Abstract
Background
The setting in which induction of labour takes place (home or inpatient) is likely to have implications for safety, women's experiences and costs.
Home induction may be started at home with the subsequent active phase of labour happening either at home or in a healthcare facility (hospital, birth centre, midwifery‐led unit). More commonly, home induction starts in a healthcare facility, then the woman goes home to await the start of labour. Inpatient induction takes place in a healthcare facility where the woman stays while awaiting the start of labour.
Objectives
To assess the effects on neonatal and maternal outcomes of third trimester home induction of labour compared with inpatient induction using the same method of induction.
Search methods
For this update, we searched the Cochrane Pregnancy and Childbirth Group's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (31 January 2020)), and reference lists of retrieved studies.
Selection criteria
Published and unpublished randomised controlled trials (RCTs) in which home and inpatient settings for induction have been compared. We included conference abstracts but excluded quasi‐randomised trials and cross‐over studies.
Data collection and analysis
Two review authors independently assessed study reports for inclusion. Two review authors carried out data extraction and assessment of risk of bias independently. GRADE assessments were checked by a third review author.
Main results
We included seven RCTs, six of which provided data on 1610 women and their babies. Studies were undertaken between 1998 and 2015, and all were in high‐ or upper‐middle income countries. Most women were induced for post dates. Three studies reported government funding, one reported no funding and three did not report on their funding source. Most GRADE assessments gave very low‐certainty evidence, downgrading mostly for high risk of bias and serious imprecision.
1. Home compared to inpatient induction with vaginal prostaglandin E (PGE) (two RCTs, 1028 women and babies; 1022 providing data).
Although women's satisfaction may be slightly better in home settings, the evidence is very uncertain (mean difference (MD) 0.16, 95% confidence interval (CI) ‐0.02 to 0.34, 1 study, 399 women), very low‐certainty evidence.
There may be little or no difference between home and inpatient induction for other primary outcomes, with all evidence being very low certainty:
‐ spontaneous vaginal birth (average risk ratio (RR) [aRR] 0.91, 95% CI 0.69 to 1.21, 2 studies, 1022 women, random‐effects method);
‐ uterine hyperstimulation (RR 1.19, 95% CI 0.40 to 3.50, 1 study, 821 women);
‐ caesarean birth (RR 1.01, 95% CI 0.81 to 1.28, 2 studies, 1022 women);
‐ neonatal infection (RR 1.29, 95% CI 0.59 to 2.82, 1 study, 821 babies);
‐ admission to neonatal intensive care unit (NICU) (RR 1.20, 95% CI 0.50 to 2.90, 2 studies, 1022 babies).
Studies did not report serious neonatal morbidity or mortality.
2. Home compared to inpatient induction with controlled release PGE (one RCT, 299 women and babies providing data).
There was no information on whether the questionnaire on women's satisfaction with care used a validated instrument, but the findings presented showed no overall difference in scores.
We found little or no difference between the groups for other primary outcomes, all also being very low‐certainty evidence:
‐ spontaneous vaginal birth (RR 0.94, 95% CI 0.77 to 1.14, 1 study, 299 women);
‐ uterine hyperstimulation (RR 1.01, 95% CI 0.51 to 1.98, 1 study, 299 women);
‐ caesarean births (RR 0.95, 95% CI 0.64 to 1.42, 1 study, 299 women);
‐ admission to NICU (RR 1.38, 0.57 to 3.34, 1 study, 299 babies).
The study did not report on neonatal infection nor serious neonatal morbidity or mortality.
3. Home compared to inpatient induction with balloon or Foley catheter (four RCTs; three studies, 289 women and babies providing data).
It was again unclear whether questionnaires reporting women's experiences/satisfaction with care were validated instruments, with one study (48 women, 69% response rate) finding women were similarly satisfied.
Home inductions may reduce the number of caesarean births, but the data are also compatible with a slight increase and are of very low‐certainty (RR 0.64, 95% CI 0.41 to 1.01, 2 studies, 159 women).
There was little or no difference between the groups for other primary outcomes with all being very low‐certainty evidence:
‐ spontaneous vaginal birth (RR 1.04, 95% CI 0.54 to 1.98, 1 study, 48 women):
‐ uterine hyperstimulation (RR 0.45, 95% CI 0.03 to 6.79, 1 study, 48 women);
‐ admission to NICU (RR 0.37, 95% CI 0.07 to 1.86, 2 studies, 159 babies).
There were no serious neonatal infections nor serious neonatal morbidity or mortality in the one study (involving 48 babies) assessing these outcomes.
Authors' conclusions
Data on the effectiveness, safety and women's experiences of home versus inpatient induction of labour are limited and of very low‐certainty. Given that serious adverse events are likely to be extremely rare, the safety data are more likely to come from very large observational cohort studies rather than relatively small RCTs.
Plain language summary
Home versus inpatient induction of labour
What is the issue?
We wanted to find out from randomised controlled trials, (RCTs) whether, after induction of labour in a hospital or healthcare facility, women preferred to go home or stay in the facility to await the start of labour. Also, to know if there was any impact on clinical outcomes for either the women or their babies.
Why is this important?
Induction of labour towards the end of pregnancy involves artificially bringing on contractions to start labour. There are risks for mother and baby from induction, but sometimes these are outweighed by the risks of continuing the pregnancy.
However, induction can be a challenging experience for women as they may feel uncomfortable, unsupported and a lack of control. The use of home induction of labour may improve women’s experiences, reduce the length of stay in hospital and lower overall costs. The safety of both the mother and baby are critical factors for consideration. Only certain forms of induction are considered suitable for home induction, for example, vaginal prostaglandins or balloon/Foley catheters.
What evidence did we find?
We searched for evidence on 31 January 2020 and found seven RCTs, six of which provided data on 1610 women and their babies. These studies were all undertaken in income‐rich countries. The certainty of the evidence was mostly very low, mainly because of the limited number of studies, some of which were small, and there was lack of clarity in the study design.
The women all received the induction with initial monitoring in hospital. Women in the home induction group were then able to go home to wait for the start of active labour, or for a set period of time. Women in the inpatient group stayed in hospital.
With vaginal prostaglandin (PGE2) for induction, we found two studies with 1022 women and their babies. There may be little or no difference in women's satisfaction between waiting for labour to become active at home or in hospital, although women tended to be more satisfied with going home to wait. For women, there may be no clear differences in the number who had a spontaneous vaginal birth, overstimulation of the uterus or a caesarean birth. For the babies, there may be a similar incidence of infection and admission to neonatal intensive care unit (NICU). The costs may possibly be less in home settings.
For induction with controlled release prostaglandin (PGE2) into the vagina, we found just one study of 299 women and their babies but the findings indicate probably little or no difference.
Using a balloon or Foley catheter for induction, we found three studies providing data on 289 women and their babies. Two studies reported on women’s satisfaction, and showed a tendency to favour home settings, but the way data were collected was unclear. There may be little or no difference in the number of spontaneous vaginal births, overstimulation of uterine contractions and babies admitted to NICU. Home induction may possibly reduce the number of caesarean births but more data are needed.
What does this mean?
The studies did not include sufficient numbers of women and babies to show clear differences in outcomes between home and inpatient induction of labour, and the certainty of the evidence was generally very low. More studies are needed, and further studies are already underway. We need more data on women's experiences and views on their care, as well as on safety and cost.
Summary of findings
Background
For most women, the onset of labour and childbirth is spontaneous. However, induction of labour is a relatively common occurrence, with approximately one in four pregnancies worldwide ending with an induction of labour (WHO 2011). With recent studies reporting that induction of labour for post‐term pregnancy results in a lower risk of caesarean section than expectant management, this trend in induction is set to continue (Grobman 2018; Middleton 2018). While the indications and methods of induction of labour have been the subject of randomised controlled trials, systematic reviews and clinical guidelines, there is an absence of evidence on the setting (home versus hospital/healthcare facility) in which induction takes place, a factor that may have significant implications for women's satisfaction and for cost (Kelly 2013).
The optimal method of induction of labour would lead to cervical ripening and a ‘spontaneous’ onset of labour, without increasing the risk of maternal or fetal complications (Calder 1998). Currently, labour may be induced, at term, using pharmacological (e.g. prostaglandins, oxytocin, misoprostol), mechanical and physical methods (e.g. rupture of membranes) or complementary/alternative methods (e.g. breast stimulation) (Alfirevic 2016; Hofmeyr 2009).
Pharmacological methods are the most common methods of induction of labour. However, pharmacological methods are not suitable for all women and can lead to increased risk of hyperstimulation, and very rarely uterine rupture (NICE 2008; WHO 2011). Traditionally, pharmacological methods of induction of labour were thought to require intermittent or continuous fetal monitoring in an inpatient setting, and so were not suitable for home induction. However, some forms of induction, both pharmacological (e.g. vaginal prostaglandins) and mechanical (e.g. Foley catheter) can be considered appropriate for the woman to return home once induction is started but labour is yet to begin (Vogel 2017). Membrane sweep is generally not considered a formal method of induction of labour but is an intervention sometimes used at home or in an outpatient setting. Membrane sweeping would not normally be used on its own as a method of induction of labour in an inpatient setting (Finucane 2020).
The use of home induction of labour potentially supports maternal satisfaction and autonomy, convenience, reduced length of stay in hospital and significant cost savings (NICE 2008; Oster 2011 ‐ part of Wilkinson 2015 ‐ OPRA; Wong 2002). Induction of labour is reported as being a challenging experience for women where they may feel a lack of control, unsupported and uncomfortable in their environment (Coates 2019). Women often find hospital a noisy busy place with a lack of privacy and rules imposed, hence the possibility of spending time at home after initiation of induction may be appealing to some women (Coates 2019). Benefits which must be balanced with maintaining maternal and fetal safety.
Description of the condition
Induction of labour should only be performed when there are clear indications that continuing with a pregnancy is of greater risk to the mother or baby than the risk of induction of labour (ACOG 2009; WHO 2011). Induction of labour is performed for a number of reasons towards the end of pregnancy, with induction of labour for post‐term (mainly after 41 weeks) the most common of these (Kelly 2013; Nippita 2015; SOGC 2013; Sue‐A‐Quan 1999). A pregnancy is deemed full term at 37 completed weeks' gestation, however, up to 10% of pregnancies will continue past 42 weeks’ gestation and are then considered “post‐term” (Finucane 2020; Middleton 2018).
Description of the intervention
Induction of labour involves artificially stimulating uterine contractions to initiate the onset of labour (Hofmeyr 2009; WHO 2011). Induction of labour is usually performed in hospital/facility settings using a range of interventions (Kelly 2013). Often involving the use of vaginal prostaglandin agents (Kelly 2013). There has been an increased interest in the use of home induction of labour in recent times. In this scenario, women are either induced at home or more commonly attend the hospital/clinic/healthcare facility to receive the induction agent and initial assessment of fetal well‐being then return home afterwards. They return to the hospital or healthcare facility when they start to contract regularly or at a given time point (unless the woman wishes a home birth). If they experience any adverse reactions, including hyperstimulation, they return to the hospital/healthcare facility straight away.
For the purpose of this review, home induction is defined as induction at home, or more commonly, after the induction process has been started in a hospital/healthcare facility the women spends time at home. Inpatient inductions are defined as induction in healthcare facilities (hospitals or birth centres, or midwifery‐led units), where the woman remains there following induction and awaiting the start of labour.
How the intervention might work
Home induction of labour potentially offers improved maternal satisfaction, autonomy and choice when compared to hospital/facility‐based policies. Home induction of labour is a potentially an efficient, low‐cost method of induction of labour.
Why it is important to do this review
Approximately twenty‐five per cent or more of all pregnancies will end in an induction of labour. Home induction of labour potentially offers a low‐cost alternative to hospital‐based induction of labour for low‐risk pregnancies, while potentially supporting choice and autonomy for women. This systematic review evaluates the available evidence to assess the efficacy and women's experiences of outpatient induction of labour compared to inpatient settings.
Objectives
To assess the effects on neonatal and maternal outcomes of third trimester home induction of labour compared with inpatient induction using the same method of induction.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished randomised controlled trials (RCTs), which compare home versus inpatient/hospital/healthcare facility settings for induction of labour. We planned to include cluster‐randomised trials (but did not identify any), but we excluded quasi‐randomised trials and cross‐over studies. We included conference abstracts.
Types of participants
Pregnant women at term (equal to or greater than 37 weeks' gestation) carrying a live baby where the woman was considered suitable for induction of labour at home, or she was able to go home after the induction to await labour.
Types of interventions
Pharmacological agents or mechanical methods of induction of labour suitable for home inductions, for example, prostaglandins, or balloon or Foley catheters. We excluded membrane sweep as this is not considered a formal method of induction and we believe there are no circumstances where membrane sweep would be carried out in an inpatient/hospital/healthcare facility setting as this would involve the woman staying overnight in the inpatient setting until labour starts (Finucane 2020).
We chose to pool data from different prostaglandins (Thomas 2014). We have kept induction with controlled‐release prostaglandin E (CR‐PGE) separate from prostaglandin induction with gel or suppository because with CR‐PGE, the PGE can be removed if complications arise (Lyrenas 2001). We have chosen to keep induction with single (generally Foley catheter) and double balloon catheters together as recent evidence indicates both kinds of balloon catheter have similar efficacy and women's satisfaction (Liu 2019).
Home induction is defined as: induction at home or where induction is carried out in healthcare facility (hospital, birth centre, midwifery‐led unit, clinic) then the women goes home to await the start of labour.
Inpatient settings are defined as: healthcare facilities, hospitals, birth centres or midwifery‐led units where the woman remains there following induction and awaiting the start of labour.
We only included studies where the same intervention for induction of labour is used in both settings.
This review does not attempt to compare the relative effects of different methods of induction of labour on maternal and neonatal outcomes within an outpatient setting. This is the topic of a separate review (Vogel 2017).
Types of outcome measures
For this update, we have chosen outcomes from the core outcome set (COS) from a recent publication (Dos Santos 2018). We separated the mode of birth into spontaneous vaginal birth, caesarean birth and instrumental vaginal birth. We have added assessments of women's experiences/satisfaction, pain, her sense of control and any cost‐effectiveness as defined by trialists.
Previous versions of this review used clinically relevant outcomes from the induction of labour generic protocol (Hofmeyr 2009), and the review assessing methods of outpatient induction of labour (Vogel 2017).
Primary outcomes
Spontaneous vaginal birth (noting data for within 24 hours or within 48 to 72 hours if reported) (COS)
Uterine hyperstimulation (with or without fetal heart (FHR) changes ‐ noting data for with or without FHR changes if reported) (COS)
Caesarean birth (COS)
Neonatal infection (COS) (up to 28 days after the birth)
Admission to neonatal intensive care unit (NICU) (COS)
Serious neonatal morbidity or mortality (e.g. seizures, birth asphyxia defined by trialists, birth trauma, neonatal encephalopathy, need for therapeutic hypothermia, disability in childhood) (COS) up to 28 days after the birth
Women's experiences/satisfaction with care (using only validated instruments) (COS) up to eight weeks after the birth
Secondary outcomes
Measures of effectiveness and satisfaction
Oxytocin administration (COS)
Pain ‐ self‐assessment
Spinal analgesia
Opioid analgesia
No pharmacological analgesia
Woman's sense of control
Need for more than one induction agent
Time from induction to birth (COS)
Length of hospital stay
Use of emergency services
Complications for the baby
Apgar score less than seven at five minutes
Meconium aspiration (COS)
Need for respiratory support (COS)
Perinatal mortality (COS)
Complications for the mother
Instrumental vaginal birth (COS)
Uterine scar dehiscence/rupture (COS)
Postpartum haemorrhage (PPH) (≥ 500 mL or as defined by trialists) (COS)
Hysterectomy (COS)
Maternal infection (COS)
Serious maternal morbidity or mortality (e.g. uterine rupture, admission to intensive care, pulmonary embolus, septicaemia, cardiorespiratory arrest) (COS) up to 28 days after the birth
Postnatal depression (COS) up to a year after the birth
Long‐term operative pelvic floor repair (COS) up to a year after the birth
Additional outcomes
Economic aspects as defined by trialists
Where formal economic evaluation was lacking, we attempted to describe potential cost savings and the impact of interventions used within an outpatient setting. Where possible, these estimates involved using some measures of effectiveness and complications in combination with estimates of healthcare provision.
Detailed definitions for outcomes
Perinatal and maternal morbidity and mortality are composite outcomes. This is not an ideal solution because some components are clearly less severe than others. It is possible for one intervention to cause more deaths but less severe morbidity. However, in the context of labour induction at term, this is unlikely. All these events will be rare, and a modest change in their incidence will be easier to detect if composite outcomes are presented. Incidence of individual components were explored as secondary outcomes (see above).
'Uterine rupture' includes all clinically significant ruptures of unscarred or scarred uteri. Trivial scar dehiscence noted incidentally at the time of surgery is excluded.
The terminology of uterine hyperstimulation is problematic (Curtis 1987). In the review, the term 'uterine hyperstimulation' is defined as uterine tachysystole (more than five contractions per 10 minutes for at least 20 minutes) and uterine hypersystole/hypertonus (a contraction lasting at least two minutes).
'Uterine hyperstimulation with FHR changes' is usually defined as uterine hyperstimulation syndrome (tachysystole or hypersystole with FHR changes such as persistent decelerations, tachycardia or decreased short‐term variability). However, due to varied reporting, there is the possibility of subjective bias in the interpretation of these outcomes. Also, it is not always clear from the trials if these outcomes are reported in a mutually exclusive manner. More importantly, continuous monitoring is not available in a home setting. Therefore, there is a risk of biased reporting of uterine hyperstimulation (with or without FHR changes). It is possible that bias will favour the home setting (i.e. by failure to recognise mild forms of hyperstimulation without continuous monitoring). On the other hand, clinicians who favour inpatient induction may, in the absence of continuous monitoring, label any maternal description of painful, frequent uterine contractions as hyperstimulation. Therefore, in the absence of blinding, hyperstimulation and other 'soft' outcomes should be interpreted with extreme caution.
If there are multiple time points for an outcome, we chose the latest time unless there was a clinical reason to use a specific time.
For outcomes using questionnaires, we used data in the data and analysis only if validated instruments were used (Nilvér 2017).
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (31 January 2020).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed; Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (31 January 2020) using the search methods detailed in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeKelly 2013.
For this update, the following methods were used for assessing the 37 reports (covering 21 studies) that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the certainty of the evidence using the GRADE approach
For this update the certainty of the evidence was assessed using the GRADE approach as outlined in the GRADE handbook in order to assess the certainty of the body of evidence relating to the following outcomes for the main comparisons.
Spontaneous vaginal birth
Uterine hyperstimulation
Caesarean birth
Neonatal infection
Admission to NICU
Serious neonatal morbidity or mortality
Women's experiences (satisfaction with care)
We used GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of certainty for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias. The findings were reported according to the GRADE guidance (Santesso 2020).
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion in this review, but we may include trials of this type in future updates. If we do, we plan to include cluster‐randomised trials in the analyses along with individually‐randomised trials. Their sample sizes will be adjusted using the methods described in Gates 2005 using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources are used, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We will also acknowledged heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials were not eligible for inclusion in this review.
Dealing with missing data
For included studies,we noted levels of attrition. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
We changed the reporting of Wilkinson 2015 ‐ OPRA (Wilkinson 2012 in previous version of review, Kelly 2013) from 'per protocol' to 'intention‐to‐treat analysis'.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 50% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Had we identified substantial heterogeneity (above 50%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we will considered the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
In future updates, if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses:
nulliparous women versus multiparous women;
membrane status (intact versus ruptured);
cervical status (unfavourable versus favourable or undefined);
induction indication, i.e. post‐dates (41 weeks or greater).
Subgroup analyses would have been restricted to the review's primary outcomes.
We planned to assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We would have reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value, but we had insufficient numbers of studies in each comparison to be able to undertake subgroup analyses.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effects of the following: 1) trial quality (high quality being low risk of selection and attrition bias); 2) the effect of including of conference abstracts in order to assess whether these make any difference to the overall result. This was in addition to the sensitivity analyses for units of analyses and missing data. However, there were insufficient data to undertake sensitivity analyses.
Results
Description of studies
Results of the search
See: Figure 1
1.
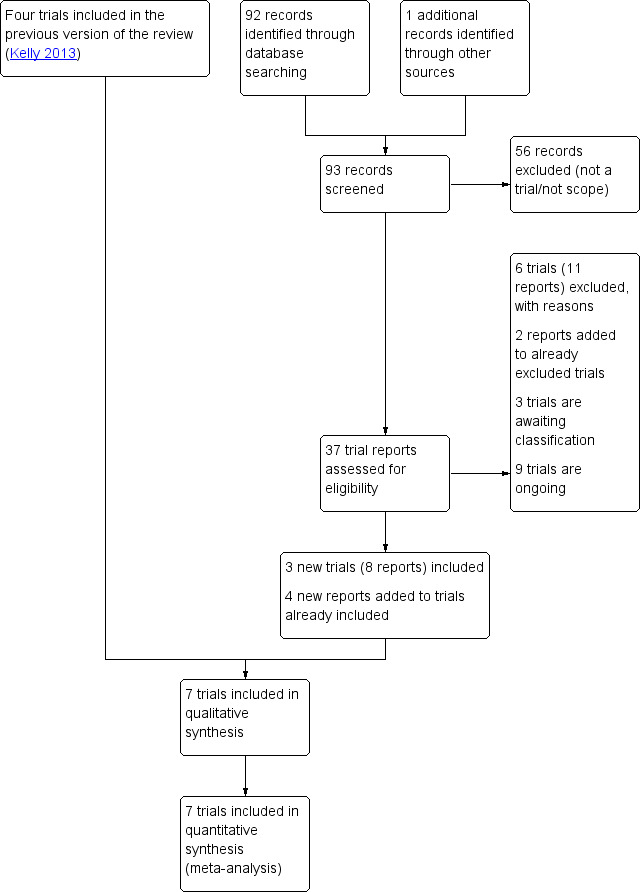
Study flow diagram.
We retrieved 37 new reports. We included three new studies (eight reports). Three were additional reports of already included studies. One of these was the full publication so the study date was changed (Wilkinson 2015 ‐ OPRA). We excluded six new studies (11 reports). A further two reports were part of two studies already excluded in the 2013 publication of this review (Kelly 2013).
There are, therefore, three new included studies, making seven studies in total (Included studies); six newly excluded studies making eight excluded studies in total (Excluded studies); three studies in awaiting classification (Studies awaiting classification), and nine studies are trial registrations for ongoing studies (Ongoing studies).
Included studies
The review now includes seven studies, although one study provided no usable data (Mohamad 2018), leaving six studies providing data on 1610 women and their babies (Biem 2003; Policiano 2017; Ryan 1998; Sciscione 2001; Wilkinson 2015 ‐ OPRA; Wilkinson 2015a ‐ COPRA). Two studies were published as a conference abstracts only (Mohamad 2018; Ryan 1998).
Two studies used vaginal prostaglandin (PGE2) induction (Ryan 1998; Wilkinson 2015 ‐ OPRA), one study used controlled‐release vaginal prostaglandin (Biem 2003), and four studies used balloon or Foley catheters for induction (Mohamad 2018; Policiano 2017; Sciscione 2001; Wilkinson 2015a ‐ COPRA).
Two studies were undertaken in Canada (Biem 2003; Ryan 1998); two in Australia (Wilkinson 2015 ‐ OPRA; Wilkinson 2015a ‐ COPRA); and one study each in the USA (Sciscione 2001), Malasyia (Mohamad 2018), and Portugal (Policiano 2017). See Characteristics of included studies.
Six of the seven studies reported studies taking place between 1998 and 2015 and the duration was about 12 to 18 months. One study did not report on the dates of the study (Ryan 1998). See Characteristics of included studies.
Three studies reported the source of their funding (Biem 2003; Wilkinson 2015 ‐ OPRA; Wilkinson 2015a ‐ COPRA), and all three studies were funded through grants from funding bodies and none reported commercial funding. One study reported having no funding (Policiano 2017); three studies did not report on funding sources (Mohamad 2018; Ryan 1998; Sciscione 2001).
Three studies reported no conflict of interest (Policiano 2017; Wilkinson 2015 ‐ OPRA; Wilkinson 2015a ‐ COPRA). Four studies did not mention conflict of interest (Biem 2003; Mohamad 2018; Ryan 1998; Sciscione 2001).
