Abstract
Background
Depression is one of the most common morbidities of the postnatal period. It has been associated with adverse outcomes for women, children, the wider family and society as a whole. Treatment is with psychosocial interventions or antidepressant medication, or both. The aim of this review is to evaluate the effectiveness of different antidepressants and to compare their effectiveness with placebo, treatment as usual or other forms of treatment. This is an update of a review last published in 2014.
Objectives
To assess the effectiveness and safety of antidepressant drugs in comparison with any other treatment (psychological, psychosocial, or pharmacological), placebo, or treatment as usual for postnatal depression.
Search methods
We searched Cochrane Common Mental Disorders's Specialized Register, CENTRAL, MEDLINE, Embase and PsycINFO in May 2020. We also searched international trials registries and contacted experts in the field.
Selection criteria
We included randomised controlled trials (RCTs) of women with depression during the first 12 months postpartum that compared antidepressant treatment (alone or in combination with another treatment) with any other treatment, placebo or treatment as usual.
Data collection and analysis
Two review authors independently extracted data from the study reports. We requested missing information from study authors wherever possible. We sought data to allow an intention‐to‐treat analysis. Where we identified sufficient comparable studies we pooled data and conducted random‐effects meta‐analyses.
Main results
We identified 11 RCTs (1016 women), the majority of which were from English‐speaking, high‐income countries; two were from middle‐income countries. Women were recruited from a mix of community‐based, primary care, maternity and outpatient settings. Most studies used selective serotonin reuptake inhibitors (SSRIs), with treatment duration ranging from 4 to 12 weeks.
Meta‐analysis showed that there may be a benefit of SSRIs over placebo in response (55% versus 43%; pooled risk ratio (RR) 1.27, 95% confidence interval (CI) 0.97 to 1.66); remission (42% versus 27%; RR 1.54, 95% CI 0.99 to 2.41); and reduced depressive symptoms (standardised mean difference (SMD) −0.30, 95% CI −0.55 to −0.05; 4 studies, 251 women), at 5 to 12 weeks' follow‐up. We were unable to conduct meta‐analysis for adverse events due to variation in the reporting of this between studies. There was no evidence of a difference between acceptability of SSRI and placebo (27% versus 27%; RR 1.10, 95% CI 0.74 to 1.64; 4 studies; 233 women). The certainty of all the evidence for SSRIs was low or very low due to the small number of included studies and a number of potential sources of bias, including high rates of attrition.
There was insufficient evidence to assess the efficacy of SSRIs compared with other classes of antidepressants and of antidepressants compared with other pharmacological interventions, complementary medicines, psychological and psychosocial interventions or treatment as usual. A substantial proportion of women experienced adverse effects but there was no evidence of differences in the number of adverse effects between treatment groups in any of the studies. Data on effects on children, including breastfed infants, parenting, and the wider family were limited, although no adverse effects were noted.
Authors' conclusions
There remains limited evidence regarding the effectiveness and safety of antidepressants in the management of postnatal depression, particularly for those with more severe depression. We found low‐certainty evidence that SSRI antidepressants may be more effective in treating postnatal depression than placebo as measured by response and remission rates. However, the low certainty of the evidence suggests that further research is very likely to have an important impact on our effect estimate. There is a continued imperative to better understand whether, and for whom, antidepressants or other treatments are more effective for postnatal depression, and whether some antidepressants are more effective or better tolerated than others.
In clinical practice, the findings of this review need to be contextualised by the extensive broader literature on antidepressants in the general population and perinatal clinical guidance, to inform an individualised risk‐benefit clinical decision. Future RCTs should focus on larger samples, longer follow‐up, comparisons with alternative treatment modalities and inclusion of child and parenting outcomes.
Plain language summary
Antidepressant treatment for postnatal depression
Review question
In this Cochrane Review, we wanted to find out how well antidepressants work for treating women with postnatal depression.
Why this is important
Postnatal depression is depression that starts within 12 months of a woman having a baby. Many women are affected. Postnatal depression can have serious short‐ and long‐term effects on the mother, the baby, and the family as a whole.
There are several ways to treat postnatal depression. These include antidepressant medication, psychological therapy, support or counselling. The type of treatment offered depends on how severe the depression is, other illnesses and the woman's choice. In general, women who are pregnant or breastfeeding are often anxious about the potential unwanted effects of antidepressant medicines on their baby.
It is important to know whether antidepressants could be an effective and acceptable treatment for women with postnatal depression.
What we did
In May 2020, we searched for studies of antidepressants for women with postnatal depression. We looked for randomised controlled trials, in which treatments were given to study participants at random. These studies give the most reliable evidence.
We included 11 studies involving 1016 women. The studies compared antidepressants with placebo (dummy pill), treatment as usual (watch and wait, regular visits with a care co‐ordinator), psychological interventions (therapy), psychosocial interventions (peer support or counselling), any other other medicines or another type of antidepressant; and complementary medicine (food supplements).
Eight of the studies were conducted in English‐speaking, high‐income countries. The length of treatment ranged from four to 24 weeks.
The outcomes we focused on were how well the treatments worked (effectiveness). This was measured by the number of people who responded well to treatment (response) or no longer met criteria for depression at the end of treatment (remission). We also looked at whether women and/or their babies experienced adverse effects with the treatment.
What did we find?
We found that women treated with antidepressants may respond slightly better and have less severe postnatal depression than women given a placebo. The number of unwanted effects experienced by women was similar between groups. There were not enough studies comparing antidepressants with other types of treatment. The most commonly studied antidepressants were from the 'SSRI' (serotonin specific reuptake inhibitor) group.
Conclusions
This review found only a few relevant studies. There is some evidence that antidepressants may work better than a dummy pill for women with postnatal depression. There is not enough evidence comparing antidepressants to other treatments for postnatal depression. Clinicians need to consider study evidence from the general population and current clinical guidelines, along with the woman's illness history and current symptoms, to make an individualised risk‐benefit treatment decision with the woman.
Certainty of the evidence
Our certainty (confidence) in the evidence is low. Some findings are based on only a few studies, with a small number of women in each treatment group. Therefore, we are not sure how reliable the results are. Our conclusions may change if more studies are conducted. Our finding that antidepressants may work better than a dummy pill is similar to findings from a larger number of studies in the general population.
Summary of findings
Background
Description of the condition
Postnatal depression, which is depression that occurs after a woman has given birth, is an important and common disorder that can have short‐ and long‐term adverse impacts on the mother, her child, and the family as a whole (Howard 2014a; Stein 2014). Perinatal suicide, which is closely linked to postnatal depression, is an important contributor to maternal mortality (Grigoriadis 2017; Khalifeh 2016; Knight 2019). Postnatal depression is associated with impaired maternal‐infant attachment, and with internalising and externalising problems in children of mothers who have postnatal depression, particularly where the depression is severe and persistent and there are familial co‐morbidities (Stein 2014). Postnatal depression has a similar epidemiology and clinical presentation to depression in the general population (Howard 2014a; Stewart 2019). It is characterised by persistent low mood and loss of pleasure or interests, occurring with associated symptoms such as changes in appetite and energy levels, disturbed sleep, and low self‐confidence (Howard 2014a; WHO 2018). The 11th revision of the International Classification for Diseases (ICD‐11; WHO 2018), and the 5th revision of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5; APA 2013), recommend the use of generic (non‐perinatal) mood disorder diagnostic categories for depression occurring in the postnatal period, in recognition of the absence of clear evidence for a distinct postnatal depressive clinical syndrome (O'Hara 2013). However, they allow for the use of a secondary perinatal diagnostic category (in ICD‐11) or specifier (in DSM‐5) for depression occurring in pregnancy or within four to six weeks after childbirth.
In the UK and internationally, research and clinical practice have most commonly defined postnatal depression as that occurring within a year of childbirth (Howard 2014a; NICE 2014; Stewart 2016; Stewart 2019), and this is the definition used in this review. However, there is no clear consensus on a definitive timeframe, and past research, practice guidelines, and diagnostic classifications have variably defined postnatal depression as depression occurring within four weeks to 12 months of delivery (O'Hara 2013; Stewart 2019). In the absence of a consensus, it has been helpfully proposed that the relevant timeframe is likely to vary according to study aim, with shorter time frames being most relevant for biological studies and longer time frames for prevention or treatment studies (O'Hara 2013).
A recent systematic review of prevalence and incidence of perinatal (i.e. antenatal and postnatal) depression estimated a pooled prevalence for postnatal depression of 9.5% (95% CI 8.9 to 10.1) in high‐income settings and 18.7% (95% CI 17.8 to 19.7) in low‐ and middle‐income settings, with no significant difference between studies using diagnostic tools (for example, a standardised structured diagnostic interview based on DSM criteria) versus those using symptom scales (such as the Edinburgh Postnatal Depression Scale (EPDS); Woody 2017). There are few incidence studies (Woody 2017), and contradictory evidence on whether depression is more likely to occur in the postnatal period than at other times in a woman’s life (Munk‐Olsen 2006; Silverman 2019; Stewart 2019); with some evidence that the risk is elevated specifically for more severe illness requiring admission (Munk‐Olsen 2009; Munk‐Olsen 2016). Recent evidence suggests that of women who experience postnatal depression, around a third also had depression in pregnancy, and a third had pre‐pregnancy depression (Wisner 2013).
Most women with postnatal depression recover within a few months but about 30% of episodes last beyond the first postnatal year (Goodman 2004). Women who have had postnatal depression in previous pregnancies also have a high risk (about 40%) of both non‐postnatal relapse and postnatal relapse in subsequent pregnancies (Cooper 1995; Wisner 2004).
It is important to distinguish postnatal depression from less severe, short‐lived conditions, such as the 'baby blues', which occur in around 50% of women and resolve spontaneously within a few days (Howard 2014a; Stewart 2019). On the other end of the severity spectrum, it is important to recognise the severe psychiatric emergency of postpartum psychosis, a rare condition affecting one to two women per 1000 in the general population, where admission is recommended to mitigate risks to mother and baby (Jones 2014). Clinically, postnatal depression is often co‐morbid with other conditions, particularly anxiety disorders (Stewart 2019).
Description of the intervention
UK national perinatal guidance recommends treatment for postnatal depression within a stepped‐care model, with antidepressant treatment being recommended for women with more severe depression, with or without combined treatment with psychological therapy (McAllister‐Williams 2017; NICE 2014). The guidance emphasises the higher threshold for antidepressant use in the perinatal period (given the uncertain risks of medication use during pregnancy and whilst breastfeeding, see below), and the importance of taking into account the woman’s preferences, illness severity, past response to treatment, and relative benefits and risks of different treatment options for mother and baby (Howard 2014b).
Antidepressant drugs are commonly divided into the classes of specific serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), and monoamine oxidase inhibitors (MAOIs), with some additional antidepressants that fall outside these classes (e.g. venlafaxine, which is a serotonin and noradrenaline reuptake inhibitor (SNRI), and mirtazapine, which is a noradrenergic and specific serotonergic antidepressant (NaSSA)). Antidepressants across different classes have a similar efficacy, so choice of antidepressant is generally guided by past response, side effect and safety profile. Additionally, in the perinatal period, choice is guided by the extent of safety data available for mothers and babies. In the general population, SSRIs are considered the first‐line antidepressant choice because they are relatively well‐tolerated and less dangerous in overdose than TCAs. In the past decade, SSRIs have been the most commonly prescribed antidepressants during pregnancy and the postnatal period, and have a relatively favourable reproductive safety profile (McAllister‐Williams 2017).
The safety of antidepressants whilst breastfeeding is an important consideration in postnatal depression treatment. Antidepressants ‐ and often their metabolites ‐ are lipid soluble and are transferred in breast milk. However, exposure to antidepressants in breastfed infants is considerably lower (5‐ to 10‐fold) than exposure in utero (Berle 2011). In general, passage of antidepressants into breast milk is low and most antidepressants are not contraindicated whilst breastfeeding (McAllister‐Williams 2017; Stewart 2019). Breastfeeding of premature or ill infants requires care and warrants discussion with paediatricians. There is some evidence from case reports that the less commonly used doxepin and bupropion may be associated with short‐term adverse effects on breastfed infants (McAllister‐Williams 2017; Stewart 2019). For all antidepressants, there is little evidence on long‐term outcomes for exposed infants (Orsolini 2015).
Due to the limitations and scarcity of the existing evidence, most manufacturers' data sheets carry warnings that antidepressants should be avoided in breastfeeding mothers. Some physicians, including general practitioners (GPs), general psychiatrists, or obstetricians, may advise women not to breastfeed when taking an antidepressant, prescribe reduced and potentially ineffective doses, or delay pharmacotherapy until after breastfeeding. However, postnatal depression has potential adverse effects for mother and baby (Howard 2014a; Stein 2014), and these need to be weighed against the uncertain but most likely small risks of medication exposure via breast milk. The choice of medication is usually guided not only by safety data but also past treatment response. Recent guidance recommends that if a mother was successfully treated for depression during her pregnancy, the same medication should be used in the postnatal period while breastfeeding, as discontinuing or switching an antidepressant treatment could lead to relapse (McAllister‐Williams 2017).
In terms of active comparators, evidence‐based psychological interventions for postnatal depression include cognitive behavioural therapy (CBT) and interpersonal therapy (IPT), whilst psychosocial interventions include peer support and non‐directive counselling (Dennis 2007). These interventions were found to be effective when compared to usual care (Dennis 2007).
How the intervention might work
There is substantial evidence showing the effectiveness of antidepressants for depression in the general population, particularly as severity of depression increases (Cipriani 2018). The previous version of this review, published in 2014, concluded that antidepressants were more effective than placebo, but highlighted the very limited evidence base on this, with high risk of bias (Molyneaux 2014). In the general population, the exact mechanism by which antidepressants have their effect is unclear. Antidepressants enhance the functional availability of monoamine transmitters (serotonin, adrenaline and dopamine) through a variety of mechanisms, including inhibition of serotonin reuptake, deactivation of monoamine oxidase and antagonism at some serotonin receptors. However, their therapeutic action is delayed relative to these pharmacological effects, and research suggests that antidepressants may act through effects on synaptic plasticity, and through functional and structural changes in brain circuits related to emotional processing (Harmer 2017; Ma 2015). Postnatal depression is likely to comprise heterogeneous disorders, and it is hypothesised that most women with postnatal depression have depression that is aetiologically similar to depression outside the perinatal period, whereas a small subgroup have depression related to specific vulnerability to postnatal risk factors, such as altered sensitivity to reproductive hormonal changes (Stewart 2019). Therefore, antidepressants are expected largely to work in a similar way for postnatal depression as for non‐perinatal depression. Recently, the US Food and Drug Administration (FDA) licensed a new pharmacological treatment specifically developed for postnatal depression (the neuromodulator, brexanolone) and this is the focus of a separate Cochrane Review (Wilson (in press)).
Why it is important to do this review
This review updates the 2014 Cochrane Review of antidepressants for the treatment of postnatal depression (Molyneaux 2014). Postnatal depression is a common problem that can have adverse short‐ and long‐term effects on the mother, her child, and the wider family; including maternal suffering, problems with mother‐infant attachment, emotional and behavioural problems in children and, rarely, maternal suicide (Howard 2014a; Khalifeh 2016; Stein 2014). In general, women who are pregnant or postnatal have a preference for psychological therapy over medication, and are often anxious about the potential adverse effects of antidepressant use on the unborn or breastfeeding baby (O'Mahen 2008). Antidepressants are recommended for the treatment of severe postnatal depression, the treatment of moderate postnatal depression that has not responded to psychological therapy, and for preventing relapse among women with a history of severe depressive illness (NICE 2014). However, there is only limited evidence on antidepressant efficacy and safety for postnatal depression (Molyneaux 2014). The 2014 Cochrane Review identified six RCTs comparing antidepressants for postnatal depression to placebo or other treatment, with high risk of bias (particularly due to dropout), very limited data comparing antidepressants to psychological therapy, and lack of safety data on child outcomes among breastfeeding mothers (Molyneaux 2014). Since Molyneaux 2014, there has been a considerable growth in perinatal mental health research and services in the UK and internationally, with the UK government investing heavily in the development of community and inpatient perinatal mental health services. There is an urgent need for updated, high‐quality evidence to inform treatment for the growing number of women accessing help for postnatal mood disorders.
We have made minor changes to the Methods of the 2014 review, which are highlighted below. They reflect either changes between the previous protocol (Hoffbrand 2001), and the 2014 review (Molyneaux 2014), or a change in understanding of the clinical context in the scientific literature. The key objectives remain unchanged to Molyneaux 2014.
Objectives
To assess the effectiveness and safety of antidepressant drugs in comparison with any other treatment (psychological, psychosocial, or pharmacological), placebo, or treatment as usual for postnatal depression.
Methods
Criteria for considering studies for this review
Types of studies
We included all published and unpublished RCTs and cluster‐RCTs. We included studies employing a cross‐over design but excluded all other study designs, including quasi‐randomised trials and non‐randomised trials.
Types of participants
Participant characteristics
Women of any age with postnatal depression, who were enrolled into a study and were not taking any antidepressant medication at the start of the study. Following a discussion of the recent scientific literature, we extended the eligible period of treatment onset from delivery to 12 months after giving birth, as opposed to six months after giving birth as used in Molyneaux 2014.
We only included those studies in which treatment was started after the birth. Trials in which treatment started antenatally (regardless of gestation) were excluded. If studies included both women who started treatment before the birth and those who started after, we included the study only if we could extract data on the women who started treatment postnatally.
Diagnosis
We used a broad definition of postnatal depression to include all women who were depressed during the first 12 months postpartum, regardless of time of onset of depression (i.e. including women whose depression started during or before pregnancy). We included studies in which women met criteria for depression by any of the following: use of a validated screening measure, for example, the Edinburgh Postnatal Depression Scale (EPDS; Cox 1987), use of standard observer‐rated depression diagnostic instrument, by a recognised diagnostic scheme (e.g. DSM‐5; APA 2013), or the ICD‐11 (WHO 2018), or by other standardised criteria, for example, the Research Diagnostic Criteria (RDC; Spitzer 1978). The threshold scores used for the respective scales were those used by the study authors.
Co‐morbidities
We included studies that enrolled participants with co‐morbid physical conditions or other psychological disorders (e.g. anxiety) provided the co‐morbidity was not the focus of the study.
Setting
We did not assign any restrictions to the type of study setting.
Types of interventions
Experimental intervention
Antidepressant medication alone or in combination with another antidepressant or treatment, initiated in at least one study arm.
We organised antidepressants into classes for the purposes of this review, for example:
SSRIs: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline;
TCAs: amitriptyline, clomipramine, desipramine, dothiepin, doxepin, imipramine, lofepramine, nortriptyline, protriptyline, trimipramine;
MAOIs; irreversible (isocarboxazid, phenelzine, tranylcipromine); reversible (brofaromine, moclobemide, tyrima);
SNRIs: duloxetine, milnacipran, venlafaxine;
other antidepressants.
Our primary analyses focused on SSRIs, as these are the most commonly used antidepressants for treatment of perinatal depression in recent routine clinical practice (McAllister‐Williams 2017; Yonkers 2014), and are the recommended first‐line antidepressant treatment in recent clinical guidance (McAllister‐Williams 2017; NICE 2014).
Comparator intervention
Placebo, any other treatment, or treatment as usual. Treatment as usual includes, but is not limited to, ‘watch and wait’, regular visits with a care co‐ordinator, or interventions aimed at addressing social risk factors). 'Any other treatment' includes, but is not limited to, psychological interventions (e.g. CBT or IPT), psychosocial interventions (e.g. peer support or non‐directive counselling) and other pharmacological interventions (e.g. another antidepressant). Complementary medicines are eligible as a comparator treatment within this group.
Brexanolone (a GABA‐A neuromodulator) was not included as a comparator intervention, as this novel treatment for postnatal depression was only recently approved by the FDA (in March 2019); and is currently only available in a small number of inpatient settings that can meet the FDA's risk mitigation measures (including medical supervision in an inpatient facility throughout the duration of its intravenous administration). We plan on conducting a separate Cochrane Review to assess its effectiveness and safety in the treatment of postnatal depression.
Types of outcome measures
We included studies that met the above inclusion criteria regardless of whether they reported the following outcomes. We describe narratively any studies that report outcomes not included here.
Primary outcomes
Response or remission of depression, using dichotomous response or remission measures as reported in the individual studies and defined by the study authors. Response is typically measured by the number of women with a reduction of at least 50% on the total score of a standardised depression scale. Remission is typically measured by the number of women whose scores fall below a predefined threshold on a standardised depression scale. We report the study authors’ definitions in this review.
-
Adverse events (or side effects) experienced by:
mother;
nursing baby.
We extracted all adverse events and data from side‐effect scales recorded in the study reports and summarise them narratively. We also report overall proportions of participants experiencing adverse effects by study arm where possible.
Secondary outcomes
Severity of depression based on rating scales (continuous data; either self‐reported, such as the EPDS (Cox 1987), or clinician‐rated, such as the Hamilton Rating Scale for Depression (HDRS; Hamilton 1967)
Acceptability of treatment both as assessed directly by questioning study participants and indirectly by the dropout rates
-
Child‐related outcomes:
neurodevelopment of the infant/child (e.g. cognitive development measured using age‐appropriate observer‐rated or parent‐reported standardised rating scales);
neglect or abuse of the baby (e.g. using the Parent‐Report Multidimensional Neglectful Behavior Scale (Kantor 2004)).
-
Parenting‐related outcomes:
maternal relationship with the baby (e.g. improved mother‐infant interactions measured using the CARE‐Index (Crittenden 1988));
overall maternal satisfaction and confidence;
the establishment or continuation of breastfeeding.
Quality of life (e.g. measured using the 36‐item Short Form (SF‐36; Ware 1992))
Timing of outcome assessment
Early phase: under five weeks
Acute phase: 5 to 12 weeks
Continuation phase: more than 12 weeks
The primary outcome of interest is the acute phase treatment response (between 5 and 12 weeks). Where this was reported, we used any additional reported early and continuation phase responses as secondary outcomes.
See Appendix 1 for descriptions of the most commonly used scales for depression.
Search methods for identification of studies
We identified all studies that described RCTs of antidepressants for postnatal depression from the specialised registers of Cochrane Common Mental Disorders (CCMD) and the Cochrane Pregnancy and Childbirth. We supplemented these with further searches of the key biomedical databases.
Cochrane Common Mental Disorders Controlled Trials Register (CCMDCTR)
The CCMD Group maintains an archived specialised register of RCTs: the CCMD Controlled Trials Register (CCMDCTR). This register contains over 40,000 reference records (reports of RCTs) for anxiety disorders, depression, bipolar disorder, eating disorders, self‐harm, and other mental disorders within the scope of this Group. The CCMDCTR is a partially trials‐based register with more than 50% of reference records tagged to around 12,500 individually PICO‐coded study records. Reports of studies for inclusion in the register were collated from (weekly) generic searches of key bibliographic databases to June 2016, which included: MEDLINE (1950 onwards), Embase (1974 onwards), PsycINFO (1967 onwards), quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), and review‐specific searches of additional databases. Reports of studies were also sourced from international trials registries, drug companies, handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses. Details of CCMD's core search strategies (used to identify RCTs) are on the Group's website, with an example of the core MEDLINE search displayed in Appendix 2.
The CCMDCTR is hosted and maintained on the new Cochrane Register of Studies (CRS). The CCMDCTR fell out of date in June 2016 when the CCMD editorial group moved from the University of Bristol to the University of York.
(Note: the CCMD Group was previously called the Cochrane Collaboration Depression, Anxiety and Neurosis (CCDAN) review group. The Group changed its name in 2015 and the re‐naming of the specialised register from CCDANCTR to CCMDCTR reflects this change).
Electronic searches
The CCMD Information Specialist searched the following biomedical databases using relevant keywords, subject headings (controlled vocabularies) and search syntax, appropriate to each resource (Appendix 3). Searches for the previous version of this review were conducted in April 2014. Search updates for this version were conducted in April 2019 and May 2020. The date of the latest search was 1 May 2020.
CCMDCTR (all years to June 2016)
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 5) via the Cochrane Library (searched 1 May 2020)
OVID MEDLINE (2014 to May 2020)
OVID Embase (2014 to May 2020)
OVID PsycINFO (2014 to May 2020)
We applied no restrictions on date, language, or publication status to the searches.
We searched the international trials registers (US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov searched 1 May 2020); and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 1 May 2020); using terms for postnatal/postpartum depression.
Searching other resources
Reference lists
We performed forward and backward citation tracking of all included studies to identify additional studies missed from the original electronic searches (for example, unpublished or in‐press citations). We did not identify any additional studies.
Personal communication
We requested information on additional ongoing or completed studies from the following sources.
Any pharmaceutical company involved in any of the included studies (as funder, sponsor, or involvement in the research)
Manufacturers of the antidepressant(s) used in any of the included studies
Authors of included studies published within the last five years
The International Marcé Society for Perinatal Mental Health
We searched Cochrane Pregnancy and Child Birth's Controlled Trials Register (CPC) in April 2014. The search did not retrieve any additional, unique studies and we searched it via CENTRAL in the Cochrane Library after this date.
Data collection and analysis
Selection of studies
We managed records retrieved by the literature search in Covidence. Two of three review authors (JB and KA or CW) independently inspected abstracts retrieved from the search. We obtained full‐text articles for any potentially relevant publications. Two of three review authors (JB and KA or CW) independently assessed the full articles for inclusion based on the defined inclusion criteria. We resolved any disagreements through discussion or by recourse to another review author (HK).
We recorded reasons for exclusion of ineligible studies. We collated multiple reports that related to the same study, so that each study rather than each report forms the unit of interest in this review.
Data extraction and management
Using Covidence, we extracted the following data from the included studies.
Methods: date of study, study design, study setting, details of blinding/allocation concealment, total duration of study, details of any 'run‐in' period, number of study centres and location, and withdrawals
Participants: total number and number of each group, inclusion and exclusion criteria, mean age, age range, severity and duration of condition, diagnostic criteria, physical and mental health comorbidities
Interventions: number of intervention groups, type of interventions and comparisons, duration of intervention and key details (e.g. dosage, adherence, quality of delivery), concomitant medications, and excluded medications
Outcomes: details of measures used to assess outcomes (e.g. details of validation), primary and secondary outcomes specified and collected, time points reported, and adverse events.
Analysis: statistical techniques used, unit of analysis for each outcome, subgroup analyses, number of participants followed up from each condition
Notes: publication type, funding for study, and notable conflicts of interest of study authors
Two of four review authors (JB, ES, CW, LR) independently extracted data from included studies. We resolved any disagreements in discussion or by recourse to another review author (HK).
We imported data into RevMan 5.4 for analysis (Review Manager 2020).
Main comparisons
We included the following main comparisons.
Antidepressants versus placebo
Antidepressants versus treatment as usual
Antidepressants versus psychological intervention
Antidepressants versus psychosocial intervention
Antidepressants versus other pharmacological intervention
Antidepressants versus complementary medicine
We present findings per antidepressant class (SSRIs, TCAs, SNRIs, MAOIs, other). We did not pool findings across studies of different antidepressant classes, since the different classes are not sufficiently homogeneous and are likely to have distinct adverse effects.
For our main analyses we focused on SSRI studies (i.e. studies that compare SSRIs versus each of the five comparison groups above).
We present findings separately for any studies reporting results on a mixture of antidepressant classes where data on individual classes are unavailable.
Assessment of risk of bias in included studies
Two of three review authors (JB, ES, or CW) independently assessed risk of bias (as high, low or unclear) for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved any disagreements in discussion or by recourse to another review author (HK).
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other bias (adherence to medication), funding source, conflicts of interest
We used RevMan 5.4 to produce 'Risk of bias' figures based on our assessment of each domain as low, high, or unclear risk (Review Manager 2020). We tried to minimise the use of the unclear category by contacting study authors for further information as needed.
Measures of treatment effect
Dichotomous data
We calculated the risk ratio (RR) and its 95% confidence interval (CI) for primary outcome dichotomous data (Bland 2000).
If possible (e.g. where individual participant‐level data were available), we planned to convert outcome measures to dichotomous data using cut‐off points on rating scales to identify those who did and did not fulfil the criteria for depression.
Continuous data
Where meta‐analysis could be conducted for continuous data, we analysed these by calculating the mean difference (MD) between groups if studies used the same outcome measure for comparison. If studies used different outcome measures to assess the same outcome, we calculated standardised mean difference (SMD) and 95% CIs.
When study authors presented 95% CIs instead of standard deviations (SD), we converted the former to SDs. If study authors did not report SDs and we could not calculate these values from available data, we asked study authors to supply the data. In the absence of data from study authors, we used the mean SD from other studies.
Where study arm‐level data were unavailable, we used mean differences and their standard error (SE) in meta‐analyses using the generic inverse variance method.
Unit of analysis issues
Cluster‐randomised trials
If any cluster‐RCTs had met the inclusion criteria for this review, we planned to extract the intra‐cluster correlation coefficient (ICC) for each study; where no such data were reported, we planned to request the information from study authors. If this information was unavailable, in line with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019), we would have used estimates from similar studies in order to 'correct' data for clustering where this had not been done. We would have used generic inverse variance methods to meta‐analyse results from cluster‐RCTs (Higgins 2019).
Studies with multiple treatment groups
Trials that have more than two arms (e.g. pharmacological intervention (A); psychological intervention (B); and control (C)) can cause issues with regards to pair‐wise meta‐analysis. In line with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019), for any studies with two or more active treatment arms, we took the following approach, dependent on whether the outcome was dichotomous or continuous. For a dichotomous outcome: we combined active treatment groups into a single arm for comparison against the control group (in relation to the number of people with events and sample sizes), or the control group was split equally. For a continuous outcome: we pooled means, SDs, and the number of participants for each active treatment group across treatment arms as a function of the number of participants in each arm to be compared against the control group.
Dealing with missing data
At some degree of loss to follow‐up, data must lose credibility (Xia 2009). The protocol for the review published in 2014 determined that studies with more than 50% loss to follow‐up would be excluded (Molyneaux 2014). However, owing to the small evidence base, the review authors decided to include studies with greater than 50% dropout. In the interest of consistency, we have taken the same approach for this review update. We have assessed the impact of data lost to follow‐up in sensitivity analyses.
In the case where included studies presented binary outcome data for women who were lost to follow‐up, we report the data. We present data on a 'once‐randomised always‐analyse' basis, assuming an intention‐to‐treat (ITT) analysis. We assumed that women lost to follow‐up had a negative outcome, with the exception of the outcome of death. For example, for the outcome of remission of depression, we assumed that this had not occurred for any of the women lost to follow‐up.
We used ITT analysis when available. We anticipated that some studies would have used a variety of imputation methods including last observation carried forward (LOCF), multiple imputation, and mixed‐effect models. All imputation methods require assumptions, which introduce uncertainty about the reliability of the results. Therefore, we indicate where studies have used imputation (and which methods) in this review.
We present ITT analysis for all primary outcomes. Where ITT analyses are unavailable for secondary outcomes, we report this in the relevant section of the results.
Assessment of heterogeneity
Where there were sufficient data for a meta‐analysis, we assessed statistical heterogeneity visually by studying the degree of overlap of the CIs for individual studies in a forest plot. We also carried out more formal assessments using the I2 statistic (Higgins 2003). The I2 statistic only provides an approximate estimate of the variability due to heterogeneity so the following overlapping bands have been used to guide our interpretation of the I2 statistic, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2020):
0% to 40% might not be important;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
75% to 100% represents considerable heterogeneity.
Assessment of reporting biases
If there had been more than 10 studies included in any meta‐analysis, we planned to generate funnel plots and inspect them visually for asymmetry. Asymmetry in the plot might be attributable to publication bias; however, there are other causes of funnel plot asymmetry (heterogeneity unrelated to publication bias) that we also planned to take into consideration.
Data synthesis
We conducted a random‐effects meta‐analysis to synthesise data from studies with comparable methods (using the same class of antidepressants and the same comparison group, e.g. placebo, listening visits) if we identified three or more studies for each comparison. We used RevMan 5.4 for meta‐analysis (Review Manager 2020).
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses to assess the effectiveness of the intervention in the following groups.
Women with mild to moderate depressive disorder (as defined by diagnostic interview or a validated scale) versus women with severe depressive disorder (as defined by diagnostic interview or a validated scale)
Women with chronic depression (onset pre‐pregnancy) versus women with onset in pregnancy versus new‐onset postnatal depression
Interventions lasting eight weeks or less versus interventions lasting more than eight weeks
We planned to compare subgroups using the formal Test for Subgroup Differences in RevMan 5.4 (Review Manager 2020).
We planned to explore and comment on any observed clinical heterogeneity, for example due to different definitions of postnatal depression or use of different diagnostic tools, in the 'Discussion' section of the review.
Sensitivity analysis
We conducted a priori sensitivity analyses (where we identified sufficient data) to explore the robustness of pooled estimates to decisions made in the systematic review. Where data were available, we assessed the effect of excluding studies with the following characteristics.
Study quality: excluding studies that had a high risk of bias in any domain
Blinding: excluding antidepressant versus placebo studies that were unblinded
-
Attrition:
excluding studies with more than 20% attrition; and
excluding studies with more than 50% attrition
Validation: excluding outcomes based on non‐validated scales from the analyses
For outcomes with both skewed data and non‐skewed data, we planned to investigate the effect of combining all data and if there was no substantive difference we left the potentially skewed data in the analyses.
Summary of findings and assessment of the certainty of the evidence
We created 'Summary of findings' tables, where we summarise findings of studies comparing SSRIs with each of the six comparison groups (i.e. placebo; treatment as usual; psychological interventions; psychosocial interventions, other pharmacological interventions and any other intervention). We have presented a separate 'Summary of Findings' table for each comparison group. We included the following outcomes: depression response, depression remission, adverse events (mother), adverse events (baby), depression severity, acceptability of treatment, and child‐related outcomes (where possible we planned to present data for 'child cognitive development'). Where possible, we have presented data for the acute phase treatment response (between five and 12 weeks) in this 'Summary of findings' table.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2020), using GRADEpro software (GRADEpro GDT). We justify all decisions to downgrade the certainty of the evidence using footnotes, and make comments to aid the reader's understanding of the review where necessary.
Two review authors (JB, LR) independently assessed the certainty of the evidence, and resolved disagreements through discussion or by consulting a third review author (HK). Judgements were justified, documented, and incorporated into reporting of results for each outcome.
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review. We avoid making recommendations for practice, and our implications for research suggest priorities for future research and outline what are the remaining uncertainties in the research area.
Results
Description of studies
This is an update of the review published in 2014 with the same title (Molyneaux 2014). We have kept the 2014 study selection criteria for this update with the exception of increasing the eligibility period to 12 months postpartum and removing the exclusion criterion based on attrition rates. These changes to the study selection methods are described in Criteria for considering studies for this review above.
Results of the search
For this version of the review, we searched all databases in April 2019 and updated in May 2020. The searches retrieved 955 records. This included a search of the international trials registers (US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov); and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch)), which we conducted in September 2019 for ongoing studies using search terms for condition (only). We conducted the update search of the trials registers via CENTRAL in the Cochrane Library in May 2020.
A separate search for systematic reviews conducted in this area returned 120 records. We did not identify any new unique studies.
The study selection process is illustrated in Figure 1.
1.
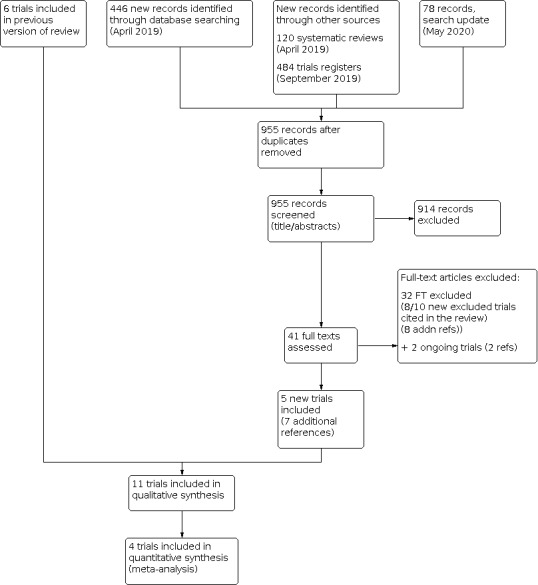
Flow diagram illustrating the study selection process
Included studies
The 2014 review (Molyneaux 2014), included six studies (Appleby 1997; Bloch 2012; Hantsoo 2013; Sharp 2010; Wisner 2006; Yonkers 2008), as well as two studies awaiting classification (Milgrom 2015; Wisner 2015), and one ongoing study (O'Hara 2019).
The 2020 review includes a total of 11 studies (33 references). This includes six included studies from the 2014 review and three studies previously identified as awaiting classification (Milgrom 2015; Wisner 2015) and ongoing (O'Hara 2019). Our update searches in 2019/2020 identified two additional studies eligible for inclusion (Chibanda 2014; Kashani 2017).
Two further studies Andriotti 2017 and IRCT20130418013058N11 are included as new, ongoing studies in this update (Characteristics of ongoing studies). We contacted the authors of both studies to confirm that they were still ongoing. Andriotti 2017 confirmed that, as of January 2020, recruitment for the study had not started. The authors of the IRCT20130418013058N11 study informed us that the study was complete but they did not respond to a request for the full results.
The 11 RCTs included in this review update provided data on 1016 women. Sample sizes ranged from 36 (Hantsoo 2013), to 254 (Sharp 2010), with the three largest studies (O'Hara 2019; Sharp 2010; Wisner 2006), contributing nearly half of the total number of included women (525/1016). All but three studies (Appleby 1997; Wisner 2006; Yonkers 2008), were published within the past 10 years. The majority of studies were conducted in English‐speaking, high‐income countries (Australia: Milgrom 2015; UK: Appleby 1997; Sharp 2010; USA: Hantsoo 2013; O'Hara 2019; Wisner 2006; Wisner 2015; Yonkers 2008). One study was conducted in Israel (high‐income country; Bloch 2012), one in Iran (upper‐middle‐income country; Kashani 2017), and one in Zimbabwe (lower‐middle‐income country; Chibanda 2014).
Participants
Four studies recruited women from community‐based or primary care settings (Appleby 1997; Chibanda 2014; Milgrom 2015; Sharp 2010), one from outpatient clinics (Kashani 2017), one from maternity wards (Bloch 2012), and three studies recruited from a range of settings that included all the above (Hantsoo 2013; O'Hara 2019; Yonkers 2008). Two studies did not report the setting (Wisner 2006; Wisner 2015). All but one study (Appleby 1997) reported a minimum age eligibility criterion. In eight studies, women had to be 18 years or older to be eligible to take part; Wisner 2006 included women aged 15 and over, Yonkers 2008 women aged 16 and over. Bloch 2012 and Wisner 2006 did not report the mean age of the participating women. In all other studies, women were in their mid 20s to early 30s.
All studies specified onset of depression within six months of delivery, with the majority recruiting women within the first three postnatal months.
Eight studies (Bloch 2012; Chibanda 2014; Hantsoo 2013; Kashani 2017; Milgrom 2015; O'Hara 2019; Wisner 2015; Yonkers 2008), used DSM‐IV criteria to establish a diagnosis of depression in eligible women. Appleby 1997 and Sharp 2010 used ICD‐10 criteria while Wisner 2006 used the HAM‐D. Additional diagnostic requirements and the use of screening tools are described in Characteristics of included studies.
All included studies specified a range of appropriate exclusion criteria. On the whole, women with current or recent severe mental illness (e.g. psychotic illness or bipolar disorder), suicidal ideation, acute physical illness, and/or substance abuse were ineligible to take part in the included studies. Some studies also excluded women with 'severe depression', for example, those requiring close monitoring or with high symptom scores (Appleby 1997; Bloch 2012; Chibanda 2014; Hantsoo 2013; Kashani 2017). Of the seven studies that provided data that could be used to characterise baseline depression severity, the mean score was consistent with 'mild depression' in three studies (Appleby 1997; Bloch 2012; Kashani 2017), and with 'moderate depression' in four studies (Chibanda 2014; Hantsoo 2013; O'Hara 2019; Yonkers 2008). Appleby 1997, Kashani 2017 and O'Hara 2019 excluded breastfeeding women (NB: O'Hara 2019 modified their eligibility criteria seven months into the study. Breastfeeding women were eligible from this point.) Hantsoo 2013; Milgrom 2015; O'Hara 2019; and Yonkers 2008 specifically excluded those women who were or were planning to become pregnant at the time of enrolment. Hantsoo 2013 and Yonkers 2008 further excluded women who had an onset of depression during pregnancy. Further details of exclusion criteria used in the individual studies can be found in Characteristics of included studies.
Interventions
With the exception of Sharp 2010, all studies used one pre‐specified antidepressant. Chibanda 2014 used the TCA amitriptyline; all other studies used SSRIs: fluoxetine (Appleby 1997; Kashani 2017); sertraline (Bloch 2012; Hantsoo 2013; Milgrom 2015; O'Hara 2019; Wisner 2006; Wisner 2015); and paroxetine (Yonkers 2008). Sharp 2010 used a pragmatic approach of comparing "antidepressant treatment" to a "wait and see" strategy and listening visits. Sharp 2010 recommended SSRIs as first‐line treatment, which the majority of women in the antidepressant group received.
Where reported, treatment duration ranged from four to 12 weeks. In the majority of studies, interventions were delivered for between four and six weeks. Details on the dosage schedules used in the included studies are reported in Characteristics of included studies.
Comparators
Six studies (Appleby 1997; Bloch 2012; Hantsoo 2013; O'Hara 2019; Wisner 2015; Yonkers 2008), used a placebo control group. Of these six studies, three (Appleby 1997; Bloch 2012; O'Hara 2019), included an additional intervention in both the antidepressant and placebo groups (see Comparison 1 for details).
Three studies included a psychological intervention comparator (Chibanda 2014: group problem‐solving therapy; Milgrom 2015: group‐CBT; O'Hara 2019: IPT). Milgrom 2015 additionally included a combined group.
Two studies included a pharmacological intervention comparator; one comparing two antidepressants (Wisner 2006), and the other comparing an antidepressant to transdermal estradiol (Wisner 2015).
We identified only one study for each of the remaining comparison groups: treatment as usual (Sharp 2010: wait and see), psychosocial interventions (Sharp 2010: listening visits), and complementary medicine (Kashani 2017: saffron).
Eight studies contributed data to one of our comparison groups, whilst three studies (O'Hara 2019; Sharp 2010; Wisner 2015), each contributed data to two of our comparison groups.
See Characteristics of included studies for further details on comparator treatment schedules.
Excluded studies
See Characteristics of excluded studies.
We excluded a total of 10 studies (11 references). Two of these were also excluded in the previous version of this review. In seven studies, antidepressants were started during pregnancy rather than postpartum (Bais 2016; Khazaie 2013; Lambregtse‐Van Den Berg; Molenaar 2016; NCT02185547; NCT02188459; Tahmasian 2013). In two studies, the same antidepressant was given in both arms (Misri 2004; Zhao 2006). Yu 2015 did not report the age of babies and therefore it was not possible to establish that the study met inclusion criteria.
Risk of bias in included studies
As prespecified in our protocol, we only included RCTs in this review update (as was the case in the previously published versions). RCTs are generally considered to offer the most robust evaluation of the effectiveness of an intervention. However, methodological shortcomings can give rise to important biases that might unduly influence the study results. We assessed the risk of such biases in the included studies using the Cochrane 'Risk of bias' tool. We present a summary below and in Figure 2. Details for each study can also be found in Figure 3 and the 'Risk of bias' tables (Characteristics of included studies).
2.

