Abstract
Background
Keratoconus is the most common corneal dystrophy. It can cause loss of uncorrected and best‐corrected visual acuity through ectasia (thinning) of the central or paracentral cornea, irregular corneal scarring, or corneal perforation. Disease onset usually occurs in the second to fourth decade of life, periods of peak educational attainment or career development. The condition is lifelong and sight‐threatening.
Corneal collagen crosslinking (CXL) using ultraviolet A (UVA) light applied to the cornea is the only treatment that has been shown to slow progression of disease. The original, more widely known technique involves application of UVA light to de‐epithelialized cornea, to which a photosensitizer (riboflavin) is added topically throughout the irradiation process.
Transepithelial CXL is a recently advocated alternative to the standard CXL procedure, in that the epithelium is kept intact during CXL. Retention of the epithelium offers the putative advantages of faster healing, less patient discomfort, faster visual rehabilitation, and less risk of corneal haze.
Objectives
To assess the short‐ and long‐term effectiveness and safety of transepithelial CXL compared with epithelium‐off CXL for progressive keratoconus.
Search methods
To identify potentially eligible studies, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2020, Issue 1); Ovid MEDLINE; Embase.com; PubMed; Latin American and Caribbean Health Sciences Literature database (LILACS); ClinicalTrials.gov; and World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). We did not impose any date or language restrictions. We last searched the electronic databases on 15 January 2020.
Selection criteria
We included randomized controlled trials (RCTs) in which transepithelial CXL had been compared with epithelium‐off CXL in participants with progressive keratoconus.
Data collection and analysis
We used standard Cochrane methodology.
Main results
We included 13 studies with 661 eyes of 567 participants enrolled; 13 to 119 participants were enrolled per study. Seven studies were conducted in Europe, three in the Middle East, and one each in India, Russia, and Turkey. Seven studies were parallel‐group RCTs, one study was an RCT with a paired‐eyes design, and five studies were RCTs in which both eyes of some or all participants were assigned to the same intervention.
Eleven studies compared transepithelial CXL with epithelium‐off CXL in participants with progressive keratoconus. There was no evidence of an important difference between intervention groups in maximum keratometry (denoted 'maximum K' or 'Kmax'; also known as steepest keratometry measurement) at 12 months or later (mean difference (MD) 0.99 diopters (D), 95% CI −0.11 to 2.09; 5 studies; 177 eyes; I2 = 41%; very low certainty evidence). Few studies described other outcomes of interest. The evidence is very uncertain that epithelium‐off CXL may have a small (data from two studies were not pooled due to considerable heterogeneity (I2 = 92%)) or no effect on stabilization of progressive keratoconus compared with transepithelial CXL; comparison of the estimated proportions of eyes with decreases or increases of 2 or more diopters in maximum K at 12 months from one study with 61 eyes was RR 0.32 (95% CI 0.09 to 1.12) and RR (non‐event) 0.86 (95% CI 0.74 to 1.00), respectively (very low certainty). We did not estimate an overall effect on corrected‐distance visual acuity (CDVA) because substantial heterogeneity was detected (I2 = 70%). No study evaluated CDVA gain or loss of 10 or more letters on a logarithm of the minimum angle of resolution (logMAR) chart. Transepithelial CXL may result in little to no difference in CDVA at 12 months or beyond. Four studies reported that either no adverse events or no serious adverse events had been observed. Another study noted no change in endothelial cell count after either procedure. Moderate certainty evidence from 4 studies (221 eyes) found that epithelium‐off CXL resulted in a slight increase in corneal haze or scarring when compared to transepithelial CXL (RR (non‐event) 1.07, 95% CI 1.01 to 1.14).
Three studies, one of which had three arms, compared outcomes among participants assigned to transepithelial CXL using iontophoresis versus those assigned to epithelium‐off CXL. No conclusive evidence was found for either keratometry or visual acuity outcomes at 12 months or later after surgery. Low certainty evidence suggests that transepithelial CXL using iontophoresis results in no difference in logMAR CDVA (MD 0.00 letter, 95% CI −0.04 to 0.04; 2 studies; 51 eyes). Only one study examined gain or loss of 10 or more logMAR letters. In terms of adverse events, one case of subepithelial infiltrate was reported after transepithelial CXL with iontophoresis, whereas two cases of faint corneal scars and four cases of permanent haze were observed after epithelium‐off CXL. Vogt's striae were found in one eye after each intervention. The certainty of the evidence was low or very low for the outcomes in this comparison due to imprecision of estimates for all outcomes and risk of bias in the studies from which data have been reported.
Authors' conclusions
Because of lack of precision, frequent indeterminate risk of bias due to inadequate reporting, and inconsistency in outcomes measured and reported among studies in this systematic review, it remains unknown whether transepithelial CXL, or any other approach, may confer an advantage over epithelium‐off CXL for patients with progressive keratoconus with respect to further progression of keratoconus, visual acuity outcomes, and patient‐reported outcomes (PROs). Arrest of the progression of keratoconus should be the primary outcome of interest in future trials of CXL, particularly when comparing the effectiveness of different approaches to CXL. Furthermore, methods of assessing and defining progressive keratoconus should be standardized. Trials with longer follow‐up are required in order to assure that outcomes are measured after corneal wound‐healing and stabilization of keratoconus. In addition, perioperative, intraoperative, and postoperative care should be standardized to permit meaningful comparisons of CXL methods. Methods to increase penetration of riboflavin through intact epithelium as well as delivery of increased dose of UVA may be needed to improve outcomes. PROs should be measured and reported. The visual significance of adverse outcomes, such as corneal haze, should be assessed and correlated with other outcomes, including PROs.
Plain language summary
What surgical procedure works best to slow the progression of keratoconus (an eye disease)?
Why is this question important? Keratoconus is a disease that affects the thin, clear outer layer of the eye, known as the cornea. Normally, the cornea is dome‐shaped. In people with keratoconus, the cornea slowly thins, and a cone‐shaped bulge develops in the center of the cornea. The disease usually begins between the ages of teens and 40, and persists throughout life. It causes blurry or distorted vision that may not be improved by wearing glasses and may result in perforation of the cornea and other visual problems.
Treatments such as glasses and contact lenses can be used to improve the vision of people with keratoconus. However, these do not slow the progression of the disease. The only treatment known to slow disease progression is ‘corneal collagen crosslinking’ (CXL).
CXL is a surgical procedure that aims to strengthen the cornea and prevent further thinning. It involves shining ultraviolet A (invisible) light rays onto eyes that have been treated with eye drops containing riboflavin (a vitamin). When the light rays meet the riboflavin, new links form between the fibers that make up the cornea.
There are two types of CXL. One type requires the removal of the cells on surface of the cornea, to make it easier for the riboflavin to reach the cornea. This procedure is called ‘epithelium‐off CXL’. The other type does not require the removal of these cells. This procedure is called ‘transepithelial CXL’. Surgeons who carry out this procedure can use chemicals to help riboflavin penetrate the cells on the surface of the cornea. They can also deliver riboflavin to the cornea using a small electrical current (iontophoresis).
Epithelium‐off CXL is the more commonly used procedure. However, transepithelial CXL could have advantages, such as faster healing and less patient discomfort. We reviewed the evidence to find out which of these two procedures is more beneficial and less risky for people with keratoconus.
How did we identify and evaluate the evidence? We searched the medical literature for studies that compared epithelium‐off CXL against transepithelial CXL. Then we compared the results and summarized the evidence from all the studies. We rated our confidence in the evidence, based on factors such as study methods and sizes, and the consistency of findings across studies.
What did we find? We found 13 studies with a total of 567 people. The studies took place in Europe, the Middle East, India, Russia, and Turkey. The shortest studies lasted six months, and the longest study lasted more than three years. Eleven studies compared transepithelial CXL without iontophoresis against epithelium‐off CXL. Three studies compared transepithelial CXL with iontophoresis against epithelium‐off CXL.
Transepithelial CXL without iontophoresis compared to epithelium‐off CXL
We do not know if one procedure is better than the other for preventing progression of keratoconus or visual loss because too few robust studies have compared the effects of these two CXL methods.
Evidence from four studies suggests that corneal hazing (clouding of the cornea) or scarring are probably more common with epithelium‐off CXL.
Transepithelial CXL with iontophoresis compared to epithelium‐off CXL
Evidence from two studies suggests that there may be little to no difference between the two procedures in changes to vision clarity. We do not know if one procedure is better than the other to prevent progression of keratoconus because two few robust studies have compared the two methods.
The evidence does not suggest that one procedure leads to more unwanted events than the other. However, our confidence in this evidence is low, because it is based on three studies that did not use robust methods.
What does this mean? Due to a lack of robust evidence, we do not know if epithelium‐off CXL or transepithelial CXL is better for slowing keratoconus progression.
Adverse events such as corneal hazing or scarring are probably more common with epithelium‐off CXL than with transepithelial CXL without iontophoresis.
We need more and larger studies to strengthen the evidence. These should compare the benefits and the risks of different CXL procedures. Studies should aim to follow patients for more than 12 months, so that long‐term effects can be compared as it can take at least that much time for corneal tissue to heal from any procedure.
How‐up‐to date is this review? The evidence in this Cochrane Review is current to January 2020.
Summary of findings
Background
Description of the condition
Keratoconus is a corneal condition that can cause loss of uncorrected and best‐corrected visual acuity through ectasia (thinning) of the central or paracentral cornea, irregular corneal scarring, or corneal perforation. It is most commonly described as a corneal dystrophy, but is influenced by environmental factors. The condition is bilateral but often asymmetric in severity (Rabinowitz 1998). Keratoconus is the most common corneal dystrophy; the prevalence in the Netherlands was recently estimated to be 1:375 (265 cases per 100,000, 95% confidence interval (CI) 260 to 270) (Godefrooij 2017a), which is six times higher than the previous estimate of 1:2000 (Kennedy 1986). Analysis of data from 4.4 million individuals enrolled in the Netherlands' largest national health insurance provider showed an annual incidence of keratoconus of 1:7500 in the relevant age category (13.3 cases per 100 000, 95% CI 11.6 to 15.2), which was also five to 10 times higher than previous population studies reported (Godefrooij 2017a). The reason for this increase is likely improved detection using corneal imaging.
Since the 1980s, when anterior corneal imaging devices became available, studies have reported keratoconus to be more prevalent among men than women, although earlier studies based on clinical examination did not show gender association. In the study from the Netherlands, 60.6% of diagnosed patients were male, and the mean age at diagnosis was 28.3 years. Disease onset usually occurs in the second to fourth decade of life, which are periods of peak educational attainment or career development in most middle‐income and high‐income countries. The condition is lifelong and sight‐threatening. Keratoconus therefore imposes a high economic burden on patients and caregivers (Rebenitsch 2011).
Studies from the United Kingdom have found that individuals with Indian, Pakistani, and Bangladeshi ethnicity have a higher prevalence of keratoconus compared with those from Northern Europe (Cozma 2005; Georgiou 2004; Pearson 2000). The differential disease burden surrounding gender, race, and ethnicity may arise from different diagnostic testing capabilities and different diagnostic criteria used in different populations over time.
Keratoconus is most commonly an isolated sporadic disorder. It has been reported to be associated with Down syndrome (Alio 2018; Rados 1948) and Leber congenital amaurosis (Elder 1994; Godel 1978), but is more often associated with atopy, wearing hard contact lenses, eye rubbing, and a positive family history of the disorder; in 13.5% of cases there is a family history of the disease (Zadnik 1998). The inheritance in these cases is believed to exhibit variable penetrance (Rabinowitz 1990). Consequently, both genetic and environmental factors probably contribute to the development of keratoconus.
Treatments such as spectacles, contact lenses, intrastromal corneal ring segments, and corneal transplantation serve to improve vision but do not slow the progression of disease, which corneal collagen crosslinking (CXL) purports to do.
Diagnosis
Aside from retinoscopic or slit‐lamp biomicroscopic findings in longer‐standing keratoconus (e.g. corneal iron ring, Vogt’s striae, Munson's sign, evidence of previous corneal hydrops), earlier stages of keratoconus are evident using modern corneal imaging. These devices include computer‐assisted videophotokeratoscopy or Scheimpflug imaging, which detects subtle abnormalities in topography of the anterior and posterior corneal surface, allowing detailed qualitative and quantitative analysis of corneal shape of the front and back surfaces. Placido‐disc‐based corneal topography provides tangential and axial dioptric power maps of the anterior corneal surface. Based on these maps, various topographic indices have been proposed for the diagnosis of preclinical (forme fruste) and clinical keratoconus and the grading of disease severity. These image‐based indices include asymmetry in dioptric power between inferior and superior hemispheres of the cornea, bow‐tie asymmetry, and skewed astigmatic axes.
Newer Scheimpflug‐based corneal tomography helps the clinician to distinguish subclinical keratoconus and keratoconus from normal corneas by examination of anterior elevation and posterior elevation at the thinnest point, change in anterior elevation, change in posterior elevation, corneal thickness at the thinnest point, location of the thinnest point, pachymetric progression, and maximum keratometry (denoted 'maximum K' or 'Kmax'; also known as the steepest keratometry measurement, maximum cone apex curvature, maximum curvature power of the whole anterior surface of the cornea, or power of the steepest point) (Cavas‐Martínez 2016).
There is no universal method to diagnose keratoconus, especially forme fruste keratoconus. The best and safest method is to collect and analyze data using different modalities and to use established clinical parameters.
Pathogenesis
Corneal collagen fibrils are organized into bundles known as lamellae, of which there are about 300 in the central cornea and 500 in the peripheral cornea. Lamellae account for the biomechanical characteristics and strength of the normal cornea. Proteoglycans are an important component of the corneal stroma matrix. Biochemical and immunohistochemical studies of these proteoglycans show differences between normal and keratoconic corneas (Meek 2005; Raiskup‐Wolf 2008). Corneal ectasia can develop in many different ways (e.g. fewer collagen lamellae than normal, fewer collagen fibrils per lamella, closer packing of collagen fibrils, or any combination of these). These conditions may arise from defects in the proteoglycans forming the extracellular matrix, destruction of previously formed components, an increased distensibility of corneal tissue causing sliding of collagen fibers or lamellae, or a combination of these mechanisms (Akhtar 2008; Hayes 2008).
Disease progression
