Correct 3D genome organization is essential for the proper functioning of the genome. Recent advances in image-based 3D genomics techniques have enabled direct tracing of chromatin folding and multiplexed imaging of nucleome architectures in single cells of several important biological systems. Here, we discuss these advances and the future directions of image-based 3D genomics.
The spatial organization of genomic DNA in the nucleus controls essential genome functions including gene expression regulation, DNA replication, mutation, repair, and recombination, and is crucially involved in many biomedical processes from development to diseases [1-3]. To characterize the spatial genome organization, the sequencing-based high-throughput chromosome conformation capture (Hi-C) technique captures the contacts between genomic regions, and has led to a series of fundamental discoveries of genomic architectures, such as the A–B compartments and topologically associating domains (TADs) [1-3], as well as allowing genome-wide profiling of promoter–enhancer interactions [3]. However, Hi-C cannot directly reveal the 3D positions of the genomic regions or the 3D chromatin folding path. Most Hi-C data provide the averaged contact frequency of many chromosome copies from different cells [1,2]. Other sequencing methods revealed genomic architectures in association with nuclear components with important functional implications, including lamina-associated domains (LADs) and nucleolus-associated domains (NADs) [1,2]. It has been challenging to combine the different sequencing-based methods to profile the multifaceted nucleome organization in the same cells. Alternatively, DNA fluorescence in situ hybridization (FISH) directly images the 3D positions of specific genomic loci and is intrinsically a single cell method [1,2]. However, traditional DNA FISH cannot systematically reveal complex chromatin folding paths. To tackle this issue, in 2016 a highly multiplexed DNA FISH technique, later termed chromatin tracing, was introduced, which enabled direct tracing of the 3D folding path of chromatin along individual chromosomes in single cells [4]. Chromatin tracing has since been applied at different genomic length scales to various cell types and model organisms, including Drosophila embryo [5,6], Caenorhabditis elegans [7], and mammalian tissue [8]. It has also been combined with multiplexed RNA and protein imaging to reveal the functional consequence of chromatin-folding changes [4-6,8], cell-type-specific organization [5,8], and the multifaceted nucleome architectures, including nucleolar and lamina associations in the same cells [7-9]. These developments offer rich information and new insights on 3D genome and nucleome organization.
Fundamental Concepts of Chromatin Tracing and Image-Based 3D Genomics
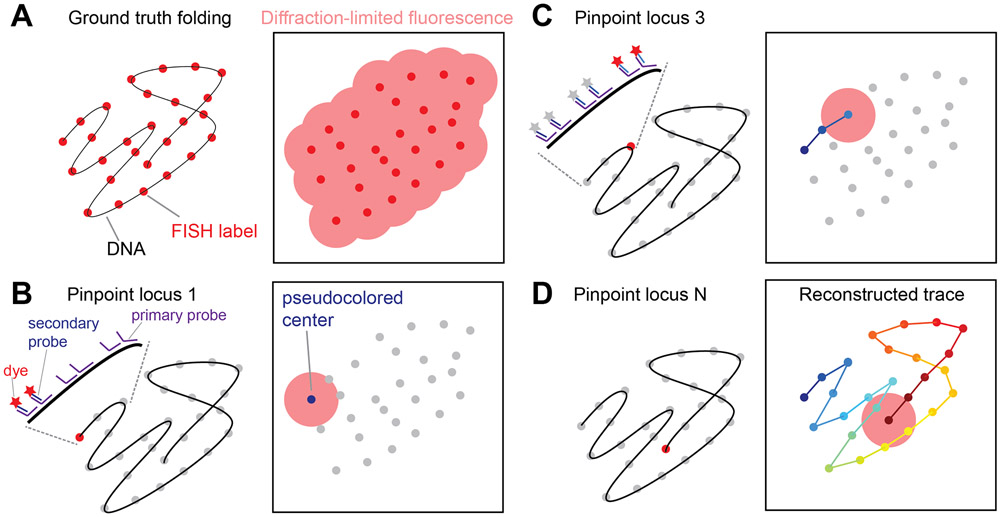
Chromatin tracing pinpoints the 3D positions of numerous genomic loci along the same chromosome, and links the 3D positions based on their genomic identities to reveal the chromatin folding path. Traditional DNA FISH does not allow chromatin tracing because: (i) when many genomic loci are visualized simultaneously with the same (or a few) fluorescence color, one cannot distinguish the genomic identities of the loci; and (ii) fluorescence from many genomic loci is often connected due to diffraction and does not allow individual loci to be resolved (Figure 1A). To tackle these issues, chromatin tracing uses a sequential imaging and reconstruction procedure. First, a library of primary FISH probes is hybridized to all genomic loci of interest. Next, fluorescently labeled secondary FISH probes are sequentially hybridized to the unique overhang regions on the primary probes associated with individual genomic loci. This way each genomic locus is visualized as a diffraction-limited spot in 3D, which allows fitting of its center position with nanoscale accuracy (Figure 1B). The fluorescence from each locus is eliminated after imaging before the next round of sequential hybridization (Figure 1C). Finally, the center positions of the imaged genomic loci are linked based on their genomic identities along the chromosome to reconstruct the effectively super-resolved 3D folding path of chromatin (Figure 1D).
