Abstract
Background
Perineal trauma is common during childbirth and may be painful. Contemporary maternity practice includes offering women numerous forms of pain relief, including the local application of cooling treatments. This Cochrane Review is an update of a review last updated in 2012.
Objectives
To evaluate the effectiveness of localised cooling treatments compared with no treatment, placebo, or other cooling treatments applied to the perineum for pain relief following perineal trauma sustained during childbirth.
Search methods
We searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (7 October 2019) and reference lists of retrieved studies.
Selection criteria
Published and unpublished randomised and quasi‐randomised trials (RCTs) that compared a localised cooling treatment applied to the perineum with no treatment, placebo, or another cooling treatment applied to relieve pain related to perineal trauma sustained during childbirth.
Data collection and analysis
Two review authors independently assessed study eligibility, extracted data and assessed the risk of bias of included studies. Data were double checked for accuracy. The certainty of the evidence was assessed using the GRADE approach.
Main results
We included 10 RCTs that enrolled 1233 women randomised to the use of one cooling treatment (ice, cold gel pad, cooling plus compression, cooling plus compression plus (being) horizontal) compared with another cooling treatment, no treatment, or placebo (water pack, compression). The included trials were at low or uncertain risk of bias overall, with the exception that the inability to blind participants and personnel to group allocation meant that we rated all trials at unclear or high risk for this domain.
We undertook a number of comparisons to evaluate the different treatments.
Cooling treatment (ice pack or cold gel pad) versus no treatment
There was limited very low‐certainty evidence that cooling treatment may reduce women's self‐reported perineal pain within four to six hours (mean difference (MD) −4.46, 95% confidence interval (CI) −5.07 to −3.85 on a 10‐point scale; 1 study, 100 participants) or between 24 and 48 hours of giving birth (risk ratio (RR) 0.73, 95% CI 0.57 to 0.94; 1 study, 316 participants). The evidence is very uncertain about the various measures of wound healing, for example, wound edges gaping when inspected five days after giving birth (RR 2.56, 95% CI 0.58 to 11.33; 1 study, 315 participants). Women generally rated their satisfaction with perineal care similarly following cooling or no treatment. The potential exception was that there may be a trivially lower mean difference of −0.1 on a five‐point scale of psychospiritual comfort with cooling treatment, that is unlikely to be of clinical importance.
Cooling treatment (cold gel pad) + compression versus placebo (gel pad + compression)
There was limited low‐certainty evidence that there may be a trivial MD of −0.43 in pain on a 10‐point scale at 24 to 48 hours after giving birth (95% CI −0.73 to −0.13; 1 study, 250 participants) when a cooling treatment plus compression from a well‐secured perineal pad was compared with the placebo. Levels of perineal oedema may be similar for the two groups (low‐certainty evidence) and perineal bruising was not observed. There was low‐certainty evidence that women may rate their satisfaction as being slightly higher with perineal care in the cold gel pad and compression group (MD 0.88, 95% CI 0.38 to 1.38; 1 trial, 250 participants).
Cooling treatment (ice pack) versus placebo (water pack)
One study reported that no women reported pain after using an ice pack or a water pack when asked within 24 hours of giving birth. There was low‐certainty evidence that oedema may be similar for the two groups when assessed at four to six hours (RR 0.96, 95% CI 0.50 to 1.86; 1 study, 63 participants) or within 24 hours of giving birth (RR 0.36, 95% CI 0.08 to 1.59). No women were observed to have perineal bruising at these times. The trialists reported that no women in either group experienced any adverse effects on wound healing. There was very low‐certainty evidence that women may rate their views and experiences with the treatments similarly (for example, satisfied with treatment: RR 0.91, 95% CI 0.77 to 1.08; 63 participants).
Cooling treatment (ice pack) versus cooling treatment (cold gel pad)
The evidence is very uncertain about the effects of using ice packs or cold gel pads on women’s self‐rated perineal pain, on perineal bruising, or on perineal oedema at four to six hours or within 24 hours of giving birth. Perineal oedema may persist 24 to 48 hours after giving birth in women using the ice packs (RR 1.69, 95% CI 1.03 to 2.7; 2 trials, 264 participants; very low‐certainty). The risk of gaping wound edges five days after giving birth may be decreased in women who had used ice packs (RR 0.22, 95% CI 0.05 to 1.01; 215 participants; very low‐certainty). However, this did not appear to persist to day 10 (RR 3.06, 95% CI 0.63 to 14.81; 214 participants). Women may rate their opinion of treatment less favourably following the use of ice packs five days after giving birth (RR 0.33, 95% CI 0.17 to 0.68; 1 study, 49 participants) and when assessed on day 10 (RR 0.82, 95% CI 0.73 to 0.92; 1 study, 208 participants), both very low‐certainty.
Authors' conclusions
There is limited very low‐certainty evidence that may support the use of cooling treatments, in the form or ice packs or cold gel pads, for the relief of perineal pain in the first two days following childbirth. It is likely that concurrent use of several treatments is required to adequately address this issue, including prescription and non‐prescription analgesia. Studies included in this review involved the use of cooling treatments for 10 to 20 minutes, and although no adverse effects were noted, these findings came from studies of relatively small numbers of women, or were not reported at all. The continued lack of high‐certainty evidence of the benefits of cooling treatments should be viewed with caution, and further well‐designed trials should be conducted.
Plain language summary
Local cooling for relieving pain from perineal trauma sustained during childbirth
We looked for evidence from randomised controlled trials on how effective localised cooling treatments are for reducing pain from damage to the area between the vagina and the anus, that is, 'perineal trauma', when giving birth.
What is the issue?
Perineal tears are common during childbirth. In addition, sometimes the person attending the birth cuts the perineum to give extra room for the baby to be born (an episiotomy).
These tears and cuts often cause pain and the mother may have difficulty walking or sitting comfortably, or to feed and care for her baby,
Why is this important?
The pain from perineal tears or cuts can decrease women’s ability to move around and causes discomfort when passing urine or faeces. This can affect her emotional well‐being. Persisting perineal pain can have longer‐term effects, such as pain during sex and problems with bowel movements and urination. Women are encouraged to use different ways to relieve the pain, including the use of cooling treatments such as ice packs or cold gel pads. It is important to know if cooling works and whether it can slow healing of the cut or tear.
This is an update of a review that was first published in 2007 and updated in 2012.
What evidence did we find?
We updated the search for evidence in October 2019. We have now found 10 randomised controlled trials to include. Nine of these studies had information from 998 women that we could use in the review.
Ice packs or cold gel pads were placed on the perineum for 10 to 20 minutes at a time in the first two days following childbirth. They were compared to no treatment (5 studies, 612 women) or placebo treatment of a gel pad (1 study) or a water bag (1 study), both at room temperature. Ice packs were compared with cold gel pads in three studies (338 women).
The trials were largely of very low quality due to concerns about how valid the findings were, with small numbers of women for each comparison, wide variations in treatment effects, and women knowing which treatment (or if no treatment) they had used. Few trials looked at the same comparisons or trials used different assessment tools or outcomes. Most of the findings come from single studies.
Women's self‐rated perineal pain following the use of the cold pad within six hours of giving birth may be less than for women who had no treatment (1 study, 100 women). There were no clear differences in self‐reported pain within 24 hours or up to 48 hours after giving birth (1 study, 316 women) or in perineal healing.
A cold gel pad with compression in comparison to a placebo may result in a very small reduction in pain 24 to 48 hours after giving birth (1 study, 250 women). Perineal wound healing may not be adversely affected by cooling. None of the women with an ice pack or a water pack at room temperature reported pain in the first 24 hours after giving birth (1 study, 63 women). No adverse effects on wound healing were reported.
Comparing ice packs with cold gel pads, there may be no difference in self‐rated perineal pain at any of the measurement times (3 studies, 338 women). One trial reported that fewer women using ice packs had gaping wound edges at day five but not at day 10 (215 women). In single studies, women rated their opinion of treatment less favourably with ice packs than with cold gel pads five days after giving birth (49 women) and when assessed on day 10 (208 women).
What does this mean?
There is only a small amount of low or very low‐quality evidence from small trials suggesting that cooling treatments may help relieve perineal pain after having a baby. Further research is needed to see if cooling affects how well the tears or cuts heal. Ice is readily available in high‐income countries but this may not be the case in low‐middle income countries. Gel pads that need to be placed in a freezer for cooling may also not be readily available in low‐middle income areas.
Summary of findings
Summary of findings 1. Cooling treatment (ice pack or cold gel pad) compared to no treatment for relieving pain from perineal trauma sustained during childbirth.
| Cooling treatment (ice pack or cold gel pad) compared to no treatment for relieving pain from perineal trauma sustained during childbirth | ||||||
| Patient or population: Women with perineal trauma (tear or episiotomy, or both) sustained during childbirth Setting: Brazil, Iran, Turkey, United Kingdom Intervention: cooling treatment (ice pack or cold gel pad) Comparison: no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with cooling treatment (ice pack or cold gel pad) | |||||
| Perineal pain within 4 ‐ 6 hours of giving birth | The mean perineal pain within 4 ‐ 6 hours of giving birth was 6.42 | MD 4.46 lower (5.07 lower to 3.85 lower) | ‐ | 100 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | Scale of 0 (no pain) to 10 (worst possible pain) |
| Perineal pain within 24 hours of giving birth ‐ Moderate + severe pain | Study population | RR 1.03 (0.82 to 1.29) | 316 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,d | ‐ | |
| 515 per 1000 | 530 per 1000 (422 to 664) | |||||
| Perineal pain within 24 hours of giving birth | The mean perineal pain within 24 hours of giving birth was 4.05 | MD 0.41 lower (1.78 lower to 0.95 higher) | ‐ | 166 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa,e,f,g | Scale of 0 (no pain) to 10 (worst possible pain) |
| Perineal pain between 24 ‐ 48 after giving birth ‐ Moderate + severe pain | Study population | RR 0.73 (0.57 to 0.94) | 316 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,d | ‐ | |
| 535 per 1000 | 390 per 1000 (305 to 503) | |||||
| Perineal pain 24 ‐ 48 hours after giving birth | The mean perineal pain between 24 ‐ 48 hours after giving birth was 4.36 | MD 0.53 lower (1.45 lower to 0.39 higher) | ‐ | 71 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,f,g | Scale of 0 (no pain) to 10 (worst possible pain) |
| Perineal oedema within 24 hours of giving birth | Study population | RR 1.00 (0.87 to 1.16) | 316 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,d,g | ‐ | |
| 723 per 1000 | 723 per 1000 (629 to 838) | |||||
| Perineal bruising within 24 hours of giving birth | Study population | RR 0.98 (0.81 to 1.19) | 316 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,d,g | ‐ | |
| 604 per 1000 | 592 per 1000 (489 to 719) | |||||
| Perineal bruising 24 ‐ 48 hours after giving birth | Study population | RR 1.13 (0.97 to 1.32) | 316 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,d,g | ‐ | |
| 673 per 1000 | 761 per 1000 (653 to 889) | |||||
| Perineal redness, oedema, bruising, discharge, wound gaping 24 ‐ 48 hours of giving birth | The mean perineal redness, oedema, bruising, discharge, wound gaping between 24‐48 hours of giving birth was 2.89 | MD 1.19 lower (2.07 lower to 0.31 lower) | ‐ | 71 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,e,h,i | The REEDA scale provides a rating of Redness, Edema, Ecchymosis (bruising), Discharge & Approximation (of skin edges): range 0 to 15, with higher scores representing increased tissue trauma |
| Adverse effects on perineal wound healing: Day 5 ‐ Wound not healing | Study population | RR 1.24 (0.34 to 4.58) | 315 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,d | ‐ | |
| 30 per 1000 | 37 per 1000 (10 to 137) | |||||
| Adverse effects on perineal wound healing: Day 5 ‐ Wound edges gaping | Study population | RR 2.56 (0.58 to 11.33) | 315 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,d | ‐ | |
| 20 per 1000 | 51 per 1000 (12 to 227) | |||||
| Maternal views and experiences of treatment ‐ Satisfaction with overall perineal care (good + very good + excellent) Day 10 | Study population | RR 1.07 (0.97 to 1.18) | 308 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,d | ‐ | |
| 840 per 1000 | 899 per 1000 (815 to 991) | |||||
| Maternal views and experiences of treatment ‐ Psychospiritual comfort within 6 hours of giving birth | The mean maternal views and experiences of treatment ‐ Psychospiritual comfort within 6 hours of giving birth was 1.97 | MD 0.1 lower (0.2 lower to 0.0) | ‐ | 100 (1 RCT) | ⊕⊕⊝⊝ LOWa,j | Scale of 1 (low comfort) to 5 (high comfort) |
| Maternal views and experiences of treatment ‐ Sociocultural comfort | The mean maternal views and experiences of treatment ‐ Sociocultural comfort was 2.94 | MD 0.01 higher (0.09 lower to 0.11 higher) | ‐ | 100 (1 RCT) | ⊕⊕⊝⊝ LOWa,j | Scale of 1 (low comfort) to 5 (high comfort) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level for unblinding of outcome assessors. bDowngraded one level due to uncertainty about selection bias. cDowngraded one level for imprecision: unequal variance intervention vs no intervention. dDowngraded two levels for very serious limitations due to incomplete outcome data. eDowngraded one level for risk of selection bias. fDowngraded one level for risk of selective reporting. gDowngraded one level due for imprecision: confidence intervals crossing line of no effect. hDowngraded one level for possible unblinding of outcome assessors. iDowngraded one level for imprecision (wide confidence intervals). jDowngraded one level for imprecision: trivial difference (less than one point on a 5‐point scale).
Summary of findings 2. Cooling treatment (cold gel pad)+compression compared to placebo (gel pad+compression) for relieving pain from perineal trauma sustained during childbirth.
| Cooling treatment (cold gel pad)+compression compared to placebo (gel pad+compression) for relieving pain from perineal trauma sustained during childbirth | ||||||
| Patient or population: Women with perineal trauma (tear or episiotomy, or both) sustained during childbirth Setting: Thailand Intervention: cooling treatment (cold gel pad)+compression Comparison: placebo (gel pad+compression) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo (gel pad+compression) | Risk with cooling treatment (cold gel pad)+compression | |||||
| Perineal pain within 4 ‐ 6 hours of giving birth | The mean perineal pain within 4 ‐ 6 hours of giving birth was 3.15 | MD 0.32 lower (0.78 lower to 0.14 higher) | ‐ | 250 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | A scale of zero (no pain) to 10 (worst possible pain) |
| Perineal pain within 24 ‐ 48 hours after giving birth | The mean perineal pain within 24 ‐ 48 hours after giving birth was 2.48 | MD 0.43 lower (0.73 lower to 0.13 lower) | ‐ | 250 (1 RCT) | ⊕⊕⊝⊝ LOWc | A scale of zero (no pain) to 10 (worst possible pain) |
| Perineal oedema 24 ‐ 48 hours after giving birth | The mean perineal oedema 24 ‐ 48 hours after giving birth was 0.176 | MD 0.15 lower (0.28 lower to 0.03 lower) | ‐ | 250 (1 RCT) | ⊕⊕⊝⊝ LOWa,d | Scale zero: none ‐ 3: > 2 cm |
| Perineal bruising 24 ‐ 48 hours after giving birth | ‐ | 250 (1 RCT) | ‐ | No women were observed to have perineal bruising | ||
| Adverse effects | ‐ | (0 studies) | ‐ | No trials reported on adverse effects | ||
| Satisfaction with perineal care | The mean satisfaction with perineal care was 6.87 | MD 0.88 higher (0.38 higher to 1.38 higher) | ‐ | 250 (1 RCT) | ⊕⊕⊝⊝ LOWa,c | Scale of zero (not satisfied) to 10 (most satisfied) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level for unblinding of outcome assessors. bDowngraded one level due to imprecision: wide confidence intervals crossing the line of no effect. cDowngraded one level for imprecision: trivial difference on a 10‐point scale. dDowngraded one level for imprecision: trivial difference on a 3‐point scale.
Summary of findings 3. Cooling treatment (ice pack) compared to placebo (water pack) for relieving pain from perineal trauma sustained during childbirth.
| Cooling treatment (ice pack) compared to placebo (water pack) for relieving pain from perineal trauma sustained during childbirth | ||||||
| Patient or population: Women with perineal trauma (tear or episiotomy, or both) sustained during childbirth Setting: Brazil Intervention: cooling treatment (ice pack) Comparison: placebo (water pack) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo (water pack) | Risk with cooling treatment (ice pack) | |||||
| Perineal pain within 24 hours after giving birth | Study population | ‐ | 63 (1 RCT) | ‐ | No participants reported perineal pain within this time frame | |
| see comment | see comment | |||||
| Perineal oedema within 4 ‐ 6 hours after giving birth | Study population | RR 0.96 (0.50 to 1.86) | 63 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | ‐ | |
| 371 per 1000 | 357 per 1000 (186 to 691) | |||||
| Perineal oedema within 24 hours after giving birth | Study population | RR 0.36 (0.08 to 1.59) | 63 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | ‐ | |
| 200 per 1000 | 72 per 1000 (16 to 318) | |||||
| Adverse effects on perineal healing | Study population | ‐ | 63 (1 RCT) | ‐ | No adverse events were reported by participants or observed by outcome assessors | |
| see comment | see comment | |||||
| Maternal views and experiences with treatment ‐ Satisfied with treatment | Study population | RR 0.91 (0.77 to 1.08) | 63 (1 RCT) | ⊕⊝⊝⊝ VERY LOWb,c,d | ‐ | |
| 943 per 1000 | 858 per 1000 (726 to 1000) | |||||
| Maternal views and experiences with treatment ‐ Would repeat treatment in future childbirth | Study population | RR 0.88 (0.75 to 1.04) | 63 (1 RCT) | ⊕⊝⊝⊝ VERY LOWb,c,d | ‐ | |
| 971 per 1000 | 855 per 1000 (729 to 1000) | |||||
| Maternal views and experiences with treatment ‐ Would recommend treatment | Study population | RR 0.89 (0.77 to 1.03) | 63 (1 RCT) | ⊕⊝⊝⊝ VERY LOWb,c,d | ‐ | |
| 1000 per 1000 | 890 per 1000 (770 to 1000) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level for imprecision: wide confidence intervals crossing the line of no effect. bDowngraded one level for small sample size. cDowngraded one level due to unblinding of outcome assessor. dDowngraded one level for imprecision: confidence intervals crossing the line of no effect.
Summary of findings 4. Cooling treatment (ice pack) compared to cooling treatment (cold gel pad) for relieving pain from perineal trauma sustained during childbirth.
| Cooling treatment (ice pack) compared to cooling treatment (cold gel pad) for relieving pain from perineal trauma sustained during childbirth | ||||||
| Patient or population: Women with perineal trauma (tear or episiotomy, or both) sustained during childbirth Setting: Iran, United Kingdom Intervention: cooling treatment (ice pack) Comparison: cooling treatment (cold gel pad) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with cooling treatment (cold gel pad) | Risk with cooling treatment (ice pack) | |||||
| Perineal pain within 4 ‐ 6 hours of giving birth ‐ Moderate + severe pain | Study population | RR 0.57 (0.26 to 1.24) | 49 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c,d | ‐ | |
| 481 per 1000 | 274 per 1000 (125 to 597) | |||||
| Perineal pain within 24 hours of giving birth ‐ Moderate + severe pain | Study population | RR 0.98 (0.78 to 1.22) | 264 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,e | ‐ | |
| 541 per 1000 | 530 per 1000 (422 to 660) | |||||
| Perineal pain within 24 hours of giving birth | The mean perineal pain within 24 hours of giving birth was 3.84 | MD 0.58 higher (0.44 lower to 1.6 higher) | ‐ | 74 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,d,f,g | Scale of 0 (no pain) to 10 (worst possible pain) |
| Perineal pain 24 ‐ 48 hours after giving birth ‐ Moderate + severe pain | Study population | RR 1.21 (0.89 to 1.65) | 263 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | ‐ | |
| 343 per 1000 | 415 per 1000 (306 to 566) | |||||
| Perineal pain 24 ‐ 48 hours of giving birth | The mean perineal pain between 24‐48 hours of giving birth was 2.97 | MD 0.86 higher (0.1 lower to 1.82 higher) | ‐ | 74 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,c,d,f | Scale of 0 (no pain) to 10 (worst possible pain) |
| Perineal oedema within 4 ‐ 6 hours of giving birth | Study population | RR 1.39 (0.93 to 2.09) | 49 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c,d | ‐ | |
| 556 per 1000 | 772 per 1000 (517 to 1000) | |||||
| Perineal oedema within 24 hours of giving birth | Study population | RR 0.97 (0.84 to 1.13) | 264 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,e | ‐ | |
| 733 per 1000 | 711 per 1000 (616 to 829) | |||||
| Perineal oedema 24 ‐ 48 hours after giving birth | Study population | RR 1.69 (1.03 to 2.77) | 264 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,h | ‐ | |
| 393 per 1000 | 663 per 1000 (404 to 1000) | |||||
| Perineal bruising within 4 ‐ 6 hours of giving birth | Study population | RR 1.23 (0.51 to 2.97) | 49 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,d,h | ‐ | |
| 259 per 1000 | 319 per 1000 (132 to 770) | |||||
| Perineal bruising within 24 hours of giving birth | Study population | RR 0.95 (0.79 to 1.14) | 264 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 5 | ‐ | |
| 644 per 1000 | 612 per 1000 (509 to 735) | |||||
| Perineal redness, oedema, bruising, discharge, wound gaping within 24 hours of giving birth | The mean perineal redness, oedema, bruising, discharge, wound gaping within 24 hours of giving birth was 2.3 | MD 0.13 lower (0.85 lower to 0.59 higher) | ‐ | 74 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,d,e | The REEDA scale assesses 5 aspects of wound healing: redness, (o)edema, ecchymosis (bruising), discharge and (wound edge) approximation on a scale of 0 (best) to 3 (worst) for each component for a maximum score of 15 |
| Adverse effects on perineal wound healing: Day 5 ‐ Wound not healing | Study population | RR 1.01 (0.26 to 3.93) | 215 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | ‐ | |
| 37 per 1000 | 37 per 1000 (10 to 146) | |||||
| Adverse effects on perineal wound healing: Day 5 ‐ Wound edges gaping | Study population | RR 0.22 (0.05 to 1.01) | 215 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,h | ‐ | |
| 83 per 1000 | 18 per 1000 (4 to 84) | |||||
| Maternal views and experiences with treatment ‐ Opinion on treatment effects (good + very good + excellent) Day 5 | Study population | RR 0.33 (0.17 to 0.68) | 49 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,d | ‐ | |
| 815 per 1000 | 269 per 1000 (139 to 554) | |||||
| Maternal views and experiences with treatment ‐ Satisfaction with overall perineal care (good + very good + excellent) Day 10 | Study population | RR 0.82 (0.73 to 0.92) | 208 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b | ‐ | |
| 934 per 1000 | 766 per 1000 (682 to 859) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level for unblinding of outcome assessors. bDowngraded two levels for very serious limitations due to incomplete outcome data. cDowngraded one level due to imprecision: wide confidence intervals crossing the line of no effect. dDowngraded one level for small sample size. eDowngraded one level due for imprecision: confidence intervals crossing line of no effect. fDowngraded one level for risk of selective reporting. gDowngraded one level for imprecision: trivial difference on a 10‐point scale. hDowngraded one level for imprecision (wide confidence intervals).
Background
Description of the condition
Perineal trauma: effects and prevention
Perineal trauma, whether by episiotomy (cutting of the perineum to enlarge the vaginal orifice during the end of the second stage of birthing) or from naturally‐occurring tears, is common during childbirth. In Australia in 2018, 55% of women sustained tears and 23% had an episiotomy (AIHW 2020). In the United Kingdom, 22% of women having a vaginal birth undergo episiotomy and 3.5% sustain third‐ or fourth‐degree tears (NMPA 2019), while average pooled estimated episiotomy rates in low‐middle income countries are 46% (95% confidence interval 35% to 55%), ranging from 10% in Cameroon to 98% in Pakistan (Aguiar 2019). The combination of spontaneous tears and episiotomy therefore encompasses a large proportion of women who sustain perineal trauma with a vaginal birth (Abedzadeh‐Kalahroudi 2019). Further sources of trauma include vaginal lacerations and trauma to the external genitalia (labia, clitoris, periurethra) (Wen 2018).
In the hours, days and months following childbirth, this trauma may be painful (East 2011; Francisco 2011; Manresa 2019) and can be increased in women with deeper trauma (> 2 cm) (Lawrence 2017). This pain can result in decreased mobility and increased discomfort with passing urine or faeces (Subki 2019), may negatively impact on the woman's ability to care for her new baby (East 2011) and affect emotional well‐being (Shoorab 2019). Perineal pain that persists beyond the immediate postpartum period may warrant further evaluation and may have longer‐term effects, such as sexual and pelvic dysfunction (Doğan 2017; Manresa 2019). A number of risk factors for severe perineal trauma have been identified, with some debate remaining. These generally include primiparity, ethnicity, with women from Southern Asia and Africa being at greater risk than others, instrumental birth, and prolonged length of second stage (for example, over two hours) (Davies‐Tuck 2015; Gibson‐Helm 2015).
A number of techniques may be applied for the prevention or minimisation of perineal trauma (Aasheim 2017), which may subsequently reduce the perineal pain associated with childbirth. Possible preventive or minimisation measures include perineal massage during pregnancy, application of warm packs to the perineum during second stage, mediolateral versus midline episiotomy, and birthing attendants' hands on the perineum during the birth of the baby's head, to name a few (Aasheim 2017). Selective use of episiotomy, for example, in situations where severe perineal trauma would otherwise occur, or for fetal indications, results in less severe perineal trauma (Jiang 2017). It remains unclear whether or not women's self‐reported perineal pain is influenced by the routine or selected use of episiotomy (Jiang 2017).
Analgesia for perineal trauma
When perineal trauma does occur, regardless of the underlying contributing factors or interventions, pain, where present, requires attention. Contemporary maternity practice includes offering the woman numerous forms of pain relief, often used in combination. Several Cochrane Reviews have considered the evidence of the effectiveness of treatments used in recent decades. These include: methods and materials used for suturing perineal tears or episiotomies (Kettle 2010; Kettle 2012); topically‐applied anaesthetics (for example, lignocaine) and a topical preparation of pramoxine/hydrocortisone (Hedayati 2005); orally or rectally administered non‐steroidal anti‐inflammatory drugs (NSAIDS) (Hedayati 2003; Wuytack 2016); ultrasound (Hay‐Smith 1998); and other oral medications such as aspirin (Molakatalla 2017) or paracetamol/acetaminophen (Chou 2013). While these treatments demonstrate varying levels of success in relieving pain from perineal trauma, they may also involve a degree of cost to the consumer, the health service, or both. Paracetamol and NSAIDS are considered safe in breastfeeding (Bisson 2019), while potentially harmful side effects of these and other medications also need to be considered. Women's satisfaction is also an important consideration of any treatment used for reducing perineal pain, although this is poorly studied (Molakatalla 2017; Wuytack 2016).
A safe, effective, low‐cost alternative, available in primary healthcare settings as well as in hospitals, and that is acceptable to childbearing women, would be attractive. The use of cryotherapy (cooling treatment) in the form of ice packs or cold gel pads, may be one such alternative.
Description of the intervention
Cooling therapy for pain relief
Cooling to reduce inflammation and provide short‐term relief of pain from localised tissue trauma has been used for over four decades (Ernst 1994; McMasters 1977), particularly for the treatment of delayed‐onset muscle soreness (DOMS), sports trauma and following surgery (Adie 2010; Martimbianco 2014; Nogueira 2019; Oakley 2013; Torres 2012; Van den Bekerom 2012). Such cryotherapy may be in the form of local application of crushed ice or frozen gel, through to whole‐body cryotherapy (WBC) or cold‐water immersion (CWI) (Bleakley 2010). A common approach, attributed to Mirkin 1978, is to incorporate cooling within a ‘bundle’ of care, typically referred to as “RICE”, involving Rest, local application of Ice, along with Compression and Elevation of the region.
Cooling treatments for perineal pain relief following childbirth have traditionally been applied intermittently in a number of ways, including: (i) solid or crushed ice applied directly to the perineum or between layers of a pad (Grant 1989; Leventhal 2010); (ii) a cold gel pack applied to the perineum (Leventhal 2010; Steen 1999); or (iii) bathing in iced water (Grant 1989).
How the intervention might work
The potential effects of cooling on wound healing
Bonica’s theory (Bonica 1990) explains the physiology of local tissue injury and the potential effect of cold therapy. The accumulation of fluid (swelling/oedema) in an inflamed, injured area occurs due to increased permeability of the dilated peripheral blood vessels. When cold is applied, the skin blood supply is reduced. In turn, reduced tissue swelling and bleeding may also reduce bruising and localised pain. The effectiveness of this theory is supported by a systematic review showing that cryotherapy substantially reduces oedema following acute trauma (Collins 2008).
Pain signalling, inflammation and vascular changes are influenced by several biochemical mediators. These include serotonin, histamine and kinins. Serotonin dilates capillaries, increases vascular permeability and contracts non‐vascular smooth muscle. Actions of histamine include increased capillary permeability, arteriolar dilation and contraction of non‐vascular smooth muscle, while kinins increase vascular permeability and vasodilation. Any mechanism that reduces these vascular responses will also reduce the effect of the mediator(s) (Dray 1995). Reducing soft tissue temperature by 10 to 15 degrees Celsius decreases local cell metabolism, reduces the oxygen requirement of the tissue and causes constriction of the peripheral blood vessels (Mac Auley 2001). Heat‐activated receptors are thought to play a significant role in inflammation‐related pain (Kettle 2012; Reid 2005). White 2013 concluded that metabolic rate and blood flow is reliably affected by tissue cooling. The use of a cooling treatment for between five and 15 minutes lowers the skin temperature and reduces swelling, thus providing analgesia (Bleakley 2012; Kuo 2013; Leventhal 2010; Machado 2016; Malanga 2015; White 2013).
White 2013 also states that although metabolic rate and blood flow seem to be reliably affected by cold, further studies are needed to investigate a dose‐dependence.
Can cooling delay wound healing or pose safety concerns?
Concern about the safety and potential harm of ice has long been considered. This debate has arisen again, triggered in part by Mirkin 2015 using his online blog to debunk his original design of the clinical tool of RICE, published in his seminal textbook (Mirkin 1978). Dubois 2020 recently created a new approach for the management of soft tissue injury which suggests avoiding the use of ice therapy and anti‐inflammatory medication due to the disruption in the inflammatory process and the potential negative effect on soft tissue repair. Inflammation and muscle regeneration are closely interconnected through complex interactions which in acute conditions are beneficial to muscle healing (Dushesne 2017). The theory may or may not translate to actual harms. An experimental randomised controlled trial showed that although cryotherapy effectively reduced inflammation mechanisms it did not negatively alter regeneration markers or collagen deposition (Ramos 2016), while in humans, icing was found to disrupt inflammation and some aspects of revascularisation in acutely‐injured skeletal muscle but showed no substantial negative effect on muscle growth (Singh 2017). There appears to be no reported ‘harm’ from cooling to muscle function, but it was shown that it does not accelerate muscle recovery following exercise‐induced muscle damage (Engelhard 2019).
Other potential risks of ice therapies include nerve palsies and ice burns. Beyond the desired localised/short‐term numbing effect, true nerve palsies are more likely to occur at bony joints where there are exposed nerve areas (knee/elbow) or after prolonged, extreme temperature cooling as seen with WBC and CWI (Lubkowska 2012; Swenson 1996). Dosage recommendations for local ice therapy were originally described, limiting exposure to 10‐minute sessions intermittently, to avoid skin damage (Mac Auley 2001). This along with other application care relevant to the injured area will further eliminate the risk of ice burns.
Why it is important to do this review
Cooling to relieve pain following childbirth‐related perineal trauma, although lacking in supporting evidence, has been and continues to be commonly used in clinical practice for over 30 years (NICE 2006; Queensland Clinical Guidelines 2018; Sleep 1988). The ongoing conflicting evidence on the efficacy of cooling treatments is complicated further by the emerging debate on potential harm such therapies may cause when used to treat sports injuries or surgical wounds, and needs to be considered for the perineal region as related to childbirth. The acceptability to women of any cooling therapy needs to be evaluated. This systematic review will provide childbearing women and their caregivers with updated evidence to inform clinical practice. This Cochrane Review is a further update of a review first published in 2007 and updated in 2012.
Objectives
To evaluate the effectiveness of localised cooling treatments compared with no treatment, placebo, or other cooling treatments applied to the perineum for pain relief following perineal trauma sustained during childbirth.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished randomised and quasi‐randomised trials that compared localised cooling treatment(s) applied to the perineum with no treatment, placebo treatment, or another cooling treatment applied to the perineum to relieve pain related to perineal trauma sustained during childbirth. We excluded cross‐over studies. We were able to confirm that all of the reports that were available in abstract form were also linked with later‐published full trial reports. If we identify any other abstracts in future updates, we will include them if they contain sufficient information to assess study eligibility and risk of bias, and if the authors confirm in writing that they reported final results that would not change in subsequent publications.
Types of participants
Women with perineal trauma (tear or episiotomy, or both) sustained during childbirth.
Types of interventions
Application of localised cooling treatment to the perineum, versus no treatment, placebo treatment or another form of cooling treatment.
Types of outcome measures
We are aware of two publications: (i) a generic protocol for the systematic review of drugs used to relieve perineal pain in the early postpartum period (Chou 2009); and (ii) the WHO postnatal care guideline summary (personal communication, L Jones 11 December 2019), that have highlighted the outcomes to be considered when consolidating guidance for safe and effective interventions during the postnatal care of mothers and babies. We have revised the outcomes in this update to align with those in these two publications where possible (see Differences between protocol and review).
Primary outcomes
(1) Perineal pain, as measured by the trial authors, for example, using a visual analogue scale, at the following time periods (or as close to the time period as possible):
within four to six hours of giving birth;
within 24 hours of giving birth;
between 24 and 48 hours of giving birth.
Secondary outcomes
(2) Perineal pain, as measured by the trial authors, for example, using a visual analogue scale, associated with activities of daily living (for example, sitting, walking, urinating, caring for baby) at the following time periods (or as close to the time period as possible):
within four to six hours of giving birth;
within 24 hours of giving birth;
between 24 and 48 hours of giving birth.
(3) Perineal oedema, as measured by the study authors, at the following time periods (or as close to the time period as possible):
within four to six hours of giving birth;
within 24 hours of giving birth;
between 24 and 48 hours of giving birth.
(4) Perineal bruising, as measured by the study authors, at the following time periods (or as close to the time period as possible):
within four to six hours of giving birth;
within 24 hours of giving birth;
between 24 and 48 hours of giving birth.
(5) Additional analgesia for relief of perineal pain:
within 24 hours of giving birth;
between 24 and 48 hours of giving birth.
(6) Adverse effects on perineal healing, such as infection or wound breakdown, as measured by the study authors.
(7) Maternal exhaustion, as measured by the study authors, at the following time periods (or as close to the time period as possible):
within four to six hours of giving birth;
within 24 hours of giving birth;
between 24 and 48 hours of giving birth.
(8) Maternal views and experiences with treatment, using a validated instrument or as otherwise measured by the study authors.
(9) Women providing any breast milk to the baby 24 to 48 hours after giving birth (via the breast or as expressed breast milk).
(10) Cost of treatment.
Search methods for identification of studies
The following methods section is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (7 October 2019).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections Included studies; Excluded studies; Studies awaiting classification;.
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (7 October 2019), using the search methods detailed in Appendix 1.
Searching other resources
We sought ongoing and unpublished trials by contacting experts in the field.
We searched the reference lists of retrieved studies.
We did not apply any language restrictions.
Data collection and analysis
For methods used in the previous version of this review, see East 2012.
For this update, we used the following methods for assessing the reports that we identified as a result of the updated search.
The following methods section is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Original review and 2012 update: two review authors (Christine East (CE), Naomi Henshall (NH), or Karen Wallace (KW)) independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We planned to resolve any disagreement through discussion or, if required, we would consult a third person (NH, KW, Lisa Begg or Paul Marchant).