Just as there are no definitive criteria for the diagnosis of keratoconus, there are no definitive criteria for its progression. Corneal changes apparent on slit‐lamp biomicroscopic examination in overt cases of keratoconus are not always present to demonstrate evidence of progression, therefore one cannot rely on clinical criteria alone. Increase in maximum keratometry (K) reading by 1 diopter (D) or more remains the most frequently reported index of disease progression (Caporossi 2010; Hersh 2011; Raiskup‐Wolf 2008; Wittig‐Silva 2008).
Other parameters used to identify or monitor disease progression include worsening of refractive or corneal astigmatism (a result of corneal ectasia that is progressing); change in uncorrected or corrected distance visual acuity, or both (O'Brart 2015; Poli 2015); and worsening of corneal topographical indices other than maximum K. These indices include simulated keratometry (Sim K) or mean keratometry (mean K). In their review of data submitted for approval of CXL, the US Food and Drug Administration (FDA) defined keratoconus progression as exhibiting one or more of the following changes over 24 months: an increase of 1.00 D or greater in the 'steepest keratometry measurement' (i.e. Kmax; not to be confused with steepest central simulated keratometry, known as steep K or K2), an increase of 1.00 D or greater in manifest cylinder, and an increase of 0.50 D or greater in manifest refraction spherical equivalent. Others have defined progression as change occurring over the 6‐ to 24‐month period.
Consequently, the definition of progressive keratoconus varies in the parameters examined, the amount of change in the parameters, and the length of time over which change is observed to document an indication for CXL, ranging from 6 to 24 months. Terminology also varies: 'maximum keratometry,' 'apical keratometry,' 'cone apex keratometry,' 'steepest keratometry,' or 'maximum cone apex curvature' have been used interchangeably.
Description of the intervention
In earlier decades corneal transplantation (keratoplasty) was performed in 10% to 20% of patients with keratoconus (Gordon 2006; Rabinowitz 1998; Tuft 1994). Although rare, keratoconus has been reported to recur in transplanted corneas (Abelson 1980). Recent studies strongly suggest that the introduction of CXL has reduced the need for corneal transplantation. In the three‐year period after the introduction of CXL in the Netherlands (2012 to 2014), 25% fewer corneal transplants were performed than in the three‐year period before its introduction (2005 to 2007) (Godefrooij 2016). At an institution in Norway, the frequency of keratoplasty was more than halved during 2013 to 2014 relative to 2005 to 2006, a decline attributed to the introduction of CXL (Sandvik 2015). Consequently, by halting or decreasing the progression of keratoconus through corneal stiffening (Wollensak 2003a), CXL has the potential to decrease the number of keratoconus patients who undergo invasive procedures (lamellar or penetrating keratoplasties), with their attendant risks of rejection, endophthalmitis, and other infections.
CXL was first approved in Europe. The first individuals with keratoconus to receive crosslinking were treated in Dresden, Germany; the CXL protocol developed by Seiler and colleagues in 1997 and used in clinical trials in Germany by 1998 is named the 'Dresden protocol' (Spoerl 1998; Spoerl 1999; Wollensak 2003a). It is an epithelium‐off procedure and is the current standard worldwide, although some investigators are altering the length of time of the treatment and treatment protocols (Haberman 2018; Kymionis 2014; Kymionis 2017; Medeiros 2016; Price 2018b; Spadea 2018; Toker 2017).
The National Institute for Health and Care Excellence (NICE) in the UK produced interventional procedures guidance (IPG) for keratoconus in 2009, updating it in 2013 to encompass keratectasia as well as keratoconus (NICE 2019). The FDA approved the Dresden protocol in 2016 for use in the USA. It involves first de‐epithelializing the central cornea (approximately 9 mm) and applying a solution of riboflavin‐dextran (0.1% riboflavin‐5‐phosphate and 20% dextran T‐500) as a photosensitizer to pre‐treat the cornea, and then repeating the application every five minutes for the duration of the 30‐minute treatment with ultraviolet A (UVA) 1 cm away from the cornea, using 370 nm UVA with an irradiance of 3 mW/cm2. The NICE IPG acknowledges that “precise timings and treatment protocols vary.” It also states: "Postoperatively, topical antibiotics and anti‐inflammatory drops are normally prescribed, with topical steroids if necessary." The original Dresden protocol did not utilize postoperative topical steroids (Wollensak 2003a); postoperative steroids were used for two weeks in the FDA trial (Hersh 2017). In some cases, a bandage contact lens may also be used for a few days. The procedure is done on one eye at a time and may be repeated when needed.
All of the seminal publications on CXL have described epithelium‐off procedures. Transepithelial CXL is a recent alternative to the standard CXL procedure where the epithelium is kept intact during CXL. This technique is theoretically associated with avoidance of issues associated with removal of corneal epithelium such as delayed re‐epithelialization and risk of microbial keratitis, reduction of pain, reduction of corneal haze, reduction of transient corneal edema, reduction of glare, and avoidance of corneal dehydration and thinning during epithelium‐off CXL, thus allowing treatment of very thin, ectatic corneas (Caporossi 2012; Greenstein 2010; Hayes 2008; Hersh 2018; Mazzotta 2007; Wollensak 2009). Because the epithelium is a barrier to diffusion of riboflavin—a large molecule—into the corneal stroma, newer studies have described methods by which to circumvent this limitation through either iontophoresis (Bikbova 2016; Buzzonetti 2015) or chemical permeability enhancers such as topical anesthetics and benzalkonium chloride, which Wollensak and colleagues were the first to describe (Koppen 2012; Vinciguerra 2016; Wollensak 2009).
How the intervention might work
Since Wollensak's publication of CXL in human trials in 2003, studies have supported the efficacy of epithelium‐off corneal collagen crosslinking (O'Brart 2013; Poli 2015; Raiskup 2015; Wittig‐Silva 2014). Natural crosslinking occurs in the cornea with age (Knox Cartwright 2011); therapeutic CXL offers a much higher level of crosslinking beyond that which occurs with age. Seiler and colleagues found significant increases in stromal stress‐strain measurements (i.e. corneal rigidity) in animal models following CXL treatment, which have been replicated in human trials (Wollensak 2003b). These trials have also shown an increase in the diameter of corneal collagen fibers, which likely contributes to decreased progression of ectasia. The resultant effect of CXL is increased resistance to both enzymatic digestion and thermal damage (Spoerl 2004a; Spoerl 2004b; Wollensak 2003b).
In short, it is theorized that CXL has an impact on keratoconus progression by strengthening and stabilizing the collagen lamellae, resulting in mechanical stiffening of the cornea. CXL may improve the patient's refractive error by reducing the irregular astigmatism caused by the biochemical instability of the cornea (Hersh 2017), and preventing the progression of corneal steepening. However, improvement of refractive error is neither guaranteed nor significant in many patients. The improvement in vision has been found to be greater when CXL is combined with intracorneal ring segments than when using the segments alone (Chan 2007).
Transepithelial CXL differs from the standard CXL procedure in that the epithelium is kept intact during CXL. The reason the corneal epithelium is removed prior to standard CXL is to facilitate stromal absorption of riboflavin, a large molecule. Retention of the epithelium in CXL offers the putative advantages of faster healing, less patient discomfort, faster visual rehabilitation, and less risk of corneal haze (Caporossi 2012; Greenstein 2010; Hayes 2008; Hersh 2018; Mazzotta 2007; Wollensak 2009). Haze seen with epithelium‐off CXL generally does not occur in transepithelial CXL. Haze is the result of keratocyte apoptosis and repopulation by myofibroblasts, leading to decreased corneal transparency and a clinically visible line demarcating the depth of the actual crosslinking effect (Kuo 1997; Mazzotta 2007; Seiler 2006; Wollensak 2007). In fact, some clinician researchers believe that aside from the main goal of stabilization of keratoconus, indicators of CXL effect are a visible demarcation line (that can appear as 'haze'), flattened keratometry, and reduced pachymetry (Doors 2009; Seiler 2006). In addition, transepithelial CXL may decrease the amount of corneal thinning that occurs during crosslinking (which can be substantial), thus allowing treatment of very thin, ectatic corneas, for which CXL may otherwise be precluded for fear of UVA damage to intraocular structures (Filippello 2012; Khairy 2014; Rosenblat 2016; Spadea 2012).
Why it is important to do this review
The question remains as to which method of CXL is more effective and safe. Although epithelium‐off CXL is more established than the transepithelial technique, an increasing number of studies of transepithelial CXL and epithelium‐off CXL are being undertaken to evaluate their comparative effectiveness. It is unclear which technique better achieves the stated goal of CXL, which is to halt or slow progression of keratoconus (as defined in various ways). Furthermore, controversy surrounds the clinical effects of transepithelial CXL compared with epithelium‐off corneal crosslinking for keratoconus (Al Fayez 2015; Kocak 2014; Leccisotti 2010; Magli 2013; Rossi 2015; Wen 2018). Research on rabbit eyes suggests that corneal biomechanical rigidity after transepithelial CXL is one‐fifth of that after epithelium‐off procedures (Wollensak 2009). In addition, the progression of keratoconus is not linear over time. Periods of stability can be interrupted by periods of progression. Because estimation of the rate of turnover of collagen and the extracellular matrix of the corneal stroma may be years or decades, long‐term follow‐up after CXL is essential to determine the longevity of effects and to identify long‐term complications (Caporossi 2013; O'Brart 2015; Poli 2015; Raiskup‐Wolf 2008; Shalchi 2015; Soeters 2015).
There are also uncertainties regarding patient satisfaction, quality of life, and cost‐effectiveness of the two interventions. By a median of 3.5 years after CXL, 89% of approximately 500 CXL patients in one center who responded to a survey reported that they believed CXL had halted their disease progression (Price 2018a). Treatment at a younger age and at a mild stage of keratoconus was associated with higher satisfaction and perceived efficacy (Price 2018a), although objectively more patients with worse disease had improvement in visual acuity and corneal flattening (Greenstein 2013). The expectations of patients with advanced stages of keratoconus may exceed what CXL can deliver, leading to lower perceived efficacy (Price 2016). Researchers calculated that CXL for progressive keratoconus is cost‐effective at a willingness‐to‐pay threshold of three times the gross domestic product (GDP) per capita. Cost‐effectiveness was strongly influenced by the assumption that CXL is effective for 10 years in the base‐case scenario. The treatment would be extremely cost‐effective if the effects last 15 years or longer (Godefrooij 2017b; Leung 2017).
Objectives
To assess the short‐ and long‐term effectiveness and safety of transepithelial CXL compared with epithelium‐off CXL for progressive keratoconus.
Methods
Criteria for considering studies for this review
Types of studies
We included data only from randomized controlled trials (RCTs) comparing transepithelial CXL with epithelium‐off CXL. Although we were primarily interested in studies in which participants had been followed for 24 months or longer, we also included studies with shorter follow‐up times. We planned to accept quasi‐randomized controlled trials (i.e. trials using quasi‐random methods to allocate participants, such as alternation, date of birth, or case record number) if no RCTs were identified. We did not include quasi‐randomized controlled trials in this review because we identified RCTs.
Types of participants
We included studies of participants with progressive keratoconus. We recorded participant characteristics (age, gender, age at onset), location of the cone, preoperative severity of disease (when described in terms other than 'progressive keratoconus'), and any comorbid conditions (e.g. Down syndrome) when this information was available. We excluded studies of participants with corneal ectasia due to other reasons (e.g. ectasia status post laser in‐situ keratomileusis). We excluded studies that enrolled participants under the age of 14 (FDA approval is for individuals 14 years of age and older).
In most publications, progressive keratoconus is defined as one or more of the following: an increase of at least 1.00 D of Kmax, not to be confused with steepest central simulated keratometry, known as steep K or K2); an increase of at least 1.00 D in manifest cylinder; or an increase of 0.5 D or more in manifest refraction spherical equivalent (MRSE) over the previous 6‐ to 24‐month period. The FDA utilized these criteria when reviewing data for CXL submitted for approval, but defined progression as change occurring over the previous 24‐month period, which is much longer than most studies. Other criteria to be considered for progressive keratoconus are 1.00 D or more increase in mean keratometry or in steepest central simulated keratometry (steep K or K2) in the previous 6‐ to 24‐month period (Sinhab 2014).
Examples of less conservative definitions of progressive keratoconus are as follows:
an increase of at least 1.00 D in maximum K or central corneal astigmatism over a six‐month period (Çerman 2015);
an increase of at least 0.5 D in maximum K, in steepest central simulated keratometry values (steep K or K2), in mean keratometry (the mean of steepest and flattest central simulated keratometry), and/or in topographic cylinder value over the previous 6 to 12 months (Soeters 2015);
reduced uncorrected distance visual acuity (UDVA) or corrected distance visual acuity (CDVA) by more than 1 logarithm of the minimum angle of resolution (logMAR) line and/or worsening of refractive or corneal astigmatism, Sim K, or Kmax by 0.75 D over the 12 to 24 months prior to CXL (O'Brart 2015).
Types of interventions
We included studies in which transepithelial CXL was compared with epithelium‐off CXL. We included studies in which different adjunctive therapy was used in both treatment arms. Riboflavin is a photosensitizer used in both methods of CXL. Chemical enhancers such as benzalkonium chloride or topical anesthetic (proparacaine as well as iontophoresis) may be used to improve transepithelial stromal absorption of riboflavin.
Types of outcome measures
Critical outcomes
The critical outcome for this review is keratometry (K), a measurement of corneal curvature that is used to assess keratoconus progression. Because K may be quantified in various ways in individual studies (e.g. maximum K versus mean K), we examined K both as a continuous outcome (change in maximum K from baseline) and as a dichotomous outcome (proportion of participants whose maximum K decreased by at least 2 D, indicating arrest or slowing of disease progression), proportion of eyes whose maximum K increased by at least 2 D from baseline, and proportion of eyes whose maximum K remained stable (Asri 2011). Our time points of interest were 12 and 24 months after corneal CXL. We extracted the available data closest to these time points.
Other important outcomes
We considered the following important outcomes at 12 months or more after CXL. When there were multiple measurements after 12 months, we extracted the measurement made at the longest follow‐up time point.
1. Visual acuity (visual acuity recorded and analyzed as the number of letters read on a chart with a logMAR scale (ETDRS 1985).
Mean change in CDVA from baseline
Proportion of participants who gained 10 or more letters from baseline (equivalent to 2 lines; 0.2 on a logMAR scale)
Proportion of participants who lost 10 or more letters (2 lines, 0.2 logMAR) from baseline
2. Patient questionnaire responses regarding subjective visual function parameters (e.g. photophobia, difficulty driving at night, difficult reading, diplopia, fluctuation in vision, glare, haloes, starbursts, dryness, pain, foreign body sensation), preferably as change from baseline.
3. Costs of the interventions as reported from the individual studies.
Adverse outcomes
We reported the following adverse outcomes at the longest follow‐up time point when presented in the included studies: proportion of participants who had central corneal opacity or haze or scar; corneal sterile infiltrate; herpetic keratitis; non‐healing or other epithelial defect lasting more than one week; eye pain or irritation; dry eye; photophobia; punctate keratitis; corneal inflammation; endothelial cell damage as indicated by decrease in endothelial cell density. Corneal stromolysis ('melt') has been described in the setting of CXL complicated by microbial infection or combined with excimer laser, but not CXL alone to date.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases for RCTs and controlled clinical trials. There were no restrictions to language or year of publication. The electronic databases were last searched on 15 January 2020.