Risk of bias graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each 'Risk of bias' item for each included study
Allocation
All but one study (Hantsoo 2013), described adequately produced randomisation sequences. This usually involved the use of computer software; several studies used permuted block randomisation. The risk of selection bias due to inadequate sequence generation was low across the included studies. However, only four studies (Kashani 2017; Milgrom 2015; Sharp 2010; Wisner 2015), described appropriate means of allocation concealment. The other studies did not report sufficient detail to allow us to judge whether or not the person carrying out the randomisation was able to anticipate the next group allocation as per the randomisation sequence. Consequently, across the included studies, the risk of selection bias due to weaknesses in allocation concealment is unclear.
Blinding
Where possible, we assessed the level of blinding separately for participants (performance bias), personnel (performance bias), and outcome assessors (detection bias).
Performance bias
For an evaluation of the risk of performance bias in the included studies it is important to bear in mind the nature of treatment and control interventions used. Three studies (Chibanda 2014; Milgrom 2015; Sharp 2010), compared antidepressants with group problem‐solving therapy, group‐CBT (and a combined group) and treatment as usual/listening visits, respectively. Due to the nature of the control interventions, blinding was not possible in these cases. The same is true for those participants in O'Hara 2019 who were part of the IPT arm. Despite blinding not being feasible or possible in these cases, the fact that personnel and participants were unblinded to treatment allocation means that there was a high risk of performance bias in these cases.
Appleby 1997; Bloch 2012; Hantsoo 2013; Kashani 2017; Wisner 2006; Wisner 2015; and Yonkers 2008 (all comparing antidepressants with placebo) reported adequate blinding of participants and personnel. Wisner 2015 used a three‐arm design (antidepressant, placebo, transdermal estradiol); appropriate strategies to blind participants and study personnel were reported for all three arms (placebo was available as both patches (to mimic estradiol) and pills so that participants did not know if they were in one of the two active treatment groups or the placebo group). O'Hara 2019 reported adequate blinding of participants and personnel for comparison between antidepressant treatment and placebo. In these instances, the risk of performance bias was low.
Detection bias
The majority of studies (Appleby 1997; Hantsoo 2013; Kashani 2017; O'Hara 2019; Wisner 2006; Wisner 2015; Yonkers 2008), reported adequate blinding of outcome assessors and were consequently at a low risk of detection bias. Three studies (Bloch 2012; Chibanda 2014; Milgrom 2015), reported insufficient detail to allow an assessment of detection bias (unclear risk of bias). Sharp 2010 explicitly stated that outcome assessors were not blinded (high risk of bias).
Incomplete outcome data
With the exception of Bloch 2012, all studies were at either high (Kashani 2017; Wisner 2006; Yonkers 2008), or unclear (Appleby 1997; Chibanda 2014; Hantsoo 2013; Milgrom 2015; O'Hara 2019; Sharp 2010; Wisner 2015), risk of attrition bias. This was mostly due to unclear or incomplete reporting of participants dropping out/lost to follow‐up and how this was addressed in analyses. Those studies judged to be at high risk of attrition bias reported imbalances in attrition between groups (Wisner 2006), a high dropout rate with reasons not reported (Yonkers 2008), or did not perform an ITT analysis (Kashani 2017).
Selective reporting
The assessment of reporting bias relies mostly on an inspection of the study protocol or registration record to assess if all pre‐specified outcomes were collected, reported, and analysed as planned. Trial protocols are not always published and trial registry entries are often poorly maintained. As such, for six out of the 11 included studies we were unable to assess the risk of reporting bias (unclear risk: Appleby 1997; Bloch 2012; Chibanda 2014; Hantsoo 2013; Wisner 2006; Wisner 2015). We judged the five remaining studies to be at high risk of reporting bias: Kashani 2017 (remission and response not defined in protocol, reported follow‐up schedule different from protocol); Milgrom 2015 (remission not defined in protocol); O'Hara 2019 (primary/secondary outcomes different between study publication and protocol); Sharp 2010 (paper does not report all outcomes specified in protocol); and Yonkers 2008 (scales reported in Methods section of paper not reported in Results).
Other potential sources of bias
Similarly, the assessment of other potential sources of bias is often hampered by incomplete reporting. Risk of bias for this domain was unclear for eight of the 11 included studies (Appleby 1997; Bloch 2012; Chibanda 2014; Hantsoo 2013; Kashani 2017; O'Hara 2019; Wisner 2015; Yonkers 2008). Two studies were at high risk of bias from group differences in attrition from the study (Sharp 2010), and low adherence (Milgrom 2015). We judged Wisner 2006 to be at low risk of bias from other sources.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Summary of findings 1. Antidepressants versus placebo for postnatal depression.
| Antidepressants versus placebo for postnatal depression | ||||||
|
Patient or population: women of any age, with PND up to 12 months Settings: any Intervention: antidepressant (SSRI) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of women (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with antidepressant | |||||
|
Depression response (acute, 5 ≤ 12 weeks) |
427 per 1000 | 543 per 1000 (414 to 719) |
RR 1.27 (0.97 to 1.66) |
205 (4 RCTs) | ⊕⊕⊝⊝a,b,c Low |
Three studies defined response as ≥ 50% reduction in EPDS or HAM‐D score from baseline. One study defined response as CGI‐I score of 1 or 2. |
|
Depression remission (acute, 5 ≤ 12 weeks) |
272 per 1000 | 419 per 1000 (269 to 655) |
RR 1.54 (0.99 to 2.41) |
205 (4 RCTs) | ⊕⊕⊝⊝a,b,c Low |
All studies defined remission as a score of ≤ 7 or ≤ 8 on EPDS or HAM‐D scales |
| Adverse events | Yonkers 2008 reported that some side effects appeared more common in the antidepressant group, no significant differences were found. Hantsoo 2013 reported side effects in 3/17 women from the sertraline group and 1/19 in the placebo group. No women dropped out due to side effects. Bloch 2012 showed no significant difference between treatment groups at week 8 (P = 0.46) or at week 12 (P = 0.94) in UKU Side Effect Rating scores, although the overall proportion of women experiencing side effects in each group was not given, neither were the details of types of side effects experienced. Appleby 1997 reported that 1 woman dropped out of the fluoxetine group and 3 women dropped out of the placebo group due to side effects, but the nature of these side effects was not reported. Side effects were only reported among women who dropped out of the study. Wisner 2015 used the Asberg rating scale for side effects. Comparing items rating moderate intensity, the only significant difference was for headaches, which were more common in the placebo (75%) group than the sertraline (43%) group (P = 0.03). For items rated 3 (severe), no significant differences between treatment groups were observed. 5 women (4/30 from sertraline group and 1/29 from placebo group) were withdrawn from the study as 4 developed hypomania (3 sertraline, 1 placebo) and 1 developed psychosis (sertraline). OHara 2019 reported that serious adverse events occurred in 10 women treated with sertraline and 7 women treated with placebo. |
⊕⊕⊝⊝d Low |
||||
|
Depression severity ‐ overall (acute, 5 ≤ 12 weeks) |
The mean depression severity score was between 7 and 13 | SMD 0.30lower (0.55 lower to 0.05 lower) |
‐ | 251 (4 RCTs) | ⊕⊕⊝⊝a,c Low |
Measured with HAM‐D or EPDS. Lower mean score = less severe depression. SMD 0.30 represents a small effect in favour of SSRI. This reflects a reduction of 1.68 on the EPDS scale or a reduction of 2.08 on the HAMD scale. |
|
Depression severity ‐ EPDS (acute, 5 ≤ 12 weeks) |
The mean EPDS score (acute phase) was between 8 and 14 | MD 3.51 lower (6.24 lower to 0.78 lower) | ‐ | 122 (2 RCTs) |
⊕⊕⊝⊝c,e Low |
Lower mean score = less severe depression |
| Treatment acceptability ‐ dropouts | 271 per 1000 |
298 per 1000 (201 to 445) |
RR 1.10 (0.74 to 1.64) |
233 (4 RCTs) |
⊕⊕⊝⊝a,b,c Low |
|
| Child‐related outcomes | See comment | ‐ | ‐ | ‐ | No study reported this outcome | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CGI: Clinical Global Impressions; EPDS: Edinburgh Postnatal Depression Scale; HAM‐D: Hamilton Rating Scale for Depression; MD: mean difference; PND: postnatal depression; RR: risk ratio; SMD: standardised mean difference; SSRI: selective serotonin reuptake inhibitors | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for high risk of attrition and reporting bias in one study, unclear risk of selection, detection, reporting and other bias in three other studies. bDowngraded one level for imprecision as the confidence interval includes no effect and appreciable harm. cDowngraded one level for imprecision as the number of participants is less than 400. dDowngraded two levels for high risk of attrition bias in one study and unclear risk of attrition bias in the remaining three studies, high risk of reporting bias in one study and unclear risk of reporting bias in the remaining three studies, unclear risk of selection bias in three studies, unclear risk of performance bias in one study and unclear risk of other bias in all four studies. eDowngraded one level for unclear risk of selection bias, attrition bias, reporting bias and other bias in two studies.
Summary of findings 2. Antidepressants versus treatment as usual for postnatal depression.
| Antidepressants compared with treatment as usual for postnatal depression | ||||||
|
Patient or population: women of any age, with PND up to 12 months Settings: any Intervention: antidepressant (based on GP or participant choice) Comparison: treatment as usual (including, but not limited to, ‘watch and wait’, regular visits with a care co‐ordinator, or interventions aimed at addressing social risk factors) | ||||||
| Outcomes | Assumed absolute effects* (95% CI) | Relative effect (95% CI) | No of women (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with treatment as usual | Risk with antidepressant | |||||
|
Depression response or remission (acute, 5 ≤ 12 weeks) |
See comment | ‐ | ‐ | ‐ | No studies reported this outcome at this time point | |
| Adverse events | No adverse events occurred | ‐ | 254 (1 RCT) |
⊕⊝⊝⊝a,b Very low |
||
|
Depression remission (early phase, < 5 weeks) |
196 per 1000 | 454 per 1000 (295to 695) | RR 2.31 (1.50 to 3.54) | 218 (1 RCT) | ⊕⊝⊝⊝a,b Very low |
Depression remission defined as EPDS score < 13 |
|
Depression severity ‐ mean EPDS score (early phase, < 5 weeks) |
The mean EPDS score was 16 | MD 2.50 lower (3.85 lower to 1.15 lower) | ‐ | 225 (1 RCT) | ⊕⊝⊝⊝a,b Very low |
Lower mean score = less severe depression |
|
Treatment acceptability ‐ dropouts (early phase, < 5 weeks) |
104 per 1000 |
178 per 1000 (95 to 336) |
RR 1.71 (0.91 to 3.23) | 254 (1 RCT) | ⊕⊝⊝⊝a,b,c Very low |
From the reported data, it was not possible to determine whether this difference in withdrawal was due to a lack of acceptability of treatment with antidepressants or to other factors. |
| Child‐related outcomes | See comment | ‐ | ‐ | ‐ | No study reported this outcome | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EPDS: Edinburgh Postnatal Depression Scale; MD: mean difference; PND: postnatal depression; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels due to high risk of performance, detection, reporting and other biases. bDowngraded one level for imprecision as the number of participants is less than 400. cDowngraded one level for imprecision as the confidence interval includes no effect and appreciable harm.
Summary of findings 3. Antidepressants versus psychological interventions for postnatal depression.
| Antidepressants compared with psychological interventions for postnatal depression | ||||||
|
Patient or population: women of any age, with PND up to 12 months Settings: any Intervention: antidepressants (SSRI or TCA) Comparison: psychological interventions (group problem solving therapy, group CBT, IPT) | ||||||
| Outcomes | Assumed absolute effects* (95% CI) | Relative effect (95% CI) | No of women (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with psychological interventions | Risk with antidepressants | |||||
|
Depression response or remission (acute phase, 5 ≤ 12 weeks) |
See comment | ‐ | ‐ | ‐ | One 3‐armed study reported no significant difference in response, defined as ≥ 50% reduction in HAM‐D‐symptoms from baseline (P = 0.05) or remission, defined as HAMD‐17 score ≤ 7 (P = 0.37), but the between‐group mean differences were not presented. | |
| Adverse events | Chibanda 2014 reported that 3 (11%) women in the amitriptyline arm discontinued due to adverse events. There were no adverse events in the women receiving group problem solving therapy. OHara 2019 reported serious adverse events in 10/56 women treated with sertraline and 8/53 women treated with IPT. Although authors state the number of instances of serious suicidal ideation, suicide attempt, and worsening neurovegetative symptoms (N = 5), infant hospitalisations (N = 11) and participant hospitalisations (N = 9), it is not possible to determine how many occurred in which arm, or indeed if any occurred in the placebo arm of this 3‐armed study. Milgrom 2015 reported no adverse events. |
⊕⊕⊝⊝a Low |
||||
|
Depression severity ‐ mean EPDS score (acute phase, 5 ≤ 12 weeks) |
The mean EPDS score was 8 | MD 2.48 higher (0.71 higher to 4.25 higher) | ‐ | 49 (1 RCT) | ⊕⊕⊝⊝b,c Low |
Lower mean score = less severe depression |
| Treatment acceptability ‐ dropouts | 100 per 1000 |
107 per 1000 (24 to 488) |
RR 1.07 (0.24 to 4.88) | 58 (1 RCT) | ⊕⊝⊝⊝b,c,d Very low |
|
| Child‐related outcomes | see comment | ‐ | ‐ | ‐ | No studies reported this outcome | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CBT: cognitive behavioural therapy; EPDS: Edinburgh Postnatal Depression Scale; HAM‐D: Hamilton Rating Scale for Depression; IPT: interpersonal therapy; PND: postnatal depression; RR: risk ratio; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressant | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for high risk of performance, reporting and other biases. bDowngraded one level for imprecision as the number of participants is less than 400. cDowngraded one level for high risk of performance bias. dDowngraded one level for imprecision as the confidence interval includes no effect and appreciable harm.
Summary of findings 4. Antidepressants versus psychosocial interventions for postnatal depression.
| Antidepressants compared with psychosocial interventions for postnatal depression | ||||||
|
Patient or population: women of any age, with PND up to 12 months Settings: any Intervention: antidepressant (based on GP or participant choice) Comparison: psychosocial intervention (listening visits) | ||||||
| Outcomes | Assumed absolute effects* (95% CI) | Relative effect (95% CI) | No of women (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with psychosocial intervention | Risk with antidepressant | |||||
|
Depression response or remission (acute phase, 5 ≤ 12 weeks) |
See comment | ‐ | ‐ | ‐ | No study reported this outcome at this time point | |
| Adverse events | No adverse events occurred | ‐ | 254 (1 RCT) |
‐ | ||
| Depression severity (acute phase, 5 ≤ 12 weeks) | See comment | ‐ | ‐ | ‐ | No study reported this outcome | |
| Treatment acceptability ‐ dropouts | 128 per 1000 |
248 per 1000 (143 to 429) |
RR 1.94 (1.12 to 3.35) | 254 (1 RCT) |
⊕⊝⊝⊝a,b Very low |
From the reported data, it was not possible to determine whether this difference in withdrawal was due to a lack of acceptability of treatment with antidepressants or to other factors. |
| Child‐related outcomes | See comment | ‐ | ‐ | ‐ | No study reported this outcome | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GP: general practitioner; PND: postnatal depression; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for high risk of performance, detection, reporting and other biases. bDowngraded one level for imprecision as the number of participants is less than 400.
Summary of findings 5. Antidepressants versus any other pharmacological intervention for postnatal depression.
| Antidepressants compared with any other pharmacological intervention for postnatal depression | ||||||
|
Patient or population: women of any age, with PND up to 12 months Settings: any Intervention: antidepressant (sertraline) Comparison: any other pharmacological intervention (nortriptyline or estradiol) | ||||||
| Outcomes | Assumed absolute effects* (95% CI) | Relative effect (95% CI) | No of women (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with any other pharmacological intervention | Risk with antidepressant | |||||
|
Depression response (acute phase, 5 ≤ 12 weeks) |
600 per 1000 | 588 per 1000 (456 to 762) | RR 0.98 (0.76 to 1.27) | 165 (2 RCTs) |
⊕⊝⊝⊝a,b,c Very low |
Both studies defined response as ≥ 50% reduction in HAM‐D from baseline. |
|
Depression remission (acute phase, 5 ≤ 12 weeks) |
413 per 1000 see comment | 404 per 1000 (281 to 582) | RR 0.98 (0.68 to 1.41) | 165 (2 RCTs) |
⊕⊝⊝⊝a,b,c Very low |
Both studies defined remission as a score ≤ 7 or 8 on the HAM‐D scale. |
| Adverse events | Both studies reported no difference between groups in the overall number of moderate or severe side effects reported using the Asberg Side Effects Rating Scale. However, in one study, some side effects were more common among women who took nortriptyline than women taking sertraline: moderate to severe thirst (P = 0.02), dry mouth (P = 0.001) and constipation (P = 0.05). Other side effects were more common in the sertraline than nortriptyline group: constant or severe headaches (P = 0.05), increased perspiration (P = 0.04) and hot flushes interrupting sleep (P = 0.04) | ‐ | ‐ | ‐ | ‐ | |
|
Depression response (early phase, < 5 weeks) |
No significant difference in response (50% reduction in HAM‐D from baseline) between sertraline and nortriptyline at week 4 (P = 0.29) | ‐ | 109 (1 RCT) |
⊕⊕⊝⊝a,b Low |
||
|
Depression remission (early phase, < 5 weeks) |
No significant difference in remission (≤ 7 or 8 on the HAM‐D scale) between sertraline and nortriptyline at week 4 (P = 0.79). | ‐ | 109 (1 RCT) |
⊕⊕⊝⊝a,b Low |
||
|
Depression severity‐ mean HAM‐D scores (acute phase, 5 ≤ 12 weeks) |
The mean HAM‐D score was 10 | MD 0.65 lower (3.18 lower to 1.87 higher) | ‐ | 165 (2 RCTs) |
⊕⊝⊝⊝a,b,c Very low |
Lower mean score = less severe depression |
| Treatment acceptability ‐dropouts | 241 per 1000 | 419 per 1000 (238 to 737) | RR 1.74 (0.99 to 3.06) | 109 (1 RCT) |
⊕⊝⊝⊝a,b,c Very low |
|
| Child‐related outcomes | see comment | ‐ | ‐ | ‐ | No study reported this outcome | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HAM‐D: Hamilton Rating Scale for Depression; MD: mean difference; PND: postnatal depression; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for high risk of attrition bias in one study. bDowngraded one level for imprecision as the number of participants is less than 400. cDowngraded one level for imprecision as the confidence interval includes no effect and appreciable harm.
Summary of findings 6. Antidepressants versus any complementary medicine.
| Antidepressants compared with any other intervention for postnatal depression | ||||||
|
Patient or population: women of any age, with PND up to 12 months Settings: any Intervention: antidepressant (fluoxetine) Comparison: any complementary medicine (saffron) | ||||||
| Outcomes | Absolute assumed effects* (95% CI) | Relative effect (95% CI) | No of women (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with any other intervention | Risk with antidepressant | |||||
|
Depression response (acute phase, 5 ≤ 12 weeks) |
406 per 1000 | 500 per 1000 (288 to 861) | RR 1.23 (0.71 to 2.12) | 64 (1 RCT) | ⊕⊝⊝⊝a,b,c Very low |
Depression response defined as ≥ 50% decrease in HAM‐D |
|
Depression remission (acute phase, 5 ≤ 12 weeks) |
188 per 1000 |
219 per 1000 (83 to 579) |
RR 1.17 (0.44 to 3.09) | 64 (1 RCT) | ⊕⊝⊝⊝a,b,c Very low |
Depression remission defined as score ≤ 7 HAM‐D |
| Adverse events | Frequencies of adverse events were not significantly different between the 2 groups. No major adverse event and no death occurred | ‐ | ‐ | ‐ | ‐ | |
| Depression severity | See comment | ‐ | ‐ | ‐ | No study reported this outcome | |
| Treatment acceptability | See comment | ‐ | ‐ | ‐ | No study reported this outcome | |
| Child‐related outcomes | See comment | ‐ | ‐ | ‐ | No study reported this outcome | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HAM‐D: Hamilton Rating Scale for Depression; PND: postnatal depression; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for high risk of attrition and reporting bias. bDowngraded one level for imprecision as the number of participants is less than 400. cDowngraded one level for imprecision as the confidence interval includes no effect and appreciable harm.
We grouped the included studies into the comparisons described in the Methods.
Comparison 1: antidepressants versus placebo (Appleby 1997; Bloch 2012; Hantsoo 2013; O'Hara 2019; Wisner 2015; Yonkers 2008)
Comparison 2: antidepressants versus treatment as usual (Sharp 2010)
Comparison 3: antidepressants versus psychological intervention (Chibanda 2014; Milgrom 2015; O'Hara 2019)
Comparison 4: antidepressants versus psychosocial intervention (Sharp 2010)
Comparison 5: antidepressants versus any other pharmacological intervention, including other antidepressants (Wisner 2006; Wisner 2015)
Comparison 6: antidepressants versus complementary medicine (Kashani 2017)
Comparison 1: antidepressants versus placebo
Six studies with 482 women contributed data to this comparison (Appleby 1997; Bloch 2012; Hantsoo 2013; O'Hara 2019; Wisner 2015; Yonkers 2008). All studies compared an SSRI against placebo: fluoxetine (Appleby 1997); sertraline (Bloch 2012; Hantsoo 2013; O'Hara 2019; Wisner 2015); and paroxetine (Yonkers 2008). In three studies (Appleby 1997; Bloch 2012; O'Hara 2019), women in both groups received another treatment in addition to the antidepressant/placebo: Appleby 1997 included CBT‐based counselling for both groups; Bloch 2012 brief dynamic psychotherapy; and O'Hara 2019 used clinical management. O'Hara 2019 and Wisner 2015 are three‐armed studies (O'Hara 2019, sertraline versus placebo versus IPT; Wisner 2015, sertraline versus placebo versus transdermal estradiol). We used data from the sertraline and placebo arms in these analyses.
Primary outcomes
1.1. Depression response or remission (acute phase)
Five studies (Bloch 2012; Hantsoo 2013; O'Hara 2019; Wisner 2015; Yonkers 2008), reported depression response during the acute phase (5 to 12 weeks or less). We could not enter data from O'Hara 2019 into the meta‐analysis as it was a three‐armed study and authors reported an F statistic for logistic regression analyses comparing these groups, but there were insufficient data to calculate an RR and 95% CI. Three studies (Bloch 2012; Hantsoo 2013; Wisner 2015), defined response as a 50% or higher reduction in EPDS or HAM‐D score from baseline, and one study (Yonkers 2008), used a CGI‐I score of 1 or 2. Where studies reported response at more than one time point during the acute phase, we included data from the latest measurement within the 5‐ to 12‐week timeframe (Bloch 2012; Wisner 2015; Yonkers 2008; eight weeks, Hantsoo 2013: six weeks). Random‐effects meta‐analysis showed that SSRIs may be associated with a small increase in response rates compared to placebo (RR 1.27, 95% CI 0.97 to 1.66; I2 = 0%; 4 studies, 205 women; Analysis 1.1).
1.1. Analysis.