The interventions examined in the seven studies all involved induction and initial monitoring in hospital, with subsequent discharge home to await the start of labour or for a fixed period of time for women in the home induction group. The comparators were all with induction, labour and birth in hospital.
Excluded studies
Eight studies were excluded from the review. Seven studies were excluded because they compared two different methods of induction of labour between home and inpatient settings (Austin 2015; Beckmann 2020; Henry 2011; Kuper 2018; Rijnders 2011; Torbenson 2015; Wise 2020). One study was excluded because it compared vaginal misoprostol versus placebo and all women went home after the induction (PonMalar 2017).
Risk of bias in included studies
See Figure 2 and Figure 3 for a summary of 'Risk of bias' assessments.
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four studies were low risk of bias for both sequence generation and allocation concealment (Biem 2003; Policiano 2017; Wilkinson 2015 ‐ OPRA; Wilkinson 2015a ‐ COPRA). One study was low risk of bias for sequence generation but unclear for allocation concealment (Sciscione 2001). Two studies were unclear risk of bias for both sequence generation and allocation concealment (Mohamad 2018; Ryan 1998). See Characteristics of included studies.
Blinding
With interventions where management in different settings are compared, it is not feasible to blind study participants to group allocation, and in the seven included studies blinding of the outcome assessors was not reported and as this would take considerable effort. We have taken this to mean these studies were also high risk of bias in outcome assessments. The lack of blinding introduces the potential for bias in the subjective outcomes in these trials and this should be kept in mind when interpreting the results. See Characteristics of included studies.
Incomplete outcome data
Five studies were low risk of attrition bias (Biem 2003; Policiano 2017; Sciscione 2001; Wilkinson 2015 ‐ OPRA; Wilkinson 2015a ‐ COPRA). Two studies were unclear on this assessment (Mohamad 2018; Ryan 1998). See Characteristics of included studies.
Selective reporting
We judged none of the studies to be low risk of selective reporting bias. We assessed four studies to be of unclear risk (Biem 2003; Mohamad 2018; Ryan 1998; Sciscione 2001), and three studies to be high risk of selective reporting bias as there were outcomes reported which were not listed in the methods and also incomplete reporting of some outcomes (Policiano 2017; Wilkinson 2015 ‐ OPRA; Wilkinson 2015a ‐ COPRA). See Characteristics of included studies.
Other potential sources of bias
We assessed two studies as low risk of other biases (Biem 2003; Wilkinson 2015a ‐ COPRA), and five studies as unclear (Mohamad 2018; Policiano 2017; Ryan 1998; Sciscione 2001; Wilkinson 2015 ‐ OPRA).
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Home compared to inpatient induction with vaginal PGE for improving birth outcomes.
| Home compared to inpatient induction with vaginal PGE for improving birth outcomes | ||||||
| Patient or population: women having induction of labour at term Setting: high‐income countries Intervention: home induction with vaginal PGE Comparison: inpatient induction with vaginal PGE | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | Comments | ||
| Without outpatient | With outpatient | Difference | ||||
| Spontaneous vaginal birth № of participants: 1022 (2 RCTs) | RR 0.91 (0.69 to 1.21) | Study population | ⊕⊝⊝⊝ VERY LOW 1 2 3 | |||
| 56.9% | 51.8% (39.3 to 68.9) | 5.1% fewer (17.6 fewer to 12 more) | ||||
| Uterine hyperstimulation № of participants: 821 (1 RCT) | RR 1.19 (0.40 to 3.50) | Study population | ⊕⊝⊝⊝ VERY LOW 4 5 6 | |||
| 1.4% | 1.7% (0.6 to 5.1) | 0.3% more (0.9 fewer to 3.6 more) | ||||
| Caesarean birth № of participants: 1022 (2 RCTs) | RR 1.01 (0.81 to 1.28) | Study population | ⊕⊝⊝⊝ VERY LOW 7 8 | |||
| 21.7% | 21.9% (17.6 to 27.8) | 0.2% more (4.1 fewer to 6.1 more) | ||||
| Neonatal infection (up to 28 days) № of participants: 821 (1 RCT) | RR 1.29 (0.59 to 2.82) | Study population | ⊕⊝⊝⊝ VERY LOW 4 5 6 | |||
| 2.7% | 3.4% (1.6 to 7.5) | 0.8% more (1.1 fewer to 4.8 more) | ||||
| Admission to NICU № of participants: 1022 (2 RCTs) | RR 1.2 (0.5 to 2.90) | Study population | ⊕⊝⊝⊝ VERY LOW 5 9 | |||
| 1.7% | 2.1% (0.9 to 5) | 0.3% more (0.9 fewer to 3.3 more) | ||||
| Serious neonatal morbidity or mortality (up to 28 days) № of participants: (0 RCTs) | not pooled | Study population | ||||
| not pooled | not pooled | not pooled | ||||
| Women's experiences (satisfaction with care) (up to 8 weeks) № of participants: 399 (1 RCT) | ‐ | The mean women's experiences (satisfaction with care) without outpatient was 0 | ‐ | MD 0.16 higher (0.02 lower to 0.34 higher) | ⊕⊝⊝⊝ VERY LOW 4 10 11 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; NICU: neonatal intensive care unit; PGE: prostaglandin E; RCT: randomised controlled trial; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level for serious limitations in risk of bias: high risk of blinding bias and selective reporting bias. Downgraded one level for serious indirectness: 1 study providing 57% of data involving 48% of women not receiving the intervention. .
2 Downgraded one level for serious heterogeneity: I2 = 77%.
3 No downgrade: although a wide CI and not a large number of participants, there are a large number of events ‐ borderline decision.
4 Downgraded two levels for very serious limitations in risk of bias: high risk of blinding bias, selective reporting bias and 100% of data involved 48% of women not receiving the intervention.
5 Downgraded two levels for very serious imprecision: very few events and wide confidence interval. .
6 No downgrade for lack of generalisability: data from only one study but of reasonable size.
7 Downgraded two levels for very serious limitations in risk of bias: high risk of blinding bias, selective reporting bias and 85% of data from study with 48% of participants not receiving the intervention.
8 Downgraded one level for serious imprecision: low number of participants and events, but reasonable confidence interval ‐ borderline decision
9 Downgraded two levels for very serious risk of bias: high risk of blinding bias (100%). 66% of data from study with unclear selection bias. 34% of data from study with high risk of selective reporting bias and 48% of participants not receiving the intervention.
10 Downgraded one level for serious imprecision: insufficient number of participants.
11 Downgraded one level for lack of generalisability: data from 1 study so lack of generalisability. Borderline decision.
Summary of findings 2. Home compared to inpatient induction with controlled release PGE for improving birth outcomes.
| Home compared to inpatient induction with controlled release PGE for improving birth outcomes | ||||||
| Patient or population: women having induction of labour at term Setting: one high‐income country Intervention: home induction with controlled release PGE Comparison: inpatient induction with controlled release PGE | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | Comments | ||
| Without outpatient | With outpatient | Difference | ||||
| Spontaneous vaginal birth № of participants: 299 (1 RCT) | RR 0.94 (0.77 to 1.14) | Study population | ⊕⊝⊝⊝ VERY LOW 1 2 3 | |||
| 59.3% | 55.8% (45.7 to 67.6) | 3.6% fewer (13.6 fewer to 8.3 more) | ||||
| Uterine hyperstimulation № of participants: 299 (1 RCT) | RR 1.01 (0.51 to 1.98) | Study population | ⊕⊝⊝⊝ VERY LOW 1 2 4 | |||
| 10.0% | 10.1% (5.1 to 19.8) | 0.1% more (4.9 fewer to 9.8 more) | ||||
| Caesarean birth № of participants: 299 (1 RCT) | RR 0.95 (0.64 to 1.42) | Study population | ⊕⊝⊝⊝ VERY LOW 1 2 4 | |||
| 24.7% | 23.4% (15.8 to 35) | 1.2% fewer (8.9 fewer to 10.4 more) | ||||
| Neonatal infection (up to 28 days) № of participants: (0 RCTs) | not pooled | Study population | ||||
| not pooled | not pooled | not pooled | ||||
| Admission to NICU № of participants: 299 (1 RCT) | RR 1.38 (0.57 to 3.34) | Study population | ⊕⊝⊝⊝ VERY LOW 1 2 4 | |||
| 5.3% | 7.4% (3 to 17.8) | 2.0% more (2.3 fewer to 12.5 more) | ||||
| Serious neonatal morbidity or mortality (up to 28 days) № of participants: (0 RCTs) | not pooled | Study population | ||||
| not pooled | not pooled | not pooled | ||||
| Mothers' experiences (satisfaction with care) (up to 8 weeks) № of participants: (0 RCT) |
‐ | The mean mothers' experiences (satisfaction with care) without outpatient was 0 | ‐ | see comment | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; NICU: neonatal intensive care unit; PGE: prostaglandin E; RCT: randomised controlled trial; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level for risk of bias: high risk of blinding bias.
2 Downgraded one level for lack of generalisability: only 1 study of 299 women .
3 Downgraded one level for serious imprecision: low number of participants and events.
4 Downgraded two levels for very serious imprecision: low number of participants, very low events and wide confidence interval.
5 Downgraded one level for serious imprecision: low number of participants..
Summary of findings 3. Home compared to inpatient induction with balloon or Foley catheter for improving birth outcomes.
| Home compared to inpatient induction with balloon or Foley catheter for improving birth outcomes | ||||||
| Patient or population: women having induction of labour at term Setting: high‐income countries Intervention: home induction with balloon or Foley catheter Comparison: inpatient induction with balloon or Foley catheter | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | Comments | ||
| Without outpatient | With outpatient | Difference | ||||
| Spontaneous vaginal birth № of participants: 48 (1 RCT) | RR 1.04 (0.54 to 1.98) | Study population | ⊕⊝⊝⊝ VERY LOW 1 2 3 | |||
| 46.7% | 48.5% (25.2 to 92.4) | 1.9% more (21.5 fewer to 45.7 more) | ||||
| Uterine hyperstimulation № of participants: 48 (1 RCT) | RR 0.45 (0.03 to 6.79) | Study population | ⊕⊝⊝⊝ VERY LOW 1 2 3 | |||
| 6.7% | 3.0% (0.2 to 45.3) | 3.7% fewer (6.5 fewer to 38.6 more) | ||||
| Caesarean birth № of participants: 159 (2 RCTs) | RR 0.64 (0.41 to 1.01) | Study population | ⊕⊝⊝⊝ VERY LOW 3 4 | |||
| 41.5% | 26.6% (17 to 42) | 15.0% fewer (24.5 fewer to 0.4 more) | ||||
| Neonatal infection (up to 28 days) № of participants: 48 (1 RCT) | not estimable | Study population | ‐ | |||
| 0.0% | 0.0% (0 to 0) | 0.0% fewer (0 fewer to 0 fewer) | ||||
| Admission to NICU № of participants: 159 (2 RCTs) | RR 0.37 (0.07 to 1.86) | Study population | ⊕⊝⊝⊝ VERY LOW 3 5 | |||
| 6.2% | 2.3% (0.4 to 11.4) | 3.9% fewer (5.7 fewer to 5.3 more) | ||||
| Serious neonatal morbidity or mortality (up to 28 days) № of participants: 48 (1 RCT) | not estimable | Study population | ‐ | |||
| 0.0% | 0.0% (0 to 0) | 0.0% fewer (0 fewer to 0 fewer) | ||||
| Mothers' experiences (satisfaction with care) (up to 8 weeks) № of participants: (0 RCTs) | ‐ | The mean mothers' experiences (satisfaction with care) without outpatient was 0 | ‐ | see comment | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; NICU: neonatal intensive care unit; PGE: prostaglandin E; RCT: randomised controlled trial; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate:: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level for serious risk of bias: high risk of blinding bias.
2 Downgraded one level for serious lack of generalisability: only 1 small study of 48 women
3 Downgraded two levels for very serious imprecision: very few participants, very few events and wide confidence interval.
4 Downgraded one level for serious risk of bias: high risk of blinding bias and 1 study contributing 78% of data unclear on allocation concealment.
5 Downgraded one level for serious risk of bias: high risk of blinding bias and 86% of data from study with unclear allocation concealment.
We have kept the data on home induction compared with inpatient induction separate for the different methods of induction of labour, including keeping separate prostaglandin E (PGE) and controlled‐release prostaglandin E (CR‐PGE) as CR‐PGE can be removed in case of complications where standard PGE cannot be removed once inserted (Lyrenas 2001).
1. Home versus inpatient induction with vaginal PGE (two studies 1028 women and their babies)
Two studies involving 1028 women and babies (1022 providing data) addressed this question (Ryan 1998; Wilkinson 2015 ‐ OPRA). The studies were undertaken in Canada (Ryan 1998) and Australia (Wilkinson 2015 ‐ OPRA).
Both studies used prostaglandin E2 (PGE2) for induction in the two settings. In one study, women allocated to home induction were able to go home after a satisfactory 40‐minute electronic fetal monitoring (EFM) trace. They were to return for reassessment in the morning or earlier if labour commenced, or if they had concerns. If a second dose of prostaglandins was required in the morning, these women were given the option of going home again. Women allocated to inpatient induction were admitted to the labour ward in the evening for induction, had EFM and were encouraged to rest overnight and reassessment was planned for the morning unless labour commenced beforehand. Nulliparous women received 2 mg of PGE2, and parous women 1 mg of PGE2 in accordance with South Australia Perinatal Guidelines (Wilkinson 2015 ‐ OPRA). The other study was a conference abstract and gave no detail of the intervention (Ryan 1998). See Characteristics of included studies.
Both studies were assessed as high risk of bias for blinding. One study was low risk for selection bias and incomplete outcome data (Wilkinson 2015 ‐ OPRA), while the other study was unclear on both these aspects (Ryan 1998). One study was assessed as high risk for selective reporting bias and also 48% of women did not receive induction of labour as most went into spontaneous labour (Wilkinson 2015 ‐ OPRA). See Figure 3; Characteristics of included studies.
Primary outcomes
Overall, we assessed the evidence to be of very low‐certainty.
Compared with induction in inpatient settings, we found home induction with vaginal PGE2 may lead to slightly better satisfaction with care for women, but the data are also compatible with no difference or slightly less satisfaction (mean difference (MD) 0.16, 95% confidence interval (CI) ‐0.02 to 0.34, 1 study, 399 women, Analysis 1.7). The certainty of the evidence was very low, downgraded for high risk of blinding bias, high protocol deviation, serious imprecision and lack of generalisability (Table 1).
1.7. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 7: Women's experiences (satisfaction with care) up to 8 weeks
We found evidence of little or no difference between home induction and inpatient induction of labour with vaginal PGE2 for the following primary outcomes:
spontaneous vaginal birth: average risk ratio (RR) [aRR] 0.91, 95% (CI) 0.69 to 1.21, 2 studies, 1022 women, random effects (Analysis 1.1). There was evidence of considerable heterogeneity (Tau² = 0.03; Chi² = 4.31, df = 1 (P = 0.04); I² 77%). The certainty of the evidence was very low, downgraded for high risk of blinding bias, high protocol deviation and considerable heterogeneity (Table 1).
uterine hyperstimulation: RR 1.19, 95% CI 0.40 to 3.50, 1 study, 821 women (Analysis 1.2). The certainty of the evidence was very low, downgraded for high risk of blinding bias, high protocol deviation and very serious imprecision (Table 1).
caesarean birth: RR 1.01, 95% CI 0.81 to 1.28, 2 studies, 1022 women (Analysis 1.3). Certainty of the evidence was very low, downgraded for high risk of blinding bias, high protocol deviation and serious imprecision (Table 1).
neonatal infection: RR 1.29, 95% CI 0.59 to 2.82, 1 study, 821 babies (Analysis 1.4). Certainty of the evidence was very low, downgraded for high risk of blinding bias, high protocol deviation and very serious imprecision (Table 1).
admission to neonatal intensive care unit (NICU): RR 1.20, 95% CI 0.50 to 2.90, 2 studies, 1022 babies (Analysis 1.5). Certainty of the evidence was very low, downgraded for high risk of blinding bias, unclear selection bias, high protocol deviation and very serious imprecision (Table 1).
serious neonatal morbidity or perinatal mortality: neither study assessed this outcome.
1.1. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 1: Spontaneous vaginal birth
1.2. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 2: Uterine hyperstimulation
1.3. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 3: Caesarean birth
1.4. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 4: Neonatal infection
1.5. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 5: Admission to NICU
Subgroup analyses: it was not possible to undertake subgroup analyses for parity and indication for induction as neither study reported on these. For membrane status and cervical status, Wilkinson 2015 ‐ OPRA included only women with intact membranes and an unfavourable cervix, whilst Ryan 1998 did not report on either of these aspects, hence the data are not mutually exclusive (Comparison 4; Comparison 5).
Sensitivity analyses: there were insufficient data to undertake sensitivity analyses.
Secondary outcomes
The certainty of the evidence for the secondary outcomes varied from low to very low.
Measures of effectiveness and satisfaction
Compared with inpatient induction, home induction with vaginal PGE2 may slightly improve a woman's sense of control, but the data are also compatible with no difference (MD 0.13, 95% CI 0.00 to 0.26, 1 study, 615 women, low‐certainty evidence, Analysis 1.13).
1.13. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 13: Woman's sense of control
Compared with inpatient setting, the home setting may make little or no difference to:
the number of women receiving oxytocin administration: RR 1.01, 95% CI 0.90 to 1.15, 2 studies, 1022 women, low‐certainty evidence (Analysis 1.8);
number of women having spinal analgesia: RR 1.01, 95% CI 0.93 to 1.10, 2 studies, 1022 women, low‐certainty evidence (Analysis 1.10).
1.8. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 8: Oxytocin administration
1.10. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 10: Spinal analgesia
We found the evidence was very uncertain for the following outcomes:
opioid analgesia: RR 1.50, 95% CI 1.22 to 1.85, 1 study, 821 women, very low‐certainty evidence (Analysis 1.11);
no pharmacological analgesia: RR 1.07, 95% CI 0.71 to 1.61, 1 study, 821 babies, very low‐certainty evidence (Analysis 1.12);
length of hospital stay (in days): MD 0.00, 95% CI ‐0.18 to 0.19, 2 studies, 1022 women, low‐certainty evidence (Analysis 1.16).
1.11. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 11: Opioid analgesia
1.12. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 12: No pharmacological analgesia
1.16. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 16: Length of hospital stay (in days)
Neither study assessed the following effectiveness outcomes: pain (self‐assessment); need for more than one induction agent; time from induction to birth; use of emergency services.
Complications for the baby
We found no clear difference between home induction and inpatient induction of labour with PGE for the following complications for the baby:
Apgar score less than seven at five minutes: RR 1.34, 95% CI 0.59 to 3.02, 2 studies, 1022 infants, very low‐certainty evidence (Analysis 1.18);
for perinatal mortality, there was one event (in the home group) reported in the 821 babies in one of the studies (Wilkinson 2015 ‐ OPRA, Analysis 1.21).
1.18. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 18: Apgar score < 7 at 5 minutes
1.21. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 21: Perinatal mortality
Neither study assessed the following complications for the baby: meconium aspiration; need for respiratory support.
Complications for the mother
We found no clear difference between home induction and inpatient induction of labour with vaginal PGE2 for the following maternal complications:
instrumental vaginal birth: aRR 1.22, 95% CI 0.67 to 2.22, 2 studies, 1022 women, random‐effects analysis, very low‐certainty evidence (Analysis 1.22);
postpartum haemorrhage (PPH) (≥ 500 mL or as defined by trialists): RR 1.10, 95% CI 0.76 to 1.58, 1 study, 821 women, very low‐certainty evidence (Analysis 1.24).
1.22. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 22: Instrumental vaginal birth
1.24. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 24: PPH (≥ 500 mL or as defined by trialists)
Neither study assessed the following maternal complication outcomes: uterine scar dehiscence/rupture: hysterectomy: maternal infection: serious maternal morbidity or mortality: postnatal depression: long‐term operative pelvic floor repair.
Additional outcomes
Economic aspects as defined by trialists
One study undertook a cost‐analysis looking at the key drivers of length of time in hospital and professional care provided as the trial demonstrated comparable clinical outcomes between home and inpatient induction of labour (Adelson 2013 part of Wilkinson 2015 ‐ OPRA). They identified a cost saving for women randomised to home induction of $319 per woman (95% CI $104 to $742) compared with women randomised to inpatient induction, and for women actually receiving the intervention the cost saving was $433 per woman (95% CI $282 to $1148). In addition, the savings were partly offset by the cost of an outpatient priming clinic, then showing a saving of $156 per woman.
The authors of the other study reported a cost saving for outpatients were $585 per women, however there is no information in the conference abstract on the methodology of this calculation (Ryan 1998).
Qualitative study on women's experiences
One study undertook qualitative research on women's experiences during the study (Wilkinson 2015 ‐ OPRA). They undertook a seven‐week postnatal questionnaire with a 76% response rate in both groups. The questionnaire on satisfaction and experiences was adapted from a validated seven‐week postnatal questionnaire (Turnbull 1996), and a thematic analysis of interviews of women who had experience of induction (Oster 2011 part of Wilkinson 2015 ‐ OPRA). There were small differences between the two groups for seven of the nine subscales with more favourable scores for women allocated to home settings than for those allocated to inpatient settings. They reported no real difference between the two groups for postpartum depression or infant feeding. There was no increase in anxiety in the outpatient group (Turnbull 2013 part of Wilkinson 2015 ‐ OPRA).
The authors of the other study reported that outpatients were more satisfied with their childbirth experience ‐ but again, no information in this conference abstract on the methodology used to assess this (Ryan 1998).
2. Home versus inpatient induction with controlled release PGE (one study, 300 women and their babies)
One study, undertaken in Canada, involving 300 women and their babies (299 providing data) addressed this issue (Biem 2003). Women received 10 mg controlled‐release PGE2 (CR‐PGE2), and were monitored in the antenatal ward for one hour prior to discharge home for the women in the home induction group. These women returned when in labour or within 12 hours. After 24 hours, if they were not in labour, they returned to hospital for induction of labour as an inpatient. Women were in telephone contact with a nurse every four hours and were given detailed instructions on seeking help if required. They were asked to remain within easy travelling distance of the hospital.
The study was low risk for selection bias, incomplete outcome data and other biases, high risk for blinding and unclear on selective reporting (Figure 3).
Primary outcomes
Overall, we assessed the evidence to be of very low‐certainty.
We found little or no difference between home induction and inpatient induction of labour with CR‐PGE2 for the following primary outcomes:
spontaneous vaginal births: RR 0.94, 95% CI 0.77 to 1.14, 1 study, 299 women (Analysis 2.1). Certainty of the evidence was very low, downgraded for high risk of blinding bias, very serious imprecision and lack of generalisability (Table 2).
uterine hyperstimulation: RR 1.01, 95% CI 0.51 to 1.98, 1 study, 299 women, (Analysis 2.2). Certainty of the evidence was very low, downgraded for high risk of blinding bias, very serious imprecision and lack of generalisability (Table 2).
caesarean birth: RR 0.95, 95% CI 0.64 to 1.42, 1 study, 299 women (Analysis 2.3). Certainty of the evidence was very low, downgraded for high risk of blinding bias, very serious imprecision and lack of generalisability (Table 2).
neonatal infection. The study did not assess this outcome.
admission to NICU: RR 1.38, 95% CI 0.57 to 3.34, 1 study, 299 babies (Analysis 2.5). Certainty of the evidence was very low, downgraded for high risk of blinding bias, very serious imprecision and lack of generalisability (Table 2).
serious neonatal morbidity or mortality. The study did not assess this outcome.
2.1. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 1: Spontaneous vaginal birth
2.2. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 2: Uterine hyperstimulation
2.3. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 3: Caesarean birth
2.5. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 5: Admission to NICU
For women's experiences/satisfaction with care, it is unclear if the questionnaire was validated
Subgroup analyses: these were not feasible as there was only one study in this comparison.
Sensitivity analyses: there were insufficient data to undertake sensitivity analyses.
Secondary outcomes
The certainty of the evidence for the secondary outcomes varied from low to very low.
Measures of effectiveness and satisfaction
We found little or no difference between home induction and inpatient induction of labour with controlled‐release PGE for the following measures of effectiveness:
oxytocin administration: RR 0.76, 95% CI 0.46 to 1.27, 1 study, 299 women, very low‐certainty evidence (Analysis 2.8);
pain ‐ self‐assessment: MD ‐0.10, 95% CI ‐0.42 to 0.22, 1 study, 299 women, very low‐certainty evidence (Analysis 2.9);
spinal analgesia: RR 1.02, 95% CI 0.91 to 1.16, 1 study, 299 women, very low‐certainty evidence (Analysis 2.10).
2.8. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 8: Oxytocin administration
2.9. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 9: Pain ‐ self assessment
2.10. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 10: Spinal analgesia
The study did not assess: opioid analgesia; no pharmacological analgesia; woman's sense of control; need for more than one induction agent; time from induction to birth; length of hospital stay; use of emergency services.
Complications for the baby
The study did not assess any of our outcomes on complications for the baby, namely: Apgar score less than seven at five minutes; meconium aspiration; need for respiratory support; perinatal mortality.
Complications for the mother
We found little or no difference between home induction and inpatient induction of labour with controlled‐release PGE for the following maternal complications:
instrumental vaginal birth: RR 1.34, 95% CI 0.83 to 2.17, 1 study, 299 women, very low‐certainty evidence (Analysis 2.22).
2.22. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 22: Instrumental vaginal birth
The study did not assess: uterine scar dehiscence/rupture; PPH (≥ 500 mL or as defined by trialists); hysterectomy; maternal infection; serious maternal morbidity or mortality; postnatal depression; long‐term operative pelvic floor repair.
Additional outcomes
Economic aspects as defined by trialists
The study did not assess economic aspects.
Qualitative study on women's experiences
The study did undertake qualitative assessment of women's experiences and satisfaction with care, but it seems their questionnaire was not a validated instrument. They found no difference in the overall mean score of women's satisfaction although more women in the home induction group rated their satisfaction as high. Pain in the initial 12 hours and anxiety were similar (Biem 2003).