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 1) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 15 January 2020) (Appendix 1)
MEDLINE Ovid (1946 to 15 January 2020) (Appendix 2)
Embase.com (1947 to 15 January 2020) (Appendix 3)
PubMed (1946 to 15 January 2020) (Appendix 4)
LILACS (Latin American and Caribbean Health Science Information database) (1982 to 15 January 2020) (Appendix 5)
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 15 January 2020) (Appendix 6)
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 15 January 2020) (Appendix 7)
Searching other resources
We searched the reference lists of reports from included trials to look for additional trials. We did not search conference abstracts for the purposes of this review, as many eyes and vision conference abstracts are routinely included in Embase.com, which we searched as part of the electronic searches.
Data collection and analysis
Selection of studies
After duplicates were removed from the merged search results, two review authors (two of IK, KL, MR) independently used Covidence to screen titles and abstracts of all records identified by the search (Covidence). The review authors classified each record as either relevant or not relevant for full‐text review. Two review authors (two of IK, KL, MR) independently assessed the full‐text copies of all records identified as relevant during title and abstract screening to determine eligibility for inclusion. All discrepancies between review authors were resolved by discussion at each stage of the screening process. We identified and grouped reports from the same study to avoid extracting data from the same study for the same outcomes more than once. We contacted the authors of trial reports (and waited two weeks for a response) in an attempt to clarify any details needed to permit a complete assessment of eligibility. We documented the reasons for exclusion for each study judged as not eligible after review of the full‐text reports. All full‐text reports were published in English. For future updates, we will first attempt to screen reports published in languages other than English for relevancy using Google Translate (translate.google.com). Whenever a clear decision cannot be made based on a translated version, we will consult colleagues who are fluent in the language to determine eligibility and, in the case the study is eligible for inclusion, to assist with data extraction.
Data extraction and management
Two review authors (two of IK, MR, SN) independently used Covidence to extract the data from included trials as proposed in the protocol (Kuo 2020). Two review authors (IK and SN) compared the extracted data and resolved any discrepancies by discussion. One review author exported data from Covidence into Review Manager 5 (Review Manager 2020), and a second review author verified the exported data.
Assessment of risk of bias in included studies
We attempted to contact the authors of reports via email if information to permit a judgement was insufficient. We assessed risk of bias using the available information when we did not receive the response within two weeks. Two review authors (two of IK, MR, SN) independently assessed the risk of bias in each included study, following the guidance in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). Specific items for consideration included random sequence generation and allocation concealment before randomization (selection bias), masking of participants and study personnel (performance bias), masking of outcome assessors (detection bias), missing data and intention‐to‐treat analysis (attrition bias), selective outcome reporting (reporting bias), and other potential sources of bias. We assigned each study for each domain as having 'low risk of bias,' 'high risk of bias,' or, when the information provided was insufficient to make an assessment, 'unclear risk of bias.' We documented the reasons for our assessments and resolved any discrepancies through discussion. Our assessments, for individual studies and overall, are provided in the 'Risk of bias' summary figures.
Measures of treatment effect
We used mean difference to compare interventions with respect to continuous outcomes, including change in maximum K, change in CDVA, and change in patient questionnaire responses regarding subjective visual function parameters. We did not analyze the cost of the interventions because no included study examined this outcome.
We used risk ratios to compare interventions for dichotomous outcomes, including proportion of participants whose maximum K decreased by at least 2 D or increased by at least 2 D, proportion of participants whose maximum K remained stable, proportion of participants who gained 10 or more logMAR letters, proportion who lost 10 or more logMAR letters, and proportion of participants who experienced adverse events.
Unit of analysis issues
We determined whether the design of each included study specified intervention on one or both eyes from each participant and whether study investigators randomized at the participant level or at the eye level. In four of the 13 included studies, both eyes of all or some participants were included (Bikbova 2016; Cifariello 2018; Lombardo 2016; Razmjoo 2014), and in one study a paired‐eyes design was employed (Stojanovic 2014). None of these studies considered intraperson correlation of outcomes in the analysis. We analyzed these data as reported. This approach was conservative, as confidence intervals were wider than they would have been if the potential within‐person correlation was accounted for. Only one eye per participant was included in the remaining eight studies.
Because certain medical treatments have the potential to influence the outcome in the contralateral eye, we excluded studies that adopted a paired design in our sensitivity analysis. In addition, keratoconus tends to have asymmetrical presentation, which would complicate interpretation of findings from studies with a paired‐eyes design. In future updates, we will extract estimates that properly account for the intraperson correlation of two eyes of a participant when both eyes have been treated whenever the required data are available.
Dealing with missing data
Where data on included studies were unclear or incomplete, we contacted the authors of reports via email. We received responses and information from several authors (Lombardo 2016; Nawaz 2015; Razmjoo 2014; Rossi 2015; Rossi 2018; Soeters 2015). When there was no response within two weeks, we analyzed the data using the available information. We did not impute missing data. Whenever the quality of the available data from a study prevented meaningful analysis, we omitted the study from quantitative analyses and reported the data in a narrative format when appropriate.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by examining participant characteristics and outcomes, carefully reviewing the study report(s), and taking into consideration potential risk of bias. We examined forest plots and the I2 values and compared effect estimates and confidence intervals among studies. We considered an I2 value greater than 60% as indicative of substantial heterogeneity and suggesting that a meta‐analysis to estimate an overall intervention effect may not be appropriate.
We anticipated that heterogeneity would be significant, arising from baseline patient characteristics, different definitions of 'progressive keratoconus,' techniques used for transepithelial and epithelium‐off CXL, and various outcome measures. Substantial heterogeneity may affect the overall strength of the evidence. We explored comparisons between transepithelial and epithelium‐off CXL techniques within subgroups to explain observed heterogeneity whenever sufficient data were available.
Assessment of reporting biases
We did not use funnel plots to assess small‐study effects, which could be due to publication bias, because fewer than 10 trials contributed data to any meta‐analysis. We judged selective reporting as part of the 'Risk of bias' assessment for each individual study.
Data synthesis
We followed the guidelines in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions for data synthesis and analysis (Deeks 2017). The results for each prespecified outcome are reported in Table 1; Table 2. We analyzed studies separately by length of follow‐up (< 12 months and ≧ 12 months). We used a random‐effects model for quantitative syntheses when three or more studies reported data for the same outcome.
Summary of findings 1. Transepithelial CXL compared with epithelium‐off CXL for progressive keratoconus.
| Transepithelial CXL compared with epithelium‐off CXL for progressive keratoconus | ||||||
|
Patient or population: participants with keratoconus Settings: tertiary care or university hospital Intervention: transepithelial CXL Comparison: epithelium‐off CXL | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Epithelium‐off CXL | Transepithelial CXL | |||||
|
Mean change in maximum K from baseline or final value—at 12 months or more (diopters) |
The mean maximum K ranged across control groups from −1.5 to −0.92 (change from baseline), or 47.76 to 55.44 (final value). | The mean maximum K in the intervention groups was −1 to 0.3 (change from baseline), or
49.75 to 56.33 (final value), and on average 0.99 higher (95% CI −0.11 to 2.09). |
‐ | 177 eyes (5 studies) | ⊕⊝⊝⊝ Very low1,2,3 | |
| Proportion of participants whose maximum K decreased by at least 2 diopters—at 12 months | 269 per 1000 | 86 per 1000 | RR 0.32 (0.09 to 1.12) | 61 participants (1 study) | ⊕⊝⊝⊝ Very low1,2 | |
| Proportion of participants whose maximum K increased by at least 2 diopters—at 12 months | 0 per 1000 | 143 per 1000 | RR (non‐event) 0.86 (0.74 to 1.00) | 61 participants (1 study) | ⊕⊝⊝⊝ Very low1,2 | |
| Proportion of participants whose keratoconus remained stable—at 12 months | 1000 per 1000 | 441 per 1000, or 800 per 1000 (data not pooled due to considerable heterogeneity (I2 = 92%)) | ‐ | 131 participants (2 studies) | ⊕⊝⊝⊝ Very Low1,2,3 | |
| Mean change in corrected distance visual acuity (logMAR) from baseline or final values—at 12 months or more | The mean corrected distance visual acuity ranged across control groups from −0.13 to −0.07 (change from baseline), or 0.05 (final value). | The mean corrected distance visual acuity in the intervention groups was −0.16 to −0.11 (change from baseline), or 0.02 (final value), and mean difference from −0.07 to 0.02 (data not pooled due to substantial heterogeneity (I2 = 70%)) | ‐ | 137 eyes (4 studies) | ⊕⊝⊝⊝ Very low1,2,3 | |
| Proportion of participants who gained 10 or more logMAR letters from baseline | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported gains of 10 or more logMAR letters. |
| Proportion of participants who lost 10 or more logMAR letters from baseline | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported losses of 10 or more logMAR letters. |
| Adverse outcomes—corneal haze or scarring | 76 per 1000 | 0 per 1000 | RR (non‐event) 1.07 (1.01 to 1.14) | 221 eyes (4 studies) |
⊕⊕⊕⊝ Moderate1 | Herpetic keratitis, sterile infiltrate, and epithelial defect observed in 1 eye each in epithelium‐off group; Vogt's striae reported in 1 eye in each intervention group. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CXL: corneal collagen crosslinking; logMAR: logarithm of the minimum angle of resolution;RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1Downgraded one level for study limitation due to high risk of performance and other biases among included studies. 2Downgraded one or two levels for imprecision due to wide confidence interval crossing line of no effect, and small sample size (as based on one study of n = 61, two studies of n = 131, four studies of n = 137, or five studies of n = 177). 3Downgraded one level for unexpected heterogeneity (I2 = 70%, or 92%) or inconsistency of results.
Summary of findings 2. Transepithelial CXL using iontophoresis compared with epithelium‐off CXL for progressive keratoconus.
| Transepithelial CXL using iontophoresiscompared with epithelium‐off CXL for progressive keratoconus | |||||
|
Patient or population: participants with progressive keratoconus Settings: tertiary care or university hospital Intervention: transepithelial CXL using iontophoresis Comparison: epithelium‐off CXL | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Epithelium‐off CXL | Transepithelial CXL using iontophoresis | ||||
|
Mean change in maximum K from baseline or final value—at 12 months or more (diopters) |
The mean maximum K ranged across control groups −1.51 (change from baseline), or 55.44 (final value). | The mean maximum K in the intervention groups was −1.05 (change from baseline), or 52.52 (final value), and mean difference from −2.92 to 0.46 (data not pooled due to substantial heterogeneity (I2 = 68%)). | ‐ | 51 eyes (2 studies) | ⊕⊝⊝⊝ Very low1,2,3 |
| Proportion of participants whose maximum K decreased by at least 2 diopters—at 24 months | 91 per 1000 | 0 per 1000 | RR (non‐event) 1.12 (0.89 to 1.40) | 31 eyes (1 study) | ⊕⊝⊝⊝ Very low1,2 |
| Proportion of participants whose maximum K increased by at least 2 diopters—at 24 months | 0 per 1000 | 0 per 1000 | RR (non‐event) 1.00 (0.87 to 1.15) | 31 eyes (1 study) | ⊕⊝⊝⊝ Very low1,2 |
| Proportion of participants whose keratoconus remained stable—at 24 months | 1000 per 1000 | 900 per 1000 | RR 0.92 (0.76 to 1.12) | 31 eyes (1 study) | ⊕⊝⊝⊝ Very low1,2 |
| Mean change in corrected distance visual acuity (logMAR) from baseline or final values at 12 months or more | The mean corrected distance visual acuity ranged across control groups −0.13 (change from baseline), or 0.03 (final value). | The mean corrected distance visual acuity in the intervention groups was −0.13 (change from baseline), or 0.04 (final value), and no mean difference (95% CI −0.04 to 0.04). | ‐ | 51 eyes (2 studies) | ⊕⊕⊝⊝ Low1,2 |
| Proportion of participants who gained 10 or more logMAR letters from baseline | 83 per 1000 | 227 per 1000 | RR 2.73 (0.36 to 20.74) | 34 eyes (1 study) | ⊕⊝⊝⊝ Very low1,2 |
| Proportion of participants who lost 10 or more logMAR letters from baseline | 0 per 1000 | 0 per 1000 | RR 1.00 (0.88 to 1.13) | 34 eyes (1 study) | ⊕⊝⊝⊝ Very low1,2 |
| Adverse outcomes | 1 subepithelial infiltrate in transepithelial CXL group; 2 faint corneal scars and 4 permanent haze in epithelium‐off CXL group; Vogt's striae observed in 1 eye in each intervention group. | ‐ | 203 eyes (3 studies) |
⊕⊕⊝⊝ Low1,2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CXL: corneal collagen crosslinking; logMAR: logarithm of the minimum angle of resolution;RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | |||||
1Downgraded one level for study limitation due to high risk of performance and other biases among included studies. 2Downgraded one or two levels for imprecision due to wide confidence interval crossing line of no effect, and small sample size (based on one study of n = 31 or 34, two studies of n = 51, or three studies of n = 203). 3Downgraded one level for unexpected heterogeneity (I2 = 68%) or inconsistency of results.
Subgroup analysis and investigation of heterogeneity
We classified studies based on techniques within transepithelial CXL, and compared epithelium‐off CXL with transepithelial CXL separately with and without use of iontophoresis (i.e. transepithelial CXL versus epithelium‐off CXL, and transepithelial CXL using iontophoresis versus epithelium‐off CXL). We also performed subgroup analyses by methods of outcome reporting to investigate observed heterogeneity. We planned to perform other subgroup analysis to account for differences in types of participants, type of riboflavin used, method of administration, how riboflavin saturation was assessed, and power and timing of UV light exposure; however, lack of data from included studies precluded these subgroup analyses.
As stated above, we excluded trials that enrolled patients with corneal ectasia that was not keratoconus and that did not report outcomes separately for participants with keratoconus.
Sensitivity analysis
We conducted sensitivity analysis to determine the impact on effect estimates of a paired‐eyes design of one included trial and any post hoc decisions made during the review process. We have reported the results of any sensitivity analysis performed and discussed our interpretation of the effects on the overall findings of the review.
Summary of findings and assessment of the certainty of the evidence
We have presented 'Summary of findings' tables for the two comparisons: 1) transepithelial CXL versus epithelium‐off CXL; and 2) transepithelial CXL using iontophoresis versus epithelium‐off CXL (Table 1; Table 2). The 'Summary of findings' tables include the following seven outcomes, assessed at 12 months after CXL whenever sufficient data were available.
