Abstract
Background
This is the first update of this review first published in 2009. When treating elevated blood pressure, doctors usually try to achieve a blood pressure target. That target is the blood pressure value below which the optimal clinical benefit is supposedly obtained. “The lower the better” approach that guided the treatment of elevated blood pressure for many years was challenged during the last decade due to lack of evidence from randomised trials supporting that strategy. For that reason, the standard blood pressure target in clinical practice during the last years has been less than 140/90 mm Hg for the general population of patients with elevated blood pressure. However, new trials published in recent years have reintroduced the idea of trying to achieve lower blood pressure targets. Therefore, it is important to know whether the benefits outweigh harms when attempting to achieve targets lower than the standard target.
Objectives
The primary objective was to determine if lower blood pressure targets (any target less than or equal to 135/85 mm Hg) are associated with reduction in mortality and morbidity as compared with standard blood pressure targets (less than or equal to 140/ 90 mm Hg) for the treatment of patients with chronic arterial hypertension.
The secondary objectives were: to determine if there is a change in mean achieved systolic blood pressure (SBP) and diastolic blood pressure (DBP associated with "lower targets" as compared with "standard targets" in patients with chronic arterial hypertension; and to determine if there is a change in withdrawals due to adverse events with "lower targets" as compared with "standard targets", in patients with elevated blood pressure.
Search methods
The Cochrane Hypertension Information Specialist searched the following databases for randomised controlled trials up to May 2019: the Cochrane Hypertension Specialised Register, CENTRAL (2019, Issue 4), Ovid MEDLINE, Ovid Embase, the WHO International Clinical Trials Registry Platform, and ClinicalTrials.gov. We also contacted authors of relevant papers regarding further published and unpublished work. The searches had no language restrictions.
Selection criteria
Randomised controlled trials (RCTs) comparing patients allocated to lower or to standard blood pressure targets (see above).
Data collection and analysis
Two review authors (JAA, VL) independently assessed the included trials and extracted data. Primary outcomes were total mortality; total serious adverse events; myocardial infarction, stroke, congestive heart failure, end stage renal disease, and other serious adverse events. Secondary outcomes were achieved mean SBP and DBP, withdrawals due to adverse effects, and mean number of antihypertensive drugs used. We assessed the risk of bias of each trial using the Cochrane risk of bias tool and the certainty of the evidence using the GRADE approach.
Main results
This update includes 11 RCTs involving 38,688 participants with a mean follow‐up of 3.7 years. This represents 7 new RCTs compared with the original version.
At baseline the mean weighted age was 63.1 years and the mean weighted blood pressure was 155/91 mm Hg.
Lower targets do not reduce total mortality (risk ratio (RR) 0.95, 95% confidence interval (CI) 0.86 to 1.05; 11 trials, 38,688 participants; high‐certainty evidence) and do not reduce total serious adverse events (RR 1.04, 95% CI 0.99 to 1.08; 6 trials, 18,165 participants; moderate‐certainty evidence). This means that the benefits of lower targets do not outweigh the harms as compared to standard blood pressure targets. Lower targets may reduce myocardial infarction (RR 0.84, 95% CI 0.73 to 0.96; 6 trials, 18,938 participants, absolute risk reduction (ARR) 0.4%, number needed to treat to benefit (NNTB) 250 over 3.7 years) and congestive heart failure (RR 0.75, 95% CI 0.60 to 0.92; 5 trials, 15,859 participants, ARR 0.6%, NNTB 167 over 3.7 years) (low‐certainty for both outcomes). Reduction in myocardial infarction and congestive heart failure was not reflected in total serious adverse events. This may be due to an increase in other serious adverse events (RR 1.44, 95% CI 1.32 to 1.59; 6 trials. 18,938 participants, absolute risk increase (ARI) 3%, number needed to treat to harm (NNTH) 33 over four years) (low‐certainty evidence).
Participants assigned to a "lower” target received one additional antihypertensive medication and achieved a significantly lower mean SBP (122.8 mm Hg versus 135.0 mm Hg, and a lower mean DBP (82.0 mm Hg versus 85.2 mm Hg, than those assigned to "standard target".
Authors' conclusions
For the general population of persons with elevated blood pressure, the benefits of trying to achieve a lower blood pressure target rather than a standard target (≤ 140/90 mm Hg) do not outweigh the harms associated with that intervention. Further research is needed to see if some groups of patients would benefit or be harmed by lower targets. The results of this review are primarily applicable to older people with moderate to high cardiovascular risk. They may not be applicable to other populations.
Plain language summary
The use of lower blood pressure targets for people with hypertension
Background
We conducted this review to find and assess all trials designed to evaluate whether lower blood pressure targets are better than standard blood pressure targets for people with hypertension.
The main objective in the treatment of hypertension is to prevent serious vascular complications. For the general population of people with hypertension, the standard treatment target has been to achieve a blood pressure of less than 140/90 mm Hg. Some clinical guidelines have recommended stricter control of blood pressure based on the assumption that achieving a lower blood pressure will produce a greater reduction in cardiovascular events.
Study Characteristics
The evidence is current to May 2019. We included 11 randomised controlled trials involving 38,688 adult participants with arterial hypertension, aged between 20 and 80 years of age, who received treatment aimed to lower blood pressure to a standard compared to a lower blood pressure target and followed for mean 3.7 years to detect differences in mortality and adverse events.
Key Results
The only significant benefits in the group assigned to 'lower' blood pressure targets was a small reduction in the incidence of heart attack and a small reduction in the incidence of congestive heart failure. However, the lower target group had an increase in the number of other serious adverse events. High‐certainty evidence showed there was no difference in death from any cause or total serious adverse events with lower as compared to standard blood pressure targets. .
For the general population of persons with elevated blood pressure the small benefits of trying to achieve a lower blood pressure target rather than a standard target (≤ 140/90 mm Hg) do not outweigh the harms. Further research is needed to see if some groups of patients would benefit or be harmed by lower targets.
Summary of findings
Summary of findings 1. Lower BP target compared to standard BP target for hypertension.
| Lower BP target compared to standard BP target for hypertension | ||||||
| Patient or population: adult patients with hypertension Setting: outpatient setting Intervention: lower BP target Comparison: standard BP target (<=140/<=90) | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| Without lower BP target | With lower BP target | Difference | ||||
| Total mortality follow‐up: mean 3.7 years № of participants: 38,688 (11 RCTs) | RR 0.95 (0.86 to 1.05) | Study population | ⊕⊕⊕⊕ HIGH | Lower blood pressure targets do not reduce mortality. | ||
| 4.2% | 4.0% (3.6 to 4.4) | 0.2% fewer (0.6 fewer to 0.2 more) | ||||
| Total serious adverse events № of participants: 18165 (6 RCTs) | RR 1.04 (0.99 to 1.08) | Study population | ⊕⊕⊕⊝ MODERATE1 | Lower blood pressure targets do not reduce total serious adverse events. | ||
| 29.1% | 30.3% (28.8 to 31.4) | 1.2% more (0.3 fewer to 2.3 more) | ||||
| Myocardial infarction № of participants: 38,198 (8 RCTs) | RR 0.84 (0.73 to 0.96) | Study population | ⊕⊕⊝⊝ LOW 1 2 | Lower blood pressure target may reduce myocardial infarction slightly. | ||
| 2.5% | 2.1% (1.9 to 2.4) | 0.4% fewer (0.7 fewer to 0.1 fewer) | ||||
| Stroke № of participants: 37,087 (7 RCTs) | RR 0.88 (0.77 to 1.01) | Study population | ⊕⊕⊝⊝ LOW 1 2 4 | It is uncertain whether the lower blood pressure target reduces stroke slightly (mainly due to systolic target) | ||
| 2.5% | 2.2% (1.9 to 2.5) | 0.3% fewer (0.6 fewer to 0 fewer) | ||||
| Congestive heart failure № of participants: 15,859 (5 RCTs) | RR 0.75 (0.60 to 0.92) | Study population | ⊕⊕⊝⊝ LOW 1 2 3 | Lower blood pressure target may reduce congestive heart failure slightly. | ||
| 2.5% | 1.9% (1.5 to 2.3) | 0.6% fewer (1 fewer to 0.2 fewer) | ||||
| Other serious adverse events follow up: mean 3.7 years № of participants: 18,938 (6 RCTs) | RR 1.44 (1.32 to 1.59) | Study population | ⊕⊕⊝⊝ LOW 1 3 | Lower blood pressure target may increase other serious adverse events (ARI 3.0%) | ||
| 6.8% | 9.8% (9.0 to 10.9) | 3.0% more (2.2 more to 4 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ARI: absolute risk increase; CI: Confidence interval; RCT: randomised controlled trial; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Information is missing for several trials
2 Trials could not be blinded
3 Wide confidence interval
4 Effect with systolic target and diastolic target was heterogeneous.
Background
Description of the condition
Epidemiological studies show a continuous direct relationship between blood pressure and adverse cardiovascular events (Prospective Studies Collaboration 2002; Prospective Studies Collaboration 2007). The relationship has a greater slope with increasing levels of blood pressure. Therefore, elevated blood pressure has been identified as one of the major risk factors for adverse cardiovascular events (Kannel 1996; Rapsomaniki 2014; Stamler 1993; Stokes 1987). Diastolic blood pressure (DBP) was originally considered the main risk component. More recently, systolic blood pressure (SBP) has been considered more important, because its prognostic value appears greater than that of DBP and it is observable over all age ranges. Diastolic blood pressure is a clear risk factor in young people but it disappears or even reverts in older people.
The absence of an apparent threshold in the association between blood pressure and cardiovascular events (Prospective Studies Collaboration 2002) implies that any numerical cut‐off value above which elevated blood pressure (hypertension) is defined is arbitrary. The standard for diagnosis of arterial hypertension is based on consensus recommendations, which attempt to predict the blood pressure above which it is expected that treatment will provide more benefit than harm. At the present time the benefits of treatment has been shown to outweigh the harms for adults 60 years of age and older with moderate to severe hypertension (Musini 2019). However, the issue has been controversial for adults with mild hypertension (140‐159/90‐99 mmHg) (Diao 2012, Sundström 2015).
The primary goal in the management of patients with elevated blood pressure is to maximise the reduction in mortality and morbidity (Mancia 2013). The benefit from drug treatment seems rather clear when treating substantially elevated blood pressure (Law 2009), but the lower threshold at which this relationship no longer applies has not been identified definitively. At the same time, the benefit from some blood pressure‐lowering drugs has been established in other conditions with normal or even low blood pressure levels, e.g. angiotensin‐converting enzyme (ACE) inhibitors and beta‐blockers in congestive heart failure, or beta‐blockers after myocardial infarction. However, in these situations, the benefit from these drugs has been established with fixed dosages, without any adjustment to the apparent blood pressure level or response; furthermore, in those conditions the benefits could be due to other pathophysiological mechanisms and not only due to the reduction in blood pressure.
Besides, the potential benefits of treating elevated blood pressure might be influenced by different factors, such as the profile of adverse effects of the antihypertensive drugs and the patient's overall cardiovascular risk (BPLTTC 2014; Jackson 2005; Thomopoulos 2014; Zanchetti 2015).
The threshold above which antihypertensive treatment benefits outweigh harms in patients with elevated blood pressure remains unclear.
Description of the intervention
The target blood pressure is used in clinical practice as the goal of antihypertensive therapy. It guides the physician in clinical practice when making treatment decisions related to the intensity of the antihypertensive regimen used for each patient. For example, if the blood pressure is higher than the target, then the practitioner would increase the antihypertensive treatment by increasing the dose or adding another drug. The standard target pressure has generally been the arbitrary threshold blood pressure above which treatment is recommended. Thus over the years, the standard SBP target declined from ≤ 160 mm Hg to a target of ≤ 140 mm Hg. Similarly, the standard DBP target has decreased from ≤ 100 mm Hg to ≤ 90 mm Hg.
How the intervention might work
It is assumed that treating to lower blood pressure targets with antihypertensive drugs will achieve the predicted reduction in cardiovascular morbidity and mortality seen in epidemiological observational studies. However, elevated blood pressure can be considered as a marker of vascular disease and aggressive reduction in blood pressure does not necessarily mean that the pathological and functional vascular abnormalities already established will be reversed. In fact, some trials not designed to compare blood pressure targets have shown that achieving lower blood pressures does not necessarily provide an additional reduction in cardiovascular mortality and morbidity (ONTARGET 2008).
Why it is important to do this review
The trend toward “the lower the pressure the better” was a dominant concept in the treatment of hypertension for many years, especially for patients considered to be at higher risk, such as people with diabetes, chronic renal disease, or ischaemic heart disease (AHA 2007; BHS 2004; ESH‐ESC 2007; JNC 7 2003; K/DOQI 2004; Laurent 2004; WHO/ISH 2003). That concept was mainly based on observational data and on retrospective analyses of outcome trials. However, the only way to prove that a lower blood pressure target is beneficial is through clinical trials where patients are randomised to different treatment targets. The first version of this Cochrane Systematic Review and meta‐analysis of randomised controlled clinical trials (Arguedas 2009) found that in the general population of patients with hypertension, treating to blood pressure targets lower than 135/85 mm Hg by pharmacological means did not result in lower mortality or cardiovascular morbidity as compared with standard targets (lower than 140 mm Hg to 160 mm Hg SBP and lower than 90 mm Hf to 100 mm Hg diastolic). Therefore, the assumption that treating to lower targets would provide a greater reduction in cardiovascular risk, as suggested by epidemiological studies, was not proven, and “the lower the better” strategy in hypertension was challenged (Arguedas 2010; Filippone 2011; Grossman 2011).
The results of that previous Cochrane Systematic Review were based mainly on diastolic targets, since systolic targets were only marginally expressed in two trials aiming for targets defined according to mean arterial blood pressure. Two additional Cochrane Reviews including only patients with diabetes (Arguedas 2013) or with established cardiovascular disease (Saiz 2020) concluded that evidence from randomised trials does not support blood pressure targets lower than the standard targets in people with elevated blood pressure and those conditions.
Due to the lack of evidence, several clinical guidelines abandoned "the lower the better" strategy, and set a general standard target of less than 140/90 mm Hg for patients with hypertension (ADA 2016; ASH/ISH 2014; JNC 8 2014; Mancia 2013; NICE 2011), with the exception related to elderly patients, for whom a higher systolic target of < 150 mm Hg was suggested in one guideline (JNC 8 2014).
However, several trials and review analyses published later re‐introduced the controversy of aiming for lower blood pressure targets (Ettehad 2016; Heimark 2018; Laurent 2016; SPRINT 2015; Xie 2016). Despite criticism (Kaul 2018), the lower target is recommended again in some clinical guidelines (AACE 2019, ACC/AHA 2017), while other guidelines maintain the standard target (NICE 2019). Finally, some other guidelines recommend a blood pressure target below 140/90 mm Hg in all patients, but also suggest a target below 130/80 mm Hg under certain circumstances such as diabetes or chronic kidney disease (ESC/ESH 2018, ADA 2019, Hypertension Canada 2020).
Attempting to achieve lower blood pressure targets has several consequences. The most obvious is the need for larger doses or an increased number of antihypertensive drugs. This has an adverse impact on patients in terms of inconvenience and costs. More drugs and higher doses will also increase adverse drug effects and could lead to higher rates of permanent treatment discontinuation (Thomopoulos 2016 b). Besides, serious adverse effects could cancel any potential benefits associated with any lower blood pressures achieved (Bangalore 2010; Dorresteijn 2012; Lund‐Johansen 2003; Ortiz 2016; Sleight 2009; Voko 1999; Zanchetti 2003).
The importance of this review is to update the 2009 review, including all randomised controlled trials (TCTs) where patients with elevated blood pressure were randomised to lower targets (< 135/85 mm Hg) as compared with the standard targets (< 140/90 mm Hg). Trials with treatment targets higher than the standard targets were excluded.
Objectives
Primary objective
To determine if there is a reduction in total mortality and morbidity associated with treatment of blood pressure to "lower targets" as compared with "standard targets" in the management of patients with chronic arterial hypertension. "Lower targets" are defined as blood pressure targets less than or equal to 135/85 mm Hg. "Standard targets" are defined as blood pressure targets less than or equal to 140/90 mm Hg.
Secondary objectives
To determine if there is a change in mean achieved systolic blood pressure (SBP) and diastolic blood pressure (DBP) associated with "lower targets" as compared with "standard targets" in patients with chronic arterial hypertension.
To determine if there is a change in withdrawals due to adverse effects with "lower targets" as compared with "standard targets", in patients with elevated blood pressure.