3. Home versus inpatient induction with balloon or Foley catheter (four studies; three studies, 289 women and their babies provided data)
We identified four studies involving 349 women and their babes addressing this issue, although one study provided no data (Mohamad 2018), hence three studies involving 289 women and their babies provided data (Policiano 2017; Sciscione 2001; Wilkinson 2015a ‐ COPRA). Three of the studies used a single balloon Foley catheter (Mohamad 2018; Policiano 2017; Sciscione 2001), and one study used a double balloon catheter (Wilkinson 2015a ‐ COPRA).
In all three studies, women in the home induction group only went home following the insertion of the catheter after a reassuring cardiotocogram (CTG) trace. They were given written instructions on when to return to hospital. In Wilkinson 2015a ‐ COPRA; women who had not started in labour by the following morning were given further induction of labour with amniotomy and oxytocin infusion on returning to hospital.
All four studies were high risk of blinding bias. Two studies were low risk of selection bias (Policiano 2017; Wilkinson 2015a ‐ COPRA), one was low risk for sequence generation and unclear for allocation concealment (Sciscione 2001), and the other study (which provided no data) was unclear for both (Mohamad 2018). Three studies were low risk on incomplete outcome data (Policiano 2017; Sciscione 2001; Wilkinson 2015a ‐ COPRA), and one was unclear (Mohamad 2018). Two studies were high risk for selective reporting bias (Policiano 2017; Wilkinson 2015a ‐ COPRA), and two were unclear (Mohamad 2018; Sciscione 2001). One study was low risk for other biases (Wilkinson 2015a ‐ COPRA), and the others were unclear (Policiano 2017; Mohamad 2018; Sciscione 2001). See Figure 3.
Primary outcomes
Overall, we assessed the evidence to be of very low certainty.
One study reported on women's satisfaction with their care comparing home induction or inpatient induction but the questionnaire seemed to be not a validated instrument (Wilkinson 2015a ‐ COPRA).
Compared with inpatient induction, home inductions with balloon or Foley catheter may:
reduce the number of caesarean births but the evidence is very uncertain and the data are also compatible with a slight increase (RR 0.64, 95% CI 0.41 to 1.01, 2 studies, 159 women, Analysis 3.3). Certainty of the evidence was very low, downgraded for high risk of blinding bias, unclear allocation concealment for 86% of data and serious imprecision (Table 3).
3.3. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 3: Caesarean birth
Compared with inpatient induction, the evidence is very uncertain regarding the effect of home inductions with balloon or Foley catheter on the following primary outcomes:
spontaneous vaginal birth: RR 1.04, 95% CI 0.54 to 1.98, 1 study, 48 women (Analysis 3.1). Certainty of the evidence was very low, downgraded for high risk of blinding bias, very serious imprecision and lack of generalisability (Table 3).
uterine hyperstimulation: RR 0.45, 95% CI 0.03 to 6.79, 1 study, 48 women (Analysis 3.2). Certainty of the evidence was very low, downgraded for high risk of blinding bias, very serious imprecision and lack of generalisability (Table 3).
neonatal infection: one study assessed this outcome but found no infections (Analysis 3.4).
admission to NICU: RR 0.37, 95% CI 0.07 to 1.86, 2 studies, 159 babies (Analysis 3.5). Certainty of the evidence was very low, downgraded for high risk of blinding bias, unclear allocation concealment for 86% of data and very serious imprecision (Table 3).
serious neonatal morbidity or mortality, one study assessed this outcome but found no serous neonatal morbidity nor mortality (Analysis 3.6).
3.1. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 1: Spontaneous vaginal birth
3.2. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 2: Uterine hyperstimulation
3.4. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 4: Neonatal infection
3.5. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 5: Admission to NICU
3.6. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 6: Serious neonatal morbidity or mortality
Subgroup analyses: it was not possible to undertake any subgroup analyses as all the studies fell into the same subgroup. For parity, the studies included mixed parity; for membrane status all the studies included only women with intact membranes; for cervical status all studies only included women with an unfavourable cervix; and for indication for induction none of the studies reported on this.
Sensitivity analyses: there were insufficient data to undertake sensitivity analyses.
Secondary outcomes
The certainty of the evidence for the secondary outcomes varied from low to very low.
Measures of effectiveness and satisfaction
Compared with inpatient induction, home induction of labour with balloon or Foley catheter may:
reduce oxytocin administration: RR 0.75, 95% CI 0.57 to 0.97, 1 study, 48 women, very low‐certainty evidence (Analysis 3.8).
increase pain as assessed by women (MD 0.90, 95% CI 0.02 to 1.78, 1 study, 111 women, very low‐certainty evidence, Analysis 3.9).
reduce the time from induction to birth (in hours) (MD ‐3.51, 95% CI ‐6.32 to ‐0.69, 3 studies, 289 women, low‐certainty evidence, Analysis 3.15).
slightly reduce the length of hospital stay (in days) (MD ‐0.50, 95% CI ‐0.71 to ‐0.30), 2 studies, 178 women, low‐certainty evidence Analysis 3.16).
3.8. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 8: Oxytocin administration
3.9. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 9: Pain ‐ self assessment
3.15. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 15: Time from induction to birth (in hours)
3.16. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 16: Length of hospital stay (in days)
Compared with inpatient induction, home induction of labour with Foley catheter may make little or no difference to the following measures of effectiveness:
spinal analgesia: RR 0.99, 95% CI 0.91 to 1.09, 2 studies, 159 women, low‐certainty evidence (Analysis 3.10).
need for more than one induction agent: RR 1.08, 95% CI 0.82 to 1.41, 1 study, 130 women, very low‐certainty evidence (Analysis 3.14).
3.10. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 10: Spinal analgesia
3.14. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 14: Need for more than one induction agent
None of the studies assessed: opioid analgesia; no pharmacological analgesia; woman's sense of control; use of emergency services.
Complications for the baby
Compared with inpatient induction, home induction with balloon or Foley catheter may make little or no difference to the following measures of complications for the baby:
Apgar score less than seven at five minutes: RR 2.35, 95% CI 0.12 to 46.22, 1 study, 48 babies, very low certainty evidence (Analysis 3.18);
meconium aspiration: RR 1.41, 95% CI 0.06 to 32.78, 1 study, 48 babies, very low certainty evidence (Analysis 3.19);
need for respiratory support: RR 1.41, 95% CI 0.06 to 32.78, 1 study, 48 babies, very low certainty evidence (Analysis 3.20);
perinatal mortality: 2 studies reported this outcome and neither had any perinatal mortality (Analysis 3.21).
3.18. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 18: Apgar score < 7 at 5 minutes
3.19. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 19: Meconium aspiration
3.20. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 20: Need for respiratory support
3.21. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 21: Perinatal mortality
Complications for the mother
The evidence is very uncertain about the effect of home induction with a catheter the following maternal complications:
instrumental vaginal birth: RR 1.67, 95% CI 0.54 to 5.11, 1 study, 48 women, very low‐certainty evidence (Analysis 3.22);
maternal infection: one study assessed this outcome but found no maternal infection (Analysis 3.26);
serious maternal morbidity or mortality: one study assessed this outcome but found no events (Analysis 3.27).
3.22. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 22: Instrumental vaginal birth
3.26. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 26: Maternal infection
3.27. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 27: Serious maternal morbidity or mortality
None of the studies assessed the following: uterine scar dehiscence/rupture; PPH (≥ 500 mL or as defined by trialists); hysterectomy; postnatal depression; long‐term operative pelvic floor repair
Additional outcomes
Economic aspects as defined by trialists
None of the studies assessed economic aspects as defined by trialists.
Qualitative study on women's experiences
One study used a four‐week postnatal questionnaire similar to, but not the same as, the validated instrument used in the OPRA trial (Wilkinson 2015 ‐ OPRA) and found more women in the home induction group reported being physically uncomfortable while waiting for labour after balloon insertion (68% versus 36%), although 91% both groups reported being satisfied with care. They also found women in the home induction group were less likely to report feeling isolated and reported a higher sense of privacy (Turnbull 2014 part of Wilkinson 2015a ‐ COPRA).
A conference abstract reported women in the home induction group fewer felt emotionally alone, more were able to rest and relax compared to the inpatient group although no menton is made of whether they used a validated measure (Mohamad 2018).
Discussion
Summary of main results
There are limited data currently available to compare the efficacy and safety of home versus inpatient induction of labour. Of the seven studies identified, we found six providing data on 1610 women and babies. Two studies used vaginal PGE2 preparation for induction (Ryan 1998; Wilkinson 2015 ‐ OPRA). One study used controlled‐release PGE2 (Biem 2003). Four studies used a catheter for induction, with three of these studies providing data, two of the studies using the Foley catheter (Policiano 2017; Sciscione 2001) and one using the double balloon catheter (Wilkinson 2015a ‐ COPRA)). Overall, the certainty of the evidence was generally very low with all studies at high risk of blinding bias.
1) Home versus inpatient induction with vaginal PGE
Two studies provided data on 1022 women and their babies. Women may have rated their satisfaction with care slightly higher in the home induction group in the one study assessing this outcome (Wilkinson 2015 ‐ OPRA), but the findings were also compatible with no difference or women slightly less satisfied (very low‐certainty evidence). Again, in this one study (Wilkinson 2015 ‐ OPRA) more women probably felt a better sense of control in the outpatient group, but the finding was also compatible with no difference (very‐low certainty of evidence). There may be more women in the home group using opioid analgesia, but the true effect may be substantially different (very low‐certainty evidence).
On the other outcomes assessed, there was little or no difference between home induction and inpatient induction of labour, but we have little confidence in these effect estimates, all of which are very low‐certainty evidence.
2) Home versus inpatient induction with controlled‐release PGE
The one study (providing data on 299 women and their babies) compared home versus inpatient induction with controlled‐release PGE (Biem 2003). This study may have identified more women with high satisfaction, but their questionnaire was not reported as a validated instrument. All other outcomes assessed showed little or no difference between the two settings, but again the true effect may be substantially different as all outcome assessments were of very low certainty of evidence.
3) Home versus inpatient induction with Foley catheter
We found four studies addressing this question, with only three providing data (involving 289 women and their babies) (Policiano 2017; Sciscione 2001; Wilkinson 2015a ‐ COPRA). The certainty of the evidence was generally very low. The qualitative evidence may suggest that women in the outpatient group may be less likely to feel isolated but more may feel uncomfortable whilst awaiting labour, however, it is not clear if the questionnaire was a validated instrument. For other outcomes, we found that compared with inpatient induction, home induction of labour may reduce the time from induction to birth and may slightly reduce the length of hospital stay (low‐certainty evidence). The home birth may also slightly increase the pain as assessed by women (very low‐certainty evidence),
Over the three comparisons, only two studies reported information on cost, both studies using vaginal prostaglandins for induction. One study reported a detailed cost‐analysis and concluded there was not a meaningful difference in cost between the settings although there was a trend in favour of home induction providing some cost saving (Adelson 2013 ‐ part of Wilkinson 2015 ‐ OPRA). The other study (a conference abstract) provided no methodology on how the assessment was made but reported cost savings for home induction (Ryan 1998).
Overall completeness and applicability of evidence
The outcomes chosen for the review update now include 20 outcomes recommended as core outcomes for assessment in studies on induction of labour (Dos Santos 2018). Other outcomes chosen reflect the evidence from the systematic review on women's experiences of induction of labour (Coates 2019).
The studies included in the review did not have enough power to detect clinically important differences between the randomised groups for most outcomes, and more information is required to assess the effectiveness and safety of methods of induction of labour comparing home and inpatient settings. In one of the included trials, three women experienced serious labour complications, and the author of this study calls for larger studies in different settings "to compare the frequency of uncommon adverse events in labour and delivery" (Biem 2003).
Interpreting some of the results from the included studies was not simple. Outcome data using time intervals when examining induction of labour are often complicated. There are a variety of start and end points used which makes comparing findings from studies difficult.
As women's convenience and labour experience is often cited as a reason for home inductions, it is surprising that there is so little information on this. Two studies collected information on maternal satisfaction from women in both arms of the trial (Biem 2003; Wilkinson 2015 ‐ OPRA). One study looked at maternal anxiety, and this may be useful additional outcome for future updates (Biem 2003). One study states that the women's preference and satisfaction were assessed but the results were not reported or published (Mohamad 2018). Wilkinson 2015a ‐ COPRA undertook a four‐week questionnaire on various aspects of women's views and reported on women's feeling of isolation, noisy surroundings, getting a good nights sleep, however, it appeared the questionnaire may not have been a validated instrument, so their overall assessment of satisfaction did not provide a score to include in this review, but they found women equally satisfied in both groups.
Cost savings are also frequently mentioned as a reason for providing less inpatient care. Again, although three studies provided some information on length of hospital stay, this did not easily translate into cost data. Without a full breakdown of health service utilisation, it is not possible to impute costs.
The included studies had strict eligibility criteria and it is likely that home induction is only suitable for selected groups of women. The criteria cited within these studies reflect suitable 'low‐risk' groups.
There are nine ongoing studies assessing induction of labour in the two settings. We will aim to update this review when further data are available.
Quality of the evidence
The included trials were of very low or low quality/certainty of the evidence, with all the outcomes chosen for GRADE assessment being of very low certainty. There was limited information regarding randomisation and/or concealment in three of the seven included studies, and ideally, these processes should be explicit to avoid the introduction of bias. When comparing the same intervention within two settings, it is not possible to blind women to the actual method of induction of labour, but where possible, researchers assessing outcomes should be blinded to the setting and the authors of the included studies did not provide any information on this.
Grade assessments show generally very low certainty of evidence, with downgrading mostly for high risk of bias and serious or very serious imprecision.
All of the studies were underpowered for the outcomes assessed. One trial recruited a significant number of women at the point of randomisation, however, only about half of the total number who entered the study received the intervention; most women going into spontaneous labour following randomisation and not requiring induction (Wilkinson 2015 ‐ OPRA). It is unclear how this may have affected the overall quality of this study and the results of this study should, therefore, be interpreted with caution. In their next study on this topic (Wilkinson 2015a ‐ COPRA), these authors randomised women after the induction catheter was inserted, so inevitably all women had induction of labour.
Potential biases in the review process
We are aware of potential biases in the review process which we have tried to minimise. Our methodology included having two people who identified the studies to include or exclude, and to do data extraction, including the assessments of risk of bias. For the GRADE assessments of certainty of the evidence, assessments were checked by another author. These are inevitably subjective assessments and so at risk of bias. There were insufficient data to enable us to undertake sensitivity analyses but with a number of ongoing studies this may be possible in a future update. It was not possible to blind clinicians nor women in any of these studies, and none of the studies reported attempting to blind outcome assessors, this inevitably reduces the certainty of the evidence.
Agreements and disagreements with other studies or reviews
A systematic review and meta‐analysis published in June 2020 asked a similar question as this review, but chose some differing aspects of methodology (Dong 2020). Both reviews included only randomised controlled trials and women ≥ 37 weeks gestation. However, in order to answer the question of whether outcomes are different in two settings, we included only studies that used same method of induction of labour in both settings. Dong 2020 chose to include studies even if both setting and induction methods were different, and chose to exclude conference abstracts. In addition, we assessed the different methods of induction in separate comparisons, whilst Dong 2020 pooled all the studies then undertook, where possible, subgroup analyses by the method of induction. Overall, both reviews had similar findings, with no notable differences in outcomes between the groups, although we are more cautious regarding the robustness of safety data.
Authors' conclusions
Implications for practice.
This review highlights the small volume of available evidence relating to home induction versus inpatient induction of labour. Conclusions regarding the efficacy, safety and cost‐effectiveness of home inductions cannot be drawn from the available evidence.
Implications for research.
Further studies are required to assess both efficacy and the potential hazards of initiating labour in and away from a hospital setting, and researchers are guided to consider the use of outcomes similar to those developed within this review. We acknowledge that the data on safety, given the rarity of serious adverse events like intrapartum death, serious brain injury and uterine rupture, are unlikely to come from randomised evidence. For this, large prospective cohort, studies will be needed. It is very important for future research to include more evidence on women's experiences and satisfaction both through qualitative studies using validated instruments alongside the trials as well as including more outcomes important to women.
What's new
| Date | Event | Description |
|---|---|---|
| 31 January 2020 | New citation required but conclusions have not changed | Three new studies included. Conclusions have not changed. |
| 31 January 2020 | New search has been performed | Search updated and 33 new reports were identified and added, covering 21 new studies. Three are included, six excluded, three are awaiting classification and nine are trial registrations for ongoing studies. We have changed 'outpatient induction' to 'home induction'. We modified the PICO and methodology by:
We changed the reporting of Wilkinson 2015 (Wilkinson 2012 in previous 2013 version of review) from 'per protocol' to 'intention‐to‐treat analysis'. |
History
Protocol first published: Issue 4, 2008 Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 14 August 2013 | New citation required and conclusions have changed | With the addition of one new trial of vaginal PGE2, there is now no evidence of a difference between the likelihood of women requiring instrumental delivery in either setting. In the previous version of this review, women in the outpatient group were more likely to have instrumental deliveries. |
| 30 June 2013 | New search has been performed | Search updated. Eight new trial reports identified. One new trial (five reports) included (Wilkinson 2015 ‐ OPRA) and one trial (three reports) excluded (Henry 2011). We have also excluded a trial previously awaiting classification (Rijnders 2011) and moved one previously ongoing study report ‐ Turnbull 2009 ‐ to the included section under Wilkinson 2015 ‐ OPRA . |
Acknowledgements
We would like to acknowledge the contribution of Therese Dowswell, Research Associate, Cochrane Pregnancy and Childbirth Group, as an author on previous versions of this review (Kelly 2009b.) Therese was supported by a grant from the National Institute for Health Research (NIHR), UK NIHR NHS Cochrane Collaboration Programme Grant Scheme award for NHS prioritised, centrally‐managed, pregnancy and childbirth systematic reviews: CPGS02.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
This review is supported by funding from the UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP) to Cochrane Pregnancy and Childbirth (University of Liverpool). HRP supports and coordinates research on a global scale, synthesizes research through systematic reviews of literature, builds research capacity in low‐ and middle‐income countries and develops dissemination tools to make efficient use of ever‐increasing research information. In addition to its cosponsors, the International Planned Parenthood Federation (IPPF) and UNAIDS are both members of HRP’s governing body.
We would also like to thank Anthony Kelly and Arpita Ghosh for their contribution as authors to earlier versions of this review.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of our international panel of consumers and the Group's Statistical Asviser. The authors are grateful to the following peer reviewers for their time and comments: Michel Boulvain, University of Geneva and GHOL, Nyon Hospital, Switzerland; Farida Elshafeey, Ain Shams University, Egypt.
Appendices
Appendix 1. Search methods for ICTRP and ClinicalTrials.gov
ICTRP
outpatient AND induction AND labor
ClinicalTrials.gov
Advanced search
outpatient | Interventional Studies | Induced; Birth
outpatient | Interventional Studies | induction of labor
Data and analyses
Comparison 1. Home versus inpatient induction with vaginal PGE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Spontaneous vaginal birth | 2 | 1022 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.69, 1.21] |
| 1.2 Uterine hyperstimulation | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.40, 3.50] |
| 1.3 Caesarean birth | 2 | 1022 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.81, 1.28] |
| 1.4 Neonatal infection | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.82] |
| 1.5 Admission to NICU | 2 | 1022 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.50, 2.90] |
| 1.6 Serious neonatal morbidity or mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.7 Women's experiences (satisfaction with care) up to 8 weeks | 1 | 399 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.02, 0.34] |
| 1.8 Oxytocin administration | 2 | 1022 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.90, 1.15] |
| 1.9 Pain ‐ self assessment | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 1.10 Spinal analgesia | 2 | 1022 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.10] |
| 1.11 Opioid analgesia | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.22, 1.85] |
| 1.12 No pharmacological analgesia | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.71, 1.61] |
| 1.13 Woman's sense of control | 1 | 615 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.00, 0.26] |
| 1.14 Need for more than one induction agent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.15 Time from induction to birth (in hours) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 1.16 Length of hospital stay (in days) | 2 | 1022 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.18, 0.19] |
| 1.17 Use of emergency services | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.18 Apgar score < 7 at 5 minutes | 2 | 1022 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.59, 3.02] |
| 1.19 Meconium aspiration | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.20 Need for respiratory support | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.21 Perinatal mortality | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.12, 74.69] |
| 1.22 Instrumental vaginal birth | 2 | 1022 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.67, 2.22] |
| 1.23 Uterine scar dehiscence/rupture | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.24 PPH (≥ 500 mL or as defined by trialists) | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.76, 1.58] |
| 1.25 Hysterectomy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.26 Maternal infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.27 Serious maternal morbidity or mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.28 Postnatal depression | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.29 Long‐term operative pelvic floor repair | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.30 Economic assessments as defined by trialists | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
1.6. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 6: Serious neonatal morbidity or mortality
1.9. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 9: Pain ‐ self assessment
1.14. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 14: Need for more than one induction agent
1.15. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 15: Time from induction to birth (in hours)
1.17. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 17: Use of emergency services
1.19. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 19: Meconium aspiration
1.20. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 20: Need for respiratory support
1.23. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 23: Uterine scar dehiscence/rupture
1.25. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 25: Hysterectomy
1.26. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 26: Maternal infection
1.27. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 27: Serious maternal morbidity or mortality
1.28. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 28: Postnatal depression
1.29. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 29: Long‐term operative pelvic floor repair
1.30. Analysis.