Mean change in maximum K from baseline
Proportion of participants whose maximum K decreased or increased by at least 2 D from baseline
Proportion of participants whose maximum K remained stable
Mean change in CDVA from baseline
Proportion of participants who gained 10 or more logMAR letters from baseline
Proportion of participants who lost 10 or more logMAR letters from baseline
Adverse outcomes
We used the GRADE approach to assess the overall certainty of evidence for each outcome. We began our assessment by judging the randomized design of each included study to confer a high certainty of evidence for each outcome, downgrading certainty to moderate, low, or very low when there was evidence of high risk of bias, inconsistency, indirectness, or imprecision. We will consider publication bias in updates to the review that include more trials in individual meta‐analyses.
Results
Description of studies
Results of the search
Our searches of the electronic databases in January 2020 yielded 3364 records. After removal of duplicates, 2785 titles and abstracts were screened. We retrieved 57 full‐text reports for further review. After full‐text screening, we included 13 studies (22 records), identified two ongoing studies (two records), listed one study (one record) as awaiting classification, and excluded 32 studies (32 records) with reasons. We contacted the investigator of one study to clarify study eligibility, but did not receive a response (ChiCTR1900021768). Two ongoing studies were reported to start in 2019 and estimated to complete in 2021 (NCT03990506) and 2024 (NCT03858036). A study selection flow diagram is shown in Figure 1.
1.
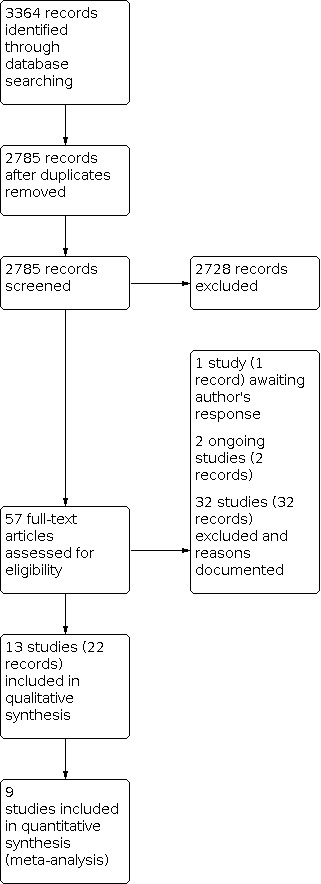
Study flow diagram.
Included studies
See: Characteristics of included studies
We included 13 studies in the review. Seven studies were parallel‐group RCTs; one study was an RCT with a paired‐eyes design (Stojanovic 2014); five studies were RCTs in which both eyes of some or all participants were assigned to the same intervention, and data from each eye were analyzed separately without taking into account intraperson correlation.
Types of participants
Seven of the 13 included studies were conducted in Europe (five in Italy, one each in the Netherlands and Norway), three in the Middle East (one each in Iran, Jordan, and Saudi Arabia), and one each in India, Russia, and Turkey. In total, 567 participants (661 eyes) with progressive keratoconus were enrolled; 13 to 119 participants were enrolled per study. The mean age of participants ranged from 23 to 30, with a median of 28 years. The composition of study populations by gender ranged from 15% to 73% women, with a median of 31%. The mean maximum K ranged from 47 to 58 D, with a median of 54 D. The mean logMAR best‐corrected distance visual acuity at baseline was 0.10 to 0.34, with a median of 0.29.
Types of interventions
Three trials described transepithelial CXL using iontophoresis (Bikbova 2016; Lombardo 2016; Rossi 2018), but only two trials provided quantifiable data for meta‐analysis (Lombardo 2016; Rossi 2018). Eleven trials described non‐iontophoresis‐assisted transepithelial CXL, including one trial that compared three arms: iontophoresis‐assisted transepithelial, transepithelial, and epithelium‐off crosslinking (Rossi 2018). Pre‐irradiation riboflavin 0.10% with dextran 15% to 20% was instilled in all eyes in trials except one (Stojanovic 2014), which used riboflavin 0.5% without dextran. Stojanovic and colleagues utilized a Merocel sponge to produce microabrasions of the superficial epithelial layers caused by friction upon blinking by the patient. These microabrasions enhance riboflavin penetration through the otherwise intact corneal epithelium (Stojanovic 2012). In addition to the use of topical anesthetic drops (which can also enhance penetration of riboflavin and were used in both transepithelial and epithelium‐off trials), two studies utilized agents to enhance penetration. These agents were benzalkonium chloride 0.02% for 30 minutes (Al Fayez 2015), and proparacaine 0.5% and gentamicin 0.3% both preserved with benzalkonium chloride 0.005% every minute for the first 5 minutes before saturation with riboflavin along with the same preserved proparacaine 0.5% every 30 seconds until saturation was confirmed at the slit lamp (Stojanovic 2014). Several trials utilized a combination drug formulated specifically for transepithelial procedures consisting of riboflavin 0.1% plus dextran 15% enhanced with amino acid TRIS (trometamol) and EDTA (ethylenediaminetetraacetic acid) prior to irradiation; others used riboflavin 0.1% alone or riboflavin 0.1% with dextran 15% to 20%. Both iontophoresis‐assisted transepithelial studies utilized riboflavin 0.1% with trometamol and EDTA, without dextran.
One trial described transepithelial CXL of the central 3 mm of the cornea, leaving the epithelium intact in the 3‐millimeter ring surrounding this central zone (Razmjoo 2014). Corneas were constantly bathed in riboflavin during irradiation in iontophoresis‐assisted transepithelial procedures. Riboflavin was not instilled during irradiation in transepithelial procedures in two trials (Al Fayez 2015; Stojanovic 2014).
Investigators of transepithelial trials without iontophoresis irradiated corneas for 30 minutes; iontophoresis‐assisted trials described irradiation for 9 minutes(Lombardo 2016) or 10 minutes( Rossi 2018). When utilized, postoperative topical steroid drops were instilled for one to four weeks after surgery; steroid drop regimens included dexamethasone 0.1%, fluorometholone 0.1%, prednisolone acetate 1%, and betamethasone. Participants in one trial did not receive any steroid drop after transepithelial CXL (Cifariello 2018). In short, investigators described different mechanisms to promote riboflavin as well as UV light absorption, and variability in the time, power (10 mW/cm2 in both trials of transepithelial CXL with iontophoresis, in contrast to 3 mW/cm2 in transepithelial CXL trials without iontophoresis), and distance between irradiation source and cornea (1 cm to 5 cm).
The epithelium‐off procedures were more uniform. Most investigators used the Dresden protocol, which employs UVA‐light diodes (370 nm) at a 1‐centimeter distance for 30 minutes using 3 mW/cm2 irradiance, which corresponds to a dose of 5.4 J/cm2. As with the transepithelial studies, the cross‐linking devices were manufactured by different companies. Pre‐irradiation riboflavin 0.1% and dextran 20% were instilled in all eyes undergoing epithelium‐off procedures, except for one trial that used 0.5% riboflavin without dextran (Stojanovic 2014). Intraoperative riboflavin was instilled every 2 to 5 minutes during irradiation in all trials. Concentration of the riboflavin was 0.1%, except for one trial that used 0.025% (Lombardo 2016). Postoperative topical steroid drop regimens varied (e.g. fluorometholone 0.1%, betamethasone, dexamethasone 0.1%, prednisolone acetate 1%) and ranged in length from one to four weeks.
Two studies did not investigate clinical parameters (Acar 2014; Mastropasqua 2013), but instead compared morphological corneal changes after epithelium‐off CXL and after transepithelial CXL without iontophoresis using in vivo confocal microscopy and/or anterior segment optical coherence tomography. One group studied whether prolonged riboflavin pre‐treatment of eyes undergoing transepithelial CXL could facilitate penetration of riboflavin through intact corneal epithelium (Acar 2014). By instilling riboflavin 0.1% along with dextran 20% and chemical enhancers every 10 minutes for 2 hours prior to transepithelial CXL, their goal was to approximate a similar depth of effect as epithelium‐off procedures. Neither study described postoperative topical steroid drop regimens (Acar 2014; Mastropasqua 2013).
Types of outcomes
Critical outcomes
All studies except for two (Acar 2014; Mastropasqua 2013) measured keratometry outcomes. However, summary data were reported from some studies without indicators of precision (e.g. standard deviation) or denominators or estimates by intervention were compared using only P values or were reported only in figures. Eight studies reported keratometry outcomes when follow‐up examinations ended at 6 months (Nawaz 2015; Razmjoo 2014), 12 months (Rossi 2015; Rossi 2018; Soeters 2015; Stojanovic 2014), or 24 months (Cifariello 2018; Lombardo 2016); data from these studies were included in the meta‐analyses.
Other important outcomes
1. Visual acuity
All studies except for two (Acar 2014; Mastropasqua 2013)assessed visual acuity, but only six studies provided data for CDVA in a form suitable for inclusion in meta‐analysis at 6 months (Nawaz 2015), 12 months (Rossi 2015; Rossi 2018; Soeters 2015; Stojanovic 2014), and 24 months (Lombardo 2016). Only one study reported the proportion of participants who gained or lost 10 or more logMAR letters from baseline (Lombardo 2016).
2. Subjective visual function
One study measured subjective symptoms among participants using the Ocular Surface Disease Index and reported scores at one month (Cifariello 2018).
3. Costs of the interventions as reported from individual studies
None of the included studies reported or evaluated the costs of the interventions compared.
Adverse outcomes
All studies except one (Mastropasqua 2013) reported intraoperative or postoperative complications.
Excluded studies
We excluded 32 studies after full‐text review and provided the reasons of exclusion in the Characteristics of excluded studies table. In summary, we excluded 20 studies that were not RCTs, six studies with the wrong participants (not participants with progressive keratoconus, or not adult participants), and two studies evaluating the wrong interventions. We excluded three studies because they were preliminary reports. We excluded one RCT because it was terminated early due to few participants.
Risk of bias in included studies
Our assessment of the risk of bias for each of 13 studies is described in the Characteristics of included studies table. A summary of 'Risk of bias' assessments is shown in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Five studies reported employing an adequate method for random sequence generation by using a computer‐generated random number table (Al Fayez 2015; Lombardo 2016; Razmjoo 2014; Rossi 2015; Rossi 2018). We judged these studies as at low risk of bias for this domain. The remaining eight studies did not specify the method for allocation sequence generation and were therefore judged to be at unclear risk of bias. Of the eight studies, reports from two studies stated that the investigators had used "odd‐even number method (randomized control trial)"(Al Zubi 2019; Nawaz 2015). We attempted to contact the study investigators by email to clarify this statement, but did not receive a reply.
We judged two studies as having a low risk of bias based on the reported use of adequate procedures for allocation concealment before assignment (Al Fayez 2015; Lombardo 2016). The remaining 11 studies provided insufficient information to permit a judgement on allocation concealment and were judged as having an unclear risk of bias for this domain.
Blinding
Given the nature of the interventions, masking the surgeons in the trials included in this review would have been challenging. Eight studies reported that participants and personnel were aware of the allocation; we judged these studies to be at high risk of performance bias (Al Fayez 2015; Cifariello 2018; Lombardo 2016; Razmjoo 2014; Rossi 2015; Rossi 2018; Soeters 2015; Stojanovic 2014). The remaining five trials provided insufficient information to permit a judgement and were judged as having an unclear risk of bias.
Incomplete outcome data
We judged seven studies in which all participants had been examined for the primary outcome (Acar 2014; Razmjoo 2014; Rossi 2015; Rossi 2018; Stojanovic 2014) or an intention‐to‐treat analysis was performed (Lombardo 2016; Soeters 2015), as having a low risk of attrition bias. The numbers of participants who were excluded or lost to follow‐up were not explicitly reported for the other six studies; in the absence of this information, we judged the risk of attrition bias for these studies as unclear (Al Fayez 2015; Al Zubi 2019; Bikbova 2016; Cifariello 2018; Mastropasqua 2013; Nawaz 2015).
Selective reporting
Three studies reported all outcomes specified in the clinical trial registries and were thus judged to be at low risk of reporting bias (Lombardo 2016; Razmjoo 2014; Stojanovic 2014). Protocols or trial registry records were not publicly available for the other 10 studies. We designated four studies as having a high risk of selective outcome reporting because either not all outcomes specified in the methods were described in the results sections, or the study failed to report key outcomes that the investigators would have been expected to measure and report for such a study, or outcomes were incompletely reported, so as to reduce precision (Al Fayez 2015; Al Zubi 2019; Bikbova 2016; Nawaz 2015). We judged the remaining six trials to have an unclear risk of reporting bias.
Other potential sources of bias
We judged three studies to have a high risk of other bias due to a baseline imbalance between intervention groups (Cifariello 2018; Rossi 2015; Soeters 2015). One study received medical devices from industry and was assessed as at high risk of bias for this domain (Lombardo 2016). We judged six studies to be at low risk of other bias (Acar 2014; Al Fayez 2015; Mastropasqua 2013; Nawaz 2015; Razmjoo 2014; Rossi 2018), and the remaining two studies as at unclear risk of bias for this domain because insufficient information was provided to permit a judgement.
Effects of interventions
Because of implicit heterogeneity, we assigned trials into two comparison groups: the three trials in which transepithelial CXL was used with iontophoresis assistance (Bikbova 2016; Lombardo 2016; Rossi 2018); and the 11 trials without iontophoresis assistance (Acar 2014; Al Fayez 2015; Al Zubi 2019; Cifariello 2018; Mastropasqua 2013; Nawaz 2015; Razmjoo 2014; Rossi 2015; Rossi 2018; Soeters 2015; Stojanovic 2014).
Comparison 1: Transepithelial CXL versus epithelium‐off CXL
As noted above, 11 studies compared outcomes among participants assigned to transepithelial CXL versus epithelium‐off CXL with length of follow‐up of 6 months (Acar 2014; Nawaz 2015; Razmjoo 2014), and 12 months or beyond (Al Fayez 2015; Al Zubi 2019; Cifariello 2018; Mastropasqua 2013; Rossi 2015; Rossi 2018; Soeters 2015; Stojanovic 2014). In three studies, both eyes of all or some participants were included (Cifariello 2018; Mastropasqua 2013; Razmjoo 2014), and one study employed a paired‐eyes design (Stojanovic 2014). None of these studies considered intraperson correlation of outcomes in the analysis. We analyzed these data as reported. This approach was conservative; confidence intervals were wider than they would have been if the potential within‐person correlation could have been accounted for. The results are summarized in Summary of findings table 1.
Keratometry outcomes
Five RCTs reported keratometry data suitable for inclusion in meta‐analyses, either as mean change in maximum K from baseline ( Rossi 2015; Soeters 2015) or mean maximum K (Cifariello 2018; Rossi 2018; Stojanovic 2014) at 12 months or more after surgery. Mean changes in maximum K ranged from a decrease of 0.5 to an increase of 1.99 D and yielded an overall estimated mean difference between interventions of 0.99 D (95% confidence interval (CI) −0.11 to 2.09; 177 eyes; I2 = 41%; Analysis 1.1; Figure 3). Although the overall estimate of mean difference favored epithelium‐off CXL, the confidence interval was consistent with no difference between the two interventions for this outcome at 12 months or later. We performed sensitivity analysis in which we excluded the study with a paired‐eyes design (Stojanovic 2014), as proposed in the review protocol; from that analysis we calculated a mean difference of 1.14 (95% CI −0.06 to 2.33). Heterogeneity remained moderate (I2 = 49%), and the confidence interval on the estimate from the remaining studies remained consistent with no difference. Subgroup analysis by the methods of outcome reporting estimated a mean difference of 1.21 (95% CI −0.48 to 2.90; I2 = 0) from three RCTs that reported mean maximum K (Cifariello 2018; Rossi 2018; Stojanovic 2014); and a mean difference of 0.90 (95% CI −0.94 to 2.74; I2 = 81%) from two RCTs that had reported mean change in maximum K from baseline (Rossi 2015; Soeters 2015). Two additional trials reported mean maximum K measured six months postsurgery (Nawaz 2015; Razmjoo 2014). The estimated overall difference in maximum K at six months was −1.02 (95% CI −2.53 to 0.49; 84 eyes; Analysis 1.1; Figure 3). In these studies with different time points (i.e. < 12 months and ≧ 12 months), the confidence interval was consistent with no difference. One study measured maximum K, but due to lack of available data we did not include this study in the meta‐analysis (Al Fayez 2015). The investigators reported that maximum K was significantly lower in the epithelium‐off group than in the transepithelial group.
1.1. Analysis.

