Abstract
Background
Many people with dementia are cared for at home by unpaid informal caregivers, usually family members. Caregivers may experience a range of physical, emotional, financial and social harms, which are often described collectively as caregiver burden. The degree of burden experienced is associated with characteristics of the caregiver, such as gender, and characteristics of the person with dementia, such as dementia stage, and the presence of behavioural problems or neuropsychiatric disturbances. It is a strong predictor of admission to residential care for people with dementia.
Psychoeducational interventions might prevent or reduce caregiver burden. Overall, they are intended to improve caregivers' knowledge about the disease and its care; to increase caregivers' sense of competence and their ability to cope with difficult situations; to relieve feelings of isolation and allow caregivers to attend to their own emotional and physical needs. These interventions are heterogeneous, varying in their theoretical framework, components, and delivery formats. Interventions that are delivered remotely, using printed materials, telephone or video technologies, may be particularly suitable for caregivers who have difficulty accessing face‐to‐face services because of their own health problems, poor access to transport, or absence of substitute care. During the COVID‐19 pandemic, containment measures in many countries required people to be isolated in their homes, including people with dementia and their family carers. In such circumstances, there is no alternative to remote delivery of interventions.
Objectives
To assess the efficacy and acceptability of remotely delivered interventions aiming to reduce burden and improve mood and quality of life of informal caregivers of people with dementia.
Search methods
We searched the Specialised Register of the Cochrane Dementia and Cognitive Improvement Group, MEDLINE, Embase and four other databases, as well as two international trials registries, on 10 April 2020. We also examined the bibliographies of relevant review papers and published trials.
Selection criteria
We included only randomised controlled trials that assessed the remote delivery of structured interventions for informal caregivers who were providing care for people with dementia living at home. Caregivers had to be unpaid adults (relatives or members of the person's community). The interventions could be delivered using printed materials, the telephone, the Internet or a mixture of these, but could not involve any face‐to‐face contact with professionals. We categorised intervention components as information, training or support. Information interventions included two key elements: (i) they provided standardised information, and (ii) the caregiver played a passive role. Support interventions promoted interaction with other people (professionals or peers). Training interventions trained caregivers in practical skills to manage care. We excluded interventions that were primarily individual psychotherapy.
Our primary outcomes were caregiver burden, mood, health‐related quality of life and dropout for any reason. Secondary outcomes were caregiver knowledge and skills, use of health and social care resources, admission of the person with dementia to institutional care, and quality of life of the person with dementia.
Data collection and analysis
Study selection, data extraction and assessment of the risk of bias in included studies were done independently by two review authors. We used the Template for Intervention Description and Replication (TIDieR) to describe the interventions. We conducted meta‐analyses using a random‐effects model to derive estimates of effect size. We used GRADE methods to describe our degree of certainty about effect estimates.
Main results
We included 26 studies in this review (2367 participants). We compared (1) interventions involving training, support or both, with or without information (experimental interventions) with usual treatment, waiting list or attention control (12 studies, 944 participants); and (2) the same experimental interventions with provision of information alone (14 studies, 1423 participants).
We downgraded evidence for study limitations and, for some outcomes, for inconsistency between studies. There was a frequent risk of bias from self‐rating of subjective outcomes by participants who were not blind to the intervention. Randomisation methods were not always well‐reported and there was potential for attrition bias in some studies. Therefore, all evidence was of moderate or low certainty.
In the comparison of experimental interventions with usual treatment, waiting list or attention control, we found that the experimental interventions probably have little or no effect on caregiver burden (nine studies, 597 participants; standardised mean difference (SMD) ‐0.06, 95% confidence interval (CI) ‐0.35 to 0.23); depressive symptoms (eight studies, 638 participants; SMD ‐0.05, 95% CI ‐0.22 to 0.12); or health‐related quality of life (two studies, 311 participants; SMD 0.10, 95% CI ‐0.13 to 0.32). The experimental interventions probably result in little or no difference in dropout for any reason (eight studies, 661 participants; risk ratio (RR) 1.15, 95% CI 0.87 to 1.53).
In the comparison of experimental interventions with a control condition of information alone, we found that experimental interventions may result in a slight reduction in caregiver burden (nine studies, 650 participants; SMD ‐0.24, 95% CI ‐0.51 to 0.04); probably result in a slight improvement in depressive symptoms (11 studies, 1100 participants; SMD ‐0.25, 95% CI ‐0.43 to ‐0.06); may result in little or no difference in caregiver health‐related quality of life (two studies, 257 participants; SMD ‐0.03, 95% CI ‐0.28 to 0.21); and probably result in an increase in dropouts for any reason (12 studies, 1266 participants; RR 1.51, 95% CI 1.04 to 2.20).
Authors' conclusions
Remotely delivered interventions including support, training or both, with or without information, may slightly reduce caregiver burden and improve caregiver depressive symptoms when compared with provision of information alone, but not when compared with usual treatment, waiting list or attention control. They seem to make little or no difference to health‐related quality of life. Caregivers receiving training or support were more likely than those receiving information alone to drop out of the studies, which might limit applicability. The efficacy of these interventions may depend on the nature and availability of usual services in the study settings.
Plain language summary
Remotely delivered information, training and support for informal caregivers of people with dementia
Review questions
We were interested in remotely delivered interventions involving information, training and support for family caregivers of people with dementia. By remotely delivered, we meant that they were provided over the telephone or on a computer or mobile electronic device. We asked whether these kinds of intervention helped caregivers more than doing nothing, and also whether interventions that included elements of training and support worked better than simple provision of information about dementia.
Background
Caring for a family member or friend with dementia can offer positive experiences, but often also has negative consequences for the caregiver. These negative consequences may be emotional, physical, social and financial, and are sometimes described as caregiver 'burden.' Many interventions have been developed to try to help caregivers in their caring role. Often, these interventions involve several components. In this review we divided the intervention components into information (increasing caregivers' knowledge about dementia), training (helping them to practice important skills for successful caring) and support (providing an opportunity to share experiences and feelings with other people). We chose to review only interventions delivered remotely, in part because we were writing during the COVID‐19 pandemic, when many countries required people to remain in their homes. However, remotely delivered interventions may also be useful in many other kinds of situations when it is difficult for caregivers to access services in person.
Study characteristics
We searched up to April 2020 for randomised controlled trials that addressed our review questions. We found 12 studies with 944 participants that compared groups of caregivers receiving usual care, or some non‐specific contact with researchers, with other groups receiving remotely delivered interventions that involved information with training, support or both. We found another 14 studies with 1423 participants that compared simple information provision with more complex interventions that involved training or support. The interventions lasted an average of 16 weeks. Three studies took place in China; all the others were from North America or Europe. About half used the telephone and about half used the Internet to deliver the interventions.
What are the main results of our review?
Compared with the usual services provided for caregivers where the studies took place, or with non‐specific contact with the researchers, we found that the information, training and support interventions probably had no important effect on caregivers' overall burden, depressive symptoms, or quality of life. Caregivers in both groups may have been equally likely to drop out of the studies for any reason. Compared to information only, interventions which included training and support may result in a slight reduction of caregiver burden, probably reduce depressive symptoms, may have little or no effect on quality of life, and probably make it more likely that caregivers drop out of the studies. We did not find any obvious effects of different intervention components, but we were not able to draw any firm conclusions about this. There was no evidence on whether the interventions improved the quality of life of the people with dementia who were being cared for. We did not find any studies that reported harmful effects of the interventions or the additional burden they might add to the caregiver's life. We do not know how the interventions would perform in countries where few health and social care services are available for people with dementia and their families, or in situations where caregivers are unable to access usual services.
How reliable are these results?
We found that most studies were well‐conducted, but because most outcomes were subjective, there was a risk that the expectations of the caregivers and researchers could have influenced results. For some outcomes, there were inconsistent results among studies. Overall, our confidence in our findings was moderate or low so the results could be affected by further research.
Summary of findings
Background
Description of the condition
It is estimated that worldwide, about 50 million people have dementia, and there are nearly 10 million new cases every year (WHO 2020). Many people with dementia are being cared for at home by an unpaid informal caregiver, who is usually a female family member, often a spouse or daughter. It is estimated that family caregivers spend five to 20 hours per day and an average of 60 hours per week caring for the person living with dementia (Marziali 2006).
As a consequence of their role as caregiver of a person with a progressive and irreversible disease, the informal caregiver may experience a range of physical, emotional, financial and social harms (Collins 2011). This situation has been widely described in the literature and is known as caregiver burden (Dillehay 1990; George 1986). Caregiver burden is associated with characteristics of the caregiver, such as gender, and characteristics of the person with dementia, such as behavioural problems, stage of dementia and the presence of neuropsychiatric disturbances (Haley 2004). It is a strong predictor of a move from home care to residential care for people with dementia (Eska 2013).
Description of the intervention
A variety of psychosocial interventions have been suggested to prevent or to reduce the negative consequences of dementia care on informal caregivers (Cooke 2001; Etters 2008; Pusey 2001; Selwood 2007). The interventions are generally heterogeneous regarding (i) their theoretical framework, (ii) the focus and the type of interventions reported (e.g. behavioural intervention, stress management, education about the disease), and (iii) the administration format (group or individual, generic or tailored to specific needs, telephone‐ or Internet‐based).
Interventions that are delivered remotely using written materials, the telephone or Internet may be particularly suitable for caregivers of people with dementia who can struggle to access face‐to‐face services because of their own health problems, poor access to transport, or difficulties finding substitute care. At the time of writing, measures used by many countries to contain the COVID‐19 pandemic require that most people, including people with dementia and their family carers, are confined to their homes, isolating themselves from direct contact with others. In such circumstances, there is no alternative to remote delivery of interventions.
There are also, however, significant barriers to remote delivery of interventions, particularly for older caregivers. They may have sensory impairments or other disabilities that make use of the necessary technologies difficult. They may also lack confidence with the technologies involved and may particularly value direct contact with others, being less familiar than many younger people with the experience of finding support in online communities.
This review assesses the efficacy and acceptability of remotely delivered interventions that aim to offer information, training and support, or a mixture of these, to informal carers of people with dementia.
Information
We considered information interventions to have two key elements: (i) they involve the provision of standardised information whose contents are determined by the professional; and (ii) the participant has a passive role (i.e. there is no interaction with the professional or active practice of skills). The intervention can address various aspects: issues related to the person with dementia (e.g. signs and symptoms, natural history, treatments and care alternatives), their care (e.g. activities of daily living, nutrition, communication with the person with dementia), the difficulties derived from their care (behavioural problems, role incompatibility, use of drugs, sleep disturbances), or resources available in the community (Gallagher‐Thompson 2010). The format of implementation can vary: leaflets, manuals, lectures or audio‐visual presentations. The nature of these interventions makes them relatively easy to deliver remotely.
Training
The key element of training interventions is to provide caregivers with the practical skills to manage the burden of care. Caregivers will play an active role in the program (e.g. through supervised practice, or role playing). The training may relate to the care of the person with dementia (e.g. recognising behavioural triggers, communicating more effectively, tailoring tasks to patients' capabilities) or to management of the caregiver's psychological stress. Training can be classified into the following categories: (i) meeting patients' basic care needs, (ii) managing behavioural problems, and (iii) managing and coping with caregiver stress (Losada Baltar 2004; Martin‐Carrasco 2014). Delivery of these elements remotely is likely to be more challenging than the simple provision of information.
Support
The key element of support interventions is the participants' active role in discussing and sharing feelings, problems or issues related to care with other caregivers and professionals (Winter 2006). This category could include two types of support: (i) peer support (e.g. participation in a caregivers' group that is not professionally facilitated), and (ii) professional support (e.g. receiving counselling from a health professional) (Mittelman 2004). Peer support groups, in particular, are usually face‐to‐face, but as technologies develop, it is increasingly possible for these to take place online.
How the intervention might work
Interventions for caregivers of people with dementia may work in a variety of ways depending on the content. Broadly speaking, they are intended to improve caregivers' knowledge about the disease and its care (Ducharme 2011; Liddle 2012); to increase their sense of competence (Laakkonen 2012) and their ability to cope with difficult situations (e.g. behavioural disturbances, communication problems) (Cheng 2012); and to relieve feelings of isolation, as well as to allow caregivers to attend to their own emotional and physical needs (Roth 2009).
Why it is important to do this review
Throughout the world, the majority of care for people with dementia is provided by family members. Anything that can be done to ease the burden of this care will be good for the caregivers and for the people they care for. It may also ease the burden on health and social care systems, for example by improving caregivers' health and by delaying institutional care for the person with dementia. All health and care systems are struggling with the magnitude of the problem presented by dementia. Remote delivery of interventions to support caregivers, if acceptable and effective, offers the opportunity to reach more people than conventional services, potentially at lower cost.
Development of remotely delivered interventions acquired a new urgency in the context of the COVID‐19 pandemic. The strain on family caregivers who are isolated with a person with dementia is hugely magnified. Not only are they cut off from their usual community and professional supports, but so is the person with dementia, whose behaviour may be more challenging as a result. Because of their age and vulnerability, these groups may experience more prolonged periods of isolation than other members of the community. Identification of effective interventions for caregivers that can be delivered remotely is needed more than ever.
This review aims to collate the best evidence about the efficacy and acceptability of these interventions to facilitate decision‐making by those designing, delivering and receiving services.
Objectives
To assess the efficacy and acceptability of remotely delivered interventions aiming to reduce the burden and improve the mood and quality of life (QoL) of informal caregivers of people with dementia.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) in which caregivers were randomly assigned to remotely delivered psychoeducational interventions that included information with support or training, or to a control condition. The control condition could be simple provision of information or waiting list, usual care or a non‐specific intervention serving as an attention control.
We excluded all non‐randomised studies and also RCTs with a cross‐over design, due to the high risk of carryover effects.
Types of participants
Eligible trials included informal caregivers of people with dementia receiving care at home. The dementia of the cared‐for person could be of any type. We accepted clinical diagnoses or diagnoses reached using formal diagnostic criteria such as the Diagnostic and Statistical Manual of Mental Disorders (DSM), the International Classification of Diseases (ICD), the National Institute on Aging ‐ Alzheimer's Association (NIA‐AA) or the Alzheimer's criteria of the National Institute of Neurological and Communicative Disorders and the Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA). Caregivers had to be unpaid adults (relatives, or members of the person's community; 18 years of age or older), of either sex, and of any ethnic or geographical origin. Studies that included caregivers of people with mixed diagnoses were included only if data on caregivers of people with dementia could be extracted separately.
Types of interventions
We included trials that assessed remote delivery of structured interventions involving training, support, information provision, or combinations of these elements, and that only targeted informal caregivers. We considered an intervention to be structured if there was a clear description of a standard set of components and how they were implemented. They could be delivered using paper, the telephone, the Internet or a mixture of these, but did not involve any face‐to‐face contact with professionals or other caregivers. These interventions had to be compared with a control condition, which could be provision of information alone or a waiting list, usual care or any non‐specific intervention serving as an attention control. Participants in waiting list groups were offered the experimental interventions once the study finished. Participants in attention control groups received a similar amount of contact with professionals to those in the experimental group, but the content of the contact involved no specific information, training or support related to dementia.
The following interventions were excluded:
interventions aimed at healthcare professionals;
interventions aimed at the people with dementia;
respite care interventions;
interventions for caregivers that are individually tailored and whose components are not well defined; and
interventions for caregivers that are predominantly psychotherapy, including interventions based entirely on the cognitive behavioural model (CBT). However, interventions that included elements of CBT as part of a larger, multimodal intervention were eligible for inclusion.
We used the following criteria to identify information, support and training. Information interventions include two key elements: (i) they provide standardised information, and (ii) the caregiver plays a passive role. Support interventions promote caregiver interaction with other people (professionals or peers). Training interventions are structured interventions intended to train caregivers in practical skills in order to prevent or alleviate the negative consequences of care giving.
We used the following operational criteria to categorise the interventions:
| Intervention | Definition | Key elements |
| Information |
Structured programmes with standardised information about issues related to care of people with dementia and caregiver burden. The contents of the intervention can include issues related to:
|
The intervention involves the provision of standardised information whose contents are determined by a professional in charge of the dementia. The participant has a passive role, and there is no interaction with the professional or active learning and practice of appropriate skills. |
| Support |
Programmes that allow the caregiver to talk, discuss or share information about their caregiver issues. There are two types of support:
|
The participant has an interactive role talking, discussing or sharing their feelings, problems or contents related to the care provided with other caregivers or professionals. |
| Training |
Structured interventions intended to empower caregivers with practical skills to manage the issues related to care. The skills to be acquired may relate to the care of the person with dementia, or to the management of the caregiver's psychological stress, according to the following categories:
|
Caregivers play an active role (e.g. through supervised practice, role playing, etc.) in the intervention provided. |
We compared (1) all interventions involving training, support or both, with or without information provision, with usual treatment, waiting list or attention control and (2) all interventions involving training, support or both, with or without information provision, with provision of information alone. We used the Template for Intervention Description and Replication (TIDieR) checklist to describe the interventions tested in each included trial, to obtain a detailed account of the assessed interventions, and to improve comparability among studies (Hoffmann 2014).
Types of outcome measures
We focused on outcomes that have been identified as relevant to evaluate effectiveness of psychosocial interventions in dementia care (Moniz‐Cook 2008).
Primary outcomes
Caregiver burden, measured by validated questionnaires such as the Zarit Burden Interview (ZBI) or the Sense of Competence Questionnaire (SCQ 27).
Caregiver mood or psychosocial well‐being, measured by validated questionnaires such as the Center for Epidemiologic Studies Depression Scale (CES‐D), the Hospital Anxiety and Depression Scale (HADS), or the General Health Questionnaire (GHQ).
Caregiver health‐related quality of life (HRQoL), measured by the EuroQoL Group's instrument (EUROQOL), the 36‐Item Short Form Health Survey (SF‐36) or the World Health Organization (WHO) Quality of Life assessment tool (WHOQOL).
Acceptability of the intervention: we used dropouts for any reason as a surrogate for the acceptability of interventions.
Secondary outcomes
Caregiver knowledge and skills.
Use of health or social resources.
Admission of the person with dementia to institutional care.
QoL of the person with dementia, measured by validated questionnaires such as the specific Quality of life in Alzheimer's disease (QOL‐AD) instrument, the Dementia Quality of Life (DQOL) instrument, or the generic EQ‐5D instrument.
Search methods for identification of studies
Electronic searches
We searched ALOIS, the Specialised Register of the Cochrane Dementia and Cognitive Improvement Group (www.medicine.ox.ac.uk/alois) on 10 April 2020. We used the following terms: (caregiv* or carer*) and (support* or information* or program* or training* or psychoeducation* or psycho‐education* or skill* or education* or tele* or video* or computer* or internet* or online or stress*). In addition, when trials are added to the ALOIS database, they are assigned a 'study aim.' One of the possible study aims is "caregiver focussed." We searched the whole database using this aim filter.
ALOIS is maintained by the Information Specialists of the Cochrane Dementia and Cognitive Improvement Group (CDCIG), and contains studies that fall within the areas of dementia prevention, dementia treatment and management, and cognitive enhancement in healthy elderly populations. The studies are identified through:
Monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL, PsycInfo and LILACS;
Monthly searches of a number of trial registers: ISRCTN; UMIN (Japan's Trial Register); the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials and the Netherlands National Trials Register, plus others);
Quarterly search of the Cochrane Central Register of Controlled Trials (CENTRAL);
Six‐monthly searches of a number of grey literature source: ISI Web of Science Core Collection.
To view a list of all sources searched for ALOIS, see About ALOIS on the ALOIS website (www.medicine.ox.ac.uk/alois).
We also ran additional searches in MEDLINE, Embase, PsycINFO, CINAHL, and CENTRAL. We searched two international trials registries, ClinicalTrials.gov at the US National Institutes of Health, and the WHO ICTRP, to ensure that the search was as comprehensive and as up‐to‐date as possible. We have presented the search strategies used and the number of results retrieved in Appendix 1.
The most recent search for this review was done on 10 April 2020. Before that date, we had run searches in December 2015, July 2016, May 2017, May 2018 and May 2019.
Searching other resources
We complemented this search with manual searches of the bibliographies of relevant review papers and published trials to locate additional relevant studies.
Data collection and analysis
Selection of studies
Pairs of review authors (two of EGF, JRR, JB) independently examined the titles and abstracts of citations obtained by the searches to identify studies that may meet the inclusion criteria. We retrieved the full‐texts of all potentially eligible articles and examined these against the inclusion and exclusion criteria, again independently and in duplicate. Studies excluded and reasons for exclusion are reported in the Characteristics of excluded studies table.
Data extraction and management
Pairs of review authors (two of EGF, JRR, BS, JB) independently extracted data using a standardised data collection form. In case of disagreement, another review author (IS) was consulted and consensus sought. For each study, extracted data included: trial registry identification, funding and potential conflicts of interest, main methodological characteristics, results. We described the key characteristics of each trial in tables, paying special attention to the characteristics of the interventions assessed. We described the interventions according to the TiDieR checklist (Hoffmann 2014).
Assessment of risk of bias in included studies
We assessed the risk of bias in included studies by following the guidance of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Pairs of review authors (two of EGF, JRR, JB) extracted the appropriate information and independently rated the risk of bias for each study and outcome. In case of disagreement, another review author (IS) was consulted and consensus sought. We assessed the following sources of bias: selection bias (including random sequence generation and allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessments); attrition bias (incomplete outcome data); and reporting bias (selective reporting).
Using 'Risk of bias' tables, we rated the risk of bias in each domain as either "low risk," "unclear risk" or "high risk" and provided an explanation for each rating. We considered trials with inadequate randomisation and lack of blinding (for outcome assessment) as being at overall "high risk of bias."
Measures of treatment effect
We used the risk ratio (RR) with its 95% confidence interval (CI) as the measure of treatment effect for dichotomous data and the mean difference (MD) with its 95% CI as the measure of treatment effect for continuous data. We used the SMD with its 95% CI for continuous outcomes only if similar outcome constructs were measured by different rating scales.
Unit of analysis issues
The unit of analysis was the individual allocated to a treatment group. We did not identify any eligible cluster‐randomised trials. If a trial reported outcomes at more than one time point (e.g. weekly or monthly outcome measures; end‐of‐trial and follow‐up outcome measures), we extracted the measure closest in time to the end of treatment and used this as a measure of the acute effect of the intervention. If a trial included more than one control or experimental group, we followed the guidance of the updated Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). When managing several eligible experimental interventions versus a unique control, we split the control sample to avoid over‐counting participants. Where there was more than one control group, we combined these if it was conceptually reasonable to do so.
Dealing with missing data
We used intention‐to‐treat (ITT) data where this was reported. If an included study imputed missing data, we reported the data imputation method.
Assessment of heterogeneity
We considered heterogeneity in study characteristics to decide if combining the results would be clinically meaningful. If we judged the studies to be too heterogeneous, then we did not conduct meta‐analyses, but reported individual results. We assessed between‐studies heterogeneity in meta‐analyses using the I2 statistic, complemented with the examination of overlapping 95% CI.
Assessment of reporting biases
We assessed small study bias using funnel plots where there were 10 or more studies in a comparison.
In the bibliographic searches, if we found any RCT included in any clinical trial register that we considered could have been completed, but for which we had not been able to locate any results, we contacted the authors to request information about the status of the trial, and the trial results, if it had been completed.
Data synthesis
If we considered trials to be sufficiently similar that it was appropriate to pool data, we conducted meta‐analyses using a random‐effects model for two main comparisons:
a comparison of all interventions involving information, training or support versus usual treatment, waiting list or attention control;
a comparison of all interventions involving training or support (with or without information) versus information alone.
Where there were sufficient data, we stratified the analyses by type of intervention.
We conducted all analyses in Review Manager version 5.3 (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
Depending on availability of data, we intended to conduct subgroup analyses according to:
the intensity/length of the intervention provided;
the mode of delivery of the intervention: telephone‐only versus Internet with or without telephone;
the characteristics of participants; and
the outcome scales used to score each construct of interest.
Sensitivity analysis
If feasible, we intended to perform sensitivity analyses to assess the influence of studies at higher risk of bias, and the impact of attrition on the robustness of the pooled results. However, the small number of included studies by group comparison precluded reliable sensitivity analyses. Another form of sensitivity analysis, the leave‐one‐study method, did not identify any specific study as highly influential on the combined results.
'Summary of findings' tables
We summarised the review findings in a 'Summary of findings' table using the online GRADEpro GDT application (GRADEpro GDT). In the table, we reported the estimated treatment effects for the review's primary outcomes and for resources use and institutionalisation. For each effect estimate, we used the GRADE approach (Schünemann 2013) to rate our confidence that the estimate is correct (high, moderate, low or very low certainty). GRADE ratings take account of study limitations, imprecision of effect estimates, inconsistency among studies, indirectness of evidence and publication bias. When assessing imprecision, we considered that an absolute value of SMD 0.50 probably represented an important between‐group difference. We considered inconsistency to be substantial enough to downgrade our confidence in the effect estimate if we found I2 to be greater than 60%.
Results
Description of studies
Results of the search
After a first assessment by the CDCIG information specialist, we assessed the remaining 2,010 potentially relevant records. Of these, 264 records remained after first assessment by the author team, and after more detailed screening of titles and abstracts, we retrieved 94 full‐text articles for assessment. We excluded 52 articles, recording the reasons for exclusion. We included 26 studies (39 references) in the qualitative synthesis and 25 studies in the quantitative synthesis. Three studies are awaiting classification because they may be eligible, but there is not enough information in their registry protocols to make an eligibility decision (see Studies awaiting classification). Another 13 studies are ongoing studies that have not yet reported results (see Ongoing studies). Figure 1 presents the flow diagram of the study selection process.
1.
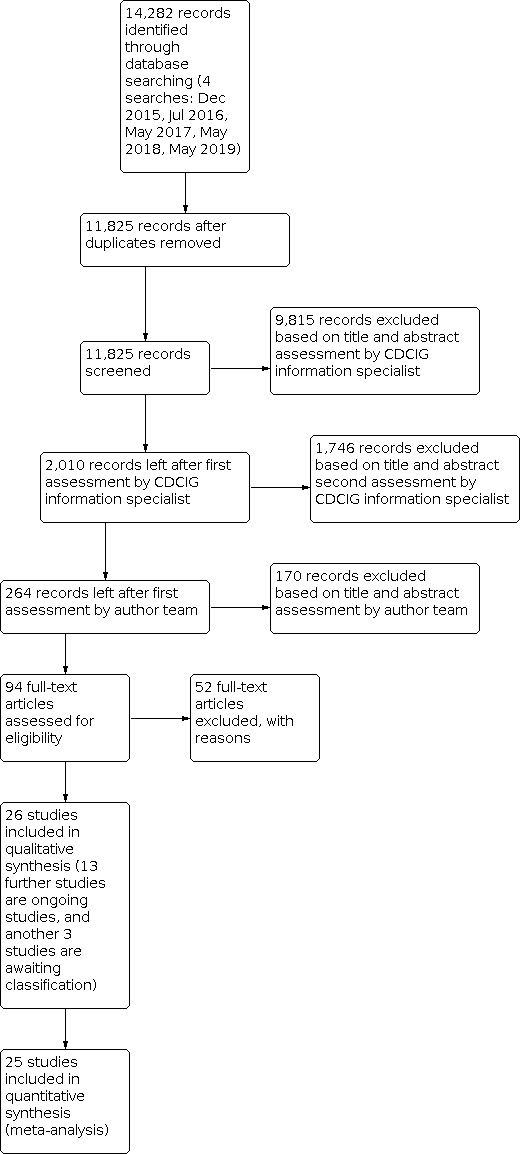
Study flow diagram.
Included studies
We identified 26 studies with 2367 participants for inclusion in the review (see Characteristics of included studies).
Setting and participants
Included studies were conducted or published over 25 years (from 1995 to 2020). Most studies were from the US (15 studies, 58%). China and the Netherlands contributed three studies each, France two studies, and Canada, Spain and the UK one study each. Studies had a median sample size of 67 participants (interquartile range (IQR) 49 to 110) and a median duration of 16 weeks (IQR 12 to 24). Only six studies (23%) provided data for a continuation follow‐up after the end of the intervention (median of 24 weeks).
Most caregivers were female (72%) with a median age of 63 years (range 51 to 72 years) . Care recipients were mainly diagnosed with dementia due to Alzheimer's disease (83%), had a median age of 78 years (range 62 to 81 years) and had been treated for dementia for a median of 38.5 months (range 30 to 53 months).
Interventions
We examined the descriptions of the experimental interventions in the included studies to look for elements meeting our definitions of information, training and support. Almost all of the experimental interventions specified provision of information to caregivers as a component of a more complex intervention. We considered this element was not present in only three studies: Davis 2004 and Dowling 2014, which we considered to be relatively pure training interventions, and Winter 2007, which we considered to be a relatively pure support intervention. However, even in these cases, it was possible that some provision of information did occur during the course of delivery of the other elements. Therefore, we constructed three subgroups of experimental intervention based on the predominant components: training with or without information, support with or without information, and interventions including both support and training elements. We considered these groups to be exploratory, recognising that all the interventions were complex in nature and that there is inevitably some overlap among the categories.
We classified the experimental intervention in 12 studies as training or training with information (Au 2015; Au 2019; Blom 2015; Czaja 2013; Davis 2004; Dowling 2014; Gant 2007; Kajiyama 2013; Martindale‐Adams 2013; Metcalfe 2019; NCT00056316; NCT03417219), in eight studies as support or support with information (Brennan 1995; Hattink 2015; Hayden 2012; Kwok 2013; Mahoney 2003; Nunez‐Naveira 2016; Torkamani 2014; Winter 2007), and in six studies as involving both training and support with information (Cristancho‐Lacroix 2015; Duggleby 2018; Gustafson 2019; Huis in het Veld 2020; Tremont 2008; Tremont 2015). Fourteen studies delivered the remote intervention by telephone and 12 using the Internet.
Eight studies included usual treatment (Brennan 1995; Cristancho‐Lacroix 2015; Mahoney 2003; Nunez‐Naveira 2016; Torkamani 2014; Tremont 2008) or waiting list (Hattink 2015; Metcalfe 2019) as the control condition. Four studies provided some form of attention control during the treatment period, with or without socially supportive telephone calls (Davis 2004; Dowling 2014; Hayden 2012; Tremont 2015). Eight studies provided specific information on dementia and related problems (Blom 2015; Duggleby 2018; Gustafson 2019; Kajiyama 2013; Kwok 2013; Martindale‐Adams 2013; NCT03417219; Winter 2007) in the control condition. Six further studies provided both information and some form of control attention (Au 2015; Au 2019; Czaja 2013; Gant 2007; Huis in het Veld 2020; NCT00056316).
The TIDieR checklist for included studies is reported in Additional tables (Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18; Table 19; Table 20; Table 21; Table 22; Table 23; Table 24; Table 25; Table 26; Table 27; Table 28 ).
1. TIDieR ‐ Au 2015.
| Study | Au 2015 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | Psychoeducation with behavioral activation (PsyED‐BA). | Psychoeducation only (PsyED). |
| WHY | Based on the Gallagher‐Thompson’s program Coping with Cargiving. The aim is to enhance the use of coping skills via behavioral activation (BA), since it would be more acceptable and helpful than change dysfunctional thoughts, overcome barriers to treatment access and train paraprofessionals to deliver some modules of the program. | General discussion about the psychoeducation program and information packet. |
| WHAT materials | Caregivers were given a printed copy of the psychoeducation program (adapted from the Chinese version of the Coping with Caregiving manual) together with an information packet with fact sheets concerning local organizations, community resources, and social and mental issues related to dementia. 1.‐ Patient‐Change Workshop, Procedures Manual, Progress Notes, Problem Behavior Tracking forms, written cueing systems for repetitive verbal behavior, audio/videotape or tactile diversion program for repetitive vocal and physical behaviours, and verbal and physical prompting procedures for continence and self‐feeding problems, Caregiver Self‐Change Recording Form. 2.‐ Self‐change Workshop, Problem Behavior Tracking forms, Caregiver‐self change recording form. |
Caregivers were given a printed copy of the psychoeducation program (adapted from the Chinese version of the Coping with Caregiving manual) together with an information packet with fact sheets concerning local organizations, community resources, and social and mental issues related to dementia. |
| PROCEDURES | Participants were contacted by telephone within the same week of the baseline assessment to start the psychoeducation program. There were a total of four weekly intervention telephone calls, each lasting about 30 minutes. ‐ In the first week, all participants were taught about the symptoms and associated behavioral changes of dementia and the possible effects on the caregivers. Participants were also invited to share their caregiving experiences. ‐ In the second week, participants learned about the physical, social, and psychological consequences of stress and being aware of their own stress. They were invited to talk about their own stress. ‐ In the third week, they learned about the principles of identifying and scheduling pleasant events for themselves. ‐ In the fourth week, participants learned about communication: communicating their own needs to seek support from their family members. They also learned about the characteristics of various different types of communications: passive, aggressive, and assertive. Participants had eight biweekly telephone sessions over the following 4 months. Each session consisted of a telephone call lasting 15 to 20 minutes. The first four sessions focused on pleasant event scheduling and the other four sessions on effective communication. The tasks involved in each of the four sessions were as follows: 1. Activity monitoring: how is the participant spending time/ communicating now? 2. Activity scheduling: schedule pleasant event/effective communications 3. Reinforcing or modifying the pleasant event and communication based on feedback or self‐evaluation 4. Activity rescheduling/revision based on changes after modification. Finally, social work services were available upon requests in outpatient departments in which the care recipients received their regular follow‐up. |
Participants were contacted by telephone within the same week of the baseline assessment to start the psychoeducation program. There were a total of four weekly intervention telephone calls, each lasting about 30 minutes. Participants had eight biweekly telephone sessions over the following 4 months. Each session consisted of a telephone call lasting 15 to 20 minutes: ‐ Participants were asked to go through the materials of the psychoeducation program and the information package. For each of the telephone session, the participants were asked to select their own topics for general discussion. If the caregiver selected pleasant event scheduling or communications, general discussion would be carried out without any BA procedures. Finally, social work services were available upon requests in outpatient departments in which the care recipients received their regular follow‐up. |
| WHO provided | Five paraprofessionals recruited trained to administer BA procedures. They were between 55 and 60 years old and had previously completed post‐secondary school training in areas related to human services (i.e., nursing or management). They had subsequently completed a 42‐hour course on Introduction to Psychology and had then received 20 hours of group training led by a social worker and a clinical psychologist (the principal investigator of the study) on BA in the context of the Copy with Caregiving program. Each paraprofessional worker was tested on a mock case before delivering the program to actual caregivers. Ongoing weekly supervision was provided by the clinical psychologist and social worker. | |
| HOW delivered | Individually over the telephone. | |
| WHERE occurred | At home. | |
| WHEN and HOW MUCH | Twelve sessions: sessions 1 to 4 were delivered weekly, sessions 5 to 12 were delivered biweekly. Each session consisted of a telephone call lasting 15 to 20 minutes. | |
| TAILORING | Interventions were comprehensively designed, not tailored to cover individual or unmeet needs. | |
| MODIFICATIONS | None described. | |
| HOW WELL planned | No described. | |
| HOW WELL actual | No described. | |
2. TIDier ‐ Au 2019.
| Study | Au 2019 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | Psychoeducation with behavioral activation (TBA). | General Monitoring (TMG). |
| WHY | Behavioral activation (BA) focuses on constructing reinforcement contingencies that increase functional behavior and self‐efficacy. Self‐efficacy refers to the person’s beliefs about the abilities to exercise control on the events affecting their lives. The intervention was adapted from the Chinese Version of the Coping with Caregiving. | General discussion about the psychoeducation program and information packet. |
| WHAT materials | Written information, including the forms for pleasant event scheduling, was mailed to participants before the program started. | Information packet. |
| PROCEDURES | Four weekly sessions of psychoeducation and eight sessions of behavioral activation. Themes of the psychoeducation component were as follows. Session 1 (week 1): · Symptoms and associated behavioral changes in dementia · Stages in dementia · Caregiving roles and demands · Effects on caregivers Session 2 (week 2): · Physical, social and psychological consequence of stress · Identifying stress reactions · Awareness of stress · Stress and well‐being Session 3 (week 3): · The effect of life events on mood · Tracking daily/ weekly events · Identifying pleasant events · Scheduling pleasant events Session 4 (week 4): · Communication needs to family members · Types of communications: passive, aggressive and assertive · Resources available in the community · Planning in the future Themes of the behavioral activation component were: · Session 1 – Review the present use of time. Using the monitoring form · Session 2 – Brain‐storm pleasant events. Scheduling pleasant activities · Session 3 – Review scheduling of events. Discuss how to improve · Session 4 – Review modifications. Consolidate gains on scheduling · Session 5 – Review present social support. Explore new sources of support · Session 6 – Examine communication skills. Explore new options · Session 7 – Review new communications. Discuss how to improve · Session 8 – Review modification. Consolidate gains on support |
All TGM participants received four weekly psycho‐education sessions over the phone with the same contents as in the TBA group. These caregivers were then assigned to eight bi‐weekly sessions of general monitoring with no BA intervention. Each of these sessions started with checking in with the caregiver through inviting them to update their caregiving situation. Caregivers were then guided to discuss one of the following topics at each session in this order: 1. caregiver’s health 2. care‐recipient’s needs 3. caregiver’s routines 4. social support. As there were a total of eight sessions, the last four sessions repeated the order of the first four. While some caregivers might report on attempts they made on their own initiative to improve their scheduling and communication, no specific attempt was made to ask them to review these attempts. |
| WHO provided | An interventionist with a degree in social work delivered all the four sessions of psycho‐education. Six paraprofessional coaches, between 50 and 60 years old and with an undergraduate degree in helping or service professions, delivered the BA or MG interventions, or carried out monitoring. A social worker and a clinical psychologist provided the training and facilitated weekly supervision separately for TBA and TGM coaches. | |
| HOW delivered | Individually over the telephone. | |
| WHERE occurred | At home. | |
| WHEN and HOW MUCH | Twelve sessions: sessions 1 to 4 were delivered weekly and sessions 5 to 12 were delivered biweekly. Each session lasted about 20 minutes. | |
| TAILORING | Interventions were comprehensively designed, not tailored to cover individual or unmeet needs. | |
| MODIFICATIONS | None described. | |
| HOW WELL planned | The program fidelity was assessed by a rating system built into recording form. At the end of each session, all interventionists, including the paraprofessionals, were asked to rate to what extent they were able to follow the protocol for each of the four PE sessions (3 = fully; 2 = adequately with at least 60% of the material covered; 1 = slightly; 0 = not at all). A similar procedure was adopted for each of the 8 sessions for both TBA and TGM. In addition, 10 cases from TBA and 10 cases from TGM were audiotaped. Interventionists’ adherence to the intervention protocol was assessed by two graduate students who had received eight hours of training on the coding scheme. The sessions were coded with reference to four core TBA strategies (activity planning, review to improve on scheduling, develop new help‐seeking communication skills and review to improve on communications) and four core TGM strategies (updating on caregiving situation, health and needs of the caregiver and the care‐recipient, daily routines and family communications). | |
| HOW WELL actual | As planned. Minimal deviations to the main components of the intervention. | |
3. TIDieR ‐ Blom 2015.
| Study | Blom 2015 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | Mastery of dementia (MoD). | Minimal Intervention (e‐bulletins). |
| WHY | A guided self‐help Internet course ‘Mastery over Dementia’ (MoD) designed to reduce caregivers’ symptoms of depression and anxiety. MoD included a combination of psycho‐education with active participation of the caregiver, management of behavioral problems, teaching coping strategies, components of cognitive behavioral therapy (cognitive reframing: changing non‐helpful into helpful thoughts), and increasing social support. | No applicable. |
| WHAT materials | Internet contents. | E‐bulletins (digital newsletters). |
| PROCEDURES | The Internet course consists of 8 lessons and a booster session with the guidance of a coach monitoring the progress of participants and evaluating the homework. Each lesson has the same structure and consists of information (text material and videos), exercises, and homework, with an evaluation at the start and end of each session. The elements of the course were presented in the following order: · coping with behavioral problems (problem solving) · relaxation · arranging help from others · changing non‐helping thoughts into helping thoughts (cognitive restructuring) · communication with others (assertiveness training). The booster session was provided a month after participants finished the eight lessons, and provided a summary of what was learned. After every lesson, participants sent their homework to a coach via a secure application. The coach sent electronic feedback to caregivers on their homework within three working days. The feedback had to be opened before the next lesson could be started. Participants were automatically reminded to start with a new lesson or to send in their homework if they were not active for a fixed period of time. All participants in this study received feedback from the same coach, a psychologist employed by a health care agency with additional training in cognitive behavioral therapy and experience in the field of dementia. |
Caregivers received a minimal intervention consisting of e‐bulletins (digital newsletters) with practical information on providing care for someone with dementia. The bulletins were sent by email according to a fixed schedule (every 3 weeks) over nearly 6 months. The topics of the bulletin, which did not overlap with the content of MoD, were: · driving · holiday breaks · medication · legal affairs · activities throughout the day · help with daily routines · grieving · safety measures in the home · possibilities for peer support. There was no contact with a coach over the length of the study. |
| WHO provided | Internet intervention. A coach monitored the progression of the participants. | No applicable. |
| HOW delivered | Online. | |
| WHERE occurred | At home. | |
| WHEN and HOW MUCH | Eight lessons plus a booster session. | The bulletins were sent by email according to a fixed schedule (every 3 weeks) over nearly 6 months. |
| TAILORING | Interventions were comprehensively designed, not tailored to cover individual or unmeet needs. | |
| MODIFICATIONS | None described. | |
| HOW WELL planned | No described. | |
| HOW WELL actual | No described. | |
4. TIDieR ‐ Brennan 1995.
| Study | Brennan 1995 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | ComputerLink. | None beyond general information to identify local resources. |
| WHY | The intervention aimed to help caregivers develop problem‐solving skills, manage their own emotions, and increase their knowledge of dementia and caregiver‐support strategies. | No applicable. |
| WHAT materials | Caregivers received a Wyse 30 terminal and a Everex modem system. | No applicable. |
| PROCEDURES | ComputerLink provided three functions: · information · decision support · communication The electronic Encyclopedia provided extensive factual information to enhance self‐care and understanding of Alzheimer’s disease and promote health management of the care recipient. The program uses a system to help caregivers to resolve problems basing in the multiattribute utility theory of von Winterfeldt & Edwards. ComputerLink permitted several options for communication among caregivers, including private mail, access to a public bulletin board, and a section of questions and answers. A nurse moderator entered the system daily and read and responded to messages. The nurse served as a moderator and facilitator. |
None beyond general information to identify local resources. |
| WHO provided | Study nurses who had completed an intervention training programme. | No applicable. |
| HOW delivered | Online. | No applicable. |
| WHERE occurred | At home. | No applicable. |
| WHEN and HOW MUCH | Participants had access to the system 24 hours a day during 12 months. | No applicable. |
| TAILORING | Intervention was comprehensively designed, not tailored to cover individual or unmeet needs. | No applicable. |
| MODIFICATIONS | None described. | No applicable. |
| HOW WELL planned | Indicators for logging in the system. | No applicable. |
| HOW WELL actual | Caregivers logged into the system 3,875 times, They accessed it a mean of 83 times over 12 months. A typical encounter lasted 13 minutes. | No applicable. |
5. TIDieR ‐ Cristancho‐Lacroix 2015.
| Study | Cristancho‐Lacroix 2015 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | Diapason. | Treatment as usual. |
| WHY | The program’s content on the web was based on cognitive theories of stress. | No applicable. |
| WHAT materials | Web materials. | No applicable. |
| PROCEDURES | · Session 1: Caregiver stress – this session presents a definition of stress, its causes and consequences on caregivers, risk factors for chronic stress, and mechanisms and effects of relaxation (includes a link to the relaxation training in the Diapason website), as well as strategies for managing stress underlining the importance of looking for respite. · Session 2: Understanding the disease – in this session, the Alzheimer’s disease diagnosis procedure, the symptoms, the progression of the illness, and the consequences on daily life activities for persons with Alzheimer’s disease (PWAD) are explained. · Session 3: Maintaining the loved ones’ autonomy – this session presents the reasons and strategies to involve loved ones in the process of care in order to stimulate the preserved functions and compensate for the lost ones. The session underlines the importance of maintaining the self‐esteem of PWAD. · Session 4: Understanding their reactions – in this session, the most frequent behavioural and psychological symptoms of dementia (BPSD) and their characteristics are succinctly described and illustrated by examples from daily life. The contextual and intrinsic factors that might be associated with them are also described. · Session 5: Coping with behavioral and emotional troubles – this session presents practical advice on how to cope vis‐à‐vis the BPSD described in the previous session. · Session 6: Communicating with loved ones – this session includes the description of the most frequent language troubles and the strategies to modulate and adapt communication to the preserved skills of PWAD. · Session 7: Improving their daily lives – this session presents strategies to facilitate the performance of activities that become difficult or impossible to execute due to apraxia, illustrating them with examples adapted to daily life. · Session 8: Avoiding falls – the session includes practical advice for maintaining and stimulating the relative’s balance and actions to adopt in the event of a fall. In addition, various actions are described to adapt the relative’s home. · Session 9: Pharmacological and non‐pharmacological interventions – this session includes a brief presentation of different interventions available for caregivers in France with pharmacological treatment as well as cognitive and psychological support. · Session 10: Social and financial support – this session presents the different stakeholders and services that may help caregivers in their daily life. The financial and social support provided by the French government is also revised. · Session 11: About the future – this session provides caregivers with information about the role of disease progression anticipation, inviting them to try and foresee solutions keeping a prospective vision, encouraging them to look for further sources of information, and social support to reduce the uncertainty of caregiving situations. · Session 12: In a nutshell – the last session encompasses a summary of the Diapason program, emphasizing the acceptance of support and help and the importance of obtaining more information to anticipate and avoid stressful circumstances. Additionally the website contains other sections that can be consulted at any time: · Relaxation training: guidelines for learning relaxation as well as 2 videos for the modelling of Schultz’s Autogenic Training and Jacobson’s method. · Life Stories: stories about 4 couples, based on testimonials of caregivers, in which difficult situations are illustrated and possible solutions to manage them are discussed (i.e., apathy of patient, caregivers’ isolation). · Glossary: a glossary for technical words (i.e., neuropsychological assessment, aphasia). · Stimulation: practical activities to stimulate autonomy and share pleasant activities with the relatives in daily life. · Forum: a private and anonymous forum to interact with peers, to express their concerns, discuss solutions to daily problems, and share their feelings and experiences. The participants use nicknames to protect their privacy. A clinical psychologist participates in the discussions if necessary (i.e., avoiding aggressive or inappropriate comments). |
No applicable. |
| WHO provided | No web‐moderate self‐provided intervention except when using the forum resource. | No applicable. |
| HOW delivered | Online. | No applicable. |
| WHERE occurred | Home. | No applicable. |
| WHEN and HOW MUCH | The length of the intervention was 3 months, with each weekly session lasting 15 to 30 minutes on average, but there was no time limit and the participants could access different website sections (i.e., relaxation training, forum) for as long as they wished at any time. | No applicable. |
| TAILORING | Intervention was comprehensively designed, not tailored to cover individual or unmeet needs. | No applicable. |
| MODIFICATIONS | None described. | No applicable. |
| HOW WELL planned | Indicators for login in the system. | No applicable. |
| HOW WELL actual | On average, participants used the website 19.7 times (SD 12.9) and for 262.2 minutes (SD 270.7) during the first 3 months. | No applicable. |
6. TIDieR ‐ Czaja 2013.
| Study | Czaja 2013 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | Named "intervention condition". | Two control conditions which were combined: attention control and information |
| WHY | The intervention condition was modelled after the REACH II intervention. | No described. |
| WHAT materials | A videophone (CISCO IP 7900) was installed in the caregivers’ homes and connected to a Digital Subscriber Line and to a secure server at the host site. Participants in this condition also received a caregiver notebook with basic information about caregiving and community resources. | Caregivers in the attention condition were provided with a notebook with information related to nutrition. The information condition group received educational materials with basic information about dementia, safety, and community resources. |
| PROCEDURES | The intervention included education and skills training. It was designed to address five caregiver risk areas (safety, social support, problem behaviours, depression, and caregiver health). The intervention was standardized with respect to the treatment domains and strategies used within the treatment domains but individualized with respect to the amount of emphasis within a treatment domain based on a risk assessment administered at baseline. Certified interventionists taught problem solving strategies to deal with the care recipients’ problem behaviours and training on stress management, healthy behavior strategies, community resources, and communication strategies. The intervention included six 1‐hour monthly sessions. The four educational seminars were brief video lectures from experts on topics relevant to caregiving (i.e., update on Alzheimer disease, caregiver depression) and were presented serially; a new video appeared monthly beginning in month 2 of the intervention.The intervention also included five videophone support group sessions, which were interspersed throughout the intervention period. The support groups were structured and included up to six caregivers and a certified group leader. During the support group sessions caregivers received topical information (related to the material covered in the individual intervention sessions) and shared experiences and concerns. | Attention condition: participants received two 1‐hour in‐home sessions and four videophone sessions. They also participated in five telephone support group sessions, which were interspersed with the individual intervention sessions. The support groups followed the same format as those for the intervention group. The content for the attention control condition was structured around nutrition and healthy eating. Information condition: participants were mailed a packet of educational materials and received a brief (<15 minute) telephone “check‐in call” at 3 months post randomisation. |
