Abstract
Background
Self‐harm (SH; intentional self‐poisoning or self‐injury regardless of degree of suicidal intent or other types of motivation) is a growing problem in most countries, often repeated, and associated with suicide. Evidence assessing the effectiveness of pharmacological agents and/or natural products in the treatment of SH is lacking, especially when compared with the evidence for psychosocial interventions. This review therefore updates a previous Cochrane Review (last published in 2015) on the role of pharmacological interventions for SH in adults.
Objectives
To assess the effects of pharmacological agents or natural products for SH compared to comparison types of treatment (e.g. placebo or alternative pharmacological treatment) for adults (aged 18 years or older) who engage in SH.
Search methods
We searched the Cochrane Common Mental Disorders Specialised Register, the Cochrane Library (Central Register of Controlled Trials [CENTRAL] and Cochrane Database of Systematic Reviews [CDSR]), together with MEDLINE. Ovid Embase and PsycINFO (to 4 July 2020).
Selection criteria
We included all randomised controlled trials (RCTs) comparing pharmacological agents or natural products with placebo/alternative pharmacological treatment in individuals with a recent (within six months of trial entry) episode of SH resulting in presentation to hospital or clinical services. The primary outcome was the occurrence of a repeated episode of SH over a maximum follow‐up period of two years. Secondary outcomes included treatment acceptability, treatment adherence, depression, hopelessness, general functioning, social functioning, suicidal ideation, and suicide.
Data collection and analysis
We independently selected trials, extracted data, and appraised trial quality. For binary outcomes, we calculated odds ratios (ORs) and their 95% confidence internals (CIs). For continuous outcomes we calculated the mean difference (MD) or standardised mean difference (SMD) and 95% CI. The overall certainty of evidence for the primary outcome (i.e. repetition of SH at post‐intervention) was appraised for each intervention using the GRADE approach.
Main results
We included data from seven trials with a total of 574 participants. Participants in these trials were predominately female (63.5%) with a mean age of 35.3 years (standard deviation (SD) 3.1 years). It is uncertain if newer generation antidepressants reduce repetition of SH compared to placebo (OR 0.59, 95% CI 0.29 to 1.19; N = 129; k = 2; very low‐certainty evidence). There may be a lower rate of SH repetition for antipsychotics (21%) as compared to placebo (75%) (OR 0.09, 95% CI 0.02 to 0.50; N = 30; k = 1; low‐certainty evidence). However, there was no evidence of a difference between antipsychotics compared to another comparator drug/dose for repetition of SH (OR 1.51, 95% CI 0.50 to 4.58; N = 53; k = 1; low‐certainty evidence). There was also no evidence of a difference for mood stabilisers compared to placebo for repetition of SH (OR 0.99, 95% CI 0.33 to 2.95; N = 167; k = 1; very low‐certainty evidence), or for natural products compared to placebo for repetition of SH (OR 1.33, 95% CI 0.38 to 4.62; N = 49; k = 1; lo‐ certainty) evidence.
Authors' conclusions
Given the low or very low quality of the available evidence, and the small number of trials identified, there is only uncertain evidence regarding pharmacological interventions in patients who engage in SH. More and larger trials of pharmacotherapy are required, preferably using newer agents. These might include evaluation of newer atypical antipsychotics. Further work should also include evaluation of adverse effects of pharmacological agents. Other research could include evaluation of combined pharmacotherapy and psychological treatment.
Plain language summary
Drugs and natural products for self‐harm in adults
We have reviewed the international literature regarding pharmacological (drug) and natural product (dietary supplementation) treatment trials in the field. A total of seven trials meeting our inclusion criteria were identified. There is little evidence of beneficial effects of either pharmacological or natural product treatments. However, few trials have been conducted and those that have are small, meaning that possible beneficial effects of some therapies cannot be ruled out.
Why is this review important?
Self‐harm (SH), which includes intentional self‐poisoning/overdose and self‐injury, is a major problem in many countries and is strongly linked with suicide. It is therefore important that effective treatments for SH patients are developed. Whilst there has been an increase in the use of psychosocial interventions for SH in adults (which is the focus of a separate review), drug treatments are frequently used in clinical practice. It is therefore important to assess the evidence for their effectiveness.
Who will be interested in this review?
Hospital administrators (e.g. service providers), health policy officers and third party payers (e.g. health insurers), clinicians working with patients who engage in SH, patients themselves, and their relatives.
What questions does this review aim to answer?
This review is an update of a previous Cochrane Review from 2015 which found little evidence of beneficial effects of drug treatments on repetition of SH. This updated aims to further evaluate the evidence for effectiveness of drugs and natural products for patients who engage in SH with a broader range of outcomes.
Which studies were included in the review?
To be included in the review, studies had to be randomised controlled trials of drug treatments for adults who had recently engaged in SH.
What does the evidence from the review tell us?
There is currently no clear evidence for the effectiveness of antidepressants, antipsychotics, mood stabilisers, or natural products in preventing repetition of SH.
What should happen next?
We recommend further trials of drugs for SH patients, possibly in combination with psychological treatment.
Summary of findings
Background
Description of the condition
Self‐harm (SH), which includes all intentional acts of self‐poisoning (such as intentional drug overdoses) or self‐injury (such as self‐cutting), regardless of degree of suicidal intent or other types of motivation (Hawton 2003), has been a growing problem in most countries. In Australia, for example, it is estimated that there are now more than 26,000 general hospitalisations for SH each year, or a rate of 116.7 per 100,000 persons (Harrison 2014), similar to rates observed in a number of other comparable countries (Canner 2018; Griffin 2014; Morthorst 2016; Ting 2012; Wilkinson 2002). However, it is notable that rates of emergency department presentations for SH are often higher than hospitalisations (Bergen 2010; Corcoran 2015). In the UK, for example, higher rates of emergency department presentations for SH in both females (442 per 100,000) and males (362 per 100,000) have been reported (Geulayov 2016). There are also many more episodes of SH occurring in the community that do not come to the attention of clinical services. Worldwide, for example, the World Health Organization (WHO) estimates that the rate of SH may be as high as 400 per 100,000, according to self‐report data (WHO 2014a).
In contrast to suicide rates, rates of hospital‐presenting SH are higher in females than in males in most countries (Canner 2018; Griffin 2014; Masiran 2017; Morthorst 2016; Ting 2012; Wilkinson 2002), with rates peaking in younger adults up to 24 years of age (Perry 2012). However, this difference decreases over the life cycle (Hawton 2008). SH is less common in older people, but tends to be associated with higher suicidal intent (Hawton 2008), with consequent greater risk of suicide (Murphy 2012).
For those who present to hospital, the most common method of SH is self‐poisoning. Overdoses of analgesics and psychotropics, especially paracetamol or acetaminophen, are common in some countries; particularly high‐income countries. Self‐cutting is the next most frequent method used by those who present to hospital. However, in the community, self‐cutting and other forms of self‐injury are far more frequent than self‐poisoning (Müller 2016).
SH is often repeated. Up to one‐quarter of those who present to hospital following SH return to the same hospital within a year (Carroll 2014; Owens 2002); although some individuals may present to another hospital. Others may not present to hospital at all given that studies identifying SH repetition via self‐report suggest that as many as one in five report further SH episodes following a hospital presentation (Carroll 2014). Repetition is more common in individuals who have a history of previous episodes of SH, personality disorder, psychiatric treatment, and alcohol or drug misuse (Larkin 2014). Risks of repeat SH may also be associated with method. Rates of repetition are higher among those who present to hospital following self‐injury alone (Carroll 2014; Lilley 2008), or combined self‐injury and self‐poisoning (Perry 2012), compared to those who present for self‐poisoning alone.
SH is associated with suicide. The risk of death by suicide within one year among people who present to hospital with SH varies across studies from nearly 1% to over 3% (Carroll 2014; Owens 2002). This variation reflects the characteristics of the population, and the background national suicide rate. In the UK, for example, during the first year after an episode of SH, the risk of suicide is around 50 times that of the general population, with a particularly high risk in men (Carroll 2014; Geulayov 2019). One quarter of these deaths are estimated to occur within one month after discharge, and almost 50% by three months (Forte 2019), although the risk of suicide appears to remain elevated for a number of years (Geulayov 2019). A history of SH is the strongest risk factor for suicide across a range of psychiatric disorders. Repetition of SH further increases the risk of suicide (Zahl 2004).
SH and suicide are the result of a complex interplay between genetic, biological, psychiatric, psychological, social, cultural, and other factors. Psychiatric disorders, particularly mood and anxiety disorders, are associated with the largest contribution to the risk of both SH (Hawton 2013), and suicide in adults (Ferrari 2014). Personality disorders, including borderline personality disorder, are also associated with SH, particularly frequent repetition. Alcohol use may also play an important role (Ferrari 2014). Both psychological and biological factors appear to further increase vulnerability to SH. Psychological factors may include difficulties in problem‐solving, low self‐esteem, impulsivity, vulnerability to having pessimistic thoughts about the future (i.e. hopelessness), and a sense of entrapment. Biological factors include disturbances in the serotonergic and stress response systems (van Heeringen 2014).
Description of the intervention
Given the high prevalence of depression in people who engage in SH, pharmacological interventions may include antidepressants, antipsychotics, and mood stabilisers (including anticonvulsants and lithium). SH also arises in the context of anxiety and general distress and thus anxiolytics (including both benzodiazepines and non‐benzodiazepine anxiolytics) may be trialled. Other pharmacological agents may also be trialled.
How the intervention might work
Antidepressants
In relation to the prevention of SH and suicidal behaviour, the primary mechanism would be the effect of antidepressants on depression. However, there might also be other relevant specific effects, such as with drugs acting on the serotonin system, it having been suggested that serotonin levels are relevant to impulsivity, which is a feature sometimes associated with suicidal behaviour (van Heeringen 2014).
While different classifications of antidepressants have been suggested, a currently accepted classification is non‐selective monoamine inhibitors (e.g., amitriptyline, imipramine, dosulepin), selective serotonin reuptake inhibitors (e.g., fluoxetine, sertraline, citalopram), monoamine oxidase inhibitors, sub‐grouped as non‐selective monoamine oxidase inhibitors (e.g., phenelzine) and monoamine oxidase A inhibitors (e.g., moclobemide), and other antidepressants (e.g,. venlafaxine, mirtazapine, trazadone) (WHO 2014b).
An earlier approach was to group antidepressants as tricyclics, newer generation antidepressants (NGAs) (while recognising that many specific drugs in this category were introduced many years ago), and other antidepressants. This approach was used in the previous version of this review (Hawton 2015). For pragmatic reasons, we have therefore continued to use this categorisation in this update.
Antidepressants are often prescribed in the same dose range used to treat major depression. However, owing to the increased risk of overdose in this population, including the likelihood that people who engage in self‐poisoning may use their own medication (Gjelsvik 2014), antidepressants associated with lower case fatality indices are generally preferred (Hawton 2010), especially in people thought to be at risk of suicide.
Antipsychotics
In people with a history of repeat SH, treatment with antipsychotics may be used to reduce heightened levels of arousal often experienced by them, especially in relation to stressful life events. By reducing this arousal, the urge to engage in SH may be reduced, although there is little evidence for their efficacy in reducing suicidal behaviour in adults (Stoffers 2010). Lower doses may be prescribed to obtain this effect than is generally used in the treatment of psychotic disorders.
Anxiolytics, including both benzodiazepines and non‐benzodiazepine anxiolytics
Given this population experiences a high prevalence of anxiety disorders (Hawton 2013) anxiolytics, including benzodiazepines and non‐benzodiazepine anxiolytics, may be used to reduce suicidal behaviour (Tyrer 2012). However, because of their GABAminergic effects, benzodiazepines may increase aggression and disinhibition (Albrecht 2014). Current evidence is that benzodiazepines are associated with increased risk of suicidal behaviour (Dodds 2017). Therefore, it is usually recommended that benzodiazepines are used very cautiously, if at all, in people at risk of SH.
Mood stabilisers (including antiepileptics)
Mood stabilisers may have a role for people diagnosed with bipolar disorder or unipolar depression, especially to prevent the recurrence of episodes of mood disorder (Cipriani 2013b). Therefore, these drugs might reduce the risk of SH. However, to date, this effect has only been found for lithium (Cipriani 2013a; Smith 2017). Lithium may reduce the risk of SH via a serotonin‐mediated reduction in impulsivity and aggression. It is also possible that the long‐term clinical monitoring, which all persons prescribed lithium must undergo might contribute to a reduction in SH (Cipriani 2013a).
Other pharmacological agents
Other pharmacological agents, particularly the N‐Methyl‐D‐aspartate receptor antagonist, ketamine, may also be trialled. Ketamine has been shown to have an antisuicidal effect, independent of its antidepressant effects (Sanacora 2017). As a result, the FDA has recently granted approval for the use of both ketamine and esketamine, as adjunctive treatments to antidepressant therapy (FDA 2019). Ketamine has been associated with reduced suicidal ideation severity in the short term in adults with treatment‐resistant mood disorders (Wilkinson 2018; Witt 2020a). However, few trials have investigated the effect of ketamine over longer time periods. The effectiveness of ketamine on SH, and potential adverse effects of ketamine administration, such as dissociation, emergence psychosis, and rebound suicidal ideation, or behaviour, or both, remain under‐studied (Witt 2020a).
Natural products
There is some interest in the use of natural products, for example dietary supplementation of omega‐3 fatty acids (fish oils; Tanskanen 2001). Omega‐3 fatty acids have been implicated in the neural network, which is shown to correlate with the lethality of recent SH (Mann 2013). Blood plasma polyunsaturated fatty acid levels have also been implicated in the serotonin‐mediated link between low cholesterol and SH, suggesting that low omega‐3 fatty acid levels may have a negative impact on serotonin function (Sublette 2006). For those in whom SH is impulsive, omega‐3 supplementation may stimulate serotonin activity, thereby reducing the likelihood of engaging in SH (Brunner 2002).
Why it is important to do this review
SH is a major social and healthcare problem. It represents significant morbidity, is often repeated, and is linked with suicide. Many countries now have suicide prevention strategies, all of which include a focus on improved management of people presenting with SH (WHO 2014a). SH is also associated with substantial healthcare costs (Sinclair 2011). In the UK, the overall median cost per episode of SH has been estimated to be £809, although costs are significantly higher for cases of combined self‐injury and self‐poisoning, compared to either self‐injury or self‐poisoning alone. These costs are mainly attributable to health‐service level contact (i.e. inpatient stay or admission to intensive care; Tsiachristas 2017).
In the UK, the National Collaborating Centre for Mental Health (NCCMH) produced the first guideline on the treatment of SH behaviours in 2004 (NCCMH 2004). This guideline focused on the short‐term physical and psychological management of SH. This guidance was updated in 2011, using interim data from a previous version of this review as the evidence‐base, and focused on the longer‐term psychological management of SH (NICE 2011). Subsequently, similar guidelines have been published by the Royal College of Psychiatrists (Royal College of Psychiatrists 2014), the Royal Australian and New Zealand College of Psychiatrists (Carter 2016), and German Professional Associations and Societies (Plener 2016), amongst others (Courtney 2019).
In 2021, the guidance contained in the 2011 NICE guidelines for the longer‐term management of SH will be due for updating. Therefore, we are updating our review (Hawton 2015), in order to provide contemporary evidence to guide clinical policy and practice.
Objectives
To assess the effects of pharmacological agents or natural products for self‐harm (SH) compared to comparison types of treatment (e.g. placebo or alternative pharmacological treatment) for adults (aged 18 years or older) who engage in SH.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised controlled trials (RCTs) of specific pharmacological agents or natural products versus placebo, or any other pharmacological comparisons in the treatment of adults with a recent (within six months of trial entry) presentation for self‐harm (SH). All RCTs (including cluster‐RCTs and cross‐over trials) were eligible for inclusion regardless of publication type or language; however, we excluded quasi‐randomised trials.
Types of participants
While exact eligibility criteria often differ both within and between regions and countries (Witt 2020b), we included participants of both sexes and all ethnicities, who were 18 years and older, with a recent (i.e. within six months of trial entry) presentation to hospital or clinical services for SH.
We defined SH as all intentional acts of self‐poisoning (such as intentional drug overdoses) or self‐injury (such as self‐cutting), regardless of degree of suicidal intent or other types of motivation (Hawton 2003). This definition includes acts intended to result in death ('attempted suicide'), those without suicidal intent (e.g. to communicate distress, to temporarily reduce unpleasant feelings, sometimes termed 'non‐suicidal self‐injury'), and those with mixed motivation. We did not distinguish between attempted suicide and non‐suicidal self‐injury in this review, because there is a high level of co‐occurrence between them, and the two cannot be distinguished in any reliable way, including on levels of suicidal intent (Klonsky 2011). Lastly, the motivations for SH are complex and can change, even within a single episode (De Beurs 2018).
We excluded trials in which participants were hospitalised for suicidal ideation only (i.e. without evidence of SH).
Types of interventions
Interventions
These included the following.
Tricyclic antidepressants (TCAs, e.g. amitriptyline).
Newer generation antidepressants (NGAs), such as selective serotonin reuptake inhibitor (SSRIs, e.g. fluoxetine), serotonin and noradrenaline reuptake inhibitors (SNRIs, e.g. venlafaxine), norepinephrine reuptake inhibitors (NRIs, e.g. reboxetine), norepinephrine‐dopamine reuptake inhibitors (NDRIs, e.g., bupropion), tetracyclic antidepressants (e.g. maprotiline), noradrenergic specific serotonergic antidepressants (NaSSAs, e.g. mirtazapine), serotonin antagonist or reuptake inhibitors (SARIs, e.g. trazodone), or reversible inhibitors of monoamine oxidase type A (RIMAs, e.g. moclobemide).
Other antidepressants, such as irreversible monoamine oxidase inhibitors (MAOIs, e.g. phenelzine).
Antipsychotics (e.g. quetiapine).
Anxiolytics, including both benzodiazepines (e.g. diazepam), and non‐benzodiazepine anxiolytics (e.g. buspirone).
Mood stabilisers, including antiepileptics (e.g. sodium valporate) and lithium.
Other pharmacological agents (e.g. ketamine).
Natural products (e.g. omega‐3 essential fatty acid supplementation).
Comparators
In pharmacological trials, where a comparison with the specific effects of a drug is being made, the comparator is typically placebo, which consists of any pharmacologically inactive treatment, such as sugar pills or injections with saline. We also included trials in which another pharmacological intervention (such as another standard pharmacological agent, reduced dose of the intervention agent, or active comparator) was used.
Combination interventions
We also planned to include combination interventions, where any pharmacological agent of any class, as outlined above, is combined with psychological therapy. However, as the focus of this review is the effectiveness of pharmacological agents for people who self harm, we only included such trials if both the intervention and control groups received the same psychological therapy, to ensure that any potential effect of the psychosocial therapy was balanced across both groups. The effectiveness of psychosocial therapy alone for adults who engage in SH behaviours is the subject of a separate review (Hawton 2016).
Types of outcome measures
For all outcomes, we were primarily interested in quantifying the effect of treatment assignment to the intervention at baseline, regardless of whether the intervention was received as intended (i.e. the intention‐to‐treat effect).
Primary outcomes
The primary outcome measure in this review was the occurrence of repeated SH over a maximum follow‐up period of two years. Repetition of SH was identified through self‐report, collateral report, clinical records, or research monitoring systems. As we wished to incorporate the maximum data from each trial, we included both self‐reported and hospital records of SH, where available. Preference was given to clinical records over self‐report where a study reported both measures. We also reported proportions of participants repeating SH, frequency of repeat episodes, and time to SH repetition (where available).
Secondary outcomes
Given increasing interest in the measurement of outcomes of importance to those who engage in SH (Owens 2020), we planned to analyse data for the following secondary outcomes (where available) over a maximum follow‐up period of two years.
Treatment acceptability
This was measured by differences in discontinuation rates for any reason.
Treatment adherence
This was assessed using a range of measures of adherence, including: pill counts, changes in blood measures, and the proportion of participants that both started and completed treatment.
Depression
This was assessed as either continuous data, by scores on psychometric measures of depression symptoms, for example total scores on the Beck Depression Inventory (BDI; Beck 1961), or scores on the depression sub‐scale of the Hospital Anxiety and Depression Scale (HADS; Zigmond 1983), or as dichotomous data as the proportion of participants who meet defined diagnostic criteria for depression.
Hopelessness
This was assessed as either continuous data, by scores on psychometric measures of hopelessness, for example, total scores on the Beck Hopelessness Scale (BHS; Beck 1974), or as dichotomous data as the proportion of participants reporting hopelessness.
General functioning
This was assessed as either continuous data, by scores on psychometric measures of general functioning, for example, total scores on the Global Assessment of Functioning (GAF; APA 2000), or as dichotomous data as the proportion of participants reporting improved general functioning.
Social functioning
This was assessed as either continuous data, by scores on psychometric measures of social functioning, for example, total scores on the Social Adjustment Scale (SAS; Weissman 1999), or as dichotomous data as the proportion of participants reporting improved social functioning.
Suicidal ideation
This was assessed as either continuous data, by scores on psychometric measures of suicidal ideation, for example, total scores on the Beck Scale for Suicidal Ideation (BSS; Beck 1988), or as dichotomous data as the proportion of participants reaching a defined cut‐off for ideation.
Suicide
This included register‐recorded deaths, or reports from collateral informants, such as family members or neighbours.
Search methods for identification of studies
Electronic searches
An information specialist searched the following databases (to 4 July 2020), using relevant subject headings (controlled vocabularies) and search syntax as appropriate for each resource: Cochrane Common Mental Disorders Specialised Register (Appendix 1), Cochrane Library (Central Register of Controlled Trials; CENTRAL), Cochrane Database of Systematic Reviews (CDSR), MEDLINE Ovid, Embase Ovid, and PsycINFO Ovid (Appendix 2).
A date restriction was applied as the search was to update an earlier version of this review (Hawton 2015). However, we did not apply any further restrictions on language or publication status to the searches.
We searched for retraction statements and errata once the included studies were selected.
We also searched the World Health Organization International Clinical Trials Registry Platform, and the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov to identify ongoing trials.
Searching other resources
Conference abstracts
In addition to conference abstracts retrieved via the main electronic search, we also screened the proceedings of recent (last five years) conferences organised by the largest scientific committees in the field:
International Association for Suicide Prevention (both global congresses and regional conferences), and;
Joint International Academy of Suicide Research and American Foundation for Suicide Prevention International Summits on Suicide Research.