2020 update: CE, Jiajia (Jessie) Liu (JL) and Emma Dorward (ED) assessed the previously‐included studies for the updated criteria and the newly‐identified studies for inclusion. We planned to resolve any disagreement through discussion or, if required, we would consult a third person (Rhiannon Whale (RW)).
Data extraction and management
We designed a form to extract data. For eligible studies, at least two review authors (2007 and 2012: CE, NH, KW, LB, PM, or JL; 2020: CE, JL, ED) extracted the data using the agreed form. We planned to resolve discrepancies through discussion or, if required, we would consult a third person from within the authorship. We double‐checked a sub‐sample of these data against the Review Manager 5 software (RevMan 2014).
When information on any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (2007 and 2012: CE, NH, KW, LB, PM, or JL; 2020: CE, JL, ED) independently assessed risks of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020a). We planned to resolve any disagreement by discussion or by involving a third assessor from within the authorship.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random‐number table; computer random‐number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
We assess the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively‐numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
We planned to consider excluding studies with over 20% missing data unless there were good reasons to include them, based on other assessments of their risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review had been reported);
high risk of bias (where not all the study’s prespecified outcomes had been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020a). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as a summary risk ratio with a 95% confidence interval.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measure the same outcome, but used different scales.
We analysed data on all participants with available data in the group to which they were allocated, regardless of whether or not they received the allocated intervention. If in the original reports participants were not analysed in the group to which they were randomised, and there was sufficient information in the trial report, we planned to attempt to restore them to the group to which they had been randomised.
The trials reported assessments of pain, oedema and bruising at different time periods and by different criteria, necessitating the use of judgement by the review authors when selecting which assessment would most closely represent our stated outcome measures. We selected the assessment closest to the upper end of the timeframe specified in our outcomes. Where pain was reported as "any" or by degrees, we selected a total of the ratings, for example, moderate, severe and unbearable.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually‐randomised trials. If we identify suitable trials in future updates, we will adjust their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020b) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Other unit‐of‐analysis issues
For this updated review, we adopted the approach recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020b) to overcome a unit‐of‐analysis error for studies that could contribute multiple, correlated comparisons. Where data were reported in three groups (intervention one, intervention two, no treatment) we combined data for the intervention groups (cold gel pads and ice packs) where this was possible with the available data (Steen 2000; Steen 2002) for the comparison with no treatment. The continuous data reported by Navvabi 2009 did not allow for this: given that in previous versions of this review, we had not identified evidence of differences in outcomes when comparing one or other of these treatments (East 2012), we elected to report only the cold gel pad data and compared this with no treatment.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were to be analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau2, I2 and Chi2 statistics. We regarded heterogeneity as substantial if I2 was greater than 30% and either Tau2 was greater than zero, or there was a low P value (less than 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
If there are 10 or more studies in a future meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it. Such analyses may include inflated measures of effect related to poor methodological quality or small sample size (Higgins 2020a).
Data synthesis
We carried out statistical analysis using the Review Manager 5 software (RevMan 2014). We used a fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials examined the same intervention, and we judged the trials’ populations and methods to be sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if we detected substantial statistical heterogeneity, we used a random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
Where we used random‐effects analyses, we present the results as the average treatment effect with 95% confidence intervals, and the estimates of Tau2 and I2.
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it. We considered whether there was clinical heterogeneity and if so, how this aligned with the PICO characteristics.
For fixed‐effect inverse variance meta‐analyses, we planned to assess differences between subgroups by interaction tests. For random‐effects and fixed‐effect meta‐analyses using methods other than inverse variance, we assessed differences between subgroups by inspection of the subgroups’ confidence intervals; non‐overlapping confidence intervals indicate a statistically significant difference in treatment effect between the subgroups.
We carried out the following subgroup analyses on the primary outcome for the one trial for which these data were available (Steen 2002):
parity (primiparity, multiparity);
mode of birth (spontaneous vaginal birth, assisted vaginal birth (forceps, vacuum))
Sensitivity analysis
We carried out sensitivity analysis to explore the potential effect of risks of bias for the very few outcomes that had more than one study contributing data, as had been undertaken in earlier versions of this review. In line with contemporary methodology, we will only undertake sensitivity analyses for primary outcomes if trials that report these are included in a future update and require such analysis.
Summary of findings and assessment of the certainty of the evidence
For this update we assessed the certainty of the evidence using the GRADE approach, as outlined in the GRADE handbook, in order to assess the certainty of the body of evidence relating to the following outcomes for the main comparisons of:
Cooling treatment (ice pack or cold gel pad) versus no treatment;
Cooling treatment (cold gel pad) + compression versus placebo (gel pad + compression);
Cooling treatment (ice pack) versus placebo (water pack);
Cooling treatment (ice pack) versus cooling treatment (cold gel pad).
for the following outcomes:
Perineal pain within four to six hours of giving birth;
Perineal pain within 24 hours of giving birth;
Perineal pain between 24 to 48 hours after giving birth;
Perineal oedema within 24 hours of giving birth;
Perineal bruising within 24 hours of giving birth;
Adverse effects on perineal healing;
Maternal views and experiences with treatment.
We did not generate 'Summary of findings' tables for the subgroups, given that only one trial contributed to these.
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5 (RevMan 2014) in order to create ’Summary of findings’ tables. We produced a summary of the intervention effect and a measure of certainty for each of the above outcomes, using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
Results of the search
See: Figure 1.
1.
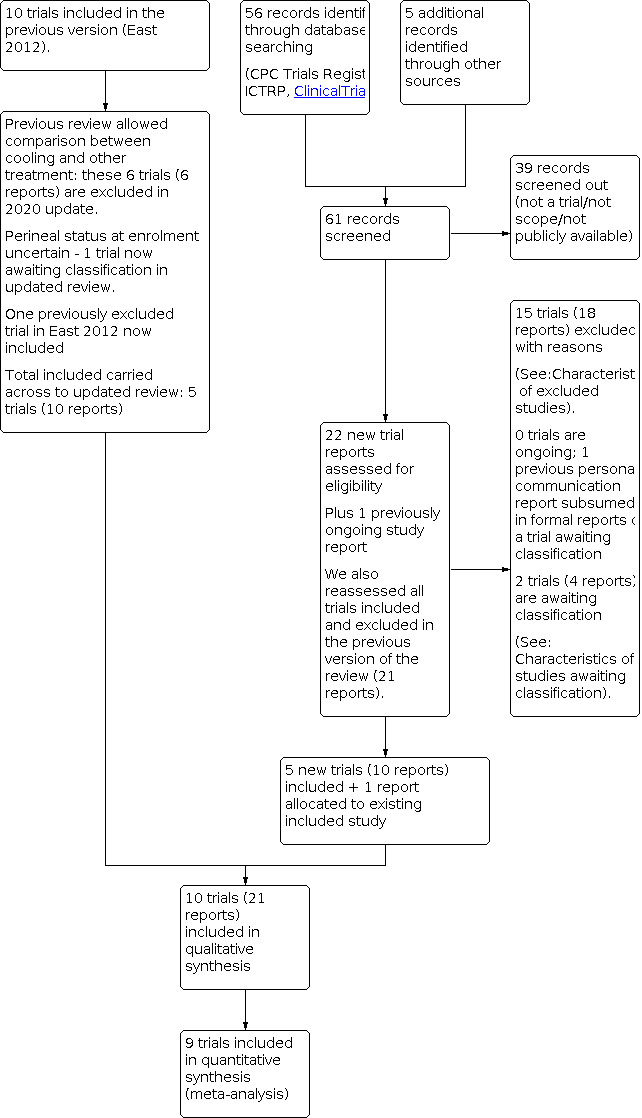
ReStudy flow diagram.
The searches for this update yielded 63 records. After screening out 41 records, there were 22 new reports. We also reassessed the 22 trial reports from the previous version of the review to meet eligibility for the fewer objectives in this update; recognition that a previously‐included study enrolled women without perineal trauma; and that a study previously excluded on the grounds of quality concerns was eligible for inclusion.
We included 10 trials (21 reports) and excluded 15 trials (18 reports). There are two trials awaiting classification (four reports), as the authors have not been able to provide detail to allow for analysis of only those women with perineal trauma (see Characteristics of studies awaiting classification). The one ongoing study in the previous version of the review was a personal communication about the trial that is now awaiting classification. We did not identify any new ongoing studies.
Included studies
We included 10 studies (Barclay 1983; Beleza 2017; East 2007; Francisco 2018; Morais 2016; Navvabi 2009; Senol 2017; Steen 2000; Steen 2002; Yusamran 2007) that enrolled 1233 women randomised to the use of one cooling treatment (ice, cold gel pad, cooling plus compression, cooling plus compression plus (being) horizontal) compared with another cooling treatment, with no treatment, or with placebo (water pack, compression). The report by Barclay 1983 did not present any outcome data in a format that could be included in the review, while others had substantial rates of attrition (see below), giving a total of 998 women contributing data to the primary outcome of perineal pain and to various of the secondary outcomes. Few trials used the same comparisons, assessment tools or outcomes. We therefore conducted five comparisons, each with between one and three trials contributing data to the continuous or dichotomous outcomes (see below). We used descriptive categories within the analyses to provide adequate detail for the reader. See Characteristics of included studies.
Design
The included studies were all randomised controlled trials.
Sample sizes
One study was a pilot to test feasibility for a larger planned trial and enrolled 16 women (East 2007). The remaining studies enrolled between 20 and 50 per treatment group (Barclay 1983; Beleza 2017; Francisco 2018; Morais 2016; Navvabi 2009; Senol 2017; Steen 2000), while two studies enrolled 125 or 150 women to each group (Steen 2002; Yusamran 2007 respectively). Of note, two studies (Steen 2000; Steen 2002) suffered considerable attrition with non‐return of completed paperwork by the clinicians (see Incomplete outcome data (attrition bias).
Setting
Studies were conducted in the birthing centres, or birthing suites or postnatal wards of hospitals, and continued to community care in Australia (Barclay 1983; East 2007), Brazil (Beleza 2017; Francisco 2018; Morais 2016), Iran (Navvabi 2009), Thailand (Yusamran 2007), Turkey (Senol 2017) and the United Kingdom (Steen 2000; Steen 2002).
Participants
Healthy primiparous or multiparous women of childbearing age with a singleton pregnancy and cephalic presentation who gave birth vaginally around term gestation were invited to participate. Some studies only included women with a normal vaginal birth (Morais 2016; Navvabi 2009), enrolled women who had an instrumental birth (Steen 2000), included both normal and assisted (vacuum or forceps) vaginal births (Beleza 2017; East 2007; Steen 2002) or did not specify mode of vaginal birth (Barclay 1983; Senol 2017; Yusamran 2007). For some studies, a minimum level of perineal trauma (second degree tear, Steen 2002) or an episiotomy were criteria for study entry (Barclay 1983; Beleza 2017; Navvabi 2009; Steen 2000; Steen 2002; Yusamran 2007), while for three studies, lesser levels of perineal trauma or even an intact perineum could qualify a woman for study entry: given that our prespecified inclusion criteria did not allow for an intact perineum, we contacted study authors to obtain data for women with some trauma; relevant data were made available by East 2007, Francisco 2018 and Morais 2016.
The authors of Senol 2017 confirmed the numbers of primiparous and multiparous women in each group and had reported data by parity: although the published report indicated that all the primiparous women had an episiotomy, detail later provided from the authors indicated that one primipara in each group had an intact perineum. We elected to retain the data in this review rather than remove them and suffer the delays in obtaining data for only the 98 women, given the potentially unlikely impact that only omitting outcomes from 2% of participants would have on the findings. Data for the 79% of multiparous women who had perineal trauma (tear or episiotomy) were not available separately from the full sample for inclusion in this version of the review.
No studies enrolled women with severe perineal trauma, i.e. third‐ or fourth‐degree laceration, as this requires more specialised treatment.
Interventions and comparisons
Cooling treatments
Ice packs were commonly used. These could be in the form of a plastic bag or a latex medical glove filled with crushed ice or filled with water then frozen, or commercially‐available packs that would be soaked in water and frozen (Beleza 2017; East 2007; Francisco 2018; Morais 2016; Navvabi 2009; Steen 2000; Steen 2002). Cold gel pads that could be placed in a freezer came in a variety of forms, from those designed specifically for the studies (Steen 2000; Steen 2002), which were commercialised and incorporated into the trial by Navvabi 2009 or the use of a commercially‐available heat/cold pack (Senol 2017; Yusamran 2007). In one study cooling was provided in the form of a "cold sitz bath" whereby ice and salt were added to a bowl of water (Barclay 1983). The addition of compression (East 2007; Sheikhan 2011) and lying horizontally for a short time (East 2007) meant that the cooling treatment was part of a bundle of care, akin to the RICE principles. An overview of the cooling treatments is provided in Table 5. Four trials compared outcomes when one cooling therapy was compared with another therapy or regimen. For example, Navvabi 2009; Steen 2000 and Steen 2002 compared the use of ice packs and cold gel pads; East 2007 compared regular application of ice packs along with compression and being horizontal, compared with ad hoc ice pack usage.
1. Summary of cooling methods and results.
| Study | Form of cooling | Additional technique | 1st application post‐birth | Length of application | Frequency | Perineal temperature | Assessment of perineal pain | Adverse effects on perineal healing |
| Iced sitz bath | ||||||||
| Barclay 1983 | 30 g salt added to 1 ‐ 2 litres cold tap water (~ 7 °C) + ice | immersion | Day 1 | 10 minutes | 3 ‐ 4 a day | not assessed | post‐treatment ‐ days 1 ‐ 5 |
not assessed |
| Ice packs | ||||||||
| Beleza 2017 | 15 cm bag filled with 150 g crushed ice | wrapped in thin cotton tissue |
within 24 hours: average of 15 hours |
20 minutes | single application | pre‐ application: 34.5 °C post‐application ‐ 10 minutes: 20.5 °C ‐ 20 minutes: 23.4 °C ‐ 1 hour: 33.8 °C |
pre‐application post‐application ‐20 minutes ‐60 minutes |
not reported |
| East 2007 | commercial product (Medichill, USA) soaked in water then frozen | within perineal pad | birth suite | 20 minutes | 2 ‐ 3 hourly for 2 ‐ 3 days |
not assessed | post‐application ‐ within 24 hours ‐ 24 ‐ 48 hours |
no women had adverse effects |
| Francisco 2018 | plastic pack filled with 250 mL water and placed in freezer – removed in the form of ice | wrapped in thin cotton gauze applied to the perineum – participant lying down |
meant of 12.7 hours after birthing; rooming‐in unit, birth centre | 10 minutes | single application | pre‐application of ice (intact and perineal trauma): mean 33.8 °C post‐application ‐ immediately after: mean 15.6 °C ‐ 2 hours later: mean 34.1 °C |
pre‐application ‐ immediately after intervention ‐ 2 hours later |
adverse effects not recorded |
| Morais 2016 | latex glove, crushed ice | wrapped in sterile wet gauze |
2 hours | 20 minutes | 6 applications at 6‐hourly intervals | reduction by 10‐15 °C at 10 ‐ 20 minutes of application |
pre‐application post application ‐ immediately ‐ 24 hours |
no women had adverse effects |
| Navvabi 2009 | “ice pack” | no detail | within 4 hours after episiotomy repair | no detail | single application | not assessed | post‐application ‐ 4 hours ‐ days 1 ‐ 2 ‐ day 5 ‐ day 10 |
wound approximation is part of REEDA scale – not able to access separate result but no between‐group differences in overall scale with other groups. |
| Steen 2000 | saline sachets frozen, for 2 hours |
in sterile gauze cover | within 4 hours after suturing |
Unpublished information from the authors noted that gel pad groups took 20 minutes to warm to perineal temperature, compared with ice packs, which melted more quickly. | as per women’s preferences for 48 hours (mean number of applications 10.4, SD 4.3) |
‐ | post‐application ‐ 0 ‐ 4 hours ‐ day 1 ‐ day 2 |
wound healing and closure of wound edges assessed days 5 and 10: findings not reported |
| Steen 2002 | saline sachets frozen, for 2 hours |
in sterile gauze cover | within 4 hours after suturing |
not described | as per women’s preferences for 4 days. Day/Median/Range 1 5 0 ‐ 8 2 26 0 ‐ 8 3 4 0 ‐ 10 4 2 0 ‐ 14 |
not assessed | pre‐application post‐application ‐ days 1‐5 ‐ day 10 ‐ day 14 |
wound healing and closure of wound edges assessed days 5, 10 and 14: low prevalence and wide confidence intervals |
| Gel pads | ||||||||
| Navvabi 2009 | gel pad (Femepad, Florri‐Femé Pharmaceuticals Ltd, UK) with “antifreeze” gel | freeze for 2 hours prior to app |
within 4 hours of episiotomy repair |
not assessed | Initial application then as per women’s preferences for 10 days | not assessed | post‐application ‐ 4 hours ‐ days 1‐2 ‐ day 5 ‐ day 10 |
wound approximation is part of REEDA scale – not able to access separate result, however, no evidence of between‐group differences in overall scale. |
| Senol 2017 | ThermoGEL, freezer 1 hour: −10 °C once in form of ice | wrapped sterile “pad” |
2 hours post‐birth | 20 minutes | at 2 hours and 6 hours | post‐1st application 24.4 (SD 0.72) °C post‐2nd application 25.5 (SD 0.61) °C |
2 hours after each application | no women had adverse effects |
| Steen 2000 | gel pad, propylene glycol (anti‐freeze), frozen for 2 hours | sterile gauze covers | within 4 hours after suturing | Unpublished information from the authors noted that gel pad groups took 20 minutes to warm to perineal temperature, compared with ice packs, which melted more quickly | as per women’s preferences for 48 hours (mean 84, SD 5.0 applications) |
not assessed | post‐application ‐ 0 ‐ 4 hours ‐ day 1 ‐ day 2 |
wound healing and closure of wound edges assessed days 5 and 10: findings not reported |
| Steen 2002 | gel pad, propylene glycol (anti‐freeze), frozen for 2 hours | sterile gauze covers | within 4 hours after suturing | not described | as per women’s preferences for 4 days. Day/Median/Range 1 20 0 ‐ 12 2 36 0 ‐ 14 3 5 0 ‐ 13 4 2 0 ‐ 14 |
not assessed | pre‐application post‐application ‐ days 1 ‐ 5 ‐ day 10 ‐ day 14 |
wound healing and closure of wound edges assessed days 5, 10 and 14: low prevalence and wide confidence intervals |
| Yusamran 2007 | adapted commercially‐available gel pack (3M Thailand Ltd) to suit perineum (11 x 9 cm), placed in 4 °C fridge‐freezer | designed cotton pack to insert gel pad and support sanitary pad – attached loops to suspend it | ‐ | 15 minutes to maintain pad temperate of 4 ‐ 12.8 °C 2 hours |
replaced every 15 minutes for 2 hours | not assessed | pre‐ and post each application in the 2 hours, then at 48 hours | wound approximation included in the overall REEDA score |
°C – degrees Celsius; cm – centimetres; hr – hour(s); REEDA score – redness, (o)edema, ecchymosis, discharge, (wound edge) approximation; SD – standard deviation
Control/no treatment or placebo groups
Control or no‐treatment groups involved 'routine' care, described by some as involving regular hygiene, use of a sanitary pad or analgesia or both on request (Barclay 1983; Beleza 2017; Senol 2017, Steen 2002). Placebo care was included in the form of a latex glove filled with water at 20 °C to 25 °C (Morais 2016) or a gel pad and compression (Yusamran 2007). Cooling treatments were therefore compared with no treatment or with the placebo.
Outcomes
Primary outcome
All included studies reported women's self‐rated perineal pain at one or more of the prespecified times of between four and six hours after giving birth, within the first 24 hours of giving birth or between 24 to 48 hours after giving birth. A scale of zero (no pain) to 10 (worst possible pain) was used by Beleza 2017; Francisco 2018; Navvabi 2009; Senol 2017 and Yusamran 2007, or 1 (no pain) to 5 (severe pain, Barclay 1983). The remaining trialists reported data as none, mild, moderate, or severe (East 2007; Morais 2016 (converted from a numeric scale: 0 to 5 none/mild or 6 to 10 moderate/severe); Steen 2000; Steen 2002). Results of pain ratings in Barclay 1983 were only available in graphical form; as the authors were unable to be provide numeric data, this study did not contribute data for this outcome.
Secondary outcomes
No studies reported on all the prespecified secondary outcomes. Perineal pain that interfered with activities of daily living was reported for some or all of sitting, walking, urinating and caring for the baby by East 2007; Senol 2017 and Steen 2002; perineal oedema and bruising were reported by Navvabi 2009; Senol 2017; Steen 2002 and Yusamran 2007. The use of additional analgesia was evaluated by East 2007; Morais 2016 and Steen 2002; while Morais 2016 and Steen 2002 reported on women providing any breast milk to their babies. Few trials reported on adverse effects of cooling, no treatment or placebo on perineal healing (Morais 2016; Senol 2017). Maternal exhaustion was reported in the form of maternal energy level in one study (East 2007) and women's views of their experience with perineal care was provided by Morais 2016; Senol 2017; Steen 2002 and Yusamran 2007. No studies conducted an economic analysis.
Timeframes
The studies were conducted across more than three decades, from the 1980s (implied for Barclay 1983), 1993 to 1994 and 1998 to 1999 for Steen 2000; Steen 2002 respectively, from 2005 to 2006 (Navvabi 2009; Yusamran 2007), 2007 to 2008 (Beleza 2017), 2008 to 2009 (East 2007) and between 2012 and 2013 (Francisco 2018; Morais 2016; Senol 2017).
Sources of funding
Funding sources were detailed in six reports (Beleza 2017; Francisco 2018; Morais 2016; Navvabi 2009; Steen 2000; Steen 2002). Lack of funding meant that the full study proposed by East 2007 did not proceed.
Declarations of interest
A potential interest was implied in the report by Steen 2000 for the gel pads co‐designed by the authors and colleagues. This was formally declared in the subsequent study by Mary Steen who holds a patent for these gel pads (Steen 2002), although it was not declared in a study co‐authored by the patent holder (Mary Steen) when it was used in the study by Navvabi 2009. The lead author of this review (CE) used the original Cochrane protocol and review to design a trial, which was subsequently registered and piloted (East 2007): two independent review authors abstracted the relevant information and included it in this review. A statement that there were no declarations of interest was provided by Beleza 2017; Francisco 2018 and Senol 2017, while declarations of interest were not included in the reports by Barclay 1983, Morais 2016 or Yusamran 2007.
Excluded studies
We excluded 15 studies. A number of studies identified in searches were not randomised, despite the relevant keywords being in the titles or abstracts (De Souza Bosco Paiva 2016; Dube 2013; Lu 2015; Pinkerton 1961) or were cross‐over studies (LaFoy 1989; Nam 1991; Ramler 1986).
In the earlier versions of this review, we included comparisons between cooling treatments and other treatments, such as warmth, Hammamelis water (witch hazel), pramoxine or pulsed electromagnetic therapy. For the 2020 update, we followed the contemporary methods of minimising the number of comparisons and did not prioritise comparisons between cooling with other non‐cooling treatments. We therefore excluded in this update the following studies that were included in the previous versions of the review for these comparisons: Gallie 2003; Hill 1989; Moore 1989; Sheikhan 2011; Thangaraju 2006). We excluded a further three studies identified in the updated search strategy that compared cooling treatment with another form of treatment (Amani 2015; Kaur 2012; NCT02024256 (See table of Characteristics of excluded studies).
Risk of bias in included studies
The included trials were at low or uncertain risk of bias overall, with the exception that the inability to blind participants and personnel to group allocation meant that we considered all trials to be at unclear or high risk for this domain. Other areas at high risk of bias included two studies in which allocation concealment was unclear, while three studies had substantial attrition bias (detailed below). Blinding of outcome assessors was generally a source of unclear risk of bias (see below). See (Figure 2 and Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was low risk of bias for all except two trials. Barclay 1983 did not provide any detail on how it was determined that women were admitted to the ward area in which the particular treatment group was used (judged to be high risk of bias). A web‐based random number table‐generating website was used by Senol 2017 and although the authors confirmed that a researcher undertook this, it remained unclear if the researcher had other responsibilities in the trial and could therefore have introduced some bias.
Blinding
Blinding of participants and clinicians was not feasible in the study designs and the risk of performance bias was generally judged as being unclear. Barclay 1983 was subject to multiple risks of bias and unblinding of both participants and personnel was likely to have contributed to these concerns. For the remaining studies, it is unclear whether outcomes reported by the participants were at risk of detection bias: one author noted the potential for participants to wish to please the researcher and thus influence the outcomes (Beleza 2017). Two trials reported blinding of outcome assessors for assessment of perineal oedema/healing (Morais 2016; Steen 2000): these were judged as low risk of detection bias when considering GRADE and unclear for women's perceptions of pain. For the remaining trials detection bias was judged as being at unclear risk of bias, particularly for objective outcomes such as perineal oedema, adverse events, rates of breastfeeding, use of additional analgesia and costs.
Incomplete outcome data
The proportions of incomplete outcome data ranged from 0% (Barclay 1983; Beleza 2017; Francisco 2018; Morais 2016; Senol 2017; Yusamran 2007), 6% to 9% (East 2007; Navvabi 2009), or higher in the two trials that required return of questionnaires once women had been seen at home daily to day 10 post‐birth (Steen 2000: 37.2% attrition; Steen 2002: 29.8% attrition). The attrition rates were dissimilar for the ice pack (42%) and gel pad groups (32.5%) in Steen 2000, while for Steen 2002 there were similar rates across the ice pack (29.3%), gel pad (28.7%) and no‐treatment groups (32.7%). We considered the potential effect of including the reported data, given our original a priori plan to consider excluding trials with more than 20% loss to follow‐up in the earlier versions of the review. The losses to follow‐up were related to challenges in obtaining the data forms from the community midwives. We considered that this would potentially contribute less bias than if the losses related to non‐return by participants rather than their clinicians. We therefore included these studies in the (meta‐) analyses.
Selective reporting
We judged the pilot trial by East 2007 to be at low risk of reporting bias, given its prospective registration and that the raw data were available to the review authors. For studies with neither registration nor published protocols that were conducted some decades ago, we considered the risk of bias to be unclear, as this would not have been a reporting requirement when seeking journal publication at that time (Barclay 1983; Steen 2000; Steen 2002; Yusamran 2007). Although studies conducted in the 2010s would often be required to be registered as a condition of publication, we determined that Senol 2017 was at unclear risk of bias, given that enrolment numbers and outcomes appeared to be consistent between the thesis and journal publication. We judged two reports to be at high risk of reporting bias, as they were registered five years after recruiting for their studies (Beleza 2017; Navvabi 2009). Two studies registered with the Brazilian Trial Registry, for one, the authors provided a download of the information, but this is not accessible publicly (Morais 2016), while for the other the authors confirmed the registration, although it is not available online (Francisco 2018). We therefore judged these as being at unclear risk of reporting bias.
Other potential sources of bias
For Barclay 1983, women had heard about the use of iced sitz baths and requested their use, as well as being influenced by some of the midwives to select this as an option. Findings were reported in the treatment groups in which they ultimately ended up, rather than the prospective expectation that there would be even numbers of women in each group. This may have introduced unknown biases in addition to those noted above.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment
Five studies (Beleza 2017; Francisco 2018; Navvabi 2009; Senol 2017; Steen 2002) enrolled 725 women and provided outcome data for 582 participants to compare cooling treatments as either an ice pack or a cold gel pad versus no treatment. Overall certainty of the evidence was very low, limited by the women, personnel and outcome assessors being unblinded to treatment allocation. Other concerns included unclear selection bias (Beleza 2017; Senol 2017) and high attrition (Steen 2000; Steen 2002). Additionally, small sample sizes for some outcomes, confidence intervals (CIs) that included the null and wide CIs contributed to concerns about the imprecision of the findings.
See: Table 1.
Primary outcome
Perineal pain
Five trial reports provided data on women’s self‐rated moderate and severe perineal pain (Steen 2002, n = 316) or using a scale of 0 (no pain) to 10 (worst possible pain) (Beleza 2017 n = 50; Francisco 2018 n = 45; Navvabi 2009 n = 71; and Senol 2017 n = 100). Women's self‐rated perineal pain immediately following removal of the cold pad and within six hours of giving birth may be lower than for women who have no treatment (Senol 2017: mean difference (MD) −4.46, 95% CI −5.07 to −3.85; 100 women; Analysis 1.1). We are left with uncertainty about the effect of cooling treatments compared with no treatment on women’s self‐reported moderate or severe perineal pain within 24 hours of giving birth (1 study, 316 women; Analysis 1.2) or on a rating from zero to 10 (MD −0.41, 95% CI −1.78 to 0.95; 3 studies, 166 women; Analysis 1.3). Reported pain may be lower between 24 and 48 hours after giving birth for women when they use cooling, compared with no treatment (risk ratio (RR) 0.73, 95% CI 0.57 to 0.94; 1 study, 316 women; Analysis 1.4). Beleza 2017 and Navvabi 2009 reported that there may be no between‐group differences for women’s self‐rated perineal pain (scale 0 no pain to 10 worst pain) within 24 hours of giving birth (MD −0.34, 95% CI −2.60 to 1.92, I2 = 57%; random effects; 2 studies, 121 women; Analysis 1.3) or within 24 and 48 hours of giving birth (MD −0.53, 95% CI −1.45 to 0.39; 1 study, 71 women, Analysis 1.5).
1.1. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 1: Perineal pain within 4‐6 hours of giving birth
1.2. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 2: Perineal pain within 24 hours of giving birth
1.3. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 3: Perineal pain within 24 hours of giving birth
1.4. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 4: Perineal pain between 24 ‐ 48 hours after giving birth
1.5. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 5: Perineal pain between 24 ‐48 hours after giving birth
Secondary outcomes
Perineal pain associated with activities of daily living
One study (Steen 2002) reported on 312 women’s self‐assessed moderate and severe perineal pain associated with sitting (Analysis 1.6; Analysis 1.7), walking (Analysis 1.8; Analysis 1.9) and feeding baby (Analysis 1.10; Analysis 1.11) within 24 hours and between 24 and 48 hours of giving birth, with no apparent between‐group differences. Senol 2017 noted that women had less pain associated with sitting, walking or breastfeeding in women following application of the cold gel pad, compared with no treatment ("p<0.001"), but no data were available to explore this further.
1.6. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 6: Pain associated with activities of daily living (sitting) within 24 hours of giving birth
1.7. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 7: Pain associated with activities of daily living (sitting) between 24 ‐ 48 hours of giving birth
1.8. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 8: Pain associated with activities of daily living (walking) within 24 hours of giving birth
1.9. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 9: Pain associated with activities of daily living (walking) between 24 ‐ 48 hours of giving birth
1.10. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 10: Pain associated with activities of daily living (feeding baby) within 24 hours of giving birth
1.11. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 11: Pain associated with activities of daily living (feeding baby) between 24 ‐ 48 hours of giving birth
Perineal oedema, bruising and wound healing
Perineal oedema was reported as a distinct outcome by Steen 2002 (n = 316), which may have favoured cooling treatments compared with no treatment between 24 and 48 hours of giving birth (RR 0.82, 95% CI 0.69 to 0.98; Analysis 1.13), although not within 24 hours of giving birth (Analysis 1.12).
1.13. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 13: Perineal oedema between 24 ‐ 48 hours after giving birth
1.12. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 12: Perineal oedema within 24 hours of giving birth
Bruising of the perineum was reported as a distinct outcome for 316 women by Steen 2002, with no between‐group differences when measured within 24 hours, or between 24 and 48 hours of giving birth (Analysis 1.14; Analysis 1.15).
1.14. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 14: Perineal bruising within 24 hours of giving birth
1.15. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 15: Perineal bruising between 24 ‐ 48 hours after giving birth
Navvabi 2009 reported a composite scale of perineal status, using the REEDA scale of zero (healthiest) to 15 (worst): this includes an assessment of redness, (o)edema, ecchymosis (bruising), discharge and approximation (wound gaping). They did not find any difference in this scale for women receiving cooling or no treatments within 24 hours of giving birth (Analysis 1.16), although those using ice packs were estimated to have a lower score than those with no treatment (Analysis 1.17). This scale was also used by Senol 2017; however, we were unable to distinguish primiparous women (had episiotomy) from multiparous women (had episiotomy, tear or intact perineum), and could not include these data in the review. Although the trialists treated this variable as continuous data, this assumption cannot be guaranteed and we advise caution in interpreting the results.
1.16. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 16: Perineal redness, oedema, bruising, discharge, wound gaping within 24 hours of giving birth
1.17. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 17: Perineal redness, oedema, bruising, discharge, wound gaping between 24 ‐ 48 hours of giving birth
Additional analgesia for relief of perineal pain
Women in both groups in Steen 2002 used non‐prescription and prescription analgesia similarly within 24 hours and between 24 and 48 hours after giving birth (Analysis 1.18; Analysis 1.19).
1.18. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 18: Additional analgesia for relief of perineal pain: within 24 hours of giving birth
1.19. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 19: Additional analgesia for relief of perineal pain between 24 ‐ 48 hours after giving birth
Adverse effects of treatment on perineal healing
No women in either group reported adverse effects of an ice pack or no treatment within four to six hours after giving birth (100 women; Senol 2017). A number of outcomes were assessed for 315 women by Steen 2002. However, we are uncertain of whether there are differences in the reported outcomes: perineal wound healing: day 5 (Analysis 1.20) ‐ wound not healing (RR 1.24, 95% CI 0.34 to 4.58), wound edges gaping (RR 2.56, 95% CI 0.58 to 11.33), any sign of infection (RR 0.47, 95% CI 0.12 to 1.82); day 10 (Analysis 1.21) ‐ wound not healing (RR 0.70, 95% CI 0.20 to 2.42), wound edges gaping (RR 0.62, 95% CI 0.22 to 1.74), any sign of infection (RR 2.33, 95% CI 0.52 to 10.42), need for wound resuture (RR 2.34, 95% CI 0.11 to 48.25), noting that there were few events for these outcomes and where GRADE assessments were conducted (Table 1) they were of very low certainty.
1.20. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 20: Adverse effects on perineal wound healing: Day 5
1.21. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 21: Adverse effects on perineal wound healing: Day 10
Maternal exhaustion
No studies reported on maternal exhaustion.
Maternal views and experiences with treatment
Steen 2002 asked women about their satisfaction with perineal care 10 days after giving birth. There may be no difference in women’s ratings of their care as good, very good or excellent for the two groups (308 women; Analysis 1.22). The Postnatal Comfort Questionnaire, using a scale of 1 (low comfort) to 5 (high comfort) and administered by Senol 2017, included two subgroups of relevance to maternal views and experiences. Women may have a trivially higher level of psychospiritual comfort when using the cooling treatment (cold gel pad) compared with no treatment (MD −0.10, 95% CI −0.20 to 0.00) and there may be no between‐group difference in their sociocultural comfort ratings (MD 0.01, 95% CI −0.09 to 0.11; 1 study, 100 women; Analysis 1.23). Beleza 2017 sought opinions from the 24 women who received cryotherapy: 96% responded that the ice bag was comfortable, 96% agreed that they would use this again, while all women reported that they were satisfied or very satisfied with the treatment.
1.22. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 22: Maternal views and experiences of treatment
1.23. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 23: Maternal views and experiences of treatment
Women providing any breast milk to the baby 24 to 48 hours after giving birth
Data from one study (Steen 2002) reported no difference in the proportion of women providing their babies with breast milk compared with no treatment (RR 0.89, 95% CI 0.73 to 1.07; 315 women; Analysis 1.24).
1.24. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 24: Women providing any breastmilk to baby 24 ‐ 48 hours after giving birth
Cost of treatment
No studies evaluated the costs of treatments.
Subgroup analyses
The authors of one study (Steen 2002) made data available that allowed us to conduct the prespecified subgroup analyses for comparisons of the effectiveness of cooling treatment (ice packs, cold gel pads) and no treatment on the primary outcome, perineal pain.
Parity
There appeared to be some difference in effect between the subgroups for perineal pain by parity within 24 hours of giving birth but not between 24 and 48 hours of giving birth (Analysis 1.25; Analysis 1.26).
1.25. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 25: Subgroup: Parity and perineal pain within 24 hours of giving birth
1.26. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 26: Subgroup: Parity and perineal pain between 24 ‐ 48 hours after giving birth
Mode of birth
There did not appear to be any difference in effect between the subgroups for perineal pain by mode of vaginal birth when measured within 24 hours or between 24 and 48 hours of giving birth (Analysis 1.27; Analysis 1.28).
1.27. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 27: Subgroup: Mode of birth and perineal pain within 24 hours of giving birth
1.28. Analysis.

