Abstract
Background
Extracranial carotid artery stenosis is the major cause of stroke, which can lead to disability and mortality. Carotid endarterectomy (CEA) with carotid patch angioplasty is the most popular technique for reducing the risk of stroke. Patch material may be made from an autologous vein, bovine pericardium, or synthetic material including polytetrafluoroethylene (PTFE), Dacron, polyurethane, and polyester. This is an update of a review that was first published in 1996 and was last updated in 2010.
Objectives
To assess the safety and efficacy of different types of patch materials used in carotid patch angioplasty. The primary hypothesis was that a synthetic material was associated with lower risk of patch rupture versus venous patches, but that venous patches were associated with lower risk of perioperative stroke and early or late infection, or both.
Search methods
We searched the Cochrane Stroke Group trials register (last searched 25 May 2020); the Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 4), in the Cochrane Library; MEDLINE (1966 to 25 May 2020); Embase (1980 to 25 May 2020); the Index to Scientific and Technical Proceedings (1980 to 2019); the Web of Science Core Collection; ClinicalTrials.gov; and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) portal. We handsearched relevant journals and conference proceedings, checked reference lists, and contacted experts in the field.
Selection criteria
Randomised and quasi‐randomised trials (RCTs) comparing one type of carotid patch with another for CEA.
Data collection and analysis
Two review authors independently assessed eligibility, risk of bias, and trial quality; extracted data; and determined the quality of evidence using the GRADE approach. Outcomes, for example, perioperative ipsilateral stroke and long‐term ipsilateral stroke (at least one year), were collected and analysed.
Main results
We included 14 trials involving a total of 2278 CEAs with patch closure operations: seven trials compared vein closure with PTFE closure, five compared Dacron grafts with other synthetic materials, and two compared bovine pericardium with other synthetic materials. In most trials, a patient could be randomised twice and could have each carotid artery randomised to different treatment groups.
Synthetic patch compared with vein patch angioplasty Vein patch may have little to no difference in effect on perioperative ipsilateral stroke between synthetic versus vein materials, but the evidence is very uncertain (odds ratio (OR) 2.05, 95% confidence interval (CI) 0.66 to 6.38; 5 studies, 797 participants; very low‐quality evidence). Vein patch may have little to no difference in effect on long‐term ipsilateral stroke between synthetic versus vein materials, but the evidence is very uncertain (OR 1.45, 95% CI 0.69 to 3.07; P = 0.33; 4 studies, 776 participants; very low‐quality evidence). Vein patch may increase pseudoaneurysm formation when compared with synthetic patch, but the evidence is very uncertain (OR 0.09, 95% CI 0.02 to 0.49; 4 studies, 776 participants; very low‐quality evidence). However, the numbers involved were small.
Dacron patch compared with other synthetic patch angioplasty Dacron versus PTFE patch materials
PTFE patch may reduce the risk of perioperative ipsilateral stroke (OR 3.35, 95% CI 0.19 to 59.06; 2 studies, 400 participants; very low‐quality evidence). PTFE patch may reduce the risk of long‐term ipsilateral stroke (OR 1.52, 95% CI 0.25 to 9.27; 1 study, 200 participants; very low‐quality evidence). Dacron may result in an increase in perioperative combined stroke and transient ischaemic attack (TIA) (OR 4.41 95% CI 1.20 to 16.14; 1 study, 200 participants; low‐quality evidence) when compared with PTFE. Early arterial re‐stenosis or occlusion (within 30 days) was also higher for Dacron patches. During follow‐up for longer than one year, more 'any strokes' (OR 10.58, 95% CI 1.34 to 83.43; 2 studies, 304 participants; low‐quality evidence) and stroke/death (OR 6.06, 95% CI 1.31 to 28.07; 1 study, 200 participants; low‐quality evidence) were reported with Dacron patch closure, although numbers of outcome events were small. Dacron patch may increase the risk of re‐stenosis when compared with other synthetic materials (especially with PTFE), but the evidence is very uncertain (OR 3.73, 95% CI 0.71 to 19.65; 3 studies, 490 participants; low‐quality evidence).
Bovine pericardium patch compared with other synthetic patch angioplasty Bovine pericardium versus PTFE patch materials
Evidence suggests that bovine pericardium patch results in a reduction in long‐term ipsilateral stroke (OR 4.17, 95% CI 0.46 to 38.02; 1 study, 195 participants; low‐quality evidence). Bovine pericardial patch may reduce the risk of perioperative fatal stroke, death, and infection compared to synthetic material (OR 5.16, 95% CI 0.24 to 108.83; 2 studies, 290 participants; low‐quality evidence for PTFE, and low‐quality evidence for Dacron; OR 4.39, 95% CI 0.48 to 39.95; 2 studies, 290 participants; low‐quality evidence for PTFE, and low‐quality evidence for Dacron; OR 7.30, 95% CI 0.37 to 143.16; 1 study, 195 participants; low‐quality evidence, respectively), but the numbers of outcomes were small. The evidence is very uncertain about effects of the patch on infection outcomes.
Authors' conclusions
The number of outcome events is too small to allow conclusions, and more trial data are required to establish whether any differences do exist. Nevertheless, there is little to no difference in effect on perioperative and long‐term ipsilateral stroke between vein and any synthetic patch material. Some evidence indicates that other synthetic patches (e.g. PTFE) may be superior to Dacron grafts in terms of perioperative stroke and TIA rates, and both early and late arterial re‐stenosis and occlusion. Pseudoaneurysm formation may be more common after use of a vein patch than after use of a synthetic patch. Bovine pericardial patch, which is an acellular xenograft material, may reduce the risk of perioperative fatal stroke, death, and infection compared to other synthetic patches. Further large RCTs are required before definitive conclusions can be reached.
Keywords: Humans; Aneurysm, False; Aneurysm, False/epidemiology; Angioplasty; Angioplasty/methods; Bias; Bioprosthesis; Blood Vessel Prosthesis; Blood Vessel Prosthesis/adverse effects; Carotid Stenosis; Endarterectomy, Carotid; Endarterectomy, Carotid/classification; Endarterectomy, Carotid/methods; Endarterectomy, Carotid/mortality; Polyethylene Terephthalates; Polyethylene Terephthalates/adverse effects; Polytetrafluoroethylene; Polytetrafluoroethylene/adverse effects; Postoperative Complications; Postoperative Complications/epidemiology; Postoperative Complications/etiology; Postoperative Complications/mortality; Postoperative Complications/prevention & control; Randomized Controlled Trials as Topic; Saphenous Vein; Stroke; Stroke/epidemiology; Stroke/etiology; Stroke/mortality; Stroke/prevention & control
Plain language summary
Patches of different types for carotid patch angioplasty
Question
What are the best types of patch materials for patients who undergo carotid patch angioplasty?
Background Carotid endarterectomy is an operation done to remove some diseased artery lining that has caused a stroke. Usually patients who need this operation are at risk of a stroke because of recent stroke symptoms or severe disease of the carotid artery. Inserting a patch at the end of the carotid operation appears to reduce the risk of further stroke and artery disease. These patches are made of synthetic material, the patient’s own vein, or other natural materials such as bovine pericardium. Vein patching is often used and is resistant to infection. However, abnormal swelling of the patch or patch rupture has been a matter of concern. Synthetic patch materials including Dacron and polytetrafluoroethylene (PTFE) have high strength and may involve lower risk of patch rupture. However, synthetic materials may confer greater risk of infection. Bovine pericardium may carry lower risk for both infection and other complications. However the best choice of material for carotid patch angioplasty procedures is still uncertain. This review aims to assess whether one type of patch is better than another for clinical outcomes (such as stroke and death) and complications (such as patch rupture or infection).
Search date We searched for studies up to 25 May 2020. Study characteristics This review identified 14 randomised controlled trials (RCTs) involving 2278 carotid endarterectomies, which compared different patch materials: seven trials compared vein closure with PTFE closure, five compared Dacron grafts with other synthetic materials, and two compared bovine pericardium with other synthetic materials. Primary endpoints were postoperative and long‐term (during at least one year) stroke on the operated side. Secondary endpoints were any stroke, transient ischaemic attack (TIA), death, artery narrowing or blockage, and other complications including artery rupture, cranial nerve palsy, wound infection or bleeding, and reoperation or abnormal swelling (pseudoaneurysm).
Key results The results of using different types of patch materials after carotid endarterectomy were as follows. • Vein patch versus synthetic material: there were no differences in the risk of stroke postoperatively or over the long term. The main concerns were that vein patches appeared to result in more abnormal swelling (pseudoaneurysm). Information on other complications was limited.
• Dacron versus other synthetic material: Dacron patches were associated with higher risk of combined perioperative stroke and TIA, early arterial re‐stenosis or occlusion, and any strokes at longer‐term follow‐up, although numbers of outcome events were small.
• Bovine pericardium patch versus other synthetic materials: there were no differences in any clinical outcomes or complications, although the numbers of outcome events were small. Information on other complications was limited.
Quality of the evidence Most evidence was of low or very low quality due to research methods and small numbers. No RCTs could be blinded for surgeons or patients due to the nature of the intervention, and most trials did not report their funding source. Most outcomes were downgraded for imprecision due to wide confidence intervals and low event rates.
Summary of findings
Summary of findings 1. Main comparison of synthetic patch versus vein patch angioplasty.
| Synthetic patch versus vein patch angioplasty for carotid endarterectomy | ||||||
|
Patient or population: patients undergoing carotid endarterectomy, whether initial indication for endarterectomy was symptomatic or asymptomatic carotid disease Settings: in hospitals with carotid centres Intervention: synthetic patch angioplasty Comparison: vein patch angioplasty | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Odds ratio (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Vein patch | Synthetic patch | |||||
| Perioperative ipsilateral stroke (< 30 days) | PTFE | OR 1.82 (0.49 to 6.78) | 590 (4 studies) | ⊝⊝⊝⊝ very lowa,b,c,d | None of these RCTs could be blinded for surgeons or patients due to the nature of the intervention All studies did not report funding sources Most studies were downgraded due to imprecision (wide confidence intervals and low event rates) |
|
| 7 per 1000 | 17 per 1000 | |||||
| Dacron | OR 2.86 (0.29 to 27.92) | 207 (1 study) | ⊝⊝⊝⊝ very lowa,b,e | |||
| 10 per 1000 | 28 per 1000 | |||||
| Total | OR 2.05 (0.66 to 6.38) | 797 (5 studies) | ⊝⊝⊝⊝ very lowa,b,c,d,e | |||
| 8 per 1000 | 20 per 1000 | |||||
| Perioperative combined stroke and TIA (< 30 days) | N/A | N/A | N/A | N/A | N/A | N/A |
| Perioperative death from all causes (< 30 days) | PTFE | OR 0.62 (0.16 to 2.41) | 609 (4 studies) | ⊕⊝⊝⊝ very lowa,b,c,d | None of these RCTs could be blinded for surgeons or patients due to the nature of the intervention Most studies did not report funding sources except 1 study, which had high risk of bias due to funding sources from the manufacturer (Hayes 2001) Most studies were downgraded due to imprecision (wide confidence intervals and low event rates) |
|
| 13 per 1000 | 7 per 1000 | |||||
| Dacron | OR 0.45 (0.10 to 2.03) | 673 (3 studies) | ⊝⊝⊝⊝ very lowa,b,e,f,g | |||
| 15 per 1000 | 6 per 1000 | |||||
| Polyester | OR 0.35 (0.01 to 8.81) | 87 (1 study) | ⊕⊝⊝⊝ lowa,b | |||
| 22 per 1000 | 0 per 1000 | |||||
| Total | OR 0.52 (0.20 to 1.34) | 1369 (8 studies) | ⊝⊝⊝⊝ very lowa,b,c,d,e,f | |||
| 15 per 1000 | 6 per 1000 | |||||
| Long‐term ipsilateral stroke | PTFE | OR 1.36 (0.47 to 3.88) | 500 (3 studies) | ⊝⊝⊝⊝ very lowa,b,c,d | None of these RCTs could be blinded for surgeons or patients due to the nature of the intervention Most studies did not report funding sources except 1 study, which had high risk of bias due to funding sources from the manufacturer (Hayes 2001) Most studies were downgraded due to imprecision (wide confidence intervals and low event rates) |
|
| 24 per 1000 | 32 per 1000 | |||||
| Dacron | OR 1.56 (0.54 to 4.50) | 276 (1 studies) | ⊝⊝⊝⊝ very lowa,b,g | |||
| 43 per 1000 | 66 per 1000 | |||||
| Total | OR 1.45 (0.69 to 3.07) | 776 (4 studies) | ⊝⊝⊝⊝ very lowa,b,c,d,g | |||
| 31 per 1000 | 44 per 1000 | |||||
| Long‐term any stroke | PTFE | OR 1.62 (0.63 to 4.18) | 609 (4 studies) | ⊕⊝⊝⊝ verylowa,b,c,d | None of these RCTs could be blinded for surgeons or patients due to the nature of the intervention Most studies did not report funding sources except 1 study, which had high risk of bias due to funding sources from the manufacturer (Hayes 2001) Most studies were downgraded due to imprecision (wide confidence intervals and low event rates) |
|
| 23 per 1000 | 36 per 1000 | |||||
| Dacron | OR 1.31 (0.60 to 2.87) | 471 (2 studies) | ⊝⊝⊝⊝ very lowa,b,f,g | |||
| 50 per 1000 | 65 per 1000 | |||||
| Polyester | OR 0.4 (0.07 to 2.18) | 87 (1 study) | ⊕⊝⊝⊝ lowa,b | |||
| 111 per 1000 | 48 per 1000 | |||||
| Total | OR 1.22 (0.70, 2.13) | 1167 (7 studies) | ⊝⊝⊝⊝ very lowa,b,c,d,f,g | |||
| 41 per 1000 | 48 per 1000 | |||||
| Long‐term stroke or death | PTFE | OR 1.02 (0.57 to 1.82) | 449 (3 studies) | ⊕⊝⊝⊝ very lowa,b,c,d | None of these RCTs could be blinded for surgeons or patients due to the nature of the intervention Most studies did not report funding sources except 1 study, which had high risk of bias due to funding sources from the manufacturer (Hayes 2001) Most studies were downgraded due to imprecision (wide confidence intervals and low event rates) |
|
| 121 per 1000 | 119 per 1000 | |||||
| Dacron | OR 1.07 (0.62 to 1.87) | 471 (2 studies) | ⊝⊝⊝⊝ very lowa,b,f,g | |||
| 120 per 1000 | 130 per 1000 | |||||
| Total | OR 1.05 (0.70, 1.56) | 920 (5 studies) | ⊝⊝⊝⊝ very lowa,b,dc,f,g | |||
| 121 per 1000 | 125 per 1000 | |||||
| Long‐term pseudoaneurysm formation | PTFE | OR 0.09 (0.02 to 0.49) | 500 (3 studies) | ⊕⊝⊝⊝ lowa,c,d | None of these RCTs could be blinded for surgeons or patients due to the nature of the intervention Most studies did not report funding sources except 1 study, which had high risk of bias due to funding sources from the manufacturer (Hayes 2001) |
|
| 56 per 1000 | 4 per 1000 | |||||
| Dacron | Not estimable | 276 (1 study) | ⊝⊝⊝⊝ very lowa,b,g | |||
| 0 per 1000 | 0 per 1000 | |||||
| Total | 776 (4 studies) | ⊝⊝⊝⊝ very lowa,b,c,d,g | ||||
| 36 per 1000 | 3 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not available; OR: odds ratio; PTFE: polytetrafluoroethylene; RCT: randomised controlled trial; TIA: transient ischaemic attack. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
| Long‐term: outcomes during long‐term follow‐up (≥ 1 year) including events during the first 30 days. | ||||||
aRisk of bias due torandomised trials did not blind surgeons and patients, and most studies did not report funding sources.
bImprecision was due to wide confidence intervals (low event rates).
cOne study had unclear risk for random sequence generation (AbuRahma 1996), and 2 studies did not report the method of allocation concealment (AbuRahma 1996; Gonzalez 1994).
dOne study did not report blinding of outcome assessment (Ricco 1996).
eOne study did not report blinding of outcome assessment with unclear risk for selection bias (Katz 1996).
fTwo studies did not report blinding of outcome assessment (Hayes 2001O'Hara 2002)
gOne study had high risk of bias due to funding sources from the manufacturer and did not report blinding of outcome assessment (Hayes 2001).
Summary of findings 2. Main comparison of Dacron patch versus other synthetic patch angioplasty.
| Dacron patch versus other synthetic patch angioplasty for carotid endarterectomy | ||||||
|
Patient or population: patients undergoing carotid endarterectomy, whether the initial indication for endarterectomy was symptomatic or asymptomatic carotid disease Settings: in hospitals with carotid centres Intervention: Dacron patch angioplasty Comparison: other synthetic patch angioplasty | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Odds ratio (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other synthetic material | Dacron patch | |||||
| Perioperative ipsilateral stroke (< 30 days) | PTFE | RR 3.35 (0.19 to 59.06) | 400 (2 studies) | ⊝⊝⊝⊝ very lowa,b,c,d | None of these RCTs could be blinded for surgeons or patients due to the nature of the intervention None of these RCTs reported funding sources and the number of patients lost to follow‐up |
|
| 10 per 1000 | 45 per 1000 | |||||
| Perioperative combined stroke and TIA (< 30 days) | PTFE | OR 4.41 (1.20 to 16.14) | 200 (1 study) | ⊕⊝⊝⊝ lowa,c | None of these RCTs could be blinded for surgeons or patients due to the nature of the intervention None of these RCTs reported funding sources and the number of patients lost to follow‐up |
|
| 30 per 1000 | 120 per 1000 | |||||
| Perioperative death from all causes (< 30 days) | PTFE | OR 1.51 (0.25 to 9.07) | 400 (2 studies) | ⊝⊝⊝⊝ very lowa,b,c,d | None of these RCTs could be blinded for surgeons or patients due to the nature of the intervention None of these RCTs reported funding sources Most studies were downgraded due to imprecision (wide confidence intervals and low event rates) |
|
| 5 per 1000 | 10 per 1000 | |||||
| Bovine pericardium | OR 3.55 (0.14 to 89.42) | 95 (1 study) | ⊝⊝⊝⊝ very lowa,b,e | |||
| 0 per 1000 | 23 per 1000 | |||||
| Polyurethane | OR 0.19 (0.01 to 4.11) | 104 (1 study) | ⊕⊝⊝⊝ lowa,b | |||
| 38 per 1000 | 0 per 1000 | |||||
| Total | OR 1.03 (0.30 to 3.57) | 599 (4 studies) | ⊝⊝⊝⊝ very lowa,b,c,d,e | |||
| 10 per 1000 | 10 per 1000 | |||||
| Long‐term ipsilateral stroke | PTFE | OR 1.52(0.25 to 9.27) | 200 (1 study) | ⊝⊝⊝⊝ very lowa,b,d | None of these RCTs could be blinded for surgeons or patients due to the nature of the intervention None of these RCTs reported funding sources Most studies were downgraded due to imprecision (wide confidence intervals and low event rates) |
|
| 20 per 1000 | 30 per 1000 | |||||
| Long‐term any stroke | PTFE | OR 16.12 (0.91 to 286.22) | 200 (1 study) | ⊝⊝⊝⊝ very lowa,b,c | None of these RCTs could be blinded for surgeons or patients due to the nature of the intervention None of these RCTs reported funding sources Most studies were downgraded due to imprecision (wide confidence intervals and low event rates) |
|
| 0 per 1000 | 7 per 1000 | |||||
| Polyurethane | OR 5.20 (0.24 to 110.95) | 104 (1 study) | ⊕⊝⊝⊝ lowa,b | |||
| 0 per 1000 | 38 per 1000 | |||||
| Total | OR 10.58 (1.34 to 83.43) | 304 (2 studies) | ⊕⊝⊝⊝ lowa,c | |||
| 0 per 1000 | 59 per 1000 | |||||
