Notes
Editorial note
An error was noted about the reason for exclusion of the study Sauerbruch 2015. Previously, it was erroneously indicated that the reason for exclusion was related to the population. The correct reason for exclusion is related to the comparison. Specifically, drug therapy with or without variceal band ligation guided by hepatic venous pressure gradient (HVPG) was not an intervention of interest for this review. The error is corrected in the review and table of excluded studies.
Abstract
Background
Approximately 40% to 95% of people with cirrhosis have oesophageal varices. About 15% to 20% of oesophageal varices bleed in about one to three years of diagnosis. Several different treatments are available, which include endoscopic sclerotherapy, variceal band ligation, beta‐blockers, transjugular intrahepatic portosystemic shunt (TIPS), and surgical portocaval shunts, among others. However, there is uncertainty surrounding their individual and relative benefits and harms.
Objectives
To compare the benefits and harms of different initial treatments for secondary prevention of variceal bleeding in adults with previous oesophageal variceal bleeding due to decompensated liver cirrhosis through a network meta‐analysis and to generate rankings of the different treatments for secondary prevention according to their safety and efficacy.
Search methods
We searched CENTRAL, MEDLINE, Embase, Science Citation Index Expanded, World Health Organization International Clinical Trials Registry Platform, and trials registers until December 2019 to identify randomised clinical trials in people with cirrhosis and a previous history of bleeding from oesophageal varices.
Selection criteria
We included only randomised clinical trials (irrespective of language, blinding, or status) in adults with cirrhosis and previous history of bleeding from oesophageal varices. We excluded randomised clinical trials in which participants had no previous history of bleeding from oesophageal varices, previous history of bleeding only from gastric varices, those who failed previous treatment (refractory bleeding), those who had acute bleeding at the time of treatment, and those who had previously undergone liver transplantation.
Data collection and analysis
We performed a network meta‐analysis with OpenBUGS using Bayesian methods and calculated the differences in treatments using hazard ratios (HR), odds ratios (OR) and rate ratios with 95% credible intervals (CrI) based on an available‐case analysis, according to National Institute of Health and Care Excellence Decision Support Unit guidance.
Main results
We included a total of 48 randomised clinical trials (3526 participants) in the review. Forty‐six trials (3442 participants) were included in one or more comparisons. The trials that provided the information included people with cirrhosis due to varied aetiologies. The follow‐up ranged from two months to 61 months. All the trials were at high risk of bias. A total of 12 interventions were compared in these trials (sclerotherapy, beta‐blockers, variceal band ligation, beta‐blockers plus sclerotherapy, no active intervention, TIPS (transjugular intrahepatic portosystemic shunt), beta‐blockers plus nitrates, portocaval shunt, sclerotherapy plus variceal band ligation, beta‐blockers plus nitrates plus variceal band ligation, beta‐blockers plus variceal band ligation, sclerotherapy plus nitrates).
Overall, 22.5% of the trial participants who received the reference treatment (chosen because this was the commonest treatment compared in the trials) of sclerotherapy died during the follow‐up period ranging from two months to 61 months. There was considerable uncertainty in the effects of interventions on mortality. Accordingly, none of the interventions showed superiority over another. None of the trials reported health‐related quality of life. Based on low‐certainty evidence, variceal band ligation may result in fewer serious adverse events (number of people) than sclerotherapy (OR 0.19; 95% CrI 0.06 to 0.54; 1 trial; 100 participants).
Based on low or very low‐certainty evidence, the adverse events (number of participants) and adverse events (number of events) may be different across many comparisons; however, these differences are due to very small trials at high risk of bias showing large differences in some comparisons leading to many differences despite absence of direct evidence.
Based on low‐certainty evidence, TIPS may result in large decrease in symptomatic rebleed than variceal band ligation (HR 0.12; 95% CrI 0.03 to 0.41; 1 trial; 58 participants). Based on moderate‐certainty evidence, any variceal rebleed was probably lower in sclerotherapy than in no active intervention (HR 0.62; 95% CrI 0.35 to 0.99, direct comparison HR 0.66; 95% CrI 0.11 to 3.13; 3 trials; 296 participants), beta‐blockers plus sclerotherapy than sclerotherapy alone (HR 0.60; 95% CrI 0.37 to 0.95; direct comparison HR 0.50; 95% CrI 0.07 to 2.96; 4 trials; 231 participants); TIPS than sclerotherapy (HR 0.18; 95% CrI 0.08 to 0.38; direct comparison HR 0.22; 95% CrI 0.01 to 7.51; 2 trials; 109 participants), and in portocaval shunt than sclerotherapy (HR 0.21; 95% CrI 0.05 to 0.77; no direct comparison) groups.
Based on low‐certainty evidence, beta‐blockers alone and TIPS might result in more, other compensation, events than sclerotherapy (rate ratio 2.37; 95% CrI 1.35 to 4.67; 1 trial; 65 participants and rate ratio 2.30; 95% CrI 1.20 to 4.65; 2 trials; 109 participants; low‐certainty evidence).
The evidence indicates considerable uncertainty about the effect of the interventions including those related to beta‐blockers plus variceal band ligation in the remaining comparisons.
Authors' conclusions
The evidence indicates considerable uncertainty about the effect of the interventions on mortality. Variceal band ligation might result in fewer serious adverse events than sclerotherapy. TIPS might result in a large decrease in symptomatic rebleed than variceal band ligation. Sclerotherapy probably results in fewer 'any' variceal rebleeding than no active intervention. Beta‐blockers plus sclerotherapy and TIPS probably result in fewer 'any' variceal rebleeding than sclerotherapy. Beta‐blockers alone and TIPS might result in more other compensation events than sclerotherapy. The evidence indicates considerable uncertainty about the effect of the interventions in the remaining comparisons. Accordingly, high‐quality randomised comparative clinical trials are needed.
Plain language summary
Prevention of rebleeding from enlarged veins in the food pipe (oesophagus) resulting from advanced liver disease
What is the aim of this Cochrane Review? To find out the best available preventive treatment for repeated bleeding from oesophageal varices (enlarged veins in the food pipe) in people with advanced liver disease (liver cirrhosis, or late‐stage scarring of the liver with complications). People with cirrhosis who had previously bled from oesophageal varices are at significant risk of death from another episode of bleeding. Therefore, it is important to provide preventive treatment to prevent rebleeding in such people, but the benefits and harms of different treatments available are currently unclear. The authors of this review collected and analysed all relevant randomised clinical trials with the aim of finding out the best treatment. They found 48 randomised clinical trials (studies where participants are randomly assigned to one of two treatment groups). During analysis of data, authors used standard Cochrane methods, which allow comparison of only two treatments at a time. Authors also used advanced techniques that allow comparison of multiple treatments at the same time (usually referred as 'network (or indirect) meta‐analysis').
Date of literature search December 2019
Key messages None of the studies were conducted without flaws, and because of this, there is moderate to very high uncertainty in the findings of this review. Approximately one in five trial participants with cirrhosis who received preventive treatment after control of initial bleeding from oesophageal varices died within five years of treatment with sclerotherapy.
What was studied in the review? This review looked at adults of any sex, age, and ethnic origin, with advanced liver disease due to various causes and previous bleeding from oesophageal varices. Participants were given different treatments for preventing further bleeding oesophageal varices. The authors excluded studies in people who had bleeding from the stomach, who had no previous bleeding from the oesophageal varices, those who failed to respond to another treatment before study entry, and those who had liver transplantation previously. The average age of participants, when reported, ranged from 40 to 63 years. The treatments used in the trials included endoscopic sclerotherapy (injecting into the enlarged veins by looking through a tube inserted through the mouth), variceal band ligation (inserting bands around the dilated veins by seeing through a tube inserted through the mouth), beta‐blockers (drugs that slow the heart and decrease the force of heart pumping resulting in decrease pressure in the blood vessels), and TIPS (transjugular intrahepatic portosystemic shunt; an artificial channel that connects the different blood vessels that carry oxygen‐depleted blood (venous system)) within the liver to reduce the pressure built‐up in the portal venous system, one of the two venous systems draining the liver), portocaval shunt (performing surgery to create the artificial channel described for TIPS) among others. The review authors wanted to gather and analyse data on death, quality of life, serious and non‐serious adverse events, recurrence of bleeding, and development of other complications of advanced liver disease. What were the main results of the review? The 48 studies included a small number of participants (3526 participants). Study data were sparse. Forty‐six studies with 3442 participants provided data for analyses. The follow‐up of the trial participants ranged from two months to five years.
The funding source for the research was unclear in 36 studies; commercial organisations funded five studies. There were no concerns regarding the source of funding for the remaining nine studies.
The review shows the following. ‐ The evidence indicates considerable uncertainty about the effect of the interventions on the risk of death ‐ Variceal band ligation might result in fewer serious adverse events than sclerotherapy ‐ The evidence indicates considerable uncertainty about the effect of the interventions on serious and non‐serious adverse events ‐ Sclerotherapy probably results in decrease in further bleeding than no treatment ‐ Beta‐blockers plus sclerotherapy and TIPS probably result in a decrease in further bleeding than sclerotherapy alone ‐ Portocaval shunt may result in a decrease in further bleeding than sclerotherapy ‐ The evidence indicates considerable uncertainty about the effect of the interventions in the remaining comparisons ‐ None of the trials reported health‐related quality of life ‐ Future well‐designed trials are needed to find out the best treatment for people with cirrhosis and previous bleeding from oesophageal varices.
Summary of findings
Background
Description of the condition
Liver cirrhosis
The liver is a complex organ with multiple functions including carbohydrate metabolism, fat metabolism, protein metabolism, drug metabolism, synthetic functions, storage functions, digestive functions, excretory functions, and immunological functions (Read 1972). Liver cirrhosis is a liver disease in which the normal microcirculation, the gross vascular anatomy, and the hepatic architecture have been variably destroyed and altered with fibrous septa surrounding regenerated or regenerating parenchymal nodules (Tsochatzis 2014; NCBI 2018a). The major causes of liver cirrhosis include excessive alcohol consumption, viral hepatitis, non‐alcohol related fatty liver disease, autoimmune liver disease, and metabolic liver disease (Williams 2014; Ratib 2015; Setiawan 2016). The global prevalence of liver cirrhosis is difficult to estimate as most estimates correspond to chronic liver disease (which includes liver fibrosis and liver cirrhosis). In studies from the USA, the prevalence of chronic liver disease varies between 0.3% to 2.1% (Scaglione 2015; Setiawan 2016); in the UK, the prevalence was 0.1% in one study (Fleming 2008). In 2010, liver cirrhosis was responsible for an estimated 2% of all global deaths, equivalent to one million deaths (Mokdad 2014). There is an increasing trend of cirrhosis‐related deaths in some countries such as the UK, while there is a decreasing trend in other countries such as France (Mokdad 2014; Williams 2014). The major cause of complications and deaths in people with liver cirrhosis is due to the development of clinically significant portal hypertension (hepatic venous pressure gradient at least 10 mmHg) (de Franchis 2015). Some of the clinical features of decompensation include jaundice, coagulopathy, ascites, variceal bleeding, hepatic encephalopathy, and renal failure (de Franchis 2015; McPherson 2016; EASL 2018). Decompensated cirrhosis is the most common indication for liver transplantation (Merion 2010; Adam 2012).
Oesophageal varices
Oesophageal varices are dilated blood vessels in the oesophagus, usually due to portal hypertension (NCBI 2018b). Presence of oesophageal varices is a feature of clinically significant portal hypertension. The prevalence of oesophageal varices varies between 40% and 95% in people with cirrhosis (Chawla 2012; McCarty 2017). The annual incidence of oesophageal varices in people with cirrhosis varies from 3% to 22% (Cales 1990; Merli 2003; D'Amico 2014).
There are many classification systems available for assessing the risk of bleeding from oesophageal varices. The classification system that is followed from a management perspective is the Baveno I consensus definition which classifies oesophageal varices as small and large (de Franchis 1992). The criteria for distinction between small and large oesophageal varices is variable (de Franchis 1992). The current UK guidelines and European Association for the Study of the Liver (EASL) guidelines on the management of variceal bleeding acknowledges this variability and suggests that small varices tend to be narrow and flatten easily with air during endoscopy as compared to medium/large varices which are usually broader and flatten with difficulty, or do not flatten at all (Tripathi 2015; EASL 2018). Other definitions for small oesophageal varices include less than 5 mm in size and less than 25% of oesophageal lumen (Abby Philips 2016). Other risk factors for bleeding from oesophageal varices include the pressure in the varices (hepatic venous pressure gradient greater than 12 mmHg), increased tension on the variceal wall as indicated by red spots or red wale markings (longitudinal red streaks on the varices) on endoscopy, and severity of the liver disease (Beppu 1981; NIEC 1988; de Franchis 2015; Tripathi 2015). Approximately 15% to 20% of people with oesophageal varices bleed in about one to three years (Gluud 2012; Qi 2015). The short‐term mortality of an episode of acute variceal bleeding is about 15% to 30% (Ioannou 2003; Gøtzsche 2008; D'Amico 2010; Rios 2015). Of those who survive, approximately 30% die in two years and approximately 20% have another episode of bleeding over two years (Qi 2016). In France, the mean in‐hospital costs of treating acute episode of bleeding was EURO 13,500 in 2007 (Thabut 2007); in the USA, the mean six‐month costs of treating people with variceal bleeding was USD 16,500 in 2000 (Zaman 2000).
Pathophysiology of oesophageal varices
In addition to causing arterial vasodilation of the splanchnic circulation (dilation of the blood vessels supplying the digestive organs in the abdomen such as the liver, pancreas, and intestines) (Gines 2009; Moore 2013), portal hypertension causes dilation of the collaterals between the portal venous system and systemic venous system (Sass 2009). One of the major locations of these collaterals is the lower end of the oesophagus and proximal part of the stomach. Therefore, portal hypertension leads to oesophageal varices (Sass 2009). According to Frank's modification of the Laplace law, the tension on the walls of blood vessels are dependent upon the diameter of the blood vessel and the pressure gradient across the walls (i.e. the difference in pressure inside the varices and the oesophageal pressure) (Herman 2015). Since both the diameter of the vessels and the pressure at which the blood flows in the varices are increased due to portal hypertension, the tension on the wall increases leading to dilation of the blood vessels at the lower end of the oesophagus and proximal part of the stomach, which in turn increases the tension further (Herman 2015). This vicious circle can eventually culminate in rupture of the varices (Sass 2009; Herman 2015).
Description of the intervention
Secondary prevention of bleeding refers to preventing re‐bleeding once the initial variceal bleed has been stopped. The various treatments include non‐cardioselective beta‐blockers such as propranolol, endoscopic variceal band ligation, sclerotherapy, nitrates, transjugular intrahepatic portosystemic shunt (TIPS), and surgical portosystemic shunt (de Franchis 2015; Tripathi 2015; Qi 2016; Garcia‐Tsao 2017; EASL 2018). Of these, the UK guidelines, the EASL guidelines, and the American Association for the Study of Liver Diseases (AASLD) guidelines indicate that non‐cardioselective beta‐blockers in combination with endoscopic band ligation should be considered as the first‐line treatment to prevent rebleeding in people with a history of variceal bleeding (de Franchis 2015; Tripathi 2015; Garcia‐Tsao 2017; EASL 2018). TIPS is considered a second‐line treatment in people who rebleed despite having received secondary prevention treatment with beta‐blockers plus endoscopic band ligation (de Franchis 2015; Tripathi 2015; Garcia‐Tsao 2017); surgical portosystemic shunt is an alternative treatment in people who are not eligible for TIPS (Tripathi 2015).
How the intervention might work
Non‐cardioselective beta‐blockers work by causing splanchnic vasoconstriction and decreasing the cardiac output, leading to decreased portal pressure and decreased flow in the collaterals, which in turn decreases the pressure inside the oesophageal varices (Tripathi 2015). TIPS and surgical portosystemic shunt are aimed at diverting blood flow from the portal system to the systemic circulation, thereby decreasing the portal pressure and reducing the pressure inside the oesophageal varices. Endoscopic variceal band ligation and sclerotherapy are local treatments aimed at obliteration of the oesophageal varices by reducing the blood flow in the oesophageal varices. Nitrates attempt to decrease the variceal pressure by vasodilation and decreased portal pressure (Tripathi 2015).
Why it is important to do this review
Considering the high mortality associated with variceal bleeding, it is important to provide optimal evidence‐based treatment to prevent rebleeding in people with a history of variceal bleeding and also improve their survival. Several different treatments are available; however, their relative efficacy and optimal combination are not known. There has been one Cochrane Review on portosystemic shunts versus endoscopic therapy for variceal rebleeding in people with a history of variceal bleeding due to cirrhosis (Brand 2018). There have been no previous network meta‐analyses on the topic. Network meta‐analysis allows for a combination of direct and indirect evidence and ranking of different interventions for different outcomes (Salanti 2011; Salanti 2012). It also allows calculation of effect estimates when no direct evidence of relative effectiveness exists and allows inclusion of all relevant interventions in the population in a single analysis allowing the relative ranking of these interventions. With this systematic review and network meta‐analysis, we aim to provide the best level of evidence for the benefits and harms of different treatments for the prevention of bleeding in people with oesophageal varices due to liver cirrhosis. We have also presented results from direct comparisons whenever possible, as well as performing the network meta‐analysis.
Objectives
To compare the benefits and harms of different initial treatments for secondary prevention of variceal bleeding in adults with previous oesophageal variceal bleed due to decompensated liver cirrhosis through a network meta‐analysis and to generate rankings of the different treatments for secondary prevention according to their safety and efficacy.
Methods
Criteria for considering studies for this review
Types of studies
We considered only randomised clinical trials (including cross‐over and cluster‐randomised clinical trials) for this network meta‐analysis irrespective of language, publication status, or date of publication. We excluded studies of other designs because of the risk of bias in such studies. Inclusion of indirect observational evidence could weaken our network meta‐analysis, but this could also be viewed as a strength for assessing rare adverse events. It is well‐established that exclusion of non‐randomised studies increases the focus on potential benefits and reduces the focus on the risks of serious adverse events and those of any adverse events. However, we did not include these studies because of the findings of this review, i.e. the treatment decision should be driven by effects on mortality and other features of decompensation rather than treatment‐related adverse events.
Types of participants
We included randomised clinical trials with adults with a history of oesophageal varices due to decompensated liver cirrhosis undergoing treatment for the prevention of rebleeding. We included trials in which people with oesophageal varices also had gastric varices, but we did not include trials in which the treatment was targeted at the gastric varices rather than oesophageal varices (as the pathophysiology and treatment for gastric only varices is different from oesophageal varices). We excluded randomised clinical trials in which participants had no previous history of bleeding or had an ongoing episode of variceal bleeding (considered in other reviews). We also excluded trials in which the participants had previously undergone liver transplantation (as the treatments used may be different in such patients compared to those who did not undergo liver transplantation). We also excluded participants who were refractory to secondary prevention treatments (as the treatments used as second line are different from those used for first line). We also excluded trials which included some participants who were eligible for this review and others who were not eligible for this review, unless separate data were available for the trial participants who were eligible for this review.
Types of interventions
We included any of the following interventions for comparison with one another, either alone or in combination:
non‐cardioselective beta‐blockers such as propranolol, carvedilol, and nadolol;
endoscopic variceal band ligation;
endoscopic variceal sclerotherapy;
nitrates;
TIPS procedure;
other forms of portosystemic shunt;
no active intervention (no intervention or placebo).
We considered 'sclerotherapy' as the reference group. Each of the above categories was considered as a 'treatment node'. We considered variations in endoscopic interventions or drugs within the same class, doses of drugs, frequency and duration of interventions as the same treatment node; therefore, we did not include trials comparing variations within treatment. We treated each different combination of the categories as different treatment nodes. All the above interventions were considered 'decision set', i.e. all the above interventions were of direct interest.
While we identified some additional interventions that are not listed above, we did not add these interventions to the list because they are no longer in use as initial treatment (first‐line therapy) of secondary prevention of bleeding from oesophageal varices.
We evaluated the plausibility of the network meta‐analysis transitivity assumption by looking at the inclusion and exclusion criteria in the studies. The transitivity assumption means that participants included in the different trials with different treatments (in this case, for secondary prevention of oesophageal variceal bleeding) can be considered to be a part of a multi‐arm randomised clinical trial and could potentially have been randomised to any of the interventions (Salanti 2012). In other words, any participant that meets the inclusion criteria is, in principle, equally likely to be randomised to any of the above eligible interventions or that potential effect‐modifiers are not systematically different across comparisons. This necessitates that information on potential effect‐modifiers such as presence or absence of other features of decompensation such as ascites are similar across comparisons.
Types of outcome measures
Primary outcomes
All‐cause mortality at maximal follow‐up (time‐to‐death).
Health‐related quality of life using a validated scale such as the EQ‐5D or 36‐Item Short Form Health Survey (SF‐36) (EuroQol 2018; Optum 2018), at maximal follow‐up.
-
Serious adverse events (during or within six months after cessation of intervention). We defined a serious adverse event as any event that would increase mortality; is life‐threatening; requires hospitalisation; results in persistent or significant disability; is a congenital anomaly/birth defect; or any important medical event that might jeopardise the person or require intervention to prevent it (ICH‐GCP 1997). However, none of the trial authors defined serious adverse events. Therefore, we used the list provided by trial authors for serious adverse events (as indicated in the protocol).
Proportion of people with one or more serious adverse events.
Number of serious adverse events per participant.
Secondary outcomes
-
Any adverse events (during or within six months after cessation of intervention). We defined an adverse event as any untoward medical occurrence not necessarily having a causal relationship with the intervention but resulting in a dose reduction or discontinuation of intervention (any time after commencement of intervention) (ICH‐GCP 1997). However, none of the trial authors defined 'adverse event'. Therefore, we used the list provided by trial authors for adverse events (as indicated in the protocol).
Proportion of people with one or more adverse events.
Number of any adverse events per participant.
Liver transplantation (time‐to‐liver transplantation at maximal follow‐up).
-
Variceal rebleeding (time‐to‐oesophageal variceal bleeding however defined by authors at maximal follow‐up).
Symptomatic variceal rebleeding (e.g. shortness of breath, shock, requiring blood transfusion).
Any variceal bleeding.
Time‐to‐other features of decompensation (maximal follow‐up).
Exploratory outcomes
Length of hospital stay (all hospital admissions until maximal follow‐up).
Number of days of lost work (in people who work) (maximal follow‐up).
Treatment costs (including the cost of the treatment and any resulting complications).
We chose the outcomes based on their importance to patients in a survey related to research priorities for people with liver diseases (Gurusamy 2019), based on feedback of the patient and public representative of this project, and based on an online survey about the outcomes promoted through Cochrane Consumer Network. Of these, the primary outcomes were considered critical outcomes, the secondary outcomes were considered important outcomes, and the exploratory outcomes were considered unimportant outcomes. We have presented the primary and secondary outcomes in the 'Summary of findings' tables.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, MEDLINE Ovid, Embase Ovid, and Science Citation Index Expanded (Web of Science) from inception to date of search for randomised clinical trials comparing two or more of the above interventions without applying any language restrictions (Royle 2003). We searched for all possible comparisons formed by the interventions of interest. To identify further ongoing or completed trials, we also searched clinicaltrials.gov, and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/) which searches various trial registers, including ISRCTN and ClinicalTrials.gov. We also searched the European Medical Agency (EMA) (www.ema.europa.eu/ema/) and USA Food and Drug Administration (FDA) (www.fda.gov) registries for randomised clinical trials. We provided the search strategies along with the date of search in Appendix 1.
Searching other resources
We searched the references of the identified trials and the existing Cochrane Review on secondary prevention of variceal bleeding in people with oesophageal varices due to liver cirrhosis (Brand 2018) to identify additional trials for inclusion.
Data collection and analysis
Selection of studies
Two review authors (KG and DRo or MC) independently identified trials for inclusion by screening the titles and abstracts of articles identified by the literature search, and sought full‐text articles of any references identified by at least one review author for potential inclusion. We selected trials for inclusion based on the full‐text articles. We listed the references that we excluded and the reasons for their exclusion in the Characteristics of excluded studies table. We also listed any ongoing trials identified primarily through the search of the clinical trial registers for further follow‐up. We resolved any discrepancies through discussion. We illustrated the study selection process in a PRISMA diagram (Figure 2).
2.
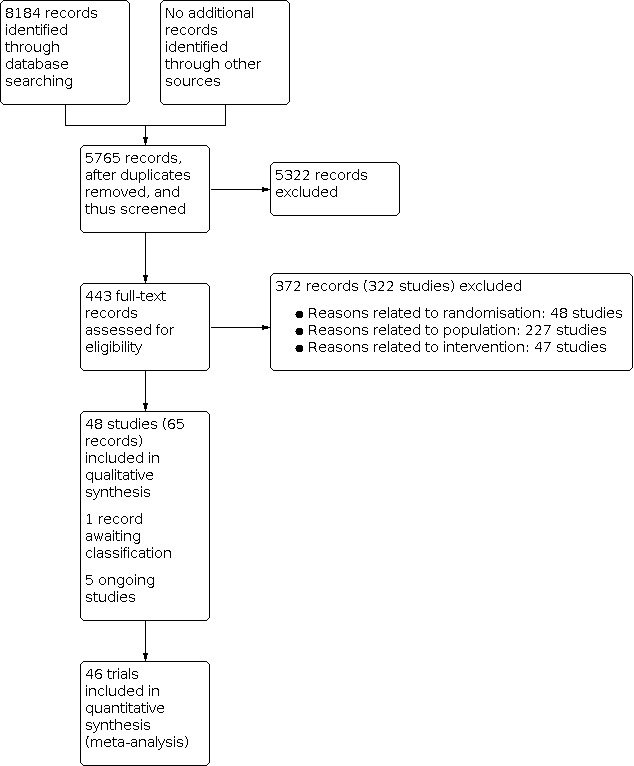
Study flow diagram Date of search 17 December 2019
Data extraction and management
Two review authors (KG, MPT, IP, AB, DRa, NW, LB, SA, TB, MC, DF) independently extracted the data below in a prepiloted Microsoft Excel‐based data extraction form (after translation of non‐English articles).
-
Outcome data (for each outcome and for each intervention group whenever applicable):
number of participants randomised;
number of participants included for the analysis;
number of participants with events for binary outcomes, mean and standard deviation for continuous outcomes, number of events and the mean follow‐up period for count outcomes, and number of participants with events and the mean follow‐up period for time‐to‐event outcomes;
natural logarithm of hazard ratio and its standard error if this was reported rather than the number of participants with events and the mean follow‐up period for time‐to‐event outcomes;
definition of outcomes or scale used if appropriate.
-
Data on potential effect modifiers:
participant characteristics such as age, sex, presence of other features of decompensation such as ascites, the aetiology for cirrhosis, and the interval between diagnosis of variceal bleeding and prophylactic treatment;
details of the intervention and control (including dose, frequency, and duration);
length of follow‐up;
information related to 'Risk of bias' assessment (see below).
-
Other data:
year and language of publication;
country in which the participants were recruited;
year(s) in which the trial was conducted;
inclusion and exclusion criteria.
We collected data at maximum follow‐up but also at short term (up to three months), and medium term (from three months to five years), if these were available.
We attempted to contact the trial authors in the case of unclear or missing information. We resolved any differences in opinion through discussion.
Assessment of risk of bias in included studies
We followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions to assess the risk of bias in included trials (Higgins 2011). Specifically, we assessed sources of bias as defined below (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Savović 2012a; Savović 2012b; Savović 2018).
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if performed by an independent person not otherwise involved in the trial.
Unclear risk of bias: the method of sequence generation was not specified.
High risk of bias: the sequence generation method was not random or only quasi‐randomised.
Allocation concealment
Low risk of bias: the allocation sequence was described as unknown to the investigators. Hence, the participants' allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit, an onsite locked computer, identical‐looking numbered sealed opaque envelopes, drug bottles or containers prepared by an independent pharmacist, or an independent investigator.
Unclear risk of bias: it was unclear if the allocation was hidden or if the block size was relatively small and fixed so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: the allocation sequence was likely to be known to the investigators who assigned the participants.
Blinding of participants and personnel
Low risk of bias: blinding of participants and key study personnel ensured, and it was unlikely that the blinding could have been broken; or rarely no blinding or incomplete blinding, but the review authors judged that the outcome was not likely to be influenced by lack of blinding.
Unclear risk of bias: any of the following: insufficient information to permit judgement of 'low risk' or 'high risk'; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding or incomplete blinding, and the outcome was likely to be influenced by lack of blinding; or blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome was likely to be influenced by lack of blinding.
Blinded outcome assessment
Low risk of bias: blinding of outcome assessment ensured, and unlikely that the blinding could have been broken; or rarely no blinding of outcome assessment, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding.
Unclear risk of bias: any of the following: insufficient information to permit judgement of 'low risk' or 'high risk'; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding of outcome assessment, and the outcome measurement was likely to be influenced by lack of blinding; or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement was likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The study used sufficient methods, such as multiple imputation, to handle missing data.
Unclear risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: the trial reported the following predefined outcomes: all‐cause mortality, adverse events, and variceal rebleeding. If the original trial protocol was available, the outcomes should have been those called for in that protocol. If we obtained the trial protocol from a trial registry (e.g. ClinicalTrials.gov), the outcomes sought should have been those enumerated in the original protocol if the trial protocol was registered before or at the time that the trial was begun. If the trial protocol was registered after the trial was begun, we did not consider those outcomes to be reliable.
Unclear risk of bias: not all predefined, or clinically relevant and reasonably expected, outcomes were reported fully, or it was unclear whether data on these outcomes were recorded or not.
High risk of bias: one or more predefined or clinically relevant and reasonably expected outcomes were not reported, despite the fact that data on these outcomes should have been available and even recorded.
Other bias
Low risk of bias: the trial appeared to be free of other components that could put it at risk of bias (e.g. inappropriate control or dose or administration of control, baseline differences, early stopping).
Uncertain risk of bias: the trial may or may not have been free of other components that could put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias (e.g. baseline differences, early stopping).
We considered a trial to be at low risk of bias if we assessed the trial to be at low risk of bias across all listed bias risk domains. Otherwise, we considered the trial to be at high risk of bias. At the outcome level, we classified an outcome to be at low risk of bias if the allocation sequence generation, allocation concealment, blinding of participants, healthcare professionals, and outcome assessors, incomplete outcome data, and selective outcome reporting (at the outcome level) were at low risk of bias for objective and subjective outcomes (Savović 2018).
Measures of treatment effect
Relative treatment effects
For dichotomous variables (e.g. proportion of participants with serious adverse events or any adverse events), we calculated the odds ratio (OR) with 95% credible interval (CrI) (or Bayesian confidence interval) (Severini 1993). For continuous variables (e.g. health‐related quality of life reported on the same scale), we calculated the mean difference (MD) with 95% Crl. We planned to use standardised mean difference (SMD) values with 95% Crl for health‐related quality of life if included trials used different scales. If we calculated the SMD, we planned to convert it to a common scale, for example, EQ‐5D or SF‐36 (using the standard deviation of the common scale) for the purpose of interpretation. For count outcomes (e.g. number of serious adverse events or number of any adverse events), we calculated the rate ratio (RaR) with 95% Crl. This assumes that the events are independent of each other, i.e. if a person has had an event, they are not at an increased risk of further outcomes, which is the assumption in Poisson likelihood. For time‐to‐event data (e.g. all‐cause mortality at maximal follow‐up), we calculated hazard ratios (HRs) with 95% Crl.
Relative ranking
We estimated the ranking probabilities for all interventions of being at each possible rank for each intervention for each outcome when network meta‐analysis was performed. We obtained the surface under the cumulative ranking curve (SUCRA) (cumulative probability), rankogram, and relative ranking table with CrI for the ranking probabilities for each outcome when network meta‐analysis was performed (Salanti 2011; Chaimani 2013).
Unit of analysis issues
The unit of analysis was the participant with a history of oesophageal variceal bleeding according to the intervention group to which the participant was randomly assigned.
Cluster‐randomised clinical trials
If we had identified any cluster‐randomised clinical trials, we planned to include cluster‐randomised clinical trials, provided that the effect estimate adjusted for cluster correlation was available, or if there was sufficient information available to calculate the design effect (which would allow us to take clustering into account). We also planned to assess additional domains of risk of bias for cluster‐randomised trials according to guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Cross‐over randomised clinical trials
If we identified any cross‐over randomised clinical trials, we planned to include only the outcomes after the period of the first intervention because the included treatments could have residual effects.
Trials with multiple intervention groups
We collected data for all trial intervention groups that met the inclusion criteria. The codes that we used for analysis accounted for the correlation between the effect sizes from studies with more than two groups.
Dealing with missing data
We performed an intention‐to‐treat analysis, whenever possible (Newell 1992); otherwise, we used the data available to us. When intention‐to‐treat analysis was not used and the data were not missing at random (for example, treatment was withdrawn due to adverse events or duration of treatment was shortened because of lack of response and such participants were excluded from analysis), this could lead to biased results; therefore, we conducted best‐worst‐case scenario analysis (assuming a good outcome in the intervention group and bad outcome in the control group) and worst‐best case scenario analysis (assuming a bad outcome in the intervention group and good outcome in the control group) as sensitivity analyses, whenever possible, for binary and time‐to‐event outcomes where binomial likelihood was used.
For continuous outcomes, we imputed the standard deviation from P values, according to guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If the data were likely to be normally distributed, we used the median for meta‐analysis when the mean was not available; otherwise, we planned to simply provide a median and interquartile range of the difference in medians. If it was not possible to calculate the standard deviation from the P value or the confidence intervals, we planned to impute the standard deviation using the largest standard deviation in other trials for that outcome. This form of imputation can decrease the weight of the study for calculation of mean differences and may bias the effect estimate to no effect for calculation of standardised mean differences (Higgins 2011).
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by carefully examining the characteristics and design of included trials. We also planned to assess the presence of clinical heterogeneity by comparing effect estimates (please see Subgroup analysis and investigation of heterogeneity) in trial reports of different drug dosages, presence of other features of decompensation, refractory or recurrent ascites, different aetiologies for cirrhosis (for example, alcohol‐related liver disease, viral liver diseases, autoimmune liver disease), and based on the co‐interventions (for example, both groups receive prophylactic antibiotics to decrease the risk of subacute bacterial peritonitis). Different study designs and risk of bias can contribute to methodological heterogeneity.
We assessed statistical heterogeneity by comparing the results of the fixed‐effect model meta‐analysis and the random‐effects model meta‐analysis, lack of overlap of 95% credible intervals of between‐study variance (tau2) with 0, and by calculating the network meta‐analysis‐specific I2 statistic using Stata/SE 15.1 (Jackson 2014). When possible, we explored substantial clinical, methodological, or statistical heterogeneity and addressed the heterogeneity in subgroup analysis (see 'Subgroup analysis and investigation of heterogeneity').
Assessment of transitivity across treatment comparisons
We assessed the transitivity assumption by comparing the distribution of the potential effect modifiers (clinical: other features of decompensation and methodological: risk of bias, year of randomisation, duration of follow‐up) across the different pairwise comparisons.
Assessment of reporting biases
For the network meta‐analysis, we planned to perform a comparison‐adjusted funnel plot. However, to interpret a comparison‐adjusted funnel plot, it is necessary to rank the studies in a meaningful way as asymmetry may be due to small sample sizes in newer studies (comparing newer treatments with older treatments), or higher risk of bias in older studies (Chaimani 2012). As there was no specific change in the risk of bias in the studies, sample size, or the control group used over time, we judged the reporting bias by the completeness of the search (Chaimani 2012). We also considered lack of reporting of outcomes as a form of reporting bias.
Data synthesis
We conducted network meta‐analyses to compare multiple interventions simultaneously for each of the primary and secondary outcomes. When two or more interventions were combined, we considered this as a separate intervention ('node'). Network meta‐analysis combines direct evidence within trials and indirect evidence across trials (Mills 2012). We obtained a network plot to ensure that the trials were connected by interventions using Stata/SE 15.1 (Chaimani 2013). We excluded any trials that were not connected to the network from the network meta‐analysis, and we reported only the direct pairwise meta‐analysis for such comparisons. We summarised the population and methodological characteristics of the trials included in the network meta‐analysis in a table based on pairwise comparisons. We conducted a Bayesian network meta‐analysis using the Markov chain Monte Carlo method in OpenBUGS 3.2.3, according to guidance from the National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU) documents (Dias 2016). We modelled the treatment contrast (i.e. log odds ratio for binary outcomes, mean difference or standardised mean difference for continuous outcomes, log rate ratio for count outcomes, and log hazard ratio for time‐to‐event outcomes) for any two interventions ('functional parameters') as a function of comparisons between each individual intervention and the reference group ('basic parameters') using appropriate likelihood functions and links (Lu 2006). We used binomial likelihood and logit link for binary outcomes, Poisson likelihood and log link for count outcomes, binomial likelihood and complementary log‐log link (a semiparametric model which excludes censored individuals from the denominator of ‘at risk’ individuals at the point when they are censored) for time‐to‐event outcomes, and normal likelihood and identity link for continuous outcomes. We used 'sclerotherapy' as the reference group across the networks, as this was the commonest intervention compared in the trials. We performed a fixed‐effect model and random‐effects model for the network meta‐analysis. We reported both models for comparison with the reference group in a forest plot when the results were different between the models. For each pairwise comparison in a table, we reported the fixed‐effect model if the two models reported similar results; otherwise, we reported the more conservative model, i.e. usually the random‐effects model.
We used a hierarchical Bayesian model using three different sets of initial values to start the simulation‐based parameter estimation to assist with the assessment of convergence, employing codes provided by NICE DSU (Dias 2016). We used a normal distribution with large variance (10,000) for treatment effect priors (vague or flat priors) centred at no effect. For the random‐effects model, we used a prior distributed uniformly (limits: 0 to 5) for the between‐trial standard deviation parameter and assumed that this variability would be the same across treatment comparisons (Dias 2016). We used a 'burn‐in' of 30,000 simulations, checked for convergence (of effect estimates and between‐study heterogeneity) visually (i.e. whether the values in different chains mixed very well by visualisation), and ran the models for another 10,000 simulations to obtain effect estimates. If we did not obtain convergence, we increased the number of simulations for the 'burn‐in' and used the 'thin' and 'over relax' functions to decrease the autocorrelation. If we still did not obtain convergence, we used alternate initial values and priors employing methods suggested by van Valkenhoef 2012. We estimated the probability that each intervention ranked at each of the possible positions based on estimated effect sizes and their corresponding uncertainty using the NICE DSU codes (Dias 2016).
Assessment of inconsistency
We assessed inconsistency (statistical evidence of the violation of the transitivity assumption) by fitting both an inconsistency model and a consistency model. We used inconsistency models employed in the NICE DSU manual, as we used a common between‐study standard deviation (Dias 2014). In addition, we used design‐by‐treatment full interaction model and inconsistency factor (IF) plots to assess inconsistency (Higgins 2012; Chaimani 2013) when applicable. We used Stata/SE 15.1 to create IF plots. In the presence of inconsistency (model fit better with inconsistency models than consistency model, 95% CrI of 'between‐design' variance did not overlap 0, and the 95% confidence intervals of inconsistency factor did not overlap 0), we assessed whether the inconsistency was due to clinical or methodological heterogeneity by performing separate analyses for each of the different subgroups mentioned in the Subgroup analysis and investigation of heterogeneity section or limited network meta‐analysis to a more compatible subset of trials when possible.
Direct comparison
We performed the direct comparisons in the randomised clinical trials using the same codes and the same technical details.
Subgroup analysis and investigation of heterogeneity
We planned to assess the differences in the effect estimates between the following subgroups and planned to investigate heterogeneity and inconsistency using meta‐regression with the help of the codes provided in NICE DSU guidance (Dias 2012a), if we included a sufficient number of trials (when there were at least two trials in at least two of the subgroups). We planned to use the following trial‐level covariates for meta‐regression.
Trials at low risk of bias compared to trials at high risk of bias.
Based on the presence of other features of decompensation (e.g. ascites).
Based on the aetiology for cirrhosis (e.g. alcohol‐related liver disease, viral liver diseases, autoimmune liver disease).
Based on the interval between the variceal bleed and the start of prophylactic treatment
Based on the cointerventions (e.g. both groups receive prophylactic antibiotics to decrease the risk of subacute bacterial peritonitis in people with low‐protein ascites).
Based on the period of follow‐up: short term: up to three months, medium term: more than three months to five years, and long term: more than five years.
Based on the definition used by authors for serious adverse events and any adverse events (ICH‐GCP 1997 compared to other definitions).
We planned to calculate a single common interaction term which assumes that each relative treatment effect compared to a common comparator treatment (i.e. sclerotherapy) is impacted in the same way by the covariate in question when applicable (Dias 2012a). If the 95% Crl of the interaction term did not overlap zero, we considered this statistically significant heterogeneity or inconsistency (depending upon the factor being used as covariate).
Sensitivity analysis
If there were post‐randomisation dropouts, we reanalysed the results using the best‐worst‐case scenario and worst‐best case scenario analyses as sensitivity analyses whenever possible. We also performed a sensitivity analysis excluding the trials in which mean or standard deviation, or both, were imputed, and we used the median standard deviation in the trials to impute missing standard deviations.
Presentation of results
We followed the PRISMA‐network meta‐analysis statement while reporting (Hutton 2015). We presented the effect estimates with 95% CrI for each pairwise comparison calculated from the direct comparisons and network meta‐analysis. We originally planned to present the cumulative probability of the treatment ranks (i.e. the probability that the intervention was within the top two, the probability that the intervention was within the top three, etc), but we did not present these because of the sparse data, which can lead to misinterpretation of results due to large uncertainty in the rankings (the CrI was 0 to 1 for all the ranks) in graphs (SUCRA) (Salanti 2011). We plotted the probability that each intervention was best, second best, third best, etc. for each of the different outcomes (rankograms), which are generally considered more informative (Salanti 2011; Dias 2012b), but we did not present these because of the sparse data which can lead to misinterpretation of results due to large uncertainty in the rankings (the CrI was 0 to 1 for all the ranks). We uploaded all the raw data and the codes used for analysis in the European Organization for Nuclear Research open source database (Zenodo) here.
Recommendations for future research
We provided recommendations for future research in the population, intervention, control, outcomes, period of follow‐up, and study design, based on the uncertainties that we identified from the existing research.
Summary of findings and assessment of the certainty of the evidence
We presented 'Summary of findings' tables for all the primary and secondary outcomes (see Primary outcomes; Secondary outcomes). We followed the approach suggested by Yepes‐Nunez and colleagues (Yepes‐Nunez 2019). First, we calculated the direct and indirect effect estimates (when possible) and 95% Crl using the node‐splitting approach (Dias 2010), that is, calculating the direct estimate for each comparison by including only trials in which there was direct comparison of interventions and the indirect estimate for each comparison by excluding the trials in which there was direct comparison of interventions (and ensuring a connected network). Next, we rated the quality of direct and indirect effect estimates using GRADE methodology which takes into account the risk of bias, inconsistency (heterogeneity), directness of evidence (including incoherence, the term used in GRADE methodology for inconsistency in network meta‐analysis), imprecision, and publication bias (Guyatt 2011a). We then presented the relative and absolute estimates of the meta‐analysis with the best certainty of evidence (Yepes‐Nunez 2019). For illustration of the absolute measures, we used weighted median (Edgeworth 1887) control group proportion or mean. We also presented the 'Summary of findings' tables in a second format presenting all the outcomes for selected interventions (Yepes‐Nunez 2019): we selected the five interventions (beta‐blockers, variceal band ligation, beta‐blockers plus sclerotherapy, no active intervention, and TIPS) which were compared in the most trials (Table 3), and in addition selected beta‐blockers plus variceal band ligation, currently recommended as standard of care by various clinical practice guidelines (de Franchis 2015; Tripathi 2015; Garcia‐Tsao 2017; EASL 2018).
1. Characteristics of included studies (ordered by comparison).
| Study name | Intervention 1 (number of participants) versus Intervention 2 (number of participants) | Included participants with other features of decompensation | Etiology of cirrhosis | Interval between variceal bleeding and treatment > 1 year | Period of recruitment | Follow‐up in months | Overall risk of bias |
| Alexandrino 1988 | Beta‐blockers (34) versus Sclerotherapy (31) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | No | Not stated | 29 | High |
| Andreani 1991 | Beta‐blockers (35) versus Sclerotherapy (40) | Not stated | Alcohol‐related cirrhosis: Not stated Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | No | 1985 ‐ 1988 | 12 | High |
| Bader 1987 | Beta‐blockers (17) versus Sclerotherapy (18) | Not stated | Alcohol‐related cirrhosis: Not stated Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | No | 1984‐1986 | 14 | High |
| Dasarathy 1992 | Beta‐blockers (46) versus Sclerotherapy (45) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | Not stated | 1996 ‐ 1990 | 12 | High |
| Dwivedi 1992 | Beta‐blockers (14) versus Sclerotherapy (16) | Yes (encephalopathy) | Alcohol‐related cirrhosis: Not stated Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | Not stated | 1986 ‐ 1987 | 7.5 | High |
| Fleig 1988 | Beta‐blockers (50) versus Sclerotherapy (55) | Not stated | Alcohol‐related cirrhosis: Not stated Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | Not stated | 1983 ‐ Not stated | 25 | High |
| Martin 1991 | Beta‐blockers (34) versus Sclerotherapy (42) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: No participants had viral‐related cirrhosis Autoimmune disease‐related cirrhosis: No participants had autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | No | 1984 ‐ 1986 | 35.6 | High |
| Rossi 1991 | Beta‐blockers (27) versus Sclerotherapy (26) | Yes (ascites) | Alcohol‐related cirrhosis: All participants had alcohol‐related cirrhosis Viral‐related cirrhosis: No participants had viral‐related cirrhosis Autoimmune disease‐related cirrhosis: No participants had autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: No participants had other‐causes for cirrhosis | Not stated | 1983 ‐ 1987 | 19 | High |
| Urbistondo 1996 | Beta‐blockers (15) versus Sclerotherapy (13) | Not stated | Alcohol‐related cirrhosis: All participants had alcohol‐related cirrhosis Viral‐related cirrhosis: No participants had viral‐related cirrhosis Autoimmune disease‐related cirrhosis: No participants had autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: No participants had other‐causes for cirrhosis | No | 1989 ‐ 1994 | 23.2 | High |
| Avgerinos 1997 | Variceal band ligation (37) versus Sclerotherapy (40) | Yes (ascites) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Participants with autoimmune disease‐related cirrhosis and without autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | No | 1992 ‐ 1993 | 15.2 | High |
| Baroncini 1997 | Variceal band ligation (57) versus Sclerotherapy (54) | Yes (encephalopathy) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Participants with autoimmune disease‐related cirrhosis and without autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | Not stated | 1993 ‐ 1995 | 16.9 | High |
| Kong 2015 | Variceal band ligation (20) versus Sclerotherapy (18) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Participants with autoimmune disease‐related cirrhosis and without autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | Not stated | 2008 ‐ 2012 | 16 | High |
| Viazis 2002 | Variceal band ligation (36) versus Sclerotherapy (37) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | Not stated | 1995 ‐ 1998 | 1.8 | High |
| Ahmad 2009 | Variceal band ligation (39) versus Beta‐blockers (39) | Yes (encephalopathy) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: No participants had autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: No participants had other‐causes for cirrhosis | No | 2003 ‐ 2005 | 9 | High |
| Kumar 2015 | Variceal band ligation (56) versus Beta‐blockers (47) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | Not stated | Not stated | 16.4 | High |
| Avgerinos 1993 | Beta‐blockers plus Sclerotherapy (45) versus Sclerotherapy (40) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Participants with autoimmune disease‐related cirrhosis and without autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | No | 1986 ‐ 1989 | 23.9 | High |
| Bertoni 1990 | Beta‐blockers plus Sclerotherapy (14) versus Sclerotherapy (14) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | Not stated | Not stated | 2 | High |
| Fornaciari 1990 | Beta‐blockers plus Sclerotherapy (14) versus Sclerotherapy (14) | Not stated | Alcohol‐related cirrhosis: Not stated Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | Not stated | Not stated | 3 | High |
| Jensen 1989 | Beta‐blockers plus Sclerotherapy (15) versus Sclerotherapy (16) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Participants with autoimmune disease‐related cirrhosis and without autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | Not stated | 1985 ‐ 1987 | 9 | High |
| Kanazawa 1991 | Beta‐blockers plus Sclerotherapy (20) versus Sclerotherapy (23) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | Not stated | 1985 ‐ 1990 | 26.7 | High |
| Lundell 1990 | Beta‐blockers plus Sclerotherapy (19) versus Sclerotherapy (22) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | Not stated | Not stated | 7.9 | High |
| Villanueva 1994 | Beta‐blockers plus Sclerotherapy (22) versus Sclerotherapy (18) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | Not stated | 1989 ‐ 1991 | 26 | High |
| Vinel 1992 | Beta‐blockers plus Sclerotherapy (39) versus Sclerotherapy (35) | Yes (encephalopathy) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | Not stated | Not stated | 3.2 | High |
| Ink 1992 | Beta‐blockers plus Sclerotherapy (65) versus Beta‐blockers (66) | Yes (encephalopathy) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | No | 1986 ‐ 1989 | 24 | High |
| Anonymous 1994 | No active intervention (107) versus Sclerotherapy (97) | Yes (not stated) | Alcohol‐related cirrhosis: All participants had alcohol‐related cirrhosis Viral‐related cirrhosis: No participants had viral‐related cirrhosis Autoimmune disease‐related cirrhosis: No participants had autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: No participants had other‐causes for cirrhosis | No | 1985 ‐ 1989 | 12 | High |
| Mckee 1994 | No active intervention (18) versus Sclerotherapy (22) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Participants with autoimmune disease‐related cirrhosis and without autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | Not stated | 1986 ‐ 1989 | 24 | High |
| Rossi 1991 | No active intervention (26) versus Sclerotherapy (26) | Yes (ascites) | Alcohol‐related cirrhosis: All participants had alcohol‐related cirrhosis Viral‐related cirrhosis: No participants had viral‐related cirrhosis Autoimmune disease‐related cirrhosis: No participants had autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: No participants had other‐causes for cirrhosis | Not stated | 1983 ‐ 1987 | 19 | High |
| Westaby 1985a | No active intervention (60) versus Sclerotherapy (56) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Participants with autoimmune disease‐related cirrhosis and without autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | Not stated | 1977 ‐ 1981 | 37 | High |
| Bonkovsky 1989 | No active intervention (10) versus Beta‐blockers (10) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | Not stated | Not stated | 12 | High |
| Esquivel Lopez 1984 | No active intervention (8) versus Beta‐blockers (11) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | Not stated | Not stated | 12 | High |
| Jiron 1993 | No active intervention (28) versus Beta‐blockers (29) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Participants with autoimmune disease‐related cirrhosis and without autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | No | 1983 ‐ 1986 | 48 | High |
| Rossi 1991 | No active intervention (26) versus Beta‐blockers (27) | Yes (ascites) | Alcohol‐related cirrhosis: All participants had alcohol‐related cirrhosis Viral‐related cirrhosis: No participants had viral‐related cirrhosis Autoimmune disease‐related cirrhosis: No participants had autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: No participants had other‐causes for cirrhosis | Not stated | 1983 ‐ 1987 | 19 | High |
| Sheen 1989 | No active intervention (18) versus Beta‐blockers (18) | Yes (encephalopathy) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | No | 1983 ‐ 1985 | 12.5 | High |
| Cabrera 1996 | TIPS (32) versus Sclerotherapy (31) | Yes (ascites) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: not stated | No | 1991 ‐ 1994 | 15 | High |
| Garcia‐Villarreal 1999 | TIPS (22) versus Sclerotherapy (24) | Yes (ascites) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | No | 1993 ‐ 1997 | 20.6 | High |
| Sanyal 1997 | TIPS (41) versus Sclerotherapy (39) | Yes (encephalopathy) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | No | 1991‐1994 | 32 | High |
| Jalan 1997 | TIPS (31) versus Variceal band ligation (27) | Yes (encephalopathy) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Participants with autoimmune disease‐related cirrhosis and without autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | No | 1993 ‐ 1995 | 16.2 | High |
| Sauer 1997 | TIPS (42) versus Beta‐blockers plus Sclerotherapy (41) | Yes (ascites) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | No | 1992‐1995 | 18 | High |
| Ahmad 2009 | Beta‐blockers plus Nitrates (35) versus Beta‐blockers (39) | Yes (encephalopathy) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: No participants had autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: No participants had other‐causes for cirrhosis | No | 2003 ‐ 2005 | 9 | High |
| Kumar 2015 | Beta‐blockers plus Nitrates (39) versus Beta‐blockers (47) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | Not stated | Not stated | 16.4 | High |
| Masliah 1997 | Beta‐blockers plus Nitrates (46) versus Beta‐blockers (49) | Not stated | Alcohol‐related cirrhosis: Not stated Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | Not stated | 1991 ‐ 1996 | 29 | High |
| Ahmad 2009 | Beta‐blockers plus Nitrates (35) versus Variceal band ligation (39) | Yes (encephalopathy) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: No participants had autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: No participants had other‐causes for cirrhosis | No | 2003 ‐ 2005 | 9 | High |
| Kumar 2015 | Beta‐blockers plus Nitrates (39) versus Variceal band ligation (56) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | Not stated | Not stated | 16.4 | High |
| Henderson 1990 | Portocaval shunt (35) versus Sclerotherapy (37) | Yes (encephalopathy) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Participants with autoimmune disease‐related cirrhosis and without autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | Not stated | 1981 ‐ 1985 | 61 | High |
| Isaksson 1995 | Portocaval shunt (24) versus Sclerotherapy (21) | Yes (encephalopathy) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | Not stated | 1982 ‐ 1989 | 65.2 | High |
| Urbistondo 1996 | Portocaval shunt (15) versus Sclerotherapy (13) | Not stated | Alcohol‐related cirrhosis: All participants had alcohol‐related cirrhosis Viral‐related cirrhosis: No participants had viral‐related cirrhosis Autoimmune disease‐related cirrhosis: No participants had autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: No participants had other‐causes for cirrhosis | No | 1989 ‐ 1994 | 23.2 | High |
| Ampelas 1987 | Portocaval shunt (24) versus Beta‐blockers (26) | Not stated | Alcohol‐related cirrhosis: Not stated Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | No | Not stated | 18 | High |
| Parelon 1989 | Portocaval shunt (24) versus Beta‐blockers (26) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | Not stated | 1982 ‐ 1985 | 39 | High |
| Urbistondo 1996 | Portocaval shunt (15) versus Beta‐blockers (15) | Not stated | Alcohol‐related cirrhosis: All participants had alcohol‐related cirrhosis Viral‐related cirrhosis: No participants had viral‐related cirrhosis Autoimmune disease‐related cirrhosis: No participants had autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: No participants had other‐causes for cirrhosis | No | 1989 ‐ 1994 | 23.2 | High |
| Argonz 2000 | Sclerotherapy plus Variceal band ligation (39) versus Variceal band ligation (41) | Yes (ascites) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Participants with autoimmune disease‐related cirrhosis and without autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | Not stated | 1994 ‐ 1997 | 12 | High |
| Baroncini 1996 | Sclerotherapy plus Variceal band ligation () versus Variceal band ligation () | Not stated | Alcohol‐related cirrhosis: Not stated Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | Not stated | 1995 ‐ 1996 | 4 | High |
| Cennamo 1998 | Sclerotherapy plus Variceal band ligation (16) versus Variceal band ligation (18) | Not stated | Alcohol‐related cirrhosis: Not stated Viral‐related cirrhosis: Not stated Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Not stated | Not stated | 1996 ‐ 1998 | 12.6 | High |
| Romero 2006 | Sclerotherapy plus Variceal band ligation (52) versus Beta‐blockers plus Nitrates (57) | Yes (ascites) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | Not stated | 1998 ‐ 2002 | 11.7 | High |
| Ahmad 2009 | Beta‐blockers plus Nitrates plus Variceal band ligation (37) versus Beta‐blockers (39) | Yes (encephalopathy) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: No participants had autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: No participants had other‐causes for cirrhosis | No | 2003 ‐ 2005 | 9 | High |
| Ahmad 2009 | Beta‐blockers plus Nitrates plus Variceal band ligation (37) versus Variceal band ligation (39) | Yes (encephalopathy) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: No participants had autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: No participants had other‐causes for cirrhosis | No | 2003 ‐ 2005 | 9 | High |
| Ahmad 2009 | Beta‐blockers plus Nitrates plus Variceal band ligation (37) versus Beta‐blockers plus Nitrates (35) | Yes (encephalopathy) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: No participants had autoimmune disease‐related cirrhosis Other‐causes for cirrhosis: No participants had other‐causes for cirrhosis | No | 2003 ‐ 2005 | 9 | High |
| García‐Pagán 2009 | Beta‐blockers plus Nitrates plus Variceal band ligation (80) versus Beta‐blockers plus Nitrates (78) | Yes (ascites) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | No | 2003 ‐ 2005 | 15 | High |
| Sauer 2002 | Beta‐blockers plus Variceal band ligation (42) versus TIPS (43) | Yes (ascites) | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | No | 1995 ‐ 1999 | 46.8 | High |
| Bertoni 1994 | Sclerotherapy plus Nitrates (37) versus Sclerotherapy (39) | Not stated | Alcohol‐related cirrhosis: Participants with alcohol‐related cirrhosis and without alcohol‐related cirrhosis Viral‐related cirrhosis: Participants with viral‐related cirrhosis and without viral‐related cirrhosis Autoimmune disease‐related cirrhosis: Not stated Other‐causes for cirrhosis: Participants with other‐causes for cirrhosis and without other‐causes for cirrhosis | No | 1990 ‐ 1992 | 2 | High |
Results
Description of studies
Results of the search
We identified 8184 records through electronic searches of CENTRAL (Wiley) (n = 1855), MEDLINE Ovid (n = 2725), Embase Ovid (n = 1034), Science Citation Index expanded (n = 1902), ClinicalTrials.gov (n = 83), WHO Trials register (n = 110), FDA (n = 36), and EMA (n = 439). After removing duplicate records, there were 5765 records. We excluded 5322 clearly irrelevant records through reading titles and abstracts. We retrieved a total of 443 full‐text records for further assessment in detail. We excluded 372 records (322 studies) for the reasons stated in the Characteristics of excluded studies. One record is awaiting classification and five records are ongoing trials. Thus, we included a total of 48 trials described in 65 records (Characteristics of included studies). The reference flow is shown in Figure 2.