| WHO provided | Interventionists. All assessors and interventionists received training and were certified before entering the field. | |
| HOW delivered | Online plus telephone. | Face to face plus telephone. |
| WHERE occurred | At home. | |
| WHEN and HOW MUCH | 6 sessions (1 hour each). | · 6 sessions (1 hour each). · 15 minutes “check‐in‐call”. |
| TAILORING | Interventions were comprehensively designed, not tailored to cover individual or unmeet needs. | |
| MODIFICATIONS | None described. | |
| HOW WELL planned | Interventionists submitted taped treatment sessions for review and feedback as part of the certification. Treatment implementation was monitored and maintained by weekly supervision meetings. Both interventionists and assessors followed a detailed manual of operations and a delivery assessment form was completed after each contact. | |
| HOW WELL actual | No described. | |
7. TIDieR ‐ Davis 2994.
| Study | Davis 2004 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | Telephone training or in‐home training (this review only includes evidence from telephone training). | Friendly, socially supportive, telephone calls. |
| WHY | The study was grounded in the stress, appraisal and coping theory of Lazarus. The premise for the caregivers skill training intervention was that improvements in caregiver's outcomes could be addressed by expanding caregiver's repertoire of effective appraisal and coping strategies through telephone training strategies | No applicable. |
| WHAT materials | Caregivers received a standardized, loose‐leaf notebook with suggestions for managing frequently encountered Alzheimer’s disease situations. They had to fill out a weekly log on problems they had managed over the past week. | Socially supportive friendly telephone calls. |
| PROCEDURES | Caregivers participated in training sessions focused on: · general problem solving · caragiver appraisal of behavior problems · written behavioral programs for managing specific problems · strategies for handling effective responses to difficult caregiving situations. They received an initial home visit of 45 minutes to introduce the trained interventionist and to familiarize with the uses of the notebook and log sheets. At each weekly contact, the interventionist reviewed the past week of caregiving problems with the caregiver, discussed the log entries and used selected notebook sections to help the caregiver manage the situation. |
Friendly callers made an initial home visit of approximately 45 minutes to introduce themselves. At the time of each contact, callers required about the caregiver's past week as well as any changes in their general health and medication regimen. |
| WHO provided | Interventionists. | |
| HOW delivered | Telephone. | |
| WHERE occurred | At home. | |
| WHEN and HOW MUCH | 12 weekly telephone contacts. | |
| TAILORING | Interventions were comprehensively designed, not tailored to cover individual or unmeet needs. | |
| MODIFICATIONS | No described. | |
| HOW WELL planned | Telephone contacts were tape recorded and reviewed to assess continued adherence. | |
| HOW WELL actual | Telephone contacts averaged 37 minutes (SD=18) | Friendly telephone calls averaged 16 minutes (SD=12). |
8. TIDieR ‐ Dowling 2014.
| Study | Dowling 2014 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | LEAF (Life Enhancing Activities for Family Caregivers). | Attention control group. |
| WHY | The positive affect intervention teaches participants a series of behavioral and cognitive “skills” for increasing positive affect coping with health‐related and other life stresses. The skills include noticing and capitalizing on positive events, gratitude, mindfulness, positive reappraisal, focusing on personal strengths, attainable goals, and acts of kindness. | No described. |
| WHAT materials | No described. | |
| PROCEDURES | Skill‐building Intervention Sessions · Session 1: Positive Events and Gratitude – Identify a positive or meaningful event in the last week and what it means to note, favour, and capitalize on positive events. Homework: write down 3 things that went well each day and why they went well. Begin a gratitude journal (writing one thing each day you are grateful for) that continued throughout the rest of the intervention. · Session 2: Mindfulness – Teach concepts of mindful attention and non‐judgment. Homework: (a) practice awareness of breathing and meditation for 10 minutes daily and (b) once a day take the time to enjoy something that you usually hurry through, do one thing at a time, and pay attention to it. Participants were encouraged to continue the breathing exercise through the remaining weeks of the intervention and to continue the gratitude journal. · Session 3: Positive Reappraisal – Discuss the meaning of positive reappraisal and how to apply it to everyday occurrences. Homework: each day think of one negative or stressful thing that happened. Practice positive reappraisal – why it may not be as bad as initially thought or something good that might come of it. Participants were encouraged to write about their experience reappraising at the end of each day and to continue their gratitude journal and mindful breathing exercises. · Session 4: Personal Strengths and Attainable Goal – Generate a list of personal strengths that can be used in everyday life. Define attainable goals and practice setting one related to self‐care. Homework: achieve attainable goal. Participants were encouraged to write about their goals at end of day and to continue their gratitude journal and mindful breathing exercises. · Session 5: Altruistic Behaviors/Acts of Kindness – Doing for Others – Discuss the positive impact of doing for others. Homework: do something nice for someone else each day. Participants were encouraged to write about their acts of kindness at end of day and to continue their gratitude journal and mindful breathing exercises. |
Participants randomised to the control group engaged in 5 one‐on‐one sessions with a facilitator. The sessions were comparable in length to the intervention sessions (approximately 1 hour) but consisted of an interview and did not have any didactic portion or skills practice. Each session began with the completion of the modified Differential Emotions Scale (DES). In addition to these affect questions, each session had qualitative and quantitative questions and activities cantered on a theme (i.e., life history, health history, diet and exercise, social networks, and meaning and spirituality), to keep the sessions different and interesting for participants. Home practice for the control group consisted of the brief daily affect reports. At the start of sessions 2 through 6, the facilitator reviewed the previous week’s affect diary with the participant. |
| WHO provided | Facilitators for the intervention sessions were trained for content and delivery. Two were clinical nurse specialists in fronto‐temporal dementia and 1 was a psychologist. | The facilitator for the control group sessions was a research associate, familiar with the content of the intervention sessions so as to not engage in the intervention content during control sessions with participants. |
| HOW delivered | Mainly by video‐conference (only 1 subject participated in‐person, all others participated remotely). | |
| WHERE occurred | At home. | |
| WHEN and HOW MUCH | 6 sessions of 1 hour each. | |
| TAILORING | Interventions were comprehensively designed, not tailored to cover individual or unmeet needs. | |
| MODIFICATIONS | None described. | |
| HOW WELL planned | All sessions were audio‐recorded digitally for both quality assurance and intervention evaluation. | |
| HOW WELL actual | No described. | |
9. TIDieR ‐ Duggleby 2018.
| Study | Duggleby 2018 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | My Tools 4 Care (MT4C). | Information. |
| WHY | The aim of the intervention, based on transition theory, was to support carers through transitions and increase their self‐efficacy, hope, and health related quality of life. The intervention had multiple components and included choice, as the carers could choose which sections they would like use, and when. User‐generated content is encouraged throughout the intervention as participants may write in sections, add stories, pictures, music etc. Sections include: · About Me · Common Changes to Expect · Frequently Asked Questions and Resources · Important Health Information (about person they are caring for). |
No applicable. |
| WHAT materials | An online version of the Toolkit entitled My Tools 4 Care (MT4C) (https://www.mytools4care.ca/). | A copy of the Alzheimer Society’s booklet The Progression of Alzheimer’s Disease, sent by email. |
| PROCEDURES | Participants received information for the MT4C website, (i.e., the website address, a unique username and password to access the site for 3 months), and an electronic copy of a Toolkit Checklist in which the participant will be asked to document their use of MT4C (i.e., time spent and content accessed over the 3 months). Participants also received an electronic copy of the Alzheimer Society’s educational booklet, The Progression of Alzheimer’s Disease. | The electronic document consists of a five‐part series that describes the stages of Alzheimer’s disease. It is written for the person with Alzheimer disease, their family and carers and is freely accessible via the Alzheimer Society of Canada website. |
| WHO provided | Whole intervention was over the Internet. | No applicable. |
| HOW delivered | Online | Emailed booklet. |
| WHERE occurred | At home. | |
| WHEN and HOW MUCH | Intervention was accessible for 3 months. | No applicable. |
| TAILORING | Interventions were comprehensively designed, not tailored to cover individual or unmeet needs. | |
| MODIFICATIONS | None described. | No applicable. |
| HOW WELL planned | Webpage recorded the time and duration of access. | No applicable. |
| HOW WELL actual | 73% of caregivers used MT4C at least once over the 3‐month period. By 3 months, participants spent most of their time on Section 2 – Common changes to expect (median 15 minutes) and Section 4 – Resources (median 10 minutes). | No applicable. |
10. TIDieR ‐ Gant 2007.
| Study | Gant 2007 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | Video/workbook/telephone coaching condition. | Education/check‐in‐call condition. |
| WHY | No described. | |
| WHAT materials | Caregivers in the video condition received a 10‐session video series, a workbook from the Dementia Caregiving Skills Program, and weekly telephone calls from a trained coach. | Participants received by mail a 37‐page booklet, Basic Dementia Care Guide and phone calls. |
| PROCEDURES | The intervention used primarily behavioural strategies: behavioural activation, behavioural management, and stress reduction through relaxation training. The workbook provided didactic and experimental materials to reinforce information presented in each video session. Participants received 12 weekly phone calls by trained research staff who served as coaches. The first 10 calls reinforced each of the video sessions, the last 2 calls served as follow‐up for further application of concepts. Coaches followed a coach manual that provided a script for reviewing didactic materials and assignments with caregivers, and for assisting them in the application of intervention concepts to their problems. During the coaching calls caregivers reported the specific behavioural strategies that they devised, written down, used, and evaluated, based on the behaviour management module that they learned to apply to their situations. | Intervention included information on dementia and suggestions for dealing with a variety of caregiving challenges. In a cover letter, procedures for maximizing the benefits of this educational booklet were provided. Caregivers then received approximately 7 biweekly telephone calls by a trained staff member. In these calls, the staff member checked on the safety of the caregiver and family member, discussed the caregiver’s use of the suggestions from the guide, and responded to questions by referring the caregiver to appropriate sections in the guide. A standardized script was used for calls to participants in this comparison condition. |
| WHO provided | Coaches following a coach manual. | |
| HOW delivered | By telephone. | |
| WHERE occurred | At home. | |
| WHEN and HOW MUCH | 12 weekly telephone calls. | 7 bi‐weekly telephone calls. |
| TAILORING | Interventions were comprehensively designed, not tailored to cover individual or unmeet needs. | |
| MODIFICATIONS | None described. | |
| HOW WELL planned | No described. | |
| HOW WELL actual | No described. | |
11. TIDieR ‐ Gustafson 2019.
| Study | Gustafson 2019 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | D‐CHESS (Dementia–Comprehensive Health Enhancement Support System). | Caregiving booklet. |
| WHY | D‐CHESS was designed to help with motivation, decision making, stress reduction, and access to services by allowing caregivers to obtain information and support without concern for location, distance, time, confidentiality, or education. | No described. |
| WHAT materials | Website. Participants were also offered the use of three commercially available sensors: a Bluetooth tracker, a GPS location tracker, and a motion sensor. The Bluetooth tracker would help locate lost items such as keys; the GPS location tracker could be carried by a patient, allowing the caregiver to monitor the patient’s location; and the motion sensor could be used to alert a caregiver if the patient entered specific areas of the home that could be hazardous. | No applicable. |
| PROCEDURES | This system offered detailed information about dementia, personal accounts of how other caregivers coped, descriptions and locations of services that caregivers may need, and links to vetted online resources. Users could anonymously pose questions to experts, receive suggestions of resources for help, communicate with other caregivers through facilitated discussion groups, and use decision aids to work through key issues. In addition, D‐CHESS services offer other elements proven effective in caregiver programs, such as assertive out‐reach, action planning, and peer and family support, as well as quality informational resources such as expert advice and vetted articles and websites. | No described. |
| WHO provided | Whole intervention was over the Internet. | No described. |
| HOW delivered | Online. | Booklet. |
| WHERE occurred | At home. | |
| WHEN and HOW MUCH | The intervention group received access to the D‐CHESS website for 6 months. | No applicable. |
| TAILORING | Interventions were comprehensively designed, not tailored to cover individual or unmeet needs. | |
| MODIFICATIONS | None described. | |
| HOW WELL planned | D‐CHESS automatically collected use data, including: number of participants logging on, number of page views, specific services used, discussion group posts, data input to decision aids, and private messages sent to the Alzheimer’s disease information specialist at the Wisconsin Alzheimer’s Disease Research Center. | No applicable. |
| HOW WELL actual | Overall, participants continued to access D‐CHESS throughout the study, with 100%, 50%, 71%, 71%, 57%, and 64% logging on during months 1 through 6, respectively. | No applicable. |
12. TIDieR ‐ Hattink 2015.
| Study | Hattink 2015 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | STAR E‐Learning (European Skills Training and Reskilling). | Waiting list. |
| WHY | The STAR training portal was designed to provide dementia care training both for informal and formal caregivers. It is designed as a multilingual e‐learning tool from experts on education, technology, and dementia care. | Participants in the control group were informed they could follow the e‐learning course after post‐test measurements were performed within the experimental intervention. |
| WHAT materials | The course content was developed from 3 perspectives: · Medical model of dementia: information on types of dementia, symptoms and diagnostics · Functional consequences in daily life and how to compensate for disabilities · Dealing with the psychosocial consequences for the person with dementia and his family |
No applicable. |
| PROCEDURES | The STAR training portal offers the following functionality: · 8 modules on different topics in dementia care (2 at basic level, 6 at intermediate to advanced level): what is dementia; living with dementia; getting a diagnosis; practical difficulties in daily life; emotional impact of dementia; support strategies; positive and empathic communication; looking after yourself · Learning Path Advisor to assess baseline knowledge and confidence, to suggest the point to start the course · Facebook and LinkedIn communities to promote peer support and provide opportunities to contact dementia care professionals The modules consist of text, videos, interactive exercises, knowledge tests, and also include references to other websites, literature, and videos. |
No applicable.. |
| WHO provided | Whole intervention was over the Internet. | No applicable. |
| HOW delivered | Online. | No applicable. |
| WHERE occurred | At home. | |
| WHEN and HOW MUCH | The intervention group received access to the STAR website for 4 months. | No applicable. |
| TAILORING | Interventions were comprehensively designed, not tailored to cover individual or unmeet needs. Caregivers can follow their own learning path. | |
| MODIFICATIONS | None described. | |
| HOW WELL planned | Pilot study to assess STAR’s usefulness, user friendliness, and impact on knowledge. | No applicable. |
| HOW WELL actual | As planned. | No applicable. |
13. TIDieR ‐ Hayden 2012.
| Study | Hayden 2012 | ||
| TIDieR item | Experimental intervention | Control intervention | |
| BRIEF NAME | The use of Internet technology for psychoeducation and support with dementia caregivers. | Customary care plus monthly brief telephone calls. | |
| WHY | Data suggest that providing education, social support, and ongoing professional consultation to families involved in the care of a relative with Alzheimer’s disease results in improvement in caregiver psychological status, and sometimes even slows the functional decline of the patient. | No applicable. | |
| WHAT materials | Customary care and access to an intensive, interactive online education and support website. | Customary care and telephone calls. | |
| PROCEDURES | · Online education and support, provided by nine brief educational streaming videos, with brief written brochures, plus non‐compulsory weekly 1‐hour professionally hosted synchronous online chats to review the educational material and problem‐solving care issues, using the rest of the group as sources of feedback. Participants received weekly telephone and email reminders about the chat sessions. · Additional focus cantered on self‐care promotion via behavioural interventions and goal attainment scaling. |
Active control condition involving supportive phone calls. | |
| WHO provided | Geriatricians, a geriatric psychiatrist, clinical psychologists, a research sociologist, and master’s and bachelor’s level support staff. Both interventions were provided by the same team. | ||
| HOW delivered | Internet. | Telephone. | |
| WHERE occurred | At home. | ||
| WHEN and HOW MUCH | 6‐month interventions. | ||
| TAILORING | Interventions were comprehensively designed, not tailored to cover individual or unmeet needs. | ||
| MODIFICATIONS | None described. | ||
| HOW WELL planned | No described. | ||
| HOW WELL actual | Reports of technological challenges encountered by many of the participants as well as constraints due to VA resources and information security policies. | ||
14. TIDieR ‐ Huis in het Veld 2020.
| Study | Huis in het Veld 2020 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | Major Self‐Management Support Intervention. | · Medium Self‐Management Support Intervention · Minor Self‐Management Support Intervention |
| WHY | The intervention is based on the 5 steps of the “5A model” of self‐management support and the person‐centered care theory of Kitwood. · assessing the state of behavior, beliefs, and motivation · advising based upon personal health risks · agreeing on a realistic set of goals · assisting in anticipating barriers and developing a specific action plan · arranging follow‐up. |
Not described. |
| WHAT materials | Emails, online videos, e‐bulletins. | Online videos and e‐bulletins. |
| PROCEDURES | The major intervention arm consists of the following elements: · 3 personal email contacts with a nurse specialized in dementia care · provision of 6 online videos about how to manage behavior changes in a relative with dementia and to improve self‐efficacy in managing with this behavior · provision of e‐bulletins with practical information about different types of behavior changes and how to manage them The nurse gave feedback on assignments and gave feedback on the plan that the family caregiver came up with in the assignments.There were 6 videos about different types of behavior changes that occur frequently (dependent behavior, aggressive behavior, suspicious behavior, apathy or indifference, nighttime restlessness, and masking behavior). Family caregivers chosen the number of videos they watch and the accompanying assignments that they do themselves, depending on their own needs and the behavior changes that occur in their relative with dementia. The behavior changes covered in the bulletins were the same as in the videos. The e‐bulletins involve assignments to help caregivers translate the generic information to their own situation and to reflect on possible causes of the behavior changes, how they want to influence the behavior, and how they want to cope with it. |
· The medium self‐management support intervention consists only of the online videos and e‐bulletins · The minor self‐management support intervention consists only of the e‐bulletins, the same as those in the major and medium support interventions |
| WHO provided | The personal email contacts were handled by a nurse with a Bachelor’s or Master’s qualification in nursing and with follow‐up training in dementia care. | |
| HOW delivered | Email plus online videos plus e‐bulletins. | Online videos plus e‐bulletins. |
| WHERE occurred | At home. | |
| WHEN and HOW MUCH | 6 weeks. | |
| TAILORING | Interventions were comprehensively designed, not tailored to cover individual or unmeet needs. | |
| MODIFICATIONS | None described. | |
| HOW WELL planned | No described. | |
| HOW WELL actual | No described. | |
15. TIDieR ‐ Kajiyama 2013.
| Study | Kajiyama 2013 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | iCare Stress Management e‐Training Program (ICC). | Education/Information‐Only Condition (EOC). |
| WHY | The iCare program is an adaptation of the psycho‐ educational program ‘Coping with Caregiving’ (CwC) developed by Gallagher‐Thompson and colleagues that aims to teach a set of core coping skills to caregivers for stress management. These include: relaxation training; learning to increase everyday pleasant activities (including if possible some pleasant activities with the person with dementia); cognitive restructuring (of unduly negative appraisals regarding caregiving and themselves); and improved communication skills on how to ask for help effectively from family members and relevant community and medical institutions. | The EOC served as a control for the purpose of obtaining new information about dementia and what to expect in caring for dementia patients. It also controlled for the involvement with relevant information on the Internet and for the general cognitive and experiential capacity required to ‘navigate’ in complex Internet materials. |
| WHAT materials | Website and video‐recordings. | |
| PROCEDURES | Educational modules had a hierarchical structure. Participants were encouraged to practice specific assignments in each module over a 7‐to‐10‐day interval before moving to the next one. The iCare program consists of the following modules: · information segment about what ‘dementia’ means and what are common problems associated with it · components on dealing with stress including techniques for relaxation, stress management, and challenging unduly negative thoughts about caregiving · behavioral activation (increasing everyday positive activities for oneself and the patient with dementia) · communication skills to improve help‐seeking with family and community institutions as well as improving ability to relate to the patient with dementia · managing difficult behaviours of the patient with dementia · review of ‘healthy habits’ (nutrition and exercise) for the caregiver along with information on national resources they can consult for further on‐going assistance At the end of each module, CGs were asked to create their own individual ‘action plans’ in the workbook requiring them to describe what they had learned by completing the module and relevant assignments and how they planned to use the information from that specific module in the future in their daily lives. |
Caregivers assigned to the EOC were exposed to a website containing similar navigational features, but with content focused on information about dementia, obtained from reputable national sources. In addition, links to certain video‐taped information were provided. |
| WHO provided | Online. | |
| HOW delivered | Online. | |
| WHERE occurred | At home. | |
| WHEN and HOW MUCH | During 3 months. | No applicable. |
| TAILORING | Interventions were comprehensively designed, not tailored to cover individual or unmeet needs. | |
| MODIFICATIONS | None described. | |
| HOW WELL planned | No described. | |
| HOW WELL actual | No described. | |
16. TIDieR ‐ Kwok 2013.
| Study | Kwok 2013 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | Psychoeducational intervention. | DVD information. |
| WHY | The intervention is based on theories of psychosocial transition and stress coping, under the framework of cognitive behavioral therapy, and focuses on: · providing emotional support · directing caregivers to appropriate resources · encouraging caregivers to attend to their own physical, emotional, and social needs · educating caregivers on strategies to cope with ongoing problems |
No described. |
| WHAT materials | Manualized psychoeducational intervention. | DVD with educational information about dementia caregiving. |
| PROCEDURES | The participants in the intervention group were educated and given advice on topics related to dementia caregiving: · knowledge of dementia · skills of communicating with the patient · management of behavioral and psychological symptoms of dementia · caregivers’ own emotional issues · resources available in the community · long‐term care plan The topics covered and the schedule of presentation were similar to typical psychoeducation interventions held “on site” at community centres. |
No applicable. |
| WHO provided | A registered social worker. | No applicable. |
| HOW delivered | By telephone. | DVD. |
| WHERE occurred | At home. | |
| WHEN and HOW MUCH | 12 sessions (approximately 30 minutes per session, one session per week). | No applicable. |
| TAILORING | Interventions were comprehensively designed, not tailored to cover individual or unmeet needs. | |
| MODIFICATIONS | None described. | |
| HOW WELL planned | No described. | |
| HOW WELL actual | No described. | |
17. TIDieR ‐ Mahoney 2003.
| Study | Mahoney 2003 | ||
| TIDieR item | Experimental intervention | Control intervention | |
| BRIEF NAME | TLC System. | Control. | |
| WHY | The theoretical model asserts that the background and context of stress (caregiving relationship), plus the stressor (care recipient’s behaviours), as mediated by caregiver mastery, resulted in manifestations of caregiver stress. | No described. | |
| WHAT materials | The technology consisted of an integrated telephone network system and an IVR computer network system. | No applicable. | |
| PROCEDURES | Caregivers dialled in from any standard touch‐tone telephone and heard the narrator greet them by name, review the menu of four module options, and provide the service they requested. The included modules were: · Weekly caregiver’s conversation: monitored the caregiver’s stress levels and provided information on how to manage the care recipient’s behavioural problems. · Personal mailbox: allowed caregivers to send and receive communications through voice mail among themselves or to communicate with a clinical nurse specialist who directly answered or triaged questions to a multidisciplinary professional panel of Alzheimer’s disease experts. · Bulletin board: in‐home telephone support group that provided personal and caregiver group voice mail, similar to a computer chat group. · Activity‐respite conversation: care recipient distraction module designed to reduce disruptive behaviours and to provide caregivers with respite time. |
No applicable. | |
| WHO provided | Internet intervention. | No applicable. | |
| HOW delivered | Telephone‐delivered. | No applicable. | |
| WHERE occurred | At home. | ||
| WHEN and HOW MUCH | The intervention group had access to the system for a 12‐month period, after which their passwords were disabled. | No applicable. | |
| TAILORING | Intervention was comprehensively designed and not tailored to cover individual or unmeet needs. However, because caregivers have diverse needs and preferences, the intervention offered multiple components with flexibility to effectively appeal to a variety of users. Participants chose the type of component, frequency, duration, and timing of the usage. | No applicable. | |
| MODIFICATIONS | None described. | ||
| HOW WELL planned | No described. | ||
| HOW WELL actual | No described. | ||
18. TIDieR ‐ Martindale‐Adams 2013.
| Study | Martindale‐Adams 2013 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | Telephone support (CONNECT). | Printed materials. |
| WHY | Content and structure of the intervention were based on the 6‐month REACH II intervention of 12 individual in‐home and telephone sessions and five telephone support group sessions. | No described. |
| WHAT materials | Session materials consisted of a Caregiver Notebook and commercially available pamphlets. The Notebook comprised 29 behaviour – management chapters of five to eight pages each (i.e., bathing, repeated questions), and 17 caregiver stress/coping chapters (i.e., assertiveness, communication, grief) based on research and practice, written in large print and at a fifth‐grade reading level. | Control caregivers received pamphlets on dementia and safety as well as telephone numbers for local resources. |
| PROCEDURES | Like REACH, the multi‐component intervention targeted caregiving risks, including risks associated with emotional and physical well‐being, safety, burden, social support, and patient behaviour management. The first six sessions covered all Caregiver Notebook chapters that were standardized to include the following topics: · introduction · basic information about dementia and financial and legal issues · safety · caregiver health and well‐being · communication · problem solving The sessions were semi‐structured telephone calls with education, skills‐building, and support. Each session began with a relaxation exercise, caregiver updates, review of strategies tried from the preceding session’s topic, and the group leader’s presentation on a behaviour management or stress and coping topic.The rest of the session included discussion and practice by the entire group on the session’s topic, selection of individual strategies to try, selection of the next session’s topic, and closure, including another signal breath relaxation exercise. |
Control caregivers received pamphlets on dementia and safety as well as telephone numbers for local resources. |
| WHO provided | Trained professionals. | |
| HOW delivered | Telephone. | Printed materials. |
| WHERE occurred | At home. | |
| WHEN and HOW MUCH | Groups met 14 times over 1 year. The support groups met bi‐weekly for 2 months and monthly thereafter for 1 year, for a total of 14 hour‐long sessions. | No described. |
| TAILORING | Interventions were comprehensively designed, not tailored to cover individual or unmeet needs. | |
| MODIFICATIONS | None described. | |
| HOW WELL planned | No described. | |
| HOW WELL actual | Of the 77 intervention caregivers, 47 (61%) had at least 75% of the 14 sessions and 59 (76.6%) completed at least half. Six caregivers (7.8%) had fewer than three sessions. | No applicable. |
19. TIDieR ‐ Metcalfe 2019.
| Study | Metcalfe 2019 | ||
| TIDieR item | Experimental intervention | Control intervention | |
| BRIEF NAME | RHAPSODY. | Wait‐list. | |
| WHY | The intervention adopts a multiple‐component model of dementia carer support inspired by the REACH II Study adapted to the care of people with young onset dementia. | No applicable. | |
| WHAT materials | Online device | No applicable. | |
| PROCEDURES | The programme features seven modules: · What is young onset dementia? · Medical perspective on young onset dementia · Frequent problems and solutions · Dealing with challenging behaviours · Family issues · How to get help · Looking after yourself |
No applicable. | |
| WHO provided | Internet intervention. | No applicable. | |
| HOW delivered | Online. | No applicable. | |
| WHERE occurred | At home. | ||
| WHEN and HOW MUCH | 6 weeks. | No applicable. | |
| TAILORING | Intervention was comprehensively designed, not tailored to cover individual or unmeet needs. | No applicable. | |
| MODIFICATIONS | None described. | No applicable. | |
| HOW WELL planned | No described. | ||
| HOW WELL actual | No described. | ||
20. TIDieR ‐ NCT00056316.
| Study | NCT00056316 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | Behavioural intervention. | Basic education. |
| WHY | No described. | |
| WHAT materials | Video, workbook and telephone calls. | 37‐page Basic Care Guide and telephone calls. |
| PROCEDURES | Multicomponent behavioral intervention using 10‐session video series, workbook, and weekly telephone coaching sessions. | Participants receive 37‐page Basic Care Guide and bi‐weekly telephone calls by a trained staff member. |
| WHO provided | Trained staff member. | |
| HOW delivered | Video plus telephone. | Booklet. |
| WHERE occurred | No described. | |
| WHEN and HOW MUCH | No described. | |
| TAILORING | No described. | |
| MODIFICATIONS | None described. | |
| HOW WELL planned | No described. | |
| HOW WELL actual | No described. | |
21. TIDieR ‐ NCT03417219.
| Study | NCT03417219 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | Education and Skill Building Rehabilitation‐mobile (ESBR‐m). | Usual care plus supplemental educational materials. |
| WHY | No described. | |
| WHAT materials | Internet contents. | Educational materials. |
| PROCEDURES | Participants randomised to the ESBR‐m group will participate in four, 90‐minute group ( 5 participants) sessions. These four sessions are supplemented with a booster session one month following the last intervention session. | Participants randomised to the usual care group will receive supplemental educational materials. |
| WHO provided | No described. | |
| HOW delivered | Internet. | Booklets. |
| WHERE occurred | At home. | |
| WHEN and HOW MUCH | Four, 90‐minute group sessions. | No applicable. |
| TAILORING | No described. | |
| MODIFICATIONS | None described. | |
| HOW WELL planned | No described. | |
| HOW WELL actual | No described. | |
22. TIDieR ‐ Nunez‐Naveira 2016.
| Study | Nunez‐Naveira 2016 | ||
| TIDieR item | Experimental intervention | Control intervention | |
| BRIEF NAME | UnderstAID. | Participants in the control group did not use the application and maintained their usual lifestyle. | |
| WHY | No described. | No applicable. | |
| WHAT materials | Smartphone, PC or Tablet. | No applicable. | |
| PROCEDURES | The understAID consists on a Learning section with a database of contents organized in 5 modules with information about 15 different topics. The topics cover information about the care of a person with dementia and caring for oneself as a caregiver, and consist of text, videos, and images and they also include references to other websites. The modules and topics included in understAID are: · Module 1, Cognitive Declines (Topics: Attention, Memory, and Orientation) · Module 2, Daily Tasks (Topics: Bathing, Incontinence, Massage and Touch, and Physical Exercises) · Module 3, Behavioural Changes (Topics: Anxiety and Agitated Behaviour, Depressive Mood, Manic Symptoms, and Emotional Control and Recognition) · Module 4, Social Activities (Communication and Apathy and Loss of Motivation) · Module 5, You as a Caregiver (Topics: Coping with Own Stress and Motivation). Additionally, it has a Social Network section where the caregivers can interact with other participants and exchange information and opinions. This section was moderated by the researchers of the study. |
No applicable. | |
| WHO provided | Internet intervention. | No applicable. | |
| HOW delivered | Online. | No applicable. | |
| WHERE occurred | At home. | ||
| WHEN and HOW MUCH | Three months. | No applicable. | |
| TAILORING | Intervention was comprehensively designed, not tailored to cover individual or unmeet needs. However, understAID also included the option of filling in an interactive customisation questionnaire with questions about the level of dementia of the person cared by the informal caregiver and about the preferences, energy, and time availability for learning of the caregiver. By answering these questions at the entry of the application, the information level showed to the informal caregivers is personalized and adjusted to their personal situation. | No applicable. | |
| MODIFICATIONS | None described. | No applicable. | |
| HOW WELL planned | No described. | No applicable. | |
| HOW WELL actual | No described. | No applicable. | |
23. TIDieR ‐ Torkamani 2014.
| Study | Torkamani 2014 | ||
| TIDieR item | Experimental intervention | Control intervention | |
| BRIEF NAME | ALADDIN. | The participants in the control group were only assessed at the study three time points, without any further contact or intervention. | |
| WHY | No described. | No applicable. | |
| WHAT materials | Internet contents. | No applicable. | |
| PROCEDURES | ALADDIN is a computerized platform designed to offer avenues of support and information to the carer. It also manages and communicates information related to the patient with dementia and their carers from their home to the clinicians, facilitating distant monitoring. ALADDIN has four key features: · ‘ALADDIN TV’ provides information and educational material about dementia, as well as musical entertainment and relaxation and exercise techniques · The ‘SOCIAL NETWORKING’ feature provides a forum for carers using ALADDIN to communicate with each other · ‘MY TASKS’ is the distant monitoring feature of ALADDIN, where carers complete questionnaires about their own and their relatives’ health. Their responses can subsequently generate clinical alerts based on set parameters, comparing new responses to previous data, resulting in the immediate detection of change by the clinicians monitoring the system · The ‘CONTACT US’ feature allows the carer to alert the clinical site and/or generate a request for contact |
No applicable. | |
| WHO provided | Internet intervention. | No applicable. | |
| HOW delivered | Online. | No applicable. | |
| WHERE occurred | At home. | ||
| WHEN and HOW MUCH | The project ran for 6 months. | ||
| TAILORING | Intervention was comprehensively designed, not tailored to cover individual or unmeet needs. However, being the primary users of ALADDIN, the caregivers chose the schedule of their tasks. | No applicable. | |
| MODIFICATIONS | None described. | No applicable. | |
| HOW WELL planned | The system was monitored twice daily by the clinical teams. | No applicable. | |
| HOW WELL actual | No described. | No applicable. | |
24. TIDieR ‐ Tremont 2008.
| Study | Tremont 2008 | ||
| TIDieR item | Experimental intervention | Control intervention | |
| BRIEF NAME | Family Intervention: Telephone Tracking – Dementia (FITT‐D). | Standard care. | |
| WHY | The intervention is delivered entirely over the telephone. Theoretical underpinnings of the intervention are based on psychosocial transition, transactional stress and coping, and a systems view of family functioning. | No applicable. | |
| WHAT materials | Treatment manual and interventions guide. Telephone. | No applicable | |
| PROCEDURES | Telephone calls included an initial call (orientation and psychoeducation), weekly calls for six weeks, 12 additional contacts every two weeks, and four monthly termination calls. Initial contacts lasted approximately 60 minutes, and follow‐up contacts lasted about 15–30 minutes. The calls focused on providing emotional support, directing caregivers to appropriate resources, encouraging caregivers to attend to their own physical, emotional and social needs, and teaching caregivers strategies to cope with ongoing problems. The intervention did not provide case management, serve as a question‐and‐answer hotline, or provide psychotherapy over the telephone. The FITT method consists of two stages: · The initial stage, Orientation and Psychoeducation, involved providing caregivers with a rationale for the FITT, an introduction to educational and resource materials, a description of what would happen during future phone contacts and an assessment of key areas thought to be instrumental in addressing caregiver burden and mental health (i.e., caregivers’ health, functioning, mood, thinking and family life). The psychoeducation component of this initial stage involved reviewing information about dementia and common psychological, emotional, psychosocial and medical effects of caregiving · The second stage, Follow‐up, involved weekly and bi‐weekly contacts in which new problems were identified, positive and negative changes in caregivers or care recipients were discussed, and psychoeducational information was reviewed and applied for particular situations. The final four follow‐up calls (monthly) addressed issues of termination by allowing caregivers to anticipate FITT contacts coming to an end and to foster reliance on the support network established during the intervention. |
No applicable. | |
| WHO provided | Two master’s level therapists who were trained in the FITT‐D procedure delivered the intervention. Doctoral‐level staff supervised therapists weekly to ensure adherence to the protocol and minimize drift. | No applicable. | |
| HOW delivered | Telephone. | No applicable. | |
| WHERE occurred | At home. | ||
| WHEN and HOW MUCH | 23 telephone contacts over 12 months. Initial contacts lasted approximately 60 minutes, and follow‐up contacts lasted about 15–30 minutes. The intervention involved approximately 12 hours of contact between the therapist and caregiver. | No applicable. | |
| TAILORING | Intervention was comprehensively designed, not tailored to cover individual or unmeet needs. | No applicable. | |
| MODIFICATIONS | None described. | No applicable. | |
| HOW WELL planned | Sessions were audio‐ taped, and two raters reviewed 30 randomly selected telephone contacts and independently completed adherence and competence scales. | No applicable. | |
| HOW WELL actual | No described. | No applicable. | |
25. TIDieR ‐ Tremont 2015.
| Study | Tremont 2015 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | Family Intervention: Telephone Tracking‐Caregiver (FITT‐C). | Telephone Support (TS). |
| WHY | The theoretical framework of FITT‐C is based on psychosocial transition, transactional stress and coping, and a systems view of family functioning. The three underlying theories of FITT‐C are geared toward enhancing coping within the caregiver through active problem solving and facilitating positive changes within the family system. | The control condition was designed to account for nonspecific therapeutic factors, such as interpersonal contact and relationship. The approach was based on a nondirective control condition. |
| WHAT materials | Telephone. | Telephone. |
| PROCEDURES | Focused on providing dementia education, emotional support, directing caregivers to appropriate resources, encouraging caregivers to attend to their physical, emotional, and social needs, and teaching caregivers strategies to cope with ongoing problems. The FITT method consisted of an initial orientation and psycho‐ education call that involved providing caregivers with a rationale of FITT, an introduction to the resource materials, a description of future telephone contacts, and education about dementia and effects of caregiving. The remaining contacts identified changes since the last call, assessed key areas for the caregiver (i.e., health, functioning, mood, social support, and family life), and provided interventions and psychoeducation to help caregivers solve problems and use family resources. The focus of the final two calls changed to identifying helpful aspects of contacts and how these functions could be met after termination. After the final call, the therapist prepared a letter briefly highlighting the progress during the intervention and encouraged the caregiver to continue to develop and use adaptive coping strategies. | The primary goal of this condition was to provide nondirective support for caregivers through empathic and reflective listening and open‐ended questioning. The role of the therapist was to provide unconditional positive regard to caregivers and to establish a supportive relationship. Therapists were discouraged from providing directive strategies, such as education, problem‐solving, advice‐giving, or task directives. However, education was not withheld if the caregiver had the wrong information. |
| WHO provided | Individuals recruited to serve as therapists had experience working with dementia patients and/or care‐ givers or psychotherapy experience working with adults. Therapists were required to be master’s level and received training in dementia and caregiving. FITT‐C therapists received additional training in the intervention method. | |
| HOW delivered | Telephone. | |
| WHERE occurred | At home. | |
| WHEN and HOW MUCH | 16 telephone contacts over 6 months. Although initial telephone contacts had standardized durations (approximately 60 minutes), follow‐up contacts varied depending on the severity of caregiver problems (15–30 minutes). | |
| TAILORING | Interventions were comprehensively designed, not tailored to cover individual or unmeet needs. | |
| MODIFICATIONS | None described. | |
| HOW WELL planned | Quality control was implemented by weekly supervision of both the FITT‐C and TS therapists. All telephone contacts were audiotaped, and a subset was reviewed during supervision sessions to ensure adherence and to better guide therapists’ intervention strategies. Any deviations from the treatment protocol were brought to the therapist’s attention for remediation. | |
| HOW WELL actual | The number of missed telephone calls from 16 planned calls was comparable between conditions with an average of 1.81 missed calls for FITT‐C and 1.22 for TS. The average call length was slightly longer for the FITT‐C group (37 minutes) compared with TS (30 minutes). | |
26. TIDieR ‐ Winter 2007.
| Study | Winter 2007 | ||
| TIDieR item | Experimental intervention | Control intervention | |
| BRIEF NAME | Telesupport group treatment. | Control condition. | |
| WHY | No described. | No applicable. | |
| WHAT materials | Conference‐calling technology (Toshiba Digital Business Telephone Model DKT2010‐S System and Centrex teleconference service) to link 5 caregivers per group for an hour weekly. | Nurses and an educational booklet. | |
| PROCEDURES | The primary goal was to enhance caregiver ability to manage daily stressors by providing emotional support and validation. Initially, facilitators focused on developing group cohesion. As groups progress, disclosure of intimate problems and personal conflicts emerge. Caregivers express emotions and share coping strategies including cognitive reframing and practical approaches to organizing care routines. They also assist each other in problem solving and share educational resources. The mutual support and validation provided by group members normalize experiences and provide a supportive social network, core to the service model. | No described. | |
| WHO provided | Telesupport groups were conducted by trained social workers. | No applicable. | |
| HOW delivered | Teleconference Group format. | No applicable. | |
| WHERE occurred | At home. | ||
| WHEN and HOW MUCH | 26 possible sessions in the 6 months following baseline. | No applicable. | |
| TAILORING | Intervention was comprehensively designed, not tailored to cover individual or unmeet needs. | No applicable. | |
| MODIFICATIONS | None described. | No applicable. | |
| HOW WELL planned | No described. | No applicable. | |
| HOW WELL actual | Telesupport group caregivers participated in an aver‐ age of 14.8 sessions, of a possible 26. | No applicable. | |
Outcome measures
Caregiver burden and caregiver mood were the most frequently reported outcomes across studies.
Caregiver burden was reported in 17 studies using four different scales: the Zarit Burden Inventory (ZBI, versions of 22‐item and 12‐item; 12 studies, 65%); the 24‐item Revised Memory and Behavior Checklist (RMBPC, four studies, 23%); the 10‐item Burden Scale for Family Caregivers (BSFC, one study) and another 6‐item scale (one study).
Caregiver mood was reported in 17 studies using five different scales: the Center for Epidemiologic Studies‐Depression (CES‐D, versions of 20‐item and 10‐item; 12 studies, 71%); the 21‐item Beck Depression Inventory (BDI, two studies, 12%); the 30‐item Geriatric Depression Scale (GDS), the 8‐item Patient Health Questionnaire (PHQ), and the SF‐12 v2 Mental Component Summary (MCS) (one study each). For 16 of these studies, the scale measures depressive symptoms; therefore in the interests of accuracy, we describe the outcome simply as depressive symptoms.
Caregiver HRQoL was reported in four studies with four different scales: the 19‐item Perceived Quality of Life Scale (PQoL), the EuroQol‐5D Visual Analogue Scale (EQ‐5D VAS), the EuroQol‐5D 5‐level (EQ‐5D‐5L), and the 36‐item Short‐Form Health Survey (SF‐36).
Caregiver knowledge and skills was reported in eight studies using six different scales; the Revised Scale for Caregiving Self‐Efficacy (RSCSE with 15‐ and 19‐item versions; three studies, 37%); the 10‐item General Self‐Efficacy Scale (GSES), the 30‐item Alzheimer's Disease Knowledge Scale (ADKS), the 32‐item Trust in Own Abilities (TOA), the 4‐item Caregiver Competence Scale (CCS), and a Visual Analogue Scale (VAS) (one study each).
Care recipient QoL was not reported in any of the included studies.
Because studies used so many different measures with different metrics to report conceptually similar outcomes, we used the SMD for continuous outcomes as the measure of effect size.
Two studies (Duggleby 2018; Huis in het Veld 2020) reported results for caregiver knowledge and skills as mean differences that could not be pooled with the raw effects extracted from the rest of studies. Both studies are included in their respective comparisons and the results reported accordingly.
Funding
All studies but one reported being funded by public grants. Funding source was not stated for Kwok 2013.
Excluded studies
We excluded 52 studies with reasons (see Characteristics of excluded studies).
Risk of bias in included studies
We performed a 'Risk of bias' assessment for each included trial. Our judgements regarding the overall risk of bias in individual studies are presented in Figure 2 and Figure 3.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All included studies were reported to be randomised. However, many studies did not describe the randomisation procedure in sufficient detail to assess risk of bias, and we considered the risk to be unclear. We asked authors for additional information, which we included, if available, in the 'Risk of bias' tables.
We judged most studies to be at unclear risk of bias for allocation concealment because of insufficient information. We asked authors for additional information, which we included, if available, in the 'Risk of bias' tables.
Blinding
Due to the characteristics of interventions included in this review, blinding of participants and personnel was, in general, not feasible. We rated all studies but Au 2019 and Blom 2015 as being at high risk of bias. Both studies asserted that participants did not know whether the intervention they received was the experimental or the control, and the data were all collected via the Internet with no intermediary interviewer.
We judged most studies to be at high risk of bias for blinding of outcome assessment because most outcomes were subjective and assessed with participant self‐report scales. We considered five studies to be at low risk of bias in this domain (Au 2015; Au 2019; Blom 2015; Huis in het Veld 2020; Tremont 2015).
Incomplete outcome data
We judged most studies to be at low or unclear risk of bias in this domain. We rated the risk as high in 10 studies because they did not provide data about the flow of participants over the study period or presented high attrition rates or differential attrition rates by intervention (Blom 2015; Czaja 2013; Davis 2004; Duggleby 2018; Gustafson 2019; Kajiyama 2013; Mahoney 2003; NCT03417219; Nunez‐Naveira 2016; Tremont 2008).
Selective reporting
About 50% of the studies did not report the availability of a protocol or clinical trial registry entry. We asked authors for additional information, which we included, if available, in the 'Risk of bias' tables. We rated most studies as being at unclear risk of bias for lacking information on prespecification of outcomes at the protocol stage and whether they were primary or secondary outcomes. We judged one study to be at high risk of bias for reporting only statistically significant results (Davis 2004).
Other potential sources of bias
We judged two studies to be at high risk of bias (Au 2015; Winter 2007). The Au 2015 study broke randomisation by adding further data to a pilot study already published; the Winter 2007 study found, after randomisation, a relevant imbalance of prognostic factors at baseline.
Effects of interventions
Summary of findings 1. Complex information, support and training interventions compared to usual treatment, waiting list or attention control informations for informal caregivers of people with dementia.
| Interventions involving training, support or both (with or without information) compared to usual treatment, waiting list or attention for informal caregivers of people with dementia | ||||||
| Participants or population: Informal caregivers of people with dementia Setting: Community Intervention: Any intervention including information, training or support Comparison: Usual treatment, waiting list or attention control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual treatment, waiting list or attention | Risk with any intervention | |||||
| Caregiver burden assessed with: self‐reported scales ‐ lower score indicates less burden follow up: median 24 weeks | ‐ | SMD 0.06 lower (0.35 lower to 0.23 higher) | ‐ | 597 (9 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Any intervention probably does not affect caregiver burden. There are no important differences in effect estimates among type of interventions:
|
| Caregiver mood assessed with: self‐reported scales ‐ lower score indicates fewer depressive symptoms follow up: median 24 weeks | ‐ | SMD 0.05 lower (0.22 lower to 0.12 higher) | ‐ | 638 (8 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Any intervention probably does not affect caregiver mood ‐ depressive symptoms. There are no important differences in effect estimates among type of interventions:
|
| Caregiver health‐related quality of life (HRQoL) assessed with: self‐reported scales ‐ higher score indicates better QoL follow up: range 6 weeks to 24 weeks | ‐ | SMD 0.1 higher (0.13 lower to 0.32 higher) | ‐ | 311 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | Any intervention probably does not affect caregiver health‐related quality of life. There are no important differences in effect estimates among type of interventions:
|
| Dropouts for any reason assessed with: number of dropouts for any reason throughout the trial follow up: median 18 weeks | Study population | RR 1.15 (0.87 to 1.53) | 661 (8 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | Any intervention probably results in little or no difference in dropouts for any reason. There are no important differences in effect estimates among type of interventions:
|
|
| 194 per 1000 | 223 per 1000 (169 to 296) | |||||
| Use of health and social resources assessed with: Frequency of use throughout the trial follow up: mean 24 weeks | Study population | Rate ratio 1.05 (0.93 to 1.19) | 4776 person‐weeks (1 RCT) | ⊕⊕⊕⊝ MODERATE 5 | Any intervention probably results in little to no difference in use of health and social resources. | |
| 204 per 1000 | 214 per 1000 (190 to 243) | |||||
| Institutional care ‐ nursing home placement assessed with: Frequency of nursing home placement throughout the trial follow up: median 24 weeks | Study population | RR 0.59 (0.11 to 3.11) | 34 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 6 7 | The evidence is very uncertain about the effect of any intervention on institutional care ‐ nursing home placement. | |
| 188 per 1000 | 111 per 1000 (21 to 583) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HRQoL: Health‐related quality of life; QoL: Quality of life; RCT: Randomised controlled trial; RR: Risk ratio; SMD: Standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for study limitations: Presence of high risk for performance and detection bias due to lack of blinding for participants, personnel, and the use of self‐reported outcome scales by unblinded participants. Also, presence of attrition bias.
2Downgraded for study limitations: Presence of high risk for performance bias.
3Downgraded for study limitations: Presence of high risk for performance and detection bias. Lack of blinding for participants might affect the overall rate of dropouts.
4Downgraded for imprecision: Few events to get precise estimates (< 300 overall). 95% CI includes a negligible effect and important benefit/harm.
5 Downgraded for imprecision: Rate ratio estimated with 1 study only including 250 participants and 4776 person‐weeks of follow up.
6Downgraded for study limitations: Unclear risk for selection bias. High risk for performance and detection bias.
7Downgraded for imprecision: Only 1 study with very few events (5 overall).
Summary of findings 2. Complex information, support and training interventions compared to information provision only for informal caregivers of people with dementia.
| Interventions involving training, support or both (with or without information) compared to control information alone for informal caregivers of people with dementia | ||||||
| Participants or population: Informal caregivers of people with dementia Setting: Community Intervention: Any intervention including information, training or support Comparison: Information | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control information | Risk with any intervention | |||||
| Caregiver burden assessed with: self‐reported scales ‐ lower score indicates less burden follow up: median 20 weeks | ‐ | SMD 0.24 lower (0.51 lower to 0.04 higher) | ‐ | 650 (9 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Any intervention may result in a slight reduction in caregiver burden. There are no important differences in effect estimates among type of interventions:
|
| Caregiver mood assessed with: self‐reported scales ‐ lower score indicates fewer depressive symptoms follow up: median 20 weeks | ‐ | SMD 0.25 lower (0.43 lower to 0.06 lower) | ‐ | 1100 (11 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Any intervention probably results in a slight improvement in caregiver mood ‐ depressive symptoms. There are no important differences in effect estimates among type of interventions:
|
| Caregiver health‐related quality of life (HRQoL) assessed with: self‐reported scales ‐ higher score indicates better QoL follow up: range 12 weeks to 24 weeks | ‐ | SMD 0.03 lower (0.28 lower to 0.21 higher) | ‐ | 257 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 3 | Any intervention may result in little or no difference in caregiver health‐related quality of life. |
| Dropouts for any reason assessed with: number of dropouts for any reason throughout the trial follow up: median 16 weeks | Study population | RR 1.51 (1.04 to 2.20) | 1266 (12 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Any intervention probably results in an increase in dropouts for any reason. There are no important differences in effect estimates among type of interventions:
|
|
| 130 per 1000 | 196 per 1000 (135 to 286) | |||||
| Use of health and social resources ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No included study reported use of health and social resources. |
| Institutional care ‐ nursing home placement assessed with: frequency of nursing home placement throughout the trial follow up: mean 12 weeks | Study population | RR 2.67 (0.12 to 60.93) | 32 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 4 5 | The evidence is very uncertain about the effect of any intervention on institutional care ‐ nursing home placement. | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HRQoL: Health‐related quality of life; QoL: Quality of life; RCT: Randomised controlled trial; RR: Risk ratio; SMD: Standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for study limitations: Presence of high risk for performance and detection bias due to the lack of blinding for participants, personnel, and the use of self‐reported outcome scales by unblinded participants. Also, presence of attrition bias.
2Downgraded for inconsistency: Unexplained important heterogeneity (I2 > 60% or not overlapping 95% confidence intervals).
3Downgraded for imprecision: Few participants to get precise estimates (< 300 overall).
4Downgraded for study limitations: Presence of high risk for performance and detection bias due to lack of blinding for participants, personnel, and the use of self‐reported outcome scales by unblinded participants.
5Downgraded for imprecision: Few events to get precise estimates (< 300 overall). 95% CI includes a negligible effect and important benefit/harm.
1 Any intervention involving training, support or both (with or without information) versus usual treatment, waiting list or attention control
Primary outcomes
1.1 Caregiver burden
Based on moderate‐certainty evidence from nine randomised studies including 597 participants, we found that remotely delivered interventions involving training, support or both (with or without information) probably do not affect caregiver burden (SMD ‐0.06, 95% CI ‐0.35 to 0.23; I2 = 60%). Negative values indicate less burden in the experimental intervention group. The range of SMD values includes no effect and small effects in favour of either experimental intervention or control. There is no evidence of important differences in effect estimates among types of interventions (Analysis 1.1; Figure 4).
1.1. Analysis.