To determine the mean number of antihypertensive drugs used to achieve the blood pressure targets
Methods
Criteria for considering studies for this review
Types of studies
We considered only randomised controlled clinical trials. Trials cannot be blinded as to blood pressure targets because the treating physicians must know the target to which each patient has been assigned in order to make the proper adjustment in the therapy to achieve the blood pressure goal.
All trials that reported any of the outcomes were included. Trials were not limited by any concomitant disease, other factor or baseline cardiovascular risk. There was no language restriction.
Types of participants
Participants were adults (>18 years) with elevated blood pressure documented in a standard way on at least two occasions, or already receiving treatment for elevated blood pressure, irrespective of the baseline blood pressure.
Types of interventions
Trials were included if individuals were randomised to a "lower" target SBP/DBP (≤ 135/85 mm Hg) as compared with a "standard" target blood pressure (≤ 140/90 mm Hg).
Types of outcome measures
This review focuses on mortality and morbidity outcomes
Primary outcomes
All‐cause mortality plus cardiovascular and non‐cardiovascular mortality separately.
Total serious adverse events (total serious morbidity and mortality).
Cardiovascular serious adverse events: myocardial infarction, stroke, congestive heart failure, end‐stage renal failure. A composite of total cardiovascular events was not possible because it was not reported consistently in the different trials.
All other serious adverse events.
Secondary outcomes
Systolic blood pressure (SBP) achieved
Diastolic blood pressure(DBP) achieved
Withdrawals due to adverse effects
Number of antihypertensive drugs needed per patient
Search methods for identification of studies
Electronic searches
Searching other resources
Electronic searches
The Cochrane Hypertension Information Specialist searched the following databases without language or publication status restrictions:
Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched 29 May 2019);
Cochrane Central Register of Controlled Trials (CENTRAL) (2019, Issue 4, 2019) via the Cochrane Register of Studies (CRS‐Web) (searched 29 May 2019);
Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) (searched 29 May 2019);
Embase Ovid (from 1974 onwards) (searched 29 May 2019);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) (searched 28 May 2019);
World Health Organization International Clinical Trials Registry Platform (https://apps.who.int/trialsearch) (searched 28 May 2019).
The Information Specialist modelled subject strategies for databases on the search strategy designed for MEDLINE. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 6, (Higgins 2019). We present search strategies for major databases in Appendix 1.
Searching other resources
The Cochrane Hypertension Information Specialist searched the Hypertension Specialised Register segment (which includes searches of MEDLINE, Embase, and Epistemonikos for systematic reviews) to retrieve existing reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Specialised Register also includes searches for controlled trials in the Allied and Complementary Medicine Database (AMED), CAB Abstracts & Global Health, CINAHL, ProQuest Dissertations & Theses and Web of Science.
We checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials.
Where necessary, we contacted authors of key papers and abstracts to request additional information about their trials.
We searched clinical study reports for additional information about relevant trials.
We searched ISI Web of Science for papers which cite studies included in the review.
Data collection and analysis
Two review authors (JAA, VL) assessed search results independently.
Selection of studies
Two reviewers (JAA, VL) independently assessed the eligibility of the trials, resolving discrepancies by discussion, or by recourse to a third individual if necessary.
Data extraction and management
Two review authors (JAA, VL) independently extracted data from the included trials. For the synthesis and analysis of the data, we used Cochrane review manager software, RevMan 5.3.5. Quantitative analyses of outcomes was based on the intention‐to‐treat principle.
Assessment of risk of bias in included studies
Two review authors (JAA, JMW) independently performed the assessment of risk of bias for each study, using the six domains of Cochrane's 'Risk of bias' tool according to the method described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
Measures of treatment effect
We used the risk ratio (RR) and a fixed‐effect model to combine outcomes across trials. We calculated absolute risk reduction (ARR) and absolute risk increase (ARI) when there was a significant difference between treatments for any outcome. We calculated the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH) in order to estimate the number of patients needed to treat to provide one additional benefit or to produce one additional harm, respectively.
Unit of analysis issues
The analysis of outcomes was based on randomised participants according to the intention‐to‐treat‐principle.
Dealing with missing data
We tried to contact authors in case of missing information in the retrieved articles.
Assessment of heterogeneity
We used the Chi2 and I2 statistics to test for heterogeneity of treatment effect between the trials (Higgins 2003). A Chi2 value less than 0.05 or an I2 value greater than 50% was considered indicative of significant heterogeneity. If significant heterogeneity existed, we attempted to explain the cause of the heterogeneity.
Assessment of reporting biases
We planned to construct a funnel plot to test for asymmetry when 10 or more studies were identified for any comparison.
Data synthesis
Two review authors analysed and reported data using RevMan.
Subgroup analysis and investigation of heterogeneity
We set up the systolic targets and diastolic targets as subgroups so it is possible to see the data separately for each target. We aimed to investigate for heterogeneity in achieved blood pressures.
Sensitivity analysis
A sensitivity analysis was performed including only trials comparing SBP <130 mm Hg versus < 140 mm Hg.
Summary of findings and assessment of the certainty of the evidence
We used Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach to assess the certainty of the supporting evidence behind each estimate of treatment effect (Schunemann 2019a ; Schunemann 2019b). We presented key findings of the review, including a summary of the amount of data, the magnitude of the effect size and the overall certainty of the evidence, in the Table 1.
Results
Description of studies
This review included 11 randomised open label trials studying 38,688 participants.
Results of the search
The search identified 8107 records. There remained 5474 publications after partial screening and removal of duplicates by the information specialist. Most of these publications were rejected after reading the abstract or the complete report. These left 28 studies that seemed appropriate for this systematic review. The detailed analysis of those 28 studies revealed 11 randomised controlled trials (RCTs) that met the inclusion criteria and 17 RCTs did not meet the inclusion criteria (Figure 1).
1.
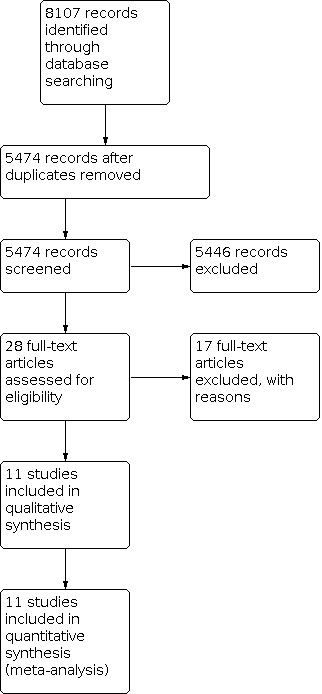
6Study flow diagram.
Included studies
Only two trials (Schrier 2002, SMAC‐AF 2017) compared clinical outcomes associated with both systolic blood pressure (SBP) and diastolic blood pressure (DBP) targets within our definitions for “lower” and “standard” targets; for analyses, those two trials were combined with trials comparing SBP targets. Five trials (ACCORD 2010; Cardio‐Sis 2008; PAST‐BP 2016; SPRINT 2015; SPS3 2013) compared clinical outcomes associated with different SBP targets within our definitions for “lower” and “standard” targets. Four trials (Toto 1995, HOT 1998, ABCD (H) 2000, REIN‐2 2005) compared clinical outcomes associated with different DBP targets meeting our definitions for “lower” and “standard” targets.
a. Methods
The included trials were open‐label RCTs. In most of the trials an independent end point committee, which was blinded to the study intervention arms, reviewed the cardiovascular events; this condition was not mentioned in three studies (PAST‐BP 2016; Schrier 2002; Toto 1995).
Some studies used a 2 x 2 factorial design. For that reason, in those studies participants were also randomised to: intensive or standard glycaemic control (ACCORD 2010), two antiplatelet agents (SPS3 2013), two different antihypertensive drugs (ABCD (H) 2000); placebo or enalapril (Toto 1995); placebo or acetylsalicylic acid (HOT 1998).
The studies included participants from more than 25 countries from Asia, Europe, North America and South America.
The mean follow‐up period varied from one to seven years. The mean weighted follow‐up was 3.7 years.
b. Participants
The total number of participants included in the 11 trials was 38,688. Given the mean follow‐up duration, that number represents 143,145 patient‐years.
The trials included people between the ages of 20 and 80 years. The weighted mean age at baseline was 63.1 years.
The inclusion criteria varied among the trials (see Characteristics of included studies table). However, an additional major cardiovascular risk factor was required to be included in most of the trials.
ACCORD 2010 and ABCD (H) 2000 only included people with diabetes. On the contrary, diabetics were excluded in SPRINT 2015 and in Cardio‐Sis 2008. The number of participants with diabetes at baseline was not reported in some of the smaller trials; with the available information, at least 7863 participants (20.3%) had diabetes at baseline.
Nephropathy was an inclusion criterion in REIN‐2 2005, Schrier 2002, and Toto 1995. A previous recent lacunar stroke was required to be included in SPS3 2013. Atrial fibrillation was an inclusion criteria in SMAC‐AF 2017.
The number of participants with established cardiovascular disease at baseline was not reported in some of the smaller trials. With the available information, at least 9153 participants (23.7%) were secondary prevention at baseline.
Many of the participants were already taking antihypertensive drugs on study entry. The baseline blood pressure required for inclusion also varied (see Characteristics of included studies table). Briefly, a specific SBP was required for inclusion in Schrier 2002 (> 140 mm Hg), Cardio‐Sis 2008 (>150 mm Hg), SMAC‐AF 2017 (>130 mm Hg), and SPRINT 2015 (between 130 mm Hg and 180 mm Hg). Similarly, a specific DBP was required for inclusion in ABCD (H) 2000 (≥ 90 mm Hg), HOT 1998 (between 100 mm Hg and 115 mm Hg), Schrier 2002 (> 90 mm Hg), and Toto 1995 (> 95 mm Hg). There were no restrictions regarding baseline blood pressure in ACCORD 2010, SPS3 2013, and REIN‐2 2005. The mean weighted blood‐pressure at baseline was 155/91 mm Hg.
c. Interventions
For trials comparing SBP targets seeTable 2.
1. Interventions in trials comparing SBP targets.
| Trial | Lower target | Standard target |
| ACCORD | < 120 mm Hg | < 140 mm Hg |
| Cardio‐Sis | < 130 mm Hg | < 140 mm Hg |
| SPS 3 | < 130 mm Hg | between 130 mm Hg and 139 mm Hg |
| SPRINT | < 120 mm Hg | < 140 mm Hg |
| PAST‐BP | < 130 mm Hg | < 140 mm Hg |
| SMAC‐AF | < 120 mm Hg | < 140 mm Hg |
| Schrier | < 120 mm Hg | between 135 mm Hg and 140 mm Hg |
Participants in ACCORD 2010 were randomly assigned to intensive therapy that targeted SBP of less than 120 mm Hg or standard therapy that targeted SBP of less than 140 mm Hg.
Participants in Cardio‐Sis 2008 were randomly assigned to tight control that targeted SBP of less than 130 mm Hg or usual control that targeted SBP of less than 140 mm Hg.
Participants in SPS3 2013 were randomly assigned to more intensive therapy that targeted SBP of less than 130 mm Hg or less intensive therapy that targeted SBP between 130 mm Hg and 149 mm Hg.
Participants in SPRINT 2015 were randomly assigned to intensive treatment that targeted SBP of less than 120 mm Hg or standard treatment that targeted SBP of less than 140 mm Hg.
Participants in PAST‐BP 2016 were randomly assigned to intensive treatment that targeted SBP of less than 130 mm Hg or 10 mm Hg reduction from baseline if it was < 140 mm Hg or standard treatment that targeted SBP of less than 140 mm Hg.
Participants in SMAC‐AF 2017 were randomly assigned to aggressive treatment that targeted SBP of less than 120 mm Hg or standard treatment that targeted SBP of less than 140 mm Hg.
Participants in Schrier 2002 were randomly assigned to rigorous therapy that targeted SBP of less than 120 mm Hg or standard therapy that targeted SBP between 135 mm Hg and 140 mm Hg.
For trials comparing DBP targets see Table 3.
2. Interventions in trials comparing DBP targets.
| Trial | Lower target | Standard target |
| ABCD‐H | < 75 mm Hg | between 80 mm Hg and 89 mm Hg |
| HOT | < 80 mm Hg and < 85 mm Hg | < 90 mm Hg |
| REIN‐2 | < 80 mm Hg | < 90 mm Hg |
| Toto | between 65 and 80 mm Hg | between 85 mm Hg and 95 mm Hg |
| SMAC‐AF | < 80 mm Hg | < 90 mm Hg |
| Schrier | < 80 mm Hg | between 85 mm Hg and 90 mm Hg |
Participants in ABCD (H) 2000 were randomly assigned to intensive treatment with a DBP goal of 75 mm Hg or moderate treatment with a DBP goal of 80 mm Hg to 89 mm Hg.
Participants in HOT 1998 were randomly assigned to two lower DBP target groups: less than or equal to 85 mm Hg, and less than or equal to 80 mm Hg as compared to a standard target less than or equal to 90 mm Hg.
Participants in REIN‐2 2005 were randomly assigned to intensified blood pressure control (< 130/80 mm Hg) or conventional blood pressure control (DBP < 90 mm Hg).
Participants in Toto 1995 were randomly assigned to strict blood pressure control (DBP between 65 mm Hg and 80 mm Hg) or conventional control (DBP between 85 mm Hg and 95 mm Hg).
d. Outcomes
The primary outcome varied among the trials. It was a composite of cardiovascular events in HOT 1998, ACCORD 2010, and SPRINT 2015. It was recurrent stroke in SPS3 2013, and progression to end‐stage renal disease (ESRD) in REIN‐2 2005. Surrogate markers of cardiac or renal function were the primary outcome in the remaining trials. All trials included individual or composite cardiovascular events as secondary outcomes. In no trial was mortality a primary outcome.
The criteria used to define outcomes could vary between studies; for example, some studies reported silent myocardial infarctions separately.
e. Additional notes
Trials comparing diastolic targets were published between 1995 and 2006, whereas trials comparing systolic targets were published between 2002 and 2017.
The types of antihypertensive drugs used varied among the trials.
Excluded studies
One thousand and ninety‐four participants, self‐identified as African‐Americans, with diminished glomerular filtration rate, were included in this randomised, open‐label, controlled trial. They were randomly assigned to a “usual”‐ or “lower‐blood pressure” group. “Usual” meant arterial pressure was defined as a mean arterial pressure between 102 mm Hg and 107 mm Hg. “Lower” mean arterial pressure was defined as a mean arterial pressure ≤ 92 mm.
This trial was not included because any given value of mean arterial pressure may represent many different combinations of SBP and DBP, and therefore cannot be precisely associated with the SBP and DBP ranges specified for this review.
A randomised, open‐label, controlled trial that included 480 diabetic patients. Participants were randomised to "intensive" or "moderate" treatment.
This trial was excluded because most of the participants were normotensive, defined as a baseline DBP between 80 mm Hg and 89 mm Hg and who were not receiving antihypertensive medications at the randomisation visit. It also included 26 patients with isolated systolic hypertension, but their distribution and their outcomes were not reported separately.
This trial included 129 type‐2 diabetic participants with a SBP < 140 mm Hg, a DBP between 80 mm Hg and 90 mm Hg, and without evidence of overt albuminuria. Participants were randomised to either intensive blood pressure control aiming for a DBP goal of 75 mm Hg or to moderate blood pressure control aiming to maintain DBP between 80 mm Hg and 90 mm Hg.
It was excluded because it only included normotensive participants.
This trial included 1000 patients with acute intracerebral haemorrhage. They were randomised to intensive treatment (SBP target of 110 mm Hg to 139 mm Hg) or to standard treatment (SBP target of 140 mm Hg to 179 mm Hg).
It was excluded for several reasons: it included only patients with a special condition different from treatment of chronic arterial hypertension, the follow‐up period (three months) was shorter than specified for this review, and the intensive treatment interval included SBP values greater than specified for our standard target.
A randomised, open‐label, controlled trial involving 2127 hypertensive patients aged 45 to 67 years. To be included, participants had to be receiving antihypertensive treatment, and their treated DBP on at least three consecutive visits were in the range between 90 mm Hg and 100 mm Hg. Participants were randomised to “intensified” or “unchanged” therapy. In the group allocated to “intensified” treatment, the purpose was to reduce DBP to less than or equal to 80 mm Hg. In the group allocated to “unchanged” therapy, the aim was to maintain theDBP in the range of 90 mm Hg to 100 mm Hg.
This study, which showed no difference in morbidity or mortality outcomes between the target groups, was excluded from this meta‐analysis because the number of patients randomised to each treatment arm was not reported.
