Abstract
Background
A frozen embryo transfer (FET) cycle is when one or more embryos (frozen during a previous treatment cycle) are thawed and transferred to the uterus. Some women undergo fresh embryo transfer (ET) cycles with embryos derived from donated oocytes. In both situations, the endometrium is primed with oestrogen and progestogen in different doses and routes of administration.
Objectives
To evaluate the most effective endometrial preparation for women undergoing transfer with frozen embryos or embryos from donor oocytes with regard to the subsequent live birth rate (LBR).
Search methods
The Cochrane Gynaecology and Fertility Group trials register, CENTRAL, MEDLINE, Embase, PsycINFO, LILACS, trials registers and abstracts of reproductive societies' meetings were searched in June 2020 together with reference checking and contact with study authors and experts in the field to identify additional studies.
Selection criteria
Randomised controlled trials (RCTs) evaluating endometrial preparation in women undergoing fresh donor cycles and frozen embryo transfers.
Data collection and analysis
We used standard methodological procedures recommended by Cochrane. We analysed all available interventions versus placebo, no treatment, or between each other. The primary review outcome was live birth rate. Secondary outcomes were clinical and multiple pregnancy, miscarriage, cycle cancellation, endometrial thickness and adverse effects.
Main results
Thirty‐one RCTs (5426 women) were included. Evidence was moderate to very low‐quality: the main limitations were serious risk of bias due to poor reporting of methods, and serious imprecision.
Stimulated versus programmed cycle
We are uncertain whether a letrozole‐stimulated cycle compared to a programmed cycle, for endometrial preparation, improves LBR (odds ratio (OR) 1.26, 95% confidence interval (CI) 0.49 to 3.26; 100 participants; one study; very low‐quality evidence).
Stimulating with follicle stimulating hormone (FSH), letrozole or clomiphene citrate may improve clinical pregnancy rate (CPR) (OR 1.63, 95% CI 1.12 to 2.38; 656 participants; five studies; I2 = 11%; low‐quality evidence). We are uncertain if they reduce miscarriage rate (MR) (OR 0.79, 95% CI 0.36 to 1.71; 355 participants; three studies; I2 = 0%; very low‐quality evidence). Endometrial thickness (ET) may be reduced with clomiphene citrate (mean difference(MD) ‐1.04, 95% CI ‐1.59 to ‐0.49; 92 participants; one study; low‐quality evidence). Other outcomes were not reported.
Natural versus programmed cycle
We are uncertain of the effect from a natural versus programmed cycle for LBR (OR 0.97, 95% CI 0.74 to 1.28; 1285 participants; four studies; I2 = 0%; very low‐quality evidence) and CPR (OR 0.79, 95% CI 0.62 to 1.01; 1249 participants; five studies; I2 = 60%; very low‐quality evidence), while a natural cycle probably reduces the cycle cancellation rate (CCR) (OR 0.60, 95% CI 0.44 to 0.82; 734 participants; one study; moderate‐quality evidence). We are uncertain of the effect on MR and ET. No study reported other outcomes.
Transdermal versus oral oestrogens
From low‐quality evidence we are uncertain of the effect transdermal compared to oral oestrogens has on CPR (OR 0.86, 95% CI 0.59 to 1.25; 504 participants; three studies; I2 = 58%) or MR (OR 0.55, 95% CI 0.27 to 1.09; 414 participants; two studies; I2 = 0%). Other outcomes were not reported.
Day of starting administration of progestogen
When doing a fresh ET using donated oocytes in a synchronised cycle starting progestogen on the day of oocyte pick‐up (OPU) or the day after OPU, in comparison with recipients that start progestogen the day prior to OPU, probably increases the CPR (OR 1.87, 95% CI 1.13 to 3.08; 282 participants; one study, moderate‐quality evidence). We are uncertain of the effect on multiple pregnancy rate (MPR) or MR. It probably reduces the CCR (OR 0.28, 95% CI 0.11 to 0.74; 282 participants; one study; moderate‐quality evidence). No study reported other outcomes.
Gonadotropin‐releasing hormone (GnRH) agonist versus control
A cycle with GnRH agonist compared to without may improve LBR (OR 2.62, 95% CI 1.19 to 5.78; 234 participants; one study; low‐quality evidence). From low‐quality evidence we are uncertain of the effect on CPR (OR 1.08, 95% CI 0.82 to 1.43; 1289 participants; eight studies; I2 = 20%), MR (OR 0.85, 95% CI 0.36 to 2.00; 828 participants; four studies; I2 = 0%), CCR (OR 0.49, 95% CI 0.21 to 1.17; 530 participants; two studies; I2 = 0%) and ET (MD ‐0.08, 95% CI ‐0.33 to 0.16; 697 participants; four studies; I2 = 4%). No study reported other outcomes.
Among different GnRH agonists
From very low‐quality evidence we are uncertain if cycles among different GnRH agonists improves CPR or MR. No study reported other outcomes.
GnRH agonists versus GnRH antagonists
GnRH antagonists compared to agonists probably improves CPR (OR 0.62, 95% CI 0.42 to 0.90; 473 participants; one study; moderate‐quality evidence). We are uncertain of the effect on MR and MPR. No study reported other outcomes.
Aspirin versus control
From very low‐quality evidence we are uncertain whether a cycle with aspirin versus without improves LBR, CPR, or ET.
Steroids versus control
From very low‐quality evidence we are uncertain whether a cycle with steroids compared to without improves LBR, CPR or MR. No study reported other outcomes.
Authors' conclusions
There is insufficient evidence on the use of any particular intervention for endometrial preparation in women undergoing fresh donor cycles and frozen embryo transfers. In frozen embryo transfers, low‐quality evidence showed that clinical pregnancy rates may be improved in a stimulated cycle compared to a programmed one, and we are uncertain of the effect when comparing a programmed cycle to a natural cycle. Cycle cancellation rates are probably reduced in a natural cycle. Although administering a GnRH agonist, compared to without, may improve live birth rates, clinical pregnancy rates will probably be improved in a GnRH antagonist cycle over an agonist cycle.
In fresh synchronised oocyte donor cycles, the clinical pregnancy rate is probably improved and cycle cancellation rates are probably reduced when starting progestogen the day of or day after donor oocyte retrieval.
Adequately powered studies are needed to evaluate each treatment more accurately.
Plain language summary
Endometrial preparation for egg donor recipients or for frozen embryo transfers
Review question
What is the most effective method for endometrial preparation in women undergoing embryo transfers with frozen embryos or embryos derived from donor oocytes?
Background
Couples undergo infertility treatments due to male factor, female factors or unexplained infertility. After an unsuccessful fresh embryo transfer cycle, a frozen‐thawed embryo transfer can be performed when frozen embryos are available. Adequate hormonal preparation of the endometrium is of utmost importance for both egg donor and frozen embryo replacement cycles to provide the optimal chances of pregnancy. Many drugs and various modes of administration have been tried by several investigators in order to optimise implantation rates and consequently improve the success rates of the embryo transfer procedures: stimulated cycles (to generate endogenous oestradiol), programmed cycles (administering exogenous oestradiol) or natural cycles (allowing the ovaries to produce oestradiol without stimulation) are some of the options; avoiding spontaneous ovulation with gonadotropin‐releasing hormone (GnRH) agonists and antagonists could have some impact; or using some other drugs such as aspirin or steroids that could potentially enhance the endometrial receptivity were also evaluated.
Study characteristics
We found 31 randomised controlled trials comparing different interventions such as the dose and route of administration of oestrogens and progestogen, the use of drugs that stop the patient from ovulating prematurely (GnRH agonists), and the use of other medications to improve the endometrium in a total of 5426 women. The evidence is current to June 2020.
Key results
We are uncertain whether a stimulated cycle (with letrozole) compared to a programmed cycle, for endometrial preparation, improves live birth. The evidence suggests that if the chance of live birth following a programmed cycle is assumed to be 24%, the chance following a stimulated cycle would be between 13% and 51%. We are also uncertain of the impact on miscarriage rate and endometrial thickness. A stimulated cycle may improve clinical pregnancy rate. Data were lacking on multiple pregnancy, cycle cancellation and other adverse effects.
We are uncertain whether a natural cycle improves the live birth rate, pregnancy rate, miscarriage rate and endometrial thickness in comparison with a programmed cycle. Data were lacking for all other outcomes.
We are uncertain if transdermal (delivered via the skin) oestrogens compared with oral (by mouth) oestrogens improve clinical pregnancy rate and miscarriage rate. Data were lacking for all other outcomes in this comparison.
Starting progestogen on the day of the donor oocyte retrieval or the day after probably increases the clinical pregnancy rate and probably reduces the cycle cancellation rate. We are uncertain if it reduces the miscarriage rate. Data were lacking for all other outcomes.
A cycle with GnRH agonist compared to without may improve live birth rate. We are uncertain of the effect of a GnRH cycle compared to no GnRH for the outcomes of clinical pregnancy rate, miscarriage rate, and endometrial thickness. No study reported on the other outcomes for this comparison.
We are uncertain if any GnRH agonist is better than other: a cycle with daily leuprolide or with deposit tryptorelin improves clinical pregnancy rate, or if daily acetate leuprolide or daily nafarelin reduces the miscarriage rate. Other outcomes were not reported.
GnRH antagonists compared to agonists probably improve clinical pregnancy rate. We are uncertain of the effect on miscarriage rate and multiple pregnancy rate. No study reported the other outcomes.
We are uncertain whether a cycle with aspirin compared to a cycle without improves live birth, clinical pregnancy rate or endometrial thickness. Data were lacking for all other outcomes.
We are also uncertain whether a cycle with steroids compared to a cycle without steroids improves live birth rate, clinical pregnancy rate or miscarriage rate. No study reported on the other outcomes.
Quality of the evidence
The evidence was of moderate to very low‐quality. The main limitations in the evidence were poor reporting of study methods, and lack of precision in the findings for live birth.
Summary of findings
Background
Description of the condition
It is estimated that about 15% of couples will fail to achieve conception after 12 months of unprotected intercourse (Smarr 2017; te Velde 2000). Ultimately, more than half of these infertile couples will undergo an assisted reproductive technology (ART) procedure such as in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI). Less than 50% of the women under 40 years of age and only 10% to 15% of those over 40 years will get pregnant through a fresh ART cycle (SART 2016). Increasingly, couples are trying to achieve conception later in their reproductive life and, given that pregnancy rates decrease as women's ages increase, more and more of these women present with subfertility problems.
When a fresh cycle is unsuccessful and frozen embryos are available, a frozen‐thawed embryo transfer may be performed. About 15% to 20% of all ART cycles performed using the woman's own oocytes use frozen embryos (De Geyter 2018; SART 2016).
Oocyte donation is a frequent treatment option that is increasingly used for infertile women given the high percentage of patients undergoing ART who are over 40 years of age in most ART programs. Twenty‐two per cent of all ART cycles in Latin America (LA Register 2015), about 10% in the USA (SART 2016), and around 10% of the fresh ART cycles reported in Europe (De Geyter 2018) are performed using donated oocytes.
Description of the intervention
Although more subfertile women undergo ART procedures every year, implantation failure of a fresh transferred embryo remains one of the most important limiting factors that prevents conception. Most women undergoing ART obtain an acceptable number of oocytes and embryos, but only few of these embryos implant after being transferred into the endometrial cavity (Garcia‐Velasco 2000). Thus, the endometrial preparation and profile is one of the main variables to be evaluated in women undergoing an embryo transfer procedure using donated oocytes, and also in frozen‐thawed embryo transfers.
In order to carry out the embryo transfer, an endometrial preparation is needed. The endometrial needs to be thickened with oestrogens and, then, some progestogen is needed to open the implantation window. There are several types of oestrogens, several dosages and several administration routes, and the luteal phase support with progestogen could be started with different timings.
On the other hand, in order to avoid a spontaneous ovulation, sometimes, gonadotropin‐releasing hormone (GnRH) agonists or antagonists are used. The reason to avoid a spontaneous ovulation is that, when ovulation occurs, progesterone starts rising, which could result in the implantation window opening too early and, therefore, miss the synchronisation with the embryo that is going to be implanted.
Finally, there are some add‐ons that have been proposed to improve the endometrial preparation. One of them is aspirin, which used in low doses (75‐325 mg/day) orally, works as an antiplatelet agent enhancing the prostacyclin synthesis. This action promotes vasodilation and, eventually, could improve the perfusion of some organs such as the endometrium (Kuo 1997). Another one is sildenafil citrate, which can be taken orally or vaginally at 25‐50 mg/day. It could also promote an improvement in the uterine blood flow by potentiating the effect of nitric oxide on vascular smooth muscles (Malinova 2013). On the other hand, steroids has also been proposed as a potentially useful add on. Steroids, such as dexamethasone 0.5 mg orally or methylprednisolone 4 mg orally are proposed to be used for a short period of 4‐5 days before the embryo transfer. They are immunomodulatory agents that could affect positively on the implantation rate by suppressing uterine natural killers cells cytotoxicity and cytokine secretion, and promoting the proliferation and invasion of trophoblast (Abdolmohammadi‐Vahid 2016).
How the intervention might work
In the implantation process an interaction between the embryo and the endometrium exists. This process seems to be affected by two crucial factors (Devroey 1998; Fox 2016). These are: endometrial receptivity and, synchronisation between the embryo developmental stage and the endometrial profile during the window of implantation (Nawroth 2005). Endometrial receptivity depends on the hormone replacement protocol used for this purpose. In normal physiology, the proliferative phase is characterised by a progressive mitotic growth of the functional endometrium in response to the increasing circulating oestrogen levels. The secretory phase commences after ovulation, when progesterone is secreted by the corpus luteum, and is responsible for the histological and molecular changes in the endometrium that occur during the luteal phase. Therefore, in simple terms, progesterone completes the endometrial preparation after adequate estrogenic priming (Steiner 2006).
Finally, there are many other interventions that have been used in order to improve the implantation rate and, this way, increase the live birth rate. These interventions promote the improvement of uterine blood flow (aspirin and sildenafil) or impact on the immunological system (steroids) (Abdolmohammadi‐Vahid 2016; Kuo 1997; Malinova 2013).
Why it is important to do this review
With artificial endometrial preparation (programmed cycle) for women undergoing embryo transfer with frozen embryos or the transfer of fresh embryos derived from donated oocytes the aim is to stimulate the growth of the endometrium in a similar fashion to the natural cycle, by the sequential administration of oestrogen and progestogen. Devroey has reported that hormonal replacement is different in women with functioning ovaries from those women with amenorrhoea; the former group may spontaneously ovulate leading to the decidualisation of endometrial cells (Devroey 1998). Due to this possibility, drugs that suppress ovarian function (such as GnRH agonists) are frequently used in conjunction with oestrogens. Different routes and doses of hormone administration have been used worldwide in order to provide adequate endometrial preparation. However, no clear evidence exists about which is the best endometrial preparation protocol for maximising the receptivity of the endometrium. This review set out to summarise and compare the evidence about the benefits and disadvantages of the different endometrial preparation methods. This is an update of the systematic review originally published in 2010 (Glujovsky 2010).
Objectives
To evaluate the most effective endometrial preparation for women undergoing embryo transfer with frozen embryos, or from using donor oocytes, with regard to the subsequent live birth rate.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs). We excluded quasi‐RCTs and cross‐over studies unless pre‐cross‐over data were available.
Types of participants
Infertile women undergoing an assisted reproductive technology (ART) procedure utilising either fresh donor cycles or frozen embryo transfers were considered.
Types of interventions
The interventions were compared with placebo, no treatment, or between different interventions, both in frozen embryo replacement cycles and in donor oocyte embryo replacement cycles.
Programmed cycle versus stimulated cycle
Programmed cycle versus natural cycle
Transdermal oestrogens versus oral oestrogens
Day of starting administration of the progestogen
GnRH agonists versus control
Among different gonadotropin‐releasing hormone (GnRH) agonists
GnRH agonists versus GnRH antagonists
Aspirin versus control
Steroids versus control
After data collection, the review authors considered that outcomes not listed in the protocol should also be included, to meet the objectives of the review. The following outcomes were added to the inclusion criteria: day of starting the progestogen (which is important as some egg donor programs delay the time to starting the progestogen in order to avoid a cycle cancellation in the case of a total failed fertilisation); programmed cycle versus cycle with ovarian stimulation (some authors claim that ovarian stimulation makes a more natural environment than artificial stimulation).
Types of outcome measures
Primary outcomes
Live birth rate (number of births of one or more living infants per number of women randomised) (Zegers‐Hochschild 2006)
Secondary outcomes
Clinical pregnancy rate (number of pregnancies with at least one sac per number of women randomised)
Miscarriage rate (number of pregnancies ending in the spontaneous loss of the embryo or fetus before 20 weeks of gestation) per woman randomised
Multiple pregnancy rate (number of pregnancies with two or more fetuses per woman randomised
Cycle cancellation rate (number of women with at least one cancelled cycle per number of women randomised)
Endometrial thickness (in millimetres), by ultrasound scan
Other adverse effects such as local adverse effects, hot flushes (at least one adverse effect (excluding miscarriage) per number of women randomised)
Search methods for identification of studies
We searched for all published and unpublished RCTs, of women undergoing embryo transfer cycles with frozen embryos or donated oocytes, without language restriction and in consultation with the Gynaecology and Fertility Group (CGF) Information Specialist.
Electronic searches
We searched following databases:
The Cochrane Gynaecology and Fertility Group (CGFG) specialised register, PROCITE platform; searched 24 June 2020 (Appendix 1);
CENTRAL via The Cochrane Register of Studies Online (CRSO), Web Platform, searched 24 June 2020 (Appendix 2) (CENTRAL now contains records from CINAHL and the trial registries; clinicaltrials.gov and the World Health Organisation International Trials Registry Platform search portal);
MEDLINE, OVID platform, searched from 1946 to 24 June 2020 (Appendix 3);
Embase, OVID platform, searched from 1980 to 24 June 2020 (Appendix 4);
PsycINFO, OVID platform, searched from 1806 to 24 June 2020 (Appendix 5);
LILACS, Web platform, searched 24 June 2020 (Appendix 6).
Searching other resources
We searched the following other resources:
We searched the National Institute of Clinical Excellence fertility assessment and treatment guidelines (Nice 2017);
We checked references of identified RCTs and relevant systematic reviews;
We personally contacted manufacturers, experts, and specialists in the field;
We handsearched the conference abstracts of the European Society of Human Reproduction and Embryology, and the American Society for Reproductive Medicine.
Data collection and analysis
Selection of studies
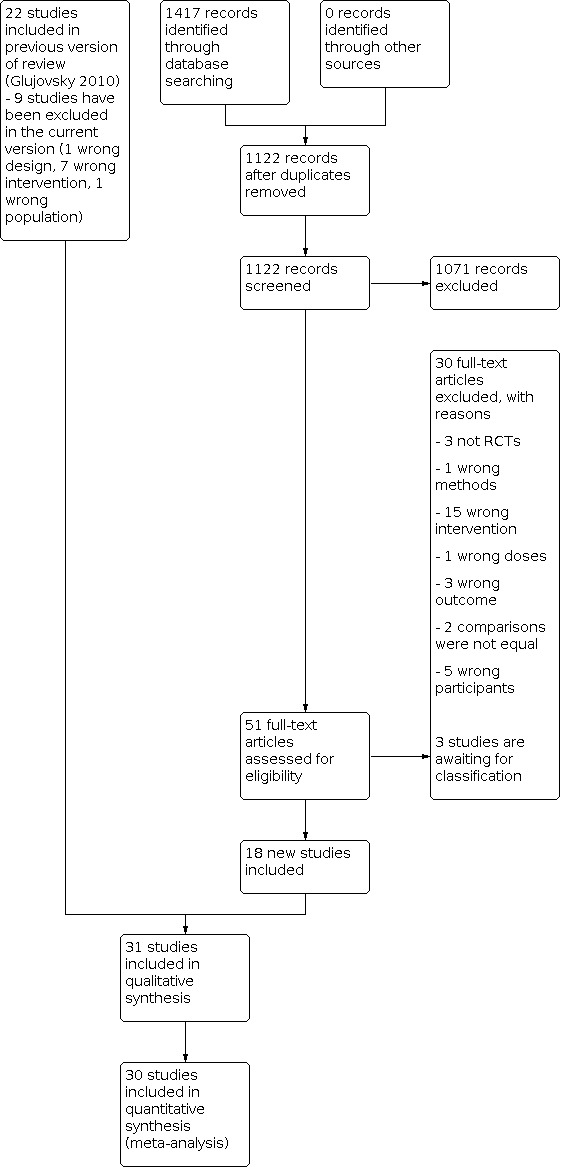
Two review authors (DG, RP) independently undertook the study selection; both are experts in subfertility. Both review authors screened the titles and abstracts of articles found in the search. They discarded studies that were clearly ineligible but were overly inclusive rather than risking the loss of relevant studies. Both review authors independently assessed whether the studies met the inclusion criteria, with disagreements being resolved by discussion. If there was still no agreement, the disagreement was settled by a third review author (CS). Further information was sought from the authors if papers contained insufficient information to make a decision about eligibility. 'Risk of bias' assessment was done using a pro forma. The selection process is documented in a PRISMA flow chart (Figure 1).
1.
Data extraction and management
The same two review authors independently extracted information from the results sections of the included studies using the pro forma's designed by the Review Group. Discrepancies were resolved by discussion. If there was still no agreement, the discrepancy was resolved by a third review author. For each included trial, information was collected regarding the location of the study, methods of the study (as per the quality assessment checklist), the participants (age range, eligibility criteria), the nature of the interventions, and data relating to the outcomes specified above. See data extraction table for details, Appendix 7. If cross‐over trials had been included, we would only have used data from the first stage.
Assessment of risk of bias in included studies
Review authors DG and RP independently assessed the risk of bias of all studies that were deemed eligible for the review using the Cochrane 'Risk of bias' assessment tool (Higgins 2011) to assess: selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other bias. The categories are briefly described in additional Table 10. Judgements were assigned as recommended in the Cochrane Handbook for Systematic Reviews of Interventions Section 8.5 (Higgins 2011), and the conclusions presented in the 'Risk of bias' tables. Disagreements were resolved by discussion. If there was still no agreement, the discrepancy was settled by a third review author (AC). We incorporated the assessment of bias judgements into the interpretation of review findings by means of sensitivity analyses.
1. Quality assessment criteria.
| Assesment | Yes | Unclear | No |
| Allocation concealment | Adequate e.g. central randomisation / allocation, sealed envelopes, etc. | Not reported / unclear | Inadequate |
| Treatment blinding | Statement that containers were identical, drugs were identical in appearance and taste | Not reported / unclear | Interventions were not identical |
| Outcome assessment | Blinded, standardised assessment | Assesment procedures not stated | Assessment not blinded or not standardised |
| Follow‐up completeness for first outcome (live birth rate) | Live birth rate reported | Pregnancy rate reported | Other outcome |
| Baseline equality | Groups balanced in terms of age and angina frequency | Balance not reported | Groups not balanced |
| Losses to follow‐up (not including early cessation of therapy followed up) | Losses of 10% or less | Not reported / unclear | Losses of more than 10% |
| Bias in the analysis: intention‐to‐treat (ITT) | ITT analysis done by the authors | Unclear | Not ITT analysis done by the authors |
| Risk of bias | All of the previous criteria met (all are assessment quality A) | One or more of the previous criteria partly met (at least one assessment quality B and no assessment quality C) | One or more of the previous criteria not met (at least one assessment quality C) |
Measures of treatment effect
For dichotomous data (e.g. live birth rates), results for each study were expressed as odds ratio (OR) with 95% confidence intervals (CIs) and combined for meta‐analysis with RevMan software.For continuous data (e.g. endometrial thickness), we calculated the mean difference (MD) and 95% CIs between treatment groups.
Unit of analysis issues
The primary analysis was per woman randomised. If studies reported only 'per cycle' data, we contacted study authors to request 'per woman randomised' data, and put them in awaiting classification if no reply was received from the authors. We counted multiple live births (e.g. twins, triplets) as one live birth event.
Dealing with missing data
We analysed data on an intention‐to‐treat basis as far as possible and attempted to collect missing data from the trial authors. When data on live birth could not be obtained, we undertook imputation and assumed that the outcome did not occur. For other outcomes, we analysed only available data. Any imputed data were subject to sensitivity analysis.
If studies reported sufficient detail to calculate the MD, but no information on the associated standard deviation (SD), we assumed the outcome had an SD equal to the highest SD from other studies within the same analysis.
Assessment of heterogeneity
Clinical and methodological characteristics of the included studies were examined by visual inspection of the forest‐plot graphs, the overlap in CIs and, more formally, by checking the results of the I2 statistic. An I2 measurement greater than 50% was taken to indicate substantial heterogeneity (Higgins 2011). When possible, and if no heterogeneity was present, the outcomes were pooled.
Assessment of reporting biases
We aimed to minimise the potential impact of publication bias and other reporting biases by ensuring a comprehensive search for eligible studies. If 10 or more studies were included in an analysis, we planned to use a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies; Higgins 2011).
Data synthesis
Data synthesis and analyses were done using the Review Manager 5.3 (Review Manager 2014). Dichotomous outcomes were reported as odds ratios (OR) and continuous outcomes as mean differences (MD), both with 95% CIs. The mean change was used in continuous outcomes, where possible, otherwise mean results at final follow‐up were used. Studies reporting change differences and end of treatment differences were entered into the same analyses. Interventions were compared on the basis of drug class. Where studies randomised recipients across arms involving comparisons of members of the same drug class, the data for arms comparing the same drug class were combined.
Heterogeneity in treatment effects across studies was assessed using inspection of forest plots, the Cochran Q test and I² quantity (where P < 0.10 and > 60%, respectively, were considered evidence of substantial heterogeneity). Where the authors considered it was reasonable to pool studies, the fixed‐effect model was used to combine study results where estimates of heterogeneity were minimal and the random‐effects method of DerSimonian and Laird (DerSimonian 1986) where heterogeneity was moderate (I2 > 40%). Where substantial heterogeneity was evident, summary estimates were not calculated, but the estimates of effect were investigated further using subgroup and sensitivity analyses.
Subgroup analysis and investigation of heterogeneity
We performed a subgroup analysis for frozen‐thawed embryo transfers and fresh transfers of embryos coming from donor oocytes. Although heterogeneity was expected due to some variables (amenorrhoea versus non‐amenorrhoea, endometrial thickness, women's age, embryo quality, embryo transfer) a subgroup analysis was only performed for studies where amenorrhoea was clearly stated, as most studies were inadequately described for other variables.
A post‐hoc subgroup analysis was performed when evaluating the stimulated cycles with clomiphene citrate for the outcome endometrial thickness. As clomiphene citrate works as a selective oestrogen receptor modulator, it is expected to result in some thinner endometrium in comparison to other stimulation protocols.
Sensitivity analysis
We performed a sensitivity analysis using fixed‐effect and random‐effects models to confirm or discard the consistency of results. For continuous data, results from each study were expressed as MD with 95% CsI and combined for meta‐analysis. Meta‐analytic methods for continuous data assume that the underlying distribution of the measurements is normal. If data were clearly skewed and results were reported in the publication as median and range, with non‐parametric tests of significance, the results would also have been reported in the 'Other data' section of the review.
Summary of findings and assessment of the certainty of the evidence
We prepared a 'Summary of findings' tables using GRADEpro and Cochrane methods (Higgins 2011; GRADEpro GDT 2015). These tables evaluated the overall quality of the body of evidence for the main review outcomes (live birth, clinical pregnancy, miscarriage, multiple pregnancy, cycle cancellation, endometrial thickness, other adverse effects) for the main review comparison (Programmed cycles versus Stimulated cycles versus Natural cycles). Additional 'Summary of findings' tables were also prepared for the main review outcomes for other important comparisons (Transdermal oestrogens versus oral oestrogens; day of starting administration of the progestogen; GnRH agonists versus control versus GnRH antagonists; low‐dose aspirin versus control and steroids versus control). We assessed the quality of the evidence using GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness and publication bias). Judgements about the quality of the evidence (high, moderate, low or very low) were made by two review authors working independently, with disagreements resolved by discussion. Judgements were justified, documented, and incorporated into reporting of results for each outcome. We extracted study data, format our comparisons in data tables and prepared 'Summary of findings' tables before writing the results and conclusions of our review.Higgins 2011
Results
Description of studies
Results of the search
The search for this update retrieved 1417 articles. Fifty‐one studies were added at this update as potentially eligible and were retrieved in full text. Considering the 22 studies that were included in the original review, 73 studies met our inclusion criteria. We excluded 39 studies (nine from the previous version and 30 from the current search). Three studies are awaiting classification until more information is received about their methods. See study tables: Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification, and PRISMA flow chart (Figure 1).
Included studies
Study design and setting
Thirty one parallel‐design randomised controlled trials (RCTs) involving 5426 women were included. All were conducted in IVF units. One of the included studies was not included in the primary analysis because it was a study with a very low number of randomised women (less than 10) and were a subgroup of women with previous cancellation due to thin endometrium (Check 2002).
Participants Five of the 31 studies were performed in women undergoing fresh donor oocyte embryo replacement cycles (Escriba 2006; Gutierrez 1999; Remohi 1994; Tocino 2007; Vidal 2009) and the remaining 26 studies involved frozen embryo replacement cycles (Agha‐Hosseini 2018; Aleyasin 2017; Nekoo 2015; Bider 1996; Check 2002; Child 2013; Dal Prato 2002; Davar 2007; Davar 2016; Davar 2020; Ding 2007; El‐Toukhy 2004; Greco 2016; Groenewoud 2016; Kahraman 2018; Lee 2008; Madani 2019; Matsuura 2014; Moffitt 1995; Movahedi 2018; Ramos 2007; Samsami 2018; Samsami 2019; Sheikhi 2018; Tehraninejad 2018; Wright 2006). Most treatment regimens varied from one study to the other. The publication dates of included studies were from 1994 to 2020.
Interventions A variety of different protocols for endometrial preparation were used. Programmed cycle (priming with oestrogens) versus stimulated cycle with follicle stimulating hormone (FSH) (Wright 2006), letrozole (Aleyasin 2017; Matsuura 2014; Samsami 2019) or clomiphene citrate (Sheikhi 2018), transdermal oestrogens versus oral oestrogens (Davar 2016; Kahraman 2018; Tehraninejad 2018). The types of gonadotropin‐releasing hormone (GnRH) agonist used were daily leuprolide acetate (Gutierrez 1999; Remohi 1994), daily variopeptyl (Davar 2020), nasal buserelin (El‐Toukhy 2004; Movahedi 2018), subcutaneous buserelin (Davar 2007; Samsami 2018), daily nafarelin (Gutierrez 1999), depot leuprolide acetate (Tocino 2007), and daily tryptorelin (Tocino 2007) and depot tryptorelin (Dal Prato 2002; Ramos 2007). Also, the use of GnRH antagonists versus GnRH agonists (Vidal 2009). Glucocorticoids used also varied from one study to the other: dexamethasone (Bider 1996) and 6‐alfa‐methylprednisolone (Moffitt 1995) Sildenafil (Check 2002) and aspirin (Madani 2019) were evaluated as well. During the data collection, we found six studies where the comparisons (starting day of progestogen, programmed cycle versus stimulated or natural cycles) had not been included in our protocol; however, we decided to include them in the review because of the importance of the results (Agha‐Hosseini 2018; Ding 2007; Escriba 2006; Greco 2016; Groenewoud 2016; Lee 2008).
Outcomes The primary outcome was live birth rate but only eight studies (Agha‐Hosseini 2018; Aleyasin 2017; Bider 1996; Child 2013; El‐Toukhy 2004; Greco 2016; Groenewoud 2016; Madani 2019) evaluated this outcome. secondary outcomes were clinical pregnancy rate (which is a proxy of the primary outcome), multiple pregnancy rate, cycle cancellation rate, miscarriage rate, and endometrial thickness (before the embryo transfer). Finally, nine different comparison types were performed. Heterogeneity was evaluated for each intervention. In those cases where we found heterogeneity, pre‐specified sensitivity and subgroup analyses were performed. We also evaluated ad hoc subgroups. We added 'Summary of findings' tables for all the outcomes.
Excluded studies
Of the 39 excluded studies, four were not RCTs (Check 1998; Nardo 2006; Neuspiller 1998; Stadtmauer 2009), one used an inadequate method of randomisation (Sathanandan 1991), 22 did not appear to use one of our specified treatments for endometrial preparation (Arun Muthuvel 2016; Bernabeu 2006; Bjuresten 2011; Boostanfar 2016; Caligara 2003; Cambiaghi 2013; Davar 2015; Eftekhar 2013; Gibbons 1998; Gogce 2015; Hershko 2016; Lan 2008; Li 2014; Lightman 1999; Llacer 2017; Moon 2004; Prapas 2009a; Prapas 2009b; Sanchez 2009; Shiotani 2006; Tesarik 2003; Zegers‐Hochschild 2000), one used doses for vaginal progestogen that were bellow the standard doses (Feliciani 2004), three did not evaluate a primary or secondary outcome stated in our protocol (Krasnow 1996; Lewin 2001; Taskin 2002), two used comparisons that were not equal (both treatment groups had more than one intervention in their treatment regimen) (Check 2004; Simon 1998) and six included participants that did not met our criteria (Davar 2016a; Davari‐Tanha 2016; Huang 2017; Weckstein 1997; Xu 2015; Zolghadri 2014). Three studies are awaiting classification because the outcomes were reported 'per cycle' and not per woman randomised, in which more than one cycle was performed on each randomised woman (Masrour 2018; Page 2005; Tur‐Kaspa 2010).
Risk of bias in included studies
Details on the quality of each individual study are described in the table 'Characteristics of included studies', where the individual quality criteria were rated for each study.
Although most authors gave a description of the randomisation method, only some of them described the allocation method. As a placebo was not used in most of the trials, there was no blinding of patients; blinding of healthcare workers was not described. Nevertheless, we do not think that lack of blindness can bias the results in this case. Live birth rate was reported in a minority of cases; the remainder of the authors used pregnancy rate, which was the most frequently reported final outcome. Even when comparability at baseline was not measured, it was generally evaluated. There were very few trials with a relevant loss to follow‐up. There was heterogeneity in intention‐to‐treat analysis and it was not done by all the authors. Finally, outcome assessment was not generally described in terms of blinding of the evaluator. Both a 'Risk of bias' table (Figure 2) and a 'Risk of bias' graph (Figure 3) are presented.
2.