Reference lists
We also checked the reference lists of all relevant RCTs, and the reference lists of major reviews that included a focus on pharmacological interventions for SH in adults (Hawton 2015).
Correspondence
We consulted the corresponding authors of trials, and other experts in the field to find out if they are aware of any ongoing or unpublished RCTs on the pharmacological treatment of adults who engage in SH that were not identified by the electronic searches.
Data collection and analysis
Selection of studies
Review authors KW, KH, and one of either SH, GR, TTS, ET, or PH, independently assessed the titles of reports identified by the electronic search for eligibility. We distinguished between:
eligible or potentially eligible trials for retrieval, in which any psychosocial intervention was compared with a comparator (e.g., placebo or alternative pharmacological treatment);
ineligible general treatment trials, not for retrieval (i.e. where there was no control treatment.
All trials identified as potentially eligible for inclusion then underwent a second screening. Pairs of review authors, working independently from one another, screened the full text of eligible or potentially eligible trials to identify whether the trial met our inclusion criteria. We resolved disagreements in consultation with the senior review author (KH). Where disagreements could not be resolved from the information reported in the trial, or where it was unclear whether the trial satisfied our inclusion criteria, we contacted corresponding trial authors for additional clarification.
We identified and excluded duplicate records, and collated multiple reports of the same trial, so that each trial, rather than each report, represented the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Liberati 2009), and completed a 'Characteristics of excluded studies' table.
Data extraction and management
Review author KW and one of either SH, or GR independently extracted data from the included trials, using a standardised extraction form. Where there were any disagreements, they were resolved in consensus discussions with KH.
Data extracted from each eligible trial included the following.
Participant information: number randomised, number lost to follow‐up or withdrawn, number analysed, mean or median age, sex composition, diagnoses, diagnostic criteria, inclusion criteria, and exclusion criteria.
Methods: trial design, total duration of the trial, details of any 'run in' period (if applicable), number of trial centres and their location, setting, and date.
Intervention(s): details of the intervention, including dose, duration, route of administration, whether concomitant medications were permitted and details of these medications, and any excluded medications.
Comparator(s): details of the comparator, including dose, duration, route of administration, whether concomitant medications were permitted and details of these medications, and any excluded medications.
Outcomes: raw data for each eligible outcome (see Types of outcome measures), details of other outcomes specified and reported, and time points at which outcomes were reported.
Notes: source of trial funding, and any notable conflicts of interest of trial authors.
We extracted both dichotomous and continuous outcomes data from eligible trials. As the use of non‐validated psychometric scales is associated with bias, we extracted continuous data only if the psychometric scale used to measure the outcome of interest had been previously published in a peer‐reviewed journal, and was not subjected to item, scoring, or other modification by the trial authors (Marshall 2000).
We planned the following main comparisons.
Tricyclic antidepressants versus placebo.
Tricyclic antidepressants versus another comparator drug or dose.
Newer generation antidepressants versus placebo.
Newer generation antidepressants versus another comparator drug or dose.
Any other antidepressants versus placebo.
Any other antidepressants versus another comparator drug or dose.
Antipsychotics versus placebo.
Antipsychotics versus another comparator drug or dose.
Anxiolytics, including benzodiazepines and non‐benzodiazepine anxiolytics, versus placebo.
Anxiolytics, including benzodiazepines and non‐benzodiazepine anxiolytics, versus another comparator drug or dose.
Mood stabilisers, including antiepileptics and lithium, versus placebo.
Mood stabilisers, including antiepileptics and lithium, versus another comparator drug or dose.
Other pharmacological agents versus placebo.
Other pharmacological agents versus another comparator drug or dose.
Natural products versus placebo.
Natural products versus another comparator drug or dose.
Assessment of risk of bias in included studies
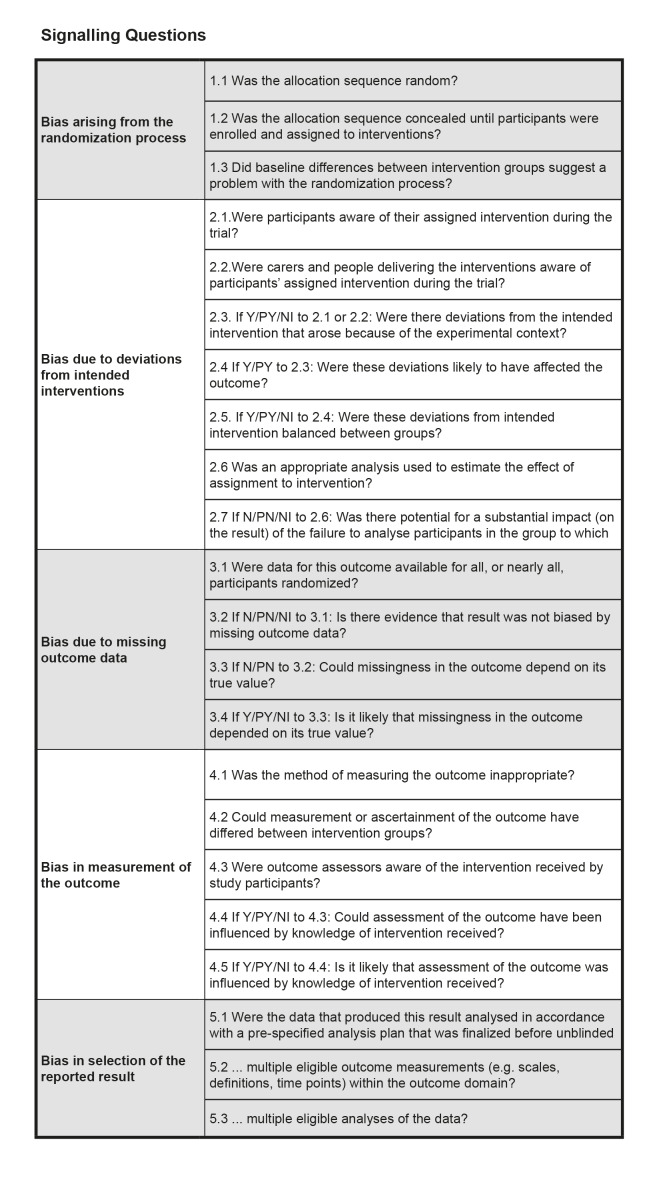
Highly biased studies are more likely to overestimate treatment effectiveness (Moher 1998). Review author KW and one of either SH, or GR independently evaluated the risk of bias for the primary outcome (i.e. repetition of SH post‐intervention) by using version 2 of the Cochrane Risk of Bias tool, RoB 2 (Sterne 2019). This tool encourages consideration of the following domains:
Bias in the randomisation process.
Deviations from the intended intervention (assignment to intervention).
Missing outcome data.
Bias in the measurement of the outcome.
Bias in the selection of the reported result.
For cluster‐RCTs, we also evaluated the following.
Bias arising from the timing of identification and recruitment of participants.
Signalling questions in the RoB 2 tool provided the basis for the tool’s domain‐level judgements about the risk of bias. Two review authors independently judged each source of potential bias low risk, high risk, or some concerns. An overall 'Risk of bias' judgement was then made for each study by combining ratings across these domains. Specifically, if any of the above domains were rated at high risk, the overall 'Risk of bias' judgement was rated at high risk. We reported this overall judgement, which can also be low risk, high risk, or some concerns, in the text of the review, and in the 'Risk of bias' tables.
Where inadequate details were provided in the original report, we contacted corresponding trial authors to provide clarification. We resolved disagreements through discussions with KH.
We entered and organised our RoB 2 assessments on an Excel spreadsheet (Microsoft Excel RoB2 Macro), and made them available as electronic supplements.
Measures of treatment effect
Dichotomous outcomes
We summarised dichotomous outcomes, such as the number of participants engaging in a repeat SH episode, or number of deaths by suicide, using the summary odds ratio (OR) and the accompanying 95% confidence interval (CI), as the OR is the most appropriate effect size statistic for summarising associations between two dichotomous groups (Fleiss 1994).
Continuous outcomes
For outcomes measured on a continuous scale, we used mean differences (MDs) and accompanying 95% CI where the same outcome measure was used. Where different outcome measures were used, we used the standardised mean difference (SMD) and its accompanying 95% CI.
We aggregated trials in a meta‐analysis only where treatments were sufficiently similar. For trials that could not be included in a meta‐analysis, we provided narrative descriptions of the results.
Hierarchy of outcomes
Where a trial measured the same outcome, for example depression, in two or more ways, we planned to use the most common measure across trials in any meta‐analysis. We also planned to report scores from other measures in a supplementary table.
Timing of outcome assessment
The primary end point for this review was post‐intervention (i.e, at the conclusion of the treatment period). We also reported outcomes for the following secondary end points (where data were available).
Between zero and six months after the conclusion of the treatment period.
Between six and 12 months after the conclusion of the treatment period.
Between 12 and 24 months after the conclusion of the treatment period.
Where there was more than one outcome assessment within a time period, we used data from the last assessment in the time period, unless different outcomes are assessed at different time points. For treatment adherence, we also planned to use within‐treatment results.
Unit of analysis issues
Zelen design trials
Trials in this area are increasingly using Zelen's method, in which consent is obtained subsequent to randomisation and treatment allocation (Witt 2020b). This design may lead to bias if, for example, participants allocated to one particular arm of the trial disproportionally refuse to provide consent for participation or, alternatively, if participants only provide consent if they are allowed to cross over to the other treatment arm (Torgerson 2004).
Although no trial included in this review used Zelen's design, should we identify a trial using Zelen's method in future updates of this review, we plan to extract data for all randomised participants as this is consistent with Zelen's original intention (Zelen 1979), and preserves randomisation. This will typically be possible for our primary outcome, repetition of SH, as this will generally be ascertained from clinical, hospital, and/or medical records. However, for certain self‐reported outcome measures, data may only be reported on the basis of those who consented to participation. We therefore also plan to conduct sensitivity analyses to investigate what impact, if any, the inclusion of these trials may have on the pooled estimate of treatment effectiveness.
Cluster‐randomised trials
Cluster randomisation, for example by clinician or general practice, can lead to overestimation of the significance of a treatment effect, resulting in an inflation of the nominal type I error rate, unless appropriate adjustment is made for the effects of clustering (Donner 2002; Kerry 1998).
Although no trial included in this review used cluster randomisation, should we identify a trial using cluster randomisation in future updates of this review, we will follow the guidance outlined in Higgins 2019a. Specifically, where possible, we will analyse data using measures that statistically accounted for the cluster design. Where this is not possible, we will analyse data using the effective sample size.
Cross‐over trials
A primary concern with cross‐over trials is the carry‐over effect, in which the effect of the intervention treatment (e.g. pharmacological, physiological, psychological) influences the participant's response to the subsequent control condition (Elbourne 2002). As a consequence, on entry to the second phase of the trial, participants may differ systematically from their initial state, despite a wash‐out phase. In turn, this may result in a concomitant underestimation of the effectiveness of the treatment intervention (Curtin 2002a; Curtin 2002b).
No trial included in this review used cross‐over methodology. However, should we identify any cross‐over trials in future updates of this trial, we will only extract data from the first phase of the trial, prior to cross‐over, to protect against the carry‐over effect.
Studies with multiple treatment arms
One trial in the current review included multiple treatment arms (Hirsch 1982). As both intervention arms in this trial investigated the effectiveness of newer generation antidepressants (i.e. mianserin or nomifensine), we combined dichotomous data from these two arms. For continuous outcomes, we combined data using the formula reported in Higgins 2011.
Studies with adjusted effect sizes
Where trials reported both unadjusted and adjusted effect sizes, we included only observed, unadjusted effect sizes.
Dealing with missing data
We did not impute missing data, as we considered that the bias that would be introduced by doing this would outweigh any benefit of increased statistical power that may have been gained by including imputed data. However, where authors omitted standard deviations (SD) for continuous measures, we contacted corresponding authors to request missing data. Where missing data could not be provided, we calculated missing SDs using other data from the trial, such as CIs, based on methods outlined in Higgins 2019b.
Assessment of heterogeneity
Between‐study heterogeneity can be assessed using either the Chi² or I² statistics. However, in this review, we only used the I² statistic to quantify inconsistency, as this is considered to be more reliable (Deeks 2019). The I² statistic indicates the percentage of between‐study variation due to chance, and can take any value from 0% to 100% (Deeks 2019).
We used the following values to denote relative importance of heterogeneity, as per Deeks 2019:
unimportant: 0% to 40%;
moderate: 30% to 60%;
substantial: 50% to 90%;
considerable: 75% to 100%.
We also took the magnitude and direction of effects and strength of evidence for heterogeneity into account (e.g. the CI for I²).
Where substantial levels of heterogeneity were found, we explored reasons for this heterogeneity (see Subgroup analysis and investigation of heterogeneity for details).
Assessment of reporting biases
Reporting bias occurs when the decision to publish a particular trial is influenced by the direction and significance of the results (Egger 1997). Research suggests, for example, that trials with statistically significant findings are more likely to be submitted for publication, and subsequently, be accepted for publication, leading to possible overestimation of the true treatment effect (Hopewell 2009).
To assess whether trials included in any meta‐analysis were affected by reporting bias, we planned to enter data into a funnel plot when a meta‐analysis includes results of at least 10 trials. Should evidence of any small study effects be identified, we planned to explore reasons for funnel plot asymmetry, including the presence of possible publication bias (Egger 1997).
Data synthesis
For the purposes of this review, we calculated the pooled odds ratio (OR) and accompanying 95% CI using the random‐effects model, as this is the most appropriate model for incorporating heterogeneity between studies (Deeks 2019). We used the Mantel‐Haenszel method for dichotomous data, and the inverse variance method for continuous data. We conducted all analyses in Review Manager 5.4 (Review Manager 2020).
Subgroup analysis and investigation of heterogeneity
Subgroup analyses
We planned to undertake the following subgroup analyses where there were sufficient data to do so:
sex (males versus females);
repeater status (first SH episode versus repeat SH episode).
Formal tests for subgroup differences were undertaken in Review Manager 5.4 (Review Manager 2020). However, it is only possible to undertake these subgroup analyses if randomisation was stratified by these factors, otherwise, there is the risk that doing so could lead to confounding. Given that randomisation was not stratified by these factors in the included studies, we found there were insufficient data to undertake these subgroup analyses in the current update.
Investigation of heterogeneity
Although no meta‐analysis was associated with substantial levels of between‐study heterogeneity (i.e., I² ≥ 75%), in future updates, should any meta‐analysis be associated with substantial levels of between‐study heterogeneity two review authors will firstly independently triple‐check data to ensure these were correctly entered. Assuming data were entered correctly, we will investigate the source of this heterogeneity using a formal statistical approach as outlined in Viechtbauer 2020.
Sensitivity analysis
We planned to undertake the following sensitivity analyses, where appropriate, to test whether key methodological factors or decisions may have influenced the main result.
Where a trial made use of Zelen's method of randomisation (see Unit of analysis issues).
Where a trial contributed to substantial between‐study heterogeneity (see Subgroup analysis and investigation of heterogeneity).
However, as no included trial made use of Zelen's method of randomisation, and furthermore, no meta‐analysis was associated with substantial levels of between‐study heterogeneity, we were unable to undertake these sensitivity analyses.
Summary of findings and assessment of the certainty of the evidence
For each comparison we planned to construct a 'Summary of findings' table for our primary outcome measure, repetition of SH post‐intervention, following the recommendations outlined in Schünemann 2019. These tables provide information concerning the overall certainty of the evidence from all included trials that measured the outcome. We assessed the quality of evidence across the following domains.
'Risk of bias' assessment.
Indirectness of evidence.
Unexplained heterogeneity or inconsistency of results.
Imprecision of effect estimates.
Potential publication bias.
For each of these domains, we downgraded the evidence from high certainty by one level (for serious) or by two levels (for very serious). For risk of bias, we downgraded this domain by one level when we rate any of the sources of risk of bias (as described in Assessment of risk of bias in included studies) at high risk for any of the studies included in the pooled estimate, or by two levels when we rate multiple studies at high risk for any of these sources. For indirectness of evidence, we considered the extent to which trials included in any meta‐analysis use proxy measures to ascertain repetition of SH; we downgraded this domain by one level if one study used proxy measures, and by two levels if multiple studies used proxy measures. For unexplained heterogeneity or inconsistency of results, we downgraded this domain by one level where the I² value indicated substantial levels of heterogeneity, or by two levels where the I² value indicated considerable levels of heterogeneity. For imprecision, we downgraded this domain by one level where the 95% CI for the pooled effect included the null value. Finally, for the potential publication bias domain, we considered any evidence of funnel plot asymmetry (if available), as well as other evidence such as suspected selective availability of data, and downgraded by one or more levels where publication bias was suspected.
We then used these domains to rate the overall certainty of evidence for the primary outcome according to the following.
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect, and may change the estimate.
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect, and may change the estimate.
Very low certainty: we are very uncertain about the estimate.
We constructed 'Summary of findings' tables using GRADEpro GDT software (GRADEpro GDT 2015).
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of the studies included in this review. Our recommendations for practice and research suggest priorities for future research, and outline the remaining uncertainties in the area.
Results
Description of studies
Results of the search
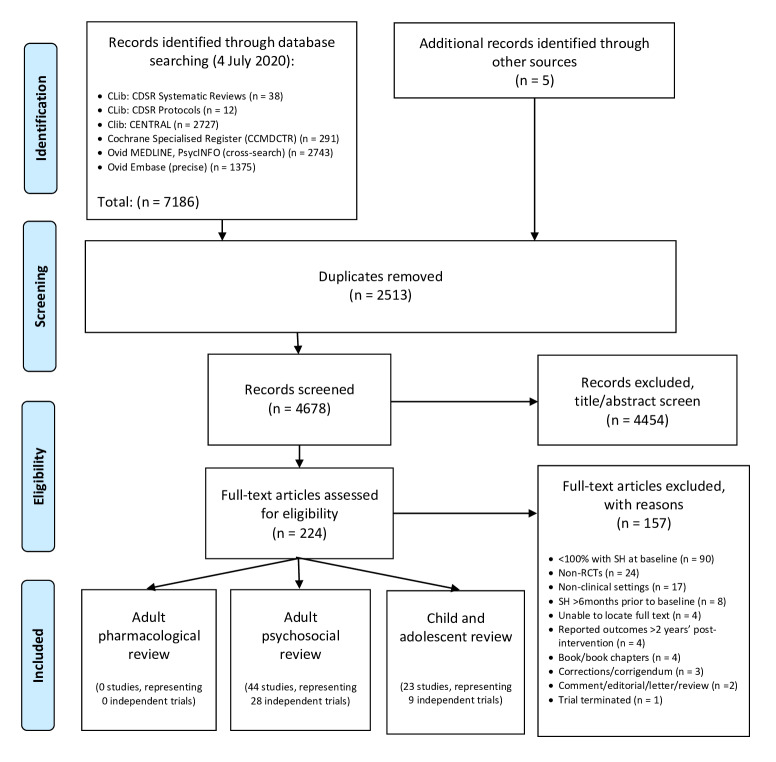
For this update, a total of 7186 records were found using the search strategy as outlined in Appendix 1 and Appendix 2. Five further records were identified following correspondence and discussion with researchers in the field. After deduplication, the initial number was reduced to 4678. Of these, 4454 were excluded following title/abstract screening, whilst a further 157 were excluded after reviewing the full texts (Figure 1).
1.