Comparison 1: Home versus inpatient induction with vaginal PGE, Outcome 30: Economic assessments as defined by trialists
Comparison 2. Home versus inpatient induction with controlled release PGE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Spontaneous vaginal birth | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.77, 1.14] |
| 2.2 Uterine hyperstimulation | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.51, 1.98] |
| 2.3 Caesarean birth | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.64, 1.42] |
| 2.4 Neonatal infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.5 Admission to NICU | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.57, 3.34] |
| 2.6 Serious neonatal morbidity or mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.7 Women's experiences (satisfaction with care) up to 8 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 2.8 Oxytocin administration | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.46, 1.27] |
| 2.9 Pain ‐ self assessment | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.42, 0.22] |
| 2.10 Spinal analgesia | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.91, 1.16] |
| 2.11 Opioid analgesia | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.12 No pharmacological analgesia | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.13 Woman's sense of control | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 2.14 Need for more than one induction agent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.15 Time from induction to birth (in hours) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 2.16 Length of hospital stay (in days) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 2.17 Use of emergency services | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.18 Apgar score < 7 at 5 minutes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.19 Meconium aspiration | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.20 Need for respiratory support | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.21 Perinatal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.22 Instrumental vaginal birth | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.83, 2.17] |
| 2.23 Uterine scar dehiscence/rupture | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.24 PPH (≥ 500 mL or as defined by trialists) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.25 Hysterectomy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.26 Maternal infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.27 Serious maternal morbidity or mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.28 Postnatal depression | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.29 Long‐term operative pelvic floor repair | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.30 Economic assessments as defined by trialists | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
2.4. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 4: Neonatal infection
2.6. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 6: Serious neonatal morbidity or mortality
2.7. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 7: Women's experiences (satisfaction with care) up to 8 weeks
2.11. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 11: Opioid analgesia
2.12. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 12: No pharmacological analgesia
2.13. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 13: Woman's sense of control
2.14. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 14: Need for more than one induction agent
2.15. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 15: Time from induction to birth (in hours)
2.16. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 16: Length of hospital stay (in days)
2.17. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 17: Use of emergency services
2.18. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 18: Apgar score < 7 at 5 minutes
2.19. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 19: Meconium aspiration
2.20. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 20: Need for respiratory support
2.21. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 21: Perinatal mortality
2.23. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 23: Uterine scar dehiscence/rupture
2.24. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 24: PPH (≥ 500 mL or as defined by trialists)
2.25. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 25: Hysterectomy
2.26. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 26: Maternal infection
2.27. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 27: Serious maternal morbidity or mortality
2.28. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 28: Postnatal depression
2.29. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 29: Long‐term operative pelvic floor repair
2.30. Analysis.