Included studies
Fourty‐eight trials were included (Esquivel Lopez 1984; Westaby 1985a; Ampelas 1987; Bader 1987; Alexandrino 1988; Fleig 1988; Bonkovsky 1989; Jensen 1989; Parelon 1989; Sheen 1989; Bertoni 1990; Fornaciari 1990; Henderson 1990; Lundell 1990; Andreani 1991; Kanazawa 1991; Martin 1991; Rossi 1991; Dasarathy 1992; Dwivedi 1992; Ink 1992; Vinel 1992; Avgerinos 1993; Jiron 1993; Anonymous 1994; Bertoni 1994; Mckee 1994; Villanueva 1994; Isaksson 1995; Baroncini 1996; Cabrera 1996; Urbistondo 1996; Avgerinos 1997; Baroncini 1997; Jalan 1997; Masliah 1997; Sanyal 1997; Sauer 1997; Cennamo 1998; Garcia‐Villarreal 1999; Argonz 2000; Sauer 2002; Viazis 2002; Romero 2006; Ahmad 2009; García‐Pagán 2009; Kong 2015; Kumar 2015). A total of 3526 participants were randomised to different interventions. The number of participants ranged from 14 to 204. A total of 3442 participants from 46 trials were included in one of more comparisons (Westaby 1985a; Ampelas 1987; Bader 1987; Alexandrino 1988; Fleig 1988; Bonkovsky 1989; Jensen 1989; Parelon 1989; Sheen 1989; Bertoni 1990; Fornaciari 1990; Henderson 1990; Lundell 1990; Andreani 1991; Kanazawa 1991; Martin 1991; Rossi 1991; Dasarathy 1992; Dwivedi 1992; Ink 1992; Vinel 1992; Avgerinos 1993; Jiron 1993; Anonymous 1994; Bertoni 1994; Mckee 1994; Villanueva 1994; Isaksson 1995; Cabrera 1996; Urbistondo 1996; Avgerinos 1997; Baroncini 1997; Jalan 1997; Masliah 1997; Sanyal 1997; Sauer 1997; Cennamo 1998; Garcia‐Villarreal 1999; Argonz 2000; Sauer 2002; Viazis 2002; Romero 2006; Ahmad 2009; García‐Pagán 2009; Kong 2015; Kumar 2015). We did not identify any cluster randomised clinical trials or cross‐over randomised clinical trials that addressed the objectives of the review.
Participants
The mean or median age of the participants in the trials ranged from 40 to 63 years in the trials that reported this information (Esquivel Lopez 1984; Westaby 1985a; Bader 1987; Alexandrino 1988; Jensen 1989; Parelon 1989; Sheen 1989; Bertoni 1990; Lundell 1990; Kanazawa 1991; Martin 1991; Rossi 1991; Dasarathy 1992; Dwivedi 1992; Ink 1992; Vinel 1992; Avgerinos 1993; Jiron 1993; Bertoni 1994; Mckee 1994; Villanueva 1994; Isaksson 1995; Baroncini 1996; Cabrera 1996; Urbistondo 1996; Avgerinos 1997; Baroncini 1997; Jalan 1997; Sanyal 1997; Sauer 1997; Garcia‐Villarreal 1999; Argonz 2000; Sauer 2002; Viazis 2002; Romero 2006; Ahmad 2009; García‐Pagán 2009; Kong 2015; Kumar 2015). The proportion of females ranged from 0.0% to 80.4% in the trials that reported this information (Esquivel Lopez 1984; Westaby 1985a; Alexandrino 1988; Bonkovsky 1989; Jensen 1989; Parelon 1989; Sheen 1989; Bertoni 1990; Lundell 1990; Kanazawa 1991; Martin 1991; Rossi 1991; Dasarathy 1992; Dwivedi 1992; Ink 1992; Vinel 1992; Avgerinos 1993; Jiron 1993; Anonymous 1994; Bertoni 1994; Mckee 1994; Villanueva 1994; Isaksson 1995; Cabrera 1996; Urbistondo 1996; Avgerinos 1997; Baroncini 1997; Jalan 1997; Sanyal 1997; Sauer 1997; Cennamo 1998; Garcia‐Villarreal 1999; Argonz 2000; Sauer 2002; Viazis 2002; Romero 2006; Ahmad 2009; García‐Pagán 2009; Kong 2015). The follow‐up period in the trials ranged from 1.8 to 65.2 months. Four trials had short‐term follow‐up (up to three months) (Bertoni 1990; Fornaciari 1990; Bertoni 1994; Viazis 2002); 42 trials had medium‐term follow‐up (three months to five years) (Esquivel Lopez 1984; Westaby 1985a; Ampelas 1987; Bader 1987; Alexandrino 1988; Fleig 1988; Bonkovsky 1989; Jensen 1989; Parelon 1989; Sheen 1989; Lundell 1990; Andreani 1991; Kanazawa 1991; Martin 1991; Rossi 1991; Dasarathy 1992; Dwivedi 1992; Ink 1992; Vinel 1992; Avgerinos 1993; Jiron 1993; Anonymous 1994; Mckee 1994; Villanueva 1994; Baroncini 1996; Cabrera 1996; Urbistondo 1996; Avgerinos 1997; Baroncini 1997; Jalan 1997; Masliah 1997; Sanyal 1997; Sauer 1997; Cennamo 1998; Garcia‐Villarreal 1999; Argonz 2000; Sauer 2002; Romero 2006; Ahmad 2009; García‐Pagán 2009; Kong 2015; Kumar 2015); and two trials had long‐term follow‐up (more than five years) (Henderson 1990; Isaksson 1995).
Nineteen trials reported the proportion of participants who had other features of decompensation: in one trial, none of the participants had other features of decompensation (Sheen 1989); in the remaining 18 trials, the proportion of participants who had other features of decompensation ranged from 11.1% to 67.2% (Henderson 1990; Rossi 1991; Dwivedi 1992; Ink 1992; Vinel 1992; Isaksson 1995; Cabrera 1996; Avgerinos 1997; Baroncini 1997; Jalan 1997; Sanyal 1997; Sauer 1997; Garcia‐Villarreal 1999; Argonz 2000; Sauer 2002; Romero 2006; Ahmad 2009; García‐Pagán 2009).
Some 39 trials reported the proportion of participants who had alcohol‐related cirrhosis: in three trials, all the participants had alcohol‐related cirrhosis (Rossi 1991; Anonymous 1994; Urbistondo 1996); in the remaining 36 trials, the proportion of participants who had alcohol‐related cirrhosis ranged from 0.7% to 97.4% (Esquivel Lopez 1984; Westaby 1985a; Alexandrino 1988; Bonkovsky 1989; Jensen 1989; Parelon 1989; Sheen 1989; Bertoni 1990; Henderson 1990; Lundell 1990; Kanazawa 1991; Martin 1991; Dasarathy 1992; Ink 1992; Vinel 1992; Avgerinos 1993; Jiron 1993; Bertoni 1994; Mckee 1994; Villanueva 1994; Isaksson 1995; Cabrera 1996; Avgerinos 1997; Baroncini 1997; Jalan 1997; Sanyal 1997; Sauer 1997; Garcia‐Villarreal 1999; Argonz 2000; Sauer 2002; Viazis 2002; Romero 2006; Ahmad 2009; García‐Pagán 2009; Kong 2015; Kumar 2015). Some 27 trials reported the proportion of participants who had viral‐related cirrhosis: in four trials, none of the participants had viral‐related cirrhosis (Martin 1991; Rossi 1991; Anonymous 1994; Urbistondo 1996); in the remaining 23 trials, the proportion of participants who had viral‐related cirrhosis ranged from 1.8% to 99.3% (Westaby 1985a; Jensen 1989; Sheen 1989; Bertoni 1990; Henderson 1990; Kanazawa 1991; Dasarathy 1992; Avgerinos 1993; Jiron 1993; Bertoni 1994; Mckee 1994; Avgerinos 1997; Baroncini 1997; Jalan 1997; Sanyal 1997; Sauer 1997; Argonz 2000; Sauer 2002; Viazis 2002; Romero 2006; Ahmad 2009; García‐Pagán 2009; Kong 2015). Some 16 trials reported the proportion of participants who had autoimmune disease‐related cirrhosis: in five trials, none of the participants had autoimmune disease‐related cirrhosis (Martin 1991; Rossi 1991; Anonymous 1994; Urbistondo 1996; Ahmad 2009); in the remaining 11 trials, the proportion of participants who had autoimmune disease‐related cirrhosis ranged from 1.3% to 21.6% (Westaby 1985a; Jensen 1989; Henderson 1990; Avgerinos 1993; Jiron 1993; Mckee 1994; Avgerinos 1997; Baroncini 1997; Jalan 1997; Argonz 2000; Kong 2015). Some 27 trials reported the proportion of participants who had other‐causes for cirrhosis: in four trials, none of the participants had other‐causes for cirrhosis (Rossi 1991; Anonymous 1994; Urbistondo 1996; Ahmad 2009); in the remaining 23 trials, the proportion of participants who had other‐causes for cirrhosis ranged from 0.9% to 47.3% (Westaby 1985a; Alexandrino 1988; Jensen 1989; Sheen 1989; Bertoni 1990; Henderson 1990; Martin 1991; Dasarathy 1992; Avgerinos 1993; Jiron 1993; Bertoni 1994; Mckee 1994; Avgerinos 1997; Baroncini 1997; Jalan 1997; Sanyal 1997; Sauer 1997; Argonz 2000; Sauer 2002; Viazis 2002; Romero 2006; García‐Pagán 2009; Kong 2015).
Interventions
A total of 12 interventions were compared in these trials (sclerotherapy, beta‐blockers, variceal band ligation, beta‐blockers plus sclerotherapy, no active intervention, TIPS, beta‐blockers plus nitrates, portocaval shunt, sclerotherapy plus variceal band ligation, beta‐blockers plus nitrates plus variceal band ligation, beta‐blockers plus variceal band ligation, sclerotherapy plus nitrates).
Forty‐four trials had two interventions (Esquivel Lopez 1984; Westaby 1985a; Ampelas 1987; Bader 1987; Alexandrino 1988; Fleig 1988; Bonkovsky 1989; Jensen 1989; Parelon 1989; Sheen 1989; Bertoni 1990; Fornaciari 1990; Henderson 1990; Lundell 1990; Andreani 1991; Kanazawa 1991; Martin 1991; Dasarathy 1992; Dwivedi 1992; Ink 1992; Vinel 1992; Avgerinos 1993; Jiron 1993; Anonymous 1994; Bertoni 1994; Mckee 1994; Villanueva 1994; Isaksson 1995; Baroncini 1996; Cabrera 1996; Avgerinos 1997; Baroncini 1997; Jalan 1997; Masliah 1997; Sanyal 1997; Sauer 1997; Cennamo 1998; Garcia‐Villarreal 1999; Argonz 2000; Sauer 2002; Viazis 2002; Romero 2006; García‐Pagán 2009; Kong 2015), three trials had three interventions (Rossi 1991; Kumar 2015; Urbistondo 1996), and one trial had four interventions (Ahmad 2009) included for this review.
Some 46 trials reported one or more outcomes for this review (Westaby 1985a; Ampelas 1987; Bader 1987; Alexandrino 1988; Fleig 1988; Bonkovsky 1989; Jensen 1989; Parelon 1989; Sheen 1989; Bertoni 1990; Fornaciari 1990; Henderson 1990; Lundell 1990; Andreani 1991; Kanazawa 1991; Martin 1991; Rossi 1991; Dasarathy 1992; Dwivedi 1992; Ink 1992; Vinel 1992; Avgerinos 1993; Jiron 1993; Anonymous 1994; Bertoni 1994; Mckee 1994; Villanueva 1994; Isaksson 1995; Cabrera 1996; Urbistondo 1996; Avgerinos 1997; Baroncini 1997; Jalan 1997; Masliah 1997; Sanyal 1997; Sauer 1997; Cennamo 1998; Garcia‐Villarreal 1999; Argonz 2000;Domagk 2000Sauer 2002; Viazis 2002; Romero 2006; Ahmad 2009; García‐Pagán 2009; Kong 2015; Kumar 2015). The important characteristics, potential effect modifiers, and follow‐up in each trial is reported in Table 3. Overall, there do not seem to be any systematic differences between the comparisons.
Funding
The source of funding for five trials was industrial organisations who would benefit from the results of the study (Fleig 1988; Bonkovsky 1989; Jensen 1989; Bertoni 1994; García‐Pagán 2009); nine trials were funded by neutral organisations who have no vested interests in the results of the study (Westaby 1985a; Henderson 1990; Lundell 1990; Avgerinos 1993; Anonymous 1994; Sanyal 1997; Sauer 1997; Romero 2006; Kong 2015); the source of funding for the remaining 34 trials was unclear (Esquivel Lopez 1984; Ampelas 1987; Bader 1987; Alexandrino 1988; Parelon 1989; Sheen 1989; Bertoni 1990; Fornaciari 1990; Andreani 1991; Kanazawa 1991; Martin 1991; Rossi 1991; Dasarathy 1992; Dwivedi 1992; Ink 1992; Vinel 1992; Jiron 1993; Mckee 1994; Villanueva 1994; Isaksson 1995; Baroncini 1996; Cabrera 1996; Urbistondo 1996; Avgerinos 1997; Baroncini 1997; Jalan 1997; Masliah 1997; Cennamo 1998; Garcia‐Villarreal 1999; Argonz 2000; Sauer 2002; Viazis 2002; Ahmad 2009; Kumar 2015).
Excluded studies
The reasons for exclusion of studies are listed in Characteristics of excluded studies. The summary of reasons for exclusion of studies are as follows.
Reasons related to randomisation: 48 studies (Orloff 1962; Berardi 1974; Orloff 1974; Paquet 1983; Adson 1984; Conn 1986; Conn 1987; Kleber 1987; Piai 1987; Terblanche 1988; Fort 1990; Gilbert 1991; Svoboda 1992; Conn 1993; Thiel 1993; Van Stiegmann 1993; Dwivedi 1995; Mino 1995; Benner 1996; Pereira 1997; Srinivasan 1997; Am. Soc. Gastro. Endo. 1998; Gong 1998; Sheikh 1998; Sung 1998; Khaitiyar 2000; Russo 2000; Shah 2001; Groszmann 2002; Maldonado 2002; Marrero 2002; Taniai 2002; Villanueva 2002; Wiest 2002; Okano 2003a; Okano 2003b; Yoshida 2004; Gonzalez‐Suarez 2006; Kuran 2006; Hua 2007; D'Amico 2008; Evrard 2008; Bosch 2013; Zhou 2013; Orloff 2014; Chen 2018; NCT03583996; Pfisterer 2018)
Reasons related to population: 226 studies (Resnick 1969; Callow 1970; Jackson 1971; Resnick 1974; Rikkers 1978; Terblanche 1979; Lebrec 1981; Reynolds 1981; Witzel 1982; Burroughs 1983; Otte 1983; Terblanche 1983; Westaby 1984; Korula 1985; Westaby 1985b; Mastai 1986; Villeneuve 1986; Westaby 1986; Gatta 1987; Queuniet 1987; Teres 1987; Dollet 1988; Dunk 1988; Johansson 1988; Kanazawa 1988; Prioton 1988; Colombo 1989; Jeng 1989; Kitano 1989; O'Connor 1989; Sotto 1989; Taupignon 1989; Tommasini 1989; Westaby 1989; Cestari 1990; Garden 1990; McKee 1990; Santambrogio 1990; Spina 1990; Taranto 1990; Braga 1991; Feu 1991; Garcia‐Pagan 1991; Kleber 1991; Planas 1991; Testa 1991; Bhargava 1992; Kitano 1992; McCormick 1992; Acharya 1993; Feu 1993; Hashizume 1993; Lo 1993; McCormick 1993; Rikkers 1993; Saraya 1993; Teres 1993; Young 1993; Berner 1994; Bolognesi 1994; El‐Tourabi 1994; Moreto 1994; Primignani 1994; Vickers 1994; Bolognesi 1995; Cirera 1995; Li 1995; Ministro 1995; Pontes 1995; Primignani 1995; Albillos 1996; Elsayed 1996; Escorsell 1996; Estevens 1996; Garcia‐Pagan 1996; Iwao 1996; Lin 1996; Nakase 1996; Nevens 1996a; Nevens 1996b; Rosemurgy 1996; Villanueva 1996; Zironi 1996; Balatsos 1997; Bhargava 1997; Durdevic 1997; Escorsell 1997; Escorsell 1997a; Fakhry 1997; Iso 1997; Jenkins 1997; Pang 1997; Rossle 1997; Saeed 1997; Sarin 1997; Sugano 1997; Bandi 1998; Barrioz 1998; D'Amico 1998; Lo 1998; Masumoto 1998; Merli 1998; Shin 1998; Siqueira 1998; Zhao 1998; Al Traif 1999; Banares 1999; Buuren 1999; de la Pena 1999; Djurdjevic 1999; Garg 1999; Gotoh 1999; Gralnek 1999; Masci 1999; Nishikawa 1999; Pena 1999; Umehara 1999; Domagk 2000; Gournay 2000; Iwakiri 2000; NCT00006161; Romero 2000; Shigemitsu 2000; Van Buuren 2000; Cheng 2001; Escorsell 2001; Lee 2001; Nakamura 2001; Narahara 2001; Pomier‐Layrargues 2001; Schepke 2001; Sugano 2001; Villanueva 2001; Bobadilla‐Diaz 2002; De 2002; Escorsell 2002; Lin 2002; Lo 2002; Schiedermaier 2002; Sen 2002; Serwah 2002; Vorobioff 2002; Bellis 2003; De 2003; Evrard 2003; Schiedermaier 2003; Liu 2004; Silva 2004; Tripathi 2004; de la Pena 2005; Farag 2005; Ferrari 2005; Kalambokis 2005; Kuwayama 2005; Lin 2005; Pena 2005; Pozzi 2005; Sarin 2005; Lin 2006; Ohmoto 2006; Santambrogio 2006; Bhuiyan 2007; ISRCTN77521636; Morales 2007; NCT00570973; Qi 2007; Vorobioff 2007; ChiCTR08000228; Fernandez 2008; Lo 2008; NCT00799851; Van Buuren 2008; Zargar 2008; Zhang 2008; Kumar 2009; Lo 2009a; Bonilha 2010; Gong 2010; Harras 2010; NCT01103154; Graupera 2011; Luo 2011; Priyadarshi 2011; Santos 2011; Copaci 2012; EUCTR2006‐006393‐14; EUCTR2012‐002489‐11; Lo 2012; NCT01640964; Wang 2012; Chen 2013; George 2013; Smith 2013; Sohn 2013; Sun 2013; Zhao 2013; EUCTR2014‐002018‐21; Mo 2014; NCT02119884; Stanley 2014; Abd Elmoety 2015; ChiCTR11001577; ChiCTR12002148; Geng 2015; Helmy 2015; ISRCTN14174793; Liao 2015; Luo 2015; NCT02508623; Chen 2016; Costa 2016; Hanno 2016; Harki 2016a; Holster 2016; Lacet 2016; Li 2016; NCT02646202; Zuckerman 2016; ChiCTR1800020322; Dong 2018; Lv 2018; NCT03687216; NCT03783065; Chen 2019; ChiCTR1900021212; Dunne 2019)
Reasons related to intervention: 48 studies (Mikkelsen 1974; Goff 1986; Rhodes 1986; Bories 1987; Terabayashi 1987; Akriviadis 1989; Palazzi 1989; Triger 1992; Fiaccadori 1993; Dehesa 1994; Magnano 1994; Nos 1995; Sarin 1995; Krige 1996; Kim 1997; Eleftheriadis 1998; Escorsell 1998; Liu 1998; Nakamura 1998; Li 2000; Li 2000a; Lo 2000; Gonzalez‐Abraldes 2001; Jiang 2001; Brensing 2002; Cipolletta 2002; Gulberg 2002; Patch 2002; Lu 2004; Zhu 2004; Baik 2005; EUCTR2005‐003557‐27; El‐Saadany 2007; Lo 2009b; Villanueva 2009; Monici 2010; Agarwala 2011; ChiCTR11000192; Agarwal 2015; Rawat 2015; Sauerbruch 2015; Abraldes 2016; ChiCTR15007655; NCT02740166; Huang 2017; Kamal 2017; Villanueva 2017; ChiCTR1800018070).
Risk of bias in included studies
The risk of bias is summarised in Figure 3, Figure 4, and in Table 4. All the trials were at unclear or high risk of bias in at least one of the domains and were considered to be at high risk of bias overall.
3.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
2. Risk of bias (ordered by comparisons).
| Study name | Intervention 1 (number of participants) versus Intervention 2 (number of participants) | Sequence generation | Allocation concealment | Blinding of patients and healthcare providers | Blinding of outcome assessors | Missing outcome bias | Selective outcome reporting | Other bias | Overall risk of bias |
| Alexandrino 1988 | Beta‐blockers (34) versus Sclerotherapy (31) | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Low | High |
| Andreani 1991 | Beta‐blockers (35) versus Sclerotherapy (40) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | High |
| Bader 1987 | Beta‐blockers (17) versus Sclerotherapy (18) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | High |
| Dasarathy 1992 | Beta‐blockers (46) versus Sclerotherapy (45) | Unclear | Unclear | Unclear | Unclear | High | Low | Low | High |
| Dwivedi 1992 | Beta‐blockers (14) versus Sclerotherapy (16) | Low | Unclear | Unclear | Unclear | High | Unclear | Low | High |
| Fleig 1988 | Beta‐blockers (50) versus Sclerotherapy (55) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | High |
| Martin 1991 | Beta‐blockers (34) versus Sclerotherapy (42) | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low | High |
| Rossi 1991 | Beta‐blockers (27) versus Sclerotherapy (26) | Unclear | Low | High | Low | Low | Unclear | Low | High |
| Urbistondo 1996 | Beta‐blockers (15) versus Sclerotherapy (13) | Low | Unclear | Unclear | Unclear | Low | Unclear | Low | High |
| Avgerinos 1997 | Variceal band ligation (37) versus Sclerotherapy (40) | Low | Unclear | Unclear | Unclear | Low | Low | Low | High |
| Baroncini 1997 | Variceal band ligation (57) versus Sclerotherapy (54) | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low | High |
| Kong 2015 | Variceal band ligation (20) versus Sclerotherapy (18) | Low | Unclear | Unclear | Unclear | Low | Low | Low | High |
| Viazis 2002 | Variceal band ligation (36) versus Sclerotherapy (37) | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Low | High |
| Ahmad 2009 | Variceal band ligation (39) versus Beta‐blockers (39) | Low | Low | Unclear | Unclear | High | Unclear | Low | High |
| Kumar 2015 | Variceal band ligation (56) versus Beta‐blockers (47) | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low | High |
| Avgerinos 1993 | Beta‐blockers plus Sclerotherapy (45) versus Sclerotherapy (40) | Unclear | Unclear | Unclear | Unclear | Low | Low | Low | High |
| Bertoni 1990 | Beta‐blockers plus Sclerotherapy (14) versus Sclerotherapy (14) | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Low | High |
| Fornaciari 1990 | Beta‐blockers plus Sclerotherapy (14) versus Sclerotherapy (14) | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Low | High |
| Jensen 1989 | Beta‐blockers plus Sclerotherapy (15) versus Sclerotherapy (16) | Low | Low | Low | Low | Unclear | Low | Low | High |
| Kanazawa 1991 | Beta‐blockers plus Sclerotherapy (20) versus Sclerotherapy (23) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | High |
| Lundell 1990 | Beta‐blockers plus Sclerotherapy (19) versus Sclerotherapy (22) | Unclear | Unclear | Unclear | Unclear | Low | Unclear | High | High |
| Villanueva 1994 | Beta‐blockers plus Sclerotherapy (22) versus Sclerotherapy (18) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | High |
| Vinel 1992 | Beta‐blockers plus Sclerotherapy (39) versus Sclerotherapy (35) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | High |
| Ink 1992 | Beta‐blockers plus Sclerotherapy (65) versus Beta‐blockers (66) | Unclear | Unclear | Unclear | Unclear | Low | Low | Low | High |
| Anonymous 1994 | No active intervention (107) versus Sclerotherapy (97) | Unclear | Unclear | Low | Low | Low | Unclear | Low | High |
| Mckee 1994 | No active intervention (18) versus Sclerotherapy (22) | Unclear | Unclear | Unclear | Unclear | Low | Low | Low | High |
| Rossi 1991 | No active intervention (26) versus Sclerotherapy (26) | Unclear | Low | High | Low | Low | Unclear | Low | High |
| Westaby 1985a | No active intervention (60) versus Sclerotherapy (56) | Unclear | Unclear | High | High | Low | Unclear | Low | High |
| Bonkovsky 1989 | No active intervention (10) versus Beta‐blockers (10) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | High |
| Esquivel Lopez 1984 | No active intervention (8) versus Beta‐blockers (11) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | High |
| Jiron 1993 | No active intervention (28) versus Beta‐blockers (29) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | High |
| Rossi 1991 | No active intervention (26) versus Beta‐blockers (27) | Unclear | Low | High | Low | Low | Unclear | Low | High |
| Sheen 1989 | No active intervention (18) versus Beta‐blockers (18) | Unclear | Unclear | Unclear | Unclear | Low | Low | Low | High |
| Cabrera 1996 | TIPS (32) versus Sclerotherapy (31) | Low | Unclear | Unclear | Unclear | Low | Low | Low | High |
| Garcia‐Villarreal 1999 | TIPS (22) versus Sclerotherapy (24) | Low | Unclear | Unclear | Unclear | Low | Unclear | Low | High |
| Sanyal 1997 | TIPS (41) versus Sclerotherapy (39) | Low | Unclear | High | High | Low | Unclear | Low | High |
| Jalan 1997 | TIPS (31) versus Variceal band ligation (27) | Unclear | Unclear | Unclear | Unclear | Low | Low | Low | High |
| Sauer 1997 | TIPS (42) versus Beta‐blockers plus Sclerotherapy (41) | Low | Low | High | High | Low | Low | Low | High |
| Ahmad 2009 | Beta‐blockers plus Nitrates (35) versus Beta‐blockers (39) | Low | Low | Unclear | Unclear | High | Unclear | Low | High |
| Kumar 2015 | Beta‐blockers plus Nitrates (39) versus Beta‐blockers (47) | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low | High |
| Masliah 1997 | Beta‐blockers plus Nitrates (46) versus Beta‐blockers (49) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | High |
| Ahmad 2009 | Beta‐blockers plus Nitrates (35) versus Variceal band ligation (39) | Low | Low | Unclear | Unclear | High | Unclear | Low | High |
| Kumar 2015 | Beta‐blockers plus Nitrates (39) versus Variceal band ligation (56) | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low | High |
| Henderson 1990 | Portocaval shunt (35) versus Sclerotherapy (37) | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Low | High |
| Isaksson 1995 | Portocaval shunt (24) versus Sclerotherapy (21) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | High |
| Urbistondo 1996 | Portocaval shunt (15) versus Sclerotherapy (13) | Low | Unclear | Unclear | Unclear | Low | Unclear | Low | High |
| Ampelas 1987 | Portocaval shunt (24) versus Beta‐blockers (26) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | High |
| Parelon 1989 | Portocaval shunt (24) versus Beta‐blockers (26) | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | High |
| Urbistondo 1996 | Portocaval shunt (15) versus Beta‐blockers (15) | Low | Unclear | Unclear | Unclear | Low | Unclear | Low | High |
| Argonz 2000 | Sclerotherapy plus Variceal band ligation (39) versus Variceal band ligation (41) | Low | Low | Unclear | Unclear | Low | Low | High | High |
| Baroncini 1996 | Sclerotherapy plus Variceal band ligation () versus Variceal band ligation () | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | High |
| Cennamo 1998 | Sclerotherapy plus Variceal band ligation (16) versus Variceal band ligation (18) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | High |
| Romero 2006 | Sclerotherapy plus Variceal band ligation (52) versus Beta‐blockers plus Nitrates (57) | Low | Low | Unclear | Unclear | Low | Low | Low | High |
| Ahmad 2009 | Beta‐blockers plus Nitrates plus Variceal band ligation (37) versus Beta‐blockers (39) | Low | Low | Unclear | Unclear | High | Unclear | Low | High |
| Ahmad 2009 | Beta‐blockers plus Nitrates plus Variceal band ligation (37) versus Variceal band ligation (39) | Low | Low | Unclear | Unclear | High | Unclear | Low | High |
| Ahmad 2009 | Beta‐blockers plus Nitrates plus Variceal band ligation (37) versus Beta‐blockers plus Nitrates (35) | Low | Low | Unclear | Unclear | High | Unclear | Low | High |
| García‐Pagán 2009 | Beta‐blockers plus Nitrates plus Variceal band ligation (80) versus Beta‐blockers plus Nitrates (78) | Low | Low | Unclear | Unclear | Low | Low | Low | High |
| Sauer 2002 | Beta‐blockers plus Variceal band ligation (42) versus TIPS (43) | Low | Low | Unclear | Unclear | Low | Unclear | Low | High |
| Bertoni 1994 | Sclerotherapy plus Nitrates (37) versus Sclerotherapy (39) | Low | Unclear | Low | Low | Low | Unclear | Low | High |
Allocation
Some 15 trials were at low risk of sequence generation bias (Jensen 1989; Dwivedi 1992; Bertoni 1994; Cabrera 1996; Urbistondo 1996; Avgerinos 1997; Sanyal 1997; Sauer 1997; Garcia‐Villarreal 1999; Argonz 2000; Sauer 2002; Romero 2006; Ahmad 2009; García‐Pagán 2009; Kong 2015); the remaining 33 trials, which did not provide sufficient information, were at unclear risk of sequence generation bias (Westaby 1985a; Alexandrino 1988; Fleig 1988; Bonkovsky 1989; Esquivel Lopez 1984; Ampelas 1987; Bader 1987; Parelon 1989; Sheen 1989; Bertoni 1990; Henderson 1990; Fornaciari 1990; Lundell 1990; Andreani 1991; Kanazawa 1991; Martin 1991; Rossi 1991; Dasarathy 1992; Ink 1992; Avgerinos 1993; Jiron 1993; Vinel 1992; Anonymous 1994; Mckee 1994; Villanueva 1994; Isaksson 1995; Baroncini 1996; Baroncini 1997; Jalan 1997; Masliah 1997; Cennamo 1998; Viazis 2002; Kumar 2015).
Some eight trials were at low risk of allocation concealment bias (Jensen 1989; Rossi 1991; Sauer 1997; Argonz 2000; Sauer 2002; Romero 2006; Ahmad 2009; García‐Pagán 2009); the remaining 40 trials, which did not provide sufficient information, were at unclear risk of allocation concealment bias (Esquivel Lopez 1984; Westaby 1985a; Ampelas 1987; Bader 1987; Alexandrino 1988; Fleig 1988; Bonkovsky 1989; Parelon 1989; Sheen 1989; Bertoni 1990; Fornaciari 1990; Henderson 1990; Lundell 1990; Andreani 1991; Kanazawa 1991; Martin 1991; Dasarathy 1992; Dwivedi 1992; Ink 1992; Vinel 1992; Avgerinos 1993; Jiron 1993; Anonymous 1994; Bertoni 1994; Mckee 1994; Villanueva 1994; Isaksson 1995; Baroncini 1996; Cabrera 1996; Urbistondo 1996; Avgerinos 1997; Baroncini 1997; Jalan 1997; Masliah 1997; Sanyal 1997; Cennamo 1998; Garcia‐Villarreal 1999; Viazis 2002; Kong 2015; Kumar 2015).
Blinding
Three trials were at low risk of performance bias as the participants and healthcare providers were blinded (Jensen 1989; Anonymous 1994; Bertoni 1994); 41 trials, which did not provide sufficient information, were at unclear risk of performance bias (Esquivel Lopez 1984; Ampelas 1987; Bader 1987; Alexandrino 1988; Fleig 1988; Bonkovsky 1989; Parelon 1989; Sheen 1989; Bertoni 1990; Fornaciari 1990; Henderson 1990; Lundell 1990; Andreani 1991; Kanazawa 1991; Martin 1991; Dasarathy 1992; Dwivedi 1992; Ink 1992; Vinel 1992; Avgerinos 1993; Jiron 1993; Mckee 1994; Villanueva 1994; Isaksson 1995; Baroncini 1996; Cabrera 1996; Urbistondo 1996; Avgerinos 1997; Baroncini 1997; Jalan 1997; Masliah 1997; Cennamo 1998; Garcia‐Villarreal 1999; Argonz 2000; Sauer 2002; Viazis 2002; Romero 2006; Ahmad 2009; García‐Pagán 2009; Kong 2015; Kumar 2015); the remaining four trials were at high risk of performance bias (Westaby 1985a; Rossi 1991; Sanyal 1997; Sauer 1997).
Four trials were at low risk of detection bias (Jensen 1989; Rossi 1991; Anonymous 1994; Bertoni 1994); 41 trials, which did not provide sufficient information, were at unclear risk of detection bias (Esquivel Lopez 1984; Ampelas 1987; Bader 1987; Alexandrino 1988; Fleig 1988; Bonkovsky 1989; Parelon 1989; Sheen 1989; Bertoni 1990; Fornaciari 1990; Henderson 1990; Lundell 1990; Andreani 1991; Kanazawa 1991; Martin 1991; Dasarathy 1992; Dwivedi 1992; Ink 1992; Vinel 1992; Avgerinos 1993; Jiron 1993; Mckee 1994; Villanueva 1994; Isaksson 1995; Baroncini 1996; Cabrera 1996; Urbistondo 1996; Avgerinos 1997; Baroncini 1997; Jalan 1997; Masliah 1997; Cennamo 1998; Garcia‐Villarreal 1999; Argonz 2000; Sauer 2002; Viazis 2002; Romero 2006; Ahmad 2009; García‐Pagán 2009; Kong 2015; Kumar 2015); the remaining three trials were at high risk of detection bias (Westaby 1985a; Sanyal 1997; Sauer 1997).
Incomplete outcome data
Some 26 trials were at low risk of attrition bias as there were no post‐randomisation dropouts or an intention‐to‐treat analysis was used (Westaby 1985a; Alexandrino 1988; Sheen 1989; Bertoni 1990; Fornaciari 1990; Henderson 1990; Lundell 1990; Rossi 1991; Ink 1992; Avgerinos 1993; Anonymous 1994; Bertoni 1994; Mckee 1994; Cabrera 1996; Urbistondo 1996; Avgerinos 1997; Jalan 1997; Sanyal 1997; Sauer 1997; Garcia‐Villarreal 1999; Argonz 2000; Sauer 2002; Viazis 2002; Romero 2006; García‐Pagán 2009; Kong 2015); 18 trials were at unclear risk of attrition bias (Esquivel Lopez 1984; Ampelas 1987; Bader 1987; Fleig 1988; Bonkovsky 1989; Jensen 1989; Andreani 1991; Kanazawa 1991; Martin 1991; Vinel 1992; Jiron 1993; Villanueva 1994; Isaksson 1995; Baroncini 1996; Baroncini 1997; Masliah 1997; Cennamo 1998; Kumar 2015) because it was not clear whether there were post‐randomisation drop‐outs or whether the post‐randomisation dropouts were related to the outcomes (if there were post‐randomisation dropouts); the remaining four trials were at high risk of attrition bias (Parelon 1989; Dasarathy 1992; Dwivedi 1992; Ahmad 2009), as the post‐randomisation dropouts were probably related to the outcomes.
Selective reporting
Some 17 trials were at low risk of selective outcome reporting bias (Jensen 1989; Sheen 1989; Martin 1991; Dasarathy 1992; Ink 1992; Avgerinos 1993; Mckee 1994; Cabrera 1996; Avgerinos 1997; Baroncini 1997; Jalan 1997; Sauer 1997; Argonz 2000; Romero 2006; García‐Pagán 2009; Kong 2015; Kumar 2015), as the outcomes were reported or the important clinical outcomes expected to be reported in such trials were reported; the remaining 31 trials were at unclear risk of selective outcome reporting bias (Esquivel Lopez 1984; Westaby 1985a; Ampelas 1987; Bader 1987; Alexandrino 1988; Fleig 1988; Bonkovsky 1989; Parelon 1989; Bertoni 1990; Fornaciari 1990; Henderson 1990; Lundell 1990; Andreani 1991; Kanazawa 1991; Rossi 1991; Dwivedi 1992; Vinel 1992; Jiron 1993; Anonymous 1994; Bertoni 1994; Villanueva 1994; Isaksson 1995; Baroncini 1996; Urbistondo 1996; Masliah 1997; Sanyal 1997; Cennamo 1998; Garcia‐Villarreal 1999; Sauer 2002; Viazis 2002; Ahmad 2009), as a protocol published prior to recruitment was not available.
Other potential sources of bias
No other potential source of bias was noted in any of the trials.
Effects of interventions
Summary of findings 1. Secondary prevention of bleeding in people with previous oesophageal variceal bleeding due to decompensated liver cirrhosis (common interventions).
| Patient or population: people with liver cirrhosis and previous oesophageal variceal bleeding Settings: secondary or tertiary care Intervention: various interventions Comparison: sclerotherapy Follow‐up period: 2 months to 65 months | ||||||||||||
| Outcomes/Interventions | Beta‐blockers | Variceal band ligation | Beta‐blockers plus Sclerotherapy | No active intervention | TIPS | Beta‐blockers plus Variceal band ligation | ||||||
| Mortality | ||||||||||||
| Sclerotherapy 225 per 1000 (22.5%) | HR 0.88 (0.66 to 1.18) Network estimate | 28 fewer per 1000 (77 fewer to 40 more) | HR 0.95 (0.62 to 1.46) Network estimate | 12 fewer per 1000 (86 fewer to 103 more) | HR 0.69 (0.43 to 1.09) Network estimate | 70 fewer per 1000 (128 fewer to 20 more) | HR 1.20 (0.83 to 1.84) Network estimate | 44 more per 1000 (39 fewer to 189 more) | HR 0.94 (0.56 to 1.59) Network estimate | 13 fewer per 1000 (100 fewer to 132 more) | HR 0.83 (0.22 to 3.05) Network estimate | 21 fewer per 1000 (107 fewer to 131 more) |
| Low certainty1,2 | Low certainty1,2 | Low certainty1,2 | Low certainty1,2 | Low certainty1,2 | Low certainty1,2 | |||||||
| Based on 493 participants (9 RCTs) | Based on 399 participants (5 RCTs) | Based on 370 participants (8 RCTs) | Based on 412 participants (4 RCTs) | Based on 189 participants (3 RCTs) | No direct RCT | |||||||
| Health‐related quality of life | ||||||||||||
| None of the trials reported heath‐related quality of life. | ||||||||||||
| Serious adverse events (number of people) | ||||||||||||
| Sclerotherapy 360 per 1000 (36%) | OR 0.47 (0.13 to 1.53) Network estimate | 150 fewer per 1000 (291 fewer to 103 more) | OR 0.19 (0.06 to 0.53) Network estimate | 265 fewer per 1000 (330 fewer to 130 fewer) | OR 1.29 (0.28 to 5.70) Network estimate | 61 more per 1000 (223 fewer to 402 more) | ‐ | ‐ | ‐ | |||
| Very low certainty1,2,3 | Low certainty1,3 | Very low certainty1,2,3 | ||||||||||
| Based on 91 participants (1 RCT) | Based on 100 participants (1 RCT) | No direct RCT | ||||||||||
| Serious adverse events (number of events) | ||||||||||||
| None of the trials reported serious adverse events (number of events). | ||||||||||||
| Any adverse events (number of people) | ||||||||||||
| Sclerotherapy 380 per 1000 (38%) | OR 11.86 (1.16 to 427.95) Network estimate | 499 more per 1000 (35 more to 616 more) | OR 0.39 (0.16 to 1.09) Network estimate | 186 fewer per 1000 (292 fewer to 21 more) | OR 1.46 (0.36 to 6.06) Network estimate | 92 more per 1000 (200 fewer to 408 more) | OR 0.22 (0.05 to 0.86) Direct estimate | 261 fewer per 1000 (350 fewer to 34 fewer) | OR 0.02 (0.00 to 0.17) Network estimate | 368 fewer per 1000 (379 fewer to 284 fewer) | ‐ | |
| Low certainty1,3 | Very low certainty1,2,3 | Very low certainty1,2,3 | Low certainty1,3 | Low certainty1,3 | ||||||||
| No direct RCT | Based on 115 participants (2 RCTs) | Based on 71 participants (2 RCTs) | Based on 40 participants (1 RCT) | No direct RCT | ||||||||
| Any adverse events (number of events) | ||||||||||||
| Sclerotherapy 581 per 1000 (58.1 per 100 participants) | ‐ | Rate ratio 0.40 (0.26 to 0.61) Network estimate | 348 fewer per 1000 (431 fewer to 226 fewer) | Rate ratio 0.93 (0.68 to 1.26) Network estimate | 44 fewer per 1000 (186 fewer to 148 more) | ‐ | Rate ratio 1.10 (0.73 to 1.66) Network estimate | 57 more per 1000 (158 fewer to 382 more) | Rate ratio 0.13 (0.06 to 0.28) Network estimate | 505 fewer per 1000 (547 fewer to 420 fewer) | ||
| Moderate certainty1 | Low certainty1,2 | Low certainty1,2 | Moderate certainty1 | |||||||||
| Based on 188 participants (2 RCTs) | Based on 128 participants (2 RCTs) | Based on 63 participants (1 RCT) | No direct RCT | |||||||||
| Liver transplantation | ||||||||||||
| Sclerotherapy 19 per 1000 (1.9%) | ‐ | HR 0.94 (0.03 to 35.27) Network estimate | 1 fewer per 1000 (18 fewer to 635 more) | HR 1.26 (0.17 to 10.10) Network estimate | 5 more per 1000 (15 fewer to 169 more) | ‐ | HR 1.52 (0.39 to 6.60) Network estimate | 10 more per 1000 (11 fewer to 104 more) | ‐ | |||
| Very low certainty1,2,3 | Very low certainty1,2,3 | Very low certainty1,2,3 | ||||||||||
| Based on 111 participants (1 RCT) | Based on 40 participants (1 RCT) | Based on 80 participants (1 RCT) | ||||||||||
| Symptomatic variceal rebleed | ||||||||||||
| Sclerotherapy 56 per 1000 (5.6%) | HR 0.46 (0.00 to 43.25) Network estimate | 30 fewer per 1000 (55 fewer to 944 more) | HR 0.43 (0.00 to 50.40) Network estimate | 31 fewer per 1000 (55 fewer to 944 more) | ‐ | HR 1.19 (0.00 to 1726.76) Network estimate | 11 more per 1000 (56 fewer to 944 more) | HR 0.05 (0.00 to 94.44) Network estimate | 53 fewer per 1000 (56 fewer to 944 more) | ‐ | ||
| Very low certainty1,2,3,4,5 | Very low certainty1,2,3,4,5 | Very low certainty1,2,3,4,5 | Very low certainty1,2,3,4,5 | |||||||||
| Based on 28 participants (1 RCT) | Based on 111 participants (1 RCT) | No direct RCT | No direct RCT | |||||||||
| Any variceal rebleed | ||||||||||||
| Sclerotherapy 473 per 1000 (47.3%) | HR 1.62 (1.14 to 2.38) Network estimate | 295 more per 1000 (67 more to 527 more) | HR 1.61 (0.72 to 3.77) Network estimate | 287 more per 1000 (132 fewer to 527 more) | HR 0.60 (0.37 to 0.95) Network estimate | 189 fewer per 1000 (300 fewer to 24 fewer) | HR 1.61 (1.01 to 2.86) Network estimate | 288 more per 1000 (5 more to 527 more) | HR 0.18 (0.08 to 0.38) Network estimate | 386 fewer per 1000 (434 fewer to 294 fewer) | ‐ | |
| Moderate certainty1 | Low certainty1,2 | Moderate certainty1 | Moderate certainty1 | Moderate certainty1 | ||||||||
| Based on 420 participants (6 RCTs) | Based on 111 participants (2 RCTs) | Based on 231 participants (4 RCTs) | Based on 296 participants (3 RCTs) | Based on 109 participants (2 RCTs) | ||||||||
| Other features of decompensation at maximal follow‐up | ||||||||||||
| Sclerotherapy 292 per 1000 (29.2 per 100 participants) | Rate ratio 2.40 (1.35 to 4.55) Network estimate | 409 more per 1000 (103 more to 1035 more) | Rate ratio 1.92 (0.31 to 10.62) Network estimate | 267 more per 1000 (201 fewer to 2807 more) | Rate ratio 0.45 (0.09 to 1.75) Network estimate | 160 fewer per 1000 (267 fewer to 218 more) | ‐ | Rate ratio 2.27 (1.19 to 4.59) Network estimate | 369 more per 1000 (56 more to 1046 more) | ‐ | ||
| Low certainty1,3 | Very low certainty1,2,3 | Very low certainty1,2,3 | Low certainty1,3 | |||||||||
| Based on 65 participants (1 RCT) | No direct RCT | No direct RCT | Based on 109 participants (2 RCTs) | |||||||||
| *Ranking was not provided because of the considerable uncertainty in the ranking. CrI: Credible interval; HR: Hazard ratio; OR: Odds ratio; RCT: randomised clinical trial. | ||||||||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||||||||
1Downgraded one level for risk of bias because the trial(s) included in the analysis was/were at high risk of bias 2Downgraded one level for imprecision because the credible intervals were wide (included clinical benefit and harms) 3Downgraded one level for imprecision because the sample size was small 4Downgraded one level for indirectness because this is based on indirect evidence only or these was evidence of statistical inconsistency 5Downgraded one level for inconsistency because there was evidence of statistical heterogeneity
Summary of findings 2. Secondary prevention of bleeding in people with previous oesophageal variceal bleeding due to decompensated liver cirrhosis (all interventions).
