Abstract
Background
Various rehabilitation treatments may be offered following surgery for flexor tendon injuries of the hand. Rehabilitation often includes a combination of an exercise regimen and an orthosis, plus other rehabilitation treatments, usually delivered together. The effectiveness of these interventions remains unclear.
Objectives
To assess the effects (benefits and harms) of different rehabilitation interventions after surgery for flexor tendon injuries of the hand.
Search methods
We searched the Cochrane Central Register of Controlled Trials, the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register, MEDLINE, Embase, two additional databases and two international trials registries, unrestricted by language. The last date of searches was 11 August 2020. We checked the reference lists of included studies and relevant systematic reviews.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs that compared any postoperative rehabilitation intervention with no intervention, control, placebo, or another postoperative rehabilitation intervention in individuals who have had surgery for flexor tendon injuries of the hand. Trials comparing different mobilisation regimens either with another mobilisation regimen or with a control were the main comparisons of interest. Our main outcomes of interest were patient‐reported function, active range of motion of the fingers, and number of participants experiencing an adverse event.
Data collection and analysis
Two review authors independently selected trials for inclusion, extracted data, assessed risk of bias and assessed the quality of the body of evidence for primary outcomes using the GRADE approach, according to standard Cochrane methodology.
Main results
We included 16 RCTs and one quasi‐RCT, with a total of 1108 participants, mainly adults. Overall, the participants were aged between 7 and 72 years, and 74% were male. Studies mainly focused on flexor tendon injuries in zone II.
The 17 studies were heterogeneous with respect to the types of rehabilitation treatments provided, intensity, duration of treatment and the treatment setting. Each trial tested one of 14 comparisons, eight of which were of different exercise regimens. The other trials examined the timing of return to unrestricted functional activities after surgery (one study); the use of external devices applied to the participant to facilitate mobilisation, such as an exoskeleton (one study) or continuous passive motion device (one study); modalities such as laser therapy (two studies) or ultrasound therapy (one study); and a motor imagery treatment (one study). No trials tested different types of orthoses; different orthosis wearing regimens, including duration; different timings for commencing mobilisation; different types of scar management; or different timings for commencing strengthening.
Trials were generally at high risk of bias for one or more domains, including lack of blinding, incomplete outcome data and selective outcome reporting. Data pooling was limited to tendon rupture data in a three trial comparison. We rated the evidence available for all reported outcomes of all comparisons as very low‐certainty evidence, which means that we have very little confidence in the estimates of effect.
We present the findings from three exercise regimen comparisons, as these are commonly used in clinical current practice.
Early active flexion plus controlled passive exercise regimen versus early controlled passive exercise regimen (modified Kleinert protocol) was compared in one trial of 53 participants with mainly zone II flexor tendon repairs. There is very low‐certainty evidence of no clinically important difference between the two groups in patient‐rated function or active finger range of motion at 6 or 12 months follow‐up. There is very low‐certainty evidence of little between‐group difference in adverse events: there were 15 overall. All three tendon ruptures underwent secondary surgery.
An active exercise regimen versus an immobilisation regimen for three weeks was compared in one trial reporting data for 84 participants with zone II flexor tendon repairs. The trial did not report on self‐rated function, on range of movement during three to six months or numbers of participants experiencing adverse events. The very low‐certainty evidence for poor (under one‐quarter that of normal) range of finger movement at one to three years follow‐up means we are uncertain of the finding of zero cases in the active group versus seven cases in the immobilisation regimen. The same uncertainty applies to the finding of little difference between the two groups in adverse events (5 tendon ruptures in the active group versus 10 probable scar adhesion in the immobilisation group) indicated for surgery.
Place and hold exercise regimen performed within an orthosis versus a controlled passive regimen using rubber band traction was compared in three heterogeneous trials, which reported data for a maximum of 194 participants, with mainly zone II flexor tendon repairs. The trials did not report on range of movement during three to six months, or numbers of participants experiencing adverse events. There was very low‐certainty evidence of no difference in self‐rated function using the Disability of the Arm, Shoulder and Hand (DASH) functional assessment between the two groups at six months (one trial) or at 12 months (one trial). There is very low‐certainty evidence from one trial of greater active finger range of motion at 12 months after place and hold. Secondary surgery data were not available; however, all seven recorded tendon ruptures would have required surgery.
All the evidence for the other five exercise comparisons as well as those of the other six comparisons made by the included studies was incomplete and, where available, of very low‐certainty.
Authors' conclusions
There is a lack of evidence from RCTs on most of the rehabilitation interventions used following surgery for flexor tendon injuries of the hand. The limited and very low‐certainty evidence for all 14 comparisons examined in the 17 included studies means that we have very little confidence in the estimates of effect for all outcomes for which data were available for these comparisons.
The dearth of evidence identified in this review points to the urgent need for sufficiently powered RCTs that examine key questions relating to the rehabilitation of these injuries. A consensus approach identifying these and establishing minimum study conduct and reporting criteria will be valuable. Our suggestions for future research are detailed in the review.
Keywords: Adolescent; Adult; Aged; Child; Female; Humans; Male; Middle Aged; Young Adult; Bias; Exercise Therapy; Exercise Therapy/adverse effects; Exercise Therapy/methods; Exoskeleton Device; Hand Injuries; Hand Injuries/rehabilitation; Hand Injuries/surgery; Immobilization; Laser Therapy; Muscle Contraction; Muscle Contraction/physiology; Postoperative Care; Postoperative Care/methods; Randomized Controlled Trials as Topic; Randomized Controlled Trials as Topic/statistics & numerical data; Range of Motion, Articular; Rupture; Rupture/rehabilitation; Rupture/surgery; Tendon Injuries; Tendon Injuries/rehabilitation; Tendon Injuries/surgery; Ultrasonic Therapy
Plain language summary
What are the best ways for recovering movement in the hand after surgery to repair flexor tendons (tendons in the hand that enable fingers to bend)?
Why is this question important?
Flexor tendons are strong smooth cords that connect the muscles in the forearm (between the hand and elbow) to the bones in the fingers. These tendons allow us to bend our fingers. (Other tendons, known as extensor tendons, allow us to straighten them.)
If flexor tendons become damaged – for example, because of a deep cut from broken glass – surgery is usually needed. The aim of surgery is to repair the tendons so that movement can be restored in the affected fingers.
After surgery, the tendons need a lengthy period of rehabilitation to recover from the injury, the surgery and to restore movement. This period typically lasts 12 weeks, though it can be longer for people with complex injuries or with complications such as joint deformities. Rehabilitation usually involves several different steps. After surgery, people often must wear a splint or other devise to stabilise or immobilise the hand and wrist. They also often must do hand exercises to stop the repaired tendons from sticking to surrounding tissue and limiting hand movement.
There are many kinds of different rehabilitation programmes, but it is unclear whether some are better than others. We set out to review the evidence from research studies, to find out:
‐ which approaches are most effective in restoring finger motion and function; and
‐ which approaches minimise the risk of adverse (unwanted) events, such as tendon ruptures, scar tissue sticking to other tissues, and joint stiffness.
How did we identify and evaluate the evidence?
First, we searched for studies in the medical literature that compared any rehabilitation approach after flexor tendon surgery against:
‐ no treatment;
‐ a placebo (dummy) treatment (in which, for example, someone thinks they may be receiving laser therapy but the machine is switched off); or
‐ another rehabilitation approach.
We then compared the results, and summarised the evidence from all the studies. Finally, we rated our confidence in the evidence, based on factors such as study methods and sizes, and the consistency of findings across studies that tested the same comparison.
What did we find?
We found 17 studies that involved a total of 1108 people who had received surgery to repair torn flexor tendons. The people were aged between 7 and 72 years, and three‐quarters of them were male.
Ten studies evaluated one each of eight different hand exercise programmes. The other seven studies evaluated a variety of other rehabilitation approaches, such as:
‐ laser therapy, in which light is directed at the tendons to encourage healing;
‐ ultrasound, in which sound waves are directed at the tendons to encourage healing; and
‐ a wearable machine (exoskeleton), designed to assist people in their movements.
We found very little evidence about the benefits and risks of different rehabilitation approaches. The evidence we did find was not robust. For example, for the three most relevant exercise comparisons we identified only:
‐ one study (84 people) that compared finger exercises against immobilisation;
‐ one study (53 people) that evaluated the effects of adding regular finger exercises (20 to 30 times every waking hour for four weeks from the first day after surgery) to ‘passive’ exercises (in which people regularly folded the fingers in the injured hand using the uninjured hand); and
‐ three studies (190 people) that evaluated the effects of adding ‘place and hold’ exercises (during which people use their uninjured hand to fold the fingers of the injured hand, and then have to hold the folded fingers in place for a few seconds without any support) to passive exercises.
The studies were too small, or reported too little robust or usable information, for us to determine which approach is best.
What does this mean?
We do not know which method works best for people to recover movement in the hand after surgery to repair flexor tendons. This is because there is not enough robust evidence about the benefits and risks of different methods.
Further research is needed to help inform clinicians’ and patients’ choice of rehabilitation after surgery for flexor tendon injuries.
How up‐to‐date is this review?
The evidence in this Cochrane Review is current to August 2020.
Summary of findings
Summary of findings 1. Early active flexion plus controlled passive exercise regimen versus early controlled passive exercise regimen for rehabilitation following surgery for flexor tendon injuries of the hand.
| Addition of active flexion exercises to controlled passive exercise regimen for rehabilitation following surgery for flexor tendon injuries of the hand | ||||||
| Patient or population: participants undergoing rehabilitation following surgery for flexor tendon injuries of the handa Setting: orthopaedic surgery department (Norway) Intervention: active flexion exercises commenced at one day post‐surgery plus standard hand therapy using modified Kleinert regimen Comparison: standard hand therapy using modified Kleinert regimen started at one day post‐surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Early controlled passive exercise regimen (modified Kleinert protocol) | Early active flexion plus early controlled passive exercise regimen | |||||
| Functional assessment using a patient reported outcome measure:
assessed with a VAS of ADL
(0 to 10; higher score = better function) Follow‐up: 6 months |
Study population | ‐ | 62 fingers (1 RCT) |
⊕⊝⊝⊝ VERY LOW b,c,d | The study reporting this outcome reported median and interquartile ranges. It found no evidence of a difference between the two groups (reported P = 0.942). It is very unlikely that a difference of 0.3 on an 11 point scale is clinically important. |
|
| See comment. Median 8.5, IQR 3.5 |
See comment. Median 8.8, IQR 1.5 | |||||
| Functional assessment using a patient reported outcome measure:
assessed with a VAS of ADL
(0 to 10; higher score = better function) Follow‐up: 12 months |
Study population | ‐ | 63 fingers (1 RCT) | ⊕⊝⊝⊝ VERY LOWb,c,d | The study reporting this outcome reported median and interquartile ranges. It found no evidence of a difference between the two groups (reported P = 0.113). It is very unlikely that a difference of 0.5 on an 11 point scale is clinically important |
|
| See comment. Median 8.8, IQR 2.9 |
See comment. Median 9.3, IQR 1.2 | |||||
| Active finger range of motion assessed with: goniometer (degrees) Follow‐up: 6 months | Study population | ‐ | 63 fingers (1 RCT) | ⊕⊝⊝⊝ VERY LOW b,c | This difference is not meaningful and could have been due to error in measurement: for goniometric measurement, the minimal detectable difference is between 12 and 30 degrees with a standard error of measurement ranging from 4 to 11 degrees per joint (Reissner 2019). | |
| Mean AROM in the control group was 134 degrees | MD 3 degrees higher (14.00 lower to 20.00 higher) | |||||
| Active finger range of motion assessed with: goniometer (degrees) Follow‐up: 12 months | Study population | ‐ | 63 fingers (1 RCT) | ⊕⊝⊝⊝ VERY LOW b,c | This difference is not meaningful and could have been due to error in measurement: for goniometric measurement, the minimal detectable difference is between 12 and 30 degrees with a standard error of measurement ranging from 4 to 11 degrees per joint (Reissner 2019). | |
| Mean AROM in the control group was 140 degrees | MD 9 degrees higher (7.04 lower to 25.04 higher) | |||||
| Adverse events: participants incurring one or more adverse events Follow‐up: 12 months |
Study population | RR 0.58 (0.23 to 1.44) |
69 fingers (1 RCT) |
⊕⊝⊝⊝ VERY LOW b,e | The 15 adverse events comprised 3 tendon ruptures, 6 wound dehiscence, 1 complex regional pain syndrome and 5 transitory swelling and tenderness of the tendon sheaf. | |
| 282 per 1000 | 164 per 1000 (65 lower to 406 higher) |
|||||
| Adverse event (tendon rupture) Follow‐up: 12 months | Study population | RR 1.73 (0.16 to 18.20) | 69 fingers (1 RCT) | ⊕⊝⊝⊝ VERY LOW b,e | All three tendon ruptures (4.3%) underwent secondary surgery. | |
| 31 per 1000 | 54 per 1000 (5 to 569) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADL: activities of daily living; AROM: active range of motion; CI: confidence interval; IQR: interquartile range; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; VAS: visual Analogue Scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aThe majority (68%) were zone II flexor tendon repairs; the rest being zone I and III. bDowngraded two levels due to very serious risk of bias reflecting performance and detection bias (no blinding) and unit of analysis errors since the data were provided for fingers not participants. cDowngraded one level due to serious imprecision reflecting the small sample size.
dDowngraded one level due to serious indirectness reflecting the reflecting the uncertain nature of the outcome.
eDowngraded two levels due to very serious imprecision reflecting the small number of events.
Summary of findings 2. Active exercise regimen compared with an immobilisation regimen following surgery for flexor tendon injuries of the hand.
| Active exercise regimen compared with an immobilisation regimen following surgery for flexor tendon injuries of the hand | ||||||
| Patient or population: participants undergoing rehabilitation following surgery for flexor tendon injuries of the handa Setting: specialist hand clinic (Brazil) Intervention: active exercise regimen commenced within one day post‐surgery for 3 weeks, all wore a dorsal splint Comparison: immobilisation in a dorsal splint for 3 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Immobilisation regimen for three weeks | Active exercise regimen commenced within one day post‐surgery | |||||
| Functional assessment using a patient reported outcome measure Follow‐up: 3 to 6 months | See comment. | See comment. | Not estimable | ‐ | See comment. | Outcome was not reported |
| Functional assessment using a patient reported outcome measure Follow‐up: more than 6 months | See comment. | See comment. | Not estimable | ‐ | See comment. | Outcome was not reported |
| Range of movement Follow‐up: 3 to 6 months | See comment. | See comment. | Not estimable | ‐ | See comment. | Outcome was not reported |
| Range of movement assessed with poor outcome (Strickland criteria) Follow‐up: 12 to 36 months | Study population | RR 0.08 (0.00 to 1.43) | 84 (1 RCT) | ⊕⊝⊝⊝ VERY LOWb,c,d | Poor outcome is < 25% of normal. The 7 (15%) cases of poor range of finger movement were all in the immobilisation group |
|
| 149 per 1000 | 12 per 1000 (0 to 213) | |||||
| Adverse events: participants incurring one or more adverse events | See comment. | See comment. | Not estimable | 84 (1 RCT) | See comment | Outcome was not reported. Only tendon rupture and indication for tenolysis data provided; see below. |
| Adverse events requiring (or indicated for) surgery | Study population | RR 0.64 (0.24 to 1.70) | 84 (1 RCT) | ⊕⊝⊝⊝ VERY LOWb,e | All five cases of tendon rupture (13.5% of 37) needing surgical repair occurred after 2 weeks in the active mobilisation group and all 10 cases of range of motion deficiency (21.3% of 47) indicating scar adhesion and need for tenolysis occurred in the immobilisation group. | |
| 213 per 1000 | 137 per 1000 (52 to 363) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group (in this table, this is directly based on the study population) and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect | ||||||
aAll were zone II flexor tendon repairs.
bDowngraded two levels for very serious risk of bias reflecting detection bias (no blinding of outcome assessors), potential selection bias and attrition bias.
cDowngraded one level for serious indirectness reflecting the unsatisfactory nature of the outcome.
dDowngraded one level because of low number of events and wide confidence intervals for this outcome.
eThese complications reflect the anticipated complications of early active mobilisation (early re‐rupture) and immobilisation (scar adhesions and contractures). It is uncertain whether tenolysis was actually done for those participants with range of motion deficit in the immobilisation group.
Summary of findings 3. Place and hold exercise regimen versus controlled passive exercise regimen following surgery for flexor tendon injuries of the hand.
| Place and hold exercise versus controlled passive exercise following surgery for flexor tendon injuries of the hand | ||||||
| Patient or population: participants undergoing rehabilitation following surgery for flexor tendon injuries of the handa Setting: specialist hand clinics (Iran and USA) and a specialist rehabilitation department (Egypt) Intervention: place and hold exercise regimenb Comparison: controlled passive exercise regimenc | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Controlled passive exercise regimen | Place and hold exercise regimen | |||||
| Functional assessment using a patient reported outcome measure: assessed with DASH questionnaire (0 to 100; higher scores = worse disability) Follow‐up: 6 months | Study population | Not estimable | 26 (1 RCT) | ⊕⊝⊝⊝ VERY LOWd,e | The study reporting this outcome reported median and interquartile ranges, finding no evidence of a difference between the two groups (reported P = 0.62). | |
| See comment. Median 15, IQR 10 to 30 |
See comment. Median 23, IQR 2 to 26 |
|||||
| Functional assessment using a patient reported outcome measure: assessed with DASH questionnaire (0 to 100; higher scores = worse disability) Follow up: 12 months | Study population | ‐ | 89 (1 RCT) | ⊕⊝⊝⊝ VERY LOWd,f | These differences are not clinically important. The recommended minimal clinically important difference for DASH is 15 (DASH/QuickDASH). | |
| Mean DASH score was 3.1 | Mean DASH score was 1.1 lower (2.77 lower to 0.57 higher) | |||||
| Range of movement Follow‐up: 3 to 6 months | See comment. | See comment. | Not estimable | ‐ | See comment. | Outcome was not reported |
| Range of movement (degrees) Follow‐up: 12 months | Study population | ‐ | 89 (102 digits) (1 RCT) | ⊕⊝⊝⊝ VERY LOWd,g | ||
| Mean ROM was 128 degrees | Mean ROM was 28 degrees higher (18.87 higher to 37.13 higher) | |||||
| Adverse events: participants incurring one or more adverse events | See comment. | See comment. | Not estimable | 84 (1 RCT) | See comment. | This outcome was not reported by the three trials testing this comparison. Only tendon rupture (see next) was commonly reported. One trial (26 participants), reporting by tendon or digit (36 digits), also reported on scar adherence (reported only in the controlled passive group), flexion contracture at the DIP and PIP joints and tendon lag; very low certainty evidence for all individual complications. |
| Adverse events requiring (or indicated for) surgery | Study populationsh | RR 0.81 (0.19 to 3.50) | 196 tendons (3 RCTs) | ⊕⊝⊝⊝ VERY LOWd,i | These data were limited to tendon rupture. We considered that all 7 tendon ruptures (3.6% of 196 tendons) would have required surgery.j | |
| 40 per 1000 | 33 per 1000 (8 to 140) |
|||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group (in this table, this is directly based on the study population) and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DASH: Disabilities of the Arm, Shoulder and Hand; DIP: distal interphalangeal; IQR: interquartile range; PIP: proximal interphalangeal; RCT: randomised controlled trial; ROM: range of movement; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect | ||||||
aThe majority were zone II flexor tendon repairs; one of the three studies making this comparison also included 7 zone I injuries and 7 zone III injuries.
bAll exercises started at 3 days from surgery. The place and hold exercise regimens varied among the three studies. In two studies, the exercises were carried out with a dorsal orthosis and in one study, the dorsal orthosis was removal when doing the exercises
cAll exercises started at 3 days from surgery. The controlled passive exercise regimens varied among the three studies. However, rubber band traction was common to all three.
dDowngraded two levels for very serious risk of bias reflecting lack of blinding (performance and detection bias)
eDowngraded one level for serious imprecision.
fDowngraded one level for serious imprecision, also reflecting the potential 'ceiling' effect as the mean values were very low and thus cannot discriminate between the two groups.
gDowngraded one level for serious imprecision as the data are presented for tendons not participants.
hControl group risk was devised from the summed data from the three studies. These results were not available for participants rather than tendons.
iDowngraded two levels for very serious imprecision reflecting a very low number of events and wide confidence interval for this outcome.
jWe did not include the 14 cases of scar adherence (70% of 20 tendons) reported in the controlled passive group of one trial because the outcome was not defined and no details of the extent or consequences were provided.
Background
Description of the condition
Tendons connect muscles to bones and enable motion at joints. The flexor tendons of the hand, which connect various flexor muscles in the forearm and hand to the bones (phalanges) in the fingers and thumb, act to bend (i.e. flex) the fingers or thumb. They are essential for complex hand function, including pinch, grip and motor dexterity. There are two flexor tendons in each finger; these connect with the flexor digitorum profundus (FDP) and the flexor digitorum superficialis (FDS) muscles in the forearm. The two flexor tendons in the thumb connect with the flexor pollicis longus (FPL) and flexor pollicis brevis (FPB) muscles. For much of the finger, flexor tendons move within tunnels called flexor sheaths. These are synovium‐lined tunnels that hold the tendons close to the bones, ensuring mechanical efficiency and preventing the tendons from 'bowstringing' across the joints. Sections of the sheaths are thickened into five fibrous bands called annular pulleys (A1 to A5) and three flexible compressible sections called cruciate pulleys (C1 to C3).
Flexor tendon injuries can be caused by open cuts (such as by broken glass) to the hand and crush injuries. They can also be caused by sudden forced extension of the fingers or thumb resulting in an avulsion injury where the tendon is, or tendons are, pulled away from the bone. Flexor tendon injuries of the hand are relatively common with 33 injuries per 100,000 person‐years (De Jong 2014). The incidence of tendon injuries is higher in males and in people aged 20 to 29 years (De Jong 2014). The FDP tendon of the fifth (little) finger is the most commonly injured tendon in isolation (Rosberg 2003).
Five anatomical zones (zones I to V) are commonly used to categorise the level of tendon injury in the fingers, hand and forearm (Verdan 1960). Zone I includes the FDP tendons from the insertion of the FDP on the distal phalanx bone to the insertions of the FDS tendons on the middle phalanx. Zone II extends between the FDS insertions to the proximal edges of the A1 pulleys. Zone III is the area in the palm of the hand between the A1 pulleys and the distal edge of the transverse carpal ligament in the wrist. Zone IV includes the tendons passing in the carpal tunnel. Zone V is the section proximal to the carpal tunnel at the wrist to the origin of the tendons at their respective muscle bellies. Injuries in zone II, where the tendons are contained within the flexor sheaths, are the most common (De Jong 2014). Often, the associated pulleys are damaged during the flexor tendon injury in this zone. The clinical issues related to an inefficient pulley system can include bowstringing, reduced composite finger flexion, stiffness and reduced grip strength (Lilly 2006).
Laceration or avulsion injury to the flexor tendons is generally managed with surgery. Acute injuries tend to be managed with primary surgical repair of the tendon. This is done by direct end‐to‐end tendon repair with multi‐strand sutures (2, 4, 6 or 8 strand) of the core of the tendon and with additional peripheral sutures around the sides of the tendon. The pattern and strength of the suture repairs prevent gapping and contribute to the strength of the repair. When primary repair of the tendon has failed (that is, a tendon ruptures) or when primary repair is not feasible due to concurrent injuries (e.g. nerve, blood vessel, bony injury or infection) or loss of tendon length, secondary surgical intervention may be advised. Secondary surgery involves either secondary repair of the failed primary tendon repair or a two‐stage reconstructive surgical process. Zone II injury is typically considered to be more difficult both to repair surgically and to rehabilitate. Repair of zone II tendons often requires more additional procedures (e.g. excising one slip of the FDS tendon or part of the A2 or A4 pulleys) than other zones, often resulting in poorer mobility and functional outcomes (Tang 2013).
Description of the intervention
This review examines the rehabilitation interventions that are prescribed after the surgical repair of both open and closed flexor tendon injuries. Rehabilitation interventions are usually provided by a physical therapist or an occupational therapist who specialises in providing hand therapy interventions. Therapists often prescribe a combination of interventions to protect the tendon repair, promote tendon healing, remedy any effects of scarring and swelling, and to regain mobility and function of the hand. Early rehabilitation in the first six to eight weeks following surgery is focused on protecting the tendon repair (Evans 2012; Strickland 2005). Intervention in this early postoperative period can include patient education, fabrication of a type of orthosis, an exercise regimen, wound care (application of dressings or topical applications), swelling management (e.g. compression therapy, elevation or cryotherapy), scar management (e.g. massage treatments, topical applications, desensitisation programmes) and electrotherapy modalities (e.g. neuromuscular electrical stimulation) (Pettengill 2005; Villecio 2010).
A therapist will fabricate an orthosis in order to protect the tendon repair. Orthoses restrict hand use and usually allow joints to move within a safe range of motion (ROM). Orthoses can be made from a variety of materials. They can also have different designs including hand‐based designs (crossing only the finger and/or thumb and wrist joints) (Peck 2014), forearm‐based designs (extending from finger and/or thumb to the proximal forearm), or they can have a dynamic component (often using an elastic traction system to mimic the action of the tendons, thus preventing strain on the repairs when moving the joints) (Evans 2012; Strickland 2005). Variations also exist with respect to the specific position of the wrist and digits within the orthosis.
Historically, post‐operative management consisted of immobilisation for three weeks post surgery based on the early animal studies by Mason and Allen in the 1940s (Mason 1941). This was based on the principle that tendons required extrinsic healing from surrounding tissues before they could be subject to gliding stresses (Peacock 1965). However, by the 1970s, surgeons such as Kleinert and Duran (Kleinert 1975; Duran 1975) proposed passive flexion either using rubber band traction systems or passive exercises based on the concepts that tendons could heal intrinsically and thus minimise adhesions. As surgical techniques and strength of repairs have continued to improve, place and hold exercises, synergistic wrist gliding exercises and active gliding exercises have been incorporated in the rehabilitation regimens. Active flexion exercises are recommended; this is where fingers are bent through a progressive arc of motion from one third to full bending, typically over a period of four weeks (Tang 2018a).There has also been a simultaneous shift of orthotic positioning of the wrist and finger/thumb joints from significant flexion to keeping the wrist in extension to allow for reduced work of flexion and mechanical efficiency (Evans 2012). Different exercise regimens are in use, often defined in terms of the types of exercise entailed. These include: immobilisation regimens, passive motion exercise regimens, place and hold exercise regimens, or active motion exercise regimens (Clancy 2013; Gelberman 1991; Hagberg 2000; Pettengill 2005). Regimens are typically named after the type of exercises that are initiated in the early postoperative period.
Immobilisation regimens: During immobilisation, therapists prescribe no exercise during a defined postoperative time period. The patient is usually placed in a dorsal extension blocking plaster cast or an orthosis including the affected wrist, fingers and thumb.
Passive motion exercise regimens: These refer to bending the finger manually with assistance from either the patient's other hand, from another person (e.g. a physical therapist) or by means of a dynamic component (e.g. elastic component such as rubber bands). The patient then actively straightens the finger into the orthosis using the muscle power of the uninjured extensor tendon. For the purpose of this review controlled passive exercise regimens imply the use of rubber band traction to hold the fingers in passive flexion.
Place and hold exercise regimens: These are exercise programmes where the injured digit is manually flexed using either the patient's uninjured hand or by another person (e.g. a physical therapist); the patient then tries to hold the flexed position by actively using the muscle strength of the injured flexor tendon.
Active motion exercise regimens: These refer to bending and straightening the fingers and thumb through an arc of motion using the patient's own muscle strength with no assistance.
Exercise regimens may comprise combinations of these regimens (for example, passive motion exercises followed by place and hold and/or active exercises) or variations of these regimens (for example, active motion through a 'mid‐range' or 'full‐range', or synergistic motion of the wrist and hand using the tenodesis effect). The timing of the commencement of the exercise programme (for example, delaying the commencement of certain types of exercises following surgery), as well as duration and frequency of exercises may also vary (Evans 2012).
Rehabilitation generally progresses over time. Interventions recommended later in the rehabilitation process include grip and pinch strengthening, functional rehabilitation (that is, prescription of graduated hand function by introducing more strenuous self‐care, domestic and work duties) and return‐to‐work interventions (for example, work hardening and job modifications). There is variability in these exercise regimens with regards to frequency and repetitions of the performed exercises as well as the intervals of progression from one to a different type of exercise. During the later stages of rehabilitation, additional orthosis can also be fabricated to prevent and manage joint deformities or tightness of soft tissue components.
Work hardening programmes are graded exercises and activities to improve strength, endurance and co‐ordination to facilitate a person's return to employment (Pettengill 2010).
The mode of delivery of the intervention is also an important consideration. People may receive early rehabilitation in a hospital or clinic setting. Later programmes may take place in the clinic setting, or alternatively the individual might perform them in the home or workplace. In each setting, the level of patient supervision differs. For example, a therapist may supervise rehabilitation in a clinic setting. Whereas, there may be limited supervisions for a tele‐rehabilitation programme, or none or limited supervision for a home exercise programme.
How the intervention might work
Over the last few decades, knowledge of tendon biology and biomechanics has improved considerably (Osei 2014; Wu 2013). This includes tendon response to injury, repair and stress as well as the mechanical characteristics of the current surgical techniques (to improve the strength of the repair whilst allowing smooth excursion of the tendons through the tunnels of the flexor tendon sheaths) (Lutsky 2015). This knowledge has, in turn, influenced rehabilitation protocols and the types of treatments offered (Groth 2004).
Advances in flexor tendon surgery such as surgical repair techniques, suture types, tendon grafting, use of antiseptic and other wound and scar limiting agents, wide‐awake surgery, have also influenced the advancement of hand rehabilitation (Bindra 2005; Tang 2018a). Various surgical repair techniques are currently used around the world and contribute to the choice of orthosis and exercise protocol prescribed, with different types of repairs being thought to withstand greater forces and therefore being able to tolerate earlier active mobilisation.
Rehabilitation aims to protect the repaired tendons, promote intrinsic tendon healing, minimise extrinsic scar tissue formation, optimise tendon gliding and restore motion and functional use (Elliot 2007Strickland 2005). The types of rehabilitation interventions recommended by healthcare providers are generally based on a number of factors that may include: the nature of the injury (e.g. traumatic open injury or closed avulsion injury), stage of the rehabilitation (e.g. immediately following the surgery versus longer term rehabilitation at three months or beyond), the strength of the repair (e.g. number of suture strands in the repair), associated injuries (e.g. concomitant nerve, bone, blood vessel or ligament damage), pre‐injury medical history or ability to comply with rehabilitation (Evans 2012).
Various rehabilitation treatments are frequently used in the early to late post‐operative stages. Education is considered very important for patient adherence to rehabilitation following surgery (Evans 2012). Advice often focuses on the importance of adhering to treatment recommendations, the level of functional activity permitted and general care of the repaired tendon and wound (Pettengill 2010). Orthoses are applied in the early post‐surgical stage. The purpose of providing orthoses is to position the wrist and fingers so that the tendon repairs are not under any tension, but still allow motion within a safe range (Pettengill 2010). Careful positioning of the hand within the orthosis is necessary. Therefore, the joint angles within the orthosis have great significance. For example, it is thought that dynamic traction designs with the metacarpal joints in 70 degrees of flexion increases the risk of proximal interphalangeal (PIP) joint flexion contracture (Burge 1990). Early designs of exercise regimens assumed that 3 mm to 5 mm of tendon excursion (i.e. the distance a tendon travels upon motion of a joint) decreased tendon adhesions that limit finger mobility (Duran 1975). Therapists often recommend protocol‐based exercise regimens to improve the tendons' gliding function by minimising adhesions (Khanna 2009), preventing joint stiffness and improving range of motion. Practitioners believe these exercises to be essential in regaining long‐term finger dexterity and hand function (Pettengill 2010). Moreover, research suggests that controlled stress on the tendons, created by either passive or active motion, facilitates healing, controls early collagen deposition and facilitates biochemical events that increase tensile strength (Buckwalter 1999; Evans 2012). However, excessive stress during motion may also pose a risk of gapping or rupture of the repaired tendon ends (Evans 2005). The timing of the interventions, especially the commencement of an exercise regimen, may influence how an intervention works (Adolfsson 1996; Evans 2012). Various studies have found that periods of immobilisation immediately following repair can result in loss of tensile strength and glide (Evans 2012). However, other authors advocate delayed mobilisation for up to three to five days to allow inflammation and oedema to subside and minimise the strain on the flexor tendon (Halikis 1997; Zhao 2004). Wound care treatments are essential in preventing infection and facilitating wound healing (Von der Heyde 2010). As therapists often prescribe early exercise, the dressings should not impede motion or place extra stress on the tendon repairs when the finger is moved. Oedema management helps to reduce the amount of swelling in the digit and hand. Oedema in the subcutaneous tissue adds significantly to the gliding resistance, whereas pulleys may add to the resistance of the swollen repaired tendon (Wu 2013). Scar management treatments may be advisable to promote optimal scar formation and prevent skin and tendon adhesions or reduce scar hypersensitivity (Jones 2005). Electrotherapy modalities, such as neuromuscular electrical stimulation, are thought to provoke stronger muscle contractions. Practice guidelines have recommended therapeutic ultrasound for promoting healing while minimising the formation of soft‐tissue and skin adhesions (Pettengill 2010). Therapists may also utilise strengthening and work hardening treatments to facilitate early return‐to‐work, leisure and sporting activities in the later stages following surgery (Pettengill 2010).
Why it is important to do this review
Flexor tendon injuries create significant impairment in terms of the functional use of the hand. They create an economic burden on our health care systems and an indirect costs related to missed workdays. A recent study in the United States estimated the cost of flexor tendon lacerations to society using a validated prevalence‐based cost of illness model (Mehrzad 2019). They reported that flexor tendon lacerations incur an estimated cost of up to USD 409.1 million annually. The estimated total direct costs per injury are USD 13,725, whereas indirect costs up to USD 112,888. Therefore, it is important to focus our efforts to improve treatments and rehabilitation protocols which decrease not only the physical and psychological burden to the individual but also financial costs that impact not only the person, but also society as a whole.
Flexor tendon injuries of the hand can result in loss of finger and thumb motion, reduced functional hand use and quality of life. The management of these injuries has evolved over several decades. At present, there is no gold standard rehabilitation programme used for rehabilitation following surgery for flexor tendon injuries. As a result, centres across the globe use a wide range of rehabilitation treatments. Clinical practice is often influenced by the results of biomechanical and biological studies (Osei 2014; Wu 2013). Instead we need to examine the high‐quality clinical evidence to establish the effectiveness and safety of rehabilitation interventions for managing flexor tendon injuries of the hand and thus identify those interventions that are most effective at restoring digital motion and function whilst minimising the risk of complications and adverse events.
Objectives
To assess the effects (benefits and harms) of different rehabilitation interventions after surgery for flexor tendon injuries of the hand.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised and quasi‐randomised (i.e. with treatment allocation method that is not strictly random, such as allocation by patient hospital identification number) controlled trials evaluating rehabilitation interventions after surgery for flexor tendon injuries of the hand.
Types of participants
We included trials of individuals who had undergone post‐surgical rehabilitation following primary and secondary repair, or reconstruction of partial or total lacerations or rupture of one or more flexor tendons in any of the flexor tendon zones of injuries. We excluded studies examining the effectiveness of tendon transfers for people with neurological conditions.
Types of interventions
We included all types of rehabilitation following surgery for flexor tendon injury of the hand. Primary interventions included orthoses to protect the repair/reconstruction, exercise regimens, scar management and hand strengthening. We also considered interventions for reducing or controlling oedema, for work hardening and desensitisation programmes. We also considered the timing of the interventions' commencement (e.g. early active motion regimens). We excluded wound care, oral pharmacological interventions and topical pain relief ointments.
The main comparisons we considered were:
different types of orthoses; e.g. dynamic orthosis versus static orthosis; comparisons of different finger and wrist positioning within the orthosis;
different orthosis wearing regimens, including duration; e.g. six weeks or shorter versus longer than six weeks;
different exercise regimens; e.g. controlled active mobilisation versus controlled passive mobilisation;
different timings for commencing mobilisation; e.g. started within the first three days versus after three days;
different types of scar management; e.g. massage versus topical applications such as silicone gel sheets;
different timings for commencing strengthening; e.g. six to 10 weeks versus after 10 weeks;
different doses for interventions, other than orthosis wearing regimen; e.g. ultrasound dose, frequency or amount of interventions.
For interventions in which a control or sham group was appropriate (such as scar management, or strengthening and work hardening), we compared the active intervention versus the control or sham group. For the exercise protocols, we selected the least aggressive protocol as the control group.
Types of outcome measures
We included studies if the protocol included the measurement of at least one clinical outcome related to function, range of motion or adverse event reporting. We assessed all outcomes as short‐term (defined as three months or less), medium term (over three months to six months) and long‐term (over six months).
Primary outcomes
Functional assessment using a patient reported outcome measure, such as Patient Rated Wrist and Hand Evaluation (PRWHE); Michigan Hand Questionnaire (MHQ); or Disability of the Arm, Shoulder and Hand (DASH)
Active finger ROM using goniometric measurement. Different classification systems have been designed that incorporate ROM and are used to describe outcomes following tendon surgery (such as, Stickland‐Glogovac classification; Strickland classification; Tang classification)
Adverse events including tendon rupture, revision surgery, scar adhesion, delayed wound healing, loss of mobility or function, joint contracture, triggering of the digit, pulley failure, persistent pain and sensory deficits. We will report the total number of participants with adverse events and for each of these events
Secondary outcomes
Passive finger ROM using goniometric measurement
Hand strength (including grip strength, pinch strength)
Return to previous activity (including return to work, education, musical instrument or sport). Return to work will be reported separately if available (including same, modified or alternate duties) for individuals working at the time of injury
Functional assessment using an objective measure (including Jebsen Hand Function Test)
Quality of life using a self‐report measure (such as Euro‐QOL, SF‐36)
Satisfaction with the result of the surgery at three months or longer
Where available, we collected resource and cost data such as health care utilisation, and insurance data related to work absence, and reported these data in the Characteristics of included studies. However, these data were not a focus of this review.
Search methods for identification of studies
Electronic searches
We conducted a comprehensive search of the following databases:
Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (18 June 2019);
Cochrane Central Register of Controlled Trials (CENTRAL) (11 August 2020 Issue 8);
MEDLINE (including Epub Ahead of Print, In‐Process and Other Non‐Indexed Citations, Daily and Versions(R) (1946 to 10 August 2020);
Embase (1980 to 11 August 2020);
AMED (1985 to 18 June 2019);
CINAHL Plus (1937 to 18 June 2019).
We also searched the following clinical trials registries for ongoing or recently completed trials:
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (18 June 2019);
US National Institutes of Health Clinical Trials search portal (ClinicalTrials.gov) (11 August 2020).
In MEDLINE, we combined subject‐specific terms with the sensitivity‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011). Search strategies for all major databases are reported in Appendix 1. We did not apply any language or date restrictions.
Searching other resources
We searched the reference lists of included studies, relevant articles on flexor tendon rehabilitation and any known systematic reviews on the topic for information on additional trials, including unpublished or ongoing studies. Where necessary, we contacted authors of key papers and abstracts to request further information about their trials.
Data collection and analysis
Review authors followed recommended strategies for data collection and analysis documented in Chapters 7 and 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors (BJ and SP) independently screened the titles and abstracts of all retrieved references. We retrieved full‐text articles of all studies that appeared to meet the eligibility criteria. The same two review authors independently screened the full‐text articles against the eligibility criteria and documented their decisions. Review authors compared their lists; a third review author (MR) was available to resolve any disagreement, which was not required. Where identification was possible, we collated multiple reports of the same study and placed these under the same study ID. We have included a PRISMA flow chart to illustrate the study selection process (Moher 2009). We attempted to contact trial authors for clarification of study methods and characteristics, where necessary, to establish trial eligibility.
Data extraction and management
Two review authors (BJ and SP) independently extracted data using a standard pre‐defined data extraction forms specifically developed for this review.
We extracted the following study characteristics.
Methods: study design, date of study, duration of study, study setting, randomisation procedure, allocation, blinding and unit of analysis.
Participants: number of participants, number of involved digits, number of injured flexor tendons, age (mean, standard deviation, range), sex, type of flexor tendon injury, baseline characteristics, time between injury and surgery, inclusion criteria, exclusion criteria, type of surgery, diabetes and smoking status.
Interventions: intervention, comparison (e.g. control or sham), co‐interventions, and care programmes provided to all participants.
Outcomes: primary outcomes, secondary outcomes specific and collected, time points of evaluation, and resource use.
Notes: funding for trial, relevant conflicts of interest related to the study of trial authors, and any unit of analysis issues.
Two review authors (SP and BJ) independently extracted outcome data from included studies. We noted in the Characteristics of included studies tables if trial authors did not report outcome data in a usable way. We resolved disagreements by discussion. Two review authors (SP or BJ) transferred data into Cochrane's statistical software, Review Manager 5 (RevMan 2014), and a third review author (MR) cross‐checked the entries.
Assessment of risk of bias in included studies
Two review authors (BJ and SP) used Cochrane's tool for assessing risk of bias to independently evaluate the risk of bias for each trial in the following eight domains (Higgins 2011):
sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias) for participant self reported outcomes;
blinding of outcome assessment (detection bias) for objective assessments;
incomplete outcome data, rated separately for data measured at less than three months, three months to six months; and grater than six months follow‐up (attrition bias);
selective outcome reporting (reporting bias);
other risk of bias (whether the unit of analysis was appropriate, checking for premature stopping of the trial and the basis for this; and for extreme baseline differences between comparison groups).
We assessed risk of bias of self‐reported and objective outcome measurements separately for the two blinding and incomplete outcome data domains. For each domain, we assigned a judgement of high, low or unclear risk of bias based on the criteria in Table 8.5.d of the Cochrane Handbook (Higgins 2011). The review authors resolved disagreement by discussion and consensus. When criteria were unclear, one review author attempted to obtain further information from the authors of the trial. Where information on risk of bias related to unpublished data or correspondence with trial authors was received, we noted this in the 'Risk of bias' tables.
Measures of treatment effect
For dichotomous data we used risk ratios (RRs) with 95% confidence intervals (CIs).
For continuous data measured with the same scale, we used mean differences (MDs) and 95% CIs. If trials used different scoring systems to measure the same underlying concept (for example, different measures of function), we planned to use the standardized mean differences (SMDs) and 95% CIs. We used final scores in preference to change scores.
Where reported in trial reports, we presented non‐parametric data, such as medians (Med) and interquartile ranges (IQR) in the text, tables or both.
Unit of analysis issues
We clarified the unit of analysis; thus, whether the number reported represents participants, hands, digits or flexor tendons. Potential unit of analysis issues arise when multiple fingers on the same hand have had separate flexor tendon injuries. Bilateral involvement may be possible. We sought information about the unit of randomisation (that is, participants, hands, involved digits or involved tendons) for studies that included participants with multiple‐digit involvement in the same hand or had bilateral injury. We examined the study reports to see whether analyses were conducted using methods that take into account the dependency of observations. If trials did not report appropriate analyses, we contacted the authors for further information and data. If such data were not available and where possible, we planned to conduct sensitivity analyses that considered the number of randomised participants with bilateral or multiple digit involvement. We also avoided unit of analysis issues related to repeated observations of the same outcome, such as by presenting separate data for different periods of follow‐up (section 9.3.1; Higgins 2011). Where a single trial reported on multiple trial arms, we included only the relevant groups of the trial. If the same meta‐analysis combined two comparisons from the trial, we planned to split the control group to avoid double‐counting. However, this was not the case in any of the included studies.
Dealing with missing data
Intention‐to‐treat (ITT) analysis was our first choice when data were available. If data for key study characteristics or primary outcomes were missing or incomplete, we contacted the trial authors to obtain these. We considered conducting sensitivity analyses when missing data were not obtainable and their absence was considered likely to introduce bias. We also planned to conduct sensitivity analyses to explore the effects of excluding best‐case and worst‐case studies from the analyses. However, we did not have sufficient studies to complete this. We also planned to calculate missing data where possible; for example, calculating standard deviations from other available data such as standard errors (Section 16.1.3.1; Higgins 2011), or from data that was presented graphically. Again, we were not able to do this for any of the included studies.
Assessment of heterogeneity
Review authors assessed clinical heterogeneity (i.e. study populations, interventions and outcomes) between studies qualitatively. We assessed statistical heterogeneity by visual inspection of the overlap of CIs on the forest plots, along with consideration of the Chi2 tests for heterogeneity and I2 statistic (Higgins 2011). We based our interpretation of the I2 value in Higgins 2011:
0% to 40% might not be important;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
75% to 100% may represent very substantial heterogeneity.
Assessment of reporting biases