Comparison 1: Cooling treatment (ice pack or cold gel pad) versus no treatment, Outcome 28: Subgroup: Mode of birth and perineal pain between 24 ‐ 48 hours after giving birth
Comparison 2: Cooling treatment (cold gel pad) + compression versus placebo (gel pad + compression)
Yusamran 2007 enrolled 250 women in a study comparing cooling using a cold gel pad plus compression from a well‐fitting sanitary pad secured in place with a belt, compared with a placebo gel pad, also with the well‐fitting pad to provide compression. Data were reported on all women enrolled. The overall evidence was of low certainty, with trivial effect sizes for some outcomes, wide CIs that included the null effect for other outcomes and the uncertainty of the potential bias introduced with unblinding.
See: Table 2.
Primary outcome
Perineal pain
The one study suggested that there may be no difference in women's self‐reported perineal pain on a scale of zero (no pain) to 10 (worst possible pain), assessed within four to six hours of giving birth (MD −0.32, 95% CI −0.78 to 0.14; 250 women; Analysis 2.1). Women may experience very slightly less perineal pain in the cooling therapy group than did those in the placebo group, when assessed between 24 and 48 hours following birth (MD −0.43, 95% CI −0.73 to −0.13; 250 women; Analysis 2.2).
2.1. Analysis.

