Abstract
Background
Tubal disease accounts for 20% of infertility cases. Hydrosalpinx, caused by distal tubal occlusion leading to fluid accumulation in the tube(s), is a particularly severe form of tubal disease negatively affecting the outcomes of assisted reproductive technology (ART). It is thought that tubal surgery may improve the outcome of ART in women with hydrosalpinges.
Objectives
To assess the effectiveness and safety of tubal surgery in women with hydrosalpinges prior to undergoing conventional in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI).
Search methods
We searched the Cochrane Gynaecology and Fertility (CGF) Group trials register, CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL, DARE, and two trial registers on 8 January 2020, together with reference checking and contact with study authors and experts in the field to identify additional trials.
Selection criteria
Randomised controlled trials (RCTs) comparing surgical treatment versus no surgical treatment, or comparing surgical interventions head‐to‐head, in women with tubal disease prior to undergoing IVF.
Data collection and analysis
We used Cochrane's standard methodological procedures. The primary outcomes were live birth rate (LBR) and surgical complication rate per woman randomised. Secondary outcomes included clinical, multiple and ectopic pregnancy rates, miscarriage rates and mean numbers of oocytes retrieved and of embryos obtained.
Main results
We included 11 parallel‐design RCTs, involving a total of 1386 participants. The included trials compared different types of tubal surgery (salpingectomy, tubal occlusion or transvaginal aspiration of hydrosalpingeal fluid) to no tubal surgery, or individual interventions to one another. We assessed no studies as being at low risk of bias across all domains, with the main limitations being lack of blinding, wide confidence intervals and low event and sample sizes. We used GRADE methodology to rate the quality of the evidence. Apart from one moderate‐quality result in one review comparison, the evidence provided by these 11 trials ranged between very low‐ to low‐quality.
Salpingectomy versus no tubal surgery
No included study reported on LBR for this comparison. We are uncertain of the effect of salpingectomy on surgical complications such as the rate of conversion to laparotomy (Peto odds ratio (OR) 5.80, 95% confidence interval (CI) 0.11 to 303.69; one RCT; n = 204; very low‐quality evidence) and pelvic infection (Peto OR 5.80, 95% CI 0.11 to 303.69; one RCT; n = 204; very low‐quality evidence). Salpingectomy probably increases clinical pregnancy rate (CPR) versus no surgery (risk ratio (RR) 2.02, 95% CI 1.44 to 2.82; four RCTs; n = 455; I2 = 42.5%; moderate‐quality evidence). This suggests that in women with a CPR of approximately 19% without tubal surgery, the rate with salpingectomy lies between 27% and 52%.
Proximal tubal occlusion versus no surgery
No study reported on LBR and surgical complication rate for this comparison. Tubal occlusion may increase CPR compared to no tubal surgery (RR 3.21, 95% CI 1.72 to 5.99; two RCTs; n = 209; I2 = 0%; low‐quality evidence). This suggests that with a CPR of approximately 12% without tubal surgery, the rate with tubal occlusion lies between 21% and 74%.
Transvaginal aspiration of hydrosalpingeal fluid versus no surgery
No study reported on LBR for this comparison, and there was insufficient evidence to identify a difference in surgical complication rate between groups (Peto OR not estimable; one RCT; n = 176). We are uncertain whether transvaginal aspiration of hydrosalpingeal fluid increases CPR compared to no tubal surgery (RR 1.67, 95% CI 1.10 to 2.55; three RCTs; n = 311; I2 = 0%; very low‐quality evidence).
Laparoscopic proximal tubal occlusion versus laparoscopic salpingectomy
We are uncertain of the effect of laparoscopic proximal tubal occlusion versus laparoscopic salpingectomy on LBR (RR 1.21, 95% CI 0.76 to 1.95; one RCT; n = 165; very low‐quality evidence) and CPR (RR 0.81, 95% CI 0.62 to 1.07; three RCTs; n = 347; I2 = 77%; very low‐quality evidence). No study reported on surgical complication rate for this comparison.
Transvaginal aspiration of hydrosalpingeal fluid versus laparoscopic salpingectomy
No study reported on LBR for this comparison, and there was insufficient evidence to identify a difference in surgical complication rate between groups (Peto OR not estimable; one RCT; n = 160). We are uncertain of the effect of transvaginal aspiration of hydrosalpingeal fluid versus laparoscopic salpingectomy on CPR (RR 0.69, 95% CI 0.44 to 1.07; one RCT; n = 160; very low‐quality evidence).
Authors' conclusions
We found moderate‐quality evidence that salpingectomy prior to ART probably increases the CPR compared to no surgery in women with hydrosalpinges. When comparing tubal occlusion to no intervention, we found that tubal occlusion may increase CPR, although the evidence was of low quality. We found insufficient evidence of any effect on procedure‐ or pregnancy‐related adverse events when comparing tubal surgery to no intervention. Importantly, none of the studies reported on long term fertility outcomes. Further high‐quality trials are required to definitely determine the impact of tubal surgery on IVF and pregnancy outcomes of women with hydrosalpinges, particularly for LBR and surgical complications; and to investigate the relative efficacy and safety of the different surgical modalities in the treatment of hydrosalpinges prior to ART.
Plain language summary
Surgical treatment for tubal disease in women due to undergo in vitro fertilisation
Review question
We reviewed the efficacy and safety of performing surgery on women with known disease of the fallopian tube, particularly hydrosalpinx (a condition in which fluid accumulates in one or both fallopian tubes, leading to poor reproductive success), before in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI). We aimed to compare all types of surgery on the fallopian tube with no surgery prior to IVF. These types of surgery include salpingectomy, where one or both fallopian tubes are removed; tubal occlusion, where the fallopian tubes are blocked using metal clips or divided with scissors and electrocautery so that the fluid from existing hydrosalpinges does not reach the cavity of the womb; and ultrasound‐guided aspiration of the hydrosalpingeal fluid through the vagina. Where evidence was available, we also aimed to compare any type of fallopian tube surgery to any other type of fallopian tube surgery.
Background
Up to one in five women who suffer with infertility are diagnosed with blockage of one or both fallopian tubes. IVF treatment is used for women with tubal disease, as the eggs and sperm are manipulated outside the body. The resulting embryos are transferred back into the cavity of the womb, without the need for open fallopian tubes. However, research has shown that in cases of tubal blockage, women may develop a condition termed hydrosalpinx, where fluid accumulates inside the tubes and may prevent the successful implantation of embryos created by IVF. Tubal surgery has therefore been suggested to treat hydrosalpinges, as it may prevent the hydrosalpingeal fluid from reaching the cavity of the womb. If this fluid reaches the womb cavity, it may negatively affect the success of assisted conception.
Study characteristics
We found 11 randomised controlled trials comparing surgery on the fallopian tube to no tubal surgery in a total of 1386 women with hydrosalpinges prior to IVF. The evidence is current to January 2020.
Key results
No studies reported on live birth rates in the main comparison of tubal surgery versus no tubal surgery. Compared to no surgery in the fallopian tube, salpingectomy probably increases the chance of clinical pregnancy. The evidence suggests that if the chance of clinical pregnancy is assumed to be 19% with no salpingectomy, the chance of clinical pregnancy following salpingectomy would be between 27% and 52%. There was a lack of sufficient data to identify an effect of the different types of tubal surgery on adverse events such as surgical complications, miscarriage and ectopic pregnancy.
Quality of the evidence
Apart from one moderate‐quality result in one review comparison, the evidence provided by these 11 trials ranged from very low‐ to low‐quality. The main limitations in this body of research were the lack of blinding (the process where the women participating in the trial, as well as the research staff, are not aware of the intervention used), inconsistency (differences in results across studies) and imprecision (random error and small size of each study) .
Summary of findings
Background
Description of the condition
Infertility is common, affecting up to one in six heterosexual couples trying to conceive. Tubal pathology, whereby there is mechanical obstruction or altered function of one or both fallopian tubes, accounts for 20% of infertility cases and has many aetiologies including pelvic infection, previous abdominal surgery and endometriosis (Evers 2002; NICE 2013). In cases of distal tubal occlusion, fluid accumulation may occur inside the fallopian tube(s), leading to the formation of hydrosalpinx, which is considered to be the most severe form of tubal disease due to its negative impact upon both natural and assisted conception outcomes (Strandell 2002).
Rather than passively reducing fecundity, tubal disease may actively contribute to early reproductive failure (Chan 2002). Such a deleterious effect has been linked to different possible mechanisms, including the presence of molecules secreted by the tubal epithelium or contained in hydrosalpingeal fluid that are toxic to gametes and embryos (Bao 2017; Mukherjee 1996; Zeyneloglu 1998); an altered nutrient environment within the fallopian tube fluid affecting the early stages of embryogenesis (Bao 2017; Dickens 1995; Koong 1998; Tay 1997); an abnormal uterotubal flow leading to impaired fertilisation, endometrial receptivity and implantation (Cheng 2015; Eytan 2001; Meyer 1997; Zeyneloglu 1998); and a direct cytotoxic effect on the endometrium resulting from the leakage of hydrosalpingeal fluid through the uterine cavity, termed hydrorrhoea (Andersen 1996; Mansour 1991; Strandell 2002).
Assisted reproductive technology (ART), whereby human gametes and embryos are manipulated outside the body with the goal of achieving a pregnancy, was first developed to circumvent the tubal stages of fertilisation and early embryogenesis in women with fallopian tube pathology (Edwards 1984). Nevertheless, since the inception of in‐vitro fertilisation (IVF) there has been a growing body of evidence demonstrating a reduction in pregnancy rates, and an increased risk of early pregnancy loss, in women with tubal disease undergoing ART, particularly where hydrosalpinx is present (Bao 2017; Camus 1999; Chu 2015; Fleming 1996; Kassabji 1994; Strandell 1994; Vandromme 1995; Van Voorhis 2019; Zeyneloglu 1998). This has generated support towards treating hydrosalpinx prior to ART, although there remains a paucity of evidence on the relative efficacy and side‐effect profile of different treatment modalities (Aboulghar 1998; Lass 1999; Van Voorhis 2019).
Description of the intervention
There is no consensus on how to best manage women with tubal disease prior to IVF. In those with hydrosalpinx, treatment options aim to remove the detrimental effect of the hydrosalpingeal fluid by either aspirating it under ultrasound guidance; draining it by means of salpingostomy; isolating the hydrosalpinges from the uterine cavity via laparoscopic or hysteroscopic proximal tubal occlusion; or most often by removing the affected fallopian tube(s) altogether (salpingectomy).
How the intervention might work
Tubal surgery prior to ART mainly aims to remove the deleterious effect of hydrosalpingeal fluid upon embryo development and endometrial receptivity. Different surgical techniques have been employed to this effect, including salpingectomy, tubal occlusion, aspiration of hydrosalpingeal fluid and salpingostomy.
Salpingectomy remains the most commonly used treatment for hydrosalpinges, and the previous version of this review concluded that the odds of ongoing pregnancy (odds ratio (OR) 2.14, 95% confidence interval (CI) 1.23 to 3.73) and of clinical pregnancy (OR 2.31, 95% CI 1.48 to 3.62) were increased with laparoscopic salpingectomy for hydrosalpinges prior to IVF in comparison to no intervention (Johnson 2010). Salpingectomy is nonetheless invasive, irreversible and may be technically difficult to perform in women with dense pelvic adhesions (Dreyer 2016). Moreover, while a recent meta‐analysis of eight non‐randomised and randomised studies concluded that salpingectomy does not appear to reduce ovarian reserve (Mohamed 2017), there is evidence demonstrating that the ovarian response to controlled stimulation in ART may be impaired in women with a history of previous salpingectomy (Fan 2016; Gelbaya 2006; Lass 1998).
Tubal occlusion is perceived to be less invasive than salpingectomy and can be achieved via hysteroscopy (by inserting intratubal devices) or laparoscopy (by applying proximal tubal clips or cauterisation). Both approaches have been widely used in female sterilisation with high success rates (Hurskainen 2010; Smith 2010) and, more recently, in women with hydrosalpinx to prevent leakage of hydrosalpingeal fluid into the endometrial cavity prior to undergoing ART (Rosenfield 2005; Stadtmauer 2000). Nevertheless, the Essure® device, previously used for tubal occlusion to achieve sterilisation or treat hydrosalpinges, has been recently discontinued worldwide due to safety concerns (Horwell 2017).
Ultrasound‐guided transvaginal aspiration of hydrosalpingeal fluid has also been proposed as an alternative to salpingectomy as it is safe, less invasive and can be performed in an outpatient setting. However, the risk of hydrosalpinx recurrence within two weeks following aspiration has been shown to be as high as 30%, suggesting its long‐term effectiveness may be low (Hammadieh 2008).
Salpingostomy involves incising the fallopian tubes via laparoscopy or laparotomy, thus allowing for the drainage of fluid where hydrosalpinx is present. The benefits of salpingostomy include a low complication rate and the potential for future natural conception (Taylor 2001). Yet a recent systematic review demonstrated that although the live birth rate (LBR) achieved by natural conception following salpingostomy was 25%, the procedure carries a 10% risk of ectopic pregnancy (Chu 2015).
Why it is important to do this review
This review update stems from the need to establish which interventions are safest and most effective in achieving a live birth in women with hydrosalpinx prior to undergoing IVF. In addition to comparing the overall effect of all forms of tubal surgery versus no intervention, there is a need to investigate the relative effectiveness and safety of individual surgical modalities in head‐to‐head comparisons.
Crucially, since the previous version of this review, new randomised controlled data on live birth rates following salpingectomy and tubal occlusion have been published. It is therefore important to reappraise the available evidence in order to best inform women and clinicians when making management decisions.
Objectives
To assess the effectiveness and safety of tubal surgery in women with tubal disease prior to undergoing conventional IVF or intracytoplasmic sperm injection (ICSI).
Methods
Criteria for considering studies for this review
Types of studies
We included all published and unpublished randomised controlled trials (RCTs) comparing women undergoing surgical treatment for tubal disease prior to IVF with a control group receiving no intervention or any other form of surgical treatment. We planned to include cross‐over trials only if phase one data could be extracted. We excluded quasi‐randomised trials.
Types of participants
We included women with a known diagnosis of tubal disease (by means of diagnostic surgery or imaging such as simple ultrasound, hysterosalpingogram or hysterosalpingo‐contrast sonography) due to undergo IVF. There were no exclusion criteria.
Types of interventions
We included all studies where any surgical procedure performed unilaterally and/or bilaterally for tubal disease (such as salpingectomy, tubal occlusion, ultrasound guided/surgical aspiration of hydrosalpinx fluid or salpingostomy) was compared with any other tubal surgery, non‐surgical intervention or no intervention.
Types of outcome measures
Primary outcomes
LBR per woman randomised, defined as the delivery of a live fetus after 22 completed weeks of gestational age (Zegers‐Hochschild 2017)
Surgical complication rate per woman randomised, e.g. intraoperative bleeding or vasomotor instability, infection, need for repeat surgery or overall complications as reported by trialists
Secondary outcomes
Clinical pregnancy rate (CPR) per woman randomised, defined as the presence of one or more gestational sacs on ultrasound (Zegers‐Hochschild 2017)
Multiple pregnancy rate per woman randomised, defined as the number of twin, triplet or higher‐order pregnancies confirmed by ultrasound or delivery
Miscarriage rate per woman randomised, defined as the spontaneous loss of an intrauterine pregnancy prior to 22 completed weeks of gestational age (Zegers‐Hochschild 2017)
Ectopic pregnancy rate per woman randomised, defined as pregnancy outside the uterine cavity as diagnosed by ultrasound, surgical identification or histopathology (Zegers‐Hochschild 2017)
Mean number of oocytes retrieved per woman randomised
Mean number of embryos obtained per woman randomised
If outcomes were not reported as above, sufficient information had to be available to convert results to the outcomes stated above.
Search methods for identification of studies
We performed searches for all published and unpublished RCTs of women diagnosed with tubal disease receiving surgical treatment prior to IVF.
Electronic searches
We searched the following electronic databases;
The Cochrane Gynaecology and Fertility Group (CGFG) Specialised Register of Controlled Trials (PROCITE platform); searched 8 January 2020 (Appendix 1).
Cochrane CENTRAL via the Cochrane Register of Studies Online (CRSO) web platform; searched 8 January 2020 (Appendix 2).
MEDLINE; searched from 1946 to 8 January 2020 (OVID platform) (Appendix 3).
Embase; searched from 1980 to 8 January 2020 (OVID platform) (Appendix 4).
PsycINFO; searched from 1806 to 8 January 2020 (OVID platform) (Appendix 5).
Cumulative Index to Nursing and Allied Health Literature (CINAHL); searched from 1961 to 8 January 2020 (EBSCO platform) (Appendix 6).
We combined the MEDLINE search with the Cochrane highly sensitive strategy for identifying randomised trials, which appears in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). Embase and PsycINFO searches were combined with trial filters developed by the Scottish Intercollegiate Guidelines Network () (www.sign.ac.uk/what-we-do/methodology/search-filters/).
We searched the following additional sources of trials.
-
Trial registers for ongoing and registered trials
ClinicalTrials.gov, a service of the US National Institutes of Health (www.clinicaltrials.gov).
World Health Organization International Clinical Trials Registry Platform search portal (www.who.int/trialsearch).
DARE (Database of Abstracts of Reviews of Effects) in the Cochrane Library at onlinelibrary.wiley.com (for reference lists from relevant non‐Cochrane reviews).
Web of Knowledge (wokinfo.com).
OpenGrey; for unpublished reports from Europe (www.opengrey.eu).
LILACS (Latin American and Caribbean Health Science Information Database); (regional.bvsalud.org).
PubMed and Google (for recent trials not yet indexed in MEDLINE).
Searching other resources
In consultation with the Cochrane Gynaecology and Fertility (CGF) Group Information Specialist, we handsearched relevant journals and conference abstracts that were not covered by the above sources, without language restrictions,
Data collection and analysis
Selection of studies
Two review authors (PM and EXG) carried out an initial screen of titles and abstracts obtained by the searches and identified potentially eligible studies. We then retrieved the full text of all potentially eligible studies. Two review authors (PM and EXG) independently examined the full‐text articles for compliance with the inclusion criteria and selected studies eligible for inclusion in the review. We corresponded with study investigators as required to clarify study eligibility, and resolved disagreements about study eligibility by discussion or through arbitration by a third reviewer (IG). We documented the selection process using a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow chart (Moher 2009) (Figure 1).
1.
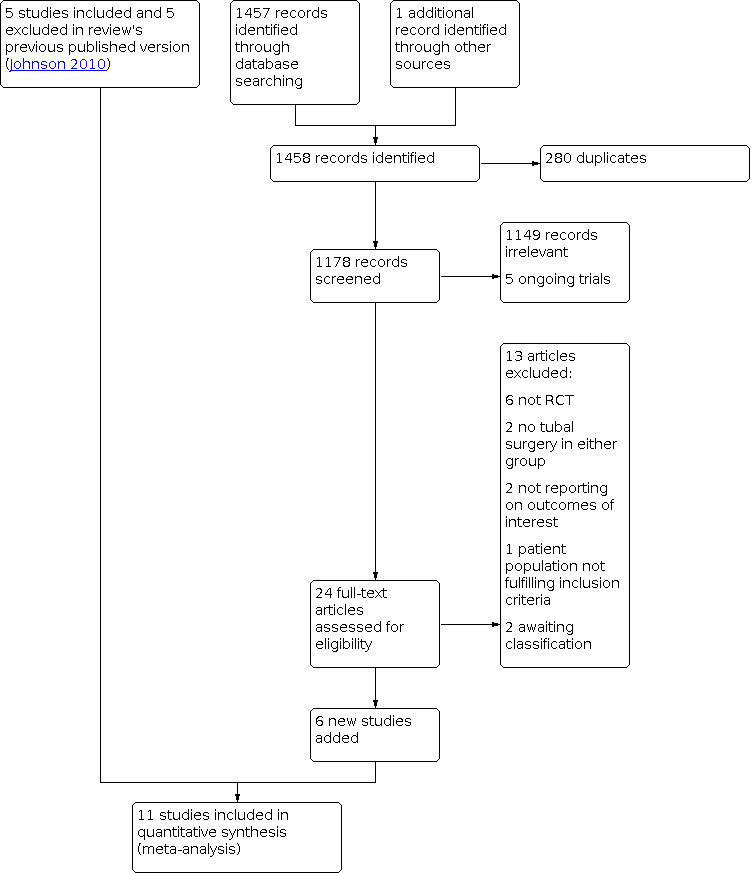
Study flow diagram.
Data extraction and management
Two review authors (PM and EXG) independently assessed study characteristics and methodological details of included studies and extracted data. We resolved differences in opinion by discussion between the two review authors or by consultation with a third review author (IG). Where studies had multiple publications, we assessed overlapping reports and collated them under a single study ID, with multiple references as required. Where we required additional information on trial methodology, original trial data or both, we contacted corresponding authors. We sent reminder correspondence, if we did not receive a reply within two weeks.
Assessment of risk of bias in included studies
Two review authors (PM and EXG) independently assessed the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool (Higgins 2011). We assessed the following parameters: allocation (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other bias. We resolved differences in opinion by consultation with a third reviewer (IG). We described all judgements fully as presented in the 'Risk of bias' table, which has been incorporated into the interpretation of review findings by means of sensitivity analyses.
Measures of treatment effect
We performed statistical analyses according to Cochrane guidance. For dichotomous data (e.g. LBR) we used the number of events in the control and intervention groups of each study to calculate Mantel‐Haenszel risk ratios (RRs) with 95% CIs. We then combined these for meta‐analysis using RevMan 5.3 software and a fixed‐effect model. For outcomes with a small number of events, we used a Peto OR with its 95% CI instead. For continuous data, we calculated mean differences (MDs) or standardized mean difference (SMD) between treatment groups and presented these with 95% CIs for all outcomes.
Unit of analysis issues
The primary analysis was done per woman randomised. We planned to summarise in an additional table data that did not allow valid analysis (e.g. 'per cycle' or 'per pregnancy' data) but did not include these in the meta‐analysis. We counted multiple birth as a single live birth event. We planned to include only first‐phase data obtained from cross‐over trials. If studies reported only 'per cycle' data, we contacted study authors to request 'per woman randomised' data.
Dealing with missing data
We analysed data on an intention‐to‐treat (ITT) basis to the extent possible and attempted to obtain missing data from the original trialists. When data on live birth or clinical pregnancy were unobtainable, we assumed that the outcome did not occur. For other outcomes, we analysed only available data. We planned to subject any imputation to sensitivity analysis.
When studies reported sufficient data to allow calculation of MDs but provided no information on standard deviation (SD), we assumed that the outcome had an SD equal to the highest SD provided by other studies included in the same analysis.
Based on the extent to which data were missing, we explored the potential impact of the missing data on the results by sensitivity analysis.
Assessment of heterogeneity
We assessed statistical heterogeneity by measurement of the I2 statistic to determine whether clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis. We considered an I2 measurement greater than 50% as an indicator of substantial heterogeneity (Higgins 2011). We explored substantial heterogeneity by conducting planned subgroup analyses as detailed below. We took any statistical heterogeneity into account when interpreting the results, especially if variation in the direction of effect was noted.
Assessment of reporting biases
We aimed to minimise the potential impact of publication bias and other reporting bias by ensuring a comprehensive search of multiple databases and grey literature. We planned that if 10 or more studies were included in the same analysis, we would produce a funnel plot to explore the impact of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies) (Higgins 2011).
Data synthesis
We combined data from the primary studies using a fixed‐effect model for the comparison between different tubal surgery techniques or no surgical intervention prior to IVF.
If studies analysing individual surgical techniques were sufficiently similar (e.g. tubal occlusion, hydrosalpinx aspiration, salpingectomy) we combined and stratified the data using a fixed‐effect model in the following comparisons:
-
Surgical treatment (all types) versus no surgery on the fallopian tube, followed by IVF. We stratified this according to the type of tubal surgery undertaken:
Salpingectomy versus no intervention on the fallopian tube, followed by IVF;
-
Occlusion of the fallopian tube versus no intervention on the fallopian tube, followed by IVF
Hysteroscopic tubal occlusion versus no intervention on the fallopian tube
Laparoscopic tubal occlusion versus no intervention on the fallopian tube;
Aspiration of hydrosalpingeal fluid versus no surgery, followed by IVF;
One tubal surgery modality (i.e. salpingectomy, tubal occlusion or aspiration of hydrosalpingeal fluid) versus any other type of tubal surgery.
In meta‐analyses, we graphically displayed an increase in the risk of a particular outcome that may be beneficial (e.g. LBR) or detrimental (e.g. adverse effects rate) to the right of the centre‐line and a decrease in the odds of an outcome to the left of the centre‐line.
Subgroup analysis and investigation of heterogeneity
Where data were available, we aimed to conduct a subgroup analysis to obtain separate evidence for primary outcomes within the following subgroups.
Age: women aged <40 years or ≥40 years. Female age is the principal limiting factor of ART success and could have affected the reported pregnancy outcomes regardless of tubal disease.
Sensitivity analysis
We planned to conduct sensitivity analyses for the primary outcome measures to determine whether conclusions were robust to arbitrary decisions made regarding eligibility and analysis. These analyses were to include consideration of whether review conclusions would have differed if:
eligibility had been restricted to studies at low risk of bias (i.e. no high or unclear risk of selection bias);
a random‐effects model had been adopted;
the unit of analysis had been per clinical pregnancy rather than per woman, for relevant outcomes (multiple pregnancy, miscarriage); or
the summary effect measure was expressed as odds ratio rather than relative risk;
studies with imputed results had been removed from the analysis.
Where we detected substantial heterogeneity, we explored clinical or methodological differences between or among studies that might have accounted for the heterogeneity.
Overall quality of the body of evidence: 'Summary of findings' table
We generated GRADE 'Summary of findings' (SoF) tables using GRADEpro software (GRADEpro GDT 2015). Two review authors (PM and EXG) prepared these tables, working independently. The two review authors resolved disagreements by discussion and consensus.
In using GRADE methodology, review authors considered several criteria to assess the quality of evidence for each outcome across the body of literature. These criteria include study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias. On the basis of these criteria, we justified, documented and incorporated into the SoF tables our judgements about evidence quality (high quality, moderate quality, low quality or very low quality) for all outcomes.
Our SoF tables evaluated the overall quality of the body of evidence for the three main review comparisons (tubal surgery versus non‐surgical or no intervention prior to IVF; tubal occlusion versus laparoscopic salpingectomy; and transvaginal aspiration of hydrosalpingeal fluid versus laparoscopic salpingectomy), and report the main review outcomes (LBR, surgical complication rate, CPR, miscarriage rate and ectopic pregnancy rate).
