Abstract
Background
Salvage systemic therapy has become the new standard of care in patients with advanced gastric and oesophago‐gastric junction (OGJ) adenocarcinoma, following disease progression on first‐line fluoropyrimidine and platinum‐containing chemotherapy. Pharmacological agents proven to be effective in this setting include both chemotherapy and biological therapy, however, the consensus on the best salvage systemic therapy has not been reached.
Objectives
To assess the effects of systemic chemotherapy and biological therapy, either alone or in combination, on overall survival (OS) and progression‐free survival (PFS) in patients with advanced gastric and OGJ adenocarcinoma, whose disease has progressed on, or relapsed after first‐line fluoropyrimidine and platinum‐containing chemotherapy. Adverse events (AEs), tumour response rate (TRR) and quality of life (QoL) associated with systemic chemotherapy and/or biological therapy were additionally assessed.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, trial registries and proceedings of the major oncology conferences up to October 2020. We additionally handsearched the reference lists of studies. No language restriction was applied.
Selection criteria
We included randomised controlled trials (RCTs) comparing salvage systemic therapy (chemotherapy and/or biological therapy) and either another type of salvage systemic therapy, placebo, best supportive care (BSC) or no treatment in patients with gastric and OGJ adenocarcinoma refractory to first‐line fluoropyrimidine and platinum‐containing chemotherapy.
Data collection and analysis
Two review authors independently performed selection of eligible studies and the primary author extracted study characteristics and outcome data from included studies. We assessed the quality and risk of bias of eligible studies according to the Cochrane Handbook for Systematic Reviews of Interventions. We expressed pooled estimates of effect using hazard ratio (HR) calculated using an inverse variance random‐effects model for time‐to‐event data, and risk ratio (RR) calculated using Mantel‐Haenszel random‐effects model for binary data. The certainty of evidence was graded using GRADEpro.
Main results
We identified 17 RCTs with 5110 participants for inclusion in this review. Tweenty‐nine studies are ongoing and twenty studies are awaiting classification. No studies examined the following comparisons: chemotherapy combined with biological therapy versus placebo, BSC or no treatment, chemotherapy combined with biological therapy versus biological therapy, biological therapy versus biological therapy and chemotherapy combined with biological therapy versus chemotherapy combined with biological therapy.
Chemotherapy versus placebo, best supportive care or no treatment
Chemotherapy probably improves OS (HR = 0.66, 95% CI 0.52 to 0.83, moderate‐certainty evidence) based on two studies involving 547 participants and improves PFS (HR = 0.57, 95% CI 0.47 to 0.69, high‐certainty evidence) based on one study involving 507 participants over placebo and BSC. Chemotherapy probably increases serious AEs (SAEs) (RR = 1.38, 95% CI 1.20 to 1.59, moderate‐certainty evidence) based on one study involving 503 participants.
Biological therapy versus placebo, best supportive care or no treatment
Biological therapy improves OS (HR = 0.55, 95% CI 0.41 to 0.73, high‐certainty evidence) and probably improves PFS (HR = 0.33, 95% CI 0.19 to 0.57, moderate‐certainty evidence) over placebo based on three studies involving 781 participants. There is currently insufficient evidence for increased SAEs from biological therapy (RR = 1.14, 95% CI 0.95 to 1.37, low‐certainty evidence) based on two studies involving 638 participants.
Chemotherapy versus biological therapy
This comparison only considered immunotherapy. There is probably no evidence of a difference for OS (HR = 0.82, 95% CI 0.66 to 1.02, moderate‐certainty evidence) between chemotherapy and immunotherapy, and immunotherapy probably reduces PFS (HR = 1.27, 95% CI 1.03 to 1.57, moderate‐certainty evidence) based on one study involving 395 participants. SAEs may be less frequent with immunotherapy compared to chemotherapy (RR = 0.41, 95% CI 0.30 to 0.57, low‐certainty evidence).
Chemotherapy combined with biological therapy versus chemotherapy
Addition of biological therapy to chemotherapy probably does not improve OS (HR = 0.93, 95% CI 0.83 to 1.04, moderate‐certainty evidence) and we are uncertain whether it improves PFS (HR = 0.87, 95% CI 0.74 to 1.02, very low‐certainty evidence) based on seven studies involving 2743 participants. We are similarly uncertain whether combined chemotherapy and biological therapy increases SAEs (RR = 1.17, 95% CI 0.95 to 1.44, very low‐certainty evidence) based on four studies involving 1618 participants.
Chemotherapy versus chemotherapy
There is no evidence of a difference for OS and PFS between irinotecan and paclitaxel (HR = 1.13, 95% CI 0.86 to 1.48, low‐certainty evidence for OS; HR = 1.14, 95% CI 0.88 to 1.48, low‐certainty evidence for PFS) based on one study involving 219 participants. Similarly, there is no evidence to indicate improved OS and PFS from addition of another chemotherapy to docetaxel (HR = 1.05, 95% CI 0.72 to 1.54, low‐certainty evidence for OS; HR = 0.75, 95% CI 0.52 to 1.09, low‐certainty evidence for PFS) based on two studies involving 121 participants. Grade ≥ 3 neutropenia occurred commonly with both mono‐ and poly‐chemotherapy except for docetaxel‐S1 and EOX chemotherapy.
Authors' conclusions
Survival outcome of patients with advanced gastric and OGJ adenocarcinoma whose disease progressed on first‐line fluoropyrimidine and platinum‐containing chemotherapy can be improved by chemotherapy and biological therapy. Biological therapy, in particular, achieves this without clear increase in SAEs or QoL impairment. Whether biological therapy is preferred over chemotherapy is still unclear and there is no evidence of a difference for OS outcome, although immunotherapy may be associated with less SAEs. Addition of biological therapy to chemotherapy and poly‐chemotherapy are associated with frequent treatment‐related toxicity without clear survival benefit.
Plain language summary
Which treatments work best for advanced stomach cancer that has not responded to standard chemotherapy?
What is advanced stomach cancer?
Gastric (stomach) cancer usually begins in the mucous‐producing cells lining the stomach. Oesophago‐gastric junction (OGJ) cancer starts where the food pipe (oesophagus) joins the stomach. Advanced cancer is cancer that has spread to nearby tissues, or to another part of the body, despite treatment.
Treatments for gastric and OGJ cancer include:
• operation to remove the cancer;
• chemotherapy (medicines that kill cancer cells);
• radiotherapy (radiation to kill cancer cells); and
• biological therapy (medicines made from proteins and other substances that occur naturally in the body).
Biological therapies include immunotherapy (medicines that help the immune system to recognise and kill cancer cells) and therapies that target something in, or surrounding, the cancer, such as the cancer's blood supply. Standard chemotherapy usually combines two medicines containing fluoropyrimidine and platinum.
When standard chemotherapy for advanced cancer has not worked, further treatment aims to slow the growth of the cancer to help people live longer. Further treatments include: other chemotherapy medicines, biological therapies, and best supportive care (care that helps a person cope with life‐limiting illness and its treatment).
Why we did this Cochrane Review
Stomach and OGJ cancer are difficult to treat. We wanted to find out which treatments work best to control these cancers and help people live longer, when standard chemotherapy has not worked.
What did we do?
We searched for studies that looked at chemotherapy and/or biological therapies for advanced stomach or OGJ cancer that had not responded to standard chemotherapy. We looked for studies in which the treatment each person received was decided at random. These studies usually give the most reliable evidence about the effects of treatments.
Search date
We included evidence published up to October 2020.
What we found
We found 17 studies in 5110 people with advanced stomach or OGJ cancer. Studies compared further chemotherapy and/or biological therapies, given by mouth or through the bloodstream (systemic), with:
• another systemic chemotherapy and/or biological therapy;
• a placebo ('dummy' treatment);
• best supportive care; and
• no treatment.
The studies looked at:
• how long people lived;
• any adverse (unwanted) effects; and
• their quality of life (well‐being).
What are the results of our review?
People probably live longer after further chemotherapy (irinotecan or trifluridine plus tipiracil) than with placebo treatment or best supportive care. But chemotherapy probably increases serious unwanted effects, including diarrhoea, fever, and lower numbers of red and white blood cells.
People may live as long after irinotecan chemotherapy as after paclitaxel chemotherapy. Adding another chemotherapy (oxaliplatin or cisplatin) to docetaxel may not affect how long people live.
People live longer after biological therapy (nivolumab, apatinib or regorafenib) than with placebo treatment. We did not find enough evidence about whether biological therapy increases unwanted effects.
People given immunotherapy (pembrolizumab) probably live as long as people given chemotherapy (paclitaxel), but may not have as many unwanted effects as with chemotherapy.
Combining chemotherapy with biological therapy probably does not help people live longer than chemotherapy alone, and we are uncertain whether it increases unwanted effects.
How reliable are these results?
We are moderately confident that chemotherapy probably helps people to live longer than placebo treatment or best supportive care. We are confident that people live longer on biological therapy than placebo treatment. We think more evidence is unlikely to change this result.
We are less confident about the results for unwanted effects. Some studies had missing data or did not report these; and in some studies people and their doctors knew which treatment was given, which could have affected the study results. These results are likely to change when more evidence becomes available.
Conclusions
If advanced stomach or OGJ cancer has not responded to standard chemotherapy, further chemotherapy or biological therapy help people to live longer than placebo treatment, best supportive care, or no treatment. However, chemotherapy is more clearly associated with unwanted effects than biological therapy.
We are unsure if biological therapies work better than chemotherapy, but they may cause fewer unwanted effects. Combining chemotherapy and biological therapies may cause more unwanted effects without giving any extra benefit.
Summary of findings
Background
A glossary of terms is provided in Appendix 1.
Description of the condition
Despite gradual decline in its occurrence during recent decades, gastric cancer remains a major health burden internationally. It is the fifth most common cancer and there were over one million incident cases in 2018 worldwide (Ferlay 2018). The condition is particularly prevalent in Eastern Asia where 60% of worldwide cases occur. Gastric cancer is the third most common cause of cancer‐related death; it contributed to 783,000 deaths in 2018 worldwide. Causes of gastric cancer are multifactorial, although infection with Helicobacter pylori (H.pylori) is considered to be the primary carcinogenic step. Other risk factors include smoking, high salt consumption, and processed meat intake as well as genetic polymorphisms in hosts (Rawla 2019). Histologically, 95% of gastric cancer demonstrates adenocarcinoma.
Oesophago‐gastric junction (OGJ) cancer affects the border between the oesophagus and the stomach, and the vast majority of this type of cancer is adenocarcinoma. Unlike gastric adenocarcinoma, the incidence of OGJ adenocarcinoma has increased significantly since 1970 in Western countries. In England its age‐standardised incidence rose by 2.6 fold between the 1970s and 1990s, before plateauing (Offman 2018). Because of its location, the definition and staging of OGJ cancer has been the source of controversy. In the eighth edition of the American Joint Committee in Cancer (AJCC) staging system, adenocarcinomas with epicentres no more than 2 cm into the gastric cardia are staged as oesophageal cancer, while those extending further are staged as stomach cancers (Rice 2017). Risk factors for OGJ cancer include smoking, gastro‐oesophageal reflux disease, Barrett's oesophagus and obesity, while a diet high in fibre and H.pylori infection are inversely associated with the condition (Buas 2013). The shifts in dietary practices towards increased fat intake and meat consumption, together with the decline in prevalence of H.pylori infection in Western countries, are thought to have contributed to the rise in OGJ cancer's incidence in these countries. Improved site classification of OGJ and gastric cardia adenocarcinoma may also explain this change (Corley 2004).
Both gastric and OGJ cancers have a dismal prognosis. The five‐year survival rates of these cancers have been reported to be 32% and 20% in the USA, respectively (SEER 2018). Their high mortalities are explained by the majority of patients with these cancers presenting with either advanced disease or relapse following curative intervention. For both gastric and OGJ cancer, where radical resection remains the only treatment offering potential cure, the conditions are considered advanced if complete resection is not possible either due to local extension or the presence of distant metastases. Surgically curable early gastric cancers are usually asymptomatic and only infrequently detected outside screening programs. In many parts of the world where screening programs do not exist, more than 50% of gastric cancer cases are diagnosed at advanced stage (Wesolowski 2009). Similarly, more than 80% of patients with OGJ cancer have advanced disease at diagnosis (Siewert 2005). Prognoses of advanced gastric and OJG cancers are poor with the five‐year survival rate being less than 10%, however, selected patients still benefit from local and systemic therapies of palliative intention (SEER 2018).
Description of the intervention
Systemic therapy refers to the treatment of cancer using pharmacological agents, whereby drugs commonly administered orally or intravenously travel through the bloodstream to reach and affect cancer cells around the body. There are currently two types of systemic pharmacological therapy utilised in the management of gastric and OGJ cancers: chemotherapy and biological therapy. While chemotherapy is a group of cytotoxic chemicals used against cancer, biological therapy involves the use of substances derived from living organisms. Biological therapy is categorised based on its mechanism of action and the main types of biological therapy currently in clinical use or having been evaluated in large clinical trials for stomach and OGJ cancers include anti‐HER2 antibody, vascular endothelial growth factor receptor (VEGFR)‐targeted therapy and immunotherapy.
Fluoropyrimidine and platinum‐containing chemotherapy has been established as the backbone of first‐line systemic therapy for both advanced gastric and OGJ cancers, with some debate as to the benefit of additional taxane or anthracycline; however, the disease eventually progresses on the treatment (Cunningham 2008; Kang 2009; Van Cutsem 2006). Gastric and OGJ cancers, which relapse shortly after the perioperative or adjuvant chemotherapy containing these two agents, are similarly considered to be resistant to this regimen. For patients whose disease has progressed on first‐line chemotherapy, best supportive care (BSC) was previously the main management approach.
Salvage systemic therapy refers to second‐line and beyond chemotherapy and biological therapy, and they are administered following failure of first‐line systemic therapy. With an increasing number of studies being published supporting its benefit, second‐line pharmacological management of advanced gastric and OGJ cancer has now become the standard of care for patients with reasonable fitness (Kanagavel 2015). Chemotherapy such as irinotecan and taxanes were the first to be demonstrated to improve survival of these patients following the failure of fluoropyrimidine and platinum‐containing therapy (Ford 2014; Kang 2012; Thuss‐Patience 2011). More recently, there has been a rapid emergence of several biological therapy agents with proven anticancer activity for advanced gastric cancer in the salvage setting, expanding the pharmacological options for these cancers (Pavlakis 2016; Wilke 2014).
How the intervention might work
Salvage systemic therapy is administered with an intention to prolong and maximise quality of life (QoL). Both salvage chemotherapy and biological therapy have been demonstrated to delay progression of cancer growth and extend survival in a proportion of patients with gastric and OGJ cancers (Ford 2014; Kang 2012; Pavlakis 2016; Thuss‐Patience 2011; Wilke 2014). These therapies can also improve or delay the deterioration of cancer‐related symptoms such as fatigue, nausea/vomiting, pain and the level of functioning experienced by patients (Al‐Batran 2014; Ford 2014; Thuss‐Patience 2011; Wilke 2014).
Salvage systemic therapy can cause treatment‐related adverse events (AEs) in some patients. The type and the extent of AEs experienced depend on the exact therapy administered and the risk factors held by individual patients. The list of such treatment‐related AEs includes anaemia, diarrhoea, fatigue, infection, loss of appetite, nausea/vomiting, neuropathy, skin rash and stomatitis. When selecting patients with gastric and OGJ cancer for salvage systemic therapy, the expected benefit of the therapy needs to be weighed against the potential toxicities.
Why it is important to do this review
There are now multiple studies supporting the benefit of salvage systemic therapy to improve the survival of patients with advanced gastric and OGJ cancer. Pharmacological agents proven to be effective in this setting include both chemotherapy and newly available biological therapy. Studies comparing the efficacy of these agents vary in size, comparator interventions, and consequently, and consensus on the best salvage systemic therapy has not been reached. There also remain uncertainties about risk‐benefit trade‐off of salvage systemic therapy due to the expected short survival of these patients and potential toxicities associated with treatment.
Objectives
Primary objective
To assess the effects of systemic chemotherapy and biological therapy, either alone or in combination, on overall survival (OS) and progression‐free survival (PFS) in patients with advanced gastric and OGJ adenocarcinoma, whose disease has progressed or relapsed on first‐line fluoropyrimidine and platinum‐containing chemotherapy.
Secondary objectives
To assess the effect of the aforementioned intervention on tumour response, AEs and QoL.
To assess the impact of patients' geographical regions (East and South‐East Asia versus the rest of the world) on the survival benefit of salvage systemic therapy for advanced gastric and OGJ adenocarcinoma.
To compare the survival benefit of salvage systemic therapy for advanced gastric and OGJ adenocarcinoma in the second versus third‐line and beyond setting.
Methods
Criteria for considering studies for this review
Types of studies
We considered both blinded and open‐label randomised studies, in which a type of salvage systemic therapy is compared with either another type of salvage systemic therapy, placebo, BSC or no treatment. Quasi‐randomised studies were accepted as long as they were parallel‐group randomised studies. We included studies reported as full text or as abstract only as well as unpublished studies, if they met the other inclusion criteria.
Types of participants
We included adults patients over 18 years of age with a histological or cytological diagnosis of locally advanced and unresectable or metastatic gastric and OGJ adenocarcinoma, whose disease had progressed on first‐line fluoropyrimidine and platinum‐containing palliative chemotherapy. Patients with disease progression on, or disease relapse within 6 months of fluoropyrimidine and platinum‐containing neoadjuvant/perioperative/adjuvant chemotherapy, who were no longer suitable for potentially curative surgery, were also included in the review.
The original intention of this systemic review was to assess the effects of systemic therapy in patients with advanced gastric carcinoma, following progression on the standard first‐line chemotherapy. The decision to include OGJ adenocarcinoma in the review, however, was made based on the fact that the majority of clinical studies on advanced gastric cancer enrol patients with OGJ cancer and extracting the proposed primary endpoints for patients with gastric cancer alone was not always possible. OGJ cancer is commonly treated using the same approach as gastric cancer and distinguishing gastric cardia cancer involving OGJ from distal oesophagus and OGJ cancer extending inferiorly to involve gastric cardia remains controversial. In the most recent eighth edition of the American Joint Committee in Cancer (AJCC) staging system, the definition of cancer location for OGJ cancer has changed from the position of the upper edge of the cancer to its epicentre and some even suggest the genetic signature of OGJ cancers may be more accurate in determining the cell of origin for cancer staging rather than its gross location (Hayakawa 2016).
We excluded studies with the following characteristics:
studies in which patients have gastric or OGJ cancer other than adenocarcinoma in histology, unless these patients can be separated out for the purpose of outcome assessment;
studies in which patients have oesophageal cancer other than OGJ cancer, unless these patients can be separated out for the purpose of outcome assessment;
studies in which 10% or more of patients previously received chemotherapy which did not contain both fluoropyrimidine and platinum as first‐line therapy, unless these patients can be separated out for the purpose of outcome assessment; and
studies which do not mention the Eastern Cooperative Oncology Group (ECOG) performance score (PS), and those in which 10% or more of patients have an ECOG PS more than 2, unless these patients can be separated out for the purpose of outcome assessment.
When studies met the above listed exclusion criteria, we contacted investigators or study sponsors in order to obtain individual patient data for the purpose of primary and secondary outcome analysis and subgroup analysis where possible.
Types of interventions
We included studies comparing systemically administered (parenteral or oral) chemotherapy and biological therapy, either alone or in combination, with or without BSC, to another systemically administered therapy, placebo, BSC or no treatment.
We allowed the following co‐interventions in both experimental and control arms of studies, provided they were not part of the randomised treatment and administered for the purposes of symptom control alone:
surgery; and
radiotherapy.
Types of outcome measures
We assessed following outcomes.
Primary outcomes
Overall survival (OS), defined as survival time from the start of the intervention until death from any cause.
Progression‐free survival (PFS), defined as survival time without disease progression from the start of the intervention.
Serious adverse events (SAEs), assessed using Common Terminology Criteria for Adverse Events (CTCAE) and defined as Grade ≥ 3.
OS and PFS were chosen as the primary outcomes as they are considered to be the most clinically relevant efficacy assessment tools for patients and clinicians to decide on administration of the intervention. Disease progression for the purpose of determining OS and PFS was defined according to Response Evaluation Criteria in Solid Tumours (RECIST) or immune‐related Response Evaluation Criteria in Solid Tumours (irRECIST). irRECIST was developed to take account of pseudo‐progression, a feature unique to immunotherapy. It involves one‐dimensional measurement as in the case of RECIST, however, confirmation of disease progression is needed minimum four weeks after the first assessment indicating disease progression.
SAEs are also important in predicting the tolerability of the intervention and for its benefit‐risk assessment. CTCAE Grade 3 AEs generally refer to any type of AEs which require medical intervention and/or hospitalisation, but are not life‐threatening, while Grade 4 AEs are those which are life‐threatening and Grade 5 AEs are those resulting in death.
Secondary outcomes
Tumour response rate (TRR), assessed using RECIST or irRECIST.
Any adverse events (AAEs), assessed using CTCAE.
QoL, assessed using validated tools.
TRR was chosen for the secondary outcomes to assess the anticancer activity of the intervention. As patients included in this review generally had very limited prognoses, AAEs and QoL associated with the intervention were thought to be important aspects of their management.
We aimed to compare the following treatment groups for the primary and secondary outcomes.
Chemotherapy versus placebo, BSC or no treatment.
Biological therapy versus placebo, BSC or no treatment.
Chemotherapy combined with biological therapy versus placebo, BSC or no treatment.
Chemotherapy versus biological therapy.
Chemotherapy combined with biological therapy versus chemotherapy.
Chemotherapy combined with biological therapy versus biological therapy.
Chemotherapy versus chemotherapy.
Biological therapy versus biological therapy.
Chemotherapy combined with biological therapy versus chemotherapy combined with biological therapy.
Reporting of the outcomes listed here was not an inclusion criterion for the review.
Search methods for identification of studies
No restrictions were placed on the language of publication when searching the electronic databases or reviewing reference lists in identified studies. We translated abstracts of non‐English language papers and assessed them for potential inclusion in the review as necessary.
Electronic searches
We searched the following electronic databases:
Cochrane Central Register of Controlled Trials (CENTRAL) (inception to Issue 9, 2020) (Appendix 2) (which includes RCTs from Cochrane Gut Group Specialised Register);
EMBASE (1974 to October 2020) (via Ovid) (Appendix 3); and
MEDLINE (1946 to October 2020) (via Ovid) (Appendix 4).
We additionally searched:
WHO ICTRP (http://www.who.int/ictrp/en/) (Appendix 5); and
ClinicalTrials.gov (https://clinicaltrials.gov/) (Appendix 6).
Proceedings of the following oncology conferences were also handsearched (2000 to October 2020):
American Society of Clinical Oncology Annual Meeting;
American Society of Clinical Oncology Gastrointestinal Cancer Symposium; and
European Society of Medical Oncology Congress.
Searching other resources
We checked the reference lists of all relevant primary studies and review articles for additional references.
Data collection and analysis
Selection of studies
Two review authors independently screened for inclusion the titles and abstracts of all the studies we identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports/publication and two review authors independently screened the full text and identified studies for inclusion. These authors also identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion or, if required, we consulted a third person. We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) and Characteristics of excluded studies (Liberati 2009).
1.
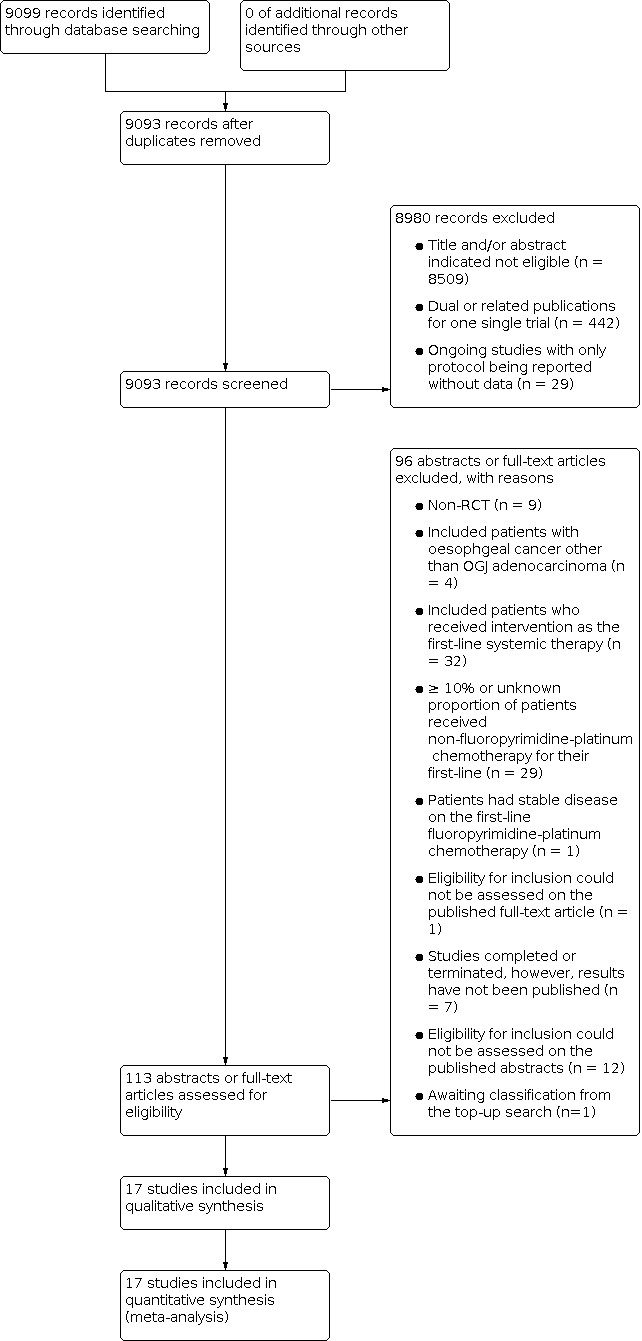
Study flow diagram.
Data extraction and management
We used a standard data collection form for study characteristics and outcome data. One review author extracted study characteristics and outcome data from included studies. We extracted the following study characteristics.
Methods: study design, total duration of study and run in, number of study centres, study setting, withdrawals, and date of study.
Participants: number, mean age, age range, geographical location, gender, staging of disease, diagnostic criteria, inclusion criteria, exclusion criteria, and ECOG PS.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for study and notable conflicts of interest of trial authors.
We noted in Characteristics of included studies if outcome data were not reported in a usable way. One review author copied the data from the data collection form into the Review Manager file. We double‐checked that the data were entered correctly by comparing the study reports against the data presented in the systematic review. A second review author spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias of each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor. We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Imbalance in baseline characteristics across treatment arms.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low or unclear risk and provided a justification for our judgment in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed (Figure 2). We considered attrition risk separately for QoL and the rest of the outcomes. Where information on risk of bias relates to unpublished data or correspondence with an author, we noted this in the 'Risk of bias' table.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Measures of treatment effect
We analysed time‐to‐event data (OS and PFS) as hazard ratios (HRs) with a 95% confidence intervals (CIs) and dichotomous data as risk ratios (RRs) with a 95% CIs. We planned to analyse continuous data as mean difference (MD) or standardised mean difference (SMD) with 95% CIs, ensuring that higher scores for continuous outcomes have the same meaning for the particular outcome, explaining the direction and reporting where the directions were reversed if this was necessary.
We undertook meta‐analysis only where this was meaningful, i.e. if the treatments, participants and underlying clinical questions were similar enough for pooling to make sense.
Where multiple study arms were reported in a single study and two comparisons (e.g. drug A versus placebo and drug B versus placebo) must be entered into the same meta‐analysis, we halved the control group to avoid double counting.
Unit of analysis issues
We included studies with a parallel group design where participants are individually assigned to one of the treatment groups with a single observation for each outcome from each participant. The unit of analysis therefore was individuals assigned to each group.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as an abstract only).
Assessment of heterogeneity
Heterogeneity secondary to clinical diversity was expected to be observed between the studies. This included variation in participants (age, ethnicity, baseline ECOG PS, volume of cancer, study eligibility), interventions (chemotherapy and/or biological therapy administered, intensity/dose, intervention in the control group) and outcomes (follow‐up duration), as well as variation in study methodology. We assessed heterogeneity among the studies in each analysis with visual inspection and statistically using the Chi‐square (Chi²) test and the I‐square (I²) statistic. We used a P value threshold of 0.10 to determine statistical significance for the Chi² test, and considered an I² of 30% or less to be low degree, 30% to 60% to be moderate degree, and 60% or more to be a high degree of heterogeneity. We recognised that there is uncertainty in the I² measurement when there are few studies in a meta‐analysis.
When we identified substantial heterogeneity, we explored possible sources of heterogeneity using the pre‐specified sensitivity and subgroup analysis described below.
Assessment of reporting biases
We attempted to contact study investigators asking them to provide missing outcome data. When this was not possible, and the missing data were thought to introduce serious bias, the impact of including such studies in the overall assessment of results was explored by a sensitivity analysis.
As we were unable to pool more than ten studies for any of the meta‐analyses performed in this review, planned assessment using funnel plots to explore possible publication biases was not carried out.
Data synthesis
We used Review Manager 5.4.1 (Review Manager 2020) for pooling of data at a study level, and statistical analysis. Given the potential heterogeneity in the included studies as described above, we used a random‐effects model for both primary and secondary outcome analysis and subgroup analysis.
'Summary of findings' tables
We created 'Summary of findings' tables using the following outcomes: OS, PFS, SAEs, TRR, AAEs and QoL. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies which contribute data to the meta‐analysis for the pre‐specified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook (Higgins 2011) and used GRADEpro software. We justified all decisions to down‐ or up‐grade the quality of studies using footnotes, and made comments to aid readers' understanding of the review where necessary. We considered whether there is any additional outcome information that was not able to be incorporated into meta‐analysis, and noted this in the comments and stated if it supports or contradicts the information from the meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We carried out the following subgroup analyses.
Line of treatment (second line versus third line and beyond).
Geographical region (East and South‐East Asia versus the rest of the world).
Extent of the disease (locally advanced versus metastatic).
Type of biological therapy (non‐immunotherapy versus immunotherapy).
Geographical region rather than ethnicity was chosen as this additionally accounts for the regional difference in the preferred first‐line systemic therapy for gastric and OGJ cancer, and also to maximise the data attainment.
The following outcomes were used in subgroup analysis:
OS; and
PFS.
We used the formal statistical test, Chi² test, with significance set at 10%, to test for subgroup interactions.
We aimed to compare the following treatment groups for the subgroup analysis.
Chemotherapy versus placebo, BSC or no treatment.
Biological therapy versus placebo, BSC or no treatment.
Chemotherapy combined with biological therapy versus placebo, BSC or no treatment.
Chemotherapy versus biological therapy.
Chemotherapy combined with biological therapy versus chemotherapy.
Chemotherapy combined with biological therapy versus biological therapy.
Sensitivity analysis
We performed sensitivity analysis to assess the effect of risk of bias and heterogeneity in the included studies. We repeated the OS and PFS analysis with the following conditions.
Use of a fixed‐effect model.
Exclusion of studies with high risk or unclear risk of bias for randomisation, blinding or attrition.
Exclusion of studies with incomplete reporting of primary outcome (HR for OS and/or PFS estimated from the test or acquired directly from investigators).
Exclusion of studies evaluating HER2‐targeted therapy in biomarker selected participants.
If any additional clinical or methodological variations suitable for sensitivity analysis arose during the review process, we considered this.
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review. We avoided making specific recommendations for practice and our implications for research gave the reader a clear sense of where the focus of any future research in the area should be and what the remaining uncertainties are.
We conducted the review according to the published protocol and reported any deviations from it in the 'Differences between protocol and review' section of the systematic review.
Results
Description of studies
We categorised the relevant studies into four categories and their characteristics were summarised see Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification and Characteristics of ongoing studies.
Results of the search
Through the electronic search in October 2020, we identified 9099 unique records; 3024 from EMBASE, 375 from CENTRAL, 1934 from MEDLINE, 3294 from WHO ICTRP and 472 from ClinicalTrials.gov. Handsearching found no relevant abstracts from conference proceedings. Of 9093 records left after removing duplicates, 8999 records were considered not suitable for further assessment; 8509 records were excluded after screening title and abstract and 442 records were excluded as they were related records for single trials. Tweenty‐nine studies are ongoing and nineteen studies are awaiting classification as there were no publication on results available (n = 7) or their abstracts provided insufficient information for screening in the absence of full‐text articles (n = 12). One study (Zhao 2019) identified in the top‐up search performed immediately prior to publication is not yet fully incorporated into the review and is added to studies awaiting classifications; it will be incorporated into the review at the next update. Ninety‐four published abstracts or full‐text articles were assessed in detail and seventy‐six of them were considered to have failed screening based on non‐RCT (n = 9), included participants with oesophageal cancer other than oesophago‐gastric junction (OGJ) adenocarcinoma (n = 4), included participants who received intervention as first‐line systemic therapy (n = 32), equal or more than 10%, or unknown proportion of participants received non‐fluoropyrimidine and platinum‐containing chemotherapy as the first‐line (n = 29), participants had stable disease on first‐line fluoropyrimidine and platinum‐containing chemotherapy (n = 1) and eligibility for inclusion could not be assessed on the published full‐text article (n = 1). Details of search results are shown in the PRISMA flow diagram (Figure 1).
Included studies
Fourteen studies (Bang 2017; Hironaka 2013; Kim 2015; Lee 2017; Ling 2018; Makiyama 2018; Pauligk 2017; Shah 2018; Shitara 2018; Shitara 2018b; Thuss‐Patience 2011; Thuss‐Patience 2017; Yi 2012; Wilke 2014) were considered to have satisfied our selection criteria and included into this review. Three studies (Kang 2017; Li 2013; Pavlakis 2016) did not provide sufficient information for screening whether more than 90% of participants from these studies received and progressed on fluoropyrimidine and platinum‐containing chemotherapy, however, whether these two agents were administered concurrently was not clear in the texts. Although no response was obtained from study authors, these studies were considered highly relevant and included in the review. A total of 17 studies with 5110 participants were considered in this review.
Study design
Eight studies had double‐blind placebo‐controlled design (Bang 2017; Kang 2017; Li 2013; Pauligk 2017; Pavlakis 2016; Shah 2018; Shitara 2018b; Wilke 2014), while another six studies had open‐label design (Hironaka 2013; Makiyama 2018; Shitara 2018; Thuss‐Patience 2011; Thuss‐Patience 2017; Yi 2012). Three studies (Kim 2015; Lee 2017; Ling 2018) did not mention blinding status, however, were most likely open‐label trials. Seven studies (Bang 2017; Kang 2017; Pavlakis 2016; Shah 2018; Shitara 2018; Shitara 2018b; Thuss‐Patience 2017) were multinational, eight studies (Hironaka 2013; Kim 2015; Lee 2017; Li 2013; Makiyama 2018; Pauligk 2017; Thuss‐Patience 2011; Yi 2012) were multi‐centre trials in a single country, and two studies (Ling 2018; Yi 2012) were single‐centre trials. Nine studies (Bang 2017; Hironaka 2013; Kang 2017; Pauligk 2017; Shah 2018; Shitara 2018; Shitara 2018b; Thuss‐Patience 2011; Wilke 2014) were phase 3, six studies (Kim 2015; Lee 2017; Li 2013; Makiyama 2018; Pavlakis 2016; Yi 2012) were phase 2, and one study (Thuss‐Patience 2017) was phase 2/3. One study (Ling 2018) did not describe the phase.
Participants
Reported median age of participants varied from 52 to 65 years with the youngest and oldest participant being 19 and 85 years old, respectively. All the studies when data were available, had more male than female participants with the proportion of male participants ranging from 65.4% to 80.8%. In total, 3379 out of 4721 participants in this review were estimated to be males with two studies (Makiyama 2018; Pauligk 2017) not providing any information on the gender of participants. The number of participants with ECOG PS 2 was low in the majority of included studies; seven studies (Bang 2017; Kang 2017; Li 2013; Pavlakis 2016; Shah 2018; Shitara 2018b; Wilke 2014) only enrolled participants with ECOG PS 0‐1 and in five studies (Hironaka 2013; Kim 2015; Lee 2017; Shitara 2018; Thuss‐Patience 2017), the proportion of participants with ECOG PS 2 was less than 6.0%. In three studies (Ling 2018; Thuss‐Patience 2011: Yi 2012) more than 10% of the study population had ECOG PS 2 with the highest being 24.3%.
Four studies (Hironaka 2013; Kim 2015; Lee 2017; Ling 2018) included participants with only gastric cancer. For nine studies (Bang 2017; Kang 2017; Pavlakis 2016; Shah 2018; Shitara 2018; Shitara 2018b; Thuss‐Patience 2011; Thuss‐Patience 2017; Wilke 2014), which reported the number of participants with gastric and OGJ adenocarcinoma, 57.5% to 97.0% of the study participants had gastric cancer. The remaining four studies (Li 2013; Makiyama 2018; Pauligk 2017; Yi 2012) enrolled participants with both gastric and OGJ adenocarcinoma, however, the number of participants with each condition was not provided.
Five studies (Hironaka 2013; Kim 2015; Lee 2017; Ling 2018; Thuss‐Patience 2011) included participants with metastatic or recurrent disease, while the other 12 studies (Bang 2017; Kang 2017; Li 2013; Makiyama 2018; Pauligk 2017; Pavlakis 2016; Shah 2018; Shitara 2018; Shitara 2018b; Thuss‐Patience 2017; Wilke 2014; Yi 2012) included participants with locally advanced unresectable disease and those with metastatic or recurrent disease. When known, the proportion of participants with metastatic disease ranged between 57.0% to 100.0%. Participants with recurrent disease were evaluated by seven studies (Hironaka 2013; Kang 2017; Kim 2015; Lee 2017; Makiyama 2018; Pauligk 2017; Pavlakis 2016) .
Nine studies (Bang 2017; Hironaka 2013; Kang 2017; Li 2013; Shitara 2018; Shitara 2018b; Thuss‐Patience 2011; Thuss‐Patience 2017; Wilke 2014) reported the number of participants who had previous gastrectomy or resection of primary tumours and the proportion such participants varied between 30.0% to 76.6%. Kim 2015 reported the proportion of participants who had received palliative operation to be 46.2%, however, it did not describe the type of surgery performed.
Study treatment was administered as second‐line systemic therapy in 12 studies (Bang 2017; Hironaka 2013; Kim 2015; Lee 2017; Ling 2018; Makiyama 2018; Shah 2018; Shitara 2018; Thuss‐Patience 2011; Thuss‐Patience 2017; Wilke 2014; Yi 2012) with 3522 participants, second‐ or third‐line in one study (Pavlakis 2016) with 147 participants, second‐ to fourth‐line in one study (Pauligk 2017) with 300 participants, and third‐line and beyond in three studies (Kang 2017; Li 2013; Shitara 2018b) with 1141 participants. Six studies (Pavlakis 2016; Shah 2018; Shitara 2018; Shitara 2018b; Thuss‐Patience 2017; Wilke 2014) were multinational trials with participating centres located worldwide, nine studies (Bang 2017; Hironaka 2013; Kang 2017; Kim 2015; Lee 2017; Li 2013; Ling 2018; Makiyama 2018; Yi 2012) recruited participants from Asian countries and two studies from Germany (Pauligk 2017; Thuss‐Patience 2011). In total, 2327 participants from 13 studies (Bang 2017; Hironaka 2013; Kang 2017; Kim 2015; Lee 2017; Li 2013; Ling 2018; Makiyama 2018; Pavlakis 2016; Shitara 2018; Shitara 2018b; Wilke 2014; Yi 2012) were enrolled from Asian countries with additional 157 participants in one study (Thuss‐Patience 2017) being enrolled from countries in the Asia‐Pacific region. The number participants enrolled from Asian countries was unknown for one study (Shah 2018), which was a multinational trial.
Three studies selected participants based on biomarker expression. Only participants with HER2 positivity were enrolled in two studies (Makiyama 2018; Thuss‐Patience 2017). In the third study (Shitara 2018), the study's predefined primary outcomes were overall survival (OS) and progression‐free survival (PFS) in PD‐L1 combined positive score ≥ 1 participants and the enrolment was restricted to participants with PD‐L1 combined positive score of ≥ 1 after 489 out of 592 participants were enrolled.
Interventions
Taxanes were the most commonly investigated single‐agent chemotherapy: paclitaxel was examined in six studies (Bang 2017; Hironaka 2013; Makiyama 2018; Pauligk 2017; Shah 2018; Wilke 2014), docetaxel in three studies (Kim 2015; Lee 2017; Yi 2012) and either paclitaxel or docetaxel in one study (Thuss‐Patience 2017). Irinotecan was assessed in two studies (Hironaka 2013; Thuss‐Patience 2011) and Trifluridine/tipiracil in one study (Shitara 2018b). Combination chemotherapy was compared with either single‐agent chemotherapy or another combination chemotherapy in five studies: docetaxel‐oxaliplatin (Kim 2015), docetaxel‐cisplatin (Lee 2017), docetaxel‐S1 (Lee 2017), EOX (Ling 2018) and FOLFIRI (Ling 2018). Five studies examined single‐agent biological therapy: nivolumab (Kang 2017), apatinib (Li 2013), regorafenib (Pavlakis 2016), pembrolizumab (Shitara 2018) and trastuzumab emtansine (Thuss‐Patience 2017). Alternatively, biological therapy was examined as an addition to taxane chemotherapy:olaparib‐paclitaxel (Bang 2017), trastuzumab‐paclitaxel (Makiyama 2018), everolimus‐paclitaxel (Pauligk 2017), napabucasin‐paclitaxel (Shah 2018), ramucirumab‐paclitaxel (Wilke 2014) and sunitinib‐docetaxel (Yi 2012).
Outcome measures
Outcomes on efficacy: overall survival (OS), progression‐free survival (PFS) and tumour response rate (TRR).
World Health Organization (WHO) criteria were used for efficacy assessment in one study (Thuss‐Patience 2011) and the method was not described in two studies published as conference abstracts (Makiyama 2018; Pauligk 2017). WHO criteria have been previously shown to be comparable to Response Evaluation Criteria in Solid Tumours (RECIST) criteria in evaluating the response of colorectal carcinoma (Choi 2005). In the remaining studies RECIST or immune‐related Response Evaluation Criteria in Solid Tumours (irRECIST) was used to assess for radiological disease progression. OS and PFS were measured in all the studies included in this review, except in Yi 2012 where time to progression (TTP) was reported in place of PFS. Three studies (Makiyama 2018; Pauligk 2017; Shah 2018) did not specify if efficacy analysis was performed on the intention‐to‐treat population. As these studies specified radiologically measurable or evaluable disease as one of their inclusion criteria, we assumed efficacy analysis was performed on the intention‐to‐treat population. One study (Thuss‐Patience 2011) reported PFS and TRR on participants in the irinotecan arm and not the best supportive care (BSC) arm. Two studies (Bang 2017; Pauligk 2017) reported hazard ratios (HRs) for survival outcomes with 97.5% confidence intervals (CIs and P values; 95% CIs were estimated under the assumption for Gaussian distribution. Three studies (Kim 2015; Lee 2017; Ling 2018) reported survival outcomes without referring to HRs and they were estimated from the published Kaplan‐Meire curves for two of the studies (Kim 2015; Lee 2017). Kaplan‐Meier curves from Ling 2018 indicated median overall survival (mOS) and median progression‐free survival (mPFS), which differed from those described in its text, raising a question on the accuracy of these Kaplan‐Meier curves and the estimated HRs from them. TRR was assessed on subpopulation of randomised participants, those with measurable disease at baseline in three studies (Hironaka 2013; Kang 2017; Thuss‐Patience 2017). One study (Pauligk 2017) did not report TRR and was excluded from meta‐analysis for this outcome. 4806, 4766 and 4328 participants were considered to have been included in OS, PFS and TRR analyses, respectively.
Outcomes on adverse events: serious adverse events (SAEs) and adverse events (AEs).
Apart from three studies (Makiyama 2018; Pauligk 2017; Shah 2018), which did not specify the assessment method for safety, National Cancer Institute : Common Terminology Criteria for Adverse Events (NCI CTCAE) version 2.0‐4.03 was used for measuring AEs and SAEs. Minimal descriptions were provided on AEs for three studies (Makiyama 2018; Pauligk 2017; Shah 2018).Two studies (Pavlakis 2016; Thuss‐Patience 2011) only reported data on SAEs alone with the second study only measuring AEs in the irinotecan arm and not the BSC arm. For the remaining studies, comprehensive results on AEs were available.
Outcomes on quality of life (QoL)
Seven studies listed patient‐reported QoL as their outcomes and assessed QoL using one or combinations of three questionnaires: European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire C30 (QLQ‐C30) in all the studies (Bang 2017; Lee 2017; Li 2013; Pavlakis 2016; Thuss‐Patience 2011; Thuss‐Patience 2017; Wilke 2014), EORTC gastric module (QLQ‐STO22) in four studies (Bang 2017; Lee 2017; Pavlakis 2016; Thuss‐Patience 2017), and the EuroQol five dimensions health status questionnaire (EQ‐5D) in two studies (Thuss‐Patience 2017; Wilke 2014). Two studies (Bang 2017; Pauligk 2017) resulted in separate publications focusing on the QoL outcomes. QoL outcomes of another study (Shitara 2018b) were similarly going to be published separately, however, this was not available at the time of preparing this review.
Excluded studies
The two commonest reasons for studies being excluded after the assessment of abstracts or full‐text articles were enrolment of participants who received intervention as first‐line systemic therapy (n = 32) and equal or more than 10%, or unknown proportion of participants having received non‐fluoropyrimidine and platinum‐containing chemotherapy as first‐line therapy (n = 29). Study authors were contacted via email when insufficient data were available for screening, however, only a few responses were received and in those occasions study data were owned by sponsors and could not be released.
Risk of bias in included studies
Potential source of bias in included studies are described in Characteristics of included studies and summarised in 'Risk of bias' summary table (Figure 2).
Allocation
Randomisation method was not described for four studies: one full‐text article (Kim 2015) and three conference abstracts (Makiyama 2018; Pauligk 2017; Shah 2018). In an additional four studies (Lee 2017; Li 2013; Ling 2018; Yi 2012), authors only reported random sequence method or allocation concealment method, but not both.
Blinding
Eight studies (Bang 2017; Kang 2017; Li 2013; Pauligk 2017; Pavlakis 2016; Shah 2018; Shitara 2018b; Wilke 2014) had double‐blind placebo‐controlled design and were considered to be at low risk of performance and detection bias, except for Shah 2018 where the data safety monitoring board recommended unblinding after an interim analysis suggested meeting the primary endpoint at final analyses was unlikely. In three of the six open‐label studies included in the review (Hironaka 2013; Shitara 2018; Yi 2012) response assessment was performed centrally by masked reviewers, therefore these studies were thought to be at high risk of performance bias, but not detection bias. Two open‐label studies (Thuss‐Patience 2011; Thuss‐Patience 2017) were considered to be at high risk of both performance and detection bias. No blinding status for outcome assessment was provided for the remaining one study (Makiyama 2018).
Imbalance in baseline characteristics (confounding)
Twelve studies (Bang 2017; Hironaka 2013; Kang 2017; Li 2013; Ling 2018; Pavlakis 2016; Shitara 2018; Shitara 2018b; Thuss‐Patience 2011; Thuss‐Patience 2017; Wilke 2014; Yi 2012) were assessed to be at low risk of confounding based on the description of stratification at randomisation and well‐balanced patient and disease characteristics at baseline. In two studies (Kim 2015; Lee 2017) the risk of confounding was unclear due to some imbalances in patient and disease characteristics at baseline. Detailed study description was not available for Makiyama 2018, Pauligk 2017, and Shah 2018, which were published as conference abstracts, to adequately assess their risk of confounding.
Efficacy and safety outcomes
All the studies performed the survival analysis on the intention‐to‐treat population and assessed to be at low risk of attrition bias, except for the three conference abstracts (Makiyama 2018; Pauligk 2017; Shah 2018) where details of analysis performed was unknown. Five studies (Hironaka 2013; Lee 2017; Li 2013; Pavlakis 2016; Yi 2012) modified their intention‐to‐treat population by removing participants who were found to be ineligible for the study after randomisation, withdrew the consent and/or did not receive any study treatment, however, in these studies the intention‐to‐treat population still constituted more than 95% of the total randomised population and considered to be at low risk of attrition bias.
Quality of life (QoL) outcomes
QoL endpoints were measured in seven studies. 84.2% of participants completed QoL questionnaires at least one post‐baseline time point in Wilke 2014, and this study was assessed to be low risk of attrition bias. For Bang 2017 and Pavlakis 2016, they were rated 65.9% and 62.6%, respectively and these studies were considered to be at high risk of attrition bias. For Lee 2017, more than 60% of participants completed baseline QoL questionnaires and for Thuss‐Patience 2011 the completion rate of the QoL questionnaire was said to be "poor". Therefore, these studies were similarly considered to be at high risk of attrition bias. For the remaining two studies (Li 2013; Thuss‐Patience 2017), detailed results including the number of participants completing the relevant questionnaires were not provided. For Shitara 2018b, in which QoL outcome is yet to be published, and for the studies which did not measure QoL outcome, their attrition bias risk was categorised as low in 'Risk of bias' summary table (Figure 2).
Selective reporting
One study (Thuss‐Patience 2011) reported OS for both treatment arms, but not PFS, TRR and AE outcomes, which were provided for the active treatment arm alone and not the BSC arm. In the same study, QoL was not reported due to poor completion rate of EORTC QLQ‐C30 questionnaire by the participants, which authors explained limited any meaningful analysis. Detailed results on AEs were not given for the two conference abstracts (Pauligk 2017; Makiyama 2018), however, all the predefined efficacy outcomes were reported in these studies. The other 14 studies in the review provided results for both AAEs and SAEs, except for Pavlakis 2016 and Thuss‐Patience 2011, which only provided the result for SAEs. Four studies (Bang 2017; Lee 2017; Li 2013; Thuss‐Patience 2017) provided variable amount of results on QoL in a descriptive manner. In two studies (Pavlakis 2016; Wilke 2014), separate articles focusing on QoL outcomes alone were published.
At least a brief summary of their protocols was available through relevant clinical trial registries for all the studies except for three studies (Kim 2015; Ling 2018; Thuss‐Patience 2011). All the efficacy outcomes relevant to this review (predefined in the case of studies with protocols available) were reported in all the studies apart from Thuss‐Patience 2011. Considering the order of importance of different outcomes based on the purpose of this review to be efficacy outcomes > safety outcomes > QoL outcomes, one study (Thuss‐Patience 2011) was assessed to be at high risk of reporting bias, three studies (Makiyama 2018; Pauligk 2017; Shah 2018) to be unclear and the rest of studies to be at low risk of reporting bias.
Other potential sources of bias
Three studies (Kim 2015; Lee 2017; Thuss‐Patience 2011) were terminated prematurely due to poor accrual, making these studies at high risk of recruitment bias. Enrolment was restricted to a biomarker‐selected population after 82.6% of the study participants had enrolled in Shitara 2018, however, this did not affect the study's primary outcome results and hence this study was considered to be still low risk of recruitment bias. Other sources of bias could not be adequately assessed in three studies (Makiyama 2018; Pauligk 2017; Shah 2018) published as conference abstracts.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings 1. Chemotherapy versus placebo, best supportive care (BSC) or no treatment.
| Chemotherapy compared to placebo, BSC or no treatment for advanced gastric and oesophago‐gastric junction adenocarcinoma | ||||||
| Patient or population: advanced gastric and oesophago‐gastric junction adenocarcinoma Setting: second‐line and beyond Intervention: chemotherapy Comparison: placebo, BSC or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with chemotherapy | Risk with placebo, BSC or no treatment | |||||
| Overall survival | mOS weighed for study size | HR 0.66 (0.52 to 0.83) | 547 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | 2 studies compared chemotherapy (irinotecan and trifluridine/tipiracil) to placebo or BSC. Heterogeneity was low (I2 = 7%, P = 0.30). | |
| 5.6 months | 3.5 months | |||||
| Progression‐free survival | mPFS | HR 0.57 (0.47 to 0.69) | 507 (1 RCT) | ⊕⊕⊕⊕ HIGH | 1 study compared trifluridine/tipiracil to placebo. | |
| 2.0 months | 1.8 months | |||||
| Serious adverse events | Study population | RR 1.38 (1.20 to 1.59) | 503 (1 RCT) | ⊕⊕⊕⊝ MODERATE 2 | 1 study compared trifluridine/tipiracil to placebo. | |
| 797 per 1,000 (693 to 918) |
577 per 1,000 | |||||
| Tumour response rate | RR 2.17 (0.63 to 7.48) | 435 (1 RCT) | ⊕⊕⊝⊝ LOW 3 | 1 study compared trifluridine/tipiracil to placebo. | ||
| 97 per 1,000 (28 to 335) | 45 per 1,000 | |||||
| Any adverse events | RR 1.04 (1.00 to 1.09) | 503 (1 RCT) | ⊕⊕⊕⊕ HIGH | 1 study compared trifluridine/tipiracil to placebo. | ||
| 972 per 1,000 (935 to 1,000) | 935 per 1,000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval ;HR: hazard ratio; mOS: median overall survival; mPFS: median progression‐free survival; RCT: randomised controlled trial; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded by one level for imprecision; 95% CI for the HR included null effect and appreciable benefit from chemotherapy.
2 Downgraded by one level for imprecision; 95% CI for the RR included null effect and appreciable harm from chemotherapy.
3 Downgraded by two levels for imprecision; the number of events analysed was small and 95% CI included both appreciable benefit and harm from chemotherapy.
Summary of findings 2. Biological therapy versus placebo, best supportive care (BSC) or no treatment.
| Biological therapy compared to placebo, BSC or no treatment for advanced gastric and oesophago‐gastric junction adenocarcinoma | ||||||
| Patient or population: advanced gastric and oesophago‐gastric junction adenocarcinoma Setting: second‐ and third‐line Intervention: biological therapy Comparison: placebo, BSC or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with biological therapy | Risk with placebo, BSC or no treatment | |||||
| Overall survival | mOS weighed for study size | HR 0.55 (0.41 to 0.73) | 781 (3 RCTs) | ⊕⊕⊕⊕ HIGH | 1 study compared nivolumab to placebo and 2 studies compared VEGFR‐targeted agents (apatinib and regorafenib) to placebo. Heterogeneity was moderate (I2 = 55%, P = 0.08). | |
| 5.2 months | 3.9 months | |||||
| Progression‐free survival | mPFS weighed for study size | HR 0.33 (0.19 to 0.57) | 781 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | 1 study compared nivolumab to placebo and 2 studies compared VEGFR‐targeted agents (apatinib and regorafenib) to placebo. Heterogeneity was high (I2 = 87%, P < 0.0001). | |
| 2.1 months | 1.3 months | |||||
| Serious adverse events | Study population | RR 1.14 (0.95 to 1.37) | 638 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 | 1 study compared nivolumab to placebo and the other study compared regorafenib to placebo. Heterogeneity was low (I2 = 3%, P = 0.31). | |
| 481 per 1,000 (401 to 578) | 422 per 1,000 | |||||
| Tumour response rate | Study population | RR 5.12 (1.23 to 21.27) | 687 (3 RCTs) | ⊕⊕⊝⊝ LOW 3 | 1 study compared nivolumab to placebo and 2 studies compared VEGFR‐targeted agents (apatinib and regorafenib) to placebo. Heterogeneity was low (I² = 13%, P = 0.33). | |
| 470 per 1,000 (113 to 1,000) | 92 per 1,000 | |||||
| Any adverse events | Study population | RR 1.08 (1.00 to 1.17) | 491 (1 RCT) | ⊕⊕⊕⊕ HIGH | 1 study compared nivolumab to placebo. | |
| 906 per 1,000 (839 to 981) | 839 per 1,000 | |||||
| Quality of life (QoL | )Similar QoL was experienced by participants treated with biological therapy and placebo, except for improved insomnia and pain associated with apatinib and regorafenib treatment, respectively. More participants receiving regorafenib experienced diarrhoea, and sore throat and mouth. | 283 (2 RCTs) | ⊕⊕⊝⊝ LOW 4 5 | QoL was assessed in 2 studies comparing VEGFR‐targeted agents (apatinib and regorafenib) to placebo, using EORTC QLQ‐C30 +/‐ QLQ‐STO22 and EQ‐5D. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HR: hazard ratio; mOS: median overall survival; mPFS: median progression‐free survival; RCT: randomised controlled trial; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded by one level for inconsistency; heterogeneity between studies was high.
2 Downgraded by two levels for imprecision; the number of events analysed was small and 95% CI included both null effect and appreciable harm from biological therapy.
3 Downgraded by two levels for imprecision; the number of events analysed was small and 95% CI was very wide.
4 Downgraded by one level for attrition bias; less participants in the placebo arm completed QoL questionnaires post baseline.
5 Downgraded by one level for imprecision; the number of events analysed was small.
Summary of findings 3. Chemotherapy versus biological therapy.
| Chemotherapy compared to biological therapy for advanced gastric and oesophago‐gastric junction adenocarcinoma | ||||||
| Patient or population: advanced gastric and oesophago‐gastric junction adenocarcinoma Setting: second‐line Intervention: biological therapy Comparison: chemotherapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with biological therapy | Risk with Chemotherapy | |||||
| Overall survival | mOS | HR 0.82 (0.66 to 1.02) | 395 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | 1 study compared pembrolizumab to paclitaxel in PD‐L1 CPS ≥ 1 population. | |
| 9.1 months | 8.3 months | |||||
| Progression‐free survival | mPFS | HR 1.27 (1.03 to 1.57) | 395 (1 RCT) | ⊕⊕⊕⊝ MODERATE 2 | 1 study compared pembrolizumab to paclitaxel in PD‐L1 CPS ≥ 1 population. | |
| 1.5 months | 4.1 months | |||||
| Serious adverse events | Study population | RR 0.41 (0.30 to 0.57) | 570 (1 RCT) | ⊕⊕⊝⊝ LOW 3 4 | 1 study compared pembrolizumab to paclitaxel. | |
| 143 per 1,000 (104 to 198) | 348 per 1,000 | |||||
| Tumour response rate | Study population | RR 1.17 (0.72 to 1.88) | 395 (1 RCT) | ⊕⊕⊝⊝ LOW 5 | 1 study compared pembrolizumab to paclitaxel in PD‐L1 CPS ≥ 1 population. | |
| 159 per 1,000 (98 to 255) |
136 per 1,000 | |||||
| Any adverse events | Study population | RR 0.63 (0.56 to 0.71) | 570 (1 RCT) | ⊕⊕⊕⊝ MODERATE 4 | 1 study compared pembrolizumab to paclitaxel. | |
| 530 per 1,000 (471 to 597) |
841 per 1,000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HR: hazard ratio; mOS: median overall survival; mPFS: median progression‐free survival; RCT: randomised controlled trial;RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by one level for imprecision; 95% CI included both null effect and appreciable benefit from biological therapy.
2 Downgraded by one level for imprecision; 95% CI included both null effect and appreciable harm from biological therapy.
3 Downgraded by one level for imprecision; the total number of events analysed was small.
4 Downgraded by one level for risk of performance bias in Shitara 2018.
5 Downgraded by two levels for imprecision; the number of events analysed was small and 95% CI included both appreciable benefit and harm from biological therapy.
Summary of findings 4. Chemmotherapy combined with biological therapy versus chemotherapy.
| Chemmotherapy compared to chemotherapy combined with biological therapy for advanced gastric and oesophago‐gastric junction adenocarcinoma | ||||||
| Patient or population: advanced gastric and oesophago‐gastric junction adenocarcinoma Setting: second‐ to fourth‐line Intervention: chemotherapy combined with biological therapy Comparison: chemotherapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with chemotherapy combined with biological therapy | Risk with chemotherapy | |||||
| Overall survival | mOS weighed for study size | HR 0.93 (0.83 to 1.04) | 2743 (7 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | 1 study each examined PARP inhibitor (olaparib), anti‐HER2 antibody (trastuzumab), cancer stem cell inhibitor (napabucasin), mTOR inhibitor (everolimus), anti‐VEGFR2 antibody (ramucirumab) and multi‐tyrosine kinase inhibitor (sunitinib) together with chemotherapy. The other study examined anti‐HER2 antibody conjugated with chemotherapy (trastuzumab‐emtansine). The control arm was taxane in all studies. Heterogeneity was moderate (I2 = 36%, P = 0.15). | |
| 8.1 months | 7.2 months | |||||
| Progression‐free survival | mPFS weighed for study size | HR 0.87 (0.74 to 1.02) | 2743 (7 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 3 4 | 1 study each examined PARP inhibitor (olaparib), anti‐HER2 antibody (trastuzumab), cancer stem cell inhibitor (napabucasin), mTOR inhibitor (everolimus), anti‐VEGFR2 antibody (ramucirumab) and multi‐tyrosine kinase inhibitor (sunitinib) together with chemotherapy. The other study examined anti‐HER2 antibody conjugated with chemotherapy (trastuzumab‐emtansine). The control arm was taxane in all studies. Heterogeneity was high (I2 = 71%, P = 0.002). | |
| 3.9 months | 3.1 months | |||||
| Serious adverse events | Study population | RR 1.17 (0.95 to 1.44) | 1618 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 5 6 | 1 study each examined PARP inhibitor (olaparib), anti‐VEGFR2 antibody (ramucirumab) and multi‐tyrosine kinase inhibitor (sunitinib) together with chemotherapy. The other study examined anti‐HER2 antibody conjugated with chemotherapy (trastuzumab‐emtansine). The control arm was taxane in all studies. Heterogeneity was high (I2 = 86%, P < 0.0001). | |
| 735 per 1,000 (686 to 791) |
613 per 1,000 | |||||
| Tumour response rate | Study population | RR 1.35 (0.99 to 1.85) | 2404 (6 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 3 4 | 1 study each examined PARP inhibitor (olaparib), anti‐HER2 antibody (trastuzumab), cancer stem cell inhibitor (napabucasin), anti‐VEGFR2 antibody (ramucirumab) and multi‐tyrosine kinase inhibitor (sunitinib) together with chemotherapy. The other study examined anti‐HER2 antibody conjugated with chemotherapy (trastuzumab‐emtansine). The control arm was taxane in all studies. Heterogeneity was high (I2 = 66%, P = 0.01). | |
| 294 per 1,000 (216 to 403) |
218 per 1,000 | |||||
| Any adverse events | Study population | RR 1.01 (1.00 to 1.03) | 1513 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 7 | 1 study each examined PARP inhibitor (olaparib) and anti‐VEGFR2 antibody (ramucirumab). The other study examined anti‐HER2 antibody conjugated with chemotherapy (trastuzumab‐emtansine). The control arm was taxane in all studies. Heterogeneity was low (I2 = 0%, P = 0.78). | |
| 987 per 1,000 (977 to 1000) |
977 per 1,000 | |||||
| Quality of life (QoL) | Median time to deterioration of EORTC QLQ‐C30 global health status for olaparib‐paclitaxel vs placebo‐paclitaxel | HR 0.88 (0.74 to 1.04) | 1154 (2 RCTs) | ⊕⊕⊝⊝ LOW 4 8 | QoL was assessed by 2 out of 7 studies using EORTC QLQ‐C30 plus QLQ‐SOT22 or EQ‐5D‐3L. 1 study each investigated PARP inhibitor (olaparib) and anti‐VEGFR2 antibody (ramucirumab). The control arm was taxane in both studies. Meta‐analysis was performed for time to deterioration of EORTC QLQ‐C30 global health status. Heterogeneity was very low (I² = 0%, P = 0.47). | |
| 3.4 months | 2.4 months | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HR: hazard ratio; mOS: median overall survival; mPFS: median progression‐free survival; RCT: randomised controlled trial; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded by one level for unclear risk of selection, attrition and reporting bias and confounders in Makiyama 2018, Pauligk 2017 and Shah 2018.
2 Downgraded by one level for risk of detection bias in Thuss‐Patience 2017.
3 Downgraded by one level for inconsistency; 95% CIs of some studies did not overlap.
4 Downgraded by one level for imprecision; 95% CI included both null effect and appreciable benefit from chemotherapy combined with biological therapy.
5 Downgraded by one level for imprecision; 95% CI included both null effect and appreciable harm from chemotherapy combined with biological therapy.
6 Downgraded by one level for risk of performance bias in Thuss‐Patience 2017 and Yi 2012.
7 Downgraded by one level for risk of performance and detection bias in Thuss‐Patience 2017.
8 Downgraded by one level for attrition bias in Bang 2017 and Wilke 2014.
Summary of findings 5. Chemotherapy versus chemotherapy.
| Chemotherapy compared to chemotherapy for advanced gastric and oesophago‐gastric junction adenocarcinoma | ||||||
| Patient or population: advanced gastric adenocarcinoma Setting: second‐line Intervention: chemotherapy Comparison: chemotherapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with chemotherapy | Risk with chemotherapy | |||||
| Overall survival (non‐taxane monotherapy vs taxane monotherapy) | mOS | HR 1.13 (0.86 to 1.48) | 219 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | 1 study compared irinotecan to paclitaxel. | |
| 8.4 months (irinotecan) | 9.5 months (paclitaxel) | |||||
| Overall survival (taxane‐containing doublet therapy vs taxane monotherapy) | mOS weighed for study size | HR 1.05 (0.72 to 1.54) | 121 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 | 1 study compared docetaxel‐oxaliplatin to docetaxel and the other study compared docetaxel‐cisplatin and docetaxel‐S1 to docetaxel. Heterogeneity was moderate (I2 = 44%, P = 0.17). | |
| 7.0 months (taxane‐containing doublet therapy) | 8.8 months (docetaxel) | |||||
| Overall survival (non‐taxane containing therapy vs another non‐taxane containing therapy) | mOS (P = 0.17) | Not applicable | 107 (1 RCT) | ⊕⊕⊕⊕ HIGH | 1 study compared FOLFIRI to EOX. | |
| 18.5 months (FOLFIRI) | 19.3 months (EOX) | |||||
| Progression‐free survival (non‐taxane monotherapy vs taxane monotherapy) | mPFS | HR 1.14 (0.88 to 1.48) | 219 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | 1 study compared irinotecan to paclitaxel. | |
| 2.3 months (irinotecan) | 3.6 months (paclitaxel) | |||||
| Progression‐free survival (taxane‐containing doublet therapy vs taxane monotherapy) | mPFS weighed for study size | HR 0.75 (0.52 to 1.09) | 121 (2 RCTs) | ⊕⊕⊝⊝ LOW 3 | 1 study compared docetaxel‐oxaliplatin to docetaxel and the other study compared docetaxel‐cisplatin and docetaxel‐S1 to docetaxel. Heterogeneity was moderate (I2 = 59%, P = 0.09). | |
| 3.4 months (taxane‐containing doublet therapy) | 1.6 months (docetaxel) | |||||
| Progression‐free survival (non‐taxane containing therapy vs another non‐taxane containing therapy) | mPFS (P = 0.46) | Not applicable | 107 (1 RCT) | ⊕⊕⊕⊕ HIGH | 1 study compared FOLFIRI to EOX. | |
| 8.1 months (FOLFIRI) | 7.4 months (EOX) | |||||
| Serious adverse effects | Grade ≥ 3 neutropenia was common occurring in > 20% of participants receiving both mono chemotherapy and polychemotherapy except for docetaxel‐S1 and EOX arms. | 448 (4 RCTs) | ⊕⊕⊕⊕ HIGH | 1 study each compared docetaxel‐cisplatin and docetaxel‐S1 to docetaxel, irinotecan to paclitaxel, docetaxel‐oxaliplatin to docetaxel and FOLFIRI to EOX. All 4 studies measured serious adverse events, however, none of them reported the total number of participants with any serious adverse events. | ||
| Tumour response rate (non‐taxane monotherapy vs taxane monotherapy) | Study population | RR 0.65 (0.34 to 1.26) | 179 (1 RCT) | ⊕⊕⊝⊝ LOW 4 | 1 study compared irinotecan to paclitaxel. | |
| 136 per 1,000 (71 to 263) (irinotecan) |
209 per 1,000 (paclitaxel) | |||||
| Tumour response rate (taxane‐containing doublet therapy vs taxane monotherapy) | Study population | RR 1.47 (0.56 to 3.89) | 121 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 5 6 | 1 study examined both docetaxel‐cisplatin and docetaxel‐S1 and the other study examined docetaxel‐oxaliplatin. Control arm was docetaxel in both studies. Heterogeneity was very low (I² = 0%, P = 0.92). | |
| 147 per 1,000 (56 to 389) (taxane‐containing doublet therapy) |
100 per 1,000 (taxane monotherapy) | |||||
| Tumour response rate (non‐taxane containing therapy vs another non‐taxane containing therapy) | Study population | RR 0.89 (0.48 to 1.67) | 107 (1 RCT) | ⊕⊕⊝⊝ LOW 7 | 1 study compared FOLFIRI to EOX chemotherapy. | |
| 254 per 1,000 (137 to 477) (FOLFIRI) |
286 per 1,000 (EOX) | |||||
| Adverse events | Neutropenia, anaemia, diarrhoea and anorexia were some of the commonly experienced adverse events across all the studies. Neuropathy was associated with paclitaxel, docetaxel‐S1 and EOX treatment. | 448 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 8 | 4 studies measured any adverse events, however, none of them reported the total number of participants with any adverse events. | ||
| Quality of life | No difference was observed for global QoL between docetaxel‐cisplatin, docetaxel‐S1 and docetaxel arms, however, worse QoL was reported for participants receiving the doublet chemotherapy for several symptom categories including physical functioning, fatigue and appetite loss. | 70 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 9 10 | 1 study comparing docetaxel‐cisplatin to docetaxel‐S1 and docetaxel reported QoL. QoL was assessed using EORTC QLQ‐C30 and gastric modules QLQ‐STO22. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HR: hazard ratio; mOS: median overall survival; mPFS: median progression‐free survival; RR: Risk ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded by two levels for imprecision; the number of events analysed was small and 95% CI included both null effect and appreciable harm from irinotecan.
2 Downgraded by two levels for imprecision; the number of events analysed was small and the 95% CI included both appreciable benefit and harm from taxane‐containing doublet therapy.
3 Downgraded by two levels for imprecision; the number of events analysed was small and 95% CI included both null effect and appreciable benefit from taxane‐containing doublet therapy.
4 Downgraded by two levels for imprecision; the number of events analysed was small and 95% CI included both appreciable benefit and harm from irinotecan.
5 Downgraded by one level for unclear risk of selection bias, detection bias and confounders in Kim 2015 and Lee 2017.
6 Downgraded by two levels for imprecision; the number of events analysed was small and 95% CI included both appreciable benefit and harm from taxane‐containing doublet therapy.
7 Downgraded by two levels for imprecision; the number events analysed was small and 95% CI included both appreciable benefit and harm from FOLFIRI.
8 Downgraded by one level for performance bias in Hironaka 2013.
9 Downgraded by one level for attrition bias in Lee 2017.
10 Downgraded by two levels for imprecision; the number of events analysed was very small.
We extracted summary data from all 17 included studies. No studies were identified for the following four pre‐specified comparisons.
Chemotherapy combined with biological therapy versus placebo, best supportive care (BSC) or no treatment.
Chemotherapy combined with biological therapy versus biological therapy.
Biological therapy versus biological therapy.
Chemotherapy combined with biological therapy versus chemotherapy combined with biological therapy
Chemotherapy versus placebo, best supportive care (BSC) or no treatment
Two studies (Shitara 2018b; Thuss‐Patience 2011) involving 547 participants compared chemotherapy versus placebo or BSC with 358 and 189 participants in each arm, respectively. Shitara 2018b examined the efficacy of trifluridine/tipiracil against placebo as third‐line and beyond treatment in metastatic gastric and oesophago‐gastric junction (OGJ) adenocarcinoma, while Thuss‐Patience 2011 examined that of irinotecan against BSC as second‐line treatment in metastatic gastric and OGJ adenocarcinoma.
Overall survival (OS)
Two studies (Shitara 2018b; Thuss‐Patience 2011) involving 547 participants contributed data to this meta‐analysis. Median OS (mOS), weighed for study size was 5.6 months in the chemotherapy arm and 3.5 months in the placebo or BSC arm. The pooled hazard ratio (HR) was 0.66 (95% confidence interval (CI) 0.52 to 0.83), indicating OS benefit of chemotherapy over placebo or BSC (Analysis 1.1). Heterogeneity between the two studies was low (I2 = 7%, P = 0.30) and sensitivity analysis using fixed‐effect model resulted in similar HR (HR = 0.66, 95% CI 0.52 to 0.83). The certainty of evidence was moderate due to imprecision. The 95% CI for the pooled HR included null effect and appreciable benefit from chemotherapy. Thuss‐Patience 2011 was associated with high risk of detection and reporting biases, however, they were considered unlikely to have impacted the OS outcome.
1.1. Analysis.