| Patient or population: people with liver cirrhosis and previous oesophageal variceal bleeding Settings: secondary or tertiary care Intervention: various interventions Comparison: sclerotherapy Follow‐up period: 2 months to 65 months Network geometry plots:Figure 1 | |||||
| Interventions | Relative effect (95% CrI) | Anticipated absolute effect* (95% CrI) | Quality of evidence | ||
| Sclerotherapy | Various interventions | Difference | |||
| Mortality Total studies: 45 Total participants: 3369 | |||||
| Sclerotherapy | Reference | ||||
| Beta‐blockers (9 RCTs; 493 participants) | HR 0.88 (0.66 to 1.18) Network estimate | 225 per 1000 | 197 per 1000 (148 to 265) | 28 fewer per 1000 (77 fewer to 40 more) | Low certainty1,2 |
| Variceal band ligation (5 RCTs; 399 participants) | HR 0.95 (0.62 to 1.46) Network estimate | 225 per 1000 | 213 per 1000 (139 to 328) | 12 fewer per 1000 (86 fewer to 103 more) | Low certainty1,2 |
| Beta‐blockers plus Sclerotherapy (8 RCTs; 370 participants) | HR 0.69 (0.43 to 1.09) Network estimate | 225 per 1000 | 155 per 1000 (97 to 245) | 70 fewer per 1000 (128 fewer to 20 more) | Low certainty1,2 |
| No active intervention (4 RCTs; 412 participants) | HR 1.20 (0.83 to 1.84) Network estimate | 225 per 1000 | 269 per 1000 (186 to 414) | 44 more per 1000 (39 fewer to 189 more) | Low certainty1,2 |
| TIPS (3 RCTs; 189 participants) | HR 0.94 (0.56 to 1.59) Network estimate | 225 per 1000 | 212 per 1000 (125 to 357) | 13 fewer per 1000 (100 fewer to 132 more) | Low certainty1,2 |
| Beta‐blockers plus Nitrates (No direct RCT) | HR 0.91 (0.53 to 1.58) Network estimate | 225 per 1000 | 204 per 1000 (118 to 356) | 21 fewer per 1000 (107 fewer to 131 more) | Low certainty1,2 |
| Portocaval shunt (2 RCTs; 100 participants) | HR 1.21 (0.68 to 2.15) Network estimate | 225 per 1000 | 273 per 1000 (154 to 484) | 48 more per 1000 (71 fewer to 259 more) | Low certainty1,2 |
| Sclerotherapy plus Variceal band ligation (No direct RCT) | HR 0.78 (0.35 to 1.76) Network estimate | 225 per 1000 | 176 per 1000 (79 to 397) | 49 fewer per 1000 (146 fewer to 172 more) | Low certainty1,2 |
| Beta‐blockers plus Nitrates plus Variceal band ligation (No direct RCT) | HR 0.89 (0.40 to 1.98) Network estimate | 225 per 1000 | 201 per 1000 (91 to 444) | 24 fewer per 1000 (134 fewer to 219 more) | Low certainty1,2 |
| Beta‐blockers plus Variceal band ligation (No direct RCT) | HR 0.83 (0.22 to 3.05) Network estimate | 225 per 1000 | 188 per 1000 (50 to 686) | 37 fewer per 1000 (175 fewer to 461 more) | Low certainty1,2 |
| Sclerotherapy plus Nitrates (1 RCT; 76 participants) | HR 0.19 (0.02 to 0.86) Network estimate | 225 per 1000 | 42 per 1000 (5 to 194) | 183 fewer per 1000 (220 fewer to 31 fewer) | Moderate certainty1 |
| Health‐related quality of life | |||||
| None of the trials reported health‐related quality of life. | |||||
| Serious adverse events (number of people) Total studies: 3 Total participants: 322 | |||||
| Sclerotherapy | Reference | ||||
| Beta‐blockers (1 RCT; 91 participants) | OR 0.47 (0.13 to 1.53) Network estimate | 360 per 1000 | 210 per 1000 (69 to 463) | 150 fewer per 1000 (291 fewer to 103 more) | Very low certainty1,2,3 |
| Variceal band ligation (1 RCT; 100 participants) | OR 0.19 (0.06 to 0.53) Network estimate | 360 per 1000 | 95 per 1000 (30 to 230) | 265 fewer per 1000 (330 fewer to 130 fewer) | Low certainty1,3 |
| Beta‐blockers plus Sclerotherapy (No direct RCT) | OR 1.29 (0.28 to 5.70) Network estimate | 360 per 1000 | 421 per 1000 (137 to 762) | 61 more per 1000 (223 fewer to 402 more) | Very low certainty1,2,3 |
| Serious adverse events (number of events) | |||||
| None of the trials reported serious adverse events (number of events). | |||||
| Any adverse events (number of people) Total studies: 11 Total participants: 859 | |||||
| Sclerotherapy | Reference | ||||
| Beta‐blockers (No direct RCT) | OR 11.86 (1.16 to 427.95) Network estimate | 380 per 1000 | 879 per 1000 (415 to 996) | 499 more per 1000 (35 more to 616 more) | Low certainty1,3 |
| Variceal band ligation (3 RCTs; 215 participants) | OR 0.39 (0.16 to 1.09) Network estimate | 380 per 1000 | 194 per 1000 (88 to 401) | 186 fewer per 1000 (292 fewer to 21 more) | Low certainty1,3 |
| Beta‐blockers plus Sclerotherapy (2 RCTs; 71 participants) | OR 1.46 (0.36 to 6.06) Network estimate | 380 per 1000 | 472 per 1000 (180 to 788) | 92 more per 1000 (200 fewer to 408 more) | Low certainty1,3 |
| No active intervention (1 RCT; 40 participants) | OR 0.22 (0.05 to 0.86) Direct estimate | 380 per 1000 | 119 per 1000 (30 to 346) | 261 fewer per 1000 (350 fewer to 34 fewer) | Low certainty1,3 |
| TIPS (No direct RCT) | OR 0.02 (0.00 to 0.17) Network estimate | 380 per 1000 | 12 per 1000 (1 to 96) | 368 fewer per 1000 (379 fewer to 284 fewer) | Low certainty1,3 |
| Beta‐blockers plus Nitrates (No direct RCT) | OR 27.58 (2.79 to 981.42) Network estimate | 380 per 1000 | 944 per 1000 (631 to 998) | 564 more per 1000 (251 more to 618 more) | Low certainty1,3 |
| Sclerotherapy plus Variceal band ligation (No direct RCT) | OR 2.46 (0.36 to 23.78) Network estimate | 380 per 1000 | 601 per 1000 (179 to 936) | 221 more per 1000 (201 fewer to 556 more) | Very low certainty1,2,3 |
| Beta‐blockers plus Nitrates plus Variceal band ligation (No direct RCT) | OR 94.92 (6.85 to 4500.75) Network estimate | 380 per 1000 | 983 per 1000 (808 to 1000) | 603 more per 1000 (428 more to 620 more) | Low certainty1,3 |
| Any adverse events (number of events) Total studies: 8 Total participants: 592 | |||||
| Sclerotherapy | Reference | ||||
| Variceal band ligation (2 RCTs; 188 participants) | Rate ratio 0.40 (0.26 to 0.61) Network estimate | 581 per 1000 | 233 per 1000 (149 to 355) | 348 fewer per 1000 (431 fewer to 226 fewer) | Moderate certainty1 |
| Beta‐blockers plus Sclerotherapy (2 RCTs; 128 participants) | Rate ratio 0.93 (0.68 to 1.26) Network estimate | 581 per 1000 | 537 per 1000 (395 to 729) | 44 fewer per 1000 (186 fewer to 148 more) | Low certainty1,2 |
| TIPS (1 RCT; 63 participants) | Rate ratio 1.10 (0.73 to 1.66) Network estimate | 581 per 1000 | 637 per 1000 (423 to 963) | 57 more per 1000 (158 fewer to 382 more) | Low certainty1,2 |
| Portocaval shunt (1 RCT; 45 participants) | Rate ratio 0.87 (0.34 to 2.28) Network estimate | 581 per 1000 | 507 per 1000 (195 to 1327) | 74 fewer per 1000 (386 fewer to 746 more) | Low certainty1,2 |
| Beta‐blockers plus Variceal band ligation (No direct RCT) | Rate ratio 0.13 (0.06 to 0.28) Network estimate | 581 per 1000 | 76 per 1000 (33 to 160) | 505 fewer per 1000 (547 fewer to 420 fewer) | Moderate certainty1 |
| Liver transplantation Total studies: 4 Total participants: 314 | |||||
| Sclerotherapy | Reference | ||||
| Variceal band ligation (1 RCT; 111 participants) | HR 0.94 (0.03 to 35.27) Network estimate | 19 per 1000 | 17 per 1000 (0 to 653) | 1 fewer per 1000 (18 fewer to 635 more) | Very low certainty1,2,3 |
| Beta‐blockers plus Sclerotherapy (1 RCT; 40 participants) | HR 1.26 (0.17 to 10.10) Network estimate | 19 per 1000 | 23 per 1000 (3 to 187) | 5 more per 1000 (15 fewer to 169 more) | Very low certainty1,2,3 |
| TIPS (1 RCT; 80 participants) | HR 1.52 (0.39 to 6.60) Network estimate | 19 per 1000 | 28 per 1000 (7 to 122) | 10 more per 1000 (11 fewer to 104 more) | Very low certainty1,2,3 |
| Symptomatic variceal rebleed Total studies: 7 Total participants: 550 | |||||
| Sclerotherapy | Reference | ||||
| Beta‐blockers (1 RCT; 28 participants) | HR 0.46 (0.00 to 43.25) Network estimate | 56 per 1000 | 25 per 1000 (0 to 1000) | 30 fewer per 1000 (55 fewer to 944 more) | Very low certainty1,2,3,4,5 |
| Variceal band ligation (1 RCT; 111 participants) | HR 0.43 (0.00 to 50.40) Network estimate | 56 per 1000 | 24 per 1000 (0 to 1000) | 31 fewer per 1000 (55 fewer to 944 more) | Very low certainty1,2,3,4,5 |
| No active intervention (No direct RCT) | HR 1.19 (0.00 to 1726.76) Network estimate | 56 per 1000 | 66 per 1000 (0 to 1000) | 11 more per 1000 (56 fewer to 944 more) | Very low certainty1,2,3,4,5 |
| TIPS (No direct RCT) | HR 0.05 (0.00 to 94.44) Network estimate | 56 per 1000 | 3 per 1000 (0 to 1000) | 53 fewer per 1000 (56 fewer to 944 more) | Very low certainty1,2,3,4,5 |
| Beta‐blockers plus Nitrates (No direct RCT) | HR 0.31 (0.00 to 183.46) Network estimate | 56 per 1000 | 17 per 1000 (0 to 1000) | 38 fewer per 1000 (56 fewer to 944 more) | Very low certainty1,2,3,4,5 |
| Portocaval shunt (2 RCTs; 100 participants) | HR 0.17 (0.00 to 8.86) Network estimate | 56 per 1000 | 9 per 1000 (0 to 492) | 46 fewer per 1000 (55 fewer to 437 more) | Very low certainty1,2,3,4,5 |
| Sclerotherapy plus Variceal band ligation (No direct RCT) | HR 0.30 (0.00 to 524.27) Network estimate | 56 per 1000 | 17 per 1000 (0 to 1000) | 39 fewer per 1000 (56 fewer to 944 more) | Very low certainty1,2,3,4,5 |
| Beta‐blockers plus Nitrates plus Variceal band ligation (No direct RCT) | HR 0.25 (0.00 to 154.01) Network estimate | 56 per 1000 | 14 per 1000 (0 to 1000) | 42 fewer per 1000 (56 fewer to 944 more) | Very low certainty1,2,3,4,5 |
| Any variceal rebleed Total studies: 23 Total participants: 1713 | |||||
| Sclerotherapy | Reference | ||||
| Beta‐blockers (6 RCTs; 420 participants) | HR 1.62 (1.14 to 2.38) Network estimate | 473 per 1000 | 767 per 1000 (540 to 1000) | 295 more per 1000 (67 more to 527 more) | Moderate certainty1 |
| Variceal band ligation (2 RCTs; 111 participants) | HR 1.61 (0.72 to 3.77) Network estimate | 473 per 1000 | 760 per 1000 (341 to 1000) | 287 more per 1000 (132 fewer to 527 more) | Low certainty1,2 |
| Beta‐blockers plus sclerotherapy (4 RCTs; 231 participants) | HR 0.60 (0.37 to 0.95) Network estimate | 473 per 1000 | 284 per 1000 (173 to 449) | 189 fewer per 1000 (300 fewer to 24 fewer) | Moderate certainty1 |
| No active intervention (3 RCTs; 296 participants) | HR 1.61 (1.01 to 2.86) Network estimate | 473 per 1000 | 761 per 1000 (478 to 1000) | 288 more per 1000 (5 more to 527 more) | Moderate certainty1 |
| TIPS (2 RCTs; 109 participants) | HR 0.18 (0.08 to 0.38) Network estimate | 473 per 1000 | 87 per 1000 (39 to 179) | 386 fewer per 1000 (434 fewer to 294 fewer) | Moderate certainty1 |
| Beta‐blockers plus nitrates (No direct RCT) | HR 2.13 (0.79 to 5.86) Network estimate | 473 per 1000 | 1000 per 1000 (373 to 1000) | 527 more per 1000 (99 fewer to 527 more) | Low certainty1,2 |
| Portocaval shunt (No direct RCT) | HR 0.21 (0.05 to 0.77) Network estimate | 473 per 1000 | 98 per 1000 (22 to 366) | 374 fewer per 1000 (451 fewer to 107 fewer) | Moderate certainty1 |
| Sclerotherapy plus variceal band ligation (No direct RCT) | HR 1.83 (0.46 to 7.40) Network estimate | 473 per 1000 | 864 per 1000 (216 to 1000) | 391 more per 1000 (256 fewer to 527 more) | Low certainty1,2 |
| Other features of decompensation Total studies: 6 Total participants: 349 | |||||
| Sclerotherapy | Reference | ||||
| Beta‐blockers (1 RCT; 65 participants) | Rate ratio 2.40 (1.35 to 4.55) Network estimate | 292 per 1000 | 701 per 1000 (395 to 1327) | 409 more per 1000 (103 more to 1035 more) | Low certainty1,3 |
| Variceal band ligation (No direct RCT) | Rate ratio 1.92 (0.31 to 10.62) Network estimate | 292 per 1000 | 559 per 1000 (90 to 3098) | 267 more per 1000 (201 fewer to 2807 more) | Very low certainty1,2,3 |
| Beta‐blockers plus Sclerotherapy (No direct RCT) | Rate ratio 0.45 (0.09 to 1.75) Network estimate | 292 per 1000 | 131 per 1000 (25 to 509) | 160 fewer per 1000 (267 fewer to 218 more) | Very low certainty1,2,3 |
| TIPS (2 RCTs; 109 participants) | Rate ratio 2.27 (1.19 to 4.59) Network estimate | 292 per 1000 | 661 per 1000 (348 to 1338) | 369 more per 1000 (56 more to 1046 more) | Low certainty1,3 |
| Sclerotherapy plus Variceal band ligation (No direct RCT) | Rate ratio 2.18 (0.04 to 129.28) Network estimate | 292 per 1000 | 635 per 1000 (11 to 37707) | 344 more per 1000 (281 fewer to 37416 more) | Very low certainty1,2,3 |
| *Ranking was not provided because of the considerable uncertainty in the ranking. CrI: Credible interval; HR: Hazard ratio; OR: Odds ratio; RCT: randomised clinical trial. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for risk of bias because the trial(s) included in the analysis was/were at high risk of bias 2Downgraded one level for imprecision because the credible intervals were wide (included clinical benefit and harms) 3Downgraded one level for imprecision because the sample size was small 4Downgraded one level for indirectness because these was evidence of statistical inconsistency 5Downgraded one level for inconsistency because there was evidence of statistical heterogeneity
The network plots (where relevant) are available in Figure 1. The inconsistency factor plots (where relevant) are available in Figure 5. The differences in the fixed‐effect versus random‐effects model, where relevant, are available in Figure 6. The model fit is available in Table 5. The effect estimates are available in Table 6.
1.
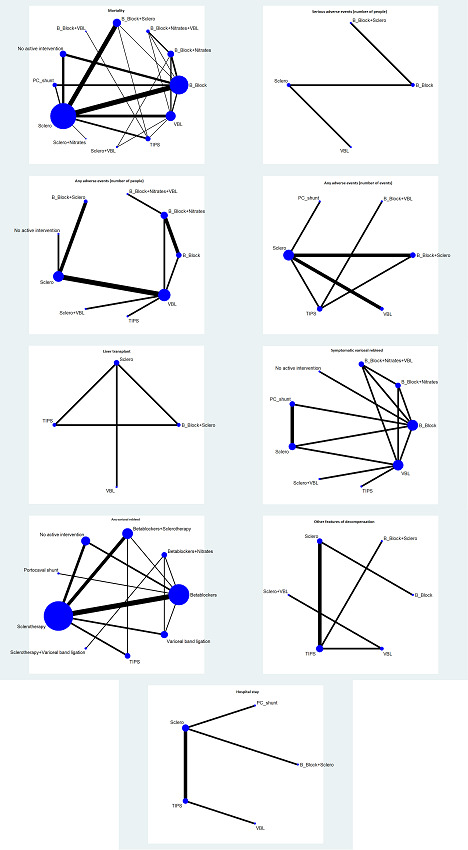
Network plots: A high resolution version of this image can be found here.
The network plots showing the outcomes for which network meta‐analysis was performed. The size of the node (circle) provides a measure of the number of trials in which the particular Intervention was included as one of the intervention groups. The thickness of the line provides a measure of the number of direct comparisons between two nodes (Interventions).
Abbreviations
B_Block = Beta‐blockers PC_shunt = Portocaval shunt Sclero = Sclerotherapy TIPS = Transjugular intrahepatic portosystemic shunt VBL = Variceal band ligation
5.

Inconsistency factor plots showing the inconsistency factors for the outcomes with direct and indirect evidence available for one or more comparisons. There was no evidence of inconsistency except for mortality and symptomatic variceal bleed (where the confidence intervals of the inconsistency factors do not overlap 0). A higher resolution image of this picture is available here.
Abbreviations
B_Block = Beta‐blockers PC_shunt = Portocaval shunt Sclero = Sclerotherapy TIPS = Transjugular intrahepatic portosystemic shunt VBL = Variceal band ligation
6.
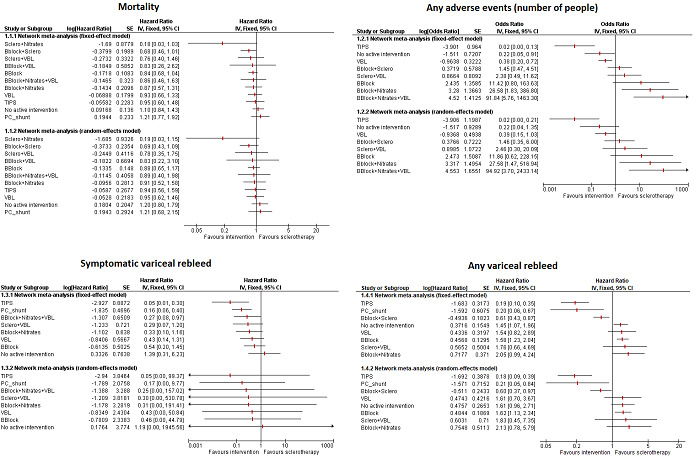
Forest plots showing the outcomes for which the random‐effects model were different from the fixed‐effect model. The more conservative random‐effects model was used. A higher resolution image of this picture is available here.
Abbreviations
BBlock = Beta‐blockers PC_shunt = Portocaval shunt Sclero = Sclerotherapy TIPS = Transjugular intrahepatic portosystemic shunt VBL = Variceal band ligation
3. Network meta‐analysis model fit.
| Mortality | Fixed‐effect model | Random‐effects model | Inconsistency model |
| Dbar | 402 | 390.4 | 391 |
| DIC | 454.3 | 451.9 | 459.3 |
| pD | 52.38 | 61.45 | 68.36 |
| Serious adverse events (number of people) | Fixed‐effect model | Random‐effects model | Inconsistency model |
| Dbar | 29.49 | 381.7 | 381.8 |
| DIC | 35.52 | 440.9 | 447.6 |
| pD | 6.026 | 59.2 | 65.82 |
| Any adverse events (number of people) | Fixed‐effect model | Random‐effects model | Inconsistency model |
| Dbar | 101.1 | 102 | 101.1 |
| DIC | 120.1 | 122 | 118.2 |
| pD | 18.97 | 20.04 | 17.14 |
| Any adverse events (number of events) | Fixed‐effect model | Random‐effects model | Inconsistency model |
| Dbar | 112.2 | 112.1 | 94.85 |
| DIC | 125.1 | 125.1 | 110.7 |
| pD | 12.96 | 12.93 | 15.87 |
| Liver transplantation | Fixed‐effect model | Random‐effects model | Inconsistency model |
| Dbar | 27.01 | 152 | 150.5 |
| DIC | 33.49 | 175.7 | 176.3 |
| pD | 6.486 | 23.75 | 25.78 |
| Symptomatic variceal rebleed | Fixed‐effect model | Random‐effects model | Inconsistency model |
| Dbar | 82.88 | 74.73 | 74.66 |
| DIC | 97.66 | 91.41 | 91.34 |
| pD | 14.77 | 16.69 | 16.68 |
| Any variceal rebleed | Fixed‐effect model | Random‐effects model | Inconsistency model |
| Dbar | 222.9 | 216.3 | 216.7 |
| DIC | 252.7 | 251.9 | 256.5 |
| pD | 29.8 | 35.63 | 39.82 |
| Other features of decompensation | Fixed‐effect model | Random‐effects model | Inconsistency model |
| Dbar | 55.92 | 55.9 | ‐ |
| DIC | 66.58 | 66.53 | ‐ |
| pD | 10.66 | 10.63 | ‐ |
Dbar: posterior mean of deviance; DIC: deviance information criteria; pD: effective number of parameters or leverage.
4. Effect estimates.
| This table is too wide to be displayed in RevMan. This table can be found here. |
The table provides the effect estimates of each pairwise comparison for the different outcomes. The top half of the table indicates the effect estimates from the direct comparisons. The bottom half of the table indicates the effect estimates from the network meta‐analysis. For network meta‐analysis, to identify the effect estimate of a comparison, say A versus B, look at the cell that occupies the row corresponding to intervention A and the column corresponding to intervention B for the effect estimate that is obtained directly. If that cell is empty (indicated by a '‐'), look at the row corresponding to intervention B and the column corresponding to intervention A. Take the inverse of this number (i.e. 1/number) to arrive at the treatment effect of A versus B. For direct comparisons, this is exactly the opposite; look at the cell that occupies the column corresponding to intervention A and the row corresponding to intervention B for the direct effect estimate. If that cell is empty, look at the column corresponding to intervention B and the row corresponding to intervention A. Take the inverse of this number to arrive at the treatment effect of A versus B. If the cell corresponding to B versus A is also missing in direct comparisons, this means that there was no direct comparison.
Statistically significant results are shown in italics. Green colour indicates that the intervention A is better than B and red colour indicates that the intervention A is worse than B.
Additional information
The credible intervals for adverse events (number of participants) is extremely wide for some comparisons. This is because of the major differences in the proportion of participants with adverse events between the interventions in some direct comparisons, which might increase even further (depending upon the data) when indirect evidence is calculated.
Because of the confusion that arose when we reported the 'no active intervention' (which is not the current standard clinical practice) as intervention versus another more common intervention used in clinical practice, we have inverted the intervention and control for comparisons involving no active intervention. This would result in differences between the effect estimates in this table and text.
The 95% credible intervals of the probability ranks were wide and included 0 and 1 in most comparisons for all the primary and secondary outcomes. This was probably because of the sparse data from small trials. Therefore, we did not present the ranking probabilities (in a table), rankograms, and SUCRA (surface under the cumulative ranking curve) plots as we considered that presenting this information would be unhelpful and potentially misleading, and it would ignore the differences in systematic errors in the trials.
The certainty of evidence was moderate, low, or very low for all the comparisons. This was because all the trials included in the comparison were at unclear or high risk of bias for at least one risk of bias domain at the outcome level (downgraded one level). For all direct comparisons, the number of events were fewer than 300 events and we downgraded one level for imprecision. For network meta‐analysis, for outcomes other than mortality, any adverse events (number of events), and any variceal rebleed, the number of events were fewer than 300 and we downgraded one level for imprecision. In comparisons where the wide credible intervals overlapped significant clinical effect and no effect, we downgraded one more level for imprecision. There was also evidence of heterogeneity (called inconsistency in the GRADE system; not to be confused with inconsistency in direct and indirect estimates in the context of network meta‐analysis) for symptomatic variceal rebleed. We downgraded one level for indirectness for symptomatic variceal rebleed as they may be incongruence for this outcome.
Mortality
Forty‐two trials (3369 participants) reported mortality (Westaby 1985a; Ampelas 1987; Bader 1987; Fleig 1988; Bonkovsky 1989; Jensen 1989; Parelon 1989; Sheen 1989; Bertoni 1990; Fornaciari 1990; Henderson 1990; Lundell 1990; Andreani 1991; Kanazawa 1991; Martin 1991; Rossi 1991; Dasarathy 1992; Dwivedi 1992; Ink 1992; Vinel 1992; Avgerinos 1993; Jiron 1993; Anonymous 1994; Bertoni 1994; Mckee 1994; Villanueva 1994; Cabrera 1996; Urbistondo 1996; Avgerinos 1997; Baroncini 1997; Jalan 1997; Sanyal 1997; Sauer 1997; Garcia‐Villarreal 1999; Argonz 2000; Sauer 2002; Viazis 2002; Romero 2006; Ahmad 2009; García‐Pagán 2009; Kong 2015; Kumar 2015). A total of 12 treatments were compared in these trials. There were 853 (25.8%) events in total. The median control group (endoscopic sclerotherapy) proportion was 22.5%.
Direct comparisons
Sclerotherapy plus nitrates had lower mortality than sclerotherapy: hazard ratio (HR) 0.18 (95% credible interval (CrI) 0.02 to 0.79; 1 trial; 76 participants; low‐certainty evidence).
There was no evidence of differences between the treatments in the remaining direct comparisons (i.e. the remaining direct comparisons were not statistically significant) as shown in Table 6 (very low‐certainty evidence).
Network meta‐analysis
All the trials were connected to the network. There was no evidence of inconsistency according to model fit and the 'between‐design' variance. However, there was evidence of inconsistency in one loop (made up of the three‐way comparison between beta‐blockers, portocaval shunt, and sclerotherapy) in the inconsistency factor plot. The random‐effects model was used because it was more conservative than the fixed‐effect model, even though the model fit was similar as fixed‐effect model. The 'between‐study variance' was 0.07 (95% CrI 0.00 to 0.27).
In the network meta‐analysis, in the following pairwise comparisons, the first intervention had lower mortality than the second intervention.
Sclerotherapy plus nitrates versus sclerotherapy: HR 0.19 (95% CrI 0.02 to 0.86); direct comparison HR 0.18 (95% CrI 0.02 to 0.79); 1 trial; 76 participants; moderate‐certainty evidence
Sclerotherapy plus nitrates versus variceal band ligation: HR 0.19 (95% CrI 0.02 to 0.97); no direct comparison; moderate‐certainty evidence
Sclerotherapy plus nitrates versus no active intervention: HR 0.15 (95% CrI 0.02 to 0.75); no direct comparison; moderate‐certainty evidence
Sclerotherapy plus nitrates versus portocaval shunt: HR 0.15 (95% CrI 0.02 to 0.79); no direct comparison; moderate‐certainty evidence
There was no evidence of differences between the treatments in the remaining comparisons in the network meta‐analysis (low‐certainty evidence). After excluding the trials including surgical portocaval shunt as one of their treatments (to assess whether the inconsistency in the inconsistency factor plot was due to the loop between beta‐blockers, portocaval shunt, and sclerotherapy), the network meta‐analysis results did not change. There was no further inconsistency in the inconsistency factor plot.
Health‐related quality of life
None of the trials reported health‐related quality of life.
Serious adverse events
None of the trials reported whether they used the ICH‐GCP 1997 definition of serious adverse events. We used the description of events as 'serious' or 'severe' adverse events or complications as serious adverse events.
Serious adverse events (number of participants)
Four trials (467 participants) reported serious adverse events (number of participants) (Sheen 1989; Dasarathy 1992; Ink 1992; Romero 2006). A total of six treatments were compared in these trials. There were 80 events in total (17.1%). The median control group proportion was 36.0%.
Direct comparisons
Variceal band ligation had lower serious adverse events (number of people) than sclerotherapy: odds ratio (OR) 0.19 (95% CrI 0.06 to 0.54; 1 trial; 100 participants; low‐certainty evidence).
Beta‐blockers plus sclerotherapy had higher serious adverse events (number of participants) than beta‐blockers: OR 2.75 (95% CrI 1.18 to 6.82; 1 trial; 131 participants; low‐certainty evidence).
There was no evidence of differences between the treatments in the remaining direct comparisons (i.e. the remaining direct comparisons were not statistically significant) (very low‐certainty evidence).
Network meta‐analysis
One trial was not connected to the network because it had treatments unconnected to network (Romero 2006); one trial was not connected to the network because it was the only trial for the comparison and had zero‐event in one of the intervention groups (Sheen 1989). The network had four connected treatments. There were no triangular or quadrangular loops; therefore, inconsistency was not checked. The fixed‐effect model was used because there was only one trial for each of the comparisons.
In the network meta‐analysis, variceal band ligation had lower serious adverse events (number of people) than sclerotherapy: OR 0.19 (95% CrI 0.06 to 0.53); direct comparison OR 0.19 (95% CrI 0.06 to 0.54); 1 trial; 100 participants; low‐certainty evidence; and beta‐blockers plus sclerotherapy had higher serious events (number of participants) than beta‐blockers: OR 2.74 (95% CrI 1.18 to 6.70); direct comparison OR 2.75 (95% CrI 1.18 to 6.82); 1 trial; 131 participants; very low‐certainty evidence.
There was no evidence of differences between the treatments in the remaining comparisons in the network meta‐analysis (very low‐certainty evidence).
Serious adverse events (number of events)
None of the trials reported serious adverse events (number of events).
Any adverse events
None of the trials reported whether they used the ICH‐GCP 1997 definition of any adverse events. We used the description of events as 'adverse events' or 'complications' as any adverse events.
Any adverse events (number of participants)
Eleven trials (895 participants) reported any adverse events (number of participants) (Jensen 1989; Sheen 1989; Mckee 1994; Villanueva 1994; Avgerinos 1997; Jalan 1997; Masliah 1997; Argonz 2000; García‐Pagán 2009; Kong 2015; Kumar 2015). A total of nine treatments were compared in these trials. There were 274 events in total (30.6%). The median control group proportion was 38.0%.
Direct comparisons
In the following direct comparisons, the first intervention had lower any adverse events (number of participants) than the second intervention.
Sclerotherapy versus no active intervention: OR 4.55 (95% CrI 1.16 to 20.00); 1 trial; 40 participants; low‐certainty evidence
Variceal band ligation versus beta‐blockers: OR 0.03 (95% CrI 0.00 to 0.21); 1 trial; 103 participants; low‐certainty evidence
TIPS versus variceal band ligation: OR 0.05 (95% CrI 0.01 to 0.25); 1 trial; 58 participants; low‐certainty evidence
In the following direct comparisons, the first intervention had higher any adverse events (number of participants) than the second intervention.
Beta‐blockers plus nitrates versus beta‐blockers: OR 2.34 (95% CrI 1.16 to 4.75); 2 trials; 181 participants; low‐certainty evidence
Beta‐blockers plus nitrates versus variceal band ligation: OR 68.37 (95% CrI 10.79 to 2071.44); 1 trial; 95 participants; low‐certainty evidence
Sclerotherapy plus variceal band ligation versus variceal band ligation: OR 6.27 (95% CrI 1.70 to 31.63); 1 trial; 80 participants; low‐certainty evidence
Beta‐blockers plus nitrates plus variceal band ligation versus beta‐blockers plus nitrates: OR 3.40 (95% CrI 1.78 to 6.71); 1 trial; 158 participants; low‐certainty evidence
There was no evidence of differences between the treatments in the remaining direct comparisons (i.e. the remaining direct comparisons were not statistically significant) as shown in Table 6 (very low‐certainty evidence).
Network meta‐analysis
One trial was not connected to the network because it was the only trial for the comparison and had zero‐events in one of the intervention groups (Sheen 1989). All treatments were connected. There was no evidence of inconsistency according to model fit or inconsistency factor plot. We were unable to obtain convergence for the 'between‐design' variance despite various measures (probably because of the sparse data). The random‐effects model was used because it was more conservative, even though the model fit was similar as fixed‐effect model. The 'between‐study variance' was 0.09 (95% CrI 0.00 to 2.71).
In the network meta‐analysis, in the following pairwise comparisons, the first intervention had lower any adverse events (number of participants) than the second intervention.
TIPS versus sclerotherapy: OR 0.02 (95% CrI 0.00 to 0.17); no direct comparison; low‐certainty evidence
Variceal band ligation versus beta‐blockers: OR 0.03 (95% CrI 0.00 to 0.29); direct comparison OR 0.03 (95% CrI 0.00 to 0.21); 1 trial; 103 participants; low‐certainty evidence
Beta‐blockers versus no active intervention: 56.49 (95% CrI 2.83 to 3016.94); no direct comparison; low‐certainty evidence
TIPS versus beta‐blockers: OR 0.00 (95% CrI 0.00 to 0.03); no direct comparison; low‐certainty evidence
TIPS versus variceal band ligation: OR 0.05 (95% CrI 0.00 to 0.35); direct comparison OR 0.05 (95% CrI 0.01 to 0.25); 1 trial; 58 participants; low‐certainty evidence
TIPS versus beta‐blockers plus sclerotherapy: OR 0.01 (95% CrI 0.00 to 0.19); no direct comparison; lo‐ certainty evidence
In the network meta‐analysis, in the following pairwise comparisons, the first intervention had higher any adverse events (number of participants) than the second intervention.
Beta‐blockers versus sclerotherapy: OR 11.86 (95% CrI 1.16 to 427.95); no direct comparison; low‐certainty evidence
Beta‐blockers plus nitrates versus sclerotherapy: OR 27.58 (95% CrI 2.79 to 981.42); no direct comparison; low‐certainty evidence
Beta‐blockers plus nitrates versus beta‐blockers plus sclerotherapy: OR 19.28 (95% CrI 1.26 to 920.58); no direct comparison; low‐certainty evidence
Beta‐blockers plus nitrates versus no active intervention: OR 133.4 (95% CrI 6.86 to 7215.6); no direct comparison; low‐certainty evidence
Beta‐blockers plus nitrates versus TIPS: OR 1474.4 (95% CrI 76.6 to 84120.0); no direct comparison; low‐certainty evidence
Beta‐blockers plus nitrates versus variceal band ligation: OR 69.76 (95% CrI 8.65 to 2199.53); direct comparison: OR 68.37 (95% CrI 10.79 to 2071.44); 1 trial; 95 participants; low‐certainty evidence
Beta‐blockers plus nitrates plus variceal band ligation versus sclerotherapy: OR 94.92 (95% CrI 6.85 to 4500.75); no direct comparison; low‐certainty evidence
Beta‐blockers plus nitrates plus variceal band ligation versus beta‐blockers: OR 7.96 (95% CrI 1.44 to 44.57); no direct comparison; low‐certainty evidence
Beta‐blockers plus nitrates plus variceal band ligation versus variceal band ligation: OR 239.61 (95% CrI 20.47 to 9701.15); no direct comparison; low‐certainty evidence
Beta‐blockers plus nitrates plus variceal band ligation versus beta‐blockers plus sclerotherapy: OR 66.22 (95% CrI 3.19 to 3944.19); no direct comparison; low‐certainty evidence
Beta‐blockers plus nitrates plus variceal band ligation versus no active intervention: OR 454.86 (95% CrI 17.78 to 31888.48); no direct comparison; low‐certainty evidence
Beta‐blockers plus nitrates plus variceal band ligation versus TIPS: OR 5084.74 (95% CrI 208.51 to 362217.45); no direct comparison; low‐certainty evidence
Beta‐blockers plus nitrates plus variceal band ligation versus sclerotherapy plus variceal band ligation: OR 38.40 (95% CrI 1.61 to 2221.64); no direct comparison; low‐certainty evidence
Sclerotherapy plus variceal band ligation versus variceal band ligation: OR 6.30 (95% CrI 1.11 to 47.18); direct comparison: OR 6.27 (95% CrI 1.70 to 31.63); 1 trial; 80 participants; low‐certainty evidence
Sclerotherapy plus variceal band ligation versus TIPS: OR 125.59 (95% CrI 9.11 to 2754.52); no direct comparison; low‐certainty evidence
There was no evidence of differences between the treatments in the remaining comparisons in the network meta‐analysis (very low‐certainty evidence).
Any adverse events (number of events)
Eleven trials (935 participants) reported any adverse events (number of events) (Kanazawa 1991; Martin 1991; Avgerinos 1993; Isaksson 1995; Cabrera 1996; Avgerinos 1997; Baroncini 1997; Sauer 1997; Sauer 2002; Romero 2006; García‐Pagán 2009). A total of 10 treatments were compared in these trials. There were 634 events in total (0.7 events per participant). The median control event rate was 0.581 events per participant.
Direct comparisons
In the following direct comparisons, the first intervention had lower any adverse events (number of events) than the second intervention.
Variceal band ligation versus sclerotherapy: rate ratio 0.40 (95% CrI 0.26 to 0.61); 2 trials; 188 participants; low‐certainty evidence
Beta‐blockers plus variceal band ligation versus TIPS: rate ratio 0.12 (95% CrI 0.06 to 0.22); 1 trial; 85 participants; low‐certainty evidence
Beta‐blockers plus nitrates plus variceal band ligation had higher any adverse events (number of events) than beta‐blockers plus nitrates: rate ratio 2.39 (95% CrI 1.51 to 3.89); 1 trial; 158 participants; low‐certainty evidence
There was no evidence of differences between the treatments in the remaining direct comparisons (i.e. the remaining direct comparisons were not statistically significant) as shown in Table 6 (very low‐certainty evidence). There was no evidence of differences in the remaining comparison not connected to the network: sclerotherapy plus variceal band ligation versus beta‐blockers plus nitrates: rate ratio 0.97 (95% CrI 0.55 to 1.70); 1 trial; 109 participants (very low‐certainty evidence).
Network meta‐analysis
Two trials were not connected to the network because they had treatments unconnected to network (Romero 2006; García‐Pagán 2009); one trial was not connected to the network because it was the only trial for the comparison and had zero‐events in one of the arms (Martin 1991). The network had six connected treatments. There was no evidence of inconsistency according to the inconsistency factor plot, but there was evidence was inconsistency according to model fit. We could not obtain convergence by design‐by‐treatment model. Fixed‐effect model was used as it had similar model fit and equivalent results as random‐effects model.
In the network meta‐analysis, in the following pairwise comparisons, the first intervention had lower any adverse events (number of events) than the second intervention.
Variceal band ligation versus sclerotherapy: rate ratio 0.40 (95% CrI 0.26 to 0.61); direct comparison: rate ratio 0.40 (95% CrI 0.26 to 0.61); 2 trials; 188 participants; moderate‐certainty evidence
Beta‐blockers plus variceal band ligation versus sclerotherapy: rate ratio 0.13 (95% CrI 0.06 to 0.28); no direct comparison; moderate‐certainty evidence
Beta‐blockers plus variceal band ligation versus variceal band ligation: rate ratio 0.33 (95% CrI 0.13 to 0.78); no direct comparison;moderate‐certainty evidence
Beta‐blockers plus variceal band ligation versus beta‐blockers plus sclerotherapy: rate ratio 0.14 (95% CrI 0.06 to 0.29); no direct comparison; moderate‐certainty evidence
Beta‐blockers plus variceal band ligation versus TIPS: rate ratio 0.12 (95% CrI 0.06 to 0.22); direct comparison: rate ratio 0.12 (95% CrI 0.06 to 0.22); 1 trial; 85 participants; moderate‐certainty evidence
Beta‐blockers plus variceal band ligation versus portocaval shunt: rate ratio 0.15 (95% CrI 0.04 to 0.51); no direct comparison; moderate‐certainty evidence.
In the network meta‐analysis, in the following pairwise comparisons, the first intervention had higher any adverse events (number of events) than the second intervention.
Beta‐blockers plus sclerotherapy versus variceal band ligation: rate ratio 2.30 (95% CrI 1.37 to 3.97); no direct comparison; moderate‐certainty evidence
TIPS versus variceal band ligation: rate ratio 2.74 (95% CrI 1.52 to 5.02); no direct comparison; moderate‐certainty evidence
There was no evidence of differences between the treatments in the remaining comparisons in the network meta‐analysis (low‐certainty evidence).
Liver transplantation
Six trials (588 participants) reported liver transplantation (Westaby 1985a; Villanueva 1994; Baroncini 1997; Sanyal 1997; Sauer 1997; García‐Pagán 2009). A total of seven treatments were compared in these trials. There were 21 events in total (3.6%). The median control group proportion was 1.9%.
Direct comparisons
There was no evidence of difference in any of the direct comparisons (i.e. there was no statistically significant difference in any of the comparisons) as shown in Table 6 (very low‐certainty evidence). There was no evidence of differences in the remaining comparison not connected to the network: beta‐blockers plus nitrates plus variceal band ligation versus beta‐blockers plus nitrates: HR 0.61 (95% CrI 0.07 to 3.90; 1 trial; 158 participants; very low‐certainty evidence).
Network meta‐analysis
One trial was not connected to the network because it had zero‐events in both arms (Westaby 1985a); one trial was not connected to the network because it had treatments unconnected to network (García‐Pagán 2009).The network had four connected treatments. There was no evidence of inconsistency according to model fit, inconsistency factor, and the 'between‐design' variance. The fixed‐effect model was used because there was only one trial for each of the comparisons. In the network meta‐analysis, there was no evidence of difference in any of the comparisons (very low‐certainty evidence).
Variceal rebleed
Symptomatic variceal rebleed
Seven trials (550 participants) reported symptomatic variceal rebleed (Sheen 1989; Henderson 1990; Urbistondo 1996; Baroncini 1997; Jalan 1997; Argonz 2000; Ahmad 2009). A total of nine treatments were compared in these trials. There were 141 (25.6%) events in total. The median control group proportion was 5.6%.
Direct comparisons
TIPS had lower symptomatic variceal rebleed than variceal band ligation: HR 0.12 (95% CrI 0.03 to 0.41; 1 trial; 58 participants; low‐certainty evidence).
There was no evidence of differences between the treatments in the remaining direct comparisons (i.e. the remaining direct comparisons were not statistically significant) as shown in Table 6 (very low‐certainty evidence).
Network meta‐analysis
All the trials were connected to the network. All treatments were connected. There was no evidence of inconsistency according to model fit and the 'between‐design' variance. However, there was evidence of inconsistency in one loop (made up of the three‐way comparison between beta‐blockers, portocaval shunt, and sclerotherapy in the inconsistency factor plot. The random‐effects model was used because it was more conservative and had better model fit. The 'between‐study variance' was 5.06 (95% CrI 0.30 to 22.71).
In the network meta‐analysis, there was no evidence of differences in any of the comparisons (very low‐certainty evidence). After excluding the trials including portocaval shunt as one of their treatments (to assess whether the inconsistency in the inconsistency factor plot was due to the loop between beta‐blockers, portocaval shunt, and sclerotherapy), the network meta‐analysis results did not change. We could not obtain an inconsistency factor plot since there was only study in each closed loop after the exclusion of the trials including portocaval shunt as one of their treatments.
Any variceal rebleed
Twenty‐two trials (1676 participants) reported any variceal rebleed (Ampelas 1987; Alexandrino 1988; Fleig 1988; Bonkovsky 1989; Jensen 1989; Lundell 1990; Martin 1991; Rossi 1991; Dasarathy 1992; Dwivedi 1992; Ink 1992; Vinel 1992; Avgerinos 1993; Anonymous 1994; Mckee 1994; Cabrera 1996; Sauer 1997; Garcia‐Villarreal 1999; Viazis 2002; Romero 2006; Kong 2015; Kumar 2015). A total of nine treatments were compared in these trials. There were 692 events in total (40.4%). The median control group proportion was 47.3%.
Direct comparisons
In the following direct comparisons, the first intervention had lower any variceal rebleed than the second intervention.
Beta‐blockers plus sclerotherapy versus beta‐blockers: HR 0.36 (95% CrI 0.18 to 0.70); 1 trial; 131 participants; low‐certainty evidence
Portocaval shunt versus beta‐blockers: HR 0.13 (95% CrI 0.03 to 0.37); 1 trial; 50 participants; low‐certainty evidence
TIPS versus beta‐blockers plus sclerotherapy: HR 0.20 (95% CrI 0.07 to 0.49); 1 trial; 83 participants; low‐certainty evidence
Beta‐blockers had higher any variceal rebleed than sclerotherapy: HR 1.68 (95% CrI 1.06 to 2.83); 6 trials; 420 participants; low‐certainty evidence.
There was no evidence of differences between the treatments in the remaining direct comparisons (i.e. the remaining direct comparisons were not statistically significant) as shown in Table 6 (very low‐certainty evidence).
Network meta‐analysis
All the trials were connected to the network. All treatments were connected. There was no evidence of inconsistency according to model fit, inconsistency factor plot, and the 'between‐design' variance. The random‐effects model was used because it was more conservative than the fixed‐effect model, even though the model fit was similar as fixed‐effect model. The 'between‐study variance' was 0.09 (95% CrI 0.00 to 0.48).
In the network meta‐analysis, in the following pairwise comparisons, the first intervention had lower any variceal rebleed than the second intervention.
Sclerotherapy versus no active intervention: HR: 0.62 (95% CrI 0.35 to 0.99); direct comparison HR 0.66 (95% CrI 0.11 to 3.13) 3 trials; 296 participants; moderate‐certainty evidence
Beta‐blockers plus sclerotherapy versus sclerotherapy: HR 0.60 (95% CrI 0.37 to 0.95); direct comparison HR 0.50 (95% CrI 0.07 to 2.96); 4 trials; 231 participants; moderate‐certainty evidence
TIPS versus sclerotherapy: HR 0.18 (95% CrI 0.08 to 0.38); direct comparison HR 0.22 (95% CrI 0.01 to 7.51); 2 trials; 109 participants; moderate‐certainty evidence
Portocaval shunt versus sclerotherapy: HR 0.21 (95% CrI 0.05 to 0.77); no direct comparison; moderate‐certainty evidence
Beta‐blockers plus sclerotherapy versus beta‐blockers: HR 0.37 (95% CrI 0.21 to 0.62); direct comparison HR 0.36 (95% CrI 0.18 to 0.70); 1 trial; 131 participants; moderate‐certainty evidence
TIPS versus beta‐blockers: HR 0.11 (95% CrI 0.05 to 0.25); no direct comparison; moderate‐certainty evidence
Portocaval shunt versus beta‐blockers: HR 0.13 (95% CrI 0.03 to 0.45); direct comparison HR 0.13 (95% CrI 0.03 to 0.37); 1 trial; 50 participants; moderate‐certainty evidence
Beta‐blockers plus sclerotherapy versus variceal band ligation: HR 0.37 (95% CrI 0.14 to 0.91); no direct comparison; moderate‐certainty evidence
Beta‐blockers plus sclerotherapy versus no active intervention: HR 0.37 (95% CrI 0.17 to 0.70); no direct comparison; moderate‐certainty evidence
TIPS versus variceal band ligation: HR 0.11 (95% CrI 0.04 to 0.33); no direct comparison; moderate‐certainty evidence
Portocaval shunt versus variceal band ligation: HR 0.13 (95% CrI 0.02 to 0.57); no direct comparison; moderate‐certainty evidence
TIPS versus beta‐blockers plus sclerotherapy: HR 0.31 (95% CrI 0.14 to 0.65); direct comparison HR 0.20 (95% CrI 0.07 to 0.49); 1 trial; 83 participants; moderate‐certainty evidence
TIPS versus no active intervention: HR 0.11 (95% CrI 0.04 to 0.27); no direct comparison; moderate‐certainty evidence
Portocaval shunt versus no active intervention: HR 0.13 (95% CrI 0.03 to 0.50); no direct comparison; moderate‐certainty evidence
Portocaval shunt versus beta‐blockers plus nitrates: HR 0.10 (95% CrI 0.02 to 0.47); no direct comparison; moderate‐certainty evidence
In the network meta‐analysis, in the following pairwise comparisons, the first intervention had higher any variceal rebleed than the second intervention.
Beta‐blockers versus sclerotherapy: HR 1.62 (95% CrI 1.14 to 2.38); direct comparison HR 1.68 (95% CrI 1.06 to 2.83); 6 trials; 420 participants; moderate‐certainty evidence
Beta‐blockers plus nitrates versus beta‐blockers plus sclerotherapy: HR 3.54 (95% CrI 1.23 to 10.82); no direct comparison; moderate‐certainty evidence
Beta‐blockers plus nitrates versus TIPS: HR 11.57 (95% CrI 3.43 to 41.93); no direct comparison; moderate‐certainty evidence
Sclerotherapy plus variceal band ligation versus TIPS: HR 9.98 (95% CrI 2.11 to 50.00); no direct comparison; moderate‐certainty evidence
Sclerotherapy plus variceal band ligation versus portocaval shunt: HR 8.88 (95% CrI 1.37 to 63.43); no direct comparison; moderate‐certainty evidence
There was no evidence of differences between the treatments in the remaining comparisons in the network meta‐analysis (low‐certainty evidence).
Other features of decompensation
Eight trials (416 participants) reported other features of decompensation (Alexandrino 1988; Jensen 1989; Sheen 1989; Cabrera 1996; Jalan 1997; Sauer 1997; Cennamo 1998; Garcia‐Villarreal 1999). A total of seven treatments were compared in these trials. There were 123 events in total (0.3 events per participant). The decompensation events included liver failure, hepatic encephalopathy, and spontaneous bacterial peritonitis (secondary to ascites). The median control event rate was 0.292 events per participant.
Direct comparisons
In the following direct comparisons, the first intervention had lower other features of decompensation than the second intervention.
Beta‐blockers versus sclerotherapy: rate ratio 2.37 (95% CrI 1.35 to 4.67); 1 trial; 65 participants; low‐certainty evidence
TIPS versus sclerotherapy: rate ratio 2.30 (95% CrI 1.20 to 4.65); 2 trials; 109 participants; low‐certainty evidence
TIPS versus beta‐blockers plus sclerotherapy: rate ratio 4.79 (95% CrI 1.53 to 18.82); 1 trial; 83 participants; low‐certainty evidence
There was no evidence of differences between the treatments in the remaining direct comparisons (i.e. the remaining direct comparisons were not statistically significant) as shown in Table 6 (very low‐certainty evidence).
Network meta‐analysis
Two trials were not connected to the network because they were the only trials for the comparison and had zero‐events in one of the arms (Jensen 1989; Sheen 1989). The network had six connected treatments. There were no triangular or quadrangular loops; therefore, inconsistency was not checked. The fixed‐effect model was used because it had equivalent results and model fit as random‐effects model.
Beta‐blockers plus sclerotherapy had lower other features of decompensation than beta‐blockers: rate ratio 0.18 (95% CrI 0.03 to 0.82); no direct comparison; low‐certainty evidence
In the network meta‐analysis, in the following pairwise comparisons, the first intervention had higher other features of decompensation than the second intervention.
Beta‐blockers versus sclerotherapy: rate ratio 2.40 (95% CrI 1.35 to 4.55); direct comparison rate ratio 2.37 (95% CrI 1.35 to 4.67); 1 trial; 65 participants; low‐certainty evidence
TIPS versus sclerotherapy: rate ratio 2.27 (95% CrI 1.19 to 4.59); direct comparison rate ratio 2.30 (95% CrI 1.20 to 4.65); 2 trials; 109 participants; low‐certainty evidence
TIPS versus beta‐blockers plus sclerotherapy: rate ratio 5.01 (95% CrI 1.57 to 23.45); direct comparison rate ratio 4.79 (95% CrI 1.53 to 18.82); 1 trial; 83 participants; low‐certainty evidence
There was no evidence of differences between the treatments in the remaining comparisons in the network meta‐analysis (very low‐certainty evidence).
Exploratory outcomes
Length of hospital stay
Six trials (413 participants) reported length of hospital stay for all admissions until maximal follow‐up) (Kanazawa 1991; Isaksson 1995; Cabrera 1996; Jalan 1997; Garcia‐Villarreal 1999; García‐Pagán 2009). The median control group mean was 29.9 days per participant. A total of seven treatments were compared in these trials.
Direct comparisons
There was no evidence of difference in any of the direct comparisons (i.e. there was no statistically significant difference in any of the comparisons) as shown in Table 6. There was no evidence of difference in the remaining comparison not connected to the network: beta‐blockers plus nitrates plus variceal band ligation versus beta‐blockers plus nitrates (mean difference (MD) 1.01 days (95% CrI ‐4.77 to 6.64)).
Network meta‐analysis
One trial was not connected to the network because it had treatments unconnected to network (García‐Pagán 2009). The network had five connected treatments. There were no triangular or quadrangular loops; therefore, inconsistency was not checked. The fixed‐effect model was used because it had equivalent results and model fit as random‐effects model.
In the network meta‐analysis, beta‐blockers plus sclerotherapy had shorter length of hospital stay than variceal band ligation: MD ‐20.19 days (95% CrI ‐36.57 to ‐3.92); no direct evidence.
There was no evidence of differences between the treatments in the remaining comparisons in the network meta‐analysis. In the sensitivity analysis of excluding the two trials in which the standard deviation was imputed, there was no evidence of difference in any of the direct comparisons or network meta‐analysis.
Work days lost
None of the trials reported work days lost.
Treatment costs
Two trials (103 participants) reported treatment costs (Isaksson 1995; Jalan 1997). A total of four treatments were compared in these trials. One trial reported treatment costs in USD (Isaksson 1995). One trial reported treatment costs in pound sterling (Jalan 1997). 'Pound sterling' was converted to USD using Purchasing Power Parities and the conversion rates on 10 March 2020). None of the trials reported information to calculate the standard deviation.
The mean treatment costs reported in the trials were as follows.
Portocaval shunt (USD 12049) versus sclerotherapy (USD 12027) (Isaksson 1995)
TIPS (USD 9958) versus variceal band ligation (USD 11894) in the two trials (Jalan 1997).
Subgroup analysis
We did not perform any subgroup analysis. This is because none of the trials were at low risk of bias, there were no separate outcome data based on clinical features such as high risk of bleeding, other features of decompensation or aetiology for cirrhosis, and none of the trial authors clearly stated whether they used ICH‐GCP 1997 for defining serious adverse events or any adverse events.
Sensitivity analysis
'Best‐worst' and 'worst‐best' scenario analyses
We performed the 'best‐worst' and 'worst‐best' scenario analyses for the sensitivity analysis related to missing outcome data. There were changes to interpretation of the results for the following analyses in the following outcomes. The 'main analysis' refers to results without any imputation of data.
Mortality
-
Sclerotherapy plus nitrates versus beta‐blockers:
main analysis: no evidence of difference between groups
worst‐best analysis: no evidence of difference between groups
best‐worst analysis: lower in sclerotherapy plus nitrates than beta‐blockers
-
Sclerotherapy plus nitrates versus variceal band ligation:
main analysis: lower in sclerotherapy plus nitrates than variceal band ligation
worst‐best analysis: no evidence of difference between groups
best‐worst analysis: lower in sclerotherapy plus nitrates than variceal band ligation
-
No active intervention versus beta‐blockers plus sclerotherapy:
main analysis: no evidence of difference between groups
worst‐best analysis: higher in no active intervention than beta‐blockers plus sclerotherapy
best‐worst analysis: no evidence of difference between groups
-
Sclerotherapy plus nitrates versus TIPS:
main analysis: no evidence of difference between groups
worst‐best analysis: no evidence of difference between groups
best‐worst analysis: lower in sclerotherapy plus nitrates than TIPS
Any variceal rebleed
-
No active intervention versus sclerotherapy:
main analysis: higher in no active intervention than sclerotherapy
worst‐best analysis: no evidence of difference between groups
best‐worst analysis: higher in no active intervention than sclerotherapy
Therefore, these results should be interpreted with caution, as they are susceptible to attrition bias resulting from post‐randomisation dropouts. There were no changes to interpretation of the results for the remaining analyses or outcomes. These outcomes and comparisons are therefore robust to post‐randomisation dropouts.
Imputation of standard deviation
We did not perform any imputation of standard deviation.
Assessment of reporting biases
Since there was no meaningful way in which to rank these studies (i.e. there was no specific change in the risk of bias in the studies, sample size, or the control group used over time), we were unable to perform the comparison‐adjusted funnel plot. Mortality was reported in most trials. However, other important outcomes such as adverse events were not reported in some trials, indicating the possibility of reporting biases.
Post hoc analyses
Following comments from clinical experts who commented that the baseline risk in the control group would have changed over the time, we performed the following analyses: baseline risk‐adjusted network meta‐analyses for mortality and any variceal rebleed, the two outcomes reported by most trials and the outcomes that determine whether an outcomes should be used. We also analysed a subset of trials published from 2000 year onwards because of the potential changes in baseline risk.
Since we could not explain the reason for the recommendations of the major gastroenterological associations in recommending combination of beta‐blockers plus variceal band ligation over beta‐blockers plus endoscopic sclerotherapy (for which there is moderate‐certainty evidence that there is a decrease in 'any variceal bleed' by using a combination of beta‐blockers plus sclerotherapy versus sclerotherapy alone or beta‐blockers alone and low‐certainty evidence that there is a decrease in 'any variceal rebleed' by using a combination of beta‐blockers plus sclerotherapy versus variceal band ligation alone), we explored whether we could establish that there is no difference in effect when endoscopic sclerotherapy or variceal band ligation are added to beta‐blockers. The answer for this question can be explored by a component network meta‐analysis approach where it is possible to assess the contribution of adding a second treatment (in this case, endoscopic sclerotherapy or variceal band ligation) to an already existing treatment (in this case, beta‐blockers) ('main effects') and assess the interaction between the additional treatment and the existing treatment ('interaction effects') (Welton 2009; Freeman 2018). However, this requires that there is at least one trial that compares beta‐blockers plus variceal band ligation with either beta‐blockers plus sclerotherapy or beta‐blockers alone to be included in the network. In the absence of any such trials, we were unable to establish that there is no difference in effect when endoscopic sclerotherapy is replaced by variceal band ligation when used in combination with beta‐blockers.
Baseline‐risk adjusted analysis
We could not obtain convergence for the baseline‐risk adjusted model for either mortality or any variceal rebleed.
Subset of trials published from the year 2000 onwards
Mortality
There was no evidence of differences in any of the comparisons.
Any variceal bleed
There was no evidence of differences in any of the comparisons.
Discussion
Summary of main results
We performed a systematic review and network meta‐analysis of the common treatments used for secondary prevention of oesophageal variceal bleeding in people with oesophageal varices due to liver cirrhosis. A total of 48 trials, including a total of 3526 participants, were included in this review. A total of 12 interventions were compared in these trials. A total of 46 trials including 3442 participants were included for one or more comparisons of this review (Westaby 1985a; Ampelas 1987; Bader 1987; Alexandrino 1988; Fleig 1988; Bonkovsky 1989; Jensen 1989; Parelon 1989; Sheen 1989; Bertoni 1990; Fornaciari 1990; Henderson 1990; Lundell 1990; Andreani 1991; Kanazawa 1991; Martin 1991; Rossi 1991; Dasarathy 1992; Dwivedi 1992; Ink 1992; Vinel 1992; Avgerinos 1993; Jiron 1993; Anonymous 1994; Bertoni 1994; Mckee 1994; Villanueva 1994; Isaksson 1995; Cabrera 1996; Urbistondo 1996; Avgerinos 1997; Baroncini 1997; Jalan 1997; Masliah 1997; Sanyal 1997; Sauer 1997; Cennamo 1998; Garcia‐Villarreal 1999; Argonz 2000; Sauer 2002; Viazis 2002; Romero 2006; Ahmad 2009; García‐Pagán 2009; Kong 2015; Kumar 2015).