| Long‐term stroke or death | PTFE | OR 6.06 (1.31 to 28.07) | 200 (1 study) | ⊕⊝⊝⊝ lowa,c | None of these RCTs could be blinded for surgeons or patients due to the nature of the intervention None of these RCTs reported funding sources and the number of patients lost to follow‐up |
|
| 20 per 1000 | 110 per 1000 | |||||
| Long‐term pseudoaneurysm formation | N/A | N/A | N/A | N/A | N/A | N/A |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not available; OR: odds ratio; PTFE: polytetrafluoroethylene; RCT: randomised controlled trial; RR: risk ratio; TIA: transient ischaemic attack. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
| Long‐term: outcomes during long‐term follow‐up (≥ 1 year) including events during the first 30 days. | ||||||
aRisk of bias due to randomised trials did not blind surgeons and patients, and most studies did not report funding sources.
bImprecision was due to wide confidence intervals (low event rates).
cOne study did not report the number of patients lost to follow‐up (AbuRahma 2002).
dOne study did not report the number of patients lost to follow‐up (AbuRahma 2007).
eOne study was at high risk of bias due to no random sequence generation and unclear allocation concealment, and did not report on blinding of outcome assessment (Marien 2002).
Summary of findings 3. Main comparison of bovine pericardium patch versus other synthetic patch angioplasty.
| Bovine pericardium patch versus other synthetic patch angioplasty for carotid endarterectomy | ||||||
|
Patient or population: patients undergoing carotid endarterectomy, whether the initial indication for endarterectomy was symptomatic or asymptomatic carotid disease Settings: in hospitals with carotid centres Intervention: bovine pericardium patch angioplasty Comparison: other synthetic patch angioplasty | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Odds ratio (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other synthetic material | Bovine pericardium patch | |||||
| Perioperative ipsilateral stroke (< 30 days) | N/A | N/A | N/A | N/A | N/A | N/A |
| Perioperative combined stroke and TIA (< 30 days) | PTFE | OR 4.17 (0.46 to 38.02) | 195 (1 study) | ⊕⊕⊕⊝ lowa,b,c | None of these RCTs could be blinded for surgeons or patients due to the nature of the intervention None of these RCTs reported funding sources Most studies were downgraded due to imprecision (wide confidence intervals and low event rates) |
|
| 41 per 1000 | 10 per 1000 | |||||
| Dacron | OR 0.22 (0.01 to 4.76) | 95 (1 study) | ⊕⊕⊝⊝ very lowa,b,d | |||
| 0 per 1000 | 39 per 1000 | |||||
| Total | OR 1.18 (0.07 to 20.39) | 290 (2 studies) | ⊕⊕⊝⊝ very lowa,b,c,d | |||
| 28 per 1000 | 20 per 1000 | |||||
| Perioperative death from all causes (< 30 days) | PTFE | OR 5.16 (0.24 to 108.83) | 195 (1 study) | ⊕⊕⊕⊝ lowa,b,c | None of these RCTs could be blinded for surgeons or patients due to the nature of the intervention None of these RCTs reported funding sources Most studies were downgraded due to imprecision (wide confidence intervals and low event rates) |
|
| 21 per 1000 | 0 per 1000 | |||||
| Dacron | OR 3.55 (0.14 to 89.42) | 95 (1 study) | ⊕⊕⊝⊝ very lowa,b,d | |||
| 23 per 1000 | 0 per 1000 | |||||
| Total | OR 4.39 (0.48 to 39.95) | 290 (2 studies) | ⊕⊕⊝⊝ very lowa,b,c,d | |||
| 21 per 1000 | 0 per 1000 | |||||
| Long‐term ipsilateral stroke | PTFE | OR 4.17 (0.46 to 38.02) | 195 (1 study) | ⊕⊕⊕⊝ lowa,b,c | None of these RCTs could be blinded for surgeons or patients due to the nature of the intervention None of these RCTs reported funding sources Most studies were downgraded due to imprecision (wide confidence intervals and low event rates) |
|
| 93 per 1000 | 31 per 1000 | |||||
| Long‐term any stroke | N/A | N/A | N/A | N/A | N/A | N/A |
| Long‐term stroke or death | N/A | N/A | N/A | N/A | N/A | N/A |
| Long‐term pseudoaneurysm formation | N/A | N/A | N/A | N/A | N/A | N/A |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; OR: odds ratio; PTFE: polytetrafluoroethylene; RCT: randomised controlled trial; TIA: transient ischaemic attack. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
| Long‐term; outcomes during long‐term follow‐up (≥ 1 year) including events during the first 30 days. | ||||||
aRisk of bias due to randomized trials did not blind surgeons and patients, and most studies did not report funding sources.
bImprecision due to wide confidence intervals (low event rates).
cOne study did not report blinding of outcome assessment (Stone 2014).
dOne study was at high risk of bias due to no random sequence generation and unclear allocation concealment, and did not report on blinding of outcome assessment (Marien 2002).
Background
Description of the condition
Stroke is one of the leading causes of mortality in the world. In the European population (GBD 2019), 1.4 million strokes occur each year (Truelsen 2006). In the UK, 150,000 first‐ever strokes occurred during the Oxfordshire Community Stroke Project from 1981 to 1986 (Bamford 1988). Stroke is the second leading cause of death (Nichols 2012). Eighty‐five per cent of strokes are ischaemic (Bamford 1988). The most common cause of ischaemic stroke is stenosis or occlusion of the atherosclerotic internal carotid artery and/or middle cerebral artery. Carotid endarterectomy (CEA) has been shown in large, well‐conducted randomised controlled trials (RCTs) to reduce the risk of stroke in patients with recently symptomatic, severe stenosis (> 70%) of the extracranial internal carotid artery (ECST 1991; ECST 1998; NASCET 1991; NASCET 1998). Some evidence suggests that CEA may be beneficial for some categories of asymptomatic patients (ACAS 1995; ACST‐1 2010). A multi‐centre RCT has shown that immediate CEA confers a 4.6% absolute risk reduction compared with medical therapy in asymptomatic patients (ACST‐1 2010).
Description of the intervention
Carotid endarterectomy is a surgical procedure undertaken to correct internal carotid stenosis from inside the carotid artery wall. In a standard endarterectomy, the most popular technique, carotid plaque is removed by a longitudinal arteriotomy. What is less clear at present is whether different surgical techniques affect the outcome, although increasing evidence suggests that carotid patch angioplasty is superior to primary closure in reducing the risk of re‐stenosis and improving both short‐ and long‐term clinical outcomes (Counsell 1998; Rerkasem 2010). Consequently, many vascular surgeons use carotid patching either routinely or selectively. However, considerable debate over the choice of patch material is ongoing.
How the intervention might work
Vein patching (with vein usually harvested from the saphenous vein and sometimes from the jugular vein) is favoured by some on the basis that a non‐randomised comparison suggested it was better for preventing stroke or death (Fode 1986). Vein patching also offers the advantages of being easily available and easy to handle, with possibly greater resistance to infection. Synthetic material such as Dacron or polytetrafluoroethylene (PTFE) is favoured by others, who feel that it offers lower risk of patch rupture ‐ Murie 1994 ‐ and aneurysmal dilatation ‐ Gonzalez 1994 ‐ and that it spares the morbidity associated with saphenous vein harvesting and leaves the vein intact, which may be required for coronary bypass grafting at a later date. It is also possible that one type of synthetic material is better than another. For example, AbuRahma 2002 found that PTFE resulted in fewer perioperative carotid thromboses and strokes than Dacron. Finally, biomaterials such as bovine pericardium are now in common use, and some evidence suggests that bovine pericardium provides faster haemostasis time than PTFE without differences in perioperative or late neurological events or re‐stenosis (Kim 2001; Stone 2014).
Why it is important to do this review
Multiple RCTs have compared outcomes between different materials for carotid patch after endarterectomy. The most reliable evidence on the best material to use comes from these trials, and it is important to synthesise the results so we can identify the best patch material.
Objectives
To assess the safety and efficacy of different types of patch materials used in carotid patch angioplasty. The primary hypothesis was that a synthetic material was associated with lower risk of patch rupture versus venous patches, but that venous patches were associated with lower risk of perioperative stroke and early or late infection, or both.
Methods
Criteria for considering studies for this review
Types of studies
We sought to identify all unconfounded randomised trials in which one type of carotid patch was compared to another. We also included quasi‐randomised trials in which allocation to different treatment regimens was not adequately concealed (e.g. allocation by alternation, date of birth, hospital number, day of the week, or by using an open random number list).
Types of participants
We considered trials that included any type of patient undergoing carotid endarterectomy as eligible, whether the initial indication for endarterectomy was symptomatic or asymptomatic carotid disease.
Types of interventions
We sought to identify all trials comparing one type of patch material with another in CEA. Currently available materials include saphenous vein (harvested from either the ankle or the groin), Dacron, polytetrafluoroethylene (PTFE) polyester or polyurethane, and bovine pericardium.
Types of outcome measures
Primary outcomes
Perioperative ipsilateral stroke (< 30 days)
Long‐term ipsilateral stroke (outcomes during long‐term follow‐up (at least one year) including events during the first 30 days)
Ipsilateral stroke describes insufficient blood flow to the cerebral hemisphere secondary to same side occlusion or severe stenosis of the internal carotid artery.
Secondary outcomes
Perioperative clinical outcome including any stroke, combined stroke and transient ischaemic attack (TIA), death from all causes, fatal stroke, stroke, or death (< 30 days)
Perioperative complications (< 30 days) including arterial rupture, cranial nerve palsy, wound infection, wound haemorrhage, early re‐stenosis or arterial occlusion, complication requiring further operation
Long‐term clinical outcome including any stroke, combined stroke and TIA, death from all causes, fatal stroke, stroke, or death (outcomes during long‐term follow‐up (at least one year) including events during the first 30 days)
Long‐term complications including infection of the endarterectomy site, arterial occlusion/re‐stenosis > 50%, pseudoaneurysm formation (outcomes during long‐term follow‐up (at least one year) including events during the first 30 days)
Search methods for identification of studies
See the methods for the Cochrane Stroke Group Specialised register. We did not use any language restrictions in the searches; we arranged translation of all possibly relevant publications when necessary.
Electronic searches
We searched the Cochrane Stroke Group's Trials Register, which was last searched by the Cochrane Stroke Group's Information Specialist on 25 May 2020. We also updated electronic searches and handsearched additional issues of relevant journals as follows.
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 5), in the Cochrane Library (searched 25 May 2020); MEDLINE Ovid (1946 to 25 May 2020); Embase Ovid (1980 to 25 May 2020); the Index to Scientific and Technical Proceedings (1980 to 25 May 2020; searched using the terms "carotid" and ("trial* or random*")); and the Web of Science Core Collection (last searched 25 May 2020).
Searching other resources
We searched the following ongoing trials in the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 25 May 2020) and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 25 May 2020) (Higgins 2016).
We handsearched the following journals including conference supplements.
Annals of Surgery (1981 to 25 May 2020).
Annals of Vascular Surgery (1994 to 25 May 2020).
Cardiovascular Surgery (now Vascular) (1994 to 25 May 2020).
European Journal of Vascular Surgery (now European Journal of Vascular and Endovascular Surgery) (1987 to 25 May 2020).
Journal of Vascular Surgery (1994 to 25 May 2020).
Stroke (1994 to 25 25 May 2020).
We reviewed the reference lists of all relevant studies. We contacted experts in the field to identify further published and unpublished studies.
For the previous version of the review, we handsearched the following journals including conference supplements.
American Journal of Surgery (1994 to 25 May 2020).
British Journal of Surgery (1985 to 25 May 2020).
World Journal of Surgery (1978 to 25 May 2020).
We handsearched abstracts of the following meetings for the years 1995 to 25 May 2020.
AGM of the Vascular Surgical Society (UK).
AGM of the Association of Surgeons of Great Britain and Ireland.
American Heart Association Stroke Conference.
Annual Meeting of the Society for Vascular Surgery (USA).
The European Stroke Conference.
Data collection and analysis
Selection of studies
Three review authors (SO, TB, and KR) independently read the titles and abstracts of records obtained from the searches, excluded obviously irrelevant studies, and selected those trials that met the inclusion criteria. We obtained the full‐text articles of potentially relevant studies. All three review authors (SO, TB, and KR) screened all documents and independently extracted data, including details of methods, participants, setting, context, interventions, outcomes, results, publications, and investigators. We resolved all disagreements through discussion and performed meta‐analysis using RevMan 5.4 (RevMan 2020).
Data extraction and management
Two review authors independently reviewed and assessed all trials (so each trial received two assessments) and double‐checked all data extracted. We recorded the following details: randomisation method, blinding of clinical and Doppler assessments, whether outcomes were reported for all participants originally randomised to each group irrespective of whether they received the operation they were allocated to or whether the participant was excluded after randomisation, and the number of participants lost to follow‐up. We sought data on the number of outcome events for all participants originally randomised to allow an intention‐to‐treat analysis. For the 14 included trials, we also extracted details about participants included in the trials, inclusion and exclusion criteria, individual patient data and participant characteristics (age, gender, indication for surgery), type of carotid patching, comparability of treatment and control groups for important prognostic factors, type of patch, type of anaesthetic, use of shunts, and use of antiplatelet therapy during follow‐up. We merged data into a single composite database and gave detailed consideration to the definition for each variable used in the original trials. Much of the above data were not available from the publications, and so we sought further information from triallists in all cases; however, we did not always receive a response. We resolved all disagreements through discussion with other review authors (BS, DPH).
Assessment of risk of bias in included studies
Two review authors (SO, BS) independently assessed risk of bias (high risk, low risk, unclear risk) using the Cochrane ’Risk of bias’ tool as described in the Cochrane Handbook for Systematic Reviews of Interventions and reported details in the ’Risk of bias’ tables (Higgins 2011). We resolved all disagreements through discussion. Risks of bias included random sequence generation and allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), and incomplete outcome data (attrition bias).
Measures of treatment effect
We used RevMan 5.4 to carry out statistical analyses to determine the estimates of effect and to describe the magnitude of the intervention effect in terms of how different outcome data were between the two groups (RevMan 2020). Types of intervention effects include ratio effect measures that compare the odds of an event between two groups (odd ratios (ORs)); every estimate is expressed with a measure of that uncertainty, including a confidence interval (CI).
For dichotomous variables, we calculated proportional risk reductions based on weighted estimate of the OR using the Peto method (APT 1994). We calculated absolute risk reductions from the crude risks of each outcome in all trials combined with 95% CIs.
Unit of analysis issues
All trials randomised the artery rather than the participant, and all included participants who had bilateral carotid endarterectomies. Therefore, it was possible for a single participant to have both types of patch material. For these participants, it would be difficult to relate death or stroke to one particular procedure. In the previous version of this review (Rerkasem 2010), for trials in which it was possible for a patient to have both procedures, death and any stroke were analysed only in those who had unilateral procedures or the same procedure to both arteries. When we could not obtain data from the authors of relevant trials, we excluded the whole trial from the analysis (Lord 1989). Of the six studies published since 1995, two ensured that participants undergoing more than one operation were assigned the same closure method (Hayes 2001; O'Hara 2002), whereas four did not (AbuRahma 1996; AbuRahma 2002; Katz 1996; Stone 2014). However, most participants who undergo more than one operation will have a period of at least 30 days between procedures; therefore, short‐term results are likely to be reliable regardless of how data are analysed. Only one of the later studies reported long‐term follow‐up (AbuRahma 1996), but the number of bilateral operations was small compared with the total number of operations carried out, and this was unlikely to bias the results significantly. Therefore, we included this trial, but this should be borne in mind when results are interpreted.
A separate analysis of only strokes ipsilateral to the operated artery was also performed for each artery. However, the total number of strokes was very similar to the number of ipsilateral strokes because in the majority of studies, all strokes were ipsilateral. Arterial complications, such as occlusion, haemorrhage from the endarterectomy site, re‐stenosis, infection at the operation site, or pseudoaneurysm formation, were analysed for all arteries rather than for participants. Analyses based on arteries assumed that for participants who had bilateral endarterectomies, outcome events in each carotid artery were independent.
Dealing with missing data
When data were missing, we contacted the corresponding author or co‐author through the address given in the publication. If this information was not available, we searched for the study group via the Internet and contacted group members for missing information.
Assessment of heterogeneity
We assessed heterogeneity between study results using the I² statistic (Higgins 2020). This examined the percentage of total variation across studies due to heterogeneity rather than to chance. Thresholds for interpretation of the I² statistic can be misleading, in that the importance of inconsistency depends on several factors. A rough guide to interpretation in the context of meta‐analyses of randomised trials is as follows.
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
