Abstract
Background
Pulmonary arterial hypertension is a devastating disease that leads to right heart failure and premature death. Endothelin receptor antagonists have shown efficacy in the treatment of pulmonary arterial hypertension.
Objectives
To evaluate the efficacy of endothelin receptor antagonists (ERAs) in pulmonary arterial hypertension.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and the reference sections of retrieved articles. The searches are current as of 4 November 2020.
Selection criteria
We included randomised trials and quasi‐randomised trials involving participants with pulmonary arterial hypertension.
Data collection and analysis
Two of five review authors selected studies, extracted data and assessed study quality according to established criteria. We used standard methods expected by Cochrane. The primary outcomes were exercise capacity (six‐minute walk distance, 6MWD), World Health Organization (WHO) or New York Heart Association (NYHA) functional class, Borg dyspnoea scores and dyspnoea‐fatigue ratings, and mortality.
Main results
We included 17 randomised controlled trials involving a total of 3322 participants. Most trials were of relatively short duration (12 weeks to six months). Sixteen trials were placebo‐controlled, and of these nine investigated a non‐selective ERA and seven a selective ERA.
We evaluated two comparisons in the review: ERA versus placebo and ERA versus phosphodiesterase type 5 (PDE5) inhibitor. The abstract focuses on the placebo‐controlled trials only and presents the pooled results of selective and non‐selective ERAs.
After treatment, participants receiving ERAs could probably walk on average 25.06 m (95% confidence interval (CI) 17.13 to 32.99 m; 2739 participants; 14 studies; I2 = 34%, moderate‐certainty evidence) further than those receiving placebo in a 6MWD. Endothelin receptor antagonists probably improved more participants' WHO functional class (odds ratio (OR) 1.41, 95% CI 1.16 to 1.70; participants = 3060; studies = 15; I2 = 5%, moderate‐certainty evidence) and probably lowered the odds of functional class deterioration (OR 0.43, 95% CI 0.26 to 0.72; participants = 2347; studies = 13; I2 = 40%, moderate‐certainty evidence) compared with placebo. There may be a reduction in mortality with ERAs (OR 0.78, 95% CI 0.58, 1.07; 2889 participants; 12 studies; I2 = 0%, low‐certainty evidence), and pooled data suggest that ERAs probably improve cardiopulmonary haemodynamics and may reduce Borg dyspnoea score in symptomatic patients. Hepatic toxicity was not common, but may be increased by ERA treatment from 37 to 67 (95% CI 34 to 130) per 1000 over 25 weeks of treatment (OR 1.88, 95% CI 0.91 to 3.90; moderate‐certainty evidence). Although ERAs were well tolerated in this population, several cases of irreversible liver failure caused by sitaxsentan have been reported, which led the licence holder for sitaxsentan to withdraw the product from all markets worldwide.
As planned, we performed subgroup analyses comparing selective and non‐selective ERAs, and with the exception of mean pulmonary artery pressure, did not detect any clear subgroup differences for any outcome.
Authors' conclusions
For people with pulmonary arterial hypertension with WHO functional class II and III, endothelin receptor antagonists probably increase exercise capacity, improve WHO functional class, prevent WHO functional class deterioration, result in favourable changes in cardiopulmonary haemodynamic variables compared with placebo. However, they are less effective in reducing dyspnoea and mortality. The efficacy data were strongest in those with idiopathic pulmonary hypertension. The irreversible liver failure caused by sitaxsentan and its withdrawal from global markets emphasise the importance of hepatic monitoring in people treated with ERAs. The question of the effects of ERAs on pulmonary arterial hypertension has now likely been answered. The combined use of ERAs and phosphodiesterase inhibitors may provide more benefit in pulmonary arterial hypertension; however, this needs to be confirmed in future studies.
Plain language summary
Endothelin receptor antagonists for pulmonary arterial hypertension
Review question
Do endothelin receptor antagonists increase how much a person is capable of exercising (exercise capacity), improve symptoms, or reduce death in people with pulmonary arterial hypertension (PAH)?
Background
Pulmonary arterial hypertension is a devastating disease characterised by an increase in pulmonary vascular resistance which leads to right heart failure and ultimately death.
Endothelin receptor antagonists are a class of strong vasodilators (medications that open (dilate) blood vessels) capable of stopping the process of cell division, which could dilate and result in a favourable pulmonary arterial structural alteration.
Study characteristics
We reviewed the evidence from randomised studies (studies in which people are assigned to one of two or more treatment groups using a random method). After a thorough search and assessment of the medical literature, we identified 17 studies with a total of 3322 participants for inclusion in the review. A vast majority of the participants had PAH without known cause (idiopathic). The evidence is current to November 2020.
Key results
Endothelin receptor antagonists probably increase exercise capacity, improve World Health Organization functional class (a measurement of how severe a person's pulmonary hypertension symptoms are), and may improve death rates and symptoms in people with PAH; however they may also increase the risk of liver damage, although this was rare. The question of the effects of endothelin receptor antagonists on PAH has now likely been answered.
Certainty of the evidence
Overall, the evidence presented is of moderate certainty due to the high occurrence of missing data.
Summary of findings
Summary of findings 1. Endothelin receptor antagonists compared to placebo for pulmonary arterial hypertension.
| Endothelin receptor antagonists compared to placebo for pulmonary arterial hypertension | ||||||
| Participant or population: pulmonary arterial hypertension Setting: clinics and hospitals Intervention: endothelin receptor antagonists Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with endothelin receptor antagonists | |||||
| Change from baseline in 6MWD (m) mean duration of study 16.3 weeks |
The weighted mean change on control was −4.56 m. | MD 25.06 higher (17.13 higher to 32.99 higher) | ‐ | 2739 (14 RCTs) | ⊕⊕⊕⊝ Moderate1 | Higher is better for 6MWD. |
| Proportion of participants with improved functional class mean duration of study 16.8 weeks |
175 per 1000 | 230 per 1000 (197 to 264) | OR 1.41 (1.16 to 1.70) | 3060 (15 RCTs) | ⊕⊕⊕⊝ Moderate1 | Participants with high OR are more likely to achieve functional improvement. |
| Change from baseline in BDI mean duration of study 14.3 weeks |
The weighted mean change on control was 0.25 higher. | MD 0.43 lower (0.90 lower to 0.04 higher) | ‐ | 788 (7 RCTs) | ⊕⊕⊝⊝Low2 | Symptoms are worse with higher score of BDI. |
| Mortality mean duration of study 30.2 weeks |
73 per 1000 | 58 per 1000 (44 to 78) | OR 0.78 (0.58 to 1.07) | 2889 (12 RCTs) | ⊕⊕⊝⊝ Low3 | Participants with lower OR are less likely to die. |
| Change from baseline in mean PAP (mmHg) mean duration of study 17.1 weeks |
The weighted mean change on control was 0.53 higher. | MD 4.65 lower (6.05 lower to 3.26 lower) | ‐ | 729 (8 RCTs) | ⊕⊕⊕⊝ Moderate4 | Participants are worse with higher pulmonary artery pressure. |
| Change from baseline in PVR (dyn/s/cm5) mean duration of study 15.7 weeks |
The weighted mean change on control was 63.55 higher. | MD 236.24 lower (333.21 lower to 139.26 lower) | ‐ | 586 (7 RCTs) | ⊕⊕⊕⊝ Moderate4 | Participants are worse with higher pulmonary vascular resistance. |
| Hepatic toxicity mean duration of study 25 weeks |
37 per 1000 | 67 per 1000 (34 to 130) | OR 1.88 (0.91 to 3.90) | 2250 (11 RCTs) | ⊕⊕⊕⊝ Moderate1 | Participants with higher OR are more likely to suffer hepatic toxicity. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 6MWD: 6‐minute walk distance; BDI: Borg dyspnoea index; CI: confidence interval; MD: mean difference; OR: odds ratio; PAP: pulmonary artery pressure; PVR: pulmonary vascular resistance; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Incomplete outcome data (attrition bias) due to missing data imbalanced between intervention and control groups in most of the included studies (−1 level). 2Attrition, publication bias and wide upper confidence interval (−2 levels). 3We downgraded the evidence for the outcome of mortality by 2 levels because the study period was relatively short and events in most of the studies few, and wide upper confidence interval of odds ratio. 4The pooled analysis used raw data from subgroups of the included studies.
Background
Description of the condition
Pulmonary arterial hypertension (PAH) is a rare disease of the distal pulmonary arteries characterised by vasoconstriction, vascular proliferation, obstructive remodelling of the pulmonary vessel wall, inflammation, and thrombosis, which lead to a progressive increase in pulmonary vascular resistance, and, ultimately, right ventricular failure and death (Galiè 2015; Thenappan 2018). PAH includes the following subcategories (Simonneau 2019): 1. idiopathic PAH; 2. heritable PAH; 3 drug‐ and toxin‐induced PAH; 4. PAH associated with (4.1 connective tissue disease; 4.2 HIV infection; 4.3 portal hypertension; 4.4 congenital heart disease; 4.5 schistosomiasis); 5. PAH long‐term responders to calcium channel blockers; 6. PAH with overt features of venous/capillaries involvement; and 7. persistent pulmonary hypertension of the newborn (PPHN) syndrome.
Considerable progress has been made in identifying the causes of PAH in recent decades. Mutations in the type II bone morphogenetic protein receptor (BMPR2) gene contributes to heritable PAH (Thenappan 2018). Epigenetic dysregulation of DNA methylation, histone acetylation, and microRNAs play a key role in disease pathogenesis (Galiè 2015). Complex changes in cytokines, cellular immunity, and autoantibodies indicate that PAH is in part an autoimmune, inflammatory disease (Guignabert 2013). Endothelial dysfunction characterised by overexpression of vasoconstrictors and downexpression of vasoactive mediators occurs in PAH as a result. Reduced production of vasoactive mediators such as nitric oxide (NO) and prostacyclin, along with overexpression of vasoconstrictors such as endothelin‐1 (ET‐1) and thromboxane A2, increase pulmonary vascular tone and promote pulmonary vascular remodelling in people with PAH. The substances targeting pulmonary vasoactive mechanisms (Galiè 2015; Klinger 2019; Thenappan 2018), used alone or in combination, therefore improve functional capacity and haemodynamics and reduce hospital admissions.
Description of the intervention
Endothelin receptor antagonists (ERAs) are a class of potent vasodilators and antimitotic substances that could specifically dilate and remodel the pulmonary arterial system (Horinouchi 2013). Two separate receptors for ET have been identified: endothelin type A (ET‐A) receptors, which are most commonly found on vascular smooth muscle cells and which induce vasoconstriction by increasing intracellular calcium; and endothelin type B (ET‐B) receptors located on endothelial cells, which stimulate the release of vasodilating agents such as NO and prostacyclin (Madonna 2015). However, ET‐B receptors also appear on vascular smooth muscle cells, where they stimulate vasoconstriction. Theoretically, either selective blocking of ET‐A receptors alone, or non‐selective blocking of both ET‐A and ET‐B receptors together, could dilate local pulmonary vessels (Klinger 2019; Thenappan 2018).
How the intervention might work
Endothelin is a potent vasoconstrictor and smooth muscle mitogen. It is overexpressed in the lungs of people with PAH, and elevated plasma endothelin concentrations are correlated with poorer prognosis (Chester 2014). Furthermore, an overproduction of endothelin may also lead to pulmonary vascular remodelling in PAH (Shao 2011). ERAs have shown efficacy in the treatment of PAH in clinical trials (AMBITION; ARIES‐1; ARIES‐2; BREATHE‐1; BREATHE‐2; COMPASS‐2; SERAPHIN; STRIDE‐1; STRIDE‐2).
Why it is important to do this review
Since bosentan, the first ERA, was approved by the US Food and Drug Administration (FDA) to treat PAH, many new agents, both selective and non‐selective ERAs, have been developed and tested in clinical trials. Importantly, the efficacy and safety of combined use of ERAs and phosphodiesterase (PDE) inhibitors have been investigated in population with PAH (AMBITION; COMPASS‐2). However, the efficacy of ERAs was not consistent in these clinical trials; a systematic review to determine the efficacy of ERAs in the treatment of PAH was thus warranted.
Objectives
To evaluate the efficacy of endothelin receptor antagonists in pulmonary arterial hypertension.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials or quasi‐randomised clinical trials.
Types of participants
We included trials involving adults and children (≥ 2 years) with PAH. The diagnosis of PAH should have been made according to European Respiratory Society/European Society of Cardiology/World Health Organization (ERS/ESC/WHO) guidelines. We included studies involving participants with PAH secondary to other diseases if data assessing the outcomes specific to the PAH component of their syndrome were available.
Types of interventions
We considered trials in which participants took an ERA alone or in combination against any comparator.
We included the following co‐interventions.
Phosphodiesterase type 5 (PDE5) inhibitors
Soluble guanylate cyclase (sGC)
Prostanoids
Nitrates
Calcium channel blockers
Non‐pulmonary hypertension‐specific medications including diuretics, anticoagulants, and oxygen
Types of outcome measures
Primary outcomes
Exercise capacity (as measured by a six‐minute walk distance (6MWD)).
World Health Organization (WHO) functional class or New York Heart Association (NYHA) functional class (WHO/NYHA).
Borg dyspnoea scores and dyspnoea‐fatigue ratings.
Mortality.
Secondary outcomes
Cardiopulmonary haemodynamics including mean pulmonary artery pressure (PAP); pulmonary vascular resistance (PVR), cardiac index, cardiac output (CO), systemic arterial oxygen saturation and systemic oxygen transport.
Pulmonary function tests.
Adverse events (e.g. hepatic toxicity).
Search methods for identification of studies
Electronic searches
The Cochrane Airways Group Information Specialist undertook literature searches on 4 November 2020 using the following sources: the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 11 of 12, November 2020) (from 1966 to November 2020), which contains the Cochrane Airways Group Register of Trials, MEDLINE Ovid SP (from 1966 to November 2020), Embase Ovid SP (from 1980 to November 2020), the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/), and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/). We applied no language restrictions. The database search strategies are shown in Appendix 1.
Searching other resources
We handsearched the reference lists of retrieved articles for additional trials. We also searched documents released from the FDA and European Medicines Agency (EMA).
Data collection and analysis
Selection of studies
Three review authors (LC, CJ, and DB) independently screened the titles and abstracts of records identified by the search for potential eligibility, categorising them as 'retrieve' or 'do not retrieve'. We retrieved all available full‐text publications. Three review authors (LC, CJ, and DB) independently assessed the full‐text reports as either eligible for inclusion, or identified and reported the reasons for exclusion (Figure 1). Any disagreements were resolved by consensus or by involving a third person/review author (GY). One final review author (LK) analysed the included and excluded texts to ensure uniform enforcement of the study protocol. We excluded duplicates and collated multiple reports of the same study (Figure 1). We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) and 'Characteristics of excluded studies' table.
1.
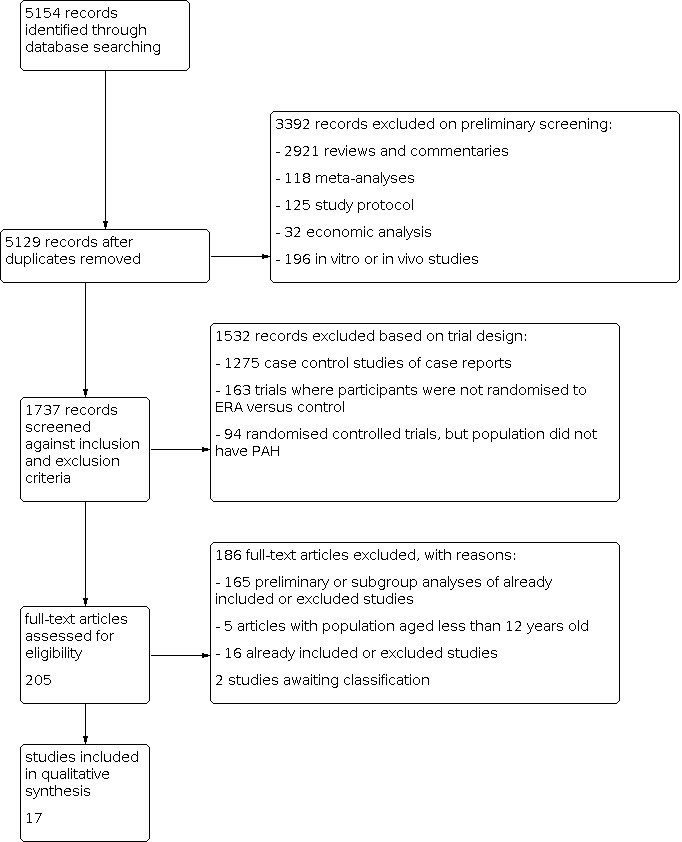
Study flow diagram.
Data extraction and management
We used Covidence to collect study characteristics and outcome data (Covidence). Three review authors (LC, CJ, and GY) extracted the following study characteristics from the included studies.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, withdrawals, and date of study.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for studies and notable conflicts of interest of trial authors.
Three review authors (LC, CJ, and GY) independently extracted the outcome data from included studies. Any disagreements were resolved through discussion or by consulting a third review author (DB) if required. One review author (LC) transferred data into the Review Manager 5 file (Review Manager 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (LK) spot‐checked study characteristics for accuracy against the study report.
Assessment of risk of bias in included studies
We independently assessed the methodological quality of included trials against the following criteria.
Was the study described as randomised?
Was the allocation concealment adequate?
Were the participants blinded?
Was there blinded outcome assessment?
Was there a description of withdrawals and dropouts?
Were the results analysed according to intention‐to‐treat?
Was there any other bias?
We graded each potential source of bias as 'low', 'high', or 'unclear', and provided a justification for our judgement in the 'Risk of bias' table. We took into account the risk of bias when considering treatment effects.
Measures of treatment effect
We treated short ordinal scales (e.g. WHO functional class, NYHA class) as dichotomous measures, arranging the data into two categories: improvement or deterioration in class. We analysed linear scales (e.g. Borg dyspnoea scores) as continuous data.
Unit of analysis issues
For studies where more than one eligible treatment arm was included, we split the data between two treatment‐control comparisons by splitting the control group and halving the sample sizes. The total numbers in the meta‐analysis thus added up to the original size of the group.
Dealing with missing data
For missing data on prespecified primary outcomes, we contacted the study investigators to request the data. If the data were not available, we performed sensitivity analyses to assess how sensitive the results were to reasonable changes in the assumptions made.
Assessment of heterogeneity
We compared the Chi2 statistic with its degrees of freedom to identify heterogeneity. We considered a statistic bigger than its degrees of freedom and I2 > 30% as evidence of heterogeneity.
Assessment of reporting biases
We conducted standard funnel plots for the primary outcome (i.e. 6MWD) to investigate the potential for the influence of publication bias on the analysis.
Data synthesis
In the absence of significant heterogeneity, we combined results using a mean difference (MD) for continuous data, and odds ratio (OR) for dichotomous data. If there was significant heterogeneity (i.e. the ratio of Chi2 statistic and its degrees of freedom was bigger than 1.0, and the I2 > 30%), we used the random‐effects model to assess treatment effect.
A number of articles reported studies where more than one eligible treatment arm was included. For these studies, we split the data between two treatment‐control comparisons by splitting the control group and halving the sample sizes. For example, STRIDE‐1 was a triple‐arm clinical study that did not provide combined 'sitaxsentan' data versus placebo on secondary outcomes such as mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance (PVR). For relevant outcomes, we entered data from both intervention arms separately for the meta‐analysis, denoting the dosage distinction in footnotes. Data for placebo participants were entered with the number of participants in each of the placebo 'groups' halved. The total numbers in the meta‐analysis thus added up to the original size of the group. The same method was used in STRIDE‐2, STRIDE‐4, and SERAPHIN (see Characteristics of included studies). STRIDE‐2 compared sitaxsentan with both a placebo arm and an open‐label bosentan arm. For this study, we used the data from participants treated with bosentan and placebo for reference in the notes, and did not pool the data with those of other studies.
The AMBITION study had triple arms, that is combination therapy with 10 mg of ambrisentan plus 40 mg of tadalafil (combination‐therapy group), 10 mg of ambrisentan plus placebo (ambrisentan monotherapy group), or 40 mg of tadalafil plus placebo (tadalafil‐monotherapy group). We pooled the data from the combination‐therapy groups versus tadalafil‐monotherapy group in the review, and compared ambrisentan monotherapy versus tadalafil in the subgroup analysis of ERAs versus phosphodiesterase inhibitors.
Subgroup analysis and investigation of heterogeneity
Non‐selective ERA (antagonising both ET‐A and ET‐B) versus selective ERA (antagonising ET‐A) only.
Participants with idiopathic PAH versus those with PAH related to other conditions.
Sensitivity analysis
When there was a significant difference in the characteristics of participants, intervention, comparator, or outcome, we conducted a sensitivity analysis to assess how the results were affected by reasonable changes to the assumptions made.
Summary of findings and assessment of the certainty of the evidence
We created a 'Summary of findings' table using the following outcomes (Table 1): change in 6MWD, improvement in WHO functional class, Borg dyspnoea scores, mortality, PAP, PVR, and risk of hepatic toxicity. We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data for the prespecified outcomes. We used the methods and recommendations described in Section 8.5 and Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020), employing GRADEpro GDT software (GRADEpro GDT). We justified all decisions to downgrade the quality of studies using footnotes and made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
Results of the search
The literature search identified 5154 titles and abstracts. Of these, we identified 17 randomised controlled trials for inclusion in this version of the review (Table 2) (AMBITION; ARIES‐1; ARIES‐2; BREATHE‐1; BREATHE‐2; BREATHE‐5; Channick 2001; COMPASS‐2; EARLY; EDITA; MAESTRO; PORTICO; SERAPH; SERAPHIN; STRIDE‐1; STRIDE‐2; STRIDE‐4). We divided four trials based on the doses given (SERAPHIN; STRIDE‐1; STRIDE‐2; STRIDE‐4); see Data synthesis for details. The process of identifying studies eligible for inclusion in the analysis is summarised in a flow diagram (Figure 1). We obtained the full‐text versions of the 17 included studies from published papers or the FDA and EMA websites. Galiè 2003 reported data from a subset of participants from the BREATHE‐1 study. Studies referenced in multiple sources were assessed for completeness of study details. When there was a discrepancy in the data between published journal papers and documents from the FDA and EMA, we used data from the FDA or EMA. We identified two potentially eligible studies in abstract form. We contacted the authors for detailed data, but received no response, therefore we assessed these studies as awaiting classification (for details see Characteristics of studies awaiting classification).
1. Main study characteristics across all studies.
| Study | N | Country | Intervention | Control | Outcomes |
| AMBITION | 747 | International | Ambrisentan + tadalafil or ambrisentan | Tadalafil + placebo | Primary outcome: time to the first event of clinical failure Secondary outcomes: change from baseline in NT‐proBNP level, 6MWD, WHO FC, and Borg dyspnoea index |
| ARIES‐1 | 201 | International | Ambrisentan (5 mg/day or 10 mg/day) | Placebo | Primary outcome: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC, Borg dyspnoea index and time to clinical worsening, plasma BNP, and SF‐36 physical functioning scale |
| ARIES‐2 | 192 | International | Ambrisentan (2.5 mg/day or 5 mg/day) | Placebo | Primary outcome: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC, Borg dyspnoea index and time to clinical worsening, plasma BNP, and SF‐36 |
| BREATHE‐1 | 213 | International | Bosentan | Placebo | Primary outcome: change from baseline in 6MWD Secondary outcomes: Borg dyspnoea index, WHO FC |
| BREATHE‐2 | 33 | International | Bosentan | Placebo | Primary outcome: change from baseline to week 16 in TPR Secondary outcomes: change in cardiac index, PVR, mPAP, mRAP, 6MWD, NYHA FC and dyspnoea‐fatigue rating |
| BREATHE‐5 | 54 | International | Bosentan | Placebo | Primary outcome: change from baseline in SpO2 and PVR Secondary outcomes: change from baseline in 6MWD, WHO FC, cardiac index, PVR, mPAP, and mRAP |
| Channick 2001 | 32 | International | Bosentan | Placebo | Primary outcome: change from baseline in 6MWD Secondary outcomes: cardiopulmonary haemodynamics (cardiac index, PVR, mPAP, mRAP), WHO FC |
| COMPASS‐2 | 334 | International | Bosentan | Placebo | Primary outcome: time to the first morbidity/mortality event Secondary outcomes: change in 6MWD, WHO FC, time to the first occurrence of death from any cause, hospitalisation for PAH or start of intravenous prostanoid therapy, atrial septostomy, or lung transplant. |
| EARLY | 185 | International | Bosentan | Placebo | Primary outcomes: PVR and change from baseline in 6MWD Secondary outcomes: time to clinical worsening, change from baseline to month 6 in WHO FC, Borg dyspnoea index, mPAP, cardiac index, RAP, and SvO2 |
| EDITA | 38 | Germany | Ambrisentan | Placebo | Primary outcome: change in mPAP Secondary outcomes: change in WHO FC, change in cardiac index, change in PVR, symptoms of SSc, quality of life (SF‐36), lung function tests, right heart dimensions and function, NT‐proBNP, measures of disease‐related progression |
| MAESTRO | 150 | International | Macitentan | Placebo | Primary outcome: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC and Borg dyspnoea index |
| PORTICO | 85 | International | Macitentan | Placebo | Primary outcome: change from baseline to PVR Secondary outcomes: change from baseline in RAP, mPAP, cardiac index, total pulmonary resistance, SvO2, NT‐proBNP, 6MWD, and WHO FC |
| SERAPH | 26 | British | Bosentan | Sildenafil | Primary outcome: change in right ventricle mass from baseline Secondary outcomes: change from baseline in 6MWD, cardiac index, Borg dyspnoea index, quality of life, and plasma BNP level from baseline |
| SERAPHIN | 742 | International | Macitentan | Placebo | Primary outcome: time from the initiation of treatment to the first event related to pulmonary arterial hypertension Secondary outcomes: change from baseline in 6MWD, percentage of participants with an improvement in WHO FC, death due to PAH or hospitalisations for PAH, and death from any cause |
| STRIDE‐1 | 178 | International | Sitaxsentan | Placebo | Primary outcome: peak oxygen consumption Secondary outcomes: change from baseline in 6MWD, NYHA FC, PAP, cardiac index, and PVR |
| STRIDE‐2 | 245 | International | Sitaxsentan | Placebo | Primary outcome: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC, Borg dyspnoea index and time to clinical worsening |
| STRIDE‐4 | 98 | International | Sitaxsentan | Placebo | Primary efficacy endpoint was the change in 6MWD from baseline to week 18. Secondary outcomes: changes in WHO FC from baseline at each assessment and time to clinical worsening, Borg dyspnoea index |
6MWD: 6‐minute walk distance; BNP: B‐type natriuretic peptide; mPAP: mean pulmonary artery pressure; mRAP: mean right atrial pressure; MVO2: mixed venous oxygen saturation; NT‐proBNP: N‐terminal pro–brain natriuretic peptide; NYHA FC: New York Heart Association functional class; PAH: pulmonary arterial hypertension; PVR: pulmonary vascular resistance; SF‐36: 36‐item Short Form Health Survey; SpO2: oxygen saturation; SSc: systemic sclerosis; SvO2: mixed venous oxygen saturation; TPR: total pulmonary resistance; WHO FC: World Health Organization functional class
Included studies
Methods
Sixteen studies were randomised and placebo‐controlled (AMBITION; ARIES‐1; ARIES‐2; BREATHE‐1; BREATHE‐2; BREATHE‐5; Channick 2001; COMPASS‐2; EARLY; EDITA; MAESTRO; PORTICO; SERAPHIN; STRIDE‐1; STRIDE‐2; STRIDE‐4). One study was a randomised head‐to‐head study of bosentan (an ERA) and sildenafil (a PDE5 inhibitor) (SERAPH). A full description of each study with clinical characteristics at baseline, inclusion/exclusion criteria, methods of randomisation, and outcomes is provided in Characteristics of included studies.
Interventions
Nine trials compared a non‐selective ERA (bosentan and macitentan) against placebo (BREATHE‐1; BREATHE‐2; BREATHE‐5; Channick 2001; COMPASS‐2; EARLY; MAESTRO; PORTICO; SERAPHIN). Seven trials compared selective ERAs (sitaxsentan or ambrisentan) against placebo (AMBITION; ARIES‐1; ARIES‐2; EDITA; STRIDE‐1; STRIDE‐2; STRIDE‐4). Three studies tested the efficacy of combination therapy versus monotherapy in participants with PAH (AMBITION; BREATHE‐2; COMPASS‐2). SERAPH compared bosentan against sildenafil (a PDE5 inhibitor).
For the purposes of the review, we specified two comparisons: comparison 1: ERA versus placebo (where ERAs are subgrouped into selective versus non‐selective); and comparison 2: ERA versus PDE5 inhibitor.
Participants
Thirteen trials recruited participants with idiopathic PAH and PAH secondary to connective tissue disease and congenital heart disease (AMBITION; ARIES‐1; ARIES‐2; BREATHE‐1; BREATHE‐2; Channick 2001; COMPASS‐2; EARLY; SERAPH; SERAPHIN; STRIDE‐1; STRIDE‐2; STRIDE‐4). Two trials tested the efficacy of ERAs in participants with Eisenmenger syndrome (BREATHE‐5; MAESTRO). One study investigated the effect of macitentan on participants with portopulmonary hypertension (PORTICO). One study investigated systemic sclerosis patients with mildly elevated mean pulmonary arterial pressure (mPAP) at rest between 21 and 24 mmHg and/or > 30 mmHg during low‐dose exercise (EDITA).
Outcomes
Seven trials used the 6MWD as the primary outcome measure (ARIES‐1; ARIES‐2; BREATHE‐1; Channick 2001; MAESTRO; STRIDE‐2; STRIDE‐4), whilst one trial used change from baseline in peripheral oxygen saturation (SpO2) and PVR as the primary outcome (BREATHE‐5). STRIDE‐1 used peak exercise VO2 as its primary outcome. EARLY and PORTICO used PVR as the primary outcome. The primary efficacy parameter in BREATHE‐2 was change from baseline to week 16 in total pulmonary resistance (TPR). SERAPH used change in right ventricle mass from baseline as its primary endpoint. Three studies used time to first event of clinical worsening as the primary endpoint (AMBITION; COMPASS‐2; SERAPHIN). The EDITA study used change in mPAP at rest between groups as the primary endpoint. Additional outcome measures used in the included studies were WHO or NYHA functional class status, Borg dyspnoea score, cardiopulmonary haemodynamics, and drug safety and tolerability. For details, see Characteristics of included studies.
Excluded studies
We excluded 101 studies, with reasons provided in the Characteristics of excluded studies table.
Risk of bias in included studies
All but two trials, EDITA; SERAPH, were reported as international, multicentre, double‐blind, randomised, placebo‐controlled trials. Both SERAPH and EDITA are single‐centre, double‐blind, randomised, placebo‐controlled trials. Randomisation in all trials was conducted according to computer‐generated random number programs. We judged allocation concealment to be adequate in all trials. All trials described dropouts and withdrawals. All but two trials, EARLY; EDITA, were statistically analysed according to the intention‐to‐treat principle. See Characteristics of included studies for a full description of the risk of bias in included studies, and Figure 2 for a 'Risk of bias' summary.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All of the included trials used computer‐generated random numbers and were placebo controlled, therefore we judged allocation concealment to be adequate.
Blinding
Eight trials reported detailed descriptions of how treating clinicians, participants, and assessors were sufficiently blinded and were therefore judged as at low risk of bias (AMBITION; BREATHE‐5; COMPASS‐2; EARLY; EDITA; MAESTRO; PORTICO; SERAPHIN). Other trials were reported to be double‐blind, but as no further information was available we judged them to be at unclear risk of bias.
Incomplete outcome data
All studies described details of dropouts and withdrawals. All trials except for two, EARLY; EDITA, performed statistical analysis based on intention‐to‐treat. However, due to the high occurrence of missing data, 13 included trials were categorized as at high risk of attrition bias. For details, see Characteristics of included studies.
Selective reporting
Funnel plots for trials comparing ERAs versus placebo (outcome of change from baseline in 6MWD) were qualitatively symmetrical and did not suggest the presence of publication bias. Nine trials reported their prespecified primary outcomes according to pre‐published protocols in ClinicalTrials.gov and were therefore considered to be at low risk of reporting bias (AMBITION; ARIES‐1; ARIES‐2; COMPASS‐2; EARLY; EDITA; MAESTRO; PORTICO; SERAPHIN). We did not have access to protocols for the other studies.
Other potential sources of bias
It should be noted that most (16 out of 17) included studies were sponsored by the drug manufacturer.
Effects of interventions
See: Table 1
Only outcomes specified in the protocol were included in the meta‐analysis. Unless otherwise stated, data used in the review were from published material or documents released from the FDA and EMA.
Comparison 1: Endothelin receptor antagonists versus placebo
Sixteen studies reported ERA versus placebo; these have been subgrouped into selective and non‐selective ERAs.
Primary outcomes
Exercise capacity
Sixteen trials assessed change from baseline in 6MWD (AMBITION; ARIES‐1; ARIES‐2; BREATHE‐1; BREATHE‐2; BREATHE‐5; Channick 2001; COMPASS‐2; EARLY; EDITA; MAESTRO; PORTICO; SERAPHIN; STRIDE‐1; STRIDE‐2; STRIDE‐4). The pooled data showed that participants treated with ERAs could walk 25 metres further than those treated with placebo (mean difference (MD) 25.06 m, 95% confidence interval (CI) 17.13 to 32.99; participants = 2739; studies = 14; I2 = 34%; Analysis 1.1, moderate‐certainty evidence). We observed significant heterogeneity between the trials.
1.1. Analysis.