Comparison 1: Chemotherapy versus placebo, BSC or no treatment, Outcome 1: Overall survival
Progression‐free survival (PFS)
Thuss‐Patience 2011 reported PFS only for the irinotecan‐treated participants and their median PFS (mPFS) was 2.5 months. Hence, Shitara 2018b alone was included in the quantitative analysis and mPFS was 2.0 months in the trifluridine/tipiracil arm and 1.8 months in the placebo arm with HR of 0.57 (95% CI 0.47 to 0.69) in favour of chemotherapy (Analysis 1.2). The certainty of evidence was high.
1.2. Analysis.

Comparison 1: Chemotherapy versus placebo, BSC or no treatment, Outcome 2: Progression‐free survival
Serious adverse events (SAEs)
SAEs were reported by both studies, however, only for the irinotecan‐treated participants by Thuss‐Patience 2011. The most common SAE associated with irinotecan was diarrhoea (26%), and neutropenic fever affected 16% of participants receiving irinotecan. No treatment‐related death was observed. In Shitara 2018b, the most frequently reported SAEs were neutropenia (34.0%) and anaemia (19.1%) in the trifluridine/tipiracil arm and abdominal pain (8.9%) and general deterioration of physical health (8.9%) in the placebo arm. Grade ≥ 3 febrile neutropenia occurred in 1.8% of participants receiving trifluridine/tipiracil. The cause of treatment‐related death was cardiopulmonary arrest (n = 1) in the trifluridine/tipiracil arm and toxic hepatitis (n = 1) in the placebo arm.
The quantitative analysis considered 503 participants from Shitara 2018b and SAEs affected 79.7% and 57.78% of those receiving trifluridine/tipiracil and placebo, respectively. Risk ratio (RR) was 1.38 (95% CI 1.20 to 1.59), indicating higher occurrence of SAEs in the trifluridine/tipiracil arm (Analysis 1.3). Heterogeneity assessment and sensitivity analysis were not performed. The certainty of evidence was moderate due to imprecision. The 95% CI for the RR included null effect and appreciable harm from chemotherapy.
1.3. Analysis.

Comparison 1: Chemotherapy versus placebo, BSC or no treatment, Outcome 3: Serious adverse events
Tumour response rate (TRR)
Thuss‐Patience 2011 reported TRR only for the irinotecan‐treated participants and this was 0%. The quantitative analysis as a result only considered 435 participants from Shitara 2018b. TRR was 4.5% and 2.1% for participants receiving trifluridine/tipiracil and placebo, respectively. RR was 2.17 (95% CI 0.63 to 7.48), indicating insufficient evidence for TRR benefit of trifluridine/tipiracil (Analysis 1.4). The certainty of evidence was low due to imprecision with the small number of events analysed and the wide 95% CI for the RR.
1.4. Analysis.

Comparison 1: Chemotherapy versus placebo, BSC or no treatment, Outcome 4: Tumour response rate
Any adverse events (AAE)
AAE outcome was provided by one study (Shitara 2018b) for 503 participants. AAEs reported in more than 30% of participants in both trifluridine/tipiracil and placebo arms were nausea, anaemia and decreased appetite. Neutropenia additionally affected 52.5% of participants in the trifluridine/tipiracil arm. AAEs affected 97.3% and 93.5% of participants receiving trifluridine/tipiracil and placebo, respectively. RR was 1.04 (95% CI 1.00 to 1.09), indicating equivalent occurrence of AAEs between the two arms (Analysis 1.5). The certainty of evidence was high.
1.5. Analysis.

Comparison 1: Chemotherapy versus placebo, BSC or no treatment, Outcome 5: Any adverse events
Quality of life (QoL)
QoL measured using EORTC QLQ‐C30 questionnaire was one of the predefined endpoints in Thuss‐Patience 2011, however, due to the poor return of the forms, the study authors decided meaningful analysis was not plausible and no result was reported.
Biological therapy versus placebo, best supportive care (BSC) or no treatment
Three studies (Kang 2017; Li 2013; Pavlakis 2016) involving 781 participants compared biological therapy versus placebo in locally advanced, metastatic or recurrent gastric and OGJ adenocarcinoma. Biological therapy and placebo were administered to 520 (190 in the VEGFR‐targeted therapy arm and 330 in the immunotherapy arm) and 261 participants, respectively. Biological therapy examined were PD‐1 inhibitor, nivolumab (Kang 2017) and two tyrosine kinase inhibitors, apatinib (Li 2013) and regorafenib (Pavlakis 2016). While apatinib selectively targets VEGFR2, regorafenib is a multi‐target tyrosine kinase inhibitor including VEGFR2. All the studies were placebo‐controlled studies. In one study (Pavlakis 2016), participants received study treatment as second‐ or third‐line systemic treatment, while in the remaining two studies (Kang 2017; Li 2013), study treatment was provided in the third‐line setting.
Overall survival (OS)
Three studies ( Kang 2017; Li 2013; Pavlakis 2016 ) involving 781 participants contributed data to this meta‐analysis. mOS weighed fro study size was 5.2 months in the biological therapy arm and 3.9 months in the placebo arm. The pooled HR was 0.55 (95% CI 0.41 to 0.73), indicating OS benefit of biological therapy over placebo (Analysis 2.1). Heterogeneity between studies was moderate (I2 = 55%, P = 0.08) and sensitivity analysis using fixed‐effect model narrowed the 95% CI (HR = 0.59, 95% CI 0.50 to 0.70). With exclusion of Li 2013, which was associated with unclear risk of selection bias, the result remained in favour of biological therapy (HR = 0.66, 95% CI 0.55 to 0.79). The certainty of evidence was high.
2.1. Analysis.

Comparison 2: Biological therapy versus placebo, BSC or no treatment, Outcome 1: Overall survival
Progression‐free survival (PFS)
Three studies (Kang 2017; Li 2013; Pavlakis 2016) involving 781 participants contributed data to this meta‐analysis. mPFS weighed fro study size was 2.1 months in the biological therapy arm and 1.3 months in the placebo arm. As with the OS analysis, we found PFS benefit of biological therapy with the pooled HR of 0.33 (95% CI 0.19 to 0.57) (Analysis 2.2). Heterogeneity between studies was high (I2 = 87%, P < 0.0001). The 95% CI narrowed on sensitivity analysis using fixed‐effect model (HR = 0.47, 95% CI 0.40 to 0.55). With exclusion of Li 2013, which was associated with unclear risk of selection bias, the 95% CI widened, although the result still indicated the PFS benefit of biological therapy (HR = 0.50, 95% CI 0.34 to 0.75). The certainty of evidence was moderate due to heterogeneity between studies and the 95% CIs of some studies did not overlap.
2.2. Analysis.