We performed an extensive literature search, and we are confident that we have identified all major relevant trials. We also contacted experts in this field. We searched for trials published in all languages, and we arranged translation of all possibly relevant publications when required. In addition, we searched all relevant ongoing clinical trials from ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) portal, and we handsearched relevant journals and reference lists. We had planned to compare study protocols with final study reports to evaluate selective reporting of outcomes. We used funnel plots to assess publication bias because more than 10 studies were included (Sterne 2011). However, none of the trials reported limits outside the 95% CI.
Data synthesis
We included in the combined analysis all participants included in the final analysis of results of the original trials, using the Mantel Haenszel method. We used the fixed‐effect model for meta‐analysis in the absence of clinical, methodological, and statistical heterogeneity. If the I² statistic was high, we also applied a random‐effects model to see whether the conclusions differed, and we noted any differences (Higgins 2003). We performed all analyses of the effects of surgery on an intention‐to‐treat basis according to randomised treatment allocation. We assessed the significance of differences between treatment groups by the log‐rank test stratified by study. We tested the significance of differences in baseline data between trials and treatment groups using the Chi² test or Student’s t‐test, as appropriate. We used Cochrane RevMan 5.4 software (RevMan 2020), as well as SPSS for Windows version 26.0, for all analyses (SPSS 2019 [Computer program]). If pooling was not possible or appropriate, we had planned to present a narrative summary (Deeks 2011).
Pooling of individual patient data
We obtained original individual patient data for the 14 included trials. We merged data on presenting events, baseline clinical data, operative details, surgical and anaesthetic techniques, perioperative events, and long‐term follow‐up into a single composite database. We gave detailed consideration to the definition for each variable used in the original trials. When definitions were identical, we merged comparable data. When possible, we resolved differences in definitions of variables between studies by reconstructing definitions to achieve comparability.
Subgroup analysis and investigation of heterogeneity
We planned to explore heterogeneity by conducting subgroup analyses. We specified the following subgroup analyses.
Age (younger than 65 years old versus 65 to 74 years old versus 75+ years old).
Gender (men versus women).
Diabetes versus no diabetes.
Hypertension versus no hypertension.
Previous myocardial infarction or angina versus no coronary artery disease.
Peripheral arterial disease (PAD) versus no PAD.
Current smoker versus non‐smoker.
Asymptomatic disease versus symptomatic disease of carotid stenosis.
Contralateral carotid stenosis versus unilateral carotid stenosis.
Contralateral carotid occlusion versus no occlusion.
Preoperative antiplatelet therapy versus no antiplatelet therapy.
Intraoperative shunt versus no shunt.
Irregular or ulcerated symptomatic carotid plaque versus smooth plaque on the pre‐randomisation angiogram.
We planned to use an established method for subgroup analyses (Deeks 2001). In the future, we will fulfil planned subgroup analyses when more studies are included in a single analysis, all with sufficient information to reveal the subgroups.
Analyses were stratified by patch type. Tests for overall effect and subgroup differences by patch type included synthetic material versus vein, Dacron versus other synthetic material, and bovine pericardium versus synthetic material. Each subgroup was analysed for perioperative events (< 30 days) and events during long‐term follow‐up (at least one year), including events during the first 30 days. So, a total of six groups of data and analyses were examined. All outcomes from 12 RCTs were collected directly from two‐arm comparisons (AbuRahma 2002; AbuRahma 2007; Albrecht-Fruh 1998; Gonzalez 1994; Grego 2003; Hayes 2001; Katz 1996; Marien 2002; Meerwaldt 2008; O'Hara 2002; Ricco 1996; Stone 2014). Only two included RCTs have multiple arms. Multiple‐arm analysis compared different types of patches and primary closure of the endarterectomy site (AbuRahma 1996; Lord 1989). One RCT used three primary comparisons between non‐patch, PTFE, and saphenous vein (AbuRahma 1996). However, individual comparison probabilities were set at P = 0.0167 on the basis of the Bonferroni method for correction for multiple comparisons. Thus, outcomes of these studies were not extracted from the subgroup analysis. We did not investigate potential effect modifiers via subgroup analysis.
Sensitivity analysis
We planned to undertake the following sensitivity analyses to explore effects of methodological features when decisions for the process undertaken in this systematic review were somewhat arbitrary or unclear.
Allocation concealment: we planned to repeat analysis and to exclude high risk of selection bias trials.
Blinding of outcome assessment: we planned to repeat data analysis and to exclude high risk of detection bias trials.
Incomplete outcome data: we planned to repeat data analysis, to identify the method of dealing with missing outcome data, and to exclude high risk of attrition bias trials.
Selective reporting: we planned to repeat data analysis, to find evidence of published findings on all study outcomes, and to exclude high risk of reporting bias trials.
Other bias: publication type: we planned to exclude trials with the absence of peer‐review.
Given that foreknowledge of treatment allocation might lead to biased treatment allocation and exaggerated treatment effects (Schulz 1995), we performed in the first version of this review separate sensitivity analyses of those trials in which allocation concealment was secure and those in which it was less secure. However, we found no significant differences between trials with different allocation techniques, and no studies in this later review were quasi‐randomised; therefore, we did not carry out sensitivity analyses for this version of the review.
Summary of findings and assessment of the certainty of the evidence
We created three Summary of findings tables for the main comparisons with GRADE Profiler 3.6 (GRADEpro 2015), which imports data from RevMan 5 (RevMan 2020). This table presents the results and the quality of the evidence of the main outcomes, using the GRADE system, which classifies the quality of evidence as high, moderate, low, and very low (Schünemann 2011). We included seven important outcomes including major outcome: 1) perioperative combined stroke and TIA (< 30 days); 2) perioperative death from all causes (< 30 days); 3) perioperative fatal stroke (< 30 days); 4) longterm any stroke; 5) longterm stroke or death, complications; 6) perioperative wound infection (< 30 days); and 7) longterm pseudoaneurysm formation.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
We identified 25,599 records through database searching and 84 additional records from other sources in 2020. These searches yielded a total of 12,560 records after de‐duplication; only 43 full‐text articles remained after title and abstract screening. Finally, upon screening the full text, we excluded all 42 full‐text articles because they did not meet the inclusion criteria. We included one new study in this update of the review (Stone 2014). The review now includes 14 RCTs of different types of patches for carotid patch angioplasty; we found no ongoing studies. See Figure 1. It is important to note that the number of studies identified in the search process was consistently smaller than the number in the previous (2010) version. This might be due to the application of new search methods (i.e. highly sensitive search strategies).
1.
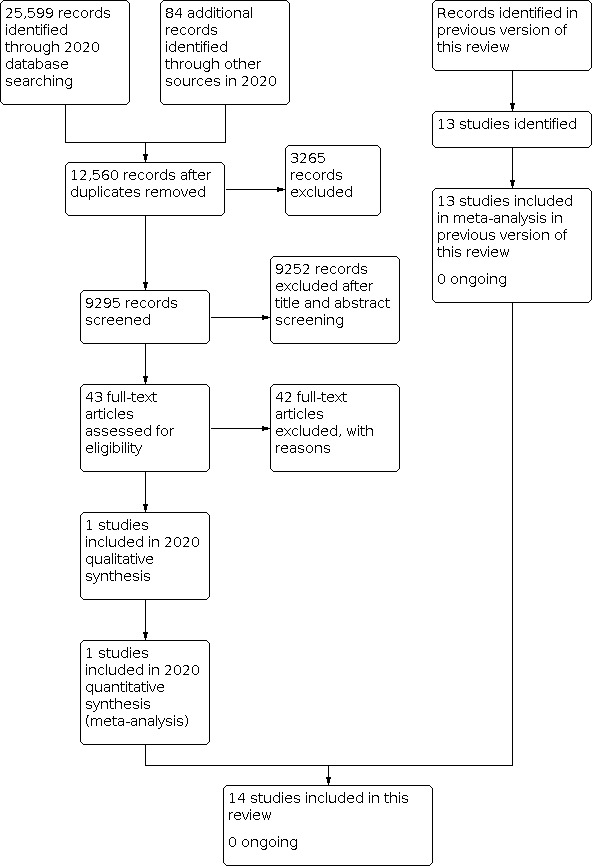
Study flow diagram.
Included studies
The previous version of this review included 13 trials involving a total of 2083 operations available for analysis (Rerkasem 2010). Since that time, many prospective and retrospective studies have examined different patch types. However, only one additional RCT of sufficient standard had been performed, and this has been included in the current review. See Characteristics of included studies.
The addition of the new trial comparing synthetic polytetrafluoroethylene (PTFE) with bovine pericardium patching increased the total number of trials to 14, with 2278 operations available for analysis (Figure 1) (Stone 2014). Seven trials compared vein closure with PTFE closure, five compared Dacron grafts with other synthetic materials, and two compared bovine pericardium with other synthetic materials. Two trials compared vein to PTFE and polyester patch (Grego 2003; Meerwaldt 2008), one compared Dacron to PTFE patching (AbuRahma 2007), and the rest compared Dacron with other synthetic materials, namely, polyurethane patch ‐ Albrecht‐Fruh 1998 ‐ and bovine pericardium ‐ Marien 2002. One pre‐1995 and one post‐1995 trial had three arms: saphenous vein patching, PTFE patching, and primary closure (AbuRahma 1996; Lord 1989). Only results from the vein patching and PTFE patching groups are included in this review. Four trials compared saphenous vein harvested from the groin with synthetic patches (Hayes 2001; Katz 1996; Lord 1989; Ricco 1996). Two trials used saphenous vein from the ankle (Gonzalez 1994; Meerwaldt 2008), one trial alternately used vein from the jugular vein and from the saphenous vein at the ankle (AbuRahma 1996), and one trial used vein from the external jugular vein (Grego 2003). One trial did not specify a site (O'Hara 2002). In all trials, operations were performed under general anaesthetic, and most were also performed with shunting. All patients received antiplatelet therapy perioperatively. One study used heparin reversal at completion of surgery in 30% of synthetic closure patients but not in vein closure patients (Katz 1996). One used heparin reversal in all patients (Gonzalez 1994), five used reversal in none (AbuRahma 1996; AbuRahma 2002; AbuRahma 2007; Grego 2003; Ricco 1996), and data were unavailable for six cases (Albrecht‐Fruh 1998; Hayes 2001; Lord 1989; Marien 2002; Meerwaldt 2008; O'Hara 2002).
Early (within 30 days) postoperative arterial occlusion or carotid thrombosis was assessed by duplex sonography or angiography in 11 trials (AbuRahma 1996; AbuRahma 2002; AbuRahma 2007; Albrecht‐Fruh 1998; Gonzalez 1994; Grego 2003; Hayes 2001; Marien 2002; Meerwaldt 2008; Ricco 1996; Stone 2014), and assessment was based on symptoms in only two trials (Katz 1996; Lord 1989). During long‐term follow‐up, re‐stenosis of the arteries was assessed by Duplex ultrasound in 11 trials (AbuRahma 1996; AbuRahma 2002; AbuRahma 2007; Albrecht‐Fruh 1998; Grego 2003; Hayes 2001; Marien 2002; Meerwaldt 2008; O'Hara 2002; Ricco 1996; Stone 2014), and assessment was done by Doppler ultrasound and intravenous digital subtraction angiography in another (Gonzalez 1994). Two trials provided only data on ipsilateral strokes, and it is unclear whether any other strokes occurred during follow‐up (Lord 1989; Ricco 1996).
In the pre‐2002 review, the average age of patients involved in the trials was about 67.5 years, 60% to 80% were men, and less than 36% of operations were performed for asymptomatic carotid disease. In the studies conducted since 2002, the average age of patients was 67.65 years, 50% to 80% were men, and 47% of the operations were performed for asymptomatic carotid disease (excluding one study that intended to operate only on symptomatic patients (Meerwaldt 2008)). One trial included only patients with narrow internal carotid arteries (< 5 mm external diameter) and excluded patients with recurrent carotid stenosis (Ricco 1996), whereas two trials excluded patients with internal carotid diameters smaller than 4 mm (AbuRahma 1996; AbuRahma 2002). All but two trials excluded patients undergoing either recurrent carotid endarterectomy or combined coronary and carotid surgery at the same time (AbuRahma 1996; AbuRahma 2002; AbuRahma 2007; Grego 2003; Hayes 2001; Katz 1996; Marien 2002; O'Hara 2002; Ricco 1996), one excluded no patients at all (Gonzalez 1994), and one did not provide information on exclusions (Lord 1989). In all but four trials, treatment groups were comparable for important prognostic factors. Two trials included more men in the synthetic group than in the vein patch group (Grego 2003; O'Hara 2002). Another two trials reported different stroke rates between the two groups (Marien 2002; Meerwaldt 2008).
Excluded studies
See Characteristics of excluded studies.
Three RCTs of carotid endarterectomy did not meet our inclusion criteria. Our outcomes, which were decided at the time of setting up the review, were clinical outcomes that do not include non‐relevant outcomes such as microemboli, number of oxidized cellulose packets, etc. So, our review addressed the potential for carotid patch angioplasty with different types of patch materials to prevent a particular clinical outcome (Mckenzie 2020). The first trial exclusion was based on lack of clinical data involving 74 patients randomised between Dacron, PTFE, and venous patch by an open random number list. The main outcomes were number of packets of oxidized cellulose used and elapsed time between removal of carotid‐occluding clamps and completion of the procedure (Carney 1987). The second trial outcome looked at microemboli perioperatively, which was not related to a clinical outcome nor to the efficacy of carotid patch material angioplasty (Chyatte 1996). The last excluded trial recorded bleeding time and microemboli perioperatively (Ruckert 2000).
Risk of bias in included studies
Included trials had several significant flaws (Figure 2; Figure 3). However, trials published after 2002 were generally of better quality than those published before that time. Up to 2002, 6 out of 10 studies reported a method of randomisation (AbuRahma 1996; AbuRahma 2002; Hayes 2001; Marien 2002; O'Hara 2002; Ricco 1996); after 2002, all 3 trials reported a method of randomisation (AbuRahma 2007; Grego 2003; Meerwaldt 2008). Adequately concealed allocation was performed in 7 of the 10 pre‐2002 trials (AbuRahma 1996; AbuRahma 2002; Gonzalez 1994; Hayes 2001; Lord 1989; O'Hara 2002; Ricco 1996), and after 2002, all four trials had adequately concealed allocation (AbuRahma 2007; Grego 2003; Meerwaldt 2008; Stone 2014). Eight trials attempted to perform blinded follow‐up (AbuRahma 1996; AbuRahma 2002; AbuRahma 2007; Grego 2003; Hayes 2001; Meerwaldt 2008; O'Hara 2002; Ricco 1996). One trial did not blind outcome assessments and followed patients only until hospital discharge (Lord 1989). As mentioned previously, one of the main flaws in all trials was that a patient undergoing bilateral carotid endarterectomy could be randomised twice and have each carotid artery randomised to different treatment groups. In these trials, it is unclear from published reports how many patients in each group underwent bilateral procedures that were different in each artery, and whether any deaths or strokes occurred in these patients. True intention‐to‐treat analyses were possible in seven trials (AbuRahma 1996; AbuRahma 2002; AbuRahma 2007; Gonzalez 1994; Hayes 2001; Lord 1989; O'Hara 2002).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Eight included studies were RCTs with adequate generation of a randomised sequence, and we assessed them to be at low risk of bias (AbuRahma 2002; AbuRahma 2007; Gonzalez 1994; Grego 2003; Hayes 2001; O'Hara 2002; Ricco 1996; Stone 2014). Five RCTs did not report the random sequence generation method, and we assessed them to be at unclear risk of bias (AbuRahma 1996; Albrecht‐Fruh 1998; Katz 1996; Lord 1989; Meerwaldt 2008). One included study was at high risk of bias due to sequence generation that was based on the last number of the patient's medical record (Marien 2002). Allocation concealment was adequate in eight trials, which we assessed to be at low risk of bias (AbuRahma 2002; AbuRahma 2007; Grego 2003; Hayes 2001; Meerwaldt 2008; O'Hara 2002; Ricco 1996; Stone 2014). In six trials, it is not clear whether allocation to groups was adequately concealed; we assessed these trials to be at unclear risk of bias (AbuRahma 1996; Albrecht‐Fruh 1998; Gonzalez 1994; Katz 1996; Lord 1989; Marien 2002).
Blinding
Because of the nature of the intervention, none of these RCTs could be blinded for surgeons or participants (AbuRahma 1996; AbuRahma 2002; AbuRahma 2007; Albrecht‐Fruh 1998; Gonzalez 1994; Grego 2003; Hayes 2001; Katz 1996; Lord 1989; Marien 2002; Meerwaldt 2008; O'Hara 2002; Ricco 1996; Stone 2014). Five studies made use of an independent external review process for all outcomes (AbuRahma 2002; AbuRahma 2007; Gonzalez 1994; Grego 2003; Meerwaldt 2008), but the clinical data presented for review were derived from the unblinded assessment discussed above and may, in theory, have been subject to bias. We assessed performance bias to be at high risk of bias in all 14 RCTs (AbuRahma 1996; AbuRahma 2002; AbuRahma 2007; Albrecht‐Fruh 1998; Gonzalez 1994; Grego 2003; Hayes 2001; Katz 1996; Lord 1989; Marien 2002; Meerwaldt 2008; O'Hara 2002; Ricco 1996; Stone 2014), and we assessed detection bias to be at unclear risk in nine RCTs, which did not address blinding of outcome assessment (AbuRahma 1996; Albrecht‐Fruh 1998; Hayes 2001; Katz 1996; Lord 1989; Marien 2002; O'Hara 2002; Ricco 1996; Stone 2014).
Incomplete outcome data
Few participants were lost to follow‐up in any of these studies. The design features of the 14 RCTs are summarised in Characteristics of included studies. Only two included trials did not report follow‐up patient data and cross‐over data between different types of arm patches (AbuRahma 2002; AbuRahma 2007). We assessed attrition bias to be at unclear risk of bias for both these trials (AbuRahma 2002; AbuRahma 2007), and we assessed it to be at low risk of bias for the other trials (AbuRahma 1996; Albrecht‐Fruh 1998; Gonzalez 1994; Grego 2003; Hayes 2001; Katz 1996; Lord 1989; Marien 2002; Meerwaldt 2008; O'Hara 2002; Ricco 1996; Stone 2014).
Selective reporting
Study authors published findings on all study outcomes. This was entirely appropriate and is very unlikely to have introduced any bias into the results. We assessed selective reporting to be at low risk of bias in all included RCTs (AbuRahma 1996; AbuRahma 2002; AbuRahma 2007; Albrecht‐Fruh 1998; Gonzalez 1994; Grego 2003; Hayes 2001; Katz 1996; Lord 1989; Marien 2002; Meerwaldt 2008; O'Hara 2002; Ricco 1996; Stone 2014). Data for analysis in this review were based on all study outcomes, and all results are included in the analysis. These data were not a subset of the original variables recorded.
Other potential sources of bias
We judged other potential sources of bias to be of low risk in all RCTs (AbuRahma 1996; AbuRahma 2002; AbuRahma 2007; Albrecht‐Fruh 1998; Gonzalez 1994; Grego 2003; Hayes 2001; Katz 1996; Lord 1989; Marien 2002; Meerwaldt 2008; O'Hara 2002; Ricco 1996; Stone 2014).
Effects of interventions
See: Table 1; Table 2; Table 3
We included in this review data from 14 trials involving 2278 operations. The results presented may differ from those in the published reports when we have obtained additional information from study authors. There was no statistical heterogeneity in any of the analyses except outcome of perioperative ipsilateral stroke, any stroke, and long‐term death; arterial occlusion between Dacron and other synthetic patch (Analysis 2.1; Analysis 2.2; Analysis 5.3; Analysis 5.5), and outcome of perioperative combined stroke and TIA, complication requiring further operation, and wound haemorrhage between bovine and other synthetic patch (Analysis 3.3; Analysis 3.6; Analysis 3.8).
2.1. Analysis.