'Risk of bias' summary: review authors' judgments about each risk of bias item for each included study.
3.

'Risk of bias' graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
Allocation
In four studies the method of concealing allocation was adequate (Dal Prato 2002; Groenewoud 2016; Madani 2019; Moffitt 1995), while in four other studies there was high risk of bias (Agha‐Hosseini 2018; El‐Toukhy 2004; Sheikhi 2018;Tehraninejad 2018) The remaining studies are unclear as the allocation concealment method was not explained and authors did not respond when they were contacted.
Blinding
Quality limitations were mainly in blinding, given that only one study stated that participants were blinded to treatment (Moffitt 1995). Because the assessed outcomes were very objective, lack of blinding of the outcome assessors did not introduce as high a risk of bias if the outcomes had been subjective. We considered that lack of blinding could only impact on cancellation rates.
Incomplete outcome data
In three studies we found a high risk of bias due to a high proportion of the participants that were not followed up (Check 2002; Groenewoud 2016; Samsami 2018).
The sample size of the analysed studies and subgroups varied from a minimum of 16 to a maximum of 354 women.
We contacted the authors for more information, as required.
Selective reporting
Only eight studies (Agha‐Hosseini 2018; Aleyasin 2017; Bider 1996; Child 2013; El‐Toukhy 2004; Greco 2016; Groenewoud 2016; Madani 2019) reported the live birth rate, our primary outcome. This could represent a potential selective reporting bias.
Other potential sources of bias
All studies except four (Gutierrez 1999; Lee 2008; Ramos 2007; Tocino 2007) reported baseline equality between groups with respect to age at stimulation, diagnosis of infertility, number of transferred embryos and number of previous pregnancies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9
Summary of findings 1. Stimulated cycle compared to programmed for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes.
| Stimulated cycle compared to programmed for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes | ||||||
| Patient or population: women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes Setting: IVF unit Intervention: stimulated cycle Comparison: programmed cycle | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with programmed | Risk with Stimulated cycle | |||||
| Live birth rate | 240 per 1000 | 285 per 1000 (134 to 507) | OR 1.26 (0.49 to 3.26) | 100 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a b | Letrozole stimulation versus programmed cycle |
| Clinical pregnancy rate | 191 per 1000 | 278 per 1000 (210 to 360) | OR 1.63 (1.12 to 2.38) | 656 (5 RCTs) | ⊕⊕⊝⊝ LOW a c | |
| Miscarriage rate | 87 per 1000 | 70 per 1000 (33 to 140) | OR 0.79 (0.36 to 1.71) | 355 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW a b | |
| Multiple pregnancy rate | Not reported in any study | |||||
| Cycle cancellation rate | Not reported in any study | |||||
| Endometrial thickness (mm) | The mean endometrial thickness (mm) was 8.7 mm | MD ‐0.05 mm (‐0.19 lower to 0.10 higher) | ‐ | 362 (2 RCTs) | ⊕⊕⊝⊝ LOW a c | Letrozole stimulation versus programmed cycle |
| Other adverse effects | Not reported in any study | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; RCT: randomised controlled trial. | ||||||
|
GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a Downgraded one level due to risk of bias. It is unclear the sequence generation and method of allocation concealment that was used.
b Downgraded two levels due to very serious imprecision. The confidence interval is too wide. The intervention could improve or reduce the outcome.
c Downgraded one level due to imprecision. The confidence interval is too wide.
Summary of findings 2. Natural cycle compared to programmed cycle for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes.
| Natural cycle compared to programmed cycle for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes | ||||||
| Patient or population: women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes Setting: IVF unit Intervention: natural cycle Comparison: programmed cycle | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with programmed cycle | Risk with Natural cycle | |||||
| Live birth rate | 233 per 1000 | 228 per 1000 (184 to 280) | OR 0.97 (0.74 to 1.28) | 1285 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW a b c | |
| Clinical pregnancy rate | 347 per 1000 | 296 per 1000 (248 to 350) | OR 0.79 (0.62 to 1.01) | 1249 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW a b d | |
| Miscarriage rate | 50 per 1000 | 32 per 1000 (13 to 82) | OR 0.64 (0.25 to 1.63) | 485 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa e | |
| Multiple pregnancy rate | Not reported in any study | |||||
| Cycle cancellation rate | 365 per 1000 | 256 per 1000 (202 to 320) | OR 0.60 (0.44 to 0.82) | 734 (1 RCT) | ⊕⊕⊕⊝ MODERATE b | |
| Endometrial thickness (mm) | The mean difference endometrial thickness (mm) was 0.42 | MD 0.22 higher (0.25 lower to 0.69 higher) | ‐ | 485 (3 RCTs) | ⊕⊕⊝⊝ LOW a d | |
| Other adverse effects | Not reported in any study | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; RCT: randomised controlled trial | ||||||
|
GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a We downgraded the evidence by one level, due to risk of bias: unclear or high risk of bias for allocation concealment.
b We downgraded the evidence by one level, due to risk of bias: high risk of attrition bias.
c We downgraded the evidence by one level, due to imprecision: the confidence interval is too wide. The intervention could improve or reduce the outcome.
d We downgraded the evidence by one level for inconsistency due to heterogeneity.
e We downgraded two levels for serious imprecision, few events.
Summary of findings 3. Transdermal oestrogens compared to oral oestrogens for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes.
| Transdermal oestrogens compared to oral oestrogens for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes | ||||||
| Patient or population: women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes Setting: IVF unit Intervention: transdermal oestrogens Comparison: oral oestrogens | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with oral oestrogens | Risk with Transdermal oestrogens | |||||
| Live birth rate | ‐ | Not reported in any study | ||||
| Clinical pregnancy rate | 506 per 1000 | 468 per 1000 (377 to 561) | OR 0.86 (0.59 to 1.25) | 504 (3 RCTs) | ⊕⊕⊝⊝ LOW a b | |
| Miscarriage rate | 119 per 1000 | 69 per 1000 (35 to 128) | OR 0.55 (0.27 to 1.09) | 414 (2 RCTs) | ⊕⊕⊝⊝ LOW a b | |
| Multiple pregnancy rate | ‐ | Not reported in any study | ||||
| Cycle cancellation rate | ‐ | Not reported in any study | ||||
| Endometrial thickness (mm) | ‐ | Not reported in any study | ||||
| Other adverse effects | ‐ | Not reported in any study | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; RCT: randomised controlled trial. | ||||||
|
GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a We downgraded the evidence by one level, due to risk of bias: unclear or high risk of bias for allocation concealment.
b We downgraded the evidence by one level, due to very wide confidence interval. The intervention could improve or reduce the outcome.
Summary of findings 4. Starting administration of the progestogen earlier compared to starting administration of the progestogen later for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes.
| Starting administration of the progestogen earlier compared to starting administration of the progestogen later for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes | ||||||
| Patient or population: women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes Setting: IVF unit Intervention: starting administration of progestogen earlier Comparison: starting administration of progestogen later | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with starting administration of the progestogen earlier | Risk with starting administration of the progestogen later | |||||
| Live birth rate | Not reported in any study | |||||
| Clinical pregnancy rate | 381 per 1000 | 536 per 1000 (433 to 634) | OR 1.87 (1.13 to 3.08) | 282 (1 RCT) | ⊕⊕⊕⊝ MODERATE a | Day before oocyte retrieval (T1) versus day of oocyte retrieval or day after(T2) in Oocyte donation |
| Miscarriage Rate | 128 per 1000 | 62 per 1000 (23 to 155) | OR 0.45 (0.16 to 1.25) | 191 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a b | Day before oocyte retrieval (T1) versus day of oocyte retrieval (T2) in Oocyte donation |
| Multiple pregnancy rate | 189 per 1000 | 144 per 1000 (79 to 249) |
0.72 (0.37 to 1.42) | 282 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a b | Day before oocyte retrieval (T1) versus day of oocyte retrieval or day after(T2) in Oocyte donation |
| Cycle cancellation rate | 38 per 1000 | 11 per 1000 (4 to 28) | OR 0.28 (0.11 to 0.74) | 282 (1 RCT) | ⊕⊕⊕⊝ MODERATE a | |
| Endometrial thickness | Not reported in any study | |||||
| Other adverse effects | Not reported in any study | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; RCT: randomised controlled trial. | ||||||
|
GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a We downgraded the evidence by one level, due to risk of bias: unclear risk of bias for allocation concealment and unclear risk of attrition bias.
b We downgraded the evidence by two levels, due to a very wide confidence interval. The intervention could improve or reduce the outcome.
Summary of findings 5. GnRH agonists compared to control for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes.
| GnRH agonists compared to control for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes | ||||||
| Patient or population: women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes Setting: IVF unit Intervention: GnRH agonists Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Risk with GnRH agonists | |||||
| Live birth rate | 85 per 1000 | 197 per 1000 (100 to 351) | OR 2.62 (1.19 to 5.78) | 234 (1 RCT) | ⊕⊕⊝⊝ LOW a b | |
| Clinical pregnancy rate | 184 per 1000 | 199 per 1000 (151 to 264) | OR 1.08 (0.82 to 1.43) | 1289 (8 RCTs) | ⊕⊕⊝⊝ LOW c d | In frozen‐embryo transfers |
| Miscarriage rate | 30 per 1000 | 26 per 1000 (11 to 58) | OR 0.85 (0.36 to 2.00) | 828 (4 RCTs) | ⊕⊕⊝⊝ LOW d e | |
| Multiple pregnancy rate | Not reported in any study | |||||
| Cycle cancellation cycles | 60 per 1000 | 30 per 1000 (13 to 69) | OR 0.49 (0.21 to 1.17) | 530 (2 RCTs) | ⊕⊕⊝⊝ LOW d e | |
| Endometrial thickness (mm) | The mean endometrial thickness (mm) was 9.4 mm | MD 0.08 mm lower (0.33 lower to 0.16 higher) | ‐ | 697 (4 RCTs) | ⊕⊕⊝⊝ LOW d e | |
| Other adverse effects | Not reported in any study | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; GnRH: gonadotropin‐releasing hormone; OR: Odds ratio; RCT: randomised controlled trial. | ||||||
|
GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a We downgraded the evidence by one level, due to high risk of bias in allocation concealment.
b We downgraded one level due to imprecision, one small study (less than 300).
c We downgraded the evidence by one level, due to unclear or high risk of bias in allocation concealment and unclear risk of bias in reporting bias.
d We downgraded the evidence by one level, due to imprecision: a wide confidence interval. The intervention could improve or reduce the outcome.
e We downgraded the evidence by one level, due to high risk of bias in randomisation method and allocation concealment.
Summary of findings 6. Among different GnRH agonists compared to placebo for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes.
| Among different GnRH agonists compared to placebo for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes | ||||||
| Patient or population: women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes Setting: IVF unit Intervention: among different GnRH agonists Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Among different GnRH agonists | |||||
| Live birth rate | Not reported in any study | |||||
| Clinical pregnancy rate | 406 per 1000 | 569 per 1000 (391 to 730) | OR 1.93 (0.62 to 5.98) | 50 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a b | tryptorelin (deposit) versus Leuprolide (daily) in Oocyte donation in ovulating recipients |
| Miscarriage rate | 143 per 1000 | 181 per 1000 (57 to 448) | OR 1.33 (0.36 to 4.87) | 68 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a b | tryptorelin (deposit) versus Leuprolide (daily) in Oocyte donation in ovulating recipients |
| Multiple pregnancy rate | Not reported in any study | |||||
| Cycle cancellation rate | Not reported in any study | |||||
| Endometrial thickness (mm) | Not reported in any study | |||||
| Other adverse effects | Not reported in any study | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; GnRH: gonadotropin‐releasing hormone; OR: Odds ratio; RCT: randomised controlled trial. | ||||||
|
GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a We downgraded the evidence by two levels, due to high risk of bias in randomisation method and in allocation concealment
b We downgraded the evidence by two levels due to very serious imprecision: the confidence interval is too wide. The intervention could improve or reduce the outcome.
Summary of findings 7. GnRH agonists compared to GnRH antagonists for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes.
| GnRH agonists compared to GnRH antagonists for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes | ||||||
| Patient or population: women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes Setting: IVF unit Intervention: GnRH agonists Comparison: GnRH antagonists | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with GnRH antagonists | Risk with GnRH agonists | |||||
| Live birth rate | Not reported in any study | |||||
| Clinical pregnancy rate | 681 per 1000 | 570 per 1000 (473 to 658) | OR 0.62 (0.42 to 0.90) | 473 (1 RCT) | ⊕⊕⊕⊝ MODERATE a | |
| Miscarriage rate | 86 per 1000 | 66 per 1000 (35 to 123) | OR 0.75 (0.38 to 1.49) | 473 (1 RCT) | ⊕⊕⊝⊝ LOW a b | |
| Multiple pregnancy rate | 254 per 1000 | 190 per 1000 (133 to 267) | OR 0.69 (0.45 to 1.07) | 473 (1 RCT) | ⊕⊕⊝⊝ LOW a b | |
| Cycle cancellation rate | Not reported in any study | |||||
| Endometrial thickness (mm) | Not reported in any study | |||||
| Other adverse effects | Not reported in any study | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; GnRH: gonadotropin‐releasing hormone; OR: Odds ratio; RCT: randomised controlled trial. | ||||||
|
GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a We downgraded the evidence by one level, due to risk of bias: unclear risk of bias in randomisation method and in allocation concealment
b We downgraded the evidence by one level, due to imprecision: wide confidence interval.
Summary of findings 8. Aspirin compared to control for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes.
| Aspirin compared to control for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes | ||||||
| Patient or population: women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes Setting: IVF unit Intervention: aspirin Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Risk with Aspirin | |||||
| Live birth rate | 100 per 1000 | 400 per 1000 (141 to 730) | OR 6.00 (1.48 to 24.30) | 60 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a b | |
| Clinical pregnancy rate | 167 per 1000 | 400 per 1000 (167 to 690) | OR 3.33 (1.00 to 11.14) | 60 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a b | |
| Miscarriage rate | Not reported in any study | |||||
| Multiple pregnancy rate | Not reported in any study | |||||
| Cycle cancellation rate | Not reported in any study | |||||
| Endometrial thickness (mm) | The mean endometrial Thickness (mm) was 9.1 mm | MD 0.4 mm lower (0.95 lower to 0.15 higher) | ‐ | 60 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a b | |
| Other adverse effects | Not reported in any study | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; RCT: randomised controlled trial. | ||||||
|
GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a Downgraded one level for risk of bias: live birth and pregnancy rates were lower than usual in the group that did not use the intervention. It is unclear if other bias exist.
b Downgraded two levels due to imprecision. It is only one very small study (n = 60). The intervention could improve or have no effect on the outcome.
Summary of findings 9. Steroids compared to control for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes.
| Steroids compared to control for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes | ||||||
| Patient or population: women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes Setting: IVF unit Intervention: steroids Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Risk with Steroids | |||||
| Live birth rate | 85 per 1000 | 58 per 1000 (13 to 224) | OR 0.66 (0.14 to 3.11) | 99 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a b | |
| Clinical pregnancy rate | 200 per 1000 | 184 per 1000 (91 to 337) | OR 0.90 (0.40 to 2.03) | 160 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW a b | |
| Miscarriage rate | 38 per 1000 | 55 per 1000 (12 to 215) | OR 1.49 (0.32 to 7.03) | 160 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW a b | |
| Multiple pregnancy rate | One study measured the multiple pregnancy rate and reported none in either group (n = 99) | |||||
| Cycle cancellation rate | Not reported in any study | |||||
| Endometrial thickness (mm) | Not reported in any study | |||||
| Other adverse effects | Not reported in any study | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; RCT: randomised controlled trial. | ||||||
|
GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a Downgraded one level due to risk of bias in allocation concealment.
b Downgraded two levels due to very serious imprecision: the confidence interval is too wide, and few events. The intervention could improve or reduce the outcome.
1) Programmed cycle (priming with oestrogens) versus stimulated cycle (with follicle stimulating hormone (FSH), letrozole or clomiphene citrate) Only one study (Wright 2006) compared the outcomes for women having a cycle in which the ovaries were stimulated with recombinant follicle stimulating hormone (rFSH) and programmed cycles in which the endometrium was stimulated with oestrogen (17‐beta oestradiol). A total of 100 women undergoing a frozen‐thaw embryo transfer were stimulated with rFSH injections (150 international units (IU)) on days six, eight, and 10 of the menstrual cycle and then continued until the endometrium was thicker than 7 mm or follicles were bigger than 16 mm to 20 mm. The 99 women in the control group, with a programmed cycle, received 17‐beta oestradiol (4 mg) daily until the thickness of the endometrium was greater than 7 mm.
Three other studies(Aleyasin 2017; Matsuura 2014; Samsami 2019) analysed 369 women and compared a stimulation with letrozole (2.5 mg to 5 mg) and a programmed cycle with oral oestrogens for a frozen embryo transfer.
One study (Sheikhi 2018) analysed 92 women and compared a stimulation with clomiphene citrate (50 mg per day for five days) and a programmed cycle with oral oestrogens for a frozen embryo transfer.
Primary outcome
1.1‐Live birth rate (Analysis 1.1)
1.1. Analysis.

Comparison 1: Programmed cycle versus stimulated cycle, Outcome 1: Live birth rate
We are uncertain whether stimulation with letrozole (followed by rFSH from the seventh day) improves the live birth rate in comparison to a programmed cycle (odds ratio (OR) 1.26, 95% confidence interval (CI) 0.49 to 3.26; participants = 100; studies = 1, very low‐quality evidence). This suggests that if the chance of live birth following a programmed cycle is assumed to be 24%, the chance following a stimulated cycle with letrozole would be between 13% and 51%.
There are no results on this outcome in studies that evaluated stimulation with FSH only.
Secondary outcomes
1.2‐ Clinical pregnancy rate (Analysis 1.2)
1.2. Analysis.