To reduce outcome reporting bias, we searched for published and unpublished studies without language restrictions. We sought trial registration documents for all trials by searching ClinicalTrials.gov and the ICTRP. We compared these with the corresponding published RCTs (Dwan 2008). We contacted the authors of unpublished trials to ask for unpublished results. Selective outcome reporting biases were appraised as part of the risk of bias assessment of each trial. We planned to investigate the likelihood of publication bias by generating funnel plots if we had pooled data from 10 trials or more. However, we did not have a sufficient number of studies to conduct this analysis.
Data synthesis
When considered appropriate, we aimed to pool results of comparable groups of trials with similar characteristics (participants, interventions, outcome measures and timing of outcome measurements) to obtain estimates of the efficacy of specific rehabilitation interventions following flexor tendon surgery. We planned to pool results in a meta‐analysis using either a fixed‐effect or a random‐effects model (depending on the level of clinical and methodological heterogeneity). Our choice of the model to report would be guided by careful consideration of the extent of heterogeneity and whether it can be explained, in addition to other factors, such as the number and size of included studies. We used 95% CIs throughout. We considered not pooling data where there is considerable heterogeneity (I2 > 75%) that cannot be explained by the diversity of methodological or clinical features among trials. Where it was inappropriate to pool data, we presented trial data in the analyses or tables for illustrative purposes and reported these in the text as a narrative synthesis.
Subgroup analysis and investigation of heterogeneity
If sufficient studies were available, we had planned to perform subgroup analyses including:
zone of the tendon repair (zone I, II, III, IV and V);
type of suture technique for surgical repair (two‐strand, four‐strand, six‐strand repairs);
primary repair, secondary repair (i.e. repair following rupture of a primary repaired tendon) versus secondary tendon reconstruction;
timing of the start of the intervention (e.g. immediate (within the first three days), three days to six weeks, six to 10 weeks, after 10 weeks);
thumb versus fingers injuries;
partial lacerations, complete lacerations and avulsion injuries (ruptures);
workers' compensation insurance versus private insurance.
We selected these subgroups because the nature of each group may influence the intervention outcome. Repair of flexor tendons in different zones are thought to have different outcomes because of the biomechanics of the flexor tendons (Rigo 2016; Stone 1989). The strength of the repair is thought to increase together with the number of strands, which in turn may influence outcomes (Lee 2015; Myer 2016). Primary and secondary repair and secondary reconstruction may have different outcomes due to the length of time after the initial injury and different method used (Freilich 2007).
Where subgroup analysis was possible and appropriate, we planned to investigate whether the results of subgroups were significantly different by inspecting the overlap of confidence intervals and performing the test for subgroup differences available in RevMan 5 (RevMan 2014).
Sensitivity analysis
If sufficient studies had been available, we planned to conduct sensitivity analyses on various aspects of trial and review methodology and the robustness of the results.These included sensitivity analyses to explore the effects of the following:
exclusion of trials at high or unclear risk of selection bias from inadequate concealment of allocation;
exclusion of trials at high or unclear risk of attrition bias from incomplete outcome data;
exclusion of trials reported only in conference proceedings and other short reports;
the choice of statistical model for pooling (fixed‐effect versus random‐effects);
exclusion of trials at risk of unit of analysis issues, relating either to body parts or outcome reporting (e.g. total complications where it is unclear whether participants had more than one reported complication).
We did not conduct any sensitivity analyses in the current version of this review.
Summary of findings and assessment of the certainty of the evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the certainty of evidence (Schünemann 2011). We used GRADEPro software (www.gradepro.org) to create 'Summary of findings' tables. We presented the results of the most important comparisons of the review in these 'Summary of findings' tables, which summarise the certainty of the evidence, the magnitude of effect of the interventions examined and the sum of available data for each outcome (Schünemann 2011). The 'Summary of findings' tables include an overall grading of evidence certainty related to each of the main outcomes.
According to GRADE, an initial judgement of 'high‐certainty evidence' is reserved for a body of evidence based on RCTs. We downgraded the certainty of evidence to 'moderate certainty', 'low certainty' or 'very low certainty', depending on the presence and extent of five factors: study limitations, inconsistency of effect, imprecision, indirectness or publication bias. We prepared 'Summary of findings' tables for those comparisons that tested interventions commonly used in clinical current practice. We selected the following primary outcomes for presentation in 'Summary of findings' tables: functional assessment using a patient‐reported outcome measure at medium‐term follow‐up (more than three to six months) and long‐term follow‐up (greater than six months); active range of motion at medium‐ and long term follow‐ups (we used total active range of movement in preference to poor outcome categories of classification systems); and total of participants with adverse events and total of participants with adverse events requiring (or indicated for) surgery.
Results
Description of studies
Results of the search
We conducted searches up to August 2020. We screened a total of 1278 records from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (26 records), CENTRAL (208 records), MEDLINE (462 records), Embase (227 records), CINAHL Plus (63 records), AMED (75 records), WHO ICTRP (138 records), and ClinicalTrials.gov (79 records) (Table 4).
1. Number of records retrieved by each search strategy.
| Database | Period Searched | Date Searched | Number of hits |
| Cochrane Bone, Joint and Musculoskeletal Trials (BJMT) Specialised Register | 18 June 2019 | 18 June 2019 | 26 |
| CENTRAL | August 2020, Issue 8 | 11 August 2020 | 208 |
| MEDLINE | January 1946 to August 2020 | 10 August 2020 | 462 |
| Embase | January 1980 to June 2019 | 18 June 2019 | 227 |
| CINAHL PLUS | January 1937 to June 2019 | 18 June 2019 | 63 |
| AMED | January 1985 to August 2020 | 10 January 2017 | 75 |
| ClinicalTrials.gov | 11 August 2020 | 79 | |
| WHO International Clinical Trials Registry Platform (ICTRP) | 18 June 2019 | 138 |
Among all searches, we identified a total of 47 articles for potential inclusion, for which we obtained full reports where possible. After linking any references pertaining to the same study under a single study ID, we identified 40 studies. Upon further analysis, we included 17 studies (Abdel Sabour 2018; Adolfsson 1996; Farzad 2014; Geetha 2014; Gelberman 1991; Gulke 2018; Hagberg 2000; Kneafsey 1994; Ozkan 2004; Poorpezeshk 2018; Rigo 2017; Scavenius 2000; Silva 2003; Stenekes 2009; Trumble 2010; Uday Raj 2018; Vialaneix 2003). We excluded 12 studies (Bainbridge 1994; Baktir 1996; Horsfall 2016; ISRCTN80184286; Kingston 2014; NCT01939808; Peck 1998; Peck 2014; Percival 1989; Stegink Jansen 1990; Xiao 2018; Yildirim 2010). Four studies are awaiting classification (Kitis 2009; Liu 2004; Naude 2019; Yavari 2009). We found seven ongoing studies from searching the WHO ICTRP (CTRI/2019/01/016821; IRCT201310138177N8; IRCT20150721023277N7; NCT03812978; NCT03850210; NCT04237415; NCT04385485). A flow diagram summarising the study selection process is shown in Figure 1.
1.
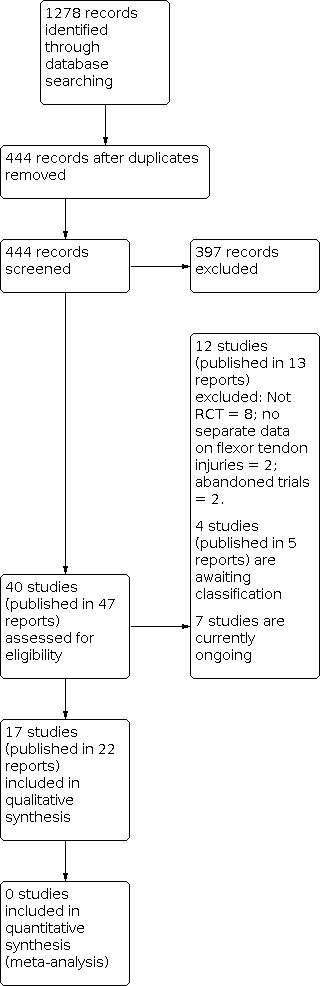
Diagram showing the flow of studies through the study selection process
Translation from German to English was obtained for one included study (Gulke 2018).
Included studies
Full descriptions of each of 17 included trials is provided in the Characteristics of included studies table. A summary of the each study's characteristics and participant details is included in Table 5.
2. Summary of study settings and participant characteristics.
| Study | Country | Setting |
Recruited Participants; Digits; Tendons |
Final follow‐up Participants; Digits; Tendons |
Zones |
Age (years) Mean (Range) |
Male | Female |
| Abdel Sabour 2018 | Egypt | Rehabilitation Department | 33; NR; 45 | 26; NR; 36 | I‐III | 26.8 (15‐60)a | 21a | 5a |
| Adolfsson 1996 | Sweden | NR | 96; 106; NR | 82; 91; 118 | II | 37a | 54a | 28a |
| Farzad 2014 | Iran | Hand therapy clinic | 54; 64; 108 | 54; 64; 108 | II | 28.5 (13‐50) | 37 | 17 |
| Geetha 2014 | India | Hospital | 106; 139; NR | 100; 131; NR | II | G1: NR (10‐45)a G2: 35 (22‐50)a |
89a | 11a |
| Gelberman 1991 | USA | Multi‐centre hospital | 51; 60; 102b | 51; 60; 102 | II | 29.4a | NR | NR |
| Gulke 2018 | Germany | Physiotherapy clinic | 62; NR;NR | 59; NR; NR | II | 29.5 (18‐60) | 44 | 18 |
| Hagberg 2000 | Sweden | NR | 100; 108; NR | NR; NR; NR | II | NR | NR | NR |
| Kneafsey 1994 | UK | Plastic surgery centre | 112; NR; NR | 80; NR; NR | I‐III | NR | NR | NR |
| Ozkan 2004 | Turkey | Hand surgery centre | 25; 41; NR | 23; 39; NR | I‐V | 24 (7‐43) | 15 | 10 |
| Poorpezeshk 2018 | Iran | Plastic surgery centre | 97; 114; 114 | 77; 92; 92 | I‐III | 27a | 60a | 17a |
| Rigo 2017 | Norway | Orthopaedic surgery centre | 53; 73; 73 | 45; 63; 63 | I‐III | 38.7 (18‐72)c | 36c | 14c |
| Scavenius 2000 | Denmark | Hand surgery centre | 39; 39; 39 | 33; 33; 33 | I‐II | NR | NR | NR |
| Silva 2003 | Brazil | Hand surgery centre | 84; NR; 152 | NR; NR; NR | II | 34 (18‐66) | NR | NR |
| Stenekes 2009 | Netherlands | Plastic surgery centre | 28; NR; NR | 25; NR; NR | All | 33.5a | 18a | 7a |
| Trumble 2010 | USA | Multi‐centre hand surgery centre | 103; 119; 238 | 89; 102; 204 | II | 29 (15‐51)a | 63a | 30a |
| Uday Raj 2018 | India | Plastic surgery centre | 30; NR; NR | 30; NR; NR | V | NR | NR | NR |
| Vialaneix 2003 | France | NR | 35; NR; NR | NR; NR NR | II | 35 | NR | NR |
NR: Not reported; G1: Group 1; G2: Group 2
aOnly reported at follow‐up (not at baseline)
bParticipants were only eligible if they were available for the 6 month follow‐up. Thus, this number is likely to have been much higher than reported here.
cExcludes 3 participants who experienced tendon ruptures after randomisation.
Design
Sixteen trials were described as randomised, although six did not describe the randomisation process used (Hagberg 2000, Kneafsey 1994, Scavenius 2000; Silva 2003; Stenekes 2009; Vialaneix 2003). Gelberman 1991 was quasi‐randomised. All trials used a parallel‐group design and allocated participants into one of two intervention arms. All studies appeared to randomise at the level of the participant. Of note is that the composition of the ultrasound intervention in Geetha 2014 was changed twice during study recruitment; this was not randomised.
Setting
The 17 included trials were conducted in 13 countries: two each in India (Geetha 2014; Uday Raj 2018), Iran (Farzad 2014; Poorpezeshk 2018), Sweden (Adolfsson 1996; Hagberg 2000) and USA (Gelberman 1991; Trumble 2010); and one each in Brazil (Silva 2003), Denmark (Scavenius 2000), Egypt (Abdel Sabour 2018), France (Vialaneix 2003), Germany (Gulke 2018), Netherlands (Stenekes 2009), Norway (Rigo 2017), Turkey (Ozkan 2004) and UK (Kneafsey 1994). There were two multicentre studies (Gelberman 1991.Trumble 2010). Three studies did not state clearly how many centres were involved (Abdel Sabour 2018; Hagberg 2000; Vialaneix 2003). The remainder were all single‐centre studies.
Studies were conducted in various clinical settings. This included rehabilitation, physiotherapy or hand therapy clinics (Abdel Sabour 2018; Farzad 2014; Gulke 2018) or hospital departments or centres such as plastic surgery, hand surgery and orthopaedic surgery (Geetha 2014; Gelberman 1991; Kneafsey 1994; Ozkan 2004; Poorpezeshk 2018; Rigo 2017; Scavenius 2000; Silva 2003; Stenekes 2009; Trumble 2010; Uday Raj 2018). Three studies did not report where the study was conducted (Abdel Sabour 2018; Hagberg 2000; Vialaneix 2003).
The earliest study was published in 1991 (Gelberman 1991).
Funding sources and potential conflicts of interest
Ten studies did not disclose funding sources or potential conflicts of interest (Adolfsson 1996; Farzad 2014; Gelberman 1991; Hagberg 2000; Kneafsey 1994; Ozkan 2004; Scavenius 2000; Silva 2003; Stenekes 2009; Vialaneix 2003). Three studies reported receiving no financial support (Abdel Sabour 2018; Rigo 2017; Uday Raj 2018). Three studies received funding to support their research (Geetha 2014; Poorpezeshk 2018; Trumble 2010). Additionally, Abdel Sabour 2018 and Trumble 2010 reported conflicts of interest but did not state what these were. Five studies stated that they had no conflicts of interest to declare (Geetha 2014; Gulke 2018; Poorpezeshk 2018; Rigo 2017; Uday Raj 2018).
Participants
A total of 1108 participants were recruited into the 17 trials. The number of participants in each trial ranged from 25 (Ozkan 2004) to 112 (Kneafsey 1994). Only 10 studies reported the sex distribution of participants (Abdel Sabour 2018; Adolfsson 1996; Farzad 2014; Geetha 2014; Gulke 2018; Ozkan 2004; Poorpezeshk 2018; Rigo 2017; Stenekes 2009; Trumble 2010). Further, while some studies reported the sex distribution at baseline, others reported those available at follow‐up or analysis. From the 608 participants for which data were available, 74% were male. Age data were reported in 13 studies (Abdel Sabour 2018; Adolfsson 1996; Farzad 2014; Geetha 2014; Gelberman 1991; Gulke 2018; Ozkan 2004; Poorpezeshk 2018; Rigo 2017; Silva 2003; Stenekes 2009; Trumble 2010; Vialaneix 2003); see Table 5. The distribution of ages for those studies in which it was reported ranged from 7 years (Ozkan 2004) to 72 years (Rigo 2017). Five studies reported including children (Abdel Sabour 2018; Farzad 2014; Geetha 2014; Ozkan 2004; Trumble 2010). However, no studies focused specifically on rehabilitation interventions for children.
Nine studies focused on flexor tendon injuries in flexor tendon zone II (Adolfsson 1996; Farzad 2014; Geetha 2014; Gelberman 1991; Gulke 2018; Hagberg 2000; Silva 2003; Trumble 2010; Vialaneix 2003). One study included injuries in zone I or II (Scavenius 2000); three studies included zones I to III (Kneafsey 1994; Poorpezeshk 2018; Rigo 2017); one study included zone I to V injuries (Ozkan 2004); one study included only zone V injuries (Uday Raj 2018) and two studies included injuries in all flexor tendon zones (Abdel Sabour 2018; Stenekes 2009).
In two studies, participants contributed one digit each with one or two tendon lacerations; participants with multiple digit lacerations were not included (Scavenius 2000; Silva 2003). In nine studies, participants contributed one or more than one digit to the study (Adolfsson 1996; Farzad 2014; Geetha 2014; Gelberman 1991; Hagberg 2000; Ozkan 2004; Poorpezeshk 2018; Rigo 2017; Trumble 2010). In the remaining six studies, it was unclear if participants with more than one digit or tendon lacerations were included in the study (Abdel Sabour 2018; Gulke 2018; Kneafsey 1994; Stenekes 2009; Uday Raj 2018; Vialaneix 2003).
Intervention and comparisons
The trials presented findings across different treatment interventions. Ten studies focused on our main comparison examining exercise regimens with the same or different orthosis designs (Abdel Sabour 2018; Farzad 2014; Hagberg 2000; Kneafsey 1994; Rigo 2017; Scavenius 2000; Silva 2003; Trumble 2010; Uday Raj 2018; Vialaneix 2003). Mobilisation regimens tested included:
early active flexion plus controlled passive exercise regimen versus early controlled passive exercise regimen (modified Kleinert protocol) (Rigo 2017);
early active flexion plus passive exercise regimen (Strickland and Small protocol) versus controlled passive exercise regimen (Kleinert protocol) (Vialaneix 2003);
active flexion plus active extension exercise regimen versus passive flexion plus active extension exercise regimen (Scavenius 2000);
active flexion exercise regimen versus controlled passive exercise regimen (Hagberg 2000);
active exercise regimen versus immobilisation regimen (Silva 2003);
early place and hold progress to tendon gliding exercise regimen (multiple treatments) versus early passive progressed to active exercise regimen (multiple treatments) (Uday Raj 2018);
place and hold exercise regimen versus controlled passive exercise regimen (Abdel Sabour 2018; Farzad 2014; Trumble 2010);
early passive flexion exercise regimen (modified Duran protocol) versus early controlled passive exercise regimen (modified Kleinert protocol) (Kneafsey 1994).
Other interventions included duration of rehabilitation programme and return to unrestricted activities (Adolfsson 1996); devices such as an exoskeleton (Gulke 2018) and a continuous passive motion device (Gelberman 1991); ultrasound therapy (Geetha 2014); laser therapy (Ozkan 2004; Poorpezeshk 2018) and motor imagery (Stenekes 2009). Rehabilitation interventions varied in intensity, duration and setting.
Outcomes
The outcomes measured in each trial are summarised in an outcome matrix in Table 6.
3. Outcome matrix showing the outcomes reported for the individual trials.
| Study ID | Function: patient‐reported | Active ROM | Adverse event | Passive ROM | Strength | Return to work | Function: objective measure |
Quality of life |
Satisfaction |
| Abdel Sabour 2018 | X | X | X | X | |||||
| Adolfsson 1996 | X | X | X | X | X | ||||
| Farzad 2014 | X | X | |||||||
| Geetha 2014 | X | X | X | ||||||
| Gelberman 1991 | X | X | |||||||
| Gulke 2018 | X | X | X | X | X | ||||
| Hagberg 2000 | X | X | |||||||
| Kneafsey 1994 | X | X | |||||||
| Ozkan 2004 | X | X | X | ||||||
| Poorpezeshk 2018 | X | X | X | ||||||
| Rigo 2017 | X | X | X | X | |||||
| Scavenius 2000 | X | X | X | ||||||
| Silva 2003 | X | X | |||||||
| Stenekes 2009 | X | X | X | ||||||
| Trumble 2010 | X | X | X | X | X | X | |||
| Uday Raj 2018 | X | X | X | ||||||
| Vialaneix 2003 | X | X | X |
Primary outcome measures
Six of the 17 studies reported our primary outcome of interest, functional status using a patient‐reported outcome measure. Three studies used a subjective assessment of overall function using a visual analogue scale (VAS) (Adolfsson 1996; Rigo 2017; Stenekes 2009). One study used the MHQ (Stenekes 2009) and three studies used the DASH outcome measure (Abdel Sabour 2018; Gulke 2018; Trumble 2010).
All studies but one (Poorpezeshk 2018) measured our primary outcome of interest, active ROM. However, ROM was reported using several different classification systems that are based on goniometric ROM measurements used to calculate categories, from a poor to an excellent overall outcome. These include the Strickland‐Glogovac (Strickland 1980), Strickland or Modified Strickland (Strickland 1985), Tang (Tang 2007), International Federation of Societies for Surgery of the Hand (IFSSHP) (Silva 2003); Lousville (Lister 1977), Tsuge (Tsuge 1977) and Buck‐Gramcko (Buck‐Gramcko 1976) classifications. Total active motion (TAM) (Kleinert 1983; ASSH 1976) calculates the total active range of motion of the digits including the metacarpophalangeal (MCP), proximal interphalangeal (PIP) and distal interphalangeal (DIP) joints. Three classification systems (Strickland‐Glogovac, Strickland, Tang) categorise outcomes as excellent, good, fair/satisfactory, poor or failure for zone II injuries (Table 7). They calculate the sum of active ranges of motion of the PIP and DIP joints (total flexion minus extension deficits). Motion is reported as a percentage of the contralateral side. If the contralateral PIP and DIP joint is not measured, the total is assumed to be 175 degrees. The IFSSH classification is similar but instead of the contralateral ROM the total active movement is calculated as a percentage of total passive movement. Classification systems (Lousville, Tsuge, Buck‐Gramcko) that are based on measurement of movement using fingertip to palm distance were not included as an outcome of interest in our review due to the lack of standardisation of this outcome measure and inherent subjectivity in its measurement. The Lousville classification system (Lister 1977) incorporates the extension deficit as well as movement measured in cm of the distance from the fingertip to the distal palmar crease. The Tsuge classification (Tsuge 1977) measures the distance between finger pulp and distal palmar crease and the angle of each joint with the fingers in maximum flexion. The Buck‐Gramcko system (Buck‐Gramcko 1976) incorporates range of motion, total extension lag and fingertip to nail distance; and has separate grading systems for digits and thumb. Where a classification system is based purely on goniometric measurement (e.g. Strickland classification systems), we report on the number of participants who had a 'poor' outcome. This is due to the inconsistency in cut‐off measurements used to classify 'good' to 'excellent' outcomes. Flexion contractures, extension deficits and joint / tendon lags are also evaluated using ROM measured with a goniometer and have been reported as adverse events for the purpose of this review.
4. Outcome classifications using active ROM.
| Classification system | Outcome (% motion achieved) | Excellent | Good |
Fair or satisfactory |
Poor | Failure |
| Strickland‐Glogovac (Strickland 1980) |
% active PIP + DIP ROM (active flexion ‐ extension deficit) compared to contralateral side or 175 degrees* | 85% to 100% | 70% to 84% | 50% to 69% | 0% to 49% | Not applicable |
| ROM (in degrees) | > 150 | 125 to 149 | 90 to 124 | < 90 | Not applicable | |
| Strickland or Modified Strickland (Strickland 1985) |
% active PIP + DIP ROM (active flexion ‐ extension deficit) compared to contralateral side or 175 degrees* | 75% to 100% | 50% to 74% | 24% to 49% | 0% to 24% | Not applicable |
| ROM (in degrees) | > 132 | 88 to 131 | 45 to 87 | < 44 | Not applicable | |
| Tang*** (Tang 2007) |
% active PIP + DIP ROM compared to contralateral side or 175 degrees* | 90% to 100% | 70% to 89% | 50% to 69% | 30% to 49% | < 30% |
| IFSSH (Silva 2003) |
% Total active motion compared to total passive motion** | 75% to 100% | 50% to 74% | 24% to 49% | 0% to 24% | Not applicable |
| Total Active Motion (ASSH 1976) |
% active MCP + PIP + DIP ROM (active flexion ‐ extension deficit) compared to contralateral side | Normal | > 75% | 50% to 75% | < 50% | < pre‐surgery |
DIP: distal interphalangeal; IFFSH: International Federation of Societies for Surgery of the Hand; MCP: metacarpophalangeal; ROM: range of movement; PIP: proximal interphalangeal
*The sum of active ROM of the PIP and DIP joints is calculated in degrees. This is done by adding the flexion achieved at the PIP and DIP joints and subtracting any extension deficits from the total. Motion is reported as a % of the contralateral side. It is assumed that 175 degrees is the sum of motion of the normal PIP and DIP joints of the unaffected side. % motion achieved = ((PIP + DIP flexion) ‐ (PIP + DIP extension deficit)) X 100 divided by 175.
** % motion achieved = (Total active motion X 100) divided by Total passive motion.
***According to Tang, the excellent and good scores are further divided into plus and minus sub‐grades based on their grip strength and quality of motion (investigator’s subjective judgement of the coordination, visual arc and speed of motion).
The studies that reported ROM using the different classification systems include: TAM (Farzad 2014; Gelberman 1991; Hagberg 2000; Scavenius 2000; Stenekes 2009; Uday Raj 2018), Strickland‐Glogovac classification (Gelberman 1991; Rigo 2017 ), Strickland classification (Farzad 2014; Geetha 2014; Gulke 2018; Ozkan 2004; Scavenius 2000; Silva 2003; Vialaneix 2003), Lousville classification(Adolfsson 1996; Vialaneix 2003; Uday Raj 2018), Tang classification (Rigo 2017), Tsuge classification (Adolfsson 1996) and Buck‐Gramcko classification (Adolfsson 1996; Ozkan 2004). The mean values for active ROM for individual joints was provided by two studies (Gulke 2018; Hagberg 2000). Individual joint active ROM for the affected digit's interphalangeal joints for each participant was provided by only one study (Gelberman 1991). One study used another non‐validated, non‐standardised classification system that was not clearly described in the paper (Kneafsey 1994). While Silva and colleagues (Silva 2003) stated they used the IFSSH system, the classification system reported was not referenced in the paper.
Fifteen of the studies reported our primary outcome of interest, adverse events (Abdel Sabour 2018; Adolfsson 1996; Farzad 2014; Geetha 2014; Gelberman 1991; Gulke 2018; Hagberg 2000; Ozkan 2004; Poorpezeshk 2018; Rigo 2017; Scavenius 2000; Silva 2003Trumble 2010; Uday Raj 2018; Vialaneix 2003). Adverse events were not reported in two studies (Kneafsey 1994, Stenekes 2009). Tendon rupture was the only adverse event consistently reported in all of the studies that reported adverse events. One study reported the timing of the ruptures (Rigo 2017) and two studies reported the intra‐operative findings of the cause of the ruptured tendon (Rigo 2017; Silva 2003). In the fifteen studies that clearly reported the presence or absence of tendon ruptures, there were a total of 41 tendon ruptures reported ranging between zero ruptures in three studies (Farzad 2014; Poorpezeshk 2018; Uday Raj 2018) to a maximum of 13 ruptures (Hagberg 2000). It was difficult for the review authors to determine how many secondary surgeries were performed in total, as several papers reported rupture as an adverse event, but did not clearly state whether these patients went on to have secondary repairs or reconstructions of the tendon ruptures. Secondary surgeries for ruptured tendons were reported by three studies (Rigo 2017; Trumble 2010; Vialaneix 2003). One study reported that one patient underwent a DIP fusion (Vialaneix 2003). Another common secondary surgery performed after a flexor tendon repair is a tenolysis, in which scar tissue is removed to allow the tendon to move freely. Only three studies reported the number of participants undergoing tenolysis (Silva 2003; Scavenius 2000; Vialaneix 2003). In these three studies, a total of 21 patients required a tenolysis. Few studies reported other adverse events including rates of infections (Gelberman 1991), delays in post‐operative wound healing (Rigo 2017; Geetha 2014 ), or diagnosis of chronic regional pain syndrome / Sudeck's disease (Rigo 2017; Gulke 2018). Flexion contracture or extension deficit was reported in five studies (Abdel Sabour 2018; Geetha 2014; Gulke 2018; Hagberg 2000; Trumble 2010).
Secondary outcome measures
Passive finger ROM using goniometric measurement was reported in one study (Poorpezeshk 2018).
Hand grip strength was reported in nine studies (Adolfsson 1996; Geetha 2014; Gulke 2018; Kneafsey 1994; Ozkan 2004; Rigo 2017; Scavenius 2000; Stenekes 2009; Uday Raj 2018).
Return to activity (including return to work) was addressed in three studies. Absence from work was recorded and compared between the two groups in one study (Adolfsson 1996). Mean and range for number of days to return to full duties without restriction was reported for each group in one study (Trumble 2010). Mean and range of days to return to work for all participants regardless of group was reported in one study (Vialaneix 2003).
Functional assessment using an objective measure was reported in one study, which used both the Jebsen‐Taylor hand function score and the Purdue Pegboard dexterity test (Trumble 2010).
Quality of life using a self‐report measure was not reported by any study.
Satisfaction with the intervention provided and whether the patients would recommend the treatment was investigated in one study (Gulke 2018). Another study measured patient satisfaction with Laser/placebo intervention using an analogue scale from 1 (dissatisfied) to 7 (completely satisfied) (Poorpezeshk 2018). Two studies reported a similar construct, satisfaction with hand function after the surgery using an analogue scale (Abdel Sabour 2018; Trumble 2010).
We obtained additional data not reported in study publications from trial authors of the following studies: Adolfsson 1996; Geetha 2014; Ozkan 2004; Rigo 2017. A number of attempts to contact authors of the other 13 trials for clarification were unsuccessful.
Excluded studies
We excluded 12 studies after review of the full‐text publication (Bainbridge 1994; Baktir 1996; Horsfall 2016; ISRCTN80184286; Kingston 2014; NCT01939808; Peck 1998; Peck 2014; Percival 1989; Stegink Jansen 1990; Xiao 2018; Yildirim 2010). Two studies (Kingston 2014; Xiao 2018) were excluded as they included participants who may have had a flexor tendon injury; however, separate data were not available for only the participants with flexor tendon injuries. Two studies found through a trial registry were abandoned before recruitment started (ISRCTN80184286; NCT01939808). The main reason for excluding the other eight studies was because a non‐randomised study design was used.
Studies awaiting classification
Four studies are awaiting classification pending either clarification from the authors or translation to English (Kitis 2009; Liu 2004; Naude 2019; Yavari 2009). Kitis 2009 compared early active mobilisation versus passive mobilisation but it is unclear whether it is a randomised trial. Liu 2004 appears to have randomised 62 children to a multi‐component intervention group consisting exercises with an occupational therapist‐play or to a control group that received general rehabilitation. This article, which was identified after editorial review, requires translation from Chinese. Naude 2019 was a pilot feasibility study that compared graded active digital flexion with a modified Duran protocol focusing on passive digital flexion in 31 participants with zone II, later extended to include zone I, II and IV flexor tendon injuries of the hand. There was a substantial loss to follow‐up, leaving only 14 participants at eight weeks follow‐up. We retained this recently identified trial in this section given its currently minimal contribution to the evidence base. Yavari 2009 examined active mobilisation commenced at 14 days versus four weeks of immobilisation in 240 people, but requires multiple clarifications, including whether this study is a randomised trial (no evidence in support of this description); an imbalance in the numbers allocated; and incorrect data and percentages that do not compute to whole numbers (indicating that these data may be incorrect).
Ongoing studies
Details of seven ongoing studies are given below:
1. CTRI/2019/01/016821: trial registered in January 2019 comparing conventional hand therapy versus an assistive device for a three month period (2 sessions per day for 60 minutes) in 120 participants in India. The main outcomes are active flexion and total active movement measured at 3 and 6 weeks, and 3, 6 and 12 months. The listed secondary outcomes include grip and pinch strength, and function using the Quick‐DASH at the same time points. This study has not yet been completed.
2. IRCT201310138177N8: trial registered in November 2013 in Iran randomising 20 participants between the ages of 15 and 60 years to either an active or passive mobilisation regimen. Although this study is reported as being completed, we have not found a published report. Outcomes include adverse events, range of motion, grip and pinch strength.
3. IRCT20150721023277N7: trial registered in August 2018 comparing early active to passive exercise regimen for zone I and II flexor tendon repairs in participants over the age of 12 years. This is randomised parallel intervention trial aiming to recruit 30 participants is being conducted in Iran and will have the following primary outcomes: satisfaction with ADL tasks and hand function. The listed secondary outcomes include: DASH, fine motor dexterity, range of motion, grip strength, and pinch strength.
4. NCT03812978: trial registered in February 2019 randomised 101 participants following FDP tendon repair in Sweden to either a smart phone application programme to improve exercise adherence or a control group. Both groups receive a standard hand therapy programme. According to the trials register, this study has completed recruitment but no data have yet been reported. The primary outcomes are related to adherence. The listed secondary outcomes include self‐efficacy and range of motion.
5. NCT03850210: trial registered in June 2015 and is now completed, but no data are yet available. This UK‐based trial randomised 60 participants over age 16 years with zone II flexor tendon repair to either a traditional long forearm based splint or a Manchester short splint that allows wrist movement. Primary outcome includes range of motion, and secondary outcome includes adverse events.
6. NCT04237415: trial registered retrospectively in January 2020, which states that recruitment for the trial has been completed. This trial, undertaken at UMUT ERASLAN, Pamukkale University, Turkey, randomised 22 participants to either EMG biofeedback or a control group. Primary outcome includes finger joint range of motion (measured at 6, 12, and 24 weeks), electrical muscle activity (at 5, 12, and 24 weeks), grip strength (12 and 24 weeks), and the Michigan Hand Questionnaire (at 5, 12 and 24 weeks).
7. NCT04385485: trial registered retrospectively in May 2020, for which trial recruitment has been completed. This Swedish trial randomised 64 participants aged 16 years and older who have had either zone I or II tendon injuries to either active rehabilitation or passive rehabilitation groups. Primary outcome includes range of motion (measured at 4, 8 and 12 weeks, and 6 and 12 months). Secondary outcomes include grip strength and pinch strength (at 6 and 12 months), adverse events including tendon rupture, need for secondary operation (within the first 12 months), function measured using the DASH (at 3, 6 and 12 months).
Risk of bias in included studies
Full details of the 'Risk of bias' assessments are presented in the Characteristics of included studies. Summaries are presented in Figure 2and Figure 3. For studies in which we rated a risk of bias as unclear, we attempted to contact the trial authors to request clarification or additional data. Where authors provided additional information or data, this has been recorded in the Characteristics of included studies. Additional information on study methods and data used in the risk of bias assessment was obtained from (Adolfsson 1996; Geetha 2014; Ozkan 2004; Rigo 2017).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We rated generation of the randomisation sequence to be at low risk of bias in 10 trials (Abdel Sabour 2018; Adolfsson 1996; Farzad 2014; Geetha 2014; Gulke 2018; Ozkan 2004; Poorpezeshk 2018; Rigo 2017; Trumble 2010; Uday Raj 2018). Three of these trials used a random envelope draw of cards created for the study (Adolfsson 1996; Rigo 2017; Trumble 2010). One trial used a computerised random number generator (Farzad 2014); two studies used stratified block randomisation (Gulke 2018; Poorpezeshk 2018); one used random card selection (Geetha 2014); one used a random number table (Ozkan 2004); and one study used a lottery system (Uday Raj 2018). One study used a quasi‐randomisation sequence by allocating participants to groups based on whether they were born in an even or odd number month (Gelberman 1991). Six studies did not clearly report the randomisation process used (Hagberg 2000, Kneafsey 1994, Scavenius 2000; Silva 2003; Stenekes 2009; Vialaneix 2003), and attempts to obtain this information from trial authors were unsuccessful.
Allocation concealment was rated to be at low risk of bias in five trials (Abdel Sabour 2018; Adolfsson 1996; Geetha 2014; Rigo 2017; Trumble 2010). Allocation concealment was rated to be at high risk of bias in Gelberman 1991, where participants were placed in one of two study groups depending upon the month in which they were born. The remaining 11 studies were rated as having unclear risk of bias, as they did not report a clear method for concealing the allocation sequence (Farzad 2014; Gulke 2018; Hagberg 2000; Kneafsey 1994; Ozkan 2004; Poorpezeshk 2018; Scavenius 2000; Silva 2003; Stenekes 2009; Uday Raj 2018; Vialaneix 2003). Attempts to clarify this with trial authors were unsuccessful.
Blinding
Five studies were rated as having low risk of performance bias (Farzad 2014; Geetha 2014; Gelberman 1991; Ozkan 2004; Scavenius 2000). Three studies achieved blinding of both participants and study personnel for self reported outcomes and were rated as having low risk of bias as these studies did not include any patient self reported outcomes (Farzad 2014; Geetha 2014; Gelberman 1991). One study (Ozkan 2004) achieved blinding of the participants, but it is unlikely that the personnel were blinded due to the nature of the intervention. Scavenius 2000 was rated as having low risk of bias as none of the outcomes were self‐reported or measured by the treatment provider. Eight included studies were not able to achieve participant blinding due to the nature of interventions and were rated as having a high risk of bias (Abdel Sabour 2018; Adolfsson 1996; Gulke 2018; Rigo 2017; Silva 2003; Stenekes 2009; Trumble 2010; Vialaneix 2003). Four studies (Hagberg 2000; Kneafsey 1994; Poorpezeshk 2018; Uday Raj 2018) were classified as having an unclear risk of bias regarding blinding of participants and personnel. One study (Uday Raj 2018) reported that participants were blinded from which group they were in, but were aware they were receiving one of the interventions. Due to the nature of the intervention, care providers could not be blinded to the intervention. It is not known how successful the blinding of the participants was considering the care providers could not be blinded. It was classified as unclear risk as attempts to clarify this with trial authors were unsuccessful. Another study with an unclear rating was Poorpezeshk 2018, which stated in the clinical trials registry that the study was "double‐blinded". However it is unclear where this blinding occurred. Due to the nature of the interventions, it is possible that the participants could have been blinded to the interventions, but this was not explicitly stated.
Blinding of outcome assessment (detection bias) was assessed in two categories, for self‐reported measures and measurements recorded by observers. For self‐reported measures, where these were available, blinding was considered to be low risk for Ozkan 2004 only. It was unclear whether blinding was achieved in two studies that did have self‐reported outcomes (Abdel Sabour 2018; Poorpezeshk 2018). In these instances, an explicit statement regarding assessor blinding was not included in the trial description, and attempts to clarify this issue with trial authors were unsuccessful. The risk was considered to be high for five studies (Adolfsson 1996; Gulke 2018; Rigo 2017; Stenekes 2009; Trumble 2010). The other nine studies did not include any self‐reported outcomes.
Blinding of outcome assessment (detection bias) for objective outcomes was deemed to be low risk of bias in four included studies (Farzad 2014; Geetha 2014; Ozkan 2004; Poorpezeshk 2018). It was unclear whether blinding of outcome assessors was achieved in nine studies (Abdel Sabour 2018; Gulke 2018; Hagberg 2000; Kneafsey 1994; Scavenius 2000; Silva 2003; Stenekes 2009; Uday Raj 2018; Vialaneix 2003). In these instances, an explicit statement regarding assessor blinding was not included in the trial description, and attempts to clarify this issue with trial authors were unsuccessful. The risk of bias from unblinded outcome assessors was considered to be high for four studies (Adolfsson 1996; Gelberman 1991; Rigo 2017; Trumble 2010). Adolfsson 1996 only blinded the assessors for the final outcome assessment at 24 weeks, but not at the earlier time points of assessment. Blinding of outcome assessors was not undertaken in the other three studies, which clearly reported that measurement of outcomes was performed by the treating therapists (Gelberman 1991; Rigo 2017; Trumble 2010).
Incomplete outcome data
Seven studies did not clearly report if any of the participants were lost to follow‐up (Gelberman 1991; Hagberg 2000; Kneafsey 1994; Ozkan 2004; Scavenius 2000; Silva 2003; Vialaneix 2003). Of the 10 studies that reported the loss to follow‐up of the participants, three included trials achieved complete follow‐up of the data set (Farzad 2014; Stenekes 2009; Uday Raj 2018). Only five studies reported number of participants lost to follow‐up for each group (Abdel Sabour 2018; Geetha 2014; Poorpezeshk 2018; Rigo 2017; Trumble 2010). Two studies (Adolfsson 1996; Gulke 2018) reported the total number of participants in the study lost to follow‐up (i.e. no specific reporting of the number lost in each group). The time interval when the loss to follow‐up occurred was not clearly reported. The biggest loss to follow‐up was noted by Poorpezeshk 2018, in which 20 participants were lost to follow‐up, all from the control group. Studies were examined for attrition bias at three time intervals: less than three months, three to six months, and greater than six months.
For outcomes measures under three months, we rated five studies as having low risk of bias (Abdel Sabour 2018; Farzad 2014; Geetha 2014; Rigo 2017; Uday Raj 2018). Seven studies were rated as unclear risk either due to insufficient data being provided in the study publications and no further data being received from the trial authors upon request (Gulke 2018; Hagberg 2000; Kneafsey 1994; Ozkan 2004; Stenekes 2009; Trumble 2010; Vialaneix 2003). Two studies did not collect data at this time point (Gelberman 1991; Silva 2003). We rated two studies at high risk of bias (Adolfsson 1996; Poorpezeshk 2018). In Adolfsson 1996, the number of participants and digits contributed to the study was provided via correspondence from the study authors. The 82 participants who were included in the analysis did not include 14 drop‐outs, eight of whom were lost to follow‐up. It is unclear from which group the 14 drop‐outs were excluded from or the reasons for lost to follow‐up. In Poorpezeshk 2018, the loss to follow‐up was 34% in the control group (with no dropouts in the intervention group), which may have influenced the effect size.
For outcomes measured between three and six months, we rated two studies as having low risk of bias (Geetha 2014; Rigo 2017). We rated six as having unclear risk of bias due to insufficient data being provided in the study publications and no further data being received from the trial authors upon request (Abdel Sabour 2018; Gulke 2018; Hagberg 2000; Kneafsey 1994; Trumble 2010; Vialaneix 2003). One study, Adolfsson 1996, was rated as having high risk of bias, as it is not clear how the dropouts were accounted for in the analysis or which groups they were in.
Only six studies measured outcomes beyond six months (Gelberman 1991; Hagberg 2000; Rigo 2017; Scavenius 2000; Silva 2003; Trumble 2010). Of these, two were rated to be at low risk of bias for completeness of outcome data (Rigo 2017; Trumble 2010). Three studies rated as having an unclear risk of bias did not provide sufficient information in their publications and attempts to obtain this were unsuccessful (Hagberg 2000; Scavenius 2000; Silva 2003). Gelberman 1991 was rated to be at high risk of bias as it was not clear how many participants dropped out of the study, and only those who were a minimum of six months following surgery were included.
Selective reporting
We rated two studies as having a low risk of bias for selective reporting (Abdel Sabour 2018; Poorpezeshk 2018). We rated seven studies as having an unclear risk of bias for selective reporting due to insufficient information being provided in the publications for the studies, and attempts to obtain this information from the trial authors were unsuccessful (Farzad 2014; Gelberman 1991; Gulke 2018; Ozkan 2004; Rigo 2017; Scavenius 2000; Trumble 2010). We assessed eight studies as having a high risk of bias for selective outcome reporting, as they did not specify results for some of the outcomes listed in the methods section of the publication, or in a published protocol (Adolfsson 1996; Geetha 2014; Hagberg 2000; Kneafsey 1994; Silva 2003; Stenekes 2009; Uday Raj 2018; Vialaneix 2003).
We compared the outcomes reported to their study design as reported on the clinical trial register where available. We were only able to retrieve trial registration documents for three studies (Abdel Sabour 2018; Geetha 2014; Poorpezeshk 2018).
Other potential sources of bias
We rated six studies to be at high risk of other bias (Abdel Sabour 2018; Geetha 2014; Rigo 2017; Scavenius 2000; Stenekes 2009; Uday Raj 2018), mainly because of unit of analysis errors. However, the unit of analysis was not always clearly stated when reporting outcomes in some of the included studies. Some measurements like grip and pinch strength are recorded per hand, whereas other outcomes may be reported per digit or per tendon repair (that is, one or more tendons can be repaired in the same digit). This means that it is very easy for a unit of analysis error to occur and can lead to errors in analysis and misleading interpretation of the study's findings. Five studies clearly reported the unit of analysis for their outcome measures and four were assessed as being at low risk of bias (Adolfsson 1996; Farzad 2014; Gelberman 1991; Trumble 2010). The other study, Rigo 2017, was rated at high risk of other bias, as strength was analysed as a finger level outcome, rather than hand/participant level outcome; hence, a unit of analysis error occurred. Eight studies were rated as having an unclear risk of bias, due to insufficient information being provided to make a judgement (Gulke 2018; Hagberg 2000; Kneafsey 1994; Ozkan 2004; Poorpezeshk 2018; Silva 2003; Uday Raj 2018; Vialaneix 2003). Four other studies were identified as being at high risk of bias and it appears as if an unit of analysis error was likely (Abdel Sabour 2018; Geetha 2014; Hagberg 2000; Stenekes 2009). Like Rigo 2017, Geetha 2014 analysed grip strength for each of the digits included in the study; however, grip strength is calculated per hand/participant and this likely to have resulted in an unit of analysis error. Similarly, it is unclear whether the number of ruptures that occurred were per person, digit or tendon in Hagberg 2000. In Stenekes 2009, pinch strength was measured for each affected digit; however, the unit of analysis appears to be affected tendons. In Abdel Sabour 2018, the unit of analysis is tendons not fingers; however, measurements such as range of motion, scar adhesion and DASH are measured at the finger or person level. A unit of analysis error appears to have occurred for these outcomes as analyses appear to have been conducted per tendon.
Geetha 2014 additionally reported outcomes of movement and strength in non‐standardised categories. These ranges for movement and strength were not uniform between the groups and not recorded at the same time interval. This made it difficult to compare the outcomes between the groups. Further, Rigo 2017 recorded grip and pinch strength as a percentage of the contralateral side, with no consideration made in the analysis for hand dominance.
Scavenius 2000 was rated as high risk as the intervention groups also received different surgical treatments which may have influenced the results.
Effects of interventions
See: Table 1; Table 2; Table 3
We were able to perform very limited meta‐analyses because of clinical heterogeneity or incomplete outcome reporting of the included trials. Summary data and effect estimates (with 95% CIs) for all trials are presented where available. If an outcome is not referred to in a subsection or table, then no data for that outcome were available in the trials. If there is no analysis reported for a particular outcome for a comparison, trialists reported insufficient data (e.g. no standard deviations for continuous outcomes) to perform the required analysis. Our attempts to obtain missing data were generally unsuccessful. Where possible, we have provided as much detail as possible from the original source, for transparency and to enhance interpretation of the findings.
There were no trials testing the following comparison categories that were prespecified as 'main comparisons' in the protocol.
different types of orthoses;
different orthosis wearing regimens, including duration;
different timings for commencing mobilisation;
different types of scar management;
different timings for commencing strengthening.
We created 'Summary of findings' tables for the following three exercise regimen comparisons, which were selected as they are commonly used in current clinical practice (Bigorre 2018; Tang 2018b).
Early active flexion plus controlled passive exercise regimen versus early controlled passive exercise regimen (modified Kleinert protocol) (Table 1).
Active exercise regimen versus immobilisation regimen (Table 2).
Place and hold exercise regimen versus controlled passive exercise regimen (Table 3).
Suitability of trials for meta‐analysis
Most comparisons were tested by single trials only. Of the two comparisons tested by two and three trials respectively, we considered it appropriate to pool only one outcome (tendon rupture).
In addition, 11 (of 17) trials reported data that could not be included in the statistical analysis, for several reasons:
omission of measures (or errors) of variability (e.g. SDs) in reports of continuous outcomes (Adolfsson 1996; Hagberg 2000; Poorpezeshk 2018; Scavenius 2000; Trumble 2010; Vialaneix 2003);
conclusions stated without support for point estimates, or frequency counts of outcomes (Hagberg 2000; Stenekes 2009);
lack of reporting of the time point of measurement (Stenekes 2009);
conclusion stated with no point estimate, or data for either group (Adolfsson 1996; Hagberg 2000; Kneafsey 1994; Scavenius 2000; Trumble 2010; Uday Raj 2018; Vialaneix 2003); and,
data were skewed and reported in a format not able to be entered into RevMan 5 (Abdel Sabour 2018); and,
comparison of data between groups were measured at different time points or used different outcome definitions (Geetha 2014).
Early active flexion plus controlled passive exercise regimen versus early controlled passive exercise regimen alone
One trial (Rigo 2017), which randomised 53 participants with 73 zone I to III FDP tendon repairs, evaluated the addition of active flexion exercises to a controlled passive exercise regimen comprising a modified Kleinert regimen, which was used in both intervention groups. Both active and passive only groups commenced the exercise regiments at one day post‐surgery. Outcomes were assessed post‐surgically at one and two months (for ROM only) and at 3, 6 and 12 months (for all outcome measures). The outcomes assessed included functional assessment using a patient reported outcome measure (injured finger use in activities of daily living (ADL) using a visual analogue scale); active finger range of motion (Strickland‐Glogovac and Tang classifications); adverse events (number of participants with tendon ruptures, complex regional pain syndrome, wound dehiscence, finger oedema) and strength (grip and pinch strength).
The main outcomes for this comparison, for which there is only very low‐certainty evidence, are presented in Table 1. For all outcomes, the evidence was downgraded two levels due to very serious risk of bias and one level due to serious imprecision, reflecting wide confidence intervals, confidence intervals crossing the line of no effect, or both. The evidence was also downgraded one level for serious indirectness for the functional assessment outcome used in this trial; there is also no information on the minimally important difference for this outcome. Unit of analysis problems resulting from the reporting by digit instead of participant means that the confidence intervals are narrower than they should be. Additionally, although the trial authors provided us with mean and SD data for continuous outcomes, it should be noted these were reported as median (Med) and interquartile range (IQR) data in the trial report. We also present the latter for the ADL results because there were greater disparities between these and the mean and SD data for this outcome.
Primary outcomes
-
Functional assessment using a patient reported outcome measure
-
The use of the injured finger(s) in ADL at 2, 3, 6 and 12 month follow‐ups was evaluated using a visual analogue scale (0 to 10; higher scores = better ADL). The Med and IQR results presented in the trial report are shown in Analysis 1.1, and the mean and SD results provided by the trial authors to us are shown, is in Analysis 1.2.
There were no significant between‐group differences at any time point reported by the authors. All differences between the two groups were under 1.0 point and thus unlikely to be clinically important.
The exploratory analyses in Analysis 1.2 indicated higher scores in the active group at two months, but not subsequently.
-
-
Active finger range of motion
Active range of motion was measured using a goniometer as a continuous outcome. Using the individual PIP and DIP joint measurements of the finger, these were summed to calculate TAM. From these data, Strickland‐Glogovac (Strickland 1980) and Tang (Tang 2007) classifications were calculated (refer to Table 7 for a definition for each of the classifications categories).
-
TAM of PIP and DIP joints (in degrees).
No evidence of between‐group differences were found between the two groups at any of the follow‐ups (Analysis 1.3).
-
Strickland classification (number of fingers that were classified as a "poor" category)
There were no significant differences between groups at any time interval (Analysis 1.4). Rigo 2017 also found no statistically significant differences in the distribution of participants in the four categories (excellent, good, fair and poor) for active range of motion for the finger digits at 2, 3, 6 or 12 months. The data at 12 months were excellent (20), good (8), fair (5) and poor (1) for the active group and excellent (14), good (6), fair (5) and poor (4) for the control group; reported P = 0.247.
-
Tang classification (number of fingers that were classified as a "failure" or "poor" result (Analysis 1.4).
There were no significant differences between groups at any time interval.
-
Adverse events
Overall, there were six adverse events reported in the active group versus nine adverse events in the control group (RR 0.58, 95% CI 0.23 to 1.44; Analysis 1.5).
-
Tendon rupture
Two FDP tendon ruptures occurred in the active group at weeks 5 and 6 respectively, whereas one FDP rupture was reported at week 12 in the control group. All ruptures underwent secondary surgery.
-
Complex regional pain syndrome
The only participant developing this adverse outcome was in the control group.
-
Wound dehiscence
Delayed wound healing or superficial infection was seen in six fingers in the control group versus none in the active group.
-
Swelling and tenderness of the tendon sheaf (all transitory)
This was seen in four fingers in the active group versus one in the control group.
1.1. Analysis.
Comparison 1: Early active flexion plus controlled passive exercise regimen versus early controlled passive exercise regimen (modified Kleinert protocol), Outcome 1: Self‐reported function for ADL using a VAS (0 to 10; higher = better)
| Self‐reported function for ADL using a VAS (0 to 10; higher = better) | ||||||
| Study | Follow‐up | Active Median (IQR) | Active N (digits) | Control Median; IQR | Control N (digits) | Reported P value |
| Rigo 2017 | At 2 months | 7.2 (3.0) | 36 | 6.5 (3.1) | 32 | P = 0.204 |
| At 3 months | 7.3 (3.6) | 36 | 7.7 (3.0) | 31 | P = 0.850 | |
| At 6 months | 8.8 (1.5) | 32 | 8.5 (3.5) | 30 | P = 0.942 | |
| At 12 months | 9.3 (1.2) | 34 | 8.8 (2.0) | 29 | P = 0.113 | |
1.2. Analysis.