Comparison 2: Perioperative events: Dacron versus other synthetic patch (< 30 days), Outcome 1: Ipsilateral stroke
2.2. Analysis.

Comparison 2: Perioperative events: Dacron versus other synthetic patch (< 30 days), Outcome 2: Any stroke
5.3. Analysis.

Comparison 5: Events during long‐term follow‐up (≥ 1 year) including events during first 30 days: Dacron versus other synthetic, Outcome 3: Death
5.5. Analysis.

Comparison 5: Events during long‐term follow‐up (≥ 1 year) including events during first 30 days: Dacron versus other synthetic, Outcome 5: Arterial occlusion/re‐stenosis > 50%
3.3. Analysis.

Comparison 3: Perioperative events: bovine versus other synthetic (< 30 days), Outcome 3: Combined stroke and TIA
3.6. Analysis.

Comparison 3: Perioperative events: bovine versus other synthetic (< 30 days), Outcome 6: Complication requiring further operation
3.8. Analysis.

Comparison 3: Perioperative events: bovine versus other synthetic (< 30 days), Outcome 8: Wound haemorrhage
Operative details
Duration of operation
The duration of the operation was not analysed statistically because evidence shows that durations were not normally distributed. Synthetic patching was associated with significantly longer operation times than vein patching in one trial: 128.0 ± 4.1 minutes versus 112.8 ± 3.5 minutes (P < 0.05) (Gonzalez 1994). However, synthetic patching was observed to have longer operation times versus vein patching with no statistically significant differences in two trials: 100 ± 21 minutes versus 93 ± 25 minutes (P = 0.1) (Meerwaldt 2008), and 105 minutes versus 89 minutes (P > 0.05) (Ricco 1996). In contrast, two other trials found vein patching was observed to have longer operation times versus either PTFE or Dacron patching, but there was no statistically significant difference in the two trials: mean times 126 ± 27 minutes versus 123 ± 28 minutes (P > 0.05) (AbuRahma 1996), and median times 105 minutes (95% confidence interval (CI) 102 to 115) versus 103 minutes (95% CI 102 to 114) (P = 0.71) (Hayes 2001).
In the first two cases, this difference was due to longer haemostasis times with PTFE. However, the difference in the second pair of trials was explained by the longer time required to harvest vein from the groin or neck. The trial that was excluded because of lack of clinical data reported that time from release of the clamps to completion of the operation was longer with PTFE patches (53 minutes) compared with both vein patching (41 minutes) and Dacron patching (45 minutes) because of excessive bleeding (Carney 1987). The trials comparing PTFE with Dacron patching found no significant differences in operation time except in two trials: mean times 119 ± 26 minutes versus 113 ± 22 minutes (P = 0.81) (AbuRahma 2002), and mean times 97.4 ± 3.7 minutes versus 95.9 ± 18.7 minutes (P = 0.61) (AbuRahma 2007). For haemostasis time, PTFE patching was associated with significantly longer haemostasis time than Dacron patching in two trials: mean times 14.4 ± 4.5 minutes versus 3.4 ± 3.8 minutes (P < 0.001) (AbuRahma 2002), and mean times 5.17 ± 5.2 minutes versus 3.73 ± 2.7 minutes (P = 0.01) (AbuRahma 2007). One new trial comparing bovine pericardium patch with PTFE found a non‐significantly longer operation time but significantly shorter haemostasis time (P < 0.0273) in patients patched with bovine pericardium than in those patched with PTFE (Stone 2014). In addition, suture line bleeding was significantly less (P < 0.001) among patients patched with bovine pericardium than among those patched with Dacron (Marien 2002). Five trials did not provide adequate data on operation time (Albrecht‐Fruh 1998; Katz 1996; Lord 1989; Marien 2002; O'Hara 2002).
1. Perioperative outcomes (outcomes within 30 days of operation)
1.1. Clinical outcomes
1.1.1. Ipsilateral stroke
Vein versus synthetic material
The evidence is very uncertain for rates of ipsilateral stroke between different patch types (odds ratio (OR) 2.05, 95% confidence interval (CI) 0.66 to 6.38; P = 0.21) (Analysis 1.1). The absolute risks of perioperative stroke (1.8%, 25/1122) were very low. The functional outcome of stroke, such as severity of neurological impairment and disability, was not assessed in any trials. The small number of events makes it unlikely that any differences in effect on outcomes between PTFE patching and vein patching would have been detected even if present.
1.1. Analysis.

Comparison 1: Perioperative events: synthetic versus vein (< 30 days), Outcome 1: Ipsilateral stroke
Dacron versus other synthetic material
The evidence is very uncertain for rates of ipsilateral stroke between different patch types (OR 3.35, 95% CI 0.19 to 59.06; P = 0.91) (Analysis 2.1).
Bovine pericardial versus other synthetic material
No trial data were provided for this comparison.
1.1.2. Any stroke
Vein versus synthetic material
The evidence is very uncertain for rates of any stroke between different patch types (OR 1.15, 95% CI 0.56 to 2.39; P = 0.7) (Analysis 1.2). All strokes were ipsilateral, but in two trials, other types of stroke were not recorded. The functional outcome of stroke, such as severity of neurological impairment and disability, was not assessed in any trials.
1.2. Analysis.

Comparison 1: Perioperative events: synthetic versus vein (< 30 days), Outcome 2: Any stroke
Dacron versus other synthetic material
Seven strokes occurred in the Dacron group compared with one in the other synthetic material group (OR 2.65, 95% CI 0.06 to 111.53; P = 0.61). There was little effect on any stroke between different patch types, but the evidence is very uncertain (Analysis 2.2).
Bovine pericardial versus other synthetic material
The evidence is very uncertain for risk of ipsilateral stroke (OR 1.50, 95% CI 0.29 to 7.79; P = 0.63) with bovine pericardium compared with the other synthetic material (Analysis 3.1).
3.1. Analysis.

Comparison 3: Perioperative events: bovine versus other synthetic (< 30 days), Outcome 1: Any stroke
1.1.3. Death from all causes
Vein versus synthetic material
The evidence is very uncertain for rates of death between different patch types (OR 0.52, 95% CI 0.20 to 1.34; P = 0.18) (Analysis 1.3). All absolute risks of death (1.0%, 14/1122) were very low. The small number of events makes it unlikely that any differences between PTFE patching and vein patching would have been detected even if present.
1.3. Analysis.

Comparison 1: Perioperative events: synthetic versus vein (< 30 days), Outcome 3: Death from all causes
Dacron versus other synthetic material
Three deaths were reported in the Dacron‐patched group and three in the other synthetic material group (OR 1.03, 95% CI 0.30 to 3.57; P = 0.96) (Analysis 2.4). There was no effect on death from all causes between different patch types, but the evidence is very uncertain.
2.4. Analysis.

Comparison 2: Perioperative events: Dacron versus other synthetic patch (< 30 days), Outcome 4: Death from all causes
Bovine pericardial versus other synthetic material
The evidence is very uncertain for risk of death (OR 4.39, 95% CI 0.48 to 39.95; P = 0.19) when bovine pericardium was compared with the other synthetic material (Analysis 3.4).
3.4. Analysis.

Comparison 3: Perioperative events: bovine versus other synthetic (< 30 days), Outcome 4: Death from all causes
1.1.4. Fatal stroke
Vein versus synthetic material
The evidence is very uncertain for rates of fatal stroke between different patch types (OR 0.27, 95% CI 0.04 to 1.66; P = 0.16) (Analysis 1.4).
1.4. Analysis.

Comparison 1: Perioperative events: synthetic versus vein (< 30 days), Outcome 4: Fatal stroke
Dacron versus other synthetic material
No trial data were reported for this comparison.
Bovine pericardial versus other synthetic material
The evidence is very uncertain for risk of fatal stroke (OR 5.16, 95% CI 0.24 to 108.83; P = 0.29) when bovine pericardium was compared with the other synthetic material (Analysis 3.2).
3.2. Analysis.

Comparison 3: Perioperative events: bovine versus other synthetic (< 30 days), Outcome 2: Fatal stroke
1.1.5. Combined stroke and death
Vein versus synthetic material
The evidence is very uncertain for rates of combined stroke and death between different patch types (OR 1.25, 95% CI 0.58 to 2.66; P = 0.57) (Analysis 1.5). All absolute risks of combined stroke and death (2.4%, 27/1122) were very low. The small number of events makes it unlikely that any differences between PTFE patching and vein patching would have been detected even if present.
1.5. Analysis.

Comparison 1: Perioperative events: synthetic versus vein (< 30 days), Outcome 5: Stroke or death
Dacron versus other synthetic material
No trial data were provided for this comparison.
Bovine pericardial versus other synthetic material
No trial data were provided for this comparison.
1.1.6. Combined stroke and TIA
Vein versus synthetic material
No trial data were provided for this comparison.
Dacron versus other synthetic material
Dacron may result in an increase in perioperative combined strokes and transient ischaemic attacks (OR 4.41, 95% CI 1.20 to 16.14; P = 0.03) when compared with PTFE (Analysis 2.3).
2.3. Analysis.

Comparison 2: Perioperative events: Dacron versus other synthetic patch (< 30 days), Outcome 3: Combined stroke and TIA
Bovine pericardial versus other synthetic material
The evidence is very uncertain for perioperative combined stroke and transient ischaemic attacks (OR 1.18, 95% CI 0.07 to 20.39; P = 0.91) when bovine pericardium was compared with another synthetic material (Analysis 3.3).
1.2. Perioperative complications
1.2.1. Cranial nerve palsy
Vein versus synthetic material
Cranial nerve palsy occurred in 3% of cases (19/630). The evidence is very uncertain in that it was more common in neither group and confidence intervals were wide (OR 1.19, 95% CI 0.53 to 2.71; P = 0.67) (Analysis 1.7).
1.7. Analysis.

Comparison 1: Perioperative events: synthetic versus vein (< 30 days), Outcome 7: Cranial nerve palsy
Dacron versus PTFE
No cranial nerve palsies were reported.
Bovine pericardium versus other synthetic material
No cranial nerve palsies were reported.
1.2.2. Wound infection
Vein versus synthetic material
Wound infection was observed to be more common in the vein group compared to the synthetic patch group, but the evidence is very uncertain (OR 0.38, 95% CI 0.12 to 1.23; P = 0.11) (Analysis 1.8). This was due to increased risk of groin wound infection, for which patients undergoing synthetic patching would not be at risk. However, no patch infections during the perioperative period were reported.
1.8. Analysis.

Comparison 1: Perioperative events: synthetic versus vein (< 30 days), Outcome 8: Wound infection
Dacron versus other synthetic patch
No wound infections were reported.
Bovine pericardium versus other synthetic material
Wound infection was reported in 3% of cases (3/97) in the synthetic material group. No wound infections were reported in the bovine pericardium patch group. Wound infection was observed to be more common in the other synthetic material group compared to the bovine pericardium patch group (OR 7.30, 95% CI 0.37 to 143.16; P = 0.19) (Analysis 3.5). Bovine pericardial patch is an acellular xenograft material that may reduce the risk of infection compared to synthetic material.
3.5. Analysis.

Comparison 3: Perioperative events: bovine versus other synthetic (< 30 days), Outcome 5: Wound infection
1.2.3. Complications requiring further reoperation
Vein versus synthetic material
Complications requiring further reoperation for any reason occurred in 2.6% (33/1263) of cases, and there was no effect on complications requiring further reoperation for any reason in the vein and synthetic patch groups, but the evidence is very uncertain (OR 1.72, 95% CI 0.85 to 3.47; P = 0.13) (Analysis 1.9). Complications requiring further reoperation were for patch rupture (one in the PTFE group, and two in the vein group) or wound haemorrhage (2.44%, 33/1350). One of the vein ruptures involved saphenous vein harvested from the groin and another from the ankle (Analysis 1.11). Two of the three patch ruptures were fatal, one in each group. The evidence is very uncertain for differences in wound haematoma between vein and synthetic patch groups (OR 1.63, 95% CI 0.81 to 3.28; P = 0.2).
1.9. Analysis.

Comparison 1: Perioperative events: synthetic versus vein (< 30 days), Outcome 9: Complication requiring further operation
1.11. Analysis.

Comparison 1: Perioperative events: synthetic versus vein (< 30 days), Outcome 11: Wound haemorrhage
Dacron versus other synthetic patch
The evidence is very uncertain for complications requiring further reoperation between Dacron and other synthetic patches (OR 5.43, 95% CI 0.92 to 31.90; P = 0.06). Eight patients required reoperation: seven in the Dacron group, and one in the other synthetic patch (PTFE) group. All re‐explorations were for suspected or proven carotid thrombosis/occlusion in seven cases, and one case was due to wound haematoma (Analysis 2.5).
2.5. Analysis.

Comparison 2: Perioperative events: Dacron versus other synthetic patch (< 30 days), Outcome 5: Complication requiring further operation
Bovine pericardium versus other synthetic material
The evidence is very uncertain for the reoperation rate (OR 0.70, 95% CI 0.08 to 6.38; P = 0.75). Complications requiring further reoperation were observed in 2.1% (3/141) of cases in the synthetic material group and in 4% (6/149) of cases in the bovine pericardium patch group. Re‐explorations were performed for wound haematoma in eight cases, and for proven carotid thrombosis/occlusion in one case (Analysis 3.6). Longer haemostasis time was reported for PTFE patching (4.9 minutes) versus bovine pericardial patching (3.09 minutes) (P = 0.027) (Stone 2014), and intraoperative suture line bleeding was less in the bovine pericardium group compared to the Dacron group (P < 0.001). In addition, total intraoperative suture line bleeding (Net (± standard error of the mean (SEM)) sponge weight) was 6.25 g and 16.34 g in the bovine pericardium group versus the Dacron group, respectively (P < 0.001) (Marien 2002).
1.2.4. Arterial occlusion
Vein versus synthetic material
The absolute risk of arterial occlusion was 0.7% (8/1155). Vein patch has little effect on arterial occlusion, but evidence of differences between vein and synthetic material is very uncertain (OR 2.16, 95% CI 0.60 to 7.78; P = 0.24) (Analysis 1.10). Five of seven studies reporting rates of arterial occlusion did so based upon perioperative duplex ultrasound, whereas two reported only symptomatic occlusions.
1.10. Analysis.

Comparison 1: Perioperative events: synthetic versus vein (< 30 days), Outcome 10: Arterial occlusion
Dacron versus other synthetic patch
Data show little effect on risk of arterial occlusion, but evidence of differences between Dacron and PTFE groups (OR 11.58, 95% CI 0.63 to 212.19; P = 0.1) is very uncertain (Analysis 2.6).
2.6. Analysis.

Comparison 2: Perioperative events: Dacron versus other synthetic patch (< 30 days), Outcome 6: Arterial occlusion
Bovine pericardium versus other synthetic material
No effect in arterial occlusion rate was seen, but the evidence is very uncertain (OR 1.01, 95% CI 0.06 to 16.39; P = 0.99). Arterial occlusion occurred in 0.7% (1/141) of cases in the synthetic material group and in 0.67% (1/149) of cases in the bovine pericardium group. One arterial occlusion was re‐explored with surgical thrombectomy and repair of an intimal flap, and another case was managed conservatively with no further neurological events reported (Analysis 3.7).
3.7. Analysis.

