Letter Digit Substitution Test, and Category Fluency (Table 7). The z‐scores of the four tests were averaged to a 'global cognitive function composite score' as described in the article S‐Connect study 2013. The subscales are comparable to those used to measure executive function from the NTB used in the LipiDiDiet study 2017. We considered this test battery to be a measure of executive function.
The Alzheimer’s Disease Assessment Scale ‐ cognitive subscale (ADAS‐Cog) comprises spoken language ability, comprehension of spoken language, recall of test instructions, word finding, following commands, naming objects, construction drawing, ideational praxis, orientation, word recall and word recognition. The score ranges from 0 to 70 points, with a higher score indicating greater impairment (Rosen 1984). The MCID is mainly estimated as 2 to 4 points (Huntley 2015; Molnar 2009; Schrag 2012; Vellas 2008).
4. Content of the cognitive test batteries.
| What is the test battery called in the studies? | What are the summaries of the test components?a | Memory score (score range)b | Executive function score (score range)b | Composite score (score range)b | Other (score range) | |
| LipiDiDiet study 2017 | Neuropsychological test battery (NTB) |
|
NTB memory domain (z‐score; 3 components)
|
NTB executive function domain (z‐score; 4 components)c
|
NTB total (composite z‐score; 16 components)
|
NTB primary endpoint (z‐score; 5 components)
|
| S‐Connect study 2013 | Cognitive test battery | Global cognitive function composite score (z‐score; 4 components) | ‐ | ‐ |
Global cognitive function composite score (z‐score; 4 components)3
|
‐ |
| Souvenir II study 2012 | Neuropsychological test battery (NTB) |
|
Memory function domain score (z‐score; 5 components)
|
Executive function domain score (z‐score; 5 components)
|
NTB total composite score (z‐score; 12 components)
|
‐ |
aThe exact terminology of the subscores is not entirely consistent in the included studies. For example, the memory score is called "NTB memory domain (z‐score)" in the LipiDiDiet study 2017 and "Memory function domain score (z‐score)" in the Souvenir II study 2012. To make the text easier to read, we have standardised the wording in the continuous text. bThe NTB subdomains and the NTB total composite scores used were composed differently in the included trials. In the continuous text we will refer to the number of integrated components and the detailed presentation in Table 8. cThe "Global cognitive function composite score (z‐score)" from the S‐Connect study 2013 is based on the same components as the "NTB executive function domain (z‐score)" from the LipiDiDiet study 2017. When reporting the results, we have maintained the classification of the authors as "Composite score" in the S‐Connect study 2013 and "Executive function score" in the LipiDiDiet study 2017.
ADAS‐cog: Alzheimer's Disease Assessment Scale ‐ Cognitive subscale; CERAD: Consortium to Establish a Registry for Alzheimer’s Disease; COWAT: Controlled Oral Word Association Test; CST: Concept Shifting Test; RAVLT: Rey Auditory Verbal Learning Test; TMT: Trail Making Test; WMS: Wechsler Memory Scale; WMS‐r: Wechsler Memory Scale Revised Edition; WMS‐VPA: Wechsler Memory Scale Verbal Paired Associates
Functional outcome measures (e.g. activities of daily living)
The Alzheimer’s Disease Cooperative Study‐Activities of Daily Living (ADCS‐ADL) (Galasko 1997) was specifically designed as part of a comprehensive test battery to assess activities of daily living in people with Alzheimer’s disease in clinical trials. It consists of 23 criteria comprising simple everyday skills and complex activities, which are rated based on an interview with an informant who knows the affected study participant well. The range is from 0 to 78, with a higher score indicating better performance. Data on the MCID for ADCS‐ADL is limited. One study group defined a threshold of a two‐point score change as meaningful in an RCT investigating vitamin E and memantine in mild‐to‐moderate AD (Dysken 2014).
The Disability Assessment for Dementia (DAD) (Gelinas 1999) is used to evaluate the performance of daily activities in community‐dwelling individuals with dementia, based on caregiver information. The instrument evaluates initiation, planning and execution of simple and complex activities. A final score is derived from the percentage of all questions rated positively, indicating that the study participant is able to perform the respective task without help. Higher scores therefore indicate better performance. We found no estimates of a meaningful change.
Combined cognitive‐functional outcome measure
The Clinical Dementia Rating scale‐Sum of Boxes (CDR‐SOB) (O'Bryant 2008) is a semi‐structured interview of patients and informants for the assessment of cognition (memory, orientation, judgement/problem‐solving) and function (community affairs, home/hobbies, personal care). The CDR‐SOB total score ranges from 0 to 18 with scores from 3 to 15.5 indicating mild‐to‐moderate dementia (O'Bryant 2008). Lower scores indicate a better performance. The MCID was recently estimated to be one scale point in MCI due to AD and two points in dementia due to AD (Andrews 2019).
Adverse effects
All three included studies addressed the safety of the product. The S‐Connect study 2013 explicitly classified safety as a secondary outcome. The others reported safety parameters. Safety assessments included at a minimum: recordings of adverse events, the monitoring of vital signs, and additional laboratory parameters.
Some secondary outcomes defined in the protocol of this review (Burckhardt 2015) (concordance with intervention, entry to institutional care, hospital admissions and mortality) were not assessed explicitly as outcomes in any of the included trials. We considered any information reported by the trial authors which was relevant to institutionalisation, hospital admission or mortality in the adverse effects section (see Effects of interventions).
Excluded studies
We excluded 21 references and listed the reasons in Figure 1. Most of the references were excluded because they delivered general descriptions of the Souvenaid clinical trial programme but unrelated to a specific study. We excluded the Souvenir I study 2010 because the treatment with Souvenaid lasted only 12 weeks. The Souvenir II study 2012 had an open‐label extension with a separate registration number (NTR 2456; Souvenir II OLE study 2015), which we excluded because all participants received the active product.
One further study focused on cerebral glucose metabolism and had corresponding biomarkers as endpoints (NL‐ENIGMA). However, among the secondary outcomes, endpoints are identified that may be relevant for this review. So far, no results for these outcomes have been published. For this reason, the study will remain in the Ongoing studies category, until it can finally be moved to the included or excluded studies.
Risk of bias in included studies
Overall, we judged the trials to be well‐designed and well‐conducted, and hence to be at low risk of bias. Our ’Risk of bias’ judgments are described in the Characteristics of included studies tables and also depicted in the ’Risk of bias’ summary and ’Risk of bias’ graph (see Figure 2; Figure 3)
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Allocation
All trial reports (Souvenir II study 2012; S‐Connect study 2013; LipiDiDiet study 2017) included details of adequate sequence generation and allocation concealment methods.
Blinding
All trials (Souvenir II study 2012; S‐Connect study 2013; LipiDiDiet study 2017) used adequate blinding methods for participants and staff by using placebo drinks with an identical taste and appearance. The randomisation code was not broken until the primary outcomes were analysed.
Incomplete outcome data
In all trials (S‐Connect study 2013; Souvenir II study 2012; LipiDiDiet study 2017), the amount of missing outcome data was equally distributed between groups, but the reasons for study discontinuation were not described in sufficient detail in the published papers. However, the overall numbers of dropouts were low (< 22% in the longer‐lasting LipiDiDiet study 2017 trial) and all trial authors provided detailed information on the reasons for dropouts in personal communications. We therefore judged the influence of missing data on the overall results as marginal. Overall, missing data were considered in intention‐to‐treat‐analyses.
Selective reporting
All included trials (S‐Connect study 2013; Souvenir II study 2012,LipiDiDiet study 2017 ) reported the primary outcomes as described in the protocols published in trial registries. In the LipiDiDiet study 2017, changes of outcome measures were explained and made prior to database lock. Relevant serious adverse events (SAEs) are presented with distribution to the groups.
Other potential sources of bias
We found no further potential sources of bias, and rated the risk as low for all three studies.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Souvenaid compared to placebo for MCI/prodromal AD.
| Souvenaid compared to placebo for MCI | ||||||
| Patient or population: People with MCI/prodromal AD Setting: community Intervention: Souvenaid Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk difference with Souvenaid | |||||
| Incidence of dementia Follow‐up: 24 months | Study population | RR 1.09 (0.82 to 1.43) | 311a (1 RCT) | ⊕⊕⊕⊝ MODERATEb | Souvenaid probably results in little to no difference in incidence of dementia | |
| 373 per 1000 | 34 more per 1000 (67 fewer to 161 more) | |||||
| Cognition (global cognitive function) Assessed with: NTB total composite z‐score (16 components) Follow‐up: 24 months |
Single study reported no significant difference between Souvenaid and placebo when missing data were considered in a modified ITT analysis (LME: MD 0.10, 95% CI −0.04 to 0.24), or based on available‐case analysis (MD 0.08, 95% CI −0.04 to 0.20)c | ‐ | 311a (1 RCT) | ⊕⊕⊕⊝ MODERATEb | Souvenaid probably results in little to no difference in cognition | |
| Memory (specific cognitive function) Assessed with: NTB memory domain z‐score (3 components) Follow‐up: 24 months |
Single study reported no significant difference between Souvenaid and placebo when missing data were considered in a modified ITT analysis (LME: MD 0.14, 95% CI −0.03 to 0.30), or based on available‐case analysis (MD 0.13, 95% CI −0.01 to 0.27)c | ‐ | 311a (1 RCT) | ⊕⊕⊕⊝ MODERATEb | Souvenaid probably results in little to no difference in memory | |
| Executive function (specific cognitive function) Assessed with: NTB executive function domain z‐score (4 components) Follow‐up: 24 months | Single study reported no significant difference between Souvenaid and placebo when missing data were considered in a modified ITT analysis (LME: MD −0.04, 95% CI −0.18 to 0.10), or based on available‐case analysis (MD −0.11, 95% CI −0.22 to 0.01)c | ‐ | 311a (1 RCT) | ⊕⊕⊕⊝ MODERATEb | Souvenaid probably results in little to no difference in executive function | |
| Functional outcome (e.g. activities of daily living) Assessed with: No study measured this outcome |
‐ | ‐ | ( 0 studies) | ‐ | ‐ | |
| Combined cognitive‐functional outcome Assessed with: CDR‐SoB Follow‐up: 24 months |
Single study reported a significant difference between Souvenaid and placebo when missing data were considered in a modified ITT analysis (LME: MD −0.60, 95% CI −1.01 to −0.19), or based on available‐case analysis (MD −0.56, 95% CI −0.95 to −0.17) | ‐ | 311a (1 RCT) | ⊕⊕⊕⊝ MODERATEb | A lower score is better. Souvenaid probably results in a slight improvement in a combined measure of cognition and function. This difference is below estimates of meaningful changes | |
| Any adverse event Follow‐up: 24 months |
879 per 1000 | 870 per 1000 (800 to 949) |
RR 0.99 (0.91 to 1.08) | 309 (1 RCT) | ⊕⊕⊕⊝ MODERATEb | Souvenaid probably results in little to no difference in any adverse events |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; ITT: Intention to treat; LME: Linear mixed effects model; MD: Mean difference; NTB: Neuropsychological Test Battery; CDR‐SoB: Clinical Dementia Rating Scale Sum of Boxes | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aNumber given is number of participants randomised. Number of participants in each analysis varied with method of handling missing participant data.
bDowngraded for imprecision. Broad 95% CI. cZ‐score.
Summary of findings 2. Souvenaid compared to placebo for mild Alzheimer's disease dementia.
| Souvenaid compared to placebo for mild Alzheimer's disease | ||||||
| Patient or population: People with mild Alzheimer's disease Setting: community Intervention: Souvenaid Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Souvenaid | |||||
| Cognition (global cognitive function) assessed with: NTB total composite z‐score (12 components) Follow‐up: 24 weeks |
Single study observed significant or no significant differences depending on the statistical model used, based on available‐case analysis (MD 0.08, 95% CI 0.00 to 0.17), or when missing data were considered in mITT analysis (MMRM P = 0.035), or mITT with another statistical model (24‐week trajectory P = 0.053)a | ‐ | 259b (1 RCT) | ⊕⊕⊕⊝ MODERATEc | It is not clear how large a difference on this scale would be clinically important. Whether or not there was a statistically significant difference between groups (P < 0.05) in cognition depended on the method used to handle missing data and/or the statistical test used | |
| Memory (specific cognitive function) Assessed with: NTB memory function domain z‐score (5 components) Follow‐up: 24 weeks |
Single study observed significant or no significant differences depending on the statistical model used based on available‐case analysis (MD 0.09, 95% CI −0.03 to 0.21), or when missing data were considered in a mITT analysis (MMRM P = 0.09), or mITT with another statistical model (24‐week trajectory P = 0.023)a | ‐ | 259b (1 RCT) | ⊕⊕⊕⊝ MODERATEc | It is not clear how large a difference on this scale would be clinically important. Whether or not there was a statistically significant difference in memory between groups (P < 0.05) depended on the method used to handle missing data and/or the statistical test used | |
| Executive function (specific cognitive function) Assessed with: NTB executive function domain z‐score (5 components) Follow‐up: 24 weeks |
Single study reported no significant difference between Souvenaid and placebo based on available‐case analysis (MD 0.04, 95% CI −0.05 to 0.13), or when missing data were considered with MMRM (P = 0.39), or with another statistical model (24‐week trajectory P = 0.69)a | ‐ | 259b (1 RCT) | ⊕⊕⊕⊝ MODERATEc | Souvenaid probably results in little to no difference in executive function | |
| Functional outcome (activities of daily living) Assessed with: DAD Follow‐up: 24 weeks |
Single study reported no significant difference between Souvenaid and placebo (P = 0.36) No further analysis was provided |
‐ | 259b (1 RCT) | ⊕⊕⊕⊝ MODERATEc | Souvenaid probably results in little to no difference in functional outcome (activities of daily living) | |
| Quality of life Assessed with: No study measured this outcome |
‐ | ‐ | (0 studies) | ‐ | ‐ | |
| Combined cognitive‐functional outcome Assessed with: No study measured this outcome |
‐ | ‐ | (0 studies) | ‐ | ‐ | |
| Any adverse events Follow‐up: 24 weeks |
605 per 1000 | 520 per 1000 | RR 0.86 (0.69 to 1.07) | 258 (1 RCT) | ⊕⊕⊕⊝ MODERATEc | Souvenaid probably results in little to no difference in any adverse events |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; MD: Mean difference; mITT: Modified intention‐to‐treat; MMRM: Mixed model of repeated measures; NTB: Neuropsychological Test Battery; DAD: Disability Assessment for Dementia | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aZ‐score.
bNumber given is number of participants randomised. Number of participants in each analysis varied with method of handling missing participant data.
cDowngraded for imprecision. Broad 95% CI.
Summary of findings 3. Souvenaid compared to placebo for mild‐to‐moderate Alzheimer's dementia.
| Souvenaid compared to placebo for mild‐to‐moderate Alzheimer's disease | ||||||
| Patient or population: People with mild‐to‐moderate Alzheimer's dementia Setting: community Intervention: Souvenaid Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Souvenaid | |||||
| Cognition (global cognitive function) Assessed with: ADAS‐cog Follow‐up: 24 weeks |
Single study reported no significant difference between Souvenaid and placebo based on available‐case analysis (MD 1.02, 95% CI −1.11 to 3.15), or when missing data were considered in a mITT analysis (MMRM: MD 0.37; P = 0.51) | ‐ | 527a (1 RCT) | ⊕⊕⊕⊝ MODERATEb | Souvenaid probably results in little to no difference in cognition | |
| Memory (specific cognitive function) Assessed with: No study measured this outcome Follow‐up: 24 weeks |
‐ | ‐ | (0 studies) | ‐ | ‐ | |
| Executive function (specific cognitive function) Assessed with: Global cognitive function composite z‐score (4 components) Follow‐up: 24 weeks |
Single study reported no significant difference between Souvenaid and placebo based on available case analysis (MD 0.08, 95% CI −0.07 to 0.23), or when missing data were considered in a mITT analysis (MMRM P = 0.32)c | ‐ | 527a (1 RCT) | ⊕⊕⊕⊝ MODERATEb | Souvenaid probably results in little to no difference in executive function | |
| Activities of daily living (functional outcome) Assessed with: ADCS‐ADL Follow‐up: 24 weeks |
Single study reported no significant difference between Souvenaid and placebo based on available case analysis (MD 0.51, 95% CI −2.4 to 3.42), or when missing data were considered in a mITT analysis (MMRM P = 0.77) | ‐ | 527a (1 RCT) | ⊕⊕⊕⊝ MODERATEb | Souvenaid probably results in little to no difference in functional outcome (activities of daily living) | |
| Quality of life, Assessed with: No study measured this outcome |
‐ | ‐ | (0 studies) | ‐ | ‐ | |
| Combined cognitive‐functional outcome Assessed with: CDR‐SoB Follow‐up: 24 weeks |
Single study reported no significant difference between Souvenaid and placebo based on available‐case analysis (MD −0.12, 95% CI −0.74 to 0.50), or when missing data were considered in a mITT analysis (MMRM P = 0.50) | ‐ | 527a (1 RCT) | ⊕⊕⊕⊝ MODERATEb | Souvenaid probably results in little to no difference in the combined cognitive‐functional outcome. | |
| Any adverse events Follow‐up: 24 weeks |
635 per 1000 | 571 per 1000 (495 to 654) |
RR 0.90 (0.78 to 1.03) | 524 (1 RCT) | ⊕⊕⊕⊝ MODERATEb | Souvenaid probably results in little to no difference in any adverse events |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; MD: Mean difference; mITT: Modified intention to treat MMRM: Mixed model for repeated measures; ADAS‐cog: Alzheimer's Disease Assessment Scale‐Cognitive Subscale; ADCS‐ADL: Alzheimer's Disease Cooperative Study Activities of Daily Living; CDR‐SoB: Clinical Dementia Rating Scale Sum of Boxes | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aNumber given is number of participants randomised. Number of participants in each analysis varied with method of handling missing participant data.
bDowngraded for imprecision. Broad 95% CI.
cZ‐score.
See Table 1; Table 2; Table 3.
Incidence of Alzheimer's disease dementia in individuals with MCI/prodromal AD (primary review outcome)
Incidence of Alzheimer's disease dementia was only investigated in the LipiDiDiet study 2017, which was the only study in which participants did not have a diagnosis of dementia at baseline. Souvenaid for 24 months probably results in little or no difference in incidence of dementia. Of all randomised participants, 41% (62 of 153) were diagnosed with dementia in the Souvenaid group and 37% (59 out of 158) in the control group. The ITT analysis based on all randomised participants showed no difference between the groups (RR 1.09, 95% CI 0.82 to 1.43; 1 trial, 311 participants; Analysis 1.1). We considered this as moderate‐quality evidence, downgraded for imprecision.
1.1. Analysis.