Overall, 22.5% of the trial participants who received endoscopic sclerotherapy died during the follow‐up period, ranging from two months to 61 months. Based on moderate‐certainty evidence, sclerotherapy plus nitrates had lower mortality than sclerotherapy (HR 0.19; 95% CrI 0.02 to 0.86; direct comparison HR 0.18; 95% CrI 0.02 to 0.78; 1 trial; 76 participants), and sclerotherapy plus nitrates had lower mortality than variceal band ligation (HR 0.19; 95% CrI 0.02 to 0.97; no direct comparison), no active intervention (HR 0.15; 95% CrI 0.02 to 0.75; no direct comparison), and portocaval shunt (HR 0.15; 95% CrI 0.02 to 0.79; no direct comparison). However, this is driven by the results of one small trial at high risk of bias, which have not been reproduced because this was the only trial on the comparison. While there is guidance to downgrade evidence when there is heterogeneity between results (Guyatt 2011b), this assessment needs a minimum of two trials. There was no evidence of heterogeneity in the network meta‐analysis. The results were robust to the exclusion of the loop which caused inconsistency. Therefore, the evidence was not downgraded for incongruence. While we followed the GRADE methodology and arrived at moderate‐certainty evidence, we consider that there is considerable uncertainty to conclude about the effectiveness of sclerotherapy plus nitrates compared to sclerotherapy on mortality, driven by the effect estimates of a single small trial at high risk of bias.
None of the trials reported health‐related quality of life. Based on low‐certainty evidence, serious events (number of participants) were lower in variceal band ligation than in sclerotherapy (OR 0.19; 95% CrI 0.06 to 0.54; direct comparison; 1 trial; 100 participants) and more in beta‐blockers plus sclerotherapy than in beta‐blockers (OR 2.75; 95% CrI 1.18 to 6.82; direct comparison; 1 trial; 131 participants).
Based on low‐certainty evidence, the adverse events (number of participants) were different across many comparisons; however, these differences are due to very small trials at high risk of bias showing large differences in some comparisons leading to many differences despite absence of direct evidence. Similarly, based on low‐certainty evidence, the adverse events (number of events) were different across many comparisons; however, these differences are due to very small trials at high risk of bias showing large differences in some comparisons leading to many differences despite absence of direct evidence.
Based on low‐certainty evidence, symptomatic variceal rebleed was lower in TIPS than in variceal band ligation (HR 0.12; 95% CrI 0.03 to 0.41; 1 trial; 58 participants).
Based on moderate‐certainty evidence, any variceal rebleed was lower in sclerotherapy than in no active intervention (HR 0.62; 95% CrI 0.35 to 0.99; direct comparison HR 0.66; 95% CrI 0.11 to 3.13; 3 trials; 296 participants), beta‐blockers plus sclerotherapy than sclerotherapy (HR 0.60; 95% CrI 0.37 to 0.95; direct comparison HR 0.50; 95% CrI 0.07 to 2.96; 4 trials; 231 participants), TIPS than sclerotherapy (HR 0.18; 95% CrI 0.08 to 0.38; direct comparison HR 0.22; 95% CrI 0.01 to 7.51; 2 trials; 109 participants), portocaval shunt than in sclerotherapy (HR 0.21; 95% CrI 0.05 to 0.77); no direct comparison), beta‐blockers plus sclerotherapy than beta‐blockers (HR 0.37; 95% CrI 0.21 to 0.62; direct comparison HR 0.36; 95% CrI 0.18 to 0.70; 1 trial; 131 participants), TIPS than beta‐blockers (HR 0.11; 95% CrI 0.05 to 0.25; no direct comparison), portocaval shunt than beta‐blockers (HR 0.13; 95% CrI 0.03 to 0.45; direct comparison HR 0.13; 95% CrI 0.03 to 0.37; 1 trial; 50 participants), TIPS than variceal band ligation (HR 0.11; 95% CrI 0.04 to 0.33; no direct comparison), TIPS than no active intervention (HR 0.11; 95% CrI 0.04 to 0.27; no direct comparison), beta‐blockers plus sclerotherapy than variceal band ligation (0.37; 95% CrI 0.14 to 0.91; no direct comparison), TIPS versus beta‐blockers plus sclerotherapy (HR 0.31; 95% CrI 0.14 to 0.65; direct comparison HR 0.20; 95% CrI 0.07 to 0.49; 1 trial; 83 participants), beta‐blockers plus sclerotherapy than no active intervention (0.37; 95% CrI 0.17 to 0.70; no direct comparison), portocaval shunt than variceal band ligation (HR 0.13; 95% CrI 0.02 to 0.57; no direct comparison), portocaval shunt than no active intervention (HR 0.13; 95% CrI 0.03 to 0.50; no direct comparison), and portocaval shunt than beta‐blockers plus nitrates (HR 0.10; 95% CrI 0.02 to 0.47; no direct comparison). Based on moderate‐certainty evidence, any variceal rebleed was higher in beta‐blockers plus nitrates than beta‐blockers plus sclerotherapy (HR 3.54; 95% CrI 1.23 to 10.82; no direct comparison), beta‐blockers plus nitrates than TIPS (HR 11.57; 95% CrI 3.43 to 41.93; no direct comparison), sclerotherapy plus variceal band ligation than TIPS (HR 9.98; 95% CrI 2.11 to 50.00; no direct comparison), and sclerotherapy plus variceal band ligation than portocaval shunt (HR 8.88; 95% CrI 1.37 to 63.43; no direct comparison).
Overall, the key information is that there is no evidence of difference in 'any' oesophageal variceal rebleed between variceal band ligation and sclerotherapy, nor was there any trial comparing 'any' variceal rebleed between beta‐blockers plus variceal band ligation versus sclerotherapy or variceal band ligation. The only trial in which there was a difference in mortality (sclerotherapy plus nitrates had lower mortality than sclerotherapy (HR 0.18; 95% CrI 0.02 to 0.78; 1 trial; 76 participants)) did not report 'any' oesophageal variceal rebleed (Bertoni 1994).
Based on very low‐certainty evidence, other features of decompensation were lower in beta‐blockers plus sclerotherapy than beta‐blockers (rate ratio 0.18; 95% CrI 0.03 to 0.82; no direct comparison). Based on very low‐certainty evidence, other features of decompensation were higher in beta‐blockers than sclerotherapy (rate ratio 2.40; 95% CrI 1.35 to 4.55; direct comparison: rate ratio 2.37; 95% CrI 1.35 to 4.67; 1 trial; 65 participants), TIPS than sclerotherapy (rate ratio 2.27; 95% CrI 1.19 to 4.59; direct comparison: rate ratio 2.30; 95% CrI 1.20 to 4.65; 2 trials; 109 participants), and TIPS than beta‐blockers plus sclerotherapy (rate ratio 5.01; 95% CrI 1.57 to 23.45; direct comparison rate ratio 4.79; 95% CrI 1.53 to 18.82; 1 trial; 83 participants). Hepatic encephalopathy events were the major other decompensation events in the TIPS group.
The evidence indicates considerable uncertainty about the effect of the interventions in the remaining comparisons.
The weighted median mortality in the sclerotherapy was 22.5% up to about five years. The sample size required to detect a relative risk reduction of 20% in the experimental group, with type I error of 5%, and type II error of 20% is 2402 participants. The prevalence of oesophageal varices varies between 40% and 95% in people with cirrhosis (Chawla 2012; McCarty 2017). Approximately 15% to 20% of people with oesophageal varices bleed in about one to three years (Gluud 2012; Qi 2015; Roccarina 2020). Therefore, it is very much possible to power studies in this population based on mortality.
In terms of population, it is probably better to perform different trials in people with oesophageal variceal bleeding based on other features of decompensation: one trial in people with ascites but without history of hepatic encephalopathy, and another in those without ascites. This is because the interventions to be compared are likely to be different in these two groups of patients. In people with history of oesophageal variceal bleeding and ascites, but without a history of encephalopathy, TIPS can be considered the intervention. This is because TIPS may decrease rebleeding (as shown by the above summary) and may increase the resolution of ascites compared to paracentesis plus fluid replacement (Benmassaoud 2020), but may increase hepatic encephalopathy as shown by this review and supported by other studies (Saab 2006; Zhou 2019). The control group in such a trial should ideally be beta‐blockers plus sclerotherapy on which plenty of trials have been conducted. But considering that it may be difficult to conduct such a trial, use of variceal band ligation as control group is probably also acceptable. The use of beta‐blockers in ascites is controversial (Njei 2016). Therefore, probably the most acceptable control group is endoscopic treatment alone.
In people without ascites, the interventions to be compared can be beta‐blockers plus sclerotherapy, variceal band ligation alone, and beta‐blockers plus variceal band ligation as most of the evidence on the effectiveness of treatment in preventing rebleeding relates to beta‐blockers plus sclerotherapy, while the variceal band ligation is associated with fewer adverse events with the potential to give equivalent results as sclerotherapy. TIPS is another option as an intervention in people without hepatic encephalopathy.
Among the ongoing trials that address the above comparisons, NCT00966082 compares variceal band ligation plus beta‐blockers versus variceal band ligation with an estimated recruitment target of 180 participants; NCT02477384 compares TIPS versus variceal band ligation plus beta‐blockers. The estimated recruitment target for this trial is 72 participants. These numbers seem too low. Therefore, further randomised clinical trials are necessary.
Future trials should assess the health‐related quality of life as this is an outcome that is considered as important by patients.
Overall completeness and applicability of evidence
There did not seem to be any restrictions based on the aetiology or the presence of other features of decompensation in the trials that provided this information, particularly for the main interventions compared in this review. Therefore, the results of the study are applicable in people with cirrhosis resulting from varied aetiologies having oesophageal varices with a previous history of bleeding.
The findings of this review are applicable only for adults with cirrhosis with oesophageal varices and are not applicable to children, people (of any age group) with gastric varices, or people with oesophageal varices due to other causes of portal hypertension such as portal vein thrombosis or schistosomiasis. The review is also not applicable in people who undergo liver transplantation. We did not find any trials comparing liver transplantation with one of these treatments; therefore, the review does provide any information about comparison of any of these treatments with liver transplantation.
Quality of the evidence
The overall certainty of evidence varied between moderate, low, or very low. One of the main reasons for this was the unclear or high risk of bias in all the trials. It is possible to perform trials at low risk of bias in certain comparisons: randomisation can be performed using standard methods, for example, web‐based central randomisation; an intention‐to‐treat analysis can be performed; and a protocol should be published prior to recruitment. However, blinding of healthcare providers and participants may not be possible if endoscopic treatments or TIPS are used as one of the interventions. However, it is possible to obtain low risk of performance bias by outlining the protocol clearly for additional treatments and hospital admissions. Outcome assessor blinding can be achieved for all comparisons by use of placebo (in pharmacological intervention trials) or a second team to assess the outcomes. If that is not possible, using clear highly reproducible criteria for outcome definitions can decrease detection bias.
Another major reason for the decreased certainty of evidence was imprecision. While some network meta‐analyses had sufficient numbers of events, none of the direct comparisons had adequate sample size. As a result, the credible intervals overlapped clinically significant benefits and clinically significant harms for most comparisons. Outcomes from ongoing trials can probably decrease the imprecision.
We used clinical outcomes; therefore, there is no issue of indirectness due to outcomes. There was no suggestion that the potential effect modifiers were systematically different across comparisons (i.e. there was no concern about the transitivity assumption) for most outcomes. However, one cannot rule out inconsistency ('incoherence' according to GRADE terminology) despite finding no evidence of this in most analyses.
There was no meaningful way to rank these studies (i.e. there was no specific change in the risk of bias in the studies, sample size, or the control group used over time); we have completed a thorough search for studies on effectiveness. However, different sets of trials were included for different outcomes: while 90% of trials reported mortality, only around 10% of trials reported serious adverse events adequately; only around 50% of trials reported variceal rebleed adequately; and only around 15% of trials described other decompensation events. These are outcomes which would have been recorded in trials of this nature, but were not reported. This may suggest reporting bias for these outcomes.
Potential biases in the review process
We selected a range of databases to search without using any language restrictions and conducted the network meta‐analysis according to NICE DSU (National Institute for Health and Care Excellence Decision Support Unit) guidance. In addition, we have analysed using the fixed‐effect model and random‐effects model and assessed and reported inconsistency whenever possible. These are the strengths of the review process.
We have excluded studies that compared variations in duration or dose in the different interventions. Hence, this review does not provide information on whether one variation is better than another.
All the trials were at high risk of bias and there was significant uncertainty in the ranking. Therefore, we could not rank the interventions in the order of effectiveness. There were sparse events for some interventions for adverse events resulting in very wide credible intervals. Therefore, direct comparisons are more reliable for these comparisons.
The potential effect modifiers in the trials that reported them were broadly similar across comparisons. The results of direct comparisons and indirect comparisons were similar for the most outcomes where we could assess this. Therefore, the concern about the transitivity assumption is low. However, this cannot be ruled out.
We included only randomised clinical trials which are known to focus mostly on benefits and do not collect and report harms in a detailed manner. A significant effort is required to identify non‐randomised studies that reported on harm. It is also challenging to assess the risk of bias in those studies. If the ongoing trials result in adequate power to find meaningful differences in mortality, a systematic review on adverse events from observational studies will likely be unnecessary.
We included the trials without applying any restrictions based on publication date. The baseline risk may have changed over time. Therefore, we attempted a post hoc analysis adjusting for baseline risk, which did not converge and performed an analysis including only trials published from 2000 onwards, which showed no evidence that any of the interventions was better than other interventions .
Agreements and disagreements with other studies or reviews
This is the first network meta‐analysis on different secondary prevention interventions as first‐line therapy in people with a previous history of oesophageal variceal bleeding. Our inclusion criteria are different from the other recent systematic reviews on secondary prevention treatments (Brand 2018; Dwinata 2019). In both systematic reviews, there was no restriction based on the previous site of variceal bleeding (Brand 2018; Dwinata 2019), while we restricted to previous history of oesophageal varices. In addition, the participants were refractory to endoscopic therapy in most trials (Brand 2018). Therefore, our conclusions differ from these reviews.
We found no evidence to support the recommendations of the major associations including AASLD (American Association for the Study of Liver Diseases), Baveno Consensus Workshop, BSG (British Society of Gastroenterology), EASL (European Association for the Study of the Liver) to use beta‐blockers plus variceal band ligation for secondary prevention of bleeding from oesophageal varices (de Franchis 2015; Tripathi 2015; Garcia‐Tsao 2017; EASL 2018). Moderate‐ or low‐certainty evidence in our review suggested that serious adverse events (number of participants) was lower in variceal band ligation than sclerotherapy, but there is considerable uncertainty in the results of the effectiveness of these treatments compared to endoscopic sclerotherapy.
To find out if variceal band ligation might have been considered to be equivalent to sclerotherapy despite the lack of power to rule out differences in effect between them, we performed a component network meta‐analysis. Our attempt at component network meta‐analysis also demonstrates that it is not possible to establish that there is no difference in effect when endoscopic sclerotherapy is replaced by variceal band ligation when used in combination with beta‐blockers using currently available information. Therefore, the reasons for the differences between our systematic review and clinical practice guidelines can be speculative at best, and we have avoided such speculations.
Authors' conclusions
Implications for practice.
The evidence indicates considerable uncertainty about the effects of the interventions on mortality. Variceal band ligation might result in fewer serious adverse events than sclerotherapy. Transjugular intrahepatic portosystemic shunt (TIPS) might result in a large decrease in symptomatic rebleed than variceal band ligation. Sclerotherapy probably results in fewer 'any' variceal rebleeding than no active intervention. Beta‐blockers plus sclerotherapy and TIPS probably result in fewer 'any' variceal rebleeding than sclerotherapy. Beta‐blockers alone and TIPS may result in more other compensation events than sclerotherapy. The evidence indicates considerable uncertainty about the effect of the interventions in the remaining comparisons.
Implications for research.
Further well‐designed randomised clinical trials are necessary. Some aspects of the design of the randomised clinical trials are as follows.
Study design Parallel, randomised clinical trial.
Participants People with liver cirrhosis and history of bleeding from oesophageal varices.
Interventions/control
In those with ascites and no hepatic encephalopathy: TIPS versus sclerotherapy or variceal band ligation
In those without ascites: beta‐blockers plus sclerotherapy, variceal band ligation alone, and beta‐blockers plus variceal band ligation. TIPS can also be considered in those without hepatic encephalopathy.
Outcomes
Primary outcome: mortality.
Secondary outcomes: health‐related quality of life, rebleeding, decompensation events, adverse events, transfusion requirements, and resource utilisation measures including length of hospital stay, costs.
Minimum length of follow‐up: three years.
Sample size
For a simple two‐arm parallel randomised clinical trial, the sample size required to detect or reject a relative risk reduction of 20% in the experimental group from the control group proportion of 22,5% mortality, with type I error of 5%, and type II error of 20%, 2402 participants are required.
Other aspects
Trials need to be conducted and reported according to the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) statement (Chan 2013) and CONSORT statement (Schulz 2010).
What's new
| Date | Event | Description |
|---|---|---|
| 6 September 2022 | Amended | An error about the reason for exclusion of the study Sauerbruch 2015 was corrected. |
History
Protocol first published: Issue 9, 2018 Review first published: Issue 3, 2021
Notes
The methods section of this protocol is based on a standard Cochrane Hepato‐Biliary Group template incorporating advice by the Complex Reviews Support Unit for a network meta‐analysis protocol (Best 2018).
Acknowledgements
We acknowledge the help and support of the Cochrane Hepato‐Biliary Group, Cochrane Central Editorial Unit, and copy editors. The authors would also like to thank the peer reviewers listed below who provided comments to improve the review.
Peer reviewers of protocol: Erwin Biecker, Germany; David Hoaglin, USA Peer reviewers of review: Erwin Biecker, Germany; David Hoaglin, USA; Roberto de Franchis, Italy; Sarika Parambath, Australia Contact Editor: Christian Gluud, Denmark Sign‐off Editor: Goran Hauser, Croatia Statistical Editor (Methods Support Unit, Editorial & Methods Department): Kerry Dwan, UK Cochrane Abdominal and Endocrine Network Editor: Rachel Richardson; UK
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in the Copenhagen Trial Unit, Centre for Clinical Intervention Research, the Capital Region of Denmark, Rigshospitalet, Copenhagen, Denmark.
This project was funded by the National Institute for Health Research (NIHR) Systematic Reviews Programme (project number 16/114/17) and was supported by the Complex Reviews Support Unit, also funded by the National Institute for Health Research (project number 14/178/29).
Department of Health Disclaimer
The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the 16/114/17 or 14/178/29 Programmes, the NIHR, the NHS, or the Department of Health.
Danish State and The Copenhagen Trial Unit Disclaimer
The views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or The Copenhagen Trial Unit.
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| Central Register of Controlled Trials (CENTRAL) in the Cochrane Library | Issue 12, 2019 | #1 MeSH descriptor: [Esophageal and Gastric Varices] explode all trees #2 *esophageal varic* #3 #1 or #2 |
| MEDLINE Ovid | January 1947 to December 2019 | 1. exp "Esophageal and Gastric Varices"/ 2. *esophageal varic*/.ti,ab. 3. 1 or 2 4. randomized controlled trial.pt. 5. controlled clinical trial.pt. 6. randomized.ab. 7. placebo.ab. 8. drug therapy.fs. 9. randomly.ab. 10. trial.ab. 11. groups.ab. 12. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 13. exp animals/ not humans.sh. 14. 12 not 13 15. 3 and 14 |
| Embase Ovid | January 1974 to December 2019 | 1. exp esophagus varices/ 2. *esophageal varic*/.ti,ab. 3. 1 or 2 4. exp crossover‐procedure/ or exp double‐blind procedure/ or exp randomized controlled trial/ or single‐blind procedure/ 5. (((((random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or double*) adj blind*) or single*) adj blind*) or assign* or allocat* or volunteer*).af. 6. 4 or 5 7. 3 and 6 |
| Science Citation Index Expanded (Web of Science) | January 1945 to December 2019 | #1 TS= (*esophageal varic*) #2 TS=(random* OR rct* OR crossover OR masked OR blind* OR placebo* OR meta‐analysis OR systematic review* OR meta‐analys*) |
| World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/Default.aspx) | December 2019 | Condition: Esophageal Varices |
| ClinicalTrials.gov | December 2019 | Interventional Studies | Esophageal Varices |
| European Medical Agency (www.ema.europa.eu/ema/) and US Food and Drug Administration (www.fda.gov) | March 2020 | Esophageal Varices AND random |
Appendix 2. Data
This table is too wide to be displayed in RevMan. This table can be found here.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmad 2009.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Pakistan
Period of recruitment: 2003‐2005
Number randomised: 160
Post‐randomisation dropouts: 10 (6.3%)
Revised sample size: 150
Reasons for post‐randomisation dropouts: intolerance to drug (8), lost to follow‐up (2)
Average age (years): 52
Females: 49 (32.7%)
Other features of decompensation: 30 (20.0%)
Alcohol‐related cirrhosis: 1 (0.7%)
Viral‐related cirrhosis: 149 (99.3%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: 0 (0.0%)
Other inclusion/exclusion criteria: Exclusion: previous endoscopic or drug therapy; any contraindication to either treatment; bleeding gastric varices or gastropathy; advanced hepatocellular carcinoma, acute on chronic liver disease or any other debilitating disease |
|
| Interventions | Group 1: beta‐blockers plus nitrates plus variceal band ligation (n = 37) Further details: propranolol: 10 mg three times a day increased over one week (duration not stated) plus isosorbide mononitrate 10 mg twice daily increasing over one week to 20 mg (duration not stated) plus variceal band ligation (Saeed Sixshooter every 3 weeks until eradication) Group 2: beta‐blockers plus nitrates (n = 35) Further details: propranolol: 10 mg three times a day increased over one week (duration not stated) plus isosorbide mononitrate 10 mg twice daily increasing over one week to 20 mg (duration not stated) Group 3: variceal band ligation (n = 39) Further details: variceal band ligation (Saeed Sixshooter every 3 weeks until eradication) Group 4: beta‐blockers (n = 39) Further details: propranolol: 10 mg three times daily increased over one week (duration not stated) | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, variceal rebleed at maximal follow‐up (symptomatic recovery) (number of patients) Follow‐up (months): 9 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned to one of the four treatment groups, using opaque, sealed envelopes, that contained a treatment assignment derived from computer‐generated random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomly assigned to one of the four treatment groups, using opaque, sealed envelopes, that contained a treatment assignment derived from computer‐generated random numbers" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation dropouts which were related to the intervention and probably related to the outcome |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available and adverse events were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Alexandrino 1988.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Portugal Period of recruitment: not stated Number randomised: 65 Post‐randomisation dropouts: 0 (0.0%) Revised sample size: 65 Average age (years): 47 Females: 16 (24.6%) Other features of decompensation: not stated Alcohol‐related cirrhosis: 52 (80.0%) Viral‐related cirrhosis: not stated Autoimmune disease‐related cirrhosis: not stated Other causes for cirrhosis: 13 (20.0%) Other inclusion/exclusion criteria: not stated | |
| Interventions | Group 1: beta‐blockers (n = 34) Further details: propranolol: reduction of pulse rate by 25%, duration not stated but probably until the follow‐up period (29 months) Group 2: sclerotherapy (n = 31) Further details: sclerotherapy: ethanolamine oleate every 3 weeks until obliteration | |
| Outcomes | Outcomes reported: variceal rebleed at maximal follow‐up (any) (number of patients), other features of decompensation at maximal follow‐up Follow‐up (months): 29 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was done by a list of random numbers allocated to sealed envelopes in two separate groups for Child's A and B patients:" Comment: further details how the random sequence was not provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was done by a list of random numbers allocated to sealed envelopes in two separate groups for Child's A and B patients:" Comment: further information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available and mortality and adverse events were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Ampelas 1987.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: France
Period of recruitment: not stated
Number randomised: 50
Post‐randomisation dropouts: not stated
Revised sample size: 50
Average age (years): not stated
Females: not stated
Other features of decompensation: not stated
Alcohol‐related cirrhosis: not stated
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated
Other inclusion/exclusion criteria: Exclusion: age >80 years, contraindications to beta‐blocker |
|
| Interventions | Group 1: portocaval shunt (n = 24) Further details: azygo‐portal disconnection Group 2: beta‐blockers (n = 26) Further details: propranolol: reduce heart rate by 25% (duration not reported) | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, variceal rebleed at maximal follow‐up (any) (number of patients) Follow‐up (months): 18 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available and adverse events were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Andreani 1991.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: France
Period of recruitment: 1985‐1988
Number randomised: 75
Post‐randomisation dropouts: not stated
Revised sample size: 75
Average age (years): not stated
Females: not stated
Other features of decompensation: not stated
Alcohol‐related cirrhosis: not stated
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated
Other inclusion/exclusion criteria: Exclusion: previous treatment of portal hypertension (not specified), contraindications to beta‐blocker |
|
| Interventions | Group 1: beta‐blockers (n = 35) Further details: propranolol: reduction of pulse rate by 25%, duration not stated but probably until the follow‐up period (29 months) Group 2: sclerotherapy (n = 40) Further details: sclerotherapy: polidocanol (no further details) | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up Follow‐up (months): 12 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available and mortality and adverse events were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Anonymous 1994.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: USA
Period of recruitment: 1985‐1989
Number randomised: 204
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 204
Average age (years): not stated
Females: 0 (0.0%)
Other features of decompensation: not stated
Alcohol‐related cirrhosis: 204 (100.0%)
Viral‐related cirrhosis: 0 (0.0%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: 0 (0.0%)
Other inclusion/exclusion criteria: Exclusion: inability to give informed consent, contraindications to upper endoscopy, a positive test for Hepatitis B surface antigen in serum, a history of sclerotherapy or shunt surgery for varices, oesophageal or gastric malignancy, myocardial infarction within the past 6 months, need for p‐adrenergic antagonist drug therapy, current bleeding from source other than oesophageal varices or a decision by the treating physician to exclude the patient |
|
| Interventions | Group 1: no active intervention (n = 107) Further details: placebo Group 2: sclerotherapy (n = 97) Further details: sclerotherapy: not stated; 0.5 to 2 mL, maximum of 20 mL per session | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, variceal rebleed at maximal follow‐up (any) (number of patients) Follow‐up (months): 12 | |
| Notes | Source of funding (quote): "Funded by the VA cooperative study branch (author responses)" Trial name/trial registry number: THE VETERANS AFFAIRS COOPERATIVE VARICEAL SCLEROTHERAPY GROUP Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was carried out using sealed envelopes prepared centrally " Comment: details on how the sequence generation was generated was not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was carried out using sealed envelopes prepared centrally " Comment: further details were not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Only the endoscopists were aware of patients’ treatment assignment; all other caregivers, and the patients as well, remained blinded" Comment: the healthcare professionals providing the care to the participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Only the endoscopists were aware of patients’ treatment assignment; all other caregivers, and the patients as well, remained blinded" Comment: the healthcare professionals who provided the care to the participants were outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available and adverse events were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Argonz 2000.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Argentina
Period of recruitment: 1994‐1997
Number randomised: 80
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 80
Average age (years): 53
Females: 18 (22.5%)
Other features of decompensation: 28 (35.0%)
Alcohol‐related cirrhosis: 46 (57.5%)
Viral‐related cirrhosis: 20 (25.0%)
Autoimmune disease‐related cirrhosis: 11 (13.8%)
Other causes for cirrhosis: 5 (6.3%)
Other inclusion/exclusion criteria: Exclusion criteria were 1) portal vein thrombosis 2) fundal gastric varices 3) hepatocellular carcinoma or any other malignant tumour 4) more than one sclerotherapy or variceal band ligation procedure after control of acute variceal bleeding |
|
| Interventions | Group 1: sclerotherapy plus variceal band ligation (n = 39) Further details: sclerotherapy: 2% 1 mL polidocanol up to 10 mL plus variceal band ligation (bard interventional products) up to 10 bands 1 to 2 weeks until eradication of varices Group 2: variceal band ligation (n = 41) Further details: variceal band ligation (bard interventional products) up to 10 bands 1 to 2 weeks until eradication of varices | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, any adverse events (number of people), variceal rebleed at maximal follow‐up (symptomatic recovery) (number of patients) Follow‐up (months): 12 | |
| Notes | Source of funding (quote): "Supported in part by a grant from Fundacion Argentina para el Estudio de la Enfermedades del Higado (FUNDHIG)"
Trial name/trial registry number: not stated
Attempts were made to contact the authors in February 2020 Individual patients had multiple cirrhosis aetiologies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The assignment was determined by means of a table of random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was carried out utilizing consecutively numbered, sealed opaque envelopes containing the treatment assignment" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Comment: no prepublished protocol was available, but the authors reported mortality, adverse events, and variceal rebleeding |
| Other bias | Low risk | Comment: no other bias noted |
Avgerinos 1993.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Greece
Period of recruitment: 1986‐1989
Number randomised: 85
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 85
Average age (years): 58
Females: 24 (28.2%)
Other features of decompensation: not stated
Alcohol‐related cirrhosis: 22 (25.9%)
Viral‐related cirrhosis: 43 (50.6%)
Autoimmune disease‐related cirrhosis: 5 (5.9%)
Other causes for cirrhosis: 15 (17.6%)
Other inclusion/exclusion criteria: Inclusion: 1) initial control of haemorrhage 2) no history of previous variceal bleeding 3) absence of hepatocellular carcinoma 4) no contraindication to propanolol such as airway obstruction, left ventricular failure or diabetes mellitus (type I) 5) no history of previous treatment for portal hypertension 6) absence of severe liver disease defined by the presence of coma, intractable ascites, or severe hyperbilirubinaemia (>85 mmol/L) |
|
| Interventions | Group 1: beta‐blockers plus sclerotherapy (n = 45) Further details: propranolol to decrease the heart rate by 25% plus sclerotherapy: 5% ethanolamine oleate, maximum 20 mL per session at weekly intervals, until varices became too small to inject Group 2: sclerotherapy (n = 40) Further details: sclerotherapy: 5% ethanolamine oleate, maximum 20 mL per session at weekly intervals, until varices became too small to inject | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, any adverse events (number of events), variceal rebleed at maximal follow‐up (any) (number of patients), variceal rebleed at maximal follow‐up (any) (number of rebleeds) Follow‐up (months): 23.9 | |
| Notes | Source of funding (quote): "This work was supported by a grant from the Faculty of Medicine, University of Athens" Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "All endoscopies and sclerotherapies were performed by a group of three experienced endoscopists who did not know which patients had received propranolol" Comment: it was not clear if these were the outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: no prepublished protocol was available, but the authors reported mortality, adverse events, and variceal rebleeding |
| Other bias | Low risk | Comment: no other bias noted |
Avgerinos 1997.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Greece
Period of recruitment: 1992‐1993
Number randomised: 77
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 77
Average age (years): 55
Females: 14 (18.2%)
Other features of decompensation: 38 (49.4%)
Alcohol‐related cirrhosis: 35 (45.5%)
Viral‐related cirrhosis: 31 (40.3%)
Autoimmune disease‐related cirrhosis: 1 (1.3%)
Other causes for cirrhosis: 10 (13.0%)
Other inclusion/exclusion criteria: Inclusion: a) control of the acute bleeding episode from oesophageal varices by endoscopic injection sclerotherapy b) no history of previous endoscopic or surgical treatment for varices c) absence of hepatocellular carcinoma |
|
| Interventions | Group 1: variceal band ligation (n = 37) Further details: variceal band ligation (Bard intervention products, Tewkesbury, Massachusetts, USA) 2 to 9 bands (average interval between sessions: 8.8 days) Group 2: sclerotherapy (n = 40) Further details: sclerotherapy (5% ethanolamine oleate) (average interval between sessions: 7.6 days) | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, any adverse events (number of people), any adverse events (number of events), variceal rebleed at maximal follow‐up (any) (number of rebleeds) Follow‐up (months): 15.2 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization sequence" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The investigators managing the patients were not aware of the treatment a patient would be assigned before randomization took place" Comment: further details were not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: no prepublished protocol was available, but the authors reported mortality, adverse events, and variceal rebleeding |
| Other bias | Low risk | Comment: no other bias noted |
Bader 1987.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: France
Period of recruitment: 1984‐1986
Number randomised: 37
Post‐randomisation dropouts: 2 (5.4%)
Revised sample size: 35
Reasons for post‐randomisation dropouts: not stated
Average age (years): 55
Females: not stated
Other features of decompensation: not stated
Alcohol‐related cirrhosis: not stated
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated
Other inclusion/exclusion criteria: Exclusion: liver cancer, contraindications to beta‐blockers, repeated haemorrhage |
|
| Interventions | Group 1: beta‐blockers (n = 17) Further details: propranolol 40 to 160 mg (average 80 mg)/day (duration not stated) Group 2: sclerotherapy (n = 18) Further details: sclerotherapy: 2% Polidocanol (no further details) | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up Follow‐up (months): 14 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: one patient in each group were excluded because of non‐compliance or complications related to treatment. it is not clear whether this would have affected the effect estimate |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available, and adverse events and oesophageal variceal rebleed were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Baroncini 1996.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Italy
Period of recruitment: 1995‐1996
Number randomised: 14
Post‐randomisation dropouts: not stated
Revised sample size: 14
Average age (years): 60
Females: not stated
Other features of decompensation: not stated
Alcohol‐related cirrhosis: not stated
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated
Other inclusion/exclusion criteria: Exclusion: other disease likely to reduce survival and hepatocellular carcinoma |
|
| Interventions | Group 1: sclerotherapy plus variceal band ligation (n = not stated) Further details: sclerotherapy (1% polidocanol maximum 20 mL per session) plus variceal band ligation (no further details) every 15 days until eradication of varices Group 2: variceal band ligation (n = not stated) Further details: variceal band ligation (no further details) every 15 days until eradication of varices Additional details: number of participants in each group was not reported | |
| Outcomes | None of the outcomes of interest were reported | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available, and none of the outcomes of interest for this review were reported |
| Other bias | Low risk | Comment: no other bias noted |
Baroncini 1997.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Italy
Period of recruitment: 1993‐1995
Number randomised: 111
Post‐randomisation dropouts: not stated
Revised sample size: 111
Average age (years): 62
Females: 36 (32.4%)
Other features of decompensation: 24 (21.6%)
Alcohol‐related cirrhosis: 15 (13.5%)
Viral‐related cirrhosis: 93 (83.8%)
Autoimmune disease‐related cirrhosis: 2 (1.8%)
Other causes for cirrhosis: 1 (0.9%)
Other inclusion/exclusion criteria: Exclusion: bleeding from gastric varices, hepatocellular carcinoma or severe diseases likely to reduce survival |
|
| Interventions | Group 1: variceal band ligation (n = 57) Further details: variceal band ligation (Bard Interventional Products), repeated every 1 to 2 weeks until eradication Group 2: sclerotherapy (n = 54) Further details: sclerotherapy: 1% polidocanol 5 mL to 9 mL, repeated every 1 to 2 weeks until eradication | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, any adverse events (number of events), liver transplantation at maximal follow‐up, variceal rebleed at maximal follow‐up (symptomatic recovery) (number of patients) Follow‐up (months): 16.9 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | Low risk | Comment: no prepublished protocol was available, but the authors reported mortality, adverse events, and variceal rebleeding |
| Other bias | Low risk | Comment: no other bias noted |
Bertoni 1990.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Italy
Period of recruitment: not stated
Number randomised: 28
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 28
Average age (years): 59
Females: 10 (35.7%)
Other features of decompensation: not stated
Alcohol‐related cirrhosis: 16 (57.1%)
Viral‐related cirrhosis: 4 (14.3%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: 8 (28.6%)
Other inclusion/exclusion criteria: Exclusion: insulin dependent diabetes, asthma, severe cardiac disease, persistent bleeding, age >75 |
|
| Interventions | Group 1: beta‐blockers plus sclerotherapy (n = 14) Further details: nadolol to reduce heart rate by 25% Group 2: sclerotherapy (n = 14) Further details: sclerotherapy: 1% polidocanol at weekly intervals until eradication | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up Follow‐up (months): 2 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available, and adverse events and oesophageal variceal rebleed were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Bertoni 1994.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Italy
Period of recruitment: 1990‐1992
Number randomised: 76
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 76
Average age (years): 62
Females: 23 (30.3%)
Other features of decompensation: not stated
Alcohol‐related cirrhosis: 20 (26.3%)
Viral‐related cirrhosis: 32 (42.1%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: 24 (31.6%)
Other inclusion/exclusion criteria: Exclusion: prolonged severe encephalopathy, advanced intra or extrahepatic tumour, resumption of beta‐blocker therapy, previous sclerotherapy, intractable ascites, imminent liver transplantation |
|
| Interventions | Group 1: sclerotherapy plus nitrates (n = 37) Further details: sclerotherapy: continuation of the sclerotherapy performed as emergency or elective procedure, until eradication of varices plus isosorbide mononitrate 50 mg/day until eradication of varices Group 2: sclerotherapy (n = 39) Further details: sclerotherapy: continuation of the sclerotherapy performed as emergency or elective procedure, until eradication of varices plus placebo | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, variceal rebleed at maximal follow‐up (any) (number of rebleeds) Follow‐up (months): 2 | |
| Notes | Source of funding (quote): "We are grateful to ‘Chiesi Farmaceutici (Parma, Italy) for supplying the trial capsules" Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised in a double‐blind fashion by means of a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients were randomised in a double‐blind fashion by means of a table of random numbers" Comment: blinding was achieved by the use of a placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients were randomised in a double‐blind fashion by means of a table of random numbers" Comment: blinding was achieved by the use of a placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available and adverse events were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Bonkovsky 1989.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: USA
Period of recruitment: not stated
Number randomised: 20
Post‐randomisation dropouts: not stated
Revised sample size: 20
Average age (years): not stated
Females: 1 (5.0%)
Other features of decompensation: not stated
Alcohol‐related cirrhosis: 13 (65.0%)
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated
Other inclusion/exclusion criteria: Exclusion: severe encephalopathy, diabetes mellitus, asthma, cardiac disease, renal insufficiency |
|
| Interventions | Group 1: no active intervention (n = 10) Further details: placebo Group 2: beta‐blockers (n = 10) Further details: atenolol 50 mg to 100 mg to decrease heart rate by 25%; duration not stated | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, variceal rebleed at maximal follow‐up (any) (number of patients) Follow‐up (months): 12 | |
| Notes | Source of funding (quote): "Supported by ICI/Stuart Pharmaceuticals" Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…were assigned randomly (by random number‐opaque envelope technique) to receive atenolol and matching placebo" Comment: the details of sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "…were assigned randomly (by random number‐opaque envelope technique) to receive atenolol and matching placebo" comment: not clear if the envelopes were sealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Patients did not know which treatment they were receiving" Comment: not clear whether healthcare providers were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available and adverse events were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Cabrera 1996.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Spain
Period of recruitment: 1991‐1994
Number randomised: 63
Post‐randomisation dropouts: 2 (3.2%)
Revised sample size: 61
Reasons for post‐randomisation dropouts: not stated
Average age (years): 56
Females: 20 (32.8%)
Other features of decompensation: 21 (34.4%)
Alcohol‐related cirrhosis: 43 (70.5%)
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated
Other inclusion/exclusion criteria: Exclusion: 1. Hepatocellular carcinoma 2. Non‐variceal bleeding 3. Chronic encephalopathy 4. Neoplastic disease 5. Portal vein thrombosis 6. End‐stage liver disease |
|
| Interventions | Group 1: TIPS (n = 32) Further details: TIPS: a 10‐mm diameter Wallstent endoprosthesis Group 2: sclerotherapy (n = 31) Further details: sclerotherapy: 1% polidocanol 10 mL to 30 mL per session, weekly for the first month and at 1 to 3 month intervals until obliteration of varices | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, any adverse events (number of events), variceal rebleed at maximal follow‐up (any) (number of patients), other features of decompensation at maximal follow‐up, length of hospital stay (days) (all admissions until maximal follow‐up), length of hospital stay (days) (all admissions until maximal follow‐up) (sensitivity analysis) Follow‐up (months): 15 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed 3 days after the variceal bleeding was controlled using computer generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: no prepublished protocol was available, but the authors reported mortality, adverse events, and variceal rebleeding |
| Other bias | Low risk | Comment: no other bias noted |
Cennamo 1998.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Italy Period of recruitment: 1996‐1998 Number randomised: 34 Post‐randomisation dropouts: not stated Revised sample size: 34 Average age (years): not stated Females: 7 (20.6%) Other features of decompensation: not stated Alcohol‐related cirrhosis: not stated Viral‐related cirrhosis: not stated Autoimmune disease‐related cirrhosis: not stated Other causes for cirrhosis: not stated Other inclusion/exclusion criteria: not stated | |
| Interventions | Group 1: variceal band ligation plus sclerotherapy (n = 16) Further details: variceal band ligation (no further details) plus sclerotherapy: 1% polidocanol up to 20 mL until eradication Group 2: variceal band ligation (n = 18) Further details: variceal band ligation (no further details) | |
| Outcomes | Outcomes reported: other features of decompensation at maximal follow‐up Follow‐up (months): 12.6 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available, and mortality, adverse events, and oesophageal variceal rebleed were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Dasarathy 1992.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: India
Period of recruitment: 1986‐1990
Number randomised: 104
Post‐randomisation dropouts: 13 (12.5%)
Revised sample size: 91
Reasons for post‐randomisation dropouts: adverse events related to propranolol, refusal to follow study protocol
Average age (years): 45
Females: 15 (16.5%)
Other features of decompensation: not stated
Alcohol‐related cirrhosis: 25 (27.5%)
Viral‐related cirrhosis: 23 (25.3%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: 43 (47.3%)
Other inclusion/exclusion criteria: Exclusion: 1) Child class A patients 2) Endoscopic diagnosis of small oesophageal varices and of varices without signs of high risk of bleeding 3) Presence of any other potential bleeding site 4) Contraindication to the use of beta‐blocking agents and previous treatment with beta‐blockers, endoscopic sclerotherapy or surgery for portal hypertension |
|
| Interventions | Group 1: beta‐blockers (n = 46) Further details: propranolol to achieve a reduction in heart rate of 25% Group 2: sclerotherapy (n = 45) Further details: sclerotherapy: 1% polidocanol at 10‐day intervals until obliteration | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, serious adverse events (number of people), variceal rebleed at maximal follow‐up (any) (number of patients), variceal rebleed at maximal follow‐up (symptomatic recovery) (number of rebleeds) Follow‐up (months): 12 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation dropouts, that are likely to be related to the intervention and the outcome |
| Selective reporting (reporting bias) | Low risk | Comment: no prepublished protocol was available, but the authors reported mortality, adverse events, and variceal rebleeding |
| Other bias | Low risk | Comment: no other bias noted |
Dwivedi 1992.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: India
Period of recruitment: 1986‐1987
Number randomised: 32
Post‐randomisation dropouts: 2 (6.3%)
Revised sample size: 30
Reasons for post‐randomisation dropouts: Complications of beta‐blockers
Average age (years): 40
Females: 7 (23.3%)
Other features of decompensation: 13 (43.3%)
Alcohol‐related cirrhosis: not stated
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated
Other inclusion/exclusion criteria: Inclusion: liver cirrhosis Exclusion: contraindications to use of beta‐blockers |
|
| Interventions | Group 1: beta‐blockers (n = 14) Further details: propranolol to decrease the heart rate by 25% Group 2: sclerotherapy (n = 16) Further details: sclerotherapy: sclerosant not stated, repeated at 3‐week intervals until obliteration | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, variceal rebleed at maximal follow‐up (any) (number of patients), variceal rebleed at maximal follow‐up (any) (number of rebleeds) Follow‐up (months): 7.5 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random number tables" |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation dropouts related to the intervention and outcomes |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available and adverse events were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Esquivel Lopez 1984.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Mexico
Period of recruitment: not stated
Number randomised: 19
Post‐randomisation dropouts: not stated
Revised sample size: 19
Average age (years): 48
Females: 2 (10.5%)
Other features of decompensation: not stated
Alcohol‐related cirrhosis: 17 (89.5%)
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated
Other inclusion/exclusion criteria: Inclusion: liver cirrhosis and variceal bleeding Exclusion: heart failure, diabetes and lung disease |
|
| Interventions | Group 1: no active intervention (n = 8) Further details: no treatment Group 2: beta‐blockers (n = 11) Further details: propranolol to maintain heart rate at 25% below normal rate | |
| Outcomes | None of the outcomes of interest were reported | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available, and mortality, adverse events, and oesophageal variceal rebleed were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Fleig 1988.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Germany
Period of recruitment: 1983‐not stated
Number randomised: 115
Post‐randomisation dropouts: 10 (8.7%)
Revised sample size: 105
Reasons for post‐randomisation dropouts: not stated, but an interim reported exclusion of 8 patients for immediate rebleeding and protocol violations
Average age (years): not stated
Females: not stated
Other features of decompensation: not stated
Alcohol‐related cirrhosis: not stated
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated
Other inclusion/exclusion criteria: Inclusion: liver cirrhosis Exclusion: noncirrhotic portal hypertension, contraindications to the use of beta‐blocking agents, previous sclerotherapy or emergency sclerotherapy in the treatment of the index bleed, severe ascites, disorientation due to severe portal‐systemic encephalopathy [grade 3 and more], patients not willing to be subject to randomisation |
|
| Interventions | Group 1: beta‐blockers (n = 50) Further details: propranolol to reduce the resting heart rate by about 25% Group 2: sclerotherapy (n = 55) Further details: sclerotherapy: 1% polidocanol approximately 40 mL per session 3 to 4 day intervals until they reduced to grade 1 varices | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, variceal rebleed at maximal follow‐up (any) (number of patients), variceal rebleed at maximal follow‐up (any) (number of rebleeds) Follow‐up (months): 25 | |
| Notes | Source of funding (quote): "K. Rainer was supported by a grant from the ICI‐Rhein Pharma, Plankstadt, Federal Republic of Germany" Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomly allocated by sealed envelopes to treatment with either sclerotherapy or propranolol" Comment: further details were not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available, but the authors reported mortality, adverse events, and variceal rebleeding |
| Other bias | Low risk | Comment: no other bias noted |
Fornaciari 1990.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Italy
Period of recruitment: not stated
Number randomised: 28
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 28
Average age (years): not stated
Females: not stated
Other features of decompensation: not stated
Alcohol‐related cirrhosis: not stated
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated
Other inclusion/exclusion criteria: Exclusion: contraindication to beta‐blocker |
|
| Interventions | Group 1: beta‐blockers plus sclerotherapy (n = 14) Further details: nadolol to reduce heart rate by 25% plus sclerotherapy: no further details Group 2: sclerotherapy (n = 14) Further details: sclerotherapy: no further details | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up Follow‐up (months): 3 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available, and adverse events and oesophageal variceal rebleed were not reported |
| Other bias | Low risk | Comment: no other bias noted |
García‐Pagán 2009.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Spain
Period of recruitment: 2003‐2005
Number randomised: 160
Post‐randomisation dropouts: 2 (1.3%)
Revised sample size: 158
Reasons for post‐randomisation dropouts: prehepatic portal hypertension, portal vein thrombosis
Average age (years): 57
Females: 40 (25.3%)
Other features of decompensation: 54 (34.2%)
Alcohol‐related cirrhosis: 100 (63.3%)
Viral‐related cirrhosis: 62 (39.2%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: 15 (9.5%)
Other inclusion/exclusion criteria: Inclusion: 1) successful treatment of the index bleed with vasoactive drugs (terlipressin or somatostatin), antibiotics and endoscopic treatment 2) age between 18 and 75 years 3) no previous randomisation in the study and 4) provided signed, informed, written consent to participate in the study Exclusion: 1) failure to fulfil entry criteria 2) pregnancy 3) known hepatocellular carcinoma 4) chronic renal failure 5) Child–Pugh score >13 or a concomitant disease with reduced life expectancy 6) contraindications to beta‐blocker or isosorbide mononitrate 7) previous treatment to prevent rebleeding with a portosystemic shunt or with combined pharmacological therapy with beta‐blocker plus isosorbide mononitrate 8) treatment with EBL in the 3 months before the index bled 9) bleeding from isolated gastric or ectopic varices and 10) portal vein thrombosis |
|
| Interventions | Group 1: beta‐blockers plus nitrates plus variceal band ligation (n = 80) Further details: nadolol maximum tolerated dose (i.e. systolic blood pressure >=95 mmHg and resting heart rate >50 beats/min plus isosorbide nitrate 10 mg to 40 mg (maximum tolerated dose: same criteria as for nadolol) plus variceal band ligation (multiband devices), repeated every 10 to 14 days until variceal eradication Group 2: beta‐blockers plus nitrates (n = 78) Further details: nadolol maximum tolerated dose (i.e. systolic blood pressure >=95 mm Hg and resting heart rate >50 beats/min plus isosorbide nitrate 10 mg to 40 mg (maximum tolerated dose: same criteria as for nadolol) | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, any adverse events (number of people), any adverse events (number of events), liver transplantation at maximal follow‐up, length of hospital stay (days) (all admissions until maximal follow‐up), length of hospital stay (days) (all admissions until maximal follow‐up) (sensitivity analysis) Follow‐up (months): 15 | |
| Notes | Source of funding (quote): "Nadolol was kindly supplied by Sanofi Winthrop (Barcelona, Spain). Isosorbide mononitrate was kindly provided by Lacer (Barcelona, Spain)." Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation sequence was generated by computer in blocks of 8 and the code was kept at the coordinating centre in sealed, consecutively numbered, opaque envelopes" |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation sequence was generated by computer in blocks of 8 and the code was kept at the coordinating centre in sealed, consecutively numbered, opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were two post‐randomisation dropouts, however, they are unrelated to the treatment |
| Selective reporting (reporting bias) | Low risk | Comment: no prepublished protocol was available, but the authors reported mortality, adverse events, and variceal rebleeding |
| Other bias | Low risk | Comment: no other bias noted |
Garcia‐Villarreal 1999.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Spain
Period of recruitment: 1993‐1997
Number randomised: 46
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 46
Average age (years): 56
Females: 9 (19.6%)
Other features of decompensation: 21 (45.7%)
Alcohol‐related cirrhosis: 33 (71.7%)
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated
Other inclusion/exclusion criteria: Inclusion: 1) endoscopically proven oesophageal variceal bleeding 2) diagnosis of cirrhosis based on clinical history and laboratory, ultrasonography, and/or liver biopsy findings 3) age between 18 and 75 years and 4) informed consent from the patient or his/her next of kin when encephalopathy was present Exclusion: 1) history of chronic encephalopathy 2) portal vein thrombosis 3) hepatocellular carcinoma 4) end‐stage liver disease defined by the presence of more than one of the following parameters: prothrombin index, 35%, bilirubin 5 mg/dL, and plasma creatinine 3 mg/dL and 5) follow‐up not possible |
|
| Interventions | Group 1: TIPS (n = 22) Further details: TIPS, performed under local anaesthesia (no further details) Group 2: sclerotherapy (n = 24) Further details: sclerotherapy: 5% ethanolamide oleate, 12 to 20 mL per session repeated every 7 to 10 days until variceal obliteration | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, variceal rebleed at maximal follow‐up (any) (number of patients), other features of decompensation at maximal follow‐up, length of hospital stay (days) (all admissions until maximal follow‐up), length of hospital stay (days) (all admissions until maximal follow‐up) (sensitivity analysis) Follow‐up (months): 20.6 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using computer‐generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available and adverse events were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Henderson 1990.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: USA
Period of recruitment: 1981‐1985
Number randomised: 72
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 72
Average age (years): not stated
Females: not stated
Other features of decompensation: 8 (11.1%)
Alcohol‐related cirrhosis: 43 (59.7%)
Viral‐related cirrhosis: 13 (18.1%)
Autoimmune disease‐related cirrhosis: 5 (6.9%)
Other causes for cirrhosis: 11 (15.3%)
Other inclusion/exclusion criteria: Inclusion: oesophageal bleeding secondary to liver cirrhosis, suitability for either distal spleno‐renal shunt or sclerotherapy, no previous sclerotherapy Exclusion: non‐cirrhotic portal hypertension related bleeding |
|
| Interventions | Group 1: portocaval shunt (n = 35) Further details: distal splenorenal shunt Group 2: sclerotherapy (n = 37) Further details: sclerotherapy: 1.5% to 2% sodium morrhuate and 0.75% to 1% sodium tetradecyl sulphate monthly intervals until obliteration | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, variceal rebleed at maximal follow‐up (symptomatic recovery) (number of patients) Follow‐up (months): 61 | |
| Notes | Source of funding (quote): "Supported by Public Health Service Research Grant AM 15736 and General Clinical Research Center Public Health Service Grant 5M01RR00039"
Trial name/trial registry number: not stated
Attempts were made to contact the authors in February 2020 Individual patients had multiple cirrhosis aetiologies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was by closed envelope with a recurring block size of four" Comment: further details were not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available and adverse events were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Ink 1992.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: France
Period of recruitment: 1986‐1989
Number randomised: 131
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 131
Average age (years): 53
Females: 30 (22.9%)
Other features of decompensation: 41 (31.3%)
Alcohol‐related cirrhosis: 126 (96.2%)
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated
Other inclusion/exclusion criteria: Inclusion: a) recent episode of bleeding from oesophageal varices that was confirmed by emergency endoscopy (endoscopic stigmata of recent variceal bleeding or oesophageal varices with no other pathological condition present to explain major upper gastrointestinal trace bleeding) b) bleeding that had stopped for at least 24 hours without any sclerotherapy session c) a Child‐Pugh score greater than 6 and d) oral acceptance to participate from the patient or from the next of kin if the patient was too ill to consent Exclusion: a) previous treatment with propranolol or sclerotherapy for portal hypertension b) contraindication to the use of propranolol because of asthma, cardiac insufficiency or use of insulin or sulfamides indicating diabetes mellitus, disturbance of atrioventricular conduction or Raynaud’s syndrome c) contraindication to sclerotherapy because of severe encephalopathy, previous oesophageal surgery, hiatal hernia longer than 4 cm or oesophageal stenosis d) existence of hepatocellular carcinoma or serious illness reducing life expectancy (for example, ongoing cancer, hepatic coma or prothrombin time less than 20%) or e) unfeasibility of regular surveillance (for reasons of distance or apparent indiscipline) |
|
| Interventions | Group 1: beta‐blockers plus sclerotherapy (n = 65) Further details: propranolol titrated to reduce the resting pulse rate by 25% (duration not stated; likely to be until follow‐up period) plus sclerotherapy 1% polidocanol 40 mL to 60 mL initially weekly and then monthly to eradicate varices Group 2: beta‐blockers (n = 66) Further details: propranolol titrated to reduce the resting pulse rate by 25% (duration not stated; likely to be until follow‐up period) | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, serious adverse events (number of people), variceal rebleed at maximal follow‐up (any) (number of patients) Follow‐up (months): 24 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the patients in each center were randomly assigned to their treatment groups by sealed opaque envelopes" Comment: further details of whether they were consecutively numbered were not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: no prepublished protocol was available, but the authors reported mortality, adverse events, and variceal rebleeding |
| Other bias | Low risk | Comment: no other bias noted |
Isaksson 1995.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Sweden
Period of recruitment: 1982‐1989
Number randomised: 45
Post‐randomisation dropouts: not stated
Revised sample size: 45
Average age (years): 52
Females: 12 (26.7%)
Other features of decompensation: 19 (42.2%)
Alcohol‐related cirrhosis: 33 (73.3%)
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated
Other inclusion/exclusion criteria: Inclusion: 1) age between 20 to 75 years at randomisation 2) the bleeding source should be oesophageal varices verified endoscopically 3) presence of portal hypertension and 4) the diagnosis of liver cirrhosis should be verified on histological examination |