Comparison 2: Cooling treatment (cold gel pad)+compression versus placebo (gel pad+compression), Outcome 1: Perineal pain within 4 ‐ 6 hours of giving birth
2.2. Analysis.

Comparison 2: Cooling treatment (cold gel pad)+compression versus placebo (gel pad+compression), Outcome 2: Perineal pain within 24 ‐ 48 hours after giving birth
Secondary outcomes
Perineal pain associated with activities of daily living
No studies evaluated this outcome.
Perineal oedema
There was less oedema (scale zero: none ‐ 3: > 2 cm) in the cooling‐therapy group compared with the placebo group when observed within 24 to 48 hours after giving birth (MD −0.15, 95% CI −0.28 to −0.03; 250 women; Analysis 2.3). Although the trialists treated this variable as continuous data, this assumption cannot be guaranteed and we advise caution in interpreting the results.
2.3. Analysis.

Comparison 2: Cooling treatment (cold gel pad)+compression versus placebo (gel pad+compression), Outcome 3: Perineal oedema between 24 ‐ 48 hours after giving birth
Perineal bruising
None of the women in either treatment group were observed to have perineal bruising when assessed within 24 to 48 hours of giving birth (250 women).
Additional analgesia for relief of perineal pain
No studies evaluated this outcome.
Adverse effects on perineal healing
No women in either group were noted to have poor wound approximation when assessed 24 to 48 hours after giving birth (250 women).
Maternal exhaustion
No studies reported on maternal exhaustion.
Maternal views and experiences with treatment
Women were slightly more satisfied with their perineal care after receiving cooling treatment compared with compression on a scale of zero (not satisfied) to 10 (most satisfied) (Analysis 2.4).
2.4. Analysis.

Comparison 2: Cooling treatment (cold gel pad)+compression versus placebo (gel pad+compression), Outcome 4: Satisfaction with perineal care
Women providing any breast milk to the baby 24 to 48 hours after giving birth
No studies provided detail of infant feeding.
Cost of treatment
No studies evaluated the costs of treatments.
Comparison 3: Cooling treatment (ice pack) versus placebo (water pack)
Morais 2016 enrolled 63 women who had experienced perineal trauma, and provided outcome data for these 63 participants to compare cooling treatment in the form of an ice pack with a placebo of a water pack. Data were provided directly by the study authors, as the published data also included women with an intact perineum (total n = 80).
We judged the certainty of the evidence as being very low, given that participants and personnel were unblinded and the small sample size. Confidence intervals that included the null and wide CIs contributed to concerns about the imprecision of the findings.
See: Table 3
Primary outcome
Perineal pain
The study authors advised that no women reported pain within four to six hours, or within 24 hours of giving birth (Morais 2016).
Secondary outcomes
Perineal pain associated with activities of daily living
No studies addressed this outcome.
Perineal oedema
There may be no difference in perineal oedema between women in the cooling versus placebo group, when assessed within four to six hours of giving birth (RR 0.96, 95% CI 0.50 to 1.86; 63 women; Analysis 3.1) or within 24 hours of giving birth (RR 0.36, 95% CI 0.08 to 1.59; 63 women; Analysis 3.2).
3.1. Analysis.

Comparison 3: Cooling treatment (ice pack) versus placebo (water pack), Outcome 1: Perineal oedema within 4 ‐ 6 hours after giving birth
3.2. Analysis.

Comparison 3: Cooling treatment (ice pack) versus placebo (water pack), Outcome 2: Perineal oedema within 24 hours after giving birth
Perineal bruising
No studies evaluated this outcome.
Additional analgesia for relief of perineal pain
There may be no between‐group difference in the proportion of women using additional analgesia within 24 hours of giving birth (RR 0.63, 95% CI 0.35 to 1.11; 63 women; Analysis 3.3).
3.3. Analysis.

Comparison 3: Cooling treatment (ice pack) versus placebo (water pack), Outcome 3: Additional analgesia for relief of perineal pain within 24 hours of giving birth
Adverse effects on perineal healing
No women were reported to have experienced adverse effects on wound healing (63 women).
Maternal exhaustion
None of the women reported that they were exhausted when assessed within four to six hours or within 24 hours of giving birth.
Maternal views and experiences with treatment
There may be no difference in the women's ratings of three aspects of their experiences with treatment (Analysis 3.4) as: (i) being satisfied with treatment (RR 0.91, 95% CI 0.77 to 1.08); (ii) would repeat the treatment in future childbirth (RR 0.88, 95% CI 0.75 to 1.04); and (iii) would recommend treatment (RR 0.89, 95% CI 0.77 to 1.03).
3.4. Analysis.

Comparison 3: Cooling treatment (ice pack) versus placebo (water pack), Outcome 4: Maternal views and experiences with treatment
Women providing any breast milk to the baby 24 to 48 hours after giving birth
All participants provided some breast milk to their babies within 24 to 48 hours of giving birth (Analysis 3.5).
3.5. Analysis.

Comparison 3: Cooling treatment (ice pack) versus placebo (water pack), Outcome 5: Women providing any breastmilk to baby 48 hours after giving birth
Cost of treatment
No studies evaluated the costs of the ice pack or water bag.
Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad)
Three trials (Navvabi 2009; Steen 2000; Steen 2002) enrolled 459 women in their studies comparing one cooling treatment (ice pack) with an alternative cooling treatment (cold gel pad), and reported outcomes for 338 of these participants.
We judged the overall certainty of evidence to be very low, noting that all participants, personnel and outcome assessors were unblinded, there were high attrition rates in the studies by Steen 2000 (37%) and Steen 2002 (29%), and selection bias was unclear for Navvabi 2009. Additionally, the small sample sizes for most outcomes and wide CIs contributed to concerns about the imprecision of the findings.
See: Table 4.
Primary outcome
Perineal pain
Three trial reports provided data on women’s self‐rated perineal pain as moderate and severe (Steen 2000, n = 49; Steen 2002, n = 215) or using a scale of 0 (no pain) to 10 (worst possible pain, Navvabi 2009 n = 74). There may be no differences between the groups in women’s self‐reported moderate or severe perineal pain within four and six hours of giving birth (RR 0.57, 95% CI 0.26 to 1.24; 1 study, 49 women; Analysis 4.1). The evidence was too uncertain to suggest whether one cooling treatment was better than the other for relieving perineal pain within 24 hours of giving birth (RR 0.98, 95% CI 0.78 to 1.22; 2 studies, 264 women; Analysis 4.2; MD 0.58, 95% CI −0.44 to 1.60; 1 study, 74 women; Analysis 4.3) or between 24 and 48 hours after giving birth (RR 1.21 95% CI 0.89 to 1.65; 2 studies, 264 women; Analysis 4.4; MD 0.86, 95% CI −0.10 to 1.82; 1 study, n = 74; Analysis 4.5).
4.1. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 1: Perineal pain within 4 ‐ 6 hours of giving birth
4.2. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 2: Perineal pain within 24 hours of giving birth
4.3. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 3: Perineal pain within 24 hours of giving birth
4.4. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 4: Perineal pain between 24 ‐ 48 hours after giving birth
4.5. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 5: Perineal pain between 24 ‐ 48 hours of giving birth
Secondary outcomes
Perineal pain associated with activities of daily living (sitting, walking, feeding baby)
One study (Steen 2002) reported on 215 women’s self‐assessed perineal pain associated with sitting (Analysis 4.6; Analysis 4.7), walking (Analysis 4.8; Analysis 4.9) and feeding baby (Analysis 4.10; Analysis 4.11) within 24 hours and between 24 and 48 hours of giving birth, with no apparent between‐group differences.
4.6. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 6: Pain associated with activities of daily living (sitting) within 24 hours of giving birth
4.7. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 7: Pain associated with activities of daily living (sitting) between 24 ‐ 48 hours after giving birth
4.8. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 8: Pain associated with activities of daily living (walking) within 24 hours of giving birth
4.9. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 9: Pain associated with activities of daily living (walking) between 24 ‐ 48 hours after giving birth
4.10. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 10: Pain associated with activities of daily living (feeding baby) within 24 hours of giving birth
4.11. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 11: Pain associated with activities of daily living (feeding baby) between 24 ‐ 48 hours after giving birth
Perineal oedema, bruising and wound healing
Perineal oedema was reported as a distinct outcome by Steen 2000 and Steen 2002, but we are uncertain of whether there are differences between women using ice packs or cold gel pads within four to six hours of giving birth (RR 1.39, 95% CI 0.93 to 2.09; 1 study, 49 women; Analysis 4.12), or within 24 hours of giving birth (RR 0.97, 95% CI 0.84 to 1.13; 2 studies, 264 women; Analysis 4.13). Women using ice packs may be more likely to have oedema between 24 and 48 hours after giving birth, compared with those using cold gel pads (RR 1.69, 95% CI 1.03 to 2.77; 2 studies, 264 women; Analysis 4.14).
4.12. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 12: Perineal oedema within 4 ‐ 6 hours of giving birth
4.13. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 13: Perineal oedema within 24 hours of giving birth
4.14. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 14: Perineal oedema between 24 ‐ 48 hours after giving birth
Bruising of the perineum was reported as a distinct outcome by Steen 2002, with no difference when using cooling treatments compared with no treatment within four to six hours of giving birth (RR 1.23, 95% CI 0.51 to 2.97; 1 study, 49 women; Analysis 4.15), within 24 hours (RR 0.95, 95% CI 0.79 to 1.14; 2 studies, 264 women; Analysis 4.16), or between 24 and 48 hours of giving birth (RR 1.07, 95% CI 0.92 to 1.25; 2 studies, 264 women; Analysis 4.17).
4.15. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 15: Perineal bruising within 4 ‐ 6 hours of giving birth
4.16. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 16: Perineal bruising within 24 hours of giving birth
4.17. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 17: Perineal bruising between 24 ‐ 48 hours after giving birth
Navvabi 2009 reported a composite scale of perineal status, using the REEDA scale of zero (healthiest) to 15 (worst): this includes an assessment of redness, (o)edema, ecchymosis (bruising), discharge and approximation (wound gaping). They reported that there may be no overall difference in this scale for women using ice packs or cold gel pads, either within 24 hours of giving birth (MD −0.13, 95% CI −0.85 to 0.59; 1 study, 74 women; Analysis 4.18) or between 24 and 48 hours after giving birth (MD 0.20, 95% CI −0.33 to 0.73; 1 study, 74 women; Analysis 4.19).
4.18. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 18: Perineal redness, oedema, bruising, discharge, wound gaping within 24 hours of giving birth
4.19. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 19: Perineal redness, oedema, bruising, discharge, wound gaping between 24 ‐ 48 hours after giving birth
Additional analgesia for relief of perineal pain
Women in both groups in Steen 2002 (215 women) used non‐prescription and prescription analgesia similarly with 24 hours and between 24 and 48 hours after giving birth (Analysis 4.20; Analysis 4.21).
4.20. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 20: Additional analgesia for relief of perineal pain: within 24 hours of giving birth
4.21. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 21: Additional analgesia for relief of perineal pain: 24 ‐ 48 hours after giving birth
Adverse effects of treatment on perineal healing
The report by Navvabi 2009 included wound edge approximation as part of the REEDA scale: this component could not be analysed separately from the overall scale, but there were no apparent between‐group differences, as noted above for "perineal oedema, bruising and wound healing".
Although wound healing was not reported in the time frame specified for this review, Steen 2002 assessed healing on days five and 10 by inspecting the approximation of the skin edges. More women using ice packs possibly had wound edges gaping at day five than those who had used the gel pads (RR 0.22, 95% CI 0.05 to 1.01; 1 study, 215 women; Analysis 4.22). However, this did not appear to persist to day 10 (RR 3.06, 95% CI 0.63 to 14.81; 1 study, 214 women; Analysis 4.23). Other assessments of wound healing did not provide any certainty of evidence for between‐group differences in terms of the wound not healing, any sign of wound infection, and by day 10 the need for wound resuture (Analysis 4.22; Analysis 4.23), noting the wide CIs for these outcomes that had few events.
4.22. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 22: Adverse effects on perineal wound healing: Day 5
4.23. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 23: Adverse effects on perineal wound healing: Day 10
Maternal exhaustion
No studies reported on maternal exhaustion.
Maternal views and experiences with treatment
Maternal views were evaluated in slightly different formats and for different time frames in the two trials that reported this outcome, and we consider them separately (Analysis 4.24). Women may be less likely to rate their opinion of treatment effects favourably following the use of ice packs compared with cold gel pads five days after giving birth (RR 0.33, 95% CI 0.17 to 0.68; 1 study, 49 women ), and may be less favourable in their ratings of ice packs than of cold gel pads when assessed on day 10 following birth (RR 0.82, 95% CI 0.73 to 0.92; 1 study, 208 women; low certainty).
4.24. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 24: Maternal views and experiences with treatment
Women providing any breast milk to the baby 24 to 48 hours after giving birth
The one study (Steen 2002) reported similar proportions of women providing breast milk to their babies when using either the ice packs or cold gel pads (RR 1.05, 95% CI 0.84 to 1.33; 1 study, 212 women; Analysis 4.25).
4.25. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 25: Women providing any breastmilk to the baby 48 hours after giving birth
Cost of treatment
No studies evaluated the costs of treatments.
Subgroup analyses
The authors of one study (Steen 2002) made data available that allowed us to conduct the prespecified subgroup analyses for comparisons of the effectiveness of the cooling treatment (ice packs, cold gel pads) on the primary outcome, perineal pain.
Parity
There appeared to be no difference in effect between the subgroups for perineal pain by parity within 24 hours of giving birth or between 24 and 48 hours of giving birth (Analysis 4.26; Analysis 4.27).
4.26. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 26: Subgroup: Parity and perineal pain within 24 hours of giving birth
4.27. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 27: Subgroup: Parity and perineal pain between 24 ‐ 48 hours after giving birth
Mode of birth
There did not appear to be any difference in effect between the subgroups for perineal pain by mode of vaginal birth when measured within 24 hours or between 24 and 48 hours of giving birth (Analysis 4.28; Analysis 4.29).
4.28. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 28: Subgroup: Mode of birth and perineal pain within 24 hours of giving birth
4.29. Analysis.

Comparison 4: Cooling treatment (ice pack) versus cooling treatment (cold gel pad), Outcome 29: Subgroup: Mode of birth and perineal pain between 24 ‐ 48 hours after giving birth
Comparison 5: Cooling treatment (ice pack, compressing, being horizontal) versus cooling treatment (ad hoc application of ice packs)
East 2007 proposed a trial comparing the use of a structured regimen involving regular (approximately six times daily) application of an ice pack with compression and lying horizontally (I.C H.), compared to the ad hoc application of ice packs. A pilot study on 20 women was conducted to assess the feasibility of the study processes. The study author provided unpublished data on the 16 women who had some degree of perineal trauma. Lack of funding meant that the full trial did not proceed. The wide CIs for each outcome reflect the small sample size.
The limitations of the study included unblinding of participants, personnel and outcome assessors, along with the very small numbers enrolled in the pilot feasibility study. We did not prepare a 'Summary of findings' table as there were so few participants in this single pilot study for this comparison.
Primary outcome
Perineal pain
There was no apparent difference in women's self‐reported perineal pain from the I.C.H. regimen compared with the ad hoc application of ice packs within 24 hours (RR 3.33, 95% CI 0.49 to 22.90; 8 women; Analysis 5.1) or between 24 and 48 hours of giving birth (RR 1.00, 95% CI 0.20 to 4.95; 12 women; Analysis 5.2).
5.1. Analysis.

Comparison 5: Cooling treatment (I.C.H. (ice, compression, (being) horizontal)) versus cooling treatment (ad hoc ice pack usage), Outcome 1: Perineal pain within 24 hours of giving birth
5.2. Analysis.

Comparison 5: Cooling treatment (I.C.H. (ice, compression, (being) horizontal)) versus cooling treatment (ad hoc ice pack usage), Outcome 2: Perineal pain between 24 ‐ 48 hours after giving birth
Secondary outcomes
Perineal pain associated with activities of daily living
There were no apparent between‐group difference in women's self‐reported moderate and severe pain for sitting, walking, urinating or caring for the baby within 24 hours of giving birth or 24 to 48 hours after giving birth (Analysis 5.3; Analysis 5.4; Analysis 5.5; Analysis 5.6; Analysis 5.7; Analysis 5.8; Analysis 5.9; Analysis 5.10).
5.3. Analysis.

Comparison 5: Cooling treatment (I.C.H. (ice, compression, (being) horizontal)) versus cooling treatment (ad hoc ice pack usage), Outcome 3: Perineal pain associated with activities of daily living (sitting) within 24 hours of giving birth
5.4. Analysis.

Comparison 5: Cooling treatment (I.C.H. (ice, compression, (being) horizontal)) versus cooling treatment (ad hoc ice pack usage), Outcome 4: Perineal pain associated with activities of daily living (sitting) between 24 ‐ 48 hours after giving birth
5.5. Analysis.

Comparison 5: Cooling treatment (I.C.H. (ice, compression, (being) horizontal)) versus cooling treatment (ad hoc ice pack usage), Outcome 5: Perineal pain associated with activities of daily living (walking) within 24 hours of giving birth
5.6. Analysis.

Comparison 5: Cooling treatment (I.C.H. (ice, compression, (being) horizontal)) versus cooling treatment (ad hoc ice pack usage), Outcome 6: Perineal pain associated with activities of daily living (walking) between 24 ‐ 48 hours after giving birth
5.7. Analysis.

Comparison 5: Cooling treatment (I.C.H. (ice, compression, (being) horizontal)) versus cooling treatment (ad hoc ice pack usage), Outcome 7: Perineal pain associated with activities of daily living (urinating) within 24 hours of giving birth
5.8. Analysis.

Comparison 5: Cooling treatment (I.C.H. (ice, compression, (being) horizontal)) versus cooling treatment (ad hoc ice pack usage), Outcome 8: Perineal pain associated with activities of daily living (urinating) between 24 ‐ 48 hours after giving birth
5.9. Analysis.

Comparison 5: Cooling treatment (I.C.H. (ice, compression, (being) horizontal)) versus cooling treatment (ad hoc ice pack usage), Outcome 9: Perineal pain associated with activities of daily living (caring for baby) within 24 hours of giving birth
5.10. Analysis.

Comparison 5: Cooling treatment (I.C.H. (ice, compression, (being) horizontal)) versus cooling treatment (ad hoc ice pack usage), Outcome 10: Perineal pain associated with activities of daily living (caring for baby) between 24 ‐ 48 hours after giving birth
Perineal oedema
The pilot study did not evaluate this outcome.
Perineal bruising
The pilot study did not evaluate this outcome.
Additional analgesia for relief of perineal pain
There were no apparent between‐group differences in the proportion of women using additional analgesia within 24 hours of giving birth (RR 0.68, 95% CI 0.31 to 1.51; 8 women; Analysis 5.11) or between 24 and 48 hours of giving birth (RR 0.80, 95% CI 0.41 to 1.56; 12 women; Analysis 5.12).
5.11. Analysis.

Comparison 5: Cooling treatment (I.C.H. (ice, compression, (being) horizontal)) versus cooling treatment (ad hoc ice pack usage), Outcome 11: Additional analgesia for relief of perineal pain: within 24 hours of giving birth
5.12. Analysis.

Comparison 5: Cooling treatment (I.C.H. (ice, compression, (being) horizontal)) versus cooling treatment (ad hoc ice pack usage), Outcome 12: Additional analgesia for relief of perineal pain: between 24 ‐ 48 hours after giving birth
Adverse effects on perineal healing
No studies evaluated this outcome.
Maternal exhaustion
The participants did not report differences in their energy levels, measured on a scale of 1 (much less than normal) to 5 (much more than normal) within 24 hours of giving birth (MD 0.70, 95% CI −0.11 to 1.51; 8 women; Analysis 5.13) or between 24 and 48 hours after giving birth (MD 0.00, 95% CI −0.92 to 0.92; 12 women; Analysis 5.14).
5.13. Analysis.

Comparison 5: Cooling treatment (I.C.H. (ice, compression, (being) horizontal)) versus cooling treatment (ad hoc ice pack usage), Outcome 13: Self‐reported energy level: within 24 hours of giving birth
5.14. Analysis.