Comparison 1: Souvenaid versus placebo in prodromal Alzheimer's disease, Outcome 1: Incidence of Alzheimer's dementia
Changes in global and specific cognitive functions (primary review outcomes)
MCI/prodromal AD
In the LipiDiDiet study 2017 a five‐component NTB z‐score (named "NTB primary endpoint z‐score" in the study) was used as the primary outcome in participants with prodromal AD. Table 7 shows the components of the individual subscores. At 24 months, the mean change from baseline in NTB primary endpoint z‐score (five components) (a higher score was better) was −0.028 (SD 0.453) in the Souvenaid group and −0.108 (SD 0.528) in the placebo group. Souvenaid probably results in little to no difference between the groups (MD 0.08, 95% CI −0.04 to 0.20; 1 trial, 275 participants) in our analysis based on available cases (Analysis 1.2). The linear mixed model (LME) analysis performed by the study authors showed no statistically significant benefit of Souvenaid in a 24‐month period, whether in a modified intention‐to‐treat analysis (LME: MD 0.10, 95% CI −0.04 to 0.24; P = 0.17; 311 participants) or in a per protocol analysis (LME: MD 0.14, 95% CI −0.02 to 0.30; P = 0.080; 295 participants). The results for the NTB total composite z‐score (16 components, Table 7) were similar ((MD 0.01, 95% CI −0.08 to 0.10; 274 participants; Analysis 1.3). We considered all analyses as moderate‐quality evidence, downgraded for imprecision.
1.2. Analysis.

Comparison 1: Souvenaid versus placebo in prodromal Alzheimer's disease, Outcome 2: Cognition: NTB primary endpoint z‐score (five components)
1.3. Analysis.

Comparison 1: Souvenaid versus placebo in prodromal Alzheimer's disease, Outcome 3: Cognition: NTB total composite z‐score (16 components)
At 24 months, the mean change from baseline in the NTB memory domain z‐score (three components, Table 7) was −0.003 (SD 0.569) in the Souvenaid group and −0.13 (SD 0.619) in the placebo group (a higher score was better). Souvenaid probably results in little to no difference between the groups (available cases) (MD 0.13, 95% CI −0.01 to 0.27; 1 trial, 274 participants; Analysis 1.4). This remained stable in the modified intention‐to‐treat analysis (LME: MD 0.14, 95% CI −0.03 to 0.30; P = 0.10; 311 participants) and per protocol analysis (LME: MD 0.18, 95% CI −0.005 to 0.37; P = 0.057; 295 participants) performed by the study authors (LipiDiDiet study 2017). We considered this as moderate‐quality evidence, downgraded once for imprecision.
1.4. Analysis.

Comparison 1: Souvenaid versus placebo in prodromal Alzheimer's disease, Outcome 4: Specific cognition: NTB memory domain z‐score (3 components)
At 24 months, the mean change from baseline in the NTB executive function domain z‐score (four components, Table 7) was −0.145 (SD 0.445) in the Souvenaid group and −0.039 (SD 0.506) in the placebo group (a higher score was better). Souvenaid probably results in little to no difference between the groups (MD −0.11, 95% CI −0.22 to 0.01; 1 trial, 274 participants; Analysis 1.5) (available cases). The models performed by the authors showed similar results in a modified ITT analysis (LME: MD z‐score −0.04, 95% CI −0.18 to 0.10) or in a per protocol analysis (LME: 0.01, 95% CI −0.14 to 0.16).We considered this as moderate‐quality evidence, downgraded once for imprecision.
1.5. Analysis.

Comparison 1: Souvenaid versus placebo in prodromal Alzheimer's disease, Outcome 5: Specific cognition: NTB executive function domain z‐score (4 components)
Mild AD dementia
Cognition was measured with the NTB in mild AD (Souvenir II study 2012). After 24 weeks, the mean change from baseline in the NTB total composite z‐score (12 components, Table 7) was 0.12 (SD 0.278) in the Souvenaid group and 0.035 (SD 0.286) in the placebo group (a higher score was better). Souvenaid probably results in little to no meaningful difference, depending on the statistical approach. There was some difference between the groups in favour of Souvenaid in our available‐case analysis (MD 0.08, 95% CI 0.00 to 0.17; 1 trial, 172 participants; Analysis 2.1), which was also observed when missing data were considered in the mixed model of repeated measure (MMRM) analysis performed by the study authors (P = 0.035; 259 participants), but not in the trajectories over 24 weeks (MMRM: P = 0.053; 259 participants). We considered this as moderate‐quality evidence, downgraded once for imprecision.
2.1. Analysis.