Comparison 1: Antidepressants vs placebo, Outcome 1: Depression response (acute phase)
Five studies (Bloch 2012; Hantsoo 2013; O'Hara 2019; Wisner 2015; Yonkers 2008), reported remission during the acute phase (5 to 12 weeks or less). We could not enter data from O'Hara 2019 into the meta‐analysis as it was a three‐armed study and authors reported an F statistic for logistic regression analyses comparing these groups, but there were insufficient data to calculate an RR and 95% CI. All studies defined remission as a score of 7 or less or 8 or less on EPDS or HAM‐D scales. Where studies reported remission at more than one time point during the acute phase, we included data from the latest measurement within the 5‐ to 12‐week timeframe (Bloch 2012; Wisner 2015; Yonkers 2008: eight weeks; Hantsoo 2013: six weeks). Random‐effects meta‐analysis showed that SSRIs may be associated with a small increase in remission rates compared to placebo (RR 1.54, 95% CI 0.99 to 2.41; I2 = 26%; 4 studies, 205 women; Analysis 1.2).
1.2. Analysis.

Comparison 1: Antidepressants vs placebo, Outcome 2: Depression remission (acute phase)
1.2. Adverse events
Six studies reported adverse events (Appleby 1997; Bloch 2012; Hantsoo 2013; OHara 2019; Wisner 2015; Yonkers 2008).
Yonkers 2008 reported decreased appetite (antidepressant 9%; placebo 6%), diarrhoea (antidepressant 11%; placebo 11%), dizziness (antidepressant 17%; placebo 9%), dry mouth (antidepressant 11%; placebo 0%), headache (antidepressant 26%; placebo 37%), nausea (antidepressant 14%; placebo 17%), and somnolence and drowsiness (antidepressant 14%; placebo 14%). Although some adverse effects appeared more common in the antidepressant group, there were no significant differences.
Three out of 17 women from the sertraline group and one out of 19 in the placebo group reported adverse effects in Hantsoo 2013. No women dropped out due to adverse effects and Hantsoo 2013 did not report any adverse events for any of the women or their breastfeeding infants.
Bloch 2012 reported a hypomanic switch in two women from the brief dynamic psychotherapy (BDP) plus sertraline group at week 8. UKU Side Effect Rating scores showed no significant difference between treatment groups at week 8 (sertraline, mean 14.0 (SD 11.76) vs placebo, mean 15.10 (SD 14.0) or at week 12 (sertraline, mean 13.72 (SD 10.47) vs placebo, mean 14.0 (SD 10.22) although the study did not give the overall proportion of women who experienced adverse effects in each group, nor the details of types of adverse effects experienced.
In Appleby 1997, one woman dropped out of the fluoxetine group and three women dropped out of the placebo group due to adverse effects, but the nature of these adverse effects was not reported. Adverse effects were only reported among women who dropped out of the study.
Wisner 2015 used the Asberg rating scale for adverse effects. Comparing items rated moderate intensity, the only significant difference was for headaches, which were more common in the placebo (75%) group than the sertraline (43%) group (P = 0.03). For items rated 3 (severe), they did not observe any significant differences between treatment groups. Five women (4/30 from sertraline group and 1/29 from placebo group) were withdrawn from the study as four developed hypomania (3 sertraline, 1 placebo) and one developed psychosis (sertraline).
In OHara 2019, 10 women treated with sertraline and seven women treated with placebo experienced serious adverse events. Although the study authors state that there were five instances of serious suicidal ideation, suicide attempt, and worsening neurovegetative symptoms, 11 instances of infant hospitalisations and nine participant hospitalisations, it is not possible to determine how many occurred in which arm, or indeed if any occurred in the IPT arm of this three‐armed study.
Secondary outcomes
1.3. Severity of depression
All six studies measured severity of depression using EPDS or HAM‐D. We were unable to enter two studies into the meta‐analysis as one did not report standard deviations (Hantsoo 2013), and another only reported the F statistic for linear regression comparing three groups (O'Hara 2019). We contacted authors of both studies for the relevant data but did not get a response. Therefore, only four studies reported data that we could use in a meta‐analysis (Appleby 1997; Bloch 2012; Wisner 2015; Yonkers 2008). Two studies reported mean EPDS scores (Appleby 1997; Bloch 2012), and three reported HAM‐D scores (Appleby 1997; Wisner 2015; Yonkers 2008). As the Appleby 1997 study used both scales, we used EPDS data in the meta‐analysis. Random‐effects meta‐analysis showed that SSRIs may be associated with reduced depressive symptoms in the acute phase (5 ≤ 12 weeks; SMD −0.30, 95% CI −0.55 to −0.05; I2 = 0%; 4 studies, 251 women; Analysis 1.3).
1.3. Analysis.

Comparison 1: Antidepressants vs placebo, Outcome 3: Severity of depression ‐ overall (acute phase)
Appleby 1997 reported geometric means and 95% CIs. Using the methods in Higgins 2008, we converted the geometric means and 95% CIs into standard means and SDs to enter into our meta‐analysis. We acknowledge that there are limitations in computing these data: it is unclear how well the formula works in small samples and usually the geometric means are used because of skewed data. Therefore, we conducted a sensitivity analysis without the data from Appleby 1997 to see how this study affected the results (see Analysis 8.1; Analysis 8.2).
8.1. Analysis.

Comparison 8: Sensitivity analysis ‐ antidepressant vs placebo, excluding studies with imputed data, Outcome 1: Severity of depression ‐ overall (acute phase)
8.2. Analysis.

Comparison 8: Sensitivity analysis ‐ antidepressant vs placebo, excluding studies with imputed data, Outcome 2: Severity of depression ‐ HAM‐D (acute phase)
Edinburgh Postnatal Depression Scale (EPDS)
Three studies (Appleby 1997; Bloch 2012; Hantsoo 2013), reported mean EPDS in the acute phase (5 ≤ 12 weeks). Hantsoo 2013 did not report SDs. We contacted the study authors for these data but did not get a response. Appleby 1997 reported the geometric mean and 95% CIs. Using the methods in Higgins 2008, we converted these to the standard mean and SDs. Meta‐analysis of two studies showed that SSRIs may be associated with a reduction in the mean EPDS score in the acute phase (MD −3.51, 95% CI −6.24 to −0.78; I2 = 5%); 2 studies, 122 women; Analysis 1.4).
1.4. Analysis.

Comparison 1: Antidepressants vs placebo, Outcome 4: Severity of depression ‐ EPDS (acute phase)
Appleby 1997 also reported geometric mean EPDS and 95% CIs in the early phase. Using the methods in Higgins 2008, we converted these to the standard mean and SDs. At week 4, the mean EPDS was 9.97 (SD 6.95) in 43 women treated with fluoxetine and 10.78 (SD 7.36) in 44 women given a placebo.
Hamilton Rating Scale for Depression (HAM‐D)
Five studies (Appleby 1997; Hantsoo 2013; O'Hara 2019; Wisner 2015; Yonkers 2008), reported mean HAM‐D scores in the acute phase. However, we could not use data from two studies: Hantsoo 2013 did not report SDs and O'Hara 2019 only reported the F statistic for linear regression. For the three studies with usable data, where outcomes were reported at more than one time point during the acute phase, we included data from the latest measurement within the 5‐ to 12‐week timeframe (Appleby 1997: 12 weeks; Wisner 2015; Yonkers 2008: eight weeks). Random‐effects meta‐analysis showed no difference between SSRIs and placebo (MD −2.02, 95% CI −4.46 to 0.42; I2 = 39%; 3 studies, 216 women; Analysis 1.5). The O'Hara 2019 three‐armed study also reported no significant differences between sertraline, placebo and IPT (P = 0.45) but did not report the between‐group mean differences.
1.5. Analysis.

Comparison 1: Antidepressants vs placebo, Outcome 5: Severity of depression ‐ HAM‐D (acute phase)
Clinical Global Impressions‐Severity (CGI‐S)
Three studies (Bloch 2012; O'Hara 2019; Yonkers 2008), reported CGI‐S in the acute phase. O'Hara 2019 reported no differences between the three treatments (sertraline, placebo and IPT; P = 0.56) but we could not enter data into a meta‐analysis as they reported between‐group mean differences but not 95% confidence intervals. Meta‐analysis of two studies indicated no difference in CGI‐S scores between SSRI and placebo (MD −0.69, 95% CI −1.87 to 0.48; I2 = 85%; 2 studies, 110 women; Analysis 1.6).
1.6. Analysis.

Comparison 1: Antidepressants vs placebo, Outcome 6: Severity of depression ‐ CGI‐S (acute phase)
Clinical Global Impressions‐Improvement (CGI‐I)
Two studies (Bloch 2012; O'Hara 2019), reported mean CGI‐I score in the acute phase. O'Hara 2019 reported no differences between the three treatments (sertraline, placebo and IPT; P = 0.38) but we could not enter data into a meta‐analysis as they reported between‐group mean differences. Bloch 2012 reported no differences in mean CGI‐I scores between sertraline (1.56 (SD 1.25)) and placebo (0.65 (SD 1.32)) at week 12 (P = 0.83)
Montgomery–Åsberg Depression Rating Scale (MADRS)
One study (Bloch 2012), reported no difference in mean MADRS score between sertraline (3.78 (SD 3.49)) and placebo (5.94 SD 6.17)) at week 12 (P = 0.21).
Inventory of Depressive Symptomatology, Self Report (IDS‐SR)
One study (Yonkers 2008), reported IDS‐SR in the acute phase. The mean IDS‐SR was significantly lower in the paroxetine group (14.0 (SD 11.6)) than the placebo group (22.6 (SD 14.1)) at week 8 (P = 0.02). However, IDS‐SR scores were significantly different between the antidepressant and placebo groups at baseline and the study authors concluded that this baseline difference was carried over at later time points, as the group‐by‐time interaction effect was not significant.
Inventory of Depression and Anxiety Symptoms (IDAS‐GD)
O'Hara 2019 reported that sertraline maybe associated with faster improvement than placebo from baseline to the 12‐week assessment but did not report the between‐group mean differences.
Beck Depression Inventory (BDI)
O'Hara 2019 reported there may be a difference between the three treatment groups for mean BDI at 12 weeks but did not report the between‐group mean differences.
Mental Health Inventory (MHI)
One study (Bloch 2012), reported MHI Wellbeing in the acute phase but there was no difference between sertraline and placebo at week 12. Bloch 2012 also reported MHI Distress but there was no difference between sertraline and placebo at week 12 (sertraline, mean 2.3 (SD 0.63) vs placebo, mean 2.69 (SD 0.78).
1.4. Treatment acceptability
Four studies measured dropouts due to any reason (Appleby 1997; Bloch 2012; Hantsoo 2013; Yonkers 2008). Meta‐analysis showed no difference between treatments (34% drop out in antidepressant group vs 27% drop out in placebo group; RR 1.10, 95% CI 0.74 to 1.64; I2 = 0%; 4 studies, 233 women; Analysis 1.7).
1.7. Analysis.

Comparison 1: Antidepressants vs placebo, Outcome 7: Treatment acceptability ‐ dropouts
Yonkers 2008 measured treatment compliance. Among the 35 women on paroxetine, seven were taking less than 80% of medication at one visit, four were non‐adherent on a second visit and one was excluded from the study for ongoing non‐adherence. Among the 35 women on placebo, 10 were non‐compliant at one visit, three were non‐compliant at two visits and one was non‐compliant on a fourth visit. Appleby 1997; Bloch 2012 and Hantsoo 2013 did not provide any details on adherence to medications in either treatment arm.
1.5. Child‐related outcomes
No data available
1.6. Parenting‐related outcomes
No data available
1.7. Quality of life
No data available
Comparison 2: antidepressants versus treatment as usual
One study (Sharp 2010; 254 women), compared antidepressants with treatment as usual and reported outcomes assessed at four weeks. There were no set antidepressants as women received prescriptions based on the GP or woman's choice. Most women were prescribed citalopram, fluoxetine or sertraline (see Characteristics of included studies table for details).
Primary outcomes
2.1. Depression response or remission (acute phase)
Sharp 2010 did not report depression response or remission during the acute phase (5 to ≦ 12 weeks). Outcomes reported at four weeks are included below as secondary outcomes.
2.2. Adverse events
Sharp 2010 did not report any adverse events or serious adverse effects of treatment. They did not report any data on adverse effects related to infants or the safety of breastfeeding.
Secondary outcomes
2.3. Depression response or remission (early phase)
Results reported by Sharp 2010 showed higher remission (EPDS score less than 13) rates in the antidepressant group compared with treatment as usual after four weeks (RR 2.31, 95% CI 1.50 to 3.54; 1 study, 218 women; Analysis 2.1).
2.1. Analysis.

Comparison 2: Antidepressants vs treatment as usual, Outcome 1: Depression remission (early phase)
2.4. Severity of depression
Sharp 2010 reported severity of depression (mean EPDS score) at four weeks (early phase). The antidepressant group had a lower mean EPDS score (i.e. less severe depression) than the treatment‐as‐usual group (MD −2.50, 95% CI −3.85 to −1.15; 1 study, 225 women; Analysis 2.2).
2.2. Analysis.

Comparison 2: Antidepressants vs treatment as usual, Outcome 2: Severity of depression ‐ EPDS (early phase)
2.5. Treatment acceptability
Sharp 2010 reported dropout rates at four weeks (early phase). Percentage dropout was greater in the antidepressant group compared with the treatment‐as‐usual group (18% dropout in antidepressant group vs 10% dropout in TAU group; RR 1.71, 95% CI 0.91 to 3.23; 1 study, 254 women; Analysis 2.3). From the reported data, it was not possible to determine whether this difference in withdrawal was due to a lack of acceptability of treatment with antidepressants or to other factors. Sharp 2010 assessed adherence using the Morisky Adherence Scale and four items adapted from a scale reported by Schroeder 2006. Trial authors reported low adherence to treatment: the percentage of women randomised to antidepressants who reported actually taking antidepressants in the previous four weeks was 56% at four weeks (59/106; only reported for the women followed up).
2.3. Analysis.

Comparison 2: Antidepressants vs treatment as usual, Outcome 3: Treatment acceptability ‐ dropouts
2.6. Child‐related outcomes
No data available
2.7. Parenting‐related outcomes
Sharp 2010 examined the effect on maternal functioning using the Maternal Adjustment and Maternal Attitudes (MAMA) Attitudes Towards Pregnancy and Baby subscale (postpartum version). They reported data at four weeks (early phase) and 18 weeks (continuation phase). Higher scores indicate a more positive attitude. The study found maternal functioning maybe better in women receiving treatment as usual at four weeks (MD 1.80, 95% CI 0.18 to 3.42; 1 study, 192 women; Analysis 2.4) or at 18 weeks (MD 1.00, 95% CI −0.45 to 2.45; 1 study, 183 women; Analysis 2.5) compared with women receiving antidepressants.
2.4. Analysis.

Comparison 2: Antidepressants vs treatment as usual, Outcome 4: Parenting‐related outcomes ‐ MAMA (early phase)
2.5. Analysis.

Comparison 2: Antidepressants vs treatment as usual, Outcome 5: Parenting‐related outcomes ‐ MAMA (continuation phase)
2.8. Quality of life
Sharp 2010 used the SF‐12 and EQ‐5D to measure quality of life. Higher scores indicated better quality of life. At four weeks, SSRIs were associated with a poorer SF‐12 mental health score than treatment as usual (MD 0.45, 95% CI 0.21 to 0.69; 1 study, 175 women; Analysis 2.6), but there was no difference on the physical health component score (MD 0.03, 95% CI −0.25 to 0.31; 1 study, 175 women; Analysis 2.7). The EQ‐5D mean scores at four weeks were also similar between SSRI and treatment as usual (utility score: MD 0.05, 95% CI −0.01 to 0.11; 1 study, 198 women; Analysis 2.8) and visual analogue scale (MD 5.10, 95% CI −1.62 to 11.82; 1 study, 204 women; Analysis 2.9).
2.6. Analysis.

Comparison 2: Antidepressants vs treatment as usual, Outcome 6: Quality of life ‐ SF‐12 mental health
2.7. Analysis.

Comparison 2: Antidepressants vs treatment as usual, Outcome 7: Quality of life ‐ SF‐12 physical health
2.8. Analysis.

Comparison 2: Antidepressants vs treatment as usual, Outcome 8: Quality of life ‐ EQ5D utility score
2.9. Analysis.

Comparison 2: Antidepressants vs treatment as usual, Outcome 9: Quality of life ‐ EQ5D visual analogue scale
Comparison 3: antidepressants versus psychological interventions
Three studies, with 265 women contributed data to this comparison (Chibanda 2014; Milgrom 2015; O'Hara 2019). Two studies compared the SSRI sertraline (Milgrom 2015; O'Hara 2019), while one study used the TCA amitriptyline (Chibanda 2014). Chibanda 2014 used group problem solving therapy as a comparison. The two remaining studies were three‐armed. Milgrom 2015 (sertraline versus group CBT versus combined sertraline/group CBT). O'Hara 2019 sertraline versus placebo versus IPT. For this comparison, data from the sertraline versus IPT arm was used for the O'Hara 2019 study and for the Milgrom 2015 study, data from the sertraline versus group CBT arm was used.
Primary outcomes
3.1. Depression response or remission (acute phase)
One study (O'Hara 2019) reported response, defined as ≥ 50% reduction in HAM‐D‐symptoms from baseline. Authors reported no statistically significant difference between the treatment groups (sertraline, placebo, IPT: P = 0.05). However, the proportion of responders in each group were not reported thus providing limited clarity on the relative efficacy of sertraline and IPT in comparison with one another and with placebo.
O'Hara 2019 also reported remission, defined as HAM‐D‐17 score ≤ 7. Authors report no statistically significant difference between the treatment groups (sertraline, placebo, IPT: P = 0.37).
3.2. Adverse events
Three study reported adverse events (Chibanda 2014; Milgrom 2015; O'Hara 2019).
Chibanda 2014 reported that 3 (11%) women in the amitriptyline arm discontinued due to adverse events. There were no adverse events in the women receiving group problem solving therapy.
In the OHara 2019 study, serious adverse events occurred in 10/56 women treated with sertraline and 8/53 women treated with IPT. Although authors state the number of instances of serious suicidal ideation, suicide attempt, and worsening neurovegetative symptoms (N=5), infant hospitalisations (N=11) and participant hospitalisations (N=9), it is not possible to determine how many occurred in which arm, or indeed if any occurred in the placebo arm of this three‐armed study.
The study by Milgrom 2015 reported no adverse events.
Secondary outcomes
3.3. Severity of depression
Edinburgh Postnatal Depression scale (EPDS)
Chibanda 2014 reported the mean EPDS scores at six weeks. Authors reported higher mean EPDS scores (i.e., more severe depression) in the amitriptyline group compared with the women who received group problem solving therapy (MD 2.48, 95% CI 0.71 to 4.25; participants = 49; studies = 1; Analysis 3.1)
3.1. Analysis.