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 1: Change from baseline in 6‐minute walk
Subgroup analysis showed that the therapeutic effect of the non‐selective ERA (bosentan or macitentan) on 6MWD was 20.51 metres (95% CI 10.03 to 31.00; participants = 1860; studies = 8; I2 = 42%), and the selective ERAs was 33.48 metres (95% CI 23.12 to 43.83; participants = 879; studies = 6; I2 = 0%) compared to placebo. The test for subgroup differences was not significant (Chi2 = 2.97, df = 1 (P = 0.08), I2 = 66.4%).
Data from BREATHE‐2 and AMBITION were insufficient to evaluate the exercise capacity test. The BREATHE‐2 study showed a remarkable increase in the 6MWD in both treatment groups (68 metres (median) in the bosentan/prostacyclin group versus 74 metres (median) in the placebo/prostacyclin group). However, no statistical difference was observed between the two groups in the 6MWD.
AMBITION showed the median change from baseline to week 24 in 6MWD to be 48.98 (interquartile range (IQR) 4.63 to 85.75) in the combined ambrisentan plus tadalafil group and 22.70 (IQR −8.25 to 66.00) in the tadalafil‐monotherapy group, favouring the combination therapy group (P< 0.01).
WHO or NYHA functional class
Fifteen trials including a total of 3184 participants reported detailed numbers of participants with improved or deteriorated WHO/NYHA functional class (AMBITION; ARIES‐1; ARIES‐2; BREATHE‐1; BREATHE‐2; BREATHE‐5; Channick 2001; COMPASS‐2; EARLY; MAESTRO; PORTICO; SERAPHIN; STRIDE‐1; STRIDE‐2; STRIDE‐4).
ERAs improved more participants' functional class than placebo (odds ratio (OR) 1.41, 95% CI 1.16 to 1.70; participants = 3060; studies = 15; I2 = 5%; Analysis 1.2, moderate‐certainty evidence) and lowered the odds of functional class deterioration (OR 0.43, 95% CI 0.26 to 0.72; participants = 2347; studies = 13; I2 = 40%; Analysis 1.3, moderate‐certainty evidence).
1.2. Analysis.

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 2: Proportion of participants with improved functional class
1.3. Analysis.

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 3: Proportion of participants with deteriorated functional class
For non‐selective ERAs, 248 of 1118 participants in the ERA group improved their functional class versus 117 to 778 participants in the placebo group (OR 1.45, 95% CI 1.13 to 1.87; participants = 1896; studies = 9; I2 = 29%; Analysis 1.2), favouring non‐selective ERAs. For selective ERAs, 260 of 880 participants in the ERA group improved their functional class versus 90 of 408 in the placebo group (OR 1.35, 95% CI 1.01 to 1.80; participants = 1164; studies = 6; I2 = 0%), favouring selective ERAs (Analysis 1.2). The test for subgroup differences was not significant (Chi2 = 0.13, df = 1 (P = 0.72), I2 = 0%).
Both non‐selective ERA and selective ERAs significantly lowered the occurrence of WHO/NYHA function deterioration in participants with PAH: non‐selective ERAs (OR 0.65, 95% CI 0.30 to 1.42; participants = 1121; studies = 7; I2 = 32%) and selective ERAs (OR 0.31, 95% CI 0.17 to 0.60; participants = 1226; studies = 6; I2 = 36%; Analysis 1.3) compared to placebo. The test for subgroup differences was not significant (Chi2 = 1.98, df = 1 (P = 0.16), I2 = 49.6%).
Borg dyspnoea score
Considering symptoms such as dyspnoea is an important aspect of assessing response to therapy. In the most commonly used Borg dyspnoea scale in PAH, lower is better, and the minimal clinically important difference in PAH is 1 unit (Khair 2016). Data from seven trials included in this review addressed this outcome (ARIES‐1; ARIES‐2; BREATHE‐1; Channick 2001; EDITA; SERAPH; STRIDE‐2). We found heterogeneity in the selective ERA trials that could not be explained in terms of clinical characteristics and methodological quality, so we pooled the data using a random‐effects model. Overall, ERAs may mildly reduce Borg dyspnoea scores by 0.43 units (MD −0.43, 95% CI −0.90 to 0.04; participants = 788; studies = 7; I2 = 49%; Analysis 1.4, low‐certainty evidence).
1.4. Analysis.