Comparison 1: Programmed cycle versus stimulated cycle, Outcome 2: Clinical pregnancy rate
Stimulating with FSH, letrozole or clomiphene citrate may improve the clinical pregnancy rate (OR 1.63, 95% CI 1.12 to 2.38; participants = 656; studies = 5; I2 = 11%, low‐quality evidence). This suggests that if the chance of a clinical pregnancy following a programmed cycle is assumed to be 19%, the chance following a stimulated cycle would be between 21% and 36%.
1.3‐ Miscarriage rate (Analysis 1.3)
1.3. Analysis.

Comparison 1: Programmed cycle versus stimulated cycle, Outcome 3: Miscarriage rate
We are uncertain whether administrating FSH, letrozole or clomiphene citrate decreases the miscarriage rate (OR 0.79, 95% CI 0.36 to 1.71; participants = 355; studies = 3; I2 = 0%, very low‐quality evidence). This suggests that if the chance of miscarriage following a programmed cycle is assumed to be 9%, the chance following a stimulated cycle would be between 3% and 14%.
Multiple pregnancy rate
Not reported.
Cycle cancellation rate
Not reported.
1.4‐ Endometrial thickness (Analysis 1.4) We are uncertain whether administrating FSH or letrozole increases the endometrial thickness compared to a programmed cycle (mean difference (MD) ‐0.05, 95% CI ‐0.19 to 0.10; participants = 362; studies = 2; I2 = 0%, low‐quality evidence). This suggests that if endometrial thickness following a programmed cycle is assumed to be 8.7 mm, following a stimulated cycle it could be between 8.4 mm and 8.9 mm.
1.4. Analysis.

Comparison 1: Programmed cycle versus stimulated cycle, Outcome 4: Endometrial thickness (mm)
When administering clomiphene citrate, endometrial thickness may be thinner in comparison with a programmed cycle (MD ‐1.04, 95% CI ‐1.59 to ‐0.49; participants = 92; studies = 1; low‐quality evidence). This suggests that if endometrial thickness following a programmed cycle is assumed to be 8.7 mm, following a stimulated cycle with clomiphene citrate it could be between 7.1 mm and 8.2 mm.
Other adverse effects
Not reported.
2) Programmed cycle (priming with oestrogens) versus natural cycle
Six studies (Agha‐Hosseini 2018; Child 2013; Greco 2016; Groenewoud 2016; Lee 2008; Sheikhi 2018) compared 830 women with a natural cycle who received an human chorionic gonadotropin (hCG) injection (10000 IU) for triggering the ovulation versus 860 women who received oral micronised estradiol (programmed cycle) between 2 mg/day up to 8 mg/day if the endometrial thickness was inadequate.
Primary outcome
2.1‐Live birth rate (Analysis 2.1) We are uncertain whether a natural cycle improves the live birth rate in comparison with a programmed cycle (OR 0.97, 95% CI 0.74 to 1.28; participants = 1285; studies = 4; I2 = 0%, very low‐quality evidence). This suggests that if the chance of live birth following a programmed cycle is assumed to be 23%, the chance following a natural cycle would be between 18% and 28%.
2.1. Analysis.

Comparison 2: Programmed cycle versus natural cycle, Outcome 1: Live birth rate
Secondary outcomes
2.2‐Clinical pregnancy rate (Analysis 2.2) We are uncertain of the effect on the clinical pregnancy rate between a programmed cycle stimulating with oestradiol, and a natural cycle (OR 0.79, 95% CI 0.62 to 1.01; participants = 1249; studies = 5; I2 = 60%, very low‐quality evidence). This suggests that if the chance of a clinical pregnancy following a programmed cycle is assumed to be 35%, the chance following a natural cycle would be between 25% and 35%.
2.2. Analysis.

Comparison 2: Programmed cycle versus natural cycle, Outcome 2: Clinical pregnancy rate
2.3‐Miscarriage rate (Analysis 2.3) We are uncertain whether a programmed cycle or natural cycle will decrease the miscarriage rate (OR 0.64, 95% CI 0.25 to 1.63; participants = 485; studies = 3; I2 = 0%, very low‐quality evidence). This suggests that if the chance of miscarriage following a programmed cycle is assumed to be 5%, the chance following a natural cycle would be between 1% and 8%.
2.3. Analysis.

Comparison 2: Programmed cycle versus natural cycle, Outcome 3: Miscarriage rate
Multiple pregnancy rate
Not reported.
2.4‐ Cycle cancellation rate
The cycle cancellation rate is probably lower with a modified natural than in a programmed cycle mainly due to insufficient endometrium thickness within the first 14 days (OR 0.60, 95% CI 0.44 to 0.82; participants = 734; studies = 1; moderate‐quality evidence). This suggests that if the chance of a cycle cancellation following a programmed cycle is assumed to be 37%, the chance following a natural cycle would be between 20% and 32%.
2.5‐Endometrial thickness (Analysis 2.5)
2.5. Analysis.

Comparison 2: Programmed cycle versus natural cycle, Outcome 5: Endometrial thickness (mm)
We are uncertain if endometrial thickness is increased when using a natural cycle or a programmed cycle (MD 0.22, 95% CI ‐0.25 to 0.69; participants = 485; studies = 3; I2 = 85%, low‐quality evidence). This suggests that if the endometrial thickness following a programmed cycle is assumed to be 9.1 mm, the thickness following a natural cycle is between 8.8 mm and 9.8 mm.
Other adverse effects
Not reported.
3) Transdermal oestrogens versus oral oestrogens
Three studies (Davar 2016; Kahraman 2018; Tehraninejad 2018) evaluated 457 women that used oestrogens either transdermal (3.9 mg to 6 mg/day) or oral (6 mg/day to 8 mg/day).
Primary outcome
Live birth rate
Not reported.
Secondary outcomes
3.1‐ Clinical pregnancy rate (Analysis 3.1)
3.1. Analysis.

Comparison 3: Transdermal oestrogens versus oral oestrogens, Outcome 1: Clinical pregnancy rate
We are uncertain if the transdermal or oral oestrogens increases the pregnancy rate (OR 0.86, 95% CI 0.59 to 1.25; participants = 504; studies = 3; I2 = 58%; low‐quality evidence). This suggests that if the chance of a clinical pregnancy following oral oestrogens is assumed to be 51%, the chance following transdermal oestrogens would be between 38% and 56%.
3.2‐ Miscarriage rate (Analysis 3.2)
3.2. Analysis.

Comparison 3: Transdermal oestrogens versus oral oestrogens, Outcome 2: Miscarriage rate
We are uncertain whether transdermal or oral oestrogens decreases the miscarriage rate (OR 0.55, 95% CI 0.27 to 1.09; participants = 414; studies = 2; I2 = 0%; low‐quality evidence). This suggests that if the chance of a miscarriage following oral oestrogens is assumed to be 12%, the chance following transdermal oestrogens would be between 4% and 13%.
Multiple pregnancy rate
Not reported.
Cycle cancellation rate
Not reported
Endometrial thickness
Not reported.
Other adverse effects
Not reported.
4) Day of starting progesterone
Two trials evaluated the outcomes for 331 women in order to identify the optimal day for starting micronised intravaginal progesterone (800mg/day) in a fresh synchronised oocyte donor program. One of the trials evaluated cycles that were transferred on day three after the oocyte retrieval (Escriba 2006). Three interventions were compared: i) starting progesterone the day before oocyte pick up (OPU); ii) starting progesterone the day of OPU; and iii) starting progesterone the day after OPU. All recipients used vaginal suppositories of progesterone and had the embryo transfer on day three. Besides, one trial (Ding 2007) evaluated the start of progesterone six days versus seven days before the frozen embryo transfer.
Primary outcome
Live birth rate
Not reported.
Secondary outcomes
4.1‐Clinical pregnancy rate (Analysis 4.1) Starting progesterone on the day of OPU may improve the clinical pregnancy rate in comparison with starting the day before the OPU (OR 1.92, 95% CI 1.08 to 3.42; participants = 191; studies = 1, low‐quality evidence). Starting progesterone on the day after OPU may improve the clinical pregnancy rate in comparison with starting the day before the OPU (OR 1.81, 95% CI 1.01 to 3.24; participants = 188; studies = 1; low‐quality evidence). When analysed together (Analysis 4.6: ad hoc), starting progesterone either on the OPU day or the day after OPU probably improves the clinical pregnancy rate in comparison to starting the day before (OR 1.87, 95% CI 1.13 to 3.08, participants = 282, studies = 1, moderate‐quality evidence). Due to a wide confidence interval, we are uncertain of the effect of starting progesterone the day of OPU or the day after on the clinical pregnancy rate (OR 0.94, 95% CI 0.53 to 1.68; participants = 99; studies = 1; low‐quality evidence) (Escriba 2006). This suggests that if the chance of a clinical pregnancy when starting the progesterone the day before OPU is assumed to be 38%, the chance when starting the day of OPU or the day after OPU may be between 43% and 64%.
4.1. Analysis.

Comparison 4: Day of starting administration of the progesterone, Outcome 1: Clinical Pregnancy Rate
4.6. Analysis.

Comparison 4: Day of starting administration of the progesterone, Outcome 6: Clinical Pregnancy Rate (by subgroups)
Due to wide confidence interval, we are uncertain of the effect of starting progesterone six days or seven days before a frozen embryo transfer on clinical pregnancy rate (OR 0.75, 95% CI 0.24 to 2.34; participants = 49; studies = 1, very low‐quality evidence) (Ding 2007).
4.2‐Miscarriage rate (Analysis 4.2) We are uncertain of the effect of starting the progesterone before or on the day of OPU (OR 0.45, 95% CI 0.16 to 1.25; participants = 191; studies = 1; very low‐quality evidence), on the day of OPU or the day after OPU (OR 2.52, 95% CI 0.85 to 7.46; participants = 185; studies = 1; very low‐quality evidence), or on the day before or after OPU (OR 1.13, 95% CI 0.33 to 3.85; participants = 188; studies = 1; very low‐quality evidence). This suggests that if the chance of a miscarriage when starting the progesterone the day before OPU is assumed to be 13%, the chance when starting the day of OPU would be between 2% and 16%.
4.2. Analysis.

Comparison 4: Day of starting administration of the progesterone, Outcome 2: Miscarriage Rate
4.3‐Multiple pregnancy rate (Analysis 4.3) We are uncertain of the effect of any of the three intervention groups on the multiple pregnancy rate (OR 0.72, 95% CI 0.37 to 1.42; participants = 282; studies = 1; very low‐quality evidence). This suggests that if the chance of a multiple pregnancy when starting the progesterone the day before OPU is assumed to be 19%, the chance when starting the day of OPU or the day after OPU is between 8% and 25%.
4.3. Analysis.

Comparison 4: Day of starting administration of the progesterone, Outcome 3: Multiple Pregnancy Rate
4.4‐Cycle cancellation rate (Analysis 4.4) Starting progesterone on the day after OPU or the day of OPU probably reduces the cancellation rate when compared to starting the day before OPU (OR 0.28, 95% CI 0.11 to 0.74; participants = 282; studies = 2; I2 = 0%; moderate‐quality evidence) (see Analysis 4.7 and Figure 4). We are uncertain of the effect on cycle cancellation rate when comparing the start of the progesterone on the day of OPU or the day after OPU (OR 0.77, 95% CI 0.17 to 3.53; participants = 185; studies = 1, low‐quality evidence). Starting the progesterone the day after the OPU may reduce the risk of cancellation in comparison to starting the progesterone the day of OPU or the day before OPU. However, the analysis ruled out a clinically relevant difference when starting the progesterone the day after the OPU in comparison to starting one or two days before, but the quality of the evidence is low (OR 0.10, 95% CI 0.01 to 1.82; participants = 282; studies = 1; I2 = 0%; low‐quality evidence) (Escriba 2006). This suggests that if the chance of a cycle cancellation when starting the progesterone the day before OPU is assumed to be 4%, the chance when starting the day of OPU or the day after OPU is probably between 0% and 3%.
4.4. Analysis.

Comparison 4: Day of starting administration of the progesterone, Outcome 4: Cycle cancellation rate
4.7. Analysis.

Comparison 4: Day of starting administration of the progesterone, Outcome 7: Cycle cancellation rate (by subgroups)
4.

Forest plot of comparison: 4 Day of starting administration of the Progesterone, outcome: 4.7 Cancelled cycles (by subgroups).
Endometrial thickness
Not reported.
Other adverse effects
Not reported.
5) GnRH agonist versus no treatment In nine studies, a total of 1358 women were included for an analysis of gonadotropin‐releasing hormone (GnRH) agonist versus control. Type of agonist and administration route were varied.
Primary outcome
5.1‐Live birth rate (Analysis 5.1) Live birth rate was described by only one trial (El‐Toukhy 2004), in which nasal buserelin was compared with no treatment in 334 women undergoing a frozen‐thaw embryo transfer. Nasal buserelin may improve live birth rate in comparison with no GnRH agonists (OR 2.62, 95% CI 1.19 to 5.78; participants= 234; studies = 1; low‐quality evidence). This suggests that if the chance of a live birth following no GnRH agonists is assumed to be 9%, the chance following GnRH agonists would be between 10% and 35%.
5.1. Analysis.

Comparison 5: GnRH agonists versus control, Outcome 1: Live Birth Rate
Secondary outcomes
5.2‐Clinical pregnancy rate (Analysis 5.2) Clinical pregnancy rate was determined by eight trials (Nekoo 2015; Dal Prato 2002; Davar 2007; Davar 2020; El‐Toukhy 2004; Movahedi 2018; Ramos 2007; Samsami 2018), which evaluated the efficacy of intramuscular diphereline, nasal and subcutaneous buserelin, subcutaneous variopeptyl, and intramuscular depot tryptorelin for women undergoing a frozen‐thaw embryo transfer. A ninth study (Remohi 1994) tested daily leuprolide acetate in oocyte donor recipients. The comparator was no treatment in all nine studies.
5.2. Analysis.

Comparison 5: GnRH agonists versus control, Outcome 2: Clinical Pregnancy Rate
Frozen‐thawed embryo transfers
We are uncertain of the effect of GnRH agonists on clinical pregnancy rate (OR 1.08, 95% CI 0.82 to 1.43; participants = 1289; studies = 8; I2 = 20%; low‐quality evidence) in frozen‐thawed embryo transfers. This suggests that if the chance of a clinical pregnancy following no GnRH agonists is assumed to be 18%, the chance following GnRH agonists would be between 15% and 26%.
Fresh oocyte donor transfers
We are uncertain of the effect of GnRH agonists on clinical pregnancy rate (OR 0.86, 95% CI 0.33 to 2.22; participants = 54; studies = 1; low‐quality evidence) in fresh oocyte donor transfers.
5.3‐ Miscarriage rate (Analysis 5.3) Three studies (Nekoo 2015; Dal Prato 2002; Samsami 2018) evaluated the miscarriage rate. We are uncertain of the effect of the GnRH on miscarriage rate (OR 0.85, 95% CI 0.36 to 2.00; participants = 828; studies = 4; I2 = 0%, low‐quality evidence). This suggests that if the chance of a miscarriage following no GnRH agonists is assumed to be 3%, the chance following GnRH agonists would be between 1% and 6%.
5.3. Analysis.

Comparison 5: GnRH agonists versus control, Outcome 3: Miscarriage Rate
Multiple pregnancy rate
Not reported.
5.4‐ Cycle cancellation rate (Analysis 5.4) Two of the five studies (Dal Prato 2002; El‐Toukhy 2004) described the cycle cancellation rates in a total of 530 women being prepared to undergo a frozen‐thaw embryo transfer. We are uncertain if the use of GnRH agonists reduces the cycle cancellation rate (OR 0.49, 95% CI 0.21 to 1.17; participants = 530; studies = 2; I2 = 0%, low‐quality evidence). This suggests that if the chance of a miscarriage following no GnRH agonists is assumed to be 6%, the chance following GnRH agonists would be between 1% and 7%.
5.4. Analysis.

Comparison 5: GnRH agonists versus control, Outcome 4: Cycle cancellation rate
5.5‐ Endometrial thickness (Analysis 5.5) In 697 women involved in four studies (Dal Prato 2002; El‐Toukhy 2004; Davar 2020; Movahedi 2018), the analysis ruled out a clinically relevant difference in the endometrial thickness, but the quality of the evidence is low (MD ‐0.08, 95% CI ‐0.33 to 0.16, four RCTs, N = 697, I2=4%, low‐quality evidence). This suggests that if the endometrial thickness following GnRH agonists is assumed to be 9.4 mm, the endometrial thickness following no treatment is probably between 9.1 mm and 9.6 mm.
5.5. Analysis.

Comparison 5: GnRH agonists versus control, Outcome 5: Endometrial Thickness (mm)
Other adverse effects
Not reported.
6) One GnRH agonist versus other types of GnRH agonists Two studies evaluated the use of different types of GnRH agonists in fresh donor oocyte recipient cycles. One study (Gutierrez 1999) reported results from a comparison between leuprolide acetate (1 mg/day subcutaneously) and nafarelin (100 μg twice a day) starting at mid‐luteal phase. The other study compared daily leuprolide acetate (0.1 mL/day) versus depot tryptorelin (3.75 mg intramuscularly) both starting 5 days before ending the contraceptive pill (Tocino 2007). A total of 118 women were analysed.
Primary outcome
Live birth rate
Not reported.
Secondary outcomes
6.1‐ Clinical pregnancy rate (Analysis 6.1) We are uncertain whether any specific GnRH agonist improves the clinical pregnancy rate. When comparing daily leuprolide acetate and deposit tryptorelin (OR 1.93, 95% CI 0.62 to 5.98; participants = 50; studies = 1; very low‐quality evidence). This suggests that if the chance of a clinical pregnancy following daily leuprolide acetate is assumed to be 41%, the chance following deposit tryptorelin would be between 39% and 73%.
6.1. Analysis.

Comparison 6: Among different GnRH agonists, Outcome 1: Clinical Pregnancy Rate
6.2‐Miscarriage rate (Analysis 6.2) We are uncertain whether any specific GnRH agonist reduces the miscarriage rate. When comparing daily leuprolide acetate and daily nafarelin (OR 1.33, 95% CI 0.36 to 4.87; participants = 68; studies = 1; very low‐quality evidence). This suggests that if the chance of a miscarriage following daily leuprolide acetate is assumed to be 6%, the chance following daily nafarelin would be between 1% and 20%.
6.2. Analysis.

Comparison 6: Among different GnRH agonists, Outcome 2: Miscarriage Rate
Multiple pregnancy rate
Not reported.
Cycle cancellation rate Not reported.
Endometrial thickness
Not reported.
Other adverse effects
Not reported.
7) GnRH agonists versus GnRH antagonists
One study (Vidal 2009) analysed 473 women who underwent preparation of the endometrium for oocyte donation with either a 7 day dosage of GnRH antagonist (cetrorelix 0.25mg) versus conventional single dose GnRH agonist (tryptorelin, 3.75 mg intramuscularly) on day 21 of the menstrual cycle.
Primary outcome
Live birth rate
Not reported.
Secondary outcomes
7.1‐ Clinical pregnancy rate
Administrating GnRH antagonists probably increases the clinical pregnancy rate (OR 0.62, 95% CI 0.42 to 0.90; participants = 473; studies = 1; moderate‐quality evidence). This suggests that if the chance of a clinical pregnancy following GnRH antagonists is assumed to be 68%, the chance following GnRH agonists would be between 47% and 66%.
7.2‐ Miscarriage rate
We are uncertain whether administrating GnRH agonist or antagonists increases the miscarriage rate (OR 0.75, 95% CI 0.38 to 1.49; participants = 473; studies = 1; low‐quality evidence). This suggests that if the chance of a miscarriage following GnRH antagonists is assumed to be 9%, the chance following GnRH agonists would be between 4% and 12%.
7.3‐ Multiple pregnancy rate
We are uncertain whether administrating GnRH agonist or antagonists reduces the multiple pregnancy rate (OR 0.69, 95% CI 0.45 to 1.07; participants = 473; studies = 1, low‐quality evidence). This suggests that if the chance of a miscarriage following GnRH antagonists is assumed to be 25%, the chance following GnRH agonists would be between 13% and 27%.
Cycle cancellation rate
Not reported.
Endometrial thickness
Not reported
Other adverse effects
Not reported.
8) Low‐dose aspirin versus no treatment One study (Madani 2019) involving 60 women undergoing a frozen embryo transfer were randomised to receive either low dose aspirin (100 mg/day orally) or placebo which was administered at the same time as estradiol valerate..
Primary outcome
8.1‐Live birth rate (Analysis 8.1) We are uncertain whether administering low‐dose aspirin increases the live birth rate (OR 6.00, 95% CI 1.48 to 24.30; participants = 60; studies = 1; very low‐quality evidence). This suggests that if the chance of a live birth following no administration of aspirin is assumed to be 10%, the chance following aspirin would be between 14% and 73%.
8.1. Analysis.

Comparison 8: Aspirin versus control, Outcome 1: Live Birth Rate
Secondary outcomes
8.2‐Clinical pregnancy rate (Analysis 8.2) It is uncertain whether the low‐dose aspirin increases the clinical pregnancy rate (OR 3.33, 95% CI 1.00 to 11.14; participants = 60; studies = 1, very low‐quality evidence). This suggests that if the chance of a clinical pregnancy following no administration of aspirin is assumed to be 17%, the chance following aspirin would be between 17% and 69%.
8.2. Analysis.

Comparison 8: Aspirin versus control, Outcome 2: Clinical Pregnancy Rate
Miscariage rate
Not reported.
Multiple pregnancy rate
Not reported
Cycle cancellation rate
Not reported.
8.3‐Endometrial thickness (Analysis 8.3) We are uncertain whether there is a clinically relevant difference between using aspirin or not (MD ‐0.40, 95% CI ‐0.95 to 0.15; participants = 60; studies = 1; very low‐quality evidence). This suggests that if the endometrial thickness following no administration of aspirin is assumed to be 9.1 mm, the chance of the endometrial thickness following administration of aspirin would be between 8.9 mm and 10 mm.
8.3. Analysis.

Comparison 8: Aspirin versus control, Outcome 3: Endometrial Thickness (mm)
Other adverse effects
Not reported.
9) Steroids versus no treatment Two studies (Bider 1996; Moffitt 1995) involving a total of 160 women who underwent a frozen‐thaw embryo transfer evaluated the impact of taking steroids for one or two days prior to embryo transfer on reproductive outcomes. One trial used dexamethasone 0.5 mg orally for five days and the other used methylprednisolone 4 mg orally for four days.
Primary outcome
9.1‐Live birth rate (Analysis 9.1)
9.1. Analysis.

Comparison 9: Steroids versus control, Outcome 1: Live Birth Rate
Live birth rate was described by one trial (Bider 1996). It is uncertain whether corticosteroids improves the live birth rate compared to control (OR 0.66, 95% CI 0.14 to 3.11; participants = 99; studies = 1; very low‐quality evidence). This suggests that if the chance of a live birth following no administration of steroids is assumed to be 9%, the chance following steroids would be between 1% and 22%.
Secondary outcomes
9.2‐Clinical pregnancy rate (Analysis 9.2) Clinical pregnancy rate was described by two trials (Bider 1996; Moffitt 1995). It is uncertain whether corticosteroids improves the clinical pregnancy rate (OR 0.90, 95% CI 0.40 to 2.03; participants = 160; studies = 2; I2 = 0%; very low‐quality evidence). This suggests that if the chance of a clinical pregnancy following no administration of steroids is assumed to be 20%, the chance following steroids would be between 9% and 34%.
9.2. Analysis.

Comparison 9: Steroids versus control, Outcome 2: Clinical Pregnancy Rate
9.3‐Miscarriage rate (Analysis 9.3) It is uncertain whether corticosteroids improves the miscarriage rate (OR 1.49, 95% CI 0.32 to 7.03; participants = 160; studies = 2; I2 = 0%; very low‐quality evidence). This suggests that if the chance of a miscarriage following no administration of steroids is assumed to be 4%, the chance following steroids would be between 1% and 22%.
9.3. Analysis.

Comparison 9: Steroids versus control, Outcome 3: Miscarriage Rate
9.4‐Multiple pregnancy rate (Analysis 9.4) Not reported.
9.4. Analysis.