Comparison 3: Antidepressants vs psychological interventions, Outcome 1: Severity of depression ‐ EPDS (acute phase)
Hamilton Rating Scale for Depression (HAM‐D)
The O'Hara 2019 study reported no statistically significant differences between the three treatment groups (sertraline, placebo and IPT; P = 0.45) but the between‐group mean differences are not reported
Clinical Global Impressions‐Severity (CGI‐I)
O'Hara 2019 reported no statistically significant difference between mean BDI at 12 weeks between the three treatment groups (sertraline, placebo and IPT; P = 0.38) but the between‐group mean differences are not reported.
Clinical Global Impressions‐Improvement (CGI‐I)
O'Hara 2019 reported no statistically significant difference between mean BDI at 12 weeks between the three treatment groups (sertraline, placebo and IPT; P = 0.55) but the between‐group mean differences are not reported.
Inventory of Depression and Anxiety Symptoms (IDAS‐GD)
O'Hara 2019 reported that sertraline showed faster improvement than IPT from baseline to the eight‐week (P = 0.01) and 12‐week assessment (P = 0.01) but the between‐group mean differences are not reported.
Beck Depression Inventory
Milgrom 2015 reported mean BDI scores in the acute phase. At 12 weeks, the mean BDI was lower in the group who received group CBT than those who received sertraline (MD 6.73, 95% CI 1.51 to 11.95; participants = 29; studies = 1; Analysis 3.2). O'Hara 2019 reported that there may be a difference in mean BDI at 12 weeks between the three treatment groups (sertraline, placebo and IPT; P = 0.06) but the between‐group mean differences are not reported.
3.2. Analysis.

Comparison 3: Antidepressants vs psychological interventions, Outcome 2: Severity of depression ‐ BDI (acute phase)
Milgrom 2015 also reported BDI in the continuation phase. At 24 weeks, the mean BDI was lower in the CBT group than in the sertraline group (MD 7.89, 95% CI 1.14 to 14.64; participants = 29; studies = 1; Analysis 3.3).
3.3. Analysis.

Comparison 3: Antidepressants vs psychological interventions, Outcome 3: Severity of depression ‐ BDI (continuation phase)
3.4. Treatment acceptability
In the Chibanda 2014 study, 3/28 (11%) women in the amitriptyline group and 3/30 (10%) of the women who received group problem solving therapy were lost to follow up (RR 1.07, 95% CI 0.24 to 4.88; participants = 58; studies = 1; Analysis 3.4).
3.4. Analysis.

Comparison 3: Antidepressants vs psychological interventions, Outcome 4: Treatment acceptability
3.5. Child‐related outcomes
No data available
3.6. Parenting‐related outcomes
Milgrom 2015 reported Parenting Stress Index (PSI) in the acute phase. There were no statistically significant differences in mean PSI scores between sertraline and group CBT at 12 weeks (MD 9.32, 95% CI ‐14.63 to 33.27; participants = 29; studies = 1; Analysis 3.5). However, at 24 weeks, sertraline was associated with an increase in stress score (MD 30.37, 95% CI 6.25 to 54.49; participants = 29; studies = 1; Analysis 3.6).
3.5. Analysis.

Comparison 3: Antidepressants vs psychological interventions, Outcome 5: Parenting‐related outcomes ‐ PSI (acute phase)
3.6. Analysis.

Comparison 3: Antidepressants vs psychological interventions, Outcome 6: Parenting‐related outcomes ‐ PSI (continuation phase)
3.7. Quality of life
No data available
Comparison 4: antidepressants versus psychosocial interventions
One study (Sharp 2010; 254 women) compared antidepressants with listening visits and reported outcomes assessed at 18 weeks. There were no set antidepressants as women received prescriptions based on GP/participant choice. Most participants were prescribed citalopram, fluoxetine or sertraline (see Characteristics of included studies table for details).
Primary outcomes
4.1. Depression response or remission (acute phase)
Sharp 2010 did not report depression response or remission during the acute phase (5 to ≤ 12 weeks). Outcomes reported at 18 weeks are included below as a secondary outcomes.
4.2. Adverse events
Sharp 2010 did not report any adverse events or serious adverse effects of treatment. They did not report any data on adverse effects related to infants or the safety of breastfeeding.
Secondary outcomes
4.3. Depression response or remission (continuation phase)
Sharp 2010 reported remission (EPDS score less than 13) rates may be lower in the sertraline group at 18 weeks (RR 1.20, 95% CI 0.95 to 1.53; 1 study, 206 women; Analysis 4.1) compared with the listening visits group.
4.1. Analysis.

Comparison 4: Antidepressants vs psychosocial interventions, Outcome 1: Depression remission (continuation phase)
4.4. Severity of depression
Sharp 2010 reported severity of depression (mean EPDS score) at 18 weeks (continuation phase). The antidepressant group had a lower mean EPDS score (i.e. less severe depression) than the listening visits group (MD −1.00, 95% CI −2.54 to 0.54; 1 study, 206 women; Analysis 4.2).
4.2. Analysis.

Comparison 4: Antidepressants vs psychosocial interventions, Outcome 2: Severity of depression ‐ EPDS (continuation phase)
4.5. Treatment acceptability
Sharp 2010 reported dropout rates at 18 weeks (continuation phase). Percentage dropout was greater in the antidepressant group compared with the listening visits group (RR 1.94, 95% CI 1.12 to 3.35; 1 study, 254 women; Analysis 4.3). From the reported data, it was not possible to determine whether this difference in withdrawal was due to a lack of acceptability of treatment with antidepressants or to other factors. In terms of adherence at 18 weeks, 64% of women randomised to antidepressants reported taking antidepressants in the previous four weeks (62/97 women followed up) and 34% of women randomised to listening visits reported taking antidepressants in the past four weeks (37/109 followed up).
4.3. Analysis.

Comparison 4: Antidepressants vs psychosocial interventions, Outcome 3: Treatment acceptability ‐ dropouts
4.6. Child‐related outcomes
No data available
4.7. Parenting‐related outcomes
Sharp 2010 examined the effect on maternal functioning using the Maternal Adjustment and Maternal Attitudes (MAMA) Attitudes Towards Pregnancy and Baby subscale (postpartum version). At 18 weeks, there was no difference in scores between the two treatment groups (MD 1.00, 95% CI −0.45 to 2.45; 1 study, 183 women; Analysis 4.4).
4.4. Analysis.

Comparison 4: Antidepressants vs psychosocial interventions, Outcome 4: Parenting‐related outcomes ‐ MAMA (continuation phase)
4.8. Quality of life
Sharp 2010 reported various quality‐of‐life measures at 18 weeks. According to the SF‐12, there were no differences in the mental health scores (MD 0.13, 95% CI −0.15 to 0.41; 1 study, 169 women; Analysis 4.5) or physical health scores (MD 0.19, 95% CI −0.09 to 0.47; 1 study, 169 women; Analysis 4.6) between the two treatment groups. Using the EQ‐5D, there were no differences in the utility scores (MD −0.01, 95% CI −0.08 to 0.06; 1 study, 194 women; Analysis 4.7) or the visual analogue scores (MD −0.80, 95% CI −9.08 to 7.48; 1 study, 194 women; Analysis 4.8).
4.5. Analysis.

Comparison 4: Antidepressants vs psychosocial interventions, Outcome 5: Quality of life ‐ SF12 mental health
4.6. Analysis.

Comparison 4: Antidepressants vs psychosocial interventions, Outcome 6: Quality of life ‐ SF12 physical health
4.7. Analysis.

Comparison 4: Antidepressants vs psychosocial interventions, Outcome 7: Quality of life ‐ EQ‐5D utility score
4.8. Analysis.

Comparison 4: Antidepressants vs psychosocial interventions, Outcome 8: Quality of life ‐ EQ‐5D visual analogue scale
Comparison 5: antidepressants versus any other pharmacological intervention
Two studies with 165 women compared antidepressants (both using sertraline) with another pharmacological intervention (Wisner 2006; Wisner 2015). In Wisner 2006 women in the control group received nortriptyline. Wisner 2015 included an active comparison group (in addition to a placebo group) that received transdermal estradiol.
Primary outcomes
5.1. Depression response or remission (acute phase)
Both studies (Wisner 2006; Wisner 2015), reported response in the acute phase, defined as 50% or higher reduction in HAM‐D from baseline to week 8 (RR 0.98, 95% CI 0.76 to 1.27; I2 = 74%; 2 studies, 165 women; Analysis 5.1).
5.1. Analysis.

Comparison 5: Antidepressant vs other pharmacological intervention, Outcome 1: Depression response ‐ acute phase
Both studies (Wisner 2006; Wisner 2015), also measured remission in the acute phase, defined as a score 7 or less, or 8 or less on the HAM‐D scale (RR 0.98, 95% CI 0.68 to 1.41; I2 = 0%; 2 studies, 165 women; Analysis 5.2).
5.2. Analysis.

Comparison 5: Antidepressant vs other pharmacological intervention, Outcome 2: Depression remission ‐ acute phase
Wisner 2006 also measured depression response (RR 0.82, 95% CI 0.56 to 1.19; 1 study, 109 women; Analysis 5.3) and remission (RR 0.92, 95% CI 0.51 to 1.67; 1 study, 109 women; Analysis 5.4) in the early phase and found there may be no difference between treatments.
5.3. Analysis.

Comparison 5: Antidepressant vs other pharmacological intervention, Outcome 3: Depression response ‐ early phase
5.4. Analysis.

Comparison 5: Antidepressant vs other pharmacological intervention, Outcome 4: Depression remission ‐ early phase
5.2. Adverse events
In Wisner 2006 there was no difference between sertraline and nortriptyline in the overall number of adverse effects reported using the Asberg Side Effects Rating Scale (P = 1.00). However, some adverse effects were more common among women who took nortriptyline than women taking sertraline: cholinergic symptoms such as moderate to severe thirst (19% to 23% with nortriptyline versus 3% to 4% with sertraline; P = 0.02), dry mouth (20% to 40% with nortriptyline versus 2% to 11% with sertraline; P = 0.001) and constipation (23% to 25% with nortriptyline versus 7% to 12% with sertraline; P = 0.05). Other adverse effects were more common in the sertraline than nortriptyline group: constant or severe headaches (10% to 15% with sertraline versus 1% to 2% with nortriptyline; P = 0.05), slight to moderate increased perspiration (35% to 40% with sertraline versus 15% to 20% with nortriptyline; P = 0.04) and hot flushes interrupting sleep (4% to 10% with sertraline versus 0% to 2% with nortriptyline; P = 0.04). Wisner 2006 reported that babies of breastfeeding mothers in the study had no adverse effects.
Wisner 2015 used the Asberg rating scale for adverse effects. Comparing items rated moderate or severe intensity, no significant differences between treatment groups were observed. Four women from the sertraline group were withdrawn from the study as they developed hypomania or psychosis, or both. None of the women treated with estradiol developed hypomania or psychosis.
Secondary outcomes
5.3. Severity of depression
Two studies (Wisner 2006; Wisner 2015), measured severity of depression. Random‐effects meta‐analysis showed there may be no difference in the mean HAM‐D score at eight weeks (MD −0.65, 95% CI −3.18 to 1.87; I2 = 29%; 2 studies, 165 women; Analysis 5.5).
5.5. Analysis.

Comparison 5: Antidepressant vs other pharmacological intervention, Outcome 5: Depression severity ‐ HAM‐D (acute phase)
Wisner 2006 also reported the proportion of women according to CGI‐S scores at week 8. In the sertraline group, 94% had a CGI‐S score of 0 to 1, 3% 2 to 3 and 3% had a CGI‐S score or 4 and above. In the nortriptyline group 94% had a CGI‐S score of 0 to 1, 2% 2 to 3 and 4% had a CGI‐S score of 4 or above (P = 1.00).
5.4. Treatment acceptability
In Wisner 2006, more women randomised to sertraline than women randomised to nortriptyline withdrew from the study in the first eight weeks (RR 1.74, 95% CI 0.99 to 3.06; 1 study, 109 women; Analysis 5.6), with a higher proportion of women lost to follow‐up or withdrawing by personal choice in the sertraline group (20% with sertraline versus 6% with nortriptyline; P = 0.03). The proportion of women withdrawing for other reasons (side effects, hypomania occurrence or clinical deterioration) did not differ significantly between the two drug groups. There were no significant differences in rates of withdrawal after week 8 (entering the continuation phase of the study). Of those eligible to enter the continuation phase, 24/32 (75%) of those randomised to sertraline and 25/40 (63%) of those randomised to nortriptyline chose to do so (P = 0.26). Adherence (assessed through serum levels) found that 14 women had minimal drug levels in their blood despite claims of compliance. There was no significant difference found in lack of compliance between women assigned to nortriptyline (9/51, 18%) and women assigned to sertraline (5/44, 11%; P = 0.29).
5.6. Analysis.

Comparison 5: Antidepressant vs other pharmacological intervention, Outcome 6: Treatment acceptability ‐ dropouts
5.5. Child‐related outcomes
No data available
5.6. Parenting‐related outcomes
No data available
5.7. Quality of life
Wisner 2006 investigated the effect of treatment with nortriptyline versus sertraline on social functioning as assessed by the Social Problems Questionnaire. No significant effect of treatment modality was found at week 8 (P = 0.62) or when the interaction of time by drug group was examined (P = 0.33). There were also no significant differences between antidepressant groups at week 24.
Comparison 6: antidepressants versus complementary medicine
One study with 64 women contributed data to this comparison, which used saffron as a comparator intervention (Kashani 2017).
Primary outcomes
6.1. Depression remission or response (acute phase)
Kashani 2017 reported that 50% (16/32) of the fluoxetine group compared to 41% (13/32) of the saffron group responded to treatment at 6 weeks (RR 1.23, 95% CI 0.71 to 2.12; 1 study, 64 women; Analysis 6.1). The study isn't large enough to conclude whether there are differences between groups.
6.1. Analysis.

Comparison 6: Antidepressants vs complementary medicine, Outcome 1: Depression response ‐ acute phase
Partial response, defined as a 25% to 50% reduction in HDRS score at week 6 occurred in 18/32 (56%) and 15/32 (47%) women treated with fluoxetine and saffron, respectively (P = 0.61)
Kashani 2017 also reported that 22% (7/32) of the fluoxetine group compared to 19% (6/32) of the saffron group were in remission at six weeks (RR 1.17, 95% CI 0.44 to 3.09; 1 study, 64 women; Analysis 6.2). The study isn't large enough to conclude whether there are differences between groups.
6.2. Analysis.

Comparison 6: Antidepressants vs complementary medicine, Outcome 2: Depression remission ‐ acute phase
6.2. Adverse events
In Kashani 2017, women in the fluoxetine group experienced more headache, dry mouth, daytime drowsiness, constipation and sweating than the saffron group. However, frequencies of adverse events were not significantly different between the two groups. No major adverse event and no death occurred.
Secondary outcomes
6.3. Severity of depression
No data available
6.4. Treatment acceptability
No data available
6.5. Child‐related outcomes
No data available
6.6. Parenting‐related outcomes
No data available
6.7. Quality of life
No data available
Subgroup analyses
Due to the small number of studies included in our meta‐analyses, we were unable to conduct any of the predefined subgroup analyses.
Sensitivity analysis
Study quality
We performed a sensitivity analysis excluding Yonkers 2008, which we judged at high risk of attrition and reporting biases. The results remained unchanged. There was no difference between SSRI and placebo for depression response (RR 1.27, 95% CI 0.91 to 1.77; I2 = 16%; 3 studies, 135 women; Analysis 7.1) nor remission in the acute phase (RR 1.35, 95% CI 0.87 to 2.11; I2 = 13%; 3 studies, 135 women; Analysis 7.2). SSRIs were associated with a reduction in the overall severity of depression (SMD −0.37, 95% CI −0.67 to −0.08; I2 = 0%; 3 studies, 181 women; Analysis 7.3) but there was no difference in mean HAM‐D score (MD −0.85, 95% CI −3.03 to 1.34; I2 = 0%; 2 studies, 146 women; Analysis 7.4) nor the mean CGI‐S score at the acute phase (MD −0.10, 95% CI −0.76 to 0.56; 1 study, 35 women; Analysis 7.5).
7.1. Analysis.

Comparison 7: Sensitivity analysis ‐ antidepressant vs placebo, excluding studies at high risk of bias, Outcome 1: Depression response (acute phase)
7.2. Analysis.

Comparison 7: Sensitivity analysis ‐ antidepressant vs placebo, excluding studies at high risk of bias, Outcome 2: Depression remission (acute phase)
7.3. Analysis.

Comparison 7: Sensitivity analysis ‐ antidepressant vs placebo, excluding studies at high risk of bias, Outcome 3: Severity of depression ‐ overall (acute phase)
7.4. Analysis.

Comparison 7: Sensitivity analysis ‐ antidepressant vs placebo, excluding studies at high risk of bias, Outcome 4: Severity of depression ‐ HAM‐D (acute phase)
7.5. Analysis.