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 4: Change from baseline in Borg dyspnoea index
Compared with placebo, both the non‐selective ERA bosentan (MD −0.27, 95% CI −1.58 to 1.03; participants = 240; studies = 3; I2 = 60%) and selective ERAs (MD −0.43, 95% CI −1.01 to 0.14; participants = 548; studies = 4; I2 = 55%; Analysis 1.4) may reduce Borg dyspnoea score. The test for subgroup differences was not significant (Chi2 = 0.05, df = 1 (P = 0.83), I2 = 0%).
MAESTRO reported that no relevant trends in change from baseline to week 16 in the Borg dyspnoea index existed between the macitentan and placebo groups, but did not provide detailed data. BREATHE‐5 reported Borg score as an outcome with limited detail.
AMBITION reported the treating effects in the study portal (ClinicalTrials.gov: NCT01178073) as median IQR: the change from baseline in Borg dyspnoea score was −1.00 (−2.00 to 0.50) in the combination therapy group; and −0.50 (−2.00 to 0.88) in the tadalafil‐monotherapy group.
Mortality
Twelve trials including 2889 participants reported a total of 193 deaths (AMBITION; ARIES‐1; ARIES‐2; BREATHE‐1; BREATHE‐2; COMPASS‐2; EARLY; MAESTRO; SERAPHIN; STRIDE‐1; STRIDE‐2), of which 113 were on active treatment and 80 on placebo. There was a non‐significant trend towards ERAs lowering mortality in participants with PAH (OR 0.78, 95% CI 0.58 to 1.07; participants = 2889; studies = 12; I2 = 0%; Analysis 1.5, low‐certainty evidence).
1.5. Analysis.

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 5: Mortality
Subgroup analysis from the pooled data showed that there was no significant difference in mortality between non‐selective ERAs and selective ERAs and placebo (OR 0.88, 95% CI 0.62 to 1.23; participants = 1759; studies = 7; I2 = 0%), whereas selective ERAs were found to significantly lower mortality (OR 0.45, 95% CI 0.21 to 0.94; participants = 1130; studies = 5; I2 = 0%). The test for subgroup differences was not significant (Chi2 = 2.62, df = 1 (P = 0.11), I2 = 61.8%).
To compare the treatment effects of subgroups (selective ERAs versus non‐selective ERAs) on mortality, we calculated the interaction between the two subgroups using a method previously described by Altman 2003. The estimated interaction effect is a relative odds ratio 0.51 (95% CI 0.22 to 1.16), indicating that there is no clear evidence of a different treatment effect between non‐selective ERAs and selective ERAs on mortality.
Secondary outcomes
Cardiopulmonary haemodynamics
Ten studies measured haemodynamics variables (BREATHE‐1; BREATHE‐2; BREATHE‐5; Channick 2001; EARLY; EDITA; MAESTRO; PORTICO; SERAPHIN; STRIDE‐1): mean pulmonary artery pressure (mPAP), pulmonary vascular resistance (mPVR), and cardiac index. Haemodynamic variables were measured by right‐heart catheterisation in seven studies (BREATHE‐2; Channick 2001; EARLY; MAESTRO; PORTICO; SERAPHIN; STRIDE‐1), compared with BREATHE‐1, which used an echocardiological method. Data from BREATHE‐1 were reported as a subgroup analysis in Galiè 2003.
Mean pulmonary artery pressure
Nine studies assessed mPAP (BREATHE‐2; BREATHE‐5; Channick 2001; EARLY; EDITA; MAESTRO; PORTICO; SERAPHIN; STRIDE‐1). We observed significant heterogeneity between the studies, therefore we pooled them using random‐effects modelling. Pooled data from eight studies showed that ERAs lowered mPAP by 4.65 mmHg (95% CI −6.05 to −3.26; participants = 729; I2 = 19%; Analysis 1.6, moderate‐certainty evidence) more than placebo (BREATHE‐5; Channick 2001; EARLY; EDITA; MAESTRO; PORTICO; SERAPHIN; STRIDE‐1).
1.6. Analysis.

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 6: Change from baseline in mean pulmonary artery pressure
The non‐selective ERA (bosentan) lowered mPAP by 5.79 mmHg (95% CI −7.30 to −4.27; participants = 519; studies = 6; I2 = 0%; Analysis 1.6) more than placebo, whereas the selective ERA lowered mPAP by 2.65 mmHg (95% CI −5.31 to 0.00; participants = 210; studies = 2; I2 = 36%; Analysis 1.6) more than placebo. There was a significant difference in the test for subgroup differences (Chi2= 4.04, df = 1 (P = 0.04), I2 = 75.2%).
BREATHE‐2 did not report data for change from baseline in mPAP, but instead as an end‐of‐treatment score: mean ± standard error (SE) was 59.2 ± 3.2 mmHg in the placebo group and 52.5 ± 2.4 mmHg in the bosentan group, which was not statistically significant (P = 0.3).
Pulmonary vascular resistance
Seven studies on 586 participants reported data of change from baseline in PVR, all of them showing that ERA significantly improved PVR compared with placebo (BREATHE‐5; Channick 2001; EARLY; EDITA; MAESTRO; PORTICO; STRIDE‐1).
We observed significant heterogeneity between studies and therefore pooled them using random‐effects modelling. Data from seven trials showed that ERAs could significantly reduce PVR by 236.24 dyn/s/cm5 (95% CI −333.21 to −139.26; participants = 586; studies = 7; I2 = 14.7%; Analysis 1.7, moderate‐certainty evidence) more than placebo (BREATHE‐5; Channick 2001; EARLY; EDITA; MAESTRO; PORTICO; STRIDE‐1).
1.7. Analysis.

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 7: Change from baseline in pulmonary vascular resistance
BREATHE‐2 and SERAPHIN reported an end‐of‐treatment score, as mean and standard deviation. The pooled data showed that participants treated with ERAs had much lower PVR than those treated with placebo (MD −288.59 dyn/s/cm5, 95% CI −472.18 to −104.99; participants = 175; studies = 2; I2 = 0%; Analysis 1.8).
1.8. Analysis.

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 8: Pulmonary vascular resistance
Three studies reported ratio of geometric mean PVR. The pooled data showed that participants receiving ERAs had a much lower ratio of geometric mean PVR (0.69, 95% CI 0.60 to 0.80; participants = 124; Analysis 1.9), favouring ERAs (MAESTRO; PORTICO; SERAPHIN).
1.9. Analysis.

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 9: Ratio of geometric mean PVR
Cardiac index
Seven studies on 718 participants reported cardiac index data (Channick 2001; EARLY; EDITA; Galiè 2003; PORTICO; SERAPHIN; STRIDE‐1). We observed significant heterogeneity between studies and therefore pooled them using a random‐effects model. Pooled data from these studies showed that ERAs could significantly increase cardiac index compared with placebo (MD 0.50 L/min/m2, 95% CI 0.35 to 0.65; participants = 718; studies = 7; I2 = 59%; Analysis 1.10) (Channick 2001; EARLY; EDITA; Galiè 2003; PORTICO; SERAPHIN; STRIDE‐1). The placebo‐corrected therapeutic effect of the non‐selective ERA on cardiac index was 0.55 L/min/m2 (95% CI 0.34 to 0.77; participants = 509; studies = 5; I2 = 70%), whilst for the selective ERA (sitaxsentan) it was 0.39 L/min/m2 (95% CI 0.23 to 0.54; participants = 209; studies = 2; I2 = 0%). The test for subgroup differences was not significant (Chi2 = 1.50, df = 1 (P = 0.22), I2 = 33.2%).
1.10. Analysis.

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 10: Change from baseline in cardiac index
Adverse events
Eleven trials including 2250 participants reported hepatic toxicity in detail (AMBITION; BREATHE‐1; BREATHE‐2; BREATHE‐5; Channick 2001; EARLY; MAESTRO; PORTICO; SERAPHIN; STRIDE‐1; STRIDE‐2). We observed heterogeneity between studies and therefore pooled them using a random‐effects model. Overall, 102 of 1357 participants receiving ERA had hepatic toxicity events compared with 33 of 893 participants receiving placebo, which was not significantly different (OR 1.88, 95% CI 0.91 to 3.90; participants = 2250; studies = 11; I2 = 53%, moderate‐certainty evidence), though the direction of effect favoured placebo.
ARIES‐1 and ARIES‐2 reported reassuring combined data for hepatic toxicity. None of the 261 participants receiving ambrisentan developed serum aminotransferase concentrations greater than three times the upper limit of normal, compared with three participants (2.3%) receiving placebo displaying elevated levels.
Subgroup analysis
Pulmonary arterial hypertension associated with connective tissue disease
The subgroup analysis of non‐selective ERAs versus selective ERAs for mortality is described above. We could not perform a subgroup analysis comparing participants with idiopathic PAH versus those with PAH related to other conditions due to insufficient information. However, Denton 2006 reported the combined data from two trials, BREATHE‐1; Channick 2001, comparing bosentan to placebo for PAH secondary to connective tissue disease (PAH‐CTD). There was no significant difference between the bosentan and placebo groups.
Seibold 2005 reported the combined data of sitaxsentan from STRIDE‐1, STRIDE‐2, and STRIDE‐4 for PAH‐CTD, which included sitaxsentan at 50 mg, 100 mg, and 300 mg once daily; 110 of 512 participants had PAH‐CTD, including 63 with systemic sclerosis (SSc), 22 with overlap/mixed connective tissue disease, and 25 with systemic lupus erythematosus. The data showed a change from baseline in 6MWD of −16 ± 15.0 metres (mean ± SE) for placebo (N = 28); −2 ± 13.4 metres for sitaxsentan 50 mg daily (N = 26); 21 ± 10.4 metres for sitaxsentan 100 mg daily (N = 39); and 2 ± 14.1 metres for sitaxsentan 300 mg daily (N = 17). Only participants treated with 100 mg daily (the dose authorised by the EMA) significantly improved in 6MWD, which was comparable to participants with idiopathic PAH treated with sitaxsentan.
Badesch 2007 reported the pooled data for ambrisentan therapy in idiopathic PAH (n = 251) and PAH‐CTD (n = 124). At week 12, the placebo‐adjusted increase in 6MWD was 58 metres (95% CI 36 to 79) in participants with idiopathic PAH and 19 metres (95% CI −10 to 48) in participants with PAH‐CTD; the placebo‐adjusted decrease in Borg dyspnoea score was −0.8 (95% CI −1.4 to −0.19) in the idiopathic PAH group and −1.0 (95% CI −1.7 to −0.18) in the PAH‐CTD group. WHO functional class deterioration was 2.4% in participants with idiopathic PAH and 3.7% in participants with PAH‐CTD in the ambrisentan group, compared with 16.5% in participants with idiopathic PAH and 20.9% in participants with PAH‐CTD in the placebo group.
In the AMBITION study, 187 participants had CTD associated with PAH, of whom 118 had SSc associated with PAH. Initial combination therapy reduced the risk of clinical failure when compared with pooled monotherapy in each subgroup: CTD‐PAH (hazard ratio 0.43, 95% CI 0.24 to 0.77) and SSc‐PAH (0.44, 95% CI 0.22 to 0.89).
In the EDITA study, 38 participants had SSc associated with mildly elevated mPAP between 21 and 24 mmHg at rest and/or > 30 mmHg during low‐dose exercise were randomly assigned to treatment with either ambrisentan 5 to 10 mg/day or placebo. After six months, the two groups did not differ in the primary endpoint (i.e. change in mPAP). However, the ambrisentan group showed significant improvements in the secondary endpoints, that is cardiac index and PVR, at rest or at peak exercise.
Pulmonary arterial hypertension associated with portopulmonary hypertension
PORTICO first reported the effect of the non‐selective ERA macitentan on portopulmonary hypertension. The study's primary outcome (i.e. PVR at the end of study) was improved in the macitentan group, but not exercise capacity or WHO functional class at the end of study.
Pulmonary arterial hypertension associated with congenital heart disease
Two trials investigated the efficacy of non‐selective ERAs on Eisenmenger syndrome (BREATHE‐5; MAESTRO). The pooled data showed that non‐selective ERAs had no impact on exercise capacity, WHO functional class, or mortality. However, ERAs did improve mPAP (MD −4.63 mmHg, 95% CI −8.03 to −1.23; participants = 90; studies = 2; I2 = 0%; Analysis 3.5) and PVR (MD −480.07 dyn/s/cm5, 95% CI −753.34 to −206.79; participants = 93; studies = 2; I2 = 0%; Analysis 3.6).
3.5. Analysis.

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 5: Change from baseline in mean pulmonary arterial pressure
3.6. Analysis.

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 6: Change from baseline in pulmonary vascular resistance
Comparison 2: Endothelin receptor antagonists versus phosphodiesterase‐5 inhibitors
Primary outcomes
Exercise capacity
Two trials tested the efficacy of ERA versus PDE5 inhibitors (AMBITION; SERAPH).
SERAPH reported that mean 6MWD increased with both treatments compared to baseline. Sildenfafil may increase exercise capacity (MD 55 m, 95% CI 0.1 to 109.9; Analysis 2.1) when compared with bosentan in participants with PAH.
2.1. Analysis.

Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 1: 6‐minute walk
AMBITION showed a median change from baseline to week 24 in 6MWD of 27.00 (IQR −14.00 to 63.25) in the ambrisentan‐monotherapy group and 22.70 (IQR −8.25 to 66.00) in the tadalafil‐monotherapy group, with no difference between groups.
WHO or NYHA functional class
Only AMBITION reported the effect of ambrisentan versus tadalafil on WHO functional class. There was no difference between groups in either WHO functional improvement or deterioration (Analysis 2.2; Analysis 2.3).
2.2. Analysis.

Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 2: Proportion of participants with improved functional class
2.3. Analysis.

Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 3: Proportion of participants with deteriorated functional class
Borg dyspnoea score
Only the SERAPH study reported detailed data on Borg dyspnoea score. A trend showed that sildenafil may reduce Borg dyspnoea score compared with bosentan in participants with PAH (Analysis 2.4).
2.4. Analysis.

Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 4: Symptoms
Mortality
A trend showed that ERAs and PDE‐5 inhibitors may reduce mortality in participants with PAH (OR 0.32, 95% CI 0.07 to 1.36; participants = 273; studies = 2; I2 = 0%; Analysis 2.5).
2.5. Analysis.

Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 5: Mortality
Secondary outcomes
Cardiac index
Only the SERAPH study reported data on cardiac index. There was no difference between treatments for this outcome (MD 0 L/min/m2, 95% CI −0.14 to 0.14; participants = 25; studies = 1; Analysis 2.6).
2.6. Analysis.

Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 6: Cardiac index
Sensitivity analysis
Including or excluding combination therapy did not make a difference in the effect of ERAs on the primary outcomes (i.e. exercise capacity, functional class, and mortality) (Table 3).
2. Sensitivity analysis: including versus excluding combination therapy.
| Outcome | Including combination therapy | Excluding combination therapy |
| Change in 6MWD, mean with 95% CI | 25.06 (17.13 to 32.99) | 25.65 (16.80 to 34.49) |
| WHO/NYHA FC improvement, OR with 95% CI | 1.41 (1.16 to 1.70) | 1.52 (1.22 to 1.91) |
| Mortality, OR with 95% CI | 0.78 (0.58 to 1.07) | 0.82 (0.58 to 1.17) |
6MWD: 6‐minute walk distance; CI: confidence interval; NYHA FC: New York Heart Association functional class; OR: odds ratio; WHO FC: World Health Organization functional class
Including or excluding PAH participants with PAH associated with congenital heart disease and portal hypertension had little impact on the effect of ERAs on primary outcomes such as exercise capacity, functional class, and mortality (Table 4).
3. Sensitivity analysis: including versus excluding PAH participants associated with SSc, or CHD, or portal hypertension.
| Outcome | Including combination therapy | Excluding combination therapy |
| Change in 6MWD, mean with 95% CI | 25.06 (17.13 to 32.99) | 27.90 (20.96 to 34.83) |
| WHO/NYHA FC improvement, OR with 95% CI | 1.41 (1.16 to 1.70) | 1.46 (1.19 to 1.78) |
| Mortality, OR with 95% CI | 0.78 (0.58 to 1.07) | 0.77 (0.57 to 1.05) |
6MWD: 6‐minute walk distance; CHD: congenital heart disease; CI: confidence interval; NYHA FC: New York Heart Association functional class; OR: odds ratio; PAH: pulmonary arterial hypertension; SSc: systemic sclerosis; WHO FC: World Health Organization functional class
Discussion
The pooled data show that ERAs probably increase exercise capacity, improve WHO functional class, prevent WHO functional class deterioration, reduce dyspnoea, and improve cardiopulmonary haemodynamic variables, and may improve mortality in participants with PAH. However, they may also increase the risk of liver damage, although this was rare.
Clinical efficacy
Minimal clinically important differences (MCID) are patient‐derived scores that reflect changes in a clinical intervention that are meaningful for the patient. The six‐minute walk test (6MWD), as a reliable tool for the assessment of exercise capacity in participants with PAH, is an independent predictor of mortality (Miyamoto 2000; Sitbon 2002). Although the pooled data showed that ERAs statistically significantly increased 6MWD by 25.06 m in participants with PAH, it is less than MCID estimated by Mathai 2012. Additionally, ERAs reduced the pooled Borg dyspnoea score by 0.43, where the MCID for PAH is 1 unit (Khair 2016).
Mortality events across the included trials were low, and we did not find a significant difference for these comparisons despite the trend favouring ERAs. The included studies were underpowered for evaluating mortality, particularly given their short duration of study period and the use of time to first event of related to PAH as primary endpoint, which led to censoring of participants earlier (BREATHE‐5; Channick 2001; STRIDE‐4). There is insufficient certainty around the observed result to exclude either a reduction in mortality or no difference between groups. Transplantation or epoprostenol remains the preferred therapy with a major reduction in mortality in PAH participants with WHO functional class IV (Barst 1996).
The exact mechanism of action of ERAs on the pulmonary vascular bed remains unclear. Vasodilatation is likely to be just a part of the mechanism since usually 70% to 80% of participants with idiopathic PAH do not respond acutely to vasodilators (Rich 1992; Sitbon 1998). PAH‐targeted therapies (e.g. prostaglandins, PDE inhibitors, ERAs, and soluble guanylate cyclase stimulators), used alone or in combination, improve exercise capacity and haemodynamics and reduce hospitalisations. However, these pulmonary vasodilators do not target key features of PAH pathogenesis and have not been shown to reduce mortality (Galiè 2015; Klinger 2019; Thenappan 2018).
Using ERAs as part of combination therapy is an attractive option to target the multiple pathophysiological mechanisms in PAH. Combination therapy might be pursued by the simultaneous initiation of two (or more) treatments or by the addition of a second (or third) treatment to a previous therapy that is considered insufficient. Several combination studies of ERAs and PDE inhibitors have been carried out in participants with PAH (AMBITION; COMPASS‐2; Zhuang 2014). One randomised controlled trial used bosentan plus sildenafil (COMPASS‐2), and another two randomised controlled trials used ambrisentan plus tadalafil (AMBITION; Zhuang 2014). The evidence consistently showed that participants with PAH receiving combination therapy could walk longer than those receiving monotherapy.
The BREATHE‐2 trials, with small sample sizes, explored the efficacy and safety of combination therapy (bosentan in combination with intravenous prostacyclin), showing a trend without statistical significance towards haemodynamic and clinical improvement of this combination in participants with PAH. There is clearly an interaction between bosentan and prostacyclin in the BREATHE‐2 trial. However, in line with the predefined subgroups in the protocol for this review, the BREATHE‐2 trial was included in the meta‐analysis of ERAs versus placebo. Nevertheless, excluding BREATHE‐2 from the meta‐analysis made little difference to the results.
Subgroup analysis
Both a non‐selective ERA and a selective ERA can dilate constricted pulmonary pre‐capillary arterioles and improve exercise capacity and symptoms. Determining the relative benefit of these agents requires head‐to‐head comparisons. A post hoc analysis of the STRIDE‐2 trial that was neither designed nor powered to detect non‐inferiority or equivalence showed a mean difference in the 18‐week change from baseline between sitaxsentan and bosentan of 1.9 metres (95% CI −22.7 to 26.5; P = 0.9). Also, the AMBITION trial reported that there was no difference on 6MWD between ambrisentan‐monotherapy group and tadalafil‐monotherapy group.
ERAs showed efficacy in participants with PAH associated with CTD in post hoc analysis. ERAs did improve cardiopulmonary haemodynamic in participants with congenital heart disease and portal hypertension, but had little impact on 6MWD and functional class.
Safety and tolerability
One major concern regarding ERAs is hepatotoxicity and its capacity to cause substantial damage to the liver. This review did not show a high occurrence of hepatic toxicity. However, there were three reported cases of irreversible liver failure induced by sitaxsentan, one in the UK in 2009 and two cases from clinical trials in India and Ukraine in 2010. The reported fatal hepatic toxicity in association with sitaxsentan was idiosyncratic, unlikely to be detected by monthly monitoring, and irreversible when sitaxsentan was discontinued. Consequently, sitaxsentan was withdrawn from the market voluntarily by its licence holder. These cases emphasise the importance of dosing and hepatic monitoring in individuals with PAH.
Summary of main results
For participants with pulmonary arterial hypertension with WHO functional class II and III, endothelin receptor antagonists probably increase exercise capacity, improve WHO functional class, prevent WHO functional class deterioration, result in favourable changes in cardiopulmonary haemodynamic variables. However, they are less effective in reducing dyspnoea and mortality.
Overall completeness and applicability of evidence
All the trials identified in the current review addressed our main outcomes (i.e. 6MWD, WHO/NYHA functional class, and mortality). All relevant types of participants, interventions, and outcomes have been investigated (Table 2). The evidence we collected is highly relevant to the review questions. For people with pulmonary arterial hypertension with WHO functional class II and III, endothelin receptor antagonists probably increase exercise capacity, improve WHO functional class, prevent WHO functional class deterioration, result in favourable changes in cardiopulmonary haemodynamic variables. However, they are less effective in reducing dyspnoea and mortality.
Certainty of the evidence
All data included in the meta‐analysis were from randomised, placebo‐controlled clinical trials, and the participants, treating clinicians, and assessors were blinded. All but two trials, EARLY; EDITA, performed analyses based on intention‐to‐treat for their prespecified primary outcomes. However, the risk of attrition bias is high in the included trials due to the high occurrence of missing data (Table 2). On this basis we consider the overall certainty of the evidence to be moderate. Overall, the results of the studies included in the review are consistent, confirming the internal validity of the results of the review.
Potential biases in the review process
Many studies used Borg dyspnoea score as an outcome but provided limited data, thus bias may be introduced into the review. We analysed hepatic toxicity as an adverse event because this was listed in the protocol and approved. However, there are other serious adverse events related to ERAs which limit its use in clinical practice.
Agreements and disagreements with other studies or reviews
Our results are consistent with recent overviews (Duo‐Ji 2017; Wang 2018). Our review is more current than these overviews and provides more robust data which further confirm the clinical efficacy of ERAs in participants with PAH.
Authors' conclusions
Implications for practice.
For people with pulmonary arterial hypertension with WHO functional class II and III, endothelin receptor antagonists probably increase exercise capacity, improve WHO functional class, prevent WHO functional class deterioration, result in favourable changes in cardiopulmonary haemodynamic variables. However, they are less effective in reducing dyspnoea and mortality. Efficacy data are strongest in participants with the idiopathic form of the disease. Limited data show that combination therapy using ERAs and PDE inhibitor may provide more benefit in PAH; however, this needs to be confirmed in future studies.
Implications for research.
The question of the effects of ERAs on PAH has now likely been answered. The effect of ERAs on dyspnoea score and mortality remains uncertain, and long‐term observational studies are warranted. Combination therapy using ERAs and PDE inhibitor may provide more benefit in PAH; however, this needs to be confirmed in future studies.
What's new
| Date | Event | Description |
|---|---|---|
| 29 March 2021 | Amended | Tracked changes in Figure 1 resolved. |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 20 January 2021 | New search has been performed | New literature search run, six new clinical studies added. Anticoagulation is not currently a recommended treatment for pulmonary arterial hypertension according to established guidelines, therefore we revised our inclusion criteria. We also revised our inclusion criteria with regard to the diagnosis of pulmonary arterial hypertension according to the new guidelines. We screened the trials again based on these changes. |
| 20 January 2021 | New citation required but conclusions have not changed | Adding five large‐scale clinical trials and one small trial to the review has made the evidence more robust; however, the conclusions remain unchanged. The review has been edited for style and the Plain language summary has been revised. |
| 2 May 2013 | New search has been performed | Literature search re‐run. |
| 2 May 2013 | New citation required and conclusions have changed | Substantive amendment. Five new studies added, and the results and conclusions updated. |
| 2 May 2013 | New citation required and conclusions have changed | The review has been updated with an additional two studies (Humbert 2004; Wilkins 2005). The additional data narrowed the confidence interval around the significant increase in the risk of improved functional class status in favour of endothelin receptor antagonists. |
Acknowledgements
We wish to thank the editorial staff of Cochrane Airways for their support. We would also like to thank Dr Sally Green, Ms Denise O'Connor, Mr Steve McDonald, Janet Piehl and other staff in the Australasian Cochrane Centre for their supervision and technical assistance in the progress of writing this review. We are also grateful to Dr Elmer Villanueva for excellent statistical assistance.
The authors and Airways Editorial Team are grateful to Theo Trandafirescu, Mount Sinai Medical Center for his peer review, as well as another peer reviewer who wishes to remain anonymous.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Database search strategies
CENTRAL (via the Cochrane Register of Studies)
#1 MESH DESCRIPTOR Hypertension, Pulmonary EXPLODE ALL #2 MESH DESCRIPTOR Pulmonary Heart Disease #3 pulmonar* near hypertensi* #4 PAH or IPAH.ti,ab. #5 {or #1‐#4} #6 MESH DESCRIPTOR Receptors, Endothelin EXPLODE ALL #7 endothelin* near receptor* #8 endothelium* near receptor* #9 bosentan #10 Sitaxentan #11 ambrisentan #12 Macitentan #13 Selexipag #14 {or #6‐#13} #15 #14 AND #5
MEDLINE Ovid SP
1. exp Hypertension, Pulmonary/ 2. Pulmonary Heart Disease/ 3. (pulmonar* adj2 hypertensi*).tw. 4. (PAH or IPAH).ab,ti. 5. or/1‐4 6. exp Receptors, Endothelin/ 7. exp Endothelin Receptor Antagonists/ 8. ((endothelin$ or endothelium$) adj2 receptor$).tw. 9. bosentan.tw. 10. Sitaxentan.tw. 11. ambrisentan.tw. 12. Macitentan.tw. 13. Selexipag.tw. 14. or/6‐13 15. 5 and 14 16. (controlled clinical trial or randomised controlled trial).pt. 17. (randomised or randomised).ab,ti. 18. placebo.ab,ti. 19. dt.fs. 20. randomly.ab,ti. 21. trial.ab,ti. 22. groups.ab,ti. 23. or/16‐22 24. Animals/ 25. Humans/ 26. 24 not (24 and 25) 27. 23 not 26 28. 15 and 27
EMBASE Ovid SP
1. exp Pulmonary Hypertension/ 2. (pulmonar* adj2 hypertensi*).tw. 3. (PAH or IPAH).ab,ti. 4. or/1‐3 5. exp endothelin receptor antagonist/ 6. ((endothelin$ or endothelium$) adj2 receptor$).tw. 7. bosentan.tw. 8. Sitaxentan.tw. 9. ambrisentan.tw. 10. Macitentan.tw. 11. Selexipag.tw. 12. or/5‐11 13. 4 and 12 14. Randomized Controlled Trial/ 15. randomisation/ 16. controlled clinical trial/ 17. Double Blind Procedure/ 18. Single Blind Procedure/ 19. Crossover Procedure/ 20. (clinica$ adj3 trial$).tw. 21. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).tw. 22. exp Placebo/ 23. placebo$.ti,ab. 24. random$.ti,ab. 25. ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).tw. 26. (crossover$ or cross‐over$).ti,ab. 27. or/14‐26 28. exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 29. human/ or normal human/ or human cell/ 30. 28 and 29 31. 28 not 30 32. 27 not 31 33. 13 and 32
ClinicalTrials.gov
| Condition | Pulmonary Arterial Hypertension OR pulmonary hypertension |
| intervention | bosentan OR Sitaxentan OR ambrisentan OR Macitentan OR Selexipag |
| study type | Interventional |
WHO ICTRP
| Condition | Pulmonary Arterial Hypertension OR pulmonary hypertension |
| intervention | bosentan OR Sitaxentan OR ambrisentan OR Macitentan OR Selexipag |
Data and analyses
Comparison 1. Endothelin receptor antagonists versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Change from baseline in 6‐minute walk | 14 | 2739 | Mean Difference (IV, Random, 95% CI) | 25.06 [17.13, 32.99] |
| 1.1.1 Non‐selective ERA | 8 | 1860 | Mean Difference (IV, Random, 95% CI) | 20.51 [10.03, 31.00] |
| 1.1.2 Selective ERA | 6 | 879 | Mean Difference (IV, Random, 95% CI) | 33.48 [23.12, 43.83] |
| 1.2 Proportion of participants with improved functional class | 15 | 3060 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.16, 1.70] |
| 1.2.1 Non‐selective ERAs | 9 | 1896 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.13, 1.87] |
| 1.2.2 Selective ERAs | 6 | 1164 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.01, 1.80] |
| 1.3 Proportion of participants with deteriorated functional class | 13 | 2347 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.26, 0.72] |
| 1.3.1 Non‐selective ERA | 7 | 1121 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.30, 1.42] |
| 1.3.2 Selective ERAs | 6 | 1226 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.17, 0.60] |
| 1.4 Change from baseline in Borg dyspnoea index | 7 | 788 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.90, 0.04] |
| 1.4.1 Non‐selective ERAs | 3 | 240 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐1.58, 1.03] |
| 1.4.2 Selective ERAs | 4 | 548 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐1.01, 0.14] |
| 1.5 Mortality | 12 | 2889 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.58, 1.07] |
| 1.5.1 Non‐selective ERAs | 7 | 1759 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.62, 1.23] |
| 1.5.2 Selective ERAs | 5 | 1130 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.21, 0.94] |
| 1.6 Change from baseline in mean pulmonary artery pressure | 8 | 729 | Mean Difference (IV, Random, 95% CI) | ‐4.65 [‐6.05, ‐3.26] |
| 1.6.1 Non‐selective ERAs | 6 | 519 | Mean Difference (IV, Random, 95% CI) | ‐5.79 [‐7.30, ‐4.27] |
| 1.6.2 Selective ERAs | 2 | 210 | Mean Difference (IV, Random, 95% CI) | ‐2.65 [‐5.31, 0.00] |
| 1.7 Change from baseline in pulmonary vascular resistance | 7 | 586 | Mean Difference (IV, Random, 95% CI) | ‐236.24 [‐333.21, ‐139.26] |
| 1.7.1 Non‐selective ERAs | 5 | 376 | Mean Difference (IV, Random, 95% CI) | ‐281.74 [‐395.85, ‐167.63] |
| 1.7.2 Selective ERAs | 2 | 210 | Mean Difference (IV, Random, 95% CI) | ‐173.73 [‐332.52, ‐14.94] |
| 1.8 Pulmonary vascular resistance | 2 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐288.59 [‐472.18, ‐104.99] |
| 1.9 Ratio of geometric mean PVR | 3 | Ratio of Geometric mean (IV, Random, 95% CI) | Subtotals only | |
| 1.9.1 Selective ERAs | 0 | Ratio of Geometric mean (IV, Random, 95% CI) | Not estimable | |
| 1.9.2 Non‐selective ERAs | 3 | Ratio of Geometric mean (IV, Random, 95% CI) | 0.69 [0.60, 0.80] | |
| 1.10 Change from baseline in cardiac index | 7 | 718 | Mean Difference (IV, Random, 95% CI) | 0.50 [0.35, 0.65] |
| 1.10.1 Non‐selective ERAs | 5 | 509 | Mean Difference (IV, Random, 95% CI) | 0.55 [0.34, 0.77] |
| 1.10.2 Selective ERAs | 2 | 209 | Mean Difference (IV, Random, 95% CI) | 0.39 [0.23, 0.54] |
| 1.11 Change from baseline in SpO 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.12 Hepatic toxicity | 11 | 2250 | Odds Ratio (M‐H, Random, 95% CI) | 1.88 [0.91, 3.90] |
| 1.12.1 Non‐selective ERAs | 9 | 1888 | Odds Ratio (M‐H, Random, 95% CI) | 2.33 [0.98, 5.56] |
| 1.12.2 Selective ERAs | 2 | 362 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.31, 2.51] |
1.11. Analysis.

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 11: Change from baseline in SpO 2
1.12. Analysis.

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 12: Hepatic toxicity
Comparison 2. Endothelin receptor antagonists versus PDE5 inhibitor.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 6‐minute walk | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 Proportion of participants with improved functional class | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3 Proportion of participants with deteriorated functional class | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.4 Symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5 Mortality | 2 | 273 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.36] |
| 2.6 Cardiac index | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Endothelin receptor antagonists in Eisenmenger syndrome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Change from baseline in 6‐minute walk | 2 | 280 | Mean Difference (IV, Random, 95% CI) | 21.49 [‐31.23, 74.21] |
| 3.1.1 Non‐selective ERA | 2 | 280 | Mean Difference (IV, Random, 95% CI) | 21.49 [‐31.23, 74.21] |
| 3.1.2 Selective ERA | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 3.2 Proportion of participants with improved functional class | 2 | 280 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.48, 1.90] |
| 3.2.1 Non‐selective ERAs | 2 | 280 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.48, 1.90] |
| 3.2.2 Selective ERAs | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.3 Proportion of participants with deteriorated functional class | 2 | 280 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.09, 4.85] |
| 3.3.1 Non‐selective ERA | 2 | 280 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.09, 4.85] |
| 3.3.2 Selective ERAs | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | Not estimable |
| 3.4 Mortality | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.4.1 Non‐selective ERAs | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.4.2 Selective ERAs | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.5 Change from baseline in mean pulmonary arterial pressure | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐4.63 [‐8.03, ‐1.23] |
| 3.5.1 Non‐selective ERAs | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐4.63 [‐8.03, ‐1.23] |
| 3.5.2 Selective ERAs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 3.6 Change from baseline in pulmonary vascular resistance | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐480.07 [‐753.34, ‐206.79] |
| 3.6.1 Non‐selective ERAs | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐480.07 [‐753.34, ‐206.79] |
| 3.6.2 Selective ERAs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 3.7 Change from baseline in SpO 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 1: Change from baseline in 6‐minute walk
3.2. Analysis.

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 2: Proportion of participants with improved functional class
3.3. Analysis.

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 3: Proportion of participants with deteriorated functional class
3.4. Analysis.

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 4: Mortality
3.7. Analysis.