This trial included 987 women with pre‐existing or gestational hypertension. Participants were randomised to tight‐control (DBP < 85 mm Hg) or less‐tight control (DBP < 100 mm Hg). This trial was excluded because it compared blood pressure targets during pregnancy and it looked at different outcomes due to the short follow‐up period. Besides, gestational hypertension is a very different condition than chronic hypertension in terms of pathogenesis and prognostic implications.
758 hypertensive diabetic patients were included in this randomised trial. This trial compared “tight control” of blood pressure (aiming at < 150/85 mm Hg), with “less tight control” (aiming at < 180/105 mm Hg). This trial was excluded from the review for the same reasons as the UKPDS 1998 trial. Furthermore, it is likely that participants in this trial represent a subgroup of patients included in UKPDS 38, because the study design is similar and the authors are the same.
In this trial 3518 hypertensive patients were randomised to usual control (125‐134/80‐84 mm Hg) or tight control (<125/< 80 mm Hg) according to blood pressure self‐measurement at home. This trial was excluded because measurements and targets are different when blood pressure is measured at home.
This trial included 4418 Japanese hypertensive patients older than 65 years. Participants were randomised to SBP < 140 mm Hg or SBP between 140 mm Hg and 160 mm Hg. This study showed no difference in morbidity or mortality outcomes between the target groups. It was not included because none of the targets in this trial were within the values considered as "lower target" in our systematic review.
This randomised controlled trial included 129 patients with type 1 diabetes mellitus and diabetic nephropathy who were randomly assigned to a mean arterial blood pressure (MAP) goal less than or equal to 92 mm Hg or a MAP goal between 100 mm Hg and 107 mm Hg. The primary outcomes in this trial were surrogate markers of renal function in order to determine the impact of assignment to different levels of blood pressure control on the course of type 1 diabetic nephropathy.
It was excluded for several reasons. Blood pressure targets were defined according to MAP. Besides, it did not provide data on any of the main outcomes defined for this systematic review. The only reported clinical event was end‐stage renal disease (ESRD). Twelve patients reached ESRD, but the distribution of those according to the blood pressure target assigned was not provided. It also reported achieved blood pressure but as mean arterial pressure, not asSBP and/orDBP achieved.
Eight hundred and forty participants with chronic renal disease were included in this randomised, open‐label, controlled trial. They were randomly assigned to a “usual”‐ or “low‐blood pressure” group. “Usual blood pressure” was defined as a mean arterial pressure ≤ 107 mm Hg for patients < 60 years of age, and ≤ 113 mm Hg for > 60 years. “Low blood pressure” was defined as a mean arterial pressure ≤ 92 mm Hg for patients < 60 years of age, and ≤ 98 mm Hg for > 60 years.
This trial was not included because any given value of mean arterial pressure may represent many different combinations of SBP and DBP, and therefore cannot be precisely associated with the SBP and DBP ranges specified for this review.
This was a randomised, open‐label, blinded‐to‐end‐point study performed in 499 American Indians with diabetes and no prior cardiovascular events. The primary end point was progression of atherosclerosis determined by ultrasonographic measurement of the common carotid artery intimal medial thickness. The incidence of clinical events was a secondary outcome. Patients were randomised to standard or aggressive treatment groups. The standard treatment was designed as a SBP target of 130 mm Hg or lower and low‐density lipoprotein cholesterol (LDL‐C) target of 100 mg/dL or lower, whereas aggressive treatment was defined as a SBP target of 115 mm Hg or lower and LDL‐C target of 70 mg/dL or lower.
This trial was not included because the dual intervention does not allow discrimination of the events specifically associated with a lower blood pressure target. Besides, both SBP targets in this trial were within the values considered as "lower targets" in our systematic review.
Two‐hundred and twenty‐two participants, with uncontrolled hypertension, preserved ejection fraction, and diastolic dysfunction, were randomised to two targeted treatment strategies: “intensive”, with a SBP target < 130 mm Hg, or “standard”, with a SBP target < 140 mm Hg. It compared changes in echocardiographic parameters for diastolic dysfunction after 24 weeks of treatment.
This trial was not included because it did not provide any information regarding mortality or cardiovascular events.
This was a randomised, open‐label, parallel study. Eighty patients with type‐2 diabetes were randomly assigned to receive conventional treatment in accordance with national guidelines in Denmark, and 80 patients to receive intensive treatment. The intensive treatment arm included stepwise implementation of behaviour modification and pharmacological therapy that targeted more strict values for SBP (< 140 mm Hg during the initial seven years and < 130 mm Hg during the last two years in the intensive treatment arm versus < 160 mm Hg and < 135 mm Hg, respectively in the conventional treatment arm) and DBP (< 85 mm Hg during the initial seven years and < 80 mm Hg during the last two years in the intensive treatment arm vs < 95 mm Hg and 85 mm Hg, respectively in the conventional treatment arm), but also more strict targets for glycosylated haemoglobin, fasting total serum cholesterol and fasting serum triglycerides, treatment with an ACE inhibitor irrespective of blood pressure, and aspirin therapy for patients with peripheral artery disease, and also aspirin therapy for patients without coronary artery disease or without peripheral artery disease during the last 2 years.
This trial was not included because the multifactorial intervention prevented any inference as to whether any difference in clinical outcomes could be attributed to a lower blood pressure target or to any of the other combined interventions.
This RCT included 1184 hypertensive diabetic patients comparing “tight control” of blood pressure with “less tight control”. The “tight control” group aimed at a blood pressure of < 150/85 mm Hg. In the “less tight control” group the target was originally set at < 200/105 mm Hg, but was reduced to < 180/105 mm Hg five years after the start of the trial.
This study was excluded because the target for SBP in the “tight control” group was higher than stated in our protocol. In addition, and more important, the targets for both SBP and DBP in the “less tight control group” were much higher than specified in the protocol for this systematic review. These “less tight” pressures are similar to the escape criteria in most placebo or no treatment controlled antihypertensive trials, and much higher than conventional goals prevalent since the 1970's.
This trial included 3260 hypertensive patients between 70 and 84 years old. They were randomised to SBP < 140 mm Hg or SBP between 141 mm Hg and 150 mm Hg. This study showed no difference in morbidity or mortality outcomes between the target groups. It was not included because neither target in this trial was within the values considered as "lower targets" in our systematic review.
This was a randomised, open‐label, blinded‐to‐end‐point study performed in 724 Chinese hypertensive patients older than 70 years. Patients were randomised to intensive treatment defined as less than 140/90 mm Hg, or standard.treatment defined as less than 150/90 mm Hg.
This trial was not included because neither target in this trial was within the values considered as "lower targets" in our systematic review.
Risk of bias in included studies
The 'Risk of bias' summary for each trial is shown in figure 2.
Allocation
In six trials (ACCORD 2010; Cardio‐Sis 2008; HOT 1998; Schrier 2002; SPRINT 2015; SPS3 2013) randomisation was performed centrally and computer‐generated and were therefore considered low risk of bias. The method of randomisation was not described in the other trials.
Blinding
None of the trials was blinded to blood pressure goal because of the need to titrate treatment to achieve the specific target (high risk of performance bias). In most of the trials an independent end point committee, which was blinded to the study intervention arms, reviewed the cardiovascular events; this condition was not mentioned in the Toto 1995 and Schrier 2002 trials (low to unclear risk of detection bias).
Incomplete outcome data
In the HOT 1998 trial, 2.6% of the patients were lost to follow‐up, and they were equally distributed between the target arms. In ACCORD 2010, 4.9% were lost to follow‐up, and their distribution is not known. In SPS3 2013 3% were lost to follow‐up, and their distribution was not reported. In Cardio‐Sis 2008 only one patient, allocated to usual control, was lost to follow‐up. In SPRINT 2015, 245 participants were lost to follow‐up; 111 were allocated to the intensive treatment group and 134 to the standard treatment. In REIN‐2 2005 6 patients (four in the conventional control group and two in the intensified control group) were lost to follow‐up (one and two of them, respectively never took study drugs). In PAST‐BP 2016, 16% of participants withdrew from the trial (20% in the intensive treatment arm and 12% in the standard treatment arm). In SMAC‐AF 2017 3 participants were lost to follow‐up; one was allocated to the intensive treatment group and two to the standard treatment. No specific information about dropouts was provided in the remaining trials reports.
Selective reporting
Some of the outcomes were not evaluated or reported in the trials. The most important example of potential selective reporting bias is total serious adverse events, because they were not uniformly recorded.
Other potential sources of bias
In Toto 1995, the exclusion of patients not able to achieve the lower target during the randomisation period is a limitation of the trial as the results are only relevant to "responders" as defined in that study.
SPRINT 2015 was terminated early for benefit. SPRINT 2015 also used a blood pressure measurement strategy that could provide blood pressure values lower than expected from traditional office measurement strategies (Agarwal 2017, Kjeldsen 2016).
Several studies were industry funded. The summary of the 'Risk of bias' judgements in shown in Figure 2.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
We present the results according to the Cochrane Hypertension Group standard hierarchy of outcomes. Several outcomes were not reported in the published trials. Missing information was requested by e‐mail sent to the main authors of each trial, but some information was not obtained. Some additional information, not included in the original published reports, was provided by the Blood Pressure Lowering Treatment Trialists' Collaboration (BPLTTC 2003). We have reported the data by pooling the results from the systolic target and the diastolic target trials below and in the Table 1. We have done this for three reasons. 1) Systolic blood pressure (SBP) and diastolic blood pressure (DBP) are not independent variables. Any intervention that affects systolic pressure also affects diastolic pressure in the same direction. 2) For most of the outcomes the results for the systolic target and diastolic target were homogeneous (see Data and analyses). 3) Pooling all the data provides a more robust estimate of the effect size.
1.1 Total mortality: systolic and diastolic targets
There was no difference in total mortality between the “lower target” and the “standard target” groups (risk ratio (RR) 0.95, 95% confidence interval (CI) 0.86 to 1.05, P = 0.32; 11 trials, 38,688 participants; high‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Low vs Standard BP Target, Outcome 1: Total mortality
1.2 Cardiovascular (CV) mortality: systolic and diastolic targets
There was no difference in CV mortality between the “lower target” group and the "standard target" groups (RR 0.90, 95% CI 0.76 to 1.06, P = 0.21; 9 trials, 37,500 participants; Analysis 1.2).
1.2. Analysis.

Comparison 1: Low vs Standard BP Target, Outcome 2: CV mortality
1.3 Non‐cardiovascular (CV) mortality: systolic and diastolic targets
There was no difference in non‐CV between the “lower target” and the “standard target” groups (RR 1.02, 95% CI 0.88 to 1.18, P = 0.82; 9 trials, 37,500 participants; Analysis 1.3).
1.3. Analysis.

Comparison 1: Low vs Standard BP Target, Outcome 3: Non‐CV mortality
1.4 Total serious adverse events (as best determined, see Discussion): systolic and diastolic targets
There was no difference in total serious adverse events between the “lower target” and the “standard target” groups (RR 1.04, 95% CI 0.99 to 1.08, P = 0.10; 6 trials, 18,165 participants; moderate‐certainty evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1: Low vs Standard BP Target, Outcome 4: Total serious adverse events
1.5 Myocardial infarction: systolic and diastolic targets
There was a reduced incidence of myocardial infarction in the “lower target” group than the "standard target" group (RR 0.84, 95% CI 0.73 to 0.96, P = 0.01; 8 trials, 38,198 participants; low‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Low vs Standard BP Target, Outcome 5: Myocardial infarction
The incidence of myocardial infarction was 1,82% in the "lower target" group and 2,55% in the "standard target group": absolute risk reduction 0.73 %, NNTB 137 for a mean of 3.7 years.
1.6 Stroke: systolic and diastolic target
There was a numerically lower incidence of stroke in the “lower target” group than the "standard target" group (RR 0.88, 95% CI 0.77 to 1.01, P = 0.07; 7 trials, 37,087, participants; low‐certainty evidence; Analysis 1.6). This was driven by the lower systolic target. For this outcome there was significant heterogeneity between the subgroups: I2 = 73%.
1.6. Analysis.

Comparison 1: Low vs Standard BP Target, Outcome 6: Stroke
1.7 Congestive heart‐failure: systolic and diastolic targets
There was a significantly lower incidence of congestive heart failure in the “lower target” group (RR 0.75, 95% CI 0.60 to 0.92, P = 0.007; 5 trials, 15,859 participants; low‐certainty evidence; Analysis 1.7), primarily due to the SPRINT 2015 trial.
1.7. Analysis.

Comparison 1: Low vs Standard BP Target, Outcome 7: Congestive heart failure
The incidence of congestive heart failure was 1,84% in the "lower target" group and 2,47% in the "standard target group": absolute risk reduction 0,63 %, NNTB 159 for a mean of 3.7 years.
1.8 End‐stage renal disease: systolic and diastolic targets
There was no difference in end‐stage renal disease between the “lower target” and the “standard target” groups (RR 1.06, 95% CI 0.83 to 1.37, P = 0.64; 6 trials, 14,768 participants; Analysis 1.8).
1.8. Analysis.

Comparison 1: Low vs Standard BP Target, Outcome 8: End‐stage renal failure
1.9 All other serious adverse events: systolic target
There was a significantly higher incidence of other serious adverse events in the "lower target" group than the "standard target" group (RR 1.44, 95% CI 1.32 to 1.59, P < 0.00001; 6 trials, 18,938 participants; low‐certainty evidence; Analysis 1.9). The incidence of all other serious adverse events was 9.8% in the “lower target” group and 6.8% in the “standard target” group: absolute risk increase 3%, NNTH 33 for 3.7 years.
1.9. Analysis.

Comparison 1: Low vs Standard BP Target, Outcome 9: All other serious adverse events
This outcome was not reported in any of the trials comparing diastolic targets.
1.10 Systolic blood pressure (SBP)achieved: systolic and diastolic targets
Heterogeneity between trials was high for this outcome, basically due to two small trials (Cardio‐Sis 2008; PAST‐BP 2016) in which the mean difference in achieved blood pressure between arms was small. Using the random‐effects model, the achieved SBP was significantly lower in the “lower target” group than in the “standard target” group: P < 0.00001.
The fixed‐effect model provides the best estimate of average magnitude of the difference between the SBP in the two groups. For trials comparing systolic targets: 122.9 mm Hg in the "lower target" group versus 135.0 mm Hg in the "standard target" group, (MD 12.10 mm Hg, 95% CI ‐12.45 to ‐11.74), P < 0.00001; 7 trials, 19,013 participants; Analysis 1.10.1).
1.10. Analysis.

Comparison 1: Low vs Standard BP Target, Outcome 10: Systolic blood pressure achieved
For trials comparing diastolic targets, the SBP achieved was also significantly lower in the “lower target” group than in the “standard target” group: 140.1 versus 143.3 mm Hg, (MD 3.29, 95% CI ‐3.63, ‐2.96), P < 0.00001; 4 trials, 19,675 participants; Analysis 1.10.2).
For all trials, the MD in SBP achieved in the two groups wasMD ‐7.52 mm Hg, 95% CI ‐7.76 to ‐7.27, P < 0,00001; 11 trials, 38,688 participants; Analysis 1.10.
1.11 Diastolic blood pressure (DBP) achieved: systolic and diastolic targets
Heterogeneity between trials was high for this outcome, basically due to two small trials (PAST‐BP 2016 and Cardio‐Sis 2008), in which the mean difference in achieved blood pressure between arms was small. Using the random‐effects mode, the achieved DBP was significantly lower in the “lower target” group than in the “standard target” group: P < 0.00001.
The fixed‐effect model provides the best estimate of average magnitude of the difference between the DBP in the two groups. For trials comparing diastolic targets: MD 82.0 mm Hg in the "lower target" group versus 85.2 mm Hg in the "standard target" group, (MD 3.2 mm Hg, 95% CI ‐3.33 to ‐3.03) (P < 0,00001; 4 trials, 19,675 participants; Analysis 1.11.2).
1.11. Analysis.

Comparison 1: Low vs Standard BP Target, Outcome 11: Diastolic blood pressure achieved
For trials comparing systolic targets, the DBP achieved was also significantly lower in the “lower target” group than in the “standard target” group: 68,3 versus 74,9 mm Hg, (MD ‐6.61, 95% CI ‐6.83, ‐6.39), P < 0.0001; 6 trials, 15,993 participants; Analysis 1.11.1). For all trials, the difference in DBP achieved in the two groups was ‐4,28 mm Hg (95% CI ‐4.41 to ‐4.16, P < 0,00001; 10 trials, 35,668 participants; Analysis 1.11).