Comparison 1: Transepithelial corneal collagen crosslinking versus epithelium‐off corneal collagen crosslinking, Outcome 1: Mean change in maximum K from baseline or final value
3.

Forest plot of comparison: 1 Transepithelial corneal collagen crosslinking versus epithelium‐off corneal collagen crosslinking, outcome: 1.1 Mean change in maximum K from baseline or final value.
Only one study reported the numbers or proportions of eyes with decreases or increases of 2 or more diopters in maximum K (Soeters 2015). At 12 months after surgery, estimated risk ratios were consistent with no or only a trivial difference between epithelium‐off CXL and transepithelial CXL in their effects on these outcomes for participants with progressive keratoconus (proportion of participants whose maximum K decreased by at least 2 D: risk ratio 0.32, 95% CI 0.09 to 1.12; Analysis 1.2; proportion of participants whose maximum K increased by at least 2 D: risk ratio (non‐event) 0.86, 95% CI 0.74 to 1.00; Analysis 1.3).
1.2. Analysis.

Comparison 1: Transepithelial corneal collagen crosslinking versus epithelium‐off corneal collagen crosslinking, Outcome 2: Proportion of participants whose maximum K decreased by at least 2 diopters
1.3. Analysis.

Comparison 1: Transepithelial corneal collagen crosslinking versus epithelium‐off corneal collagen crosslinking, Outcome 3: Proportion of participants whose maximum K increased by at least 2 diopters
Estimated effects of all keratometry outcomes are very low certainty due to risk of performance and other biases (−1), imprecision (−1), and inconsistency (−1) among studies.
Progression of keratoconus
Two studies reported data regarding the progression of keratoconus (Al Fayez 2015; Soeters 2015). We did not combine the data from these two studies because considerable heterogeneity was detected (I2 = 92%). In the Soeters 2015 study, 7 (20%) of 35 eyes experienced progression of keratoconus at 12 months or longer after surgery in the transepithelial CXL arm versus none of 26 eyes in the epithelium‐off arm. The estimated risk ratio for stable keratoconus (i.e. no progression) was 0.81 (95% CI 0.68 to 0.96; 61 eyes; Analysis 1.4). Nineteen (56%) of 34 eyes in the transepithelial CXL arm and none of 36 eyes in the epithelium‐off arm experienced progression of keratoconus at 36 months after surgery in another study (Al Fayez 2015) with the estimated risk ratio for stable keratoconus of 0.45 (95%CI 0.31 to 0.65; 70 eyes; Analysis 1.4). These two studies suggest that epithelium‐off CXL may have a small effect on this outcome compared to transepithelial CXL, but the evidence is very uncertain; the evidence for this estimate is of very low certainty due to high risk of performance and other biases (−1), imprecision (−1) and unexplained heterogeneity (−1).
1.4. Analysis.