Study Flow Diagram
Included studies
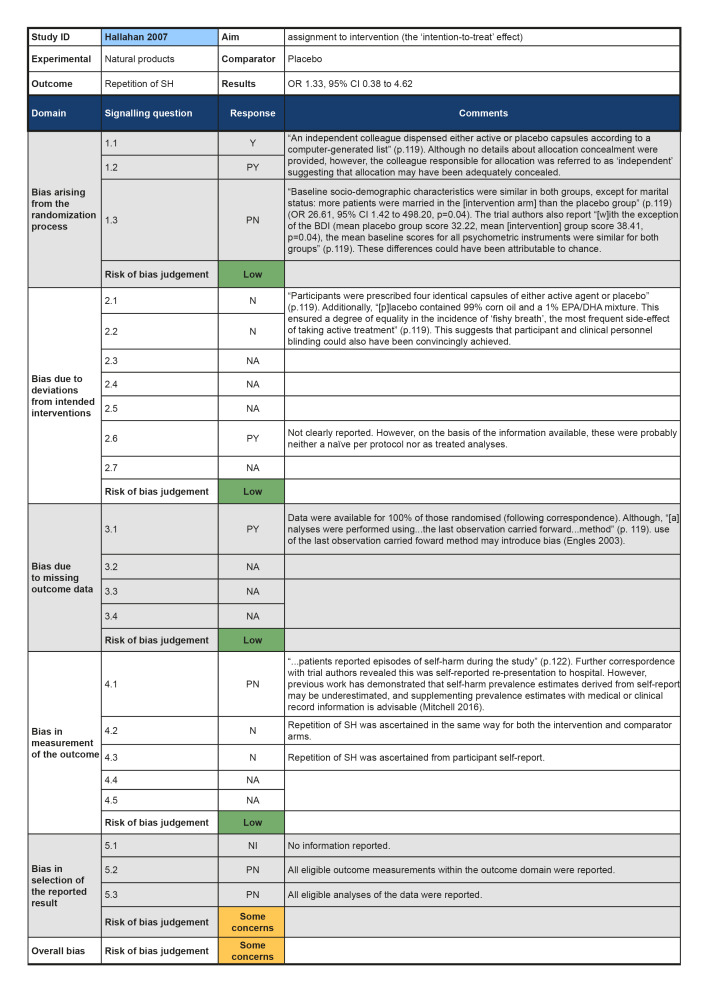
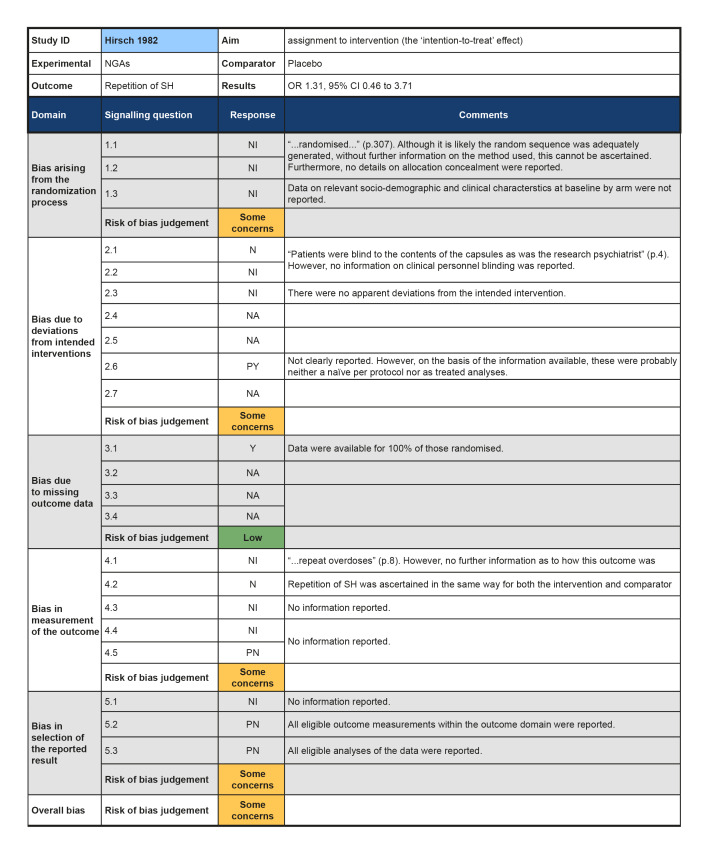
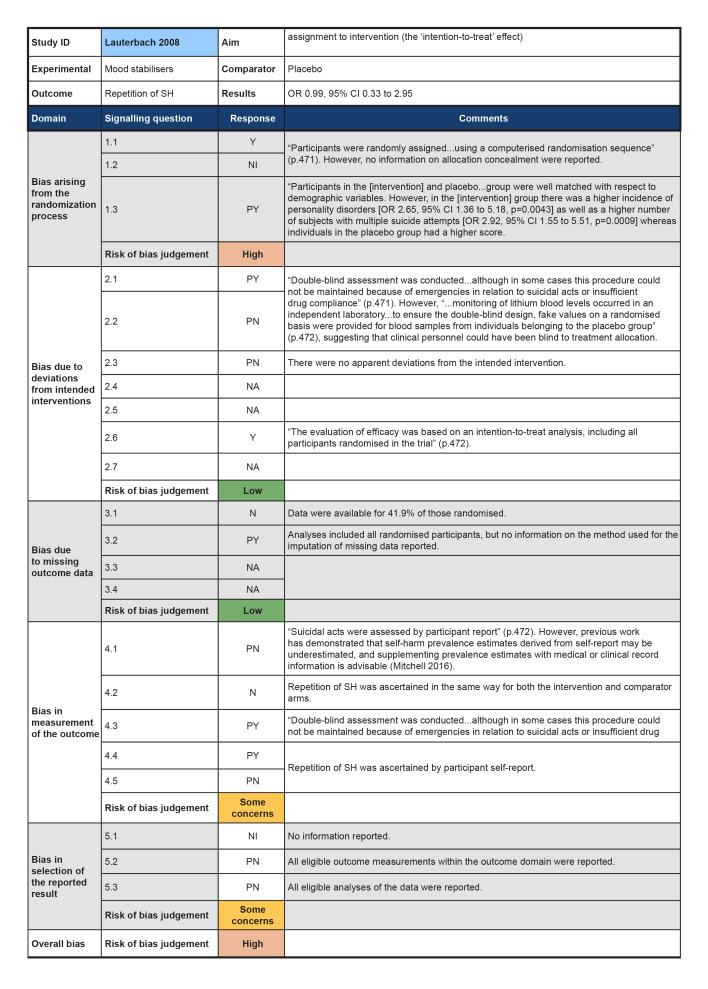
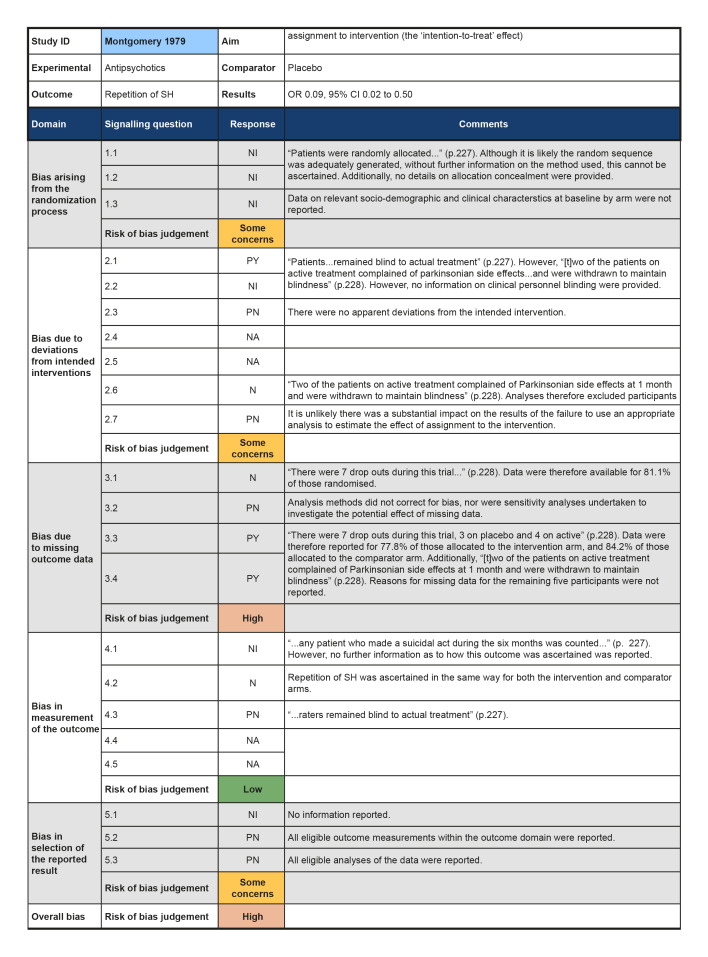
In the previous version of this review (Hawton 2015), seven trials of pharmacological interventions for self‐harm (SH) in adults were included. The present update did not locate any additional trials of pharmacological interventions or natural products for SH in adults. The present review therefore includes seven non‐overlapping trials (Battaglia 1999; Hallahan 2007; Hirsch 1982; Lauterbach 2008; Montgomery 1979; Montgomery 1983; Verkes 1998). No further reports provided any additional data on these trials.
Two of these trials have not been published (Montgomery 1979; Hirsch 1982). Unpublished data were obtained from study authors for three of these trials (Battaglia 1999; Hallahan 2007; Verkes 1998) (see the Characteristics of included studies tables for further information on these trials).
Design
Of these seven trials, five were placebo‐controlled RCTs (Hallahan 2007; Hirsch 1982; Lauterbach 2008; Montgomery 1983; Verkes 1998). The remaining two trials compared the effectiveness of the intervention agent to an active comparator drug/dose (Battaglia 1999; Montgomery 1979). All seven trials employed a simple randomisation procedure based on individual allocation to the intervention and comparator arms.
Setting
Of the seven independent RCTs included in this review, three were from the UK (Hirsch 1982; Montgomery 1979; Montgomery 1983), and one was from each of the USA (Battaglia 1999), Germany (Lauterbach 2008), the Netherlands (Verkes 1998), and the Republic of Ireland (Hallahan 2007).
Although all participants were identified following a hospital admission for SH, five trials did not clearly specify if treatment was delivered on an inpatient or outpatient basis. For the remaining two trials (Lauterbach 2008; Verkes 1998), participants were treated in outpatient settings.
Participants and participant characteristics
The included trials comprised a total of 574 participants. All had engaged in at least one episode of SH prior to trial entry. A history of SH prior to the index episode (i.e. a history of multiple episodes of SH) was a requirement for participation in five trials (Battaglia 1999; Hallahan 2007; Lauterbach 2008; Montgomery 1979; Montgomery 1983).
Information on the methods of SH for the index episode was not reported in the majority of trials (Battaglia 1999; Hallahan 2007; Montgomery 1979; Montgomery 1983; Verkes 1998). In one trial, only those who had engaged in self‐poisoning (i.e, not illicit substances or poison) were eligible to participate (Hirsch 1982), whilst in the remaining trial (Lauterbach 2008), a variety of different methods were used, including: self‐poisoning (73.2%), self‐injury (14.4%), jumping from a height (2.5%), and attempted hanging, attempted shooting, or attempted drowning (5.0%). The methods used by the remaining 4.9% of participants in this trial were not reported. Whilst the predominance of participants engaging in self‐poisoning in these two trials is reflective of the typical pattern observed in those who present to hospital, in the community, SH more often involves self‐cutting and other forms of self‐injury (Müller 2016).
All trials included both male and female participants. Of the six trials that reported information on sex, the majority of participants were female (63.5%), reflecting the typical pattern for SH (Hawton 2008). Of the five trials that reported information on age, the weighted mean age of participants at trial entry was 35.3 years (standard (SD): 3.1 years). Two trials included a small number of adolescent participants (i.e. those under 18 years of age), but the precise number was not reported in either (Hallahan 2007; Hirsch 1982).
In the five trials that reported information on psychiatric diagnoses (Battaglia 1999; Hallahan 2007; Lauterbach 2008; Montgomery 1983; Verkes 1998), participants were most commonly diagnosed with major depression (34.5%), followed by any personality disorder (29.3%), and substance use disorder (24.8%). Around one‐in‐five (22.0%) were diagnosed with borderline personality disorder specifically. Information on comorbid diagnoses were reported in one trial (Lauterbach 2008). For this trial, the most common comorbidity was for any personality disorder (33.%), followed by substance use disorder (8.4%), and any anxiety disorder (7.2%). In a second trial (Verkes 1998), one‐quarter (25.3%) of the sample were diagnosed with more than one psychiatric disorder from the following: any anxiety disorder, any depressive disorder, dysthymia, any dissociative disorder, any adjustment disorder, and alcohol use disorder. However, the proportion diagnosed with each comorbid condition was not reported.
Interventions
The trials included in this review investigated the effectiveness of various pharmacological agents.
Newer generation antidepressants (NGAs) versus placebo (Hirsch 1982; Montgomery 1983; Verkes 1998).
Antipsychotics versus placebo (Montgomery 1979).
Antipsychotics versus another comparator drug/dose (Battaglia 1999).
Mood stabilisers, including antiepileptics, versus placebo (Lauterbach 2008).
Natural products (omega‐3 essential fatty acid; n‐3EFA) versus placebo (Hallahan 2007).
Outcomes
Primary outcome
All trials reported data on the primary outcome of this review, repetition of SH. In two trials this was based on self‐reported information (Battaglia 1999; Lauterbach 2008), and in two further trials on re‐presentation to hospital (Hallahan 2007; Hirsch 1982). For the remaining three trials the source of information for this outcome was unclear (Montgomery 1979; Montgomery 1983; Hirsch 1982).
Secondary outcomes
Treatment acceptability
Treatment acceptability was measured as the proportion of participants that discontinued treatment for any reason in six trials (Battaglia 1999; Hirsch 1982; Lauterbach 2008; Montgomery 1979; Montgomery 1983; Verkes 1998). For the remaining trial, only data on the proportion of participants that discontinued treatment due to the development of adverse effects was reported (Hallahan 2007).
Treatment adherence
Treatment adherence was assessed using pill counts in the two trials that reported data on this outcome (Hallahan 2007; Verkes 1998).
Depression
Depression was assessed using the Beck Depression Inventory (BDI) (Hallahan 2007; Verkes 1998) or the Hamilton Depression Rating Scale (HDRS; Hamilton 1960) (Hallahan 2007; Lauterbach 2008).
Hopelessness
Hopelessness was assessed using the Beck Hopelessness Scale (BHS) (Lauterbach 2008; Verkes 1998).
General functioning
No trial reported data on general functioning.
Social functioning
No trial reported data on social functioning.
Suicidal ideation
Suicidal ideation was assessed using the sub‐scale of the Modified Overt Aggression Scale (MOAS; Sorgi 1991) in one trial (Hallahan 2007), and the Scale for Suicidal Ideation (SSI) in one further trial (Lauterbach 2008).
Suicide
It was unclear how suicide was ascertained in any of the trials that reported data on this outcome.
Excluded studies
A total of 157 studies were excluded from this update. The most common reason for exclusion was that not all trial participants had engaged in SH within six months of trial entry (90 studies). Reasons for exclusion for the remaining studies are reported in Figure 1.
Details on the reasons for exclusion for those trials related to pharmacological interventions for SH in adults identified by this update are reported in the Characteristics of excluded studies section.
Ongoing studies
Of the two ongoing trials identified in the previous version of this review (Hawton 2015), one of oral lithium was subsequently terminated (NCT01928446), whilst the second, of oral ketamine, upon publication, did not meet inclusion criteria (Domany 2019). Two ongoing studies were identified in this update (see Characteristics of ongoing studies section for further information on these trials).
Studies awaiting classification
We identified one trial that is awaiting classification (see Characteristics of studies awaiting classification section for further information on this trial).
Risk of bias in included studies
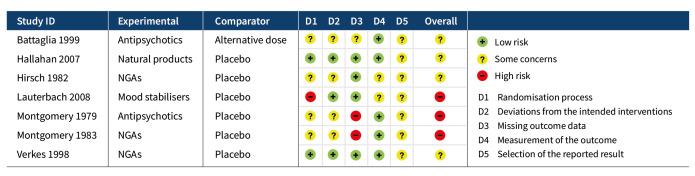
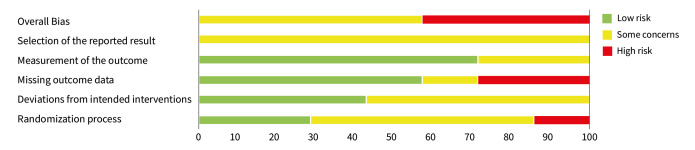
Risk of bias was evaluated for the primary outcome repetition of SH at post‐intervention. The results of the 'Risk of bias' assessments can be seen in Figure 2 and Figure 3. Full 'Risk of bias' assessments, including the evidence we used to justify our ratings, are available in Appendix 3.
2.
Results of 'Risk of bias' assessments for each study
3.
Summary of 'Risk of bias' assessments
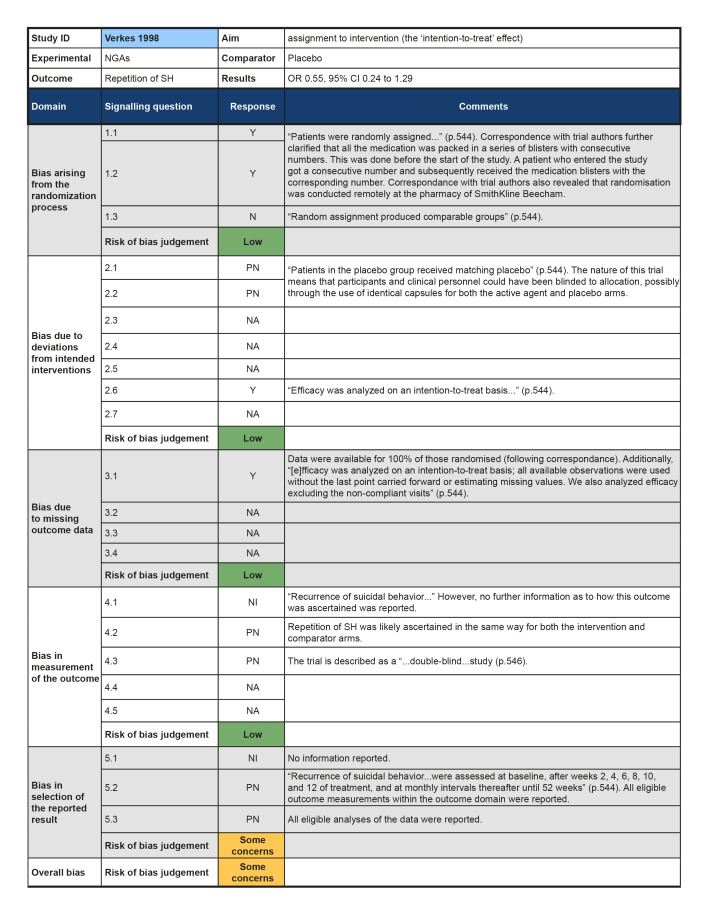
Bias arising from randomisation process
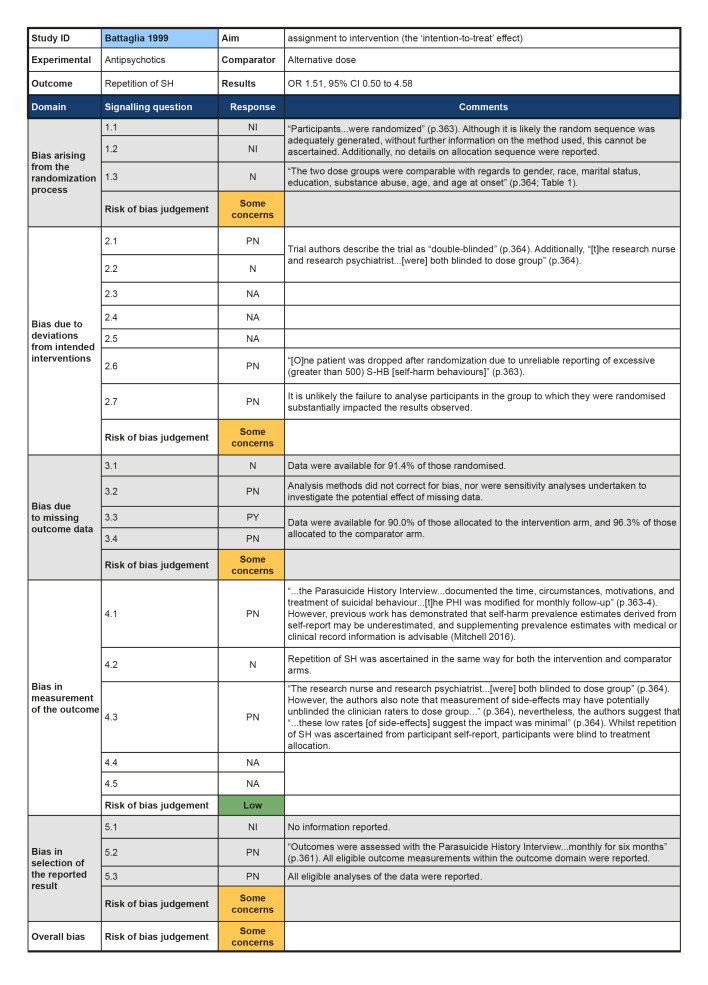
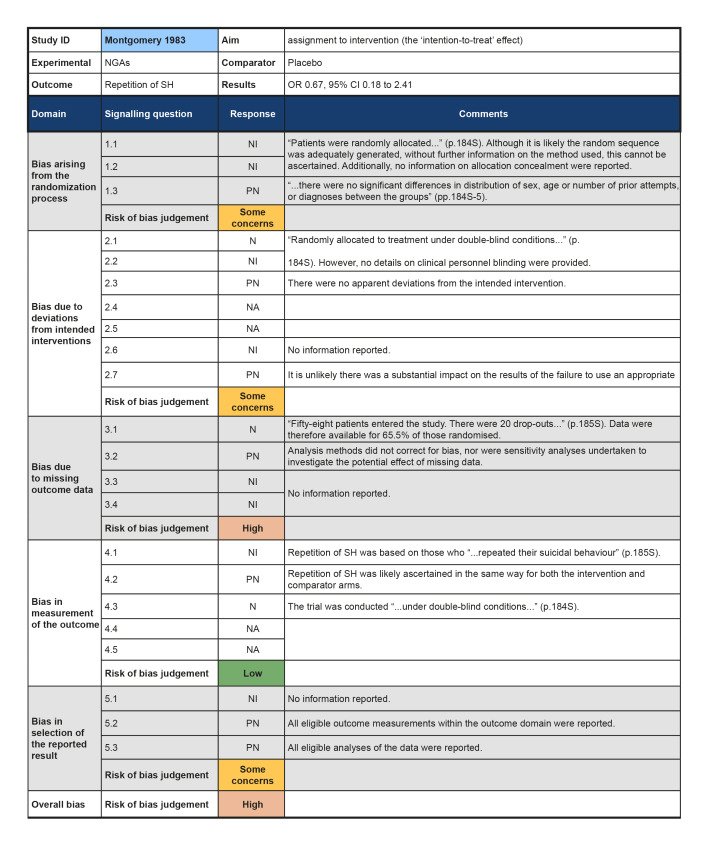
Although all trials used random allocation to assign participants to the intervention and comparator arms, only two trials were rated as low risk of bias for this domain (Hallahan 2007; Verkes 1998). There were some concerns regarding bias arising from the randomisation process for over half (57.1%) of the trials included in this review. For some older trials, insufficient information on the method used to generate the randomisation sequence was reported. Additionally, no information on allocation concealment was reported in a number of these trials (Battaglia 1999; Hirsch 1982; Montgomery 1979; Montgomery 1983). One trial was rated as high risk of bias for this domain (Lauterbach 2008). For this trial, a very significantly greater proportion of those assigned to the intervention arm were diagnosed with a personality disorder and had a history of multiple suicide attempts, whilst those assigned to the comparator arm had higher scores on the Suicide Intent Scale at baseline. Given these differences, there may have been a problem with the randomisation process.
Bias due to deviations from intended interventions
Three trials were rated as low risk of bias for this domain as participants and clinical personnel were blind to allocation, no deviations from the intended intervention were apparent, and analyses were conducted on an intention‐to‐treat (ITT) basis (Hallahan 2007; Lauterbach 2008; Verkes 1998). For the remaining trials, either no specific information on participant and clinical personnel were reported (Hirsch 1982; Montgomery 1983), or analyses excluded eligible trial participants post‐intervention (Battaglia 1999; Montgomery 1979). These four trials (57.1%) were therefore rated as at some concerns for this domain.
Bias due to missing outcome data
Over half (57.1%) of the trials included in this review were at low risk of bias for those domain. One trial (14.3%) was rated as some concerns for this domain as greater than 5% of the data were missing at the post‐intervention assessment, there was some evidence of a larger proportion of missing data for the intervention arm as compared to the comparator arm, and further, sensitivity analyses were not undertaken to understand the impact missing data may have had on the estimate of treatment effectiveness (Battaglia 1999). Two trials (28.6%) were rated as high risk of bias for this domain as greater than 5% of the data were missing at the post‐intervention assessment and either no information on causes of missingness were reported, or alternatively, missingness may have been related to the development of side‐effects (Montgomery 1979; Montgomery 1983).