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 7: Change from baseline in SpO 2
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AMBITION.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline characteristics Combination (ambrisentan plus tadalafil) therapy
Ambrisentan monotherapy
Tadalafil monotherapy
|
|
| Interventions |
Intervention characteristics Combination (ambrisentan plus tadalafil) therapy
Ambrisentan monotherapy
Tadalafil monotherapy
|
|
| Outcomes | 6MWD Mortality WHO FC improved WHO FC deteriorated |
|
| Identification |
Sponsorship source: Gilead Sciences and GlaxoSmithKline Country: Italy Setting: International, multicentre study Comments: p. 843, the end of study Author's name: Nazzareno Galiè Institution: Department of Experimental, Diagnostic, and Specialty Medicine, University of Bologna Email: nazzareno.galie@unibo.it Address: Massarenti 9, Bologna 40138, Italy |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: Randomisation was performed centrally by the study sponsors using an interactive voice‐response system. p. 836, the first paragraph in section "study procedures" |
| Allocation concealment (selection bias) | Low risk | "Matching placebo tablets were administered to maintain blinding" in appendix materials. p. 22 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Judgement comment: "Matching placebo tablets were administered to maintain blinding" in appendix materials. p. 22 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data were missing in 3 intervention groups and missing data were not balanced. 21.7% (55/253) participants discontinued from the combination therapy group, 34.1% (43/126) participants from the ambrisentan group, and 24.0% (29/121) participants from the tadalafil group. |
| Selective reporting (reporting bias) | Low risk | Reported as per protocol published at ClinicalTrials.gov. |
ARIES‐1.
| Study characteristics | ||
| Methods | Multicentre, randomised, placebo‐controlled, parallel, double‐blind trial. Randomisation was conducted centrally according to a computer‐generated random number. | |
| Participants |
Baseline characteristics Placebo
Ambrisentan (5 mg/day)
Ambrisentan (10 mg/day)
|
|
| Interventions | Participants were randomised to receive placebo or ambrisentan 5 or 10 mg orally daily for 12 weeks.
|
|
| Outcomes | Primary outcomes: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC, Borg dyspnoea index and time to clinical worsening, plasma BNP and 36‐item Short Form Health Survey (SF‐36) physical functioning scale |
|
| Identification | ‐ | |
| Notes | The combined data of ARIES‐1 and ARIES‐2 showed that 21 participants discontinued in the placebo group in ARIES‐1 and ARIES‐2, 6 participants in the ambrisentan 2.5 mg group, 9 participants in the ambrisentan 5 mg group, and 5 participants in the ambrisentan 10 mg group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A central randomisation scheme stratified by PAH cause (idiopathic versus other PAH causes) was used to assign participants." |
| Allocation concealment (selection bias) | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data were missing in the intervention groups, and missing data were not balanced. A total of 41 participants in ARIES‐1 and ARIES‐2 studies discontinued prematurely during the 12‐week treatment period: 21 (15.9%) receiving placebo and 20 (7.6%) receiving ambrisentan. |
| Selective reporting (reporting bias) | Low risk | Prespecified primary outcome was reported in the results. ClinicalTrials.gov Identifier: NCT00091598 |
ARIES‐2.
| Study characteristics | ||
| Methods | Multicentre, randomised, placebo‐controlled, parallel, double‐blind trial. Randomisation was conducted centrally according to a computer‐generated random number. | |
| Participants |
Baseline characteristics Placebo
Ambrisentan (5 mg/day)
Ambrisentan (5 mg/day)
|
|
| Interventions | Participants were randomised to receive placebo or ambrisentan 2.5 or 5 mg daily for 12 weeks.
|
|
| Outcomes | Primary outcomes: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC, Borg dyspnoea index and time to clinical worsening, plasma BNP and 36‐item Short Form Health Survey (SF‐36) physical functioning scale |
|
| Identification | ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A central randomisation scheme stratified by PAH cause (idiopathic versus other PAH causes) was used to assign participants." |
| Allocation concealment (selection bias) | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data were missing in the intervention groups, and missing data were not balanced. A total of 41 participants in ARIES‐1 and ARIES‐2 studies discontinued prematurely during the 12‐week treatment period: 21 (15.9%) receiving placebo and 20 (7.6%) receiving ambrisentan. |
| Selective reporting (reporting bias) | Low risk | Prespecified primary outcome was reported in the results. ClinicalTrials.gov Identifier: NCT00091598 |
BREATHE‐1.
| Study characteristics | ||
| Methods | Multicentre, randomised, placebo‐controlled, parallel, double‐blind trial. Randomisation was conducted according to a computer‐generated random number. | |
| Participants |
Baseline characteristics Placebo
Bosentan
|
|
| Interventions | Intervention group: 62.5 mg bosentan twice daily for the first 4 weeks followed by the target dose (125 mg or 250 mg twice daily) for 12 weeks Control group: placebo
|
|
| Outcomes | Primary outcomes: change from baseline in 6MWD Secondary outcomes: Borg dyspnoea index; WHO FC |
|
| Identification | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised allocation by pharmacy‐controlled randomisation |
| Allocation concealment (selection bias) | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9% participants discontinued from the placebo group due to suffering clinical worsening of symptoms of PAH or syncope occurred, as compared with no participants in the bosentan group. The missing data were not balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. |
BREATHE‐2.
| Study characteristics | ||
| Methods | Multicentre, randomised, placebo‐controlled, parallel, double‐blind trial. Randomisation was conducted according to a computer‐generated random number. | |
| Participants |
Baseline characteristics Placebo/epoprostenol
Bosentan/epoprostenol
|
|
| Interventions | 33 participants with PAH started prostacyclin treatment (2 ng/kg/min starting dose, up to 14 ± 2 ng/kg/min at week 16) and were randomised for 16 weeks in a 2:1 ratio to bosentan (62.5 mg twice daily for 4 weeks then 125 mg twice daily) or placebo.
|
|
| Outcomes | Primary outcomes: change from baseline to week 16 in TPR Secondary outcomes: change in cardiac index, PVR, mPAP, and mRAP, 6MWD, NYHA FC and dyspnoea‐fatigue rating | |
| Identification | ‐ | |
| Notes | The standard deviations of change from baseline in 6MWD were estimated from figures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised allocation by pharmacy‐controlled randomisation |
| Allocation concealment (selection bias) | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data were missing (1/11 in the placebo group versus 4/22 in the bosentan group), and missing data were not balanced. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. |
BREATHE‐5.
| Study characteristics | ||
| Methods | Multicentre, randomised, placebo‐controlled, parallel, double‐blind trial. Randomisation was conducted according to a computer‐generated random number. | |
| Participants |
Baseline characteristics Placebo
Bosentan
|
|
| Interventions | Intervention group: 62.5 mg bosentan twice daily for the first 4 weeks followed by the target dose (125 mg twice daily) for 12 weeks. Participants who did not tolerate the target dose of 125 mg twice daily could be down titrated to the starting dose (62.5 mg twice daily). Control group: placebo
|
|
| Outcomes | Primary outcomes: change from baseline in SpO2 and PVR index Secondary outcomes: change from baseline in 6MWD, WHO FC, cardiac index, PVR, PAP, and RAP |
|
| Identification | ‐ | |
| Notes | Standard deviations were estimated from standard errors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was controlled by study medication packaging (Almedica HPS AG, Reinach, Switzerland)." |
| Allocation concealment (selection bias) | Low risk | "The investigators, participants, monitors, and sponsor personnel remained blinded to the treatment until closure of the clinical database." p. 49 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The investigators, participants, monitors, and sponsor personnel remained blinded to the treatment until closure of the clinical database." p. 49 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data were missing. 2/17 (11.8%) in the placebo group versus 2/37 (5.4%) in the bosentan group discontinued from the study. Missing data were balanced. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. |
Channick 2001.
| Study characteristics | ||
| Methods | Multicentre, randomised, parallel trial comparing bosentan with placebo for 12 weeks. Randomisation was computer generated with a block size of 3. | |
| Participants |
Baseline characteristics Placebo
Bosentan
|
|
| Interventions | Intervention group: 62.5 mg bosentan twice daily for the first 4 weeks followed by the target dose (125 mg twice daily) Control group: placebo
|
|
| Outcomes | Primary outcomes: 6MWD Secondary outcomes: cardiopulmonary haemodynamics (cardiac index, PVR, mPAP, and mRAP), WHO FC |
|
| Identification | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was computer generated using Drug Labelling System with a block size of three." |
| Allocation concealment (selection bias) | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data were missing (2/11) in the placebo group, but not in the bosentan group (0/21). Missing data were not balanced. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. |
COMPASS‐2.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline characteristics Bosentan
Placebo
|
|
| Interventions |
Intervention characteristics Bosentan
Placebo
|
|
| Outcomes | Mortality WHO FC improved WHO FC worsened Change in 6MWD Hepatic toxicity |
|
| Identification |
Sponsorship source: Actelion Pharmaceuticals Ltd. Setting: International, multicentre, university‐based hospital Author's name: Vallerie McLaughlin Institution: University of Michigan Health System Email: vmclaugh@umich.edu Address: Department of Internal Medicine, Division of Cardiovascular Medicine, University of Michigan Health System, Ann Arbor, MI 48109‐5853, USA |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "COMPASS‐2 was a prospective, international, randomised, double‐blind, placebo‐controlled, event‐driven trial" |
| Allocation concealment (selection bias) | Low risk | "COMPASS‐2 was a prospective, international, randomised, double‐blind, placebo‐controlled, event‐driven trial (www.clinicaltrials.gov identifier number NCT00303459)." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 25.7% participants were discontinued from the placebo group and 31.4% from the bosentan group. |
| Selective reporting (reporting bias) | Low risk | Reported according to the protocol published at ClinicalTrials.gov. |
EARLY.
| Study characteristics | ||
| Methods | Multicentre, randomised, placebo‐controlled, parallel, double‐blind trial | |
| Participants |
Baseline characteristics Placebo
Bosentan
|
|
| Interventions | Participants treated with either bosentan at an initial dose of 62.5 mg twice daily, uptitrating to 125 mg twice daily after 4 weeks, or remaining at 62.5 mg twice daily if bodyweight < 40 kg, or placebo for a 6‐month double‐blind treatment period.
|
|
| Outcomes | Primary outcomes: PVR and change from baseline in 6MWD Secondary outcomes: time to clinical worsening, change from baseline to month 6 in WHO FC, Borg dyspnoea index, mPAP, cardiac index, RAP, and mixed venous oxygen saturation |
|
| Identification | ‐ | |
| Notes | Standard deviations were estimated from confidence intervals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned in a one to one ratio to receive either bosentan using a centralised integrated voice recognition system." |
| Allocation concealment (selection bias) | Low risk | "This system assigned a unique randomisation number to each participant and designated the correct blinded study medication to be dispensed, both at the start of study treatment and at each scheduled visit. This code was accessible only to authorised individuals who were not involved in the conduct or analysis of the study, until the time of unblinding." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "This system assigned a unique randomisation number to each participant and designated the correct blinded study medication to be dispensed, both at the start of study treatment and at each scheduled visit. This code was accessible only to authorised individuals who were not involved in the conduct or analysis of the study, until the time of unblinding." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12.9% (12/93) participants discontinued bosentan, and 10.8% (10/92) participants discontinued placebo. Missing data were balanced. |
| Selective reporting (reporting bias) | Low risk | Prespecified primary outcome reported in the results. ClinicalTrials.gov Identifier: NCT00091715 |
EDITA.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline characteristics Placebo
Ambrisentan (10 mg/day)
|
|
| Interventions |
Intervention characteristics Placebo
Ambrisentan (10 mg/day)
|
|
| Outcomes | Change in mean PAP Change in WHO FC Change in cardiac index Change in PVR Symptoms of SSc Quality of life (SF‐36) Lung function tests, right Heart dimensions and function NT‐proBNP Measures of disease‐related progression |
|
| Identification |
Sponsorship source: GlaxoSmithKline Setting: Single‐centre, university‐based hospital Author's name: Ekkehard Grünig Institution: Centre for Pulmonary Hypertension, Thoraxklinik at Heidelberg University Hospital Email: ekkehard.gruenig@med.uni‐heidelberg.de Address: Röntgenstrasse 1, 69126 Heidelberg, Germany |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: "The EDITA study was a single‐center (PH Center, Thoraxklinik at Heidelberg University Hospital, Heidelberg, Germany) investigator‐initiated trial using a prospective, randomised, double‐blind (patient and investigator), parallel group, placebo‐controlled, phase IIA clinical study design. Patients were randomised 1:1 to either ambrisentan or placebo by simple randomisation." p. 3 in the study section |
| Allocation concealment (selection bias) | Low risk | "Placebo tablets with the same color and shape as ambrisentan were provided by GlaxoSmithKline." p. 3 in the study section |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Placebo tablets with the same color and shape as ambrisentan were provided by GlaxoSmithKline." p. 3 in the study section |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data were missing in the intervention group and placebo group, and missing data were not balanced. 2/19 participants discontinued from the intervention group, and 4/19 participants discontinued from the placebo group. |
| Selective reporting (reporting bias) | Low risk | Reported as per protocol published at ClinicalTrials.gov. |
Galiè 2003.
| Study characteristics | ||
| Methods | This trial report is on a subset of participants from the BREATHE‐1 study. | |
| Participants |
Baseline characteristics Placebo
Bosentan
|
|
| Interventions | Same as BREATHE‐1 | |
| Outcomes | Cardiac index | |
| Identification | ‐ | |
| Notes | This is a subgroup analysis of BREATHE‐1 study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See BREATHE‐1. |
| Allocation concealment (selection bias) | Low risk | See BREATHE‐1. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | See BREATHE‐1. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 out of 29 participants in the placebo group and 1 out of 56 in the bosentan group discontinued the study medication. The missing data were not balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. |
MAESTRO.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline characteristics Macitentan
Placebo
|
|
| Interventions |
Intervention characteristics Macitentan
Placebo
|
|
| Outcomes | 6MWD Mortality WHO FC improved WHO FC deteriorated mPAP PVR Change from baseline in 6MWD SPO2 Change in SPO2 Change in PAP Change in PVR |
|
| Identification |
Sponsorship source: Actelion Pharmaceuticals Ltd. Country: International study Setting: Tertiary referral hospital Author's name: Michael A Gatzoulis Institution: The Royal Brompton Hospital Email: m.gatzoulis@rbht.nhs.uk Address: Sydney Street, London, SW3 6NP, UK |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was based on a prespecified randomisation schedule using randomisation lists generated by an independent Contract Research Organization (Almac Clinical Technologies) and their centralized randomisation system, via an Interactive Voice Response System or Interactive Web Response System." |
| Allocation concealment (selection bias) | Low risk | "Participants and sites remained blinded to their previous treatment allocation." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Participants and sites remained blinded to their previous treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 97% participants in the macitentan group and 98% participants in the placebo group completed the study. |
| Selective reporting (reporting bias) | Low risk | Reported as per protocol published at ClinicalTrials.gov. |
PORTICO.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline characteristics Macitentan (10 mg)
Placebo
|
|
| Interventions |
Intervention characteristics Macitentan (10 mg)
Placebo
|
|
| Outcomes | Change in 6MWD Mortality WHO FC improved Change in mPAP Change in cardiac index Change in PVR WHO FC deteriorated |
|
| Identification |
Sponsorship source: Actelion Pharmaceuticals Ltd. Country: International study Author's name: Olivier Sitbon Email: olivier.sitbon@u‐psud.fr |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned in a 1:1 ratio, with block sizes of four, via an interactive voice and web response system (by independent contract research organisation Almac) to receive either macitentan 10 mg or matching placebo orally once a day." Judgement comment: p. 596, the first paragraph in section "randomisation and masking" |
| Allocation concealment (selection bias) | Low risk | "Participants were randomly assigned in a 1:1 ratio, with block sizes of four, via an interactive voice and web response system (by independent contract research organisation Almac) to receive either macitentan 10 mg or matching placebo orally once a day." Judgement comment: p. 596, the first paragraph in section "randomisation and masking" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Confirmed with the study sponsor: matching placebo tablets were administered to maintain blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9.4% participants in the macitentan group and 2.4% participants in the placebo group discontinued the study. Missing data were observed and imbalanced between groups. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: there was no obvious selective outcome reporting based on the protocol reported in ClinicalTrials.gov. |
SERAPH.
| Study characteristics | ||
| Methods | Single‐centre, randomised, head‐to‐head, double‐blind trial | |
| Participants |
Baseline characteristics Sildenafil
Bosentan
|
|
| Interventions | Intervention group: bosentan (62.5 mg twice daily for first 4 weeks, then uptitrated to 125 mg twice daily)
Control group: sildenafil (50 mg twice daily during the first 4 weeks, then uptitrated to 50 mg 3 times daily)
|
|
| Outcomes | Primary outcome: change in right ventricle mass from baseline Secondary outcomes: change from baseline in 6MWD, cardiac index, Borg dyspnoea index, quality of life, and plasma B‐type natriuretic peptide level from baseline | |