Comparison 1: Early active flexion plus controlled passive exercise regimen versus early controlled passive exercise regimen (modified Kleinert protocol), Outcome 2: Self‐reported function using VAS for ADLs (0 to 10; higher scores = better); secondary analyses
1.3. Analysis.

Comparison 1: Early active flexion plus controlled passive exercise regimen versus early controlled passive exercise regimen (modified Kleinert protocol), Outcome 3: Active finger range of motion (degrees)
1.4. Analysis.

Comparison 1: Early active flexion plus controlled passive exercise regimen versus early controlled passive exercise regimen (modified Kleinert protocol), Outcome 4: Finger range of movement ‐ Poor outcome
1.5. Analysis.

Comparison 1: Early active flexion plus controlled passive exercise regimen versus early controlled passive exercise regimen (modified Kleinert protocol), Outcome 5: Adverse events
Secondary outcomes
One secondary outcome, strength, was reported for Rigo 2017 (Table 6).
-
Strength
-
Grip strength (percentage of the contralateral hand)
There was no evidence of important differences between two groups at 3, 6 or 12 months follow‐ups in grip strength (Analysis 1.6).
-
Pinch strength (percentage of the contralateral hand)
Pinch strength was better at three and six months in the active group; however, this advantage did not persist at 12 months (Analysis 1.6).
-
1.6. Analysis.

Comparison 1: Early active flexion plus controlled passive exercise regimen versus early controlled passive exercise regimen (modified Kleinert protocol), Outcome 6: Strength (% of the contralateral hand or digit)
Early active flexion plus passive exercise regimen (Strickland and Small protocol) versus controlled passive exercise regimen (Kleinert protocol)
One trial, Vialaneix 2003, randomised 35 participants who had a primary flexor tendon repair in zone II into two intervention groups listed in the heading. At the third post‐operative day, 16 participants randomised to the active group commenced early passive flexion, active flexion and active extension within the orthosis using the Strickland exercise regimen (Strickland 2000; Small 1989) and 19 participants commenced early controlled passive mobilisation using rubber band traction, according to the Kleinert regimen (Kleinert 1967).
Outcomes were measured at an average of 18 months following the surgery, but also reported at 8, 12 and 24 weeks. Outcomes measured included active finger range of motion (Strickland classification); fingertip to palm distance (not an outcome of interest for this review); adverse events (subsequent surgical procedures) and return to previous activity (total duration of time off work after the surgery for a subgroup of 15 manual workers).
This trial was reported as a conference abstract only and provided very limited and incomplete data. The certainly of the available evidence for this comparison is very low, reflecting downgrading two levels for very serious risk of bias and two levels for very serious imprecision.
Primary outcomes
-
Functional assessment using a patient reported outcome measure
Not reported
-
Active finger range of motion
-
Using the Strickland classification, the total percentages of participants achieving "good‐excellent" results in the two groups were reported at 8, 12 and 24 weeks. These are shown below. Some of the percentages do not correspond to whole numbers if the numbers randomised (16 versus 19) are used. The authors reported that the study did not show any significant difference in "functional outcome" between the two groups.
At 8 weeks (percentage of participants with good or excellent outcomes): 75% active group versus 95% controlled passive group (probably corresponds to 18/19 versus 12/16)
At 12 weeks (percentage of participants with good or excellent outcomes): 81% active group versus 94% controlled passive group
At 24 weeks (percentage of participants with good or excellent outcomes): 92% active group versus 100% controlled passive group
-
-
Adverse events
These were not reported. However, subsequent surgery required in eight participants comprised five tenolysis, one DIP fusion and two flexor tendon grafts due to late rupture. No breakdown was provided by treatment group.
Secondary outcomes
No secondary outcomes were reported for individual groups for Vialaneix 2003 (Table 6).
Active flexion plus active extension exercise regimen (plus modified Kessler suture surgical technique) versus passive flexion plus active extension exercise regimen (plus grasping suture and external pull‐out know surgical technique)
Scavenius 2000 randomised 39 participants with zone I and II flexor tendon repairs into either an active flexion plus active extension exercise regimen group (active group) or passive flexion plus active extension exercise regimen group (passive group). Each group also received a different surgical technique with the active group having a Modified Kessler suture (Ti‐cron 4.0) repair, and the passive group receiving a grasping suture (Prolene 2.0) and external pull‐out knot technique. Participants had flexor tendon repairs to the thumb (n = 6) or digits (n = 33) in flexor tendon zone I or II. The active group (n = 20) also performed active extension exercises but performed active flexion exercises (instead of passive) which was described as a Mantero protocol. The passive group (n = 19) performed active extension and controlled passive flexion exercises (using a protocol reported by study authors to have been developed "by May E. et al").
The outcomes assessed at one year following surgery included active finger range of motion (TAM and Strickland classification for the FDP repairs of the digits only; that is, this outcome was not calculated for the thumb tendon repairs) and adverse events (tendon ruptures, scar adhesions requiring tenolysis surgery).
This trial was reported as a conference abstract only and provided very limited and incomplete data. Data were missing for the number of participants in each group in the analyses and no measures of variability were provided for range of motion. The certainly of the available evidence for this comparison is very low, reflecting downgrading two levels for very serious risk of bias, two levels for very serious imprecision and one level for serious indirectness (as the groups received different surgical techniques).
Primary outcomes
-
Functional assessment using a patient reported outcome measure
Not reported.
-
Active finger range of motion
-
TAM for the digits (numbers in each group not reported)
Scavenius 2000 reported that there was no significant difference between the two groups in mean TAM as a percentage of normal values: active group was 70.1% compared with the passive group at 75.7%.
-
Strickland classification
Scavenius 2000 reported the differences in the percentages of participants in the four categories (excellent, good, fair and poor) for active range of motion for the finger digits was not significant. Data for the two groups were for excellent (25%), good (8%), fair (67%) and poor (0%) for the active group and excellent (27%), good (33%), fair (40%) and poor (0%) for the passive group.
-
-
Active thumb IP joint motion (numbers in each group not reported)
Scavenius 2000 reported the mean range of motion for the repaired thumb tendons was 51.7 degrees in the active group and 66.7 degrees in the passive group (between group difference reported as not significant: reported P = 0.7).
-
Adverse events.
-
Adverse events were recorded for each group were the number of participants who experienced a tendon rupture and the number of participants with scar adhesions that required surgery (tenolysis surgical procedure). Data for these are presented Analysis 2.1.
Tendon ruptures. All three tendon ruptures occurred in the active group.
Tenolysis surgery was required for four participants in the active group and two participants in the passive group.
-
2.1. Analysis.

Comparison 2: Early active flexion + active extension exercise regimen (+ modified Kessler suture surgical technique) versus passive flexion + active extension exercise regimen (+ grasping suture and external pull‐out knot surgical technique), Outcome 1: Adverse events
Secondary outcomes
No secondary outcomes were reported for Scavenius 2000 (Table 6).
Active flexion exercise regimen versus controlled passive exercise regimen
One trial, Hagberg 2000, which was reported in a published abstract only, randomised 100 participants who had direct tendon repair for zone II lacerations in 108 digits. They compared an early active mobilisation group (active group) with an early controlled passive mobilisation using rubber band traction group (controlled passive group). The exercise regimen and orthosis were continued for three weeks following the tendon repair, after which participants were allowed to commence active mobilisation. No further details on the trial participants, including the numbers in each group, were provided.
The outcomes reported in the abstract were limited to active finger range of motion (TAM and DIP joint flexion) and adverse events (tendon ruptures; extension deficit) at one year. The data were incomplete and unusable and it is unclear whether the results applied to participants or digits. The certainly of the available evidence for this comparison is very low, reflecting downgrading two levels for very serious risk of bias and two levels for very serious imprecision.
Primary outcomes
-
Functional assessment using a patient reported outcome measure
Not reported.
-
Active finger range of motion
-
TAM
The median range of motion was 245 degrees in the early active group versus 250 degrees in the controlled passive group. The authors stated there was no statistically significant difference between the groups.
-
DIP active motion
The median active motion was 65 degrees in the active group and 58 degrees in the controlled passive group. The authors stated there was no statistically significant difference between the groups.
-
-
Adverse events
-
Tendon ruptures
There were 13 tendon ruptures overall, five of which occurred in the active group and eight in the controlled passive group.
-
Extension deficit of the DIP joint.
The median extension deficit was 5 degrees in both groups.
-
Secondary outcomes
No secondary outcomes were reported for Hagberg 2000 (Table 6).
Active exercise regimen versus immobilisation regimen
One study, Silva 2003, compared an early active exercise regimen (active group) with an immobilisation regimen (immobilisation group) following a zone II flexor tendon repairs in 84 people (152 tendons). Both groups received a dorsal blocking orthosis to protect the flexor tendon repair for three weeks following surgery. The active group commenced an exercise programme 12 hours after surgery. The program consisted of 10 hourly motions of active flexion‐extension during a 16 hour waking day. The immobilisation group did not perform any exercises in the orthosis. After three weeks, the orthosis was discarded by both groups.
Outcomes were evaluated at a mean of 22 months post surgery (range 12 to 36 months). Outcomes assessed included finger range of motion (to calculate outcomes as per the IFSSH and Strickland classifications (described in Table 7) and adverse events (indication for tenolysis surgery; tendon ruptures and repair of these).
The main outcomes for this comparison, for which only very low certainty evidence is available, are presented in Table 2.
Primary outcomes
-
Functional assessment using a patient reported outcome measure
Not reported.
-
Active finger range of motion
Only the number of participants for each of the IFSSH or Strickland classification groups were reported. For the analysis, we present the numbers in the poor categories of the two outcomes. At 12 months or more post‐surgery, the active exercise group had fewer poor outcomes than the immobilisation group when rated using either the IFSSH (1/37 versus 15/47; RR 0.08, 95% CI 0.01 to 0.61) or Strickland's classification (0/37 versus 7/47; RR 0.08, 95% CI 0.00 to 1.43; very low‐certainty evidence); seeAnalysis 3.1.
-
Trial authors reported that according to the IFSSH classification (% of participants in this category(number of digits)):
Active exercise group: excellent 0% (n = 0); good 65% (n = 24); satisfactory 32% (n = 12); poor 3% (n = 1).
Immobilisation group: excellent 0% (n = 0); good 43% (n = 20); satisfactory 25% (n = 12); poor 32% (n = 15).
-
Trial authors reported the following according to the Strickland's classification (% of participants in this category(number of digits)):
Active exercise group: excellent 62% (n = 23); good 35% (n = 13); satisfactory 3% (n = 1); poor 0% (n = 0).
Immobilisation group: excellent 47% (n = 22); good 23% (n = 11); satisfactory 15% (n = 7); poor 15% (n = 7).
-
Adverse events
The overall number of participants experiencing one or more adverse events was not reported.
Tendon ruptures was reported in 13.5% of the participants in the active group (5 of 37 participants), all of which occurred in the second week after commencing active mobilisation (Analysis 3.2). All participants underwent immediate surgical rerepair performed in the same way as the primary tendon suture and were returned to the early active exercise regimen. No participants in the Immobilisation group experienced a tendon rupture.
3.1. Analysis.

Comparison 3: Active exercise regimen versus immobilisation regimen, Outcome 1: Range of movement at 12+ months: poor outcome
3.2. Analysis.

Comparison 3: Active exercise regimen versus immobilisation regimen, Outcome 2: Adverse events
-
Tenolysis surgery was not required by participants in the active group compared with 21% participants (10 of 47 participants) of the immobilisation group, whose range of motion deficit fulfilled the indication criterion for tenolysis (Analysis 3.2). However, it is not clear whether these went on to have tenolysis.
-
Actual or indicated secondary surgery for tendon rupture or a range of motion deficit indicating scar adhesions
Secondary surgery was either tendon repair in the active group or indication for tenolysis in the immobilisation group (5/37 versus 10/47; RR 0.64, 95% CI 0.24 to 1.70; very low‐certainty evidence; Analysis 3.2).
Secondary outcomes
One secondary outcome was reported for Silva 2003 (Table 6).
-
Passive finger range of motion
Although measured to calculate the IFFSH classification, these data were not reported separately in the publication.
Early place and hold progressed to tendon gliding exercise regimen versus early passive progressed to active exercise regimen
One randomised study of 30 participants with zone V flexor tendon repairs evaluated the benefits between two types of multiple treatment exercise regimens (Uday Raj 2018). One group received of early mobilisation with place and hold exercises commenced in the first week which progressed to a graduated tendon gliding exercise program at four weeks (place and hold group). The second intervention group received a passive exercise regimen which was progressed to full active exercises of all finger joints at four weeks (passive group). Both groups received the same standard hand therapy treatments and both were placed in dorsal blocking splints (although at different positions for the wrist‐ place and hold at 20 to 30 degrees and passive at 45 to 50 degrees).
Outcomes were evaluated at 4 and 12 weeks for range of motion; and at 12 weeks for grip strength. Outcomes included active finger range of motion (TAM); active finger tip to distal palmar crease distance (not an outcome of interest for this review); wrist active range of motion (not an outcome of interest for this review); adverse events (tendon ruptures) and strength (grip strength measured using a Jamar Dynamometer). The certainly of the available evidence for this comparison is very low, reflecting downgrading by two levels for very serious risk of bias, two levels for very serious imprecision for tendon rupture and one level for serious indirectness, reflecting the inadequate description of outcome assessment.
Primary outcomes
-
Functional assessment using a patient‐reported outcome measure
Not reported.
-
Active finger range of motion
Data for active ROM was reported as a percentage difference in mean values of TAM between the 12 week and initial measurement at four weeks, and reported for individual digits (i.e. index, middle, ring and small digit data were reported separately). Requests to the trial authors for more information, including final ROM data, were unsuccessful.
-
Adverse events
Only tendon rupture was reported; there were none in either group.
Secondary outcomes
One secondary outcome was reported for Uday Raj 2018 (Table 6).
-
Strength
Grip strength was measured at 12 weeks post surgery using a Jamar hand‐held dynamometer, and reported as the percentage difference in strength between the participant's affected hand and their unaffected hand. The place and hold group had significantly greater difference between their affected and non affected sides than the passive group (MD 6.90%, 95% CI 4.86 to 8.94; Analysis 4.1).
4.1. Analysis.

Comparison 4: Early place and hold progressed to tendon gliding exercise regimen versus early passive progressed to active exercise regimen, Outcome 1: Grip strength at 12 weeks (% difference between normal and affected hands)
Place and hold exercise regimen versus controlled passive exercise regimen
Three heterogeneous trials compared place and hold exercise (place and hold group) versus controlled passive exercise (controlled passive group) (Abdel Sabour 2018; Farzad 2014; Trumble 2010).
Abdel Sabour 2018 recruited 33 participants (45 tendons) following a 2‐strand flexor tendon repair; this study included zones I, II and II flexor tendon injuries. Both groups were placed in a dorsal blocking orthosis with the wrist positioned in 20 degrees flexion and MCP joints in 70 degrees flexion. Exercises commenced three days following the repair. The place and hold exercise regimen consisted of passive flexion of the affected finger and then the participant tried to maintain the flexed posture through contraction of the involved muscle for five seconds. Controlled passive exercise regimen consisted of passive finger flexion achieved by the modified Kleinert rubber band traction system. Both groups performed additional active extension plus passive range of motion of each digit. The place and hold group were allowed to progress to active wrist tenodesis glides but the time interval has not been specified. They performed 25 repetitions of each exercise every waking hour for the first six weeks post‐surgery. At six weeks, the orthosis was discarded. Outcomes, which were assessed from six weeks up to six months, included functional assessment using a patient reported outcome measure (DASH score); adverse events (tendon rupture; scar adherence; flexion contracture; extension lag) and satisfaction with the result of the surgery.
Farzad 2014 randomised 54 participants (64 digits; 108 tendons) with zone II flexor tendon injuries into the two exercise regimen groups. Participants in both groups were placed in a dorsal blocking orthosis with their wrist positioned at 0 to 30 degrees of flexion and their MCP joints in 70 to 90 degrees flexion. Exercises were commenced three days following a 2‐strand flexor tendon repair. Participants of the place and hold group were advised to passively flex their fingers using the other hand with their wrist in 30 degrees of extension (out of the orthosis), and then hold the finger position actively for 3 to 5 seconds, performing 10 repetitions four times a day. In the controlled passive group, flexion was caused by rubber band traction within the dorsal blocking orthosis. Patients performed active finger extension within the dorsal blocking orthosis, performing 10 repetitions every waking hour. At three weeks, both groups progressed to active exercises. Outcomes, which were assessed at eight weeks by an independent blinded assessor, included active finger range of motion (TAM) of PIP and DIP joints combined, Strickland classification (described in Table 7) and adverse events (tendon ruptures).
Trumble 2010 randomised 103 participants (119 digits) who had undergone 4‐strand zone II flexor tendon repair to a place and hold regimen using a tenodesis orthosis or to a passive exercise regimen using an orthosis with rubber band traction. Participants of the place and hold group were placed in a dorsal blocking orthosis for six weeks and were also provided with a tenodesis orthosis (using a wrist hinge) to perform exercises during the first four weeks post‐operation. Place and hold finger exercises were initiated on day three, active flexor tendon gliding exercises at week four, and composite wrist and finger flexion exercises were introduced at week five post‐surgery. Participants of the controlled passive group performed a combination of Kleinert and Duran protocols. This included being placed in a rubber band traction orthosis and coming out of the orthosis to perform passive flexion‐extension and active interphalangeal extension during the first three weeks post‐operation. Place and hold exercises were introduced at three weeks post‐operation and active finger flexion commenced at six weeks. Range of motion and flexion contracture were evaluated at 6, 12, 26 and 52 weeks. All other outcomes were assessed at one year post‐surgery. These included functional assessment using a patient‐reported outcome measure (DASH score); active finger range of motion (PIP and DIP joints); adverse events (tendon ruptures, flexion contracture); return to previous activity (total days from injury to return to work on full duties); functional assessment using an objective measure (Jebsen‐Taylor hand function score, Perdue Pegboard test); satisfaction with the result of the surgery (numerical analogue scale from 1 (dissatisfied) to 10 (completely satisfied).
The main outcomes for this comparison, for which only very low certainty evidence is available, are presented in Table 3.
Primary outcomes
-
Functional assessment using a patient reported outcome measure (DASH score, 0 to 100 with a higher score indicating more disability); see Analysis 5.1.
-
This was reported in two trials but at different times (6 months and 12 months).
Reflecting the non‐normal distribution of the results, Abdel Sabour 2018 reported Med and IQR and found no evidence of a difference between the two groups at six months: place and hold group Med 23, IQR 2 to 26; passive group Med 15, IQR 10 to 30; reported P = 0.62. They also reported mean and standard deviations in their discussion, which are shown in an exploratory analysis, again showing no evidence of a difference between the two groups (MD ‐1.10, 95% CI ‐14.44 to 12.24; 26 participants; very low‐certainty evidence; downgraded one level for serious risk of bias and two levels for very serious imprecision).
Trumble 2010 found no important difference in function between the two groups at one year (MD ‐1.10, 95% CI ‐2.77 to 0.57; 89 participants; very low‐certainty evidence; downgraded two levels for very serious risk of bias and one level for imprecision, reflecting potential ceiling effects given the very low DASH scores indicating minimal upper limb disability).
-
-
Active finger range of motion; see Analysis 5.2
-
Active finger range of motion was reported using a continuous range of motion outcome (TAM) in Abdel Sabour 2018 and Trumble 2010, and also as a dichotomous outcome by categorising the continuous outcome into four categories (Poor; Good; Satisfactory; Excellent) using the Strickland Classification System in Abdel Sabour 2018. Active range of motion was not directly reported in Abdel Sabour 2018 but goniometry data would have been used to assess contracture and deformity (see adverse events).
Farzad 2014 found the TAM at PIP and DIP joint was significantly better in the place and hold group compared with the controlled passive group at eight weeks (MD 32.00 degrees, 95% CI 15.5 to 48.5; 64 digits; very low certainty evidence).
Trumble 2010 found greater active range of motion in the place and hold group at 6, 12, 26 weeks and, as shown in Analysis 5.2, at 52 weeks: MD 28.00 degrees, 95% CI 18.87 to 37.13; 102 digits; very low certainty evidence). The number of participants or digits included in the analyses for the 6, 12 and 26 week time points were not reported.
In Farzad 2014, no digits in the place and hold group had a "poor" outcome according to the Stickland classification system compared with nine in the controlled passive group at eight weeks (0/31 versus 9/33; RR 0.06, 95% CI 0.00 to 0.92; Analysis 5.3). The numbers of digits in each category were as follows: place and hold group: excellent: 45% (n = 14); good 32% (n = 10); satisfactory 23% (n = 7); poor 0% (n = 0); controlled passive group: excellent: 24% (n = 8); good 18% (n = 6); satisfactory 30% (n = 10); poor 27% (n = 9).
-
Adverse events
5.1. Analysis.

Comparison 5: Place and hold exercise regimen versus Controlled passive exercise regimen, Outcome 1: Function (self‐reported): DASH scores (0 to 100; higher score = more disability)
5.2. Analysis.

Comparison 5: Place and hold exercise regimen versus Controlled passive exercise regimen, Outcome 2: Range of movement (Total Active Movement) (degrees)
5.3. Analysis.

Comparison 5: Place and hold exercise regimen versus Controlled passive exercise regimen, Outcome 3: Range of movement (Strickland Criteria) at 8 weeks: Poor outcome
-
The available data for these, reported by tendons (digits) rather than participants, are presented in Analysis 5.4. There were no data for overall numbers of participants incurring one or more adverse events. Only Abdel Sabour 2018 reported on adverse events other than tendon rupture.
Three tendon ruptures were reported for Abdel Sabour 2018, none in Farzad 2014 and four in Trumble 2010. There was no evidence of a difference between groups (3/96 participants or tendons versus 4/100; RR 0.81, 95% CI 0.19 to 3.50; 196 participants). All four participants in Trumble 2010 underwent surgery to re‐repair the tendons using a two‐stage surgical reconstruction; they were all excluded from final analysis.
Abdel Sabour 2018 reported no tendons had scar adherence in the place and hold group compared with 14 in the controlled passive group (0/16 versus 14/20; RR 0.04, 95% CI 0.00 to 0.66). Specific criteria for assessing scar adherence were not provided in this study and it is unclear whether further intervention was undertaken.
Abdel Sabour 2018 measured flexion contracture at DIP joint and PIP joint and then as a combined DIP + PIP joint measure. The results probably applied to follow‐up at six weeks.There were fewer DIP joint contractures in the place and hold group: 1/16 versus 10 of 20 tendons (RR 0.13, 95% CI 0.02 to 0.88); as well as fewer PIP joint contractures in the place and hold group (3/16 versus 8/20; RR 0.47, 95% CI 0.15 to 1.48). Abdel Sabour 2018 reported significantly lower combined DIP and PIP flexion deformity, measured in degrees, at six weeks in the place and hold group but we are unsure of these data and have not reported these here. Although assessed in Trumble 2010, data for flexion contractures were not reported.
Tendon lag was calculated in Abdel Sabour 2018 by measuring the difference between the passive and active range of motion at the PIP joint and DIP joint to calculate the FDS and FDP tendon lag respectively. Presence of a lag was reported (number of patients with lag as a percentage of participants in that group). Tendon lag is generally reported as an arc of motion (in degrees).There were fewer digits with FDP tendon lag in the place and hold group (2/16 versus 15/20; RR 0.25, 95% CI 0.06 to 0.98) as well as there were fewer digits with FDS tendon lag (2/16 versus 10/20; RR 0.17, 95% CI 0.04 to 0.62).
5.4. Analysis.