Comparison 7: Sensitivity analysis ‐ antidepressant vs placebo, excluding studies at high risk of bias, Outcome 5: Severity of depression ‐ CGI‐S (acute phase)
Blinding
We planned on conducting sensitivity analyses excluding studies that were unblinded. We were unable to conduct this due to the small number of studies included in our meta‐analyses.
Attrition
We planned on conducting sensitivity analyses excluding studies with more than 20% and more than 50% attrition. We were unable to complete this due to the small number of studies included in our meta‐analyses.
Validation
We planned on conducting sensitivity analyses excluding studies with outcomes based on non‐validated scales. We were unable to complete this due to the small number of studies included in our meta‐analyses.
Trials with imputed data
We did not plan this sensitivity analysis a priori. However, we decided to conduct it due to the limitations in using data from the Appleby 1997 study, which reported geometric means and 95% CIs. For the outcome, overall severity of depression in the acute phase, excluding the Appleby 1997 study made little difference to the result. Antidepressants may still be associated with less severe depression than placebo (SMD −0.40, 95% CI −0.71 to −0.09; I2 = 0%; 3 studies, 164 women; Analysis 8.1).
Discussion
Summary of main results
We identified 11 RCTs with 1016 women that evaluated the efficacy and safety of antidepressant medication in the treatment of postnatal depression. Ten of these used only one antidepressant, nine of which involved an SSRI (six with sertraline, two with fluoxetine and one with paroxetine). Treatment duration ranged from 4 to 12 weeks (where reported).
Six studies compared SSRI to placebo, although three of these also included psychological interventions or clinical management in both treatment and placebo arms. On meta‐analysis of the four studies (205 women) with available data on response and remission, there was low‐certainty evidence of a small benefit of SSRIs over placebo in response and remission at 5 to 12 weeks' follow‐up; and some limited evidence of a greater reduction in depression severity. However, the certainty of this evidence was low (using the GRADE criteria); as discussed below.
There were insufficient data to facilitate meta‐analysis for any of the other comparisons.
One study compared the SSRI fluoxetine to the complementary medicine of saffron (68 women), and found no evidence of a difference in response or remission rates. Another two of the studies (165 women) involving SSRIs compared sertraline to the other pharmacological interventions of a TCA antidepressant: nortriptyline (Wisner 2006), and transdermal estradiol (Wisner 2015), and found no evidence of a difference between groups in response or remission rates or depression severity at 5 to 12 weeks' follow‐up. Of the three studies involving psychological interventions (Chibanda 2014; Milgrom 2015; O'Hara 2019; 265 women), data on the primary outcome of response or remission were only available for one study comparing the SSRI sertraline to IPT and placebo (O'Hara 2019; 162 women). This study was unable to detect a difference between either sertraline, IPT or placebo. Authors did not provide sufficient data to draw conclusions on the relative efficacy of sertraline and IPT with one another or compared to placebo.
One study (254 women) provided comparisons between both treatment as usual and psychosocial intervention (Sharp 2010). There was some evidence for higher remission rates at four weeks' follow‐up in the antidepressant group versus treatment as usual but less evidence at 18 weeks' follow‐up after the control group began receiving listening visits.
Adverse effects were reported by a substantial proportion of women and were characteristic of the class of antidepressant used, for example nausea and headaches with SSRIs and cholinergic side effects such as dry mouth and constipation with TCAs. However, it was not always clear from reporting of adverse events if there were differences between women treated with antidepressants versus the comparator, making it difficult to evaluate the relative impact that such events may have had on treatment acceptability within the study between groups. Nonetheless, there was no evidence for an increased burden of adverse events in women treated with antidepressants compared with placebo, treatment as usual or other interventions. Likewise, there was no evidence to suggest differential treatment acceptability between groups, although Sharp 2010 reported greater dropout rates at four weeks' follow‐up in the antidepressant group (18%), versus treatment as usual (10%), which may be an indicator of acceptability. While withdrawal rates from the studies were often high, many of the studies did not differ between treatment groups in this regard.
None of the 11 RCTs reported any child‐related outcomes, although two of the studies using sertraline and nortriptyline (Hantsoo 2013; Wisner 2006) reported no adverse events related to breastfeeding on the infant (Hantsoo 2013). Two of the studies used outcome measures of parenting. Sharp 2010 found evidence of a difference in maternal adjustment favouring treatment‐as‐usual groups or listening visit groups over antidepressants in the early phase but not in the continuation phase, whilst Milgrom 2015 found no evidence for a difference in parenting stress between women treated with sertraline versus group CBT in the early phase but evidence of a difference in the continuation phase.
In summary, there is limited, low‐certainty evidence that there may be a small benefit of SSRI antidepressants compared to placebo in the treatment of postnatal depression; and that antidepressants are likely to be as safe and acceptable as placebo. While most of the safety data relate to women, with fewer data pertaining to safety for the breastfeeding infant, the few studies that reported such data did not report any adverse effects related to breastfeeding. There is insufficient evidence on the relative efficacy, safety and acceptability of other classes of antidepressant compared to placebo. There is also insufficient evidence on the relative efficacy, safety and acceptability of SSRIs compared to other classes of antidepressants and of antidepressants compared to other pharmacological interventions, complementary medicines, psychological and psychosocial interventions or treatment as usual. There are also very limited data on child‐ and parenting‐related outcomes of antidepressant treatment for postnatal depression.
Overall completeness and applicability of evidence
Since the last update of this review in 2014 (Molyneaux 2014), there remains limited evidence on the efficacy and safety of antidepressants for postnatal depression. We identified only 11 studies in total, and it is unclear whether this is related to lack of focus on this population or difficulty in recruiting postnatal woman (for example due to parenting pressures or reluctance to use antidepressants during this period). We could only conduct meta‐analysis for one of the comparisons between SSRI antidepressants and placebo. There were insufficient data to conduct meta‐analyses for any of the other comparisons, with evidence often consisting of single studies. There remains limited evidence on the impact of antidepressant treatment for postnatal depression on parenting‐ and child‐related outcomes, including breastfeeding, with limited or absent reporting of outcomes in breastfeeding infants and other studies excluding breastfeeding women.
While the majority of studies were conducted in English‐speaking, high‐income countries, we identified two new studies from middle‐income countries, eligible for inclusion since the last update of this review in 2014, so the extent to which they enhance the global applicability of our findings is limited. There remains an evidence gap in studies conducted in low‐ and middle‐income countries.
While we increased the eligible period of treatment onset to 12 months after giving birth, as opposed to six months used in the last update of this review in 2014, the majority of women were recruited to studies within the first three postnatal months. While onset of postnatal depression likely peaks at three months postpartum, there remains an elevated risk during the first postnatal year (Gavin 2005), with perinatal services in the UK treating women up to one year postpartum. Indeed about 30% of postnatal episodes last beyond the first postnatal year (Goodman 2004). However, the maximum treatment duration in any of the included studies was three months. Thus there is a need for research to provide further understanding of whether there is differing efficacy of antidepressants dependent on duration and time of onset of postnatal depression.
Severity of postnatal depression is a very important consideration, since greater depression severity is a key indication for treatment with antidepressants (NICE 2014). There is clear evidence from non‐pregnant adults that antidepressants are more effective compared with placebo for individuals with at least moderate depression severity, and for those with chronic symptoms (Cipriani 2018; Cleare 2015; Kirsch 2008), yet many indicators of more severe depression were exclusion criteria in the included studies, for example suicidal ideation or high depression symptom scores, and several studies included women with mild depression. Other exclusions such as severe mental illness and common co‐morbidities such as substance abuse and physical ill health may limit the applicability of the evidence to those women most in need of postnatal depression treatment.
None of the studies reported direct evidence regarding treatment acceptability, that is, evidence obtained by directly asking participating women about their treatment experience. We used reported data on dropout rates as a proxy measure for treatment acceptability. This approach is used in other Cochrane Reviews (Brown 2019), but it is not without its problems. Women who drop out of the study might continue treatment, while women who stop taking their allocated study medication might not be considered dropouts if they continue to attend follow‐up visits. Conceptually, treatment dropout is different from study dropout. However, in practice it is often not possible to tell the two apart. Furthermore, attrition might be a more accurate measure of treatment acceptability when psychological or psychosocial treatments are concerned. Adherence to medication treatments is difficult to measure accurately as it is usually reliant on participant self‐report. Future research might benefit from a more direct measurement of treatment acceptability, which might also be explored in qualitative work.
Quality of the evidence
There are a number of limitations of the included studies that impact on the quality of the evidence that they provide. We judged the overall certainty of the evidence in the meta‐analysis for the comparison of SSRIs with placebo to be low (using the GRADE criteria).
Some of the included studies did not report a power calculation and the largest sample size was 254, so the evidence base is likely limited by small, underpowered studies. There are a number of other potential sources of bias in our included studies. We identified performance bias as a possible issue in those studies not using a placebo and for which, as a result, blinding was not possible. Thus participants' (or indeed research personnel’s) personal preference for either treatment or beliefs about effectiveness of certain types of treatment may have influenced their self‐report outcome measures.
In many of the studies, inadequate reporting of how incomplete outcome data were handled and failure to publish a study protocol also introduces the possibility of attrition and reporting biases respectively, particularly in light of the high rates of attrition noted in a number of the studies (range in included studies 0% to 61.4%, median 19.4%). Although studies that contributed primary outcomes to the meta‐analysis of SRRIs versus placebo conducted ITT analysis, not all the studies employed it and they did not use it for all the secondary outcomes. Given the high dropout rates noted, this is a potential source of bias if reasons for dropout, such as clinical deterioration, differed between treatment groups.
Potential biases in the review process
Our review employed a thorough search strategy to provide a comprehensive synthesis of the evidence to date. We made significant efforts to obtain raw data if not provided in the published manuscript. Nonetheless, it remains possible that publication bias may have influenced our findings and could not be assessed due to insufficient numbers of studies.
We planned to conduct a number of sensitivity and subgroup analyses to explore sources of variation within our results. However, the small number of included studies precluded many of these. We were able to explore the impact that risk of bias had on our meta‐analysis of SSRIs versus placebo and found that removal of a study judged to be at high risk of attrition and reporting biases had little impact on the pooled estimates for response and remission.
There remain a number of possible sources of heterogeneity between all studies considered in this review, including different study settings, inclusion and exclusion criteria and diagnostic criteria for postnatal depression. However, in meta‐analyses of response and remission rates for SSRIs versus placebo, heterogeneity was low (I2= 0% for response and 26% for remission), suggesting that it might not be important in this comparison (Deeks 2020) .
Agreements and disagreements with other studies or reviews
It is perhaps surprising, given the increased investment in perinatal mental health service provision in the UK and internationally since this review was last updated in 2014, that we did not identify more eligible RCTs for inclusion in this updated review. There have been a few evidence syntheses since 2014 pertaining to the treatment of postnatal depression with antidepressants but these relate to systematic reviews of international clinical guidelines (Molenaar 2018), and economic evaluations (Gurung 2018).
In the general population, there is good‐quality evidence that antidepressants are more effective than placebo for moderate to severe depression, with small to moderate effect sizes reported in recent reviews (Cipriani 2018; Cleare 2015; Kirsch 2008). For example, a recent systematic review and network meta‐analysis of the efficacy and acceptability of 21 antidepressants synthesised evidence from 522 studies with 11,647 participants, and found that all antidepressants were more effective than placebo, in terms of both response and remission (Cipriani 2018). Whilst many antidepressant RCTs in the general population specify at least moderate depression severity for inclusion, several studies in our review had mean baseline depression scores indicating mild depression, and some excluded women with more severe depression; and this may explain the weaker evidence and smaller effect sizes for a benefit of SSRIs over placebos found in this review. Another moderator of efficacy is setting, with past reviews indicating that patients in primary versus secondary care settings gain more benefit from both treatment and placebo, with less difference between treatment groups (Cleare 2015). Several studies in this review were based in primary care or general maternity settings, and this may partially explain the small difference between antidepressant and placebo groups. Finally, in the included studies, several of the placebo‐controlled studies also included another intervention (including psychological interventions), and there may be a smaller benefit of 'add‐on treatment' versus sole treatment with antidepressants.
Systematic reviews in the general population (Cleare 2015; Cuijpers 2013), have found evidence that psychological therapy (and particularly CBT) is as effective as antidepressants for the treatment of mild to moderate depression.
However, there is unclear evidence for the comparative efficacy of CBT and antidepressants in severe depression.
We found insufficient data to confirm these conclusions yet in postnatal depression.
An important consideration is patient preference ‐ where in general population studies there is evidence that following patient preference for antidepressant or psychological therapy improves adherence and may improve outcomes, at least in primary care settings (Cleare 2015). There is some qualitative evidence that women have a preference for psychological over pharmacological therapy in the perinatal period (McAllister‐Williams 2017), but no evidence on patient preference and its effect on outcome in the RCTs included in this review.
The present review is the most recent and comprehensive in evaluating the efficacy and safety of antidepressant medication for the treatment of postnatal depression. There is limited, low‐quality evidence that antidepressants may be associated with greater response and remission rates than placebo. However, the evidence is insufficient for women with more severe depression and for many of our intended treatment comparisons. The limitations of the included studies reduce the conclusiveness of our findings.
Authors' conclusions
Implications for practice.
The findings from this review have somewhat limited direct implications for clinical practice given the small number of included studies, their methodological weaknesses and the paucity of evidence for women with more severe postnatal depression. However, their relevance to clinical practice could be considered within the broader relevant literature; including RCT evidence for depression in the general population and current clinical perinatal guidelines.
Our conclusion that there is a possible small benefit of SSRIs over placebo for women with postnatal depression is in line with more extensive RCT evidence for depression in the general population. Whereas we were unable to assess antidepressant efficacy by depression severity, general population studies find greater antidepressant benefit for those with more severe depression. The evidence reported in this review regarding antidepressants versus psychological interventions for postnatal depression is of too low certainty, and limited by poor study reporting, to draw any meaningful conclusions. The acceptability of different treatment modalities and their impact on child and parenting outcomes are important drivers for treatment choices by women with postnatal depression, and remain key evidence gaps for clinical practice.
The evidence base to guide clinicians on the efficacy of antidepressants in their management of women with postnatal depression remains unclear, particularly for women with severe postnatal depression. Evidence from this review, alongside current clinical guidelines and reference to evidence from the general adult population, should inform an individualised risk‐benefit discussion with the woman seeking treatment for postnatal depression.
Implications for research.
The current evidence base is limited by the small number of RCTs with underpowered samples and a number of other potential biases, including high attrition. The focus of future research in this area should be on expanding the evidence base to include evaluations of the efficacy and safety of the full range of antidepressants relative to placebo, treatment as usual and other treatment options. Greater understanding is also needed of what antidepressants work for which women, and how treatment effect may be modified by illness severity, other clinical characteristics and socio‐demographic factors. Untreated postnatal depression has important child and parenting adverse effects, and future studies should include child and parenting outcome measures.
What's new
| Date | Event | Description |
|---|---|---|
| 29 January 2021 | New search has been performed | This is an update of the Cochrane Review with the same title published by Molyneaux et al. in 2014 (Molyneaux 2014). A new protocol was published in March 2020 to reflect advances in the area since publication of the previous review/protocol (Brown 2020). |
| 29 January 2021 | New citation required and conclusions have changed | 2 additional included studies and 2 ongoing studies added in this update. |
History
Protocol first published: Issue 3, 2020 Review first published: Issue 2, 2021
Acknowledgements
We thank the CCMD editorial team. We thank Sarah Dawson (CCMD Information Specialist) and Reinhard Wentz of the Institute of Child Health for assistance with developing the search strategy.
We are grateful to the authors of the previous versions of this Cochrane Review (Hoffbrand 2001; Molyneaux 2014), including Helen Crawley, Sara Hoffbrand, Amar Karia, and Helen McGeown.
We thank Huisi Wang for the translation of Yu 2015 through Cochrane Task Exchange.
We and the CCMD editorial team thank the following editors and peer reviewers for their time and comments: Mohan Bairwa, Ndi Euphrasia Ebai‐Atuh, Nick Meader, Simone Vigod, Verity Westgate and Gillian Worthy. We also thank Cochrane Copy Edit Support for assistance.
CRG funding acknowledgement: the National Institute for Health Research (NIHR) is the largest single funder of the CCMD Group.
Disclaimer: the views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the NIHR, the NHS, or the Department of Health and Social Care.
Appendices
Appendix 1. Scales for measuring depression
Beck Anxiety Inventory (BAI) is a 21‐item self‐administered instrument that measures the severity of anxiety.
BAI (0‐7: minimal level of anxiety, 8‐15 mild anxiety, 16‐25 moderate anxiety, 26‐63 severe anxiety) (taken from description of Milgrom 2015).
Beck Depression Inventory (BDI and BDI‐II) is a 21‐item self‐administered instrument that measures the symptoms of depression.
BDI‐II (14‐19: mild depression, 20‐28: moderate depression, 29‐63: severe depression) (taken from description of Milgrom 2015)
Clinical Interview Schedule ‐ Revised (CIS‐R) is a structured diagnostic interview schedule for the diagnosis of common mental disorders. The CIS‐R is widely used in population and primary care surveys to provide estimates of depression.
Clinical Global Improvement Scale (CGI) is a clinician‐rated scale that assesses changes in symptoms. The scales are rated on a scale of 1 = very much improved; 2 = much improved; 3 = minimally improved; 4 = no change; 5 = minimally worse; 6 = much worse or 7 = very much worse. Each component of the CGI is rated separately and the scales do not yield a global score.
CGI‐Severity of Illness measure is a clinician‐rated scale that assesses the severity of symptoms. The CGI‐Severity of Illness is rated on a scale of 1 = not at all ill; 2 = borderline mentally ill; 3 = mildly ill; 4 = moderately ill; 5 = markedly ill; 6 = severely ill or 7 = extremely ill. Each component of the CGI is rated separately and the scales do not yield a global score.
Edinburth Postnatal Depression Scale (EPDS) is a 10‐item self administered screen for perinatal depression, validated in 20 languages. For each item, women are asked to select 1 of 4 responses that most closely describe how they have felt over the past 7 days. Each response has a value of 0‐3; scores for the 10 items are summed to give a total score between 0 and 30. The EPDS is the most widely used screening instrument for postpartum depression and has a positive predictive value for postnatal major depression of 9% to 64% (with a cut‐off score of 9/10) or 17% to 100% (with a cut‐off of 12/13). A cut‐off score of 12/13 is used in most studies to indicate postpartum depression. The EPDS does not discriminate levels of depression and additional information is required to meet diagnostic criteria for depression.
EuroQol 5 Dimension (EQ‐5D) is a preference‐based measure of health‐related quality of life measured on 5 dimensions (i.e. mobility, self‐care, usual activities, pain/discomfort and anxiety/depression), each rated on 3 levels (i.e. no problems, some problems and severe problems). Participants are classified into 1 of 243 health states, each associated with a score that can be used to calculate quality‐adjusted life years. The measure has been extensively used in health economic evaluations and its psychometric properties are adequate.
Global Assessment Scale (GAS) is a rating scale for evaluating the overall functioning of a person during a specified time period on a continuum from psychological or psychiatric sickness to health.
Golombok Rust Inventory of Marital State (GRIMS) is a 28‐item self‐complete questionnaire that assesses the quality of the relationship between a married or co‐habiting couple.
Hamilton Rating Scale for Anxiety (HAM‐A) is a clinician‐rated screening instrument that assesses the presence and severity of anxiety. Total scores are obtained by summing the score of each item, 0‐4 (symptom is absent, mild, moderate or severe). For the 14‐item HAM‐A version total scores range from 0 to 56. A score of 0‐13 is indicative of no anxiety; 14‐17 is indicative of mild anxiety; 18‐24 is indicative of moderate anxiety and 25‐30 is indicative of severe anxiety.
Hamilton Rating Scale for Depression (HAM‐D or HDRS) is a clinician‐rated screening instrument that assesses the presence and severity of depression. Total scores are obtained by summing the score of each item, 0‐4 ((symptom is absent, mild, moderate or severe) or 0‐2 (absent, slight or trivial, or clearly present). For the 17‐item HAM‐D version, total scores range from 0 to 54. A score of 0‐6 is indicative of no depression, 7‐17 is indicative of mild depression, 18‐24 is indicative of moderate depression and ≥ 25 is indicative of severe depression. For most raters, a total score of ≤ 7 after treatment is a typical indicator of remission and a decrease of 50% or more from baseline is considered an indicator of a clinically significant change.
Inventory of Depression and Anxiety Symptoms (IDAS‐GD) is a self‐report instrument that assesses the symptoms of major depression.
Montgomery‐Åsberg Depression Rating Scale (MADRS) is a diagnostic instrument that measures the severity of depressive episodes. Each response has a value of 0‐6; scores for the 10 items are summed to give a total score between 0 and 60. A score of 0‐6 is indicative of no depression, 7‐19 is indicative of mild depression; 20‐34 is indicative of moderate depression and ≥ 35 is indicative of severe depression.
Maternal Adjustment and Maternal Attitudes (MAMA) is a self‐administered questionnaire that examines perceptions of maternal adjustment and attitudes towards marital relationships and the baby. The postnatal subscale of the MAMA questionnaire comprises 12 items rated on a 4‐point scale from 1 = "not at all" to 4 = "very much".
Parenting Stress Index (PSI) is a self‐report measure of parenting stress, comprised of 101 items. It includes three domains: child characteristics, parent characteristics, and situational/demographic life stress.
12‐Item Short Form (SF‐12) is a 12‐item self‐complete questionnaire that measures functional health and well‐being. The measure is a widely used and well‐validated generic measure of functional quality of life.
Social Problems Questionnaire (SPQ) is a 33‐item self report questionnaire that covers 10 areas or domains, including housing conditions; occupation; financial status; social and leisure activities; contacts with relatives, friends and neighbours; family functioning; child‐parent interaction; relationship with spouse or partner and legal matters. The individual items are rated on a 4‐point scale ranging from 0 (no social difficulties/satisfactory adjustment) to 3 (severe social difficulties/very poor adjustment).
Appendix 2. Specialised Register: CCMD's core MEDLINE search strategy
The search strategy listed below is the weekly OVID MEDLINE search used to inform the Cochrane Common Mental Disorders (CCMD) Group’s Specialised Register. It is based on a list of terms for all conditions within the scope of the CCMD Group plus a sensitive RCT filter. 1. [MeSH Headings]: eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/
2. [Title/ Author Keywords]: (eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).ti,kf.
3. [RCT filter]: (controlled clinical trial.pt. or randomised controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomised controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.)
4. (1 and 2 and 3)
Records were screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs were tagged to the appropriate study record.
Appendix 3. Database search strategy
Cochrane Common Mental Disorders Controlled Trials Register (CMDCTR‐Studies and Reference Registers) (all available years) & Cochrane Central Register of Controlled Trials (CENTRAL) ℅ CRS (all years)
#1 MESH DESCRIPTOR Antidepressive Agents EXPLODE ALL AND INREGISTER
#2 MESH DESCRIPTOR Neurotransmitter Uptake Inhibitors EXPLODE ALL AND INREGISTER
#3 MESH DESCRIPTOR Monoamine Oxidase Inhibitors EXPLODE ALL AND INREGISTER
#4 (Antidepressant Agent):EMT AND INREGISTER
#5 ("Serotonin Receptor Affecting Agent" or "Serotonin Uptake Inhibitor" or "Serotonin Noradrenalin Reuptake Inhibitor" or "Triple Reuptake inhibitor"):EMT AND INREGISTER
#6 ("Dopamine Receptor Affecting Agent" or "Dopamine Uptake Inhibitor/"):EMT AND INREGISTER
#7 ("Adrenergic Receptor Affecting Agent" or "Noradrenalin Uptake Inhibitor"):EMT AND INREGISTER
#8 ("Neurotransmitter Uptake Inhibitors"):EMT AND INREGISTER
#9 ("Monoamine Oxidase Inhibitor"):EMT AND INREGISTER
#10 (antidepress* or "anti depress*" or MAOI* or "monoamine oxidase inhibit*" or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) and (uptake or reuptake or re‐uptake)) or noradrenerg* or antiadrenergic or "anti adrenergic" or SSRI* or SNRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic* or psychotropic*) AND INREGISTER
#11 Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or (Bupropion or Amfebutamone) or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine) or Clorgyline or Clovoxamine or (CX157 or Tyrima) or Demexiptiline or Deprenyl or (Desipramine* or Pertofrane) or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or St John*) or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or Lorpiprazole or (Lu AA21004 or Vortioxetine) or Lu AA24530 or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Mepiprazole or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or (Tryptophan not depletion) or Venlafaxine or Viloxazine or Vilazodone or Vortioxetine or Viqualine or Zimelidine AND INREGISTER
#12 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #11)
#13 (postpartum or post‐partum or "post partum" or postnatal* or post‐natal* or "post natal*" or perinatal* or peri‐natal* or "peri natal*" or puerp* or intrapartum or intra‐partum or "intra partum" or antepartum or ante‐partum or "ante partum") AND INREGISTER
#14 (pregnan* or maternity or birth) and depress* AND INREGISTER
#15 (#13 OR #14)
#16 (#12 AND #15)
Ovid MEDLINE® and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily
<RCTs 2014 onwards> <Systematic Reviews (all years)>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp Antidepressive Agents/
2 exp Neurotransmitter Uptake Inhibitors/
3 exp Monoamine Oxidase Inhibitors/
4 (antidepress* or anti depress* or MAOI* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) and (uptake or reuptake or re‐uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic* or psychotropic*).ti,ab,kf.
5 (Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or (Bupropion or Amfebutamone) or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine) or Clorgyline or Clovoxamine or (CX157 or Tyrima) or Demexiptiline or Deprenyl or (Desipramine* or Pertofrane) or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or St John*) or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or Lorpiprazole or (Lu AA21004 or Vortioxetine) or Lu AA24530 or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Mepiprazole or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or (Tryptophan not depletion) or Venlafaxine or Viloxazine or Vilazodone or Vortioxetine or Viqualine or Zimelidine).mp.
6 or/1‐5
7 Depression, Postpartum/
8 ((postpartum* or post partum* or postnatal* or post natal* or perinatal* or peri natal* or puerp* or intrapartum* or intra partum* or antepartum* or ante partum*) adj3 (depress* or dysthymi* or adjustment disorder* or mood disorder* or affective disorder* or affective symptom*)).ti,ab,kf.
9 (7 or 8)
10 (6 and 9)
11 controlled clinical trial.pt.
12 randomized controlled trial.pt.
13 clinical trials as topic/
14 (randomi#ed or randomi#ation or randomi#ing).ti,ab,kf.
15 (RCT or "at random" or (random* adj3 (administ* or allocat* or assign* or class* or cluster or crossover or cross‐over or control* or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or pragmatic or quasi or recruit* or split or subsitut* or treat*))).ti,ab,kf.
16 placebo.ab,ti,kf.
17 trial.ti.
18 (control* adj3 group*).ab.
19 (control* and (trial or study or group*) and (waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,kf,hw.
20 ((single or double or triple or treble) adj2 (blind* or mask* or dummy)).ti,ab,kf.
21 double‐blind method/ or random allocation/ or single‐blind method/
22 or/11‐21
23 exp animals/ not humans.sh.
24 (22 not 23)
25 (10 and 24)
26 (2014* or 2015* or 2016* or 2017* or 2018* or 2019* or 2020*).yr,dt,ed,ep.
27 (25 and 26)
28 (systematic or structured or evidence or trials or studies).ti. and ((review or overview or look or examination or update* or summary).ti. or review.pt.)
29 (0266‐4623 or 1469‐493X or 1366‐5278 or 1530‐440X or 2046‐4053).is.
30 meta‐analysis.pt. or (meta‐analys* or meta analys* or metaanalys* or meta synth* or meta‐synth* or metasynth*).ti,ab,kf,hw.
31 ((systematic or meta) adj2 (analys* or review)).ti,kf. or ((systematic* or quantitativ* or methodologic*) adj5 (review* or overview*)).ti,ab,kf,sh. or (quantitativ$ adj5 synthesis$).ti,ab,kf,hw.
