Abstract
Background
It has been suggested that low serum zinc levels may be associated with suboptimal outcomes of pregnancy, such as prolonged labour, atonic postpartum haemorrhage, pregnancy‐induced hypertension, preterm labour and post‐term pregnancies, although these associations have not yet been established. This is an update of a review first published in 1997 and subsequently updated in 2007, 2012 and 2015.
Objectives
1. To compare the effects on maternal, fetal, neonatal and infant outcomes in healthy pregnant women receiving zinc supplementation versus no zinc supplementation, or placebo. 2. To assess the above outcomes in a subgroup analysis reviewing studies performed in women who are, or are likely to be, zinc‐deficient.
Search methods
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (3 July 2020), and reference lists of retrieved studies.
Selection criteria
Randomised trials of zinc supplementation versus no zinc supplementation or placebo administration during pregnancy, earlier than 27 weeks' gestation. We excluded quasi‐randomised controlled trials. We intended to include studies presented only as abstracts, if they provided enough information or, if necessary, by contacting authors to analyse them against our criteria; we did not find any such studies.
Data collection and analysis
Three review authors applied the study selection criteria, assessed trial quality and extracted data. When necessary, we contacted study authors for additional information. We assessed the certainty of the evidence using GRADE.
Main results
For this update, we included 25 randomised controlled trials (RCTs) involving over 18,000 women and their babies. The overall risk of bias was low in half of the studies. The evidence suggests that zinc supplementation may result in little or no difference in reducing preterm births (risk ratio (RR) 0.87, 95% confidence interval (CI) 0.74 to 1.03; 21 studies, 9851 participants; low‐certainty evidence). Further, zinc supplementation may make little or no difference in reducing the risk of stillbirth (RR 1.22, 95% CI 0.80 to 1.88; 7 studies, 3295 participants; low‐certainty evidence), or perinatal deaths (RR 1.10, 95% CI 0.81 to 1.51; 2 studies, 2489 participants; low‐certainty evidence). It is unclear whether zinc supplementation reduces neonatal death, because the certainty of the evidence is very low. Finally, for other birth outcomes, zinc supplementation may make little or no difference to mean birthweight (MD 13.83, 95% CI ‐15.81 to 43.46; 22 studies, 7977 participants; low‐certainty evidence), and probably makes little or no difference in reducing the risk of low birthweight (RR 0.94, 95% CI 0.79 to 1.13; 17 studies, 7399 participants; moderate‐certainty evidence) and small‐for‐gestational age babies when compared to placebo or no zinc supplementation (RR 1.02, 95% CI 0.92 to 1.12; 9 studies, 5330 participants; moderate‐certainty evidence). We did not conduct subgroup analyses, as very few studies used normal zinc populations.
Authors' conclusions
There is not enough evidence that zinc supplementation during pregnancy results in improvements in maternal or neonatal outcomes. Future research to address ways of improving the overall nutritional status of pregnant women, particularly in low‐income regions, and not looking at zinc in isolation, should be an urgent priority.
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Bias; Dietary Supplements; Infant, Low Birth Weight; Infant, Small for Gestational Age; Perinatal Death; Perinatal Death/prevention & control; Pregnancy Outcome; Premature Birth; Premature Birth/epidemiology; Premature Birth/prevention & control; Randomized Controlled Trials as Topic; Stillbirth; Stillbirth/epidemiology; Zinc; Zinc/administration & dosage; Zinc/blood
Plain language summary
Zinc supplementation for improving pregnancy and infant outcome
What is the issue?
In low‐ and middle‐income countries, many women have poor diets and are deficient in key micronutrients that are required for good health. This is especially concerning during pregnancy, when energy and nutrient needs are greater for both the mother and the growing baby. Zinc plays a critical role in normal growth and development. Deficiency in zinc could lead to adverse health outcomes, such as being born too soon or too small.
This is an update of a review first published in 1997 and subsequently updated in 2007, 2012 and 2015.
Why is this important?
Although severe zinc deficiency is rare, it is estimated that mild‐to‐moderate deficiency is common in several regions of the world. Studies of human pregnancy and zinc supplementation, including those from low‐ and middle‐income countries, have failed to document a consistent beneficial effect on fetal growth, length of gestation, and early newborn survival.
What evidence did we find?
We searched for studies in July 2020. This updated review now includes 25 randomised controlled trials, involving over 18,000 women and their babies. We found that zinc supplementation in pregnancy may make little to no difference in reducing the risk of preterm births, stillbirths, or deaths around the time of birth, when compared to no zinc supplementation or placebo. Zinc supplementation may make little or no difference to the birthweight of babies, and probably makes little or no difference to the number of babies born either with a low birthweight or small for their gestational age, when compared with no zinc supplementation, or with giving a placebo. We cannot be sure whether zinc supplementation reduces death in newborns, because the certainty of the evidence is very low.
What does this mean?
There is not enough evidence that zinc supplementation during pregnancy results in better outcomes for women and their babies. Finding ways to improve women's overall nutritional status, particularly in low‐income areas, will do more to improve the health of mothers and babies than supplementing pregnant women with zinc alone. This should be an urgent research priority for the future.
Summary of findings
Summary of findings 1. Zinc supplementation compared to no zinc (with or without placebo) for improving pregnancy and infant outcome.
| Zinc supplementation compared to no zinc (with or without placebo) for improving pregnancy and infant outcomes | ||||||
| Patient or population: Pregnant women (healthy and probable zinc deficiency) Setting: Global Intervention: Zinc supplementation Comparison: No zinc (with or without placebo) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no zinc (with or without placebo) | Risk with zinc supplementation | |||||
| Preterm birth (< 37 weeks) | Study population | RR 0.87 (0.74 to 1.03) | 9851 (21 RCTs) | ⊕⊕⊝⊝ LOWa,b | ‐ | |
| 122 per 1000 | 106 per 1000 (91 to 126) | |||||
| Stillbirth | Study population | RR 1.22 (0.80 to 1.88) | 3295 (7 RCTs) | ⊕⊕⊝⊝ LOWa,b | ‐ | |
| 21 per 1000 | 26 per 1000 (17 to 40) | |||||
| Neonatal death | Study population | RR 2.44 (0.40 to 14.83) | 1965 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | ‐ | |
| 24 per 1000 | 59 per 1000 (10 to 358) | |||||
| Perinatal death | Study population | RR 1.10 (0.81 to 1.51) | 2489 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | ‐ | |
| 58 per 1000 | 63 per 1000 (47 to 87) | |||||
| Birthweight | The mean birthweight was 0 | MD 13.83 higher (15.81 lower to 43.46 higher) | ‐ | 7977 (22 RCTs) | ⊕⊕⊝⊝ LOWa,b | ‐ |
| Small‐for‐gestational age or IUGR | Study population | RR 1.02 (0.92 to 1.12) | 5330 (9 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ | |
| 224 per 1000 | 228 per 1000 (206 to 251) | |||||
| Low Birthweight (< 2500 g) | Study population | RR 0.94 (0.79 to 1.13) | 7399 (17 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ | |
| 133 per 1000 | 125 per 1000 (105 to 150) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; IUGR: intrauterine growth restriction; MD: mean difference; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded −1 for study design: Most studies contributing data had design limitations. bDowngraded −1 for imprecision: Wide confidence interval (CI) crossing the line of no effect. cDowngraded −1 for inconsistency: Heterogeneity (I2 value) is between 50% and 90%, which is considered substantial.
Background
Description of the condition
The overall nutritional status of the mother during pregnancy is a significant contributor to both maternal and perinatal mortality and morbidity (Koblinsky 1995). This is likely to be even more crucial in developing countries, where anaemia and infections, such as malaria and hookworm, further compound the issue (Gibson 2006).
Description of the intervention
Zinc is known to play an important role in many biological functions, including protein synthesis and nucleic acid metabolism (King 2006). Although severe zinc deficiency is now considered rare, mild‐to‐moderate deficiency may be relatively common throughout the world (Sanstead 1991). In a review of the literature published between 1970 and 1991, Parr 1996 noted that, on average, pregnant and lactating women worldwide consumed 9.6 mg zinc a day, well below the recommended 15 mg daily, during the last two trimesters of pregnancy (Sanstead 1996; WHO 1996). In animal studies, zinc deficiency during the early stages of pregnancy is associated with reduced fertility (Apgar 1970), fetal neurological malformations and growth retardation (McKenzie 1975). Deficiency in later stages of pregnancy negatively affects neuronal growth and may also be associated with impaired brain function and behavioural abnormalities (Golub 1995).
How the intervention might work
In humans, pregnant women with acrodermatitis enteropathica (an inherited defect in zinc absorption from the bowel) show an association with increased risk of congenital malformations and pregnancy losses (Verburg 1974). Numerous reports have noted low serum zinc levels to be linked with abnormalities of labour such as prolonged labour and atonic postpartum haemorrhage (Prema 1980), pregnancy‐induced hypertension (Jameson 1976; Jameson 1993), preterm labour (Jones 1981) and post‐term pregnancies (Simmer 1985). Others (Cherry 1981; Chesters 1982) have failed to show any such association.
Some researchers have also reported an association between low zinc and small‐for‐gestational age babies, and poor perinatal outcome (Kiilholma 1984a; Kiilholma 1984b). Kirksey 1994 reported low maternal serum zinc levels during pregnancy to be associated with an increased risk of low birthweight and preterm birth. Low‐birthweight babies have higher rates of morbidity and mortality due to infectious disease and impaired immunity, and thus it is possible that zinc deficiency may also affect infant growth and well‐being.
Why it is important to do this review
Studies of the effects of zinc supplementation have differed in their findings. These inconsistencies in study findings could be due to a lack of consensus on accurate assessment of zinc status (Aggett 1991) and to differences in the populations studied. Randomised controlled trials of zinc supplementation in pregnancy would help to address the association, if any, between zinc deficiency and pregnancy outcome and neonatal and infant health and well‐being.
The fetal nervous system develops progressively during pregnancy, influencing motor and autonomic functions. Change in the pattern of fetal heart rate and movements monitored electronically have been related to fetal neurobehavioural development (DiPietro 1996), with atypical neurodevelopment being shown in fetuses that exhibit other indicators of neurologic compromise (Hepper 1995). In a publication from Egypt, Kirksey 1991 also reported a positive association between maternal zinc status during the second trimester of pregnancy and newborn behaviour.
It is plausible that the effect of zinc supplementation would vary among different population groups, depending on their nutritional status, with any effect likely to be more apparent in women from the developing world. The World Health Organization (WHO) and United Nations Children's Education Fund (UNICEF) promote the antenatal use of multiple‐micronutrient supplementation, including zinc, to all pregnant women where there are population‐level micronutrient deficiencies and in the context of rigorous research (WHO 2020).
The aim of this review is to systematically consider all randomised controlled trials of zinc supplementation in pregnancy, and to evaluate the role of zinc as it relates to pregnancy, labour and birth as well as to maternal and infant health and well‐being.
Objectives
To compare the effects on maternal, fetal, neonatal and infant outcomes in healthy pregnant women receiving zinc supplementation versus no zinc supplementation, or placebo.
To assess the above outcomes in a subgroup analysis reviewing studies performed in women who are, or are likely to be, zinc‐deficient.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials of zinc supplementation versus no zinc supplementation or placebo administration during pregnancy, earlier than 27 weeks' gestation. We exclude quasi‐randomised controlled trials. We intended to include studies presented only as abstracts, if they provided enough information or, if necessary, by contacting authors to analyse them against our criteria; we did not find any such studies.
Types of participants
Normal pregnant women with no systemic illness. Women may have had normal zinc levels, or they may have been, or were likely to have been, zinc‐deficient.
Types of interventions
Routine zinc supplementation versus no zinc supplementation, or placebo.
Types of outcome measures
We have included outcomes related to clinical complications of pregnancy for maternal, fetal, neonatal and infant outcomes. We have not included data related to biochemical outcomes or studies reporting only biochemical outcomes.
Primary outcomes
Maternal and pregnancy outcomes
Preterm labour or birth (less than 37 weeks), or both
Neonatal outcomes
Stillbirth Neonatal death Perinatal death Birthweight Small‐for‐gestational age (birthweight less than 10th centile for gestational age) Low birthweight (less than 2.5 kg)
Secondary outcomes
Maternal and pregnancy outcomes
Antepartum haemorrhage Pregnancy‐induced hypertension Prelabour rupture of membranes Post‐term pregnancy Induction of labour Any maternal infection Meconium in liquor Caesarean section Instrumental vaginal birth Retained placenta Postpartum haemorrhage Smell dysfunction Taste dysfunction
Fetal neurodevelopmental assessment
Baseline fetal heart rate Baseline variability Number of accelerations Number of fetal movements Fetal activity level (minutes) Movement amplitude
Neonatal outcomes
Gestational age at birth High birthweight (more than 4.5 kg) Apgar score of less than five at five minutes Head circumference Hypoxia Neonatal sepsis Neonatal jaundice Respiratory distress syndrome Neonatal intraventricular haemorrhage Necrotising enterocolitis Neonatal length of hospital stay Congenital malformation (non‐prespecified outcome) Chest circumference (non‐prespecified outcome) Crown‐heel length (non‐prespecified outcome) Neonatal birth length (non‐prespecified outcome) Neonatal mid‐upper arm circumference (non‐prespecified outcome)
Infant/child outcomes
Episodes of disease Weight‐for‐age Z‐score Weight‐for‐height Z‐score Mid‐upper arm circumference Mental development index Psychomotor development index Other measures of infant or child development Head circumference (non‐prespecified outcome) Chest circumference (non‐prespecified outcome)
Search methods for identification of studies
The following Methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (3 July 2020).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register, including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included, Excluded or Ongoing).
We also searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (3 July 2020) using the search methods detailed in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, see Mori 2012.
For this update, we used the following methods, which are based on a standard template used by the Cochrane Pregnancy and Childbirth Group, to assess the eight new reports that we identified as a result of the updated search.
Selection of studies
For this update, two review authors (Bianca Carducci (BC) and Emily Keats (EK)), independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreements through discussion.
Data extraction and management
We designed a form to extract data. For eligible studies in this update, two review authors (BC and EK) extracted data using the agreed form. We planned to resolve any discrepancies through discussion or, if required, we would have consulted the third review author. We entered data into Review Manager 5 software (RevMan 2019) and checked them for accuracy.
When information about any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
BC and EK independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random‐number table; computer random‐number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment, and assessed whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively‐numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as a summary risk ratio with a 95% confidence interval.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. If necessary, we planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually‐randomised trials. We would have adjusted their sample sizes or standard errors using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. Had we used ICCs from other sources, we would have reported this and conducted sensitivity analyses to investigate the effect of variation in the ICC. We included one cluster‐randomised trial (Nepal 2003), with analyses adjusted for clustering using a design effect of 1.2.
We synthesised the relevant information from Nepal 2003 and the individually‐randomised trials. We considered it reasonable to combine the results from both as there was little heterogeneity between the study designs, and we considered an interaction between the effect of intervention and the choice of randomisation unit unlikely.
If necessary, we would have acknowledged heterogeneity in the randomisation unit and performed a subgroup analysis to investigate the effects of the randomisation unit.
We do not consider cross‐over trials to be eligible for this review.
Dealing with missing data
We noted levels of attrition in the included studies. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses as far as possible on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they had been allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau2, I2 and Chi2 statistics. We regarded heterogeneity as substantial if I2 was greater than 30% and either the Tau2 was greater than zero, or there was a low P value (less than 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
When there were 10 or more studies in a meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually, and used formal tests for funnel plot asymmetry. We performed exploratory analyses to investigate any asymmetry we detected.
Data synthesis
We carried out statistical analysis using the Review Manager 5 software (RevMan 2019). We used a random‐effects meta‐analysis to produce an overall summary when we considered an average treatment effect across trials to be clinically meaningful for combining data as the underlying treatment effects differed between trials, or we detected substantial statistical heterogeneity, or both. The random‐effects summary was treated as the average range of possible treatment effects, and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful we did not combine trials. We present the results as the average treatment effect with a 95% confidence interval, and the estimates of Tau2 and I2.
Subgroup analysis and investigation of heterogeneity
We planned to identify substantial heterogeneity, and investigate it using subgroup analyses and sensitivity analyses. In future updates, we will consider whether an overall summary is meaningful, and where it is, will use random‐effects analysis to produce it.
We plan to carry out the following subgroup analysis by incorporating zinc status as subgroups as part of the primary comparison.
Risk of populations (population with no or low risk of zinc deficiency versus population with assumed risk of zinc deficiency).
Study settings (studies conducted in high‐income settings versus low‐income settings).
We consider studies at risk of zinc deficiency if they met one of the criteria below.
Biochemical status – According to IZiNCG, zinc deficiency is defined as a serum zinc level of less than 56 μg/dl (or 8.56 umol/L) during the first trimester, or less than 50 μg/dl (or 7.65 umol/L) during the second or third trimester (International Zinc Nutrition Consultative Group., Assessing population zinc status with serum zinc concentration. IZiNCG Technical Brief, 2007(2)); or
Dietary assessment – According to the Institute of Medicine, the RDA for pregnant women is 12 mg/day (Food and Nutrition Board, Institute of Medicine. Zinc. Dietary reference intakes for vitamin A, vitamin K, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, D.C.: National Academy Press; 2001:442‐501); or
Estimated country prevalence of zinc deficiency (Wessells 2012).
We will use the primary outcomes in the subgroup analysis.
We will assess differences between subgroups by interaction tests. For random‐effects and fixed‐effect meta‐analyses using methods other than inverse variance, we will assess differences between subgroups by interaction tests.
We did not perform this subgroup analysis, due to an imbalance in numbers of studies and women in each subgroup, meaning the analyses would not provide meaningful results.
Sensitivity analysis
We carried out sensitivity analysis to explore the effects of adequate allocation concealment, but found that restricting to only trials with adequate allocation concealment made very little difference to the results for the primary outcomes.
Summary of findings and assessment of the certainty of the evidence
For this update we used the GRADE approach (Schünemann 2009) in order to assess the quality of the body of evidence relating to the following primary outcomes for the main comparisons:
Preterm labour or birth (less than 37 weeks), or both
Stillbirth
Neonatal death
Perinatal death
Birthweight
Small‐for‐gestational age (birthweight less than 10th centile for gestational age)
Low birthweight (less than 2.5 kg)
We used the GRADEprofiler (GRADE 2014) to import data from Review Manager 5.4 (RevMan 2019) in order to create 'Summary of findings' tables. We produced a summary of the intervention effect and a measure of certainty for each of the above outcomes using the GRADE approach. This uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
Results of the search
See: Figure 1.
1.
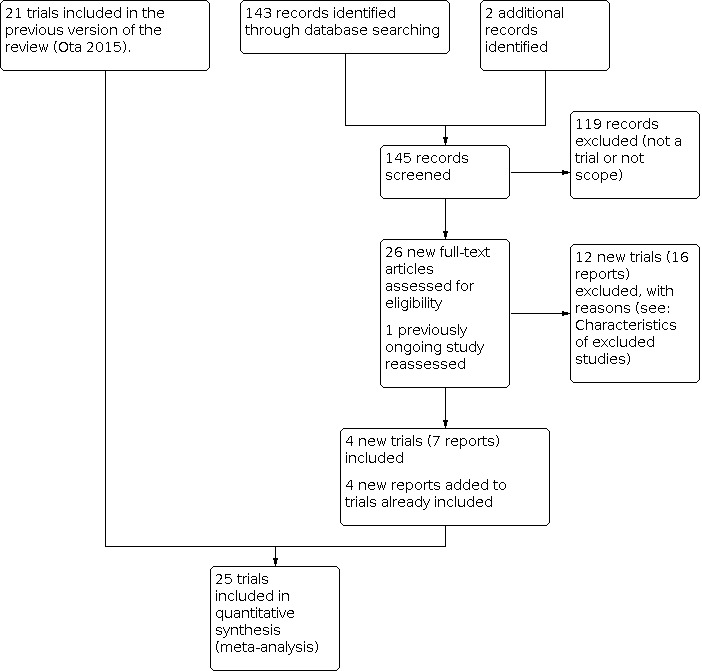
Study flow diagram.
In this update, we assessed 26 new reports and one trial that was classified as ongoing in the previous version of the review. We included four new randomised controlled trials (seven reports) (Bangladesh 2016; Iran 2009; Iran 2016; Tanzania 2017), and added four additional reports to trials already included. We excluded 12 trials (16 reports).
Included studies
We included a total of 25 randomised controlled trials (RCTs) involving over 18,000 women, at varying gestational ages at baseline, and their babies in this updated review (see the Characteristics of included studies tables for details).
Participants, settings and trial dates
Sixteen studies included women from low‐ and middle‐income settings. For this update, we added two studies from Iran (Iran 2009; Iran 2016), one from Tanzania (Tanzania 2017) and one from Bangladesh (Bangladesh 2016), and added data for a previously‐included trial (Egypt 2014). One of the four studies in the higher‐income or mixed‐income settings only recruited women at risk of giving birth to small‐for‐gestational age babies (UK 1991a). Trials were conducted between 1977 and 2014.
Baseline zinc concentrations and nutritional status
In this update, all four newly‐added trials included healthy pregnant women, although likely zinc‐deficient (Bangladesh 2016; Iran 2009; Iran 2016; Tanzania 2017). Previously three trials were included with women of normal zinc concentrations (UK 1991a; UK 1991b; USA 1989) and 18 trials with low zinc concentrations (Bangladesh 2000; Chile 2001; China 2001; Denmark 1996; Egypt 2014; Ghana 2009; Indonesia 2001a; Indonesia 2001b; Iran 2010; Nepal 2003; Pakistan 2005; Peru 1999; Peru 2004; S Africa 1985; UK 1989; USA 1983; USA 1985; USA 1995).
Types of interventions
In 11 trials iron‐folic acid (IFA) was provided as a co‐intervention to both the treatment and control groups (or iron‐folic acid‐alone group) (Egypt 2014; Ghana 2009; Indonesia 2001a; Iran 2009; Iran 2016; Nepal 2003; Pakistan 2005; Peru 1999; Peru 2004; Tanzania 2017; UK 1991b), while one trial provided only iron to both treatment and control groups (Chile 2001). Furthermore, 10 trials compared zinc with placebo (Bangladesh 2000; Bangladesh 2016; China 2001; Denmark 1996; Indonesia 2001b; Iran 2010; S Africa 1985; UK 1989; UK 1991a; USA 1989). Three trials provided both treatment and control groups with a multiple micronutrient supplement (USA 1983; USA 1985; USA 1995) (seeCharacteristics of included studies table).