Comparison 5: Place and hold exercise regimen versus Controlled passive exercise regimen, Outcome 4: Adverse events
Secondary outcomes
Two secondary outcomes were reported for Abdel Sabour 2018, three for Trumble 2010 but none for Farzad 2014 (Table 6). The evidence was of very low certainty, downgraded at least one level for serious risk of bias and two levels for very serious imprecision, for all secondary outcomes.
-
Passive finger range of motion
This was measured and used to calculate the tendon lag for Abdel Sabour 2018, as reported in adverse events above, but separate data were not provided.
-
Return to previous activity
Excluding the four participants with tendon ruptures, Trumble 2010 reported that the participants in the place and hold group returned to full‐duty work without restriction significantly earlier (P < 0.05) with an average of 82 days (range 68 to 94 days) compared with an average of 103 days (range 76 to126 days) in the controlled passive group.
-
Functional assessment using objective measures
At one year, Trumble 2010 found little difference between the groups in the results of the Jebsen‐Taylor test (MD ‐3.00 seconds, 95% CI ‐9.38 to 3.38; Analysis 5.5) or Purdue pegboard scores (MD 1.00 pegs, 95% CI ‐2.54 to 4.54, Analysis 5.6).
-
Satisfaction with the result of the surgery
Patients’ satisfaction with their hand function after surgery at three months or longer was evaluated by Abdel Sabour 2018 using a numerical analogue scale ranging from 0 (completely dissatisfied) to 10 (completely satisfied). Reflecting the non‐normal distribution of the data, Abdel Sabour 2018 reported the following findings for 26 participants: place and hold group Med 6, IQR 4 to 8.5; passive group Med 4, IQR 2 to 7; reported P = 0.049.
Trumble 2010 found little difference between the two groups in satisfaction scores, rated using a numerical rating scale from 1 (dissatisfied) to 10 (completely satisfied), at one year (MD 1.20, 95% CI ‐0.50 to 2.90; Analysis 5.7).
5.5. Analysis.

Comparison 5: Place and hold exercise regimen versus Controlled passive exercise regimen, Outcome 5: Function (observed): Jebsen Taylor at 52 weeks (seconds)
5.6. Analysis.

Comparison 5: Place and hold exercise regimen versus Controlled passive exercise regimen, Outcome 6: Function (observed): Purdue pegboard at 52 weeks (pegs)
5.7. Analysis.

Comparison 5: Place and hold exercise regimen versus Controlled passive exercise regimen, Outcome 7: Satisfaction with hand function (0 to 10: complete satisfaction) at 6 months
Early passive flexion exercise regimen (modified Duran protocol) versus early controlled passive exercise regimen (modified Kleinert protocol)
Kneafsey 1994 compared controlled passive flexion exercise regimen using a modified Duran, Strickland and Glogovac protocol (passive group) with a controlled passive flexion plus active extension using a modified Kleinert protocol exercise regimen (controlled passive group) in 112 participants with either zones I, II or III tendon lacerations. Participants in the passive group performed isolated and composite flexion in the orthosis without the rubber band traction, and both active and passive extension within the orthosis. Participants in the controlled passive group performed active extension exercises, and fingers were maintained in passive flexion using rubber band traction orthosis between exercises. The sole report of this trial was a published conference abstract reporting an interim analysis of the first 80 participants.
Outcomes measured, up to a possible six months, included active finger range of motion and strength (power grip, pinch grip and maximum finger pressure) measured using a Jamar dynamometer. No data were provided and the timing of the follow‐up to which statistical significance testing applied is unclear.
Primary outcomes
-
Functional assessment using a patient reported outcome measure
Not reported.
-
Active finger range of motion
The authors reported that there was no statistical significance difference in outcome between the two groups (P > 0.05).
-
Adverse events
Not reported.
Secondary outcomes
One secondary outcome was reported for Kneafsey 1994 (Table 6).
-
Strength
The authors reported that there was no statistical significance difference in outcome between the two groups (P > 0.05).
Unrestricted activity at 8 weeks post‐surgery versus unrestricted activity at 10 weeks post‐surgery
Adolfsson 1996 randomised 96 participants (106 digits) with zone II flexor tendon lacerations to either unrestricted hand activity commenced at eight versus ten weeks following surgery. Results were reported for 82 participants (91 digits). All participants received standardised interventions for the first six weeks, which included a forearm based orthosis extending to the PIP joint; initial four weeks of passive flexion with rubber band traction and active extension exercises within the orthosis; followed by two weeks of active exercises within the orthosis. The participants were then randomised at six weeks into the two different programmes that advised a gradual increase in loading to unrestricted activities to be commenced at either eight weeks (8‐week group) or ten weeks (10‐week group).
In Adolfsson 1996, active range of motion data and distance from the fingertip and middle of the pulp to the distal palmar crease data were used to calculate the Buck‐Gramcko (Buck‐Gramcko 1976), Louisville (Lister 1977) and Tsuge (Tsuge 1977) classification systems for the fingers; and the Buck‐Gramcko score for FPL repairs (Buck‐Gramcko 1976). None of these results were relevant to this review. Other outcome measures recorded at 16 weeks included subjectively rated functional assessment of hand function using a visual analogue scale; strength (grip strength using a Jamar dynamometer) and return to previous activity (whether they were absent from work or not). The authors did not provide measures of variability for continuous outcomes.
The certainty of the available evidence for this comparison is very low, reflecting downgrading by two levels for very serious risk of bias and two levels for very serious imprecision.
Primary outcomes
-
Functional assessment using a patient‐reported outcome measure
Subjective assessment of function, which was measured using a participant's subjective assessment of their own hand function using a visual analogue scale, was equivalent in both groups with a mean value of 88%.
-
Active finger range of motion
Comparison between the two groups of active ROM was recorded by the trial authors using three different classification scales, none of which are relevant to our review, as they do not rely solely on goniometric measurement. However, Adolfsson 1996 found no significant differences between the groups at any time interval.
-
Adverse events
-
Tendon rupture
One FDP tendon in the 8‐week group ruptured at seven weeks after the repair; no tendons ruptured in the 10‐week group (Analysis 6.1).
-
6.1. Analysis.

Comparison 6: Unrestricted activity at 8 weeks post‐surgery versus unrestricted activity at 10 weeks, Outcome 1: Adverse event (tendon rupture)
Secondary outcomes
Two secondary outcomes were reported for Adolfsson 1996 (Table 6).
-
Strength (percentage of the strength of the contralateral hand)
Adolfsson 1996 reported the mean grip strength at 16 weeks post surgery was 72% of the uninjured hand in the 8‐week group compared with 76% in the 10‐week group.
-
Return to previous activity
Of those participants working at the time of injury, 31 participants in the 8‐week group returned to work on average 2.1 weeks earlier than the 29 participants in the 10‐week group.
Exoskeleton versus physiotherapy
One randomised study (Gulke 2018) evaluated the benefit of applying an exoskeleton (exoskeleton group) compared with physiotherapy (physiotherapy group) following zone II flexor tendon repairs of both the FDP and FDS tendons in the index, middle or ring fingers in 62 participants. All participants were placed in a modified Kleinert orthosis with rubber band traction on the second day post surgery and commenced active and passive (as required) extension of the fingers within the orthosis (10 repetitions/hour). From the second week post‐surgery, patients were randomised to the exoskeleton group or the physiotherapy group. The exoskeleton group had the device applied by the physiotherapist for 30 minutes three times a week. No other treatments were provided. The physiotherapy group received physiotherapy treatment but the type of treatment and dose was not specified. It is assumed that the physiotherapy intervention was likely to be multi‐modal, consisting of various treatments. Both groups received treatment three times a week until function was deemed by the doctor to be satisfactory.
Outcomes were evaluated at 6, 12 and 18 weeks post‐surgery. Outcomes evaluated included functional assessment using a patient reported outcome measure (DASH score); active finger range of motion (isolated PIP and DIP joint ROM; TAM of the MCP, PIP and DIP joint; Strickland classification); adverse events (extension deficit; tendon ruptures; complex regional pain syndrome); strength (grip and pinch strength); satisfaction with the rehabilitation intervention (not an outcome of interest for this review). Please refer to Table 7 for a description of how the categories for the Strickland Classification (Strickland 2005) were defined in this study.
This article was published in German, and a translation of the article was obtained.
The certainty of the evidence is very low, being downgraded by two levels for very serious risk of bias and one or two levels for serious or very serious imprecision.
Primary outcomes
-
Functional assessment using a patient reported outcome measure
-
The DASH score was used to measure function and disability. This score ranges from 0 to 100 with a higher score indicating more disability.
Whilst both groups improved over time, there is very low‐certainty evidence of no clinically important between‐group differences at either 12 weeks (MD ‐2.80, 95% CI ‐7.63 to 2.03; 59 participants) or 18 weeks (MD ‐0.70, 95% CI ‐4.53 to 3.13); see Analysis 7.1.
-
-
Active finger range of motion
-
Isolated PIP joints and the DIP joints (as well as MCP joints, which are not the focus of this review) of the affected finger were measured and reported. These measurements were summed to calculate TAM of the affected finger.These measurements were also used to categorise the participants into four outcome groups (Poor; Fair; Good; Excellent) according to the Strickland classification (Strickland 2005).
PIP joint active finger range of motion: there is very low‐certainty evidence of little or no between‐group difference in this outcome at any time interval (Analysis 7.2).
DIP joint active finger range of motion: there is very low‐certainty evidence of little or no between‐group difference in this outcome at any time interval (Analysis 7.3).
TAM of the affected finger: there is very low‐certainty evidence of little or no between‐group difference in this outcome at any time interval (Analysis 7.4).
None of the participants in either group had a poor rating according to the Strickland classification at 18 weeks post‐surgery. In the exoskeleton group, the results were: excellent 80% (n = 24); good 18% (n = 5); satisfactory 3% (n = 1); poor 0% (n = 0). In the physiotherapy group, the results were: excellent 62% (n = 18); good 17% (n = 5); satisfactory 20% (n = 6); poor 0% (n = 0).
-
-
Adverse events
-
Three types of adverse events were recorded: tendon ruptures, diagnosis of complex regional pain syndrome (CRPS) and extension deficit. Data for the first two outcomes are shown in Analysis 7.5.
One participant of the physiotherapy group suffered a tendon rupture.
One participant of the exoskeleton group was diagnosed with CRPS at six weeks.
The number of participants with extension deficit at the PIP joint was not stated. This outcome was reported only as a continuous outcome (degrees of deficit).
-
7.1. Analysis.

Comparison 7: Exoskeleton versus physiotherapy, Outcome 1: Function (self‐reported): DASH scores (0 to 100; higher score = more disability)
7.2. Analysis.

Comparison 7: Exoskeleton versus physiotherapy, Outcome 2: Active finger range of motion of PIP joint (degrees)
7.3. Analysis.

Comparison 7: Exoskeleton versus physiotherapy, Outcome 3: Active finger range of motion of DIP joint (degrees)
7.4. Analysis.

Comparison 7: Exoskeleton versus physiotherapy, Outcome 4: Active finger range of motion (Total Active Movement) (degrees)
7.5. Analysis.

Comparison 7: Exoskeleton versus physiotherapy, Outcome 5: Adverse events
Secondary outcomes
One secondary outcome was reported for Gulke 2018 (Table 6).
-
Strength
-
Grip strength (kg)
At 18 weeks, there were no significant differences between groups (Analysis 7.6).
-
Pinch strength between thumb and injured finger (kg)
At 18 weeks, there were no significant differences between groups (Analysis 7.6).
-
7.6. Analysis.

Comparison 7: Exoskeleton versus physiotherapy, Outcome 6: Strength at 18 weeks
Continuous passive motion device versus controlled passive progressed to active exercise regimen (Modified Kleinert)
Gelberman 1991, a quasi‐randomised trial of 51 participants (60 digits) with zone II flexor tendon repairs, compared continuous passive motion (CPM) device (CPM group) with controlled passive exercise regimen using rubber band traction (controlled passive group). Participants began therapy on the first postoperative day. Digits were protected in dorsal‐blocking orthosis for a minimum of six weeks. The CPM group had the device attached to the protective orthosis on the first day following surgery to allow 60 degrees arc of PIP joint and a 30 to 40 degree arc of DIP joint motion. The CPM group used the device in isolation for 8 to 12 hours a day in the first four weeks. Active movements were introduced at four weeks to be performed in addition to the motion provided by the CPM device. The controlled passive group was placed in a similar dorsal blocking orthosis but with rubber band traction was applied. Patients performed active extension and passive flexion to the palmar crease for the first four weeks, when active flexion was introduced. Gelberman 1991 noted frequent problems with power failures and mechanical breakages of the CPM devices early on as well as issues with adherence; these were alleviated by increased time in patient education, both initially and at intervals throughout the rehabilitation period.
Outcomes were assessed by treating therapists at a minimum of six months post surgery (mean 10.8 months, range: 6 to 38 months). Outcomes included active finger range of motion (TAM and Strickland‐Glogovac classification (Strickland 1980)) and adverse events (infection; tendon rupture).
The evidence for all reported outcomes was of very low certainty, downgraded by two levels for very serious risk of bias and two levels for very serious imprecision.
Primary outcomes
-
Functional assessment using a patient‐reported outcome measure
Not reported.
-
Active finger range of motion
-
TAM was calculated by summing the total active motion of the MCP, PIP and DIP joints at six months post‐surgery. This was then used to categorise participants into groups according to the Strickland‐Glogovac classification (see below).
There is very low certainty evidence of higher TAM values in the CPM group (MD 20.48 degrees, 95% CI 0.89 to 40.07; 59 digits; Analysis 8.1).
-
Fewer digits in the CPM group had a poor outcome according to the Strickland‐Glogovac classification (1/29 versus 8/30; RR 0.13, 95% CI 0.02 to 0.97; Analysis 8.2). The data for each of the categories are detailed below:
CPM group (% of the entire group in this category(number of digits)): excellent 35% (n = 10); good 31% (n = 9); fair 31% (n = 9); poor 3% (n = 1).
Controlled passive group (% of the entire group in this category(number of digits)): excellent 27% (n = 8); good 23% (n = 7); fair 23% (n = 7); poor 27% (n = 8).
-
-
Adverse events
-
Gelberman 1991 reported on tendon rupture and infections for each group (Analysis 8.3).
One participant in the controlled passive group ruptured both FDP and FDS tendons at four weeks post‐surgery and underwent immediate secondary surgery. His data were not included in the final analysis.
No infections occurred in either group.
-
8.1. Analysis.

Comparison 8: Continuous passive motion device versus controlled passive progressed to active exercise regimen (Modified Kleinert), Outcome 1: Active finger range of motion (degrees)
8.2. Analysis.

Comparison 8: Continuous passive motion device versus controlled passive progressed to active exercise regimen (Modified Kleinert), Outcome 2: Range of movement at > 6 months (Strickland criteria) ‐ poor outcome
8.3. Analysis.

Comparison 8: Continuous passive motion device versus controlled passive progressed to active exercise regimen (Modified Kleinert), Outcome 3: Adverse event
Secondary outcomes
No secondary outcomes were reported for Gelberman 1991 (Table 6).
Ultrasound therapy versus control
One study of 106 participants (139 digits with zone II flexor tendon injuries), Geetha 2014, compared the effect of daily ultrasound therapy lasting five minutes on the repair site in the initial three weeks of rehabilitation versus a control group. Both groups were immobilised a dorsal plaster of Paris cast for three weeks with wrist in neutral and MCP joints in 70 degrees flexion and commenced the same mobilisation from three weeks. Over the five‐year duration of the trial, three different ultrasound regimens with different frequencies, intensities and timing were tested in turn. Details of these are provided in Characteristics of included studies. These three groups were neither randomised nor concurrent and we have pooled the data from the three ultrasound groups in the analyses.
Outcomes assessed included change in active finger ROM (combined measurement summing PIP and DIP joint ROM between 3 to 12 weeks, measured in degrees; Strickland Classification (Strickland 1980) at 3 months); grip strength (measured at 12 weeks); and adverse events (wound dehiscence, tendon ruptures, extension lag). Grip strength was inappropriately reported for digits, not participants. The results for ROM were reported for involved fingers instead of participants and thus resulted in unit of analysis errors.
The evidence for all outcomes reported for this comparison was of very low certainty, reflecting downgrading by two levels for very serious risk of bias, one level for serious indirectness and at least one level for serious imprecision, given the low numbers of events and participants.
Primary outcomes
-
Functional assessment using a patient reported outcome measure
Not reported.
-
Active finger range of motion
Change in active ROM between three weeks and three months (PIP and DIP ROM combined), which was reported separately for the three ultrasound groups and control group, was presented as the summed mean totals of combined PIP and DIP joint flexion range or motion (TAM). These are entered into RevMan 5 as three subgroups. We split the numbers of the control group into three in order to provide a pooled estimate for this outcome, which favours ultrasound: MD 26.47 degrees, 95% CI 19.70 to 33.24; 131 digits; Analysis 9.1
9.1. Analysis.

Comparison 9: Ultrasound versus control, Outcome 1: Improvement in active range of movement (Difference between 3 and 12 weeks; Total Active Motion measured in degrees)
-
Trial authors reported that according to the Strickland's classification (% of participants in this category(number of digits)):
Ultrasound (91 digits): excellent 20% (n = 18); good 57% (n = 52); fair (satisfactory) 5% (n = 5); poor 18% (n = 16).
Immobilisation group (40 digits): excellent 5% (n = 2); good 20% (n = 8); fair (satisfactory) 10% (n = 4); poor 65% (n = 26).
Based on the above data, and adding the two cases with rupture as a poor outcome in the ultrasound group, there were fewer digits with a poor ROM outcome in the ultrasound group: 16/93 versus 26/40; RR 0.26, 95% CI 0.16 to 0.44; 133 digits; Analysis 9.2).
-
9.2. Analysis.

Comparison 9: Ultrasound versus control, Outcome 2: Active finger range of motion (Strickland classification) at 3 months ‐ Poor outcome
-
Adverse events
Adverse events included tendon ruptures and wound dehiscence which were reported as the number of participants with this adverse event in each group (Analysis 9.3). Data for extension lag deficits were not usable and hence the overall numbers of participants with adverse events were not calculated.
-
Wound dehiscence
Wound dehiscence was observed in four participants allocated to ultrasound. Ultrasound therapy was stopped and the four participants were excluded from the study. No control group participants experienced wound dehiscence.
-
Tendon rupture
Two participants allocated ultrasound experienced tendon rupture after three weeks.
Extension lag deficits were also measured by the trial investigators. However, the available data were not usable for the following reasons. The method for calculating extension lag was not defined and was used interchangeably with flexion deformities in the publication. Extension lag was also expressed in a non‐standardised format as ranges of extension deficit (measured goniometrically) and these ranges differed between the groups. The time intervals for when this motion was achieved also differed between the groups; some data were reported at eight weeks and some at 12 weeks.
9.3. Analysis.

Comparison 9: Ultrasound versus control, Outcome 3: Adverse events
Secondary outcomes
One secondary outcome was reported for Geetha 2014 (Table 6).
-
Grip strength
Grip strength was inappropriately reported as number of digits rather than participants achieving a certain percentage of contralateral grip strength. This percentage threshold also varied between groups. Thus the reported data are unusable.
Low level laser therapy versus control (placebo)
Two heterogeneous trials compared low level laser therapy with a placebo control (Ozkan 2004; Poorpezeshk 2018).
Ozkan 2004 randomised 25 participants (41 digits) with flexor tendon injuries in zones I to V to either GaAs laser therapy (laser group) or a placebo control group. All participants received the Washington rehabilitation program for 12 weeks after surgery. In the laser group, whirlpool and infrared GaAs diode laser of 100 Hz frequency was applied from day 8 to 21 post surgery in 21 digits (13 participants). The control group, 20 digits (12 participants) received the same intervention with the machine switched off (i.e. placebo). Review‐relevant outcomes, which were measured at 12 weeks post surgery, include: active finger range of motion (total active motion, Strickland classification (Strickland 1980)); adverse events (tendon ruptures); and grip strength.
Poorpezeshk 2018, which included 97 participants (114 fingers) with flexor tendon injuries in zones I to III, randomised between red and infrared low level laser therapy (laser group) and a placebo control group, to examine the adjuvant effect of low‐level laser therapy on recovery of tendon injury in patients. Post‐operative care appeared to have consisted of four weeks of immobilisation in a plaster brace. Ten sessions of laser or sham therapy were provided from the second post‐operative day, two to three times a week. The probe was placed over the repair site using contact method. Outcomes were assessed by two independent blinded assessors. Review‐relevant outcomes include adverse events (infection; tendon rupture) and weekly measurement of passive range of motion of the PIP and DIP joints. Final assessments were recorded at four weeks post‐surgery.
We rated the available evidence as very low certainty, downgraded one level for serious risk of bias and two levels for very serious imprecision.
Primary outcomes
-
Functional assessment using a patient reported outcome measure
Not reported in either trial.
-
Active range of motion
Total active motion was reported in Ozkan 2004 only. Ozkan 2004, which did not state how this outcome was derived, did not find evidence of a between‐group difference (MD ‐14.17 degrees, 95% CI ‐36.48 to 8.14; 41 digits; Analysis 10.1).
Strickland's classification (number of digits (% of total digits)): One digit in each group was rated as a "poor" outcome based on the Strickland classification (Analysis 10.2). Data for all groups Strickland categories for the laser group were: excellent 60% (n = 12); good 25% (n = 5); fair 10% (n = 2); poor 5% (n = 1); and for the placebo group: excellent 58% (n = 11); good 16% (n = 3); fair 21% (n = 4); poor 5% (n = 1).
-
Adverse events
-
Between the two trials, two adverse effects, both tendon rupture, were observed in Ozkan 2004 and one, a wound infection, in Poorpezeshk 2018 (Analysis 10.3).
One participant of the laser group of Poorpezeshk 2018 experienced a wound infection, which was managed with conservative treatment.
One tendon rupture was reported in each group of Ozkan 2004; both were excluded from the analysis for other outcome measures. No tendon rupture was reported in either group of Poorpezeshk 2018.
-
10.1. Analysis.

Comparison 10: Low‐level laser therapy versus placebo control, Outcome 1: Active finger range of motion (Total active motion (degrees)) at 12 weeks
10.2. Analysis.

Comparison 10: Low‐level laser therapy versus placebo control, Outcome 2: Range of movement at 12 weeks: poor outcome
10.3. Analysis.

Comparison 10: Low‐level laser therapy versus placebo control, Outcome 3: Adverse events
Secondary outcomes
Two secondary outcomes were reported. These were passive finger range of motion reported in Poorpezeshk 2018 and strength in Ozkan 2004 (Table 6).
-
Passive finger range of motion
-
In Poorpezeshk 2018, total passive range of motion at the PIP and DIP joints were measured by a goniometer and recorded after the first session of laser therapy, and at weekly intervals until four weeks.
The trial investigators reported that at four weeks, total passive range of motion was greater in the laser group with a mean (SD) of 235 degrees (0.0) compared with 110 degrees(10) for the control group (P < 0.001). Given that an SD 0.0 is unlikely to be correct, these data should be considered unreliable.
-
-
Strength
In Ozkan 2004, grip strength was calculated as a percentage loss in the affected hand compared with the unaffected side at 12 weeks post surgery. There was very low certainty evidence of little difference between the two groups (MD ‐4.55, 95% CI ‐9.29 to 0.19; Analysis 10.4).
10.4. Analysis.

Comparison 10: Low‐level laser therapy versus placebo control, Outcome 4: Grip strength at 12 weeks (% lost using uninjured hand as comparison)
Motor imagery intervention versus control
Stenekes 2009, which included any zone of flexor tendon injury, compared kinaesthetic motor imagery of finger flexion movements (motor imagery group) with a control group in 25 participants. Standard care provided to both groups included six weeks using a Kleinert dorsal blocking orthosis; with only passive finger flexion allowed for the first four weeks and then 'place and hold' flexion exercises from four to six weeks. During these six weeks, the motor imagery intervention comprised eight sessions of motor imagery (10 repetitions of mental active flexion, held for three seconds followed by imagined finger extension and stretch) on a daily basis.
Outcomes included functional assessment using a patient‐reported outcome measure (MHQ; a single item question on hand skills using VAS); active finger range of motion (TAM); and strength (grip and pinch strength). Stenekes 2009 also measured preparation time of finger flexion and kinematic analysis including drawing accuracy and speed (which were not outcomes of interest of this review). Final follow‐up was at 12 weeks. The authors did not provide data for functional assessment or ROM outcomes.
The evidence for all outcomes reported for this comparison was of very low certainty, reflecting downgrading by two levels for very serious risk of bias, and at least one level for serious imprecision, given the low numbers of participants.
Primary outcomes
-
Functional assessment using a patient‐reported outcome measure
Function was measured using two different questionnaires including the MHQ and a one‐item question on hand skills using a VAS. These were completed for each hand for each participant. Stenekes 2009 reported that there was no significant between group difference for either outcome.
-
Active finger range of motion
TAM was calculated per hand and recorded as a percentage of the other side. Stenekes 2009 reported that there was no significant difference between groups in TAM.
-
Adverse events
Not reported.
Secondary outcomes
One secondary outcome was reported for Stenekes 2009 (Table 6).
-
Strength
-
Grip strength
At 12 weeks, no significant difference in grip strength was found between the two groups (Analysis 11.1).
-
Pinch strength
At 12 weeks, no significant difference in pinch strength was found between the two groups (Analysis 11.1).
-
11.1. Analysis.