Comparison 2: Biological therapy versus placebo, BSC or no treatment, Outcome 2: Progression‐free survival
Serious adverse events (SAEs)
The result on SAEs was available for all three studies. In Li 2013, one death was possibly related to SAEs from apatinib. The most common SAEs associated with apatinib were hypertension and hand‐foot syndrome, being observed in 8.5% to 10.9% and 4.3% to 13.0% of participants receiving apatinib, respectively. In Kang 2017, treatment‐related deaths were reported in 1.5% of participants receiving nivolumab. These events included acute hepatitis (n = 1), cardiac arrest (n = 1), death of unknown cause (n = 1), exertional dyspnoea (n = 1), and pneumonia (n = 1). The most common SAE associated with nivolumab was anaemia (11.5%). In Pavlakis 2016, treatment‐related death occurred in 2.1% of participants receiving regorafenib, which included one case of hepatic failure and one case of unresolved Grade 3 abdominal pain. The most common SAEs associated with regorafenib was hypertension occurring in 10.3% of participants in the regorafenib arm.
Two studies (Kang 2017; Pavlakis 2016) involving 638 participants reported the number of participants with any SAEs. 47.3% of participants receiving biological therapy experienced any SAEs compared to 42.2% of participants receiving placebo. The pooled RR was 1.14 (95% CI 0.95 to 1.37) and there was insufficient evidence to decide whether biological therapy increases the incidence of SAEs (Analysis 2.3). The observed heterogeneity between the two studies was low (I2 = 3%, P = 0.31). Sensitivity analysis using fixed‐effect model did not change the result (HR = 1.13, 95% CI 0.94 to 1.36). The certainty of evidence was low due to imprecision; the total number of events in the analysis was small and the 95% CI for the pooled RR included null effect and appreciable harm from biological therapy.
2.3. Analysis.

Comparison 2: Biological therapy versus placebo, BSC or no treatment, Outcome 3: Serious adverse events
Tumour response rate (TRR)
Three studies (Kang 2017; Li 2013; Pavlakis 2016) involving 687 participants contributed data to this meta‐analysis. The pooled TRR was 9.2% in the biological therapy arm and 0.4% in the placebo arm. When studies examining VEGFR‐targeted therapy and immunotherapy were separated, TRR was 6.3% and 11.2%, respectively. The pooled RR was 5.12 (95% CI 1.23 to 21.27), in favour of biological therapy (Analysis 2.4). The observed heterogeneity was low (I² = 13%, P = 0.33). The certainty of evidence was low due to imprecision; the number of events analysed was small and the 95% CI for the pooled RR was very wide.
2.4. Analysis.

Comparison 2: Biological therapy versus placebo, BSC or no treatment, Outcome 4: Tumour response rate
Any adverse events (AAEs)
AAE outcome was available for two studies (Kang 2017; Li 2013). In Kang 2017, AAEs reported in 15% or more of participants in the nivolumab arm were abdominal pain, decreased appetite, nausea, diarrhoea and pruritis. In Li 2013, the most common AAEs were hypertension, hand‐foot syndrome, elevated aminotransferases and leucopenia. They were experienced by 39.1% to 40.4%, 25.5% to 45.7%, 19.2% to 41.3% and 39.1% to 48.9% of participants in the apatinib arms, respectively.
One study (Kang 2017) involving 491 participants reported the number of participants with AAEs. The occurrence of AAEs was 90.9% for participants receiving nivolumab compared to 83.9% for those receiving placebo with RR of 1.08 (95% CI 1.00 to 1.17) (Analysis 2.5). Nivolumab was not associated with increased AAEs. The certainty of evidence was high.
2.5. Analysis.

Comparison 2: Biological therapy versus placebo, BSC or no treatment, Outcome 5: Any adverse events
Quality of life (QoL)
Two studies reported on QoL. In Li 2013, EORTC QLQ‐C30 was used and the descriptive result was provided. The number of participants completing the questionnaire was not reported. The significant change in QoL over the course of treatment was only found for insomnia, where after two cycles of treatment, insomnia significantly improved in participants receiving apatinib compared to those receiving placebo (P = 0.002). There was a trend for improved cognitive function in the apatinib arm compared to the placebo arm after two cycles of treatment (P = 0.067). Pavlakis 2016 assessed QoL associated with regorafenib and placebo treatment using EORTC QLQ‐C30, QLQ‐STO22, EQ‐5D and the patient disease and treatment assessment (PTDATA) form. 92.5% of the randomised participants completed the baseline QoL assessment with 40.8% of placebo‐ and 32.3% of regorafenib‐receiving participants providing no post‐baseline information. At Week four, there was more reporting of diarrhoea by participants receiving regorafenib (regorafenib‐to‐placebo difference of 14.0%, CI 2 to 24, P = 0.019). Pain score at Week 8 between the two treatment arms differed by 10 in favour of regorafenib treatment, however, this was not statistically significant. There was a lower incidence of cough (P = 0.004) and higher incidence of sore mouth or throat (P = 0.05) in the regorafenib arm. The overall certainty of evidence was low due to attrition bias and imprecision related to the small number of participants analysed.
Chemotherapy versus biological therapy
One study (Shitara 2018) involving 592 participants compared chemotherapy versus any biological therapy in participants with metastatic or locally advanced unresectable gastric and OGJ adenocarcinoma as second‐line therapy. 296 participants were in each arm. Biological therapy examined was PD‐1 inhibitor, pembrolizumab and this was compared to paclitaxel. The study selected participants based on biomarker expression; PD‐L1 combined positive score (CPS) ≥ 1. The study initially enrolled participants independent of their PD‐L1 CPS, which was later restricted to those with PD‐L1 CPS ≥ 1 and its predefined primary endpoints were OS and PFS in participants with PD‐L1 CPS ≥ 1. We, therefore, analysed participants with PD‐L1 CPS ≥ 1 for our efficacy outcomes and their overall population for safety outcomes.
Overall survival (OS)
One study (Shitara 2018) involving 395 participants contributed data to the analysis. mOS for the PD‐L1 CPS ≥ 1 population was 9.1 months in the pembrolizumab arm and 8.3 months in the paclitaxel arm. The HR was 0.82 (95% CI 0.66 to 1.02), indicating insufficient evidence for OS benefit of pembrolizumab over paclitaxel (Analysis 3.1). For the overall population (n = 592), mOS was 6.7 months in the pembrolizumab arm and 8.3 months in the paclitaxel arm with HR of 0·94 (95% CI 0·79 to 1·12). The certainty of evidence was moderate due to imprecision; the 95% CI for the HR included both null effect and appreciable benefit from biological therapy.
3.1. Analysis.

Comparison 3: Chemotherapy versus biological therapy, Outcome 1: Overall survival
Progression‐free survival (PFS)
One study (Shitara 2018) involving 395 participants contributed data to the analysis. mPFS for the PD‐L1 CPS ≥ 1 population was 1.5 months for the pembrolizumab arm and 4.1 months for the paclitaxel arm. The HR was 1.27 (95% CI 1.03 to 1.57) in favour of paclitaxel (Analysis 3.2). PFS analysis on the overall population resulted in HR of 1·49 (95% CI 1·25 to 1·77) and mPFS for each arm remained unchanged. The certainty of evidence was moderate due to imprecision; the 95% CI for the HR included both null effect and appreciable harm from pembrolizumab.
3.2. Analysis.

Comparison 3: Chemotherapy versus biological therapy, Outcome 2: Progression‐free survival
Serious adverse events (SAEs)
One study (Shitara 2018) involving 570 participants contributed data to this analysis. Treatment‐related death occurred in 1.0% of participants in the pembrolizumab arm (one case of colitis, interstitial lung disease, and unspecified death each) and < 1% in the paclitaxel arm (one case of pulmonary embolism). The most common treatment‐related SAEs were anaemia (2.4%) and fatigue (2.4%) in the pembrolizumab arm and neutropenia (7.2%) and fatigue (4.7%) in the paclitaxel arm. Immune‐mediated SAEs due to pembrolizumab were rare; hepatitis (1.4%), hypophysitis (< 1%) and pneumonitis (< 1%).
Participants receiving pembrolizumab (14.3%) experienced SAEs compared to 34.8% of those receiving paclitaxel. RR was 0.41 (95% CI 0.30 to 0.57) in favour of pembrolizumab (Analysis 3.3). The certainty of evidence was low due to risk of bias and imprecision; the study was associated with risk of performance bias and the number of events included in the analysis was small.
3.3. Analysis.

Comparison 3: Chemotherapy versus biological therapy, Outcome 3: Serious adverse events
Tumour response rate (TRR)
One study (Shitara 2018) involving 395 participants contributed data to this analysis. TRR for the PD‐L1 CPS ≥ 1 population was 15.8% in the pembrolizumab arm and 13.6% in the paclitaxel arm with RR of 1.17 (95% CI 0.72 to 1.88), implying insufficient evidence for TRR benefit from pembrolizumab or paclitaxel (Analysis 3.4). The certainty of evidence was low due to imprecision; the number of events included in the analysis was small and the 95% CI for the RR included both null effect and appreciable benefit of biological therapy.
3.4. Analysis.

Comparison 3: Chemotherapy versus biological therapy, Outcome 4: Tumour response rate
Any adverse events (AAEs)
One study (Shitara 2018) involving 570 participants contributed data to this analysis. AAEs affecting more than 10% of participants were fatigue (11.9%) in the pembrolizumab arm and alopecia (40.2%), fatigue (23.2%), nausea (18.1%), decreased appetite (15.6%), diarrhoea (13.8%), anaemia (14.1%) and peripheral neuropathy (14.5%) in the paclitaxel arm. Immune mediated AAEs were observed in 18.4% of participants receiving pembrolizumab and 7.6% of those receiving paclitaxel in this study. The incidence of AAEs was 52.7% for pembrolizumab‐treated participants and 84.1% for paclitaxel‐treated participants. RR was 0.63 (95% CI 0.56 to 0.71) in favour of pembrolizumab (Analysis 3.5). The certainty of evidence was moderate due to risk of performance bias.
3.5. Analysis.

Comparison 3: Chemotherapy versus biological therapy, Outcome 5: Any adverse events
Chemotherapy combined with biological therapy versus chemotherapy
Seven studies (Bang 2017; Makiyama 2018; Pauligk 2017; Shah 2018; Thuss‐Patience 2017; Wilke 2014; Yi 2012) involving 2743 participants compared chemotherapy combined with biological therapy versus chemotherapy in participants with locally advanced, metastatic or recurrent gastric and OGJ adenocarcinoma as second‐line therapy. One thousand, four hundred and twenty‐eight and 1315 participants received chemotherapy combined with biological therapy and chemotherapy alone, respectively. The type of biological therapy used varied between studies; poly ADP ribose polymerase (PARP) inhibitor, olaparib (Bang 2017), monoclonal antibody against HER2, trastuzumab (Makiyama 2018), mTOR kinase inhibitor, everolimus (Pauligk 2017), cancer stem cell inhibitor, napabucasin (Shah 2018), monoclonal antibody against VEGFR2, ramucirumab (Wilke 2014) and multi‐targeted tyrosine kinase inhibitor, sunitinib (Yi 2012). In one study (Thuss‐Patience 2017), trastuzumab‐emtansine, which is trastuzumab conjugated with a cytotoxic agent DM1 was administered in the chemotherapy combined with biological therapy arm. Taxanes were used in the chemotherapy arm for all the studies. Three studies (Makiyama 2018; Pauligk 2017; Shah 2018) were conference abstracts, while others were full‐text articles. In Makiyama 2018 and Thuss‐Patience 2017, participants were enrolled based on HER2 positivity. Shah 2018 reported the total number of participants randomised, but not the number of participants in each treatment arm and whether efficacy analysis was performed on the intention‐to‐treat population was not known. The number of participants, hence, in each treatment arm was estimated based on the described 1:1 randomisation and the study was only included in the meta‐analysis for efficacy outcomes.
Overall survival (OS)
Seven studies (Bang 2017; Makiyama 2018; Pauligk 2017; Shah 2018; Thuss‐Patience 2017; Wilke 2014; Yi 2012) involving 2743 participants contributed data to this meta‐analysis. mOS weighed for study size was 8.1 months in the chemotherapy combined with biological therapy arm and 7.2 months in the chemotherapy arm. In two studies (Bang 2017; Pauligk 2017), 95% CIs associated with HR for OS outcome were not provided; Bang 2017 reported the HR with 97.5% CIs and Pauligk 2017 with a P value; 95% CIs were estimated under the assumption for Gaussian distribution.
The pooled HR was 0.93 (95% CI 0.83 to 1.04), indicating equivalent OS benefit between the two arms (Analysis 4.1). The observed heterogeneity was moderate (I2 = 36%, P = 0.15). Sensitivity analysis using fixed‐effect model made little difference to the result (HR = 0.92, 95% CI 0.84 to 1.00). The result changed little with exclusion of three studies (Makiyama 2018; Pauligk 2017; Shah 2018) published as conference abstracts with unclear risk of bias (HR = 0.88, 95% CI 0.75 to 1.04), and exclusion of Bang 2017 and Pauligk 2017 for which 95% CIs were estimated (HR = 0.97, 95% CI 0.84 to 1.13). Removal of Makiyama 2018 and Thuss‐Patience 2017, which investigated paclitaxel‐trastuzumab and trastuzumab emtansine, respectively in the HER2‐positive population, made the small OS benefit become apparent (HR = 0.89, 95% CI 0.80 to 0.99). The certainty of evidence was moderate due to Makiyama 2018 , Pauligk 2017 and Shah 2018 being associated with unclear risk of selection, attrition and reporting bias as well as confounders.
4.1. Analysis.

Comparison 4: Chemotherapy combined with biological therapy versus chemotherapy, Outcome 1: Overall survival
Progression‐free survival (PFS)
Seven studies (Bang 2017; Makiyama 2018; Pauligk 2017; Shah 2018; Thuss‐Patience 2017; Wilke 2014; Yi 2012) involving 2743 participants contributed data to this meta‐analysis. Yi 2012 reported TTP defined as the interval between the date of the first study treatment and the date of documented disease progression in place of PFS. As more than 90% of participants in both docetaxel‐sunitinib and docetaxel arms experienced disease progression in the study, we accepted this TTP outcome for the PFS analysis. mPFS weighed for study size was 3.9 months in the chemotherapy combined with biological therapy arm and 3.1 months in the chemotherapy arm. As with OS, no 95% CIs were provided by Bang 2017 and Pauligk 2017 for their HR and they were estimated.
The pooled HR was 0.87 (95% CI 0.74 to 1.02), indicating insufficient evidence for PFS benefit of chemotherapy combined with biological therapy (Analysis 4.2). The observed heterogeneity was high (I2 = 71%, P = 0.002). On sensitivity analysis using fixed‐effect model, PFS benefit of combined chemotherapy and biological therapy became apparent (HR = 0.85, 95% CI 0.79 to 0.93). Exclusion of three studies (Makiyama 2018; Pauligk 2017; Shah 2018) published as conference abstracts with unclear risk of bias widened the 95% CI (HR = 0.82, 95% CI 0.63 to 1.07). When two studies (Bang 2017; Pauligk 2017) with unpublished 95% CIs for their HRs as well as Yi 2012, which reported TTP instead of PFS, were removed, the 95% CI widened further (HR = 0.89, 95% CI 0.68 to 1.18). Exclusion of Makiyama 2018 and Thuss‐Patience 2017, which investigated paclitaxel‐trastuzumab and trastuzumab emtansine, respectively in the HER2‐positive population made PFS benefit of combined chemotherapy and biological therapy became apparent (HR =0.82, 95% CI 0.68 to 0.98). The certainty of evidence was very low due to risk of detection bias in Thuss‐Patience 2017 as well as unclear risk of bias in several domains for Makiyama 2018 , Pauligk 2017 and Shah 2018 as described for the OS outcome. Inconsistency and imprecision were additionally detected; the 95% CIs of some studies did not overlap and the 95% CI for the pooled HR included PFS benefit of chemotherapy combined with biological therapy as well as null effect.
4.2. Analysis.

Comparison 4: Chemotherapy combined with biological therapy versus chemotherapy, Outcome 2: Progression‐free survival
Serious adverse events (SAEs)
Four studies (Bang 2017; Thuss‐Patience 2017; Wilke 2014; Yi 2012) involving 1618 participants contributed data to this meta‐analysis. In Bang 2017, two treatment‐related deaths occurred; liver injury (n = 1) in the olaparib‐paclitaxel arm and cardiac failure (n = 1) in the placebo‐paclitaxel arm. The most common SAE was neutropenia in both arms; 29.8% in the olaparib‐paclitaxel arm and 22.8% in the placebo‐ paclitaxel arm. In Thuss‐Patience 2017, treatment‐related deaths were reported in 4% of participants in both arms; gastric haemorrhage (n = 1), upper gastrointestinal haemorrhage (n = 1), atypical pneumonia (n = 1), septic shock (n = 1), aspiration pneumonia (n = 1), pulmonary alveolar haemorrhage (n = 1) and pneumonia (n = 2) in the trastuzumab emtansine arm and unexplained cause of death (n = 1), respiratory failure (n = 1) and pneumonia (n = 2) in the taxane arm. The most common SAEs in participants receiving trastuzumab emtansine were anaemia (26.3%) and thrombocytopenia (11.2%), while in participants treated with taxanes they were neutropenia (38.7%) and anaemia (18.0%). In Wilke 2014, 2% of participants from ramucirumab‐paclitaxel and placebo‐paclitaxel arms experienced treatment‐related death; details of underlying AEs were septic shock, malabsorption, gastrointestinal haemorrhage, death of unknown origin, pulmonary embolism and sepsis in the ramucirumab‐paclitaxel arm and acute renal failure, cardiac failure, febrile neutropenia, septic shock, pulmonary embolism and cerebral haemorrhage in the placebo‐paclitaxel arm. The most common SAE was again neutropenia; 40.7% in the ramucirumab‐paclitaxel arm and 18.8% in the placebo‐paclitaxel arm. In Yi 2012, neutropenia was frequent in both the docetaxel‐sunitinib and docetaxel arms, and 26.8% and 16.3% of participants in each arm experienced febrile neutropenia, respectively. Shah 2018, reported 69.2% and 59.7% of participants receiving napabucasin‐paclitaxel and placebo‐paclitaxel, respectively experienced SAEs. As the study did not provide the number of participants analysed, it was not included in the meta‐analysis.
The total incidence of SAEs were 72.6% in the chemotherapy combined with biological therapy arm, compared to 61.3% in the chemotherapy alone arm. The pooled RR was 1.17 (95% CI 0.95 to 1.44) and there was insufficient evidence to decide whether addition of biological therapy to chemotherapy increases occurrence of SAEs (Analysis 4.3). The heterogeneity between the studies was high (I2 = 86%, P < 0.0001). Sensitivy analyses using fixed‐effect model caused the chemotherapy combined with biological therapy arm to be associated with more SAEs (RR = 1.20, 95% CI 1.12 to 1.29). Similarly, exclusion of Thuss‐Patience 2017 and Yi 2012, which were associated with performance bias, resulted in the pooled RR to be in favour of chemotherapy (RR = 1.29, 95% CI 1.19 to 1.39). The certainty of evidence was very low due to risk of bias, inconsistency and imprecision. Thuss‐Patience 2017 and Yi 2012 are both open‐label studies with risk of performance bias. The 95% CIs of some studies did not overlap and the 95% CI associated with the pooled RR included both null effect and appreciable harm of chemotherapy combined with biological therapy.
4.3. Analysis.

Comparison 4: Chemotherapy combined with biological therapy versus chemotherapy, Outcome 3: Serious adverse events
Tumour response rate (TRR)
Six studies (Bang 2017; Makiyama 2018; Shah 2018; Thuss‐Patience 2017; Wilke 2014; Yi 2012) involving 2404 participants contributed data to this meta‐analysis. Makiyama 2018 and Shah 2018 only reported TRR without the exact number of participants who had complete and partial responses and no reference was made if the analysis was performed on the intention‐to‐treat population. Hence, the number of events was estimated from the number of participants randomised.
We observed the pooled RR of 1.35 (95% CI 0.99 to 1.85), indicating insufficient evidence for TRR benefit of adding biological therapy to chemotherapy (Analysis 4.4). The heterogeneity observed was high (I2 = 66%, P = 0.01). The certainty of evidence was very low due to risk of detection bias in Thuss‐Patience 2017, inconsistency with 95% CIs of some studies not overlapping and imprecision with the 95% CI for the pooled RR including both null effect and appreciable benefit from chemotherapy combined with biological therapy.
4.4. Analysis.

Comparison 4: Chemotherapy combined with biological therapy versus chemotherapy, Outcome 4: Tumour response rate
Any adverse events (AAEs)
Five studies (Bang 2017; Shah 2018; Thuss‐Patience 2017; Wilke 2014; Yi 2012) reported the result on AAEs. In Bang 2017, the most common AEs in the olaparib‐paclitaxel arm were neutropenia (46.9%), alopecia (42.7%) and anaemia (38.5%), and in the placebo‐paclitaxel group, they were alopecia (44.8%) and neutropenia (41.7%). In Thuss‐Patience 2017, the most common AAEs were alopecia (51.4%), neutropenia (50.4%), fatigue (47.7%) and peripheral neuropathy (36.9%) in the taxane arm and anaemia (35.8%) and fatigue (30.3%) in the trastuzumab emtansine arm. Haemorrhoage was experienced by 11.7% of participants receiving taxane and 28.1% of those receiving trastuzumab emtansine. In Wilke 2014, the most common AAEs were neutropenia (54.4%), fatigue (56.9%) and neuropathy (45.9%), in the ramucirumab‐paclitaxel arm and fatigue (43.8%) in the placebo‐paclitaxel arm. In Yi 2012, the most common AAEs was anaemia in both docetaxel‐sunitinib and docetaxel arm (91.1% versus 93.9%). Addition of sunitinib to docetaxel increased incidence of stomatitis (51.8%), diarrhoea (35.7%) and hand‐foot syndrome (53.6%).
Three studies (Bang 2017,Thuss‐Patience 2017; Wilke 2014) involving 1513 participants contributed data to the meta‐analysis. The rate of AAEs were 98.6% in the chemotherapy combined with biological therapy arm and 97.7% in the chemotherapy alone arm. The pooled RR was 1.01 (95% CI 1.00 to 1.03), indicating equivalent occurrence of AAEs between the two arms (Analysis 4.5). We observed low heterogeneity between the studies (I2 = 0%, P = 0.78). The certainty of evidence was moderate due to risk of performance and detection bias in Thuss‐Patience 2017.
4.5. Analysis.

Comparison 4: Chemotherapy combined with biological therapy versus chemotherapy, Outcome 5: Any adverse events
Quality of life (QoL)
QoL data were reported by three studies (Bang 2017; Thuss‐Patience 2017; Wilke 2014). Bang 2017 reported time to deterioration of EORTC QLQ‐C30 global health status. For overall population, median time to deterioration of global health status was 3.4 months in the olaparib‐paclitaxel arm and 2.4 months in the placebo‐paclitaxel arm with HR of 0.82 (95% CI 0.64 to 1.05). Thuss‐Patience 2017 measured QoL using EORTC QLQ‐C30, QLQ‐STO22 and EQ‐5D and described it to be similar between the treatment arms. Wilke 2014 assessed QoL using EORTC QLQ‐C30 and EQ‐5D‐3L. HR for global health status measured using QLQ‐C30 was 0.93 (95% CI 0.73 to 1.18). A consistently higher proportion of participants in the ramucirumab‐paclitaxel arm experienced stable or improved QoL parameters at each assessment, compared with those in the placebo‐paclitaxel arm. The compliance of questionnaires were similar across the two treatment arms throughout the study; 97.6% and 97.9% at baseline and 70.0% and 69.2% at Week 36.
Meta‐analysis on time to deterioration of EORTC QLQ‐C30 global health status was performed on data from 1154 participants in the two studies (Bang 2017; Wilke 2014). The pooled HR was 0.88 (95% CI 0.74 to 1.04) and whether chemotherapy combined with biological therapy improves QoL experienced could not be decided (Analysis 4.6). Heterogeneity assessed was very low (I² = 0%, P = 0.47). The certainty of evidence was low due to risk of attrition bias in both studies and the 95% CI for the pooled HR including both null effect and appreciable benefit from chemotherapy combined with biological therapy.
4.6. Analysis.

Comparison 4: Chemotherapy combined with biological therapy versus chemotherapy, Outcome 6: QoL
Chemotherapy versus chemotherapy
Four studies (Hironaka 2013; Kim 2015; Lee 2017; Ling 2018) involving 447 participants compared chemotherapy to another chemotherapy in participants with advanced or recurrent gastric adenocarcinoma as second‐line treatment. Chemotehrpay regimens examined varied widely between studies. One study (Hironaka 2013) compared non‐taxane monotherapy (irinotecan) versus taxane monotherapy with 108 participants in the irinotecan arm and 111 in the paclitaxel arm. Two studies (Kim 2015 ; Lee 2017 ) compared taxane‐containing doublet therapy (docetaxel‐oxaliplatin, docetaxel‐cisplatin and docetaxel‐S1) versus taxane monotherapy with 71 participants in the taxane‐containing doublet therapy arm and 50 in the taxane monotherapy arm. One study (Ling 2018) compared non‐taxane containing doublet therapy (FOLFIRI) to triplet therapy (capecitabine‐epirubicin‐oxaliplatin) with 51 participants in the FOLFIRI arm and 56 in the EOX arm. To enable meaningful analysis, efficacy outcomes are discussed under following three categories: non‐taxane monotherapy versus taxane monotherapy, taxane‐containing doublet chemotherapy versus taxane monotherapy, and non‐taxane containing chemotherapy versus another non‐taxane containing chemotherapy.
Overall survival (OS)
Four studies (Hironaka 2013; Kim 2015; Li 2013; Ling 2018) involving 447 participants reported OS outcomes. For two of the studies (Kim 2015 ; Lee 2017) OS outcomes were provided without HRs and they were estimated from the published Kaplan‐Meier curves using a previously described method (Tierney 2007). Ling 2018 similarly reported OS outcome without HR, however, the mOS in the text did not correspond with the mOS obtained from the published Kaplan‐Meier curve, raising a question on the accuracy of this Kaplan‐Meier curve and the estimated HR. This study, therefore, was not considered for the quantitative analysis.
Non‐taxane monotherapy versus taxane monotherapy
One study (Hironaka 2013) involving 219 participants contributed data to this analysis. Reported mOS was 8.4 months in the irinotecan arm and 9.5 months in the paclitaxel arm. HR was 1.13 (95% CI 0.86 to 1.48), indicating insufficient evidence for OS benefit of paclitaxel over irinotecan (Analysis 5.1). The certainty of evidence was low due to imprecision secondary to the limited number of participants analysed and the 95% CI for the HR including both null effect and appreciable harm from irinotecan. The study was at risk of performance bias, but this is unlikely to have affected the outcome.
5.1. Analysis.

Comparison 5: Chemotherapy versus chemotherapy, Outcome 1: Overall survival (non‐taxane monotherapy vs taxane monotherapy)
Taxane‐containing doublet therapy vs taxane monotherapy
Two studies (Kim 2015; Lee 2017) involving 121 participants contributed data to this meta‐analysis. mOS weighed for study size was 7.0 months in the taxane‐containing doublet arm and 8.8 months in the taxane monotherapy arm. The pooled HR was 1.05 (95% CI 0.72 to 1.54), indicating insufficient evidence for OS benefit of taxane‐containing doublet therapy or taxane monotherapy (Analysis 5.2). There was moderate heterogeneity between studies (I2 = 44%, P = 0.17). Sensitivity analysis using fixed‐effect mode resulted in similar HR (HR = 1.07, 95% CI 0.81 to 1.42). Exclusion of Kim 2015, which was associated with unclear risk of selection bias, caused the HR to widen (HR = 1.11, 95% CI 0.61 to 2.00). The certainty of evidence was low due to imprecision; the number of participants analysed was small and the 95% CI for the pooled HR included both appreciable benefit and harm from taxane‐containing doublet therapy.
5.2. Analysis.

Comparison 5: Chemotherapy versus chemotherapy, Outcome 2: Overall survival (taxane‐containing doublet therapy vs taxane monotherapy)
Non‐taxane containing chemotherapy vs another non‐taxane containing chemotherapy
Ling 2018 involving 107 participants compared the efficacy of FOLFIRI and EOX chemotherapy and observed equivalent OS outcome from the two regimens; reported mOS was 18.5 and 19.3 months, respectively (P = 0.170).
Progression‐free survival (PFS)
Four studies (Hironaka 2013; Kim 2015; Li 2013; Ling 2018) involving 447 participants reported PFS outcomes. For the same reasons described in the OS section, HRs associated with PFS outcomes were estimated from the published Kaplan‐Meier curves for Kim 2015 and Lee 2017, and Ling 2018 was not considered for the quantitative analysis.
Non‐taxane monotherapy versus taxane monotherapy
One study (Hironaka 2013) involving 219 participants contributed data to this analysis. mPFS was 2.3 months in the irinotecan arm and 3.6 months in the paclitaxel arm. We could not decide between irinotecan and paclitaxel in terms of effects on PFS (HR = 1.14, 95% CI 0.88 to 1.48) (Analysis 5.3). The certainty of evidence was low due to imprecision secondary to the limited number of participants analysed and the 95% CI for the HR including both null effect and the appreciable harm from irinotecan.
5.3. Analysis.

Comparison 5: Chemotherapy versus chemotherapy, Outcome 3: Progression‐free survival (non‐taxane monotherapy vs taxane monotherapy)
Taxane‐containing doublet therapy versus taxane monotherapy
Two studies (Kim 2015; Lee 2017) involving 121 participants contributed data to this meta‐analysis. mPFS weighed for study size was 3.4 months in the taxane‐containing doublet therapy arm and 1.6 months in the taxane monotherapy arm. The pooled HR was 0.75 (95% CI 0.5 to 1.09), indicating insufficient evidence for PFS benefit of taxane‐containing doublet therapy over taxane monotherapy (Analysis 5.4). Hetereogeniety between studies was moderate (I2 = 59%, P = 0.09). Sensitivity analysis using fixed‐effect mode resulted in the HR to become in favour of taxane containing doublet therapy (HR = 95% CI 0.59 to 0.95). Exclusion of Kim 2015, which was associated with unclear risk of selection bias resulted in the 95% CI to widen (HR = 0.86 95% CI 0.54 to 1.38). The certainty of evidence was low due to imprecision; the number of events analysed was small and the 95% CI for the HR included both null effect and appreciable benefit from taxane‐containing doublet therapy.
5.4. Analysis.

Comparison 5: Chemotherapy versus chemotherapy, Outcome 4: Progression‐free survival (taxane‐containing doublet therapy vs taxane monotherapy)
Non‐taxane containing chemotherapy versus another non‐taxane containing chemotherapy
Ling 2018 involving 107 participants compared the efficacy of FOLFIRI and EOX and observed equivalent PFS outcome from the two regimens; the reported mPFS was 8.1 and 7.4 months, respectively (P = 0.460).
Serious adverse events (SAEs)
All four studies measured SAEs, however, none of them reported the total number of participants with any SAEs. Meta‐analysis was not possible for this outcome.
In Hironaka 2013, treatment‐related death was observed in two patients (1.8%) in the irinotecan arm with causes being serious pneumonia (n = 1) and gastric perforation (n = 1). The most common Grade 3‐4 AEs were neutropenia and anaemia in both arms; 28.7% and 21.3% in the paclitaxel arm and 39.1% and 30.0% in the irinotecan arm. In Kim 2015, the most common SAEs were neutropenia (32.0%) in the docetaxel‐oxaliplatin arm with 20.0% of participants in this arm developing febrile neutropenia. There was one possible treatment‐related death in Lee 2017 in the docetaxel‐cisplatin arm (peritonitis without neutropenia) and for the docetaxel‐S1 arm (pneumonia with neutropenia). The most common Grade 3‐4 SAEs in this study was neutropenia with febrile neutropenia affecting 8.3%, 4.3% and 8.7% of participants in the docetaxel‐cisplatin, docetaxel‐S1 and docetaxel arms, respectively. In Ling 2018, neutropenia was again the most common SAE, being observed in 33.3% and 7.1% of participants receiving FOLFIRI and EOX, respectively. The certainty of evidence was considered to be high as none of risk of bias recognised for the included studies was thought to have significantly impacted the reporting of SAEs.
Tumour response rate
Four studies (Hironaka 2013; Kim 2015; Lee 2017; Ling 2018) involving 407 participants reported TRR data.
Non‐taxane monotherapy vs taxane monotherapy
One study (Hironaka 2013) involving 179 participants with more than one measurable lesions at baseline contributed data to this analysis. TRR was 13.6% in the irinotecan arm and 20.9% in the paclitaxel arm. RR was 0.65 (95% CI 0.34 to 1.26), implying insufficient evidence for TRR benefit of paclitaxel or irinotecan (Analysis 5.5). The certainty of evidence was low due to imprecision; the number events analysed was small and the 95% CI for the RR included both appreciable benefit and harm from paclitaxel.
5.5. Analysis.

Comparison 5: Chemotherapy versus chemotherapy, Outcome 5: Tumour response rate (non‐taxane monotherapy vs taxane monotherapy)
Taxane‐containing doublet therapy versus taxane monotherapy
Two studies (Kim 2015; Lee 2017) involving 121 participants contributed data to this meta‐analysis. We observed TRR of 12.7% in the taxane‐containing doublet therapy arm and 10.0% in the taxane monotherapy arm. The pooled RR was 1.47 (95% CI 0.56 to 3.89) and whether taxane‐containing doublet therapy increases or decreases TRR could not be decided (Analysis 5.6). Heterogeneity observed between the two studies was low (I² = 0%, P = 0.92). The certainty of evidence was very low due to risk of bias and imprecision. There was unclear risk of selection and detection bias as well as confounders in both studies. The number of events analysed was small and the 95% CI for the pooled RR included both appreciable benefit and harm from taxane‐containing doublet chemotherapy.
5.6. Analysis.

Comparison 5: Chemotherapy versus chemotherapy, Outcome 6: Tumour response rate (taxane‐containing doublet therapy vs taxane monotherapy)
Non‐taxane containing chemotherapy versus another non‐taxane containing chemotherapy
One study (Ling 2018) involving 107 participants contributed data to this analysis. TRR was 25.5% in the FOLFIRI arm and 28.6 % in the EOX arm. RR was 0.89 (95% CI 0.48 to 1.67), indicating insufficient evidence for TRR benefit of both arms (Analysis 5.7). The certainty of evidence was low due to imprecision; the number of events analysed was small and the 95% CI for the RR included both appreciable benefit and harm from FOLFIRI.
5.7. Analysis.

Comparison 5: Chemotherapy versus chemotherapy, Outcome 7: Tumour response rate (non‐taxane containing therapy vs another non‐taxane containing therapy)
Any adverse events (AAEs)
None of the four studies which compared chemotherapy to another chemotherapy reported the total number of participants with AAEs to allow meta‐analysis on this outcome.
In Hironaka 2013, neutropenia and anaemia were the most commonly observed AAEs and observed in 70.0% and 78.7%, 76.4% and 63.9% of participants in the irinotecan and paclitaxel arms, respectively. Febrile neutropenia occurred more frequently in the irinotecan‐treated participants, affecting 9.1% of them compared to 2.8% in the paclitaxel arm. Sensory neuropathy occurred in 57.4% of participants receiving paclitaxel. In Kim 2015, the most common AAE was neutropenia in both arms; it affected 40.0% and 14.8% of participants in the docetaxel‐oxaliplatin and the docetaxel arm, respectively. In Lee 2017, anaemia and anorexia affected the majority of participants regardless of their treatment arm. Peripheral neuropathy was another frequently observed AEs, occurring in 41.7%, 52.2% and 43.5% of participants receiving docetaxel‐cisplatin, docetaxel‐S1 and docetaxel, respectively. Diarrhoea occurred more frequently in docetaxel and docetaxel‐S1 arms (47.8%) than docetaxel‐cisplatin arm (25.0%). In Ling 2018, neutropenia, anaemia, thrombocytopenia and diarrhoea were much more common in the FOLFIRI arm occurring in 66.7%,66.7% and 88.2%, and 25.5% of participants in the FOLFIRI arm. The certainty of evidence was moderate due to the risk of performance bias in Hironaka 2013.
Quality of life
Quality of life was reported by one study (Lee 2017) using EORTC QLQ‐C30 and gastric modules QLQ‐STO22. More than 60% of the participants in each arm completed the baseline QoL questionnaire and at least one post‐treatment questionnaire, and QoL compliance was similar in the three arms. Although there was no significant difference in the global QoL scores between the three arms, the combination arms had poorer QoL scores compared to the docetaxel alone arm in several domains including physical functioning, fatigue and appetite loss. The certainty of evidence was very low due to the risk of attrition bias and very small number of participants analysed from one study.
Subgroup analysis by line of treatment (second‐line versus third‐line and beyond)
Of the 17 studies included in this review,12 studies (Bang 2017; Hironaka 2013; Kim 2015; Lee 2017; Ling 2018; Makiyama 2018; Shah 2018; Shitara 2018; Thuss‐Patience 2011; Thuss‐Patience 2017; Wilke 2014; Yi 2012) investigated study treatments in the second‐line setting, one study (Pavlakis 2016) in the second‐ or third‐line setting, one study (Pauligk 2017) in the second‐ to fourth‐line setting and three studies (Kang 2017; Li 2013; Shitara 2018b) in the third‐line and beyond setting. Subgroup analysis by line of treatment was only possible for chemotherapy versus placebo, BSC or no treatment on OS, and biological therapy versus placebo, BSC or no treatment on PFS.
Chemotherapy versus placebo, best supportive care (BSC) or no treatment: overall survival (OS)
Two studies (Shitara 2018b; Thuss‐Patience 2011) involving 547 participants contributed data to this subgroup analysis; 40 and 507 participants received second‐ and third‐line treatment, respectively. Chemotherapy provided OS benefit regardless of line of treatment over placebo, BSC or no treatment; HR was 0.48 (95% CI 0.25 to 0.92) in second‐line and 0.69 (95% CI 0.56 to 0.85) in third‐line (Analysis 1.6). Test for subgroup difference did not reach statistical significance (I² = 7.3%, P = 0.30).
1.6. Analysis.

Comparison 1: Chemotherapy versus placebo, BSC or no treatment, Outcome 6: Subgroup analysis by line of treatment: overall survival
Biological therapy versus placebo, best supportive care (BSC) or no treatment: progression‐free survival (PFS) (VEGFR‐targeted therapy)
Two studies (Li 2013; Pavlakis 2016) involving 288 participants contributed data to this subgroup analysis; 62 and 226 participants were in the second‐ and third‐line setting, respectively. PFS benefit was achieved by biological therapy regardless of line of treatment with the observed benefit being larger for the third‐line setting. HR in the second‐line setting was 0.49 (95% CI 0.28 to 0.86) and that in the third‐line setting was 0.24 (95% CI 0.17 to 0.34) (Analysis 2.6). Test for subgroup difference reached statistical significance (I² = 78.5%, P = 0.03), while the 95% CIs for the two subgroups overlapped.
2.6. Analysis.