|
| Interventions | Group 1: portocaval shunt (n = 24) Further details: mesocaval shunt Group 2: sclerotherapy (n = 21) Further details: 1% ethoxysclerol up to 30 mL twice a week initially and monthly until varices were eradicated | |
| Outcomes | Outcomes reported: any adverse events (number of events), length of hospital stay (days) (all admissions until maximal follow‐up), treatment costs Follow‐up (months): 65.2 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomization was done within the Child's groups and by using closed envelopes" Comment: further details were not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available, and mortality and oesophageal variceal rebleed were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Jalan 1997.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: UK
Period of recruitment: 1993‐1995
Number randomised: 58
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 58
Average age (years): 58
Females: 21 (36.2%)
Other features of decompensation: 16 (27.6%)
Alcohol‐related cirrhosis: 47 (81.0%)
Viral‐related cirrhosis: 4 (6.9%)
Autoimmune disease‐related cirrhosis: 5 (8.6%)
Other causes for cirrhosis: 2 (3.4%)
Other inclusion/exclusion criteria: Inclusion: liver cirrhosis, age 18‐75 years, first episode of oesophageal varices haemorrhage Exclusion: bleeding from other varices, previous endoscopic treatment for variceal bleeding, hepatorenal failure, hepatic or extra‐hepatic malignancy, portal vein thrombosis, failure to give consent |
|
| Interventions | Group 1: TIPS (n = 31) Further details: TIPS using 1 or 2 12 mm expandable metal stents Group 2: variceal band ligation (n = 27) Further details: variceal band ligation, single band; repeated weekly until eradication | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, any adverse events (number of people), variceal rebleed at maximal follow‐up (symptomatic recovery) (number of patients), other features of decompensation at maximal follow‐up, variceal rebleed at maximal follow‐up (symptomatic recovery) (number of rebleeds), length of hospital stay (days) (all admissions until maximal follow‐up), treatment costs, length of hospital stay (days) (all admissions until maximal follow‐up) (sensitivity analysis) Follow‐up (months): 16.2 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Treatment assignment was achieved using the closed‐envelope method" Comment: further details were not available |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Treatment assignment was achieved using the closed‐envelope method" Comment: further details were not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: no prepublished protocol was available, but the authors reported mortality, adverse events, and variceal rebleeding |
| Other bias | Low risk | Comment: no other bias noted |
Jensen 1989.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Denmark
Period of recruitment: 1985‐1987
Number randomised: 31
Post‐randomisation dropouts: not stated
Revised sample size: 31
Average age (years): 47
Females: 4 (12.9%)
Other features of decompensation: not stated
Alcohol‐related cirrhosis: 26 (83.9%)
Viral‐related cirrhosis: 2 (6.5%)
Autoimmune disease‐related cirrhosis: 1 (9.7%)
Other causes for cirrhosis: 2 (6.5%)
Other inclusion/exclusion criteria: Inclusion: liver cirrhosis, 1st variceal bleeding (no previous bleeding) Exclusion: contraindications to use of beta‐blockers |
|
| Interventions | Group 1: beta‐blockers plus sclerotherapy (n = 15) Further details: propranolol 160 mg slow release for 6 months plus sclerotherapy (sclerosant not stated) at monthly intervals to obliterate varices Group 2: sclerotherapy (n = 16) Further details: sclerotherapy (sclerosant not stated) at monthly intervals to obliterate varices plus placebo for 6 months | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, any adverse events (number of people), variceal rebleed at maximal follow‐up (any) (number of patients), other features of decompensation at maximal follow‐up, variceal rebleed at maximal follow‐up (any) (number of rebleeds) Follow‐up (months): 9 | |
| Notes | Source of funding (quote): "The Inderal was provided by ICI, Pharmaceutical Division, UK" Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomized in a double‐blind manner by a computer‐generated randomization schedule" |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients were randomized in a double‐blind manner by a computer‐generated randomization schedule" Comment: although the precise method of allocation concealment was not reported, the allocation was probably concealed using a placebo |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patients were randomized in a double‐blind manner by a computer‐generated randomization schedule" Comment: blinding was achieved by the use of a placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patients were randomized in a double‐blind manner by a computer‐generated randomization schedule" Comment: blinding was achieved by the use of a placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | Low risk | Comment: no prepublished protocol was available, but the authors reported mortality, adverse events, and variceal rebleeding |
| Other bias | Low risk | Comment: no other bias noted |
Jiron 1993.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Chile
Period of recruitment: 1983‐1986
Number randomised: 57
Post‐randomisation dropouts: not stated
Revised sample size: 57
Average age (years): 54
Females: 23 (40.4%)
Other features of decompensation: not stated
Alcohol‐related cirrhosis: 41 (71.9%)
Viral‐related cirrhosis: 1 (1.8%)
Autoimmune disease‐related cirrhosis: 1 (1.8%)
Other causes for cirrhosis: 14 (24.6%)
Other inclusion/exclusion criteria: Inclusion: liver cirrhosis, variceal bleeding happened within 1 week and no more than 15 days, oesophageal varices grade >1, absence of contraindications to the use of beta‐blockers |
|
| Interventions | Group 1: no active intervention (n = 28) Further details: placebo Group 2: beta‐blockers (n = 29) Further details: propranolol in increasing doses from 40 mg/ day until 25% decrease in baseline heart rate was reached (duration not reported) | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up Follow‐up (months): 48 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available, and adverse events and oesophageal variceal rebleed were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Kanazawa 1991.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Japan
Period of recruitment: 1985‐1990
Number randomised: 43
Post‐randomisation dropouts: 4 (9.3%)
Revised sample size: 39
Reasons for post‐randomisation dropouts: lost to follow‐up
Average age (years): 52
Females: 8 (18.6%)
Other features of decompensation: not stated
Alcohol‐related cirrhosis: 20 (46.5%)
Viral‐related cirrhosis: 20 (46.5%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated
Other inclusion/exclusion criteria: Inclusion: patients with vomiting blood as the chief complaint, presence of oesophageal varices in which haemostasis is obtained on endoscopy, liver biopsy diagnosed cirrhosis Exclusion: liver cancer on ultrasound, CT or angiography |
|
| Interventions | Group 1: beta‐blockers plus sclerotherapy (n = 20) Further details: propranolol started at 30 mg titrated to reduce the heart rate by 25% plus sclerotherapy: ethanolamine oleate, repeated weekly to reduce it to F1 Group 2: sclerotherapy (n = 23) Further details: sclerotherapy: ethanolamine oleate, repeated weekly to reduce it to F1 | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, any adverse events (number of people), any adverse events (number of events), length of hospital stay (days) (all admissions until maximal follow‐up) Follow‐up (months): 26.7 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The subjects were divided into two groups by the envelope method" Comment: further details were not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there were post‐randomisation dropouts; it was not clear whether these were related to the intervention and outcomes |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available and oesophageal variceal rebleed was not reported |
| Other bias | Low risk | Comment: no other bias noted |
Kong 2015.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: China
Period of recruitment: 2008‐2012
Number randomised: 38
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 38
Average age (years): 53
Females: 15 (39.5%)
Other features of decompensation: not stated
Alcohol‐related cirrhosis: 3 (7.9%)
Viral‐related cirrhosis: 20 (52.6%)
Autoimmune disease‐related cirrhosis: 4 (10.5%)
Other causes for cirrhosis: 8 (21.1%)
Other inclusion/exclusion criteria: Inclusion: liver cirrhosis, high variceal pressure, previous variceal bleeding Exclusion: portal vein thrombosis, treatment with beta‐blockers, previous endoscopic treatment of varices (ligation or sclerotherapy), multifocal hepatocellular carcinoma, severe clotting defects, hepatic encephalopathy grade Ⅲ and Ⅳ, previous surgical portosystemic shunts or TIPS were also excluded from the study |
|
| Interventions | Group 1: variceal band ligation (n = 20) Further details: variceal band ligation, super 7 multiple band ligator, every 2 to 3 weeks until all oesophageal varices were obliterated or were significantly reduced to small residual varices (F1) Group 2: sclerotherapy (n = 18) Further details: sclerotherapy: 1% lauromacrogol, every 1 to 2 weeks until all oesophageal varices were obliterated or were significantly reduced to small residual varices (F1) | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, any adverse events (number of people), variceal rebleed at maximal follow‐up (any) (number of patients) Follow‐up (months): 16 | |
| Notes | Source of funding (quote): "Educational and Health Department of Anhui Province, No. KJ2010A158, No. KJ2012Z189 and No. 2010B018; and National Natural Science Foundation of China, No. 81271736" Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "consecutively numbered envelopes that contained the treatment assignments, which were generated by a system using computer allocated random digit numbers" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "consecutively numbered envelopes that contained the treatment assignments, which were generated by a system using computer allocated random digit numbers" Comment: not clear whether the envelopes were sealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: no prepublished protocol was available, but the authors reported mortality, adverse events, and variceal rebleeding |
| Other bias | Low risk | Comment: no other bias noted |
Kumar 2015.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: India Period of recruitment: not stated Number randomised: 142 Post‐randomisation dropouts: not stated Revised sample size: 142 Average age (years): 44 Females: not stated Other features of decompensation: not stated Alcohol‐related cirrhosis: 84 (59.2%) Viral‐related cirrhosis: not stated Autoimmune disease‐related cirrhosis: not stated Other causes for cirrhosis: not stated Other inclusion/exclusion criteria: not stated | |
| Interventions | Group 1: beta‐blockers plus nitrates (n = 39) Further details: propranolol plus isosorbide‐5‐mononitrate (no further details) Group 2: variceal band ligation (n = 56) Further details: variceal band ligation (no further details) Group 3: beta‐blockers (n = 47) Further details: carvedilol (no further details) | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, any adverse events (number of people), variceal rebleed at maximal follow‐up (any) (number of patients) Follow‐up (months): 16.4 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | Low risk | Comment: no prepublished protocol was available, but the authors reported mortality, adverse events, and variceal rebleeding |
| Other bias | Low risk | Comment: no other bias noted |
Lundell 1990.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Sweden
Period of recruitment: not stated
Number randomised: 41
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 41
Average age (years): 57
Females: 19 (46.3%)
Other features of decompensation: not stated
Alcohol‐related cirrhosis: 26 (63.4%)
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated
Other inclusion/exclusion criteria: Inclusion: patients admitted with bleeding from oesophageal varices Exclusion: patients who had previously received sclerotherapy |
|
| Interventions | Group 1: beta‐blockers plus sclerotherapy (n = 19) Further details: sclerotherapy: 1% aethoxysclerol at monthly intervals until obliteration plus propranolol to decrease heart rate by 25% Group 2: sclerotherapy (n = 22) Further details: sclerotherapy: 1% aethoxysclerol at monthly intervals until obliteration | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, variceal rebleed at maximal follow‐up (any) (number of patients) Follow‐up (months): 7.9 | |
| Notes | Source of funding (quote): "This study was supported by grants from the Swedish Research Council (Project 17 X‐760)" Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available and adverse events were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Martin 1991.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: France
Period of recruitment: 1984‐1986
Number randomised: 76
Post‐randomisation drop outs: not stated
Revised sample size: 76
Average age (years): 53
Females: 12 (15.8%)
Other features of decompensation: not stated
Alcohol‐related cirrhosis: 74 (97.4%)
Viral‐related cirrhosis: 0 (0.0%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: 2 (2.6%)
Other inclusion/exclusion criteria: Exclusion: 1) previous treatment with propranolol or sclerotherapy 2) age >75 years 3) contraindications to beta‐blockers 4) hepatocellular carcinoma |
|
| Interventions | Group 1: beta‐blockers (n = 34) Further details: propranolol dose resulting in a reduction in heart rate by at least 25% at rest and stable with effort Group 2: sclerotherapy (n = 42) Further details: sclerotherapy 3% polidocanol mixed with radio‐opaque material, repeated every 3 weeks until obliteration of varices | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, any adverse events (number of events), variceal rebleed at maximal follow‐up (any) (number of patients) Follow‐up (months): 35.6 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were randomized into 2 groups by random drawing with sealed envelopes" Comment: further details were not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | Low risk | Comment: no prepublished protocol was available, but the authors reported mortality, adverse events, and variceal rebleeding |
| Other bias | Low risk | Comment: no other bias noted |
Masliah 1997.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: France Period of recruitment: 1991‐1996 Number randomised: 95 Post‐randomisation dropouts: not stated Revised sample size: 95 Average age (years): not stated Females: not stated Other features of decompensation: not stated Alcohol‐related cirrhosis: not stated Viral‐related cirrhosis: not stated Autoimmune disease‐related cirrhosis: not stated Other causes for cirrhosis: not stated Other inclusion/exclusion criteria: not stated | |
| Interventions | Group 1: beta‐blockers plus nitrates (n = 46) Further details: propranolol plus isosorbide mononitrate (no further details) Group 2: beta‐blockers (n = 49) Further details: propranolol alone, no further details | |
| Outcomes | Outcomes reported: any adverse events (number of people) Follow‐up (months): 29 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available, and mortality and oesophageal variceal rebleed were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Mckee 1994.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Scotland
Period of recruitment: 1986‐1989
Number randomised: 40
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 40
Average age (years): 59
Females: 17 (42.5%)
Other features of decompensation: not stated
Alcohol‐related cirrhosis: 27 (67.5%)
Viral‐related cirrhosis: 5 (12.5%)
Autoimmune disease‐related cirrhosis: 4 (10.0%)
Other causes for cirrhosis: 4 (10.0%)
Other inclusion/exclusion criteria: Inclusion: patients who were referred for the first time to the hospital with suspected variceal bleeding Exclusion: age <65, Child's grade A or B |
|
| Interventions | Group 1: sclerotherapy (n = 22) Further details: no further details Group 2: no active intervention (n = 18) Further details: no treatment (on demand sclerotherapy, when they developed bleeding) | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, any adverse events (number of people), variceal rebleed at maximal follow‐up (any) (number of patients) Follow‐up (months): 24 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Sealed, numbered envelopes" Comment: further details such as opaqueness or consecutive numbers were not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: no prepublished protocol was available, but the authors reported mortality, adverse events, and variceal rebleeding |
| Other bias | Low risk | Comment: no other bias noted |
Parelon 1989.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: France
Period of recruitment: 1982‐1985
Number randomised: 55
Post‐randomisation dropouts: 5 (9.1%)
Revised sample size: 50
Reasons for post‐randomisation dropouts: died before procedure or titration could be achieved
Average age (years): 56
Females: 9 (18.0%)
Other features of decompensation: not stated
Alcohol‐related cirrhosis: 33 (66.0%)
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated
Other inclusion/exclusion criteria: Exclusion: 1) age >80 years 2) severe visceral illness (cardiac, respiratory or renal failure) 3) contraindications to surgical intervention and/or limited life expectancy 4) contraindication to beta‐blockers |
|
| Interventions | Group 1: portocaval shunt (n = 24) Further details: porto‐azygos anastomosis Group 2: beta‐blockers (n = 26) Further details: propranolol to reduce the heart rate by 25% | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up Follow‐up (months): 39 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation dropouts, which were probably related to the intervention and the outcome |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available, and adverse events and oesophageal variceal rebleed were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Romero 2006.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Argentina
Period of recruitment: 1998‐2002
Number randomised: 109
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 109
Average age (years): 52
Females: 37 (33.9%)
Other features of decompensation: 40 (36.7%)
Alcohol‐related cirrhosis: 67 (61.5%)
Viral‐related cirrhosis: 28 (25.7%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: 27 (24.8%)
Other inclusion/exclusion criteria: Inclusion criteria: 1) cirrhosis 2) the index variceal bleeding episode, demonstrated by emergency endoscopy, in the prior 3 months without any other evidence of bleeding within this time 3) informed consent signed by the patient Exclusion criteria: 1) portal vein thrombosis 2) fundal gastric varices 3) any malignant tumour 4) more than one endoscopic treatment after the control of acute variceal bleeding 5) creatinine > or =1.6 mg/dL 6) contraindications to receive beta‐blockers 7) bacterial infection and/or encephalopathy 8) inability to attend follow‐up visits |
|
| Interventions | Group 1: sclerotherapy plus variceal band ligation (n = 52) Further details: variceal band ligation using single band device initially and for later patients using a multiband ligator every two weeks until obliteration of varices plus sclerotherapy, one or two sessions at the end of ligation sessions for any residual varices Group 2: beta‐blockers plus nitrates (n = 57) Further details: nadolol dosage to achieve a 25% decrease in resting heart rate or until 55 bpm plus isosorbide mono nitrate starting with 10 mg twice daily, which was increased to 40 mg twice daily unless hypotension (systolic <90 mmHg) or severe headache occurred | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, serious adverse events (number of people), any adverse events (number of events), variceal rebleed at maximal follow‐up (any) (number of patients), variceal rebleed at maximal follow‐up (any) (number of rebleeds) Follow‐up (months): 11.7 | |
| Notes | Source of funding (quote): "This study was supported in part by a grant from Fundacion Argentina para el Estudio de las Enfermedades del Higado (FUNDHIG)"
Trial name/trial registry number: not stated
Attempts were made to contact the authors in February 2020 Individual patients had multiple cirrhosis aetiologies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a table of random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was carried out utilizing consecutively numbered, opaque, sealed envelopes containing the treatment assignment" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: no prepublished protocol was available, but the authors reported mortality, adverse events, and variceal rebleeding |
| Other bias | Low risk | Comment: no other bias noted |
Rossi 1991.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: France
Period of recruitment: 1983‐1987
Number randomised: 79
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 79
Average age (years): 54
Females: 19 (24.1%)
Other features of decompensation: 46 (58.2%)
Alcohol‐related cirrhosis: 79 (100.0%)
Viral‐related cirrhosis: 0 (0.0%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: 0 (0.0%)
Other inclusion/exclusion criteria: Inclusion criteria: 1) cirrhosis confirmed histologically or suggested by biochemical and clinical data 2) age <75 years 3) bleeding from oesophageal varices Exclusion criteria: 1) hepatic carcinoma 2) life expectancy <1 year (i.e. Child Pugh Score >13) 3) another cause of upper gastrointestinal bleeding, gastric or duodenal ulcer, gastric varices or severe congestive gastropathy 4) contraindication to beta‐blockers 5) previous course of sclerotherapy or any treatment known to alter portal haemodynamics 6) if the patient had required a transfusion of more than 6 units of blood within the first 24 hours 7) patients expected to have a low level of compliance or refused to participate |
|
| Interventions | Group 1: sclerotherapy (n = 26)
Further details: sclerotherapy 1% polidocanol, a total of 30 to 45 mL was injected, and repeated every 5 to 7 days until obliteration of varices
Group 2: beta‐blockers (n = 27)
Further details: propranolol titrated until a 20% to 25% reduction in resting heart rate was achieved up to a maximum of 160 mg/day Group 3: no active intervention (n = 26) Further details: no treatment |
|
| Outcomes | Outcomes reported: mortality at maximal follow‐up, variceal rebleed at maximal follow‐up (any) (number of patients) Follow‐up (months): 19 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Low risk | Quote: "eligible patients were randomly assigned to treatment using a consecutively numbered series of sealed individual opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients were not blinded since the two treatment procedures were different, and therefore, the control group was not given placebo. Consequently, physicians were also not blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The person assessing the outcome did not belong to the center where the trial took place and did not know which treatment had been given" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available and adverse events were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Sanyal 1997.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: USA
Period of recruitment: 1991‐1994
Number randomised: 80
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 80
Average age (years): 50
Females: 26 (32.5%)
Other features of decompensation: 16 (20.0%)
Alcohol‐related cirrhosis: 33 (41.3%)
Viral‐related cirrhosis: 36 (45.0%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: 11 (13.8%)
Other inclusion/exclusion criteria: Inclusion: active bleeding stopped for at least 72 hours Exclusion: portal vein thrombosis, evident hepatoma on ultrasound, end‐stage cancer or systemic diseases with life expectancy under 1 yr, pregnancy, history of non‐compliance to treatment, failure to obtain consent |
|
| Interventions | Group 1: TIPS (n = 41) Further details: TIPS: wallstent; the stents were dilated with an 8‐mm balloon catheter Group 2: sclerotherapy (n = 39) Further details: sclerotherapy 5% ethanolamine oleate 10 to 30 mL per session, initially at weekly intervals for 1st month and then every 1 to 3 months until obliteration | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, liver transplantation at maximal follow‐up Follow‐up (months): 32 | |
| Notes | Source of funding (quote): "In part by an award from the National Institutes of Health to the Clinical Research Center at the Medical College of Virginia (RR‐00065) and an award from the American College of Gastroenterology" Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization code" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "sealed opaque envelope" Comments: Further details were not available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients were not blinded (author replies)" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Investigators were also not blinded (author replies)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available, and adverse events and oesophageal variceal rebleed were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Sauer 1997.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Germany
Period of recruitment: 1992‐1995
Number randomised: 83
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 83
Average age (years): 56
Females: 35 (42.2%)
Other features of decompensation: 21 (25.3%)
Alcohol‐related cirrhosis: 51 (61.4%)
Viral‐related cirrhosis: 23 (27.7%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: 9 (10.8%)
Other inclusion/exclusion criteria: Exclusion: gastric varices, previous endoscopic or surgical treatment of varices, neoplastic disease or severe co‐morbid condition with expected survival less than 6 months, septicaemia, portal vein thrombosis, uncontrolled bleeding, contraindication to propanolol |
|
| Interventions | Group 1: TIPS (n = 42) Further details: TIPS stents were dilated to 8 mm to 12 mm Group 2: beta‐blockers plus sclerotherapy (n = 41) Further details: propranolol at oral doses which reduced the resting heart rate by approximately 25% plus sclerotherapy 5% ethanolamine oleate 10 to 30 mL per session, initially at weekly intervals for 1st month and then every 1 to 3 months until obliteration | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, any adverse events (number of events), liver transplantation at maximal follow‐up, variceal rebleed at maximal follow‐up (any) (number of patients), other features of decompensation at maximal follow‐up, variceal rebleed at maximal follow‐up (any) (number of rebleeds) Follow‐up (months): 18 | |
| Notes | Source of funding (quote): "The study was funded exclusively by institutional resources (author replies)" Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using computer‐generated random numbers by an independent person not involved in the treatment of patients" |
| Allocation concealment (selection bias) | Low risk | Quote: "using computer‐generated random numbers by an independent person not involved in the treatment of patients" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "None of the participants (patients, healthcare professionals or assessors) were blinded (author replies)" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "None of the participants (patients, healthcare professionals or assessors) were blinded (author replies)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: no prepublished protocol was available, but the authors reported mortality, adverse events, and variceal rebleeding |
| Other bias | Low risk | Comment: no other bias noted |
Sauer 2002.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Germany
Period of recruitment: 1995‐1999
Number randomised: 85
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 85
Average age (years): 54
Females: 35 (41.2%)
Other features of decompensation: 34 (40.0%)
Alcohol‐related cirrhosis: 53 (62.4%)
Viral‐related cirrhosis: 21 (24.7%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: 11 (12.9%)
Other inclusion/exclusion criteria: Exclusion: gastric varices, previous endoscopic or surgical treatment of varices, neoplastic disease or severe co‐morbid condition with expected survival less than 6 months, encephalopathy grade 3 or 4, septicaemia, portal vein thrombosis, uncontrolled bleeding, contraindication to propanolol |
|
| Interventions | Group 1: beta‐blockers plus variceal band ligation (n = 42) Further details: propranolol at oral doses which reduced the resting heart rate by approximately 25% plus variceal band ligation, was performed initially at intervals of 1‐2 weeks until the varices disappeared Group 2: TIPS (n = 43) Further details: TIPS stents were dilated to 8‐12 mm | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, any adverse events (number of events) Follow‐up (months): 46.8 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was carried out as early as possible, using computer‐generated random numbers, by an independent person not involved in the treatment of the patients" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was carried out as early as possible, using computer‐generated random numbers, by an independent person not involved in the treatment of the patients" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available and oesophageal variceal rebleed was not reported |
| Other bias | Low risk | Comment: no other bias noted |
Sheen 1989.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: China
Period of recruitment: 1983‐1985
Number randomised: 36
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 36
Average age (years): 44
Females: 5 (13.9%)
Other features of decompensation: 0 (0.0%)
Alcohol‐related cirrhosis: 16 (44.4%)
Viral‐related cirrhosis: 16 (44.4%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: 8 (22.2%)
Other inclusion/exclusion criteria: Exclusion: previous treatment with endoscopic sclerotherapy, heart or lung disease, hepatocellular carcinoma, refusal to participate |
|
| Interventions | Group 1: no active intervention (n = 18) Further details: no treatment Group 2: beta‐blockers (n = 18) Further details: propranolol in increasing dosages until the heart rate was reduced by approximately 25% | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, serious adverse events (number of people), any adverse events (number of people), variceal rebleed at maximal follow‐up (symptomatic recovery) (number of patients), other features of decompensation at maximal follow‐up Follow‐up (months): 12.5 | |
| Notes | Source of funding (quote): "This work was supported in part by a grant from the Prosperous Foundation, Taipei" Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Quote: "simple randomization by sealed envelope were carried out" Comment: further details were not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: no prepublished protocol was available, but the authors reported mortality, adverse events, and variceal rebleeding |
| Other bias | Low risk | Comment: no other bias noted |
Urbistondo 1996.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: USA
Period of recruitment: 1989‐1994
Number randomised: 43
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 43
Average age (years): 47
Females: 5 (11.6%)
Other features of decompensation: not stated
Alcohol‐related cirrhosis: 43 (100.0%)
Viral‐related cirrhosis: 0 (0.0%)
Autoimmune disease‐related cirrhosis: 0 (0.0%)
Other causes for cirrhosis: 0 (0.0%)
Other inclusion/exclusion criteria: Exclusion criteria: other etiologies of liver disease besides alcohol |
|
| Interventions | Group 1: beta‐blockers (n = 15) Further details: propranolol titrated to obtain 25% reduction in heart rate from baseline or less than 60 beats per minute Group 2: sclerotherapy (n = 13) Further details: sclerotherapy: 1.5% sodium tetradecyl sulphate 12 mL to 16 mL per session, twice a week initially and then once a week until obliteration Group 3: portocaval shunt (n = 15) Further details: distal splenorenal shunt | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, variceal rebleed at maximal follow‐up (symptomatic recovery) (number of patients) Follow‐up (months): 23.2 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available and adverse events were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Viazis 2002.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Greece
Period of recruitment: 1995‐1998
Number randomised: 73
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 73
Average age (years): 63
Females: 32 (43.8%)
Other features of decompensation: not stated
Alcohol‐related cirrhosis: 28 (38.4%)
Viral‐related cirrhosis: 32 (43.8%)
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: 13 (17.8%)
Other inclusion/exclusion criteria: Inclusion: patients admitted to hospital, age >18, established cirrhosis, bleeding of varices controlled by one session of endoscopic variceal sclerotherapy plus or minus somatostatin, variceal rebleeding requiring endoscopic treatment with 42 days after admission, informed consent Exclusion: variceal rebleeding requiring endoscopic treatment within 42 days after admission, history of previous chronic endoscopic or surgical treatment for varices and portal hypertension |
|
| Interventions | Group 1: variceal band ligation (n = 36) Further details: variceal band ligation using multiband ligator repeated at 7‐ to 10‐day intervals until variceal eradication was achieved Group 2: sclerotherapy (n = 37) Further details: sclerotherapy using up to 20 mL of ethanolamine repeated at 7‐ to 10‐day intervals until variceal eradication was achieved | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, variceal rebleed at maximal follow‐up (any) (number of patients) Follow‐up (months): 1.8 | |
| Notes | Source of funding (quote): "Dr Nikos Viazis was supported by a grant from the Hellenic Society of Gastroenterology" Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Quote: "After randomization according to a sealed envelope technique" Comment: further details were not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Oesophageal manometry and pH monitoring were performed by a physician who was not aware of the type of endoscopic treatment the patients had received" Comment: not clear if patients and other healthcare professionals involved in treatment were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Oesophageal manometry and pH monitoring were performed by a physician who was not aware of the type of endoscopic treatment the patients had received" Comment: not clear if patients and other healthcare professionals involved in assessment of clinical outcomes were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available and adverse events were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Villanueva 1994.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: Spain
Period of recruitment: 1989‐1991
Number randomised: 40
Post‐randomisation dropouts: not stated
Revised sample size: 40
Average age (years): 57
Females: 17 (42.5%)
Other features of decompensation: not stated
Alcohol‐related cirrhosis: 20 (50.0%)
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated
Other inclusion/exclusion criteria: Exclusion: age <16 or >75, non cirrhotic portal hypertension, hepatocellular carcinoma, portal vein thrombosis, Child Pugh class C, life expectancy under 1 year, contraindication to beta‐blockers, already taking beta‐blocker, bleeding from gastric varices or other sources of bleeding |
|
| Interventions | Group 1: beta‐blockers plus sclerotherapy (n = 22) Further details: nadolol dose titrated until a 25% reduction in baseline heart rate was achieved, without decreasing below 55 beats per minute plus sclerotherapy 10 to 20 mL of 5% ethanolamine in each session, initially twice weekly and later at monthly intervals Group 2: sclerotherapy (n = 18) Further details: sclerotherapy 10 to 20 mL of 5% ethanolamine in each session, initially twice weekly and later at monthly intervals | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, any adverse events (number of people), liver transplantation at maximal follow‐up Follow‐up (months): 26 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomization was carried out through the system of sealed envelopes, which were opened just before the beginning of the elective treatment" Comment: not clear whether the sealed envelopes were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available and oesophageal variceal rebleed was not reported |
| Other bias | Low risk | Comment: no other bias noted |
Vinel 1992.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: France
Period of recruitment: not stated
Number randomised: 75
Post‐randomisation dropouts: 1 (1.3%)
Revised sample size: 74
Reasons for post‐randomisation dropouts: not stated
Average age (years): 56
Females: 16 (21.6%)
Other features of decompensation: 17 (23.0%)
Alcohol‐related cirrhosis: 66 (89.2%)
Viral‐related cirrhosis: not stated
Autoimmune disease‐related cirrhosis: not stated
Other causes for cirrhosis: not stated
Other inclusion/exclusion criteria: Exclusion: Previous treatment with propanolol, sclerotherapy, shunt or deconnection surgery, hepatocellular carcinoma or contraindication to beta‐blockers |
|
| Interventions | Group 1: beta‐blockers plus sclerotherapy (n = 39) Further details: propranolol adjusted to decrease resting heart rate by 25% plus sclerotherapy using 1% polidocanol repeated every week initially and then every 2 weeks until obliteration Group 2: sclerotherapy (n = 35) Further details: sclerotherapy using 1% polidocanol repeated every week initially and then every 2 weeks until obliteration | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, variceal rebleed at maximal follow‐up (any) (number of patients), variceal rebleed at maximal follow‐up (any) (number of rebleeds) Follow‐up (months): 3.2 | |
| Notes | Source of funding: not stated Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomized into two groups using sealed opaque envelopes" Comment: further details such as whether they were consecutively numbered were not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: one participant was excluded: there reason for exclusion was not stated; therefore, it is not possible to determine whether the dropout was related to the intervention or outcome |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available and adverse events were not reported |
| Other bias | Low risk | Comment: no other bias noted |
Westaby 1985a.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | Country: UK
Period of recruitment: 1977‐1981
Number randomised: 116
Post‐randomisation dropouts: 0 (0.0%)
Revised sample size: 116
Average age (years): 53
Females: 52 (44.8%)
Other features of decompensation: not stated
Alcohol‐related cirrhosis: 63 (54.3%)
Viral‐related cirrhosis: 10 (8.6%)
Autoimmune disease‐related cirrhosis: 25 (21.6%)
Other causes for cirrhosis: 18 (15.5%)
Other inclusion/exclusion criteria: Exclusion: rapid deterioration, previous surgery for varices, HBV, or physician refused patient enter study |
|
| Interventions | Group 1: no active intervention (n = 60) Further details: no treatment Group 2: sclerotherapy (n = 56) Further details: sclerotherapy: sclerosant not stated, repeated every 3 weeks until variceal obliteration | |
| Outcomes | Outcomes reported: mortality at maximal follow‐up, liver transplantation at maximal follow‐up Follow‐up (months): 37 | |
| Notes | Source of funding (quote): "Through King’s College Hospital " Trial name/trial registry number: not stated Attempts were made to contact the authors in February 2020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Quote: "sealed envelope" Comment: further details were not available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "not possible for endoscopists to be blinded (author replies)" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "not blinded (author replies)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prepublished protocol was available, and adverse events and oesophageal variceal rebleed were not reported |
| Other bias | Low risk | Comment: no other bias noted |
CT: computerised tomography; EBL: endoscopic band ligation; HBV: hepatitis B virus; TIPS: transjugular intrahepatic portosystemic shunt.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abd Elmoety 2015 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Abraldes 2016 | Not a comparison of interest for this review |
| Acharya 1993 | Not a population of interest for this review |
| Adson 1984 | Not a RCT |
| Agarwal 2015 | Not a comparison of interest for this review |
| Agarwala 2011 | Not a comparison of interest for this review |
| Akriviadis 1989 | Not a comparison of interest for this review |
| Albillos 1996 | Not a population of interest for this review |
| Al Traif 1999 | Not a population of interest for this review |
| Am. Soc. Gastro. Endo. 1998 | Not a RCT |
| Baik 2005 | Not a comparison of interest for this review |
| Balatsos 1997 | Not a population of interest for this review |
| Banares 1999 | Not a population of interest for this review |
| Bandi 1998 | Not a population of interest for this review |
| Barrioz 1998 | Not a population of interest for this review |
| Bellis 2003 | Not a population of interest for this review |
| Benner 1996 | Not a RCT |
| Berardi 1974 | Not a RCT |
| Berner 1994 | Not a population of interest for this review |
| Bhargava 1992 | Not a population of interest for this review |
| Bhargava 1997 | Not a population of interest for this review |
| Bhuiyan 2007 | Not a population of interest for this review |
| Bobadilla‐Diaz 2002 | Not a population of interest for this review |
| Bolognesi 1994 | Not a population of interest for this review |
| Bolognesi 1995 | Not a population of interest for this review |
| Bonilha 2010 | Not a population of interest for this review |
| Bories 1987 | Not a comparison of interest for this review |
| Bosch 2013 | Not a RCT |
| Braga 1991 | Not a population of interest for this review |
| Brensing 2002 | Not a RCT |
| Burroughs 1983 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Buuren 1999 | Not a population of interest for this review |
| Callow 1970 | Not a population of interest for this review |
| Cestari 1990 | Not a population of interest for this review |
| Chen 2013 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Chen 2016 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Chen 2018 | Not a RCT |
| Chen 2019 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Cheng 2001 | Not a population of interest for this review |
| ChiCTR08000228 | Not a population of interest for this review |
| ChiCTR11000192 | Not a comparison of interest for this review |
| ChiCTR11001577 | Not a population of interest for this review |
| ChiCTR12002148 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| ChiCTR15007655 | Not a comparison of interest for this review |
| ChiCTR1800018070 | Not a comparison of interest for this review |
| ChiCTR1800020322 | Not a population of interest for this review |
| ChiCTR1900021212 | Not a population of interest for this review |
| Cipolletta 2002 | Not a comparison of interest for this review |
| Cirera 1995 | Not a population of interest for this review |
| Colombo 1989 | Not a population of interest for this review |
| Conn 1986 | Not a RCT |
| Conn 1987 | Not a RCT |
| Conn 1993 | Not a RCT |
| Copaci 2012 | Not a population of interest for this review |
| Costa 2016 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| D'Amico 1998 | Not a population of interest for this review |
| D'Amico 2008 | Not a RCT |
| De 2002 | Not a population of interest for this review |
| De 2003 | Not a population of interest for this review |
| Dehesa 1994 | Not a comparison of interest for this review |
| de la Pena 1999 | Not a population of interest for this review |
| de la Pena 2005 | Not a population of interest for this review |
| Djurdjevic 1999 | Not a population of interest for this review |
| Dollet 1988 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Domagk 2000 | Not a population of interest for this review (Not clear if participants had active bleeding) |
| Dong 2018 | Not a population of interest for this review |
| Dunk 1988 | Not a population of interest for this review |
| Dunne 2019 | Not a population of interest for this review |
| Durdevic 1997 | Not a population of interest for this review |
| Dwivedi 1995 | Not a RCT |
| Eleftheriadis 1998 | Not a comparison of interest for this review |
| El‐Saadany 2007 | Not a comparison of interest for this review |
| Elsayed 1996 | Not a population of interest for this review |
| El‐Tourabi 1994 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Escorsell 1996 | Not a population of interest for this review |
| Escorsell 1997 | Not a population of interest for this review |
| Escorsell 1997a | Not a population of interest for this review |
| Escorsell 1998 | Not a comparison of interest for this review |
| Escorsell 2001 | Not a population of interest for this review |
| Escorsell 2002 | Not a population of interest for this review |
| Estevens 1996 | Not a population of interest for this review |
| EUCTR2005‐003557‐27 | Not a comparison of interest for this review |
| EUCTR2006‐006393‐14 | Not a population of interest for this review |
| EUCTR2012‐002489‐11 | Not a population of interest for this review |
| EUCTR2014‐002018‐21 | Not a population of interest for this review |
| Evrard 2003 | Not a population of interest for this review |
| Evrard 2008 | Not a RCT |
| Fakhry 1997 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Farag 2005 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Fernandez 2008 | Not a population of interest for this review |
| Ferrari 2005 | Not a population of interest for this review |
| Feu 1991 | Not a population of interest for this review |
| Feu 1993 | Not a population of interest for this review |
| Fiaccadori 1993 | Not a comparison of interest for this review |
| Fort 1990 | Not a RCT |
| Garcia‐Pagan 1991 | Not a population of interest for this review |
| Garcia‐Pagan 1996 | Not a population of interest for this review |
| Garden 1990 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Garg 1999 | Not a population of interest for this review |
| Gatta 1987 | Not a population of interest for this review |
| Geng 2015 | Not a population of interest for this review |
| George 2013 | Not a population of interest for this review |
| Gilbert 1991 | Not a RCT |
| Goff 1986 | Not a comparison of interest for this review |
| Gong 1998 | Not a RCT |
| Gong 2010 | Not a population of interest for this review |
| Gonzalez‐Abraldes 2001 | Not a comparison of interest for this review |
| Gonzalez‐Suarez 2006 | Not a RCT |
| Gotoh 1999 | Not a population of interest for this review |
| Gournay 2000 | Not a population of interest for this review |
| Gralnek 1999 | Not a population of interest for this review |
| Graupera 2011 | Not a population of interest for this review |
| Groszmann 2002 | Not a RCT |
| Gulberg 2002 | Additional treatments neither equal nor randomised between groups |
| Hanno 2016 | Not a population of interest for this review |
| Harki 2016a | Not a population of interest for this review |
| Harras 2010 | Not a population of interest for this review |
| Hashizume 1993 | Not a population of interest for this review |
| Helmy 2015 | Not a population of interest for this review |
| Holster 2016 | Not a population of interest for this review |
| Hua 2007 | Not a RCT |
| Huang 2017 | Not a comparison of interest for this review |
| Iso 1997 | Not a population of interest for this review |
| ISRCTN14174793 | Not a population of interest for this review |
| ISRCTN77521636 | Not a population of interest for this review |
| Iwakiri 2000 | Not a population of interest for this review |
| Iwao 1996 | Not a population of interest for this review |
| Jackson 1971 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Jeng 1989 | Not a population of interest for this review |
| Jenkins 1997 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Jiang 2001 | Not a comparison of interest for this review |
| Johansson 1988 | Not a population of interest for this review |
| Kalambokis 2005 | Not a population of interest for this review |
| Kamal 2017 | Not a comparison of interest for this review |
| Kanazawa 1988 | Not a population of interest for this review |
| Khaitiyar 2000 | Not a RCT |
| Kim 1997 | Additional treatments neither equal nor randomised between groups |
| Kitano 1989 | Not a population of interest for this review |
| Kitano 1992 | Not a population of interest for this review |
| Kleber 1987 | Not a RCT |
| Kleber 1991 | Not a population of interest for this review |
| Korula 1985 | Not a population of interest for this review |
| Krige 1996 | Not a comparison of interest for this review |
| Kumar 2009 | Not a population of interest for this review |
| Kuran 2006 | Not a RCT |
| Kuwayama 2005 | Not a population of interest for this review |
| Lacet 2016 | Not a population of interest for this review |
| Lebrec 1981 | Not a population of interest for this review |
| Lee 2001 | Not a population of interest for this review |
| Li 1995 | Not a population of interest for this review |
| Li 2000 | Not a comparison of interest for this review |
| Li 2000a | Not a comparison of interest for this review |
| Li 2016 | Not a population of interest for this review |
| Liao 2015 | Not a population of interest for this review |
| Lin 1996 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Lin 2002 | Not a population of interest for this review |
| Lin 2005 | Not a population of interest for this review |
| Lin 2006 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Liu 1998 | Not a comparison of interest for this review |
| Liu 2004 | Not a population of interest for this review |
| Lo 1993 | Not a population of interest for this review |
| Lo 1998 | Not a population of interest for this review |
| Lo 2000 | Not a comparison of interest for this review |
| Lo 2002 | Not a population of interest for this review |
| Lo 2008 | Not a population of interest for this review |
| Lo 2009a | Not a population of interest for this review |
| Lo 2009b | Not a comparison of interest for this review |
| Lo 2012 | Not a population of interest for this review |
| Lu 2004 | Not a comparison of interest for this review |
| Luo 2011 | Not a population of interest for this review |
| Luo 2015 | Not a population of interest for this review |
| Lv 2018 | Not a population of interest for this review |
| Magnano 1994 | Not a comparison of interest for this review |
| Maldonado 2002 | Not a RCT |
| Marrero 2002 | Not a RCT |
| Masci 1999 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Mastai 1986 | Not a population of interest for this review |
| Masumoto 1998 | Not a population of interest for this review |
| McCormick 1992 | Not a population of interest for this review |
| McCormick 1993 | Not a population of interest for this review |
| McKee 1990 | Not a population of interest for this review |
| Merli 1998 | Not a population of interest for this review |
| Mikkelsen 1974 | Not a comparison of interest for this review |
| Ministro 1995 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Mino 1995 | Not a RCT |
| Mo 2014 | Not a population of interest for this review |
| Monici 2010 | Not a comparison of interest for this review |
| Morales 2007 | Not a population of interest for this review |
| Moreto 1994 | Not a population of interest for this review |
| Nakamura 1998 | Not a comparison of interest for this review |
| Nakamura 2001 | Not a population of interest for this review |
| Nakase 1996 | Not a population of interest for this review |
| Narahara 2001 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| NCT00006161 | Not a population of interest for this review |
| NCT00570973 | Not a population of interest (only those who failed initial treatment were included) |
| NCT00799851 | Not a population of interest for this review |
| NCT01103154 | Not a population of interest for this review |
| NCT01640964 | Not a population of interest for this review |
| NCT02119884 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| NCT02508623 | Not a population of interest for this review |
| NCT02646202 | Not a population of interest for this review |
| NCT02740166 | Not a comparison of interest for this review |
| NCT03583996 | Not a RCT |
| NCT03687216 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| NCT03783065 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Nevens 1996a | Not a population of interest for this review |
| Nevens 1996b | Not a population of interest for this review |
| Nishikawa 1999 | Not a population of interest for this review |
| Nos 1995 | Not an intervention of interest for this review |
| O'Connor 1989 | Not a population of interest for this review |
| Ohmoto 2006 | Not a population of interest for this review |
| Okano 2003a | Not a RCT |
| Okano 2003b | Not a RCT |
| Orloff 1962 | Not a RCT |
| Orloff 1974 | Not a RCT |
| Orloff 2014 | Not a RCT |
| Otte 1983 | Not a population of interest for this review |
| Palazzi 1989 | Not a comparison of interest for this review |
| Pang 1997 | Not a population of interest for this review |
| Paquet 1983 | Not a RCT |
| Patch 2002 | Not a population of interest for this review |
| Pena 1999 | Not a population of interest for this review |
| Pena 2005 | Not a population of interest for this review |
| Pereira 1997 | Not a RCT |
| Pfisterer 2018 | Not a RCT |
| Piai 1987 | Not a RCT |
| Planas 1991 | Not a population of interest for this review |
| Pomier‐Layrargues 2001 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Pontes 1995 | Not a population of interest for this review |
| Pozzi 2005 | Not a population of interest for this review |
| Primignani 1994 | Not a population of interest for this review |
| Primignani 1995 | Not a population of interest for this review |
| Prioton 1988 | Not a population of interest for this review |
| Priyadarshi 2011 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Qi 2007 | Not a population of interest for this review |
| Queuniet 1987 | Not a population of interest for this review |
| Rawat 2015 | Not a comparison of interest for this review |
| Resnick 1969 | Not a population of interest for this review |
| Resnick 1974 | Not a population of interest for this review |
| Reynolds 1981 | Not a population of interest for this review |
| Rhodes 1986 | Not a comparison of interest for this review |
| Rikkers 1978 | Not a population of interest for this review |
| Rikkers 1993 | Not a population of interest for this review |
| Romero 2000 | Not a population of interest for this review |
| Rosemurgy 1996 | Not a population of interest for this review |
| Rossle 1997 | Not a population of interest for this review |
| Russo 2000 | Not a RCT |
| Saeed 1997 | Not a population of interest for this review |
| Santambrogio 1990 | Not a population of interest for this review |
| Santambrogio 2006 | Additional treatments neither equal nor randomised between groups |
| Santos 2011 | Not a population of interest for this review |
| Saraya 1993 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Sarin 1995 | Not a comparison of interest for this review |
| Sarin 1997 | Not a population of interest for this review |
| Sarin 2005 | Not a population of interest for this review |
| Sauerbruch 2015 | Not a comparison of interest for this review |
| Schepke 2001 | Not a population of interest for this review |
| Schiedermaier 2002 | Not a population of interest for this review |
| Schiedermaier 2003 | Not a population of interest for this review |
| Sen 2002 | Not a population of interest for this review |
| Serwah 2002 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Shah 2001 | Not a RCT |
| Sheikh 1998 | Not a RCT |
| Shigemitsu 2000 | Not a population of interest for this review |
| Shin 1998 | Not a population of interest for this review |
| Silva 2004 | Not a population of interest for this review |
| Siqueira 1998 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Smith 2013 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Sohn 2013 | Not a population of interest for this review |
| Sotto 1989 | Not a population of interest for this review |
| Spina 1990 | Not a population of interest for this review |
| Srinivasan 1997 | Not a RCT |
| Stanley 2014 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Sugano 1997 | Not a population of interest for this review |
| Sugano 2001 | Not a population of interest for this review |
| Sun 2013 | Not a population of interest for this review |
| Sung 1998 | Not a RCT |
| Svoboda 1992 | Not a RCT |
| Taniai 2002 | Not a RCT |
| Taranto 1990 | Not a population of interest for this review |
| Taupignon 1989 | Not a population of interest for this review |
| Terabayashi 1987 | Not a comparison of interest for this review |
| Terblanche 1979 | Not a population of interest for this review |
| Terblanche 1983 | Not a population of interest for this review |
| Terblanche 1988 | Not a RCT |
| Teres 1987 | Not a population of interest for this review |
| Teres 1993 | Not a population of interest for this review |
| Testa 1991 | Not a population of interest for this review |
| Thiel 1993 | Not a RCT |
| Tommasini 1989 | Not a population of interest for this review |
| Triger 1992 | Not a comparison of interest for this review |
| Tripathi 2004 | Not a population of interest for this review |
| Umehara 1999 | Not a population of interest for this review |
| Van Buuren 2000 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Van Buuren 2008 | Not a population of interest for this review |
| Van Stiegmann 1993 | Not a RCT |
| Vickers 1994 | Not a population of interest for this review |
| Villanueva 1996 | Not a population of interest for this review |
| Villanueva 2001 | Not a population of interest for this review |
| Villanueva 2002 | Not a RCT |
| Villanueva 2009 | Not a comparison of interest for this review |
| Villanueva 2017 | Not a comparison of interest for this review |
| Villeneuve 1986 | Not a population of interest for this review |
| Vorobioff 2002 | Not a population of interest for this review |
| Vorobioff 2007 | Not a population of interest for this review |
| Wang 2012 | Not a population of interest for this review |
| Westaby 1984 | Not a population of interest for this review |
| Westaby 1985b | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Westaby 1986 | Not a population of interest for this review |
| Westaby 1989 | Not clear if the studies included non‐cirrhotic participants or gastric variceal bleeding |
| Wiest 2002 | Not a RCT |
| Witzel 1982 | Not a population of interest for this review |
| Yoshida 2004 | Not a RCT |
| Young 1993 | Not a population of interest for this review |
| Zargar 2008 | Not a population of interest for this review |
| Zhang 2008 | Not a population of interest for this review |
| Zhao 1998 | Not a population of interest for this review |
| Zhao 2013 | Not a population of interest for this review |
| Zhou 2013 | Not a RCT |
| Zhu 2004 | Not a comparison of interest for this review |
| Zironi 1996 | Not a population of interest for this review |
| Zuckerman 2016 | Not a population of interest for this review |
Characteristics of studies awaiting classification [ordered by study ID]
Jirón 1992.
| Methods | Not stated |
| Participants | Not stated |
| Interventions | Group 1: sclerotherapy Further details: not stated Group 2: beta‐blocker Further details: propranolol |
| Outcomes | Not stated |
| Notes | Not available |
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐IIR‐16007964.
| Study name | Sequential therapy with oesophageal varice ligation and sclerotherapy compared with ligation alone on the obliteration and recurrence of oesophageal varices in patients with decompensated cirrhosis‐A randomized double‐blinded controlled study |
| Methods | Randomised clinical trial |
| Participants | Inclusion/exclusion criteria Inclusion: 1) Consecutive patients with decompensated cirrhosis and oesophageal varices, with the age between 18‐65 years old; 2) patients underwent endoscopic varices ligation therapy within 1 year, with current diameter of oesophageal varices<=0.6 cm; 3) all the enrolled patients signed informed consent Exclusion: 1) With liver cancer (>stage B in Barcelona Clinic Liver Cancer staging classification) or other malignancy; 2) with gastric varices or other ectopic varices; 3) with severe illness in heart, brain, lung or kidney, such as stroke, uremia, acute coronary syndrome, respiratory failure or type I hepatorenal syndrome; 4) no tolerance to endoscopy; 5) transjugular intrahepatic portosystemic stent shunt or surgery before entry; 6) total bilirubin >170 umol/L or Child‐Pugh score >13; 7 without cirrhosis; 8 use of sclerotherapy or cyanoacrylate 1 year before entry |
| Interventions | Group 1: endoscopic variceal ligation Further details: undergoing regular ligation therapy until the obliteration of varice Group 2: sclerotherapy plus endoscopic variceal ligation Further details: undergoing regular sclerotherapy with diameter of varices≤0.6cm after ligation until the obliteration of varices |
| Outcomes | Planned outcomes: Primary:
Secondary:
|
| Starting date | From 1 March 2016 |
| Contact information | Anjiang Wang waj1103b@163.com 17 Yongwai Main Street, West Nanjing Road, Donghu District, Nancang, Jiangxi, China |
| Notes |
NCT00966082.
| Study name | EBL versus EBL and propranolol for the prevention of variceal rebleeding in pts with previous variceal treatment |
| Methods | Randomised clinical trial |
| Participants | Inclusion/exclusion criteria Inclusion:
Exclusion:
|
| Interventions | Group 1: endoscopic variceal ligation Further details: perform endoscopic band ligation until eradication of oesophageal varices, and then follow‐up endoscopy with 3‐6 months interval Group 2: endoscopic variceal ligation plus betablocker Further details: perform endoscopic band ligation until eradication of oesophageal varices, and then follow‐up endoscopy with 3‐6 months interval, with propranolol |
| Outcomes | Primary: Rebleeding from oesophageal varices (Time Frame: 2 years) Secondary: Upper gastrointestinal bleeding; significant oesophageal variceal bleeding; mortality; adverse events (Time Frame: 2 years) |
| Starting date | First posted 26 August 2009 |
| Contact information | Soon Ho Um umsh@korea.ac.kr Yeon Seok Seo drseo@korea.ac.kr |
| Notes |
NCT02477384.
| Study name | 8mm‐TIPS versus endoscopic variceal ligation (EVL) plus propranolol for prevention of variceal rebleeding |
| Methods | Randomised clinical trial |
| Participants | Inclusion/exclusion criteria Inclusion:
Exclusion:
|
| Interventions | Group 1: 8mm‐TIPS Further details: patients in this group would underwent TIPS placement with 8mm‐diameter ePTFE‐covered stents Group 2: endoscopic variceal ligation plus betablocker Further details: patients in this group would underwent sequential endoscopic variceal ligation and propranolol treatment |
| Outcomes | Primary:
Secondary:
|
| Starting date | First posted 22 June 2015 |
| Contact information | Xuefeng Luo West China Hospital Chengdu, Sichuan, China, 610041 |
| Notes |
NCT03094234.
| Study name | 8mm‐TIPS versus endoscopic variceal ligation (EVL) plus propranolol for prevention of variceal rebleeding in patients with Child A cirrhosis |
| Methods | Randomised clinical trial |
| Participants | Inclusion/exclusion criteria Inclusion: cirrhosis patients who had bled from oesophageal varices (≥5 days and ≤28 days) Child‐Pugh A Exclusion: presence of gastric varices, non‐cirrhotic portal hypertension, portal vein thrombosis, history of hepatic encephalopathy, total bilirubin ≥51.3 umol/L, previous treatment of TIPS or surgery, proven malignancy including hepatocellular carcinoma, contraindications to TIPS, EVL or propranolol, end‐stage renal disease under renal replacement therapy, cardiorespiratory failure, pregnancy or patients not giving informed consent for endoscopic procedure |
| Interventions | Group 1: 8 mm‐TIPS Further details: patients in this group would underwent TIPS placement with 8 mm‐diameter ePTFE‐covered stents Group 2: endoscopic variceal ligation plus beta‐blocker Further details: patients in this group would underwent sequential endoscopic variceal ligation and propranolol treatment |
| Outcomes | Primary:
Secondary:
|
| Starting date | First posted 29 March 2017 |
| Contact information | xuefeng luo luo_xuefeng@yeah.net |
| Notes |
NCT04074473.
| Study name | Impact of nonselective beta‐blocker on acute kidney injury in cirrhotic patients with oesophageal varices |
| Methods | Randomised clinical trial |
| Participants | Inclusion/exclusion criteria Inclusion: Age of 20 to 85 years Cirrhotic patients with oesophageal varices regardless of bleeding event or not will be enrolled in this study. Exclusion: Terminal stage hepatocellular carcinoma/other malignancy/stroke or active sepsis/chronic kidney disease stage 4 under renal replacement therapy/contraindications to non‐selective beta‐blockers/a history of non‐selective beta‐blockers use, sclerotherapy, banding ligation, transjugular intrahepatic porto‐systemic shunt, or shunt surgery/serum total bilirubin >10 mg/dL/refractory ascites/hepato‐renal syndrome/ pregnancy/severe heart failure (NYHA Fc III/IV)/bronchial asthma or chronic obstructive pulmonary disease/second or third degree atrioventricular block/severe hypotension/refusal to participate |
| Interventions | Group 1: betablocker Further details: propranolol 10 mg twice daily initially and titrate dosage every week to achieve 25% drop of heart rate (keep heart rate> 55 or systemic blood pressure>90 mmHg) Group 2: oesophageal variceal ligation Further details: oesophageal variceal ligation every 3‐4 weeks to achieve variceal eradication under endoscopy. After eradication, follow‐up endoscopy every 3 months and variceal ligation again if recurrence Group 3: oesophageal variceal ligation (DC inderal after EV eradication) Further details: patients randomised to banding ligation group discontinue propranolol after eradication of oesophageal varices |
| Outcomes | Outcomes planned Primary:
Secondary:
|
| Starting date | Actual study start date 5 November 2015 |
| Contact information | Ming‐Chih Hou mchou@vghtpe.gov.tw Han‐Chieh Lin hclin@vghtpe.gov.tw |
| Notes |
EBL: endoscopic band ligation; ePTFE: expanded polytetrafluoroethylene; EVL: endoscopic variceal ligation; HBV: hepatitis B virus; NYHA: New York Heart Association; pts: patients;TIPS: transjugular intrahepatic portosystemic shunt.