Comparison 3: Perioperative events: bovine versus other synthetic (< 30 days), Outcome 7: Arterial occlusion
2. Long‐term outcomes (outcomes during long‐term follow‐up (at least one year) including events during the first 30 days)
Three trials did not follow patients for at least one year; these have been excluded from these analyses (Katz 1996; Lord 1989; Marien 2002).
2.1. Clinical outcomes
2.1.1. Long‐term ipsilateral stroke
Vein versus synthetic material
Data show a small differences in effect on ipsilateral stroke rate between vein and other synthetic material, but the evidence is very uncertain (OR 1.45, 95% CI 0.69 to 3.07; P = 0.33). The overall risk of ipsilateral stroke was 3.7% (29/776) (Analysis 4.1).
4.1. Analysis.

Comparison 4: Events during long‐term follow‐up (≥ 1 year) including events during first 30 days: synthetic versus vein, Outcome 1: Ipsilateral stroke
Dacron versus other synthetic patch
One trial performed this comparison, which showed little effect on long‐term ipsilateral stroke between Dacron and PTFE patches, but the evidence is very uncertain (OR 1.52, 95% CI 0.25 to 9.27; P = 0.65) (Analysis 5.1). The overall risk of ipsilateral stroke was 2.5% (5/200).
5.1. Analysis.

Comparison 5: Events during long‐term follow‐up (≥ 1 year) including events during first 30 days: Dacron versus other synthetic, Outcome 1: Ipsilateral stroke
Bovine pericardium versus other synthetic material
Only one trial performed this comparison, which showed that bovine pericardium may result in a slight increase in long‐term ipsilateral stroke compared to PTFE (OR 4.17, 95% CI 0.46 to 38.02; P = 0.21) (Analysis 6.1). The overall risk of ipsilateral stroke was 2.6% (5/195).
6.1. Analysis.

Comparison 6: Events during long‐term follow‐up (≥ 1 year) including events during first 30 days: bovine versus other synthetic, Outcome 1: Ipsilateral stroke
2.1.2. Long‐term stroke
Vein versus synthetic material
There were 52 recorded strokes of any type during follow‐up (overall risk 4.5%). Data show little difference in effects on long‐term stroke between synthetic patching and vein patching, and confidence intervals were wide. So, the evidence is very uncertain (OR 1.22, 95% CI 0.70 to 2.13; P = 0.49) (Analysis 4.2). The functional outcome of stroke was not assessed in any of the trials reporting long‐term outcomes.
4.2. Analysis.

Comparison 4: Events during long‐term follow‐up (≥ 1 year) including events during first 30 days: synthetic versus vein, Outcome 2: Any stroke
Dacron versus other synthetic patch
Dacron may result in a large increase in long‐term any stroke with Dacron compared with PTFE and polyurethane patching (OR 10.58, 95% CI 1.34 to 83.43; P = 0.03) (Analysis 5.2).
5.2. Analysis.

Comparison 5: Events during long‐term follow‐up (≥ 1 year) including events during first 30 days: Dacron versus other synthetic, Outcome 2: Any stroke
Bovine pericardium versus other synthetic material
No strokes were reported.
2.1.3. Death from all causes
Vein versus synthetic material
Data show little difference in effect on case fatality between the two groups (overall risk 8.4%), but the evidence is very uncertain (OR 0.89, 95% CI 0.59 to 1.36; P = 0.59) (Analysis 4.3).
4.3. Analysis.

Comparison 4: Events during long‐term follow‐up (≥ 1 year) including events during first 30 days: synthetic versus vein, Outcome 3: Death from all causes
Dacron versus other synthetic patch
Only two trials reported this outcome, with no effect on death rate between Dacron and other synthetic materials, but the evidence is very uncertain (OR 0.73, 95% CI 0.03 to 18.52; P = 0.85) (Analysis 5.3). The overall risk of death was 3.7% (29/776).
Bovine pericardium versus other synthetic material
No deaths from any cause were reported.
2.1.4. Long‐term any stroke or death
Vein versus synthetic material
There was no difference in effect on stroke or death between the PTFE group (10.31%, 57/457) and the vein patch group (10.19%, 56/463) (OR 1.05, 95% CI 0.70 to 1.56; P = 0.82), but the evidence is very uncertain (Analysis 4.5).
4.5. Analysis.

Comparison 4: Events during long‐term follow‐up (≥ 1 year) including events during first 30 days: synthetic versus vein, Outcome 5: Stroke or death
Dacron versus other synthetic patch
Only one study reported this outcome, for which the evidence suggests Dacron results in a greater increase in any stroke or death rate when compared with PTFE (OR 6.06, 95% CI 1.31 to 28.07; P = 0.02) (Analysis 5.4). The overall risk of ipsilateral stroke was 6.5% (13/200).
5.4. Analysis.

Comparison 5: Events during long‐term follow‐up (≥ 1 year) including events during first 30 days: Dacron versus other synthetic, Outcome 4: Stroke or death
Bovine pericardium versus other synthetic material
No stroke or death was reported.
2.2. Long‐term complications (during long‐term follow‐up (at least one year) including events during the first 30 days)
2.2.1. Occlusion/re‐stenosis greater than 50%
Vein versus synthetic material
Only 73 arteries (6.4%) became re‐stenosed or occluded during follow‐up, so it remains unclear whether PTFE patching reduces this risk. There was no effect on arterial occlusion or re‐stenosis greater than 50% between vein and synthetic material, but the evidence is very uncertain (OR 0.97, 95% CI 0.62 to 1.55; P = 0.91) (Analysis 4.7).
4.7. Analysis.

Comparison 4: Events during long‐term follow‐up (≥ 1 year) including events during first 30 days: synthetic versus vein, Outcome 7: Arterial occlusions/re‐stenosis > 50%
Dacron versus other synthetic patch
Dacron patch may increase the risk of re‐stenosis when compared with other synthetic materials (especially PTFE), but the evidence is very uncertain (OR 3.73, 95% CI 0.71 to 19.65; P = 0.12) (Analysis 5.5).
Bovine pericardium versus other synthetic material
Only one trial performed this comparison, which may result in little to no difference (OR 0.20, 95% CI 0.01 to 4.18; P = 0.30) in occlusion or re‐stenosis greater than 50% between the bovine pericardium group and the PTFE group. Only two arteries (2%) became re‐stenosed in the bovine pericardium group, and none in the PTFE group, during three years of follow‐up (Analysis 6.3).
6.3. Analysis.

Comparison 6: Events during long‐term follow‐up (≥ 1 year) including events during first 30 days: bovine versus other synthetic, Outcome 3: Arterial occlusion/re‐stenosis > 50%
2.2.2. Infection of endarterectomy site
Vein versus synthetic material
One artery with a PTFE patch developed an infected false aneurysm at seven months, which was successfully excised. No other late graft infections were reported (Analysis 4.6). There was no difference in effect on infection of the endarterectomy site between vein or synthetic material, but the evidence is very uncertain (OR 2.76, 95% CI 0.11 to 69.42; P = 0.54).
4.6. Analysis.

Comparison 4: Events during long‐term follow‐up (≥ 1 year) including events during first 30 days: synthetic versus vein, Outcome 6: Infection at endarterectomy site
Dacron versus other synthetic patch
No trials performed this comparison.
Bovine pericardium versus other synthetic material
No infection rate was reported for any of the included trials.
2.2.3. Pseudoaneurysm
Vein versus synthetic material
Data from four trials were available, but no trial provided an adequate definition of a pseudoaneurysm (AbuRahma 1996; Gonzalez 1994; Hayes 2001; Ricco 1996). Two trials showed reductions in the risk of pseudoaneurysm with PTFE patching (0.8%) compared with vein patching (11.9%) (OR 0.09, 95% CI 0.02 to 0.49) (Analysis 4.8) (Gonzalez 1994; Ricco 1996), and two trials did not report any pseudoaneurysms in either group (AbuRahma 1996; Hayes 2001). Moreover, the clinical significance of reduced risk with PTFE patching was unclear. None of the pseudoaneurysms ruptured or were associated with ipsilateral stroke; in one trial, all dilatations appeared within one month of surgery, and none were progressive (Gonzalez 1994). Synthetic patch may reduce pseudoaneurysm formation when compared with vein patch, but the evidence is very uncertain (OR 0.09, 95% CI 0.02 to 0.49; P = 0.005) (Analysis 4.8)
4.8. Analysis.

Comparison 4: Events during long‐term follow‐up (≥ 1 year) including events during first 30 days: synthetic versus vein, Outcome 8: Pseudoaneurysm formation
Dacron versus other synthetic patch
No trial data were provided for this comparison.
Bovine pericardium versus other synthetic material
No pseudoaneurysms were reported for any of the included trials.
Discussion
Summary of main results
Benefit of selection of type of patch for carotid patch angioplasty procedure
The results of this systematic review in 2010 were inconclusive because very few patients had been included in randomised comparisons of different types of patches. Despite an increase in the number of patients included in this current analysis, data were still insufficient to allow us to draw useful conclusions. We found no differences in effects on the risk of stroke or death suffered by patients receiving synthetic or venous patches (either perioperatively or during long‐term follow‐up). The risk of major arterial complications, such as rupture or infection, was very low (< 1%) in all groups, and we found no data to support the belief that synthetic patching may reduce the risk of patch rupture. Comparison of wound infection rates between vein and synthetic patches revealed slightly lower rates with synthetic patches, mainly due to vein harvest surgical site infections. Vein patches may increase the risk of pseudoaneurysm formation compared with synthetic patches. However, the numbers involved were small, and so the clinical significance of this finding is uncertain. Furthermore, the definition of pseudoaneurysm may not have been consistent between trials, and the clinical implications of this are unclear because no complications were related to pseudoaneurysm formation. It has been suggested that reversal of perioperative anticoagulation may reduce the risk of postoperative bleeding but may increase the risk of perioperative stroke. Inconsistency in rates of anticoagulation reversal between the included studies may, therefore, confound interpretation of the results.
Compared to other synthetic patches, Dacron may result in an increase in perioperative combined stroke and transient ischaemic attacks with no effect on ipsilateral stroke or any stroke within 30 days. Early arterial re‐stenosis or occlusion was also higher for Dacron patches. During follow‐up for longer than one year, more stroke and stroke/death occurred with Dacron patch closure, although the numbers of outcome events were small. Dacron patch closure may increase the risk of long‐term arterial re‐stenosis or occlusion, but the evidence is very uncertain. Bovine pericardium patch may decrease the incidences of perioperative fatal stroke or death compared to other synthetic grafts (polytetrafluoroethylene (PTFE) and Dacron), but the evidence is very uncertain because the numbers of outcomes were small. In addition, bovine pericardial patch is an acellular xenograft material that may reduce the risk of infection compared to synthetic material.
Overall completeness and applicability of evidence
Increasing evidence supports the use of routine or selective patching over primary closure during carotid endarterectomy (Counsell 1998). However, this review has shown that little reliable evidence is available to guide surgeons on which patch material to use. Synthetic patches offer the advantage of sparing the morbidity and time associated with vein harvesting (e.g. poor wound healing, pain) and ensure that the vein is available for future coronary bypass grafting if required. However, use of PTFE in patching may increase operation time (by several minutes) mainly due to increased bleeding through suture holes. This may be less of a problem with Dacron patching (Carney 1987). In addition, the trial by Carney and Lilly suggests that surgeons preferred the handling qualities of Dacron or vein to PTFE, which they found less compliant (Carney 1987). Dacron may, therefore, be preferable to PTFE, although some people believe it carries greater risk of thrombosis (Lord 1989), and it may be more prone to infection (Schmitt 1986). Furthermore, the only good quality randomised trials that have compared the use of Dacron and other synthetic material in carotid endarterectomy found benefit of PTFE over Hemoshield Dacron graft in terms of 30‐day stroke rate and postoperative re‐stenosis greater than 50% (AbuRahma 2002; AbuRahma 2007). However, researchers also noted longer haemostasis time associated with PTFE. Bovine pericardium patch showed less haemostasis time (Stone 2014), with less suture line bleeding, compared to other synthetic material patches (Marien 2002), with no difference in wound haemorrhage between the two groups.
Quality of the evidence
The quality of evidence for all outcomes was very low to low because risk of bias and imprecision were present in the included studies. None of these randomised controlled trials (RCTs) could be blinded for surgeons or patients due to the nature of the intervention.
Comparison between vein and synthetic patches shows that two studies may not have been true RCTs because random sequence generation and allocation concealment were unclear (AbuRahma 1996; Lord 1989); three studies did not address blinding of outcome assessments (AbuRahma 1996; Lord 1989; Ricco 1996); one study had unclear risk of selection bias and blinding of outcome assessments (Katz 1996); three studies did not address blinding of outcome assessment (Hayes 2001; Katz 1996; O'Hara 2002); and one study had unclear risk of selection bias (Katz 1996).
Comparison between Dacron and other synthetic patches shows that two studies did not report the number of patients lost to follow‐up (AbuRahma 2002; AbuRahma 2007); one study had high risk of bias due to no random sequence generation with unclear allocation concealment, and because it did not address blinding of outcome assessments (Marien 2002).
Comparison between bovine pericardium and other synthetic patches shows that one study did not address blinding of outcome assessments (Stone 2014); one study had high risk of bias due to no random sequence generation with unclear allocation concealment, and because it did not address blinding of outcome assessments (Marien 2002).
Differences between trials with selected patient criteria, details of operative techniques, selective versus routine shunt during operation, and timing of follow‐up of patients led to inadequate quality of evidence for each outcome.
In summary, the quality of evidence for perioperative any stroke, any stroke, combined stroke and transient ischaemic attack (TIA), and death from all causes in the comparison between bovine pericardium and other synthetic patch was very low because the included study did not use random sequence generation, had unclear allocation concealment, and did not address blinding of outcome assessments; this study also had a small sample size (Marien 2002). Most studies had wide confidence intervals for most outcomes because low event rates led to imprecision (AbuRahma 1996; AbuRahma 2007; Albrecht‐Fruh 1998; Carney 1987; Chyatte 1996; Gonzalez 1994; Grego 2003; Hayes 2001; Katz 1996; Lord 1989; Marien 2002; Meerwaldt 2008; O'Hara 2002; Ricco 1996; Ruckert 2000; Stone 2014). So, the meta‐analyses require additional studies with more participants to reach optimal information size.
Potential biases in the review process
Attempts to obtain all relevant data were successful. Selection bias, performance bias, detection bias, attrition bias, and other bias were identified in all 14 RCTs. We searched systematically for all studies in all languages. We did not perform subgroup analyses of pooled data from included studies. We have reported all of the analyses that we performed.
Agreements and disagreements with other studies or reviews
In 2013, a systematic review of 13 RCTs was published (Ren 2013). Nine trials involving 1946 carotid endarterectomies (CEAs) compared venous patch with synthetic patch materials including bovine pericardium patch (AbuRahma 1996; Gonzalez 1994; Grego 2003; Hayes 2001; Katz 1996; Lord 1989; Meerwaldt 2008; O'Hara 2002; Ricco 1996). Two trials involving 400 CEAs compared Dacron patch with PTFE patch (AbuRahma 2002; AbuRahma 2007). Data show little to no effect on perioperative and long‐term stroke, death, re‐stenosis, or wound infection in CEA with venous patch versus synthetic patch material. However, some evidence shows that PTFE was superior to Dacron in terms of TIA and stroke, with 50% re‐stenosis to occlusion of the carotid artery and carotid thrombosis in CEA. One RCT of 95 CEAs in 92 patients compared bovine pericardium with Dacron patch and demonstrated a decrease in intraoperative suture line bleeding with bovine pericardium compared with Dacron patch (P < 0.001) (Marien 2002). Haemostasis time in CEA with PTFE patch was longer than with venous patch (P < 0.0001), and was longer than with Dacron patch (P < 0.0001). Pseudoaneurysm formation outcomes were not reported in this study. Ren 2013 included evidence up to November 2012; therefore it did not include the recent prospective randomised trial of PTFE versus bovine pericardium patching in CEA (Stone 2014). Mean haemostasis time was 4.90 minutes for PTFE patch versus 3.09 minutes for bovine pericardium patch (P = 0.027). However, haemorrhage or re‐exploration due to neck haematoma was not different. The result of Ren 2013 was concordant with the result of our updated systematic review, in which PTFE patch performed better than Dacron patch, but high‐quality studies to determine the optimal material for patching are scarce.
In 2018, a systematic review and meta‐analysis was carried out to compare perioperative stroke, death, myocardial infarction, wound infection, carotid thrombosis, and re‐stenosis for carotid patch angioplasty after CEA in people suffering from carotid artery stenosis (Texakalidis 2018). This meta‐analysis shows that neither synthetic nor venous patch material is superior to the other in terms of perioperative and long‐term primary and secondary outcomes. However, some evidence suggests that PTFE patches may be superior to Dacron grafts in terms of perioperative stroke and TIA rates, as well as re‐stenosis and occlusion. Bovine pericardium patch might be better than other synthetic patches in terms of incidence of death and neck haematoma. However, evidence regarding effects of bovine patch on death and wound haemorrhage is very uncertain.
Previous and recent systematic reviews of RCTs were inconclusive due to a small quantity of data, high heterogeneity, and lack of high‐quality studies (Ren 2013; Texakalidis 2018). In addition, a modern type of PTFE patch described in AbuRahma 2007 is reported to have better outcomes than the conventional PTFE patch (AbuRahma 1996; Gonzalez 1994; Grego 2003; Lord 1989; Ricco 1996). Each generation of synthetic patches has been modified in an attempt to improve performance. However, no further RCTs have examined bovine pericardium patch or the new‐generation synthetic patch. Additional high‐quality RCTs are required to determine optimal materials for carotid patch angioplasty.
Authors' conclusions
Implications for practice.
Because little to no difference in effect was observed for perioperative and long‐term ipsilateral stroke risk between synthetic patch materials and vein or bovine pericardium patches, the results of this review do not support the use of any one material over any other for carotid endarterectomy. Among the synthetic materials, more complications (re‐stenosis or occlusion) were noted with Dacron, but evidence is very uncertain due to very low quality. However, the low quality of evidence suggests that the Dacron patch may result in an increase in perioperative combined stroke and TIA, long‐term any stroke, stroke, or death. Estimates of effect may be biased because of lack of blinding of assessors for the outcome.
Implications for research.
Further RCTs comparing one type of patch with another are required; they should include large numbers of patients and multiple arms with good methodological design to reach optimal information size. More robust RCTs comparing different types of graft material for patching in patients with carotid endarterectomy are required. Currently, no ongoing RCTs are comparing types of patches.
What's new
| Date | Event | Description |
|---|---|---|
| 25 May 2020 | New citation required but conclusions have not changed | From the new trial comparing synthetic polytetrafluoroethylene (PTFE) with bovine pericardium patching, the sample size and the number of outcome events are too small to allow conclusions. There was no significant difference in perioperative fatal stroke, death, and infection between bovine pericardium and other synthetic patches |
| 25 May 2020 | New search has been performed | We have updated the searches for this review. We have included 1 new trial comparing synthetic polytetrafluoroethylene (PTFE) with bovine pericardium patching. This trial provides more data on the short‐term outcome between synthetic (PTFE) and bovine pericardium patch materials. The review now includes 14 randomised controlled trials with data for 2278 operations |
History
Protocol first published: Issue 3, 1996 Review first published: Issue 3, 1996
| Date | Event | Description |
|---|---|---|
| 9 September 2008 | Amended | Converted to new review format |
| 28 February 2003 | New search has been performed | Differences between this review and the previous version: 4 new trials comparing synthetic with venous patching have recently been added to the review (AbuRahma 1996; Hayes 2001; Katz 1996; O'Hara 2002). Data on a total of 1280 operations are now available for analysis. A further trial, 'Jugular vein versus PTFE patch for carotid endarterectomy', by Deriu and Grego, is due to be reported in the next year, has been added to the 'Awaiting assessment' section, and will be included in the review as soon as possible |
Acknowledgements
We thank the following: Hazel Fraser for searching the Cochrane Stroke Group trials register and for providing regular updates of newly identified RCTs; and Joshua Cheyne for searching the Cochrane Central Register of Controlled Trials (CENTRAL). We also thank the previous authors of this review, particularly Dr Rick Bond.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1MeSH descriptor: [Endarterectomy, Carotid] #2MeSH descriptor: [Carotid Arteries] #3MeSH descriptor: [Carotid Artery Diseases] #4MeSH descriptor: [Carotid Arteries] #5MeSH descriptor: [Carotid Artery Diseases] #6carotid:ti,ab,kw (Word variations have been searched) #7#4 or #5 or #66360 #8MeSH descriptor: [Endarterectomy] #9(endarterectom* or surg*):ti,ab,kw (Word variations have been searched)178437 #10#8 or #9178437 #11#7 and #102050
Appendix 2. MEDLINE Ovid search strategy
1. Endarterectomy, carotid/ 2. exp carotid arteries/su 3. exp carotid artery diseases/su 4. exp carotid arteries/ 5. exp carotid artery diseases/ 6. carotid.tw. 7. 4 or 5 or 6 8. endarterectomy/ 9. (endarterectom$ or surg$).tw. 10. 8 or 9 11. 7 and 10 12. 1 or 2 or 3 or 11 13. randomized controlled trial/ 14. randomized controlled trials as topic/ 15. controlled clinical trial/ 16. controlled clinical trials as topic/ 17. random allocation/ 18. clinical trial/ 19. exp clinical trials as topic/ 20. (clin$ adj25 trial$).tw. 21. random$.tw. 22. research design/ 23. intervention studies/ 24. control$.tw. 25. patch$.tw. 26. 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 27. 12 and 26 28. limit 27 to humans
Appendix 3. Embase Ovid search strategy
1. carotid endarterectomy/ 2. carotid artery surgery/ 3. exp carotid artery disease/su 4. exp carotid artery/ 5. exp carotid artery disease/ 6. 4 or 5 7. artery surgery/ or endarterectomy/ or vascular surgery/ or surgery/ 8. 6 and 7 9. (carotid adj5 (endarterect$ or surgery)).tw. 10. 1 or 2 or 3 or 8 or 9 11. Clinical trial/ 12. randomized controlled trial/ 13. controlled study/ 14. randomization/ 15. random$.tw. 16. Prospective study/ 17. "Evaluation and follow up"/ or Follow up/ 18. versus.tw. 19. prospective.tw. 20. types of study/ 21. methodology/ 22. comparative study/ 23. ((intervention or experiment$) adj5 group$).tw. 24. Parallel design/ 25. intermethod comparison/ 26. (controls or control group$).tw. 27. (control$ adj trial$).tw. 28. patch$.tw. 29. or/11‐28 30. 10 and 29
Appendix 4. Search strategies for other databases
Database: ClinicalTrials.gov
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) INFLECT EXACT "Interventional" [STUDY‐TYPES] AND carotid endarterectomy [DISEASE]
Database: WHO International Clinical Trials Registry Platform (ICTRP; 23 October 2019)
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) carotid AND patch OR carotid AND angioplasty
Data and analyses
Comparison 1. Perioperative events: synthetic versus vein (< 30 days).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Ipsilateral stroke | 5 | 797 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.66, 6.38] |
| 1.1.1 PTFE | 4 | 590 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.49, 6.78] |
| 1.1.2 Dacron | 1 | 207 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.86 [0.29, 27.92] |
| 1.2 Any stroke | 9 | 1459 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.56, 2.39] |
| 1.2.1 PTFE | 5 | 699 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.59, 6.67] |
| 1.2.2 Dacron | 3 | 673 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.39, 3.57] |
| 1.2.3 Polyester | 1 | 87 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.33] |
| 1.3 Death from all causes | 8 | 1369 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.20, 1.34] |
| 1.3.1 PTFE | 4 | 609 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.16, 2.41] |
| 1.3.2 Dacron | 3 | 673 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.10, 2.03] |
| 1.3.3 Polyester | 1 | 87 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.81] |
| 1.4 Fatal stroke | 6 | 1122 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.04, 1.66] |
| 1.4.1 PTFE | 3 | 449 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.99] |
| 1.4.2 Dacron | 3 | 673 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 2.17] |
| 1.5 Stroke or death | 6 | 1122 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.58, 2.66] |
| 1.5.1 PTFE | 3 | 449 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.42, 4.80] |
| 1.5.2 Dacron | 3 | 673 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.44, 3.02] |
| 1.6 Arterial rupture | 6 | 1068 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.13, 3.54] |
| 1.6.1 PTFE | 4 | 590 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.13, 7.23] |
| 1.6.2 Dacron | 2 | 478 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.66] |
| 1.7 Cranial nerve palsy | 4 | 717 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.53, 2.71] |
| 1.7.1 PTFE | 2 | 359 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.28, 5.76] |
| 1.7.2 Dacron | 1 | 271 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.32, 3.21] |
| 1.7.3 Polyester | 1 | 87 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.26, 10.42] |
| 1.8 Wound infection | 3 | 673 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.12, 1.23] |
| 1.8.1 PTFE | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.8.2 Dacron | 3 | 673 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.12, 1.23] |
| 1.9 Complication requiring further operation | 7 | 1263 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.85, 3.47] |
| 1.9.1 PTFE | 4 | 590 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.19, 4.97] |
| 1.9.2 Dacron | 3 | 673 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.89, 4.28] |
| 1.10 Arterial occlusion | 7 | 1155 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.60, 7.78] |
| 1.10.1 PTFE | 4 | 590 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.50 [0.48, 13.12] |
| 1.10.2 Dacron | 2 | 478 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.11, 70.30] |
| 1.10.3 Polyester | 1 | 87 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.06, 17.72] |
| 1.11 Wound haemorrhage | 8 | 1350 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.81, 3.28] |
| 1.11.1 PTFE | 4 | 590 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.06, 15.67] |
| 1.11.2 Dacron | 3 | 673 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.77, 3.72] |
| 1.11.3 Polyester | 1 | 87 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.26, 10.42] |
1.6. Analysis.