| Identification | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The medication was blinded in identical‐looking gelatin capsules and randomised using a computer generated random list by the Hammersmith Hospital pharmacy." |
| Allocation concealment (selection bias) | Low risk | "The medication was blinded in identical‐looking gelatin capsules and randomised using a computer generated random list by the Hammersmith Hospital pharmacy." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The medication was blinded in identical‐looking gelatin capsules and randomised using a computer generated random list by the Hammersmith Hospital pharmacy." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant discontinued sildenafil because of death, and none discontinued bosentan during the study. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. |
SERAPHIN.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline characteristics Placebo
Macitentan (3 mg)
Macitentan (10 mg)
|
|
| Interventions |
Intervention characteristics Placebo
Macitentan (3 mg)
Macitentan (10 mg)
|
|
| Outcomes | Change in 6MWD Mortality WHO FC improved Change in mPAP Change in cardiac index Change in PVR |
|
| Identification |
Sponsorship source: Actelion Pharmaceuticals Setting: Muticentre, international, university‐based hospital Author's name: Tomás Pulido Institution: Ignacio Chávez National Heart Institute Email: tpulido@prodigy.net.mx Address: Ignacio Chávez National Heart Institute, Juan Badiano 1, 4th Fl., Mexico City, 14080 Mexico |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned via an interactive voice and web response system. |
| Allocation concealment (selection bias) | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "An independent clinical event committee adjudicated, in a blinded fashion" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 59.2% participants discontinued placebo versus 45.7% participants discontinued macitentan. The missing data were not balanced. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: clinicaltrials.gov/ct2/show/NCT00660179 |
STRIDE‐1.
| Study characteristics | ||
| Methods | Multicentre, randomised, placebo‐controlled, parallel, double‐blind trial. Randomisation was conducted centrally according to a computer‐generated random number. | |
| Participants |
Baseline characteristics Placebo
Sitaxsentan 100 mg
Sitaxsentan 300 mg
|
|
| Interventions | Intervention group: 100 mg once daily or 300 mg once daily Control group: placebo
|
|
| Outcomes | Primary outcomes: peak oxygen consumption Secondary outcomes: 6MWD, NYHA FC, PAP, cardiac index, and PVR |
|
| Identification | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed centrally and stratified by centre in blocks according to a computer‐generated random number table." |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed centrally and stratified by centre in blocks according to a computer‐generated random number table." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Confirmed with the study sponsor: matching placebo tablets were administered to maintain blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8.3% (5/60) participants discontinued placebo versus 5.9% (7/118) participants sitaxsentan. The missing data was few and balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. |
STRIDE‐2.
| Study characteristics | ||
| Methods | Multicentre, randomised, placebo‐controlled, parallel, double‐blind trial. Randomisation was conducted centrally according to a computer‐generated random number. | |
| Participants |
Baseline characteristics Placebo
Sitaxsentan 50 mg
Sitaxsentan 100 mg
Bosentan
|
|
| Interventions | Participants were randomised to receive placebo, sitaxsentan 50 mg or 100 mg orally once daily, or open‐label bosentan for 18 weeks.
|
|
| Outcomes | Primary outcomes: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC, Borg dyspnoea index and time to clinical worsening |
|
| Identification | ‐ | |
| Notes | STRIDE‐2 trial compared sitaxsentan with both a placebo arm and an open‐label bosentan arm, therefore we have listed data for participants treated with bosentan and placebo for reference; however, we did not pool these data with the other data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed centrally according to a computer‐generated random number table. Randomization was 1:1:1:1." |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed centrally and stratified by centre in blocks according to a computer‐generated random number table." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Confirmed with the study sponsor: matching placebo tablets were administered to maintain blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 17.7% (5/60) participants discontinued placebo versus 8.2% (20/245) sitaxsentan. The missing data were imbalanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. |
STRIDE‐4.
| Study characteristics | ||
| Methods | Multicentre, randomised, placebo‐controlled, parallel, double‐blind trial | |
| Participants |
Baseline characteristics Placebo
Sitaxsentan 50 mg
Sitaxsentan 100 mg
|
|
| Interventions | Participants were randomised to receive placebo, sitaxsentan 50 mg, or sitaxsentan 100 mg orally once daily for 18 weeks.
|
|
| Outcomes | Primary outcomes: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC, Borg dyspnoea index and time to clinical worsening |
|
| Identification | ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed centrally and stratified by centre in blocks according to a computer‐generated random number table." |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed centrally and stratified by centre in blocks according to a computer‐generated random number table." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Confirmed with the study sponsor: matching placebo tablets were administered to maintain blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12.5% (4/32) participants discontinued placebo versus 10.9% (7/64) sitaxsentan. The missing data were balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. |
6MWD: 6‐minute walk distance; BNP: B‐type natriuretic peptide; CAD: coronary artery disease; CHD: congenital heart disease; CTD: connective tissue disease; mPAP: mean pulmonary artery pressure; mRAP: mean right atrial pressure; NYHA FC: New York Heart Association functional class; PAH: pulmonary arterial hypertension; PAP: pulmonary artery pressure; PDE5: phosphodiesterase type 5; PH: pulmonary hypertension; PVR: pulmonary vascular resistance; RAP: right atrial pressure; SD: standard deviation; SF‐36: 36‐item Short Form Health Survey; SpO2: oxygen saturation; SSc: systemic sclerosis; WHO FC: World Health Organization functional class
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahn 2014 | Participants did not have PAH. |
| ASSET‐2 | Participants did not have PAH. |
| Atsmon 2013 | RCT, but populations did not have PAH. |
| Barst 2011 | Participants were not randomised to an ERA. |
| Baughman 2014 | Participants did not have PAH. |
| Benza 2007 | Participants were not randomised to an ERA. |
| Bose 2011 | Participants did not have PAH. |
| Bruderer 2014 | Participants did not have PAH. |
| BUILD‐2 | Participants did not have PAH. |
| Burgess 2008 | Participants did not have PAH. |
| Cardenas 2020 | Participants did not have PAH. |
| Chin 2020 | Participants were not randomised to an ERA. |
| Coyne 2005 | Participants did not have PAH. |
| Danaietash 2019 | Participants did not have PAH. |
| Denault 2013 | Participants did not have PAH. |
| Dhaun 2011 | Participants did not have PAH. |
| Escribano‐Subias 2019 | Participants did not have PAH. |
| Faoro 2009 | Participants did not have PAH. |
| Fatima 2018 | Participants did not have PAH. |
| Frey 2008 | Participants did not have PAH. |
| Galiè 2005 | Participants were not randomised to an ERA. |
| Galiè 2009a | Participants were not randomised to an ERA. |
| Galiè 2009b | Long‐term follow‐up data of RCT using sitaxsentan |
| Galiè 2010 | Participants were not randomised to an ERA. |
| Ghofrani 2010 | Participants were not randomised to an ERA. |
| Ghofrani 2017 | Participants did not have PAH. |
| Gillies 2011 | Participants did not have PAH. |
| Gillies 2013 | Participants did not have PAH. |
| Givertz 2000 | Participants did not have PAH. |
| Gomberg‐Maitland 2005 | Participants were not randomised to an ERA. |
| Gotti 2014 | Participants did not have PAH. |
| Gotzkowsky 2010 | Participants did not have PAH. |
| Grander 2014 | Participants did not have PAH. |
| Grill 2020 | Participants did not have PAH. |
| Gutierrez 2013 | Participants did not have PAH. |
| Han 2017 | Mixed population used. |
| Hill 2018 | Participants did not have PAH. |
| Hoeper 2006 | Participants were not randomised to an ERA. |
| Howard 2019 | Participants did not have PAH. |
| Huez 2009 | Participants did not have PAH. |
| Hurst 2016 | Participants did not have PAH. |
| Iversen 2010 | Participants were not randomised to an ERA. |
| Jais 2008 | Participants did not have PAH. |
| Kalra 2002 | Participants did not have PAH. |
| Kaluski 2008 | Participants did not have PAH. |
| Kefford 2010 | Participants did not have PAH. |
| Keir 2013 | Participants did not have PAH. |
| Keir 2014 | Participants did not have PAH. |
| King 2008 | Participants did not have PAH |
| Kiowski 1995 | Participants did not have PAH. |
| Koller 2017 | Participants did not have PAH. |
| Korn 2004 | Participants did not have PAH. |
| Lee 2009 | Participants were not randomised to an ERA. |
| Lindegger 2014 | Participants did not have PAH. |
| Mazzanti 2013 | Duplicate publications |
| McLaughlin 2003 | Participants were not randomised to an ERA. |
| McLaughlin 2006 | Participants were not randomised to an ERA. |
| McLaughlin 2010 | Participants were not randomised to an ERA. |
| Mereles 2006 | Participants were not randomised to an ERA. |
| Metersky 2011 | Participants did not have PAH. |
| Modesti 2006 | Participants did not have PAH. |
| Naeije 2010 | Participants did not have PAH. |
| Nakahara 2010 | Participants did not have PAH. |
| NCT00077584 | Participants did not have PAH. |
| Okour 2019 | Participants did not have PAH. |
| Oudiz 2007 | Long‐term follow‐up data of RCT using sitaxsentan |
| Oudiz 2009 | Long‐term follow‐up data of RCT using sitaxsentan |
| Palazzini 2010 | Participants did not have PAH. |
| Raghu 2013 | Participants did not have PAH. |
| Raghu 2015 | Participants did not have PAH. |
| Reesink 2010 | Participants did not have PAH. |
| Robbins 2006 | Participants were not randomised to an ERA. |
| Schmetterer 1998 | Participants did not have PAH. |
| Schmidt 2001 | Participants did not have PAH. |
| Schuuring 2013 | Participants did not have PAH. |
| Seheult 2009 | Participants did not have PAH. |
| Seibold 2010 | Participants did not have PAH. |
| Sfikakis 2007 | Participants did not have PAH. |
| Shang 2013 | Participants did not have PAH. |
| Shang 2016 | Participants did not have PAH. |
| Sharma 2014 | Participants did not have PAH. |
| Shenoy 2011 | Participants did not have PAH. |
| Sidharta 2010 | Participants did not have PAH. |
| Sidharta 2013a | Participants did not have PAH. |
| Sidharta 2013b | Participants did not have PAH. |
| Sidharta 2014 | Participants did not have PAH. |
| Simonneau 2010 | Participants were not randomised to an ERA. |
| Simonneau 2017 | Participants did not have PAH. |
| Spence 2008 | Participants did not have PAH. |
| Spence 2009 | Participants did not have PAH. |
| Stavros 2010 | Participants did not have PAH. |
| Stolz 2008 | Participants did not have PAH. |
| Surie 2013 | Participants did not have PAH. |
| Tanaka 2017 | Participants did not have PAH. |
| Tanaka 2019 | Participants did not have PAH. |
| Vachiery 2018 | Participants did not have PAH. |
| Van Der Zander 2006 | Participants did not have PAH. |
| Van Giersbergen 2005 | Participants did not have PAH. |
| Webb 2017 | Participants did not have PAH. |
| Worthington 2010 | Participants were not randomised to an ERA. |
| Wrishko 2008 | Participants did not have PAH. |
ERA: endothelin receptor antagonists; PAH: pulmonary arterial hypertension; RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Dwivedi 2018.
| Methods | Randomisation method was not clear. |
| Participants | Pulmonary arterial hypertension in systemic sclerosis Inclusion criteria:
Exclusion criteria:
|
| Interventions | Sildenafil 20 mg and bosentan 62.5 mg versus sildenafil 20 mg and placebo |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Notes |
Rinaldi 2018.
| Methods | Randomisation method was not clear. |
| Participants | PAH patients with WHO FC II or III |
| Interventions |
Intervention characteristics Combination (ambrisentan plus tadalafil) therapy
Ambrisentan monotherapy
Tadalafil monotherapy
|
| Outcomes | Change in mPAP Change in RAP Change in PVR Change from baseline in 6MWD Change in MVO2 |
| Notes |
6MWD: 6‐minute walk distance; ERA: endothelin receptor antagonist; mPAP: mean pulmonary artery pressure; MVO2: mixed venous oxygen saturation; NYHA FC: New York Heart Association functional class; PAH: pulmonary arterial hypertension; PAP: pulmonary artery pressure; PDE5: phosphodiesterase type 5; PVR: pulmonary vascular resistance; RAP: right atrial pressure; NT‐proBNP; N‐terminal pro B‐type natriuretic peptide; SSc: systemic sclerosis; TPR: total pulmonary resistance; WHO FC: World Health Organization functional class
Differences between protocol and review
The previous version of this review stated that studies would be excluded if the participants were not taking anticoagulants. This is no longer recommended, therefore we have removed this restriction, and included both studies of participants who were not taking anticoagulants as well as those who were.
Contributions of authors
CL: protocol initiation, study assessment, data extraction and entry, interpretation and write‐up. Overall responsibility for maintaining the review.
JC: protocol development, study assessment, data extraction, interpretation and write‐up.
YG: study assessment, data extraction, interpretation and write‐up.
BD: study assessment, interpretation and write‐up.
KL: study assessment, interpretation and write‐up.
Contributions of editorial team
Chris Cates (Coordinating Editor) checked the data entry prior to the full write‐up of the review; edited the protocol; advised on methodology; and approved the protocol prior to publication.
Hayley Barnes (Contact Editor): edited the review; advised on methodology, interpretation, and content.
Emma Dennett (Managing Editor): co‐ordinated the editorial process; advised on interpretation and content; edited the review.
Emma Jackson (Assistant Managing Editor): conducted peer review; edited the reference and other sections of the protocol and the review.
Elizabeth Stovold (Information Specialist): designed the search strategy; ran the searches; edited the Search methods section.
Sources of support
Internal sources
-
All authors, Other
The authors declare that no such funding was received for this systematic review.
External sources
-
Wold Health Organization (WHO), Other
WHO sponsored Dr Chao Liu to receive evidence‐based practice training at Monash University in Australia.
Declarations of interest
This systematic review was supported by a grant from the World Health Organization whilst CL was in residence at the Australasian Cochrane Centre. Dr Chao Liu participated in training from the Australasian Cochrane Centre (including the Cochrane Review Completion Program) whilst in Australia.
Chao Liu: none known Junmin Chen: none known Yanqiu Gao: none known Bao Deng: none known Kunshen Liu: none known
Edited (no change to conclusions)
References
References to studies included in this review
AMBITION {published and unpublished data}
- Galie N, Barbera JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. New England Journal of Medicine 2015;373(9):834-44. [DOI: 10.1056/NEJMoa1413687] [DOI] [PubMed] [Google Scholar]
ARIES‐1 {published data only}
- Galiè N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation 2008;117(23):3010-9. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration Center for Drug Evaluation and Research. Letairis (Ambrisentan) Tablets: Medical Review(s). www.accessdata.fda.gov/drugsatfda_docs/nda/2019/022081Orig1s039.pdf (accessed prior to 10 March 2020).
ARIES‐2 {published data only}
- Galiè N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation 2008;117(23):3010-9. [DOI] [PubMed] [Google Scholar]
BREATHE‐1 {published data only}
- Rubin LJ, Badesch DB, Barst RJ, Galiè N, Black CM, Keogh A. Bosentan therapy for pulmonary arterial hypertension. New England Journal of Medicine 2002;346(12):896-903. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration Center for Drug Evaluation and Research. Tracleer (Bosentan) Tablets: Medical Review(s). www.accessdata.fda.gov/drugsatfda_docs/nda/2001/21-290_Tracleer.cfm (accessed prior to 12 March 2020).
BREATHE‐2 {published data only}
- Humbert M, Barst RJ, Robbins IM, Channick RN, Galiè N, Boonstra A, et al. Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. European Respiratory Journal 2004;24(3):353-9. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration Center for Drug Evaluation and Research. Tracleer (Bosentan) Tablets: Medical Review(s). www.accessdata.fda.gov/drugsatfda_docs/nda/2001/21-290_Tracleer.cfm (accessed prior to 12 March 2020).
BREATHE‐5 {published data only}
- Galiè N, Beghetti M, Gatzoulis MA, Granton J, Berger RM, Lauer A, et al. Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation 2006;114(1):48-54. [DOI] [PubMed] [Google Scholar]
Channick 2001 {published data only}
- Badesch DB, Bodin F, Channick RN, Frost A, Rainisio M, Robbins IM, et al. Complete results of the first randomized, placebo-controlled study of bosentan, a dual endothelin receptor antagonist, in pulmonary arterial hypertension. Current Therapeutic Research, Clinical and Experimental 2002;63(4):227-46. [Google Scholar]
- Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet 2001;358(9288):1119-23. [DOI] [PubMed] [Google Scholar]
COMPASS‐2 {published data only}
- McLaughlin V, Channick RN, Ghofrani HA, Lemarie JC, Naeije R, Packer M, et al. Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. European Respiratory Journal 2015;46(2):405-13. [DOI: 10.1183/13993003.02044-2014] [DOI] [PubMed] [Google Scholar]
EARLY {published and unpublished data}
- Galiè N, Rubin L, Hoeper M, Jansa P, Al-Hiti H, Meyer G, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet 2008;371(9630):2093-100. [DOI] [PubMed] [Google Scholar]
EDITA {published data only}
- Pan Z, Marra AM, Benjamin N, Eichstaedt CA, Blank N, Bossone E, et al. Early treatment with ambrisentan of mildly elevated mean pulmonary arterial pressure associated with systemic sclerosis: a randomized, controlled, double-blind, parallel group study (EDITA study). Arthritis Research & Therapy 2019;21:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Galiè 2003 {published data only}
- Galiè N, Hinderliter AL, Torbicki A, Fourme T, Simonneau G, Pulido T, et al. Effects of the oral endothelin-receptor antagonist bosentan on echocardiographic and doppler measures in patients with pulmonary arterial hypertension. Journal of the American College of Cardiology 2003;41(8):1380-6. [DOI] [PubMed] [Google Scholar]
MAESTRO {published data only}
- Gatzoulis MA, Landzberg M, Beghetti M, Berger RM, Efficace M, Gesang S, et al. Evaluation of macitentan in patients with Eisenmenger Syndrome. Circulation 2019;139(1):51-63. [DOI: 10.1161/CIRCULATIONAHA.118.033575] [DOI] [PMC free article] [PubMed] [Google Scholar]
PORTICO {published data only}
- Krowka MJ, Cottreel E, De Groote P, Hoeper MM, Kim N, Martin N, et al. Efficacy and safety of macitentan in patients with portopulmonary hypertension: the randomized, placebo controlled portico trial. Hepatology. Conference 2018;Suppl 1:70A-71. [DOI: 10.1002/hep.30256] [DOI] [Google Scholar]