Figure 1.
Concept of Chromatin Tracing. (A) Conventional DNA fluorescence in situ hybridization (FISH) cannot distinguish and resolve many genomic loci along the same chromatin. (B, C) In chromatin tracing, primary FISH probes are hybridized to all genomic loci of interest. Then dye-labeled secondary FISH probes help sequentially visualize the genomic loci as individual diffraction-limited spots, the centers of which can be pinpointed with nanoscale accuracy. The procedures to pinpoint locus 1 (B) and locus 3 (C) are shown. (D) After all loci are visualized and pinpointed, their center positions are linked according to their genomic identities to reconstruct the chromatin trace.
Chromatin Tracing at Different Length Scales and in Different Organisms
Chromatin tracing was first demonstrated at TAD-to-chromosome scale in human cell culture through imaging the center positions of tens of TADs along whole chromosomes, and resolving the folding of these chromosomes with megabase resolution [4]. With this technique, the work discovered that the previously proposed fractal-globule model cannot describe chromosome organization at large scale; that A and B compartments are spatially segregated, stable structures in individual chromosome copies; and that active and inactive X chromosomes adopt drastically different folding schemes [4].
To visualize chromatin structures at sub-TAD scale, the genomic resolution of chromatin tracing was increased to 30 kb, which led to the identification of highly variant TAD-like structures in single chromosomes in cultured human cells [10]. Strikingly, the maintenance of TAD-like structures is independent of cohesin, an important component for establishing looped TADs [10]. Another sub-TAD scale chromatin tracing work showed that maternal and paternal homolog chromosomes are organized in different manners at this scale [11].
Recently, chromatin tracing has been applied to tissues of model organisms. In Drosophila embryos, a work traced the conformation of bithorax complex locus with a genomic resolution of 2–10 kb, and detected distinct promoter–enhancer interactions in different segments [5]. Another work traced chromatin conformation changes during zygotic genome activation in Drosophila embryos with ~10-kb resolution [6]. Chromatin tracing was also applied to C. elegans embryos, and revealed that early embryonic chromosomes in C. elegans adopt an unconventional barbell-like structure facilitated by lamina association [7]. Recently, chromatin tracing was adapted to mouse tissue section and demonstrated in a multiscale fashion across four orders of magnitude of genomic lengths from 5 kb to whole chromosomes, identifying de novo organization of promoter–enhancer interactions, TADs, compartments, and chromosome territories in mouse fetal liver [8].
Recent progress has also increased the genomic coverage and throughput of chromatin tracing. Here, a key concept is to assign each genomic locus a unique combinatorial barcode that is decoded by sequential imaging. A recent work detected barcodes by in situ sequencing, and visualized in total 36 genomic loci on six chromosomes in human cell culture [12]. A recent work traced in total more than 1000 genomic loci along all human chromosomes in cell culture, achieving genome-wide chromatin tracing with megabase resolution [9]. These improvements of chromatin tracing are summarized in Figure 2.
Figure 2.
Milestones of Chromatin Tracing. The genomic resolution of chromatin tracing has been improved from megabases (Mb) to kilobases (kb), allowing chromatin organization to be characterized at different scales and in a multiscale fashion in the same cell. The application of chromatin tracing has been expanded from human cell culture to various model organisms. Chromatin tracing is also combined with imaging of other biomolecules to distinguish histone variant distributions, profile RNAs, and study laminar and nucleolar associations of chromatin, which yields more comprehensive understanding of nucleome architectures and their functional relevance. The genomic throughput of chromatin tracing has been improved from tracing tens of genomic loci along a chromosome to genome-wide tracing of 1000 loci. Abbreviation: TAD, topologically associating domain.
