Abstract
Background
Despite substantial improvements in the success of assisted reproduction techniques (ART), live birth rates may remain consistently low, and practitioners may look for innovative treatments to improve the outcomes. The injection of embryo culture supernatant in the endometrial cavity can be undertaken at various time intervals before embryo transfer. It provides an altered endometrial environment through the secretion of factors considered to facilitate implantation. It is proposed that injection of the supernatant into the endometrial cavity prior to embryo transfer will stimulate the endometrium and provide better conditions for implantation to take place. An increased implantation rate would subsequently increase rates of clinical pregnancy and live birth, but current robust evidence on the efficacy of injected embryo culture supernatant is lacking.
Objectives
To evaluate the effectiveness and safety of endometrial injection of embryo culture supernatant before embryo transfer in women undergoing ART.
Search methods
Our search strategies were designed with the help of the Cochrane Gynaecology and Fertility Group Information Specialist. We sought to identify all published and unpublished randomised controlled trials (RCTs) meeting inclusion criteria. Searches were performed on 2 December 2019.
We searched the Cochrane Gynaecology and Fertility Group Specialised Register of controlled trials, CENTRAL, MEDLINE, Embase, CINAHL, trials registries and grey literature. We made further searches in the UK National Institute for Health and Care Excellence (NICE) fertility assessment and treatment guidelines. We handsearched reference lists of relevant systematic reviews and RCTs, together with searches of PubMed and Google for any recent trials that have not yet been indexed in the major databases. We had no language or location restrictions.
Selection criteria
We included RCTs testing the use of endometrial injection of embryo culture supernatant before embryo transfer during an ART cycle, compared with the non‐use of this intervention, the use of placebo or the use of any other similar drug.
Data collection and analysis
Two review authors independently selected studies, assessed risk of bias, extracted data from studies and attempted to contact the authors where data were missing. We pooled studies using a fixed‐effect model. Our primary outcomes were live birth/ongoing pregnancy and miscarriage. We performed statistical analysis using Review Manager 5. We assessed evidence quality using GRADE methods.
Main results
We found five RCTs suitable for inclusion in the review (526 women analysed). We made two comparisons: embryo culture supernatant use versus standard care or no intervention; and embryo culture supernatant use versus culture medium.
All studies were published as full‐text articles. Data derived from the reports or through direct communication with investigators were available for the final meta‐analysis performed. The GRADE evidence quality of studies ranged from very low‐quality to moderate‐quality. Factors reducing evidence quality included high risk of bias due to lack of blinding, unclear risk of publication bias and selective outcome reporting, serious inconsistency among study outcomes, and serious imprecision due to wide confidence intervals (CIs) and low numbers of events.
Comparison 1. Endometrial injection of embryo culture supernatant before embryo transfer versus standard care or no intervention:
One study reported live birth only and two reported the composite outcome live birth and ongoing pregnancy. We are uncertain whether endometrial injection of embryo culture supernatant before embryo transfer during an ART cycle improves live birth/ongoing pregnancy rates compared to no intervention (odds ratio (OR) 1.11, 95% CI 0.73 to 1.70; 3 RCTs; n = 340, I2 = 84%; very low‐quality evidence). Results suggest that if the chance of live birth/ongoing pregnancy following placebo or no treatment is assumed to be 42%, the chance following the endometrial injection of embryo culture supernatant before embryo transfer would vary between 22% and 81%.
We are also uncertain whether the endometrial injection of embryo culture supernatant could decrease miscarriage rates, compared to no intervention (OR 0.89, 95% CI 0.44 to 1.78, 4 RCTs, n = 430, I2 = 58%, very low‐quality evidence). Results suggest that if the chance of miscarriage following placebo or no treatment is assumed to be 9%, the chance following injection of embryo culture supernatant would vary between 3% and 30%.
Concerning the secondary outcomes, we are uncertain whether the injection of embryo culture supernatant prior to embryo transfer could increase clinical pregnancy rates (OR 1.13, 95% CI 0.80 to 1.61; 5 RCTs; n = 526, I2 = 0%; very low‐quality evidence), decrease ectopic pregnancy rates (OR 0.32, 95% CI 0.01 to 8.24; n = 250; 2 RCTs; I2 = 41%; very low‐quality evidence), decrease multiple pregnancy rates (OR 0.70, 95% CI 0.26 to 1.83; 2 RCTs; n = 150; I2 = 63%; very low‐quality evidence), or decrease preterm delivery rates (OR 0.63, 95% CI 0.17 to 2.42; 1 RCT; n = 90; I2 = 0%; very low‐quality evidence), compared to no intervention. Finally, there may have been little or no difference in foetal abnormality rates between the two groups (OR 3.10, 95% CI 0.12 to 79.23; 1 RCT; n = 60; I2 = 0%; low‐quality evidence).
Comparison 2. Endometrial injection of embryo culture supernatant versus endometrial injection of culture medium before embryo transfer
We are uncertain whether the use of embryo culture supernatant improves clinical pregnancy rates, compared to the use of culture medium (OR 1.09, 95% CI 0.48 to 2.46; n = 96; 1 RCT; very low‐quality evidence). No study reported live birth/ongoing pregnancy, miscarriage, ectopic or multiple pregnancy, preterm delivery or foetal abnormalities.
Authors' conclusions
We are uncertain whether the addition of endometrial injection of embryo culture supernatant before embryo transfer as a routine method for the treatment of women undergoing ART can improve pregnancy outcomes. This conclusion is based on current available data from five RCTs, with evidence quality ranging from very low to moderate across studies. Further large well‐designed RCTs reporting on live births and adverse clinical outcomes are still required to clarify the exact role of endometrial injection of embryo culture supernatant before embryo transfer.
Plain language summary
Endometrial injection of embryo culture supernatant for subfertile women in assisted reproduction
Review question:
Researchers in the Cochrane Collaboration reviewed the evidence about the effectiveness and safety of endometrial injection of embryo culture supernatant before embryo transfer in women undergoing assisted reproduction.
Background:
Assisted reproduction techniques (ART) includes techniques used for treating subfertility, and in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) are the most common. Despite both clinical and laboratory efforts and improvements in the success of these treatments, pregnancy rates remain relatively low. In IVF, eggs are retrieved from a woman's ovaries and placed in a dish with sperm from her partner or from a donor, in a liquid called embryo culture medium. In ICSI, a single sperm cell is injected into a woman's egg, and then the egg goes into the culture medium.
Various factors allow a communication of embryo with maternal tissues. These could affect the receptivity of the lining of the womb (endometrium). Researchers have proposed that injecting the upper layer of the embryo culture's liquid, called supernatant, into the womb before embryo transfer, might stimulate the lining of the womb and facilitate the embryo's attachment to it. This could improve live birth rates and other ART outcomes.
Although injection before embryo transfer seems to be an encouraging procedure, its effectiveness and safety remain controversial, as there is not much available evidence about outcomes. In this Cochrane Review, we summarised the relevant evidence. We aimed for our conclusions to be as robust as possible, and also to identify any limitations to the evidence.
Study characteristics:
In consultation with the Cochrane Gynaecology and Fertility Group Information Specialist, we performed a comprehensive literature search of the standard medical databases, from each databases' earliest records to December 2019. We searched for all randomised controlled trials (RCTs) (studies in which participants are assigned to a treatment group using a random method) that investigated the effectiveness of endometrial injection of embryo culture supernatant before embryo transfer, during a cycle with IVF or ICSI, versus any other intervention or no intervention (usual care). IVF and ICSI are the two major modalities of ART. We searched for studies irrespective of language and country of origin. Two review authors independently selected and evaluated studies, extracted data, and attempted to contact the authors of studies for which data were missing. Five studies comprising 526 women met our review's inclusion criteria. We identified no ongoing studies.
Key results:
We are uncertain whether the routine use of endometrial injection of embryo culture supernatant before embryo transfer has a positive effect in women undergoing assisted reproduction, compared to usual care, for improving both live birth or ongoing pregnancy and miscarriage rates. We found that the live birth or ongoing pregnancy rate with usual care was 42%, but it varied between 35% and 55% when supernatant was injected. The risk of miscarriage was found to be 9% for usual care, and between 4% and 15% with the injection. We found similar conclusions for the rates of clinical pregnancy, multiple pregnancy and ectopic pregnancy, as well as for preterm delivery and foetal abnormalities. No single outcome measure in our review's included RCTs demonstrated a clear benefit with its use. There is also insufficient evidence to support the routine use of endometrial injection of embryo culture supernatant compared to culture media before embryo transfer.
Quality of the evidence:
The quality of the evidence was very low for nearly all outcomes. Evidence quality was low for foetal abnormalities. The main limitations of the included RCTs were poorly reported study methods, wide variations in the characteristics of the included studies and statistical imprecision due to the small study numbers and few numbers of events reported.
Summary of findings
Background
Description of the condition
Subfertility is a major problem among all populations and affects 10% to 15% of couples of reproductive age (Gnoth 2005). The average age of women who give birth to their first child is rising. Overall, it is almost 29 years of age in Europe, and in Mediterranean countries such as Greece, Italy, Malta and Spain it is one year older (Eurostat 2015; Eurostat 2019). The proportion of women who give birth to their first child between the ages of 30 and 39 is 59.4% in Spain and 51.9% in Greece. Similarly, in the USA, from 2000 to 2014, for all birth orders, the mean age of mothers has increased. Age at first birth has had the largest increase, from 24.9 years in 2000 to 26.3 years in 2014 (CDC 2016). A similar pattern is observed across Europe (Rendall 2010).
Assisted reproduction techniques (ART) include the techniques used for treating subfertility, and is defined as "all interventions that include the in vitro handling of both human oocytes and sperm or of embryos for the purpose of reproduction" (Zegers‐Hochschild 2017). It is estimated that the number of births worldwide as a result of in vitro fertilisation (IVF) has already exceeded eight million births (ESHRE 2018). However, pregnancy rates after IVF have remained stable in the past decade, at around 30% for cases undergoing intracytoplasmic sperm injection (ICSI) (Van Steirteghem 1993; Kuczyński 2001; Ben Rhouma 2003; Motteram 2015; EIM 2017). Advancing maternal age at primigravidity increases the proportion of couples who need assisted reproduction. Bearing in mind the significant impact of subfertility on a couple's quality of life, every effort should be made to increase their chance of live birth. Many interventions have been investigated to overcome this situation (Farquhar 2015), but with conflicting results and no firm consensus (Carney 2012; Lensen 2018; Siristatidis 2018). Some modalities that have been assessed for improving ART outcomes are endometrial injury (Nastri 2015), administration of granulocyte‐colony stimulating growth factor (Kamath 2020), aspirin (Siristatidis 2016), hyaluronic acid (Bontekoe 2014), antioxidants (Showell 2017) and pre‐treatment with androgens (Nagels 2015). In this context, current research focuses on enhanced understanding of cellular and molecular pathways involved in the process of implantation and the potential for targeted interventions to improve implantation rates.
Although there have been continuous efforts to improve understanding of human implantation in order to achieve a balance between regulation and dysregulation of endometrial function, and to facilitate the transfer of high‐quality embryos in subfertile women undergoing assisted reproduction, implantation rates remain limited. There is a lack of useful tests to assess the function of the healthy endometrium and in vivo peri‐implantation events remain in a “black box” (Macklon 2017). Moreover, there are reports pointing out that implantation failure, and especially repeated failure, is linked to failure to properly synchronize the embryo with the woman's "personalized window of implantation" (Valdes 2017).
Description of the intervention
Successful implantation is a result of a complex sequence of physiological events that must be synchronised in order for the zygote to travel through the salpinx and reach the endometrial cavity at a specific time (the 'implantation window'). This synchronisation necessitates a timely cross‐talk between the zygote and the endometrium (Lopata 1996).
For this purpose, an embryo culture supernatant is derived from the patient’s own embryos' culture media and injected into the uterine cavity in an IVF cycle with cleavage stage embryos or blastocysts. Embryos are taken off and put in a separate dish with fresh media, and then the supernatant (culture medium) is aspirated with an embryo transfer catheter. The supernatant injection (approximately 20 μL) is performed transcervically into the endometrial cavity, close to the fundus, using a separate IVF catheter (Goto 2007). The intervention is a straightforward, feasible technique that is easily accomplished before embryo transfer.
How the intervention might work
The rationale for the intervention is that it provides an altered and optimum endometrial environment that might mimic in vivo conditions, through the secretion of embryonic factors, which are considered to facilitate implantation.
Studies suggest the human embryo secretes various factors during its growth and prior to implantation, which seem to contribute to cross‐talk with the maternal tissues, thus modulating endometrial receptivity (Teh 2016). Specifically, reports suggest that the human pre‐implantation embryo produces various factor that modulate implantation potential, including immunosuppressive factors and vascular endothelial growth factor (VEGF), human leukocyte antigen G (HLA‐G), interleukins (including IL‐1 and IL‐8), leukaemia inhibitory factor (LIF), monocyte chemoattractant protein 1 (MCP‐1) and Regulated on Activation, Normal T Cell Expressed and Secreted (RANTES) (Dinarello 1994; Giudice 1995; Tazuke 1996; Krüssel 2000; Spandorfer 2000; Caballero‐Campo 2002; Achache 2006; Desai 2006). Moreover, it downregulates the human mucin gene 1 (MUC‐1), which naturally creates a barrier to the endometrial‐embryo attachment (Meseguer 2001). At the same time, the endometrium regulates other receptors, e.g. for oestrogen and progesterone, in an effort to improve the endometrial receptivity for the process of implantation (Tazuke 1996; Tehraninejad 2012; Teh 2016).
There is evidence to associate a negative impact of ovarian hyperstimulation on embryo‐endometrial synchrony and subsequent pregnancy outcome (Maheshwari 2012; Teh 2016). It is proposed that injection of the supernatant into the endometrial cavity prior to embryo transfer would stimulate the endometrium and provide better conditions (e.g. transfer of some cytokines released in the culture media in the uterine cavity) for implantation to take place. An increased implantation rate would subsequently increase rates of clinical pregnancy and live birth.
Why it is important to do this review
Injection of embryo culture supernatant prior to embryo transfer seems to be a promising procedure, but there is uncertainty as to its effectiveness and safety. In an initial report, the endometrial culture medium was cryopreserved along with blastocysts and transferred warmed two days before the frozen embryo transfer; authors reported significant improvements in implantation and pregnancy rates (Goto 2007). Further studies conducted using both cleavage and blastocyst stage fresh and frozen embryo transfer cycles reported conflicting results (Goto 2009; Zhu 2010; Prapas 2012; Kamath 2015; Hamdi 2018).
There is thus an emerging need to summarise current evidence and provide a clear view on the effectiveness of this practice in order to encourage or disprove its clinical application. In this Cochrane Review, authors systematically reviewed and synthesised the relevant evidence and identified any gaps or limitations in our current understanding. Assessment of the methodological quality of existing and ongoing trials may encourage the conduct of more studies on this topic.
Objectives
To evaluate the effectiveness and safety of endometrial injection of embryo culture supernatant before embryo transfer in women undergoing ART.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised controlled trials (RCTs) that assessed the effectiveness and safety of endometrial injection of embryo culture supernatant before embryo transfer in women undergoing IVF or ICSI. Quasi‐randomised and crossover trials were excluded, unless first phase results were present. We had no limitations in terms of language or study setting.
Types of participants
Women and couples undergoing IVF or ICSI cycles (both fresh and frozen). Oocyte donation cycles were excluded, in order to ensure that the quality of the oocyte could not affect the final outcome due to the intervention examined.
Types of interventions
Endometrial injection of embryo culture supernatant before embryo transfer, during an assisted reproductive cycle with IVF or ICSI (fresh or frozen), versus any other intervention or no intervention (usual care).
Types of outcome measures
Primary outcomes
Effectiveness
Live birth or (in studies not reporting live birth) ongoing pregnancy per woman or couple randomised.
Live birth is defined as the delivery of a live foetus after 20 completed weeks of gestational age. Ongoing pregnancy is defined as the presence of a foetal heart on ultrasound scan after 12 weeks of gestation per woman or couple randomised. cumulative live birth was also to be reported, if data were available.
Safety
Miscarriage rates per woman or couple randomised.
Miscarriage is defined as the loss of pregnancy before 20 completed weeks of gestational age.
Secondary outcomes
Effectiveness
Clinical pregnancy rate per woman or couple randomised;
Clinical pregnancy is defined as the presence of a foetal heart on ultrasound scan at seven weeks of gestation.
Safety
-
Adverse events per woman or couple randomised:
multiple and ectopic pregnancy rates;
foetal growth restriction;
preterm delivery (< 37 weeks of gestation);
foetal abnormality rate (chromosomal, congenital and anatomical).
Search methods for identification of studies
We searched for published and unpublished RCTs that assess the impact of endometrial injection of embryo culture supernatant before the embryo transfer, during a cycle with IVF or ICSI, in consultation with the Cochrane Gynaecology and Fertility Group's Information Specialist.
Electronic searches
We searched the following databases:
the Cochrane Gynaecology and Fertility Group Specialised Register; PROCITE platform, searched 2 December 2019 (Appendix 1)
CENTRAL; OVID platform, searched 2 December 2019 (Issue October 2019) (Appendix 2)
MEDLINE; OVID platform, searched from 1946 to 2 December 2019 (Appendix 3),
Embase; OVID platform, searched from 1980 to 2 December 2019 (Appendix 4)
CINAHL Plus; EBSCO platform, searched from 1961 to 2 December 2019 (Appendix 5).
All searches were carried out without any language, setting or date restriction.
We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying RCTs that appears in the Cochrane Handbook of Systematic Reviews of Interventions (Chapter 6, 6.4.11; Lefebvre 2011). We combined the Embase and CINAHL searches with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/methodology/filters.html#random).
We searched the World Health Organization's International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/Default.aspx) and the ClinicalTrials.gov registry for ongoing and registered trials (Appendix 6). We also searched OpenGrey (www.opengrey.eu/) for grey literature. We consulted experienced clinicians to learn of any ongoing or existing studies that we had not identified.
Searching other resources
We examined the references lists of all studies (included and excluded) and relevant reviews in order to identify further relevant articles. We also contacted experts in the field to find any additional studies.
Data collection and analysis
We entered data into Review Manager 5 (RevMan 5) (RevMan 2014). We conducted statistical analysis in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors (DV and ES) independently screened the titles and abstracts of the publications identified by the literature search strategy. We excluded studies that did not meet the inclusion criteria and retrieved the full‐text articles of the remaining publications. We evaluated these independently to identify RCTs eligible for inclusion. Any potential disagreements related to study eligibility were resolved by discussion with another review author (CSS). All excluded studies were listed after full‐text assessment in the ‘Characteristics of excluded studies' tables, and we documented the study selection process in a PRISMA flow chart (Figure 1).
1.
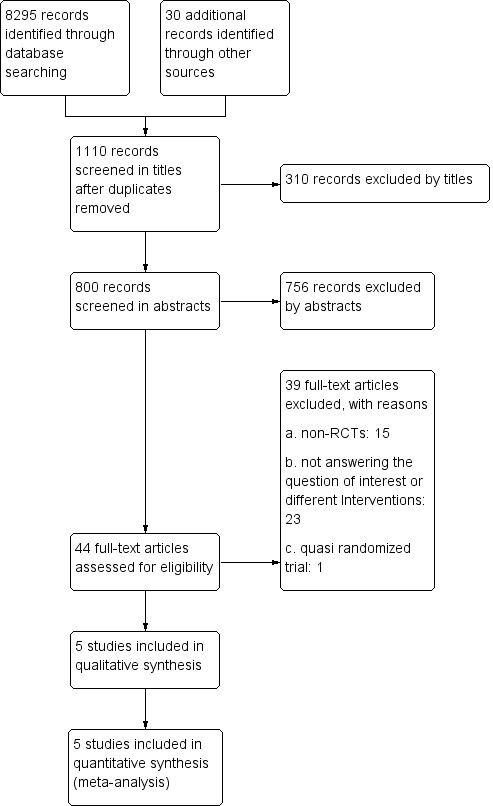
Study flow diagram.
Data extraction and management
Two review authors (DV and ES) independently extracted study characteristics and outcome data from the included studies using a pre‐designed data extraction form. All authors searched for detailed information on participants, interventions, comparators, outcomes, study design, funding sources and declarations of interest for the primary investigators. For studies with multiple publications, we used the main RCT report as the reference and we supplemented it with additional data from the secondary publications.