Comparison 1: Transepithelial corneal collagen crosslinking versus epithelium‐off corneal collagen crosslinking, Outcome 4: Proportion of participants whose keratoconus remained stable
Visual acuity outcomes
Four included studies reported change in corrected distance visual acuity (CDVA) from baseline to 12 months or a later final examination (Rossi 2015; Rossi 2018; Soeters 2015; Stojanovic 2014). The estimated overall mean change in CDVA was −0.07 to 0.03 among the four studies (Analysis 1.5; Figure 4). Statistical heterogeneity (I2 = 70%) indicated that calculation of an overall effect was inappropriate. In sensitivity analysis excluding the trial with a paired‐eyes design (Stojanovic 2014), heterogeneity remained substantial (I2 = 80%), leading us to conclude that the study with a paired‐eyes design did not explain the heterogeneity. One additional study reported change in CDVA from baseline to six months (Nawaz 2015): estimated mean difference was 0.01 (95% CI −0.03 to 0.05; 40 eyes; Analysis 1.5), corresponding to a few letters of a line of Snellen visual acuity. The difference between interventions for change in CDVA may be neither clinically nor statistically significant at either 6 months or 12 months or beyond. The same trend may be observed in two studies that measured CDVA but were not included in the meta‐analysis due to lack of data (Al Fayez 2015; Al Zubi 2019). The evidence for the overall estimates is of very low certainty due to high risk of performance and other biases (−1), imprecision (−1), and unexplained heterogeneity (−1).
1.5. Analysis.

Comparison 1: Transepithelial corneal collagen crosslinking versus epithelium‐off corneal collagen crosslinking, Outcome 5: Mean change in corrected distance visual acuity (logMAR) from baseline or final values
4.

Forest plot of comparison: 1 Transepithelial corneal collagen crosslinking versus epithelium‐off corneal collagen crosslinking, outcome: 1.5 Mean change in corrected distance visual acuity (logMAR) from baseline or final values.
No study reported gains or losses of 10 or more letters of CDVA.
Subjective visual function
Only the Cifariello 2018 study compared transepithelial CXL with epithelium‐off CXL and measured a subjective outcome based on participant responses to a standard questionnaire, the Ocular Surface Disease Index. Mean scores at one‐month postintervention favored transepithelial CXL: mean difference −2.30 (95% CI −3.62 to −0.98; n = 40; Analysis 1.6). The evidence for this estimate is of low certainty due to risk of bias (−1) and imprecision (−1).
1.6. Analysis.

Comparison 1: Transepithelial corneal collagen crosslinking versus epithelium‐off corneal collagen crosslinking, Outcome 6: Patient questionnaire of subjective visual function parameters (Ocular Surface Disease Index) at 1 month
Costs of the interventions
No study reported costs of either intervention.
Adverse outcomes
All eleven studies reported information about adverse outcomes. The investigators of four studies reported either no adverse events or no serious adverse events (Al Fayez 2015; Razmjoo 2014; Rossi 2015; Stojanovic 2014). One study noted no change in endothelial cell count after either procedure (Rossi 2015).
Eye pain among participants in the epithelium‐off CXL group early in the postoperative period was mentioned in reports from two studies (Nawaz 2015; Rossi 2018). Corneal haze or scarring was reported in four eyes from one study (Al Zubi 2019), two eyes from another study (Nawaz 2015), and one from each of two studies (Cifariello 2018; Soeters 2015); all eight eyes had been assigned to epithelium‐off CXL. Of the eight affected participants, one had deep stromal haze at six months (Soeters 2015). According to the summary estimate of four studies, eyes undergoing epithelium‐off CXL somewhat more often experienced corneal haze or scarring (risk ratio (non‐event) 1.07, 95% CI 1.01 to 1.14; 221 eyes; I2 = 0%; Analysis 1.7; Figure 5). Herpetic keratitis, sterile infiltrate, and an epithelial defect were observed in one eye each in the epithelium‐off group in one study (Soeters 2015). In Cifariello 2018, one eye in each intervention group developed Vogt's striae. We graded the certainty of evidence as moderate for adverse outcomes, downgrading by one level for high risk of bias (−1).
1.7. Analysis.

Comparison 1: Transepithelial corneal collagen crosslinking versus epithelium‐off corneal collagen crosslinking, Outcome 7: Adverse outcomes—corneal haze or scarring
5.

Forest plot of comparison: 1 Transepithelial corneal collagen crosslinking versus epithelium‐off corneal collagen crosslinking, outcome: 1.7 Adverse outcomes—corneal haze or scarring.
Comparison 2: Transepithelial CXL using iontophoresis versus epithelium‐off CXL
Three studies compared outcomes among participants assigned to transepithelial CXL using iontophoresis versus those assigned to epithelium‐off CXL at the end of follow‐up of 12 months (Rossi 2018), or 24 months (Bikbova 2016; Lombardo 2016). Two studies included both eyes of some participants (Bikbova 2016; Lombardo 2016). Neither trial considered the non‐independence of the eyes of bilaterally affected participants in the analysis; thus, the confidence interval is wider than it should be. The results are summarized in Summary of findings table 2.
Keratometry outcomes
Two studies reported analyzeable data for change in maximum K from baseline to 12 months or longer after surgery (Lombardo 2016; Rossi 2018). The estimates of the mean difference in change were inconsistent: the estimate from Lombardo 2016 favored epithelium‐off CXL, but the estimate from Rossi 2018 favored transepithelial CXL using iontophoresis (Analysis 2.1; Figure 6). We did not estimate an overall effect on this outcome because substantial heterogeneity was detected (I2 = 68%).
2.1. Analysis.

Comparison 2: Transepithelial corneal collagen crosslinking using iontophoresis versus epithelium‐off corneal collagen crosslinking, Outcome 1: Mean change in maximum K from baseline or final value at 12 months or more
6.

Forest plot of comparison: 2 Transepithelial corneal collagen crosslinking using iontophoresis versus epithelium‐off corneal collagen crosslinking, outcome: 2.1 Mean change in maximum K from baseline or final value at 12 months or more.
The certainty of the evidence for this outcome is thus very low due to imprecision of estimates for all outcomes (−1), unexpected heterogeneity (−1), and risk of bias (−1) in the studies from which data have been reported.
Progression of keratoconus
Only one study reported data for a decrease in maximum K by 2 or more diopters (Lombardo 2016). One eye (9%) of 11 eyes in the epithelium‐off CXL arm, and none of 20 eyes in the transepithelial CXL with iontophoresis arm, had experienced such a decrease by 24 months (risk ratio (non‐event) 1.12, 95% CI 0.89 to 1.40; 31 eyes; Analysis 2.2). At 24 months, no eye in either intervention group had an increase in maximum K by 2 or more diopters (risk ratio (non‐event) 1.00, 95% CI 0.87 to 1.15; 31 eyes; Analysis 2.3). This study was the only one to report progression of keratoconus for this comparison of interventions. Two eyes (10%) among 20 eyes in the transepithelial CXL with iontophoresis arm versus none of 11 eyes in the epithelium‐off arm experienced progression of keratoconus (risk ratio for stable keratoconus 0.92, 95% CI 0.76 to 1.12; 31 eyes; Analysis 2.4).
2.2. Analysis.

Comparison 2: Transepithelial corneal collagen crosslinking using iontophoresis versus epithelium‐off corneal collagen crosslinking, Outcome 2: Proportion of participants whose maximum K decreased by at least 2 diopters
2.3. Analysis.