32 (integrative research review* or research integration).tw. or scoping review?.ti,kf. or (review.ti,kf,pt. and (trials as topic or studies as topic).hw.) or (evidence adj3 review*).ti,ab,kf.
33 review.pt. and ((medline or medlars or embase or pubmed or scisearch or psychinfo or psycinfo or psychlit or psyclit or cinahl or electronic database* or bibliographic database* or computeri#ed database* or online database* or pooling or pooled or mantel haenszel or peto or dersimonian or der simonian or fixed effect or ((hand adj2 search*) or (manual* adj2 search*))).tw,hw. or (retraction of publication or retracted publication).pt.)
34 or/28‐33
35 (10 and 34)
36 (26 and 35)
***************************
Ovid Embase
<RCTs 2014 onwards> <Systematic Reviews (all years)> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp Antidepressant Agent/ 2 Serotonin Receptor Affecting Agent/ or Serotonin Uptake Inhibitor/ or Serotonin Noradrenalin Reuptake Inhibitor/ or Triple Reuptake inhibitor/ 3 Dopamine Receptor Affecting Agent/ or Dopamine Uptake Inhibitor/ 4 Adrenergic Receptor Affecting Agent/ or Noradrenalin Uptake Inhibitor/ 5 Neurotransmitter Uptake Inhibitors/ 6 exp Monoamine Oxidase Inhibitor/ 7 (antidepress* or anti depress* or MAOI* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) and (uptake or reuptake or re‐uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic* or psychotropic*).ti,ab,kw. 8 (Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or (Bupropion or Amfebutamone) or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine) or Clorgyline or Clovoxamine or (CX157 or Tyrima) or Demexiptiline or Deprenyl or (Desipramine* or Pertofrane) or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or St John*) or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or Lorpiprazole or (Lu AA21004 or Vortioxetine) or Lu AA24530 or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Mepiprazole or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or (Tryptophan not depletion) or Venlafaxine or Viloxazine or Vilazodone or Vortioxetine or Viqualine or Zimelidine).mp. 9 (1 or 2 or 3 or 4 or 5 or 6 or 7 or 8) 10 postnatal depression/ 11 ((postpartum* or post partum* or postnatal* or post natal* or perinatal* or peri natal* or puerp* or intrapartum* or intra partum* or antepartum* or ante partum*) adj3 (depress* or dysthymi* or adjustment disorder* or mood disorder* or affective disorder* or affective symptom*)).ti,ab,kw. 12 (10 or 11) 13 (9 and 12) 14 randomized controlled trial/ 15 randomization.de. 16 controlled clinical trial/ and (Disease Management or Drug Therapy or Prevention or Rehabilitation or Therapy).fs. 17 *clinical trial/ 18 placebo.de. 19 placebo.ti,ab. 20 trial.ti. 21 (randomi#ed or randomi#ation or randomi#ing).ti,ab,kw. 22 (RCT or "at random" or (random* adj3 (administ* or allocat* or assign* or class* or cluster or control* or crossover or cross‐over or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or pragmatic or quasi or recruit* or split or subsitut* or treat*))).ti,ab,kw. 23 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$ or dummy)).mp. 24 (control* and (study or group?) and (waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,kw,hw. 25 or/14‐24 26 ((animal or nonhuman) not (human and (animal or nonhuman))).de. 27 (25 not 26) 28 (13 and 27) 29 (2014* or 2015* or 2016* or 2017* or 2018* or 2019* or 2020*).yr,dc. 30 (28 and 29) 31 systematic review/ or meta analysis/ or network meta‐analysis/ 32 ((systematic or structured or evidence or trials or studies) and (review or overview or look or examination or update* or summary)).ti. 33 (0266‐4623 or 1469‐493X or 1366‐5278 or 1530‐440X or 2046‐4053).is. 34 (systematic review? or evidence report* or technology assessment?).jw. 35 (meta‐analys* or meta analys* or metaanalys* or meta synth* or meta‐synth* or metasynth*).ti,ab,kw,hw. 36 ((systematic or meta) adj2 (analys* or review)).ti,kw. or ((systematic* or quantitativ* or methodologic*) adj5 (review* or overview*)).ti,ab,kw,sh. or (quantitativ* adj5 synthes*).ti,ab,kw,hw. 37 exp "clinical trial (topic)"/ and review.ti,kw,pt. 38 (integrative research review* or research integration).ti,ab,kw. or scoping review?.ti,kw. or (evidence adj3 review*).ti,ab,kw. 39 review.pt. and (medline or medlars or embase or pubmed or scisearch or psychinfo or psycinfo or psychlit or psyclit or cinahl or electronic database* or bibliographic database* or computeri#ed database* or online database* or pooling or pooled or mantel haenszel or peto or dersimonian or der simonian or fixed effect or ((hand adj2 search*) or (manual* adj2 search*))).ti,ab,kw,hw. 40 review.pt. and ((evidence based adj (medicine or practice)) or (outcome? adj (assessment or research)) or treatment outcome).hw. 41 or/31‐40 42 (13 and 41) 43 (29 and 42) ***************************
Ovid PsycINFO
<RCTs 2014 onwards> <Systematic Reviews (all years)> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 Psychopharmacology/ or Neuropsychopharmacology/ 2 "3340".cc. 3 exp Antidepressant Drugs/ 4 Neurotransmitter Uptake Inhibitors/ or exp serotonin norepinephrine reuptake inhibitors/ or exp serotonin reuptake inhibitors/ 5 exp Monoamine Oxidase Inhibitors/ 6 exp Tricyclic Antidepressant Drugs/ 7 (antidepress* or anti depress* or MAOI* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) and (uptake or reuptake or re‐uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic*).mp. 8 Drug Therapy/ 9 (Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or (Bupropion or Amfebutamone) or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine) or Clorgyline or Clovoxamine or (CX157 or Tyrima) or Demexiptiline or Deprenyl or (Desipramine* or Pertofrane) or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or St John*) or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or Lorpiprazole or (Lu AA21004 or Vortioxetine) or Lu AA24530 or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Mepiprazole or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or (Tryptophan not depletion) or Venlafaxine or Viloxazine or Vilazodone or Vortioxetine or Viqualine or Zimelidine).ti,ab,id,hw. 10 or/1‐9 11 postpartum depression/ or postnatal period/ 12 ((postpartum* or post partum* or postnatal* or post natal* or perinatal* or peri natal* or puerp* or intrapartum* or intra partum* or antepartum* or ante partum*) adj3 (depress* or dysthymi* or adjustment disorder* or mood disorder* or affective disorder* or affective symptom*)).ti,ab,id. 13 (11 or 12) 14 (10 and 13) 15 clinical trials.sh. 16 (randomi#ed or randomi#ation or randomi#ing).ti,ab,id. 17 (RCT or at random or (random* adj3 (administ* or allocat* or assign* or class* or control* or crossover or cross‐over or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or recruit* or split or subsitut* or treat*))).ti,ab,id. 18 (control* and (trial or study or group) and (placebo or waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,id,hw. 19 ((single or double or triple or treble) adj2 (blind* or mask* or dummy)).ti,ab,id. 20 trial.ti. 21 placebo.ti,ab,id,hw. 22 treatment outcome.md. 23 treatment effectiveness evaluation.sh. 24 mental health program evaluation.sh. 25 or/15‐24 26 (14 and 25) 27 (2014* or 2015* or 2016* or 2017* or 2018* or 2019* or 2020*).yr,an. 28 (26 and 27) 29 (meta analysis or "systematic review").md. 30 meta analysis/ 31 ((systematic or structured or evidence or trials or studies) and (review or overview or look or examination or update* or summary)).ti. 32 (meta‐analys* or meta analys* or metaanalys* or meta synth* or meta‐synth* or metasynth*).ti,ab,id,hw. (31678) 33 ((systematic or meta) adj2 (analys* or review)).ti,id. or ((systematic* or quantitativ* or methodologic*) adj5 (review* or overview*)).ti,ab,id,sh. or (quantitativ* adj5 synthes*).ti,ab,id,hw. 34 (integrative research review* or research integration).ti,ab,id. or scoping review?.ti,id. or (evidence adj3 review*).ti,ab,id. (16928) 35 literature review.sh. and (medline or medlars or embase or pubmed or scisearch or psychinfo or psycinfo or psychlit or psyclit or cinahl or electronic database* or bibliographic database* or computeri#ed database* or online database* or pooling or pooled or mantel haenszel or peto or dersimonian or der simonian or fixed effect or ((hand adj2 search*) or (manual* adj2 search*))).ti,ab,kw,hw. 36 ((systematic or structured or evidence or trials or studies) adj3 review*).ti,ab,id. and (evidence based practice or treatment outcomes or mental health program evaluation).sh. 37 or/29‐36 38 (14 and 37) 39 (27 and 38) ***************************
Data and analyses
Comparison 1. Antidepressants vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Depression response (acute phase) | 4 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.97, 1.66] |
| 1.2 Depression remission (acute phase) | 4 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.99, 2.41] |
| 1.3 Severity of depression ‐ overall (acute phase) | 4 | 251 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.55, ‐0.05] |
| 1.4 Severity of depression ‐ EPDS (acute phase) | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐3.51 [‐6.24, ‐0.78] |
| 1.5 Severity of depression ‐ HAM‐D (acute phase) | 3 | 216 | Mean Difference (IV, Random, 95% CI) | ‐2.02 [‐4.46, 0.42] |
| 1.6 Severity of depression ‐ CGI‐S (acute phase) | 2 | 110 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.87, 0.48] |
| 1.7 Treatment acceptability ‐ dropouts | 4 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.74, 1.64] |
Comparison 2. Antidepressants vs treatment as usual.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Depression remission (early phase) | 1 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [1.50, 3.54] |
| 2.2 Severity of depression ‐ EPDS (early phase) | 1 | 225 | Mean Difference (IV, Random, 95% CI) | ‐2.50 [‐3.85, ‐1.15] |
| 2.3 Treatment acceptability ‐ dropouts | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.91, 3.23] |
| 2.4 Parenting‐related outcomes ‐ MAMA (early phase) | 1 | 192 | Mean Difference (IV, Random, 95% CI) | 1.80 [0.18, 3.42] |
| 2.5 Parenting‐related outcomes ‐ MAMA (continuation phase) | 1 | 183 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐0.45, 2.45] |
| 2.6 Quality of life ‐ SF‐12 mental health | 1 | 175 | Mean Difference (IV, Random, 95% CI) | 0.45 [0.21, 0.69] |
| 2.7 Quality of life ‐ SF‐12 physical health | 1 | 175 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.25, 0.31] |
| 2.8 Quality of life ‐ EQ5D utility score | 1 | 198 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.01, 0.11] |
| 2.9 Quality of life ‐ EQ5D visual analogue scale | 1 | 204 | Mean Difference (IV, Random, 95% CI) | 5.10 [‐1.62, 11.82] |
Comparison 3. Antidepressants vs psychological interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Severity of depression ‐ EPDS (acute phase) | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 2.48 [0.71, 4.25] |
| 3.2 Severity of depression ‐ BDI (acute phase) | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 6.73 [1.51, 11.95] |
| 3.3 Severity of depression ‐ BDI (continuation phase) | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 7.89 [1.14, 14.64] |
| 3.4 Treatment acceptability | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.24, 4.88] |
| 3.5 Parenting‐related outcomes ‐ PSI (acute phase) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 9.32 [‐14.63, 33.27] |
| 3.6 Parenting‐related outcomes ‐ PSI (continuation phase) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 30.37 [6.26, 54.48] |
Comparison 4. Antidepressants vs psychosocial interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Depression remission (continuation phase) | 1 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.95, 1.53] |
| 4.2 Severity of depression ‐ EPDS (continuation phase) | 1 | 206 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐2.54, 0.54] |
| 4.3 Treatment acceptability ‐ dropouts | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [1.12, 3.35] |
| 4.4 Parenting‐related outcomes ‐ MAMA (continuation phase) | 1 | 183 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐0.45, 2.45] |
| 4.5 Quality of life ‐ SF12 mental health | 1 | 169 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.15, 0.41] |
| 4.6 Quality of life ‐ SF12 physical health | 1 | 169 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.09, 0.47] |
| 4.7 Quality of life ‐ EQ‐5D utility score | 1 | 194 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.08, 0.06] |
| 4.8 Quality of life ‐ EQ‐5D visual analogue scale | 1 | 194 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐9.08, 7.48] |
Comparison 5. Antidepressant vs other pharmacological intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Depression response ‐ acute phase | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.76, 1.27] |
| 5.2 Depression remission ‐ acute phase | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.68, 1.41] |
| 5.3 Depression response ‐ early phase | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.56, 1.19] |
| 5.4 Depression remission ‐ early phase | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.51, 1.67] |
| 5.5 Depression severity ‐ HAM‐D (acute phase) | 2 | 165 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐3.18, 1.87] |
| 5.6 Treatment acceptability ‐ dropouts | 1 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.99, 3.06] |
Comparison 6. Antidepressants vs complementary medicine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Depression response ‐ acute phase | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.71, 2.12] |
| 6.2 Depression remission ‐ acute phase | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.44, 3.09] |
Comparison 7. Sensitivity analysis ‐ antidepressant vs placebo, excluding studies at high risk of bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Depression response (acute phase) | 3 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.91, 1.77] |
| 7.2 Depression remission (acute phase) | 3 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.87, 2.11] |
| 7.3 Severity of depression ‐ overall (acute phase) | 3 | 181 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.67, ‐0.08] |
| 7.4 Severity of depression ‐ HAM‐D (acute phase) | 2 | 146 | Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐3.03, 1.34] |
| 7.5 Severity of depression ‐ CGI‐S (acute phase) | 1 | 35 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.76, 0.56] |
Comparison 8. Sensitivity analysis ‐ antidepressant vs placebo, excluding studies with imputed data.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Severity of depression ‐ overall (acute phase) | 3 | 164 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.71, ‐0.09] |
| 8.2 Severity of depression ‐ HAM‐D (acute phase) | 2 | 129 | Mean Difference (IV, Random, 95% CI) | ‐2.92 [‐6.54, 0.71] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Appleby 1997.
| Study characteristics | ||
| Methods |
Study design: RCT, double‐blind Location: UK (Manchester) Setting: community setting No. of centres: 2 large hospitals in an urban health district Dates of study: recruitment May 1993‐February 1995 Total duration of study: 23 months; recruitment (20 months), follow‐up (12 weeks) Recruitment: women on maternity wards were asked to allow assessment of their mood in their homes 6‐8 weeks later Randomisation method: computer‐generated random numbers Analysis by ITT: yes (LOCF), in addition to analysis by completion Power calculation: none stated |
|
| Participants |
Inclusion criteria: women who scored ≥ 10 on the EPDS at the screening visit were assessed with the CIS‐R and eligible to participate if they scored ≥ 12, as well as satisfying research diagnostic criteria for major or minor depressive disorder Exclusion criteria: chronic (> 2 years) or resistant depression, current drug or alcohol misuse, severe illness requiring close monitoring or hospital admission, breastfeeding Number recruited: 87 Number dropped out: 26 Number analysed: 87 (additional completers' analysis with 61 participants): fluoxetine + 1 counselling session N = 22; fluoxetine + 6 counselling sessions N = 21; placebo + 1 counselling session N = 23; placebo + 6 counselling sessions N = 21 Age (mean) years: fluoxetine + 1 counselling session 25.7; fluoxetine + 6 counselling sessions 26.6; placebo + 1 counselling session 23.1; placebo + 6 counselling sessions 26.0 Severity of PND, mean (range) score: CIS‐R: fluoxetine 28.2 (26.4‐30.1); placebo 28.3 (26.6‐30.1), EPDS: fluoxetine 17.2 (16.2‐18.2); placebo 16.9 (15.8‐18.1), HAM‐D: fluoxetine 13.2 (13‐15.5); placebo 13.9 (12.5‐15.4) Duration of PND: not reported Physical health co‐morbidities: not reported Mental health co‐morbidities: not reported Ethnicity: no details Socioeconomic status: no details |
|
| Interventions | Women were randomly assigned to 1 of 4 groups
Counselling was derived from CBT and structured to offer reassurance and practical advice on areas of concern to depressed mothers |
|
| Outcomes |
Primary outcome: depressive symptoms as measured by mean scores on the CIS‐R, the HAM‐D (week 1 and 12 only) and EPDS Time points: week 1, 4 and 12 |
|
| Notes | Funding source not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were allocated to one of four treatment groups by using computer generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No details on allocation concealment given |
| Blinding of participants (performance bias) | Low risk | "The counselling was delivered by a psychologist… supervised by a second psychiatrist, both were blind to drug treatment, as were trial subjects" |
| Blinding of personnel (performance bias) | Low risk | "The counselling was delivered by a psychologist… supervised by a second psychiatrist, both were blind to drug treatment, as were trial subjects" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The assessment interviews were conducted by a psychiatrist blind to subject treatment group" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Drop‐out rates were similar in the four groups. Drop outs were younger than subjects who completed the study and more likely to have an unemployed partner and to have a planned pregnancy, but the groups did not differ on initial psychiatric morbidity scores, employment, obstetric complications, parity, family history, or personal history of depression, including postnatal depression" Of 87 total participants, 14/43 from the fluoxetine plus counselling group dropped out and 12/44 of the placebo plus counselling group dropped out Details of dropout timings and reasons were reported, but mainly "no reason given". Lack of improvement was the reason for 3 dropouts in the fluoxetine group but 0 in the placebo group. In contrast, 3 women in the placebo group but only 1 woman in the intervention group dropped out due to side effects |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable |
| Other bias | Unclear risk | No details given on adherence to medication No funding details reported |
Bloch 2012.
| Study characteristics | ||
| Methods |
Study design: RCT, double‐blind, placebo‐controlled Location: Israel Setting: maternity ward and baby care centre No. of centres: 1 (Tel Aviv Sourasky Medical Center) Dates of study: not reported Total duration of study: 8 weeks Recruitment: patients were referred from maternity wards or from baby care centres Randomisation method: pharmacy‐generated random serial numbers Analysis by ITT: yes (LOCF) Power calculation: yes |
|
| Participants |
Inclusion criteria: aged 18‐45 years; met criteria for current MDD during screening and baseline visit according to DSM‐IV (Structured Clinical Interview for DSM‐IV Axis I disorders), onset of depression within 2 months of delivery Exclusion criteria: MADRS score ≥ 30, suicidal ideation (MADRS item 10 score ≥ 5), psychotic symptoms or bipolar disorder, current depressive episode > 6 months, current treatment with antidepressants, 2 failed adequate trials of antidepressants, major physical illness, alcoholism or drug use Number recruited: 42 Number dropped after baseline assessment: 2 (both from placebo + BDP* group) Number dropped out by week 8: 4 from sertraline + BDP group; 3 from placebo + BDP group (not including the 2 dropped out after baseline assessment) Number analysed: 40 (2 participants who dropped out after baseline excluded): sertraline N = 20; placebo N = 20 Age: no data Severity of PND mean (SD) score: MADRS: sertraline 19.8 (4.98); placebo 19.8 (4.64) EPDS: sertraline 18.4 (4.83); placebo 16.05 (4.84) CGI‐S: sertraline 3.7 (0.66); placebo 3.8 (0.62) CGI‐I: sertraline 2.65 (1.18); placebo 2.45 (1.10) MHI‐wellbeing: sertraline 2.08 (0.74); placebo 2.01 (0.64) MHI‐distress: sertraline 4.16 (0.77); placebo 3.83 (0.86) Duration of PND: not reported Physical health co‐morbidities: not reported Mental health co‐morbidities: anxiety diagnosis: sertraline N = 5; placebo N = 4, past depression: sertraline N = 4; placebo N = 5 Ethnicity: no data Socioeconomic status: sertraline + BDP group: high income: 7/20 (35%), middle income: 10/20 (50%), low income: 3/20 (15%); placebo + BDP group: high income: 4/20 (20%), middle income: 7/20 (35%), low income 9/20 (45%) |
|
| Interventions | Women were randomly assigned to 1 of 2 groups
*BDP is a time‐limited psychotherapeutic intervention that aims to enhance the patient's insights about repetitive circumstances. |
|
| Outcomes |
Primary outcome: continuous change in depressive symptoms as measured by the MADRS and EPDS during 8‐week randomisation phase Secondary outcomes: continuous change in MADRS and EPDS during open phase of the study (weeks 8‐12), proportion of women meeting response and remission status at week 8 (response defined as > 50% reduction in MADRS or EPDS scores during treatment and remission as a final score of < 10 on the MADRS or < 7 on the EPDS) Other secondary ratings: measurements of symptom severity using the CGI‐I and CGI‐S, assessment of global mental health with the MHI and assessment of adverse effects using the UKU Side Effect Rating Scale Time points: weeks 0, 2, 4, 6, 8, 12 |
|
| Notes | This study was funded by an Independent Investigator Award to Dr Bloch from the National Alliance for Research on Schizophrenia and Depression, Great Neck, New York. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The institution's pharmacy‐generated random patient serial numbers with active versus placebo ratio 1:1 were issued to the researchers and randomly assigned to eligible patients by the psychiatrist after the informed consent was signed". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information given to be certain of allocation concealment |
| Blinding of participants (performance bias) | Low risk | "The second group received dummy pills daily, identical in appearance to the active pills, according to the same protocol as the active group" |
| Blinding of personnel (performance bias) | Low risk | "The managing psychiatrist was blinded to treatment condition... The managing psychiatrist was also asked at the end of the full protocol to document her assessment of whether the patient received active or placebo pills, and indeed, was unable to correctly guess this factor in every instance" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given on who assessed outcomes so unclear whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Seven patients discontinued medication between weeks 4 and 8, three from the placebo group and four from the active group. Discontinuation was due to lack of motivation (n = 4: placebo group, n = 2; sertraline group, n = 2) and clinical deterioration (n = 3: placebo group, n = 1; sertraline group, n = 2)." 42 participants were originally in the study, 2 participants dropped out of the placebo group immediately after the baseline. 40 participants are included in the ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient detail in protocol |
| Other bias | Unclear risk | "A pill count was conducted to monitor compliance. Protocol violation was defined as <80% compliance by pill count" It is unclear whether the 7 patients who discontinued medication are the only participants with low compliance "The compliance for psychotherapy was good: in the sertraline group, 92% of the psychotherapy sessions were attended compared to 87% in the placebo group (P = NS) [not significant]" |
Chibanda 2014.
| Study characteristics | ||
| Methods |
Study design: RCT Location: Zimbabwe Setting: urban primary care clinics in Chitungwiza, a peri‐urban community with a population of 1.5 million located on the outskirts of the city of Harare No. of centres: 2 Dates of study: not reported Total duration of study: 6 weeks Recruitment: patients attending routine postnatal appointment Randomisation method: computer‐generated random numbers Analysis by ITT: no Power calculation: not reported |
|
| Participants |
Inclusion criteria: women aged ≥ 18; baby aged 6‐7 weeks at 6‐week postnatal check‐up; local resident; meeting criteria for major depression according to DSM‐IV Exclusion criteria: resident elsewhere; unable to give informed consent; had psychosis, severe depression and/or suicidal ideation Number recruited: 58 Number dropped out: amitriptyline: N = 6; group problem‐solving: N = 3 Number analysed: 49; amitriptyline N = 22, group problem‐solving N = 27 Age, mean (SD) years: amitriptyline: 24 (4.4); group problem‐solving: 25 ( 5.4) Severity of PND, mean (SD) EPDS score: amitriptyline:17.9 (3.9); group problem‐solving: 17.3 (3.7) Duration of PND: not reported Physical health co‐morbidities: HIV‐positive: amitriptyline N = 6; group problem‐solving N = 8 Mental health co‐morbidities: not reported Ethnicity: no data Socioeconomic status: mean years spent in education (SD) amitriptyline group: 11.2 (4.5); group problem‐solving group: 10.6 (4.6) |
|
| Interventions | Women were randomly assigned to 1 of 2 groups
Group problem‐solving therapy was delivered by peer counsellors who received supervision by psychiatrists. |
|
| Outcomes |
Primary outcome: EPDS scores Time points: 6 weeks post‐treatment |
|
| Notes | "The author(s) received no financial support for the research, authorship, and/or publication of this article." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not reported |
| Blinding of participants (performance bias) | High risk | No attempts to blind participants reported and very unlikely given nature of study |
| Blinding of personnel (performance bias) | High risk | No attempts to blind personnel reported and very unlikely given nature of study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Of the 58 postpartum mothers who received the intervention, 49 (85%) completed 6 weeks of treatment with group PST (n = 27) or pharmacotherapy (n = 22). Overall, 9 (15%) women were lost to follow‐up at 6 weeks including 3 (10%) from group PST and 6 (21%) from the pharmacotherapy group, respectively." Numbers are relatively small and both groups affected. Reasons not clearly reported. Dropouts and losses to follow‐up were not included in the analyses. |
| Selective reporting (reporting bias) | Unclear risk | Unable to locate study protocol using the registration number given in the paper |
| Other bias | Unclear risk | No details given on adherence to medication |
Hantsoo 2013.
| Study characteristics | ||
| Methods |
Study design: RCT, double‐blind, placebo‐controlled Location: USA Setting: clinical and community No. of centres: 1 Dates of study: 1994‐2004 Total duration of study: 6 weeks Recruitment: via local obstetrician‐gynaecologists, paediatricians, mental health professionals, postnatal depression support groups, and advertisements in local newspapers Randomisation method: unclear Analysis by ITT: yes (LOCF for response and remission analyses) and by evaluable group Power calculation: yes |
|
| Participants |
Inclusion criteria: aged 18‐45 years, depression onset reported within 3 months after delivery, no psychotropic medication for ≥ 5 weeks, and given birth within the last 12 months to an infant without serious medical issues. Participants were required to have a diagnosis of postnatal depression based on the SCID, to score ≥ 18 and < 32 on the 19‐item HAM‐D and to have at least "moderate" symptoms on the severity of illness rating of the CGI scale. Only English speaking women were eligible. Exclusion criteria: onset of MDD during pregnancy (indicated on the SCID), screened positive for thyroid disease (unless thyroid condition stable), drug or alcohol dependence in the last 6 months or positive urine drug test during screening, current or history of psychotic disorder (Axis I, including bipolar type I), active suicidal ideation, any significant medical conditions, planning to become pregnant or past failed trial of sertraline. Number recruited: 38 (36 randomised after the placebo run‐in week: 2 participants had > 30% decline in HAM‐D scores during the run‐in week and were removed from the study as per protocol) Number dropped out: 7 dropped out by week 7 (final week) Number analysed: 36 analysed on an ITT basis; sertraline N = 17; placebo N = 19. Repeated analyses with evaluable group had at least 3 post‐randomisation assessments (33 women); Age, mean (SD) years: total sample 30.8 (4.0), with no between‐group differences; sertraline: 29.6 (4.0); placebo: 31.7 (3.7) Severity of PND, mean (SD) score: HAM‐D: sertraline 20.6 (2.8); placebo 23.2 (3.9) EPDS: sertraline 18.8 (2.6); placebo 20.8 (5.7) Duration of PND, mean (SD) weeks: sertraline 15.5 (13.6); placebo 13.5 (12.3) Physical health co‐morbidities: not reported Mental health co‐morbidities: mean (SD) HAM‐A scores: sertraline 21.3 (6.5); placebo 24.5 (5.8) Ethnicity: sertraline: 16 white, 1 Hispanic; placebo: 18 white, 1 Hispanic Education,mean (SD) years: sertraline 14.4 (2.0); placebo: 14.0 (1.2) |
|
| Interventions | All participants underwent a 1‐week single‐blind placebo lead‐in. Participants who still met the inclusion criteria and had had < 30% reduction in HAM‐D scores were randomly assigned to 1 of 2 groups
|
|
| Outcomes |
Primary outcomes: response in psychiatric symptoms: treatment response was defined as a score of ≤ 10 on the HAM‐D, at least a 50% decrease in HAM‐D score from baseline, and a score of "much improved" or "very much improved" on the improvement scale of the CGI; remission defined as per criteria for response to treatment in addition to a HAM‐D score ≤ 7 Secondary outcomes: trends over time in depressive symptoms as rated by the HAM‐D and the EPDS, and in anxiety symptoms as rated by the HAM‐A. The predominant interest was the treatment group by linear time interaction Time points: 6 weeks post‐treatment |
|
| Notes | This study was funded by Pfizer (New York, NY, USA), the National Institute of Mental Health (P50 MH099910 and K23 MH01830) and the National Institute of Drug Abuse (K24 DA03031). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After the lead‐in, all the subjects... were randomised to a 6‐week, double‐blind trial" |
| Allocation concealment (selection bias) | Unclear risk | No details given on allocation concealment |
| Blinding of participants (performance bias) | Low risk | 1 participant was excluded after the study began due to accidental unblinding |
| Blinding of personnel (performance bias) | Low risk | "A research pharmacist was responsible for creating a blinding table and distributing the study drug; all other study personnel remained blind to subject treatment status" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All other study personnel remained blind to subject treatment status" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "A total of 17 women were randomised to the sertraline group and 19 were randomised to placebo, for a total of 36 women in the intent‐to‐treat group. The reasons for failure to the full 7 weeks included clinical deteriorating (n = 3, all in the placebo group), loss to follow‐up (n = 3), and accidental unblinding (n = 1)" There could have been an underestimation of treatment effect as women dropping out for clinical deterioration were all in the control group |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable |
| Other bias | Unclear risk | No details given on adherence to medication Funding by Pfizer ‐ but it is unclear if they provided the study medication |
Kashani 2017.
| Study characteristics | ||
| Methods |
Study design: RCT, double‐blind Location: Iran Setting: outpatient clinics No. of centres: 3 (Yas Women General Hospital, Arash Hospital, Baharloo Hospital) Dates of study: September‐December 2015 Total duration of study: 6 weeks Recruitment: outpatient clinic Randomisation method: permuted randomisation block (blocks of 4, allocation ratio 1:1) Analysis by ITT: no Power calculation: yes |
|
| Participants |
Inclusion criteria: women 18–45 years of age; diagnosis of postpartum depression based on DSM‐IV; 4–12 weeks after childbirth; mild to moderate depression with HDRS score ≥10 and ≤18 Exclusion criteria: women with psychotic depression, history of suicidal or infanticidal thoughts, a history of bipolar disorder, substance or alcohol dependence (with the exception of nicotine dependence), lactation, hypothyroidism, acute medical illness, patients suffering from any DSM‐IV diagnosis other than postpartum depression Number recruited: 68 Number dropped out: fluoxetine: N = 2; saffron: N = 2 Number analysed: 64; fluoxetine N = 32; saffron N = 32 Age, mean (SD) years: fluoxetine: 32.09 (4.99); saffron: 29.21 (7.69) Severity of PND, mean (SD) HDRS score: fluoxetine 16.65 (1.12); saffron 16.53 (1.48) Duration of PND, mean (SD): not reported Physical health co‐morbidities: not reported Mental health co‐morbidities: history of depression: fluoxetine N = 9; saffron N = 7 Ethnicity: no data Socioeconomic status: education fluoxetine group: primary school 2/32, high school diploma 24/32, university degree 4/32; saffron group: primary school: 4/32, high school diploma 26/32, university degree 2/32 |
|
| Interventions | Women were randomly assigned to 1 of 2 groups
|
|
| Outcomes |