Comparison 2: Souvenaid versus placebo in mild dementia due to Alzheimer's disease, Outcome 1: Cognition: NTB total composite z‐score (twelve components)
Memory was assessed as the primary outcome in participants with mild AD (Souvenir II study 2012) by using the NTB memory function domain z‐score (five components, Table 7) (a higher score was better). After 24 weeks, the mean change from baseline in the Souvenaid group was 0.202 (SD 0.395) and 0.111 (SD 0.463) in the placebo group. Souvenaid probably results in little to no meaningful difference, depending on the statistical approach. There was no difference between the groups after 24 weeks (MD 0.09, 95% CI −0.03 to 0.21; 1 trial, 206 participants) when we calculated the mean difference based on available cases (Analysis 2.2). The result is similar to the analysis performed by the authors where the change from baseline at week 24 was calculated in a MMRM based on the ITT population (MMRM: P = 0.090; 259 participants). The authors also considered missing data for the differences in the trajectories over time and in a sensitivity analysis with multiple imputations (MIs). The difference in the trajectories over 24 weeks showed a significant difference in favour of Souvenaid in the NTB memory function domain z‐score (MMRM; P = 0.023; 259 participants). This result remained stable when the NTB total composite z‐score (12 components, Table 7) was calculated by multiple imputations when one or two NTB items were missing (P = 0.032) (Souvenir II study 2012). We considered this as moderate‐quality evidence, downgraded once for imprecision.
2.2. Analysis.

Comparison 2: Souvenaid versus placebo in mild dementia due to Alzheimer's disease, Outcome 2: Specific cognition: NTB memory function domain z‐score (five components)
At 24 weeks the mean change from baseline in NTB executive function domain z‐score (five components, Table 7) was 0.048 (SD 0.333) in the Souvenaid group and 0.006 (SD 0.323) in the placebo group (a higher score was better). Souvenaid probably results in little to no difference in executive function (specific cognitive function) on the NTB executive function domain z‐score (MD 0.04, 95% CI −0.05 to 0.13; 1 trial, 192 participants; Analysis 2.3) (available‐case analysis). The models based on the ITT population performed by the authors showed similar results for the change from baseline after 24 weeks (MMRM: P = 0.386; 259 participants) and in the trajectories over 24 weeks (MMRM: P = 0.69; 259 participants) (Souvenir II study 2012). We considered this as moderate‐quality evidence, downgraded once for imprecision.
2.3. Analysis.

Comparison 2: Souvenaid versus placebo in mild dementia due to Alzheimer's disease, Outcome 3: Specific cognition: NTB executive function domain z‐score (five components)
Mild‐to‐moderate AD dementia
The S‐Connect study 2013 assessed cognitive function in people with mild‐to‐moderate AD dementia using the ADAS‐cog as the primary outcome (lower score is better, score range 0 ‐ 70). After 24 weeks, the mean score in the Souvenaid group was 25.44 (SD 11.56) and the mean score in the placebo group was 24.42 (SD 10.95). Souvenaid probably results in little to no difference in cognition. Our available‐case analysis (MD 1.02, 95% CI −1.11 to 3.15; 1 trial, 428 participants; Analysis 3.1) and the ITT analysis performed by the authors (MMRM: MD 0.37; P = 0.51; 527 participants) showed no statistically significant difference between the groups. At 24 weeks, the mean global cognitive function composite z‐score (four components, Table 7) was 0.09 (SD 0.74) in the Souvenaid group and 0.01 (SD 0.71) in the placebo group (a higher score was better). In the available‐case analysis, there was no difference between the groups for cognition on global cognitive function composite z‐score (four components, Table 7) (z‐score) (MD 0.08, 95% CI −0.07 to 0.23; 1 trial, 364 participants). The ITT analysis also showed no difference (MMRM: P = 0.323; 527 participants). We considered both analyses as moderate‐quality evidence, downgraded once for imprecision.
3.1. Analysis.

Comparison 3: Souvenaid versus placebo in mild to moderate dementia due to Alzheimer's disease, Outcome 1: Cognition: ADAS‐Cog
Changes in functional outcome measures (e.g. activities of daily living) (primary review outcome)
MCI/prodromal AD
We found no evidence in this population.
Mild AD dementia
In the Souvenir II study 2012 functional ability was measured on the DAD scale (no means presented) in participants with mild AD. The authors report no difference between the groups after 24 weeks (Mann‐Whitney U test: P = 0.361; N of participants unclear). We downgraded the evidence once for imprecision.
Mild‐to‐moderate AD dementia
Souvenaid probably results in little to no difference in activities of daily living. Daily‐living function was measured with the ADCS‐ADL (a higher score was better, score range 0 ‐ 78) in mild‐to‐moderate AD. After 24 weeks, the mean in the Souvenaid group was 54.66 (SD 15.56) and the mean in the placebo group was 54.15 (15.91). There was no evidence for a difference between the groups in our available‐case analysis (MD 0.51, 95% CI −2.40 to 3.42; 1 trial, 451 participants) (Analysis 3.3) and in an ITT analysis performed by the study authors (MMRM: P = 0.77; 527 participants; S‐Connect study 2013). We considered this as moderate‐quality evidence, downgraded once for imprecision.
3.3. Analysis.

Comparison 3: Souvenaid versus placebo in mild to moderate dementia due to Alzheimer's disease, Outcome 3: Functional outcomes: ADCS‐ADL
Combined cognitive‐functional outcome measure (primary outcome)
Combined cognition‐function was measured with the CDR‐SoB in two of the studies as a secondary outcome.
MCI/prodromal AD
The LipiDiDiet study 2017 used the CDR‐SoB (a higher score was worse, score range 0 ‐ 18) as a secondary outcome. In this trial Souvenaid may slightly improve overall cognitive‐functional performance. After 24 months, the mean change from baseline was 0.56 (SD 1.32) in the Souvenaid group and 1.12 (SD 1.72) in the placebo group. The difference was statistically significant in favour of Souvenaid. This effect was stable in our available‐case analysis (MD −0.56, 95% CI −0.95 to −0.17; 1 trial, 230 participants; Analysis 1.6), in a modified ITT analysis (LME: MD −0.60, 95% CI −1.01 to −0.19; P = 0.005; 311 participants) and in a per protocol analysis (LME: −0.72, 95% CI −1.16 to −0.28; P = 0.002; 295 participants), both published by the study authors. We consider this as moderate‐quality evidence, downgrade once for imprecision.
1.6. Analysis.

Comparison 1: Souvenaid versus placebo in prodromal Alzheimer's disease, Outcome 6: Combined cognitive‐function: CDR‐SoB
Mild AD dementia
We found no evidence for Souvenaid in this population.
Mild‐to‐moderate AD dementia
The S‐Connect study 2013 measured this combined outcome with the CDR‐SoB (a higher score was worse, score range 0 ‐ 18) after 24 weeks, and observed a mean of 6.89 (SD 3.35) in the Souvenaid group and 7.01 (SD 3.419) in the placebo group. Souvenaid probably results in little to no difference between the groups. There was no statistically significant difference between the groups in our available‐case analysis (MD −0.12, 95% CI −0.74 to 0.50; 1 trial, 450 participant; Analysis 3.4) and when missing data were considered in an ITT analysis (MMRM: P = 0.50; 527 participants; S‐Connect study 2013) performed by the study authors. We considered this as moderate‐quality evidence, downgraded once for imprecision.
3.4. Analysis.

Comparison 3: Souvenaid versus placebo in mild to moderate dementia due to Alzheimer's disease, Outcome 4: Combined cognitive‐functional outcome: CDR‐SoB
Adverse events and adverse effects (primary review outcome)
The European Medicines Agency (EMA) recommends an on‐treatment follow‐up of at least six months after short‐term trials to demonstrate safety (EMA 2018). With a study duration of 24 weeks, the S‐Connect study 2013 and the Souvenir II study 2012 did not fully meet this requirement. The LipiDiDiet study 2017 had a treatment duration of 24 months.
All included studies describe the intervention as well‐tolerated. Compliance with the study product seemed to be high (LipiDiDiet study 2017: 93.4%; S‐Connect study 2013: 94.1%; Souvenir II study 2012: 97.1%). The following results are all reported in an 'as treated' population.
For adverse events and adverse effects, we recorded under general safety the number of participants who had at least one (serious) adverse event. Some study participants had more than one adverse event. It was not possible to show which adverse events occurred together and whether there were frequent combinations.
All but one of the serious adverse events were considered to be unrelated to the use of the study product. The one serious adverse event (confusion) reported in the S‐Connect study 2013 that was considered to be possibly related to the use of the study product was in the placebo group. In the terminology of the PRISMA harms checklist (Zorzela 2016) and the Cochrane Handbook (Peryer 2020), we consider this as a serious adverse effect.
None of the studies reported full causality assessment according to the PRISMA harms recommendations (Zorzela 2016).
MCI/prodromal AD
None of the serious adverse events in the LipiDiDiet study 2017 were regarded as related to the study products. Some of the adverse events which contributed to study discontinuation seemed to be related to study product (adverse effects). This related to five participants in the active group (two with eczema, one each with abdominal pain upper, regurgitation, or lactose intolerance) and four participants in the control group (one each with diarrhoea, hypersensitivity, urticaria, or lactose intolerance and weight gain).
Adverse effects which did not lead to study discontinuation were not explicitly identified as such. The project co‐ordinator informed us that if they had noticed adverse events which had been traceable to the intervention they would have reported them.
In the LipiDiDiet study 2017 the safety analysis is also presented for all participants who received at least one dose of the study product. The proportion of participants affected by at least one adverse event during the 24 months was nearly equal in the Souvenaid group (132/152; 86.4%) and the placebo group (138/157; 87.9%) (RR 0.99, 95% CI 0.91 to 1.08; 309 participants). The frequency of at least one serious adverse event showed only small differences between the Souvenaid group (34/152; 22.4%) and the placebo group (30/157; 19.1%) (RR 1.17, 95% CI 0.76 to 1.81; 309 participants; Analysis 1.7). We downgraded the evidence once for imprecision.
1.7. Analysis.

Comparison 1: Souvenaid versus placebo in prodromal Alzheimer's disease, Outcome 7: General safety
The overall dropout rate was equally distributed between the Souvenaid group (21.7%) and control group (21.0%) (RR 1.03, 95% CI 0.67 to 1.58; 309 participants). The dropout rates due to adverse events (RR 1.55, 95% CI 0.57 to 4.25; 309 participants) and serious adverse events (RR 2.07, 95% CI 0.38 to 11.11; 309 participants) were also similar, but the very wide confidence intervals indicate the imprecision of the results (Analysis 1.7). The LipiDiDiet study 2017 reported two hospital admissions within the study period of 24 months, but there were serious adverse events (e.g. cardiac operation) that would have led to additional hospital admissions beyond those reported. The LipiDiDiet study 2017 had five deaths within 24 months, four in the Souvenaid group (one each due to infection and bronchial carcinoma, and two due to respiratory failure) and one in the placebo group (sudden death). The deaths were probably not due to the experimental or control interventions.The details of the adverse events of the LipiDiDiet study 2017 are presented in Table 8.
5. Detailed adverse events in people with prodromal Alzheimer's Disease (24‐month follow‐up, AST analysis).
| Outcome | intervention group n = 152 | control group n = 157 | RR and 95% CI |
| Specific safety: most common serious adverse events | |||
| Myocardial infarction | 0 | 2 | 0.21 (0.01 to 4.27) |
| Fall | 2 | 1 | 2.07 (0.19 to 22.55) |
| Intervertebral disc protrusion | 0 | 2 | 0.21 (0.01 to 4.27) |
| Osteoarthritis | 0 | 3 | 0.15 (0.01 to 2.83) |
| Syncope | 3 | 0 | 7.23 (0.38 to 138.78) |
| (Major) depression | 1 | 3 | 0.34 (0.04 to 3.27) |
| Cardiac operation | 0 | 2 | 0.21 (0.01 to 4.27) |
| Hospitalisation | 2 | 0 | 5.16 (0.25 to 106.68) |
| Circulatory collapse | 2 | 0 | 5.16 (0.25 to 106.68) |
| Specific safety: most common adverse events | |||
| Vertigo | 6 | 12 | 0.52 (0.20 to 1.34) |
| Diarrhoea | 7 | 14 | 0.52 (0.21 to 1.24) |
| Cystitis | 4 | 9 | 0.46 (0.14 to 1.46) |
| Nasopharyngitis | 7 | 16 | 0.45 (0.19 to 1.07) |
| Respiratory tract infection | 7 | 9 | 0.80 (0.31 to 2.10) |
| Urinary tract infection | 7 | 9 | 0.80 (0.31 to 2.10) |
| Fall | 11 | 8 | 1.42 (0.59 to 3.43) |
| Arthralgia | 4 | 9 | 0.46 (0.14 to 1.46) |
| Back pain | 10 | 5 | 2.07 (0.72 to 5.90) |
| Headache | 9 | 12 | 0.77 (0.34 to 1.79) |
| Cough | 2 | 10 | 0.21 (0.05 to 0.93) |
Mild AD dementia
In the Souvenir II study 2012 84.1% of the reported adverse events were assessed as being "not related" or "unlikely to be related" to the intervention (82.5% active group; 85.4% control group). Further details of the remaining 15.9% adverse events, which may be related to the interventions and therefore would be adverse effects, are not reported.
Regarding general safety, the Souvenir II study 2012 showed the following results in the participants who received at least one unit of the study product (all participants, treated population): The proportion of the participants affected by at least one adverse event during 24 weeks was lower in the Souvenaid group (67/129; 51.9%) than in the in the placebo group (78/129; 60.5%) (RR 0.86, 95% CI 0.69 to 1.07; 258 participants). Serious adverse events were higher in the Souvenaid group (10/129; 7.8%) than in the placebo group (6/129; 4.7%) (RR 1.67, 95% CI 0.62 to 4.45; 258 participants). The overall dropout rate was also a little higher in the Souvenaid group (RR 1.32, 95% CI 0.58 to 3.03; 259 participants). Few participants terminated the study due to adverse events or serious adverse events (2.3% in the intervention group and 1.65% in the placebo group) (RR 1.49, 95% CI 0.25 to 8.76; 259 participants; Analysis 2.4). Data on hospital admissions were not provided. There were no deaths during the 24 weeks of the study (Souvenir II study 2012).
2.4. Analysis.