Nepal 2003 was a cluster‐RCT, with analyses not adjusted for clustering in the presented study reports. We therefore performed additional calculations for these study results.
Dosage of zinc supplementation
The dose of daily zinc supplementation ranged from 5 mg (China 2001) to 50 mg zinc a day (Iran 2009). Some women in S Africa 1985 had doses of up to 90 mg zinc per day.
Duration of supplementation
Women were supplemented from before conception (Nepal 2003), as early as eight weeks of gestation (Tanzania 2017), and as late as 26 weeks of gestation (USA 1983; USA 1985). One study provided zinc supplements up to six months postpartum (Bangladesh 2016).
Adherence to treatment
Three studies (Chile 2001; Denmark 1996; Iran 2009) excluded women who did not comply with their treatment (85%, 60% and 70% compliance respectively) and the other 22 studies included or probably included women in the analysis who did not comply. Of the latter group, two studies (UK 1991a; USA 1983) presented at least some results separately for those women who complied and those who did not comply. Adherence was generally reported to be over 70%, except for Pakistan 2005; UK 1989; UK 1991a and Bangladesh 2016, where it was 50% to nearly 70%.
Funding sources
Funding sources varied across the studies. Bangladesh 2000 was funded by Royal Netherlands Government (activity number RISC, BD009602) and the ICDDR,B Centre for Health and Population Research; Bangladesh 2016 by the Nestle Foundation; Chile 2001 was partially funded by FONDECYT grant 068092; Denmark 1996 by lrege Frk. K. Rasmussens mindele‐ gat and Sygesikringen “danmarks” sundhedsfond.; Ghana 2009 was funded by Public Health Research Group of Edith Cowan University; Indonesia 2001b was funded by MotherCare, John Snow Inc., Washington, USA and UNICEF, Jakarta, Indonesia; Gadjah Mada University and the University of Newcastle, Newcastle, Australia; and infrastructure support from the Community Health and Nutrition Research Laboratories, Medical School, Gadjah Mada University and Ministry of Health, Republic of Indonesia through the Third Community Health and Nutrition Development Project Loan from the World Bank (IBRD Loan No. 3550‐IND); Iran 2009 was funded by the Research Fund of Aradabil University of Medical Sciences; Iran 2016 received financial support from the Research Deputy at the Guilan University of Medical Sciences; Nepal 2003 the US Agency for International Development (USAID) and additional support from the Unicef Country Office, Kathmandu, Nepal, and the Bill and Melinda Gates Foundation; Pakistan 2005 from Saving Newborn Lives of Save the Children, USA; Peru 1999 was supported by DAN‐5116‐a‐00‐8051‐00 and HRN‐A‐00‐97‐00015‐00, cooperative agreements between USAID/OHN and The Johns Hopkins University; Peru 2004 by the Nestle Research Foundation, Lausanne, Switzerland; S Africa 1985 by the Ross Laboratories, Columbus Ohio; South African Sugar Association and the South African Medical Research Council; Tanzania 2017 by grants from the National Institute of Child Health and Human Development (NICHD R01 HD057941‐01 and K24 HD 058795); UK 1989 and UK 1991a by the Smith Klein and French and Thames Laboratories Ltd.; USA 1983 by the Science and Education Administration of the USDA under Grant 5901‐0410‐8‐0105‐0 from the Competitive Research Grants Office; USA 1985 by the Science and Education Administration of the USDA under Grant 78‐59‐2065‐0‐1‐105‐1 from the Competitive Research Grants Office; USA 1989 was supported by a US Department of Agriculture, Sciences and Education Administration, Human Nutrition Extramural Research Grant to the Tulane University Medical Center; a cooperative agreement (7USC, 427, 250A, 1624, 2201) between the US Department of Agriculture Agricultural Research Service, Human Nutrition Research Center, Grand Forks, ND, and Tulane University Medical Center; Tulane Universityl and the General Nutrition Corporation, Fargo, ND, which provided the placebo and zinc supplement.; USA 1995 was supported by grants HD27289 and HD28119 from the National Institutes of Health, Bethesda, Md and by research contract DHHS 282‐92‐0055 from the Agency for Health Care Policy and Research, Rockville, Md. China 2001; Egypt 2014; Indonesia 2001a; Iran 2010; and UK 1991b did not disclose funding sources.
Declarations of interest
Bangladesh 2000; Bangladesh 2016; Egypt 2014; Indonesia 2001a; Indonesia 2001b; Iran 2016; Nepal 2003; Peru 2004 all declared no conflicts of interest. The remaining studies did not disclose whether or not there were any conflicts to declare.
Excluded studies
We excluded 28 studies in total. See table of Characteristics of excluded studies for details.
Nine studies were excluded because they were not RCTs (An 2001; Appelbaum 1979; Hambidge 1983; Hambidge 2017; India 1993; Kynast 1986; Naher 2012; Nishiyama 1999; Nogueira 2003). Most of the excluded studies were because the participants had health or pregnancy issues which therefore did not satisfy our inclusion criteria: seven studies only included women with impaired glucose tolerance (Asemi 2019a; Asemi 2019b; Heidarzadeh 2017; Karamali 2015; Karamali 2016; Ostadmohammadi 2019; Roshanravan 2020); two studies included women with HIV (Fawzi 2005; Villamor 2006), or were anaemic (Mahmoudian 2005; Yalda 2010), and single studies included women with night blindness (Christian 2001); who were postpartum (Fard 2017); had depression (Page 2018); or had preterm premature rupture of membranes (PPROM) (Shahnazi 2017). Three studies were excluded because the intervention was a micronutrient including a combination of supplements (France 2004; Makola 2003) or did not include a zinc supplement (Van Vliet 2001). One study was excluded because the only outcomes measured were inflammatory markers (Mesdaghinia 2019).
Risk of bias in included studies
Risk of bias for the included studies is summarised in Figure 2 and Figure 3.
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was considered adequate in 12 trials (Bangladesh 2000; Egypt 2014; Ghana 2009; Indonesia 2001b; Iran 2010; Iran 2016; Nepal 2003; Peru 2004; Tanzania 2017; UK 1989; UK 1991a; USA 1995), with most using computer‐generated random numbers. The remaining 13 trials did not adequately describe the method of random‐number generation and were judged to be at unclear risk of selection bias (Bangladesh 2016; Chile 2001; China 2001; Denmark 1996; Indonesia 2001a; Iran 2009; Pakistan 2005; Peru 1999; S Africa 1985; UK 1991b; USA 1983; USA 1985; USA 1989). Allocation concealment was considered adequate in 15 trials (China 2001; Denmark 1996; Egypt 2014; Indonesia 2001b; Iran 2009; Iran 2010; Iran 2016; Nepal 2003; Peru 1999;Peru 2004; S Africa 1985;Tanzania 2017; UK 1989;USA 1985; USA 1983). Allocation concealment was rated as unclear in 10 trials: Bangladesh 2000; Bangladesh 2016; Chile 2001; Ghana 2009; Indonesia 2001a; Pakistan 2005; UK 1991a; UK 1991b; USA 1989; USA 1995 (method not described or not clearly described); and in Indonesia 2001a there was third‐party randomisation but no details of how allocations were concealed.
Blinding
All trials stated that both investigators and mothers were blinded or that the trial was double‐blinded.
Blinding of outcome assessors was not well described but was likely to have happened in most trials (at least for short‐term outcomes), as most were placebo‐controlled. Sixteen trials (Bangladesh 2000; Bangladesh 2016; Indonesia 2001b; Iran 2009; Iran 2016; Nepal 2003; Pakistan 2005; Peru 1999; Peru 2004; S Africa 1985; Tanzania 2017; UK 1989; UK 1991a; USA 1985; USA 1989; USA 1995) were at low risk of detection bias and nine were unclear (Chile 2001; China 2001; Denmark 1996; Egypt 2014; Ghana 2009; Indonesia 2001a; Iran 2010; UK 1991b; USA 1983).
Incomplete outcome data
Losses to follow‐up ranged from 1% in UK 1989 to 40% in Denmark 1996. Attrition bias was judged to be at high risk in only five trials (Bangladesh 2016; Chile 2001; Denmark 1996; Iran 2010; Tanzania 2017) and unclear in 12 due to lack of sufficient information to make a clear judgement (Bangladesh 2000; Ghana 2009; Indonesia 2001a; Nepal 2003; Pakistan 2005; Peru 1999; S Africa 1985; UK 1991b; USA 1983; USA 1985; USA 1989; USA 1995).
Selective reporting
Selective reporting bias was mostly rated as unclear, with four RCTs judged to be at high risk due to expected outcomes not being reported, or reported incompletely (Peru 2004; Tanzania 2017; UK 1991b; USA 1985).
Other potential sources of bias
Other sources of bias were not generally evident, although several trials reported some baseline imbalances (China 2001; Iran 2010) and one had restricted analyses (Denmark 1996) and so were judged unclear.
Effects of interventions
See: Table 1
Zinc supplementation versus no zinc (with or without placebo)
We included 25 RCTs involving over 18,000 women and their babies. Only three trials involved women with normal zinc levels (UK 1991a; USA 1989; UK 1991b). The remaining 22 trials included women with low levels of zinc (Bangladesh 2000; Chile 2001; China 2001; Denmark 1996; Egypt 2014; Ghana 2009; Indonesia 2001b; Indonesia 2001a; Iran 2010; Nepal 2003; Pakistan 2005; Peru 1999; Peru 2004; S Africa 1985; UK 1989; USA 1983; USA 1985; USA 1995), or with probable zinc deficiency (Bangladesh 2016; Iran 2009; Iran 2016; Tanzania 2017).
Primary outcomes
When compared to placebo, zinc supplementation may have little or no effect on reducing the risk of preterm births (risk ratio (RR) 0.87, 95% confidence interval (CI) 0.74 to 1.03; 21 studies, 9851 participants; I2 = 44%; low‐quality evidence; Analysis 1.1). Further, zinc supplementation may make little or no difference in reducing the risk of stillbirths (RR 1.22, 95% CI 0.80 to 1.88; 7 studies, 3295 participants; low‐certainty evidence;Analysis 1.2), or perinatal deaths (RR 1.10, 95% CI 0.81 to 1.51; 2 studies, 2489 participants; low‐certainty evidence;Analysis 1.4). It is uncertain whether zinc supplementation reduces neonatal death because the certainty of the evidence is very low (Analysis 1.3). Finally, for other birth outcomes, zinc supplementation may make little or no difference in mean birthweight (MD 13.83, 95% CI −15.81 to 43.46; 22 studies, 7977 participants; I2 = 42%; low‐certainty evidence;Analysis 1.5), and probably makes little or no difference to the risk of low birthweight (RR 0.94, 95% CI 0.79 to 1.13; 17 studies, 7399 participants; I2 = 36%; moderate‐certainty evidence;Analysis 1.7) or small‐for‐gestational‐age babies as compared to placebo (RR 1.02, 95% CI 0.92 to 1.12; 9 studies, 5330 participants; moderate‐certainty evidence;Analysis 1.6). Subgroup analyses were not conducted, as very few studies used normal zinc populations.
1.1. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 1: Preterm birth (< 37 weeks)
1.2. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 2: Stillbirth
1.4. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 4: Perinatal death
1.3. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 3: Neonatal death
1.5. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 5: Birthweight
1.7. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 7: Low Birthweight (< 2500 g)
1.6. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 6: Small‐for‐gestational age or IUGR
Secondary outcomes
Maternal and pregnancy outcomes
Zinc supplementation may make little or no difference to pregnancy hypertension or pre‐eclampsia (RR 0.79, 95% CI 0.52 to 1.20; 10 studies, 3999 participants; Analysis 1.9) or prelabour rupture of membranes (RR 0.95, 95% CI 0.81 to 1.13; 4 studies, 2410 participants; Analysis 1.10). It is uncertain whether zinc supplementation improves or reduces antepartum haemorrhage (studies = 1; Analysis 1.8), post‐term birth (studies = 3; Analysis 1.11), meconium in liquor (studies = 2; Analysis 1.14), instrumental vaginal birth (studies = 1; Analysis 1.16), retention of placenta (studies = 1; Analysis 1.17), and smell dysfunction or taste dysfunction (1 study each; Analysis 1.19; Analysis 1.20) because the certainty of this evidence is very low. In one trial of women at risk for small‐for‐gestational‐age babies (UK 1991a), fewer women in the zinc group than in the no‐zinc group were induced (RR 0.27, 95% CI 0.10 to 0.73; 1 study, 52 women; Analysis 1.12).
1.9. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 9: Pregnancy hypertension or pre‐eclampsia
1.10. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 10: Prelabour rupture of membranes
1.8. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 8: Antepartum haemorrhage
1.11. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 11: Post‐term birth
1.14. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 14: Meconium in liquor
1.16. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 16: Instrumental vaginal birth
1.17. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 17: Retention of placenta
1.19. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 19: Smell dysfunction
1.20. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 20: Taste dysfunction
1.12. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 12: Induction of labour
In addition, compared to placebo, zinc supplementation may make little or no difference in maternal infections (RR 0.94, 95% CI 0.72 to 1.23; 4 studies, 1891 participants; Analysis 1.13), caesarean section (RR 0.89, 95% CI 0.65 to 1.20; 8 studies, 2608 participants; I2 = 66%; Analysis 1.15), or postpartum haemorrhage (RR 0.54, 95% CI 0.12 to 2.36; 4 studies, 1115 participants; I2 = 84%; Analysis 1.18). The heterogeneity in caesarean section (I2 = 66%) may in part be due to the income level of the countries where the trials were conducted. Women in high‐income settings who were supplemented with zinc saw a trend towards a reduction in caesarean sections, while the opposite was true for women in low‐income settings.
1.13. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 13: Any maternal infection
1.15. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 15: Caesarean section
1.18. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 18: Postpartum haemorrhage
Fetal neurodevelopmental assessment
In one RCT of 176 babies (Peru 2004), four measures of fetal heart rate (fetal heart rate (Analysis 1.21), number of fetal movement bouts (Analysis 1.24), fetal activity level (Analysis 1.25), and fetal movement amplitude (Analysis 1.26)) showed no evidence of differences between the zinc and no‐zinc groups, while fetal heart rate variability (MD 0.60, 95% CI 0.04 to 1.16; 1 study, 176 participants; Analysis 1.22) and number of fetal accelerations were higher in the zinc groups (MD 1.90, 95% CI 0.91 to 2.89; 1 study, 176 participants; Analysis 1.23).
1.21. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 21: Fetal heart rate (beats/minute)
1.24. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 24: Number of fetal movement bouts
1.25. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 25: Fetal activity level
1.26. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 26: Fetal movement amplitude
1.22. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 22: Fetal heart rate variability (beats/minute)
1.23. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 23: Number of fetal accelerations
Neonatal outcomes
Zinc supplementation may make little or no difference to gestational age at birth (MD −0.01, 95% CI −0.13 to 0.10; 11 studies, 4627 participants; Analysis 1.27), high birthweight or large‐for‐gestational age (RR 0.96, 95% CI 0.65 to 1.42; 4 studies, 1636 participants; I2 = 40%; Analysis 1.28; RR 1.09, 95% CI 0.80 to 1.48; 1 study; 1206 participants; Analysis 1.29, respectively), or neonatal head circumference (cm) (MD 0.05 cm, 95% CI −0.10 to 0.20; 12 studies, 5344 participants; I2 = 72%; Analysis 1.31).
1.27. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 27: Gestational age at birth
1.28. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 28: High birthweight (> 3500 g)
1.29. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 29: Large‐for‐gestational age
1.31. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 31: Neonatal head circumference
It is uncertain whether zinc supplementation improves or reduces the following outcomes: Apgar scores less than 8, 7 or 5 at five minutes (Analysis 1.30), neonatal hypoxia (Analysis 1.32), jaundice (Analysis 1.34), neonatal intraventricular haemorrhage (Analysis 1.36), necrotising enterocolitis (Analysis 1.37), or neonatal hospital stay (Analysis 1.38). Each of these outcomes was only available from one or two RCTs. Zinc supplementation may make little or no difference to congenital malformations (RR 0.67, 95% CI 0.33 to 1.35; 5 studies, 1106 participants; Analysis 1.39) but may slightly reduce neonatal sepsis (RR 0.17, 95% CI 0.03 to 0.98; 2 studies, 736 participants; Analysis 1.33), and respiratory distress syndrome (RR 0.46, 95% CI 0.23 to 0.90; 4 studies, 1684 participants; I2 = 62%; Analysis 1.35).
1.30. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 30: Five‐minute Apgar score
1.32. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 32: Neonatal hypoxia (blue or floppy)
1.34. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 34: Neonatal jaundice
1.36. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 36: Neonatal intraventricular haemorrhage
1.37. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 37: Necrotising enterocolitis
1.38. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 38: Neonatal hospital stay
1.39. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 39: Congenital malformation
1.33. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 33: Neonatal sepsis
1.35. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 35: Respiratory distress syndrome
Zinc supplementation may make little or no difference to neonatal chest circumference (MD 0.01 cm, 95% CI −0.16 to 0.18; 3 studies, 1427 participants; Analysis 1.40), crown‐heel length (MD 0.08, 95% CI −0.20 to 0.36; 4 studies, 2149 participants; I2 = 37%; Analysis 1.41), birth length (MD −0.02 cm, 95% CI −0.17 to 0.14; 8 studies, 4540 participants; Analysis 1.42), or mid‐upper arm circumference (non‐prespecified) (MD 0.73 mms, 95% CI −0.37 to 1.83; 3 studies, 1826 participants; Analysis 1.43).
1.40. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 40: Neonatal chest circumference
1.41. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 41: Crown‐heel length
1.42. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 42: Neonatal Birth Length
1.43. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 43: Neonatal mid‐upper arm circumference
Infant/child outcomes
In one RCT of 410 infants (Bangladesh 2000), the zinc group (196 infants) had fewer episodes per infant of acute diarrhoea over six months (MD −0.40 episodes, 95% CI −0.79 to −0.01; Analysis 1.44). No clear differences were seen for episodes of persistent dysentery (Analysis 1.45), cough (Analysis 1.46), acute lower respiratory infection (Analysis 1.47) or impetigo (Analysis 1.48) over the same period.
1.44. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 44: Diarrhoea (episodes/infant over 6 months)
1.45. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 45: Dysentery (episodes/infant over 6 months)
1.46. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 46: Cough (episodes/infant over 6 months)
1.47. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 47: Acute lower respiratory infection (episodes/infant over 6 months)
1.48. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 48: Impetigo (episodes/infant over 6 months)
Infant weight‐for‐age (Z‐score) were higher in the control group when compared with the zinc group at six months (MD ‐0.34, 95% CI ‐0.57 to ‐0.12; 2 studies, 235 participants) and 13 months (MD ‐0.40, 95% CI ‐0.70 to ‐0.10; 1 study, 168 participants; Bangladesh 2000) (Analysis 1.49). No evidence of difference was seen for weight‐for‐height at six months in one RCT of 67 infants (Indonesia 2001a) (Analysis 1.50). Zinc supplementation may make little or no difference to infant mid‐upper arm circumference (MD 1.00 mm, 95% CI −0.55 to 2.55; 1 study, 410 participants; Analysis 1.51).
1.49. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 49: Infant weight‐for‐age (Z‐score)
1.50. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 50: Infant weight‐for‐height (Z‐score)
1.51. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 51: Infant mid‐upper arm circumference
Zinc supplementation may make little or no difference to infant head circumference (MD −0.10 cm, 95% CI −0.38 to 0.18; 1 study, 410 participants; Analysis 1.66), or infant chest circumference (MD −0.20 cm, 95% CI −0.56 to 0.16; 1 study, 410 participants; Analysis 1.67).
1.66. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 66: Infant head circumference
1.67. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 67: Infant chest circumference
Measures of infant development
Three RCTs (Bangladesh 2000; Peru 2004; USA 1995) measured child development outcomes. A subset of 168 infants from Bangladesh 2000 assessed at 13 months found that the zinc group had worse mental development (MD −3.30, 95% CI −6.51 to −0.09; 1 study, 168 participants; Analysis 1.52), psychomotor development index scores (MD −7.00, 95% CI −11.92 to −2.08; 1 study, 168 participants; Analysis 1.53), emotional tone (MD −0.65, 95% CI −1.24 to −0.06; 1 study, 168 participants; Analysis 1.55) and co‐operation (MD −0.60, 95% CI −1.16 to −0.04; 1 study, 168 participants; Analysis 1.57) than the control group, with infant approach (Analysis 1.54), activity (Analysis 1.56), and vocalisation (Analysis 1.58) showing no clear differences. The US RCT (USA 1995) followed up 355 infants at five years, finding no evidence of differences between zinc and control groups for differential abilities (Analysis 1.59), visual or auditory sequential memory scores (Analysis 1.60; Analysis 1.61), Knox cube (Analysis 1.62), gross motor scale (Analysis 1.63) and grooved pegboard scores (Analysis 1.64). The trial in Peru (Peru 2004) reported the intelligence quotient of infants at 54 months, which showed no evidence of difference (Analysis 1.65).
1.52. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 52: Infant mental development index
1.53. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 53: Infant psychomotor development index
1.55. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 55: Infant emotional tone
1.57. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 57: Infant co‐operation
1.54. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 54: Infant approach
1.56. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 56: Infant activity
1.58. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 58: Infant vocalisation
1.59. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 59: Differential abilities score at 5 years
1.60. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 60: Visual sequential memory score
1.61. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 61: Auditory sequential memory score
1.62. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 62: Knox cube score
1.63. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 63: Gross motor scale score
1.64. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 64: Grooved pegboard score
1.65. Analysis.

Comparison 1: Zinc supplementation versus no zinc (with or without placebo), Outcome 65: Intelligence quotient of infants at 54 months
Reporting bias
There are five outcomes with meta‐analyses including more than 10 studies (Analysis 1.1; Analysis 1.5; Analysis 1.7; Analysis 1.27; Analysis 1.31). Although there was no evidence of reporting bias in preterm birth, birthweight and head circumference, the distribution of the results of low birthweight and gestational age at birth are asymmetrical (Figure 4; Figure 5). This means there is a possibility of reporting bias and warrants careful interpretation of the results. The result for effectiveness by zinc could have been overestimated.
4.

Funnel plot of comparison: 1 Zinc supplementation versus no zinc (with or without placebo), outcome: 1.8 Low Birth Weight (< 2500 g).