Primary outcome: improvement of depressive symptoms using HDRS Secondary outcomes: improvement in HDRS score at each time point, partial responders (25%‐50% reduction in HDRS score), responders (≥ 50% reduction in HDRS score), remitters (HDRS score ≤ 7), time needed to respond to treatment, response rate (≥ 50% reduction in HDRS score) and remission rate (HDRS score ≤ 7) compared between groups Adverse events: 25‐item checklist Time points: week 1, 3 and 6 |
|
| Notes | Funded through a grant from Tehran University of Medical Sciences (Grant no: 23222). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An independent party, who was not involved elsewhere in the trial, randomized codes by permuted randomization block (blocks of 4, allocation ratio 1:1)." |
| Allocation concealment (selection bias) | Low risk | "Concealment of allocation was performed using sequentially numbered, sealed opaque envelopes. An aluminium foil inside the envelopes kept them impermeable to intense light." |
| Blinding of participants (performance bias) | Low risk | Study participant, research investigator and the rater were all blind to the treatment allocation. Saffron and fluoxetine capsules were indistinguishable in their shape, size, texture, colour and odour." |
| Blinding of personnel (performance bias) | Low risk | Study participant, research investigator and the rater were all blind to the treatment allocation. Saffron and fluoxetine capsules were indistinguishable in their shape, size, texture, colour and odour." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study participant, research investigator and the rater were all blind to the treatment allocation. Saffron and fluoxetine capsules were indistinguishable in their shape, size, texture, colour and odour." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 34 randomised to each group but data presented for only 32. Not ITT. 2 excluded from each group due to shift from moderate to severe depression |
| Selective reporting (reporting bias) | High risk | Remission, response and adverse events are not specified as outcomes in protocol (and therefore protocol does not define remission or response). Protocol states HDRS will be measured at weeks 2, 4 and 6, not weeks 1, 3 and 6 |
| Other bias | Unclear risk | No adherence data reported. No other bias identified, including financial conflicts or other COIs. Limited detail on recruitment of the small sample of women ‐ possible risk of sampling bias |
Milgrom 2015.
| Study characteristics | ||
| Methods |
Study design: RCT, parallel 3‐group Location: Australia (Melbourne) Setting: community‐based (maternal and child health centres) No. of centres: not reported Dates of study: not reported Total duration of study: 3 years Recruitment: community screening programme at a routine 12‐week postnatal check‐up Randomisation method: variable‐length permuted blocks randomisation schedule Analysis by ITT: yes (using multiple imputation) Power calculation: yes |
|
| Participants |
Inclusion criteria: between 19 and 40 years of age and had an infant > 2 months and < 8 months of age, born after a full‐term pregnancy, with no congenital abnormalities; a screening EPDS score of ≥ 13; DSM‐IV diagnosis of a depressive disorder with postnatal onset. Exclusion criteria: positive serum pregnancy test; a concurrent psychiatric disorder (excepting co‐morbid anxiety); a recent history of antidepressant usage (within the last month); a history of major allergy or drug allergy; a history of substance abuse; prior non‐response to sertraline, or prior non‐response to adequate trials of two SSRIs; a predisposition to headache, migraine or nausea; tobacco habit > 10 cigarettes per day; caffeine consumption > 6 cups of coffee/tea or cola‐flavoured drinks per day; ongoing dental work; extreme levels of depression (psychotic); or suicidal intent Number recruited: 45 Number dropped out: 0 Number analysed: 45; sertraline N = 15; group‐CBT N = 14; sertraline + group‐CBT N = 16 Age, mean (SD) years: sertraline 31.6 (3.2); group‐CBT 28.5 (3.2); sertraline + group‐CBT 30.1 (5.5) Severity of PND, mean (SD) EPDS score: sertraline 16.8 (4.5); group‐CBT 16.6 (4.2); sertraline + group‐CBT 17.2 (3.7) Duration of PND: not reported Physical health co‐morbidities: not reported Mental health co‐morbidities: 3 participants had a primary diagnosis of mixed anxiety/ depressive disorder (not reported by treatment arm) Ethnicity: no data Socioeconomic status: household income sertraline group: up to AUD 50,000 35.7%, up to or over AUD 80,000 57.2%, not reported 7.1%; group‐CBT group: up to AUD 50,000 43.7%, up to or over AUD 80,000 37.5%, not reported 18.8%; sertraline + group‐CBT group: up to AUD 50,000 50%, up to or over AUD 80,000 25%, not reported 25% |
|
| Interventions | Women were randomly assigned to 1 of 3 groups:
Authors described the group CBT sessions as a "manualised, replicable treatment". |
|
| Outcomes |
Primary outcome: BDI‐II (14‐19: mild depression, 20‐28: moderate depression, 29‐63: severe depression) Secondary outcomes: BAI (0‐7: minimal level of anxiety, 8‐15 mild anxiety, 16‐25 moderate anxiety, 26‐63 severe anxiety), PSI (scores > 260 reflect clinically significant parenting dysfunction) Time points: 12 and 24 weeks post‐treatment |
|
| Notes | The study was funded through a grant from Pfizer Inc. and by the Kinsman Fund. Neither funding body had any input into the study design, the analysis or interpretation of results, or the decision to publish the findings. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "variable‐length permuted blocks randomisation schedule" |
| Allocation concealment (selection bias) | Low risk | "A variable‐length permuted blocks randomisation schedule, generated and administered by an independent person, was prepared prior to commencement of the study and operated via telephone." |
| Blinding of participants (performance bias) | High risk | Blinding not reported. Unlikely to be blinded given nature of study |
| Blinding of personnel (performance bias) | High risk | Blinding not reported. Unlikely to be blinded given nature of study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not reported but trial registry entry describes that outcome assessors were blinded (clinicaltrials.gov/ct2/show/NCT02122393) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis using multiple imputation but it is unclear how many (if any) participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | All specified outcomes reported but 12‐week measurement not specified in protocol and remission analysis (including definition of remission) not mentioned in protocol |
| Other bias | High risk | Low adherence in antidepressant and combined arms. Study funded by manufacturer of sertraline preparation used in 2 of the treatment arms but no conflict of interest declared by study authors |
O'Hara 2019.
| Study characteristics | ||
| Methods |
Study design: RCT, placebo‐controlled, three‐arm parallel group Location: USA Setting: mixed setting including community, obstetric‐gynaecology, paediatric, and mental health clinics, partial hospitalisation programmes No. of centres: 2 (Women and Infants Hospital (WIH) in Rhode Island and the University of Iowa) Dates of study: not reported Total duration of study: 12 weeks Recruitment: not reported Randomisation method: permuted blocks, stratified by site Analysis by ITT: yes Power calculation: not reported |
|
| Participants |
Inclusion criteria: given birth within previous 6 months, primary DSM‐IV diagnosis of major depressive episode using the SCID, 17‐item HAM‐D score ≥ 15, delivery of a healthy infant, at least 36‐weeks' gestation, 18‐50 years, willing to use acceptable birth control methods. 7 months after recruitment began, the intake criteria were modified to include women in the first year postpartum. Approximately 2½ years after recruitment began HAM‐D‐17 scores for inclusion were decreased from ≥ 15 to ≥ 12 in order to increase recruitment. Exclusion criteria: current or past diagnosis of bipolar disorder, schizophrenia or other psychotic disorder, diagnosis of alcohol or drug abuse or dependence in the past year, current psychotic symptoms, acute suicidal or homicidal risk based on SCID interview, current illegal drug use (based on self‐report or positive urine screen), ongoing treatment with antidepressant medication or psychotherapy, previous trial of IPT with a certified IPT therapist, previous adequate trial of sertraline (i.e. at least 8 weeks of at least 100 mg daily), Contraindications to the use of sertraline (liver transaminase level > 1.25 times normal), abnormal TSH (<0.4 μU/mL or >5.5 μU/mL), Currently pregnant (based on a urine screen). 7 months after recruitment began, the intake criteria were modified to include breast feeding women and the use of sleep medication on an as needed basis (i.e. up to three times per week) was permitted. Number recruited: 162 Number dropped out: sertraline N = 16; placebo N = 9; IPT N = 10 Number analysed: 162: sertraline N = 56; placebo N = 53; IPT N = 53 Age, mean(SD) years: sertraline 28.2 (5.6); placebo 27.3 (5.1); IPT 26.3 (6.1) Severity of PND, mean (SD) HAM‐17 score: sertraline 21.8 (4.7); placebo 22.0 (4.7); IPT 21.9 (4.4) Duration of PND: not reported Physical health co‐morbidities: not reported Mental health co‐morbidities: not reported Ethnicity: % BAME: sertraline 37.5; placebo 47.2; IPT 45.3 Socioeconomic status: no data |
|
| Interventions | Women were randomly assigned to 1 of 3 groups:
Authors described the interpersonal psychotherapy sessions as a manualised intervention. Therapists were supervised monthly by one of the authors using video recordings. |
|
| Outcomes |
Primary outcome: HAMD‐17 Secondary outcomes: BDI, IDAS‐GD, CGI, PPAQ Time points: 4, 8, and 12 weeks post‐treatment |
|
| Notes | This work was funded by NIMH grants R01MH074636 (PI:Stuart) and R01MH074919 (PI:Zlotnick) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned (using the method of permuted blocks) to either IPT (N = 53), sertraline (N = 56), or pill placebo (N = 53). Randomization was stratified by study site (Iowa and WIH) and further stratified within the Iowa site by identification method (use of birth records or direct clinical referral)." |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not reported |
| Blinding of participants (performance bias) | Unclear risk | Antidepressant v placebo: low risk ‐ personnel (and presumably participants) were blind to treatment assignment for placebo vs. sertraline Antidepressant v psychotherapy: high risk ‐ personnel (and presumably participants) were blind to treatment assignment for placebo vs. sertraline. But clinicians were unblinded. |
| Blinding of personnel (performance bias) | Unclear risk | Antidepressant v placebo: low risk ‐ personnel (and presumably participants) were blind to treatment assignment for placebo vs. sertraline Antidepressant v psychotherapy: high risk ‐ personnel (and presumably participants) were blind to treatment assignment for placebo vs. sertraline. But clinicians were unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All interview assessments were conducted by trained research assistants blinded to treatment condition and were conducted separate from treatment sessions." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Adverse events: unclear risk ‐ slightly more participants lost to follow‐up in antidepressant group (16/56) than placebo (9/53) and psychotherapy (10/53). Some reasons for dropout were reported but majority were 'unknown'. Depression severity: high risk ‐ slightly more participants lost to follow‐up in antidepressant group (16/56) than placebo (9/53) and psychotherapy (10/53). Some reasons for dropout were reported but majority were 'unknown'. All participants included in analyses in paper but data were not extractable. Data extracted from clinicaltrials.gov are completers only Response and remission: low risk ‐ all participants included in analyses. |
| Selective reporting (reporting bias) | High risk | HAM‐A listed as secondary outcome measure in protocol but IDAS‐GD is measured/reported in paper instead and is only outcome measure with significant group x time interaction (although not extracted for this review). In paper data are reported for 4‐, 8‐ and 12‐week time points, where protocol only specifies 12 weeks, 6 months and 9 months (from baseline). |
| Other bias | Unclear risk | Sertraline and placebo pills were provided by Pfizer, Inc. but no funding provided by pharmaceutical company. 1 author is the owner of and receives compensation from IPT institute. No data reported on medication adherence among completers. More participants in antidepressant group did not receive intervention or discontinued (32.2%) compared with placebo (24.5%) and IPT (26.4%) |
Sharp 2010.
| Study characteristics | ||
| Methods |
Study design: RCT Location: UK Setting: community‐based No. of centres: 77 general practices based in Bristol (N = 21), south London (N = 21) and Manchester (N = 35) Dates of study: January 2005‐March 2008 Total duration of study: recruitment (January 2005‐August 2007), 18‐week follow up completed by March 2008 Recruitment: GP practice, collaborating GPs and health visitors were also able to refer women who became depressed between 6 and 26 weeks postnatally Randomisation method: web‐based randomisation programme Analysis by ITT: ITT, multiple imputation and complete‐case analysis all employed Power calculation: yes |
|
| Participants |
Inclusion criteria: women aged ≥ 18 years who had a recent live birth and were living with their baby were eligible for screening phase. After screening, deemed to be eligible if: score of ≥ 13 on baseline EPDS, ICD‐10 primary diagnosis of depression on the CIS‐R, proficient in English at a level to complete all research assessments and recently delivered baby was < 26 weeks old Exclusion criteria: stillbirth or neonatal death, baby > 26 weeks old, baby fostered or adopted. Women were also not eligible if they had psychosis, alcohol or drug abuse, were already receiving treatment for depression or were actively suicidal Number recruited: 254 Number dropped out by week 4: antidepressants: 23/129, treatment as usual: 13/125 Number dropped out by week 18: antidepressants: 32/129, listening visits: 16/125 Number analysed: 218 primary analysis on an ITT basis at 4 weeks, 206 primary analysis on an ITT basis at 18 weeks, also analysed as all 254 randomised. Antipressants N = 129, treatment as usual N = 125 Age, mean (SD) years; 29.3 (6.3). Age not reported by individual treatment arm. Severity of PND, mean (SD) score: EPDS: antidepressants 17.3 (3.3); treatment as usual 17.7 (3.5) CIS‐R: antidepressants 25.9 (7.3); treatment as usual 26.0 (7.9) Duration of PND: not reported Physical health co‐morbidities: not reported Mental health co‐morbidities: not reported Ethnicity: white 196 (77.8%), black 29 (11.5%), Asian 13 (5.2%), other 14 (5.6%) Socioeconomic status: highest educational qualification: none: 36 (14.8%), GCSE (school exams taken at 16) 67 (27.5%), A level (school exams taken at 18) 32 (13.1%), NVQ (National Vocational Qualification) 48 (19.7%), degree 61 (25.0%) |
|
| Interventions | Women were randomly assigned to 1 of 2 groups
|
|
| Outcomes |
Primary outcome: assessment of remission of postnatal depression using EPDS < 13 at follow‐up Secondary outcomes: change in depressive symptoms (EPDS) as continuous variable, physical and mental health assessment (SF‐12), assessment of maternal functioning (MAMA), health‐related quality of life (EQ‐5D), quality of marital relationships (GRIMS). If women had a male partner he was asked to complete the following: assessment of relationship with partner (GRIMS), assessment of paternal functioning (PAPA), general health assessment (GHQ and SF‐12) Time points: immediately post‐treatment, 4 and 8 weeks post‐treatment |
|
| Notes | This study was funded by the National Institute for Health Research Health Technology Assessment programme. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Before the baseline home visit, the women’s trial identification number, date of birth and trial centre were entered into a web‐based randomisation program" "The randomisation sequences was generated using a computer program with block sizes of six, eight and ten, varied randomly" |
| Allocation concealment (selection bias) | Low risk | "After eligibility had been determined and consent had been obtained at the home visit, the researcher telephoned the remote computerised randomisation service and responded to a series of questions by keying numbers (e.g. patient identification number, baseline EPDS score) of the telephone keypad" "The methods of sequence generation were concealed from the researchers involved in enrolling and randomising the women into the trial" |
| Blinding of participants (performance bias) | High risk | "Participants, researchers and those delivering the interventions were not blinded to the treatment allocation" |
| Blinding of personnel (performance bias) | High risk | "Participants, researchers and those delivering the interventions were not blinded to the treatment allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Participants, researchers and those delivering the interventions were not blinded to the treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "More women in the antidepressant group withdrew or were lost to follow‐up [4 weeks: antidepressants 23 (18%), listening visits 13 (10%) P = 0.090; 18 weeks: antidepressants 32 (25%), listening visits 16 (13%) P = 0.015]" Reasons for dropout are not given and characteristics of dropouts are not given separately by intervention group Sensitivity analyses (including multiple imputation) were performed to examine the impact of missing data. The imputation of missing data had no material effect on the results |
| Selective reporting (reporting bias) | High risk | All primary outcomes reported. Some evidence of selective outcome reporting from the protocol where the HOME measure and Bayley Scale of Infant Developments are prespecified but not reported in the main paper. Paternal measures are also detailed in the protocol; these are detailed in the methods of the main paper and it is stated that they will be discussed in a separate report, but this could not be identified |
| Other bias | High risk | "At 4 weeks only 59 (56%) of the 106 women followed up among those randomised to the antidepressants and who completed the [adherence] questionnaire reported taking any antidepressants. In the listening visits groups seven (6%) of the 112 women followed up also reported taking antidepressants... At the 18‐week time point, the numbers in each group who reported taking antidepressants during the previous 4 weeks were 62 (64% of the 97 followed up) and 37 (34% of 109 followed up) in the antidepressants and listening visits groups, respectively" |
Wisner 2006.
| Study characteristics | ||
| Methods |
Study design: RCT, double‐blind Location: USA Setting: no details No. of centres: 3 (Cleveland, Ohio; Louisville, Kentucky; Pittsburgh, Pennsylvania) Dates of study: not reported Total duration of study: 24 weeks; acute phase (8 weeks) continuation phase (16 weeks) Recruitment: not reported Randomisation method: block randomisation with a sequence generated in SPSS Analysis by ITT: yes for primary outcomes (response and remission), LOCF Power calculation: yes |
|
| Participants |
Inclusion criteria: women aged 15‐45 years with major depression within 4 weeks of birth. Women with chronic depression (an episode on major depression beginning before the index pregnancy) were also included after additional funding was obtained part‐way through the study. Mothers had to present for treatment within 3 months of delivery and score ≥ 18 on the HAM‐D Exclusion criteria: presence of any other Axis I disorder except generalised anxiety disorder or panic disorder, contraindications to TCA treatment, and concurrent psychiatric treatment Number recruited: 109 Number dropped out: 23 from the sertraline group (42%), 13 from the nortriptyline group (24%) Number analysed: ITT and analyses presented for 95 women who took the assigned medication for at least 1 week and provided at least 1 week of follow‐up data and 83 women who provided at least 3 weeks of follow‐up data Age: no data Severity of PND: specific data not reported. Authors stated that "women randomly assigned to SERT versus NTP did not differ on initial HRSD, CGI, GAS, and the SPQ composite score". Duration of PND: not reported Physical health co‐morbidities: not reported Mental health co‐morbidities: not reported Ethnicity: significantly more non‐white women were randomly assigned to sertraline (40%) than nortriptyline (19%) (Fisher exact test; P = 0.02). There were no other demographic differences between the 2 drug groups at baseline Socioeconomic status: not reported |
|
| Interventions | Women were randomly assigned to 1 of 2 groups
|
|
| Outcomes |
Primary outcomes: response to treatment at 8 weeks (50% reduction in HAM‐D from baseline); remission of depression (HAM‐D < 7 at week 8); continuous change in HAM‐D; severity of symptoms of depression (CGI scale at week 8); overall functioning as measured by the GAS; issues in income, housing, relationships and work (SPQ) Secondary outcome: side effects on the Asberg Side Effects Rating Scale in addition to time to withdrawal due to side effects, obsessions and compulsions measured with the YBOCS, emergence of mania was screening for safety reasons using the Mania Rating Scale (derived from the Schedule for Affective Disorders and Schizophrenia) Time points: weekly intervals for weeks 1‐8, then again at week 24 |
|
| Notes | This study was funded by the National Institute of Mental Health. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomised 1:1 to either nortriptyline or sertraline in block of 8 to 12 with a sequence generated by SPSS" |
| Allocation concealment (selection bias) | Unclear risk | No details given on allocation concealment |
| Blinding of participants (performance bias) | Low risk | "Prescriptions were assembled by the research pharmacist. The nortriptyline and sertraline were delivered in 2 doses, with breakfast and at bedtime. The opaque, inert gelatine capsules contained either sertraline (AM)/placebo(HS) or placebo(AM)/nortriptyline (HS)" |
| Blinding of personnel (performance bias) | Low risk | "The primary staff (side effects monitor, mood symptom rater, and study psychiatrist) were blind to drug assignment until project completion. The medication monitoring function (nurse) was separate from (and blind to) the mood monitoring (interviewer)" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The primary staff (side effects monitor, mood symptom rater, and study psychiatrist) were blind to drug assignment until project completion" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Significantly more women who took sertraline compared with nortriptyline withdrew from the study in the first 8 weeks (23/55 [42%] versus 13/54 [24%], respectively [P = 0.02]). The proportion of women who were lost to follow‐up or withdrew by personal choice differed significantly (sertraline, 20%, vs. nortriptyline, 6%; Wilcoxon χ21 = 4.86; P = 0.03). Other reasons for withdrawal (side effects, hypomania occurrence, or clinical deterioration) did not differ between the 2 drug groups" It is unclear why the difference in withdrawal between study groups was so high ‐ but likely to cause bias in results |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable |
| Other bias | Low risk | "Fourteen women had minimal drug in their blood despite claims of compliance. The results remained the same when data from these 14 women were removed. Drug assignment in the 14 women was distributed similarly between nortriptyline (n = 9/51, 18%) and sertraline (n = 5/44, 11%; Fisher exact test, P = 0.29)" |
Wisner 2015.
| Study characteristics | ||
| Methods |
Study design: RCT, 3‐armed Location: USA Setting: University of Pittsburgh within an academic psychiatry specialty programme in women's health No. of centres: 1 Dates of study: not reported Total duration of study: 8 weeks Recruitment: not reported Randomisation method: stratified by breastfeeding status, infant age (adjusted for gestational age at birth), randomisation tabled created in SAS using a random 3‐ and 6‐block design Analysis by ITT: yes, using LOCF for response and remission and repeated measures mixed linear model for depression severity. Unclear if 1 participant in placebo arm was excluded as they were ineligible and did not receive intervention. Power calculation: study underpowered (stopped early) |
|
| Participants |
Inclusion criteria: 2‐13 weeks postpartum, score of 18 on Structured Interview Guide for the SIGH‐ADS, Meeting diagnostic criteria for major depressive disorder according to the Structured Clinical Interview for DSM4. Women between 18 and 40 years of age, planning to use or using a non‐estrogen‐containing birth control regimen, without major medical problems, and had normal lipid profiles relative to the postpartum period, nor using other therapies for depression, including antidepressants, psychotherapy, light therapy, or herbal remedies, negative urine drug screen. Breastfeeding women were eligible for inclusion. Exclusion criteria: women with bipolar disorder, a previous psychotic episode, or substance abuse within the last 6 months, heavy smoking, a thromboembolic event, hypercoagulability, current or past history of breast, uterine, or ovarian cancer, or first degree relatives with thromboembolic events Number recruited: 85 Number dropped out: sertraline: N = 8; placebo: N = 7; estradiol: N = 7 Number analysed: 84 or 85 (reporting unclear regarding one woman from the placebo group who was ineligible and should not have been randomised) Age, mean (SD) years: sertraline: 26.2 (5.9); placebo: 27.3 (5.4); estradiol: 26.2 (6.0) Severity of PND: not reported Duration of PND: not reported Physical health co‐morbidities: not reported Mental health co‐morbidities: not reported Ethnicity: % BAME: sertraline: 36.7; placebo: 27.6; estradiol: 46.2 Socioeconomic status: sertraline: < high school: 2/30, high school: 9/30, technical: 13/30, college: 2/30, postgraduate: 3/30; transdermal estradiol < high school: 4/26, high school: 8/26, technical: 8/26, college: 3/26, postgraduate: 3/26; placebo: < high school: 4/29, high school: 6/29, technical: 9/29, college: 8/29, postgraduate: 2/29 |
|
| Interventions | Women were randomly assigned to 1 of 3 groups
|
|
| Outcomes |
Primary outcomes: response (reduction of baseline SIGH‐ADS score by ≥ 50%) or remission (Exit SIGH‐ADS score ≤ 8) Adverse events: Asberg Side Effects Scale |
|
| Notes | Funding: NIH Grant R01 MH057102 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation, stratified by breastfeeding status and infant age. Randomisation tables created using software |
| Allocation concealment (selection bias) | Low risk | Patient assigned using software. Randomisation assignment made within the system and stored in a password‐protected part of the database |
| Blinding of participants (performance bias) | Low risk | All participants received opaque capsules and transdermal patches, with placebo identical to medication. Procedure for maintaining blinding during disbursement of medications reported in detail. Medication monitoring by the study psychiatrist was separate from (and blind to) mood symptom monitoring. |
| Blinding of personnel (performance bias) | Low risk | Primary study staff blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All evaluators remained blind to the participant's drug assignment until the study was completed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "last observation carried forward for the primary outcomes of response and remission," Slightly higher % of participants lost to follow‐up for antidepressant arm (17%) than placebo (10%) or estradiol (8%). Reasons not reported. ITT analysis but using LOCF, so possible bias in favour of antidepressants. Unclear if 1 randomised participant in placebo arm excluded from analysis or not due to being ineligible for study. |
| Selective reporting (reporting bias) | Unclear risk | No mention of a registered protocol. Trial stopped early |
| Other bias | Unclear risk | Several authors received financial support and/or compensation from pharmaceutical companies. It is unclear which pharmaceutical company supplied the sertraline preparation used in the study. No data on adherence to medication among completers reported. |
Yonkers 2008.
| Study characteristics | ||
| Methods |
Study design: RCT, parallel‐group Location: USA Setting: community/secondary care No. of centres: 4: Yale University School of Medicine, Bridgeport Hospital, University of Texas Southwestern Medical Center, and Massachusetts General Hospital Dates of study: 1997‐2004 Total duration of study: 7 years Recruitment: women were recruited by advertisement or referral from obstetric care providers Randomisation method: predetermined with a computer‐generated schedule in blocked sets of 4 and was stratified by site Analysis by ITT: yes (LOCF for response and remission analyses) Power calculation: yes |
|
| Participants |
Inclusion criteria: aged ≥ 16 years, met diagnostic criteria for MDD with an onset in the 3 months post‐delivery, had given birth within the previous 9 months and had a score on the 17‐item HAM‐D of at least 16 at the initial visit. Women who were breastfeeding were allowed to participate Exclusion criteria: onset of MDD prior to delivery, current suicidal ideation with intent, current (within the last 6 months) alcohol or drug abuse or dependence, current psychotic symptoms, lifetime diagnosis of schizophrenia, bipolar disorder or schizoaffective disorder, currently receiving treatment (pharmacotherapy or psychotherapy) for a psychiatric disorder, currently pregnant, unwilling to be randomised or unable to attend treatment visits at a participating site Number recruited: 70 women (35 active treatment, 35 placebo) Number dropped out by final week (week 8 ± 7 days): paroxetine: 20/35 (57%); placebo: 23/35 (66%) Number analysed: paroxetine N = 35; placebo N = 35. ITT analysis and evaluation at week 8 for results from 17 women in paroxetine group and 14 women in the placebo group Age, mean (SD) years: paroxetine: 26.1 (6.5); placebo: 25.9 (6.5) Severity of PND, mean (SD) score: HRSD‐17: paroxetine 23.6 (4.7); placebo 24.7 (5.0) IDS‐SR: paroxetine 38.6 (8.4); placebo 42.8 (8.4) CGI‐S: paroxetine 4.2 (1.0); placebo 4.5 (0.9) Duration of PND: not reported Physical health co‐morbidities: not reported Mental health co‐morbidities: paroxetine N = 15 (46.9%); placebo N = 15 (53.1%). Comorbid psychiatric conditions = lifetime alcohol abuse, alcohol dependence, drug abuse, drug dependence, anxiety disorder Ethnicity: paroxetine: white: 18 (51.4%), black: 5 (14.3%), Hispanic: 11 (31.4%), other 1 (2.9%); placebo: white: 16 (45.7%), black: 4 (11.4%), Hispanic: 14 (40.0%), other 1 (2.9%) Socioeconomic status: paroxetine: < 12 years of education: 11 (37.9%), > 12 years of education: 18 (62.1%); placebo: < 12 years of education: 15 (53.6%), > 12 years of education: 13 (46.4%) |
|
| Interventions | Women were randomly assigned to 1 of 2 groups
|
|
| Outcomes |
Primary outcome: change in depressive symptoms measured by the HAM‐D, CGI and the Inventory of Depressive Symptomatology ‐ Self‐report scale Secondary outcomes: rates of remission, defined as a HAM‐D score of ≤ 8, and response, defined as a CGI‐Improvement scale score of 1 or 2; predictors of remission defined as above; Social Adjustment as measured by the SAS; SF‐36 Time points: weeks 1, 2, 3, 4, 6 and for a final visit, at week 8 (± 7 days) |
|
| Notes | This study was supported by a Collaborative Research Trial, Investigator‐Initiated grant from GlaxoSmithKline to Drs Yonkers and Cohen and by National Institute of Mental Health grant MH01648 to Dr Yonkers. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomly assigned to take identical capsules of either paroxetine or placebo. Random assignment was predetermined with a computer‐generated schedule in blocked sets of 4 and was stratified by site. A study statistician was responsible for random assignment" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details provided to be sure of allocation concealment |
| Blinding of participants (performance bias) | Low risk | "Subjects were instructed to take 1 capsule (10mg of immediate‐release paroxetine or identical placebo)" |
| Blinding of personnel (performance bias) | Low risk | "A study statistician was responsible for random assignment, and remaining study staff were blind to group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "..remaining study staff were blind to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Seventy women qualified for the study, and 31 completed study treatment… Subjects withdrew from the active treatment for the following reasons: 1 due to an adverse event (nausea), 6 due to lack of efficacy, including 1 subject who was psychiatrically hospitalised, 6 who were lost to follow‐up, 5 who felt well and no longer desired treatment, 1 who became pregnant and 1 who was noncompliant In subjects randomly assigned to placebo, 4 left the study because of perceived adverse events (rash, nausea, diarrhoea, headache), 7 discontinued because of lack of efficacy, including 1 subject who required hospitalisation, 9 were lost to follow‐up, 2 improved and no longer desired treatment, and 1 subject moved" "Given the high rate of dropout, we explored additional models to assess the robustness of remission results. These models first assumed that all dropouts were remitters and then that they were all non‐remitters. In both models, treatment with paroxetine remained significantly better than treatment with placebo" Dropout numbers are similar in the 2 groups and some reasons account for similar numbers across the 2 groups but for a substantial proportion 'lost to follow up' the reason for dropout is unknown. Sensitivity analyses only performed for the primary outcome |