With the aim of retrieving additional data or methodological details where necessary, we contacted authors of the included studies via email. We sent a reminder if needed (a second email 15 days after the first communication, if we received insufficient data or no reply). Requested information included missing data, potential subgroups that were not reported in the published manuscript and elaboration on methods for the quality assessment. We resolved any potential disagreements through consensus involving one of the other review authors (CSS or VK). One review author imported data into RevMan 5, and a second review author validated the imported values against the data extraction form.
Assessment of risk of bias in included studies
Two review authors (DV and ES) independently assessed risk of bias in the included studies using the Cochrane ‘Risk of bias’ assessment tool for selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias and other types of bias (Higgins 2011). A priori, we considered that a degree of bias might inevitably exist in the studies, not only due to the difficulty in blinding clinical staff and embryologists, but also due to the unavoidable interaction between clinicians and patients, given the nature of clinical processes required for the best possible treatment outcomes. Considering these factors, we proceeded with a meticulous evaluation of the methods of each study, and requested further specifications from study authors in order to clarify our evaluations. We also examined the possibility of selective outcome reporting through comparison of outcomes reported in study protocols or in their articles' methods sections with the actual reported outcomes. We expected that pre‐specified primary outcomes would be appropriately described and that adverse events would be reported clearly. Our intention was to group multiple outcomes, if necessary. We resolved any disagreements through discussion with another review author (CSS). We explicitly reported our judgements of risk of bias in the ‘Risk of bias in included studies’ table in the ‘Characteristics of included studies’ section, with relevant information supporting our assessments.
Measures of treatment effect
All defined outcomes were binary (dichotomous), and we used the numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel odds ratios (ORs). We used Peto ORs for outcomes with low event rates, when needed, as described in the ‘Data synthesis' section. We reversed the direction of effect of individual studies, if required, to ensure consistency across trials. We presented 95% CIs for the ORs.
Unit of analysis issues
We expected that all studies would have the woman (or couple) as the unit of randomisation. When data were not reported per woman (or couple), e.g. if studies reported data "per cycle," we made every effort either to extract the data from the text or retrieve them through correspondence with the study authors, or both. If we were unsuccessful, our intention was to summarise data in narrative analysis and in additional tables, as necessary.
We counted multiple live births (e.g. twins, triplets) as single live birth events.
Dealing with missing data
We evaluated included studies to determine whether missing data were randomly distributed. Where data were missing, we contacted trial authors to retrieve as much information as possible. Where this was unobtainable, we undertook imputation of individual values for our primary effectiveness outcome live birth/ongoing pregnancy only. Live birth/ongoing pregnancy was assumed not to have occurred in participants not reporting this. For other outcomes, we analysed the available data.
Assessment of heterogeneity
We initially considered whether the clinical and methodological characteristics of the included studies are consistent enough to provide a clinically meaningful results through data pooling in a meta‐analysis.
We assessed statistical heterogeneity with the I2 statistic. We considered an I2 statistic value of 30% to 60% to suggest moderate heterogeneity, and a value of 60% to 90% to suggest substantial heterogeneity across studies (Higgins 2011). In case of substantial heterogeneity with important clinical impact for a specific outcome, we explored possible explanations through subgroup and sensitivity analyses (where data were available).
Assessment of reporting biases
We aimed to minimise the potential impact of publication bias and other reporting biases by means of a thorough search for published and unpublished data, We planned to use a funnel plot to explore publication bias if we had combined data from 10 or more RCTs in a given meta‐analysis.
Data synthesis
All outcomes were dichotomous (binary). We combined data from similar RCTs, using a fixed‐effect Mantel‐Haenszel model. We reported the pooled ORs with their 95% CIs. Where events were rare, and if all relevant criteria were fulfilled, we considered the Peto method for pooling the data. An increase in the odds of the outcome is displayed graphically in the meta‐analyses to the right of the centre‐line, and a decrease in the odds of an outcome to the left of the centre‐line.
We planned to carry out pooled analyses for the following comparisons: women or couples randomised to receive endometrial injection of embryo culture supernatant before the embryo transfer, during a cycle with IVF or ICSI, versus women/couples randomised to receive one of the following comparators:
any other intervention during a cycle of IVF or ICSI (e.g. endometrial injury or plasma infusion, or medical adjuncts given prior to embryo transfer);
sham or placebo‐type intervention; or
usual care (no additional intervention) during a cycle of IVF or ICSI.
Subgroup analysis and investigation of heterogeneity
Clinical heterogeneity, given the diversity in interventions, may affect the results. Differences in the cross‐talk between endometrium and embryo could affect the outcome of the endometrium culture injection at different times before embryo transfer. In addition, the quality of the embryo on day 3 or day 5 may be different; the blastocyst is considered to be more viable with higher rates of successful implantation and the number of previous ART cycles reflects the potential of each woman to conceive (more unsuccessful attempts reduce the possibilities). Where there was substantial heterogeneity (I2 > 60%), we determined effects for the primary and the most clinically important secondary outcomes within the following subgroups, if data were sufficient for any meaningful analyses:
age of the woman (≤ 37 years, 38 to 41 years, ≥ 42 years);
day of embryo transfer (early cleavage or blastocyst);
type of cycle (frozen or fresh); and
time of endometrium culture injection before embryo transfer (e.g. ≥ 1 day and ≥ 1 hour before the transfer).
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes of live birth/ongoing pregnancy and miscarriage, as well as for the most clinically important secondary outcomes of clinical pregnancy, ectopic pregnancy and multiple pregnancy, in order to determine whether the conclusions are robust to arbitrary decisions made regarding the eligibility and analysis. These analyses included consideration of whether the review conclusions would have differed if:
1. a random‐effects model had been used;
2. the summary effect measure had been risk ratio (RR) rather than OR;
3. studies at high or unclear risk of selection bias (random sequence generation and allocation concealment) or reporting bias and studies at high risk of bias in any other domain had been excluded;
4. the type of studies (full text or abstract) had been considered;
5. the type of denominator in miscarriage (per pregnancy or per woman) had been considered; and
6. the primary outcome had been restricted to live birth or included both live birth and ongoing pregnancy rate.
Overall quality of the evidence: ‘Summary of findings' table
We prepared two ‘Summary of findings' tables using the browser‐based version of GRADEpro (GRADEpro GDT 2015). This table evaluates the overall quality of the body of evidence for the main review comparison (endometrial injection of embryo culture supernatant versus no intervention, or versus any other intervention) on the primary and most important secondary outcomes (live birth/ongoing pregnancy, miscarriage, clinical pregnancy and adverse events) using GRADE criteria on study limitations, consistency of effect, imprecision, indirectness and publication bias. We justified, documented and incorporated judgements about evidence quality into reporting of results for each outcome. Two review authors (ES and DV) independently assessed the quality of the evidence, and resolved any disagreements by consulting a third review author (CSS). We prepared two different ‘Summary of findings' tables according to the comparisons (intervention versus no intervention or usual care; and intervention versus stimulation of the endometrium with culture media).
We extracted study data, formatted our comparisons in data tables and prepared the ‘Summary of findings' tables before writing the results and conclusions of our review.
Results
Description of studies
Results of the search
Through the initial database search and other sources, we identified 8325 articles. After removing duplicate records, and screening 1110 titles and the remaining 800 abstracts, we identified 44 studies as possibly eligible, and retrieved the full‐text report. From these, we excluded 39 studies, with reasons for exclusion. We identified and selected five studies for analysis. We found no studies awaiting further assessment nor any ongoing studies. We have displayed the summary details in the relevant figure (Figure 1).
Included studies
Five RCTs including 526 women in total met the inclusion criteria for this review (Goto 2009; Zhu 2010; Prapas 2012; Tehraninejad 2012; Kamath 2015). We selected these for qualitative and quantitative analysis.
We sent emails to authors of all included studies (with appropriate reminders) to obtain additional data.
In accordance with our pre‐specified criteria and the available data, we were able to include all five included studies in our meta‐analysis (Goto 2009; Zhu 2010; Prapas 2012;Tehraninejad 2012; Kamath 2015).
Study design and setting
All five studies were set as RCTs, conducted in IVF centres (private or university‐based) in China, Greece, India, Iran and Japan. Sample size was small in three of the five included studies (Zhu 2010,Tehraninejad 2012; Kamath 2015), while two studies were relatively larger: Goto 2009 randomised 144 women and Prapas 2012 randomised 400 women. All studies were conducted in single centres. All studies were published as full articles.
Participants
Baseline characteristics of the participants did not differ significantly across studies or intervention groups, Four studies included subfertile women, younger than 38 years old, who were eligible for IVF or ICSI treatment, and excluded women with risk factors for negative clinical outcomes, such as hydrosalpinx or endometriosis. One study (Prapas 2012) had a higher mean (standard deviation (SD)) age of included women (37.2 (5.2) for experimental, 35.8 (5.8) for control group). Three studies (Zhu 2010; Tehraninejad 2012; Kamath 2015) reported the mean (SD) duration of couple infertility, which ranged from 5.1 (2.8) years (Zhu 2010) to 8.07 (3.53 years) (Kamath 2015). Three studies reported basal FSH levels, which ranged from 5.1 (2.3) mIU/mL (Zhu 2010) to 6.7 (2.3) mIU/mL (Tehraninejad 2012).
Type of infertility was reported by two studies (Zhu 2010; Kamath 2015). Infertility was primary in 55% and 57% and secondary in 45% and 33% of the included women, respectively. In Prapas 2012, the 200 oocyte donor cycles were evenly distributed across both groups and the study was included. After communication with study authors, we obtained and only included the 200 cycles for whom non‐donor oocytes were used. Concerning the employment of fresh or frozen strategies, one study (Goto 2009) described frozen‐thaw embryo transfer for the purposes of the study.
Data availability
In all five studies, data on all randomised women were available for analysis. All studies reported no losses after randomisation and thorough follow‐up.
Interventions
In the intervention groups of all studies, before the embryo transfer, the embryo culture supernatant was injected into the uterine cavity, using an embryo transfer catheter placed just beyond the internal os, prior to embryo transfer.
In all studies, the comparison was between injection of embryo culture supernatant before embryo transfer, versus no injection prior to transfer (Goto 2009; Zhu 2010; Prapas 2012; Tehraninejad 2012; Kamath 2015). One study had an extra comparison, examining injection of embryo culture supernatant before embryo transfer versus injection of culture medium before embryo transfer (Goto 2009).
In two studies, embryos were transferred either on day 3 or day 5 (Prapas 2012, Zhu 2010), while in the remaining studies embryos were transferred on day 5 (Goto 2009; Tehraninejad 2012; Kamath 2015).
In one study (Goto 2009) authors used frozen thawed blastocysts and embryo culture medium and in another (Kamath 2015) authors used vitrified warmed blastocysts.
Outcomes
Rates of live birth or ongoing pregnancy were reported in three studies (340 women analysed; Prapas 2012; Tehraninejad 2012; Kamath 2015). One study reported on live birth rates only (Kamath 2015), while two studies reported on live birth or ongoing pregnancy rates as a combined outcome (Prapas 2012; Tehraninejad 2012). Miscarriage rates were reported in four studies (430 women; Zhu 2010; Prapas 2012; Tehraninejad 2012; Kamath 2015).
Regarding the secondary outcomes, clinical pregnancy was reported in all five studies. Ectopic pregnancy was reported in one study (190 women; Prapas 2012). Multiple pregnancy was reported in two studies (150 women; Zhu 2010; Kamath 2015).
The preterm delivery rate was reported in one study (90 women; Tehraninejad 2012). Foetal abnormality was reported in one study (60 women; Kamath 2015).
Excluded studies
Of the 800 records we identified after removal of duplicates, we excluded 766 studies on the basis of the abstract (Figure 1). Of the remaining 44 papers, all full texts were retrieved. Of these, we subsequently excluded 38 as non‐RCTs, or because they did not explore the outcomes of interest pre‐specified in this review. We excluded one quasi‐randomised RCT (Goto 2007) (see Characteristics of excluded studies).
Studies awaiting assessment
No studies are awaiting further classification.
Ongoing studies
We found no ongoing studies related to the objective of this review.
Risk of bias in included studies
The risk of bias is described in detail in the 'Risk of bias' table in Characteristics of included studies, and presented in Figure 2 and Figure 3. The decision has been made after sending emails to the study authors in an attempt to retrieve any further data.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
One study had a published registered protocol in international databases (Kamath 2015), and one study had an ethical approval obtained, reported as a specific number in the text (Tehraninejad 2012). The domains for whom high or unclear risk was noted were blinding of assessment (Goto 2009; Zhu 2010; Tehraninejad 2012; Kamath 2015) and blinding of participants (Goto 2009; Zhu 2010; Kamath 2015).
Allocation
Random sequence generation
All five studies were introduced as RCTs. We assessed all five of them to be at low risk of selection bias for random sequence generation, as the investigators used computer generated randomization sequences for the selection of the women or, in one case, coloured‐marble lots drawn by a blinded technician.
Allocation concealment
We considered four studies (Zhu 2010; Prapas 2012; Tehraninejad 2012; Kamath 2015) to be at low risk of bias in this domain, having used sealed, consecutively numbered opaque envelopes or by blinded personnel, so that patients and investigators could not foresee the interventions. Concerning Goto 2009, in the absence of the relevant data in the published report, we tried to contact the investigators, but we did not receive any additional information. We judged this domain to be at unclear risk of bias (Goto 2009).
Blinding
Two studies were considered to be at low risk of performance bias because they described clearly blinding of patients, physicians and laboratory personnel (Prapas 2012; Tehraninejad 2012). The remaining three studies were at high risk of performance bias, due to lack of blinding of participants and personnel or because in the absence of details, we deemed that the method used was likely to have affected the results (Goto 2009; Zhu 2010; Kamath 2015).
We assessed two studies to be at low risk of detection bias (Goto 2009; Prapas 2012), according to information provided by the investigators through direct communication. Two studies were at unclear risk of detection bias, due to lack of sufficient information to reach a conclusion (Tehraninejad 2012; Kamath 2015). We judged one study to be at high risk of detection bias due to the complete absence of details concerning blinding of outcome assessment. We deemed that the method used was likely to have affected the results (Zhu 2010).
Incomplete outcome data
All five studies were assessed to be at low risk of attrition bias, as no losses through follow‐up were included and all subjects initially randomised were included in the statistical analysis of the studies' selected outcomes.
Selective reporting
We assessed one study to be at low risk of reporting bias (Kamath 2015) due to the adequate presentation of favourable and adverse clinical outcomes, and to the existence of a registered protocol in the Indian trial registry (CTRI/2013/01/003280), published before the study was conducted. We assessed three studies (Zhu 2010; Prapas 2012; Tehraninejad 2012) to be at unclear risk of reporting bias, as all pre‐specified outcomes of the study were adequately reported, including reporting of adverse events. However, these studies did not have published protocols pre‐specifying the study objectives. We assessed one study to be at high risk of reporting bias, as the investigators did not report the primary adverse event (miscarriage) (Goto 2009). This would be expected from a study that focused on embryological outcomes, avoiding a longer follow‐up that could reveal clinically important results, such as live births or ongoing pregnancies (Goto 2009).
Other potential sources of bias
We assessed one study to be at unclear risk of other bias because of a lack of sufficient information to determine otherwise (Goto 2009).
All other four studies were judged at low risk of other bias, because we could not detect further methodological gaps and we had no rationale to question another source inducing bias in the results (Zhu 2010; Prapas 2012; Tehraninejad 2012; Kamath 2015)
Effects of interventions
Summary of findings 1. Embryo culture supernatant injection compared to no intervention for subfertile women in assisted reproduction.
| Embryo culture supernatant injection compared to no intervention for subfertile women in assisted reproduction | ||||||
| Patient or population: Subfertile women undergoing assisted reproduction Setting: IVF Units Intervention: Embryo culture supernatant injection before embryo transfer Comparison: No intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no intervention | Risk with embryo culture supernatant | |||||
| Live birth/ongoing pregnancy | 419 per 1,000 | 419 per 1,000 (218 to 808) | OR 1.11 (0.73 to 1.7) | 340 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1,2,3 | |
| Miscarriage | 88 per 1,000 | 102 per 1,000 (29 to 303) | OR 0.89 (0.44 to 1.78) | 430 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 1,2,3 | |
| Clinical pregnancy | 513 per 1,000 | 544 per 1,000 (462 to 636) | OR 1.13 (0.80 to 1.61) | 526 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW1,2,3 | |
| Ectopic pregnancy | 8 per 1,000 | 3 per 1,000 (0 to 61) | OR 0.32 (0.01 to 8.24) | 250 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW1,2,3 | |
| Multiple pregnancy | 147 per 1,000 | 107 per 1,000 (43 to 239) | OR 0.70 (0.26 to 1.83) | 150 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW1,2,3 | |
| Preterm delivery | 133 per 1,000 | 88 per 1,000 (25 to 271) | OR 0.63 (0.17 to 2.42) | 90 (1 RCT) | ⊕⊝⊝⊝ VERY LOW2,4 | |
| Foetal abnormalities | 0 per 1,000 | 0 per 1,000 (0 to 0) | OR 3.10 (0.12 to 79.23) | 60 (1 RCT) | ⊕⊕⊕⊝ LOW4 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Downgraded one level for imprecision; wide CIs and low number of events. 2 Downgraded one level due to studies at high risk of bias and unclear risk of publication bias 3 Downgraded one level due to high heterogeneity 4Downgraded two levels for imprecision; wide CIs and low number of events.
Summary of findings 2. Embryo culture supernatant compared to culture medium injection for subfertile women in assisted reproduction.
| Embryo culture supernatant compared to culture medium injection for subfertile women in assisted reproduction | ||||||
| Patient or population: Subfertile women undergoing assisted reproduction Setting: IVF Units Intervention: Embryo culture supernatant injection before embryo transfer Comparison: Culture medium injection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with culture medium injection | Risk with embryo culture supernatant | |||||
| Live birth/ongoing pregnancy | Not reported in any study in this comparison | |||||
| Miscarriage | Not reported in any study in this comparison | |||||
| Clinical pregnancy | 583 per 1,000 | 604 per 1,000 (402 to 775) | OR 1.09 (0.48 to 2.46) | 96 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1,2 | |
| Ectopic pregnancy | Not reported in any study in this comparison | |||||
| Multiple pregnancy | Not reported in any study in this comparison | |||||
| Preterm delivery | Not reported in any study in this comparison | |||||
| Foetal abnormalities | Not reported in any study in this comparison | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Downgraded two levels for imprecision; wide CIs and low number of events 2 Downgraded one level due to studies at high risk of bias and unclear risk of publication bias
Comparison 1: Embryo culture supernatant versus no intervention
Primary outcomes
1.1 Live birth or ongoing pregnancy rate per woman randomised
This outcome was reported in three out of five studies (Prapas 2012; Tehraninejad 2012; Kamath 2015). We are uncertain whether the endometrial injection of embryo culture supernatant injected before embryo transfer improves live birth or ongoing pregnancy rates compared to the use of placebo/no intervention (OR 1.11, 95% CI 0.73 to 1.70; 3 RCTs; n = 340; I2 = 84%; very low‐quality evidence). Our findings suggest that if the chance of live birth/ongoing pregnancy following placebo or no treatment is assumed to be 42%, the chance following the endometrial injection of embryo culture supernatant before embryo transfer is estimated to be between 22 and 81% (Analysis 1.1; Figure 4).
1.1. Analysis.