Comparison 1: Any intervention versus usual treatment, waiting list or attention, Outcome 1: Caregiver burden
4.

Forest plot of comparison: 1 Any intervention versus usual treatment, waiting list or attention, outcome: 1.1 Caregiver burden.
Support/information or support interventions versus control interventions: based on low‐certainty evidence from three randomised studies including 156 participants, the evidence suggests little or no difference in caregiver burden (SMD 0.12, 95% CI ‐0.43 to 0.66) (Figure 4, upper panel). The range of SMD values includes no effect and moderate effects in favour of either experimental or control interventions.
Training/information or training interventions versus control interventions: based on low‐certainty evidence from three randomised studies including 109 participants, the evidence suggests little or no difference in caregiver burden (SMD ‐0.14, 95% CI ‐0.78 to 0.50) (Figure 4, middle panel). The range of SMD values includes no effect, a large effect in favour of the experimental interventions or a moderate effect in favour of the control interventions.
Interventions including both training and support elements versus control interventions: the evidence from three randomised studies including 332 participants is very uncertain about the effect of interventions including both training and support on caregiver burden (SMD ‐0.17, 95% CI ‐0.70 to 0.36) (Figure 4, lower panel). The range of SMD values includes no effect, a large effect in favour of the experimental interventions and a moderate effect in favour of the control interventions.
1.2 Caregiver depressive symptoms
Based on moderate‐certainty evidence from eight randomised studies including 638 participants, we found that remotely delivered interventions involving training, support or both (with or without information) probably do not affect caregiver depressive symptoms (SMD ‐0.05, 95% CI ‐0.22 to 0.12; I2 = 9%). Negative values indicate fewer depressive symptoms in the experimental intervention group. The range of SMD values includes no effect, a small effect in favour of the experimental interventions and a negligible effect in favour of the control interventions. There is no evidence of important differences in effect estimates among types of interventions (Analysis 1.2; Figure 5).
1.2. Analysis.

Comparison 1: Any intervention versus usual treatment, waiting list or attention, Outcome 2: Caregiver mood
5.

Forest plot of comparison: 1 Any intervention versus usual treatment, waiting list or attention, outcome: 1.2 Caregiver mood.
Support/information or support interventions versus control interventions: based on low‐certainty evidence from four randomised studies including 283 participants, the evidence suggests that the experimental interventions may have little or no effect on caregiver depressive symptoms (SMD ‐0.06, 95% CI ‐0.38 to 0.25) (Figure 5, upper panel). The range of SMD values includes no effect and small effects in favour of either experimental or control interventions.
Training/information or training interventions versus control interventions: based on low‐certainty evidence from one randomised study including 23 participants, the evidence suggests that the experimental interventions may have little or no effect on caregiver depressive symptoms (SMD 0.02, 95% CI ‐0.80 to 0.84) (Figure 5, middle panel). The range of SMD values includes no effect and large effects in favour of either experimental or control interventions.
Interventions involving both training and support elements versus control interventions: based on low‐certainty evidence from three randomised studies including 332 participants, the evidence suggests that the experimental interventions may have little or no effect on caregiver depressive symptoms (SMD ‐0.06, 95% CI ‐0.32 to 0.19) (Figure 5, lower panel). The range of SMD values includes no effect, a small effect in favour of the experimental interventions and a very small effect in favour of the control interventions.
1.3 Caregiver HRQoL
Based on moderate‐certainty evidence from two randomised studies including 311 participants, remotely delivered interventions involving training, support or both (with or without information) probably do not affect caregiver HRQoL (SMD 0.10, 95% CI ‐0.13 to 0.32; I2 = 0%). Positive values indicate better HRQoL in the experimental intervention group. The range of SMD values include no effect, a small effect in favour of the experimental interventions and a negligible effect in favour of the control interventions. There is no evidence of important differences in effect estimates among types of interventions (Analysis 1.3; Figure 6).
1.3. Analysis.

Comparison 1: Any intervention versus usual treatment, waiting list or attention, Outcome 3: Caregiver HRQoL
6.

Forest plot of comparison: 1 Any intervention versus usual treatment, waiting list or attention, outcome: 1.3 Caregiver HRQoL.
Training/information or training interventions versus control interventions: based on low‐certainty evidence from one randomised study including 61 participants, the evidence suggests that there may be little or no difference in caregiver HRQoL (SMD ‐0.12, 95% CI ‐0.62 to 0.38) (Figure 6, upper panel). The range of SMD values includes no effect, a small effect in favour of the experimental interventions and a moderate effect in favour of the control interventions.
Interventions including both training and support elements versus control interventions: based on moderate‐certainty evidence from one randomised study including 250 participants, the experimental intervention probably has little or no effect on caregiver HRQoL (SMD 0.15, 95% CI ‐0.10 to 0.40) (Figure 6, lower panel). The range of SMD values includes no effect, a moderate effect in favour of the experimental intervention and a negligible effect in favour of the control intervention.
1.4 Dropouts for any reason
Based on low‐certainty evidence from eight randomised studies including 661 participants, remotely delivered interventions involving training, support or both (with or without information) probably result in little or no difference in dropouts for any reason (RR 1.15, 95% CI 0.87 to 1.53; I2 = 0%). The risk for the experimental intervention group was 223 per 1000 (95% CI 169 to 296) versus 194 per 1000 for the control group. There is no evidence of important differences in risk of dropout among types of interventions (Analysis 1.4; Figure 7).
1.4. Analysis.

Comparison 1: Any intervention versus usual treatment, waiting list or attention, Outcome 4: Dropouts for any reason
7.

Forest plot of comparison: 1 Any intervention versus usual treatment, waiting list or attention, outcome: 1.4 Dropouts for any reason.
Support/information or support interventions versus control interventions: based on low‐certainty evidence from three randomised studies including 232 participants, the evidence suggests that the experimental interventions may not affect the risk of dropping out of the study for any reason (RR 1.01, 95% CI 0.59 to 1.73) (Figure 7, upper panel). The risk of dropout in the experimental intervention group was 174 per 1000, versus 172 per 1000 for the control group.
Training/information or training interventions versus control interventions: based on low‐certainty evidence from two randomised studies including 70 participants, the evidence suggests that the experimental interventions may not affect the risk of dropping out for any reason (RR 1.42, 95% CI 0.50 to 4.04) (Figure 7, middle panel). The risk of dropout in the experimental intervention group was 365 per 1000, versus 257 per 1000 for the control group.
Interventions including both training and support elements versus control interventions: based on low‐certainty evidence from three randomised studies including 359 participants, the evidence suggests that the experimental interventions may not affect the risk of dropping out of the study for any reason (RR 1.10, 95% CI 0.75 to 1.63) (Figure 7, lower panel). The risk for intervention was 215 per 1000, versus 195 per 1000 for control.
Secondary outcomes
1.5 Caregiver knowledge and skills
Based on low‐certainty evidence from four randomised studies including 223 participants (Cristancho‐Lacroix 2015; Hattink 2015; Metcalfe 2019; Nunez‐Naveira 2016), the evidence suggests that remotely delivered interventions involving training, support or both (with or without information) may make little or no difference to caregiver knowledge and skills (SMD 0.20, 95% CI ‐0.10 to 0.50; I2 = 21%). Positive values favour the experimental intervention group. The range of SMD values includes no effect, a moderate effect in favour of the experimental interventions and a negligible effect in favour of the control interventions. There is no evidence of important differences in effect estimates among types of intervention (Analysis 1.5).
1.5. Analysis.

Comparison 1: Any intervention versus usual treatment, waiting list or attention, Outcome 5: Caregiver knowledge and skills
Support/information or support interventions versus control interventions: based on low‐certainty evidence from two randomised studies including 120 participants, the evidence suggests that the experimental interventions may result in little or no difference in caregiver knowledge and skills (SMD 0.18, 95% CI ‐0.18 to 0.54). The range of SMD values includes no effect, a moderate effect in favour of the experimental interventions and a small effect in favour of the control interventions.
Training/information or training interventions versus control interventions: based on low‐certainty evidence from one randomised study including 54 participants, the evidence suggests that the experimental intervention may make little or no difference to caregiver knowledge and skills (SMD ‐0.12, 95% CI ‐0.65 to 0.42). The range of SMD values includes no effect and moderate effects in favour of either the experimental or control intervention.
Interventions including both training and support elements versus control interventions: based on low‐certainty evidence from one randomised study including 49 participants, the evidence suggests that the experimental intervention may result in an increase in caregiver knowledge and skills (SMD 0.61, 95% CI 0.04 to 1.18). The SMD values range from a negligible to a large effect in favour of the experimental intervention.
1.6 Use of health and social resources
Based on moderate‐certainty evidence from one randomised study including 250 participants (Tremont 2015), a remotely delivered intervention involving training, support or both (with or without information) probably results in little or no difference in use of health and social resources (Rate Ratio 1.05, 95% CI 0.93 to 1.19) (Analysis 1.6).
1.6. Analysis.

Comparison 1: Any intervention versus usual treatment, waiting list or attention, Outcome 6: Use of health and social resources
1.7 Admission to institutional care (nursing home placement)
Due to very low‐certainty evidence from one randomised study including 34 participants (Hayden 2012), the evidence is very uncertain about the effect of a remotely delivered interventions involving training, support or both (with or without information) on institutional care (nursing home) placement (RR 0.59, 95% CI 0.11 to 3.11) (Analysis 1.7).
1.7. Analysis.

Comparison 1: Any intervention versus usual treatment, waiting list or attention, Outcome 7: Institutional care ‐ nursing home placement
1.8 Care recipient QoL
We found no studies that examined the QoL of the cared‐for person with dementia.
2 Any intervention involving training, support or both (with or without information) versus information alone
Primary outcomes
2.1 Caregiver burden
Based on low‐certainty evidence from nine studies including 650 participants, remotely delivered interventions involving training, support or both (with or without information) may result in a slight reduction in caregiver burden (SMD ‐0.24, 95% CI ‐0.51 to 0.04; I2 = 62%) compared to a control condition of information provision alone. Negative values indicate lower burden in the experimental intervention group. The SMD values range from a moderate effect in favour of the experimental interventions to a negligible effect in favour of the control interventions (information provision). There is no evidence of important differences in effect estimates among types of interventions (Analysis 2.1; Figure 8).
2.1. Analysis.

Comparison 2: Any intervention versus control information, Outcome 1: Caregiver burden
8.