Comparison 2: Biological therapy versus placebo, BSC or no treatment, Outcome 6: Subgroup analysis by line of treatment: progression‐free survival
Subgroup analysis by geographical region (East and South‐East Asia versus rest of the world)
Of the 17 studies included in this review, nine studies (Bang 2017; Hironaka 2013; Kang 2017; Kim 2015; Lee 2017; Li 2013; Ling 2018; Makiyama 2018; Yi 2012) were conducted in East and South‐East Asia, two studies (Pauligk 2017; Thuss‐Patience 2011) in Europe and six studies (Pavlakis 2016; Shah 2018; Shitara 2018; Shitara 2018b; Thuss‐Patience 2017; Wilke 2014) worldwide. Subgroup analysis by geographical region was only possible for chemotherapy versus placebo, BSC or no treatment on OS and PFS, biological therapy versus placebo, BSC or no treatment on PFS, and chemotherapy combined with biological therapy versus chemotherapy on OS and PFS.
Chemotherapy versus placebo, best supportive care (BSC) or no treatment: overall survival (OS) and progression‐free survival (PFS)
Two studies (Shitara 2018b; Thuss‐Patience 2011) involving 547 participants contributed data to this subgroup analysis for OS; 73 participants were treated in East and South‐East Asia and 474 in the rest of the world. OS benefit of chemotherapy was observed only for participants from the rest of the world (HR = 0.65, 95% CI 0.52 to 0.80) and was unclear for those from East and South‐East Asia (HR = 0.77, 95% CI 0.46 to 1.29) (Analysis 1.7). The test for subgroup difference did not reach statistical significance (I² = 0.0%, P = 0.37).
1.7. Analysis.

Comparison 1: Chemotherapy versus placebo, BSC or no treatment, Outcome 7: Subgroup analysis by geographical region: overall survival
One study (Shitara 2018b) involving 507 participants contributed data to this subgroup analysis for PFS; 73 participants were from East and South‐East Asia and 434 from the rest of the world. PFS benefit was demonstrated for participants from both East and South‐East Asia and the rest of the world. HR was 0.33 (95% CI 0.19 to 0.57) for East and South‐East Asia and 0.61 (95% CI 0.49 to 0.76) for the rest of the world (Analysis 1.8). The test for subgroup difference was significant (I² = 75.5%, P = 0.04) and the observed PFS benefit was larger for participants from East and South‐East Asia with overlapping 95% CIs for the two subgroups.
1.8. Analysis.

Comparison 1: Chemotherapy versus placebo, BSC or no treatment, Outcome 8: Subgroup analysis by geographical region: progression‐free survival
Biological therapy versus placebo, best supportive care (BSC) or no treatment: progression‐free survival ({FS) (VEGFR‐targeted therapy)
Two studies (Li 2013; Pavlakis 2016) involving 288 participants contributed data to this subgroup analysis; 195 participants were treated in East and South‐East Asia and 93 in the rest of the world. HR for participants from East and South‐East Asia was 0.17 (95% 0.12 to 0.24), indicating PFS benefit of VEGFR‐targeted therapy over placebo (Analysis 2.7). PFS benefit was also observed for participants from the rest of the world (HR = 0.61, 95% CI 0.39 to 0.95). The test for subgroup difference reached statistical significance (I² = 94.7%, P = 0.0001), with the observed PFS benefit larger for participants from East and South‐East Asia.
2.7. Analysis.

Comparison 2: Biological therapy versus placebo, BSC or no treatment, Outcome 7: Subgroup analysis by geographical region: progression‐free survival (VEGFR‐targeted therapy)
Chemotherapy versus biological therapy: overall survival (OS)
One study (Shitara 2018) involving 395 participants contributed data to this subgroup analysis; 104 participants were from East and South‐East Asia and 291 from the rest of the world. The data indicated insufficient evidence for OS benefit of pembrolizumab or paclitaxel for participants from East and South‐East Asia (HR = 0.90, 95% CI 0.59 to 1.37) (Analysis 3.6). OS benefit of pembrolizumab in participants from the rest of the world was similarly unclear (HR = 0.81, 95% 0.61 to 1.08). The test for subgroup difference did not reach statistical significance (I² = 0.0%, P = 0.69).
3.6. Analysis.

Comparison 3: Chemotherapy versus biological therapy, Outcome 6: Subgroup analysis by geographical region: overall survival
Chemotherapy combined with biological therapy versus chemotherapy: overall survival (OS) and progression‐free survival (PFS)
Six studies (Bang 2017; Makiyama 2018; Pauligk 2017;Thuss‐Patience 2017; Wilke 2014; Yi 2012) involving 2030 participants contributed data to the subgroup analysis for OS; 1099 participants were treated in East and South‐East Asia and 931 in the rest of the world. HR for participants from East and South‐East Asia was 0.95 (95% CI 0.78 to 1.16), indicating the lack of OS benefit of chemotherapy combined with biological therapy, unlike participants from the rest of the world who were shown to have OS benefit (HR = 0.84, 95% CI 0.73 to 0.97) (Analysis 4.7). The test for subgroup difference did not reach statistical significance (I² = 0.7%, P = 0.32).
4.7. Analysis.

Comparison 4: Chemotherapy combined with biological therapy versus chemotherapy, Outcome 7: Subgroup analysis by geographical region: overall survival
Five studies (Bang 2017; Makiyama 2018; Pauligk 2017; Wilke 2014; Yi 2012) involving 1684 participants also contributed data to the subgroup analysis for PFS; 942 participants were from East and South‐East Asia and 742 were from the rest of the world. HR for participants from East and South‐East Asia was 0.79 (95% CI 0.68 to 0.92), indicating PFS benefit of chemotherapy combined with biological therapy (Analysis 4.8). Participants from the rest of the world similarly received PFS benefit from chemotherapy combined with biological therapy (HR = 0.74, 95% CI 0.57 to 0.95). The test for subgroup difference did not reach statistical significance (I² = 0.0%, P = 0.67).
4.8. Analysis.

Comparison 4: Chemotherapy combined with biological therapy versus chemotherapy, Outcome 8: Subgroup analysis by geographical region: progression‐free survival
Subgroup analysis by extent of disease (locally advanced versus metastatic disease)
Of the 17 studies included in this review, 12 studies (Bang 2017; Kang 2017; Li 2013; Ling 2018; Makiyama 2018; Pauligk 2017; Shah 2018; Shitara 2018; Thuss‐Patience 2011; Thuss‐Patience 2017; Wilke 2014; Yi 2012) enrolled participants with either locally advanced and metastatic disease with some additionally including recurrent disease. The remaining five studies (Hironaka 2013; Kim 2015; Lee 2017; Pavlakis 2016; Shitara 2018b) enrolled metastatic and recurrent disease. Subgroup analysis was only possible for chemotherapy combined with biological therapy versus chemotherapy.
Chemotherapy combined with biological therapy versus chemotherapy: overall survival (OS)
Two studies (Bang 2017; Thuss‐Patience 2017) involving 859 participants contributed data to this subgroup analysis. Only 14 participants with locally advanced disease were included and the remaining 845 participants had metastatic disease. HR for participants with locally advanced disease was 1.56 (95% CI 0.31 to 7.85) and whether chemotherapy combined with biological therapy improves or worsens OS could not be decided (Analysis 4.9). There was similarly insufficient evidence to decide between chemotherapy combined with biological therapy and chemotherapy alone in terms of effects on OS for participants with metastatic disease (HR = 0.93, 95% CI 0.64 to 1.35). The test for subgroup difference did not reach statistical significance (I² = 0.0%, P = 0.54).
4.9. Analysis.

Comparison 4: Chemotherapy combined with biological therapy versus chemotherapy, Outcome 9: Subgroup analysis by extent of disease: overall survival
Subgroup analysis by type of biological therapy (non‐immunotherapy versus immunotherapy)
As immunotherapy is associated with clinical response patterns not observed with other biological therapy including its durable response, an unplanned subgroup analysis was performed based on the types of biological therapy. Of the 17 studies included in this review, 11 studies (Bang 2017; Kang 2017; Li 2013; Makiyama 2018; Pauligk 2017; Pavlakis 2016; Shah 2018; Shitara 2018; Thuss‐Patience 2017; Wilke 2014; Yi 2012) examined biological therapy and two of them (Kang 2017; Shitara 2018) assessed immunotherapy. Subgroup analysis was only possible for biological therapy versus placebo, BSC or no treatment.
Biological therapy versus placebo, best supportive care (BSC) or no treatment: overall survival (OS) and progression‐free survival (PFS)
Three studies (Kang 2017; Li 2013; Pavlakis 2016) involving 781 participants contributed data to this subgroup analysis for OS; 288 participants were assessed in the two studies (Li 2013; Pavlakis 2016) examining VEGFR‐targeted therapy and 493 participants were assessed in one study (Kang 2017) examining immunotherapy. The mOS, weighed for study size was 5.2 months in the VEGFR‐targeted therapy arm and 3.5 months in the placebo arm for Li 2013 and Pavlakis 2016, and 5.3 months in the immunotherapy arm and 4.4 months in the placebo arm for Kang 2017. OS benefit was observed for both VEGFR‐targeted therapy (HR = 0.50, 95% CI 0.31 to 0.79) and immunotherapy (HR = 0.63, 95% 0.51 to 0.78) (Analysis 2.8). The test for subgroup difference did not reach statistical significance (I² = 0.0%, P = 0.37).
2.8. Analysis.

Comparison 2: Biological therapy versus placebo, BSC or no treatment, Outcome 8: Subgroup analysis by types of biological therapy: overall survival
Three studies (Kang 2017; Li 2013; Pavlakis 2016) involving 781 participants contributed data to this subgroup analysis for PFS. 288 participants were assessed in the two studies (Li 2013; Pavlakis 2016) examining VEGFR‐targeted therapy and 493 participants were assessed in one study (Kang 2017) examining immunotherapy. mPFS, weighed for study size was 3.5 months in the VEGFR‐targeted therapy arm and 1.1 months in the placebo arm for Li 2013 and Pavlakis 2016, and 1.6 months in the immunotherapy arm and 1.5 months in the placebo arm for Kang 2017. As with OS subgroup analysis, PFS benefit was observed for both VEGFR‐targeted therapy (HR = 0.26, 95% CI 0.15 to 0.45) and immunotherapy (HR = 0.60, 95% CI 0.49 to 0.73) (Analysis 2.9). The test for subgroup difference reached statistical significance (I² = 87.4%, P < 0.005) with the observed PFS benefit larger for participants receiving VEGFR‐targeted therapy.
2.9. Analysis.