Comparison 5: Cooling treatment (I.C.H. (ice, compression, (being) horizontal)) versus cooling treatment (ad hoc ice pack usage), Outcome 14: Self‐reported energy level: between 24 ‐ 48 hours of giving birth
Maternal views and experiences with treatment
Women in each treatment group rated their experiences with treatment as being effective or extremely effective similarly, both within 24 hours of giving birth (RR 0.41, 95% CI 0.11 to 1.48; 8 women; Analysis 5.15) and within 24 and 48 hours after giving birth (RR 1.18, 95% CI 0.76 to 1.83; 12 women; Analysis 5.16).
5.15. Analysis.

Comparison 5: Cooling treatment (I.C.H. (ice, compression, (being) horizontal)) versus cooling treatment (ad hoc ice pack usage), Outcome 15: Maternal views of effectiveness of ice packs: within 24 hours of giving birth
5.16. Analysis.

Comparison 5: Cooling treatment (I.C.H. (ice, compression, (being) horizontal)) versus cooling treatment (ad hoc ice pack usage), Outcome 16: Maternal views of effectiveness of ice packs: between 24 ‐ 48 hours of giving birth
Women providing any breast milk to the baby 24 to 48 hours after giving birth
There was no between‐group difference in the proportion of women providing some breast milk to their babies between 24 and 48 hours after giving birth (RR 0.69, 95% CI 0.31 to 1.57; 7 women; Analysis 5.17).
5.17. Analysis.