Comparison 1: Embryo culture supernatant vs no intervention, Outcome 1: live birth/ongoing pregnancy
4.

Forest plot of comparison: embryo culture supernatant versus standard care, outcome: 1.1 live birth/ongoing pregnancy.
We observed considerable heterogeneity (I2 = 84%). Only one study (Tehraninejad 2012) reported an outcome favouring the intervention, while the other two reported a benefit for no intervention. To assess heterogeneity, studies were stratified according to mean age of participants, as pre‐specified in our protocol.
Ιn the subgroup of women of ≤ 37 years of age (Tehraninejad 2012; Kamath 2015), we are uncertain whether embryo culture injection increases live births (OR 1.36, 95% CI 0.73 to 2.54; 2 RCTs; n = 150; I2 = 92%; very low‐quality evidence). Concerning the other three criteria, two studies reported a blastocyst transfer and fresh cycles (Prapas 2012; Tehraninejad 2012) and one study had frozen cycles (Kamath 2015). In the subgroup analysis according to the timing of intervention, one study (Kamath 2015) reported an embryo culture supernatant injection performed two days prior to embryo transfer. In the < 1 day subgroup analysis (Prapas 2012;Tehraninejad 2012), we are uncertain whether the intervention had an effect on live birth or ongoing pregnancy (OR 1.44, 95% CI 0.90 to 2.30; 2 RCTs; n = 280; I2 = 84%; very low‐quality evidence). In the subgroup analysis according to the type of cycle (fresh or frozen), one study reported frozen cycles (Kamath 2015). We are uncertain whether there was a significant effect of the intervention on the outcome of live birth/ongoing pregnancy (OR 0.30, 95% CI 0.10 to 0.92; 1 RCT; n = 60).
The sensitivity analysis of the studies, using a random‐effects model (OR 1.05, 95% CI 0.31 to 3.53; 3 RCTs; n = 340; I2 = 84%; very low‐quality evidence), did not alter the results. There were insufficient data to perform a sensitivity analysis according to the risk of bias assessment, or type of studies (all studies were in full text) or the restriction of the primary outcome to live birth only (all studies reported live birth and ongoing pregnancy as a single outcome).
1.2 Miscarriage rate per woman randomised
This outcome was reported in four studies (Zhu 2010; Prapas 2012; Tehraninejad 2012; Kamath 2015). We are uncertain whether the endometrial injection of embryo culture supernatant before embryo transfer improves the miscarriage rate compared to no intervention or placebo (OR 0.89, 95% CI 0.44 to 1.78; 4 RCTs; n = 430; I2 = 58%; very low‐quality evidence). Results suggest that if the chance of miscarriage following placebo or no treatment is assumed to be 8.8%, the chance following injection of embryo culture supernatant is estimated to be between 2.9% and 30.3% (Analysis 1.2; Figure 5).
1.2. Analysis.

Comparison 1: Embryo culture supernatant vs no intervention, Outcome 2: miscarriage
5.

Forest plot of comparison: embryo culture supernatant versus standard care, outcome: 1.2 miscarriage.
In subgroup analyses, in the age subgroups, we are uncertain whether endometrial injection of supernatant improved miscarriage rates (years old subgroup: OR 0.86, 95% CI 0.40 to 1.85; 3 RCTs; n = 240; I2 = 72%; very low‐quality evidence). In the timing of injection subgroup analysis, we are uncertain whether the intervention improved miscarriage rates (1 to 24 hours subgroup: OR 0.47, 95% CI 0.20 to 1.14; 2 RCTs; n = 280; I2 = 58%; very low‐quality evidence). Finally, in the type of cycle (fresh or frozen) subgroup analysis, we are uncertain whether the intervention improved miscarriage rates in the fresh cycle (OR 0.57, 95% CI 0.25 to 1.27; 3 RCTs; n = 370) and frozen cycle (OR 7.25, 95% CI 0.82 to 64.46; 1 RCT, n = 60).
Sensitivity analysis of the studies using a random‐effects model (OR 1.19, 95% CI 0.31 to 4.52; 4 RCTs; n = 430; I2 = 58%; very low‐quality evidence) did not alter the results. There was insufficient data to perform a sensitivity analysis according to the risk of bias assessment, or type of studies (all studies were in full text) or the type of denominator for miscarriage (per pregnancy or per woman), as all studies used 'per woman'.
Secondary outcomes
1.3 Clinical pregnancy rates per woman or couple randomised
This outcome was reported in all five studies (Goto 2009; Zhu 2010; Prapas 2012; Tehraninejad 2012; Kamath 2015). We are uncertain whether the endometrial injection of embryo culture supernatant before embryo transfer improves clinical pregnancy rates compared with placebo (OR 1.13, 95% CI 0.80 to 1.61; 5 RCTs; n = 566; I2 = 0%; very low‐quality‐evidence). Figure 6
6.

Forest plot of comparison: embryo culture supernatant versus standard care, outcome: 1.3 clinical pregnancy.
Similarly, through subgroup analysis, we are uncertain whether there is a difference in clinical pregnancy between the experimental and the control group in the ≤ 37‐year‐old subgroup (OR 1.26, 95% CI 0.80 to 1.96; 4 RCTs; n = 336; I2 = 0%; very low‐quality‐evidence), or by stratification according to the timing of intervention (1 to 24 hours subgroup: OR 1.19, 95% CI 0.78 to 1.81; 3 RCTs; n = 376; I2= 0%; very low‐quality evidence). In subgroup analysis according to type of cycle (fresh or frozen) two studies reported frozen cycles (Goto 2009; Kamath 2015). We are uncertain as to whether there was a significant effect of injection on clinical pregnancy rates (OR 1.05, 95% CI 0.56 to 1.98; 2 RCTs; n = 156; I2 = 0%).
The sensitivity analysis of the studies (Goto 2009; Zhu 2010; Prapas 2012; Tehraninejad 2012; Kamath 2015) using a random‐effects model (OR 1.13, 95% CI 0.79 to 1.61; 5 RCTs; n = 526; I2 = 0%, very low‐quality evidence) did not alter the results. There was insufficient data to perform a sensitivity analysis according to the risk of bias assessment as none of the studies (Goto 2009; Zhu 2010; Prapas 2012; Tehraninejad 2012; Kamath 2015) fulfilled the pre‐specified inclusion criteria (low risk of selection and reporting bias, low or unclear risk in all other domains, There were also insufficient data to perform a sensitivity analysis according to the type of studies (all studies were in full text).
1.4 Ectopic pregnancy
This outcome was reported in two studies (Prapas 2012; Kamath 2015). We are uncertain whether there was a difference between the intervention group and the control group with regard to ectopic pregnancy (OR 0.32, 95% CI 0.01 to 8.24; n = 250; 2 RCTs, I2 = 41%; very low‐quality evidence). Only one event was recorded in the control group in one of the studies included (Kamath 2015), while the experimental arm of Kamath 2015 and both arms in Prapas 2012 exhibited zero events (Figure 7).
7.

Forest plot of comparison: embryo culture supernatant versus standard care, outcome: 1.4 ectopic pregnancy.
Stratification of patients by age could not generate a meaningful comparison, as this outcome was mentioned only in two studies; one of them had patients younger than 37 years old (Kamath 2015), while the other one had patients older than 37 years old (Prapas 2012).
The sensitivity analysis of the studies using a random‐effects model (OR 0.32, 95% CI 0.01 to 8.24; 2 RCTs; n = 250; I2 = 0%, very low‐quality evidence) did not alter the results. There was insufficient data to perform a sensitivity analysis according to the risk of bias assessment or the type of studies (all studies were in full text).
1.5 Multiple pregnancy
This outcome was reported in two studies (Zhu 2010; Kamath 2015). We are uncertain whether the endometrial injection of embryo culture supernatant before embryo transfer improves the incidence of multiple pregnancies compared to no intervention/placebo (OR 0.70, 95% CI 0.26 to 1.83, 2 RCTs, n = 150, I2 = 63%, very low‐quality evidence; Figure 8).
8.

Forest plot of comparison: embryo culture supernatant versus standard care, outcome: 1.5 multiple pregnancy.
In Zhu 2010, multiple embryos were transferred per woman. In Kamath 2015, stimulation of endometrium embryo transfer (SEET) was performed.
Stratification of patients by age was not applicable, as both studies reporting this outcome included patients younger than 37 years old. Through the pre‐specified subgroup analysis according to type of cycle (fresh or frozen), we are uncertain whether endometrial injection of embryo culture supernatant affected multiple pregnancy rates: fresh cycle (OR 1.75, 95% CI 0.39 to 7.81; 1 RCT; n = 90) and frozen cycle (OR 0.31, 95% CI 0.07 to 1.29; 1 RCT, n = 60).
The sensitivity analysis of the studies using a random‐effects model (OR 0.72, 95% CI 0.13 to 4.00; 2RCTs; n = 150; I2 = 63%, very low‐quality evidence) did not alter the results. There was insufficient data to perform a sensitivity analysis according to the risk of bias assessment or the type of studies (all studies were in full text).
1.6 Preterm delivery
This outcome was reported in one study (Tehraninejad 2012). We are uncertain whether endometrial injection improved preterm delivery (OR 0.63, 95% CI 0.17 to 2.42, 1 RCT, n = 90, I2 = 0%, studies = 1, very low‐quality evidence) Figure 9
9.

Forest plot of comparison: embryo culture supernatant versus standard care, outcome: 1.6 preterm delivery.
There was insufficient data to perform any meaningful sensitivity analysis.
1.7 Foetal abnormalities
This outcome was reported in one study (Kamath 2015).There may have been little or no difference in foetal abnormalities between the intervention group and the control group (OR 3.10, 95% CI 0.12 to 79.23, 1 RCT, n = 60, I2 = 0%, low‐quality evidence). Figure 10. Peto OR was 7.39 (0.15, 372.38).
10.