Comparison 2: Biological therapy versus placebo, BSC or no treatment, Outcome 9: Subgroup analysis by types of biological therapy: progression‐free survival
Discussion
This review and meta‐analysis included only parallel‐group randomised studies. The majority of studies reported adequate randomisation and allocation concealment. Six studies were open‐label, however, in some of them the tumour response rate (TRR) was assessed centrally by blinded reviewers or investigators were kept blinded from the data until the analysis was complete. Treatment arms in individual studies were generally well‐balanced for patient and disease characteristics and at low risk of reporting bias. Attrition bias was a problem in all the studies reporting quality of life (QoL) outcomes.
Summary of main results
Chemotherapy versus placebo, best supportive care (BSC) or no treatment
The meta‐analysis was only possible for overall survival (OS) outcome. Chemotherapy (irinotecan and trifluridine/tipiracil) provided probable OS benefit over placebo and BSC with prolongation of median overall survival (mOS). Chemotherapy (trifluridine/tipiracil) prolonged progression‐free survival (PFS) slightly while there was insufficient evidence to decide whether it increases TRR. Subgroup analysis by line of treatment indicated OS benefit of chemotherapy in both the second‐ and third‐line settings. OS benefit of chemotherapy was only observed in the rest of the world and there was insufficient evidence in East and South‐East Asia. PFS benefit was seen regardless of geographical region with the magnitude of benefit being larger in East and South‐East Asia. OS benefit of chemotherapy was accompanied by the probable higher incidence of serious adverse events (SAEs). The rate of Grade ≥ 3 febrile neutropenia was 1.8% in participants receiving trifluridine/tipiracil. The most common SAE associated with irinotecan was diarrhoea and Grade ≥ 3 febrile neutropenia occurred in 16% of irinotecan‐receiving participants. There was no difference in the rate of any adverse events (AAEs) between the two arms.
Chemotherapy probably improves OS in patients with metastatic gastric and : oesophago‐gastric junction (OGJ) adenocarcinoma in both the second‐ and third‐line settings and may be considered in those with good performance status. Trifluridine/tipiracil may be preferred over irinotecan given the lower rate of Grade ≥ 3 febrile neutropenia.
Biological therapy versus placebo, best supportive care (BSC) or no treatment
The meta‐analysis showed biological therapy provided OS and probably slight PFS benefits over placebo with possibly improved TRR. Subgroup analysis by the types of biological therapy showed no difference for OS benefit, however, PFS benefit was larger from vascular endothelial growth factor receptor (VEGFR)‐targeted therapy compared to immunotherapy while TRR was larger for immunotherapy. Subgroup analysis by line of treatment indicated PFS benefit of VEGFR‐targeted therapy applies to both the second‐ and third‐line settings with the magnitude of benefit being larger in the third‐line setting. PFS benefit of VEGFR‐target therapy was observed regardless of geographical region with larger benefit being demonstrated in East and South‐East Asia. There was insufficient evidence to decide between biological therapy and placebo in terms of effects on SAEs. SAEs affected 67.0% of participants receiving regorafenib and 41.5% of those receiving nivolumab. The occurrence of AAEs was equivalent between the immunotherapy and placebo arms. Narrative analysis of the two studies reporting QoL outcome suggested overall similar QoL experienced by participants treated with VEGFR‐targeted therapy and placebo.
Apatinib, regorafenib and nivolumab are all reasonable biological therapy options with OS and probably slight PFS benefits over placebo. Toxicity of nivolumab deferred from that of apatinib and regorafenib due to its action of mechanism. Based on the lower incidence of SAEs associated with nivolumab, this agent may be preferred for frailer patients with advanced gastric and OGJ adenocarcinoma, who are still keen to explore salvage systemic therapy.
Chemotherapy versus biological therapy
There was insufficient evidence to indicate OS benefit of pembrolizumab over paclitaxel for participants with PD‐L1 PCS ≥ 1 gastric and OGJ adenocarcinoma. Paclitaxel probably improved PFS in this population and there was insufficient evidence to decide between the two arms in terms of effects on TRR. In the subgroup analysis by geographical region, there was insufficient evidence for OS benefit of pembrolizumab in East and South‐East Asia or the rest of the world. SAEs affected 34.8% of participants in the paclitaxel arm and the rate was more than twice of that observed in the pembrolizumab arm. AAEs were similarly observed more frequently in the paclitaxel arm.
Pembrolizumab is a reasonable alternative to paclitaxel for patients with PD‐L1 CPS ≥ 1 status, especially if toxicity of chemotherapy is a problem. It may provide an additional therapeutic option upon development of resistance to taxane chemotherapy in this population of patients.
Chemotherapy combined with biological therapy versus chemotherapy
Biological therapy assessed in this comparison included six agents all from different therapeutic groups. OS outcome was probably equivalent between combined chemotherapy and biological therapy, and chemotherapy alone with uncertainty to whether combined chemotherapy and biological therapy improves PFS or TRR. Exclusion of studies evaluating HER2‐targeted therapy resulted in slight OS and PFS benefits of combined chemotherapy and biological therapy. Subgroup analysis by geographical region demonstrated small OS benefit of chemotherapy combined with biological therapy only in the rest of the world and not in East and South‐East Asia, although the test for subgroup difference did not reach statistical significance. The subgroup analysis by geographical region showed PFS benefit of combined chemotherapy and biological therapy for all regions. There was insufficient evidence to decide between chemotherapy combined with biological therapy and chemotherapy alone in terms of effects on OS for participants with both locally advanced and metastatic disease. SAEs affected 72.6% and 61.3% of participants in the chemotherapy combined with biological therapy and chemotherapy alone arms, respectively and it was uncertain whether combined biological therapy increases occurrence of SAEs. There was insufficient evidence to decide whether time to deterioration of EORTC QLQ‐C30 global health status improves with combined chemotherapy and biological therapy.
Combining chemotherapy and biological therapy probably achieves equivalent OS benefit to chemotherapy alone, except it may slightly improve OS and PFS outcomes when the therapy works through mechanisms other than those involving HER2 receptors. Combined chemotherapy and biological therapy is associated with common occurrence of SAEs, making this type of treatment less favoured as salvage therapy for patients with advanced gastric and OGJ adenocarcinoma.
Chemotherapy versus chemotherapy
Chemtoehrapy regimens assessed included irinotecan, taxane monotherapy, docetaxel‐containing doublet chemotherapy and non‐taxane containing poly‐chemotherapy. We could not decide between irinotecan and paclitaxel in terms of effects on OS or PFS and reported mOS and median progression‐free survival (mPFS) were not far apart. There was insufficient evidence to indicate OS and PFS benefits of addition of another chemotherapy to docetaxel. There was similarly insufficient evidence to decide between FOLFIRI and EOX in terms of effects on TRR. Treatment‐related deaths occurred in 1.8% of irinotecan‐receiving participants and 4.2% to 4.3% of participants receiving docetaxel‐containing doublet chemotherapy. Grade ≥ 3 neutropenia was common occurrence among participants receiving both mono‐and poly‐chemotherapy except for docetaxel‐S1 and EOX treatment and docetaxel‐oxaliplatin was associated with 20.0% risk of febrile neutropenia. No difference was observed for the global QoL between the docetaxel‐cisplatin, docetaxel‐S1 and docetaxel arms, however, worse QoL was reported for participants receiving the doublet chemotherapy for several symptom categories.
There is no evidence of a difference between irinotecan and paclitaxel in terms of effects on OS and PFS outcomes, however, irinotecan is associated with more treatment‐related deaths. Poly‐chemotherapy increased toxicity, particularly treatment‐related death and Grade ≥ 3 neutropenia without obvious improvement in efficacy outcomes.
Overall completeness and applicability of evidence
Seventeen studies were chosen based on the pre‐specified selection criteria for this review. Thirteen studies, which were 12conference abstracts and one full‐text article, could not be included in the review as an insufficient amount of information was available for adequate eligibility assessment. Authors of these studies were contacted via email, however, we received either no reply or were advised study data were owned by independent organisations, commonly pharmaceutical companies, who could not release them. Additional 29 studies were identified to be relevant to this review, but are ongoing.
The median age of participants in the studies included in this review varied between 52 and 65 years old and it was thought to be in line with typical gastric cancer patients. 71.6% of participants in the review were estimated to be males and this proportion was consistent with the available literature (Ajani 2017). The majority of the participants in the review was ECOG PS ≤ 1 and excluding Makiyama 2018 and Pauligk 2017, studies, which did not describe well the characteristics of patients, participants with ECOG PS 2 comprised only 1.0% of the review population. This was felt to be lower than typical gastric cancer patients in our clinical practice. When known, the proportion of participants with metastatic disease in studies included in the review ranged 89.5% to 100% and this rate was consistent with our clinical practice. The only exception was Ling 2018 where only 57.0% of participants had metastatic gastric cancer.
No studies were found under the following comparisons; chemotherapy combined with biological therapy versus placebo, BSC or no treatment, chemotherapy combined with biological therapy versus biological therapy, biological therapy versus another biological therapy, and chemotherapy combined with biological therapy versus another chemotherapy combined with biological therapy. Neither quantitative or narrative analysis was performed for these comparisons.
For many of the preplanned outcomes there was only one study providing the data and more than half the meta‐analyses performed in this review contained data from only two or three studies, frequently examining heterogenous regimens, making the generalisation of the result difficult. For comparisons between chemotherapy versus another chemotherapy, we were unable to combine the data from all applicable studies for efficacy outcomes due to the difference in the regimens examined.
Three studies (Makiyama 2018; Shitara 2018; Thuss‐Patience 2017) selected the study population based on tumour biomarker expression. Response to trastuzumab has been previously linked to HER2 positivity (Bang 2010). The association of response to nivolumab and PD‐L1 expression has been more recently reported by Janjigian 2017 with an increase in the response to nivolumab with or without ipilimumab in patients with gastric, oesophageal and OGJ cancer showing PD‐L1 ≥ 1%. Efficacy analysis for the comparison of chemotherapy versus biological therapy was hence performed on the participants with PD‐L1 CPS ≥ 1% from Shitara 2018. It is not possible to apply the findings from these three studies to the general population with advanced gastric and OGJ adenocarcinoma.
Five studies (Kang 2017; Li 2013; Pauligk 2017; Pavlakis 2016; Shitara 2018b) included participants who had failed two or more lines of systemic treatment and their proportion was 19.9% for studies providing the number of patients in various lines of treatment. Two subgroup analyses performed indicated survival outcome to be not affected by line of treatment, however, for the regimens which were only examined in the second‐line setting, if their reported efficacy applies to patients in the third‐line and beyond setting is unclear.
Of the seven studies (Bang 2017; Lee 2017; Li 2013; Pavlakis 2016; Thuss‐Patience 2011; Thuss‐Patience 2017; Wilke 2014), which intended to measure QoL, two studies provided no or minimal results; Thuss‐Patience 2011 did not report QoL outcome as attainment of participants was too poor to achieve meaningful analysis and Thuss‐Patience 2017 only described QoL outcome to be similar between the two treatment arms without detailed data. For the remaining studies, despite EORTC QLQ‐C30 being used for QoL assessment by all of them, there was inconsistency in their analysis methods, making it difficult to collectively evaluate the findings for the purpose of this review. The only meta‐analysis performed for QoL outcome was an unplanned analysis on time to deterioration of global health status measured using EORTC QLQ‐C30, which was reported by Bang 2017 and Wilke 2014. The survival benefit of systemic therapy for advanced gastric and OGJ cancer in the salvage setting is not large and the largest OS benefit, calculated as the difference in mOS weighed for study size between the treatment arms was 2.1 months for the comparison between chemotherapy versus placebo, BSC or no treatment. Considering the cost to healthcare systems and the potential psychological stress treatment may impose on patients, sustainment of QoL is an important goal for both clinicians and patients embarking on salvage systemic therapy and this outcome should be evaluated in more clinical trials.
Quality of the evidence
The majority of studies included in this review were at low risk of randomisation and allocation bias. Six studies (Hironaka 2013; Makiyama 2018; Shitara 2018; Thuss‐Patience 2011; Thuss‐Patience 2017; Yi 2012) were open‐label with no blinding of study treatment to participants. Although it is unlikely that the knowledge of study treatment by participants altered the OS, PFS, SAE and TRR outcomes, it is suspected to have affected AAE outcome due to performance bias. In two studies (Thuss‐Patience 2011; Thuss‐Patience 2017), outcome assessment was not blinded. Thuss‐Patience 2011 only reported OS outcome and hence, it is unlikely to have been affected by detection bias. In Thuss‐Patience 2017, however, all the outcomes other than OS were at risk of detection bias. Atteninment bias affected the majority of studies reporting QoL outcome. For the three studies published as conference abstracts (Makiyama 2018; Pauligk 2017; Shitara 2018b) and three other studies (Kim 2015; Li 2013; Ling 2018), risk of bias for several domains was unclear. Ling 2018, the only study published in a non‐English language in this review, was not associated with increased study bias compared to the rest of studies published in English, however, inconsistency in the survival outcomes detected between the text and the published Kaplan‐Meier curves was a concern for the certainty of data presented.
Because of the small number of participants considered and the low occurrence of events, certainty of evidence was downgraded for imprecision in some of the meta‐analyses performed in this review. This was particularly the case with the TRR analyses. For none of the comparisons examined in this review, except for that between chemotherapy combined with biological therapy versus chemotherapy, more than 300 events of tumour response, which are generally considered adequate to prevent imprecision, were achieved. The majority of the analyses on the primary outcomes had sufficient number of events. Heterogeneity which was assessed by the Chi² test and the I² statistic was frequently detected and was partly due to the diversity in the regimens used across studies. For those meta‐analyses which were not associated with heterogeneity, imprecision due to a wide 95% CI associated with hazard ratio (HR) or risk ratio (RR) was often observed. Overall, the certainty of evidence for analyses on OS and SAEs, the two outcomes commonly considered most important in the clinical practice were of moderate to high, with the exception of that on SAE outcome for biological therapy versus placebo, chemotherapy versus biological therapy, and chemotherapy combined with biological therapy versus chemotherapy as well as OS outcome for chemotherapy versus another chemotherapy.
Potential biases in the review process
Publication bias was a concern throughout this review process as studies which did not identify the treatment to be effective may not have been published. To minimise publication bias, our literature search included unpublished or ongoing trials and no restriction on language was applied.
Agreements and disagreements with other studies or reviews
Two reviews on related topics have been published in recent times. Glady 2016 published a narrative review of randomised controlled trials (RCTs) examining systemic therapy for advanced gastric cancer in second‐ and third‐line settings. The major difference from our review was that we specified included patients to have experienced disease progression on the first‐line fluoropyrimidine and platinum‐containing chemotherapy. Additionally, some patients considered in Glady 2016 had oesophageal cancer other than OGJ adenocarcinoma. Consequently, it contained more randomised studies. AEs and QoL associated with systemic therapy were not considered. As demonstrated by our review, it reported survival benefit of mono‐chemotherapy and biological therapy with VEGFR‐targeted therapy over placebo or BSC and authors concluded BSC should not be accepted as gold standard in management of advanced gastric cancer beyond the first‐line treatment. Glady 2016 reviewed three RCTs for the comparison of chemotherapy combined with biological therapy versus chemotherapy alone and reported variable survival benefit depending on the choice of biological agents. In our review, meta‐analysis for the same comparison demonstrated small OS and PFS benefit of combining chemotherapy with biological therapy only when studies examining HER2‐targeted therapy were excluded. This type of treatment was additionally associated with increased SAEs compared to chemotherapy alone.
ter Veer 2016 reviewed phase 2 and 3 RCTs of systemic therapy in advanced oesophago‐gastric cancer again in the salvage setting. Unlike our review, which focused on gastric and OGJ adenocarcinoma alone, patients with non‐OGJ oesophageal adenocarcinoma were included in this review. Difference in risk factors, gene expression and tumour biology have been previously suggested between OGJ adenocarcinoma arising from distal oesophagus and gastric cardia (Marsman 2005). Differential efficacy of chemotherapy was suggested in Chau 2009. Overall, their findings were consistent with results of this review. ter Veer 2016 reported OS benefit of chemotherapy and equivalent OS and PFS outcomes from taxane‐ and irinotecan‐based chemotherapy. The nature of survival benefit from biological therapy over placebo or BSC depended on the agent investigated with apatinib showing both OS and PFS benefit. The review also reported no difference in the OS outcome when taxane‐ or irinotecan‐containing doublet chemotherapy was compared to taxane or irinotecan monotherapy. We observed unclear TRR benefit when platinum or S1 was added to taxane with increased incidence of serious infective complications including febrile neutropenia. Unlike our review, which showed OS and PFS benefit of addition of biological therapy to chemotherapy in the meta‐analysis evaluating five non‐trastuzumab‐based biological agents all from different groups, ter Veer 2016 found ramucirumab and olaparib to be the only biological therapy with survival benefit when combined with chemotherapy.
Authors' conclusions
Implications for practice.
Salvage systemic therapy, both chemotherapy and biological therapy, improves survival outcome of patients with advanced gastric and oesophago‐gastric junction (OGJ) adenocarcinoma following disease progression on fluoropyrimidine and platinum‐containing first‐line chemotherapy. Although our review indicated insufficient evidence to decide between irinotecan and paclitaxel monotherapies in terms of effects on overall survival (OS) and progression‐free survival (PFS) outcomes, paclitaxel is associated with fewer treatment‐related deaths and Grade ≥ 3 marrow suppression. Paclitaxel, therefore, may be preferred over irinotecan at least in the second‐line setting, unless its specific toxicity such as neuropathy contraindicates its use. Trifluridine/tipiracil probably has both OS and PFS benefits over placebo in the third‐ and fourth‐line settings and this drug may be used following progression of disease on paclitaxel and/or irinotecan again in appropriately selected patients.
Biological therapy is an alternative option to chemotherapy and in the second‐line setting, both nivolumab and vascular endothelial growth factor receptor (VEGFR)‐targeted therapy such as apatinib and regorafenib were evaluated. In the third‐line setting our finding was limited to regorafenib. Only immunotherapy was compared to both placebo and chemotherapy in our review and there was no evidence of a difference for OS outcome between immunotherapy and paclitaxel with immunotherapy being possibly associated with reduced risk of SAEs, although the certainty of evidence for serious adverse event (SAE) analysis was low. Nivolumab and pembrolizumab may be reasonable options in patients who are vulnerable to toxicity associated with chemotherapy and tyrosine kinase inhibitors, bearing mind that OS benefit of pembrolizumab was evaluated in those with PD‐L1 CPS ≥ 1.
Addition of biological therapy to chemotherapy probably does not improve OS outcome unless biological therapy acts through a mechanism other than those involving HER2 receptors based on a sensitivity analysis, and the effect is not affected by the geographical regions. Addition of biological therapy to chemotherapy is associated with frequent SAEs, however, it is uncertain whether it increases SAEs as the certainty of evidence for this analysis was very low. Combined chemotherapy and biological therapy may not be a preferred treatment for advanced gastric and OGJ adenocarcinoma in the salvage setting. Based on the available evidence it is uncertain whether poly‐chemotherapy improves OS, PFS or tumour response rate (TRR), and it rather increases Grade ≥ 3 neutropenia. Use of poly‐chemotherapy is generally not supported.
Implications for research.
Although our review demonstrated survival benefit of both chemotherapy and biological therapy in patients with gastric and OGJ adenocarcinoma in the salvages setting, whether VEGFR‐target therapy such as apatinib and regorafenib is as efficacious as irinotecan and taxane monotherapy remains uncertain. This is because neither agent was examined by the study comparing chemotherapy and biological therapy in this review. The reported median overall survival (mOS) and median progression‐free survival (mPFS) were longer for irinotecan and taxane chemotherapy, however, future studies directly comparing these agents are needed to confirm this.
The number of studies included in this review was small, partly due to our strict inclusion criteria. We defined included participants to have either gastric or OGJ adenocarcinoma, but not non‐OGJ oesophageal adenocarcinoma, and to have experienced disease progression on fluoropyrimidine and platinum‐containing first‐line chemotherapy. We felt these inclusion criteria are necessary to maximise the applicability of our findings to daily clinical practice. Difference in efficacy of systemic therapy has been suggested for different locations along oesophago‐gastric tract (Chau 2009) and combination of fluoropyrimidine and platinum has now become the standard first‐line treatment for advanced gastric cancer including OGJ adenocarcinoma worldwide (Cunningham 2008; Kang 2009). As a result, each systemic regimen was only examined by a single study except for taxane and irinotecan monotherapy which were administered to participants in 10 and two studies, respectively.
The current trend in phase 3 cancer clinical trials is that new cancer therapeutics are examined in a single or a very small number of sufficiently‐powered international RCTs before being accepted as the new standard of care. This means the large number of patients are examined under the common protocol, potentially allowing for meaningful sub‐analyses such as those by ethnicity, geographical region, age group and biomarker expression. Careful consideration in determining inclusion and exclusion criteria is required so that the population examined represents patients encountered in daily clinical practice. Additionally, inclusion and reporting of quality of life (QoL) outcome is strongly encouraged for future studies given the small absolute survival benefit of systemic therapy for advanced gastric and OGJ adenocarcinoma in the salvage setting.
Immunotherapy is characterised by the unconventional patterns of response or progression and this has raised statistical issues around the optimal study design, endpoints and statistical method for analysis in clinical studies evaluating them (Ferrara 2018). Studies with survival endpoints have traditionally been designed based on a proportional hazards model under the exponential distribution assumption presuming anything which affects the hazards does so by the same ratio at all times. Long‐term survival and delayed clinical effects of immunotherapy, however, as seen from the survival curves of nivolumab (Kang 2017) and pembrolizumab (Shitara 2018) in our review, promote conventional proportional hazards model to underestimate study duration and the statistical power required (Chen 2013). Traditional survival endpoints used in clinical trials such as OS and PFS with HR are sub‐optimal to capture key attributes of immunotherapy as mPFS can potentially underestimate the clinical benefit of immunotherapy with delayed clinical effect, while mOS frequently fails to provide information concerning the small subset of patients with prolonged survival benefit. This explains the opposing direction of HR associated with OS and PFS of pembrolizumab (Shitara 2018), which influenced our survival meta‐analysis for the comparison of chemotherapy versus biological therapy. Use of an alternative statistical method and survival measures have been proposed for future clinical trials concerning immunotherapy (Chen 2013). Statistical approach in performing meta‐analysis involving such studies may additionally need to be revised.
History
Protocol first published: Issue 2, 2016 Review first published: Issue 11, 2020
Acknowledgements
The methods section of this protocol is based on a standard template used by Cochrane Gut Group.
We would to thank the following editors and peer referees who provided comment to improve the review: Paul Moayyedi (editor), Sarah Rhode (editor), Takeshi Kanno (editor), Kohei Shitara (peer referee), Luigi Marano (peer referee) and Marilyn Walsh (consumer reviewer). We thank Heather Maxwell for copy editing the review.
We thank Karin Dearness and Yuhong Yuan, ex‐ and present Managing Editors, Cochrane Gut Group, for providing administrative and logistical support for the conduct of the current review. Yuhong Yuan additionally provided assistance in developing and executing the search strategies and in translation of a non‐English publication to English.
Appendices
Appendix 1. Glossary of terms
Adenocarcinoma ‐ cancer of gland‐like structure
Adjuvant ‐ referring to treatment that is given after the primary treatment, usually surgery, to lower the risk of cancer recurrence
Asymptomatic ‐ without symptoms
Barrett's oesophagus ‐ a serious result of acid reflux disease, which changes the normal lining of the oesophagus to tissue that resembles the lining of the intestine
Biological therapy – anticancer treatment using substances derived from living organisms
Carcinogenic ‐ causing or tending to cause cancer
Gastric cardia ‐ upper portion of the stomach into which the contents of the oesophagus empty
Chemotherapy – anticancer treatment using cytotoxic chemicals
Common Terminology Criteria for Adverse Events (CTCAE) ‐ a set of criteria for the standardised classification of adverse effects of drugs used in cancer therapy
Cytological – referring to the study of cells using a microscope
Eastern Cooperative Oncology Group (ECOG) performance score (PS) – the scale developed by ECOG to describe a patient’s level of functioning in terms of their ability to care for themselves, daily activity, and physical ability
Febrile neutropenia ‐ development of a fever, sometimes with other signs of infection, in a patient with an abnormally low number of a specific type of white blood cell
Fluoropirimide, platinum, taxane, anthracycline ‐ types of chemotherapy
Gastric ‐ stomach
Genetic polymorphisms ‐ natural variations in a gene, DNA sequence or chromosome that have no adverse effects on the individual and occur with fairly high frequency in the general population
Heliocobacter pylori ‐ a type of bacterium that causes inflammation and ulcers in the stomach or small intestine
Histological – referring to the structure, especially the microscopic structure of the cancer
Immune‐related Response Evaluation Criteria In Solid Tumors (irRECIST) ‐ a set of published rules that provide better assessment of the effect of immunotherapeutic agents
Immunotherapy ‐ anticancer treatment that boosts the body's natural defences to fight cancer
Metastasis/metastases ‐ spread of cancer to parts of the body other than where it originally started
Mortality ‐ death rate
Neoadjuvant ‐ referring to treatment that is given as a first step to shrink cancer before the main treatment, which is usually surgery, is given
Neuropathy ‐ dysfunction of one or more nerves in the arms or legs, typically causing numbness and weakness
Oesophago‐gastric junction (OGJ), esophago‐gastric junction (EGJ), gastro‐oesophageal junction (GOJ) or gastro‐esophageal junction (GEJ) ‐ the border between the oesophagus and the stomach
Overall survival (OS) ‐ the length of time from either the date of diagnosis or the start of treatment that patients diagnosed with the disease are still alive
Palliative ‐ referring to treatment that lessens symptoms without curing
Parenteral ‐ administration of drugs by intravenous, subcutaneous or intramuscular infection/infusion
Perioperative ‐ around the time of surgery
Pharmacological ‐ drug related
Progression‐free survival (PFS) ‐ the length of time during and after the treatment of a disease that a patient lives with the disease but it does not get worse
Radical – referring to aggressive treatment that aims at the complete cure of a disease rather than relief of symptoms
Resection ‐ surgical removal of all or part of an organ
Response evaluation criteria in solid tumors (RECIST) ‐ a set of published rules that define when tumors in cancer patients improve ("respond"), stay the same ("stabilize"), or worsen ("progress") during treatment
Salvage – referring to treatment that is given after the cancer has not responded to other treatments
Stomatitis ‐ inflammation of the lining of the mouth
Systemic ‐ affecting the entire body
Time to progression (TTP) ‐ the length of time from the date of diagnosis or the start of treatment for a disease until the disease starts to get worse or spread to other parts of the body; unlike progression‐free survival, time to progression does not count patients who die from other causes
Toxicity/toxicities – the extent to which something is poisonous or harmful
Appendix 2. CENTRAL search strategy
MeSH descriptor: [Stomach Neoplasms] explode all trees
((gastric or stomach or gastro‐oesophageal junction or gastro‐esophageal junction or oesophago‐gastric junction or esophagogastric junction or GE junction or GEJ or GOJ or OJG or EGJ) near/4 (cancer* or carcin* or malig* or tumor* or tumour* or neoplas* or adenocarcinoma)):ti,ab,kw (Word variations have been searched)
#1 or #2
(advanced or inoperable or unopera* or unresectable or nonresect* or non resect*):ti,ab,kw (Word variations have been searched)
MeSH descriptor: [Neoplasm Metastasis] explode all trees
(metastatic or metastasis or metastases):ti,ab,kw (Word variations have been searched)
#4 or #5 or #6
#3 and #7
MeSH descriptor: [Drug Therapy] explode all trees
MeSH descriptor: [Biological Therapy] explode all trees
MeSH descriptor: [Antineoplastic Agents] explode all trees
MeSH descriptor: [Antibodies, Monoclonal] explode all trees
MeSH descriptor: [Immunotherapy] explode all trees
MeSH descriptor: [Protein Kinase Inhibitors] explode all trees
(chemo* or immunotherap*):ti,ab,kw (Word variations have been searched)
((biological or molecular or targeted or antineoplastic) near/3 (therap* or treat* or strateg* or agent* or manag*)):ti,ab,kw (Word variations have been searched)
(tyrosine kinase inhibitor* or monoclonal antibod* or mTOR inhibitor* or mammalian target of rapamycin inhibitor*):ti,ab,kw (Word variations have been searched)
(ramucirumab or ipilimumab or everolimus):ti,ab,kw (Word variations have been searched)
#9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18
#8 and #19
MeSH descriptor: [Salvage Therapy] explode all trees
MeSH descriptor: [Retreatment] explode all trees
(second line or third line or later line or rescue or salvage or refractory or reintroduction or recapture):ti,ab,kw (Word variations have been searched)
(retreat* or re‐treat*):ti,ab,kw (Word variations have been searched)
((after or following) near/3 (first line or withdraw* or withdr* or cessation or stop* or discontin* or de‐escalation)):ti,ab,kw (Word variations have been searched)
((after or following or therap* or treat* or manage* or strateg*) near/6 (fail* or recurrent or recurrence or relapse* or progress*)):ti,ab,kw (Word variations have been searched)
#21 or #22 or #23 or #24 or #25 or #26
#20 and #27
Appendix 3. EMBASE search strategy (Ovid)
exp stomach tumor/
((gastric or stomach or gastro‐oesophageal junction or gastro‐esophageal junction or oesophago‐gastric junction or esophagogastric junction or GE junction or GEJ or GOJ or OJG or EGJ ) adj4 (cancer* or carcin* or malig* or tumor* or tumour* or neoplas* or adenocarcinoma)).tw,kw.
1 or 2
(advanced or inoperable or unopera* or unresectable or nonresect* or non resect*).tw,kw.
exp metastasis/ or (metastatic or metastasis or metastases).tw,kw.
exp advanced cancer/
or/4‐6
3 and 7
exp chemotherapy/
exp biological therapy/
exp antineoplastic agent/
exp molecular therapy/
exp protein tyrosine kinase inhibitor/
exp monoclonal antibody/
exp "mammalian target of rapamycin inhibitor"/
exp cancer immunotherapy/
exp ramucirumab/
exp ipilimumab/
(chemo* or immunotherap*).ti,ab,kw.
((biological or molecular or targeted or antineoplastic) adj3 (therap* or treat* or strateg* or agent* or manag*)).tw,kw.
(tyrosine kinase inhibitor* or monoclonal antibod* or mTOR inhibitor* or mammalian target of rapamycin inhibitor* ).tw,kw.
(ramucirumab or ipilimumab or everolimus).tw,kw.
or/9‐22
8 and 23
exp salvage therapy/
(second line or third line or later line or rescue or salvage or refractory or reintroduction or recapture).tw,kw.
(retreat* or re‐treat*).tw,kw.
((after or following) adj3 (first line or withdraw* or withdr* or cessation or stop* or discontin* or de‐escalation)).tw,kw.
exp Retreatment/
((after or following or therap* or treat* or manage* or strateg*) adj6 (fail* or recurrent or recurrence or relapse* or progress*)).tw,kw.
or/25‐30
24 and 31
random:.tw.
clinical trial:.mp.
exp health care quality/
double‐blind:.mp.
placebo:.tw.
blind:.tw.
or/33‐38
exp animal/ not human.sh.
39 not 40
32 and 41
Lines 33‐39. RCT filter. Combined maximizes sensitivity and maximizes specificity version: https://hiru.mcmaster.ca/hiru/hedges/All‐EMBASE.htm
Appendix 4. MEDLINE search strategy (Ovid)
exp Stomach Neoplasms/
((gastric or stomach or gastro‐oesophageal junction or gastro‐esophageal junction or oesophago‐gastric junction or esophagogastric junction or GE junction or GEJ or GOJ or OJG or EGJ) adj4 (cancer* or carcin* or malig* or tumor* or tumour* or neoplas* or adenocarcinoma)).tw,kw.
1 or 2
(advanced or inoperable or unopera* or unresectable or nonresect* or non resect*).tw,kw.
exp Neoplasm Metastasis/ or (metastatic or metastasis or metastases).tw,kw.
4 or 5
3 and 6
exp Biological Therapy/
exp Antineoplastic Agents/
exp Protein Kinase Inhibitors/
exp Antibodies, Monoclonal/
exp Immunotherapy/
(chemo* or tor immunotherap*).tw,kw.
((biological or molecular or targeted or antineoplastic) adj3 (therap* or treat* or strateg* or agent* or manag*)).tw,kw.
(tyrosine kinase inhibitor* or monoclonal antibod* or mTOR inhibitor* or mammalian target of rapamycin inhibitor* ).tw,kw.
(ramucirumab or ipilimumab or everolimus).tw,kw.
or/8‐16
7 and 17
exp Salvage Therapy/
(second line or third line or later line or rescue or salvage or refractory or reintroduction or recapture).tw,kw.
(retreat* or re‐treat*).tw,kw.
((after or following) adj3 (first line or withdraw* or withdr* or cessation or stop* or discontin* or de‐escalation)).tw,kw.
exp Retreatment/
((after or following or therap* or treat* or manage* or strateg*) adj6 (fail* or recurrent or recurrence or relapse* or progress*)).tw,kw.
or/19‐24
18 and 25
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/27‐34
exp animals/ not humans.sh.
35 not 36
26 and 37
Lines 27‐36. RCT filter: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision); Ovid format.
Appendix 5. WHO ICTRP search strategy
gastric cancer OR stomach cancer (conditions); and
gastro‐oesophageal cancer OR gastro‐esophageal cancer OR gastro‐oesophageal junction cancer OR gastro‐esophageal junction cancer OR oesophago‐gastric cancer OR esophago‐gastric cancer (conditions)
Appendix 6. ClinicalTrials.gov search strategy
adult (18‐64) AND older adult (65+) (age group);
advanced gastric cancer (condition or disease); and
drug (intervention/treatment)
Data and analyses
Comparison 1. Chemotherapy versus placebo, BSC or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Overall survival | 2 | 547 | Hazard Ratio (IV, Random, 95% CI) | 0.66 [0.52, 0.83] |
| 1.2 Progression‐free survival | 1 | 507 | Hazard Ratio (IV, Random, 95% CI) | 0.57 [0.47, 0.69] |
| 1.3 Serious adverse events | 1 | 503 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.20, 1.59] |
| 1.4 Tumour response rate | 1 | 435 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [0.63, 7.48] |
| 1.5 Any adverse events | 1 | 503 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [1.00, 1.09] |
| 1.6 Subgroup analysis by line of treatment: overall survival | 2 | 547 | Hazard Ratio (IV, Random, 95% CI) | 0.66 [0.52, 0.83] |
| 1.6.1 Second line | 1 | 40 | Hazard Ratio (IV, Random, 95% CI) | 0.48 [0.25, 0.92] |
| 1.6.2 Third line and beyond | 1 | 507 | Hazard Ratio (IV, Random, 95% CI) | 0.69 [0.56, 0.85] |
| 1.7 Subgroup analysis by geographical region: overall survival | 2 | 547 | Hazard Ratio (IV, Random, 95% CI) | 0.66 [0.54, 0.81] |
| 1.7.1 East and South‐East Asia | 1 | 73 | Hazard Ratio (IV, Random, 95% CI) | 0.77 [0.46, 1.29] |
| 1.7.2 Rest of the world | 2 | 474 | Hazard Ratio (IV, Random, 95% CI) | 0.65 [0.52, 0.80] |
| 1.8 Subgroup analysis by geographical region: progression‐free survival | 1 | 507 | Hazard Ratio (IV, Random, 95% CI) | 0.52 [0.33, 0.82] |
| 1.8.1 East and South‐East Asia | 1 | 73 | Hazard Ratio (IV, Random, 95% CI) | 0.33 [0.19, 0.57] |
| 1.8.2 Rest of the world | 1 | 434 | Hazard Ratio (IV, Random, 95% CI) | 0.61 [0.49, 0.76] |
Comparison 2. Biological therapy versus placebo, BSC or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Overall survival | 3 | 781 | Hazard Ratio (IV, Random, 95% CI) | 0.55 [0.41, 0.73] |
| 2.2 Progression‐free survival | 3 | 781 | Hazard Ratio (IV, Random, 95% CI) | 0.33 [0.19, 0.57] |
| 2.3 Serious adverse events | 2 | 638 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.95, 1.37] |
| 2.4 Tumour response rate | 3 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 5.12 [1.23, 21.27] |
| 2.5 Any adverse events | 1 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [1.00, 1.17] |
| 2.6 Subgroup analysis by line of treatment: progression‐free survival | 2 | 288 | Hazard Ratio (IV, Random, 95% CI) | 0.28 [0.18, 0.44] |
| 2.6.1 Second‐line | 1 | 62 | Hazard Ratio (IV, Random, 95% CI) | 0.49 [0.28, 0.86] |
| 2.6.2 Third‐line and beyond | 2 | 226 | Hazard Ratio (IV, Random, 95% CI) | 0.24 [0.17, 0.34] |
| 2.7 Subgroup analysis by geographical region: progression‐free survival (VEGFR‐targeted therapy) | 2 | 288 | Hazard Ratio (IV, Random, 95% CI) | 0.24 [0.11, 0.50] |
| 2.7.1 East and South‐East Asia | 2 | 195 | Hazard Ratio (IV, Random, 95% CI) | 0.17 [0.12, 0.24] |
| 2.7.2 Rest of the world | 1 | 93 | Hazard Ratio (IV, Random, 95% CI) | 0.61 [0.39, 0.95] |
| 2.8 Subgroup analysis by types of biological therapy: overall survival | 3 | 781 | Hazard Ratio (IV, Random, 95% CI) | 0.55 [0.41, 0.73] |
| 2.8.1 VEGFR‐targeted therapy | 2 | 288 | Hazard Ratio (IV, Random, 95% CI) | 0.50 [0.31, 0.79] |
| 2.8.2 Immunotherapy | 1 | 493 | Hazard Ratio (IV, Random, 95% CI) | 0.63 [0.51, 0.78] |
| 2.9 Subgroup analysis by types of biological therapy: progression‐free survival | 3 | 781 | Hazard Ratio (IV, Random, 95% CI) | 0.33 [0.19, 0.57] |
| 2.9.1 VEGFR‐targeted therapy | 2 | 288 | Hazard Ratio (IV, Random, 95% CI) | 0.26 [0.15, 0.45] |
| 2.9.2 Immunotherapy | 1 | 493 | Hazard Ratio (IV, Random, 95% CI) | 0.60 [0.49, 0.73] |
Comparison 3. Chemotherapy versus biological therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Overall survival | 1 | 395 | Hazard Ratio (IV, Random, 95% CI) | 0.82 [0.66, 1.02] |
| 3.2 Progression‐free survival | 1 | 395 | Hazard Ratio (IV, Random, 95% CI) | 1.27 [1.03, 1.57] |
| 3.3 Serious adverse events | 1 | 570 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.30, 0.57] |
| 3.4 Tumour response rate | 1 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.72, 1.88] |
| 3.5 Any adverse events | 1 | 570 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.56, 0.71] |
| 3.6 Subgroup analysis by geographical region: overall survival | 1 | 395 | Hazard Ratio (IV, Random, 95% CI) | 0.84 [0.66, 1.06] |
| 3.6.1 East and South‐East Asia | 1 | 104 | Hazard Ratio (IV, Random, 95% CI) | 0.90 [0.59, 1.37] |
| 3.6.2 Rest of the world | 1 | 291 | Hazard Ratio (IV, Random, 95% CI) | 0.81 [0.61, 1.08] |
Comparison 4. Chemotherapy combined with biological therapy versus chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Overall survival | 7 | 2743 | Hazard Ratio (IV, Random, 95% CI) | 0.93 [0.83, 1.04] |
| 4.2 Progression‐free survival | 7 | 2743 | Hazard Ratio (IV, Random, 95% CI) | 0.87 [0.74, 1.02] |
| 4.3 Serious adverse events | 4 | 1618 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.95, 1.44] |
| 4.4 Tumour response rate | 6 | 2404 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.99, 1.85] |
| 4.5 Any adverse events | 3 | 1513 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [1.00, 1.03] |
| 4.6 QoL | 2 | 1154 | Hazard Ratio (IV, Random, 95% CI) | 0.88 [0.74, 1.04] |
| 4.7 Subgroup analysis by geographical region: overall survival | 6 | 2030 | Hazard Ratio (IV, Random, 95% CI) | 0.88 [0.78, 0.99] |
| 4.7.1 East and South‐EastAsia | 5 | 1099 | Hazard Ratio (IV, Random, 95% CI) | 0.95 [0.78, 1.16] |
| 4.7.2 Rest of the world | 3 | 931 | Hazard Ratio (IV, Random, 95% CI) | 0.84 [0.73, 0.97] |
| 4.8 Subgroup analysis by geographical region: progression‐free survival | 5 | 1684 | Hazard Ratio (IV, Random, 95% CI) | 0.77 [0.68, 0.87] |
| 4.8.1 East and South‐East Asia | 4 | 942 | Hazard Ratio (IV, Random, 95% CI) | 0.79 [0.68, 0.92] |
| 4.8.2 Rest of the world | 2 | 742 | Hazard Ratio (IV, Random, 95% CI) | 0.74 [0.57, 0.95] |
| 4.9 Subgroup analysis by extent of disease: overall survival | 2 | 859 | Hazard Ratio (IV, Random, 95% CI) | 0.95 [0.68, 1.33] |
| 4.9.1 Locally advanced | 1 | 14 | Hazard Ratio (IV, Random, 95% CI) | 1.56 [0.31, 7.85] |
| 4.9.2 Metastatic | 2 | 845 | Hazard Ratio (IV, Random, 95% CI) | 0.93 [0.64, 1.35] |
Comparison 5. Chemotherapy versus chemotherapy.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bang 2017.
| Study characteristics | ||
| Methods | International double‐blind placebo‐controlled phase 3 RCT In the overall population, median duration of follow‐up for OS was 11.1 months in olaparib‐paclitaxel group and 9.9 months in placebo‐paclitaxel group In ATM‐negative participants, median duration of follow‐up for OS was 12.6 months for olaparib‐paclitaxel group and 15.4 months for placebo‐paclitaxel group |
|
| Participants | Diagnosis: advanced gastric cancer including OGJ cancer Previous treatment status: disease progression following or during first‐line chemotherapy consisting of only 5‐fluoropyrimidine and platinum doublet combination Number of participants: 525 enrolled during 3rd September 2013 and 28th March 2016 Median age: 58 (49 to 67) years in Arm A and 59 (50 to 65) years in Arm B Number of male participants: 359 ECOG PS status: 224 PS0, 299 PS1 and 2 unknown Extent of disease: 9 locally advanced, 514 metastatic and 2 unknown Location of primary cancer: 14 OGJ, 509 gastric and 2 unknown Study locations: 58 centres in 4 countries in Asia (China, Japan, South Korea, and Taiwan) Other relevant key eligibility criteria were: life expectancy of at least 16 weeks; at least 1 measurable or non‐measurable lesion; acceptable bone marrow, liver, and renal function. Participants who had received more than 1 previous chemotherapy regimen for the treatment of advanced gastric cancer and those with history of previous treatment with PARP inhibitor or taxane were excluded. Of 525 participants randomised, 1 participant in each arm withdrew consents before receiving study treatment. |
|
| Interventions | Arm A (263 participants): oral olaparib 100 mg twice daily continuously in combination with intravenous paclitaxel 80 mg/m² on Day 1, 8, 15 of 28‐day cycle Arm B (262 participants): oral placebo twice daily continuously in combination with intravenous paclitaxel 80 mg/m² on Day 1, 8, and 15 of 28‐day cycle |
|
| Outcomes | Primary outcome: OS Secondary outcome: PFS, ORR, time to deterioration of health‐related QoL, safety, tolerability |
|
| Notes | QLQ‐STO22 (HRQoL22‐question scale developed specifically for participants with gastric cancer) was one of secondary outcomes, which was going to be reported separately, but publication not yet available. AstraZeneca provided organisational support and had a role in study design, data collection, data analyses, data interpretation, and the writing of the report. NCT01924533 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation scheme was produced by the Global Randomisation system at AstraZeneca that generates random numbers and randomisation codes were assigned sequentially as participants became eligible for randomisation |
| Allocation concealment (selection bias) | Low risk | Randomisation scheme was loaded into an interactive voice and web response system database |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Olaparib and matching placebo treatment were masked to participants, investigators and study monitors |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Olaparib and matching placebo treatment were masked to participants, investigators and study monitors |
| Imbalance in baseline characteristics across arms (confounding) | Low risk | No stratification factors were included in the study. Demographic and baseline disease characteristics were generally well‐balanced between the treatment arms in the overall population, and no major differences were noted. In the ATM‐negative population minor imbalances existed; 48% and 37% of participants had diffuse histology type, 46% and 33% of participants had > 2 metastases at baseline and 67% and 54% of had undergone a gastrectomy in the olaparib‐paclitaxel and placebo‐paclitaxel arm, respectively. |
| Incomplete outcome data (attrition bias) for outcomes other than QoL | Low risk | Response assessment was performed on intention‐to‐treat population (all participants assigned to treatment) while adverse events were assessed on safety population (all participants who received ≥ 1 dose) with 1 participant in Arm B (placebo) counted in Arm A (olaparib). |
| Incomplete outcome data (attrition bias) for QoL outcome | High risk | Only time to deterioration of health‐related‐QoL measured by EORTC QLQ‐C30 has been so far published. 93% and 66% of the participants provided QLQ‐C30 data at baseline and ≥ 1 post‐baseline time point, respectively. |
| Selective reporting (reporting bias) | Low risk | All the relevant predefined outcomes were reported |
| Other bias | Low risk | No obvious potential source of bias |
Hironaka 2013.
| Study characteristics | ||
| Methods | Multicentre open‐label phase 3 RCT Media follow‐up period 17.6 months |
|
| Participants | Diagnosis: histologically‐confirmed metastatic or recurrent gastric adenocarcinoma Previous treatment status: disease progression confirmed by CT, endoscopy or other imaging techniques during or within 1 month after last dose of first line chemotherapy with fluoropyrimidine plus platinum (in case of treatment with adjuvant or neoadjuvant chemotherapy consisting of fluoropyrimidine plus platinum, disease progression during treatment or within 6 months after treatment completion was allowed) Number of participants: 219 enrolled during August 2007 and August 2010 Median age: 64.5 (37 to 75) years in Arm A and 65 (38 to 75) years in Arm B Number of male participants: 171 ECOG PS status: 211 PS0‐1 and 8 PS2 Study locations: 37 centres in Japan Other relevant key eligibility criteria were: adequate bone marrow, hepatic and renal functions. Participants with prior history of chemotherapy with taxanes or irinotecan and those with severe peritoneal metastases were excluded. Of 223 participants randomly allocated, 5 in paclitaxel arm and 2 in irinotecan arm discontinued study treatment due to withdrawal of consent. |