Results
Description of studies
Results of the search
In the previous version of this review (Johnson 2010), the search strategy identified 103 potentially relevant citations. Five full‐text studies were included in the quantitative synthesis and meta‐analysis (Dechaud 1998; Hammadieh 2008; Kontoravdis 2006; Moshin 2006; Strandell 1999).
For this review update, our electronic searches on 8 January 2020 identified 1457 studies. We identified one additional article via ClinicalTrials.gov (www.clinicaltrials.gov). Following the removal of 280 duplicates, we screened the abstracts of 1178 articles, of which we excluded 1149 as they were clearly not relevant (Characteristics of excluded studies). Five studies were ongoing trials that had not yet reported their results (ChiCTR‐IOR‐16008961; IRCT2014011116161N1; ISRCTN40458453; NCT03521128; PACTR201709002555574) (Characteristics of ongoing studies). We assessed the full text of the remaining 24 studies and excluded 13 references for the following reasons: six were not RCTs (Bao 2016; Harb 2014; Kuzmin 2014; Mardesic 1999; Savic 1999; Yu 2018); two did not feature tubal surgery as an intervention (De Angelis 2010; Kang 2001); one did not focus on the patient population included in this review (Dias Pereira 1999); two did not report on IVF outcomes (Darwish 2006; Mossa 2005); and two studies are awaiting classification due to unclear design (Goldstein 1998) or because it was not clear what surgical intervention had been performed (Lindig 2002). The trialists did not respond to correspondence by the time of publication.
Eleven studies met the inclusion criteria for this review (An 2015; Dechaud 1998; Dreyer 2016; Fouda 2011; Fouda 2015; Hammadieh 2008; Kontoravdis 2006; Labib 2016; Moshin 2006; Strandell 1999; Vignarajan 2019) and were included in our quantitative meta‐analysis. We present the PRISMA study flow diagram in Figure 1.
Included studies
Study design and setting
The previous version of this review included a total of five RCTs and analysed the outcomes of 646 women (Dechaud 1998; Hammadieh 2008; Kontoravdis 2006; Moshin 2006; Strandell 1999). In this update, we included six additional parallel‐design RCTs (An 2015; Dreyer 2016; Fouda 2011; Fouda 2015; Labib 2016; Vignarajan 2019). Of the 11 trials included in the final meta‐analysis, nine have been published as full articles (An 2015; Dechaud 1998; Dreyer 2016; Fouda 2011; Fouda 2015; Hammadieh 2008; Kontoravdis 2006; Strandell 1999; Vignarajan 2019) and two as conference abstracts (Labib 2016; Moshin 2006). A total of 1386 women with tubal disease were analysed in this update on an intention‐to‐treat basis.
Of the included studies, two analysed the efficacy and adverse events of salpingectomy compared to no tubal surgery in women with hydrosalpinges prior to undergoing ART (Dechaud 1998; Strandell 1999); two compared transvaginal aspiration of hydrosalpingeal fluid versus no aspiration (Fouda 2011; Hammadieh 2008); two trials had three randomisation groups, assessing the effect of tubal occlusion in comparison with salpingectomy and no tubal surgery for hydrosalpinges (Kontoravdis 2006; Moshin 2006); three studies compared salpingectomy with tubal occlusion (Dreyer 2016; Labib 2016; Vignarajan 2019); and one article compared salpingectomy to transvaginal aspiration of hydrosalpingeal fluid (Fouda 2015). In one trial (An 2015), 217 women were randomised to one of three groups: Group A underwent transvaginal aspiration of hydrosalpinges in addition to auricular point sticking; Group B received transvaginal aspiration of hydrosalpinges alone; and Group C underwent no intervention. Only groups B and C were included in this analysis. We identified no studies comparing tubal occlusion with aspiration of hydrosalpingeal fluid. Furthermore, our searches did not identify any RCTs where one of the intervention arms underwent salpingostomy for the treatment of tubal disease prior to ART.
Eight of the included studies were single‐centre trials and were carried out in China (An 2015), Egypt (Fouda 2011; Fouda 2015; Labib 2016), France (Dechaud 1998), Moldova (Moshin 2006) and the United Kingdom (Hammadieh 2008). Of the three multicentre studies included, one was a multinational trial carried out in Denmark, Iceland and Sweden (Strandell 1999); and the remaining two were conducted in Greece (Kontoravdis 2006) and in the Netherlands (Dreyer 2016).
An 2015 was the largest trial included in this review, analysing 217 women. Dechaud 1998 was the smallest study, assessing 60 participants.
Loss to follow‐up occurred and was accounted for by An 2015 (n = 12), Dreyer 2016 (n = 4), Fouda 2011 (n = 3) and Kontoravdis 2006 (n = 3). There were no reported cases of loss to follow‐up in the trials by Dechaud 1998, Fouda 2015, Hammadieh 2008, Labib 2016, Moshin 2006, Strandell 1999 and Vignarajan 2019.
Participants
The 11 trials included in this review analysed a total of 1386 women with tubal disease. Of these, 502 underwent salpingectomy; 294 underwent tubal occlusion; 256 were subjected to transvaginal aspiration of hydrosalpingeal fluid; and 334 had no tubal surgery.
Participant age was stated in all of the included studies, and the upper age limit for inclusion did not exceed 41 years in any of the trials. There were no significant differences in the baseline characteristics between the study groups in the trials by Dechaud 1998, Fouda 2011, Fouda 2015, Hammadieh 2008, Kontoravdis 2006 and Vignarajan 2019. An 2015, Labib 2016 and Moshin 2006 did not refer to any differences in baseline characteristics between the trial groups, and did not provide additional details upon further correspondence with the trial authors. Strandell 1999 reported a significantly higher rate of bilateral hydrosalpinges amongst the salpingectomy group (P = 0.02) but no difference in all other baseline parameters such as age and rate of primary infertility.
The presence of unilateral or bilateral hydrosalpinges constituted an inclusion criterion in ten trials (An 2015; Dreyer 2016; Fouda 2011; Fouda 2015; Hammadieh 2008; Kontoravdis 2006; Labib 2016; Moshin 2006; Strandell 1999; Vignarajan 2019), while Dechaud 1998 also included women in whom features of salpingitis isthmica nodosa were identified by hysterosalpingogram (HSG) or laparoscopy.
An 2015, Dechaud 1998, Dreyer 2016 and Strandell 1999 diagnosed tubal disease either by HSG or laparoscopy; Kontoravdis 2006 diagnosed hydrosalpinges with HSG in all participants; Fouda 2011, Fouda 2015, Hammadieh 2008 and Moshin 2006 stated that a diagnosis of hysterosalpinges was made by ultrasound but did not specify whether contrast was used; Vignarajan 2019 diagnosed hydrosalpinges with HSG or transvaginal 2D ultrasound; and Labib 2016 did not specify how they diagnosed hydrosalpinges.
Of the included studies, three (Hammadieh 2008; Kontoravdis 2006; Strandell 1999) included couples with concurrent male factor infertility undergoing ICSI, although the proportions of these couples were similar in groups within the studies. Dreyer 2016 also included couples with male factor infertility, although only three participants underwent ICSI. On further correspondence, the study authors confirmed that none had severe male factor.
Three studies (Hammadieh 2008; Kontoravdis 2006; Moshin 2006) did not specify their exclusion criteria.
No subgroup analyses were prespecified apart from in the trial of Kontoravdis 2006, where IVF treatment outcomes were analysed in the subgroups of women with bilateral hydrosalpinges and ultrasound‐visible hydrosalpinges.
Ten studies (An 2015; Dechaud 1998; Fouda 2011; Fouda 2015; Hammadieh 2008; Kontoravdis 2006; Labib 2016; Moshin 2006; Strandell 1999; Vignarajan 2019) used gonadotropin‐releasing hormone (GnRH) agonists to achieve pituitary desensitisation, while Dreyer 2016 used GnRH agonists or antagonists. Except for Dechaud 1998 and Strandell 1999, all studies reported results over one IVF cycle, although only three trials (Dreyer 2016; Fouda 2011; Kontoravdis 2006) specifically stated that they analysed the first cycle following the allocated intervention.
With the exception of three trials (An 2015; Labib 2016; Moshin 2006), all studies reported on the timing of IVF after the intervention. Of the trials assessing efficacy of salpingectomy or tubal occlusion, four (Dreyer 2016; Kontoravdis 2006; Moshin 2006; Strandell 1999) had a time interval from surgery to IVF of at least two to three months, while in the trial by Vignarajan 2019 IVF was performed no later than 12 weeks following tubal surgery. The trial of Dechaud 1998 had a wider range of time from intervention to IVF, varying from one month to 17 months.
Interventions
Seven studies assessed laparoscopic salpingectomy in one of the intervention arms (Dechaud 1998; Dreyer 2016; Fouda 2015; Kontoravdis 2006; Labib 2016; Strandell 1999; Vignarajan 2019). Four trials assessed transvaginal aspiration of hydrosalpingeal fluid (An 2015; Fouda 2011; Fouda 2015; Hammadieh 2008); of these, three reported that the intervention was performed immediately after oocyte retrieval (Fouda 2011; Fouda 2015; Hammadieh 2008), while An 2015 did not allude to the timing of transvaginal aspiration of hydrosalpingeal fluid. Tubal occlusion was performed laparoscopically in three trials (Kontoravdis 2006; Labib 2016; Vignarajan 2019) and hysteroscopically in one (Dreyer 2016), although the Essure® clips used by Dreyer 2016 have since been discontinued by the manufacturer due to safety concerns. Of the analysed trials, seven included a study arm where no tubal surgery was performed (An 2015; Dechaud 1998; Fouda 2011; Hammadieh 2008; Kontoravdis 2006; Moshin 2006; Strandell 1999).
Of the trials assessing salpingectomy, one (Dechaud 1998) performed laparoscopic bilateral salpingectomy regardless of whether tubal disease was unilateral or bilateral; and five (Dreyer 2016; Fouda 2015; Labib 2016; Strandell 1999; Vignarajan 2019) carried out unilateral or bilateral salpingectomy depending on whether unilateral or bilateral hydrosalpinges were present.
All three trials where laparoscopic tubal occlusion was performed used bipolar diathermy applied to the isthmic segment at two separate sites, without draining the hydrosalpinges (Kontoravdis 2006; Labib 2016; Vignarajan 2019). Dreyer 2016 undertook hysteroscopic bilateral tubal occlusion by placing Essure® micro‐inserts into the proximal end of the Fallopian tube with a special delivery system. Moshin 2006 compared salpingectomy with proximal tubal occlusion and no tubal surgery, although the authors did not specify which surgical routes were employed (i.e. laparoscopic or open salpingectomy; and laparoscopic or hysteroscopic occlusion). We have therefore pooled the results from Moshin 2006 exclusively for the meta‐analysis evaluating tubal surgery (all methods) versus no tubal surgery.
No studies analysed salpingostomy as an intervention to treat tubal disease.
Outcomes
Primary outcomes
None of the included studies investigating the main comparison of tubal surgery (all methods) versus no tubal surgery reported on the primary outcome of live birth per woman randomised. For the comparison of tubal occlusion versus laparoscopic salpingectomy, two studies reported on the primary outcome of live birth per woman randomised (Dreyer 2016; Vignarajan 2019). Dreyer 2016 compared hysteroscopic proximal tubal occlusion with laparoscopic salpingectomy, while Vignarajan 2019 compared laparoscopic tubal occlusion with laparoscopic salpingectomy.
Complication rates were reported as pelvic infection rates and complications directly attributable to surgery (e.g. conversion to laparotomy). Five trials reported on rates of pelvic infection (Dreyer 2016; Fouda 2011; Fouda 2015; Hammadieh 2008; Strandell 1999), and three reported on rates of surgical complications (Dreyer 2016; Fouda 2015; Strandell 1999). Of the surgical complications described, Dreyer 2016 reported one case of umbilical incision infection; Fouda 2015 reported zero cases of surgical complications; and Strandell 1999 reported one case of conversion to laparotomy.
Secondary outcomes
All of the included trials reported on clinical pregnancy rates per woman randomised except for Dechaud 1998, where 'pregnancy' was not defined as a viable, clinical or biochemical pregnancy. Nevertheless, Dechaud 1998 reported on ongoing pregnancy rates, and these numbers were extrapolated for the purposes of meta‐analysis for the outcome 'clinical pregnancy rate'.
An 2015 was the only trial reporting on multiple pregnancy rates.
The rates of miscarriage were reported by all trials apart from Labib 2016 and Moshin 2006, while three trials did not report on the rates of ectopic pregnancy (Labib 2016; Moshin 2006; Vignarajan 2019).
All studies reported outcome rate data as absolute frequencies per woman randomised apart from Kontoravdis 2006, whose outcome data were converted from percentages to absolute numbers by the review authors; and Dechaud 1998, where cumulative pregnancy rates were reported for those who underwent more than one ART cycle, although it was possible to extract data per woman randomised for quantitative analysis.
Following the trial by Strandell 1999, a subsequent analysis was published in 2011 with cumulative results from multiple treatment cycles in the original study population. While the 2011 study carried out both an ITT analysis and an analysis per woman treated, 24 out of 77 women who had initially been randomised to no surgical intervention eventually underwent salpingectomy after one or two failed IVF cycles. The previous version of this review considered the follow‐up data to be unsuitable for meta‐analysis, and we are in agreement. For the 2020 update we have therefore maintained the use of data from the original publication in 1999.
The mean number of oocytes and embryos per woman randomised were reported by five trials (Fouda 2011; Fouda 2015; Hammadieh 2008; Kontoravdis 2006; Moshin 2006). Dechaud 1998 and Strandell 1999 reported these rates per cycle and per treated woman, respectively, and we have therefore not included them in our quantitative analysis. We included the mean number of embryos reported by Dreyer 2016 in our meta‐analysis, but the number of oocytes was reported as median ± interquartile range (IQR) and was therefore not included. Vignarajan 2019 reported on the mean number of oocytes only, while An 2015 and Labib 2016 did not refer to the number of oocytes or embryos obtained in their trials.
The included studies and their methodological details are summarised comprehensively in the Characteristics of included studies table.
Author correspondence
We contacted An 2015, Dreyer 2016, Hammadieh 2008, Labib 2016, Lindig 2002, Strandell 1999 and Vignarajan 2019 to obtain and clarify data. To date, we have received responses from all except for An 2015 and Lindig 2002.
Excluded studies
We excluded 13 references for the following reasons.
Wrong study design (six studies).
No tubal surgery in either group (two studies).
Wrong outcomes (two studies).
Wrong patient population (one study).
Awaiting classification (two studies).
Risk of bias in included studies
We assessed risk of bias in all included studies as demonstrated in Figure 2 and Figure 3. Detailed information can be found in Characteristics of included studies.
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Random sequence generation
Nine studies utilised adequate methods for random sequence generation, such as computer‐generated random number tables, and we therefore deemed them to be at low risk of bias (An 2015; Dreyer 2016; Fouda 2011; Fouda 2015; Hammadieh 2008; Kontoravdis 2006; Labib 2016; Strandell 1999; Vignarajan 2019). Although Dechaud 1998 and Moshin 2006 stated that randomisation occurred, the authors did not specify by which methods, and so we judged these studies to be at unclear risk of bias.
Allocation concealment
Eight studies reported adequate methods for allocation concealment, such as sequentially numbered, sealed opaque envelopes, and we therefore considered them to be at low risk of bias (Dreyer 2016; Fouda 2011; Fouda 2015; Hammadieh 2008; Kontoravdis 2006; Labib 2016; Moshin 2006; Strandell 1999). The remaining three studies provided no relevant details, and so we judged them to be at unclear risk of bias (An 2015; Dechaud 1998; Vignarajan 2019).
Blinding
Blinding of participants and personnel (performance bias)
Vignarajan 2019 was the only study reporting blinding of participants and personnel. We judged it to be at low risk of performance bias. In seven of the included trials, no blinding was performed (An 2015; Dreyer 2016; Fouda 2011; Fouda 2015; Hammadieh 2008; Labib 2016; Strandell 1999) and so we considered these studies to be at high risk of performance bias. No details of blinding were provided for three trials (Dechaud 1998; Kontoravdis 2006; Moshin 2006) and we considered these studies to be at an unclear risk of performance bias.
Blinding of outcome assessment (detection bias)
None of the included studies reported on blinding of outcome assessment, and we therefore judged them all to be at unclear risk of detection bias (An 2015; Dechaud 1998; Dreyer 2016; Fouda 2011; Fouda 2015; Hammadieh 2008; Kontoravdis 2006; Labib 2016; Moshin 2006; Strandell 1999; Vignarajan 2019).
Incomplete outcome data
Three trials reported outcomes on fewer women than the number originally randomised, and we thus judged them to be at high risk of attrition bias (An 2015; Fouda 2011; Kontoravdis 2006). The remaining eight studies analysed all randomised women on an ITT basis (Dechaud 1998; Dreyer 2016; Fouda 2015; Hammadieh 2008; Labib 2016; Moshin 2006; Strandell 1999; Vignarajan 2019) and so we judged them to be at low risk of attrition bias.
Selective reporting
Ten studies reported on a priori outcomes, and we judged these studies to be at low risk of reporting bias (Dechaud 1998; Dreyer 2016; Fouda 2011; Fouda 2015; Hammadieh 2008; Kontoravdis 2006; Labib 2016; Moshin 2006; Strandell 1999; Vignarajan 2019). An 2015 did not include an a priori statement of outcomes to be studied, and so we deemed it to be at unclear risk of bias.
Other potential sources of bias
We deemed seven studies to be at low risk of other bias (Dechaud 1998; Dreyer 2016; Fouda 2011; Fouda 2015; Hammadieh 2008; Kontoravdis 2006; Strandell 1999). We deemed three studies to be at unclear risk of other bias, mostly due to a lack of information on participants' baseline characteristics (An 2015; Labib 2016; Moshin 2006). We considered and one study to be at high risk of other bias due to recruitment ending prematurely (Vignarajan 2019).
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Tubal surgery versus no surgery for tubal disease in women due to undergo in vitro fertilisation.
| Tubal surgery compared to no surgery for tubal disease in women due to undergo in vitro fertilisation | ||||||
| Patient or population: tubal disease in women due to undergo in vitro fertilisation Setting: assisted reproduction clinic Intervention: tubal surgery Comparison: no tubal surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no tubal surgery | Risk with tubal surgery | |||||
| Live birth rate | No studies reported on this outcome for the main comparison. | |||||
| Surgical complication rate ‐ conversion to laparotomy | ||||||
| Salpingectomy (all methods) | 0 per 1,000 | 0 per 1,000 (0 to 0) | Peto OR 5.80 (0.11 to 303.69) | 204 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,d | We are uncertain of the effect of salpingectomy on the rate of conversion to laparotomy. |
| Surgical complication rate ‐ pelvic infection | ||||||
| Salpingectomy (all methods) | 0 per 1,000 | 0 per 1,000 (0 to 0) | Peto OR 5.80 (0.11 to 303.69) | 204 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,d | We are uncertain of the effect of salpingectomy on the rate of pelvic infection. |
| Transvaginal aspiration of hydrosalpingeal fluid | 0 per 1,000 | 0 per 1,000 (0 to 0) | Not estimable | 176 (1 RCT) | ‐ | There were insufficient data to estimate differences between groups. |
| Clinical pregnancy rate | ||||||
| Salpingectomy (all methods) | 186 per 1,000 | 376 per 1,000 (268 to 524) | RR 2.02 (1.44 to 2.82) | 455 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | Salpingectomy probably increases clinical pregnancy rate. |
| Tubal occlusion (all methods) | 123 per 1,000 | 396 per 1,000 (212 to 740) | RR 3.21 (1.72 to 5.99) | 209 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | Tubal occlusion may increase clinical pregnancy rate. |
| Transvaginal aspiration of hydrosalpingeal fluid | 178 per 1,000 | 297 per 1,000 (196 to 453) | RR 1.67 (1.10 to 2.55) | 311 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | We are uncertain whether transvaginal aspiration of hydrosalpingeal fluid increases clinical pregnancy rate. |
| Miscarriage rate | ||||||
| Salpingectomy (all methods) | 53 per 1,000 | 48 per 1,000 (18 to 126) | Peto OR 0.91 (0.33 to 2.52) | 329 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | Salpingectomy may have little or no difference in miscarriage rate. |
| Tubal occlusion (all methods) | 67 per 1,000 | 40 per 1,000 (4 to 411) | Peto OR 0.55 (0.04 to 8.43) | 65 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,d | We are uncertain of the effect of tubal occlusion on miscarriage rate. |
| Transvaginal aspiration of hydrosalpingeal fluid | 44 per 1,000 | 56 per 1,000 (21 to 148) | Peto OR 1.27 (0.44 to 3.66) | 311 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | We are uncertain of the effect of transvaginal aspiration of hydrosalpingeal fluid on miscarriage rate. |
| Ectopic pregnancy rate | ||||||
| Salpingectomy (all methods) | 23 per 1,000 | 8 per 1,000 (1 to 55) | Peto OR 0.29 (0.04 to 2.11) | 329 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | Salpingectomy may reduce ectopic pregnancy rate. |
| Tubal occlusion (all methods) | 0 per 1,000 | 0 per 1,000 (0 to 0) | Peto OR 3.67 (0.04 to 384.48) | 65 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,d | We are uncertain of the effect of tubal occlusion on miscarriage rate. |
| Transvaginal aspiration of hydrosalpingeal fluid | 15 per 1,000 | 10 per 1,000 (2 to 61) | Peto OR 0.59 (0.08 to 4.61) | 311 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | We are uncertain of the effect of transvaginal aspiration of hydrosalpingeal fluid on ectopic pregnancy rate. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IVF/ICSI: in vitro fertilisation/intracytoplasmic sperm injection; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for imprecision: wide confidence intervals.
bDowngraded one level for imprecision: low number of participants.
cDowngraded one level for risk of bias: at least one study with two domains at high risk of bias.
dDowngraded one level for imprecision: single small study.
Summary of findings 2. Laparoscopic proximal tubal occlusion versus laparoscopic salpingectomy for tubal disease in women due to undergo in vitro fertilisation.
| Laparoscopic proximal tubal occlusion versus laparoscopic salpingectomy for tubal disease in women due to undergo in vitro fertilisation | ||||||
| Patient or population: tubal disease in women due to undergo in vitro fertilisation Setting: assisted reproduction clinic Intervention: proximal tubal occlusion Comparison: laparoscopic salpingectomy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with laparoscopic salpingectomy | Risk with proximal tubal occlusion | |||||
| Live birth rate | ||||||
| Laparoscopic proximal tubal occlusion vs laparoscopic salpingectomy | 268 per 1,000 | 325 per 1,000 (204 to 523) | RR 1.21 (0.76 to 1.95) | 165 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | We are uncertain of the effect of laparoscopic proximal tubal occlusion on live birth rate compared to laparoscopic salpingectomy. |
| Surgical complication rate ‐ wound infection | No study reported on this outcome for laparoscopic proximal tubal occlusion. | |||||
| Surgical complication rate ‐ pelvic infection | No study reported on this outcome for laparoscopic proximal tubal occlusion. | |||||
| Clinical pregnancy rate | ||||||
| Laparoscopic proximal tubal occlusion vs laparoscopic salpingectomy | 410 per 1,000 | 332 per 1,000 (254 to 439) | RR 0.81 (0.62 to 1.07) | 347 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,c,d | We are uncertain of the effect of laparoscopic proximal tubal occlusion on clinical pregnancy rate compared to laparoscopic salpingectomy. |
| Miscarriage rate | ||||||
| Laparoscopic proximal tubal occlusion vs laparoscopic salpingectomy | 30 per 1,000 | 23 per 1,000 (5 to 98) | Peto OR 0.74 (0.16 to 3.34) | 265 (2 RCTs) | ⊕⊕⊝⊝ Lowa,c | Laparoscopic proximal tubal occlusion may reduce miscarriage rate slightly compared to laparoscopic salpingectomy. |
| Ectopic pregnancy rate | ||||||
| Laparoscopic proximal tubal occlusion vs laparoscopic salpingectomy | 0 per 1,000 | 0 per 1,000 (0 to 0) | Peto OR 7.39 (0.15 to 372.38) | 100 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | We are uncertain of the effect of laparoscopic proximal tubal occlusion on ectopic pregnancy rate compared to laparoscopic salpingectomy. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IVF/ICSI: in vitro fertilisation/intracytoplasmic sperm injection; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level for imprecision: low number of participants.
bDowngraded one level for imprecision: single small study.
cDowngraded one level for imprecision: wide confidence intervals.
dDowngraded one level for inconsistency: high degree of heterogeneity.
Summary of findings 3. Transvaginal aspiration of hydrosalpingeal fluid versus laparoscopic salpingectomy for tubal disease in women due to undergo in vitro fertilisation.
| Transvaginal aspiration of hydrosalpinx versus laparoscopic salpingectomy for tubal disease in women due to undergo in vitro fertilisation | ||||||
| Patient or population: tubal disease in women due to undergo in vitro fertilisation Setting: assisted reproduction clinic Intervention: transvaginal aspiration of hydrosalpinx Comparison: laparoscopic salpingectomy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with laparoscopic salpingectomy | Risk with transvaginal aspiration of hydrosalpingeal fluid | |||||
| Live birth rate | No studies reported on this outcome. | |||||
| Surgical complication rate | 0 per 1,000 | 0 per 1,000 (0 to 0) | not estimable | 160 (1 RCT) | ‐ | There were insufficient data to estimate differences between groups. |
| Clinical pregnancy rate | 400 per 1,000 | 276 per 1,000 (176 to 428) | RR 0.69 (0.44 to 1.07) | 160 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | We are uncertain of the effect of transvaginal aspiration of hydrosalpingeal fluid on clinical pregnancy rate compared to laparoscopic salpingectomy. |
| Miscarriage rate | 38 per 1,000 | 38 per 1,000 (8 to 180) | Peto OR 1.00 (0.20 to 5.08) | 160 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | We are uncertain of the effect of transvaginal aspiration of hydrosalpingeal fluid on miscarriage rate compared to laparoscopic salpingectomy. |
| Ectopic pregnancy rate | 0 per 1,000 | 0 per 1,000 (0 to 0) | Peto OR 7.39 (0.15 to 372.38) | 160 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | We are uncertain of the effect of transvaginal aspiration of hydrosalpingeal fluid on ectopic pregnancy rate compared to laparoscopic salpingectomy. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IVF/ICSI: in vitro fertilisation/intracytoplasmic sperm injection; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level for imprecision: low number of participants.
bDowngraded one level for imprecision: single small study.
cDowngraded one level for imprecision: wide confidence intervals.
I. Comparison of surgical treatment of hydrosalpinges (all methods) versus no tubal surgery
Primary outcomes
1.0 Live birth rate
None of the included studies reported on the outcome of live birth rate (LBR) for this comparison.
1.1 Surgical complication rate ‐ conversion to laparotomy
We are uncertain of the effect of salpingectomy on the rate of conversion to laparotomy (Peto OR 5.80, 95% CI 0.11 to 303.69; one RCT; n = 204; very low‐quality evidence; Analysis 1.1). Sensitivity analysis based on a random‐effects model showed the same estimates as those obtained with the fixed‐effect model. We found no studies reporting on other types of tubal surgery, such as tubal occlusion or transvaginal aspiration of hydrosalpingeal fluid versus no tubal surgery.
1.1. Analysis.