1.12 Withdrawals due to adverse effects: diastolic target
Only the REIN‐2 2005 trial of diastolic targets reported the total number of withdrawals due to adverse effects in each treatment arm, and there was no statistical difference between the groups but the confidence interval was very large (RR 2.00, CI 95% 0.51 to 7.87, P = 0.32; 1 trial, 318 participants; Analysis 1.12).
1.12. Analysis.

Comparison 1: Low vs Standard BP Target, Outcome 12: Withdrawals due to adverse events
1.13 Number of antihypertensive drugs used per patient: systolic and diastolic targets
The number of antihypertensive drugs used per patient was reported in six trials comparing systolic targets. Among trials comparing diastolic targets, only theREIN‐2 2005 trial reported that number, which was similar to the combined result of the six trials comparing systolic targets.
Overall, the mean number of antihypertensive drugs used was significantly greater in the “lower target” groups than the "standard target" groups: 2.89 versus 1.89 (MD 1.00, 95% CI 0.96 to 1.04, P < 0.00001; 6 trials, 17,902 participants; Analysis 1.13).
1.13. Analysis.

Comparison 1: Low vs Standard BP Target, Outcome 13: Number of antihypertensive drugs used per patient
Sensitivity analysis comparing SBP < 130 mm Hg versus < 140 mm Hg
It is possible that trying to achieve a very strict SBP target (<120 mm Hg) could produce an excess amount of adverse events associated with the more intensive antihypertensive therapy and, therefore, could negatively affect the benefits/harms relationship. For that reason we performed a sensitivity analysis including only trials targeting SBP < 130 mm Hg versus trials targeting < 140 mm Hg.
This comparison is limited to 4660 participants from Cardio‐Sis 2008, .SMAC‐AF 2017 and SPS3 2013 trials. The main results are shown in the following table. The only significant result was an increase in "other serious adverse events" associated with the lower target.
| Outcomes | RR (CI 95%) | P |
| Total mortality | 1.06 (0.82 to 1.37) | 0.67 |
| Cardiovascular mortality | 0.87 (0.56 to 1.34) | 0.53 |
| Non‐cardiovascular mortality | 1.21 (0.78 to 1.88) | 0.40 |
| Total serious adverse events | 1.05 (0.92 to 1.20) | 0.46 |
| Myocardial infarction | 0.88 (0.58 to 1.33) | 0.55 |
| Stroke | 0.82 (0.65 to 1.02) | 0.08 |
| Heart failure | 0.42 (0.11 to 1.63) | 0.21 |
| Other serious adverse events | 1.87 (1.34 to 2.61) | 0.0002 |
Discussion
Summary of main results
The objective in using antihypertensive drugs in patients with elevated blood pressure is to reduce morbidity and mortality. It is not known how much blood pressure has to be lowered in order to optimise that objective. Many epidemiological studies have shown a continuous direct linear relationship between blood pressure and the incidence of cardiovascular events, but the lower threshold for this relationship has not been established (Prospective Studies Collaboration 2002). More aggressive treatment in patients with elevated blood pressure aiming at lower blood pressure targets assumes that the benefits of attempting to achieve those lower blood pressure targets through antihypertensive drug therapy outweigh the harms caused by the intensive treatment. Evidence from randomised controlled trials (RCTs) and their meta‐analysis can suggest what may be expected in groups of patients similar to those studied in the RCTs, but cannot predict the balance of benefits or harms in any individual.
This systematic review and meta‐analysis of RCTs summarises the presently available evidence from trials that evaluated clinical outcomes associated with prespecified "lower blood pressure targets" as compared with "standard blood pressure targets". We found 11 trials including 38,688 patients, with a mean follow‐up period of 3.7 years that met the inclusion criteria for this review.
Because pharmacological treatment decreases both systolic blood pressure (SBP) and diastolic blood pressure (DBP), we have reported the pooled data for both in the Table 1. However, we established subgroups for systolic and diastolic targets in order to see the data for each target group separately.
On average, participants assigned to the "lower target" received one additional antihypertensive medication and achieved a 7.5 mm Hg lower SBP and a 4.3 mm Hg lower DBP than those assigned to the “standard target”. The achieved blood pressure data were highly heterogeneous.
The most important findings of this review are that high‐certainty evidence demonstrates that lower targets do not reduce total mortality (risk ratio (RR) 0.95, 95% confidence interval (CI) 0.86 to 1.05, P = 0.32) and do not reduce total serious adverse events (RR 1.04, 95% CI 0.99 to 1.08, P = 0.10; moderate‐certainty evidence). According to the USA Food and Drug Administration (FDA )definition, a serious adverse event includes any of the following conditions: death, is life‐threatening, requires inpatient hospitalisation or causes prolongation of existing hospitalisation, results in persistent or significant disability/incapacity, may have caused a congenital anomaly/birth defect, or requires intervention to prevent permanent impairment or damage. This means that on average, the benefits of lower targets do not outweigh the harms as compared to standard blood pressure targets. Thus in the treatment of patients with hypertension the standard blood pressure targets remain appropriate for most people with hypertension.
As can be seen in the Table 1, lower targets did reduce myocardial infarction (RR 0.84, 95% CI 0.73 to 0.96, P = 0.01, absolute risk reduction (ARR) 0.7% over 3.7 years), and congestive heart failure (RR 0.75, 95% CI 0.60 to 0.92, P = 0.007, (ARR 0.6% over 3.7 years. However, we judged both outcomes to be low‐certainty evidence due to the high risk of bias particularly lack of blinding bias, for ascertainment of these outcomes.
The reason the reduction in myocardial infarction and congestive heart failure was not reflected in total serious adverse events is most likely due the fact that treating to lower targets increased other serious adverse events as this review has demonstrated (RR 1.44, 95% CI 1.32 to 1.59, P < 0.00001, absolute risk increase (ARI) 3% over 3.7 years). This is reinforced by examination of serious adverse event data from the two largest trials. In ACCORD 2010, there was a significant increase in other serious adverse events attributed to blood‐pressure medications: RR 2.58 (95% CI 1.70 to 3.91), P < 0.00001, absolute risk increase 2%, which means that one extra serious adverse event occurred for every 50 patients treated intensively for 4.7 years. Serious adverse events attributed to blood pressure medications in ACCORD 2010 included hypotension, syncope, bradycardia or arrhythmia, hyperkalaemia, angioedema, and renal failure. In SPRINT 2015, serious adverse events classified as possibly or definitely related to the intervention were also increased in the low target group: RR 1.87 (95% CI 1.50 to 2.33), P < 0.001, absolute risk increase 2.2%, which means one extra serious adverse event occurred for every 46 patients treated for 3.3 years. In SPRINT 2015, the larger number of adverse events related to the intervention in the lower blood pressure group was mainly due to a 1.2% absolute increase in acute kidney injury or acute renal failure.
Overall completeness and applicability of evidence
For the general population of people with elevated blood pressure, trying to achieve a lower blood pressure target is not currently justified based on evidence from randomised trials. However, we cannot rule out that some patient populations might benefit from aiming for lower targets. As a partial answer to that question, a Cochrane Review of blood pressure targets in people with hypertension and diabetes mellitus also concluded that there was no net health benefit from lower blood pressure targets (Arguedas 2013). For people with hypertension and cardiovascular disease, an updated review also found no net health benefit for lower blood pressure targets, as compared with standard blood pressure targets (Saiz 2020).
Analysing the individual data of participants in those trials might be useful to detect some characteristics capable of better identifying patients amongst whom a lower blood pressure target might confer net benefits (Attar 2019). While such post hoc analyses cannot be applied directly to clinical practice, they can generate hypotheses leading to design and conduct of randomised trials comparing blood pressure targets in populations with specific characteristics. Conversely, individual patient data could be useful to identify groups of patients at greater risk of experiencing serious adverse events, who could be excluded from future trials of lower blood pressure targets.
The conventional measurement of blood pressure in clinical practice to establish blood pressure targets provides no information on other variables that observational studies have associated with prognosis, such as blood pressure variability or changes during sleep. There is no available evidence from RCTs that used ambulatory blood pressure monitoring to evaluate the relationship between blood pressure targets and clinical outcomes.
Finally, based on the baseline characteristics of the participants included in the studies, the results of this review are primarily applicable to older people with moderate to high cardiovascular risk. They may not be applicable to other populations.
Quality of the evidence
The main potential bias in the trials included in this review is the fact that studies could not be blinded, which leads to a high risk of performance and detection bias. However, it is possible to blind the individuals measuring the blood pressure and adjudicating the outcomes. For the most part this was not done.
The SPRINT 2015 trial had a decisive influence on the reduction detected in myocardial infarction and congestive heart failure, but it was also one of the trials with higher risk of bias. Because it was stopped early for benefit, the benefits are likely to have been exaggerated (Bassler 2010, Viele 2016). SPRINT 2015 also used a blood pressure measurement technique that could provide blood pressure values lower than expected with the traditional office measurement technique.
There was high heterogeneity in achieved blood pressures. Heterogeneity was related mainly to the small differences in mean blood pressure between treatment arms in two trials (Cardio‐Sis 2008; PAST‐BP 2016). This suggests some problem of adherence to the protocols during conduct of these trials. They were small, and their exclusion does not change our conclusions.
Overall, there was underreporting of some outcomes, especially of total people with at least one serious adverse event.
Potential biases in the review process
The manner in which we handled serious adverse events could have led to bias and deserves discussion. The total number of people with at least one serious adverse event was reported for the SPRINT 2015. It was not reported in the ACCORD 2010 trial. Using the information available for ACCORD 2010, we calculated the number of people who experienced at least one serious adverse event as the sum of primary or secondary outcomes (total mortality, non‐fatal myocardial infarction, non‐fatal stroke, non‐fatal heart failure, non‐fatal heart failure, end stage renal disease or need for dialysis) plus other serious adverse events related to the intervention. According to the ACCORD 2010 investigators, those were the only serious adverse events collected in a consistent manner throughout the trial. The authors of SMAC‐AF 2017 provided total serious adverse event information by email, in response to our request. In SPS3 2013, we calculated people with at least one serious adverse event as the sum of deaths and serious cardiovascular events reported in the published version plus additional information on other serious adverse events provided by the principal author. PAST‐BP 2016 reported emergency admissions, which was used as a reasonable surrogate for the total number of people who experienced at least one serious adverse event. It was not possible to obtain or to calculate the total number of people with at least one serious adverse event in the remaining trials.
SPRINT 2015 and Cardio‐Sis 2008 reported the outcome of "other SAEs". The ACCORD 2010 investigators elected to restrict analysis and reporting of serious adverse event data to events judged related to blood pressure medications, because those were the only events collected in a consistent manner throughout the trial and subject to safety officer and Data and Safety Monitoring Board (DSMB) review. The information from SPS3 2013 was provided by the main author of the trial as a subset of total people with at least one other serious adverse events. We calculated all other serious adverse events in PAST‐BP 2016P and SMAC‐AF 2017S as total serious adverse events minus serious adverse events previously considered in this Cochrane Review (total mortality, myocardial infarction, stroke, congestive heart failure, and end‐stage renal disease).
Another potential limitation is that we excluded two RCTs that used mean blood pressure as the target (AASK 2002; MDRD 1995). We excluded these trials because we could not be precise as to whether they met the systolic and diastolic targets specified for this review. We performed a sensitivity analysis adding those trials and it did not have any effect on the risk ratio (RR) effect estimates for any of the outcomes of our review.
Agreements and disagreements with other studies or reviews
Publication of theSPRINT 2015 trial led to several commentaries as to whether lower blood pressure targets are preferable ( Drazen 2015; Laurent 2016; Lonn 2016; Oparil 2016; Perkovic 2015; Sexton 2017;Yeh 2015). The main argument in favour was the unexpected reduction in total mortality observed in that trial, while the main objections related to safety concerns. The reduction in mortality in SPRINT 2015 is an outlier in our meta‐analysis, and we do not know to what degree this could be explained by its early termination for benefit. It is known that stopping trials early for benefit may lead to an exaggeration of the benefit (Bassler 2010).
Several meta‐analyses and reviews have evaluated blood pressure targets. Some of them came to conclusions similar to ours (Arguedas 2013; Brunstrom 2016; Chi 2018; Marianpilla 2016; Tsai 2017), while others did not (Bangalore 2017; Bundy 2017; Ettehad 2016; Lv 2012; Malhotra 2017; Thomopoulos 2016 a; Verdecchia 2016; Xie 2016). Important methodological differences underlie the systematic reviews that reached conclusions different from ours. These include one or several of the following factors:
a. They compared “more intensive” versus “less intensive” blood pressure‐lowering treatment without defining any specific value for the targets. As a result, they included old trials in which the standard targets were inappropriately high according to current medical practice (e.g. < 180/105 mm Hg), or trials comparing targets defined by mean arterial pressure.
b. The analyses were limited to benefits without reporting harms.
c. The analyses of outcomes were based on “achieved” rather than on “targeted” blood pressures. Using this approach leads to loss of randomisation and the analysis is therefore susceptible to all the biases associated with observational studies (Gueyffier 2001; MacMahon 2001; Zanchetti 2014). People who achieve lower blood pressures are likely to be different, pathophysiologically and clinically, from people who do not.
d. The inclusion of trials not designed to compare outcomes specifically associated with different blood pressure targets. Most of those trials used fixed‐dose approaches to test different hypotheses not related with specific blood pressure targets. Because of this, other factors could potentially influence the results.
e. The results were obtained through indirect comparisons from network meta‐analysis, which may be less reliable than direct comparisons of treatment effects (Cipriani 2013).
It has been suggested that tight blood pressure control could provide greater benefits if implemented early (Marianpilla 2016; Parati 2011;Zanchetti 2009), or in people at high risk of stroke, such as those with a history of cerebrovascular disease (Mancia 2011). However, these interesting arguments mentioned in some clinical guidelines (AACE 2019; ADA 2016; Kernan 2014) are not supported by solid evidence, and they should be properly evaluated and proved before being implemented in clinical practice.
Authors' conclusions
Implications for practice.
For the general population of people with elevated blood pressure the benefits of trying to achieve a lower blood pressure target rather than a standard target (≤ 140/90 mm Hg) do not outweigh the harms associated with that intervention.
Implications for research.
Identification of specific types of patients who might benefit from lower blood pressure targets in order to be evaluated in a clinical trial specifically designed for that objective.
Identification of specific types of patients who are more susceptible to serious adverse events related to lower blood pressure targets.
Evaluation of blood pressure targets in young, low risk hypertensive patients.
What's new
| Date | Event | Description |
|---|---|---|
| 30 November 2020 | New citation required and conclusions have changed | Substantial update with stronger conclusions |
| 30 November 2020 | New search has been performed | Four new included studies were added in this updated review |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 3, 2009
| Date | Event | Description |
|---|---|---|
| 28 March 2020 | New citation required and conclusions have changed | 7 new RCTs were included in this update and conclusions are more certain |
| 2 March 2020 | Amended | Decision to not include total cardiovascular events as a composite outcome as it was not reported consistently in the different trials |
| 18 November 2011 | New search has been performed | Minor numerical typographical errors corrected. |
| 12 August 2008 | Amended | Converted to new review format. |
| 11 November 2003 | Amended | Minor changes included in the protocol |
Acknowledgements
We are very grateful to the following people and institutions.
Dr. Fiona Turnbull, from the Blood Pressure Lowering Treatment Trialists' Collaboration, for providing useful additional information not available from the published reports of the trials.
Dr. William Cushman and Mr. Greg Evans, from the ACCORD Co‐ordinating Center, for providing useful additional information not available in the published report of the ACCORD trial.
Dr. Ratika Parkash and Karen A. Giddens, for providing useful additional information not available in the published report of the SMAC‐AF trial.
Dr. Oscar Benavente, for providing useful additional information not available in the published report of the SPS3 trial.
The Therapeutics Initiative, Department of Anesthesiology, Pharmacology & Therapeutics, University of British Columbia.
Department of Clinical Pharmacology and Toxicology, School of Medicine, Faculty of Medicine, University of Costa Rica.