Comparison 11: Motor imagery versus control, Outcome 1: Strength (kg)
Subgroup and sensitivity analyses, and assessment of publication bias
We could not perform any subgroup analyses in this review. Clinical heterogeneity of interventions and outcomes, or paucity of specified subgroups, meant that these analyses were not possible. Furthermore, sensitivity analyses were not performed as no meta‐analyses were conducted. Also, we were unable to generate funnel plots to assess small study effects. We consider the risk of publication bias to be low as many of the published studies reported statistically non‐significant results. However, whilst it is possible that some unpublished studies with non‐significant results exist, their inclusion in the review would be unlikely to change our conclusions.
Discussion
Summary of main results
The objective of this systematic review was to determine the effectiveness and safety of various rehabilitation treatments to optimise outcomes following surgery for flexor tendon injuries of the hand compared with no treatment, a control or another rehabilitation treatment. We have considered the results of 17 studies investigating different rehabilitation interventions following flexor tendon surgeries of the hand in a total of 1108 mainly adult participants. Most studies focused on zone II flexor tendon repairs. Only four studies included participants with zones IV and V. Five studies included children as well as adult participants. Most of the participants (74% overall where reported) were male.
The 17 studies were heterogeneous with respect to the types of rehabilitation treatments provided, intensity, duration of treatment and the treatment setting. The comparisons were confined to two comparison categories listed in our protocol. Ten studies focused on our main comparison examining exercise regimens with the same or different orthosis designs. These studies mainly focused on the type of exercises and orthoses prescribed at commencement of the rehabilitation phase. The comparisons of the other seven studies fitted loosely in our final category testing 'different doses for interventions, other than orthosis wearing regimen' that we expanded to include comparisons of a non‐exercise rehabilitation intervention versus control (no or placebo intervention). We found no studies examining the effectiveness of other adjunctive treatments in the early phase of rehabilitation, such as scar management, early oedema management, wound care, orthosis types (especially wrist and finger joint positions and inclusion of the wrist were found.
We rated the evidence available for all reported outcomes of all comparisons as very low certainty, which means that we have very little certainty in the estimates of effect.
Different exercise regimens
The 10 studies testing mobilisation strategies were heterogeneous with respect to the types of rehabilitation treatments provided, intensity, duration of treatment and the treatment setting. Three of these studies, however, tested a similar comparison. We presented the findings of the following three exercise regimen comparisons in 'Summary of findings' tables, as they are commonly used in current clinical practice.
Early active flexion plus controlled passive exercise regimen versus early controlled passive exercise regimen (modified Kleinert protocol)
The evidence for the single trial making this comparison is presented in Table 1. The trial recruited 53 participants with mainly zone II flexor tendon repairs but reported data for a maximum of 69 fingers,Standard care was provided to all participants. There is very low‐certainty evidence of no clinically important difference between the two groups in patient‐rated function at 6 or 12 months follow‐up. The same applies to active finger range of motion at the two follow‐up times. There is very low‐certainty evidence of little difference in adverse events: the 15 adverse events comprised three tendon ruptures, six wound dehiscence, one complex regional pain syndrome and five transitory swelling and tenderness of the tendon sheaf. All three tendon ruptures (4.3%) underwent secondary surgery.
Active exercise regimen versus immobilisation regimen for three weeks
The evidence for the single trial making this comparison is presented in Table 2. The trial reports data for 84 participants with zone II flexor tendon repairs, The trial did not report on self‐rated function, on range of movement in the medium term (three to six months) or on numbers of participants experiencing one or more adverse events. The very low‐certainty evidence for poor (under one‐quarter of normal) range of finger movement at one to three years follow‐up reflects the trial's very few events, which means we are uncertain of the finding of zero cases in the active group versus seven cases in the immobilisation regimen. The same uncertainty applies to the finding of little difference between the two groups in adverse events or indicated for surgery. All five cases of tendon rupture (13.5% of 37) needing surgical repair occurred after two weeks in the active mobilisation group and all 10 cases of range of motion deficiency (21.3% of 47) indicating scar adhesion and need for tenolysis occurred in the immobilisation group.
Place and hold exercise regimen versus controlled passive exercise regimen
The evidence for the three heterogeneous trials making this comparison is presented in Table 3. The trials reported data for a maximum of 194 participants with mainly zone II flexor tendon repairs. The place and hold regimens using orthoses differed among the three trials as did the passive exercise regimens, although all used rubber band traction. The trials did not report on range of movement in the medium term (three to six months) or on numbers of participants experiencing one or more adverse events. The very low‐certainty evidence means we are uncertain of the findings of no difference in self‐rated function using the DASH between the two groups at six months (data from one trial) or at 12 months (data from one trial). There is very low‐certainty evidence from one trial of greater active finger range of motion at 12 months after place and hold. Secondary surgery data were not available; however, we considered that all seven recorded tendon ruptures would have required surgery.
Other exercise comparisons
There was limited and very low‐certainty evidence available for the following five comparisons, each of which was tested by small single trials. In the following, we focus on the outcomes for which data for primary outcomes were presented in an analysis.
One trial of 35 participants with zone II flexor tendon repairs compared early active flexion plus passive exercise regimen (Strickland and Small protocol) versus controlled passive exercise regimen (Kleinert protocol). No usable data were available for this comparison, which was published only as a conference abstract.
One trial of 39 participants with zone I and II flexor tendon repairs compared active flexion plus active extension exercise regimen versus passive flexion plus active extension exercise regimen. Although presented in an analysis, the number of adverse events of tendon rupture (3) and need for tenolysis surgery (4) are too few to draw any conclusions. This trial was published only as a conference abstract.One trial of 100 participants with zone II flexor tendon repairs compared active flexion exercise regimen versus controlled passive exercise regimen. No usable data were available for this comparison, which was published only as a conference abstract,
One trial of 30 participants with zone V flexor tendon repairs compared early place and hold progress to tendon gliding exercise regimen (multiple treatments) versus early passive progressed to active exercise regimen (multiple treatments). No usable data for primary outcomes were available for this comparison.
One trial reporting an interim analysis of 80 participants with zones I, II or III tendon lacerations compared early passive flexion exercise regimen (modified Duran protocol) versus early controlled passive exercise regimen (modified Kleinert protocol). No usable data were available for this comparison.
Other comparisons tested in the included trials
The other interventions tested were the duration of rehabilitation programme and return to unrestricted activities (one trial); devices such as an exoskeleton (one trial) and a continuous passive motion device (one trial); ultrasound therapy (one trial); laser therapy (two trials) and motor imagery (one trial). Rehabilitation interventions varied in intensity, duration and setting. All the evidence for the limited outcome results available for these comparisons was rated as very low‐certainty evidence. In the following, we focus on the outcomes for which data for primary outcomes were presented in an analysis.
One trial reporting results for 96 participants with zone II flexor tendon repairs compared unrestricted activity at 8 weeks post‐surgery versus unrestricted activity at 10 weeks post‐surgery. The only results presented in an analysis was one tendon rupture.
One trial of 62 participants with zone II flexor tendon repairs compared applying an exoskeleton versus physiotherapy reported results at 12 and 18 weeks; thus short‐ and medium term follow‐up. For both follow‐up times, there is very low‐certainty evidence of no clinically important between‐group differences in function assessed using DASH or in active finger range of motion. There was one case of tendon rupture and one of complex regional pain syndrome.
One quasi‐randomised trial of 51 participants with zone II flexor tendon repairs compared a continuous passive motion device (CPM) versus controlled passive progressed to active exercise regimen. There is very low‐certainty evidence of marginally higher total active range of motion values in the CPM group at six months. There was one tendon rupture and no infections reported.
One trial of 106 participants with zone II flexor tendon repairs compared ultrasound (there were three different ultrasound regimens applied without prior specification in a protocol during the study) versus no ultrasound. Standard care was provided to all participants. There is very low‐certainty evidence of a greater improvement in total active finger motion after ultrasound at three months. Although the two cases of tendon rupture and four cases of wound dehiscence all occurred in the ultrasound group, these events were too few to draw any conclusions.
Two heterogeneous trials, including 25 participants with zones I to V repairs and 97 participants with zones I to III repairs, compared low level laser therapy with a placebo control. Standard care was provided to all participants. There is very low‐certainty evidence from the smaller trial of no between group difference in active finger range of motion at 12 weeks. There were two tendon ruptures and one wound infection reported.
One trial, published only as a conference abstract, of 25 participants with any zone of flexor tendon injury, compared motor imagery of finger flexion movements with a control group. Standard care was provided to all participants. No usable data were available for our primary outcomes.
Overall completeness and applicability of evidence
Completeness of the evidence
The data available for this review are very limited. The 17 heterogeneous and often poorly reported studies investigated different rehabilitation interventions following flexor tendon surgeries of the hand in a total of 1108 mainly adult participants. The trials tested one of 14 comparisons, 10 of which tested different exercise regimens. Of note, we identified no trials testing several of the 'main comparisons' listed in our protocol: namely, different types of orthoses; different orthosis‐wearing regimens, including duration; different timings for commencing mobilisation; different types of scar management; or different timings for commencing strengthening. Data pooling was undertaken for one outcome (7 cases of tendon rupture in a total of 196 participants or digits) in one comparison tested by three trials (place and hold exercise regimen versus controlled passive exercise regimen). We did not pool data for laser therapy, the only other multi‐trial comparison of tested by more than one trial. Although, with one exception, studies reported on finger range of motion, only six of the 17 studies reported on functional status using a patient‐reported outcome measure. Data were absent or incomplete for most other outcome measures.
Applicability of the evidence
The evidence in this review is limited also in its applicability. In terms of the study populations, it should be noted that most studies focused on zone II tendon repairs. Several studies did not report demographic data, such as gender and age, details of the type of surgery, and sufficiently detailed participant eligibility criteria. Where the populations were described, these were generally young adults and mostly male (Table 5).
Partly reflecting the limited reports, such as conference abstracts only, of several studies, the limited information on the interventions hinders applicability. While the interventions in most of the exercise trials were prescribed at commencement of the rehabilitation phase, the details of the exercise regimens with regard to specific exercises provided, their frequency and repetitions were not generally adequately described. Details of how these programmes were progressed after the first six weeks, through exercise regimens, splinting for contractures, strengthening programmes and return to restricted and or unrestricted activity were lacking.
Many of the studies also reported using concomitant treatments at the same time as the study intervention, possibly confounding the effects of the study intervention. Of particular note is Scavenius 2000, where a different surgical repair technique, which is not a rehabilitation modality, was used in the two groups. When assessing applicability of such trials, it is important to consider that such co‐interventions, whether applied to all participants or differently between the two groups, may have affected the trial findings.
Longer‐term outcomes (greater than six months) were often not collected and thus we are uncertain about the harms and benefits of these treatments at these later time points. Outcomes were not reported in a consistent way. Range of motion was reported using several different classification systems. Different classification systems used within the same studies often achieved different outcomes which makes interpretation of the data challenging. While it is attractive to be able to state how many participants received an excellent versus a poor outcome, these systems use different thresholds, so comparison across classification systems is not possible. Therefore use of goniometric measurement for affected digits (individual joint flexion/extension measurements and TAM) is easier to compare and to interpret the clinical significance across groups within a study and across studies comparing the same interventions. This also allows for interpretation of other important adverse events such as flexion contractures and active lag of the gliding tendons from adhesion formation using an objective goniometric cut‐off, rather than subjective impressions.
In particular, while studies reported on the number of tendon ruptures, it would be useful for clinicians to know details about these ruptures, including information on what stage of rehabilitation that the rupture occurred; if there was a cause identified; how many of these ruptures proceeded to have secondary surgeries; whether they were directly repaired or proceeded to have two stage tendon reconstructions; and the final outcomes that they achieved.
Many studies did not report their trial protocols a priori, or conform to the CONSORT statement for reporting their trial's findings.
Quality of the evidence
We presented ’Summary of findings’ tables only for trials addressing the primary questions of the review using the GRADE approach to assess the quality of all the evidence examined (Schünemann 2011). The certainty of the evidence was very low for the three selected comparisons (Table 1; Table 2; Table 3). Most of the evidence was downgraded for a combination of two out of three reasons. Evidence was based on small, single trials, leading to concerns about imprecise effect estimates. Even with the ability to pool three studies to examine the effectiveness of place and hold exercises versus controlled passive exercise regimens, in most outcomes we were only able to derive meaningful data from one of the studies (Table 3). Methodological quality varied across studies, but in general was of low to very low quality. All studies were small, ranging from 25 participants (Stenekes 2009) to a maximum of 100 participants (Geetha 2014; Hagberg 2000). According to the GRADE approach (Schünemann 2011), the overall certainty of the evidence for all outcomes for all intervention comparisons was very low.
Trials were downgraded due to serious risk of bias. Lack of reporting of randomisation sequence generation and allocation concealment, lack of blinding of assessors (performance and detection bias), and attrition bias seriously impacted the quality of the included trials. One of the largest issues was the inadequacy of reporting of study design and methods employed in the trials, leading to unclear risk of bias assessments. Attempts to obtain this information from the authors was unsuccessful in most cases. Inconsistency and publication bias were not rated, given the very limited pooling of data from the few trials making the same comparisons. Trials were largely downgraded further due to serious imprecision and indirectness. Serious imprecision reflecting wide confidence intervals, including those crossing the line of no effect, as well as unit of analysis errors reduced our confidence in the effect estimates. Further downgrading for serious indirectness reflected the inadequacy and unsatisfactory nature (including low reliability and validity) of some of the outcome measures.
Among other quality issues, unit of analysis errors are especially important to identify. Randomisation usually occurs at the patient level. However the unit of analysis in flexor tendon surgery rehabilitation outcomes can be patients, digits or tendons (i.e. two tendons can be injured in the same digit). Frequently reported outcomes such as grip strength are measured per patient, whereas active ROM is reported per digit. Seven studies were clear in their unit of analysis and accounted for these differences, three committed unit of analysis errors, and in seven studies it was unclear if a unit of analysis error may have occurred.
Potential biases in the review process
Although review authors attempted to minimise bias in the selection of studies for this review, collection of published data and analysis, our searches were limited to electronic databases and clinical trials registries. Therefore, we may have missed any unpublished studies. Furthermore, it was difficult to obtain all relevant data required for a systematic review from the authors of the included studies, often because of the length of time that had passed since some of the studies were completed, or no contact information was available for the authors. It was also difficult to assess selective outcome reporting for studies for which study protocols or trial registry was not available or accessible, and for which study authors did not adequately report the methods used.
In addition, unit of analysis errors or concerns (tendon/digit/participant) were identified in some studies. For studies that included participants with multiple tendon or digit repairs but did not account for this in the analysis, bias may have been introduced.
Changes were made to the protocol published a priori. These have been reported explicitly in Differences between protocol and review.
Agreements and disagreements with other studies or reviews
To our knowledge, five other systematic reviews have been published on this topic (Chesney 2011; Neiduski 2018; Starr 2013; Thien 2004; Woythal 2019). Thien 2004 is a previous Cochrane Review published in 2004 and thus out of date; we excluded one paper (Percival 1989) previously included in Thien 2004 as it was not a randomised trial.
The other four systematic reviews included outcomes from both randomised and non‐randomised studies.
Chesney 2011 reviewed flexor tendon rehabilitation protocols for zone II flexor tendon injuries of the hand. The reviewers concluded that there was weak evidence to support both early active motion regimens and regimens that combined passive mobilisation and active extension. These exercise regimens provided superior results with regard to ROM while maintaining an acceptable low rate of tendon rupture. The review included 15 studies comprising of three RCTs, two quasi experimental studies and 10 case series. One of the RCTs, Su 2005, investigated the effects of a stainless steel tendon repair device which is not considered a rehabilitation treatment using our inclusion criteria.
Neiduski 2018 reported that place and hold exercise regimens appear to provide better outcomes than passive flexion regimens for patients with two to six‐strand repairs of flexor tendon injuries, as examined by nine studies. However, they also included non‐randomised study designs, and hence are likely to have serious risk of bias impacting our certainty of this evidence.
Starr 2013 included flexor tendon injuries in all zones with the focus on comparing rehabilitation protocols between early passive and early active range of motion. They included four RCTs, eight quasi‐experimental comparative studies and 22 prospective and retrospective case series. They too included Su 2005. They found that early passive range of motion protocols had a statistically significantly decreased risk for tendon rupture but an increased risk for postoperative decreased ROM compared with early active motion protocols.
Woythal 2019 conducted a systematic review that aimed to examine the effectiveness of splints, with or without wrist immobilisation, following surgery for flexor tendon injury. They found no studies that met their inclusion criteria (randomised trials0 and hence qualitatively summarised the most relevant studies. They concluded that it is impossible to currently provide evidence‐based recommendations for or against immobilising the wrist following flexor tendon repairs.
Findings of our review are consistent with the other systematic reviews in concluding that evidence is insufficient and inconclusive to determine which rehabilitation regimens are safe and most effective in restoring function and motion following flexor tendon surgeries of the hand. All of these reviews have a narrower scope than ours in that they restricted their study eligibility criteria according to the type of exercise regimens used, whereas our review has focused on all types of interventions used by therapists for rehabilitation following flexor tendon surgery. Therefore, to our knowledge, ours is the most comprehensive review of rehabilitation interventions following flexor tendon surgery, and includes several trials not included in the other reviews (Abdel Sabour 2018; Geetha 2014; Gulke 2018; Ozkan 2004; Poorpezeshk 2018; Rigo 2017; Silva 2003; Stenekes 2009; Uday Raj 2018; Vialaneix 2003).
One study in Persian language (Yavari 2009) is awaiting classification while we are seeking further clarification from the authors on participant numbers and method of randomisation. Another study is awaiting classification (Kitis 2009) as we await further clarification from the author whether the trial was randomised or not. Five trials are currently ongoing (CTRI/2019/01/016821; NCT03812978; IRCT201310138177N8; IRCT20150721023277N7; NCT03850210) and will likely be included in future updates of this review. These studies are examining interventions of interest and are sufficiently large enough to change the findings of future updates of this review.
Authors' conclusions
Implications for practice.
The evidence from randomised controlled trials on the effects of rehabilitation interventions following surgery for flexor tendon injuries of the hand is very limited and of very low certainty, and is thus insufficient to inform practice and patient decisions. There is a notable lack of evidence from randomised controlled trials on the effectiveness of adjunctive treatments in the early phase of rehabilitation, such as scar management, early oedema management, wound care, and orthosis types (especially wrist and finger joint positions and inclusion of the wrist). There is incomplete and invariably very low‐certainty evidence for all 14 comparisons examined in the 17 included studies. This means we are uncertain of the estimates of effect for all outcomes for which data were available for all eight comparisons of different exercise regimens, and for all six other comparisons that evaluated the timing of return to unrestricted functional activities post surgery; the use of external devices applied to the participant to facilitate mobilisation, such as an exoskeleton or a continuous passive motion device; modalities such as laser therapy or ultrasound therapy; and motor imagery treatment.
Implications for research.
High‐quality randomised controlled trials are needed to assess the effectiveness and safety of rehabilitation treatments delivered after flexor tendon surgery. Specifically, studies with large samples and the power to detect statistically significant differences between groups in important patient‐centred outcomes such as function, return to work and range of motion are needed to determine the effects of interventions in improving postoperative outcomes. Whilst we acknowledge that this is not always practical, replicating studies to allow meta‐analysis would enable us to be more certain of the safety and effectiveness of certain interventions.
The identification and selection of priority areas for future research requires input from others, including consultation with patients as to their preferences and values. In the following, we make some suggestions to contribute to the discussions.
Areas for future research
Many exercise and orthosis regimens that are currently being used in practice have not been the focus of randomised trials. The idea of applying controlled stress to a repaired tendon has existed for over 40 years (Clancy 2013). Previous exercise regimens stress loaded the tendon with passive forces. Newer exercise regimens advocate the use of alternative interventions to provide active loading of the healing tendon (Evans 2012) such as place and hold exercise regimens; active flexion exercise regimens (free or through an arc of motion); isolated DIP joint motion; synergistic wrist exercises and out of orthosis exercises in the early post‐operative phase. These interventions are provided with great variability in terms of the timing of interventions; the type of exercise; the frequency and repetitions of the exercises performed within the orthosis; the position of the hand within the orthosis; and the orthosis design. Despite early active regimens being widely recommended (Tang 2018b), these rehabilitation treatments have not yet been evaluated for their effectiveness or safety, and therefore, should be included in future trials. Further various orthoses designs and timing of cessation of wearing the orthoses varies widely. Whilst this likely depends on the strength (two strand, four strand or six strand) and type of the repair, this too needs to be the focus of future trials.
Newer areas of potential research also include advances in rehabilitation as a result of wide‐awake surgery, which allows rehabilitation to commence potentially even earlier (Tang 2018a), as well as the recent increase as a result of the COVID‐19 pandemic in tele‐rehabilitation and use of other remote methods of providing care, a model of care that seems likely to continue in the longer term.
Other areas of research for future trials are the timing of commencement of the interventions; early phase interventions such as oedema management; wound and scar management; and orthosis design and position of hand within the orthosis, including orthotics used in the later stages of rehabilitation.
Current studies mainly focus on zone II injuries. We recommend further studies investigating effectiveness of interventions in other zones of injury. For example, no studies examined outcomes following zone I flexor digitorum profundus avulsion injuries and few examined thumb flexor pollicis longus injuries. Most studies included only simple tendon lacerations and nerve repairs. It is important to establish the effectiveness of these interventions and exercise regimens for more complex injuries to guide clinical practice.
Another potentially fertile area of research is on rehabilitation treatments specifically for children. This reflects that exercise and orthosis wearing regimens can be complex and not feasible or appropriate for younger children.
Rehabilitation protocols can be both complex and time consuming. For example, many exercise protocols require exercise sessions to be conducted hourly or multiple times a day over a 6 to 12 week programme, which may be difficult to accommodate with other domestic and work activities. Studies of fidelity, adherence to, and acceptability of intervention protocols by care providers and patients are also warranted. These studies could be useful adjuncts to intervention trials; in particular, consideration should be given to collection of adherence data in intervention trials.
Outcomes
Researchers should use patient‐centred outcome measures, such as function and quality of life, in addition to objective measurements, as recommended by the World Health Organization (Larson 2019). Researchers should use the best available evidence on psychometric properties of outcome measures for the constructs they measure in their studies and, where possible, select instruments that have been validated for use in rehabilitation following flexor tendon surgery (Marks 2020).
Consistency in the reporting of outcome data is also important. In particular, range of motion should be reported using goniometric measurements to allow comparison between trials, rather than the plethora of classification systems available. If using a classification system, such as those advocated by the American Society of Hand Therapists, or the International Federation of Societies for Hand Therapy and Hand Surgery, trial authors should clearly state which classification system they are using and an explicit definition for each category for that classification system. As many of these types of injuries interfere with functional use of the hand, and as a consequence, the ability to work following surgery, trials should also consider including return‐to‐work or sickness absence outcomes. This is of utmost interest to key stakeholders such as insurance providers, workers' compensation, as well as workers themselves. Where possible, outcomes should be measured in the longer term to establish the long term effectiveness and potential harms of the various treatments being prescribed.
General trial design and reporting
Reporting of trials should conform to the CONSORT criteria for design and reporting of non‐pharmacological studies (Boutron 2008) and subsequent developments. This includes the adequate reporting of interventions (Hoffmann 2014). Study authors should place trial information on appropriate clinical trials registers to ensure transparent reporting of methods planned for their study. Studies need to at a minimum report the severity of injury, flexor tendon zone and type of surgical repair when examining the effectiveness of different rehabilitation treatments. Clear information should be provided regarding the methods of randomisation and allocation concealment, blinding of participants, personnel and outcome assessors, and reporting of attrition. Trials should attempt to blind participants and outcome assessors when possible. The unit of analysis should be clearly reported as participant, hand, finger, thumb or tendon to help avoid unit‐of‐analysis errors. Moreover, trialists should be careful to include in the study report appropriate summary and measures of variability data for all outcomes prespecified in their methods, thereby helping to avoid selective reporting bias. A full account of post‐protocol changes will also help in this regard.
Interventions need to be reported in sufficient detail to ensure both transparency of reporting and replication of their intervention by both researchers and practitioners. Furthermore, other care provided to all participants or those of individual groups needs to be described, including measures taken to ensure standardisation, where appropriate.
Consistent reporting of outcomes, including timing of outcome assessments, will allow for meta‐analysis of similar outcomes in future reviews. Range of motion and tendon ruptures are commonly reported outcomes following flexor tendon surgery. Inclusion of patient‐rated outcome measures reporting function, patient satisfaction, quality of life measures and self‐reported adherence to the interventions is recommended. Reporting is required also on returning to activities especially work on full duties, modified duties, return to sport, return to musical instruments and return to education.
In particular, to aid interpretation and application, trial authors should also report demographic details and rehabilitation setting information.
History
Protocol first published: Issue 1, 2017 Review first published: Issue 1, 2021
Acknowledgements
We thank Helen Handoll and Joanne Elliott for their editorial feedback and support on this review. We also thank Maria Clarke for her help in running and reporting the searches. We are grateful to the external referees, Megan Blakeway, Christina Jerosch‐Herold and Jason Wong, for their helpful comments on the penultimate draft of the review. We thank Nicole Pitcher for her help with the plain language summary.
We thank the trial authors who took the time to respond to requests for further information about their trials, including Ali Kitis, K. Geetha, and Lars Adolfsson.
We are grateful to Hebatullah M. Abdulazeem and Martina Löwe for translation of articles.
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
CENTRAL (CRS Web)
1 MESH DESCRIPTOR Tendon Injuries AND CENTRAL: TARGET 2 MESH DESCRIPTOR Hand Injuries EXPLODE ALL AND CENTRAL: TARGET 3 #1 OR #2 4 (flexor*): AB,EH,KW,KY,MC,MH,TI,TO 5 #3 AND #4 6 MeSH descriptor Rupture AND CENTRAL: TARGET 7 MeSH descriptor Lacerations AND CENTRAL: TARGET 8 MeSH descriptor Wounds and Injuries AND CENTRAL: TARGET 9 (rupture* or lacerat* or injur* or repair*): AB,EH,KW,KY,MC,MH,TI,TO 10 #6 OR #7 OR #8 OR #9 11 (flexor near5 tendon*): AB,EH,KW,KY,MC,MH,TI,TO 12 (flexor digitorum or flexor pollicis): AB,EH,KW,KY,MC,MH,TI,TO 13 #11 OR #12 14 #10 AND #13 15 #5 or #14
MEDLINE (Ovid Online)
1 Tendon Injuries/ or Hand Injuries/ or Finger Injuries/ 2 flexor*.ti,ab. 3 1 and 2 4 Rupture/ or Lacerations/ or "Wounds and Injuries"/ 5 (rupture* or lacerat* or injur*).ti,ab. 6 in.fs. 7 4 or 5 or 6 8 (flexor adj5 tendon*).ti,ab. 9 (flexor digitorum or flexor pollicis).ti,ab. 10 8 or 9 11 7 and 10 12 3 or 11 13 randomised controlled trial.pt. 14 Controlled clinical trial.pt. 15 randomised.ab. 16 placebo.ab. 17 Drug therapy.fs. 18 randomly.ab. 19 trial.ab. 20 groups.ab. 21 or/13‐20 22 exp Animals/ not Humans/ 23 21 not 22 24 12 and 23
.pt. denotes a Publication Type term; .ab. denotes a word in the abstract; .fs. denotes a 'floating' subheading; / denotes a Medical Subject Heading (MeSH) term; .ti. denotes a word in the title.
Embase (Ovid Online)
1 Flexor Tendon Injury/ 2 Tendon Injury/ or Tendon Rupture/ or Finger Injury/ or Hand Injury/ 3 flexor*.ti,ab. 4 2 and 3 5 Rupture/ or Laceration/ or Injury/ or Avulsion Injury/ 6 (rupture* or lacerat* or injur*).ti,ab. 7 5 or 6 8 Flexor Tendon/ 9 (flexor adj5 tendon*).ti,ab. 10 (flexor digitorum or flexor pollicis).ti,ab. 11 8 or 9 or 10 12 7 and 11 13 1 or 4 or 12 14 exp randomised Controlled Trial/ or exp Single Blind Procedure/ or exp Double Blind Procedure/ or Crossover Procedure/ 15 (random* or RCT or placebo or allocat* or crossover* or 'cross over' or trial or (doubl* adj1 blind*) or (singl* adj1 blind*)).ti,ab. 16 14 or 15 17 (exp Animal/ or animal.hw. or Nonhuman/) not (exp Human/ or Human cell/ or (human or humans).ti.) 18 16 not 17 19 13 and 18
CINAHL (Ebsco)
S1 (MH "Tendon Injuries, Finger") S2 (MH "Tendon Injuries") OR (MH "Hand Injuries") OR (MH "Finger Injuries") S3 (MH "Finger Flexor Tendons") S4 TI flexor* OR AB flexor* S5 S3 OR S4 S6 S2 AND S5 S7 TX (MH "Rupture") OR (MH "Wounds and Injuries") OR (MH "Tears and Lacerations") S8 TI ( rupture* or lacerat* or injur* ) OR AB ( rupture* or lacerat* or injur* ) S9 S7 OR S8 S10 TI flexor N5 tendon* OR AB flexor N5 tendon* S11 TI ( flexor digitorum or flexor pollicis ) OR AB ( flexor digitorum or flexor pollicis ) S12 S3 OR S10 OR S11 S13 S9 AND S12 S14 S1 OR S6 OR S13 S15 PT Clinical Trial S16 (MH "Clinical Trials+") S17 TI clinical trial* OR AB clinical trial* S18 TI ( (single blind* or double blind*) ) OR AB ( (single blind* or double blind*) ) S19 TI random* OR AB random* S20 S15 OR S16 OR S17 OR S18 OR S19 S21 S14 AND S20
AMED (Ovid Online)
1 Hand injuries/ or Finger injuries/ or Tendon injuries/ 2 flexor*.ti,ab. 3 1 and 2 4 "Wounds and Injuries"/ or Rupture/ 5 (rupture* or lacerat* or injur*).ti,ab. 6 4 or 5 7 (flexor adj5 tendon*).ti,ab. 8 (flexor digitorum or flexor pollicis).ti,ab. 9 7 or 8 10 6 and 9 11 3 or 10 12 randomised controlled trial.pt. 13 Controlled clinical trial.pt. 14 randomised Controlled Trials/ 15 Random Allocation/ 16 Double‐Blind Method/ 17 or/12‐16 18 exp Animals/ not Humans/ 19 17 not 18 20 clinical trial.pt. 21 exp Clinical trials/ 22 (clinic* adj25 trial*).tw. 23 ((singl* or doubl* or trebl* or trip*) adj (mask* or blind*)).tw. 24 Placebos/ 25 placebo*.tw. 26 random*.tw. 27 exp Research design/ 28 (latin adj square).tw. 29 or/20‐28 30 29 not 18 31 30 not 19 32 11 and 31
ClinicalTrials.gov
flexor AND tendon
WHO ICTRP
flexor AND tendon*
Data and analyses
Comparison 1. Early active flexion plus controlled passive exercise regimen versus early controlled passive exercise regimen (modified Kleinert protocol).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Self‐reported function for ADL using a VAS (0 to 10; higher = better) | 1 | Other data | No numeric data | |
| 1.2 Self‐reported function using VAS for ADLs (0 to 10; higher scores = better); secondary analyses | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.1 ADL VAS at 2 months (0=worst; 10=best) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.2 ADL VAS at 3 months (0=worst; 10=best) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.3 ADL VAS at 6 months (0=worst; 10=best) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.4 ADL VAS at 12 months (0=worst; 10=best) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3 Active finger range of motion (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3.1 Total Active Movement at 1 month (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3.2 Total Active Movement at 2 months (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3.3 Total Active Movement at 3 months (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3.4 Total Active Movement at 6 months (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3.5 Total Active Movement at 12 months (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.4 Finger range of movement ‐ Poor outcome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4.1 Strickland classification at 1 month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4.2 Strickland classification at 2 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4.3 Strickland classification at 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4.4 Strickland classification at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4.5 Strickland classification at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4.6 Tang classification at 1 month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4.7 Tang classification at 2 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4.8 Tang classification at 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4.9 Tang classification at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4.10 Tang classification at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5.1 Tendon rupture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5.2 Complex Regional Pain Syndrome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5.3 Wound dehiscence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5.4 Transitory swelling + tenderness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5.5 Any adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.6 Strength (% of the contralateral hand or digit) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.1 Grip Strength at 3 months (% of the contralateral hand) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.2 Grip Strength at 6 months (% of the contralateral hand) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.3 Grip Strength at 12 months (% of the contralateral hand) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.4 Pinch Strength at 3 months (% of the contralateral digit) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.5 Pinch Strength at 6 months (% of the contralateral digit) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.6 Pinch Strength at 12 months (% of the contralateral digit) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Early active flexion + active extension exercise regimen (+ modified Kessler suture surgical technique) versus passive flexion + active extension exercise regimen (+ grasping suture and external pull‐out knot surgical technique).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1.1 Tendon ruptures | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1.2 Scar adhesions requiring surgery (Tenolysis) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Active exercise regimen versus immobilisation regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Range of movement at 12+ months: poor outcome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.1 IFSSH criteria | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.2 Strickland criteria | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.1 Tendon ruptures | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.2 Movement restrictions indicating need for tenolysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.3 Secondary surgery (actual or indicated) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4. Early place and hold progressed to tendon gliding exercise regimen versus early passive progressed to active exercise regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Grip strength at 12 weeks (% difference between normal and affected hands) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 5. Place and hold exercise regimen versus Controlled passive exercise regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Function (self‐reported): DASH scores (0 to 100; higher score = more disability) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1.1 DASH at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1.2 DASH at 52 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.2 Range of movement (Total Active Movement) (degrees) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.2.1 At 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.2.2 At 52 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.3 Range of movement (Strickland Criteria) at 8 weeks: Poor outcome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.4 Adverse events | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.4.1 Tendon rupture | 3 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.19, 3.50] |
| 5.4.2 Scar adherence | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.66] |
| 5.4.3 Flexion contracture of the DIP joint | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.02, 0.88] |
| 5.4.4 Flexion contracture of the PIP joint | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.15, 1.48] |
| 5.4.5 FDS tendon lag | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.06, 0.98] |
| 5.4.6 FDP tendon lag | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.04, 0.62] |
| 5.5 Function (observed): Jebsen Taylor at 52 weeks (seconds) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.6 Function (observed): Purdue pegboard at 52 weeks (pegs) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.7 Satisfaction with hand function (0 to 10: complete satisfaction) at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 6. Unrestricted activity at 8 weeks post‐surgery versus unrestricted activity at 10 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Adverse event (tendon rupture) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 7. Exoskeleton versus physiotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Function (self‐reported): DASH scores (0 to 100; higher score = more disability) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1.1 DASH at 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1.2 DASH at 18 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.2 Active finger range of motion of PIP joint (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.2.1 PIP joint range of motion at 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.2.2 PIP joint range of motion at 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.2.3 PIP joint range of motion at 18 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.3 Active finger range of motion of DIP joint (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.3.1 DIP joint range of motion at 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.3.2 DIP joint range of motion at 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.3.3 DIP joint range of motion at 18 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.4 Active finger range of motion (Total Active Movement) (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.4.1 TAM at 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.4.2 TAM at 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.4.3 TAM at 18 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.5 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.5.1 Tendon rupture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.5.2 CRPS (complex regional pain syndrome) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.6 Strength at 18 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.6.1 Grip strength (kg) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.6.2 Pinch strength (kg) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 8. Continuous passive motion device versus controlled passive progressed to active exercise regimen (Modified Kleinert).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Active finger range of motion (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1.1 TAM at > 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.2 Range of movement at > 6 months (Strickland criteria) ‐ poor outcome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.3 Adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.3.1 Tendon rupture at >6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.3.2 Infection at >6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 9. Ultrasound versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Improvement in active range of movement (Difference between 3 and 12 weeks; Total Active Motion measured in degrees) | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | 26.47 [19.70, 33.24] |
| 9.1.1 1MHz; 0.7W/cm2 increased to 1w/cm2 | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 31.50 [19.94, 43.06] |
| 9.1.2 1 MHz; 0.3w/cm2 increased to 1.0w/cm2 | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 23.80 [11.89, 35.71] |
| 9.1.3 3Mhz; 0.5w/cm2 increased to 0.7w/cm2 | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 23.90 [12.20, 35.60] |
| 9.2 Active finger range of motion (Strickland classification) at 3 months ‐ Poor outcome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.3 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.3.1 Tendon rupture at 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.3.2 Wound dehiscence <3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 10. Low‐level laser therapy versus placebo control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Active finger range of motion (Total active motion (degrees)) at 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.2 Range of movement at 12 weeks: poor outcome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.2.1 Strickland scoring system at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.3 Adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.3.1 Tendon rupture | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.3.2 Wound infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.4 Grip strength at 12 weeks (% lost using uninjured hand as comparison) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 11. Motor imagery versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Strength (kg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1.1 Grip strength (kg) at 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1.2 Pinch strength (kg) at 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdel Sabour 2018.
| Study characteristics | ||
| Methods |
Design: parallel group randomised controlled trial. Setting: single centre trial. Physical Medicine and Rehabilitation Department, El Demerdash Hospital, Ain Shams University, Egypt. Study conducted in 2015 to 2016 Unit of randomisation: participant Unit of analysis: tendon |
|
| Participants |
Details of sampling frame: Total eligible: 53 participants Total excluded pre‐randomisation: 20 participants Baseline characteristics: Total randomised: 33 participants (45 tendons) Place and hold group randomised: 15 participants (21 tendons) Controlled passive group randomised: 18 participants (24 tendons) Sex distribution: 21 males; 5 females Place and hold group: not reported Passive group: not reported Age: mean (range): Mean 26.8 years (15 to 60 years) Place and hold group: not reported Controlled passive group: not reported Flexor tendon zones: zone I: 7; zone II: 22; zone III: 7 Inclusion criteria:
Exclusion criteria:
Surgical technique for flexor repair: All flexor tendons were two‐strand repairs. The wound was extended using Bruner incisions, and a flap was raised to expose the tendon preserving the functionally important A2 and A4 pulleys. Pull out suture was made for zone I injury with short distal stump (< 1 cm). The suture materials were 3/0 or 4/0 prolene for core suture modified Kessler technique and 5/0 or 6/0 prolene for epitendinous sutures. Associated digital nerve and arterial injuries were repaired by the 8/0 or 9/0 Ethibond. Characteristics of participants lost to follow‐up/dropouts and included in analysis: Total lost to follow‐up: 7 participants (9 tendons) Place and hold group lost to follow‐up: 4 participants (5 tendons) Controlled passive group lost to follow‐up 3 participants (4 tendons) Total available for follow‐up: 26 participants (36 tendons) Total analysed: 26 participants (36 tendons) Place and hold group: 11 participants (16 tendons) Controlled passive group: 15 participants (20 tendons) |
|
| Interventions |
Intervention 1: Place and hold exercise regimen Components of the intervention: exercise regimen was commenced at three days after tendon repair. Place and hold mobilisation regimen consisting of passive digit flexion of the affected finger and then the participant tries to maintain the flexed posture through active contraction of the involved muscle for five seconds (i.e. place the finger in the desired flexed position and then participant attempts to hold the finger using their flexor muscles in the same position); individual passive range of motion for all joints; passive flexion active extension. These were progressed to active tenodesis exercises. Dose: 25 repetitions of each exercise. Frequency of administration: every waking hour for the first six weeks post‐surgery. Intervention 2: Controlled passive exercise regimen Components of the intervention: passive exercise regimen using the modified Kleinert method consisting of composite passive flexion and active extension of the digits plus passive range of motion to each joint of each finger. Exercise regimen was commenced at three days after tendon repair. Dose: 25 repetitions of each exercise Frequency of administration: every waking hour for the first six weeks post‐surgery. Both groups: Components of the intervention: dorsal blocking orthosis with wrist in 20° flexion, MCP joint in 70° flexion and IP joints in full extension. Dose: orthosis worn all of the time. Frequency of administration: worn all of the time for six weeks post‐surgery. At six weeks, the orthosis was discarded. |
|
| Outcomes | Outcomes were assessed at six weeks through to six months post‐surgery:
|
|
| Funding and conflicts of interest statements | Funding source: not reported Conflicts of interest: authors report "There are conflicts of interest", but none are described. | |
| Notes |
Trial registered: PACTR201708002483416 Unit of analysis is tendons not fingers/participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: 'We randomised patients into two groups by random sequence‐generating website' as per clinical trials registry: www.randomizer.org." Comment: the randomisation sequence appears to have been generated using an adequate method. |