Comparison 1: Tubal surgery (all methods) vs no tubal surgery, Outcome 1: Surgical complication rate ‐ conversion to laparotomy
1.2 Surgical complication rate ‐ pelvic infection
We are uncertain of the effect of salpingectomy on the rate of pelvic infection (Peto OR 5.80, 95% CI 0.11 to 303.69; one RCT; n = 204; low‐quality evidence; Analysis 1.2). There were no cases of pelvic infection in the study analysing transvaginal aspiration of hydrosalpingeal fluid versus no intervention and we were thus unable to identify a difference between groups for this comparison (Peto OR not estimable; one RCT; n = 176; Analysis 1.2). Sensitivity analysis based on a random‐effects model showed the same estimates as those obtained with the fixed‐effect model. We found no studies reporting on tubal occlusion versus no tubal surgery for this outcome.
1.2. Analysis.

Comparison 1: Tubal surgery (all methods) vs no tubal surgery, Outcome 2: Surgical complication rate ‐ pelvic infection
Secondary outcomes
1.3 Clinical pregnancy rate
We found moderate‐quality evidence that salpingectomy probably improves CPR in women with tubal disease compared to no tubal surgery (RR 2.02, 95% CI 1.44 to 2.82; four RCTs; n = 455; I2 = 43%; moderate‐quality evidence; Analysis 1.3; Figure 4). This suggests that with a CPR of approximately 19% (186 per 1000) without tubal surgery, the equivalent CPR with salpingectomy lies between 27% and 52% (268 to 524 per 1000). Additionally, we found that tubal occlusion may increase CPR compared to no tubal surgery (RR 3.21, 95% CI 1.72 to 5.99; two RCTs; n = 209; I2 = 0%; low‐quality evidence; Analysis 1.3; Figure 4). This suggests that with a CPR of approximately 12% (123 per 1000) without tubal surgery, the equivalent CPR with tubal occlusion lies between 21% and 74% (212 to 740 per 1000). Finally, we are uncertain whether transvaginal aspiration of hydrosalpingeal fluid increases CPR compared to no tubal surgery (RR 1.67, 95% CI 1.10 to 2.55; three RCTs; n = 311; I2 = 0%; very low‐quality evidence; Analysis 1.3; Figure 4). This suggests that with a CPR of approximately 18% (178 per 1000) without tubal surgery, the equivalent CPR with transvaginal aspiration of hydrosalpingeal fluid lies between 20% and 45% (196 to 453 per 1000).
1.3. Analysis.

Comparison 1: Tubal surgery (all methods) vs no tubal surgery, Outcome 3: Clinical pregnancy rate
4.

Forest plot of comparison: Tubal surgery (all methods) versus no tubal surgery, outcome: 8.1 Clinical pregnancy rate.
1.4 Multiple pregnancy rate
We are uncertain whether transvaginal aspiration of hydrosalpingeal fluid affects the multiple pregnancy rate compared to no tubal surgery (Peto OR 2.15, 95% CI 0.59 to 7.85; one RCT; n = 135; very low‐quality evidence; Analysis 1.4). Similar results are obtained if the multiple pregnancy rate is expressed per clinical pregnancy (Peto OR 2.05, 95% CI 0.45 to 9.42; one RCT; n = 38; very low‐quality evidence; Analysis 1.9).
1.4. Analysis.

Comparison 1: Tubal surgery (all methods) vs no tubal surgery, Outcome 4: Multiple pregnancy rate
1.9. Analysis.

Comparison 1: Tubal surgery (all methods) vs no tubal surgery, Outcome 9: Multiple pregnancy rate (per clinical pregnancy)
1.5 Miscarriage rate
Salpingectomy may have little or no difference in miscarriage rate versus no tubal surgery (Peto OR 0.91, 95% CI 0.33 to 2.52; three RCTs; n = 329; I2 = 0%; low‐quality evidence; Analysis 1.5). We are uncertain of the effect of tubal surgery on miscarriage rate with tubal occlusion (Peto OR 0.55, 95% CI 0.04 to 8.43; one RCT; n = 65; very low‐quality evidence; Analysis 1.5) and transvaginal aspiration of hydrosalpingeal fluid (Peto OR 1.27, 95% CI 0.44 to 3.66; three RCTs; n = 311; I2 = 0%; very low‐quality evidence; Analysis 1.5) versus no tubal surgery. Similar results are obtained if the miscarriage rate is expressed per clinical pregnancy for salpingectomy (Peto OR 0.45, 95% CI 0.14 to 1.48; three RCTs; n = 106; I2 = 0%; low‐quality evidence; Analysis 1.10), tubal occlusion (Peto OR 0.04, 95% CI 0.00 to 2.45; one RCT; n = 22; very low‐quality evidence; Analysis 1.10) and transvaginal aspiration of hydrosalpingeal fluid (Peto OR 0.65, 95% CI 0.19 to 2.27; three RCTs; n = 78; I2 = 0%; very low‐quality evidence; Analysis 1.10).
1.5. Analysis.

Comparison 1: Tubal surgery (all methods) vs no tubal surgery, Outcome 5: Miscarriage rate
1.10. Analysis.

Comparison 1: Tubal surgery (all methods) vs no tubal surgery, Outcome 10: Miscarriage rate (per clinical pregnancy)
1.6 Ectopic pregnancy rate
Salpingectomy may reduce ectopic pregnancy rate versus no surgery (Peto OR 0.29, 95% CI 0.04 to 2.11; three RCTs; n = 329; I2 = 0%; low‐quality evidence; Analysis 1.6). We are uncertain of the effect of tubal occlusion (Peto OR 3.67, 95% CI 0.04 to 384.48; one RCT; n = 65; very low‐quality evidence; Analysis 1.6) and transvaginal aspiration of hydrosalpingeal fluid (Peto OR 0.59, 95% CI 0.08 to 4.61; three RCTs; n = 311; I2 = 0%; very low‐quality evidence; Analysis 1.6) on ectopic pregnancy rate versus no tubal surgery.
1.6. Analysis.

Comparison 1: Tubal surgery (all methods) vs no tubal surgery, Outcome 6: Ectopic pregnancy rate
1.7 Mean number of oocytes
Tubal surgery may have no difference in the mean number of oocytes with salpingectomy (MD 0.79, 95% CI ‐0.87 to 2.45; two RCTs; n = 191; I2 = 0%; low‐quality evidence; Analysis 1.7) and tubal occlusion (MD 0.54, 95% CI ‐0.80 to 1.88; two RCTs; n = 244; I2 = 0%; low‐quality evidence; Analysis 1.7) versus no tubal surgery. We are uncertain of the effect of tubal surgery on the mean number of oocytes with transvaginal aspiration of hydrosalpingeal fluid (MD 0.96, 95% CI ‐0.67 to 2.59; two RCTs; n = 176; I2 = 0%; very low‐quality evidence; Analysis 1.7) versus no tubal surgery .
1.7. Analysis.

Comparison 1: Tubal surgery (all methods) vs no tubal surgery, Outcome 7: Mean number of oocytes
1.8 Mean number of embryos
Salpingectomy may have no difference in the mean number of embryos versus no tubal surgery (MD 0.31, 95% CI ‐1.10 to 1.72; two RCTs; n = 191; I2 = 0%; low‐quality evidence; Analysis 1.8). Additionally, we are uncertain of the effect of tubal surgery on the mean number of embryos with tubal occlusion (MD 0.26, 95% CI ‐1.07 to 1.58; two RCTs; n = 209; I2 = 0%; very low‐quality evidence; Analysis 1.8) and transvaginal aspiration of hydrosalpingeal fluid (MD 0.98, 95% CI ‐0.24 to 2.19; two RCTs; n = 176; I2 = 28%; very low‐quality evidence; Analysis 1.8) versus no tubal surgery.
1.8. Analysis.

Comparison 1: Tubal surgery (all methods) vs no tubal surgery, Outcome 8: Mean number of embryos
II. Comparison of proximal tubal occlusion (all methods) versus laparoscopic salpingectomy for hydrosalpinges
Laparoscopic proximal tubal occlusion versus laparoscopic salpingectomy
Primary outcomes
2.1.1 Live birth rate
We are uncertain of the effect of laparoscopic proximal tubal occlusion on LBR compared to laparoscopic salpingectomy (RR 1.21, 95% CI 0.76 to 1.95; one RCT; n = 165; very low‐quality evidence; Analysis 2.1; Figure 5). Sensitivity analysis using OR to express the summary effect measure showed estimates similar to those obtained with RR for laparoscopic proximal tubal occlusion versus laparoscopic salpingectomy (OR 1.31, 95% CI 0.67 to 2.57). No study was at low risk of bias in this comparison, hence the planned sensitivity analysis could not be performed.
2.1. Analysis.

Comparison 2: Proximal tubal occlusion (all methods) vs laparoscopic salpingectomy, Outcome 1: Live birth rate
5.

Forest plot of comparison: 2 Proximal tubal occlusion (all methods) vs laparoscopic salpingectomy, outcome: 2.1 Live birth rate.
2.2.1 Surgical complication rate ‐ wound infection
None of the included studies reported on the effect of laparoscopic proximal tubal occlusion versus laparoscopic salpingectomy on wound infection.
2.3.1 Surgical complication rate ‐ pelvic infection rate
None of the included studies reported on the effect of laparoscopic proximal tubal occlusion versus laparoscopic salpingectomy on pelvic infection rate.
Secondary outcomes
2.4.1 Clinical pregnancy rate
We are uncertain whether CPR is reduced by laparoscopic proximal tubal occlusion (RR 0.81, 95% CI 0.62 to 1.07; three RCTs; n = 347; I2 = 77%; very low‐quality evidence; Analysis 2.4; Figure 6) compared to laparoscopic salpingectomy.
2.4. Analysis.

Comparison 2: Proximal tubal occlusion (all methods) vs laparoscopic salpingectomy, Outcome 4: Clinical pregnancy rate
6.

Forest plot of comparison: 2 Proximal tubal occlusion (all methods) vs laparoscopic salpingectomy, outcome: 2.4 Clinical pregnancy rate.
2.5.1 Multiple pregnancy rate
None of the included studies reported on the effect of laparoscopic proximal tubal occlusion versus laparoscopic salpingectomy on multiple pregnancy rate.
2.6.1 Miscarriage rate
Laparoscopic proximal tubal occlusion may reduce the miscarriage rate slightly compared to laparoscopic salpingectomy (Peto OR 0.74, 95% CI 0.16 to 3.34; two RCTs; n = 265; I2 = 0%; low‐quality evidence; Analysis 2.6). Similar results are obtained if the miscarriage rate is expressed per clinical pregnancy for laparoscopic tubal occlusion (Peto OR 0.82, 95% CI 0.17 to 3.86; two RCTs; n = 95; I2 = 0%; low‐quality evidence; Analysis 2.11).
2.6. Analysis.

Comparison 2: Proximal tubal occlusion (all methods) vs laparoscopic salpingectomy, Outcome 6: Miscarriage rate
2.11. Analysis.

Comparison 2: Proximal tubal occlusion (all methods) vs laparoscopic salpingectomy, Outcome 11: Miscarriage rate (per clinical pregnancy)
2.7.1 Ectopic pregnancy rate
We are uncertain of the effect of laparoscopic proximal tubal occlusion on ectopic pregnancy rate compared to laparoscopic salpingectomy (Peto OR 7.39, 95% CI 0.15 to 372.38; one RCT; n = 100; very low‐quality evidence; Analysis 2.7).
2.7. Analysis.

Comparison 2: Proximal tubal occlusion (all methods) vs laparoscopic salpingectomy, Outcome 7: Ectopic pregnancy rate
2.8.1 Mean number of oocytes
We are uncertain whether laparoscopic proximal tubal occlusion impacts on the mean number of oocytes compared to laparoscopic salpingectomy (MD 0.4, 95% CI ‐0.67 to 1.48; two RCTs; n = 265; I2 = 17%; very low‐quality evidence; Analysis 2.8).
2.8. Analysis.

Comparison 2: Proximal tubal occlusion (all methods) vs laparoscopic salpingectomy, Outcome 8: Mean number of oocytes
2.9.1 Mean number of embryos
We are uncertain whether the mean number of embryos is affected by laparoscopic proximal tubal occlusion (MD 0.17, 95% CI ‐1.38 to 1.72; one RCT; n = 100; very low‐quality evidence; Analysis 2.9) compared to laparoscopic salpingectomy.
2.9. Analysis.

Comparison 2: Proximal tubal occlusion (all methods) vs laparoscopic salpingectomy, Outcome 9: Mean number of embryos
Hysteroscopic proximal tubal occlusion compared to laparoscopic salpingectomy
One study reported hysteroscopic proximal tubal occlusion versus laparoscopic salpingectomy (Dreyer 2016). The Essure® device used in that study has since been discontinued by the manufacturer.
Primary outcomes
2.1.2 Live birth rate
Hysteroscopic proximal tubal occlusion may reduce LBR compared to laparoscopic salpingectomy (RR 0.46, 95% CI 0.24 to 0.89; one RCT; n = 85; low‐quality evidence; Analysis 2.1; Figure 5). Sensitivity analysis based on a random‐effects model showed the same estimates as those obtained with the fixed‐effect model. Sensitivity analysis using OR to express the summary effect measure showed estimates similar to those obtained with RR for hysteroscopic proximal tubal occlusion versus laparoscopic salpingectomy (OR 0.31, 95% CI 0.12 to 0.81).
2.2.2 Surgical complication rate ‐ wound infection
We are uncertain of the effect of hysteroscopic proximal tubal occlusion on surgical complication rate compared to laparoscopic salpingectomy (Peto OR 0.14, 95% CI 0.00 to 6.98; one RCT; n = 85; very low‐quality evidence; Analysis 2.2). Sensitivity analysis based on a random‐effects model showed the same estimates as those obtained with the fixed‐effect model.
2.2. Analysis.

Comparison 2: Proximal tubal occlusion (all methods) vs laparoscopic salpingectomy, Outcome 2: Surgical complication rate ‐ wound infection
2.3.2 Surgical complication rate ‐ pelvic infection rate
We are uncertain of the effect of hysteroscopic proximal tubal occlusion on pelvic infection rate compared to laparoscopic salpingectomy (Peto OR 7.57, 95% CI 0.15 to 381.46; one RCT; n = 85; very low‐quality evidence; Analysis 2.3). Sensitivity analysis based on a random‐effects model showed the same estimates as those obtained with the fixed‐effect model.
2.3. Analysis.

Comparison 2: Proximal tubal occlusion (all methods) vs laparoscopic salpingectomy, Outcome 3: Surgical complication rate ‐ pelvic infection
Secondary outcomes
2.4.2 Clinical pregnancy rate
We are uncertain whether CPR is reduced by hysteroscopic proximal tubal occlusion (RR 0.53, 95% CI 0.32 to 0.89; one RCT; n = 85; very low‐quality evidence; Analysis 2.4; Figure 6) compared to laparoscopic salpingectomy.
2.5.2 Multiple pregnancy rate
We are uncertain of the effect of hysteroscopic proximal tubal occlusion on multiple pregnancy rate compared to laparoscopic salpingectomy (Peto OR 0.14, 95% CI 0.00 to 6.98; one RCT; n = 85; very low‐quality evidence; Analysis 2.5). Similar results are obtained if the multiple pregnancy rate is expressed per clinical pregnancy (Peto OR 0.22, 95% CI 0.00 to 13.62; one RCT; n = 38; very low‐quality evidence; Analysis 2.10).
2.5. Analysis.

Comparison 2: Proximal tubal occlusion (all methods) vs laparoscopic salpingectomy, Outcome 5: Multiple pregnancy rate
2.10. Analysis.