Forest plot of comparison: embryo culture supernatant versus standard care, outcome: 1.7 foetal abnormalities.
There were insufficient data to perform any meaningful sensitivity analysis.
Other analyses
We performed a sensitivity analysis using a random effects model for the primary outcomes and for the most clinically important secondary outcomes.
(1) Live birth or ongoing pregnancy rate per woman randomised
The sensitivity analysis of the studies (Prapas 2012; Tehraninejad 2012; Kamath 2015) did not alter the results (OR 1.05, 95% CI 0.31 to 3.53; 3 RCTs; n = 340; I2 = 84%; very low‐quality evidence).
(2) Miscarriage rate per woman randomised
The sensitivity analysis of the studies (Zhu 2010; Prapas 2012; Tehraninejad 2012; Kamath 2015) did not alter the results (OR 1.19, 95% CI 0.31 to 4.52; 4 RCTs; n = 430; I2 = 58%; very low‐quality evidence).
Similarly, the effect did not change for the secondary outcomes, either for clinical pregnancy (Goto 2009; Zhu 2010; Prapas 2012; Tehraninejad 2012; Kamath 2015) (OR 1.13, 95% CI 0.79 to 1.61; 5 RCTs; n = 526; I2 = 0%, very low‐quality evidence) or for ectopic pregnancy (Prapas 2012; Kamath 2015) (OR 0.32, 95% CI 0.01 to 8.24; 2 RCTs; n = 250; I2 = 0%, very low‐quality evidence) or for multiple pregnancy (Zhu 2010; Kamath 2015) (OR 0.72, 95% CI 0.13 to 4.00; 2 RCTs; n =150; I2 = 63%, very low‐quality evidence).
Each of the remaining outcomes, preterm delivery (Tehraninejad 2012) and foetal abnormalities (Kamath 2015), were reported in one study. We could not perform a meaningful sensitivity analysis.
We also performed a sensitivity analysis using RR to measure magnitude of effect (pooled RR) in the primary outcomes and the most clinically important secondary outcomes. This analysis was not mentioned in the review protocol, but it was conducted towards a more extensive investigation of the heterogeneity detected. The change is stated in the section Differences between protocol and review.
(1) Live birth or ongoing pregnancy rate per woman randomised
The sensitivity analysis of the studies (Prapas 2012; Tehraninejad 2012; Kamath 2015) did not alter the results (RR 1.00, 95% CI 0.52 to 1.93; 3 RCTs; n = 340; I2 = 82%; very low‐quality evidence).
(2) Miscarriage rate per woman randomised
The sensitivity analysis of the studies (Zhu 2010; Prapas 2012; Tehraninejad 2012; Kamath 2015) did not alter the results (RR 1.17, 95% CI 0.36 to 3.82; 4 RCTs; n = 430; I2 = 54%; very low‐quality evidence).
Similarly, the effect did not change for the secondary outcome of clinical pregnancy (Goto 2009; Zhu 2010; Prapas 2012; Tehraninejad 2012; Kamath 2015) (RR 1.06, 95% CI 0.90 to 1.24; 5 RCTs; n = 526; I2 = 0%; very low‐quality evidence). This was also true for the secondary outcomes of ectopic pregnancy (Prapas 2012; Kamath 2015) (RR 0.33, 95% CI 0.01 to 7.87; 2 RCTs; n = 250; I2 = 0%; very low‐quality evidence) and multiple pregnancy (Zhu 2010; Kamath 2015) (RR 0.73, 95% CI 0.31 to 1.71; 2 RCTs; n = 150; I2 = 60%; very low‐quality evidence) .
Each of the remaining outcomes, preterm delivery (Tehraninejad 2012) and foetal abnormalities (Kamath 2015) were reported in one study respectively.Thus, we could not perform a meaningful sensitivity analysis.
There was insufficient evidence to perform further sensitivity analyses in terms of risk of bias assessment, type of study, type of denominator in miscarriage (per woman or per pregnancy) or by restriction of the primary outcome to live birth only.
We conducted the planned analyses using Peto ORs and the results were as follows. For miscarriage, Peto OR was 0.90 (95% CI 0.46 to 1.79; 4 RCTs; n = 430; I2 = 58%; very low‐quality evidence). For ectopic pregnancy, Peto OR was 0.14 (95% CI 0.003 to 7.04; 2 RCTs; n = 250, very low‐quality evidence). For multiple pregnancy, Peto OR was 0.70 (95% CI 0.27 to 1.82; 2 RCTs; n =150; I2 = 63%; very low‐quality evidence). For foetal abnormalities, Peto OR was 7.39 (95% CI 0.15 to 372.38; 1 RCT; n = 60, moderate quality evidence).
Comparison 2: Embryo culture supernatant versus culture media
Primary outcomes
2.1 Live birth or ongoing pregnancy rate per woman randomised
There were no trials addressing this outcome.
2.2 Miscarriage rate per woman randomised
There were no trials addressing this outcome.
Secondary outcomes
2.3 Clinical pregnancy rates per woman or couple randomised
Only one trial addressed this outcome (Goto 2009). We are uncertain whether the use of embryo culture supernatant increases clinical pregnancy rates (OR 1.09, 95% CI 0.48 to 2.46; n = 96; 1 RCT; very low‐quality evidence), compared to the use of culture media before embryo transfer (Figure 11).
11.

Forest plot of comparison: 2 Embryo culture supernatant vs. stimulation of endometrium, outcome: 2.1 clinical pregnancy.
2.4 Ectopic pregnancy
There were no trials addressing this outcome.
2.5 Multiple pregnancy
There were no trials addressing this outcome.
2.6 Preterm delivery
There were no trials addressing this outcome.
2.7 Foetal abnormalities
There were no trials addressing this outcome.
Discussion
Summary of main results
This Cochrane review compared the effectiveness and safety of endometrial injection of embryo culture supernatant before embryo transfer versus any other intervention in use, or versus no intervention, for improving rates of live birth or ongoing pregnancy and miscarriage in women undergoing ART. We included five RCTs comprising 526 participants. We made two comparisons: use of embryo culture supernatant versus standard care or no intervention; and injection of embryo culture supernatant versus injection of culture medium before embryo transfer.
In the first comparison, the overall quality of evidence ranged from very low to moderate. We found very low‐quality evidence from three RCTs of no clear difference in the rates of live birth or ongoing pregnancy between the group of embryo culture supernatant injection and the group receiving standard care or no intervention (Goto 2009; Zhu 2010; Prapas 2012; Tehraninejad 2012; Kamath 2015). We found very low‐quality evidence from four RCTs of no significant difference in miscarriage rates between the two groups. In addition, we found very low‐quality evidence from five RCTs of no clear difference in clinical pregnancy rates between the embryo culture supernatant injection technique group and the control group. There were quite sparse data for the rest of the secondary outcomes, In particular, for the secondary outcomes of ectopic and multiple pregnancy, we collected very low‐quality data from two studies of no significant difference in effect between the two groups. We also found very low‐quality data from one study in preterm delivery rates with no clear difference between intervention and control group. Finally, we retrieved low quality data for the secondary outcome of foetal abnormalities from one study showing no clear difference between embryo culture supernatant injection and standard procedure. Thus we were uncertain whether the intervention improves the clinical outcomes examined. The effect estimate was not affected when we used a random‐effects model and pooled RR for both primary and important secondary outcomes. Similarly, subgroup analyses (where applicable, according to data availability) in terms of age, time of injection and type of cycle, did not substantially alter the results.
In the second comparison, we found very low‐quality evidence from one RCT demonstrating no clear difference in clinical pregnancy rates between the group that received injection of embryo culture supernatant and the group that received injection of culture medium prior to embryo transfer.There were no trials addressing the other outcomes in order to attempt further assessments.
Overall, the limited data and the very low quality of the evidence for most outcomes did not allow us to reach robust conclusions about the effectiveness and safety of endometrial injection of embryo culture supernatant on top of standard procedures (no intervention) or other types of interventions.
Overall completeness and applicability of evidence
The objectives of this review were sufficiently addressed by the studies analysed. We included five RCTs with data relevant to the review question. Thus, we were able to proceed not only with the qualitative but also with the quantitative analysis of the data retrieved, regarding the evaluation of embryo culture supernatant intrauterine injection prior to embryo transfer in comparison to standard care or no intervention. There were RCT data available to address the primary outcome measures of live birth/ongoing pregnancy and miscarriage per allocated couple or woman randomised. Similarly, there were sufficient data to address the secondary outcomes of clinical pregnancy and of ectopic or multiple pregnancy, considered as adverse effects.. There were sparse data on the outcomes of preterm delivery and foetal abnormalities, as each of them was mentioned only in one study. As for the planned comparison of the use of embryo culture supernatant with culture medium injection before embryo transfer, we were able to identify only one study, which addressed only the secondary outcome of clinical pregnancy. Consequently, there were no data available on the primary outcomes for this comparison.
Participants eligible for inclusion were women younger than 40 years old, with a good prognosis and good quality embryos available at day 2 or 3. They were due for a fresh or a frozen/vitrified‐thawed blastocyst transfer and had supernumerary embryos available for cryopreservation. Participants included could also have previously unsuccessful fresh blastocyst transfers, or a successful previous outcome previously and interested in having another child. Specific categories of patients such as women undergoing fresh transfer due to high risk of OHSS, women with recurrent implantation failures, known endometriosis, hydrosalpinx or uterine anomalies, were excluded from the trials. Given the study populations, the results of this review are widely applicable for women identified as normal responders for ART. However, there is a gap in the literature for other subgroups of subfertile women, such as those excluded from the study populations above. As the results of this systematic review demonstrated no significant efficacy and safety of injection of embryo culture supernatant in improving ART outcomes based on studies of ambiguous quality evidence, more evidence is needed to reach robust conclusions.
In addition to the published data collected, we also made multiple efforts to retrieve extra details on the trials through communication with authors. Unfortunately, we obtained only a small amount of evidence. Important information is still missing in many cases, which hindered our ability to perform more and better quality statistical analyses as well as to assess these studies overall.
Quality of the evidence
We found 44 potentially eligible studies. From these, five studies were eligible for inclusion and further analysis. We collected published data and retrieved additional details through direct communication with most authors of the original studies.
Concerning the first comparison (embryo culture supernatant use versus standard care or no intervention) the overall quality of the evidence ranged from very low to moderate. Limitations included serious risk of bias associated with poor reporting of methods and selective reporting, imprecision and high heterogeneity and unclear risk of publication bias.
In particular, we found very low‐quality evidence for the primary outcome of live birth or ongoing pregnancy that was reported in three studies, as we judged one study to be at high risk of bias, detected serious imprecision, low numbers of events and wide confidence intervals, compatible with benefit in either arm, or no difference between the groups, and identified substantial heterogeneity across studies. We also found very low‐quality evidence from four trials for the primary adverse event of miscarriage. Evidence quality was graded down for imprecision, wide confidence intervals, low number of events and serious heterogeneity,
Moreover, we found very low‐quality evidence for the secondary outcome of clinical pregnancy, for the same reasons as for live birth or ongoing pregnancy. We detected very low‐quality evidence from two trials for ectopic pregnancy due to imprecision, wide confidence intervals, low number of events and substantial heterogeneity across studies, Furthermore, the quality of evidence for the secondary outcome of multiple pregnancy described in two studies was very low for all the same reasons, including studies at high risk of bias, serious imprecision and heterogeneity. As for the secondary outcome of preterm delivery, mentioned in one study, we found very low‐quality evidence due to all factors mentioned above. Finally, we found low quality of evidence from one trial for the secondary outcome of foetal abnormalities, because of very serious imprecision and low numbers of events reported.
Concerning the second comparison (embryo culture supernatant use versus media culture use) the overall quality of the evidence for the secondary outcome of clinical pregnancy was very low. The reasons for this assessment included serious imprecision and wide CIs, high risk of bias, substantial heterogeneity and unclear publication bias. There were no data on the remaining both primary and secondary outcomes examined in this review.
Potential biases in the review process
We made every effort to identify all eligible studies, following standard procedures. Only two trial authors responded sufficiently to our requests for additional information (Prapas 2012; Kamath 2015). We retrieved only a few additional data through direct communication, while several aspects of the trials remained quite obscure to us, given that we did not receive a response with valuable clarifications from most authors. This lack of information affected our judgements substantially and subsequently became a reason for downgrading the quality of our evidence.
Agreements and disagreements with other studies or reviews
This current review aimed to establish whether the use of embryo culture supernatant before embryo transfer may play a beneficial role in improving pregnancy outcomes in women undergoing ART. Our review showed that there is no benefit of its use for ART treatment.
A similar review to date addressing this comparison, ended up with similar results (Kamath 2017). Authors included five RCTs and combined data from only two for the meta‐analysis. Due to clinical heterogeneity; they concluded that they did not find any improvement in clinical pregnancy rate with the use of embryo culture supernatant prior to embryo transfer compared to no intervention in women undergoing ART.
Authors' conclusions
Implications for practice.
Alhough data about the potential action of factors facilitating implantation in an IVF cycle exist in literature, we are uncertain if the addition of endometrial injection of embryo culture supernatant before embryo transfer as a routine method for the treatment of women undergoing ART can improve pregnancy outcomes. This conclusion is based on available data from five RCTs, with varying quality of evidence, ranging from very low quality to moderate quality, for different outcomes assessing its effectiveness and safety across studies. More rigorous studies that report on important clinical outcomes such as live births, as well as on adverse events such as miscarriage, are still required.
Implications for research.
We aimed to provide a clear overview of the effectiveness and safety of endometrial injection of embryo culture supernatant before embryo transfer in women undergoing ART. We identified five RCTs suitable for inclusion in the review, all published in the form of full texts and including a total of 526 women. Three out of the five included studies were small in terms of the study population, while two were relatively larger.
Overall, we collected data of very low‐quality evidence that did not allow us to make safe judgements leading to robust conclusions. Properly conducted RCTs with appropriate endpoints (live‐birth and miscarriage rates must be the primary outcomes, also avoiding the combination with ongoing pregnancy) that compare the use of endometrial injection of embryo culture supernatant before embryo transfer with the use of a placebo intervention in ART are required in order to give definite answers and avoid misconceptions. Appropriate study design in terms of randomisation, blinding and collecting results, as well as unbiased study reporting, are required to minimise bias and obtain consistent and objective findings. Sufficient power through sample size calculation is also needed, based on current data and estimated differences in outcomes. Participants included in the trials were mainly young women, identified as normal responders to ovarian stimulation. Other subgroups, such as high or poor responders, or even women with recurrent implantation failures, could be included in the trials. Accurate documentation of the randomisation, allocation concealment, and blinding methods is highly desirable, so that risks of bias could be eliminated and the quality of the conclusions could be at high levels. In addition to the primary outcomes of live birth and miscarriage, study protocols should include the reporting of other adverse effects, and of crucial secondary outcomes. Finally, studies on frozen‐thawed cycles should also been performed, as such strategies (e.g. freeze‐all policy) have become very popular for most of the population seeking for assisted reproduction (Zandstra 2018).
History
Protocol first published: Issue 6, 2018 Review first published: Issue 8, 2020
Acknowledgements
We thank Helen Nagels (Managing Editor), Marian Showell (Information Specialist), and the editorial board of the Cochrane Gynaecology and Fertility Group for their invaluable assistance in developing this review. In addition, we thank the authors of included studies for supplying further information in response to our queries (Kamath 2015; Prapas 2012). We also thank Vasilis Pergialiotis for his contribution in the formation of the initial idea and Costantinos Dapofoulos for his participation in the initial steps of this review (help with selection of studies).
We thank Mohan Kamath, Rui Wang, Bryan Woodward and Nada Ata Allah for providing referee comment on this review.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Specialised Register search strategy
Searched 2 December 2019
Procite platform
Keywords CONTAINS "IVF" or "ICSI" or "ET" or "intracytoplasmic sperm injection techniques" or "intracytoplasmic sperm injection" or "in‐vitro fertilisation " or "in vitro fertilization" or "Embryo Transfer" or "ovarian stimulation" or "ovarian stimulation controlled ovarian stimulation" or "ovulation induction" or "ovulation stimulation" or "superovulation" or "superovulation induction" or "ovarian hyperstimulation" or "poor responders" or "poor responder" or "poor prognostic patients" or "controlled ovarian hyperstimulation" or "controlled ovarian stimulation" or "COH" or Title CONTAINS "IVF" or "ICSI" or "ET" or "intracytoplasmic sperm injection techniques" or "intracytoplasmic sperm injection" or "in‐vitro fertilisation " or "in vitro fertilization" or "Embryo Transfer" or "ovarian stimulation" or "ovarian stimulation controlled ovarian stimulation" or "ovulation induction" or "ovulation stimulation" or "superovulation" or "superovulation induction" or "ovarian hyperstimulation"
AND
Keywords CONTAINS "uterine cavity injection" or "intrauterine flushing" or "Intrauterine injection" or "intrauterine instillation" or "flushing media" or "Flushing‐Outcome" or "stimulation of endometrium embryo transfer" or "endometrial preparation" or "endometrial priming" or "endometrial receptivity" or "endometrial stimulation" or "embryo culture supernatant" or Title CONTAINS "uterine cavity injection" or "intrauterine flushing" or "Intrauterine injection" or "intrauterine instillation" or "flushing media" or "Flushing‐Outcome" or "stimulation of endometrium embryo transfer" or "endometrial preparation" or "endometrial priming" or "endometrial receptivity" or "endometrial stimulation" or "embryo culture supernatant"
239 records
Appendix 2. CENTRAL search strategy
Searched 2 December 2019 (Issue October 2019)
OVID platform
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (2310) 2 embryo transfer$.tw. (3222) 3 vitro fertili?ation.tw. (3088) 4 ivf‐et.tw. (601) 5 icsi.tw. (2742) 6 intracytoplasmic sperm injection$.tw. (1192) 7 (blastocyst adj2 transfer$).tw. (409) 8 ivf.tw. (5735) 9 or/1‐8 (8683) 10 (inject* adj5 culture*).tw. (162) 11 (inject* adj5 medi*).tw. (3225) 12 (supernatant adj5 embryo*).tw. (11) 13 (supernatant adj5 blastocyst*).tw. (7) 14 (supernatant adj5 endometri*).tw. (8) 15 (supernatant adj5 uter*).tw. (8) 16 (endometri* adj5 inject*).tw. (128) 17 (flush* adj5 endometri*).tw. (55) 18 (flush* adj5 uter*).tw. (53) 19 (culture* adj5 uter*).tw. (59) 20 (culture* adj5 endometri*).tw. (87) 21 (inject* adj5 uter*).tw. (262) 22 exp Embryo Culture Techniques/ (105) 23 (stimulat* adj2 endomet*).tw. (192) 24 (culture* adj5 supernatant*).tw. (256) 25 (flush* adj5 supernatant*).tw. (3) 26 (transfer* adj5 supernatant).tw. (18) 27 (inject* adj5 supernatant).tw. (16) 28 (intrauter* adj5 inject*).tw. (359) 29 (intrauter* adj5 flush*).tw. (10) 30 (intrauter* adj5 supernatant*).tw. (1) 31 (intrauter* adj5 culture).tw. (12) 32 (instillation adj5 culture).tw. (5) 33 (instillation adj5 uter*).tw. (16) 34 or/10‐33 (4784) 35 9 and 34 (473)
Appendix 3. MEDLINE search strategy
Searched from 1946 to 2 December 2019
OVID platform
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (40473) 2 embryo transfer$.tw. (11563) 3 vitro fertili?ation.tw. (22472) 4 ivf‐et.tw. (2262) 5 icsi.tw. (8087) 6 intracytoplasmic sperm injection$.tw. (6967) 7 (blastocyst adj2 transfer$).tw. (994) 8 ivf.tw. (22860) 9 or/1‐8 (54566) 10 (inject* adj5 culture*).tw. (2568) 11 (inject* adj5 medi*).tw. (13789) 12 (supernatant adj5 embryo*).tw. (82) 13 (supernatant adj5 blastocyst*).tw. (5) 14 (supernatant adj5 endometri*).tw. (32) 15 (supernatant adj5 uter*).tw. (33) 16 (endometri* adj5 inject*).tw. (302) 17 (flush* adj5 endometri*).tw. (123) 18 (flush* adj5 uter*).tw. (857) 19 (culture* adj5 uter*).tw. (1041) 20 (culture* adj5 endometri*).tw. (2095) 21 (inject* adj5 uter*).tw. (1217) 22 exp Embryo Culture Techniques/ (3516) 23 (stimulat* adj2 endomet*).tw. (584) 24 (culture* adj5 supernatant*).tw. (26853) 25 (flush* adj5 supernatant*).tw. (5) 26 (transfer* adj5 supernatant).tw. (234) 27 (inject* adj5 supernatant).tw. (585) 28 (intrauter* adj5 inject*).tw. (590) 29 (intrauter* adj5 flush*).tw. (15) 30 (intrauter* adj5 supernatant*).tw. (2) 31 (intrauter* adj5 culture).tw. (36) 32 (instillation adj5 culture).tw. (28) 33 (instillation adj5 uter*).tw. (66) 34 or/10‐33 (53315) 35 randomized controlled trial.pt. (495253) 36 controlled clinical trial.pt. (93439) 37 randomized.ab. (461382) 38 randomised.ab. (92232) 39 placebo.tw. (208416) 40 clinical trials as topic.sh. (189290) 41 randomly.ab. (322085) 42 trial.ti. (208353) 43 (crossover or cross‐over or cross over).tw. (82565) 44 or/35‐43 (1315695) 45 exp animals/ not humans.sh. (4646734) 46 44 not 45 (1210602) 47 9 and 34 and 46 (258)
Appendix 4. Embase search strategy
Searched from 1980 to 2 December 2019
OVID platform
1 exp embryo transfer/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ (67110) 2 embryo$ transfer$.tw. (20553) 3 in vitro fertili?ation.tw. (29456) 4 icsi.tw. (15688) 5 intracytoplasmic sperm injection$.tw. (9334) 6 (blastocyst adj2 transfer$).tw. (2298) 7 ivf.tw. (39359) 8 assisted reproduct$.tw. (21944) 9 ovulation induc$.tw. (5492) 10 superovulat$.tw. (3769) 11 COH.tw. (2379) 12 infertil$.tw. (81454) 13 subfertil$.tw. (6743) 14 (ovari$ adj2 induction).tw. (327) 15 exp infertility therapy/ (96516) 16 exp ovulation induction/ (14072) 17 exp ovary hyperstimulation/ (9251) 18 (ovar$ adj2 hyperstimulation).tw. (7325) 19 (ovar$ adj2 stimulat$).tw. (10952) 20 or/1‐19 (183851) 21 exp embryo culture/ and (uter* or endometri*).tw. (733) 22 (inject* adj5 culture*).tw. (3219) 23 (inject* adj5 medi*).tw. (18596) 24 (supernatant adj5 embryo*).tw. (77) 25 (supernatant adj5 blastocyst*).tw. (12) 26 (supernatant adj5 endometri*).tw. (35) 27 (supernatant adj5 uter*).tw. (29) 28 (endometri* adj5 inject*).tw. (459) 29 (flush* adj5 endometri*).tw. (138) 30 (flush* adj5 uter*).tw. (882) 31 (culture* adj5 uter*).tw. (1055) 32 (culture* adj5 endometri*).tw. (2546) 33 (inject* adj5 uter*).tw. (1410) 34 (stimulat* adj2 endomet*).tw. (762) 35 (culture* adj5 supernatant*).tw. (34121) 36 (flush* adj5 supernatant*).tw. (7) 37 (transfer* adj5 supernatant).tw. (367) 38 (inject* adj5 supernatant).tw. (784) 39 (intrauter* adj5 inject*).tw. (789) 40 (intrauter* adj5 flush*).tw. (16) 41 (intrauter* adj5 supernatant*).tw. (4) 42 (intrauter* adj5 culture).tw. (42) 43 (instillation adj5 culture).tw. (33) 44 (instillation adj5 uter*).tw. (66) 45 or/21‐44 (64521) 46 20 and 45 (2869) 47 Clinical Trial/ (950403) 48 Randomized Controlled Trial/ (577437) 49 exp randomization/ (85057) 50 Single Blind Procedure/ (37266) 51 Double Blind Procedure/ (164812) 52 Crossover Procedure/ (61320) 53 Placebo/ (330107) 54 Randomi?ed controlled trial$.tw. (216707) 55 Rct.tw. (34913) 56 random allocation.tw. (1956) 57 randomly.tw. (423550) 58 randomly allocated.tw. (33740) 59 allocated randomly.tw. (2478) 60 (allocated adj2 random).tw. (810) 61 Single blind$.tw. (23733) 62 Double blind$.tw. (197359) 63 ((treble or triple) adj blind$).tw. (1048) 64 placebo$.tw. (294279) 65 prospective study/ (566102) 66 or/47‐65 (2343245) 67 case study/ (65451) 68 case report.tw. (385667) 69 abstract report/ or letter/ (1071531) 70 or/67‐69 (1512744) 71 66 not 70 (2290809) 72 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5841365) 73 71 not 72 (2131887) 74 46 and 73 (406)
Appendix 5. CINAHL Plus search strategy
Searched from 1961 to 2 December 2019
EBSCO platform
S29 S9 AND S28 109 S28 S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 4,358 S27 TX(instillation N5 uter*) 7 S26 TX(instillation N5 culture) 2 S25 TX(intrauter* N5 culture) 10 S24 TX(intrauter* N5 inject*) 138 S23 TX(inject* N5 supernatant) 21 S22 TX(transfer* N5 supernatant) 14 S21 TX(culture* N5 supernatant*) 789 S20 TX(stimulat* N2 endomet*) 100 S19 TX(inject* N5 uter*) 113 S18 TX(culture* N5 endometri*) 117 S17 TX(culture* N5 uter*) 34 S16 TX(flush* N5 uter*) 10 S15 TX(flush* N5 endometri*) 12 S14 TX(endometri* N5 inject*) 47 S13 TX(supernatant N5 endometri*) 5 S12 TX (supernatant N5 embryo*) 6 S11 TX (inject* N5 medi*) 2,849 S10 TX (inject* N5 culture*) 162 S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 10,536 S8 (MM "Embryo Transfer") 1,093 S7 TX blastocyst* N3 transfer* 361 S6 TX embryo* N3 transfer* 3,058 S5 TX ovar* N3 hyperstimulat* 830 S4 TX ovari* N3 stimulat* 991 S3 TX IVF or TX ICSI 4,961 S2 (MM "Fertilization in Vitro") 3,405 S1 TX vitro fertilization 6,919
Appendix 6. World Health Organization International Trials Registry Platform and ClinicalTrials.gov registry
Searched 2 December 2019
Web platform
Keywords or Title CONTAINS
"IVF" or "ART" and "endometrial injection" or "embryo culture supernatant"
Data and analyses
Comparison 1. Embryo culture supernatant vs no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 live birth/ongoing pregnancy | 3 | 340 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.73, 1.70] |
| 1.1.1 Age ≤ 37 | 2 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.73, 2.54] |
| 1.1.2 Age > 37 | 1 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.53, 1.68] |
| 1.2 miscarriage | 4 | 430 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.44, 1.78] |
| 1.2.1 Age ≤ 37 | 3 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.40, 1.85] |
| 1.2.2 Age > 37 | 1 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.21, 5.31] |
| 1.3 clinical pregnancy | 5 | 526 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.80, 1.61] |
| 1.3.1 Age ≤ 37 | 4 | 336 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.81, 1.96] |
| 1.3.2 Age > 37 | 1 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.54, 1.68] |
| 1.4 ectopic pregnancy | 2 | 250 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.24] |
| 1.5 multiple pregnancy | 2 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.26, 1.83] |
| 1.6 preterm delivery | 1 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.17, 2.42] |
| 1.7 foetal abnormalities | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.10 [0.12, 79.23] |
1.3. Analysis.