Forest plot of comparison: 2 Any intervention versus control information, outcome: 2.1 Caregiver burden.
Support/information or support interventions versus information alone: based on low‐certainty evidence from two randomised studies including 119 participants, the evidence suggests that the experimental interventions may have little or no effect on caregiver burden (SMD ‐0.03, 95% CI ‐0.39 to 0.33) (Figure 8, upper panel). The range of SMD values includes no effect and small effects in favour of either experimental or control interventions.
Training/information or training interventions versus information alone: based on low‐certainty evidence from six randomised studies including 506 participants, the evidence suggests intervention may reduce caregiver burden (SMD ‐0.31, 95% CI ‐0.69 to 0.07) (Figure 8, middle panel). The range of SMD values includes no effect, a large effect in favour of the experimental interventions and a negligible effect in favour of control interventions.
Interventions involving both support and training elements with information versus information alone: based on low‐certainty evidence from one randomised study including 25 participants, the evidence suggests that the interventions may have little or no effect on caregiver burden (SMD ‐0.11, 95% CI ‐0.90 to 0.68) (Figure 8, lower panel). The SMD values range from a large effect in favour of the experimental intervention to a moderate effect in favour of the control intervention.
2.2 Caregiver depressive symptoms
Based on moderate‐certainty evidence from 11 randomised studies including 1100 participants, remotely delivered interventions involving training, support or both (with or without information) probably result in a slight improvement in caregiver depressive symptoms (SMD ‐0.25, 95% CI ‐0.43 to ‐0.06; I2 = 53%) compared to a control condition of information alone. Negative values indicate fewer depressive symptoms in the experimental intervention group. SMD values range from a negligible effect to a moderate effect in favour of the experimental interventions. There is no evidence of important differences in effect estimates among types of interventions (Analysis 2.2; Figure 9). We detected no small study effects, that can indicated publication bias (analyses not shown).
2.2. Analysis.

Comparison 2: Any intervention versus control information, Outcome 2: Caregiver mood
9.

Forest plot of comparison: 2 Any intervention versus control information, outcome: 2.2 Caregiver mood.
Support/information or support interventions versus information alone: the evidence from one randomised study including 94 participants is inconclusive about the effect of the experimental intervention on caregiver depressive symptoms (SMD ‐0.28, 95% CI ‐0.69 to 0.13) (Figure 9, upper panel). The range of SMD values includes no effect, a moderate effect in favour of the experimental intervention and a small effect in favour of the control intervention.
Training/information or training interventions versus information alone: based on low‐certainty evidence from eight randomised studies including 816 participants, the evidence suggests the experimental intervention may improve caregiver depressive symptoms (SMD ‐0.31, 95% CI ‐0.54 to ‐0.08) (Figure 9, middle panel). SMD values range from a negligible to a moderate effect in favour of the experimental interventions.
Interventions involving both support and training elements with information versus information alone: based on low‐certainty evidence from two randomised studies including 190 participants, the evidence suggests that the intervention may result in little or no difference to caregiver depressive symptoms (SMD 0.04, 95% CI ‐0.24 to 0.33) (Figure 9, lower panel). The range of SMD values includes no effect and small effects in favour of either experimental or control interventions.
2.3 Caregiver HRQoL
Based on low‐certainty evidence from two randomised studies including 257 participants, remotely delivered interventions involving training, support or both (with or without information) may result in little or no difference in caregiver HRQoL when compared with information alone (SMD ‐0.03, 95% CI ‐0.28 to 0.21; I2 = 0%). Positive values indicate better HRQoL in the experimental intervention group. The range of SMD values includes no effect and small effects in favour of either experimental or control interventions (Analysis 2.3; Figure 10).
2.3. Analysis.

Comparison 2: Any intervention versus control information, Outcome 3: Caregiver HRQoL
10.

Forest plot of comparison: 2 Any intervention versus control information, outcome: 2.3 Caregiver HRQoL.
2.4 Dropouts for any reason
Based on moderate‐certainty evidence from 12 randomised studies including 1266 participants, remotely delivered interventions involving training, support or both (with or without information) probably result in an increase in dropouts for any reason (RR 1.51, 95% CI 1.04 to 2.20; I2 = 37%). The risk of dropout from the experimental interventions was 196 per 1000 (95% CI 135 to 286) versus 130 per 1000 for the control interventions (information alone). There is no evidence of important differences in risk among types of interventions (Analysis 2.4; Figure 11). We detected no small study effects, which can indicate publication bias (analyses not shown).
2.4. Analysis.

Comparison 2: Any intervention versus control information, Outcome 4: Dropouts for any reason
11.

Forest plot of comparison: 2 Any intervention versus control information, outcome: 2.4 Dropouts for any reason.
Support/information or support interventions versus information alone: based on low‐certainty evidence from one randomised study including 42 participants, the evidence suggests that the experimental intervention may have little or no effect on dropouts for any reason (RR 1.10, 95% CI 0.17 to 7.09) (Figure 11, upper panel). The risk of dropping out from the experimental interventions was 100 per 1000 versus 91 per 1000 for the control intervention.
Training/information or training interventions versus information alone: based on moderate‐certainty evidence from nine randomised studies including 994 participants, the experimental interventions likely result in a large increase in dropouts for any reason (RR 1.51, 95% CI 0.97 to 2.33) (Figure 11, middle panel). The risk of dropping out from the experimental interventions was 204 per 1000 versus 135 per 1000 for the control interventions.
Interventions involving both support and training elements with information versus information alone: based on very low‐certainty evidence from two randomised studies including 230 participants, the evidence is very uncertain about the effect of the experimental interventions on dropouts for any reason (RR 1.25, 95% CI 0.25 to 6.22) (Figure 11, lower panel). The risk of dropping out from the experimental interventions was 144 per 1000 versus 115 per 1000 for the control interventions.
Secondary outcomes
2.5 Caregiver knowledge and skills
Based on low‐certainty evidence from two randomised studies including 70 participants, the evidence was inconclusive about any effect of remotely delivered interventions involving training, support or both (with or without information) on caregiver knowledge and skills compared to a control condition of information alone (SMD 0.18, 95% CI ‐0.29 to 0.65; I2 = 0%). Positive values indicate better caregiver knowledge and skills score in the experimental intervention group. The range of SMD values include no effect, a large effect in favour of the experimental interventions and a moderate effect in favour of the control interventions (Analysis 2.5).
2.5. Analysis.

Comparison 2: Any intervention versus control information, Outcome 5: Caregiver knowledge and skills
Another two studies (Duggleby 2018; Huis in het Veld 2020), whose results we were unable to include in the meta‐analysis, also reported data on this outcome. Evidence from Duggleby 2018 including 199 participants suggests that the experimental intervention has little or no effect on caregiver knowledge and skills assessed with the 10‐item GSES (MD 0.22, 95% CI ‐0.78 to 1.22). Evidence from Huis in het Veld 2020 including 54 participants also suggests little or no effect of the experimental intervention on caregiver knowledge and skills assessed with the 32‐item TOA (MD ‐2.16, 95% CI ‐6.86 to 2.53).
2.6 Use of health/social resources
We did not find any studies that examined the use of health and social resources.
2.7 Admission to institutional care (nursing home placement)
Based on very low‐certainty evidence from one randomised study including 32 participants (Gant 2007), the evidence is very uncertain about the effect of the experimental intervention on admission to institutional care (nursing home placement) (RR 2.67, 95% CI 0.12 to 60.93) (Analysis 2.6).
2.6. Analysis.