Comparison 2: Proximal tubal occlusion (all methods) vs laparoscopic salpingectomy, Outcome 10: Multiple pregnancy rate (per clinical pregnancy)
2.6.2 Miscarriage rate
We are uncertain of the effect of hysteroscopic proximal tubal occlusion on the miscarriage rate versus laparoscopic salpingectomy (Peto OR 2.03, 95% CI 0.21 to 20.04; one RCT; n = 85; very low‐quality evidence; Analysis 2.6). Similar results are obtained if the miscarriage rate is expressed per clinical pregnancy for hysteroscopic tubal occlusion (Peto OR 4.59, 95% CI 0.40 to 53.35; one RCT; n = 38; very low‐quality evidence; Analysis 2.11).
2.7.2 Ectopic pregnancy rate
There were no cases of ectopic pregnancy in the included study and we were thus unable to estimate the effect of hysteroscopic proximal tubal occlusion versus laparoscopic salpingectomy (Peto OR not estimable; one RCT; n = 85; Analysis 2.7).
2.8.2 Mean number of oocytes
None of the included studies reported on the effect of hysteroscopic proximal tubal occlusion versus laparoscopic salpingectomy.
2.9.2 Mean number of embryos
We are uncertain whether the mean number of embryos is affected by hysteroscopic proximal tubal occlusion compared to laparoscopic salpingectomy (MD 0.10, 95% CI ‐1.77 to 1.97; one RCT; n = 85; very low‐quality evidence; Analysis 2.9).
III. Comparison of transvaginal aspiration of hydrosalpingeal fluid versus laparoscopic salpingectomy for hydrosalpinges
Primary outcomes
3.0 Live birth rate
None of the included studies reported on the outcome of LBR for this comparison.
3.1 Surgical complication rate
There was insufficient evidence to determine whether transvaginal aspiration of hydrosalpingeal fluid affects the surgical complication rate in comparison to laparoscopic salpingectomy for hydrosalpinges (Peto OR not estimable; one RCT; n = 160; Analysis 3.1). Sensitivity analysis using a random‐effects model was not possible as the OR was not estimable.
3.1. Analysis.

Comparison 3: Transvaginal aspiration of hydrosalpingeal fluid vs laparoscopic salpingectomy, Outcome 1: Surgical complication rate
Secondary outcomes
3.2 Clinical pregnancy rate
We are uncertain of the effect of transvaginal aspiration of hydrosalpingeal fluid on CPR compared to laparoscopic salpingectomy (RR 0.69, 95% CI 0.44 to 1.07; one RCT; n = 160; very low‐quality evidence; Analysis 3.2).
3.2. Analysis.

Comparison 3: Transvaginal aspiration of hydrosalpingeal fluid vs laparoscopic salpingectomy, Outcome 2: Clinical pregnancy rate
Multiple pregnancy rate
None of the included studies reported on the outcome of multiple pregnancy rate for this comparison.
3.3 Miscarriage rate
We are uncertain of the effect of transvaginal aspiration of hydrosalpingeal fluid on miscarriage rate compared to laparoscopic salpingectomy (Peto OR 1.00, 95% CI 0.20 to 5.08; one RCT; n = 160; very low‐quality evidence; Analysis 3.3). Similar results are obtained if the miscarriage rate is expressed per clinical pregnancy (Peto OR 1.53, 95% CI 0.28 to 8.45; one RCT; n = 54; very low‐quality evidence; Analysis 3.7).
3.3. Analysis.

Comparison 3: Transvaginal aspiration of hydrosalpingeal fluid vs laparoscopic salpingectomy, Outcome 3: Miscarriage rate
3.7. Analysis.

Comparison 3: Transvaginal aspiration of hydrosalpingeal fluid vs laparoscopic salpingectomy, Outcome 7: Miscarriage rate (per clinical pregnancy)
3.4 Ectopic pregnancy rate
We are uncertain of the effect of transvaginal aspiration of hydrosalpingeal fluid on ectopic pregnancy rate compared to laparoscopic salpingectomy (Peto OR 7.39, 95% CI 0.15 to 372.38; one RCT; n = 160; very low‐quality evidence; Analysis 3.4).
3.4. Analysis.

Comparison 3: Transvaginal aspiration of hydrosalpingeal fluid vs laparoscopic salpingectomy, Outcome 4: Ectopic pregnancy rate
3.5 Mean number of oocytes
We are uncertain of the effect of transvaginal aspiration of hydrosalpingeal fluid on the mean number of oocytes compared to laparoscopic salpingectomy (MD 0.34, 95% CI ‐0.85 to 1.53; one RCT; n = 160; very low‐quality evidence; Analysis 3.5).
3.5. Analysis.

Comparison 3: Transvaginal aspiration of hydrosalpingeal fluid vs laparoscopic salpingectomy, Outcome 5: Mean number of oocytes
3.6 Mean number of embryos
We are uncertain of the effect of transvaginal aspiration of hydrosalpingeal fluid on the mean number of embryos compared to laparoscopic salpingectomy (MD 0.35, 95% CI ‐0.70 to 1.40; one RCT; n = 160; very low‐quality evidence; Analysis 3.6).
3.6. Analysis.