Comparison 1: Embryo culture supernatant vs no intervention, Outcome 3: clinical pregnancy
1.4. Analysis.

Comparison 1: Embryo culture supernatant vs no intervention, Outcome 4: ectopic pregnancy
1.5. Analysis.

Comparison 1: Embryo culture supernatant vs no intervention, Outcome 5: multiple pregnancy
1.6. Analysis.

Comparison 1: Embryo culture supernatant vs no intervention, Outcome 6: preterm delivery
1.7. Analysis.

Comparison 1: Embryo culture supernatant vs no intervention, Outcome 7: foetal abnormalities
Comparison 2. Embryo culture supernatant vs. stimulation of endometrium.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Clinical pregnancy | 1 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.48, 2.46] |
2.1. Analysis.

Comparison 2: Embryo culture supernatant vs. stimulation of endometrium, Outcome 1: Clinical pregnancy
Comparison 3. Subgroup analysis, type of cycle.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 live birth/ongoing pregnancy | 3 | 340 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.73, 1.70] |
| 3.1.1 fresh cycle | 2 | 280 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.90, 2.30] |
| 3.1.2 frozen cycle | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.10, 0.92] |
| 3.2 Miscarriage | 4 | 430 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.44, 1.78] |
| 3.2.1 fresh cycle | 3 | 370 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.25, 1.27] |
| 3.2.2 frozen cycle | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.25 [0.82, 64.46] |
| 3.3 clinical pregnancy | 5 | 526 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.80, 1.61] |
| 3.3.1 fresh cycle | 3 | 370 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.77, 1.78] |
| 3.3.2 frozen cycle | 2 | 156 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.56, 1.98] |
| 3.4 multiple pregnancy | 2 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.26, 1.83] |
| 3.4.1 fresh cycle | 1 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.39, 7.81] |
| 3.4.2 frozen cycle | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.07, 1.29] |
3.1. Analysis.

Comparison 3: Subgroup analysis, type of cycle, Outcome 1: live birth/ongoing pregnancy
3.2. Analysis.

Comparison 3: Subgroup analysis, type of cycle, Outcome 2: Miscarriage
3.3. Analysis.

Comparison 3: Subgroup analysis, type of cycle, Outcome 3: clinical pregnancy
3.4. Analysis.

Comparison 3: Subgroup analysis, type of cycle, Outcome 4: multiple pregnancy
Comparison 4. Subgroup analysis, age.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Live birth/ongoing pregnancy | 3 | 340 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.73, 1.70] |
| 4.1.1 Age ≤ 37 | 2 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.73, 2.54] |
| 4.1.2 Age > 37 | 1 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.53, 1.68] |
| 4.2 Miscarriage | 4 | 430 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.44, 1.78] |
| 4.2.1 Age ≤ 37 | 3 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.40, 1.85] |
| 4.2.2 Age > 37 | 1 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.21, 5.31] |
| 4.3 Clinical Pregnancy | 5 | 526 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.80, 1.61] |
| 4.3.1 Age ≤ 37 | 4 | 336 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.81, 1.96] |
| 4.3.2 Age > 37 | 1 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.54, 1.68] |
4.1. Analysis.

Comparison 4: Subgroup analysis, age, Outcome 1: Live birth/ongoing pregnancy
4.2. Analysis.

Comparison 4: Subgroup analysis, age, Outcome 2: Miscarriage
4.3. Analysis.

Comparison 4: Subgroup analysis, age, Outcome 3: Clinical Pregnancy
Comparison 5. Subgroup analysis, timing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Live birth/ongoing pregnancy | 3 | 340 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.73, 1.70] |
| 5.1.1 1 to 24 hours | 2 | 280 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.90, 2.30] |
| 5.1.2 ≥ 1 day | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.10, 0.92] |
| 5.2 Miscarriage | 4 | 430 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.44, 1.78] |
| 5.2.1 ≥ 1 day | 2 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.42 [0.91, 21.46] |
| 5.2.2 1 to 24 hours | 2 | 280 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.20, 1.14] |
| 5.3 Clinical Pregnancy | 5 | 526 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.80, 1.61] |
| 5.3.1 ≥ 1 day | 2 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.53, 1.90] |
| 5.3.2 1 to 24 hours | 3 | 376 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.79, 1.81] |
5.1. Analysis.

Comparison 5: Subgroup analysis, timing, Outcome 1: Live birth/ongoing pregnancy
5.2. Analysis.

Comparison 5: Subgroup analysis, timing, Outcome 2: Miscarriage
5.3. Analysis.