The majority of trials included in this review were rated as at low risk of bias for this outcome (71.4%). However, two trials were rated as some concerns for this domain; this was typically either because insufficient information was reported on how repetition of SH was ascertained (Hirsch 1982), or because repetition of SH was ascertained from self‐reported information and participant blinding was incomplete due to safety considerations (Lauterbach 2008).Bias in measurement of the outcome
Bias in selection of the reported result
All trials included in this review were rated as at some concerns for this domain as these trials had been published prior to the International Committee of Medical Journal Editors' (ICMJE) requirement in 2015 that all trials be pre‐registered in a publicly available clinical trials registry. It was therefore difficult to determine whether data had been analysed according to a pre‐specified plan, although there were no apparent departures from the analyses outlined in the methods section of these trials (Battaglia 1999; Hallahan 2007; Hirsch 1982; Lauterbach 2008; Montgomery 1979; Montgomery 1983; Verkes 1998).
Overall bias
As a consequence, just under half of the trials (42.9%) included in this review were rated as at high risk of bias overall, whilst the remainder (57.1%) were rated as at some risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings 1. Newer generation antidepressants (NGAs) compared to placebo for self‐harm in adults.
| Newer generation antidepressants (NGAs) compared to placebo for self‐harm in adults | ||||||
| Patient or population: Self‐harm in adults Intervention: Newer generation antidepressants (NGAs) Comparison: Placebo | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| Without Newer generation antidepressants (NGAs) | With Newer generation antidepressants (NGAs) | Difference | ||||
| Repetition of SH by post‐intervention (NGA class) № of participants: 129 (2 RCTs) | OR 0.59 (0.29 to 1.19) | Study population | ⊕⊝⊝⊝ VERY LOW 1 2 3 | The evidence is very uncertain about the effect of newer generation antidepressants (NGAs) on repetition of self‐harm by post‐intervention. | ||
| 50.0% | 37.1% (22.5 to 54.3) | 12.9% fewer (27.5 fewer to 4.3 more) | ||||
| Repetition of SH by post‐intervention (NGA class) ‐ Mianserin vs. Placebo № of participants: 38 (1 RCT) | OR 0.67 (0.18 to 2.41) | Study population | ⊕⊝⊝⊝ VERY LOW 1 2 3 | The evidence is very uncertain about the effect of newer generation antidepressants (NGAs) on repetition of self‐harm by post‐intervention by NGA class (i.e., mianserin vs. placebo). | ||
| 57.1% | 47.2% (19.4 to 76.3) | 10.0% fewer (37.8 fewer to 19.1 more) | ||||
| Repetition of SH by post‐intervention (NGA class) ‐ Paroxetine vs. Placebo № of participants: 91 (1 RCT) | OR 0.55 (0.24 to 1.29) | Study population | ⊕⊕⊝⊝ LOW 2 3 | Further research is very likely to have an important impact on our confidence in the estimate of the effect of newer generation antidepressants (NGAs) on repetition of self‐harm by post‐intervention by NGA class (paroxetine vs. placebo), and may change the estimate. | ||
| 46.7% | 32.5% (17.4 to 53) | 14.2% fewer (29.3 fewer to 6.4 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded this domain by one level as we rated any of the sources of risk of bias (as described in Assessment of risk of bias in included studies) at high risk for one of the trials included in the pooled estimate.
2 We downgraded this domain as these were relatively older agents and, in one trial, no information on how SH was ascertained was reported.
3 We downgraded this domain by one level as the 95% CI for the pooled effect included the null value.
Summary of findings 2. Antipsychotics compared to placebo for self‐harm in adults.
| Antipsychotics compared to placebo for self‐harm in adults | ||||||
| Patient or population: Self‐harm in adults Intervention: Antipsychotics Comparison: Placebo | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| Without Antipsychotics | With Antipsychotics | Difference | ||||
| Repetition of SH by post‐intervention № of participants: 30 (1 RCT) | OR 0.09 (0.02 to 0.50) | Study population | ⊕⊕⊝⊝ LOW 1 2 | Further research is very likely to have an important impact on our confidence in the estimate of the effect of antipsychotics as compared to placebo on repetition of self‐harm by post‐intervention, and may change the estimate. | ||
| 75.0% | 21.3% (5.7 to 60) | 53.7% fewer (69.3 fewer to 15 fewer) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded this domain by one level as we rated any of the sources of risk of bias (as described in Assessment of risk of bias in included studies) at high risk for one of the trials included in the pooled estimate.
2 We downgraded this domain as this was a relatively older agent and, for one trial, no information on how SH was ascertained was reported.
Summary of findings 3. Antipsychotics compared to another comparator drug or dose for self‐harm in adults.
| Antipsychotics compared to another comparator drug or dose for self‐harm in adults | ||||||
| Patient or population: Self‐harm in adults Intervention: Antipsychotics Comparison: Another comparator drug/dose | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| Without Antipsychotics | With Antipsychotics | Difference | ||||
| Repetition of SH by post‐intervention № of participants: 53 (1 RCT) | OR 1.51 (0.50 to 4.58) | Study population | ⊕⊕⊝⊝ LOW 1 2 | Further research is very likely to have an important impact on our confidence in the estimate of the effect of antipsychotics as compared to another comparator drug or dose on repetition of self‐harm by post‐intervention, and may change the estimate. | ||
| 34.6% | 44.4% (20.9 to 70.8) | 9.8% more (13.7 fewer to 36.2 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded this domain as this was a relatively older agent.
2 We downgraded this domain by one level as the 95% CI for the pooled effect included the null value.
Summary of findings 4. Mood stabilisers, including antiepileptics and lithium compared to placebo for self‐harm in adults.
| Mood stabilisers, including antiepileptics and lithium compared to placebo for self‐harm in adults | ||||||
| Patient or population: Self‐harm in adults Intervention: Mood stabilisers, including antiepileptics and lithium Comparison: Placebo | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| Without Mood stabilisers, including antiepileptics and lithium | With Mood stabilisers, including antiepileptics and lithium | Difference | ||||
| Repetition of SH by post‐intervention № of participants: 167 (1 RCT) | OR 0.99 (0.33 to 2.95) | Study population | ⊕⊝⊝⊝ VERY LOW 1 2 3 | The evidence is very uncertain about the effect of mood stabilisers, including antiepileptics and lithium, on repetition of self‐harm by post‐intervention. | ||
| 8.4% | 8.4% (2.9 to 21.4) | 0.1% fewer (5.5 fewer to 12.9 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded this domain by one level as we rated any of the sources of risk of bias (as described in Assessment of risk of bias in included studies) at high risk for one of the trials included in the pooled estimate.
2 We downgraded this domain as previous work has demonstrated that self‐harm prevalence estimates derived from self‐report may be underestimated, and supplementing prevalence estimates with medical or clinical record information is advisable (Mitchell 2016).
3 We downgraded this domain by one level as the 95% CI for the pooled effect included the null value.
Summary of findings 5. Natural products compared to placebo for self‐harm in adults.
| Natural products compared to placebo for self‐harm in adults | ||||||
| Patient or population: Self‐harm in adults Intervention: Natural products Comparison: Placebo | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| Without Natural products | With Natural products | Difference | ||||
| Repetition of SH by post‐intervention № of participants: 49 (1 RCT) | OR 1.33 (0.38 to 4.62) | Study population | ⊕⊕⊝⊝ LOW 1 2 | Further research is very likely to have an important impact on our confidence in the estimate of the effect of natural products as compared to placebo on repetition of self‐harm by post‐intervention, and may change the estimate. | ||
| 25.9% | 31.8% (11.7 to 61.8) | 5.8% more (14.2 fewer to 35.9 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded this domain as previous work has demonstrated that self‐harm prevalence estimates derived from self‐report may be underestimated, and supplementing prevalence estimates with medical or clinical record information is advisable (Mitchell 2016).
2 We downgraded this domain by one level as the 95% CI for the pooled effect included the null value.
Comparison 1: Tricyclic antidepressants versus placebo
There were no eligible trials in which tricyclic antidepressants were compared with placebo identified by this review.
Comparison 2: Tricyclic antidepressants versus another comparator drug or dose
There were no eligible trials in which tricyclic antidepressants were compared with another comparator drug or dose identified by this review.
Comparison 3: Newer generation antidepressants (NGAs)versus placebo
Three trials evaluated the effectiveness of different NGAs in adults (weighted mean age: 35.6 ± 6.8 years; 51.2% female) admitted to general hospitals following SH. The first compared 30 mg to 60mg mianserin or 75 mg to 150mg nomifensine against placebo (Hirsch 1982, N = 114), the second compared 30mg mianserin against placebo (Montgomery 1983, N = 58), and the third compared 40 mg paroxetine per day plus weekly/fortnightly supportive psychotherapy to placebo plus supportive psychotherapy (Verkes 1998, N = 91). We acknowledge that these antidepressants are from different drug classes (i.e. tetracyclic, atypical, and selective serotonin reuptake inhibitors (SSRIs), respectively); however, we have combined results for these agents into one comparison in order to address the question of whether antidepressant treatment using NGAs might be of general benefit in this patient population. We have also subgrouped the individual agents in a post hoc analysis.
Primary outcome
3.1 Repetition of SH
While data from two trials did not show that NGAs may reduce risk of repetition of SH (23/63 versus 33/66; odds ratio (OR) 0.59, 95% CI 0.29 to 1.19; N = 129; k = 2; I2 = 0%; Analysis 1.1), the direction of effect favoured NGAs over placebo but the pooled estimate was imprecise. However, the overall risk of bias was high for one trial (Montgomery 1979) and there were some concerns for the other trial (Verkes 1998). According to GRADE criteria, we judged the evidence to be of very low certainty.
1.1. Analysis.

Comparison 1: Newer generation antidepressants (NGAs), Outcome 1: Repetition of SH by post‐intervention (NGA class)
There was also no evidence of an effect for mianserin or nomifensine at 12 weeks in a single trial (15/76 versus 6/38; OR 1.31, 95% confidence interval (CI) 0.46 to 3.71; N = 114; k = 1; I² = not applicable).
A post‐hoc analysis was conducted combining data from all three of these trials at the final follow‐up assessment (i.e. 12 weeks for Hirsch 1982, six months for Montgomery 1983, and 12 months for Verkes 1998) in order to investigate whether there is any evidence of a difference by agent. To assess the efficacy of each agent, the intervention arms in Hirsch 1982 were separated into nomifensine versus placebo (n = 76) and mianserin versus placebo (n = 76) using the approach outlined in Higgins 2011. However, there was no evidence of a difference between agents (test for subgroup differences: Chi² = 1.25, df = 2, P = 0.53, I² = 0%; Analysis 1.2).
1.2. Analysis.

Comparison 1: Newer generation antidepressants (NGAs), Outcome 2: Repetition of SH at final follow‐up (by NGA class)
Secondary outcomes
3.2 Treatment acceptability
There was no evidence of an effect for NGAs on treatment acceptability in two trials (Analysis 1.3). In the third trial, just over one‐third (34.5%) of participants discontinued treatment; however, results were not disaggregated by trial arm (Montgomery 1983).
1.3. Analysis.

Comparison 1: Newer generation antidepressants (NGAs), Outcome 3: Treatment acceptability
3.3 Treatment adherence
Data on treatment adherence was reported in one trial (Verkes 1998); however, no numerical data were provided. However, the trial authors report that "...analysis of capsule counts at each visit revealed no statistically significant differences between treatments" (p.545).
3.4 Depression
Two trials reported outcome data for depression (Hirsch 1982; Verkes 1998). Although mean scores on the Hamilton Depression Rating Scale(HDRS) were reported in Hirsch 1982, insufficient information was provided to enable calculation of accompanying SDs via imputation. In Verkes 1998, no numerical data were provided. However, the trial authors report there was "...no significant treatment effect" for this outcome by the post‐intervention assessment (p.545).
3.5 Hopelessness
Information on hopelessness was reported in one trial (Verkes 1998). Once again, however, no numerical data were provided. However, the trial authors state there was also "...no significant treatment effect" for this outcome by the post‐intervention assessment (p.545).
3.6 General functioning
No data available.
3.7 Social functioning
No data available.
3.8 Suicidal ideation
No data available.
3.9 Suicide
Numbers of suicides were reported for two trials (Hirsch 1982; Verkes 1998). In the first, one suicide occurred in the placebo group by the post‐intervention period (Verkes 1998); however, there was no evidence of an effect for NGAs on suicide in this trial (0/46 versus 1/45; OR 0.32, 95% CI 0.01 to 8.04; N = 91; k = 1; I² = not applicable). In the second, no participant died by suicide by the six month follow‐up period (Hirsch 1982).
Subgroup analyses
No included trial stratified randomisation by sex or repeater status.
Sensitivity analyses
Not applicable.
Comparison 4: Newer generation antidepressants versus another comparator drug or dose
There were no eligible trials in which NGAs were compared with another comparator drug or dose identified by this review.
Comparison 5: Any other antidepressants versus placebo
There were no eligible trials in which any other antidepressants were compared with placebo identified by this review.
Comparison 6: Any other antidepressants versus another comparator drug or dose
There were no eligible trials in which any other antidepressants were compared with another comparator drug or dose identified by this review.
Comparison 7: Antipsychotics versus placebo
The effectiveness of 'prophylactic' injections of the depot antipsychotic flupenthixol was compared to placebo in one small trial of adults (mean age 35.3 years, SD not reported; 70.3% female) without depression or schizophrenia and who were admitted to a general hospital following SH (Montgomery 1979, N = 37).
Primary outcome
7.1 Repetition of SH
Flupenthixol may reduce repetition of SH compared with placebo by post‐intervention based on evidence from one trial (3/14 versus 12/16; OR 0.09, 95% CI 0.02 to 0.50; N =30; k = 1; I² = not applicable). According to GRADE criteria, we judged the evidence to be of low certainty.
Secondary outcomes
7.2 Treatment acceptability
There was no evidence of an effect for flupenthixol on treatment acceptability by the post‐intervention assessment (4/18 versus 3/19; OR 1.52, 95% CI 0.29 to 8.01; N = 37; k = 1; I² = not applicable).
7.3 Treatment adherence
There was no evidence of an effect for the number of participants who completed the full course of treatment (14/18 versus 16/19; OR 0.66, 95% CI 0.12 to 3.45; N = 37; k = 1; I²=not applicable).
7.4 Depression
No data available.
7.5 Hopelessness
No data available.
7.6 General functioning
No data available.
7.7 Social functioning
No data available.
7.8 Suicidal ideation
No data available.
7.9 Suicide
No data available.
Subgroup analyses
No included trial stratified randomisation by sex or repeater status.
Sensitivity analyses
Not applicable.
Comparison 8: Antipsychotics versus another comparator drug or dose
A single small trial investigated the effectiveness of lo‐ dose (i.e. 12 mg/day) fluphenazine compared to ultra‐low dose (i.e, 1.5 mg/day) fluphenazine in adults (mean age: 30.4 ± 7.0 years; 43.9% female) admitted to an emergency psychiatric unit following a suicide attempt (Battaglia 1999, N = 58). However, "one patient was dropped after randomization due to unreliable reporting of excessive (greater than 500) S‐HB [self‐harm behaviours]" (p.363).
The authors of this trial report that "[t]he 'ultra‐low' (1.5 mg) was chosen to represent the extreme low end of possible pharmacologic effect for fluphenazine treatment" (p.363).
Primary outcome
8.1 Repetition of SH
There was no evidence of an effect on repetition of SH by post‐intervention for low‐dose fluphenazine in this trial (12/27 versus 9/26; OR 1.51, 95% CI 0.50 to 4.58; N = 53; k = 1; I ² =not applicable). According to GRADE criteria, we judged the evidence to be of low certainty.
Secondary outcomes
8.2 Treatment acceptability
There was no evidence of an effect for low‐dose fluphenazine on treatment acceptability (18/30 versus 15/27; OR 1.20, 95% CI 0.42 to 3.44; N=57; k=1; I²=not applicable).
8.3 Treatment adherence
No data available.
8.4 Depression
No data available.
8.5 Hopelessness
No data available.
8.6 General functioning
No data available.
8.7 Social functioning
No data available.
8.8 Suicidal ideation
No data available.
8.9 Suicide
No participant died by suicide in either arm by the post‐intervention period.
Subgroup analyses
No included trial stratified randomisation by sex or repeater status.
Sensitivity analyses
Not applicable.
Comparison 9: Anxiolytics, including benzodiazepines and non‐benzodiazepine anxiolytics, versus placebo
There were no eligible trials in which anxiolytics (including benzodiazepines and non‐benzodiazepine anxiolytics) were compared with placebo identified by this review.
Comparison 10: Anxiolytics, including benzodiazepines and non‐benzodiazepine anxiolytics, versus another comparator drug or dose
There were no eligible trials in which anxiolytics (including benzodiazepines and non‐benzodiazepine anxiolytics) were compared with another comparator drug or dose identified by this review.
Comparison 11: Mood stabilisers, including antiepileptics and lithium, versus placebo
In a single trial, the effectiveness of lithium was compared to placebo in adults (mean age: 39.4 ± 9.5 years; 57.5% female) who had engaged in SH in the context of a depressive spectrum disorder (Lauterbach 2008, N = 167).
Primary outcome
11.1 Repetition of SH
There was no evidence of an effect on repetition of SH by the post‐intervention period in a single trial of lithium (7/84 versus 7/83; OR 0.99, 95% CI 0.33 to 2.95; N = 167; k = 1; I² = not applicable). According to GRADE criteria, we judged the evidence to be of very low certainty. Please note that these ORs differ modestly from those reported by the trial authors in correspondence; however, there is no material difference in either the overall direction, magnitude, or significance of these results.
Secondary outcomes
11.2 Treatment acceptability
There was no evidence of an effect in favour of lithium for treatment acceptability, as measured by the proportion of participants who discontinued treatment, at both the six month (i.e. during treatment) (30/84 versus 32/83; OR 0.89, 95% CI 0.47 to 1.66; N = 167; k = 1; I² = not applicable) and by the post‐intervention (48/84 versus 49/83; OR 0.93, 95% CI 0.50 to 1.71; N = 167; k = 1; I² = not applicable) assessments.
11.3 Treatment adherence
No data available.
11.4 Depression
There was also no evidence of an effect for lithium on depression scores at post‐intervention (mean 8.48, SD 7.50, N = 31 versus mean 8.87, SD 8.10, N = 33; mean difference (MD) ‐0.39, 95% CI ‐4.21 to 3.43; N = 64; k = 1; I² = not applicable). It should be noted that these(MDs differ modestly from those reported by the authors; however, there is no material difference in either the overall direction, magnitude, or significance of these results.
11.5 Hopelessness
There was no evidence of an effect for lithium on hopelessness scores at post‐intervention (mean 8.88, SD 5.40, n = 26 versus mean 9.04, SD 6.10, n = 25; MD ‐0.16, 95% CI ‐3.33 to 3.01; N = 51; k = 1; I²=not applicable). These MDs also differ modestly from those reported by the authors; however, there is no material difference in either the overall direction, magnitude, or significance of these results.
11.6 General functioning
No data available.
11.7 Social functioning
No data available.
11.8 Suicidal ideation
There was no evidence of an effect for lithium on the number of patients reporting suicidal ideation, defined as a score of greater than zero on the SSI, at post‐intervention (8/31 versus 11/32; OR 0.66, 95% CI 0.22 to 1.97; N = 63; k =1; I² = not applicable) follow‐up assessments. These ORs differ modestly from those reported by the trial authors in correspondence; however, there is no material difference in either the overall direction, magnitude, or significance of these results.
11.9 Suicide
There was no evidence of an effect for lithium on suicides (0/85 versus 3/83; OR 0.1, 95% CI 0.0 to 2.7; N = 167; k = 1; I² = not applicable).
Subgroup analyses
No included trial stratified randomisation by sex or repeater status.
Sensitivity analyses
Not applicable.
Comparison 12: Mood stabilisers, including antiepileptics and lithium, versus another comparator drug or dose
There were no eligible trials in which mood stabilisers (including antiepileptics and lithium) were compared with another comparator drug or dose identified by this review.
Comparison 13: Other pharmacological agents versus placebo
There were no eligible trials in which other pharmacological agents were compared with placebo identified by this review.
Comparison 14: Other pharmacological agents versus another comparator drug or dose
There were no eligible trials in which other pharmacological agents were compared with another comparator drug or dose identified by this review.
Comparison 15: Natural products versus placebo
One trial investigated the effectiveness of dietary supplementation with omega‐3 essential fatty acid (n‐3EFA) as compared to placebo in adults (mean age 30.6 years, SD not reported; 65.3% female) admitted to accident and emergency facilities following an episode of SH (Hallahan 2007, N = 49).
Primary outcome
15.1 Repetition of SH
There was no evidence of an effect for natural products at post‐intervention in a single small trial (7/22 versus 7/27; OR 1.33, 95% CI 0.38 to 4.62; N = 49; k = 1; I² = not applicable). According to GRADE criteria, we judged the evidence to be of low certainty.
Additionally, there was no difference between groups in the mean number of SH episodes per participant between those receiving the supplement and those receiving placebo (mean 0.41 versus mean 0.41). However, insufficient information was provided to enable imputation of SDs.
Secondary outcomes
15.2 Treatment acceptability
There was no evidence of an effect for natural products on treatment acceptability as measured by the proportion of participants who discontinued treatment for any cause (3/22 versus 7/27; OR 0.45, 95% CI 0.10 to 2.00; N = 49; k=1; I² = not applicable), although the trial authors note that "[n]o patients discontinued the study because of adverse events" (Hallahan 2007, p.120).
15.3 Treatment adherence
There was no evidence of an effect for natural products on treatment adherence as measured by pill counts (19/22 versus 20/27; OR 2.22, 95% CI 0.50 to 9.85; N = 49; k = 1; I² = not applicable).
15.4 Depression
Mean and SD scores on the BDI and HRSD were reported as adjusted improvement scores. The authors report there were "significant improvements in BDI scores at 12 weeks [i.e. post‐intervention] (p = 0.004) in the [omega‐3] group. Moreover, more patients in the [omega‐3] group attained more than 50% (p = 0.001) and 70% (p = 0.001) reduction (response and remission, respectively) in symptoms...Similar data were observed for the HRSD" (Hallahan 2007, p.119).
15.5 Hopelessness
No data available.
15.6 General functioning
No data available.