Comparison 2: Transepithelial corneal collagen crosslinking using iontophoresis versus epithelium‐off corneal collagen crosslinking, Outcome 3: Proportion of participants whose maximum K increased by at least 2 diopters
2.4. Analysis.

Comparison 2: Transepithelial corneal collagen crosslinking using iontophoresis versus epithelium‐off corneal collagen crosslinking, Outcome 4: Proportion of participants whose keratoconus remained stable
The evidence for this estimate is of very low certainty due to high risk of bias (−1) and imprecision (−2).
Visual acuity outcomes
Data for all three outcomes specified for CDVA were reported by Lombardo 2016; in addition, Rossi 2018 reported data for mean change in CDVA. The overall estimate for mean change in logMAR CDVA from baseline to 12 months or later was mean difference 0.00 (95% CI −0.04 to 0.04, 51 eyes; Analysis 2.5; Figure 7). The confidence interval is consistent with no or only a trivial difference (2 letters or fewer) between the two interventions. However, confidence in the estimate is low due to imprecision (−1) and high risk of performance and other bias (−1). One study measured CDVA at 24 months or beyond, but due to lack of data was not included in the synthesis (Bikbova 2016); the authors reported that the difference in mean CDVA between groups was statistically significant in favor of transepithelial CXL.
2.5. Analysis.

Comparison 2: Transepithelial corneal collagen crosslinking using iontophoresis versus epithelium‐off corneal collagen crosslinking, Outcome 5: Mean change in corrected distance visual acuity (logMAR) from baseline or final values at 12 months or more
7.

Forest plot of comparison: 2 Transepithelial corneal collagen crosslinking using iontophoresis versus epithelium‐off corneal collagen crosslinking, outcome: 2.5 Mean change in corrected distance visual acuity (logMAR) from baseline or final values at 12 months or more.
As reported in Lombardo 2016, 5 eyes of 22 in the transepithelial CXL with iontophoresis arm gained 10 or more logMAR letters (0.2 logMAR) from baseline versus 1 of 12 eyes in the epithelium‐off CXL arm (Analysis 2.6); no eye in either intervention arm suffered a loss of 10 logMAR letters from baseline (Analysis 2.7). We judged the certainty of evidence as very low, downgrading for high risk of bias (−1) and imprecision due to small sample size and wide confidence intervals (−2).
2.6. Analysis.

Comparison 2: Transepithelial corneal collagen crosslinking using iontophoresis versus epithelium‐off corneal collagen crosslinking, Outcome 6: Proportion of participants who gained 10 or more logMAR letters from baseline
2.7. Analysis.