Comparison 5: Subgroup analysis, timing, Outcome 3: Clinical Pregnancy
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Goto 2009.
| Study characteristics | ||
| Methods | Study design: RCT Setting: Private IVF clinic Period:NS |
|
| Participants | Patient(s): 144 women in their first ART cycle who had at least one blastocyst but who, to prevent the development of ovarian hyperstimulation syndrome (OHSS), had not yet undergone fresh embryo transfer in stimulated cycles. Their blastocysts were therefore cryopreserved for frozen‐thaw embryo transfer in the study cycle. Exclusion criteria: Patients with hydrosalpinx 48 women in the BT group (23 with low‐grade blastocysts, 25 with high‐grade blastocysts) 48 women in the ST group who had culture medium injected into the uterus before BT (19 with low‐grade blastocysts, 29 with high‐grade blastocysts) 48 women in the SEET group (23 with low‐grade blastocysts, 25 with high‐grade blastocysts) Baseline characteristics Age of patient (years) Period of infertility (months) Basal FSH level (mIU/mL) No. of oocytes retrieved No. of oocytes fertilised |
|
| Interventions | Injection of embryo culture supernatant and injection of culture medium | |
| Outcomes | Main outcome measure(s): No. of chemical pregnancies Implantation rate per embryo (%) No. of clinical pregnancies Clinical pregnancy rate per transfer (%) Low grade blastocysts High grade blastocysts |
|
| Notes | 48 women in the BT group who underwent BT (control), 48 women in the stimulation group (ST) who had culture medium injected into the uterus before BT, and 48 women in the SEET group who had ECS injected into the uterus before BT. A single frozen‐thawed blastocyst was transferred in the hormonal replacement cycle in the study. Written informed consent was obtained from the patients, and the entire procedure was examined and approved by the Institutional Review Board (IRB) of Hanabusa Women’s Clinic. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised into three groups using colour‐marble lots drawn by a technician blinded to patient information |
| Allocation concealment (selection bias) | Unclear risk | Method not described in detail |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described, but unlikely to be biased as the outcome measures are not likely to be influenced by lack of blinding; the outcomes, which are dichotomous, refer to clinical events and their measurement cannot vary between different investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors report no missing outcome data |
| Selective reporting (reporting bias) | High risk | No follow‐up until live birth in published data, no miscarriages reported in published data. The study was focused on embryological data. No protocol registered |
| Other bias | Unclear risk | Consistent with other projects of the same researchers, but insufficient details to make a judgement |
Kamath 2015.
| Study characteristics | ||
| Methods | Study design: RCT Setting: university level infertility centre in India Period: The duration of the study was 3 years (from September 2011 to June 2014) |
|
| Participants | Eligible 72 women, 12 declined to participate Inclusion criteria: all consecutive women who were due for a fresh blastocyst transfer and had supernumerary embryos available for cryopreservation, patients with previously unsuccessful fresh blastocyst transfers and those who did not undergo fresh transfer due to high risk of OHSS, women who had a successful outcome previously and were interested in having another child. 60 women undergoing vitrified warmed blastocyst transfers, were randomised to SEET (n = 30) or control (n = 30) Baseline characteristics: Study group (n = 30) Age (Years) 29.9 (4.0) Body Mass Index (kg/m2) 24.51 (3.36) Duration of infertility (years) 7.55 (3.36) Total dose of gonadotrophins (IU) 1914.17 (900.35) Estradiol levels on trigger day (pg/ml) 3282.42 (2154.68 Number of MII oocytes 13.05 (5.34) Number of fertilised oocytes 10.59 (4.38) Number of blastocysts transferred 2.1 (0.5) Number of good quality blastocysts transferred (≥ 3AA) 1.8 (0.5) Control group (n = 30) Age (Years) 29.8 (4.3) Body Mass Index (kg/m2) 24.49 (3.26) Duration of infertility (years) 8.58 (3.62) Total dose of gonadotrophins (IU) 1792.50 (764.52) Estradiol levels on trigger day (pg/ml) 2737.62 (1353.83) Number of MII oocytes 13.53 (5.22) Number of fertilised oocytes 10.28 (3.64) Number of blastocysts transferred 1.9 (0.6) Number of good quality blastocysts transferred (≥ 3AA) 1.7 (0.6) Type of infertility Primary 14 Secondary 16 Previous ART attempt Yes 13 No 17 ART protocol Antagonist 13 Long protocol 17 Ultralong protocol 0 Type of infertility Primary 19 Secondary 11 Previous ART attempt Yes 9 No 21 ART protocol Antagonist 10 Long protocol 16 Ultralong protocol 4 |
|
| Interventions | Uterine flushing with supernatant embryo culture medium in vitrified warmed blastocyst transfer cycles compared to direct transfer In women allocated to the SEET group thawed supernatant embryo culture medium was injected into the uterine cavity transcervically two days prior to the planned blastocyst transfer. In the control group, direct vitrified warmed blastocyst transfer was performed. |
|
| Outcomes | Main outcome measure(s): Positive (βhCG‐ biochemical) pregnancy rate per embryo transfer (primary) Clinical pregnancy rate per embryo transfer (singleton‐ twins‐ triplet) Implantation rate per embryo Multiple pregnancy rate per clinical pregnancy Miscarriage rate per clinical pregnancy Live birth rate per embryo transfer |
|
| Notes | Study funding: Research Grant, Christian Medical College, Vellore. Conflict of interest None to declare. Authorship role KG and MSK conceived and designed the study. MM and MSK analysed and interpreted the data. MM, MSK and KG wrote the manuscript. KB, NNV, AJ, MM, MK and MSK contributed to data collection and/or performed procedures. All the authors contributed to write the manuscript. All the authors approved the final version of the manuscript. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women entering the trial were randomly distributed, using a computer generated randomisation sequence (blocks of 6) into two groups The randomisation sequence was generated by a statistician from the institutional biostatistics department |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was achieved by using consecutively numbered opaque sealed envelope. Once the women were planned for vitrified thawed transfer, the envelope was opened |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Authors report the lack of blinding as one of the limitations of the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to reach a conclusion |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no drop outs in the trial, as reported by authors All women who entered the trial were available for analysis with no loss to follow up reported (30 in each group) |
| Selective reporting (reporting bias) | Low risk | Adverse events reported ‐ expected outcomes analysed‐ published protocol (CTRI/2013/01/003280) |
| Other bias | Low risk | The study was registered ‐ methodological rationale ‐ distribution of tasks The study was approved by the institutional review board. The trial was registered with the clinical trial registry of India (CTRI/2013/01/003280) The small sample size was based and adequately powered to detect differences on the clinical pregnancy rate |
Prapas 2012.
| Study characteristics | ||
| Methods | Study design: RCT
Setting: Iakentro IVF centre Period: patients enrolled from June 2009 through November 2010 A total of 400 cycles, of which 200 IVF/ICSI and 200 oocyte donor (OD), were randomly assigned to have their uterine cavity injected (group I) or not (group II) |
|
| Participants | Group I (injection of the uterine cavity with embryo culture supernatant) Group I consisted of two subgroups, IA (ET on day 3) and IB (ET on day 5) Group II (no injection) Group II consisted of IIA (ET on day 3) and IIB (ET on day 5) After the exclusion of OD cycles (based on data from direct communication), the remaining IVF/ICSI 190 cases (200 cases allocated, 10 excluded because of difficult ET) were allocated into the intervention and control groups as follows; flushing group (study group) 93 cases ‐ non‐flushing group (control) 97 cases Inclusion criteria: All women had a history of at least one previous unsuccessful IVF/ET. age ≤ 38 years for the IVF women and ≤ 50 years for the oocyte receivers, without known endometriosis, hydrosalpinx or uterine anomalies, including small submucosal myomas or polyps. Baseline characteristics of the participants: flushing group i (n = 188) BMI 24.3±4.9 Age (mean ±SD) 37.2±5.2 Number of oocytes (mean ±SD) 13.4±5.0 Endometrial thickness (mean ±SD) 10.9±2.1 Mean embryo transferred (mean ±SD) 2.20±0.70 Embryo quality (mean ±SD) 1.84±0.94 Fertilisation rate non‐flushing group ii (n = 196) BMI 24.4±4.7 Age (mean ±SD) 35.8±5.8 Number of oocytes (mean ±SD) 13.3±4.8 Endometrial thickness (mean ±SD) 10.8±2.6 Mean embryo transferred (mean ±SD) 2.24±0.69 Embryo quality (mean ±SD) 1.82±0.93 Fertilisation rate |
|
| Interventions | Injection of embryo culture supernatant into the uterine cavity, 30 min before the embryo transfer on either day 3 or 5, in IVF/ICSI versus simple air insertion (as a placebo intervention used in the control group) | |
| Outcomes | Main outcome measure(s): Clinical pregnancy rate per transfer Implantation rate Miscarriage rate Ectopic pregnancy rate A pregnancy test was performed 15 days after the ET, and, if positive, an ultrasound scan was scheduled after two weeks to determine the number and status of implanted embryos. The concurrency of a positive beta‐ HCG test and a foetal heartbeat (seen by ultrasound) was defined as a clinical pregnancy. |
|
| Notes | The study was approved by the Institutional Review Board.
Four hundred women were allocated into group I or II when they were called to be informed about the day of their embryo transfer. All cases had a mock transfer in a cycle previous to IVF and if difficulty was encountered a cervical dilatation was performed. All cases included in the statistical analysis had at least one good quality embryo. Separate details on LBR were obtained after communication with the authors for the 200 non‐donor cycles. These cycles were eligible |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence generated from a computerised random number table |
| Allocation concealment (selection bias) | Low risk | Computerized allocation performed by a third party (midwife not further involved in the procedures) before initiation of the intervention (day of hcg injection) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Data from personal communication with author:"The doctor who performed the ET was blinded about the necessity of injection or not until the moment of ET. In all cases, there were two insertions, the first was embryo culture or simple air (flushing/non‐flushing group) and the second was ET" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | According to information from personal communication with author, the clinician who performed the outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors report no drop‐ outs / losses to follow‐up In addition, due to difficult transfer 12 cases from group I and 4 cases from group II were not included in the statistical analysis |
| Selective reporting (reporting bias) | Unclear risk | Adverse events and clinical outcomes were provided by authors after communication‐ no registered protocol |
| Other bias | Low risk | There is insufficient rationale or evidence that any problem would introduce bias |
Tehraninejad 2012.
| Study characteristics | ||
| Methods | Study design: RCT
Setting: Valiasr department of Imam Hospital complex Period: Patients enrolled from January 2010 to March 2011 |
|
| Participants | 94 couples eligible, 4 cases withdrew before randomisation, 4 cases randomly divided into only BT or SEHB groups. All cases in each group had high‐grade blastocysts Ιnclusion criteria: Infertility period of less than 10 years, more than four embryos available on day 2 in the oocyte retrieval cycle, and no previous history of ART cycles Exclusion criteria: age over 38 years for women, hydrosalpinx BT or stimulation (SEHB) groups 45 Baseline characteristics: SEHB group (45) Mean age of patients (years) 30.7 ± 5.9 Mean duration of infertility (years) 7.9 ± 5.2 Mean BMI (kg/m2) 26.1 ± 3.6 FSH level (mIU/mL) 6.2 ± 2.4 Mean number of oocytes fertilised 9.4 ± 4.2 Mean number of blastocysts transferred 1.9 ± 0.3 Mean endometrial thickness 9.9 ± 0.9 BT group (45) Mean age of patients (years) 31.7 ± 5.1 Mean duration of infertility (years) 8.1 ± 4.1 Mean BMI (kg/m2) 25.5 ± 2.5 FSH level (mIU/mL) 7.2 ± 2.2 Mean number of oocytes fertilised 9.1 ± 3.5 Mean number of blastocysts transferred 1.9 ± 0.1 Mean endometrial thickness 9.3 ± 1.9 |
|
| Interventions | Stimulation of endometrium with high‐grade blastocyst culture supernatant perfusion before blastocyst transfer | |
| Outcomes | Implantation rates, pregnancy rates, abortion, preterm and term delivery rates were compared between the two groups.
Number of chemical pregnancies (%)
Number of clinical pregnancies
Implantation rate per embryo (%)
Number of term deliveries
Number of preterm deliveries
Number of abortions A detectable gestational sac was considered as a characteristic of clinical pregnancy, and we calculated the implantation rate by dividing the number of gestational sacs by the number of embryos transferred to the uterine cavity. Abortion, preterm or term delivery, and multiple gestations were considered as pregnancy outcomes in both groups. |
|
| Notes | Two physicians categorized blastocysts as good or poor grade blastocysts, according to the criteria of Gardner et al. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple random allocation ‐ computer generated |
| Allocation concealment (selection bias) | Low risk | The computer generated random allocation to group I or group II was performed by a blinded technician, thus a third party was no further involved in the procedure |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors report that specialists who saw patients and did operations in each group were blinded to patient groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Incomplete ‐ there are concerns about altering results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors report that 90 patients (45 in SEHB and 45 in BT only group) were followed up until pregnancy outcome was recorded |
| Selective reporting (reporting bias) | Unclear risk | Both primary and secondary outcomes including adverse events analysed adequately ‐ no registered protocol |
| Other bias | Low risk | Complete information of the contributors, support and conflict of interest was provided All patients were asked to fill in an informed consent form before procedure although the study had been approved by ethics committee of Tehran University of Medical Sciences (ID number: IRCT138902232576N2) |
Zhu 2010.
| Study characteristics | ||
| Methods | Study design: RCT Setting: A reproductive medical centre. Period: The subjects were recruited and studied for a period of 6 months from January to June 2008. | |
| Participants | Patients: 90 women enrolled out of 96 eligible ones, aged 23–39 years, with a period of infertility of 3–8 years, due to the following infertility factors: tubal (n 1⁄4 54), unexplained (n 1⁄4 3), male (n 1⁄4 22), endometriosis (n 1⁄4 9), other (n 1⁄4 2). Inclusion criterion; women undergoing IVF or ICSI treatment for the first time. Exclusion criteria: women who had prior IVF or ICSI treatment and those who underwent a natural cycle IVF and had no appropriate embryo for ET. study group (45) control group (45) Baseline characteristics: Study group (45) Age (y) 31.9 ± 3.9 Duration of infertility (y) 5.3 ± 2.3 Serum hormone LH (IU/L) 5.5 ± 3.6 FSH (IU/L) 5.2 ± 2.4 PRL(mg/L) 10.7 ± 4.6 E (pmol/L) 293 ± 49 T (nmol/L) 1.7 ± 0.4 Infertility factors, {n (%)} Tubal 29 (64.4) Unexplained 1 (2.2) Male 10 (22.2) Endometriosis 4 (8.9) Others 1 (2.2) Infertility, {n (%)} Primary 26 (57.8) Secondary 19 (42.2) Number of dominant follicles 11.2 ±5.4 Number of retrieved oocytes 7.9 ± 3.3 Oocyte retrieval rate 70.5 % Number of maturation oocytes 6.9 ± 2.4 Oocyte maturation rate 87.3 % Number of fertilized oocytes 6.2 ±1.6 IVF 6.2 ± 1.9 ICSI 6.3 ± 1.4 Fertilization rate 78.5 % IVF 76.9 % ICSI 80.7 % Number of cleavage 5.7 ± 1.5 Cleavage rate 72.2 % Endometrium thickness 9.8 ± 1.3 on HCG day (mm) Number of grade 1–2 embryo 2.8 ± 0.9 Rate of grade 1–2 embryo (%) 49.1 Number of transfer embryo 2.2 ± 0.4 Control group (45) Age (y) 32.7 ± 4.4 Duration of infertility (y) 4.9 ± 1.7 Serum hormone LH (IU/L) 5.8 ± 2.9 FSH (IU/L) 4.8 ± 2.1 PRL(mg/L) 11.4 ± 5.6 E (pmol/L) 301 ± 43 T (nmol/L) 2.0 ± 0.7 Infertility factors, {n (%)} Tubal 25 (55.6) Unexplained 2 (4.4) Male 12 (26.7) Endometriosis 5 (11.1) Others 1 (2.2) Infertility, {n (%)} Primary 31 (68.9) Secondary 14 (31.1) Number of dominant follicles 10.8±4.6 Number of retrieved oocytes 7.7 ± 2.9 Oocyte retrieval rate 71.3 % Number of maturation oocytes 6.7 ± 1.8 Oocyte maturation rate 87.0 % Number of fertilized oocytes 5.8 ±1.8 IVF 6.0 ± 1.6 ICSI 5.6 ± 1.9 Fertilization rate 75.3 % IVF 77.3 % ICSI 73.7 % Number of cleavage 5.5 ± 1.4 Cleavage rate 71.5 % Endometrium thickness 10.1 ± 1.5 on HCG day (mm) Number of grade 1–2 embryo 2.6 ± 0.7 Rate of grade 1–2 embryo (%) 47.3 Number of transfer embryo 2.3 ± 0.3 |