15.7 Social functioning
No data available.
15.8 Suicidal ideation
There may be evidence of a reduction in the proportion of participants reporting suicidal ideation at the post‐intervention assessment for natural products compared with placebo (8/22 versus 19/27; OR 0.24, 95% CI 0.07 to 0.80; N = 49; k = 1; I² = not applicable).
15.9 Suicide
No participant died by suicide in either arm during the treatment period.
Subgroup analyses
No included trial stratified randomisation by sex or repeater status.
Sensitivity analyses
Not applicable.
Comparison 16: Natural products versus another comparator drug or dose
There were no eligible trials in which natural products were compared with another comparator drug or dose identified by this review.
Discussion
This review included seven trials, and failed to identify any newer trials since the previous version (Hawton 2015). Previously, we commented on the paucity of evidence on which to make firm conclusions about the most effective form of pharmacological treatment for patients who have recently engaged in self‐harm (SH). This update reinforces these conclusions.
Summary of main results
Newer generation antidepressants
On the basis of data from two trials in which different classes of antidepressants (i.e. mianserin and paroxetine) were evaluated in SH patients, the evidence remains uncertain as to whether (newer generation antidepressants NGAs) have any effect on repetition of SH compared with placebo by the post‐intervention assessment (Montgomery 1983; Verkes 1998). There was no apparent effect by the 12‐week assessment in a further trial of a different NGA (i.e. nomifensine) (Hirsch 1982). However, it should be noted that the trials were relatively small and the confidence intervals around the point estimate of the treatment effect were relatively wide. This therefore increases uncertainty about the estimates found for antidepressants in this review. Additionally, as only one death by suicide was recorded in these trials, it was not possible to determine whether there is an effect of NGAs on suicide in adults who engage in SH.
We combined results of three trials of antidepressants from different drug classes in this review (i.e. tetracyclic, atypical, and selective serotonin reuptake inhibitors (SSRIs)). Although we acknowledge that these agents have different mechanisms of action, we chose to combined them for the purposes of meta‐analysis in this review on the basis that their potential impacts on SH through reducing levels of depression are likely to be similar and to establish whether there is evidence of a generalised effect of antidepressants in this clinical population. A post‐hoc analysis, furthermore, suggested that no one antidepressant agent was superior to the others in reducing repetition of SH.
Antipsychotics
Based on data from a single relatively old and small trial in patients with a history of multiple episodes of SH without depression or schizophrenia, use of the depot antipsychotic medication flupenthixol may reduce repetition of SH compared with placebo by the post‐intervention assessment (Montgomery 1979).
Based on another single trial, there is probably little or no effect of low‐dose fluphenazine as compared with ultra‐low dose fluphenazine on repetition of SH at post‐intervention (Battaglia 1999).
Mood stabilisers, including lithium
On the basis of data from a single trial of lithium versus placebo (Lauterbach 2008), the evidence remains uncertain as to whether lithium has any effect on repetition of SH compared with placebo. However, the authors claimed there was an effect for suicide. This was based on a post‐hoc analysis of very limited data (i.e. there being no suicides in the lithium treated group, and three in the placebo group), and by taking into account exposure time in each group.
Natural products
Based on a single trial, there is probably little or no effect of omega‐3 essential fatty acids compared with placebo on repetition of SH at post‐intervention. However, fewer patients who received the supplement reported suicidal ideation at follow‐up (Hallahan 2007).
Overall completeness and applicability of evidence
Completeness of evidence
There have been few trials of pharmacological interventions for SH patients (we identified just seven), especially when compared with the number of trials of psychosocial interventions. Therefore, our conclusions are limited to a small range of pharmacological agents.
Three trials focused on drugs from three different classes of antidepressants (Hirsch 1982; Montgomery 1983; Verkes 1998). This is consistent with the high prevalence of depression found in SH patients presenting to clinical services (Hawton 2013), and with evidence that antidepressants are commonly prescribed to SH patients (Carr 2016). However, these trials included relatively older agents (i.e. mianserin, nomifensine, and paroxetine), one of which (i.e. nomifensine) is no longer used in the UK. The antidepressants now most commonly recommended for the treatment of adults diagnosed with moderate to severe depression are SSRIs (Qaseem 2016; Malhi 2015; NICE 2009), but only one drug from this class (i.e. paroxetine) has been specifically evaluated in SH patients to date. Increasingly, serotonin and noradrenaline reuptake inhibitors (SNRIs) are also used for the treatment of depression but no agents from this antidepressant class have so far been evaluated in this clinical population. In addition, there is little evidence that antidepressants reduce the risk of suicide, except in older adults (Stone 2009).
Unfortunately, presence of publication bias could not be evaluated as no meta‐analysis in the present review included 10 or more trials. However, it is notable that one trial was never published in full (Hirsch 1982), whilst a second was not published in a peer‐reviewed journal (Montgomery 1979). Therefore, we cannot rule out the possibility that publication bias may have affected the studies within this review. This is a problem that commonly affects clinical data (Easterbrook 1991).
Whilst all of the included trials reported information on repetition of SH, publication bias may have been more common for the secondary outcomes assessed by this review. However, formal testing of publication bias was not possible due to the small number of trials. None of the trials included further information on adverse effects of pharmacological therapy, other than further SH and suicidal behaviour.
Applicability of evidence
The majority of participants in these trials were female, reflecting the typical pattern for SH in hospital‐presenting populations (Hawton 2008). As no included trial stratified randomisation by sex, however, we were unable to undertake subgroup analyses to investigate whether there was any difference in treatment response between females and males. Given that there are some differences in the motives for SH in as compared to females (Claes 2007), further work on the treatment needs and preferences of males who engage in SH, as well as their experiences of clinical services, and how these may differ from females who engage in SH, is warranted.
The majority of trials included either patients who had all engaged in intentional drug overdoses or self‐poisoning, or samples where the majority had, again reflecting the typical pattern observed in patients who present to general hospitals following SH (Hawton 2007). However, there are other important patient subgroups, such as those who engage in self‐cutting, who may have different treatment needs (Hawton 2004). None of the trials included in this review specifically focused on these patients; although it should be noted that method switching is common in those who engage in repeat episodes of SH (Witt 2019). Five of the seven trials focused on those with a history of repeated SH, which is a particular issue in this clinical population given the association of individuals with a history of repeat episodes having a greater risk of suicide (Zahl 2004). However, no trial investigated impacts of pharmacological interventions for those with an initial episode of SH versus those engaging in repeated SH. We were therefore unable to undertake subgroup analyses to investigate the impact of these interventions by repeater status.
This review focused exclusively on those who engaged in SH. As a result, we have excluded trials in which participants were diagnosed with conditions such as borderline personality disorder but where SH was not required for trial entry. We also excluded trials in which participants engaged in repetitive self‐injurious behaviour in the context of an intellectual disability or developmental disorder (e.g, an autism spectrum disorder). Readers interested in the use of pharmacological interventions for these patient groups are referred to the relevant reviews (Rana 2013; Stoffers‐Winterling 2020).
Quality of the evidence
Certainty of evidence, as assessed using the GRADE approach, was generally low to very low suggesting that further research is likely to have an important impact on our confidence in the estimate of treatment effectiveness, and may in fact change the estimates. This is particularly likely to affect results for those interventions that so far have only been assessed in single trials.
Additionally, using the Cochrane 'Risk of bias' tool, version 2 (Sterne 2019), all trials included in this review possessed some concerns or a high risk of bias in relation to at least one aspect of trial design, with weaknesses most commonly observed with selection of the reported result and measurement of the outcome.
In 2015, the International Committee of Medical Journal Editors (ICMJE) recommended all clinical trials should be pre‐registered in a public trials registry (Witt 2020b). However, as all trials included in this review were published prior to 2015, it was difficult to determine whether data were analysed in accordance with a pre‐specified analysis plan; although no substantial departures were noted for any of these trials. Future trials should provide sufficient detail within the clinical trial register to determine how key outcome(s) are defined and measured to aide in the determination as to whether there has been any substantive changes to the proposed analysis plan, and if so, the reasons for any such departures.
There were also some concerns relating to bias in the measurement of the outcome. This was typically because repetition of SH was based on self‐reported information. Given that around two‐thirds of SH episodes recorded in medical and clinical records are not reported by participants, prevalence estimates derived from self‐reported information alone may underestimate the true rate of SH (Mitchell 2016). By supplementing data on self‐reported SH with information from clinical or medical records, future trials could compare results based on self‐reported information with that obtained from objective sources to investigate what impact, if any, this bias may have had on the estimate of treatment effectiveness.
Lastly, the trials included in this review were, in general, relatively small to detect significant differences in proportions of patients who engage in a repeat episode of SH. We have previously calculated that trials in this field may need to recruit up to a minimum of 1862 participants per arm to detect a significant effect for repetition of SH with 80% power at the conventional alpha level (Witt 2020b). Future trials should therefore supply a priori power calculations to justify their sample size.
Potential biases in the review process
We are confident we have identified all relevant trials of pharmacological interventions for SH in adults. However, we cannot rule out the possibility that some relevant outcome data may be missing from this review. Although data on repetition of SH were available for all of the included trials, limited data were available on secondary outcomes. Only three trials included information on depression, and two on hopelessness and suicidal ideation. Information on suicide was only published in one trial (Lauterbach 2008), and had to be requested from trial authors for the remaining trials. Nevertheless, by using the random‐effects model in all analyses, our results possess greater generalisability than if we had used the fixed‐effect model (Erez 1996).
Agreements and disagreements with other studies or reviews
This review is an update of the 2015 Cochrane Review on pharmacological interventions for SH in adults (Hawton 2015). The previous review included seven trials of four different approaches, finding that there was little evidence of beneficial effects of drug treatments on repetition of SH.
We identified only one other comprehensive review of pharmacotherapy for SH (Smith 2005). This included a wide range of evidence, not just from randomised controlled trials (RCTs). Whilst there were encouraging findings, particularly with regards to mood stabilisers and antipsychotics in this review, effects were less strong in randomised as compared to non‐randomised trials. Two further reviews considered pharmacological interventions for borderline personality disorder including participants both with and without a history of SH (Turner 2014; Stoffers‐Winterling 2020). Whilst one concluded there was some evidence of benefit for atypical antipsychotics in reducing SH on the basis of findings from a single RCT (Turner 2014), the second concluded there was little robust evidence of benefit for any one particular pharmacological agent for SH in this clinical population (Stoffers‐Winterling 2020).
Given the positive effects found for lithium with regard to SH repetition in patients diagnosed with affective disorders (Cipriani 2013a; Smith 2017), there may be a role for lithium for some SH patients. While the negative results of the Lauterbach 2008 trial, which included patients with an 'affective spectrum disorder', would appear to be contrary to this suggestion, it should be noted that there were no suicides in the lithium‐treated group in this trial. Alternatively, as studies in which beneficial effects for lithium have been found have focused on patients diagnosed with either depression or bipolar disorder, rather than those with a history of SH specifically, it may also be that the primary indication for lithium in the prevention of SH is in patients with either of these disorders, rather than SH alone.
Authors' conclusions
Implications for practice.
It is not possible to reach firm conclusions regarding pharmacological interventions for those who engage in self‐harm (SH). While depression may be common in these patients, we found only uncertain evidence that newer generation antidepressants (NGAs) prevent repetition of SH. In addition, the agents evaluated in these trials are older and one, nomifensine, is no longer used in the UK. While there may be low‐certainty evidence of benefit in a single early trial of the depot antipsychotic flupenthixol in those with a history of multiple episodes of SH, this requires replication, preferably involving more modern antipsychotic agents. Additionally, further exploration of the potential mechanism of this effect (e.g. it is possible that depot delivery was a reason for this apparent effect though maintaining stable blood plasma levels) is required.
Clinicians treating SH patients with pharmacological agents must be aware of the extra risks of overdose in this population (Gjelsvik 2014), and especially the relative toxicity of the different agents that might be used. Pharmacological agents associated with lower case fatality indices should therefore be preferred (Hawton 2010).
Implications for research.
While the results of this review did not indicate any benefit of NGAs, the high prevalence of depression in patients who self‐harm (Hawton 2013), the strong association between both depression and SH and suicide, and the frequency with which antidepressants are prescribed to patients following SH (Carr 2016), suggest that there should be further evaluation of antidepressants in this clinical population. This would preferably involve the use of more modern and less toxic antidepressants, which should also be combined with psychosocial interventions. Further evaluation of the potential of lithium is also warranted given encouraging results of trials in patients with affective disorders (i.e. both with and without SH) (Cipriani 2013a; Smith 2017), as well as the uncertainty around the impact of lithium on suicide in the one trial included in this review. Emerging evidence also suggests that lithium may have a superior antisuicidal effect as compared to other mood stabilisers (Hayes 2016). Favourable findings of intravenous ketamine and nasal esketamine in relation to short‐term reductions in suicidal ideation in those with treatment‐resistant depression also suggest a potential role for these agents in SH prevention (Witt 2020a), possibly combined with psychosocial interventions that may help to enhance and sustain these effects.
In view of the paucity of andomised controlled trials (RCTs) of pharmacological agents in patients who engage in SH, perhaps reflecting difficulties in conducting these trials due to safety considerations, valuable information about the potential impacts of pharmacological treatments on suicidal behaviour might be gained from the analysis of linked population‐wide registry data in which information about prescriptions, hospital presentations for SH, and suicide are routinely recorded (House 2020). Additionally, future trials in clinically‐diverse populations (e.g. those with treatment‐resistant depression, suicidal ideation, etc.) could consider stratifying randomisation by presence of SH. That way, relevant data relating to repetition of SH in those with a history of SH could be included in future updates of this review.
Any pharmacological interventions for adults who engage in SH should include a range of outcome measures, not just SH and suicide, but also acceptability, adherence, and attitudes to treatment as these may help to identify contributors to any apparent benefit or lack of impact. In particular, the inclusion of outcomes that matter to those who engage in SH is required to further inform intervention development (Owens 2020). It is also important that adverse effects of treatment medication, both short‐ and long‐term, are carefully evaluated, including possible use of the medication in episodes of further SH (Ferrey 2018; Gjelsvik 2014).
Additionally, from a service planning perspective, future trials should also include economic evaluations in order to determine which interventions may be most feasible to routinely implement in health service settings (Bustamante‐Madsen 2018).
What's new
| Date | Event | Description |
|---|---|---|
| 3 March 2021 | Amended | Minor text edit to add clarity to risk of bias methods and results. |
History
Protocol first published: Issue 7, 2020 Review first published: Issue 12, 2020
| Date | Event | Description |
|---|---|---|
| 2 February 2021 | Amended | Typo corrected and additional risk of bias table added. |
| 13 January 2021 | Amended | Typo corrected in Table 5. |
| 7 January 2021 | New citation required and conclusions have changed | A new protocol including updated methodology was applied (Witt 2020c). No new studies were included in this review compared to the earlier version (Hawton 2015). |
| 7 January 2021 | New search has been performed | This review updates and replaces the Cochrane Review 'Pharmacological interventions for self‐harm in adults' (Hawton 2015). |
| 1 July 2020 | Amended | We updated the protocol developed for Hawton 2015 |
Risk of bias
Risk of bias for analysis 1.1 Repetition of SH by post‐intervention (NGA class).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.1.1 Mianserin vs. Placebo | ||||||||||||
| Montgomery 1983 | Some concerns | "Patients were randomly allocated..." (p.184S). Although it is likely the random sequence was adequately generated, without further information on the method used, this cannot be ascertained. Additionally, no information on allocation concealment were reported. | Some concerns | "Randomly allocated to treatment under double‐blind conditions..." (p.184S). However, no details on clinical personnel blinding were provided. Additionally, whilst there were no apparent deviations from the intended intervention, no information was reported on the method used to estimate the effect of assignment to intervention. | High risk of bias | "Fifty‐eight patients entered the study. There were 20 drop‐outs..." (p.185S). Data were therefore available for 65.5% of those randomised. Analysis methods did not correct for bias, nor were sensitivity analyses undertaken to investigate the potential effect of missing data and no information on causes of missingness in the outcome were reported. | Low risk of bias | Repetition of SH was based on those who "...repeated their suicidal behaviour" (p.185S). However, no further information as to how this outcome was ascertained was reported. However, repetition of SH was likely ascertained in the same way for both the intervention and comparator arms and the trial was conducted "...under double‐blind conditions..." (p.184S). | Some concerns | No information reported. | High risk of bias | Overall, this trial was rated as high risk of bias as missingness in the outcome likely depended on its true value and analyses did not correct for bias, nor were sensitivity analyses undertaken to investigate the potential effect of missing data. |
| Subgroup 1.1.2 Paroxetine vs. Placebo | ||||||||||||
| Verkes 1998 | Low risk of bias | "Patients were randomly assigned..." (p.544). Correspondence with trial authors further clarified that all the medication was packed in a series of blisters with consecutive numbers. This was done before the start of the study. A patient who entered the study got a consecutive number and subsequently received the medication blisters with the corresponding number. Correspondence with trial authors also revealed that randomisation was conducted remotely at the pharmacy of SmithKline Beecham. Additionally, "[r]andom assignment produced comparable groups" (p.544). | Low risk of bias | "Patients in the placebo group received matching placebo" (p.544). The nature of this trial means that participants and clinical personnel could have been blinded to allocation, possibly through the use of identical capsules for both the active agent and placebo arms. Additionally, "[e]fficacy was analyzed on an intention‐to‐treat basis..." (p.544). | Low risk of bias | Data were available for 100% of those randomised. Additionally, "[e]fficacy was analyzed on an intention‐to‐treat basis; all available observations were used without the last point carried forward or estimating missing values. We also analyzed efficacy excluding the non‐compliant visits" (p.544). | Low risk of bias | "Recurrence of suicidal behavior..." However, no further information as to how this outcome was ascertained was reported. However, repetition of SH was likely ascertained in the same way for both the intervention and comparator arms. Additionally, the trial is described as a "...double‐blind...study" (p.546). | Some concerns | No information was reported on whether this result was analysed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis. Although, "[r]ecurrence of suicidal behavior...were assessed at baseline, after weeks 2, 4, 6, 8, 10, and 12 of treatment, and at monthly intervals thereafter until 52 weeks" (p.544) and all eligible outcome measurements within the outcome domain were reported. Additionally, all eligible analyses of the data were reported. | Some concerns | Overall, this trial was rated as some concerns for risk of bias as no specific information was reported as to whether the data that produced this result analysed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis. |
Acknowledgements
The authors thank the following trial authors for providing us with unpublished data: Brian Hallahan and John Rush.
The authors and the CCMD Editorial Team are also grateful to the following peer reviewers for their time and comments: Gregory Carter, Nick Meader, Hector Pardo‐Hernandez, Sue Rees and Mark Sinyor. They would also like to thank Cochrane Copy Edit Support for the team's help and Phil Roberts from the University of York for graphic design support in the production of Figure 4 to 11.
This project has previously been supported by the National Co‐ordinating Centre for NHS Service Delivery and Organisation R&D (NCCSDO). KH is funded by Oxford Health NHS Foundation Trust. He is a National Institute for Health Research (NIHR) Emeritus Senior Investigator and used personal funding from NIHR to help support this update.
The NIHR is the largest single funder of the CCMD Group.
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Appendices
Appendix 1. Cochrane Common Mental Disorders Group Specialized Register
The Cochrane Common Mental Disorders Group (CCMD) maintains an archived controlled trials register known as the CCMDCTR. This specialized register contains over 40,000 reference records (reports of RCTs) for anxiety disorders, depression, bipolar disorder, eating disorders, self‐harm, and other mental disorders within the scope of this Group. The CCMDCTR is a partially studies‐based register with more than 50% of reference records tagged to around 12,500 individually PICO‐coded study records. Reports of studies for inclusion in the register were collated from (weekly) generic searches of key bibliographic databases to June 2016, which included: MEDLINE (1950 onwards), Embase (1974 onwards), PsycINFO (1967 onwards), quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), and review‐specific searches of additional databases. Reports of studies were also sourced from international trials registries, drug companies, the handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses. Details of CCMD's core search strategies (used to identify RCTs) are on the Group's website, with an example of the core MEDLINE search displayed below.
[MeSH Headings]: eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/ OR [Title/ Author Keywords]: (eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).tw,kf. AND [RCT filter]: (controlled clinical trial.pt. or randomised controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomised controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.)