Comparison 2: Any intervention versus control information, Outcome 6: Institutional care ‐ nursing home placement
2.8 Care recipient QoL
We did not find any studies that examined the QoL of the cared‐for person with dementia.
Discussion
Summary of main results
Our review included 26 studies with a total of 2367 participants. We conducted two comparisons:
Interventions involving training, support or both (with or without information) versus usual treatment, waiting list or attention control;
Interventions involving training, support or both (with or without information) versus information alone.
Interventions involving training, support or both (with or without information) versus usual treatment, waiting list or attention control
Primary outcomes
In this comparison, we found that the experimental interventions probably make little or no difference to caregiver burden (nine studies, 597 participants), caregiver depressive symptoms (eight studies, 638 participants) or caregiver HRQoL (two studies, 311 participants), and probably have little or no effect on dropouts for any reason (eight studies, 661 participants).
Secondary outcomes
We found that the experimental interventions may have little or no effect on caregiver knowledge and skills (four studies, 223 participants), or on use of health and social resources (one study, 250 participants). We found that the evidence is very uncertain about their effect on admission to institutional care (nursing home placement). We did not find studies that examined the QoL of the care recipient.
Interventions involving training, support or both (with or without information) versus information alone
Primary outcomes
In this comparison, we found that interventions including training or support compared to information alone may result in a slight reduction in caregiver burden (nine studies, 650 participants), probably result in a slight reduction in caregiver depressive symptoms (11 studies, 1100 participants), may make little or no difference to caregiver HRQoL (two studies, 257 participants), and probably result in a large increase in dropouts for any reason (12 studies, 1266 participants).
Secondary outcomes
We found ino evidence of an effect of the experimental interventions on caregiver knowledge and skills and very uncertain evidence about the effect on institutional care (nursing home placement) (one study, 32 participants). We did not find studies that examined the use of health and social resources or the QoL of the cared‐for person with dementia.
Overall completeness and applicability of evidence
We believe that we are likely to have identified all the studies that address the efficacy and safety of remotely delivered psychoeducational interventions for caregivers of people with dementia. Our search for evidence was extensive, using the Specialised Register of the CDCIG, as well as running complementary searches in appropriate electronic databases, clinical trials registries, and hand‐searching references from included studies.
The average characteristics of caregivers (mostly females over 60 years of age) and care recipients (83% Alzheimer's diagnosis) are representative of the patient‐caregiver dyads that usually access community health and social care facilities.
There was significant diversity in the outcomes measured in the included trials. Of our primary outcomes, caregiver burden and caregiver mood were most frequently assessed, but assessment of mood in most studies relied on scales of depressive symptoms. Few studies used validated measures of caregiver anxiety, which would also be of interest. Caregivers' HRQoL was assessed in only four trials. We did not find explicit reporting of adverse effects of the interventions and the additional burden they might add to the caregiver's life. Although acceptability is a complex, multi‐dimensional construct, it is difficult to measure and we were only able to approximate it using measures of overall dropout from the studies as a surrogate.
Ten studies reported, if not acceptability, quality control indicators associated with uptake of the interventions, fidelity of implementation or user satisfaction with the programs. As described below, these indicators and assessment areas varied very widely across studies and so we did not attempt to combine them. Au 2019 assessed program fidelity using an unblinding rating system after the end of each session. Brennan 1995 assessed service use by counting service contacts. Cristancho‐Lacroix 2015 assessed the percentage of participants finishing the protocol. Davis 2004 assessed treatment implementation with audiotaped sessions and weekly caregiving logs, among other treatment delivery and receipt checks. Duggleby 2018 assessed if the intervention was used at least once, Martindale‐Adams 2013 assessed percentage of participants attending a minimum number of program sessions. Metcalfe 2019 assessed technology acceptance measuring the perceived usefulness, the perceived ease of use, computer self‐efficacy and behavioural intentions. Nunez‐Naveira 2016 assessed the feasibility of the intervention with three self‐completed questionnaires developed for the study. Torkamani 2014 assessed the web platform with satisfaction questionnaires. Tremont 2015 assessed the intervention with a treatment satisfaction questionnaire.
There are two issues that may compromise the applicability of results from this review: its reliance mostly on populations from high‐income countries with distinct health and social services at community level, and the limitation of the available evidence to the acute effects of interventions, assessed at a median of 16 weeks. Little evidence comes from studies conducted in non‐Western countries (only three studies conducted in China), and most studies were conducted in the United States (15 of 26) which, unlike European countries (eight studies), lacks a universal public health system. We also lack sufficient information to determine whether results apply over longer follow‐up periods, or if effects could be delayed in time, as the developed skills and coping strategies might help caregivers as the dementia progresses. In fact, very few studies (six of 26) presented data to assess effects over time after the end of the experimental interventions (median follow‐up time 24 weeks). These studies describe per protocol results (completers only) rather than intention‐to‐treat results. These missing data limit the applicability of our review.
In summary, evidence is lacking for several important outcomes.The applicability of the evidence from our review may be restricted to populations of high‐income countries, and to the acute effects of interventions.
Quality of the evidence
We used GRADE methods to rate the overall certainty of the evidence for each outcome. All results were downgraded for study limitations. The most widespread risk of bias was the risk of performance bias due to self‐rating of subjective outcomes by participants who were not blind to the treatment. Randomisation methods were not always reported in sufficient detail to allow risk of bias to be assessed. In some studies, there was a risk of attrition bias. We also downgraded some results for inconsistency between studies.
For the comparison of any intervention versus usual treatment, waiting list or attention, we consider the certainty of evidence to be low to moderate. Evidence certainty is low for dropouts for any cause, and moderate for caregiver burden, caregiver mood (depressive symptoms), and caregiver HRQoL (Table 1).
For the comparison of any intervention versus control information, we consider the certainty of evidence to be low to moderate. Evidence certainty is low for caregiver burden and caregiver HRQoL, and moderate for caregiver mood (depressive symptoms) and dropouts for any cause (Table 2).
Potential biases in the review process
We conducted this systematic review in accordance with Cochrane's current standards. However, the following potential limitations in the review process deserve mention:
The complexity and heterogeneity of the interventions in our review, and their incompletely documented methodology and results, present notable disadvantages when trying to summarise the evidence of complex interventions on caregiver well‐being (Belle 2003; Gaugler 2017). We have used the TIDieR template (Hoffmann 2014) to describe interventions, and following consultation with the Cochrane Dementia and Cognitive Improvement Group, we have classified experimental interventions into three groups for the purposes of subgroup analyses (support alone or with information, training alone or with information, and interventions including both support and training). This was because all but three studies that provided support or training also included provision of information. Further, we have classified control interventions in two categories (one grouping together usual treatment, waiting list and attention control, and the other including information provision only). Pooled analyses for each comparison grouped together all experimental interventions in an overall category labelled 'any treatment'. This inclusive category does not present misclassification bias. However, subgroup analyses by type of experimental intervention may present misclassification issues, which usually tend to bias effects to the null hypothesis of no difference.
This review has examined efficacy at a median time of 16 weeks. There is no information on longer effects of the interventions. Only six studies reported any data for longer follow‐up (median of 24 weeks). However, these studies form a selected subset of all included studies and present data for completers only, which may introduce additional bias because of attrition and missing information. Therefore, we have not conducted a follow‐up analysis.
We contacted study authors of the included trials where the publicly available information for methodological aspects of the studies and/or reported results was unclear or missing. We received responses from some authors (acknowledged in Characteristics of included studies), but many others were impossible to locate or did not respond to our information requests.
An unexpected finding of this review is that, contrary to what might have been anticipated, psychoeducational interventions for caregivers seem to show slightly more efficacy on decreasing burden and improving mood of caregivers,when compared with a control intervention that involved the simple provision of structured information about dementia, than when compared with usual treatment, being on a waiting list, or having non‐specific attention. It is possible that the differential dropout rates we have found in the comparison of experimental interventions with information alone might contribute to this unforeseen result. Since most studies reported outcomes for completers only and experimental interventions were associated with a higher risk of dropout, especially in the studies using an information‐only control, it is conceivable that attrition bias might have inflated effect sizes in favour of interventions in this comparison.
Since most studies have been run in high income countries with developed health and social care systems, it is also possible that usual treatment control groups had access at least to basic information and support as part of standard care services or from other community sources (charities, self‐help groups). If this were the case, usual treatment might represent a level of care that could be efficacious enough to narrow the differences with any other intervention. In support of this argument, it is worth noting that in the first comparison, most studies included usual treatment (n = 6) or attention controls (n = 4), and only two studies (Hattink 2015; Metcalfe 2019) included a waiting list control group.
Agreements and disagreements with other studies or reviews
Other recent reviews have concluded that the efficacy of interventions including information and support or training, is relatively small with absolute average effects of about SMD 0.20 on caregivers' burden ( Abrahams 2018; Williams 2019a), caregivers' mood‐depression (Abrahams 2018), and caregivers' QoL (Abrahams 2018). These effect sizes are slightly higher than those previously reported for remotely delivered interventions (Internet‐based or telephone‐based), but well within the range of variability for effect sizes. Reviews addressing caregiver burden in dementia (Deeken 2019) and dementia among several other chronic conditions (stroke survivors, non‐small cell lung cancer and brain injury) (Ploeg 2018), and reviewing technology‐based and Web‐based interventions respectively, report effect sizes of SMD ‐0.13 (95% CI ‐0.24 to ‐0.02) in favour of any intervention and SMD 0.03 in favour of control (95% CI ‐0.31 to 0.36). Our values overlap with those results: SMD ‐0.06 (95% CI ‐0.35 to 0.23) for comparison versus usual treatment, waiting list or attention control and SMD ‐0.24 (95% CI ‐0.51 to 0.04) for comparison versus control information. For caregiver mood (mainly assessed with depression questionnaires), Deeken 2019 (in dementia caregivers) and Sherifali 2018 (in other chronic condition caregivers) report effect sizes of SMD ‐0.20 (95% CI ‐0.31 to ‐0.10) and SMD ‐0.19 (95% CI ‐0.43 to 0.05) in favour of any intervention, and our values also agree with those results: SMD ‐0.05 (95% CI ‐0.22 to 0.12) for comparison versus usual treatment, waiting list or attention control and SMD ‐0.25 (95% CI ‐0.43 to ‐0.06) for comparison versus control information. For caregiver QoL in chronic conditions (mostly dementia but also stroke, lung cancer and brain injury), Sherifali 2018 reports a negligible effect size of SMD 0.01 in favour of any intervention but with a very wide confidence interval that covers a moderate effect in both directions, in favour and against intervention (95% CI ‐0.49 to 0.51). Our results are also compatible: SMD 0.10 in favour of any intervention (95% CI ‐0.13 to 0.32) for comparison versus usual treatment, waiting list or attention control and SMD ‐0.03 (95% CI ‐0.28 to 0.21) for comparison versus control information. We have not found effect sizes from other reviews dealing with the outcome of dropouts for any reason.
Two qualitative reviews (Egan 2018; Hopwood 2018) indicate positive responses to the use of Internet‐based interventions by caregivers, and that they might be efficacious to improve mental health and reduce caregiver burden. However, they also highlight problems with determining the effectiveness of Internet interventions: the marked methodological diversity across studies (Egan 2018), and the need for high‐quality studies to increase the quality of evidence (Hopwood 2018).
An earlier Cochrane Review that partially overlaps with ours (Lins 2014) found that telephone counselling for informal carers of people with dementia was efficacious for decreasing depressive symptoms (SMD ‐0.32; 95% CI ‐0.63 to ‐0.01) and caregiver burden (SMD ‐0.45; 95% CI ‐0.90 to 0.01). These results are the most favourable we have seen reported for any remotely delivered intervention. The review is restricted to a comparison of telephone counselling with no treatment, usual care, or friendly calls for chatting. It is worth noting that all but one of the studies included in that review are also included in ours. For depressive symptoms, Tremont 2008 and Winter 2007 overlap with ours, as well as Davis 2004, Tremont 2008, and Winter 2007 for caregiver burden. However, we did not include another study that Lins 2014 included for both outcomes (Finkel 2007). Hence, the reviews are not entirely comparable.
In summary, given the large variability of effect sizes reported by other reviews, our results seem consistent with those previously published for caregiver burden, mood and QoL. We cannot compare our results for acceptability of interventions (dropouts for any cause) with other reviews, which did not study this outcome.
Authors' conclusions
Implications for practice.
Remotely delivered, complex interventions involving information, support or training for informal caregivers of people with dementia may slightly reduce caregiver burden and depressive symptoms when compared with provision of information alone, but not when compared with usual treatment, waiting list or attention control. In settings where social and health resources are well developed and available to caregivers of people with dementia, these interventions may not add significantly to usual care. We cannot comment on the efficacy of these interventions in settings with less‐developed health and social care systems or in situations when usual care services cannot be accessed.
Implications for research.
Overall, remotely delivered interventions involving training or support seem to be less acceptable than control interventions, as assessed by the number of participants dropping out. This might limit their applicability in community settings. It is therefore important to investigate causes for dropouts, including any difficulties related to the delivery of the interventions, such as the use of online platforms, or the nature of learning materials. It is also important to investigate adverse effects as possible causes for dropouts, including the additional burden they might add to a caregiver's life. Our results for important outcomes such as caregiver quality of life and improvement in caregiver knowledge and skills were very imprecise, at least in part because few studies measured them. These outcomes should be included in future studies, without neglecting core outcomes such as caregiver burden and mood, including both depression and anxiety. Mixed methods study designs, incorporating qualitative elements, may be particularly useful for these complex and heterogeneous interventions in order to aid interpretation of quantitative data on efficacy and acceptability or dropout. Given the rapid increase in the numbers of people with dementia in low‐income countries, research into interventions to improve the situation of family caregivers in these settings would be very valuable.
History
Protocol first published: Issue 2, 2007 Review first published: Issue 12, 2020
Acknowledgements
Study partially funded with a research grant from the University of the Basque Country, UPV/EHU (GIU14/27).
Gabriel Martínez (School of Medicine and Dentistry, Universidad de Antofagasta, Antofagasta, Chile) participated in the development of the original review protocol that gave rise to the current review.
We would like to thank peer reviewers Kieren Egan and Ralph Möhler and consumer reviewer Cathie Hofstetter for their comments and feedback.
Appendices
Appendix 1. Source Search strategy Hits retrieved ALOIS (www.medicine.ox.ac.uk/alois)Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| ALOIS (www.medicine.ox.ac.uk/alois) [Date of most recent search: 10 April 2020] |
Advanced search: Study aim: Caregiver focused AND study design: RCT OR CCT OR Unclear | Dec 2015: 183 Jul 2016: 2 May 2017: 3 May 2018: 4 May 2019: 207 |
| MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) [Date of most recent search: 10 April 2020] |
1 Dementia/ 2 Delirium/ 3 Wernicke Encephalopathy/ 4 Delirium, Dementia, Amnestic, Cognitive Disorders/ 5 ("benign senescent forgetfulness" or ("normal pressure hydrocephalus" and "shunt*") or ("organic brain disease" or "organic brain syndrome") or ((cerebral* or cerebrovascular or cerebro‐vascular) adj2 insufficien*) or (cerebr* adj2 deteriorat*) or (chronic adj2 (cerebrovascular or cerebro‐vascular)) or (creutzfeldt or jcd or cjd) or (lewy* adj2 bod*) or (pick* adj2 disease) or alzheimer* or binswanger* or deliri* or dement* or huntington* or korsako*).tw. 6 1 or 2 or 3 or 4 or 5 7 Caregivers/ 8 (caregiver* or care‐giver* or carer* or daughter* or dependents or families* or family* or folk* or kinship or parent* or relatives or spouse*).tw. 9 7 or 8 10 Audiovisual aids/ 11 Books/ 12 Consumer Health Information/ 13 Health Education/ 14 Health Information Systems/ 15 Health Literacy/ 16 Health promotion/ 17 Information Dissemination/ 18 Pamphlets/ 19 Social Media/ 20 Telephone/ 21 ("Patient information" or "social media" or Audiovisual* or Audio‐visual* or Book* or Leaflet* or Lecture* or Manuals).tw. 22 Education/ 23 Training Support/ 24 (Education or Train or Training).tw. 25 Community Networks/ 26 Homemaker Services/ 27 Respite Care/ 28 Self‐Help Groups/ 29 Social Support/ 30 ((caregiver* or care‐giver* or carer* or daughter* or dependents or families* or family* or folk* or kin* or parent* or relatives or spouse*) adj5 Support).tw. 31 (counsel* or respite).tw. 32 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 33 6 and 9 and 32 34 (randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab. 35 33 and 34 36 dementia/ 37 delirium/ 38 Wernicke Encephalopathy/ 39 cognitive defect/ 40 ("benign senescent forgetfulness" or ("normal pressure hydrocephalus" and "shunt*") or ("organic brain disease" or "organic brain syndrome") or ((cerebral* or cerebrovascular or cerebro‐vascular) adj2 insufficien*) or (cerebr* adj2 deteriorat*) or (chronic adj2 (cerebrovascular or cerebro‐vascular)) or (creutzfeldt or jcd or cjd) or (lewy* adj2 bod*) or (pick* adj2 disease) or alzheimer* or binswanger* or deliri* or dement* or huntington* or korsako*).tw. 41 36 or 37 or 38 or 39 or 40 42 Caregivers/ 43 (caregiver* or care‐giver* or carer* or daughter* or dependents or families* or family* or folk* or kinship or parent* or relatives or spouse*).tw. 44 42 or 43 45 audiovisual aid/ 46 book/ 47 consumer health information/ 48 health education/ 49 medical information system/ 50 health literacy/ 51 health promotion/ 52 information dissemination/ 53 publication/ 54 social media/ 55 telephone/ 56 ("Patient information" or "social media" or Audiovisual* or Audio‐visual* or Book* or Leaflet* or Lecture* or Manuals).tw. 57 education/ 58 (Education or Train or Training).tw. 59 community care/ 60 home care/ 61 respite care/ 62 self help/ 63 social support/ 64 ((caregiver* or care‐giver* or carer* or daughter* or dependents or families* or family* or folk* or kin* or parent* or relatives or spouse*) adj5 Support).tw. 65 (counsel* or respite).tw. 66 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 67 randomized controlled trial/ 68 controlled clinical trial/ 69 random$.ti,ab. 70 randomization/ 71 intermethod comparison/ 72 placebo.ti,ab. 73 (compare or compared or comparison).ti. 74 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 75 (open adj label).ti,ab. 76 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 77 Double‐Blind Method/ 78 parallel group$1.ti,ab. 79 (crossover or cross over).ti,ab. 80 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 81 (assigned or allocated).ti,ab. 82 (controlled adj7 (study or design or trial)).ti,ab. 83 (volunteer or volunteers).ti,ab. 84 trial.ti. 85 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 86 41 and 44 and 66 and 85 87 (2015* or 2016*).em. 88 86 and 87 89 Dementia/ 90 Delirium/ 91 Wernicke Encephalopathy/ 92 Delirium, Dementia, Amnestic, Cognitive Disorders/ 93 ("benign senescent forgetfulness" or ("normal pressure hydrocephalus" and "shunt*") or ("organic brain disease" or "organic brain syndrome") or ((cerebral* or cerebrovascular or cerebro‐vascular) adj2 insufficien*) or (cerebr* adj2 deteriorat*) or (chronic adj2 (cerebrovascular or cerebro‐vascular)) or (creutzfeldt or jcd or cjd) or (lewy* adj2 bod*) or (pick* adj2 disease) or alzheimer* or binswanger* or deliri* or dement* or huntington* or korsako*).tw. 94 89 or 90 or 91 or 92 or 93 95 Caregivers/ 96 (caregiver* or care‐giver* or carer* or daughter* or dependents or families* or family* or folk* or kinship or parent* or relatives or spouse*).tw. 97 95 or 96 98 Audiovisual aids/ 99 Books/ 100 Consumer Health Information/ 101 Health Education/ 102 Health Information Systems/ 103 Health Literacy/ 104 Health promotion/ 105 Information Dissemination/ 106 Pamphlets/ 107 Social Media/ 108 Telephone/ 109 ("Patient information" or "social media" or Audiovisual* or Audio‐visual* or Book* or Leaflet* or Lecture* or Manuals).tw. 110 Education/ 111 Training Support/ 112 (Education or Train or Training).tw. 113 Community Networks/ 114 Homemaker Services/ 115 Respite Care/ 116 Self‐Help Groups/ 117 Social Support/ 118 ((caregiver* or care‐giver* or carer* or daughter* or dependents or families* or family* or folk* or kin* or parent* or relatives or spouse*) adj5 Support).tw. 119 (counsel* or respite).tw. 120 98 or 99 or 100 or 101 or 102 or 103 or 104 or 105 or 106 or 107 or 108 or 109 or 110 or 111 or 112 or 113 or 114 or 115 or 116 or 117 or 118 or 119 121 94 and 97 and 120 122 (randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab. 123 121 and 122 |
Dec 2015: 1282 Jul 2016: 166 May 2017: 159 May 2018: 169 May 2019: 164 |
| Embase 1974‐2019 May 14 (Ovid SP) [Date of most recent search: 10 April 2020] |
1 Dementia/ 2 Delirium/ 3 Wernicke Encephalopathy/ 4 Delirium, Dementia, Amnestic, Cognitive Disorders/ 5 ("benign senescent forgetfulness" or ("normal pressure hydrocephalus" and "shunt*") or ("organic brain disease" or "organic brain syndrome") or ((cerebral* or cerebrovascular or cerebro‐vascular) adj2 insufficien*) or (cerebr* adj2 deteriorat*) or (chronic adj2 (cerebrovascular or cerebro‐vascular)) or (creutzfeldt or jcd or cjd) or (lewy* adj2 bod*) or (pick* adj2 disease) or alzheimer* or binswanger* or deliri* or dement* or huntington* or korsako*).tw. 6 1 or 2 or 3 or 4 or 5 7 Caregivers/ 8 (caregiver* or care‐giver* or carer* or daughter* or dependents or families* or family* or folk* or kinship or parent* or relatives or spouse*).tw. 9 7 or 8 10 Audiovisual aids/ 11 Books/ 12 Consumer Health Information/ 13 Health Education/ 14 Health Information Systems/ 15 Health Literacy/ 16 Health promotion/ 17 Information Dissemination/ 18 Pamphlets/ 19 Social Media/ 20 Telephone/ 21 ("Patient information" or "social media" or Audiovisual* or Audio‐visual* or Book* or Leaflet* or Lecture* or Manuals).tw. 22 Education/ 23 Training Support/ 24 (Education or Train or Training).tw. 25 Community Networks/ 26 Homemaker Services/ 27 Respite Care/ 28 Self‐Help Groups/ 29 Social Support/ 30 ((caregiver* or care‐giver* or carer* or daughter* or dependents or families* or family* or folk* or kin* or parent* or relatives or spouse*) adj5 Support).tw. 31 (counsel* or respite).tw. 32 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 33 6 and 9 and 32 34 (randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab. 35 33 and 34 36 dementia/ 37 delirium/ 38 Wernicke Encephalopathy/ 39 cognitive defect/ 40 ("benign senescent forgetfulness" or ("normal pressure hydrocephalus" and "shunt*") or ("organic brain disease" or "organic brain syndrome") or ((cerebral* or cerebrovascular or cerebro‐vascular) adj2 insufficien*) or (cerebr* adj2 deteriorat*) or (chronic adj2 (cerebrovascular or cerebro‐vascular)) or (creutzfeldt or jcd or cjd) or (lewy* adj2 bod*) or (pick* adj2 disease) or alzheimer* or binswanger* or deliri* or dement* or huntington* or korsako*).tw. 41 36 or 37 or 38 or 39 or 40 42 Caregivers/ 43 (caregiver* or care‐giver* or carer* or daughter* or dependents or families* or family* or folk* or kinship or parent* or relatives or spouse*).tw. 44 42 or 43 45 audiovisual aid/ 46 book/ 47 consumer health information/ 48 health education/ 49 medical information system/ 50 health literacy/ 51 health promotion/ 52 information dissemination/ 53 publication/ 54 social media/ 55 telephone/ 56 ("Patient information" or "social media" or Audiovisual* or Audio‐visual* or Book* or Leaflet* or Lecture* or Manuals).tw. 57 education/ 58 (Education or Train or Training).tw. 59 community care/ 60 home care/ 61 respite care/ 62 self help/ 63 social support/ 64 ((caregiver* or care‐giver* or carer* or daughter* or dependents or families* or family* or folk* or kin* or parent* or relatives or spouse*) adj5 Support).tw. 65 (counsel* or respite).tw. 66 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 67 randomized controlled trial/ 68 controlled clinical trial/ 69 random$.ti,ab. 70 randomization/ 71 intermethod comparison/ 72 placebo.ti,ab. 73 (compare or compared or comparison).ti. 74 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 75 (open adj label).ti,ab. 76 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 77 Double‐Blind Method/ 78 parallel group$1.ti,ab. 79 (crossover or cross over).ti,ab. 80 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 81 (assigned or allocated).ti,ab. 82 (controlled adj7 (study or design or trial)).ti,ab. 83 (volunteer or volunteers).ti,ab. 84 trial.ti. 85 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 86 41 and 44 and 66 and 85 |
Dec 2015: 1857 Jul 2016: 379 May 2017: 331 May 2018: 574 May 2019: 465 |
| PSYCINFO 1806‐May week 2 2019 (Ovid SP) [Date of most recent search: 10 April 2020] |
1 dement*.ti,ab. 2 alzheimer*.ti,ab. 3 exp Dementia/ 4 (lewy* adj2 bod*).ti,ab. 5 (frontotemporal* or FTD or FTLD).ti,ab. 6 2 or 3 or 4 or 5 7 Caregivers/ 8 (caregiver* or care‐giver* or carer* or daughter* or dependents or families* or family* or folk* or kinship or parent* or relatives or spouse*).ti,ab. 9 7 or 8 10 Educational Audiovisual Aids/ 11 book/ 12 exp Health Education/ 13 Health Literacy/ 14 Health Promotion/ 15 Information Dissemination/ 16 exp Social Media/ 17 exp Telephone Systems/ 18 ("Patient information" or "social media" or Audiovisual* or Audio‐visual* or Book* or Leaflet* or Lecture* or Manuals).ti,ab. 19 Education/ 20 (Education or Train or Training).ti,ab. 21 Community Mental Health Services/ 22 Community Services/ 23 Home Care/ 24 Respite Care/ 25 Support Groups/ 26 Self Help Techniques/ 27 Social Support/ 28 ((caregiver* or care‐giver* or carer* or daughter* or dependents or families* or family* or folk* or kin* or parent* or relatives or spouse*) adj5 Support).ti,ab. 29 (counsel* or respite).ti,ab. 30 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 31 exp Clinical Trials/ 32 randomly.ab. 33 randomi?ed.ti,ab. 34 placebo.ti,ab. 35 groups.ab. 36 "double‐blind*".ti,ab. 37 "single‐blind*".ti,ab. 38 RCT.ti,ab. 39 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 40 6 and 9 and 30 and 39 |
Dec 2015: 835 Jul 2016: 81 May 2017: 71 May 2018: 77 May 2019: 59 |
| CINAHL (EBSCOhost) [Date of most recent search: 10 April 2020] |
S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S20 (MH "Caregivers") OR (MH "Caregiver Support") S21 TX caregiver* or care‐giver* or carer* or daughter* or dependents or families* or family* or folk* or kinship or parent* or relatives or spouse* S22 S20 OR S21 S23 (MH "Books") S24 (MH "Consumer Health Information") S25 (MH "Health Education") S26 (MH "Health Information Systems") OR (MH "Home Health Care Information Systems") S27 (MH "Health Promotion") S28 (MH "Pamphlets") S29 (MH "Social Media") S30 (MH "Telephone") S31 TX "Patient information" or "social media" or Audiovisual* or Audio‐visual* or Book* or Leaflet* or Lecture* or Manuals S32 (MH "Education") S33 TX Education or Train or Training S34 (MH "Community Networks") S35 (MH "Homemaker Services") S36 (MH "Respite Care") S37 TX caregiver* or care‐giver* or carer* or daughter* or dependents or families* or family* or folk* or kin* or parent* or relatives or spouse* S38 TX support S39 S37 AND S38 S40 TX counsel* or respite S41 S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S39 OR S40 S42 S19 AND S41 S43 S22 AND S42 S44 (MH "Randomized Controlled Trials") S45 AB randomly S46 AB placebo S47 AB groups S48 AB randomised S49 TI randomised S50 TI randomized S51 AB randomized S52 AB "double‐blind*" S53 TI "double‐blind*" S54 TI "single‐blind*" S55 AB "single‐blind*" S56 AB RCT S57 S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 S58 S43 AND S57 |
Dec 2015: 1364 Jul 2016: 179 May 2017: 129 May 2018: 275 May 2019: 442 |
| Web of Science Core Collection [Date of most recent search: 10 April 2020] |
(dement* OR alzheimer* OR "lew* bod*" OR frontotemporal OR FTD OR FTLD OR "severe* cognit* impair*") AND TOPIC: (cargiver* OR care‐giver* OR carer* OR daughter* OR dependents OR families* OR family* OR folk* OR kinship OR parent* OR relatives OR spouse*) AND TOPIC: ("Patient information" OR "social media" OR Audiovisual* OR Audio‐visual* OR Book* OR Leaflet* OR Lecture* OR Manuals OR Education OR Train OR Training OR counsel* OR respite) AND TOPIC: (random* OR trial OR placebo OR "double‐blind*" OR "single‐blind*" OR RCT OR "control group*") Timespan: All years. Search language=Auto |
Dec 2015: 1171 Jul 2016: 207 May 2017: 267 May 2018: 220 May 2019: 160 |
| LILACS (BIREME) [Date of most recent search: 10 April 2020] |
Information OR informações or INFORMAÇÃO or educación OR educação OR education OR manual OR Capacitación OR training [Words] and "cuidador familiar" OR caregiver OR cônjuge OR filha OR hija OR daughter OR family OR familiar [Words] and dementia OR Demencia OR demência OR alzheimers OR Alzheimer [Words] | Dec 2015: 131 Jul 2016: 10 May 2017: 6 May 2018: 6 May 2019: 5 |
| CENTRAL (The Cochrane Library, issue 5 of 12, 2019) [Date of most recent search: 10 April 2020] |
#1 MeSH descriptor: [Dementia] explode all trees #2 MeSH descriptor: [Delirium, Dementia, Amnestic, Cognitive Disorders] explode all trees #3 alzheimer* or binswanger* or deliri* or dement* or huntington* or korsako* #4 #1 or #2 or #3 #5 MeSH descriptor: [Caregivers] explode all trees #6 caregiver* or care‐giver* or carer* or daughter* or dependents or families* or family* or folk* or kinship or parent* or relatives or spouse* #7 #5 or #6 #8 MeSH descriptor: [Audiovisual Aids] explode all trees #9 MeSH descriptor: [Books] explode all trees #10 MeSH descriptor: [Consumer Health Information] explode all trees #11 MeSH descriptor: [Information Dissemination] explode all trees #12 MeSH descriptor: [Health Promotion] explode all trees #13 MeSH descriptor: [Health Literacy] explode all trees #14 MeSH descriptor: [Health Information Systems] explode all trees #15 MeSH descriptor: [Pamphlets] explode all trees #16 MeSH descriptor: [Social Media] explode all trees #17 MeSH descriptor: [Telephone] explode all trees #18 "Patient information" or "social media" or Audiovisual* or Audio‐visual* or Book* or Leaflet* or Lecture* or Manuals #19 MeSH descriptor: [Education] explode all trees #20 MeSH descriptor: [Training Support] explode all trees #21 Education or Train or Training #22 MeSH descriptor: [Community Networks] explode all trees #23 MeSH descriptor: [Homemaker Services] explode all trees #24 MeSH descriptor: [Respite Care] explode all trees #25 MeSH descriptor: [Self‐Help Groups] explode all trees #26 MeSH descriptor: [Social Support] explode all trees #27 counsel* or respite #28 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 #29 #4 and #7 and #28 |
Dec 2015: 1220 Jul 2016: 52 May 2017: 108 May 2018: 276 May 2019: 505 |
| Clinicaltrials.gov (www.clinicaltrials.gov) [Date of most recent search: 10 April 2020] |
Search terms: dementia AND condition: carer OR caregiver [Interventional studies] All dates, all status | Dec 2015: 113 Jul 2016: 8 May 2017: 58 May 2018: 92 May 2019: 88 |
| ICTRP Search Portal (http://apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] [Date of most recent search: 109 Aperil 2020] |
(Title: dementia or Alzheimer or alzheimers or lewy) AND (condition: carer or caregiver) All dates, all status | Dec2015: 12 Jul 2016: 11 May 2017: 15 May 2018: 46 May 2019: 38 |
| TOTAL before de‐duplication and first assessment | Dec 2015: 8168 Jul 2016: 1095 May 2017: 1147 May 2018: 1739 May 2019: 2133 TOTAL: 14,282 |
|
| TOTAL after de‐duplication | Dec 2015: 7211 Jul 2016: 827 May 2017: 908 May 2018: 1321 May 2019: 1558 TOTAL: 11,825 |
|
| TOTAL after first assessment by CDCIG information specialist | Dec 2015: 1028 Jul 2016: 183 May 2017: 125 May 2018: 252 May 2019: 422 TOTAL: 2,010 |
|
Data and analyses
Comparison 1. Any intervention versus usual treatment, waiting list or attention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Caregiver burden | 9 | 597 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.35, 0.23] |
| 1.1.1 Support/information plus support | 3 | 156 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.43, 0.66] |
| 1.1.2 Training/information plus training | 3 | 109 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.78, 0.50] |
| 1.1.3 Training and support | 3 | 332 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.70, 0.36] |
| 1.2 Caregiver mood | 8 | 638 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.22, 0.12] |
| 1.2.1 Support/information plus support | 4 | 283 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.38, 0.25] |
| 1.2.2 Training/information plus training | 1 | 23 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.80, 0.84] |
| 1.2.3 Training and support | 3 | 332 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.32, 0.19] |
| 1.3 Caregiver HRQoL | 2 | 311 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.13, 0.32] |
| 1.3.1 Training/information plus training | 1 | 61 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.62, 0.38] |
| 1.3.2 Training and support | 1 | 250 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.10, 0.40] |
| 1.4 Dropouts for any reason | 8 | 661 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.87, 1.53] |
| 1.4.1 Support/information plus support | 3 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.59, 1.73] |
| 1.4.2 Training/information plus training | 2 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.50, 4.04] |
| 1.4.3 Training and support | 3 | 359 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.75, 1.63] |
| 1.5 Caregiver knowledge and skills | 4 | 223 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.10, 0.50] |
| 1.5.1 Support/information plus support | 2 | 120 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.18, 0.54] |
| 1.5.2 Training/information plus training | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.65, 0.42] |
| 1.5.3 Training and support | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | 0.61 [0.04, 1.18] |
| 1.6 Use of health and social resources | 1 | Rate Ratio (IV, Random, 95% CI) | 1.05 [0.93, 1.19] | |
| 1.7 Institutional care ‐ nursing home placement | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.11, 3.11] |
Comparison 2. Any intervention versus control information.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Caregiver burden | 9 | 650 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.51, 0.04] |
| 2.1.1 Support/information plus support | 2 | 119 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.39, 0.33] |
| 2.1.2 Training/information plus training | 6 | 506 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.69, 0.07] |
| 2.1.3 Training and support | 1 | 25 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.90, 0.68] |
| 2.2 Caregiver mood | 11 | 1100 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.43, ‐0.06] |
| 2.2.1 Support/information plus support | 1 | 94 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.69, 0.13] |
| 2.2.2 Training/information plus training | 8 | 816 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.54, ‐0.08] |
| 2.2.3 Training and support | 2 | 190 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.24, 0.33] |
| 2.3 Caregiver HRQoL | 2 | 257 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.28, 0.21] |
| 2.4 Dropouts for any reason | 12 | 1266 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.04, 2.20] |
| 2.4.1 Support/information plus support | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.17, 7.09] |
| 2.4.2 Training/information plus training | 9 | 994 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.97, 2.33] |
| 2.4.3 Training and support | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.25, 6.22] |
| 2.5 Caregiver knowledge and skills | 2 | 70 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.29, 0.65] |
| 2.5.1 Support/information plus support | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.47, 0.81] |
| 2.5.2 Training/information plus raining | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.51, 0.88] |
| 2.6 Institutional care ‐ nursing home placement | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [0.12, 60.93] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Au 2015.
| Study characteristics | ||
| Methods |
Design: Multicentre, parallel randomised study. No. of participating centres: Two centres (United Christian Hospital; Prince of Whales Hospital; Hong Kong, China) Study dates: Not reported. |
|
| Participants |
Inclusion criteria: Informal caregivers aged 25 or older, caring for a familiar diagnosed of Alzheimer's disease for at least 3 months. Exclusion criteria: Participants who showed signs of severe intellectual deficits, demonstrated suicidal ideation, exhibited evidence of psychotic disorders, or could not read or speak fluent Chinese/Cantonese. No. of participants randomised to interventions: ‐ PsyED with BA (n = 51). ‐ PsyED only (n = 45). Baseline characteristics: ‐ Caregivers: Mean age for participants was 56.2 years (SD = 10.6). Most caregivers were females (76 of 93, 82%), spouses or children of AD patients (85 participants of 93, 91%). The mean time for caring was 10.4 hours/day (SD = 8.9). The mean CES‐D score was 13.5 (SD = 8.0). ‐ Care recipients: Mean age was 80.9 years (SD = 6.6). There were 60 females of 93 (64.5%) and 33 males of 93 (35.5%). Mean MMSE was 15.7 (SD = 5.6). |
|
| Interventions |
Experimental: Psychoeducation with telephone‐assisted behavioral activation (PsyED‐BA). All participants received a psychoeducation program via telephone for 4 weeks. Then, the experimental group received eight biweekly telephone follow‐up calls of BA practice focused on pleasant event scheduling and improving communications. Control: Psychoeducation only (PsyED). All participants received a psychoeducation program via telephone for 4 weeks. Then, the control group received eight biweekly telephone follow‐up calls of general discussion of psychoeducation and related information. Length of intervention: 4 months. |
|
| Outcomes |
Primary outcomes: ‐ Center for Epidemiologic Studies‐Depression Scale (CES‐D) (range scores 0 to 30, higher scores denote more depressive symptoms). Secondary outcomes: ‐ None reported Outcomes assessment: At 4 months. |
|
| Notes |
Clinical trial registration code: Not reported. Funding: Food and Health Bureau of the Hong Kong SAR Government (HKPolyU‐2011‐K‐JB3). Conflicts of interest: None declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Study described as randomised, however the process of randomization is not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is no information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: There is not information on blinding procedures for participants, professionals were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Research staff carrying out the assessments and interventions had no knowledge of the allocation. All assessments at the three time points were carried out by research staff not involved in executing the PsyED program or the subsequent interventions". Comment: Intervention components and procedures were comparable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Data recorded from 93 caregivers of 96 randomised, and the study provides reasons for discontinuation on treatment by arm. |
| Selective reporting (reporting bias) | Unclear risk | Comment: There is not clinical trial registration and/or available protocol. |
| Other bias | High risk | Comment: Break of randomization. Data added to a former pilot study already published. |
Au 2019.
| Study characteristics | ||
| Methods |
Design: Parallel randomised controlled trial. No. of participating centres: Two: United Christian Hospital and Prince of Wales Hospital, Hong Kong, China. Study dates: January 2015 to June 2016. |
|
| Participants |
Inclusion criteria: Primary caregivers (spouse, kin, or sibling) aged 25 or above caring for a care recipient diagnosed with mild to moderate Alzheimer's disease. Providing at least 14 hours of care per week for at least 3 months. Exclusion criteria: Presence of signs of the following conditions: severe intellectual deficits, suicidal ideation, psychotic disorders, or lack of the ability to read Chinese and speak Cantonese. No. of participants randomised to intervention: ‐ TBA (n=56). ‐ TGM (n=55). Baseline characteristics: ‐ Caregivers: Mean age was 57.1 years (SD=10.2); 90 females and 21 males. The average years of caregiving were 4 (1.8) with an average of 10.9 (7.6) hours/day spent in caregiving. Mean score of burden (22‐item ZBI) at baseline 30.9 (SD=17.2). ‐ Care recipients: 69 participants (62%) presented moderate dementia, 42 participants (38%) presented severe dementia. |
|
| Interventions |
Experimental: Telephone‐administered psycho‐education with behavioral activation intervention (TBA). Control: Telephone general monitoring. Length of intervention: Four‐month. |
|
| Outcomes |
Primary outcomes: ‐ Depressive symptoms (CES‐D). Secondary outcomes: ‐ Caregiver burden (Zarit Burden Scale). Outcomes assessment: An average of about 5 months since baseline. |
|
| Notes |
Clinical trial registration code: NCT03552159. Funding: Research Council of the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region (Health Care Promotion Fund 0310015). Conflicts of interest: None declared. Fidelity / adherence: Authors report that 60% of participants fully covered the program and 40% adequately covered the program. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocation sequence was obtained by random number generation by a staff who was not involved in enrolling/assigning participants... block randomization was used to achieve balance between the numbers of participants in both arms". |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation was concealed in sequentially numbered sealed envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "... both assessors and participants were blinded to intervention condition". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assessments were carried out by research assistants who were blind to the group allocation of participants". Comment: Intervention components and procedures were comparable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: The study presents reasons for losses to follow‐up without relevant differences between arms, also ITT analysis was done. |
| Selective reporting (reporting bias) | Low risk | Comment: Study registration available (NCT03552159), all prespecified results are reported. |
| Other bias | Low risk | Comment: No other source of bias detected. |
Blom 2015.
| Study characteristics | ||
| Methods |
Design: Parallel randomised study. No. of participant centres: Dutch Alzheimer’s Society; The Netherlands. Study dates: Between 2010 and 2012. |
|
| Participants |
Inclusion criteria: Family caregivers of people with dementia with at least some symptoms of depression or anxiety or feelings of burden (CES‐D > 4 or HADS‐A > 3 or a burden score of at least 6 on a scale ranging from 0 to 10). Exclusion criteria: Caregivers with a high level of depression or anxiety (CES‐D > 15 or HADS‐A > 7) or who had suicidal thoughts were first evaluated by an elderly care physician for evaluation and to refer those who were in need of immediate medical treatment. No. of participants randomised to interventions: ‐ Internet course Mastery over Dementia (MoD) (n = 151). ‐ Minimal intervention (digital newsletters, e‐bulletins) (n = 100). Baseline characteristics: ‐ Caregivers: Mean age for participants was 61.2 years (SD = 12.4). Most caregivers were females (170 of 245, 69%), spouses of dementia patients (143 participants of 245, 58%). The mean CES‐D score was 17.4 (SD = 9.4). ‐ Care recipients: Mean age for patients was 75.9 years (SD = 9.4). Most were females (148 of 245, 60%). The mean severity of dementia measured by IQ‐CODE was 58.9 (SD = 5.7). |
|
| Interventions |
Experimental: Internet course Mastery over Dementia (MoD). The Internet course consists of 8 lessons and a booster session with the guidance of a coach monitoring the progress of participants and evaluating the homework. Control: Digital newsletters (e‐bulletins). Caregivers received a minimal intervention consisting of digital newsletters with practical information on providing care for someone with dementia. The bulletins were sent by email according to a fixed schedule (every 3 weeks) over nearly 6 months. Lenght of intervention: 5 ‐ 6 months. |
|
| Outcomes |
Primary outcomes: ‐ Depression, assessed by the Center for Epidemiologic Studies Depression Scale (CES‐D), (scores range 0 to 30, higher scores denote more depressive symptoms). Secondary outcomes: ‐ Anxiety, assessed by the 7‐item anxiety subscale of the Hospital Anxiety and Depression Scale (HADS) (scores range 0 to 21, higher scores denote more anxiety symptoms). ‐ Role overload, assessed by the Self‐Perceived Pressure from Informal Care scale (SPPIC), (range score 0 to 9, higher scores denote higher role overload). ‐ Caregiver perceived stress, assessed by the Dutch version of the Revised Memory and Behavioral Problem Checklist (RMBPC), (mean product score ranging from 0 to 16, higher scores represent higher burden). ‐ Sense of competence, assessed by the SSCQ scale (score range 0 to 7, higher scores represent higher sense of competence). ‐ Severity of dementia (IQCODE), (score range 0 to 64, higher scores denote higher severity). Outcomes assessment: At 5 ‐ 6 months. |
|
| Notes |
Clinical trial registration code: Dutch trial register (NTR2051). Funding: Supported by the Alzheimer’s Society in the Netherlands, the health care provider Geriant and the VU University. Conflicts of interest: None declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A researcher not connected to the study used a computerized random‐number generator for block randomization with variable sizes (experimental group=3: comparison group = 2)". |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is no information on procedures to conceal random allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants did not know whether the intervention they received was the experimental or the comparison intervention. The data were all collected via the Internet with no intermediary interviewer." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The data were all collected via the Internet with no intermediary interviewer". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There are differential reasons for follow‐up between trial arms; 59 of 151 participants (39.1%) were lost to follow‐up in the intervention group, whereas 11 of 100 participants (11%) were lost to follow‐up in the control intervention. |