5.

Funnel plot of comparison: 1 Zinc supplementation versus no zinc (with or without placebo), outcome: 1.27 Gestational age at birth.
Discussion
Summary of main results
This review of 25 randomised controlled trials, including over 18,000 women and their babies, has not provided compelling evidence for routine zinc supplementation during pregnancy to mitigate maternal and neonatal outcomes.
The evidence suggests that zinc supplementation may result in little or no difference in reducing the risk of preterm birth, stillbirths, or perinatal deaths. It is uncertain whether zinc supplementation reduces neonatal death because the certainty of evidence is very low. In terms of other birth outcomes, zinc supplementation may make little or no difference in mean birthweight, and probably makes little or no difference in reducing the risk of low birthweight and small‐for‐gestational age babies as compared to placebo or no zinc supplementation.
The suggestion of reporting bias from the funnel plots on low birthweight and gestational age at birth warrants further investigation, as the results of effectiveness of zinc supplementation could have been overestimated. Subgroup analysis involving women who are healthy (normal zinc) compared to those that are or are likely to be zinc‐deficient, could not be conducted as most studies used populations with presumed zinc deficiency.
Overall completeness and applicability of evidence
Previous Cochrane Reviews on micronutrient and iron‐folic acid supplementation in pregnancy showed a probable slight reduction in preterm birth with MMN and iron‐folic acid supplementation as compared to iron with or without folic acid (Risk Ratio: 0.95, 95% Confidence Interval: 0.90 to 1.01, moderate quality of evidence) (Keats 2019). Although dosage of zinc may play a role, no dose‐response pattern was evident in this review (with the possible exception of pre‐eclampsia). It is possible that zinc used in conjunction with iron‐folic acid may dilute the effect of supplementation. The intrauterine growth effect seen in UK 1991a, where women were selected on the basis of being at risk for giving birth to a small‐for‐gestational‐age baby, has not been replicated. In the Bangladesh 2000 study, where the incidence of small‐for‐gestational‐age was 75% and low birthweight was 43%, supplementation with 30 mg zinc daily did not improve pregnancy outcomes. This is most likely due to the presence of other concurrent nutrient deficiencies. Peru 1999, Bangladesh 2000 and USA 1995 studies attempted to assess the neurodevelopmental effect of zinc supplementation on infants. The inconsistencies in their results probably reflect the dependence of such outcomes on many variables.
Quality of the evidence
The overall risk of bias was low in half of the studies.
We assessed the certainty of the evidence using GRADE, comparing the effects of zinc supplementation versus placebo/no intervention during pregnancy (Table 1). The GRADE certainty of the evidence was low for preterm birth, perinatal death, stillbirth, and birthweight, downgraded by two levels due to most studies having design limitations, and wide 95% CIs crossing the line of no effect. Neonatal death was considered to be very‐low certainty for limitations in study designs, a wide 95% CI crossing the line of no effect, and the presence of high heterogeneity (I2 = 54%). Small‐for‐gestational‐age and low birthweight were considered to be moderate certainty of evidence, downgraded by just one level because of study design limitations.
Potential biases in the review process
We followed the Cochrane Pregnancy and Childbirth Group search strategies and review process to reduce potential biases.
Agreements and disagreements with other studies or reviews
Zinc is likely to be only one micronutrient in the overall picture of maternal nutrition prior to and during the course of pregnancy. A Cochrane Review on micronutrient supplementation concluded that there is a reduction for low birthweight and small‐for‐gestational‐age babies with multiple‐micronutrient supplements compared with iron‐folic acid supplementation, and potentially a small benefit for preterm birth (Keats 2019). Addressing the underlying problem of poor nutrition is critical in order to make any significant impact on morbidity and mortality. This includes proximal and intermediate factors of malnutrition, including low socioeconomic status (Peru 1999). Villar 2003 indicated that while zinc supplementation may be promising, "it is unlikely that any specific nutrient on its own ... will prevent .... preterm delivery or death during pregnancy". The WHO and UNICEF promote antenatal use of multiple‐micronutrient supplementation, including zinc, to all pregnant women where there are population‐level micronutrient deficiencies and in the context of rigorous research (WHO 2020).
Although improving birthweight, particularly in women from low‐income countries, is desirable, data from Nepal 2003 promote a degree of caution. In the overall Nepal 2003 study, multiple‐micronutrient supplementation (but not other combinations of micronutrients) compared with controls was associated with more babies with a birthweight above 3.3 kg; and this high birthweight was associated with an increased risk of symptoms of birth asphyxia (RR 0.96, 95% CI 0.65 to 1.42).
Despite uncertainty about the effects of maternal zinc supplementation, many pharmaceutical companies have added zinc to their multivitamin preparations. Lack of any significant benefit from zinc supplementation of mothers suggests that we should now not waste valuable resources looking at zinc in isolation. Furthermore, infant micronutrient supplementation (including zinc) may be more effective than maternal supplementation (Lassi 2016; Shrimpton 2005). Any future research aimed at improving outcomes related to maternal nutrition should address ways of modifying the overall nutritional status of pregnant women, particularly in developing countries. This may not come from the scientific but from the political community, where more resources need to be put into improving the overall socioeconomic status of impoverished populations, and also to improve the status of the women in such populations.
Authors' conclusions
Implications for practice.
The findings of this review suggest that routine zinc supplementation probably made little or no difference in reducing the risk of preterm births in women of probable low zinc status.
There is not enough evidence to show that routine zinc supplementation in women results in an effect on other clinically relevant outcomes, including mortality (perinatal and neonatal), birthweight, small‐for‐gestational‐age or low birthweight.
Implications for research.
There appeared to be inconsistency between trials for some pregnancy outcomes. The reduction in preterm birth needs further assessment, probably in association with protein‐calorie nutrition and co‐supplementation iron‐folic acid. Many studies have demonstrated some positive response in biochemical parameters such as serum zinc status of mother or baby, or both, with supplementation (Bangladesh 2000; Peru 1999), as have studies of iron supplementation in pregnancy to reduce maternal iron deficiency, iron‐deficiency anemia and anemia at term (Peña‐Rosas 2015). It is now crucial to focus on the impact of any intervention on outcomes that are of clinical significance and particularly those that may be related to maternal, fetal, neonatal and infant mortality and morbidity. This is relevant because of the limited resources, both financial and human, currently available worldwide, but in particular in the developing countries where such morbidity and mortality are high. Future research aimed at improving outcomes related to maternal nutrition should address ways of modifying the overall nutritional status of pregnant women, particularly in low‐income regions, and avoiding looking at zinc in isolation. Future research should also address other interventions such as work reduction in populations of pregnant women at high risk of nutritional deficiency.
What's new
| Date | Event | Description |
|---|---|---|
| 3 July 2020 | New citation required and conclusions have changed | Addition of studies, corrections, reclassifications and further analyses. Zinc deficiency does not reduce the risk of preterm births in women with or at risk of zinc deficiency. |
| 3 July 2020 | New search has been performed | Search updated; we added 4 new randomised controlled trials and 1 previously included trial with data to this review. |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 3, 1997
| Date | Event | Description |
|---|---|---|
| 15 September 2015 | Amended | Added additional information to Characteristics of included studies and Characteristics of excluded studies tables. |
| 31 October 2014 | New search has been performed | Search updated. Seven new reports identified from the updated search: two reports of one new trial included (Egypt 2014a); one new trial excluded (Naher 2012) and four new reports of existing trials added. Methods have been updated. A 'Summary of findings' table incorporated. |
| 31 October 2014 | New citation required but conclusions have not changed | The inclusion of one new trial (Egypt 2014a) did not change the conclusions. |
| 9 November 2011 | New citation required but conclusions have not changed | New authors helped to update this review. |
| 9 November 2011 | New search has been performed | Search updated. Three new trials included (China 2001; Ghana 2009; Iran 2010) and four new trials excluded (Mahmoudian 2005; Van Vliet 2001; Villamor 2006; Yalda 2010). |
| 1 July 2011 | Amended | Search updated. Thirteen trial reports added to Studies awaiting classification. |
| 6 November 2008 | Amended | Converted to new review format. |
| 20 December 2006 | New search has been performed | Search updated. Nine new studies have been added to the original seven included studies, plus one previously excluded study (USA 1985) has now been included, making a total of 17 studies included in the 2006 update. A total of 11 studies have been excluded in this update and two studies have been placed in Studies awaiting classification. The Background and Methods sections have been expanded in this update, and additional outcomes have been added. The title has been changed from 'Zinc supplementation in pregnancy' to 'Zinc supplementation for improving pregnancy and infant outcome'. The conclusions regarding the effect of zinc supplementation on reducing preterm birth have been slightly strengthened. |
Acknowledgements
As part of the pre‐publication editorial process, this review was commented on by three peers (an editor and two referees who are external to the editorial team) and the Group's Statistical Adviser. The authors are grateful to the following peer reviewers for their time and comments: Menna Kanel, Egypt; Theresa Lawrie.
This project was supported by the National Institute for Health Research (NIHR), via Evidence Synthesis Programme funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
S Osendarp, for providing information about unpublished trials. We also thank Sally J Reynolds and Becky Ann Davie for their support for the 'Risk of bias' assessment in this update.
We would like to acknowledge Erika Ota, Philippa Middleton, Kassam Mahomed, Celine Miyazaki, Rintaro Mori and Ruoyan Tobe‐Gai for their contribution to previous versions of this review.
Appendices
Appendix 1. Search methods for ICTRP and ClinicalTrials.gov
ICTRP
zinc AND pregnancy
zinc AND pregnant
zinc AND antenatal
zinc AND prenatal
ClinicalTrials.gov
Advanced search
pregnancy | Interventional Studies | zinc
Data and analyses
Comparison 1. Zinc supplementation versus no zinc (with or without placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Preterm birth (< 37 weeks) | 21 | 9851 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.74, 1.03] |
| 1.2 Stillbirth | 7 | 3295 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.80, 1.88] |
| 1.3 Neonatal death | 3 | 1965 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [0.40, 14.83] |
| 1.4 Perinatal death | 2 | 2489 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.81, 1.51] |
| 1.5 Birthweight | 22 | 7977 | Mean Difference (IV, Random, 95% CI) | 13.83 [‐15.81, 43.46] |
| 1.6 Small‐for‐gestational age or IUGR | 9 | 5330 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.92, 1.12] |
| 1.7 Low Birthweight (< 2500 g) | 17 | 7399 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.79, 1.13] |
| 1.8 Antepartum haemorrhage | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.8.1 Second trimester | 1 | 1206 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.57, 4.45] |
| 1.8.2 Third trimester | 1 | 1206 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.39, 2.33] |
| 1.9 Pregnancy hypertension or pre‐eclampsia | 10 | 3999 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.52, 1.20] |
| 1.9.1 Pre‐eclampsia | 6 | 2568 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.62, 1.42] |
| 1.9.2 Pregnancy hypertension | 5 | 994 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.20, 2.14] |
| 1.9.3 Pregnancy hypertension or pre‐eclampsia | 1 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.20, 1.45] |
| 1.10 Prelabour rupture of membranes | 4 | 2410 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.81, 1.13] |
| 1.11 Post‐term birth | 3 | 1554 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.74, 1.59] |
| 1.12 Induction of labour | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.10, 0.73] |
| 1.13 Any maternal infection | 4 | 1891 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.23] |
| 1.14 Meconium in liquor | 2 | 1385 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.86, 1.56] |
| 1.15 Caesarean section | 8 | 2608 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.65, 1.20] |
| 1.16 Instrumental vaginal birth | 1 | 1206 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.79, 1.59] |
| 1.17 Retention of placenta | 1 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 6.62 [0.83, 52.71] |
| 1.18 Postpartum haemorrhage | 4 | 1115 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.12, 2.36] |
| 1.19 Smell dysfunction | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.55, 1.86] |
| 1.20 Taste dysfunction | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.36, 1.50] |
| 1.21 Fetal heart rate (beats/minute) | 1 | 176 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐3.31, 0.91] |
| 1.22 Fetal heart rate variability (beats/minute) | 1 | 176 | Mean Difference (IV, Random, 95% CI) | 0.60 [0.04, 1.16] |
| 1.23 Number of fetal accelerations | 1 | 176 | Mean Difference (IV, Random, 95% CI) | 1.90 [0.91, 2.89] |
| 1.24 Number of fetal movement bouts | 1 | 176 | Mean Difference (IV, Random, 95% CI) | 1.70 [‐2.53, 5.93] |
| 1.25 Fetal activity level | 1 | 176 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐2.66, 2.06] |
| 1.26 Fetal movement amplitude | 1 | 176 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.79, 1.19] |
| 1.27 Gestational age at birth | 11 | 4627 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.13, 0.10] |
| 1.28 High birthweight (> 3500 g) | 4 | 1636 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.65, 1.42] |
| 1.29 Large‐for‐gestational age | 1 | 1206 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.80, 1.48] |
| 1.30 Five‐minute Apgar score | 4 | 2304 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.61, 1.49] |
| 1.30.1 Less than 8 | 1 | 486 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.32, 2.38] |
| 1.30.2 Less than 7 | 1 | 528 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.49, 1.84] |
| 1.30.3 Less than 5 | 2 | 1290 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.46, 2.20] |
| 1.31 Neonatal head circumference | 12 | 5344 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.10, 0.20] |
| 1.32 Neonatal hypoxia (blue or floppy) | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 5.25 [0.66, 41.86] |
| 1.33 Neonatal sepsis | 2 | 736 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.03, 0.98] |
| 1.34 Neonatal jaundice | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.06, 13.56] |
| 1.35 Respiratory distress syndrome | 4 | 1684 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.23, 0.90] |
| 1.36 Neonatal intraventricular haemorrhage | 1 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.14, 6.86] |
| 1.37 Necrotising enterocolitis | 1 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.18, 21.34] |
| 1.38 Neonatal hospital stay | 1 | 580 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐2.39, 0.19] |
| 1.39 Congenital malformation | 5 | 1106 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.33, 1.35] |
| 1.40 Neonatal chest circumference | 3 | 1427 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.16, 0.18] |
| 1.41 Crown‐heel length | 4 | 2149 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.20, 0.36] |
| 1.42 Neonatal Birth Length | 8 | 4340 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.17, 0.14] |
| 1.43 Neonatal mid‐upper arm circumference | 3 | 1826 | Mean Difference (IV, Random, 95% CI) | 0.73 [‐0.37, 1.83] |
| 1.44 Diarrhoea (episodes/infant over 6 months) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.44.1 Acute diarrhoea | 1 | 410 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.79, ‐0.01] |
| 1.44.2 Persistent diarrhoea | 1 | 410 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.13, 0.13] |
| 1.45 Dysentery (episodes/infant over 6 months) | 1 | 410 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.12, 0.00] |
| 1.46 Cough (episodes/infant over 6 months) | 1 | 410 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.56, 0.16] |
| 1.47 Acute lower respiratory infection (episodes/infant over 6 months) | 1 | 410 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.34, 0.14] |
| 1.48 Impetigo (episodes/infant over 6 months) | 1 | 410 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.24, 0.04] |
| 1.49 Infant weight‐for‐age (Z‐score) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.49.1 Z‐score at 6 months | 2 | 235 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.57, ‐0.12] |
| 1.49.2 Z‐score at 13 months | 1 | 168 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.70, ‐0.10] |
| 1.50 Infant weight‐for‐height (Z‐score) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.82, 0.02] |
| 1.51 Infant mid‐upper arm circumference | 1 | 410 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐0.55, 2.55] |
| 1.52 Infant mental development index | 1 | 168 | Mean Difference (IV, Random, 95% CI) | ‐3.30 [‐6.51, ‐0.09] |
| 1.53 Infant psychomotor development index | 1 | 168 | Mean Difference (IV, Random, 95% CI) | ‐7.00 [‐11.92, ‐2.08] |
| 1.54 Infant approach | 1 | 168 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.38, 0.58] |
| 1.55 Infant emotional tone | 1 | 168 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.24, ‐0.06] |
| 1.56 Infant activity | 1 | 168 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.43, 0.23] |
| 1.57 Infant co‐operation | 1 | 168 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.16, ‐0.04] |
| 1.58 Infant vocalisation | 1 | 168 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.54, 0.38] |
| 1.59 Differential abilities score at 5 years | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.59.1 Non‐verbal ability | 1 | 355 | Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐5.70, 0.90] |
| 1.59.2 Verbal ability | 1 | 355 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐2.56, 1.96] |
| 1.59.3 General conceptual ability, IQ | 1 | 355 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐3.74, 1.54] |
| 1.60 Visual sequential memory score | 1 | 355 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐2.24, 0.64] |
| 1.61 Auditory sequential memory score | 1 | 355 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.65, 1.85] |
| 1.62 Knox cube score | 1 | 355 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.19, 0.39] |
| 1.63 Gross motor scale score | 1 | 355 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐4.79, 0.79] |
| 1.64 Grooved pegboard score | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.64.1 Dominant hand | 1 | 355 | Mean Difference (IV, Random, 95% CI) | 2.50 [‐1.26, 6.26] |
| 1.64.2 Non‐dominant hand | 1 | 355 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐2.71, 5.11] |
| 1.65 Intelligence quotient of infants at 54 months | 1 | 181 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐3.33, 2.53] |
| 1.66 Infant head circumference | 1 | 410 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.38, 0.18] |
| 1.67 Infant chest circumference | 1 | 410 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.56, 0.16] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bangladesh 2000.
| Study characteristics | ||
| Methods | A double‐blind, randomised, placebo‐controlled trial | |
| Participants | 559 pregnant women between 12 and 16 weeks' gestation, from Dhaka city slums The 446 women who completed follow‐up had a mean baseline serum zinc level of 15.3 (SD 4.3) µmol/L (similar to those lost to follow‐up) Energy intakes were low at 4 months' gestation (median 6065 kJ/day). A total of 559 pregnant women from selected areas of Dhaka city slums were identified between 12 and 16 weeks of gestation through an established pregnancy identification system between March and June 1996. Women were included if they remain at or near their residences in Dhaka for the delivery, without established medical risk for reduced or excessive birthweight (e.g. hypertension, renal disease, or diabetes). Energy intakes were low at 4 months' gestation (median 6065 kJ/day) | |
| Interventions |
Zinc was given twice the recommended daily intake during the last 2 trimesters of pregnancy The zinc tablets contained (31.0 mg Zn/tablet; range: 28.6 – 32.6) and placebo tablets contained (0.0 mg Zn/tablet; range: 0.0 – 0.1) was verified and confirmed by 2 independent laboratories. The placebo was a cellulose tablet indistinguishable from the zinc supplement in both appearance and taste. Health workers provided a 1‐week supply of zinc or placebo tablets (ACME Ltd, Dhaka) to the houses of the women weekly and instructed her to consume 1 tablet daily between meals and not together with other vitamin or mineral supplements Compliance was assessed by counting the remaining tablets in each strip at the next visit. Unannounced compliance checks between regular visits were performed monthly in subsamples of 10% of the study participants |
|
| Outcomes |
Maternal outcomes Serum zinc concentrations at 7 months' gestation Haemoglobin concentrations at 7 months' gestation Blood pressure at 7 months' gestation Neonatal outcomes Birthweight Low birthweight, < 2500 g, < 2000 g, < 1500 g Stillbirth Gestational age (weeks) Premature birth,< 37 weeks, < 32 weeks Small‐for‐gestational age Length (cm) Head circumference (cm) Chest circumference (cm) Mid‐upper arm circumference (mm) |
|
| Notes | Adherence: percentage of days during follow‐up that a woman reported having consumed a supplement was 86% Final sample size of 410 infants was sufficient to detect a 110 g difference in birthweight Dates of study: March 1996 ‐ October 1997 Funding sources: Royal Netherlands Government (activity number RISC, BD009602) and the ICDDR,B Centre for Health and Population Research, which receives its support from United Nations agencies, international organizations and foundations, medical research organizations, and donor governments. Declarations of interest: authors declare no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated random letter assignment." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomly assigned" Comment: no details given about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both investigators and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specifically mentioned but assessors were also likely to have been blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 113/559 (20.2%) women were lost to follow‐up before birth; (55 (20.4%) in the zinc group and 58 (20.0%) in the placebo group) ‐ most (60) due to migration out of the area By 13 months follow‐up, 383 (68.5%) infants remained in the trial, with only 168 of these infants being included in the 13‐month analysis |
| Selective reporting (reporting bias) | Unclear risk | Some primary outcomes such as mode of birth not reported |
| Other bias | Low risk | No apparent source of other bias |
Bangladesh 2016.
| Study characteristics | ||
| Methods | This double‐blind, RCT was conducted in 2 urban slum areas, Kamrangir Char and Hazaribagh, in Dhaka, Bangladesh | |
| Participants | Zinc group (n = 28), Placebo group (n = 28)
Pregnant women between 11 ‐ 13 weeks of gestation were eligible for enrolment Exclusion criteria included: systemic or chronic disease, previous complicated pregnancies or abortion and congenital anomaly. Informed written consent was obtained from participating mothers |
|
| Interventions | 1. Zinc group (n = 28) received a tablet to be consumed daily, containing 20 mg of zinc sulfate 2. Placebo group (n = 28) received a cellulose placebo tablet to be consumed daily | |
| Outcomes |
Neonatal outcomes Low birthweight (< 2500 g) Preterm birth Serum/plasma zinc concentrations (umol/L) |
|
| Notes | Compliance with tablet consumption was assessed by counting the remaining tablets in the bubble packs during weekly home visits
Dates of study: 2011 ‐ 2013 Funding Sources: Nestle Foundation Declarations of Interest: authors declare no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "placebo‐controlled, double‐blind and randomised trial" Comment: Insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment process to permit judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Probable. Quote: "Zinc and placebo tablets were identical in color, shape, size, odor and test." Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Probable. Quote: "Zinc and placebo tablets were identical in color, shape, size, odor and test." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Seventy percentage of the mother‐infant pairs completed the study protocol; the high number lost to follow‐up could be due to the >1‐year‐long study period since enrolment and the high migration rate of the slum population in Dhaka." Comment: Reasons for attrition were reported. Attrition and exclusions were balanced across the treatment arms |
| Selective reporting (reporting bias) | Low risk | All outcomes presented in the Methods section were reported in the paper |
| Other bias | Low risk | Study flow chart n values do not align with Table 1 n values |
Chile 2001.
| Study characteristics | ||
| Methods | A double‐blind, randomised, placebo‐controlled trial | |
| Participants | 804 pregnant adolescents were recruited from 5 Primary Care Centres in southern urban slums in Santiago, Chile. They were selected from their prenatal clinic visits before 20 weeks of gestation and aged < 19 years at the estimated time of delivery. The pregnant adolescents identified with chronic diseases, drug abuse, mental retardation, illiteracy or those with pregnancies due to incest or rape were not considered. Subgroup of 220 randomly selected pregnant adolescents at their 28 ‐ 30 weeks of gestation with a low zinc intake (7.4 (SD 2.3 mg)) at the initial admission were evaluated for dietary nutrient intake. Women showed adequate protein intakes but a relatively low mean energy intake | |
| Interventions |
Zinc‐supplemented group (S) received 20 mg of Zn capsules daily (sulphate), or the placebo group (P) received an equivalent capsule of a placebo containing lactose. The group codes changed twice during the study and were kept by the pharmacist who prepared the capsules until the end of computational analysis for double‐blinding procedure. The individuals who ingested less than 50% of the capsules in any month of the study were excluded. All participants received 40 mg iron (sulphate) supplements daily Compliance with zinc intake was evaluated by counting the remaining capsules during the monthly visits |
|
| Outcomes |
Maternal outcomes Pre‐eclampsia Plasma zinc Hair zinc Maternal oedema Maternal cholestasis Red blood cell membrane alkaline phosphatases Plasma alkaline phosphatases Neonatal outcomes Low birthweight Birthweight Gestational age at birth Preterm birth Spontaneous abortions Length at birth Head circumference |
|
| Notes | Adherence: non‐adherers were excluded from analysis; this included individuals who ingested less than 50% of zinc supplements in any month of the study Dates of study: not reported Funding sources: partially funded by FONDECYT grant 068092 Declarations of interest: not disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" Comment: no further details reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomly assigned" Comment: pharmacist kept codes; no further details reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind fashion" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up: 297/804 (37%) ‐ failure to come to visits (137), taking less than 15 zinc capsules in any 1 month (115), spontaneous abortion (12), intervention began after 20 weeks' gestation (10), absence of pregnancy (7), change of address (6), apparent intolerance to zinc or placebo (6), twin pregnancy (4) |
| Selective reporting (reporting bias) | Unclear risk | Not all expected maternal primary outcomes reported, but most primary infant outcomes specified in this review were reported |
| Other bias | Low risk | No apparent risk of other bias |
China 2001.
| Study characteristics | ||
| Methods | A double‐blind, randomised, placebo‐controlled four‐armed trial | |
| Participants | 146 pregnant women, less than 12 weeks' gestation, who were living in southwest Shanghai, Maqiao countryside and were attending the prenatal clinic at Maqiao Primary Health Care Center were selected for the study The people living in this area were uneducated with nutritional knowledge, and took cereal‐based diet with a little or even no milk or milk products; they were therefore presumed to have mild‐to‐moderate zinc deficiency according to Chinese recommended dietary allowance. The zinc content of drinking water in this area was considered negligible and no women received folic acid, iron supplementation or any commercial nutrition products during this trial |
|
| Interventions | For the zinc treatment groups, zinc lactate in capsule were given daily.