Records were screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs were tagged to the appropriate study record
An information specialist with CCMD cross‐searched the CCMDCTR‐Studies and References register using the following terms (all fields):
(suicid* or parasuicid* or "auto mutilat*" or automutilat* or "self destruct*" or selfdestruct* or self‐harm* or selfharm* or "self immolat*" or selfimmolat* or "self inflict*" or selfinflict* or "self injur*" or selfinjur* or selfmutilat* or "self mutilat*" or "self poison*" or selfpoison* or (self adj2 (cut or cuts or cutting or cutter? or burn or burns or burning or bite or bites or biting or hit or hits or hitting)) or "head bang*" or headbang* or "over dose*" or overdos* or NSSI* or nonsuicid* or non‐suicid*) (2015‐June‐2016) n=291
Appendix 2. MEDLINE, Embase, PsycINFO Ovid search strategy
An information specialist with CCMD searched the main bibliographic, biomedical databases using the terms listed below from January 2015 to 4‐July‐2020. [N.B. CCMDCTR is current to June 2016 only]
Search summary
Date‐of‐search: 4‐July‐2020
Cochrane Library (CDSR) Systematic Reviews, n=38
Cochrane Library (CDSR) Protocols, n=12
Cochrane Library CENTRAL, n=2727
Cochrane Specialised Register (CCMDCTR), n=291
Ovid MEDLINE, PsycINFO (cross‐search), n=2743
Ovid Embase (precise), n=1375
Total=7186 Duplicates removed, n=2483 To screen, n=4703 [Cochrane Library CENTRAL‐Trial Register Records (removed) n=1969] Cochrane Library (Issue 7 of 12, 2020) [Date limited, 2015 onwards] #1 MeSH descriptor: [Self‐Injurious Behavior] explode all trees #2 (overdose* and prevent*):kw or (overdos* near/3 prevent*):ti,ab #3 ((nonfatal or non‐fatal) near/2 (overdose* or over dose*)):ti,ab,kw #4 (NSSI* or ((nonsuicid* or non‐suicid*) near/2 (self* or injur*))):ti,ab #5 (suicid* or parasuicid* or (auto next mutilat*) or automutilat* or (self next destruct*) or selfdestruct* or self‐harm* or selfharm* or (self next harm*) or (self next immolat*) or selfimmolat* or (self next inflict*) or selfinflict* or (self next injur*) or selfinjur* or selfmutilat* or (self next mutilat*) or (self next poison*) or selfpoison* or (self near/2 (cut or cuts or cutting or cutter* or burn or burns or burning or bite or bites or biting or hit or hits or hitting)) or (head next bang*) or headbang*):ti,ab,kw #6 (#1 or #2 or #3 or #4 or #5) Limited 2015 to date CDSR‐reviews (38); CDSR‐protocols (12); CENTRAL (2727); CENTRAL‐TR (1969) *************************** PsycINFO/MEDLINE cross‐search
Ovid APA PsycInfo <1806 to June Week 5 2020>, Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily <1946 to July 02, 2020> [Date limited, 2015 onwards] Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 Automutilation/ or Self‐injurious Behavior/ or Self‐destructive Behavior/ or Self‐mutilation/ or Self‐inflicted Wounds/ (19601) 2 Suicidal Behavior/ or Suicide/ or Suicidal Ideation/ or Attempted Suicide/ or Suicide, Attempted/ or Self Poisoning/ or Suicide Prevention/ or Suicide Prevention Centers/ or Suicidology/ (97875) 3 (suicid* or parasuicid* or auto mutilat* or automutilat* or self destruct* or selfdestruct* or self‐harm* or selfharm* or self immolat* or selfimmolat* or self inflict* or selfinflict* or self injur* or selfinjur* or selfmutilat* or self mutilat* or self poison* or selfpoison* or (self adj2 (cut or cuts or cutting or cutter? or burn or burns or burning or bite or bites or biting or hit or hits or hitting)) or head bang* or headbang*).ti,ab,kf,kw,id. (164244) 4 (NSSI? or ((nonsuicid* or non‐suicid*) adj2 (self* or injur*))).ti,ab,kf,kw,id. (3469) 5 (Overdose/ or Drug Overdose/ or Drug Overdoses/) and prevent*.af. (3529) 6 ((nonfatal or non‐fatal) adj2 (overdose? or over dose?)).mp. (571) 7 or/1‐6 (183505) 8 Randomized Controlled Trial/ (509272) 9 Randomized Controlled Trial.pt. (508805) 10 Randomization/ (103117) 11 Random Allocation/ (103117) 12 Controlled Clinical Trial/ (93744) 13 Controlled Clinical Trial.pt. (93744) 14 Double‐blind Method/ or Single‐blind Method/ (186347) 15 (randomi#ed or randomi#ation or randomi#ing).ti,ab,kf,kw,id. (726423) 16 (RCT or "at random" or (random* adj3 (administ* or allocat* or assign* or class* or cluster or crossover or cross‐over or control* or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or pragmatic or quasi or recruit* or split or subsitut* or treat*))).ti,ab,kf,kw,id. (667814) 17 trial.ti. (251482) 18 placebo/ or (placebo and (allocat* or assign* or control* or group*)).ti,ab,kf,kw,id. (204672) 19 (control* adj3 group*).ab. (634930) 20 (control* and (trial or study or group*) and (waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,kf,kw,id. (32182) 21 ((single or double or triple or treble) adj2 (blind* or mask* or dummy)).ti,ab,kf,kw,id. (200109) 22 treatment effectiveness evaluation/ (24511) 23 or/8‐22 (1833008) 24 7 and 23 (9906) 25 (2015* or 2016* or 2017* or 2018* or 2019* or 2020*).yr,dc,dp,dt,ep,ez. (7909383) 26 24 and 25 (3732) 27 remove duplicates from 26 (2743) *************************** Ovid Embase <1974 to 2020 Week 26> [Date limited, 2015 onwards] Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 Automutilation/ (17795) 2 suicidal behavior/ or self immolation/ or self poisoning/ or suicidal ideation/ or suicide/ or suicide attempt/ (101066) 3 Drug Overdose/ and prevent*.af. (4897) 4 (suicid* or parasuicid* or auto mutilat* or automutilat* or self destruct* or selfdestruct* or self‐harm* or selfharm* or self immolat* or selfimmolat* or self inflict* or selfinflict* or self injur* or selfinjur* or selfmutilat* or self mutilat* or self poison* or selfpoison* or (self adj2 (cut or cuts or cutting or cutter? or burn or burns or burning or bite or bites or biting or hit or hits or hitting)) or head bang* or headbang*).ti,kw. (62383) 5 (NSSI? or ((nonsuicid* or non‐suicid*) adj2 (self* or injur*))).ti,ab,kw. (1786) 6 ((nonfatal or non‐fatal) adj2 (overdose? or over dose?)).mp. (418) 7 or/1‐6 (125188) 8 randomized controlled trial/ (608057) 9 randomization.de. (87068) 10 controlled clinical trial/ and (Disease Management or Drug Therapy or Prevention or Rehabilitation or Therapy).fs. (253859) 11 *clinical trial/ (17606) 12 placebo.de. (351476) 13 placebo.ti,ab. (307193) 14 trial.ti. (301646) 15 (randomi#ed or randomi#ation or randomi#ing).ti,ab,kw. (917476) 16 (RCT or "at random" or (random* adj3 (administ* or allocat* or assign* or class* or cluster or control* or crossover or cross‐over or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or pragmatic or quasi or recruit* or split or subsitut* or treat*))).ti,ab,kw. (769731) 17 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$ or dummy)).mp. (309225) 18 (control* and (study or group?) and (waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,kw,hw. (39665) 19 or/8‐18 (1732296) 20 ((animal or nonhuman) not (human and (animal or nonhuman))).de. (5683745) 21 19 not 20 (1575127) 22 7 and 21 (8502) 23 (2015* or 2016* or 2017* or 2018* or 2019* or 2020*).yr,dc,dp. (9329153) 24 22 and 23 (3277) 25 limit 24 to exclude medline journals (353) 26 *Automutilation/ (7770) 27 *suicidal behavior/ or *self immolation/ or *self poisoning/ or *suicidal ideation/ or *suicide/ or *suicide attempt/ (49956) 28 *Drug Overdose/ and prevent*.af. (984) 29 4 or 5 or 6 or 26 or 27 or 28 (72349) 30 21 and 29 (2692) 31 23 and 30 (1132) 32 25 or 31 (1375) ***************************
Appendix 3. Full 'Risk of bias' assessments for each study
Full 'Risk of bias' assessments for each study, including the evidence we used to justify our ratings can be found in Figure 7; Figure 8; Figure 9; Figure 10; Figure 11; Figure 12; and Figure 13.
4.
'Risk of bias' assessment for Battaglia 1999
5.
'Risk of bias' assessment for Hallahan 2007
6.
'Risk of bias' assessment for Hirsch 1982
7.
'Risk of bias' assessment for Lauterbach 2008
8.
'Risk of bias' assessment for Montgomery 1979
9.
'Risk of bias' assessment for Montgomery 1983
10.
'Risk of bias' assessment for Verkes 1998
See Figure 14 for the signalling questions.
11.
Data and analyses
Comparison 1. Newer generation antidepressants (NGAs).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Repetition of SH by post‐intervention (NGA class) | 2 | 129 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.29, 1.19] |
| 1.1.1 Mianserin vs. Placebo | 1 | 38 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.18, 2.41] |
| 1.1.2 Paroxetine vs. Placebo | 1 | 91 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.24, 1.29] |
| 1.2 Repetition of SH at final follow‐up (by NGA class) | 3 | 243 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.42, 1.35] |
| 1.2.1 Mianserin vs. Placebo | 2 | 95 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.39, 2.99] |
| 1.2.2 Nomifensine vs. Placebo | 1 | 57 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.17, 3.81] |
| 1.2.3 Paroxetine vs. Placebo | 1 | 91 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.24, 1.29] |
| 1.3 Treatment acceptability | 2 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.29, 1.71] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Battaglia 1999.
| Study characteristics | ||
| Methods | Double‐blind RCT. Participants were individually assigned to either 'low' dose (i.e., 12.5 mg/monthly) or 'ultra‐low' dose (i.e., 1.5 mg/monthly) of intramuscular fluphenazine decanoate injections. Follow‐up period: 6 months. N lost to follow‐up: 5/58 (8.6%) for repetition of SH. |
|
| Participants |
Number of total participants: 58 participants were randomised, 30 were allocated to the intervention arm (i.e., 12.5 mg/monthly of intramuscular fluphenazine decanoate) and 28 to the control arm (i.e., 1.5 mg/monthly of intramuscular fluphenazine decanoate). Profile of participants: all (n = 58; 100%) had multiple episodes of SH prior to trial entry. Almost one‐half (n = 28; 44%) were female. The majority were diagnosed with substance misuse disorders (n = 45; 79%), followed by any mood disorder (n = 20; 35%), and any anxiety disorder (n = 17; 29%). Source of participants: patients presenting to a psychiatric hospital screened for a history of suicide attempts. Inclusion criteria: i) aged between 18‐65 years; ii) receiving treatment for a suicide attempt that occurred within 30 days prior to trial entry; iii) ≥ 2 prior suicide attempts; iv) able to read English. Exclusion criteria: i) allergic/hypersensitive to fluphenazine; ii) diagnosed with tardive dyskinesia; iii) a history of neuroleptic malignant syndrome; iv) diagnosed with narrow angle glaucoma; v) diagnosed with schizophrenia; vi) diagnosed with any terminal illness with less than 1 year life expectancy; vii) pregnant or of childbearing age and not using effective birth control; viii) current/expected to continue with treatment using medications with psychotropic effects. |
|
| Interventions |
Intervention: 12.5 mg/monthly of intramuscular fluphenazine decanoate administered by intramuscular injection. Control: 1.5 mg/monthly of intramuscular fluphenazine decanoate administered by intramuscular injection. Concomitant medication(s): benztropine was administered, as necessary, to prevent extra‐pyramidal symptoms. It is unclear what proportion of the intervention and control groups were using concomitant medications, however. Length of treatment: six months. Location: Dallas, Texas, USA. |
|
| Outcomes |
Primary outcome(s): repetition of SH according to self‐report. Secondary outcome(s): i) adverse effects, as measured by the Abnormal Involuntary Movement Scale; ii) alcohol and other drug use, as measured by an idiosyncratic checklist developed by the authors; iii) suicide (unclear how ascertained). |
|
| Notes |
Funding: “This research was supported in part by a grant from The National Institute of Mental Health (MH‐53799) and by Mental Health Connections, a partnership between Dallas County Mental Health Mental Retardation and the Department of Psychiatry at the University of Texas South‐Western Medical Centre. Funding was from the Texas State Legislature and Dallas Country Mental Health and Mental Retardation” (Battaglia 1999, p.370). Conflict(s) of interest: no details provided. Other: data on suicides were obtained following correspondence with authors. |
|
Hallahan 2007.
| Study characteristics | ||
| Methods | Double‐blind placebo‐controlled RCT. Participants were individually assigned via a computer generated list to either 2128mg/day of EPA plus DHA (i.e., participants received four capsules each containing 305mg EPA and 227 DHA) or four capsules containing 99% corn oil and a 1% EPA/DHA mixture (to control for the 'fishy breath' side effect associated with the intervention treatment). Follow‐up period: 12 weeks. N lost to follow‐up: 0/49 (0%) for repetition of SH. |
|
| Participants |
Number of total participants: 49 participants were randomised, 22 were allocated to the intervention arm (i.e., 2128 mg/day of EPA plus DHA) and 27 to the control arm (i.e., four capsules containing 99% corn oil and a 1% EPA/DHA mixture). Profile of participants: all (n = 49; 100%) had multiple episodes of SH prior to trial entry. The majority were female (n = 32; 65%). The majority were diagnosed with any personality disorder (n = 40; 81.6%), followed by borderline personality disorder specifically (n = 35; 71.4%), and alcohol misuse disorder ( n = 20; 41%). Around one‐half (n = 26; 53%) were taking psychotropic medication. Source of participants: patients presenting to hospital following an episode of SH. Inclusion criteria: i) presenting to hospital following an episode of SH; ii) at least one previous episode of SH; iii) living inside the catchment area. Exclusion criteria: i) current history of addiction, substance abuse, psychosis, or any eating disorder; ii) currently receiving psychotherapy; iii) history of dyslipidaemia; iv) involved with any treatment, diet, or illness known to interfere with lipidorn‐3EFA metabolism; v) weight loss greater than 10% over the previous 3 months; vi) taking supplements containing n‐3 EFAs or have consumed fish more than once per week; vii) changes to, or introduction of, psychotropic medication during the previous 6 weeks. |
|
| Interventions |
Intervention: four capsules containing 305 mg EPA and 227mg DHA. Total dose equalled 2128 mg per day of EPA plus DHA. Control: four capsules per day consisting of 99% corn oil and a 1% EPA/DHA mixture. Concomitant medication(s): were permitted, however, details of these were not provided. Around one‐half of both the intervention (n = 13; 59.1%) and placebo (n = 13; 55.6%) groups were using concomitant psychiatric medications. Length of treatment: 12 weeks. Location: Dublin, Republic of Ireland. |
|
| Outcomes |
Primary outcome(s): i) repetition of SH according to hospital records. Secondary outcome(s): i) depression, as measured by the BDI; ii) suicidal ideation, as measured by the Overt Aggression Scale, Modified (OAS‐M); iii) suicide (unclear how ascertained); iv) treatment adherence, as measured by pill counts; v) aggressive behaviour, as measured by the OAS‐M; vi) impulsivity, as measured by the Immediate and Delayed Memory Tasks; vii) daily stresses, as measured by the Perceived Stress Scale and the Daily Hassles and Uplifts Scale. |
|
| Notes |
Funding: “B.H. received salary support from the Department of Psychiatry, University of Illinois at Chicago, USA” (Hallahan 2007, p.122). Conflict(s) of interest: “Pronova (now Epax) AS, Lysaker, Norway, provided the active preparation and placebo but were not otherwise involved in the study” (Hallahan 2007, p.118). No other conflicts of interest were stated. |
|
Hirsch 1982.
| Study characteristics | ||
| Methods | Double‐blind placebo‐controlled RCT. Participants were individually assigned to either 30‐60mg/day of mianserin, 75‐150mg/day of nomifensine, or placebo (no further information on placebo provided). Follow‐up period: 12 weeks. N lost to follow‐up: 0/114 (0%) for repetition of SH. |
|
| Participants |
Number of total participants: 114 participants, 38 were allocated to the mianserin arm, 38 were allocated to the nomifensine arm, and 38 to the placebo arm. Profile of participants: not stated. Source of participants: patients admitted to hospital following an episode of intentional drug overdose. Inclusion criteria: i) General Health Questionnaire (GHQ) score of over 20. Exclusion criteria: i) currently receiving antidepressant or antipsychotic medication. |
|
| Interventions |
Intervention: oral administration of either 30 mg/day tp 60 mg/day of mianserin or 75 mg/day t 150 mg/ day of nomifensine. Control: placebo. No further information provided. Concomitant medication(s): no information on whether concomitant medication(s) were permitted was provided. Length of treatment: six weeks. Location: London, UK. |
|
| Outcomes |
Primary outcome(s): i) repetition of SH (unclear how ascertained). Secondary outcome(s): i) depression, as measured by the HDRS, ii) adverse events, as measured by the Symptoms Emergent Checklist; iii) life events, as measured by the Brief Life Events Scale; iv) general health, as measured by the GHQ; v) treatment adherence, as measured by pill counts; vi) suicide (unclear how ascertained). |
|
| Notes |
Funding: no details on funding provided. Conflict(s) of interest: no details on conflicts of interest provided. Other: data on depression and treatment adherence had to be excluded due to an inability to collect unpublished data from the trial authors for these outcomes. |
|
Lauterbach 2008.
| Study characteristics | ||
| Methods | Double‐blind placebo‐controlled RCT. Participants were individually assigned via a computerised sequence to either oral administration of lithium carbonate or placebo (no further information on placebo provided). Follow‐up period: 14 weeks. N lost to follow‐up: 22/169 (13.0%) at one month; 50/169 (29.6%) at three months; 62/169 (36.7%) at six months; 97/169 (57.4%) at 12 months. |
|
| Participants |
Number of total participants: 167 participants, 84 were allocated to the lithium arm, and 83 were allocated to the placebo arm. Profile of participants: 44.3% (n = 74) had multiple episodes of SH prior to trial entry. Over one‐half (n = 96; 57.5%) were female. Around three‐quarters (n = 123; 76%) were diagnosed with major depression. Source of participants: patients admitted to one of five participating emergency departments following a suicide attempt. Inclusion criteria: i) at least 18 years of age; ii) suicide attempt within three months prior to first drug administration; iii) suicide attempt occurred within the context of a depressive spectrum disorder; iv) able to complete the screening and baseline assessment protocols; v) able to provide written informed consent. Exclusion criteria: i) diagnosed with a disorder associated with frequent suicidal behaviour (e.g., schizophrenia, borderline personality disorder, severe SH and/or substance‐related disorders, including current substance addictions); ii) diagnosed with any disorder indicated for long‐term lithium treatment; iii) diagnosed with any disorder for which lithium treatment is contraindicated; iv) any other contraindications (e.g., pregnant, breast‐feeding, etc.) |
|
| Interventions |
Intervention: oral administration of lithium carbonate according to a fixed schedule of dose augmentation (i.e., 200 mg/week) until an effective blood level of between 0.6 mm/L to 0.8 mmol/L had been achieved. For most participants, this level was achieved in 34 weeks. Participants received, in addition, TAU involving consultations with physicians in the community and referral to psychiatric treatment as necessary. Control: oral administration of a placebo capsule in addition to TAU involving consultations with physicians in the community and referral to psychiatric treatment as necessary. Ingredients for the placebo capsule are not provided. Concomitant medication(s): were permitted, however, details of these were not provided including details on the proportion of the intervention and placebo groups using concomitant medications. Length of treatment: 12 months. Location: Germany. |
|
| Outcomes |
Primary outcome(s): i) repetition of SH according to self‐report; ii) suicide (unclear how ascertained). Secondary outcome(s): i) depression, as measured by the HDRS; ii) hopelessness, as measured by the BHS; iii) suicidal ideation, as measured by the SSI. |
|
| Notes |
Funding: “This research was supported by grants 01GI 9920 and 01GI 0220 from the German Ministry for Education and Research within the promotional emphasis 'German Research Network on Depression’ (subproject 1.2), and German Research Foundation grant LA 1975/2‐1 to Erik Lauterbach. Additional funding was granted by Sanofi‐Aventis” (Lauterbach 2008, p.477). Conflict(s) of interest: “Dr. Ahrens has received a research grant from Sanofi Aventis” (Lauterbach 2008, p.477). Other: data for depression, hopelessness, and suicidal ideation had to be requested from the trial authors. |
|
Montgomery 1979.
| Study characteristics | ||
| Methods | Double‐blind placebo‐controlled RCT. Participants were individually assigned to either an intramuscular injection of 20 mg/month flupenthixol decanoate or placebo (no further information on placebo provided). Follow‐up period: six months. N lost to follow‐up: 7/37 (18.9%) for repetition of SH. |
|
| Participants |
Number of total participants: 37 participants, 18 were allocated to intramuscular flupenthixol decanoate and 19 to placebo. Profile of participants: all (n = 37; 100%) had multiple episodes of SH prior to trial inclusion. The majority (n = 26; 70.3%) were female. Source of participants: patients admitted to a general hospital following a suicidal act. Inclusion criteria: i) documented history of two or more episodes of SH. Exclusion criteria: i) diagnosis of depression or schizophrenia; ii) diagnosis of an organic illness. |
|
| Interventions |
Intervention: intramuscular administration of 20 mg/month of flupenthixol decanoate. Control: placebo. No further information provided. Concomitant medication(s): no information on whether concomitant medication(s) were permitted was provided. Length of treatment: six months. Location: Maidstone, UK. |
|
| Outcomes |
Primary outcome(s): i) repetition of SH (unclear how ascertained). Secondary outcome(s): i) treatment adherence, as measured by the number of participants who completed the full course of treatment; ii) adverse effects (unclear how ascertained). |
|
| Notes |
Funding: no details on funding were provided. Conflict(s) of interest: no details on author interests were provided. |
|
Montgomery 1983.
| Study characteristics | ||
| Methods | Double‐blind placebo‐controlled RCT. Participants were individually assigned to either 30mg/day mianserin or placebo (no further information on placebo provided). Follow‐up period: six months. N lost to follow‐up: 0/58 (0%) for repetition of SH. |
|
| Participants |
Number of total participants: 58 participants, 17 were allocated to the mianserin arm, and 21 to the placebo arm. Profile of participants: all (n = 58; 100%) had multiple episodes of SH prior to trial entry. Two‐thirds (n = 38; 66%) were female. All (n = 58; 100%) had been diagnosed with borderline or histrionic personality disorder. Source of participants: patients admitted to a medical ward following SH. Inclusion criteria: i) multiple previous episodes of SH; ii) diagnosis of personality disorder. Exclusion criteria: i) diagnosis of depression or schizophrenia. |
|
| Interventions |
Intervention: oral administration of 3 0mg/day mianserin. Control: placebo. No further information provided. Concomitant medication(s): no information on whether concomitant medication(s) were permitted was provided. Length of treatment: six months. Location: London, UK. |
|
| Outcomes |
Primary outcome(s): i) repetition of SH (unclear how ascertained). Secondary outcome(s): i) depression, as measured by the MADRS; ii) treatment adherence, as measured by pill counts. |
|
| Notes |
Funding: no details on funding were provided. Conflict(s) of interest: no details on author interests were provided. Other: data on depression had to be excluded due to an inability to collect unpublished data from the trial authors for this outcome. |
|
Verkes 1998.