| Selective reporting (reporting bias) | High risk | The SAS and SF‐36 were included in the methods but not reported in the results |
| Other bias | Unclear risk | "Pill counts revealed that, among women assigned to paroxetine, 7 were noncompliant (took less than 80% of prescribed pills at 1 visit, and 4 were non‐compliant at 2 visits. One subject assigned to active treatment was discontinued due to on‐going lack of compliance; of the remaining subject, no others fell below the 80% compliance rate at more than 2 visits. Among subjects assigned to placebo, 10 were noncompliant at 1 visit, 3 were noncompliant during at least 2 visits, and 1 was noncompliant on 4 occasions" The potential bias was unclear as we do not know whether non‐compliant women were taking 0% or 79% of their medication. It is also not clear whether the numbers of non‐compliant participants were reported for the study as a whole (26/70 women) or only for those who did not drop out (26/31 women) Funded by GlaxoSmithKline but it is unclear if they provided the study medication. |
BAME: Black, Asian and minority ethnic; BAI: Beck Anxiety Inventory; BDI: Beck Depression Inventory; BDP: brief dynamic psychotherapy; CBT: cognitive behavioural therapy; CGI‐I: Clinical Global Impressions‐Improvement; CGI‐S: Clinical Global Impressions‐Severity; CIS‐R: Revised Clinical Interview Schedule; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; EPDS: Edinburgh Postnatal Depression Scale; EQ‐5D: EuroQol‐5 Dimenion; GAS: Global Assessment Scale; GHQ: General Health Questionnaire; GP: general practitioner; GRIMS: Golombok Rust Inventory of Marital State; HAM‐A: Hamilton Rating Scale for Anxiety; HAM‐D: Hamilton Rating Scale for Depression; HDRS/HRSD: Hamilton Rating Scale for Depression; ICD‐10: International Classification of Disease Tenth Revision; IDAS‐GD: Inventory of Depression and Anxiety Symptoms, General Depression scale; IPT: interpersonal psychotherapy; ITT: intention to treat; LOCF: last observation carried forward; MADRS: Montgomery‐Åsberg Depression Rating Scale; MAMA: Maternal Adjustment and Maternal Attitudes; MDD: major depressive disorder; MHI: Mental Health Index; PAPA: Preschool Age Psychiatric Assessment; PND: postnatal depression; PPAQ: Postpartum Adjustment Questionnaire; PSI: Parenting Stress Index; RCT: randomised controlled trial; SAS: Social Adjustment Scale; SCID: Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; SD: standard deviation; SF‐12: 12‐item Short Form; SIGH‐ADS: Structured Interview Guide for the Hamilton Depression Rating Scale—Atypical Depression Symptoms Version; SPQ: Social Problems Questionnaire; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; TSH: thyroid‐stimulating hormone; YBOCS: Yale‐Brown Obsessive Compulsive Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bais 2016 | Drug treatment started in pregnancy not postpartum |
| Khazaie 2013 | Drug treatment started in pregnancy not postpartum |
| Lambregtse‐Van Den Berg | Drug treatment started in pregnancy not postpartum |
| Misri 2004 | Not comparing antidepressants with another intervention ‐ both arms had same antidepressant (paroxetine vs paroxetine + CBT) |
| Molenaar 2016 | Drug treatment started in pregnancy not postpartum |
| NCT02185547 | Drug treatment started in pregnancy not postpartum |
| NCT02188459 | Drug treatment started in pregnancy not postpartum |
| Tahmasian 2013 | Drug treatment started in pregnancy not postpartum |
| Yu 2015 | Could not establish if participants met inclusion criteria |
| Zhao 2006 | Not comparing antidepressants with another intervention ‐ both arms had same antidepressant (fluoxetine vs fluoxetine + shugan powder) |
CBT: cognitive behavioural therapy
Characteristics of ongoing studies [ordered by study ID]
Andriotti 2017.
| Study name | ASSERT trial ‐ How to assess the safety and efficacy of a high frequency rTMS in postpartum depression? A multicenter, double blinded, randomized, placebo‐controlled clinical trial |
| Methods | 3‐arm RCT |
| Participants | Inclusion criteria: women aged 18 year or over; moderate to severe PPD (HAMD‐17 score >18) |
| Interventions | Women were randomised to:
|
| Outcomes | Primary outcome: reduction in HAMD‐17 score |
| Starting date | Unclear |
| Contact information | Tomas Andriotti Email: tomas.andriotti@me.com |
| Notes | Results comparing arm 1 (rTMS + sertraline) with arm 3 (rTMS + placebo) will potentially be eligible for inclusion in an update of this review. Contact with the authors in January 2020 revealed that the study had not yet started. |
IRCT20130418013058N11.
| Study name | The effect of crocin and sertraline in mild to moderate postpartum depression in two treatment groups (sertraline and crocin); a randomized placebo controlled clinical trial |
| Methods | Double blind, 2‐arm RCT |
| Participants |
Inclusion criteria: patients with PPD according to DSM‐V Scale; 14 score of BDI; age range 18‐45 years old; have a healthy, live and single baby; not receiving any psychiatric medication 5 weeks before the start of the study; written consent for participation Exclusion criteria: other psychiatric disorders such as behavioral disorder, bipolar disorder and schizophrenia, drug abuse; history of chronic and systematic diseases; restrictive living conditions that cause the patient to not complete the course of treatment and need another drug; sensitivity to saffron; having other symptoms of psychosis or suicidal tendencies or any other mental disorder; medical problems such as pre‐eclampsia and diabetes during pregnancy; symptoms of psychosis and thoughts of suicide or child abuse |
| Interventions | Women were randomised to:
|
| Outcomes |
Primary outcomes: depression (BDI) at 1, 2 and 3 months after randomisation Secondary outcomes: side effects of crocin |
| Starting date | 3 Sepetmber 2018 |
| Contact information | Seyed Ahmad Mohajeri Pharmaceutical Research Center, School Of Pharmacy, Mashhad University of Medical Sciences, Iran (Islamic Republic of) Email: mohajeria@mums.ac.ir |
| Notes |
BDI: Beck Depression Inventory; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; HAMD‐17: Hamilton Depression Rating Scale, 17‐item version; Hz: Hertz; PPD: postpartum depression; RCT: randomised controlled trial; rTMS: repetitive Transcranial Magnetic Stimulation
Differences between protocol and review
We added the comparison group 'complementary medicine', that included a single study comparing antidepressants to saffron. This is not a difference from the protocol, since this study is eligible for inclusion under the 'any other treatment' category. However, it did not fit into the three (non‐exhaustive) example subgroups that were given in the protocol: psychological, psychosocial or other pharmacological interventions. These subgroups were chosen on the basis of past literature. As we only identified one eligible study outside of these prespecified subgroups, we added the new subgroup of 'complementary medicine' for this study.
We added a sensitivity analysis for studies with imputed data, This was not planned a priori but we felt it was appropriate to add it as we used data from a study where we had to convert geometric means and 95% confidence intervals into standard means and standard deviations to use in our meta‐analysis. This comes with limitations and it was important to investigate how using data from this study affected the results.
Contributions of authors
Kylee Trevillion (KT), Louise M Howard (LH), and Emma Molyneaux (EM) developed the protocol methodology and background as part of previous versions of this Cochrane Review (Hoffbrand 2001; Molyneaux 2014). Jennifer Valeska Elli Brown (JB), Lindsay Robertson (LR) and Emily South (ES) performed the data extraction and 'Risk of bias' assessments. Hind Khalifeh (HK) Louise Howard (LH) and Claire Wilson (CW) were available to discuss disagreements in data extraction and 'Risk of bias' assessments. CCMD Editor Nick Meader (NM) provided advice for statistical analyses conducted by JB and LR. JB and LR contributed to GRADE assessments and constructed 'Summary of findings' tables. All review authors approved the final review prior to publication.
Sources of support
Internal sources
-
King's College London, UK
Authors' host institution
-
University of York, UK
Authors' host institution ES: time on this project is supported by the University of York.
External sources
-
National Institute for Health Research (NIHR), UK
JB: time on this review was funded by Cochrane Infrastructure funding to Cochrane Common Mental Disorders.
KA: is funded by a NIHR Doctoral Research Fellowship (NIHR‐DRF‐2016‐09‐042).
LR: time on this review was funded by Cochrane Infrastructure funding to Cochrane Common Mental Disorders.
KT: currently funded by the NIHR Policy Research Programme and the NIHR Health Services and Delivery Research Programme (Grant Reference Number 16/117/03).
LMH: work is supported by the NIHR Mental Health Biomedical Research Centre at the South London and Maudsley NHS Foundation Trust and King’s College London; and the NIHR South London ARC (Applied Research Collaboration).
HK: supported by the NIHR Mental Health Biomedical Research Centre at the South London and Maudsley NHS Foundation Trust and King's College London.
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
-
Tommy's ‐ registered charity, UK
EM: was supported by Tommy's baby charity.
-
Medical Research Council (MRC), UK
CW: is funded by a Clinical Research Training Fellowship (MR/P019293/1).
Declarations of interest
JB: no conflicts of interest CW: no conflicts of interest KA: no conflicts of interest LR: no conflicts of interest ES: no conflicts of interest EM: no conflicts of interest KT: no conflicts of interest LH: has worked for the National Institute for Health and Care Excellence (NICE) Scientific Advice on pharmacological treatment for postnatal depression. HK: no conflicts of interest
New
References
References to studies included in this review
Appleby 1997 {published data only}
- Appleby L, Warner R, Whitton A, Faragher B. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ 1997;314(7085):932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby L. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. In: 9th Congress of the Association of European Psychiatrists. Copenhagen, Denmark, 1998. [DOI] [PMC free article] [PubMed]
- Warner R, Appleby L, Whitton A, Faragher B. A controlled study of fluoxetine and cognitive behavioural counselling in the treatment of post-natal depression. In: Royal College of Psychiatrists Winter Meeting. Cardiff, UK, 1997. [DOI] [PMC free article] [PubMed]
Bloch 2012 {published data only}
- Bloch M, Meiboom H, Lorberblat M, Ahoronov I, Bluvshtein I, Ben-Itzhak S, et al. Treatment of postpartum depression with psychotherapy and add-on sertraline: a double-blind, randomised, placebo-controlled study. European Neuropsychopharmacology 2011;21 Suppl 3:S259-60. [Google Scholar]
- Bloch M, Meiboom H, Lorberblatt M, Bluvstein I, Aharonov I, Schreiber S. The effect of sertraline add-on to brief dynamic psychotherapy for the treatment of postpartum depression: a randomized, double-blind, placebo-controlled study. Journal of Clinical Psychiatry 2012;73(2):235-41. [DOI] [PubMed] [Google Scholar]
Chibanda 2014 {published data only}
- Chibanda D, Shetty AK, Tshimanga M, Woelk G, Stranix-Chibanda L, Rusakaniko S. Group problem-solving therapy for postnatal depression among HIV-positive and HIV-negative mothers in Zimbabwe. Journal of the International Association of Providers of AIDS Care 2014;13(4):335-41. [DOI: 10.1177/2325957413495564] [DOI] [PubMed] [Google Scholar]
Hantsoo 2013 {published data only}
- Epperson CN, McDougle CJ, Ward-O'Brien D, Price LH. A controlled study of antidepressant treatment of postpartum depression. In: 149th Annual Meeting of the American Psychiatric Association. New York, USA, 1996.
- Hantsoo L, Ward-O'Brien D, Czarkowski KA, Gueorguieva R, Price LH, Epperson CN. A randomized, placebo-controlled, double-blind trial of sertraline for postpartum depression. Psychopharmacology 2014;231(5):939-48. [DOI: 10.1007/s00213-013-3316-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kashani 2017 {published data only}
- IRCT201509201556N82. Comparison of saffron and fluoxetine in the treatment of mild to moderate post partum depression: a double–blind clinical trial. en.irct.ir/trial/931?revision=931 (first received 21 September 2015).
- Kashani L, Eslatmanesh S, Saedi N, Niroomand N, Ebrahimi M, Hosseinian M, et al. Comparison of saffron versus fluoxetine in treatment of mild to moderate postpartum depression: a double-blind, randomized clinical trial. Pharmacopsychiatry 2017;50(2):64-8. [DOI: 10.1055/s-0042-115306] [DOI] [PubMed] [Google Scholar]
Milgrom 2015 {published data only}
- Milgrom J, Gemmill AW, Ericksen J, Burrows G, Buist A, Reece J. Treatment of postnatal depression with cognitive behavioural therapy, sertraline and combination therapy: a randomised controlled trial. Australian and New Zealand Journal of Psychiatry 2015;49(3):236-45. [DOI: 10.1177/0004867414565474] [DOI] [PubMed] [Google Scholar]
- NCT02122393. A randomised trial of sertraline, cognitive behaviour therapy & combined therapy for postnatal depression. clinicaltrials.gov/ct2/show/NCT02122393 (first received 24 April 2014).
O'Hara 2019 {published data only}
- NCT00602355. Effectiveness of sertraline alone and interpersonal psychotherapy alone in treating women with postpartum depression. clinicaltrials.gov/ct2/show/NCT00602355 (first received 28 January 2008).
- O'Hara MW, Pearlstein T, Stuart S, Long JD, Mills JA, Zlotnick C. A placebo controlled treatment trial of sertraline and interpersonal psychotherapy for postpartum depression. Journal of Affective disorders 2019;245:524-32. [DOI: 10.1016/j.jad.2018.10.361] [DOI] [PubMed] [Google Scholar]
Sharp 2010 {published data only}
- Chew-Graham C, Chamberlain E, Turner K, Folkes L, Caulfield L, Sharp D. GPs' and health visitors' views on the diagnosis and management of postnatal depression: a qualitative study. British Journal of General Practice 2008;58(548):169-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew-Graham CA, Sharp D, Chamberlain E, Folkes L, Turner KM. Disclosure of symptoms of postnatal depression, the perspectives of health professionals and women: a qualitative study. BMC Family Practice 2009;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard L, Chew-Graham CA, Tylee A, Lewis G, Anderson I, Abel K, et al. The RESPOND trial: a randomized evaluation of antidepressants and support for women with postnatal depression. Archives of Women's Mental Health 2011;14 Suppl 1:Meeting Abstracts. The Marcé International Society International Biennial General Scientific Meeting. [DOI: 10.1007/s00737-010-0203-1] [DOI] [Google Scholar]
- Howard LM, Flach C, Mehay A, Sharp D, Tylee A. The prevalence of suicidal ideation identified by the Edinburgh Postnatal Depression Scale in postpartum women in primary care: findings from the RESPOND trial. BMC Pregnancy and Childbirth 2011;11:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Chew-Graham CA, Tylee A, Lewis G, Howard L, Anderson I, et al. A pragmatic randomised controlled trial to compare antidepressants with a community-based psychosocial intervention for the treatment of women with postnatal depression: the RESPOND trial. Health Technology Assessment 2010;14(43):1-181. [DOI] [PubMed] [Google Scholar]
- Turner KM, Chew-Graham C, Folkes L, Sharp D. Women's experiences of health visitor delivered listening visits as a treatment for postnatal depression: a qualitative study. Patient Education and Counselling 2010;78(2):234-9. [DOI] [PubMed] [Google Scholar]
- Turner KM, Sharp D, Folkes L, Chew-Graham C. Women's views and experiences of antidepressants as a treatment for postnatal depression: a qualitative study. Family Practice 2008;25(6):450-5. [DOI] [PubMed] [Google Scholar]
- Wan MW, Sharp DJ, Howard LM, Abel KM. Attitudes and adjustment to the parental role in mothers following treatment for postnatal depression. Journal of Affective Disorders 2011;131(1-3):284-90. [DOI] [PubMed] [Google Scholar]
Wisner 2006 {published data only}
- Lanza di Scalea T, Hanusa BH, Wisner KL. Sexual functioning and symptom remission in women with postpartum depression after antidepressant treatment. Journal of Clinical Psychiatry 2009;70(3):423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon MC, Wisner K, Hanusa BH, Philips A. Role functioning and symptom remission in women with postpartum depression after antidepressant treatment. Archives of Psychiatric Nursing 2003;17(6):276-83. [DOI] [PubMed] [Google Scholar]
- Logsdon MC, Wisner K, Hanusa BH. Does maternal role functioning improve with antidepressant treatment in women with postpartum depression. Journal of Women's Health 2009;18(1):85-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KL, Hanusa BH, Perel JM, Peindl KS, Piontek CM, Sit DKetal. Postpartum depression: a randomized trial of sertraline versus nortriptyline. Journal of Clinical Psychopharmacology 2006;26(4):353-60. [DOI] [PubMed] [Google Scholar]
Wisner 2015 {published data only}
- NCT00744328. Postpartum depression: transdermal estradiol versus sertraline (E2SERT). clinicaltrials.gov/ct2/show/NCT00744328 (first received 29 August 2008).
- Pinheiro E, Bogen DL, Hoxha D, Wisner KL. Transdermal estradiol treatment during breastfeeding: maternal and infant serum concentrations. Archives of Women's Mental Health 2016;19(2):409-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner K. Estradiol for postpartum depression: translational challenges. Neuropsychopharmacology 2014;39:S32. [Google Scholar]
- Wisner KL, Sit DK, Moses-Kolko EL, Driscoll KE, Prairie BA, Stika CS, et al. Transdermal estradiol treatment for postpartum depression: a pilot, randomized trial. Journal of Clinical Psychopharmacology 2015;35(4):389-95. [DOI: 10.1097/JCP.0000000000000351] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yonkers 2008 {published data only}
- Heath AC, Yonkers KA, Rush AJ. Paroxetine in the treatment of postpartum depression. In: 153rd Annual Meeting of the American Psychiatric Association. Chicago, USA, 2000.
- Heath AC, Yonkers KA, Rush AJ. Paroxetine in the treatment of postpartum depression. In: 155th Annual Meeting of the American Psychiatric Association. Philadelphia, USA, 2002.
- Yonkers KA, Lin H, Howell HB, Heath AC, Cohen LS. Pharmacologic treatment of postpartum women with new-onset major depressive disorder: a randomized controlled trial with paroxetine. Journal of Clinical Psychiatry 2008;69(4):659-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bais 2016 {published data only}
- Bais B, Kamperman AM, Van der Zwaag MD, Dieleman GC, Harmsen van der Vliet-Torij HW, Bijma HH, et al. Bright light therapy in pregnant women with major depressive disorder: study protocol for a randomized, double-blind, controlled clinical trial. BMC Psychiatry 2016;16(1):381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khazaie 2013 {published data only}
- Khazaie H, Ghadami MR, Knight DC, Emamian F, Tahmasian M. Insomnia treatment in the third trimester of pregnancy reduces postpartum depression symptoms: a randomized clinical trial. Psychiatry Research 2013;210(3):901-5. [DOI] [PubMed] [Google Scholar]
Lambregtse‐Van Den Berg {published data only}
- Lambregtse van-den Berg M, Burger H, Bockting CL, Bonsel GJ, Duvekot JJ, Torij HW. Stop or go? Tapering antidepressants in pregnancy: a pragmatic multicenter RCT to investigate risk and benefits for mother and child. Archives of Women's Mental Health 2015;18(2):403-34. [Google Scholar]
Misri 2004 {published data only}
- Misri S, Reebye P, Corral M, Milis L. The use of paroxetine and cognitive-behavioral therapy in postpartum depression and anxiety: a randomized controlled trial. Journal of Clinical Psychiatry 2004;65(9):1236-41. [DOI] [PubMed] [Google Scholar]
- Misri S, Reebye P, Milis L, Shah S. The impact of treatment intervention on parenting stress in postpartum depressed mothers: a prospective study. American Journal of Orthopsychiatry 2006;76(1):115-9. [DOI] [PubMed] [Google Scholar]
Molenaar 2016 {published data only}
- Molenaar NM, Brouwer ME, Bockting CL, Bonsel GJ, Van der Veere CN, Torij HW, et al. Stop or go? Preventive cognitive therapy with guided tapering of antidepressants during pregnancy: study protocol of a pragmatic multicentre non-inferiority randomized controlled trial. BMC Psychiatry 2016;16(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02185547 {published data only}
- NCT02185547. Effects and consequences for mother and child from treatment for depression. clinicaltrials.gov/show/NCT02185547 (first received 9 July 2014).
NCT02188459 {published data only}
- NCT02188459. Placebo-controlled trial of bupropion for smoking cessation in pregnant women. clinicaltrials.gov/show/NCT02188459 (first received 11 July 2014).
Tahmasian 2013 {published data only}
- Tahmasian M, Khazaie H, Ghadami M, Knight D, Emamian F. Insomnia treatment in the third trimester of pregnancy prevents postpartum depression: a randomized clinical trial. Sleep Medicine 2013;1:e39. [DOI] [PubMed] [Google Scholar]
- Yu H, Liu C, Wang X. The therapeutic effects of paroxetine addition of psychological intervention on post-partum depression. Progress in Obstetrics and Gynecology 2006;15(4):289-91. [Google Scholar]
Yu 2015 {published data only}
- Yu SJ, Li XQ, Feng XM, Cao WF. Therapeutic efficacy of accupuncture at the thirteen ghost points for postpartum depression and its effects on the quality of life. Shanghai Journal of Accupuncture and Moxibustion 2015;34(1):14-6. [Google Scholar]
Zhao 2006 {published data only}
- Zhao X, Lin H. Study on postpartum depression treated with combination of fluoxetine and Chaihu Shugan powder. Liaoning Journal of Traditional Chinese Medicine 2006;33(5):586-7. [Google Scholar]
References to ongoing studies
Andriotti 2017 {published data only (unpublished sought but not used)}
- Andriotti T, Stavale R, Nafee T, Fakhry S, Mohamed MM, et al. ASSERT trial - How to assess the safety and efficacy of a high frequency rTMS in postpartum depression? A multicenter, double blinded, randomized, placebo-controlled clinical trial. Contemporary Clinical Trial Communications 2017;5:86-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT20130418013058N11 {published data only}
- IRCT20130418013058N11. The effect of crocin and sertraline in mild to moderate postpartum depression in two treatment groups (sertraline and crorin); a randomized placebo controlled clinical trial. en.irct.ir/trial/32820 (first received 16 December 2018).
Additional references
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM–5). 5th edition. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
Berle 2011
- Berle JØ, Spigset O. Antidepressant use during breastfeeding. Current Women's Health Reviews 2011;7(1):28-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bland 2000
- Bland JM, Altman DG. Statistics notes: the odds ratio. BMJ 2000;320(7247):1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2019
- Brown JV, Walton N, Meader N, Todd A, Webster LA, Steele R, et al. Pharmacy-based management for depression in adults. Cochrane Database of Systematic Reviews 2019, Issue 12. Art. No: CD013299. [DOI: 10.1002/14651858.CD013299.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipriani 2018
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 2018;391(10128):1357-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cleare 2015
- Cleare A, Pariante CM, Young AH, Anderson IM, Christmas D, Cowen PJ, et al, Members of the Consensus Meeting. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. Journal of Psychopharmacology 2015;29(5):459-525. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 10 December 2019. Available at covidence.org.
Cox 1987
- Cox J, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry 1987;150:782-6. [DOI] [PubMed] [Google Scholar]
Crittenden 1988
- Crittenden PM. Relationships at risk. In: Belsky J, Nezworski T, editors(s). Clinical Implications of Attachment. Hillsdale, NJ: Lawrence Erlbaum Associates, 1988:136-74. [Google Scholar]
Cuijpers 2013
- Cuijpers P, Berking M, Andersson G, Quigley L, Kleiboer A, Dobson KS. A meta-analysis of cognitive-behavioural therapy for adult depression, alone and in comparison with other treatments. Canadian Journal of Psychiatry 2013;58(7):376-85. [DOI] [PubMed] [Google Scholar]
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Dennis 2007
- Dennis CL, Hodnett ED. Psychosocial and psychological interventions for treating postpartum depression. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD006116. [DOI: 10.1002/14651858.CD006116.pub2] [DOI] [PubMed] [Google Scholar]
Gavin 2005
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstetrics & Gynaecology 2005;106(5):1071-83. [DOI] [PubMed] [Google Scholar]
Goodman 2004
- Goodman JH. Postpartum depression beyond the early postpartum period. Journal of Obstetrics, Gynecology & Neonatal Nursing 2004;33:410-20. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), (accessed prior to 3 March 2020). Available at gradepro.org.
Grigoriadis 2017
- Grigoriadis S, Wilton AS, Kurdyak PA, Rhodes AE, VonderPorten EH, Levitt A, et al. Perinatal suicide in Ontario, Canada: a 15-year population-based study. Canadian Medical Association Journal 2017;189(34):E1085-92. [DOI: 10.1503/cmaj.170088] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurung 2018
- Gurung B, Jackson LJ, Monahan M, Butterworth R, Roberts TE. Identifying and assessing the benefits of interventions for postnatal depression: a systematic review of economic evaluations. BMC Pregnancy and Childbirth 2018;18(1):179. [DOI: 10.1186/s12884-018-1738-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamilton 1967
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology 1967;6(4):278-96. [DOI: 10.1111/j.2044-8260.1967.tb00530.x] [DOI] [PubMed] [Google Scholar]
Harmer 2017
- Harmer CJ, Duman RS, Cowen PJ. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry 2017;4(5):409-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JP, White IR, Anzures-Cabrera J. Meta-analysis of skewed data: combining results reported on log-transformed or raw scales. Statistics in Medicine 2008;27(29):6072-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2019
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. Available from www.training.cochrane.org/handbook.
Howard 2014a
- Howard LM, Molyneaux E, Dennis C-L, Rochat T, Stein A, Milgrom J. Non-psychotic mental disorders in the perinatal period. Lancet 2014;384(9956):1775-88. [DOI: 10.1016/S0140-6736(14)61276-9] [DOI] [PubMed] [Google Scholar]
Howard 2014b
- Howard LM, Megnin-Viggars O, Symington I, Pilling S, Guideline Development Group. Antenatal and postnatal mental health: summary of updated NICE guidance. BMJ 2014;349:g7394. [DOI: 10.1136/bmj.g7394] [DOI] [PubMed] [Google Scholar]
Jones 2014
- Jones I, Chandra PS, Dazzan P, Howard LM. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet 2014;384(9956):1789-99. [DOI] [PubMed] [Google Scholar]
Kantor 2004
- Kantor GK, Holt MK, Mebert CJ, Straus MA, Drach KM, Ricci LR, et al. Development and preliminary psychometric properties of the Multidimensional Neglectful Behavior Scale-Child Report. Child Maltreatment 2004;9(5):409-28. [DOI] [PubMed] [Google Scholar]
Khalifeh 2016
- Khalifeh H, Hunt IM, Appleby L, Howard LM. Suicide in perinatal and non-perinatal women in contact with psychiatric services: 15 year findings from a UK national inquiry. Lancet Psychiatry 2016;3(3):233-42. [DOI] [PubMed] [Google Scholar]
Knight 2019
- Knight M, Bunch K, Tuffnell D, Shakespeare J, Kotnis R, Kenyon S, et al (Eds), on behalf of MBRRACE-UK. Saving lives, improving mothers’ care - lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2015-17. In: Saving Lives, Improving Mothers’ Care. Oxford: University of Oxford National Perinatal Epidemiology Unit, 2019. [Google Scholar]
Ma 2015
- Ma Y. Neuropsychological mechanism underlying antidepressant effect: a systematic meta-analysis. Molecular Psychiatry 2015;20(3):311-9. [DOI] [PubMed] [Google Scholar]
McAllister‐Williams 2017
- McAllister-Williams RH, Baldwin DS, Cantwell R, Easter A, Gilvarry E, Glover V, et al. British Association for Psychopharmacology consensus guidance on the use of psychotropic medication preconception, in pregnancy and postpartum 2017. Journal of Psychopharmacology 2017;31(5):519-52. [DOI] [PubMed] [Google Scholar]
Molenaar 2018
- Molenaar NM, Kamperman AM, Boyce P, Bergink V. Guidelines on treatment of perinatal depression with antidepressants: an international review. Australian & New Zealand Journal of Psychiatry 2018;52(4):320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munk‐Olsen 2006
- Munk-Olsen T, Laursen TM, Pedersen CB, Mors O, Mortensen PB. New parents and mental disorders: a population-based register study. JAMA 2006;296(21):2582-9. [DOI] [PubMed] [Google Scholar]
Munk‐Olsen 2009
- Munk-Olsen T, Laursen TM, Mendelson T, Pedersen CB, Mors O, Mortensen PB. Risks and predictors of readmission for a mental disorder during the postpartum period. Archives of General Psychiatry 2009;66(2):189-95. [DOI] [PubMed] [Google Scholar]
Munk‐Olsen 2016
- Munk-Olsen T, Maegbaek ML, Johannsen BM, Liu X, Howard LM, di Florio A, et al. Perinatal psychiatric episodes: a population-based study on treatment incidence and prevalence. Translational Psychiatry 2016;6(10):e919. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2014
- National Institute for Health and Care Excellence. Antenatal and postnatal mental health: clinical management and service guidance. Clinical guideline [CG192]. Published date: December 2014. Last updated: February 2020. London: NICE, 2014. [Google Scholar]
O'Hara 2013
- O'Hara MW, McCabe JE. Postpartum depression: current status and future directions. Annual Review of Clinical Psychology 2013;9:379-407. [DOI] [PubMed] [Google Scholar]
O'Mahen 2008
- O'Mahen HA, Flynn HA. Preferences and perceived barriers to treatment for depression during the perinatal period. Journal of Women's Health 2008;17(8):1301-9. [DOI] [PubMed] [Google Scholar]
Orsolini 2015
- Orsolini L, Bellantuono C. Serotonin reuptake inhibitors and breastfeeding: a systematic review. Human Psychopharmacology 2015;30(1):4-20. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Schroeder 2006
- Schroeder K, Fahey T, Hay AD, Montgomery A, Peters TJ. Adherence to antihypertensive medication assessed by self-report was associated with electronic monitoring compliance. Jounral of Clinical Epidemiology 2006;59:650-1. [DOI] [PubMed] [Google Scholar]
Schünemann 2020
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, et al, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Silverman 2019
- Silverman ME, Reichenberg A, Lichtenstein P, Sandin S. Is depression more likely following childbirth? A population-based study. Archives of Women's Mental Health 2019;22(2):253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spitzer 1978
- Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: rationale and reliability. Archives of General Psychiatry 1978;35(6):773-82. [DOI] [PubMed] [Google Scholar]
Stein 2014
- Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, et al. Effects of perinatal mental disorders on the fetus and child. Lancet 2014;384(9956):1800-19. [DOI] [PubMed] [Google Scholar]
Stewart 2016
- Stewart DE, Vigod S. Postpartum depression. New England Journal of Medicine 2016;375(22):2177-86. [DOI] [PubMed] [Google Scholar]
Stewart 2019
- Stewart DE, Vigod SN. Postpartum depression: pathophysiology, treatment, and emerging therapeutics. Annual review of medicine. Annual Review of Medicine 2019;70:183-96. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PubMed] [Google Scholar]
WHO 2018
- World Health Organization. International classification of diseases for mortality and morbidity statistics (11th Revision). 18 June 2018. Available at www.who.int/classifications/icd/en/ (accessed prior to 3 March 2020).
Wilson (in press)
- Wilson CA, Robertson L, Brown JVE, Ayre K, Khalifeh H. Brexanolone for postnatal depression. Cochrane Database of Systematic Reviews (in press). [DOI] [PMC free article] [PubMed]
Wisner 2013
- Wisner KL, Sit DK, McShea MC, Rizzo DM, Zoretich RA, Hughes CL, et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry 2013;70(5):490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woody 2017
- Woody CA, Ferrari AJ, Siskind DJ, Whiteford HA, Harris MG. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. Journal of Affective Disorders 2017;219:86-92. [DOI] [PubMed] [Google Scholar]
Xia 2009
- Xia J, Adams C, Bhagat N, Bhagat V, Bhoopathi P, El-Sayeh H, et al. Losing participants before the trial ends erodes credibility of findings. Psychiatric Bulletin 2009;33(7):254-7. [Google Scholar]
Yonkers 2014
- Yonkers KA, Blackwell KA, Glover J, Forray A. Antidepressant use in pregnant and postpartum women. Annual Review of Clinicial Psychology 2014;10:369-92. [DOI: 10.1146/annurev-clinpsy-032813-153626] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Brown 2020
- Brown JVE, Wilson CA, Ayre K, South E, Molyneaux E, Trevillion K, et al. Antidepressant treatment for postnatal depression. Cochrane Database of Systematic Reviews 2020, Issue 3. Art. No: CD013560. [DOI: 10.1002/14651858.CD013560] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffbrand 2001
- Hoffbrand SE, Howard L, Crawley H. Antidepressant treatment for post-natal depression. Cochrane Database of Systematic Reviews 2001, Issue 1. Art. No: CD002018. [DOI: 10.1002/14651858.CD002018] [DOI] [Google Scholar]
Molyneaux 2014
- Molyneaux E, Howard LM, McGeown HR, Karia AM, Trevillion K. Antidepressant treatment for postnatal depression. Cochrane Database of Systematic Reviews 2014, Issue 9. Art. No: CD002018. [DOI: 10.1002/14651858.CD002018.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]