Appendices
Appendix 1. Search Strategies
Database: Ovid MEDLINE(R) 1946 to Present with Daily Update Search Date: 31 May 2019 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 hypertension/ 2 hypertens$.tw,kw. 3 exp blood pressure/ 4 (blood pressure or bloodpressure).tw,kw. 5 or/1‐4 6 ((goal? or intensive$ or strict$ or target$ or tight$) adj4 (antihypertensive? or hypertensive? or bp or control or dbp or diastolic or pressure? or sbp or systolic or treat$)).tw,kw. 7 randomized controlled trial.pt. 8 controlled clinical trial.pt. 9 randomized.ab. 10 placebo.ab. 11 clinical trials as topic/ 12 randomly.ab. 13 trial.ti. 14 or/7‐13 15 animals/ not (humans/ and animals/) 16 14 not 15 17 5 and 6 and 16 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Cochrane Hypertension Specialised Register via Cochrane Register of Studies (CRS‐Web) Search Date: 31 May 2019
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 (goal* or intensive* or strict* or target* or tight*) NEAR4 (antihypertensive* or hypertensive* or bp or control or dbp or diastolic or pressure* or sbp or systolic or treatment*) AND INSEGMENT #2 RCT:DE AND INSEGMENT #3 Review:MISC2 AND INSEGMENT #4 #2 OR #3 AND INSEGMENT #5 #1 AND #4 AND INSEGMENT ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Cochrane Central Register of Controlled Trials via Cochrane Register of Studies (CRS‐Web) Search Date: 31 May 2019
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 MESH DESCRIPTOR hypertension EXPLODE ALL AND CENTRAL:TARGET #2 hypertens*:ti,ab AND CENTRAL:TARGET #3 MESH DESCRIPTOR blood pressure EXPLODE ALL AND CENTRAL:TARGET #4 (blood pressure OR bloodpressure) AND CENTRAL:TARGET #5 #1 OR #2 OR #3 OR #4 AND CENTRAL:TARGET #6 (goal* or intensive* or strict* or target* or tight*) NEAR4 (antihypertensive* or hypertensive* or bp or control or dbp or diastolic or pressure* or sbp or systolic or treatment*) AND CENTRAL:TARGET #7 #5 AND #6 AND CENTRAL:TARGET #8 #7 NOT *:MH AND CENTRAL:TARGET #9 #7 NOT *:EM AND CENTRAL:TARGET #10 #8 AND #9
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Embase <1974 to 2019 May 31> Search Date: 31 May 2019 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp hypertension/ 2 hypertens$.tw,kw. 3 blood pressure.mp. 4 or/1‐3 5 ((goal? or intensive$ or strict$ or target$ or tight$) adj2 (antihypertensive? or hypertensive? or bp or control or dbp or diastolic or pressure? or sbp or systolic or treatment$)).tw,kw. 6 randomized controlled trial/ 7 crossover procedure/ 8 double‐blind procedure/ 9 (randomi?ed or randomly).tw. 10 (crossover$ or cross‐over$).tw. 11 placebo.ab. 12 (doubl$ adj blind$).tw. 13 assign$.ab. 14 allocat$.ab. 15 or/6‐14 16 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 17 15 not 16 18 4 and 5 and 17 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: ClinicalTrials.gov Search Date: 31 May 2019 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Search Terms: (goal OR intensive OR strict OR target OR tight) AND (randomized) Study type: Interventional Studies Conditions: hypertension Outcome Measures: blood pressure ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: WHO International Clinical Trials Registry Platform (ICTRP) Search Date: 31 May 2019 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ goal AND blood pressure AND randomized intensive AND blood pressure AND randomized strict AND blood pressure AND randomized target AND blood pressure AND randomized tight AND blood pressure AND randomized
Data and analyses
Comparison 1. Low vs Standard BP Target.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Total mortality | 11 | 38688 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.86, 1.05] |
| 1.1.1 Systolic target | 7 | 19013 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.04] |
| 1.1.2 Diastolic target | 4 | 19675 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.86, 1.20] |
| 1.2 CV mortality | 9 | 37500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.76, 1.06] |
| 1.2.1 Systolic target | 6 | 17902 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.65, 1.02] |
| 1.2.2 Diastolic target | 3 | 19598 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.80, 1.29] |
| 1.3 Non‐CV mortality | 9 | 37500 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.88, 1.18] |
| 1.3.1 Systolic target | 6 | 17902 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.23] |
| 1.3.2 Diastolic target | 3 | 19598 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.81, 1.27] |
| 1.4 Total serious adverse events | 6 | 18165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.99, 1.08] |
| 1.4.1 Systolic target | 5 | 17827 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.99, 1.08] |
| 1.4.2 Diastolic target | 1 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.90, 2.15] |
| 1.5 Myocardial infarction | 8 | 38198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.73, 0.96] |
| 1.5.1 Systolic target | 6 | 18938 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.73, 1.01] |
| 1.5.2 Diastolic target | 2 | 19260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.61, 1.02] |
| 1.6 Stroke | 7 | 37087 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.77, 1.01] |
| 1.6.1 Systolic target | 5 | 17827 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.67, 0.94] |
| 1.6.2 Diastolic target | 2 | 19260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.84, 1.34] |
| 1.7 Congestive heart failure | 5 | 15859 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.92] |
| 1.7.1 Systolic target | 4 | 15389 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.59, 0.91] |
| 1.7.2 Diastolic target | 1 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.40, 2.43] |
| 1.8 End‐stage renal failure | 6 | 14768 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.83, 1.37] |
| 1.8.1 Systolic target | 4 | 14353 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.71, 1.36] |
| 1.8.2 Diastolic target | 2 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.83, 1.82] |
| 1.9 All other serious adverse events | 6 | 18938 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.32, 1.59] |
| 1.9.1 Systolic target | 6 | 18938 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.32, 1.59] |
| 1.10 Systolic blood pressure achieved | 11 | 38688 | Mean Difference (IV, Fixed, 95% CI) | ‐7.52 [‐7.76, ‐7.27] |
| 1.10.1 Systolic target | 7 | 19013 | Mean Difference (IV, Fixed, 95% CI) | ‐12.10 [‐12.45, ‐11.74] |
| 1.10.2 Diastolic target | 4 | 19675 | Mean Difference (IV, Fixed, 95% CI) | ‐3.29 [‐3.63, ‐2.96] |
| 1.11 Diastolic blood pressure achieved | 10 | 35668 | Mean Difference (IV, Fixed, 95% CI) | ‐4.28 [‐4.41, ‐4.16] |
| 1.11.1 Systolic target | 6 | 15993 | Mean Difference (IV, Fixed, 95% CI) | ‐6.61 [‐6.83, ‐6.39] |
| 1.11.2 Diastolic target | 4 | 19675 | Mean Difference (IV, Fixed, 95% CI) | ‐3.18 [‐3.33, ‐3.03] |
| 1.12 Withdrawals due to adverse events | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.51, 7.86] |
| 1.12.1 Diastolic target | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.51, 7.86] |
| 1.13 Number of antihypertensive drugs used per patient | 7 | 18240 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [0.96, 1.04] |
| 1.13.1 Systolic target | 6 | 17902 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [0.96, 1.04] |
| 1.13.2 Diastolic target | 1 | 338 | Mean Difference (IV, Fixed, 95% CI) | 0.85 [0.59, 1.11] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ABCD (H) 2000.
| Study characteristics | ||
| Methods | Randomised, open‐label clinical trial. Patients were randomised to intensive versus moderate blood pressure control. They were also allocated to either nisoldipine or enalapril as the initial antihypertensive medication. If the target blood pressure was not achieved with increasing doses, then open‐labelled antihypertensive medications were added in a step‐wise fashion, initially with metoprolol, then hydrochlorothiazide or additional drugs, but not a calcium channel blocker or ACE inhibitor. Blood pressure recordings were obtained at peak drug levels and were an average of three seated readings obtained at each visit. The follow‐up period was 5 years. | |
| Participants | 470 patients, between the ages of 40 and 74 years, with type 2diabetes mellitus diagnosed. All of them had a DBP equal to or higher than 90 mm Hg without taking antihypertensive medications. They could not have had a myocardial infarction or a cerebrovascular accident within the previous 6 months, had coronary artery bypass surgery within the previous 3 months, had unstable angina pectoris within the previous 6 months, had congestive heart failure NYHA class III or IV, demonstrated an absolute need for ACE inhibitors or CCB, and/or had a serum creatinine level > 3 mg/dL. | |
| Interventions | Diastolic BP targets Patients were randomised into two treatment arms consisting of intensive treatment with a DBP goal of 75 mm Hg, and moderate treatment with a DBP goal of 80 mm Hg to 89 mm Hg. |
|
| Outcomes | The primary end point was the change in 24‐hour creatinine clearance. Secondary end points included cardiovascular events, retinopathy, clinical neuropathy, and urinary albumin excretion | |
| Notes | Patients were also randomised to either nisoldipine or enalapril as the initial antihypertensive medication. Study funded by a grant from Aventis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants assigned to quote: “moderate treatment” had a greater prevalence of established vascular disease |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and investigators not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An independent end point committee, which was blinded to the study intervention arms, reviewed all cardiovascular events. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data on losses to follow‐up were not reported |
| Selective reporting (reporting bias) | High risk | Not all outcomes reported |
| Other bias | Unclear risk | Funding not reported |
ACCORD 2010.
| Study characteristics | ||
| Methods | Randomised and multicentre trial performed in the USA and Canada. The entire ACCORD trial enrolled 10,251 participants with type 2 diabetes mellitus considered to be at high risk. All participants were randomly assigned to either intensive or standard glycaemic control. In addition, 4733 participants were also randomly assigned (in a 2‐by‐2 factorial design) to either intensive or standard blood‐pressure control (the ACCORD blood‐pressure trial). The mean follow‐up was 4.7 years |
|
| Participants | 4733 participants were included in the ACCORD BP trial. Participants were eligible if they had type 2 diabetes mellitus and a glycated haemoglobin level of 7.5% or more, and were 40 years of age or older with cardiovascular disease or 55 years of age or older with anatomical evidence of a substantial amount of atherosclerosis, albuminuria, left ventricular hypertrophy, or at least two additional risk factors for cardiovascular disease (dyslipidaemia, hypertension, smoking, or obesity). Participants with a SBP between 130 mm Hg and 180 mm Hg who were taking three or fewer antihypertensive medications and who had the equivalent of a 24‐hour protein excretion rate of less than 1.0 g were also eligible for the blood pressure trial. Exclusion criteria included a body‐mass index of more than 45, a serum creatinine level of more than 1.5 mg per dL, and other serious illness |
|
| Interventions | Systolic BP targets Intensive therapy was defined by a target SBP of less than 120 mm Hg, whereas standard therapy targeted a SBP of less than 140 mm Hg. There was no specific drug regimen to achieve the target blood pressure. However, all the antihypertensive regimens were to include drug classes that had been shown to result in a reduction in cardiovascular events among participants with diabetes |
|
| Outcomes | The primary outcome was the first occurrence of a major cardiovascular event, which was defined as the composite of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death. Prespecified secondary outcomes included the combination of the primary outcome plus revascularisation or hospitalisation for congestive heart failure, the combination of a fatal coronary event, nonfatal myocardial infarction, or unstable angina; nonfatal myocardial infarction; fatal or nonfatal stroke, nonfatal stroke, death from any cause, death from cardiovascular causes, and hospitalisation or death due to heart failure | |
| Notes | Study supported by contracts from the National Institutes of Health and the Centers for Disease Control and Prevention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and investigators not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An independent Endpoint Committee, which was blinded to the study intervention arms, reviewed all cardiovascular events. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The vital status for 5% of randomised participants was unknown at the end of the trial. Their distribution is not reported |
| Selective reporting (reporting bias) | High risk | The ACCORD investigators elected to restrict analysis and reporting of SAE data to events related to blood pressure medications because those were the only events collected in a consistent manner throughout the trial and subject to safety officer and DSMB review. |
| Other bias | Low risk | The trial was sponsored by the National Heart, Lung, and Blood Institute from the USA. No other funding reported |
Cardio‐Sis 2008.
| Study characteristics | ||
| Methods | Randomised, open, multicentre trial performed in 44 centres in Italy. Patients were followed up every 4 months. Blood pressure was the average of three consecutive readings at every visit with standard mercury sphygmomanometers. Analysis was by intention‐to‐treat with all available data. The mean duration of follow‐up was 2 years |
|
| Participants | 1111 non‐diabetic patients were included. Participants were eligible if they were aged 55 years or older, with a SBP > 150 mm Hg, who had been receiving antihypertensive treatment for at least 12 weeks. They also had at least one additional risk factor (cigarette smoking, total cholesterol ≥ 5.2 mmol/L, HDL cholesterol < 1.0 mmol/L LDL cholesterol ≥ 3.4 mmol/L, family history of premature cardiovascular disease in first degree relative, previous transient ischaemic attack or stroke, or established coronary or peripheral artery disease. Exclusion criteria included a fasting glucose ≥ 7.0 mmol/L, those with a history of diabetes mellitus, any disease reducing life expectancy, renal dysfunction (serum creatinine ≥ 176.8 µmol/L), clinically relevant hepatic or haematological disorders, valvular heart disease, disorders confusing the electrocardiographic diagnosis of LVH, atrial fibrillation and substance misuse. |
|
| Interventions | Systolic BP targets Patients were allocated to tight (< 130 mm Hg) or usual control (<140 mm Hg) of SBP. Antihypertensive drug treatment included various combinations of previous drugs plus drugs made available for the purpose of the study. The choice of drugs was left to the discretion of the investigators. In the tight control group, one SBP reading higher than 130 mm Hg at any visit led to intensification of treatment. Conversely, in the usual‐control group, achievement of a SBP below 130 mm Hg entailed downtitration of treatment. |
|
| Outcomes | The primary outcome was the prevalence of electrocardiographic LVH at the final 2‐year visit. The main prespecified secondary outcome was a composite of all‐cause mortality, fatal or non‐fatal myocardial infarction, fatal or non‐fatal stroke, transient ischaemic attack, congestive heart failure NYHA III or IV requiring admission to hospital, angina pectoris with objective evidence of myocardial ischaemia, new‐onset atrial fibrillation, coronary revascularisation, aortic dissection, occlusive peripheral arterial disease, and renal failure requiring dialysis. Other predefined secondary outcomes were the single components of the main secondary outcome and difference between groups in the achieved SBP. | |
| Notes | Study supported by the Associazione Nazionale Medici Cardiologi Ospedalieri and funded by several pharmaceutical companies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random function |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and investigators not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An Independent Clinical Event Committee, masked to the group allocation, evaluated all clinical events. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A greater percentage of participants assigned to tight control were not available for 0ne‐year follow‐up visit |
| Selective reporting (reporting bias) | High risk | Not all outcomes reported |
| Other bias | High risk | Funded by the pharmaceutical industry |
HOT 1998.
| Study characteristics | ||
| Methods | Randomised, open‐label, controlled trial, with blinded endpoint evaluation (PROBE) design. Patients were randomly assigned to one of three DBP target groups: less or equal than 90 mm Hg, less or equal than 85 mm Hg, or less or equal than 80 mm Hg. Randomisation took into consideration the following baseline variables: age, sex, previous antihypertensive therapy, smoking, previous myocardial infarction, previous coronary heart disease, previous stroke and diabetes mellitus. Blood pressure was measured three times, by an oscillometric semiautomatic device, with the patient in the sitting position after 5 minutes of rest. All patients were given the same therapeutic approach, organized in the following steps in order to achieve the target blood pressure. 1. starting therapy was felodipine 5 mg once a day 2. angiotensin enzyme (ACE) inhibitors or beta‐blockers were added 3. increased dose of felodipine to 10 mg once a day 4. doubling the dose of the ACE inhibitor or beta‐blocker 5. adding a diuretic The average follow‐up was 3.8 years. | |
| Participants | 19,193 hypertensive patients, aged 50 to 80 years, were initially included, but the study population was composed by 18,790 patients because 403 of them were excluded early in the trial because of the suspicion of incorrect inclusion. Baseline DBP between 100 mm Hg and 115 mm Hg was an inclusion criterion. 1501 non‐insulin treated diabetic patients were included and the event rates were reported separately in them. Main exclusion criteria were malignant hypertension, secondary hypertension, DBP > 115 mm Hg, stroke or myocardial infarction within 12 months prior to randomisation, decompensated congestive heart failure, other serious concomitant diseases which could affect survival during the next 2 to 3 years, patients who required a beta‐blocker, ACE inhibitor or diuretic for reasons other than hypertension, patients who required antiplatelet or anticoagulant therapy, and insulin treated diabetics. | |
| Interventions | Diastolic BP targets Patients were randomly assigned to one of threeDBP target groups: less or equal than 90 mm Hg, less or equal than 85 mm Hg, or less or equal than 80 mm Hg. |
|
| Outcomes | The outcomes measured were: total and cardiovascular mortality, all (fatal and non‐fatal) myocardial infarctions including silent infarctions, all (fatal and non‐fatal) strokes, and major cardiovascular events (all myocardial infarctions plus all strokes plus other cardiovascular deaths). | |
| Notes | Patients were also randomly assigned to acetylsalicylic acid 75 mg daily or placebo.