- Sitbon O, Bosch J, Cottreel E, Csonka D, Groote P, Hoeper MM, et al. Macitentan for the treatment of portopulmonary hypertension (PORTICO): a multicentre, randomised, double-blind, placebo-controlled, phase 4 trial. Lancet Respiratory Medicine 2019;7:594-604. [DOI] [PubMed] [Google Scholar]
SERAPH {published data only}
- Wilkins MR, Paul GA, Strange JW, Tunariu N, Gin-Sing W, Banya WA, et al. Sildenafil versus endothelin receptor antagonist for pulmonary hypertension (SERAPH) study. American Journal of Respiratory and Critical Care Medicine 2005;171(11):1292-7. [DOI] [PubMed] [Google Scholar]
SERAPHIN {published and unpublished data}
- Pulido T, Adzerikho I, Channick RN, Delcroix M, Galie N, Ghofrani HA, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. New England Journal of Medicine 2013;369(9):809-18. [DOI: 10.1056/NEJMoa1213917] [DOI] [PubMed] [Google Scholar]
STRIDE‐1 {published and unpublished data}
- Barst RJ, Langleben D, Frost E, Horn EM, Oudiz R, Shapiro S, et al. Sitaxsentan therapy for pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine 2004;169:441-7. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. EPARs for authorised medicinal products for human use: Thelin. European Public Assessment Report. www.emea.europa.eu/humandocs/PDFs/EPAR/thelin/H-679-en6.pdf (accessed 22 March 2008).
- Therapeutic Goods Administration (TGA), Australia. Public summary documents by product: Thelin. www.health.gov.au/internet/main/publishing.nsf/Content/pbac-psd-sitaxentan-sodium-july07 (accessed 25 April 2008).
STRIDE‐2 {published data only}
- Barst RJ, Langleben D, Badesch D, Frost A, Lawrence EC, Shapiro S, et al. Treatment of pulmonary arterial hypertension with the selective endothelin-A receptor antagonist sitaxsentan. Journal of the American College of Cardiology 2006;47(10):2049-56. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. EPARs for authorised medicinal products for human use: Thelin. European Public Assessment Report. www.ema.europa.eu/en/medicines/human/EPAR/thelin (accessed prior to 22 March 2020).
- Therapeutic Goods Administration (TGA), Australia. Public summary documents by product: Thelin. www.health.gov.au/internet/main/publishing.nsf/Content/pbac-psd-sitaxentan-sodium-july07 (accessed 25 April 2008).
STRIDE‐4 {published data only}
- European Medicines Agency. EPARs for authorised medicinal products for human use: Thelin. European Public Assessment Report. www.ema.europa.eu/en/medicines/human/EPAR/thelin (accessed 22 March 2008).
References to studies excluded from this review
Ahn 2014 {published data only}
- Ahn LY, Kim SE, Yi S, Dingemanse J, Lim KS, Jang IJ, et al. Pharmacokinetic-pharmacodynamic relationships of macitentan, a new endothelin receptor antagonist, after multiple dosing in healthy Korean subjects. American Journal of Cardiovascular Drugs 2014;14(5):377-85. [DOI] [PubMed] [Google Scholar]
ASSET‐2 {published data only}
- Barst RJ, Mubarak KK, Machado RF, Ataga KI, Benza RL, Castro O, et al. Exercise capacity and haemodynamics inpatients with sickle cell disease with pulmonary hypertension treated with bosentan: results of the ASSET studies. British Journal of Haematology 2010;149(3):426-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atsmon 2013 {published data only}
- Atsmon J, Dingemanse J, Shaikevich D, Volokhov I, Sidharta PN. Investigation of the effects of ketoconazole on the pharmacokinetics of macitentan, a novel dual endothelin receptor antagonist, in healthy subjects. Clinical Pharmacokinetics 2013;52(8):685-92. [DOI: 10.1007/s40262-013-0063-8] [DOI] [PubMed] [Google Scholar]
Barst 2011 {published data only}
- Barst RJ, Oudiz RJ, Beardsworth A, Brundage BH, Simonneau G, Ghofrani HA, et al. Tadalafil monotherapy and as add-on to background bosentan in patients with pulmonary arterial hypertension. Journal of Heart and Lung Transplantation 2011;30(6):632-43. [DOI] [PubMed] [Google Scholar]
Baughman 2014 {published data only}
- Baughman RP, Culver DA, Cordova FC, Padilla M, Gibson KF, Lower EE, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest 2014;145(4):810-7. [DOI] [PubMed] [Google Scholar]
Benza 2007 {published data only}
- Benza RL, Mehta S, Keogh A, Lawrence EC, Oudiz RJ, Barst RJ. Sitaxsentan treatment for patients with pulmonary arterial hypertension discontinuing bosentan. Journal of Heart and Lung Transplantation 2007;26(1):63-9. [DOI] [PubMed] [Google Scholar]
Bose 2011 {published data only}
- Bose N, Bena J, Trunick C, Petrich J, Bork DJ, Krishnan G, et al. Evaluation of the effect of ambrisentan on digital microvascular flow in patients with systemic sclerosis using laser doppler perfusion imaging. Arthritis and Rheumatism 2011;63(10 Suppl 1):S276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruderer 2014 {published data only}
- Bruderer S, Hurst N, Kaufmann P, Dingemanse J. Multiple-dose up-titration study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of selexipag, an orally available selective prostacyclin receptor agonist, in healthy subjects. Pharmacology 2014;94(3-4):148-56. [DOI] [PubMed] [Google Scholar]
BUILD‐2 {unpublished data only}
- NCT00070590. Efficacy and safety of oral bosentan in pulmonary fibrosis associated with scleroderma. clinicaltrials.gov/ct2/show/NCT00070590 (first received 8 October 2003).
Burgess 2008 {published data only}
- Burgess G, Hoogkamer H, Collings L, Dingemanse J. Mutual pharmacokinetic interactions between steady-state bosentan and sildenafil. European Journal of Clinical Pharmacology 2008;64(1):43-50. [DOI] [PubMed] [Google Scholar]
Cardenas 2020 {published data only}
- Cardenas KPC, Velazquez JER, Escobar JJO, Chirinos J, Pendela M. Bioequivalence and tolerability of ambrisentan: a pharmacokinetic study in Mexican healthy male subjects. European Journal of Drug Metabolism and Pharmacokinetics 2020;45:611-8. [DOI] [PubMed] [Google Scholar]
Chin 2020 {published data only}
- Chin KM, Sitbon O, Doelberg M, Gibbs JSR, Hoeper MM, Martin N, et al. Efficacy and safety of initial triple oral versus initial double oral combination therapy in patients with newly diagnosed pulmonary arterial hypertension (PAH): results of the randomized controlled triton study. American Journal of Respiratory and Critical Care Medicine 2020;201:A2928. [Google Scholar]
Coyne 2005 {published data only}
- Coyne TC, Garces PC, Kramer W. No clinical interaction between sitaxsentan and sildenafil. In: American Thoracic Society 2005 International Conference, 2005 May 20-25; San Diego (CA). 2005. [[A57] [Poster: K70]]
Danaietash 2019 {published data only}
- Danaietash P, Verweij P, Flamion B, Menard J, Bellet M. Efficacy and safety of various doses of the new dual endothelin receptor antagonist aprocitentan in the treatment of hypertension. European Heart Journal 2019;40(Suppl 1):ehz747.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Denault 2013 {published data only}
- Denault AY, Pearl RG, Michler RE, Rao V, Tsui SS, Seitelberger R, et al. Tezosentan and right ventricular failure in patients with pulmonary hypertension undergoing cardiac surgery: the TACTICS trial. Journal of Cardiothoracic and Vascular Anesthesia 2013;27(6):1212-7. [DOI] [PubMed] [Google Scholar]
Dhaun 2011 {published data only}
- Dhaun N, Johnston NR, Goddard J, Webb DJ. Chronic selective endothelin a receptor antagonism reduces serum uric acid in hypertensive chronic kidney disease. Hypertension 2011;58(2):e11-2. [DOI] [PubMed] [Google Scholar]
Escribano‐Subias 2019 {published data only}
- Escribano-Subias P, Bendjenana H, Curtis PS, Lang I, Vonk Noordegraaf A. Ambrisentan for treatment of inoperable chronic thromboembolic pulmonary hypertension (CTEPH). Pulmonary Circulation 2019;9(2):2045894019846433. [DOI: 10.1177/2045894019846433] [DOI] [PMC free article] [PubMed] [Google Scholar]
Faoro 2009 {published data only}
- Faoro V, Boldingh S, Moreels M, Martinez S, Lamotte M, Unger P, et al. Bosentan decreases pulmonary vascular resistance and improves exercise capacity in acute hypoxia. Chest 2009;135(5):1215-22. [DOI] [PubMed] [Google Scholar]
Fatima 2018 {published data only}
- Fatima N, Arshad S, Quddusi AI, Rehman A, Nadeem A, Iqbal I. Comparison of the efficacy of sildenafil alone versus sildenafil plus bosentan in newborns with persistent pulmonary hypertension. Journal of Ayub Medical College, Abbottabad 2018;30(3):333-6. [PubMed] [Google Scholar]
Frey 2008 {published data only}
- Frey R, Muck W, Unger S, Artmeier-Brandt U, Weimann G, Wensing G. Single-dose pharmacokinetics, pharmacodynamics, tolerability, and safety of the soluble guanylate cyclase stimulator BAY 63-2521: an ascending-dose study in healthy male volunteers. Journal of Clinical Pharmacology 2008;48(8):926-34. [DOI] [PubMed] [Google Scholar]
Galiè 2005 {published data only}
- Galiè N, Badesch D, Oudiz R, Simonneau G, McGoon MD, Keogh AM, et al. Ambrisentan therapy for pulmonary arterial hypertension. Journal of the American College of Cardiology 2005;46(3):529-35. [DOI] [PubMed] [Google Scholar]
Galiè 2009a {published data only}
- Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation 2009;119(22):2894-903. [DOI] [PubMed] [Google Scholar]
Galiè 2009b {published data only}
- Galiè N, Naeije R, Burgess G, Dilleen M. 3-year survival of patients treated with sitaxentan sodium (Thelin) for pulmonary arterial hypertension. European Heart Journal 2009;Abstract Supplement:262. [Google Scholar]
Galiè 2010 {published data only}
- Galiè N, Badesch D, Fleming T, Simonneau G, Rubin L, Ewert R, et al. Effects of inhaled aviptadil (vasoactive intestinal peptide) in patients with pulmonary arterial hypertension (PAH): results from a phase II study. European Heart Journal 2010;3122(Suppl 1):22, 347. [Google Scholar]
Ghofrani 2010 {published data only}
- Ghofrani HA, Morrell NW, Hoeper MM, Olschewski H, Peacock A, Barst RJ, et al. Long term use of imatinib In patients with severe pulmonary arterial hypertension [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181:A2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghofrani 2017 {published data only}
- Ghofrani HA, Simonneau G, D'Armini AM, Fedullo P, Howard LS, Jais X, et al. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respiratory Medicine 2017;5(10):785-94. [DOI] [PubMed] [Google Scholar]
Gillies 2011 {published data only}
- Gillies HC, Wang X, Staehr P, Zack J. PAH therapy in HIV: lack of drug-drug interaction between ambrisentan and ritonavir. American Journal of Respiratory and Critical Care Medicine 2011;183:A5913. [Google Scholar]
Gillies 2013 {published data only}
- Gillies H, Henig N, Pederson P, Shao L, Chien J, O'Riordan T, et al. A placebo-controlled study of ambrisentan in subjects with idiopathic pulmonary fibrosis (ARTEMIS-IPF). Life Sciences 2013;93(25-6):e59. [Google Scholar]
Givertz 2000 {published data only}
- Givertz MM, Colucci WS, LeJemtel TH, Gottlieb SS, Hare JM, Slawsky MT, et al. Acute endothelin A receptor blockade causes selective pulmonary vasodilation in patients with chronic heart failure. Circulation 2000;101(25):2922-7. [DOI] [PubMed] [Google Scholar]
Gomberg‐Maitland 2005 {published data only}
- Gomberg-Maitland M, McLaughlin V, Gulati M, Rich S, Arbor A. Efficacy and safety of sildenafil and atorvastatin added to bosentan as therapy for pulmonary arterial hypertension. In: American Thoracic Society 2005 International Conference; 2005 May 20-25; San Diego (CA). 2005. [[A57] [Poster: K18]]
Gotti 2014 {published data only}
- Gotti E, Manes A, Palazzini M, Bernabe C, Bachetti C, Conficoni E, et al. A randomized open label study comparing first-line treatment with bosentan or sildenafil in chronic thromboembolic pulmonary hypertension (CTEPH). European Heart Journal 2014;35:208. [Google Scholar]
Gotzkowsky 2010 {published data only}
- Gotzkowsky SK, Dingemanse J, Lai A, Mottola D, Laliberte K. Lack of a pharmacokinetic interaction between oral treprostinil and bosentan in healthy adult volunteers. Journal of Clinical Pharmacology 2010;50(7):829-34. [DOI] [PubMed] [Google Scholar]
Grander 2014 {published data only}
- Grander W, Koller K, Steringer-Mascherbauer R, Ebner CH. Endothelin receptor blockade in heart failure with diastolic dysfunction and pulmonary hypertension. European Heart Journal 2014;35:692. [DOI] [PubMed] [Google Scholar]
Grill 2020 {published data only}
- Grill S, Bruderer S, Sidharta PN, Antonova M, Globig S, Carlson J, et al. Bioequivalence of macitentan and tadalafil given as fixed-dose combination or single-component tablets in healthy subjects. British Journal of Clinical Pharmacology 2020;86(12):2424-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gutierrez 2013 {published data only}
- Gutierrez MM, Nicolas LB, Donazzolo Y, Dingemanse J. Relative bioavailability of a newly developed pediatric formulation of bosentan vs. the adult formulation. International Journal of Clinical Pharmacology and Therapeutics 2013;51(6):529-36. [DOI] [PubMed] [Google Scholar]
Han 2017 {published data only}
- Han X, Zhang Y, Dong L, Fang L, Chai Y, Niu M, et al. Treatment of pulmonary arterial hypertension using initial combination therapy of bosentan and iloprost. Respiratory Care 2017;62(4):489-96. [DOI: 10.4187/respcare.05280] [DOI] [PubMed] [Google Scholar]
Hill 2018 {published data only}
- Hill KD, Maharaj A, Thompson E, Li JS, Pearson E, Hornik C. A double blind, randomized, controlled pharmacokinetic and pharmacodynamics trial of ambrisentan after Fontan surgery. Circulation 2018;138:A14968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoeper 2006 {published data only}
- Hoeper MM, Leuchte H, Halank M, Wilkens H, Meyer FJ, Seyfarth HJ, et al. Combining inhaled iloprost with bosentan in patients with idiopathic pulmonary arterial hypertension. European Respiratory Journal 2006;28(4):691-4. [DOI] [PubMed] [Google Scholar]
Howard 2019 {published data only}
- Howard LS, Ghofrani HA, D'Armini AM, Fedullo P, Gesang S, Jais X, et al. Long-term safety, tolerability and efficacy of macitentan in patients with inoperable chronic thromboembolic pulmonary hypertension: the merit-1 study and its open-label extension merit-2. In: American Journal of Respiratory and Critical Care Medicine. Vol. 201. 2019:A6068.
Huez 2009 {published data only}
- Huez S, Faoro V, Moreels M, Bastin R, Retailleau K, Lamotte M, et al. Role of pulmonary hypertension and right ventricular dysfunction in aerobic exercise capacity limitation in normal volunteers at high altitude. Acta Cardiologica 2009;64(1):114. [Google Scholar]
Hurst 2016 {published data only}
- Hurst N, Pellek M, Dingemanse J, Sidharta PN. Lack of pharmacokinetic interactions between macitentan and a combined oral contraceptive in healthy female subjects. American College of Clinical Pharmacology 2016;56(6):669-74. [DOI] [PubMed] [Google Scholar]
Iversen 2010 {published data only}
- Iversen K, Jensen AS, Jensen TV, Vejlstrup NG, Sondergaard L. Combination therapy with bosentan and sildenafil in Eisenmenger syndrome: a randomized, placebo-controlled, double-blinded trial. European Heart Journal 2010;31(9):1124-31. [DOI] [PubMed] [Google Scholar]
Jais 2008 {published data only}
- Jais X, D'Armini AM, Jansa P, Torbicki A, Delcroix M, Ghofrani HA, et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. Journal of the American College of Cardiology 2008;52(25):2127-34. [DOI] [PubMed] [Google Scholar]
Kalra 2002 {published data only}
- Kalra PR, Moon JC, Coats AJ. Do results of the ENABLE (Endothelin Antagonist Bosentan for Lowering Cardiac Events in Heart Failure) study spell the end for non-selective endothelin antagonism in heart failure? International Journal of Cardiology 2002;85(2-3):195-7. [DOI] [PubMed] [Google Scholar]
Kaluski 2008 {published data only}
- Kaluski E, Cotter G, Leitman M, Milo-Cotter O, Krakover R, Kobrin I, et al. Clinical and hemodynamic effects of bosentan dose optimization in symptomatic heart failure patients with severe systolic dysfunction, associated with secondary pulmonary hypertension - a multi-center randomized study. Cardiology 2008;109(4):273-80. [DOI] [PubMed] [Google Scholar]
Kefford 2010 {published data only}
- Kefford RF, Clingan PR, Brady B, Ballmer A, Morganti A, Hersey P. A randomized, double-blind, placebo-controlled study of high-dose bosentan in patients with stage IV metastatic melanoma receiving first-line dacarbazine chemotherapy. Molecular Cancer 2010;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keir 2013 {published data only}
- Keir G, Corte T, Parfitt L, Maher T, Marino P, Renzoni E, et al. Bosentan in pulmonary hypertension associated with fibrotic idiopathic interstitial pneumonia: a randomized, double-blind, placebo-controlled study. European Respiratory Journal 2013;42:1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keir 2014 {published data only}
- Keir G, Corte T, Dimopoulos K, Gatzoulis M, Marino P, Renzoni E, et al. Non-invasive haemodynamic measurement to detect treatment response in pulmonary hypertension associated with fibrotic idiopathic interstitial pneumonia [Abstract]. European Respiratory Journal 2014;44(Suppl 58):P2335. [Google Scholar]
King 2008 {published data only}
- King TE Jr, Behr J, Brown KK, du Bois RM, Lancaster L, Andrade JA, et al. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine 2008;177(1):75-81. [DOI] [PubMed] [Google Scholar]
Kiowski 1995 {published data only}
- Kiowski W, Sutsch G, Hunziker P, Muller P, Kim J, Oechslin E, et al. Evidence for endothelin-1-mediated vasoconstriction in severe chronic heart failure. Lancet 1995;346(8977):732-6. [DOI] [PubMed] [Google Scholar]
Koller 2017 {published data only}
- Koller B, Steringer-Mascherbauer R, Ebner CH, Weber T, Ammer M, Eichinger J, et al. Pilot study of endothelin receptor blockade in heart failure with diastolic dysfunction and pulmonary hypertension (BADDHY-Trial). Heart, Lung and Circulation 2017;26(5):433-41. [DOI] [PubMed] [Google Scholar]
Korn 2004 {published data only}
- Korn JH, Mayes M, Matucci CM, Rainisio M, Pope J, Hachulla E, et al. Digital ulcers in systemic sclerosis: prevention by treatment with bosentan, an oral endothelin receptor antagonist. Arthritis and Rheumatism 2004;50(12):3985-93. [DOI] [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee TM, Chen CC, Shen HN, Chang NC. Effects of pravastatin on functional capacity in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Clinical Science 2009;116(6):497-505. [DOI] [PubMed] [Google Scholar]
Lindegger 2014 {published data only}
- Lindegger N, Sidharta PN, Reseski K, Dingemanse J. Macitentan, a dual endothelin receptor antagonist for the treatment of pulmonary arterial hypertension, does not affect cardiac repolarization in healthy subjects. Pulmonary Pharmacology and Therapeutics 2014;29(1):41-8. [DOI] [PubMed] [Google Scholar]
Mazzanti 2013 {published data only}
- Mazzanti G, Palazzini M, Leci E, Dardi F, Rinaldi A, D'Adamo A, et al. A randomized open label study comparing first-line treatment with bosentan or sildenafil in pulmonary arterial hypertension (PAH): long-term results. American Journal of Respiratory and Critical Care Medicine 2013;187:A3535. [Google Scholar]
McLaughlin 2003 {published data only}
- McLaughlin VV, Gaine SP, Barst RJ, Oudiz RJ, Bourge RC, Frost A, et al. Efficacy and safety of treprostinil: an epoprostenol analog for primary pulmonary hypertension. Journal of Cardiovascular Pharmacology 2003;41(2):293-9. [DOI] [PubMed] [Google Scholar]
McLaughlin 2006 {published data only}
- McLaughlin VV, Oudiz RJ, Frost A, Tapson VF, Murali S, Channick RN, et al. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine 2006;174(11):1257-63. [DOI] [PubMed] [Google Scholar]
McLaughlin 2010 {published data only}
- McLaughlin VV, Benza RL, Rubin LJ, Channick RN, Voswinckel R, Tapson VF, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. Journal of the American College of Cardiology 2010;55(18):1915-22. [DOI] [PubMed] [Google Scholar]