Comparison 9: Steroids versus control, Outcome 4: Multiple Pregnancy Rate
Cycle cancellation rate
Not reported.
Other adverse effects
Not reported.
There were not enough studies with low risk of bias for randomisation method and allocation concealment to make a sensitivity analysis by quality of evidence.
Discussion
Summary of main results
This systematic review of endometrial preparation has demonstrated that few properly performed studies exist comparing the pre‐specified interventions. However, some few conclusions can be retrieved.
In frozen embryo transfers, low‐quality evidence showed that clinical pregnancy rates may be improved in a stimulated cycle compared to a programmed one, and we are uncertain of the effect when comparing a programmed cycle to a natural cycle. Cycle cancellation rates are probably reduced in a natural cycle. Although administering a gonadotropin‐releasing hormone (GnRH) agonist, compared to without, may improve live birth rates, clinical pregnancy rates will probably be improved in a GnRH antagonist cycle over an agonist cycle.
In fresh synchronised oocyte donor cycles, clinical pregnancy rate is probably improved and cancellation rates are probably reduced when starting progestogen the day of or day after donor oocyte retrieval.
Although frozen embryo replacement and oocyte donation cycles have been used clinically for several years (Leeton 1986; Mohr 1985), there is no agreement about the optimal way to prepare the endometrium prior to and immediately following embryo transfer.
More double‐blinded randomised controlled trials (RCTs) with adequate power are required in the future in order to determine if any of the interventions assessed in our review do enhance the chances of live births among those infertile women undergoing frozen embryo transfer or oocyte donation cycles.
Overall completeness and applicability of evidence
The exhaustive search strategy of published and unpublished studies and the limited body of evidence make it unlikely that any relevant study was omitted. Although patients, settings, and interventions could be representative of clinical practice, applicability could be limited by other sources as we found insufficient evidence.
The evidence provided by this review applies to women who are going to have a frozen embryo transfer or a fresh synchronised embryo transfer of embryos derived from donor oocytes. These are interventions in everyday practice, for the general population, and should not be applied to specific cases such as women with a thin endometrium. As most studies do not report live birth rates, we do not have enough evidence, though some of the information could be carefully extrapolated from secondary outcomes such as clinical pregnancy rate.
Quality of the evidence
The quality of the included studies limited our conclusions. The present review shows that endometrial preparation for frozen embryo transfers and fresh embryo transfers in oocyte donation cycles have been evaluated in many different ways. We investigated the need to use GnRH agonists for ovarian suppression, different types and modes of endometrial estrogenic stimulation, different types and timing of initiating progestogen, and other peripheral interventions such as the use of low‐dose aspirin or steroids prior to embryo transfer procedures. More than one intervention may impact on clinical results and so we included RCTs where only one intervention was evaluated.
Overall, the quality of the evidence was low to very low because of methodological limitations, with some moderate‐quality evidence. Few authors described the allocation method, there was no blinding of patients, and blinding of healthcare‐workers was not described. Live birth rate was reported in a minority of cases. Finally, outcome assessment was not generally described in terms of blinding of the evaluator (seeFigure 2; Figure 3).
Potential biases in the review process
Since we followed the updated version of the for Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we are confident the review process was not biased. Although the search strategy was comprehensive, there could be some studies that could have been missed.
Agreements and disagreements with other studies or reviews
Five studies compared different types of GnRH agonists but none demonstrated statistically significant differences. Endometrial development should be analysed in future studies in order to determine if depot and daily formulations can impact in a different way on endometrial implantation. We did not find any other review of this particular topic.
Authors' conclusions
Implications for practice.
There is insufficient evidence to support the use of any particular intervention in endometrial preparation that clearly improves treatment outcomes for women undergoing embryo transfers with frozen embryos or embryos derived from donor oocytes. The results of this review should be carefully considered because of the heterogeneity between some trials. However, we still could draw some potential conclusions given the moderate‐ to low‐quality evidence. Clinical pregnancy rates may be improved in a stimulated cycle compared to a programmed cycle, and cycle cancellation rates are probably reduced in a natural cycle in comparison with programmed cycles. The use of gonadotropin‐releasing hormone (GnRH) analogues, compared to without, may improve live birth rates (some data showed that GnRH antagonists may be more effective than GnRH agonists). In fresh synchronised oocyte donor cycles, starting progestogen the day of, or day after donor oocyte retrieval probably improves the live birth rate and probably reduces the cycle cancellation rate. Finally, we did not find benefits with the other medications evaluated.
Implications for research.
There is little comparative information on each of the treatments that might impact on endometrial preparation for women undergoing embryo transfers with frozen embryos or embryos derived from donor oocytes. Adequately powered randomised controlled trials (RCTs) are needed, with a longer follow‐up to report live birth rates, and to evaluate each of the treatments more accurately. More studies are needed to analyse if stimulated, programmed or natural cycles are different or not. And more studies are needed to specifically evaluate if GnRH agonist use is associated with fewer cycle cancellations, to see if this medication impacts on the development of the endometrium, and to see if GnRH antagonists are better than agonists. More studies are needed to confirm the timing of the administration of progestogen and to analyse if adding low‐dose aspirin to the endometrial preparation is beneficial or not. Furthermore, more studies are needed to evaluate if testing the endometrial receptivity could help identify the appropriate day to start progestogen. Finally, more studies would help to determine if there is a subgroup of women who may benefit from any specific medication.
What's new
| Date | Event | Description |
|---|---|---|
| 24 June 2020 | New search has been performed | New search has been performed with an update to the review and literature. Gabriel Fiszbajn is no longer an author as he could not participate in the update. His contribution is described in the Acknowledgements. Andrea Quinteiro Retamar was included as an author and participated in the update. Contact details were also updated. |
| 21 August 2019 | New citation required and conclusions have changed | Some conclusions have changed, and new comparisons were added; 18 new studies were included and 39 studies were excluded. Nine studies from the previous review version were excluded as they no longer meet the inclusion criteria for this review update. |
| 21 August 2019 | Amended | The review has a new outline: studies that evaluated luteal phase support were excluded as it will not be evaluated in this review any more; and some comparisons were excluded i.e. those in which the intervention is progesterone versus nothing or different types of progesterone (but we included day to start administration of progesterone in egg donor cycles). |
History
Protocol first published: Issue 1, 2007 Review first published: Issue 1, 2010
| Date | Event | Description |
|---|---|---|
| 27 January 2010 | Amended | Minor editing made to text |
| 8 April 2008 | Amended | Converted to new review format. |
| 18 February 2008 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Marian Showell who contributed to the update of the search strategy. Daniel Comande, librarian, who contributed to the search strategy and identification of the cited studies. Paul Bergh MD, Antonio Pellicer MD and Mark Sauer MD have been consulted as experts in the field in order to be thorough and include all the studies that should be part of this review. We wish to also thank Katie Stocking and Drs Elizabeth Glanvlle and Emily Liu for peer review comments on the draft full review.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group specialised register search strategy
PROCITE platform
Searched 24 June 2020
Keywords CONTAINS "Oocyte donation" or "oocyte donors" or "oocyte recipient‐age" or "frozen embryo transfer" or "frozen embryos" or "frozen‐thawed cycle" or "frozen‐thawed embryo transfer" or "frozen‐thawed embryos" or "FET" or "cryopreserved embryos" or "cryopreserved‐thawed embryos" or "embryo vitrification" or Title CONTAINS "Oocyte donation" or "oocyte donors" or "oocyte recipient‐age" or "frozen embryo transfer" or "frozen embryos" or "frozen‐thawed cycle" or "frozen‐thawed embryo transfer" or "frozen‐thawed embryos" or "FET" or "cryopreserved embryos" or "cryopreserved‐thawed embryos" or "embryo vitrification"
AND
Keywords CONTAINS "endometrial development" or "endometrial preparation" or "endometrial priming" or "endometrial receptivity" or "endometrial proliferation" or "endometrial response" or "endometrial thickness" or "utrogestan" or "Endometrin" or "estrogen" or "Sildenafil" or "Glucocorticoids" or "GnRH a" or "GnRH agonist" or "GnRH analog" or "GnRH analogue" or "GnRH analogues" or "GnRHa" or "GnRHa‐gonadotropin" or "Gonadorelin" or "Leuprolide" or "leuprolide acetate" or "Nafarelin" or "Progesterone" or "Luteinising hormone releasing hormone" or "luteinizing hormone" or "luteinizing hormone supplementation" or "Lutenising hormone releasing hormone" or "HCG" or "human chorionic gonadotropin" or "Piroxicam" or "oestrodiol" or "estradiol" or "rFSH" or "recombinant FSH" or "Steroids" or "steroid pretreatment" or "low‐dose aspirin" or "aspirin" or "stimulated cycle" or "natural cycle" or "natural cycles" or "pituitary desensitisation" or "pituitary desensitization" or "indomethacin"
(376 records)
Appendix 2. CENTRAL via The Cochrane Register of Studies Online (CRSO)
Web platform
Searched 24 June 2020
#1 (pituitary adj2 suppress*):TI,AB,KY 234
#2 (artificial cycle*):TI,AB,KY 67
#3 (stimulated cycle*):TI,AB,KY 184
#4 (natural* cycle*):TI,AB,KY 299
#5 aspirin:TI,AB,KY 13312
#6 (acetylsalicylic acid):TI,AB,KY 5093
#7 sildenafil:TI,AB,KY 1924
#8 antibiotic*:TI,AB,KY 28369
#9 steroid*:TI,AB,KY 28651
#10 gonadorelin:TI,AB,KY 709
#11 GnRHa:TI,AB,KY 464
#12 (GnRH agonist*):TI,AB,KY 1594
#13 MESH DESCRIPTOR Gonadotropin‐Releasing Hormone EXPLODE ALL TREES 2578
#14 MESH DESCRIPTOR Leuprolide EXPLODE ALL TREES 678
#15 MESH DESCRIPTOR Nafarelin EXPLODE ALL TREES 77
#16 (gonadotropin‐releasing hormone*):TI,AB,KY 2264
#17 (GnRH analogue*):TI,AB,KY 322
#18 (GnRH a):TI,AB,KY 386
#19 lhrh:TI,AB,KY 666
#20 rec‐FSH:TI,AB,KY 40
#21 (recombinant follicle stimulating hormone):TI,AB,KY 287
#22 rFSH:TI,AB,KY 458
#23 leuprolide:TI,AB,KY 988
#24 nafarelin:TI,AB,KY 140
#25 progesterone*:TI,AB,KY 6853
#26 glucocorticoid*:TI,AB,KY 8106
#27 (luteinizing hormone):TI,AB,KY 3484
#28 indomethacin:TI,AB,KY 2872
#29 (estradiol or oestradiol):TI,AB,KY 10656
#30 piroxicam:TI,AB,KY 1202
#31 estrogen:TI,AB,KY 11038
#32 corticosteroid*:TI,AB,KY 19549
#33 hcg:TI,AB,KY 2956
#34 (human chorionic gonadotropin*):TI,AB,KY 1012
#35 (endometrin or utrogestin):TI,AB,KY 30
#36 (endometri* adj2 prepar*):TI,AB,KY 218
#37 (uter* adj2 receptiv*):TI,AB,KY 40
#38 (endometri* adj2 receptiv*):TI,AB,KY 291
#39 (endometri* adj2 thick*):TI,AB,KY 1554
#40 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 120828
#41 FET:TI,AB,KY 404
#42 (frozen embryo*):TI,AB,KY 408
#43 (egg dona*):TI,AB,KY 51
#44 (frozen thaw*):TI,AB,KY 486
#45 (oocyte* adj2 don*):TI,AB,KY 353
#46 (thaw* adj2 cycle*):TI,AB,KY 227
#47 (cryopreserv* adj2 embryo*):TI,AB,KY 356
#48 (cryopreserv* adj2 blastocyst*):TI,AB,KY 21
#49 nidation:TI,AB,KY 351
#50 (embryo* adj2 implant*):TI,AB,KY 1001
#51 MESH DESCRIPTOR Cryopreservation EXPLODE ALL TREES 545
#52 MESH DESCRIPTOR Vitrification EXPLODE ALL TREES 40
#53 vitrification:TI,AB,KY 362
#54 cryopreservation:TI,AB,KY 983
#55 #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 3457
#56 #40 AND #55 1420
Appendix 3. MEDLINE search strategy
OVID platform
Searched from 1946 to 24 June 2020
1 (pituitary adj2 suppress$).tw. (1114) 2 artificial cycle$.tw. (168) 3 stimulated cycle$.tw. (775) 4 natural cycle$.tw. (1328) 5 aspirin.tw. (48032) 6 acetylsalicylic acid.tw. (8901) 7 sildenafil.tw. (6571) 8 antibiotic$.tw. (332582) 9 steroid$.tw. (231804) 10 gonadorelin.tw. (216) 11 GnRHa.tw. (1568) 12 GnRH agonist$.tw. (4450) 13 exp gonadotropin‐releasing hormone/ or exp leuprolide/ or exp nafarelin/ (32156) 14 gonadotropin‐releasing hormone$.tw. (14080) 15 GnRH analogue$.tw. (1480) 16 GnRH a.tw. (1084) 17 sildenafil.tw. (6571) 18 lhrh.tw. (6329) 19 rec‐FSH.tw. (53) 20 recombinant follicle stimulating hormone.tw. (479) 21 rFSH.tw. (614) 22 leuprolide.tw. (1884) 23 nafarelin.tw. (259) 24 progesterone$.tw. (82786) 25 glucocorticoid$.tw. (67560) 26 luteinizing hormone.tw. (29065) 27 indomethacin.tw. (35656) 28 (estradiol or oestradiol).tw. (93099) 29 piroxicam.tw. (2992) 30 estrogen.tw. (118624) 31 corticosteroid$.tw. (101899) 32 hcg.tw. (24712) 33 human chorionic gonadotropin$.tw. (13998) 34 (endometrin or utrogestin).tw. (14) 35 (endometri$ adj2 prepar$).tw. (570) 36 (uter$ adj2 receptiv$).tw. (796) 37 (endometri$ adj2 receptiv$).tw. (1676) 38 (endometri$ adj2 thick$).tw. (3091) 39 or/1‐38 (1017621) 40 FET.tw. (3288) 41 frozen embryo$.tw. (1607) 42 egg dona$.tw. (405) 43 frozen thaw$.tw. (5139) 44 (oocyte$ adj2 don$).tw. (2421) 45 (thaw$ adj2 cycle$).tw. (3408) 46 (oocyte$ adj2 recipient$).tw. (533) 47 (cryopreserv$ adj2 embryo$).tw. (2176) 48 nidation.tw. (512) 49 (embryo$ adj2 implant$).tw. (6323) 50 or/40‐49 (23181) 51 50 and 39 (4339) 52 randomized controlled trial.pt. (508061) 53 controlled clinical trial.pt. (93724) 54 randomized.ab. (483710) 55 placebo.tw. (214488) 56 clinical trials as topic.sh. (191681) 57 randomly.ab. (335695) 58 trial.ti. (220488) 59 (crossover or cross‐over or cross over).tw. (85078) 60 or/52‐59 (1326942) 61 (animals not (humans and animals)).sh. (4676244) 62 60 not 61 (1220054) 63 51 and 62 (485)
Appendix 4. Embase search strategy
OVID platform
Searched from 1980 to 24 June 2020
1 (pituitary adj2 suppress$).tw. (1219) 2 artificial cycle$.tw. (235) 3 stimulated cycle$.tw. (1149) 4 natural cycle$.tw. (2089) 5 aspirin.tw. (110276) 6 acetylsalicylic acid.tw. (11089) 7 sildenafil.tw. (10312) 8 antibiotic$.tw. (413013) 9 steroid$.tw. (300613) 10 gonadorelin.tw. (335) 11 GnRHa.tw. (2333) 12 GnRH agonist$.tw. (6693) 13 exp exp gonadorelin/ or exp leuprolide/ or exp nafarelin/ (11780) 14 gonadotropin‐releasing hormone$.tw. (15789) 15 GnRH analogue$.tw. (2302) 16 GnRH a.tw. (1387) 17 sildenafil.tw. (10312) 18 lhrh.tw. (7286) 19 rec‐FSH.tw. (107) 20 recombinant follicle stimulating hormone.tw. (654) 21 rFSH.tw. (1182) 22 leuprolide.tw. (2830) 23 nafarelin.tw. (336) 24 progesterone$.tw. (90966) 25 glucocorticoid$.tw. (84702) 26 luteinizing hormone.tw. (27513) 27 indomethacin.tw. (38550) 28 (estradiol or oestradiol).tw. (101236) 29 piroxicam.tw. (4210) 30 estrogen.tw. (142938) 31 corticosteroid$.tw. (142746) 32 hcg.tw. (31147) 33 human chorionic gonadotropin$.tw. (14799) 34 (endometrin or utrogestin).tw. (106) 35 (endometri$ adj2 prepar$).tw. (990) 36 (uter$ adj2 receptiv$).tw. (1120) 37 (endometri$ adj2 receptiv$).tw. (2898) 38 (endometri$ adj2 thick$).tw. (5367) 39 or/1‐38 (1303046) 40 FET.tw. (4647) 41 frozen embryo$.tw. (3143) 42 egg dona$.tw. (866) 43 frozen thaw$.tw. (6523) 44 (oocyte$ adj2 don$).tw. (4468) 45 (thaw$ adj2 cycle$).tw. (4623) 46 (oocyte$ adj2 recipient$).tw. (779) 47 (cryopreserv$ adj2 embryo$).tw. (3460) 48 nidation.tw. (284) 49 (embryo$ adj2 implant$).tw. (8913) 50 or/40‐49 (32238) 51 50 and 39 (7349) 52 Clinical Trial/ (965973) 53 Randomized Controlled Trial/ (603591) 54 exp randomization/ (87107) 55 Single Blind Procedure/ (39191) 56 Double Blind Procedure/ (170262) 57 Crossover Procedure/ (63315) 58 Placebo/ (337422) 59 Randomi?ed controlled trial$.tw. (229960) 60 Rct.tw. (37375) 61 random allocation.tw. (2011) 62 randomly allocated.tw. (35202) 63 allocated randomly.tw. (2544) 64 (allocated adj2 random).tw. (815) 65 Single blind$.tw. (24707) 66 Double blind$.tw. (202693) 67 ((treble or triple) adj blind$).tw. (1148) 68 placebo$.tw. (302824) 69 prospective study/ (606319) 70 or/52‐69 (2191222) 71 case study/ (69825) 72 case report.tw. (403092) 73 abstract report/ or letter/ (1099122) 74 or/71‐73 (1561443) 75 70 not 74 (2137697) 76 75 and 51 (1231)
Appendix 5. PsycINFO search strategy
OVID platform
Searched from 1806 to 24 June 2020
1 (pituitary adj2 suppress$).tw. (37) 2 artificial cycle$.tw. (4) 3 stimulated cycle$.tw. (4) 4 natural cycle$.tw. (59) 5 aspirin.tw. (1183) 6 acetylsalicylic acid.tw. (212) 7 sildenafil.tw. (599) 8 antibiotic$.tw. (2841) 9 steroid$.tw. (9950) 10 gonadorelin.tw. (3) 11 GnRHa.tw. (51) 12 GnRH agonist$.tw. (75) 13 gonadotropin‐releasing hormone$.tw. (775) 14 GnRH analogue$.tw. (26) 15 GnRH a.tw. (11) 16 sildenafil.tw. (599) 17 lhrh.tw. (215) 18 rec‐FSH.tw. (0) 19 recombinant follicle stimulating hormone.tw. (3) 20 rFSH.tw. (1) 21 leuprolide.tw. (84) 22 nafarelin.tw. (1) 23 progesterone$.tw. (4291) 24 glucocorticoid$.tw. (6211) 25 luteinizing hormone.tw. (1387) 26 indomethacin.tw. (681) 27 (estradiol or oestradiol).tw. (6555) 28 piroxicam.tw. (45) 29 estrogen.tw. (7044) 30 corticosteroid$.tw. (3112) 31 hcg.tw. (106) 32 human chorionic gonadotropin$.tw. (96) 33 (endometri$ adj2 prepar$).tw. (1) 34 (uter$ adj2 receptiv$).tw. (3) 35 (endometri$ adj2 thick$).tw. (14) 36 exp Gonadotropic Hormones/ (4220) 37 or/1‐36 (36686) 38 FET.tw. (69) 39 frozen embryo$.tw. (27) 40 egg dona$.tw. (123) 41 frozen thaw$.tw. (4) 42 (oocyte$ adj2 don$).tw. (60) 43 (thaw$ adj2 cycle$).tw. (19) 44 (oocyte$ adj2 recipient$).tw. (2) 45 (cryopreserv$ adj2 embryo$).tw. (23) 46 (embryo$ adj2 implant$).tw. (52) 47 or/38‐46 (361) 48 37 and 47 (13)
Appendix 6. LILACS search strategy
Web platform
Searched 24 June 2020
((MH Donación de Oocito OR MH Implantación del Embrión OR MH Criopreservación) OR (Pt Cryopreserv$ OR Pt frozen thaw$)) AND ((MH Aspirina OR MH Esteroides OR MH Agentes Antibacterianos OR MH Gonadorelina) OR ((Pt Sildenafil OR Pt Antibiotic$ OR Pt Gonado$ OR Pt GnRH$ OR PT lhrh$)) OR (MH Implantación del Embrión/efectos de drogas)) AND ((Pt ENSAYO CONTROLADO ALEATORIO OR Pt ENSAYO CLINICO CONTROLADO OR Mh ENSAYOS CONTROLADOS ALEATORIOS OR Mh DISTRIBUCIÓN ALEATORIA OR Mh METODO DOBLE CIEGO OR Mh METODO SIMPLECIEGO OR Pt ESTUDIO MULTICÉNTRICO) or ((tw ensaio or tw ensayo or tw trial) and (tw azar or tw acaso or tw placebo or tw control$ or tw aleat$ or tw random$ or (tw duplo and tw cego) or (tw doble and tw ciego) or (tw double and tw blind)) and tw clinic$)) AND NOT ((Ct ANIMALES OR Mh ANIMALES OR Ct CONEJOS OR Ct RATÓN OR MH Ratas OR MH Primates OR MH Perros OR MH Conejos OR MH Porcinos) AND NOT (Ct HUMANO AND Ct ANIMALES)) [Palavras]
Appendix 7. Data extraction form
| Reviewer 1 |
| Reviewer 1 |
| Reviewer 2 |
| # Covidence |
| Last Name Year of report |
| Title |
| Intervention |
| Live Birth Rate (n/N) |
| Clinical Pregnancy Rate (n/N) |
| Miscarriage Rate (n/N) |
| Multiple PR (n/N) |
| Cycle cancellation rate (n/N) |
| Endometrial thickness (mm) |
| Other adverse effects (n/N) |
| Comparator |
| Live Birth Rate (n/N) |
| Clinical Pregnancy Rate (n/N) |
| Miscarriage Rate (n/N) |
| Multiple PR (n/N) |
| Cycle cancellation rate (n/N) |
| Endometrial thickness (mm) |
| Other adverse effects (n/N) |
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
| Adequate sequence generation? |
| Allocation concealment? |
| Blinding? |
| Incomplete outcome data addressed |
| Free of selective reporting? |
| Free of other bias? |
| Risk of bias |
Data and analyses
Comparison 1. Programmed cycle versus stimulated cycle.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Live birth rate | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.49, 3.26] |
| 1.1.1 Stimulated with letrozole | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.49, 3.26] |
| 1.2 Clinical pregnancy rate | 5 | 656 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.12, 2.38] |
| 1.2.1 Stimulated with FSH | 1 | 199 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.47, 2.66] |
| 1.2.2 Stimulated with Letrozole | 3 | 365 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.24, 3.04] |
| 1.2.3 Stimulated with Clomiphene Citrate | 1 | 92 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.25, 3.22] |
| 1.3 Miscarriage rate | 3 | 355 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.36, 1.71] |
| 1.3.1 Stimulated with Letrozole | 2 | 263 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.41, 2.13] |
| 1.3.2 Stimulated with Clomiphene Citrate | 1 | 92 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.09] |
| 1.4 Endometrial thickness (mm) | 3 | 454 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.25, 0.03] |
| 1.4.1 Stimulated with FSH | 1 | 199 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.29, 0.29] |
| 1.4.2 Stimulated with Letrozole | 1 | 163 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.22, 0.10] |
| 1.4.3 Stimulated with Clomiphene Citrate | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐1.59, ‐0.49] |
Comparison 2. Programmed cycle versus natural cycle.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Live birth rate | 4 | 1285 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.74, 1.28] |
| 2.2 Clinical pregnancy rate | 5 | 1249 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.62, 1.01] |
| 2.3 Miscarriage rate | 3 | 485 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.25, 1.63] |
| 2.4 Cycle cancellation rate | 1 | 734 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.44, 0.82] |
| 2.5 Endometrial thickness (mm) | 3 | 485 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.25, 0.69] |
2.4. Analysis.

Comparison 2: Programmed cycle versus natural cycle, Outcome 4: Cycle cancellation rate
Comparison 3. Transdermal oestrogens versus oral oestrogens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Clinical pregnancy rate | 3 | 504 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.59, 1.25] |
| 3.2 Miscarriage rate | 2 | 414 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.27, 1.09] |
Comparison 4. Day of starting administration of the progesterone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Clinical Pregnancy Rate | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1.1 Day before oocyte retrieval (T1) versus day of oocyte retrieval (T2) (Oocyte donation) | 1 | 191 | Odds Ratio (M‐H, Random, 95% CI) | 1.92 [1.08, 3.42] |
| 4.1.2 Day of oocyte retrieval (T1) versus day after oocyte retrieval (T2) (Oocyte donation) | 1 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.53, 1.68] |
| 4.1.3 Day before oocyte retrieval (T1) versus day after oocyte retrieval (T2) (Oocyte donation) | 1 | 188 | Odds Ratio (M‐H, Random, 95% CI) | 1.81 [1.01, 3.24] |
| 4.1.4 Six days (T1) versus seven days (T2) before embryo transfer | 1 | 49 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.24, 2.34] |
| 4.2 Miscarriage Rate | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.2.1 Day before oocyte retrieval (T1) versus day of oocyte retrieval (T2) (Oocyte donation) | 1 | 191 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.16, 1.25] |
| 4.2.2 Day of oocyte retrieval (T1) versus day after oocyte retrieval (T2) (Oocyte donation) | 1 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 2.52 [0.85, 7.46] |
| 4.2.3 Day before oocyte retrieval (T1) versus day after oocyte retrieval (T2) (Oocyte donation) | 1 | 188 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.33, 3.85] |
| 4.3 Multiple Pregnancy Rate | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.3.1 Day before oocyte retrieval (T1) versus day of oocyte retrieval (T2) (Oocyte donation) | 1 | 191 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.29, 1.32] |
| 4.3.2 Day of oocyte retrieval (T1) versus day after oocyte retrieval (T2) (Oocyte donation) | 1 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 1.37 [0.65, 2.88] |
| 4.3.3 Day before oocyte retrieval (T1) versus day after oocyte retrieval (T2) (Oocyte donation) | 1 | 188 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.39, 1.89] |
| 4.3.4 Day before oocyte retrieval (T1) versus day of or day after oocyte retrieval (T2) (Oocyte donation) | 1 | 282 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.37, 1.42] |
| 4.4 Cycle cancellation rate | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.4.1 Day before oocyte retrieval (T1) versus day of oocyte retrieval (T2) (Oocyte donation) | 1 | 191 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.10, 1.01] |
| 4.4.2 Day of oocyte retrieval (T1) versus day after oocyte retrieval (T2) (Oocyte donation) | 1 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.17, 3.53] |
| 4.4.3 Day before oocyte retrieval (T1) versus day after oocyte retrieval (T2) (Oocyte donation) | 1 | 188 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.07, 0.89] |
| 4.5 Cycle cancellation rate because of failed fertilization (by subgroups) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.5.1 Day before or day of oocyte retrieval (T1) versus day after of oocyte retrieval (T2) (Oocyte donation) | 1 | 282 | Odds Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 1.82] |
| 4.6 Clinical Pregnancy Rate (by subgroups) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.6.1 Day before oocyte retrieval (T1) versus day of oocyte retrieval (T2) (Oocyte donation) | 1 | 191 | Odds Ratio (M‐H, Random, 95% CI) | 1.92 [1.08, 3.42] |
| 4.6.2 Day before oocyte retrieval (T1) versus day after oocyte retrieval (T2) (Oocyte donation) | 1 | 188 | Odds Ratio (M‐H, Random, 95% CI) | 1.81 [1.01, 3.24] |
| 4.6.3 Day before oocyte retrieval (T1) versus day of or after oocyte retrieval (T2) (Oocyte donation) | 1 | 282 | Odds Ratio (M‐H, Random, 95% CI) | 1.87 [1.13, 3.08] |
| 4.7 Cycle cancellation rate (by subgroups) | 1 | 282 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.11, 0.74] |
| 4.7.1 Day before oocyte retrieval (T1) versus day of oocyte retrieval (T2) (Oocyte donation) | 1 | 143 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.09, 1.19] |
| 4.7.2 Day before oocyte retrieval (T1) versus day after oocyte retrieval (T2) (Oocyte donation) | 1 | 139 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.06, 1.00] |
4.5. Analysis.