| Allocation concealment (selection bias) | Low risk | Quote: as per the clinical trials registry, "allocation was determined by the holder of the sequence who is situated offsite". Comment: it appears that the allocation sequence was concealed by keeping the sequence off site until time of recruitment. An adequate method was used to conceal the allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not reported but due to the nature of the intervention (participation in an exercise programme) it is unlikely participants were blinded to the intervention group they were assigned. Due to the nature of the intervention, healthcare providers could not be blinded to the intervention. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Comment: non‐blinded participants, who may have had different expectations about the benefits of the intervention they received when rating the Disabilities of the Arm, Shoulder and Hand questionnaire and satisfaction. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: it is not stated whether the outcome assessors were blinded for range of motion and adverse events. We attempted to contact the authors but did not receive a response. |
| Incomplete outcome data (less than 3 months) (attrition bias) All outcomes | Low risk | Quote: "A total of 33 patients (45 tendons) were enrolled in the study, and only 26 (36 tendons) continued in the study, as seven patients were lost to follow‐up." Data for each group is also reported. Comment: the number of participants who dropped out are included in the CONSORT diagram and results section. Data are not clearly reported in the manuscript, and it is unknown at what time point the data reported was collected and when these participants were lost to follow up. The reasons for those lost to follow‐up is also not described. However, the number of drop‐outs per group were similar (3 in the passive group versus 4 in the PAH group. So it is unlikely this has biased the results. |
| Incomplete outcome data (3 to 6 months) (attrition bias) All outcomes | Unclear risk | Quote: "We conducted our study for 12 weeks….". However, the DASH appears to have been conducted at six months. Comment: it is unclear what data were collected beyond 12 weeks. Clarification from the authors was not received on request for further data. The authors do not report when adverse events were measured. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes that were reported in the clinical trials register and in the methods section of the paper are reported in the results. |
| Other bias (outcomes appropriately analysed) | High risk | Comment: the authors have used the unit of analysis as tendons. However, some of the outcomes that were measured such as range of motion, scar adhesion and DASH are measured at the finger or person level. A unit of analysis error appears to have occurred for these outcomes as measurements are per tendon. No further sources of bias were detected. |
Adolfsson 1996.
| Study characteristics | ||
| Methods |
Study design: parallel group randomised controlled trial Setting: Sweden Unit of randomisation: participant Unit of analysis: digit; thumbs analysed separately |
|
| Participants |
Details of sampling frame: Total eligible: 96 participants (106 digits) Total excluded pre‐randomisation: 0* Baseline characteristics: Total randomised: 96 participants (106 digits, 81 fingers and 25 thumbs) Sex distribution at baseline: 68 males; 28 females Age: not reported in baseline characteristics Flexor tendon zone: zone II: 106 digits Inclusion criteria:
Exclusion criteria:
Surgical technique for the flexor tendon repair: Surgery within 24 hours of injury. Repaired with a modified Kessler suture using 4/0 Maxon (Davis and Geck) and a running circumferential 6/0 Prolene (Ethicon) suture. Characteristics of participants lost to follow‐up/dropouts and included in analysis: Total drop‐outs: 14 participants (six ruptured in the first three weeks and eight were lost to follow‐up)* Total available for follow‐up: 82 participants (68 fingers and 23 thumbs) Total included in analysis: 82 participants (68 fingers and 23 thumbs) 8‐week group: 38 participants (45 digits (35 fingers; 10 thumbs); 15 fingers contributed 2 tendons) 10‐week group: 44 participants (46 digits (33 fingers; 13 thumbs); 12 fingers contributed 2 tendons) Sex distribution at follow‐up (only reported for those included in analysis): 54 males; 28 females 8‐week group: 28 males; 10 females 10‐week group: 26 males; 18 females Age: mean ± SD (range)(only reported for those included in analysis): 8‐week group: mean 36 years 10‐week group: mean 38 years |
|
| Interventions |
Intervention 1: Unrestricted activity from 8 weeks post‐surgery Components of the intervention: at 6 weeks, participants were instructed in a programme to gradually increase the load on the involved hand, allowing unrestricted activity at eight weeks after the surgery. Dose: not reported. Frequency of administration: not reported. Intervention 2: Unrestricted activity from 10 weeks post‐surgery Components of the intervention: at 6 weeks, participants were instructed in a programme with slower gradual increase of load on the involved hand allowing unrestricted activity at 10 weeks. Dose: not reported. Frequency of administration: not reported. Both groups: Components of the intervention: all participants received the same therapy for the first six weeks: dorsal blocking orthosis with transverse palmar component and rubber band traction; week one to four passive exercise regimen; week 5 to 6 active exercise regimen. Immobilised in a dorsal plaster orthosis from below elbow to the fingertips with the wrist in 30 degrees flexion and the MP joints in > 70 degrees flexion. A passive flexion‐active extension regime was used according to the modified programme described by Karlander et al in 1993. On the first post‐operative day the wound dressing was reduced and another orthosis from below elbow to the PIP joints was applied. Rubber bands were attached to the nails of four fingers. Following FPL repairs a dorsal orthosis was applied from below elbow over the thumb with the wrist in 30 degrees of flexion, and a rubber band was attached to the thumb only. During the first 4 weeks passive flexion through traction on the rubber band and active extension exercises. During weeks five to six, active flexion and extension without load commented, still keeping the dorsal orthosis between exercises. Participants were randomised and enrolled into the study between four and six weeks.* Dose: orthosis: full time wear except for exercises. Exercise regimen: 6 repetitions of each exercise. Frequency of administration: for the first six weeks: orthosis applied immediately after surgery, worn all the time except for exercises. Exercise regimen: 10 times per day. |
|
| Outcomes | Outcomes were assessed at 8, 16, 24 weeks for intervention 1 group, and 10, 16, 24 weeks for intervention 2 group. Thus, groups were measured on the commencement of the intervention and were only measured at the same time points at 16 and 24 weeks.
Goniometric measurements were then used to calculate the following classifications:
|
|
| Funding and conflicts of interest statements | Funding source: Not reported Conflicts of interest: Not reported | |
| Notes | * Data provided or clarified by correspondence from the authors
**Fingers and thumbs were grouped together when randomised, but analysed separately for the ROM outcomes. ***Outcomes were measured at different time points in the groups, except for the 6 month time interval. Hence, only six months outcomes were reported in the paper, and in this review. ****Incomplete data reporting for these outcomes prevented their inclusion in this review's analyses. No clinical trial registration found. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After the 6th week the patients were randomised into two groups…."; and correspondence received from the authors "Randomization was performed by an independent OT, using concealed envelopes at four weeks after surgery. 120 envelopes had been prepared beforehand. The envelopes were prepared, randomly mixed and then delivered in a pile to the OTs who consecutively picked them up in the order they came. " Comment: the randomisation sequence appears to have been generated using an adequate method. |
| Allocation concealment (selection bias) | Low risk | Quote: "After the 6th week the patients were randomised into two groups…."; and correspondence received from the authors "Randomization was performed by an independent OT, using concealed envelopes at four weeks after surgery. 120 envelopes had been prepared beforehand." Comment: information provided by the authors confirms that the allocation sequence was concealed prior to randomisation. An adequate method was used to conceal the allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The rehabilitation was supervised by any OT on duty who was not blinded to the allocation and performed the intermediate measurements at 8, 10 and 16 weeks." Comment: not reported but due to the nature of the intervention (participation in graded hand function) it is unlikely participants were blinded to the intervention group they were assigned. Due to the nature of the intervention, care providers could not be blinded to the intervention. Participants were not blind to treatment, and may have had different expectations about the benefits of each intervention, self‐reported outcome (function). |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: non‐blinded participants, who may have had different expectations about the benefits of the intervention the received (function using VAS). |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Quote: "The rehabilitation was supervised by any OT on duty who was not blinded to the allocation and performed the intermediate measurements at 8, 10 and 16 weeks. The assessor (author 2 or 3) doing the final examination after 24 weeks was however blinded to the rehabilitation program at that point." Comment: the outcome assessment was blinded at 24 weeks, but not at the earlier time points. |
| Incomplete outcome data (less than 3 months) (attrition bias) All outcomes | High risk | Comment: the number of participants and digits contributed to the study was provided via correspondence from the authors. The 82 participants who were included in the analysis does not include (n = 14) drop‐outs. Eight of these were lost to follow‐up. It is unclear from which group the 14 drop‐outs were excluded from or the reasons for lost to follow‐up.This drop‐out rate may have had an impact on the results. No further data could be obtained from the authors. |
| Incomplete outcome data (3 to 6 months) (attrition bias) All outcomes | High risk | Comment: the number of participants and digits contributed to the study was provided via correspondence from the authors. The 82 patients who were included in the analysis does not include 14 drop‐outs. Eight of these were lost to follow‐up. It is unclear from which group the 14 drop‐outs were excluded from or the reasons for lost to follow‐up. This drop‐out rate may have had an impact on the results. No further data could be obtained from the authors. |
| Selective reporting (reporting bias) | High risk | Comment: outcomes were measured at various time points between 6 and 12 weeks. However, these data are not reported in the results section of the publication. Also, without a trial protocol is unclear whether other outcomes were measured but not reported. Correspondence from the authors indicated that these data are no longer available. |
| Other bias (outcomes appropriately analysed) | Low risk | Comment: randomisation occurred at the participant level. However, some of the outcomes are reported at the participant level (function, strength and work), some at the digit level (range of motion), and some at the tendon level (e.g. ruptures). The number of participants, fingers, thumbs and tendons in each group appears to be clearly stated in the manuscript. No other sources of bias were detected. |
Farzad 2014.
| Study characteristics | ||
| Methods |
Study design: parallel group randomised controlled trial Setting: single centre; Hand Therapy Clinic, Tehran, Iran Unit of randomisation: participant Unit of analysis: digit |
|
| Participants |
Details of sampling frame: Total eligible: 70 participants Total excluded pre‐randomisation: 16 participants Baseline Characteristics: Total randomised: 54 participants (64 digits) Passive group: 28 participants (33 digits) Place and hold group: 26 participants (31 digits) Sex distribution: 37 males; 17 females Passive group: 19 males; 9 females PAH group: 18 males; 8 females Age: mean ± SD (range) Passive group: 28 ± 9 years (17 to 50 years) PAH group: 29 ± 8 years (13 to 47 years) Flexor tendon zone: zone II: 64 digits Inclusion criteria:
Exclusion criteria:
Surgical technique for flexor tendon repair: FDP tendon repair: 2‐strand modified Kessler core 3‐0 prolene and simple running Epitendinous suture using 5‐0 prolene. FDS tendon repair: 2 x figure‐of‐eight sutures using 4‐0 prolene. Characteristics of participants lost to follow‐up/dropouts and included in analysis: Total available for follow‐up: 54 participants (64 fingers) Total analysed: 54 participants (64 fingers) Passive group: 28 participants (33 fingers) PAH group: 26 participants (31 fingers) |
|
| Interventions |
Intervention 1: Controlled passive exercise regimen Components of the intervention: orthosis: dorsal blocking orthosis with rubber band traction (attached to a hook placed on the fingernail and passed under a pulley to cause passive flexion of the IP joints) ‐ the rubber band traction was the only difference between groups and was used to perform the controlled passive exercises. Exercise regimen: passive flexion caused by the rubber band traction followed by active finger extension within the dorsal blocking orthosis. Exercises commenced three days after swelling reduced (this means exercises were started at different time points for each participant). Dose: orthosis: worn full time. Exercises: minimum of 10 repetitions per exercise. Frequency of administration: orthosis: worn full time. Exercises: every waking hour for 21 days Intervention 2: Place and hold exercise regimen Components of the intervention: orthosis: as described in both groups. Exercise regimen: patients were advised to passively flex their fingers using the other hand with the wrist in 30 degrees of extension (out of the orthosis), and then hold the finger position actively, holding for three to five seconds. Dose: orthosis: worn full time except for exercises. Exercises: 10 repetitions per exercise Frequency of administration: orthosis: worn full time except for exercises. Exercises: four times per day for 21 days. Both groups: Orthosis: protection of the tendons using a dorsal static blocking orthosis with wrist positioned at 0 to 30 degrees of flexion and the MCP joints in 70 to 90 degrees flexion. Exercises: commenced three days after swelling reduced (this means exercises were started at different time points for each participant). At 21 days, all patients were allowed to actively flex their fingers. At four weeks, gliding exercises (extension of the MP joints while holding IPs in flexion, flexion of the MP joints with IPs in extension and composite flexion). At six weeks, blocking exercises (flexion of the PIP joint while the MCP joint is kept in extension; flexion of the DIP joint when MCP and PIP joints are held in extension) and resistive exercises (e.g. therapist) were initiated. Exercises commenced three days after swelling reduced (this means exercises were started at different time points for each participant). Dose: not reported. Frequency of administration: not reported. |
|
| Outcomes | Outcomes measured at eight weeks post‐surgery: by an independent research therapist, blinded to group allotment and not involved in the care of the participants.
|
|
| Funding and conflicts of interest statements | Funding source: not reported Conflicts of interest: not reported. | |
| Notes | *Unit of Analysis is fingers NOT participants. No clinical trials registration found. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " After providing informed consent, patients referred in the first week after surgery to the hand rehabilitation clinic were randomised equally to either place and active hold or modified Kleinert according to a computerized random number generator." Comment: the randomisation sequence appears to have been generated using an adequate method. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information was insufficient to reveal whether the allocation sequence was adequately concealed until interventions were assigned. We attempted to contact the authors but did not receive a response. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: there were no self‐reported outcome measures used in this study. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Eight weeks after surgery an independent research therapist not involved in the care of the patients and blinded to group allotment evaluated patients." Comment: the outcome assessor was blinded to the intervention groups. |
| Incomplete outcome data (less than 3 months) (attrition bias) All outcomes | Low risk | Comment: the data set probably was complete as no withdrawals were reported throughout the study period. |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcome measures specified in the methods were reported in the results section. However, without a trial protocol, it is unclear whether other outcomes were assessed but not reported. |
| Other bias (outcomes appropriately analysed) | Low risk | Comment: range of motion was measured for each digit, and was reported with the unit being digits. Other outcomes appeared to have been measured at the participant level as appropriate. It is unlikely that a unit of analysis error occurred. No further sources of bias were detected. |
Geetha 2014.
| Study characteristics | ||
| Methods |
Study design: parallel group randomised controlled trial (allocation to the three non‐concurrent intervention groups was not randomised) Setting: single centre; Medical College Hospital, Chennai, India Unit of randomisation: participant Unit of analysis: digit |
|
| Participants |
Details of sampling frame: Total eligible: 106 participants (139 digits) Total excluded pre‐randomisation: not reported Baseline characteristics: Total randomised: 72 participants (99 digits) ultrasound groups; 34 (40 digits) control group Total lost to follow‐up in all groups: 6 participants (8 digits) Sex distribution: not reported at baseline Age: not reported at baseline Flexor tendon zone: zone II: 139 digits Inclusion criteria:
Exclusion criteria:
Surgical technique: Operations performed by senior residents. 2‐strand modified Kessler Mason suture for tendon repair. Characteristics of participants lost to follow‐up/dropouts and included in analysis: Total available for follow‐up: ultrasound (US) groups: 66 participants (91 digits) Total available for follow‐up: control group: 34 participants (40 digits) Number included in analyses: Total analysed: ultrasound group: 66 participants (93 digits)
Total analysed: control group: 34 participants (40 digits) Attrition: dropouts and exclusions:
Sex distribution at follow‐up: Of 100 followed up: 89 males; 11 females US group: 59 males; 7 females Control group: 30 males; 4 females Age: mean ± SD (range) age at follow‐up: US group 1: incomplete information (range 10 to 45 years listed for 42 of 66 followed‐up participants) Control group: 35 years (22 to 50 years) |
|
| Interventions | Both intervention groups were immobilised a dorsal plaster of Paris cast for three weeks with wrist in neutral and MCP joints in 70 degrees flexion and commenced the same mobilisation from three weeks. Intervention: Ultrasound Components of the intervention: ultrasound: with the orthosis in place, the dressings were removed. The ultrasound coupling gel was applied to the zone II region. The ultrasound treatment head was placed over the site of the tendon repair and gently moved in order to "iron out the irregularities in the near field and to avoid standing waves due to reflection". Care was taken not to cause undue movements to the repaired finger. After ultrasound therapy, the dressings were reapplied. Standard hand therapy: as described below was commenced at three weeks. Dose: the dosage of the ultrasound changed twice during the five year recruitment period; this was not randomised and appears to have been selected by the therapist. Frequency of administration: 5 minutes. Not reported how many sessions were performed and how often they were performed.
The ultrasound group also received the same standard hand therapy programme as the control. Control: Standard hand therapy programme Components of the intervention: orthosis: dorsal plaster of Paris orthosis with wrist in neutral position, MCPs in 70 degrees flexion and IPJs in extension. Hand therapy: three to six weeks, orthosis removed, scar massage; exercise regimen consisting of active exercises, blocking exercises and place hold exercises. Between six to eight weeks: exercise regimen consisting of additional passive stretching and resisted exercises. After eight weeks: lift weights; allowed to return‐to‐work Dose: orthosis: full time; hand therapy: once per day. Frequency of administration: orthosis: full time for three weeks; hand therapy: daily from three to at least eight weeks. |
|
| Outcomes | Outcomes were assessed at three months post‐surgery:
|
|
| Funding and conflicts of interest statements | Funding source: Medical College Hospital Chenai Conflicts of interest: none declared | |
| Notes | *All outcomes were measured at 3 months. Although the methods stated that range of motion was also measured weekly from 3 weeks, this was not reported in the results section. ** A unit of analysis error appears to have occurred. This is a per hand level measure where the unit of analysis used was digits. Clinical Trials Registry of India; Ref: CTRI/2013/04/003576 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were asked to draw a card indiscriminately from an envelope containing a pack of cards labelled as ultrasound or immobilisation in 2:1 ratio"; and from correspondence received from Geetha: "There were two ultrasound cards for one immobilisation card. Specifically, out of 15 cards, 10 were ultrasound and 5 were immobilisation. Patients were asked to select a card at random from the envelope." However, comparisons are made between the three ultrasound groups, where randomisation did not occur, and it appears that the therapist selected which ultrasound dose was applied to the participant. Comment: the randomisation sequence appears to have been generated using an adequate method between the ultrasound and the control groups. It is important to note that within the ultrasound group, patients received one of three different ultrasound regimens, which was not randomised. |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients were asked to draw a card indiscriminately from an envelope containing a pack of cards labelled as ultrasound or immobilisation in 2:1 ratio."; and from correspondence received from Geetha: "Patients were asked to select a card at random from the envelope. The cards were kept in a large opaque envelope. They were not visible to the patient or to the person performing the randomisation." Comment: although the cards could have potentially be seen during the selection from the one large opaque envelope, the authors reported that precautions were taken to blind the patient and the person performing the randomisation. Thus, it appears an adequate method was used to conceal the allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: there were no self‐reported outcomes. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Results were assessed by an independent observer who was not involved in the study" and from email correspondence from Geetha, "The assessment was done by the physiatrist who was not part of the study. She was blinded to the intervention group of the patients." Comment: the outcome assessor was blinded to the intervention group. |
| Incomplete outcome data (less than 3 months) (attrition bias) All outcomes | Low risk | Comment: no data were collected before 3 months. However, there were few dropouts at 3 months. |
| Incomplete outcome data (3 to 6 months) (attrition bias) All outcomes | Low risk | Comment: participants who were lost to follow‐up or were withdrawn from the study due to an adverse event are clearly reported. Withdrawals and how they were dealt with are clearly reported. Also, the number of participants that dropped out from each group were low (range 1 to 3). |
| Selective reporting (reporting bias) | High risk | Comment: range of motion data were collected at weekly intervals, but only 12 week data were reported in the results section. However it is likely that these data were recorded between 3 to 11 weeks, and therefore it is unlikely to have practical implications. However, without a trial protocol, it is unclear whether other outcomes were assessed but not reported. Additionally, ROM and grip strength is reported in ranges; no means or standard deviations were reported. Authors also used different range classifications that are not consistent across the four groups and are also recorded at different time intervals. |
| Other bias (outcomes appropriately analysed) | High risk | Comment: grip strength was calculated in a non‐standardised way: % of contralateral side. In the grip strength analysis, it appears that a unit of analysis error has also occurred. The grip strength is reported per digit; however, this is at a per hand not per digit level outcome. For range of motion, authors also appeared to use different range classifications that are not consistent across the four groups and are also recorded at different time intervals. |
Gelberman 1991.
| Study characteristics | ||
| Methods |
Study design: parallel group quasi‐randomised trial Setting: multi‐centre; three hospital sites in USA Unit of randomisation: participant Unit of analysis: digit |
|
| Participants |
Details of sampling frame: Total eligible: not reported Total excluded pre‐randomisation: not reported Baseline characteristics: Total randomised: unclear whether those analysed were also the same number as those randomised Intervention: unclear whether those analysed were also the same number as those randomised Control: unclear whether those analysed were also the same number as those randomised Sex distribution: Not reported Age: mean: Intervention: 26.2 years Control: 32.8 years Flexor tendon zone: zone II: 60 digits (analysed) Inclusion criteria:
Exclusion criteria:
Surgical technique for flexor tendon repair: Flexor tendons were exposed through palmar zig‐zag incisions. The proximal tendon stumps were isolated by either flexing the wrist and digits or probing the tendon sheaths with a blunt tendon passer. The tendon stumps were delivered atraumatically into the tendon sheath defect. A funnel shaped enlargement was created in the tendon sheath through the non critical membranous region when necessary to accomplish repair, as described by Lister. The tendons were repaired in the manner described by Kessler and Missim, with 4‐0 braided Dacron sutures (Ethicon, Somerville, New Jersey) under a magnification factor of 3.5. A continuous 6‐0 nylon epitenon suture was used to invaginate the free tendon ends. Digital sheath defects were not repaired. Similar operative technique was reported to have been used across all three sites. Characteristics of participants lost to follow‐up/dropouts and included in analysis: Total available for follow‐up: 51 participants (60 digits, 102 tendons) Total lost to follow‐up: not reported Total analysed: 51 participants (60 digits, 102 tendons) CPM group: 26 participants (29 digits, 48 tendons) Passive group: 25 participants (31 digits, 54 tendons) |
|
| Interventions |
Intervention: Continuous passive‐motion (CPM) machine
Week 1 to 4: Intervention components: CPM, light dressings, orthosis: dorsal extension lock orthosis fabricated extending from the proximal forearm to the proximal interphalangeal joints positioned with wrist flexed 30 degrees and metacarpophalangeal joints flexed 45 degrees to be used during CPM use. Palmar straps supporting the forearm, wrist, transverse palmar arch, and proximal phalanges maintained the extremities securely in the orthosis. A second dorsal extension‐block orthosis, which extended to the fingertips, was fabricated during the first therapy session. This was worn if the CPM machine was not in use. In addition to being taught how to apply, operate, and remove the CPM machine, participants were instructed in early motion exercises that were to be performed if the device malfunctioned. The exercises were the same as those taught to participants of group 2. Technical description of the CPM device and use: CPM 5000, Sutter Biomedical, San Diego, California. Participant was attached to the CPM and adjusted so that the interphalangeal joints could be moved through an arc of flexion and extension. The goal was to achieve a 60 degree arc of proximal interphalangeal joint motion and a 30 to 40 degree arc of distal interphalangeal joint motion. Typically, it required two therapy sessions to achieve this goal. They were given charts on which to log the length of each CPM session. Therapists verified the record by comparing it with the elapsed‐time read‐out located on the CPM device. The patient was instructed to remove the CPM drive bar from the fingertips and to form a fist gently ten times every two hours during the day. The drive bar was then reattached and the CPM machine reactivated. Passive and active exercises were continued. Dose: CPM: Both rate and force parameters of CPM motion were maintained at the medium setting: 160 cycles of interphalangeal joint flexion and extension per hour (i.e. one cycle every 25 seconds). Frequency of administration: commenced one day post‐surgery. Participants were instructed to wear the device for eight to 12 hours a day for six weeks. Week 5 to 6: Intervention components: CPM alternated with active exercise regimen. The CPM and splinting were discontinued six weeks postoperatively. Exercise regimen: active motion was allowed 4 weeks postoperatively. At 6 weeks, isolated interphalangeal joint blocking exercises and gentle composite extension. Dose: CPM: both rate and force parameters of CPM motion were maintained at the medium setting: 160 cycles of interphalangeal joint flexion and extension per hour (i.e. one cycle every 25 seconds). Frequency of administration: commenced one day post‐surgery. Participants were instructed to wear the device for 8 to 12 hours a day for six weeks. Week 8 to 12: Intervention components: resistive exercises commenced. The exercise regimen gradually progressed to full activity by the 12th postoperative week. Control: Controlled passive progressed to active exercise regimen Intervention components: orthosis: dorsal blocking orthosis applied on the first postoperative day, positioned with wrist in 30 degree flexion, the metacarpophalangeal joints in 60 to 70 degree flexion, and the interphalangeal joints either in neutral or flexion through rubber band traction. The protective orthosis was discontinued at six weeks. Exercise regimen: Week 1 to 4: patients performed active extension exercises to the confines of the orthosis and passive flexion to the distal palmar crease. Week 4: active finger flexion initiated. Week 6 to 8: progressive resistive exercises gradually introduced and progressed until full activity achieved. Week 12: no restrictions. Dose: the rehabilitation protocol varied slightly in cycle number and duration depending upon the facility at which the patient was treated. The approximate number of cycles of interphalangeal flexion/extension ranged from 120 to 300 cycles a day for the first 6 weeks. Frequency of administration: the rehabilitation protocol varied slightly in cycle number and duration depending upon the facility at which the patient was treated. Both groups: All patients begun therapy at one day post operation. Orthosis: post‐operatively, bulky long‐arm dressings with dorsal plaster splints were applied, with the wrist in 30 degrees flexion and the metacarpophalangeal joints in 70 degrees flexion. |
|
| Outcomes | Outcomes were assessed at a minimum of 6 months post‐surgery (mean follow‐up period was 10.8 months (6 to 38 months)):
|
|
| Funding and conflicts of interest statements | Funding source: not reported Conflicts of interest: not reported | |
| Notes | Only patients with a minimum follow‐up time of six months from repair to re‐examination were included. The authors did not report on how many were eligible or randomised into the study, only those whose data were analysed.
Checks of the raw data provided indicated that the authors reported standard errors; we converted these to standard deviations. No clinical trial registration found. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Postoperatively, patients were placed in one of two study groups depending upon the month in which they were born. Group 1 included those patients born in even‐numbered months (February, April, June, August, October, and December). Group 2 consisted of those patients born in odd numbered months (January, March, May, July, September, and November)." Comment: the sequence appears to have been generated using a quasi‐randomised method. |
| Allocation concealment (selection bias) | High risk | Quote: "Postoperatively, patients were placed in one of two study groups depending upon the month in which they were born. Group 1 included those patients born in even‐numbered months (February, April, June, August, October, and December). Group 2 consisted of those patients born in odd numbered months (January, March, May, July, September, and November)." Comment: due to the use of quasi‐randomisation, the allocation sequence was not concealed prior to randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: there were no self‐reported outcomes. |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Quote: "Final evaluations were performed by the therapists…" Comment: the treating therapists performed the outcome evaluations and thus could not be blinded to the intervention. |
| Incomplete outcome data (over 6 months) (attrition bias) | High risk | Quote: "Only patients with a minimum follow‐up time of six months from repair to re‐examination were included." Comment: the number of participants who were randomised into the study is not reported. It is unclear how many participants dropped‐out, and how this might have affected the data analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes prespecified in the methods section of the publication, were reported in the results section of the publication. However, without a trial protocol, it is unclear whether other outcomes were assessed but not reported. |
| Other bias (outcomes appropriately analysed) | Low risk | Comment: participants were randomised per person, but some of the statistical analyses were conducted per digit. The authors state the number of participants/digits/tendons in the analysis. It is unlikely that this would likely impact the outcomes. No further sources of bias were identified. |
Gulke 2018.
| Study characteristics | ||
| Methods |
Study design: parallel group randomised controlled trial Setting: single‐centre; Physiotherapy clinic in Germany Unit of randomisation: participant Unit of analysis: unclear |
|
| Participants |
Details of sampling frame: Total eligible: not reported Total excluded pre‐randomisation: not reported Baseline characteristics: Total randomised: 62 participants (digits not reported) Exoskeleton group: 31 (probably) Exercise group: 31 (probably) Sex distribution: 44 males; 18 females (not reported by group) Age: mean (range): Mean 29.5 years (18 to 60 years) (not reported by group) Flexor tendon zone: zone II: 62 participants Inclusion criteria:
Exclusion criteria:
Surgical technique for flexor repair: Tendons were repaired using absorbable PDS sutures. Two‐strand core Kirchmayer ‐ Kessler sutures (4.0 PDS) and simple continuous epitendinous (6.0 PDS) was used to repair the tendon. In the distal zone II, the radial and ulnar slip of the FDS was repaired using a Z suture (4.0 PDS) or analogue to FDP. Characteristics of participants lost to follow‐up and included in analysis: Total excluded from analysis: 3 participants (2 complications and 1 lost to follow‐up) Total available at 18 weeks follow‐up: 59 participants (digits not reported). Total available at earlier time‐points not reported. |
|
| Interventions |
Intervention: Exoskeleton Intervention components: this group commenced use of exoskeleton at two weeks post surgery, three times a week for 30 min administered by the physiotherapists at the hospital. Exoskeleton was attached dorsally on the finger with Velcro with the wrist held in 30 degrees flexion. Due to the circular motion arms of the exoskeleton, the pressure on the extension side of the finger was always perpendicular to the axis of motion. In the beginning of each session, ROM was measured and the exoskeleton was adjusted to the individual finger. Control: Physiotherapy Intervention components: participants only received the treatments common to both groups (see below). Dose: the duration of the treatment was variable. Frequency of administration: three times a week. Physiotherapy was stopped when the doctor found that the participant had free function or the patient was satisfied with the functional result. Both groups: Intervention components: modified Kleinert orthosis consisting of a dorsal blocking splint with rubber band traction applied to the fingertips. All participants were in the hospital for four days post surgery and provided with education and an exercise regimen. Exercises included finger active and passive (if required) extension of the finger in the orthosis. If passive flexion through dynamic pull of the orthosis could not be achieved, patient was advised to assist full flexion with the unaffected hand. Scar management was initiated 2 weeks after surgery following removal of stitches. Arm was bathed in lukewarm chamomile tea, scar massaged, moisturised and orthosis was reapplied. All treatments including were completed in 30 degrees wrist flexion. Dose: exercises: 10 x each exercise. Scar management: 10 minutes. Chamomile tea bath: not reported. Frequency of administration: exercises: every hour, for six weeks duration. Scar management: three times per day. Chamomile tea bath: not reported. Moisturiser application: not reported. |
|
| Outcomes | Outcomes were assessed at 6, 12 and 18 weeks post‐flexor tendon repair (at following the commencement of treatments):
|
|
| Funding and conflicts of interest statements | Funding source: not reported Conflicts of interest: none reported | |
| Notes | German language paper ‐ data extracted by translators (see Acknowledgements for translators) No clinical trial registration found. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: patients were assigned to one of two groups using standardised controlled block randomisation. The allocation was based on a randomisation sheet, created by an established randomisation program. The randomisation sequence appears to have been generated using an adequate method. |
| Allocation concealment (selection bias) | Unclear risk | Comment: it appears that the randomisation sequence was kept on a randomisation sheet. However, it is unclear who held this sheet (e.g. external person versus a member of the research team) and whether this sheet was kept for the duration of the recruitment using a concealed method. We attempted to contact the authors but did not receive a response by the time of publication. Therefore, it is unclear what method was used to conceal the random allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of the intervention they received. Given the nature of the interventions, trial personnel (treaters) were not blind to treatment, and may have had different expectations about the benefits of the treatments they were delivering. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: non‐blinded participants, who may have had different expectations about the benefits of the intervention they received. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: it was not reported whether the outcome assessors were blinded to the intervention. We attempted to contact the authors but had not received a response by the time of publication. |
| Incomplete outcome data (less than 3 months) (attrition bias) All outcomes | Unclear risk | Comment: participants lost to follow‐up and due to complications have been reported as overall numbers for both groups combined. We are therefore unsure in which group the three excluded participants were assigned and at which time point. In addition, how many participants were randomised to each group is not reported in the publication; we have assumed it was 31 in each group. We attempted to contact the authors to clarify but had not received a response. However, it appears that these were excluded from the analysis, and were likely to be accounted for. |
| Incomplete outcome data (3 to 6 months) (attrition bias) All outcomes | Unclear risk | Comment: participants lost to follow‐up and due to complications have been reported as overall numbers for both groups combined. We are therefore unsure in which group the three excluded participants were assigned and at which time point. In addition, how many participants were randomised to each group is not reported in the publication; we have assumed it was 31 in each group. We attempted to contact the authors to clarify but had not received a response. However, it appears that these were excluded from the analysis, and were likely to be accounted for. |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes prespecified in the methods section of the publication, were reported in the results section of the publication. Also without a trial protocol, it is unclear whether other outcomes were assessed but not reported. |
| Other bias (outcomes appropriately analysed) | Unclear risk | Comment: participants contributed more than one tendon and data appears to have been reported at the participant level. Therefore, a unit of analysis error may have occurred. We attempted to contact the authors to clarify but had not received a response by the time of publication. No other sources of bias were identified. |
Hagberg 2000.
| Study characteristics | ||
| Methods |
Study design: parallel group randomised trial* Setting: Sweden Unit of randomisation: participant Unit of analysis: unclear |
|
| Participants |
Details of sampling frame: Total eligible: not reported Total excluded pre‐randomisation: not reported Baseline characteristics: Total randomised: 100 participants (108 digits) Active group: not reported Controlled passive group: not reported Sex distribution: not reported Age: not reported Flexor tendon zone: zone II: 108 digits Inclusion criteria:
Exclusion criteria: Not reported. Surgical technique for the flexor tendon repair: Direct tendon repair in zone II. Characteristics of participants lost to follow‐up and included in analysis: Not reported |
|
| Interventions |
Intervention 1: Early active flexion exercise regimen Intervention component: active flexion exercise regimen for the first three weeks post‐surgery. No other treatments reported in the publication.* Dose: not reported Frequency of administration: not reported Intervention 2: Controlled passive exercise regimen Intervention components: early controlled passive exercise regimen with rubber band traction (assumed to be attached to a dorsal blocking split). No other treatments reported in the publication.* Dose: not reported Frequency of administration: not reported Both groups: all participants were permitted active mobilisation at 3 weeks after surgery. |
|
| Outcomes | Outcomes were measured at 3, 4, 6, 8 weeks; 4 months and 1 year:
|
|
| Funding and conflicts of interest statements | Funding source: not reported Conflicts of interest: not reported | |
| Notes | *This was a conference proceeding. Hence, very little information was reported in the publication. No other publications of the trial were found. No clinical trial registration or publication of the full trial found. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "One hundred consecutive patients with flexor tendon laceration in 108 digits were stratified according to type of injuries after having direct tendon repair and were randomised to either early active mobilisation or early controlled mobilisation with rubber band traction." Comment: there is insufficient information in the publication to judge whether the allocation sequence was generated in a random manner. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "One hundred consecutive patients with flexor tendon laceration in 108 digits were stratified according to type of injuries after having direct tendon repair and were randomised to either early active mobilisation or early controlled mobilisation with rubber band traction." Comment: there is insufficient information in the publication to judge whether the allocation sequence was adequately concealed prior to the randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: due to the nature of the interventions, it is not likely that the participants or the intervention personnel would have been blinded to the intervention. However, no self‐reported measures were reported in the results. As this was a conference proceeding, we are unsure whether there were additional outcomes included. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: it is not reported in the publication whether the outcome assessors were blinded to the intervention. We were unable to contact the authors to clarify. |
| Incomplete outcome data (less than 3 months) (attrition bias) All outcomes | Unclear risk | Comment: data were collected at 3, 4, 6 and 8 weeks. Yet, no outcome data were reported for less than 12 weeks in the publication. This was a conference abstract and attempts to contact the authors were unsuccessful and a full paper of the study has not been published. |
| Incomplete outcome data (3 to 6 months) (attrition bias) All outcomes | Unclear risk | Comment: it is unclear in the publication the flow through of participants from baseline, to randomisation, to outcome measurement at follow up. The numbers of participants randomised to each group are not provided and no information is provided on whether all of these participants continued through the study to the 12 month follow‐up. Data were collected at multiple intervals but was not reported except at the one‐year interval. This was a conference abstract and attempts to contact the authors were unsuccessful and a full paper of the study has not been published. |
| Incomplete outcome data (over 6 months) (attrition bias) | Unclear risk | Comment: It is unclear in the publication the flow through of participants from baseline, to randomisation, to outcome measurement at follow‐up. The numbers of participants randomised to each group are not provided and no information is provided on whether all of these participants continued through the study to the 12 month follow‐up. Data were reported for the one year follow‐up; however, there is no reporting of the number of randomised versus followed‐up participants, or dropouts, at this time point. This was a conference abstract and attempts to contact the authors were unsuccessful and a full paper of the study has not been published. |
| Selective reporting (reporting bias) | High risk | Comment: the conference abstract does not contain a detailed methods section. It is unclear whether selective outcome reporting occurred. Also without a trial protocol, it is unclear whether other outcomes were assessed but not reported. In addition, no standard deviations or p‐values for the outcomes are reported in the abstract. |
| Other bias (outcomes appropriately analysed) | Unclear risk | Comment: it is unclear in the results whether the number of ruptures that occurred was per person, digit or tendon. A unit of analysis error may have occurred, but this is unclear. |
Kneafsey 1994.
| Study characteristics | ||
| Methods |
Study Design: parallel group randomised trial* Setting: single‐centre; Welsh Plastic Surgery Centre, UK Unit of randomisation: participant Unit of analysis: unclear |
|
| Participants |
Details of sampling frame: Total eligible: not reported Total excluded pre‐randomisation: not reported Baseline characteristics: Total randomised: 112 participants Passive: not reported Controlled passive: not reported Sex distribution: not reported Age: not reported Flexor tendon zone: not reported Inclusion criteria:
Exclusion criteria:
Surgical technique: Strickland (1985) repairs in zones I to III. Characteristics of participants lost to follow‐up and included in analysis: Total available for follow‐up: not reported Total drop‐outs: not reported Total analysed: 80 participants (interim analysis) Passive: not reported Controlled passive: not reported |
|
| Interventions |
Intervention 1: Early passive exercise regimen (modified Duran protocol) Components of the intervention: exercise regimen: early passive flexion without rubber band traction, controlled passive mobilisation regimen (cited as using the modified Duran, Strickland and Glogovac regimen 1990). Isolated and composite passive flexion in the orthosis without the rubber band traction, and both active and passive extension within the orthosis. Dose: not reported Frequency of administration: not reported Intervention 2: Early controlled passive exercise regimen (modified Kleinert protocol) Components of the intervention: exercise regimen: early controlled passive flexion using rubber band traction, controlled passive flexion with active extension regimen (modified Kleinert Regime, May et al 1992). Active extension exercises and fingers were maintained in passive flexion using rubber band traction orthosis. The rubber band traction allowed the passive movements to be controlled. Dose: not reported Frequency of administration: not reported Both groups: Components of the intervention: participants were seen within 72 hours of surgery prior to leaving the hospital, and subsequently continued physiotherapy under supervision on an out‐patient basis. In addition, participants were reviewed on a weekly basis by medical staff. Dose: not reported Frequency of administration: not reported |
|
| Outcomes | Outcomes were measured at 3 and 6 month intervals; additional active ROM was recorded at 6 weeks.
|
|
| Funding and conflicts of interest statements |
Funding source: not reported Conflicts of interest: not reported |
|
| Notes | *This was a conference proceeding reporting an interim analysis. Hence, very little information was reported in the publication. The authors were contacted to provide more information about the study methods and results, but no response from the authors was received. No other publications on this study were found. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A prospective, randomised study was set up at the Welsh Regional Plastic Surgery Centre in July 1992." Comment: there is insufficient information to determine whether the randomisation sequence was adequately generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A prospective, randomised study was set up at the Welsh Regional Plastic Surgery Centre in July 1992." Comment: information was insufficient to reveal the adequacy of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: due to the nature of the interventions, it is unlikely that the participants or intervention personnel were blinded to the intervention. However, no self‐reported measures were reported. However, since this was a conference abstract outcomes there is insufficient information to make a judgement. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: there is insufficient information to determine whether the outcome assessors were blinded, or not, to the group assignments. |
| Incomplete outcome data (less than 3 months) (attrition bias) All outcomes | Unclear risk | Comment: there is no information provided about the flow of participants through the study, reasons for exclusions, attrition or for being excluded from the analysis. |
| Incomplete outcome data (3 to 6 months) (attrition bias) All outcomes | Unclear risk | Comment: there is no information is provided about the flow of participants through the study, reasons for exclusions, attrition or for being excluded from the analysis. |
| Selective reporting (reporting bias) | High risk | The only data reported are quote: "combination of Kleinert and Strickland grading (So et al, 1990). Statistical analysis carried out on the first 80 patients using the paired students t test shows no significant difference in outcome." Comment: no means, standard deviations of p‐values are reported. Very little data are provided in this conference proceeding. |
| Other bias (outcomes appropriately analysed) | Unclear risk | Comment: insufficient information is provided in the publication to determine whether a unit of analysis error may have occurred, or whether standardised methods for measuring the outcome were used. |
Ozkan 2004.
| Study characteristics | ||
| Methods |
Study design: parallel group randomised controlled trial Setting: single‐centre; Hand surgery unit, Uludag University Medical Faculty, Bursa, Turkey Unit of randomisation: participant Unit of analysis: digit |
|
| Participants |
Details of sampling frame: Total eligible: not reported Total excluded pre‐randomisation: not reported Baseline characteristics: Total randomised: 25 participants (41 digits) Laser group: 13 participants (21 digits) Control group: 12 participants (20 digits) Sex distribution: Randomised: 15 males; 10 females Laser group: 8 males; 4 females* Control group: 6 males; 6 females* Age: mean ± SD (range): 23.75 (range: 7 to 43) years Laser group: 23.75 ± 2.56 years Control group: 24.0 ± 3.03 years Flexor tendon zone: Zone I: 4 (Group 1: 2; Group 2: 2) Zone II: 13 (Group 1: 6; Group 2: 7) Zone III: 8 (Group 1: 3; Group 2: 5) Zone IV: 3 (Group 1: 3; Group 2: 0) Zone V: 11 (Group 1: 6; Group 2: 5) Inclusion criteria:
Exclusion criteria:
Surgical technique for flexor tendon repair: No details reported. Characteristics of participants lost to follow‐up/drop‐outs and included in analysis: Total available for follow‐up: 25 (41 digits) Total drop‐outs: 2 tendon ruptures were excluded Total analysed: 23 (39 digits) |
|
| Interventions |
Intervention: Low level laser therapy (LLLT) Components of the intervention: laser: following a whirlpool treatment, laser was applied to four different points with 1 cm intervals along the injury zone. The head of the instrument was held perpendicular to and in slight contact with the skin. Technical description of the laser device: the infrared‐27 GaAs diode laser instrument (Roland Series Elettronica Pagani) with the wavelength of 904 nm, frequency range of 5–7000 Hz, and maximum power of 27 W, 50 W, or 2734 W. Dose: frequency: 100 Hz for 130 second duration Frequency of administration: once per day for 10 weekdays during a two‐week period. Control: Placebo Components of the intervention: placebo laser treatment was given by using the same instrument as the intervention group, and placing its head in the same way on the hand but not turning it on. Dose: 130 second duration Frequency of administration: once per day for 10 weekdays during a two‐week period. Both groups: Components of the intervention: orthosis: modified Kleinert orthosis with a palmar pulley was applied to the injured hand of each patient three days after surgery. Exercise regimen: Washington exercise regimen was implemented for 12 weeks post‐operatively. Additional components: whirlpool (35 °C was applied to the injured hand of the patient for 15 minutes) and laser commenced from days 8 to 21. Dose: not reported Frequency of administration: not reported |
|
| Outcomes | Outcomes were assessed weekly up to 12 weeks:
|
|
| Funding and conflicts of interest statements | Funding source: not reported Conflicts of interest: not reported | |
| Notes | * There is an inconsistency in the number of males and females in each group compared with the total number reported in the paper. Authors were contacted for clarification, but no response was received. **Not an outcome of interest for this review No clinical trial registration found. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: correspondence received from the authors states that "it was a randomised controlled study. The patients were randomised into two groups using random‐number table." Comment: an adequate methods was used to generate the randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were assigned into two groups by a second observer other than the one who made the evaluation throughout the study." Comment: tt appears that someone who was not involved in the treatments assigned the groups. However, there is still insufficient information to determine who this person was and whether an adequate method was used to conceal the allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: study is described as "a placebo‐controlled double‐blind prospective study model". Comment: participants are likely to have been blinded to the intervention as the placebo group received the same treatment without the machine being switched on. It may not have been possible for the personnel (treaters) providing the intervention to be blinded due to the nature of the intervention, if they were required to change the settings on the laser machine. However, since none of the self‐reported outcomes are outcomes of interest for this review, risk of bias is likely not have occurred. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Quote: study is also described as " a placebo‐controlled double‐blind prospective study model". Comment: it is not clearly reported at what level the blinding occurred. Due to the nature of the intervention, the participants were likely to have been blinded. It is unlikely that the care providers were blinded as they would need to set the parameters on the laser machine. It is unclear whether participants were provided with any information from the treating personnel that would make them perceive the laser they received as superior to the placebo. However, since the self‐reported measures were also not outcomes of interest for this review, this is unlikely to have biased the results. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The patients were assigned into two groups by a second observer other than the one who made the evaluation throughout the study." Study is also described as " a placebo‐controlled double‐blind prospective study model". Comment: it appears that the person who conducted outcome evaluations was blinded to the intervention. |
| Incomplete outcome data (less than 3 months) (attrition bias) All outcomes | Unclear risk | Comment: one exclusion reported in each group due to tendon rupture, but no attrition due to drop‐out mentioned in either group. Although not reported, this does not mean that it did not occur. |
| Selective reporting (reporting bias) | Unclear risk | Comment: all data for the outcomes mentioned in the methods section are reported in the results section. Also without a trial protocol, it is unclear whether other outcomes were assessed but not reported. |
| Other bias (outcomes appropriately analysed) | Unclear risk | Comment: too little information is provided in the publication to know whether a unit of analysis error may have occurred. Outcomes are reported for digits and it is unclear if grip strength was measured per participant or per digit and how this was accounted for in the analysis. There is also a discrepancy in the number of participants reported in total (25 patients) and the total in gender distribution (24 patients). |
Poorpezeshk 2018.
| Study characteristics | ||
| Methods |
Study Design: parallel group randomised controlled trial Setting: single‐centre; Department of Plastic Surgery, 15 Khordad Hospital of Shahid Beheshti University of Medical Sciences, Tehran, Iran; 2015 to 2016 Unit of randomisation: participant Unit of analysis: participant |
|
| Participants |
Details of sampling frame: Total eligible: not reported Total excluded pre‐randomisation: not reported Baseline characteristics: Total randomised: 97 participants (114 fingers) Laser: 39 participants (46 fingers) Control: 58 participants (68 fingers) Sex distribution: not reported at baseline Age: not reported at baseline Flexor tendon zone: not reported at baseline Inclusion criteria:
Exclusion criteria:
Surgical technique: All tendons were repaired with four‐strand repairs. Primary repair was performed under general or regional anaesthesia, 6 to 24 hours following the patient’s admission. The surgery protocol under loop magnification was Brunner incision, repairing flexor tendons by four‐strand modified Kessler core suture method; periphery running suture was performed with 4/0 Nylon, and digital nerve repairing with 10/0 Nylon in 32 patients. Characteristics of participants lost to follow‐up/drop‐outs and included in analysis: Total drop‐outs: 20 participants, all in control group Total available for 4‐week follow‐up: 77 participants (92 fingers) Total available for 4‐week follow‐up: laser group: 39 participants (46 fingers) Total available for 4‐week follow‐up: control group: 38 participants (46 fingers) Sex distribution at follow‐up: 60 males; 17 females Laser: 31 males; 8 females Control: 29 males; 9 females Mean ± SD age: Laser: 27.85 +/‐ 9.26 Control: 26.72 +/‐ 9.69 Flexor tendon zone distribution: Zone I: 6 (Laser: 2; Control: 4) Zone II: 67 (Laser: 37; Control: 30) Zone III: 19 (Laser: 5; Control: 14) |
|
| Interventions |
Intervention: Low level laser therapy (LLLT) Components of the intervention: LLLT as commenced at day two post‐surgery, within the plaster brace. Technical specification of the LLLT device: Mustang 2000 Laser device (Technical Co., Moscow, Russia) with two probes of red (KLO4) and infrared laser (LO7). The laser probes were placed over the repairing site in the contact method. Red and infrared laser were used to accelerate tendon healing. Dose: the setting for the red laser was continuous mode, 660 nm, and 2 J/cm2. Infrared laser in pulsed mode, wave 810 nm, 100 Hz, 5.85 J/Cm2. Specification for the LLLT applied: Peak power output 15 W; power density 15 W/cm2; wave length 890 nm; pulse frequency 100 Hz; spot size 0.002 cm2; pulsed duration 130 ns; duration of exposure for each point 60 sec; energy density 5.85 J/cm2. Frequency of administration: 2 to3 times per week, for 10 sessions over four‐week period. Control: Placebo Components of the intervention: placebo low level laser therapy with the power off. Dose: placebo dose with machine off. Frequency of administration: 2 to 3 times per week, for 10 sessions over a four‐week period. Both groups: Components of the intervention: orthosis: plaster brace with 10 degrees wrist, 90 degrees MCP joint and zero degrees IP joint flexion. Exercise regimen: Kleinert rehabilitation regimen was started within the first 24 hours. Dose: orthosis: full time for four weeks. Exercise regimen: not reported Frequency of administration: orthosis: full time for four weeks. Exercise regimen: not reported |
|
| Outcomes | Outcome assessment was recorded at four weeks.
|
|
| Funding and conflicts of interest statements |
Funding source: Vice Chancellor for research Shahid Beheshti University of Medical Sciences Conflicts of interest: none declared |
|
| Notes | Trial registered: IRCT2017050233783N1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " Using the unequal treatment allocation method, patients were randomly divided into two groups… We used the stratified block randomisation scheme with an allocation ratio of 0.6:0.4 to determine the unequal sample size…" Comment: the randomisation sequence appears to have been generated using an adequate method. |
| Allocation concealment (selection bias) | Unclear risk | Comment: it is unclear how the allocation sequence was concealed, if at all, until the intervention was assigned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: although not explicitly stated in the publication, the clinical trials registry states that the study was "double‐blinded". However, there is insufficient information in the publication to know at which level this blinding occurred. Due to the nature of the interventions, it is possible that the participants could have been blinded to the interventions, but this was not explicitly stated. It is also possible that the personnel (treaters) were blinded but this was not explicitly stated. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Comment: although not explicitly stated, the clinical trials registry states that the study was "double‐blinded". However, there is insufficient information in the publication to know at which level this blinding occurred. Due to the nature of the interventions, it is possible that the participants could have been blinded to the interventions, but this was not explicitly stated. It is also possible that the care providers were blinded but this was not explicitly stated. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The two observers, blind to the LLLT group, assessed the data independently." Comment: it appears that the outcome assessors were blind to the intervention assignment. |
| Incomplete outcome data (less than 3 months) (attrition bias) All outcomes | High risk | Quote: "Of 58 patients in the control group, 20 patients did not come back for follow up and 38 patients were treated with involvement of total 46 fingers. None has attended a hand therapy clinic" Comment: participants lost to follow‐up are outlined in the CONSORT diagram and described in the text. Reasons for lost to follow‐up were stated. This was also accounted for in the analysis and the researchers blocked randomisation at a ratio of 0.6:0.4. However, although documented, this loss to follow‐up (34% of participants in the control group) is high. |
| Selective reporting (reporting bias) | Low risk | Comment: all data for the outcomes mentioned in the methods and clinical trials registry are reported in the results section. It appears that earlier weekly data may have been collected and not reported. However, it is unlikely that this impacts our judgement of the clinical effectiveness of the treatment. |
| Other bias (outcomes appropriately analysed) | Unclear risk | Comment: it is unclear whether a unit of analysis error for the range of motion measures has occurred, as it reports participants but this is a digit level measurement. No further sources of bias were identified. |
Rigo 2017.
| Study characteristics | ||
| Methods |
Study design: parallel group randomised trial Setting: single‐centre; Department of Orthopaedic Surgery, Oslo, Norway Unit of randomisation: participant Unit of analysis: digit and participant (where appropriate) |
|
| Participants |
Details of sampling frame: Total eligible: 53 patients (73 fingers) Total excluded pre‐randomisation: 0 patients Baseline characteristics: Total randomised: 53 participants (73 fingers) Active flexion: 24 participants (39 fingers) Controlled passive: 29 participants (33 fingers) Sex distribution: 36 males; 14 females Active flexion: 18 males; 4 females Controlled passive: 18 males; 10 females Age: mean (range): Active: 37 years (18 to 66 years) Controlled passive: 40 years (19 to 72 years) Flexor tendon zone: Zone I: 18 (Active: 12; Control: 6) Zone II: 47 (Active: 25; Control: 22) Zone III: 4 (Active: 0; Control: 4) Inclusion criteria:
Exclusion criteria:
Surgical technique: Tendon repairs were performed one to four days after injury. The wound was extended in a zig‐zag fashion and the sheath was opened in the palmar midline with limited pulley release at the site of the repair. The FDP tendon was directly repaired with a two‐strand core suture in a side‐locking loop configuration using 3‐0 braided poly blend polyethylene (FiberWire; Arthrex Co., Naples, FL, USA). The repair was completed with a running epitendinous suture with 5‐0 monofilament nylon (Dermalon; Covidien Ltd, Mansfield, MA, USA) in an interlocking horizontal mattress suture fashion. This repair configuration is similar to the Silfverskold repair, with the differences in use of FiberWire as the suture material and locking loops of the core suture placed on the side instead of the volar surface of the tendon and in use of Dona’s interlocking horizontal mattress suture instead of cross‐stitches in making peripheral sutures. In cases with avulsion or a distal tendon stump too short for placement of a suture, the tendon was reattached with transverse intraosseous loop technique. The core suture was identical to the end‐to‐end repair. Characteristics of participants lost to follow‐up/drop‐outs: Total drop‐outs: 8 participants (9 fingers) Total excluded from analysis for 12 months follow‐up: 8 participants (9 fingers) Active flexion: 4 participants (2 ruptures and 2 lost to follow‐up) Controlled passive: 4 participants (1 rupture and 3 lost to follow‐up) Total available for 12 months follow‐up: 45 participants (63 fingers) Active flexion: 20 participants (34 fingers) Controlled passive: 25 participants (29 fingers) Number of digits included in analyses*: 1 month: active flexion: 37; controlled passive: 42 2 months: active flexion: 36; controlled passive: 32 3 months: active flexion: 36; controlled passive: 31 6 months: active flexion: 32; controlled passive: 30 12 months: active flexion: 34; controlled passive: 29 |
|
| Interventions |
Intervention: Active flexion plus controlled passive exercise regimen Components of intervention: exercise regimen: additional warm up exercises from day 1 post surgery with additional active extension and passive flexion hourly, followed by active unresisted finger flexions with the rubber bands released. Standard care as below. Dose: 10 repetitions of active extension/passive flexion; 10 to 20 active flexion. Frequency of administration: every waking hour for four weeks (starting from day one post‐surgery). Control: Controlled passive exercise regimen (modified Kleinert protocol) As described below. Both groups: Components of the intervention: dressings: bandages removed on first post‐operative day and spray on dressing applied. Orthosis: standardised dorsal blocking plaster orthosis applied in 0 to 20 degrees wrist flexion and 50 to 80 degrees MCP joint flexion. Splint extended to PIP joint distally. Rubber bands were attached to the nails of the injured finger and pulley placed in the palm (as per modified Kleinert regimen). Exercise regimen: weeks 1 to 4: full passive flexion using other hand, and active extension. Six weeks: active flexion exercises initiated for Standard care group and continued for Intervention group. Graded functional use: Simple activities of daily living (ADL) allowed at six weeks and gradual increasing in load to allow full gripping at 12 weeks. Dose: orthosis: worn full time for four weeks. Exercise regimen: 20 to 30 repetitions each exercise. Graded functional use: not reported. Frequency of administration: orthosis: worn full time for four weeks. Exercise regimen: every waking hour. Graded functional use: not reported. |
|
| Outcomes | Outcomes were assessed at 1, 2, 3, 6, 12 months after surgery:
|
|
| Funding and conflicts of interest statements | Funding source: none received Conflicts of interest: none declared | |
| Notes | The unit of analysis was fingers for the appropriate outcome measures, and participants for appropriate outcomes measures. No clinical trial registration found. Additional data was provided by correspondence from the authors including a data table for AROM, grip and pinch strengths, and VAS ADLs with means and SDs for 1, 2, 3, 6 and 12 months. *Analyses were conducted per digit. We used the number of digits as described in Table 3 of the publication for all our analyses conducted in RevMan. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We randomised with closed envelopes without any external identification, concealing the allocation until opening. At inclusion, every patient chose an envelope, which was opened after the repair was completed and the orthosis with rubber bands was applied,"; and correspondence received from authors: "The envelops were mixed like cards, then the patients themselves chose one from the deck of envelops, that means the sequence was random." Comment: it appears that the sequence was generated in a random manner. |
| Allocation concealment (selection bias) | Low risk | Quote: "We randomised with closed envelopes without any external identification, concealing the allocation until opening. At inclusion, every patient chose an envelope, which was opened after the repair was completed and the orthosis with rubber bands was applied." Comment: allocation was concealed in opaque envelopes. An adequate method was used to conceal the allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: non‐blinded participants who may have had different expectations about the benefits of the intervention they received when assessing functional use. |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Quote: "Two therapists (not blinded to group allocation) performed the registrations." Comment: the therapists who conducted the outcome assessments were not blinded to group allocation. |
| Incomplete outcome data (less than 3 months) (attrition bias) All outcomes | Low risk | Comment: information is provided about the flow of participants through the study, reasons for exclusions, attrition or for being excluded from the analysis. Loss to follow‐up was minimal and unlikely to have influenced the study findings. |
| Incomplete outcome data (3 to 6 months) (attrition bias) All outcomes | Low risk | Comment: information is provided about the flow of participants through the study, reasons for exclusions, attrition or for being excluded from the analysis. Loss to follow‐up was minimal and unlikely to have influenced the study findings. |
| Incomplete outcome data (over 6 months) (attrition bias) | Low risk | Comment: information is provided about the flow of participants through the study, reasons for exclusions, attrition or for being excluded from the analysis. Loss to follow‐up was minimal and unlikely to have influenced the study findings. |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcome measures reported in the methods section were reported in the results section of the publication. However, without a trial protocol, it is unclear whether other outcomes were assessed but not reported. |
| Other bias (outcomes appropriately analysed) | High risk | Comment: it appears that an unit of analysis error has occurred for the measurement of grip strength. Authors reported that they analysed all outcomes per finger digit, however grip strength is a participant level variable. No further sources of bias were found. Furthermore, grip and pinch strength were measured as a percentage of the contralateral side with no controlling for hand dominance. |
Scavenius 2000.
| Study characteristics | ||
| Methods |
Study Design: parallel group randomised trial Setting: single‐centre; Department of Hand Surgery, Copenhagen University Hospital, Denmark Unit of randomisation: participant Unit of analysis: participant (only contributed one digit) |
|
| Participants |
Details of sampling frame: Total eligible: not reported Total excluded pre‐randomisation: not reported Baseline characteristics: Total randomised: 39 participants (39 digits; 39 tendons) Active: 19 tendons (thumb and digits) Passive: 20 tendons (thumb and digits) Sex distribution: not reported Age: not reported Flexor tendon zone: Zone I: 7 (includes one FPL) Zone II: 32 (includes 5 FPL) Inclusion criteria:
Exclusion criteria:
Surgical technique for the flexor repair: Primary tendon repair was performed within three days of injury. Each group received a different surgical technique. Active group: modified Kessler suture (Ti‐cron 4.0) Passive group: grasping suture (Prolene 2.0) and external pull‐out knot technique. Characteristics of participants lost to follow‐up/drop‐outs and included in analysis: Total drop‐outs: 6 (lost to follow‐up: 3; tendon ruptures: 3) Total excluded from analysis: 6 (lost to follow‐up: 3; tendon ruptures: 3) |
|
| Interventions |
Intervention 1: Active flexion plus active extension exercise regimen (plus modified Kessler suture surgical technique) Components of the intervention: this group's flexor tendon was repaired with a modified Kessler suture (Ti‐cron 4.0). Exercise regimen: early active controlled mobilisation, active extension and active flexion exercises (described as the May et al. protocol, but no citation or further description provided) Dose: not reported Frequency of administration: not reported Intervention 2: Passive flexion plus active extension exercise regimen (plus grasping suture and external pull‐out knot surgical technique) Components of the intervention: this group's flexor tendons were repaired with a grasping suture (Prolene 2.0) and external pull‐out knot technique. Exercise regimen: early passive exercise regimen, active extension and passive flexion exercises (described as the Mantero protocol, but no citation of further description provided). Dose: not reported Frequency of administration: not reported |
|
| Outcomes | Outcomes were measured at one‐year post surgery:
|
|
| Funding and conflicts of interest statements |
Funding source: not reported Conflicts of interest: not reported |
|
| Notes | This was a conference proceeding. Hence, very little information was reported in the publication. The authors were contacted to provide more information about the study methods and results, but no response from the authors was received. No clinical trials registration or publication of the full trial was found. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This study was conducted as a prospective randomised design…..." Comment: there is insufficient information in the publication to judge whether the allocation sequence was generated in a random manner. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "This study was conducted as a prospective randomised design…..." Comment: there is insufficient information in the publication to judge whether the allocation sequence was adequately concealed prior to the randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: due to the nature of the interventions, it is not likely that the participants or the intervention personnel would have been blinded to the intervention. However, none of the outcomes were self‐reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: there is insufficient information to determine whether the outcome assessors were blinded to the intervention. |
| Incomplete outcome data (over 6 months) (attrition bias) | Unclear risk | Comment: the conference abstract details that six participants were either lost to follow‐up or had tendon ruptures and therefore were not included in the one year follow‐up. It is not reported which group these drop‐outs occurred in and whether this biased the results. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the outcomes and methods for measurement are not detailed in the methods section of the conference abstract. It is unclear whether selective outcome reporting occurred. Also, without a trial protocol, it is unclear whether other outcomes were assessed but not reported. |
| Other bias (outcomes appropriately analysed) | High risk | Comment: groups received different surgical interventions that may have biased the results in favour of one group over another. As it appears that each participant only contributed one digit, it does not appear that an unit of analysis error has occurred. No further sources of bias were identified. |
Silva 2003.
| Study characteristics | ||
| Methods |
Study design: parallel group randomised controlled trial Setting: single‐centre; Service of Hand Surgery and Microsurgery, Clinica SOS Mao, Porto Alegre, Brazil Unit of randomisation: participant Unit of analysis: unclear |
|
| Participants |
Details of sampling frame: Total eligible: not reported Total excluded pre‐randomisation: not reported Baseline characteristics: Total randomised: 84 participants (84 digits; 152 tendons) Active group: 37 participants (37 digits; 68 tendons) Immobilisation group: 47 participants (47 digits; 84 tendons) Sex distribution: not reported Age: mean (range): Group 1: 32 (20 to 64) years Group 2: 35 (18 to 66) years Flexor tendon zone: zone II: 84 digits Inclusion criteria:
Exclusion criteria:
Surgical technique for the flexor tendon repair: Surgery was performed under axillary block and tourniquet. Brunner’s zigzag palmar incisions. Tendon repair was a combined Kessler’s modified technique using 3‐0 synthetic monofilament. A2, A3, A4 pulleys were preserved. Tendon sheath was not sutured. Arterial and nerve injuries, when present, were repaired with 9‐0 synthetic monofilament. All surgeries were performed 7 to 21 days after traumatic injury. Characteristics of participants lost to follow‐up/drop‐outs and included in analysis: Total available for follow‐up: not reported Total drop‐outs: not reported Total analysed: not reported |
|
| Interventions |
Intervention: Active exercise regimen Components of the intervention: exercise regimen: early active mobilisation initiated at 12 hours after tendon repair ‐ active flexion/extension exercises. Orthosis: dorsal splint protection (as per both groups). After re‐operation for an adverse event, the patient was then placed again on the same exercise regimen. Participants were reviewed weekly during the first month and post‐operative follow‐up ranged from 12 to 36 months. Dose: 10 repetitions of each movement. Frequency of administration: every waking hour for 16 hours per day. Intervention: Immobilisation regimen Components of the intervention: hand immobilised with a dorsal splint for three weeks; as per both groups (detailed below) Both groups: Components of the intervention: hand immobilised in a dorsal orthosis positioned in 30 to 60 degrees wrist flexion and the MCP joints in 90 degrees flexion with the IP joints extended. The orthosis was removed at week 3 post‐surgery. Participants were seen weekly at the clinic during the first month. Dose: orthosis: worn full time Frequency of administration: orthosis: worn full time for three weeks |
|
| Outcomes | Outcomes were recorded at a minimum of 12 months (mean 22 months, range 12 to 36 months) post‐surgery:
|
|
| Funding and conflicts of interest statements | Funding source: not reported Conflicts of interest: not reported | |
| Notes | There is some difficulty identifying the unit of analysis for the analysis: e.g. tenolysis unit of analysis is “cases”. We have assumed that since each participant only offered one digit to the study that a case represents both digit and person. The number of participants in each group was calculated manually from the data provided in the publication. No clinical trial registration found. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were prospectively randomised, and divided into two groups." Comment: there is insufficient information in the publication to judge whether the allocation sequence was generated in a random manner. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were prospectively randomised, and divided into two groups." Comment: there is insufficient information in the publication to judge whether the allocation sequence was adequately concealed until the interventions were assigned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: it is not reported whether the outcome assessors were blinded to the intervention, or who the outcome assessors were. |
| Incomplete outcome data (over 6 months) (attrition bias) | Unclear risk | Comment: it is unclear in the publication the flow through of participants from baseline, to randomisation, to outcome measurement at 12 months. It is unclear whether the number of participants reported includes any drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: all outcome measures reported in the methods section were reported in the results section of the publication. However, without a trial protocol, it is unclear whether other outcomes were assessed but not reported. Further, no means or standard deviations for the range of movement measurements were reported. |
| Other bias (outcomes appropriately analysed) | Unclear risk | Comment: randomisation occurred at the participant level. However, it is sometimes unclear whether some of the outcomes were reported at the tendon level (some participants had two tendons repaired) or at the participant or digit level. Therefore, a unit of analysis error may have occurred. |
Stenekes 2009.
| Study characteristics | ||
| Methods |
Study Design: parallel group randomised controlled trial Setting: single‐centre; Department of Plastic Surgery, Netherlands; 2003 to 2005 Unit of randomisation: participant Unit of analysis: unclear |
|
| Participants |
Details of sampling frame: Total eligible: not reported Total excluded pre‐randomisation: not reported Baseline characteristics: Total randomised: 28 participants Total excluded post‐randomisation: 3 participants (2 fractures intra‐op, 1 participant did not have a tendon injury) Sex distribution: not reported at baseline Age: not reported at baseline Flexor tendon zone: not reported Inclusion criteria:
Exclusion criteria:
Surgical technique: not reported Characteristics of participants lost to follow‐up/drop‐outs and included in analysis: Total available for follow‐up: 25 participants Total drop‐outs: 0 Total crossed over group: 1 participant allocated motor imagery was crossed over to the control Total analysed: 25 participants Intervention (baseline/follow‐up) n = 13/12* Control (baseline/follow‐up) n = 12/13* Sex distribution at follow‐up: Motor imagery: 9 males; 3 females Control: 9 males; 4 females Age: mean ± SD (range) at follow‐up: Motor imagery: 36.1 ± 11.3 years Control: 31.1 ± 10.0 years |
|
| Interventions |
Intervention: Motor imagery (MI) Components of the intervention: participants were instructed to perform active flexion and extension movements mentally during the immobilisation period. The instructions were as follows: “Try to imagine as vividly as possible that you slowly clench your fingers and bend the wrist of your splinted hand. Host this image for 3 seconds. Next, imaging that you straighten your wrist and stretch your fingers. Repeat these imaginary movements 10 times (1 session).” Participants entered the actual number of sessions they performed on a form at the end of each day. Dose: each imagined movement was performed 10 times held for three seconds Frequency of administration: 8 sessions per day Control: Standard care hand therapy There are no actual specific details of what the control group was provided but it is assumed that they received the treatment that both groups were provided (below), and no additional treatments were provided. Both groups: Participants of both groups underwent their regular treatment. Components of the intervention: orthosis: relative immobilisation using a Kleinert orthosis. with a wrist band to enable the fingers to be held in a flexed position. Exercise regimen: during the first four weeks postoperatively, only passive flexion of the finger joints was allowed. At 4 to 6 weeks: place‐and‐hold exercises were also practiced: exercises in which the patient flexes their fingers passively with the help of the hand. The fingers are released and the patient is to hold the fingers in the flexed position. Dose:orthosis: 6 weeks full‐time wear; wrist band worn only at night. Frequency of administration: exercise regimen: not reported |
|
| Outcomes | Outcomes were assessed at 6, 7, 8, 10, and 12 weeks postoperatively
|
|
| Funding and conflicts of interest statements | Funding source: not reported Conflicts of interest: not reported | |
| Notes | Participants of the motor imagery group had significantly more injured tendons per person (2.3 ± 0.5) compared with the control group (1.5 ± 1.0). One participant allocated motor imagery was found to have a low VMIQ score (< 72) and was crossed‐over into the control group. However, the participant flow diagram in the trial report does not make this clear. *There also appears to be an error in Figure 1 (page 555) of the trial report with respect to the number of participants at baseline and follow‐up in each group. This could reflect that one participant was crossed‐over from the motor imagery group to the control group; but this is not made clear. No clinical trial registration found. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After inclusion, subjects were admitted at random to either the control group of the motor imagery group…." Comment: it is unclear how the random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "After inclusion, subjects were admitted at random to either the control group of the motor imagery group…." Comment: it is unclear how the random allocation sequence was concealed until the interventions were assigned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: non‐blinded participants, who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: there is insufficient information to determine whether the outcome assessor was blinded to the intervention group. |
| Incomplete outcome data (less than 3 months) (attrition bias) All outcomes | Unclear risk | Comment: there are some discrepancies with the PRISMA flow chart numbers and the numbers of participants at baseline and 12 week follow‐up in the publication. It is unclear how any dropouts were accounted for in the analysis. |
| Selective reporting (reporting bias) | High risk | Comment: in the methods section, most outcomes were recorded at 6, 7, 8 and 10 weeks. However, only the 12 week data are reported in the publication. Also, without a trial protocol, it is unclear whether other outcomes were assessed but not reported. Means and standard deviations for main outcomes were also not reported. |
| Other bias (outcomes appropriately analysed) | High risk | Comment: it is unclear whether a unit of analysis error may have occurred with the pinch strength measurements: in the methods it states that pinch strength was measured for each digit, and for a number of participants more than one tendon was damaged. |
Trumble 2010.
| Study characteristics | ||
| Methods |
Study design: parallel group randomised trial Setting: multi‐centre; eight hand surgery centres, Washington, USA Unit of randomisation: participant Unit of analysis: participant and digit (as appropriate) |
|
| Participants |
Details of sampling frame: Total eligible: 103 participants (119 digits) Total excluded pre‐randomisation: 0 participants (0 digits) Baseline characteristics: Total randomised: 103 participants (119 digits) Place and hold group: 52 participants (61 digits) Controlled passive group: 51 participants (58 digits) Sex distribution: not reported at baseline Age: not reported at baseline Flexor tendon zone: zone II: 119 digits Inclusion criteria:
Exclusion criteria:
Surgical technique: Patients had operation within 48 hours of injury. Skin incision left to discretion of surgeon – most were Bruner incision for oblique lacerations, mid‐axial incisions for transverse lacerations. All repairs were 4‐strand using Strickland technique with 3‐0 polyester for two core sutures, and 6‐0 monofilament Prolene for running epitendinous suture. No repair of the tendon sheath was done. The two slips of the FDS were repaired with a simple core Kessler sutures with 3‐0 polyester. When nerves were injured, they were repaired with microsurgery. All participants had zone II repairs– multiple fingers. Characteristics of participants lost to follow‐up/drop‐outs and included in analysis: Total available for 12‐month follow‐up: 93 (106 digits) Place and hold group: 47 participants (54 digits) Controlled passive group: 46 participants (52 digits) Total analysed (excluding tendon ruptures): 89 participants (102 digits) at 12‐month follow‐up Place and hold group: 46 patients (52 digits) Controlled passive group: 44 patients (50 digits) Sex distribution at follow up: 63 males; 30 females Age: mean (range) at follow up: Mean 29 years (15 to 51 years) Place and hold: 28 (16 to 51) years Controlled passive: 32 (15 to 49) years |
|
| Interventions |
Intervention 1: Place and hold exercise regimen 72 hours to 4 weeks post‐surgery: Components of the intervention: exercise regimen: place and hold and passive exercise programmes using a hinged orthosis (specific for the exercise regimen) that allows for wrist extension while still maintaining the MCP joints in flexion initiated on day 3 post‐surgery. Participant completed place and hold exercise regimen within the orthosis ‐ passively, the wrist is placed into 30 degrees extension, while fingers are pushed passively intro flexion; participant gently contracts the finger flexors to attempt to hold the flexed position, and then relaxes and allows the wrist to drop into flexion and the fingers to extend. Orthosis: between therapy sessions, patients were managed with a static dorsal blocking orthosis that maintained the wrist and MCPJs in flexion. Dose: each place and hold exercise is held for five seconds. Number of repetitions not reported. Orthosis: dorsal blocking splint is worn full time between exercise sessions. Frequency of administration: exercise regimen: Hourly. Orthosis: dorsal blocking splint is worn between exercise sessions. 2 to 4 weeks: Components of the intervention: exercise regimen: place and hold and passive exercises that were now performed without the tenodesis orthosis. Orthosis: between therapy sessions, participants continue to wear dorsal blocking orthosis. Dose: each place and hold exercise is held for five seconds. Number of repetitions not reported. Orthosis: dorsal blocking splint is worn full time between exercise sessions. Frequency of administration: exercise regimen: hourly. Orthosis: dorsal blocking splint is worn between exercise sessions. 4 weeks: Components of the intervention: exercise regimen: tenodesis exercises without an orthosis. Active movement from full fist, to hook fist to straight fist to full finger extension commenced. Orthosis: between therapy sessions, participants continue to wear dorsal blocking orthosis. Dose: each place and hold exercise is held for five seconds. Number of repetitions not reported. Orthosis: dorsal blocking splint is worn full time between exercise sessions. Frequency of administration: exercise regimen: not reported. Orthosis: dorsal blocking splint is worn between exercise sessions. 5 weeks: Components of the intervention: exercise regimen: week 4 exercises plus active wrist and finger flexion following by wrist and finger extension. Orthosis: between therapy sessions, participants continue to wear dorsal blocking orthosis. Dose: number of repetitions not reported. Orthosis: dorsal blocking splint is worn full time between exercise sessions. Frequency of administration: exercise regimen: not reported. Orthosis: dorsal blocking splint is worn between exercise sessions. 6 weeks: Components of the intervention: exercise regimen: active finger flexion exercises with joint blocking are added to the regimen. Buddy taping may be applied to facilitate flexion. Orthosis: discontinue wear. Dose: exercise regimen: not reported Frequency of administration: exercise regimen: not reported 7 weeks: Components of the intervention: exercise regimen: passive extension exercises and extension splints may be used when indicated. Dose: as needed. Frequency of administration: as needed. 9 weeks: Components of the intervention: light strengthening commenced. Dose: not reported. Frequency of administration: not reported. 10 to 14 weeks: Components of the intervention: progressive strengthening programme to regain pre‐operative strength. Unrestricted activity allowed at 14 weeks. Dose: not reported. Frequency of administration: not reported. Intervention 2: Controlled passive exercise regimen Combined protocols from both the Duran (passive exercise program) and Kleinert (rubber band traction) passive motion rehabilitation program allowed patients to come out of the rubber‐band traction to perform the passive Duran therapy with the therapist. Only the injured digit was placed in the Kleinert rubber‐band traction. 24 hours to 3 weeks: Components of the intervention: orthosis: long arm dorsal blocking orthosis. Exercise regimen: all participants commenced an active extension and passive flexion exercise regimen. Oedema management: participants were provided with compressive wraps within 24 to 72 hours post‐surgery. Dose: orthosis: full‐time wear for 72 hours. Exercise regimen: not reported. Oedema management: not reported. Frequency of administration: orthosis: full‐time wear from immediately post‐surgery until commenced hand therapy (within 72 hours). Exercise regimen: not reported. Oedema management: not reported 3 to 6 weeks: Components of the intervention: exercise regimen: place and hold exercises commenced. Orthosis: rubber band traction applied to a dorsal blocking orthosis and wrist position changed to neutral. Dose: not reported Frequency of administration: not reported 6 to 9 weeks: Components of the intervention: exercise regimen: passive extension of isolated joints, combined joint finger extension exercises with wrist flexed. and light function commenced. Orthosis: wean from dorsal blocking splint. Dose: not reported Frequency of administration: not reported 9 to 12 weeks: Components of the intervention: exercise regimen: blocking exercises for PIP and DIPJs, progressive resistive exercises. Orthosis: commence static progressive splinting and/or gentle extension splinting for joint contractures as needed. Dose: not reported Frequency of administration: not reported 12 to 14 weeks: Components of the intervention: light to moderate resistive activities. Dose: not reported Frequency of administration: not reported 16 weeks: No precautions Both groups: 24 to 72 hours post‐surgery: Components of the intervention: orthosis: Long arm dorsal blocking orthosis. Exercise regimen: all participants commenced an active extension and passive flexion exercise regimen. Oedema management: participants were provided with compressive wraps within 24 to 72 hours post‐surgery. Dose: orthosis: full‐time wear for 72 hours. Exercise regimen: not reported. Oedema management: not reported. Frequency of administration: orthosis: full‐time wear from immediately post‐surgery until commenced hand therapy (within 72 hours). Exercise regimen: not reported. Oedema management: not reported. (Full protocol available in the appendix of the publication) |
|
| Outcomes | Outcomes measured at 6, 12, 26, 52 weeks:
|
|
| Funding and conflicts of interest statements | Funding source: Orthopaedic Research and Education Foundation (external funding agency) Conflicts of interest: authors reported that one or more of the authors had financial disclosures related to the project to declare. | |
| Notes | No clinical trial registration found. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each centre had a research coordinator who enrolled the patients and randomised the treatments. The research coordinator performed the randomisation by drawing a card indiscriminately from an envelope with an equal number of cards labelled active or passive before the patient started therapy." Comment: the randomisation sequence appears to have been generated using an adequate method. |
| Allocation concealment (selection bias) | Low risk | Quote: the research coordinator performed the randomisation by drawing a card indiscriminately from an envelope with an equal number of cards labelled active or passive before the patient started therapy." Comment: the allocation sequence appears to have been adequately concealed prior to assignment of interventions. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: insufficient information to determine from the publication. However, given the nature of the interventions (participation in an exercise programme with orthoses), participants could not be blinded to treatment, and may have had different expectations about the benefits of each intervention. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: non‐blinded participants, who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes. |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Quote: "The therapist who performed the therapy also recorded the measurements of motion, sensation and dexterity so they could not be blinded…" Comment: the outcomes assessors were not blinded to the group allocations. |
| Incomplete outcome data (less than 3 months) (attrition bias) All outcomes | Unclear risk | Comment: only range of motion data were collected at the six week time point. It is unclear whether this is a complete data set or participants were lost to attrition at these time points, as the number of participants included in the assessments are not reported. Just because it is not reported we can not assume it is a full data set. |
| Incomplete outcome data (3 to 6 months) (attrition bias) All outcomes | Unclear risk | Comment: it is unclear how many participants were loss to follow‐up at 3 and 6 month evaluation. Only range of motion was measured at the 3 and 6 month evaluation. |
| Incomplete outcome data (over 6 months) (attrition bias) | Low risk | Quote: "Ten patients (13 digits) were lost to follow up, resulting in 93 patients and 106 injured digits who completed the 12‐month follow up evaluation." Comment: the number of participants and digits lost to follow‐up were reported. However, it is unclear how these participants differed to those who were analysed at the earlier time points for range of motion, or how these drop outs may have affected the data. Although, the loss to follow‐up appears to be accounted for the 12 months evaluation at which time the majority of the outcomes were measured. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the outcomes were reported as per the pre‐specified methods section. However, without a trial protocol, it is unclear whether other outcomes were assessed but not reported. |
| Other bias (outcomes appropriately analysed) | Low risk | Comment: it is unlikely that a unit of analysis error occurred as outcomes reported per person are reported as such (e.g. DASH, dexterity measures, satisfaction). Range of motion was calculated per digit. The authors disclosed funding for the project; however, it does not appear that this would have influenced the results in any way as it appears to have been from a non‐commercial granting agency. No further sources of risk of bias were identified. |
Uday Raj 2018.
| Study characteristics | ||
| Methods |
Study Design: randomised trial Setting: single‐centre; Department of Plastic and Reconstructive Surgery of Nizam’s Institute of Medical Sciences, Hyderabad, India Unit of randomisation: participant Unit of analysis: unclear |
|
| Participants |
Details of sampling frame: Total eligible: 46 participants (digits: not reported) Total excluded pre‐randomisation: 16 participants (digits: not reported) Baseline characteristics: Total randomised: 30 participants (digits: not reported) Place and hold: 15 participants (digits: not reported) Passive: 15 participants (digits: not reported) Sex distribution: not reported Age: not reported Flexor tendon zone: zone V: 46 participants Inclusion criteria:
Exclusion criteria:
Surgical technique for flexor tendon repair: Not reported Characteristics of participants lost to follow‐up/drop‐outs and included in analysis Total lost to follow up/drop‐outs: 0 participants Total available for 12‐week follow‐up: 30 participants (digits: not reported) Total analysed: 30 participants (digits: not reported) |
|
| Interventions |
Intervention 1: Early place and hold progressed to tendon gliding exercise regimen (multiple treatments) Week 1 to 3: Place and hold exercise regimen Components of the intervention: orthosis: dorsal blocking splint (wrist in 20° to 30°, MCP in 50° to 70°, IPJs in extension). Exercise regimen: within the orthosis: (1) active PIP and DIP joint extension; (2) isolated passive PIP and DIP flexion, followed by composite passive finger exercise; (3) place and hold exercise. Dose: exercise regimen: (1) 50 repetitions; (2) 5 to 10 repetitions; (3) 2 to 5 repetitions Frequency of administration: exercise regimen: hourly Weeks 4 to 5: Graduated tendon gliding exercise regimen Components of the intervention: orthosis: ceased early only if there is poor movement/scarring. Exercise regimen: (1) MCP joint blocked in flexion and gentle progressive IP joint extension; (2) FDS/FDP tendon gliding; (3) upgrade at five weeks to include non‐resistive blocking exercises Dose: exercise regimen: not reported Frequency of administration: exercise regimen: not reported Week 6: Full passive stretching exercise regimen Components of the intervention: orthosis: ceased. Exercise regimen: passive stretching exercise regimen for all joints Dose: exercise regimen: not reported Frequency of administration: exercise regimen: not reported Intervention 2: Early passive progressed to active exercise regimen (multiple treatments) Week 1 to 3: Passive exercise regimen Components of the intervention: orthosis: dorsal blocking splint (wrist in 40 to 50 degrees, MCP 50 to 70 degrees, IPJs in extension). Exercise regimen: within the orthosis: passive exercise to all IP joints Dose: exercise regimen: not reported Frequency of administration: exercise regimen: hourly Week 4 to 6: active exercise regimen Components of the intervention: exercise regimen: active MCP and IP joint exercises Dose: exercise regimen: not reported Frequency of administration: exercise regimen: hourly Both groups: Components of the intervention: orthosis: each group received a dorsal blocking splint but at different wrist positions. Orthosis was discarded at four weeks unless there was excellent motion and in this case, orthosis was work for an additional 1 to 2 weeks. Exercise regimen: shoulder and elbow (no forearm) in all planes; (2) At week 4 ‐ active wrist exercises. Oedema reduction: elevation. Pain relief: TENS. Scar care: at week 3 (massage inside orthosis). Electrical stimulation: to promote tendon glide. Graded functional activity and strengthening regimen: commenced at week 6 and progressed to normal function by week 12. Dose: orthosis: full time for six weeks. Exercise regimen: not reported. Oedema reduction: not reported. TENS: not reported. Scar care: not reported. Electrical stimulation: not reported. Graded functional activity: not reported. Frequency of administration: orthosis: worn full time. Scar care: during therapy sessions (unknown frequency). Oedema reduction: not reported. TENS: not reported. Electrical stimulation: not reported. Graded functional activity and strengthening regimen: not reported. NB: There is insufficient information in the publication to determine when the exercise regimen commenced during the first week post‐operation. |
|
| Outcomes | Outcomes were measured at 4 and 12 weeks:
|
|
| Funding and conflicts of interest statements | Funding source: self‐funded Conflicts of interest: none declared | |
| Notes | Outcomes were reported as differences between the 12th week and baseline measures or 4th week and baseline for range of motion (TAM). Participants contributed one or more tendons to the studies. The number of digits reported and the unit of analysis for each outcome is not reported. There is also insufficient information to determined the unit of analysis from the publication. A unit of analysis error may have occurred. No clinical trials registration found. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " Thirty subjects who fit the criteria were selected and randomly divided into two groups of 15 each by lottery method…" Comment: the randomisation sequence appears to have been generated using an adequate method. |