All capsules were prepared by Laboratory, Second Military Medical University with indistinguishable appearance. Women were instructed to take a single capsule per day 1 hour before or 3 hours after the evening meal. The content of the capsules and the code of the capsule bottles were not known by the investigator or the pregnant women. Only 156 women were followed up under antenatal care |
|
| Outcomes | For the purposes of this review, Group A, B and C were combined as an intervention group and Group D served as a control group Maternal outcomes Caesarean section Weight gain Duration of labour Oxygen demand Forceps Neonatal outcomes Small‐for‐gestational age/Intrauterine growth restriction Gestational age at birth Neonatal sepsis Low birthweight Congenital malformation Stillbirth Preterm birth Apgar score Chest circumference Neck circumference Head circumference Crown‐heel length Ponderal index |
|
| Notes | Dates of study: September 1995 ‐ December 1997 Funding sources: not disclosed Declarations of interest: not disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description other than the allocation was made randomly |
| Allocation concealment (selection bias) | Low risk | All capsules were prepared by pharmacy and allocation was concealed for both investigators and women |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All capsules were prepared by pharmacy and both investigators and enrolled pregnant women were concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts for maternal and neonatal clinical outcomes reported |
| Selective reporting (reporting bias) | Unclear risk | There is no information on protocol published prior to this trial and no information to make appropriate judgements on this |
| Other bias | Unclear risk | It was reported that obstetric and physical background data between the groups were not significantly different, although actual data were not reported |
Denmark 1996.
| Study characteristics | ||
| Methods | A double‐blinded, randomised placebo‐controlled trial | |
| Participants | Normal healthy middle‐class women (at least 18 years old) less than 20 weeks pregnant confirmed by scan for their first visit and booked for delivery at Kolding hospital and Horsens hospital, Denmark. Any known intolerance towards zinc, diabetes mellitus, thyrotoxicosis or earlier rhesus immunization were excluded from the trials. The women were thought likely to be zinc‐deficient by the previous study project 'Pregnancy, environment and way of life' in Denmark |
|
| Interventions |
Women received 2 tablets of Zinclet@ (44 mg elemental zinc in total) or 2 placebo tablets containing inert substances. They were indistinguishable in appearance and taste. The tablets were prepared by the Gunnar Kjems Aps company Women were advised to take 2 tablets daily after breakfast and to avoid taking tablets possibly containing iron together with those of the study, as iron reduces zinc uptake. Women were excluded later, if there were any side‐effects caused by the tablets, if they wanted to stop or if she had not taken the tablets for 14 days in all |
|
| Outcomes |
Maternal
outcomes Prelabour rupture of membranes; Pre‐eclampsia; Antepartum haemorrhage Caesarean section Green amniotic fluid; Mean loss of blood during delivery Neonatal outcomes Preterm labour <36 weeks Low 5‐minute Apgar score Large‐for‐gestational age Small‐for‐gestational age Birthweight (not able to be used in graphs since no SDs provided) Birth length |
|
| Notes | Adherence: non‐adherers were excluded from the final analysis; reasons included side‐effects from tablets, if a woman wished to stop or if a woman had not taken the tablets for 14 days in all. The authors noted that women did not differ in basic characteristics. There were however, significantly more smokers in the non‐adherers group and thus the numbers in the final analysis related to labour and birth have also excluded smokers Dates of study: June 1991 ‐ January 1993 Funding sources: lrege Frk. K. Rasmussens mindele‐ gat and Sygesikringen “danmarks” sundhedsfond Declarations of interest: not disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed in successive groups of 10 active and 10 placebos; no further details reported |
| Allocation concealment (selection bias) | Low risk | The code was not broken until the end of the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators and mothers were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 794/2000 (39.7%); 415 in zinc group and 379 in placebo group |
| Selective reporting (reporting bias) | Unclear risk | Not all expected maternal primary outcomes reported, but most primary infant outcomes specified in this review were reported |
| Other bias | Unclear risk | Analyses relating to labour and birth excluded smokers |
Egypt 2014.
| Study characteristics | ||
| Methods | A double‐blind, randomised, 3‐arm, parallel‐group placebo‐controlled trial | |
| Participants | 1055 healthy pregnant women from low‐ and middle‐income pregnant populations attending 2 antenatal care centres were screened for low level of zinc serum. Of these women, 675 had low zinc serum level and were eligible for the trial in Alexandria, Egypt The ages ranged between 20 and 45 years, with gestational age below 16 weeks with normal course of pregnancy were included for the trial Women identified through interviews to be on any other form of zinc supplements at any dosage, or risk of having reduced or excessive birthweight of infants (e.g. diabetes, hypertension, renal and heart disease) were excluded |
|
| Interventions |
Zinc supplements were provided from 16 weeks until delivery, and a subgroup of 100 women were monitored for their dietary intake. Of the 675 women, 597 of women completed the study |
|
| Outcomes | We used the zinc‐only and placebo arms Maternal outcomes Weight gain Serum zinc Haemoglobin level Neonatal outcomes Birthweight Preterm birth Congenital malformation Stillbirth Head circumference Chest circumference Arm circumference Early neonatal morbidity Respiratory distress Infection |
|
| Notes | The mean maternal dietary intake of zinc was 7 mg/day in the 3 groups Dates of study: February 2007 ‐ September 2009 Funding sources: Not disclosed Declarations of Interest: None to disclose |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to 1 of the 3 study groups. An independent statistician generated the allocation sequence using computer‐generated random numbers, using Excel software. After obtaining informed consent for enrolment, the investigators randomly assigned participants till the required sample size was met. There was no stratification during the randomisation |
| Allocation concealment (selection bias) | Low risk | The assignment of participants to study conditions was carried out at the study centres. A co‐worker wrote the treatment allocations (A, B and C) on sequentially‐numbered opaque plastic boxes. 1 box containing 30 capsules of a given random number was prepared for monthly use. Thus, 5 boxes were totally numbered for each participant. Additional packs were available for non‐compliant or replaced cases. The assigned box number was transferred to a folder prepared for each woman where data such as any complications/withdrawal/ non‐compliance were recorded. 2 copies of randomisation lists were prepared and kept by 2 independent staff not involved in the study. They kept them until collection and analysis of data were completed. Deciphering of group labels took place after completing the analysis and commenting on the results |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All study personnel, except the statistician who generated the sequence, and the participants were blinded to the allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “a structured interview was administered to mothers to collect the following data.” Comment: Insufficient information on whether the interviewer was blinded to make a judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No description of the women who dropped out from study, although dropout rates are in balance (intervention 88% versus placebo 89%) |
| Selective reporting (reporting bias) | Low risk | All outcomes measured were reported |
| Other bias | Low risk | The report appears to be free of other sources of bias |
Ghana 2009.
| Study characteristics | ||
| Methods | A double‐blind RCT | |
| Participants | N = 299 for intervention and n = 301 for control allocated 400 pregnant women in Ghana earlier than 16 weeks of gestation who presented themselves for antenatal care and have been screened for their gestational age in the Wa Regional Hospital of the Upper West Region in Ghana Women who were receiving zinc supplements at any dosage level or were severely anaemic (i.e., Hb < 7.0 g/dL) were excluded. The iron‐zinc and iron‐only supplements were pre‐coded and supplied by Nutricaps pharmaceutical company in the USA. The supplements (in the form of capsules) were of the same shape, colour and taste and packaging. The women were advised to take the supplements at least 2 hours before or after meals, and at night, just before going to bed. Compliance was monitored by interviewing all participants after having being enrolled for 4 weeks using structured questionnaire to check the frequency and dosage of supplement intake. A subsample of 213 women at recruitment were assessed for serum ferritin but only 173 were repeated at 34 ‐ 36 weeks' gestation |
|
| Interventions |
Both groups received malaria chemoprophylaxis in the form of sulphadoxine pyrimethamine, and 400 μg folic acid. The supplements were taken every other day from enrolment until delivery |
|
| Outcomes |
Neonatal outcomes Intrauterine growth restriction/small‐for‐gestational age Low birthweight Preterm birth Birthweight Haemoglobin concentration Serum ferritin concentration Plasma zinc concentration |
|
| Notes | Dates of study: September 2005 ‐ November 2006 Funding sources: Public Health Research Group of Edith Cowan University; midwives of the antenatal unit and the labour ward of Wa Regional Hospital, Ghana Declarations of interest: not disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computer‐generated random number |
| Allocation concealment (selection bias) | Unclear risk | Opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The capsules for both intervention and placebo were the same |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 27 out of 299 of the intervention group and 30 out of 301 of the control group were lost to follow‐up and excluded from the analysis |
| Selective reporting (reporting bias) | Unclear risk | It was not clear if a protocol of this trial had been published prior to the study; no maternal outcomes reported |
| Other bias | Low risk | Baseline characteristics were compared, with no significant difference seen between groups |
Indonesia 2001a.
| Study characteristics | ||
| Methods | A 4‐arm double‐blinded RCT. A follow‐up study of the infants with a factorial design | |
| Participants | 230 pregnant women were recruited before 20 weeks of gestational age, from 13 adjacent villages in Bogor District, Indonesia. Of 230 women, 179 women remained until delivery and only 170 women were enrolled for follow‐up of infant and mother until 6 months postpartum study. Each woman was supplemented daily during pregnancy until delivery. Women had mean plasma zinc concentrations of about 11 µmol/L Exclusion criteria at enrolment were twin pregnancy and congenital abnormalities |
|
| Interventions | Iron + folate acid: (n = 41)
Iron + folate acid + β‐carotene: (n = 43)
Iron + folate acid + zinc: (n = 44)
Iron + folate acid + β‐carotene + zinc (n = 42) All women received iron and folic acid (30 mg iron as ferrous fumarate/d and 0.4 mg pteroylglutamic acid/d). In addition, 1 group of women received ß‐carotene (4.5 mg as water‐soluble granulate/d; ß‐carotene group), 1 group received zinc (30 mg zinc as sulphate/day; zinc group), 1 group received ß‐carotene plus zinc (4.5 mg ß‐carotene and 30 mg zinc/d; ß‐carotene + zinc group), and 1 group received only iron and folic acid (control group). Capsules were prepared by the pharmacy of the Gelderse Vallei Hospital (Ede, Netherlands) and given a letter code; the micronutrients were indistinguishable from each other. Compliance, expressed as a proportion of the intended supplements consumed during pregnancy, did not differ among the groups, with a mean compliance of > 80% in all groups, and 90% of the women taking > 50% of the intended dose |
|
| Outcomes |
Maternal outcomes Caesarean section Prolonged labour Retention of placenta Postpartum haemorrhage Infection 6‐month serum zinc Neonatal outcomes Birthweight Preterm birth Low birthweight Congenital malformation Stillbirths Neonatal deaths Blue/floppy (neonatal hypoxia) Jaundice Fever/not drinking Umbilical infection 6‐month Z‐scores 6‐month haemoglobin Plasma retinol Plasma zinc |
|
| Notes | Adherence: mean adherence was over 80% Dates of study: July 1998 ‐ January 1999 Funding sources: not disclosed Declarations of interest: authors declare no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Supplements were prepared by a third party (hospital pharmacy in the Netherlands), but no detail given of how the contents of the bottles were concealed from the investigators or the participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as being "double‐blind"; probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up: 50/229 (22%) women before giving birth; 136 newborns completed follow‐up at 6 months |
| Selective reporting (reporting bias) | Unclear risk | Not all expected maternal primary outcomes reported, but most primary infant outcomes specified in the review were reported |
| Other bias | Low risk | No apparent risk of other bias |
Indonesia 2001b.
| Study characteristics | ||
| Methods | A double‐blinded, randomised placebo‐controlled trial. A factorial design 4‐arm (Zibuvita study) trial and then included a follow‐up study of the infant (the Zinak and Pronak study) | |
| Participants | 5736 women who live in Purworejo district of central Java were identified as pregnant from the Indonesian Ministry of Health surveillance. Of these pregnant women, 2173 women at a gestational age of less than (120 days) 17 weeks were eligible for the trial study. After losses to follow‐up, only 2098 delivery and 1956 neonates were analysed in the Zinak and Pronak study. The follow‐up of the children was from birth up to 2 years of age | |
| Interventions | Vitamin A (2400RE): n = 484/527 (91.8%)
Zinc (20 mg): n = 477/531 (89.8%)
Vitamin A (2400RE) + zinc (20 mg): n = 495/543 (91.2%)
Placebo: n = 500/523 (95.6%) Women were randomly allocated to 3 treatment groups and placebo group. The treatment groups were given micronutrient capsules from the date of inclusion in the study until delivery. The capsule contained either 2400 RE of vitamin A (as retinyl palmitate) or 20 mg of ZnSO4, or the same dose of both vitamin A and ZnSO4, or placebo. All capsules also contained 2 mg of DL‐a‐tocopherol as an antioxidant and 350 mg of soya bean oil, 20 mg of beeswax and 8 mg of lecithin as capsule filler. The supplements were manufactured by Tishcon Corp. (Westbury, NY, USA) and they were packaged in plastic strips in identical, opaque pink capsules as sufficient supplements for 2 weeks or 1 month. Fieldworkers distributed capsules and monitored compliance at the home of the women by counting the unused capsules |
|
| Outcomes |
Maternal outcomes
Pregnancy weight Neonatal outcomes Birthweight Low birthweight; Stillbirths Neonatal deaths; Blue/floppy (neonatal hypoxia); Fever/not drinking Umbilical infection 6‐month Z‐scores 6‐month haemoglobin, plasma retinol, plasma zinc Birth size (weight and length) Small‐for‐gestational age |
|
| Notes | Adherence: mean adherence ranged from 71% to 73% across the 4 arms of the study Dates of study: September 1995 ‐ December 1999 Funding sources: sources of financial support: MotherCare, John Snow Inc., Washington, USA and UNICEF, Jakarta, Indonesia; Gadjah Mada University and the University of Newcastle, Newcastle, Australia; and infrastructure support from the Community Health and Nutrition Research Laboratories, Medical School, Gadjah Mada University and Ministry of Health, Republic of Indonesia through the Third Community Health and Nutrition Development Project Loan from the World Bank (IBRD Loan No. 3550‐IND). The development of this manuscript was supported by the Centre for Global Health at Umeå University, FAS and the Swedish Council for Working Life and Social Research (grant number 2006‐1512) Declarations of interest: authors declare no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pseudo‐random number‐generator in blocks of 12 |
| Allocation concealment (selection bias) | Low risk | Treatment allocation sequence was prepared and held at a remote site |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All investigators, field and laboratory staff and participants were blinded to the treatment code |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but likely to have been done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 519 of the 1008 women had pregnancies ending between 1 April and 31 October 1997; data available for 503/519 (97%) of these women |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available and there was not enough information to make this judgement |
| Other bias | Low risk | No apparent risk of other bias |
Iran 2009.
| Study characteristics | ||
| Methods | This double‐blind RCT was conducted in an urban setting of the Ardabil District (province) of Iran | |
| Participants | 196 pregnant women between 16‐20 weeks of gestation were recruited from urban healthcare centres, and provided informed consent. Exclusion criteria included: hypertension, diabetes, renal disease, history of prematurity, PROM or low birthweight infants. Zinc group (n = 98), Placebo group (n = 98) | |
| Interventions |
Participants in both arms received 1 mg of folic acid and 30 mg of ferrous sulphate tablets daily, to be consumed at night. The zinc sulphate and placebo tablets were consumed midmorning |
|
| Outcomes |
Maternal outcomes Pre‐eclampsia and eclampsia PROM Hypertension Neonatal outcomes Gestational age at birth Birthweight Height Head circumference Low birthweight Preterm birth Low birthweight Stillbirth |
|
| Notes | Information on compliance was assessed and recorded monthly by trained midwives. Women whose compliance was less than 70% were excluded from analysis Dates of study: not reported, Dates of recruitment: April 2004 to March 2005 Funding sources: Research Fund of Aradabil University of Medical Sciences Declarations of Interest: not disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was double‐blind and the women were randomly assigned to receive either 50 mg daily elemental zinc as zinc sulphate (n = 98), or placebo (n = 98)." Comment: Insufficient information to permit a judgement |
| Allocation concealment (selection bias) | Low risk | Allocation probable. Quote: "The zinc sulphate and placebo capsules were made by the Alhavi Company. The placebo capsules were similar to the zinc sulphate capsules in both shape and blister packing. The zinc sulphate was coded as "A" and the placebo as "B", however, both interviewers and participants remained blind of these codes until the study was completed." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Probable. Quote: "The zinc sulphate and placebo capsules were made by the Alhavi Company. The placebo capsules were similar to the zinc sulphate capsules in both shape and blister packing. The zinc sulphate was coded as "A" and the placebo as "B", however, both interviewers and participants remained blind of these codes until the study was completed" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Probable. Quote: "The zinc sulphate and placebo capsules were made by the Alhavi Company. The placebo capsules were similar to the zinc sulphate capsules in both shape and blister packing. The zinc sulphate was coded as "A" and the placebo as "B", however, both interviewers and participants remained blind of these codes until the study was completed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and exclusion were equally distributed across arms Quote: "A total of 196 subjects were recruited and randomly assigned in two groups. Six refused to participate (3 in zinc and 3 in placebo group), remaining (190) were included in the study. Subjects who consumed placebo or supple‐ ment irregularly (on fewer than 21 days per month or 70% compliance) were excluded; 11 (5.8%) were excluded (6.3% in the zinc group and 5.3% in placebo group NS), they withdrew prior to the outcome being measured, but 93.2% of the participants consumed Zinc supplement or placebo regularly." |
| Selective reporting (reporting bias) | Low risk | All outcomes presented in the methods section were reported in the paper |
| Other bias | Low risk | None |
Iran 2010.
| Study characteristics | ||
| Methods | A double‐blind, randomised, placebo‐controlled trial | |
| Participants | 110 healthy pregnant women with a previous preterm delivery who were receiving prenatal care from obstetrics and gynaecology outpatient clinics of Isfahan University of Medical Sciences were recruited for the trial. The healthy pregnant women were aged 18 – 35 years, at 12–16 weeks' gestational age at delivery, height > 150 cm, weight > 45 kg, non‐smoker, no complicated pregnancy, but with history of preterm delivery, carrying a singleton fetus, living in Isfahan and willing to continue current medications for the duration of the study |
|
| Interventions | Intervention: 50 mg/day Zn as Zn sulphate (n = 42)
Placebo: (n = 42) The treatment group received (50 mg/day Zn as Zn sulphate) produced by a local pharmaceutical company, Alhavi Pharmaceutical Laboratory, Tehran, Iran, from the day of reporting (12th – 16th weeks of gestation) until delivery, and the control group received placebo. Both groups administered capsules orally before meals once a day. The capsules were distributed monthly during prenatal visit. Compliance with study treatment was established by asking the women about missed doses and by counting unused sachets. The doses used are safe during pregnancy |
|
| Outcomes |
Maternal outcomes
Caesarean section
Pre‐eclampsia Neonatal outcomes Small‐for‐gestational age/intrauterine growth restriction Gestational age at birth Preterm birth Low birthweight |
|
| Notes | Dates of study: January 2007 ‐ June 2008 Funding sources: not disclosed Declarations of interest: not disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomised according to a pre‐existing list produced by a computer programme |
| Allocation concealment (selection bias) | Low risk | Neither the woman nor physician who assessed the outcome were aware of treatment type that the woman was receiving. The masking of the active and placebo treatments was preserved by creating treatments that looked identical. The hospital pharmacist was informed of all randomisation assignments and was responsible for labelling the study drug and maintaining a master list linking the women and their treatment assignments |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | As above |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 42 out of allocated 55 women in the intervention group and 42 out of 55 women in the control group were analysed (26% lost to follow‐up in each group) |
| Selective reporting (reporting bias) | Unclear risk | Not enough information to make this judgement. No information on whether the protocol had been published prior to the trial |
| Other bias | Unclear risk | No significant baseline differences except for higher haemoglobin concentrations in the zinc group (MD 0.5 g/dL) |
Iran 2016.