24% of all investigators‐reported events were rejected by the Clinical Event Committee. Several pharmaceutical companies were among the principal sponsors of the trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and investigators not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An Independent Clinical Event Committee, masked to the group allocation, evaluated all clinical events. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data on losses to follow‐up was not reported |
| Selective reporting (reporting bias) | High risk | Not all outcomes reported |
| Other bias | High risk | Industry funded |
PAST‐BP 2016.
| Study characteristics | ||
| Methods | Randomised, open‐label, controlled trial. Patients were randomly assigned to an intensive blood pressure target or a standard target. BP was measured by using an automated sphygmomanometer. BP was measured in a standardised way, with the patient seated for five minutes and then six measurements taken at one minute intervals. The reported number was the average of the second and third measurements. The average follow‐up was 1 year. | |
| Participants | 529 patients were considered for inclusion if they were in the practice's TIA/stroke register. They were excluded if their baseline SBP was less than 125 mm Hg, they were already taking three or more antihypertensive agents, they had a greater than 20 mm Hg postural change in SBP on standing, they were already being treated to a 130 mm Hg SBP target. 379 participants were included in the analysis | |
| Interventions | Systolic BP targets Intensive blood pressure target was defined as < 130 mm Hg or a 10 mm Hg reduction if baseline pressure was < 140 mm Hg, whereas standard target was < 130 mm Hg. |
|
| Outcomes | The primary outcome was change in SBP between baseline and one year. Clinical events were identified through review of the general practice records. They included fatal and non‐fatal stroke, myocardial infarction, fatal coronary heart disease, or other cardiovascular death, emergency hospital admissions, and deaths. | |
| Notes | Funded by the National Institute for Health Research in England | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and investigators not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Clinical events were identified through review of the general practice records, but they were not evaluated by investigators |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16% of patients withdrew from the trial (20% in the intensive target arm and 12% in the standard target arm). Despite that, all patients were followed‐up for clinical events and deaths |
| Selective reporting (reporting bias) | High risk | Not all outcomes reported |
| Other bias | High risk | A significantly greater number of patients (109 versus 57, P = 0.005) in the intensive target group did not have their BP treatment increased when the BP was above target, mainly due to symptoms attributed to BP drugs and patient not wanting treatment to be intensified |
REIN‐2 2005.
| Study characteristics | ||
| Methods | Multicentre, randomised, controlled trial. Before randomisation, patients were treated with antihypertensive drugs (apart from ACE inhibitors, angiotensin‐II‐receptor antagonists, and dihydropyridine calcium‐channel blockers) to maintain DBP at less than 90 mm Hg. Participants were then randomly assigned to either conventional blood‐pressure control (DBP < 90 mm Hg, irrespective of SBP) or intensified blood‐pressure control. To achieve the intensified blood‐pressure level, patients received add‐on therapy with the dihydropyridine calcium‐channel blocker felodipine 5 mg/day, and up‐titrated the dose after a week to 10 mg/day according to blood pressure response. In both arms up‐ and down‐titration of concomitant drugs was allowed to maintain the target blood pressure and to avoid symptomatic hypotension. Blood pressure was measured 1 week, 2 weeks, and 3 weeks after randomisation, and every 3 months thereafter. Additional measurements were done within 1 week after any change in antihypertensive therapy. The blood pressure was the mean of three values taken 2 minutes apart, after 5 minutes rest in the sitting position. on the same arm by a standard sphygmomanometer. The time of day when blood pressure was measured was not reported. The median follow‐up was 19 months. | |
| Participants | 338 patients, who had non‐diabetic nephropathy and persistent proteinuria, and who had not received ACE‐inhibition therapy for at least 6 weeks. Persistent proteinuria was defined as urinary protein excretion exceeding 1 g per 24 hours for at least 3 months without evidence of urinary‐tract infection or overt heart failure (NYHA class III‐IV). Patients with proteinuria of 1‐3 g per 24 hours were included if their creatinine clearance was less than 45 mL/min per 1.73 m2; those with a proteinuria of 3 g per 24 hours or more were included if their creatinine clearance was less than 70 mL/min per 1.73 m2. Exclusion criteria were treatment with corticosteroids, non‐steroidal antiinflammatory drugs, or immunosupressive drugs; acute myocardial infarction or cerebrovascular accident in the previous 6 months, severe uncontrolled hypertension, evidence or suspicion of renovascular disease, obstructive uropathy, type 1 diabetes mellitus, collagen disease, cancer, higher serum aminotransferase concentrations, or chronic cough, history of allergy, or poor tolerance to ACE inhibitors or dihydropiridine calcium‐channel blockers, pregnancy, breastfeeding. | |
| Interventions | Systolic/diastolic BP targets Participants were randomly assigned to either "conventional" (diastolic < 90 mm Hg) or intensified (systolic/diastolic < 130/80 mm Hg) blood‐pressure control. |
|
| Outcomes | The primary outcome was progression to end‐stage renal disease. Other outcomes were GFR decline, residual proteinuria, fatal and non‐fatal cardiovascular events. | |
| Notes | After the first interim analysis, done as per protocol, an independent adjudicating panel stated that the study had to be stopped for futility because the outcomes were similar in both arms despite more effective blood‐pressure reduction in the intensified blood‐pressure control arm. The trial was undertaken by the Mario Negri Institute for Pharmacological Research. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and investigators not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were followed even if target BP was not achieved |
| Selective reporting (reporting bias) | High risk | Not all outcomes reported |
| Other bias | High risk | Terminated early. The study was supported in part by Aventis Pharma S.A. |
Schrier 2002.
| Study characteristics | ||
| Methods | Randomized trial performed in a single centre in the USA. All 75 participants were sequentially randomised with stratification by renal function to rigorous or standard BP control via computer‐generated randomisation codes. In all participants, the mean of three sitting BP measurements was used to determine BP level. Dose adjustments were made weekly until the desired BP goal was reached. The mean follow‐up was 7 years |
|
| Participants | 75 patients with autosomal dominant polycystic kidney disease were included in the trial. They had hypertension (BP > 140/90 mm Hg) and LVH. Participants were eligible if they were between 20 and 60 years of age, had creatinine clearance more than 30 ml(min per 1.73m2, and men had left ventricular mass index (LVMI) > 125 g/m, and women had LVMI 110 g/m. The following participants were excluded: participants who could not tolerate the study medications, participants with > 3 g urinary protein per day or those with a second renal diagnosis, participants who required antiarrhythmic medications, lactating or pregnant participants or subjects taking oral contraceptive medications, participants with underlying psychiatric disorders, and participants who, by the discretion of the investigator, were thought to be unable to comply with the guidelines of the protocol. Additionally, participants with LVH due to primary causes other than hypertension were excluded from the trial. |
|
| Interventions | Systolic/diastolic BP targets Participants were randomised to either rigorous (<120/80 mm Hg) or standard (135 mm Hg to 140/85 to 90 mm Hg) BP control. The initial antihypertensive drug was either enalapril or amlodipine, at escalating doses. If more medications were needed to achieve the BP goal, hydrochlorothiazide, clonidine, spironolactone, or some combination of these were added as necessary. Rarely, other antihypertensive medications were added at the discretion of the study physician. |
|
| Outcomes | The primary outcome was the decline of glomerular filtration rate and in mean left ventricular mass index from baseline to year 7. | |
| Notes | Funded by the Department of Health and Human Services, National Institute of Diabetes, Digestive and Kidney Diseases, and the National Institutes of Health from the USA. Pfizer Inc. provided part of the funding too. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Via computer‐generated randomisation codes |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and investigators not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 participants dropped out of the study, with no difference between group assigned |
| Selective reporting (reporting bias) | High risk | Not all outcomes reported |
| Other bias | Unclear risk | Role of Pfizer Inc. is not clear. |
SMAC‐AF 2017.
| Study characteristics | ||
| Methods | Randomised, parallel, open‐label clinical trial with blinded end‐point evaluation performed in 13 tertiary centres in Canada | |
| Participants | 184 participants who had a baseline BP > 130/80 mm Hg, symptomatic paroxysmal or persistent atrial fibrillation and were scheduled to undergo catheter ablation. Patients with moderate to severe renal dysfunction or prior intolerance to an angiotensin receptor II antagonist were excluded. | |
| Interventions | Systolic/diastolic BP targets Participants were randomly assigned to aggressive BP (target < 120/80 mm Hg) or standard BP (target < 140/90 mm Hg) treatment. Titration of medications in the aggressive BP treatment group occurred at 2‐week intervals through telephone follow‐up |
|
| Outcomes | The primary outcome was time to symptomatic atrial fibrillation or atrial flutter. | |
| Notes | The study was sponsored by the Nova Scotia Health Research Foundation and the Canadian Institutes of Health Research. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and investigators not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A blinded event committee adjudicated end‐points. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Eleven randomised patients could not be included for several reasons. Their distribution was not reported. Three participants were lost to follow‐up: 1 was allocated to the intensive treatment group and 2 to the standard treatment. |
| Selective reporting (reporting bias) | High risk | Not all outcomes reported |
| Other bias | Low risk | |
SPRINT 2015.
| Study characteristics | ||
| Methods | Randomised, controlled, multicentrE, open‐label trial conducted at 102 clinical sites in the USA and Canada. An independent data and safety monitoring board monitored unblinded trial results and safety events. | |
| Participants | 9361 participants were included in the trial. Participants were required to meet all the following criteria: an age of at least 50 years, a SBP of 130 to mm Hg180 mm Hg, and an increased risk of cardiovascular events. Increased risk was defined by one of he following: clinical or subclinical cardiovascular disease other than stroke; chronic kidney disease, with an estimated glomerular filtration rate of 20 mL to less than 60 mL per minute per 1,73 m2 of body surface area; a 10‐year risk of cardiovascular disease of 15% or greater on the basis of the Framingham risk score; or an age of 75 years or older. Patients with diabetes mellitus or prior stroke were excluded. | |
| Interventions | Systolic BP targets Eligible participants were assigned to a SBP target of either less than 140 mm Hg (the standard‐treatment group) or less than 120 mm Hg (the intensive‐treatment group). The baseline antihypertensive regimens were adjusted on the basis of the study‐group assignment. All major classes of antihypertensive agents were included in the formulary and were provided at no cost to the participants. Participants were seen monthly for the first 3 months and every 3 months thereafter. Medications for participants in the intensive‐treatment group were adjusted on a monthly basis to target a SBP of less than 120 mm Hg. For participants in the standard‐treatment group, medications were adjusted to target a SBP of 135 mm Hg to 139 mm Hg, and the dose was reduced if SBP was less than 130 mm Hg on a single visit or less than 135 mm Hg on two consecutive visits. Dose adjustments was based on a mean of three blood‐pressure measurements at an office visit while the patient was seated and after 5 minutes of quiet rest. |
|
| Outcomes | The primary outcome was the composite of myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, acute decompensated heart failure, and death from cardiovascular causes. Secondary outcomes included the individual components of the primary composite outcome, death from any cause, and the composite of the primary outcome or death from any cause. Renal outcomes were also assessed. | |
| Notes | The study was terminated early. The median follow‐up was 3.3 years of the planned average of 5 years. The study was funded by the National Institutes of Health. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was stratified according to clinical site." Baseline BP was almost identical in the 2 groups. |
| Allocation concealment (selection bias) | Unclear risk | No explanation as to how they concealed allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Participants and study personnel were aware of the study‐group assignments." The differences in achieved BP are unrealistic given only one drug, mean difference between‐group and very suggestive of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome adjudicators were not aware of study‐group assignments but there is nothing to suggest they adjudicated all outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | More patients lost to follow‐up in standard target group. |
| Selective reporting (reporting bias) | Low risk | All important outcomes reported including total people with at least one serious adverse event. |
| Other bias | High risk | Role of Takeda and Arbour Pharmaceuticals in addition to providing drugs is not clear. Terminated early for benefit. It used a BP measurement strategy that could provide BP values lower than expected with the traditional office measurement strategies |
SPS3 2013.
| Study characteristics | ||
| Methods | Randomised and multicentre clinical trial performed in 81 centres in North America, Latin America, and Spain. Patients were randomised according to a two‐by‐two multifactorial design to two antiplatelet regimens and two target ranges. Analysis was done by intention‐to‐treat. The mean follow‐up was 3.7 years |
|
| Participants | 3020 participants were included in the SPS3 trial. Participants were eligible if they were aged 30 years or older, were normotensive or hypertensive, had had a recent (within 180 days), symptomatic; MRI‐confirmed lacunar stroke, and were without surgically amenable ipsilateral carotid artery stenosis or high‐risk cardioembolic sources. Main exclusion criteria included disabling stroke, previous intracranial haemorrhage from non‐traumatic causes, or cortical ischaemic stroke. | |
| Interventions | Systolic BP targets Patients were randomised to two blood‐pressure‐control groups with targets of 130‐149 mm Hg or less than 130 mm Hg. Treatment was open label. To avoid lowering of blood pressure soon after an acute stroke, participants were randomised at least 2 weeks after the index stroke. Blood pressure was measured three times at every visit and the average measurement was used to decide hypertension status. After randomisation, if patients had blood pressure outside the assigned target range, they were initially seen at least monthly for measurement of blood pressure and adjustment of medications. Antihypertensive medications were prescribed by the local study physician. At least one drug from each of the major classes of antihypertensive medications was available. |
|
| Outcomes | The primary outcome was reduction in all strokes. Secondary outcomes included acute myocardial infarction, need for acute admission to hospital for a major vascular event, and death, classified as vascular, non‐vascular, or unknown. All reported efficacy outcomes were confirmed by a central adjudication committee that was unaware of treatment assignment. Safety outcomes were serious adverse events related to hypotension and blood pressure management | |
| Notes | Funded by the National Institutes of Health‐National Institute of Neurological Disorders and Stroke (NIH‐NINDS) from the USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated with a permuted‐block design |
| Allocation concealment (selection bias) | Low risk | Treatment assignments were stored electronically |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and investigators not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All reported outcomes were confirmed by a central adjudication committee that was unaware of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3% of participants were lost to follow‐up and an additional 15% ended follow‐up early. Their distribution is not reported |
| Selective reporting (reporting bias) | High risk | Not all outcomes reported |
| Other bias | Low risk | No other apparent bias |
Toto 1995.