Comparison 3: Transvaginal aspiration of hydrosalpingeal fluid vs laparoscopic salpingectomy, Outcome 6: Mean number of embryos
IV. Tubal occlusion (all methods) versus aspiration of hydrosalpingeal fluid
No studies reported on this comparison.
V. Laparoscopic salpingectomy versus any other method of salpingectomy
No studies reported on this comparison.
VI. Laparoscopic tubal occlusion versus hysteroscopic tubal occlusion
No studies reported on this comparison.
Discussion
Summary of main results
This is the third update of a Cochrane Review that aimed to determine whether tubal surgery affects reproductive outcomes in women with tubal disease prior to undergoing ART. The first and second updates were conducted in 2004 and 2010, respectively. In addition to the five RCTs analysed in the previous version of this review (Dechaud 1998; Hammadieh 2008; Kontoravdis 2006; Moshin 2006; Strandell 1999), we included five new published manuscripts (An 2015; Dreyer 2016; Fouda 2011; Fouda 2015; Vignarajan 2019) and one new conference paper (Labib 2016) in the current version.
Tubal surgery versus no tubal surgery
In the previous version of this review, the authors identified no studies reporting on LBR. This remains the case in the current version for the main comparison of tubal surgery versus no surgery in women with tubal disease prior to undergoing ART. Nevertheless, our findings suggest that tubal surgery in the form of salpingectomy probably increases the rate of clinical pregnancy in comparison to no intervention in women undergoing ART. The evidence resulting from trials comparing tubal occlusion to no tubal surgery was of low or very low quality, mainly due to a low number of events (often deriving from a single study) and wide CIs of included studies, although our meta‐analysis indicated that tubal occlusion may also increase CPR in comparison to no intervention. There was no clear evidence of a difference between the groups for surgical complication rate, multiple pregnancy rate, miscarriage rate, ectopic pregnancy rate, mean number of oocytes and mean number of embryos. A subgroup analysis for women younger and older than 40 years old was not possible due to a lack of data.
Proximal tubal occlusion versus laparoscopic salpingectomy
Although Dreyer 2016 and Vignarajan 2019 reported for the first time on LBR in women undergoing tubal occlusion (with hysteroscopy and laparoscopy, respectively) versus laparoscopic salpingectomy, the evidence was judged to be of low and very low quality mainly due to low event rates and wide CIs. Overall, however, the evidence suggests that hysteroscopic proximal tubal occlusion may decrease LBR compared to laparoscopic salpingectomy, although this is not the case for laparoscopic proximal tubal occlusion versus laparoscopic salpingectomy, with no evidence found of a difference in the latter comparison. Furthermore, there was no clear evidence of a difference between the groups for surgical complication rate, CPR, multiple pregnancy rate, miscarriage rate, ectopic pregnancy rate, mean number of oocytes and mean number of embryos. We did not conduct a subgroup analysis based on age due to the paucity of data. It is important to reiterate that the Essure® device used by Dreyer 2016 for hysteroscopic proximal tubal occlusion has now been discontinued and that no other hysteroscopic devices have been studied since in RCTs analysing the treatment of hydrosalpinges prior to IVF/ICSI.
Transvaginal aspiration of hydrosalpinx versus laparoscopic salpingectomy
When transvaginal aspiration of hydrosalpingeal fluid was compared to laparoscopic salpingectomy, the evidence was insufficient to conclude whether or not there was a difference between groups in live birth rate, surgical complication rate, clinical pregnancy rate, multiple pregnancy rate, ectopic pregnancy rate, mean number of oocytes and mean number of embryos.
Overall completeness and applicability of evidence
In this review, we included 11 studies with 1386 women. The study populations were broadly similar in terms of age and tubal disease. All of the included trials defined tubal disease as the presence of hydrosalpinx in imaging studies or at the time of laparoscopy, with the exception of Dechaud 1998, where tubal diverticula were, in isolation or concurrently to hydrosalpinx, sufficient to make a diagnosis of tubal disease. Given the study populations, the results of this Cochrane Review will be largely applicable to women who have been found to have hydrosalpinges.
Although we were able to include six new trials in the current update, the relatively high number of surgical modalities investigated in the included studies (salpingectomy, tubal occlusion and transvaginal aspiration of hydrosalpingeal fluid) versus no surgical intervention, and the existence of four trials investigating head‐to‐head comparisons between different interventions (Dreyer 2016; Fouda 2015; Labib 2016; Vignarajan 2019), increased the number of analysable comparisons but limited our ability to pool data. Most studies investigating salpingectomy versus no tubal surgery or other intervention clearly stated that salpingectomy was performed laparoscopically, with the exception of Moshin 2006, a conference abstract that did not define the mode of salpingectomy (laparoscopy or laparotomy). In the interest of accuracy, we decided to differentiate between laparoscopic and hysteroscopic tubal occlusion for the purposes of meta‐analysis, given that the rates of adverse events are known to be higher with abdominal surgery.
Despite the known detrimental effect of hydrosalpingeal fluid upon the interface between the implanting embryo and the endometrium, none of the included trials reported on hydrosalpinx size. In addition, none of the included studies commented on the presence or absence of ultrasound‐visible fluid in the endometrial cavity.
Although Hammadieh 2008 alluded to the potential impact of hydrosalpinx re‐accumulation on IVF outcomes following transvaginal aspiration of hydrosalpingeal fluid, the authors did not specify the amount of time elapsed between aspiration and embryo transfer in their trial. Furthermore, of the four trials investigating transvaginal aspiration of hydrosalpingeal fluid (An 2015; Fouda 2011; Fouda 2015; Hammadieh 2008), all reported that transvaginal aspiration was performed immediately after oocyte retrieval, except for An 2015 where no mention of timing was made. Only two of the trials (Fouda 2011 and Fouda 2015) specified the amount of time between oocyte retrieval and embryo transfer (2‐3 days, when it is possible that re‐accumulation of hydrosalpinges would have not yet occurred).
Only two of the included studies reported on the primary outcome of LBR per woman randomised (Dreyer 2016; Vignarajan 2019). Nevertheless, all included studies reported on CPR, and we believe that it is reasonable to postulate that the studied interventions are unlikely to affect second and third trimester pregnancy outcomes. It is clear, however, that there remains a paucity of evidence on live birth rates for all of the interventions studied.
In addition, our findings indicate low rates of complications associated with the studied interventions, although only four studies reported on complication rates (Dreyer 2016; Fouda 2011; Fouda 2015; Hammadieh 2008). This precludes any statistically significant comparisons between groups in the outcomes pertaining to adverse events and may to an extent result from overall low participant numbers. Most of the included studies were underpowered for the analyses of either LBR or CPR, with the exception of Fouda 2011 and Kontoravdis 2006, whose authors clearly stated that a power calculation had been performed and followed. Labib 2016 also stated that a power calculation was performed and followed, although the authors reported results in fewer women than those required by their power calculation. It is unclear whether An 2015 and Moshin 2006 performed a power calculation, while in the remaining six trials a power calculation was performed but not followed, mainly due to slow recruitment (Dechaud 1998; Dreyer 2016; Fouda 2015; Hammadieh 2008; Strandell 1999; Vignarajan 2019). It is therefore likely that the included studies were significantly underpowered to detect differences for surgical complication rates, a much rarer outcome than live birth or clinical pregnancy. In addition, larger and more geographically diverse studies are required to ascertain whether region‐specific causes of tubal disease (e.g. genital tuberculosis and pelvic inflammatory disease leading to significant intraabdominal adhesions) may affect the rates of complications associated with abdominal surgery.
Quality of the evidence
The methodological quality of the included studies varied. Of the 11 included trials, nine were published RCTs (An 2015; Dechaud 1998; Dreyer 2016; Fouda 2011; Fouda 2015; Hammadieh 2008; Kontoravdis 2006; Strandell 1999; Vignarajan 2019) and two were conference abstracts (Labib 2016; Moshin 2006). Correspondence with authors resulted in additional details and data being obtained from Labib 2016, while further data for Moshin 2006 had been provided by the trialists for the previous version of this review. The risk of bias for individual studies is summarised in Figure 2 and Figure 3.
Overall, we identified significant potential for performance bias in the included studies. Vignarajan 2019, where laparoscopic salpingectomy was compared with laparoscopic tubal occlusion, was the only trial clearly stating that blinding of both participants and personnel was undertaken. Six trials stated that participants and personnel were not blinded, mainly due to the nature of the studied intervention (surgery versus no surgery, or abdominal versus hysteroscopic surgery) (An 2015; Dreyer 2016; Fouda 2011; Fouda 2015; Hammadieh 2008; Labib 2016). In the remaining three studies, the authors did not clearly state whether blinding of participants and personnel was performed (Dechaud 1998; Kontoravdis 2006; Moshin 2006).
We also identified significant potential for detection bias in the included trials. None of the included studies specifically stated that there had been blinding of outcome assessors, and we therefore judged them all to be an unclear risk of detection bias (An 2015; Dechaud 1998; Dreyer 2016; Fouda 2011; Fouda 2015; Hammadieh 2008; Kontoravdis 2006; Labib 2016; Moshin 2006; Strandell 1999; Vignarajan 2019
Although patient and personnel blinding may present with challenges in the context of surgical trials, blinding of outcome assessors would have been possible in all of the included studies. Nevertheless, it is likely that many of the measured outcomes would not differ had participants and/or personnel been blinded to the intervention.
The potential for attrition bias was significant in three of the included studies, due to incomplete reporting (An 2015; Fouda 2011; Kontoravdis 2006).
The overall low number of participants and events significantly contributed to downgrading of evidence. As discussed above, power calculations were undertaken but not followed by most of the included trials. Larger, multicentre trials are required to investigate the effect of the different modalities of tubal surgery in women with tubal disease prior to undergoing ART.
We rated the quality of the evidence based on the GRADE criteria. Apart from one moderate‐quality result in one review comparison, the quality of the trials was judged to be low or very low. See Table 1, Table 2 and Table 3.
Potential biases in the review process
In this review, we made every effort to identify all eligible studies. We conducted systematic searches of multiple databases, as well as trial registries, to identify unpublished and ongoing studies. However, it is possible that our searches did not identify all unpublished studies. Data were not available to analyse for our predefined subgroup analyses. We conducted sensitivity analysis where possible, as pre‐specified in our review protocol. We contacted trial authors for missing information where needed. While the majority of authors responded to our correspondence, the requested data were not always available or complete. We were unable to construct a funnel plot due to the small number of included studies. Finally, BWJM was one of the lead investigators in Dreyer 2016 and is also a co‐author in this review.
Agreements and disagreements with other studies or reviews
The previous version of this review was published ten years ago (Johnson 2010). Since then, one new head‐to‐head comparison between salpingectomy and transvaginal aspiration of hydrosalpingeal fluid has been studied by Fouda 2015, while the remaining new trials added to evidence from previously studied comparisons (An 2015; Dreyer 2016; Fouda 2011; Labib 2016; Vignarajan 2019). Additionally, we rated the quality of the evidence in this update based on the GRADE criteria, which were not used in the previous versions of this review.
In this update, the evidence attesting to the effect of salpingectomy versus no surgery was of moderate quality for the outcome of clinical pregnancy, and revealed an effect size similar to that demonstrated in the previous version (Johnson 2010).
The evidence on tubal occlusion versus no tubal surgery was of low or very low quality in this update, although it suggests that, similarly to the results of Johnson 2010, tubal occlusion may increase the clinical pregnancy rate in comparison to no surgery, while there was no evidence of a difference in miscarriage and ectopic pregnancy rate.
In spite of two new RCTs comparing transvaginal aspiration of hydrosalpingeal fluid to no tubal surgery (An 2015; Fouda 2011), the evidence on transvaginal aspiration of hydrosalpingeal fluid is of very low quality for all of the assessed outcomes, in line with the previous version of this review where there was insufficient evidence of an effect.
Crucially, for the first time we included studies that reported on live birth rates (Dreyer 2016; Vignarajan 2019), although the quality of the evidence was judged to be low or very low. Further randomised trials assessing this outcome are required, with larger numbers of participants and, where possible, adequate blinding of participants, personnel and outcome assessors.
Our review did not identify RCTs evaluating salpingostomy as a treatment modality for women with tubal disease prior to undergoing ART. We are therefore unable to add to the systematic review of retrospective observational studies by Chu 2015, which demonstrated a poled live birth rate of 25% in women conceiving naturally following salpingostomy and a pooled ectopic pregnancy rate of 10%.
We also report, for the first time, on the number of oocytes and embryos per woman randomised in the included studies. Our findings did not show evidence of a difference in all of the comparisons analysed.
Authors' conclusions
Implications for practice.
We found moderate‐quality evidence that salpingectomy probably increases the clinical pregnancy rate (CPR) in women with tubal disease prior to undergoing assisted reproductive technology (ART), although there is an overall paucity of data on the safety of the studied interventions (salpingectomy, tubal occlusion and transvaginal aspiration of hydrosalpingeal fluid). In addition, although the evidence of the effect of laparoscopic tubal occlusion on CPR versus no tubal surgery was of low‐quality, our findings suggest that laparoscopic tubal occlusion may still be better than no surgery. When making informed choices about treatment options, women would benefit from receiving advice about the overall lack of good‐quality evidence on the efficacy and safety of tubal surgery prior to ART.
Implications for research.
More high‐quality randomised controlled trials (RCTs) are needed to investigate the efficacy and safety of tubal surgery in its multiple iterations prior to ART in women with tubal disease. We specifically suggest that further studies should focus on live birth rate (LBR) and surgical complication rate as the primary outcomes of choice. Moreover, while blinding of participants and personnel may be difficult due to the nature of the interventions studied, future trials should ensure adequate blinding, including of those assessing outcomes.
Overall, we identified RCTs investigating three different modalities of surgical intervention for tubal disease: salpingectomy, tubal occlusion and transvaginal aspiration of hydrosalpingeal fluid. Future studies should indeed compare additional head‐to‐head interventions, specifically between laparoscopic salpingectomy and transvaginal aspiration of hydrosalpingeal fluid, given that the latter may represent a less invasive procedure. Furthermore, while we found low‐quality evidence suggesting that hysteroscopic proximal tubal occlusion with Essure® may decrease the live birth rate when compared to laparoscopic salpingectomy, the Essure® device has been discontinued by the manufacturer in the USA and in the UK due to long‐term safety concerns (Horwell 2017) and thus Essure® is no longer an option in clinical practice. We did not identify any RCTs comparing tubal occlusion using devices other than Essure® with transvaginal aspiration of hydrosalpingeal fluid, and would advocate this as an important comparison to address in future trials.
Finally, the relative paucity of multicentre trials in this review may have contributed to low participant and event rates, thus affecting the quality of the evidence presented. We recommend that future studies involve as many centres as possible, in order to maximise recruitment in a timely manner and generate adequately powered data.
What's new
| Date | Event | Description |
|---|---|---|
| 19 August 2020 | New search has been performed | The addition of six new studies and two new comparisons has led to a change in the conclusions of this review. |
| 19 August 2020 | New citation required and conclusions have changed | This review was updated in January 2020. PM led a team of new authors including EXG, CB and IEG. Previous authors including NJ, SVV, AS and BWJM provided significant input to the current update. Six new trials were included in this review: An 2015; Dreyer 2016; Fouda 2011; Fouda 2015; Labib 2016 and Vignarajan 2019. In addition to strengthening existing data on different head‐to‐head comparisons, the new trials have also provided novel data on the following comparisons: salpingectomy versus tubal occlusion (Dreyer 2016; Labib 2016; Vignarajan 2019); and salpingectomy versus transvaginal aspiration of hydrosalpingeal fluid (Fouda 2015). |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 25 May 2008 | New citation required but conclusions have not changed | This review was updated in August 2008. New authors have joined the team: SVV, AS and BWJM. As compared to 2004; study identification and selection was performed by a second reviewer based upon a slightly adjusted search. Three new trials were included: Kontoravdis 2006; Moshin 2006;and Hammadieh 2008. Therefore this review now includes studies reporting the effect of ultrasound guided aspiration of hydrosalpinges and the efficacy of tubal occlusion. A previously included trial was excluded: Goldstein 1998a. One ongoing study was found: Darwish 2005,2006, 2007; this preliminary study assessed the feasibility of hysteroscopic tubal occlusion with electrocautery. Follow‐up time was not long enough to assess results of pregnancy. Data extraction and data analysis of the three newly included studies was completed by the second reviewer (SVV) in consensus with the first reviewer. Outcomes were extracted according to stricter definitions of Live birth, ongoing pregnancy, viable‐clinical or biochemical pregnancy. Results were imported in Revman 5 and analysis was undertaken. The Cochrane manuscript was rewritten and edited by the same panel and two newly added authors: B.W. Mol and A. Strandell. |
| 29 April 2008 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Helen Nagels, CGF Managing Editor, for her support and guidance in the preparation of this review; Marian Showell, Cochrane Information Specialist, for her help in preparing and conducting the search strategies; and Ms Xiaojing Wu for assessing two Chinese manuscripts and extracting relevant data. We also thank Martin Sowter for his significant contributions to previous versions of this systematic review, and the peer reviewers (Dr Vivienne Moore, Dr Vanessa Jordan and Dr Mohan Kamath) whose helpful comments made this a better piece of work.
Finally, we wish to thank the trial authors who contributed to this review by supplying additional details of their research in all the versions of this review, particularly Evangelos Makrakis, Herve Déchaud, Nahed Hammadieh, Vaeceslav Moshin, Kareem Labib and Kim Dreyer.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility specialised register search strategy
PROCITE platform
Searched 8 January 2020
Keywords CONTAINS "IVF" or "ICSI" or "in‐vitro fertilisation " or "in‐vitro fertilisation procedure" or "in‐vitro fertilisation procedure failure" or "in‐vitro fertilisation techniques" or "in vitro fertilization" or "intracytoplasmic sperm injection" or "intracytoplasmic sperm injection techniques" or Title CONTAINS "IVF" or "ICSI" or "in‐vitro fertilisation " or "in‐vitro fertilisation procedure" or "in‐vitro fertilisation procedure failure" or "in‐vitro fertilisation techniques" or "in vitro fertilization" or "intracytoplasmic sperm injection" or "intracytoplasmic sperm injection techniques" AND Keywords CONTAINS "tubal anastomosis" or "tubal disorders" or "tubal factor" or "tubal occlusion" or "tubal infertility" or "tubal occlusion ‐ proximal" or "tubal patency" or "tubal reconstruction" or "tubal subfertility" or "tuboplasty" or "tube drainage" or "Fallopian tube obstruction" or "Fallopian Tube Fixation" or "falloscopy" or "Salpingolysis" or "salpingotomy" or "hydrosalpinges" or "hydrosalpingies" or "Hydrosalpinx" or "hydrotubation" or "Aspiration" or "salpingectomy" or "*Salpingostomy‐" or Title CONTAINS" tubal anastomosis" or "tubal disorders" or "tubal factor" or "tubal occlusion" or "tubal infertility" or "tubal occlusion ‐ proximal" or "tubal patency" or "tubal reconstruction" or "tubal subfertility" or "tuboplasty" or "tube drainage" or "Fallopian tube obstruction" or "Fallopian Tube Fixation" or "falloscopy" or "Salpingolysis" or "salpingotomy" or "hydrosalpinges" or "hydrosalpingies" or "Hydrosalpinx" or "hydrotubation" or "Aspiration" or "salpingectomy" or "*Salpingostomy‐"
399 records
Appendix 2. CENTRAL via the CENTRAL Register of Studies Online (CRSO) search strategy
Web platform
Searched 8 January 2020
#1 MESH DESCRIPTOR Reproductive Techniques, Assisted EXPLODE ALL TREES 3081 #2 (in vitro fertilisation or in vitro fertilization):TI,AB,KY 3265 #3 (ivf or icsi):TI,AB,KY 6238 #4 (intracytoplasmic sperm injection*):TI,AB,KY 1878 #5 (assisted reproducti* techn*):TI,AB,KY 853 #6 (fertil* or subfertil* or infertil*):TI,AB,KY 13167 #7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 15101 #8 MESH DESCRIPTOR Fallopian Tube Diseases EXPLODE ALL TREES 306 #9 (Fallopian* adj3 Disease*):TI,AB,KY 75 #10 salping*:TI,AB,KY 751 #11 (tub* adj5 (ligation* or electrocauter* or occlusion* or occluded or block* or clamp* or factor* or adhesion* or clip*)):TI,AB,KY 1228 #12 (fallopian* adj5 (ligation* or electrocauter* or occlusion* or occluded or block* or clamp* or factor* or adhesion* or clip*)):TI,AB,KY 34 #13 ((fallopian* or tub*) adj5 Filshie):TI,AB,KY 10 #14 hydrosalpin*:TI,AB,KY 130 #15 ((tube or tubes or tubal) adj5 disease*):TI,AB,KY 235 #16 (surg* adj5 (tube or tubes or tubal)):TI,AB,KY 892 #17 (surg* adj5 fallopian*):TI,AB,KY 36 #18 (laparoscop* adj5 (tube or tubes or tubal)):TI,AB,KY 250 #19 (laparoscop* adj5 fallopian*):TI,AB,KY 16 #20 ((ultrasound guided) adj5 aspiration*):TI,AB,KY 430 #21 (aspirat* adj5 (tube or tubes or tubal)):TI,AB,KY 158 #22 ((tube or tubes or tubal) adj5 interrupt*):TI,AB,KY 12 #23 essure*:TI,AB,KY 26 #24 hysteroscop*:TI,AB,KY 1380 #25 #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 4976 #26 #7 AND #25 900
Appendix 3. MEDLINE seach strategy
OVID platform
Searched from 1946 to 8 January 2020
1 (in vitro fertilisation or in vitro fertilization).tw. (22614) 2 (ivf or icsi).tw. (26771) 3 intracytoplasmic sperm injections.tw. (32) 4 exp reproductive techniques, assisted/ or exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ or exp zygote intrafallopian transfer/ (67958) 5 (ART or embryo transfer or et).tw. (362139) 6 assisted reproducti* techn*.tw. (9868) 7 pregnan$.tw. (490159) 8 (fertil$ or subfertil$ or infertil$).tw. (203471) 9 or/1‐8 (1015727) 10 exp Fallopian Tube Diseases/ (7783) 11 (Fallopian$ adj3 Disease$).tw. (70) 12 salping$.tw. (9762) 13 (fallopian adj3 (ligation or electrocauter$ or occlusion$ or occluded or block$ or clamp$ or factor$ or adhesion$)).tw. (343) 14 (tub$ adj5 adhesion$).tw. (1297) 15 (tub$ adj3 occlusion$).tw. (1780) 16 (tub$ adj3 disease$).tw. (11067) 17 (tub$ adj3 factor$).tw. (4555) 18 (tub$ adj5 block$).tw. (3299) 19 (tub$ adj5 (clamp$ or clip$)).tw. (765) 20 (tub$ adj3 electrocaut$).tw. (30) 21 (tub$ adj5 Filshie$).tw. (28) 22 (tub$ adj3 ligation$).tw. (2217) 23 hydrosalpin$.tw. (941) 24 (surg$ adj5 tub$).tw. (9787) 25 (surg$ adj5 fallopian$).tw. (307) 26 (laparoscop$ adj5 (tube$ or tubal)).tw. (2202) 27 (ultrasound guided adj5 aspiration$).tw. (4584) 28 (aspirat$ adj5 (tube$ or tubal)).tw. (1514) 29 ((tube$ or tubal) adj5 interrupt$).tw. (259) 30 essure$.tw. (297) 31 (clip$ adj5 fallopian$).tw. (34) 32 hysteroscop$.tw. (6655) 33 or/10‐32 (60460) 34 9 and 33 (11496) 35 randomized controlled trial.pt. (498115) 36 controlled clinical trial.pt. (93508) 37 randomized.ab. (465872) 38 placebo.tw. (209671) 39 clinical trials as topic.sh. (189737) 40 randomly.ab. (324757) 41 trial.ti. (210828) 42 (crossover or cross‐over or cross over).tw. (83014) 43 or/35‐42 (1290969) 44 (animals not (humans and animals)).sh. (4627622) 45 43 not 44 (1186322) 46 45 and 34 (713)
Appendix 4. Embase search strategy
OVID platform
Searched from 1980 to 8 January 2020
1 exp embryo transfer/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ (67401) 2 (in vitro fertilisation or in vitro fertilization).tw. (29596) 3 (ivf or icsi or embryo transfer$).tw. (54369) 4 intracytoplasmic sperm injection.tw. (9351) 5 assisted reproducti* techn*.tw. (14957) 6 (fertil$ or subfertil$ or infertil$).tw. (245336) 7 or/1‐4 (92086) 8 (tub$ adj3 disease$).tw. (14047) 9 (fallopian$ adj5 (disease$ or ligation or electrocauter$ or occlusion$ or occluded or block$ or clamp$ or factor$ or adhesion$)).tw. (720) 10 (tub$ adj5 adhesion$).tw. (1614) 11 (tub$ adj5 occlusion$).tw. (2361) 12 (tub$ adj5 block$).tw. (3862) 13 (tub$ adj3 factor$).tw. (5582) 14 (tub$ adj3 (clamp$ or clip$)).tw. (639) 15 (tub$ adj3 electrocaut$).tw. (30) 16 (ultrasound guided adj5 aspiration$).tw. (7158) 17 (laparoscop$ adj5 (tube$ or tubal)).tw. (2898) 18 (aspirat$ adj5 (tube$ or tubal)).tw. (2037) 19 (tub$ adj3 ligation$).tw. (2455) 20 hydrosalpin$.tw. (1392) 21 (surg$ adj5 tub$).tw. (9972) 22 (surg$ adj5 fallopian$).tw. (378) 23 salping$.tw. (13962) 24 (aspirat$ adj5 hydrosalpin$).tw. (31) 25 ((tube$ or tubal) adj3 interrupt$).tw. (170) 26 (clip$ adj5 fallopian$).tw. (19) 27 essure$.tw. (690) 28 exp uterine tube disease/ or exp hydrosalpinx/ or exp salpingitis/ or exp tuboovarian abscess/ or exp uterine tube abscess/ or exp uterine tube occlusion/ (9340) 29 or/8‐28 (67900) 30 29 and 7 (3349) 31 Controlled study/ or randomized controlled trial/ (7185101) 32 double blind procedure/ (165323) 33 single blind procedure/ (37498) 34 crossover procedure/ (61641) 35 drug comparison/ (75079) 36 placebo/ (331164) 37 random$.ti,ab,hw,tn,mf. (1686177) 38 latin square.ti,ab,hw,tn,mf. (4783) 39 crossover.ti,ab,hw,tn,mf. (94664) 40 cross‐over.ti,ab,hw,tn,mf. (30380) 41 placebo$.ti,ab,hw,tn,mf. (430231) 42 ((doubl$ or singl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).ti,ab,hw,tn,mf. (293371) 43 (comparative adj5 trial$).ti,ab,hw,tn,mf. (61481) 44 (clinical adj5 trial$).ti,ab,hw,tn,mf. (1532589) 45 or/31‐44 (8833311) 46 nonhuman/ (6016793) 47 animal/ not (human/ and animal/) (954934) 48 or/46‐47 (6956883) 49 45 not 48 (5671301) 50 49 and 30 (1059)
Appendix 5. PsycINFO search strategy
OVID platform
Searched from 1806 to 8 January 2020
1 exp Infertility/ or exp Reproductive Technology/ (3425) 2 (in vitro fertilisation or in vitro fertilization).tw. (732) 3 (ivf or icsi or et or embryo transfer).tw. (137844) 4 intracytoplasmic sperm injection.tw. (56) 5 or/1‐4 (140834) 6 (tub$ adj3 disease$).tw. (301) 7 (tub$ adj5 adhesion$).tw. (9) 8 (tub$ adj5 occlusion$).tw. (10) 9 (tub$ adj5 block$).tw. (83) 10 (fallopian$ adj3 block$).tw. (2) 11 hydrosalpin$.tw. (0) 12 (surg$ adj5 tub$).tw. (88) 13 (surg$ adj5 fallopian$).tw. (2) 14 salping$.tw. (89) 15 essure$.tw. (2) 16 or/6‐15 (580) 17 5 and 16 (34) 18 random.tw. (57037) 19 control.tw. (436161) 20 double‐blind.tw. (22568) 21 clinical trials/ (11528) 22 placebo/ (5444) 23 exp Treatment/ (1025667) 24 or/18‐23 (1415570) 25 17 and 24 (22)