| Study characteristics | ||
| Methods | Double‐blind placebo‐controlled RCT. Participants were individually assigned to either 40mg/day paroxetine plus weekly/fortnightly TAU or placebo (no further information on placebo provided) plus TAU. Follow‐up period: 12 months. N lost to follow‐up: 0/91 (0%) for repetition of SH data. |
|
| Participants |
Number of total participants: 91 participants, 46 were allocated to the paroxetine arm, and 45 were allocated to the placebo arm. Profile of participants: all (n = 91; 100%) had engaged in multiple episodes of SH prior to trial entry. Over one‐half (n = 54; 59.3%) were female. The majority (n = 84; 92%) were diagnosed with a personality disorder, followed by an alcohol use disorder (n = 40; 44%), any depressive disorder (n = 23; 25.3%), adjustment disorder (n = 19; 20.9%), any dissociative disorder (n = 8; 8.8%), dysthymia (n = 6; 6.5%), any anxiety disorder (n = 4; 4.4%). A minority (n = 15; 16.5%) had no formal psychiatric diagnosis. Source of participants: patients were recruited from outpatient departments in accident and emergency wards of university hospitals. Inclusion criteria: i) aged 18 years and over; ii) previous history of SH. Exclusion criteria: i) current diagnosis of major depression. |
|
| Interventions |
Intervention: oral administration of paroxetine 40 mg/day, plus weekly or fortnightly psychotherapy TAU. Control: placebo (no further information provided) plus weekly or fortnightly psychotherapy TAU. Concomitant medication(s): one‐half (n = 24; 52.2%) of the intervention group used concomitant benzodiazepine medications during the trial, compared to 62.2% (n = 28) of those allocated to placebo. Length of treatment: 12 months. Location: Leiden and Rotterdam, the Netherlands. |
|
| Outcomes |
Primary outcome(s): i) repetition of SH (unclear how ascertained). Secondary outcome(s): i) depression, as measured by the BDI; ii) hopelessness, as measured by the BHS; iii) anger, as measured by the State‐Trait Anger Expression Inventory; iv) treatment adherence, as measured by pill counts and platelet serotonin levels; v) adverse events (unclear how ascertained); vi) side effects (unclear how ascertained); vii) suicide (unclear how ascertained). |
|
| Notes |
Funding: “Supported by grant 89‐110 CRO 012859 from the Dutch Ministry of Welfare, Health, and Cultural Affairs and a grant from SmithKline Beecham Pharmaceuticals” (Verkes 1998, p.543). Conflict(s) of interest: no details on author interests are provided. Other: trial authors note that “the number of previous suicide attempts...was manifestly associated with the risk of another suicide attempt.... With adjustment for this important predictive characteristic, paroxetine proved to reduce the recurrence of suicidal behaviour significantly” (Verkes 1998, p.544‐555). |
|
BDI: Beck Depression Inventory; BHS: Beck Hopelessness Scale; DHA: docosahexaenoic acid; EFA: essential fatty acid; EPA: eicosapentaenoic acid; GHQ: General Health Questionnaire; HDRS: Hamilton Depression Rating Scale; MADRS: Montgomery Åsberg Depression Rating Scale; mmol/L: millimoles per litre; mg: milligrams; RCT: randomised controlled trial; SH: self‐harm; SSI: Scale for Suicidal Ideation; TAU: treatment as usual.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bozzatello 2017 | At baseline, < 100% of the sample had engaged in SH. |
| Crawford 2018 | At baseline, 53.3% had engaged in SH within six months of trial entry. |
| Domany 2019 | At baseline, < 100% of the sample had engaged in SH. |
| Grant 2016 | Participants were diagnosed with skin‐picking disorder. |
| Grunebaum 2012 | At baseline, < 100% of the sample had engaged in SH. |
| Marriott 2016 | Study protocol. However, unlikely that all participants will have engaged in SH at baseline. |
| McCall 2018 | At baseline, < 100% of the sample had engaged in SH. |
| NCT00065936 | Trial registration record last verified 1 May 2003. Current trial status therefore unknown. |
| NCT00533117 | At baseline, < 100% had engaged in SH within six months of trial entry. |
| NCT00539188 | Trial terminated early due to poor participant adherence. |
| NCT01103180 | Trial terminated due to feasibility problems in identifying and recruiting participants. |
| NCT01928446 | Trial terminated early due to Data Monitoring Committee (DMC) recommendations. |
| NCT02039479 | Trial registration record last verified 1 March 2018. Current trial status therefore unknown. |
| Oquendo 2011 | At baseline, < 100% had engaged in SH within six months of trial entry. |
| Yovell 2016 | At baseline, 64.5% of the sample had engaged in SH. |
DMC: data monitoring committee; SH: self‐harm.
Characteristics of studies awaiting classification [ordered by study ID]
NCT00834834.
| Methods |
Study name: Comparing treatments for self‐injury and suicidal behavior in people with borderline personality disorder. Study design: Single (outcome assessor) blind, active‐controlled, RCT. Participants were individually assigned to either either up to 40 mg/day fluoxetine, up to 60mg/day citalopram, or six months of manualised DBT, consisting of weekly (60 min) sessions of individual therapy and weekly (90 min) sessions of group‐based therapy sessions. Follow‐up period: 12 months. |
| Participants |
Number of total participants: 84 participants, 28 were allocated to the fluoxetine arm, 28 were allocated to the citalopram arm, and 28 were allocated to the DBT arm. Source of participants: patients were recruited from outpatient departments. Inclusion criteria: i) aged 18 to 65 years; ii) suicide attempt in the two months prior to trial entry; ii) ≥1 additional suicide attempt and/or episode of SH (including NSS) in the year prior to trial entry; iii) reports current suicidal ideation; iv) able to be managed on an outpatient basis; v) not currently receiving optimum psychiatric treatment and agrees to notify study staff if any psychiatric care outside this study is sought; vi) has a stable living arrangement; vii) sufficient language ability; viii) willing and judged to be clinically able to undergo wash‐out of psychotropic medications, except for occasional benzodiazepines use, for two to six weeks before treatment; ix) willing to use an effective method of birth control (female participants). Exclusion criteria: i) meets DSM‐IV criteria for mental retardation, bipolar I disorder, schizophrenia, delusional disorder, schizophreniform disorder, schizoaffective disorder, or psychosis NOS; ii) requires treatment for an acute medical illness, including severe substance dependence and anorexia; iii) clinically too unstable to be managed in an outpatient setting; iv) requiring care other than that permitted by the protocol; v) failed adequate trials of fluoxetine and citalopram for an episode of major depression in the two years prior to trial entry; vi) history of severe allergies, adverse drug reactions, or known allergy to fluoxetine or citalopram; vii) has a heart pacemaker body implant, and/or other metal implants that may present a risk to the participant and/or interfere with the fMRI scan; viii) has claustrophobia or significant discomfort in enclosed space; ix) diagnosed with hypertension, cardiovascular disease, or abnormal EKGs; x) diagnosed with Raynaud's disorder; xi) pregnant. |
| Interventions |
Intervention (fluoxetine): participants received a starting dose of 20 mg/day increased over four weeks, depending on tolerability, to a maximum of 40 mg/day. Intervention (citalopram): participants received a starting dose as determined by the study psychiatrist increased to up to a maximum of 60 mg/day. Active comparator: manualised DBT consisting of weekly (60 min) individual therapy sessions and weekly (90 min) group therapy sessions delivered over a six month period. Length of treatment: six months. |
| Outcomes |
Primary outcome(s): i) frequency of repeat episodes of SH ascertained using the Columbia Suicide History Interview (CSHI). Secondary outcome(s): i) repetition of SH ascertained using the CSHI. |
| Notes |
Source(s) of funding: National Institute of Mental Health (NIMH). Conflict(s) of interest: none reported. Other: whilst preliminary data for this trial has been published on ClinicalTrials.gov, we were unable to confirm trial eligibility despite several efforts to contact the trial authors. |
CSHI: Columbia Suicide History Inventory; DBT: dialectical behaviour therapy; DSM‐IV: Diagnostic and Statistical Manual of Mental disorders, Fourth version; EKG: electrocardiogram; fMRI: functional Magnetic Resonance Imagining; mg: milligrams; NOS: not otherwise specified; NSSI: non‐suicidal self‐injury; SH: self‐harm.
Characteristics of ongoing studies [ordered by study ID]
NCT04005053.
| Study name | Identifying biological signatures of N‐Acetylcysteine for non‐suicidal self‐injury in adolescents and young adults. |
| Methods | Triple‐blind placebo‐controlled RCT. Assignment: parallel, individual‐level. |
| Participants |
Inclusion criteria: i) aged 16 to 24 years; ii) at least one episode of NSSI in the two months preceding trial entry; iii) ≥5 past episodes of NSSI with evidence of significant tissue damage (e.g., scars); iv) if in receipt of any psychotropic medications, participants need to be dose‐stable for at least one month prior to trial entry; v) able to understand and comply with study procedures. Exclusion criteria: i) any MRI contraindications (e.g. metal plates, claustrophobia, braces, implanted devices); ii) current serious medical illness; iii) current substance use disorder (except tobacco use); iv) diagnosed with any psychosis; v) diagnosed with any neurodevelopmental disorder; vi) have taken N‐acetylcysteine or glutathione regularly in the 6 months preceding study entry; vii) currently pregnant, planning to become pregnant, breast‐feeding, or unwilling to use contraception; viii) allergy/sensitivity to N‐acetylcysteine; ix) unable or unwilling to provide written informed consent. |
| Interventions |
Intervention 1: oral low‐dose (3600 mg/day) N‐acetylcysteine delivered over an 8‐week period. Intervention 2: oral high‐dose (5400 mg/day) N‐acetylcysteine delivered over an 8‐week period. Control: oral placebo delivered over an 8‐week period. |
| Outcomes |
Primary outcome(s): i) glutathione concentrations in the anterior cingulate cortex, as measured by the number of participants who achieve a 5% increase in glutathione concentrations according to MRS. Secondary outcome(s): i) glutathione reduced‐to‐oxidized ratio, as measured by the number of participants who achieve an increase of at least 50% in the reduced‐to‐oxidized ratio of glutathione in the blood; ii) glutathione concentrations in the anterior cingulate cortex, as measured by the number of participants who achieve a 5% decrease in glutathione concentrations according to MRS. |
| Starting date | 1 August, 2019. |
| Contact information | Principal investigator: Assoc/Prof Kathryn Cullen, Department of Psychiatry, University of Minnesota, MN, USA (rega0026@umn.edu). |
| Notes | We are grateful to Assoc/Prof Cullen for confirming the above details were correct, 6 August, 2020. |
NCT04242914.
| Study name | The effect of intravenous ketamine on non suicidal self injuries. |
| Methods | Double‐blind placebo‐controlled RCT. Assignment: parallel, individual‐level. |
| Participants |
Inclusion criteria: i) females; ii) aged between 18 and 65 years; iii) inpatients on the psychiatric ward of a general hospital; iv) self‐reporting NSSI and/or active NSSI on hospital admission or in the preceding week. Exclusion criteria: i) males; ii) unable to provide written informed consent in Hebrew; iii) pregnant or breast‐feeding; iv) history of substance misuse; v) diagnosed with a psychotic disorder and/or major physical condition, including hypertension, arrthymias, or a severe or active neurological condition; vi) previous treatment involving ketamine in which no improvement was observed. |
| Interventions |
Intervention: intravenous infusion of ketamine (0.5mg/kg) in addition to midazolam (0.03mg/kg) over 40 minutes. Control: intravenous infusion of midazolam (0.03mg/kg) over 40 minutes. |
| Outcomes |
Primary outcome(s): i) NSSI, as measured by the Brief Non‐Suicidal Self‐Injury Assessment. Secondary outcome(s): i) ketamine biomarkers, as measured by levels of interleukin 6, high sensitive C‐Reactive Protein, and brain dendritic neurotrophic factor; ii) depression, as measured by the BDI; iii) anxiety, as measured by the DASS; iv) suicidal ideation, as measured by the C‐SSRS and the BSSI; v) impulsivity, as measured by the BIS‐11; vi) well‐being, as measured by the WHO‐5; vii) NSSI attitudes, as measured by the VAS; viii) adverse effects, as measured by an idiosyncratic scale developed by the authors. |
| Starting date | 25 February, 2019. |
| Contact information | Principal investigator: Dr Lior Dvorak, Tel‐Aviv Sourasky Medical Center, Tel‐Aviv, Israel (liordv@tlvmc.gov.il). |
| Notes | We are grateful to Dr Dvorak for confirming the above details were correct, 6 August, 2020. |
BDI: Beck Depression Inventory; BIS‐11: Barratt Impulsiveness Scale, 11 item; BSSI: Beck Scale of Suicidal Ideation;C‐SSRS: Columbia Suicide Severity Rating Scale; DASS: Depression Anxiety Stress Scale; kg: kilograms; mg: milligrams; MRI: magnetic resonance imaging; MRS: magnetic resonance spectroscopy; NSSI: non‐suicidal self‐injury; RCT: randomised controlled trial; VAS: Visual Analogue Scale; WHO‐5: World Health Organization, Five Well‐Being Index.
Differences between protocol and review
None.
Contributions of authors
KH had the idea for the review. All authors screened studies for inclusion. KGW, SEH, GR, and KH extracted data and assessed risk of bias for included studies. KGW, SEH, and TLTS conducted the statistical analyses. KGW and KH wrote the initial version of the review and all authors contributed to the writing of drafts. All authors approved the final version of the review for publication.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Health and Medical Research Council, Australia
KW is supported by an Emerging Leadership 1 Fellowship awarded by the NHMRC (APP1177787).
-
National Institute of Health Research (NIHR), UK
KW received support from the NIHR (Award number: NIHR 131985).
Declarations of interest
KGW: is an editor for the Cochrane Common Mental Disorders Group, and senior editor for the Self‐Harm and Suicide Satellite of the group. SEH: is the joint co‐ordinating editor of the Cochrane Common Mental Disorders Group. She is funded by an Auckland Medical Research Foundation Douglas Goodfellow Repatriation Fellowship to develop and test a digital intervention for young people who engage in self‐harm. She is the Principal Clinical Advisor of the Suicide Prevention Office of the Ministry of Health for the New Zealand Government. GR: no declarations of interest to report in relation to this review PH: no declarations of interest to report in relation to this review TLTS: no declarations of interest to report in relation to this review ET: no declarations of interest to report in relation to this review KH: no declarations of interest to report in relation to this review
Edited (no change to conclusions)
References
References to studies included in this review
Battaglia 1999 {published data only}
- Battaglia J, Wolff TK, Wagner-Johnson DS, Rush AJ, Carmody TJ, Basco MR. Structured diagnostic assessment and depot fluphenazine treatment of multiple suicide attempters in the emergency department. International Clinical Psychopharmacology 1999;14(6):361-72. [DOI] [PubMed] [Google Scholar]
Hallahan 2007 {published data only}
- Hallahan B, Hibbeln RJ, Davis JM, Garland MR. Omega-3 fatty acid supplementation in patients with recurrent self-harm: single-centre double-blind randomised controlled trial. British Journal of Psychiatry 2007;190:118-22. [DOI] [PubMed] [Google Scholar]
Hirsch 1982 {published and unpublished data}
- Draper R, Hirsch SR. Treatment of parasuicide patients with mianserin, nomifensine and placebo: a double blind placebo controlled trial. Unpublished manuscript 1982.
- Hirsch S, Walsh C, Draper R. Parasuicide: a review of treatment interventions. Journal of Affective Disorders 1982;4:299-311. [DOI] [PubMed] [Google Scholar]
Lauterbach 2008 {published and unpublished data}
- Lauterbach E, Ahrens B, Felber W, Müller-Oerlinghausen B, Kilb B, Bischof G, et al. Suicide prevention by lithium (SUPLI): challenges of a multi-center prospective study. Archives of Suicide Research 2005;9:27-34. [DOI] [PubMed] [Google Scholar]
- Lauterbach E, Felber W, Müller-Oerlinghausen B, Ahrens B, Bronisch T, Meyer T, et al. Adjunctive lithium treatment in the prevention of suicidal behaviour in depressive disorders: a randomised, placebo-controlled, 1-year trial. Acta Psychiatrica Scandinavica 2008;118:469-79. [DOI] [PubMed] [Google Scholar]
Montgomery 1979 {published data only}
- Montgomery S, Montgomery D, Jayanthi-Rani S, Roy D, Shaw P, McAuley R. Maintenance therapy in repeat suicidal behaviour: a placebo controlled trial. Proceedings of the 10th International Congress for Suicide Prevention and Crisis Intervention. Ottawa, Canada 1979;1:227-9. [Google Scholar]
Montgomery 1983 {published data only}
- Montgomery S, Roy D, Montgomery D. The prevention of recurrent suicidal acts. British Journal of Clinical Pharmacology 1983;15:183s-188s. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verkes 1998 {published data only}
- Verkes RJ, Mast RC, Hengeveld MW, Tuyl JP, Zwinderman AH, Kempen GM. Reduction by paroxetine of suicidal behavior in patients with repeated suicide attempts but not major depression. American Journal of Psychiatry 1998;1555:543-7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bozzatello 2017 {published data only}
- Bozzatello P, Rocca P, Uscinska M, Bellino S. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: An open-label randomized controlled trial. CNS Drugs 2017;31:809-19. [DOI] [PubMed] [Google Scholar]
Crawford 2018 {published data only}
- Crawford MJ, Sanatinia R, Barrett B, Cunningham G, Dale O, Ganguli P, et al. Lamotrigine for people with borderline personality disorder: A RCT. Health Technology Assessment 2018;22:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford MJ, Sanatinia R, Barrett B, Cunningham G, Dale O, Ganguli P, et al. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. American Journal of Psychiatry 2018;175:756-64. [DOI] [PubMed] [Google Scholar]
Domany 2019 {published data only (unpublished sought but not used)}
- Domany Y, Bleich-Cohen M, Tarrasch R, Meidan R, Litvak-Lazar O, Stoppleman N, et al. Repeated oral ketamine for out-patient treatment of resistant depression: randomised, double-blind, placebo-controlled, proof-of-concept study. British Journal of Psychiatry 2019;214:20-6. [DOI] [PubMed] [Google Scholar]
Grant 2016 {published data only}
- Grant JE, Chamberlain SR, Redden SA, Leppink EW, Odlaug BL, Kim SW. N-Acetylcysteine in the treatment of excoriation disorder: A randomized clinical trial. JAMA Psychiatry 2016;73:490-6. [DOI] [PubMed] [Google Scholar]
Grunebaum 2012 {published data only}
- Grunebaum MF, Ellis SP, Duan N, Burke AK, Oquendo MA, Mann JJ. Pilot randomized clinical trial of an SSRI vs bupropion: effects on suicidal behavior, ideation, and mood in major depression. Neuropsychopharmacology 2012;37:697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunebaum MF, Keilp JG, Ellis SP, Sudol K, Bauer N, Burke AK, et al. SSRI versus bupropion effects on symptom clusters in suicidal depression: Post-hoc analysis of a randomized clinical trial. Journal of Clinical Psychiatry 2013;74:872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilp JG, Ellis SP, Gorlyn M, Burke AK, Oquendo MA, Mann JJ, et al. Suicidal ideation declines with improvement in the subjective symptoms of major depression. Journal of Affective Disorders 2018;227:65-70. [DOI] [PubMed] [Google Scholar]
- Parris MS, Marver JE, Chaudhury SR, Ellis SP, Metts AV, Keilp JG, et al. Effects of anxiety on suicidal ideation: exploratory analysis of a paroxetine versus bupropion randomized trial. International Clinical Psychopharmacology 2018;33:249-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marriott 2016 {published data only}
- Marriott BP, Hibbeln JR, Killeen TK, Magruder KM, Holes-Lewis K, Tolliver BK, et al. Design and methods for the Better Resiliency Among Veterans and non-Veterans with Omega-3's (BRAVO) study: a double blind, placebo-controlled trial of omega-3 fatty acid supplementation among adult individuals at risk of suicide. Contemporary Clinical Trials 2016;47:325-33. [DOI] [PubMed] [Google Scholar]
McCall 2018 {published data only}
- McCall WV, Pillai A, Case D, McCloud L, Nolla T, Branch F, et al. A pilot, randomized clinical trial of bedtime doses of prazosin versus placebo in suicidal posttraumatic stress disorder in patients with nightmares. Journal of Clinical Psychopharmacology 2018;38:618-21. [DOI] [PubMed] [Google Scholar]
NCT00065936 {unpublished data only}
- NCT00065936. Self-injury: diagnosis and treatment. clinicaltrials.gov/ct2/show/NCT00065936 (first received 5 August 2003).
NCT00533117 {unpublished data only}
- NCT00533117. Treating suicidal behavior and self-mutilation in people with borderline personality disorder. www.clinicaltrials.gov/ct2/show/NCT00533117 (first received 28 August 2017).
NCT00539188 {unpublished data only}
- NCT00539188. N-Acetylcysteine in adjunct to DBT for the treatment of self-injurious behavior in BPD. clinicaltrials.gov/ct2/show/NCT00539188 (first received 4 October 2007).
NCT01103180 {unpublished data only}
- NCT01103180. SSRIs and self-harm in borderline personality disorder. clinicaltrials.gov/ct2/show/NCT01103180 (first received 14 June 2019).
NCT01928446 {unpublished data only}
- NCT01928446. Lithium for suicidal behavior in mood disorders (Li+). clinicaltrials.gov/ct2/show/NCT01928446 (first received 26 August 2013).
NCT02039479 {unpublished data only}
- NCT02039479. Randomized, placebo-controlled multicenter trial of lithium plus treatment as usual (TAU) for acute suicidal ideation and behavior in patients with suicidal major depressive episode. clinicaltrials.gov/ct2/show/NCT02039479 (first received 17 January 2014).
Oquendo 2011 {published data only}
- Oquendo MA, Galfalvy HC, Currier D, Grunebaum MF, Sher L, Sullivan GM, et al. Treatment for suicide attempters with bipolar disorder: a randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. American Journal of Psychiatry 2011;168:1050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yovell 2016 {published data only}
- Yovell Y, Bar G, Mashiah M, Baruch Y, Briskman I, Asherov J, et al. Ultra-low-dose buprenorphine as a time-limited treatment for severe suicidal ideation: a randomized controlled trial. American Journal of Psychiatry 2016;173:491-8. [DOI] [PubMed] [Google Scholar]
- Yovell Y, Bar G. Erratum for: ultra-low-dose buprenorphine as a time-limited treatment for severe suicidal ideation: a randomized trial. American Journal of Psychiatry 2016;173:198. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00834834 {published data only}
- NCT00834834. Comparing treatments for self-injury and suicidal behavior in people with borderline personality disorder. clinicaltrials.gov/ct2/show/NCT00834834 (first received 3 February 2009).
- Stoffers-Winterling J, Storebø OJ, Lieb K. Pharmacotherapy for borderline personality disorder:aAn update of published, unpublished and ongoing studies. Current Psychiatry Reports 2020;22:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT04005053 {unpublished data only}
- NCT04005053. Identifying biological signatures of N-Acetylcysteine for non-suicidal self-injury in adolescents and young adults. clinicaltrials.gov/ct2/show/NCT04005053 (first received 2 July 2019).