Comparison 2: Souvenaid versus placebo in mild dementia due to Alzheimer's disease, Outcome 4: General safety (follow up 24 weeks, all subjects treated population)
None of the effects described in the Souvenir II study 2012 reached statistical significance. We consider the evidence for adverse events as moderate, downgraded once for imprecision. We present detailed adverse events in Table 9.
6. Detailed adverse events in people with mild dementia due to Alzheimer's Disease (24‐week follow‐up, AST analysis).
| Adverse event | intervention group n = 129 | control group n = 129 | RR and 95% CI |
| body as a whole | 11 | 20 | 0.51 (0.23 to 1.11) |
| central and peripheral nervous system disorders | 11 | 18 | 0.57 (0.26 to 1.27) |
| gastro‐intestinal system disorders | 22 | 30 | 0.68 (0.37 to 1.25) |
| metabolic and nutritional disorders | 13 | 9 | 1.49 (0.62 to 3.63) |
| musculo‐skeletal system disorders | 10 | 9 | 1.12 (0.44 to 2.86) |
| psychiatric disorders | 15 | 16 | 0.93 (0.44 to 1.97) |
| respiratory system disorders | 10 | 15 | 0.64 (0.28 to 1.48) |
| skin and appendages disorders | 4 | 10 | 0.38 (0.12 to 1.25) |
| other (e.g. falls to surgical intervention) | 8 | 8 | 1.00 (0.36 to 2.75) |
Mild‐to‐moderate AD dementia
in the S‐Connect study 2013 there are no details on adverse events that might be related to the intervention (i.e. on adverse effects).
Regarding general safety, the S‐Connect study 2013 showed the following results in the participants who received at least one unit of the study product (all participants, treated population): The proportion of the participants affected by at least one adverse event during 24 weeks was lower in the Souvenaid group (150/264; 56.8%) than in the in the placebo group (165/260; 63.5%) (RR 0.90, 95% CI 0.78 to 1.03; 1 trial, 524 participants). Serious adverse events were fewer in the Souvenaid group (26/264; 9.8%) than in the placebo group (34/260; 13.1%) (RR 0.78, 95% CI 0.49 to 1.26; 524 participants). The overall dropout rate was similarly distributed in both groups (RR 0.94, 95% CI 0.62 to 1.42; 527 participants). Few participants terminated the study due to adverse events or serious adverse events. The relative effect in favour of the intervention group appears at first sight to be large, but it is based on small absolute numbers (2/265.in the intervention group and 4/262 in the placebo group) (RR 0.49, 95% CI 0.09 to 2.68; 527 participants; Analysis 3.5). Data on hospital admissions were not provided. One death due to malignant mesothelioma in the control group was reported in the S‐Connect study 2013.
3.5. Analysis.