Comparison 4: Day of starting administration of the progesterone, Outcome 5: Cycle cancellation rate because of failed fertilization (by subgroups)
Comparison 5. GnRH agonists versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Live Birth Rate | 1 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.62 [1.19, 5.78] |
| 5.1.1 Nasal Buserelin versus No treatment (Frozen‐embryo transfer) | 1 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.62 [1.19, 5.78] |
| 5.2 Clinical Pregnancy Rate | 9 | 1358 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.81, 1.39] |
| 5.2.1 Leuprolide acetate 1 mg versus No treatment (Oocyte donation) | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.33, 2.22] |
| 5.2.2 Nasal Buserelin versus No treatment (Frozen‐embryo transfer) | 2 | 334 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.01, 3.26] |
| 5.2.3 Tryptorelin (deposit) versus No treatment (Frozen‐embryo transfer) | 2 | 415 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.55, 1.41] |
| 5.2.4 Subcutaneous Buserelin versus no treatment | 2 | 297 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.60, 2.33] |
| 5.2.5 Diphereline 3.75 mg IM versus no treatment | 1 | 176 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.34, 1.34] |
| 5.2.6 Variopeptyl (daily) versus No treatment (Frozen‐embryo transfer) | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.42, 4.54] |
| 5.3 Miscarriage Rate | 4 | 828 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.36, 2.00] |
| 5.3.1 Tryptorelin (deposit) versus No treatment (Frozen‐embryo transfer) | 2 | 415 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.29, 2.96] |
| 5.3.2 Diphereline 3.75 mg IM versus no treatment | 1 | 176 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.08, 2.43] |
| 5.3.3 Subcutaneous Buserelin versus no treatment | 1 | 237 | Odds Ratio (M‐H, Random, 95% CI) | 1.50 [0.25, 9.14] |
| 5.4 Cycle cancellation rate | 2 | 530 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.21, 1.17] |
| 5.4.1 Nasal Buserelin (Frozen‐embryo transfer) | 1 | 234 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.16, 1.95] |
| 5.4.2 Tryptorelin (deposit) versus No treatment (Frozen‐embryo transfer) | 1 | 296 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.13, 1.47] |
| 5.5 Endometrial Thickness (mm) | 4 | 697 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.33, 0.16] |
| 5.5.1 Nasal Buserelin (Frozen‐embryo transfer) | 2 | 334 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.99, 0.72] |
| 5.5.2 Tryptorelin (deposit) versus No treatment (Frozen‐embryo transfer) | 1 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.43, 0.23] |
| 5.5.3 Variopeptyl (daily) versus No treatment (Frozen‐embryo transfer) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.82, 0.34] |
| 5.6 Clinical Pregnancy Rate with most used GnRH agonists | 2 | 365 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.51, 1.33] |
| 5.6.1 Leuprolide acetate 1 mg versus No treatment (Oocyte donation) | 1 | 69 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.33, 2.22] |
| 5.6.2 Tryptorelin (deposit) versus No treatment (Frozen‐embryo transfer) | 1 | 296 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.46, 1.42] |
5.6. Analysis.

Comparison 5: GnRH agonists versus control, Outcome 6: Clinical Pregnancy Rate with most used GnRH agonists
Comparison 6. Among different GnRH agonists.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Clinical Pregnancy Rate | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1.1 Nafareline (daily) vs Leuprolide (daily) (Oocyte donation ‐ ovulating recipients) | 1 | 68 | Odds Ratio (M‐H, Random, 95% CI) | 1.26 [0.49, 3.27] |
| 6.1.2 Leuprolide (daily) vs Tryptorelin (deposit) (Oocyte donation ‐ ovulating recipients) | 1 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 1.93 [0.62, 5.98] |
| 6.2 Miscarriage Rate | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.2.1 Leuprolide vs Nafareline (Oocyte donation ‐ non‐amenorrhea recipients) | 1 | 68 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.36, 4.87] |
Comparison 7. GnRH agonists versus GnRH antagonists.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Clinical pregnancy rate | 1 | 473 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.42, 0.90] |
| 7.2 Miscarriage rate | 1 | 473 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.38, 1.49] |
| 7.3 Multiple Pregnancy Rate | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
7.1. Analysis.

Comparison 7: GnRH agonists versus GnRH antagonists, Outcome 1: Clinical pregnancy rate
7.2. Analysis.

Comparison 7: GnRH agonists versus GnRH antagonists, Outcome 2: Miscarriage rate
7.3. Analysis.