Comparison 2: Home versus inpatient induction with controlled release PGE, Outcome 30: Economic assessments as defined by trialists
Comparison 3. Home versus inpatient induction with balloon or Foley catheter.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Spontaneous vaginal birth | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.54, 1.98] |
| 3.2 Uterine hyperstimulation | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.03, 6.79] |
| 3.3 Caesarean birth | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.41, 1.01] |
| 3.4 Neonatal infection | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.5 Admission to NICU | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.07, 1.86] |
| 3.6 Serious neonatal morbidity or mortality | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.7 Women's experiences (satisfaction with care) up to 8 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 3.8 Oxytocin administration | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.57, 0.97] |
| 3.9 Pain ‐ self assessment | 1 | 111 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [0.02, 1.78] |
| 3.10 Spinal analgesia | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.09] |
| 3.11 Opioid analgesia | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.12 No pharmacological analgesia | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.13 Woman's sense of control | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 3.14 Need for more than one induction agent | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.82, 1.41] |
| 3.15 Time from induction to birth (in hours) | 3 | 289 | Mean Difference (IV, Fixed, 95% CI) | ‐3.51 [‐6.32, ‐0.69] |
| 3.16 Length of hospital stay (in days) | 2 | 178 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐0.71, ‐0.30] |
| 3.17 Use of emergency services | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.18 Apgar score < 7 at 5 minutes | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [0.12, 46.22] |
| 3.19 Meconium aspiration | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.06, 32.78] |
| 3.20 Need for respiratory support | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.06, 32.78] |
| 3.21 Perinatal mortality | 2 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.22 Instrumental vaginal birth | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.54, 5.11] |
| 3.23 Uterine scar dehiscence/rupture | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.24 PPH (≥ 500 mL or as defined by trialists) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.25 Hysterectomy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.26 Maternal infection | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.27 Serious maternal morbidity or mortality | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.28 Postnatal depression | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.29 Long‐term operative pelvic floor repair | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.30 Economic assessments as defined by trialists | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
3.7. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 7: Women's experiences (satisfaction with care) up to 8 weeks
3.11. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 11: Opioid analgesia
3.12. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 12: No pharmacological analgesia
3.13. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 13: Woman's sense of control
3.17. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 17: Use of emergency services
3.23. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 23: Uterine scar dehiscence/rupture
3.24. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 24: PPH (≥ 500 mL or as defined by trialists)
3.25. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 25: Hysterectomy
3.28. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 28: Postnatal depression
3.29. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 29: Long‐term operative pelvic floor repair
3.30. Analysis.