Comparison 3: Souvenaid versus placebo in mild to moderate dementia due to Alzheimer's disease, Outcome 5: General safety (follow up 24 weeks, all subjects treated population)
None of the effects described in the S‐Connect study 2013 reached statistical significance. We consider the evidence for adverse events as moderate, downgraded once for imprecision. We present detailed adverse events in Table 10.
7. Detailed adverse events in people with mild‐to‐moderate dementia due to Alheimer's Disease (24‐week follow‐up, AST analysis).
| Adverse event | intervention group n = 264 | control group n = 260 | RR and 95% CI |
| body as a whole | 24 | 33 | 0.69 (0.39 to 1.20) |
| central and peripheral nervous system disorders | 27 | 21 | 1.30 (0.71 to 2.36) |
| gastro‐intestinal system disorders | 41 | 38 | 1.07 (0.67 to 1.73) |
| metabolic and nutritional disorders | 19 | 19 | 0.98 (0.51 to 1.90) |
| musculo‐skeletal system disorders | 24 | 15 | 1.63 (0.84 to 3.19) |
| psychiatric disorders | 32 | 43 | 0.70 (0.42 to 1.14) |
| respiratory system disorders | 50 | 42 | 1.21 (0.77 to 1.90) |
| skin and appendages disorders | 8 | 18 | 0.42 (0.18 to 0.98) |
| urinary system disorders | 25 | 19 | 1.33 (0.71 to 2.47) |
| other (e.g. falls to surgical intervention) | 20 | 27 | 0.71 (0.39 to 1.30) |
In total, the number of body system‐specific adverse events that occurred in the 24 months of the LipiDiDiet study 2017 were mostly lower than the event rate in the two shorter studies with 24 weeks follow‐up.
Non‐cognitive symptoms associated with dementia and quality of life (secondary review outcomes)
None of the studies assessed either non‐cognitive symptoms associated with dementia or quality of life.
Discussion
Summary of main results
We included three RCTs which investigated Souvenaid in 1097 participants at different stages of AD. The progression from MCI (prodromal AD) to dementia due to AD was measured in only one trial, which lasted over a period of 24 months. All of the studies measured cognitive function. The two RCTs which included participants with very mild or mild‐to‐moderate AD dementia also measured everyday function, whereas combined cognition‐function was only measured in prodromal AD and mild‐to‐moderate AD dementia. All studies reported adverse events.
We found no convincing evidence of a benefit for Souvenaid on any of our primary or secondary outcomes, including moderate‐quality evidence that Souvenaid does not affect incidence of dementia over two years in participants with prodromal AD at baseline.
The evidence for combined cognitive‐functional outcomes is conflicting. The largest trial showed a small benefit in participants with prodromal AD. The difference proved stable under several statistical assumptions for missing participant data. However, taking recently‐published MCID estimates for CDR‐SoB in this population (Andrews 2019) into account, the difference is not clinically meaningful. There was little to no difference in participants with mild‐to‐moderate AD dementia.
Adverse effects observed were low in all trials. There were a number of adverse events, but to make a connection with Souvenaid or to reject it would put too much strain on the available data. We downgraded the evidence for imprecision because of wide confidence intervals.
Quality of life or other secondary review outcomes were not reported for any of the trials.
Overall completeness and applicability of evidence
The trials investigated Souvenaid in an appropriate dose (according to the recommendation of the manufacturer). All trials included community‐dwelling participants with prodromal AD, very mild and mild‐to‐moderate AD dementia who seem to accurately represent the population seen in clinical practice. According to the nutritional parameters reported, we further assumed that the participants were at low risk of nutritional deficiencies. Based on the available data, it was not possible to investigate Souvenaid in subgroups with nutritional deficiencies. We therefore cannot rule out that people with AD and poorer baseline nutritional status may benefit from Souvenaid. Nor can we draw any conclusions about its effects in people with advanced dementia.
All trials investigated relevant endpoints for people with AD, as recommended by an expert group of the European Medicines Agency (EMA 2018). They assessed cognition with different test batteries which may affect the comparability. It is assumed that these tests are sensitive enough to detect changes in people at the milder end of the disease spectrum, but so far there is no consensus about the validity of these tests for this purpose. However, all trials also assessed biomarkers, which we have not included since the utility of these is also still under debate (EMA 2018). Data permitting, we would have performed several meta‐analyses at endpoints reported in the LipiDiDiet study 2017 and the Souvenir II study 2012. We also planned several sensitivity analyses to test this approach. This plan followed the recommendation of the EMA and we think that from a clinical point of view, these populations are similar enough to be investigated together. Apart from the diagnoses, both studies included drug‐naïve participants with similar MMSE scores. We specified in our protocol that we would pool data from comparable time points if outcomes were measured at more than one time point. However, we were not able to get six‐month data which we could use for a meta‐analysis. Both the sponsor Nutricia and the authors of LipiDiDiet study 2017 declined to provide the data, with the argument that no meaningful scientific hypothesis exists for pooling intervention effects in these populations at this time point. Both lines of argument might be reasonable. There are scientific uncertainties in the field of AD about the optimal duration of follow‐up for a trial and the demarcation of disease stages in this population (EMA 2018).
Quality of the evidence
We assessed all three RCTs as being at low risk of bias in all domains according to the Cochrane 'Risk of bias' tool. By using the GRADE approach, we rated the overall quality of evidence for all outcomes as moderate. However, the evidence is based on single RCTs with some conflicting results.
Potential biases in the review process
We prespecified inclusion of domain‐specific cognitive outcomes without defining an explicit hierarchical order.
We excluded one RCT due to a short‐term follow‐up period of only 12 weeks. This study (Souvenir I study 2010) observed some beneficial effects. However, we do not see that the inclusion of this trial would have changed our overall conclusions. Two of the included studies were industry‐funded. There were too few studies to use the funnel‐plot method to look for indications of unpublished studies, so we cannot rule out this possibility.
We did not include biomarker measures, due to uncertain diagnostic validity in the field of dementia. We found no convincing evidence on patient‐relevant dementia measures and decided not to compensate for this lack of evidence with biomarkers. We might change this approach in the future, if and when they are recommended as primary outcomes in dementia trials. We assessed beneficial and adverse effects in the same review and did not conduct a special search for studies assessing the unintended effects of the product. In so doing, we have taken the adverse events from our included studies, which are very low. Including data from non‐randomised trials or other populations might have changed the numbers but probably not the certainty of the evidence.
Agreements and disagreements with other studies or reviews
A systematic review by Onakpoya 2017 investigated Souvenaid in Alzheimer's disease. Their search strategy, inclusion criteria and methods for meta‐analysis differed slightly from ours. The authors included the trial with a follow‐up of 12 weeks. They have further conducted meta‐analyses by combining different measures. None of the meta‐analyses showed a difference between Souvenaid and the placebo. Altogether, the review's findings are in line with ours. To our knowledge, no systematic review included the recent LipiDiDiet study 2017, which investigated the incidence of AD dementia as a secondary outcome.
Authors' conclusions
Implications for practice.
Currently, there is no evidence that Souvenaid is useful for preventing progression from prodromal AD to dementia. Furthermore, we found no convincing evidence for the efficacy of Souvenaid in the treatment of symptoms of dementia due to Alzheimer’s disease. These results were based on moderate‐quality evidence. There are advantages for some outcomes, especially in early stages of the disease, but they are small and not consistent across all outcomes. Adverse effects and adverse events of Souvenaid seem to be uncommon. The evidence is drawn from three studies and data were insufficient for meta‐analysis, so that our ability to draw conclusions about any effects is limited. The effects on more severe AD dementia remain unknown.
Implications for research.
One study reported some positive results for memory in very mild AD dementia, but we did not replicate this finding using a different statistical approach. Another reported significant beneficial effects in a combined measure of cognition and function (CDR‐SoB) in participants with prodromal AD; this result was robust to different statistical models. These results should be replicated and investigated further, especially in participants at the very mild end of the AD continuum.
Souvenaid is a nutritional intervention. Future trials should investigate people with nutritional deficiencies or provide prespecified subgroup analyses for people at risk of malnutrition. From a review author's and a reader's perspective, it would be helpful if similar instruments were used to measure patient‐relevant outcomes. With regard to further study design features, we refer to current recommendations on the clinical investigation of medicines in AD (i.e. EMA 2018; FDA 2018), which we think can also guide trials investigating nutritional supplements.
History
Protocol first published: Issue 5, 2015 Review first published: Issue 12, 2020
Acknowledgements
We acknowledge the work of the Cochrane Dementia and Cognitive Improvement Group (CDCIG) Information Specialists, Anna Noel‐Storr who designed the search strategy and Candida Fenton who provided the search results. We also acknowledge Jenny McCleery (Co‐ordinating Editor of the CDCIG) for her helpful editing and her valuable methodological comments. We also thank the peer reviewers Janice M. Ranson and Caryl Nowson for their thorough peer review, and consumer reviewer Catherine Hofstetter who gave us valuable feedback. Thanks also to Sue Marcus (Managing Editor of CDCIG) for her excellent support. We gratefully acknowledge Hanna Mass for her thorough proofreading and editing as a native speaker.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) [date of latest search: 24 June 2020] |
Souvenaid OR Fortasyn | Feb 2016: 7 Jan 2017: 0 Mar 2018: 0 May 2019: 0 June 2020: 2 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) [date of latest search: 24 June 2020] |
1. dement*.ti,ab. 2. exp *Dementia/ 3. alzheimer*.ti,ab. 4. (AD or "ADD").ti. 5. 1 or 2 or 3 or 4 6. souvenaid.ti,ab. 7. "Fortasyn Connect*".ti,ab. 8. ("medical food" and memory).ti,ab. 9. 6 or 7 or 8 10. 5 and 9 |
Feb 2016: 24 Jan 2017: 2 Mar 2018: 5 May 2019: 2 June 2020: 4 |
| 3. EMBASE 1974 ‐ present [date of latest search: 24 June 2020] |
1. dement*.ti,ab. 2. exp *Dementia/ 3. alzheimer*.ti,ab. 4. (AD or "ADD").ti. 5. 1 or 2 or 3 or 4 6. souvenaid.ti,ab. 7. "Fortasyn Connect*".ti,ab. 8. ("medical food" and memory).ti,ab. 9. 6 or 7 or 8 10. 5 and 9 |
Feb 2016: 104 Jan 2016: 9 Mar 2018: 25 May 2019: 9 June 2020: 14 |
| 4. PSYCINFO 1967 ‐ present [date of latest search: 24 June 2020] |
1. dement*.ti,ab. 2. exp *Dementia/ 3. alzheimer*.ti,ab. 4. (AD or "ADD").ti. 5. 1 or 2 or 3 or 4 6. souvenaid.ti,ab. 7. "Fortasyn Connect*".ti,ab. 8. ("medical food" and memory).ti,ab. 9. 6 or 7 or 8 10. 5 and 9 |
Feb 2016: 10 Jan 2017: 1 Mar 2018: 3 May 2019: 5 June 2020: 1 |
| 5. CINAHL (EBSCOhost) [date of latest search: 24 June 2020] |
S1. TX dement* OR SU Dementia OR TX alzheimer* OR TI (AD or "ADD") S2. TX souvenaid OR TX "Fortasyn Connect*" OR TX ("medical food" and memory) S3. (S1 AND S2) |
Feb 2016: 2 Jan 2017: 0 Mar 2018: 0 May 2019: 3 June 2020: 2 |
| 6. ISI Web of Science – all databases [includes: Web of Science (1945‐present); BIOSIS Previews (1926‐present); MEDLINE (1950‐present); Journal Citation Reports] [date of latest search: 24 June 2020] |
(dement* OR alzheimer* OR AD OR ADD) AND TOPIC: (souvenaid OR "Fortasyn Connect*" OR ("medical food" and memory)) Timespan=All years Search language=Auto |
Feb 2016: 50 Jan 2017: 6 Mar 2018: 12 May 2019: 6 June 2020: 8 |
| 7. LILACS (BIREME) [date of latest search: 24 June 2020] |
dement* OR alzheimer* OR AD OR ADD [Words] and souvenaid OR "Fortasyn Connect*" OR "medical food" [Words] | Feb 2016: 0 Jan2017: 0 Mar 2018: 0 May 2019: 0 June 2020: 0 |
| 8. CENTRAL (The Cochrane Library) http://crso.cochrane.org/SearchSimple.php [date of latest search: 24 June 2020] |
#1 Dementia:MH #2 dement*:TI,AB,KY #3 alzheimer:MH #4 alzheimer*:TI,AB,KY #5 (AD or "ADD"):TI,AB,KY #6 #1 OR #2 OR #3 OR #4 OR #5 #7 souvenaid:TI,AB,KY #8 ("Fortasyn Connect*"):TI,AB,KY #9 ("medical food" and memory):TI,AB,KY #10 #7 OR #8 OR #9 #11 #6 AND #10 |
Feb 2016: 25 Jan 2016: 5 Mar 2018: 9 May 2019: 15 June 2020: 14 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) [date of latest search: 24 June 2020] |
Advanved search: [Condition: dementia OR "mild cognitive impairment" OR MCI OR alzheimers OR Alzheimer] AND [Intervention: souvenaid OR fortasyn] No date restrictions No trial status restrictions |
Feb 2016: 0 Jan 2017: 0 Mar 2018: 0 May 2019: 0 June 2020: |
| 10. ICTRP Search Portal (http://apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] [date of latest search: 10 May 2019. n.b. database unavailable 24 June 2020] |
dement* OR alzheimer* OR AD OR ADD in the Condition And souvenaid OR Fortasyn in the Intervention |
Feb 2016: 7 Jan 2017: 0 Mar 2018: 1 May 2019: 0 |
| TOTAL before de‐duplication | Feb 2016: 229 Jan 2017: 23 Mar 2018: 55 May 2019: 39 June 2020: 45 TOTAL: 391 |
|
| TOTAL after de‐duplication | Feb 2016: 187 Jan 2017: 12 Mar 2018: 23 May 2019: 31 June 2020: 30 TOTAL: 283 |
|
Data and analyses
Comparison 1. Souvenaid versus placebo in prodromal Alzheimer's disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Incidence of Alzheimer's dementia | 1 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.82, 1.43] |
| 1.2 Cognition: NTB primary endpoint z‐score (five components) | 1 | 275 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.04, 0.20] |
| 1.3 Cognition: NTB total composite z‐score (16 components) | 1 | 274 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.08, 0.10] |
| 1.4 Specific cognition: NTB memory domain z‐score (3 components) | 1 | 274 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.01, 0.27] |
| 1.5 Specific cognition: NTB executive function domain z‐score (4 components) | 1 | 274 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.22, 0.01] |
| 1.6 Combined cognitive‐function: CDR‐SoB | 1 | 230 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐0.95, ‐0.17] |
| 1.7 General safety | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 Any adverse events | 1 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.08] |
| 1.7.2 Any serious adverse events | 1 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.76, 1.81] |
| 1.7.3 Dropout overall | 1 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.67, 1.58] |
| 1.7.4 Dropout due to adverse events | 1 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.57, 4.25] |
| 1.7.5 Dropout due to serious adverse events | 1 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.38, 11.11] |
Comparison 2. Souvenaid versus placebo in mild dementia due to Alzheimer's disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Cognition: NTB total composite z‐score (twelve components) | 1 | 172 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [0.00, 0.17] |
| 2.2 Specific cognition: NTB memory function domain z‐score (five components) | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.03, 0.21] |
| 2.3 Specific cognition: NTB executive function domain z‐score (five components) | 1 | 192 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.05, 0.13] |
| 2.4 General safety (follow up 24 weeks, all subjects treated population) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.4.1 Any adverse event | 1 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.07] |
| 2.4.2 Any serious adverse event | 1 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.62, 4.45] |
| 2.4.3 Dropout overall | 1 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.58, 3.03] |
| 2.4.4 Dropout due to (serious) adverse events | 1 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.25, 8.76] |
Comparison 3. Souvenaid versus placebo in mild to moderate dementia due to Alzheimer's disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Cognition: ADAS‐Cog | 1 | 428 | Mean Difference (IV, Fixed, 95% CI) | 1.02 [‐1.11, 3.15] |
| 3.2 Cognition: Global cognitive function composite score z‐score (4 components) | 1 | 364 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.07, 0.23] |
| 3.3 Functional outcomes: ADCS‐ADL | 1 | 451 | Mean Difference (IV, Fixed, 95% CI) | 0.51 [‐2.40, 3.42] |
| 3.4 Combined cognitive‐functional outcome: CDR‐SoB | 1 | 450 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.74, 0.50] |
| 3.5 General safety (follow up 24 weeks, all subjects treated population) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.5.1 Any adverse event | 1 | 524 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.78, 1.03] |
| 3.5.2 Any serious adverse event | 1 | 524 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.49, 1.26] |
| 3.5.3 Dropout overall | 1 | 527 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.62, 1.42] |
| 3.5.4 Dropout due to (serious) adverse events | 1 | 527 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.68] |
3.2. Analysis.