Multimodal Imaging of 3D Genome, Nucleome, and Transcriptome
An advantage of imaging-based 3D genomics is the ease of multimodal imaging of RNAs, proteins, and other biomolecules together with chromatin folding. The advantages of multimodal imaging are multifold. First, it reveals how chromatin folding is associated with gene expression. Along this line, multiple works combined chromatin tracing with RNA FISH. A work in Drosophila embryos showed that transcription burst is only weakly associated with promoter–enhancer contacts for multiple genes, suggesting that sustained promoter–enhancer contacts are not required for the transcription of these genes [5]. Another study observed changes in TAD internal organization associated with sna gene transcription [6]. In mouse fetal liver, A–B compartment changes were shown to be associated with transcription changes, and promoter–enhancer contact associated with essential Scd2 gene expression was identified [8]. A–B compartment changes associated with transcription burst were also observed in human cell culture [9].
A second advantage is to allow cell type-specific analyses in a complex tissue environment. To this end, single-cell RNA profiles of cell type marker genes were used to distinguish cell types in mouse fetal livers and Drosophila embryos, and de novo cell-type-specific promoter–enhancer interactions were identified in both tissues [5,8], as well as distinct cell-type-specific A–B compartmentalization schemes in mouse fetal liver [8].
A third advantage is to visualize chromatin state and other nucleome architectures together with chromatin folding. The initial chromatin tracing work [4] used coimmunofluorescence labeling of a histone variant to distinguish and separately trace the active and inactive X chromosomes in female human cells. Two recent studies measured the spatial associations of traced genomic loci with other nuclear components including nucleoli, lamina, and nuclear speckles [8,9]. As expected, nucleoli and lamina associations are correlated with compartment B chromatin [8,9], while nuclear speckle associations are linked to compartment A chromatin [9]. Furthermore, across different fetal liver cell types, the nucleolar and lamina association levels are only partially explained by A–B compartments, with closely related cell types (erythroblasts and proerythroblasts) having nearly identical A–B compartmentalization schemes but systematically different nucleolar and lamina associations [8]. Figure 2 summarizes the multiplexed imaging of nucleome architectures and cellular components.
We expect further improvements on the genomic resolution and throughput of chromatin tracing to achieve kilobase-resolution, genome-wide chromatin tracing, which may be achieved by increasing the number of imaging rounds and improving the barcode scheme to avoid imaging spatially adjacent loci simultaneously, and by super-resolution or expansion microscopy to resolve adjacent loci in the same image. We also expect the purpose of chromatin tracing to be expanded to profile the epigenome in ways conceptually similar to the demonstrated coimaging of histone variant distribution in chromatin tracing [4]. Most importantly, we expect chromatin tracing to be applied to a wide variety of biomedical contexts. Particularly, adaptation to fresh frozen or formalin-fixed paraffin-embedded clinical samples will open up opportunities to study chromatin folding and nucleome architectures in human health and disease, and may lead to new diagnosis, prognosis, and treatment strategies.
In summary, chromatin tracing illuminates the 3D genome and nucleome, and is a powerful tool to dissect the rich information beyond the genome sequences.
References:
- 1.Bonev B and Cavalli G (2016) Organization and function of the 3D genome. Nat. Rev. Genet 17, 661–678 [DOI] [PubMed] [Google Scholar]
- 2.Gibcus JH and Dekker J (2013) The hierarchy of the 3D genome. Mol. Cell 49, 773–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoenfelder S and Fraser P (2019) Long-range enhancer–promoter contacts in gene expression control. Nat. Rev. Genet 20, 437–455 [DOI] [PubMed] [Google Scholar]
- 4.Wang S et al. (2016) Spatial organization of chromatin domains and compartments in single chromosomes. Science 353, 598–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mateo LJ et al. (2019) Visualizing DNA folding and RNA in embryos at single-cell resolution. Nature 568, 49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardozo Gizzi AM et al. (2019) Microscopy-based chromosome conformation capture enables simultaneous visualization of genome organization and transcription in intact organisms. Mol. Cell 74, 212–222.e5 [DOI] [PubMed] [Google Scholar]
- 7.Sawh AN et al. (2020) Lamina-dependent stretching and unconventional chromosome compartments in early C. elegans embryos. Mol. Cell 78, 96–111.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu M et al. (2020) Multiplexed imaging of nucleome architectures in single cells of mammalian tissue. Nat. Commun 11, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su J-H et al. (2020) Genome-scale imaging of the 3D organization and transcriptional activity of chromatin. Cell 182, 1641–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bintu B et al. (2018) Super-resolution chromatin tracing reveals domains and cooperative interactions in single cells. Science 362, eaau1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nir G et al. (2018) Walking along chromosomes with super-resolution imaging, contact maps, and integrative modeling. PLoS Genet. 14, e1007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen HQ et al. (2020) 3D mapping and accelerated super-resolution imaging of the human genome using in situ sequencing. Nat. Methods 17, 822–832 [DOI] [PMC free article] [PubMed] [Google Scholar]