Comparison 5: Cooling treatment (I.C.H. (ice, compression, (being) horizontal)) versus cooling treatment (ad hoc ice pack usage), Outcome 17: Women providing any breastmilk to the baby between 24 ‐ 48 hours after giving birth
Cost of treatment
The pilot study did not evaluate the cost of treatments.
Discussion
Summary of main results
Ten trials considered the potential for cooling treatments to improve maternal perineal pain and other outcomes: nine of these trials contributed data from 998 women for this updated review. Across the studies primiparous and multiparous women experiencing normal vaginal births and assisted vaginal deliveries were included. No women with severe perineal trauma (3rd/4th degree tears) were included.
We considered the primary outcome of women's self‐reported perineal pain ratings at four to six hours, within 24 hours, and 24 to 48 hours after giving birth.
The included studies provided some reports of the secondary outcomes of perineal pain interfering with activities of daily living, perineal oedema and bruising, additional analgesic use, adverse effects including perineal wound healing, maternal exhaustion, women's views/experiences with treatments (or no treatment) and breast milk provision to babies.
Few trials used similar comparisons, assessment tools and outcome measures, making it inherently difficult to draw conclusions. For the purpose of this review update we extracted five comparisons from the trials. These included cooling compared to either no treatment or a placebo treatment, or comparisons between different cooling modalities. It is important to note that gaining true participant/outcome assessor blinding of participants for this clinical intervention is difficult if not impossible, with such limitations being acknowledged by the trial authors. Blinding of outcome assessors was also problematic, with the women themselves reporting the primary outcome of pain and their experiences with treatments (or no treatment).
Primary outcome: perineal pain
There was very low‐certainty evidence from relatively small studies to evaluate the effectiveness of cooling treatments on women's self‐reported perineal pain.
When comparing perineal pain following the use of cooling treatments versus no treatment, there was very low‐certainty evidence to suggest that cooling was more favourable at four to six hours after having the baby, or at 24 to 48 hours after giving birth.
When cooling was compared with a placebo option, one study that compared the use of a cold gel pad + compression with an unchilled gel pad + compression found a trivial difference of 0.43 points on a 10‐point scale that favoured the cold gel pad + compression group within 24 and 48 hours of giving birth. Another placebo option was the use of a bag of water at room temperature, as the comparator to a bag of ice: the authors advised that no women experienced perineal pain in either group, thus providing no opportunity to evaluate this outcome further.
Looking at different methods of cooling, it remains uncertain if there was any evidence of an effect that favoured one form of cryotherapy over another on women's self‐reported perineal pain within 24 hours or 24 to 48 hours after giving birth.
Secondary outcomes
The evidence available is of very low certainty for most outcomes and so we are unable to determine the impact of different interventions on women's recovery from childbirth‐related perineal trauma. Women did not report different levels of perineal pain when they engaged in activities such as sitting, walking, urinating or caring for the baby, or exhaustion for any of the comparisons, for those trials that reported on this outcome. Nor did women appear to use additional analgesia differently following perineal cooling treatments, no treatments or placebos.
There was probably a reduction in perineal oedema following the use of cooling treatments compared with no treatment or a placebo within 24 to 48 hours after giving birth, and it is possible that this improved with the use of ice packs compared with cold gel pads (very low‐certainty evidence). Although not widely reported, there was very low‐certainty evidence of no difference in perineal bruising when applying or not applying cooling therapies. This was the case both for bruising used as a distinct outcome (Steen 2002) and as part of a composite scale on perineal status using the REEDA scale (Navvabi 2009).
Data on adverse effects on perineal wound healing were available for the comparisons between cooling and no treatment, cooling and placebo (unchilled gel pad), and ice pack compared with cold gel pad. Only one study provided very low‐quality certainty of evidence that there was probably less wound gaping five days after giving birth following the use of ice packs, compared with cold gel pads, with very wide 95% confidence intervals that suggest a very large potential effect (as little as 5% likelihood in the ice‐pack group) through to 1% more likelihood in the cold gel pad group: this trend did not appear to persist to day 10. Furthermore, none of the other wound‐healing indicators showed a difference between groups, influenced by the low numbers of those with adverse events and wide confidence intervals around the measures of effect. Two studies assessed wound gaping as a component of the REEDA scale. Extraction of this individual component of data for analysis could not be done, but the evidence from these studies did not suggest an overall difference in between‐group measures at 24 to 48 hours after giving birth.
Women generally rated their satisfaction and experiences with perineal care very favourably, regardless of the use of cooling, no treatment or placebo. When two cooling methods, ice packs and cold gel pads, were compared, however, there was limited very low‐certainty evidence that women preferred the cold gel pads over the ice packs: this finding needs to be interpreted cautiously, given that one of the investigators was the developer and patent‐holder of the gel pads used, as well as acknowledging the small sample sizes, lack of blinding and wide confidence intervals around the measures of effect. We considered the very low‐certainty evidence of a mean difference of −0.43 on a 10‐point scale when assessed between 24 and 48 hours after giving birth to be a trivial finding, favouring the use of a cooling treatment plus compression over some compression.
The ability of women to supply breast milk to their babies appeared not to be affected by any modalities of treatment.
No studies reported an economic analysis of the treatments.
What is the future for cryotherapy overall?
Safe dosage recommendations of cooling (Mac Auley 2001) appear to have been applied in the methodology of the trials in this review.
Literature investigating the effects of cryotherapy/cooling in sporting or post‐surgical environments describes a dose dependency (Bleakley 2006; Kuo 2013; Machado 2016). The same could be presumed for perineal trauma post‐childbirth, which highlights the need to look at a dose‐response relationship to tissue temperature and other outcome measures. In more recent studies (Beleza 2017; Morais 2016; Senol 2017; Yusamran 2007) we are beginning to see this taken into account, but assessment of this is beyond the scope of this current review. It appears there is now a pressing need for this dose‐dependency data to examine the cooling threshold or optimal window beyond which effectiveness can be seen while still reducing the risk of adverse effects.
Overall completeness and applicability of evidence
The studies enrolled relatively small numbers of women and may not represent all models of postnatal perineal care globally. The use of the combined principles of rest, ice, compression and elevation have not been well explored. Neither has there been adequate reporting of adverse effects from cooling treatments, that are not only important in this setting, but remain an area of concern with the use of cooling in the treatment of other injuries (Dubois 2020; Dushesne 2017; Singh 2017).
We await data from trialists that would allow inclusion of their findings for participants with perineal trauma and not for those with an intact perineum (Leventhal 2011; Oliveira 2012). Informal review of the published data for all women in these trials suggests that their ultimate inclusion would not change the overall findings of the review.
While ice is readily available in high‐income countries, this may or may not be the case in low‐middle income countries. Gel pads for cooling are suitable for use, cleaning and re‐use by only one woman, cost money to acquire and require access to a freezer: they are therefore available to many in high‐income countries and possibly less so in low‐middle income areas. The developer and patent‐holder has examined the effectiveness of cold gel pads in three trials (Steen 2000; Steen 2002; Navvabi 2009). Another commercially‐available cold gel pad was adapted for use in the trial undertaken by Yusamran 2007. There is limited certainty of the evidence for the safety and effectiveness of cold gel pads for the relief of perineal pain following childbirth.
Quality of the evidence
Included studies were generally at unclear to high risk of bias. Most of the outcomes assessed using GRADE had a rating of very low‐certainty evidence, with downgrading attributed to the high risk of bias associated with the inability to blind outcome assessments, with the participants providing data for many of the outcomes. One study had a very low sample size, being only a pilot study (n = 16; East 2007) and most of the remaining studies enrolled fewer than 100 women. Downgrading for imprecision was undertaken when there was high attrition and/or the confidence intervals included the null effect or were judged to be wide. Two trials were retrospectively registered (Beleza 2017; Navvabi 2009) and details of the registrations for Francisco 2018 and Morais 2016 are not publicly accessible online. There were very few outcomes examined by two or more trialists for each comparison, meaning that heterogeneity was limited and inconsistency of findings could not be judged.
Potential biases in the review process
The comprehensive search strategy was accompanied by rigorous screening, appraisal and data extraction methodology. Two other review authors performed data extraction and conducted the 'Risk of bias' assessments for the one study that was led by one of the review authors (East 2007). We contacted study authors to clarify uncertainties where at all possible. We therefore consider the risk of other biases in the review process to be low.
Agreements and disagreements with other studies or reviews
We are not aware of any other published systematic reviews or non‐randomised studies that have examined the potential for cooling treatments to relieve perineal pain following childbirth.
Authors' conclusions
Implications for practice.
There is limited very low‐certainty evidence to support the use of cooling treatments, in the form or ice packs or cold gel pads, for the relief of perineal pain in the first two days following childbirth. It is likely that concurrent use of several treatments is required to adequately address this issue, including prescription and non‐prescription analgesia (East 2011). Studies included in this review involved the use of cooling treatments for 10 to 20 minutes, and although no adverse effects were noted, these findings came from studies of relatively small numbers of women, or adverse events were not reported at all. The use of cooling therapies for the management of postnatal perineal pain is common practice and positively accepted by women, midwives, doctors and therapists alike. The continued lack of high‐certainty evidence of the benefits of cooling treatments should be viewed with caution, and further well‐designed trials should be conducted before we consider changing current clinical practices.
Implications for research.
The effectiveness of cooling treatments for relief of perineal pain may be better assessed in larger studies that report the treatment regimens and examine the findings according to parity, mode of birth and degree of perineal trauma. Outcome assessors should be blinded to group allocation for evaluation of oedema, bruising and adverse effects on wound healing. Studies that use cold gel pads would ideally be conducted by investigators who have no commercial interest in these devices and/or ensuring that personnel caring for the women and the women themselves are not aware of any such commercial arrangements when providing their ratings for the various outcomes. Future studies may further consider the effects of removing the cooling treatment after a set period of time, replacing the cooling device at regular intervals and considering potential adverse effects of prolonged cooling, along with examining the effects of incorporating rest, compression and 'elevation' (lying down, rather than sitting or standing).
Given the limited evidence to support the use of cooling treatments and the general lack of effective alternatives compared with cooling identified in this review, future studies may ethically consider the use of a no‐topical treatment group, to compare the potential effectiveness of agents that provide a greater degree of cooling that can be sustained for longer than current methods.
What's new
| Date | Event | Description |
|---|---|---|
| 7 October 2019 | New search has been performed | Search updated: 24 trial reports assessed for eligibility. Four new trials (nine reports) added to included studies. Six studies previously included are now excluded and one previously excluded is now included. We have updated the objectives and outcomes to reflect current methodologies: fewer outcomes were reviewed in fewer comparisons. The update includes 10 trials, excludes 15 trials and has two trials awaiting classification. |
| 7 October 2019 | New citation required but conclusions have not changed | The conclusions remained unchanged in this update, compared with previous versions of the review. |
History
Protocol first published: Issue 1, 2007 Review first published: Issue 4, 2007
| Date | Event | Description |
|---|---|---|
| 8 August 2012 | Amended | We have corrected the following errors. Error in misreporting Leventhal 2011a as having 23% missing data now removed from text. Error in stating that measured temperature may have been from the ice pack, when the review authors had not understood that this recording was taken after the ice pack was removed (Leventhal 2011a). Note that skin temperature measured by Leventhal 2011a was considerably less than normal temperature and can be challenged by other literature. As this discussion is beyond the scope of this review, we have removed the comment. |
| 1 February 2012 | New search has been performed | Search updated. Six new studies identified. Five have been included (Leventhal 2011a; Nawabi 2009a; Sheikhan 2011a; Thangaraju 2006a; Yasumran 2007a). One trial is ongoing (Sao Paulo 2011). This updated review is now comprised of 10 included studies (involving 1825 women). The conclusions have not changed. |
| 1 February 2012 | New citation required but conclusions have not changed | Updated. |
| 5 October 2011 | Amended | Search and review updated |
| 10 November 2008 | Amended | Contact details updated. |
| 18 February 2008 | Amended | Converted to new review format. |
| 11 July 2007 | New citation required and conclusions have changed | Substantive amendment |
Notes
Nil
Acknowledgements
We acknowledge Mary Steen and Michelle Briggs for a previous version of the protocol, which was also authored by Paul Marchant. We thank Philippa Middleton for her helpful assistance in compiling the protocol and the original review.
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser. The authors are grateful to the following peer reviewer for her time and comments: Andrea Deussen, Adelaide Medical School, Robinson Research Institure, The University of Adelaide, Australia.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
Appendices
Appendix 1. Search methods for ICTRP and ClinicalTrials.gov
ICTRP
cooling AND episiotomy
cooling AND perineum
ice AND episiotomy
ice AND perineum
cryotherapy AND episiotomy
cryotherapy AND perineum
ClinicalTrials.gov
Advanced search
Interventional Studies | Episiotomy | cryotherapy
episiotomy | Interventional Studies | cooling
perineum | Interventional Studies | cooling
perineum | Interventional Studies | ice
episiotomy | Interventional Studies | ice
Appendix 2. Methods used in the previous version of this review and changed for the 2020 update
Objective
To evaluate the effectiveness and side effects of localised cooling treatments compared with: (i) no treatment; (ii) other cooling treatments; and (iii) non‐cooling treatments applied to the perineum following perineal trauma sustained during childbirth. To meet this objective, we examined the effect of these treatments on pain, bruising and oedema and considered how each of these affected activities such as daily living, breastfeeding and attending to baby. We also considered other factors, including depression and women's views of and experience with treatments for perineal pain relief.
Outcomes
Primary outcomes (1) Perineal pain, as measured by the trial authors, at the following time periods (or as close to the time period as possible): ‐ within four to six hours of giving birth; ‐ within 24 hours of giving birth; ‐ between 24 and 72 hours of giving birth; ‐ between three and 14 days after giving birth; ‐ three months after giving birth.
(2) Perineal pain, as measured by the trial authors, associated with activities of daily living (for example, sitting, walking, urinating, caring for baby) at the following time periods (or as close to the time period as possible): ‐ within four to six hours of giving birth; ‐ within 24 hours of giving birth; ‐ between 24 and 72 hours of giving birth; ‐ between three and 14 days after giving birth; ‐ three months after giving birth.
(3) Painful sexual intercourse at three months postpartum.
(4) Additional analgesia for relief of perineal pain: ‐ need for and timing of additional analgesia in hospital; ‐ need for and type of additional analgesia after discharge from hospital.
(5) Perineal oedema, as measured by the study authors, at the following time periods (or as close to the time period as possible): ‐ within four to six hours of giving birth; ‐ within 24 hours of giving birth; ‐ between 24 and 72 hours of giving birth; ‐ between three and 14 days after giving birth.
(6) Perineal bruising, as measured by the study authors, at the following time periods (or as close to the time period as possible): ‐ within four to six hours of giving birth; ‐ within 24 hours of giving birth; ‐ between 24 and 72 hours of giving birth; ‐ between three and 14 days after giving birth.
(7) Adverse effects on perineal healing, as measured by the study authors.
(8) Side effects severe enough to discontinue treatment.
(9) Cost of treatment.
(10) Women breastfeeding at: ‐ discharge from postpartum care; ‐ six weeks postpartum.
(11) Adverse effects on mother‐baby interactions, as measured by the study authors.
(12) Maternal views and experiences with treatment, as measured by the study authors.
(13) Maternal length of postnatal stay.
(14) Effects on maternal quality of life, as measured by the study authors.
(15) Women with postnatal depression.
(16) Maternal exhaustion, as measured by the study authors, at the following time periods (or as close to the time period as possible): ‐ within 24 hours of giving birth; ‐ between 24 and 72 hours of giving birth; ‐ between three and 14 days after giving birth; ‐ three months after giving birth.
Data and analyses
Comparison 1. Cooling treatment (ice pack or cold gel pad) versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Perineal pain within 4‐6 hours of giving birth | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐4.46 [‐5.07, ‐3.85] |
| 1.2 Perineal pain within 24 hours of giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Moderate + severe pain | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.29] |
| 1.3 Perineal pain within 24 hours of giving birth | 3 | 166 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐1.78, 0.95] |
| 1.4 Perineal pain between 24 ‐ 48 hours after giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 Moderate + severe pain | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.57, 0.94] |
| 1.5 Perineal pain between 24 ‐48 hours after giving birth | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.45, 0.39] |
| 1.6 Pain associated with activities of daily living (sitting) within 24 hours of giving birth | 1 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.98, 1.09] |
| 1.7 Pain associated with activities of daily living (sitting) between 24 ‐ 48 hours of giving birth | 1 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.95, 1.05] |
| 1.8 Pain associated with activities of daily living (walking) within 24 hours of giving birth | 1 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.94, 1.08] |
| 1.9 Pain associated with activities of daily living (walking) between 24 ‐ 48 hours of giving birth | 1 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.09] |
| 1.10 Pain associated with activities of daily living (feeding baby) within 24 hours of giving birth | 1 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.84, 1.55] |
| 1.11 Pain associated with activities of daily living (feeding baby) between 24 ‐ 48 hours of giving birth | 1 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.63, 1.21] |
| 1.12 Perineal oedema within 24 hours of giving birth | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.87, 1.16] |
| 1.13 Perineal oedema between 24 ‐ 48 hours after giving birth | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 1.14 Perineal bruising within 24 hours of giving birth | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.81, 1.19] |
| 1.15 Perineal bruising between 24 ‐ 48 hours after giving birth | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.97, 1.32] |
| 1.16 Perineal redness, oedema, bruising, discharge, wound gaping within 24 hours of giving birth | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐1.14, 0.38] |
| 1.17 Perineal redness, oedema, bruising, discharge, wound gaping between 24 ‐ 48 hours of giving birth | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐1.19 [‐2.07, ‐0.31] |
| 1.18 Additional analgesia for relief of perineal pain: within 24 hours of giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.18.1 Non‐prescription analgesia | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.60, 1.24] |
| 1.18.2 Prescription analgesia | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.82, 1.89] |
| 1.19 Additional analgesia for relief of perineal pain between 24 ‐ 48 hours after giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.19.1 Non‐prescription analgesia | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.58, 1.27] |
| 1.19.2 Prescription analgesia | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.87, 2.08] |
| 1.20 Adverse effects on perineal wound healing: Day 5 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.20.1 Wound not healing | 1 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.34, 4.58] |
| 1.20.2 Wound edges gaping | 1 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [0.58, 11.33] |
| 1.20.3 Any sign of infection | 1 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.12, 1.82] |
| 1.21 Adverse effects on perineal wound healing: Day 10 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.21.1 Wound not healing | 1 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.20, 2.42] |
| 1.21.2 Wound edges gaping | 1 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.22, 1.74] |
| 1.21.3 Any sign of infection | 1 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.52, 10.42] |
| 1.21.4 Wound resutured | 1 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.34 [0.11, 48.25] |
| 1.22 Maternal views and experiences of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.22.1 Satisfaction with overall perineal care (good + very good + excellent) Day 10 | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.97, 1.18] |
| 1.23 Maternal views and experiences of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.23.1 Psychospiritual comfort within six hours of giving birth | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.20, 0.00] |
| 1.23.2 Sociocultural comfort | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.09, 0.11] |
| 1.24 Women providing any breastmilk to baby 24 ‐ 48 hours after giving birth | 1 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.73, 1.07] |
| 1.25 Subgroup: Parity and perineal pain within 24 hours of giving birth | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.25.1 Moderate + severe pain: primiparous women | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.76, 1.21] |
| 1.25.2 Moderate + severe pain: multiparous women | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.74, 2.16] |
| 1.26 Subgroup: Parity and perineal pain between 24 ‐ 48 hours after giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.26.1 Moderate + severe pain: primiparous women | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.50, 0.84] |
| 1.26.2 Moderate + severe pain: multiparous women | 1 | 115 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.59, 1.80] |
| 1.27 Subgroup: Mode of birth and perineal pain within 24 hours of giving birth | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.27.1 Moderate + severe perineal pain: spontaneous vaginal birth | 1 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.87, 1.60] |
| 1.27.2 Moderate + severe pain: assisted vaginal birth | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.56, 1.00] |
| 1.28 Subgroup: Mode of birth and perineal pain between 24 ‐ 48 hours after giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.28.1 Moderate + severe pain: spontaneous vaginal birth | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.52, 1.02] |
| 1.28.2 Moderate + severe pain: assisted vaginal birth | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.50, 0.93] |
Comparison 2. Cooling treatment (cold gel pad)+compression versus placebo (gel pad+compression).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Perineal pain within 4 ‐ 6 hours of giving birth | 1 | 250 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐0.78, 0.14] |
| 2.2 Perineal pain within 24 ‐ 48 hours after giving birth | 1 | 250 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.73, ‐0.13] |
| 2.3 Perineal oedema between 24 ‐ 48 hours after giving birth | 1 | 250 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.28, ‐0.03] |
| 2.4 Satisfaction with perineal care | 1 | 250 | Mean Difference (IV, Fixed, 95% CI) | 0.88 [0.38, 1.38] |
Comparison 3. Cooling treatment (ice pack) versus placebo (water pack).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Perineal oedema within 4 ‐ 6 hours after giving birth | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.50, 1.86] |
| 3.2 Perineal oedema within 24 hours after giving birth | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.08, 1.59] |
| 3.3 Additional analgesia for relief of perineal pain within 24 hours of giving birth | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.35, 1.11] |
| 3.4 Maternal views and experiences with treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.4.1 Satisfied with treatment | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.77, 1.08] |
| 3.4.2 Would repeat treatment in future childbirth | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.75, 1.04] |
| 3.4.3 Would recommend treatment | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.03] |
| 3.5 Women providing any breastmilk to baby 48 hours after giving birth | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.94, 1.06] |
Comparison 4. Cooling treatment (ice pack) versus cooling treatment (cold gel pad).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Perineal pain within 4 ‐ 6 hours of giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1.1 Moderate + severe pain | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.26, 1.24] |
| 4.2 Perineal pain within 24 hours of giving birth | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.2.1 Moderate + severe pain | 2 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.78, 1.22] |
| 4.3 Perineal pain within 24 hours of giving birth | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 0.58 [‐0.44, 1.60] |
| 4.4 Perineal pain between 24 ‐ 48 hours after giving birth | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.4.1 Moderate + severe pain | 2 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.89, 1.65] |
| 4.5 Perineal pain between 24 ‐ 48 hours of giving birth | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 0.86 [‐0.10, 1.82] |
| 4.6 Pain associated with activities of daily living (sitting) within 24 hours of giving birth | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.94, 1.03] |
| 4.7 Pain associated with activities of daily living (sitting) between 24 ‐ 48 hours after giving birth | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.92, 1.03] |
| 4.8 Pain associated with activities of daily living (walking) within 24 hours of giving birth | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.89, 1.04] |
| 4.9 Pain associated with activities of daily living (walking) between 24 ‐ 48 hours after giving birth | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.85, 1.03] |
| 4.10 Pain associated with activities of daily living (feeding baby) within 24 hours of giving birth | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.68, 1.28] |
| 4.11 Pain associated with activities of daily living (feeding baby) between 24 ‐ 48 hours after giving birth | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.73, 1.63] |
| 4.12 Perineal oedema within 4 ‐ 6 hours of giving birth | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.93, 2.09] |
| 4.13 Perineal oedema within 24 hours of giving birth | 2 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.84, 1.13] |
| 4.14 Perineal oedema between 24 ‐ 48 hours after giving birth | 2 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.03, 2.77] |
| 4.15 Perineal bruising within 4 ‐ 6 hours of giving birth | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.51, 2.97] |
| 4.16 Perineal bruising within 24 hours of giving birth | 2 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.79, 1.14] |
| 4.17 Perineal bruising between 24 ‐ 48 hours after giving birth | 2 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.92, 1.25] |
| 4.18 Perineal redness, oedema, bruising, discharge, wound gaping within 24 hours of giving birth | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.85, 0.59] |
| 4.19 Perineal redness, oedema, bruising, discharge, wound gaping between 24 ‐ 48 hours after giving birth | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.33, 0.73] |
| 4.20 Additional analgesia for relief of perineal pain: within 24 hours of giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.20.1 Non‐prescription analgesia | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.63, 1.51] |
| 4.20.2 Prescription analgesia | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.56, 1.31] |
| 4.21 Additional analgesia for relief of perineal pain: 24 ‐ 48 hours after giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.21.1 Non‐prescription analgesia | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.70, 1.84] |
| 4.21.2 Prescription analgesia | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.43, 1.05] |
| 4.22 Adverse effects on perineal wound healing: Day 5 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.22.1 Wound not healing | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.26, 3.93] |
| 4.22.2 Wound edges gaping | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 1.01] |
| 4.22.3 Any sign of infection | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.08 [0.50, 166.67] |
| 4.23 Adverse effects on perineal wound healing: Day 10 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.23.1 Wound not healing | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.09, 2.70] |
| 4.23.2 Wound edges gaping | 1 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.63, 14.81] |
| 4.23.3 Any sign of infection | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.45, 5.31] |
| 4.23.4 Wound resutured | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.06, 15.93] |
| 4.24 Maternal views and experiences with treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.24.1 Opinion on treatment effects (good + very good + excellent) Day 5 | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.17, 0.68] |
| 4.24.2 Satisfaction with overall perineal care (good + very good + excellent) Day 10 | 1 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.73, 0.92] |
| 4.25 Women providing any breastmilk to the baby 48 hours after giving birth | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.84, 1.33] |
| 4.26 Subgroup: Parity and perineal pain within 24 hours of giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.26.1 Moderate + severe pain: primiparous women | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.68, 1.18] |
| 4.26.2 Moderate + severe pain: multiparous women | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.69, 2.05] |
| 4.27 Subgroup: Parity and perineal pain between 24 ‐ 48 hours after giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.27.1 Moderate + severe pain: primiparous women | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.72, 1.57] |
| 4.27.2 Moderate + severe pain: multiparous women | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.78, 3.02] |
| 4.28 Subgroup: Mode of birth and perineal pain within 24 hours of giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.28.1 Moderate + severe pain: spontaneous vaginal birth | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.68, 1.26] |
| 4.28.2 Moderate + severe pain: assisted vaginal birth | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.66, 1.53] |
| 4.29 Subgroup: Mode of birth and perineal pain between 24 ‐ 48 hours after giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.29.1 Moderate + severe pain: spontaneous vaginal birth | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.83, 2.09] |
| 4.29.2 Moderate + severe pain: assisted vaginal birth | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.66, 1.67] |
Comparison 5. Cooling treatment (I.C.H. (ice, compression, (being) horizontal)) versus cooling treatment (ad hoc ice pack usage).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Perineal pain within 24 hours of giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1.1 Moderate + severe pain | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.49, 22.90] |
| 5.2 Perineal pain between 24 ‐ 48 hours after giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.2.1 Moderate + severe pain | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.20, 4.95] |
| 5.3 Perineal pain associated with activities of daily living (sitting) within 24 hours of giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.3.1 Moderate + severe pain | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.50 [0.72, 153.07] |
| 5.4 Perineal pain associated with activities of daily living (sitting) between 24 ‐ 48 hours after giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.4.1 Moderate + severe pain | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.05, 2.37] |
| 5.5 Perineal pain associated with activities of daily living (walking) within 24 hours of giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.5.1 Moderate + severe pain | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.49, 22.90] |
| 5.6 Perineal pain associated with activities of daily living (walking) between 24 ‐ 48 hours after giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.6.1 Moderate + severe pain | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.14, 1.77] |
| 5.7 Perineal pain associated with activities of daily living (urinating) within 24 hours of giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.7.1 Moderate + severe pain | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.50 [0.24, 85.12] |
| 5.8 Perineal pain associated with activities of daily living (urinating) between 24 ‐ 48 hours after giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.8.1 Moderate + severe pain | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.15, 61.74] |