| Study characteristics | ||
| Methods | The study was a 2 X 2 factorial, randomised controlled trial. Patients were randomised to either placebo or enalapril and to either "strict" or "conventional" blood pressure ranges. Before randomisation, DBP was lowered to 80 mm Hg or less over a 3 to 6 months initial assessment period. Patients able to achieve that target were randomised and included in the study. To achieve the target DBP, a stepped‐care approach with antihypertensive medications was used: a diuretic was the initial drug, followed by a beta‐blocker, hydralazine or minoxidil, and clonidine, alpha‐methyldopa or a alpha‐1 blocker. With the exception of the diuretic, the maximum dose of each agent was used before moving to the next step. In patients assigned to "conventional" group, DBP was allowed to increase to the 85 to 95 mm Hg range, whereas in patients assigned to the "strict" group the intention was to maintain DBP in the 65 mm Hg to 80 mm Hg range. Blood pressure was measured in the supine position with a mercury sphygmomanometer after a minimum of 5 minutes rest. Three measurements were taken at 2‐minute intervals. The mean of those measurements was used. Mean follow‐up was 40.5 ± 1.8 months in the "strict" group, and 42.2 ± 2.1 months in the "conventional" group. | |
| Participants | 87 patients with hypertensive nephrosclerosis were initially considered for the trial. Their age ranged from 25 to 73 years. The inclusion criteria were a DBP higher than or equal to 95 mm Hg, a serum creatinine greater than 1.6 mg/dL but lower than 7.0 mg/dL and a glomerular filtration rate less than or equal to 70 mL/min/1.73m2, history of long‐standing hypertension, an inactive urine sediment, a protein excretion rate lower than 2 g per day, no physical or biochemical evidence for a humoral‐mediated cause for hypertension. Exclusion criteria were diabetes mellitus, a recent history (in the previous 4 months) of malignant hypertension, stroke or myocardial infarction, acute renal failure of any cause, analgesic abuse, polycystic kidney disease, systemic lupus erythematosus, scleroderma, rapidly progressive glomerulonephritis, evidence of significant hepatic impairment (AST and ALT greater than 2.5 X normal, or serum total bilirubin > 1.5 mg/dL), mental incapacity, pregnancy or lactation, primary aldosteronism, renovascular hypertension, pheochromocytoma. Based on the initial assessment period, 77 patients were classified as "responders" and 10 patients were "non‐responders". Since they were not randomised, "non‐responder" patients were not included in this study. |
|
| Interventions | Diastolic BP targets "Responder" patients were randomised to either placebo or enalapril, in a double‐blind design. They were also randomised to either "strict" or "conventional" blood pressure ranges. "Strict" was defined as a DBP lower than 80 mm Hg, whereas "conventional" was defined as a DBP between 85 mm Hg and 95 mm Hg. After randomisation, the blinded study drug was titrated to maximum allowable dose and the unblinded antihypertensive agents were back‐titrated as needed to achieve and maintain blood pressure control. |
|
| Outcomes | The primary outcome was the rate of decline in glomerular filtration rate, measured by the renal clearance of 125I‐iothalamate. Other outcomes were death, end‐stage renal disease and 50% decline in glomerular filtration rate or doubled serum creatinine (from baseline). | |
| Notes | Assignment to enalapril versus placebo did not change the results of the blood pressure control. The study was supported in part by Merck, Sharp and Dohme. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and investigators not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | High risk | Not all outcomes reported |
| Other bias | High risk | The initial assessment period selected responder participants. Supported by pharmaceutical industry. |
ACE: angiotensin‐converting‐enzyme; ALT: alanine aminotransferase ; AST: aspartate aminotransferase;CCB: calcium channel blocker; DBP: diastolic blood pressure;DSMB: Data and Safety Monitoring Board; HDL: high‐density lipoprotein; LDL: low‐density lipoprotein; LVH: left ventricular hypertrophy; MRI: magnetic resonance imaging; LVH: left ventricular hypertrophy; NYHA: New York Heart Association;SAE: severe adverse effect: SBP: systolic blood pressure; TIA: transient ischaemic attack;
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| AASK 2002 | Trial compared targets defined by mean arterial pressure and therefore cannot be precisely associated with the SBP and DBP ranges specified for this review. |
| ABCD‐2V 2006 | Trial only included normotensive diabetic patients, defined as a baseline SBP < 140 mm Hg and a baseline DBP between 80 mm Hg and 90 mm Hg. |
| ABCD‐N 2002 | Trial only included normotensive diabetic patients, defined as a baseline DBP between 80 mm Hg and 89 mm Hg and who were not receiving antihypertensive medications at the randomisation visit. It included 26 patients with isolated systolic hypertension, but their distribution and their outcomes were not reported separately. |
| ATACH‐2 2016 | This trial was excluded because it compared targets for SBP when treating acute hypertensive response in patients with intracerebral haemorrhage, which is a different condition than treating chronic arterial hypertension. |
| BBB 1994 | The number of patients randomised to each treatment target was not reported and not provided by the authors. |
| CHIPS 2015 | The trial included women with pre‐existing or gestational hypertension; gestational hypertension is a very different condition in terms of pathogenesis and prognostic implications. This trial compared blood pressure targets during pregnancy, and due to the short follow‐up period, it looked at different outcomes. |
| HDS 1996 | The higher blood pressure target in this trial (aiming for systolic < 180 mm Hg and diastolic < 105 mm Hg) was much higher than the standard target interval defined in our protocol. |
| HOMED‐BP 2012 | Both home blood pressure targets were lower than the standard targets in our review. |
| JATOS 2008 | This trial was not included because both blood pressure targets in this trial were within the values considered as "standard targets" in our systematic review. |
| Lewis 1999 | No usable data for any of the outcomes defined in this systematic review were reported. |
| MDRD 1995 | This trial compared targets defined by mean arterial pressure and therefore cannot be precisely associated with the SBP and DBP ranges specified for this review. |
| SANDS 2008 | This trial used a dual intervention, lower blood pressure and lower LDL cholesterol plus both SBP targets were within the values considered as "lower targets" in this systematic review. |
| Solomon 2010 | This trial was not included because it did not provide any information regarding mortality or cardiovascular events. |
| Steno‐2 2003 | The multifactorial intervention in the two treatment groups prevented any inference as to whether any difference in clinical outcomes could be attributed to a lower blood pressure target or to any of the other combined interventions. |
| UKPDS 1998 | The higher blood pressure target in this trial (aiming for systolic < 180 mm Hg and diastolic < 105 mm Hg) was much higher than the standard target interval defined in our protocol. |
| VALISH 2010 | This trial was not included because both targets in this trial were within the values considered as "standard targets" in our systematic review. |
| Wei 2013 | This trial was not included because both targets in this trial were within the values considered as "standard targets" in our systematic review. |
DBP: diastolic blood pressure; LDL: low‐density lipoprotein; SBP: systolic blood pressure
Differences between protocol and review
In the protocol we did not separate the trials according to systolic or diastolic targets. In the review we have set up the systolic targets and diastolic targets as subgroups so it is possible to see the data separately for each target.
Contributions of authors
Jose Agustín Arguedas developed the basis for the protocol. He was primarily responsible for identifying and assessing studies, data extraction and analyses and writing the review. Viriam Leiva independently verified the trials for inclusion and the data entry. James Wright formulated the idea for the review and assisted in methodological issues and writing the review.
Sources of support
Internal sources
-
Departments of Anesthesiology, Pharmacology & Therapeutics and Medicine, Faculty of Medicine, University of British Columbia, Canada
In kind costs for space and maintenance
-
Universidad de Costa Rica, Costa Rica
In kind costs.
External sources
-
British Columbia Ministry of Health, Canada
Ongoing grant to the Therapeutics Initiative
Declarations of interest
JAA has lectured on this subject in activities organised by Astra‐Zeneca and MSD, neither of which participated in the content of the talks or in the preparation of this work.
VL and JMW have no conflict to declare.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
ABCD (H) 2000 {published and unpublished data}
- Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic complications in patients with hypertension and type 2 diabetes. Diabetes Care 2000;23(suppl 2):B54-B64. [PubMed] [Google Scholar]
ACCORD 2010 {published data only (unpublished sought but not used)}
- The ACCORD Study Group. Effects of intensive blood-pressure control in type-2 diabetes mellitus. New England Journal of Medicine 2010;362:1575-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cardio‐Sis 2008 {published data only (unpublished sought but not used)}
- Verdecchia P, Staessen JA, Angeli F, on behalf of the Cardio-Sis Investigators. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open label randomised trial. Lancet 2009;374:525-33. [DOI] [PubMed] [Google Scholar]
HOT 1998 {published and unpublished data}
- Hansson L, for the HOT Study Group. The Hypertension Optimal Treatment Study (The HOT Study). Blood Pressure 1993;2:62-8. [DOI] [PubMed] [Google Scholar]
- Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt D, Julius S, et al, for the HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomized trial. Lancet 1998;351(9118):1755-62. [DOI] [PubMed] [Google Scholar]
- Hansson L, Zanchetti A, for the HOT Study Group. The Hypertension Optimal Treatment (HOT) Study: 12-month data on blood pressure and tolerability with special reference to age and gender. Blood Pressure 1995;4:313-9. [DOI] [PubMed] [Google Scholar]
- Hansson L, Zanchetti A, for the HOT Study Group. The Hypertension Optimal Treatment (HOT) Study: 24-month data on blood pressure and tolerability. Blood Pressure 1997;6:313-7. [DOI] [PubMed] [Google Scholar]
- Hansson L, Zanchetti A for the HOT Study. The Hypertension Optimal Treatment (HOT) Study. Patient characteristics: randomization, risk profiles, and early blood pressure results. Blood Pressure 1994;3:322-7. [DOI] [PubMed] [Google Scholar]
- Hansson L. The Hypertension Optimal Treatment study and the importance of lowering blood pressure. Journal of Hypertension 1999;17(suppl 1):S9-S13. [PubMed] [Google Scholar]
- Zanchetti A, Hansson L, Clement D, Elmfeldt D, Julius S, Rosenthal T, et al HOT Study Group. Benefits and risks of more intensive blood pressure lowering in hypertensive patients of the HOT study with different risk profiles: does a J-shaped curve exist in smokers? Journal of Hypertension 2003;21(4):787-804. [DOI] [PubMed] [Google Scholar]
- Zanchetti A, Hansson L, Dahlöf B, Elmfeldt D, Kjeldsen S, Kolloch R, et al on behalf of the HOT Study Group. Effects of individual factors on the incidence of cardiovascular events in the treated hypertensive patients of the Hypertension Optimal Treatment Study. Journal of Hypertension 2001;19(6):1149-59. [DOI] [PubMed] [Google Scholar]
PAST‐BP 2016 {published data only (unpublished sought but not used)}
- Mant J, McManus RJ, Roalfe A, Fletcher K, Taylor C, Martin U, et al. Different systolic blood pressure targets for people with history of stroke or transient ischaemic attack: PAST-BP (Prevention After Stroke-Blood Pressure) randomized controlled trial. BMJ 2016;352:i708. [DOI] [PMC free article] [PubMed] [Google Scholar]
REIN‐2 2005 {published data only (unpublished sought but not used)}
- Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene-Iordache B, Turturro M, et al, REIN-2 Study Group. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised, controlled trial. Lancet 2005;365(9463):939-46. [DOI] [PubMed] [Google Scholar]
Schrier 2002 {published data only (unpublished sought but not used)}
- Schrier R, McFann K, Johnson A, Chapman A, Edelstein C, Brosnahan G, et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: results of a seven year prospective randomized trial. Journal of the American Society of Nephrology 2002;13(7):1733-9. [DOI] [PubMed] [Google Scholar]
SMAC‐AF 2017 {published data only (unpublished sought but not used)}
- Parkash R, Wells GA, Sapp JL, Healey JS, Tardif JC, Greiss I, et al. Effect of aggressive blood pressure control on the recurrence of atrial fibrillation after catheter ablation. A randomized, open label clinical trial (SMAC-AF Substrate Modification with Aggressive Blood Pressure Control). Circulation 2017;135(19):1788-98. [DOI] [PubMed] [Google Scholar]
SPRINT 2015 {published data only (unpublished sought but not used)}
- The SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A randomized trial of intensive versus standard blood-pressure control. New England Journal of Medicine 2015;373(22):2103-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
SPS3 2013 {published data only (unpublished sought but not used)}
- The SPS3 Study Group, Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013;382(9891):507-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toto 1995 {published data only}
- Toto RD, Mitchell HC, Smith RD, Lee HC, McIntire D, Pettinger WA. "Strict" blood pressure control and progression of renal disease in hypertensive nephrosclerosis. Kidney International 1995;48(3):851-9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
AASK 2002 {published data only}
- Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al, African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease. JAMA 2002;288(19):2421-31. [DOI] [PubMed] [Google Scholar]
ABCD‐2V 2006 {published data only}
- Estacio RO, Coll JR, Tran ZV, Schrier RX. Effect of intensive blood pressure control with valsartan on urinary albumin excretion in normotensive patients with type 2 diabetes. American Journal of Hypertension 2006;19(12):1241-8. [DOI] [PubMed] [Google Scholar]
ABCD‐N 2002 {published data only}
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney International 2002;61:1086-97. [DOI] [PubMed] [Google Scholar]
ATACH‐2 2016 {published data only}
- Qureshi AI, Palesch YY, Barsan WG, Hanley DF, Hsu CY, Martin RL, et al, ATACH-2 Trial Investigators and the Neurological Emergency Treatment Trials Network. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. New England Journal of Medicine 2016;375(11):1033-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
BBB 1994 {published data only}
- Hansson L for the BBB Study Group. The BBB Study Group: the effect of intensified antihypertensive treatment on the level of blood pressure, side-effects, morbidity and mortality in "well treated" hypertensive patients. Blood Pressure 1994;3(4):248-54. [DOI] [PubMed] [Google Scholar]
- The BBB Study Group. The BBB study: a prospective randomized study of intensified antiihypertensive treatment. Journal of Hypertension 1988;6(9):693-7. [DOI] [PubMed] [Google Scholar]
CHIPS 2015 {published data only}
- Magee LA, Dadelszen P, Rey E, Ross S, Asztalos E, Murphy KE, et al. Less-tight versus tight control of hypertension in pregnancy. New England Journal of Medicine 2015;372(5):407-17. [DOI] [PubMed] [Google Scholar]
HDS 1996 {published data only}
- Hypertension in Diabetes Study Group. Hypertension in Diabetes Study IV. Therapeutic requirements to maintain tight blood pressure control. Diabetologia 1996;39(12):1554-61. [DOI] [PubMed] [Google Scholar]
HOMED‐BP 2012 {published data only}
- Asayama K, Ohkubo T, Metoki H, Obara T, Inoue R, Kikuya M, et al. Cardiovascular outcomes in the first trial of antihypertensive therapy guided by self-measured home blood pressure. Hypertension Research 2012;35:1102-10. [DOI] [PubMed] [Google Scholar]
JATOS 2008 {published data only}
- JATOS Study Group. Principal results of the Japanese Trial to Assess Optimal Systolic blood pressure in elderly hypertensive patients (JATOS). Hypertension Research 2008;31(12):2115-27. [DOI] [PubMed] [Google Scholar]
Lewis 1999 {published data only}
- Lewis JB, Berl T, Bain RP, Rohde RD, Lewis EJ. Effect on intensive blood pressure control on the course of type 1 diabetic nephropathy. American Journal of Kidney Diseases 1999;34(5):809-17. [DOI] [PubMed] [Google Scholar]
MDRD 1995 {published data only}
- Peterson JC, Adler S, Burkart KM, Greene T, Hebert LA, Hunsicker LG, et al. Blood pressure control, proteinuria, and the progression of renal disease: the Modification of Diet in Renal Disease Study. Annals of Internal Medicine 1995;123(10):754-62. [DOI] [PubMed] [Google Scholar]
- Sarnak MJ, Greene T, Wang X, Beck G, Kusek JW, Collins AJ, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the Modification of Diet in Renal Disease Study. Annals of Internal Medicine 2005;142(5):342-51. [DOI] [PubMed] [Google Scholar]
SANDS 2008 {published data only}
- Howard BV, Roman MJ, Devereux RB, Fleg JL, Galloway JM, Henderson JA, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes. JAMA 2008;299(14):1678-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomon 2010 {published data only}
- Solomon SD, Verma A, Desai A, Hassanein A, Izzo J, Oparil S, et al. Effect of intensive versus standard blood pressure lowering on diastolic function in patients with uncontrolled hypertension and diastolic dysfunction. Hypertension 2010;55(2):241-8. [DOI] [PubMed] [Google Scholar]
Steno‐2 2003 {published data only}
- Goede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. New England Journal of Medicine 2003;348(5):383-93. [DOI] [PubMed] [Google Scholar]
UKPDS 1998 {published data only}
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703-13. [PMC free article] [PubMed] [Google Scholar]
VALISH 2010 {published data only}
- Ogihara T, Saruta T, Rakugi H, Matsuoka H, Shimamoto K, Shimada K, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010;56(2):196-202. [DOI] [PubMed] [Google Scholar]
Wei 2013 {published data only}