| Allocation concealment (selection bias) | Unclear risk | Comment: there is insufficient information to determine how the random allocation sequence was concealed until the interventions were assigned. Clarification from the authors has been requested but was not received at the time of publication. Although a lottery method was used, it is unclear when the randomisation using this method occurred. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: " Patients were blinded for being in either of the groups." Comment: it appears that participants were blinded from which group they were in, but were aware they were receiving one of the interventions. Due to the nature of the intervention, treaters could not be blinded to the intervention. It is not known how successful the blinding of the participants was considering the treaters could not be blinded. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: it is not reported who conducted the outcome assessments and whether they were blinded to the participant's intervention assignment. |
| Incomplete outcome data (less than 3 months) (attrition bias) All outcomes | Low risk | Quote: "No tendon ruptures or subject drop‐outs were recorded." Comment: there was no attrition. Data were reported on a complete data set. |
| Selective reporting (reporting bias) | High risk | Comment: all outcomes described in the methods section of the paper are reported in the results section of the paper. No clinical trials registry was identified in the publication. Data are reported as the improvement from the first ROM measurement taken at week 1 to week 12, and then compared between groups. Baseline measurements are not reported nor does it state whether these were accounted for in the analysis. Mean and standard deviation scores for the different time points are not reported. |
| Other bias (outcomes appropriately analysed) | High risk | Comment: it is unclear whether a unit of analysis error may have occurred as the numbers of participants, hands and digits in each group included in the analysis are not reported. The authors had no conflicts of interest or funding sources. No further sources of bias detected. Outcomes were not appropriately analysed and were reported in a way that was not able to be interpreted in a meaningful way by the review authors. |
Vialaneix 2003.
| Study characteristics | ||
| Methods |
Study Design: parallel group randomised trial Setting: France Unit of randomisation: participant Unit of analysis: unclear |
|
| Participants |
Details of sampling frame: Total eligible: not reported Total excluded pre‐randomisation: not reported Baseline characteristics: Total randomised: 35 participants (digits: not reported) Active group: 16 participants Controlled passive group: 19 participants Sex distribution: not reported Age: mean: 35 years Flexor tendon zone: zone II: 35 participants Inclusion criteria:
Exclusion criteria:
Surgical technique: Primary repair was performed as an emergency procedure using a 4/0 absorbable Tsuge core suture and a peripheral epitenon running suture (6/0 Prolene). Characteristics of participants lost to follow‐up/drop‐outs and included in analysis Total lost to follow up/drop‐outs: not reported Total available for follow‐up: not reported Total analysed: not reported |
|
| Interventions |
Intervention 1: Early active flexion plus passive exercise regimen (Strickland and Small protocol) Components of the intervention: exercise regimen: Passive flexion, active flexion and active extension within the orthosis using the Strickland exercise regimen. Authors cited these papers for their intervention protocol, Strickland 2000; Small 1989. Dose: exercise regimen: not reported Frequency of adminstration: exercise regimen: not reported Intervention 2: Controlled passive exercise regimen (modified Kleinert protocol) Components of the intervention: exercise regimen: controlled passive mobilisation using rubber band traction. Authors stated using the Kleinert protocol as referenced in Kleinert (Kleinert 1967). Dose: exercise regimen: not reported Frequency of administration: exercise regimen: not reported Both groups: Components of the intervention: rehabilitation started at three days post‐operation for both groups (other intervention components not reported). Dose: exercise regimen: not reported Frequency of administration: exercise regimen: not reported |
|
| Outcomes | Outcomes were measured at 8, 12 and 24 weeks:
|
|
| Funding and conflicts of interest statements | Funding source: not reported Conflicts of interest: not reported | |
| Notes | This was a conference proceeding. Hence, very little information was reported in the publication. No other publications of the trial were found. The authors were contacted to provide more information about the study methods and results, but no response from the authors was received. Insufficient information is provided in the publication to determine whether or not a unit of analysis error may have occurred. However, very little data are included in the abstract. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This prospective randomised study was designed to compare two methods of early mobilisation (Kleinert versus Strickland protocol) after primary repair of flexor tendons." Comment: there is insufficient information in the publication to judge whether the allocation sequence was generated in a random manner. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "This prospective randomised study was designed to compare two methods of early mobilisation (Kleinert versus Strickland protocol) after primary repair of flexor tendons." Comment: there is insufficient information in the publication to judge whether the allocation sequence was adequately concealed prior to the randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: it is not reported whether the outcome assessors were blinded to the intervention. |
| Incomplete outcome data (less than 3 months) (attrition bias) All outcomes | Unclear risk | Comment: the participant flow was not reported and is unclear. It is unclear whether the number of participants reported were those that were randomised into the study and/or those available at follow‐up. Clarification from the authors has been requested but was not received at the time of publication. |
| Incomplete outcome data (3 to 6 months) (attrition bias) All outcomes | Unclear risk | Comment: the participant flow was not reported and is unclear. It is unclear whether the number of participants reported were those that were randomised into the study and/or those available at follow‐up. Clarification from the authors has been requested but was not received at the time of publication. |
| Selective reporting (reporting bias) | High risk | Comment: the outcomes mentioned in the methods section are not fully reported in the results section of the conference abstract. Also, without a trial protocol, it is unclear whether other outcomes were assessed but not reported. Fingertip to palm distances and percentages of patients receiving fair or poor outcomes are not reported. Adverse events and mean duration for inability to work are reported for overall participants and not for the individual groups. |
| Other bias (outcomes appropriately analysed) | Unclear risk | Comment: very limited information is given in the conference abstract regarding the number of digits and tendons contributed to the study for each participant. The unit of analysis is unclear. |
ADL: activities of daily living; AROM: active range of motion; CPM: continuous passive motion; CRPS: complex regional pain syndrome; DASH: Disabilities of the Arm, Shoulder and Hand questionnaire; DIP: distal interphalangeal; FDP: flexor digitorum profundus; FDS: flexor digitorum superficialis; FPL: flexor pollicis longus; IF: index flexion; IP: interphalangeal; IPJ: interphalangeal joint; LF: little flexion; LLLT: low level laser therapy; MCP: metacarpophalangeal; MF: middle flexion; MI: motor imagery; NR: not reported; OT: occupational therapist; PAH: place and hold; PDS: polydioxanone suture; PIP: proximal interphalangeal; RCT: randomised controlled trial; RF: ring flexion; ROM: range of motion; RTW: return to work; SD: standard deviation; TAM: total active motion; VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bainbridge 1994 | Not a randomised trial. |
| Baktir 1996 | Not a randomised trial. |
| Horsfall 2016 | Not a randomised trial. |
| ISRCTN80184286 | Study never started recruitment and was abandoned. |
| Kingston 2014 | No separate analysis for participants with flexor tendon injuries. Not our outcome of interest (compliance). |
| NCT01939808 | Study never started recruitment and was abandoned. |
| Peck 1998 | Not a randomised trial. |
| Peck 2014 | Not a randomised trial. |
| Percival 1989 | Not a randomised trial. |
| Stegink Jansen 1990 | Not a randomised trial. |
| Xiao 2018 | Included participants had traumatic hand injuries involving bone or/and flexor tendon. No separate analysis for participants with flexor tendon injuries. |
| Yildirim 2010 | Not a randomised trial. |
Characteristics of studies awaiting classification [ordered by study ID]
Kitis 2009.
| Methods | Experimental study, design unclear |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention 1: Controlled active extension x 12/hour. Passive flexion maintained using the Kleinert splint. Intervention 2: Passive flexion and passive extension, performed in a dorsal blocking orthosis. |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes | It is unclear whether the study was a randomised trial. We received two emails from the trial authors, one stating that this is a randomised trial, and one stating that this was NOT a randomised trial. We are awaiting further clarification from the authors. |
Liu 2004.
| Methods | Randomised parallel group controlled trial |
| Participants | 62 children with injury of hand flexor tendon |
| Interventions |
Intervention: Rehabilitation exercises by occupational therapy with comprehensive analysis, case treatment, genearch (spl) training and learning from playing Control: Routine comprehensive training |
| Outcomes | Not known |
| Notes | We identified this article post‐editorial review. This article needs to be translated from Chinese. Some information is included in Pedro (https://search.pedro.org.au/search‐results/record‐detail/11056). |
Naude 2019.
| Methods | Randomised parallel group intervention trial; blinded outcome assessor |
| Participants |
Details of sampling frame: Total eligible: 86 Total randomised: 31 Active intervention group: 17 Passive intervention group: 14 Total analysed: 14 (17 participants dropped out of the trial and/or were not able to be reached for follow‐up) Active Intervention group analysed: 6 Passive intervention group analysed: 8 Sex distribution: 23 males; 8 females Active group: 13 males; 4 females Passive group: 10 males; 4 females Mean ± SD (range) age: Active group: 30 (18 to 50) years Passive group: 29 (20 to 41) years Inclusion criteria:
Exclusion criteria:
Surgical technique: Four strand tendon repair, using a 3/0 braided material, oversewn with a continuous nylon 6/0 suture. Setting: Single centre trial, Occupational Therapy Hand Service Department, within a tertiary academic state hospital (Tygerberg Hospital, Cape Town, South Africa) |
| Interventions |
Intervention 1: Active rehabilitation Components of the intervention: Exercise regimen: Controlled graded active digital flexion. Dose: Graded programme. Frequency of administration: 5 to 6 45‐minute sessions over 8 to 10 weeks Intervention 2: Passive rehabilitation Components of the intervention: Exercise regimen: Modified Duran protocol focusing on passive digital flexion. Active finger flexion is not introduced until 4 to 5 weeks post repair. Dose: Graded programme. Frequency of administration: 5 to 6 45‐minute sessions over 8 to 10 weeks Both groups: Components of the intervention: Orthosis: Dorsal orthosis (position: wrist in neutral to 20 degrees extension, MCP joints in 80 degrees flexion, IPJs in full extension). |
| Outcomes | Outcome data were collected at eight weeks post‐surgery.
|
| Notes | This was a pilot feasibility trial and hence was not powered to test the effectiveness of the interventions. There was a substantial loss to follow‐up (45%) over the eight week intervention period from both groups. |
Yavari 2009.
| Methods | Parallel group quasi‐randomised clinical trial |
| Participants |
Details of sampling frame: Total eligible: 240 patients (252 tendons) Total excluded pre‐randomisation: Not reported Total randomised: 240 patients (252 tendons) Intervention group: 48 patients (67 tendons) Control group: 192 patients (185 tendons) Sex distribution: 186 males; 54 females Intervention group: 36 males; 12 females Control group: 150 males; 42 females Mean ± SD (range) age: Intervention group: 30.6 ± 8 years Control group: 33 ± 12 years Inclusion criteria:
Exclusion criteria:
Surgical technique: Four strand tendon repair Setting: Single centre trial, Tehran Khordad Hospital, Iran |
| Interventions |
Intervention Group: Patients were mobilised at day 14 after surgery, performed active movements and underwent physiotherapy for three months. Control Group: Patients were immobilised for four weeks after surgery. From four weeks, active movements and physiotherapy were done for three months (similar to intervention group). |
| Outcomes |
|
| Notes | The control group has 192 patients with only 185 tendons. We assume this is an error in reporting as there cannot be more number of patients than tendons. There also is a big difference in participant numbers between the two groups. This study does not appear to be randomised (no evidence in support of this description); imbalance in the numbers allocated; incorrect data and percentages that don't compute to whole numbers indicate these data are incorrect. We are awaiting clarification from the authors. A second publication was identified |
DASH: Disability of the Arm, Shoulder and Hand; IPJ: interphalangeal joint; FDP: flexor digitorum profundus; FDS: flexor digitorum superficialis; MCP: metacarpophalangeal;
Characteristics of ongoing studies [ordered by study ID]
CTRI/2019/01/016821.
| Study name | A randomised control trial comparing the functional outcomes in patients with post tendon repair mobilised by conventional hand therapy versus assistive device. |
| Methods | Randomised, parallel group, active controlled trial, interventional design. |
| Participants |
Target sample size: 120 participants Inclusion criteria:
Exclusion criteria:
Setting: Department of Orthopaedics, Kasturba Medical College, Manipal, India. |
| Interventions |
Intervention 1: Mobilisation with assistive device Intervention 2: Mobilisation with conventional hand therapy Both groups will have intervention provided for a period of three months and two sessions daily one in the morning and one in the evening each session will be for a period of 60 minutes. |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | 1 January 2019 |
| Contact information | Ashwath Acharya Phone: 8217736961 Email: anmacharya@gmail.com |
| Notes |
WHO Clinical trials registry: CTRI/2019/01/016821 URL: www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=29865 |
IRCT201310138177N8.
| Study name | Comparison of active and passive rehabilitation on outcomes of the flexor tendons repair in zone II of the hand. |
| Methods | Parallel group single blinded randomised controlled trial. |
| Participants |
Sample size: 20 participants
Inclusion criteria:
Exclusion criteria:
Setting: Hazrat Fatima Hospital, Seyed Jamaleddin Asadabadi Ave, Tehran, Iran (Islamic Republic of). |
| Interventions |
Intervention 1: Active rehabilitation. Full range of active flexion and extension of the operated finger under supervision of the occupational therapist; three movements in one session per day for three weeks. Intervention 2: Passive rehabilitation. Full range of passive flexion of the finger (rubber band) and active extension by the patient under supervision of the occupational therapist; three movements in three sessions per day for three weeks. |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | 6 November 2013 |
| Contact information | Mohammad Javad Fatemi Phone: +98 21 8871 7272 Email: fatemi@sina.tums.ac.ir |
| Notes |
WHO clinical trials registry: IRCT201310138177N8. URL: en.irct.ir/trial/8628. |
IRCT20150721023277N7.
| Study name | The investigation of the effect of two methods of early active and passive motion on hand function and satisfaction in patients with flexor tendon injury in zone I and II. |
| Methods | Randomised double blinded parallel group interventional study design. |
| Participants |
Target sample size: 30 participants Inclusion criteria:
Exclusion criteria:
Setting: School of Rehabilitation, Shahid Shahnazari Street, Madar Square, Mirdamad Blvd, Tehran 15459‐13487 Tehran, Iran (Islamic Republic of). |
| Interventions |
Intervention 1: "The clients will be referred to the occupational therapist within 24‐24 hours after surgery. In this group, regular rehabilitation programs and the Strickland protocol will be implemented. The treatment sessions will be performed by the therapist for 45 minutes, 3 days a week for 8 weeks. In this method, two splints are used: 1: Dorsal Block Splint, which is made of plaster, and wrist joint in 20 degrees of flexion, metacarpophalangeal at 50 degrees of flexion and interphalangeal in the extension and used most of the time, 2: Splint is a thermoplastic training. The wrist part is hinged and allows full flexion to the wrist, metacarpophalangeal and interphalangeal joints, but limits the wrist at 30 degrees of extension, metacarpophalangeal at 60 degrees and interphalangeal at 25 degrees of flexion. Week 4‐0: Every hour the exercises will be performed by 15 times repetitive exercises in orthosis Dorsal Block and the flexion Place and hold practice with 15 repetitions in orthosis, These exercises will be presented in writing and videos recorded for them at home. Week 8‐4: orthosis training will be lifted, but orthosis Dorsal Block will be covered except during training, and exercises will be done every 2 hours. At 6‐5 weeks, the blocking and hook fist exercises will be performed. 7‐8 weeks of progressive resistance exercises and daily routine activities will be added. At week 14, complete resistance exercises and heavy daily activities will be performed." Intervention 2: "The client will be referred to the occupational therapist within 24‐24 hours after surgery. A therapist will be performed a traditional rehabilitation program modified Duran protocol of 40 minutes, three days per week, for eight weeks. In this group, a dorsal block orthosis will be used, which will place the wrist joint at 20° flexion and metacarpophalangeal at 50° flexion and allowing the interphalangeal joints to be fully extended." |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | 14 August 2018 |
| Contact information | Laleh Lajevardi Phone: +98 21 2222 8051 Email: lajevardi.l@iums.ac.ir |
| Notes |
WHO Clinical trials registry: IRCT20150721023277N7. URL: en.irct.ir/trial/28585. We were unsuccessful in our attempts to obtain more information on this study. |
NCT03812978.
| Study name | Mobile application for improving rehabilitation after flexor tendon repair |
| Methods | Parallel group randomised controlled trial. |
| Participants |
Sample size: 101 participants
Inclusion criteria:
Exclusion criteria:
Setting: Karolinska Institutet, Sweden. |
| Interventions |
Control Group : Standard treatment. Rehabilitation according to early active motion. Experimental Group: Standard treatment and intervention (smart phone application). Intervention group also received a smart phone app including; exercise videos, push‐notifications for exercise, exercise diary, written information on the surgery, rehabilitation, questions and answers. |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | 1 March 2017 |
| Contact information | Marianne Arner and Jonas Svingen Email: jonas.svingen@ki.se Phone: +46709360278 |
| Notes |
WHO Clinical trials registry: NCT03812978. URL: https://clinicaltrials.gov/show/NCT03812978. |
NCT03850210.
| Study name | The Manchester Short Splint in the rehabilitation of zone II flexor tendon repairs |
| Methods | Parallel group randomised controlled trial. |
| Participants |
Sample size: 60 participants
Inclusion criteria:
Exclusion criteria:
Setting: Manchester University NHS Foundation Trust, Manchester, United Kingdom. |
| Interventions |
Intervention: Device: Short splint that permits maximal wrist flexion and up to 45° of wrist extension with a block to 30° of MCP joint extension. Active control: Device: Traditional long splint which is a forearm‐based dorsal thermoplastic splint that immobilises the wrist in neutral position with a block to 30° of MCP joint extension. |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | 1 June 2015 |
| Contact information | Vivien Lees, Bradley Tallon Email: vivienlees@live.com; bradley.tallon@mft.nhs.uk Phone: 0161 291 6648;01612915757 |
| Notes |
WHO Clinical trials registry: NCT03850210. URL: https://clinicaltrials.gov/show/NCT03850210. |
NCT04237415.
| Study name | Electromyographic (EMG) biofeedback training in Zone I‐III flexor tendon injuries |
| Methods | Parallel group randomised controlled trial |
| Participants |
Sample size: 22 participants
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: EMG biofeedback training + modified Duran protocol, same physiotherapist three times a week for 12 weeks Control: modified Duran protocol, same physiotherapist three times a week for 12 weeks |
| Outcomes |
Primary outcomes:
|
| Starting date | 10 October 2016 |
| Contact information | Umut Eraslan & Ali Kitis, Pamukkale University, Turkey |
| Notes |
Clinicaltrials.gov: https://clinicaltrials.gov/ct2/show/NCT04237415 This trial was retrospectively reported. This study has a reported completion date of 21 March 2019. |
NCT04385485.
| Study name | Primary flexor tendon repair in zone I and II: a prospective randomised trial of passive mobilisation with place‐and‐hold, compared with true active motion therapy |
| Methods | Parallel group randomised intervention trial; blinded outcome assessor |
| Participants |
Sample size: 64 participants
Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention 1: Active rehabilitation: Participant sees an occupational therapist 1 to 3 days after surgery. An orthosis is fabricated to immobilise the wrist and work as an extension block for the MCP joints, and is worn day and night for 4 weeks. Another splint that immobilises the DIP and PIP joints is work whenever the participant is not exercising. During active exercise the participant follows a strict protocol with both active and passive training and increasing number of repetitions for 3 months. Intervention 2: Passive rehabilitation: At 1 to 3 days after surgery, the participant is provided with a new plaster that immobilises the wrist and works as an extension block for the MCP joints. The occupational therapist attaches rubber bands to the nails of all fingers, and the training is done passively with active hold according to a strict protocol. This passive rehabilitation is done over 4 weeks with, other rehabilitation up to 3 months. |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | 14 July2014 |
| Contact information | Jan Friden, Professor, Sahlgrenska University Hospital, Sweden |
| Notes |
clinicaltrials.gov: This trial was retrospectively reported. This study has a reported completion date of 30 August 2018. |
AISEQ: Athlete Injury Self Efficacy Questionnaire; DIP: distal interphalangeal; EMG: electromyography FDP: flexor digitorum profundus; FDS: flexor digitorum superficialis; MCP: metacarpophalangeal; PIP: proximal interphalangeal; SIRAS: Sport Injury Adherence Scale
Differences between protocol and review
Types of interventions
We included the comparison of different doses for interventions, other than orthosis‐wearing regimen; e.g. ultrasound dose, frequency or amount of interventions in types of intervention.
Types of outcome measures
For our second primary outcome (active finger ROM using goniometric measurement), we allowed classification of ROM using a categorical outcome.
We modified our definitions for timing of outcome measurement by inserting a medium term period (3 to 6 months) before the long term period, which now starts at over six months instead of over three months.
Assessment of risk of bias in the included studies
Linked with our revised definitions of timing of follow‐up assessment, we limited the second group to up to six months, and added in a third group for outcomes measured for greater than six months.
Contributions of authors
SP and BJ wrote the protocol. SP and BJ completed the searches, eligibility, risk of bias assessments and data extraction. SP completed the data analysis, and the GRADE tables. All authors contributed to, and approved, the final version of the review.
Contributions of the editorial base
Helen Handoll (Co‐ordinating Editor): edited the review; advised on methodology and review content; and approved the final version for publication. Joanne Elliott (Managing Editor and Information Specialist): developed search strategies, coordinated the editorial process; advised on content; and edited the review. Maria Clarke (Information Specialist): ran the search and edited the search methods section.
Sources of support
Internal sources
-
Harvard T.H. Chan School of Public Health, Center for Work, Health, and Well‐being, USA
Host institution for Dr. Susan E Peters
-
Brisbane Hand and Upper Limb Research Institute, Australia
Host institution for Professor Mark Ross
-
Sunshine Coast University Hospital, Queensland Health & Advanced Hand Clinic, Maroochydore, Queensland, Australia
Host institutions for Bhavana Jha
External sources
None, Other
Declarations of interest
SP: none known. BJ: none known. MR: none known.
New
References
References to studies included in this review
Abdel Sabour 2018 {published data only}
- *.Abdel Sabour HM, Labib A, Sallam AA, Elbanna M. Comparative study between early active and passive rehabilitation protocols following two-strand flexor tendon repair: can two-strand flexor tendon repair withstands early active rehabilitation? Egyptian Rheumatology and Rehabilitation 2018;45:125-32. [Google Scholar]
- PACTR201708002483416. Comparative study between early active and early passive rehabilitation protocols following 2-strand flexor tendon repair. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201708002483416 (first received 29 July 2017).
Adolfsson 1996 {published data only}
- *.Adolfsson L, Soderberg G, Larsson M, Karlander LE. The effects of a shortened postoperative mobilization programme after flexor tendon repair in zone 2. Journal of Hand Surgery (British Volume) 1996;21(1):67-71. [DOI] [PubMed] [Google Scholar]
- Adolfsson L. Request for further information on your study for a Cochrane Systematic Review [personal communication]. Email to: S Peters 19 December 2017.
- Adolfsson L. Request for further information on your study for a Cochrane Systematic Review [personal communication]. Email to: S Peters 2 December 2017.
Farzad 2014 {published data only}
- Farzad M, Layeghi F, Asgari A, Ring DC, Karimlou M, Hosseini SA. A prospective randomized controlled trial of controlled passive mobilization vs. place and active hold exercises after zone 2 flexor tendon repair. Hand Surgery 2014;19(1):53-9. [DOI] [PubMed] [Google Scholar]
Geetha 2014 {published and unpublished data}
- CTRI/2013/04/003576. A clinical trial to study the effects of the topically administered ultrasound to the operated field in improving the active flexion of the fingers, after repair of the injured flexor tendons in the fingers and hand. who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2013/04/003576 (first received 22 April 2013).
- *.Geetha K, Hariharan NC, Mohan J. Early ultrasound therapy for rehabilitation after zone II flexor tendon repair. Indian Journal of Plastic Surgery 2014;47(1):85-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha K. Request for information on your study to include in a Cochrane Systematic Review [personal communication]. Email to: S Peters 16 December 2017.
Gelberman 1991 {published data only}
- Gelberman RH, Nunley JA 2nd, Osterman AL, Breen TF, Dimick MP, Woo SL. Influences of the protected passive mobilization interval on flexor tendon healing. A prospective randomized clinical study. Clinical Orthopaedics and Related Research 1991;264:189-6. [PubMed] [Google Scholar]
Gulke 2018 {published data only}
- Gulke J, Mentzel M, Krischak G, Gulkin D, Dornacher D, Wachter N. Early functional passive mobilization of flexor tendon injuries of the hand (zone 2): exercise with an exoskeleton compared to physical therapy. [Die fruhfunktionelle passive mobilisation nach beugesehnenverletzung (zone 2) an der hand: beubung mit einem exoskelett im vergleich zur physiotherapie]. Unfallchirurg 2018;121(7):560-8. [DOI] [PubMed] [Google Scholar]
Hagberg 2000 {published data only}
- Hagberg L, Abrahamsson S, Pihlstrom S, Carlsson I. Active flexion versus rubber band traction for early controlled mobilisation following flexor tendon repair in zone II: a controlled clinical study [Abstract]. Journal of Hand Surgery (British Volume) 2000;24(Suppl 1):52. [Google Scholar]
Kneafsey 1994 {published data only}
- Kneafsey B, O'Shaughnessy M, Vidal P, Milling MAP, Sykes PJ. Controlled mobilization after flexor tendon repair: a prospective comparison of two methods. Journal of Hand Surgery (British Volume) 1994;19:37-8. [Google Scholar]
Ozkan 2004 {published data only}
- Altan L. Request for information on your study to include in a Cochrane Systematic Review [personal communication]. Email to: S Peters 17 October 2018.
- *.Ozkan N, Altan L, Bingol U, Akin S, Yurtkuran M. Investigation of the supplementary effect of GaAs laser therapy on the rehabilitation of human digital flexor tendons. Journal of Clinical Laser Medicine and Surgery 2004;22(2):105-10. [DOI] [PubMed] [Google Scholar]
Poorpezeshk 2018 {published data only}
- Irct2017050233783N. Early low level laser therapy improved passive range of motion and decreased pain in patients with flexor tendon injury. https://www.irct.ir/trial/25972 (first received 20 June 2017). [Irct2017050233783N] [DOI] [PubMed]
- *.Poorpezeshk N, Ghoreishi SK, Bayat M, Pouriran R, Yavari M. Early low-level laser therapy improves the passive range of motion and decreases pain in patients with flexor tendon injury. Photomedicine and Laser Surgery 2018;36(10):530-5. [DOI] [PubMed] [Google Scholar]
Rigo 2017 {published data only}
- Rigo I. Request for information on your study to include in a Cochrane Systematic Review [personal communication]. Email to: S Peters 12 October 2018.
- *.Rigo IZ, Haugstvedt JR, Rokkum M. The effect of adding active flexion to modified Kleinert regime on outcomes for zone 1 to 3 flexor tendon repairs. A prospective randomized trial. Journal of Hand Surgery (European Volume) 2017;42(9):920-9. [DOI] [PubMed] [Google Scholar]
- Zhou X, Chen J. Commentary on: The effect of adding active flexion to modified Kleinert regime on outcomes for zone 1 to 3 flexor tendon repairs. A prospective randomized trial. Journal of Hand Surgery (European Volume) 2017;42(9):930-1. [DOI] [PubMed] [Google Scholar]
Scavenius 2000 {published data only}
- Scavenius M, Soe-Nielsen N, Boeckstyns M, Sassene I. Early active versus early passive regimen after primary tendon repair [Abstract]. In: Danish Orthopaedic Society Autumn Meeting; 2000 Oct 26-27. Copenhagen, Denmark: www.actaorthopscand.org/Pages/ACTAhome.html (accessed 23 August 2002), 2000.
Silva 2003 {published data only}
- Silva JB, Calcagnotto G, Oliveira CGD, Fisher H. A randomised prospective study on early active mobilization after zone II flexor tendon repair. Revista Brasileira de Ortopedia 2003;38(10):581-8. [Google Scholar]
Stenekes 2009 {published data only}
- Stenekes MW, Geertzen JH, Nicolai JPA, De Jong BM, Mulder T. Effects of motor imagery on hand function during immobilization after flexor tendon repair. Archives of Physical Medicine and Rehabilitation 2009;90(4):553-9. [DOI] [PubMed] [Google Scholar]
Trumble 2010 {published data only}
- Idler RS, Trumble TE, Strickland JW, Stern PJ. Randomized prospective trial of active vs. passive motion after Zone II flexor tendon repair. In: American Society for Surgery of the Hand 55th Annual Meeting; 2000 Oct 5-7. Seattle, USA, 2000.
- *.Trumble TE, Vedder NB, Seiler JG, Hanel DP, Diao E, Pettrone S. Zone-II flexor tendon repair: a randomized prospective trial of active place-and-hold therapy compared with passive motion therapy. Journal of Bone and Joint Surgery. American Volume 2010;92:1381-9. [DOI] [PubMed] [Google Scholar]
Uday Raj 2018 {published data only}
- Uday Raj J, Praveen D, Mukunda Reddy D, Srikanth R. Effectiveness of Kleinert’s controlled motion protocol on tendon gliding following zone 5 flexor tendon repair. Indian Journal of Physiotherapy and Occupational Therapy 2018;12(1):128-34. [Google Scholar]
Vialaneix 2003 {published data only}
- *.Vialaneix J, Petry D, Dap F, Dautel G. Prospective study after primary repair of flexor tendons in zone II: comparison of two methods of early mobilization (Klienert versus Strickland protocol). In: 58th American Society for Surgery of the Hand Meeting; 2003 Oct 3-5. Chicago, USA, 2003.
References to studies excluded from this review
Bainbridge 1994 {published data only}
- Bainbridge LC, Robertson C, Elliot D. A comparison of post-operative mobilization of flexor tendon repairs with "passive flexion-active extension" and "controlled active motion" techniques. Journal of Hand Surgery (British and European Volume) 1994;19:517-21. [DOI] [PubMed] [Google Scholar]
Baktir 1996 {published data only}
- Baktir A, Turk CY, Sahin KV, Kardas Y. Flexor tendon repair in zone 2 followed by early active mobilization. Journal of Hand Surgery (British and European Volume) 1996;21:624-8. [DOI] [PubMed] [Google Scholar]
Horsfall 2016 {published data only}
- Horsfall G. Pilot study comparing two Early Active Motion (EAM) regimens for surgically repaired flexor tendons, zone 1-IV, Wrist Synergistic EAM vs. Modified Belfast Static Wrist EAM. Journal of Hand Therapy 2016;29(3):362-3. [Google Scholar]
ISRCTN80184286 {published data only}
- *.ISRCTN80184286. Mobilization of the thumb flexor tendon. isrctn.com/ISRCTN80184286 (first received 12 September 2003).
- Scott P. Study status [personal communication]. Email to: S Peters 3 August 2017.
Kingston 2014 {published data only}
- ACTRN12608000530325. A clinical trial to investigate whether providing a home exercise program on DVD improves compliance with patients who have hand injuries. apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12608000530325 (first received 15 October 2008).
- *.Kingston GA, Williams G, Gray MA, Judd J. Does a DVD improve compliance with home exercise programs forpeople who have sustained a traumatic hand injury? Results of afeasibility study. Disability and Rehabilitation: Assistive Technology 2014;9(3):188-94. [DOI] [PubMed] [Google Scholar]
NCT01939808 {published and unpublished data}
- NCT01939808. Comparison of the effect of splinting the wrist in extension versus neutral positioning during rehabilitation following zone I/II flexor tendon repair. clinicaltrials.gov/ct2/show/NCT01939808 (first received 11 September 2013).
- Scott P. Email correspondence. Email to: S Peters 3 August 2018.
Peck 1998 {published data only}
- Peck FH, Bucher CA, Watson JS, Roe A. A comparative study of two methods of controlled mobilization of flexor tendon repairs in zone 2. Journal of Hand Surgery (British and European Volume) 1998;23:41-5. [DOI] [PubMed] [Google Scholar]
Peck 2014 {published data only}
- Peck FH, Roe AE, Ng CY, Duff C, McGrouther DA, Lees VC. The Manchester short splint: a change to splinting practice in the rehabilitation of zone II flexor tendon repairs. Hand Therapy 2014;19(2):47-53. [Google Scholar]
Percival 1989 {published data only}
- Percival NJ, Sykes PJ. Flexor pollicis longus tendon repair: a comparison between dynamic and static splintage. Journal of Hand Surgery (British volume) 1989;14B:412-5. [DOI] [PubMed] [Google Scholar]
Stegink Jansen 1990 {published data only}
- Stegink Jansen CW, Minerbo GM. A comparison between early dynamically controlled mobilization and immobilization after flexor tendon repair in zone 2 of the hand: preliminary results. Journal of Hand Therapy 1990;3(1):20-5. [Google Scholar]
Xiao 2018 {published data only}
- Xiao X, Huang J, Chen Z, Xia X, Wang S, Yang Z. Effects of computer-assisted wrist/hand training on the improvement of hand function in traumatic hand injuries. International Journal of Clinical and Experimental Medicine 2018;11(2):1208-6. [Google Scholar]
Yildirim 2010 {published data only}
- Yildirim A, Nas K. Evaluation of postoperative early mobilization in patients with repaired flexor tendons of the wrist, the spaghetti wrist. Journal of Back and Musculoskeletal Rehabilitation 2010;23:193-200. [DOI: 10.3233/BMR20100266] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kitis 2009 {published data only}
- *.Kitis A, Buker N, Kara IG. Comparison of two methods of controlled mobilisation of repaired flexor tendons in zone 2. Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery 2009;43:160-5. [DOI] [PubMed] [Google Scholar]
- Kitis A. Clarification on your study to be included in a Cochrane review [personal communication]. Email to: S Peters 30 May 2017.
Liu 2004 {published data only}
- Liu AH. Effect of the functional exercise on the delicate activities of hand in children after surgical repair for injury of hand flexor tendon. Chinese Journal of Clinical Rehabilitation 2004;8(36):8300-1. [Google Scholar]
Naude 2019 {published data only}
- Naude AB, Klerk S. Introducing early active mobilisation following flexor tendon repair in the context of care in a developing country: A randomised feasibility study. South African Journal of Occupational Therapy 2019;49(2):48-56. [Google Scholar]
Yavari 2009 {published data only}
- Yavari M, Sadeghi M, Mozaffari N. Evaluation of the results of early mobilization following flexor tendon repair of fingers. Research in Medicine: Journal of Research in Medical Sciences 2009;33(1):5. [Google Scholar]
- *.Yavari M, Sadeghi SM, Mozaffari N. Evaluation of the results of early mobilization following flexor tendon repair of fingers [بررسي تاثير حركت زودرس برعملكرد ترميم تاندون هاي فلكسور انگشتان دست]. Pajouhesh Dar Pezeshki (Journal of the Faculty of Medicine) 2009;33(1):35-8. [Google Scholar]
References to ongoing studies
CTRI/2019/01/016821 {published data only}CTRI/2019/01/016821
- CTRI/2019/01/016821. Comparison of conventional hand therapy and assistive device in the rehabilitation of flexor tendon injuries. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2019/01/016821 (first received 1 January 2019).
IRCT201310138177N8 {published data only}IRCT201310138177N8
- IRCT201310138177N8. Comparison of active and passive rehabilitation on outcomes of the flexor tendons repair in fingers of the hand. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201310138177N8 (first received 6 November 2013).
IRCT20150721023277N7 {published data only}IRCT20150721023277N7
- IRCT20150721023277N7. The investigation of the effect of two methods of early active and passive motion in flexor tendon injury in zone 1and 2. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20150721023277N7 (first received 14 August 2014).
NCT03812978 {published data only}NCT03812978
- NCT03812978. Mobile application for improving rehabilitation after flexor tendon repair. who.int/trialsearch/Trial2.aspx?TrialID=NCT03812978 (first received 15 January 2019).
NCT03850210 {published data only}NCT03850210
- NCT03850210. The Manchester short splint in the rehabilitation of zone II flexor tendon repairs. https://clinicaltrials.gov/ct2/show/NCT03850210 (first received 21 February 2019).
NCT04237415 {published data only}
- NCT04237415. Electromyographic (EMG) biofeedback training in zone I-III flexor tendon injuries. clinicaltrials.gov/show/NCT04237415 (first received 23 January 2020).
NCT04385485 {published data only}
- NCT04385485. Clinical trial comparing active and passive rehabilitation after flexor tendon injury. clinicaltrials.gov/show/NCT04385485 (first received 13 May 2020).
Additional references
ASSH 1976
- Clinical Assessment Committee Report. American Society for Surgery of the Hand 1976.
Bigorre 2018
- Bigorre N, Delaquaize F, Degez F, Celerier S. Primary flexor tendons repair in zone 2: Current trends with GEMMSOR survey results. Hand Surgery and Rehabilitation 2018;37(5):281-8. [DOI] [PubMed] [Google Scholar]
Bindra 2005
- Bindra RR. Evolution of concepts in flexor tendon surgery of the hand. In: Walsh WR, editors(s). Repair and Regeneration of Ligaments, Tendons, and Joint Capsule. Totowa, New Jersey: Humana Press, 2005:87-106. [Google Scholar]
Boutron 2008
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P, CONSORT Group. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Annals of Internal Medicine 2008;148(4):295-309. [DOI] [PubMed] [Google Scholar]
Buck‐Gramcko 1976
- Buck-Gramcko D, Dietrich FE, Gogge S. Evaluation criteria for follow-up examinations of flexor tendon surgery [Bewertungskriterien bei Nachuntersuchungen yon Beugensehnenweiderherstellungen]. Handchirurgie 1976;8(2):65-9. [PubMed] [Google Scholar]
Buckwalter 1999
- Buckwalter JA, Grodzinsky AJ. Loading of healing bone, fibrous tissue and muscle: implications for orthopaedic practice. Journal of the American Academy of Orthopedic Surgeons 1999;7(5):291-9. [DOI] [PubMed] [Google Scholar]
Burge 1990
- Burge PD, Brown M. Elastic band mobilisation after flexor tendon repair; splint design and risk of flexion contracture. Journal of Hand Surgery (British Volume) 1990;15:443-8. [DOI] [PubMed] [Google Scholar]
Chesney 2011
- Chesney A, Chauhan A, Kattan A, Farrokhyar F, Thoma A. Systematic review of flexor tendon rehabilitation protocols in zone II of the hand. Plastic and Reconstructive Surgery 2011;127(4):1583-92. [DOI] [PubMed] [Google Scholar]
Clancy 2013
- Clancy SP, Mass DP. Current flexor and extensor tendon motion regimes: a summary. Hand Clinics 2013;29(2):295-309. [DOI] [PubMed] [Google Scholar]
De Jong 2014
- De Jong JP, Nguyen JT, Sonnema AJM, Nguyen EC, Amadio PC, Moran SL. The incidence of acute traumatic tendon injuries in the hand and wrist: a 10-year population-based study. Clinics in Orthopedic Surgery 2014;6(2):196-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duran 1975
- Duran R, Houser R. Controlled passive motion following flexor tendon repairing zones 2 and 3. In: Hunter JM, Schneider LH, editors(s). AAOS Symposium on Tendon Surgery in the Hand. St Louis, MO: CV Moscby, 1975:105-14. [Google Scholar]
Dwan 2008
- Dwan K, Altman D, Arnaiz JA, Bloom J, Chan A, Cronin E et al. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLOS One 2008;3(8):e3081. [DOI: 10.1371/journal.pone.00381] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elliot 2007
- Elliot D, Barbieri CH, Evans RB, Mass D, Tang JB. IFSSH Flexor Tendon Committee Report 2007. Journal of Hand Surgery (European Volume) 2007;32(3):346-56. [DOI] [PubMed] [Google Scholar]
Evans 2005
- Evans RB. Zone I flexor tendon rehabilitation with limited extension and active flexion. Journal of Hand Therapy 2005;18:128-40. [DOI] [PubMed] [Google Scholar]
Evans 2012
- Evans RB. Managing the injured tendon: current concepts. Journal of Hand Therapy 2012;25(2):173-89. [DOI] [PubMed] [Google Scholar]
Freilich 2007
- Freilich AM, Chhabra AB. Secondary flexor tendon reconstruction. A review. Journal of Hand Surgery 2007;32:1436-42. [DOI] [PubMed] [Google Scholar]
Groth 2004
- Groth GN. Pyramid of progressive force exercises to the injured flexor tendon. Journal of Hand Therapy 2004;17(1):31-42. [DOI] [PubMed] [Google Scholar]
Halikis 1997
- Halikis MN, Kubota H, Aoki M. Effect of immobilization, immediate mobilization, and delayed mobilization on the resistance to digital flexion using a tendon injury model. Journal of Hand Surgery (American Volume) 1997;22:464-72. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Jones 2005
- Jones L. Scar management in hand therapy - is our practice evidence based? British Journal of Hand Therapy 2005;10(2):40-6. [Google Scholar]
Khanna 2009
- Khanna A, Gougoulias N, Maffulli N. Modalities in prevention of flexor tendon adhesion in the hand: what have we achieved so far? British Medical Bulletin 2009;90:85-109. [DOI] [PubMed] [Google Scholar]
Kleinert 1967
- Kleinert HE, Kutz JE, Ashbell TS, Martinez E. Primary repair of lacerated flexor tendon in "no man's land". Journal of Bone and Joint Surgery (American Volume) 1967;49:577. [Google Scholar]
Kleinert 1975
- Kleinert HE, Kutz JE, Cohen MJ. Primary repair of zone 2 flexor tendon lacerations. In: American Academy of Orthopaedic Surgeons: Symposium on Tendon Surgery in the Hand. St. Louis, MO: Mosby, 1975:91-104. [Google Scholar]
Kleinert 1983
- Kleinert HE, Verdan C. Report of the committee on tendon injuries (International Federation of Societies for Surgery of the Hand). Journal of Hand Surgery (American Volume) 1983;8(5):794-8. [DOI] [PubMed] [Google Scholar]
Larson 2019
- Larson E, Sharma J, Bohren MA, Tunçalp Ö. When the patient is the expert: measuring patient experience and satisfaction with care. Bulletin of the World Health Organization 2019;97:563-9. [DOI: 10.2471/BLT.18.225201] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2015
- Lee HI, Lee JS, Kim TH, Chang S, Park MJ, Lee GJ. Comparison of flexor tendon suture techniques including 1 using 10 strands. Journal of Hand Surgery 2015;40(7):1369-76. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Lilly 2006
- Lilly SI, Messer TM. Complications after treatment of flexor tendon injuries. Journal of the American Academy of Orthopaedic Surgeons 2006;14(7):387-96. [DOI] [PubMed] [Google Scholar]
Lister 1977
- Lister GD, Kleinert HE, Kutz JE, Atasoy E. Primary flexor tendon repair followed by immediate controlled mobilization. Journal of Hand Surgery (American) 1977;2:441-51. [DOI] [PubMed] [Google Scholar]
Lutsky 2015
- Lutsky KF Giang EL, Matzon JL. Flexor tendon injury, repair and rehabilitation. Orthopedic Clinics of North America 2015;46(1):67-76. [DOI] [PubMed] [Google Scholar]
Marks 2020
- Marks M. Which patient-reported outcomes shall we use in hand surgery? Journal of Hand Surgery (European Volume) 2020;45(1):5-11. [DOI: 10.1177/1753193419882875] [DOI] [PubMed] [Google Scholar]
Mason 1941
- Mason ML, Allen HS. The rate of healing of tendons: an experimental study of tensile strength. Annals of Surgery 1941;113(3):424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehrzad 2019
- Mehrzad R, Mookerjee V, Schmidt S, Jehle CC, Kiwanuka E, Liu PY. The economic impact of flexor tendon lacerations of the hand in the United States. Annals of Plastic Surgery 2019;83(4):419-23. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 2009;339(7716):332-6. [PMC free article] [PubMed] [Google Scholar]
Myer 2016
- Myer C, Fowler JR. Flexor tendon repair: healing, biomechanics, and suture configurations. Orthopaedic Clinics of North America 2016;47(1):219-26. [DOI] [PubMed] [Google Scholar]
Neiduski 2018
- Neiduski RL, Powell RK. Flexor tendon rehabilitation in the 21st century: a systematic review. Journal of Hand Therapy 2018;32(2):165-74. [DOI: 10.1016/j.jht.2018.06.001] [DOI] [PubMed] [Google Scholar]
Osei 2014
- Osei DA, Stepan JG, Calfee RP, Thomopoulos S, Boyer MI, Potter R, et al. The effect of suture caliber and number of core suture strands on zone II flexor tendon repair: a study in human cadavers. Journal of Hand Surgery (American Volume) 2014;39(2):262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peacock 1965
- Peacock Jr EE. Biological principles in the healing of long tendons. Surgical Clinics of North America 1965;45:461-76. [DOI] [PubMed] [Google Scholar]
Pettengill 2005
- Pettengill KM. The evolution of early mobilization of the repaired flexor tendon. Journal of Hand Therapy 2005;18:157-68. [DOI] [PubMed] [Google Scholar]
Pettengill 2010
- Pettengill KM, Strien G. Postoperative management of flexor tendon injuries. In: Skirven TM, Osterman AL, Fedorczyk JM, Amadio PC, editors(s). Rehabilitation of the Hand and Upper Extremity. 6th edition. Philadelphia: Elsevier Mosby, 2010:457-9. [Google Scholar]
Reissner 2019
- Reissner L, Fischer G, List R, Taylor WR, Giovanoli P, Calcagni M. Minimal detectable difference of the finger and wrist range of motion: comparison of goniometry and 3D motion analysis. Journal of Orthopaedic Surgery and Research 2019;14(173):https://doi.org/10.1186/s13018-019-1177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rigo 2016
- Rigo IZ, Rokkum M. Predictors of outcome after primary flexor tendon repair in zone 1, 2 and 3. Journal of Hand Surgery (European Volume) 2016;41(8):793-801. [DOI] [PubMed] [Google Scholar]
Rosberg 2003
- Rosberg HE, Carlsson KS, Hojgard S, Lindgren B, Lundborg G, Dahlin LB. What determines the costs of repair and rehabilitation of flexor tendon injuries in zone II? A multiple regression analysis of data from southern Sweden. Journal of Hand Surgery (British Volume) 2003;28(2):106-12. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Small 1989
- Small JO, Brennen MD, Colville J. Early active mobilisation following flexor tendon repair in zone 2. Journal of Hand Surgery (British Volume) 1989;14:383-91. [DOI] [PubMed] [Google Scholar]
Smith 1973
- Smith HB. Smith hand function evaluation. American Journal of Occupational Therapy 1973;27(5):244-51. [PubMed] [Google Scholar]
Starr 2013
- Starr HM, Snoddy M, Hammond KE, Seiler JG. Flexor tendon repair rehabilitation protocols: a systematic review. Journal of Hand Surgery (American Volume) 2013;38:1712-7. [DOI] [PubMed] [Google Scholar]
Stone 1989
- Stone RG, Spencer EL, Almquist EE. An evaluation of early motion management following primary flexor tendon repair: Zones 1-3. Journal of Hand Therapy 1989;2(4):223-30. [Google Scholar]
Strickland 1980
- Strickland JW, Glogovac SV. Digital function following flexor tendon repair in Zone II: a comparison of immobilization and controlled passive motion techniques. Journal of Hand Surgery (American) 1980;5(6):537-43. [DOI] [PubMed] [Google Scholar]
Strickland 1985
- Strickland JW. Results of flexor tendon surgery. Hand Clinics 1985;1(1):167-79. [PubMed] [Google Scholar]
Strickland 2000
- Strickland 2000. Development of flexor tendon surgery: 25 years of progress. Journal of Hand Surgery 2000;25(2):214-35. [DOI] [PubMed] [Google Scholar]
Strickland 2005
- Strickland JW. The scientific basis for advances in flexor tendon surgery. Journal of Hand Therapy 2005;18(2):94-110. [DOI] [PubMed] [Google Scholar]
Su 2005
- Su BW, Solomons M, Barrow A, Senoge ME, Gilberti M, Lubbers L, et al. Device for zone-II flexor tendon repair: a multicenter, randomized, blinded, clinical trial. Journal of Bone and Joint Surgery (American Volume) 2005;87(5):923-35. [DOI: 10.2106/JBJS.C.01483] [DOI] [PubMed] [Google Scholar]
Tang 2007
- Tang JB. Indications, methods, postoperative motion and outcome evaluation of primary flexor tendon repairs in zone 2. Journal of Hand Surgery (British Volume) 2007;32(2):118-29. [DOI] [PubMed] [Google Scholar]
Tang 2013
- Tang JB, Amadio PC, Boyer MI, Savage R, Zhao C, Sandow M, et al. Current practice of primary flexor tendon repair. A global view. Hand Clinics 2013;29(2):179-89. [DOI] [PubMed] [Google Scholar]
Tang 2018a
- Tang JB. New developments are improving flexor tendon repair. Plastic and Reconstructive Surgery 2018;141:1427-37. [DOI] [PubMed] [Google Scholar]
Tang 2018b
- Tang JB. Recent evolutions in flexor tendon repairs and rehabilitation. Journal of Hand Surgery (European Volume) 2018;43(5):469-73. [DOI] [PubMed] [Google Scholar]
Tsuge 1977
- Tsuge K, Ikuta Y, Matsuishi Y. Repair of flexor tendons by intratendinous tendon suture. Journal of Hand Surgery 1977;2(6):436-40. [DOI] [PubMed] [Google Scholar]
Verdan 1960
- Verdan CE. Primary repair of flexor tendon. Journal of Bone and Joint Surgery (American Volume) 1960;42:647-57. [PubMed] [Google Scholar]
Villecio 2010
- Villeco JP. Edema therapist's management. In: Skirven TM, Osterman AL, Fedorczyk JM, Amadio PC, editors(s). Rehabilitation of the Hand and Upper Extremity. 6th edition. Philadelphia: Elsevier Mosby, 2010:64. [Google Scholar]
Von der Heyde 2010
- Von der Heyde RL. Wound classification and management. In: Skirven TM, Osterman AL, Fedorczyk JM, Amadio PC, editors(s). Rehabilitation of the Hand and Upper Extremity. 6th edition. Philadelphia: Elsevier Mosby, 2010:219-33. [Google Scholar]
Woythal 2019
- Woythal L, Holmer P, Brorson S. Splints, with or without wrist immobilization, following surgical repair of flexor tendon lesions of the hand: a systematic review. Hand Surgery Rehabilitation 2019;38(4):217-22. [DOI] [PubMed] [Google Scholar]
Wu 2013
- Wu YF, Tang JB. Tendon healing, edema, and resistance to flexor tendon gliding: clinical implications. Hand Clinics 2013;29(2):167-78. [DOI] [PubMed] [Google Scholar]
Zhao 2004
- Zhao C, Amadio PC, Paillard P. Digital resistance and tendon strength during the first week after flexor digitorum profundus tendon repair in a canine model in vivo. Journal of Bone and Joint Surgery (American Volume) 2004;86:320-27. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Thien 2004
- Thien TB Becker JH, Theis JC. Rehabilitation after surgery for flexor tendon injuries of the hand. Cochrane Database of Systematic Reviews 2004, Issue 4. Art. No: CD003979. [DOI: 10.1002/14651858.CD003979.pub2] [DOI] [PubMed] [Google Scholar]