| Study characteristics | ||
| Methods | A randomised control trial conducted in an urban setting in Rasht (Guilan Province), in Iran | |
| Participants | Healthy pregnant women (n = 540) at 16 weeks gestation who presented at the antenatal care clinic, and did not have high‐risk pregnancies were eligible. Exclusion criteria included age under 18, or over 40 years, height < 145 cm, BMI < 15 kg/m2 or > 30 kg/m2 before pregnancy, smoking, multifetal pregnancy, pregnancies assisted by reproductive technology, uterine anomalies, leiomyoma, and any established medical risk for reduced or excessive birthweight (including hypertension, renal diseases, diabetes, and other contributing chronic disease). Eligible women must also have had no history of miscarriage, intrauterine death, stillbirth, low birthweight babies in previous pregnancies, premature birth, pre‐eclampsia and/or macrosomia | |
| Interventions |
|
|
| Outcomes |
Maternal outcomes Pre‐eclampsia PROM Weight gain during pregnancy Neonatal outcomes Birthweight Head circumference Length Gestational age at birth Low birthweight Macrosomia Spontaneous abortion Apgar scores at 5 minutes |
|
| Notes | Dates of study: January 2010 ‐ January 2012 Funding sources: financial support provided by the Research Deputy at the Guilan University of Medical Sciences Declarations of interest: authors declare no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated randomized list using a size four permuted block was used. Random sequences were prepared by a researcher with no clinical involvement in the trial. A gynecologist from the clinic allocated participants to each group by using random sequences." |
| Allocation concealment (selection bias) | Low risk | Quote: "A computer‐generated randomized list using a size four permuted block was used. Random sequences were prepared by a researcher with no clinical involvement in the trial. A gynecologist from the clinic allocated participants to each group by using random sequences." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Outcome assessors and data analysts were kept blinded to the allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Outcome assessors and data analysts were kept blinded to the allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 61 women were lost to follow‐up because they declined to continue participating. Overall 235/270 receiving zinc, and 244/270 not receiving zinc completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes presented in the methods section were reported in the paper |
| Other bias | Low risk | None |
Nepal 2003.
| Study characteristics | ||
| Methods | A double‐blind, cluster‐randomised, controlled trial (factorial design) with 1 ‐ 5 treatment arms | |
| Participants | 4926 pregnant women and 4130 liveborn infants in a rural plains district of Sarlahi, community in Nepal, which had 426 sectors (communities of about 100 ‐ 150 households) ‐ only 2 of the 5 arms (total of 1659 infants) used in this review. This is the same area of Nepal in which we previously recorded evidence of vitamin A, iron, and zinc deficiency among pregnant women Women who were currently pregnant, breastfeeding a baby less than 9 months old, menopausal, sterilised or widowed were excluded. Supplementation began before conception |
|
| Interventions | The sectors were randomly assigned to 1 of 5 treatment arms The control group was vitamin A (1000 µg retinol equivalents) FA group, vitamin A + folic acid (400 µg) FAFe group, vitamin A + folic acid + iron (60 mg) FAFeZn group, vitamin A + folic acid + iron + zinc (30 mg) MN group, vitamin A + folic acid + iron+ zinc + other micronutrients (10 µg vitamin D, 10 mg vitamin E, 1.6 mg thiamine, 1.8 mg riboflavin, 20 mg niacin, 2.2 mg vitamin B‐6, 2.6 µg vitamin B‐12, 100 mg vitamin C, 65 µg vitamin K, 2.0 mg Cu, 100 mg Mg) The supplements were provided by UNICEF, identical in shape, size, and colour, arrived in Nepal in opaque, sealed, and labelled bottles coded 1 – 5. The code allocation was kept locked at the Johns Hopkins University, Baltimore. The investigators, field staff, and participants were blinded to the codes throughout the study. |
|
| Outcomes | We used the following arms for zinc versus placebo comparison (n values are live births + stillbirths). Zinc: zinc + iron + folate (n = 858).
No zinc: iron + folate (n = 801) Neonatal outcomes Preterm birth Stillbirth Perinatal death Neonatal death; Birthweight Chest circumference Head circumference Length Low birthweight Small‐for‐gestational age |
|
| Notes | Adherence: mean adherence was 88%.
RRs adjusted for the cluster‐design effects were presented for each of the 5 arms of the RCT Dates of study: December 1998 ‐ April 2001 Funding sources: US Agency for International Development (USAID) and additional support from the Unicef Country Office, Kathmandu, Nepal, and the Bill and Melinda Gates Foundation Declarations of interest: authors declare no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised sectors by "drawing numbered identical chips from a hat" (in blocks of 5 within each community) |
| Allocation concealment (selection bias) | Low risk | Supplements were of identical shape, size and colour and arrived in Nepal in opaque, sealed and labelled bottles coded 1 ‐ 5. The code allocation was kept locked at the Johns Hopkins University, Baltimore |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, investigators, field staff and statisticians were all blinded to the codes throughout the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators, field staff and statisticians were all blinded to the codes throughout the study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 155/827 (19%) of infants in the zinc group and 167/872 (19%) in the non‐zinc group were lost to follow‐up or excluded from analysis (infant died, mother refused, home was inaccessible, birthweight was measured more than 72 hours after birth or missing data) |
| Selective reporting (reporting bias) | Low risk | Most expected outcomes were reported with some exceptions such as mode of birth and postpartum haemorrhage |
| Other bias | Low risk | No apparent evidence of other sources of bias apart from a small imbalance between groups in maternal weight (which was adjusted for in the analyses) |
Pakistan 2005.
| Study characteristics | ||
| Methods | A double‐blind, RCT | |
| Participants | By simple random sampling, 250 women from 2 urban hospitals and 1 rural community in Pakistan at 10 ‐ 16 weeks' gestation were recruited. 242 women completed the study. The mean (SD) age of the women was 25.7 (4.8) years (range 16 – 40). Women with known systemic disease were excluded. Serum zinc at enrolment was mean 71.51 µg/dL (SD 21) in the zinc group and 74.09 (SD 23.2) in the placebo group |
|
| Interventions |
The supplement was a 20 mg of zinc sulphate powder capsule filled with glucose, and a similar capsule as placebo. The supplement were given to the women from the time of booking to the end of their gestational week. Routine supplements of folic acid and iron were also given. The dietary zinc intake was taken into account by a food diary and various food items were assigned a score. Women were followed up at monthly intervals by trained staff. Compliance was ensured by health visitors and pills were counted out every month before a new supply was issued, to double check the consumption of the medicine. All women had routine supplements of folic acid and iron |
|
| Outcomes | Neonatal outcomes Preterm birth (<37 weeks) Occipitofrontal circumference Low birthweight Abortion/intrauterine death Birthweight Length | |
| Notes | Adherence: about 65% of women had good adherence, which was similar in both groups Dates of study: April 2003 ‐ April 2004 Funding sources: Saving Newborn Lives of Save the Children, USA Declarations of interest: not disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "simple random sampling with preassigned code." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women and health workers were blinded to content of medication |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but likely to have been done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up: 15% (actual figures not given, but paper notes that losses were non‐differential) |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available. Not enough information to make this judgement |
| Other bias | Low risk | No apparent risk of other bias |
Peru 1999.
| Study characteristics | ||
| Methods | A double‐blind RCT | |
| Participants | 1295 women with a low‐risk pregnancy (uncomplicated and eligible for vaginal delivery), at the Hospital Materno Infantil in Lima, Peru, and low zinc intake who were carrying a singleton fetus, and had lived in coastal Peru for ≥ 6 months before pregnancy were recruited for the study. These women indicated with low zinc intake living in this region and at 10 to 24 weeks' gestation. The study protocol was approved by the institutional review boards of the Instituto de Investigación Nutricional (IIN) and The Johns Hopkins School of Hygiene and Public Health |
|
| Interventions |
1 group received a daily supplements containing 60 mg Fe (ferrous sulphate) and 250 µg folate with 15 mg of Zn (zinc sulphate), and the other received the same Fe supplement but without an additional 15 mg Zn (zinc sulphate). Women were asked to take 1 pill daily midmorning with a vitamin C–containing drink or water according to the Peruvian guideline Supplementation began at gestation week 10 – 24 and continued until 4 weeks postpartum. The supplements were produced by a local pharmaceutical company (Instituto Quimioterápico, SA, Lima, Peru) in coded blister packages To verify the formulation of the supplements and the integrity of the coding scheme, samples of each supplement type were analysed by the IIN laboratory twice during the study |
|
| Outcomes | Maternal outcomes Duration of pregnancy Serum and urinary zinc concentrations Haemoglobin Serum ferritin Neonatal outcomes Birthweight Preterm birth (< 33 weeks, 33 ‐ 36 weeks, 37 ‐ 42 weeks, > 42 weeks) Very preterm birth (< 33 weeks) Post‐term birth (> 42 completed weeks) Low birthweight High birthweight Cord vein zinc Cord vein haemoglobin Cord vein serum ferritin Crown‐heel length Head circumference Chest circumference Calf circumference Mid‐upper arm circumference Biceps, subcapsular and calf skinfold thickness | |
| Notes | Adherence: mean of about 85% of capsules consumed, which was similar across the groups
Adjustments for baseline differences in maternal age and in‐home electricity were made by multiple regression Dates of study: 1995 ‐ 1997 Funding sources: supported by DAN‐5116‐a‐00‐8051‐00 and HRN‐A‐00‐97‐00015‐00, cooperative agreements between USAID/OHN and The Johns Hopkins University Declarations of interest: not disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported |
| Allocation concealment (selection bias) | Low risk | Coded blister packages were prepared by a local pharmaceutical company, and allocation was thus concealed by use of this third party |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators, other health personnel and women were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but likely to have been done due to use of placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 21.5% (279/1295) women lost to follow‐up by time of giving birth ‐ 18 (1%) were found to live in another community and therefore not eligible to participate; 92 (7%) declined to participate; 71 (5%) moved out of the study area; 30 (2%) miscarried; 58 (4%) left the study for other reasons; 10 (1%) were subsequently found to have twin pregnancies or to have developed pregnancy complications |
| Selective reporting (reporting bias) | Unclear risk | No information on if the protocol had been published prior to the trial |
| Other bias | Low risk | No apparent risk of other bias |
Peru 2004.
| Study characteristics | ||
| Methods | A double‐blind RCT | |
| Participants | 242 (low‐income) Peruvian pregnant women at 10 to 16 weeks’ gestation receiving prenatal care at the Hospital Materno Infantil San Jose in Lima, Peru were recruited. The maternal dietary zinc intake is approximately 8 mg/d,12 an intake much lower than recommended intakes at that time of 15 mg/d (US Recommended Dietary Allowance) in this region. Women who were with singleton pregnancy, and had lived in coastal Peru for at least 6 months before becoming pregnant were included in the study Exclusions was made according to a protocol for fetal neurobehavioural assessment |
|
| Interventions |
Women were randomly assigned to receive a daily supplement containing 60 mg iron (ferrous sulphate) and 250 mg folic acid, with 25 mg zinc (zinc sulphate) or the same supplement but without 25 mg zinc (zinc sulphate) The supplements were manufactured in Lima, had the same appearance and taste, and both study personnel and participants were masked to treatment assignment. The supplements were distributed in blister packs at monthly intervals, beginning at entry into the study at 10 to 16 weeks’ gestation and continuing until 1 month postpartum. Adherence with supplementation was checked bi‐weekly by health workers who visited the women in their homes and observed the number of tablets remaining in each blister pack. The level of adherence was calculated as the percentage of tablets taken over the number of days in the study. They used a standard questionnaire with specific questions about potential benefits or side effects of supplement consumption |
|
| Outcomes | Neonatal and infant outcomes Fetal heart rate measures Preterm birth with complications Gestational age at birth Birthweight Length Biparietal diameter Abdominal circumference Femur diaphysis length Infant feeding Infant growth Child development at 54 months Dietary and nutritional status at 54 months Mean arterial pressure at 54 months BMI at 54 months Haemoglobin concentration at 54 months Plasma zinc concentration at 54 months C‐reactive protein concentration at 54 months Home Observation for the Measurement of the Environment (HOME) Scale assessment at 54 months Heart rate measures at 54 months | |
| Notes | Adherence: mean adherence rate was 87% (86% in the zinc group and 88% in the no‐zinc group) Dates of study: 1998 ‐ 2000 Funding sources: Nestle Research Foundation, Lausanne, Switzerland. MM was also supported in part by the Consiglio Nazionale delle Ricerche, Italy Declarations of interest: authors declare no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomly assigned in blocks of 2 using computer‐generated lists from Johns Hopkins and sent to Peru |
| Allocation concealment (selection bias) | Low risk | The randomisation code was made by the pharmaceutical company and maintained in a sealed and secured envelope in Lima; supplements had the same appearance and taste |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both study personnel and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specifically stated, but we have assumed that outcome assessors were blinded and remained blinded for the longer‐term analyses |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 222/242 (90.1%) women completed the protocol and 195 (80.6%) were included in the analysis of birth outcomes: 94 (78%) zinc and 101 (84%) no zinc. The 47 lost were made up of 20 change of address, declining to continue in the study, or travel and 27 exclusions for significant obstetric or medical complications At 54‐month follow‐up, there were 205 eligible children (includes children of 10 mothers excluded from the initial analysis), and evaluations were completed for 184 (90%) of these children: (86 (87%) from the zinc group and 98 (92%) from the non‐zinc group |
| Selective reporting (reporting bias) | High risk | A number of birth outcomes such as postpartum haemorrhage, stillbirth or neonatal death, low birthweight or Apgar scores were not reported; and preterm birth was only reported as preterm birth with complications which were treated as study exclusions |
| Other bias | Low risk | No apparent source of other bias although the study was designed primarily to assess neonatal and infant outcomes (see selective reporting above) |
S Africa 1985.
| Study characteristics | ||
| Methods | A double‐blind, randomised, placebo‐controlled, 4‐arm trial. | |
| Participants | 127 black women before 20th week of pregnancy for antenatal medical care with expectation of spontaneous vaginal delivery and a willingness to attend the clinic at Kwa‐Mashu Polyclinc near Durban, South Africa, daily until delivery to eat dietary supplements under supervision were selected for the study. Free transportation was provided daily to the clinic. At entry, each woman had a detailed medical and socioeconomic history, physical examination, assessment of the week of pregnancy by date of LMP and ultrasonic measurements. Serum levels of proteins, cholesterol, triglyceride, carotene, vitamin A, vitamin C, phosphorus, alkaline phosphatase, calcium and magnesium were also measured An experienced dietitian calculated dietary intake before starting the supplements from a 24‐hour, quantitative, dietary recall history The recorded diets were deficient in energy, protein, the B vitamins, calcium and iron among these women Women in the zinc group in this study had a significantly lower mean weight than the women in the placebo group |
|
| Interventions |
Women were randomly assigned to 1 of 4 groups. Before supplementation the women in 3 of the 4 groups had similar body weights. Primigravidas were equally distributed by chance among the 4 groups. Group 1 received placebo pills and group 2, 30 ‐ 90 mg zinc gluconate daily. Groups 3 and 4 were given food supplements from the 20th week of pregnancy to delivery, Monday through Friday These supplements were designed to correct dietary deficiencies detected in the dietary recall histories, particularly deficiencies in energy and protein Group 3 women received a high bulk supplement, a mixture of beans and maize in a 1.2: 1 ratio as mush with added vitamins. Group 4 women received a low bulk supplement, a porridge containing 100 g dry skimmed milk, maize flour, vitamins and minerals. It differed from the group 3 supplement in its 36 g of animal protein and in its higher levels of several vitamins and calcium |
|
| Outcomes | We used the zinc‐only and placebo arms Maternal outcomes Maternal weight gain Levels of constituents of serum samples: Albumin, cholesterol, triacylglycerol, carotene, vitamin A, vitamin C, phosphorus, alkaline phosphatase, calcium, magnesium Neonatal outcomes Gestational age at birth Birthweight |
|
| Notes | Adherence: figures for adherence were not given, but the authors commented that it was high, due to free transportation to the clinic where the supplements or placebo were consumed under supervision
Groups given dietary supplements are not included in the analysis Dates of study: recruitment began January ‐ March 1977 Funding sources: Ross Laboratories, Columbus Ohio; South African Sugar Association and the South African Medical Research Council Declarations of interest: not disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported |
| Allocation concealment (selection bias) | Low risk | Randomisation by numbered packets prepared at the pharmacy, code held by pharmacy until the end of the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding by use of placebo until end of study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but likely to have been blinded due to use of placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up: 10% (exact figures not given) of women before giving birth, principally due to moving out of the area |
| Selective reporting (reporting bias) | Unclear risk | Not enough information to make this judgement. No information on if the protocol had been published prior to the trial |
| Other bias | Low risk | No apparent risk of other bias |
Tanzania 2017.
| Study characteristics | ||
| Methods | A double‐blind, randomised, placebo‐controlled trial conducted in 8 antenatal care clinics in the urban Temeke and Ilala districts of Dar es Salaam, Tanzania | |
| Participants | Participants (n = 2500) were pregnant women in their first trimester of pregnancy, primigravida or secundigravida. Eligible participants intended to stay in Dar es Salaam for at least 6 weeks after delivery, and were HIV‐negative | |
| Interventions |
According to standard of care, participants were also given 60 mg of iron (daily) and 5 mg of folic acid (daily), and 1500 mg of sulfadoxine and 75 mg of pyrimethamine as pregnancy malaria prophylaxis |
|
| Outcomes | We used the zinc‐only and placebo arms Maternal outcomes Placental malaria Haemoglobin Maternal anaemia Severe maternal anaemia Placental weight Maternal hospitalisations during pregnancy Maternal death Neonatal outcomes Gestational age at birth Stillbirth and miscarriage Perinatal death (> 28 weeks of gestational to 7 days after birth) Infant mortality at 6 weeks Birthweight Low birthweight Very low birthweight Preterm birth Small‐for‐gestational age (INTERGROWTH‐21st standard and Alexander growth standard) |
|
| Notes | Authors were contacted to obtain disaggregated data for zinc versus placebo groups Dates of study: July 2010 ‐ September 2013. Follow‐up was completed June 2014 Funding sources: grants from the National Institute of Child Health and Human Development (NICHD R01 HD057941‐01 and K24 HD 058795 [Christopher Duggan]) Declarations of interest: not disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Probable. Quote: "Allocation to treatment groups was performed according to a computer‐generated randomization sequence using blocks of size 20 by a scientist not involved in the data collection." |
| Allocation concealment (selection bias) | Low risk | Probable. Quote: "Allocation to treatment groups was performed according to a computer‐generated randomization sequence using blocks of size 20 by a scientist not involved in the data collection." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Probable. Quote: "Neither the participants nor the study personnel had access to the masking information" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Probable. Quote: "Each study clinic was issued regimen bottles that were pre‐labelled according to this sequence by study pharmacists who had no contact with participants. At enrolment, each participant was assigned to the next numbered bottle at their site. The study remained blinded until all trial assessments and database cleaning were completed, at which time study staff analyzing the data were given access to treatment assignments." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | > 20% loss to follow‐up. Reasons for loss to follow‐up were reported |
| Selective reporting (reporting bias) | High risk | Results for vitamin A supplementation were combined with the results from the vitamin A‐zinc combined arm. Similarly, the results for zinc supplementation were combined with the results from the vitamin A‐zinc combined arm. This prevented us from reporting disaggregated results for the vitamin A‐only group, zinc‐only group and the vitamin A‐and‐zinc‐combined group. We contacted authors for disaggregated data |
| Other bias | Low risk | None |
UK 1989.
| Study characteristics | ||
| Methods | A double‐blind, randomised, placebo‐controlled trial | |
| Participants | 500 women who were less than 20 weeks pregnant at the first visit booking for delivery at Southmead Hospital, Bristol were recruited for study. An ultrasound scan was done in 95% of cases. At the end of the visit potential volunteers were seen by the research midwife, who explained the study fully. Median zinc concentrations at enrolment were 1.192 µmol/10 x 10 cells in the zinc group and 1.147 in the placebo group 494 women remained to complete the study. |
|
| Interventions |
Women were randomly allocated to receive a capsule containing 20 mg elemental zinc (66 mg zinc sulphate) oral capsule containing inert substances (sucrose, maize starch, purified talc, kaolin, gelatin) but which was indistinguishable in appearance and taste from the one containing zinc The capsules were prepared by Smith Kline and French Ltd The mothers were advised to take 1 capsule daily after breakfast. Serum haemoglobin and ferritin concentrations were measured in all women at the first visit. Iron and folate supplementation was advised only if the haemoglobin concentration was < 100 g/l or the serum ferritin concentration was l< 10 µg/l. Enough supplementation capsule supply was provided to last until the next visit The research midwives visited the women at the 28 ‐ 32 weeks and again on the day of delivery During the study and after delivery clinical details were recorded by the research midwife by interview as well as from the case records. Compliance was assessed by the regularity with which the study capsules were taken. Those who took the supplement daily or on most days were regarded as compliers, and the rest were regarded as non‐compliers |
|
| Outcomes |
Maternal outcomes Prelabour rupture of membranes Pregnancy hypertension Any maternal infection ‐ (pre or postdelivery) Caesarean section Postpartum haemorrhage Neonatal outcomes Low birthweight (< 2500 g) Birthweight (2500 ‐ 3499 g), (> 3500 g) Small‐for‐gestational age (< 10th centile) Preterm birth (< 37 weeks) Post‐term birth (> 42 weeks) Stillbirth/neonatal death Miscarriage Congenital malformations Apgar score at 1 minute < 6 Apgar score at 5 minutes < 8 Head circumference Crown‐heel length Ponderal index |
|
| Notes | Adherence: adherence levels were not reported, but non‐adherers were included in study results. At 28 to 32 weeks' gestation, just over half the women claimed to be taking the supplement every day, and nearly two‐thirds were doing so by the time of giving birth. Although results were not presented separately for adherers and non‐adherers, the authors state that no significant differences between them were found, apart from a significantly lower risk of postpartum infection among the adherers Dates of study: recruitment began September 1985 ‐ March 1986 Funding sources: Smith Klein and French and Thames laboratories Declarations of interest: not disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation tables |
| Allocation concealment (selection bias) | Low risk | Bottles prepared by drug company and labelled A/B |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of placebo; code not broken until the end of the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but likely to have been done due to use of placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up: 6/500 (1%) ‐ 4 women moved and 2 miscarried |
| Selective reporting (reporting bias) | Low risk | Most of the outcomes specified in the review were reported |
| Other bias | Low risk | No apparent risk of other bias |
UK 1991a.