Appendix 6. CINAHL search strategy
EBSCO platform
Searched from 1961 to 8 January 2020
S47 S23 AND S46 57 S46 S45 NOT S44 619,293 S45 S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 647,524 S44 S42 NOT S43 166,276 S43 MH (human) 2,017,924 S42 S39 OR S40 OR S41 188,872 S41 TI (animal model*) 2,869 S40 MH (animal studies) 109,882 S39 MH animals+ 86,771 S38 AB (cluster W3 RCT) 313 S37 MH (crossover design) OR MH (comparative studies) 249,647 S36 AB (control W5 group) 98,074 S35 PT (randomized controlled trial) 86,214 S34 MH (placebos) 11,559 S33 MH (sample size) AND AB (assigned OR allocated OR control) 3,750 S32 TI (trial) 98,127 S31 AB (random*) 278,212 S30 TI (randomised OR randomized) 96,059 S29 MH cluster sample 3,987 S28 MH pretest‐posttest design 39,169 S27 MH random assignment 56,787 S26 MH single‐blind studies 12,993 S25 MH double‐blind studies 43,175 S24 MH randomized controlled trials 89,156 S23 S7 AND S22 268 S22 S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 9,851 S21 TX essure* 216 S20 TX (surg* N5 filshie*) 1 S19 TX (aspirat* N5 (tube or tubes or tubal)) 352 S18 TX (ultrasound guided N5 aspiration*) 1,193 S17 TX (laparoscop* N5 (tube or tubes or tubal)) 340 S16 TX (surg* N3 tubal) 156 S15 TX (surg* N3 tubes) 1,484 S14 TX (surg* N3 tube) 1,484 S13 TX (surg* N5 fallopian*) 524 S12 TX hydrosalpin* 146 S11 TX(tub* N5 Filshie*). 5 S10 TX(tub* N3 (adhesion* or ligation* or electrocauter* or occlusion* or occluded or block* or clamp* or factor* or disease*)) 6,495 S9 TX(fallopian N3 (adhesion* or ligation* or electrocauter* or occlusion* or occluded or block* or clamp* or factor* or disease*)) 459 S8 (MM "Fallopian Tube Diseases+") 405 S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 13,277 S6 TX ivf or TX icsi 5,015 S5 TX in vitro fertilization 7,063 S4 TX in vitro fertilisation 7,063 S3 TX intracytoplasmic sperm injection* 903 S2 (MM "Fertilization in Vitro") 3,435 S1 (MM "Reproduction Techniques+") 8,981
Appendix 7. Items of data extraction
The following characteristics were assessed: (1) Trial characteristics (a) Study design: (i) Randomised controlled trial (ii) Patient recruitement. (iii) Patient sampling (iv) Patient in‐ and exclusion criteria (v) Duration of follow‐up (vi) Type of follow‐up
(c) Size of study: (i) Number of women recruited (ii) Number of women randomised (iii) Number of women excluded (iv) Number of women withdrawn and lost to follow‐up (v) Number of women analysed
(d) Study setting: (i) Single‐centre or multicentre (ii) Location (iii) Timing and duration
(e) Criteria for surgical treatment prior to IVF: (i) Tubal disease (ii) Hydrosalpinx (iii) Either of the above plus previous failed IVF
(2) Characteristics of the study participants (a) Baseline characteristics (i) Age (ii) Primary or secondary infertility (iii) Duration of infertility (iv) Investigative work‐up ‐ baseline follicle‐stimulating hormone (FSH), semen analysis, diagnosis of tubal pathology, confirmatory test of ovulation (v) Other contributory causes to infertility than tubal disease (vi) Previous treatments ‐ IVF and other treatments
(b) Treatment characteristics (i) IVF protocol (ii) Time from surgery to IVF (iii) Proportion undergoing intracytoplasmic sperm injection (ICSI) (iv) Number of oocytes retrieved at IVF (iii) Fertilisation rate (iv) Number of embryos transferred
(3) Interventions (a) Timing of surgical intervention (b) Nature of surgical intervention (c) Absence of other interventions in treatment and control group
(4) Outcomes (a) Primary (i) Live birth rate (b) Secondary (i) Ongoing pregnancy rate (ii) Viable pregnancy rate (iii) Clinical pregnancy rate (iv) Biochemical pregnancy rate (v) Ectopic pregnancy rate (vi) Miscarriage rate (vii) Multiple pregnancy rate (viii) Surgical complication rate (ix) Ovarian response to IVF
Appendix 8. Assessment of risk of bias
We assesed the following bias risk domains, according to the following terms:
(1) Study size bias ‐ was a power calculation performed and adhered to? (2) Selection bias ‐ was allocation concealment was not performed by any of the following methods: central computer randomisation, on site assignment, or sealed opaque envelopes? (3) Detection bias ‐ were both the physician performing IVF and the outcome assessor blinded? And was the length of follow‐up was adequate to detect the stated outcome measure(s)? Blinding of patient blinding was not applied as a quality criterion; as this sham tubal surgical intervention is not ethical and less relevant as patients cannot influence the occurrence or the detection of pregnancy. (4) Attrition bias ‐ was loss to follow‐up of patients accounted for and dealt with an intention‐to‐treat analysis? (5) Selective reporting bias ‐were there no suggestions of selective reporting? (7) Funding bias ‐ Was there no source of funding of the trial, or was this stated?
All quality items were scored with yes (suggesting the absence or a low risk of bias), no or unclear (suggesting a risks of bias of the certain item).
Data and analyses
Comparison 1. Tubal surgery (all methods) vs no tubal surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Surgical complication rate ‐ conversion to laparotomy | 1 | 204 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.80 [0.11, 303.69] |
| 1.1.1 Salpingectomy (all methods) vs no tubal surgery | 1 | 204 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.80 [0.11, 303.69] |
| 1.2 Surgical complication rate ‐ pelvic infection | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Salpingectomy (all methods) vs no tubal surgery | 1 | 204 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.80 [0.11, 303.69] |
| 1.2.2 Transvaginal aspiration of hydrosalpingeal fluid vs no tubal surgery | 2 | 176 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.3 Clinical pregnancy rate | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 Salpingectomy (all methods) vs no tubal surgery | 4 | 455 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.44, 2.82] |
| 1.3.2 Tubal occlusion (all methods) vs no tubal surgery | 2 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [1.72, 5.99] |
| 1.3.3 Transvaginal aspiration of hydrosalpingeal fluid vs no tubal surgery | 3 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.10, 2.55] |
| 1.4 Multiple pregnancy rate | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 Transvaginal aspiration of hydrosalpingeal fluid vs no tubal surgery | 1 | 135 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.15 [0.59, 7.85] |
| 1.5 Miscarriage rate | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 Salpingectomy (all methods) vs no tubal surgery | 3 | 329 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.33, 2.52] |
| 1.5.2 Tubal occlusion (all methods) vs no tubal surgery | 1 | 65 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.04, 8.43] |
| 1.5.3 Transvaginal aspiration of hydrosalpingeal fluid vs no tubal surgery | 3 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.44, 3.66] |
| 1.6 Ectopic pregnancy rate | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.6.1 Salpingectomy (all methods) vs no tubal surgery | 3 | 329 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.04, 2.11] |
| 1.6.2 Tubal occlusion (all methods) vs no tubal surgery | 1 | 65 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.67 [0.04, 384.48] |
| 1.6.3 Transvaginal aspiration of hydrosalpingeal fluid vs no tubal surgery | 3 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.08, 4.61] |
| 1.7 Mean number of oocytes | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 Salpingectomy (all methods) vs no tubal surgery | 2 | 191 | Mean Difference (IV, Fixed, 95% CI) | 0.79 [‐0.87, 2.45] |
| 1.7.2 Tubal occlusion (all methods) vs no tubal surgery | 2 | 244 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [‐0.80, 1.88] |
| 1.7.3 Transvaginal aspiration of hydrosalpingeal fluid vs no tubal surgery | 2 | 176 | Mean Difference (IV, Fixed, 95% CI) | 0.96 [‐0.67, 2.59] |
| 1.8 Mean number of embryos | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.8.1 Salpingectomy (all methods) vs no tubal surgery | 2 | 191 | Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐1.10, 1.72] |
| 1.8.2 Tubal occlusion (all methods) vs no tubal surgery | 2 | 209 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐1.07, 1.58] |
| 1.8.3 Transvaginal aspiration of hydrosalpingeal fluid vs no tubal surgery | 2 | 176 | Mean Difference (IV, Fixed, 95% CI) | 0.98 [‐0.24, 2.19] |
| 1.9 Multiple pregnancy rate (per clinical pregnancy) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.9.1 Transvaginal aspiration of hydrosalpingeal fluid vs no tubal surgery | 1 | 38 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.05 [0.45, 9.42] |
| 1.10 Miscarriage rate (per clinical pregnancy) | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.10.1 Salpingectomy (all methods) vs no tubal surgery | 3 | 106 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.45 [0.14, 1.48] |
| 1.10.2 Tubal occlusion (all methods) vs no tubal surgery | 1 | 22 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.04 [0.00, 2.45] |
| 1.10.3 Transvaginal aspiration of hydrosalpingeal fluid vs no tubal surgery | 3 | 78 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.19, 2.27] |
Comparison 2. Proximal tubal occlusion (all methods) vs laparoscopic salpingectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Live birth rate | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 Proximal tubal occlusion (laparoscopy) vs laparoscopic salpingectomy | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.76, 1.95] |
| 2.1.2 Proximal tubal occlusion (hysteroscopy) vs laparoscopic salpingectomy | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.24, 0.89] |
| 2.2 Surgical complication rate ‐ wound infection | 1 | 85 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.98] |
| 2.2.1 Proximal tubal occlusion (hysteroscopy) vs laparoscopic salpingectomy | 1 | 85 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.98] |
| 2.3 Surgical complication rate ‐ pelvic infection | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 Proximal tubal occlusion (hysteroscopy) vs laparoscopic salpingectomy | 1 | 85 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.57 [0.15, 381.46] |
| 2.4 Clinical pregnancy rate | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.4.1 Proximal tubal occlusion (laparoscopy) vs laparoscopic salpingectomy | 3 | 347 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.62, 1.07] |
| 2.4.2 Proximal tubal occlusion (hysteroscopy) vs laparoscopic salpingectomy | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.32, 0.89] |
| 2.5 Multiple pregnancy rate | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.5.1 Proximal tubal occlusion (hysteroscopy) vs laparoscopic salpingectomy | 1 | 85 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.98] |
| 2.6 Miscarriage rate | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.6.1 Proximal tubal occlusion (laparoscopy) vs laparoscopic salpingectomy | 2 | 265 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.16, 3.34] |
| 2.6.2 Proximal tubal occlusion (hysteroscopy) vs laparoscopic salpingectomy | 1 | 85 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.03 [0.21, 20.04] |
| 2.7 Ectopic pregnancy rate | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.7.1 Proximal tubal occlusion (laparoscopy) vs laparoscopic salpingectomy | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.15, 372.38] |
| 2.7.2 Proximal tubal occlusion (hysteroscopy) vs laparoscopic salpingectomy | 1 | 85 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.8 Mean number of oocytes | 2 | 265 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.67, 1.48] |
| 2.8.1 Proximal tubal occlusion (laparoscopy) vs laparoscopic salpingectomy | 2 | 265 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.67, 1.48] |
| 2.9 Mean number of embryos | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.9.1 Proximal tubal occlusion (laparoscopy) vs laparoscopic salpingectomy | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐1.38, 1.72] |
| 2.9.2 Proximal tubal occlusion (hysteroscopy) vs laparoscopic salpingectomy | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐1.77, 1.97] |
| 2.10 Multiple pregnancy rate (per clinical pregnancy) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.10.1 Proximal tubal occlusion (hysteroscopy) vs laparoscopic salpingectomy | 1 | 38 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.00, 13.62] |
| 2.11 Miscarriage rate (per clinical pregnancy) | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.11.1 Proximal tubal occlusion (laparoscopy) vs laparoscopic salpingectomy | 2 | 95 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.82 [0.17, 3.86] |
| 2.11.2 Proximal tubal occlusion (hysteroscopy) vs laparoscopic salpingectomy | 1 | 38 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.59 [0.40, 53.35] |
Comparison 3. Transvaginal aspiration of hydrosalpingeal fluid vs laparoscopic salpingectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Surgical complication rate | 1 | 160 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 3.2 Clinical pregnancy rate | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.44, 1.07] |
| 3.3 Miscarriage rate | 1 | 160 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.20, 5.08] |
| 3.4 Ectopic pregnancy rate | 1 | 160 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.15, 372.38] |
| 3.5 Mean number of oocytes | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [‐0.85, 1.53] |
| 3.6 Mean number of embryos | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [‐0.70, 1.40] |
| 3.7 Miscarriage rate (per clinical pregnancy) | 1 | 54 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.53 [0.28, 8.45] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
An 2015.
| Study characteristics | ||
| Methods | Parallel RCT | |
| Participants | Country: China Site: Center for Reproductive Medicine, Gansu Provincial Hospital of Maternity and Children Healthcare, Lanzhou, China. Participants: 229 women with recently diagnosed hydrosalpinx by either HSG or laparoscopy prior to undergoing IVF‐ET, of which Group A (94 women) underwent transvaginal aspiration of hydrosalpinx and auricular point sticking (a form of acupuncture); Group B (89 women) underwent transvaginal aspiration of hydrosalpinx only; and Group C (46 women) underwent no intervention. Mean age: range 20 to 40 years. Inclusion: women with hydrosalpinx diagnosed by contrast imaging or laparoscopy, aged 20 to 40 years, wishing to conceive. Exclusion: patients with any of the following ‐ acute pelvic infection; high blood pressure; endocrine disease (e.g. diabetes, hyperthyroidism, hyperprolactinaemia, Stein‐Leventhal syndrome); endometriosis; and adenomyosis. IVF protocol: A daily injection of GnRH agonist at a dose of 3.75 mg i.m. was administered from day 2 of the menstrual cycle. On day 7 of the cycle, a transvaginal ultrasound was performed to measure endometrial thickness. Following 30 days of GnRH agonist injections, 2 mg estradiol valerate was administered p.o. once daily for four days and increased by 1 mg at a time until endometrial thickness reached 8 to 10 mm. No details were provided regarding oocyte collection, embryo transfer or luteal phase support. |
|
| Interventions | Group A underwent transvaginal aspiration of hydrosalpinx and auricular point sticking; Group B underwent transvaginal aspiration of hydrosalpinx only; and Group C received no intervention. No further details of the interventions are provided. |
|
| Outcomes |
|
|
| Notes | We did not obtain responses after emailing the authors to obtain clarification and further details of their methodology and outcomes. The authors did not specify a trial registration number. It is unclear whether power calculation was undertaken. There is no mention of any funding sources involved in the study. It is unclear whether an intention‐to‐treat analysis was performed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random digit table" |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding performed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) Intention to treat for primary outcome? | High risk | Out of 229 women randomised, only 217 were analysed. No explanation was given for the 12 participants whose data were not analysed. |
| Selective reporting (reporting bias) | Unclear risk | Not specified. |
| Other bias | Unclear risk | Apart from stating that participants' age and duration of infertility did not differ across all groups, no further mention is made regarding differences in demographic characteristics. |
Dechaud 1998.
| Study characteristics | ||
| Methods | Parallel RCT | |
| Participants | Country: France Site: Single centre ‐ Department of Obstetrics and Gynecology, Centre Hospitalier Universitaire Arnaud de Villeneuve, Montpellier, France. Participants: 60 women with severe tubal infertility diagnosed by either HSG or laparoscopic surgery, not suitable for tubal repair, were included (30 underwent laparoscopic salpingectomy, and 30 did not receive any treatment prior to undergoing IVF). Mean age ± SD: 30.6 ± 3.3 (control group) and 31.7 ± 4.5 (salpingectomy group). Inclusion: women with severe tubal infertility, not suitable for tubal repair, with all other fertility investigations normal. HSG criteria for severe tubal disease included extensive inflammatory disease in the proximal part of the tube with diverticula extending to > 2 cm of the isthmus (salpingitis isthmica nodosa) or a hydrosalpinx with poor prognosis due to abnormal mucosal folds or irregular walls. Laparoscopic criteria for severe tubal pathology included the presence of proximal nodes or an inflamed and thick‐walled hydrosalpinx. Adhesions alone were considered insufficient to make a diagnosis of severe tubal pathology. Exclusion: patients older than 40 years; additional causes of infertility; tubal pathology suitable for repair by tubal catheterization, laparoscopic surgery or microsurgical techniques; severe tubal pathology requiring bilateral salpingectomy as part of treatment; lack of patient consent for salpingectomy or randomisation. IVF protocol: All participants underwent pituitary desensitization with GnRH agonist (Decapeptyl LP 3.75 mg i.m.; Ipsen, Paris, France) starting on day 1 or 2 of the menstrual cycle and administered once daily for 14 days. Pituitary desensitization was assessed at the end of the 14 days of GnRH agonist injections by ultrasound (no follicles measuring > 10 mm in diameter) and serum estradiol levels (< 60 pg/mL). All women then received a 7‐day course of i.m. injections of hMG (Humegon; Organon, Paris, France) at a dose of 300 IU once daily and an additional injection of 150 IU once daily. The dose of hMG was adjusted according to daily ultrasound monitoring of follicle development and serum estradiol levels. Once an estradiol level above 1500 pg/mL and at least three follicles measuring more than 17 mm in diameter were identified, hCG at a dose of 5000 IU i.m. was administered. Oocyte retrieval occurred under ultrasound guidance 35 hours after hCG administration. Embryo transfer took place 48 hours after oocyte retrieval in all women for whom embryos were obtained after IVF. The number of embryos transferred was determined according to the age of the participant, the level of ovarian response, the fecundity of the oocytes, embryo morphology and counselling of the couple. |
|
| Interventions | All included women underwent laparoscopy. On the day before surgery, informed consent for potential bilateral salpingectomy was obtained from all participants. At the time of laparoscopy, those with a surgical diagnosis of tubal disease not suitable for repair were randomised to undergo either adhesiolysis and bilateral salpingectomy OR adhesiolysis only. | |
| Outcomes |
|
|
| Notes | For the purposes of meta‐analysis, the review authors used the ongoing pregnancy rate as clinical pregnancy rate. The authors did not specify a trial registration number. A power calculation was performed but not adhered to as the number calculated (322 participants in each group) could not be achieved in the trial setting. No statement regarding competing interests. Presence or absence of funding is not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "the patients were assigned randomly either to undergo bilateral salpingectomy or not to undergo salpingectomy" |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) Intention to treat for primary outcome? | Low risk | Intention to treat analysis is unclear, however the number randomised is the same as the number analysed. No statement regarding loss to follow‐up or withdrawals. |
| Selective reporting (reporting bias) | Low risk | No suggestion of selective reporting. |
| Other bias | Low risk | There were no significant differences in participant characteristics. |
Dreyer 2016.
| Study characteristics | ||
| Methods | Parallel RCT | |
| Participants | Country: The Netherlands Site: Two‐centre study performed in one academic hospital (VU University Medical Centre, Amsterdam, the Netherlands) and one teaching hospital (Spaarne Gasthuis, Hoofddorp, the Netherlands). Participants: 85 women with unilateral or bilateral hydrosalpinges visible at ultrasound and confirmed with HSG or at laparoscopy who were scheduled for an IVF/ICSI treatment, of which 42 were allocated to hysteroscopic proximal tubal occlusion by intratubal device placement (Essure®) and 43 were allocated to laparoscopic salpingectomy prior to ART. Mean age ± SD: 32.6 ± 4.5 years (Essure®) and 32.0 ± 4.5 years (Laparoscopic salpingectomy). Inclusion: Women aged 18 to 41 years old with a diagnosis of unilateral or bilateral hydrosalpinx (defined as a distally occluded Fallopian tube which became pathologically dilated during tubal patency testing) confirmed by HSG or at laparoscopy prior to undergoing ART. Exclusion: pelvic inflammatory disease within the previous 6 months; hydrosalpinges with evidence of proximal blockage; women in whom laparoscopic salpingectomy was precluded by a frozen pelvis diagnosed at a previous laparoscopy; women with type 0 or 1 fibroids interfering with Essure® insertion; and refusal to undergo Essure® insertion. IVF protocol: All participants underwent IVF/ICSI 12 weeks after treatment of hydrosalpinges. GnRH agonists or antagonists were used to achieve pituitary down‐regulation. Further details of the local IVF protocol are not provided. The authors analysed the first IVF/ICSI cycle (including fresh and all frozen‐thawed embryo transfers) following the treatment of hydrosalpinges. |
|
| Interventions | Participants were randomised to undergo either hysteroscopic proximal tubal occlusion with Essure® intratubal devices OR laparoscopic salpingectomy. Hysteroscopic proximal tubal occlusion with Essure® intratubal devices: all Essure® devices (Bayer HealthCare Pharmaceuticals, Inc., Whippany, NJ, USA) were inserted in an outpatient setting with antibiotic prophylaxis (Doxycycline 200 mg for 5 days). A rigid hysteroscope (5.5 mm with 5‐Fr working channel, Olympus Netherlands B.V.) was used and the Essure® micro‐inserts were placed in the proximal end of the Fallopian tube (unilateral or bilateral according to the presence of hydrosalpinx). A maximum of three coils were allowed to protrude into the uterine cavity. A follow‐up HSG was performed 12 weeks after Essure® to confirm proximal occlusion of the hydrosalpinges. Laparoscopic salpingectomy: depending on whether one or two hydrosalpinges were present, a unilateral or bilateral salpingectomy was performed. In those diagnosed with extensive pelvic adhesions at laparoscopy precluding salpingectomy, proximal tubal ligation using a two‐site isthmic diathermy technique was performed as an alternative to salpingectomy. Conversion to laparotomy was not allowed. All participants who underwent laparoscopy received perioperative antibiotic prophylaxis with cefuroxime 1500 mg i.v. and metronidazole 500 mg i.v. |
|
| Outcomes |
|
|
| Notes | Further data on participant characteristics (specifically with regards to the severity of male factor infertility in eligible participants) and outcomes were obtained through written correspondence with the authors. The study was prospectively registered on the Dutch Trial Register (NTR 2073). A power calculation was performed but not followed. The calculated sample size required a total of 426 participants per group, which the authors did not consider feasible. Instead, only women with hydrosalpinges large enough to be visible on ultrasound were included. One author received 'non‐financial support from Conceptus Inc'. The Essure® device has been withdrawn by the manufacturer since publication of Dreyer 2016 and is no longer available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization list with block sizes of four" |
| Allocation concealment (selection bias) | Low risk | "independent data manager" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The study was unblinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) Intention to treat for primary outcome? | Low risk | "Women who were randomised, but never started IVF/ICSI treatment were included for the ITT analyses". Loss to follow‐up was accounted for according to patient allocation: 2 women allocated to Essure® were lost to follow‐up; and another 2 allocated to salpingectomy were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | No suggestion of selective reporting. |
| Other bias | Low risk | There were no differences in baseline characteristics between the two groups. |
Fouda 2011.
| Study characteristics | ||
| Methods | Parallel RCT | |
| Participants | Country: Egypt Site: Single centre ‐ Assisted conception unit of Ahmed Elgazzar hospital, Cairo, Egypt. Participants: 110 women with unilateral or bilateral hydrosalpinges diagnosed by ultrasound, of which 55 patients underwent ultrasound‐guided aspiration of hydrosalpinx prior to IVF‐ET (Group A) and 55 women received no intervention prior to IVF‐ET (Group B). Mean age ± SD: 28.16 ± 3.62 (Group A) and 29.38 ± 4.03 (Group B). Inclusion: women aged 18‐37 years with unilateral or bilateral hydrosalpinges visible by ultrasound; body mass index 19‐29 kg/m2; infertility lasting longer than 1 year; normal basal LH, FSH and prolactin concentrations; and normal semen analysis. Exclusion: patients with uterine fibroids requiring surgical removal; endometriosis; male‐factor infertility requiring ICSI; previous IVF cycles; history of recurrent miscarriage; known endocrinologic disorders; and the presence of systemic disease contraindicating pregnancy. IVF protocol: All included women underwent IVF with a long GnRH agonist protocol using Leuprorelin acetate (Lucrin, Abbott, Australasia) at a daily dose of 1 mg s.c., starting 1 week before the anticipated date of the next menstrual period (usually day 21 of the cycle preceding the IVF‐ET cycle). Once pituitary downregulation was achieved (ultrasound confirming the absence of cysts in the ovaries and endometrial thickness < 5 mm; and serum estradiol < 50 pg/mL), the dose of Leuprorelin acetate was halved and controlled ovarian stimulation was commenced. Where pituitary downregulation failed to occur after 21 days of GnRH agonist therapy, the cycle was cancelled. Controlled ovarian stimulation was performed using highly purified urinary FSH (HP‐uFSH) (Fostimon, IBSA) at a starting dose which varied from 225 IU/day to 300 IU/day according to participant age, basal FSH level and antral follicle count. Following 5 days of HP‐uFSH therapy, dose adjustments were undertaken according to serum estradiol levels and follicle development. Once three or more follicles measuring more than 17 mm in diameter were identified on ultrasound, HP‐uFSH and Leuprorelin acetate were stopped and hCG (Pregnyl; N.V. Organon, OSS, Holland) was administered i.m. at a dose of 10,000 IU. Oocyte retrieval was performed with ultrasound guidance under deep sedation 36 ± 2 h following hCG administration. |
|
| Interventions | Group A: Following oocyte retrieval, an aspiration needle was inserted into the hydrosalpinx under ultrasound guidance to aspirate the hydrosalpingeal fluid, which was sent for microbiology analysis. Antibiotic prophylaxis was undertaken with a single dose of Azithromycin 1000 mg orally and 1 g Ceftriaxone i.m. given 2 h prior to oocyte retrieval. Group B: no intervention. Embryo transfer took place 3 days after oocyte retrieval (no more than three embryos transferred per cycle). A urine pregnancy test was performed at 2 weeks and an ultrasound examination carried out at 5 weeks after embryo transfer to diagnose clinical pregnancy. Luteal phase support was undertaken with progesterone vaginal capsules (Utrogestan, Safe Pharma, Egypt) at a dose of 20 mg three times daily, starting on the day of oocyte retrieval until fetal cardiac activity was identified on ultrasound at 5 weeks or pregnancy was ruled out by a negative beta‐hCG serum test. |