|
| Interventions | Arm A (111 participants): intravenous paclitaxel 80 mg/m² on Day 1, 8, 15 of 28‐day cycle Arm B (112 participants): intravenous irinotecan 150 mg/m² on Day 1 and 15 of 28‐day cycle |
|
| Outcomes | Primary outcome: OS Secondary outcome: PFS, RR, safety |
|
| Notes | The study was funded by Yakult Pharmaceutical Industry and Daiichi Sankyo UMIN000001252 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using minimisation method |
| Allocation concealment (selection bias) | Low risk | Random assignment was carried out centrally at the data centre |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An independent statistician and data analysis centre performed primary analysis for OS and all investigators remained blinded to data until analysis was completed. |
| Imbalance in baseline characteristics across arms (confounding) | Low risk | Random assignment was carried out using minimisation method with the following adjustment factors; institution, ECOG PS (0‐1 vs 2) & measurable lesions (presence vs absence). Baseline characteristics of patients in the intention‐to‐treat population were well‐balanced between the two treatment arms. |
| Incomplete outcome data (attrition bias) for outcomes other than QoL | Low risk | Survival analyses were performed on intention‐to‐treat population (all randomly assigned participants who met eligibility criteria with those found to be ineligible after random assignment being excluded), whereas safety analyses were performed on safety population (all randomly assigned participants who received ≥ 1 dose of study treatment). Response rate was assessed on participants with ≥ 1 measurable lesion at baseline. |
| Incomplete outcome data (attrition bias) for QoL outcome | Low risk | Not relevant |
| Selective reporting (reporting bias) | Low risk | All the relevant predefined outcomes were reported |
| Other bias | Low risk | No obvious potential source of bias |
Kang 2017.
| Study characteristics | ||
| Methods | International double‐blind placebo‐controlled phase 3 RCT Medial follow‐up in surviving participants was 8.87 months (IQR 6.57 to 12.37) in nivolumab arm and 8.59 months (IQR 5.65 to 11.37) in placebo arm |
|
| Participants | Diagnosis: histologically‐confirmed unresectable advanced or recurrent gastric or OGJ adenocarcinoma Previous treatment status: refractory to or intolerant of standard therapy and previous treatment with ≥ 2 chemotherapy regimens in advanced or recurrent setting. All participants had previous treatment with fluoropyrimidine and 94% of participants in Arm A (nivolumab) and 96% of participants in Arm B (placebo) had received platinum, however, the proportion of participants who had fluoropyrimidine‐platinum chemotherapy regimen was not documented. Number of participants: 493 enrolled during Nov 4, 2014, and Feb 26, 2016, Median age: 62 (54 to 69) years in Arm A and 61 (53 to 68) years in Arm B Number of male participants: 348 ECOG PS status: 143 PS0 and 350 PS1 Location of primary cancer: 42 OGJ, 407 gastric and 32 unknown Study locations: 49 centres in 3 countries in Asia (Japan, South Korea, and Taiwan) Other relevant key eligibility criterion was life expectancy of at least 3 months. Participants with following conditions were excluded: ongoing or previous autoimmune disease or interstitial lung disease, acute diverticulitis or gastrointestinal ulcerative disease or other uncontrolled or clinically significant medical disorders; brain metastasis that were symptomatic or required treatment; previous treatment anti‐PD‐1, anti‐PD‐L1, anti‐PD‐L2, anti‐CD137 or anti‐CTLA‐4 antibodies were also excluded. Of 493 participants randomly allocated, 1 in the placebo group withdrew consent before receiving treatment. |
|
| Interventions | Arm A (330 participants): intravenous nivolumab 3 mg/kg fortnightly Arm B (163 participants): intravenous placebo fortnightly |
|
| Outcomes | Primary outcome: OS Secondary outcome: PFS, ORR, DCR, duration of response, TTR, best overall response, maximum percentage change from baseline in the sum of diameters of target lesions, safety |
|
| Notes | Participants could continue study treatment following first disease progression if following criteria were met: evidence of investigator‐assessed clinical benefit, tolerance of study drug, stable PS, continuation of treatment would not delay an intervention to prevent serious complications of disease progression, participants provided written informed consent before continuing study treatment. Study was funded by Ono Pharmaceutical and Bristol‐Myers Squibb and the assistance from Ono Pharmaceutical included statistical support and provision of medical monitors. NCT02267343 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed via an interactive web response system |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed via an interactive web response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind placebo‐controlled study |
| Imbalance in baseline characteristics across arms (confounding) | Low risk | Randomisation was stratified according to country (Japan vs Korea vs Taiwan), ECOG PS (0 vs 1), and the number of organs with metastases (< 2 vs ≥ 2). Baseline patient and disease characteristics were balanced across treatment arms. |
| Incomplete outcome data (attrition bias) for outcomes other than QoL | Low risk | Survival analyses were performed in intention‐to‐treat population (all participants who were randomly assigned), whereas safety analyses were performed in safety population (all participants who received at least 1 dose of study treatment). Response rate was assessed on response‐evaluable population (all participants with measurable target lesions at baseline). |
| Incomplete outcome data (attrition bias) for QoL outcome | Low risk | Not relevant |
| Selective reporting (reporting bias) | Low risk | All the relevant predefined outcomes were reported |
| Other bias | Low risk | No obvious potential source of bias |
Kim 2015.
| Study characteristics | ||
| Methods | Multicentre phase 2 RCT | |
| Participants | Diagnosis: histologically‐confirmed metastatic or recurrent gastric adenocarcinoma Previous treatment status: objective radiographic disease progression either during first‐line chemotherapy or within 6 months after last dose of cisplatin‐based adjuvant chemotherapy. 96.6% of participants in Arm A (docetaxel) and 96% of participants in Arm B (docetaxel‐oxaliplatin) had fluoropyrimidine‐platinum containing chemotherapy regimen. Number of participants: 52 enrolled during January 2009 to January 2012 Median age: 54 (Arm A) and 59 (Arm B) years Number of male participants: 42 ECOG PS status: 8 PS0, 42 PS1 and 2 PS2 Study locations: 6 centres in South Korea Other relevant key eligibility criteria were: at least 1 measurable lesion, life expectancy > 12 weeks; adequate organ function. Participants with following conditions were excluded: previous exposure to docetaxel or oxaliplatin; symptomatic peripheral neuropathy of Grade ≥ 2; brain metastasis; history of other tumour except BCC or successfully treated cervical carcinoma; another serious medical condition; hypersensitivity to atropine; pregnant/breast‐feeding. |
|
| Interventions | Arm A (27): intravenous docetaxel 36 mg/m² on Day 1 and 8 of 21‐day cycle up to max 9 cycles Arm B (25): intravenous docetaxel 36 mg/m² on Day 1 and 8 and intravenous oxaliplatin on Day 1 of 21‐day cycle up to max 9 cycles |
|
| Outcomes | Primary outcome: ORR Secondary outcome: PFS, OS |
|
| Notes | Trial was terminated early due to poor px accrual rate. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Imbalance in baseline characteristics across arms (confounding) | Unclear risk | Randomisation was stratified by ECOG PS (0‐1 vs 2). Higher proportion of participants in the docetaxel arm had metastases of lymph nodes and bladder/ureter at base line; 44.4% and 14.8% in the docetaxel arm, respectively and 32% and 4% in the docetaxel‐oxaliplatin arm, respectively. They were considered unlikely to affect the outcome of this review. 32% of participants in the docetaxel‐oxaliplatin arm had received paclitaxel‐S1‐cisplatin previously, compared to 22.2% of participants in the docetaxel arm. |
| Incomplete outcome data (attrition bias) for outcomes other than QoL | Low risk | Both efficacy and safety analyses were performed on intention‐to‐treat population (all enrolled participants) |
| Incomplete outcome data (attrition bias) for QoL outcome | Low risk | Not relevant |
| Selective reporting (reporting bias) | Low risk | No protocol was available, however, all important outcomes were reported |
| Other bias | High risk | Trial was terminated early due to poor px accrual rate |
Lee 2017.
| Study characteristics | ||
| Methods | Multicentre phase 2 RCT Median follow‐up time 7.3 months (range 1.6 to 31.5) |
|
| Participants | Diagnosis: histologically‐confirmed metastatic gastric adenocarcinoma Previous treatment status: disease progression during or within 6 months of completion of first‐line chemotherapy with S1‐ or capecitabine‐cisplatin; Number of participants: 69 Median age: 56 (34 to 68) (Arm A), 55 (38 to 74) (Arm B) and 55 (39 to 68) (Arm C) years Number of male participants: 52 ECOG PS status: 4 PS0, 61 PS1 and 4 PS2 Study locations: 3 centres in South Korea Other relevant key eligibility criteria were: measurable lesion; adequate major organ function. Participants with following conditions were excluded: concurrent uncontrolled medical conditions; other malignancies within past 3 years; prior treatment with taxane; pre‐existing Grade ≥ 2 neuropathy. Of 72 participants randomly allocated, 1 in docetaxel‐S‐1 arm withdrew consent before receiving treatment. |
|
| Interventions | Arm A (23 participants): intravenous docetaxel 75 mg/m² on Day 1 of 21‐day cycle Arm B (23 participants): intravenous docetaxel 60 mg/m² on Day 1 and intravenous cisplatin 60 mg/m² on Day 1 of 21‐day cycle Arm C (23 participants):intravenous docetaxel 60 mg/m² on Day 1 and oral S1 30 mg/m² on Day 1 to 14 of 21‐day cycle Study treatment was continued until disease progression, unacceptable toxicity or withdrawal of consent by participants. |
|
| Outcomes | Primary outcome: ORR Secondary outcome: PFS, OS, safety, QoL |
|
| Notes | Trial was terminated early due to slow recruitment. Study was supported by the Research Institute and Hospital, National Cancer Center, Republic of Korea. NCT00980603 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using random permutation method |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Imbalance in baseline characteristics across arms (confounding) | Unclear risk | Randomisation was stratified according to study site, ECOG PS (0‐1 vs 2), and best response to first‐line chemotherapy. Smaller proportion of participants in the docetaxel‐S1 arm were males; 60.9% in docetaxel‐sunitinib arm compared to 78.3% and 87% in the docetaxel and docetaxel‐cisplatin arms, respectively. 95.7% of participants in the docetaxel arm had upfront metastatic disease compared to 78.3% in the docetaxel and docetaxel‐cisplatin arms. More participants in the docetaxel‐S1 arm had ≥ 2 metastases at baseline. |
| Incomplete outcome data (attrition bias) for outcomes other than QoL | Low risk | Efficacy analyses were performed on modified intention‐to‐treat population (all randomised participants excluding those who were deemed ineligible or never started study treatment), whereas safety analyses were performed on safety population (all participants who received at least 1 dose of study treatment).1 participant in Arm B (docetaxel‐cisplatin) was considered ineligible and excluded from efficacy analyses, but included in safety analyses. 2 participants in Arm C did not receive study treatment and excluded from both efficacy and safety analyses. Modified intention‐to‐treat population was 95.8% of randomised participants. |
| Incomplete outcome data (attrition bias) for QoL outcome | High risk | More than 60% of participants in each arm completed baseline QoL questionnaire and at least one post‐treatment questionnaire. Although it was described compliance was similar in 3 arms, the exact proportion of participants completing QoL questionnaire at different time points were not provided. |
| Selective reporting (reporting bias) | Low risk | All the relevant predefined outcomes were reported |
| Other bias | High risk | Trial was terminated early due to slow recruitment. |
Li 2013.
| Study characteristics | ||
| Methods | Multicentre double‐blind placebo‐controlled phase 2 RCT | |
| Participants | Diagnosis: histologically‐confirmed advanced or metastatic gastric cancer including OGJ adenocarcinoma Previous treatment status: lack of response or intolerance to at least 2 chemotherapy including both platinum and fluoropyrimidine. Whether participants had received fluoropyrimidine and platinum concurrently was not described. Number of participants: 141 with enrolment starting June 2009 and participants being observed until October 2010 Median age: 55 (Arm A), 53 (Arm B), 54 (Arm C) years Number of male participants: 109 Extent of disease: 5 locally advanced, 136 metastatic Study locations: 15 centres in China Other relevant key eligibility criteria were: at least 1measurable lesion; acceptable haematologic, hepatic and renal function. Participants with uncontrolled BP on medication (> 140/90 mmHg), bleeding tendency or those receiving thrombolytics or anticoagulants were excluded. |
|
| Interventions | Arm A (47 participants): oral apatinib 850 mg once daily continuously on 28‐day cycle Arm B (46 participants): oral apatinib 425 mg twice daily continuously on 28‐day cycle Arm C (48 participants): oral placebo 2 tablets in the morning and 1 tablet in the evening continuous on 28‐day cycle Study treatment was continued until disease progression, intolerable toxicity or withdrawal of consent by participants. of 141 participants randomly assigned, 6 in placebo arm, 7 in apatinib 850 mg arm and 6 in apatinib 425 mg arm withdrew consent. |
|
| Outcomes | Primary outcome: PFS Secondary outcome: DCR, ORR, OS, QoL, safety |
|
| Notes | Fudan University Shanghai Cancer Center funded Manette Marais, PhD to assist with writing and editing the article. NCT00970138 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Random assignment was centrally managed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo‐controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind placebo‐controlled trial |
| Imbalance in baseline characteristics across arms (confounding) | Low risk | Randomisation was stratified according to the number of metastatic organs. The baseline characteristics of patients in the different groups were similar with regard to age, sex ratio, surgical history, disease stage, and number of metastatic organs. |
| Incomplete outcome data (attrition bias) for outcomes other than QoL | Low risk | Both response and safety analyses were performed on intention‐to‐treat population (all randomised participants excluding those who withdrew consent before receiving study treatment) . The intention‐to‐treat population was 97.2% of the randomly assigned participants. |
| Incomplete outcome data (attrition bias) for QoL outcome | Unclear risk | No information on the number of participants who completed QoL questionnaire or the results of QoL questionnaire was provided. |
| Selective reporting (reporting bias) | Low risk | All the relevant predefined outcomes were reported |
| Other bias | Low risk | No obvious potential source of bias |
Ling 2018.
| Study characteristics | ||
| Methods | Single‐centre RCT Study enrolment commenced 1st January 2010 and follow‐up ended 26th September 2015 |
|
| Participants | Disease: advanced GC confirmed by oesophago‐gastro‐duodenoscopy or post‐surgical pathology, including those inoperable or relapsed/metastasis after surgery Previous treatment status: disease progression after DCF chemotherapy Number of participants: 107 enrolled between 1st January 2010 and 1st June 2013 Median age: 53 (31 to 71) years Number of male participants: 70 ECOG PS status: 81 PS0‐1 and 26 PS2 Extent of disease: 46 locally advanced and 61 metastatic Study locations: single centre in China Other relevant key eligibility criteria were: adequate routine bloods including liver and kidney function; normal ECG |
|
| Interventions | Arm A (56 participants): intravenous epirubicin 50 mg/m² on Day 1, intravenous oxaliplatin 85 mg/m² on Day 1 and oral capecitabine 625 mg/m² twice daily D1‐14 of 21‐day cycle Arm B (51 participants): intravenous irinotecan 180 mg/m² on Day 1, intravenous leucovorin 400 mg/m² on Day 1, intravenous 5‐FU, 400mg/m² on Day 1 over 24 hours of 14‐day cycle |
|
| Outcomes | Endpoints: RR, DCR, safety, PFS, OS | |
| Notes | Text in Chinese. No HR was reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a digit randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess on the published full text |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to assess on the published full text |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unable to assess on the published full text |
| Imbalance in baseline characteristics across arms (confounding) | Low risk | The baseline characteristics of patients in the different groups were similar with regard to age, sex ratio, ECOG PS, surgical history, disease stage, histological subtype, and number of metastatic organs. |
| Incomplete outcome data (attrition bias) for outcomes other than QoL | Low risk | Efficacy analyses were performed on intention‐to‐treat population (all the randomised participants) |
| Incomplete outcome data (attrition bias) for QoL outcome | Low risk | Not relevant |
| Selective reporting (reporting bias) | Low risk | No protocol was available, however, all important outcomes were reported |
| Other bias | Low risk | No obvious potential source of bias |
Makiyama 2018.
| Study characteristics | ||
| Methods | Multicentre open‐label phase 2 RCT | |
| Participants | Diagnosis: HER2‐positive advanced unresectable or recurrent G/GEJ cancer Previous treatment status: progression first‐line chemotherapy with trastuzumab + fluoropyrimidine + platinum; ECOG0‐2 Number of participants: 89 enrolled between December 2012 to October 2016 Study locations: 8 centres in Japan Other relevant key eligibility criteria were: ECOG PS 0‐2; expected survival ≥ 90 days; measurable or evaluable lesion, adequate bone marrow and kidney/liver functions; left ventricular Ejection fraction (LVEF) ≥ 50% measured by echocardiography or multi‐gated acquisition scan (MUGA) |
|
| Interventions | Arm A (44 participants): intravenous paclitaxel 80 mg/m² on Day 1, 8, 15 of 28‐day cycle and trastuzumab 8 mg/kg followed by 6 mg/kg on Day 1 of 21‐day cycle Arm B (45 participants): intravenous paclitaxel 80 mg/m² on Day 1, 8, 15 of 28‐day cycle |
|
| Outcomes | Primary endpoint: PFS Secondary endpoints: OS, RR, safety and translational biomarker research |
|
| Notes | Only abstract published UMIN000009297 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unable to assess on the published abstract |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess on the published abstract |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unable to assess on the published abstract |
| Imbalance in baseline characteristics across arms (confounding) | Unclear risk | Unable to assess on the published abstract |
| Incomplete outcome data (attrition bias) for outcomes other than QoL | Unclear risk | Unable to assess on the published abstract |
| Incomplete outcome data (attrition bias) for QoL outcome | Low risk | Not relevant |
| Selective reporting (reporting bias) | Unclear risk | All the relevant predefined efficacy outcomes were reported, however, safety outcomes were very limited |
| Other bias | Unclear risk | Unable to assess on the published abstract |
Pauligk 2017.
| Study characteristics | ||
| Methods | Multicentre double‐blind placebo‐controlled Phase 3 RCT | |
| Participants | Disease: patients with inoperable locally advanced, metastatic or recurrent gastric carcinoma or adenocarcinoma of the esophagogastric junction (EGJ) Previous treatment status: progression after treatment with a fluoropyrimidine/platinum‐containing regimen Number of participants: 300 with enrolment commencing October 2011 Mean age: 62 years Study locations: in Germany Other relevant key eligibility criteria were: At least one measurable or evaluable lesion; ECOG PS 0‐2; adequate bone marrow, liver and renal functions. Participants with Paclitaxel refractory disease or unstable CNS disease were excluded. |
|
| Interventions | Arm A (150 participants): intravenous paclitaxel 80 mg//m² on Day 1, 8 and 15 and oral everolimus on Day 1‐28 of 28‐day cycle Arm B (150 participants): intravenous paclitaxel 80 mg/m² on Day 1, 8 and 15 and oral placebo of 28‐day cycle |
|
| Outcomes | Primary endpoint: OS Secondary endpoints: ORR, DCR, PFS and toxicity |
|
| Notes | Only abstract published NCT01248403 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unable to assess on the published abstract |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess on the published abstract |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo‐controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind placebo‐controlled trial |
| Imbalance in baseline characteristics across arms (confounding) | Unclear risk | Unable to assess on the published abstract |
| Incomplete outcome data (attrition bias) for outcomes other than QoL | Unclear risk | Unable to assess on the published abstract |
| Incomplete outcome data (attrition bias) for QoL outcome | Low risk | Not relevant |
| Selective reporting (reporting bias) | Unclear risk | All the relevant predefined efficacy outcomes were reported, but safety outcomes were very limited |
| Other bias | Unclear risk | Unable to assess on the published abstract |
Pavlakis 2016.
| Study characteristics | ||
| Methods | International double‐blind placebo‐controlled phase 2 RCT Kaplan‐Meier estimate of median follow‐up was 17.1 months (95% CI 14.6 to 19.4) |
|
| Participants | Diagnosis: metastatic or locally recurrent OGJ or stomach adenocarcinoma or undifferentiated histology Previous treatment status: refractory to ≤ 2 lines chemotherapy including prior fluoropyrimidine and platinum. Whether participants had received fluoropyrimidine and platinum concurrently was not described. Number of participants: 147 enrolled between 7th November 2012 and 25th February 2014 Mean age: 63 (31 to 81) years in Arm A and 62 (32‐85) years in Arm B Number of male participants: 118 ECOG PS status: 62 PS0 and 85 PS1 Location of primary cancer: 56 OGJ and 91 gastric Study locations: 53 centres in 4 countries worldwide (Australia, New Zealand, Canada and South Korea) Other relevant key eligibility criterion was measurable disease according to RECIST v1.1 by CT within 21 days before random assignment. Participants with poorly controlled hypertension, prior anti‐VEGF therapy and uncontrolled CNS disease were excluded. Of 152 randomly allocated participants, 4 in regorafenib arm and 2 in placebo arm discontinued treatment due to withdraw of consent. |
|
| Interventions | Arm A (97 participants): oral regorafenib 160 mg once daily on Day 1‐21 of 28‐day cycle Arm B (50 participants): oral placebo once daily on Day 1‐21 of 28‐day cycle Study treatment was continued until progression or prohibitive toxicity. |
|
| Outcomes | Primary outcome: PFS Secondary outcome: ORR, clinical benefit status at 2 months (defined as receiving treatment and remaining progression‐free during Week 6‐10), OS, adverse events, QoL |
|
| Notes | Participants receiving placebo who were subsequently unblinded because of documented PD were able to receive open‐label regorafenib provided they continued to meet protocol=specified safety and PS criteria. 29 pxs (58%) receiving placebo chose regorafenib Rx after PD. Supported by unrestricted research grant from Bayer HealthCare Pharmaceuticals to the Australasian Gastro‐Intestinal Trials Group to conduct the study independently and by National Health and Medical Research Council (NHMRC) Program Grant ACTRN12612000239864 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a permuted‐blocks method and an independent statistician monitored random assignment system. |
| Allocation concealment (selection bias) | Low risk | Random assignment was performed centrally via a web‐based system, maintaining allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo‐controlled trial: regorafenib and matching placebo were provided by manufacturer to sites, labelled with unique kit numbers, which were assigned to participants on random assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind placebo‐controlled trial: responses were assessed locally using RECIST v1.1, blinded central review of all RECIST source documents was undertaken for all participants for date of progression and 13% of participants were randomly selected for blinded central review by 2 independent radiologists. |
| Imbalance in baseline characteristics across arms (confounding) | Low risk | Randomisation was stratified by lines of prior chemotherapy for advanced disease (one v two) and geographic region (Australia, New Zealand or Canada vs South Korea). The groups were balanced at baseline including for median plasma VEGF (≤ 0.14 vs > 0.14 pg/mL). |
| Incomplete outcome data (attrition bias) for outcomes other than QoL | Low risk | Efficacy and safety analyses was performed on all the randomised participants excluding those not meeting inclusion criteria. 3 participants in Arm A (regorafenib) were excluded based on absence of measurable disease (2) and more than 2 lines of previous chemotherapy and 2 participants in Arm B (placebo) were excluded as their primary tumours were distal oesophageal in origin. |
| Incomplete outcome data (attrition bias) for QoL outcome | High risk | 96% and 67% of the participants who consented to participate in the QoL sub‐study completed a baseline QoL assessment, and at least one post‐baseline QoL assessment. |
| Selective reporting (reporting bias) | Low risk | All the relevant predefined outcomes were reported |
| Other bias | Low risk | No obvious potential source of bias |
Shah 2018.
| Study characteristics | ||
| Methods | Multinational double‐blind placebo‐controlled phase 3 RCT | |
| Participants | Disease: cytologically‐ or histologically‐confirmed advanced gastric or OGJ adenocarcinoma that is metastatic or locally advanced and unresectable Previous treatment status: progression on one prior line of therapy containing a fluoropyrimidine/platinum doublet Number of participants: 714 with enrolment commencing October 2014 Number of male participants: 72.1% Location of primary cancer: 25.4% OGJ and 74.6% gastric Study locations: 263 locations in 22 countries worldwide Other relevant key eligibility criteria were: patients with either measurable disease or non‐measurable evaluable disease were eligible ; ECOG PS 0‐1; adequate bone marrow, liver and renal functions. Participants with following conditions were excluded: prior taxanes in the neoadjuvant or adjuvant setting with progression occurring within 6 months of completion of taxane therapy or any taxanes in the metastatic setting; any known symptomatic brain metastases requiring steroids; peripheral neuropathy ≥ CTCAE Grade 2 at baseline. |
|
| Interventions | Arm A (estimated to be 357 participants): oral napabucasin 480 mg two times daily and intravenous paclitaxel 80 mg/m2 intravenously on Days 1, 8, and 15 of every 4‐week cycle Arm B (estimated to be 357 participants): oral placebo two times daily and intravenous paclitaxel 80 mg/m2 on Days 1, 8, and 15 of every 4‐week cycle Study treatment was continued until disease progression, death, intolerable toxicity or patient/investigator decision to stop treatment |
|
| Outcomes | Primary endpoint: OS Secondary endpoints: PFS, ORR, DCR and safety |
|
| Notes | Only abstract published NCT02178956 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unable to assess on the published abstract |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess on the published abstract |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind placebo‐controlled trial, however, at interim analysis of 380 events, the data safety monitoring board recommended trial unblinding when meeting the primary endpoint at final analyses appeared unlikely, though no safety concerns of clinical significance were identified. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind placebo‐controlled trial, however, at interim analysis of 380 events, the data safety monitoring board recommended trial unblinding when meeting the primary endpoint at final analyses appeared unlikely, though no safety concerns of clinical significance were identified. |
| Imbalance in baseline characteristics across arms (confounding) | Unclear risk | Unable to assess on the published abstract |
| Incomplete outcome data (attrition bias) for outcomes other than QoL | Unclear risk | Unable to assess on the published abstract |
| Incomplete outcome data (attrition bias) for QoL outcome | Low risk | Not relevant |
| Selective reporting (reporting bias) | Unclear risk | All the relevant predefined efficacy outcomes were reported |
| Other bias | Unclear risk | Unable to assess on the published abstract |
Shitara 2018.
| Study characteristics | ||
| Methods | International open‐label phase 3 RCT Median follow‐up was 7·9 months (IQR 3·4 to 14·6) in the total population and 8·5 months (3·7 to 15·7) in the population with a PD‐L1 CPS ≥ 1. |
|
| Participants | Diagnosis: histologically‐ or cytologically‐confirmed adenocarcinoma of stomach or OGJ that was metastatic or locally advanced but unresectable Previous treatment status: disease progression per RECIST v1.1 after first‐line chemotherapy with a platinum and fluoropyrimidine (as well as with trastuzumab in participants with HER2‐positive tumours) Number of participants: 592 enrolled between 4th June 4 2015 and 26th July 2016 Mean age: 62·5 (54 to 70) years in Arm A and 60·0 (53 to 68) years in Arm B Number of male participants: 410 ECOG PS status: 164 PS0, 327 PS1 and 1 PS2 Extent of disease: 6 locally advanced and 586 metastatic Location of primary cancer: 185 OGJ and 407 gastric Study locations: 140 centres in 30 countries worldwide Other relevant key eligibility criterion was availability of a tumour sample for PD‐L1 assessment. Participants with following conditions were excluded: squamous or undifferentiated histology; previous treatment with any PD‐1, PD‐L1 or PD‐L2 inhibitor; active autoimmune disease that necessitated systemic treatment. Of 592 participants randomly assigned, 9 (6 with CPS ≥ 1) in pembrolizumab arm and 20 (13 with CPS ≥ 1) in paclitaxel arm discontinued treatment due to withdrawal of consent. |
|
| Interventions | Arm A (296 participants with 196 PD‐L1 combined positive score ≥ 1): intravenous pembrolizumab 200 mg on Day 1 of 21‐day cycle Arm B (296 participants with 199 PD‐L1 combined positive score ≥ 1): intravenous paclitaxel 80 mg/m² on Day 1, 8, 15 of 28‐day cycle Study treatment was continued until disease progression, intolerable toxicity, doctor decision or withdrawal of consent by participants. |
|
| Outcomes | Primary outcome: OS and PFS (assessed per RECIST by masked and independent central review) in PD‐L1 combined positive score ≥ 1 participants Secondary outcome: RR, duration of response, OS and PFS in total population, PFS (assessed per RECIST by local investigators and per irRECIST by masked and independent central review) in PD‐L1 combined positive score ≥ 1 participants and total population, TTP, safety |
|
| Notes | After 489 participants were enrolled, the independent data monitoring committee recommended that enrolment to be restricted to participants with PD‐L1 combined positive score of ≥ 1. Study was funded by Merck Sharp & Dohme, a subsidiary of Merck & Co., who participated in study design, data analysis and interpretation, and manuscript writing. NCT02370498 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation schedule was generated by system vendor using a computerised random list generator |
| Allocation concealment (selection bias) | Low risk | Random allocation was performed using a central interactive voice‐response and integrated web‐response system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients, treating doctors, the external data monitoring committee and sponsor representatives were not masked to treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Response assessment was performed by the central radiological reviewers who were masked to treatment assignment |
| Imbalance in baseline characteristics across arms (confounding) | Low risk | Enrolment of the first 125 patients was stratified by geographical region (Europe, Israel, North America, and Australia vs Asia vs rest of world) and ECOG performance status (0 vs 1). Following a protocol amendment, enrolment of the remaining 467 patients was stratified by geographical region (Europe, Israel, North America, and Australia vs Asia vs rest of the world), time to progression on first‐line therapy (< 6 months vs ≥ 6 months), and PD‐L1 CPS (< 1 vs ≥ 1). Baseline demographics and disease characteristics were generally balanced between groups in the total population and the population with a PD‐L1 CPS of 1 or higher. |
| Incomplete outcome data (attrition bias) for outcomes other than QoL | Low risk | Efficacy analyses were performed on intention‐to‐treat population (all randomised participants irrespective of whether they received treatment), while safety analyses were performed on safety population (all participants who received at least 1 dose of study treatment) |
| Incomplete outcome data (attrition bias) for QoL outcome | Low risk | Not relevant |
| Selective reporting (reporting bias) | Low risk | All the relevant predefined outcomes were reported |
| Other bias | Low risk | No obvious potential source of bias |
Shitara 2018b.
| Study characteristics | ||
| Methods | Multinational double‐blind placebo‐controlled phase 3 RCT | |
| Participants | Diagnosis: histologically‐confirmed, non‐resectable, metastatic gastric adenocarcinoma including adenocarcinoma of GOJ Previous treatment status: previously received two or more standard‐of‐care regimens and had experienced radiological disease progression within 3 months of the last dose of the last previous therapy or were unable to tolerate their last previous therapy; previous regimens must have included a fluoropyrimidine, a platinum agent and a taxane or irinotecan, or both; participants with HER2‐positive tumours must have received previous anti‐HER2 therapy, if available Number of participants: 507 enrolled between 24th February 2016 and 5th January 2018 Mean age: 64 (56 to 70) in Arm A and 63 (56 to 69) in Arm B Number of male participants: 369 ECOG PS status: 191 PS0 and 316 PS2 Location of primary cancer: 145 OGJ, 360 gastric and 2 both OGJ and gastric Study locations: 110 centres in 17 countries worldwide Other relevant key eligibility criteria were: adequate bone marrow, kidney and liver functions; measurable and non‐measurable disease according to RECIST v1.1. Participants with following conditions were excluded: any other active malignancy, CNS metastasis, active infection, serious organ dysfunction, autoimmune disorders; a history of organ transplantation requiring immunosuppressive therapy. |
|
| Interventions | Arm A (337 participants): oral TAS‐102 35 mg/m2 twice daily on Day 1‐5 and Day 8‐12 of each 28‐day cycle Arm B (170 participants): oral placebo twice daily on Day 1‐5 and Day 8‐12 of each 28‐day cycle Study treatment was continued until disease progression, intolerable toxicity, or patient withdrawal. Of 507 participants randomly assigned, 2 participants in placebo arm withdrew consent before receiving treatment. Further 14 participants in TAS‐102 arm and 4 participants in placebo arm discontinues treatment due to withdrawal of consent. |
|
| Outcomes | Primary endpoint: OS Secondary endpoints: PFS, ORR, DCR, safety, time to deterioration of ECOG PS and health‐related QoL |
|
| Notes | Study was funded by Taiho Oncology and Taiho Pharmaceutical., who had a role in study design, data collection, data analysis, and data interpretation, and employees of the study funder had roles in writing the report. NCT02500043 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed via a dynamic allocation method (biased coin) |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed via with an interactive‐voice web‐response system, which assigned study medication by assigning a kit number to that patient |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, investigators and study‐site personnel were masked to treatment assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Those assessing outcomes and those analysing the data were masked to treatment assignment |
| Imbalance in baseline characteristics across arms (confounding) | Low risk | Randomisation was stratified by region (Japan vs rest of world), ECOG performance status (0 vs 1), and previous treatment with ramucirumab (yes vs no). Baseline demographic and disease characteristics were generally balanced between the 2 arms. |
| Incomplete outcome data (attrition bias) for outcomes other than QoL | Low risk | OS and PFS were analysed in the intention‐to‐treat population, while safety analysis population included all patients who received at least 1 dose of study treatment |
| Incomplete outcome data (attrition bias) for QoL outcome | Low risk | Not relevant |
| Selective reporting (reporting bias) | Low risk | All the relevant predefined outcomes except for QoL (to be published separately) were reported |
| Other bias | Low risk | No obvious potential source of bias |
Thuss‐Patience 2011.
| Study characteristics | ||
| Methods | Multicentre open‐label phase 3 RCT The participants were randomised between October 2002 and December 2006. Follow‐up was complete when the last participant died on the 10th of October 2007. |
|
| Participants | Diagnosis: measurable and/or evaluable histologically‐proven adenocarcinoma of stomach or OGJ which were metastatic or locally advanced disease with surgical incurability Previous treatment status: 1 palliative chemotherapy with documented objective imaging proven progression during or within 6 months after the end of first‐line chemotherapy. All participants in Arm A (irinotecan) and 90% of participants in Arm B (BSC) had fluoropyrimidine‐platinum containing chemotherapy for first line. Number of participants: 40 enrolled during October 2002 and December 2006 Mean age: 58 (43 to 73) years in Arm A and 55 (35 to 72) years in Arm B Number of male participants: 29 ECOG PS status: 31 PS0‐1 and 9 PS2 Extent of disease: 40 metastatic Location of primary cancer: 17 OGJ and 23 gastric Study locations: 10 centres in Germany Participants with following conditions were excluded: prior second malignancy; uncontrolled infection; CNS metastasis; other severe medical illnesses; major operation within the last 2 weeks; previous treatment with irinotecan; chronic diarrhoea; parallel treatment with any experimental or any other kind of antineoplastic therapy. |
|
| Interventions | Arm A (21 participants): intravenous Irinotecan 250 mg/m² in the first cycle which had to be increased to 350 mg/m² in subsequent cycles on Day 1 of 21‐day cycle Study treatment was continued until objective or clinical tumour progression, side effects, participants wish or maximum of 10 cycles. Arm B (19 participants): best supportive care |
|
| Outcomes | Primry outcome: OS Secondary outcome: ORR, TTP, toxicity |
|
| Notes | Study was closed prematurely due to poor accrual. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation blocks of 4 for each stratification arm were determined by using a coin |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed centrally |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Imbalance in baseline characteristics across arms (confounding) | Low risk | Randomisation was stratified according toECOG PS, pretreatment and timing of progression under first‐line treatment. Participants were well‐balanced between the 2 arms for localisation of the primary tumour, histological subtype, number of metastatic sites and response to first‐line chemotherapy, in addition to stratification factors. |
| Incomplete outcome data (attrition bias) for outcomes other than QoL | Low risk | OS analysis was performed on the intention‐to‐treat population (all the randomised participants) |
| Incomplete outcome data (attrition bias) for QoL outcome | High risk | QoL was not reported due to poor completion rate of questionnaire |
| Selective reporting (reporting bias) | High risk | PFS, ORR and toxicity data were reported for irinotecan treated participants only and QoL was not published due to poor completion rate of questionnaire |
| Other bias | High risk | Study was closed prematurely due to poor accrual and the most frequent reason for low recruitment reported from different centres was patient refusal of randomisation. |
Thuss‐Patience 2017.
| Study characteristics | ||
| Methods | International open‐label adaptive phase 2/3 RCT Duration of median follow‐up was 17.5 months (IQR12.1 to 23.0) for trastuzumab emtansine 2.4mg/kg arm and 15.4 months for taxane arm |
|
| Participants | Diagnosis: centrally tested HER2‐positive, measurable and/or evaluable advanced gastric cancer including OGJ adenocarcinoma Previoius treatment status: disease progression either during or after first‐line treatment with combination of at least platinum and fluoropyrimidine given concurrently Number of participants: 345 enrolled during 3rd September 2012 and 30th June 2015 (pre‐planned interim was performed on 14th October 2013) Mean age: 62.0 (19 to 79) years in Arm A 62.0 (27 to 80) in Arm B Number of male participants: 272 ECOG PS status: 142 PS0, 201 PS1 and 1 PS2 Extent of disease: 14 locally advanced and 331 metastatic Location of primary cancer: 110 OGJ and 235 gastric Study locations: 107 centres in 28 countries worldwide Participants with following conditions were excluded: another malignancy within previous 5 years; brain metastasis; peripheral neuropathy Grade ≥ 2; cardiopulmonary dysfunction; concurrent serious uncontrolled or known infection with HIV, active Hepatitis B or C. Of 345 participants randomly assigned, 2 participants in trastuzumab emtansine 2.4 mg/kg arm and 3 participants in taxane arm withdrew consent before receiving treatment. Further 9 participants and 11 participants in each arm discontinued treatment due to withdrawal of consent. |
|
| Interventions | Arm A (228 participants): intravenous trastuzumab emtansine 2.4 mg/kg on Day 1 of 7‐day cycle Arm B (117 participants): intravenous docetaxel 75 mg/m² on Day 1 of 21‐day cycle or intravenous paclitaxel 80 mg/m² on Day 1 of 7‐day cycle Study treatment was continued until progressive disease, intolerable toxicity, initiation of another anti‐cancer therapy, participants' and/or physicians' decision to discontinue study treatment or study termination. |
|
| Outcomes | Primary outcome: OS Secondary outcome: PFS, ORR, duration of response, safety, patient‐reported outcome, pharmacokinetics |
|
| Notes | The phase 2 part of the trial examined two dosing regimens of trastuzumab emtansine (2·4 mg/kg weekly compared with 3·6 mg/kg every 3 weeks) to allow selection of the most appropriate dose for assessment in the phase 3 part, which compared the efficacy and safety of the selected dose of trastuzumab emtansine with taxane treatment. F Hoffmann‐La Roche funded the study; they provided the study drugs and was involved in study design, protocol development, regulatory and ethics approvals, safety monitoring and reporting, data management and data analysis and interpretation. NCT01641939 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a permuted block randomisation procedure |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed via an interactive voice response system or interactive web response system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither participants nor investigators were masked to treatment assignment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Response assessments were determined by investigators who were not masked to treatment assignment |
| Imbalance in baseline characteristics across arms (confounding) | Low risk | Randomisation was stratified for world region (Asia Pacific; western Europe, USA, and Canada; other [eastern Europe, Latin America, and remaining countries]), previous HER2‐targeted therapy (yes vs no), and previous gastrectomy (yes vs no). Baseline characteristics were generally well‐balanced. |
| Incomplete outcome data (attrition bias) for outcomes other than QoL | Low risk | Survival analyses were performed on intention‐to‐treat population (all participants assigned to treatment), while safety analyses were performed on safety population (all subjects who received at least 1 dose of study treatment). Response rate was assessed on participants with measurable disease at base line. |
| Incomplete outcome data (attrition bias) for QoL outcome | Unclear risk | Detailed result of patient‐reported outcome including the number of participants who completed the relevant questionnaires was not reported |
| Selective reporting (reporting bias) | Low risk | All the relevant predefined outcomes except for patient‐related outcomes were reported |
| Other bias | Low risk | No obvious potential source of bias |
Wilke 2014.
| Study characteristics | ||
| Methods | International double‐blind placebo‐controlled phase 3 RCT Median follow‐up for OS of 7.9 months (IQR 4·2 to 13·0). |
|
| Participants | Diagnosis: metastatic or non‐resectable locally advanced gastric or OGJ adenocarcinoma Previous treatment status: objective radiological or clinical disease progression during or within 4 months of last dose of first‐line platinum and fluoropyrimidine doublet with and without anthracycline Number of participants: 665 enrolled during 23rd December 2010 and 23rd September 2012 Median age: 61 (25 to 83) years in Arm A and 61 (24 to 84) years in Arm B Number of male participants: 472 ECOG PS status: 261 PS1 and 404 PS2 Location of primary cancer: 137 OGJ and 528 gastric Study locations: 170 centres in 27 countries worldwide Other relevant key eligibility criterion was measurable or non‐measurable evaluable disease. Participants with following conditions were excluded: squamous or undifferentiated gastric cancer; gastrointestinal perforation, fistulae or any arterial thromboembolic even within 6 months; any significant gastrointestinal bleeding or any significant venous thromboembolism within 3 months before randomisation or poorly controlled hypertension. Of 665 participants randomly assigned, 23 in ramucirumab‐paclitaxel arm and 13 in placebo‐paclitaxel arm discontinued treatment due to withdrawal of consent. |
|
| Interventions | Arm A (330 participants): intravenous ramucirumab 8 mg/kg on Day 1 and 15 and intravenous paclitaxel 80 mg/m² IV D1, 8 and 15 of 28 day cycle Arm B (335 participants): intravenous placebo 8 mg/kg IV D1 and 15 and intravenous paclitaxel 80 mg/m² IV D1, 8 and 15 of 28 day cycle Study treatment was continued until disease progression, unacceptable toxicity or withdrawal of consent by participants. |
|
| Outcomes | Primary outcome: OS Secondary outcome: PFS, ORR, DCR, patient‐reported outcomes, immunogenicity of ramucirumab and safety |
|
| Notes | Eli Lilly provided study drug and collaborated with investigators on protocol, study design, data gathering, analysis and interpretation and writing and preparation of this report. NCT01170663 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was generated using the permuted blocks method within each stratum by a statistician not involved in the study activities. |
| Allocation concealment (selection bias) | Low risk | The sequence was programmed into a centralised interactive voice or web‐response system. Study sites enrolled participants by accessing the centralised interactive voice or web‐response system, which then assigned a unique identification number to each participant and randomly assigned participants to one of 2 treatment groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded placebo‐controlled study |
| Imbalance in baseline characteristics across arms (confounding) | Low risk | Randomisation was stratified ed by geographic region (region 1, Europe, Israel, Australia, and the USA; region 2, Argentina, Brazil, Chile, and Mexico; and region 3, Japan, South Korea, Hong Kong, Singapore, and Taiwan), time to progression after first dose of first‐line therapy (< 6 months vs ≥ 6 months), and disease measurability (measurable vs non‐measurable). Baseline characteristics of patients and their tumours were generally well‐balanced between the groups. |
| Incomplete outcome data (attrition bias) for outcomes other than QoL | Low risk | Efficacy analyses were performed on intention‐to‐treat population (all randomly assigned participants irrespective of whether participants received study treatment), while safety analyses included all participants who received at least 1 dose of any study treatment. |