| Study characteristics | ||
| Methods | A double‐blind, randomised, placebo‐controlled 2‐arm trial | |
| Participants | 56 pregnant women between 15 ‐ 25 weeks of pregnancy were selected at St Thomas' Hospital, London, UK. To fulfil the criteria for eligibility, women with low maternal pre‐pregnancy weight (less than 95% ideal body weight) were selected For both arms of this study, Asian women and primigravidae who smoked more than 5 cigarettes per day with previous small‐for‐gestational‐age baby were set as criteria. For the zinc supplement group, women with previous small‐for‐gestational‐age baby, Asian, with low pre‐pregnancy weight and primigravidae who smoked were included. The placebo group were women with low pre‐pregnancy weight, previous small‐for‐gestational‐age baby, Asian, primigravidae who smoked. Social class was allocated from the classification of the Office of Population Censuses and Surveys (1980); classes 4 ‐ 7 were grouped as lower socioeconomic |
|
| Interventions |
Coded placebo and non‐placebo tablets were prepared by Thames Laboratories Ltd, UK. The effervescent Zn tablets were 22.5 mg |
|
| Outcomes |
Maternal outcomes Pregnancy hypertension Post‐term labour Induction of labour Caesarean section Neonatal outcomes Small‐for‐gestational age Preterm birth Low birthweight Birthweight > 3500 g Congenital malformations Stillbirth/neonatal death. |
|
| Notes | Adherence was 43% in the zinc group and 67% in the placebo group; outcomes were presented separately for adherers and non‐adherers Dates of study: not disclosed Funding sources: Smith Klein and French and Thames Laboratories Ltd Declarations of interest: not disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table, no mention of how the numbers were generated but probably adequately done |
| Allocation concealment (selection bias) | Unclear risk | Quote: "coded placebo or non‐placebo tablet or 22.5 mg effervescent zinc...was randomly prescribed." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blind Quote: "all clinical decisions were made by staff in the labour and delivery wards who were unaware of the trial details". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported, but probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/60 (7%); 2 women moved home, 1 termination of pregnancy, 1 miscarriage (all in the placebo group) |
| Selective reporting (reporting bias) | Unclear risk | Trial did not report all of the primary outcomes expected or specified for this review |
| Other bias | Low risk | No apparent source of other bias |
UK 1991b.
| Study characteristics | ||
| Methods | A double‐blind, RCT | |
| Participants | 152 women resident in Scunthorpe Health District who booked for care before the 18th week of gestation, and who were booked for delivery at either Scunthorpe Maternity Home or at Scunthorpe General Hospital, were recruited for the study 134 women completed the trial |
|
| Interventions |
Women were randomly assigned to 2 groups, X and Y The supplements were prepared by Smith Kline and French. The spansules contained 150 mg of FeSO4 and 0 ‐ 5 mg of folic acid for Group X. For Group Y, 62 mg of zinc sulphide was added to the 150 mg of FeSO4 and 0 ‐ 5 mg of folic acid. Participants were asked to take 1 spansule per day. Haematological tests were undertaken on entering the study and at the 28th week of gestation |
|
| Outcomes | Neonatal outcomes Birthweight (< 2899 g, 2900 ‐ 3399 g, 3400 ‐ 3799 g, > 3800 g) Congenital malformations Stillbirth/neonatal death | |
| Notes | Adherence was not reported Dates of study: February 1983 ‐ July 1988 Funding sources: not disclosed Declarations of interest: not disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up: 18/152 (12%) due to GI effects, aborted or woman moved, leaving 72 in the zinc group and 62 in the control group |
| Selective reporting (reporting bias) | High risk | No maternal outcomes reported |
| Other bias | Low risk | No apparent risk of other bias |
USA 1983.
| Study characteristics | ||
| Methods | A double‐blind RCT | |
| Participants | 213 Hispanic women of Mexican descent 17 years of age or older who were not over 27 weeks of gestational age. Women without diabetes or heart, renal or thyroid disease were selected for study. Women specifically selected on the basis of being at high risk for low zinc status ‐ at baseline, 81% of women had recalled dietary intakes providing < 2/3 RDA All participants agreed to take the test vitamin and mineral supplement as prescribed, return to the clinic for 2 interviews with the research nutritionist, allow 3 blood and 2 hair samples to be taken, and permit the nutritionist to obtain information from their clinic, delivery, and infant records |
|
| Interventions |
Women were randomly assigned into 2 groups. The treatment group received a daily vitamin and mineral supplement as a single capsule providing about 20 mg of zinc as zinc acetate. The control group received a similar supplement without zinc. The capsules were indistinguishable in appearance. The capsule bottles were labelled A or B (Balancel Forte, Meyer Laboratories, Ft Lauderdale, FL). Meyer Laboratory provided the supervisor of the clinic pharmacy with the information necessary to identify the capsules containing zinc. The women were instructed to take the capsule with their evening meal to reduce the possible transitory effect on zinc concentrations in serum samples which were collected in the morning All supplements were formulated to provide 8000 IU vitamin A, 400 IU vitamin D, 30 IU vitamin E, 2 mg thiamin mononitrate, 2 mg riboflavin, 20 mg niacinamide, 5 mg pyridoxine HCI, 1 mg folic acid, 10 µg vitamin B12 (cyanocobalamin), 10 mg D‐calcium pantothenate, 60 mg vitamin C, 100 mg calcium (as carbonate), 20 mg iron (as ferrous fumarate), 50 mg of magnesium (as oxide), 1 mg of manganese (as sulphate), and 150 µg iodine (as potassium iodide) per day |
|
| Outcomes |
Maternal outcomes Pregnancy hypertension PROM Placental weight Serum copper Serum albumin Serum Vitamin A Haemoglobin Plasma folacin RBC folacin ALA dehydratase Serum ribonuclease EGPT index Caesarean section Incidences of infection (vaginitis, asymptomatic bacteruria, cystitis, cervicitis, chorioamnionitis) Length of labour (< 3 or >20 hours) Excessive blood loss during delivery Post‐term pregnancy (> 42 weeks gestation) Neonatal outcomes Birthweight Preterm birth Low birthweight Spontaneous abortion (< 20 weeks) Perinatal death Congenital malformations Apgar scores < 4 (1 min after birth), (5 min after birth) Hair zinc (2 months of age), (6 months of age) |
|
| Notes | Adherence: defined as a woman who was in the study long enough to take supplements for more than 60 days and who returned to the pharmacy for 1 or more refills of 60 capsules. According to this definition, 82% overall (90% (81/90) in the control group and 75% (65/87) in the zinc group) were adherent in those 177 women who were not lost to follow‐up Dates of study: June 1979 ‐ September 1980 Funding sources: Science and Education Administration of the USDA under Grant 5901‐0410‐8‐0105‐0 from the Competitive Research Grants Office Declarations of interest: not disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "randomly assigned" ‐ not definitively stated but likely to have been third party randomisation. Paper stated that "code was not broken until the study was completed". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind"; "capsules were indistinguishable." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 36/213 (16.9%) lost to follow‐up (3 spontaneous abortions < 20 weeks, 2 sets of twins, 31 records that could not be located). The breakdown was 20/107 (18.7%) lost from the zinc group and 16/106 (15.1%) from the placebo group. Breakdown of reasons was not reported except for spontaneous abortions ‐ 1 in the zinc group and 2 in the control group |
| Selective reporting (reporting bias) | Unclear risk | A number of primary maternal, pregnancy and neonatal outcomes were not reported (e.g. caesarean section, postpartum haemorrhage, perinatal death) |
| Other bias | Low risk | No apparent source of other bias |
USA 1985.
| Study characteristics | ||
| Methods | A double‐blind, RCT | |
| Participants | 138 Hispanic teenagers who were under 17 years of age and were not over 27 weeks' gestation and attending prenatal clinic at Los Angeles were recruited for the study The mean dietary zinc intakes among these women were about 50% of the RDA according to LMP. These teenagers did not have diabetes, heart, renal or thyroid disease. All participants agreed to take the test vitamin and mineral supplement as prescribed, return to the clinic for 2 interviews with the research nutritionist, allow 3 blood and 2 hair samples to be taken, and permit the nutritionist to obtain the study details provided by the staff from their clinic, delivery, and infant records |
|
| Interventions |
Treatment group received daily vitamin and mineral supplement in a single capsule providing (20 mg) of zinc. The control group received a capsule that did not contain zinc. The supplements comprised 8000 IU vitamin A, 400 IU vitamin D, 30 IU vitamin E, 2 mg thiamin mononitrate, 2 mg riboflavin, 20 mg niacinamide, 5 mg pyridoxine HCl, 1 mg folic acid, 10 µg vitamin B12 (cyanocobalamin), 10 mg pantothenic acid, 60 mg vitamin C, 100 mg calcium (as carbonate), 20 mg iron (as ferrous fumarate), 50 mg magnesium (as oxide), 1 mg manganese (as sulphate) and 150 µg iodine (as potassium iodide). The capsules between the 2 groups were identical in taste and appearance by (Pharmavite Pharmaceuticals Coporation, Pacoima, CA). Participantss were instructed to take the capsule with their evening meal rather than at breakfast. In addition, 108 mg iron/day was prescribed routinely at 20 weeks' gestation |
|
| Outcomes |
Maternal outcomes Serum zinc Serum copper Serum albumin Haemoglobin Serum ferritin Plasma folacin RBC folacin Serum Vitamin A Placental weight Pregnancy‐induced hypertension Meconium‐stained amniotic fluid Neonatal outcomes Infant weight Birthweight > 2500 g Fetal death Preterm birth Apgar scores Major or minor anomalies |
|
| Notes | Adherence: defined as those in study long enough to take supplements for more than 60 days and who then returned to the pharmacy for 1 or more refills of 60 capsules = 93% of teenagers who returned for a final interview. No significant difference in adherence rates between the groups, so results were not presented separately for adherers and non‐adherers Dates of study: June 1979 ‐ September 1980 Funding sources: Science and Education Administration of the USDA under Grant 78‐59‐2065‐0‐1‐105‐1 from the Competitive Research Grants Office Declarations of interest: not disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" ‐ not further described |
| Allocation concealment (selection bias) | Low risk | Third party (dispensed by clinic pharmacy) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Capsules were identical in composition and indistinguishable in taste and appearance, and the code was not broken until the end of the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but likely to have been blinded due to the use of a placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Birthweight data not available for 31/138 (22%); due to 2 spontaneous abortions and 29 records that could not be located |
| Selective reporting (reporting bias) | High risk | Data for outcomes such as perinatal death and preterm birth were collected but not fully reported (only that no significant differences were found) |
| Other bias | Low risk | No apparent source of other bias |
USA 1989.
| Study characteristics | ||
| Methods | A double‐blind, randomised, 2‐arm placebo‐controlled trial. Women in the groups were of low weight, normal weight and high weight | |
| Participants | The pregnant adolescent women who were at risk for zinc deficiency and enrolled in the prenatal clinic of Charity Hospital of New Orleans, a large urban state‐supported hospital serving area women without access to private maternity care, were considered for the trial. At the first clinic visit, the pregnant adolescent woman attended the a nutrition lecture presented by a nurse, and data of their characteristics and background information were collected. At the second visit, 652 low‐income pregnant adolescent women who were at less than 25 weeks' gestation (average age 17.6 years; range 13.5 to 19.6) were recruited for the trial. Women were grouped by their weight percentile, and treatment group Total of 556 completed the study |
|
| Interventions |
Women were randomly assigned to receiving tablets of 30 mg Zn as gluconate (the Z group) or as the placebo (P) group containing cellulose A sample of blood was taken for chemical analysis and the previous 24‐hour dietary assessments were repeated 8 ‐ 10 weeks after enrolment. The course of the pregnancy was documented by the physician at each prenatal visit. Compliance with the treatment regimen was assessed by a tablet count at each clinic visit and questioning after delivery when details of labour and delivery events were collected. |
|
| Outcomes |
Maternal outcomes
Weight gain
Placental weight
Caesearan section
Labour length
Arm circumference
Triceps skinfold thickness Neonatal outcomes Gestational age at birth Birthweight Low birthweight (< 2500 g) Preterm birth Birth length Head circumference Chest circumference Respiratory assistance |
|
| Notes | Reported compliance was good ‐ 87% consumed 6 or 7 tablets per week Dates of study: not disclosed Funding sources: supported by a US Department of Agriculture, Sciences and Education Administration, Human Nutrition Extramural Research Grant to the Tulane University Medical Center; a cooperative agreement (7USC, 427, 250A, 1624, 2201) between the US Department of Agriculture Agricultural Research Service, Human Nutrition Research Center, Grand Forks, ND, and Tulane University Medical Center; Tulane Universityl and the General Nutrition Corporation, Fargo, ND, which provided the placebo and zinc supplement Declarations of interest: not disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomly assigned." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind"; "identical‐appearing tablets". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Neither the subjects nor the investigators were informed of tablet identity until after completion of the data collection." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up: 10.9% (71/652) at entry and 14.7% (96/652) (cumulative) at birth. Breakdown of losses by group was not reported, nor were reasons for losses |
| Selective reporting (reporting bias) | Unclear risk | A number of primary maternal, pregnancy and neonatal outcomes were not reported (e.g. caesarean, postpartum haemorrhage, perinatal death) |
| Other bias | Low risk | No apparent source of other bias |
USA 1995.
| Study characteristics | ||
| Methods | A double‐blind, randomised, placebo‐controlled trial | |
| Participants | 5058 medically indigent African‐American women receiving prenatal care in 4 Jefferson County (Alabama) Health Department clinics considered for selection The pregnant women who were both nulliparous and multiparous ranged between 13 and 44 years of age were included for the selection criteria. Of these women, 589 at 14 ‐ 23 weeks' gestation were selected for the randomisation trial based on a plasma zinc level below the estimated median for gestational age for the population at the time of enrolment in prenatal care Only 580 women's data completed for analysis due to 9 women had insufficient outcome data |
|
| Interventions |
Women were randomly assigned to both the zinc supplement and placebo groups. Both group received a daily prenatal multivitamin/mineral tablet not containing zinc but containing folic acid, iron, and other minerals. The tablets were produced by Mission Pharmacal, San Antonio, Texas. Zinc supplement and placebo were prepared in a capsules by Rempak, Carteret, New Jersy, and only the zinc supplement contained 25 mg of zinc (zinc sulphate). Women were asked to take 1 of each supplement daily, but the time was not specified The zinc content of the tablets was verified independently in our laboratory. Prior to this study, pregnant women in this care system received only folic acid and iron supplementation For each woman, compliance was defined as the percentage of zinc tablets consumed compared with the number of days enrolled in the project prior to delivery During the study, women in both the zinc supplement and placebo groups were provided with a daily prenatal multivitamin/mineral tablet not containing zinc but folic acid, iron and other minerals |
|
| Outcomes |
Maternal outcomes Pregnancy hypertension Neonatal outcomes Preterm birth Low birthweight Small‐for‐gestational age Stillbirth Neonatal death Neonatal sepsis Crown‐heel length Child mental and psychomotor development at 5 years |
|
| Notes | Adherence: mean was 78% of days for both groups. Adherence was defined as the percentage of zinc tablets consumed compared with the number of days enrolled in the project prior to birth Dates of study: March 1991 ‐ August 1993 Funding sources: supported by grants HD27289 and HD28119 from the National Institutes of Health, Bethesda, Md and by research contract DHHS 282‐92‐0055 from the Agency for Health Care Policy and Research, Rockville, Md Declarations of interest: not disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "both caregivers and subjects were blind regarding the content of the supplement." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but likely to have been done due to the use of a placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up: samples unavailable from 24.7% (143/580 women; 63/294 (21.4%) in the zinc group and 80/286 (28%) in the placebo group At 5 years of age, results were available for 355/580 children (61%) |
| Selective reporting (reporting bias) | Unclear risk | Not enough information to make this judgement. No information on if the protocol had been published prior to the trial |
| Other bias | Low risk | No apparent source of other bias |
BMI: body mass index; dL: decilitre; g: gram; GI: gastrointestinal; IU: international units; kJ: kilojoule; L: litre; LMP: last menstrual period; mg: milligram; PROM: premature rupture of membranes; RCT: randomised controlled trial; RDA: recommended daily allowance; RR: risk ratio; SD: standard deviation; µg: micrograms; µmol: micromoles
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| An 2001 | 313 healthy pregnant women at their fifth month of gestation enrolled in the hospital for prenatal care in Beijing, China, were given fortified biscuits containing (vitamin D), (vitamin D + calcium), (vitamin D + calcium + iron), (vitamin D + calcium + iron + zinc). Only 1 woman selected from the same hospital (no fortified biscuits given) as control comparison. This was a quasi‐randomised study, in which there was no randomised sequence generation performed and allocation was by the order of hospital visits |
| Appelbaum 1979 | The effect of diet supplementation throughout pregnancy on third‐trimester amniotic fluid growth‐supporting activity was studied in 100 African women; 32 were given zinc supplementation, 22 each animal and vegetable supplements, respectively, and 24 served as controls. Zinc level was measured among the groups and the inhibitory of zinc was conducted by in vitro testing of the amniotic fluid collected. The study design did not indicate a randomised controlled trial and the outcome was the zinc level of amniotic fluid only |
| Asemi 2019a | The population selected for the trial were not in a healthy state. Women had impaired glucose tolerance |
| Asemi 2019b | The population selected for the trial were not in a healthy state. Women had impaired glucose tolerance. |
| Christian 2001 | A placebo‐controlled trial in Nepal was conducted on 202 women reported to be night‐blind during pregnancy. They were randomly assigned in a double‐blind manner, stratified on vitamin A, ß‐carotene, or placebo receipt, to receive 25 mg Zn or placebo daily for 3 weeks. The participant selection was in women who had night blindness and there were no prespecified outcomes reported related to pregnancy except for vision restoration of pregnant women |
| Fard 2017 | The population selected for the trial were postpartum. Trial was investigating effect on zinc on postpartum depression |
| Fawzi 2005 | Pregnant women who were HIV‐infected, who resided in Dar es Salaam, Tanzania, at the time of the baseline interview, and who intended to stay in the city until delivery were considered. 400 HIV‐infected pregnant women between 12 and 27 weeks of gestation were randomly assigned to daily oral supplementation with either 25 mg Zn or placebo between recruitment and 6 weeks after delivery. The population selected for the trial were not in a healthy state. |
| France 2004 | Healthy pregnant women (n = 100) receiving prenatal care between 12 and 16 weeks of gestation in the Obstetric Departments of Grenoble and Lyon Hospitals in France participated in a double‐blind, randomised, placebo‐controlled trial. The intervention was micronutrients supplement or placebo. The micronutrients contained vitamin C (60 mg), ß‐carotene (4.8 mg), vitamin E (10 mg), thiamin (1.4 mg), riboflavin (1.6 mg), niacin (15 mg), pantothenic acid (6 mg), folic acid (200 mg), cobalamin (1 mg), Zn (15 mg as citrate), Mg (87.5 mg as glycerophosphate), Ca (100 mg as carbonate). Zinc was not given separately as the main intervention |
| Hambidge 1983 | A longitudinal study (monthly intervals) in 46 pregnant middle‐income women. 10 of the women received a daily supplement of 15 mg Zn and the rest of the women did not receive any zinc supplement. The design was an observational study with no mention of randomisation or allocation to zinc or no‐zinc groups |
| Hambidge 2017 | A longitudinal, observational study using convenience sampling. This study was not randomised |
| Heidarzadeh 2017 | The population selected for the trial were not in a healthy state. Women had impaired glucose tolerance |
| India 1993 | 90 pregnant women were randomly assigned to control (A) and 120 pregnant women were randomly assigned to zinc‐treated (B) groups. Group B women were administered a single daily dose of 45 mg zinc as a 200 mg zinc sulphate tablet (Zinfate, Yash Pharma) from the day of reporting till delivery. The control‐group women were not provided with zinc supplementation. The total number of women finally selected in group A serving as control was 62, and that in Gp. B was 106. The study design was quasi‐randomised and with large discrepancies in numbers of participants at baseline and follow‐up |
| Karamali 2015 | The population selected for the trial were not in a healthy state. Women had impaired glucose tolerance |
| Karamali 2016 | The population selected for the trial were not in a healthy state. Women had impaired glucose tolerance |
| Kynast 1986 | A randomly‐selected study group of 179 pregnant women and a control group of 345 pregnant women were given zinc aspartate. This study investigates the prophylactic effectiveness of zinc replacement in reducing the overall complication rate for both mother and fetus, and in particular for large‐for‐date and small‐for‐date infants. The study design was quasi‐randomised, with allocation done by alternation |
| Mahmoudian 2005 | A total of 118 anaemic women were recruited in this randomised controlled trial. Both groups received 100 mg elemental iron daily. The intervention group received an additional dose of 15 mg zinc every day for a period of 12 weeks, while the control group received placebo. The participants were all anaemic women and outcomes were haemoglobin concentration only |
| Makola 2003 | This study was a randomised, placebo‐controlled double‐blind effectiveness trial of a micronutrient‐fortified dietary supplement conducted in pregnant women in Tanzania. Pregnant women who believed that they were between 12 and 34 weeks pregnant were invited to participate in the study. The intervention was micronutrient supplement (orange‐flavoured micronutrient‐fortified powdered beverage mix containing 11 micronutrients, including zinc) in comparison to placebo. Women with gestation greater than 26 weeks were included in the study, and zinc was not provided separately as a supplemental intervention |
| Mesdaghinia 2019 | This randomised, double‐blind, placebo‐controlled, clinical trial was conducted among 52 women at risk for IUGR according to abnormal uterine artery Doppler waveform. Participants were randomly assigned to take either 233 mg zinc gluconate (containing 30 mg zinc) supplements (n = 26) or placebo (n = 26) for 10 weeks from 17 to 27 weeks of gestation. Primary outcomes of interest were inflammatory markers. Excluded based on outcomes not measured |
| Naher 2012 | 200 pregnant women, age ranging between 18 ‐ 40 years and gestational age ranging from 37 ‐ 42 weeks were selected for a cross‐sectional study in Bangladesh. Among them, 100 were advised to take 61.8 mg zinc daily and the others did not. The study design was an observational study and not a randomised controlled trial |
| Nishiyama 1999 | 38 Japanese women at the second trimester of pregnancy had haemoglobin concentrations below 11.0 g/dL and 32 of 38 had normocytic erythrocytes. These women were divided into 3 groups, and were compared for their haematological status and serum IGF‐I levels before and after iron (Group A) or Zn (Group B) or iron plus Zn (Group C) supplementation. The women were anaemic and the study design was a clinical controlled trial where women could choose 1 of 3 intervention groups |
| Nogueira 2003 | 74 low‐income pregnant adolescents in Brazil ranging from 13 ‐ 18 years of age received supplementation of (folic acid + iron), (folic acid + zinc sulphate + iron) or only iron. The pregnant adolescents were divided into 5 groups. The study method was based on longitudinal design and zinc plasma concentration was measured to support the folic acid metabolism |
| Ostadmohammadi 2019 | The population selected for the trial were not in a healthy state. Women had impaired glucose tolerance |
| Page 2018 | At assessment participants are classified as either having a history of depression or onset of depression in pregnancy, and as either users or non‐users of antidepressants. The population selected for the trial were not in a healthy state |
| Roshanravan 2020 | The population selected for the trial were not in healthy state. Women had impaired glucose tolerance |
| Shahnazi 2017 | In this randomised, double‐blind, controlled clinical trial, 108 healthy pregnant women (at gestational age of 16 ‐ 30 weeks) with a history of PPROM and singleton pregnancy were selected by convenience sampling method in the Midwifery Clinic of Shahid Akbarabadi hospital in Tehran, Iran, between 2014 and 2015. The population selected for the trial were not in a healthy state |
| Van Vliet 2001 | The study was an open, randomised study, using a cross‐over approach for 2 sources of vitamin A (i.e. liver paste and retinyl palmitate containing oil) and a parallel approach for 3 dose levels of vitamin A (i.e. 3.0, 7.5, and 15 mg vitamin A) for women between 19 ‐ 47 years of age. Pregnant women were excluded from the study. The intervention did not use zinc supplements or any zinc constituent supplements |
| Villamor 2006 | Pregnant women with HIV and the HIV status of their babies was assessed at birth and at 6 weeks postpartum at Dar es Salaam Tanzania. Women 12 – 27 weeks of gestation were randomly assigned to receive a daily oral dose of 25 mg zinc or placebo from the day of the first prenatal visit until 6 weeks post‐delivery. All the participants were infected with HIV |
| Yalda 2010 | Single‐blind randomised clinical controlled trial conducted in Kurdistan region, Iraq. 100 anaemic pregnant women were selected to receive, first group (A), supplemented daily with 120 mg iron and second group (B) received 120 mg iron + 22.5 mg zinc. The pregnant women were all diagnosed with anaemia and the outcome was to assess the improvement of anaemic condition |
Differences between protocol and review
We have updated our methods to reflect the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) for 'Risk of bias' procedures, and Higgins 2020 for all other methods.