| Selective reporting (reporting bias) | Low risk | Comment: There is a clinical trial registration and a published protocol for the study. |
| Other bias | Low risk | Comment: None detected. |
Brennan 1995.
| Study characteristics | ||
| Methods |
Design: Parallel randomised study. No. of participating centres: One centre. Study dates: Not reported. |
|
| Participants |
Inclusion criteria: Caregivers with (i) primary responsibility as a family caregiver for a person with Alzheimer's disease who was living at home, (ii) a local telephone exchange, and (iii) the ability to read and write English. Exclusion criteria: None stated. No. of participants randomised to interventions: ‐ComputerLink (n = 51). ‐ Control (n = 51). Baseline characteristics: Caregivers: Predominantly female (67%), educated at or beyond high school (86%), with a median age of 64 years. Care recipients: Predominantly spouses (68%) or parents (28%), who had required care for an average of 34 months (SD = 26.8) at the start of the study. |
|
| Interventions |
Experimental: ComputerLink, a computer network providing information, communication, and decision‐support functions for caregivers. Control: Placebo training experience identifying local services and resources. Length of intervention: 12 months. |
|
| Outcomes |
Primary outcomes: ‐ Decision‐making confidence (modification of a previous scale). ‐ Decision‐making skills (investigator‐developed self‐report instrument). Secondary outcomes: ‐ The Instrumental and Expressive Social Support Scale (IESS). A 27‐item scale to measure the perceived adequacy of available support from the social network. ‐ Burden, assessed with the Impact of Caregiving Scale (higher scores indicate greater burden). ‐ Depression, assessed with the 20‐item Center for Epidemiological Studies Depression Scale (CES‐D). Scores range from 0 to 60, higher scores indicate a greater level of depression. ‐ Caregiver contact with community and medical services. Outcomes assessment: At 12 months. |
|
| Notes |
Clinical trial registration code: Not reported. Funding: National Institute of Aging (AG 8617). Conflicts of interest: None declared. Fidelity / adherence: Authors reported an average of 2 service contacts/week per participant. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Block randomization, blocks of 10 (information provided by authors). |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is no information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Intervention cannot be blinded to participants and/or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Self‐reported outcomes (information provided by authors). Intervention components and procedures were not comparable with participants not blind to interventions and subjective participant‐reported outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 4 participants of 51 randomised to intervention (8%) dropped out, 2 participants of 51 randomised to control intervention (4%) dropped out. |
| Selective reporting (reporting bias) | Unclear risk | Comment: There is not clinical registration and/or protocol available. |
| Other bias | Low risk | Comment: None detected. |
Cristancho‐Lacroix 2015.
| Study characteristics | ||
| Methods |
Design: Parallel randomised study. No. of participant centres:1 day care centre in Paris, France. Study dates: Between 2011 and 2014. |
|
| Participants |
Inclusion criteria: French‐speaking caregivers of community‐dwelling persons with Alzheimer’s disease (DSM criteria). Caregivers had to spend at least 4 hours per week with their relative, be aged 18 years or older, scored 12 or more on the Perceived Stress Scale (PSS‐14), and have access to a computer with Internet connection. Exclusion criteria: Professional caregivers. No. of participants randomised to interventions: ‐ Password‐protected website program (Diapason) (n = 25). ‐ Usual care (information) (n = 24). Baseline characteristics: Caregivers: Mean age for participants was 61.6 years (SD = 11.6). Most caregivers were females (32 of 49, 65%), spouses or daughters/sons of AD patients (47 participants of 49, 96%). The mean self‐perceived stress (PSS‐14) was 24.3 (SD = 7.9). Care recipients: The mean time since onset of symptoms was 4.4 years (SD = 3.3). The mean MMSE score was 18.7 (SD = 5.0). |
|
| Interventions |
Experimental: Usual care plus the Diapason program. Twelve thematic sessions were sequentially and weekly unblocked once the previous one was entirely viewed. Each session included theoretical and practical information, videos of health professionals, and a practice guide for applying the session’s content in real life. Participants could access different website sections (i.e., relaxation training, forum) for as long as they wished at any time. Intervention targeted (1) caregivers’ beliefs about illness and the caregiving role, (2) caregivers’ skills to manage daily life difficulties, and (3) caregivers’ social support and help‐seeking behavior to obtain respite or financial support, and to meet and discuss with peers through a forum. Control: Usual care. Information about the illness during their semiannual follow‐up with their geriatrician. The control group participants were given access to the Diapason program at the end of the study. Lenght of intervention: 3 months. |
|
| Outcomes |
Primary outcomes: ‐ Perceived stress of caregivers, measured by the item Perceived Stress Scale (PSS‐14), (score range 0 to 56, higher scores indicate higher stress levels). Secondary outcomes: ‐ Self‐efficacy, measured by the Revised Scale for Caregiving Self‐Efficacy (RSCS), (3 self‐efficacy domains with a score range from 0 to 100 each one, higher scores indicate a higher degree of confidence for each situation: obtaining respite, responding to disruptive behaviour, and controlling upsetting thoughts). ‐ Perception and reaction to cognitive or behavioral symptoms, evaluated with the Revised Memory and Behavior Problems Checklist (RMBPC), (score range 0 to 96, higher scores indicate greater perceived burden). ‐ Subjective burden, evaluated with the Zarit Burden Interview (ZBI), (score range 0 to 88, higher scores denote greater burden). ‐ Depressive symptoms, measured with a version of the Beck Depression Inventory (BDI‐II), (range score 0 to 63, higher scores indicate higher levels of depressive symptoms). ‐ Self‐perceived health, measured with the Nottingham Health Profile (NHP), (range score 0 to 100, lower scores indicate less QoL). Outcomes assessment: At 3 months (final assessment), and 6 months (follow‐up assessment). |
|
| Notes |
Clinical trial registration code: NCT01430286. Funding: French Health Ministry (Projet de Recherche en Qualité Hospitalière 2009‐PREQHOS 2009) and Fondation Méderic Alzheimer project grants 2012‐2014. Conflicts of interest: Some authors and researchers were involved in the development of the Diapason web program. Fidelity / adherence: Authors reported that 71% of participants completed the protocol and validated at least 10 of 12 outline sessions. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were recruited and randomised offline in 2 parallel groups based on a computer‐generated randomization list using blocking and stratification by sex and relationship (spouses vs non‐spouses)". |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is no information on procedures to conceal random allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Study described as unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Intervention components and procedures were not comparable with participants not blind to intervention and subjective participant‐reported outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 8 participants of 25 (32%) dropped out from the intervention arm, 7 participants of 24 (29.2%) dropped out from the control arm. Reasons for discontinuation on treatment reported. |
| Selective reporting (reporting bias) | Low risk | Comment: There is a clinical trial registration (NCT01430286). |
| Other bias | Low risk | Comment: None detected. |
Czaja 2013.
| Study characteristics | ||
| Methods |
Design: Parallel randomised study. No. of participating centres: Recruitment from a variety of sources (memory disorder clinics, social service agencies, churches, community centres, newspaper advertisements, and community presentations). Study dates: Not reported. |
|
| Participants |
Inclusion criteria: Caregivers 21 years or older, living or sharing cooking facilities with the patient, and having had provided care for a minimum of 4 hours/day for at least the past 6 months. Having a telephone, planning to remain in the area for at least 6 months, and competency in either English or Spanish. Dementia patients (physician diagnosis of AD or other dementia) with a MMSE score < 24 and presenting at least one limitation in activities of daily living, or two limitations in instrumental activities of daily living. Exclusion criteria: Caregiver or patient presenting an illness or disability that would prohibit participation, or if the patient had an MMSE score of 0 and was bedridden. No. of participants randomised to interventions: ‐ Technology‐based multi‐component psychosocial intervention (n = 38). ‐ Attention control (n = 36). ‐ information alone (n = 36) Baseline characteristics: Caregivers: Mean age for participants was 60.2 years (SD = 13.0). Most caregivers were females (84%), spouses, sons or daughter of patients (88.2%). On average caregivers had been providing care for 5.6 years (SD = 5.2). Care recipients: Mean age for patients was 79.5 years (SD = 9.4). Patients had average scores of about 11. |
|
| Interventions |
Experimental: Technology‐based multi‐component standardised psychosocial intervention including six 1‐hour monthly sessions, two in‐home sessions, and four sessions delivered by videophone. Control: ‐ Attention control condition: Participants received the same amount of contact as those in the intervention condition but the content was structured around nutrition and healthy eating. ‐ information alone: Participants received a packet of educational materials (basic information about dementia, caregiving, safety, and community resources) and a brief (< 15 minute) telephone "check‐in call" at 3 months post randomization. Length of intervention: 5 months. |
|
| Outcomes |
Primary outcomes: ‐ Depression, assessed with CES‐D (score range 0 to 30, higher scores denote more depressive symptoms). ‐ Burden, assessed with the Revised Memory and Behavior Problems Checklist (RMBPC), (score range 0 to 96, higher scores represent higher perceived stress). ‐ Positive aspects of caregiving (9‐item instrument; score range 0 to 36, higher scores indicate more positive feelings about caregiving). ‐ Social support (10‐item instrument; responses scored on a 4‐point scale, 0 = never to 3 = very often). Secondary outcomes: None stated. Outcomes assessment: At 5‐month. |
|
| Notes |
Clinical trial registration code: Not reported. Funding: Langeloth Foundation, Cisco Corp., AT&T, and the Administration on Aging. Conflicts of interest: None declared. Other: Results report combined data for both control groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Study described as randomised, however no information on the randomization procedure is described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Interventions cannot be blinded to participants. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "A certified assessor blind to group assignment administered the follow‐up assessment". Comment: The information‐only control group presents components and procedures not comparable to the rest of trial arms. Unmasked study with participants not blind to interventions and subjective participant‐reported outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Differential drop out rates by treatment arms. Two participants of 38 (5.3%) dropped out from the intervention arm, 2 participants of 36 (5.5%) dropped out from the attention control arm, but 7 participants of 36 (19.4%) dropped out from the control intervention. |
| Selective reporting (reporting bias) | Unclear risk | Comment: There is not clinical trial registration and/or available protocol. |
| Other bias | Low risk | Comment: None detected. |
Davis 2004.
| Study characteristics | ||
| Methods |
Design: Multicentric parallel randomised study. No. of participating centres: Geriatric clinics and home care agencies in central Alabama, USA. Study dates: Not reported. |
|
| Participants |
Inclusion criteria: Caregivers living with the care recipients, having primary caregiving responsibility for an average of at least 4 hours/day, be able to speak and read English, available by phone, and no participating in any other caregiver study. AD patients diagnosed by physicians, aged 50 years or older, with a MMSE score of 24 or less, and presenting 4 or more behavior problems (RMBPC). Exclusion criteria: Caregiver or care recipient hospitalised for 2 or more weeks during the 24‐week study period, and patients presenting also DSM‐IV diagnosis of alcoholism or schizophrenia. No. of participants randomised to interventions: ‐ Telephone training (n = 23). ‐ In‐home training (n = 24). ‐ Friendly calls (n = 24). Baseline characteristics: Caregivers: Mean age for participants was 67.1 years (SD = 13.6). Most caregivers were females, spouses, sons or daughter of AD patients. The mean caregiving burden (SCB‐A) was 35.8 (SD = 12.4). Care recipients: Mean age for patients was 74.1 years (SD = 11.8) |
|
| Interventions |
Experimental: ‐ Telephone training included standardised skill training through 12 weekly telephone contacts. ‐ In‐home training included standardised skill training through 12 weekly in‐home contacts. Control: 12 weekly friendly (socially supportive) phone calls. Length of intervention: 12 weeks. |
|
| Outcomes |
Primary outcomes: ‐ Caregiver burden (SCB‐A), with higher scores denoting greater burden. ‐ Caregiver distress (SCB‐B), with higher scores denoting greater stress/burden. ‐ Caregiver depression, assessed by the Geriatric Depression Rating Scale (GDRS), (score range 0 to 30, higher scores indicate more depressive symptoms). ‐ Caregiver social support (ISEL), (range score 0 to 30, higher scores indicate more perceived social support). ‐ Caregiver life satisfaction (LSI‐Z), with higher scores denoting more life satisfaction. Secondary outcomes: None stated. Outcomes assessment: At 12 weeks (final assessment), and at 24 weeks (follow‐up assessment). |
|
| Notes |
Clinical trial registration code: Not reported. Funding: National Institute of Nursing Research (K01 NR 00095), and REACH Project (5U01NR 04261). Conflicts of interest: None declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Study described as randomised but there is not information on the randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is no information on procedures to conceal random allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Interventions were not blinded to participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Intervention components and procedures were comparable between trial arms, and data collection was independent of the intervention staff, however by the time of follow up assessments it is possible that the data collectors of subjective participant‐reported outcomes knew the group assignment of the caregiver dyad. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Comparisons of drop‐outs between groups were relevant (In home‐training 25%, Telephone training 56,52, and Friendly calls 33,33%). |
| Selective reporting (reporting bias) | High risk | Comment: There is no clinical trial register identification and/or available protocol, however only significant outcome variables from those recorded in the methods section are reported. |
| Other bias | Low risk | Comment: None detected. |
Dowling 2014.
| Study characteristics | ||
| Methods |
Design: Parallel randomised controlled trial. No. of participating centres: One, the UCSF Memory and Aging Center. Study dates: Recruitment between July 2011 and March 2012. |
|
| Participants |
Inclusion criteria: Caregivers needed to reside with and be the primary care provider for a family member with frontotemporal dementia (FTD), speak and read English, have Internet access at speeds consistent with video communication. Exclusion criteria: Caregivers with evidence of severe cognitive impairment or active psychosis. No. of participants randomised to interventions: ‐ Life Enhancing Activities for Family Caregivers (LEAF), (n = 12). ‐ Attention control (n = 12). Baseline characteristics: Caregivers: Mean age for participants was 59.6 years (SD = 8.1). Most caregivers were female (69%), and spousal caregivers (87%). The length of time as a caregiver was 4.2 years (SD = 2.1), The average burden assessed with the ZBI was 47.1 (SD = 13.5). Care recipients: Persons diagnosed with FTD. |
|
| Interventions |
Experimental: LEAF program delivered across 5 skill‐building sessions with one‐on‐one in‐person sessions with the facilitator (n = 1), or with one‐on‐one Internet video‐conference with the facilitator (n = 11). Control: Five one‐on‐one sessions with the facilitator. The sessions were comparable in length to the intervention sessions but consisted of an interview and did not have any didactic portion of skills practice. Length of intervention: 5 weeks. |
|
| Outcomes |
Primary outcomes: ‐ Positive and negative affect, assessed with the 20‐item version of the Differential Emotions Scale (DES). ‐ Depresive mood, assessed with the 20‐item Center for Epidemiologic Studies Depression Scales (CES‐D), (range score 0 to 60, higher scores indicate more depressive symptoms). ‐ Caregiver stress, assessed with the 10‐item Perceived Stress Scale (PSS), (scores range from 0 to 40, higher scores indicate greater stress). ‐ Caregiver distress, assessed with the Neuropsychiatric Inventory (NPI), (higher scores indicate greater caregiver distress). ‐ Caregiver burden, assessed with the 22‐item Zarit Burden Interview (ZBI), (scores range from 0 to 88, higher scores denote higher burden). Secondary outcomes: None stated. Outcomes assessment: At 6 weeks (final assessment), and at 10 weeks (follow‐up assessment). |
|
| Notes |
Clinical trial registration code: NCT01825681. Funding: Grant from the Atlantic Philanthropies Foundation. Conflicts of interest: None declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomised to the intervention or control conditions determined by a computer‐generated random number sequence." |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is no information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Participants and personnel were not blinded to interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Intervention components and procedures were comparable between study arms, however this is an unmasked study with participants not blind to interventions and subjective participant‐reported outcomes. Moreover, intervention facilitators were not blind to intervention condition. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Overall, 4 participants of 24 (16.7%) were lost to follow up. |
| Selective reporting (reporting bias) | Low risk | Comment: Clinical trial registration NCT01825681 |
| Other bias | Low risk | Comment: None detected. |
Duggleby 2018.
| Study characteristics | ||
| Methods |
Design: Randomised parallel trial. No. of participating centres: Multicenter. Study dates: June 2015 to April 2017. |
|
| Participants |
Inclusion criteria: Carers (18+ years) of community‐living care recipients with Alzheimer's disease and related dementia (ADRD) and two or more chronic conditions, and 65+ years. Carers were all English speaking and either a family or fried of the care recipient, with access to a computer and a valid email address Exclusion criteria: Non‐English speaking carers; care recipient under the age of 65 years, who did not have ADRD and chronic conditions, or who was not a community‐living care recipient. No. of participants randomised to interventions: ‐ My Tools 4 Care (MT4C) (n=101). ‐ Educational control group as usual care (n=98). Baseline characteristics: Caregivers: Mean age was 63.6 years (SD=11.6), 81% were female (161 of 199), most were spouse or partners (189 of 199, 95%), with a mean duration of care of 4.3 years (SD=4.2). Care recipients: Mean age was 80.3 years (SD=7.7), most were males (104 of 199, 52%). |
|
| Interventions |
Experimental: MT4C is a Web‐based intervention intended to support carers . Control: Educational group Length of intervention: 3 months. |
|
| Outcomes |
Primary outcomes: ‐ mental component summary score (MCS) from the SF‐12v2. Scores range from 0 to 100, with higher scores indicating a better health‐related quality of life. Secondary outcomes: ‐ physical component summary score (PCS) from the SF‐12v2. Scores range from 0 to 100, with higher scores indicating a better health‐related quality of life. ‐ General Self‐Efficacy Scale (GSES). Total scores range from 10 to 40, with higher scores indicating a greater level of self‐efficacy. ‐ Herth Hope Index (HHI). Total scores range from 12 to 48, with higher scores indicating a greater hope. ‐ Health and Social Services Utilization Inventory (HSSUI). Outcomes assessment: At 3 and 6 months after baseline. |
|
| Notes |
Clinical trial registration code: NCT02428387. Funding: Canadian Institutes of Health Research (TTF 128261). Conflicts of interest: No competing interests declared. Fidelity / adherence: Authors report that 73% of caregivers used the intervention at least once over the 3‐month intervention period. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A biostatistician...generated group allocations using stratified permuted block randomization". |
| Allocation concealment (selection bias) | Low risk | Quote: "Random number sequences were fed... into RedCap, a secure, password‐protected Web‐based randomization service... which allocated clients to the two groups according to a random sequence". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Given the nature of the study, the research team was unblinded to group allocation". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Data collection forms were emailed to participants following the first interview. However, outcomes were participant‐reported and intervention components and procedures were not comparable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: intention‐to‐treat principles were used in all analyses. However, randomised groups presented differential attrition; at 3‐month 23 participants of 101 (23%) were lost to follow‐up in the intervention group whereas 9 participants of 98 (9%) were lost in the comparison group. Rates at 6‐month follow‐up were 27% (28/101) and 17% (17/98) respectively. |
| Selective reporting (reporting bias) | Low risk | Comment: study is registered (NCT02428387) and protocol published (Duggleby 2017). Reported outcomes agree with those described in registry and protocol. |
| Other bias | Low risk | Comment: none detected. |
Gant 2007.
| Study characteristics | ||
| Methods |
Design: Parallel group randomised study. No. of participant centres: One. Missouri, USA. Study dates: Not reported. |
|
| Participants |
Inclusion criteria: Primary caregivers (men) for a family member diagnosed with dementia; living in same residence as care recipient; caregiver between ages of 30 and 85; no plans to place the family member in a nursing home or hospital services within the next 6 months; visual acuity sufficient to read a book; auditory ability to hear voice on the telephone and TV; access to telephone, TV, and VCR; an established relationship with a clinic or primary care physician with whom caregiver had visited within the last year; at least 2 care recipient memory or behavior problems that occurred in the past week, and at least a moderate level of distress (upset or annoyance) following 2 or more care recipient behaviours. Exclusion criteria: Current alcohol abuse or serious suicidal ideation; history of suicide attempt; no current involvement in another caregiver intervention project. No. of participants randomised to interventions: ‐ Video intervention (video/workbook/telephone coaching condition) (n = 17). ‐ Educational booklet, check‐in‐call condition (n = 15). Baseline characteristics: Caregivers: Mean age for participants was 71.6 years (SD = 9.7). All caregivers were men and most were spouse (88%). Care recipients: Mean age for patients with dementia was 76.1 years (SD = 7.6). |
|
| Interventions |
Experimental (video/workbook/telephone coaching condition): Participants received a 10‐session video series, a workbook adapted for men from the Dementia Caregiving Skills Program, and 12 weekly telephone calls from a trained coach. Control (education/check‐in‐call condition): Participants received by mail a 37‐page booklet, Basic Dementia Care Guide, which included information on dementia and suggestions for dealing with a variety of caregiving challenges. Caregivers then received approximately 7 biweekly telephone calls by a trained staff member. Length of intervention: 12 weeks. |
|
| Outcomes |
Primary outcomes: ‐ Memory and behavior problems, assessed by the RMBPC scale (score range 0 to 96, higher scores reflect higher levels of perceived caregiver burden). ‐ Self‐efficacy, assessed by the 15‐item Revised Scale for Caregiving Self‐ Efficacy (score range 0 to 100, higher scores denote more self‐efficacy). ‐ Positive and negative emotions of the caregiver, assessed by the Positive and Negative Affect Scale (score range 10 to 50, higher scores denote either more positive or negative emotions). ‐ target complaints interview (3 most upset situations assessed with a Likert scale (1: slightly or not at all, 5: extremely). Secondary outcomes: None stated for the caregiver. Outcomes assessment: At 12 weeks. |
|
| Notes |
Clinical trial registration code: Not reported. Funding: Grants from the Missouri Alzheimer’s Disease and Related Disorders Research Board, and the National Institute of Mental Health. Conflicts of interest: None declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Use of an online random number generator, Research Randomizer available at randomizer.org (information provided by authors). |
| Allocation concealment (selection bias) | Low risk | Comment: The randomization sequence was concealed for project coordinator (information provided by authors). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Participants and personnel were not blinded to interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Assessment interviews were conducted by the trained project staff who were blind to condition". Comment: However, intervention components and procedures were not comparable wit participants not blind to intervention and subjective participant‐reported outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 32 randomised caregivers, 4 did not complete the program; all 4 of these withdrawals were from the video group. Reasons for withdrawal included insufficient interest and time (n = 3), as well as nursing home placement of the care recipient (n = 1). For the purposes of outcome analyses, an intent‐to‐treat approach (pre‐intervention scores carried forward) was used to handle the missing data." |
| Selective reporting (reporting bias) | Unclear risk | Comment: There is no available protocol or clinical trial registry. |
| Other bias | Low risk | Comment: None detected. |
Gustafson 2019.
| Study characteristics | ||
| Methods |
Design: Parallel randomised clinical trial. No. of participating centres: One. Wisconsin, USA. Study dates: Not reported. |
|
| Participants |
Inclusion criteria: Primary caregivers for a patient with dementia, providing care in the patient's home, and able to read English. Exclusion criteria: No stated. No. of participants randomised to intervention: ‐ D‐CHESS (n=16). ‐ Control group (n=15). Baseline characteristics: ‐ Caregivers: 19 females and 12 males. ‐ Care recipients: Mean Clinical Dementia Rating score 1.55 (SD=0.93), range from 0.5 (very mild dementia) to 3.0 (severe dementia). |
|
| Interventions |
Experimental: Dementia‐Comprehensive Health Enhancement Support System (D‐CHESS). Control: Emailed informative book without any further training. Length of intervention: 6 months. |
|
| Outcomes |
Primary outcomes: ‐ Caregiver burden assessed with a 6‐item caregiver load scale. ‐ Family conflict assessed with a 12‐item adapted scale. ‐ Satisfaction with care decisions assessed with the 6‐item Satisfaction with Decision Scale. ‐ Social support assessed with the 14‐item MOS Social Support Survey. ‐ Anxiety assessed with the 7‐item Generalised Anxiety Disorder scale. ‐ Depression assessed with the 8‐item Patient Health Questionnaire. ‐ Loneliness assessed with the 20‐item UCLA Loneliness Scale. ‐ Coping competence assessed with an ad‐hoc 9‐item scale. Secondary outcomes: ‐ Not stated. Outcomes assessment: At 6 months. |
|
| Notes |
Clinical trial registration code: Not reported. Funding: Internal funds; University of Wisconsin‐Madison. Conflicts of interest: Authors state nothing to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The randomization procedure is not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is no information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: The D‐CHESS intervention group received access to the website and any of three commercially available sensors (a Bluetooth tracker, a GPS location tracker, and a motion sensor). It makes unlikely to mask interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Intervention components and procedures were not comparable in an unmasked study with participants not blind to interventions and subjective participant‐reported outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "... one participant in the control group was a significant outlier on five of the eight outcome variables; this participant was removed from all analyses." |
| Selective reporting (reporting bias) | Low risk | Comment: There is no available protocol or clinical trial registration number, however the study reports all outcomes described in its method section independently of their significance. |
| Other bias | Low risk | Comment: None detected. |
Hattink 2015.
| Study characteristics | ||
| Methods |
Design: Parallel group randomised trial. No. of participating centres: Multinational study (Netherlands, UK). Study dates: May 2013 to March 2014. |
|
| Participants |
Inclusion criteria: Informal caregivers, volunteers, and professional caregivers of a person with dementia, who were sufficiently computer literate to utilize the STAR website. Exclusion criteria: Not reported. No. of participants randomised to interventions: ‐ STAR intervention (n = 27 lay people ‐ informal caregivers plus volunteers). ‐ Wait‐list control group (n = 32). Baseline characteristics: Caregivers: Mean age for participants was 53.9 years (SD = 13), most were female (71%), 63% of participants were caring 2 or more years. Care recipients: The study does not provide information. |
|
| Interventions |
Experimental: E‐learning tool: European Skills Training and Reskilling (STAR) project. Control: No intervention but free access to STAR after research ended. Lenght of intervention: 2 to 4 months. |
|
| Outcomes |
Primary outcomes: ‐ Knowledge on dementia assessed with the 30‐item Alzheimer's Disease Knowledge Scale (ADKS). ‐ Attitudes toward dementia assessed with 2 questions from the Alzheimer's disease survey. ‐ Approaches to dementia assessed with the Approaches to Dementia Questionnaire. ‐ Usefulness and user friendliness assessed with an ad hoc 29‐item questionnaire. Secondary outcomes: ‐ Empathy assessed with the 28‐item Interpersonal Reactivity Index. ‐ Quality of life assessed with 2 distinct questions ‐ only administered to informal caregivers. ‐ Burden assessed with 1 question ‐ only administered to informal caregivers. ‐ Sense of competence assessed with the 7‐item Short Sense of Competence Questionnaire ‐ only administered to informal caregivers. Outcomes assessment: 2 to 4 months. |
|
| Notes |
Clinical trial registration code: Not reported. Funding: European Union Leonardo da Vinci Life Long Learning Programme (no. 510364‐2010) and the BAVO Foundation in the Netherlands. Conflicts of interest: None declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Interventions were randomly assigned by strata using randomization software. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is no information to judge allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Interventions could not be blinded. In fact, participants randomly allocated to the STAR intervention were invited to take part in a Facebook community. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Outcome assessments were based on standardized self‐assessed questionnaires delivered online but filled with participants unblinded to interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: With a high total dropouts at post‐test (39%), reasons are recorded and no relevant differences appeared between completers and dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes described are reported. |
| Other bias | Unclear risk | Comment: The study reports data grouped for informal caregivers and volunteers together as lay people because of the relatively small number of volunteers. |
Hayden 2012.
| Study characteristics | ||
| Methods |
Design: Randomised parallel trial. No. of participating centres: One. Study dates: 8/2007 to 6/2012. |
|
| Participants |
Inclusion criteria: Patient with a diagnosis of Alzheimer's disease under current treatment, living in community and ambulatory, with age 50‐95 years. Caregiver age 18‐90 years; patient and caregiver residing within 2 hours of Los Angeles; home Internet access. Exclusion criteria: Patient living in residential setting; acute illness or chronic disease in patient or caregiver; patient or caregiver plans to leave area within the year. No. of participants randomised to interventions: ‐ Caregiver website support (n = 29). ‐ Caregiver brief supportive phone calls (n = 24). Baseline characteristics: Caregivers: Mean age for participants was 67.4 years (SD = 11.6). Most caregivers were females (81%), partners or spouses of AD patients (60%). Care recipients: Mean age was 81.1 years (SD = 7.4). |
|
| Interventions |
Experimental: Caregiver website support (customary care plus access to an intensive, interactive online education and support website information for 6 months). Control: Caregiver brief supportive phone calls (customary care plus monthly brief telephone calls with project staff). Length of intervention: 6 months. |
|
| Outcomes |
Primary outcomes: ‐ Caregiver burden assessed with the 12‐item Zarit Short Burden Scale. Range of scores from 12 to 60; higher scores mean greater caregiver burden. ‐ Patient problematic behavioral patterns assessed with the 24‐item Revised Memory and Behavior Problem Checklist (RMPBPC). Range of scores from 0 to 96; higher scores mean greater frequency of problematic behaviours. ‐ Caregiver negative reactions to problematic behavioral patterns assessed with the Negative Reactions Scale from the RMBPC. Range of scores from 0 to 96; higher scores mean greater degree of distress. ‐ Caregiver depression assessed with the 21‐item Beck Depression Inventory. Range of scores from 0 to 63; higher scores mean increased endorsement of depressive symptoms. Secondary outcomes: ‐ Patient medication adherence. ‐ Number of participants placed in assisted living or nursing homes 12 months from baseline. Outcomes assessment: At 6‐ and 12‐month post‐randomisation. |
|
| Notes |
Clinical trial registration code: NCT00416078. Funding: Veteran's Affairs Health Services Research and Development (Grant IIR 05‐107). Conflicts of interest: None declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Study described as randomised but without information on the randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is no information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Interventions cannot be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: There is no information on outcome assessment, but intervention components and procedures are not comparable with subjective participant‐reported outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There is a high attrition rate but similar between arms. 11 of 29 participants (38%) dropped out from the experimental arm, 8 of 24 participants (33%) dropped out from the control arm. |
| Selective reporting (reporting bias) | Low risk | Comment: Trial registered at ClinicalTrials.gov (NCT00416078). |
| Other bias | Low risk | Comment: None detected. |
Huis in het Veld 2020.
| Study characteristics | ||
| Methods |
Design: Parallel randomised controlled trial. No. of participating centres: One. VU University Medical Center, Amsterdam, Netherlands. Study dates: March 2017 ‐ August 2017. |
|
| Participants |
Inclusion criteria: Caregiver aged at least 18 years and relative of a person with dementia living at home. Must have regular contact with the care recipient (at least once a week) and access to the Internet, as well as be able to read and write Dutch. Exclusion criteria: Not stated. No. of participants randomised to intervention: ‐ Major self‐management support intervention (n=27). ‐ Medium self‐management support intervention (n=27). ‐ Minor self‐management support intervention (n=27). Baseline characteristics: ‐ Caregivers: Mean age 56.5 years (SD=12.5), primarily female (71 of 81, 88%). ‐ Care recipients: Mean age 75.1 (SD=9.9), more often male (42 of 81, 52%), with Alzheimer's disease being the most prevalent for of dementia (47 of 81, 57%). |
|
| Interventions |
Experimental: Three personal e‐mail contacts with a nurse specialised in dementia care plus six online videos, and six electronic bulletins (e‐bulletins). Control: ‐ Medium intervention: Six online videos and six e‐bulletins. ‐ Minor intervention: Only six e‐bulletins. Length of intervention: 12 weeks. |
|
| Outcomes |
Primary outcomes: ‐ Self‐efficacy measured with the 32‐item Trust in Own Abilities (TOA). Secondary outcomes: ‐ Presence and number of behaviours and mood problems of the person with dementia assessed with the Revised Memory and Behavioral Problem Checklist (RMBPC). ‐ Impact of providing family care assessed with the 11‐item Dyadic Relationship Scale (DRS). Outcomes assessment: At 12 weeks. |
|
| Notes |
Clinical trial registration code: NL6090 (old code NTR6237). Funding: Netherlands Organization for Health Research and Development. Conflicts of interest: None declared. Other: Results extracted for the comparison major intervention (support) versus medium intervention (information). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Block randomization with block sizes of 6 and 9 to achieve balance in the allocation of participants to intervention arms. |
| Allocation concealment (selection bias) | Low risk | Comment: Once randomised, participants received and e‐mail communication from the principal investigator (who was not related with the randomization procedure) containing elements of the intervention arm. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Participant and researcher blinding is not possible due to the sort of e‐health intervention they are receiving. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: Intervention components and procedures were comparable and subjective participant‐reported outcomes were administered through the Internet. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Attrition rate at 12 weeks (as assessed by returning outcome questionnaires) is relatively low (15 participants of 81, 18.5%). |
| Selective reporting (reporting bias) | Low risk | Comment: There is a trial registration and trial protocol. All outcomes prespecified in the protocol are reported. |
| Other bias | Low risk | Comment: None detected. |
Kajiyama 2013.
| Study characteristics | ||
| Methods |
Design: Parallel group randomised study. No. of participant centres: One, in Stanford; USA. Study dates:From May 2011 to January 2012. |
|
| Participants |
Inclusion criteria: Caregivers at least 21 years of age or older; caring for an individual with a clinical diagnosis of dementia; and with access to the Internet on any type of computer or had access to a DVD player. Exclusion criteria: Caregivers that scored > 30 on the CES‐D scale; and if they were engaged in caregiving activities for less than an average of 8 hours/week. No. of participants randomised to interventions: ‐ iCare protocol (ICC) (n = 75). ‐ Education/Information‐Only Condition (EOC) (n = 75). Baseline characteristics: Caregivers: Mean age for participants was 56.1 years (SD = 11.4). Most were female (83%) , spouse, partner or child of the patient (89%). The mean number of hours of care was 66.9 hours per week (SD = 40.6). The mean score for CES‐D was 15.9 (SD = 8.0). Care recipients: Mean age for patients was 77.8 years (SD = 9,9). |
|
| Interventions |
Experimental: iCare Condition (ICC), online iCare Stress Management e‐Training Program developed from the "Coping with Caregiving" program. Control: Education/Information‐Only Condition (EOC). Participants were exposed to a website containing similar navigational features, but with content focused on information about dementia. In addition, links to video‐taped information were provided. Written materials from various health agencies were also provided in a booklet format. Thus, extensive information using ‘state‐of‐the‐art’ media strategies was made available to these participants, but without a format designed to enhance the development of specific skills to deal with stress from caregiving. Lenght of intervention: 3 months. |
|
| Outcomes |
Primary outcomes: ‐ Caregiver stress, assessed by the 10‐item Perceived Stress Scale (PSS) (range scores 0 to 40, higher scores denote more perceived stress. Secondary outcomes: ‐ Caregiver level of bother due to disruptive behaviours, assessed by the Revised Memory and Behavior Problems Checklist (RMBPC) (range scores 0 to 96, higher scores denote more perceived stress). ‐ Caregiver level of depression, assessed by the Center for Epidemiologic Studies Depression Scale (CES‐D) (range scores 0 to 30, higher scores denote more depressive symptoms). ‐ Quality of life, assessed by the Perceived quality of life (PQoL). Outcomes assessment: At 3 months. |
|
| Notes |
Clinical trial registration code: NCT01378195. Funding: National Institute on Aging (Award No. R44AG032762). Conflicts of interest: None declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Study described as randomised but there is no information on the randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is no information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Intervention cannot be blinded to participants and/or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Outcome assessment was done by self‐report questionnaires, but Intervention components and procedures were not comparable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: High level of attrition and also differential. 29 participants of 75 randomised to intervention (38.7%) were lost to follow up, 18 participants of 75 randomised to control intervention (24%) were lost to follow up. |
| Selective reporting (reporting bias) | Low risk | Comment: This is a registered trial (NCT01378195). Article include the outcomes recorded in the trial registration. |
| Other bias | Low risk | Comment: None detected. |
Kwok 2013.
| Study characteristics | ||
| Methods |
Design: Parallel group randomised study. No. of participant centres: One, Hong Kong, China. Study dates: Recruitment between February 2011 and March 2012. |
|
| Participants |
Inclusion criteria: Caregivers of care recipients having clinical diagnosis of dementia of any stage, and being the primary caregivers. Exclusion criteria: Caregivers under the age of 18 years and those exhibiting intellectual impairment. No. of participants randomised to interventions: ‐ Telephone‐delivered psychoeducation group (n = 20). ‐ Control group (n = 22). Baseline characteristics: Caregivers: Most caregivers were female (71%), and spouse or child of patients (89%), 60% of caregivers presented an age between 31 and 50 years. Care recipients: Most patients were female (74%). |
|
| Interventions |
Experimental: A 12‐session psychoeducation program delivered by registered social workers over the telephone (approximately 30 minutes per session, one session per week). Control: DVD with educational information about dementia caregiving. Lenght of intervention: 12 weeks. |
|
| Outcomes |
Primary outcomes: ‐ Caregiver burden, measured by the Zarit Burden Interview (ZBI), (score range 0 to 88, higher scores indicate greater burden). ‐ Caregiver self‐efficacy, measured by the Revised Scale for Caregiving Self‐efficacy, (continuous scale from 0% to 100%, higher scores indicated greater sense of self‐efficacy). Secondary outcomes: ‐ Care recipients stage of dementia, indexed by the Global Deterioration Scale, (7 stages from 1 = no cognitive decline, to 7 = severe dementia). ‐ Care recipients cognitive functioning, assessed by the Abbreviated Mental Test. ‐ Behavioral problems of care recipients, measured by the participants using the Cohen–Mansfeld Agitation Inventory (scores range 29 to 203, higher scores indicate greater agitated behaviours). Outcomes assessment: At 12 weeks. |
|
| Notes |
Clinical trial registration code: Not reported. Funding: None declared. Conflicts of interest: None declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The participants were randomly assigned to either a psychoeducation group (intervention group) or a control group using a computerized randomization program." |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is no information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Interventions were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Pretests were carried out by a research assistant or by self‐administration of the caregivers. Post‐tests were administered to the participants approximately 3 months after pretest by a research assistant blind to the group assignment of the participants." Comment: Intervention components and procedures were not comparable throughout the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Ther is a low and non differential attrition: two participants of 20 (10%) dropped out from the intervention group, 2 participants of 22 (9.1%) dropped out from the control group. |
| Selective reporting (reporting bias) | Unclear risk | Comment: There is no trial registration and/or available protocol. |
| Other bias | Low risk | Comment: None detected. |
Mahoney 2003.
| Study characteristics | ||
| Methods |
Design: Parallel group randomised study. No. of participant centres: One, in Boston; USA. Study dates: Participants were recruited over a 22‐month period between 1997 and 1999. |
|
| Participants |
Inclusion criteria: Caregivers over 21 years of age, providing 4 or more hours/day of assistance or supervision for a minimum of 6 months to a family member with AD who had at least two impairments of instrumental activities of daily living (e.g., driving, shopping, or managing money) or one activity of daily living (e.g., toileting, bathing, eating), and exhibited at least one AD‐related disturbing behavior. Caregivers were required to have touch‐tone telephone service, and a touch‐tone phone was provided if needed. Exclusion criteria: Caregivers with plans to institutionalise the family member within 6 months, were participating in another clinical intervention study for caregivers, were terminally ill, or refused consent. No. of participants randomised to interventions: ‐ Resources to Enhance Alzheimer's Caregiver Health for Telephone‐Linked Care (REACH for TLC) (n = 49). ‐ Usual care (n = 51). Baseline characteristics: Caregivers: Mean age for participants was 62.6 years (SD = 12.7). Most were female (78%), and spouses, child or sibling of the care recipient (97%). Care recipients: Mean age for patients was 77.5 years (SD = 8.5); 48% were female. The mean MMSE score was 11.4 (SD = 8.3). |
|
| Interventions |
Experimental (REACH for TLC): A multisystem telecommunications based approach to help caregivers manage Alzheimer’s related disturbing behaviours. They received yearlong access to an IVR‐mediated system. The system provided caregiver stress monitoring and counselling information, personal voice‐mail linkage to AD experts, a voice‐mail telephone support group, and a distraction call for care recipients. Control: Usual care group. Length of intervention: 12 month access to the IVR‐mediated system; 18 month follow‐up. |
|
| Outcomes |
Primary outcomes: ‐ Caregiver bother, measured by the RMBPC scale (score range 0 to 96, higher scores denote greater perceived burden). ‐ Caregiver anxiety, measured by the State Anxiety Inventory (STAI), (10‐item with response options from 1 = not at all to 4 = very much, with 4 items scaled negatively; higher scores denote greater anxiety). ‐ Caregiver depression, assessed with the CES‐D scale (range score 0 to 60, higher scores indicate more depressive symptoms). Secondary outcomes: None stated. Outcomes assessment: At 6 (intermediate assessment), 12 (final assessment), and 18 months (follow‐up assessment). |
|
| Notes |
Clinical trial registration code: Not reported. Funding: Supported by the National Institutes of Health (NIH) National Institute on Aging under Research Grant AG13255‐01‐06. This study was part of the NIH‐sponsored Resources to Enhance Alzheimer’s Caregiver Health (REACH) multisite collaborative project, 1995–2001. Conflicts of interest: None declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Computer‐generated random assignment. |