Comparison 7: GnRH agonists versus GnRH antagonists, Outcome 3: Multiple Pregnancy Rate
Comparison 8. Aspirin versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Live Birth Rate | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.00 [1.48, 24.30] |
| 8.1.1 Aspirin vs No treatment | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.00 [1.48, 24.30] |
| 8.2 Clinical Pregnancy Rate | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.33 [1.00, 11.14] |
| 8.2.1 Aspirin vs No treatment | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.33 [1.00, 11.14] |
| 8.3 Endometrial Thickness (mm) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.95, 0.15] |
| 8.3.1 Aspirin vs No treatment | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.95, 0.15] |
Comparison 9. Steroids versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Live Birth Rate | 1 | 99 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.14, 3.11] |
| 9.1.1 Dexamethasone (Frozen‐embryo transfer) | 1 | 99 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.14, 3.11] |
| 9.2 Clinical Pregnancy Rate | 2 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.40, 2.03] |
| 9.2.1 Dexamethasone (Frozen‐embryo transfer) | 1 | 99 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.33, 3.42] |
| 9.2.2 6‐alpha‐methylprednisolone (Frozen‐embryo transfer) | 1 | 61 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.25, 2.38] |
| 9.3 Miscarriage Rate | 2 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 1.49 [0.32, 7.03] |
| 9.3.1 Dexamethasone (Frozen‐embryo transfer) | 1 | 99 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.05, 14.84] |
| 9.3.2 6‐alpha‐methylprednisolone (Frozen‐embryo transfer) | 1 | 61 | Odds Ratio (M‐H, Random, 95% CI) | 1.86 [0.29, 12.01] |
| 9.4 Multiple Pregnancy Rate | 1 | 99 | Odds Ratio (M‐H, Random, 95% CI) | Not estimable |
| 9.4.1 Dexamethasone (Frozen‐embryo transfer) | 1 | 99 | Odds Ratio (M‐H, Random, 95% CI) | Not estimable |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agha‐Hosseini 2018.
| Study characteristics | ||
| Methods | The enrolled women were divided randomly into two groups to undergo either a modified natural cycle (NC) FET (group A) or artificial cycle (AC) FET (group B) using computerised software in a 1:1 fashion. | |
| Participants | All women who were aged between 18 and 40 years and had regular menses (25 to 34 days) and who had at least two cryopreserved embryos derived from intracytoplasmic sperm injection (ICSI) treatment cycles from January 2012 to December 2014 were enrolled. Women with endometriosis, immune diseases, recurrent abortion, donated sperm or oocyte, uterine abnormality, ovarian cyst or previous ovarian surgery, history of previous IVF failure, and any known contraindications or allergy for oral estradiol or progesterone therapy were excluded from participating in the study. In addition, patients were excluded if their clinical history included percutaneous epididymal sperm aspiration or testicular sperm extraction. | |
| Interventions | 85 patients were considered as group A (NC FET) and 85 were classified as group B (AC‐FET) and were assigned to receive the related protocol. NC FET: An ultrasound examination was performed on days 10 to 12 of the cycle after a spontaneous menses to detect the leading follicle. When at least one dominant follicle reached ≥18 mm in diameter and the thickness of the endometrium was at least 8 mm, a bolus of 10.000 IU of human chorionic gonadotropin (hCG) (Pregnyl; N.V. Organon, Oss, the Netherlands) was injected intramuscularly for the induction of ovulation and the embryos were thawed and transferred 4 days later. Artificial cycle FET: from the 21st day of the previous cycle, 500 μg/day of buserelin acetate (Suprecur; Hoechst UK Ltd, Hounslow, UK) was subcutaneously injected. Oral estradiol valerate (Progynova, Bayer, Germany) was then administered from day 2 of the next cycle from 2 mg/day to 2 mg/day ×4. The E dosage was adjusted based on the endometrial thickness as assessed using transvaginal ultrasound. | |
| Outcomes | A total of 63 clinical pregnancies occurred in the NC‐FET and the AC‐FET groups [33 (38.9%) versus 30 (35.3%) clinical pregnancies; P = 0.4, respectively]. | |
| Notes | Trial registration: not mentioned Funding: this work was financially supported by the Research Deputy of Tehran University of Medical Sciences (Grant Number: 94‐01‐01‐2051) Conflict of interest: the authors declare that there are no conflicts of interest that could be perceived as prejudicing the impartially of the research reported. Study dates: cycles from January 2012 to December 2014 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The enrolled women were divided randomly into two groups to undergo either a modified natural cycle FET (group A) or artificial cycle FET (group B) using computerized software in a 1:1 fashion" |
| Allocation concealment (selection bias) | High risk | The treating physicians (n = 2) gave treatment based on the allocated chart |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 of 85 women discontinued the intervention in the group of AC‐FET |
| Selective reporting (reporting bias) | Unclear risk | No protocol was found |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Aleyasin 2017.
| Study characteristics | ||
| Methods | This randomised clinical trial included 100 women (18 to 42 years) randomly assigned to two groups based on Bernoulli distribution. | |
| Participants | Inclusion criteria were all women (18 to 42 years old) who were undergoing endometrial preparation for first frozen embryo transfer. Infertile couples with male infertility undergone testicular sperm extraction (TESE) or percutaneous epididymal sperm aspiration (PESA); severe endometriosis (stage 3 or 4); uterine myoma ≥ 4 cm, and fresh embryo transfer were excluded. 50 women were allocated to each arm. |
|
| Interventions | Group I received GnRH agonist (Buserelin, 500 μg subcutaneously) from the previous mid luteal cycle, then estradiol valerate (2 mg/ daily orally) was started on the second day and was increased until the observation of 8 mm endometrial thickness. Group II received letrozole (Iran hormone, Iran, 5 mg/daily) on the second day of the cycle for five days, then HMG 75 IU was injected on the seventh day. After the observation of 18 mm follicle in transvaginal ultrasound, human chorionic gonadotropin (hCG) (Ferring, Germany, 10,000 IU, and IM) was injected for ovulation induction. | |
| Outcomes | The main outcome was the live birth rate. The rate of live birth, implantation, chemical, and clinical pregnancy, abortion, cancellation and endometrial thickness were compared between two groups. |
|
| Notes | Trial registration: IRCT201306256689N3 Funding: no Conflict of interest: Quote: "There is no conflict of interest in this article" Study dates: between February 2014 and February 2016 Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly method based on Bernoulli distribution |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of follow‐up. |
| Selective reporting (reporting bias) | Low risk | Protocol IRCT201306256689N3 |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Bider 1996.
| Study characteristics | ||
| Methods | Single‐centre trial. Trial design: parallel. Allocation: participants were randomised using a computer‐generated randomisation list. Assignment method was not stated. Blinding: not stated. Follow‐up: until live birth. | |
| Participants | N = 99. Women mean age at time of oocyte retrieval was not reported. Their mean age at time of embryo transfer was 33.7 years for dexamethasone group and 32.9 years for control group. Cause of infertility: tubal factor in 100%. | |
| Interventions | Dexamethasone (0.5 mg orally nightly for five days, beginning on the day of ovulation) versus no treatment. | |
| Outcomes | Pregnancy rate (dexamethasone versus no treatment): 13.5% versus 12.8%. | |
| Notes | Dexamethasone group transferred a mean of 2.8 embryos and the control group transferred 2.1 embryos. Trial registration: no Funding: no Conflict of interest: no Study dates: during a 23‐month period that ended in December 1994 Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were treated according to a computer‐based randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There are no losses of follow‐up (<10%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Check 2002.
| Study characteristics | ||
| Methods | The present study randomly assigned women attempting frozen embryo transfer (ET) | |
| Participants | Women attempting frozen embryo transfer (ET) who failed to attain an 8 mm endometrial thickness following graduating dosages of oral E2. Sixteen patients with a mean number of 1.5 attempted but cancelled frozen ET cycles were selected. The mean endometrial thickness for those cycles following oral E2 treatment was 6.6 mm and was 6.3 mm on cycles immediately prior to the study. | |
| Interventions | Treatment with oral E2 plus 25 mg 4 times per day of sildenafil from day 3 to 9 of cycle (vaginal wash day 10) or oral E2 plus 2 mg 2 times per day of vaginal E2 from day 2 to peak thickness. | |
| Outcomes | Seven women randomised to vaginal E2 had a mean endometrial thickness of 7.2 mm. | |
| Notes | Trial registration: not stated Funding: not stated Conflict of interest: not stated Study dates: not described Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The present study randomly assigned women attempting frozen embryo transfer (ET) |
| Allocation concealment (selection bias) | Unclear risk | The present study randomly assigned women attempting frozen embryo transfer (ET) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No attempts to blind were done. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Frozen ET was attempted in only 6 of the 16 women (4 with sildenafil and 2 with vaginal E2) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | It is an abstract from a meeting a there is no enough information to discard other bias |
Child 2013.
| Study characteristics | ||
| Methods | Prospective open randomised controlled trial. Pilot study. | |
| Participants | Regular ovulatory cycles; age less than 40 years at original IVF. 159 women were randomised (80 Natural; 79 HRT) | |
| Interventions | Women were randomised to Natural FET (Group 1) or HRT FER (Group 2) for a single cycle. Natural FET. Embryo transfer was scheduled 5‐7 days after a positive urine LH surge. 1 or 2 embryos were replaced under abdominal ultrasound guidance. Following a positive urine pregnancy test scans were performed at 6 and 10 weeks gestation. No drugs were used. HRT FET. Nasal nafarelin was started on cycle D21 followed by estradiol valerate (2mg orally increasing to 6mg/day) when pituitary suppression was confirmed. After 2 weeks of estradiol and an endometrial thickness of at least 7mm progesterone pessaries were started and the embryo transfer undertaken. HRT was continued until 10 weeks gestation. | |
| Outcomes | Live birth rate per cycle. Live birth rates were 26.3% (Natural) and 31.7% (HRT) per randomised patient (P = NS) | |
| Notes | Trial registration: no Funding: Oxford Fertility Unit Conflict of interest: no Study dates: not described Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is not described how randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | It is not described how allocation was performed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis and less than 10% of follow‐up loss. 159 women were randomised (80 Natural; 79 HRT) and 145 had embryo transfer and completed the study (72 Natural; 73 HRT) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Dal Prato 2002.
| Study characteristics | ||
| Methods | Single‐centre trial. Trial design: parallel. Allocation: on an individual basis by using sealed envelopes and randomly assigned sequential numbers. Blinding: not stated. Follow‐up: until pregnancy. Therefore live birth rate not included in outcomes. | |
| Participants | N = 296. Women mean age at time of oocyte retrieval was 32.9 years for tryptorelin group and 32.5 years for the no‐treatment group. Their mean age at time of embryo transfer was 34.6 years for tryptorelin group and 33.9 years for the no‐treatment group. Cause of infertility: not stated. | |
| Interventions | A single IM injection of tryptorelin 3.75 mg was administered in the mid‐luteal phase of the cycle to the tryptorelin group. No injection was given to the other group of women. | |
| Outcomes | Pregnancy rate (tryptorelin versus no treatment): 19.1% versus 22.6%. | |
| Notes | Both groups transferred a mean of 2.1 embryos. Trial registration: no Funding: no Conflict of interest: no Study dates: From April 1999 to September 2000 Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was performed on an individual basis by using sealed envelopes containing the name of one of the two treatments". This sentence does not explain the method of randomisation, which is not stated. |
| Allocation concealment (selection bias) | Low risk | Assignment was made when an eligible patient agreed to participate. Each envelope and allocation was sequentially numbered to avoid allocating a patient out of sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol was found |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Davar 2007.
| Study characteristics | ||
| Methods | Single‐centre trial. Trial design: parallel. Allocation: randomisation by computer‐generated list. Allocation method: not stated. Blinding: no. Follow‐up: until pregnancy. Therefore live birth rate not included in outcomes. | |
| Participants | N = 60. Women mean age at time of oocyte retrieval was 28.1 years for buserelin group and 27.8 years for the no‐treatment group. | |
| Interventions | Group A commenced steroid supplementation without prior pituitary desensitization. Group B had pituitary suppression prior to steroid hormone administration with Buserelin acetate starting in mid‐luteal phase (day 21) at a dose of 0.5 mg, subcutaneously, and continued until day 11 of the cycle. Both groups received increasing doses of valerate estradiol from 2 to 6 mg/day. orally. | |
| Outcomes | Pregnancy rate (buserelin versus no treatment): 10% versus 6.6% | |
| Notes | Cryopreservation was performed on pro nuclear stage. Transfer was performed when endometrial thickness was at least 8 mm. Trial registration: no Funding: no Conflict of interest: no Study dates: between January 2005 and October 2006 Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study population was randomly divided into two groups according to a computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | No information was found about the allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol was found |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Davar 2016.
| Study characteristics | ||
| Methods | Patients were randomly allocated to oral estradiol or 17 beta‐estradiol transdermal patch | |
| Participants | A total number of 90 patients who underwent frozen‐thawed embryo transfer cycles were enrolled in this study | |
| Interventions | In the study group with transdermal route (n = 45), 100 μg of 17‐B estradiol transdermal patch (Novartis, Turkey) was applied every other day from the second day of menstruation cycle, and each patch was removed after four days. In the control group with oral route (n = 45), at the time of cycle, 6 mg of oral estradiol valerate (Aburaihan, Iran) was started daily. | |
| Outcomes | Clinical pregnancy rate per transfer (36.4% versus 28.6%, respectively, P = 0.29) | |
| Notes | Trial registration: IRCT2012112610328N2 Funding: Vice chancellor of Yazd Research and Clinical Center for Infertility Conflict of interest: the authors declare they have no conflict of interest Study dates: between April 2012 and Jan 2013 Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states "Randomized" |
| Allocation concealment (selection bias) | Unclear risk | No information listed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol was found |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Davar 2020.
| Study characteristics | ||
| Methods | Computer‐generated randomisation | |
| Participants | 67 infertile women with history of idiopathic RIF (at least two implantation failures). All women with endometrial polyp, uterine myoma, and uterine anomaly were excluded from the study | |
| Interventions | The case group (n = 34) received 0.1 mg/day of the GnRH agonist (Variopeptyl, VarianDarou, Iran), SC, from day 21 of the cycle preceding the actual FET cycle. On the second day of the cycle, the dose of GnRH agonist was reduced to 0.05 mg and 6 mg/day oral estradiol valerate (2 mg, Aburaihan Co., Tehran, Iran) was also started. When the endometrial thickness reached to 7.5 mm, vaginal supplementation of Cyclogest® pessaries (Cox Pharmaceuticals, Barnstaple, UK) at 400 mg twice daily was started and the GnRH agonist was also stopped. The control group (n = 33), received 6 mg/day oral estradiol valerate (2 mg, Aburaihan Co., Tehran, Iran) from the second day of the cycle without the GnRH agonist. In the two groups, frozen‐thawed embryos were transferred on the fourth day of progesterone treatment. |
|
| Outcomes | Clinical pregnancy was approved by the detection of a fetal heartbeat 2 wk after positive β‐hCG. | |
| Notes | in Yazd Reproductive Sciences Institute, Yazd, Iran between August and November 2017 IRCT: 201708292604N3 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% of missing outcomes |
| Selective reporting (reporting bias) | Low risk | IRCT: 201708292604N3 |
| Other bias | Low risk | No other risk of bias was reported |
Ding 2007.
| Study characteristics | ||
| Methods | Frozen‐thawed blastocyst transfer was randomly performed on either 6th or 7th day of progestogen administration. | |
| Participants | Frozen‐thawed blastocyst transfers during 49 consecutive cycles were randomised into two groups according to the number of days of progestogen administration | |
| Interventions | In Group 1 (23 cycles), frozen blastocysts were thawed and transferred on the 6th and in Group 2 (26 cycles) on the 7th day of progestogen administration | |
| Outcomes | After transfer, the presence of intrauterine gestational sac was defined as clinical pregnancy (Clinical PR) and the presence of fetal cardiac activity as ongoing pregnancy (Ongoing PR). | |
| Notes | Trial registration: not stated Funding: not stated Conflict of interest: not stated Study dates: not described Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Frozen‐thawed blastocyst transfer was randomly performed on either 6th or 7th day of progesterone administration |
| Allocation concealment (selection bias) | Unclear risk | Frozen‐thawed blastocyst transfer was randomly performed on either 6th or 7th day of progesterone administration |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No attempts to blind were done. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated if there were missing data |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | It is an abstract from a meeting and there is not enough information to discard other bias |
El‐Toukhy 2004.
| Study characteristics | ||
| Methods | Single‐centre study. Trial design: parallel. Allocation: 117 women were allocated to each arm. Participants were randomised using a computer‐generated randomisation list. Woman enrolment and assignment to their treatment groups was carried out at the time of participation by medical staff not involved in the study. Blinding: not stated. Follow‐up: until live birth. | |
| Participants | N = 234. Women mean age at time of embryo transfer was 32.8 years for buserelin group and 33.2 years for the no treatment group. Cause of infertility: not stated. | |
| Interventions | Group A (n = 117) had pituitary suppression prior to steroid hormone administration, while group B (n = 117) commenced steroid supplementation without prior pituitary desensitization. (Fig. 1) Pituitary suppression in group A patients was performed using buserelin nasal spray (Superfact, Hoechst UK Ltd., Hounslow, Middlesex, UK) starting in the mid‐luteal phase (day 21) of the menstrual cycle. On day 1 of subsequent menstruation, oestrogen stimulation was initiated using oral estradiol valerate 6 mg daily in two divided doses (Climaval, Novartis Pharmaceuticals, Surrey, UK). Group B patients started oestrogen stimulation on day 1 of menstruation using the same dose of Climaval (6mg/day). In both groups, patients remained on this dose for 12±14 days, after which endometrial thickness was evaluated using an ultrasound scanner with a 6.5 MHz probe (Hitachi EUB 525, Tokyo, Japan). The dose of Climaval was increased to 8mg/day for a further 7±12 days if endometrial thickness was less than 8mm. When endometrial thickness had reached 8mm or more, micronized progesterone pessaries (Cyclogest, Shire Pharmaceuticals Ltd., Hants, UK) 400 mg twice daily were commenced and buserelin nasal spray stopped in group A patients. | |
| Outcomes | Live birth rate (buserelin versus no treatment): 20% versus 8.5% (P = 0.01). | |
| Notes | Buserelin group transferred a mean of 2.3 embryos and the control group transferred 2.2 embryos. Trial registration: no Funding: no Conflict of interest: no Study dates: between January 1998 and July 2001 Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a computer‐generated randomisation list |
| Allocation concealment (selection bias) | High risk | Quote: "woman enrolment and assignment to their treatment groups was carried out at the time of participation by medical staff not involved in the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol was found |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Escriba 2006.
| Study characteristics | ||
| Methods | Single‐centre study. Trial design: parallel. Allocation: a computer‐based randomisation divided recipients into three groups. Blinding: not stated. Follow‐up: until pregnancy. Therefore live birth rate not included in outcomes. | |
| Participants | N = 282. Oocyte recipients mean age was 39.4 years in both groups. Donor mean age was not reported. Cause of infertility: age 47.6%; poor responders 25.6%; implantation failure 8.5%; severe endometriosis 8.5%; other causes: 9.8%. |
|
| Interventions | The protocol for steroid replacement included pituitary desensitization with a single intramuscular ampule administration of 3.75 mg of tryptorelin (Decapeptyl depot 3.75, Ipsen Pharma, Madrid, Spain) in the mid‐luteal phase of the menstrual cycle. Hormonal replacement therapy was initiated when ultrasound confirmed ovarian quiescence during the following menstruation. Two milligrams of E2 valerate (Progynova, Schering Spain, Madrid, Spain) were administered daily for the first 8 days, 4 mg for the next 3 days, and 6 mg from thereon. After 13 days of E2 valerate administration, endometrial thickness and pattern were tested. If a three‐layer pattern was observed in a 7 mm endometrium, the aforementioned dose of E therapy was continued at least until the pregnancy test was performed. If the endometrium was not seen to be sufficiently developed, doses of E2 valerate were increased to 8 mg/day. Daily administration of 800 mg/day of micronized intravaginal P (Progeffik, Laboratories Effik S.A., Madrid, Spain) was initiated according to the randomisation. The first group (group A) began P supplementation the day before oocyte retrieval. In the second group (group B) P was administered from the day of oocyte retrieval. The third group (group C) began P one day after retrieval, once fertilization had been confirmed. |
|
| Outcomes | Pregnancy rate ("before" versus "day of" versus "After"): 38.1% versus 54.2% versus 52.7%. Cancellation rate: 12.4% (8 out of 12 were because of fertilisation failure) versus 4.2% (1 of 4 were because of fertilisation failure) versus 3.3% (0 out of 3 were because of fertilisation failure). | |
| Notes | All embryo transfers were done on day 3. Endometrium was prepared with oral estradiol and intravaginal micronised progesterone. Trial registration: no Funding: no Conflict of interest: no Study dates: between September 2003 and September 2004 Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐based randomisation divided recipients into three groups |
| Allocation concealment (selection bias) | Unclear risk | Not clear how allocation concealment was done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol was found |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Greco 2016.
| Study characteristics | ||
| Methods | 236 patients undergoing infertility treatment were randomised in 1:1 ratio; 118 received a frozen‐thawed single euploid blastocyst transfer in a modified natural cycle and 118 in an artificial cycle with GnRH‐agonist pituitary suppression | |
| Participants | Inclusion criteria: maternal age <42 years, regular menstrual cycle, normal intrauterine cavity on pretreatment assessment, the presence of at least one vitrified euploid blastocyst obtained after intracytoplasmic sperm injection (ICSI) followed by preimplantation genetic diagnosis by aCGH, and a consent to undergo a frozen‐thawed single transfer in a modified‐NC or after hormonal endometrium preparation. Exclusion criteria were as follows: ovulation disorders, BMI >29 kg/m2, endometriosis grade ≥III according to the American Fertility Society criteria, and the use of testicular sperm for ICSI. | |
| Interventions | In the artificial protocol, GnRH‐agonist (buserelin acetate; Suprefact®; Hoechst, Marion Roussel, Milan, Italy) was started at the dose of 0.2 mg twice daily on day 21 of the previous menstrual cycle. When serum estradiol concentrations were <40 pg/mL and progesterone <1.5 ng/mL and no ovarian cystic structures were observed by transvaginal ultrasound, increasing doses of oral estradiol valerate (Progynova, Bayer, New Zealand limited, Auckland) were given. In general, patients started estradiol at a dose of 2 mg twice a day. This dose was increased every 3–5 days up to a maximum dose of 2 mg three times a day. Serial serum estradiol measurements and transvaginal ultrasound evaluation of the endometrium were monitored. After adequate endometrial proliferation (>7 mm), serum estradiol (>200 pg) and progesterone concentration (<1.5 ng/mL) were documented, GnRH‐agonist treatment was stopped, and treatment with intramuscular progesterone (Prontogest, IBSA, Lodi, Italy), 50 mg/day, was initiated. In cases of pregnancy, estradiol and progesterone were continued until the 12th gestational week. In modified‐natural cycle, all patients assessed on cycle day 3 the FSH, LH, estradiol, and progesterone levels in order to check if they corresponded to the early follicular phase. Subsequently, serum estradiol and LH levels and transvaginal ultrasound evaluation of the endometrium were performed serially according to the physician’s decision starting from day 8 of the cycle. Criteria for hCG administration included the following: mean diameter of dominant follicle of at least 17 mm, the endometrial thickness >7 mm, serum estradiol >200 pg, serum progesterone <1.5 ng/mL, and absence of a spontaneous LH surge. Final oocyte maturation was induced using 10.000 IU of hCG (Gonasi, 10.000 IU, IBSA, Lodi, Italy). The spontaneous LH surge was defined as LH concentration rise by 180 % above the latest available value. In these cases, hCG was not administrated and the cycles were cancelled. Intramuscular administration of progesterone at dose of 50 mg/day (Prontogest, IBSA, Lodi, Italy) was started in all patients 2 days after hCG. |
|
| Outcomes | The primary end‐points were the clinical pregnancy and implantation rates | |
| Notes | Trial registration: NCT02378584 Funding: no Conflict of interest: no Study dates: started on February 2015 and completed on September 2015 Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Two hundred thirty‐six patients were included in the study and randomized in two groups according to computer‐generated, not cancelled, simple randomization list with allocation assignment 1.1" |
| Allocation concealment (selection bias) | Unclear risk | Allocation method is not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We consider that blinding was not likely to influence findings |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We consider that blinding was not likely to influence findings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of follow‐up. |
| Selective reporting (reporting bias) | Low risk | NCT02378584 |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Groenewoud 2016.
| Study characteristics | ||
| Methods | This is a multicentre, non‐inferiority, randomised controlled trial. Patients were randomised based on a 1:1 allocation to either one cycle of modified natural cycle (mNC‐FET) or artificial cycle (AC‐FET). | |
| Participants | 18 to 40 years old, had to have a regular menstruation cycle between 26 and 35 days and frozen‐thawed embryos to be transferred had to derive from one of the first three IVF or IVF‐ICSI treatment cycles. Patients with a uterine anomaly, a contraindication for one of the prescribed medications in this study or patients undergoing a donor gamete procedure were excluded from participation. 1032 patients were included. | |
| Interventions | Patients undergoing modified NC‐FET (mNC‐FET) attended for ultrasound evaluation of the dominant follicle from Day 10 to 12 of their menstrual cycle. Ultrasound monitoring continued until the dominant follicle reached 16 – 20 mm in diameter. When the follicle had reached a size indicating maturity, hCG (5000 IU Pregnylw or 250 mg Ovitrellew, Merck, Kenilworth, USA) was given subcutaneously to trigger ovulation. No minimal endometrial thickness to precede treatment was appointed in the protocol and no additional endocrine monitoring was performed. Patients did not receive luteal support. In AC‐ FET cycles, oral oestrogen (Progynova 2 mg, three times daily; Bayer, Leverkusen, Germany) was commenced on the first or second day of the cycle with the aim of supporting endometrial proliferation and suppressing follicle growth. After 12 to 14 days, vaginal ultrasound examination was performed to confirm that no dominant follicle had emerged and to measure endometrial thickness. When the endometrial thickness reached ≥8 mm, vaginal micronised progesterone 200 mg three times daily was given. If the endometrial thickness was considered inadequate, the oestrogen dosage was raised to 8 mg daily and ultrasound examination was repeated after 1 week. If the endometrium remained,8 mm, the FET treatment cycle was cancelled. |
|
| Outcomes | Live birth rate (LBR) after mNC‐FET was 11.5% (57/495) versus 8.8% in AC‐FET (41/464). Main difference for cancellation in programmed cycle was insufficient endometrium thickness, while main reason for cancellation in natural cycle was spontaneous ovulation. | |
| Notes | Trial registration: Netherlands trial register, number NTR 1586 Funding for this study was provided by an unrestricted educational grant was awarded by Merck Sharp Dohme (MSD). MSD had no input or influence on the realisation of the study protocol or execution of the study. Nor did MSD play any role in the analysis and interpretation of the data as well as the preparation and approval of this manuscript. N.S.M. is supported by the National Institute for Health Research, Biomedical Research Centres (NIHR BRC) Southampton in Nutrition. Conflict of interest: all reported competing interests are outside the submitted work. Study dates: from February 2009 to April 2014 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Stratified randomisation with variable block sizes (ranging 2 – 12) was used in order to achieve a balanced 1:1 allocation. Stratification was based on the origin of the frozen embryos (IVF versus ICSI) and fertility clinic. To ensure allocation concealment, a web‐based randomisation module using a computerized list was used. The nature of the treatment interventions precluded blinding of patients and treating physicians" |
| Allocation concealment (selection bias) | Low risk | quote: "Stratified randomisation with variable block sizes (ranging 2 – 12) was used in order to achieve a balanced 1:1 allocation. Stratification was based on the origin of the frozen embryos (IVF versus ICSI) and fertility clinic. To ensure allocation concealment, a web‐based randomisation module using a computerized list was used. The nature of the treatment interventions precluded blinding of patients and treating physicians." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | For cancellation rate, absence of blinding was important as in natural cycles no minimal endometrial thickness to precede treatment was appointed but in the artifical cycles they needed 8 mm to go ahead. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 29% did not receive the intervention |
| Selective reporting (reporting bias) | Low risk | Netherlands trial registry (number NTR 1586) |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Gutierrez 1999.
| Study characteristics | ||
| Methods | Single‐centre trial.. Trial design: parallel. Allocation: method of randomisation and assignment was not stated. Blinding: not stated. Follow‐up: until pregnancy. Therefore live birth rate not included in outcomes. | |
| Participants | N = 68. Neither donor nor recipient mean age was reported. All the recipients were ovulating women. Cause of infertility: not stated. | |
| Interventions | 1 mg/day SC of leuprolide acetate was given to one group of women and 100 μg twice a day daily of nafareline acetate was given to the other group of recipients, starting at mid‐luteal phase. | |
| Outcomes | Pregnancy rate (leuprolide versus nafareline): 48.5% versus 54.3%. | |
| Notes | Data were obtained from the abstract. Trial registration: no Funding: no Conflict of interest: no Study dates: not stated Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not stated |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kahraman 2018.
| Study characteristics | ||
| Methods | The RCT was designed as a non‐inferiority study | |
| Participants | Women with irregular menses or anovulatory cycles undergoing frozen thawed single blastocyst transfer cycles without GnRHa suppression, having at least one day 5 (n = 290) or day 6 (n = 12) vitrified blastocyst in the Assisted Reproductive Technologies and Reproductive Genetics Centre at Istanbul Memorial Hospital. Exclusion criteria were the following: female age above 38 years, two or more previous unsuccessful cycles, a history of two or more early pregnancy losses, severe endometriosis, severe uterine malformation, azoospermia, and a history of familial thrombophilia or an abnormality in the thrombophilia tests. In addition, women with polycystic ovarian syndrome with more than 30 cumulus oocyte complexes retrieved at the pick‐up were considered to be ineligible and therefore were excluded. | |
| Interventions | 154 patients were allocated to receive 3.9 mg estradiol transdermal patch (Climara®, Bayer Turk, Turkey), whereas 160 women were allocated to endometrial preparation with a fixed dose of 2 mg three times per day oral of estradiol tablets (total 6 mg) (Estrofem®, Novo Nordisk, Denmark). If endometrial thickness was 7 mm or more, progesterone vaginal gel (Crinone® 8%; Merck Serono, Switzerland) twice a day, was started on the same day. Otherwise, oestrogen administration was continued to day 21. In cases where development had continued satisfactorily, embryo transfer was carried out 5 days after the commencement of progesterone administration. | |
| Outcomes | The viable clinical pregnancy rate was also higher in the oral ERT group (69.9% versus 61%), although it did not reach statistical significance (P = 0.103). The clinical miscarriage rate was higher in the oral ERT group (19.3% versus 14.6% in the patch group) (P = 0.387). | |
| Notes | Trial registration: NCT03155048 Funding: no Conflict of interest: the authors declare that they have no conflict of interest. Study dates: between May 2017 and October 2017 Authors were contacted but no proper explanation was found. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | www.randomization.com was used for randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% of loss of follow‐up |