Outcomes have been separated into 'Primary' and 'Secondary' outcomes.
We have added congenital malformation, neonatal and infant chest circumference, neonatal head circumference, neonatal mid‐upper arm circumference, crown‐heel length, and birth length to our secondary outcomes.
We conducted a subgroup analysis for pregnancy hypertension and pre‐eclampsia.
GIven the number of trials identified and the standard methods for the Cochrane Pregnancy and Childbirth Group, we have excluded quasi‐randomised controlled trials.
Contributions of authors
B Carducci and EC Keats screened for eligibility, assessed risks of bias and extracted data for the newly included studies in the update. BC conducted the analyses and prepared the second version of this manuscript. EC Keats and ZA Bhutta read and approved the draft of the update.
Sources of support
Internal sources
Discipline of Obstetrics and Gynaecology, The University of Adelaide, Australia
National Center for Child Health and Development, Japan
External sources
-
Ministry of Health, Labour and Welfare, Japan
Health Labour Sciences Research Grant (No.13800128)
The Evidence and Programme Guidance, Department of Nutrition for Health and Development, World Health Organization, Switzerland
Declarations of interest
Bianca Carducci: none known
Emily C Keats: none known
Zulfiqar A Bhutta: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Bangladesh 2000 {published data only}
- Darmstadt GL, Osendarp SJ, Ahmed S, Feldman C, Van JM, Baqui AH, et al. Effect of antenatal zinc supplementation on impetigo in infants in Bangladesh. Pediatric Infectious Disease Journal 2012;31(4):407-9. [DOI] [PubMed] [Google Scholar]
- Hamadani JD, Fuchs GJ, Osendarp SJ, Huda SN, Grantham-McGregor SM. Zinc supplementation during pregnancy and effects on mental development and behaviour of infants: a follow-up study. Lancet 2002;360(9329):290-4. [DOI] [PubMed] [Google Scholar]
- Osendarp S. Zinc supplementation in Bangladeshi women and infants: effects on pregnancy outcome, infant growth, morbidity and immune response [thesis]. Wageningen: Wageningen University, 2001. [Google Scholar]
- Osendarp SJ, Raaij JM, Darmstadt GL, Baqui AH, Hautvast JG, Fuchs GJ. Zinc supplementation during pregnancy and effects on growth and morbidity in low birthweight infants: a randomised placebo controlled trial. Lancet 2001;357(9262):1080-5. [DOI] [PubMed] [Google Scholar]
- Osendarp SJ, Van Raaij JM, Arifeen SE, Wahed MA, Baqui AH, Fuchs GJ. A randomized, placebo-controlled trial of the effect of zinc supplementation during pregnancy outcome in Bangladeshi urban poor. American Journal of Clinical Nutrition 2000;71(1):114-9. [DOI] [PubMed] [Google Scholar]
Bangladesh 2016 {published data only}
- Ahmad SM, Hossain MB, Monirujjaman M, Islam S, Huda MN, Kabir Y, et al. Maternal zinc supplementation improves hepatitis B antibody responses in infants but decreases plasma zinc level. European Journal of Nutrition 2016;55(5):1823-9. [DOI] [PubMed] [Google Scholar]
Chile 2001 {published data only}
- Castillo-Duran C, Marin V, Alcazar LS, Iturralde H, Ruz MO. Controlled trial of zinc supplementation in Chilean pregnancy adolescents. Nutrition Research 2001;21:715-24. [Google Scholar]
China 2001 {published data only}
- Xie L, Chen X, Pan J. The effects of zinc supplementation to Chinese rural pregnant women and their pregnancy outcome. Journal of Shanghai Second Medical University 2001;13(2):119-24. [Google Scholar]
- Yuan W, Geng G, Chen A, Wu J, Zhang Z, Gao E. Effects of zinc supplementation of rural pregnant women on the growth of offspring in early childhood. Fudan University Journal of Medical Sciences 2004;31(5):496-501. [Google Scholar]
Denmark 1996 {published data only}
- Jønsson B, Hauge B, Larsen MF, Hald F. Zinc supplementation during pregnancy: a double blind randomised controlled trial. Acta Obstetricia et Gynecologica Scandinavica 1996;75(8):725-9. [DOI] [PubMed] [Google Scholar]
Egypt 2014 {published data only}
- Naem NE, El-Sayed NM, Nossier SA, Abu Zeid AA. Zinc status and dietary intake of pregnant women, Alexandria, Egypt. Journal of the Egyptian Public Health Association 2014;89(1):35-41. [DOI] [PubMed] [Google Scholar]
- Nossier SA, Naeim NE, El-Sayed NA, Abu Zeid AA. The effect of zinc supplementation on pregnancy outcomes: a double-blind, randomised controlled trial, Egypt. British Journal of Nutrition 2015;114(2):274-85. [DOI] [PubMed] [Google Scholar]
- PACTR201303000453309. Does zinc supplementation improve pregnancy outcome of women in Alexandria, Egypt? www.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201303000453309 2011.
Ghana 2009 {published data only}
- Saaka M, Oosthuizen J, Beatty S. Effect of joint iron and zinc supplementation on malarial infection and anaemia. East African Journal of Public Health 2009;6(1):55-62. [DOI] [PubMed] [Google Scholar]
- Saaka M, Oosthuizen J, Beatty S. Effect of prenatal zinc supplementation on birthweight. Journal of Health, Population & Nutrition 2009;27(5):619-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaka M. Combined iron and zinc supplementation improves haematologic status of pregnant women in Upper West Region of Ghana. Ghana Medical Journal 2012;46(4):225-33. [PMC free article] [PubMed] [Google Scholar]
Indonesia 2001a {published data only}
- Dijkhuizen MA, Wieringa FT, West CE, Muhilal. Zinc plus β-carotene supplementation of pregnant women is superior to β-carotene supplementation alone in improving vitamin A status in both mothers and infants. American Journal of Clinical Nutrition 2004;80(5):1299-307. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen MA, Wieringa FT. Vitamin A, iron and zinc deficiency in Indonesia: micronutrient interactions and effects of supplementation [thesis]. Wageningen: Wageningen University, 2001. [Google Scholar]
- Wieringa FT, Dijkhuizen MA, Muhilal, Van der Meer JW. Maternal micronutrient supplementation with zinc and beta-carotene affects morbidity and immune function of infants during the first 6 months of life. European Journal of Clinical Nutrition 2010;64(10):1072-9. [DOI] [PubMed] [Google Scholar]
Indonesia 2001b {published data only}
- Hakimi M, Dibley MJ, Surjono A, Nurdiati D. Impact of vitamin A and zinc supplementation on puerperal sepsis: a randomized controlled trial in rural Indonesia. In: Nurdiati D, editors(s). Nutrition and Reproductive Health in Central Java, Indonesia; an Epidemiological Approach [PhD thesis]. Umea, Sweden: Umea University Medical Dissertations, 2001. [Google Scholar]
- Prawirohartono EP, Nystrom L, Ivarsson A, Stenlund H, Lind T. The impact of prenatal vitamin A and zinc supplementation on growth of children up to 2 years of age in rural Java, Indonesia. Public Health Nutrition 2011;14(12):2197-206. [DOI] [PubMed] [Google Scholar]
- Prawirohartono EP, Nystrom L, Nurdiati DS, Hakimi M, Lind T. The impact of prenatal vitamin A and zinc supplementation on birth size and neonatal survival - a double-blind, randomized controlled trial in a rural area of Indonesia. International Journal for Vitamin and Nutrition Research 2013;83(1):14-25. [DOI] [PubMed] [Google Scholar]
Iran 2009 {published data only}
- Aminisani N, Ehdaivand F, Shamshirgaran SM, Mohajery M, Pourfarzi F, Sadeghiyeh Ahari MD. Zinc supplementation during pregnancy: a randomized controlled trial. Iranian Journal of Pharmacology & Therapeutics 2009;8(2):67-71. [Google Scholar]
Iran 2010 {published data only}
- Danesh A, Janghorbani M, Mohammadi B. Effects of zinc supplementation during pregnancy on pregnancy outcome in women with history of preterm delivery: a double-blind randomized, placebo-controlled trial. Journal of Maternal-Fetal and Neonatal Medicine 2010;23(5):403-8. [DOI] [PubMed] [Google Scholar]
Iran 2016 {published data only}
- IRCT138707161306N1. Assessment the effect of zinc supplementation on adverse outcomes of pregnancy, a randomized controlled clinical trial. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT138707161306N1 (accessed 6 December 2010).
- Zahiri Sorouri Z, Sadeghi H, Pourmarzi D. The effect of zinc supplementation on pregnancy outcome: a randomized controlled trial. Journal of Maternal-Fetal & Neonatal Medicine 2016;29(13):2194-8. [DOI] [PubMed] [Google Scholar]
Nepal 2003 {published data only}
- Christian P, Jiang T, Khatry SK, LeClerq SC, Shrestha SR, West KP Jr. Antenatal supplementation with micronutrients and biochemical indicators of status and subclinical infection in rural Nepal. American Journal of Clinical Nutrition 2006;83(4):788-94. [DOI] [PubMed] [Google Scholar]
- Christian P, Khatry SK, Katz J, Pradhan EK, LeClerq SC, Ram Shrestha S, et al. Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomised community trial. BMJ 2003;326(7389):571-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian P, Shrestha J, LeClerq SC, Khatry SK, Jiang T, Wagner T, et al. Supplementation with micronutrients in addition to iron and folic acid does not further improve the hematologic status of pregnant women in Nepal. Journal of Nutrition 2003;133(11):3492-8. [DOI] [PubMed] [Google Scholar]
- Christian P, West KP, Khatry SK, Leclerq SC, Pradhan EK, Katz J, et al. Effects of maternal micronutrient supplementation on fetal loss and infant mortality: a cluster-randomized trial in Nepal. American Journal of Clinical Nutrition 2003;78(6):1194-202. [DOI] [PubMed] [Google Scholar]
- Stewart CP, Christian P, Schulze KJ, Leclerq SC, West KP, Khatry SK. Antenatal micronutrient supplementation reduces metabolic syndrome in 6- to 8-year-old children in rural Nepal. Journal of Nutrition 2009;139(8):1575-81. [DOI] [PubMed] [Google Scholar]
Pakistan 2005 {published data only}
- Hafeez A, Mahmood G, Hassan M, Batool T, Hayat H, Mazhar F, et al. Serum zinc levels and effects of oral supplementation in pregnant women. JCPSP - Journal of the College of Physicians and Surgeons Pakistan 2005;15(10):612-5. [PubMed] [Google Scholar]
- Hafeez A, Mehmood G, Mazhar F. Oral zinc supplementation in pregnant women and its effect on birth weight: a randomised controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90:F170-F171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peru 1999 {published data only}
- Caulfield LE, Donangelo CM, Chen P, Junco J, Merialdi M, Zavaleta N. Red blood cell metallothionein as an indicator of zinc status during pregnancy. Nutrition 2008;24(11-12):1081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield LE, Zavaleta N, Figueroa A, Zulema L. Maternal zinc supplementation does not affect size at birth or pregnancy duration in Peru. Journal of Nutrition 1999;129(8):1563-8. [DOI] [PubMed] [Google Scholar]
- Caulfield LE, Zavaleta N, Figueroa A. Adding zinc to prenatal iron and folate supplements improves maternal and neonatal zinc status in a Peruvian population. American Journal of Clinical Nutrition 1999;69(6):1257-63. [DOI] [PubMed] [Google Scholar]
- Iannotti LL, Zavaleta N, Leon Z, Huasquiche C, Shankar AH, Caulfield LE. Maternal zinc supplementation reduces diarrheal morbidity in Peruvian infants. Journal of Pediatrics 2010;156(6):960-4, 964.e1- 964.e2. [DOI] [PubMed] [Google Scholar]
- Iannotti LL, Zavaleta N, Leon Z, Shankar AH, Caulfield LE. Maternal zinc supplementation and growth in Peruvian infants. American Journal of Clinical Nutrition 2008;88(1):154-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merialdi M, Caulfield LE, Zavaleta N, Figueroa A, DiPietro JA. Adding zinc to prenatal iron and folate tablets improves fetal neurobehavioral development. American Journal of Obstetrics and Gynecology 1999;180(2 Pt 1):483-90. [DOI] [PubMed] [Google Scholar]
- Mispireta ML, Caulfield LE, Zavaleta N, Merialdi M, Putnick DL, Bornstein MH, et al. Effect of maternal zinc supplementation on the cardiometabolic profile of Peruvian children: results from a randomized clinical trial. Journal of Developmental Origins of Health and Disease 2017;8(1):56-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien KO, Zavaleta N, Caulfield LE, Wen J, Abrams SA. Prenatal iron supplements impair zinc absorption in pregnant Peruvian women. Journal of Nutrition 2000;130(9):2251-5. [DOI] [PubMed] [Google Scholar]
- O'Brien KO, Zavaleta N, Caulfield LE, Yang D-X, Abrams SA. Influence of prenatal iron and zinc supplements on supplemental iron absorption, red blood cell incorporation, and iron status in pregnant Peruvian women. American Journal of Clinical Nutrition 1999;69(3):509-15. [DOI] [PubMed] [Google Scholar]
- Zavaleta N, Caulfield LE, Figueroa A, Chen P. Patterns of compliance with prenatal iron supplementation among Peruvian women. Maternal and Child Nutrition 2014;10(2):198-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavaleta N, Caulfield LE, Garcia T. Changes in iron status during pregnancy in Peruvian women receiving prenatal iron and folic acid supplements with or without zinc. American Journal of Clinical Nutrition 2000;71(4):956-61. [DOI] [PubMed] [Google Scholar]
Peru 2004 {published data only}
- Caulfield LE, Putnick DL, Zavaleta N, Lazarte F, Albornoz C, Chen P, et al. Maternal gestational zinc supplementation does not influence multiple aspects of child development at 54 mo of age in Peru. American Journal of Clinical Nutrition 2010;92(1):130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield LE, Zavaleta N, Chen P, Lazarte F, Albornoz C, Putnick DL, et al. Maternal zinc supplementation during pregnancy affects autonomic function of Peruvian children assessed at 54 months of age. Journal of Nutrition 2011;141(2):327-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merialdi M, Caulfield LE, Zavaleta N, Figueroa A, Costigan KA, Dominici F, et al. Randomized controlled trial of prenatal zinc supplementation and fetal bone growth. American Journal of Clinical Nutrition 2004;79(5):826-30. [DOI] [PubMed] [Google Scholar]
- Merialdi M, Caulfield LE, Zavaleta N, Figueroa A, Dominici F, DiPietro JA. Randomized controlled trial of prenatal zinc supplementation and the development of fetal heart rate. American Journal of Obstetrics and Gynecology 2004;190(4):1106-12. [DOI] [PubMed] [Google Scholar]
S Africa 1985 {published data only}
- Ross SM, Nel E, Naeye RL. Differing effects of low and high bulk maternal dietary supplements during pregnancy. Early Human Development 1985;10(3-4):295-302. [DOI] [PubMed] [Google Scholar]
Tanzania 2017 {published data only}
- Darling AM, Mugusi FM, Etheredge AJ, Gunaratna NS, Abioye AI, Aboud S, et al. Vitamin A and zinc supplementation among pregnant women to prevent placental malaria: a randomized, double-blind, placebo-controlled trial in Tanzania. American Journal of Tropical Medicine and Hygiene 2017;96(4):826-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor RA, Abioye AI, Darling AM, Hertzmark E, Aboud S, Premji Z, et al. Prenatal zinc and vitamin A reduce the benefit of iron on maternal hematologic and micronutrient status at delivery in Tanzania. Journal of Nutrition 2020;150(2):240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchik H, Darling AM, Abioye AI, Tesha F, Smith ER, Sudfeld CR, et al. Prenatal nutrition, stimulation, and exposure to punishment are associated with early child motor, cognitive, and socioemotional development in Dar es Salaam, Tanzania. Annals of Nutrition and Metabolism 2017;71(Suppl 2):626, Abstract no: 144-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
UK 1989 {published data only}
- James DK, Golding J, Mahomed K, McCabe R. A randomised double blind placebo controlled trial of zinc supplementation in pregnancy. In: Proceedings of 27th Autumn meeting of British Association of Perinatal Medicine; 1989; UK. 1989.
- Mahomed K, James DK, Golding J, McCabe R. Failure to taste zinc sulphate solution does not predict zinc deficiency in pregnancy. European Journal of Obstetrics & Gynecology and Reproductive Biology 1993;48(3):169-75. [DOI] [PubMed] [Google Scholar]
- Mahomed K, James DK, Golding J, McCabe R. Zinc supplementation during pregnancy: a double blind randomised controlled trial. BMJ 1989;299(6703):826-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
UK 1991a {published data only}
- Simmer K, Lort-Phillips L, James C, Thompson RP. A double blind trial of zinc supplementation in pregnancy. European Journal of Clinical Nutrition 1991;45(3):139-44. [PubMed] [Google Scholar]
UK 1991b {published data only}
- Robertson JS, Heywood B, Atkinson SM. Zinc supplementation during pregnancy. Journal of Public Health Medicine 1991;13(3):227-9. [DOI] [PubMed] [Google Scholar]
USA 1983 {published data only}
- Hunt IF, Murphy NJ, Cleaver AE, Faraji B, Swendseid ME, Coulson AH, et al. Zinc supplementation during pregnancy: effects on selected blood constituents and on progress and outcome of pregnancy in low-income women of Mexican descent. American Journal of Clinical Nutrition 1984;40(3):508-21. [DOI] [PubMed] [Google Scholar]
- Hunt IF, Murphy NJ, Cleaver AE, Faraji B, Swendseid ME, Coulson AM, et al. Zinc supplementation during pregnancy: zinc concentration of serum and hair from low-income women of Mexican descent. American Journal of Clinical Nutrition 1983;37(4):572-82. [DOI] [PubMed] [Google Scholar]
USA 1985 {published data only}
- Hunt IF, Murphy NJ, Cleaver AE, Faraji B, Swendseid ME, Browdy BL, et al. Zinc supplementation during pregnancy in low income teenagers of Mexican descent: effects on selected blood constituents and on progress and outcome of pregnancy. American Journal of Clinical Nutrition 1985;42(5):815-28. [DOI] [PubMed] [Google Scholar]
USA 1989 {published data only}
- Cherry FF, Sandstead HH, Rojas P, Johnson LK, Batson HK, Wang XB. Adolescent pregnancy: associations among body weight, zinc nutriture, and pregnancy outcome. American Journal of Clinical Nutrition 1989;50(5):945-54. [DOI] [PubMed] [Google Scholar]
USA 1995 {published data only}
- Goldenberg R, Tamura T, Neggers Y, Copper R, Johnston K, DuBard M, et al. Maternal zinc supplementation increases birthweight and head circumference. American Journal of Obstetrics and Gynecology 1995;172(1 Pt 2):368. [Google Scholar]
- Goldenberg RL, Tamura T, Neggers Y, Cooper RL, Johnston KE, DuBard MB, et al. The effect of zinc supplementation on pregnancy outcome. JAMA 1995;274(6):463-8. [DOI] [PubMed] [Google Scholar]
- Hogg B, Tamura T, Johnston K, DuBard M, Goldenberg RL. Homocysteine levels in pregnancy induced hypertension (PIH), preeclampsia (PE) and intrauterine growth retardation (IUGR) [abstract]. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S90. [DOI] [PubMed] [Google Scholar]
- Hogg BB, Tamura T, Johnston KE, DuBard MB, Goldenberg RL. Second-trimester plasma homocysteine levels and pregnancy-induced hypertension, preeclampsia, and intrauterine growth restriction. American Journal of Obstetrics and Gynecology 2000;183(4):805-9. [DOI] [PubMed] [Google Scholar]
- Neggers YH, Goldenberg RL, Tamura T, Johnston KE, Copper RL, DuBard M. Plasma and erythrocyte zinc concentrations and their relationship to dietary zinc intake and zinc supplementation during pregnancy in low-income African-American women. Journal of the American Dietetic Association 1997;97(11):1269-74. [DOI] [PubMed] [Google Scholar]
- Tamura T, Goldenberg RL, Ramey SL, Nelson KG, Chapman VR. Effect of zinc supplementation of pregnant women on the mental and psychomotor development of their children at 5 y of age. American Journal of Clinical Nutrition 2003;77(6):1512-6. [DOI] [PubMed] [Google Scholar]
- Tamura T, Goldenberg RN, Johnston KE, DuBard MB. Effect of smoking on plasma ferritin concentrations in pregnant women. Clinical Chemistry 1995;41(8):1190-1. [PubMed] [Google Scholar]
- Tamura T, Olin KL, Goldenberg RL, Johnston KE, Dubard MB, Keen CL. Plasma extracellular superoxide dismutase activity in healthy pregnant women is not influenced by zinc supplementation. Biological Trace Element Research 2001;80(2):107-13. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
An 2001 {published data only}
- An H, Yin S, Xu Q. Effects of supplementing calcium, iron and zinc on the fetus development and growth during pregnancy. Chung-Hua Yu Fang i Hsueh Tsa Chih [Chinese Journal of Preventive Medicine] 2001;35(6):370-3. [PubMed] [Google Scholar]
Appelbaum 1979 {published data only}
- Appelbaum PC, Ross SM, Dhupelia I, Naeye RL. The effect of diet supplementation and addition of zinc in vitro on the growth-supporting property of amniotic fluid in African women. American Journal of Obstetrics and Gynecology 1979;135(1):82-4. [PubMed] [Google Scholar]
Asemi 2019a {published data only}
- Asemi Z, IRCT20170513033941N57. The effects of combined zinc and vitamin E supplementation on pregnancy outcomes, inflammatory factors, oxidative stress biomarkers and gene expression related to inflammation in women with gestational diabetes. en.irct.ir/trial/38412 (first received 16 April 2019).