- Wei Y, Jin Z, Shen G, Zhao X, Yang W, Zhong Y, et al. Effects of intensive antihypertensive treatment on Chinese hypertensive patients older than 70 years. Journal of Clinical Hypertension (Greenwich) 2013;15(6):420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
AACE 2019
- Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 Executive Summary. Endocrine Practice 2019;25(1):69-100. [DOI] [PubMed] [Google Scholar]
ACC/AHA 2017
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71(6):e13-e115. [DOI] [PubMed] [Google Scholar]
ADA 2016
- American Diabetes Association. Cardiovascular disease and risk management. Diabetes Care 2016;39(Suppl1):S60-71. [DOI] [PubMed] [Google Scholar]
ADA 2019
- American Diabetes Association. Cardiovascular disease and risk management: standards of medical care in diabetes - 2019. Diabetes Care 2019;42(suppl 1):S103-S123. [DOI] [PubMed] [Google Scholar]
Agarwal 2017
- Agarwal R. Implications of blood pressure measurement technique for implementation of Systolic Blood Pressure Intervention Trial (SPRINT). Journal of the American Heart Association 2017;6(2):e004536. [DOI] [PMC free article] [PubMed] [Google Scholar]
AHA 2007
- Rosendorff C, Black HR, Cannon CP, Gersh BJ, Gore J, Izzo JL Jr, et al. Treatment of hypertension in the prevention and management of ischemic heart disease. A scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation 2007;115(21):2761-88. [DOI] [PubMed] [Google Scholar]
Arguedas 2010
- Arguedas JA. Blood pressure targets: are clinical guidelines wrong? Current Opinion in Cardioliology 2010;25(4):350-4. [DOI] [PubMed] [Google Scholar]
Arguedas 2013
- Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No: CD008277. [DOI: 10.1002/14651858.CD008277.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
ASH/ISH 2014
- Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, et al. Clinical practice guideline for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. Journal of Hypertension 2014;32(1):3-15. [DOI] [PubMed] [Google Scholar]
Attar 2019
- Attar A, Sayadi M, Jannati M. Effect of intensive blood pressure lowering on cardiovascular outcomes based on cardiovascular risk: a secondary analysis of the SPRINT trial. European Journal of Preventive Cardiology 2019;26:238-45. [DOI] [PubMed] [Google Scholar]
Bangalore 2010
- Bangalore S, Messerli FH, Wun CC, Zuckerman AL, DeMicco D, Kostis JB, et al. J-curve revisited: an analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. European Heart Journal 2010;31(23):2897-908. [DOI] [PubMed] [Google Scholar]
Bangalore 2017
- Bangalore S, Toklu B, Gianos E, Schwartzbard A, Weintraub H, Ogedegbe G, et al. Optimal systolic blood pressure target after SPRINT: Insights from a network meta-analysis of randomized trials. Annals of Internal Medicine 2017;130:707-19. [DOI] [PubMed] [Google Scholar]
Bassler 2010
- Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, et al. Stopping randomized trials early for benefit and estimation of treatment effects. Systematic review and meta-regression analysis. JAMA 2010;303:1180-7. [DOI] [PubMed] [Google Scholar]
BHS 2004
- Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF, et al, BHS guidelines working party, for the British Hypertension Society. British Hypertension Society Guidelines for hypertension management 2004 (BHS-IV): summary. BMJ 2004;328:634-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
BPLTTC 2003
- Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003;362:1527-35. [DOI] [PubMed] [Google Scholar]
BPLTTC 2014
- Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet 2014;384:591-8. [DOI] [PubMed] [Google Scholar]
Brunstrom 2016
- Brunstrôm M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ 2016;352:i717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bundy 2017
- Bundy JA, Li C, Stuchlik P, Bu X, Kelly TN, Mills KT, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality. A systematic review and network meta-analysis. JAMA Cardiology 2017;2(2):775-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chi 2018
- Chi G, Jamil A, Jamil U, Balouch MA, Marszalek J, Kahe F, et al. Effect of intensive versus standard blood pressure control on major adverse cardiac events and serious adverse events: a bivariate analysis of randomized controlled trials. Clinical and Experimental Hypertension 2018;40:160-7. [DOI: 10.1080/10641963.2018.1462373] [DOI] [PubMed] [Google Scholar]
Cipriani 2013
- Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Annals of Internal Medicine 2013;159:130-7. [DOI] [PubMed] [Google Scholar]
Diao 2012
- Diao D, Wright JM, Cundiff DK, Gueyffier F. Pharmacotherapy for mild hypertension. Cochrane Database of Systematic Reviews 2012, Issue 8. Art. No: CD006742. [DOI: 10.1002/14651858.CD006742.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dorresteijn 2012
- Dorresteijn JA, Graaf Y, Spiering W, Grobbee DE, Bots ML, Visseren FL, Secondary Manifestations of Arterial Disease Study Group. Relation between blood pressure and vascular events and mortality in patients with manifest vascular disease. J-curve revisited. Hypertension 2012;59(1):14-21. [DOI] [PubMed] [Google Scholar]
Drazen 2015
- Drazen JM, Morrissey S, Campion EW, Jarcho JA. A SPRINT to the finish. New England Journal of Medicine 2015;373:2174-5. [DOI] [PubMed] [Google Scholar]
ESC/ESH 2018
- The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). 2018 ESC/ESH Guidelines for the management of arterial hypertension. European Heart Journal 2018;39:3021-104. [DOI] [PubMed] [Google Scholar]
ESH‐ESC 2007
- Mancia G, De Backer G, Domiiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the management of arterial hypertension. The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Journal of Hypertension 2007;25(6):1105-87. [DOI] [PubMed] [Google Scholar]
Ettehad 2016
- Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957-67. [DOI] [PubMed] [Google Scholar]
Filippone 2011
- Filippone EJ, Foy A, Newman E. Goal directed antihypertensive therapy: lower may not always be better. Cleveland Clinic Journal of Medicine 2011;78:123-33. [DOI] [PubMed] [Google Scholar]
Grossman 2011
- Grossman E. Blood pressure: the lower, the better. The con side. Diabetes Care 2011;34 (suppl 2):308-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gueyffier 2001
- Gueyffier F. Observational epidemiological studies: values and limitations. Journal of Hypertension 2001;21:673-5. [DOI] [PubMed] [Google Scholar]
Heimark 2018
- Heimark S, Mariampillai JE, Narkiewicz K, Nilsson PM, Kjeldsen SE. Which target blood pressure in year 2018? Evidence form recent clinical trials. High Blood Pressure & Cardiovascular Prevention 2018;25(2):151-8. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Hypertension Canada 2020
- Rabi DE, McBrien KA, Sapir-Pichhadze R, Nakhla M, Ahmed SB, Dumanski SM, et al. Hypertension Canada's 2020 Comprehensive guidelines for the prevention, diagnosis, risk assessment, and treatment of hypertension in adults and children. Canadian Journal of Cardiology 2020;36(5):596-624. [DOI] [PubMed] [Google Scholar]
Jackson 2005
- Jackson R, Lawes CM, Bennett DA, Milne RJ, Rodgers A. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual’s absolute cardiovascular risk. Lancet 2005;365:434-41. [DOI] [PubMed] [Google Scholar]
JNC 7 2003
- Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560-72. [DOI] [PubMed] [Google Scholar]
JNC 8 2014
- James PA, Oparil S, Carter BL, et al. Evidence based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507-20. [DOI] [PubMed] [Google Scholar]
K/DOQI 2004
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. American Journal of Kidney Disease 2004;43 (5 Suppl 1):S1-290. [PubMed] [Google Scholar]
Kannel 1996
- Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA 1996;275:1571-6. [PubMed] [Google Scholar]
Kaul 2018
- Kaul S. How strong is the evidence to support blood pressure treatment goal of 130/80 mm Hg? Circulation 2018;138:2594-6. [DOI] [PubMed] [Google Scholar]
Kernan 2014
- Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45(7):2160-236. [DOI] [PubMed] [Google Scholar]
Kjeldsen 2016
- Kjeldsen SE, Lund-Johansen P, Nilsson PM, Mancia G. Unattended blood pressure measurements in the systolic blood pressure intervention trial: implications for entry and achieved blood pressure values compared with other trials. Hypertension 2016;67(5):808-12. [DOI] [PubMed] [Google Scholar]
Laurent 2004
- Laurent S. Guidelines from the British Hypertension Society. The lower the pressure the better. BMJ 2004;328:593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laurent 2016
- Laurent S, Boutouyrie P. Blood pressure lowering trials: wrapping up the topic? Lancet 2016;387:923-4. [DOI] [PubMed] [Google Scholar]
Law 2009
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lonn 2016
- Lonn EM, Yusuf S. Should patients with cardiovascular risk factors receive intensive treatment of hypertension to < 120/80 mm Hg target? An antagonist view from the HOPE-3 Trial (Heart Outcomes Evaluation-3). Circulation 2016;134:1311-3. [DOI] [PubMed] [Google Scholar]
Lund‐Johansen 2003
- Lund-Johansen P. Intensive blood pressure treatment: beneficial for all but the smoking hypertensives? Journal of Hypertension 2003;21:697-700. [DOI] [PubMed] [Google Scholar]
Lv 2012
- Lv JC, Neal B, Ehteshami P, Ninomiya T, Woodward M, Rodgers A, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: a systematic review and meta-analysis. PLOS Medicine 2012;9:e1001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacMahon 2001
- MacMahon S, Collins R. Reliable assessment of the effects of treatment on mortality and major morbidity, II: observational studies. Lancet 2001;357:455-62. [DOI] [PubMed] [Google Scholar]
Malhotra 2017
- Malhotra R, Nguyen HA, Benavente O, Mete M, Howard BV, Mant J, et al. Association between more intensive vs less intensive blood pressure lowering and risk of mortality in chronic kidney disease stages 3 to 5. A systematic review and meta-analysis. JAMA Internal Medicine 2017;177:1498-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mancia 2011
- Mancia G, Grassi G, Zanchetti A. Antihypertensive treatment and blood pressure in diabetic and non-diabetic patients. The lower the better? Diabetes Care 2011;34 (Suppl 2):304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mancia 2013
- Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension; the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Journal of Hypertension 2013;31(7):1281-357. [DOI] [PubMed] [Google Scholar]
Marianpilla 2016
- Mariampillai JE, Eskås PA, Heimark S, Kjeldsen SE, Narkiewicz K, Mancia G. A case for less intensive blood pressure control: It matters to achieve target blood pressure early and sustained below 140/90 mm Hg. Progress in Cardiovascular Diseases 2016;59:209-18. [DOI] [PubMed] [Google Scholar]
Musini 2019
- Musini VM, Tejani AM, Bassett K, Puil L, Wright JM. Pharmacotherapy for hypertension in adults 60 years or older. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD000028. [DOI: 10.1002/14651858.CD000028.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management: clinical guideline 127. https://www.nice.org.uk/guidance/cg127/chapter/1-recommendations (accessed Jan 15, 2016).
NICE 2019
- National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. www.nice.org.uk/guidance/ng136, 2019. [PubMed] [Google Scholar]
ONTARGET 2008
- ONTARGET Investigators. Telmisartan, ramipril or both in patients at high risk for vascular events. New England Journal of Medicine 2008;358:1547-59. [DOI] [PubMed] [Google Scholar]
Oparil 2016
- Oparil S, Lewis CE. Should patients with cardiovascular risk factors receive intensive treatment of hypertension to < 120/80 mm Hg target? A protagonist view from the SPRINT Trial (Systolic Blood Pressure Intervention Trial). Circulation 2016;134:1308-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ortiz 2016
- Ortiz E, James PA. Let's not sprint to judgement about new blood pressure goals. Annals of Internal Medicine 2016;164:692-3. [DOI] [PubMed] [Google Scholar]
Parati 2011
- Parati G, Bilo G, Ochoa JE. Benefits of tight blood pressure control in diabetic patients with hypertension. Importance of early and sustained implementation of effective treatment strategies. Diabetes Care 2011;34 (Suppl 2):S297-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perkovic 2015
- Perkovic V, Rodgers A. Redefining blood-pressure targets. SPRINT starts the marathon. New England Journal of Medicine 2015;373:2175-8. [DOI] [PubMed] [Google Scholar]
Prospective Studies Collaboration 2002
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903-13. [DOI] [PubMed] [Google Scholar]
Prospective Studies Collaboration 2007
- Prospective Studies Collaboration. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007;370:1829-39. [DOI] [PubMed] [Google Scholar]
Rapsomaniki 2014
- Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet 2014;383:1899-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saiz 2020
- Saiz LC, Gorricho J, Garjón J, Celaya MC, Erviti J, Leache L. Blood pressure targets for the treatment of people with hypertension and cardiovascular disease. Cochrane Database of Systematic Reviews 2020, Issue 9. Art. No: CD010315. [DOI: 10.1002/14651858.CD010315.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schunemann 2019a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidenceIn: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Schunemann 2019b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Sexton 2017
- Sexton DJ, Canney M, O'Connell MD, Moore P, Little MA, O'Seaghdha CM, et al. Injurious falls and syncope in older community-dwelling adults meeting inclusion criteria for SPRINT. JAMA International Medicine 2017;177:1385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sleight 2009
- Sleight P, Redon J, Verdecchia P, Mancia G, Gao P, Fagard R, et al, ONTARGET investigators. Prognostic value of blood pressure in patients with high vascular risk in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial study. Journal of Hypertension 2009;27:1360-9. [DOI] [PubMed] [Google Scholar]
Stamler 1993
- Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Archives of Internal Medicine 1993;153(5):598-615. [DOI] [PubMed] [Google Scholar]
Stokes 1987
- Stokes J 3rd, Kannel WB, Wolf PA, Cupples LA, D'Agostino RB. The relative importance of selective risk factors for various manifestations of cardiovascular disease among men and women from 35 to 64 years. 30 years of follow-up in the Framingham Study. Circulation 1987;75(Suppl V):V65-V73. [PubMed] [Google Scholar]
Sundström 2015
- Sundström J, Arima H, Jackson R, Turnbull F, Rahimi K, Chalmers J, et al. Effects of blood pressure reduction in mild hypertension: a systematic review and meta-analysis. Annals of Internal Medicine 2015;162:184-91. [DOI] [PubMed] [Google Scholar]
Thomopoulos 2014
- Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 3. Effects in patients at different levels of cardiovascular risk – overview and meta-analyses of randomized trials. Journal of Hypertension 2014;32:2305-14. [DOI] [PubMed] [Google Scholar]
Thomopoulos 2016 a
- Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels – updated overview and meta-analyses of randomized trials. J Hypertension 2016;34:613-22.. [DOI] [PubMed] [Google Scholar]
Thomopoulos 2016 b
- Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering treatment in hypertension: 8. Outcome reductions vs discontinuations because of adverse drug events. Journal of Hypertension 2016;34:1451-63. [DOI] [PubMed] [Google Scholar]
Tsai 2017
- Tsai WC, Wu HY, Peng YS, Yang JY, Chen HY, Chiu YL, et al. Association of intensive blood pressure control and kidney disease progression in nondiabetic patients with chronic kidney disease. A systematic review and meta-analysis. JAMA Intern Med 2017;177:792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verdecchia 2016
- Verdecchia P, Angeli F, Gentile G, Reboldi G. More versus less intensive blood pressure-lowering strategy. Cumulative evidence and trial sequential analysis. Hypertension 2016;68:642-53. [DOI] [PubMed] [Google Scholar]
Viele 2016
- Viele K, McGlothlin A, Broglio K. Interpretation of clinical trials that stopped early. JAMA 2016;315:1646-7. [DOI] [PubMed] [Google Scholar]
Voko 1999
- Vokó Z, Bots ML, Hofman A, Koudstaal PJ, Witteman JC, Breteler MM. J-shaped relation between blood pressure and stroke in treated hypertensives. Hypertension 1999;34(6):1181-5. [DOI] [PubMed] [Google Scholar]
WHO/ISH 2003
- World Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. Journal of Hypertension 2003;21:1983-92. [DOI] [PubMed] [Google Scholar]
Xie 2016
- Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet 2016;387:435-43. [DOI] [PubMed] [Google Scholar]
Yeh 2015
- Yeh JS. Blood-pressure control. New England Journal of Medicine 2015;373:2180-2. [DOI] [PubMed] [Google Scholar]
Zanchetti 2003
- Zanchetti A, Hansson L, Clement D, Elmfeldt D, Julius S, Rosenthal T, et al, HOT Study Group. Benefits and risks of more intensive blood pressure lowering in hypertensive patients of the HOT study with different risk profiles: does a J-shaped curve exist in smokers? Journal of Hypertension 2003;21(4):797-804. [DOI] [PubMed] [Google Scholar]
Zanchetti 2009
- Zanchetti A. Bottom blood pressure or bottom cardiovascular risk? How far can cardiovascular risk be reduced? Journal of Hypertension 2009;27:1509-20. [DOI] [PubMed] [Google Scholar]
Zanchetti 2014
- Zanchetti A, Liu L, Mancia G, Parati G, Grassi G, Stramba-Badiale M, et al. Blood pressure and low-density lipoprotein-cholesterol lowering for prevention of strokes and cognitive decline: a review of available trial evidence. Journal of Hypertension 2014;32(9):1741-50. [DOI] [PubMed] [Google Scholar]
Zanchetti 2015
- Zanchetti A, Thomopoulos C, Parati G. Randomized controlled trials of blood pressure lowering in hypertension. A critical reapparaisal. Circulation Research 2015;116(6):1058-73. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Arguedas 2003
- Arguedas JA, Perez MI, Wright JM. Treatment blood pressure targets for hypertension. Cochrane Database of Systematic Reviews 2003, Issue 3. Art. No: CD004349. [DOI: 10.1002/14651858.CD004349] [DOI] [PubMed] [Google Scholar]
Arguedas 2004
- Arguedas JA. Blood pressure targets in the treatment of patients with elevated blood pressure. Master's Thesis, Department of Pharmacology & Therapeutics, University of British Columbia July 2004.
Arguedas 2009
- Arguedas JA, Perez MI, Wright JM. Treatment blood pressure targets for hypertension. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD004349. [DOI: 10.1002/14651858.CD004349] [DOI] [PubMed] [Google Scholar]