Comparison 3: Home versus inpatient induction with balloon or Foley catheter, Outcome 30: Economic assessments as defined by trialists
Comparison 4. Home versus inpatient induction with vaginal PGE (subgroup by membrane status).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Spontaneous vaginal birth | 2 | 1022 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.69, 1.21] |
| 4.1.1 Intact membranes | 1 | 821 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.17] |
| 4.1.2 Ruptures membranes | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 4.1.3 Membrane status not reported | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.61, 0.99] |
| 4.2 Uterine hyperstimulation | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.40, 3.50] |
| 4.2.1 Intact membranes | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.40, 3.50] |
| 4.2.2 Ruptures membranes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.2.3 Membrane status not reported | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.3 Caesarean birth | 2 | 1022 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.81, 1.28] |
| 4.3.1 Intact membranes | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.25] |
| 4.3.2 Ruptures membranes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.3.3 Membrane status not reported | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.70, 2.20] |
| 4.4 Neonatal infection | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.82] |
| 4.4.1 Intact membranes | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.82] |
| 4.4.2 Ruptures membranes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.4.3 Membrane status not reported | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.5 Admission to NICU | 2 | 1022 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.50, 2.90] |
| 4.5.1 Intact membranes | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.21, 5.01] |
| 4.5.2 Ruptures membranes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.5.3 Membrane status not reported | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.45, 3.74] |
| 4.6 Serious neonatal morbidity or mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.6.1 Intact membranes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.6.2 Ruptures membranes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.6.3 Membrane status not reported | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.7 Women's experiences (satisfaction with care) up to 8 weeks | 1 | 399 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.02, 0.34] |
| 4.7.1 Intact membranes | 1 | 399 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.02, 0.34] |
| 4.7.2 Ruptured membranes | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 4.7.3 Membrane status not reported | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
4.1. Analysis.