| Incomplete outcome data (attrition bias) for QoL outcome | Low risk | The compliance of questionnaires defined as the number of participants who completed the questionnaires divided by the expected number of participants at that time points were similar across the two treatment arms throughout the study; 97.6% and 97.9% at baseline and 70% and 69.2% at Week 36. 84.2% of participants completed QoL questionnaires at least one post‐baseline time point. |
| Selective reporting (reporting bias) | Low risk | All the relevant predefined outcomes were reported |
| Other bias | Low risk | No obvious potential source of bias |
Yi 2012.
| Study characteristics | ||
| Methods | Single centre open‐label phase 2 RCT | |
| Participants | Diagnosis: unresectable or metastatic adenocarcinoma of stomach or OGJ progressed Previous treatment status: disease progression after a first‐line fluoropyrimidine‐platinum regimen Number of participants:105 enrolled during December 2008 and February 2011 Mean age: 54.0 (20 to 72) years in Arm A and 52 (36 to 70) years in Arm B Number of male participants: 73 ECOG PS status: 5 PS0, 71 PS1, 9 PS2 and 20 unknown Extent of disease: 11 locally advanced and 94 metastatic Study locations: single centre in South Korea Other relevant key criteria were: measurable or evaluable disease according to RECIST ver 1.1 and adequate organ function incl. bone marrow, liver and kidney. Participants with following conditions were excluded: severe comorbid illness and/or active infections; Grade ≥ 3 haemorrhage according to CTCAE v3.0 within prior 4 weeks; pregnant or lactating women; active CNS metastasis not controllable with radiotherapy or corticosteroids. |
|
| Interventions | Arm A (56 participants): intravenous docetaxel 60 mg/m² on Day 1 and oral sunitinib 37.5 mg once daily continuously of 21‐day cycle Arm B (49 participants): intravenous docetaxel 60 mg/m² on Day 1 of 21‐day cycle Study treatment was continued until tumour progression, unacceptable toxicity or consent withdrawal by participants. |
|
| Outcomes | Primary endpoint: TTP Secondary endpoints: OS, ORR, DCR, toxicity |
|
| Notes | Pfizer provided sunitinib, but was not involved in the accrual or analysis of the data or in the preparation of the manuscript NCT01238055 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a random permuted block design |
| Allocation concealment (selection bias) | Unclear risk | Allocation method was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All treatment responses were centrally reviewed while blinded to treatment arm |
| Imbalance in baseline characteristics across arms (confounding) | Low risk | Random assignment was stratified by ECOG performance status (0, 1 vs 2). Demographics of the two arms, including median age, sex ratio, ECOG performance scale, laboratory findings (except serum alkaline phosphatase level), and sites of metastatic lesions, were not significantly different. |
| Incomplete outcome data (attrition bias) for outcomes other than QoL | Low risk | Both efficacy and safety analyses were performed on all the randomised participants excluding 2 participants in Arm A (docetaxel‐sunitinib) who withdrew consent before receiving treatment. |
| Incomplete outcome data (attrition bias) for QoL outcome | Low risk | Not relevant |
| Selective reporting (reporting bias) | Low risk | All the relevant predefined outcomes were reported except for PFS, however, this is unlikely to affect the results of this review |
| Other bias | Low risk | No obvious potential source of bias |
ATM: ataxia‐telangiectasia mutated protein; BSC: best supportive care; CNS: central nervous system; CT: computed tomography; CTCAE: Common Terminology Criteria for Adverse Events; ECG: electrocardiogram; ECOG: Eastern Cooperative Oncology Group; IQR: interquartile range; ; IV: intravenous; OGJ: oesophago‐gastric junction;ORR: overall response rate;OS: overall survival; PARP: poly ADP ribose polymerase; PFS: progression‐free survival; PS: performance score; QoL: quality of life; RCT: randomised controlled trial; RR: response rate; TRR: tumour response rate; TTP: time to progression; TTR: time to response; vs: versus.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ajani 2010 | Included participants who received intervention as first‐line systemic therapy |
| Al‐Batran 2013 | Included participants who received intervention as first‐line systemic therapy |
| Bando 2016 | Included participants who received intervention as first‐line systemic therapy |
| Bang 2015 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Bang 2017b | Participants had stable disease on first‐line fluoropyrimidine‐platinum chemotherapy |
| Bang 2018 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Belloni 2015 | Non‐RCT |
| Boku 2009 | Included participants who received intervention as first‐line systemic therapy |
| Chon 2009 | Non‐RCT |
| Cohen 2014 | Included participants with oesophageal cancer other than OGJ adenocarcinoma |
| Cui 2015 | Non‐RCT |
| Enzinger 2016 | Included participants who received intervention as first‐line systemic therapy |
| Ford 2014 | Included participants with oesophageal cancer other than OGJ adenocarcinoma |
| Fuchs 2014 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Fujii 2011 | Included participants who received intervention as first‐line systemic therapy |
| Furue 1985 | Included participants who received intervention as first‐line systemic therapy |
| Fushida 2016 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Goto 1989 | Included participants who received intervention as first‐line systemic therapy |
| Guo 2019 | Non‐RCT |
| Higuchi 2014 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Inokuchi 2007 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Janjigian 2018 | Includedparticipants with oesophageal cancer other than OGJ adenocarcinoma |
| Kang 2012 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Kelly 2020 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Klein 1992 | Included participants who received intervention as first‐line systemic therapy |
| Koizumi 2001 | Non‐RCT |
| Kurokawa 2014 | Included participants who received intervention as first‐line systemic therapy |
| Lee 2012 | Non‐RCT |
| Lee 2018 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Li 2016 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Li 2016b | Included participants who received intervention as first‐line systemic therapy |
| Li 2018 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Loehrer 1994 | Included participants who received intervention as first‐line systemic therapy |
| Lorenzen 2015 | Included participants with oesophageal cancer other than OGJ adenocarcinoma |
| Luelmo 2008 | Included participants who received intervention as first‐line systemic therapy |
| Maruta 2007 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Moehler 2016 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Morita 2007 | Included participants who received intervention as first‐line systemic therapy |
| Murad 1993 | Included participants who received intervention as first‐line systemic therapy |
| Murakami 2001 | Non‐RCT |
| Nakanishi 2016 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Nakano 1999 | Included participants who received intervention as first‐line systemic therapy |
| Nishikawa 2015 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Nishikawa 2015c | Included participants who received intervention as first‐line systemic therapy |
| Nishina 2016 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Ochiai 1992 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Ohtsu 2013 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Popov 2008 | Included participants who received intervention as first‐line systemic therapy |
| Pyrhönen 1995 | Included participants who received intervention as first‐line systemic therapy |
| Qiao 2019 | Non‐RCT |
| Qu 2019 | Eligibility for inclusion could not be assessed on the published full‐text article |
| Roy 2013 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Sadighi 2006 | Included participants who received intervention as first‐line systemic therapy |
| Sang 2017 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Satoh 2014 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Satoh 2015 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Schnitzler 1986 | Included participants who received intervention as first‐line systemic therapy |
| Shirao 2013 | Included participants who received intervention as first‐line systemic therapy |
| Shitara 2017 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Sym 2013 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Tabernero 2017 | Included participants who received intervention as first‐line systemic therapy |
| Tanabe 2015 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Tebbutt 2002 | Included participants who received intervention as first‐line systemic therapy |
| Tebbutt 2010 | Included participants who received intervention as first‐line systemic therapy |
| Tebbutt 2016 | Included participants who received intervention as first‐line systemic therapy |
| Tsavaris 1996 | Included participants who received intervention as first‐line systemic therapy |
| Unknown 1992 | Included participants who received intervention as first‐line systemic therapy |
| Van Cutsem 2015 | Included participants who received intervention as first‐line systemic therapy |
| Van Cutsem 2017 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Wu 2020 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Yamamura 1998 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Yoshino 2016 | Included participants who received intervention as first‐line systemic therapy |
| Zhao 2009 | Included participants who received intervention as first‐line systemic therapy |
| Zhong 2007 | Included participants who received intervention as first‐line systemic therapy |
| Zhou 2015 | ≥10% or unknown proportion of participants received non‐fluoropyrimidine‐platinum chemotherapy for first‐line |
| Zhu 2016 | Non‐RCT |
OGJ: oesophago‐gastric junction; RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
Chung 2020.
| Methods | Open‐label Phase 3 RCT |
| Participants | Asian patients with advanced PD‐L1–positive (combined positive score [CPS] ≥1) gastric cancer that progressed after platinum + fluoropyrimidine chemotherapy |
| Interventions | Arm A: pembrolizumab Arm B: paclitaxel |
| Outcomes | Primary endpoint: OS and PFS |
| Notes | Inadequte amount of data available for inclusion in the review on the published abstract. Study was discontinued after the result of KEYNOTE‐061 became available (pembrolizumab did not significantly prolong OS compared to paclitaxel). NCT03019588 |
Denlinger 2016.
| Methods | Multicentre open‐label Phase 2 RCT |
| Participants | Patients with histologically‐ or cytologically‐confirmed HER2 expressing metastatic or locally advanced adenocarcinoma of the distal oesophagus, GE junction or stomach |
| Interventions | Arm A: MM‐111 + paclitaxel + trastuzumab Arm B: paclitaxel + trastuzumab |
| Outcomes | Primary endpoint: PFS |
| Notes | Eligibility for inclusion in the review cannot be assessed on the published abstract. The study was closed early by the Data Monitoring Committee based on lack of efficacy within the ITT. NCT01774851 |
EUCTR2012‐002321‐30.
| Methods | Single‐blinded Phase 2 RCT |
| Participants | Patients with histologically‐proven non‐resectable or metastatic oesophagus, cardia or gastric adenocarcinoma, who have progressed after first‐line treatment with platinum containing chemotherapy |
| Interventions | Arm A: irinotecan Arm B: bevacizumab + irinotecan |
| Outcomes | Primary endpoint: PFS |
| Notes | No publication available |
Ikeda 2009.
| Methods | Phase 2/3 RCT |
| Participants | Patients with advanced or recurrent gastric cancer, who had not received any chemotherapy except one regimen (not including taxanes) |
| Interventions | Arm A: docetaxel plus S1 Arm B: 5FU plus CDDP |
| Outcomes | RR, TTF, PFS, OS and toxicity |
| Notes | Eligibility for inclusion in the review cannot be assessed on the published abstract. |
Janjigian 2012.
| Methods | Phase 2 RCT |
| Participants | advanced p53wt (≤ 20% nuclear IHC staining using D07 antibody) gastric adenocarcinoma and disease progression on non‐irinotecan chemotherapy regimen |
| Interventions | Arm A: irinotecan plus flavopiridol Arm B: irinotecan |
| Outcomes | Primary endpoint: PFS |
| Notes | Eligibility for inclusion in the review cannot be assessed on the published abstract. |
JPRN‐UMIN000003156.
| Methods | Open‐label Phase 2 RCT |
| Participants | Inoperable or recurrent gastric cancer patients |
| Interventions | Arm A: TS‐1 based therapy + LNT Arm B: TS‐1 based therapy |
| Outcomes | Primary endpoint: TTF Secondary endpoints: OS, PFS, QoL, RR, safety and compliance |
| Notes | No publication available. The study was terminated. |
JPRN‐UMIN000008827.
| Methods | Open‐label phase 2 RCT |
| Participants | Patients with gastric cancer, which is resistant to standard therapies |
| Interventions | Arm A: personalised peptide + Juzen‐taiho‐to Arm B: personalised peptide |
| Outcomes | Primary endpoint: immune‐enhancing effects Secondary endpoints: peptide‐specific CTL responses in PBMCs, safety and OS |
| Notes | No publication available |
Kan 2018.
| Methods | RCT |
| Participants | HER‐2‐positive advanced gastric cancer patients |
| Interventions | Arm A: XELOX or FOLFOX4 + trastuzumab Arm B: XELOX or FOLFOX4 |
| Outcomes | OS, PFS and ORR |
| Notes | Eligibility for inclusion in the review cannot be assessed on the published abstract. |
Kang 2019.
| Methods | International double‐blinded Phase 3 RCT |
| Participants | Patients with advanced/metastatic adenocarcinoma of the stomach or gastroesophageal junction after failure of ≥ 2 prior lines of chemotherapy |
| Interventions | Arm A: rivoceranib Arm B: placebo |
| Outcomes | Primary endpoint: OS Secondary endpoints: PFS, ORR, DCR, QoL, safety and tolerability |
| Notes | Eligibility for inclusion in the review cannot be assessed on the published abstract. NCT03042611 |
Kelly 2018.
| Methods | Multicentre, open‐label Phase Ib/II RCT |
| Participants | Patients with histologically‐ or cytologically‐confirmed recurrent or metastatic gastric or gastroesophageal junction adenocarcinoma who have progressed on, or are refractory to standard regimens |
| Interventions | Arm A: durvalumab and tremelimumab Arm B: durvalumab Arm C: tremelimumab |
| Outcomes | Primary endpoints: ORR, PFS Secondary endpoints: safety, tolerability, DCR, DOR and OS |
| Notes | Eligibility for inclusion in the review cannot be assessed on the published abstract. Study is ongoing. NCT02340975 |
Kojima 2019.
| Methods | Open‐label Phase 3 RCT |
| Participants | Patients with advanced/metastatic squamous cell carcinoma (SCC) and adenocarcinoma of the esophagus or Siewert type I adenocarcinoma of the EGJ, who progressed on no more or less than one previous line of standard therapy |
| Interventions | Arm A: pembrolizumab Arm B: paclitaxel, docetaxel or irinotecan |
| Outcomes | Primary endpoint: OS Secondary endpoints: PFS, ORR, toxicity |
| Notes | Eligibility for inclusion in the review cannot be assessed on the published abstract. NCT02564263 |
NCT00683787.
| Methods | Phase 2 RCT |
| Participants | Patients with histologically confirmed metastatic gastric adenocarcinoma or gastroesophageal junction cancer |
| Interventions | Arm A: docetaxel Arm B: docetaxel + vandetanib 100mg once daily Arm C: docetaxel + vandetanib 300mg once daily |
| Outcomes | Primary endpoint: ORR Secondary endpoint: PFS, OS and toxicity |
| Notes | No publication available. The study was terminated due to low accrual. |
NCT00991952.
| Methods | Multicentre double‐blinded Phase 2 RCT |
| Participants | Patients with pathologically‐confirmed unresectable or metastatic carcinoma of the stomach or GEJ, who have received one prior chemotherapy regimen |
| Interventions | Arm A: alvocidib + irinotecan Arm B: irinotecan |
| Outcomes | Primary endpoint: ORR |
| Notes | No publication available |
NCT01579578.
| Methods | Multicentre double‐blind placebo‐controlled phase 2a RCT |
| Participants | Patients with histological diagnosis of gastric adenocarcinoma (including adenocarcinoma of the gastro‐oesophageal junction), who have radiologically confirmed progression following 1st line fluoropyrimidine and platinum based treatment for metastatic gastric cancer |
| Interventions | Arm A: AZD8931 plus paclitaxel Arm B: placebo plus paclitaxel |
| Outcomes | Primary endpoint: change in tumour size at 8 weeks Secondary endpoints: PFS, OS, ORR, DCR and safety |
| Notes | No publication available. Prermaturely terminated. |
NCT01813253.
| Methods | Multinational open‐label phase 3 RCT |
| Participants | Participants with EGFR overexpressed advanced gastric or gastroesophageal junction cancer, who experienced disease progression during or within 6 months after the last dose of fluoropyrimidine/platinum‐containing first line therapy |
| Interventions | Arm A: irinotecan plus nimotuzumab Arm B: irinotecan |
| Outcomes | Primary endpoint: OS Secondary endpoints: PFS, ORR, DCR and toxicity |
| Notes | No publication available. The study was terminated. |
Nishikawa 2015b.
| Methods | Phase 2 RCT |
| Participants | Patients with AGC who confirmed disease progression by imaging after the first‐line therapy with S1 or SP |
| Interventions | Arm A: irinotecan Arm B: paclitaxel Arm C: irinotecan plus S‐1 Arm D: paclitaxel plus S‐1 |
| Outcomes | Primary endpoint: OS Secondary endpoints: PFS, ORR and safety |
| Notes | Eligibility for inclusion in the review cannot be assessed on the published abstract. |
Shah 2019.
| Methods | Open‐label Phase 2 RCT |
| Participants | Patients with unresectable or recurrent gastric or gastroesophageal adenocarcinoma who progressed on at least 1 prior systemic therapy or line of treatment for unresectable/metastatic disease |
| Interventions | Arm A: andecaliximab + nivolumab Arm B: nivolumab |
| Outcomes | primary endpoint: ORR Secondary endpoints: PFS, OS and occurrence of adverse events |
| Notes | Eligibility for inclusion in the review cannot be assessed on the published abstract. NCT02864381 |
Wang 2017.
| Methods | RCT |
| Participants | Patients with advanced or metastatic gastric cancer after failure of first‐line chemotherapy |
| Interventions | Arm A: docetaxel plus apatinib Arm B: docetaxel |
| Outcomes | Primary endpoint: PFS Secondary endpoints: DCR, OS and ORR |
| Notes | Eligibility for inclusion in the review cannot be assessed on the published abstract. |
Yeh 2012.
| Methods | Open‐label Phase 2 RCT |
| Participants | Patients with radiologically confirmed gastric or gastro‐esophageal junction adenocarcinoma, who had progressed following 1 prior line of chemotherapy |
| Interventions | Arm A: AUY922 Arm B: docetaxel Arm C: irinotecan |
| Outcomes | Primary endpoint: PFS Secondary endpoints: OS, ORR, safety and tolerability |
| Notes | Eligibility for inclusion in the review cannot be assessed on the polished abstract. |
Zhao 2019.
| Methods | Multicentre open‐label Phase 2 RCT |
| Participants | Patients with pathological diagnosis of gastric cancer or gastroesophageal junction adenocarcinoma who failed after the first line treatment of 5‐Fu and platinum or relapsed half year after using 5‐Fu and platinum as postoperative adjuvant chemotherapy |
| Interventions | Arm A: paclitaxel + raltitrexed Arm B: paclitaxel |
| Outcomes | PFS |
| Notes | Considered eligible on the published abstract and to be included in the review at future update NCT02072317 |
CDDP: cisplatin; GEJ: gastroesophageal junction; IHC: Immunohistochemistry; ITT: intention‐to‐treat;LNT: linear no‐threshold model; ORR: overall response rate;OS: overall survival; PFS: progression‐free survival; QoL: quality of life; RCT: randomised controlled trial; RR: response rate; TRR: tumour responses rate.
Characteristics of ongoing studies [ordered by study ID]
Aanur 2017.
| Study name | A phase 2, Fast Real‐time Assessment of Combination Therapies in Immuno‐ONcology study in participants with Advanced gastric cancer (FRACTION‐Gastric Cancer) |
| Methods | Open‐label, adaptive phase 2 RCT |
| Participants | Patients with advanced GC or gastroesophageal junction (GEJ) cancer |
| Interventions | Nivolumab and ipilimumab vs nivolumab and relatlimab vs nivolumab and BMS‐986205 |
| Outcomes | Primary endpoints: ORR, DOR and PFS at 24 weeks Secondary endpoint: safety |
| Starting date | November 2016 |
| Contact information | Praveen Aanur, Fox Chase Cancer Center |
| Notes | NCT02935634 |
Al‐Batran 2020.
| Study name | Ramucirumab Plus Irinotecan / Leucovorin / 5‐FU Versus Ramucirumab Plus Paclitaxel in Patients With Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction, Who Failed One Prior Line of Palliative Chemotherapy ‐ The Phase II/III RAMIRIS Study |
| Methods | Open‐label Phase 2/3 RCT |
| Participants | Patients with metastatic or locally advanced gastric adenocarcinoma including adenocarcinoma of OGJ, who progressed during or within 6 months of first‐line platinum and fluoropyrimidine doublet with or without anthracycline or docetaxel (Phase 2) or who progressed during or within 6 months of first‐line platinum, fluoropyrimidine‐containing therapy and have received a taxane with the first‐line or during their adjuvant or neoadjuvant therapy or both (Phase 3). |
| Interventions | Arm A: FOLFIRI + Ramucirumab Arm B: Paclitaxel + Ramucirumab |
| Outcomes | Primary endpoints: OS rate, OS, ORR Secondary endpoints: PFS, ORR, DCR, toxicity and QoL |
| Starting date | May 2017 |
| Contact information | Sylvie Lorenzen, sylvie.lorenzen@mri.tum.de |
| Notes |
EUCTR2015‐000897‐36.
| Study name | A prospective, multicentre, double‐blind, randomized, placebo‐controlled, phase 3 study to evaluate efficacy and safety of masitinib with irinotecan in patients with advanced‐stage esophagogastric adenocarcinoma who have relapsed after first‐line chemotherapy |
| Methods | Multicentre double‐blind placebo‐controlled phase 3 RCT |
| Participants | Patient with histologically‐ or cytologically‐ advanced‐stage, recurrent esophagogastric adenocarcinoma, who have failed to one prior cycle cancer therapy |
| Interventions | Arm A: masitinib + irinotecan Arm B: placebo + irinotecan |
| Outcomes | Primary endpoint: OS Secondary endpoint: PFS, TTP, ORR, DCR, QoL and safety |
| Starting date | April 2016 |
| Contact information | Laurent Guy, AB science, laurent.guy@ab‐science.com |
| Notes |
EUCTR2015‐001605‐14.
| Study name | A Randomised, double‐blind, placebo‐controlled, multi‐centre phase II study to assess the efficacy and safety of second‐line Olaparib in combination with paclitaxel, in Western patients with advanced gastric and gastro‐oesophageal junction cancer |
| Methods | Multicentre double‐blind placebo‐controlled phase 2 RCT |
| Participants | Patients with advanced gastric or gastro‐oesophageal junction cancer (HER2 positive or negative) which has progressed following first‐line treatment |
| Interventions | Arm A: olaparib + paclitaxel Arm B: placebo + paclitaxel |
| Outcomes | Primary endpoint: OS Secondary endpoints: PFS, OS in ATM‐negative participants, ORR, QoL, TTR and DOR |
| Starting date | July 2015 |
| Contact information | Ye mong To, The Royal Marsden NHS Foundation Trust, yemong.to@rmh.nhs.uk |
| Notes |
EUCTR2018‐002374‐46.
| Study name | A randomised phase II trial assessing REGorafenib combined with IRInotecan as second‐line treatment in patients with metastatic gastro‐oesophageal adenocarcinomas |
| Methods | Open‐label phase 2 RCT |
| Participants | Patients with gastro‐oesophageal adenocarcinomas, GOJ (Siewert II and III) and gastric adenocarcinomas |
| Interventions | Arm A: regorafenib and irinotecan Arma B: irinotecan |
| Outcomes | Primary outcome: OS Secondary outcomes: OS rate, PFS, PFS rate, ORR, DCR, safety, QoL |
| Starting date | First received 10 October 2018 |
| Contact information | Laure Monard, UNICANCER |
| Notes |
Hirata 2020.
| Study name | WJOG10617G Randomized phase II trial of weekly paclitaxel + ramucirumab versus weekly nab‐paclitaxel + ramucirumab for unresectable advanced or recurrent gastric/esophagogastric junction adenocarcinoma with peritoneal dissemination refractory to first‐line therapy including a fluoropyrimidine (P‐SELECT) |
| Methods | Multicentre open‐label Phase 2 RCT |
| Participants | Patients with either unresectable or recurrent gastric or esophagogastric junction adenocarcinoma with peritoneal metastasis. |
| Interventions | Arm A: paclitaxel + ramucirumab Arm B: Nab‐paclitaxel + ramucirumab |
| Outcomes | Primary endpoint: OS Secondary endpoints: PFS, ORR, DCR, ascities response/control, TTF, QoL and toxicity |
| Starting date | October 2018 |
| Contact information | Naomi Yukawa, Keio University Hospital, yukawa703@keio.jp |
| Notes |
Manjii 2018.
| Study name | A Phase Ib/II, open‐label, multicenter, randomized, umbrella study evaluating the efficacy and safety of multiple immunotherapy‐based treatment combinations in patients with locally advanced unresectable or metastatic gastric or gastroesophageal junction cancer (Morpheus‐Gastric Cancer) |
| Methods | Multicentre open‐label phase 1b/2 RCT |
| Participants | 2 cohorts of patients will be enrolled: advanced unresectable or metastatic GC who have not received prior chemotherapy or have progressed on platinum‐ or fluoropyrimidine‐based chemotherapy |
| Interventions | Arm A: ramucirumab + paclitaxel Arm A: atezolizumab + cobimetinib Arm B: atezolizumab + PEGylated recombinant human hyaluronidase (PEGPH20) Arm C: atezolizumab + BL‐8040 Arm D: atezolizumab + linagliptin |
| Outcomes | Primary endpoints: investigator‐assessed ORR and safety Secondary endpoints: PFS, OS, DCR and DOR |
| Starting date | October 2017 |
| Contact information | Gulam Abbas Manji, Columbia University Medical Center/ New York‐Presbyterian Hospital |
| Notes | NCT03281369 |
NCT00509964.
| Study name | A randomized phase II trial of irinotecan monotherapy versus irinotecan, leucovorin and 5‐FU (ILF) combination chemotherapy in patients with advanced gastric cancer failing prior chemotherapy |
| Methods | Phase 2 RCT |
| Participants | Patients with inoperable, recurrent or metastatic histologically‐confirmed gastric cancer, who failed after one or more prior chemotherapy for advanced disease |
| Interventions | Arm A: irinotecan Arm B: irinotecan + leucovorin + 5FU |
| Outcomes | Primary endpoint: RR Secondary endpoint: safety |
| Starting date | May 2007 |
| Contact information | Dong Bok Shin, MD, PhD hematoma@gilhospital.com |
| Notes |
NCT01836120.
| Study name | A study of raltitrexed plus docetaxel versus docetaxel as second‐line chemotherapy in subjects with gastric cancer |
| Methods | Double‐blind RCT |
| Participants | Patients with histologically‐ or cytologically‐confirmed gastric cancer with first‐line chemotherapy failure (required containing 5‐fluorouracil) |
| Interventions | Arm A: raltitrexed plus docetaxel Arm B: docetaxel |
| Outcomes | Primary endpoint: PFS Secondary endpoints: ORR and OS |
| Starting date | April 2013 |
| Contact information | Not provided |
| Notes |
NCT02401971.
| Study name | Irinotecan plus thalidomide in second line advanced gastric cancer: a multicenter, randomized, controlled and prospective trial |
| Methods | Open‐label RCT |
| Participants | Patients with diagnosis of advanced gastric cancer |
| Interventions | Arm A: thalidomide plus irinotecan Arm B: irinotecan |
| Outcomes | Primary endpoint: TTP Secondary endpoint: CRR and OS |
| Starting date | August 2014 |
| Contact information | Wang Jufeng,Henan Cancer Hospital, 13783583966@163.com |
| Notes |
NCT02409199.
| Study name | A randomized, multicenter study to evaluate the efficacy and safety of apatinib versus docetaxel in patients with previously treated locally advanced or metastatic gastric cancer, including adenocarcinoma of the gastroesophageal junction |
| Methods | Open‐label RCT |
| Participants | Patients with advanced gastric cancer |
| Interventions | Arm A: apatinib Arm B: docetaxel |
| Outcomes | Primary endpoint: PFS Secondary endpoints: OS, ORR, DCR, QoL and safety |
| Starting date | June 2015 |
| Contact information | Liu Tianshu, Doctor, Zhongshan Hospital Affiliated to Fudan University, liu.tianshu@zs-hospital.sh.cn |
| Notes |
NCT02461407.
| Study name | A randomized, double‐blind, placebo‐controlled, multicenter clinical trial to compare the efficacy and safety of anlotinib versus placebo in patients with gastric cancer(ALTER0503) |
| Methods | Double‐blind placebo‐controlled multi‐centre RCT |
| Participants | Pathologically‐confirmed advanced gastric adenocarcinoma (including gastroesophageal junction adenocarcinoma) |
| Interventions | Arm A: anlotinib Arm B: placebo |
| Outcomes | Primary endpoint: OS Seconary endpoints: PFS, ORR and DCR |
| Starting date | June 2015 |
| Contact information | Ruihua Xu, Doctor, xurh@sysucc.org.cn |
| Notes |
NCT02485015.
| Study name | The randomized, controlled, multicenter clinical trial of apatinib plus cik as the third line therapy for patients with advanced gastric cancer |
| Methods | Open‐label phase 2 RCT |
| Participants | Patients with advanced gastric cancer |
| Interventions | Arm A: apatinib Arm B: apatinib plus cytokine‐induced‐killer cells |
| Outcomes | Primary endpoints: OS Seconary endpoints: DFS and toxicity |
| Starting date | June 2015 |
| Contact information | Not provided |
| Notes |
NCT02596256.
| Study name | Apatinib plus docetaxel versus docetaxel as second‐line treatment in advanced gastric cancer stage II randomized controlled clinical studies |
| Methods | Open‐label RCT |
| Participants | Heavily pretreated patients with advanced gastric cancer |
| Interventions | Arm A: apatinib Arm B: docetaxel |
| Outcomes | Primary endpoint: PFS |
| Starting date | April 2016 |
| Contact information | Dai, Guanghai, Department Director, Chinese PLA General Hospital |
| Notes |
NCT02632201.
| Study name | A clinical study of adoptive cellular immunotherapy using pluripotent killer t cells expressing antibodies for human epidermal growth factor receptor‐2 (HER2) in treating patients with HER2‐positive advanced gastric cancer with liver metastasis |
| Methods | Open‐label RCT |
| Participants | Patients with advanced Her2 high‐expressing gastric cancer with liver metastasis |
| Interventions | Arm A: PIK‐HER2 Arm B: DC‐PMAT |
| Outcomes | Primary endpoint: OS Secondary endpoints: PFS and QoL |
| Starting date | September 2015 |
| Contact information | Qijun Qian, PHD, Eastern Hepatobiliary Surgery Hospital, qianqj@sino-gene.cn |
| Notes |
NCT02862561.
| Study name | Clinical study using precision cell immunotherapy combined with chemotherapy in advanced gastric cancer |
| Methods | Open‐label phase 1/2 RCT |
| Participants | Advanced gastric cancer |
| Interventions | Arm A: cisplatin plus 5FU Arm B: cisplatin plus 5FU plus precision cells (DC) |
| Outcomes | Primary endpoints: OS and PFS Secondary endpoint: QoL |
| Starting date | August 2016 |
| Contact information | Naiyan Han, Shanghai International Medical Center |
| Notes |
NCT02873520.
| Study name | Clinical study using precision cell immunotherapy combined with chemotherapy in advanced gastric cancer |
| Methods | Open‐label RCT |
| Participants | Advanced gastric cancer patients |
| Interventions | Arm A: cisplatin Arm B: cisplatin + Precision Cell Immunotherapy |
| Outcomes | Primary endpoints: OS, PFS and QoL |
| Starting date | August 2016 |
| Contact information | Bi Wang, Ningbo No.5 Hospital (Ningbo Cancer Hospital), biwang0218@126.com |
| Notes |
NCT02898077.
| Study name | A randomized, multicenter, double‐blind, placebo‐controlled, phase 3 study of weekly paclitaxel with or without ramucirumab (IMC‐1121B) in patients with advanced gastric or gastroesophageal junction adenocarcinoma, refractory to or progressive after first‐line therapy with platinum and fluoropyrimidine |
| Methods | Double‐blind placebo‐controlled phase 3 RCT |
| Participants | Patients with histopathologically‐ or cytologically‐confirmed diagnosis of gastric or gastroesophageal junction (GEJ) adenocarcinoma, who have experienced documented objective radiographic or symptomatic disease progression during first‐line therapy |
| Interventions | Arm A: ramucirumab + paclitaxel Arm B: placebo + paclitaxel |
| Outcomes | Primary endpoint: OS Secondary endpoints: PFS, TTP, ORR, DOR and QoL |
| Starting date | March 2017 |
| Contact information | Eli Lilly and Company |
| Notes |
NCT03030937.
| Study name | A randomized, parallel control, exploratory trial to compare apatinib plus irinotecan versus single irinotecan as second‐line treatment in subjects with advanced gastric cancer or adenocarcinoma of esophagogastric junction |
| Methods | |
| Participants | Patients with advanced gastric cancer or adenocarcinoma of oesophagogastric junction who failed one lines of chemotherapy. |
| Interventions | Arm A: apatinib + irinotecan Arm B: irinotecan |
| Outcomes | Primayr endpoints: PFS, adverse event Secondary endpoints: ORR, DCR, OS and QoL |
| Starting date | Februrary 2017 |
| Contact information | Jianwei Yang, Fujian Cancer Hospital, swzcq62@163.com |
| Notes |
NCT03144843.
| Study name | A phase ii multicenter, randomized, double‐blind study of apatinib combined with paclitaxol versus placebo with paclitaxol as second line therapy for advanced gastric / esophagogastric junction adenocarcinoma with peritoneal metastasis |
| Methods | Multicenters double‐blind phase 2 RCT |
| Participants | Patients with histologically‐confirmed advanced or metastatic adenocarcinoma of gastric cancer(AGC) , including adenocarcinoma of the gastroesophageal junction, who have received one prior chemotherapy regimen for AGC |
| Interventions | Arm A: apatinib + paclitaxel Arm B: placebo + paclitaxel |
| Outcomes | Primary endpoint: PFS Secondary endpoints: OS, ORR, DCR and safety |
| Starting date | January 2017 |
| Contact information | Fenghua Wang, Professor, Sun Yat‐sen University |
| Notes |
NCT03223376.
| Study name | A phase III study to evaluate the efficacy and safety of fruquintinib in combination with paclitaxel versus paclitaxel alone in second line gastric cancer |
| Methods | Multicenter double‐blind, placebo‐controlled Phase 3 RCT |
| Participants | Patients with advanced gastric cancer who have progressed after first‐line standard chemotherapy |
| Interventions | Arm A: fruquintinib + paclitaxel Arm B: placebo + paclitaxel |
| Outcomes | Primary endpoint: OS Secondary endpoints: PFS, ORR, DCR and safety |
| Starting date | October 2017 |
| Contact information | Songhua Fan, MD, songhuaf@hmplglobal.com |
| Notes |
NCT03760822.
| Study name | Second‐line chemotherapy with ramucirumab +/‐ paclitaxel in elderly advanced gastric or gastroesophageal junction cancer patients (SOCRATE) |
| Methods | Open‐labelled phase 2 RCT |
| Participants | Patients with unresectable, locally advanced or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma |
| Interventions | Arm A: ramucirumab + paclitaxel Arm B: ramucirumab |
| Outcomes | Primary endpoints: survival rate at 6 months and QoL (assessed with EORTC QLQ‐ELD14) Secondary endpoints: QoL (assessed with EORTC QLQC30), OS, toxicity, time to treatment failure, PFS, ORR |
| Starting date | November 2018 |
| Contact information | Flore GEILLON, flore.geillon@u-bourgogne.fr |
| Notes |
NCT03959293.
| Study name | A Randomized Phase II Study Evaluating FOLFIRI + Durvalumab vs FOLFIRI + Durvalumab and Tremelimumab in Second‐line Treatment of Patients With Advanced Gastric or Gastro‐oesophageal Junction Adenocarcinoma |
| Methods | Open‐label Phase 2 RCT |
| Participants | Patients with advanced‐stage unresectable adenocarcinoma of the stomach or the GEJ who failed platinum‐based 1st line therapy with or without trastuzumab, recurred after surgery with neo‐adjuvant and/or adjuvant platinum‐based chemotherapy (within 6 months of the end of chemotherapy) or progressed during neo‐adjuvant and/or adjuvant platinum‐based chemotherapy |
| Interventions | Arm A: FOLFIRI + durvalumab Arm B: FOLFIRI + durvalumab + tremelimumab |
| Outcomes | Primary endpoint; PFS rate Secondary endpoints: PFS rate, OS, toxicity, TTP, PFS, best ORR and DCR |
| Starting date | July 2019 |
| Contact information | Daniel Gonzalez, daniel.gonzalez@u-bourgogne.fr |
| Notes |
NCT04294784.
| Study name | A Multi‐center, Open‐label, Randomized Controlled Clinical Study of Nab‐paclitaxel Plus SHR‐1210(PD‐1 Inhibitor)Versus Nab‐paclitaxel as Second‐line Treatment in Advanced or Recurrent Gastric and Esophagogastric Adenocarcinoma |
| Methods | Multicentre open‐label Phase 2 RCT |
| Participants | Patients with recurrent or metastatic gastric and esophagogastric adenocarcinoma who failed first‐line standard therapy excluding paclitaxel, docetaxel or any other similar medicine |
| Interventions | Arm A: Nab‐paclitaxel + SHR‐1210 (PD‐1 inhibitor) |
| Outcomes | Arm B: Nab‐paclitaxel |
| Starting date | May 2020 |
| Contact information | Xianglin Yuan, xlyuan1020@163.com |
| Notes |
NCT04342910.
| Study name | A Study of Camrelizumab (SHR‐1210) Combined With Apatinib Versus Paclitaxel or Irinotecan in Participants With Advanced Gastric/Gastroesophageal Junction Adenocarcinoma Progressed After First‐line Chemotherapy |
| Methods | Triple‐blinded Phase 3 RCT |
| Participants | Patients with metastatic or locally advanced, unresectable gastric or gastroesophageal junction adenocarcinoma who progressed on or after prior first‐line therapy containing any platinum/fluoropyrimidine or platinum/taxane doublet |
| Interventions | Arm A: apatinib + camrelizumab (SHR‐1210) Arm B: paclitaxel or Irinotecan |
| Outcomes | Primary endpoint: OS in PD‐L1 positive patients Secondary endpoints: OS, PFS, TTP, TTF, ORR, DOR, DCR, TTR, toxicity, dose intensity and pharmacokinetics |
| Starting date | June 2020 |
| Contact information | Jiangsu HengRui Medicine Co., Ltd. |
| Notes |
NCT04486651.
| Study name | A Randomized, Double‐blinded, Multicenter, Phase III Clinical Study of HX008 (Recombinant Humanized Anti‐PD‐1 Monoclonal Antibody Injection) Plus Irinotecan Versus Placebo Plus Irinotecan as Second‐line Treatment in Advanced Gastric Cancer |
| Methods | Multicentre double‐blinded Phase 3 RCT |
| Participants | Patients with locally advanced unresectable or metastatic adenocarcinoma of stomach or the esophagogastric junction who progressed during or after first‐line therapy containing platinum and/or fluoropyrimidine therapy |
| Interventions | Arm A: irinotecan + HX008 Arm B: irinotecan + placebo |
| Outcomes | Primary endpoints: OS in all participants and OS in participants with PD‐L1 CPS≥1 Secondary endpoints: PFS, ORR, DCR, DOR |
| Starting date | September 2020 |
| Contact information | Jing Huang, huangjingwg@163.com |
| Notes |
NCT04555304.
| Study name | A Randomized, Multicenter, Double‐Blind, Placebo‐Controlled, Phase 2 Study of Weekly Paclitaxel With or Without KH903 in Patients With Advanced Gastric or Gastroesophageal Junction Adenocarcinoma, Refractory to or Progressive After First‐Line Therapy With Platinum and Fluoropyrimidine |
| Methods | Multicentre double‐blinded placebo‐controlled Phase 2 RCT |
| Participants | Patients with unresectable, locally advanced or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma who progressed during first‐line therapy, or within 4 months after the last dose of first‐line therapy with any platinum or/and fluoropyrimidine doublet for unresectable or metastatic disease.Second line chemotherapy is suitable for paclitaxel |
| Interventions | Arm A: paclitaxel + KH903 Arm B: paclitaxel + placebo |
| Outcomes | Primary endpoint: PFS Secondary endpoints: ORR, DOR, DCR and toxicity |
| Starting date | September 2020 |
| Contact information | Yi Ba, bayi@timuch.com |
| Notes |
Sakai 2018.
| Study name | An intergroup phase III trial of ramucirumab plus irinotecan in third or more line beyond progression after ramucirumab for advanced gastric cancer |
| Methods | Open‐labelled phase 3 RCT |
| Participants | Patients with inoperable, locally advanced or metastatic adenocarcinoma of gastric or gastroesophageal junction |
| Interventions | Arm A: ramucirumab + irinotecan Arm B: irinotecan |
| Outcomes | Primary endpoint: OS Secondary endpoints: PFS, TTF, RR, DCR and adverse events |
| Starting date | Februrary 2017 |
| Contact information | Daisuke Sakai, Osaka University Graduate School of Medicine |
| Notes | UMIN000023065 |
Sjoquist 2017.
| Study name | Integrate II: A randomised phase 3 double‐blind placebo‐controlled study of regorafenib in refractory advanced gastro‐oesophageal cancer (AGOC)—an international study organized by the Australasian Gastrointestinal Trials Group (AGITG) |
| Methods | Double‐blind placebo‐controlled phase 3 RCT |
| Participants | Patients with refractory advanced gastro‐oesophageal cancer |
| Interventions | Arm A: regorafenib Arm B: placebo |
| Outcomes | Primary endpoint: OS Secondary endpoints: PFS, ORR, QoL and safety |
| Starting date | November 2016 |
| Contact information | Australasian Gastro‐Intestinal Trials Group |
| Notes | NCT02773524 |
ATM: ataxia‐telangiectasia mutated protein; CPS: combined positive score; DCR: disease control rate; DOR: duration of response; ORR: overall response rate;OS: overall survival; PFS: progression‐free survival; PS: performance score; QoL: quality of life; RCT: randomised controlled trial; RR: response rate; TTF: time to treatment failure; TTP: time to progression; TTR: time to response; vs: versus.
Differences between protocol and review
The original published protocol accepted both parallel‐group and cluster‐randomised studies, however, prior to commencing the literature search we decided to exclude cluster‐randomised studies to maintain the consistency in the unit of analysis. After literature search, no cluster‐randomised studies meeting our other selection criteria were identified. Although the published protocol defined the included studies to be randomised controlled trials (RCTs) comparing systemic therapy with another systemic therapy, placebo, best supportive care (BSC) or no treatment, we included Thuss‐Patience 2017 where participants in the control arm were allowed to have either docetaxel or paclitaxel, chosen by their treating physicians. Both docetaxel and paclitaxel belong to the taxane family of chemotherapy and they are suggested to have similar therapeutic efficacy with different toxicity profiles in advanced gastric cancer (Park 2006).
For 'Risk of bias' assessment, in addition to the domains predefined in the published protocol, we assessed for imbalances between the treatment arms as a source of confounder. Definition of both overall survival (OS) and progression‐free survival (PFS) varied across studies; some studies measured OS and PFS from the time of randomisation, while others from the time of commencement of intervention. As we were unable to obtain numerical results for all included studies based on a single definition of OS and PFS, both definitions were accepted in the final review for these outcomes.
Two additional subgroup analyses were undertaken in the final review: subgroup analysis by extent of disease to assess the impact of disease burden on survival outcomes and subgroup analysis by the types of biological therapy to acknowledge the difference in the patterns of response and progression between immunotherapy and biological therapy, which is not immunotherapy. Chemotherapy combined with biological therapy versus chemotherapy was added to the list of comparisons to perform subgroup analysis in the final review. Sensitivity analysis was performed only for OS and PFS and not serious adverse vents (SAEs) in the final review under the pre‐specified conditions in the published protocol at minimum. Sensitivity analysis excluding studies evaluating HER2‐targeted therapy in biomarker selected participants was performed in the final review.
The comparison of chemotherapy or biological therapy versus placebo, BSC or no treatment was not included in the final review, as this does not add new information to the findings achieved through the comparison between chemotherapy versus placebo, BSC or no treatment, and biological therapy versus placebo, BSC or no treatment.
Contributions of authors
Conceiving the protocol: Yoko Tomita
Designing the protocol: Yoko Tomita
Coordinating the protocol: Yoko Tomita
Designing search strategies: Yoko Tomita
Searching and selection of studies: Yoko Tomita, Rachael Chang
Writing the protocol: Yoko Tomita
Performing statistical analysis and overseeing the statistical methodology: Max Moldovan
Providing general advice on the protocol: Timothy Price, Amanda Townsend, Amy HC Hsieh
Sources of support
Internal sources
Department of Medical Oncology, The Queen Elizabeth Hospital, Adelaide, SA, Australia
External sources
No sources of support supplied
Declarations of interest
YT: none known.
RC: none known.
YY: none known.
MM: none known.
AH: none known.
AT: receives clinical trial grant funding from Amgen (for a study outside of the scope of this review). Amgen does not make any treatments included in this review. She is an academic member of the University of Adelaide.
TP: is a board member of the Australasian Gastro‐Intestinal Trials Group and an academic member of the University of Adelaide.
New
References
References to studies included in this review
Bang 2017 {published data only}
- Bang YJ, Xu RH, Chin K, Lee KW, Park SH, Rha SY, et al. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy (GOLD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncology 2017;18(12):1637-51. [PMID: 29103871 ] [DOI] [PubMed] [Google Scholar]
Hironaka 2013 {published data only}
- Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. Journal of Clinical Oncology 2013;31(35):4438-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kang 2017 {published data only}
- Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390(10111):2461-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kim 2015 {published data only}
- Kim JY, Ryoo HM, Bae SH, Kang BW, Chae YS, Yoon S, et al. Multi-center randomized phase II study of weekly docetaxel versus weekly docetaxel-plus-oxaliplatin as a second-line chemotherapy for patients with advanced gastric cancer. Anticancer Research 2015;35(6):3531-6. [PMID: ] [PubMed] [Google Scholar]
Lee 2017 {published data only}
- Lee KW, Kim BJ, Kim MJ, Han HS, Kim JW, Park YI, et al. A multicenter randomized phase II Study of docetaxel vs. docetaxel plus cisplatin vs. docetaxel plus S-1 as second-line chemotherapy in metastatic gastric cancer patients who had progressed after cisplatin plus Either S-1 or capecitabine. Cancer Research and Treatment 2017;49(3):706-16. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. Journal of Clinical Oncology 2013;31(26):3219-25. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ling 2018 {published data only}
- Ling S, Bo L. Efficacy and safety of second-line chemotherapy with EOX and FOLFIRI in advanced gastric cancer. Journal of Xi'an Jiaotong University (Medical Sciences) 2018;39(4):546-50. [DOI: 10.7652/jdyxb201804020] [DOI] [Google Scholar]
Makiyama 2018 {published data only}
- Makiyama A, Sagara K, Kawada J, Kashiwada T, Hosokawa A, Horie Y, et al. T-ACT (WJOG7112G): A randomized phase II study of weekly paclitaxel ± trastuzumab in patients with HER2-positive advanced gastric or gastro-esophageal junction cancer refractory to trastuzumab combined with fluoropyrimidine and platinum. Journal of Clinical Oncology 2018;36(15_suppl):4011. [DOI: 10.1200/JCO.2017.35.4_suppl.TPS218 ] [Google Scholar]
Pauligk 2017 {published data only}
- Pauligk C, Lorenzen S, Goetze T, Riera JK, Hegewisch SB, Seraphin J, et al. A randomized, double-blind, multi-center phase III study evaluating paclitaxel with and without RAD001 in patients with gastric or esophagogastric junction carcinoma who have progressed after therapy with a fluoropyrimidine/platinum-containing regimen (RADPAC). Annals of Oncology 2017;28(suppl_5):mdx369.054. [DOI: ] [Google Scholar]
Pavlakis 2016 {published data only}
- Martin AJ, Gibbs E, Sjoquist K, Pavlakis N, Simes J, Price T, et al. Health‑related quality of life associated with regorafenib treatment in refractory advanced gastric adenocarcinoma. Gastric Cancer 2018;21((3)):473-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Pavlakis N, Sjoquist KM, Martin AJ, Tsobanis E, Yip S, Kang YK, et al. Regorafenib for the treatment of advanced gastric cancer (INTEGRATE): a multinational placebo-controlled phase II trial. Journal of Clinical Oncology 2016;34(23):2728-35. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2018 {published data only}
- Shah MA, Shitara K, Lordick F, Bang YJ, Tebbutt NC, Metges JP, et al. The BRIGHTER trial: A phase 3 randomized double-blind study of napabucasin (NAPA) plus paclitaxel (PTX) versus placebo (PBO) plus PTX in patients (pts) with pretreated advanced gastric and gastroesophageal junction (GEJ) adenocarcinoma. Journal of Clinical Oncology 2018;36(15_suppl):4010. [DOI: 10.1200/JCO.2018.36.15_suppl.4010] [DOI] [Google Scholar]
Shitara 2018 {published data only}
- Shitara K, Özgüroğlu M, Bang YJ, Bartolomeo MD, Mandalà M, Ryu MH, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018;392(10142):123-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shitara 2018b {published data only}
- Shitara K, Doi T, Dvorkin M, Mansoor W, Arkenau HT, Prokharau A, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncology 2018;19(11):1437-48. [PMID: 30355453] [DOI] [PubMed] [Google Scholar]
Thuss‐Patience 2011 {published data only}
- Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer--a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). European Journal of Cancer 2011;47(15):2306-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Thuss‐Patience 2017 {published data only}