|
| Outcomes |
|
|
| Notes | The study was registered in clinicaltrials.gov (NCT01040351) on 28 December 2009. Power calculation performed and followed. The authors declared no conflict of interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was achieved through computer generated randomization list" |
| Allocation concealment (selection bias) | Low risk | "sequentially numbered, opaque, otherwise identical sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) Intention to treat for primary outcome? | High risk | Loss to follow‐up was accounted for (a total of 3 women did not undergo ART following intervention or no intervention), however an intention to treat analysis was not performed. |
| Selective reporting (reporting bias) | Low risk | No suggestion of selective reporting. |
| Other bias | Low risk | No significant differences in participant characteristics. |
Fouda 2015.
| Study characteristics | ||
| Methods | Parallel RCT | |
| Participants | Country: Egypt Site: Single centre ‐ Assisted conception unit of the Aljazeera (Al Gazeera) hospital, Giza, Egypt. Participants: 160 patients with unilateral or bilateral hydrosalpinx visible on ultrasound, of which 80 were allocated to undergo uni‐ or bilateral laparoscopic salpingectomy and 80 underwent aspiration of hydrosalpinges under ultrasound guidance. Mean age + SD: 28.14 + 3.67 (salpingectomy) and 27.55 + 3.52 (aspiration of hydrosalpinges). Inclusion: women aged 18 to 37 years with unilateral or bilateral hydrosalpinges visible by ultrasound. Exclusion: age < 18 and > 37 years; FSH ≥ 12 IU/L; uterine fibroids requiring surgical treatment; irregular menstrual cycles; previous IVF; BMI <19 or >30; endometriosis; recurrent pregnancy loss; and systemic disease contraindicating pregnancy. IVF protocol: Controlled ovarian stimulation entailed administration the GnRH agonist triptorelin (Decapeptyl, Ipsen, Slough, United Kingdom) starting one week before the anticipated day of a menstrual period, at a dose of 0.1 mg/day. Pituitary down‐regulation was confirmed in those whose serum estradiol level was < 50 pg/mL and endometrial thickness < 5 mm on Day 3 of the next menstrual cycle. Highly purified urinary FSH (HP‐uFSH) (Fostimon, IBSA, Switzerland) was commenced once pituitary down‐regulation was confirmed, with a starting dose between 150 and 300 IU according to ovarian reserve indicators such as age, antral follicle count and basal FSH. The daily dose of HP‐uFSH was adjusted 5 days after starting stimulation depending on follicle development as assessed by ultrasound and serum estradiol levels. Both triptorelin and HP‐uFSH were continued up to and including the day of hCG (Pregnyl; N.V. Organon, Oss, The Netherlands) administration. When 3 or more follicles measuring ≥ 18 mm were identified on ultrasound, ovulation was triggered with 10000 IU hCG. Oocyte retrieval was performed under deep sedation and ultrasound guidance 34 to 36 hours after hCG administration. Both groups received Azithromycin 1000 mg orally and 1 g Cefotaxime i.m. prior to oocyte retrieval. Embryo transfer (maximum 3 embryos) occurred on Day 2 or Day 3 post oocyte retrieval and serum hCG levels were measured 14 days after embryo transfer to diagnose pregnancy. An ultrasound was performed 5 weeks after embryo transfer to confirm pregnancy viability and count the number of gestational sacs in the uterine cavity. Luteal phase support was provided with progesterone suppositories 200 mg twice daily (Prontogest, Marcyrl Pharmaceutical Industries, El Obour, Egypt) starting from the day of oocyte retrieval until 12 weeks of pregnancy or a negative pregnancy test. |
|
| Interventions | Women were allocated to undergo laparoscopic salpingectomy or aspiration of hydrosalpinges. In the laparoscopic salpingectomy group, bilateral salpingectomy was performed using bipolar electrocoagulation, and proximal tubal occlusion with distal fenestration of hydrosalpinx was done in women with extensive pelvic adhesions instead of salpingectomy. A minimum period of 2 months was advised between surgery and oocyte retrieval. Women in the aspiration group underwent aspiration of hydrosalpinx immediately following oocyte retrieval. An aspiration needle was inserted into the hydrosalpinx and suction was applied to aspirate the entirety of the fluid, which was sent for microbiology analysis. A maximum of 3 embryos were transferred per participant 2 or 3 days after oocyte retrieval. |
|
| Outcomes |
|
|
| Notes | The study was registered in clinicaltrials.gov (NCT02008240) on 8 December 2013. A power calculation was performed but not followed as the number calculated (1150 participants in each group) could not be achieved in the trial setting. The authors declared no conflict of interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated randomization list" |
| Allocation concealment (selection bias) | Low risk | "sequentially numbered sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) Intention to treat for primary outcome? | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | No suggestion of selective reporting. |
| Other bias | Low risk | No significant differences in participant characteristics. |
Hammadieh 2008.
| Study characteristics | ||
| Methods | Parallel RCT | |
| Participants | Country: United Kingdom Site: Single centre ‐ Assisted Conception Unit (ACU) of Birmingham Women's Hospital, United Kingdom. Participants: 66 women with unilateral or bilateral hydrosalpinges diagnosed by ultrasound during controlled ovarian stimulation, of which 32 were allocated to undergo ultrasound‐guided aspiration of hydrosalpinx and 34 were allocated to the control group (no aspiration). Mean age ± SD: 33.4 ± 4.5 (aspiration) and 33.9 ± 4.7 (no aspiration). Inclusion: healthy women ≤ 39 years with an ultrasound diagnosis of uni‐ or bilateral hydrosalpinges during controlled ovarian stimulation or who were on the waiting list for elective salpingectomy prior to undergoing IVF. Exclusion: not specified. IVF protocol described in Hughes 1992. |
|
| Interventions | Participants allocated to the aspiration group underwent transvaginal ultrasound‐guided aspiration of hydrosalpinx immediately following oocyte collection, under deep sedation. The aspiration needle was inserted into the hydrosalpinx and suction applied to achieve complete drainage of the fluid contained in the fallopian tube(s) as confirmed by ultrasound, and hydrosalpinx fluid was sent for microbiology culture and sensitivity. In women with bilateral hydrosalpinges, the procedure was performed on both tubes with different sterile needles on each side to avoid cross‐contamination. Antibiotic cover was provided with the intraoperative administration of i.v. amoxicillin 1 g and clavulanic acid 200 mg (or metronidazole 400 mg three times daily for 5 days in those allergic to penicillin), followed by oral azithromycin 500 mg once daily for three days postoperatively. | |
| Outcomes |
|
|
| Notes | This trial was prospectively registered (NCT00566956). A power calculation was performed but not followed as the number calculated (total 158 women, 79 participants per group) could not be achieved in 3 years and the trial was stopped after nearly 4 years of recruitment as several women opted for salpingectomy instead. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer algorithm" |
| Allocation concealment (selection bias) | Low risk | Randomisation by computer algorithm using a third party administrator just prior to oocyte retrieval procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) Intention to treat for primary outcome? | Low risk | Intention to treat analysis performed (as there were no drop‐outs or losses). |
| Selective reporting (reporting bias) | Low risk | No suggestion of selective reporting. |
| Other bias | Low risk | Both groups were comparable in terms of age, cause of infertility and stimulation regimen. |
Kontoravdis 2006.
| Study characteristics | ||
| Methods | Parallel RCT | |
| Participants | Country: Greece Site: Two centres in Athens ‐ Second Department of Obstetrics and Gynecology, University of Athens, Aretaieion Hospital; and the Centre for Human Reproduction. Participants: 115 patients randomised to one of three groups ‐ 50 underwent unilateral or bilateral laparoscopic proximal tubal occlusion before IVF (Group A); 50 underwent laparoscopic unilateral or bilateral salpingectomy before IVF (Group B); and 15 received no intervention prior to IVF (Group C). Mean age ± SD: 31 ± 4.5 (Group A), 29.8 ± 3.4 (Group B), 34 ± 5.3 (Group C). Inclusion: women aged ≤ 41 years with unilateral or bilateral hydrosalpinges confirmed by HSG and Day 2/3 FSH levels ≤ 12 mIU/mL; presence of spermatozoa in semen; suitability for IVF/ICSI treatment; no contraindication for laparoscopic surgery; no previous IVF attempt; no other pelvic pathology. Exclusion criteria: not specified. IVF protocol: women in groups A and B underwent IVF treatment once two completed menstrual cycles had passed since surgery. All subjects receiving IVF underwent controlled ovarian stimulation with a long protocol. Down‐regulation was achieved with a GnRH analogue administered s.c. from the midluteal phase of the previous cycle; stimulation ensued with recombinant FSH administered s.c. at a daily dose of 150 to 300 IU based on serial ultrasound measurements of follicle growth and serum estradiol quantification. Oocyte retrieval was performed 35 h after the administration of 10000 of hCG. Embryo transfers were performed on Day 3, and the number of embryos transferred varied according to the woman's age and embryo availability/quality. Luteal phase support entailed the vaginal administration of progesterone 600 mg daily; oral doxycycline 100 mg twice daily for 6 days following oocyte retrieval; and oral prednisolone 5 mg three times per day for 6 days following oocyte retrieval. |
|
| Interventions | Women in Group A underwent laparoscopic unilateral or bilateral salpingectomy by transection of the mesosalpinx as close to the fallopian tube as possible. The tube was then removed at 1‐1.5 cm from the cornual junction. Laparoscopic proximal tubal occlusion was performed in patients in Group B by applying bipolar diathermy to the isthmic segment at two separate sites, without draining the hydrosalpinx. |
|
| Outcomes |
|
|
| Notes | The authors did not specify a trial registration number. A power calculation was performed and followed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated randomization in blocks" |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified. The previous version of the review states: "The operator and the IVF performer were the same person in some cases". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) Intention to treat for primary outcome? | High risk | 112 women analysed of 115 randomised. Group A: 2 women did not proceed to IVF treatment after tubal occlusion. Group B: 1 woman did not proceed to IVF treatment after salpingectomy. Intention to treat analysis not explicitly stated. |
| Selective reporting (reporting bias) | Low risk | No suggestions of selective outcome reporting. |
| Other bias | Low risk | There were no significant differences in demographic characteristics between groups. |
Labib 2016.
| Study characteristics | ||
| Methods | Parallel RCT | |
| Participants | Country: Egypt Site: single centre ‐ Ain Shams University Maternity Hospital, Egypt. Participants: 82 patients with bilateral hydrosalpinx were randomised to undergo laparoscopic salpingectomy (n = 41) or proximal tubal occlusion and division (n = 41). Mean age ± SD: 29.4 ± 3.19 years (salpingectomy group) and 30.14 ± 3.14 years (tubal occlusion and division group). Inclusion: age 25 to 35 years; regular menstrual cycles; no previous abdominal surgery; HSG findings of bilateral hydrosalpinges; normal baseline hormonal profile; previous one or more failed trials of IVF‐ET. Exclusion: age < 25 or > 35 years; previous laparoscopy or laparotomy; known ovulatory dysfunction due to polycystic ovary syndrome; presence of endometriosis. IVF protocol: all patients underwent a long GnRH agonist protocol according to local practice. No further details provided by the authors. |
|
| Interventions | Laparoscopic salpingectomy VERSUS proximal tubal occlusion and division. Laparoscopic salpingectomy was performed using bipolar diathermy. The mesosalpinx was transected just below the fallopian tube to minimize any compromise to the collateral blood supply of the ipsilateral ovary. Adhesiolysis was also performed. Laparoscopic tubal occlusion and division entailed transection of the fallopian tube 1 to 1.5 cm from the cornual end. Proximal tubal occlusion was performed using bipolar diathermy applied at two sites separated by ~1cm on the isthmic portion of the affected tube, and the hydrosalpinx was not drained. |
|
| Outcomes |
|
|
| Notes | This is a conference abstract, and additional information was provided by the first author via written correspondence. The authors did not specify a trial registration number. A power calculation was performed and a total of 84 women were required. However, the authors report a sample of 82 women only (41 randomly allocated to each group). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computer‐generated randomisation system as clarified by written correspondence from the first author. |
| Allocation concealment (selection bias) | Low risk | Allocation was blinded, as clarified by written correspondence from the first author. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Correspondence with the authors revealed that no blinding was undertaken. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) Intention to treat for primary outcome? | Low risk | There was no loss to follow‐up. Analyses were performed according to the intention to treat principle. |
| Selective reporting (reporting bias) | Low risk | No suggestion of selective outcome reporting. |
| Other bias | Unclear risk | Not specified. |
Moshin 2006.
| Study characteristics | ||
| Methods | Parallel RCT | |
| Participants | Country: Moldova Site: single academic centre ‐ Center for Reproductive Health and Genetics, Chisinau, Moldova. Participants: 204 patients recruited, randomised and analysed (Group 1 ‐ 66 women randomised to no surgical treatment; Group 2 ‐ 60 women randomised to salpingectomy; Group 3 ‐ 78 randomised to proximal tubal occlusion); each woman underwent one cycle of IVF as stated by the authors. Age: mean/median not specified; range 22 to 35 years. Inclusion: women with hydrosalpinges diagnosed by ultrasound. Exclusion: not specified. IVF protocol: Ovarian stimulation in a long course GnRH analogue protocol (Decapeptyl or Dipherelin) with a fixed dose (225 IU daily) of recombinant FSH for stimulation, starting on day 3 of the cycle. Ovarian response was monitored by ultrasound and serum E2 concentration. Ovulation was triggered with 10000 IU of hCG (Pregnyl) when the leading follicles reached 18 to 20 mm. Oocyte retrieval was carried out 36 hours after hCG administration. Retrieved oocytes were evaluated and fertilised by conventional insemination. Embryos were transferred on day 3 after insemination. Utrogestan 400 mg/day was administered vaginally from the day of oocyte pick‐up until 12th week of pregnancy. |
|
| Interventions | Salpingectomy versus proximal tubal clamping of hydrosalpinges versus no intervention. The authors did not specify whether salpingectomy was performed laparoscopically or via laparotomy. Additionally, no details on the tubal clamping procedure were provided. | |
| Outcomes |
|
|
| Notes | As data extraction on the abstract was limited, queries were resolved by contacting the author for the previous version of this review. The authors did not specify a trial registration number. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified. |
| Allocation concealment (selection bias) | Low risk | By opaque numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) Intention to treat for primary outcome? | Low risk | Not stated in abstract, but the number of patients randomised was the same as the number of patients analysed. Previous correspondence clarified there was no loss to follow‐up or withdrawal. |
| Selective reporting (reporting bias) | Low risk | No suggestions of selective reporting. |
| Other bias | Unclear risk | Not specified. |
Strandell 1999.
| Study characteristics | ||
| Methods | Parallel RCT | |
| Participants | Country: Denmark, Iceland and Sweden Site: Multicentre trial conducted across 9 Nordic IVF sites. Participants: 204 women with hydrosalpinx were included and randomised to undergo either laparoscopic salpingectomy or no intervention prior to IVF. Age (mean ± SD): 31.8 ± 3.6 years (laparoscopic salpingectomy group) and 31.8 ± 3.7 years (no intervention group). Inclusion: presence of unilateral or bilateral hydrosalpinges as diagnosed by HSG or laparoscopy; suitability for IVF treatment; no contraindications to laparoscopy; age < 39 years at the time of randomisation. Exclusion: previous IVF treatment and the presence of cavity‐distorting uterine fibroids; male‐factor infertility requiring ICSI was accepted in centres where conventional IVF and ICSI success rates were identical. IVF protocol: although regimens varied between centres, a long protocol with GnRH‐agonist given nasally or s.c. was generally used, followed by controlled ovarian stimulation with either HMG of HP‐/rFSH. Transvaginal ultrasound‐guided oocyte retrieval ensued. Although not more than 2 embryos were routinely transferred, this was occasionally increased to 3. |
|
| Interventions | Laparoscopic unilateral or bilateral salpingectomy was performed in the intervention group, depending on whether one or two hydrosalpinges were identified. Where technical difficulties were encountered (e.g. due to extensive adhesions), a proximal ligation and distal fenestration was performed instead. While in the intervention group 2 months were advised between surgery and IVF treatment in order to allow for the wash‐out of hydrosalpingeal fluid, in the control group women underwent ART immediately following randomisation. | |
| Outcomes |
|
|
| Notes | A subsequent analysis of cumulative data obtained from subsequent cycles in both groups was published by the trialists in 2001. Although an intention‐to‐treat analysis was performed in the follow‐up data, 24 women who had initially been assigned to the control group eventually underwent surgery after failed IVF cycles and thus their outcomes were not included in this review. The authors did not specify a trial registration number. A power calculation was performed but not followed as the sample size required (300) could not be reached within the duration of the study. The study was supported by grants from the Göteborg Medical Society, the Hjalmar Svensson Foundation and a society named "Ordensällskapet W:6". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed with sealed opaque envelopes in blocks of 10 to 30. |
| Allocation concealment (selection bias) | Low risk | Randomisation performed with sealed opaque envelopes in blocks of 10 to 30. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding was performed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) Intention to treat for primary outcome? | Low risk | An intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | No suggestions of selective reporting. |
| Other bias | Low risk | There were no significant differences in baseline characteristics between groups apart from the rate of bilateral hydrosalpinges at inclusion, which was higher in the salpingectomy group (59% versus 41%, P = 0.02). |
Vignarajan 2019.
| Study characteristics | ||
| Methods | Parallel RCT | |
| Participants | Country: India Site: single centre ‐ Reproductive Medicine Unit, Department of Obstetrics and Gynecology, All India Institute of Medical Sciences, New Delhi, India. Participants: 165 women with bilateral hydrosalpinges were randomised into two groups. Group A included 83 patients who underwent laparoscopic bilateral proximal tubal occlusion; Group B included 82 women who underwent laparoscopic bilateral salpingectomy. There was no control group. Mean age ± SD: 29.3 ± 2.6 years (Group A ‐ proximal tubal occlusion) and 29.4 ± 3.2 (Group B ‐ laparoscopic bilateral salpingectomy). Inclusion: women with bilateral hydrosalpinx diagnosed by HSG or ultrasound; age < 39 years; normal uterine cavity. Exclusion: history of endometriosis; prior ovarian surgery; polycystic ovary syndrome; poor ovarian reserve (FSH > 12 mIU/ml, AMH < 1.0 ng/ml);, adenomyosis; uterine synechiae; and a thin endometrium affecting implantation (not defined). IVF protocol: IVF cycles were undertaken within 12 weeks of tubal surgery. All women underwent a long protocol of pituitary downregulation with GnRH agonist (Leuprolide‐Luprofact, Cadila Healthcare Ltd, Ahmedabad, India) at a daily dose of 0.5 mg from day 21 of the previous cycle. Complete pituitary desensitisation was assessed 14 days after starting GnRH‐agonist and confirmed where serum estradiol < 50 pg/mL, LH < 3 IU/L, no follicles > 10 mm in diameter and endometrial thickness < 5 mm on ultrasound. Recombinant FSH (Gonal F; Merck Serono, Mumbai, India) was administered at a dose of 150 to 300 IU/day varying in accordance to patient age, BMI, AFC and serum AMH. Serial follicle tracking was undertaken to monitor ovarian response and adjust gonadotrophin doses as required. Ovulation was triggered with recombinant hCG (250 mcg, Ovitrel; Merck Serono, Mumbai, India) when at least 3 follicles measuring ≥ 18 mm were identified on ultrasound. Oocyte retrieval was performed 36 hours after maturation trigger, and conventional IVF or ICSI was performed. A fertilisation check was done 16 to 18 hours after insemination, and up to a maximum of 2 good‐quality embryos were transferred on Day 3 or 5 under ultrasound guidance. Progesterone 100 mg i.m. (Susten, Sun Pharma, India) was administered daily for luteal support. Serum beta hCG was checked 16 days after embryo transfer and those with a positive result underwent ultrasound 4 weeks after transfer. |
|
| Interventions | Group A underwent laparoscopic bilateral proximal tubal occlusion by applying bipolar diathermy to the isthmic segment at two separate sites. Hydrosalpinges were not drained. Group B underwent laparoscopic bilateral salpingectomy by transecting the fallopian tubes 1 cm away from the cornual end with bipolar diathermy. |
|
| Outcomes |
|
|
| Notes | Additional trial design and outcome data were obtained through correspondence with the trial authors. This trial was prospectively registered (CTRI/2016/08/007220). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | IVF personnel were blinded as to which surgical intervention participants had undergone prior to ART. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) Intention to treat for primary outcome? | Low risk | Loss of follow‐up was accounted for. Although 6 women allocated to undergo laparoscopic salpingectomy could not receive the intervention due to dense adhesions, an intention‐to‐treat analysis was performed on both groups. |
| Selective reporting (reporting bias) | Low risk | There was no suggestion of selective reporting. |
| Other bias | High risk | Both groups were comparable in terms of age, BMI, type of infertility, duration of infertility, history of genital tuberculosis, ovarian reserve parameters and stimulation regimen. A power calculation was performed but not followed as the number calculated (total 660 women, 330 patients per group) could not be achieved in a single centre. Recruitment was hence stopped prematurely. |
AFC: antral follicle count; AMH: anti‐müllerian hormone; ART: assisted reproductive technology; BMI: body mass index; ET: embryo transfer; FSH: follicle‐stimulating hormone; GnRH: gonadotropin‐releasing hormone; hCG: human chorionic gonadotropin;hMG: human menopausal gonadotropin; HP: highly purified; HSG: hysterosalpingogram; ICSI: intracytoplasmic sperm injection; i.m.: intramuscularly; IQR: interquartile range; IU: international units; i.v.: intravenously; IVF: in vitro fertilisation; IVF‐ET: in vitro fertilisation and embryo transfer; LH: luteinising hormone; n: number of events or participants; p.o.: orally; RCT: randomised controlled trial; SD: standard deviation; uFSH: urinary follicle‐stimulating hormone.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bao 2016 | This was a prospective cohort study. |
| Darwish 2006 | Although this was an RCT, the authors never reported on IVF/ICSI outcomes. |
| De Angelis 2010 | The intervention in this RCT was metroplasty in women with a septate uterus, therefore no tubal surgery was undertaken. |
| Dias Pereira 1999 | This RCT compared systemic multi‐dose intramuscular methotrexate with laparoscopic salpingostomy in the treatment of tubal pregnancy. Although the authors assessed fertility outcomes 18 months later, we excluded this study on the basis that its original population had a diagnosis of ectopic pregnancy, not a preexisting tubal abnormality. |
| Harb 2014 | This was a systematic review and meta‐analysis. |
| Kang 2001 | This was an RCT where 120 women with tubal obstruction diagnosed by HSG or laparoscopy were randomised into three groups: Group A received Chinese herbal medicine and antibiotics; Group B received Chinese herbal medicine only; and Group C underwent injection of placental tissue fluid. As none of the interventions involved tubal surgery, this manuscript was excluded. |
| Kuzmin 2014 | This was not an RCT. |
| Mardesic 1999 | This was not a randomised trial and patients served as their own controls. |
| Mossa 2005 | This was a randomised trial comparing spontaneous pregnancy rates after open versus laparoscopic distal tuboplasty. As no IVF/ICSI outcomes were reported, this study was excluded. |
| Savic 1999 | This was not an RCT. |
| Yu 2018 | This was a retrospective study. |
HSG: hysterosalpingogram; ICSI: intracytoplasmic sperm injection; IVF: in vitro fertilisation; RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
Goldstein 1998.
| Methods | Prospective study ‐ unclear design. |
| Participants | Women with hydrosalpinx undergoing IVF. |
| Interventions | Surgical treatment "designed for decompressing the hydrosalpinx and disconnecting the diseased tube from the uterus" versus medical treatment (daily administration of 400 mg progesterone suppository from day 20 of the menstrual cycle) versus no treatment. |
| Outcomes | Miscarriage rate (n/n, %) Ectopic pregnancy rate (n/n, %) Delivery rate (n/n, %) Post‐operative complication rate (n/n, %) |
| Notes | In this study patients appear to have served as their own controls. In the previous version of this review, we were unsuccessful in obtaining clarification from the study authors as to whether randomisation had been performed. |
Lindig 2002.
| Methods | RCT |
| Participants | 40 women randomised to undergo salpingostomy by microsurgical techniques (n = 20) or no intervention (n = 20) prior to IVF. Inclusion: not specified. Exclusion: not specified. |
| Interventions | Salpingostomy by microsurgical techniques versus no intervention prior to IVF. |
| Outcomes | Intrauterine pregnancy rate per woman randomised and per embryo transfer (n/n, %) Implantation rate (not defined) (%) Miscarriage rate (not defined) (%) Ectopic pregnancy rate (not defined) (%) |
| Notes | This is a conference abstract. The nature of the intervention ("salpingostomy by microsurgical techniques") is unclear and correspondence with the senior author was unsuccessful. The trial authors did not respond to written correspondence. |
IVF: in vitro fertilisation; n: number of events or participants; RCT: Randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐IOR‐16008961.
| Study name | An assessor‐blind, open‐label, randomised, parallel‐group, non‐inferiority study to compare the clinical pregnancy rate of interventional ultrasound sclerotherapy to surgical intervention on women with hydrosalpinx before in vitro fertilization and embryo transfer |
| Methods | Parallel RCT |
| Participants | Inclusion criteria: 1. Patients with unilateral or bilateral hydrosalpinges visible by ultrasound; 2. Aged between 20 to 37 years; 3. Period of infertility > 1 year; 4. Body mass index between 19 and 29; 5. Normal basal luteinizing hormone (LH), follicle stimulating hormone (FSH) and prolactin concentrations; 6. AFC = 7; 7. AMH level 0.24 to 11.78ng/ml; 8. Midluteal phase gonadotropin‐releasing hormone agonist (GnRH‐a) long down‐regulation protocol; 9. Normal recent semen analysis (according to WHO criteria). Exclusion criteria: 1. Uterine fibroid requiring surgical removal; 2. Endometriosis or adenomyosis; 3. Male factor of infertility requiring ICSI; 4. Previous IVF cycles; 5. History of recurrent miscarriage; 6. Endocrinologic disorders; 7. Presence of systemic disease contraindicating pregnancy. |