Comparison 3: Souvenaid versus placebo in mild to moderate dementia due to Alzheimer's disease, Outcome 2: Cognition: Global cognitive function composite score z‐score (4 components)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
LipiDiDiet study 2017.
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled, multicentre trial Trial duration from April 2009 to June 2015 |
|
| Participants | Countries: Finland, Germany, the Netherlands, and Sweden Diagnosis: prodromal Alzheimer’s disease (mean MMSE 26) Follow‐up: 24 months Inclusion criteria: age 55 – 85 years; fulfilled criteria for prodromal Alzheimer’s disease as defined by a) disorder of episodic memory, and b) evidence for underlying Alzheimer's disease pathology (Dubois 2007); MMSE score > 24 or ≥ 20 if education level ≤ 6 years; use of non‐prescription/prescription cognitive enhancers (e.g. ginkgo) and statins on a stable dose for at least 3 months prior to baseline; and availability of a responsible caregiver Exclusion criteria: participants with dementia according to DSM IV; historical use of donepezil, rivastigmine, galantamine, or memantine, use of omega‐3 preparations, alcohol or drug abuse, major depressive disorders (DSMIV) or other concomitant serious conditions, intake of vitamins B6, B12, folic acid, vitamin C, or vitamin E of more than 200% of the recommended daily intake; MRI or CT scan consistent with a diagnosis of stroke, intracranial bleeding, mass lesion, or normal pressure hydrocephalus (minimal white matter changes and up to 2 lacunar infarcts judged to be clinically insignificant were allowed). Participants who progressed to dementia during the trial could remain in the trial and start approved Alzheimer’s disease medication, according to the clinician’s judgment (switching to Souvenaid was allowed) Setting: participants were recruited from memory clinics and had routine assessments in the year before screening (not further described) Total number of participants randomised: n = 311 (n = 153 Souvenaid group / n = 158 control group) Per protocol population (24 months): n = 295 (n = 142 Souvenaid group / n = 153 control group) Baseline characteristics:
|
|
| Interventions | Intervention 1: Souvenaid 125 ml drink/once daily for 24 months which contains Fortasyn Connect™ (DHA, EPA, phospholipids, choline, UMP, vitamin B12, B6, and folate, vitamins C and E, and selenium) (see Table 4) Intervention 2: Isocaloric drink 125 ml/once daily for 24 months, similar in appearance and flavours (vanilla and strawberry) |
|
| Outcomes | Primary (24 months):
Secondary (24 months):
Safety and tolerability Surrogate parameter (i.e. MRI assessed are not included in this review) |
|
| Methods of AE Assessment |
|
|
| Notes | Funding and methods used to control bias resulting from conflict of interest: see Table 5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
LipiDiDiet study 2017: Quote: "Eligible participants were randomly assigned (1:1) to receive either the active or control product once daily according to a randomisation list, which was computer generated by Nutricia Research, stratified by site, and in block sizes of four." p. 967 |
| Allocation concealment (selection bias) | Low risk |
LipiDiDiet study 2017: Quote: "Sealed opaque envelopes were available for each participant. After acceptance of a participant to the trial, the envelope with the lowest unused number was opened at the site, containing the code for that participant." p.967 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
LipiDiDiet study 2017: Quote: "The active and control products were isocaloric and similar in appearance and flavours (vanilla and strawberry). All study personnel and participants, including the investigators and study‐site staff, were masked to treatment assignment." p.967 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Blinding of efficacy outcomes: appropriate for 24 months data Blinding of safety and efficacy interims outcomes: high risk LipiDiDiet study 2017: Quote: "All study personnel and participants, including the investigators and study‐site staff, were masked to treatment assignment. Only the trial‐independent statistician and the independent data monitoring committee, who reviewed interim data for safety and efficacy purposes, were partially unmasked.” p.967 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis and sensitivity analysis considering missing data not at random provided |
| Selective reporting (reporting bias) | Low risk | Secondary outcomes for MMSE, ADAS‐Cog, MADRS and Alzheimer’s Disease Cooperative Study‐Activities of Daily Living (ADCS‐ADL) were planned in trials registry the study protocol was changed in the same month of trial completion (June 2015) but "prior to database lock" (supplement p.5 to 6). The secondary outcomes were reduced and re‐categorised which was justified by new AD research and respective references |
| Other bias | Low risk | |
S‐Connect study 2013.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled trial Trial duration from March 2009 to March 2011 | |
| Participants | Country: USA Diagnosis: mild‐to‐moderate Alzheimer’s disease (mean MMSE 20) Follow‐up: 24 weeks Inclusion criteria: age ≥ 50 years; diagnosis of probable AD (NINCDS‐ADRA); MMSE score between 14 and 24 inclusive; use of US Food and Drug Administration‐approved AD medication on a stable dose for at least 4 months prior to baseline; and availability of a responsible study partner Exclusion criteria: diagnosis of a neurological/psychiatric disease significantly contributing to cognitive difficulties other than AD; depression; recent use of potent anticholinergic agents, antipsychotics, omega‐3 fatty acid‐containing supplements and/or oily fish consumption more than twice a week, high‐energy or high‐protein nutritional supplements or medical foods, vitamins B, C and/or E containing supplements at > 100% of daily value, or other investigational products; recent change in lipid‐lowering medications, antidepressants, or antihypertensives; alcohol or drug abuse in the opinion of the investigator; or institutionalisation in a nursing home Setting: participants were recruited from community or clinic setting but not institutionalised in a nursing home Total number of participants: n = 527 (n = 265 Souvenaid group / n = 262 control group) Per protocol population: n = 451 (n = 228 Souvenaid group / n = 223 control group) Baseline characteristics:
|
|
| Interventions | Intervention 1: Souvenaid 125 mL drink/once daily for 24 weeks which contains Fortasyn Connect™ (DHA, EPA, phospholipids, choline, UMP, vitamin B12, B6, and folate, vitamins C and E, and selenium) (see Table 4). Participants chose vanilla or strawberry flavours based on personal taste preferences Intervention 2: Iso‐caloric control product 125 ml/once daily for 24 weeks. Product lacked Fortasyn Connect™, but was otherwise identical to the active product with identical taste profile and appearance. Participants chose vanilla or strawberry flavours based on personal taste preferences |
|
| Outcomes | Primary (24 weeks):
Secondary (24 weeks):
Nutritional blood parameters assessed are not included in this review |
|
| Methods of AE Assessment |
|
|
| Notes | Funding and methods used to control bias resulting from conflict of interest: see Table 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
S‐Connect study 2013: Quote: "Allocation to active or control product was performed through a central randomization procedure in the Electronic Data Capture system using four different randomization codes (A, B, C, and D)." p. 2 |
| Allocation concealment (selection bias) | Low risk |
S‐Connect study 2013: Quote: "Allocation to active or control product was performed through a central randomization procedure in the Electronic Data Capture system using four different randomization codes (A, B, C, and D)." p. 2 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
S‐Connect study 2013: Quote: "Participants, study partners, and study staff were masked to study group assignment during the trial. Unmasking did not occur until initial statistical modeling of the primary outcome was complete." p. 2 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
S‐Connect study 2013: Quote: "Participants, study partners, and study staff were masked to study group assignment during the trial. Unmasking did not occur until initial statistical modeling of the primary outcome was complete." p. 2 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Provided ITT The reasons for discontinuation from 70 participants are unclear apart from SEA but drop outs are balanced in numbers and overall drop out 14% (IG 14%, CG 15%). |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as described in protocol, study conclusion congruent,with results and relevant AEs/SAEs are named with distribution to group |
| Other bias | Low risk | The model specifications for the outcomes measured with "trajectories over time" could be resolved by personal communication with the sponsor |
Souvenir II study 2012.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled trial Randomisation between November 2009 and May 2011. We got no information on exact trial duration from the Sponsor Nutricia |
|
| Participants | Countries: Europe (The Netherlands, Germany, Belgium, Spain, Italy, and France) Diagnosis: "drug‐naïve" patients with very mild Alzheimer’s disease (mean MMSE 25) Follow‐up: 24 weeks Inclusion criteria: diagnosis of probable Alzheimer's disease (NINCDS‐ADRA); recent MRI or CT had shown no evidence of any other potential causes of dementia; MMSE ≥ 20; age ≥ 50 years; written informed consent; and availability of a responsible caregiver Exclusion criteria: neurological disease other than Alzheimer's disease; cholinesterase inhibitor or NMDA‐receptor antagonist use within 3 months prior to baseline; Depression Scale (15‐item) score of > 6; use within 2 months prior to baseline of: Omega‐3 fatty acid containing supplements or regular consumption of oily fish (> twice/week) within 2 months prior to baseline, use of atropine, scopolamine, tolterodine, hyoscyamine, biperiden, benztropine, trihexyphenidyl, oxybutynin, antipsychotics, vitamins B, C, and/or E (> 200% of the recommended daily intake), consumption of high‐energy and/or high‐protein nutritional supplements, a change in dose of lipid‐lowering medications, antidepressants, antihypertensives, or the use of other investigational products within 1 month prior to baseline; excessive alcohol intake or drug abuse; nursing home institutionalisation; investigator uncertainty regarding the willingness or ability of the patient to comply with the protocol Setting: Community; participants recruited from Alzheimer's disease centres (The Netherlands (n = 9), Germany (n = 5), Belgium (n = 4), Spain (n = 3), Italy (n = 3), and France (n = 3)) Total number of participants: n = 259 (n = 130 Souvenaid group / n = 129 control group) Per protocol population: n = 238 (n = 118 Souvenaid group / n = 120 control group) Baseline characteristics:
|
|
| Interventions | Intervention 1: Souvenaid 125 mL drink/once daily for 24 weeks which contains Fortasyn Connect™ (DHA, EPA, phospholipids, choline, UMP, vitamin B12, B6, and folate, vitamins C and E, and selenium) (see Table 4) Intervention 2: Iso‐caloric control product 125 ml/once daily that lacked Fortasyn Connect™, but that was otherwise identical to the active product with identical taste profile and appearance |
|
| Outcomes | Primary (24 weeks):
Secondary (24 weeks):
Safety and tolerability parameters assessed but not explicitly named as outcomes; Electroencephalography and nutritional blood parameters not included in this review |
|
| Methods of AE Assessment |
|
|
| Notes | Funding and methods used to control bias resulting from conflict of interest: see Table 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Souvenir II study 2012: Quote: “Allocation to the study groups was performed using a central randomization procedure in the Electronic Data Capture system, using four different randomization codes (A, B, C, and D)” p. 228 |
| Allocation concealment (selection bias) | Low risk |
Souvenir II study 2012: Quote: “Allocation to the study groups was performed using a central randomization procedure in the Electronic Data Capture system, using four different randomization codes (A, B, C, and D)." " The investigator, study‐site staff, Danone Research staff, study staff of the Clinical Research Organisation, patients, and caregivers were all blinded to the study products.” p. 228 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Souvenir II study 2012: Quote: “The investigator, study‐site staff, Danone Research staff, study staff of the Clinical Research Organisation, patients, and caregivers were all blinded to the study products.” p. 228 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Souvenir II study 2012: Quote: “The randomization code was not broken until initial statistical modeling of the primary outcome was complete.” p. 228 Quote: "Data analysis was conducted by staff of Danone Research and an outside statistician (JWR Twisk) independently and again by staff at Rush Alzheimer’s Disease Center (S Leurgans, RC Shah, DA Bennett, W Fan) who received the whole data set and preformed a statistical analysis blinded to study treatment on the primary outcome measure" p. 234 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up 8.1% almost equal distributed |