| 5.9 Perineal pain associated with activities of daily living (caring for baby) within 24 hours of giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.9.1 Moderate + severe pain | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.03, 9.46] |
| 5.10 Perineal pain associated with activities of daily living (caring for baby) between 24 ‐ 48 hours after giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.10.1 Moderate + severe pain | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.06, 4.15] |
| 5.11 Additional analgesia for relief of perineal pain: within 24 hours of giving birth | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.31, 1.51] |
| 5.12 Additional analgesia for relief of perineal pain: between 24 ‐ 48 hours after giving birth | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.41, 1.56] |
| 5.13 Self‐reported energy level: within 24 hours of giving birth | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.11, 1.51] |
| 5.14 Self‐reported energy level: between 24 ‐ 48 hours of giving birth | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.92, 0.92] |
| 5.15 Maternal views of effectiveness of ice packs: within 24 hours of giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.15.1 Quite effective + extremely effective | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.11, 1.48] |
| 5.16 Maternal views of effectiveness of ice packs: between 24 ‐ 48 hours of giving birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.16.1 Quite effective + extremely effective | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.76, 1.83] |
| 5.17 Women providing any breastmilk to the baby between 24 ‐ 48 hours after giving birth | 1 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.31, 1.57] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barclay 1983.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Study setting: Woden Valley Hospital, Canberra, Australia Women admitted to the postnatal ward who had an episiotomy |
|
| Interventions | Of relevance to this review, the cooling intervention was an iced sitz bath: 30 g salt added to 1 ‐ 2 litres of cold tap water, to which the contents of a jug of ice were added by the woman, to help her control how quickly the temperature lowered. Women sat in the dish for 10 minutes, 3 ‐ 4 times daily (n = 40). The comparison group was hygiene only ("no treatment"): women were instructed in basic hygiene, including showering 3 times a day and drying the perineum carefully (n = 20). Of note, the investigators also considered the following interventions that were in common use at the time. The numbers of women in these groups are not counted in the overall numbers for this review: warm sitz bath (n = 30); ray lamp (n = 21) or witch hazel solution (n = 30) |
|
| Outcomes | Subjective perception of pain (scale of 1 = none to 5 = severe); objective assessment of healing, infection and analgesia usage | |
| Notes | Additional data (numbers for the graphs) and clarification of group assignment were sought by email in July 2006. The lead author responded and explained about the contamination of the groups but was unable to provide further data Dates of study: not stated Proposed number of participants: 120 Funding sources: none stated Declarations of interest: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "A four‐bed room was set aside for each method. Every woman with an episiotomy admitted to that room joined that particular method" (p16) |
| Allocation concealment (selection bias) | High risk | Unblinded. Women could request alternative treatments to those assigned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The intention was to have 30 women in each group, but 10 women from the no‐treatment group received iced sitz baths. The data were analysed by treatment received, rather than by intention to treat |
| Selective reporting (reporting bias) | Unclear risk | No protocol published |
| Other bias | High risk | Women had heard about the effectiveness or otherwise of treatments and requested them, hence 40 women were analysed for the iced sitz baths group, an additional 10 women than "allocated" to that group, who had been assigned to the hygiene‐only (usual treatment) group. Midwives also had a preference for the iced sitz baths, which contaminated the study groups. Findings were reported according to treatment used (i.e. "per protocol"), rather than treatment assigned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and midwives were not blinded to the treatment. Evidence of this resulting in bias was seen with women requesting to have the cold sitz baths |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Women rated their own pain. Staff made the remaining judgements of wound healing/infection. No mention is made of blinding a researcher to abstract the data from the woman's record |
Beleza 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Study setting: Reference Centre of Women's Health of Ribeirao Preto, Sao Paulo, Brazil Primiparous women over 18 years of age with a low‐risk pregnancy who are literate, have no cognitive defects within 6 ‐ 24 hours of having a vaginal birth with episiotomy and where anti‐inflammatory medication, if taken, has worn off by the time of assessment. |
|
| Interventions | Intervention: single perineal application of a bag of 150 g crushed ice for 20 minutes (n = 24) Control: "routine maternity care", being anti‐inflammatory analgesia on request (n = 26) |
|
| Outcomes | Self‐reported pain using a numerical rating scale (0 (least) to 10 (worst)): (i) pre‐randomisation; (ii) 20 minutes after the 1st evaluation; and (iii) 1 hour after the 2nd evaluation Intervention group only ‐ women's satisfaction with the cryotherapy |
|
| Notes | Authors clarified queries about: (i) access to analgesia post‐randomisation in each group: no women used analgesia during the treatment period (email 22 Dec 2019); and (ii) timing of the treatment and evaluations, given that recruitment could occur up to 24 hours after giving birth: authors advised (email 7 Jan 2020) that the mean (SD) time for recruitment was 15.92 hours (6.28) for the control group and 16.17 (6.37) hours for the intervention group Dates of study: July 2007 to January 2008 Funding sources: Ribeirao Preto School of Nursing of the University of Sao Paulo, Brazil Declarations of interest: "Nothing to declare" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computer‐generated randomisation list from www.randomization.com |
| Allocation concealment (selection bias) | High risk | The sole researcher accessed the randomisation group and acknowledged that they could have known the allocation prior to randomising the participant |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The consort flowchart suggests that outcome data were available for all participants, but the trial was registered 5 years after the study was undertaken, although prior to publication of the findings |
| Selective reporting (reporting bias) | High risk | The trial was registered 5 years after the study was undertaken and prior to publication of the findings |
| Other bias | Low risk | None noted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind the researcher or the participants. The researcher noted that this "... may have caused some bias related to the tendency of participants to please the physiotherapist" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The same researcher who undertook the group allocation and treatment also asked the women to rate their pain. The researcher noted that this "... may have caused some bias related to the tendency of participants to please the physiotherapist" |
East 2007.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Study setting: Royal Women's Hospital, Melbourne, Australia Women having a vaginal birth who are willing and able to give informed consent and sustained perineal trauma (episiotomy/tear) or labial tears during childbirth |
|
| Interventions | Intervention: ice pack, compression, being horizontal (I.C.H): an ice pack to the perineum for 20 minutes every 2 ‐ 3 hours for 2 ‐ 3 days, wear well‐fitting pads and underwear and lie down for 20 minutes (n = 7 in the pilot study) Control: ad‐hoc application of ice packs to the perineum (n = 9 in the pilot study) |
|
| Outcomes | Self‐reported perineal pain, perineal pain associated with activities of daily living, perineal bruising, perineal oedema, self‐reported energy level: rating 1 "much less than normal" to 5 "much more than normal", additional analgesic, impact of perineal pain on attending to baby, side effects of treatment within 24 hours; between 24 and 48 hours | |
| Notes | This is a trial registration document. The trial registration was initially registered on 30 October 2007 and was updated on 19 January 2016. A pilot study was undertaken to consider the feasibility of the protocol (n = 20 including intact perineum; n = 16 with perineal trauma and used in this review). The full study did not proceed due to lack of funding Additional information provided by author:
Dates of study: October 2008 to February 2009 Funding sources: funding not secured, therefore trial recruitment did not continue beyond the pilot study Declarations of interest: the lead author of this systematic review (CE) was the lead on this pilot RCT. This trial registry was reviewed by E Dorward and J Liu |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "A researcher not otherwise involved in the study will generate the sequence using block randomisation (1:1 control: intervention), stratified for number of children born (vaginally), mode of birth and degree of perineal trauma" |
| Allocation concealment (selection bias) | Low risk | Following informed consent and within the first 2 hours after giving birth, women are randomly allocated to 1 of the 2 treatment groups using consecutively numbered sealed, opaque envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The target sample size is 880 but actual recruitment is 20 (16 with perineal trauma). The full recruitment did not proceed due to funding issues The author did not have an opportunity to measure bruising or oedema in the pilot phase |
| Selective reporting (reporting bias) | Low risk | The author reported on all the data collected |
| Other bias | Unclear risk | None noted |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Women and clinicians were unblinded to treatment allocation, due to the nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participants self‐reported perineal pain. It was designed that a researcher assessing perineal oedema and bruising was blinded to group allocation, but data on perineal bruising or oedema were not collected |
Francisco 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Study setting: Midwfie‐led, alongside, no‐profit birth centre, Sao Paulo City, Brazil. Inclusion criteria: women aged 18 years or older, no previous birth and within 6 ‐ 24 hours of a normal vaginal birth following full‐term, singleton pregnancy with cephalic presentation. Perineal pain score ≥ 3 on numeric rating scale (0 ‐ 10) and having no analgesia for 3 hours prior to randomisation Exclusion criteria: 3rd or 4th degree perineal trauma, perineal haematoma, perineal oedema Post‐randomisation exclusion: analgesia within 2 hours of randomisation. |
|
| Interventions | Intervention group: ice pack: water‐filled plastic bag frozen and wrapped in thin cotton gauze. Applied to perineum with participant lying down. (n = 35) Control group: no treatment. (n = 35) Women in the control group could use an ice pack after the evaluation time frame |
|
| Outcomes | Perineal pain, perineal temperature | |
| Notes | Email correspondence 23 December 2019 and 8 January 2020 requesting data on women without intact perineum (tears and episiotomy only). On 10 January 2020, authors advised data will be provided as soon as possible. No data received by 29 January 2020 3 Mar 2020, additional data provided by author for perineal pain before, immediately after and 2 hours post‐ice pack application for participants with perineal trauma only 8 July 2020, authors confirmed that women were not able to use oral analgesia (routine treatment) in either the intervention or control groups during the follow‐up period. Both groups of women were able to access oral analgesia after the follow‐up period 8 July 2020, authors confirmed trial was registered at start of recruitment and that website detail currently unavailable (therefore noted to be "personal communication" rather than a referenced publication); confirmed that no protocol was published Dates of study: December 2012 to February 2013 Funding sources: Sao Paulo Research Foundation, National Council for Scientific and Technological Development Declarations of interest: "The authors declare no conflicts of interest to [disclose]" p.e340 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study used computer‐generated random sampling |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, sealed and opaque envelopes prepared by a professional not involved in the research |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In the original research paper, the outcome group data were reported for 69 out of 70 participants. Additional information received 3 March 2020 provide data for the 45 participants reported to have perineal trauma. However, the trial registration is not available online and no protocol was published |
| Selective reporting (reporting bias) | Unclear risk | The trial registration details are not publicly available online at 8 July 2020 and authors confirm no protocol published |
| Other bias | Low risk | None noted |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were unblinded, due to the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Women assessed their pain and as such were unblinded to treatment. The person who assessed perineal temperature was blinded to the kind of intervention that has been delivered to the women. To ensure this, she was not allowed to enter the maternity ward until the end of ice pack application and the participants were instructed not to comment about the group to which they were assigned |
Morais 2016.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Study setting: low‐risk maternity ward at Instituto de Medicina Integral Professor Fernando Figueira (IMIP), Recife, Pernambuco, Brazil Inclusion criteria: singleton pregnancy, cephalic presentation, normal vaginal birth between 37 ‐ 42 weeks’ gestation Exclusion criteria: received intrapartum analgesia, episiotomy, assisted vaginal birth, uterine curettage, active perineal haemorrhage, previous perineal injury before giving birth |
|
| Interventions | Intervention: cryotherapy group: latex glove filled with crushed ice, wrapped in wet surgical dressing, applied for 20 minutes with ice added as necessary to maintain the temperature of the perineal body at 10 ◦C to 15 ◦C, with 60 minutes between applications. First application at 2 hours after giving birth and repeated 6 times, with perineal temperature measured at zero, 10 and 20 minutes Total number randomised: 40 including intact perineum (28 with perineal trauma ‐ data used for this review) Placebo group: latex medical glove filled with water at 20 ◦C to 25 ◦C, wrapped in wet surgical dressing, applied for 20 minutes with 60 minutes between applications. First application at 2 hours after giving birth and repeated 6 times, with perineal temperature measured at zero, 10 and 20 minutes Total number randomised: 40 including intact perineum (35 with perineal trauma ‐ data used for this review) |
|
| Outcomes | Primary outcome: perineal pain, measured using the "Combined scale for assessing pain", which combines the use of a numeric scale of zero (no pain) to 10 (extreme pain) and a faces scale: results between 0 and 5 were analysed as none/mild while values between 6 and 10 were coded as moderate/severe. Other outcomes included perineal oedema, use of additional analgesia and adverse effects of cryotherapy. Not all data could be obtained for women with perineal trauma only. The authors provided additional unpublished data for maternal views of treatments and provision of breast milk for feeding baby | |
| Notes | Additional information sought and provided by authors for:
Dates of study: May to October 2012 Source of funding: Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) Declarations of interest: not noted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Notes from main publication and as clarified by the authors 28 January 2020: Random Allocation Software, version 1.0 used to generate list of random numbers by a collaborator who was not involved in data collection. Sealed numbered envelopes then created |
| Allocation concealment (selection bias) | Low risk | Authors clarified (28 January 2020) envelope only opened after participant consent signed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data available for all those enrolled, but it is unclear whether or not the trial was registered prior to undertaking the study |
| Selective reporting (reporting bias) | Unclear risk | The trial registration was referenced in the main RCT publication, but although the website is visible, the registration detail is not available publicly. The authors provided a copy of the registration download by email and advise that it was registered prior to seeking ethical approval for the study |
| Other bias | Low risk | No other bias noted |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind the participants fully or the researcher applying the treatment, although for both, the glove was wrapped in wet surgical dressings to “… disguise the treatments being provided” p 327 (quote) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Women (unblinded) rated their pain. The researcher who obtained the outcome data was blinded to group allocation. The same researcher was retained for the duration of the study |
Navvabi 2009.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Study setting: Mashhad, Iran 121 participants Inclusion criteria: Iranian nationality; primiparous; age 16 ‐ 35 years; lives with a partner; ability to read and write (Farsi) at level of 5th primary; term, singleton fetus 37 to 42 weeks; birthweight 2500 ‐ 4000 g; normal delivery; mediolateral episiotomy; have an address and phone number. Exclusion criteria: medical or obstetric complications; smoker; alcohol or cigarette consumption; use of perineal stretching or massage during pregnancy or labour; use of lubricant during delivery; second stage longer than 120 minutes; retained placenta; postpartum haemorrhage; haematoma; stillbirth; fetal anomaly; admission to neonatal intensive care unit All participants birthed in lithotomy, had an episiotomy, repaired with chromic catgut. All received oral analgesia within 4 hours of the episiotomy repair and self‐administered further doses of oral analgesia |
|
| Interventions | Randomisation within 4 hours of episiotomy repair Ice pack group (n = 40): the report implies that this was a one‐off application. No details provided about duration of application. Ice packs measured 12 x 5 cm, filled with water Gel pad group (n = 41): the report implies that this was a one‐off application. No details provided about duration of application. Gel pads contained propylene glycol (antifreeze), measured 23 x 5 x 1.5 cm (Femepad, Florri‐Femé Pharmaceuticals Ltd, UK) No treatment group (n = 40). |
|
| Outcomes | Self‐reported perineal pain on a numeric rating scale (NRS), 0 (no pain) to 10 (worst pain) Use of analgesia on day 10 Women's satisfaction with oral analgesia and with local treatment (e.g. ice pack or gel pad) on day 10 (NRS) Wound healing: REEDA scale of redness, oedema, ecchymosis (i.e. bruising), discharge and approximation of skin edges. Categories are assigned a total score of 0 to 15, with higher scores representing increased tissue trauma The influence of pain on activities such as sitting, urination and holding the baby were rated on a scale of 0 to 10 |
|
| Notes | Study data were provided to us by the author in 2007. At that time, we elected to await inclusion in the review until the publication was available. The findings were published in 2009 and 2011 and were included in the 2012 update of this Cochrane Review. Where data were unavailable from the publication and previously‐provided data aligned with the published data, data missing from the 2009 publication were incorporated (to correct the typesetting error in Table 1 of the publication and to obtain the SD of women's satisfaction with perineal care) Dates of study: October 2005 to February 2006 Funding sources: Mashhad University of Medical Science Declarations of interest: none stated. However, one of the study authors (M Steen‐Greaves) holds the patent for the cold gel pad used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Flow chart of eligible and enrolled women was provided. Data were reported for 110 of the 121 women randomised. However, the trial was registered 5 years after completing recruitment |
| Selective reporting (reporting bias) | High risk | The trial was registered 5 years after recruitment was completed |
| Other bias | Unclear risk | None identified. It remains unknown if having one of the authors being the developer and patent‐owner for the gel pads contributed bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were unblinded, due to the nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Women (unblinded) self‐assessed their pain and satisfaction. Outcome assessors (for the REEDA scale) were blinded, but it was noted that it may have been possible for the assessors to see the ice pack or gel pad in the woman's hospital room. One of the trial authors holds a patent on the gel pad: it is unclear if women may have known this and unconsciously influenced their outcome ratings |
Senol 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Study setting: Mersin Obstetrics and Gynecology Hospital, Turkey Women aged ≥ 18 years, having 1st, 2nd or 3rd baby, singleton pregnancy, cephalic presentation, gave birth vaginally more that 1 hour previously, at term gestation, baby healthy, not receiving oral analgesia within 4 hours of giving birth Data included in this review only include the 100 primiparous women (all reported to have had an episiotomy in the published article (subsequently revealed to be 49 of the 50 women in each group)). Data for the 78 multiparous women with a tear or episiotomy 78 (of the 100 enrolled) were not available for analysis separately and were not included in this review |
|
| Interventions | Intervention group (50 primiparous, 50 multiparous): cold gel pad wrapped in sterile pad. Applied for 20 minutes within 2 hours of giving birth and again 4 hours after the initial application Control group (50 primiparous, 50 multiparous): hygienic absorbent maternity pad |
|
| Outcomes | Prior to and immediately following each of the applications
Cold gel pad group: side effects only |
|
| Notes | Email sent 6 January 2020 requesting clarification of perineal status at enrolment Responses received 30 January 2020. Some relevant detail provided on outcomes, but no data for multiparous women with perineal trauma only. Further email 31 January requesting details of personnel preparing the random sequence generation and sealed randomisation envelopes, and whether the latter were opaque or sequentially‐numbered. Response 7 February advised that the researchers prepared the random‐numbers table and the envelopes. They noted that individuals did not know which group they would be allocated to in advance Numbers reported in this review only relate to the 100 primiparous. If the separate data for multiparous women with perineal trauma become available, this detail will be considered in a future update of this review Dates of study: 2013 Funding sources: none declared, although this could be available information in the Turkish thesis Declaration of interest: “The authors declared no potential conflicts of interest with respect to research, authorship, and/or publication of this article” p 281 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random‐number table generated from randomizer.org. The authors clarified that this was prepared by the researchers |
| Allocation concealment (selection bias) | Unclear risk | Group allocation by sealed envelopes: pink for primiparous women (n = 100) and blue for multiparous women (n = 100). Authors advised that the researchers prepared the envelopes and numbered them and that the individuals participating in the study did not know which group they would be in before opening the envelope |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No evidence to suggest incomplete data. Authors confirmed no published trial registration or protocol; this would normally have been expected for CONSORT reporting standards for a trial published at this time. Detail in the thesis aligns with the numbers in the publication |
| Selective reporting (reporting bias) | Unclear risk | Authors confirmed no published trial registration or protocol; this would normally have been expected for CONSORT reporting standards for a trial published at this time. Detail in the thesis aligns with the outcomes in the publication |
| Other bias | Unclear risk | No evidence of other bias. This cannot be fully assessed as there was no published protocol or trial registration |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were unblinded, due to the nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Women and clinicians reporting outcomes were unblinded, due to the nature of the treatment |
Steen 2000.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 120 participants
Study setting: St James University Hospital, Leeds, UK Inclusion criteria: women, 20 ‐ 35 years, English‐speaking, primigravid, term fetus, cephalic presentation, instrumental delivery, episiotomy sutured with Vicryl Exclusion criteria: any medical disorder, retained placenta, multiple pregnancy |
|
| Interventions | Cooling treatment 1: ice packs (n = 38). Normal saline sachets frozen prior to use, covered with sterile gauze prior to use Cooling treatment 2: cold gel pads (n = 40). Developed by a midwife and an obstetrician specifically for the trial. High thermal capacity cellulose‐based gel plus propylene glycol anti‐freeze within a heat‐welded soft plastic sachet. Measured 5 x 23 x 1.5 cm. Frozen prior to use. Reusable by the individual. Covered in sterile gauze before use Women chose (i) the initial time of application, within 4 hours of suturing; and (ii) how many times treatment was reapplied for up to 48 hours after suturing Unpublished information from the authors noted that gel pad groups took 20 minutes to warm to perineal temperature, compared with ice packs, which melted more quickly Of note, a 3rd group used pramoxine/hydrocortisone topical aerosol foam (Epifoam) (n = 42), a steroidal anti‐inflammatory. Foam placed on sterile gauze and applied directly to perineum. These numbers are not counted in the review |
|
| Outcomes | Perineal oedema and bruising assessed within 4 hours of suturing and at 24 and 48 hours. Wherever possible, the same midwife made the assessments for each woman. Perineal healing assessed at 5 and 10 days after giving birth. Pain self‐assessed using 10‐point visual analogue scale within first 4 hours, then at 24 and 48 hours, with assistance from the midwife assessors, then at 5 days by community midwives. At day 5, the community midwives asked the women to complete a 5‐point rating of their opinions of the benefits of treatments | |
| Notes | 12 experienced, hospital‐based midwives underwent training in the use of a visual assessment tool for oedema and bruising, with significant inter‐rater reliability
Perineal pain assessed by 10‐point visual analogue scale, analysed as continuous numerical data. Women rated benefits of treatment on a 5‐point ordinal rating scale Dates of study: September 1993 to March 1994 Funding sources: Elizabeth Clark Charitable Trust Declarations of interest: although not written specifically as declarations of interests, the report clearly states that 2 of the authors "developed and manufactured 100 maternity gel pads for the trial" (p. 50), when they were unable to have these produced by a medical company |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by a specially‐commissioned software programme for the delivery suite computer (implies appropriate random sequence generation) |
| Allocation concealment (selection bias) | Low risk | Randomisation by a specially‐commissioned software programme for the delivery suite computer |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data sheets containing all details and outcomes for each woman were initiated in hospital and sent to community midwives for follow‐up of the 78 women in their homes; if the data sheet was not returned, all details for that participant were unavailable for analysis. With further post‐randomisation refusals by participants and exclusions (on advice received at the time of analysis), the overall attrition was 29/78 (37.2%): Ice pack group: 16/38 (42%); 10 data sheets not returned, 2 refusals, 4 exclusions Gel pad group: 13/40 (32.5%); 9 data sheets not returned, 4 exclusions Information was sought and provided about the length of time that ice packs or gel pads were in place |
| Selective reporting (reporting bias) | Unclear risk | The publication noted that wound healing was assessed, but no detail was provided on this outcome This cannot be fully assessed as there was no published protocol or trial registration |
| Other bias | Unclear risk | This cannot be fully assessed as there was no published protocol or trial registration. Potential but unknown impact of author developing the gel pad |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Women self‐evaluated pain and it is unclear whether the novelty of the gel pad, or potential knowledge that the lead investigator designed it, could have influenced this, given that the report also notes that midwives were asked by non‐participants if they could use the gel pad Evaluation of perineal oedema and bruising was made by trained midwife assessors, with 'every attempt' made to blind them to treatment allocation |
Steen 2002.
| Study characteristics | ||
| Methods | Randomised controlled trial Intention‐to‐treat analysis. | |
| Participants | 450 participants
Study setting: St James University Hospital, Leeds, UK Inclusion criteria: women aged 16 ‐ 45 years, English‐speaking, cephalic presentation, term, singleton fetus, normal or instrumental birth, episiotomy or second degree perineal tear sutured with Vicryl Rapide |
|
| Interventions | Ice pack group (n = 150): normal saline sachets frozen, covered with sterile gauze prior to use Cold gel pad group (n = 150): maternity gel pads developed for the trial by a midwife and an obstetrician. Made from high thermal capacity cellulose‐based gel plus propylene glycol anti‐freeze, within a heat welded soft plastic sachet. Frozen prior to use and reusable by the individual Covered with sterile gauze prior to use No‐treatment group (n = 150): no application of ice packs or gel pads All groups bathed and used additional analgesia as required |
|
| Outcomes | Self‐assessed pain, midwives' assessments of bruising, oedema, wound healing; maternal satisfaction | |
| Notes | Additional information was sought and provided by the authors about blinding of assessors, whether the initial assessment was undertaken prior to treatment allocation and rates of wound healing. Raw data were sought and provided in 2007: permission was sought and confirmed 7 February 2020 for continued/further analysis of these data. This allowed calculation of event rates for perineal pain for the subgroup analyses, pain that interfered with breastfeeding, breastfeeding, perineal wound‐healing outcomes, frequency of cooling treatment usage Dates of study: August 1998 to June 1999 Funding sources: Executive Northern and Yorkshire Regional fellowship and a Smith and Nephew Nursing Foundation fellowship Declarations of interest: M Steen has a patent for the gel pad device. At the time of reporting the study, no royalties had been received from this patent |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, block size 15. |
| Allocation concealment (selection bias) | Low risk | Randomisation by a specially commissioned software programme for the delivery‐suite computer during data entry of delivery details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data sheets containing all details and outcomes for each woman were initiated in hospital and sent to community midwives for follow‐up of the 450 women in their homes; if the data sheet was not returned, all details for that participant were unavailable for analysis. With further post‐randomisation withdrawals, the overall attrition was 136/450 (30.2%): Ice pack group: 44/150 (29.3%); 43 data sheets not returned and 1 withdrawal Gel pad group: 43/150 (28.7%); 42 data sheets not returned and 1 withdrawal No treatment group: 49/150 (32.7%); 49 data sheets not returned |
| Selective reporting (reporting bias) | Unclear risk | This cannot be assessed as there was no published protocol or trial registration |
| Other bias | Unclear risk | None identified. This cannot be fully assessed as there was no published protocol or trial registration. M Steen has a patent for the gel pad device. At the time of reporting the study, no royalties had been received from this patent and it remains unknown if this contributed any bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were unblinded. The lead investigator holds a patent on the gel pad: it is unclear if women may have known this and unconsciously influenced their outcome ratings of perineal pain and satisfaction with perineal treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details were provided of blinding of outcome assessors (wound healing) |
Yusamran 2007.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 250 participants Study setting: Siriraj Hospital, Bangkok, Thailand Inclusion criteria: 1st delivery, over 37 weeks' gestation, normal labour, mediolateral episiotomy (2nd degree), sutured with subcuticular catgut, received 10 ‐ 20 mL 1% lignocaine Exclusion criteria: antepartum, intrapartum or postpartum complications, including: temperature 38 °C, serious perineal trauma (as judged by the obstetrician), vaginal infection before delivery, antepartum or intrapartum antibiotics, instrumental delivery, 2nd stage > 60 minutes, problems with cold treatment (e.g. poor circulation), physical condition that could impact on perineal recovery (e.g. malnutrition, anaemia, diabetes), postpartum haemorrhage, manual removal of placenta, known sensitivity to lignocaine |
|
| Interventions | Intervention group: compression with cold gel pad (n = 125) ‐ placed in freezer Control group: compression with gel pad (n = 125) Gel pads were modified from existing 11 x 25 cm to 11 x 9 cm (edge sealed by hot air banding equipment) and inserted in a cotton pack that was then suspended from a belt, to provide compression |
|
| Outcomes | Self‐reported pain on visual analogue scale, analysed on the scale of zero to 10 (most painful), at 15, 30, 45, 60 minutes (before and after application), prior to leaving delivery suite and 48 hours after delivery REEDA scale (redness, edema, ecchymosis, discharge, approximation) with results provided for each category and the total score Satisfaction with compression on visual analogue scale 0 ‐ 10 (10 most satisfied) |
|
| Notes | We contacted the authors to clarify the results of pain at 2 days, given an apparent typographical error in the published report. Data included in the review are the corrected data, provided by the authors 16 June 2011 Dates of study: August 2005 to April 2006. Funding sources: not noted Declarations of interest: not noted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table prepared by an off‐site research associate |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were presented for 249 ‐ 250 of the 250 reported to be enrolled |
| Selective reporting (reporting bias) | Unclear risk | This cannot be assessed as there was no published protocol or trial registration |
| Other bias | Unclear risk | None identified. This cannot be fully assessed as there was no published protocol or trial registration |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were unblinded. It is unclear whether or not personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amani 2015 | Compared cooling treatment with topical olive oil (not placebo, no treatment or another cooling treatment) |
| De Souza Bosco Paiva 2016 | Not a randomised trial |
| Dube 2013 | Not a randomised trial; had noted "quasi experimental approach" in abstract |