Comparison 1: Perioperative events: synthetic versus vein (< 30 days), Outcome 6: Arterial rupture
Comparison 2. Perioperative events: Dacron versus other synthetic patch (< 30 days).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Ipsilateral stroke | 2 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 3.35 [0.19, 59.06] |
| 2.1.1 PTFE | 2 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 3.35 [0.19, 59.06] |
| 2.2 Any stroke | 2 | 295 | Odds Ratio (M‐H, Random, 95% CI) | 2.65 [0.06, 111.53] |
| 2.2.1 PTFE | 1 | 200 | Odds Ratio (M‐H, Random, 95% CI) | 16.12 [0.91, 286.22] |
| 2.2.2 Bovine pericardium | 1 | 95 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.02, 9.52] |
| 2.3 Combined stroke and TIA | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.41 [1.20, 16.14] |
| 2.3.1 PTFE | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.41 [1.20, 16.14] |
| 2.4 Death from all causes | 4 | 599 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.30, 3.57] |
| 2.4.1 PTFE | 2 | 400 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.25, 9.07] |
| 2.4.2 Bovine pericardium | 1 | 95 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.55 [0.14, 89.42] |
| 2.4.3 Polyurethane | 1 | 104 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.11] |
| 2.5 Complication requiring further operation | 2 | 295 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.43 [0.92, 31.90] |
| 2.5.1 PTFE | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.32 [0.75, 53.48] |
| 2.5.2 Bovine pericardium | 1 | 95 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.55 [0.14, 89.42] |
| 2.6 Arterial occlusion | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.58 [0.63, 212.19] |
| 2.6.1 PTFE | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.58 [0.63, 212.19] |
| 2.7 Early re‐stenosis or occlusion | 2 | 400 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.26 [1.88, 28.04] |
| 2.7.1 PTFE | 2 | 400 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.26 [1.88, 28.04] |
2.7. Analysis.

Comparison 2: Perioperative events: Dacron versus other synthetic patch (< 30 days), Outcome 7: Early re‐stenosis or occlusion
Comparison 3. Perioperative events: bovine versus other synthetic (< 30 days).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Any stroke | 2 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.29, 7.79] |
| 3.1.1 PTFE | 1 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.10 [0.32, 30.29] |
| 3.1.2 Dacron | 1 | 95 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.02, 9.52] |
| 3.2 Fatal stroke | 2 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.16 [0.24, 108.83] |
| 3.2.1 PTFE | 1 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.16 [0.24, 108.83] |
| 3.2.2 Dacron | 1 | 95 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.3 Combined stroke and TIA | 2 | 290 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.07, 20.39] |
| 3.3.1 PTFE | 1 | 195 | Odds Ratio (M‐H, Random, 95% CI) | 4.17 [0.46, 38.02] |
| 3.3.2 Dacron | 1 | 95 | Odds Ratio (M‐H, Random, 95% CI) | 0.22 [0.01, 4.76] |
| 3.4 Death from all causes | 2 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.39 [0.48, 39.95] |
| 3.4.1 PTFE | 1 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.16 [0.24, 108.83] |
| 3.4.2 Dacron | 1 | 95 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.55 [0.14, 89.42] |
| 3.5 Wound infection | 1 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.30 [0.37, 143.16] |
| 3.5.1 PTFE | 1 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.30 [0.37, 143.16] |
| 3.6 Complication requiring further operation | 2 | 290 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.08, 6.38] |
| 3.6.1 PTFE | 1 | 195 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.06, 1.64] |
| 3.6.2 Dacron | 1 | 95 | Odds Ratio (M‐H, Random, 95% CI) | 3.55 [0.14, 89.42] |
| 3.7 Arterial occlusion | 2 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.06, 16.39] |
| 3.7.1 PTFE | 1 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.06, 16.39] |
| 3.7.2 Dacron | 1 | 95 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.8 Wound haemorrhage | 2 | 290 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.08, 6.38] |
| 3.8.1 PTFE | 1 | 195 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.06, 1.64] |
| 3.8.2 Dacron | 1 | 95 | Odds Ratio (M‐H, Random, 95% CI) | 3.55 [0.14, 89.42] |
Comparison 4. Events during long‐term follow‐up (≥ 1 year) including events during first 30 days: synthetic versus vein.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Ipsilateral stroke | 4 | 776 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.69, 3.07] |
| 4.1.1 PTFE | 3 | 500 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.47, 3.88] |
| 4.1.2 Dacron | 1 | 276 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.54, 4.50] |
| 4.2 Any stroke | 7 | 1167 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.70, 2.13] |
| 4.2.1 PTFE | 4 | 609 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.63, 4.18] |
| 4.2.2 Dacron | 2 | 471 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.60, 2.87] |
| 4.2.3 Polyester | 1 | 87 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.07, 2.18] |
| 4.3 Death from all causes | 7 | 1167 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.59, 1.36] |
| 4.3.1 PTFE | 4 | 609 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.50, 1.59] |
| 4.3.2 Dacron | 2 | 471 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.47, 1.66] |
| 4.3.3 Polyester | 1 | 87 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.14, 8.00] |
| 4.4 Fatal stroke | 4 | 725 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.26, 2.88] |
| 4.4.1 PTFE | 3 | 449 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.15, 8.08] |
| 4.4.2 Dacron | 1 | 276 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.17, 3.44] |
| 4.5 Stroke or death | 5 | 920 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.70, 1.56] |
| 4.5.1 PTFE | 3 | 449 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.57, 1.82] |
| 4.5.2 Dacron | 2 | 471 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.62, 1.87] |
| 4.6 Infection at endarterectomy site | 2 | 236 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.76 [0.11, 69.42] |
| 4.7 Arterial occlusions/re‐stenosis > 50% | 8 | 1238 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.62, 1.55] |
| 4.7.1 PTFE | 5 | 750 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.32, 1.22] |
| 4.7.2 Dacron | 2 | 401 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.78, 2.87] |
| 4.7.3 Polyester | 1 | 87 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.8 Pseudoaneurysm formation | 4 | 776 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.02, 0.49] |
| 4.8.1 PTFE | 3 | 500 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.02, 0.49] |
| 4.8.2 Dacron | 1 | 276 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
4.4. Analysis.

Comparison 4: Events during long‐term follow‐up (≥ 1 year) including events during first 30 days: synthetic versus vein, Outcome 4: Fatal stroke
Comparison 5. Events during long‐term follow‐up (≥ 1 year) including events during first 30 days: Dacron versus other synthetic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Ipsilateral stroke | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.25, 9.27] |
| 5.2 Any stroke | 2 | 304 | Odds Ratio (M‐H, Fixed, 95% CI) | 10.58 [1.34, 83.43] |
| 5.2.1 PTFE | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 16.12 [0.91, 286.22] |
| 5.2.2 Polyurethane | 1 | 104 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.20 [0.24, 110.95] |
| 5.3 Death | 2 | 304 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.03, 18.52] |
| 5.3.1 Polyurethane | 1 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 0.13 [0.01, 1.06] |
| 5.3.2 PTFE | 1 | 200 | Odds Ratio (M‐H, Random, 95% CI) | 3.27 [1.02, 10.52] |
| 5.4 Stroke or death | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.06 [1.31, 28.07] |
| 5.4.1 PTFE | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.06 [1.31, 28.07] |
| 5.5 Arterial occlusion/re‐stenosis > 50% | 3 | 490 | Odds Ratio (M‐H, Random, 95% CI) | 3.73 [0.71, 19.65] |
| 5.5.1 PTFE | 2 | 386 | Odds Ratio (M‐H, Random, 95% CI) | 6.28 [1.32, 29.91] |
| 5.5.2 Polyurethane | 1 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.21] |
Comparison 6. Events during long‐term follow‐up (≥ 1 year) including events during first 30 days: bovine versus other synthetic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Ipsilateral stroke | 1 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.17 [0.46, 38.02] |
| 6.1.1 PTFE | 1 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.17 [0.46, 38.02] |
| 6.2 Death | 1 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.16 [0.24, 108.83] |
| 6.2.1 PTFE | 1 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.16 [0.24, 108.83] |
| 6.3 Arterial occlusion/re‐stenosis > 50% | 1 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.18] |
| 6.3.1 PTFE | 1 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.18] |
6.2. Analysis.