| Selective reporting (reporting bias) | Low risk | Outcomes presented as described in published study protocol. |
| Other bias | Low risk | |
AD: Alzheimer's disease; ADAS‐Cog: Alzheimer’s Disease Assessment Scale ‐cognitive subscale; ADCS‐ADL: Alzheimer's Disease Cooperative Study Activities of Daily Living; AE: Adverse events; BMI: body mass index; CT: computer‐assisted tomography; DHA: Docosapentaenoic acid; DSM: Diagnostic and Statistical Manual of Mental Disorders; EPA: Eicosapentaenoic acid; ITT: Intention to treat; MADRS: Montgomery–Åsberg Depression Rating Scale; MMSE: Mini Mental State Examination; MRI: Magnetic resonance imaging; NTB: Neuropsychological Test Battery; NMDA: N‐methyl‐D‐aspartate. SAE: Serious adverse events; SD: Standard deviation; UMP: Uridine‐5'‐monophosphate
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Souvenir II OLE study 2015 | Open‐label extension of the Souvenir II study 2012, all participants received the active product |
| Souvenir I study 2010 | Treatment with Souvenaid lasted only 12 weeks |
Characteristics of ongoing studies [ordered by study ID]
NL‐ENIGMA.
| Study name | NL‐ENIGMA |
| Methods | Randomised controlled trial |
| Participants | 40 patients with mild cognitive impairment or mild dementia due to Alzheimer's disease |
| Interventions | Medical food Souvenaid or placebo for a period of 24 weeks |
| Outcomes | Primary: Quantitative absolute and relative (FDG) uptake over 24 weeks, as assessed by 18F‐FDG‐PET using Alzheimer's disease regions of interest (ROIs) Secondary: • Semi‐quantitative Standardized Uptake Value (SUV) and SUV ratio over 24 weeks, assessed by 18F‐FDG‐PET using Alzheimer's disease Regions of Interest (ROIs) using different time windows after injection • MRI biomarkers • Blood biomarkers • CSF markers (optional) • Cognition: Rey Auditory Verbal Learning Test immediate and delayed recall and recognition test ‐ Executive functioning using TMT‐A and TMT‐B Semi‐quantitative Standardized Uptake Value (SUV) and SUV ratio over 24‐weeks |
| Starting date | |
| Contact information | |
| Notes | We contacted N. Scheltens and the sponsor Nutricia in April 2019. According to N. Scheltens results will be presented at a conference in 2019. patient‐relevant outcomes are only investigated as secondary outcomes in this trial. We therefore decided to wait until the trial is published |
CSF: cerebrospinal fluid; MRI: magnetic resonance imaging
Differences between protocol and review
In our protocol (Burckhardt 2015), we listed combined cognitive‐functional measures like the CDR under the primary outcome 'Dementia severity measures'. However, these measures are not used exclusively to stage dementia severity. We therefore changed the outcome to 'Combined cognitive‐functional outcomes'.
We deviated from our plan to rate all outcomes with a consumer group for GRADE. In another review conducted by our group (Burckhardt 2016), we experienced very low variance within the ratings of the outcomes which we attributed to the low number of rating participants. In consideration of this and our limited resources, we did not further prioritise the predefined outcomes for this review.
In the protocol we have written that we would investigate the adverse effects as a primary outcome. In order to be able to present the safety aspects of the study products with favourable and unfavourable outcomes in a balanced way, as well as to take account of reporting standards of potential harms, we have extended this primary outcome to adverse events and adverse effects.
We intended to present the proportion of individuals with changes in the scale measures of the primary outcomes (i.e. more or less than four scale points for ADAS‐cog) if data were available. However, considering variable scale measures and non‐significant effects within the primary outcomes of the trials, we did not request those data from the study authors.
We performed none of the predefined meta‐analyses, sensitivity analyses or subgroup analyses, due to a lack of data. We presented all outcomes grouped by the stage of AD.
Max Herke and Tobias Wustman contributed to the protocol of this review, but left the team for personal reasons. Andreas Wienke joined the team for this review.
Contributions of authors
MB: correspondence; project management, drafting review versions; selection of randomised controlled trials (RCTs); extraction of data; assessing risks of bias; data entry, data analysis; GRADE; interpretation of data/analyses.
AF: selection of RCTs; extraction of data; assessing 'Risk of bias' data entry, data analysis; interpretation of data/analyses.
AW: interpretation of data/analyses.
GL: GRADE; interpretation of data/analyses.
SW: description of condition section, interpretation of data/analyses.
MB wrote the remaining sections of the review, which were complemented and commented by all authors.
Sources of support
Internal sources
-
Roux Program of Martin‐Luther University Halle‐Wittenberg, Medical Faculty, Germany
University Grant
External sources
-
NIHR, UK
This review was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health
Declarations of interest
Marion Burckhardt ‐ none known. Andreas Wienke ‐ none known. Astrid Fink ‐ none known. Stefan Watzke ‐ none known. Gero Langer ‐ none known.
New
References
References to studies included in this review
LipiDiDiet study 2017 {published data only}
- Freund-Levi Y, Visser PJ, Kivipelto M, Kamphuis PJ, Wieggers RL, Hartmann T, et al. Baseline characteristics of subjects with prodromal Alzheimer's disease. European Journal of Neurology 2012;19(Suppl 1):112. [Google Scholar]
- Freund-Levi Y, Visser PJ, Kivipelto M, Kamphuis PJ, Wieggers RL, Hartmann T, et al. Update of the lipididiet study. Journal of Nutrition, Health & Aging 2012;16(9):859. [Google Scholar]
- Freund-Levi Y, Visser PJ, Kivipelto M, Wieggers RL, Hartmann T, Soininen H. Baseline characteristics of subjects with prodromal Alzheimer's disease. Annals of Neurology 2012;72(Suppl 16):S53-4. [Google Scholar]
- Freund-Levi Y, Visser PJ, Kivipelto M, Wieggers RL, Hartmann T, Soininen H. The lipididiet study. Alzheimer's & Dementia 2012;8(4 Suppl):P602. [Google Scholar]
- Hendrix SB, Soininen H, Van Hees AM, Ellison N, Visser PJ, Solomon A, et al. Alzheimer's disease composite score: a post-hoc analysis using data from the LipiDiDiet trial in prodromal Alzheimer's disease. Journal of Prevention of Alzheimers Disease 2019;6(4):232-6. [DOI] [PubMed] [Google Scholar]
- Hendrix SB, Soininen H, Visser PJ, Solomon A, Kivipelto M, Hartmann T, on behalf of the LipiDiDiet clinicalstudy group. ADCOMS: a post-hoc analysis using data from the LipiDiDiet trial in prodromal Alzheimer’s disease. In: 11th Clinical Trials on Alzheimer’s disease, 2018 October 24-27; Barcelona. Barcelona, 2018.
- NTR1603. LipiDiDiet Trial. Medical Nutrition in prodromal Alzheimer's disease, a double blind controlled 24 month study with one, two, three and four 12 month Extension studies. www.trialregister.nl/trial/1683 (first receveid 29 August 2009).
- Soininen H, Solomon A, Visser PJ, Hendrix SB, Blennow K, Kivipelto M, et al. 24-month intervention with a specifc multinutrient in people with prodromal Alzheimer’s disease (LipiDiDiet):a randomised, double-blind, controlled trial. Lancet 2017;16(12):965-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soininen H, Visser PJ, Kivipelto M, Hartmann T. The lipididiet trial-effects of fortasyn connect in prodromal Alzheimer's disease. Neuro-degenerative Diseases 2017;17(Suppl 1):323. [Google Scholar]
- Soininen H, Visser PJ, Kivipelto M, Van Hees A, Hartmann T. Status of a clincial study investigating the effects of a specific nutrition combination in prodromal Alzheimer's disease. Neuro-degenerative Diseases 2015;15(Suppl 1):177. [Google Scholar]
- Soininen H, Visser PJ, Kivipelto M, Van Hees A, Rouws C, Hartmann T. Clinical trial investigating the effects of Souvenaid in prodromal Alzheimer disease. Neurobiology of Aging 2014;35(Suppl 1):S20. [Google Scholar]
- Soininen H, Visser P, Kivipelto M, Hartmann T. A clinical trial investigating the effects of fortasyn connect (souvenaid) in prodromal Alzheimer's disease: results of the lipididiet study. Neurobiology of Aging 2016;39(Suppl 1):S23. [Google Scholar]
- Visser PJ, Soininen H, Kivipelto M, Freund-Levi Y, Kamphuis PJ, Wieggers RL, et al. A randomized controlled trial investigating the effects of souvenaid in prodromal Alzheimer's disease. Alzheimer's & Dementia 2013;9(4 Suppl):669-70. [Google Scholar]
S‐Connect study 2013 {published data only}
- NTR1683. S-Connect. A randomized controlled trial to assess the efficacy of a medical food in patients with mild to moderate Alzheimer's disease using Alzheimer's disease medication. www.trialregister.nl/trial/1683 (first receveid 23 February 2009).
- Shah RC, Kamphuis PJ, Leurgans S, Swinkels SH, Sadowsky CH, Bongers A, et al. The S-Connect study: results from a randomized, controlled trial of Souvenaid in mild-to-moderate Alzheimer's disease. Alzheimer's Research & Therapy 2013;5(6):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Souvenir II study 2012 {published data only}
- NTR1863. Souvernir II. A randomized controlled trial to assess the efficacy of a Food for Special Medical Purposes (FSMP) in patients with mild Alzheimer’s disease. www.trialregister.nl/trial/1863 (first received 23 February 2009).
- Scheltens P, Twisk J, Blesa R, Scarpini E, Von Arnim C, Bongers A, et al. Souvenaid improves memory in drug-naïve patients with mild Alzheimer's disease. Journal of Alzheimer's Disease 2012;29(Suppl 1):37-8. [Google Scholar]
- Scheltens P, Twisk JW, Blesa R, Scarpini E, Von Arnim CA, Bongers A, et al. Efficacy of Souvenaid in mild Alzheimer's disease: results from a randomized, controlled trial. Journal of Alzheimer's Disease 2012;31(1):225-36. [DOI] [PubMed] [Google Scholar]
- Vellas B, Kamphuis P, Harrison J, De Deyn P, Von Arnim C, Vlesa R, et al. The Souvenir II study: rationale and study design. Alzheimer's & Dementia 2011;7(4 Suppl):791.
References to studies excluded from this review
Souvenir II OLE study 2015 {published data only}
- NTR2456. Souvenir II Open label Extension study. An open label extension study to evaluate long term use of a Food for Special Medical Purposes (FSMP) in patient with mild Alzheimer's disease who completed the Souvenir II study. www.trialregister.nl/trial/2456 (first receveid 15 October 2010).
- Olde Rikkert MG, Verhey FR, Blesa R, Von Arnim CA, Bongers A, Harrison J, et al. Tolerability and safety of souvenaid in patients with mild Alzheimer's disease: results of multi-center, 24-week, open-label extension study. Journal of Alzheimer's Disease 2015;44(2):471-80. [DOI] [PubMed] [Google Scholar]
Souvenir I study 2010 {published data only}
- Kamphuis PJ, Verhey FR, Olde Rikkert MG, Twisk JW, Swinkels SH, Scheltens P. Effect of a medical food on body mass index and activities of daily living in patients with Alzheimer's disease. Journal of Nutrition, Health & Aging 2011;15(8):672-6. [DOI] [PubMed] [Google Scholar]
- Kamphuis PJ, Verhey FR, Olde Rikkert MG, Twisk JW, Swinkels SH, Scheltens P. Efficacy of a medical food on cognition in Alzheimer's disease: results from secondary analyses of a randomized, controlled trial. Journal of Nutrition, Health & Aging 2011;15(8):720. [DOI] [PubMed] [Google Scholar]
- Scheltens P, Kamphuis PJ, Verhey FR, Olde Rikker MG, Wurtman RJ, Wilkinson D, et al. Efficacy of a medical food in mild Alzheimer's disease. Alzheimer's & Dementia 2010;6(1):1-10. [DOI] [PubMed] [Google Scholar]
- Scheltens P, Olde Rikkert MG, Verhey FR, Kamphuis PJ, Wilkinson D, Kurz A. The efficacy of Souvenaid® in mild Alzheimer's disease: design of a randomised, controlled, double-blind, parallel group, multi-centre, multi-country clinical trial. European Journal of Neurology 2008;15(s3):224. [Google Scholar]
- Scheltens P, Verhey FR, Olde Rikkert MG, Kamphuis PJ, Wilkinson D, Kurz A. The efficacy of a medical food (Souvenaid) in Alzheimer's disease. Results from the first trial and design of future trials. Alzheimer's & Dementia 2009;5(4 Suppl.):258-9. [Google Scholar]
References to ongoing studies
NL‐ENIGMA {published data only}
- NTR4493. NL-Enigma. A study exploring the effect of a nutritional intervention on brain activity in patients with mild cognitive impairment or mild dementia due to Alzheimer's disease. www.trialregister.nl/trial/4493 (first received 04 August 2014).
- Scheltens NM, Briels CT, Yaqub M, Barkhof F, Boellaard R, Van der Flier WM, et al. Exploring effects of Souvenaid on cerebral glucose metabolism in Alzheimer's disease. Alzheimer's & Dementia 2019;5:492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltens NM, Van Berckel BN, Boellaard R, Barkhof F, Van Der Flier WM, Kamphuis PJ, et al. A Dutch 24-week randomised controlled study exploring the Effect of a Nutritional Intervention on brain Glucose Metabolism in early Alzheimer's disease (NL-ENIGMA); Rationale and design. European Geriatric Medicine 2014;5(Suppl 1):S91. [Google Scholar]