Comparison 4: Home versus inpatient induction with vaginal PGE (subgroup by membrane status), Outcome 1: Spontaneous vaginal birth
4.2. Analysis.

Comparison 4: Home versus inpatient induction with vaginal PGE (subgroup by membrane status), Outcome 2: Uterine hyperstimulation
4.3. Analysis.

Comparison 4: Home versus inpatient induction with vaginal PGE (subgroup by membrane status), Outcome 3: Caesarean birth
4.4. Analysis.

Comparison 4: Home versus inpatient induction with vaginal PGE (subgroup by membrane status), Outcome 4: Neonatal infection
4.5. Analysis.

Comparison 4: Home versus inpatient induction with vaginal PGE (subgroup by membrane status), Outcome 5: Admission to NICU
4.6. Analysis.

Comparison 4: Home versus inpatient induction with vaginal PGE (subgroup by membrane status), Outcome 6: Serious neonatal morbidity or mortality
4.7. Analysis.

Comparison 4: Home versus inpatient induction with vaginal PGE (subgroup by membrane status), Outcome 7: Women's experiences (satisfaction with care) up to 8 weeks
Comparison 5. Home versus inpatient induction with vaginal PGE (subgroup by cervical status).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Spontaneous vaginal birth | 2 | 1022 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.69, 1.21] |
| 5.1.1 Unfavourable cervix | 1 | 821 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.17] |
| 5.1.2 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 5.1.3 Cervical status not defined | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.61, 0.99] |
| 5.2 Uterine hyperstimulation | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.40, 3.50] |
| 5.2.1 Unfavourable cervix | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.40, 3.50] |
| 5.2.2 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.2.3 Cervical status not defined | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.3 Caesarean birth | 2 | 1022 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.81, 1.28] |
| 5.3.1 Unfavourable cervix | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.25] |
| 5.3.2 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.3.3 Cervical status not defined | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.70, 2.20] |
| 5.4 Neonatal infection | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.82] |
| 5.4.1 Unfavourable cervix | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.82] |
| 5.4.2 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.4.3 Cervical status not defined | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.5 Admission to NICU | 2 | 1022 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.50, 2.90] |
| 5.5.1 Unfavourable cervix | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.21, 5.01] |
| 5.5.2 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.5.3 Cervical status not defined | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.45, 3.74] |
| 5.6 Serious neonatal morbidity or mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.6.1 Unfavourable cervix | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.6.2 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.6.3 Cervical status not defined | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.7 Women's experiences (satisfaction with care) up to 8 weeks | 1 | 399 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.02, 0.34] |
| 5.7.1 Unfavourable cervix | 1 | 399 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.02, 0.34] |
| 5.7.2 Favourable cervix | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 5.7.3 Cervical status not defined | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
5.1. Analysis.

Comparison 5: Home versus inpatient induction with vaginal PGE (subgroup by cervical status), Outcome 1: Spontaneous vaginal birth
5.2. Analysis.

Comparison 5: Home versus inpatient induction with vaginal PGE (subgroup by cervical status), Outcome 2: Uterine hyperstimulation
5.3. Analysis.

Comparison 5: Home versus inpatient induction with vaginal PGE (subgroup by cervical status), Outcome 3: Caesarean birth
5.4. Analysis.

Comparison 5: Home versus inpatient induction with vaginal PGE (subgroup by cervical status), Outcome 4: Neonatal infection
5.5. Analysis.

Comparison 5: Home versus inpatient induction with vaginal PGE (subgroup by cervical status), Outcome 5: Admission to NICU
5.6. Analysis.

Comparison 5: Home versus inpatient induction with vaginal PGE (subgroup by cervical status), Outcome 6: Serious neonatal morbidity or mortality
5.7. Analysis.