Comparison 6: Events during long‐term follow‐up (≥ 1 year) including events during first 30 days: bovine versus other synthetic, Outcome 2: Death
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AbuRahma 1996.
| Study characteristics | ||
| Methods | R: uncertain technique Both duplex and clinical FU blinded: unclear Cross‐overs: 4 patients in vein group underwent primary closure Exclusions during trial: 4 cross‐over and 3 jugular vein patients had saphenous vein patch Lost to FU: 3% | |
| Participants | USA 357 patients, 399 operations in 3 arms: 130 vein, 134 PTFE, and 135 primary closure 50% men Mean age 68 years 33% asymptomatic Comparability: age, sex, vascular risk factors, % asymptomatic disease similar in each group | |
| Interventions | Rx: PTFE patch Control: alternating saphenous vein patch (from ankle) and jugular vein Routine shunting for all and GA 325 mg daily aspirin was started within 24 hours of surgery for all patients | |
| Outcomes | Death, ipsilateral stroke, ipsilateral TIA, and ipsilateral RIND at 30 days and 48 months Duplex evidence of re‐stenosis > 50% during FU |
|
| Notes | Ex: 12 CEA with ICA < 4 mm or combined CABG or redo CEA surgery (3%)
FU: mean 30 months Funding sources for the study: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information about the sequence generation process was insufficient |
| Allocation concealment (selection bias) | Unclear risk | Information available to permit a judgement was insufficient |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants was not reported Because of the nature of the intervention (type of patch), this RCT could not be blinded for surgeons |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants (3%) were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Study authors published findings on all study outcomes |
| Other bias | Unclear risk | – |
AbuRahma 2002.
| Study characteristics | ||
| Methods | R: computer‐generated sealed envelopes with block of 10 randomisation Both duplex and clinical FU blinded Cross‐overs: none Exclusions during trial: redo CEAs (23 cases), CEAs with concomitant coronary artery bypass grafting (14 cases) Lost to FU: unclear | |
| Participants | USA 180 patients, 200 operations in 2 arms: 100 Dacron, 100 PTFE closure 53% men Mean age 68 years 39% asymptomatic Comparability: age, sex, vascular risk factors, % asymptomatic disease similar in each group | |
| Interventions | Rx: PTFE patch (GORE‐TEX) Control: collagen‐impregnated Dacron (Hemashield) Routine shunting for all and GA 325 mg daily aspirin was started within 24 hours of surgery for all patients | |
| Outcomes | Death, ipsilateral stroke, ipsilateral TIA, and ipsilateral RIND at 30 days Duplex evidence of re‐stenosis > 50% during follow‐up |
|
| Notes | Ex: CEA with ICA < 4 mm or combined CABG or redo CEA surgery
FU: 30 days and long‐term mean 36 months Funding sources for the study: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization envelopes were generated in blocks of 10 and placed in a closed container” |
| Allocation concealment (selection bias) | Low risk | “Using sealed opaque envelopes, each containing a slip of paper with the procedure assignment” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants was not reported Because of the nature of the intervention (type of patch), this RCT could not be blinded for surgeons |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “All patients were examined postoperatively by a physician who was blinded to the type of closure, were observed clinically, and underwent immediate postoperative color duplex ultrasound scanning” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not report this outcome |
| Selective reporting (reporting bias) | Low risk | Study authors published findings on all study outcomes |
| Other bias | Unclear risk | – |
AbuRahma 2007.
| Study characteristics | ||
| Methods | R: computer‐generated sealed envelopes with block of 10 randomisation Both duplex and clinical FU blinded Cross‐overs: none Exclusions during trial: concomitant CABG (3 cases) or redo carotid endarterectomy (2 cases) Lost to FU: unclear | |
| Participants | USA 200 patients, 200 operations in 2 arms: 100 PTFE, 100 Dacron closure 49.5% men Mean age 67.9 years 45% asymptomatic carotid disease Comparability: age, sex, vascular risk factors, % asymptomatic disease similar in each group | |
| Interventions | Rx: PTFE patch (ACUSEAL) Control: Dacron patch (Hemashield‐Fitnesse) Routine shunting for all and GA Postoperative aspirin 325 mg for all | |
| Outcomes | Perioperative outcomes: neurological event, ipsilateral stroke, ipsilateral TIA, mortality, re‐stenosis, thrombosis, combined perioperative neurological event Long‐term outcomes: ipsilateral stroke, all ipsilateral TIA, all TIA and strokes, all‐cause mortality, stroke mortality, all TIA‐stroke‐death rate, > 70% stenosis | |
| Notes | Ex: combined CABG or redo CEA surgery
FU: 30 days and long‐term mean 21 months Funding sources for the study: not applicable |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization envelopes were generated in blocks of 10 and placed in closed containers” |
| Allocation concealment (selection bias) | Low risk | “Using sealed opaque envelopes, each containing a slip of paper with the patch assignment” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants was not reported Because of the nature of the intervention (type of patch), this RCT could not be blinded for surgeons |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “All patients underwent immediate postoperative color duplex ultrasound scanning (CDUS), clinical observation, and examination by a physician who was blinded to the type of patch” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not report this outcome |
| Selective reporting (reporting bias) | Low risk | Study authors published findings on all study outcomes |
| Other bias | Unclear risk | – |
Albrecht‐Fruh 1998.
| Study characteristics | ||
| Methods | R: unclear Both duplex and clinical FU: unclear Cross‐overs: no | |
| Participants | Germany
52 patients, 52 operations
67% men
Mean age 67.1 years GA Intraluminal shunt was used dependent on SEP changes % asymptomatic carotid disease unclear but data indicate that it was similar in both groups Comparability: age, sex, vascular risk factors, % asymptomatic disease similar in each group |
|
| Interventions | Rx: Dacron patch Control: polyurethane patch % shunted: unclear Postoperative aspirin: unclear | |
| Outcomes | Perioperative outcome: bleeding time in suture hole Long‐term outcomes: stroke, re‐stenosis | |
| Notes | Ex: pregnancy, emergency operation, infection
FU: 30 days and long‐term outcome (1 year) Funding sources for the study: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants was not reported Because of the nature of the intervention (type of patch), this RCT could not be blinded for surgeons |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Study authors published findings on all study outcomes |
| Other bias | Unclear risk | – |
Gonzalez 1994.
| Study characteristics | ||
| Methods | R: open random number list (artery randomised) Clinical and DSA FU blind Cross‐overs: none Exclusions during trial: none Patients lost to FU: none | |
| Participants | Spain 84 patients, 95 operations 88% men Mean age 69 years 28% asymptomatic carotid disease Comparability: age, sex, vascular risk factors, % asymptomatic disease similar in each group | |
| Interventions | Rx: PTFE patch Control: saphenous vein patch (from ankle) Routine shunting for all Perioperative and postoperative aspirin (325 mg) for all | |
| Outcomes | Death < 30 days, at 1 year, and at end of FU Stroke < 30 days, at 1 year, and at end of FU Perioperative occlusion (intravenous DSA) Perioperative wound haemorrhage, infection, cranial nerve palsy Re‐stenosis > 50% or occlusion at 1 year and at end of FU (intravenous DSA) | |
| Notes | Ex: none
FU: mean 29 months Funding sources for the study: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Open random number list was used |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants was not reported Because of the nature of the intervention (type of patch), this RCT could not be blinded for surgeons |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This study was performed and was interpreted without knowledge of whether patients had vein patch angioplasty or PTFE patch angioplasty |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Study authors published findings on all study outcomes |
| Other bias | Unclear risk | – |
Grego 2003.
| Study characteristics | ||
| Methods | R: sealed envelopes with block randomisation Both duplex and clinical FU blinded Cross‐overs: no | |
| Participants | Italy 80 patients, 80 operations 61.9% men Mean age 70.25 years 30.6% asymptomatic carotid disease Comparability: age, vascular risk factors, % asymptomatic disease similar in each group except sex | |
| Interventions | Rx: PTFE patch Control: external jugular vein patch 100% shunted Postoperative: day 3 start aspirin, 100 mg for all | |
| Outcomes | Perioperative outcomes: blood loss, time to haemostasis, relevant neurological complication, TIA, mortality Long‐term outcomes: relevant neurological complication, stroke, mortality | |
| Notes | Ex: combined cardiac surgery, surgery to treat recurrent re‐stenosis, lack of external jugular vein
FU: perioperative outcome and long term (≥ 3 years) Funding sources for the study: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The envelopes were put in a container in blocks of 20 (10 EJV; 10 PTFE)" |
| Allocation concealment (selection bias) | Low risk | "Using sealed opaque envelopes containing indications for EJV or PTFE patching" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants was not reported Because of the nature of the intervention (type of patch), this RCT could not be blinded for surgeons |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All patients were examined postoperatively by a vascular surgeon, who was blinded to the type of procedure performed" "All patients underwent clinical vascular examination and a color duplex US scanning with a 7.5 mHz probe, with the vessel insonated at a 60‐degree angle. All examinations were performed by a single operator (M.A.), who was blinded to the type of closure" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants (5.6%) were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Study authors published findings on all study outcomes |
| Other bias | Unclear risk | – |
Hayes 2001.
| Study characteristics | ||
| Methods | R: computer‐generated sealed envelopes Both duplex and clinical FU blinded: unclear Cross‐overs: 3 patients randomised to vein had Dacron due to unsuitable vein Exclusions during trial: 3 cross‐overs; 5 patients had carotid bypass procedure performed; 1 patient had internal carotid artery ligation (after randomised) Lost to FU: 2 patients | |
| Participants | UK 273 patients, 276 operations in 2 arms: 136 patients in the thin‐walled Dacron patch (Hemashield Finesse) group and 137 patients in the vein group 67% men Mean age 70 years 11% asymptomatic Comparability: age, sex, vascular risk factors, % asymptomatic disease similar in each group | |
| Interventions | Rx: thin‐walled Dacron patch (Hemashield Finesse) Control: vein (great saphenous vein of groin area) Routine shunting for all and GA Perioperative antiplatelet: continued antiplatelet Postoperative dextran given selectively according to Doppler‐detected emboli | |
| Outcomes | Death, disabling stroke, non‐disabling stroke, cranial nerve injury, duplex evidence of re‐stenosis at 30 days and at 3 years | |
| Notes | Ex: patients with carotid bypass procedure performed, internal carotid artery ligation, unsuitable vein (Dacron patch inserted) 20 patients excluded before randomisation, 10 refusal, 6 previous bilateral varicose vein surgery, 1 severe claudication, 1 osteomyelitis, 1 repeat CEA, 1 combined common carotid angioplasty and CEA FU: 30 days and 3 years 2 patients refused long‐term duplex follow‐up Funding sources for the study: financial support from the UK Stroke Association through awarding of research grants associated with this project. Boston Scientific funded the salary of the theatre technician, who monitored all patients with TCD in the postoperative period |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Computer‐generated, random treatment methods (vein or Dacron patch) were consecutively numbered” |
| Allocation concealment (selection bias) | Low risk | “Sealed in opaque envelopes, and were allocated consecutively, immediately after induction of anesthesia” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants was not reported Because of the nature of the intervention (type of patch), this RCT could not be blinded for surgeons |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants (0.7%) were lost to follow‐up "Two patients refused to attend follow‐up clinics after 12 months had elapsed. Their clinical status, but not duplex scanning, was monitored out to 3 years by telephone review with the family doctor" |
| Selective reporting (reporting bias) | Low risk | Study authors published findings on all study outcomes |
| Other bias | High risk | Financial support from the UK Stroke Association through awarding of research grants associated with this project. Boston Scientific funded the salary of the theatre technician, who monitored all patients with TCD in the postoperative period |
Katz 1996.
| Study characteristics | ||
| Methods | R: uncertain technique | |
| Participants | USA 190 patients, 207 operations 49% men Mean age 71 years 47% asymptomatic Comparability: age, sex, vascular risk factors | |
| Interventions | Rx: knitted Dacron graft
Control: saphenous vein harvested from groin
Heparin reversed in most Dacron patients but in none of vein patients
All GA with continuous intra‐arterial pressure monitoring All patients shunted Dextran given perioperatively Postoperative aspirin for all |
|
| Outcomes | Death or ipsilateral stroke < 30 days Perioperative wound haemorrhage or infection | |
| Notes | Ex: 17 patients: 7 refusal, 6 absent vein graft, 4 combined CABG
FU: 30 days only Funding sources for the study: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants was not reported Because of the nature of the intervention (type of patch), this RCT could not be blinded for surgeons |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Study authors published findings on all study outcomes |
| Other bias | Unclear risk | – |
Lord 1989.
| Study characteristics | ||
| Methods | R: artery randomised Probably neither duplex nor clinical FU blind Cross‐overs: 4, but not known from which group Exclusions during trial: 4 cross‐overs Patients lost to FU: none | |
| Participants | Australia Number of patients unknown, 90 operations 62% men Mean age 63 years % asymptomatic carotid disease unknown Comparability: age, sex, vascular risk factors, % symptomatic disease similar in each group | |
| Interventions | Rx: PTFE patch Control: saphenous vein patch (from groin) 17% shunted (selective shunt) Postoperative aspirin for all | |
| Outcomes | Ipsilateral stroke < 30 days Perioperative occlusion (intravenous DSA) Perioperative wound haemorrhage | |
| Notes | Ex: unknown
FU: until hospital discharge Funding sources for the study: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants was not reported Because of the nature of the intervention (type of patch), this RCT could not be blinded for surgeons |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Study authors published findings on all study outcomes |
| Other bias | Unclear risk | – |
Marien 2002.
| Study characteristics | ||
| Methods | R: based on the last number of patient's medical record: odd number given bovine pericardium and even number given Dacron patches Blinded duplex and clinical FU: unclear Concealment: no Cross‐overs: none | |
| Participants | USA 92 patients, 95 operations 61% men Mean age 65.9 years 46.3% asymptomatic carotid disease Comparability: age, sex, vascular risk factors, % asymptomatic disease similar in each group, but % stroke is higher in bovine patch pericardium | |
| Interventions | Rx: bovine pericardium patch Control: Dacron patch 100 % shunted Postoperative for all patients: continue antiplatelet | |
| Outcomes | Perioperative outcome: suture line blood loss, neck haematoma, TIA, stroke, death | |
| Notes | Ex: concomitant CABG and recurrent carotid stenosis
FU: perioperative Funding sources for the study: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Based on the last number of patient's medical record" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants was not reported Because of the nature of the intervention (type of patch), this RCT could not be blinded for surgeons |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Study authors published findings on all study outcomes |
| Other bias | Unclear risk | – |
Meerwaldt 2008.
| Study characteristics | ||
| Methods | R: sealed envelopes Both duplex and clinical FU blinded Cross‐overs: none Lost to FU: none | |
| Participants | Netherlands 87 patients, 87 operations 79.3% men Mean age 66.5 years 0% asymptomatic carotid disease Comparability: age, sex, vascular risk factors similar in each group except the percentage of regressive stroke; non‐regressive stroke and bilateral carotid stenosis higher in bovine pericardium group | |
| Interventions | Rx: polyester patch (Fluoropassiv) Control: vein patch (ankle vein) 9.2% shunted Postoperative for all patients: continued dipyridamole 150 mg | |
| Outcomes | Perioperative period: death, TIA, regressive stroke, asymptomatic recurrent stenosis/acute thrombosis, local wound complication (e.g. nerve damage, infection, pain) At 6 weeks and at 2 years: death, stroke, re‐stenosis, acute thrombosis | |
| Notes | Ex: known allergies to patch product, previous ipsilateral carotid surgery, bilateral carotid endarterectomy, progressive neurological events 1 month before surgery (crescendo TIA), hospitalisation for heart failure in previous 6 months
FU: perioperative, 6 weeks, 2 years Funding sources for the study: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Low risk | "Using sealed opaque envelopes containing indication for venous or Fluoropassivä patch" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants was not reported Because of the nature of the intervention (type of patch), this RCT could not be blinded for surgeons |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All examinations were performed by an operator blinded for type of patch used" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "No patients were lost to follow‐up" |
| Selective reporting (reporting bias) | Low risk | Study authors published findings on all study outcomes |
| Other bias | Unclear risk | – |
O'Hara 2002.
| Study characteristics | ||
| Methods | R: computer‐generated sealed envelopes (artery randomised) Probably neither duplex nor clinical FU blinded Cross‐overs: none Exclusions during trial: 9 patients did not undergo allocated treatment Patients lost to FU: 4 | |
| Participants | USA 195 patients, 207 operations 74% men Mean age 69 years 58% asymptomatic Comparability: other than a higher incidence of males in the synthetic patch groups, age, vascular risk factors, and % symptomatic disease were similar in each group | |
| Interventions | Rx: knitted Dacron graft Control: saphenous vein harvest site not specified Anaesthesia and shunt use not specified | |
| Outcomes | Death or any stroke < 30 days Reoperation Any hospital complication Recurrent stenosis on follow‐up | |
| Notes | Ex: patients were excluded from randomisation including (1) declined to participate after receiving full disclosure, (2) an adequate segment of the saphenous vein was known to be unavailable in either groin before CEA, (3) the proposed CEA represented a carotid reoperation, (4) scheduled to be combined with a cardiac procedure, (5) there was any evidence of local or systemic sepsis 9 patients allocated to surgery failed to have it FU: 30 days and median long‐term duplex FU at 18 months Funding sources for the study: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Prospectively randomized either to autogenous saphenous vein (ASV) patching or to synthetic patching (with knitted Dacron graft) before operation according to a computer generated randomization scheme" |