Differences between protocol and review
Title change. The protocol had the following title: "Secondary prevention of bleeding in people with previous oesophageal variceal bleeding due to decompensated liver cirrhosis: a network meta‐analysis"
We clarified that we are evaluating the initial treatments rather than treatment of refractory bleeding after secondary prevention interventions.
We have added information about the definitions of treatment nodes and the ‘decision set’ to improve clarity.
We used the 'sclerotherapy' (endoscopic sclerotherapy) as the reference group (changed from 'non cardioselective beta‐blockers'), as sclerotherapy was the commonest intervention compared in the trials.
We removed the sentence 'We excluded such quasi‐randomised studies.' from the two bias risk domains on randomisation, as we write in Types of studies that we would not include non‐randomised studies.
We have replaced 'For profit' bias domain has been replaced with an Other bias domain. This was based on the guidance from CHBG.
We did not perform Trial Sequential Analysis because the risk of false positive results with Bayesian meta‐analysis is usually less or at least equivalent to Trial Sequential Analysis.
We used the latest guidance from the GRADE Working group (Yepes‐Nunez 2019) rather than the previous guidance (Puhan 2014) for presenting the 'Summary of findings' table.
The trials did not report the proportion of people with other episodes of decompensation but reported the number of episodes of decompensation. Therefore, we treated this as a count outcome and used the Poisson likelihood to calculate the rate ratio.
In the absence of a protocol published prior to the start of the study, we classified the risk of bias as low for selective reporting bias only when mortality, adverse events, and rebleeding were reported, as we anticipated these outcomes to be routinely measured in clinical trials of this nature.
We used 30,000 iterations (instead of 10,000 iterations) as a minimum for burn‐in of the simulation sampler used to estimate quantities in the statistical models to ensure convergence of the simulation sampler.
We did not present some information such as ranking probability tables, rankograms, and surface area under the curve (SUCRA plots) because of the concern about the misinterpretation of the results. We have highlighted this clearly within the text of the review along with the reasons for not presenting them.
We performed additional analyses following peer reviewer comments. The rationale for the additional analyses and impact on results are provided in the main text.
Contributions of authors
Protocol
Conceiving the protocol: KG Designing the protocol: KG Coordinating the protocol: KG Designing search strategies: KG Writing the protocol: KG Providing general advice on the protocol: ET Securing funding for the protocol: KG Both authors approved of the current protocol version
Performing previous work that was the foundation of the current study: not applicable
Review
Co‐ordinating the review: KG Study selection: KG, Danielle R, MC Data extraction: KG, Davide R, MPT, AB, LP, NW, LB, SA, TB, MC, DF Writing the review: KG, LB Providing advice on the review: SF, AJS, NC, EJM, MC, CSP, BRD, ET Securing funding for the review: KG All authors gave their final approval of the current review version to be published.
Sources of support
Internal sources
-
University College London, UK
Writing equipment, software, etc
External sources
-
National Institute for Health Research, UK
Payment for writing reviews, writing equipment, and software
Declarations of interest
None known for any of the authors.
Edited (no change to conclusions)
References
References to studies included in this review
Ahmad 2009 {published data only}
- Ahmad I, Khan AA, Alam A, Butt AK, Shafqat F, Sarwar S, et al. Propranolol, isosorbide mononitrate and endoscopic band ligation - alone or in varying combinations for the prevention of esophageal variceal rebleeding. Journal of the College of Physicians and Surgeons Pakistan 2009;19(5):283-6. [PubMed] [Google Scholar]
Alexandrino 1988 {published data only}
- Alexandrino PT, Alves MM, Pinto Correia J. Propranolol or endoscopic sclerotherapy in the prevention of recurrence of variceal bleeding. A prospective, randomized controlled trial. Journal of Hepatology 1988;7(2):175-85. [DOI] [PubMed] [Google Scholar]
Ampelas 1987 {published data only}
- Ampelas M, Guiry P, Feneyrou B, Bories P, Daures JP, Bauret P, et al. Pragmatic controlled study on the prevention of recurrent hemorrhage caused by the rupture of esophageal varices in cirrhotic patients: propranolol or disconnection of the azygos-portal by clip. Gastroenterologie Clinique et Biologique 1987;11(2):206a. [Google Scholar]
Andreani 1991 {published data only}
- Andreani T, Poupon R, Balkau B, Capron D, Desaint B, Barbare J, et al. Compared efficacy of propranolol and endoscopic sclerosis of esophageal varicose veins in the prevention of digestive haemorrhage relapse in people with cirrhosis. A controlled study. Gastroenterologie Clinique et Biologique 1991;15(2):A215. [Google Scholar]
Anonymous 1994 {published data only}
- The veterans affairs cooperative variceal sclerotherapy group. Sclerotherapy for male alcoholic cirrhotic patients who have bled from esophageal varices: results of a randomized, multicenter clinical trial. Hepatology (Baltimore, Md.) 1994;20(3):618-25. [PubMed] [Google Scholar]
Argonz 2000 {published data only}
- Argonz J, Kravetz D, Suarez A, Romero G, Bildozola M, Gonzalez B. Banding plus sclerotherapy is more effective than banding alone in preventing variceal rebleeding. Hepatology (Baltimore, Md.) 1996;24(4 Pt 2):208a. [Google Scholar]
- Argonz J, Kravetz D, Suarez A, Romero G, Bildozola M, Passamonti M, et al. Variceal band ligation and variceal band ligation plus sclerotherapy in the prevention of recurrent variceal bleeding in cirrhotic patients: a randomized, prospective and controlled trial. Gastrointestinal Endoscopy 2000;51(2):157-63. [DOI] [PubMed] [Google Scholar]
Avgerinos 1993 {published data only}
- Avgerinos A, Rekoumis G, Klonis C, Papadimitriou N, Gouma P, Pournaras S, et al. Propranolol in the prevention of recurrent upper gastrointestinal bleeding in patients with cirrhosis undergoing endoscopic sclerotherapy. A randomized controlled trial. Journal of Hepatology 1993;19(2):301-11. [DOI] [PubMed] [Google Scholar]
Avgerinos 1997 {published data only}
- Avgerinos A, Armonis A, Manolakopoulos S, Poulianos G, Rekoumis G, Sgourou A, et al. Endoscopic sclerotherapy versus variceal ligation in the long-term management of patients with cirrhosis after variceal bleeding. A prospective randomized study. Journal of Hepatology 1997;26(5):1034-41. [DOI] [PubMed] [Google Scholar]
Bader 1987 {published data only}
- Bader R, Claude P, Fos P, Khamlu A, Sondag D. Endoscopic sclerotherapy versus propranolol in the prevention of recurrent hemorrhage of esophageal varices or cirrhosis. Preliminary results of a randomized prospective study. Annales de Gastroenterologie et d'Hepatologie 1987;23(1):50-1. [Google Scholar]
- Bader R, Claude P, Planchon F, Sontag D. Preventive treatment of recurrent hemorrhage of esophageal varices in all cirrhotic subjects - endoscopic sclerosis or propanolol - preliminary-results of a randomized prospective study. Gastroenterologie Clinique et Biologique 1986;10(1):76-7. [Google Scholar]
Baroncini 1996 {published data only}
- Baroncini D, Piemontese A, Milandri GL, Borioni D, Billi P, Cennamo V. Ligation alone vs ligation in combination with sclerotherapy for the treatment of oesophageal varices: preliminary results of a perspective study. Italian Journal of Gastroenterology 1996;28(Suppl 2):49. [Google Scholar]
Baroncini 1997 {published data only}
- Baroncini D, dal Monte PP, Borioni D, Billi P, Milandri GL, D'Imperio N. Prospective, randomised comparison of ligation and sclerotherapy for oesophageal varices preliminary results. Gut 1994;35(Suppl 4):A11. [Google Scholar]
- Baroncini D, Dal Monte PP, Cennamo V, Billi P, D'Imperio N. Prospective, randomised comparison of ligation and sclerotherapy for oesophageal varices. Preliminary results. Italian Journal of Gastroenterology 1994;26(Suppl 2):15. [Google Scholar]
- Baroncini D, Milandri GL, Borioni D, Piemontese A, Cennamo V, Billi P, et al. A prospective randomized trial of sclerotherapy versus ligation in the elective treatment of bleeding esophageal varices. Endoscopy 1997;29(4):235-40. [DOI] [PubMed] [Google Scholar]
- Baroncini D, Milandri GL, Borioni D, Piemontese A, Cennamo V, Billi P, et al. Sclerotherapy versus ligation in the elective treatment of esophageal varices: results of a three years fu in a prospective randomized trial. Gastroenterology 1997;112(4):A1220. [DOI] [PubMed] [Google Scholar]
- Baroncini D, Piemontese A, Dal`Monte PP, Milandri GL, Boriono D, Cennamo V, et al. Results of Mid-term follow up of a prospective, randomized study of comparison of ligation and sclerotherapy for oesophageal varices (abstract). Endoscopy 1995;27(7):S95-96. [Google Scholar]
- Baroncini D, Piemontese A, Milandri G, Borioni D, Cennamo V, Billi P, et al. Variceal ligation compared with sclerotherapy in elective treatment: preliminary results of a prospective randomized study [Italian]. Giornale Italiano di Endoscopia Digestiva 1996;19(1):39-45. [Google Scholar]
Bertoni 1990 {published data only}
- Bertoni G, Fornaciari G, Beltrami M, Conigliaro R, Grazia Mortilla M, Ricci E, et al. Nadolol for prevention of variceal rebleeding during the course of endoscopic injection sclerotherapy: a randomized pilot study. Journal of Clinical Gastroenterology 1990;12(3):364-5. [PubMed] [Google Scholar]
Bertoni 1994 {published data only}
- Bertoni G, Sassatelli R, Fornaciari G, Briglia R, Tansini P, Grisendi A, et al. Oral isosorbide-5-mononitrate reduces the rebleeding rate during the course of injection sclerotherapy for esophageal varices. Scandinavian Journal of Gastroenterology 1994;29(4):363-70. [DOI] [PubMed] [Google Scholar]
Bonkovsky 1989 {published data only}
- Bonkovsky HL, Bonkovsky ML, Anderson PB, Rothstein RI, Erkkinen JF. Atenolol for prevention of rebleeding from esophageal varices in hepatic cirrhosis: results of a controlled, randomized pilot study. American Journal of Gastroenterology 1989;84(6):681-3. [PubMed] [Google Scholar]
Cabrera 1996 {published data only}
- Cabrera J, Maynar M, Granados R, Gorriz E, Reyes R, Pulido-Duque JM, et al. Transjugular intrahepatic portosystemic shunt versus sclerotherapy in the elective treatment of variceal hemorrhage. Gastroenterology 1996;110(3):832-9. [DOI] [PubMed] [Google Scholar]
Cennamo 1998 {published data only}
- Cennamo V, Baroncini D, Piemontese A, Billi P, Macchia S, Milandri GL, et al. Comparison of endoscopic band ligation alone and combined with low dose sclerotherapy for endoscopic treatment of oesophageal varices. A prospective randomized trial. Preliminary results. Italian Journal of Gastroenterology and Hepatology 1998;30(Suppl 2):A191. [Google Scholar]
Dasarathy 1992 {published data only}
- Dasarathy S, Dwivedi M, Bhargava DK, Sundaram KR, Ramachandran K. A prospective randomized trial comparing repeated endoscopic sclerotherapy and propranolol in decompensated (Child class-B and class-C) cirrhotic-patients. Hepatology (Baltimore, Md.) 1992;16(1):89-94. [DOI] [PubMed] [Google Scholar]
Dwivedi 1992 {published data only}
- Dwivedi M, Bhargava DK, Ramachandran K. Endoscopic sclerotherapy versus propranolol in prevention of recurrent variceal bleeding in patients with Child's B and C cirrhosis: a preliminary report. Indian Journal of Gastroenterology 1992;11(2):68-70. [PubMed] [Google Scholar]
Esquivel Lopez 1984 {published data only}
- Esquivel Lopez A, Lopez Fuertes F, Perches Vega A, Salgado-Escobar JL. Effects of propranolol in the patient with liver cirrhosis and hemorrhage caused by esophageal varices. Revista de Gastroenterologia de Mexico 1984;49(4):211-4. [PubMed] [Google Scholar]
Fleig 1988 {published data only}
- Fleig WE, Stance EF, Hunecke R. Prevention of recurrent bleeding in cirrhotics with recent variceal hemorrhage: prospective, randomized comparison of propranolol and sclerotherapy. Hepatology (Baltimore, Md.) 1987;7(2):355-61. [DOI] [PubMed] [Google Scholar]
- Fleig WE, Stange EF, Schomerus HD, Hunecke R, Jenss D, Schonborn W, et al. Propanolol against esophageal wall sclerosis - 1st results of a randomized study of cirrhotic patients with recent variceal bleeding. Zeitschrift Fur Gastroenterologie 1985;23(9):427. [Google Scholar]
- Fleig WE, Stange EF, Schonborn W, Wordehoff D, Preclik G, Nuber R, et al. Comparison of propranolol and sclerotherapy of the esophageal wall for preventing recurrence of variceal bleeding in patients with cirrhosis of the liver - results of a randomized long-term study. Zeitschrift Für Gastroenterologie 1988;26(9):457. [Google Scholar]
Fornaciari 1990 {published data only}
- Fornaciari G, Bertoni G, Beltrami M, Castagnetti E, Conigliaro R, Gumina C, et al. Nadolol for prevention of variceal rebleeding during the course of oesophageal injection sclerotherapy: a randomized pilot trial. Italian Journal of Gastroenterology 1990;22:171. [PubMed] [Google Scholar]
García‐Pagán 2009 {published data only}
- García-Pagán JC, Villanueva C, Albillos A, Bañares R, Morillas R, Abraldes JG, et al. Nadolol plus isosorbide mononitrate alone or associated with band ligation in the prevention of recurrent bleeding: a multicentre randomised controlled trial. Gut 2009;58(8):1144-50. [DOI] [PubMed] [Google Scholar]
Garcia‐Villarreal 1999 {published data only}
- Garcia-Villarreal L, Martinez-Lagares F, Sierra A, Guevara C, Marrero JM, Jimenez E, et al. Transjugular intrahepatic portosystemic shunt versus endoscopic sclerotherapy for the prevention of variceal rebleeding after recent variceal hemorrhage. Hepatology (Baltimore, Md.) 1999;29(1):27-32. [DOI] [PubMed] [Google Scholar]
Henderson 1990 {published data only}
- Henderson JM, Kutner MH, Millikan WJ Jr, Galambos JT, Riepe SP, Brooks WS, et al. Endoscopic variceal sclerosis compared with distal splenorenal shunt to prevent recurrent variceal bleeding in cirrhosis. A prospective, randomized trial. Annals of Internal Medicine 1990;112(4):262-9. [DOI] [PubMed] [Google Scholar]
- Warren WD, Henderson JM, Millikan WJ, Galambos JT, Brooks WS, Riepe SP, et al. Distal splenorenal shunt versus endoscopic sclerotherapy for long-term management of variceal bleeding. Preliminary report of a prospective, randomized trial. Annals of Surgery 1986;203(5):454-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ink 1992 {published data only}
- Ink O, Martin T, Poynard T, Reville M, Anciaux ML, Lenoir C, et al. Does elective sclerotherapy improve the efficacy of long-term propranolol for prevention of recurrent bleeding in patients with severe cirrhosis? A prospective multicenter, randomized trial. Hepatology (Baltimore, Md.) 1992;16(4):912-9. [DOI] [PubMed] [Google Scholar]
Isaksson 1995 {published data only}
- Isaksson B, Hultberg B, Hansson L, Bengtsson F, Jeppsson B. Effect of mesocaval interposition shunting and repeated sclerotherapy on blood levels of gastrointestinal regulatory peptides, amino acids, and lysosomal enzymes--a prospective randomised trial. Liver 1999;19(1):3-11. [DOI] [PubMed] [Google Scholar]
- Isaksson B, Jeppsson B, Bengtsson F, Hannesson P, Herlin P, Bengmark S. Mesocaval shunt or repeated sclerotherapy: effects on rebleeding and encephalopathy-a randomized trial. Surgery 1995;117(5):498-504. [DOI] [PubMed] [Google Scholar]
- Isaksson B, Thorell LH, Bengtsson F, Rosen I, Jeppsson B. Hepatic encephalopathy verified by psychometric testing and EEG in cirrhotic patients: effects of mesocaval interposition shunt or sclerotherapy. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2005;7(1):65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jalan 1997 {published data only}
- Jalan R, Forrest EH, Stanley AJ, Redhead DN, Forbes J, Dillon JF, et al. A randomized trial comparing transjugular intrahepatic portosystemic stent-shunt with variceal band ligation in the prevention of rebleeding from esophageal varices. Hepatology (Baltimore, Md.) 1997;26(5):1115-22. [DOI] [PubMed] [Google Scholar]
Jensen 1989 {published data only}
- Jensen LS, Krarup N. Propanolol prevent rebleeding from esophageal varices during endoscopic sclerotherapy before variceal eradication (abstract). Endoscopy 1988;20(Suppl 2):34. [Google Scholar]
- Jensen LS, Krarup N. Propranolol in prevention of rebleeding from oesophageal varices during the course of endoscopic sclerotherapy. Scandinavian Journal of Gastroenterology 1989;24(3):339-45. [DOI] [PubMed] [Google Scholar]
- Jensen LS, Krarup N. Propranolol may prevent recurrence of oesophageal varices after obliteration by endoscopic sclerotherapy. Scandinavian Journal of Gastroenterology 1990;25(4):352-6. [DOI] [PubMed] [Google Scholar]
Jiron 1993 {published data only}
- Jirón MI, Soto JR, Wolff C, Armas R. Prevention of digestive hemorrhage recurrence in hepatic cirrhosis with propranolol. A 4 years' follow-up study. Revista Medica de Chile 1993;121(2):133-8. [PubMed] [Google Scholar]
Kanazawa 1991 {published data only}
- Kanazawa H, Watari A, Matsusaka S, Tada N, Miyata K, Saitoh H, et al. Prospective controlled study of elective sclerotherapy plus oral propranolol for prevention of recurrent bleeding in cirrhotics with recent variceal hemorrhage. Japanese Journal of Gastroenterology 1991;88(6):1341-8. [PubMed] [Google Scholar]
Kong 2015 {published data only}
- Kong DR, Wang JG, Chen C, Yu FF, Wu Q, Xu JM. Effect of intravariceal sclerotherapy combined with esophageal mucosal sclerotherapy using small-volume sclerosant for cirrhotic patients with high variceal pressure. World Journal of Gastroenterology 2015;21(9):2800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 2015 {published data only}
- Kumar P, Kumar R, Saxena KN, Misra SP, Dwivedi M. Secondary prophylaxis of variceal hemorrhage: a comparative study of band ligation, carvedilol and propranolol plus isosorbide mononitrate. Indian Journal of Gastroenterology 2015;34(Suppl 1):A54. [Google Scholar]
Lundell 1990 {published data only}
- Lundell L, Leth R, Lind T, Lonroth H, Sjovall M, Olbe L. Evaluation of propranolol for prevention of recurrent bleeding from esophageal varices between sclerotherapy sessions. Acta Chirurgica Scandinavica 1990;156(10):711-5. [PubMed] [Google Scholar]
Martin 1991 {published data only}
- Martin T, Taupignon A, Lavignolle A, Perrin D, Bodic L. Prevention of recurrent hemorrhage in patients with cirrhosis. Results of a controlled trial of propranolol versus endoscopic sclerotherapy. Gastroenterologie Clinique et Biologique 1991;15(11):833-7. [PubMed] [Google Scholar]
Masliah 1997 {published data only}
- Masliah C, Gournay J, Martin T, Schnee M, Graf E, Perrin D. Isorbide 5-mononitrate with propranolol versus propranolol alone after hemorrhage by esophageal varices rupture: a randomized study. Gastroenterologie Clinique et Biologique 1997;21(2):A87. [Google Scholar]
Mckee 1994 {published data only}
- McKee RF, Garden OJ, Anderson JR, Carter DC. A trial of elective versus on demand sclerotherapy in "poor risk" patients with variceal haemorrhage. Endoscopy 1994;26(5):474-7. [DOI] [PubMed] [Google Scholar]
Parelon 1989 {published data only}
- Parelon G, Guiry P, Daures JP, Bories P, Feneyrou B, Ampelas M, et al. Prevention of recurrent hemorrhage caused by the rupture of esophageal varices in cirrhotic patients. A controlled study of propranolol and clip ligation of the esophagus. Presse Medicale 1989;18(35):1743-7. [PubMed] [Google Scholar]
Romero 2006 {published data only}
- Romero G, Kravetz D, Argonz J, Vulcano C, Suarez A, Fassio E, et al. Comparative study between nadolol and 5-isosorbide mononitrate vs. endoscopic band ligation plus sclerotherapy in the prevention of variceal rebleeding in cirrhotic patients: a randomized controlled trial. Alimentary Pharmacology & Therapeutics 2006;24(4):601-11. [DOI] [PubMed] [Google Scholar]
Rossi 1991 {published data only}
- Rossi V, Cales P, Burtin P, Charneau J, Person B, Pujol P, et al. Prevention of recurrent variceal bleeding in alcoholic cirrhotic-patients - prospective controlled trial of propranolol and sclerotherapy. Journal of Hepatology 1991;12(3):283-9. [DOI] [PubMed] [Google Scholar]
Sanyal 1997 {published data only}
- Sanyal AJ, Freedman AM, Luketic VA, Purdum PP, Shiffman ML, Cole PE, et al. Transjugular intrahepatic portosystemic shunts compared with endoscopic sclerotherapy for the prevention of recurrent variceal hemorrhage. A randomized, controlled trial. Annals of Internal Medicine 1997;126(11):849-57. [DOI] [PubMed] [Google Scholar]
Sauer 1997 {published data only}
- Sauer P, Theilmann L, Benz C, Roeren T, Richter G, Stremmel W, et al. TIPS versus endoscopic sclerosis + propranolol in the prevention of relapsing bleeding from oesophageal varices: a randomised study. Zeitschrift Fur Gastroenterologie 1997;35(5):425. [Google Scholar]
- Sauer P, Theilmann L, Stremmel W, Benz C, Richter GM, Stiehl A. Transjugular intrahepatic portosystemic stent shunt versus sclerotherapy plus propranolol for variceal rebleeding. Gastroenterology 1997;113(5):1623-31. [DOI] [PubMed] [Google Scholar]
Sauer 2002 {published data only}
- Sauer P, Hansmann J, Richter GM, Stremmel W, Stiehl A. Endoscopic variceal ligation plus propranolol vs. transjugular intrahepatic portosystemic stent shunt: a long-term randomized trial. Endoscopy 2002;34(9):690-7. [DOI] [PubMed] [Google Scholar]
Sheen 1989 {published data only}
- Sheen IS, Chen TY, Liaw YF. Randomized controlled study of propranolol for prevention of recurrent esophageal varices bleeding in patients with cirrhosis. Liver 1989;9(1):1-5. [DOI] [PubMed] [Google Scholar]
Urbistondo 1996 {published data only}
- Urbistondo M, Torres EA, Castro F, Oharriz J, Medina R, Molina A, et al. Prevention of recurrent esophageal bleeding and survival in patients with alcoholic cirrhosis: a randomized study. Puerto Rico Health Sciences Journal 1996;15(3):195-9. [PubMed] [Google Scholar]
Viazis 2002 {published data only}
- Viazis N, Armonis A, Vlachogiannakos J, Rekoumis G, Stefanidis G, Papadimitriou N, et al. Effects of endoscopic variceal treatment on oesophageal function: a prospective, randomized study. European Journal of Gastroenterology & Hepatology 2002;14(3):263-9. [DOI] [PubMed] [Google Scholar]
Villanueva 1994 {published data only}
- Villanueva C, Martinez FJ, Torras X, Sainz S, Soriano G, Gonzalez D, et al. Nadolol as an adjuvant to sclerotherapy of esophageal varices for prevention of recurrent hemorrhaging. Revista Espanola de Enfermedades Digestivas 1994;86(1):499-504. [PubMed] [Google Scholar]
Vinel 1992 {published data only}
- Vinel JP, Lamouliatte H, Cales P, Combis JM, Roux D, Desmorat H, et al. Propranolol reduces the rebleeding rate during endoscopic sclerotherapy before variceal obliteration. Gastroenterology 1992;102(5):1760-3. [DOI] [PubMed] [Google Scholar]
Westaby 1985a {published data only}
- Clark AW, Macdougall BR, Westaby D, Mitchell KJ, Silk DB, Strunin L, et al. Prospective controlled trial of injection sclerotherapy in patient with cirrhosis and recent variceal haemorrhage. Lancet 1980;2(8194):552-4. [DOI] [PubMed] [Google Scholar]
- MacDougall BR, Westaby D, Theodossi A. Increased long-term survival in variceal haemorrhage using injection sclerotherapy. Results of controlled trial. Lancet 1982;1(8264):124-7. [DOI] [PubMed] [Google Scholar]
- Westaby D, Macdougall BR, Williams R. Improved survival following injection sclerotherapy for esophageal varices: final analysis of a controlled trial. Hepatology (Baltimore, Md.) 1985;5(5):827-30. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abd Elmoety 2015 {published data only}
- Abd EA, Hamza Y, Zeid A, Kamal A, Ibrahim R, Sidkey A, et al. Endoscopic variceal ligation followed by argon plasma coagulation versus endoscopic variceal ligation alone: a randomized controlled trial. Journal of Hepatology 2015;62:S365. [DOI] [PubMed] [Google Scholar]
- Abd Elmoety A, Hamza Y, Zeid A, Kamal A. Endoscopic variceal ligation followed by argon plasma coagulation versus endoscopic variceal ligation alone: a randomized controlled trial. United European Gastroenterology Journal 2015;3(5 Suppl 1):A284. [DOI] [PubMed] [Google Scholar]
Abraldes 2016 {published data only}
- Abraldes JG, Villanueva C, Aracil C, Turnes J, Hernandez-Guerra M, Genesca J, et al. Addition of simvastatin to standard therapy for the prevention of variceal rebleeding does not reduce rebleeding but increases survival in patients with cirrhosis. Gastroenterology 2016;150(5):1160-70. [DOI] [PubMed] [Google Scholar]
Acharya 1993 {published data only}
- Acharya SK, Dasarathy S, Saksena S, Pande JN. A prospective randomised study to evaluate propranolol in patients undergoing long-term endoscopic sclerotherapy. Journal of Hepatology 1993;19(2):291-300. [DOI] [PubMed] [Google Scholar]
Adson 1984 {published data only}
- Adson MA, Van Heerden JA, Ilstrup DM. The distal splenorenal shunt. Archives of Surgery 1984;119(5):609-14. [DOI] [PubMed] [Google Scholar]
Agarwal 2015 {published data only}
- Agarwal A, Kumar S, Kumar SS, Kate V. Antibiotic prophylaxis in the prevention of rebleeding in acute variceal hemorrhage: a randomized trial. Journal of Pharmacology & Pharmacotherapeutics 2015;6(1):24-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Agarwala 2011 {published data only}
- Agarwala V, Prakash G, Singh R, Paliwal P, Tiwari S, Juber S, et al. Evaluation of treatment with carvedilol in comparison to propranolol in primary/secondary prophylaxis of gastroesophageal variceal bleeding. Indian Journal of Gastroenterology 2011;30(1 Suppl 1):A46. [Google Scholar]
Akriviadis 1989 {published data only}
- Akriviadis E, Korula J, Gupta S, Ko Y, Yamada S. Frequent endoscopic variceal sclerotherapy increases risk of complications. Prospective randomized controlled study of two treatment schedules. Digestive Diseases and Sciences 1989;34(7):1068-74. [DOI] [PubMed] [Google Scholar]
Albillos 1996 {published data only}
- Albillos A, Garcia-Pagan JC, Iborra J, Bandi JC, Cacho G, Paramo MP. Propranolol plus prazosin versus propranolol plus isosorbide 5-mononitrate in the treatment of portal hypertension. Hepatology (Baltimore, Md.) 1996;24(4):206a. [Google Scholar]
Al Traif 1999 {published data only}
- Al Traif I, Fachartz FS, Al-Jumah A, Al-Johani M, al-Omair A, al-Bakr F, et al. Randomized trial of ligation versus combined ligation and sclerotherapy for bleeding esophageal varices. Gastrointestinal Endoscopy 1999;50(1):1-6. [DOI] [PubMed] [Google Scholar]
Am. Soc. Gastro. Endo. 1998 {published data only}
- American Society for Gastrointestinal Endoscopy. The role of endoscopic therapy in the management of variceal hemorrhage. Gastrointestinal Endoscopy 1998;48(6):697-8. [DOI] [PubMed] [Google Scholar]
Baik 2005 {published data only}
- Baik SK, Jeong PH, Ji SW, Yoo BS, Kim HS, Lee DK, et al. Acute hemodynamic effects of octreotide and terlipressin in patients with cirrhosis: a randomized comparison. American Journal of Gastroenterology 2005;100(3):631-5. [DOI] [PubMed] [Google Scholar]
Balatsos 1997 {published data only}
- Balatsos V, Delis V, Germanopoulos A, Konstandinidis A, Pantes A, Skandalis N. Endoscopic ligation plus sclerotherapy vs ligation alone for esophageal variceal bleeding. Endoscopy 1997;29(Suppl 7):E44. [Google Scholar]
Banares 1999 {published data only}
- Banares R, Moitinho E, Piqueras B, Casado M, Garcia-Pagan JC, Diego A, et al. Carvedilol, a new nonselective beta-blocker with intrinsic anti- Alpha1-adrenergic activity, has a greater portal hypotensive effect than propranolol in patients with cirrhosis. Hepatology (Baltimore, Md.) 1999;30(1):79-83. [DOI] [PubMed] [Google Scholar]
Bandi 1998 {published data only}
- Bandi JC, Garcia-Pagan JC, Escorsell A, Francois E, Moitinho E, Rodes J, et al. Effects of propranolol on the hepatic hemodynamic response to physical exercise in patients with cirrhosis. Hepatology (Baltimore, Md.) 1998;28(3):677-82. [DOI] [PubMed] [Google Scholar]
Barrioz 1998 {published data only}
- Barrioz T, Borderie C, Ingrand P, Strock P, Silvain C, Beauchant M. Octreotide infusion increases the lower esophageal sphincter tone in cirrhotic patients: a potential role on variceal blood flow. Hepatology (Baltimore, Md.) 1996;24(4):207a. [Google Scholar]
- Barrioz T, Borderie C, Strock P, Ingrand P, Fort E, Silvain C, et al. Effects of octreotide on lower esophageal sphincter in patients with cirrhosis and portal hypertension. Digestive Diseases and Sciences 1998;43(7):1566-71. [DOI] [PubMed] [Google Scholar]
Bellis 2003 {published data only}
- Bellis L, Berzigotti A, Abraldes JG, Moitinho E, García-Pagán JC, Bosch J, et al. Low doses of isosorbide mononitrate attenuate the postprandial increase in portal pressure in patients with cirrhosis. Hepatology (Baltimore, Md.) 2003;37(2):378-84. [DOI] [PubMed] [Google Scholar]
Benner 1996 {published data only}
- Benner KG. Endoscopic versus TIPS therapy for the prevention of variceal hemorrhage: is TIPS tops? Hepatology (Baltimore, Md.) 1996;24(6):1537-9. [DOI] [PubMed] [Google Scholar]
Berardi 1974 {published data only}
- Berardi RS. Vascular complications of superior mesenteric artery infusion with pitressin in treatment of bleeding esophageal varices. American Journal of Surgery 1974;127(6):757-61. [DOI] [PubMed] [Google Scholar]
Berner 1994 {published data only}
- Berner JS, Gaing AA, Sharma R, Almenoff PL, Muhlfelder T, Korsten MA. Sequelae after esophageal variceal ligation and sclerotherapy: a prospective randomized study. American Journal of Gastroenterology 1994;89(6):852-8. [PubMed] [Google Scholar]
Bhargava 1992 {published data only}
- Bhargava DK, Singh B, Dogra R, Dasarathy S, Sharma MP. Prospective randomized comparison of sodium tetradecyl sulfate and polidocanol as variceal sclerosing agents. American Journal of Gastroenterology 1992;87(2):182-6. [PubMed] [Google Scholar]
Bhargava 1997 {published data only}
- Bhargava DK, Pokharna R. Endoscopic variceal ligation versus endoscopic variceal ligation and endoscopic sclerotherapy: a prospective randomized study. American Journal of Gastroenterology 1997;92(6):950-3. [PubMed] [Google Scholar]
Bhuiyan 2007 {published data only}
- Bhuiyan MM, Rahman MM, Kibria MG, Hasan M. Comparative study of endoscopic band ligation and sclerotherapy for treatment of oesophageal varices in cirrhotic patients. Bangladesh Medical Research Council Bulletin 2007;33(1):31-9. [PubMed] [Google Scholar]
Bobadilla‐Diaz 2002 {published data only}
- Bobadilla-Diaz J, Castro-Narro GI, Caza-Chavez E, Ibarra-Palomino J, Sanchez-Cortes E, Gonzalez-Huezo S, et al. Prospective study of endoscopic variceal ligation (EVL) plus endoscopic sclerotherapy vs EVL alone for the treatment of esophageal varices. Journal of Hepatology 2002;36(Suppl 1):198. [Google Scholar]
Bolognesi 1994 {published data only}
- Bolognesi M, Sacerdoti D, Merkel C, Gatta A. Duplex doppler sonographic evaluation of splanchnic and renal effects of single agent and combined therapy with nadolol and isosorbide-5-mononitrate in cirrhotic patients. Journal of Ultrasound in Medicine 1994;13(12):945-52. [DOI] [PubMed] [Google Scholar]
Bolognesi 1995 {published data only}
- Bolognesi M, Sacerdoti D, Merkel C, Caregaro L, Bellon S, Gatta A. Duplex doppler ultrasonography allows a multiorgan noninvasive approach to splanchnic pharmacodynamics in patients with cirrhosis. Bildgebung 1995;62(2):138-43. [PubMed] [Google Scholar]
Bonilha 2010 {published data only}
- Bonilha D, Correia L M, Gomes GF, Brito J D, Costa PP, Lenz L, et al. Endoscopic variceal ligation (EVL) alone versus endoscopic variceal ligation plus propranolol in variceal bleeding prophylaxis in cirrhotic patients. Gastrointestinal Endoscopy 2010;71:AB171-2. [Google Scholar]
Bories 1987 {published data only}
- Bories P, Ampelas M, Fenneyroy B, Guiry P, Daures JP, Bauret P, et al. Prevention of the recurrence of bleeding from oesophageal varices in cirrhosis: propranolol versus oesophageal clip, a randomized trial. Journal of Hepatology 1987;5(Suppl 1):S13. [Google Scholar]
Bosch 2013 {published data only}
- Bosch J. Carvedilol for preventing recurrent variceal bleeding: waiting for convincing evidence. Hepatology (Baltimore, Md.) 2013;57(4):1665-7. [DOI] [PubMed] [Google Scholar]
Braga 1991 {published data only}
- Braga M, Ravelli P, Missale G, Lancini GP, Cestari R. Portal hypertensive patients: effect of oral administration of propranolol on intravascular oesophageal variceal pressure (IOVP). A single-blind random study. Giornale Italiano di Endoscopia Digestiva 1991;14(4):331-40. [Google Scholar]
Brensing 2002 {published data only}
- Brensing KA, Horsch M, Textor J, Schiedermaier P, Raab P, Schepke M, et al. Hemodynamic effects of propranolol and nitrates in cirrhotics with transjugular intrahepatic portosystemic stent-shunt. Scandinavian Journal of Gastroenterology 2002;37(9):1070-6. [DOI] [PubMed] [Google Scholar]
Burroughs 1983 {published data only}
- Burroughs AK, Jenkins WJ, Sherlock S, Dunk A, Walt RP, Osuafor TO, et al. Controlled trial of propranolol for the prevention of recurrent variceal hemorrhage in patients with cirrhosis. New England Journal of Medicine 1983;309(25):1539-42. [DOI] [PubMed] [Google Scholar]
Buuren 1999 {published data only}
- Buuren HR, Groeneweg M, Vleggaar FP, Lesterhuis W, Tilburg AJ, Hop WC. The results of a randomized controlled trial evaluating TIPS and endoscopic therapy in cirrhotic patients with gastro-esophageal variceal bleeding. European Journal of Gastroenterology & Hepatology 1999;11(12):A85. [Google Scholar]
Callow 1970 {published data only}
- Callow AD, Resnick RH, Chalmers TC, Ishihara AM, Garceau AJ, O'Hara ET. Conclusions from a controlled trial of the prophylactic portacaval shunt. Surgery 1970;67(1):97-103. [PubMed] [Google Scholar]
Cestari 1990 {published data only}
- Cestari R, Braga M, Missale G, Ravelli P, Burroughs AK. Haemodynamic effect of triglycyl-lysine-vasopressin (glypressin) on intravascular oesophageal variceal pressure in patients with cirrhosis. A randomized placebo controlled trial. Journal of Hepatology 1990;10(2):205-10. [DOI] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen S, Li X, Wei B, Tang C. A prospective evaluation of multi-factors on the efficacy of transjugular intrahepatic portosystemic shunt in the patients with hepatitis B virus related hepatic cirrhosis. Gastroenterology 2012;142(5 Suppl 1):S949. [Google Scholar]
- Chen S, Li X, Wei B, Tong H, Zhang MG, Huang ZY, et al. Recurrent variceal bleeding and shunt patency: prospective randomized controlled trial of transjugular intrahepatic portosystemic shunt alone or combined with coronary vein embolization. Radiology 2013;268(3):900-6. [DOI] [PubMed] [Google Scholar]
Chen 2016 {published data only}
- Chen J, Zeng XQ, Ma LL, Li B, Tseng YJ, Lian JJ, et al. Randomized controlled trial comparing endoscopic ligation with or without sclerotherapy for secondary prophylaxis of variceal bleeding. European Journal of Gastroenterology & Hepatology 2016;28(1):95-100. [DOI] [PubMed] [Google Scholar]
Chen 2018 {published data only}
- Chen PW, Wang HM. Randomized controlled trial of scleroligation versus band ligation for eradication of gastroesophageal varices. Gastrointestinal Endoscopy 2018;87(3):904. [DOI] [PubMed] [Google Scholar]
Chen 2019 {published data only}
- Chen H, Wang Y, Jiang L, Chen W, Weng H, Xu G. A randomized controlled trial of endoscopic ultrasound guided selective variceal devascularization versus standard endoscopic variceal obturation for secondary prophylaxis of gastroesophageal variceal bleeding in a tertiary teaching hospital. Gastroenterology 2019;156(6):S1511-12. [Google Scholar]
Cheng 2001 {published data only}
- Cheng YS, Pan S, Lien GS, Suk FM, Wu MS, Chen JN, et al. Adjuvant sclerotherapy after ligation for the treatment of esophageal varices: a prospective, randomized long-term study. Gastrointestinal Endoscopy 2001;53(6):566-71. [DOI] [PubMed] [Google Scholar]
ChiCTR08000228 {published data only}
- ChiCTR08000228. Multicenter clinical trial of transjugular intrahepatic portosystemic shunt creation in the management of cirrhosis and portal hypertension. www.chictr.org.cn/showproj.aspx?proj=9299 (date of registration 10 January 2008).
ChiCTR11000192 {published data only}
- ChiCTR11000192. Clinical trial of transjugular intrahepatic portosystemic shunt (TIPS) combined with variceal embolization in the prevention of rebleeding in cirrhotic patients. www.chictr.org.cn/showprojen.aspx?proj=9333 (date of registration 26 January 2011).
ChiCTR11001577 {published data only}
- ChiCTR11001577. Randomized controlled study of TIPS versus EBL plus propranolol for preventing variceal rebleeding in advanced cirrhotic patients combined with portal vein thrombosis. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-TRC-11001577 (date of last refreshed on 25 March 2015).
ChiCTR12002148 {published data only}
- ChiCTR12002148. Randomized control trial of carvedilol versus variceal band ligation for the prevention of variceal bleed in HCC. www.chictr.org.cn/com/25/hvshowproject.aspx?id=2671 (date of last refreshed 25 March 2015).
ChiCTR15007655 {published data only}
- ChiCTR15007655. The effect of rubber bands placed at each endoscopic session on esophageal variceal obliteration and recurrence in patients with cirrhosis. www.chictr.org.cn/hvshowproject.aspx?id=6327 (date of last refreshed 20 February 2016).
ChiCTR1800018070 {published data only}
- ChiCTR1800018070. Clinical study of rehoci combined with long-acting octreotide in reducing the risk of rebleeding in patients with cirrhosis and portal hypertension. www.chictr.org.cn/showprojen.aspx?proj=30575 (date of registration 29 August 2018).
ChiCTR1800020322 {published data only}
- ChiCTR1800020322. Secondary prophylaxis of esophageal and gastric variceal varices bleeding in cirrhotic portal hypertension under HVPG guidance: a prospective, multicenter, randomized controlled study. www.chictr.org.cn/hvshowproject.aspx?id=14265 (date of last refreshed 23 December 2018).
ChiCTR1900021212 {published data only}
- ChiCTR1900021212. Randomized controlled clinical trial for secondary prevention strategy of cirrhosis based on NSBB response stratification. www.chictr.org.cn/hvshowproject.aspx?id=15016 (date of last refreshed 2 January 2019).
Cipolletta 2002 {published data only}
- Cipolletta L, Bianco MA, Rotondano G, Marmo R, Meucci C, Piscopo R. Argon plasma coagulation (APC) prevents variceal recurrence after endoscopic band ligation (EBL) of esophageal varices: preliminary results of a randomized trial. Gastrointestinal Endoscopy 2001;53(5):AB66. [DOI] [PubMed] [Google Scholar]
- Cipolletta L, Bianco MA, Rotondano G, Marmo R, Meucci C, Piscopo R. Argon plasma coagulation prevents variceal recurrence after band ligation of esophageal varices: preliminary results of a prospective randomized trial. Gastrointestinal Endoscopy 2002;56(4):467-71. [DOI] [PubMed] [Google Scholar]
Cirera 1995 {published data only}
- Cirera I, Feu F, Luca A, Garcia-Pagan JC, Fernandez M, Escorsell A, et al. Effects of bolus injections and continuous infusions of somatostatin and placebo in patients with cirrhosis: a double-blind hemodynamic investigation. Hepatology (Baltimore, Md.) 1995;22(1):106-11. [PubMed] [Google Scholar]
Colombo 1989 {published data only}
- Colombo M, Franchis R, Tommasini M, Sangiovanni A, Dioguardi N. Beta-blockade prevents recurrent gastrointestinal bleeding in well-compensated patients with alcoholic cirrhosis: a multicenter randomized controlled trial. Hepatology (Baltimore, Md.) 1989;9(3):433-8. [DOI] [PubMed] [Google Scholar]
- Colombo M, Sangiovanni A, Tommasini MA, De Franchis R. Beta-blockers in the prophylaxis of recurrent hemorrhage in cirrhotic patients. Random multicentric study. Argomenti di Gastroenterologia Clinica 1990;3(2):93-100. [Google Scholar]
Conn 1986 {published data only}
- Conn HO. Vasopressin and nitroglycerin in the treatment of bleeding varices: the bottom line. Hepatology (Baltimore, Md.) 1986;6(3):523-5. [DOI] [PubMed] [Google Scholar]
Conn 1987 {published data only}
- Conn HO. Endoscopic sclerotherapy versus percutaneous transhepatic obliteration of varices: a conceptual approach. Gastroenterology 1987;93(6):1428-31. [DOI] [PubMed] [Google Scholar]
Conn 1993 {published data only}
- Conn HO. Sclerotherapy versus beta blockade: unanticipated anomalies of experimental design. Gastroenterology 1993;105(5):1575-7. [DOI] [PubMed] [Google Scholar]
Copaci 2012 {published data only}
- Copaci I, Micu L, Mindrut E, Voiculescu M. Endoscopic variceal ligation alone versus endoscopic variceal ligation plus carvedilol in variceal bleeding prophylaxis in cirrhotic patients. Journal of Hepatology 2012;56:S261-2. [Google Scholar]
Costa 2016 {published data only}
- Costa LC, Neto JB, Ribeiro LT, Oliveira FS, Wyszomirska RF, Strauss E. Schistosomal portal hypertension: randomized trial comparing endoscopic therapy alone or preceded by esophagogastric devascularization and splenectomy. Annals of Hepatology 2016;15(5):738-44. [DOI] [PubMed] [Google Scholar]
D'Amico 1998 {published data only}
- D'Amico G, Politi F, D'Antoni A, Giannuoli G, Pasta L, Vizziani G, et al. Second study shows that octreotide may prevent early rebleeding in cirrhosis. BMJ (Clinical Research Ed.) 1998;316(7140):1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico G, Politi F, Morabito A, D'Antoni A, Guerrera D, Giannuoli G, et al. Octreotide compared with placebo in a treatment strategy for early rebleeding in cirrhosis, a double blind, randomized pragmatic trial. Hepatology (Baltimore, Md.) 1998;28(5):1206-14. [DOI] [PubMed] [Google Scholar]
D'Amico 2008 {published data only}
- D'Amico G, Luca A. TIPS is a cost effective alternative to surgical shunt as a rescue therapy for prevention of recurrent bleeding from esophageal varices. Journal of Hepatology 2008;48(3):387-90. [DOI] [PubMed] [Google Scholar]
De 2002 {published data only}
- De BK, Das D, Sen S, Biswas PK, Mandal SK, Majumdar D, et al. Acute and 7-day portal pressure response to carvedilol and propranolol in cirrhotics. Journal of Gastroenterology and Hepatology 2002;17(2):183-9. [DOI] [PubMed] [Google Scholar]
De 2003 {published data only}
- De BK, Bandyopadhyay K, Das TK, Das D, Biswas PK, Majumdar D, et al. Portal pressure response to losartan compared with propranolol in patients with cirrhosis. American Journal of Gastroenterology 2003;98(6):1371-6. [DOI] [PubMed] [Google Scholar]
Dehesa 1994 {published data only}
- Dehesa M, Vargas A, Blanco R, Blancas JM, Paz V, Turcios S. A randomized controlled study of gastro-gastro anastomosis vs sclerotherapy in the prevention of recurrent variceal hemorrhage. A preliminary report. Hepatology (Baltimore, Md.) 1994;19(4):58. [Google Scholar]
de la Pena 1999 {published data only}
- la Pena J, Rivero M, Sanchez E, Fabrega E, Crespo J, Pons-Romero F. Variceal ligation compared with endoscopic sclerotherapy for variceal hemorrhage: prospective randomized trial. Gastrointestinal Endoscopy 1999;49(4):417-23. [DOI] [PubMed] [Google Scholar]
de la Pena 2005 {published data only}
- la Pena J, Brullet E, Sanchez-Hernandez E, Rivero M, Vergara M, Martin-Lorente JL, et al. Variceal ligation plus nadolol compared with ligation for prophylaxis of variceal rebleeding: a multicenter trial. Hepatology (Baltimore, Md.) 2005;41(3):572-8. [DOI] [PubMed] [Google Scholar]
Djurdjevic 1999 {published data only}
- Djurdjevic D, Janosevic S, Dapcevic B, Vukcevic V, Djordjevic D, Svorcan P, et al. Combined ligation and sclerotherapy versus ligation alone for eradication of bleeding esophageal varices: a randomized and prospective trial. Endoscopy 1999;31(4):286-90. [DOI] [PubMed] [Google Scholar]
Dollet 1988 {published data only}
- Dollet JM, Champigneulle B, Evangelista M, Bigard MA, Gaucher P. Sclerotherapy versus propranolol after first variceal haemorrhage in alcoholic cirrhosis. Lancet 1985;2(8446):97. [DOI] [PubMed] [Google Scholar]
- Dollet JM, Champigneulle B, Patris A, Bigard MA, Gaucher P. Endoscopic sclerotherapy versus propranolol after hemorrhage caused by rupture of esophageal varices in patients with cirrhosis. Results of a 4-year randomized study. Gastroenterologie Clinique et Biologique 1988;12(3):234-9. [PubMed] [Google Scholar]
Domagk 2000 {published data only}
- Domagk D, Kucharzik T, Heindel WL, Domschke W, Menzel J. Endoscopic variceal banding compared with transjugular intrahepatic portosystemic shunt in the prevention of esophageal variceal rebleeding. A randomized trial. Gastrointestinal Endoscopy 2000;51(4):AB129. [Google Scholar]
Dong 2018 {published data only}
- Dong HD. Esophageal fibrosis formation in cirrhosis patients after eradication of esophageal varices by endoscopic band ligation. Journal of Gastroenterology and Hepatology 2018;33(Suppl 4):154. [Google Scholar]
Dunk 1988 {published data only}
- Dunk AA, Moore J, Symon A, Dickie A, Sinclair TS, Mowat NA, et al. The effects of propranolol on hepatic encephalopathy in patients with cirrhosis and portal hypertension. Alimentary Pharmacology & Therapeutics 1988;2(2):143-51. [DOI] [PubMed] [Google Scholar]
Dunne 2019 {published data only}
- Dunne P, Sinha R, Stanley A, Lachlan N, Ireland H, Shams A, et al. Use of early-tipss in patients with oesophageal variceal bleeding, a UK dual-centre randomised control trial. Gut 2019;68:A105-6. [Google Scholar]
Durdevic 1997 {published data only}
- Durdevic D, Golubovic G, Lukacevic S, Grgov S, Vukcevic V, Dordevic D. Combination of endoscopic ligation and low-volume sclerotherapy versus ligation alone in eradication of bleeding esophageal varices: a multicenter, prospective and randomized trial. Archives of Gastroenterohepatology 1997;16(3):73-6. [Google Scholar]
Dwivedi 1995 {published data only}
- Dwivedi M, Misra SP. Propranolol or sclerotherapy to prevent variceal rebleeding. National Medical Journal of India 1995;8(5):222. [PubMed] [Google Scholar]
Eleftheriadis 1998 {published data only}
- Eleftheriadis E, Kotzampassi K, Koufogiannis D. Modulation of intravariceal pressure with pentoxifylline: a possible new approach in the treatment of portal hypertension. American Journal of Gastroenterology 1998;93(12):2431-5. [DOI] [PubMed] [Google Scholar]
El‐Saadany 2007 {published data only}
- El-Saadany M, El-Meneesy A, Hamed M. Argon plasma coagulation following endoscopic injection sclerotherapy for the prevention of esophageal variceal recurrence and rebleeding. Journal of Hepatology 2007;46(Suppl 1):S92-3. [Google Scholar]
Elsayed 1996 {published data only}
- Elsayed SS, Farag FM, Azzam F, Awad M, Hamid M, Shiha G. Sclerotherapy versus sclerotherapy and propranol in the prevention of rebleeding from oesophageal varices: a randomized study. Endoscopy 1994;26(4):403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsayed SS, Farag FM, Azzam F, Awad M, Hamid M, Shiha G. Sclerotherapy versus sclerotherapy and propranolol in the prevention of rebleeding from oesophageal varices: a randomized study. Gut 1994;35(Suppl 4):A25-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsayed SS, Shiha G, Hamid M, Farag FM, Azzam F, Awad M. Sclerotherapy versus sclerotherapy and propranolol in the prevention of rebleeding from oesophageal varices: a randomised study. Gut 1996;38(5):770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Tourabi 1994 {published data only}
- El-Tourabi H, el-Amin AA, Shaheen M, Woda SA, Homeida M, Harron DW. Propranolol reduces mortality in patients with portal hypertension secondary to schistosomiasis. Annals of Tropical Medicine and Parasitology 1994;88(5):493-500. [DOI] [PubMed] [Google Scholar]
Escorsell 1996 {published data only}
- Escorsell A, Feu F, Bordas JM, Garcia-Pagan JC, Luca A, Bosch J, et al. Effects of isosorbide-5-mononitrate on variceal pressure and systemic and splanchnic haemodynamics in patients with cirrhosis. Journal of Hepatology 1996;24(4):423-9. [DOI] [PubMed] [Google Scholar]
Escorsell 1997 {published data only}
- Escorsell A, Bandi JC, Moitinho E, Feu F, Garcia-Pagan JC, Bosch J, et al. Time profile of the haemodynamic effects of terlipressin in portal hypertension. Journal of Hepatology 1997;26(3):621-7. [DOI] [PubMed] [Google Scholar]
Escorsell 1997a {published data only}
- Escorsell A, Bordas JM, Feu F, Garcia-Pagan JC, Gines A, Bosch J, et al. Endoscopic assessment of variceal volume and wall tension in cirrhotic patients: effects of pharmacological therapy. Gastroenterology 1997;113(5):1640-6. [DOI] [PubMed] [Google Scholar]
Escorsell 1998 {published data only}
- Escorsell A, Bordas JM, Arbol LR, Jaramillo JL, Planas R, Bañares R, et al. Randomized controlled trial of sclerotherapy versus somatostatin infusion in the prevention of early rebleeding following acute variceal hemorrhage in patients with cirrhosis. Variceal Bleeding Study Group. Journal of Hepatology 1998;29(5):779-88. [DOI] [PubMed] [Google Scholar]
Escorsell 2001 {published data only}
- Escorsell A, Bandi JC, Andreu V, Moitinho E, Garcia-Pagan JC, Bosch J, et al. Desensitization to the effects of intravenous octreotide in cirrhotic patients with portal hypertension. Gastroenterology 2001;120(1):161-9. [DOI] [PubMed] [Google Scholar]
Escorsell 2002 {published data only}
- Escorsell A, Baares R, Garcia-Pagan JC, Gilabert R, Moitinho E, Piqueras B, et al. TIPS versus drug therapy in preventing variceal rebleeding in advanced cirrhosis: a randomized controlled trial. Hepatology (Baltimore, Md.) 2002;35(2):385-92. [DOI] [PubMed] [Google Scholar]
Estevens 1996 {published data only}
- Estevens J, Guerreiro H, Belo T, Inacio C, Sousa D, Caldeira P, et al. Octreotide versus placebo in elective sclerotherapy of esophageal varices. Gastroenterology 1996;110(4):A15. [Google Scholar]
EUCTR2005‐003557‐27 {published data only}
- EUCTR2005-003557-27. Covered transjugular intrahepatic portosystemic stent shunt versus optimized medical treatment for the secondary prevention of variceal bleeding in cirrhosis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2005-003557-27-DE (first posted 25 November 2005).