| Allocation concealment (selection bias) | Low risk | Quote: "After the completion of the baseline data, the interviewer opened an envelope that contained the group assignment." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Interventions cannot be blinded to participants and/or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Research assistants were not blind to interventions and intervention components and procedures were not comparable with subjective participant‐reported outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There are high number of drop‐outs for relevant outcomes (>50%) in both groups at 18 months. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes and comparisons reported in the REACH protocol are presented. |
| Other bias | Low risk | Comment: None detected. |
Martindale‐Adams 2013.
| Study characteristics | ||
| Methods |
Design: Parallel randomised study. No. of participating centres: One, Veterans Affairs Medical Center, Memphis, Tennessee, USA. Study dates: October 2004 to September 2007. |
|
| Participants |
Inclusion criteria: Caregivers reporting stress or difficulty with care, living with care recipient, and providing 4 or more hours/day of supervision or care for at least 6 months. Care recipients had a dementia diagnosis or MMSE score of 23 or less and at least 1 ADL or 2 IL limitations. One member of the dyad had to be a veteran receiving services at VAMC Memphis. Exclusion criteria: Planned nursing home admission within 6 months. No. of participants randomised to interventions: ‐ Telephone Support for Dementia Caregivers (n = 77). ‐ information alone (n = 77). Baseline characteristics: Caregivers: Mean age for participants was 65.6 years (SD = 12.4). Most caregivers were female (83.7%). The mean length of time caregiving was 48.9 months (SD = 38.5). The mean caregiving hours on duty was 16.4 (SD = 8.6), and the mean caregiving time was 6.5 hours/day (SD = 4.9). The mean score for burden was 17.05 (SD = 8.7). Care recipients: Mean age for patents was 77.4 years (SD = 7.4), and most were male (87.6%). The mean MMSE score was 15.4 (SD = 7.5). |
|
| Interventions |
Experimental: Telephone Support for Dementia Caregivers included 12 individual in‐home and telephone sessions and 5 telephone support group sessions. The support groups met bi‐weekly for 2 months and monthly thereafter for 1 year, for a total of 14 hour‐long sessions. The sessions were semi‐structured telephone calls with education, skills‐building, and support. Control: Control caregivers received pamphlets on dementia and safety and information for local resources. Length of intervention: 12 months. |
|
| Outcomes |
Primary outcomes: ‐ General health, assessed with one question from the SF‐36. ‐ Caregiver burden, assessed with the ZBI scale (12‐item), (score range 0 to 48, higher scores denote greater burden). ‐ Depression, assessed by the CES‐D scale, (score range 0 to 30, higher scores denote more depressive symptoms). ‐ Overall mental health, assessed by the General Well‐Being Scale 22‐item (scoring is 1 = definitely true, all of the time to 5 = definitely false, not at all; higher scores indicate greater well‐being). ‐ Bother, assessed with the RMBPC scale (score range 0 to 96, higher scores represent higher perceived burden). ‐ Care recipient health, assessed with one question from the SF‐36 (1 = poor to 5 = excellent). Secondary outcomes: None stated. Outcomes assessment: At 6 (intermediate assessment), and 12 months (final assessment). |
|
| Notes |
Clinical trial registration code: NCT00119561. Funding: Veterans Health Administration, Health Services Research and Development Service (IIR‐03‐287‐1), US Department of Veteran Affairs; and Memphis Veterans Affairs Medical Center. Conflicts of interest: None declared. Fidelity / adherence: 61% of participants took part in at least 75% of the support group sessions of the program. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Study described as randomised but there is no information on the randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is no information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Interventions cannot be blinded. Study described as open trial in the protocol. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Intervention components and procedures were not comparable in an unmasked study with participants not blind to interventions and subjective participant‐reported outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There is a low attrition rate and similar between groups: 8 participants of 77 (10.4%) were lost to follow up in the intervention group, 7 participants of 77 (9.1%) were lost to follow up in the control group. |
| Selective reporting (reporting bias) | Low risk | Comment: Clinical Trials Identifier: NCT00119561. |
| Other bias | Low risk | Comment: None detected. |
Metcalfe 2019.
| Study characteristics | ||
| Methods |
Design: Parallel randomised controlled trial. No. of participating centres: International multicenter trial including three centres: Klinikum rechts der Isar, München, Germany; Université Pierre et Marie Curie, Paris, France; Bradford School of Dementia Studies, Bradford; UK. Study dates: First enrolment in the year 2016. |
|
| Participants |
Inclusion criteria: Primary informal carers of patients with dementia in Alzheimer's disease or behavioural‐variant frontotemporal dementia and an onset before the age of 65 years; age over 18 years; basic computer literacy and access to computer and the Internet. Exclusion criteria: Carers of people with other dementia. No. of participants randomised to intervention: ‐ RHAPSODY programme immediate access (n=30). ‐ Wait‐list control (n=30). Baseline characteristics: ‐ Caregivers: Mean age 57.4 years (SD=10.1), 61% females, mean caregiving years 2.2 (SD=1.5). ‐ Care recipients: Mean age 61.7 years (SD=4.9), 49% females. |
|
| Interventions |
Experimental: Web‐based e‐learning programme (RHAPSODY). Control: Standard treatment (usually one single counselling session), wait‐list control group. Length of intervention: 6 weeks. |
|
| Outcomes |
Primary outcomes: ‐ Self‐efficacy assessed with the Revised Scale for Caregiving Self‐Efficacy (RSCSE). ‐ Disease‐related knowledge assessed with the Coping with the Caregiving Role Questionnaire, self‐designed. Secondary outcomes: ‐ Stress assessed with the Perceived Stress Scale (PSS). ‐ Health‐related quality of life (EuroQol EQ‐5D‐5L). ‐ Caregiver burden assessed with the Burden Scale for Family Caregivers (BSFC). ‐ Patient symptoms assessed with the REvided Memory and Behaviour Checklist (RMBC). ‐ Technology acceptance assessed with the Technology Acceptance Model (TAM) adapted. Outcomes assessment: At 6 weeks and at 12 weeks (when wait‐list control have crossed to e‐learning for 6 weeks). |
|
| Notes |
Clinical trial registration code: DRKS00009891. Funding: Public grants from German, French, and UK agencies. Conflicts of interest: None declared. Other: Care recipients present early‐onset dementia (onset of symptoms before the age of 65 years). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated randomization and closed envelopes were used to assign participants to group A (immediate access to the online programme) or group B (wait‐list control with delayed access to the programme)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Computer‐generated randomization and closed envelopes were used to assign participants to group A (immediate access to the online programme) or group B (wait‐list control with delayed access to the programme)." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Unblinded study as reported by authors. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: There is no information on blinding of outcome assessors. This is an unmasked study with participants not blind to interventions and subjective participant‐reported outcomes, with components and intervention procedures not comparable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Three participants (one at each intervention site) of 61 dropped out during the study. |
| Selective reporting (reporting bias) | Low risk | Comment: There is a clinical registration number and protocol. All prespecified outcomes are reported. |
| Other bias | Low risk | Comment: None detected. |
NCT00056316.
| Study characteristics | ||
| Methods |
Design: Randomised parallel trial. No. of participating centres: One. Study dates: Recruitment from 10/2001 to 3/2004. |
|
| Participants |
Inclusion criteria: Female caregivers, 50 years and older, living with the dementia patient in the same home. Exclusion criteria: Insulin dependent diabetes, thyroid disease. No. of participants randomised to interventions: ‐ Behavioral skills training (n = 33). ‐ Basic education (n = 41). Baseline characteristics: Caregivers: Mean age for participants (all females) was 60.9 years (SD = 10.8). Care recipients: There is no available information in the registered protocol. |
|
| Interventions |
Experimental: Behavioral skills training, multicomponent behavioral intervention using 10‐session video series, workbook, and weekly telephone coaching sessions. Control: Basic education, Basic Care Guide booklet and bi‐weekly telephone calls by a trained staff member. Length of intervention: 10 weeks. |
|
| Outcomes |
Primary outcomes: ‐ Beck Depression Inventory II (21‐item BDI, total range from 0 to 63 points; higher scores mean greater severity). Secondary outcomes: ‐ Negative Affect Scale. Outcomes assessment: At (approximately) 12 weeks, and 3 and 6 months post‐intervention. |
|
| Notes |
Clinical trial registration code: NCT00056316. Funding: NIMH Grant/Contract R21MH061956. Conflicts of interest: None declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Study described as randomised but without information on the randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is no information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Interventions cannot be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Outcomes assessor was blind to randomised interventions, but intervention components and procedures were not comparable with participants not blind to interventions and subjective participant‐reported outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 3 participants of 33 (9%) dropped out from the experimental group, 3 participants of 41 (7%) dropped out from the control group. |
| Selective reporting (reporting bias) | Low risk | Comment: Trial registered at ClinicalTrials.gov (NCT00056316). |
| Other bias | Low risk | Comment: None detected. |
NCT03417219.
| Study characteristics | ||
| Methods |
Design: Parallel randomised controlled trial. No. of participating centres: VA Palo Alto Health Care System, Palo Alto, CA, USA. Study dates: 2015 ‐2017. |
|
| Participants |
Inclusion criteria: Caregivers of 18 years and older with reported distress associated to caregiving, providing at least 7 hours of care per week over the past 3 months, living with a Veteran diagnosed with dementia, and proficient in spoken and written English. Exclusion criteria: Current or lifetime history of any psychiatric disorder with psychotic features, prominent suicidal or homicidal ideation, met DSM‐IV criteria for drug or alcohol abuse or dependence (except nicotine) within the past six months, presence of alcohol intoxication or alcohol withdrawal during study recruitment or participation, diagnosis of probable or possible dementia, a Telephone Cognitive Screen score of <20, participation in another caregiver intervention study within the past year, lack of access to telephone and Internet services in the home, illness that would prevent study participation, planned transfer of care recipient to another caregiver or nursing home within 6 months, currently living with an implantable cardioverter defibrillator or pacemaker, and known pregnancy at time of consent. No. of participants randomised to intervention: ‐ Education and Skill Building Rehabilitation‐mobile (ESBR‐m, n=8). ‐ Usual care (n=8). Baseline characteristics: ‐ Caregivers: Mean age 66.06 years (SD=10.04), 13 females and 3 males ‐ Care recipients: No information provided. |
|
| Interventions |
Experimental: Education and skill building rehabilitation‐mobile (ESBR‐m). Control: Usual care plus supplemental educational materials. Length of intervention: 3 months. |
|
| Outcomes |
Primary outcomes: ‐ Caregiver burden: 22‐item Zarit Burden Interview (ZBI). ‐ Depressive symptoms: 20‐item Center for Epidemiological Studies‐Depression (CES‐D). ‐ Stress: 14‐item Terceived Stress Scale (PSS). Secondary outcomes: ‐ None stated. Outcomes assessment: At 4 months. |
|
| Notes |
Clinical trial registration code: NCT03417219. Funding: VA office of Research and Development. Conflicts of interest: None declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Sequential randomization procedure modified to assure comparability at baseline for dementia subtype. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is no information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: The study is presented as open label because of the impossibility to mask interventions throughout the study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: There is no information on blinding of outcome assessors beyond baseline, however this is an unmasked study with participants not blind to interventions and subjective participant‐reported outcomes. Moreover, interventions components and procedures are not comparable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: The study aimed to recruit 40 participants but closed with 16 (40% of sample target). Of those participants, all allocated to ESBR‐m completed the study (8/8) whereas 3 of participants allocated to usual care did not completed the study (3/8). There is no information on reasons for no completion. |
| Selective reporting (reporting bias) | Low risk | Comment: There is a trial registration and published protocol: All prespecified outcomes are reported. |
| Other bias | Low risk | Comment: None detected. |
Nunez‐Naveira 2016.
| Study characteristics | ||
| Methods |
Design: Multicentre, multinational parallel randomised study. No. of participating centres: Several centres from Spain (n = 2), Denmark (n = 2), and Poland (n = 4). Study dates: Not reported. |
|
| Participants |
Inclusion criteria: Taking care of a person diagnosed with dementia by a specialist or a neurologist according to standardised criteria (ICD‐10, DSM‐IV‐TR, NINCDS‐ADRDA), being the primary caregiver, and suffering burden according to the ZBI 22‐item scale (total score of 24 or more). Exclusion criteria: To present conditions that might prevent the evaluation of the participant or the interaction with the platform: cognitive impairment, illiterate, severe hearing and visual or motoric problems. No. of participants randomised to interventions: ‐ Application understAID (n = 36). ‐ Control (n = 41). Baseline characteristics: Caregivers: Mainly women (64%) with a mean ZBI score of 44.92 (SD = 11). Care recipients: The most frequent GDS stages were GDS4 (31%) and GDS5 (36%), followed by GD6 (26.2%) whereas only 6.6% were severely demented (GDS7). |
|
| Interventions |
Experimental: The understAID application developed as an information and communication technology (ICT) and accessible through any device with Internet connection. Control: Participants in the control group did not use the application and maintained their usual lifestyle. Length of intervention: 3 months. |
|
| Outcomes |
Primary outcomes: ‐ Depressive symptoms, assessed by the CES‐D scale (score range 0 to 60, higher scores indicate more depressive symptoms). ‐ Sense of competence, assessed with the Caregiver Competence Scale (CCS), (maximum score of 16 points, higher scores indicate more feelings of competence). ‐ Caregiving satisfaction, assessed with the Revised Caregiving Satisfaction Scale (RCSS), (maximum score of 30 points, higher scores indicate more feelings of satisfaction). Secondary outcomes: None stated. Outcomes assessment: At 3 months. |
|
| Notes |
Clinical trial registration code: Not reported. Funding: European Comission grant no. AAL‐2012‐5‐107, and national funding agencies from Spain, Denmark, and Poland. Conflicts of interest: None declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned to the experimental or to the control group by using a computer‐based random number generator." |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is no information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Participants and personnel were not blinded to interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Outcome assessors were not blind and intervention components and procedures are not comparable.. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Differential attrition. Six participants of 36 (16.7%) dropped out from the experimental group, 10 participants of 41 (24.4%) dropped out from the control group. |
| Selective reporting (reporting bias) | Unclear risk | Comment: There is not clinical trial registration and/or available protocol. |
| Other bias | Low risk | Comment: None detected. |
Torkamani 2014.
| Study characteristics | ||
| Methods |
Design: Parallel group randomised study. No. of participant centres: Three European sites: the National Hospital for Neurology and Neurosurgery, London, UK; Badalona Serveis Assistencials, Badalona, Spain; and the Psychiatric Hospital of Attica, Athens, Greece. Study dates: Not reported. |
|
| Participants |
Inclusion criteria: None stated for caregivers. Care recipients should be hospital outpatients with a diagnosis of dementia; living at home with a full time carer, presenting a Barthel Index score of at least 35 (indicating some degree of independence), and a MMSE of at least 9 and no more than 21 (indicating moderate to mild cognitive impairment). Exclusion criteria: None specified. No. of participants randomised to interventions: ‐ Computerised information platform (n = 30). ‐ Control group without intervention (n = 30). Baseline characteristics: Caregivers: Mean age for participants was 60.7 years (SD = 13.9). No other information is available. Care recipients: Mean age for patients was 78.03 years (SD = 6.91), with a mean disease duration of 3.2 years (SD = 2.18), and a mean MMSE score of 19.32 (SD = 5.0). |
|
| Interventions |
Experimental: ALADDIN, a computerised information platform to offer support and information to the carer, and to manage distant monitoring with caregivers and clinicians. Control: Assessment at 3 time points without any further contact or intervention. Lenght of intervention: 6 months. |
|
| Outcomes |
Primary outcomes: ‐ Caregiver burden, assessed by the Zarit Burden Interview (ZBI), (score range 0 to 88; higher scores denote greater burden). ‐ Caregiver depression, assessed by the Beck Depression Inventory (BDI; score range 0 to 63, higher scores denote more depressive symptoms) and the Zung Depression Self Rating Scale (ZDSR, score range 20 to 80, higher scores represent more severe depression). ‐ Caregiver quality of life, assessed by the EuroQoL (EQ5D, summary index value of 0‐1, lower scores represent poorer QoL), and the Quality of Life Scale (QOLS, score range 16 to 112, higher scores indicate better QoL). Secondary outcomes: Not stated. Outcomes assessment: At 3 (intermediate assessment) and 6 months (final assessment). |
|
| Notes |
Clinical trial registration code: Not reported. Funding: Grant from the Ambient Assisted Living, European Commission. Conflicts of interest: None declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Study reported as randomised but there is no information on the randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is no information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Participants and personnel were not blinded to interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: There is no information on blinding of outcome assessors. Moreover, this is an unmasked study with participants not masked to interventions, subjective participant‐reported outcomes and not comparable intervention components and procedures. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: There is no information to figure out the flowchart of the study. |
| Selective reporting (reporting bias) | Unclear risk | Comment: There is no trial registry and/or available protocol. |
| Other bias | Low risk | Comment: None detected. |
Tremont 2008.
| Study characteristics | ||
| Methods |
Design: Parallel group randomised study. No. of participant centres: One in New England, USA. Study dates: Not reported. |
|
| Participants |
Inclusion criteria: Caregivers of 21 or older years of age; living with a relative with dementia in the community; and providing a minimum of 4 hours of supervision or direct care per day for at least six months prior to enrolment. Care recipients should had a medical diagnosis of dementia (DSM criteria); a Clinical Dementia Rating (score of 1 (mild) or 2 (moderate); and be aged 50 or older. Exclusion criteria: Presence of a significant psychiatric illness (e.g., schizophrenia, bipolar disorder) or cognitive impairment. No. of participants randomised to interventions: ‐ Family Intervention Telephone Tracking‐Dementia (FITT‐D) (n = 32). ‐ Standard care (n = 28). Baseline characteristics: Caregivers: Mean age for participants was 63.3 years (SD = 11.8), providing an average of 44.3 moths of care (SD = 37.5), with a mean of 18.7 hours/day of care (SD = 7.8). All were spousal caregivers or adult child caregivers. The ZBI mean score for burden was 33.0 (SD = 15.8). Care recipients: Mean age for patients was 75.6 years (SD = 9.9). Mean time since dementia diagnosis was 38.6 months (SD =36.9). |
|
| Interventions |
Experimental: FITT‐D, a multi‐component intervention delivered in 23 telephone contacts over 12 months. Control: Standard care. Lenght of intervention: 12 months. |
|
| Outcomes |
Primary outcomes: ‐ Burden (Zarit Burden Interview) (score range 0 to 88, higher scores denote higher burden). ‐ Caregiver depression, assessed by the Geriatric Depression Scale (GDS) (score range 0 to 30, higher scores denote more depressive symptoms). ‐ Caregiver reaction to memory and behavior problems, assessed by the Revised Memory and Behavior Problem Checklist (RMBPC), (score range 0 to 96, higher scores reflect higher levels of perceived caregiver burden). Secondary outcomes: ‐ Caregivers Alzheimer’s Disease Knowledge Test. ‐ Caregivers SF 36 General Health. ‐ Caregivers Self Efficacy Scale. ‐ Family Assessment Device. ‐ Multidimensional Scale of Perceived Social Support. Outcomes assessment: At 12 months |
|
| Notes |
Clinical trial registration code: Not reported Funding: Grant from the National Institute of Mental Health (MH62561). Conflicts of interest: None declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Caregivers were urn randomised to groups to ensure that the two conditions were balanced on dementia severity (Clinical Dementia Rating (CDR) 1 or 2), caregiver gender, and relationship type (spouse versus other)." |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is no information on allocation concealment throughout the trial.. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Participants and personnel were not blinded to interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The research assistant was blind to group membership." Comment: Intervention components and procedures are not comparable what makes blinding of outcome assessment for subjective participant‐reported outcomes highly unlikely even if using blinded research assistants. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: High and differential attrition. 16 participants of 32 (50%) dropped out from the experimental group, 10 participants of 28 (35.7%) dropped out from the control group. |
| Selective reporting (reporting bias) | Unclear risk | Comment: There is no trial registration and/or available protocol. |
| Other bias | Low risk | Comment: None detected. |
Tremont 2015.
| Study characteristics | ||
| Methods |
Design: Parallel randomised controlled study. No. of participating centres: 2 local hospital memory clinics and recruitment from the community. USA. Study dates: Enrollment from March 2008 to August 2012. |
|
| Participants |
Inclusion criteria: Caregivers of patients formally diagnosed with any type of dementia by a neurologist, geriatrician, or psychiatrist (DSM‐IV criteria). Family member or other adult in caregiver role for at least 6 months, and who provided at least 4 hours/day of supervision or direct assistance for the person with dementia. Caregivers presenting at least two of the following experiences in a screening interview (feeling overwhelmed and stressed; sad mood/depression; anger or frustration; loss of contact with family and friends; conflict in family or family stress; neglecting their own health; demands or pressures of caregiving are too much; exhaustion and fatigue; and not taking care of their own needs or other significant responsibilities). Care recipients living in the community, and there was no plan for the care recipient to be placed in long term care or the caregiver to end their role within 6 months of study enrolment. Exclusion criteria: Presenting major acute medical illness; English not as primary language, cognitive impairment; or no access to a telephone. Care recipients living in nursing homes or assisted living centres. Care recipients with other major medical conditions affecting independent functioning. No. of participants randomised to interventions: ‐ FITT‐C (n = 133). ‐ Non‐directive telephone support (n = 117). Baseline characteristics: Caregivers: Mean age for participants was 62.72 years (SD = 12.99).Most caregivers were female (78%) and spouse or adult child of the care recipient (93%). The mean length of caregiving was 44.79 months (SD = 34.26), and the mean ZBI score was 38.18 (SD = 14.12). Care recipients: Mean age for patients was 78.06 years (SD = 10.06). The mean time since diagnosis was 30.60 months (SD = 30.77). A 77% presented a diagnosis of Alzheimer's disease. |
|
| Interventions |
Experimental: Family Intervention: Telephone Tracking‐Caregiver (FITT‐C), an entirely telephone‐based psychosocial intervention. Its aims are to enhance coping with the caregiver through active problem‐solving and facilitating positive changes within the family system. Caregivers received 16 telephone contacts distributed over 6 months. Control: Non‐directive telephone support with same number of sessions and time period of the FITT‐C intervention. Length of intervention: 6 months. |
|
| Outcomes |
Primary outcomes: ‐ Burden (Zarit Burden Interview) (score range 0 to 88, higher scores denote higher burden). ‐ Depressive symptoms (CES‐D) (score range 0 to 30, higher scores denote more depressive symptoms). ‐ Burden (RMBPC‐Reaction), (score range 0 to 96, higher scores reflect higher levels of perceived caregiver burden). Secondary outcomes: ‐ Family assessment device (FAS), (general functioning subscale, 12 items rated along a 4‐point Likert scale from strongly disagree to strongly agree). ‐ Self‐efficacy Questionnaire (SEQ) (10 items presented on a 0‐10 scale with higher score reflecting more self‐efficacy). ‐ Positive aspects of caregiving (PAC) (responses are provided on a 5‐point Likert‐type scale with higher scores representing more positive appraisals). ‐ health‐related quality of life (EuroQoL) (5 questions and an overall rating using a three‐category response format: no problem / moderate problem / severe problem). Outcomes assessment: At 6 months. |
|
| Notes |
Clinical trial registration code: NCT 00735800. Funding: Grant from the National Institute of Nursing Research (NR010559). Conflicts of interest: None declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned to receive FITT‐C (n = 133) or TS (n = 117) using an urn randomization procedure, to balance the two conditions on non‐treatment variables that might affect outcome". |
| Allocation concealment (selection bias) | Unclear risk | Comment: There is no information on allocation concealment throughout the trial. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Interventions cannot be blinded to participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Outcome assessments were conducted face‐to‐face at the baseline (before random assignment) and by telephone at the 6‐month assessment by a research assistant who was blind to group membership." Comment: Intervention components and procedures had a comparable frequency. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 19 participants of 133 (14.3%) dropped out from the experimental group, 18 participants of 117 (15.4%) dropped out from the control group. Reasons for dropping out were similar between groups. |
| Selective reporting (reporting bias) | Low risk | Comment: Trial registered at ClinicalTrials.gov (NCT00735800). |
| Other bias | Low risk | Comment: None detected. |
Winter 2007.
| Study characteristics | ||
| Methods |
Design: parallel group randomised trial. No. of participant centres: A service organization, Supportive Older Women’s Network, and an academic research centre in Philadelphia; USA. Study dates: Not reported. |
|
| Participants |
Inclusion criteria: Female caregivers, 50 years of age or older, providing care for a minimum of 6 months to a relative with a physician’s diagnosis of ADRD, and having weekly access to a telephone for at least 1 hour. Exclusion criteria: Male caregivers. No. of participants randomised to interventions: ‐ Telesupport (n = 58). ‐ Usual care (n = 45). Baseline characteristics: Caregivers: Mean age for participants was 66.6 years (SD = 9.1), a 42% were spouses of care recipients. Average years of caregiving were 4.4 (SD = 4.0). Caregivers presented a CES‐D score of 15.1 (SD = 10.9). Care recipients: Mean age for patients was 80.2 years (SD = 9.1). |
|
| Interventions |
Experimental: Telesupport treatment; professionally led telephone‐based support groups conducted by trained social workers who used conference‐ calling technology service to link 5 caregivers per group for an hour weekly. Caregivers used their own telephones with no charge. Control: Usual care, control group. Lenght of intervention: 6 months. |
|
| Outcomes |
Primary outcomes: ‐ Caregiver depression, assessed by the 20‐item Centers for Epidemiological Studies–Depression Scale (CES‐D), (score range 0 to 30, higher scores denote more depressive symptoms). ‐ Caregiver burden, assessed by the 22‐item Zarit burden scale, (score range 0 to 88, higher scores denote higher burden). ‐ Personal gains, were measured with a 6‐item scale adapted from Kaye’ Gain Through Group Involvement Scale, (score range 6 to 18, higher scores represent more personal gain). Secondary outcomes: None stated. Outcomes assessment: At 6 months. |
|
| Notes |
Clinical trial registration code: Not reported Funding: Funds from the Alzheimer’s Association. Conflicts of interest: None declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Study reported as randomised but without information on the randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The Supportive Older Women’s Network was notified of caregivers randomised to intervention and contacted caregivers to enrol them in a group." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Participants and personnel were not blinded to interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Intervention components and procedures were not comparable. This and the unblinded participants and subjective participant‐reported outcomes makes blinding outcome assessments highly unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: There is no data on attrition by treatment arms. Overall, 22 participants of 103 (21.4%) dropped out. |
| Selective reporting (reporting bias) | Unclear risk | Comment: There is no trial registration and/or available protocol. |
| Other bias | High risk | Comment: There is an imbalance in numbers randomised (58 to experimental group, 45 to control group), and differences in age at baseline (participants randomised to experimental group are older, about a mean of 5 years, than those randomised to control group). |
AAL: Active Assisted Living; AD: Alzheimer Disease; ADKS: Alzheimer’s Disease Knowledge Scale; ADL: Activities of Daily Living; ADRD: Alzheimer’s Disease and Related Dementias; BA: Behavioral activation; BDI: Beck Depression Inventory; BDI: Beck Depression Inventory; BSFC: Burden Scale for Family Caregivers; CA: California; CCS: Caregiver Competence Scale; CDR: Clinical Dementia Rating; CES‐D: Center for Epidemiological Studies – Depression scale; D‐CHESS: Dementia–Comprehensive Health Enhancement Support System; DES: Differential Emotions Scale; DRS: Dyadic Relationship Scale; DSM: Diagnostic and Statistical Manual; DVD: Digital Versatile Disc; EOC: Education Only Condition; EQ: EuroQol; ESBR‐m: Education and Skill Building Rehabilitation‐mobile ; FAS: family Assessment Service; FITT‐C: Family Intervention Telephone Tracking‐Caregiver; FITT‐D: Family Intervention Telephone Tracking‐Dementia; FTD: Frontotemporal Dementia, GDRS: Geriatric depression Rating scale; GDS: Global Deterioration Scale; GPS: Global Positioning System; GSES: General‐Self‐Efficacy Scale; HADS‐A: Hospital Anxiety and Depression Scale‐ anxiety; HADS: Hospital Anxiety and Depression Scale; HHI: Herth Hope Index; HSSUI: Health and Social Services Utilization Inventory; ICC: iCare Condition; ICD: International Statistical Classification of Diseases and Related Health Problems; ICT: Information and Communication technology; IESS: Instrumental and Expressive Social Support Scale; IQ; CODE: Informant Questionnaire on Cognitive Decline in the Elderly; ISEL: Interpersonal Support Evaluation List; ITT: Intention to treat ; IVR: Interactive Voice Response; LEAF: Life Enhancing Activities for Family; LSI‐Z: Life Satisfaction Inventory‐Z; MCS: Mental Component Summary; MMSE: Mini Mental State Examination; MoD: Mastery over Dementia; MT4C: My Tools 4 care; NHP: Nottingham Health Profile; NIH: National Institutes of Health; NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and the Alzheimer`s Disease and Related Disorders Association; NPI: Neuropsychiatric Inventory; PAC: Positive aspects of caregiving; PQoL: Perceived Quality of Life; PSS: Perceived stress scale; PsyED: Psychoeducation; QoL: Quality of Life; QOLS: Quality of life Scale; RCSE: Revised Scale for Caregiving Self‐Efficacy; RCSS: Revised Caregiving Satisfaction Scale; REACH: Resources for Enhancing Alzheimer's Caregiver Health; RMBPC: Revised Memory and Behavioral Problem Checklist; RSCS: Revised Scale for Caregiving Self‐Efficacy; SCB: Screen for Caregiver Burden; SD: standard deviation; SEQ: Self‐efficacy Questionnaire; SF: Short Form; SPPIC: Self‐perceived Pressure from Informal Care Scale; SSCQ: Short Sense of Competence Questionnaire; STAI: State ‐Trait Anxiety Inventory; STAR: Skills Training and Reskilling; TAM: Technology Acceptance Model; TBA: Telephone behavioral activation; TGM: Telephone general monitoring; TLC: Telephone‐Linked Care; TOA: Trust in Own Abilities; TV: Television; UCSF: University of California, San Francisco; UK: United Kingdom; USA: United States of America; VA: Veterans Affairs; VCR: videocassette recorder; VU: Vrije Universiteit; ZBI: Zarit burden interview.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Au 2014 | Wrong intervention: telephone‐assisted pleasant‐event scheduling (TAPES) intervention includes a psychotherapy intervention. |
| Barnes 2018 | Wrong intervention: individualised one‐to‐one cognitive behavioural approach. |
| Beauchamp 2005 | Wrong population: care receivers do not present a diagnosis of dementia (DSM/ICD/NINCDS‐ADRDA criteria). |
| Behrndt 2019 | Wrong intervention: involves an intervention with the person with dementia (ISRCTN16412551). |
| Berwig 2019 | Wrong intervention: telephone intervention follows a face‐to‐face intervention (DRKS00013736). |
| Boots 2017 | Wrong intervention: intervention combines face‐to‐face coaching with tailored web‐based modules (NTR4748). |
| Bradley 2020 | Wrong intervention: detection and management of dementia (ACTRN12618001485224). |
| Chang 2004 | Wrong study design: corrupted randomisation (caregivers recruited from a support group were assigned to the same group (experimental or attention only). |
| Chiatti 2013 | Wrong intervention: intervention arms are case management (NCT01700556). |
| Chiu 2010 | Wrong intervention: the aim of intervention is to assess usability issues with caregiver email use. |
| Eisdorfer 2003 | Wrong intervention: the arm of structural ecosystems therapy plus computer‐telephone integrated system does not fit criteria for remote intervention. |
| Finkel 2007 | Wrong intervention: no eligible control intervention (study compared two information strategies delivered online or with printed materials). |
| Gallagher‐Thompson 2007 | Wrong intervention: Includes a comparison of an in‐home behavioral management program (IHBMP) versus a telephone support condition (TSC); TSC is not the experimental but the control intervention. |
| Gallagher‐Thompson 2008 | Wrong intervention: Includes a comparison of CBT‐based intervention (Coping with Caregiving ‐ CwC) versus a minimal telephone‐based control condition (TSC); TSC is not the experimental but the control intervention. |
| Gallagher‐Thompson 2010 | Wrong population: care receivers do not present a diagnosis of dementia (DSM/ICD/NINCDS‐ADRDA criteria). |
| Gallagher‐Thompson 2015 | Wrong intervention: no eligible control intervention (study compared two information strategies). |
| Gies 2014 | Wrong intervention: the study assesses the feasibility of using a psychometric instrument administered in a web‐based format versus a face‐to‐face interview. |
| Glueckauf 2012 | Wrong intervention: intervention are telephone‐based CBT and face‐to‐face CBT. |
| Goodman 1990 | Wrong study design: study not totally randomised and also presenting with cross‐over issues. |
| Hepburn 2007 | Wrong intervention: experimental intervention (CD‐ROM) is not remote (no delivered by telephone or Internet). |
| Hicken 2016 | Wrong intervention: no eligible control intervention (study compared telephone and Internet‐based interventions). |
| Hughes 2015 | Wrong population: health care providers. |
| IRCT2017080915426N4 | Wrong intervention: mixed remote and face‐to‐face interventions. |
| ISRCTN15654731 | Wrong intervention: dyadic intervention. |
| ISRCTN41346609 | Wrong intervention: computerised cognitive behaviour therapy versus online information package. |
| Kales 2018 | Wrong intervention: individualised prescription based on 900 evidence‐based strategies (NCT02420535). |
| Kurz 2010 | Wrong intervention: intervention is not remote (face‐to‐face intervention). |
| Kwok 2014 | Wrong study design: within‐subjects pretest‐posttest design. |
| Lai 2013 | Wrong intervention: 8 participants with face‐to‐face intervention and 3 with online intervention. |
| Marziali 2006 | Wrong population: caregivers of persons with neurodegenerative disease (unknown frequency of dementia among them). |
| Mavandadi 2017 | Wrong study design: study is not randomised and do not present comparative concurrent arms (NCT02463708). |
| Mavandadi 2017a | Wrong intervention: intervention is dyadic. |
| Moskowitz 2019 | Wrong intervention: intervention is focused on management of emotions and includes psychotherapeutic procedures. |
| NCT02483520 | Wrong intervention: individualised intervention with in‐home video monitoring to manage challenging behaviours of persons with dementia. |
| NCT02585232 | Wrong intervention. Dyadic intervention (NCT02585232. |
| NCT03308032 | Wrong comparison: process evaluation in the design of a self‐directed online training (NCT03308032). |
| NCT03378050 | Wrong intervention: individualised telephone‐based intervention to address caregiver identified problems and unmet needs (NCT03378050). |
| NCT03901456 | Wrong intervention: care plan tailored to caregivers (NCT03901456). |
| NCT04037501 | Wrong intervention: care plan tailored to caregivers (NCT04037501). |
| NCT04165213 | Wrong population: training of long‐term care staff (NCT04165213). |
| NCT04226872 | Wrong population: support of caregivers when care receivers are in long‐term care facilities (NCT04226872). |
| Possin 2019 | Wrong intervention: dyadic intervention (NCT02213458). |
| Schinköthe 2014 | Wrong intervention: CBT telephone intervention. |
| Steffen 2000 | Wrong intervention: mixed remote and face‐to‐face interventions. |
| Töpfer 2018 | Wrong intervention: individual therapy with CBT sessions. |
| UMIN000031151 | Wrong intervention: face‐to‐face intervention (UMIN000031151). |
| Van Mierlo 2015 | Wrong population: mixed informal and professional caregivers allocated randomly to interventions on case manager level. |
| van Rijn 2019 | Wrong intervention: no eligible control intervention (study compared telephone counselling versus e‐learning platform ‐ NTR5521). |
| Whitlatch 2019 | Wrong intervention: intervention is not remote. |
| Williams 2010 | Wrong study design: non‐randomised trial. |
| Williams 2019 | Wrong intervention: tailored strategy for dementia care. |
| Wray 2010 | Wrong population: intervention focuses on care receivers. |
Characteristics of studies awaiting classification [ordered by study ID]
NCT03119259.
| Methods | Parallel randomised controlled trial. |
| Participants | Caregivers of people with dementia. |
| Interventions | I‐CARE support versus usual care. |
| Outcomes | ‐ Caregiver burden (NPI‐Caregiver Distress) ‐ Health care utilization (acute care) |
| Notes | ClinicalTrials.gov Identifier: NCT03119259. PI: Richard Holden (rjholden@iupui.edu) |
NCT03689179.
| Methods | Parallel randomised controlled trial. |
| Participants | Caregivers of people with dementia. |
| Interventions | Virtual coaching versus wait list control. |
| Outcomes | ‐ Anxiety symptoms (7‐item anxiety scale of HADS) ‐ Caregiver burden (24‐item Caregiver Burden Inventory) ‐ Depressive symptoms (8‐item PROMIS Depression scale) |
| Notes | ClinicalTrials.gov Identifier: NCT03689179. PI: Rebecca Utz, University of Utah. |
NCT04026919.
| Methods | Might be a parallel randomised controlled trial (describes randomization procedure) but also a single group trial as reported in the intervention model. We contacted author who said it is a randomised controlled trial but confidentiality agreements preclude her to give us more information. We have some reservations and prefer to categorize the study as awaiting classification until more information is unveiled or until publication. |
| Participants | Caregivers of people with dementia. |
| Interventions | Online support program versus no treatment. |
| Outcomes | ‐ Caregiver burden (7‐item reduced Zarit Burden Interview) ‐ Anxiety and depression (14‐item HADS) |
| Notes | ClinicalTrials.gov Identifier: NCT04026919. PI: Igone Etxeberria (igone.etxeberria@ehu.eus). |
HADS: Hospital Anxiety and Depression Scale; NPI: Neuropsychiatric Inventory; PI: Principal Investigator.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12615000509561.
| Study name | An online program for carers of people with dementia on management of behavioural and psychological symptoms. |
| Methods | Parallel randomised controlled trial. |
| Participants | Caregivers of people with dementia.. |
| Interventions | Online educational programme versus educational website of Alzheimer's Australia. |
| Outcomes | ‐ Neuropsychiatric Inventory Questionnaire (NPI‐Q) ‐ carer distress scale ‐ Zarit Burden Inventory |
| Starting date | May 2015. |
| Contact information | Prof. Lee‐Fay Low(lee‐fay.low@sydney.edu.au). |
| Notes | Australian New Zealand Clinical Trials Registry ACTRN12615000509561. |
ACTRN12619001662156.
| Study name | Can online dementia education improve stress and quality of life for dementia carers? A randomised controlled trial. |
| Methods | Parallel randomised controlled trial. |
| Participants | Caregivers of people with dementia. |
| Interventions | 7‐week MOOC course on dementia versus simple information from the Dementia Australia website. |
| Outcomes | ‐ Quality of life for caregivers (C‐DEMQOL) ‐ Quality of life for care recipients (DEM‐QOL) ‐ Caregiver knowledge & skills (Dementia Knowledge Assessment Scale) ‐ Caregiver knowledge & skills (Dementia Literacy Assessment Model) |
| Starting date | January 2020. |
| Contact information | Dr Sarang Kim (sarang.kim@utas.edu.au). |
| Notes | Australian New Zealand Clinical Trials Registry ACTRN12619001662156. |
Berwig 2017.
| Study name | Talking time: telephone support groups for informal caregivers of people with dementia. |
| Methods | Parallel randomised controlled trial. |
| Participants | Caregivers of people with dementia. |
| Interventions | Telephone‐based structured support groups versus usual care. |
| Outcomes | ‐ Mental Component Summary of the General Health Questionnaires Short Form 12 (SF‐12) ‐ Caregiver reaction scale ‐ Patient Health Questionnaire ‐ 9 items (PHQ‐9) |
| Starting date | Study completed May 2017. No results posted or published yet. |
| Contact information | Martin.Berwig@medizin.uni‐leipzig.de |
| Notes | ClinicalTrials.gov Identifier: NCT02806583. |
Dam 2017.
| Study name | Effectiveness of an online social support intervention for caregivers of people with dementia: the study protocol of a randomised controlled trial. |
| Methods | Parallel randomised controlled trial. |
| Participants | Caregivers of people with dementia. |
| Interventions | Inlife social support intervention versus waiting‐list control group. |
| Outcomes | ‐ Hospital Anxiety and Depression Scale (HADS) ‐ Care related Quality of Life scale (CarerQol) ‐ Perceived Stress Scale (PSS) |
| Starting date | October 2016. |
| Contact information | m.devugt@maastrichtuniversity.nl |
| Notes | Dutch trial register, NTR6131. |
ISRCTN16021595.
| Study name | A psycho‐educational intervention for family caregivers of patients with dementia using a mobile application. |
| Methods | Parallel randomised controlled trial. |
| Participants | Caregivers of people with dementia. |
| Interventions | Psychoeducation versus standard routine care. |
| Outcomes | ‐ Zarit Burden Inventory (ZBI) ‐ Centre for Epidemiologic Studies Depression Scale (CES‐D) ‐ Gain in Alzheimer care instrument (GAIN) |
| Starting date | January 2014. |
| Contact information | Dr Chuen Chai Dennis Seow (dennis.seow.c.c@sgh.com.sg). |
| Notes | ISRCTN16021595. |
Kovaleva 2018.
| Study name | Testing Tele‐Savvy: protocol for a randomised controlled trial. |
| Methods | Three arms parallel randomised trial. |
| Participants | Caregivers of people with dementia. |
| Interventions | Tele‐Savvy intervention condition / attention control condition / usual care. |
| Outcomes | ‐ Zarit Burden Inventory (ZBI) ‐ Centre for Epidemiological Studies Depression Scale (CES‐D) ‐ Caregiver assessment of behavioral skill ‐ Alzheimer's Disease Related Quality of Life (ADRQL) |
| Starting date | May 2017 (recruiting; estimated study completion date November 2020). |
| Contact information | PI: Kenneth Hepburn, Emory University. Contact: Rachel Nash (rachel.nash@emory.edu). |
| Notes | ClinicalTrials.gov Identifier: NCT03033875. |
Mehta 2018.
| Study name | Effectivenness of iSupport for informal caregivers of people with dementia: a randomised controlled trial. |
| Methods | Parallel randomised controlled trial. |
| Participants | Caregivers of people with dementia. |
| Interventions | Online support programme (iSupport) versus psycho‐education about dementia (minimal intervention). |
| Outcomes | ‐ Perceived Stress Scale (PSS) ‐ Centre for Epidemiological Studies Depression Scale (CES‐D) ‐ Zarit Burden Scale (ZBS) |
| Starting date | September 2017. |
| Contact information | kala.mehta@ucsf.edu |
| Notes | NTR6593. Clinical Trials Registry‐India: CTRI/2017/02/007876. |
NCT02106065.
| Study name | VA cultivating access to resources, education, and skills for dementia caregivers (VA CARES). |
| Methods | Parallel randomised controlled trial. |
| Participants | Caregivers of people with dementia. |
| Interventions | Education and skill‐building rehabilitation (ESBR) versus usual care. |
| Outcomes | ‐ Zart Burden Interview (ZBI) ‐ Center for Epidemiological Studies Depression (CES‐D) ‐ Long‐term care placement status |
| Starting date | February 2014 (Study completion date: September 2019). |
| Contact information | Blake K Scanlon (Blake.Scanlon@va.gov). |
| Notes | ClinicalTrials.gov Identifier: NCT02106065. |
NCT03260608.
| Study name | Effectiveness of a psychoeducation and support protocol by phone in the aid of caregivers of patients with dementia. |
| Methods | Parallel randomised controlled trial. |
| Participants | Caregivers of people with dementia. |
| Interventions | Telesupport versus usual care. |
| Outcomes | ‐ Zarit Burden Interview (ZBI) ‐ Beck Depression Inventory (BDI) ‐ Quality of Life Questionnaire ‐ WHOQOL |
| Starting date | August 2017 (Estimated study completion date: June 2020). |
| Contact information | Artur S Schuh (schuh.afs@gmail.com); Andressa H Hermes‐Pereira (andressahpereira@gmail.com). |
| Notes | ClinicalTrials.gov Identifier: NCT03260608. |
NCT03819816.
| Study name | Development and evaluation of the DEA app (DEA). |
| Methods | Parallel randomised controlled trial. |
| Participants | Caregivers of people with dementia. |
| Interventions | 3‐module support app versus standard information. |
| Outcomes | ‐ Caregiver burden (Burden Scale for Family Caregivers) ‐ Carevier quality of life (12‐item Short‐Form Health Survey) ‐ Caregiver quality of life (Adult Carer Quality of Life Questionnaire) |
| Starting date | January 2019 (Estimated study completion date: November 2019). |
| Contact information | Valentin Ritschl, Medical University of Vienna (valentin.ritschl@meduniwien.ac.at). |
| Notes | ClinicalTrials.gov Identifier: NCT03819816. |
NCT04104568.
| Study name | Effectiveness of an online training and support program (iSupport) for informal dementia caregivers (iSupportPT). |