Comparison 5: Home versus inpatient induction with vaginal PGE (subgroup by cervical status), Outcome 7: Women's experiences (satisfaction with care) up to 8 weeks
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Biem 2003.
| Study characteristics | ||
| Methods | RCT | |
| Participants |
Inclusion criteria.
Exclusion criteria
|
|
| Interventions |
Intervention: home IOL with vaginally controlled release PGE2
Comparator: inpatient IOL with vaginally controlled release PGE2
Comparison and subgroups
|
|
| Outcomes | Satisfaction with care, length of hospital stay, length of labour, mode of delivery, labour interventions, maternal, fetal and neonatal complications. | |
| Notes |
Trial setting: Royal University Hospital, a tertiary care obstetrical centre in Saskatoon, Saskatchewn, Canada. Trial dates: July 1999 to September 2001 Sources of trial funding:the study was supported by a Clinical Teching and Research Grant from University of Saskatchewn, and an unrestricted research grant from Ferring Inc. Toronto, Canada. Dr R Tuffnell was supported by the 2000‐2001 Medical Education Fllowship of the Royal College of Physicians and Surgeons of Canada, and Dr J Biem was supported by a Canadian Institutes of Health Research regional partnership award. Trial authors' declarations of interest: not mentioned in trial report Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...computer‐generated table of random numbers..." |
| Allocation concealment (selection bias) | Low risk | Quote:"...sequential sealed opaque envelopes...opened immediately after the insertion of the CR‐PGE2." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is not possible to blind clinicians nor women to the location, and this may have influenced subjective outcomes although not objective outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported but outcome assessors/clinicians most probably not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 woman withdrew from the study after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes listed in methods section are reported on in the results. However, we have not assessed the trial protocol. |
| Other bias | Low risk | None apparent. No baseline imbalance apparent. |
Mohamad 2018.
| Study characteristics | ||
| Methods | RCT | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: home IOL with transcervical Foley catheter
Comparator: inpatient IOL with transcervical Foley catheter
Comparison and subgroups
|
|
| Outcomes | As reported in Results: oxytocin use, duration from amniotomy to birth, mode of birth, birth within 24 hours of induction, maternal and neonatal outcomes. The labour, maternal and fetal outcomes with women's satisfaction survey were analysed after birth | |
| Notes |
Setting: no information but authors from Universiti Kebangsaan, Malasyia Medical Centre, Malasyia Trial dates: August 2017 – May 2018 Sources of trial funding: not mentioned in the conference abstract Trial authors' declarations of interest: not mentioned in the conference abstract Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is not possible to blind clinicians nor women to the location, and this may have influenced subjective outcomes although not objective outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information provided, but the clinical outcomes are assessed in labour ward so cannot be blinded, the qualitative data on women’s views could be blinded but we do not know. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information, only that 60 women were randomised – no data on the number of women in the outcome assessments. |
| Selective reporting (reporting bias) | Unclear risk | No information in Methods on what outcomes are to be measures, only reports findings on outcomes. |
| Other bias | Unclear risk | This is a conference abstract with no information on which to judge the methodology used. |
Policiano 2017.
| Study characteristics | ||
| Methods | RCT – blocked randomisation in 10s. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: home IOL with Foley catheter
Comparator: inpatient IOL with Foley catheter
Comparison and subgroups
|
|
| Outcomes | Primary: change of Bishop score (difference between BS before and after application of FC). To detect a difference of at least 1 point change in BS between groups maintaining a power of 80% with an a level of 0.05 we calculated a sample of 60 patients in each group. Secondary: induction‐to‐delivery time, inpatient time, mode of delivery, cervix length change (difference between cervix length measured by transvaginal ultrasound before and after application of FC), failed induction rate, tachysystole with fetal decelerations, intrapartum and postpartum fever 380C, maternal pain, maternal and neonatal morbidity and mortality | |
| Notes |
Setting: tertiary hospital (from trial registration form). Authors from Lisbon, Portugal Trial dates: January 2014 to December 2015 Sources of trial funding: the authors report no financial support (sponsors were Hospital de Santa Maria, Lisbon, Portugal) Trial authors' declarations of interest: the authors report no conflict of interest. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:“…computer‐generated random numbers.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Allocation was initially concealed. An envelope was opened for all consecutive participants to reveal their group assignment at the time when they were recruited into the study.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of location was not possible for this comparison, and this may have influenced subjective outcomes although not objective outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information provided so it is very likely the clinician giving care knew the allocation, particularly for the assessment of change in Bishop score and many clinical outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data collections appear complete. |
| Selective reporting (reporting bias) | High risk | Appears not to report fully on mode of birth – gave vaginal births but no differentiation between spontaneous and instrumental. Only gave caesarean section for failed induction, not overall. Do not appear to report ‘failed induction’ though maybe can use PG induction data for this. We did not assess the trial protocol. |
| Other bias | Unclear risk | Baseline characteristics were similar. Block randomisation of 10 may possibly have introduced some bias. No other biases were identified. |
Ryan 1998.
| Study characteristics | ||
| Methods | RCT | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: home IOL with PGE2
Comparator: inpatient IOL with PGE2
Comparison and subgroups
|
|
| Outcomes | Need for oxytocin; spontaneous vaginal birth; instrumental vaginal birth; caesarean birth; epidural anaesthesia; fetal distress; admission to NICU; Apgar < 7 at 5 minutes; time L&D (hours); time antepartum+L&D; time AP+L&D+ODU; time postpartum; time total hospitalised. | |
| Notes |
Setting: not reported but authors are from Unversity of Toronto, Canada Trial dates: not mentioned in trial report Sources of trial funding: not mentioned in trial report Trial authors' declarations of interest: not mentioned in trial report Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomised". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is not really possible to blind participants and personnel to the location and this may have influenced subjective outcomes although not objective outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported so unlikely to have been attempted |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No loss to follow‐up apparent but little information provided. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only available. |
| Other bias | Unclear risk | Abstract only available. |
Sciscione 2001.
| Study characteristics | ||
| Methods | RCT | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: home IOL with Foley catheter
Comparator: inpatient IOL with Foley catheter
Comparison and subgroups
|
|
| Outcomes | Primary outcome: Bishop score. | |
| Notes |
Trial setting: 2 tertiary hospitals, Christiana Hospital (Delaware) or Thomas Jefferson University Hospital (Pennsylvania), in USA. Trial dates: May 1998 to December 1999. Sources of trial funding: not mentioned in the trial report Trial authors' declarations of interest: not mentioned in the trial report Additional information: |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...computer‐generated random number table.." |
| Allocation concealment (selection bias) | Unclear risk | Sequentially numbered envelopes, but not described as 'opaque' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is not possible to blind participants and personnel to the location, and this may have influenced subjective outcomes although not objective outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention as to whether outcome assessor was blinded so most likely not as it takes considerable effort. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete data for main outcomes. Only the outpatient group was followed up in the postnatal period and there was high attrition (40%) for this longer‐term follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes listed in methods seem to all be reported in results, but we did not assess the trial protocol. |
| Other bias | Unclear risk | Not clear how many of the women approached were eligible for this trial. Not clear how women were managed as regards oxytocin and this may have had an impact on results. Not clear how many women in the outpatient group were surveyed in the postnatal period; figures differ between the main study paper and an abstract reporting survey results. |
Wilkinson 2015 ‐ OPRA.
| Study characteristics | ||
| Methods | RCT; 2 centres; parallel; 1:1; stratified by site and parity | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: home IOL with vaginal PGE2
Comparator: inpatient IOL with vaginal PGE2
Comparison and subgroups
|
|
| Outcomes | Trial registration reports: Primary
Secondary
|
|
| Notes |
Trial setting: 2 tertiary referral hospitals in South Australia. Accounting for 39% of all births in South Australia – around 20,000 annual births Trial dates: August 2008 to May 2011 Sources of trial funding: quote: "The “Outpatient Priming for Induction of Labour Trial” was funded by the National Health and Medical Research Council of Australia, Project Grant 519236." Trial authors' declarations of interest: the authors report no conflict of interest. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “…computer generated…” |
| Allocation concealment (selection bias) | Low risk | Quote: ”…allocation was permanently assigned to the woman by a web‐based system…” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote:“Blinding of the intervention was not possible after randomisation.” and this may have influenced subjective outcomes although not objective outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not specifically reported and the outcomes assessed (with the exception of cost) were probably not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/827 (< 1%) women were excluded after randomisation (but 52% did not receive the IOL). For postnatal questionnaire losses vary by outcome measure and are off the order 25% to 50%. |
| Selective reporting (reporting bias) | High risk | The trial publication includes more outcomes then are listed in the trial registration form. The methods section of the publication does not list the outcomes to be assessed. |
| Other bias | Unclear risk | 48% of women did not receive their allocated intervention. Whilst there is a similar loss in each group, the size of the loss needs to be considered and it is unclear if this may have influenced the results. The authors did an intention‐to‐treat analysis and also a per protocol analysis and the findings were similar. |
Wilkinson 2015a ‐ COPRA.
| Study characteristics | ||
| Methods | RCT, 2:1 randomisation, stratified by parity, with randomly allocated block sizes. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: home IOL with double balloon catheter
Comparator:inpatient IOL with double balloon catheter
Comparison and subgroups
|
|
| Outcomes | Oxytocin use; length of active labour; uterine hyperstimulation; pain scores; anxiety scores; maternal satisfaction; feasibility measures; economic assessment | |
| Notes |
Trial setting: Women's and Children's Hopital, Adelaide, the largest maternity teaching hospital in South Australia. Trial dates: October 2012 to July 2013 Sources of trial funding: quote: “The study was funded by a Women’s and Children’s Foundation Research Project Grant for 2012. The funding body had no role in the design, conduct or analysis or the study, nor in the decision to submit the manuscript for publication.” Trial authors' declarations of interest: quote: “The authors declare that they have no competing interests.” Additional information
Quote:"Four weeks after the birth, a de‐identified questionnaire similar to the validated instrument used in the OPRA trial was mailed to women, with a two week follow‐up for non‐responders. The questionnaire sought information on satisfaction with care, preparedness, the induction environment and specific items relating to the catheter ripening process. Responses were organized in a 5‐point Likert scale response format. Two additional free text response questions asked women their feelings on having the catheter in place and positive and negative aspects of this method."..."More outpatient women reported being physically uncomfortable while waiting for ripening to work (68 %) than inpatient women (36 %).Women who experienced outpatient ripening were less likely to report feeling isolated (9 % compared with 30 % of inpatients) or feeling emotionally alone (9 % vs.36 % inpatients) during the ripening process."..."Overall, women in both groups were equally satisfied with the care they received for their ripening and felt the baby was safe (91 % both groups)." We have not included this data in the forest plots as it is unclear whether the questionnaire was validated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:“A computer generated list with randomly allocated block sizes was prepared and sequentially numbered.“ |
| Allocation concealment (selection bias) | Low risk | Quote:“Allocation assignments were placed and sealed in opaque envelopes by a person not otherwise involved in the conduct of the trial and were securely held in the area where randomization occurred. Envelopes were only opened after participant details were recorded” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Reported as an open study and not possible to blind participants and personnel to the location, and this may have influenced subjective outcomes although not objective outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not specifically reported so most probably outcome assessment was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all 48 women reported |
| Selective reporting (reporting bias) | High risk | No clinical outcomes for measurement were reported in the methods, only acceptability to women. Trial registration lists a number of outcomes yet many more reported. We did not assess the trial protocol. |
| Other bias | Low risk | Baseline characteristics similar. With the small number of women in this pilot study run in a single hospital. No apparent other biases. |
BS: Bishop Score CR‐PGE2: controlled release PGE2 CS: caesarean section CTG: cardiotocography EFM: electronic fetal monitoring FC: Foley catheter IOL: induction of labour IUGR: intrauterine growth restriction L&D: labour and delivery NICU: neonatal intensive care unit PGE: prostaglandin E RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Austin 2015 | Different method of induction for the 2 arms: outpatient was Foley catheter and inpatient IOL was vaginal PGE2. |
| Beckmann 2020 | Different method of induction for the 2 arms: outpatient IOL was balloon catheter and Inpatient IOL was vaginal prostaglandin. |
| Henry 2011 | Different method of induction for the 2 arms: outpatient IOL was intracervical Foley catheter and Inpatient IOL was intravaginal PGE2 gel. |
| Kuper 2018 | Different method of induction for the 2 arms: outpatient IOL was Foley catheter and inpatient IOL was Foley catheter + oxytocin infusion. |
| PonMalar 2017 | This study compared vaginal misoprostol with placebo, both groups of women being sent home after induction and normal CTG. So not comparing the different settings. |
| Rijnders 2011 | Different method of induction for the 2 arms: outpatient IOL was amniotomy and Inpatient IOL was at the clinician's discretion so could be: amniotomy, balloon catheter, vaginal prostaglandin. |
| Torbenson 2015 | Different method of induction for the 2 arms: outpatient IOL was with Foley catheter and Inpatient IOL was with either Foley catheter or vaginal prostaglandin. Also this is a preference trial so some women randomised to groups and some chose. |
| Wise 2020 | Different method of induction for the 2 arms: outpatient IOL was balloon catheter and Inpatient IOL was vaginal prostaglandin. |
CTG: cardiotocograph IOL: induction of labour PGE2: prostaglandin E2
Characteristics of studies awaiting classification [ordered by study ID]
Denona 2018 TR.
| Methods | Prospective comparative pilot study |
| Participants | Pregnant women aged 18 to 40 years who have not given birth before, |
| Interventions | Outpatient induction with Cook Cervical Ripening Balloon versus inpatient induction with Cook Cervical Ripening Balloon |
| Outcomes | Primary: women's satisfaction. |
| Notes | Described as a 'prospective comparative pilot study' so it is unclear if it is randomised or not. We will write to enquire. |
Hedriana 2019 TR.
| Methods | RCT |
| Participants | Nulliparous women having IOL with Foley catheter |
| Interventions | Women given IOL with intracervical balloon placed in the outpatient clinic and sent home versus women for IOL admitted to labour ward. |
| Outcomes | Prmary: duration of time from admission for IOL to birth of baby. |
| Notes | Not clear if both the inpatient group were given Foley catheter. We will write to enquire: Herman L Hedriana, MD 916‐734‐6219 hlhedriana@ucdavis.edu |
Mullin 2014 TR.
| Methods | RCT |
| Participants | Women ≥ 37 weeks' gestation having IOL |
| Interventions | Women will undergo outpatient IOL and the catheter will be deflated and removed within 10 minutes of placement versus women will undergo IOL using the standard Foley bulb and Pitocin method. |
| Outcomes | Primary: time from hospital admission to discharge. |
| Notes | It is unclear if both groups are having the same method of IOL. We will write to enquire. |
IOL: induction of labour RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Ausbeck 2018.
| Study name | Outpatient Foley for starting induction of labour at term in nulliparous women: a randomised‐controlled study |
| Methods | RCT |
| Participants | Nulliparous women at term having IOL with Foley catheter |
| Interventions | Induction by transcervical Foley catheter in outpatient setting versus induction by transcervical Foley catheter in inpatient setting |
| Outcomes | Primary: total time from admission to birth. |
| Starting date | May 2018 |
| Contact information | Elizabeth B Ausbeck, MD., University of Alabama at Birmingham, Birmingham, Alabama, United States, 35233 |
| Notes |
Kohari 2018.
| Study name | Inpatient versus outpatient Foley cervical ripening study |
| Methods | RCT |
| Participants | Women > 37 weeks' gestation having IOL |
| Interventions | Outpatients with a transcervical Foley catheter versus inpatients with a transcervical Foley catheter |
| Outcomes | Primary: antepartum time of Foley catheter extrusion before woman being brought to labour |
| Starting date | January 2019 |
| Contact information | Katherine Kohari, MD, Yale University. Olga Grechukhina, MD, olga.grechukhina@yale.edu |
| Notes |
Mishra 2007.
| Study name | A prospective RCT of outpatient versus inpatient labour induction with vaginal controlled‐release prostaglandin‐E2: effectiveness and satisfaction. |
| Methods | RCT |
| Participants | Primagravida at term with singleton baby |
| Interventions | Outpatient versus inpatient labour induction with vaginal controlled‐release prostaglandin‐E2 |
| Outcomes | Primary: 1) Effectiveness defined in terms of proportion of women in labour or delivered within 24 hours; requirement for additional intervention, e.g. epidural 2) Satisfaction defined as proportion of women with high mean ratings after 12 hours insertion/after delivery |
| Starting date | August 2006 |
| Contact information | Dr N Mishra, Royal Surrey County Hospital NHS Trust, Egerton Road, Guildford, GU2 7XX, United Kingdom |
| Notes | Trial completed 27 September 2009. |
Nichols 2019.
| Study name | A trial of Cervidil (dinoprostone, prostaglandin E2 (PGE2), insert) for outpatient pre‐induction of cervical ripening in women at 39.0‐41.6 weeks' gestation |
| Methods | RCT |
| Participants | Women, 39 0/7 and 41 6/7 weeks' gestation, undergoing IOL, living within 20 minutes of the facility |
| Interventions | Outpatient induction with dinoprostone (10 mg) vaginal insert versus inpatient induction with dinoprostone (10 mg) vaginal insert |
| Outcomes | Primary: time of admission to completion of dilation and total cost of Induction. |
| Starting date | August 2019 |
| Contact information | John Nichols, DO, Intermountain Health Care, Inc. Dixie Regional Medical Center, Saint George, Utah, United States, 84790. jnicholsdo@gmail.com. Briana Crook, CRC, bri.crook@imail.org |
| Notes |
Pierce‐Williams 2018.
| Study name | Inpatient versus outpatient transcervical Foley catheter use for cervical ripening: a randomised controlled trial |
| Methods | RCT |
| Participants | Women at least 37 weeks' gestation, undergoing IOL, with an unfavourable cervix, defined as a Bishop score ≤ 6 |
| Interventions | Outpatient Foley catheter versus inpatient Foley catheter |
| Outcomes | Primary: difference in time on labour and delivery and difference in cost. |
| Starting date | January 2017 |
| Contact information | Rebecca Pierce‐Williams, LifeBridge Health, Sinai Hospital of Baltimore, Baltimore, Maryland, United States, 21224. bmokhiber@gmail.com |
| Notes |
Pin 2018.
| Study name | Outpatient versus inpatient Foley catheter induction of labour in multiparous women: a randomised trial |
| Methods | RCT |
| Participants | Women aged over 18 years at term with previous successful vaginal delivery beyond 28 weeks with unripe cervix who need IOL. |
| Interventions | Outpatient IOL with Foley catheter versus inpatient IOL with Foley catheter |
| Outcomes | Primary: women's satisfaction with care; percentage of women delivering during “working hours” (8am to 5pm). |
| Starting date | February 2019 |
| Contact information | Dr. Tan Yi Pin, University of Malaya Medical Centre, Lembah Pantai, Kuala Lumpur 59100, Malaysia. (Mobile number): + 60 175398075. Also Dr Shuhaina Binti Shuib, shuhainashuib@yahoo.com |
| Notes |
Rinne 2016.
| Study name | Outpatient versus inpatient double balloon catheter for induction of labour: a randomised trial |
| Methods | RCT |
| Participants | Women with uncomplicated singleton pregnancies, > 37 and < 41 + 5 gestation, living within half an hour of the hospital |
| Interventions | Outpatiient induction with double balloon catheter versus inpatient induction with double balloon catheter |
| Outcomes | Primary: pain measured by visual analogue scale after double balloon catheter |
| Starting date | June 2016 |
| Contact information | Kirsi M Rinne, Turku University Hospital, University of Turku, Turku, Finland, 20520 kirsi.rinne@tyks.fi. Also Päivi ML Polo, paivi.polo@tyks.fi. |
| Notes |
Saad 2018.
| Study name | Induction of labour in women with unfavourable cervix: randomised controlled trial comparing outpatient to inpatient cervical ripening using Dilapan‐S® (HOMECARE) |
| Methods | RCT |
| Participants | Pregnant woman whose plan of care is IOL |
| Interventions | Outpatient induction with Dilapan‐S versus inpatient induction with Dilapan‐S |
| Outcomes | Primary: hospital stay and healthcare cost. |
| Starting date | November 2018 |
| Contact information | Antonio Saad, MD, University of Texas Medical Branch, Galveston, US, afsaad@utmb.edu. Also Ashley Salazar, assalaza@utmb.edu |
| Notes |
Shrivastava 2016.
| Study name | Patient satisfaction during outpatient versus inpatient Foley catheter induction of labour |
| Methods | RCT |
| Participants | Women with singleton pregnancies, ≥ 39 weeks' gestation |
| Interventions | Outpatient IOL with Foley catheter versus inpatient IOL with Foley catheter |
| Outcomes | Primary: women's satisfaction |
| Starting date | November 2016 |
| Contact information | Vineet Shrivastava, MD. Also, Deysi Caballero, LVN, CRC, Miller Women's and Children's Hospital Long Beach, Long Beach, California, United States, 90806, dcaballero@memorialcare.org, |
| Notes |
IOL: induction of labour RCT: randomised controlled trial
Differences between protocol and review
Methods have been updated to the current standard methods for the Cochrane Pregnancy and Childbirth Group (2019).
We have changed 'outpatient induction' to 'home induction' and provided definitions for these.
We have made the following further modifications:
we changed the gestational age for inclusion from > 35 weeks' gestation to ≥ 37 weeks' gestation;
we now exclude quasi‐randomised controlled trials;
we changed the outcomes to include those recommended by the Core Outcome Sets (COS) publication (Dos Santos 2018) and include further outcomes to assess women's experiences;
we removed the additional 'Risk of bias' assessments and used, the now standard, 'Performance bias' assessment;
we have changed the outcome 'Neonatal morbidity and perinatal mortality' to 'Neonatal morbidity and mortality';
we have clarified that we will only use qualitative data if they are gathered using a validated instrument.
We added an additional search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
Contributions of authors
For the 2013 update, Anthony Kelly and Arpita Ghosh prepared the text and Zarko Alfirevic commented on drafts.
For the 2020 update, Zarko Alfirevic commented on changes to the protocol and drafts versions of the review. All other authors agreed the inclusion/exclusion decisions, undertook some data extraction and some assessment of risk of bias. G Gyte undertook GRADE assessments and entered the data into RevMan with other authors checking data entry and GRADE assessments. All authors contributed to drafting and checking the text of the review.
Sources of support
Internal sources
The University of Liverpool, UK
External sources
-
World Health Organization (WHO) and the UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Switzerland
This review is supported by funding to Cochrane Pregnancy and Childbirth (University of Liverpool)
Declarations of interest
Alfirevic Z: is the Co‐ordinating Editor of Cochrane Pregnancy and Childbirth and has not been involved in the editorial processing or any editorial decisions relating to this review.
Finucane E: none known.
Gyte G: I have received royalties from John Wiley & Son in respect of ‘A Cochrane Pocket Handbook – Pregnancy and Childbirth' Hofmeyr GJ et al. 2008.
Osoti A: none known.
Pileggi V: none known.
Plachcinski R: Independent PPI member of the Trial Steering Committee for the SOLVE trial: A randomised controlled trial of a Synthetic Osmotic cervical dilator for induction of Labour in comparison to dinoprostone Vaginal insErt. Funded by Medicem International, Czech Republic (www.medicem.com)
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Biem 2003 {published data only}
- Biem SR, Turnell RW, Olatunbosun O, Tauh M, Biem HJ. A randomized controlled trial of outpatient versus inpatient labour induction with vaginal controlled-release prostaglandin-E2: effectiveness and satisfaction. Journal of Obstetrics & Gynaecology Canada: JOGC 2003;25(1):23-31. [DOI] [PubMed] [Google Scholar]
Mohamad 2018 {published data only}
- Mohamad A, Ismail NA, Rahman RA, Kalok AH, Ahmad S. A comparison between in-patient and out-patient balloon catheter cervical ripening: a prospective randomised controlled trial in PPUKM. Medical Journal of Malaysia 2018;73:22. [Google Scholar]
Policiano 2017 {published data only}
- Policiano C, NCT02842879. Outpatient versus inpatient cervix priming with Foley catheter. https://clinicaltrials.gov/ct2/show/NCT02842879 (first received 25 July 2016). [DOI] [PubMed]
- Policiano C, Pimenta M, Martins D, Clode N. Outpatient versus inpatient cervix priming with Foley catheter: a randomized trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2017;210:1-6. [DOI] [PubMed] [Google Scholar]
Ryan 1998 {published data only}
- Ryan G, Oskamp M, Seaward PG, Barrett J, Barrett H, O'Brien K, et al. Randomized controlled trial of inpatient vs. outpatient administration of prostaglandin E2, gel for induction of labour at term [SPO Abstract 303]. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S92. [Google Scholar]
Sciscione 2001 {published data only}
- Pollock M, Maas B, Muench M, Sciscione A. Patient acceptance of outpatient pre-induction cervical ripening with the foley bulb. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S136. [Google Scholar]
- Sciscione AC, Muench M, Pollock M, Jenkins TM, Tildon-Burton J, Colmorgen GH. Transcervical foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstetrics & Gynecology 2001;98:751-6. [DOI] [PubMed] [Google Scholar]
Wilkinson 2015a ‐ COPRA {published data only}
- Adelson P, Wilkinson C, Turnbull D. Pilot trial of balloon catheter cervical priming: Women and clinician views of inpatient and outpatient applications. Journal of Paediatrics and Child Health 2014;50(Suppl 1):62-3. [Google Scholar]
- Turnbull D, ACTRN12612001184864. A pilot randomised controlled trial of outpatient balloon catheter priming for induction of labour. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612001184864 (first received 30 October 2012).
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy and Childbirth 2015;15(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson C, Adelson P, Turnbull D. A pilot randomised controlled trial of outpatient compared to inpatient cervical priming using double balloon catheters-clinical results of the copra study. Journal of Paediatrics and Child Health 2014;50(Suppl 1):37, Abstract no: A241. [Google Scholar]
- Wilkinson C, Adelson P, Turnbull D. Outpatient compared to inpatient cervical ripening with a double balloon catheter. A pilot randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2015;122(Suppl S1):231. [DOI] [PubMed] [Google Scholar]
Wilkinson 2015 ‐ OPRA {published data only}
- Adelson L, Wedlock R, Wilkinson S, Howard K, Bryce L, Turnbull A. A cost analysis of inpatient compared with outpatient prostaglandin E2 cervical priming for induction of labour: results from the OPRA trial. Australian Health Review 2013;37(4):467-73. [DOI] [PubMed] [Google Scholar]
- Adelson P, Wedlock G, Wilkinson C, Bryce R, Turnbull D. A cost analysis of outpatient priming for induction of labour induction: Results from the outpatient priming for induction of labour trial (OPRA). Journal of Paediatrics and Child Health 2013;49 Suppl 2:27. [DOI] [PubMed] [Google Scholar]
- Oster C, Adelson P, Wilkinson C, Turnbull D. Inpatient versus outpatient cervical priming for induction of labour: Therapeutic landscapes and women’s preferences. Health and Place 2011;17(1):379-85. [DOI: 10.1016/j.healthplace.2010.12.001] [DOI] [PubMed] [Google Scholar]
- Turnbull D, Adelson P, Oster C, Bryce R, Fereday J, Wilkinson C. Psychosocial outcomes of a randomized controlled trial of outpatient cervical priming for induction of labor. Birth 2013;40(2):75-80. [DOI] [PubMed] [Google Scholar]
- Turnbull D, Adelson P, Stamp G, Fereday J, Ryan P, Bryce R, et al. A two-centre randomised controlled trial of outpatient cervical priming for induction of labour: Psychosocial outcomes. Journal of Paediatrics and Child Health 2012;48(Suppl 1):61. [Google Scholar]
- Turnbull D, Oster C, Adelson P, Scalzi D, Grauenhorst S, Bryce R, et al. A two-center randomised controlled trial of outpatient cervical priming for induction of labour: A qualitative examination confirming psychosocial benefit. Journal of Paediatrics and Child Health 2013;49 Suppl 2:100. [Google Scholar]
- Turnbull D. A multicentre randomised controlled trial comparing outpatient and inpatient cervical priming with intravaginal prostaglandins for induction of labour. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=82815 (accessed 8 January 2009).
- Wilkinson C, Bryce R, Adelson P, Coffey J, Coomblas J, Ryan P, et al. Two center RCT of outpatient versus inpatient cervical ripening for induction of labour with PGE2. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S137. [Google Scholar]
- Wilkinson C, Bryce R, Adelson P, Coffey J, Coomblas J, Turnbull D. Clinical results of a randomized controlled trial of outpatient cervical priming for induction of labor with prostaglandin E2. Journal of Paediatrics and Child Health 2013;49 Suppl 2:16-7. [Google Scholar]
- Wilkinson C, Bryce R, Adelson P, Turnbull D. A randomised controlled trial of outpatient compared with inpatient cervical ripening with prostaglandin E (OPRA study). BJOG: an international journal of obstetrics and gynaecology 2015;122:94-106. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Austin 2015 {published data only}
- Austin K, Chambers GM, De Abreu Lourenco R, Madan A, Susic D, Henry A. Cost-effectiveness of term induction of labour using inpatient prostaglandin gel versus outpatient Foley catheter. Australian & New Zealand Journal of Obstetrics & Gynaecology 2015;55(5):440-5. [DOI] [PubMed] [Google Scholar]
Beckmann 2020 {published data only}
- Beckmann M, ACTRN12614000039684. Prostaglandin Inpatient iNduction of labour Compared with BALLOon Outpatient iNduction of labour: a randomised controlled trial. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12614000039684 (first received 19 December 2013).
- Beckmann M, Acreman M, Schmidt E, Merollini KM, Miller Y. Women's experience of induction of labor using PGE2 as an inpatient versus balloon catheter as an outpatient. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2020;249:1-6. [CENTRAL: CN-02118449] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Beckmann M, Gibbons K, Flenady V, Kumar S. Induction of labour using prostaglandin E2 as an inpatient versus balloon catheter as an outpatient: a multicentre randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2020;127(5):571-9. [CENTRAL: CN-02073515] [EMBASE: 630016333] [PMID: ] [DOI] [PubMed] [Google Scholar]
Henry 2011 {published data only}
- Henry A, ACTRN12609000420246. An evaluation of Outpatient Foley (intracervical) catheter versus Inpatient Prostaglandin Vaginal Gel (PGE2) on the induction of labour at term. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12609000420246 (first received 10 May 2009).
- Henry A, Madan A, Reid R, Tracy S, Sharpe V, Austin K, et al. Outpatient Foley catheter versus inpatient Prostin gel for cervical ripening: the FOG (Foley or Gel) trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2011;51:473-4. [Google Scholar]
- Henry A, Madan A, Reid R, Tracy SK, Austin K, Welsh A, et al. Outpatient Foley catheter versus inpatient prostaglandin E2 gel for induction of labour: a randomised trial. BMC Pregnancy and Childbirth 2013;13:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry A, Reid R, Madan A, Tracy S, Sharpe V, Welsh A, et al. Satisfaction survey: outpatient Foley catheter versus inpatient Prostin gel for cervical ripening. Australian and New Zealand Journal of Obstetrics and Gynaecology 2011;51:474. [Google Scholar]
Kuper 2018 {published data only}
- Kuper SG, Jauk VC, George DM, Edwards RK, Szychowski JM, Mazzoni SE, et al. Outpatient Foley catheter for induction of labor in parous women a randomized controlled trial. Obstetrics and Gynecology 2018;132(1):94-101. [DOI] [PubMed] [Google Scholar]
- Kuper SG, NCT02756689. Outpatient Foley for starting induction of labor at term. https://clinicaltrials.gov/ct2/show/NCT02756689 (first received 29 April 2016).
- Subramaniam A, Blanchard CT, Kuper SG, Jauk VC, Szychowski JM, Tita AT, et al. 660: Outpatient versus inpatient cervical ripening in obese parous women. American Journal of Obstetrics and Gynecology 2019;220(1):S437. [Google Scholar]
PonMalar 2017 {published data only}
- PonMalar J, Benjamin SJ, Abraham A, Rathore S, Jeyaseelan V, Mathews JE. Randomized double-blind placebo controlled study of preinduction cervical priming with 25 microg of misoprostol in the outpatient setting to prevent formal induction of labour. Archives of Gynecology and Obstetrics 2017;295(1):33-8. [DOI] [PubMed] [Google Scholar]
Rijnders 2011 {published data only}ISRCTN47736435
- Rijnders ME, ISRCTN47736435. Costs and effects of amniotomy at home for induction of post term pregnancy. http://www.isrctn.com/ISRCTN47736435 (9 January 2006).
- Rijnders ME. A randomised controlled trial of amniotomy at home for induction between 292 and 294 days gestation. In: Interventions in Midwife Led Care in the Netherlands to Achieve Optimal Birth Outcomes: Effects and Women's Experiences. Amsterdam: University of Amsterdam, 2011. [Google Scholar]
Torbenson 2015 {published data only}
- Torbenson VE, NCT02546193. Outpatient Foley catheter compared to usual inpatient care for cervical ripening: a randomized controlled trial. https://clinicaltrials.gov/ct2/show/NCT02546193 (first received 10 September 2015).
Wise 2020 {published data only}
- Wise M, ACTRN12616000739415. Comparison of low-risk pregnant women undergoing induction of labour at term by outpatient balloon or inpatient prostaglandin in order to assess caesarean section rate; a randomised controlled trial. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616000739415 (first received 15 March 2016).
- Wise MR, Marriott J, Battin M, Thompson JM, Stitely M, Sadler L. Outpatient balloon catheter vs inpatient prostaglandin for induction of labour (OBLIGE): a randomised controlled trial. Trials 2020;21(1):190. [CENTRAL: CN-02129785] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Denona 2018 TR {published data only}ISRCTN10109823
- Denona B, ISRCTN10109823. Comparison on patient satisfaction with Cervical Ripening Balloon using inpatient and outpatient protocol. http://www.isrctn.com/ISRCTN10109823 (first received 24 April 2018).
Hedriana 2019 TR {published data only}
- Hedriana H, NCT03934918. Outpatient cervical preparation to reduce induction duration in nulliparous term singleton vertex women: a randomized controlled trial. https://clinicaltrials.gov/ct2/show/NCT03934918 (first received 2 May 2019).
Mullin 2014 TR {published data only}
- Mullin PM, NCT02210598. Outpatient labor induction with the transcervical Foley balloon: a randomized trial comparing outpatient immediate removal Foley versus standard inpatient Foley induction. https://clinicaltrials.gov/ct2/show/NCT02210598 (first received 6 August 2014).
References to ongoing studies
Ausbeck 2018 {published data only}
- Ausbeck EB, NCT03472937. Outpatient Foley for starting induction of labor at term in nulliparous women: a randomized-controlled study. https://clinicaltrials.gov/ct2/show/NCT03472937 (first received 21 March 2018).
Kohari 2018 {published data only}
- Kohari K, NCT03725397. Inpatient versus outpatient Foley cervical ripening study. https://clinicaltrials.gov/ct2/show/NCT03725397 (first received 31 October 2018).
Mishra 2007 {published data only}ISRCTN71488751
Nichols 2019 {published data only}
- Nichols J, NCT03806231. A trial of Cervidil (dinoprostone, prostaglandin E2 (PGE2), insert) for outpatient pre-induction of cervical ripening in women at 39.0-41.6 weeks gestation. https://clinicaltrials.gov/ct2/show/NCT03806231 (first received 16 January 2019).
Pierce‐Williams 2018 {published data only}
- Pierce-Williams R, NCT03769610. Inpatient versus outpatient transcervical Foley catheter use for cervical ripening: a randomized controlled trial. https://clinicaltrials.gov/ct2/show/NCT03769610 (first received 7 December 2018).
Pin 2018 {published data only}ISRCTN13534944
- Pin TY, ISRCTN13534944. Induction of labour with a foley catheter in pregnant women with previous successful vaginal birth in outpatient vs inpatient settings. http://www.isrctn.com/ISRCTN13534944 (first received 18 December 2018).
Rinne 2016 {published data only}
- Rinne KM, NCT02793609. Outpatient versus inpatient double balloon catheter for induction of labor: a randomised trial. https://clinicaltrials.gov/ct2/show/NCT02793609 (first received 8 June 2016).
Saad 2018 {published data only}
- Saad A, NCT03665688. Induction of labor in women with unfavorable cervix: randomized controlled trial comparing outpatient to inpatient cervical ripening using Dilapan-S® (HOMECARE). https://clinicaltrials.gov/ct2/show/NCT03665688 (first received 11 September 2018).
Shrivastava 2016 {published data only}
- Shrivastava V, NCT02975167. Patient satisfaction during outpatient versus inpatient Foley catheter induction of labor. https://clinicaltrials.gov/ct2/show/NCT02975167 (first received 29 November 2016).
Additional references
ACOG 2009
- American College of Obstetricians and Gynecologists. Induction of Labor. ACOG Practice Bulletin 2009;114:386-97.
Alfirevic 2016
- Alfirevic Z, Keeney E, Dowswell T, Welton N, Medley N, Dias S, et al. Which method is best for the induction of labour? A systematic review, network meta-analysis and cost-effectiveness analysis. Health Technology Assessment 2016;20(65):1-584. [DOI] [PMC free article] [PubMed]
Calder 1998
- Calder AA. Nitric oxide--another factor in cervical ripening. Human Reproduction 1998;13(2):250-1. [DOI] [PubMed] [Google Scholar]
Coates 2019
- Coates R, Cupples G, Scamell A, McCourt C. Women’s experiences of induction of labour: qualitative systematic review and thematic synthesis. Midwifery 2019;69:17-28. [DOI] [PubMed] [Google Scholar]
Curtis 1987
- Curtis P, Evans S, Resnick J. Uterine hyperstimulation. The need for standard terminology. Journal of Reproductive Medicine 1987;32:91-5. [PubMed] [Google Scholar]
Dong 2020
- Dong S, Khan M, Hashimi F, Charny C, D’Souza R. Inpatient versus outpatient induction of labour: a systematic review and meta-analysis. BMC Pregnancy and Childbirth 2020;20:382-92. [DOI: 10.1186/s12884-020-03060-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dos Santos 2018
- Dos Santos F, Drymiotou S, Antequera Martin A, Mol BW, Gale C, Devane D, et al. Development of a core outcome set for trials on induction of labour: an international multistakeholder Delphi study. British Journal of Obstetrics and Gynaecology 2018;125:1673–80. [DOI] [PubMed] [Google Scholar]
Finucane 2020
- Finucane EM, Murphy DJ, Biesty LM, Gyte GM, Cotter AM, Ryan EM, et al. Membrane sweeping for induction of labour. Cochrane Database of Systematic Reviews 2020, Issue 2. Art. No: CD000451. [DOI: 10.1002/14651858.CD000451.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gates 2005
- Gates S. Methodological Guidelines. In: The Editorial Team. Pregnancy and Childbirth Group. About The Cochrane Collaboration (Collaborative Review Groups (CRGs)) 2005, Issue 2.
Grobman 2018
- Grobman, W, Rice M, Reddy U, Tita A, Silver R, Mallett G, et al. A randomized trial of elective induction of labor at 39 weeks compared with expectant management of low-risk nulliparous women. American Journal of Obstetrics and Gynecology 2018;218(1):S601. [DOI: 10.1016/j.ajog.2017.12.016] [DOI] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hofmeyr 2009
- Hofmeyr GJ, Alfirevic Z, Kelly AJ, Kavanagh J, Thomas J, Neilson JP, et al. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD002074. [DOI: 10.1002/14651858.CD002074.pub2] [DOI] [Google Scholar]
Liu 2019
- Liu X, Wang Y, Zhang F, Zhong X, Ou R, Luo X, et al. Double versus single-balloon catheters for labour induction and cervical ripening: a meta-analysis. BMC Pregnancy and Childbirth 2019;19:358-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lyrenas 2001
- Lyrenas S, Clason I, Ulmsten U. In vivo controlled release of PGE2 from a vaginal insert (0.8 mm, 10 mg) during induction of labour. British Journal of Obstetrics and Gynaecology 2001;108:169-78. [DOI] [PubMed] [Google Scholar]
Middleton 2018
- Middleton P, Shepherd E, Crowther CA. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD004945. [DOI: 10.1002/14651858.CD004945.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2008
- National Institute of Health and Care Excellence. Inducting labour: Clinical Guideline. National Collaborating Centre for Women’s and Children’s Health 2008;RCOG Press, London:Available at https://www.nice.org.uk/guidance/cg70/chapter/4-research-recommendations.
Nilvér 2017
- Nilvér H, Begley C, Berg M. Measuring women’s childbirth experiences: a systematic review for identification and analysis of validated instruments. BMC Pregnancy and Childbirth 2017;17:203-22. [DOI: 10.1186/s12884-017-1356-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nippita 2015
- Nippita T, Trevena J, Patterson J, Ford J, Morris J, Roberts C. Variation in hospital rates of induction of labour: a population-based record linkage study. BMJ Open 2015;5:e008755. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Santesso 2020
- Santesso N, Glenton C, Dahm P, Gardner P, Akl E, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [DOI: 10.1016/j.jclinepi.2019.10.014. Epub 2019 Nov 9.PMID: 31711912] [DOI] [PubMed] [Google Scholar]
SOGC 2013
- Society of Obstetricians and Gynaecologists of Canada. Induction of Labour SOGC Clinical Practice Guideline. Society of Obstetricians and Gynaecologists of Canada 2013;296:Available at: https://sogc.org/wp-content/uploads/2013/08/September2013-CPG296-ENG-Online_REV-D.pdf (accessed 4th April 2017).
Sue‐A‐Quan 1999
- Sue-A-Quan AK, Hannah ME, Cohen MM, Foster GA, Liston RM. Effect of labour induction on rates of stillbirth and cesarean section in post-term pregnancies. Canadian Medical Association Journal 1999;160(8):1145-9. [PMC free article] [PubMed] [Google Scholar]
Thomas 2014
- Thomas J, Fairclough A, Kavanagh J, Kelly AJ. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database of Systematic Reviews 2014, Issue 6. Art. No: CD003101. [DOI: 10.1002/14651858.CD003101.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turnbull 1996
- Turnbull D, Holmes A, Shields N, Cheyne H, Twaddle S, Gilmour WH, et al. Randomised, controlled trial of efficacy of midwife-managed care. Lancet 1996;348(9022):213-8. [DOI] [PubMed] [Google Scholar]
Vogel 2017
- Vogel JP, Osoti AO, Kelly AJ, Livio S, Norman JE, Alfirevic Z. Pharmacological and mechanical interventions for labour induction in outpatient settings. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD007701. [DOI: 10.1002/14651858.CD007701.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. WHO Recommendations for Induction of Labour. World Health Organization Guidelines 2011:http://apps.who.int/iris/bitstream/10665/44531/1/9789241501156_eng.pdf (accessed 4th April 2017).
Wong 2002
- Wong SF, Hui SK, Choi H, Ho LC. Does sweeping of membranes beyond 40 weeks reduce the need for formal induction of labour? British Journal of Obstetrics and Gynaecology 2002;109:632-6. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kelly 2008
- Kelly AJ, Alfirevic Z, Dowswell T. Outpatient versus inpatient induction of labour for improving birth outcomes. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD007372. [DOI: 10.1002/14651858.CD007372] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2009b
- Kelly AJ, Alfirevic Z, Dowswell T. Outpatient versus inpatient induction of labour for improving birth outcomes. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD007372. [DOI: 10.1002/14651858.CD007372.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2013
- Kelly AJ, Alfirevic Z, Ghosh A. Outpatient versus inpatient induction of labour for improving birth outcomes. Cochrane Database of Systematic Reviews 2013, Issue 11. Art. No: CD007372. [DOI: 10.1002/14651858.CD007372.pub3] [DOI] [PubMed] [Google Scholar]