EUCTR2006‐006393‐14 {published data only}
- EUCTR2006-006393-14. Double-blind, randomised, parallel-group, placebo-controlled, multi-centre phase ii clinical study on the efficacy and safety of different doses of udenafil in cirrhotic patients with portal hypertension preceded by an open-label pilot phase - udenafil tablets vs. placebo in portal hypertension. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2006-006393-14-LT (first posted 2 February 2012).
EUCTR2012‐002489‐11 {published data only}
- EUCTR2012-002489-11. An investigational study to assess the safety and efficacy of a new investigational drug in subjects with compensated liver cirrhosis secondary to non-alcoholic steatohepatitis (NASH). www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2012-002489-11-GB (first posted 29 October 2012).
EUCTR2014‐002018‐21 {published data only}
- EUCTR2014-002018-21. 5-Metyl-tetrahydrofolate in the treatment of portal hypertension in cirrhotics in pharmacologic prophylaxis of variceal bleeding with beta-blockers: a double-blind randomized controlled trial. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2014-002018-21-IT (first posted 16 October 2014).
Evrard 2003 {published data only}
- Evrard S, Dumonceau JM, Delhaye M, Golstein P, Deviere J, Le Moine O. Endoscopic histoacryl obliteration vs. propranolol in the prevention of esophagogastric variceal rebleeding: a randomized trial. Endoscopy 2003;35(9):729-35. [DOI] [PubMed] [Google Scholar]
Evrard 2008 {published data only}
- Evrard S, Le Moine O, Deviere J. The role of nonselective beta-blockers in the prevention of gastric variceal rebleeding. Endoscopy 2008;40(2):168. [DOI] [PubMed] [Google Scholar]
Fakhry 1997 {published data only}
- Fakhry S, Omar M, Mustafa I, ElBeheiry N, Hunter S. Endoscopic sclerotherapy versus endoscopic variceal ligation in the management of bleeding esophageal varices: a final report of a prospective randomized study in schistisomal hepatic fibrosis. Hepatology (Baltimore, Md.) 1997;26(4):33. [Google Scholar]
- Fakhry S, Omar M, Nouh A, Al-Ghannam M, Serry M, Attia M, et al. Endoscopic sclerotherapy versus endoscopic variceal ligation in the management of bleeding esophageal varices: a preliminary report of a prospective randomized study in schistosomal hepatic fibrosis. Hepatology (Baltimore, Md.) 1995;22(4):251a. [Google Scholar]
Farag 2005 {published data only}
- Farag M, Omar N, Salama M, Waked I, Saleh SM. Efficacy of a single endoscopic session of saturation band ligation in prevention of re-bleeding from esophageal varices. Journal of Hepatology 2005;42(Suppl 2):27. [Google Scholar]
Fernandez 2008 {published data only}
- Fernandez PF, Jimenez SM, Garcia MJ, Rebollo BJ, Herrerias GJ. Splanchnic hemodynamic effects of somatostatin and octreotide in cirrhotic patients. A Doppler ultrasonographic study. Revista Espanola de Enfermedades Digestivas 2008;100(9):552-9. [DOI] [PubMed] [Google Scholar]
Ferrari 2005 {published data only}
- Ferrari AP, Paulo GA, Macedo CM, Araujo I, Della LE. Efficacy of absolute alcohol injection compared with band ligation in the eradication of esophageal varices. Arquivos de Gastroenterologia 2005;42(2):72-6. [DOI] [PubMed] [Google Scholar]
Feu 1991 {published data only}
- Feu F, Bordas JM, Garcia-Pagán JC, Bosch J, Rodés J. Double-blind investigation of the effects of propranolol and placebo on the pressure of esophageal varices in patients with portal hypertension. Hepatology (Baltimore, Md.) 1991;13(5):917-22. [PubMed] [Google Scholar]
Feu 1993 {published data only}
- Feu F, Bordas JM, Luca A, Garcia-Pagan JC, Escorsell A, Bosch J, et al. Reduction of variceal pressure by propranolol: comparison of the effects on portal pressure and azygos blood flow in patients with cirrhosis. Hepatology (Baltimore, Md.) 1993;18(5):1082-9. [PubMed] [Google Scholar]
Fiaccadori 1993 {published data only}
- Fiaccadori F, Pedretti G, Biraghi M, Arcidiacono R. Terlypressin and endoscopic sclerotherapy control variceal bleeding and prevent early rebleeding in cirrhotic patients. Current Therapeutic Research, Clinical and Experimental 1993;54(5):519-28. [Google Scholar]
Fort 1990 {published data only}
- Fort E. Haemodynamic effect of triglycyl-lysine vasopressin (glypressin) on intravascular oesophageal variceal pressure in patients with cirrhosis. A randomized placebo controlled trial. Gastroenterologie Clinique et Biologique 1990;14(11):898-9. [PubMed] [Google Scholar]
Garcia‐Pagan 1991 {published data only}
- García-Pagán JC, Feu F, Bosch J, Rodés J. Propranolol compared with propranolol plus isosorbide-5-mononitrate for portal hypertension in cirrhosis. A randomized controlled study. Annals of Internal Medicine 1991;114(10):869-73. [DOI] [PubMed] [Google Scholar]
Garcia‐Pagan 1996 {published data only}
- Garcia-Pagan JC, Escorsell A, Feu F, Bandi JC, Moitinho E, Casado M, et al. Propranolol plus molsidomine vs propranolol alone in the treatment of portal hypertension in patients with cirrhosis. Journal of Hepatology 1996;24(4):430-5. [DOI] [PubMed] [Google Scholar]
Garden 1990 {published data only}
- Garden OJ, Mills PR, Birnie GG, Murray GD, Carter DC. Propranolol in the prevention of recurrent variceal hemorrhage in cirrhotic patients. A controlled trial. Gastroenterology 1990;98(1):185-90. [DOI] [PubMed] [Google Scholar]
Garg 1999 {published data only}
- Garg PK, Joshi YK, Tandon RK. Comparison of endoscopic variceal sclerotherapy with sequential endoscopic band ligation plus low-dose sclerotherapy for secondary prophylaxis of variceal hemorrhage: a prospective randomized study. Gastrointestinal Endoscopy 1999;50(3):369-73. [DOI] [PubMed] [Google Scholar]
Gatta 1987 {published data only}
- Gatta A, Merkel C, Sacerdoti D, Bolognesi M, Caregaro L, Zuin R, et al. Nadolol for prevention of variceal rebleeding in cirrhosis: a controlled clinical trial. Digestion 1987;37(1):22-8. [DOI] [PubMed] [Google Scholar]
Geng 2015 {published data only}
- Geng Q, Xiang X, Wang K, Ying H. Efficacy of endoscopic variceal ligation combined with sclerotherapy for treatment of esophageal variceal bleeding. Chinese Journal of Gastroenterology 2015;20(4):241-3. [Google Scholar]
George 2013 {published data only}
- George J, Thomas V. Propranalol is not effective in preventing the progression to severe portal hypertensive gastropathy in cirrhotic patients who had undergone variceal eradication-a randomised controlled trial. Journal of Gastroenterology and Hepatology 2014;29:11. [Google Scholar]
- George J. Propranalol is ineffective in preventing PHG progression in cirrhotics who had variceal eradication. Journal of Gastroenterology and Hepatology 2013;28:770. [Google Scholar]
Gilbert 1991 {published data only}
- Gilbert DA, Buelow RG, Chung RS, Cunningham JT, Foutch PG, Laine LA, et al. Technology assessment status evaluation: endoscopic band ligation of varices. Gastrointestinal Endoscopy 1991;37(6):670-2. [DOI] [PubMed] [Google Scholar]
Goff 1986 {published data only}
- Goff JS, Ayres SJ, Hollstromtarwater K. A randomized trial of sclerotherapy vs standard therapy for bleeding esophageal-varices - long-term follow-up. Gastroenterology 1986;90(5):1728. [Google Scholar]
Gong 1998 {published data only}
- Gong XH, Zuge CD. The effect of sandostatin used in small dose in treatment of hemorrhage of esophageal varicose. Journal of Practical Hepatology 1998;3(3):174-5. [Google Scholar]
Gong 2010 {published data only}
- Gong WD, Xue K, Chu YK, Wang Q, Yang W, Quan H, et al. Percutaneous transhepatic embolization of gastroesophageal varices combined with partial splenic embolization for variceal bleeding and hypersplenism: a comparison with surgery. Journal of Interventional Radiology 2010;19(2):105-9. [Google Scholar]
Gonzalez‐Abraldes 2001 {published data only}
- Gonzalez-Abraldes J, Albillos A, Baares R, Ruiz DA, Moitinho E, Rodriguez C, et al. Liver, pancreas, and biliary tract: randomized comparison of long-term losartan versus propranolol in lowering portal pressure in cirrhosis. Gastroenterology 2001;121(2):382-8. [DOI] [PubMed] [Google Scholar]
Gonzalez‐Suarez 2006 {published data only}
- Gonzalez-Suarez B, Guarner C, Villanueva C, Minana J, Soriano G, Gallego A, et al. Pharmacologic treatment of portal hypertension in the prevention of community-acquired spontaneous bacterial peritonitis. European Journal of Gastroenterology and Hepatology 2006;18(1):49-55. [DOI] [PubMed] [Google Scholar]
Gotoh 1999 {published data only}
- Gotoh Y, Iwakiri R, Sakata Y, Koyama T, Noda T, Matsunaga C, et al. Evaluation of endoscopic variceal ligation in prophylactic therapy for bleeding of oesophageal varices: a prospective, controlled trial compared with endoscopic injection sclerotherapy. Journal of Gastroenterology and Hepatology 1999;14(3):241-4. [DOI] [PubMed] [Google Scholar]
Gournay 2000 {published data only}
- Gournay J, Masliah C, Martin T, Perrin D, Galmiche JP. Isosorbide mononitrate and propranolol compared with propranolol alone for the prevention of variceal rebleeding. Hepatology (Baltimore, Md.) 2000;31(6):1239-45. [DOI] [PubMed] [Google Scholar]
Gralnek 1999 {published data only}
- Gralnek IM, Jensen DM, Kovacs TO, Jutabha R, Machicado GA, Gornbein J, et al. The economic impact of esophageal variceal hemorrhage: cost-effectiveness implications of endoscopic therapy. Hepatology (Baltimore, Md.) 1999;29(1):44-50. [DOI] [PubMed] [Google Scholar]
Graupera 2011 {published data only}
- Graupera I, Colomo A, Aracil C, Puente A, Hernandez-Gea V, Poca M, et al. RCT evaluating HVPG-guided therapy vs beta-blockers plus nitrates and endoscopic ligation to prevent variceal rebleeding. Hepatology (Baltimore, Md.) 2011;54:460a. [Google Scholar]
Groszmann 2002 {published data only}
- Groszmann RJ, Garcia-Tsao G. Endoscopic variceal banding vs. pharmacological therapy for the prevention of recurrent variceal hemorrhage: what makes the difference? Gastroenterology 2002;123(4):1388-91. [DOI] [PubMed] [Google Scholar]
Gulberg 2002 {published data only}
- Gulberg V, Schepke M, Geigenberger G, Holl J, Brensing KA, Waggershauser T, et al. Transjugular intrahepatic portosystemic shunting is not superior to endoscopic variceal band ligation for prevention of variceal rebleeding in cirrhotic patients: a randomized, controlled trial. Scandinavian Journal of Gastroenterology 2002;37(3):338-43. [DOI] [PubMed] [Google Scholar]
Hanno 2016 {published data only}
- Hanno AF, Aboelkheir HF, Alwazzan DA, Abdelrahman MI. Medical versus endoscopic treatment of oesophageal varices in liver cirrhosis. Gut and Gastroenterology 2018;1(2):sciaeon.org/articles/Medical-Versus-Endoscopic-Treatment-of-Oesophageal-Varices-in-Liver-Cirrhosis.pdf. [Google Scholar]
Harki 2016a {published data only}
- Harki J, Holster IL, Polinder S, Moelker A, Buuren H, Kuipers EJ, et al. Self-reported quality of life in patients treated with covered transjugular intrahepatic portosystemic shunt (TIPS) versus endoscopic plus b-blocker treatment for secondary prevention of gastro-oesophagealvariceal bleeding. Journal of Hepatology 2016;64(2 Suppl 1):S661. [Google Scholar]
- Harki J, Holster IL, Polinder S, Moelker A, Buuren HR, Kuipers EJ, et al. Cost effectiveness of covered transjugular intrahepatic portosystemic shunt (TIPS) versus endoscopic treatment for secondary prevention of gastro-oesophageal variceal bleeding. Gastroenterology 2016;150(4 Suppl 1):S1160. [Google Scholar]
Harras 2010 {published data only}
- Harras F, Sheta ES, Shehata M, El Saadany S, Selim M, Mansour L. Endoscopic band ligation plus argon plasma coagulation versus scleroligation for eradication of esophageal varices. Journal of Gastroenterology and Hepatology 2010;25(6):1058-65. [DOI] [PubMed] [Google Scholar]
Hashizume 1993 {published data only}
- Hashizume M, Ohta M, Ueno K, Tanoue K, Kitano S, Sugimachi K. Endoscopic ligation of esophageal varices compared with injection sclerotherapy: a prospective randomized trial. Gastrointestinal Endoscopy 1993;39(2):123-6. [DOI] [PubMed] [Google Scholar]
Helmy 2015 {published data only}
- Helmy H, Zaghla HE, Abdel-Razek W, Abbasy M, Elzohry HA, El-Fert AY, et al. Propranolol for prevention of recurrence of varices after endoscopic eradication. Hepatology (Baltimore, Md.) 2015;62:575a. [Google Scholar]
Holster 2016 {published data only}
- Holster IL, Moelker A, Tjwa ET, Wils A, Kuipers EJ, Pattynama P, et al. Early transjugular intrahepatic portosystemic shunt (TIPS) as compared to endoscopic treatment reduces rebleeding but not mortality in cirrhotic patients with a 1st or 2nd episode of variceal bleeding: a multicentre randomized controlled trial. United European Gastroenterology Journal 2013;1(1 Suppl 1):A84. [Google Scholar]
- Holster IL, Tjwa E, Moelker A, Wils A, Hansen BE, Vermeijden JR, et al. Covered transjugular intrahepatic portosystemic shunt versus endoscopic therapy plus beta-blocker for prevention of variceal rebleeding. Hepatology (Baltimore, Md.) 2016;63(2):581-9. [DOI] [PubMed] [Google Scholar]
Hua 2007 {published data only}
- Hua YX, Yan ZP, Cheng YD, Qiao DL, Zhou B, Chen SW, et al. Therapeutic effects of percutaneous transhepatic variceal embolization combined with partial splenic embolization for portal hypertension. Journal of Interventional Radiology 2007;16(10):665-8. [Google Scholar]
Huang 2017 {published data only}
- Huang XQ, Ma LL, Chen SY. Transparent cap-assisted endoscopic management of endoscopic sclerotherapy in esophageal varices: a randomized controlled trial. Journal of Gastroenterology and Hepatology 2017;32:27-8. [DOI] [PubMed] [Google Scholar]
- NCT02361593. Transparent cap-assisted endoscopic sclerotherapy [Transparent cap-assisted endoscopic sclerotherapy(lauromacrogol injection) in esophageal varices: a randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT02361593 (first received 12 February 2015).
Iso 1997 {published data only}
- Iso Y, Kawanaka H, Tomikawa M, Matsumata T, Kitano S, Sugimachi K. Repeated injection sclerotherapy is preferable to combined therapy with variceal ligation to avoid recurrence of esophageal varices: a prospective randomized trial. Hepato-Gastroenterology 1997;44(14):467-71. [PubMed] [Google Scholar]
ISRCTN14174793 {published data only}
- ISRCTN14174793. A trial to investigate whether giving albumin to patients with advanced liver cirrhosis will reverse immune suppression and improve outcome from infection. www.isrctn.com/ISRCTN14174793 (first posted 18 March 2015).
ISRCTN77521636 {published data only}
- ISRCTN77521636. Transjugular intrahepatic porto-systemic shunt (TIPS) with Gore-tex® covered stent-graft versus endoscopic treatment for secondary prevention of gastro-oesophageal variceal bleeding. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN77521636 (first posted 30 May 2007).
Iwakiri 2000 {published data only}
- Iwakiri R, Koyama T, Hirano M, Uchida Y, Ishibashi S, Kuwahara A, et al. Endoscopic injection sclerotherapy for esophageal varices prolonged survival of patients with hepatocellular carcinoma complicating liver cirrhosis. Gastrointestinal Endoscopy 2000;51(5):569-72. [DOI] [PubMed] [Google Scholar]
Iwao 1996 {published data only}
- Iwao T, Toyonaga A, Oho K, Shigemori H, Sakai T, Tayama C, et al. Effect of vasopressin on esophageal varices blood flow in patients with cirrhosis: comparisons with the effects on portal vein and superior mesenteric artery blood flow. Journal of Hepatology 1996;25(4):491-7. [DOI] [PubMed] [Google Scholar]
Jackson 1971 {published data only}
- Jackson FC, Perrin EB, Felix WR, Smith AG. A clinical investigation of the portacaval shunt. V. Survival analysis of the therapeutic operation. Annals of Surgery 1971;174(4):672-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeng 1989 {published data only}
- Jeng YS. Endoscopic injection sclerotherapy and propranolol in the prevention of recurrent variceal bleeding: a controlled randomized trial. Gastroenterological Endoscopy 1989;31(3):611-9. [Google Scholar]
Jenkins 1997 {published data only}
- Jenkins SA, Baxter JN, Critchley M, Kingsnorth AN, Makin CA, Ellenbogen S, et al. Randomised trial of octreotide for long term management of cirrhosis after variceal haemorrhage. BMJ (Clinical Research Ed.) 1997;315(7119):1338-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiang 2001 {published data only}
- Jiang X, Wang J, Chen S, Zhu C. A randomized controlled clinical trial in the prevention of esophageal variceal bleeding. Chinese Journal of Gastroenterology 2001;6(2):90-3. [Google Scholar]
Johansson 1988 {published data only}
- Johansson S, Herlin P, Jeppsson B, Joelsson B, Bengmark S. Repeated sclerotherapy or mesocaval shunt for prevention of rebleeding from esophageal-varices - a randomized study. British Journal of Surgery 1988;75(12):1234. [Google Scholar]
Kalambokis 2005 {published data only}
- Kalambokis G, Economou M, Paraskevi K, Konstantinos P, Pappas C, Katsaraki A, et al. Effects of somatostatin, terlipressin and somatostatin plus terlipressin on portal and systemic hemodynamics and renal sodium excretion in patients with cirrhosis. Journal of Gastroenterology and Hepatology 2005;20(7):1075-81. [DOI] [PubMed] [Google Scholar]
Kamal 2017 {published data only}
- Kamal A, Abd Elmoety AA, Hamza Y, Zeid A. Endoscopic variceal ligation followed by argon plasma coagulation against endoscopic variceal ligation alone: a randomized controlled trial. Journal of Clinical Gastroenterology 2017;51(1):49-55. [DOI] [PubMed] [Google Scholar]
Kanazawa 1988 {published data only}
- Kanazawa H, Matsusaka S, Tada N, Miyata K, Tsukui T, Kuroda H, et al. Effect of nadolol on systemic and hepatic hemodynamics, azygos blood flow and esophageal varices in patients with cirrhosis. Prospective, randomized comparison with propranolol. Therapeutic Research 1988;9(6):413-21. [Google Scholar]
Khaitiyar 2000 {published data only}
- Khaitiyar JS, Luthra SK, Prasad N, Ratnakar N, Daruwala DK. Transjugular intrahepatic portosystemic shunt versus distal splenorenal shunt--a comparative study. Hepato-Gastroenterology 2000;47(32):492-7. [PubMed] [Google Scholar]
Kim 1997 {published data only}
- Kim JB, Lee OY, Han DS, Sohn JH, Choi HS, Yoon BC. The comparison of propranolol monotherapy and propranolol, isosorbide-5-mononitrate and metoclopramide triple therapy after endoscopic sclerotherapy in patients with esophageal variceal bleeding. Korean Journal of Hepatology 1997;3(2):114-22. [Google Scholar]
Kitano 1989 {published data only}
- Kitano S, Hashizume M, Yamaga H, Wada H, Iso Y, Iwanaga T, et al. Human thrombin plus 5 per cent ethanolamine oleate injected to sclerose oesophageal varices: a prospective randomized trial. British Journal of Surgery 1989;76(7):715-8. [DOI] [PubMed] [Google Scholar]
Kitano 1992 {published data only}
- Kitano S, Iso Y, Hashizume M, Yamaga H, Koyanagi N, Wada H, et al. Sclerotherapy vs. esophageal transection vs. distal splenorenal shunt for the clinical management of esophageal varices in patients with child class A and B liver function: a prospective randomized trial. Hepatology (Baltimore, Md.) 1992;15(1):63-8. [DOI] [PubMed] [Google Scholar]
- Kitano S, Ohta M, Ueno K, Hashizume M, Iso Y, Koyanagi N, et al. Surgical versus non-surgical treatment in patients with esophageal varices--a prospective randomized study. Journal of Japan Surgical Society 1992;93(9):1156-8. [PubMed] [Google Scholar]
Kleber 1987 {published data only}
- Kleber G, Sauerbruch T, Fischer G, Paumgartner G. Transmural esophageal variceal pressure (increased with somatostatin infusion, not increased with placebo infusion). Zeitschrift Fur Gastroenterologie 1987;25(1):40. [Google Scholar]
Kleber 1991 {published data only}
- Kleber G, Sauerbruch T, Fischer G, Geigenberger G, Paumgartner G. Reduction of transmural oesophageal variceal pressure by metoclopramide. Journal of Hepatology 1991;12(3):362-6. [DOI] [PubMed] [Google Scholar]
Korula 1985 {published data only}
- Korula J, Balart LA, Radvan G, Zweiban BE, Larson AW, Kao HW, et al. A prospective, randomized controlled trial of chronic esophageal variceal sclerotherapy. Hepatology (Baltimore, Md.) 1985;5(4):584-9. [DOI] [PubMed] [Google Scholar]
- Korula J, Yamada S, Balart LA, Radvan G, Zweiban B, Gourdi M, et al. A prospective randomised controlled trial of chronic esophageal variceal sclerotherapy (EVS). Hepatology (Baltimore, Md.) 1983;3(5):825. [DOI] [PubMed] [Google Scholar]
Krige 1996 {published data only}
- Krige JE, Bornman PC, Goldberg PA, Terblanche J. Endoscopic sclerotherapy compared with esophagogastric devascularization and transection in the long-term management of bleeding esophageal varices: a prospective randomized controlled trial. Scandinavian Journal of Gastroenterology 1996;31(Suppl 220):159. [Google Scholar]
- Krige JE, Bornman PC, Terblanche J. Sclerotherapy compared to surgery for long-term bleeding oesophageal varices: a prospective randomized controlled trial. Hepatology (Baltimore, Md.) 1996;23(1):I-25. [Google Scholar]
- Krige JE, Goldberg PA, Bornman PC, Terblanche J. Endoscopic sclerotherapy compared with oesophagogastric devascularisation and transection in the long-term management of bleeding oesophageal varices: preliminary evaluation of a prospective randomized controlled trial. South African Medical Journal 1992;82(7):39. [Google Scholar]
Kumar 2009 {published data only}
- Kumar A, Jha SK, Sharma P, Dubey S, Tyagi P, Sharma BC, et al. Addition of propranolol and isosorbide mononitrate to endoscopic variceal ligation does not reduce variceal rebleeding incidence. Gastroenterology 2009;137(3):892-901. [DOI] [PubMed] [Google Scholar]
Kuran 2006 {published data only}
- Kuran S, Oguz D, Parlak E, Asil M, Cicek B, Kilic M, et al. Secondary prophylaxis of esophageal variceal treatment: endoscopic sclerotherapy, band ligation and combined therapy--long-term results. Turkish Journal of Gastroenterology 2006;17(2):103-9. [PubMed] [Google Scholar]
Kuwayama 2005 {published data only}
- Kuwayama H, Nishiki R. Randomized controlled trial of long-term proton pump inhibitor (PPI) in the prevention of esophageal variceal bleeding in cirrhotic patients. American Journal of Gastroenterology 2005;100(9):S278. [Google Scholar]
Lacet 2016 {published data only}
- Lacet CM, Neto JB, Ribeiro LT, Oliveira FS, Wyszomirska RF, Strauss E. Schistosomal portal hypertension: randomized trial comparing endoscopic therapy alone or preceded by esophagogastric devascularization and splenectomy. Annals of Hepatology 2016;15(5):738-44. [DOI] [PubMed] [Google Scholar]
Lebrec 1981 {published data only}
- Lebrec D, Nouel O, Bernuau J, Bouygues M, Rueff B, Benhamou JP. Propranolol in prevention of recurrent gastrointestinal bleeding in cirrhotic patients. Lancet 1981;1(8226):920-1. [DOI] [PubMed] [Google Scholar]
- Lebrec D, Poynard T, Bernuau J, Bercoff E, Nouel O, Capron JP, et al. A randomised controlled study of propranolol for prevention of recurrent gastrointestinal bleeding in patients with cirrhosis. Drugs 1989;37(Suppl 2):30-4. [DOI] [PubMed] [Google Scholar]
- Lebrec D, Poynard T, Hillon P, Benhamou JP. Propranolol for prevention of recurrent gastrointestinal bleeding in patients with cirrhosis: a controlled study. New England Journal of Medicine 1981;305(23):1371-4. [DOI] [PubMed] [Google Scholar]
- Lebrec D. Beta adrenergic inhibitors and portal hypertension [Inhibiteurs beta-adrenergiques et hypertension portale]. Therapie 1985;40(6):403-6. [PubMed] [Google Scholar]
Lee 2001 {published data only}
- Lee WC, Lin HC, Yang YY, Hou MC, Lee FY, Chang FY, et al. Hemodynamic effects of a combination of prazosin and terlipressin in patients with viral cirrhosis. American Journal of Gastroenterology 2001;96(4):1210-6. [DOI] [PubMed] [Google Scholar]
Li 1995 {published data only}
- Li D, Lu H, Li X, Quan Q, Li X, Lu W. Calcium channel blockers in cirrhotic patients with portal hypertension. Chinese Medical Journal 1995;108(11):803-8. [PubMed] [Google Scholar]
Li 2000 {published data only}
- Li X, Chen D, Zou J. A randomized controlled study of ligustrazine in combination with propranolol for prevention of recurrent esophageal varices bleeding. Chung Hua Kan Tsang Ping Tsa Chih 2000;8(2):99-101. [PubMed] [Google Scholar]
Li 2000a {published data only}
- Li XS, Shen DM, Zou JZ, Liu CA, Zhang L. Low dose propranolol in combination with ligustrazine for prevention of recurrent esophageal varices bleeding: a randomly controlled experimental and clinical study. World Chinese Journal of Digestology 2000;8(2):135-8. [PubMed] [Google Scholar]
Li 2016 {published data only}
- Li B, Chen S, Zhang C, Wei Y. Effects of terlipressin and high-dose octreotide on portal and systemic hemodynamics of cirrhosis patients with gastroesophageal varices: a multicenter randomized controlled trail. Journal of Gastroenterology and Hepatology 2016;31:393. [Google Scholar]
Liao 2015 {published data only}
- Liao WC, Chen PH, Hou MC, Chang CJ, Su CW, Lin HC, et al. Endoscopic ultrasonography assessment of para-esophageal varices predicts efficacy of propranolol in preventing recurrence of esophageal varices. Journal of Gastroenterology 2015;50(3):342-9. [DOI] [PubMed] [Google Scholar]
Lin 1996 {published data only}
- Lin HC, Tsai YT, Huang YT, Shieh WZ, Hou MC, Lee FY. Hemodynamic effects of a combination of octreotide and terlipressin in patients with cirrhosis. Hepatology (Baltimore, Md.) 1996;24(4):539a. [Google Scholar]
Lin 2002 {published data only}
- Lin HC, Yang YY, Hou MC, Huang YT, Lee WC, Lee FY, et al. Hemodynamic effects of a combination of octreotide and terlipressin in patients with viral hepatitis related cirrhosis. Scandinavian Journal of Gastroenterology 2002;37(4):482-7. [DOI] [PubMed] [Google Scholar]
Lin 2005 {published data only}
- Lin XF, Wu JM, Lin XY, Chen MX, Zhu QH, Wu XL. Effects of isosorbide-5-mononitrate on esophageal manometry of cirrhotic patients with esophageal varices. Chung Hua Kan Tsang Ping Tsa Chih 2005;13(8):611-2. [PubMed] [Google Scholar]
Lin 2006 {published data only}
- Lin N, Liu B, Xu RY, Fang HP, Deng MH. Splenectomy with endoscopic variceal ligation is superior to splenectomy with pericardial devascularization in treatment of portal hypertension. World Journal of Gastroenterology 2006;12(45):7375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 1998 {published data only}
- Liu TS, Wang JY, Yao LQ, Zhao NQ, Fu ZJ. Randomized clinical trial on assessment of preventive treatment for esophageal varices rebleeding. Chinese Journal of Hepatology 1998;6(2):74-6. [Google Scholar]
Liu 2004 {published data only}
- Liu B, Lin N, Xu RY. Splenectomy combined with endoscopic variceal ligation in treating portal hypertension. Journal of Central South University Medical Sciences 2004;29(1):87-9. [PubMed] [Google Scholar]
Lo 1993 {published data only}
- Lo GH, Lai KH, Lee SD, Tsai YT, Lo KJ. Does propranolol maintain post-sclerotherapy variceal obliteration? A prospective randomized study. Journal of Gastroenterology and Hepatology 1993;8(4):358-62. [DOI] [PubMed] [Google Scholar]
Lo 1998 {published data only}
- Lo GH, Lai KH, Cheng JS, Lin CK, Huang JS, Hsu PI, et al. The additive effect of sclerotherapy to patients receiving repeated endoscopic variceal ligation: a prospective, randomized trial. Hepatology (Baltimore, Md.) 1998;28(2):391-5. [DOI] [PubMed] [Google Scholar]
Lo 2000 {published data only}
- Lo GH, Lai KH, Cheng JS, Chen MH, Huang HC, Hsu PI, et al. Endoscopic variceal ligation plus nadolol and sucralfate compared with ligation alone for the prevention of variceal rebleeding: a prospective, randomized trial. Hepatology (Baltimore, Md.) 2000;32(3):461-5. [DOI] [PubMed] [Google Scholar]
Lo 2002 {published data only}
- Lo GH, Chen WC, Chen MH, Hsu PI, Lin CK, Tsai WL, et al. Banding ligation versus nadolol and isosorbide mononitrate for the prevention of esophageal variceal rebleeding. Gastroenterology 2002;123(3):728-34. [DOI] [PubMed] [Google Scholar]
Lo 2008 {published data only}
- Lo GH, Chen WC, Lin CK, Tsai WL, Chan HH, Chen TA, et al. Improved survival in patients receiving medical therapy as compared with banding ligation for the prevention of esophageal variceal rebleeding. Hepatology (Baltimore, Md.) 2008;48(2):580-7. [DOI] [PubMed] [Google Scholar]
Lo 2009a {published data only}
- Lo GH, Chen WC, Chan HH, Tsai WL, Hsu PI, Lin CK, et al. A randomized, controlled trial of banding ligation plus drug therapy versus drug therapy alone in the prevention of esophageal variceal rebleeding. Journal of Gastroenterology and Hepatology 2009;24(6):982-7. [DOI] [PubMed] [Google Scholar]
Lo 2009b {published data only}
- ISRCTN28353453. A controlled trial of terlipressin plus banding ligation versus terlipressin alone in the management of acute esophageal variceal bleeding. www.isrctn.com/ISRCTN28353453 (first posted 18 February 2006).
- Lo GH, Chen WC, Wang HM, Lin CK, Chan HH, Tsai WL, et al. Low-dose terlipressin plus banding ligation versus low-dose terlipressin alone in the prevention of very early rebleeding of oesophageal varices. Gut 2009;58(9):1275-80. [DOI] [PubMed] [Google Scholar]
Lo 2012 {published data only}
- Lo GH, Chen WC, Wang HM, Yu HC. Randomized, controlled trial of carvedilol versus nadolol plus isosorbide mononitrate for the prevention of variceal rebleeding. Journal of Gastroenterology and Hepatology 2012;27(11):1681-7. [DOI] [PubMed] [Google Scholar]
Lu 2004 {published data only}
- Lu HY, Liu XY, Huang FZ, Nie WP, Ren SP, Huang RL. Laser inducing mucosal fibrosis for preventing recurrence of esophageal varices. Chung-Hua Wai Ko Tsa Chih 2004;42(24):1513-5. [PubMed] [Google Scholar]
Luo 2011 {published data only}
- Luo H, Huang X, Huang F, Liu X. Laparoscope and endoscope for portal hypertension. Journal of Central South University Medical Sciences 2011;36(8):786-90. [DOI] [PubMed] [Google Scholar]
Luo 2015 {published data only}
- Luo X, Wang Z, Tsauo J, Zhou B, Zhang H, Li X. Advanced cirrhosis combined with portal vein thrombosis: a randomized trial of TIPS versus endoscopic band ligation plus propranolol for the prevention of recurrent esophageal variceal bleeding. Radiology 2015;276(1):286-93. [DOI] [PubMed] [Google Scholar]
Lv 2018 {published data only}
- Lv Y, Qi X, He C, Wang Z, Yin Z, Niu J, et al. Covered TIPS versus endoscopic band ligation plus propranolol for the prevention of variceal rebleeding in cirrhotic patients with portal vein thrombosis: a randomised controlled trial. Gut 2018;67(12):2156-68. [DOI] [PubMed] [Google Scholar]
Magnano 1994 {published data only}
- Magnano A, Beneventano G, Giannone N, Pillera S, Aprile G, Cosentino S, et al. Effect of triglycil-lysine-vasopressin on intravascular oesophageal variceal pressure in post-hepatic cirrhotic patients. A double-blind randomized placebo controlled trial. Giornale Italiano di Endoscopia Digestiva 1994;17(3):143-8. [Google Scholar]
Maldonado 2002 {published data only}
- Maldonado D, Barkin JS. Combined therapy for secondary prophylaxis of variceal rebleeding? American Journal of Gastroenterology 2002;97(8):2134-6. [DOI] [PubMed] [Google Scholar]
Marrero 2002 {published data only}
- Marrero JA, Scheiman JM. Prevention of recurrent variceal bleeding: as easy as A.P.C.? Gastrointestinal Endoscopy 2002;56(4):600-3. [DOI] [PubMed] [Google Scholar]
Masci 1999 {published data only}
- Masci E, Stigliano R, Mariani A, Bertoni G, Baroncini D, Cennamo V, et al. Prospective multicenter randomized trial comparing banding ligation with sclerotherapy of esophageal varices. Hepato-Gastroenterology 1999;46(27):1769-73. [PubMed] [Google Scholar]
Mastai 1986 {published data only}
- Mastai R, Grande L, Bosch J, Bruix J, Rigau J, Kravetz D, et al. Effects of metoclopramide and domperidone on azygos venous blood flow in patients with cirrhosis and portal hypertension. Hepatology (Baltimore, Md.) 1986;6(6):1244-7. [DOI] [PubMed] [Google Scholar]
Masumoto 1998 {published data only}
- Masumoto H, Toyonaga A, Oho K, Iwao T, Tanikawa K. Ligation plus low-volume sclerotherapy for high-risk esophageal varices: comparisons with ligation therapy or sclerotherapy alone. Journal of Gastroenterology 1998;33(1):1-5. [DOI] [PubMed] [Google Scholar]
McCormick 1992 {published data only}
- McCormick PA, Biagini MR, Dick R, Greenslade L, Chin J, Cardin F, et al. Ocreotide inhibits the meal-induced increases in the portal venous-pressure of cirrhotic-patients with portal-hypertension - a double-blind, place-controlled study. Hepatology (Baltimore, Md.) 1992;16(5):1180-6. [PubMed] [Google Scholar]
McCormick 1993 {published data only}
- McCormick PA, Greenslade L, Chin J, Dick R, McIntyre N, Burroughs AK. The effect of the combination of octreotide and metoclopramide on azygos blood-flow in cirrhotic-patients with portal-hypertension. European Journal of Gastroenterology & Hepatology 1993;5(10):839-43. [Google Scholar]
McKee 1990 {published data only}
- McKee R. A study of octreotide in oesophageal varices. Digestion 1990;45(Suppl 1):60-4. [DOI] [PubMed] [Google Scholar]
- McKee RF, Garden OJ, Anderson JR, Carter DC. A comparison of SMS 201-995 and oesophageal tamponade in the control of acute variceal haemorrhage. HPB Surgery 1992;6(1):7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee RF. Sandostatin therapy of acute oesophageal variceal bleeding. Digestion 1993;54(Suppl 1):27-9. [DOI] [PubMed] [Google Scholar]
Merli 1998 {published data only}
- Meddi P, Merli M, Lionetti R, De-Santis A, Valeriano V, Masini A, et al. Cost analysis for the prevention of variceal rebleeding: a comparison between transjugular intrahepatic portosystemic shunt and endoscopic sclerotherapy in a selected group of Italian cirrhotic patients. Hepatology (Baltimore, Md.) 1999;29(4):1074-7. [DOI] [PubMed] [Google Scholar]
- Merli M, Salerno F, Riggio O, de-Franchis R, Fiaccadori F, Meddi P, et al. Transjugular intrahepatic portosystemic shunt versus endoscopic sclerotherapy for the prevention of variceal bleeding in cirrhosis: a randomized multicenter trial. Gruppo Italiano Studio TIPS (G.I.S.T.). Hepatology (Baltimore, Md.) 1998;27(1):48-53. [DOI] [PubMed] [Google Scholar]
Mikkelsen 1974 {published data only}
- Mikkelsen WP. Therapeutic portacaval shunt. Preliminary data on controlled trial and morbid effects of acute hyaline necrosis. Archives of Surgery 1974;108(3):302-5. [DOI] [PubMed] [Google Scholar]
Ministro 1995 {published data only}
- Ministro P, Rosa A, Pontes JM, Baranda J, Souto P, Amaro P, et al. Endoscopic ligation of esophageal varices compared with injection sclerotherapy: a prospective randomized trial. Endoscopy 1995;27(7):S73. [DOI] [PubMed] [Google Scholar]
Mino 1995 {published data only}
- Mino G, Jaramillo JL. Ligation or endoscopic sclerotherapy of esophageal varices. Gastroenterologia y Hepatologia 1995;18(7):384-7. [PubMed] [Google Scholar]
Mo 2014 {published data only}
- Mo CY, Li SL. Short-term effect of carvedilol vs propranolol in reduction of hepatic venous pressure gradient in patients with cirrhotic portal hypertension. World Chinese Journal of Digestology 2014;22(27):4146-50. [Google Scholar]
Monici 2010 {published data only}
- Monici LT, Meirelles-Santos JO, Soares EC, Mesquita MA, Zeitune JM, Montes CG, et al. Microwave coagulation versus sclerotherapy after band ligation to prevent recurrence of high risk of bleeding esophageal varices in Child-Pugh's A and B patients. Journal of Gastroenterology 2010;45(2):204-10. [DOI] [PubMed] [Google Scholar]
Morales 2007 {published data only}
- Morales GF, Pereira Lima JC, Hornos AP, Marques DL, Costa CS, Lima Pereira L, et al. Octreotide for esophageal variceal bleeding treated with endoscopic sclerotherapy: a randomized, placebo-controlled trial. Hepato-Gastroenterology 2007;54(73):195-200. [PubMed] [Google Scholar]
Moreto 1994 {published data only}
- Moreto M, Zaballa M, Ojembarrena E, Ibanez S, Suarez MJ, Setien F, et al. Combined (short-term plus longterm) sclerotherapy v short-term only sclerotherapy: a randomised prospective trial. Gut 1994;35(5):687-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakamura 1998 {published data only}
- Nakamura S, Mitsunaga A, Murata Y, Suzuki S, Hayashi N. Endoscopic variceal electrocoagulation for recurrent esophageal varices. In: Montori A, Lirici MM, Montori J, editors(s). 6th World Congress of Endoscopic Surgery. Bologna: Monduzzi Editore, 1998:A329-32. [Google Scholar]
Nakamura 2001 {published data only}
- Nakamura S, Mitsunaga A, Murata Y, Suzuki S, Hayashi N. Endoscopic induction of mucosal fibrosis by argon plasma coagulation (APC) for esophageal varices: a prospective randomized trial of ligation plus APC vs. ligation alone. Endoscopy 2001;33(3):210-5. [DOI] [PubMed] [Google Scholar]
Nakase 1996 {published data only}
- Nakase H, Kawasaki T, Komori H, Sakatani N, Sawai S, Chiba T. Endoscopic variceal ligation versus endoscopic injection sclerotherapy: comparison of hepatic and renal function. American Journal of Gastroenterology 1996;91(10):2170-3. [PubMed] [Google Scholar]
Narahara 2001 {published data only}
- Narahara Y, Kanazawa H, Kawamata H, Tada N, Saitoh H, Matsuzaka S, et al. A randomized clinical trial comparing transjugular intrahepatic portosystemic shunt with endoscopic sclerotherapy in the long-term management of patients with cirrhosis after recent variceal hemorrhage. Hepatology Research 2001;21(3):189-98. [DOI] [PubMed] [Google Scholar]
NCT00006161 {published data only}
- NCT00006161. Decompression intervention of variceal rebleeding trial (DIVERT). clinicaltrials.gov/ct2/show/NCT00006161 (first received 9 August 2000).
NCT00570973 {published data only}
- NCT00570973. Band ligation versus transjugular intrahepatic portosystemic stent shunt (TIPS) in cirrhotics with recurrent variceal bleeding non responding to medical therapy [A randomized, controlled, multicentric trial comparing endoscopic band ligation versus transjugular intrahepatic portosystemic stent shunt in cirrhotic patients with recurrent variceal bleeding non responding to medical therapy]. clinicaltrials.gov/show/NCT00570973 (first received 11 December 2007).
NCT00799851 {published data only}
- NCT00799851. A randomized controlled trial comparing band ligation and cyanoacrylate injection for esophageal varices [Endoscopic treatment of esophageal varices in advanced liver disease patients: a randomized controlled trial comparing band ligation and cyanoacrylate injection]. clinicaltrials.gov/show/NCT00799851 (first received 1 December 2008).
NCT01103154 {published data only}
- NCT01103154. A trial of nadolol plus isosorbide mononitrate versus carvedilol for the prevention of variceal rebleeding [A controlled trial of nadolol plus isosorbide mononitrate vs. Carvedilol for the prevention of variceal rebleeding]. clinicaltrials.gov/show/NCT01103154 (first received 14 April 2010).
NCT01640964 {published data only}
- NCT01640964. An exploratory haemodynamic study in patients with compensated cirrhosis and portal hypertension [An exploratory study to investigate the haemodynamic effects of serelaxin (RLX030) in patients with compensated cirrhosis and portal hypertension]. clinicaltrials.gov/ct2/show/NCT01640964 (first received 16 July 2012).
NCT02119884 {published data only}
- NCT02119884. Hemodynamic effects of terlipressin and high dose octreotide [Hemodynamic effects of terlipressin and high dose octreotide on patients with liver cirrhosis related esophageal varices: a randomized, placebo-controlled multicenter trial]. clinicaltrials.gov/ct2/show/NCT02119884 (first received 22 April 2014).
NCT02508623 {published data only}
- NCT02508623. Effect of administration of rifaximin on the increased portal pressure of patients with liver cirrhosis and esophageal varices already treated with propranolol [Effect of administration "add on" of rifaximin on portal hypertension of patients with liver cirrhosis and esophageal varices in standard therapy with propranolol]. clinicaltrials.gov/ct2/show/NCT02508623 (first received 27 July 2015).
NCT02646202 {published data only}
- NCT02646202. Scleroligation for eradication of gastroesophageal varices [Scleroligation is a safe and effective new technique for eradication of gastroesophageal varices]. clinicaltrials.gov/ct2/show/NCT02646202 (first received 5 January 2016).
NCT02740166 {published data only}
- NCT02740166. Preventing recurrent bleeding after eradication of esophageal varices [Banding ligation plus propranolol versus banding ligation to prevent rebleeding of esophageal varices]. clinicaltrials.gov/ct2/show/NCT02740166 (first received 15 April 2016).
NCT03583996 {published data only}
- NCT03583996. The SHUNT-V study for varices. clinicaltrials.gov/ct2/show/NCT03583996 (first received 12 July 2018).
NCT03687216 {published data only}
- NCT03687216. Hvpg-guided therapy versus evl plus nsbb in second prophylaxis of esophageal variceal bleeding [A single-center randomized controlled study of secondary prophylaxis of cirrhosis related esophagogastric variceal hemorrhage treated with hvpg-guided therapy or standard esophageal variceal ligation plus beta-blocker]. clinicaltrials.gov/ct2/show/NCT03687216 (first received 27 September 2018).
NCT03783065 {published data only}
- NCT03783065. HVPG-guided laparoscopic versus endoscopic therapy for variceal rebleeding in portal hypertension: a multicenter randomized controlled trial (CHESS1803). clinicaltrials.gov/show/NCT03783065 (first received 20 December 2018).