Asemi 2019b {published data only}
- Asemi Z, IRCT20170513033941N56. The effect of combined magnesium and vitamin E on pregnancy outcomes, inflammatory factors and oxidative stress biomarkers in patients with gestational diabetes. en.irct.ir/trial/38417 (first received 15 April 2019).
Christian 2001 {published data only}
- Christian P, Khatry SK, LeClerq SC, Shrestha SR, Kimbrough-Pradhan E, West KP Jr. Iron and zinc interactions among pregnant Nepali women. Nutrition Research 2001;21(1-2):141-8. [Google Scholar]
- Christian P, Khatry SK, Yamini S, Stallings R, LeClerq SC, Shrestha SR, et al. Zinc supplementation might potentiate the effect of vitamin A in restoring night vision in pregnant Nepalese women. American Journal of Clinical Nutrition 2001;73(6):1045-51. [DOI] [PubMed] [Google Scholar]
Fard 2017 {published data only}
- Fard FE, Mirghafourvand M, Mohammad-Alizadeh Charandabi S, Farshbaf-Khalili A, Javadzadeh Y, Asgharian H. Effects of zinc and magnesium supplements on postpartum depression and anxiety: a randomized controlled clinical trial. Women & Health 2017;57(9):1115-28. [DOI] [PubMed] [Google Scholar]
Fawzi 2005 {published data only}
- Fawzi WW, Villamor E, Msamanga GI, Antelman G, Aboud S, Urassa W, et al. Trial of zinc supplements in relation to pregnancy outcomes, hematologic indicators, and T cell counts among HIV-1-infected women in Tanzania. American Journal of Clinical Nutrition 2005;81(1):161-7. [DOI] [PubMed] [Google Scholar]
France 2004 {published data only}
- Hininger I, Favier M, Arnaud J, Faure H, Thoulon JM, Hariveau E, et al. Effects of a combined micronutrient supplementation on maternal biological status and newborn anthropometrics measurements: a randomized double-blind, placebo-controlled trial in apparently healthy pregnant women. European Journal of Clinical Nutrition 2004;58(1):52-9. [DOI] [PubMed] [Google Scholar]
Hambidge 1983 {published data only}
- Hambidge KM, Krebs NF, Jacobs MA, Favier A, Guyette L, Ikle DN. Zinc nutritional status during pregnancy: a longitudinal study. American Journal of Clinical Nutrition 1983;37(3):429-42. [DOI] [PubMed] [Google Scholar]
Hambidge 2017 {published data only}
- Hambidge KM, Miller LV, Westcott J, Kemp JF, Krebs NF, Mazariegos M, et al. Upregulation of zinc absorption matches increases in physiologic requirements for zinc in women consuming high- or moderate-phytate diets during late pregnancy and early lactation. Journal of Nutrition 2017;147(6):1079-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heidarzadeh 2017 {published data only}
- Heidarzadeh Z, Samimi M, Seifati SM, Ashkezari MD, Ahmadi S, Mahmoodi S, et al. The effect of zinc supplementation on expressed levels of peroxisome proliferator-activated receptor gamma and glucose transporter type 1 genes in newborns of women with gestational diabetes mellitus. Biological Trace Element Research 2017;175(2):271-7. [DOI] [PubMed] [Google Scholar]
India 1993 {published data only}
- Garg HK, Singal KC, Arshad Z. Zinc taste test in pregnant women and its correlation with serum zinc level. Indian Journal of Physiology and Pharmacology 1993;37(4):318-22. [PubMed] [Google Scholar]
- Garg HK, Singhal KC, Arshad Z. A study of the effect of oral zinc supplementation during pregnancy on pregnancy outcome. Indian Journal of Physiology and Pharmacology 1993;37(4):276-84. [PubMed] [Google Scholar]
- Garg HK, Singhal KC, Arshad Z. Effect of oral zinc supplementation on copper and haemoglobin levels in pregnant women. Indian Journal of Physiology and Pharmacology 1994;38(4):272-6. [PubMed] [Google Scholar]
Karamali 2015 {published data only}
- Karamali M, Heidarzadeh Z, Seifati SM, Samimi M, Tabassi Z, Hajijafari M, et al. Zinc supplementation and the effects on metabolic status in gestational diabetes: a randomized, double-blind, placebo-controlled trial. Journal of Diabetes and Its Complications 2015;29(8):1314-9. [DOI] [PubMed] [Google Scholar]
Karamali 2016 {published data only}
- Karamali M, Heidarzadeh Z, Seifati SM, Samimi M, Tabassi Z, Talaee N, et al. Zinc supplementation and the effects on pregnancy outcomes in gestational diabetes: a randomized, double-blind, placebo-controlled trial. Experimental & Clinical Endocrinology & Diabetes 2016;124(1):28-33. [DOI] [PubMed] [Google Scholar]
Kynast 1986 {published data only}
- Kynast G, Saling E. Effect of oral zinc application during pregnancy. Gynecologic and Obstetric Investigation 1986;21(3):117-23. [DOI] [PubMed] [Google Scholar]
Mahmoudian 2005 {published data only}
- Mahmoudian A, Khademloo MD. The effect of simultaneous administration of zinc sulfate and ferrous sulfate in the treatment of anemic pregnant women. Journal of Research in Medical Sciences 2005;10(4):205-9. [Google Scholar]
Makola 2003 {published data only}
- Makola D, Ash DM, Tatala SR, Latham MC, Ndossi G, Mehanso H. A micronutrient-fortified beverage prevents iron deficiency, reduces anemia and improves the hemoglobin concentration of pregnant Tanzanian women. Journal of Nutrition 2003;133(5):1339-46. [DOI] [PubMed] [Google Scholar]
Mesdaghinia 2019 {published data only}
- IRCT20170513033941N33. Effect of zinc supplementation in treatment of pregnant women at risk for intrauterine growth restriction [Clinical trial of the effect of zinc supplementation compared with the placebo on metabolic profiles in pregnant women at risk for intrauterine growth restriction]. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20170513033941N33 (first received 03 June 2018). [CENTRAL: CN-01909693]
- Mesdaghinia E, Naderi F, Bahmani F, Chamani M, Ghaderi A, Asemi Z. The effects of zinc supplementation on clinical response and metabolic profiles in pregnant women at risk for intrauterine growth restriction: a randomized, double-blind, placebo-controlled trial. Journal of Maternal-fetal & Neonatal Medicine 2019 July [Epub ahead of print]:1-7. [CENTRAL: CN-01960439] [EMBASE: 628468000] [PMID: ] [DOI] [PubMed]
Naher 2012 {published data only}
- Naher K, Nahar K, Aziz MA, Hossain A, Rahman R, Yasmin N. Maternal serum zinc level and its relation with neonatal birth weight. Mymensingh Medical Journal : MMJ 2012;21(4):588-93. [PubMed] [Google Scholar]
Nishiyama 1999 {published data only}
- Nishiyama S, Kiwaki K, Miyazaki Y, Hasuda T. Zinc and IGF-I concentrations in pregnant women with anemia before and after supplementation with iron and/or zinc. Journal of the American College of Nutrition 1999;18(3):261-7. [DOI] [PubMed] [Google Scholar]
Nogueira 2003 {published data only}
- Nogueira ND, Macedo AD, Parente JV, Cozzolino SM. Nutritional profile of newborns of adolescent mothers supplemented with iron, in different concentrations, zinc and pholic acid. Revista de Nutricao 2002;15:193-200. [Google Scholar]
- Nogueira Ndo N, Parente JV, Cozzolino SM. Changes in plasma zinc and folic acid concentrations in pregnant adolescents submitted to different supplementation regimens. Cadernos de Saude Publica 2003;19(1):155-60. [DOI] [PubMed] [Google Scholar]
Ostadmohammadi 2019 {published data only}
- Ostadmohammadi V, Aghadavod E, Dastorani M, Asemi Z, Samimi M, Zarezade Mehrizi M, et al. The effect of zinc and vitamin e cosupplementation on metabolic status and its related gene expression in patients with gestational diabetes. Journal of Maternal-Fetal and Neonatal Medicine 2019;32(24):4120-7. [DOI] [PubMed] [Google Scholar]
Page 2018 {published data only}ISRCTN80468181
- Page N, ISRCTN80468181. Can taking a zinc supplement during pregnancy reduce the symptoms of depression both before and after the baby is born? www.isrctn.com/ISRCTN80468181 (first received 12 June 2018).
Roshanravan 2020 {published data only}
- Esfanjani AT, IRCT201212265670N6. Effect of zinc supplementation on insulin resistance, metabolic and inflammatory indices in non diabetic pregnant women with impaired glucose tolerance. en.irct.ir/trial/6190 (first received 10 April 2013).
- Roshanravan N, Alizadeh M, Asghari JM, Alamdari NM, Mohammadi H, Farrin N, et al. Effect of prenatal zinc supplementation on adipose tissue-derived hormones and neonatal weight, height and head circumference in women with impaired glucose tolerance test: randomized clinical controlled trial. International Journal of Diabetes in Developing Countries 2019/07;39(3):471-7. [CENTRAL: CN-01976064] [EMBASE: 2002411021] [Google Scholar]
- Roshanravan N, Tarighat-Esfanjani A, Kheirouri S, Alizadeh M, Alamdari NM, Mousavi R. The effects of zinc supplementation on inflammatory parameters in pregnant women with impaired glucose tolerance: a randomized placebo controlled clinical trial. Progress in Nutrition 2018;20:330-6. [Google Scholar]
Shahnazi 2017 {published data only}
- IRCT201311296709N14. The effect of zinc supplementation on prevention of preterm premature rupture of the membranes in high risk pregnant women: a randomized controlled clinical trial. en.irct.ir/trial/7127 (first received 5 August 2014).
- Shahnazi M, Khalili AF, Azimi S. Effect of zinc supplement on prevention of PPROM and improvement of some pregnancy outcomes in pregnant women with a history of PPROM: a randomized double-blind controlled trial. Iranian Red Crescent Medical Journal 2017;19(3):e41931. [Google Scholar]
Van Vliet 2001 {published data only}
- Van Vliet T, Boelsma E, De Vries AJ, Van den Berg H. Retinoic acid metabolites in plasma are higher after intake of liver paste compared with a vitamin a supplement in women. Journal of Nutrition 2001;131(12):3197-203. [DOI] [PubMed] [Google Scholar]
Villamor 2006 {published data only}
- Villamor E, Aboud S, Koulinska IN, Kupka R, Urassa W, Chaplin B, et al. Zinc supplementation to HIV-1-infected pregnant women: effects on maternal anthropometry, viral load, and early mother-to-child transmission. European Journal of Clinical Nutrition 2006;60(7):862-9. [DOI] [PubMed] [Google Scholar]
Yalda 2010 {published data only}
- Yalda MA, Ibrahiem AA. The effect of combined supplementation of iron and zinc versus iron alone on anemic pregnant patients in Dohuk. Jordan Medical Journal 2010;44(1):9-16. [Google Scholar]
Additional references
Aggett 1991
- Aggett PJ. The assessment of zinc status: a personal view; workshop on assessment of zinc status. Proceedings of the Nutrition Society 1991;50:9-17. [DOI] [PubMed] [Google Scholar]
Apgar 1970
- Apgar J. Effect of zinc deficiency in maintenance of pregnancy in the rat. Journal of Nutrition 1970;100(4):470. [DOI] [PubMed] [Google Scholar]
Cherry 1981
- Cherry FF, Bennett EA, Bazzono GS, Johnson LK, Fosmire GJ, Batson HK. Plasma zinc in hypertension/toxaemia and other reproductive variables in adolescent pregnancy. American Journal of Clinical Nutrition 1981;34(11):194-201. [DOI] [PubMed] [Google Scholar]
Chesters 1982
- Chesters JK. Metabolism and biochemistry of zinc. In: Prasad AS, editors(s). Clinical Biochemistry and Nutritional Aspects of Trace Elements. New York: Alan R Liss, 1982. [Google Scholar]
DiPietro 1996
- DiPietro JA, Hodgson DM, Costigan KA, Johnson TR. Fetal neurobehavioral development. Child Development 1996;67(5):2553-7. [PubMed] [Google Scholar]
Gibson 2006
- Gibson RS. Zinc: the missing link in combating micronutrient malnutrition in developing countries. Proceedings of the Nutrition Society 2006;65:51-60. [DOI] [PubMed] [Google Scholar]
Golub 1995
- Golub MS, Keen CL, Gershwin ME, Hendrickx AG. Developmental zinc deficiency and behaviour. Journal of Nutrition 1995;125(Suppl):2263S-71S. [DOI] [PubMed] [Google Scholar]
GRADE 2014 [Computer program]
- GRADEpro [Computer program on www.gradepro.org]. Version 2014. McMaster University, 2014.
Hepper 1995
- Hepper PG. The behavior of the fetus as an indicator of neural functioning. In: Lecaneut J, Fifer W, Krasnegor N, Smotherman W, editors(s). Fetal Development: a Psychological Perspective. Hillsdale (NJ): Lawrence Erlbaum Associates, 1995:405-17. [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Jameson 1976
- Jameson S. Effect of zinc deficiency in human reproduction. Acta Medica Scandinavica Supplement 1976;593:3. [PubMed] [Google Scholar]
Jameson 1993
- Jameson S. Zinc status in pregnancy: the effect of zinc therapy on perinatal mortality, prematurity and placental ablation. Annals of the New York Academy of Science 1993;678:178-92. [DOI] [PubMed] [Google Scholar]
Jones 1981
- Jones RB, Keeling PW, Hilton PJ, Thompson RP. The relationship between leucocyte and muscle zinc in health and disease. Clinical Sciences 1981;60(2):237-9. [DOI] [PubMed] [Google Scholar]
Keats 2019
- Keats EC, Haider BA, Tam E, Bhutta ZA. Multiple‐micronutrient supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No: CD004905. [DOI: 10.1002/14651858.CD004905.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kiilholma 1984a
- Kiilholma P, Gronroos M, Erkkola P, Pakarinen P, Nanto V. The role of calcium, iron, copper and zinc in preterm delivery and premature rupture of membranes. Gynecologic and Obstetric Investigation 1984;17(4):194-201. [DOI] [PubMed] [Google Scholar]
Kiilholma 1984b
- Kiilholma P, Erkkola P, Pakarinen P, Gronroos M. Trace metals in post date pregnancy. Gynecologic and Obstetric Investigation 1984;18(1):45-6. [DOI] [PubMed] [Google Scholar]
King 2006
- King JC, Cousins RJ. Zinc. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors(s). Modern Nutrition in Health and Disease. 10 edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2006:271-285. [Google Scholar]
Kirksey 1991
- Kirksey A, Rahmanifar A, Wachs TD, McCabe GP, Bessily NS, Bishry Z, et al. Determinants of pregnancy outcome and newborn behaviour in a semirural Egyptian population. American Journal of Clinical Nutrition 1991;54(4):657-67. [DOI] [PubMed] [Google Scholar]
Kirksey 1994
- Kirksey A, Wachs TD, Yunis F, Srinath U, Rahmanifar A, McCabe GP, et al. Relation of maternal zinc nutriture to pregnancy outcome and infant development in an Egyptian village. American Journal of Clinical Nutrition 1994;60(5):782-92. [DOI] [PubMed] [Google Scholar]
Koblinsky 1995
- Koblinsky MA. Beyond maternal mortality - magnitude, interrelationship, and consequences of women's health, pregnancy related complications and nutritional status on pregnancy outcomes. International Journal of Gynecology & Obstetrics 1995;48 Suppl:S21-32. [DOI] [PubMed] [Google Scholar]
Lassi 2016
- Lassi ZS, Moin A, Bhutta ZA. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database of Systematic Reviews 2016, Issue 12. Art. No: CD005978. [DOI: 10.1002/14651858.CD005978.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McKenzie 1975
- McKenzie JM, Forsmire CJ. Zinc deficiency during the latter third of pregnancy: effect on fetal rat brain, liver and placenta. Journal of Nutrition 1975;105(11):1466-73. [DOI] [PubMed] [Google Scholar]
Parr 1996
- Parr RM. Assessment of dietary intakes. In: Trace Elements in Human Nutrition and Health. Geneva: World Health Organization, 1996:265-88. [Google Scholar]
Peña‐Rosas 2015
- Peña-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD004736. [DOI: 10.1002/14651858.CD004736.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prema 1980
- Prema K, Ramalagshmi BA, Neelakumari S. Serum copper and zinc in pregnancy. Indian Medical Research 1980;71:547-53. [PubMed] [Google Scholar]
RevMan 2019 [Computer program]
- Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2019.
Sanstead 1991
- Sandstead HH. Zinc deficiency: a public health problem? American Journal of Diseases of Children 1991;145(8):853-9. [DOI] [PubMed] [Google Scholar]
Sanstead 1996
- Sandstead HH, Smith JC. Deliberations and evaluations of approaches, endpoints and paradigms for determining zinc dietary recommendations. Journal of Nutrition 1996;126:2410S-8S. [DOI] [PubMed] [Google Scholar]
Schünemann 2009
- Schünemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391-400. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shrimpton 2005
- Shrimpton R, Gross R, Darnton-Hill I, Young M. Zinc deficiency: what are the most appropriate interventions? BMJ 2005;330(7487):347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simmer 1985
- Simmer K, Thompson RP. Maternal zinc and intrauterine growth retardation. Clinical Sciences 1985;68(4):395-9. [DOI] [PubMed] [Google Scholar]
Verburg 1974
- Verburg DI, Burd LJ, Hoxtill EO, Merrill LK. Acrodermatitis enteropathica and pregnancy. Obstetrics & Gynecology 1974;44(2):233-7. [PubMed] [Google Scholar]
Villar 2003
- Villar J, Merialdi M, Gulmezoglu AM, Abalos E, Carroli G, Kulier R, et al. Nutritional interventions during pregnancy for the prevention or treatment of maternal morbidity and preterm delivery: an overview of randomized controlled trials. Journal of Nutrition 2003;133:1606S-25S. [DOI] [PubMed] [Google Scholar]
Wessells 2012
- Wessells KR, Brown KH. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PlOS One 2012;7(11):e50568. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1996
- World Health Organization Committee. Zinc. In: Trace Elements in Human Nutrition and Health. Geneva: WHO, 1996:72-104. [Google Scholar]
WHO 2020
- World Health Organization. WHO antenatal care recommendations for a positive pregnancy experience. Nutritional interventions update: Multiple micronutrient supplements during pregnancy_Nutritional interventions update: multiple micronutrient supplements during pregnancy (who.int). Geneva: World Health Organization, 2020. [PubMed] [Google Scholar]
References to other published versions of this review
Mahomed 1995
- Mahomed K. Routine zinc supplementation in pregnancy (revised 28 April 1993). In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM] The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Mahomed 1997
- Mahomed K. Zinc supplementation in pregnancy. Cochrane Database of Systematic Reviews 1997, Issue 3. Art. No: CD000230. [DOI: 10.1002/14651858.CD000230] [DOI] [PubMed] [Google Scholar]
Mahomed 2006
- Mahomed K. Zinc supplementation in pregnancy. Cochrane Database of Systematic Reviews 2006, Issue 3. Art. No: CD000230. [DOI: 10.1002/14651858.CD000230.pub2] [DOI] [PubMed] [Google Scholar]
Mahomed 2007
- Mahomed K, Bhutta ZA, Middleton P. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No: CD000230. [DOI: 10.1002/14651858.CD000230.pub3] [DOI] [PubMed] [Google Scholar]
Mori 2012
- Mori R, Ota E, Middleton P, Tobe-Gai R, Mahomed K, Bhutta ZA. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database of Systematic Reviews 2012, Issue 7. Art. No: CD000230. [DOI: 10.1002/14651858.CD000230.pub4] [DOI] [PubMed] [Google Scholar]
Ota 2015
- Ota E, Mori R, Middleton P, Tobe‐Gai R, Mahomed K, Miyazaki C, et al. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD000230. [DOI: 10.1002/14651858.CD000230.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]