|
| Interventions | Uterine cavity injection of day 2 embryo culture supernatant before day 3 embryo transfer | |
| Outcomes | Main outcome measure(s):
Embryo implantation rate Pregnancy rate (Clinical pregnancy / singleton‐ twins) Abortion rate |
|
| Notes | All of the aforementioned clinical and laboratory procedures were identical between the study group and the control group. In order to avoid a selection bias, we excluded the women who had prior IVF or ICSI treatment and those who underwent a natural cycle IVF. We selected only those who were experiencing IVF or ICSI treatment for the first time, for whom there was a steady pregnancy rate of 45%–50% in our reproductive centre. All of the baseline characteristics between the study group and the control group were also not statistically significant differences, and the results of both groups in oocyte maturation, fertilization,embryo cleavage, and grade were identical. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors report that both selection and enrolment for each subject was decided collectively by three physicians First, 90 random numbers generated by means of computer were randomly divided into the study group and the control group All subjects enrolled chronologically were arranged according to the sequence of random numbers. As a result, the 90 subjects were randomly divided into the study group and the control group |
| Allocation concealment (selection bias) | Low risk | The random allocation sequence was concealed in a closed and dark‐coloured envelope until the day of oocyte pick‐up Randomization occurred after patients agreed to participate in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was not blinded, as reported by authors |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The IVF or ICSI procedures were performed by the same physicians and laboratory technicians The treating sequence of the subjects was the same as the enrolment sequence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors report that there were no patients lost to follow‐up and no patients refused to be treated 96 eligible subjects, 4 opted not to participate when counselled about the uncertainty of effectiveness, and 2 declined to randomise Finally, 90 eligible patients were enrolled |
| Selective reporting (reporting bias) | Unclear risk | Adverse events (abortion) reported‐ clinical outcomes reported‐ ‐ no registered protocol |
| Other bias | Low risk | There were no points to doubt the quality of the study The study was approved by the Institutional Review Board of Shen‐Zhen Maternity and Child Healthcare Hospital. All subjects signed an informed consent form |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abraham 2018 | Comparison of a single‐step medium or sequential culture media |
| Akbari 2017 | Non‐RCT, in vitro study |
| Alhilali 2019 | Non‐RCT, cohort study of IL‐5 as a predictor of ICSI outcome |
| Berkkanoglu 2006 | Comparison: Flushing vs. No Flushing with culture media (Cervical canal irrigation in both groups ) |
| Cuman 2013 | Non‐RCT, in vitro study |
| de los Santos 2015 | Non‐RCT, in vitro study |
| Eftekhar 2018 | Use of platelet rich plasma and HRT vs. HRT only |
| Fawzy 2018 | Comparison of temperatures (36.5 C versus 37 C) for human embryo culture |
| Giacomini 2017 | Non‐RCT, in vitro study |
| Goto 2007 | Quasi‐randomized study |
| Goto 2018 | Non‐RCT |
| Hafezi 2018 | Intrauterine human chorionic gonadotropin flushing |
| Hambiliki 2011 | Comparison of 2 different media systems on embryo morphology and development |
| Hamdi 2018 | Flushing the endometrial cavity with follicular fluid |
| Hashish 2014 | Flushing the endometrial cavity with follicular fluid |
| Herbemont 2018 | Post‐warming culture duration comparison |
| Inoue 2014 | Retrospective study, co‐administration of prednisolone |
| IRCT20160815029374N5 | Intrauterine infusion of autologous platelet‐rich plasma on day 11 of freeze embryo transfer cycle |
| Kapiteijn 2006 | Non‐RCT, in vitro study |
| Kapiteijn 2008 | Non‐RCT |
| Khan 1991 | Use of human albumin as a replacement for serum |
| Kulmann 2018 | Culture of cumulus‐oocyte complexes (COCs) after oocyte pick‐up (OPU) in media supplemented with amino acids |
| Labied 2019 | Paraffin and mineral oil covering on early human embryo culture |
| Le Saint 2019 | Autologous endometrial cell co‐culture vs. conventional embryo culture |
| Letterie 2003 | Non‐RCT |
| Lopez 2019 | Endometrial use of autologous platelet‐derived microparticles |
| Madkour 2018 | Addition of autologous follicular fluid / IVM results |
| Mayer 2015 | Intracervical and intravaginal application of seminal plasma on the endometrium |
| Melnick 2015 | Retrospective cohort study |
| Nazari 2019 | Autologous platelet ‐rich plasma therapy |
| NCT04077970 | Intrauterine Flushing With Follicular Fluid Plus Granulosa Cells |
| Obidniak 2017 | Autologous platelet ‐rich plasma therapy |
| Ozcan 2019 | Use of microfluidic sperm sorting for the sperm selection |
| Reilly 2019 | Use of Lipiodol prior to fresh embryo transfer |
| Sigalos 2018 | Use of two different volumes (20‐25 vs 40‐45 mul) of media used for embryo transfer |
| Tabiasco 2009 | Non‐RCT |
| Valojerdi 2006 | use of Embryo Glue |
| von Wolff 2009 | Intracervical and intravaginal application of seminal plasma on the endometrium |
| Zadehmodarres 2017 | Endometrial administration of autologous platelet‐rich plasma, Non‐RCT |
Differences between protocol and review
In the protocol, we stated that we would conduct a sensitivity analyses for the primary outcomes in order to confirm robust conclusions. One of the considerations likely to alter the effect estimate was the inclusion of studies only without serious risk of bias (studies at low risk of bias with respect to randomisation methods and not at high risk of bias in any domain) in the statistical analysis.
In accordance with latest updates in authors' guidance on the rationale for conducting a sensitivity analysis, we reexamined our considerations and excluded studies at high or unclear risk of selection bias (random sequence generation and allocation concealment), studies at high or unclear risk of selective reporting bias and studies at high risk of bias in any other domain.
Furthermore, in accordance with recent updates in CGF guidance, we performed an additional sensitivity analysis using relative risk as the measure of effect.
Moreover, the sensitivity analysis was applied not only on the primary outcomes but also on the clinically important secondary outcomes (clinical, ectopic and multiple pregnancy).
Finally, the subgroup analysis was expanded on clinically important secondary outcomes, where relevant data were available.
Contributions of authors
CS designed and drafted the protocol and the full review, participated in the revisions and is the guarantor of the review. ES and DV contributed to the design and drafting of the protocol, selected the studies and DV participated in two revisions of the review. VK participated in the last major revision and the final selection of studies. All authors critically reviewed the manuscript for content, and approved the final version for publication.
Sources of support
Internal sources
None, Other
External sources
None, Other
Declarations of interest
ES, VK, DV and CS have no conflicts of interests to disclose.
New
References
References to studies included in this review
Goto 2009 {published data only}
- Goto S, Kadowaki T, Hashimoto H, Kokeguchi S, Shiotani M. Stimulation of endometrium embryo transfer can improve implantation and pregnancy rates for patients undergoing assisted reproductive technology for the first time with a high-grade blastocyst. Fertility and Sterility 2009;92(4):1264-1268. [DOI] [PubMed] [Google Scholar]
Kamath 2015 {published data only}
- Kamath MS, Mascarenhas M, B K, Vasani NN, Joshi A, K M, George K. Uterine flushing with supernatant embryo culture medium in vitrified warmed blastocyst transfer cycles: a randomized controlled trial. Journal of Assisted Reproduction and Genetics 2015;32(2):225-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prapas 2012 {published data only}
- Prapas Y, Petousis S, Panagiotidis Y, Gullo G, Kasapi L, Papadeothodorou A, Prapas N. Injection of embryo culture supernatant to the endometrial cavity does not affect outcomes in IVF/ICSI or oocyte donation cycles: a randomized clinical trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2012;162(2):169-173. [DOI] [PubMed] [Google Scholar]
Tehraninejad 2012 {published data only}
- Tehraninejad ES, Tanha FD, Ghajarzadeh M, Zandieh Z, Aziminekoo E, Zanjani HR. Stimulation of the endometrium with high-grade blastocyst culture supernatant (SEHB) can improve pregnancy outcome for couples undergoing intracytoplasmic sperm injection (ICSI): a randomized clinical trial. Archives of Gynecology and Obstetrics 2012;285(4):1167-1171. [DOI] [PubMed] [Google Scholar]
Zhu 2010 {published data only}
- Zhu W, Li X, Fu Z, Tang Z, Chen X, Zhou Y, Geng Q. Injection of day 2 embryo culture supernatant into the uterine cavity did not improve the pregnancy rate of day 3 embryo transfer in patients who underwent in vitro fertilization-embryo transfer: a randomized clinical trial. Fertility and Sterility 2010;93(7):2216-2221. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abraham 2018 {published data only}
- Abraham M, De Vos A, Mateizel I, Tournaye H, Verheyen G. In: Human Reproduction. Vol. 33. 2018:i214-215.
Akbari 2017 {published data only}
- Akbari H, Eftekhar Vaghefi SH, Shahedi A, Habibzadeh V, Mirshekari TR, Ganjizadegan A, Mollaei H, Ahmadi M, Nematollahi-Mahani SN. Mesenchymal Stem Cell-Conditioned Medium Modulates Apoptotic and Stress-Related Gene Expression, Ameliorates Maturation and Allows for the Development of Immature Human Oocytes after Artificial Activation. Genes 2017;8:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alhilali 2019 {published data only}
- Jabbar M, Alhilaliab S, Parhama A, Attaranzadehcd A, Amiriancd M, Azizzadehe M. IL-5 in follicular fluid as a negative predictor of the intracytoplasmic sperm injection outcome. Cytokine 2019;113:265-271. [DOI] [PubMed] [Google Scholar]
Berkkanoglu 2006 {published data only}
- Berkkanoglu M, Isikoglu M, Seleker M, Ozgur K. Flushing the endometrium prior to the embryo transfer does not affect the pregnancy rate. Reproductive Biomedicine Online 2006;13(2):268-271. [DOI] [PubMed] [Google Scholar]
Cuman 2013 {published data only}
- Cuman C, Menkhorst EM, Rombauts LJ, Holden S, Webster D, Bilandzic M, Osianlis T, Dimitriadis E. Preimplantation human blastocysts release factors that differentially alter human endometrial epithelial cell adhesion and gene expression relative to IVF success. Human Reproduction 2013;28(5):1161-1171. [DOI] [PubMed] [Google Scholar]
de los Santos 2015 {published data only}
- los Santos MJ, Gámiz P, los Santos JM, Romero JL, Prados N, Alonso C, et al. he Metabolomic Profile of Spent Culture Media from Day-3 Human Embryos Cultured under Low Oxygen Tension. PLoS ONE 2015;10(11):e0142724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eftekhar 2018 {published data only}
- Eftekhar M, Neghab N, Naghshineh E, Khani P. Can autologous platelet rich plasma expand endometrial thickness and improve pregnancy rate during frozen-thawed embryo transfer cycle? A randomized clinical trial. Taiwanese Journal of Obstetrics and Gynecology 2018;57(6):810-813. [DOI] [PubMed] [Google Scholar]
Fawzy 2018 {published data only}
- Fawzy M, Emad M, Gad MA, Sabry M, Kasem H, Mahmoud M, Bedaiwy MA. Comparing 36.5[degrees]C with 37[degrees]C for human embryo culture: a prospective randomized controlled trial. Reproductive Biomedicine Online 2018;36(6):620-626. [DOI] [PubMed] [Google Scholar]
Giacomini 2017 {published data only}
- Giacomini E, Vago R, Sanchez AM, Podini P, Zarovni N, Murdica V, Rizzo R, Bortolotti D, Candiani M, Viganò P. Secretome of in vitro cultured human embryos contains extracellular vesicles that are uptaken by the maternal side. Scientific Reports 2017;7(1):5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goto 2007 {published data only}
- Goto S, Kadowaki T, Hashimoto H, Kokeguchi S, Shiotani M. Stimulation of endometrium embryo transfer (SEET): injection of embryo culture supernatant into the uterine cavity before blastocyst transfer can improve implantation and pregnancy rates. Fertility and Sterility 2007;88(5):1339-1343. [DOI] [PubMed] [Google Scholar]
Goto 2018 {published data only}
- Goto S. Embryonic Modulation of Endometrial Receptivity. Journal of Mammalian Ova Research 2018;35(2):53-59. [Google Scholar]
Hafezi 2018 {published data only}
- Hafezi M, Madani T, Arabipoor A, Zolfaghari Z, Sadeghi M, Ramezanali F. The effect of intrauterine human chorionic gonadotropin flushing on live birth rate after vitrified-warmed embryo transfer in programmed cycles: a randomized clinical trial. Archives of Gynecology and Obstetrics 2018;297(6):1571-1576. [DOI] [PubMed] [Google Scholar]
Hambiliki 2011 {published data only}
- Hambiliki F, Sandell P, Yaldir F, Stavreus-Evers A. A prospective randomized sibling-oocyte study of two media systems for culturing cleavage-stage embryos-impact on fertilization rate. Journal of Assisted Reproduction and Genetics 2011;28(4):335-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamdi 2018 {published data only}
- Hamdi K, Nouri M, Farzaneh S, Mirza-Aghdazadeh-Attari M, Naghavi-Behzad M, Mohammadi S. Effect of flushing the endometrial cavity with follicular fluid on implantation rates in sub-fertile women undergoing invitro fertilization: a randomized clinical trial. Journal of family and reproductive health. 12(4):184-190 2018;12(4):184-190. [PMC free article] [PubMed] [Google Scholar]
Hashish 2014 {published data only}
- Hashish NM, Badway HS, Abdelmoty HI, Mowafya A, Youssef MAFM. Does flushing the endometrial cavity with follicular fluid after oocyte retrieval affect pregnancy rates in subfertile women undergoing intracytoplasmic sperm injection? A randomized controlled trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2014;176:153-157. [DOI] [PubMed] [Google Scholar]
Herbemont 2018 {published data only}
- Herbemont C, Chekroune S, Bonan S, Cedrin-Durnerin I, Vivot A, Sonigo C, Boujenah J, Grynberg M, Sifer C. Impact of post-warming culture duration on clinical outcomes of vitrified good-quality blastocyst transfers: a prospective randomized study. Fertility and Sterility 2018;110(7):1290-1297. [DOI] [PubMed] [Google Scholar]
Inoue 2014 {published data only}
- Inoue T, Ono Y, Yonezawa Y, Kishi J, Emi N. Improvement of Live Birth Rate Follow- ing Frozen-Thawed Blastocyst Transfer by Combination of Prednisolone Administration and Stimulation of Endometrium Embryo Transfer. Open Journal of Obstetrics and Gynecology 2014;4:745-750. [Google Scholar]
IRCT20160815029374N5 {published data only}
- PRP in infertility [The effect of intrauterine infusion of platelet-rich plasma and number of infusion on increasing the thickness of endometrium and pregnancy rate in patients with thin endometrium]. https://en.irct.ir/trial/29901 (first received 11 May 2019).
Kapiteijn 2006 {published data only}
- Kapiteijn K, Koolwijk P, Weiden RMF, Nieuw Amerongen G, Plaisier M, Hinsbergh VWM, Helmerhorst FM. Human embryo–conditioned medium stimulates in vitro endometrial angiogenesis. Fertility and Sterility 2006;85(Suppl 1):1232-1239. [DOI] [PubMed] [Google Scholar]
Kapiteijn 2008 {published data only}
- Kapiteijn K, van der Weiden RM, Helmerhorst FM. Injection of embryo culture supernatant prior to blastocyst transfer—cofactor? Fertility and Sterility 2008;89(4):1027–1028. [DOI] [PubMed] [Google Scholar]
Khan 1991 {published data only}
- Khan I, Staessen C, Devroey P, Van Steirteghem AC. Human serum albumin versus serum: a comparative study on embryo transfer medium. Fertility and Sterility 1991;56(1):98-101. [PubMed] [Google Scholar]