Nevens 1996a {published data only}
- Nevens F, Sprengers D, Feu F, Bosch J, Fevery J. Measurement of variceal pressure with an endoscopic pressure sensitive gauge: validation and effect of propranolol therapy in chronic conditions. Journal of Hepatology 1996;24(1):66-73. [DOI] [PubMed] [Google Scholar]
Nevens 1996b {published data only}
- Nevens F, Van SW, Yap SH, Fevery J. Assessment of variceal pressure by continuous non-invasive endoscopic registration: a placebo controlled evaluation of the effect of terlipressin and octreotide. Gut 1996;38(1):129-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nishikawa 1999 {published data only}
- Nishikawa Y, Hosokawa Y, Doi T, Endo H, Tanimizu M, Hyodo I, et al. Evaluation of endoscopic injection sclerotherapy with and without simultaneous ligation for the treatment of esophageal varices. Journal of Gastroenterology 1999;34(2):159-62. [DOI] [PubMed] [Google Scholar]
Nos 1995 {published data only}
- Nos P, Sala T, Pertejo V, Berenguer M, Garrigues V, Pons V, et al. Endoscopic sclerotherapy versus oesophageal transection in the prevention of variceal rebleeding. European Journal of Gastroenterology & Hepatology 1995;7(3):231-5. [PubMed] [Google Scholar]
O'Connor 1989 {published data only}
- O'Connor KW, Lehman G, Yune H, Brunelle R, Christiansen P, Hast J, et al. Comparison of three nonsurgical treatments for bleeding esophageal varices. Gastroenterology 1989;96(3):899-906. [PubMed] [Google Scholar]
- O'Connor KW, Lehman GA, Christiansen PA, Weddle R, McHenry R, Hawes R, et al. Effect of hemodynamic status at time of randomization on the nonsurgical treatment of bleeding esophageal-varices. Clinical Research 1986;34(2):A444. [Google Scholar]
Ohmoto 2006 {published data only}
- Ohmoto K, Yoshioka N, Tomiyama Y, Shibata N, Takesue M, Yoshida K, et al. Improved prognosis of cirrhosis patients with esophageal varices and thrombocytopenia treated by endoscopic variceal ligation plus partial splenic embolization. Digestive Diseases and Sciences 2006;51(2):352-8. [DOI] [PubMed] [Google Scholar]
Okano 2003a {published data only}
- Okano H, Shiraki K, Inoue H, Kawakita T, Deguchi M, Sugimoto K, et al. Long-term follow-up of patients with liver cirrhosis after endoscopic esophageal varices ligation therapy: comparison with ethanol injection therapy. Hepato-Gastroenterology 2003;50(54):2013-6. [PubMed] [Google Scholar]
Okano 2003b {published data only}
- Okano H, Shiraki K, Inoue H, Kawakita T, Deguchi M, Sugimoto K, et al. Long-term follow-up of patients with liver cirrhosis after endoscopic ethanol injection sclerotherapy for esophageal varices. Hepato-Gastroenterology 2003;50(53):1556-9. [PubMed] [Google Scholar]
Orloff 1962 {published data only}
- Orloff MJ. A comparative study of emergency transesophageal ligation and nonsurgical treatment of bleeding esophageal varices in unselected patients with cirrhosis. Surgery 1962;52(1):103-16. [PubMed] [Google Scholar]
Orloff 1974 {published data only}
- Orloff MJ, Chandler JG, Charters AC, Condon JK, Grambort DE, Modafferi TR, et al. Emergency portacaval shunt treatment for bleeding esophageal varices. Prospective study in unselected patients with alcoholic cirrhosis. Archives of Surgery 1974;108(3):293-9. [DOI] [PubMed] [Google Scholar]
Orloff 2014 {published data only}
- Orloff MJ. Fifty-three years' experience with randomized clinical trials of emergency portacaval shunt for bleeding esophageal varices in cirrhosis: 1958-2011. JAMA Surgery 2014;149(2):155-69. [DOI] [PubMed] [Google Scholar]
Otte 1983 {published data only}
- Otte JB, Reynaert M, de-Hemptinne B, Geubel A, Carlier M, Jamart J, et al. Arterialization of the portal vein. Preliminary results of a prospective randomized study. Acta Gastro-Enterologica Belgica 1983;46(3-4):161-8. [PubMed] [Google Scholar]
Palazzi 1989 {published data only}
- Palazzi A, Laurenzi F, Tiburzi C, Ottaviani O. Usefulness of supplemental treatment with glypressin during the eradication phase of esophageal varices using endoscopic sclerotherapy. Minerva Dietologica e Gastroenterologica 1989;35(4):257-60. [PubMed] [Google Scholar]
Pang 1997 {published data only}
- Pang ZF, Jia YY, Zhang QL, Zhang SH. A comparison of long-term results between ligation sclerosis and simple ligation under endoscopy in esophageal varices. Chinese Journal of Digestive Endoscopy 1997;14(5):281. [Google Scholar]
Paquet 1983 {published data only}
- Paquet KJ, Feussner H, Koussouris P. Late results of endoscopic esophageal sclerotherapy in acute and recurrent variceal hemorrhage. Langenbecks Archiv Für Chirurgie 1983;361:772. [Google Scholar]
Patch 2002 {published data only}
- Patch D, Sabin CA, Goulis J, Gerunda G, Greenslade L, Merkel C, et al. A randomized, controlled trial of medical therapy versus endoscopic ligation for the prevention of variceal rebleeding in patients with cirrhosis. Gastroenterology 2002;123(4):1013-9. [DOI] [PubMed] [Google Scholar]
Pena 1999 {published data only}
- Peña J, Rivero M, Sanchez E, Fábrega E, Crespo J, Pons-Romero F. Variceal ligation compared with endoscopic sclerotherapy for variceal hemorrhage: prospective randomized trial. Gastrointestinal Endoscopy 1999;49(4):417-23. [DOI] [PubMed] [Google Scholar]
Pena 2005 {published data only}
- Peña J, Brullet E, Sanchez-Hernández E, Rivero M, Vergara M, Martin-Lorente JL, et al. Variceal ligation plus nadolol compared with ligation for prophylaxis of variceal rebleeding: a multicenter trial. Hepatology (Baltimore, Md.) 2005;41(3):572-8. [DOI] [PubMed] [Google Scholar]
Pereira 1997 {published data only}
- Pereira SP, Wilkinson ML. Prospective randomized trial of endoscopic sclerotherapy versus variceal band ligation for esophageal varices: influence on gastropathy, gastric varices and variceal recurrence. Gastrointestinal Endoscopy 1997;46(4):384-5. [DOI] [PubMed] [Google Scholar]
Pfisterer 2018 {published data only}
- Pfisterer N, Dexheimer C, Fuchs EM, Bucsics T, Schwabl P, Mandorfer M, et al. Beta-blockers do not increase efficacy of band ligation in primary prophylaxis but they improve survival in secondary prophylaxis of variceal bleeding. Alimentary Pharmacology & Therapeutics 2018;47(7):966-79. [DOI] [PubMed] [Google Scholar]
Piai 1987 {published data only}
- Piai G, Mattera D, Minieri M, Sauro A, Mazzacca G. A prospective controlled study of endoscopic oesophageal variceal sclerotherapy in advanced cirrhosis. Italian Journal of Gastroenterology 1987;19:257-60. [Google Scholar]
Planas 1991 {published data only}
- Planas R, Boix J, Broggi M, Cabre E, Gomes-Vieira MC, Morillas R, et al. Portacaval shunt versus endoscopic sclerotherapy in the elective treatment of variceal hemorrhage. Gastroenterology 1991;100(4):1078-86. [DOI] [PubMed] [Google Scholar]
- Planas R, Boix J, Dominguez M, Abad A, Quer JC, Leon R. Prophylactiv sclerosis of esophageal varices (EV) prospective trial. Journal of Hepatology 1989;9(Suppl 1):S73. [Google Scholar]
Pomier‐Layrargues 2001 {published data only}
- Pomier-Layrargues G, Villeneuve JP, Deschênes M, Bui B, Perreault P, Fenyves D, et al. Transjugular intrahepatic portosystemic shunt (TIPS) versus endoscopic variceal ligation in the prevention of variceal rebleeding in patients with cirrhosis: a randomised trial. Gut 2001;48(3):390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pontes 1995 {published data only}
- Pontes JM, Leitao MC, Portela FA, Rosa AM, Ministro P, Freitas DS. Endoscopic ultrasonography in the treatment of oesophageal varices by endoscopic sclerotherapy and band ligation: do we need it? European Journal of Gastroenterology & Hepatology 1995;7(1):41-6. [PubMed] [Google Scholar]
Pozzi 2005 {published data only}
- Pozzi M, Grassi G, Ratti L, Favini G, Dell'Oro R, Redaelli E, et al. Cardiac, neuroadrenergic, and portal hemodynamic effects of prolonged aldosterone blockade in postviral child A cirrhosis. American Journal of Gastroenterology 2005;100(5):1110-6. [DOI] [PubMed] [Google Scholar]
- Pozzi M, Ratti L, Grassi G, Favini G, Redaelli E, Quarti TF, et al. Portal pressure gradient (HVPG) reduction after long-term >K-canrenoate in postviral child a cirrhosis with small esophageal varices (F1): lack of sympathoinhibitory effects. Journal of Hepatology 2004;40(Suppl 1):72. [Google Scholar]
Primignani 1994 {published data only}
- Primignani M, Carpinelli L, Vazzoler MC, De-Lima MC, Nolte A, Marcelli R, et al. Short-term effect of octreotide on intraoesophageal variceal pressure: a double-blind placebo-controlled study. European Journal of Gastroenterology & Hepatology 1994;6(11):1027-31. [Google Scholar]
Primignani 1995 {published data only}
- Primignani M, Andreoni B, Carpinelli L, Capria A, Rocchi G, Lorenzini I, et al. Sclerotherapy plus octreotide versus sclerotherapy alone in the prevention of early rebleeding from esophageal varices: a randomized, double-blind, placebo-controlled, multicenter trial. New Italian Endoscopic Club. Hepatology (Baltimore, Md.) 1995;21(5):1322-7. [PubMed] [Google Scholar]
Prioton 1988 {published data only}
- Prioton JB, Feneyrou B, Daures JP, Michel H, Bories P, Blanc F. Long-term results of a randomized trial of portal disconnection of the oesophagus with a clip and medical treatment in cirrhotic patients with bleeding oesophageal varices. Annales de Gastroenterologie et d'Hepatologie 1988;24(1):1-5. [PubMed] [Google Scholar]
Priyadarshi 2011 {published data only}
- Priyadarshi BP, Prakash G, Kushwaha JS, Giri R, Agarwala V, Singh RK, et al. Endoscopic ligation versus medical management to prevent rebleeding in esophageal varices. Indian Journal of Gastroenterology 2011;1:A48. [Google Scholar]
Qi 2007 {published data only}
- Qi YF, Zhu SH, Liu XY, Liu B, Li RZ, Zhou P, et al. Endoscopic variceal ligation combined with Hassab's procedure in preventing the recurrence of esophageal varices. Journal of Central South University Medical Sciences 2007;32(3):368-72. [PubMed] [Google Scholar]
Queuniet 1987 {published data only}
- Queuniet AM, Czernichow P, Lerebours E. Controlled trial of propranolol for the prevention of recurrent gastrointestinal bleeding in patients with cirrhosis. Gastroenterologie Clinique et Biologique 1987;11(1):41-7. [PubMed] [Google Scholar]
Rawat 2015 {published data only}
- Rawat R, Gupta V, Mouli P, Shalimar, Saraya A. To compare endoscopic variceal ligation plus carvedilol versus endoscopic variceal ligation plus propranolol on hepatic vein pressure gradient reduction at 1 month in patients with first episode of esophageal varix bleed: open label randomized trial. Journal of Clinical and Experimental Hepatology 2015;5:S28. [Google Scholar]
Resnick 1969 {published data only}
- Resnick RH, Chalmers TC, Ishihara AM, Garceau AJ, Callow AD, Schimmel EM, et al. A controlled study of the prophylactic portacaval shunt. A final report. Annals of Internal Medicine 1969;70(4):675-88. [DOI] [PubMed] [Google Scholar]
Resnick 1974 {published data only}
- Resnick RH, Iber FL, Ishihara AM, Chalmers TC, Zimmerman H. A controlled study of the therapeutic portacaval shunt. Gastroenterology 1974;67(5):843-57. [PubMed] [Google Scholar]
Reynolds 1981 {published data only}
- Reynolds TB, Donovan AJ, Mikkelsen WP, Redeker AG, Turrill FL, Weiner JM. Results of a 12-year randomized trial of portacaval shunt in patients with alcoholic liver disease and bleeding varices. Gastroenterology 1981;80(5):1005-11. [PubMed] [Google Scholar]
Rhodes 1986 {published data only}
- Rhodes JM, Dawson J, Cockel R, Hawker P, Dykes P, Bradby GV, et al. A randomized controlled trial of variceal compression as an adjunct to endoscopic variceal sclerosis. Scandinavian Journal of Gastroenterology 1986;21(10):1217-20. [DOI] [PubMed] [Google Scholar]
Rikkers 1978 {published data only}
- Rikkers LF, Rudman D, Galambos JT, Fulenwider JT, Millikan WJ, Kutner M, et al. A randomized, controlled trial of the distal splenorenal shunt. Annals of Surgery 1978;188(3):271-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rikkers 1993 {published data only}
- Rikkers LF, Jin G, Burnett DA, Buchi KN, Cormier RA. Shunt surgery versus endoscopic sclerotherapy for variceal hemorrhage: late results of a randomized trial. American Journal of Surgery 1993;165(1):27-32. [DOI] [PubMed] [Google Scholar]
Romero 2000 {published data only}
- Romero G, Kravetz D, Argonz J, Bildozola M, Suarez A, Terg R. Terlipressin is more effective in decreasing variceal pressure than portal pressure in cirrhotic patients. Journal of Hepatology 2000;32(3):419-25. [DOI] [PubMed] [Google Scholar]
Rosemurgy 1996 {published data only}
- Rosemurgy AS, Bloomston M, Clark WC, Thometz DP, Zervos EE. H-graft portacaval shunts versus TIPS: ten-year follow-up of a randomized trial with comparison to predicted survivals. Annals of Surgery 2005;241(2):238-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemurgy AS, Goode SE, Zwiebel BR, Black TJ, Brady PG. A prospective trial of transjugular intrahepatic portasystemic stent shunts versus small-diameter prosthetic H-graft portacaval shunts in the treatment of bleeding varices. Annals of Surgery 1996;224(3):378-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemurgy AS, Serafini FM, Zweibel BR, Black T J, Kudryk BT, Nord H J, et al. Transjugular intrahepatic portosystemic shunt vs. small-diameter prosthetic H-graft portacaval shunt: extended follow-up of an expanded randomized prospective trial. Journal of Gastrointestinal Surgery 2000;4(6):589-97. [DOI] [PubMed] [Google Scholar]
- Rosemurgy AS, Zervos EE, Bloomston M, Durkin AJ, Clark WC, Goff S. Post-shunt resource consumption favors small-diameter prosthetic H-graft portacaval shunt over TIPS for patients with poor hepatic reserve. Annals of Surgery 2003;237(6):820-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemurgy AS, Zervos EE, Goode S E, Black TJ, Zwiebel BR. Differential effects on portal and effective hepatic blood flow. A comparison between transjugular intrahepatic portasystemic shunt and small-diameter H-graft portacaval shunt. Annals of Surgery 1997;225(5):601-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemurgy AS 2nd, Bloomston M, Zervos EE, Goode SE, Pencev D, Zweibel B, et al. Transjugular intrahepatic portosystemic shunt versus H-graft portacaval shunt in the management of bleeding varices: a cost-benefit analysis. Surgery 1997;122(4):794-9. [DOI] [PubMed] [Google Scholar]
Rossle 1997 {published data only}
- Rossle M, Deibert P, Haag K, Ochs A, Olschewski M, Siegerstetter V, et al. Randomised trial of transjugular-intrahepatic-portosystemic shunt versus endoscopy plus propranolol for prevention of variceal rebleeding. Lancet 1997;349(9058):1043-9. [DOI] [PubMed] [Google Scholar]
Russo 2000 {published data only}
- Russo MW, Zacks SL, Sandler RS, Brown RS. Cost-effectiveness analysis of transjugular intrahepatic portosystemic shunt (TIPS) versus endoscopic therapy for the prevention of recurrent esophageal variceal bleeding. Hepatology (Baltimore, Md.) 2000;31(2):358-63. [DOI] [PubMed] [Google Scholar]
Saeed 1997 {published data only}
- Saeed ZA, Stiegmann GV, Ramirez FC, Reveille RM, Goff JS, Hepps KS, et al. Endoscopic variceal ligation is superior to combined ligation and sclerotherapy for esophageal varices: a multicenter prospective randomized trial. Hepatology (Baltimore, Md.) 1997;25(1):71-4. [DOI] [PubMed] [Google Scholar]
Santambrogio 1990 {published data only}
- Santambrogio R, Opocher E, Bruno S, Chiesa A, Gagliano G, Rossi S, et al. The noninvasive assessment of the effects of penbutolol on liver hemodynamics in cirrhotic patients using angioscintigraphy. A randomized controlled double-blind study. Recenti Progressi in Medicina 1990;81(11):705-9. [PubMed] [Google Scholar]
- Santambrogio R, Opocher E, Bruno S, Chiesa A, Gagliano G, Rossi S, et al. The noninvasive assessment of the effects of penbutolol on liver hemodynamics in cirrhotic patients using angioscintigraphy. A randomized controlled double-blind study. Recenti Progressi in Medicina 1990;81(11):705-9. [PubMed] [Google Scholar]
Santambrogio 2006 {published data only}
- Santambrogio R, Opocher E, Costa M, Bruno S, Ceretti AP, Spina GP. Natural history of a randomized trial comparing distal spleno-renal shunt with endoscopic sclerotherapy in the prevention of variceal rebleeding: a lesson from the past. World Journal of Gastroenterology 2006;12(39):6331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Santos 2011 {published data only}
- Santos MM, Tolentino LH, Rodrigues RA, Nakao FS, Rohr MR, de-Paulo GA, et al. Endoscopic treatment of esophageal varices in advanced liver disease patients: band ligation versus cyanoacrylate injection. European Journal of Gastroenterology and Hepatology 2011;23(1):60-5. [DOI] [PubMed] [Google Scholar]
Saraya 1993 {published data only}
- Saraya A, Sarin SK. Effects of intravenous nitroglycerin and metoclopramide on intravariceal pressure: a double-blind, randomized study. American Journal of Gastroenterology 1993;88(11):1850-3. [PubMed] [Google Scholar]
Sarin 1995 {published data only}
- Sarin SK, Saraya A. Effects of intravenous nitroglycerin and nitroglycerin and metoclopramide on intravariceal pressure: a double blind, randomized study. American Journal of Gastroenterology 1995;90(1):48-53. [PubMed] [Google Scholar]
Sarin 1997 {published data only}
- Sarin SK, Govil A, Jain A, Guptan RC, Murthy NS. Randomized prospective trial of endoscopic sclerotherapy (EST) vs variceal ligation (EVL) for bleeding esophageal-varices - influence on gastropathy, gastric varices and recurrence. Gastroenterology 1995;108(4):A1163. [DOI] [PubMed] [Google Scholar]
- Sarin SK, Govil A, Jain AK, Guptan RC, Issar SK, Jain M, et al. Prospective randomized trial of endoscopic sclerotherapy versus variceal band ligation for esophageal varices: influence on gastropathy, gastric varices and variceal recurrence. Journal of Hepatology 1997;26(4):826-32. [DOI] [PubMed] [Google Scholar]
Sarin 2005 {published data only}
- Sarin SK, Wadhawan M, Gupta R, Shahi H. Evaluation of endoscopic variceal ligation (EVL) versus propanolol plus isosorbide mononitrate/nadolol (ISMN) in the prevention of variceal rebleeding: comparison of cirrhotic and noncirrhotic patients. Digestive Diseases and Sciences 2005;50(8):1538-47. [DOI] [PubMed] [Google Scholar]
Sauerbruch 2015 {published data only}
- Sauerbruch T, Mengel M, Dollinger M, Zipprich A, Rossle M, Panther E, et al. Prevention of rebleeding from esophageal varices in patients with cirrhosis receiving small-diameter stents versus hemodynamically controlled medical therapy. Gastroenterology 2015;149(3):660-8. [DOI] [PubMed] [Google Scholar]
Schepke 2001 {published data only}
- Schepke M, Werner E, Biecker E, Schiedermaier P, Heller J, Neef M, et al. Hemodynamic effects of the angiotensin II receptor antagonist irbesartan in patients with cirrhosis and portal hypertension. Gastroenterology 2001;121(2):389-95. [DOI] [PubMed] [Google Scholar]
Schiedermaier 2002 {published data only}
- Schiedermaier P, Harrison P, Arthur M, Grandt D, Sutton R, Drewe J, et al. Effect of the somatostatin analogue lanreotide on meal-stimulated portal blood flow in patients with liver cirrhosis. Digestion 2002;65(1):56-60. [DOI] [PubMed] [Google Scholar]
Schiedermaier 2003 {published data only}
- Schiedermaier P, Koch L, Stoffel-Wagner B, Layer G, Sauerbruch T. Effect of propranolol and depot lanreotide SR on postprandial and circadian portal haemodynamics in cirrhosis. Alimentary Pharmacology & Therapeutics 2003;18(8):777-84. [DOI] [PubMed] [Google Scholar]
Sen 2002 {published data only}
- Sen S, De BK, Biswas PK, Biswas J, Das D, Maity AK. Hemodynamic effect of spironolactone in liver cirrhosis and propranolol-resistant portal hypertension. Indian Journal of Gastroenterology 2002;21(4):145-8. [PubMed] [Google Scholar]
Serwah 2002 {published data only}
- Serwah AA, Habba MR, AbdelHamid AS. Endoscopic variceal ligation compared with endoscopic sclerotherapy for bleeding oesophageal varices: a randomised controlled trial. Journal of Hepatology 2004;40:73. [Google Scholar]
- Serwah AA, Habba MR, AbdelHamid AS. Endoscopic variceal ligation compared with endoscopic sclerotherapy for variceal bleeding. Journal of Hepatology 2002;36(Suppl 1):63. [Google Scholar]
- Serwah AH, Habba MR, Abdel-Hamid A. Endoscopic variceal ligation compared with endoscopic sclerotherapy for bleeding oesophageal varices (OV): a randomized controlled trial. Journal of Clinical Epidemiology 1998;51(Suppl 1):25s. [Google Scholar]
Shah 2001 {published data only}
- Shah SR. The difficulties in carrying out this study comparing three established modalities of preventing recurrent variceal hemorrhage in patients with portal hypertension. Annals of Surgery 2001;234(2):263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheikh 1998 {published data only}
- Sheikh RA, Trudeau WL. Randomised trial of octreotide for long term management of cirrhosis after variceal hemorrhage. Gastrointestinal Endoscopy 1998;48(3):328-30. [PubMed] [Google Scholar]
Shigemitsu 2000 {published data only}
- Shigemitsu T, Yoshida T, Harada T, Takeo Y, Nakamura H, Okita K. Endoscopic injection sclerotherapy with ligation versus endoscopic injection sclerotherapy alone in the management of esophageal varices: a prospective randomized trial. Journal of Hepato-Gastroenterology 2000;47(33):733-7. [PubMed] [Google Scholar]
Shin 1998 {published data only}
- Shin KH, Lee JS, Yoon JH, Han CJ, Lee HS, Kim CY. Comparison of endoscopic variceal ligation versus combined ligation and sclerotherapy for bleeding esophageal varices. Korean Journal of Hepatology 1998;4(2):143-50. [Google Scholar]
Silva 2004 {published data only}
- Silva PG, Segovia MR, Backhouse EC, Palma M, Marquez S, Iturriaga RH. Effects of acute octreotide infusion on renal function in patients with cirrhosis and portal hypertension. Revista Medica de Chile 2004;132(2):144-50. [DOI] [PubMed] [Google Scholar]
Siqueira 1998 {published data only}
- Siqueira ES, Rohr MR, Brandt CQ, Ferrari AP. Esophageal-varices - sclerotherapy or banding ligation - A double-blind, prospective, randomized study in schistossomiasis. Gastrointestinal Endoscopy 1995;41(4):358. [Google Scholar]
- Siqueira ES, Rohr MR, Libera ED, Castro RR, Ferrari AP. Band ligation or sclerotherapy as endoscopic treatment for oesophageal varices in schistosomotic patients: results of a randomized study. HPB Surgery 1998;11(1):27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2013 {published data only}
- Smith L, Dickson S, Hayes PC, Forrest EH, Gaya DR, Mills PR, et al. Multicentre randomised controlled study comparing carvedilol with endoscopic band ligation in the prevention of variceal rebleeding. Journal of Hepatology 2013;58:S255. [DOI] [PubMed] [Google Scholar]
- Smith LA, Dickson S, Hayes PC, Tripathi D, Ferguson JW, Forrest EH, et al. Multicentre randomised controlled study comparing carvedilol with endoscopic band ligation in the prevention of variceal rebleeding. Gut 2013;62:A1-2. [DOI] [PubMed] [Google Scholar]
Sohn 2013 {published data only}
- Sohn JH, Kim TY, Um SH, Seo YS, Baik SK, Kim MY, et al. A randomized, multi-center, phase IV open-label study to evaluate and compare the effect of carvedilol versus propranolol on reduction in portal pressure in patients with cirrhosis: an interim analysis. Hepatology (Baltimore, Md.) 2013;58(4 Suppl 1):990a. [Google Scholar]
Sotto 1989 {published data only}
- Sotto A, Castro R, Glez CJ. Propranolol in the prevention of digestive bleeding in cirrhotic patients. Acta Gastroenterologica Latinoamericana 1989;19(1):15-20. [PubMed] [Google Scholar]
Spina 1990 {published data only}
- Spina GP, Santambrogio R, Opocher E, Cosentino F, Zambelli A, Rubis PG, et al. Distal splenorenal shunt versus endoscopic sclerotherapy in the prevention of variceal rebleeding. First stage of a randomized, controlled trial. Annals of Surgery 1990;211(2):178-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Srinivasan 1997 {published data only}
- Srinivasan R, Minocha A. Esophageal variceal sclerotherapy or band ligation: is two better than one? American Journal of Gastroenterology 1997;92(8):1394-5. [PubMed] [Google Scholar]
Stanley 2014 {published data only}
- Stanley AJ, Dickson S, Hayes PC, Forrest EH, Mills PR, Tripathi D, et al. Multicentre randomised controlled study comparing carvedilol with variceal band ligation in the prevention of variceal rebleeding. Journal of Hepatology 2014;61(5):1014-9. [DOI] [PubMed] [Google Scholar]
Sugano 1997 {published data only}
- Sugano S, Suzuki T, Nishio M, Makino H, Okajima T. Chronic splanchnic hemodynamic effects of low-dose transdermal nitroglycerin versus low-dose transdermal nitroglycerin plus spironolactone in patients with cirrhosis. Digestive Diseases and Sciences 1997;42(3):529-35. [DOI] [PubMed] [Google Scholar]
Sugano 2001 {published data only}
- Sugano S, Yamamoto K, Sasao K, Ishii K, Watanabe M, Tanikawa K. Daily variation of azygos and portal blood flow and the effect of propranolol administration once an evening in cirrhotics. Journal of Hepatology 2001;34(1):26-31. [DOI] [PubMed] [Google Scholar]
Sun 2013 {published data only}
- Sun J. A clinical study of endoscopic treatment mothods in esophageal varices. Journal of Gastroenterology and Hepatology 2013;28:85-6. [Google Scholar]
Sung 1998 {published data only}
- Sung JJ. Sclerotherapy or banding for oesophageal varices? HPB surgery 1998;11(2):131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Svoboda 1992 {published data only}
- Svoboda P, Ochmann J, Kantorová I, Sefr R. The ACE inhibitor, enalapril, in portal hypertension. A prospective placebo controlled study. Casopis Lekaru Ceskych 1992;131(6):170-3. [PubMed] [Google Scholar]
- Svoboda P, Ochmann J, Kantorova I. Effect of enalapril treatment and sclerotherapy of esophageal varices on hepatic hemodynamics in portal hypertension. Journal of Hepato-Gastroenterology 1992;39(6):549-52. [PubMed] [Google Scholar]
Taniai 2002 {published data only}
- Taniai N, Onda M, Tajiri T, Yoshida H, Mamada Y. Combined endoscopic and radiologic intervention to treat esophageal varices. Hepato-Gastroenterology 2002;49(46):984-8. [PubMed] [Google Scholar]
Taranto 1990 {published data only}
- Taranto D, Suozzo R, de-Sio I, Romano M, Caporaso N, Del-Vecchio BC, et al. Effect of metoclopramide on transmural oesophageal variceal pressure and portal blood flow in cirrhotic patients. Digestion 1990;47(1):56-60. [DOI] [PubMed] [Google Scholar]
Taupignon 1989 {published data only}
- Taupignon A, Martin T, Lavignolle A, Perrin D, Bodic L. Propranolol and endoscopic sclerosis comparison in recurrent treatment of esophageal varices rupture in patient with cirrhosis. A two-year results of a controlled study. Gastroenterologie Clinique et Biologique 1989;13(2):167. [Google Scholar]
Terabayashi 1987 {published data only}
- Terabayashi H, Ohnishi K, Tsunoda T, Nakata H, Saito M, Tanaka H, et al. Prospective controlled trial of elective endoscopic sclerotherapy in comparison with percutaneous transhepatic obliteration of esophageal varices in patients with nonalcoholic cirrhosis. Gastroenterology 1987;93(6):1205-9. [DOI] [PubMed] [Google Scholar]
Terblanche 1979 {published data only}
- Terblanche J, Northover JM, Bornman P. A prospective controlled trial of sclerotherapy in the long term management of patients after esophageal variceal bleeding. Surgery Gynecology and Obstetrics 1979;148(3):323-33. [PubMed] [Google Scholar]
Terblanche 1983 {published data only}
- Terblanche J, Bornman P, Yakoob H, Bane R, Wright J, Kirsch RE. Prospective randomized controlled trial of sclerotherapy after esophageal variceal bleeding. Gastroenterology 1980;79(5):1128. [Google Scholar]
- Terblanche J, Bornman PC, Kahn D, Jonker MA, Campbell JA, Wright J, et al. Failure of repeated injection sclerotherapy to improve long-term survival after oesophageal variceal bleeding. A five-year prospective controlled clinical trial. Lancet 1983;2(8363):1328-32. [DOI] [PubMed] [Google Scholar]
- Terblanche J, Jonker MA, Bornman PC, Wright J, Kirsch RE. A 5-year prospective randomized controlled clinical-trial of sclerotherapy after esophageal variceal bleeding. South African Journal of Surgery 1982;20(3):176-7. [Google Scholar]
Terblanche 1988 {published data only}
- Terblanche J. Shunt surgery versus endoscopic sclerotherapy for long-term treatment of variceal bleeding. Early results of a randomized trial. HPB Surgery 1988;1(1):85-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Teres 1987 {published data only}
- Teres J, Bordas JM, Bravo D. Sclerotherapy vs. distal splenorenal shunt in the elective treatment of variceal hemorrhage: a randomized controlled trial. Hepatology (Baltimore, Md.) 1987;7(3):430-6. [DOI] [PubMed] [Google Scholar]
Teres 1993 {published data only}
- Teres J, Bosch J, Bordas JM, Garcia-Pagan JC, Feu F, Cirera I, et al. Propranolol versus sclerotherapy in preventing variceal rebleeding: a randomized controlled trial. Gastroenterology 1993;105(5):1508-14. [DOI] [PubMed] [Google Scholar]
Testa 1991 {published data only}
- Testa R, Rodriguez G, Dagnino F, Grasso A, Gris A, Marenco S, et al. Effects of beta-adrenoceptor antagonists on cerebral blood-flow of cirrhotic-patients with portal-hypertension. Journal of Clinical Pharmacology 1991;31(2):136-9. [DOI] [PubMed] [Google Scholar]
Thiel 1993 {published data only}
- Thiel DH, Dindzans VJ, Schade RR, Rabinovitz M, Gavaler JS. Prophylactic versus emergency sclerotherapy of large esophageal varices prior to liver transplantation. Digestive Diseases and Sciences 1993;38(8):1505-10. [DOI] [PubMed] [Google Scholar]
Tommasini 1989 {published data only}
- Tommasini M, De-Franchis R, Sangiovanni A, Colombo M. Beta-blockers in the secondary prevention of gastrointestinal haemorrhage in well-compensated cirrhotics. A multicentre randomised controlled study. Drugs 1989;37(Suppl 2):35-41. [DOI] [PubMed] [Google Scholar]
Triger 1992 {published data only}
- Triger DR, Johnson AG, Brazier JE, Johnston GW, Spencer EF, McKee R, et al. A prospective trial of endoscopic sclerotherapy v oesophageal transection and gastric devascularisation in the long term management of bleeding oesophageal varices. Gut 1992;33(11):1553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triger DR, Johnson AG, Spencer EF, Jonston GW, McKee R, Anderson JR, et al. A controlled trial comparing endoscopic sclerotherapy with oesophagogastric devascularisation and transection in the long term management of bleeding oesophageal varices. Gut 1990;31(5):A592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tripathi 2004 {published data only}
- Tripathi D, Helmy A, Lui HF, Stanley AJ, Forrest E, Redhead DN, et al. Randomized controlled trial of transjugular intra-hepatic portosystemic stent-shunt (TIPSS) versus TIPSS and variceal band ligation (VBL) in the prevention of variceal re-bleeding. Journal of Hepatology 2001;34(1):69. [Google Scholar]
- Tripathi D, Lui HF, Helmy A, Dabos K, Forrest E, Stanley AJ, et al. Randomised controlled trial of long term portographic follow up versus variceal band ligation following transjugular intrahepatic portosystemic stent shunt for preventing oesophageal variceal rebleeding. Gut 2004;53(3):431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Umehara 1999 {published data only}
- Umehara M, Onda M, Tajiri T, Toba M, Yoshida H, Yamashita K. Sclerotherapy plus ligation versus ligation for the treatment of esophageal varices: a prospective randomized study. Gastrointestinal Endoscopy 1999;50(1):7-12. [DOI] [PubMed] [Google Scholar]
Van Buuren 2000 {published data only}
- Van-Buuren HR, Groeneweg M, Vleggaar FP, Lesterhuis W, van-Tilburg AJ, Hop WC, et al. The results of a randomized controlled trial evaluating TIPS and endoscopic therapy in cirrhotic patients with gastro-esophageal variceal bleeding. Journal of Hepatology 2000;32:71. [Google Scholar]
Van Buuren 2008 {published data only}
- Van-Buuren HR, Wils A, Rauws EA, Van-Hoek B, Drenth JP, Kuipers EJ, et al. Dutch study on the optimal treatment strategy for patients with a first or second occurrence of gastro-oesophageal variceal bleeding: the TIPS-TRUE trial. Nederlands Tijdschrift voor Geneeskunde 2008;152(11):643-5. [PubMed] [Google Scholar]
Van Stiegmann 1993 {published data only}
- Van-Stiegmann G. Sclerotherapy versus propranolol after a variceal bleed. HPB Surgery 1993;7(2):178-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vickers 1994 {published data only}
- Vickers C, Rhodes J, Chesner I, Hillenbrand P, Dawson J, Cockel R, et al. Prevention of rebleeding from oesophageal varices: two-year follow up of a prospective controlled trial of propranolol in addition to sclerotherapy. Journal of Hepatology 1994;21(1):81-7. [DOI] [PubMed] [Google Scholar]
- Vickers C, Rhodes J, Hillenbrand P, Bradby H, Hawker P, Dykes P, et al. Prospective controlled trial of propranolol and sclerotherapy for prevention of rebleeding from oesophageal varices. Gut 1987;28(10):A1359. [DOI] [PubMed] [Google Scholar]
Villanueva 1996 {published data only}
- Pomier-Layrargues G, Villeneuve JP, Willems B, Huet PM, Marleau D. Effects of propranolol and placebo on hepatic hemodynamics in cirrhotic-patients with bleeding esophageal-varices. Gastroenterology 1985;88(5):1685. [Google Scholar]
- Pomier-Layrargues G, Villeneuve JP, Willems B, Huet PM, Marleau D. Systemic and hepatic hemodynamics after variceal hemorrhage: effects of propranolol and placebo. Gastroenterology 1987;93(6):1218-24. [DOI] [PubMed] [Google Scholar]
- Villanueva C, Balanzo J, Novella MT, Soriano G, Sainz S, Torras X, et al. Nadolol plus isosorbide mononitrate compared with sclerotherapy for the prevention of variceal rebleeding. New England Journal of Medicine 1996;334(25):1624-9. [DOI] [PubMed] [Google Scholar]
Villanueva 2001 {published data only}
- Villanueva C, Minana J, Ortiz J, Gallego A, Soriano G, Torras X, et al. Endoscopic ligation compared with combined treatment with nadolol and isosorbide mononitrate to prevent recurrent variceal bleeding. New England Journal of Medicine 2001;345(9):647-55. [DOI] [PubMed] [Google Scholar]
Villanueva 2002 {published data only}
- Villanueva C, Minana J, Ortiz J, Gallego A, Soriano G, Torras X. Endoscopic ligation compared with combined treatment with nadolol and isosorbide mono-nitrates to prevent recurrent variceal bleeding. Gastrointestinal Endoscopy 2002;55(6):761-4. [PubMed] [Google Scholar]
Villanueva 2009 {published data only}
- Villanueva C, Aracil C, Colomo A, Lopez-Balaguer JM, Piqueras M, Gonzalez B, et al. Clinical trial: a randomized controlled study on prevention of variceal rebleeding comparing nadolol plus ligation vs. hepatic venous pressure gradient-guided pharmacological therapy. Alimentary Pharmacology & Therapeutics 2009;29(4):397-408. [DOI] [PubMed] [Google Scholar]
Villanueva 2017 {published data only}
- Villanueva C, Graupera I, Aracil C, Alvarado E, Minana J, Puente A, et al. A randomized trial to assess whether portal pressure guided therapy to prevent variceal rebleeding improves survival in cirrhosis. Hepatology (Baltimore, Md.) 2017;65(5):1693-707. [DOI] [PubMed] [Google Scholar]
Villeneuve 1986 {published data only}
- Villeneuve JP, Pomier-Layrargues G, Infante-Rivard C. Propranolol for the prevention of recurrent variceal hemorrhage: a controlled trial. Hepatology (Baltimore, Md.) 1986;6(6):1239-43. [DOI] [PubMed] [Google Scholar]
Vorobioff 2002 {published data only}
- Vorobioff JD, Gamen M, Kravetz D, Picabea E, Villavicencio R, Bordato J, et al. Effects of long-term propranolol and octreotide on postprandial hemodynamics in cirrhosis: a randomized, controlled trial. Gastroenterology 2002;122(4):916-22. [DOI] [PubMed] [Google Scholar]
Vorobioff 2007 {published data only}
- Vorobioff JD, Ferretti SE, Zangroniz P, Gamen M, Picabea E, Bessone FO, et al. Octreotide enhances portal pressure reduction induced by propranolol in cirrhosis: a randomized, controlled trial. American Journal of Gastroenterology 2007;102(10):2206-13. [DOI] [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang JG, Kong DR. Efficacy of endoscopic intervention alone versus endoscopic intervention plus propranolol in the prophylaxis of esophageal variceal rebleeding. World Chinese Journal of Digestology 2012;20(30):2944-50. [Google Scholar]
Westaby 1984 {published data only}
- Westaby D, Melia WM, Macdougall BR, Hegarty JE, Williams R. Injection sclerotherapy for esophageal-varices - a prospective randomized trial of different treatment schedules. Gut 1984;25(2):129-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Westaby 1985b {published data only}
- Westaby D, Melia WM, Macdougall BR, Hegarty JE, Gimson A E, Williams R. B1 selective adrenoreceptor blockade for the long term management of variceal bleeding. A prospective randomised trial to compare oral metoprolol with injection sclerotherapy in cirrhosis. Gut 1985;26(4):421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Westaby 1986 {published data only}
- Westaby D, Melia W, Hegarty J. Use of propranolol to reduce the rebleeding rate during injection sclerotherapy prior to variceal obliteration. Hepatology (Baltimore, Md.) 1986;6(4):673-5. [DOI] [PubMed] [Google Scholar]
Westaby 1989 {published data only}
- Westaby D, Polson R, Gimson A, Hayes P, Williams R. Comparison of propranolol with injection sclerotherapy in prevention of rebleeding from oesophageal varices in cirrhotic patients. Drugs 1989;37(Suppl 2):42-6. [DOI] [PubMed] [Google Scholar]
- Westaby D, Polson RJ, Gimson AE, Hayes PC, Hayllar K, Williams R. A controlled trial of oral propranolol compared with injection sclerotherapy for the long-term management of variceal bleeding. Hepatology (Baltimore, Md.) 1990;11(3):353-9. [DOI] [PubMed] [Google Scholar]
Wiest 2002 {published data only}
- Wiest R, Lock G, Messmann H. Endoscopic ligation compared with combined treatment with nadolol and isosorbide mononitrate to prevent recurrent variceal bleeding. Zeitschrift fur Gastroenterologie 2002;40(7):547-9. [DOI] [PubMed] [Google Scholar]
Witzel 1982 {published data only}
- Witzel L, Wolbergs ED, Krankenhaus D. Prospective controlled study of para- and intravariceal injection sclerotherapy of oesophageal varices. Scandinavian Journal of Gastroenterology 1982;17:A81. [Google Scholar]
Yoshida 2004 {published data only}
- Yoshida H, Tajiri T, Mamada Y, Taniai N, Hirahata A, Kawano Y, et al. Comparison of characteristics of recurrent esophageal varices after endoscopic ligation versus endoscopic ligation plus sclerotherapy. Journal of Hepato-Gastroenterology 2004;51(56):457-61. [PubMed] [Google Scholar]
Young 1993 {published data only}
- Young MF, Sanowski RA, Rasche R. Comparison and characterization of ulcerations induced by endoscopic ligation of esophageal varices versus endoscopic sclerotherapy. Gastrointestinal Endoscopy 1993;39(2):119-22. [DOI] [PubMed] [Google Scholar]
Zargar 2008 {published data only}
- Zargar SA, Shah OJ, Wani AH, Javid G, Shah NA, Ajaz S, et al. Shunt surgery versus endoscopic band ligation for the treatment of esophageal variceal bleeding: a prospective randomized trial. Journal of Gastroenterology and Hepatology 2008;23:A73. [Google Scholar]
Zhang 2008 {published data only}
- Zhang CQ, Liu FL, Liang B, Sun ZQ, Xu HW, Xu L, et al. A modified percutaneous transhepatic variceal embolization with 2-octyl cyanoacrylate versus endoscopic ligation in esophageal variceal bleeding management: randomized controlled trial. Digestive Diseases and Sciences 2008;53(8):2258-67. [DOI] [PubMed] [Google Scholar]
Zhao 1998 {published data only}
- Zhao Y, Gu X. Endoscopic variceal ligation versus endoscopic sclerotherapy: comparison of complications and rebleeding. In: Montori A, Lirici MM, Montori J, editors(s). 6th World Congress of Endoscopic Surgery. Bologna: Monduzzi Editore, 1998:A365-8. [Google Scholar]
Zhao 2013 {published data only}
- Zhao Y. Efficacy of APC for preventing variceal recurrence after band ligation of esophageal varices. Journal of Gastroenterology and Hepatology 2013;28:86. [Google Scholar]
Zhou 2013 {published data only}
- Zhou J, Wu Z, Wu J, Wang X, Li Y, Wang M, et al. Transjugular intrahepatic portosystemic shunt (TIPS) versus laparoscopic splenectomy (LS) plus preoperative endoscopic varices ligation (EVL) in the treatment of recurrent variceal bleeding. Surgical Endoscopy 2013;27(8):2712-20. [DOI] [PubMed] [Google Scholar]
Zhu 2004 {published data only}
- Zhu Q, Jiang XH, Yi F, Jiang Y, Sun YW, Yuan YZ, et al. The effects of octreotide on portal hemodynamics in patients with liver cirrhosis. Chinese Journal of Internal Medicine 2004;43(1):16-8. [PubMed] [Google Scholar]
Zironi 1996 {published data only}
- Zironi G, Rossi C, Siringo S, Galaverni C, Gaiani S, Piscaglia F, et al. Short- and long-term hemodynamic response to octreotide in portal hypertensive patients: a double-blind, controlled study. Liver 1996;16(4):225-34. [DOI] [PubMed] [Google Scholar]
Zuckerman 2016 {published data only}
- Zuckerman MJ, Jia Y, Hernandez JA, Kolli VR, Norte A, Amin H, et al. A prospective randomized study on the risk of bacteremia in banding versus sclerotherapy of esophageal varices. Frontiers in Medicine 2016;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Jirón 1992 {published data only}
- Jirón MI, Soto JR, Vargas L, Parrochia E, Maiza E, Wolff C. Recurrence of bleeding esophageal varices: sclerotherapy versus propranolol controlled treatment. Gastroenterología Latinoamericana 1992;3:34. [Google Scholar]
References to ongoing studies
ChiCTR‐IIR‐16007964 {published data only}
- ChiCTR-IIR-16007964. Sequential therapy with esophageal varice ligation and sclerotherapy compared with ligation alone on the obliteration and recurrence of esophageal varices in patients with decompensated cirrhosis - A randomized double-blinded controlled study. www.chictr.org.cn/hvshowproject.aspx?id=6365 (date of last refreshed on 22 February 2016).
NCT00966082 {published data only}
- NCT00966082. EBL versus EBL and propranolol for the prevention of variceal rebleeding in pts with previous variceal treatment. clinicaltrials.gov/ct2/show/NCT00966082 (first received 26 August 2009).
NCT02477384 {published data only}
- NCT02477384. 8mm-TIPS versus endoscopic variceal ligation (EVL) plus propranolol for prevention of variceal rebleeding. clinicaltrials.gov/ct2/show/NCT02477384 (first received 22 June 2015).
NCT03094234 {published data only}
- NCT03094234. 8mm-TIPS versus endoscopic variceal ligation (EVL) plus propranolol for prevention of variceal rebleeding in patients with Child A cirrhosis. clinicaltrials.gov/ct2/show/NCT03094234 (first received 29 March 2017).
NCT04074473 {published data only}
- NCT04074473. Impact of nonselective beta-blocker on acute kidney injury in cirrhotic patients with esophageal varices. clinicaltrials.gov/ct2/show/NCT04074473 (first received 30 September 2019).
Additional references
Abby Philips 2016
- Abby Philips C, Sahney A. Oesophageal and gastric varices: historical aspects, classification and grading: everything in one place. Gastroenterology Report 2016;4(3):186-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Adam 2012
- Adam R, Karam V, Delvart V, O'Grady J, Mirza D, Klempnauer J, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). Journal of Hepatology 2012;57(3):675-88. [DOI] [PubMed] [Google Scholar]
Benmassaoud 2020
- Benmassaoud A, Freeman SC, Roccarina D, Plaz Torres MC, Sutton AJ, Cooper NJ, et al. Treatment for ascites in adults with decompensated liver cirrhosis: a network meta-analysis. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD013123. [DOI: 10.1002/14651858.CD013123.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beppu 1981
- Beppu K, Inokuchi K, Koyanagi N, Nakayama S, Sakata H, Kitano S, et al. Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointestinal Endoscopy 1981;27(4):213-8. [DOI] [PubMed] [Google Scholar]
Best 2018
- Best LM, Freeman S, Sutton AJ, Hawkins N, Tsochatzis E, Gurusamy KS. Treatment for hepatorenal syndrome in people with decompensated liver cirrhosis: a network meta-analysis. Cochrane Database of Systematic Reviews 2018, Issue 9. Art. No: CD013103. [DOI: 10.1002/14651858.CD013103] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brand 2018
- Brand M, Prodehl L, Ede CJ. Surgical portosystemic shunts versus transjugular intrahepatic portosystemic shunt for variceal haemorrhage in people with cirrhosis. Cochrane Database of Systematic Reviews 2018, Issue 10. Art. No: CD001023. [DOI: 10.1002/14651858.CD001023.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cales 1990
- Cales P, Desmorat H, Vinel JP, Caucanas JP, Ravaud A, Gerin P, et al. Incidence of large oesophageal varices in patients with cirrhosis: application to prophylaxis of first bleeding. Gut 1990;31(11):1298-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2012
- Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161-76. [DOI] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLOS One 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2013
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza-Jeric K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chawla 2012
- Chawla S, Katz A, Attar BM, Gupta A, Sandhu DS, Agarwal R. Platelet count/spleen diameter ratio to predict the presence of esophageal varices in patients with cirrhosis: a systematic review. European Journal of Gastroenterology and Hepatology 2012;24(4):431-6. [DOI] [PubMed] [Google Scholar]
D'Amico 2010
- D'Amico G, Pagliaro L, Pietrosi G, Tarantino I. Emergency sclerotherapy versus vasoactive drugs for bleeding oesophageal varices in cirrhotic patients. Cochrane Database of Systematic Reviews 2010, Issue 3. Art. No: CD002233. [DOI: 10.1002/14651858.CD002233.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Amico 2014
- D'Amico G, Pasta L, Morabito A, D'Amico M, Caltagirone M, Malizia G, et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Alimentary Pharmacology & Therapeutics 2014;39(10):1180-93. [DOI] [PubMed] [Google Scholar]
de Franchis 1992
- Franchis R, Pascal JP, Ancona E, Burroughs AK, Henderson M, Fleig W, et al. Definitions, methodology and therapeutic strategies in portal hypertension. A consensus development workshop, Baveno, Lake Maggiore, Italy, April 5 and 6, 1990. Journal of Hepatology 1992;15(1-2):256-61. [DOI] [PubMed] [Google Scholar]
de Franchis 2015
- Franchis R, Baveno VIF. Expanding consensus in portal hypertension: report of the Baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. Journal of Hepatology 2015;63(3):743-52. [DOI] [PubMed] [Google Scholar]
Dias 2010
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Statistics in Medicine 2010;29(7-8):932-44. [DOI] [PubMed] [Google Scholar]
Dias 2012a
- Dias S, Sutton AJ, Welton NJ, Ades AE. NICE DSU technical support document 3: heterogeneity: subgroups, meta-regression, bias and bias-adjustment, September 2011 (last updated April 2012). nicedsu.org.uk/wp-content/uploads/2016/03/TSD3-Heterogeneity.final-report.08.05.12.pdf (accessed 6 May 2020).
Dias 2012b
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU technical support document 1: introduction to evidence synthesis for decision making, April 2011 (last updated April 2012). https://pdfs.semanticscholar.org/dfcb/3fbc7a7eaf1ca1b7b9be3c872ec3d77c846f.pdf (accessed 6 May 2020).
Dias 2014
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU technical support document 4: inconsistency in networks of evidence based on randomised controlled trials, May 2011 (last updated April 2014). nicedsu.org.uk/wp-content/uploads/2016/03/TSD4-Inconsistency.final_.15April2014.pdf (accessed 6 May 2020). [PubMed]
Dias 2016
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials, August 2011 (last updated September 2016). www.ncbi.nlm.nih.gov/pubmedhealth/PMH0088912/pdf/PubMedHealth_PMH0088912.pdf (accessed 6 May 2020). [PubMed]
Dwinata 2019
- Dwinata M, Putera DD, Adda'i MF, Hidayat PN, Hasan I. Carvedilol vs endoscopic variceal ligation for primary and secondary prevention of variceal bleeding: systematic review and meta-analysis. World Journal of Hepatology 2019;11(5):464-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
EASL 2018
Edgeworth 1887
- Edgeworth, FY. On Observations relating to several quantities. Hermathena 1887;6(13):285-79. [Google Scholar]
EuroQol 2018
- EuroQol. EQ-5D Instruments | About EQ-5D, 2018. euroqol.org/eq-5d-instruments/ (accessed 6 May 2020).
Fleming 2008
- Fleming KM, Aithal GP, Solaymani-Dodaran M, Card TR, West J. Incidence and prevalence of cirrhosis in the United Kingdom, 1992-2001: a general population-based study. Journal of Hepatology 2008;49(5):732-8. [DOI] [PubMed] [Google Scholar]
Freeman 2018
- Freeman SC, Scott NW, Powell R, Johnston M, Sutton AJ, Cooper NJ. Component network meta-analysis identifies the most effective components of psychological preparation for adults undergoing surgery under general anesthesia. Journal of Clinical Epidemiology 2018;98:105-16. [DOI] [PubMed] [Google Scholar]
Garcia‐Tsao 2017
- Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology (Baltimore, Md.) 2017;65(1):310-35. [DOI] [PubMed] [Google Scholar]
Gines 2009
- Gines P, Schrier RW. Renal failure in cirrhosis. New England Journal of Medicine 2009;361(13):1279-90. [DOI] [PubMed] [Google Scholar]
Gluud 2012
- Gluud LL, Krag A. Banding ligation versus beta-blockers for primary prevention in oesophageal varices in adults. Cochrane Database of Systematic Reviews 2012, Issue 8. Art. No: CD004544. [DOI: 10.1002/14651858.CD004544.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurusamy 2019
- Gurusamy K, Walmsley M, Davidson BR, Frier C, Fuller B, Madden A, et al. Top research priorities in liver and gallbladder disorders in the United Kingdom. BMJ Open 2019;9(3):e025045. [DOI] [PMC free article] [PubMed]
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294-302. [DOI] [PubMed] [Google Scholar]
Gøtzsche 2008
- Gøtzsche PC, Hróbjartsson A. Somatostatin analogues for acute bleeding oesophageal varices. Cochrane Database of Systematic Reviews 2008, Issue 3. Art. No: CD000193. [DOI: 10.1002/14651858.CD000193.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Herman 2015
- Herman J, Baram M. Blood and volume resuscitation for variceal hemorrhage. Annals of the American Thoracic Society 2015;12(7):1100-2. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2012
- Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Research Synthesis Methods 2012;3(2):98-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutton 2015
- Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Annals of Internal Medicine 2015;162(11):777-84. [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice CFR and ICH Guidelines. Vol. 1. Philadelphia (PA): Barnett International/PAREXEL, 1997. [Google Scholar]
Ioannou 2003
- Ioannou GN, Doust J, Rockey DC. Terlipressin for acute esophageal variceal hemorrhage. Cochrane Database of Systematic Reviews 2003, Issue 1. Art. No: CD002147. [DOI: 10.1002/14651858.CD002147] [DOI] [PubMed] [Google Scholar]
Jackson 2014
- Jackson D, Barrett JK, Rice S, White IR, Higgins JP. A design-by-treatment interaction model for network meta-analysis with random inconsistency effects. Statistics in Medicine 2014;33(21):3639-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Annals of Internal Medicine 2001;135(11):982-9. [DOI] [PubMed] [Google Scholar]
Lu 2006
- Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. Journal of the American Statistical Association 2006;101(474):447-59. [Google Scholar]
McCarty 2017
- McCarty TR, Afinogenova Y, Njei B. Use of wireless capsule endoscopy for the diagnosis and grading of esophageal varices in patients with portal hypertension: a systematic review and meta-analysis. Journal of Clinical Gastroenterology 2017;51(2):174-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
McPherson 2016
- McPherson S, Lucey MR, Moriarty KJ. Decompensated alcohol related liver disease: acute management. BMJ (Clinical Research Ed.) 2016;352:i124. [DOI] [PubMed] [Google Scholar]
Merion 2010
- Merion RM. Current status and future of liver transplantation. Seminars in Liver Disease 2010;30(4):411-21. [DOI] [PubMed] [Google Scholar]
Merli 2003
- Merli M, Nicolini G, Angeloni S, Rinaldi V, De Santis A, Merkel C, et al. Incidence and natural history of small esophageal varices in cirrhotic patients. Journal of Hepatology 2003;38(3):266-72. [DOI] [PubMed] [Google Scholar]
Mills 2012
- Mills EJ, Ioannidis JP, Thorlund K, Schünemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta-analysis. JAMA 2012;308(12):1246-53. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352(9128):609-13. [DOI] [PubMed] [Google Scholar]
Mokdad 2014
- Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Medicine 2014;12:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2013
- Moore CM, Van Thiel DH. Cirrhotic ascites review: pathophysiology, diagnosis and management. World Journal of Hepatology 2013;5(5):251-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCBI 2018a
- National Center for Biotechnology Information. Liver cirrhosis. www.ncbi.nlm.nih.gov/mesh/68008103 (accessed 6 May 2020).
NCBI 2018b
- National Center for Biotechnology Information. Esophageal and gastric varices. www.ncbi.nlm.nih.gov/mesh/68004932 (accessed 6 May 2020).
Newell 1992
- Newell DJ. Intention-to-treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837-41. [DOI] [PubMed] [Google Scholar]
NIEC 1988
- The North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. New England Journal of Medicine 1988;319(15):983-9. [DOI] [PubMed] [Google Scholar]
Njei 2016
Optum 2018
- Optum. Patient-reported outcomes | What we do | SF Health Surveys | SF-36v2 Health Survey, 2018. campaign.optum.com/optum-outcomes/what-we-do/health-surveys/sf-36v2-health-survey.html (accessed on 14 April 2018).
Puhan 2014
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ (Clinical Research Ed.) 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
Qi 2015
- Qi XS, Bao YX, Bai M, Xu WD, Dai JN, Guo XZ. Nonselective beta-blockers in cirrhotic patients with no or small varices: a meta-analysis. World Journal of Gastroenterology 2015;21(10):3100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qi 2016
- Qi X, Tian Y, Zhang W, Zhao H, Han G, Guo X. Covered TIPS for secondary prophylaxis of variceal bleeding in liver cirrhosis: a systematic review and meta-analysis of randomized controlled trials. Medicine 2016;95(50):e5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ratib 2015
- Ratib S, Fleming KM, Crooks CJ, Walker AJ, West J. Causes of death in people with liver cirrhosis in England compared with the general population: a population-based cohort study. American Journal of Gastroenterology 2015;110(8):1149-58. [DOI] [PubMed] [Google Scholar]
Read 1972
- Read AE. Clinical physiology of the liver. British Journal of Anaesthesia 1972;44(9):910-7. [DOI] [PubMed] [Google Scholar]
Rios 2015
- Ríos CE, Seron P, Gisbert JP, Bonfill CX. Endoscopic injection of cyanoacrylate glue versus other endoscopic procedures for acute bleeding gastric varices in people with portal hypertension. Cochrane Database of Systematic Reviews 2015, Issue 5. Art. No: CD010180. [DOI: 10.1002/14651858.CD010180.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roccarina 2020
- Roccarina D, Best LM, Freeman SC, Roberts D, Cooper NJ, Sutton AJ, et al. Primary prevention of bleeding in people with oesophageal varices due to liver cirrhosis: a network meta-analysis. Cochrane Database of Systematic Reviews 2020, Issue Under peer review. Art. No: CD013121. [DOI: 10.1002/14651858.CD013121] [DOI] [PMC free article] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591-603. [DOI] [PubMed] [Google Scholar]
Saab 2006
- Saab S, Nieto JM, Lewis SK, Runyon BA. TIPS versus paracentesis for cirrhotic patients with refractory ascites. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No: CD004889. [DOI: 10.1002/14651858.CD004889.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163-71. [DOI] [PubMed] [Google Scholar]
Salanti 2012
- Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80-97. [DOI] [PubMed] [Google Scholar]
Sass 2009
- Sass DA, Chopra KB. Portal hypertension and variceal hemorrhage. Medical Clinics of North America 2009;93(4):837-53, vii-viii. [DOI] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials: combined analysis of meta-epidemiological studies. Health Technology Assessment 2012;16(35):1-82. [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials. Annals of Internal Medicine 2012;157(6):429-38. [DOI] [PubMed] [Google Scholar]
Savović 2018
- Savović J, Turner RM, Mawdsley D, Jones HE, Beynon R, Higgins JP, et al. Association between risk-of-bias assessments and results of randomized trials in Cochrane reviews: the ROBES Meta-Epidemiologic Study. American Journal of Epidemiology 2018;187(5):1113-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scaglione 2015
- Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, et al. The epidemiology of cirrhosis in the united states: a population-based study. Journal of Clinical Gastroenterology 2015;49(8):690-6. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ (Clinical Research Ed.) 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Setiawan 2016
- Setiawan VW, Stram DO, Porcel J, Lu SC, Le Marchand L, Noureddin M. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: the multiethnic cohort. Hepatology (Baltimore, Md.) 2016;64(6):1969-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Severini 1993
- Severini TA. Bayesian interval estimates which are also confidence intervals. Journal of the Royal Statistical Society. Series B (Methodological) 1993;55(2):533-40. [Google Scholar]
Stata/SE 15.1 [Computer program]
- Stata/SE. Version 15.1. Texas, USA: StataCorp LLC, 2017. www.stata.com/.
Thabut 2007
- Thabut D, Hammer M, Cai Y, Carbonell N. Cost of treatment of oesophageal variceal bleeding in patients with cirrhosis in France: results of a French survey. European Journal of Gastroenterology and Hepatology 2007;19(8):679-86. [DOI] [PubMed] [Google Scholar]
Tripathi 2015
- Tripathi D, Stanley AJ, Hayes PC, Patch D, Millson C, Mehrzad H, et al. U.K. Guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut 2015;64(11):1680-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsochatzis 2014
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383(9930):1749-61. [DOI] [PubMed] [Google Scholar]
van Valkenhoef 2012
- Valkenhoef G, Lu G, Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Research Synthesis Methods 2012;3(4):285-99. [DOI] [PubMed] [Google Scholar]
Welton 2009
- Welton NJ, Caldwell DM, Adamopoulos E, Vedhara K. Mixed treatment comparison meta-analysis of complex interventions: psychological interventions in coronary heart disease. American Journal of Epidemiology 2009;169(9):1158-65. [DOI] [PubMed] [Google Scholar]
Williams 2014
- Williams R, Aspinall R, Bellis M, Camps-Walsh G, Cramp M, Dhawan A, et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet 2014;384(9958):1953-97. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yepes‐Nunez 2019
- Yepes-Nunez JJ, Li SA, Guyatt G, Jack SM, Brozek JL, Beyene J, et al. Development of the summary of findings table for network meta-analysis. Journal of Clinical Epidemiology 2019;115:1-13. [DOI] [PubMed] [Google Scholar]
Zaman 2000
- Zaman A, Goldberg RJ, Pettit KG, Kaniecki DJ, Benner K, Zacker C, et al. Cost of treating an episode of variceal bleeding in a VA setting. American Journal of Gastroenterology 2000;95(5):1323-30. [DOI] [PubMed] [Google Scholar]
Zhou 2019
- Zhou GP, Sun LY, Wei L, Qu W, Zeng ZG, Liu Y, et al. Comparision between portosystemic shunts and endoscopic therapy for prevention of variceal re-bleeding: a systematic review and meta-analysis. Chinese Medical Journal 2019;132(9):1087-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Gurusamy 2018a
- Gurusamy KS, Tsochatzis E. Secondary prevention of bleeding in people with previous oesophageal variceal bleeding due to decompensated liver cirrhosis: a network meta-analysis. Cochrane Database of Systematic Reviews 2018, Issue 9. Art. No: CD013122. [DOI: 10.1002/14651858.CD013122] [DOI] [PMC free article] [PubMed] [Google Scholar]