- Scheltens NM, Van Berckel BN, Boellaard R, Barkhof F, Van Der Flier WM, Kamphuis PJ, et al. Rationale and design of the nl-enigma study: a Dutch 24-week randomised controlled study to explorethe effect of nutritional intervention on brain glucose metabolism in early Alzheimer disease. Alzheimer's & Dementia 2014;10(4 Suppl):P61. [Google Scholar]
Additional references
Albert 2011
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia 2011;7(3):270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alix 2011
- Alix JJ, Domingues AM. White matter synapses: form, function, and dysfunction. Neurology 2011;76(4):397-404. [PMID: ] [DOI] [PubMed] [Google Scholar]
Andrade‐Moraes 2013
- Andrade-Moraes CH, Oliveira-Pinto AV, Castro-Fonseca E, Da Silva CG, Guimaraes DM, Szczupak D, et al. Cell number changes in Alzheimer's disease relate to dementia, not to plaques and tangles. Brain 2013;136(Pt 12):3738-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andrews 2019
- Andrews JS, Desai U, Kirson NY, Zichlin ML, Ball DE, Matthews BR. Disease severity and minimal clinically important differences in clinical outcome assessments for Alzheimer's disease clinical trials. Alzheimers Dementia (N Y) 2019;5:354-63. [DOI: 10.1016/j.trci.2019.06.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bloom 2014
- Bloom GS. Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurology 2014;71(4):505-8. [DOI] [PubMed] [Google Scholar]
Bråne 2001
- Bråne G, Gottfries CG, Winblad B. The Gottfries-Bråne-Steen Scale: validity, reliability and application in anti-dementia drug trials. Dementia and Geriatric Cognitive Disorders 2001;12(1):1-14. [DOI] [PubMed] [Google Scholar]
Burckhardt 2016
- Burckhardt M, Herke M, Wustmann T, Watzke S, Langer G, Fink A. Omega-3 fatty acids for the treatment of dementia. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No: CD009002. [DOI: 10.1002/14651858.CD009002.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cansev 2008
- Cansev M, Wurtman RJ, Sakamoto T, Ulus IH. Oral administration of circulating precursors for membrane phosphatides can promote the synthesis of new brain synapses. Alzheimer's & Dementia 2008;4(1 Suppl 1):S153-68. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2 edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc., 1988. [Google Scholar]
Doecke 2012
- Doecke JD, Laws SM, Faux NG, Wilson W, Burnham SC, Lam CP, et al, Alzheimer's Disease Neuroimaging Initiative, Australian Imaging Biomarker and Lifestyle Research Group. Blood-based protein biomarkers for diagnosis of Alzheimer disease. Archives of Neurology 2012;69(10):1318-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
DSM III‐R
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd revised edition. Washington D.C.: American Psychiatric Association, 1987. [Google Scholar]
DSM IV
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edition. Washington D.C.: American Psychiatric Association, 1994. [Google Scholar]
Dubois 2007
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria.. Lancet Neurology 2007;6(8):734-46. [DOI: 10.1016/s1474-4422(07)70178-3] [DOI] [PubMed] [Google Scholar]
Dubois 2014
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurology 2014;13(6):614-29. [DOI: 10.1016/S1474-4422(14)70090-0] [DOI] [PubMed] [Google Scholar]
Dubois 2016
- Dubois B, Hamper H, Feldman HH, Scheltens P, Aisen P, Anrieu S, et al. Preclinical Alzheimer's disease: definition, natural history, and diagnostic criteria. Alzheimer's & Dementia 2016;12(3):292-323. [DOI: 10.1016/j.jalz.2016.02.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dysken 2014
- Dysken MW, Sano M, Asthana S, Vertrees JE, Pallaki M, Llorente M, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 2014;311(1):33-44.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
EMA 2008
- European Medicines Agency. Guideline on Medicinal Products for the Treatment of Alzheimer's Disease and Other Treatments. www.ema.europa.eu 2008.
EMA 2018
- European Medicines Agency: Committee for Medicinal Products for Human Use (CHMP). Guideline on the clinical investigation of medicines for the treatment of Alzheimer’s disease. CPMP/EWP/553/95 Rev.2. www.ema.europa.eu 2018.
Endnote 2011 [Computer program]
- EndNote X5. Thomson Reuters, 2011.
FDA 2018
- US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation (CBER). Early Alzheimer’s Disease: Developing Drugs For Treatment, Guidelines for Industry. Draft guideline. www.fda.gov/media/110903/download 2018.
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12(3):189-98. [DOI] [PubMed] [Google Scholar]
Galasko 1997
- Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Disease and Associated Disorders 1997;11(Suppl 2):33-9. [PubMed] [Google Scholar]
Gelinas 1999
- Gelinas L, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia. Amercian Journal of Occupational Therapy 1999;53(5):471-81. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395-400. [DOI] [PubMed] [Google Scholar]
Harrison 2007
- Harrison J, Minassian SL, Jenkins L, Black RS, Koller M, Grundman M. A neuropsychological test battery for use in Alzheimer disease clinical trials. Archives of Neurology 2007;64(9):1323-9. [DOI] [PubMed] [Google Scholar]
Harrison 2011
- Harrison J, Caveney A. Newsletter Number 46.Patient Reported Outcomes: 10 Years of the Neuropsychological TestBattery (NTB). http://www.pro-newsletter.com/images/PDF/pronl_46_11042012.pdf 2011 (assessed April 2017):21-4.
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011]) The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011.. Available from www.handbook.cochrane.org..
Huntley 2015
- Huntley JD, Gould RL, Liu K, Smith M, Howard RJ. Do cognitive interventions improve general cognition in dementia? A meta-analysis and meta-regression. BMJ Open 2015;5(4):e005247. [DOI] [PMC free article] [PubMed] [Google Scholar]
IQWIG 2008
- Institute for Quality and Efficiency in Health Care. Ginkgohaltige Präparate bei Alzheimer Demenz. Köln: Institute for Quality and Efficiency in Health Care, 2008. [Google Scholar]
Jack 2011
- Jack CR Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia 2011;7(3):257-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kryscio 2014
- Kryscio RJ, Abner EL, Cooper GE, Fardo DW, Jicha GA, Nelson PT, et al. Self-reported memory complaints: Implications from a longitudinal cohort with autopsies. Neurology 2014;83(15):1359-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larson 2013
- Larson EB, Yaffe K, Langa KM. New insights into the dementia epidemic. New England Journal of Medicine 2013;369(24):2275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
Lopes 2013
- Lopes da Silva S, Vellas B, Elemans S, Luchsinger J, Kamphuis P, Yaffe K, et al. Plasma nutrient status of patients with Alzheimer's disease: systematic review and meta-analysis. Alzheimer's & Dementia 2013;10(4):485-502. [DOI] [PubMed] [Google Scholar]
McKhann 2011
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia 2011;7(3):263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mi 2013
- Mi W, Van Wijk N, Cansev M, Sijben JW, Kamphuis PJ. Nutritional approaches in the risk reduction and management of Alzheimer's disease. Nutrition 2013;29(9):1080-9. [DOI] [PubMed] [Google Scholar]
Molnar 2009
- Molnar FJ, Man-Son-Hing M, Fergusson D. Systematic review of measures of clinical significance employed in randomized controlled trials of drugs for dementia. Journal of the American Geriatrics Society 2009;57(3):536-46. [DOI] [PubMed] [Google Scholar]
Monsell 2014
- Monsell SE, Mock C, Hassenstab J, Roe CM, Cairns NJ, Morris JC, et al. Neuropsychological changes in asymptomatic persons with Alzheimer disease neuropathology. Neurology 2014;83(5):434-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nutricia 2014
- Nutricia. Souvenaid Consumer Website. www.mysouvenaid.co.uk (accessed 10 June 2014).
O'Bryant 2008
- O'Bryant SE, Waring SC, Cullum CM, Hall J, Lacritz L, Massman PJ, et al. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer's research consortium study. Archives of Neurology 2008;65(8):1091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Onakpoya 2017
- Onakpoya IJ, Heneghan CJ. The efficacy of supplementation with the novel medical food, Souvenaid, in patients with Alzheimer's disease: a systematic review and meta-analysis of randomized clinical trials. Nutritional Neuroscience 2017;20(4):219-27. [DOI] [PubMed] [Google Scholar]
Page 2020
- Page MJ, Cumpston M, Chandler J, Lasserson T. Chapter III: Reporting the review. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Peryer 2020
- Peryer G, Golder S, Junqueira D, Vohra S, Loke YK. Chapter 19: Adverse effects. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Petersen 1999
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology 1999;56(3):303-8. [DOI] [PubMed] [Google Scholar]
Prince 2009
- Prince M, Jackson J (editors). World Alzheimer Report. London: Alzheimer’s Disease International, 2009. [Google Scholar]
Prince 2014
- Prince M, Albanese E, Guerchet M, Prina M. Nutrition and Dementia. A review of Available Research. London: Alzheimer's Disease International, 2014. [Google Scholar]
Querfurth 2010
- Querfurth HW, LaFerla FM. Alzheimer's Disease. New England Journal of Medicine 2010;362(4):329-44. [DOI] [PubMed] [Google Scholar]
Revicki 2008
- Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. Journal of Clinical Epidemiology 2008;61(2):102-9. [DOI] [PubMed] [Google Scholar]
RevMan 2019 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2019.
Ritchie 2014
- Ritchie C, Smailagic N, Noel-Storr AH, Takwoingi Y, Flicker L, Mason SE, et al. Plasma and cerebrospinal fluid amyloid beta for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database of Systematic Reviews 2014, Issue 6. Art. No: CD008782. [DOI: 10.1002/14651858.CD008782.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosen 1984
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. American Journal of Psychiatry 1984;141(11):1356-64. [DOI] [PubMed] [Google Scholar]
Scheff 2007
- Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 2007;68(18):1501-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schmidt 1996
- Schmidt M. Rey Auditory Verbal Learning Test™ (RAVLT™). A Handbook. www.wpspublish.com/ravlt-rey-auditory-verbal-learning-test 1996.
Schneider 1997
- Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, et al. Validity and reliability of the Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change. The Alzheimer's Disease Cooperative Study. Alzheimer Disease and Associated Disorders 1997;11 Suppl 2:S22-32. [DOI] [PubMed] [Google Scholar]
Schrag 2012
- Schrag A, Schott JM, Alzheimer's Disease Neuroimaging Initiative. What is the clinically relevant change on the ADAS-Cog? Journal of Neurology, Neurosurgery and Psychiatry 2012;83(2):171-3. [DOI] [PubMed] [Google Scholar]
Schünemann 2020
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Sperling 2011
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia 2011;7(3):280-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
United Nations 2017
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2017 (ST/ESA/SER.A/408).. New York: United Nations, 2017. [Google Scholar]
Vellas 2008
- Vellas B, Andrieu S, Sampaio C, Coley N, Wilcock G. Endpoints for trials in Alzheimer's disease: a European task force consensus. Lancet Neurology 2008;7(5):436-50. [DOI] [PubMed] [Google Scholar]
Wechsler 2010
- Wechsler 2010. Wechsler Memory Scale - Fourth UK Edition (WMS-IV UK). Pearson.
WHO 1992
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Description and Diagnostic Guidelines. Geneva: World Health Organization, Division of Mental Health, 1992. [Google Scholar]
WHO 2012
- World Health Organization, Alzheimer's Disease International. Dementia. A Public Health Priority. www.who.int/mental_health/publications/dementia_report_2012/en/ 2012. [ISBN 978 92 4 156445 8]
WHO 2019
- World Health Organization. Fact sheet: Dementia. www.who.int/news-room/fact-sheets/detail/dementia 2019.
Williams 2010
- Williams JW, Plassman BL, Burke J, Holsinger T, Benjamin S. Preventing Alzheimer’s Disease and Cognitive Decline. Evidence Report/Technology Assessment No. 193. (Prepared by the Duke Evidence-based Practice Center under Contract No. HHSA 290-2007-10066-I.) AHRQ Publication No. 10-E005 2010. [PMC free article] [PubMed]
Wimo 2015
- Wimo A, Prince M. World Alzheimer Report 2015. The Global Impact of Dementia. The worldwide costs of dementia. London: Alzheimer’s Disease International, 2015. [Google Scholar]
Zorzela 2016
- Zorzela L, Loke YK, Ioannidis JP, Golder S, Santaguida P, Altman DG, et al, PRISMA Harms Group. PRISMA harms checklist: improving harms reporting in systematic reviews. BMJ 2016;352:i157. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Burckhardt 2015
- Burckhardt M, Herke M, Wustmann T, Fink A, Watzke S, Langer G. Souvenaid for Alzheimer's disease. Cochrane Database of Systematic Reviews 2015, Issue 5. Art. No: CD011679. [DOI: 10.1002/14651858.CD011679] [DOI] [PMC free article] [PubMed] [Google Scholar]