NCT04242914 {unpublished data only}
- NCT04242914. The effect of intravenous ketamine on non suicidal self injuries. clinicaltrials.gov/ct2/show/NCT04242914 (first received 27 January 2020).
Additional references
Albrecht 2014
- Albrecht B, Staiger PK, Hall K, Miller P, Best D, Lubman DI. Benzodiazepine use and aggressive behaviour: a systematic review. Australian and New Zealand Journal of Psychiatry 2014;48:1096-114. [DOI] [PubMed] [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561. [DOI] [PubMed] [Google Scholar]
Beck 1974
- Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: the Hopelessness Scale. Journal of Consulting and Clinical Psychology 1974;42:861-5. [DOI] [PubMed] [Google Scholar]
Beck 1988
- Beck AT, Steet RA, Ranieri WF. Scale for Suicidal Ideation: psychometric properties of a self-report version. Journal of Clinical Psychology 1988;44:499-505. [DOI] [PubMed] [Google Scholar]
Bergen 2010
- Bergen H, Hawton K, Waters K, Cooper J, Kapur N. Epidemiology and trends in non-fatal self-harm in three centres in England, 2000-2007. British Journal of Psychiatry 2010;197:493-8. [DOI] [PubMed] [Google Scholar]
Brunner 2002
- Brunner J, Parhofer KG, Schwandt P, Bronisch T. Cholesterol, essential fatty acids, and suicide. Pharmacopsychiatry 2002;35:1-5. [DOI] [PubMed] [Google Scholar]
Bustamante‐Madsen 2018
- Bustamante-Madsen L, Eddleston M, Schultz-Hansen K, Konradsen F. Quality assessment of economic evaluations of suicide and self-harm interventions. Crisis 2018;39:82-95. [DOI] [PubMed] [Google Scholar]
Canner 2018
- Canner JK, Giuliano K, Selvaraiah S, Hammond ER, Schneider EB. Emergency department visits for attempted suicide and self harm in the USA, 2006-2013. Epidemiology and Psychiatric Services 2018;27:94-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carr 2016
- Carr MJ, Ashcroft DM, Kontopantelis E, While D, Awenat Y, Cooper J, et al. Clinical management following self-harm in a UK-wide primary care cohort. Journal of Affective Disorders 2016;197:182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carroll 2014
- Carroll R, Metcalfe C, Gunnell D. Hospital presenting self-harm and risk of fatal and non-fatal repetition: systematic review and meta-analysis. PLOS One 2014;9:e89944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carter 2016
- Carter G, Page A, Large M, Hetrick S, Milner AJ, Bendit N, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Australian and New Zealand Journal of Psychiatry 2016;50:939-1000. [DOI] [PubMed] [Google Scholar]
Cipriani 2013a
- Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ 2013;346:f3646. [DOI] [PubMed] [Google Scholar]
Cipriani 2013b
- Cipriani A, Reid K, Young AH, Macritchie K, Geddes J. Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No: CD003196. [DOI: 10.1002/14651858.CD003196.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Claes 2007
- Claes L, Wandereycken W, Vertommen H. Self-injury in female versus male psychiatric patients: A comparison of characteristics, psychopathology and aggression regulation. Personality and Individual Differences 2007;42:611-21. [Google Scholar]
Corcoran 2015
- Corcoran P, Griffin E, O'Carroll A, Cassidy L, Bonner B. Hospital-treated deliberate self-harm in the western area of Northern Ireland. Crisis 2015;36:83-90. [DOI] [PubMed] [Google Scholar]
Courtney 2019
- Courtney DB, Duda S, Szatmari P, Henderson J, Bennett K. Systematic review and quality appraisal of practice guidelines for self-harm in children and adolescents. Suicide and Life-Threatening Behavior 2019;49:707-23. [DOI] [PubMed] [Google Scholar]
Curtin 2002a
- Curtin F, Elbourne D, Altman DG. Meta-analysis combining parallel and cross-over clinical trials. III. The issue of carry-over. Statistics in Medicine 2002;21:2161-73. [DOI] [PubMed] [Google Scholar]
Curtin 2002b
- Curtin F, Altman DG, Elbourne D. Meta-analysis combining parallel and cross-over trials. I. Continuous outcomes. Statistics in Medicine 2002;21:2131-44. [DOI] [PubMed] [Google Scholar]
De Beurs 2018
- De Beurs D, Vancayseele N, Borkulo C, Portzky G, Heeringen K. The association between motives, perceived problems and current thoughts of self-harm following an episode of self-harm: a network analysis. Journal of Affective Disorders 2018;240:262-70. [DOI] [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Dodds 2017
- Dodds TJ. Prescribed benzodiazepines and suicide risk: A review of the literature. Primary Care Companion CNS Disorders 2017;19:16r02037. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta-analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971-80. [DOI] [PubMed] [Google Scholar]
Easterbrook 1991
- Easterbrook PJ, Gopalan R, Berlin JA, Matthews DR. Publication bias in clinical research. Lancet 1991;337:867-72. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Christoph M. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31:140-9. [DOI] [PubMed] [Google Scholar]
Engles 2003
- Engles JM, Diehr P. Imputation of missing longitudinal data: A comparison of methods. Journal of Clinical Epidemiology 2003;56:968-76. [DOI] [PubMed] [Google Scholar]
Erez 1996
- Erez A, Bloom MC, Wells MT. Using random rather than fixed effects models in meta-analysis: Implications for situation specificity and validity generalization. Personnel Psychology 1996;49:275-306. [Google Scholar]
FDA 2019
- US Food and Drug Administration. FDA approves new nasal spray medication for treatment-resistant depression. www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified Accessed 18 March 2020.
Ferrari 2014
- Ferrari AJ, Norman RE, Freedman G, Baxter AJ, Pirkis JE, Harris MG, et al. The burden attributable to mental and substance use disorders as risk factors for suicide: findings from the Global Burden of Disease Study 2010. PLOS One 2014;9:e91936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferrey 2018
- Ferrey AE, Geulayov G, Casey D, Wells C, Fuller A, Bankhead C, et al. Relative toxicity of mood stabilisers and antipsychotics: Case fatality and fatal toxicity associated with self-poisoning. BMC Psychiatry 2018;18:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleiss 1994
- Fleiss JL. Measures of effect size for categorical data. In: Cooper H, Hedges LV, editors(s). The Handbook of Research Synthesis. New York, NY: Russell Sage Foundation, 1994. [Google Scholar]
Forte 2019
- Forte A, Buscaioni A, Fiorillo A, Pompii A, Baldessarini R. Suicidal risk following hospital discharge: a review. Harvard Review of Psychiatry 2019;27:209-16. [DOI] [PubMed] [Google Scholar]
Geulayov 2016
- Geulayov G, Kapur N, Turnbull P, Clements C, Waters K, Ness J, et al. Epidemiology and trends in non-fatal self-harm in three centres in England, 2000-2012: findings from the Multicentre Study of Self-harm in England. BMJ Open 2016;6:e010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geulayov 2019
- Geulayov G, Casey D, Bale L, Brand F, Clements C, Farooq B, et al. Suicide following presentation to hospital for non-fatal self-harm in the Multicentre Study of Self-Harm: a long-term follow-up study. Lancet Psychiatry 2019;6:1021-30. [DOI] [PubMed] [Google Scholar]
Gjelsvik 2014
- Gjelsvik B, Heyerdahl F, Lunn D, Hawton K. Change in access to prescribed medication following an episode of deliberate self-poisoning: a multilevel approach. PLOS One 2014;9:e98086. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 26 June 2020. Available at gradepro.org.
Griffin 2014
- Griffin E, Arensman E, Corcoran P, Wall A, Williamson E, Perry I. National Registry of Deliberate Self Harm Annual Report. Cork, Ireland: National Suicide Research Foundation, 2014. [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry 1960;23:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harrison 2014
- Harrison JE, Henley G, AIHW. Suicide and hospitalised self-harm in Australia: trends and analysis. Injury research and statistics series no. 93. Cat. no. INJCAT 169. Canberra: AIHW 2014.
Hawton 2003
- Hawton K, Hariss L, Hall S, Simkin S, Bale E, Bond A. Deliberate self-harm in Oxford, 1990-2000: a time of change in patient characteristics. Psychological Medicine 2003;33:987-96. [DOI] [PubMed] [Google Scholar]
Hawton 2004
- Hawton K, Harriss L, Simkin S, Bale E, Bond A. Self-cutting: patient characteristics compared with self-poisoners. Suicide and Life-Threatening Behavior 2004;34:199-208. [DOI] [PubMed] [Google Scholar]
Hawton 2007
- Hawton K, Bergen H, Casey D, Simkin S, Palmer B, Cooper J, et al. Self-harm in England: a tale of three cities. Multicentre Study of Self-Harm. Social Psychiatry and Psychiatric Epidemiology 2007;42:513-21. [DOI] [PubMed] [Google Scholar]
Hawton 2008
- Hawton K, Harriss L. The changing gender ratio in occurrence of deliberate self-harm across the lifecycle. Crisis 2008;29:4-10. [DOI] [PubMed] [Google Scholar]
Hawton 2010
- Hawton K, Bergen H, Simkin H, Cooper K, Water K, Gunnell D, et al. Toxicity of antidepressants: rates of suicide relative to prescribing and non-fatal overdose. British Journal of Psychiatry 2010;196:354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawton 2013
- Hawton K, Saunders KE, Topiwala A, Haw C. Psychiatric disorders in patients presenting to hospital following self-harm: a systematic review. Journal of Affective Disorders 2013;151:821-30. [DOI] [PubMed] [Google Scholar]
Hawton 2016
- Hawton K, Witt KG, Taylor Salisbury TL, Arensman E, Gunnell D, Hazell P, et al. Psychosocial interventions for self-harm in adults. Cochrane Database of Systematic Reviews 2016;5:CD012189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayes 2016
- Hayes JF, Pitman A, Marston L, Walters K, Geddes JR, King M, et al. Self-harm, unintentional injury, and suicide in biolar disorder during maintenance mood stabilizer treatment: A UK population-based electronic health records study. JAMA Psychiatry 2016;73:630-7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019a
- Higgins JP, Eldridge S, Li T, editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Higgins 2019b
- Higgins JPT, Li T, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hopewell 2009
- Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: MR000006. [DOI: 10.1002/14651858.MR000006.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
House 2020
- House A. Measuring outcomes in self-harm trials: what is important and what is achievable? British Journal of Psychiatry Open 2020;6:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerry 1998
- Kerry SM, Bland JM. Analysis of a trial randomised in clusters. BMJ 1998;316:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klonsky 2011
- Klonsky ED. Non-suicidal self-injury in United States adults: prevalence, socio-demographics, topography and functions. Psychological Medicine 2011;41:1981-6. [DOI] [PubMed] [Google Scholar]
Larkin 2014
- Larkin C, di Blasi Z, Arensman E. Risk factors for repetition of self-harm: a systematic review of prospective hospital-based studies. PLOS One 2014;9:e84282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lilley 2008
- Lilley R, Owens D, Horrocks J, House A, Noble R, Bergen H, et al. Hosptial care and repetition following self-harm: multicentre comparison of self-poisoning and self-injury. British Journal of Psychiatry 2008;192:440-5. [DOI] [PubMed] [Google Scholar]
Malhi 2015
- Malhi GS, Bassett D, Boyce P, Bryant R, Fitzgerald PB, Fritz K, et al. Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines for Mood Disorders. Australian and New Zealand Journal of Psychiatry 2015;49:1-185. [DOI] [PubMed] [Google Scholar]
Mann 2013
- Mann JJ. The serotonergic system in mood disorders and suicidal behaviour. Philosophical Transactions of The Royal Society (B Biological Sciences) 2013;368:20120537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adamns C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249-52. [DOI] [PubMed] [Google Scholar]
Masiran 2017
- Masiran R, Haniff J, Ali NH, Hamid AMA. Rates and profiles of self-harm presenting to Malaysian general hospitals: data from the Ministry of Health in 2011. Malaysian Journal of Medicine and Health Sciences 2017;13:39-45. [Google Scholar]
Microsoft Excel RoB2 Macro [Computer program]
- Version 6. Risk of Bias 2 Development Group, 22 August 2019. Available at: sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/current-version-of-rob-2.
Mitchell 2016
- Mitchell AJ, Hussain S, Leaver J, Rajan C, Jones A, Malcolm N, et al. Is there a difference between hospital-verified and self-reported self-harm? Implications for repetition. General Hospital Psychiatry 2016;43:12-6. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609-13. [DOI] [PubMed] [Google Scholar]
Morthorst 2016
- Morthorst BT, Soegaard B, Nordentoft M, Erlangsen A. Incidence rates of deliberate self-harm in Denmark, 1994-2011: a nationwide register study. Crisis 2016;37:256-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Müller 2016
- Müller A, Claes L, Brähler E, Zwann M. Prevalence and correlates of self-harm in the German general population. PLOS One 2016;11:e0157928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2012
- Murphy E, Kapur N, Webb R, Purandare N, Hawton K, Bergen H, et al. Risk factors for repetition and suicide following self-harm in older adults: multicentre cohort study. British Journal of Psychiatry 2012;200:399-404. [DOI] [PubMed] [Google Scholar]
NCCMH 2004
- National Collaborating Centre for Mental Health. Clinical Guideline 16. Self-harm: The short-term physical and psychological management and secondary prevention of self-harm in primary and secondary care. London, UK: National Institute for Clinical Excellence, 2004. [Google Scholar]
NICE 2009
- National Institute for Health and Care Excellence. Accessed 20 July 2020 from: https://www.nice.org.uk/guidance/cg90/resources/depression-in-adults-recognition-and-management-pdf-975742636741. National Institute for Health and Care Excellence, 2009. [Google Scholar]
NICE 2011
- National Institute for Health and Clinical Excellence. Self-harm: Longer-term management [National Clinical Practice Guideline Number 133]. Accessed 26 June 2020 from: https://www.nice.org.uk/guidance/cg133/evidence/full-guideline-pdf-184901581. London, UK: National Institute for Health and Clinical Excellence, 2011. [Google Scholar]
Owens 2002
- Owens D, Horrocks J, House A. Fatal and non-fatal repetition of self-harm: systematic review. British Journal of Psychiatry 2002;181:193-9. [DOI] [PubMed] [Google Scholar]
Owens 2020
- Owens C, Fox F, Redwood S, Davies R, Foote L, Salisbury N, et al. Measuring outcomes in trials of interventions for people who self-harm: qualitative study of service users' views. British Journal of Psychiatry Open 2020;6:e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perry 2012
- Perry IJ, Corcoran P, Fitzgerald AP, Keeley HS, Reulback U, Arensman E. The incidence and repetition of hospital-treated deliberate self-harm: findings from the world's first national registry. PLOS One 2012;7:e31663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Plener 2016
- Plener PL, Brunner R, Fegert JM, Groschwitz RC, In-Albon T, Kaess M, et al. Treating nonsuicidal self-injury (NSSI) in adolescents: consensus based German guidelines. Child and Adolescent Psychiatry and Mental Health 2016;10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qaseem 2016
- Qaseem A, Barry MJ, Kansagara D. Nonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder: a clinical practice guideline from the American College of Physicians. Annals of Internal Medicine 2016;164:350-9. [DOI] [PubMed] [Google Scholar]
Rana 2013
- Rana F, Gormez A, Varghese S. Pharmacological interventions for self-injurious behaviour in adults with intellectual disabilities. Cochrane Database of Systematic Reviews 2013;4:CD009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Royal College of Psychiatrists 2014
- Royal College of Psychiatrists. Managing Self-Harm in Young People (Guideline number CR192). London, UK: Royal College of Psychiatrists, 2014. [Google Scholar]
Sanacora 2017
- Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, et al, the American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry 2017;74:399-405. [DOI] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Sinclair 2011
- Sinclair JMA, Gray A, Rivero-Arias O, Saunders KE, Hawton K. Healthcare and social services resource use and costs of self-harm patients. Social Psychiatry and Psychiatric Epidemiology 2011;46:263-71. [DOI] [PubMed] [Google Scholar]
Smith 2005
- Smith BD. Self-mutilation and pharmacotherapy. Psychiatry 2005;2:28-37. [PMC free article] [PubMed] [Google Scholar]
Smith 2017
- Smith KA, Cipriani A. Lithium and suicide in mood disorders: Updated meta-review of the scientific literature. Bipolar Disorders 2017;19:1-12. [DOI] [PubMed] [Google Scholar]
Sorgi 1991
- Sorgi P, Ratey J, Knoedler DW, Markert RJ, Reichman M. Rating aggression in the clinical setting: A retrospective adaption of the Overt Aggression Scale: Preliminary results. Journal of Neuropsychiatry and Clinical Neuroscience 1991;3:S52-56. [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:I4898. [DOI] [PubMed] [Google Scholar]
Stoffers 2010
- Stoffers J, Völlm BA, Rücker G, Timmer A, Huband N, Lieb K. Pharmacological interventions for borderline personality disorder. Cochrane Database of Systematic Reviews 2010, Issue 6. Art. No: CD005653. [DOI: 10.1002/14651858.CD005653.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoffers‐Winterling 2020
- Stoffers-Winterling J, Storebø OJ, Lieb K. Pharmacotherapy for borderline personality disorder: An update of published, unpublished and ongoing studies. Current Psychiatry Reports 2020;22:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stone 2009
- Stone M, Laughren T, Jones ML, Levenson M, Holland PC, Hughes A, et al. Risk of suicidality in clinical trials of antidepressants in adults: Analysis of proprietary data submitted to US Food and Drug Administration. BMJ 2009;339:b2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sublette 2006
- Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. American Journal of Psychiatry 2006;163:1100-02. [DOI] [PubMed] [Google Scholar]
Tanskanen 2001
- Tanskanen A, Hibbeln JR, Hintikka J, Haatainen K, Honkalampi K, Viinamaki H. Fish consumption, depression, and suicidality in a general population. Archives of General Psychiatry 2001;58:512-3. [DOI] [PubMed] [Google Scholar]
Ting 2012
- Ting SA, Sulivan AF, Boudreaux ED, Miller I, Camargo CA. Trends in US emergency department visits for attempted suicide and self-inflicted injury, 1993-2008. General Hospital Psychiatry 2012;34:557-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Torgerson 2004
- Torgerson D. The use of Zelen's design in randomised trials. British Journal of Obstetrics and Gynaecology 2004;111:2. [DOI] [PubMed] [Google Scholar]
Tsiachristas 2017
- Tsiachristas A, McDaid D, Casey D, Brand F, Leal J, Park A-L, et al. General hospital costs in England of medical and psychiatric care for patients who self-harm: a retrospective analysis. Lancet 2017;4:759-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2014
- Turner BJ, Austin SB, Chapman AL. Treating nonsuicidal self-injury: A systematic review of psychological and pharmacological interventions. Canadian Journal of Psychiatry 2014;59:576-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tyrer 2012
- Tyrer P. Why benzodiazepine are not going away: commentary on benzodiazepines for anxiety disorders. Advances in Psychiatric Treatment 2012;18:259-62. [Google Scholar]
van Heeringen 2014
- Heeringen K, Mann JJ. The neurobiology of suicide. Lancet Psychiatry 2014;1:63-72. [DOI] [PubMed] [Google Scholar]
Viechtbauer 2020
- Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Research Synthesis Methods 2020;1:112-25. [DOI] [PubMed] [Google Scholar]
Weissman 1999
- Weissman MM, Staff MHS. Social Adjustment Scale - Self-Report (SAS-SR) User's Manual. North Tonawanda, NY: Multi-Health Systems, 1999. [Google Scholar]
WHO 2014a
- World Health Organization. Preventing Suicide: a Global Imperative. Geneva, Switzerland: World Health Organization, 2014. [Google Scholar]
WHO 2014b
- World Health Organization Collaborating Centre for Drug Statistics and Methodology. Guidelines for ATC Classification and DDD Assignment. Oslo, Norway: World Health Organization, 2014. [Google Scholar]
Wilkinson 2002
- Wilkinson S, Taylor G, Templeton L, Mistral W, Salter E, Bennett P. Admissions to hospital for deliberate self-harm in England 1995-2000: an analysis of Hospital Episode Statistics. Journal of Public Health 2002;24:179-83. [DOI] [PubMed] [Google Scholar]
Wilkinson 2018
- Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. American Journal of Psychiatry 2018;175:150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Witt 2019
- Witt K, Daly C, Arensman E, Pirkis J, Lubman D. Patterns of self-harm methods over time and the association with methods used a repeat episodes of non-fatal self-harm and suicide: A systematic review. Journal of Affective Disorders 2019;245:250-64. [DOI] [PubMed] [Google Scholar]
Witt 2020a
- Witt K, Potts J, Hubers A, Grunebaum MF, Murrough JW, Loo C, et al. Ketamine for suicidal ideation in adults with psychiatric disorders: a systematic review and meta-analysis of treatment trials. Australian and New Zealand Journal of Psychiatry 2020;54:29-45. [DOI] [PubMed] [Google Scholar]
Witt 2020b
- Witt K, Townsend E, Arensman E, Gunnell D, Hazell P, Taylor Salisbury T, et al. Psychosocial interventions for people who self-harm: methodological issues involved in trials to evaluate effectiveness. Archives of Suicide Research 2020;24(sup2):S32-93. [DOI: 10.1080/13811118.2019.1592043] [DOI] [PubMed]
Zahl 2004
- Zahl DL, Hawton K. Repetition of deliberate self-harm and subsequent suicide risk: long-term follow-up study of 11 583 patients. British Journal of Psychiatry 2004;185:70-5. [DOI] [PubMed] [Google Scholar]
Zelen 1979
- Zelen M. A new design for randomized clinical trials. New England Journal of Medicine 1979;300:1242-5. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67:361-70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hawton 2015
- Hawton K, Witt KG, Taylor Salisbury TL, Arensman E, Gunnell D, Hazell P, et al. Pharmacological interventions for self-harm in adults. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD011777. [DOI: 10.1002/14651858.CD011777] [DOI] [PMC free article] [PubMed] [Google Scholar]
Witt 2020c
- Witt KG, Hawton K, Hetrick SE, Taylor Salisbury TL, Townsend E, Hazell P. Pharmacological interventions for self-harm in adults. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013669. [DOI: 10.1002/14651858.CD013669] [DOI] [PMC free article] [PubMed] [Google Scholar]