Kulmann 2018 {published data only}
- Kulmann MIR, Martello C, Bos-Mikich A, Pagnoncelli N, Frantz G, Dutra C, Frantz N. Amino acids supplemen- tation in culture of cumulus-oo- cyte complexes: does it matter? In: JBRA Assisted Reproduction. Vol. 22. 22st Annual Congress of the SBRA, Brasilia/DF, 01-04 August 2018, 2018:267.
Labied 2019 {published data only}
- Labied S, Jouan C, Wenders F, Ravet S, Gaspard O, Thonon F, Gridelet V, Henry L, Perrier d'Hauterive S, Nisolle M. Comparison between paraffin and mineral oil covering on early human embryo culture: a prospective randomized study. Systems Biology in Reproductive Medicine 2019;65(1):81-86. [DOI] [PubMed] [Google Scholar]
Le Saint 2019 {published data only}
- Le Saint C, Crespo K, Bourdiec A, Bissonnette F, Buzaglo K, Couturier B, Bisotto S, Phillips SJ, Stutz M, Gouze J-N, Sampalis JS, Hamamah S, Kadoch IJ. Autologous endometrial cell co-culture improves human embryo development to high-quality blastocysts: a randomized controlled trial. Reproductive Biomedicine Online 2019;38(3):321-329. [DOI] [PubMed] [Google Scholar]
Letterie 2003 {published data only}
- Letterie G, Marshall L, Angle M. Intrauterine reflux of media during cervical irrigation at embryo transfer. Fertility and Sterility 2003;79(6):1444-1445. [DOI] [PubMed] [Google Scholar]
Lopez 2019 {published data only}
- Lopez EG, Bernardo LG, Fernandez FG. Improvement of endometrial receptivity through the use of autologous platelet-derived microparticles. Fertility and Sterility 2019;112(3):e331-e332. [Google Scholar]
Madkour 2018 {published data only}
- Madkour A, Bouamoud N, Kaarouch I, Louanjli N, Saadani B, Assou S, Aboulmaouahib S, Sefrioui O, Amzazi S, Copin H, Benkhalifa M. Follicular fluid and supernatant from cultured cumulus-granulosa cells improve in vitro maturation in patients with polycystic ovarian syndrome. Fertility and Sterility 2018;110(4):710-719. [DOI] [PubMed] [Google Scholar]
Mayer 2015 {published data only}
- Mayer RB, Ebner T, Yaman C, Hartl J, Sir A, Krain V, Oppelt P, Shebl O. Influence of intracervical and intravaginal seminal plasma on the endometrium in assisted reproduction: a double-blind, placebo-controlled, randomized study. Ultrasound in Obstetrics and Gynecology 2015;45(2):132-138. [DOI] [PubMed] [Google Scholar]
Melnick 2015 {published data only}
- Melnick AP, Murphy EM, Masbou AK, Sapra KJ, Rosenwaks Z, Spandorfer SD. Autologous endometrial coculture biopsy: is timing everything? Fertility and Sterility 2015;104(1):104-109.e1. [DOI] [PubMed] [Google Scholar]
Nazari 2019 {published data only}
- Nazari L, Salehpour S, Hosseini MS, Hashemi Moghanjoughi P. The effects of autologous platelet-rich plasma in repeated implantation failure: a randomized controlled trial. Human Fertility 2019:1-5. [DOI] [PubMed] [Google Scholar]
NCT04077970 {published data only}
- Intrauterine flushing with follicular fluid plus granulosa cells [Evaluation of implantation and clinical pregnancy after intrauterine flushing of infertile patients with follicular fluid plus granulosa cells - randomized controlled trial]. https://clinicaltrials.gov/ct2/show/NCT04077970 (first received 4 September 2019).
Obidniak 2017 {published data only}
- Obidniaka D, Gzgzyanb A, Feoktistovc A, Niaurid D. In: Randomized controlled trial evaluating efficacy of autologous platelet -rich plasma therapy for patients with recurrent implantation failure. Vol. 108. 2017:e370.
Ozcan 2019 {published data only}
- Ozcan P, Takmaz T, Kocer Yazici MG, Alagoz OA, Yesiladali M, Ficicioglu CN, Sevket O. Does the use of microfluidic sperm sorting for the sperm selection improve IVF success rates in male factor infertility? Fertility and Sterility 2019;112(3):e282-e283. [DOI] [PubMed] [Google Scholar]
Reilly 2019 {published data only}
- Reilly SJ, Glanville EJ, Dhorepatil B, Prentice LR, Mol BW, Johnson NP. The IVF-LUBE trial - a randomized trial to assess LipiodolÆ uterine bathing effect in women with endometriosis or repeat implantation failure undergoing IVF. Reproductive Biomedicine Online 2019;38(3):380-386. [DOI] [PubMed] [Google Scholar]
Sigalos 2018 {published data only}
- Sigalos GA, Michalopoulos Y, Kastoras AG, Triantafyllidou O, Vlahos NF. Low versus high volume of culture medium during embryo transfer: a randomized clinical trial. Journal of Assisted Reproduction and Genetics 2018;35(4):693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tabiasco 2009 {published data only}
- Tabiasco J, Perrier d'Hauterive S, Thonon F, Parinaud J, Léandri R, Foidart JM, Chaouat G, Munaut C, Lombroso R, Selva J, Bergère M, Hammoud I, Kozma N, Aguerre-Girr M, Swales AK, Sargent IL, Le Bouteiller P, Lédée N. Soluble HLA-G in IVF/ICSI embryo culture supernatants does not always predict implantationsuccess: a multicentre study. Reproductive Biomedicine Online 2009;18(3):374-381. [DOI] [PubMed] [Google Scholar]
Valojerdi 2006 {published data only}
- Valojerdi MR, Karimian L, Yazdi PE, Gilani MA, Madani T, Baghestani AR. Efficacy of a human embryo transfer medium: a prospective, randomized clinical trial study. Journal of Assisted Reproduction and Genetics 2006;23(5):207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
von Wolff 2009 {published data only}
- Wolff M, Rösner S, Thöne C, Pinheiro RM, Jauckus J, Bruckner T, Biolchi V, Alia A, Strowitzki T. Intravaginal and intracervical application of seminal plasma in in vitro fertilization or intracytoplasmic sperm injection treatment cycles--a double-blind, placebo-controlled, randomized pilot study. Fertility and Sterility 2009;91(1):167-172. [DOI] [PubMed] [Google Scholar]
Zadehmodarres 2017 {published data only}
- Zadehmodarres S, Salehpour S, Saharkhiz N, Nazari L. Treatment of thin endometrium with autologous platelet-rich plasma: a pilot study. JBRA Assisted Reproduction 2017;21(1):54-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Achache 2006
- Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Human Reproduction Update 2006;12:731–746. [DOI: 10.1093/humupd/dml004] [DOI] [PubMed] [Google Scholar]
Ben Rhouma 2003
- Ben Rhouma K, Marrakchi H, Khouja H, Attalah K, Ben Miled E, Sakly M. Outcome of intracytoplasmic injection of fresh and frozen-thawed testicular spermatozoa. A comparative study. Journal of Reproductive Medicine 2003;48(5):349-54. [PMID: ] [PubMed] [Google Scholar]
Bontekoe 2014
- Bontekoe S, Johnson N, Blake D. Adherence compounds in embryo transfer media for assisted reproductive technologies. Cochrane Database of Systematic Reviews 2014;2:Art. No.: CD007421. [DOI: 10.1002/14651858.CD007421.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caballero‐Campo 2002
- Caballero-Campo P, Domínguez F, Coloma J, Meseguer M, Remohí J, Pellicer A, Simón C. Hormonal and embryonic regulation of chemokines IL-8, MCP-1 and RANTES in the human endometrium during the window of implantation. Molecular Human Reproduction 2002;8(4):375-384. [PMID: ] [DOI] [PubMed] [Google Scholar]
Carney 2012
- Carney SK, Das S, Blake D, Farquhar C, Seif MM, Nelson L. Assisted hatching on assisted conception (in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI). Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD001894. [DOI: 10.1002/14651858.CD001894.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2016
- Mathews TJ, Hamilton BE. Mean Age of Mothers is on the Rise: United States, 2000–2014. NCHS Data Brief 2016;232. [https://www.cdc.gov/nchs/data/databriefs/db232.htm] [PubMed]
Desai 2006
- Desai N, Filipovits J, Goldfarb J. Secretion of soluble HLA-G by day 3 human embryos associated with higher pregnancy and implantation rates: assay of culture media using a new ELISA kit. Reproductive Biomedicine Online 2006;13(2):272-277. [DOI: 10.1016/S1472-6483(10)60626-8] [DOI] [PubMed] [Google Scholar]
Dinarello 1994
- Dinarello CA. The interleukin-1 family: 10 years of discovery. FASEB Journal 1994;8(15):1314-1325. [PMID: ] [PubMed] [Google Scholar]
EIM 2017
- Calhaz-Jorge C, De Geyter C, Kupka MS, Mouzon J, Erb K, Mocanu E, Motrenko T, Scaravelli G, Wyns C, Goossens V. Assisted reproductive technology in Europe, 2013: results generated from European registers by ESHRE. Human Reproduction 2017;32(10):1957–1973. [DOI: ] [DOI] [PubMed] [Google Scholar]
ESHRE 2018
- European Society of Human Reproduction and Embryology. More than 8 million babies born from IVF since the world's first in 1978: European IVF pregnancy rates now steady at around 36 percent, according to ESHRE monitoring. ScienceDaily 3 July 2018.
Eurostat 2015
- Eurostat. Women in the EU gave birth to their first child at almost 29 years of age on average. ec.europa.eu/eurostat/documents/2995521/6829228/3-13052015-CP-EN.pdf/7e9007fb-3ca9-445f-96eb-fd75d6792965 (accessed 15 May 2015).
Eurostat 2019
- Fertility statistics. https://ec.europa.eu/eurostat/statistics-explained/index.php/Fertility_statistics March 2019.
Farquhar 2015
- Farquhar C, Rishworth JR, Brown J, Nelen WLDM, Marjoribanks J. Assisted reproductive technology: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2015;7:Art. No.: CD010537. [DOI: 10.1002/14651858.CD010537.pub4] [DOI] [PubMed] [Google Scholar]
Giudice 1995
- Giudice LC. Endometrial growth factors and proteins. Seminars in Reproductive Endocrinology 1995;13(2):93-101. [DOI: 10.1055/s-2007-1016348] [DOI] [Google Scholar]
Gnoth 2005
- Gnoth C, Godehardt E, Frank-Herrmann P, Friol K, Tigges J, Freundl G. Definition and prevalence of subfertility and infertility. Human Reproduction 2005;20(5):1144-1147. [DOI: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed dd Month yyyy. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org. [http://www.gradepro.org ]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kamath 2017
- Kamath MS, Mascarenhas M, Kirubakaran R, Nair R, Kulkarni A. Use of embryo culture supernatant to improve clinical outcomes in assisted reproductive technology: a systematic review and meta-analysis. Human Fertility (Cambridge) 2017;21(2):90-97. [DOI: ] [DOI] [PubMed] [Google Scholar]
Kamath 2020
- Kamath MS, Kirubakaran R, Sunkara SK. Granulocyte-colony stimulating factor administration for subfertile women undergoing assisted reproduction. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD013226. [DOI: 10.1002/14651858.CD013226.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krüssel 2000
- Krüssel J, Behr B, Hirchenhain J, Wen Y, Milki AA, Cupisti S, Bielfeld P, Polan ML. Expression of vascular endothelial growth factor mRNA in human preimplantation embryos derived from tripronuclear zygotes. Fertility and Sterility 2000;74(6):1220-1226. [DOI: 10.1016/S0015-0282(00)01581-8] [DOI] [PubMed] [Google Scholar]
Kuczyński 2001
- Kuczyński W, Dhont M, Grygoruk C, Grochowski D, Wolczyński S, Szamatowicz M. The outcome of intracytoplasmic injection of fresh and cryopreserved ejaculated spermatozoa - a prospective randomized study. Human Reproduction 2001;16(10):2109-2113. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lensen 2018
- Lensen SF, Wilkinson J, Leijdekkers JA, La Marca A, Mol BWJ, Marjoribanks J, Torrance H, Broekmans FJ. Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI). Cochrane Database of Systematic Reviews 2018;2:No.: CD012693. [DOI: 10.1002/14651858.CD012693.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopata 1996
- Lopata A. Blastocyst-endometrial interaction: an appraisal of some old and new ideas. Molecular Human Reproduction 1996;2(7):519-525. [PMID: ] [DOI] [PubMed] [Google Scholar]
Macklon 2017
- Macklon N. Recurrent implantation failure is a pathology with a specific transcriptomic signature. Fertility and Sterility 2017;108(1):9-14. [DOI: 10.1016/j.fertnstert.2017.05.028] [DOI] [PubMed] [Google Scholar]
Maheshwari 2012
- Maheshwari A, Pandey S, Shetty A, Hamilton M, Bhattacharya S. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertility and Sterility 2012;98(2):368-77.e1-9. [DOI: 10.1016/j.fertnstert.2012.05.019] [DOI] [PubMed] [Google Scholar]
Meseguer 2001
- Meseguer M, Aplin JD, Caballero-Campo P, O'Connor JE, Martín JC, Remohí J, et al. Human endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocyst. Biology of Reproduction 2001;64(2):590-601. [DOI: ] [DOI] [PubMed] [Google Scholar]
Motteram 2015
- Motteram C, Vollenhoven B, Hope N, Osianlis T, Rombauts LJ. Live birth rates after combined adjuvant therapy in IVF-ICSI cycles: a matched case-control study. Reproductive Biomedicine Online 2015;30(4):340-348. [DOI: 10.1016/j.rbmo.2014.12.004] [DOI] [PubMed] [Google Scholar]
Nagels 2015
- Nagels HE, Rishworth JR, Siristatidis CS, Kroon B. Androgens (dehydroepiandrosterone or testosterone) for women undergoing assisted reproduction. Cochrane Database of Systematic Reviews 2015, Issue 11. Art. No: CD009749. [DOI: 10.1002/14651858.CD009749.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nastri 2015
- Nastri CO, Lensen SF, Gibreel A, Raine‐Fenning N, Ferriani RA, Bhattacharya S, Martins WP. Endometrial injury in women undergoing assisted reproductive techniques. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD009517. [DOI: 10.1002/14651858.CD009517.pub3] [DOI] [PubMed] [Google Scholar]
Rendall 2010
- Rendall M, Aracil E, Bagavos C, Couet C, Derose A, Digiulio P, Lappegard T, Robert-Bobée I, Rønsen M, Smallwood S, Verropoulou G. Increasingly heterogeneous ages at first birth by education in Southern-European and Anglo-American family-policy regimes: A seven-country comparison. Population Studies (Camb) 2010;64(3):209-227. [DOI: 10.1080/00324728.2010.512392] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Showell 2017
- Showell MG, Mackenzie‐Proctor R, Jordan V, Hart RJ. Antioxidants for female subfertility. Cochrane Database of Systematic Reviews 2017;7:Art. No.: CD007807. [DOI: 10.1002/14651858.CD007807.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siristatidis 2016
- Siristatidis CS, Basios G, Pergialiotis V, Vogiatzi P. Aspirin for in vitro fertilisation. Cochrane Database of Systematic Reviews 2016;11:Art. No.: CD004832. [DOI: 10.1002/14651858.CD004832.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siristatidis 2018
- Siristatidis CS, Sertedaki E, Vaidakis D, Varounis C, Trivella M. Metabolomics for improving pregnancy outcomes in women undergoing assisted reproductive technologies. Cochrane Database of Systematic Reviews 2018;3:Art. No.: CD011872. [DOI: 10.1002/14651858.CD011872.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spandorfer 2000
- Spandorfer SD, Neuer A, Liu HC, Bivis L, Clarke R, Veeck L, et al. Interleukin-1 levels in the supernatant of conditioned media of embryos grown in autologous endometrial coculture: correlation with outcome after in vitro fertilization. American Journal of Reproductive Immunology 2000;43(1):6-11. [10.1111/j.8755-8920.2000.430102.x ] [DOI] [PubMed] [Google Scholar]
Tazuke 1996
- Tazuke SI, Giudice LC. Growth factors and cytokines in endometrium,embryonic development, and maternal: embryonic interactions. Seminars in Reproductive Endocrinology 1996;14(3):231-245. [DOI: 10.1055/s-2007-1016333] [DOI] [PubMed] [Google Scholar]
Teh 2016
- Teh WT, McBain J, Rogers P. What is the contribution of embryo-endometrial asynchrony to implantation failure? Journal of Assisted Reproduction and Genetics 2016;33(11):1419–1430. [DOI: 10.1007/s10815-016-0773-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Valdes 2017
- Valdes CT, Schutt A, Simon C. Implantation failure of endometrial origin: it is not pathology, but our failure to synchronize the developing embryo with a receptive endometrium. Fertility and Sterility 2017;108(1):15-18. [DOI: 10.1016/j.fertnstert.2017.05.033] [DOI] [PubMed] [Google Scholar]
Van Steirteghem 1993
- Van Steirteghem AC, Liu J, Joris H, Nagy Z, Janssenswillen C, Tournaye H, et al. Higher success rate by intracytoplasmic sperm injection than by subzonal insemination. Report of a second series of 300 consecutive treatment cycles. Human Reproduction 1993;8(7):1055-1060. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zandstra 2018
- Zandstra H, Brentjens LBPM, Spauwen B, Touwslager RNH, Bons JAP, Mulder AL, Smits LJM, Hoeven MAHBM, Golde RJT, Evers JLH1, Dumoulin JCM, Van Montfoort APA. Association of culture medium with growth, weight and cardiovascular development of IVF children at the age of 9 years. Human Reproduction 2018;33(9):1645-1656. [DOI: 10.1093/humrep/dey246] [DOI] [PubMed] [Google Scholar]
Zegers‐Hochschild 2017
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, Mouzon J, Sokol R, Rienzi, Sunde LA, Schmidt L, Cooke ID, Simpson JL, Poel S. The International Glossary on Infertility and Fertility Care, 2017. Human Reproduction 2017;32(9):1786-1801. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]