| Allocation concealment (selection bias) | Low risk | "Patch assignment was done with sealed, sequenced envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants was not reported Because of the nature of the intervention (type of patch), this RCT could not be blinded for surgeons |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants (2%) were lost to follow‐up "Follow‐up examination was complete for 191 patients (203 operations) in the randomized cohort" |
| Selective reporting (reporting bias) | Low risk | Study authors published findings on all study outcomes |
| Other bias | Unclear risk | – |
Ricco 1996.
| Study characteristics | ||
| Methods | R: opaque, sequentially numbered, sealed envelopes (artery randomised) Follow‐up blinding attempted Cross‐overs: none Exclusions during trial: none Patients lost to FU: 3 PTFE, 4 vein patch | |
| Participants | France 124 patients, 141 operations 80% men Mean age 63.5 years 33% asymptomatic carotid disease Comparability: age, sex, vascular risk factors, % asymptomatic disease were similar in each group | |
| Interventions | Rx: PTFE patch Control: saphenous vein patch (90% from groin) 83% shunted Postoperative aspirin (250 mg) for all | |
| Outcomes | Death < 30 days, at 1 year, and at end of FU Ipsilateral stroke < 30 days, at 1 year, and at end of FU Perioperative occlusion (Duplex) Perioperative wound haemorrhage Re‐stenosis > 50% or occlusion at 1 year and at end of FU (Duplex) | |
| Notes | Ex: external artery diameter > 5 mm and internal diameter > 3.5 mm, recurrent stenosis
FU: mean 53 months Funding sources for the study: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequentially numbered randomisation |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants was not reported Because of the nature of the intervention (type of patch), this RCT could not be blinded for surgeons |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants (5%) were lost to follow‐up; patients lost to FU: 3 PTFE, 4 vein patch |
| Selective reporting (reporting bias) | Low risk | Study authors published findings on all study outcomes |
| Other bias | Unclear risk | – |
Stone 2014.
| Study characteristics | ||
| Methods | R: computer‐generated sealed envelopes Both duplex and clinical FU blinded: unclear Cross‐overs: no Exclusions during trial: 4 patients, 3 interposition graft, and 1 total occluded ICA (after randomised) Lost to FU: none | |
| Participants | UK 195 patients, 195 operations in 2 arms: 97 patients in ACUSEAL group and 98 patients in bovine pericardium (Vascu‐Guard) group 55% men Mean age 67 years 67% asymptomatic Comparability: age, sex, vascular risk factors, % asymptomatic disease similar in each group, except more current smokers in the ACUSEAL group and more patients with congestive heart failure in the Vascu‐Guard group | |
| Interventions | Rx: PTFE (ACUSEAL) Control: bovine pericardium (Vascu‐Guard) Routine shunting for all and GA Perioperative antiplatelet: continued antiplatelet | |
| Outcomes | Stroke within 30 days, TIA within 30 days, neck haematoma, surgical site infection, death, duplex evidence of re‐stenosis at 30 days and at 6 months | |
| Notes | Ex: 5 patients were excluded from the trial for the following reasons: for 3, patch was too short and required interposition repair; 1 patient’s disease was too extensive; 1 patient’s ICA was totally occluded 1 patient as excluded before randomisation FU: 30 days and 6 months Funding sources for the study: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Generated in blocks of 10 and placed in a closed container" |
| Allocation concealment (selection bias) | Low risk | "Using sealed opaque envelopes, each containing a slip of paper with the procedure assignment" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants was not reported. Because of the nature of the intervention (type of patch), this RCT could not be blinded for surgeons |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Study authors published findings on all study outcomes |
| Other bias | Unclear risk | – |
CABG: carotid artery bypass graft. CEA: carotid endarterectomy. DSA: digital subtraction angiography. Ex: exclusion criteria. FU: follow‐up. GA: general anaesthesia. ICA: internal carotid artery. PTFE: polytetrafluoroethylene. R: concealment of allocation. RCT: randomised controlled trial. RIND: reversible ischaemic neurological deficit. Rx: treatment. SEP: somatosensory evoked potential. TCD: transcranial Doppler. TIA: transient ischaemic attack.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Carney 1987 | Lack of clinical data No clinical outcomes recorded (e.g. death, stroke, arterial complications) 74 patients randomised between Dacron, PTFE, and venous patch Randomisation by open random number list Main outcomes were number of packets of oxidised cellulose used and elapsed time between removal of the carotid occluding clamps and the end of the procedure, which not directly refer and was non‐relevant to bleeding during surgery |
| Chyatte 1996 | The outcome was microemboli perioperatively, which was not related to clinical efficacy of carotid patch material angioplasty No clinical outcomes were recorded (e.g. death, stroke, arterial complications), but researchers looked at microemboli perioperatively |
| Ruckert 2000 | Outcomes were bleeding time and microemboli, which were not particular clinical outcomes of carotid patch material angioplasty No clinical outcomes were recorded (e.g. death, stroke, arterial complications), but researchers looked at bleeding time, microemboli perioperatively Although there is a re‐stenosis result, study authors did not report whether this was a perioperative or long‐term outcome |
PTFE: polytetrafluoroethylene.
Differences between protocol and review
We removed the sensitivity analysis because no significant difference was found between trials with different allocation techniques; because no studies in this later review were quasi‐randomised, we did not carry out sensitivity analyses for this version of the review.
Contributions of authors
Saritphat Orrapin, Thoetphum Benyakorn, Kittipan Rerkasem: refined the protocol, performed searches, selected studies for inclusion, extracted and entered data, and wrote the review.
Boonying Siribumrungwong, Dominic PJ Howard, Kittipan Rerkasem: refined the protocol, co‐ordinated the project, and commented on the design of the protocol and on the final manuscript.
Declarations of interest
Saritphat Orrapin: none known.
Thoetphum Benyakorn: none known.
Boonying Siribumrungwong: none known.
Dominic PJ Howard: none known.
Kittipan Rerkasem: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
AbuRahma 1996 {published data only}
- *.AbuRahma AF, Khan JH, Robinson PA, Saiedy S, Short YS, Boland JP, et al. Prospective randomized trial of carotid endarterectomy with primary closure and patch angioplasty with saphenous vein, jugular vein, and polytetrafluoroethylene: perioperative (30 day) results. Journal of Vascular Surgery 1996;24:998-1007. [DOI] [PubMed] [Google Scholar]
- AbuRahma AF, Robinson PA, Saiedy S, Kahn JH, Boland JP. Prospective randomized trial of carotid endarterectomy with primary closure and patch angioplasty with saphenous vein, jugular vein, and polytetrafluoroethylene: long-term follow-up. Journal of Vascular Surgery 1998;27(2):222-32. [DOI] [PubMed] [Google Scholar]
AbuRahma 2002 {published data only}
- *.AbuRahma A, Hannay S, Khan JH, Robinson PA, Hudson JK, Davis EA. Prospective randomised study of carotid endarterectomy with polytetrafluoroethylene versus collagen-impregnated Dacron (Hemashield) patching: perioperative (30-day) results. Journal of Vascular Surgery 2002;35:125-30. [DOI] [PubMed] [Google Scholar]
- AbuRahma AF, Hopkins ES, Robinson PA, Deel JT, Agarwal S. Prospective randomized trial of carotid endarterectomy with polytetrafluoroethylene versus collagen-impregnated Dacron (Hemashield) patching: late follow-up. Annals of Surgery 2003;237:885-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
AbuRahma 2007 {published data only}
- AbuRahma AF, Stone P, Deem S, Dean LS, Keiffer T, Deem E. Proposed duplex velocity criteria for carotid restenosis following carotid endarterectomy with patch closure. Journal of Vascular Surgery 2009;50:286-91. [DOI] [PubMed] [Google Scholar]
- Aburahma AF, Stone PA, Elmore M, Flaherty SK, Armistead L, AbuRahma Z. Prospective randomized trial of ACUSEAL (Gore-Tex) vs Finesse (Hemashield) patching during carotid endarterectomy: long-term outcome. Journal of Vascular Surgery 2008;48(1):99-103. [DOI] [PubMed] [Google Scholar]
- *.AbuRahma AF, Stone PA, Flaherty SK, AbuRahma Z. Prospective randomized trial of ACUSEAL (Gore-Tex) versus Hemashield-Finesse patching during carotid endarterectomy: early results. Journal of Vascular Surgery 2007;45:881-4. [DOI] [PubMed] [Google Scholar]
Albrecht‐Fruh 1998 {published data only}
- Albrecht-Fruh G, Ktenidis K, Horsch S. Dacron versus polyurethane (PUR) patch angioplasty in carotid surgery. In: Horsch S, Ktenidis K, editors(s). Perioperative Monitoring in Carotid Surgery. Darmstadt Steinkopff Springer, 1998:153-7. [Google Scholar]
Gonzalez 1994 {published and unpublished data}
- *.Gonzalez-Fajardo JA, Perez JL, Mateo AM. Saphenous vein patch versus polytetrafluoroethylene patch after carotid endarterectomy. Journal of Cardiovascular Surgery 1994;35:523-8. [PubMed] [Google Scholar]
- Perez-Burckhardt JL, Gonzalez-Fajardo JA, Mateo AH. Saphenous vein patch versus polytetrafluoroethylene patch after carotid endarterectomy. International Angiology 1995;14 Suppl 1:346. [PubMed] [Google Scholar]
Grego 2003 {published data only}
- Grego F, Antonello M, Lepidi S, Bonvini S, Deriu GP. Prospective, randomized study of external jugular vein patch versus polytetrafluoroethylene patch during carotid endarterectomy: perioperative and long-term results. Journal of Vascular Surgery 2003;38:1232-40. [DOI] [PubMed] [Google Scholar]
Hayes 2001 {published data only}
- *.Hayes PD, Allroggen H, Steel S, Thompson MM, London NJ, Bell PR, et al. Randomized trial of vein versus Dacron patching during carotid endarterectomy: influence of patch type on postoperative embolization. Journal of Vascular Surgery 2001;33(5):994-1000. [DOI] [PubMed] [Google Scholar]
- Naylor R, Hayes PD, Payne DA, Allroggen H, Steel S, Thompson MM, et al. Randomized trial of vein versus Dacron patching during carotid endarterectomy: long-term results. Journal of Vascular Surgery 2004;39(5):985-93. [DOI] [PubMed] [Google Scholar]
Katz 1996 {published data only}
- Katz SG, Kohl RD. Does the choice of material influence early morbidity in patients undergoing carotid patch angioplasty? Surgery 1996;119:297-301. [DOI] [PubMed] [Google Scholar]
Lord 1989 {published and unpublished data}
- Lord RSA, Raj TB, Stary DL, Nash PA, Graham AR, Goh KH. Comparison of saphenous vein patch, polytetrafluoroethylene patch, and direct arteriotomy closure after carotid endarterectomy. Part I: Perioperative results. Journal of Vascular Surgery 1989;9:521-9. [PubMed] [Google Scholar]
Marien 2002 {published data only}
- Marien BJ, Raffetto JD, Seidman CS, LaMorte WW, Menzoian JO. Bovine pericardium vs Dacron for patch angioplasty after carotid endarterectomy. A prospective randomized study. Archives of Surgery 2002;137:785-8. [DOI] [PubMed] [Google Scholar]
Meerwaldt 2008 {published data only}
- Meerwaldt R, Lansink KW, Blomme AM, Fritschy WM. Prospective randomized study of carotid endarterectomy with Fluoropassiv thin wall carotid patch versus venous patch. European Journal of Vascular and Endovascular Surgery 2008;36:45-52. [DOI] [PubMed] [Google Scholar]
O'Hara 2002 {published data only}
- O'Hara PJ, Hertzer NR, Mascha EJ, Krajewski LP, Clair DG, Ouriel K. A prospective, randomized study of saphenous vein patching versus synthetic patching during carotid endarterectomy. Journal of Vascular Surgery 2002;35(2):324-30. [DOI] [PubMed] [Google Scholar]
Ricco 1996 {published and unpublished data}
- *.Ricco JB, Demarque C, Bouin-Pineau MH, Camiade C. Vein patch versus PTFE patch for carotid endarterectomy. A randomized trial in a selected group of patients. Manuscript submitted to the European Journal of Vascular Surgery 1996.
- Ricco JB, Gauthier JB, Bodin-Pineau MH. Enlargement patch after carotid endarterectomy. Polytetrafluoroethylene (PTFE) or saphenous patch? Results of a randomized study after 3 years (abstract). In: Paper presented at The French Language Society of Vascular Surgery, Annual Congress of Reims. 19-20 June 1992.
Stone 2014 {published data only}
- Stone PA, AbuRahma AF, Mousa AY, Phang D, Hass SM, Modak A, et al. Prospective randomized trial of ACUSEAL versus Vascu-Guard patching in carotid endarterectomy. Annals of Vascular Surgery 2014;28(6):1530-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Carney 1987 {published data only}
- Carney WI, Lilly MP. Intraoperative evaluation of PTFE, Dacron and autologous vein as carotid patch materials. Annals of Vascular Surgery 1987;1:583-6. [DOI] [PubMed] [Google Scholar]
Chyatte 1996 {published data only (unpublished sought but not used)}
- Chyatte D. Perioperative microemboli during carotid endarterectomy: differences between synthetic and saphenous vein patches. Cerebrovascular Diseases 1996;6 Suppl 2:2 (Abstract 17). [Google Scholar]
Ruckert 2000 {published data only}
- Ruckert RI, Obitz JG, Schwenk W, Walter M. Prospective randomized comparison of different patch materials in carotid surgery. Acta Chirurgica Austriaca 2000;32:19. [Google Scholar]
Additional references
ACAS 1995
- Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995;273(18):1421-8. [PubMed] [Google Scholar]
ACST‐1 2010
- Halliday AH, Harrison M, Hayter E, Kong X, Mansfield A, Marro J. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet 2010;376:1074-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
APT 1994
- Antiplatelet Trialists Collaboration. Collaborative overview of trials of antiplatelet therapy - I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81-106. [PMC free article] [PubMed] [Google Scholar]
Bamford 1988
- Bamford JM, Sandercock PAG, Dennis MS, Warlow C, Jones L, McPherson K, et al. A prospective study of acute cerebro-vascular disease in the community. The Oxfordshire Community Stroke Project 1981-1986. (I) Methodology, demography and incident cases of first ever stroke. Journal of Neurology, Neurosurgery, and Psychiatry 1988;51:1373-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Counsell 1998
- Counsell C, Salinas R, Warlow C, Naylor R. Patch angioplasty compared to primary closure in carotid endarterectomy. Cochrane Database of Systematic Reviews 1998, Issue 1. Art. No: CD000160. [DOI: 10.1002/14651858.CD000160] [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors(s). Systematic Reviews in Health Care. 2nd edition. London, UK: BMJ Books, 2001:200. [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011:www.handbook.cochrane.org. [Google Scholar]
ECST 1991
- European Carotid Surgery Trialists' Collaborative Group. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70-99%) or with mild (0-29%) carotid stenosis. Lancet 1991;337:1235-43. [PubMed] [Google Scholar]
ECST 1998
- European Carotid Surgery Trialists Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC Eurpean Carotid Surgery Trial (ECST). Lancet 1998;351:1379-87. [PubMed] [Google Scholar]
Fode 1986
- Fode NC, Sundt Jr TM, Robertson JT, Peerless SJ, Shields CB. Multicenter retrospective review of results and complications of carotid endarterectomy in 1981. Stroke 1986;17:370-6. [DOI] [PubMed] [Google Scholar]
GBD 2019
- The Global Burden of Diseases, Injuries, and Risk Factors Study 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurology 2019;18(5):439-58. [DOI: 10.1016/S1474-4422(19)30034-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- GRADEpro GDT. GRADE Working Group. Hamilton (ON): McMaster University, 2015 (accessed 18 January 2017).
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2016
- Higgins JPT, Lasserson T, Chandler J, Tovey D, Churchill R. Standards for the conduct of new Cochrane Intervention Reviews. In: Higgins JPT, Lasserson T, Chandler J, Tovey D, Churchill R, editors(s). Methodological Expectations of Cochrane Intervention Reviews. London: Cochrane, 2016:Available from http://methods.cochrane.org/resources. [Google Scholar]
Higgins 2020
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated Sepetember 2020). Cochrane, 2020:Available from www.training.cochrane.org/handbook/current/chapter-08. [Google Scholar]
Kim 2001
- Kim GE, Kwon TW, Cho YP, Kim DK, Kim HS. Carotid endarterectomy with bovine patch angioplasty: a preliminary report. Cardiovascular Surgery 2001;9(5):458-62. [DOI] [PubMed] [Google Scholar]
Mckenzie 2020
- McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV, Thomas J. Chapter 3: Defining the criteria for including studies and how they will be grouped for the synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook. [Google Scholar]
Murie 1994
- Murie JA, John TG, Morris PJ. Carotid endarterectomy in Great Britain and Ireland: practice between 1984 and 1992. British Journal of Surgery 1994;81:827-31. [DOI] [PubMed] [Google Scholar]
NASCET 1991
- North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. New England Journal of Medicine 1991;325:445-53. [DOI] [PubMed] [Google Scholar]
NASCET 1998
- North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with symptomatic moderate or severe stenosis. New England Journal of Medicine 1998;339:1415-25. [DOI] [PubMed] [Google Scholar]
Nichols 2012
- Nichols M, Townsend N, Luengo-Fernandez R, Leal J, Scarborough P, Rayner M. European cardiovascular disease statistics 2012. Sophia Antipolis: European Heart Network, Brussels, European Society of Cardiology.
Ren 2013
- Ren S, Li S, Wen J, Zhang W, Liu P. Systematic review of randomized controlled trials of different types of patch materials during carotid endarterectomy. PLOS ONE 2013;8(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- Review Manager (RevMan). Version 5.4.1. The Cochrane Collaboration, 2020.
Schmitt 1986
- Schmitt DD, Bandyk DF, Pequet AJ, Towne JB. Bacterial adherence to vascular prostheses. A determinant of graft infectivity. Journal of Vascular Surgery 1986;3:732-40. [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011:Available from www.handbook.cochrane.org. [Google Scholar]
SPSS 2019 [Computer program] [Computer program]
- SPSS Base 26.0 for Windows. SPSS Inc. Chicago (IL): SPSS Inc, 2019.
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011:Available from www.handbook.cochrane.or. [Google Scholar]
Texakalidis 2018
- Texakalidis P, Giannopoulos S, Charisis N, Giannopoulos S, Karasavvidis T, Koullias G, et al. A meta-analysis of randomized trials comparing bovine pericardium and other patch materials for carotid endarterectomy. Journal of Vascular Surgery 2018;68:1241-56. [DOI] [PubMed] [Google Scholar]
Truelsen 2006
- Truelsen B, Piechowski-Jozwiak T, Bonita R, Mathersa C, Bogousslavsky J, Boysen G. Stroke incidence and prevalence in Europe. European Neurology 2006;13:581-98. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bond 2004
- Bond R, Rerkasem K, Naylor R, Rothwell PM. Patches of different types for carotid patch angioplasty. Cochrane Database of Systematic Reviews 2004, Issue 2. Art. No: CD000071. [DOI: 10.1002/14651858.CD000071.pub2] [DOI] [PubMed] [Google Scholar]
Counsell 2000
- Counsell C, Warlow C, Naylor R. Patches of different types for carotid patch angioplasty. Cochrane Database of Systematic Reviews 2000, Issue 1. Art. No: CD000071. [DOI: 10.1002/14651858.CD000071] [DOI] [PubMed] [Google Scholar]
Rerkasem 2010
- Rerkasem K, Rothwell PM. Patches of different types for carotid patch angioplasty. Cochrane Database of Systematic Reviews 2010, Issue 3. Art. No: CD000071. [DOI: 10.1002/14651858.CD000071] [DOI] [PMC free article] [PubMed] [Google Scholar]