| Selective reporting (reporting bias) | Low risk | ClinicalTrials.gov identifier: NCT03155048 |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Lee 2008.
| Study characteristics | ||
| Methods | Single‐centre trial. Trial design: parallel. Allocation method: not stated. Blinding: No. Follow‐up: until pregnancy. Therefore live birth rate not included in outcomes. |
|
| Participants | Eighty four (N = 84) frozen thaw embryo transfers cycles were randomised into two groups: Group A ( natural cycle) consist of 39 cycles and Group B (artificial cycles) consist of 45 cycles. | |
| Interventions | Comparison of pregnancy rate and implantation rate of frozen thawed embryo transfer cycles, between natural and artificial (hormone treated) cycles. | |
| Outcomes | Pregnancy rate (natural cycle vs artificial cycle): 74.4% versus 40.4%. | |
| Notes | Natural cycle group received an hCG injection when the leader follicle got to 18 mm to 20 mm. Then, they received vaginal progesterone supplementation, 90 mg every other day, started on the day after embryo transfer. Artificial cycle group received oral micronised estradiol, 2 mg three times daily, introduced on cycle day 2; progesterone 100 mg in oil was administered via IM injection 4 days before the embryo transfer. Cryopreservation was performed by vilification. Trial registration: no Funding: no Conflict of interest: no Study dates: not stated Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is not described how randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | It is not described how allocation was performed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Madani 2019.
| Study characteristics | ||
| Methods | This study was a pilot randomised, double‐blind placebo‐controlled trial, performed in Royan Institute for Reproductive Biomedicine from May 2012 to February 2015. Randomisation was performed by a third party using computer‐generated random numbers (SPSS version 18.0) prepared by the statistician. Concealed allocation was done by the Epidemiology Research Center. | |
| Participants | Eligibility criteria of inclusion were as follow: age of under 40 years, long or antagonist protocol, frozen‐thawed embryos available for another transfer, no history of uterine surgery, no uterine disorders, no endometriosis, no history of recurrent abortion (≥ 2 abortions) and no contraindications to aspirin. Overall, 60 available eligible women who were candidates for FET entered the study. | |
| Interventions | Women were randomly assigned (1:1) to two groups with either one dose of 100 mg oral aspirin (study group; n = 30) or placebo (control group; n = 30) on a daily basis. For the endometrial preparation, first, all patients received OCP‐LD from the 5th day of their previous menstrual cycle and 500 μg/day Buserelin (Aventis) as a GnRH agonist was administered subcutaneously from the 17th day of the cycle until pituitary desensitization (as confirmed by basal ultrasonography and serum E2 and LH levels) was obtained. Then, 2 mg of oral estradiol valerate (Aburaihan Co.) per day was initiated on the 2nd day of the cycle and the dose increased until the optimal endometrial thickness was obtained. Participants assigned to the study group received 100 mg aspirin (Pars Darou Co.) and those assigned to control group received placebo simultaneously, at the time of initiation of estradiol valerate administration. For endometrial thickness measurement, transvaginal ultrasound (Sonoline G20, Siemens Medical Solutions) was performed every 4 days from the 7th day of the cycle. After observation of at least 7‐mm endometrial thickness, 100 mg progesterone in oil (Aburaihan Co.) was administered intramuscularly or a twice‐daily dose of 400mg progesterone (800 mg) was given vaginally. |
|
| Outcomes | The study group had significantly higher rates of clinical pregnancy, implantation and live birth compared to the control group (P = 0.042; P = 0.031 and P = 0.007, respectively) | |
| Notes | Trial registration: no Funding: no Conflict of interest: the present study has no conflict of interest Study dates: from May 2012 to February 2015 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by a third party using computer‐generated random numbers (SPSS version 18.0) prepared by the statistician" |
| Allocation concealment (selection bias) | Low risk | Concealed allocation was done by the Epidemiology Research Center |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions from analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Live birth and pregnancy rates were very low in the group that did not use aspirin, which raise some concerns to generalise. Authors were contacted but no proper explanation was found. |
Matsuura 2014.
| Study characteristics | ||
| Methods | Prospective RCT in a private ART centre | |
| Participants | 102 infertile women under 42 years of age were treated with hormone replacement cycle undergoing the frozen‐thawed single blastocyst transfer | |
| Interventions | Group A (n=55) : The estradiol supplementation was initiated on day 2 from the menstruation cycle, thereafter the dydrogesterone was added. The frozen‐thawed single blastocyst was transferred to uterine cavity on day 20. Group B (n=47) : Taking the estradiol initiated with letrozole (2.5mg), 3 consecutive days together. |
|
| Outcomes | Clinical and ongoing pregnancy rates. Ongoing pregnancy rate was significantly higher in the Group B with Group A (38.3% versus 20.0%, P < 0.05) | |
| Notes | 2 mg of oral estradiol valerate (Aburaihan Co.) per day was initiated on the 2nd day of the cycle and the dose increased until the optimal endometrial thickness was obtained Trial registration: no Funding: no Conflict of interest: no Study dates: not stated Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses of follow‐up (<10%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Moffitt 1995.
| Study characteristics | ||
| Methods | Multicentre. Trial design: parallel. Allocation: randomised by pharmacist who equally packaged the tablets/placebo and randomly assigned sequential numbers. Blinding: double‐blind, placebo control. Follow‐up: until live birth, however the paper was written before all women delivered. Therefore live birth rate not included in outcomes. | |
| Participants | 61 cryopreservation patients. women mean age at time of oocyte retrieval was not reported. Their mean age at time of embryo transfer was 34.3 years for 6‐alpha‐methylprednisolone group and 33.6 years for control group. Cause of infertility: variety of causes. | |
| Interventions | In cryopreservation cycles, thawed embryos were transferred in either a natural cycle or a programmed cycle. For the natural cycle, monitoring began 2 to 3 days before the anticipated date of ovulation as judged by knowledge of previous cycle lengths. Thawing was performed on the day of ovulation as defined by the day after the peak of the serum LH surge or the day of disappearance of the dominant follicle by US. Natural cycles were supplemented with 25 mg 1M P in oil starting the day of transfer. Patients undergoing a programmed cycle applied a 0.1 mg transdermal E2 patch (Estraderm; CIBA Pharmaceuticals, Summit, NJ) on the day of menses and replaced it every 2 days. The Estraderm dose was increased to 0.2 on day 9, to 0.3 on day 11, and to 0.4 on day 13. From day 15 on, a constant dose of 0.2 mg was used for the entire luteal phase. Progesterone in oil was administered daily (50 mg 1M) starting on day 15. Group A received Methylprednisolone (4 mg orally nightly for four days, beginning the day before embryo thaw) and group B received placebo. |
|
| Outcomes | Pregnancy rate (methylprednisolone versus placebo): 25% versus 30.3%. | |
| Notes | Trial registration: no Funding: no Conflict of interest: no Study dates: from January to September 1993 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised by pharmacist who equally packaged the tablets/ placebo and randomly assigned sequential numbers |
| Allocation concealment (selection bias) | Low risk | Randomised by pharmacist who equally packaged the tablets/ placebo and randomly assigned sequential numbers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo control |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Movahedi 2018.
| Study characteristics | ||
| Methods | Participants were randomly allocated into two treatment groups | |
| Participants | 100 women with a functioning ovary and with a normal cavity of uterus were enrolled in the study. All participants were 25 to 38 years old and were eligible for infertility treatments. The analysis was confined to FET cycles which were not donor‐related. All participants who had previously undergone intracytoplasmic sperm injection (ICSI) cycle with embryo cryopreservation and the transfer of their frozen‐thawed embryos prospectively participated in this interventional study. We excluded patients above 39 years old, and those whose FSH was above 11, had endometriosis and hypothalamic amenorrhoea. | |
| Interventions | In study group (A) 60 patients used oral contraceptive‐ LD (manufactured by Aburaihan Co., Tehran‐ Iran) the month before the embryo transfer, GnRH agonist suppression (buserelin: Superfact‐manufactured by Aventis Pharma Deutschland) 0.5mg/day was administered from the day 21 of the cycle. The control group (B) was composed of 40 patients who did not use GnRH agonist. In both groups endometrial preparation was achieved by the use of estradiol valerate pill 2 mg (manufactured by Aburaihan Co. Tehran‐Iran), which were started from the second day of the menstruation and were used every day, with initial dose of 2 mg/day and after 3 days increased to 4 mg/day and after 3 days again increased to 6 mg/day. Trans‐vaginal ultrasound (TVU) was performed on the 13th day of estradiol treatment. Stimulation characteristics and protocol for the collection ICSI cycle were standard. If endometrial thickness (ET) was measured (EM) ≥ 7 mm, progesterone was added to the estradiol regimen and if the ET was < 7 mm, 2 mg estradiol valerate was added for four days before repeating TVU and starting progesterone (Cyclogest 400 mg pessaries manufactured by Actavis, Barnstaple, UK) was used vaginally, at a dose of 800 mg/day. | |
| Outcomes | Clinical pregnancy (positive fetal heart on TVU) | |
| Notes | IRCT201109224572N2 July 2008 to April 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is not stated if there were missing outcome reports |
| Selective reporting (reporting bias) | Low risk | IRCT201109224572N2 |
| Other bias | Low risk | No other risks of bias were found |
Nekoo 2015.
| Study characteristics | ||
| Methods | Computerised random allocation program: patients were randomly divided into two groups with different endometrial preparation regimen for frozen embryo transfer from January 2010 to February 2011 | |
| Participants | infertile patients (male factor) aged 20 to 37 years who had regular menstrual cycles and previously undergone IVF or ICSI with the same induction protocol with embryo cryopreservation | |
| Interventions | In both groups, oral Estradiol Valerate was taken at 4 mgdailyfromday2today5,at6mgperdayfrom day 6 to the day of the pregnancy test. In day 13 of cycle, an ultrasound examination was performed. After ultrasound confirmation of endometrial thickness (≥8 mm) and if a periovulatory follicle was not present at the day 13 ultrasound, progesterone in cyclogest supp (400 mg /Bd) was added. The dose of estradiol would be increased to 8 mg per day if the endometrial thickness was less than 8mm. In group A (93 patients), diphereline (3.75 mg IM), as a depot GnRH agonist was administered in the mid‐luteal phase (day 21). In the other group B (n = 83) commenced steroid supplementation without prior pituitary desensitisation. |
|
| Outcomes | Clinical pregnancy rate was 22.6% in the GnRH agonist group and 30.1% in the non‐GnRH agonist group | |
| Notes | Trial registration: no Funding: no Conflict of interest: Quote: "There is no conflict of interests among the authors" Study dates: from January 2010 to February 2011 Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "according to computerized random allocation program were randomly divided into two groups with different endometrial preparation regimen" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol was found |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Ramos 2007.
| Study characteristics | ||
| Methods | Single‐centre trial. Trial design: parallel. Allocation: not stated. Blinding: no. Follow‐up: until evolutive pregnancy. Live birth rate not included in outcomes. |
|
| Participants | N = 119. Women in treatment for frozen‐thawed embryo transfer were randomised for this study and all of them used oral contraceptives pills the month before the embryo transfer. Average patients´ age was 35 years old, and the number of embryo transfer was 1.3. Endometrial thickness previous progesterone was 8 mm . | |
| Interventions | 119 patients in treatment for frozen‐thawed transfer were randomised for this study and all of them used oral contraceptive pills the month before the embryo transfer, starting on day one of the cycle. 66 patients were randomised to the group A, which used GnRH agonist suppression (tryptorelin 3.75 mg depot, 1 ampoule IM) the day of contraceptive pill number 16. The group B was composed of 53 patients randomised who did not use GnRH agonist. In both groups endometrial preparation was achieved by the use of estradiol transdermical patches, which were started from the second day of the menstruation and used every other day, with an initial dose of 100 mg/d and after two days increased to 200 mg/d. Progesterone was used vaginally, at a dose of 800 mg/d, starting after at least 11 days of transdermical estradiol, on the day 0 of embryo development previous ultrasound examination and serum estradiol and progesterone levels | |
| Outcomes | Pregnancy rate (Group A tryptorelin, estradiol and+ progesterone versus group B estradiol plus progesterone alone ):25.6% versus 24.3%. Ongoing pregnancy rate: 14.1%versus 17.1%, Miscarriage rate: 11.5% versus 7.1% | |
| Notes | GnRH agonist suppression (tryptorelin 3.75 mg depot, 1 ampoule IM) was given on the day of contraceptive pill number 16 (in the previous cycle). In both groups endometrial preparation was performed with estradiol transdermic patches, which were started from the second day of menstruation and used every other day, with an initial dose of 100 mg/day and after two days increased to 200 mg/day. Progesterone was used vaginally, at a dose of 800 mg/day, started at least 11 days after transdermic estradiol was started. Trial registration: no Funding: no Conflict of interest: no Study dates: not stated Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is not described how randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | It is not described how allocation was performed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | We did not consider that blinding was likely to influence findings |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information to evaluate this |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Remohi 1994.
| Study characteristics | ||
| Methods | Single‐centre trial. Trial design: parallel. Allocation: not stated. Blinding: not stated. Follow‐up: until pregnancy. Therefore live birth rate not included in outcomes. | |
| Participants | N = 69. Oocyte recipients mean age was 37.1 years for daily leuprolide acetate group and 37.9 years for the no‐treatment group. Donor mean age were 31.4 years and 31.1 years, respectively. Cause of infertility: poor responders 85%; premature ovarian failure 15%. | |
| Interventions | One group of recipients was desensitised with daily subcutaneous administration of 1 mg leuprolide acetate and the other group was not desensitised. | |
| Outcomes | Pregnancy rate (leuprolide daily versus no treatment): 54.5% versus 58.3%. | |
| Notes | Trial registration: no Funding: no Conflict of interest: no Study dates: not stated Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was not stated how performed |
| Allocation concealment (selection bias) | Unclear risk | It was not stated how performed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data available |
| Selective reporting (reporting bias) | Unclear risk | No abstract available |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Samsami 2018.
| Study characteristics | ||
| Methods | A total of 237 women were randomly allocated in two groups (119 and 118 cases in groups A and B, respectively). | |
| Participants | All individuals with established medical diagnosis of infertility who referred to Shahid Faghighi hospital, affiliated to Shiraz University of Medical Sciences, Shiraz, Iran for IVF from November 2014 to November 2015 were invited to participate in the study. Inclusion criteria for this study were as follows: ‐history of infertility ‐ age 20‐39 years ‐ couples undergoing ART with their own gamete ‐ couples having frozen embryo available for transfer Participants with the following criteria were excluded from the study: ‐ high‐grade endometriosis ‐ existence of myoma or adhesion in uterus ‐ BMI more than 29 or less than 18 kg/m2 ‐ oocyte donation cycles |
|
| Interventions | In group A, 0.5 mg of Buserelin acetate (a GnRH agonist) (Suprefact, Hoechst AG, Germany) was injected SC daily starting on the 21st day of menstrual cycle (mid‐luteal phase). On the first day of menstruation, GnRH agonist dose was reduced to 0.3 mg/day SC. Participants in group B did not receive GnRH agonist for pituitary down‐regulation. In both groups, Estradiol Valerate (Abureyhan, Iran) was administered orally, starting on the second day of the target cycle with a dosage of 6 mg/day for endometrial preparation. | |
| Outcomes | Ongoing pregnancy until 12th week of gestation was achieved in 18 cases in group A and 16 patients in group B | |
| Notes | Thawed embryos were transferred on the 3rd day following progesterone administration Trial registration: IRCT2017052834184N1 Funding: Shiraz University of Medical Sciences (grant No 5665) Conflict of interest: authors declared no conflict of interests Study dates: from November 2014 to November 2015 Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation codes |
| Allocation concealment (selection bias) | Unclear risk | No data about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 37 out of the 237 randomised women were not analysed |
| Selective reporting (reporting bias) | Low risk | Registration ID in RCT: IRCT2017052834184N1 |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Samsami 2019.
| Study characteristics | ||
| Methods | Randomisation table | |
| Participants | Women aged 18 to 42 years having frozen embryos, normal uterine cavity, normal endometrium without any endometrial polyp or sub‐mucosal myoma (according to normal hysterosalpingography, saline infusion sonography or 2 hysteroscopy), and BMI < 35 kg/m2. The exclusion criteria were other maternal medical disease and hydrosalpinx. | |
| Interventions | 167 women, 82 in the letrozole group and 87 in the HR group. Stimulated: participants were prescribed 5 mg letrozole/day from the third day to the seventh day of the menstrual cycle. Follicular development was monitored by vaginal ultrasonography starting on the 10th day of the menstrual cycle; if the follicular diameter was ≥ 17 mm and the endometrial thickness reached 7 mm to 9 mm, they were given 10,000 units of HCG, and 36 to 48 hours after that, they were progesterone ampoule 100 mg was given IM/day. Programmed: the participants were prescribed oral estradiol valerate (2 mg three times a day) starting on the second to the third day of menstrual cycle, and on the 10th day, the endometrial thickness was monitored by vaginal ultrasonography. If the endometrial thickness was 7 mm to 9 mm and three‐line pattern, oestrogen was continued and progesterone therapy was initiated 100 mg IM/day |
|
| Outcomes | Ultrasonography was performed 28 to 30 days after the embryo transfer, having a fetal heart beat was defined as a clinical pregnancy | |
| Notes | For a period of 12 months, commencing on January 2018 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost of follow‐up < 10% |
| Selective reporting (reporting bias) | Low risk | IRCT: 20190327043121N1 |
| Other bias | Low risk | No other bias reported |
Sheikhi 2018.
| Study characteristics | ||
| Methods | The randomisation was done at the start of the cycle us‐ing sequential numbering based on a computer‐generated list that had been prepared at the Statistics Center of the Babol University of Medical Science and sent to us. Then, the participants were randomly assigned to either modified natural cycle with HCG (n = 31), mildly hormonally stimulated cycle (n = 30) or artificial regimen (n = 62), in a ratio 1:1:2. The participant and the infertility expert were not blinded for treatment allocation. | |
| Participants | A total of 131 patients submitted to vitrified thawed blastocyst transfer in our IVF laboratory were invited from March 2015 to January 2016. Women undergoing vitrification thawed blastocyst transfer (VTBT) were eligible for the study when they were normo‐ovulatory women, between 20 to 40 years of age, with "19<BMI <30." The exclusion criteria included women with PCOS, basal FSH>10 IU/mL and basal E2 <70 pg/mL, those with untreated thyroid disorders, severe endometriosis, recurrent implantation failure, uterine pathology, recurrent abortion, repeated implantation failure, smokers, athletes and patients who had used any medication in the two previous months that could interfere with the normal function of the hypothalamic‐pituitary‐gonadal axis. |
|
| Interventions | We used the natural cycle with HCG for the patients in this group; no medication was administered during the endometrial preparation. The follicles were monitored by TVS until the dominant follicles reached a diameter of 18 mm to 20 mm and endometrium thickness > 8 mm. Then, 10,000 IU of human chorionic gonadotropin (CG, Daroupakhsh, Iran) was administered for ovulation. The mild hormonally‐stimulated group with clomiphene citrate (Clomid, Iran Hormone Company) was administered 50 mg daily from day 3 of the menstrual cycle for 5 days. If during TVS a follicle 18 mm to 20 mm was visible, ovulation was deemed to have occurred. "Then, 10,000 IU of urinary" The mild hormonally‐stimulated group with clomiphene citrate (Clomid, Iran Hormone Company) was administered 50 mg daily from day 3 of the menstrual cycle for 5 days. If during TVS a follicle 18 mm to 20 mm was visible, ovulation was deemed to have occurred. Then, 10, 000 IU of urinary HCG was administered and the blastocyst were transferred 36 to 38 hours after HCG. The Artificial cycles began on the third day of the menstrual cycle or progesterone withdrawal. The dose of oral estradiol valerate (E2) (Aburaihan Pharmaceutical Co., Tehran, Iran) was 2mg bid (4mg/day). A higher initial dose of estradiol (6mg) was administered if the patient showed inadequate endometrial thickness in a previous cycle. TVS was carried out on day 10. If the endometrial thickness reached 8 mm and further, 50mg progesterone was given IM for 3 days (Aburaihan Pharmaceutical Co., Tehran, Iran) and estradiol was continued as well, then the blastocysts were transferred on the fourth day of progesterone administration. If the endometrial thickness was 8mm or less on day 10, the dose of estradiol valerate was increased to 4mg twice/day and the blastocyst were transferred 4‐5 days following initiation of progesterone administration if the signs of ovulation were observed upon TVS. If the endometrial thickness did not reach 8 mm up to day 20, or the ovulation was not confirmed, the cycle was cancelled |
|
| Outcomes | A clinical pregnancy was defined as the visualisation of a gestational sac with fetal heart activity on TVS in week five of gestation. | |
| Notes | IRCT: 201408021760N36 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequential numbering based on a computer‐generated list that had been prepared at the Statistics Center of the Babol University of Medical Science |
| Allocation concealment (selection bias) | High risk | Quote: "The participant and the infertility expert were not blinded for treatment allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | MIssing outcomes less than 10% |
| Selective reporting (reporting bias) | Low risk | IRCT: 201408021760N36 |
| Other bias | Low risk | No other risk of bias were found |
Tehraninejad 2018.
| Study characteristics | ||
| Methods | Prospective, randomised, single‐blind clinical trial. The study was carried out in Reproductive Health Research Center, Tehran University of Medical Sciences between September 2015 and November 2016. | |
| Participants | One hundred volunteers for FET cycle (due to premature ovarian failure, ovarian hyper stimulation syndrome (OHSS) or other reasons) | |
| Interventions | All participants received GnRH agonist (Decapeptyl, Ferring, Switzerland) 0.1 mg subcutaneously from the 21st day of the cycle and it was continued for at least 14 days. In the first day of menstrual cycle suppression of ovary have been confirmed by ultrasonography. In group I estradiol valerate tablet (8 mg/day) (Progynova, Schering, Berlin, Germany) and in group II topical estradiol gel (6 mg/day) (Oestrogel, 17B estradiol 0.06% gel, Besins, France) was started from the first day of menstruation. One week after the start of estradiol, ultrasonography was performed to estimate endometrial thickness and repeated if necessary. On day 13 of menstrual cycle, the second ultrasound was performed and the thickness of the endometrium was estimated. If endometrial thickness was more than 8 mm, based on the embryonic age, progesterone (Cyclogest, 400 mg, Cox Pharmaceuticals, Barnstaple, UK,) was given for four to six days before embryo transfer. | |
| Outcomes | 24% of participants in group II and 16% of women in group I had a clinical pregnancy | |
| Notes | Trial registration: IRCT2016092429951N1 Funding: no Conflict of interest: the authors report no conflicts of interest Study dates: between September 2015 and November 2016 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | High risk | Participants have been allocated by a nurse |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss of follow‐up |
| Selective reporting (reporting bias) | Low risk | Registration ID in IRCT: IRCT2016092429951N1 |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Tocino 2007.
| Study characteristics | ||
| Methods | Singl‐ centre trial. Trial design: parallel. Allocation method: not stated. Blinding: no Follow‐up: until pregnancy. Therefore live birth rate not included in outcomes. |
|
| Participants | Forty‐eight (N = 48) recipients for oocyte donation, were randomised into two groups: Group A (N = 23) used daily GnRH agonist suppression (Leuprolide Acetate 0.1 mL/day) and Group B (N = 23) used a GnRH agonist depot (tryptoreline 3.75 mg/IM) | |
| Interventions | Forty‐eight (N = 48) recipients for oocyte donation, received oral contraceptive pills the month before the embryo transfer, and were randomised into two groups: Group A (N = 23) used daily GnRH agonist suppression (Leuprolide Acetate 0.1 mL/day) starting 5 days before ending the contraceptive pill. Group B (N = 23) used a GnRH agonist depot (tryptoreline, 3.75 mg/IM) 5 days before ending the contraceptive pill. In both groups the endometrial preparation was achieved by using estradiol transdermic patches, started from the second day of the menstruation and increasing the doses | |
| Outcomes | Pregnancy rate (daily leuprolide acetate versus depot tryptoreline): 65% versus 50%, Implantation rate: 48.8% versus 36.1%, Abortion rate (6.7% versus 40%) | |
| Notes | In both groups, the average patient age was 38 years old and the mean number of embryos transferred was 1.8. the endometrial thickness and estradiol levels previous to donation were similar in both groups. Trial registration: no Funding: no Conflict of interest: no Study dates: between September 2006 and March 2007 Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of how allocation and randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | No description of how allocation and randomisation was performed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% of follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Vidal 2009.
| Study characteristics | ||
| Methods | A prospective, blinded, RCT was carried out in our centre (EudraCT: 2007‐000212‐89) from January, 2007 to September, 2009 | |
| Participants | The population randomised was 560 patients. The inclusion criteria were as follows: recipients with preserved ovarian function under 45 years old, BMI < 28 kg/m2, 1st or 2nd egg donation cycle, 1 to 2 good embryos transferred. Exclusion criteria were uterine diseases (polyps, myomas, Müllerian defects, adenomyosis), severe male factor (motile sperm < 5mill.), abnormal FISH spermatozoa, thrombophilia and recurrent pregnancy losses. | |
| Interventions | To compare a new approach for endometrial priming with the 7 day dosage of GnRH antagonist (cetrorelix 0.25 mg) in an oocyte donation programme with the conventional single dose GnRH agonist (tryptorelin, 3.75 IM) on day 21st of the menstrual cycle | |
| Outcomes | Ongoing pregnancy rate (OPR) was the primary endpoint. Implantation rate (IR), ectopic, and miscarriage rates were the secondary outcome measures. Clinical pregnancy rate in antagonists group: 68.1% and in agonist group: 56.8 % | |
| Notes | Trial registration: no Funding: no Conflict of interest: no Study dates: from January, 2007 to September, 2009 Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Population randomised: 560. From the total number of patients randomly allocated to each group, 473 underwent embryo transfer, and received 7 days GnRH antagonists (group A, 232 patients) or a single IM injection of 3.75 mg tryptorelin (group B, 241 patients) or A total of 87 dropped out of the study due to insufficient endometrial preparation or to transfer cancellation. |
| Selective reporting (reporting bias) | Low risk | EudraCT: 2007‐000212‐89 |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Wright 2006.
| Study characteristics | ||
| Methods | Single‐centre trial. Trial design: parallel. Allocation: not stated. Blinding: not stated. Follow‐up: until pregnancy. Therefore live birth rate not included in outcomes. | |
| Participants | N=199. Women's mean age at time of oocyte retrieval was not reported. Their mean age at time of embryo transfer was 34 years for both groups. Cause of infertility: not stated. | |
| Interventions | Stimulated women received recombinant FSH injections 150 IU on days 6, 8 and 10 of the menstrual cycle, and continued until endometrium was thicker than 7 mm or follicles were bigger than 16 mm to 20 mm. Women who had artificial cycles received 17‐beta‐estradiol 4 mg per day until endometrium over 7 mm. | |
| Outcomes | Pregnancy rate (FSH versus estradiol): 12.1% versus 11%. | |
| Notes | Both groups received progesterone 300 mg in vaginal suppositories. Trial registration: not stated Funding: not stated Conflict of interest: not stated Study dates: not stated Authors were contacted for missing information ‐ no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used was not stated |
| Allocation concealment (selection bias) | Unclear risk | Method used was not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not consider that blinding was likely to influence finding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We did not consider that blinding was likely to influence findings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses to follow‐up (<1 0%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | The study appears to be free of other sources of bias |
ART: assisted reproductive technology; β‐HCG: beta human chorionic gonadotropin; BMI: body mass index; FET: frozen‐thawed embryo transfer; FSH: follicle stimulating hormone; GnRH: gonadotropin‐releasing hormone; HMG: human chorionic gonadotropin; HRT: hormone replacement therapy; IVF: in vitro fertilisation; ICSI: intracytoplasmic sperm injection; IM: intramuscular; IU: international unit; IVF: in vitro fertilisation; RCT: randomised controlled trial; RIF: recurrent implantation failure; SC: subcutaneous; TVU: trans‐vaginal ultrasound.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arun Muthuvel 2016 | Wrong intervention: the study is about luteal phase support |
| Bernabeu 2006 | Wrong intervention: indomethacin was not used for endometrial preparation; it was used just before embryo transfer. |
| Bjuresten 2011 | Wrong intervention:the study is about luteal phase support |
| Boostanfar 2016 | Wrong intervention: participants did not use one of our specified treatments for endometrial preparation: Women were randomised to a single injection of 150 μg of corifollitropin alfa or daily 300 IU of recombinant follicle‐stimulating hormone for the first 7 days of controlled ovarian stimulation (COS) in a gonadotropin‐releasing hormone (GnRH) antagonist protocol. |
| Caligara 2003 | Wrong intervention: the study is about luteal phase support |
| Cambiaghi 2013 | Wrong intervention: the study is not about endometrial preparation. |
| Check 1998 | Wrong study design:the study was not a randomised controlled trial. |
| Check 2004 | Wrong intervention: the study compares different kinds of stimulation (sildenafil versus oestradiol) |
| Davar 2015 | Wrong intervention: the study evaluated the effects of single dose GnRH agonist as luteal support |
| Davar 2016a | Wrong population: participants did not meet our criteria: women with a history of thin endometrium |
| Davari‐Tanha 2016 | Wrong population: participants did not meet our criteria: women with a history of recurrent implantation failure |
| Eftekhar 2013 | Wrong intervention: the study is about luteal phase support |
| Feliciani 2004 | Wrong intervention: doses used for vaginal progesterone were below the standard doses |
| Gibbons 1998 | Wrong intervention: the study is about luteal phase support |
| Gogce 2015 | Wrong intervention: participants did not use one of our specified treatments for endometrial preparation: GnRH agonists in the luteal phase of Artificial Cycle Frozen‐Thawed Embryo Transfers |
| Hershko 2016 | Wrong intervention: the study is about luteal phase support |
| Huang 2017 | Wrong population: participants did not meet our criteria: women with history of recurrent implantation failure |
| Krasnow 1996 | The study does not evaluate our primary or secondary outcomes: the outcomes were endometrial histology and beta‐3‐integrin expression |
| Lan 2008 | Wrong intervention: the study is about luteal phase support |
| Lewin 2001 | The study does not evaluate our primary or secondary outcomes: histological outcome |
| Li 2014 | Wrong intervention: in the intervention group (letrozole) more than one intervention is applied (i.e. ovulation triggering with human chorionic gonadotropin or with tryptorelin |
| Lightman 1999 | Wrong intervention: the study concerns luteal phase support |
| Llacer 2017 | Wrong intervention: the study concerns luteal phase support |
| Moon 2004 | Wrong intervention: piroxicam was not used for endometrial preparation, it was used just before embryo transfer. |
| Nardo 2006 | Wrong study design: It is not a randomised controlled trial. |
| Neuspiller 1998 | Wrong study design: the study is a quasi‐randomised study |
| Prapas 2009a | Wrong intervention: this study evaluates the use of hCG, which was not stated in our protocol |
| Prapas 2009b | Wrong intervention: no comparison included in our protocol was evaluated |
| Sanchez 2009 | Wrong intervention: this study evaluated when to start the oestradiol replacement, which is not an intervention that was stated in our protocol. |
| Sathanandan 1991 | Wrong study design: randomisation was not adequate. |
| Shiotani 2006 | Wrong intervention: this study evaluates the use of hCG, which was not stated in our protocol. |
| Simon 1998 | There was a co‐intervention that was not similar in the two arms, which does not make it possible to analyse if the observed result was caused by the intervention or by the co‐intervention. |