| Gallie 2003 | Compared cooling treatment with pulse electromagnetic energy (not placebo, no treatment or another cooling treatment) |
| Hill 1989 | Compared cooling treatment with warm perineal pack or warm sitz bath (not placebo, no treatment or another cooling treatment) |
| Kaur 2012 | Trial registration for a study comparing cooling treatment with warm black tea bags (not placebo, no treatment or another cooling treatment) |
| LaFoy 1989 | Cross‐over trial. This was included in the original 2007 review: the 2012 update changed to exclude cross‐over trials |
| Lu 2015 | Not randomised. Women chose the group they wished to participate in. Had "quasi‐randomised controlled trial" in title |
| Moore 1989 | Compared cooing treatment with Epifoam or Hammamelis water (not placebo, no treatment or another cooling treatment) |
| Nam 1991 | Cross‐over trial. The 2012 update changed to exclude cross‐over trials |
| NCT02024256 | Compared pads soaked in cold water (potentially a cooling treatment) with pads soaked in magnesium sulfate solution (not placebo, no treatment or another cooling treatment) |
| Pinkerton 1961 | This letter to the Editor described the use of an ice‐pack and is not a randomised controlled trial |
| Ramler 1986 | Cross‐over trial. The update of this review in 2012 now excludes cross‐over trials |
| Sheikhan 2011 | Compared cooing treatment with warm sitz bath + povidone iodine (not placebo, no treatment or another cooling treatment) |
| Thangaraju 2006 | Compared cooling treatment with regular oral analgesia (not placebo, no treatment [e.g. routinely available analgesia by request, rather than prescribed regularly] or another cooling treatment) |
Characteristics of studies awaiting classification [ordered by study ID]
Leventhal 2011.
| Methods | Randomised controlled trial |
| Participants | 117 participants Inclusion criteria: maternal age 18 years or older, full‐term, nulliparous, live, singleton fetus, cephalic presentation, perineal pain score of over 3 on a numeric scale, within 2 to 48 hours of giving birth, no analgesia in the 2 hours prior to randomisation Exclusion criteria: pain score of 1 or 2; 2 or more births; maternal age less than 17 years |
| Interventions | Ice pack group (n = 38): plastic sack 8 x 16 cm, filled with 250 mL water, then frozen and wrapped in fine cotton cloth Placebo group (n = 38): plastic sack 8 x 16 cm, filled with 250 mL water, wrapped in fine cotton cloth No treatment group (n = 38). No ice or water pack applied |
| Outcomes | Perineal pain by numeric rating scale (NRS), 0 = no pain, to 10, worst pain: (1) prior to intervention; (2) 20 minutes after intervention; (3) 40 minutes after intervention; (4) 60 minutes after intervention Perineal temperature recorded every 5 minutes during the application of the ice pack or water pack |
| Notes | Authors contacted 9 January 2020 seeking data only from women with some degree of perineal trauma (publication includes intact perineum). Response 16 January 2020 from corresponding author that she does not have access to the data since retiring. No other authors' contact details yet identified Study location: Amparo Maternal, Brazil Additional information extracted from trial registration details (including inclusion and exclusion criteria) Dates of study: January and February 2008 Funding sources: Research Support Foundation of the Sao Paulo state (FAPESP), Brazil Declarations of interest: "The authors have no conflicts of interest to disclose" p.146 |
Oliveira 2012.
| Methods | Randomised controlled trial comparing 2 time frames for ice pack application. Additional third group allocated to ice treatment in a separate randomised controlled trial, with the same inclusion/exclusion criteria, ice applied for a different time frame (Leventhal 2011) |
| Participants | Women aged 18 and over, having had a normal birth of their first baby, singleton pregnancy, cephalic presentation Scoring 3 or more on a numeric scale (0, no pain to 10, worst imaginable pain) at recruitment No analgesia past 6 hours prior to recruitment No clinical obstetric concerns, e.g. haematomas, perineal oedema, haemorrhoids No comprehension or communication challenges Baby healthy |
| Interventions | Ice pack applied to the perineum for 10 (n = 38) or 15 (n = 39) in the new study or for 20 minutes (n = 38) in a previous study (Leventhal 2011) |
| Outcomes | 30% decrease in perineal pain on the numerical scale, from immediately prior to immediately after the cryotherapy treatment Reduction in perineal temperature, expected to be 10 º to 15 °C at 10 ‐ 15 minutes following ice pack application |
| Notes | Author contacted 9 January 2020 to establish perineal status for participants. Response received 15 January 2020 stating that she no longer has access to the data since retiring. No other authors' contact details yet determined |
Differences between protocol and review
The protocol and original review had included cross‐over studies. We excluded such studies in the previous update of this review (East 2012) and retained this approach in the current update, for the reasons noted in the Methods section.
We made a number of changes for the 2020 update to align with the evolution of the systematic review methodology over time:
Objectives: We edited these to compare: (i) one form of cooling with no treatment; (ii) cooling with placebo; or (iii) two different cooling treatments: we did not conduct the comparisons that were in the previous version of the review between a cooling treatment and other forms of treatment. This was to streamline the review process to better align with the topic. The previous objectives are provided in Appendix 2.
Types of studies: in previous versions of this review (e.g. East 2012), we excluded studies available only in abstract form. For this 2020 update, we were able to confirm that all of the reports that were available in abstract form were also linked with later‐published full trial reports. If we identify any other abstracts in future updates, we will include them if they contain sufficient information to assess study eligibility and risk of bias and if the authors confirm in writing that they reported final results that would not change in subsequent publications. Additionally, we had previously excluded the study by Barclay 1983, due to concerns about quality. We have now included this study as it met the inclusion criteria, although it did not contribute data to the meta‐analysis.
The change in objectives (and therefore in the comparisons with a cooling treatment) meant that we excluded comparisons between cooling treatments and some alternate form of treatment in the original and previous versions of the review (East 2012) in this 2020 update. This included the following treatments: pulsed electromagnetic therapy (Gallie 2003), warmth (Hill 1989; Sheikhan 2011), regular administration of paracetamol (Thangaraju 2006), hammamelis water (Moore 1989) and Pramoxine/Hydrocortiusone foam (Steen 2000; Moore 1989).
Outcomes: there has been a trend towards examining fewer outcomes in Cochrane Reviews over recent years. We proposed in our earlier update (East 2012) that we would reconsider using the outcomes listed in the generic protocol for reporting trials of pharmacological analgesia for the relief of perineal pain by Chou 2009. We therefore reduced the number of outcomes and the multiple time frames for which they could be considered from the previous versions of this review (see Appendix 2).
Contributions of authors
Protocol and Review 2007 ; updated review 2012 (East 2012): Christine East compiled the protocol, reviewed trials, abstracted data and wrote the review and its updates. Lisa Begg, Naomi Henshall and Karen Wallace performed data abstraction. Paul Marchant provided statistical advice, particularly on the issue of dealing with studies that had three comparison groups and provision of additional data for one study (Steen 2002). All co‐authors previewed and contributed to the content of the review and its update.
Updated Review 2020 : Christine East continued to author, with the remaining original authors declining or not otherwise being available to continue. Three new authors joined the review team. JiaJia (Jessie) Liu, Emma Dorward and Rhianonn Whale joined the authorship and assessed trials for inclusion, abstracted data and contributed to the data interpretation and general text. All co‐authors previewed and contributed to the content of the review and its update.
Sources of support
Internal sources
-
Queensland Health Nursing Research Grant, Australia
For the initial protocol and review, 2007
External sources
No sources of support supplied
Declarations of interest
Protocol and Review 2007; updated Review 2012 (East 2012)
Christine E East: received AUD 25,000 grant from the Queensland Nursing Council to support the 2007 protocol and review. Christine E East conducted an unpublished pilot study comparing the use of cold gel pads with ice packs in 2009. The study was not pursued due to lack of funding resources. It had not been considered for inclusion in the early versions of this review.
Lisa Begg: no interests to declare.
Paul R Marchant: collaborated in randomised controlled trials comparing the effectiveness of two cooling treatments for the relief of perineal pain following childbirth. These reports were included in the review (Steen 2000; Steen 2002) but were reviewed by other authors.
Karen Wallace: no interests to declare.
Updated review 2020
Christine E East: conducted an unpublished pilot study comparing the use of cold gel pads with ice packs in 2009. The study was not pursued due to lack of funding resources. It had been overlooked in previous versions of the review, given the pilot status.
Jiajia Liu: no interests to declare.
Emma Dorward: no interests to declare.
Rhiannon Whale: no interests to declare.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Barclay 1983 {published data only}
- Barclay L, Martin N. A sensitive area (Care of the episiotomy in the post-partum period). Australian Journal of Advanced Nursing 1983;1(1):12-9. [PubMed] [Google Scholar]
Beleza 2017 {published data only}
- ACTRN12613000052730. Effects of cryotherapy in perineal pain after childbirth with episiotomy. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=362921&isReview=true (first received 22 November 2012).
- Beleza AC, Ferreira C, Meirelles M, Santos C, Nakanno AM. The effect of cryotherapy in perineal pain after vaginal childbirth with episiotomy. In: 16th International WCPT Congress; 2011 June 20-23; Amsterdam, Holland. 2011.
- *.Beleza AC, Ferreira CH, Driusso P, Dos Santos CB, Nakano AM. Effect of cryotherapy on relief of perineal pain after vaginal childbirth with episiotomy: a randomized and controlled clinical trial. Physiotherapy 2017;103(4):453-8. [DOI] [PubMed] [Google Scholar]
East 2007 {unpublished data only}
- ACTRN12607000557437. Randomised controlled trial of two local cooling regimes for relieving pain from perineal trauma sustained during childbirth. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=82371 (first received 30 October 2007).
Francisco 2018 {published data only}
- East C. Co-interventions used in treatment and controls and trial registration dates [personal communication]. Email to: A Franciso 8 July 2020.
- East C. Data on outcomes provided [personal communication]. Emai to: A Francisco 3 March 2020.
- *.Francisco AA, De Oliveira SM, Steen M, Nobre MR, De Souza EV. Ice pack induced perineal analgesia after spontaneous vaginal birth: randomized controlled trial. Women and Birth 2018;31(5):e334-40. [DOI] [PubMed] [Google Scholar]
- Francisco AA, Oliveira SM, Bick D. Effect of ice to relieve perineal pain on daily activities of postpartum women. International Confederation of Midwives 30th Triennial Congress. Midwives: Improving Women’s Health; Prague, Czech Republic 2014;June 1-4:P053. [Google Scholar]
Morais 2016 {published and unpublished data}
- East C. Data on women with perineal trauma only, details of allocation concealment and trial registration [personal communication]. Email to: I Morais 28 January 2020.
- *.Morais I, Lemos A, Katz L, Melo LF, Maciel MM, Amorim MM. Perineal pain management with cryotherapy after vaginal delivery: a randomized clinical trial. Revista Brasileira De Ginecologia e Obstetricia 2016;38:325-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto AH, Amorim MM, Morais I, Lemos A, Leal N. Cryotherapy for the control of perineal pain following vaginal delivery. A randomized clinical trial. Obstetrics and Gynecology 2015;125(5 Suppl):64S-65S. [Google Scholar]
Navvabi 2009 {published and unpublished data}
- Navvabi RS, Kerman-Saravi F, Soroneh RM, Abedian Z. Cold and reduced episiotomy pain interfere with mood and daily activity. Shiraz Electronic Medical Journal 2011;12(2):87-92. [Google Scholar]
- *.Navvabi S, Abedian Z, Steen-Greaves M. Effectiveness of cooling gel pads and ice packs on perineal pain. British Journal of Midwifery 2009;17(11):724-9. [Google Scholar]
- Steen-Greaves M, Navvabi-Rigi S-D. Evaluation the effectiveness of cooling gel pads and ice packs on perineal pain and wound healing after episiotomy. irct.ir/trial/7477 (first received 11 August 2011) 2011.
Senol 2017 {published and unpublished data}
- *.Senol DK, Aslan E. The effects of cold application to the perineum on pain relief after vaginal birth. Asian Nursing Research 2017;11(4):276-82. [DOI] [PubMed] [Google Scholar]
- Senol DK. Normal Doğum Sonrasi Perineal Bölgeye Yapilansoğuk Uygulamanin Ağriyi Azaltmadaki Etkisi (The effect of cold application on reducing pain to the perineal region after normal delivery) [Doctoral Thesis]. Instanbul: Instanbul University Health Sciences Unit, 2014. [Google Scholar]
Steen 2000 {published data only}
- Griffith-Jones M, Steen M, Walker J, Cooper K. Randomised controlled trial of a new maternity cooling gel pad against ice packs and epifoam in the management of perineal oedema, bruising and pain after instrumental vaginal delivery. British Journal of Obstetrics and Gynaecology 1998;105(Suppl 17):88. [Google Scholar]
- *.Steen M, Cooper K, Marchant P, Griffiths-Jones M, Walker J. A randomised controlled trial to compare the effectiveness of icepacks and epifoam with cooling maternity gel pads at alleviating postnatal perineal trauma. Midwifery 2000;16:48-55. [DOI] [PubMed] [Google Scholar]
- Steen M, Cooper K. Perineal trauma: a localised cooling treatment for alleviating perineal oedema, bruising and pain following an episiotomy. In: "What works": research and practice in nursing; 1998 June 25; Leeds, UK. 1998.
Steen 2002 {published and unpublished data}
- Steen M, Marchant P. Alleviating perineal trauma - the APT study. RCM Midwives Journal 2001;4(8):256-8. [Google Scholar]
- Steen M, Marchant P. Ice packs and cooling gel pads versus no localised treatment for relief of perineal pain: a randomised controlled trial. Evidence Based Midwifery 2007;5(1):16-22. [DOI] [PubMed] [Google Scholar]
- *.Steen M. A randomised controlled trial to evaluate the effectiveness of localised cooling treatments in alleviating perineal trauma: the APT study. MIDIRS Midwifery Digest 2002;12(3):373-6. [Google Scholar]
Yusamran 2007 {published data only}
- Yasumran C, Titapant V, Kongjeera A. Relief perineal pain after perineorrhahy by cold gel pack pad: a randomized controlled trial. Thai Journal of Nursing Research 2007;11(2):87-95. [Google Scholar]
References to studies excluded from this review
Amani 2015 {published data only}
- *.Amani R, Kariman N, Mojab F, Alavi H, Majidi S. Comparison of the effects of cold compress with gel packs and topical olive oil on episiotomy wound healing. Journal of Babol University of Medical Sciences 2015;17(11):7-12. [Google Scholar]
- IRCT201409164529N12. Comparison the effect of cooling gel pads and topical olive oil on the intensity of episiotomy pain and wound healing in primiparous women referring to hospitals affiliated with the Gilan university of Medical Sciences in 2014. irct.ir/trial/4833 (first received 30 October 2014).
De Souza Bosco Paiva 2016 {published data only}
- *.De Souza Bosco Paiva C, De Oliveira SM, Francisco AA, Da Silva RL, De Paula Batista Mendes E, Steen M. Length of perineal pain relief after ice pack application: a quasi-experimental study. Women and Birth 2016;29(2):117-22. [DOI] [PubMed] [Google Scholar]
- RBR-9vmkcb. Duration of the analgesic effect of cryotherapy on perineal pain after childbirth: clinical trial. www.ensaiosclinicos.gov.br/rg/RBR-9vmkcb/ (first received 30 January 2013).
Dube 2013 {published data only}
- Dube J. Effect of application of ice on episiotomy. Asian Journal of Nursing Education and Research 2013;3(4):207-10. [Google Scholar]
Gallie 2003 {published data only}
- Gallie M, Pourghazi S, Grant JM. A randomized trial of pulsed electromagnetic energy compared with ice-packs for the relief of postnatal perineal pain. Journal of the Association of Chartered Physiotherapists in Women's Health 2003;93:10-4. [Google Scholar]
Hill 1989 {published data only}
- Hill PD. Effects of heat and cold on the perineum after episiotomy/laceration. Journal of Obstetric, Gynecologic and Neonatal Nursing 1989;18(2):124-9. [DOI] [PubMed] [Google Scholar]
Kaur 2012 {published data only}
- Kaur S, Janzen M. Reduction of perineal pain after vaginal birth with black tea: pilot randomized study. clinicaltrials.gov/ct2/show/NCT01626287 (first received 22 June 2012).
LaFoy 1989 {published data only}
- LaFoy J, Geden E. Postepisiotomy pain: warm versus cold sitz bath. Journal of Obstetric, Gynecologic and Neonatal Nursing 1989;18(5):399-403. [DOI] [PubMed] [Google Scholar]
Lu 2015 {published data only}
- Lu Y-Y, Su M-L, Gau M-L, Lin K-C, Au H-K. The efficacy of cold-gel packing for relieving episiotomy pain - a quasi-randomised control trial. Contemporary Nurse 2015;50(1):26-35. [DOI: 10.1080/10376178.2015.1010257] [DOI] [PubMed] [Google Scholar]
Moore 1989 {published data only}
- Moore W, James DK. A random trial of three topical analgesic agents in the treatment of episiotomy pain following instrumental vaginal delivery. Journal of Obstetrics and Gynaecology 1989;10:35-9. [Google Scholar]
Nam 1991 {published data only}
- Nam HK, Park YS. A study on comparisons of ice bag and heat lamp for the relief of perineal discomfort. Kanho Hakhoe Chi 1991;21(1):27-40. [DOI] [PubMed] [Google Scholar]
NCT02024256 {published data only}
- NCT02024256. Comparison between treatment with gauze soaked with cold magnesium sulfate solution against gauze soaked with cold water for treatment of perineal swelling following vaginal delivery - a double blind placebo controlled trial. clinicaltrials.gov/ct2/show/NCT02024256 (first received 31 December 2013).
Pinkerton 1961 {published data only}
- Pinkerton JH, Beard RW. Ice-packs after episiotomy. British Medical Journal 1961;1:1536-7. [Google Scholar]
Ramler 1986 {published data only}
- Ramler D, Roberts J. A comparison of cold and warm sitz baths for relief of postpartum perineal pain. Journal of Obstetric, Gynecologic and Neonatal Nursing 1986;15(6):471-4. [DOI] [PubMed] [Google Scholar]
Sheikhan 2011 {published data only}
- IRCT138807192248N3. The effect of cold therapy and the essential lavender oil on pain intensity and healing of episiotomy among primiparous women. irct.ir/trial/1878 (first received 26 April 2010).
- *.Sheiken F, Jahdi F, Khoie EM, Alizadeh NS, Sheikhan H, Haghani H. Episiotomy discomforts relief using cold gel pads in primiparous Iranian women (A comparative study). Research Journal of Medical Sciences 2011;5(3):150-4. [Google Scholar]
Thangaraju 2006 {published data only}
- Thangaraju P, Moey CB. Perineal cold pads versus oral analgesics in the relief of postpartum perineal wound pain. Singapore General Hospital Proceedings 2006;15(1):8-12. [Google Scholar]
References to studies awaiting assessment
Leventhal 2011 {published data only}
- ACTRN12608000439347. Ice pack for relieving perineal pain after normal delivery. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12608000439347 (first received 3 September 2008).
- *.Leventhal LC, De Oliveira SM, Nobre MR, Da Silva FM. Perineal analgesia with an ice pack after spontaneous vaginal birth: a randomized controlled trial. Journal of Midwifery and Women's Health 2011;56(2):141-6. [DOI] [PubMed] [Google Scholar]
Oliveira 2012 {published data only}
- ACTRN12608000551392. Comparing three duration times of cryotherapy (10, 15 and 20 minutes) in pregnant women for relieving pain from perineal trauma after birth. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12608000551392 (first received 5 September 2008).
- *.Oliveira SM, Silva FM, Riesco ML, Latorre M do R, Nobre MR. Comparison of application times for ice packs used to relieve perineal pain after normal birth: a randomised clinical trial. Journal of Clinical Nursing 2012;21(23-24):3382-91. [DOI] [PubMed] [Google Scholar]
Additional references
Aasheim 2017
- Aasheim V, Nilsen AB, Lukasse M, Reinar LM. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database of Systematic Reviews 2017, Issue 6. Art. No: CD006672. [DOI: 10.1002/14651858.CD006672.pub3] [DOI] [PubMed] [Google Scholar]
Abedzadeh‐Kalahroudi 2019
- Abedzadeh-Kalahroudi M, Talebian A, Sadat Z, Mesdaghinia E. Perineal trauma: incidence and its risk factors. Journal of Obstetrics and Gynaecology 2019;39(2):206-11. [DOI: 10.1080/01443615.2018.1476473] [DOI] [PubMed] [Google Scholar]
Adie 2010
- Adie S, Naylor JM, Harris IA. Cryotherapy after total knee arthroplasty a systematic review and meta-analysis of randomized controlled trials. Journal of Arthroplasty 2010;25(5):709-15. [DOI] [PubMed] [Google Scholar]
Aguiar 2019
- Aguiar M, Farley A, Hope L, Amin A, Shah P, Manaseki-Holland S. Birth-related perineal trauma in low- and middle-income countries: a systematic review and meta-analysis. Maternal and Child Health Journal 2019;23(8):1048-70. [DOI: 10.1007/s10995-019-02732-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
AIHW 2020
- Australian Institute of Health and Welfare. Australia’s Mothers and Babies 2018—in brief. Perinatal Statistics series no. 36. Cat. no. PER 108. Sydney: AIHW National Perinatal Statistics Unit, 2020. [Google Scholar]
Bisson 2019
- Bisson DL, Newell SD, Laxton C, on behalf of the Royal College of Obstetricians and Gynaecologists. Antenatal and Postnatal Analgesia. Scientific Impact Paper No. 59. BJOG: an international journal of obstetrics and gynaecology 2019;126(4):e115-124. [DOI] [PubMed] [Google Scholar]
Bleakley 2006
- Bleakley CM, McDonough SM, Macauley DC, Bjordal J. Cryotherapy for acute ankle sprains: a randomised controlled study of two different icing protocols. British Journal of Sports Medicine 2006;40(8):700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bleakley 2010
- Bleakley CM, Davison GW. What is the biochemical and physiological rationale for using cold-water immersion in sports recovery? A systematic review. British Journal of Sports Medicine 2010;44(3):179-87. [DOI] [PubMed] [Google Scholar]
Bleakley 2012
- Bleakley C, McDonough S, Gardner E, Baxter GD, Hopkins JT, Davison GW. Cold-water immersion (cryotherapy) for preventing and treating muscle soreness after exercise. Cochrane Database of Systematic Reviews 2012, Issue 2. Art. No: CD008262. [DOI: 10.1002/14651858.CD008262.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonica 1990
- Bonica JJ. The Management of Pain. 2nd edition. Philadelphia: Lea and Felbinger, 1990. [Google Scholar]
Chou 2009
- Chou D, Abalos E, Gyte GM, Gülmezoglu AM. Drugs for perineal pain in the early postpartum period: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD007734. [DOI: 10.1002/14651858.CD007734] [DOI] [Google Scholar]
Chou 2013
- Chou D, Abalos E, Gyte GM, Gülmezoglu AM. Paracetamol/acetaminophen (single administration) for perineal pain in the early postpartum period. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No: CD008407. [DOI: 10.1002/14651858.CD008407.pub2] [DOI] [PubMed] [Google Scholar]
Collins 2008
- Collins NC. Is ice right? Does cryotherapy improve outcome for acute soft tissue injury? Emergency Medicine Journal 2008;25(2):65-8. [DOI] [PubMed] [Google Scholar]
Davies‐Tuck 2015
- Davies-Tuck M, Biro MA, Mockler J, Steward L, Wallace EM, East CE. Maternal Asian ethnicity and the risk of anal sphincter injury. ACTA Obstet Gynecol 2015;94(3):308-15. [DOI] [PubMed] [Google Scholar]
Doğan 2017
- Doğan B, Gün İ, Özdamar Ö, Yılmaz A, Muhçu M. Long-term impacts of vaginal birth with mediolateral episiotomy on sexual and pelvic dysfunction and perineal pain. Journal of Maternal-fetal & Neonatal Medicine 2017;30(4):457-60. [DOI] [PubMed] [Google Scholar]
Dray 1995
- Dray A. Inflammatory mediators of pain. British Journal of Anaesthesia 1995;75(2):125-31. [DOI] [PubMed] [Google Scholar]
Dubois 2020
- Dubois B, Esculier J-F. Soft-tissue injuries simply need PEACE and LOVE. British Journal of Sports Medicine 2020;54(2):72-3. [DOI] [PubMed] [Google Scholar]
Dushesne 2017
- Duchesne E, Dufresne SS, Dumont NA. Impact of inflammation and anti-inflammatory modalities on skeletal muscle healing: From fundamental research to the clinic. Physical Therapy in Sport 2017;97(8):807-17. [DOI] [PubMed] [Google Scholar]
East 2011
- East CE, Sherburn M, Nagle C, Said J, Forster D. Perineal pain following childbirth: prevalence, effects on postnatal recovery and analgesia usage. Midwifery 2011;DOI:10.1016/j.midw.2010.11.009:5 pages. [DOI] [PubMed] [Google Scholar]
Engelhard 2019
- Engelhard D, Hofer P, Annaheim S. Evaluation of the effect of cooling strategies on recovery after surgical intervention. BMJ Open Sport & Exercise Medicine 2019;5:e000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ernst 1994
- Ernst E, Fialka V. Ice freezes pain? A review of the clinical effectiveness of analgesic cold therapy. Journal of Pain and Symptom Management 1994;9(1):56-9. [DOI] [PubMed] [Google Scholar]
Francisco 2011
Gibson‐Helm 2015
- Gibson-Helm ME, Teede HJ, Cheng I-H, Block AA, Knight M, East CE, et al. Maternal health and pregnancy outcomes comparing migrant women born in humanitarian and nonhumanitarian source countries: a retrospective, observational study. Birth: Issues in Pregnancy Care 2015;42(2):116-24. [DOI] [PubMed] [Google Scholar]
Grant 1989
- Grant A, Sleep J. Relief of perineal pain and discomfort after childbirth. In: Chalmers I, Enkin M, Keirse MJ, editors(s). Effective Care in Pregnancy and Childbirth. Vol. 2. Oxford: Oxford University Press, 1989:1347-58. [Google Scholar]
Hay‐Smith 1998
- Hay-Smith EJ. Therapeutic ultrasound for postpartum perineal pain and dyspareunia. Cochrane Database of Systematic Reviews 1998, Issue 3. Art. No: CD000495. [DOI: 10.1002/14651858.CD000495] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hedayati 2003
- Hedayati H, Parsons J, Crowther CA. Rectal analgesia for pain from perineal trauma following childbirth. Cochrane Database of Systematic Reviews 2003, Issue 3. Art. No: CD003931. [DOI: 10.1002/14651858.CD003931] [DOI] [PubMed] [Google Scholar]
Hedayati 2005
- Hedayati H, Parsons J, Crowther CA. Topically applied anaesthetics for treating perineal pain after childbirth. Cochrane Database of Systematic Reviews 2005, Issue 2. Art. No: CD004223. [DOI: 10.1002/14651858.CD004223.pub2] [DOI] [PubMed] [Google Scholar]
Higgins 2020a
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook. [ISBN: 9781119536628]
Higgins 2020b
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Jiang 2017
- Jiang H, Qian X, Carroli G, Garner P. Selective versus routine use of episiotomy for vaginal birth. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD000081. [DOI: 10.1002/14651858.CD000081.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kettle 2010
- Kettle C, Johanson RB. Absorbable synthetic versus catgut suture material for perineal repair. Cochrane Database of Systematic Reviews 2010, Issue 6. Art. No: CD000006. [DOI: 10.1002/14651858.CD000006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kettle 2012
- Kettle C, Dowswell T, Ismail KM. Continuous and interrupted suturing techniques for repair of episiotomy or second-degree tears. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD000947. [DOI: 10.1002/14651858.CD000947.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuo 2013
- Kuo CC, Lin CC, Lee WJ, Huang WT. Comparing the antiswelling and analgesic effects of three different ice pack therapy durations: a randomized controlled trial on cases with soft tissue injuries. Journal of Nursing Research 2013;21(3):186-94. [DOI] [PubMed] [Google Scholar]
Lawrence 2017
- Lawerence L, Rogers R, Borders N, Teaf D, Qualls C. The effect of perineal lacerations on pelvic floor function and anatomy at six months postpartum in a prospective cohort of nulliparous women. Birth 2017;43(4):293-302. [DOI: 10.1111/birt.12258] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leventhal 2010
- Leventhal LC, Bianchi RC, De Oliveira SM. Clinical trial comparing three types of cryotherapy in non-pregnant women. Revista da Escola de Enfermagem da USP 2010;44(2):339-45. [DOI] [PubMed] [Google Scholar]
Lubkowska 2012
- Lubkowska A. Cryotherapy: physiological considerations and applications to physical therapy. In: Brettany-Saltikov j, Paz-Laurido B, editors(s). Physical Therapy Perspectives in the 21st Century - Challenges and Possibilities. www.intechopen.com: Intertech Open, 2012. [Google Scholar]
Mac Auley 2001
- Mac Auley DC. Ice therapy: how good is the evidence? International Journal of Sports Medicine 2001;22(5):379-84. [DOI] [PubMed] [Google Scholar]
Machado 2016
- Machado A, Ferreira P, Micheletti J, De Almeida AC, Lemes IR, Vanderlei FM, et al. Can water temperature and immersion time influence the effect of cold water immersion on muscle soreness? A systematic review and meta-analysis. Sports Medicine 2016;46(4):503-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malanga 2015
- Malanga GA, Yan N, Stark J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgraduate Medicine 2015;127(1):57-65. [DOI: 10.1080/00352481.2015.992719] [DOI] [PubMed] [Google Scholar]
Manresa 2019
- Manresa M, Pereda A, Bataller E, Terre-Rull C, Ismail KM, Webb SS. Incidence of perineal pain and dyspareunia following spontaneous vaginal birth: a systematic review and meta-analysis. International Urogynecology Journal 2019;30(6):853-68. [DOI: 10.1007/s00192-019-03894-0] [DOI] [PubMed] [Google Scholar]
Martimbianco 2014
- Martimbianco AL, Gomes da Silva BN, De Carvalho AP, Silva V, Torloni MR, Peccin MS. Effectiveness and safety of cryotherapy after arthroscopic anterior cruciate ligament reconstruction. A systematic review of the literature. Physical Therapy in Sport 2014;15(4):261-8. [DOI] [PubMed] [Google Scholar]
McMasters 1977
- McMasters WC. A literary review on ice therapy in injuries. American Journal of Sports Medicine 1997;5(3):124-6. [DOI] [PubMed] [Google Scholar]
Mirkin 1978
- Mirkin G, Hoffman M. The Sports Medicine Book. New York: Little Brown & Co, 1978. [Google Scholar]
Mirkin 2015
- Mirkin G. Why ice delays recovery. www.drmirkin.com 2015.
Molakatalla 2017
- Molakatalla S, Shepherd E, Grivell RM. Aspirin (single dose) for perineal pain in the early postpartum period. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD012129. [DOI: 10.1002/14651858.CD012129.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2006
- National Institue for Health and Care Excellence (NICE. Postnatal care up to 8 weeks after birth: Clinical guideline CG37. www.nice.org.uk/guidance/cg37 2006, last updated February 2015 (accessed 21 January 2020).
NMPA 2019
- NMPA Project Team. National Maternity and Perinatal Audit: Clinical Report 2019. Based on births in NHS maternity services between 1 April 2016 and 31 March 2017. maternityaudit.org.uk/FilesUploaded/NMPA%20Clinical%20Report%202019.pdf 2019.
Nogueira 2019
- Nogueira NM, Felappi CJ, Lima CS, Medeiros DM. Effects of local cryotherapy for recovery of delayed onset muscle soreness and strength following exercise-induced muscle damage: systematic review and meta‑analysis. Sports Sciences for Health 2019;16:1-11. [DOI: 10.1007/s11332-019-00571-z] [DOI]
Oakley 2013
- Oakley ET, Pardeiro RB, Powell JW, Millar AL. The effects of multiple daily applications of ice to the hamstrings on biochemical measures, signs, and symptoms associated with exercise induced muscle damage. Journal of Strength and Conditioning Research 2013;27(10):2743-51. [DOI] [PubMed] [Google Scholar]
Queensland Clinical Guidelines 2018
- Queensland Clinical Guidelines. Perineal Care. www.health.qld.gov.au/__data/assets/pdf_file/0022/142384/g-pericare.pdf 2018.
Ramos 2016
- Ramos GV, Pinheiro CM, Messa SB, Delfino GB, Marqueti RC, Salvini FT, et al. Cryotherapy reduces inflammatory response without altering muscle regeneration process and extracellular matrix remodelling of rat muscle. Scientific Reports 2016;6:18525:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reid 2005
- Reid G. ThermoTRP channels and cold sensing: what are they and what do they do? European Journal of Physiology 2005;451:250-63. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shoorab 2019
- Shoorab NJ, Mirteimouri M, Taghipour A, Roudsari RL. Women’s experiences of emotional recovery from childbirth-related perineal trauma: a qualitative content analysis. IJCBNM 2019;7(3):181-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2017
- Singh DP, Barani Lonbani Z, Woodruff MA, Parker TJ, Steck R, Peake JM. Effects of topical icing on inflammation, angiogenisis, revascularization and myofiber regeneration in skeletal muscle following contusion injury. Frontiers in Physiology 2017;8(93):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sleep 1988
- Sleep J, Grant A. Relief of perineal pain following childbirth: a survey of midwifery practice. Midwifery 1988;4(3):118-22. [DOI] [PubMed] [Google Scholar]
Steen 1999
- Steen M, Cooper K. A new device for the treatment of perineal wounds. Journal of Wound Care 1999;8(2):87-90. [DOI] [PubMed] [Google Scholar]
Subki 2019
- Subki AH, Fakeeh MM, Hindi MM, Nas AL, Almaymuni AD, Abduljabba HS. Fecal and urinary incontinence associated with pregnancy and childbirth. Materia Sociomedica 2019;31(3):202-6. [DOI: 10.5455/msm.2019.31.202-206] [DOI] [PMC free article] [PubMed] [Google Scholar]
Swenson 1996
- Swenson C, Sward L, Karlsson J. Cryotherapy in sports medicine. Scandinavian Journal of Medicine & Science in Sports 1996;6(4):193-200. [DOI] [PubMed] [Google Scholar]
Torres 2012
- Torres R, Ribeiro F, Alberto DJ, Cabri JM. Evidence of the physiotherapeutic interventions used currently after exercise induced muscle damage: systematic review and meta-analysis. Physical Therapy in Sport 2012;13:101-14. [DOI] [PubMed] [Google Scholar]
Van den Bekerom 2012
- Van den Bekerom MP, Struijs PA, Blankevoort L, Welling L, Van Dijk CN, Kerkhoffs GM. What is the evidence for rest, ice, compression, and elevation therapy in the treatment of ankle sprains in adults? Journal of Athletic Training 2012;47(4):435-43. [DOI: 10.4085/1062-6050-47.4.14] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wen 2018
- Wen Q, Muraca GM, Ting J, Coad S, Lik KI, Lisonkova S. Temporal trends in severe maternal and neonatal trauma during childbirth: a population-based observational study. BMJ Open 2018;8(3):1-10. [DOI: 10.1136/bmjopen-2017-020578] [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2013
- White GE, Wells GD. Cold-water immersion and other forms of cryotherapy: physiological changes potentially. Extreme Physiology and Medicine 2013;2(1:26):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wuytack 2016
- Wuytack F, Smith V, Cleary BJ. Oral non-steroidal anti-inflammatory drugs (single dose) for perineal pain in the early postpartum period. Cochrane Database of Systematic Reviews 2016, Issue 7. Art. No: CD011352. [DOI: 10.1002/14651858.CD011352.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
East 2007
- East CE, Begg L, Henshall NE, Marchant P, Wallace K. Local cooling for relieving pain from perineal trauma sustained during childbirth. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD006304. [DOI: 10.1002/14651858.CD006304.pub2] [DOI] [PubMed] [Google Scholar]
East 2007a
- East CE, Marchant P, Begg L, Henshall NE. Local cooling for relieving pain from perineal trauma sustained during childbirth. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No: CD006304. [DOI: 10.1002/14651858.CD006304] [DOI] [PubMed] [Google Scholar]
East 2012
- East CE, Begg L, Henshall NE, Marchant PR, Wallace K. Local cooling for relieving pain from perineal trauma sustained during childbirth. Cochrane Database of Systematic Reviews 2012, Issue 5. Art. No: CD006304. [DOI: 10.1002/14651858.CD006304.pub3] [DOI] [PubMed] [Google Scholar]