| Interventions | Control group: surgery group (not specified). Experimental group: ultrasound sclerotherapy. |
| Outcomes | 1. Clinical pregnancy rate (not defined) 2. Live birth rate (not defined) 3. Pregnancy loss rate (not defined) 4. Ectopic pregnancy rate (not defined) 5. Ongoing pregnancy in a transfer cycle (not defined). |
| Starting date | Recruitment pending |
| Contact information | duxin@jarodx.com |
| Notes | Written correspondence to the authors remained unanswered up to the date of publication of this review. |
IRCT2014011116161N1.
| Study name | The comparison of the effect of laparoscopy salpingectomy versus laparoscopy proximal tubal on ovarian reserve and outcome of infertility problems in infertile women with hydrosalpinx |
| Methods | Parallel RCT |
| Participants | 60 women under 40 years old with infertility and hydrosalpinx and FSH < 12 mIU/mL Exclusion criteria: smoking; previous history of surgery; endometriosis; autoimmune disease; other causes of female infertility. |
| Interventions | As described in the published protocol, in the salpingectomy group cautery bipolar will be applied to the meso‐tube completely sticking to the tube so that it is removed; the tube in corneal end will also be cauterised and removed. In those assigned to tubal occlusion, proximal tubal occlusion will be undertaken at the level of the cornea with three‐point bipolar diathermy and the tube will be separated from the uterus with scissors. |
| Outcomes |
|
| Starting date | 22 January 2014. Last updated on 22 February 2018. |
| Contact information | l‐hosseini@tums.ac.ir |
| Notes | Although the study's recruitment status is advertised as "completed" in the WHO International Clinical Trials Registry Platform, written correspondence to the authors remained unanswered up to the date of publication of this review. |
ISRCTN40458453.
| Study name | The comparison of pregnancy outcomes in hydrosalpinx patients treated with salpingectomy and proximal tubal occlusion prior to in vitro fertilization embryo transfer: a randomised controlled study |
| Methods | Single‐centre prospective randomised controlled study. |
| Participants | 100 women with tubal disease prior to IVF. Inclusion criteria: Women with tubal infertility planning to undergo IVF; 18‐41 years old. Exclusion criteria: Endometriosis; prior ovarian surgery; diminished ovarian reserve; polycystic ovarian syndrome (PCOS). |
| Interventions | Group 1: Modified laparoscopic “core‐pulling” salpingectomy. All salpingectomies performed laparoscopically. Group 2: Modified laparoscopic proximal tubal occlusion. Proximal tubal occlusion also performed laparoscopically. |
| Outcomes | 1. Clinical pregnancy, defined as gestation sac and/or fetal pole measured using ultrasound scan at 22 days after embryo transfer 2. Ongoing pregnancy, defined as a fetal heartbeat measured on ultrasound beyond 10 weeks of gestation 3. Implantation rate, defined as the number of gestational sacs on ultrasound divided by the number of embryos transferred, measured using ultrasound scan at 22 days after embryo transfer 4. Ectopic pregnancy at any extrauterine site (considered as an implanted embryo), measured using ultrasound scan at 22 days and 35 days after embryo transfer 5. Miscarriage, measured during the first trimester 6. Live birth rate, measured at birth 7. Ovarian reserve, measured using FSH levels on cycle day 2‐3 before and 3 months after the laparoscopic surgery |
| Starting date | 1 January 2017 |
| Contact information | zhangsongying@zju.edu.cn |
| Notes | Although the study's recruitment status is advertised as "completed" in the WHO International Clinical Trials Registry Platform, written correspondence to the authors remained unanswered up to the date of publication of this review. |
NCT03521128.
| Study name | Comparing radiological tubal blockage versus laparoscopic salpingectomy in infertile women with hydrosalpinx during in vitro fertilisation treatment |
| Methods | Prospective open‐label RCT. |
| Participants | Inclusion: ‐ Women aged 20‐43 years at the time of IVF/ICSI treatment. ‐ Unilateral or bilateral hydrosalpinx visible on pelvic ultrasound and hysterosalpingogram. ‐ At least one frozen embryo or blastocyst available for transfer. Exclusion: ‐ History of pelvic inflammatory disease within 6 months. ‐ Hysterosalpinx with proximal tubal blockage on hysterosalpingogram. ‐ Frozen pelvis from previous laparoscopy. ‐ Women with fibroids interfering with radiological tubal blockage. ‐ Women undergoing preimplantation genetic testing. |
| Interventions | Radiological tubal blockage versus laparoscopic salpingectomy. |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | May 2018 |
| Contact information | ShangHai Ji Ai Genetics & IVF Institute China |
| Notes |
PACTR201709002555574.
| Study name | Impact of transvaginal aspiration of hydrosalpinx on ICSI outcome: RCT |
| Methods | Prospective parallel randomised trial. |
| Participants | 150 women with hydrosalpinx. Inclusion criteria: age 20 to 39 years. Exclusion criteria: active PID. |
| Interventions | Transvaginal aspiration of hydrosalpinges versus no aspiration. |
| Outcomes | 1. Ongoing pregnancy rate (not defined) 2. Clinical pregnancy rate 3. Implantation rate |
| Starting date | Pending recruitment |
| Contact information | adel.nada29@gmail.com |
| Notes | Written correspondence to the authors remained unanswered up to the date of publication of this review. |
AFC: antral follicle count;AMH: anti‐müllerian hormone; FSH: follicle‐stimulating hormone;GnRHa: gonadotropin‐releasing hormone agonist; hCG: human chorionic gonadotropin;ICSI: intracytoplasmic sperm injection; IVF: in vitro fertilisation; LH: luteinising hormone; PCOS: polycystic ovarian syndrome; PID: pelvic inflammatory disease; RCT: Randomised controlled trial; WHO: World Health Organization.
Differences between protocol and review
In the current version of the review, ongoing pregnancy rate and viable pregnancy rate were combined with clinical pregnancy rate, as ongoing pregnancy is currently not a recognised outcome by The International Glossary on Infertility and Fertility Care 2017 (Zegers‐Hochschild 2017). We no longer report on biochemical pregnancy rates, contrary to the previous version of this review, because clinical pregnancy and live birth rates are more clinically relevant outcomes.
We added the following outcomes to the meta‐analysis:
Mean number of oocytes, to investigate concerns of tubal surgery affecting the ovarian blood supply;
Mean number of embryos, to investigate whether tubal surgery had an impact upon fertilisation.
We added a subgroup analysis for women younger or older than 40 years to account for the effect of age on expected ovarian response, although this was not possible due to a lack of data.
We carried out analyses of dichotomous outcomes using RR, instead of OR, in this review, in line with Cochrane guidance recommending RR as it is a more easily interpretable measure of relative effect (Higgins 2011).
We have also updated the list of outcomes according to the most recent edition of the Cochrane Handbook of Systematic Reviews of Interventions. Additionally, we have used GradePRO to grade the evidence and produce a Summary of Findings Table for the main comparison of tubal surgery versus no tubal surgery.
Contributions of authors
PM was the lead author in writing the full review update and was involved in preparing all sections of the review. EXG was involved in data extraction for the review. CB and IEG made substantial editorial amendments to the review.
NJ conceptualised the protocol and the review primarily, and carried out the search, selection of trials and risk of bias assessment as a first reviewer and first author in the first version of the review and as a second reviewer in the previous update.
SVV updated the review in 2010 as a first reviewer performing the search, selection of trials and risk of bias assessment. She proofread the current review.
Ben Willem Mol and Annika Strandell proofread the updated review.
Sources of support
Internal sources
University of Auckland, School of Medicine, Auckland, New Zealand
External sources
None, Other
Declarations of interest
PM has no interests to declare.
EXG has no interests to declare.
NJ works as a gynaecologist at Auckland Gynaecology Group and Fertility Specialist at Repromed Auckland. Within the last 3 years NJ has had consultancy with Guerbet, Myovant Sciences, Vifor Pharma and Roche Diagnostics and has received research funding from Guerbet, AbbVie and Myovant Sciences.
SVV ‐ at the time of the update in 2010 ‐ was a medical student of the faculty of Health, Medicine and Life Sciences of the University of Maastricht. She is now a resident in Obstetrics and Gynaecology at the Erasmus Medical Center, Rotterdam, the Netherlands. She has no financial conflicts of interest.
AS led one of the trials included in this review (Strandell 1999). She is a gynaecologist at Sahlgrenska Hospital, associate professor at the University of Gothenburg and employed at the regional centre for Health Technology Assessment in Göteborg, Sweden. She has received fees from Guerbet for an expert consultancy.
BWJM is an author on one of the trials included in this review (Dreyer 2016) and confirms he took no part in selecting this study, extracting data or assessing risk of bias. In addition, BWJM is supported by a NHMRC Investigator grant (GNT1176437) and reports consultancy for ObsEva, Merk KGaA, iGenomix and Guerbet.
CMB has received research support by Bayer, Volition Rx, Roche Diagnostics and MDNA Life Sciences. He has also received consultancy fees from ObsEva, AbbVie and Myovant.
IEG's institution has received research support from Finox and Bayer.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
An 2015 {published data only}
- An JX, Ni YL, Liu XL, Gao XH, Wang Y. Effects of transvaginal aspiration of hydrosalpinx combined auricular point sticking on IVF-ET outcomes. Zhongguo Zhong Xi Yi Jie He Za Zhi 2015;35(6):682-5. [PubMed] [Google Scholar]
Dechaud 1998 {published data only}
- Dechaud H, Daures JP, Arnal F, Humeau C, Hedon B. Does previous salpingectomy improve implantation and pregnancy rates in patients with severe tubal factor infertility who are undergoing in vitro fertilization? A pilot prospective randomized study. Fertility and Sterility 1998;69(6):1020-5. [DOI] [PubMed] [Google Scholar]
- Dechaud H, Daures JP, Arnal F, Humeau C, Hedon B. Salpingectomy before undergoing IVF can increase implantation rates in severe tubal infertility patients: a prospective randomised study. In: Human Reproduction Abstracts of 13th Annual Meeting of the ESHRE. Vol. 12. 1997:23-4.
Dreyer 2016 {published data only}
- Dreyer K, Lier MC, Emanuel MH, Twisk JW, Mol BW, Schats R, Hompes PG, Mijatovic V. Hysteroscopic proximal tubal occlusion versus laparoscopic salpingectomy as a treatment for hydrosalpinges prior to IVF or ICSI: an RCT. Human Reproduction 2016;31(9):2005-16. [DOI] [PubMed] [Google Scholar]
Fouda 2011 {published data only}
- Fouda UM, Sayed AM. Effect of ultrasound-guided aspiration of hydrosalpingeal fluid during oocyte retrieval on the outcomes of in vitro fertilisation-embryo transfer: a randomised controlled trial (NCT01040351). Gynecological Endocrinology 2011;27(8):562-7. [DOI] [PubMed] [Google Scholar]
Fouda 2015 {published data only}
- Fouda UM, Sayed AM, Abdelmoty HI, Elsetohy KA. Ultrasound guided aspiration of hydrosalpinx fluid versus salpingectomy in the management of patients with ultrasound visible hydrosalpinx undergoing IVF-ET: a randomized controlled trial. BMC Women's Health 2015;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hammadieh 2008 {published data only}
- Hammadieh N, Afnan M, Sharif K, Evans J, Amso N The effect of hydrosalpinx on IVF outcome: a prospective, randomised controlled trial of vaginal ultrasound-guided hydrosalpinx aspiration during egg collection. In: Fertility and Sterility. Vol. 80(Suppl 3). 2003:S131-132, Abstract no: P-35.
- Hammadieh N, Coomarasamy A, Ola B, Papaioannou S, Afnan M, Sharif K. Ultrasound-guided hydrosalpinx aspiration during oocyte collection improves pregnancy outcome in IVF: a randomized controlled trial. Human Reproduction 2008;23(5):1113-7. [DOI] [PubMed] [Google Scholar]
Kontoravdis 2006 {published data only}
- Kontoravdis A, Makrakis E, Pantos K, Botsis D, Deligeoroglu E, Creatsas G. Proximal tubal occlusion and salpingectomy result in similar improvement in in vitro fertilization outcome in patients with hydrosalpinx. Fertility and Sterility 2006;Vol 86(6):1642-9. [DOI] [PubMed] [Google Scholar]
Labib 2016 {published and unpublished data}
- Labib K, Elmansy H, Fahmy A, Shafik A, Samy M, Emeira M. The effect laparoscopic salpingectomy versus laparoscopic proximal tubal disconnection on serum AMH levels and pregnancy rate following IVF/ICSI. In: Gynaecological Surgery. Vol. 13 suppl 1, S144. 2016.
Moshin 2006 {published data only}
- Moshin V, Hotineanu A. Reproductive outcome of the proximal tubal occlusion prior to IVF in patients with hydrosalpinx. In: Human reproduction. Supplement 1: Abstracts of the 22nd Annual Meeting of ESHRE, Prague. Czech republic, 18-21 June, 2006 edition. Vol. 21. June 2006:i193-i194.
Strandell 1999 {published data only}
- Strandell A, Lindhard A, Waldenstrom U, Thorburn J, Janson PO, Hamberger L. Hydrosalpinx and IVF outcome: a prospective randomized multicentre trial in Scandinavia on salpingectomy prior to IVF. Human Reproduction 1999;14(11):2762-9. [DOI] [PubMed] [Google Scholar]
- Strandell A, Lindhard A, Waldenstrom U, Thorburn J, Janson PO, Hamberger L. Hydrosalpinx and IVF outcome: cumulative results after salpingectomy in a randomised controlled trial. Human Reproduction 2001;16:2403-10. [DOI] [PubMed] [Google Scholar]
Vignarajan 2019 {published data only}
- Vignarajan CP, Malhotra N, Singh N. Ovarian reserve and assisted reproductive technique outcomes after laparoscopic proximal tubal occlusion or salpingectomy in women with hydrosalpinx undergoing in vitro fertilization: a randomized controlled trial. The Journal of Minimally Invasive Gynecology 2019;26(6):1070-5. [DOI: 10.1016/j.jmig.2018.10.013 ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bao 2016 {published data only}
- Bao HC, Wang XR, Wang MM, Hao CF. Core-pulling salpingectomy: a novel surgery for hydrosalpinx prior to in vitro fertilization end embryo transfer. International Journal of Clinical and Experimental Medicine 2016;9(10):19778-84. [Google Scholar]
Darwish 2006 {published data only}
- Darwish A, El Saman A. Hysteroscopic versus laparoscopic tubal occlusion of hydrosalpinges prior to IVF/ICSI. In: Middle East Fertility Society Journal. Vol. 10 Suppl 1:18-9. 2005.
- Darwish A, El Saman A. Hysteroscopic vs. laparoscopic tubal occlusion of hydrosalpinges prior to IVF/ICSI. In: Human Reproduction. Vol. 21(Suppl):i134. 2006.
De Angelis 2010 {published data only}
- De Angelis C, Antinori M, Cerusico V, Antinori S. Hysteroscopic surgery prior to IVF. In: Reproductive Biomedicine Online. Vol. 20 Suppl 3:S81. 2010.
Dias Pereira 1999 {published data only}
- Dias Pereira G, Hajenius PJ, Mol BW, Ankum WM, Hemrika DJ, Bossuyt PM, Veen F. Fertility outcome after systemic methotrexate and laparoscopic salpingostomy for tubal pregnancy. Lancet 1999;353(9154):724-5. [DOI] [PubMed] [Google Scholar]
- Dias Pereira G, Hajenius PJ, Mol BW, Ankum WM, Veen F. Fertility outcome after systemic methotrexate and laparoscopic salpingostomy for tubal pregnancy. In: Fertility and Sterility. Vol. 70(3):S411. 1998. [DOI] [PubMed]
Harb 2014 {published data only}
- Harb H, Al-Rshoud F, Coomarasamy A. The effect of presence and management of hydrosalpinx on miscarriage in IVF. In: Fertility and Sterility. Vol. 102(3):e298. 2014.
Kang 2001 {published data only}
- Kang JL, Xia W, He QY. Clinical study on treatment of oviduct obstruction by integrative traditional Chinese and Western medicine. Chinese journal of integrated traditional and Western medicine 2001;21(6):416-18. [PubMed] [Google Scholar]
Kuzmin 2014 {published data only}
Mardesic 1999 {published data only}
- Mardesic T, Muller P, Huttelová R, Zvárová J, Hulvert J, Voboril J, et al. Effect of salpingectomy on the results of IVF in women with tubal sterility--prospective study. Ceska Gynekol. 2001;66(1):259-64. [PubMed] [Google Scholar]
- Mardesic T, Muller P, Voboril J, Hulvert J, Huttelova R, Becvarova V, et al. The influence of salpingectomy of hydrosalpinges visible on ultrasound on IVF results. A pilot prospective randomized study. In: Abstracts of 11th World Congress on In Vitro Fertilization and Human Reproductive Genetics. Sydney, Australia, 9-14 May, 1999:156.
Mossa 2005 {published data only}
- Mossa B, Patella A, Ebano V, Pacifici E, Mossa S, Marziani R. Microsurgery versus laparoscopy in distal tubal obstruction hysterosalpingographically or laparoscopically investigated. Clinical and Experimental Obstetrics and Gynecology 2005;32(3):169-71. [PubMed] [Google Scholar]
Savic 1999 {published data only}
- Savic B, Milacic D, Peako N. Hydrosalpingeal fluid aspiration during oocyte retrieval has beneficial effect on outcome of in-vitro fertilization-embryo transfer. Human Reproduction 1999;14(1):310. [Google Scholar]
Yu 2018 {published data only}
- Yu X, Cai H, Zheng X, Feng J, Guan J. Tubal restorative surgery for hydrosalpinges in women due to in vitro fertilization. Archives of Gynecology and Obstetrics 2018;297(5):1169-73. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Goldstein 1998 {published data only}
- Goldstein DB, Sasaran LH, Stadtmauer L, Popa R. Selective salpingostomy-salpingectomy (SSS) and medical treatment prior to IVF in patients with hydrosalpinx. Fertility and Sterility 1998;70(1):S320. [Google Scholar]
Lindig 2002 {published data only (unpublished sought but not used)}
- Lindig T, Kleinstein J. Impact of correction of hydrosalpinges on assisted reproductive technique results. In: Human Reproduction. Vol. 17(1):142. 2002.
References to ongoing studies
ChiCTR‐IOR‐16008961 {unpublished data only}
- Xin Du. An assessor-blind, open-label, Randomized, Parallel-group, Non-inferiority Study to compare the clinical pregnancy rate of interventional ultrasound sclerotherapy to surgical intervention on women with hydrosalpinx before in vitro fertilization and embryo transfer. http://www.chictr.org.cn/showproj.aspx?proj=15073 2016.
IRCT2014011116161N1 {unpublished data only}
- Reyhaneh Hosseini. The comparison of the effect of laparoscopy Salpingectomy versus laparoscopy proximal tubal on ovarian reserve and outcome of infertility problems in infertile women with hydrosalpinx. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2014011116161N1.
ISRCTN40458453 {unpublished data only}
- Zhang Songying. The comparison of pregnancy outcomes in hydrosalpinx patients treated with salpingectomy and proximal tubal occlusion prior to in vitro fertilization embryo transfer: a randomized controlled study. http://isrctn.com/ISRCTN40458453.
NCT03521128 {unpublished data only}
- NCT03521128. Comparing radiological tubal blockage versus laparoscopic salpingectomy in infertile women with hydrosalpinx during in vitro fertilization treatment [A randomized trial comparing radiological tubal blockage versus laparoscopic salpingectomy in infertile women with hydrosalpinx during in vitro fertilization treatment]. clinicaltrials.gov/ct2/show/NCT03521128 (first received 11 May 2018).
PACTR201709002555574 {unpublished data only}
- Adel Nada. Impact of transvaginal aspiration of hydrosalpinx on ICSI outcome:RCT. https://pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=2555.
Additional references
Aboulghar 1998
- Aboulghar M. Controversies in the modern management of hydrosalpinx. Human Reproduction Update 1998;4(6):882-90. [DOI] [PubMed] [Google Scholar]
Andersen 1996
- Andersen AN, Lindhard A, Loft A, Ziebe S, Andersen CY. The infertile patient with hydrosalpinges: IVF with or without salpingectomy? Human Reproduction 1996;11:2081-4. [DOI] [PubMed] [Google Scholar]
Bao 2017
- Bao H, Qu Q, Huang X, Wang M, Wang X, Hao C. Impact of hydrosalpinx fluid on early human embryos. Systems Biology in Reproductive Medicine 2017;63:279-84. [DOI] [PubMed] [Google Scholar]
Camus 1999
- Camus E, Poncelet C, Goffinet F, Wainer B, Merlet F, Nisand I, et al. Pregnancy rates after in-vitro fertilization in cases of tubal infertility with and without hydrosalpinx: a meta-analysis of published comparative studies. Human Reproduction May 1999;14(5):1243-9. [DOI] [PubMed] [Google Scholar]
Chan 2002
- Ajonuma LC, Ng EHY, Chan HC. New insights into the mechanisms underlying hydrosalpinx fluid formation and its adverse effect on IVF outcome. Human Reproduction Update 2002;8(3):255-64. [DOI] [PubMed] [Google Scholar]
Cheng 2015
- Cheng F, Li T, Wang QL, Zhou HL, Duan L, Cai X. Effects of hydrosalpinx on ultrasonographic parameters for endometrial receptivity during the window of implantation measured by power color Doppler ultrasound. International Journal of Clinical and Experimental Medicine 2015;8(4):6103-8. [PMC free article] [PubMed] [Google Scholar]
Chu 2015
- Chu J, Harb HM, Gallos ID, Dhillon R, Al-Rshoud FM, Robinson L, Coomarasamy A. Salpingostomy in the treatment of hydrosalpinx: a systematic review and meta-analysis. Human Reproduction 2015;30(8):1882-95. [DOI] [PubMed] [Google Scholar]
Dickens 1995
- Dickens CJ, Maguiness SD, Comer MT, Palmer S, Rutherford AJ, Leese HJ. Human tubal fluid: formation and composition during vascular perfusion of the Fallopian tube. Human Reproduction 1995;10:505-8. [DOI] [PubMed] [Google Scholar]
Edwards 1984
- Edwards RG, Fishel SB, Cohen J, Fehilly CB, Purdy JM, Slater JM, et al. Factors influencing the success of in vitro fertilization for alleviating human infertility. Journal of in Vitro Fertilization and Embryo Transfer 1984;1(1):3-23. [DOI] [PubMed] [Google Scholar]
Evers 2002
- Evers JL. Female subfertility. The Lancet 2002;360(9327):151-9. [DOI] [PubMed] [Google Scholar]
Eytan 2001
- Eytan O, Azem F, Gull I, Wolman I, Elad D, Jaffa AJ. The mechanism of hydrosalpinx in embryo implantation. Human Reproduction 2001;12:2662-7. [DOI] [PubMed] [Google Scholar]
Fan 2016
- Fan M, Ma L. Effect of salpingectomy on ovarian response to hyperstimulation during in vitro fertilization: a meta-analysis. Fertility and Sterility 2016;106(2):322-29. [DOI] [PubMed] [Google Scholar]
Fleming 1996
- Fleming C, Hull MGR. Impaired implantation after in vitro fertilisation treatment associated with hydrosalpinx. British Journal of Obstetrics and Gynaecology 1996;103:268-72. [DOI] [PubMed] [Google Scholar]
Gelbaya 2006
- Gelbaya TA, Nardo LG, Fitzgerald CT, Horne G, Brison DR, Lieberman BA. Ovarian response to gonadotropins after laparoscopic salpingectomy or the division of fallopian tubes for hydrosalpinges. Fertility and Sterility 2006;85(5):1464-8. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 24 May 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Horwell 2017
- Horwell DH. End of the road for Essure?®. Journal of Family Planning and Reproductive Health Care 2017;43:240-1. [DOI] [PubMed] [Google Scholar]
Hughes 1992
- Hughes EG, Fedorkow DM, Daya S, Sagle MA, Van de Koppel P, Collins JA. The routine use of gonadotropin-releasing hormone agonists prior to in vitro fertilization and gamete intrafallopian transfer: a meta-analysis of randomized controlled trials.. Fertility and Sterility 1992;58:888-96. [DOI] [PubMed] [Google Scholar]
Hurskainen 2010
- Hurskainen R, Hovi SL, Gissler M, Grahn R, Kukkonen-Harjula K, Nord-Saari M, Makela M. Hysteroscopic tubal sterilization: a systematic review of the Essure system. Fertility and Sterility 2010;94(1):16-9. [DOI] [PubMed] [Google Scholar]
Kassabji 1994
- Kassabji M, Sims JA, Butler L, Muasher SJ. Reduced pregnancy outcomes in patients with unilateral or bilateral hydrosalpinx after in vitro fertilization. European Journal of Obstetrics & Gynecology and Reproductive Biology 1994;56(2):129-32. [DOI] [PubMed] [Google Scholar]
Koong 1998
- Koong MK, Jun JH, Song SJ, Lee HJ, Song IO, Kang IS. A second look at the embryotoxicity of hydrosalpingeal fluid: an in-vitro assessment in a murine model. Human Reproduction 1998;13(10):2852-6. [DOI] [PubMed] [Google Scholar]
Lass 1998
- Lass A, Ellenbogen A, Croucher C, et al. Effect of salpingectomy on ovarian response to superovulation in an in vitro fertilization-embryo transfer program. Fertility & Sterility 1998;70:1035-8. [DOI] [PubMed] [Google Scholar]
Lass 1999
- Lass A. What is the preferred treatment for hydrosalpinges? The ovary's perspective. Human Reproduction 1999;14(7):1674-7. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. vailable from handbook.cochrane.org.
Mansour 1991
- Mansour RT, Aboulghar MA, Serrour GI, Riad R. Fluid accumulation of the uterine cavity before embryo transfer: a possible hindrance for implantation. Journal of In vitro fertilisation and Embryo Transfer 1991;8:157-9. [DOI] [PubMed] [Google Scholar]
Meyer 1997
- Meyer WR, Castelbaum AJ, Somkuti S, Sagoskin AW, Doyle M, Harris JE, et al. Hydrosalpinges adversely affect markers of endometrial receptivity. Human Reproduction 1997;12:1393-8. [DOI] [PubMed] [Google Scholar]
Mohamed 2017
- Mohamed AA, Yosef AH, James C, Al-Hussaini TK, Bedaiwy MA, Amer SAKS. Ovarian reserve after salpingectomy: a systematic review and meta-analysis. Acta Obstetricia et Gynecologica Scandinavica 2017;96(7):795-803. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement.. PLoS Medicine 2009;6(7):e1000097. [DOI]: 10.1371/journal.pmed1000097]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mukherjee 1996
- Mukhurjee T, Copperman AB, McCaffrey C. Hydrosalpinx fluid has embryotoxic effects on murine embryogenesis: a case for prophylactic salpingectomy. Fertility & Sterility 1996;66:851-3. [DOI] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Care Excellence. Fertility problems: assessment and treatment. https://www.nice.org.uk/guidance/cg156 February 2013 (Updated September 2017). [PubMed]
Rosenfield 2005
- Rosenfield RB, Stones RE, Coates A, Matteri RK, Hesla JS. Proximal occlusion of hydrosalpinx by hysteroscopic placement of microinsert before in vitro fertilization embryo transfer. Fertility & Sterility 2005;83(5):1547. [DOI] [PubMed] [Google Scholar]
Smith 2010
- Smith RD. Contemporary hysteroscopic methods for female sterilization. International Journal of Gynaecology and Obstetrics 2010;108(1):79-84. [DOI] [PubMed] [Google Scholar]
Stadtmauer 2000
- Stadtmauer LA, Riehl RM, Toma SK, Talbert LM. Cauterization of hydrosalpinges before in vitro fertilization is an effective surgical treatment associated with improved pregnancy rates. American Journal of Obstetrics & Gynecology 2000;183:367-71. [DOI] [PubMed] [Google Scholar]
Strandell 1994
- Strandell A, Waldenstrom U, Nilsson L, Hamberger L. Hydrosalpinx reduces in-vitro fertilisation / embryo transfer pregnancy rates. Human Reproduction 1994;9:861-3. [DOI] [PubMed] [Google Scholar]
Strandell 2002
- Strandell A, Lindhard A. Why does hydrosalpinx reduce fertility; the importance of hydro salpingeal fluid. Human Reproduction 2002;17(5):1141-5. [DOI] [PubMed] [Google Scholar]
Tay 1997
- Tay JI, Rutherford AJ, Killick SR, Maguiness SD, Partridge RJ, Leese HJ. Human tubal fluid: production, nutrient composition and response to adrenergic agents. Human Reproduction 1998;70:492-9. [DOI] [PubMed] [Google Scholar]
Taylor 2001
- Taylor RC, Berkowitz J, McComb PF. Role of laparoscopic salpingostomy in the treatment of hydrosalpinx. Fertility and Sterilty 2001;75:594-600. [DOI] [PubMed] [Google Scholar]
Van Voorhis 2019
- Van Voorhis BJ, Mejia RB, Schlaff WD, Hurst BS. Is removal of hydrosalpinges prior to in vitro fertilization the standard of care? Fertility and Sterility 2019;111(4):652-6. [DOI] [PubMed] [Google Scholar]
Vandromme 1995
- Vandromme J, Chasse E, Lejeune B, Van Rysselberge M, Delvigne A, Leroy F. Hydrosalpinges in in vitro fertilisation: an unfavourable prognostic feature. Human Reproduction 1995;10:576-9. [DOI] [PubMed] [Google Scholar]
Zegers‐Hochschild 2017
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID, Simpson JL, van der Poel S. The International Glossary on Infertility and Fertility Care, 2017. Fertility and Sterility 2017;108(3):393-406. [DOI] [PubMed] [Google Scholar]
Zeyneloglu 1998
- Zeyneloglu HB, Arici A, Olive DL. Adverse effects of hydrosalpinx on pregnancy rates after in vitro fertilization - embryo transfer. Fertility & Sterility 1998;70:492-9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Johnson 2010
- Johnson N, Voorst S, Sowter MC, Strandell A, Mol BW. Surgical treatment for tubal disease in women due to undergo in vitro fertilisation. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD002125. [DOI: 10.1002/14651858.CD002125.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