| Stadtmauer 2009 | Wrong study design:this is not a randomised controlled trial because randomisation was not done for embryo transfer. It was done for endometrial biopsy and just some of them (less than 10) decided to have a transfer. |
| Taskin 2002 | The study does not evaluate our primary or secondary outcomes: excluded because the outcome was not clinical (but histological). |
| Tesarik 2003 | Wrong intervention: this study evaluates the use of hCG, which was not stated in our protocol |
| Weckstein 1997 | Wrong population: women with a history of thin endometrium |
| Xu 2015 | Wrong population: women with a history of thin endometrium |
| Zegers‐Hochschild 2000 | Wrong intervention: the study is about luteal phase support |
| Zolghadri 2014 | Wrong population: women with a history of thin endometrium |
HCG: human chorionic gonadotropin;IU: international unit.
Characteristics of studies awaiting classification [ordered by study ID]
Masrour 2018.
| Methods | After initial screening and fulfilment of inclusion and exclusion criteria, research participants were randomised, using a table of random numbers, to one of two groups: natural FET (n = 100) and HRT FET (n = 100). All participants intending to commence the intervention and meeting the study criteria were invited to the fertility unit between days 1 and 3 of their monthly cycle for baseline scan and study enrolment. At the first visit those who wished to take part, completed written consent form and were randomly assigned into mentioned groups. |
| Participants | Women aged 19 to 39 years, planning an FET cycle at the Infertility Clinic of Shahid Akbar Abadi hospital were invited to take part in this trial. They were eligible to participate if they were assisted reproductive techniques such as IVF and ICSI with frozen embryo transfer cycles due to male factor, had a regular menstrual cycle, serum levels of follicle stimulating hormone (FSH) less than 10 IU/dL and a normal serum prolactin level. Women were excluded from participating in the trial if they were allergic to estradiol or progesterone, had uterine anomaly, polycystic ovary syndrome (PCOS), endometriosis stage III/IV, preimplantation genetic diagnosis cycles, tubal factor and history of receiving donated oocytes. |
| Interventions | The women in the hormonal group were administered 4 mg to 6 mg of oestrogen in the form of oral estradiol valerate (Progynova®; Schering, Madrid, Spain) on the third day of their cycles as the intervention after a transvaginal ultrasound. A second transvaginal ultrasound was performed after 10 to 12 days of oestrogen treatment. If endometrial thickness was at least 8 mm, embryo transfer (Only blastocyst embryos) were planned. Natural micronised progesterone (Utrogestan®; Seid, Madrid, Spain) was vaginally administered at a dose of 400 mg/12 hours for 3 or 5 complete days before embryo transfer, depending on the cleavage stage of embryos (embryo age +1 day). Progesterone supplementation continued if pregnancy occurred until 12 weeks of pregnancy. |
| Outcomes | Chemical pregnancy (based on hormone levels of β‐HCG) was considered as the primary outcome and clinical pregnancy (existing fetal heartbeat) was considered as the secondary outcome. |
| Notes | IRCT code: IRCT2017081335670N1 |
Page 2005.
| Methods | Patients randomised to the stimulated protocol began rec‐FSH every other day beginning on day 4 of their menstrual cycle. Ultrasound and hormone assays were performed beginning on day 9 to 10. Rec‐hCG was administered when the endometrial thickness was ≥ 7 mm and a follicle reached 16 mm to 20 mm. Vaginal P was begun the following day. Patients randomised to the artificial cycle began taking oral 17‐beta estradiol (E2) 2 mg twice per day beginning on day 1 of their menstrual cycle. Ultrasound and hormone assays were begun on day 9 to 10; if the endometrial thickness was < 7 mm on day 9 to 10, patients were switched to vaginal E2 2 mg once per day. Patients began vaginal micronised P once the endometrial thickness was > 7 mm. |
| Participants | 165 women with functional ovaries undergoing 199 FET cycles were randomised |
| Interventions | Patients randomised to the stimulated protocol began rec‐FSH every other day beginning on day 4 of their menstrual cycle. Ultrasound and hormone assays were performed beginning on day 9 to 10. Rec‐hCG was administered when the endometrial thickness was ≥ 7 mm and a follicle reached 16 mm to 20 mm. Vaginal P was begun the following day. Patients randomised to the artificial cycle began taking oral 17‐beta estradiol (E2) 2 mg twice per day beginning on day 1 of their menstrual cycle. Ultrasound and hormone assays were begun on day 9 to 10; if the endometrial thickness was < 7 mm on day 9 to 10, patients were switched to vaginal E2 2 mg once per day. Patients began vaginal micronised P once the endometrial thickness was > 7 mm. |
| Outcomes | Endometrial thickness |
| Notes |
Tur‐Kaspa 2010.
| Methods | Patients undergoing FET or ED cycles were equally randomised for down regulation with daily antagonist injection (Cetrotide, EMD Serono, Inc.) from day 9 to 11 of oestrogen treatment or with mid‐luteal daily agonist injection (Lupron, Tap Pharmaceuticals). |
| Participants | Recipients for frozen embryo transfer (FET) and egg donation (ED) cycles |
| Interventions | Daily antagonist injection (Cetrotide, EMD Serono, Inc.) from day 9 to 11 of oestrogen treatment or with mid‐luteal daily agonist injection (Lupron, Tap Pharmaceuticals) |
| Outcomes | The primary end‐points for this interim analysis were embryo implantation and clinical pregnancy rates. Secondary end‐points were patient satisfaction and ongoing pregnancy/delivery rate. |
| Notes | www.ClinicalTrials.gov (NCT00460642) |
Outcomes that were reported only 'per cycle' and not per randomised woman, in which more than one cycle was performed on each randomised woman. No reply from the authors yet.
β‐HCG: beta human chorionic gonadotropin; BMI: body mass index; FET: frozen‐thawed embryo transfer; FSH: follicle stimulating hormone; HRT: hormone replacement therapy; IVF: in vitro fertilisation; ICSI: intracytoplasmic sperm injection; IM: intramuscular; IU: international unit; IVF: in vitro fertilisation.
Differences between protocol and review
Administration of progesterone in relation to oocyte retrieval was not pre‐specified but was added as a comparison when the review was being prepared, after protocol publication. The authors considered that this was a clinically significant comparison.
Authors also added, in this review update, the route of administration for oestrogens and comparisons between GnRH agonists and antagonists as they considered relevant.
Studies comparing progesterone versus nothing or placebo, and studies comparing different routes of administering progesterone, were excluded from this review.
Studies with clomiphene citrate were analysed as a post‐hoc subgroup for the outcome endometrial thickness.
Contributions of authors
Demian Glujovsky: conceived the protocol of the review, and coordinated the whole review process.
Romina Pesce: conceived the protocol of the review and participated in the selection, assessment and data extraction.
Agustin Ciapponi: supervised the methods. He resolved discrepancies of selection or assessment and conducted the analysis.
Carlos Sueldo: provided general advice on the protocol. He assisted in writing the review and resolved discrepancies of selection or assessment.
Andrea Quinteiro: participated in the update by carrying out the screening, assessment of eligibility and data extraction.
Roger Hart: provided general advice on and assistance with the review and contributed to writing the review.
Gabriel Fiszbajn: conceived the review and participated in the selection, assessment and data extraction of the first published review in 2010.
Sources of support
Internal sources
None form of support is provided to the authors of this review, Argentina
External sources
None form of support is provided to the authors of this review, Argentina
Declarations of interest
Demian Glujovsky: None
Romina Pesce: None
Carlos Sueldo: None
Andrea Quinteiro Retamar: None
Roger Hart: RH is the Medical Director of Fertility Specialists of Western Australia and has equity interests in Western IVF and has received research grant funding from Ferring Pharmaceuticals and Merck.
Agustin Ciapponi: None
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Agha‐Hosseini 2018 {published data only}
- Agha-Hosseini M, Hashemi L, Aleyasin A, Ghasemi M, Sarvi F, Nashtaei MS, et al. Natural cycle versus artificial cycle in frozen-thawed embryo transfer: a randomized prospective trial. Turkish Journal of Obstetrics and Gynecology 2018;15(1):12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aleyasin 2017 {published data only}
- Aleyasin A, Aghahosseini M, Safdarian L, Noorzadeh M, Fallahi P, Rezaeian Z, et al. Can letrozole plus HMG protocol improve pregnancy outcomes in frozen-thawed embryo transfer? An RCT. International Journal of Reproductive Biomedicine 2017;15(2):83-6. [PMC free article] [PubMed] [Google Scholar]
Bider 1996 {published data only}
- Bider D, Amoday I, Yonesh M, Yemini Z, Mashiach S, Dor J. Glucocorticoid administration during transfer of frozen-thawed embryos: a prospective, randomized study. Fertility and Sterility 1996;66(1):154-6. [DOI] [PubMed] [Google Scholar]
Check 2002 {published data only}
- Check J, Lee G, Nazari A, Davies E, Choe J. Neither sildenafil nor vaginal estradiol improved endometrial thickness in women with thin endometria after taking oral estradiol in graduating dosage. Annual Meeting of the Pacific Coast Reproductive Society (abstract) 2002. [PubMed]
Child 2013 {published data only}
- Child T, McVeigh E, Turner K, Mounce G. A randomized controlled trial of natural versus GnRH-agonist/HRT regimes for frozen embryo replacement. Fertility and Sterility 2013;100(3):S146. [DOI] [PubMed] [Google Scholar]
- Mounce G, McVeigh E, Turner K, Child TJ. Randomized, controlled pilot trial of natural versus hormone replacement therapy cycles in frozen embryo replacement in vitro fertilization. Fertility and sterility 2015;104(4):915-20. [DOI] [PubMed] [Google Scholar]
Dal Prato 2002 {published data only}
- Dal Prato L, Borini A, Cattoli M, Bonu MA, Sciajno R, Flamigni C. Endometrial preparation for frozen-thawed embryo transfer with or without pretreatment with gonadotropin-releasing hormone agonist. Fertility and Sterility 2002;77(5):956-60. [DOI] [PubMed] [Google Scholar]
Davar 2007 {published data only}
- Davar R, Eftekhar M, Tayebi N. Transfer of cryopreserved-thawed embryos in a cycle using exogenous steroids with or without prior gonadotrophin-realising hormone agonist. Journal of Medical Sciences 2007;7(5):880-3. [Google Scholar]
Davar 2016 {published data only}
- Davar R, Janati S, Mohseni F, Khabazkhoob M, Asgari S. A comparison of the effects of transdermal estradiol and estradiol valerate on endometrial receptivity in frozen-thawed embryo transfer cycles: a randomized clinical trial. Journal of Reproduction and Infertility 2016;17(2):97-103. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Davar 2020 {published data only}
- Davar R, Dashti S, Omidi M. Endometrial preparation using gonadotropin-releasing hormone agonist prior to frozen-thawed embryo transfer in women with repeated implantation failure: an RCT. International Journal of Reproductive BioMedicine 2020;18(5):319-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ding 2007 {published data only}
- Ding J, Rana N, Dmowski W. Length of progesterone treatment before transfer and implantation rates of frozen-thawed blastocysts. ASRM annual meeting 2007.
El‐Toukhy 2004 {published data only}
- El-Toukhy T, Taylor A, Khalaf Y, Al-Darazi K, Rowell P, Seed P, et al. Pituitary suppression in ultrasound-monitored frozen embryo replacement cycles. A randomised study. Human Reproduction (Oxford, England) 2004;19(4):874-9. [DOI] [PubMed] [Google Scholar]
Escriba 2006 {published data only}
- Escriba MJ, Bellver J, Bosch E, Sanchez M, Pellicer A, Remohi J. Delaying the initiation of progesterone supplementation until the day of fertilization does not compromise cycle outcome in patients receiving donated oocytes: a randomized study. Fertility and Sterility 2006;86(1):92-7. [DOI] [PubMed] [Google Scholar]
Greco 2016 {published data only}
- Greco E, Litwicka K, Arrivi C, Varricchio MT, Caragia A, Greco A, et al. The endometrial preparation for frozen-thawed euploid blastocyst transfer: a prospective randomized trial comparing clinical results from natural modified cycle and exogenous hormone stimulation with GnRH agonist. Journal of Assisted Reproduction and Genetics 2016;33(7):873-84. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Groenewoud 2016 {published data only}
- Groenewoud ER, Cohlen BJ, Al-Oraiby A, Brinkhuis EA, Broekmans FJ, Bruin JP, et al. A randomized controlled, non-inferiority trial of modified natural versus artificial cycle for cryo-thawed embryo transfer. Human Reproduction (Oxford, England) 2016;31(7):1483-92. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gutierrez 1999 {published data only}
- Gutierrez A, Hemandez F, Mendoza S, Monroy E, Perez-Petia E, Gallardo E. Nafarelin acetate vs leuprolide acetate in women with ovarian function undergoing oocyte donation. 55th Annual Meeting of the American Society for Reproductive Medicine 1999.
Kahraman 2018 {published data only}
- Kahraman S Cetinkaya CP Sahin Y Oner G. Transdermal versus oral estrogen: clinical outcomes in patients undergoing frozen-thawed single blastocyst transfer cycles without GnRHa suppression, a prospective randomized clinical trial. Journal of Assisted Reproduction and Genetics 2019;36(3):453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2008 {published data only}
- Lee S, Kwon H, Kim J, Lee J, Jung Y, Jung J, et al. Comparison of clinical outcome of frozen-thawed embryo transfer cycles between natural and artificial (hormone-treated) cycles. 24th Annual Meeting of the ESHRE 2008.
Madani 2019 {published data only}
- Madani T, Ahmadi F, Jahangiri N, Bahmanabadi A, Bagheri Lankarani N. Does low-dose aspirin improve pregnancy rate in women undergoing frozen-thawed embryo transfer cycle? A pilot double-blind, randomized placebo-controlled trial. Journal of Obstetrics and Gynaecology Research 2019;45(1):156-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Matsuura 2014 {published data only}
- Matsuura K, Ikegami M, Nagase Y, Wada T, Matsuura T. Clinical and ongoing pregnancy rates are improved by the addition of letrozole in the hormone replacement cycles undergoing the frozen-thawed single blastocyst transfer. Fertility and Sterility 2014;102(3):e229. [Google Scholar]
Moffitt 1995 {published data only}
- Moffitt D, Queenan JT Jr, Veeck LL, Schoolcraft W, Miller CE, Muasher SJ. Low-dose glucocorticoids after in vitro fertilization and embryo transfer have no significant effect on pregnancy rate. Fertility and Sterility 1995;63(3):571-7. [DOI] [PubMed] [Google Scholar]
Movahedi 2018 {published data only}
- Movahedi S, Aleyasin A, Agahosseini M, Safdarian L, Abroshan S, Khodaverdi S, et al. Endometrial preparation for women undergoing embryo transfer frozen-thawed embryo transfer with and without pretreatment with gonadotropin releasing hormone agonists. Journal of Family and Reproductive Health 2018;12(4):191-6. [PMC free article] [PubMed] [Google Scholar]
Nekoo 2015 {published data only}
- Nekoo EA, Chamani M, Tehrani ES, Rashidi BH, Tanha FD, Kalantari V. Artificial endometrial preparation for frozen-thawed embryo transfer with or without pretreatment with depot gonadotropin releasing hormone agonist in women with regular menses. Journal of Family and Reproductive Health 2015;9(1):1-4. [PMC free article] [PubMed] [Google Scholar]
Ramos 2007 {published data only}
- Ramos J, Caligara C, Tocino A, Rodrıguez I, Carranza F, Fernandez-Sanchez M. Prospective randomized study to compare frozen thawed embryo transfer cycles outcomes in women with functioning ovaries and HRT for endometrium preparation with or without prior GnRH suppression. ASRM Annual Meeting 2007.
Remohi 1994 {published data only}
- Remohi J, Gutierrez A, Vidal A, Tarin JJ, Pellicer A. The use of gonadotrophin-releasing hormone analogues in women receiving oocyte donation does not affect implantation rates. Human Reproduction (Oxford, England) 1994;9(9):1761-4. [DOI] [PubMed] [Google Scholar]
Samsami 2018 {published data only}
- Samsami A, Chitsazi Z, Namazi G. Frozen thawed embryo transfer cycles; a comparison of pregnancy outcomes with and without prior pituitary suppression by GnRH agonists: an RCT. International Journal of Reproductive Biomedicine 2018;16(9):587-94. [PMC free article] [PubMed] [Google Scholar]
Samsami 2019 {published data only}
- Samsami A, Ghasmpour L, Davoodi S, Alamdarloo SM, Rahmati J, Karimian A, et al. Frozen embryo transfer: Endometrial preparation by letrozole versus hormone replacement cycle: A randomized clinical trial. International Journal of Reproductive Biomedicine 2019;17(12):915-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheikhi 2018 {published data only}
- Sheikhi O, Golsorkhtabaramiri M, Esmaeilzadeh S, Mahouti T, Heidari FN. Reproductive outcomes of vitrified blastocyst transfer in modified natural cycle versus mild hormonally stimulated and artificial protocols: a randomized control trial. JBRA Assisted Reproduction 2018;22(3):221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tehraninejad 2018 {published data only}
- Tehraninejad ES, Kabodmehri R, Rashidi BH, Jafarabadi M, Keikha F, Masomi M, et al. Trans dermal estrogen (oestrogel) for endometrial preparation in freeze embryo transfer cycle: an RCT. International Journal of Reproductive Biomedicine 2018;16(1):51. [PMC free article] [PubMed] [Google Scholar]
Tocino 2007 {published data only}
- Tocino A, Caligara C, Ramos J, Carranza F, Gonzalez A, Fernandez-Sanchez M. Prospective randomized study to compare use of daily vs. depot GnRH analogue in endometrial preparation for oocyte donations cycles in recipients. ASRM Annual Meeting 2007.
Vidal 2009 {published data only}
- Vidal C, Giles J, Remoh J, Simn C, Garrido N, Bellver J, et al. The use of GnRH antagonist in endometrial priming improves oocyte donation outcome. Human Reproduction 2009;92(3):S255. [Google Scholar]
Wright 2006 {published data only}
- Wright KP, Guibert J, Weitzen S, Davy C, Fauque P, Olivennes F. Artificial versus stimulated cycles for endometrial preparation prior to frozen-thawed embryo transfer. Reproductive Biomedicine Online 2006;13(3):321-5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arun Muthuvel 2016 {published data only}
- Arun Muthuvel V, Sanjeeva Reddy N. Comparison of aqueous subcutaneous vs vaginal progesterone in frozen embryo transfer (FET) cycles. Fertility and Sterility 2016, 2016;106(3):e71. [Google Scholar]
Bernabeu 2006 {published data only}
- Bernabeu R, Roca M, Torres A, Ten J. Indomethacin effect on implantation rates in oocyte recipients. Human Reproduction (Oxford, England) 2006;21(2):364-9. [DOI] [PubMed] [Google Scholar]
Bjuresten 2011 {published data only}
- Bjuresten K, Landgren BM, Hovatta O, Stavreus-Evers A. Luteal phase progesterone increases live birth rate after frozen embryo transfer. Fertility and Sterility 2011;95(2):534-7. [DOI] [PubMed] [Google Scholar]
Boostanfar 2016 {published data only}
- Boostanfar R, Gates D, Guan Y, Gordon K, McCrary Sisk C, Stegmann BJ. Efficacy and safety of frozen-thawed embryo transfer in women aged 35 to 42 years from the PURSUE randomized clinical trial. Fertility and Sterility 2016;106(2):300-305.e5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Caligara 2003 {published data only}
- Caligara C, Ruiz S, Terrero M, Mantrana E, Calderon G, Navarro J. Vaginal versus intramuscular progesterone in oocyte donation replacement therapy. ASRM Aannual Mmeeting 2003.
Cambiaghi 2013 {published data only}
- Cambiaghi AS, Leao RB, Alvarez AV, Nascimento PF. Intrauterine injection of human chorionic gonadotropin before embryo transfer may improve clinical pregnancy and implantation rates in blastocysts transfers. Fertility and Sterility 2013;100(3):S121. [Google Scholar]
Check 1998 {published data only}
- Check JH, Dietterich C, Lurie D, Nazari A, Chuong J. A matched study to determine whether low-dose aspirin without heparin improves pregnancy rates following frozen embryo transfer and/or affects endometrial sonographic parameters. Journal of Assisted Reproduction and Genetics 1998;15(10):579-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Check 2004 {published data only}
- Check JH, Graziano V, Lee G, Nazari A, Choe JK, Dietterich C. Neither sildenafil nor vaginal estradiol improves endometrial thickness in women with thin endometria after taking oral estradiol in graduating dosages. Clinical & Experimental Obstetrics & Gynecology 2004;31(2):99-102. [0390-6663: (Print)] [PubMed]
Davar 2015 {published data only}
- Davar R, Mojtahedi MF, Miraj S. Effects of single dose GnRH agonist as luteal support on pregnancy outcome in frozen-thawed embryo transfer cycles: an RCT. Iranian Journal of Reproductive Medicine 2015;13(8):483-8. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Davar 2016a {published data only}
- Davar R, Miraj S, Farid Mojtahedi M. Effect of adding human chorionic gonadotropin to frozen thawed embryo transfer cycles with history of thin endometrium. International Journal of Reproductive Biomedicine 2016;14(1):53-6. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Davari‐Tanha 2016 {published data only}
- Davari-Tanha F, Tehraninejad ES, Ghazi M, Shahraki Z. The role of G-CSF in recurrent implantation failure: a randomized double blind placebo control trial. International Journal of Reproductive Biomedicine 2016;14(12):737-42. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Eftekhar 2013 {published data only}
- Eftekhar M, Rahsepar M, Rahmani E. Effect of progesterone supplementation on natural frozen-thawed embryo transfer cycles: a randomized controlled trial. International Journal of Fertility and Sterility 2013;7(1):13-20. [PMC free article] [PubMed] [Google Scholar]
Feliciani 2004 {published data only}
- Feliciani E, Ferraretti A, Balicchia B, Grieco N, Magli M, Gianaroli L. A prospective randomised study comparing the effect of intravaginal progesterone and intramuscular progesterone in frozen/thawed embryo transfer (FET) cycles. 20th Annual Meeting of the ESHRE 2004.
Gibbons 1998 {published data only}
- Gibbons WE, Toner JP, Hamacher P, Kolm P. Experience with a novel vaginal progesterone preparation in a donor oocyte program. Fertility and Sterility 1998;69(1):96-101. [DOI] [PubMed] [Google Scholar]
- Toner JP. Vaginal delivery of progesterone in donor oocyte therapy. Human Reproduction 2000;15(1):166-71. [DOI] [PubMed] [Google Scholar]
Gogce 2015 {published data only}
- Gogce M, Benchaib M, Hadj S, Bordes A, du Menildot P, Lornage J, et al. [Administering GnRH Agonists in the luteal phase of Artificial Cycle Frozen-Thawed Embryo Transfers. A prospective randomized study [Administration d'agonistes de la GnRH (Gonadotrophin Releasing Hormone) en phase luteale des protocoles substitutifs de transferts d'embryons congeles: etude prospective randomisee]. Gynecologie, Obstetrique & Fertilite 2015;43(11):728-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hershko 2016 {published data only}
- Hershko Klement A, Samara N, Weintraub A, Mitri F, Bentov Y, Chang P, et al. Intramuscular versus vaginal progesterone administration in medicated IVF frozen embryo transfer (FET) cycles: a randomised clinical trial. Human Reproduction 2016;31(Supplement 1):40-4. [DOI] [PubMed] [Google Scholar]
Huang 2017 {published data only}
- Huang P, Wei L, Li X. A study of intrauterine infusion of human chorionic gonadotropin (hCG) before frozen-thawed embryo transfer after two or more implantation failures. Gynecological Endocrinology : the official journal of the International Society of Gynecological Endocrinology 2017;33(1):67-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Krasnow 1996 {published data only}
- Krasnow JS, Lessey BA, Naus G, Hall LL, Guzick DS, Berga SL. Comparison of transdermal versus oral estradiol on endometrial receptivity. Fertility and Sterility 1996;65(2):332-6. [DOI] [PubMed] [Google Scholar]
Lan 2008 {published data only}
- Lan VT, Tuan P, Canh L, Tuong H, Howles CM. Comparison of the efficacy and tolerability of two formulations of vaginal progesterone for luteal phase support in frozen embryo transfer cycles. In: ASRM Annual Meeting. 2007.
- Lan VT, Tuan PH, Canh LT, Tuong HM, Howles CM. Progesterone supplementation during cryopreserved embryo transfer cycles: efficacy and convenience of two vaginal formulations. Reproductive Biomedicine Online 2008;17(3):318-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lewin 2001 {published data only}
- Lewin A, Fatum M, Shufaro Y, Simon A, Reubinoff B, Laufer N, Safran A. Artificial endometrial preparation for frozen–thawed embryo transfer using oral oestradiol and a new low-dose vaginal progesterone preparation: Endometrin tablets. 17th Annual meeting of the ESHRE 2001.
Li 2014 {published data only}
- Li SJ, Zhang YJ, Chai XS, Nie MF, Zhou YY, Chen J L, et al. Letrozole ovulation induction: an effective option in endometrial preparation for frozen-thawed embryo transfer. Archives of Gynecology and Obstetrics 2014;289(3):687-93. [DOI] [PubMed] [Google Scholar]
Lightman 1999 {published data only}
- Lightman A, Kol S, Itskovitz-Eldor J. A prospective randomized study comparing intramuscular with intravaginal natural progesterone in programmed thaw cycles. Human Reproduction (Oxford, England) 1999;14(10):2596-9. [DOI] [PubMed] [Google Scholar]
Llacer 2017 {published data only}
- Llacer J, Garcia-Hernandez EM, Moliner B, Luque L, Ten J, Bernabeu R. Subcutaneous progesterone for endometrial preparation in substituted cycles for oocyte donation recipients: a randomized controlled trial. Oxford University Press 2017;32:i58. [Google Scholar]
Moon 2004 {published data only}
- Moon HS, Park SH, Lee JO, Kim KS, Joo BS. Treatment with piroxicam before embryo transfer increases the pregnancy rate after in vitro fertilization and embryo transfer. Fertility and Sterility 2004;82(4):816-20. [DOI] [PubMed] [Google Scholar]
Nardo 2006 {published data only}
- Nardo LG, Sallam HN. Progesterone supplementation to prevent recurrent miscarriage and to reduce implantation failure in assisted reproduction cycles. Reproductive Biomedicine Online 2006;13(1):47-57. [DOI] [PubMed] [Google Scholar]
Neuspiller 1998 {published data only}
- Neuspiller F, Levy M, Remohi J, Ruiz A, Simon C, Pellicer A. The use of long- and short-acting forms of gonadotrophin-releasing hormone analogues in women undergoing oocyte donation. Human Reproduction (Oxford, England) 1998;13(5):1148-51. [DOI] [PubMed] [Google Scholar]
Prapas 2009a {published data only}
- Prapas N, Tavaniotou A, Panagiotidis Y, Prapa S, Kasapi E, Goudakou M, et al. Low-dose human chorionic gonadotropin during the proliferative phase may adversely affect endometrial receptivity in oocyte recipients. Gynecological Endocrinology 2009;25(1):53-9. [DOI] [PubMed] [Google Scholar]
Prapas 2009b {published data only}
Sanchez 2009 {published data only}
- Sanchez Ribas I, Castilln G, Garrigos V, Riqueros M, Florensa M, Ballesteros A. Which is the best moment to start hormonal replacemente therapy in recipient in a synchronised oocyte donation programme? Fertility and Sterility 2009;92(3):S135-6. [Google Scholar]
Sathanandan 1991 {published data only}
- Sathanandan M, Macnamee MC, Rainsbury P, Wick K, Brinsden P, Edwards RG. Replacement of frozen-thawed embryos in artificial and natural cycles: a prospective semi-randomized study. Human Reproduction (Oxford, England) 1991;6(5):685-7. [DOI] [PubMed] [Google Scholar]
Shiotani 2006 {published data only}
- Shiotani M, Goto S, Kokeguchi S, Matsunaga M, Watanabe J, Hashimoto H, et al. Is hCG supplementation beneficial for cryopreserved-thawed embryo transfer in estrogen/progesterone replacement cycles? Human Reproduction 2006;21(Suppl):i82.
Simon 1998 {published data only}
- Simon A, Hurwitz A, Zentner BS, Bdolah Y, Laufer N. Transfer of frozen-thawed embryos in artificially prepared cycles with and without prior gonadotrophin-releasing hormone agonist suppression: a prospective randomized study. Human Reproduction (Oxford, England) 1998;13(1O):2712-7. [DOI] [PubMed] [Google Scholar]
Stadtmauer 2009 {published data only}
- Stadtmauer L, Harrison D D, Boyd J, Bocca S, Oehninger S. Pilot study evaluating a progesterone vaginal ring for luteal-phase replacement in donor oocyte recipients. Fertility & Sterility 2009;92(5):1600-5. [DOI] [PubMed] [Google Scholar]
Taskin 2002 {published data only}
- Taskin O, Akkoyunlu G, Simsek M, Demir R, Onoglu A, Sadik S. Comparing the effects of GnRH-a on endometrial receptivity in patients undergoing ART and prepared frozen embryo transfer cycles. ASRM Annual Meeting 2002.
Tesarik 2003 {published data only}
- Tesarik J, Hazout A, Mendoza C. Luteinizing hormone affects uterine receptivity independently of ovarian function.. Reproductive Biomedicine Online 2003;7(1):59-64. [DOI] [PubMed] [Google Scholar]
Weckstein 1997 {published data only}
- Weckstein LN, Jacobson A, Galen D, Hampton K, Hammel J. Low-dose aspirin for oocyte donation recipients with a thin endometrium: prospective, randomized study. Fertility and Sterility 1997;68(5):927-30. [DOI] [PubMed] [Google Scholar]
Xu 2015 {published data only}
- Xu B, Zhang Q, Hao J, Xu D, Li Y. Two protocols to treat thin endometrium with granulocyte colony-stimulating factor during frozen embryo transfer cycles. Reproductive Biomedicine Online 2015;30(4):349-58. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zegers‐Hochschild 2000 {published data only}
- Zegers-Hochschild F, Balmaceda JP, Fabres C, Alam V, Mackenna A, Fernandez E, et al. Prospective randomized trial to evaluate the efficacy of a vaginal ring releasing progesterone for IVF and oocyte donation. Human Reproduction (Oxford, England) 2000;15(10):2093-7. [DOI] [PubMed] [Google Scholar]
Zolghadri 2014 {published data only}
- Zolghadri J, Haghbin H, Dadras N, Behdin S. Vagifem is superior to vaginal Premarin in induction of endometrial thickness in the frozen-thawed cycle patients with refractory endometria: a randomized clinical trial. Iranian Journal of Reproductive Medicine 2014;12(6):415-20. [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Masrour 2018 {published data only}
- Masrour MJ, Ashtary F. The study of natural versus hormone replacement therapy cycles in frozen embryo transfer in infertile couples on pregnancy outcome: a double blind randomized control trial. Acta Medica Mediterranea, 2018 2018;34(6):1765. [Google Scholar]
Page 2005 {published data only}
- Page K, Guibert J, Weitzen S, Davy C, Fauque P, Olivennes F. A prospective randomized trial evaluating endometrial preparation for implantation of frozen/thawed embryos using an artificial cycle versus a stimulated cycle. ASRM Annual Meeting 2005.
Tur‐Kaspa 2010 {published data only}
- Tur-Kaspa I, Najeemuddin R, Cohen A, Tkachenko N, Fowler M. GnRH antagonist (Cetrotide) instead of agonist to prepare recipients for embryo transfer: a prospective, randomized, controlled trial. Fertility and Sterility 2010;94(4):S2 Abstract no. O-06. [Google Scholar]
Additional references
Abdolmohammadi‐Vahid 2016
- Abdolmohammadi-Vahid Samaneh, Danaii Shahla, Hamdi Kobra, Jadidi-Niaragh Farhad, Ahmadi Majid, Yousefi Mehdi. Novel immunotherapeutic approaches for treatment of infertility. Biomedicine & Pharmacotherapy 2016;84:1449-1459. [DOI] [PubMed] [Google Scholar]
De Geyter 2018
- De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, et al. ART in Europe, 2014: results generated from European registries by ESHRE: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Human Reproduction (Oxford, England) 2018;33(9):1586–601. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird M. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
Devroey 1998
- Devroey P, Pados G. Preparation of endometrium for egg donation. Human Reproduction Update 1998;4(6):856-61. [DOI] [PubMed] [Google Scholar]
Fox 2016
- Fox C, Morin S, Jeong JW, Scott RT Jr, Lessey BA. Local and systemic factors and implantation: what is the evidence? Fertility and Sterility 2016;105(4):873-84. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia‐Velasco 2000
- Garcia-Velasco JA, Martinez-Salazar FJ, Grimalt L. Implantation failure [Falla de implantacion]. Reproducción asistida del siglo XXI, Cuaderno de Medicina Reproductiva 2000;6(2):211-30. [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- Hamilton (ON): GRADE Working Group, McMaster University, GRADEpro GDT. GRADE Working Group. Hamilton (ON): GRADE Working Group, McMaster University,, 2015.
Higgins 2011
- Higgins JP, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from handbook.cochrane.org 2011.
Kuo 1997
- Kuo HC, Hsu CC, Wang ST, Huang KE. Aspirin improves uterine blood flow in the peri-implantation period.. J Formos Med Assoc. 1997;96(4):253-7. [PubMed] [Google Scholar]
LA Register 2015
- Red Latinoamericana de Reproducción Asistida. Latin American Register of Assisted Reproduction [Registro Latinoamericano de Reproduccion Asistida]. http://www.redlara.com/PDF_RED/Paper-RLA-2015.pdf (accessed 17 July 2019).
Leeton 1986
- Leeton J, Chan LK, Trounson A, Harman J. Pregnancy established in an infertile patient after transfer of an embryo fertilized in vitro where the oocyte was donated by the sister of the recipient. Journal of In Vitro Fertilization and Embryo Transfer: IVF 1986;3(6):379-82. [0740-7769 (Print)] [DOI] [PubMed] [Google Scholar]
Malinova 2013
- Malinova M, Abouyta T, Krasteva M. The effect of vaginal sildenafil citrate on uterine blood flow and endometrium in the infertile women. Akusherstvo i ginekologiia 2013;52 Suppl 1:26‐30. [PubMed] [Google Scholar]
Mohr 1985
- Mohr LR, Trounson A, Freemann L. Deep-freezing and transfer of human embryos. Journal of In Vitro Fertilization and Embryo Transfer: IVF 1985;2(1):1-10. [DOI] [PubMed] [Google Scholar]
Nawroth 2005
- Nawroth F, Ludwig M. What is the 'ideal' duration of progesterone supplementation before the transfer of cryopreserved-thawed embryos in estrogen/progesterone replacement protocols? Human Reproduction (Oxford, England) 2005;20(5):1127-34. [DOI] [PubMed] [Google Scholar]
Nice 2017
- National Institute for Health and Care Excellence. Fertility problems: assessment and treatment. www.nice.org.uk/guidance/cg156. [PubMed]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
SART 2016
- National Center for Chronic Disease Prevention and Health Promotion of the Centers for Disease Control and Prevention Division of Reproductive Health. 2016 Assisted Reproductive Technology Fertility Clinic Success Rates Report. ftp://ftp.cdc.gov/pub/Publications/art/ART-2016-Clinic-Report-Full.pdf (accessed 17 July 2019).
Smarr 2017
- Smarr MM, Sapra KJ, Gemmill A, Kahn LG, Wise LA, Lynch CD, et al. Is human fecundity changing? A discussion of research and data gaps precluding us from having an answer. Human Reproduction (Oxford, England) 2017;32(3):499-504. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Steiner 2006
- Steiner AZ, Paulson RJ. Oocyte donation. Clinical Obstetrics and Gynecology 2006;49(1):44-54. [DOI] [PubMed] [Google Scholar]
te Velde 2000
- te Velde ER, Eijkemans R, Habbema HD. Variation in couple fecundity and time to pregnancy, an essential concept in human reproduction. Lancet 2000;355(9219):1928-9. [DOI] [PubMed] [Google Scholar]
Zegers‐Hochschild 2006
- Zegers-Hochschild F, Nygren KG, Adamson GD, Mouzon J, Lancaster P, Mansour R, et al. The International Committee Monitoring Assisted Reproductive Technologies (ICMART) glossary on ART terminology. Fertility and Sterility 2006;86(1):16-9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Glujovsky 2007
- Glujovsky D, Pesce R, Fiszbajn G, Sueldo C, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No: CD006359. [DOI: 10.1002/14651858.CD006359] [DOI] [PubMed] [Google Scholar]
Glujovsky 2010
- Glujovsky D, Pesce R, Fiszbajn G, Sueldo C, Hart R J, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD006359. [DOI: 10.1002/14651858.CD006359.pub2] [DOI] [PubMed] [Google Scholar]