Mereles 2006 {published data only}
- Mereles D, Ehlken N, Kreuscher S, Ghofrani S, Hoeper MM, Halank M, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation 2006;114(14):1482-9. [DOI] [PubMed] [Google Scholar]
Metersky 2011 {published data only}
- Metersky M, Coyle T. The effect of the ET-1 receptor antagonist, bosentan, on patients with poorly controlled asthma: a 17 week, double-blind, placebo-controlled crossover pilot study. Chest 2011;140(4):919A. [DOI] [PubMed] [Google Scholar]
Modesti 2006 {published data only}
- Modesti PA, Vanni S, Morabito M, Modesti A, Marchetta M, Gamberi T, et al. Role of endothelin-1 in exposure to high altitude: Acute Mountain Sickness and Endothelin-1 (ACME-1) study. Circulation 2006;114(13):1410-6. [DOI] [PubMed] [Google Scholar]
Naeije 2010 {published data only}
- Naeije R, Huez S, Lamotte M, Retailleau K, Neupane S, Abramowicz D, et al. Pulmonary artery pressure limits exercise capacity at high altitude. European Respiratory Journal 2010;36(5):1049-55. [DOI] [PubMed] [Google Scholar]
Nakahara 2010 {published data only}
- Nakahara N, Wakamatsu A, Shimamura R, Nohda S, Miki S, Hirama T. Pharmacokinetics of ambrisentan, a novel drug for treatment of Pulmonary Arterial Hypertension (PAH), in Japanese subjects. Japanese Journal of Clinical Pharmacology and Therapeutics 2010;41(6):301-8. [Google Scholar]
NCT00077584 {unpublished data only}
- NCT00077584. Efficacy and safety of oral bosentan on healing/prevention of digital (finger) ulcers in patients with scleroderma patients (RAPIDS-2) [A randomized, double-blind, placebo-controlled, multi-center study to assess the effect of bosentan on healing and prevention of ischemic digital ulcers in patients with systemic sclerosis]. clinicaltrials.gov/ct2/show/NCT00077584 (first received 11 February 2004).
Okour 2019 {published data only}
- Okour M, Puri A, Chen G, Port K, Berni A, Khindri S, et al. A phase I study to show the relative bioavailability and bioequivalence of fixed-dose combinations of ambrisentan and tadalafil in healthy subjects. Clinical Therapeutics 2019;41:1110-27. [DOI] [PubMed] [Google Scholar]
Oudiz 2007 {published data only}
- Oudiz RJ. Long-term ambrisentan therapy provides sustained benefit in patients with pulmonary arterial hypertension. Chest 2007;132(4):474a. [Google Scholar]
Oudiz 2009 {published data only}
- Oudiz RJ, Galiè N, Olschewski H, Torres F, Frost A, Ghofrani HA, et al. Long-term ambrisentan therapy for the treatment of pulmonary arterial hypertension. Journal of the American College of Cardiology 2009;54(21):1971-81. [DOI] [PubMed] [Google Scholar]
Palazzini 2010 {published data only}
- Palazzini M, Mazzanti G, Gotti E, Bulatovic I, Manes A, Marinelli A, et al. A randomized open label study comparing bosentan to sildenafil first-line treatment in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A3357. [Google Scholar]
Raghu 2013 {published data only}
- Raghu G, Behr J, Brown KK, Egan JJ, Kawut SM, Flaherty KR, et al. Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Annals of Internal Medicine 2013;158(9):641-9. [DOI] [PubMed] [Google Scholar]
Raghu 2015 {published data only}
- Raghu G, Nathan SD, Behr J, Brown KK, Egan JJ, Kawut SM, et al. Pulmonary hypertension in idiopathic pulmonary fibrosis with mild-to-moderate restriction. European Respiratory Journal 2015;46(5):1370-7. [DOI] [PubMed] [Google Scholar]
Reesink 2010 {published data only}
- Reesink HJ, Surie S, Kloek JJ, Tan HL, Tepaske R, Fedullo PF, et al. Bosentan as a bridge to pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. Journal of Thoracic and Cardiovascular Surgery 2010;139(1):85-91. [DOI] [PubMed] [Google Scholar]
Robbins 2006 {published data only}
- Robbins IM, Kawut SM, Yung D, Reilly MP, Lloyd W, Cunningham G, et al. A study of aspirin and clopidogrel in idiopathic pulmonary arterial hypertension. European Respiratory Journal 2006;27(3):578-84. [DOI] [PubMed] [Google Scholar]
Schmetterer 1998 {published data only}
- Schmetterer L, Dallinger S, Bobr B, Selenko N, Eichler H-G, Wolzt M. Systemic and renal effects of an ET(A) receptor subtype-specific antagonist in healthy subjects. British Journal of Pharmacology 1998;124(5):930-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt 2001 {published data only}
- Schmidt A, Bayerle-Eder M, Pleiner H, Zeisner C, Wolzt M, Mayer G, et al. The renal and systemic hemodynamic effects of a nitric oxide-synthase inhibitor are reversed by a selective endothelin A receptor antagonist in men. Nitric Oxide 2001;5(4):370-6. [DOI] [PubMed] [Google Scholar]
Schuuring 2013 {published data only}
- Schuuring MJ, Vis JC, Dijk AP, Melle JP, Vliegen HW, Pieper PG, et al. Impact of bosentan on exercise capacity in adults after the Fontan procedure: a randomized controlled trial. European Journal of Heart Failure 2013;15(6):690-8. [DOI] [PubMed] [Google Scholar]
Seheult 2009 {published data only}
- Seheult RD, Ruh K, Foster GP, Anholm JD. Prophylactic bosentan does not improve exercise capacity or lower pulmonary artery systolic pressure at high altitude. Respiratory Physiology and Neurobiology 2009;165(2-3):123-30. [DOI] [PubMed] [Google Scholar]
Seibold 2010 {published data only}
- Seibold JR, Denton CP, Furst DE, Guillevin L, Rubin LJ, Wells A, et al. Randomized, prospective, placebo-controlled trial of bosentan in interstitial lung disease secondary to systemic sclerosis. Arthritis and Rheumatism 2010;62(7):2101-8. [DOI] [PubMed] [Google Scholar]
Sfikakis 2007 {published data only}
- Sfikakis PP, Papamichael C, Stamatelopoulos KS, Tousoulis D, Fragiadaki KG, Katsichti P, et al. Improvement of vascular endothelial function using the oral endothelin receptor antagonist bosentan in patients with systemic sclerosis. Arthritis and Rheumatism 2007;56(6):1985-93. [DOI] [PubMed] [Google Scholar]
Shang 2013 {published data only}
- Shang XK, Li YP, Liu M, Zhou HM, Peng T, Deng XX, et al. Efficacy of endothelin receptor antagonist bosentan on the long-term prognosis in patients after Fontan operation. Zhonghua Xin Xue Guan Bing Za Zhi 2013;41(12):1025-8. [PubMed] [Google Scholar]
Shang 2016 {published data only}
- Shang XK, Lu R, Zhang X, Zhang CD, Xiao SN, Liu M, et al. Efficacy of bosentan in patients after fontan procedures: a double-blind, randomized controlled trial. Journal of Huazhong University of Science and Technology [Medical Sciences] 2016;36(4):534-40. [DOI] [PubMed] [Google Scholar]
Sharma 2014 {published data only}
- Sharma SK, Ajmani S, Sharma A, Singh S, Sinha S, Vishnubhatla S, et al. Comparison of efficacy of different treatment regimens in pulmonary hypertension secondary to lung disease and/or hypoxia [Abstract]. American Journal of Respiratory and Critical Care Medicine 2014;189:A1892. [Google Scholar]
Shenoy 2011 {published data only}
- Shenoy PD, Agarwal V. Tadalafil: a new role in Raynaud's phenomenon? International Journal of Clinical Rheumatology 2011;6(2):115-7. [Google Scholar]
Sidharta 2010 {published data only}
- Sidharta PN, Atsmon J, Dingemanse J. Investigation of the effect of ketoconazole on the pharmacokinetics of macitentan in healthy male subjects. British Journal of Clinical Pharmacology 2010;70(6):930-1. [Google Scholar]
Sidharta 2013a {published data only}
- Sidharta PN, Giersbergen PL, Dingemanse J. Safety, tolerability, pharmacokinetics, and pharmacodynamics of macitentan, an endothelin receptor antagonist, in an ascending multiple-dose study in healthy subjects. Journal of Clinical Pharmacology 2013;53(11):1131-8. [DOI] [PubMed] [Google Scholar]
Sidharta 2013b {published data only}
- Sidharta PN, Lindegger N, Reseski K, Dingemanse J. Macitentan, a novel dual endothelin receptor antagonist, does not prolong the QT/QTC interval in a thorough QTC study in healthy subjects. Clinical Pharmacology and Therapeutics 2013;93(Suppl 1):S108-9. [Google Scholar]
Sidharta 2014 {published data only}
- Sidharta PN, Dietrich H, Dingemanse J. Investigation of the effect of macitentan on the pharmacokinetics and pharmacodynamics of warfarin in healthy male subjects. Clinical Drug Investigation 2014;34(8):545-52. [DOI] [PubMed] [Google Scholar]
Simonneau 2010 {published data only}
- Simonneau G, Lang I, Torbicki A, Hoeper MM, Delcroix M, Karlocai K, et al. Efficacy, safety and tolerability of ACT-293987, a novel oral, non-prostanoid, prostaglandin I2 (IP) receptor agonist: results from a phase IIa study in pulmonary arterial hypertension (PAH). American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A2515. [Google Scholar]
Simonneau 2017 {published data only}
- Simonneau G, Jing ZC, D'Armini AM, Fedullo P, Howard L, Jais X, et al. Macitentan for inoperable chronic thromboembolic pulmonary hypertension (CTEPH): results from the randomised controlled MERIT study. European Respiratory Journal 2017;50(Suppl 61):OA1984. [Google Scholar]
Spence 2008 {published data only}
- Spence R, Mandagere A, Dufton C, Venitz J. Pharmacokinetics and safety of ambrisentan in combination with sildenafil in healthy volunteers. Journal of Clinical Pharmacology 2008;48(12):1451-9. [DOI] [PubMed] [Google Scholar]
Spence 2009 {published data only}
- Spence R, Mandagere A, Harrison B, Dufton C, Boinpally R. No clinically relevant pharmacokinetic and safety interactions of ambrisentan in combination with tadalafil in healthy volunteers. Journal of Pharmaceutical Sciences 2009;98(12):4962-74. [DOI] [PubMed] [Google Scholar]
Stavros 2010 {published data only}
- Stavros F, Kramer WG, Wilkins MR. The effects of sitaxentan on sildenafil pharmacokinetics and pharmacodynamics in healthy subjects. British Journal of Clinical Pharmacology 2010;69(1):23-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stolz 2008 {published data only}
- Stolz D, Rasch H, Linka A, Di Valentino M, Meyer A, Brutsche M, et al. A randomised, controlled trial of bosentan in severe COPD. European Respiratory Journal 2008;32(3):619-28. [DOI] [PubMed] [Google Scholar]
Surie 2013 {published data only}
- Surie S, Reesink HJ, Marcus JT, Plas MN, Kloek JJ, Vonk-Noordegraaf A, et al. Bosentan treatment is associated with improvement of right ventricular function and remodeling in chronic thromboembolic pulmonary hypertension. Clinical Cardiology 2013;36(11):698-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanaka 2017 {published data only}
- Tanaka Y, Hino M, Gemma A. Potential benefit of bosentan therapy in borderline or less severe pulmonary hypertension secondary to idiopathic pulmonary fibrosis - an interim analysis of results from a prospective, single-center, randomized, parallel-group study. BMC Pulmonary Medicine 2017;17(1):200. [DOI: 10.1186/s12890-017-0523-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanaka 2019 {published data only}
- Tanaka Y, Hino M, Aoyama J, Kosaihira S, Okano T, Seike M, et al. Interim report: long-term influence of bosentan on prognosis, activities of daily living (ADL), cardiac function and pulmonary function in patients with pulmonary hypertension secondary to COPD. In: Respirology. Vol. 24. 2019:239.
Vachiery 2018 {published data only}
- Vachiery J-L, Delcroix M, Al-Hiti H, Efficace M, Hutyra M, Lack G, et al. Macitentan in pulmonary hypertension due to left ventricular dysfunction. European Respiratory Journal 2018;51(2):1880. [DOI] [PubMed] [Google Scholar]
Van Der Zander 2006 {published data only}
- Van Der Zander K, Houben AJHM, Webb DJ, Udo E, Kietselaer B, Hofstra L, et al. Selective endothelin B receptor blockade does not influence BNP-induced natriuresis in man. Kidney International 2006;69(5):864-8. [DOI] [PubMed] [Google Scholar]
Van Giersbergen 2005 {published data only}
- Van Giersbergen PLM, Dingemanse J. Comparative investigation of the pharmacokinetics of bosentan in Caucasian and Japanese healthy subjects. American College of Clinical Pharmacology 2005;45(1):42-7. [DOI] [PubMed] [Google Scholar]
Webb 2017 {published data only}
- Webb DJ, Coll B, Heerspink HJL, Andress D, Pritchett Y, Brennan JJ, et al. Longitudinal assessment of the effect of atrasentan on thoracic bioimpedance in diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. Drugs in R&D 2017;17(3):441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Worthington 2010 {published data only}
- Worthington A, Collins N, Haddad R. Safety and feasibility of inhaled iloprost on exercise capacity in patients with pulmonary arterial hypertension. Heart Lung and Circulation 2010;19(2):1. [Google Scholar]
Wrishko 2008 {published data only}
- Wrishko RE, Dingemanse J, Yu A, Darstein C, Phillips DL, Mitchell MI. Pharmacokinetic interaction between tadalafil and bosentan in healthy male subjects. Journal of Clinical Pharmacology 2008;48(5):610-8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Dwivedi 2018 {published data only}
- Dwivedi P, Sharma SK, Dhir V, Rohit MK, Behera D, Jain S. AB0797 A randomised double blind placebo controlled trial to compare the efficacy of initial combination therapy vs monotherapy for pulmonary arterial hypertension in systemic sclerosis. Annals of the Rheumatic Diseases 2018;77(Suppl 2):1531. [Google Scholar]
Rinaldi 2018 {published data only}
- Rinaldi A, Dardi F, Albini A, Gotti E, Monti E, Palazzini M, et al. Haemodynamic and exercise effects of different types of initial oral combination therapy in pulmonary arterial hypertension. In: European Heart Journal. Vol. 39. 2018:P6341.
Additional references
Altman 2003
- Altman DG, Bland JM. Statistics notes: interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Badesch 2007
- Badesch DB, the Aries Study Group. Ambrisentan therapy for pulmonary arterial hypertension: a comparison by PAH etiology. Chest 2007;132(4):488-9. [Google Scholar]
Barst 1996
- Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. New England Journal of Medicine 1996;334(5):296-302. [DOI] [PubMed] [Google Scholar]
Chester 2014
- Chester AH, Yacoub MH. The role of endothelin-1 in pulmonary arterial hypertension. Global Cardiology Science and Practice 2014;2014(2):62-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence
- Veritas Health InnovationCovidence Systematic Review Software. Melbourne, Australia: Veritas Health Innovation. Available at www.covidence.org.
Denton 2006
- Denton CP, Humbert M, Rubin L, Black CM. Bosentan treatment for pulmonary arterial hypertension related to connective tissue disease: a subgroup analysis of the pivotal clinical trials and their open-label extensions. Annals of the Rheumatic Diseases 2006;65(10):1336-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duo‐Ji 2017
- Duo-Ji MM, Long ZW. Comparative efficacy and acceptability of endothelin receptor antagonists for pulmonary arterial hypertension: a network meta-analysis. International Journal of Cardiology 2017;234:90-8. [DOI] [PubMed] [Google Scholar]
Galiè 2015
- Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. European Respiratory Journal 2015;46(4):903. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 20 Novmber 2020. Hamilton (ON): McMaster University (developed by Evidence Prime).
Guignabert 2013
- Guignabert C, Tu L, Le HM, Ricard N, Sattler C, Seferian A, et al. Pathogenesis of pulmonary arterial hypertension: lessons from cancer. European Respiratory Review 2013;22(130):543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Horinouchi 2013
- Horinouchi T, Terada K, Higashi T, Miwa S. Endothelin receptor signaling: new insight into its regulatory mechanisms. Journal of Pharmacological Sciences 2013;123(2):85-101. [DOI] [PubMed] [Google Scholar]
Khair 2016
- Khair RM, Nwaneri C, Damico RL, Kolb T, Hassoun PM, Mathai SC. The minimal important difference in Borg dyspnea score in pulmonary arterial hypertension. Annals of the American Thoracic Society 2016;13(6):842-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klinger 2019
- Klinger JR, Elliott CG, Levine DJ, Bossone E, Duvall L, Fagan K, et al. Therapy for pulmonary arterial hypertension in adults: update of the CHEST guideline and Expert Panel Report. CHEST 2019;155(3):565-86. [DOI] [PubMed] [Google Scholar]
Madonna 2015
- Madonna R, Cocco N, De Caterina R. Pathways and drugs in pulmonary arterial hypertension – focus on the role of endothelin receptor antagonists. Cardiovascular Drugs Therapy 2015;29(5):469-79. [DOI] [PubMed] [Google Scholar]
Mathai 2012
- Mathai SC, Puhan MA, Lam D, Wise RA. The minimal important difference in the 6-minute walk test for patients with pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine 2012;186(5):428-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miyamoto 2000
- Miyamoto S, Nagaya N, Satoh T, Kyotani S, Sakamaki F, Fujita M, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. American Journal of Respiratory and Critical Care Medicine 2000;161:487-92. [DOI] [PubMed] [Google Scholar]
Review Manager 2014
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rich 1992
- Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. New England Journal of Medicine 1992;327(2):76-81. [DOI] [PubMed] [Google Scholar]
Seibold 2005
- Seibold J, Badesch D, Galiè N, Langleben D, Naeije R, Simonneau G, et al. Sitaxsentan, a selective endothelin-a receptor antagonist, improves exercise capacity in pulmonary arterial hypertension (PAH) associated with connective tissue disease (CTD). CHEST 2005;128(4):219S.
Shao 2011
- Shao D, Park JE, Wort SJ. The role of endothelin-1 in the pathogenesis of pulmonary arterial hypertension. Pharmacological Research 2011;63(6):504-11. [DOI] [PubMed] [Google Scholar]
Simonneau 2019
- Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. European Respiratory Journal 2019;53(1):1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sitbon 1998
- Sitbon O, Humbert M, Jagot JL, Taravella O, Fartoukh M, Parent F, et al. Inhaled nitric oxide as a screening agent for safely identifying responders to oral calcium-channel blockers in primary pulmonary hypertension. European Respiratory Journal 1998;12:265-70. [DOI] [PubMed] [Google Scholar]
Sitbon 2002
- Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Herve P, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. Journal of the American College of Cardiology 2002;40(4):780-8. [DOI] [PubMed] [Google Scholar]
Thenappan 2018
- Thenappan T, Ormiston ML, Ryan JJ, Archer SL. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ 2018;360:j5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2018
- Wang S, Yu M, Zheng X, Dong S. A Bayesian network meta-analysis on the efficacy and safety of eighteen targeted drugs or drug combinations for pulmonary arterial hypertension. Drug Delivery 2018;25(1):1898-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhuang 2014
- Zhuang Y, Jiang B, Gao H, Zhao W. Randomized study of adding tadalafil to existing ambrisentan in pulmonary arterial hypertension. Hypertension Research 2014;37(6):507-12. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Liu 2004
- Liu C, Chen J. Endothelin receptor antagonists for pulmonary arterial hypertension. Cochrane Database of Systematic Reviews 2004, Issue 4. Art. No: CD004434. [DOI: 10.1002/14651858.CD004434.pub2] [DOI] [PubMed] [Google Scholar]
Liu 2006
- Liu C, Chen J. Endothelin receptor antagonists for pulmonary arterial hypertension. Cochrane Database of Systematic Reviews 2006, Issue 3. Art. No: CD004434. [DOI: 10.1002/14651858.CD004434.pub3] [DOI] [PubMed] [Google Scholar]
Liu 2009
- Liu C, Chen J, Gao Y, Deng B, Liu K. Endothelin receptor antagonists for pulmonary arterial hypertension. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD004434. [DOI: 10.1002/14651858.CD004434.pub4] [DOI] [PubMed] [Google Scholar]
Liu 2013
- Liu C, Chen J, Gao Y, Deng B, Liu K. Endothelin receptor antagonists for pulmonary arterial hypertension. Cochrane Database of Systematic Reviews 2013, Issue 2. Art. No: CD004434. [DOI: 10.1002/14651858.CD004434.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]