- Thuss-Patience PC, Shah MA, Ohtsu A, Van Cutsem E, Ajani JA, Castro H, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncology 2017;18(5):640-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wilke 2014 {published data only}
- Al-Batran SE, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, et al. Quality-of-life and performance status results from the phase III RAINBOW study of ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated gastric or gastroesophageal junction adenocarcinoma. Annals of Oncology 2016;27((4)):673-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncology 2014;15(11):1224-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yi 2012 {published data only}
- Yi JH, Lee J, Lee J, Park SH, Park JO, Yim DS, et al. Randomised phase II trial of docetaxel and sunitinib in patients with metastatic gastric cancer who were previously treated with fluoropyrimidine and platinum. British Journal of Cancer 2012;106(9):1469-74. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ajani 2010 {published data only}
- Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. Journal of Clinical Oncology 2010;28(9):1547-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Al‐Batran 2013 {published data only}
- Al-Batran SE, Pauligk C, Homann N, Hartmann JT, Moehler M, Probst S, et al. The feasibility of triple-drug chemotherapy combination in older adult patients with oesophagogastric cancer: a randomised trial of the Arbeitsgemeinschaft Internistische Onkologie (FLOT65+). European Journal of Cancer 2013;49(4):835-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bando 2016 {published data only}
- Bando H, Yamada Y, Tanabe S, Nishikawa K, Gotoh M, Sugimoto N, et al. Efficacy and safety of S-1 and oxaliplatin combination therapy in elderly patients with advanced gastric cancer. Gastric Cancer 2016;19(3):919-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bang 2015 {published data only}
- Bang YJ, Im SA, Lee KW, Cho JY, Song EK, Lee KH, et al. Randomized, double-blind phase II trial with prospective classification by ATM protein level to evaluate the efficacy and tolerability of olaparib plus paclitaxel in patients with recurrent or metastatic gastric cancer. Journal of Clinical Oncology 2015;33(33):3858-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bang 2017b {published data only}
- Bang YJ, Cho JY, Kim YH, Kim JW, Di Bartolomeo M, Ajani JA, et al. Efficacy of sequential ipilimumab monotherapy versus best supportive care for unresectable locally advanced/metastatic gastric or gastroesophageal junction cancer. Clinical Cancer Research 2017;23(19):5671-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bang 2018 {published data only}
- Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, et al. Phase 3, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment for patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Annals of Oncology 2018;29(10):2052-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Belloni 2015 {published data only}
- Belloni P, Cozzi C, Toniolo D, Zannier F, Candido P, Della Torre S, et al. Second-line chemotherapy in advanced gastric cancer patients. Annals of Oncology 2015;26(suppl_6):Pages vi. [DOI: 10.1093/annonc/mdv344.44] [DOI] [Google Scholar]
Boku 2009 {published data only}
- Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncology 2009;10(11):1063-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chon 2009 {published data only}
- Chon HJ, Rha SY, Im CK, Kim C, Hong MH, Kim HR, et al. Docetaxel versus paclitaxel combined with 5-FU and leucovorin in advanced gastric cancer: combined analysis of two phase II trials. Cancer Research and Treatment 2009;41(4):196-204. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2014 {published data only}
- Cohen SJ, Feng Y, Catalano PJ, Mitchell EP, O'Dwyer PJ, Lubner SJ, et al. Randomized phase II study of paclitaxel with or without the anti-IGF-IR antibody cixutumumab (IMC-A12) as second-line treatment for patients with metastatic esophageal or GE junction cancer. Journal of Clinical Oncology 2014;32(15_suppl):4020. [DOI: 10.1200/jco.2014.32.15_suppl.4020] [DOI] [Google Scholar]
Cui 2015 {published data only}
- Cui J, Li L, Wang C, Jin H, Yao C, Wang Y, et al. Combined cellular immunotherapy and chemotherapy improves clinical outcome in patients with gastric carcinoma. Cytotherapy 2015;17.(7):979-88. [PMID: ] [DOI] [PubMed] [Google Scholar]
Enzinger 2016 {published data only}
- Enzinger PC, Burtness BA, Niedzwiecki D, Ye X, Douglas K, Ilson DH, et al. CALGB 80403 (Alliance)/E1206: a randomized phase II study of three chemotherapy regimens plus cetuximab in metastatic esophageal and gastroesophageal junction cancers. Journal of Clinical Oncology 2016;34(23):2736-42. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ford 2014 {published data only}
- Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncology 2014;15(1):78-86. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fuchs 2014 {published data only}
- Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383(9911):31-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fujii 2011 {published data only}
- Fujii M, Kim YH, Satoh T, Hosaka H, Kim T, Tsuji A, et al. Randomized phase III study of S-1 alone versus S-1 plus docetaxel (DOC) in the treatment for advanced gastric cancer (AGC): the START trial update. Journal of Clinical Oncology 2011;29(15_suppl):4016. [DOI: 10.1200/jco.2011.29.15_suppl.4016] [DOI] [Google Scholar]
Furue 1985 {published data only}
- Furue H, Uchino H, Orita K, Kimura T, Goto Y, Kondo T, et al. Clinical evaluation of schizophyllan (SPG) in advanced gastric cancer (the second report)--a randomized controlled study. Gan To Kagaku Ryoho 1985;12(6):1272-7. [PMID: ] [PubMed] [Google Scholar]
Fushida 2016 {published data only}
- Fushida S, Kinoshita J, Kaji M, Oyama K, Hirono Y, Tsukada T, et al. Paclitaxel plus valproic acid versus paclitaxel alone as second- or third-line therapy for advanced gastric cancer: a randomized Phase II trial. Drug Design, Development and Therapy 2016;10:2353–8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goto 1989 {published data only}
- Goto Y, Toyota T, Asaki S, Satoh J, Kikuchi T, Koizumi M, et al. Randomized-controlled study of treatment with UFT-MMC or UFT-ACR in advanced gastric cancer. Tohoku Study Group of Cancer Treatment for the Digestive Organs. Gan To Kagaku Ryoho 1989;16(6):2227-33. [PMID: ] [PubMed] [Google Scholar]
Guo 2019 {published data only}
- Guo Y, Tang J, Huang XE, Cao J. Efficacy and toxicity of apatinib combined with or without chemotherapy for patients with advanced or metastatic chemotherapy-refractory gastric adenocarcinoma: a prospective clinical study. Medicine (Baltimore) 2019;98(6):e13908. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higuchi 2014 {published data only}
- Higuchi K, Tanabe S, Shimada K, Hosaka H, Sasaki E, Nakayama N, et al. Biweekly irinotecan plus cisplatin versus irinotecan alone as second-line treatment for advanced gastric cancer: a randomised phase III trial (TCOG GI-0801/BIRIP trial). European Journal of Cancer 2014;50(8):1437-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
Inokuchi 2007 {published data only}
- Inokuchi M, Yamashita T, Yamada H, Kojima K, Sekita Y, Kawano T, et al. Second-line chemotherapy in gastric cancer following S-1 with CPT-11 chemotherapy performed as clinical trial. Gan To Kagaku Ryoho 2007;34(6):875-9. [PMID: ] [PubMed] [Google Scholar]
Janjigian 2018 {published data only}
- YJanjigian YY, Bendell J, Calvo E, Kim JW, PA Ascierto, Sharma P, et al. CheckMate-032 Study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. Journal of Clinical Oncology 2018;36(28):2836-44. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2012 {published data only}
- Kang JH, Lee SI, Lim DH, Park KW, Oh SY, Kwon HC, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. Journal of Clinical Oncology 2012;30(13):1513-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kelly 2020 {published data only}
- Kelly RJ, Lee J, Bang YJ, Almhanna K, Blum-Murphy M, Catenacci DVT, et al. Safety and Efficacy of Durvalumab and Tremelimumab Alone or in Combination in Patients with Advanced Gastric and Gastroesophageal Junction Adenocarcinoma. Clinical Cancer Research 2020;26(4):846-854. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klein 1992 {published data only}
- Klein HO, Buyse MB, Wils JA. Prospective randomized trial using 5-fluorouracil, adriamycin and methotrexate (FAMTX) versus FAM for treatment of advanced gastric cancer. Onkologie 1992;15:364–7. [Google Scholar]
Koizumi 2001 {published data only}
- Koizumi W. Capecitabine (Ro09-1978) for therapy of advanced and recurrent gastric cancer. Nihon Rinsho 2001 Apr;59(Suppl 4):398-403. [PMID: ] [PubMed] [Google Scholar]
Kurokawa 2014 {published data only}
- Kurokawa Y, Sugimoto N, Miwa H, Tsuda M, Nishina S, Okuda H, et al. Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1). British Journal of Cancer 2014 Mar;110:1163-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2012 {published data only}
- Lee S, Kang JH, Lim DH, Park KW, Oh SY, Hwang IG, et al. Combined analysis of randomized controlled trial (RCT) and patient-preference trial (PPT) evaluating second-line chemotherapy (SLC) in advanced gastric cancer (AGC). Journal of Clinical Oncology 2012;30(15_suppl):4064. [DOI: 10.1200/jco.2012.30.15_suppl.4064 ] [Google Scholar]
Lee 2018 {published data only}
- Lee KW, Maeng CH, Kim TY, Zang DY, Kim YH, Hwang IG, et al. A phase III study to compare the efficacy and safety of paclitaxel versus irinotecan in patients with metastatic or recurrent gastric cancer who failed in first-line therapy (KCSG ST10-01). Oncologist 2018;23:1-12. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2016 {published data only}
- Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. Journal of Clinical Oncology 2016;34(13):1448-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Li 2016b {published data only}
- Li JW, Huang CZ, Yuan JH, Chen QH. Comparison of efficacy of modified EOX and FOLFIRI regimens in treatment of metastatic gastric cancer. [Chinese]. World Chinese Journal of Digestology 2016;24(12):1866-73. [Google Scholar]
Li 2018 {published data only}
- Li J, Jia Y, Qin Y. Clinical effect of apatinib combined with chemotherapy for advanced gastric cancer as second-line and above treatment. [Chinese]. Chinese Journal of Cancer Biotherapy 2018;25(11):1135-9. [Google Scholar]
Loehrer 1994 {published data only}
- Loehrer PJ, Harry D, Chlebowski RT. 5-fluorouracil vs. epirubicin vs. 5-fluorouracil plus epirubicin in advanced gastric carcinoma. Investigational New Drugs 1994;12(1):57-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lorenzen 2015 {published data only}
- Lorenzen S, Riera Knorrenschild J, Haag GM, Pohl M, Thuss-Patience P, Bassermann F, et al. Lapatinib versus lapatinib plus capecitabine as second-line treatment in human epidermal growth factor receptor 2-amplified metastatic gastro-oesophageal cancer: a randomised phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. European Journal of Cancer 2015;5(5):569-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Luelmo 2008 {published data only}
- Luelmo S, Polee M, Van Bochove A, Pruijt H, Ouwerkerk J, Sleeboom H, et al. Randomized phase II study of cisplatin and high-dose 5-fluorouracil/leucovorin or paclitaxel and high-dose 5-fluorouracil/leucovorin in locally advanced or metastatic gastric cancer and adenocarcinomas of the gastrooesophageal junction. Annals of Oncolgy 2008;19(suppl_8):viii173-viii174. [DOI: 10.1093/annonc/mdn509] [DOI] [Google Scholar]
Maruta 2007 {published data only}
- Maruta F, Ishizone S, Hiraguri M, Fujimori Y, Shimizu F, Kumeda S, et al. A clinical study of docetaxel with or without 5'DFUR as a second-line chemotherapy for advanced gastric cancer. Medical Oncology 2007;24(1):71-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Moehler 2016 {published data only}
- Moehler M, Gepfner-Tuma I, Maderer A, Thuss-Patience PC, Ruessel J, Hegewisch-Becker S, et al. Sunitinib added to FOLFIRI versus FOLFIRI in patients with chemorefractory advanced adenocarcinoma of the stomach or lower esophagus: a randomized, placebo-controlled phase IIAIO trial with serum biomarker program. BMC Cancer 2016;15:699. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morita 2007 {published data only}
- Morita S, Baba H, Tsuburaya A, Takiuchi H, Matsui T, Maehara Y, et al. A randomized phase II selection trial in patients with advanced/recurrent gastric cancer: Trial for Advanced Stomach Cancer (TASC). Japanese Journal of Clinical Oncology 2007;37(6):469-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Murad 1993 {published data only}
- Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 1993;72(1):37-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Murakami 2001 {published data only}
- Murakami M. MTX/5FU sequential therapy for advanced and recurrent gastric cancer. Nihon Rinsho 2001;59(Suppl 4):368-74. [PMID: ] [PubMed] [Google Scholar]
Nakanishi 2016 {published data only}
- Nakanishi K, Kobayashi D, Mochizuki Y, Ishigure K, Ito S, Kojima H, et al. Phase II multi-institutional prospective randomized trial comparing S-1 plus paclitaxel with paclitaxel alone as second-line chemotherapy in S-1 pretreated gastric cancer (CCOG0701). International Journal of Clinical Oncology 2016;21(3):557-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nakano 1999 {published data only}
- Nakano H, Namatame K, Nemoto H, Motohashi H, Nishiyama K, Kumada K. A multi-institutional prospective study of lentinan in advanced gastric cancer patients with unresectable and recurrent diseases: effect on prolongation of survival and improvement of quality of life. Kanagawa Lentinan Research Group. Hepatogastroenterology 1999;46(28):2662-8. [PMID: ] [PubMed] [Google Scholar]
Nishikawa 2015 {published data only}
- Nishikawa K, Fujitani K, Inagaki H, Akamaru Y, Tokunaga S, Takagi M, et al. Randomised phase III trial of second-line irinotecan plus cisplatin versus irinotecan alone in patients with advanced gastric cancer refractory to S-1 monotherapy: TRICS trial. European Journal of Cancer 2015 May;51(7):808-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nishikawa 2015c {published data only}
- Nishikawa K, Yoshino S, Morita S Takahashi T, Sakata K, Nagao J, et al. A randomized phase III study of S-1 alone versus S-1 plus immunomodulator lentinan for unresectable or recurrent gastric cancer (JFMC36-0701). European Journal of Cancer 2015 Sep;51:S442. [DOI] [PubMed] [Google Scholar]
Nishina 2016 {published data only}
- Nishina T, Boku N, Gotoh M, Shimada Y, Hamamoto Y, Yasui H, et al. Randomized phase II study of second-line chemotherapy with the best available 5-fluorouracilregimen versus weekly administration of paclitaxel in far advanced gastric cancer with severe peritoneal metastases refractory to 5-fluorouracil-containing regimens (JCOG0407). Gastric Cancer 2016 Jul;19(3):902-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ochiai 1992 {published data only}
- Ochiai T, Isono K, Suzuki T, Koide Y, Gunji Y, Nagata M, et al. Effect of immunotherapy with lentinan on patients' survival and immunological parameters in patients with advanced gastric cancer: results of a multi-centre randomized controlled study. International Journal of Immunotherapy 1992;8(3):161-9. [Google Scholar]
Ohtsu 2013 {published data only}
- Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung HC, Pan HM, et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. Journal of Clinical Oncology 2013;31(31):3935-43. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Popov 2008 {published data only}
- Popov IP, Jelić SB, Krivokapić ZV, Jezdić SD, Pesko PM, Micev MT, et al. Bimonthly 24 h infusion of high-dose 5-fluorouracil vs EAP regimen in patients with advanced gastric cancer. a randomized phase II study. Medical Oncology 2008;25(1):73-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pyrhönen 1995 {published data only}
- Pyrhönen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. British Journal of Cancer 1995;71(3):587-91. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Qiao 2019 {published data only}
- Qiao G, Wang X, Zhou L, Zhou X, Song Y, Wang S, et al. Autologous Dendritic Cell-Cytokine Induced Killer Cell Immunotherapy Combined with S-1 Plus Cisplatin in Patients with Advanced Gastric Cancer: A Prospective Study. Clin Cancer Res 2019;25(5):1494-1504. [PMID: ] [DOI] [PubMed] [Google Scholar]
Qu 2019 {published data only}
- Qu SX, Qiu JN, Zhang YD, Liu YM, Du C, Qi XH, et al. Comparison between paclitaxel liposome combined with capecitabine and DCF regimen in the treatment of advanced gastric cancer. Chinese Journal of Cancer Prevention and Treatment 2019;26(11):790-793. [Google Scholar]
Roy 2013 {published data only}
- Roy AC, Park SR, Cunningham D, Kang YK, Chao Y, Chen LT, et al. A randomized phase II study of PEP02 (MM-398), irinotecan or docetaxel as a second-line therapy in patients with locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma. Annals of Oncology 2013;24(6):1567-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sadighi 2006 {published data only}
- Sadighi S, Mohagheghi MA, Montazeri A, Sadighi Z. Quality of life in patients with advanced gastric cancer: a randomized trial comparing docetaxel, cisplatin, 5-FU (TCF) with epirubicin, cisplatin, 5-FU (ECF). BMC Cancer 2006;5(6):274. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sang 2017 {published data only}
- Sang KL, Moon HS, Sung EK, Jin SJ, Joo YC, Eun SK, et al. A randomized trial of FOLFIRI versus docetaxel and cisplatin as a second-line chemotherapy after failure of first-line chemotherapy in advanced gastric cancer. Annals of Oncology 2017;28(suppl_3):iii134. [DOI: 10.1093/annonc/mdx261] [DOI] [Google Scholar]
Satoh 2014 {published data only}
- Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. Journal of Clinical Oncology 2014 Jul;32(19):2039-49. [PMID: ] [DOI] [PubMed] [Google Scholar]
Satoh 2015 {published data only}
- Satoh T, Lee KH, Rha SY, Sasaki Y, Park SH, Komatsu Y, et al. Randomized phase II trial of nimotuzumab plus irinotecan versus irinotecan alone as second-line therapy for patients with advanced gastric cancer. Gastric Cancer 2015 Oct;18(4):824-32. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schnitzler 1986 {published data only}
- Schnitzler G, Queisser W, Heim ME, König H, Katz R, Fritze D, et al. Phase III study of 5-FU and carmustine versus 5-FU, carmustine, and doxorubicin in advanced gastric cancer. Cancer Treatment Reports 1986;70(4):477-9. [PMID: ] [PubMed] [Google Scholar]
Shirao 2013 {published data only}
- Shirao K, Boku N, Yamada Y, Yamaguchi K, Doi T, Goto M, et al. Randomized phase III study of 5-fluorouracil continuous infusion vs. sequential methotrexate and 5-fluorouracil therapy in far advanced gastric cancer with peritoneal metastasis (JCOG0106). Japanese Journal of Clinical Oncology 2013;43(10):972-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shitara 2017 {published data only}
- Shitara K, Takashima A, Fujitani K, Koeda K, Hara H, Nakayama N, et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterology & Hepatology 2017;3(4):277287. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sym 2013 {published data only}
- Sym SJ, Hong J, Park J, Cho EK, Lee JH, Park YH, et al. A randomized phase II study of biweekly irinotecan monotherapy or a combination of irinotecan plus 5-fluorouracil/leucovorin (mFOLFIRI) in patients with metastatic gastric adenocarcinoma refractory to or progressive after first-line chemotherapy. Cancer Chemotherapy and Pharmacology 2013;71(2):481-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tabernero 2017 {published data only}
- Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Cheng K, et al. Pertuzumab (P) + trastuzumab (H) + chemotherapy (CT) for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (mGC/GEJC): final analysis of a phase III study (JACOB). Annals of Oncology 2017;28(suppl_5):v209-v268. [DOI] [PubMed] [Google Scholar]
Tanabe 2015 {published data only}
- Tanabe K, Fujii M, Nishikawa K, Kunisaki C, Tsuji A, Matsuhashi N, et al. Phase II/III study of second-line chemotherapy comparing irinotecan-alone with S-1 plus irinotecan in advanced gastric cancer refractory to first-line treatment with S-1 (JACCRO GC-05). Annals of Oncology 2015 Sep;26(9):1916-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tebbutt 2002 {published data only}
- Tebbutt NC, Norman A, Cunningham D, Iveson T, Seymour M, Hickish T, et al. A multicentre, randomised phase III trial comparing protracted venous infusion (PVI) 5-fluorouracil (5-FU) with PVI 5-FU plus mitomycin C in patients with inoperable oesophago-gastric cancer. Annals of Oncology 2002;13(10):1568-75. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tebbutt 2010 {published data only}
- Tebbutt NC, Cummins MM, Sourjina T, Strickland A, Van Hazel G, Ganju V, et al. Randomised, non-comparative phase II study of weekly docetaxel with cisplatin and 5-fluorouracil or with capecitabine in oesophagogastric cancer: the AGITG ATTAX trial. British Journal of Cancer 2010;102(3):475-81. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tebbutt 2016 {published data only}
- Tebbutt NC, Price TJ, Ferraro DA, Wong N, Veillard AS, Hall M, et al. Panitumumab added to docetaxel, cisplatin and fluoropyrimidine in oesophagogastric cancer: ATTAX3 phase II trial. British Journal of Cancer 2016;114(5):505-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsavaris 1996 {published data only}
- Tsavaris NB, Tentas K, Kosmidis P, Mylonakis N, Sakelaropoulos N, Kosmas C, et al. 5-Fluorouracil, epirubicin, and mitomycin C versus 5-fluorouracil, epirubicin, mitomycin C, and leucovorin in advanced gastric carcinoma. A randomized trial. American Journal of Clinical Oncology 1996;19(5):517-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Unknown 1992 {published data only}
- No authors listed. A randomized, comparative study of combination chemotherapies in advanced gastric cancer: 5-fluorouracil and cisplatin (FP) versus 5-fluorouracil, cisplatin, and 4'-epirubicin (FPEPIR). Kyoto Research Group for Chemotherapy of Gastric Cancer (KRGCGC). Anticancer Research 1992;12(6B):1983-8. [PMID: ] [PubMed] [Google Scholar]
Van Cutsem 2015 {published data only}
- Van Cutsem E, Boni C, Tabernero J, Massuti B, Middleton G, Dane F, et al. Docetaxel plus oxaliplatin with or without fluorouracil or capecitabine in metastatic or locally recurrent gastric cancer: a randomized phase II study. Annals of Oncology 2015;26(1):149-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Cutsem 2017 {published data only}
- Van Cutsem E, Bang YJ, Mansoor W, Petty RD, Chao Y, Cunningham D, et al. A randomized, open-label study of the efficacy and safety of AZD4547 monotherapy versus paclitaxel for the treatment of advanced gastric adenocarcinoma with FGFR2 polysomy or gene amplification. Annals of Oncology 2017;28(6):1316-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wu 2020 {published data only}
- Wu Q, Fu Y, Wen W, Xi T, Zhao G. Efficacy and prognosis analyses of apatinib combined with S-1 in third-line chemotherapy for advanced gastric cancer. JBUON 2020;25(2):987-994. [PMID: ] [PubMed] [Google Scholar]
Yamamura 1998 {published data only}
- Yamamura Y, Miyazaki I, Ogawa M, Yonemura Y, Tanemura H, Kito T, et al. A randomized controlled trial with methotrexate (MTX), 5-fluorouracil (5-FU) and pirarubicin (THP) vs 5-FU alone in advanced or recurrent gastric carcinoma. Tokai Hokuriku THP Study Group. Gan To Kagaku Ryoho 1998;25(10):1543-8. [PMID: ] [PubMed] [Google Scholar]
Yoshino 2016 {published data only}
- Yoshino S, Nishikawa K, Morita S, Takahashi T, Sakata K, Nagao J, et al. Randomised phase III study of S-1 alone versus S-1 plus lentinan for unresectable or recurrent gastric cancer (JFMC36-0701). European Journal of Cancer 2016;65:164-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhao 2009 {published data only}
- Zhao W, Ji Z. Comparison of XELOX (oxaliplatin plus capecitabine) and FOLFOX4 (oxaliplatin plus 5-fluorouracil/calcium folinate) in the treatment of advanced gastric cancer. Chinese Journal of Clinical Oncology 2009;36(18):1044-1046. [Google Scholar]
Zhong 2007 {published data only}
- Zhong Q, Feng Y, Jia ZF, Xia XT. Application of FOLFOX4 regimen in the treatment of advanced gastric carcinoma. [Chinese]. Chinese Journal of Cancer Prevention and Treatment 2007;14(8):610-2. [Google Scholar]
Zhou 2015 {published data only}
- Zhou D, Cao G, Cheng Z, Ji H, Fu W. The clinical efficacy of docetaxel combined with modified FOLFOX regimen in the treatment of advanced gastric cancer. [Chinese]. Anti-Tumor Pharmacy 2015;5(1):38-41. [DOI: 10.3969/j.issn.2095-1264.2015.006] [DOI] [Google Scholar]
Zhu 2016 {published data only}
- Zhu YP, Sheng LL, Wang L. Analysis on clinical efficacy of docetaxel combined with cisplatin and irinotecan combined with cisplatin in second-line treatment of patients with advanced gastric cancer. [Chinese]. Chinese Journal of Cancer Prevention and Treatment 2016;23(11):732-8. [Google Scholar]
References to studies awaiting assessment
Chung 2020 {published data only}
- Chung HC, Kang YK, Chen Z, Bai Y, Wan Ishak WZ, Shim BY, et al. Pembrolizumab vs paclitaxel as second-line treatment for Asian patients with PD-L1–positive advanced gastric or gastroesophageal cancer (GC) in the phase III KEYNOTE-063 trial. Journal of Clinical Oncology May 2020;38(15_suppl):e16586-e16586. [DOI: 10.1200/JCO.2020.38.15_suppl.e16586] [DOI] [Google Scholar]
Denlinger 2016 {published data only}
- Denlinger CS, Maqueda MA, Watkins DJ, Sym SJ, Bendell JC, Park SH, et al. Randomized phase 2 study of paclitaxel (PTX), trastuzumab (T) with or without MM-111 in HER2 expressing gastroesophageal cancers (GEC). Journal of Clinical Oncology 2016;34(15_suppl):4043-4043.. [DOI: 10.1200/JCO.2016.34.15_suppl.4043] [DOI] [Google Scholar]
EUCTR2012‐002321‐30 {published data only}
- EUCTR2012-002321-30. Randomized phase II trial with irinotecan as monotherapy compared to irinotecan and bevacizumab (BevIri) for patients with platinum resistant non-resectable esophagus-, cardia or gastric cancer. Available from https://www.clinicaltrialsregister.eu/ctr-search/trial/2012-002321-30/DK. First received November 2, 2012.
Ikeda 2009 {published data only}
- Ikeda R, Yoshida K, Satou Y, Takahashi M, Une Y, Yamamoto W et al. Randomized phase II/III study of docetaxel/S-1 (DS-1) versus CDDP/5FU (FUP) in advanced or recurrent gastric cancer: updated phase II results. Journal of Clinical Oncology 2009;27(15S):4595. [DOI: 10.1200/jco.2009.27.15s.4595] [DOI] [Google Scholar]
Janjigian 2012 {published data only}
- Janjigian YY, Tang LH, Shibata S, Kelsen DP, Segal M, Cheng C, et al. A multicenter random assignment phase II study of irinotecan and flavopiridol versus irinotecan alone for patients with p53 wild-type gastric adenocarcinoma (NCI 8060). Journal of Clinical Oncology 2012;30(15_suppl):e14586. [DOI: 10.1200/jco.2012.30.15_suppl.e14586 ] [Google Scholar]
JPRN‐UMIN000003156 {published data only}
- JPRN-UMIN000003156. Randomized phase II trial of TS-1 based therapy vs TS-1 based therapy + LNT for advanced or recurrent gastric cancer. Available from https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000003828. First received Februrary 9, 2010.
JPRN‐UMIN000008827 {published data only}
- JPRN-UMIN000008827. Phase II randomised clinical trial of personalized peptide vaccination in combination with Juzen-taiho-to for gastric cancer patients resistant to standard therapies. Available from https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000010352. First received August 13, 2012.
Kan 2018 {published data only}
- Kan J. Clinical study on the combined targeted therapy of her-2 protein positive chemotherapy in advanced gastric cancer in Xining. High Altitude Medicine and Biology 2018;19(4):pp A424. [DOI: 10.1089/ham.2018.29015.abstracts] [DOI] [Google Scholar]
Kang 2019 {published data only}
- Kang YK, Kang WK, Di Bartolomeo M, Chau I, Yoon HH, Cascinu S, et al. LBA43 - Randomized phase III ANGEL study of rivoceranib (apatinib) + best supportive care (BSC) vs placebo + BSC in patients with advanced/metastatic gastric cancer who failed ≥2 prior chemotherapy regimens. Annals of Oncology Oct 2019;30(Suppl 5):v877-v878. [DOI: 10.1093/annonc/mdz394.034] [DOI] [Google Scholar]
Kelly 2018 {published data only}
- Kelly RJ, Lee J, Bang YJ, Almhanna K, Blum Murphy MA, Catenacci DV, et al. Safety and efficacy of durvalumab in combination with tremelimumab, durvalumab monotherapy, and tremelimumab monotherapy in patients with advanced gastric cancer. Journal of Clinical Oncology 2018;36(15_suppl):4031. [DOI: 10.1200/JCO.2018.36.15_suppl.4031 ] [Google Scholar]
Kojima 2019 {published data only}
- Kojima T, Muro K, Francois E, Hsu CH, Moriwaki T, Kim SB, et al. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: Phase III KEYNOTE-181 study. Journal of Clinical Oncology 2019;37(4_suppl):2-2. [DOI: 10.1200/JCO.2019.37.4_suppl.2] [DOI] [PubMed] [Google Scholar]
NCT00683787 {published data only}
- NCT00683787. Multicenter randomized phase II trial of docetaxel with/without VANDETANIB for advanced gastroesophageal cancer. Availble from https://clinicaltrials.gov/ct2/show/NCT00683787. First received May 23, 2008.
NCT00991952 {published data only}
- NCT00991952. Irinotecan hydrochloride with or without Alvocidib in treating patients with advanced stomach or gastroesophageal junction cancer that cannot be removed by surgery. Available from https://clinicaltrials.gov/ct2/show/NCT00991952 First received October 8, 2009.
NCT01579578 {published data only}
- NCT01579578. A phase IIa multi-centre randomised double-blind placebo-controlled study to assess the efficacy, safety and pharmacokinetics of AZD8931 in combination with paclitaxel versus paclitaxel alone in patients with metastatic, gastric or gastro-oesophageal junction, cancer who progress following first line therapy and are ineligible for treatment with trastuzumab by HER2 status (SAGE). Available from https://clinicaltrials.gov/ct2/show/NCT01579578. First received April 18, 2012.
NCT01813253 {published data only}
- NCT01813253. A randomized, open-label, Japan-Korea-Taiwan Collaborative phase 3 study to compare the efficacy of nimotuzumab and irinotecan combination therapy versus irinotecan monotherapy as second line treatment in subjects with advanced or recurrent gastric and gastroesophageal junction cancer. Available from https://clinicaltrials.gov/ct2/show/NCT01813253. First received March 18, 2013.
Nishikawa 2015b {published data only}
- Nishikawa K, Imamura H, Kawase T, Gotoh M, Kimura Y, Ueda S, et al. A randomized phase II factorial design trial of CPT-11 versus PTX versus each combination with S-1 in patients with advanced gastric cancer refractory to S-1: final results of OGSG0701. Journal of Clinical Oncology 2015;33(3_suppl):117. [DOI: 10.1200/jco.2015.33.3_suppl.117] [DOI] [Google Scholar]
Shah 2019 {published data only}
- Shah MA, Metges JP, Cunningham D, Shiu KK, Wyrwicz L, Thai D, et al. A phase II, open-label, randomized study to evaluate the efficacy and safety of andecaliximab combined with nivolumab versus nivolumab alone in subjects with unresectable or recurrent gastric or gastroesophageal junction adenocarcinoma. Journal of Clinical Oncology 2019;37(4_suppl):75. [DOI: 10.1200/JCO.2019.37.4_suppl.75] [DOI] [Google Scholar]
Wang 2017 {published data only}
- Wang Z, Dai G, Zhou Y, Hu Hi, Zhang P, Gou M. Apatinib combined with docetaxel in second-line treatment of advanced gastric cancer: a prospective randomized controlled clinical study. Annals of Oncology 2017;28(suppl_10):x57-x76. [Google Scholar]
Yeh 2012 {published data only}
- Yeh KH, Chen JS, Sobrero A, Singhal N, De Dosso S, Kang YK, et al. Safety and tolerability data from a phase II study of AUY922 compared with chemotherapy in patients with advanced gastric cancer. Annals of Oncology Jun 2012;23(suppl_4):P-0303. [Google Scholar]
Zhao 2019 {published data only}
- Zhao X, Guo W, Chen Z, Zhang X, Zhu X, Zhang W, et al. Comparison of efficacy and safety of second-line palliative chemotherapy with paclitaxel plus raltitrexed and paclitaxel alone in patients with metastatic gastric adenocarcinoma: A randomized phase II trial. Journal of Clinical Oncology 2019;37(15_suppl):4054. [DOI: 10.1200/JCO.2019.37.15_suppl.4054] [DOI] [Google Scholar]
References to ongoing studies
Aanur 2017 {published data only}
- Aanur P, Gutierrez M, Joseph RK, Ajani JA, Ku GY, Denlinger CS, et al. FRACTION (Fast Real-time Assessment of Combination Therapies in Immuno-Oncology)-gastric cancer (GC): a randomized, open-label, adaptive, phase 2 study of nivolumab in combination with other immuno-oncology (IO) agents in patients with advanced GC. Journal of Clinical Oncology 2017;35(15_suppl):TPS4137-TPS4137. [DOI: 10.1200/JCO.2017.35.15_suppl.TPS4137 ] [Google Scholar]
Al‐Batran 2020 {published data only}
- Lorenzen S, Thuss-Patience PC, Pauligk C, Goekkurt E, Ettrich TJ, Lordick F, et al. FOLFIRI plus ramucirumab versus paclitaxel plus ramucirumab as second-line therapy for patients with advanced or metastatic gastroesophageal adenocarcinoma with or without prior docetaxel: Results from the phase II RAMIRIS Study of the AIO. Journal of Clinical Oncology 2020;38(15_suppl):4514-4514. [10.1200/JCO.2020.38.15_suppl.4514] [DOI] [PubMed] [Google Scholar]
EUCTR2015‐000897‐36 {published data only}
- EUCTR2015-000897-36. A prospective, multicentre, double-blind, randomized, placebo-controlled, phase 3 study to evaluate efficacy and safety of masitinib with irinotecan in patients with advanced-stage esophagogastric adenocarcinoma who have relapsed after first-line chemotherapy. Available from https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2015-000897-36. First received Februrary 23, 2016.
EUCTR2015‐001605‐14 {published data only}
- EUCTR2015-001605-14. A randomised, double-blind, placebo controlled, multi-centre phase II study to assess the efficacy and safety of 2nd line olaparib in combination with paclitaxel, in western patients with advanced gastric and gastro-oesophageal junction cancer. Available from https://www.clinicaltrialsregister.eu/ctr-search/trial/2015-001605-14/GB. First received July 17, 2015.
EUCTR2018‐002374‐46 {published data only}
- EUCTR2018-002374-46. A randomised phase II trial assessing regorafenib combined with irinotecan as second-line treatment in patients with metastatic gastro-oesophageal adenocarcinomas. Available from https://www.clinicaltrialsregister.eu/ctr-search/trial/2018-002374-46/FR. First received August 24, 2018.
Hirata 2020 {published data only}
- Hirata K, Hamamoto Y, Ando M, Imamura CK, Yoshimura K, Yamazaki K, et al. Weekly paclitaxel plus ramucirumab versus weekly nab-paclitaxel plus ramucirumab for unresectable advanced or recurrent gastric cancer with peritoneal dissemination refractory to first-line therapy-the P-SELECT trial (WJOG10617G)-a randomised phase II trial by the West Japan Oncology Group. BMC Cancer 2020;20(1):548. [32532230] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manjii 2018 {published data only}
- Manji GA, Bendell JC, Oh DY, Kim KP, Macarulla T, Ponz-Sarvisé M, et al. MORPHEUS: a phase Ib/II multi-trial platform evaluating the efficacy and safety of cancer immunotherapy (CIT)-based combinations in patients (pts) with gastric or pancreatic cancer. Journal of Clinical Oncology 2018;36(4_suppl):TPS530-TPS530. [DOI: 10.1200/JCO.2018.36.4_suppl.TPS530 ] [Google Scholar]
NCT00509964 {published data only}
- NCT00509964. A randomized phase II trial of irinotecan monotherapy versus Irinotecan, leucovorin and 5-FU (ILF) combination chemotherapy in patients with advanced gastric cancer failing prior chemotherapy. Available from https://clinicaltrials.gov/ct2/show/NCT00509964. First received August 1, 2007.
NCT01836120 {published data only}
- NCT01836120. A study of raltitrexed plus docetaxel versus docetaxel as second-line chemotherapy insSubjects with gastric cancer. Available from https://clinicaltrials.gov/ct2/show/NCT01836120. First received April 19, 2013.
NCT02401971 {published data only}
- NCT02401971. Irinotecan plus thalidomide in second line advanced gastric cancer: a multicenter, randomized, controlled and prospective trial. Available from https://clinicaltrials.gov/ct2/show/NCT02401971. First received March 30, 2015.
NCT02409199 {published data only}
- NCT02409199. A randomized, multicenter study to evaluate the efficacy and safety of apatinib versus docetaxel in patients with previously treated locally advanced or metastatic gastric cancer, including adenocarcinoma of the gastroesophageal junction. Available from https://clinicaltrials.gov/ct2/show/NCT02409199. First received April 6, 2015.
NCT02461407 {published data only}
- NCT02461407. A randomized, double-blind, placebo-controlled, multicenter clinical trial to compare the efficacy and safety of anlotinib versus placebo in patients with gastric cancer (ALTER0503). Available from https://clinicaltrials.gov/ct2/show/NCT02461407. First received June 3, 2015.
NCT02485015 {published data only}
- NCT02485015. The randomized, controlled, multicenter clinical trial of apatinib plus cik as the third line therapy for patients with advanced gastric cancer. Available from https://clinicaltrials.gov/ct2/show/NCT02485015. First received June 30, 2015.
NCT02596256 {published data only}
- NCT02596256. Apatinib plus docetaxel versus docetaxel as second-line treatment in advanced gastric cancer stage II randomized controlled clinical studies. Available from https://clinicaltrials.gov/ct2/show/NCT02596256. First received November 4, 2015.
NCT02632201 {published data only}
- NCT02632201. A clinical study of adoptive cellular immunotherapy using pluripotent killerT cells expressing antibodies for human epidermal growth factor receptor-2 (HER2) in treating patients with HER2-positive advanced gastric cancer with liver metastasis. Available from https://clinicaltrials.gov/ct2/show/NCT02632201. First received December 16, 2015.
NCT02862561 {published data only}
- NCT02862561. Clinical study using precision cell immunotherapy combinedwWith chemotherapy in advanced gastric cancer. Available from https://clinicaltrials.gov/ct2/show/NCT02862561. First received August 11, 2016.
NCT02873520 {published data only}
- NCT02873520. Clinical study using precision cell immunotherapy combined with chemotherapy in advanced gastric cancer. Available from https://clinicaltrials.gov/ct2/show/NCT02873520. First received August 19, 2016.
NCT02898077 {published data only}
- NCT02898077. A randomized, multicenter, double-blind, placebo-controlled, phase 3 study of weekly paclitaxel with or without ramucirumab (IMC-1121B) in patients with advanced gastric or gastroesophageal junction adenocarcinoma, refractory to or progressive after first-line therapy with platinum and fluoropyrimidine. Available from https://clinicaltrials.gov/ct2/show/NCT02898077. First received September 13, 2016.
NCT03030937 {published data only}
- NCT03030937. A randomized, parallel control, exploratory trial to compare apatinib plus irinotecan versus single irinotecan as second-line treatment in subjects with advanced gastric cancer or adenocarcinoma of esophagogastric junction. Available from https://clinicaltrials.gov/ct2/show/NCT03030937. First received January 25, 2017.
NCT03144843 {published data only}
- NCT03144843. A phase II multicenter, randomized, double-blind study of apatinib combined with paclitaxol versus placebo with paclitaxol as second line therapy for advanced gastric / esophagogastric junction adenocarcinoma with peritoneal metastasis. Available from https://clinicaltrials.gov/ct2/show/NCT03144843. First received May 9, 2017.
NCT03223376 {published data only}
- NCT03223376. A phase III Study to evaluate the efficacy and safety of fruquitinib in combination with paclitaxel versus paclitaxel alone in second line gastric cancer. Available from https://clinicaltrials.gov/ct2/show/NCT03223376. First received July 21, 2017.
NCT03760822 {published data only}
- NCT03760822. Second-line chemotherapy with ramucirumab +/- paclitaxel in elderly advanced gastric or gastroesophageal junction cancer patients (SOCRATE). Available from https://clinicaltrials.gov/ct2/show/NCT03760822. First received November 30, 2018.
NCT03959293 {published data only}
- NCT03959293. A Randomized Phase II Study Evaluating FOLFIRI + Durvalumab vs FOLFIRI + Durvalumab and Tremelimumab in Second-line Treatment of Patients With Advanced Gastric or Gastro-oesophageal Junction Adenocarcinoma. Available from https://clinicaltrials.gov/ct2/show/NCT03959293 First received May 22, 2019. [Google Scholar]
NCT04294784 {published data only}
- NCT04294784. A Multi-center, Open-label, Randomized Controlled Clinical Study of Nab-paclitaxel Plus SHR-1210(PD-1 Inhibitor)Versus Nab-paclitaxel as Second-line Treatment in Advanced or Recurrent Gastric and Esophagogastric Adenocarcinoma. Available from https://clinicaltrials.gov/ct2/show/NCT04294784 First received March 4, 2020. [Google Scholar]
NCT04342910 {published data only}
- NCT04342910. A Study of Camrelizumab (SHR-1210) Combined With Apatinib Versus Paclitaxel or Irinotecan in Participants With Advanced Gastric/Gastroesophageal Junction Adenocarcinoma Progressed After First-line Chemotherapy. Available from https://clinicaltrials.gov/ct2/show/NCT04342910 First received April 13, 2020. [Google Scholar]
NCT04486651 {published data only}
- NCT04486651. A Randomized, Double-blinded, Multicenter, Phase III Clinical Study of HX008 (Recombinant Humanized Anti-PD-1 Monoclonal Antibody Injection) Plus Irinotecan Versus Placebo Plus Irinotecan as Second-line Treatment in Advanced Gastric Cancer. Available from https://clinicaltrials.gov/ct2/show/NCT04486651 First received July 24, 2020. [Google Scholar]
NCT04555304 {published data only}
- NCT04555304. A Randomized, Multicenter, Double-Blind, Placebo-Controlled, Phase 2 Study of Weekly Paclitaxel With or Without KH903 in Patients With Advanced Gastric or Gastroesophageal Junction Adenocarcinoma, Refractory to or Progressive After First-Line Therapy With Platinum and Fluoropyrimidine. Available from https://clinicaltrials.gov/ct2/show/NCT04555304 First received September 18, 2020. [Google Scholar]
Sakai 2018 {published data only}
- Sakai D, Boku N, Kodera Y, Komatsu Y, Fujii M, Iwasa S, et al. An intergroup phase III trial of ramucirumab plus irinotecan in third or more line beyond progression after ramucirumab for advanced gastric cancer (RINDBeRG trial). Journal of Clinical Oncology 2018;36(15_suppl):TPS4138-TPS4138. [Google Scholar]
Sjoquist 2017 {published data only}
- Sjoquist KM, Pavlakis N, Martin AJ, Tsobanis E, Yip S, Bang YJ, et al. Integrate II: A randomised phase 3 double-blind placebo-controlled study of regorafenib in refractory advanced gastro-oesophageal cancer (AGOC)—an international study organized by the Australasian Gastrointestinal Trials Group (AGITG). Journal of Clinical Oncology 2017;35(15_suppl):TPS4136-TPS4136. [DOI: 10.1200/JCO.2017.35.15_suppl.TPS4136 ] [Google Scholar]
Additional references
Ajani 2017
- Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S. Gastric adenocarcinoma. Nature Reviews Disease Primers 2017;1(3):17036. [PMID: ] [DOI] [PubMed] [Google Scholar]
Al‐Batran 2014
- Al-Batran SE, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, et al. RAINBOW: a global, phase III, randomized, double-blind study of ramucirumab plus paclitaxel versus placebo plus paclitaxel patients with previously treated gastric or gastroesophageal junction (GEJ) adenocarcinoma: Quality-of-life (QoL) results. Journal of Clinical Oncology 2014;32(15_suppl,):4058-4058. [Google Scholar]
Bang 2010
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376(9742):687-97. [PMID: ] [DOI] [PubMed] [Google Scholar]
Buas 2013
- Buas, MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Seminars in Radiation Oncology 2013;23(1):3-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chau 2009
- Chau I, Norman AR, Cunningham D, Oates J, Hawkins R, Iveson T, et al. The impact of primary tumour origins in patients with advanced oesophageal, oesophago-gastric junction and gastric adenocarcinoma--individual patient data from 1775 patients in four randomised controlled trials. Annals of Oncology 2009;20(5):885-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chen 2013
- Chen TT. Statistical issues and challenges in immuno-oncology. Journal for Immunotherapy of Cancer 2013;21(1):18. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2005
- Choi JH, Ahn MJ, Rhim HC, Kim JW, Lee GH, Lee YY, et al. Comparison of WHO and RECIST criteria for response in metastatic colorectal carcinoma. Cancer Research and treatment 2005;37(5):290-3. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Corley 2004
- Corley DA, Kubo AI. Influence of site classification on cancer incidence rates: an analysis of gastric cardia carcinomas. Journal of the National Cancer Institute 2004;96(18):1383-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cunningham 2008
- Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. New England Journal of Medicine 2008;358(1):36-46. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ferlay 2018
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. Available from: https://gco.iarc.fr/today, accessed [05 Aug 2019] November 2018.
Ferrara 2018
- Ferrara R, Pilotto S, Caccese M, Grizzi G, Sperduti I, Giannarelli D, et al. Do immune checkpoint inhibitors need new studies methodology? Journal of Thoracic Disease 2018;10(Suppl 13):S1564–S1580. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glady 2016
- Galdy S, Cella CA, Spada F, Murgioni S, Frezza AM, Ravenda SP, et al. Systemic therapy beyond first-line in advanced gastric cancer: an overview of the main randomized clinical trials. Critical Reviews in Oncology/Hematology 2016;99:1-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2015 (developed by Evidence Prime, Inc.). Available from gradepro.org.
Hayakawa 2016
- Hayakawa Y, Sethi N, Sepulveda AR, Bass AJ, Wang TC. Oesophageal adenocarcinoma and gastric cancer: should we mind the gap? Nature Reviews Cancer 2016;16(5):305-18. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Janjigian 2017
- Janjigian YY, Ott PA, Calvo E, Kim JW, Ascierto PA, Sharma P, et al. Nivolumab ± ipilimumab in pts with advanced (adv)/metastatic chemotherapy-refractory (CTx-R) gastric (G), esophageal (E), or gastroesophageal junction (GEJ) cancer: CheckMate 032 study. Journal of Clinical Oncology 2017;35(15_suppl):4014. [Google Scholar]
Kanagavel 2015
- Kanagavel D, Fedyanin M, Tryakin A, Tjulandin S. Second-line treatment of metastatic gastric cancer: current options and future directions. World Journal of Gastroenterology 2015;21(41):11621-35. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2009
- Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Annals of Oncology 2009;20(4):666-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI: 10.1136/bmj.b2700] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marsman 2005
- Marsman WA, Tytgat GN, ten Kate FJ, Lanschot JJ. Differences and similarities of adenocarcinomas of the esophagus and esophagogastric junction. Journal of Surgical Oncology 2005;92(3):160-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Offman 2018
- Offman J, Pesola F, Sasieni P. Trends and projections in adenocarcinoma and squamous cell carcinoma of the oesophagus in England from 1971 to 2037. British Joural of Cancer 2018;118(10):1391–8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2006
- Park SH, Lee WK, Chung M, Lee Y, Han SH, Bang SM, et al. Paclitaxel versus docetaxel for advanced gastric cancer: a randomized phase II trial in combination with infusional 5-fluorouracil. Anticancer Drugs 2006;17(2):225-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rawla 2019
- Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Przegla̜d Gastroenterologiczny 2019;14(1):26-38. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration Review Manager (RevMan) Version 5.4.1. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2020.
Rice 2017
- Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Annals of Cardiothoracic Surgery 2017;6(2):119-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
SEER 2018
- Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M et al (eds). SEER Cancer Statistics Review, 1975-2016. Available from https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission 2019.
Siewert 2005
- Siewert JR, Feith M, Stein HJ. Biologic and clinical variations of adenocarcinoma at the esophago-gastric junction: relevance of a topographic-anatomic subclassification. Journal of Surgical Oncology 2005;90(3):139-46. [PMID: ] [DOI] [PubMed] [Google Scholar]
ter Veer 2016
- ter Veer E, Haj Mohammad N, Valkenhoef G, Ngai LL, Mali RM, Oijen MG, et al. Second- and third-line systemic therapy in patients with advanced esophagogastric cancer: a systematic review of the literature. Cancer Metastasis Review 2016;35(3):439-56. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Steward LA, Ghersi D, Budett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8(16):https://doi.org/10.1186/1745-6215-8-16. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Cutsem 2006
- Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. Journal of Clinical Oncology 2006;24(31):4991-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wesolowski 2009
- Wesolowski R, Lee C, Kim R. Is there a role for second-line chemotherapy in advanced gastric cancer? Lancet 2009;10:903-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Tomita 2016
- Tomita Y, Hsieh AHC, Yuan Y, Townsend A, Price T. Salvage systemic therapy for advanced gastric and oesophago‐gastric junction adenocarcinoma. Cochrane Database of Systematic Reviews 2016, Issue 2. Art. No: CD012078. [DOI: 10.1002/14651858.CD012078] [DOI] [PMC free article] [PubMed] [Google Scholar]