| Methods | Parallel randomised controlled trial. |
| Participants | Caregivers of people with dementia. |
| Interventions | iSupport versus education‐only e‐book. |
| Outcomes | ‐ Caregiver burden (22‐item Zarit Burden Interview) ‐ Symptoms of depression & anxiety (14‐item HADS) ‐ Caregiver quality of life (WHOQOL‐BREF) ‐ Caregiver knowledge & skills (10‐item Generalised Self‐efficacy Scale) |
| Starting date | January 2020 (Estimated study completion date: January 2021). |
| Contact information | Soraia T Sousa, Universidade de Porto (teles.s.soraia@gmail.com). Constança Paúl, Universidade de Porto (paul@icbas.up.pt). |
| Notes | ClinicalTrials.gov Identifier: NCT04104568. |
NCT04330482.
| Study name | Translating a dementia caregiver intervention into a mobile application. |
| Methods | Parallel randomised controlled trial. |
| Participants | Caregivers of people with dementia. |
| Interventions | Mobile health app versus Internet information links. |
| Outcomes | ‐ Acceptability of the intervention (completion rates) ‐ Caregiver mood (CES‐D) ‐ Caregiver burden (ZBI) |
| Starting date | January 2020 (Estimated study completion date: May 2021). |
| Contact information | Geoffrey Tremont, Rhode Island Hospital (gtremont@lifespan.org). |
| Notes | ClinicalTrials.gov Identifier: NCT04330482. |
Pinto‐Bruno 2019.
| Study name | An online minimally guided intervention to support family and other unpaid carers of people with dementia: protocol for a randomised controlled trial. |
| Methods | Parallel randomised controlled trial. |
| Participants | Caregivers of people with dementia. |
| Interventions | iSupport versus waiting list control group. |
| Outcomes | ‐ Caregiver mood (CES‐D) ‐ Caregiver burden (ZBI) |
| Starting date | January 2019 (expected publication of results June 2020). |
| Contact information | Ángel C Pinto‐Bruno, Vrije Universiteit Amsterdam (a.c.pintobruno@vu.nl). |
| Notes | Netherlands Trial Register: NL6417. |
ADRQL: Alzheimer’s Disease Related Quality of Life; BDI: Beck Depression Inventory; CES‐D: Center for Epidemiological Studies – Depression scale; ESBR: Education and Skill Building Rehabilitation; HADS: Hospital Anxiety and Depression Scale; MOOC: Massive Online Open Course; NPI‐Q: Neuropsychiatric Inventory Questionnaire; PHQ: Patient Health Questionnaire; PSS: Perceived Stress Scale; SF: Short Form; WHOQOL: World Health Organization Quality of Life; ZBI: Zarit Burden Interview; ZBS: Zarit Burden Scale.
Differences between protocol and review
In a significant change from the published protocol on which this review was based, we amended the criteria for eligibility of studies in order to focus on remotely delivered interventions. This change was made in the context of the COVID‐19 pandemic for reasons explained more fully in the Background section.
We moved the outcome 'number of dropouts for any reason,' as a surrogate for intervention acceptability, from secondary to primary outcomes before data analysis in consultation with the Cochrane Dementia and Cognitive Improvement Group (CDCIG). This was done because Cochrane recommends that primary outcomes should include at least one harm‐related outcome. We did not anticipate that harms would be reported in many included studies, but considered that reasons for dropout would be likely to include some 'harms' (such as unacceptable additional burden of the intervention), among other reasons.
The published protocol did not specify the exact comparisons to be made. After reviewing previous literature, communicating with experts in the field and discussion with the CDCIG, we defined two main comparisons. Because many studies of interventions for caregivers use an information‐only intervention as a comparator for a more complex intervention, these comparisons were of complex interventions versus either (i) waiting list, usual care or a non‐specific intervention serving as an attention control, or (ii) a simple information intervention. Because almost all complex interventions include an element of information‐provision (i.e. there are very few pure training or pure support interventions), we then defined the following subgroups within the complex interventions: support or support and information, training or training and information, and multicomponent interventions including support and training with or without information. These decisions were made prior to data analysis.
We did not proceed to additional subgroup analyses and sensitivity analyses as planned in the original protocol because of insufficient information. When data allowed, we ran stratified analysis by intervention subgroup as defined above for each combination of comparison and outcome.
Contributions of authors
All review authors (EGF, JB, JRR, BSZ, IS, JM) contributed to the design, development, and drafting the protocol.
Pairs of review authors (EGF, JB, JRR, GM, BSZ, JM) screened the studies for eligibility, obtained full text studies and performed data abstraction.
Pairs of review authors (EGF, JB, JRR, IS) assessed the risk of bias and rated the quality of evidence (GRADE).
JB and BSZ conducted data analysis; EGF, JRR and JM assisted with data interpretation.
All review authors (EGF, JB, JRR, BSZ, IS, JM) contributed to the final text.
Sources of support
Internal sources
-
Iberoamerican Cochrane Centre, Spain
International Cochrane Centre
External sources
-
NIHR, UK
This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health
Declarations of interest
EGF: none known
JB: none known
JRR: none known
BSZ: none known
IS: none known
JM: none known
New
References
References to studies included in this review
Au 2015 {published data only}
- Au A, Gallagher-Thompson D, Wong M-K, Leung J, Chan W-C, Chan CC, et al. Behavioral activation for dementia caregivers: scheduling pleasant events and enhancing communications. Clinical Interventions in Aging 2015;10:611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au A. Developing volunteer-assisted behavioral activation teleprograms to meet the needs of Chinese dementia caregivers. Clinical Gerontologist 2015;38:190-202. [DOI: 10.1080/07317115.2015.1008118] [DOI] [Google Scholar]
Au 2019 {published data only}
- Au A, Yip H-M, Lai S, Ngai S, Cheng S-T, Losada A, et al. Telephone-based behavioral activation intervention for dementia family caregivers: outcomes and mediation effect of a randomized controlled trial. Patient Education and Counseling 2019;102:2049-59. [DOI: 10.1016/j.pec.2019.06.009] [DOI] [PubMed] [Google Scholar]
Blom 2015 {published data only}
- Blom MM, Bosmans JE, Cuijpers P, Zarit SH, Pot AM. Effectiveness and cost-effectiveness of an internet intervention for family caregivers of people with dementia: design a a randomized controlled trial. BMC Psychiatry 2013;13(17):1-7. [DOI: 10.1186/1471-244X-13-17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom MM, Zarit SH, Groot Zwaaftink RBM, Cuijpers P, Pot AM. Effectiveness of an Internet intervention for family caregivers of people with dementia: results of a randomized controlled trial. PLoS One 2015;10(2):1-11. [DOI: 10.1371/journal.pone.0116622] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brennan 1995 {published data only}
- Brennan PF, Moore SM, Smyth KA. ComputerLink: electronic support for the home caregiver. ANS. Advances in Nursing Science 1991;13(4):14-27. [DOI] [PubMed] [Google Scholar]
- Brennan P F, Moore S M, Smyth K A. The effects of a special computer network on caregivers of persons with Alzheimer's disease. Nursing Research 1995;44(3):166-72. [PubMed] [Google Scholar]
Cristancho‐Lacroix 2015 {published data only}
- Cristancho-Lacroix V, Wrobel J, Cantegreil-Kallen I, Dub T, Rouquette A, Rigaud AS. A web-based psychoeducational program for informal caregivers of patients with Alzheimer's disease: a pilot randomized controlled trial. Journal of Medical Internet Research 2015;17(5):e117. [DOI: 10.2196/jmir.3717] [DOI] [PMC free article] [PubMed] [Google Scholar]
Czaja 2013 {published data only}
- Czaja SJ, Loewenstein D, Schulz R, Nair SN, Perdomo D. A videophone psychosocial intervention for dementia caregivers. American Journal of Geriatric Psychiatry 2013;21(11):1071-81. [DOI: 10.1016/j.jagp.2013.02.019] [DOI] [PubMed] [Google Scholar]
Davis 2004 {published data only}
- Davis LL, Burgio LD, Buckwalter KC, Weaver M. A comparison of in-home and telephone-based skill training interventions with caregivers of persons with dementia. Journal of Mental Health and Aging 2004;10(1):31-44. [Google Scholar]
Dowling 2014 {published data only}
- Dowling GA, Merrilees J, Mastick J, Chang VY, Hubbard E, Moskowitz JT. Life enhancing activities for family caregivers of people with frontotemporal dementia. Alzheimer Disease and Associated Disorders 2014;28:175-81. [DOI] [PubMed] [Google Scholar]
- Life Enhancing Activities for Family Caregivers (LEAF). NCT01825681 2013. [https://clinicaltrials.gov/show/NCT01825681]
Duggleby 2018 {published data only}
- Duggleby W, Ploeg J, McAiney C, Fisher K, Swindle J, Chambers T, et al. Study protocol: pragmatic randomized control trial of an internet-based intervention (My Tools 4 Care) for family carers. BMC Geriatrics 2017;17(181):1-9. [DOI: 10.1186/s12877-017-0581-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby W, Ploeg J, McAiney C, Peacock S, Fisher K, Ghosh S, et al. Web-based intervention for family carers of persons with dementia and multiple chronic conditions (My Tools 4 Care): pragmatic randomized controlled trial. Journal of Medical Internet Research 2018;20(6):e10484. [DOI: 10.2196/10484] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gant 2007 {published data only}
- Gant JR, Steffen AM, Lauderdale SA. Comparative outcomes of two distance-based interventions for male caregivers of family members with dementia. American Journal of Alzheimers Disease and Other Dementias 2007;22(2):120-8. [DOI: 10.1177/1533317506298880] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gustafson 2019 {published data only}
- Gustafson Jr DH, Gustafson Sr DH, Cody OJ, Chih M-Y, Johnston DC, Asthana S. Pilot test of a computer-based system to help family caregivers of dementia patients. Journal of Alzheimer's Disease 2019;70:541-52. [DOI: 10.3233/JAD-190052] [DOI] [PubMed] [Google Scholar]
Hattink 2015 {published data only}
- Hattink B, Meiland F, Roest H, Kevern P, Abiuso F, Bengtsson J, et al. Web-Based STAR E-Learning Course Increases Empathy and Understanding in Dementia Caregivers: Results from a Randomized Controlled Trial in the Netherlands and the United Kingdom. Journal of Medical Internet Research 2015;17(10):e241. [DOI: 10.2196/jmir.4025] [http://courses.startraining.eu/] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayden 2012 {unpublished data only}
- NCT00416078. Online caregiver psychoeducation and support: improving outcomes in AD/ADRD. NCT00416078 2006.
Huis in het Veld 2020 {published data only}
- Huis in het Veld JG, Willemse BM, Asch IFM, Groot Zwaaftink RBM, Verkade P-J, Twisk JWR, et al. Online self-management support for family caregivers dealing with behavior changes in relatives with dementia (Part 2): randomized controlled trial. Journal of Medical Internet Research 2020;22(2):e13001. [DOI: 10.2196/13001] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huis in het Veld JG, Willemse BM, Asch IFM, Groot Zwaaftink RBM, Verkade P-J, Veldhuijzen NJ, et al. Online self-management support for family caregivers to help them manage behavior changes in their relative with dementia: study protocol for a randomized controlled trial and a process evaluation. Journal of Medical Internet Research Research Protocols 2017;6(11):e239. [DOI: 10.2196/resprot.8365] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kajiyama 2013 {published data only}
- Kajiyama B, Thompson LW, Eto-Iwase T, Yamashita M, Di Mario J, Marian Tzuang Y, Gallagher-Thompson D. Exploring the effectiveness of an Internet-based program for reducing caregiver distress using the iCare Stress Management e-Training Program. Aging Mental Health 2013;17(5):544-54. [DOI: 10.1080/13607863.2013.775641] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01378195. iCare Stress Management e-Training for Dementia Family Caregivers. NCT01378195 2013. [https://clinicaltrials.gov/show/nct01378195]
Kwok 2013 {published data only}
- Kwok T, Wong B, Ip I, Chui K, Young D, Ho F. Telephone-delivered psychoeducational intervention for Hong Kong Chinese dementia caregivers: a single-blinded randomized controlled trial. Clinical Interventions in Aging 2013;8:1191-7. [DOI: 10.2147/CIA.548264] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahoney 2003 {published data only}
- Mahoney DF, Tarlow BJ, Jones RN. Effects of an automated telephone support system on caregiver burden and anxiety: findings from the REACH for TLC intervention study. Gerontologist 2003;43(4):556-67. [DOI] [PubMed] [Google Scholar]
Martindale‐Adams 2013 {published data only}
- Martindale-Adams J, Nichols LO, Burns R, Graney MJ, Zuber J. A trial of dementia caregiver telephone support. Canadian Journal of Nursing Research 2013;45(4):30-48. [DOI] [PubMed] [Google Scholar]
Metcalfe 2019 {published data only}
- Diehl-Schmid J, Metcalfe A, Jones B, Schwertel U, Kurz A. A multi-center randomised controlled pilot study of an e-learning course for family caregivers of patients with young onset dementia. In: Neuro-degenerative diseases conference. 2017.
- Kurz A, Bakker C, Böhm M, Diehl-Schmid J, Dubois B, Ferreira C, et al. RHAPSODY-Internet-based support for caregivers of people with young onset dementia: program design and methods of a pilot study. International Psychogeriatrics / IPA 2016;28(12):2091-9. [DOI: 10.1017/S1041610216001186] [DOI] [PubMed] [Google Scholar]
- Metcalfe A, Boucault S, Aloui S, Hergueta T, Dubois M. The feasibility of an internet based support program for informal caregivers of adults with early-onset dementia: a multi-centre RCT study in England, France and Germany. In: Alzheimer's International Conference, AAIC 2017; UK. 2017:P1168.
- Metcalfe A, Jones B, Mayer J, Gage H, Oyebode J, Boucault S, et al. Online information and support for carers of people with young-onset dementia: a multi-site randomised controlled pilot study. International Journal of Geriatric Psychiatry 2019;34(10):1455-64. [DOI: 10.1002/gps.5154] [DOI] [PubMed] [Google Scholar]
- RHAPSODY: a pilot study evaluating the usability and benefits of an online learning program for carers of people with young onset dementia. DRKS00009891. [http://www.drks.de/DRKS00009891]
NCT00056316 {unpublished data only}
- NCT00056316. Reducing depression in dementia caregivers. ClinicalTrials.gov 2003. [NCT00056316]
NCT03417219 {unpublished data only}
- NCT03417219. Mobile tablet education to advance caregiver health (MTEACHing). NCT03417219 2018.
Nunez‐Naveira 2016 {published data only}
- Nunez-Naveira L, Alonso-Bua B, Labra C, Gregersen R, Maibom K, Mojs E, et al. UnderstAID, an ICT Platform to Help Informal Caregivers of People with Dementia: A Pilot Randomized Controlled Study. BioMed Research International 2016;2016(5726465). [DOI: 10.1155/2016/5726465] [DOI] [PMC free article] [PubMed] [Google Scholar]
Torkamani 2014 {published data only}
- Torkamani M, McDonald L, Saez Aguayo I, Kanios C, Katsanou MN, Madeley L, et al. A randomized controlled pilot study to evaluate a technology platform for the assisted living of people with dementia and their carers. Journal of Alzheimers Disease 2014;41(2):515-23. [DOI: 10.3233/JAD-132156] [DOI] [PubMed] [Google Scholar]
Tremont 2008 {published data only}
- Tremont G, Davis JD, Bishop DS, Fortinsky RH. Telephone-delivered psychosocial intervention reduces burden in dementia caregivers. Dementia (London) 2008;7(4):503-520. [DOI: 10.1177/1471301208096632] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tremont 2015 {published data only (unpublished sought but not used)}
- Tremont G, Davis J, Papandonatos GD, Grover C, Ott BR, Fortinsky RH, et al. A telephone intervention for dementia caregivers: background, design, and baseline characteristics. Contemporary Clinical Trials 2013;36:338-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremont G, Davis JD, Ott BR, Galioto R, Crook C, Papandonatos GD, et al. Randomized trial of the Family Intervention: Telephone Tracking-Caregiver for dementia caregiver: use of community and healthcare resources. Journal of the American Geriatric Society 2017;65(5):924-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremont G, Davis JD, Papandonatos GD, Ott BR, Fortinsky RH, Gozalo P, et al. Psychosocial telephone intervention for dementia caregivers: a randomized, controlled trial. Alzheimer's & Dementia 2015;11:541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winter 2007 {published data only}
- Winter L, Gitlin LN. Evaluation of a telephone-based support group intervention for female caregivers of community-dwelling individuals with dementia. American Journal of Alzheimers Disease and Other Dementias 2007;21(6):391-7. [DOI: 10.1177/1533317506291371] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Au 2014 {published data only}
- Au A, Wong M K, Leung L M, Leung P, Wong A. Telephone-assisted pleasant-event scheduling to enhance well-being of caregivers of people with dementia: a randomised controlled trial. Hong Kong Medical Journal 2014;20(Suppl 3):S30-S33. [PubMed] [Google Scholar]
Barnes 2018 {published data only}
- Barnes CJ, Markham C. A pilot study to evaluate the effectiveness of an individualized and cognitive behavioural communication intervention for informal carers of people with dementia: the Talking Sense programme. International Journal of Language & Communication Disorders 2018;53(3):615-27. [DOI: 10.1111/1460-6984.12375] [DOI] [PubMed] [Google Scholar]
- NCT01481363. The talking sense communication programme for dementia carers. NCT01481363 2011. [https://clinicaltrials.gov/show/nct01481363]
Beauchamp 2005 {published data only}
- Beauchamp N, Irvine A B, Seeley J, Johnson B. Worksite-based Internet multimedia program for family caregivers of persons with dementia. Gerontologist 2005;45(6):793-801. [DOI] [PubMed] [Google Scholar]
Behrndt 2019 {published data only}
- Behrndt E-M, Straubmeier M, Seidl H, Vetter C, Luttenberger K, Graessel E. Brief telephone counselling is effective for caregivers who do not experience any major life events - caregiver-related outcomes of the German day-care study. BMC Health Services Research 2019;19(20). [DOI: 10.1186/s12913-018-3853-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath C, Luttenberger K, Graessel E, Scheel J, Pendergrass A, Behrndt E-M. Can brief telephone interventions reduce caregiver burden and depression in caregivers of people with cognitive impairment? - long-term results of the German day-care study (RCT). BMC Geriatrics 2019;19(196). [DOI: 10.1186/s12877-019-1207-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berwig 2019 {published data only}
- Berwig M, Lessing S, Deck R. Telephone-based aftercare groups for family carers of people with dementia: study protocol of the Talking Time - REHAB project. BMC Health Services Research 2019;19(183). [DOI: 10.1186/s1293-019-4003-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boots 2017 {published data only}
- Boots LMM, Vugt ME, Kempen GIJM, Verhey FRJ. Effectiveness of a blended care self-management program for caregivers of people with early-stage dementia (Partner in Balance): randomized controlled trial. Journal of Medical Internet Research 2018;20(7):e10017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots LMM, Vugt ME, Kempen GIJM, Verhey FRJ. Effectiveness of the blended care self-management program "Partner in Balance" for early-stage dementia caregivers: study protocol for a randomized controlled trial. Trials 2016;17:231. [DOI: 10.1186/s13063-016-1351-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots LMM, Vugt ME, Smeets CMJ, Kempen GIJM, Verhey FRJ. Implementation of the blended care self-management program for caregivers of people with early-stage dementia (Partner in Balance): process evaluation of a randomized controlled trial.. Journal of Medical Internet Research 2017;19(12):e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradley 2020 {published data only}
- Bradley K, Smith R, Hughson J-A, Atkinson D, Bessarab D, Flicker L, et al. Let's CHAT (community health approaches to) dementia in Aboriginal and Torres Strait Islander communities: protocol for a stepped wedge cluster randomised controlled trial. BMC Healt Services Research 2020;20(208). [DOI: 10.1186/s12913-020-4985-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2004 {published data only}
- Chang B L, Nitta S, Carter P A, Markham Y K. Perceived helpfulness of telephone calls--providing support for caregivers of family members with dementia. Journal of Gerontological Nursing 2004;30(9):14-21. [DOI] [PubMed] [Google Scholar]
Chiatti 2013 {published data only}
- Chiatti C, Furneri G, Rimland J M, Demma F, Bonfranceschi F, Cassetta L, et al. The economic impact of moderate stage Alzheimer's disease in Italy: Evidence from the UP-TECH randomized trial. International Psychogeriatrics 2015;27(9):1563-72. [DOI] [PubMed] [Google Scholar]
- Chiatti C, Masera F, Rimland JM, Cherubini A, Scarpino O, Spazzafumo L, et al. The UP-TECH project, an intervention to support caregivers of Alzheimer's disease patients in Italy: study protocol for a randomized controlled trial. Trials 2013;14:155. [NCT01700556] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiatti C, Rimland JM, Bonfranceschi F, Maseray F, Bustacchini S, Cassetta L, et al. The UP-TECH project, an intervention to support caregivers of Alzheimer's disease patients in Italy: preliminary findings on recruitment and caregiving burden in the baseline population. Aging and Mental Health 2015;19(6):517-25. [DOI] [PubMed] [Google Scholar]
Chiu 2010 {published data only}
- Chiu TML. Usage and non-usage behaviour of ehealth services among chinese canadians caring for a family member with dementia. Thesis 2010.
Eisdorfer 2003 {published data only}
- Eisdorfer C, Czaja SJ, Loewenstein DA, Rubert MP, Argüelles S, Mitrani VB, et al. The effect of a family therapy and technology-based intervention on caregiver depression. The Gerontologist 2003;43(4):521-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finkel 2007 {published data only}
- Finkel S, Czaja SJ, Schulz R, Martinovich Z, Harris C, Pezzuto D. E-care: a telecommunications technology intervention for family caregivers of dementia patients. American Journal of Geriatric Psychiatry 2007;15(5):443-8. [DOI] [PubMed] [Google Scholar]
Gallagher‐Thompson 2007 {published data only}
- Gallagher-Thompson D, Gray HL, Tang PC, Pu CY, Leung LY, Wang PC, et al. Impact of in-home behavioral management versus telephone support to reduce depressive symptoms and perceived stress in Chinese caregivers: results of a pilot study. American Journal of Geriatric Psychiatry 2007;15(5):425-34. [DOI] [PubMed] [Google Scholar]
Gallagher‐Thompson 2008 {published data only}
- Gallagher-Thompson D, Gray HL, Dupart T, Jimenez D, Thompson LW. Effectiveness of Cognitive/Behavioral Small Group Intervention for Reduction of Depression and Stress in Non-Hispanic White and Hispanic/Latino Women Dementia Family Caregivers: Outcomes and Mediators of Change. Journal of Rational-Emotive Cognitive-Behavior Therapy 2008;26(4):286-303. [DOI: 10.1007/s10942-008-0087-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallagher‐Thompson 2010 {published data only}
- Gallagher-Thompson D, Wang P-C, Liu W, Cheung V, Peng R, China D, et al. Effectiveness of a psychoeducational skill training DVD program to reduce stress in Chinese American dementia caregivers: Results of a preliminary study. Aging and Mental Health 2010;14(3):263-73. [DOI] [PubMed] [Google Scholar]
Gallagher‐Thompson 2015 {published data only}
- Gallagher-Thompson D, Tzuang M, Hinton L, Alvarez P, Rengifo J, Valverde I, et al. Effectiveness of a fotonovela for reducing depression and stress in Latino dementia family caregivers. Alzheimer Disease and Associated Disorders 2015;29(2):146-53. [DOI: 10.1097/WAD/0000000000000077] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gies 2014 {published data only}
- Gies C, Pierce L, Steiner V, Bijl J, Salvador D. Web-based psychosocial assessment for caregivers of persons with dementia: a feasibility study. Rehabilitation Nursing 2014;39(2):102-9. [DOI] [PubMed] [Google Scholar]
Glueckauf 2012 {published data only}
- Glueckauf RL, Davis WS, Willis F, Sharma D, Gustafson DJ, Hayes J, et al. Telephone-based, cognitive-behavioral therapy for African American dementia caregivers with depression: initial findings. Rehabilitation Psychology 2012;57(2):124-39. [DOI] [PubMed] [Google Scholar]
Goodman 1990 {published data only}
- Goodman C. Evaluation of a model self-help telephone program: impact on natural networks. Social Work 1990;35(6):556-62. [DOI] [PubMed] [Google Scholar]
- Goodman CC, Pynoos J. A model telephone information and support program for caregivers of Alzheimer's patients. Gerontologist 1990;30(3):399-404. [DOI] [PubMed] [Google Scholar]
Hepburn 2007 {published data only}
- Hepburn K, Lewis M, Tornatore J, Sherman CW, Bremer KL. The Savvy Caregiver program: the demonstrated effectiveness of a transportable dementia caregiver psychoeducation program. Journal of Gerontological Nursing 2007;33(3):30-6. [DOI] [PubMed] [Google Scholar]
Hicken 2016 {published data only}
- Hicken BL, Daniel C, Luptak M, Grant M, Kilian S, Rupper RW. Supporting caregivers of rural veterans electronically (SCORE). The Journal of Rural Health 2017;33:305-13. [DOI: 10.1111/jrh.12195] [DOI] [PubMed] [Google Scholar]
Hughes 2015 {published data only}
- Hughes ML, Lowe DA, Shine HE, Carpenter BD, Balsis S. Using the Alzheimer's Association Web Site to Improve Knowledge of Alzheimer's Disease in Health Care Providers. American Journal of Alzheimers Disease and Other Dementias 2015;30(1):98-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT2017080915426N4 {unpublished data only}
- IRCT2017080915426N4. Effects of informational support on elder family caregivers. IRCT2017080915426N4 2017. [http://en.irct.ir/trial/14691]
ISRCTN15654731 {unpublished data only}
- ISRCTN15654731. Evaluation of benefits of the CAREGIVERSPRO-MMD platform giving support and assistance to people living with dementia and their primary caregiver. ISRCTN15654731 2017. [http://isrctn.com/isrctn15654731]
ISRCTN41346609 {unpublished data only}
- ISRCTN41346609. Computerised cognitive behaviour therapy for dementia carers. isrctn.com/ISRCTN41346609 (date of registration 28/02/2013).
Kales 2018 {published data only}
- Gitlin LN, Kales HC, Marx K, Stanislawski B, Lyketsos C. A randomized trialof a web-based platform to help families manage dementia-related behavioral symptoms: the WeCareAdvisorTM. Contemporary Clinical Trials 2017;62:27-36. [DOI: 10.1016/j.cct.2017.08.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kales HC, Gitlin LN, Stanislawski B, Kim HM, Marx K, Turnwald M, et al. Effect of the WeCareAdvisorTM on family caregiver outcomes in dementia: a pilot randomized controlled trial. BMC Geriatrics 2018;18:113. [DOI: 10.1186/s12877-018-0801-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kales HC, Gitlin LN, Stanislawski B, Marx K, Turnwald M, Watkins DC, et al. The development of a caregiver-focused, web-based program to assess and manage behavioral and psychological symptoms of dementia. Alzheimer Disease and Associated Disorders 2017;31(3):263-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurz 2010 {published data only}
- Kurz A, Wagenpfeil S, Hallauer J, Schneider-Schelte H, Jansen S. Evaluation of a brief educational program for dementia carers: The AENEAS Study. International Journal of Geriatric Psychiatry 2010;25(8):861-9. [DOI: 10.1002/gps.2428] [DOI] [PubMed] [Google Scholar]
Kwok 2014 {published data only}
- Kwok T, Au A, Wong B, Ip I, Mak V, Ho F. Effectiveness of online cognitive behavioral therapy on family caregivers of people with dementia. Clinical Intervention in Aging 2014;9:631-636. [DOI: 10.2147/CIA.556337] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lai 2013 {published data only}
- Lai CKY, Wong LF, Liu K-H, Lui W, Chan MF, Yap LSY. Online and onsite training for family caregivers of people with dementia: Results from a pilot study. International Journal of Geriatric Psychiatry 2013;28:107-8. [DOI: 10.1002/gps.3798] [DOI] [PubMed] [Google Scholar]
Marziali 2006 {published data only}
- Marziali E, Donahue P. Caring for others: internet video-conferencing group intervention for family caregivers of older adults with neurodegenerative disease. The Gerontologist 2006;46(3):398-403. [DOI] [PubMed] [Google Scholar]
Mavandadi 2017 {published data only}
- Mavandadi S, Wray LO, DiFilippo S, Streim J, Oslin D. Evaluation of a telephone-delivered, community-based collaborative care management program for caregivers of older adults with dementia. The American Journal of Geriatric Psychiatry 2017;25(9):1019-28. [DOI: 10.1016/j.jagp.2017.03.015] [DOI] [PubMed] [Google Scholar]
Mavandadi 2017a {published data only}
- Mavandadi S, Wright EM, Graydon MM, Oslin DW, Wray LO. A randomized pilot trial of a telephone-based collaborative care management program for caregivers of individuals with dementia. Psychological Services 2017;14(1):102-11. [DOI: 10.1037/ser0000118] [DOI] [PubMed] [Google Scholar]
Moskowitz 2019 {published data only}
- Moskowitz JT, Cheung EO, Snowberg K, Verstaen A, Merrilees J, Salsman JM, et al. Randomized controlled trial of a facilitated online positive emotion regulation intervention for dementia caregivers. Health Psychology 2019;38(5):391-402. [DOI: 10.1037/hea0000680] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01825681. Life Enhancing Activities for Family Caregivers (LEAF). ClinicalTrial.gov 2013. [NCT01825681]
- Verstaen A, Moskowitz JT, Snowberg KE, Merrilees J, Dowling GA. Life Enhancing Activities for Family Caregivers of people with dementia: protocol for a randomized controlled trial of a positive affect skills intervention. Open Acces Journal of Clinical Trials 2018;10:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02483520 {published data only}
- NCT02483520. Supporting Family Caregivers With Technology for Dementia Home Care. NCT02483520 2014. [Google Scholar]
NCT02585232 {published data only}
- NCT02585232. Optimizing Dementia Care Through Collaborative Recovery Interventions. NCT02585232 2016. [Google Scholar]
NCT03308032 {unpublished data only}
- NCT03308032. Self-directed online training for Chinese caregivers. NCT03308032 2017. [https://clinicaltrials.gov/show/nct03308032]
NCT03378050 {unpublished data only}
- NCT03378050. A trial of a multi-component individualized telephone-based support intervention for adult-child caregivers caring for parents with dementia. NCT03378050 2017. [https://clinicaltrials.gov/show/nct03378050]
NCT03901456 {unpublished data only}
- Gaugler JE, Louwagie K. Care to plan: a tailored resource for family members of persons with dementia. NCT03901456 2019.
NCT04037501 {unpublished data only}
- Keller A, Thyrian JR. Effectiveness of a care management system to reduce unmeet needs of informal caregivers of people with dementia (GAP). NCT04037501 2019.
NCT04165213 {unpublished data only}
- Unsigned. The case for care of persons with dementia in their environments (COPE) in programs of all-inclusive care of the elderly PACE service settings. NCT04165213 2019.
NCT04226872 {unpublished data only}
- Duggleby W, O'Rourke H. Supporting family caregivers of persons living with dementia: effectiveness ans sustainability of MT4C-In Care. NCT04226872 2020.
Possin 2019 {published data only}
- Possin KL, Dulaney S, Merrilees J, Bonasera S, Chiong W, Lee K, et al. The dementia care ecosystem: Preliminary findings from a comprehensive telephone-based dementia care trial. Conference Publication 2016;Conference: American Geriatrics Society 2016 Annual Scientific Meeting. Long Beach, CA United States. Conference Publication::S199. [Google Scholar]
- Possin KL, Merrilees JJ, Dulaney S, Bonasera SJ, Chiong W, Lee K, et al. Effect of collaborative dementia care via telephone and internet on quality of life, caregiver well-being, and health care use. The Care Ecosystem randomized clinical trial. JAMA Internal Medicine 2019;179(12):1658-67. [DOI: 10.1001/jamainternmed.2019.4101] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schinköthe 2014 {published data only}
- Schinköthe D, Wilz G. The assessment of treatment integrity in a cognitive behavioral telephone intervention study with dementia caregivers. Clinical Gerontologist 2014;37(3):211-34. [Google Scholar]
Steffen 2000 {published data only}
- Steffen AM. Anger Management for Dementia Caregivers: A Preliminary Study Using Video and Telephone Interventions. Behaviour Therapy 2000;31:281-99. [Google Scholar]
Töpfer 2018 {published data only}
- Töpfer NF, Wilz G. TeleTAnDem increases the psychosocial resource utilization of dementia caregivers. GeroPsych: The Journal of Gerontopsychology and Geriatric Psychiatry 2018;31(4):173-83. [DOI: 10.1024/1662-9647/a000197] [DOI] [Google Scholar]
UMIN000031151 {unpublished data only}
- UMIN000031151. A psycho-educational program to enhance stress coping in dementia caregivers: a randomized controlled trial. upload.umin.ac.jp/cgi-open-bin/icdr_e/ctr_view.cgi?recptno=R000035583 (date of disclosure of the study information 2018/02/05). [https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?Recptno=r000035583]
Van Mierlo 2015 {published data only}
- Van Mierlo LD, Meiland FJM, Van de Ven PM, Van Hout HPJ, Dröes R-M. Evaluation of DEM-DISC, customized e-advice on health and social support services for informal carers and case managers of people with dementia; a cluster randomized trial. International Psychogeriatrics 2015;27(8):1365-78. [DOI] [PubMed] [Google Scholar]
van Rijn 2019 {published data only}
- Rijn A, Meiland F, Dröes R-M. Linking two new E-health caregiver interventions to meeting centres for people with dementia and their carers; a process evaluation. Aging & Mental Health 2020;24(8):1316-1325. [DOI: 10.1080/13607863.2019.1617243] [DOI] [PubMed] [Google Scholar]
Whitlatch 2019 {published data only}
- Whitlatch CJ, Heid AR, Femia EE, Orsulic-Jeras S, Szabo S, Zarit SH. The Suport, Health, Activities, Resources, and Education Program for early stage dementia: results from a randomized controlled trial. Dementia (London) 2019;18(6):2122-39. [DOI: 10.1177/1471301217743033] [DOI] [PubMed] [Google Scholar]
Williams 2010 {published data only}
- Williams VP, Bishop-Fitzpatrick L, Lane JD, Gwyther LP, Ballard EL, Vendittelli AP, et al. Video-based coping skills (VCS) to reduce health risk and improve psychological and physical well-being in Alzheimer's disease family caregivers. Psychosomatic Medicine 2010;72(9):897-904. [DOI: 10.1097/PSY.0b013e3181fc2d09] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2019 {published data only}
- Shaw CA, Williams KN, Perkhounkova Y, Hein M, Coleman CK. Effects of a video-based intervention on caregiver confidence for managing dementia care challenges: findings from the FamTechCare clinical trial. Clinical Gerontologist 2020;43(5):508-517. [DOI: 10.1080/07317115.2020.1729917] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KN, Perkhounkova Y, Shaw CA, Hein M, Vidoni ED, Coleman CK. Supporting family caregivers with technology for dementia home care: a randomized controlled trial. Innovation in Aging 2019;3(3):1-19. [DOI: 10.1093/geroni/igz037] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wray 2010 {published data only}
- NCT00105638. Telehealth education program for caregivers of veterans with dementia. NCT00105638.
- Wray LO, Shulan MD, Toseland RW, Freeman KE, Vásquez BE, Gao J. The effect of telephone support groups on costs of care for veterans with dementia. The Gerontologist 2010;50(5):623-31. [DOI: 10.1093/geront/gnq040] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT03119259 {unpublished data only}
- NCT03119259. iCare-AD: A Randomized Controlled Trial to Test the Efficacy of a Mobile Health Technology Application in Reducing the Behavioral and Psychological Symptoms of Patients With Alzheimer's Dementia and the Distress of Their Informal Caregivers. NCT03119259 2017. [https://clinicaltrials.gov/show/nct03119259]
NCT03689179 {unpublished data only}
- Utz R. Virtual coaching to maximize dementia caregivers' respite time use (TLC). NCT03689179 2018.
NCT04026919 {unpublished data only}
- Etxeberria I. Online support program for dementia caregivers (OKenCASA). NCT04026919 2019.
References to ongoing studies
ACTRN12615000509561 {unpublished data only}
- ACTRN12615000509561. An online program for carers of people with dementia on management of behavioural and psychological symptoms. ACTRN12615000509561 2015. [Google Scholar]
ACTRN12619001662156 {unpublished data only}
- Kim S. Can online dementia education improve stress and quality of life for dementia carers? A randomised controlled trial. ACTRN12619001662156 2019.
Berwig 2017 {published and unpublished data}
- Berwig M, Dichter MN, Albers B, Wermke K, Trutschel D, Seismann-Petersen S, et al. Feasibility and effectiveness of a telephone-based social support intervention for informal caregivers of people with dementia: study protocol of the TALKING TIME project. BMC Health Services Research 2017;17(1):280. [DOI: 10.1186/s12913-017-2231-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02806583. Talking Time: Telephone Support Groups for Informal Caregivers of People With Dementia: A Randomized Controlled Trial. NCT02806583 2015. [DOI: 10.1186/s12913-017-2231-2] [DOI] [Google Scholar]
Dam 2017 {published data only}
- Dam AEH, Vugt ME, Boxtel MPJ, Verhey FRJ. Effectiveness of an online social support intervention for caregivers of people with dementia: the study protocol of a randomised controlled trial. Trials 2017;18(395). [DOI: 10.1186/s13063-017-2097-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN16021595 {unpublished data only}
- ISRCTN16021595. A psycho-educational intervention for family caregivers of patients with dementia using a mobile application. ISRCTN16021595. [ISRCTN16021595]
Kovaleva 2018 {published and unpublished data}
- Kovaleva MA, Bilsborough E, Griffiths PC, Nocera J, Higgins M, Epps F, et al. Testing Tele-Savvy: protocol for a randomized controlled trial. Research in Nursing & Health 2018;2:107-20. [DOI: 10.1002/nur.21859] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03033875. Testing Tele-Savvy, an On-line Psychoeducation Program for Dementia Family Caregivers. NCT03033875 2017. [Google Scholar]
Mehta 2018 {published and unpublished data}
- CTRI/2017/02/007876. A study to test the effectiveness of an internet-based program to support caregivers of persons with dementia. CTRI/2017/02/007876 2017. [http://www.ctri.nic.in/clinicaltrials/pmaindet2.php?Trialid=16362]
- Mehta KM, Gallagher-Thompson D, Varghese M, Loganathan S, Baruah U, Sehher K, et al. iSupport, an online training and support program for caregivers of people with dementia: study protocol for a randomized controlled trial in India. Trials 2018;19(271). [DOI: 10.1186/s13063-018-2604-9] [CTRI/2017/02/007876] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTR6593. Effectiveness of iSupport for informal caregivers of people with dementia: a randomised controlled trial. NTR6593 2017. [http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=6593]
NCT02106065 {unpublished data only}
- NCT02106065. VA cultivating access to resources, education, and skills for dementia caregivers. NCT02106065 2014. [https://clinicaltrials.gov/show/nct02106065]
NCT03260608 {unpublished data only}
- NCT03260608. Effectiveness of a psychoeducation and support protocol by phone in the aid of caregivers of patients with dementia. NCT03260608 2017. [https://clinicaltrials.gov/show/nct03260608]
NCT03819816 {unpublished data only}
- Ritschl V. Development and evaluation of the DEA app (DEA). NCT03819816 2019.
NCT04104568 {unpublished data only}
- Sousa ST, Paúl C. Effectiveness of an online training and support program (iSupport) for informal dementia caregivers (iSupportPT). NCT04104568 2019. [DOI] [PMC free article] [PubMed]
NCT04330482 {unpublished data only}
- Tremont G. Translating a dementia caregiver intervention into a mobile application. NCT04330482 2020.
Pinto‐Bruno 2019 {published data only}
- Pinto-Bruno Ac, Margriet A, Kleiboer A, Droes R-M, Straten A. An online minimally guided intervention to support family and other unpaid carers of people with dementia: protocol for a randomized controlled trial. Journal of Medical Internet Research Research Protocols 2019;8(10):e14106. [DOI: 10.2196/14106] [NL6417] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abrahams 2018
- Abrahams R, Liu KPY, Bissett M, Fahey P, Cheung KSL, Bye R, et al. Effectiveness of interventions for co-residing family caregivers of people with dementia: systematic review and meta-analysis. Australian Occupational Therapy Journal 2018;65:208-24. [DOI: 10.1111/1440-1630.12464] [DOI] [PubMed] [Google Scholar]
Belle 2003
- Belle SH, Schulz R, Zhang S, Gitlin LN, Mendelsohn AB, Czaja SJ, et al. Using a new taxonomy to combine the uncombinable: integrating results across diverse interventions. Psychology Aging 2003;18(3):396-405. [DOI: 10.1037/0882-7974.18.3.396] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2012
- Cheng ST, Lau RW, Mak EP, Ng NS, Lam LC, Fung HH, et al. A benefit-finding intervention for family caregivers of persons with Alzheimer disease: study protocol of a randomized controlled trial. Trials 2012;13:98. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2011
- Collins LG, Swartz K. Caregiver care. American Family Physician 2011;83(11):1309-17. [PubMed] [Google Scholar]
Cooke 2001
- Cooke DD, McNally L, Mulligan KT, Harrison MJ, Newman SP. Psychosocial interventions for caregivers of people with dementia: a systematic review. Aging & Mental Health 2001;5(2):120-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
Deeken 2019
- Deeken F, Rezo A, Hinz M, Discher R, Rapp MA. Evaluation of technology-based interventions for informal caregivers of patients with dementia - a meta-analysis of randomized controlled trials. American Journal of Geriatric Psychiatry 2019;27(4):426-45. [DOI: 10.1016/j.jagp.2018.12.003] [DOI] [PubMed] [Google Scholar]
Dillehay 1990
- Dillehay RC, Sandys MR. Caregivers for Alzheimer's patients: what we are learning from research. International Journal of Aging and Human Development 1990;30(4):263-85. [DOI] [PubMed] [Google Scholar]
Ducharme 2011
- Ducharme FC, Lévesque LL, Lachance LM, Kergoat MJ, Legault AJ, Beaudet LM, et al. "Learning to become a family caregiver" efficacy of an intervention program for caregivers following diagnosis of dementia in a relative. The Gerontologist 2011;51(4):484-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Egan 2018
- Egan KJ, Pinto-Bruno AC, Bighelli I, Berg-Weger M, Straten A, Albanese E, et al. Online training and support programs designed to improve mental health and reduce burden among caregivers of people with dementia: a systematic review. Journal of the American Medical Directors Association 2018;19(3):200-6. [DOI: 10.1016/j.jamda.2017.10.023] [DOI] [PubMed] [Google Scholar]
Eska 2013
- Eska K, Graessel E, Donath C, Schwarzkopf L, Lauterberg J, Holle R. Predictors of institutionalization of dementia patients in mild to moderate stages: a 4-year prospective analysis. Dementia Geriatric Cognitive Disorders Extra 2013;3(1):426-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Etters 2008
- Etters L, Goodall D, Harrison BE. Caregiver burden among dementia patient caregivers: a review of the literature. Journal of the American Academy of Nurse Practitioners 2008;20(8):423-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gaugler 2017
- Gaugler JE, Jutkowitz E, Shippee TP, Brasure M. Consistency of dementia caregiver intervention classification: an evidence-based synthesis. International Psychogeriatrics 2017;29(1):19-30. [DOI: 10.1017/S1041610216001514] [DOI] [PMC free article] [PubMed] [Google Scholar]
George 1986
- George L, Gwyther LP. Caregiver well-being: a multidimensional examination of family caregivers of demented adults. The Gerontologist 1986;26(3):253-9. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime, Inc.) GRADEpro Guideline Development Tool. McMaster University (developed by Evidence Prime, Inc.), 2020. Available from gradepro.org.
Haley 2004
- Haley WE, Gitlin LN, Wisniewski SR, Mahoney DF, Coon DW, Winter L, et al. Well-being, appraisal, and coping in African-American and Caucasian dementia caregivers: findings from the REACH study. Aging & Mental Health 2004;8(4):316-29. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane-handbook.org.
Higgins 2020
- Higgins JPT, Thomas J (editors). Cochrane Handbook for Systematic Reviews of Interventions (second edition). Cochrane, Wiley Blackwell 2020.
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. British Medical Journal 2014;348:g1687. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hopwood 2018
- Hopwood J, Walker N, McDonagh L, Rait G, Walters K, Iliffe S, et al. Internet-based interventions aimed at supporting family caregivers of people with dementia: systematic review. Journal of Medical Internet Research 2018;20(6):e216. [DOI: 10.2196/jmir.9548] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laakkonen 2012
- Laakkonen ML, Hölttä EH, Savikko N, Strandberg TE, Suominen M, Pitkälä KH. Psychosocial group intervention to enhance self-management skills of people with dementia and their caregivers: study protocol for a randomized controlled trial. Trials 2012;13:133. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liddle 2012
- Liddle J, Smith-Conway ER, Baker R, Angwin AJ, Gallois C, Copland DA, et al. Memory and communication support strategies in dementia: effect of a training program for informal caregivers. International Psychogeriatrics / IPA 2012;24(12):1927-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lins 2014
- Lins S, Hayder-Beichel D, Rücker G, Motschall E, Antes G, Meyer G, Langer G. Efficacy and experiences of telephone counselling for informal carers of people with dementia. Cochrane Database of Systematic Reviews 2014;9(CD009126). [DOI: 10.1002/14651858.CD009126.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Losada Baltar 2004
- Losada Baltar A, Izal Fernández de Trocóniz M, Montorio Cerrato I, Márquez González M, Pérez Rojo G. [Differential efficacy of two psychoeducational interventions for dementia family caregivers] [Eficacia diferencial de dos intervenciones psicoeducativas para cuidadores de familiares con demencia]. Revista de Neurologia 2004;38(8):701-8. [PMID: ] [PubMed] [Google Scholar]
Martin‐Carrasco 2014
- Martin-Carrasco M, Domínguez-Panchón AI, González-Fraile E, Muñoz-Hermoso P, Ballesteros J, EDUCA Group. Effectiveness of a psychoeducational intervention group program in the reduction of the burden experienced by caregivers of patients with dementia: the EDUCA-II randomized trial. Alzheimer Disease and Associated Disorders 2014;28(1):79-87. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mittelman 2004
- Mittelman MS, Roth DL, Coon DW, Haley WE. Sustained benefit of supportive intervention for depressive symptoms in caregivers of patients with Alzheimer's disease. American Journal of Psychiatry 2004;161(5):850-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Moniz‐Cook 2008
- Moniz-Cook E, Vernooij-Dassen M, Woods R, Verhey F, Chattat R, De Vugt M, et al. A European consensus on outcome measures for psychosocial intervention research in dementia care. Aging & Mental Health 2008;12(1):14-29. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ploeg 2018
- Ploeg J, Ali MU, Markle-Reid M, Valaitis R, Bartholomew A, Fitzpatrick-Lewis D, et al. Caregiver-focused, web-based interventions: systematic review and meta-analysis (part 2). Journal of Medical Internet Research 2018;20(10):e11247. [DOI: 10.2196/11247] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pusey 2001
- Pusey H, Richards D. A systematic review of the effectiveness of psychosocial interventions for carers of people with dementia. Aging & Mental Health 2001;5(2):107-19. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roth 2009
- Roth DL, Perkins M, Wadley VG, Temple EM, Haley WE. Family caregiving and emotional strain: associations with quality of life in a large national sample of middle-aged and older adults. Quality of Life Research 2009;18:679-88. [DOI: 10.1007/s11136-009-9482-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). Introduction to GRADE Handbook. Handbook for grading the quality of evidence and strength of recommendations using the GRADE approach. Updated October 2013. The GRADE Working Group, 2013. Available from www.guidelinedevelopment.org/handbook (accessed 16 April 2015).
Selwood 2007
- Selwood A, Johnston K, Katona C, Lyketsos C, Livingston G. Systematic review of the effect of psychological interventions on family caregivers of people with dementia. Journal of Affective Disorders 2007;101(1-3):75-89. [DOI] [PubMed] [Google Scholar]
Sherifali 2018
- Sherifali D, Ali MU, Ploeg J, Markle-Reid M, Valaitis R, Bartholomew A, et al. Impact of Internet-based interventions on caregiver mental health: systematic review and meta-analysis. Journal of Medical Internet Research 2018;20(7):e10668. [DOI: 10.2196/10668] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2020
- Anonymous. Dementia. Web Page. [WEB PAGE: https://www.who.int/news-room/fact-sheets/detail/dementia]
Williams 2019a
- Williams F, Moghaddam N, Ramsden S, De Boos D. Interventions for reducing levels of burden amongst informal carers of persons with dementia in the community. A systematic review and meta-analysis of randomised controlled trials. Aging and Mental Health 2019;23(12):1629-42. [DOI: 10.1080/13607863.2018.1515886] [DOI] [PubMed] [Google Scholar]
Winter 2006
- Winter L, Gitlin LN. Evaluation of a telephone-based support group intervention for female caregivers of community-dwelling individuals with dementia. American Journal of Alzheimer's Disease and Other Dementias 2006;21(6):391-7. [PMID: ] [DOI] [PubMed] [Google Scholar]