Comparison 2: Transepithelial corneal collagen crosslinking using iontophoresis versus epithelium‐off corneal collagen crosslinking, Outcome 7: Proportion of participants who lost 10 or more logMAR letters from baseline
Subjective visual function
No study reported data for subjective visual function or quality of life after surgery.
Costs of the interventions
No study reported data regarding costs of either intervention.
Adverse outcomes
All three studies (203 eyes in total) in this comparison reported information about adverse outcomes. Bikbova 2016 reported that no eye had experienced epithelial damage or reduced epithelial cell density. Lombardo 2016 reported that Vogt's striae were observed in one eye in each arm, and subepithelial infiltrate was observed in one eye in the transepithelial CXL with iontophoresis arm. Rossi 2018 mentioned only early eye pain among some participants in the epithelium‐off CXL group. In the epithelium‐off CXL arm, two eyes with faint corneal scars (16.7%) were observed by three months in Lombardo 2016; four participants (5%) had “permanent haze” in Bikbova 2016. We judged the certainty of evidence as low, downgrading for high risk of bias (−1) and for imprecision due to small sample size and wide confidence intervals (−1).
Discussion
Summary of main results
In this review, we have reported outcome data from 13 RCTs that compared transepithelial CXL versus epithelium‐off CXL for progressive keratoconus. We separately compared transepithelial CXL using iontophoresis versus epithelium‐off CXL and transepithelial CXL without iontophoresis versus epithelium‐off CXL. We included eight studies in meta‐analyses performed to estimate the relative effect of these interventions on our primary outcome of mean change in maximum K from baseline or final value of maximum K. Based on two studies that ended at six months (both without iontophoresis), there was no evidence of difference between transepithelial CXL and epithelium‐off CXL. When we examined data from six studies that reported outcomes at 12 months or later, there was again no evidence of a difference in the effects of the interventions on outcomes. Furthermore, change in CDVA from baseline at 6 and 12 months or later was no different between the techniques. No study reported a loss of 10 or more letters (0.2 logMAR units) of CDVA. Regarding subjective visual function, in one trial unmasked personnel administered the Ocular Surface Disease Index one month after surgery, with scores favoring transepithelial CXL without iontophoresis; however, imprecision and risk of bias lowered the certainty of evidence for a difference. No study reported the costs of either intervention. Adverse events such as corneal haze and temporary postoperative pain were not reported for any eye in the transepithelial CXL group. Haze or scarring occurred in 14 of 191 eyes in the epithelium‐off CXL group; most resolved by 6 months, but 4 eyes had “permanent haze” (Bikbova 2016). It is not clear how significant this haze was to participants' quality of vision.
As the goal of CXL is absence of significant steepening, we also examined data from the included trials for decrease or increase in maximum K of at least 2 D and for stability of maximum K (no steepening, or steepening by no more than 1 D). Two studies that compared transepithelial CXL versus epithelium‐off CXL reported data regarding keratoconus progression (Al Fayez 2015; Soeters 2015). Of these three keratometric outcomes, the CI was consistent with a difference in effects only for keratoconus progression. There was a 19% (Soeters 2015) or 55% (Al Fayez 2015) lower risk of progression for epithelium‐off CXL compared with transepithelial CXL, but risk of bias, imprecision, and unexplained heterogeneity lessen the certainty of evidence for this outcome. For the comparison of transepithelial CXL with iontophoresis versus epithelium‐off CXL, there was no evidence of difference with respect to progression of keratoconus or increase or decrease in maximum K of at least 2 D.
Overall completeness and applicability of evidence
All of the included studies evaluated transepithelial CXL versus epithelium‐off CXL, but lack of precision, a high risk of bias for multiple 'Risk of bias' domains for several studies (indicative of lack of methodologic rigor), and substantial heterogeneity often precluded the estimation of an overall effect. We explored comparisons between transepithelial and epithelium‐off CXL techniques within subgroups in an attempt to explain observed heterogeneity whenever sufficient data were available.
At the most elementary methodologic level, the authors of study reports often did not define their methods for measuring and reporting outcomes. For example, the target outcome for CXL is to halt or slow progression of keratoconus; however, when this outcome was reported, the report authors often failed to provide a detailed definition of progressive keratoconus as used in their studies. The period of time prior to CXL during which progression was noted could be 6 months, 12 months, or not reported. Baseline characteristics of participants and eyes, when reported, varied among the studies, possibly in part due to differences in measurement devices. The same variability in devices was seen in mean maximum K (Figure 3). Baseline and final measurements were obtained using topography/tomography units based on different technologies: Placido disc (CSO and Visante used by Rossi 2015 and Rossi 2018, and Lombardo 2016, respectively), scanning‐slit (Orbscan II used by Nawaz 2015), and Scheimpflug (Pentacam used by Cifariello 2018; Razmjoo 2014; Soeters 2015). Consequently, lack of a standard definition of progressive keratoconus and different measurement devices, as well as diverse perioperative, intraoperative, and postoperative protocols, increased heterogeneity and limited the applicability of evidence.
Results of studies with 6 months of follow‐up or less may be less clinically relevant than results from studies with 12 or more months of follow‐up given the time needed for corneal remodeling after kerato‐refractive procedures. Longer follow‐up is also important because progression of keratoconus is not linear over time. Patients can show stable keratometry values at the one‐year follow‐up visit and progression two years after transepithelial CXL (Caporossi 2013). Because stability at one year may be part of the natural disease course and not related to CXL, studies with longer follow‐up may be essential to assess the efficacy of CXL.
One reason for modification of transepithelial CXL is that in some non‐RCTs, transepithelial CXL alone has been less effective than epithelium‐off CXL, which shows stability 6 to 10 years after CXL (O'Brart 2015; Poli 2015; Raiskup-Wolf 2008). To enhance penetration of riboflavin through intact epithelium, some investigators have used iontophoresis, riboflavin formulations with trometamol and EDTA (ethylenediaminetetraacetic acid), or benzalkonium chloride or created corneal microabrasions. The plethora of techniques for transepithelial CXL may explain the high level of statistical heterogeneity in the results of some analyses. When all transepithelial CXL (including studies with iontophoresis) study arms were compared with epithelium‐off CXL study arms for mean change in maximum K from baseline at 6 or 12 months or longer, statistical heterogeneity was 50%. This level of heterogeneity indicated that calculation of an overall effect may be inappropriate. However, despite performing subgroup analysis to examine mean change in maximum K in two studies of transepithelial CXL without iontophoresis versus epithelium‐off CXL with 12 months of follow‐up (Rossi 2015; Soeters 2015), statistical heterogeneity remained significant (I2 = 81%). The mean changes and standard deviations reported in these two studies suggest either very different magnitude of effect of CXL despite almost identical transepithelial and epithelium‐off CXL protocols or very different baseline characteristics of participants and eyes, or both.
More experience has been obtained internationally with epithelium‐off CXL (specifically, the original Dresden protocol) than with any transepithelial CXL technique, but transepithelial CXL protocols are evolving rapidly. As observed in this systematic review, corneal haze was reported as an adverse effect of epithelium‐off CXL that can be temporary (lasting three to four months for three participants), 'deep stromal' at six months, or 'permanent' (Bikbova 2016). Although this finding may be significant to study personnel, this haze may not be visually significant to the patient. This distinction, along with patient‐reported outcomes, should be investigated in future studies of CXL.
Quality of the evidence
Overall, we graded the certainty of evidence for all comparisons of interventions to be very low or low due to prevalent risk of bias and imprecision related to the small number of participants enrolled in most trials.
The included studies had limitations in study design and implementation. Eleven (85%) RCTs had at least one domain that was judged as having unclear (indeterminate) risk of bias because sufficient information was not provided to permit an informed judgement. Risk of bias was unclear or high for most domains for most studies. We judged eight (62%) studies as at high risk of performance and detection biases; the most common bias was performance bias. Given the nature of the interventions, it would have been difficult to mask study personnel and participants. It is likely participants (and study personnel) were able to tell which eyes underwent epithelium‐off CXL because of participants' postprocedural pain. In addition, study personnel would be able to distinguish epithelium‐off versus transepithelial CXL when assessing participants' eyes at the slit‐lamp. The second most common bias was reporting bias (selective reporting). All studies measured keratometry outcomes and visual acuity (both key outcomes for this systematic review), but few provided data in a format suitable for inclusion in meta‐analysis. Furthermore, 8 (62%) and 10 (77%) studies did not provide adequate information on the method of random sequence generation and allocation concealment, respectively.
We judged five studies (38%) as at high risk of reporting bias because not all outcomes specified in the study protocols or methods sections were summarized in the results sections of reports or the report authors failed to report key outcomes, or reported outcomes incompletely without indicators of precision such as standard deviations, or depicted them in figures only without stating numerical values. We contacted the study authors to seek clarification and additional data to ensure that the review was as complete as possible when outcomes of interest were reported inadequately. We received the information and data for several studies through personal communication (Al Fayez 2015; Lombardo 2016; Nawaz 2015; Razmjoo 2014; Rossi 2015; Rossi 2018; Soeters 2015).
A third issue was inappropriate methods of data analysis when both eyes of participants were included in a study. Reports from five RCTs in which both eyes of some or all participants were assigned to the same interventions and one RCT with a paired‐eyes design did not describe or refer to appropriate analytical methods to handle correlations between eyes. We chose to analyze these data as reported rather than exclude them from the review, which thus affected the estimates of confidence intervals for outcomes from those studies.
Potential biases in the review process
We used standard Cochrane Review methodology to minimize bias and followed Methodological Expectations of Cochrane Intervention Reviews (MECIR) standards for the reporting of new Cochrane Intervention Reviews (editorial-unit.cochrane.org/mecir) in conducting this review and reporting our findings. An Information Specialist performed a highly sensitive search to identify studies. None of the authors has any financial conflicts of interest.
Agreements and disagreements with other studies or reviews
Three groups of authors have reported meta‐analyses in which they examined the same question as in this review (Kobashi 2018; Li 2017; Wen 2018), none of which provided convincing evidence of the superiority of one of the three CXL methods with respect to maximum K or visual acuity. Transepithelial CXL techniques may be associated with less than 0.1 logMAR improvement in visual acuity (Kobashi 2018; Li 2017), corresponding to less than 1 line on a Snellen visual acuity chart. Compared with our review, the three meta‐analyses:
included fewer studies, some of which are included in our systematic review, and others which were not RCTs (Wen 2018);
did not separate transepithelial CXL without iontophoresis from transepithelial CXL with iontophoresis (Kobashi 2018; Li 2017; Wen 2018);
included studies of eyes with all types of corneal ectasia, not just progressive keratoconus (Kobashi 2018); and
included one study of CXL in a pediatric population (Wen 2018).
Authors' conclusions
Implications for practice.
The arrest of progression of keratoconus, not reduction in maximum K, should be the primary outcome of interest in clinical trials of corneal collagen crosslinking (CXL), particularly comparative effectiveness trials in which one approach to CXL is evaluated relative to another. Absence of significant steepening indicates a successful outcome for CXL. The relative change in maximum keratometry between transepithelial CXL and epithelium‐off CXL is a reasonable proxy for the relative efficacy of each procedure. Due to imprecision, frequent indeterminate risk of bias, and inconsistency among studies in this systematic review, it remains to be seen whether transepithelial CXL, or any other approach, confers an advantage over epithelium‐off CXL for individuals with progressive keratoconus with respect to progression of keratoconus, visual acuity outcomes, and patient‐reported visual function. Adverse outcomes such as corneal haze were reported to have occurred in more eyes after epithelium‐off CXL, but more details are needed from future studies to complement clinical assessments of haze and other outcomes with assessments of the visual significance of this haze to the patient.
Implications for research.
Further research is required to determine which technique most effectively stabilizes progressive keratoconus (the goal of CXL) with an acceptable risk profile of adverse outcomes or whether, in fact, there is no important difference between techniques. Stabilization of progressive keratoconus must be documented with concomitant follow‐up longer than 12 months, which is essential for corneal healing and remodeling. Stabilization of progressive keratoconus may be measured in terms of proportions of eyes in which steepening does not progress during a postsurgery follow‐up period of longer than 12 months. Clinical investigators should agree upon keratometric cut‐off value(s) for steepening and correlate them with other outcomes to determine prognostic importance and visual significance to the patient.
The relative change in maximum keratometry is a reasonable proxy for the relative efficacy of CXL approaches. This choice, rather than final keratometry values, may be reasonable when reported with indicators of variability, such as standard deviations. Use of final keratometry values as an outcome is inherently problematic because of the range of topography/tomography devices in use, which are based on different technologies.
Preoperative definitions of 'progressive keratoconus,' when reported, have varied widely among investigators and measurement devices for topography/tomography. Perioperative, intraoperative, and postoperative care should be standardized to permit meaningful comparisons of CXL methods. Methods to increase penetration of riboflavin through intact epithelium other than iontophoresis include chemical adjuvants and mechanical injury to the epithelium as described in studies included in this review, but the most effective method is not known. The presence of dextran in riboflavin solution instilled prior to and during irradiation in most transepithelial studies reduces penetration of riboflavin through the epithelium (Wollensak 2009); iontophoresis‐assisted transepithelial CXL studies utilized riboflavin without dextran. These perioperative and intraoperative considerations require further investigation. In addition, the corneal epithelium and Bowman layer decrease the passage of ultraviolet A (UVA) light. One group of investigators reported that the amount of blockage was approximately 20% to 30% (Podskochy 2004). The total dose of UVA energy may therefore need to be increased for transepithelial CXL to be effective, by means of either longer duration of CXL or higher power. Penetration of riboflavin into corneal stroma remains important; some corneal surgeons have suggested using a femtosecond laser to create a 'pocket' or laser in‐situ keratomileusis (LASIK)‐like flap in or under which riboflavin would be placed (Kanellopoulos 2009). Other investigators have begun to appreciate the role of oxygen diffusion in CXL. A suggested change to the set duration of constant UVA irradiation is to cycle UVA irradiation so as to replenish oxygen depleted during CXL (Kamaev 2012; Mazzotta 2014). Clinical investigators will also need to determine the most effective postoperative topical steroid regimen, as these regimens affect corneal wound‐healing and represent additional variables that introduce heterogeneity into quantitative analysis (Clearfield 2017).
Future studies should be rigorously designed; investigators should report adequate information by following the CONSORT statement for randomized controlled trials and analyzing outcome data appropriately to provide evidence of high certainty. Trial investigators should register their studies prospectively and make the protocol available.
What's new
| Date | Event | Description |
|---|---|---|
| 15 April 2021 | Amended | Edits made to Main results (Abstract) and Types of participants (Results/Included studies) ‐ 723 eyes of 578 participants corrected to 661 eyes of 567 participants. Edit to Plain language summary ‐ 578 people corrected to 567. |
History
Protocol first published: Issue 12, 2019 Review first published: Issue 3, 2021
Acknowledgements
We thank Lori Rosman, Information Specialist for Cochrane Eyes and Vision (CEV), who created and executed the electronic search strategies.
We thank the following peer reviewers for their comments: Renee Bovelle (Howard University), Shannon Shoaf (consumer), and one peer reviewer who wishes to remain anonymous.
We thank Peter Hersh, MD, medical monitor of the clinical studies that led to US Food and Drug Administration approval of corneal collagen crosslinking; and Andrew Morgenstern, OD, of the International Keratoconus Academy of Eye Care Professionals, for their critical review during the drafting of the protocol. This review was managed by CEV@US and was signed off for publication by Tianjing Li and Richard Wormald.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Keratoconus] explode all trees #2 (keratocon* OR (conical NEAR/2 cornea*) OR (corneal NEAR/2 ectasia*) OR keratectas*) #3 #1 OR #2 #4 MeSH descriptor: [Cross‐Linking Reagents] explode all trees #5 (cross link* or crosslink* or CXL) #6 MeSH descriptor: [Collagen] explode all trees and with qualifier(s): [radiation effects ‐ RE] #7 Collagen #8 MeSH descriptor: [Anti‐Infective Agents] explode all trees #9 (Anti Infective* or Antiinfective* or Antimicrobial* or Anti Microbial* or Microbicid*) #10 MeSH descriptor: [Riboflavin] explode all trees #11 (Riboflavin* or "Vitamin G" or "Vitamin B2" or "Vitamin B 2" or Beflavin or beflavine or flavaxin or hyrye or lactoflavin or lactoflavine or ovoflavin or pabriflan or riboflavine or ribovel or "83‐88‐5") #12 MeSH descriptor: [Ultraviolet Therapy] explode all trees #13 MeSH descriptor: [Ultraviolet Rays] explode all trees #14 (Ultraviolet* or Ultra‐Violet or UV Ray* or UV light* or "UV‐A" or UVA or Actinotherap* or Actinic Ray*) #15 MeSH descriptor: [Photosensitizing Agents] explode all trees #16 Photosensitiz* #17 MeSH descriptor: [Photochemotherapy] explode all trees #18 (Photochemotherap* or Photodynamic*) #19 MeSH descriptor: [Cornea] explode all trees and with qualifier(s): [radiation effects ‐ RE] #20 MeSH descriptor: [Cornea] explode all trees and with qualifier(s): [drug effects ‐ DE] #21 (chemical NEXT enhancer*) OR iontophor* #22 {OR #4‐#21} #23 #3 AND #22
Appendix 2. MEDLINE Ovid search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp Keratoconus/ 13. (keratocon* or (conical adj2 cornea*) or (corneal adj2 ectasia*) or keratectas*).tw. 14. 12 or 13 15. exp Cross‐Linking Reagents/ 16. (cross link* or crosslink* or CXL).tw. 17. exp Collagen/re [Radiation Effects] 18. Collagen.tw. 19. exp Anti‐Infective Agents/ 20. (Anti Infective* or Antiinfective* or Antimicrobial* or Anti Microbial* or Microbicid*).tw. 21. exp Riboflavin/ 22. (Riboflavin* or "Vitamin G" or "Vitamin B2" or "Vitamin B 2" or Beflavin or beflavine or flavaxin or hyrye or lactoflavin or lactoflavine or ovoflavin or pabriflan or riboflavine or ribovel or "83‐88‐5").tw. 23. exp Ultraviolet Therapy/ 24. exp Ultraviolet Rays/ 25. (Ultraviolet* or Ultra‐Violet or UV Ray* or UV light* or "UV‐A" or UVA or Actinotherap* or Actinic Ray*).tw. 26. exp Photosensitizing Agents/ 27. Photosensitiz*.tw. 28. exp Photochemotherapy/ 29. (Photochemotherap* or Photodynamic*).tw. 30. exp Cornea/de, re [Drug Effects, Radiation Effects] 31. (chemical enhancer* or iontophor*).tw. 32. or/15‐31 33. 14 and 32 34. 11 and 33
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'keratoconus'/exp #34 (keratocon* OR (conical* NEAR/2 cornea*) OR (corneal* NEAR/2 ectasia*) OR keratectas*):ab,ti,kw #35 #33 OR #34 #36 'cross linking reagent'/exp #37 ('cross link*' OR crosslink* OR cxl):ab,ti,kw #38 'collagen'/exp #39 collagen:ab,ti,kw #40 'antiinfective agent'/exp #41 ('anti infective*' OR antiinfective* OR antimicrobial* OR 'anti microbial*' OR microbicid*):ab,ti,kw #42 'riboflavin'/exp #43 (riboflavin* OR 'vitamin g' OR 'vitamin b2' OR 'vitamin b 2' OR beflavin OR beflavine OR flavaxin OR hyrye OR lactoflavin OR lactoflavine OR ovoflavin OR pabriflan OR riboflavine OR ribovel OR '83‐88‐5'):ab,ti,kw,tn #44 'ultraviolet phototherapy'/exp #45 'ultraviolet radiation'/exp #46 (ultraviolet* OR 'ultra violet' OR 'uv ray*' OR 'uv light*' OR 'uv‐a' OR uva OR actinotherap* OR 'actinic ray*'):ab,ti,kw #47 'photosensitizing agent'/exp #48 photosensitiz*:ab,ti,kw #49 'photochemotherapy'/exp #50 (photochemotherap* OR photodynamic*):ab,ti,kw #51 'cornea'/exp/dd_ae,dd_an,dd_cm,dd_it,dd_dt,dd_to #52 ('chemical enhancer*' OR iontophor*):ab,ti,kw,tn #53 #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 #54 #35 AND #53 #55 #32 AND #54
Appendix 4. PubMed search strategy
1. ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) 2. (keratocon*[tw] OR conical cornea*[tw] OR corneal ectasia*[tw] OR keratectas*[tw] NOT Medline[sb] 3. (cross link*[tw] OR crosslink*[tw] OR CXL[tw]) NOT Medline[sb] 4. Collagen[tw] NOT Medline[sb] 5. (Anti Infective*[tw] OR Antiinfective*[tw] OR Antimicrobial*[tw] OR Anti Microbial*[tw] OR Microbicid*[tw]) NOT Medline[sb] 6. (Riboflavin*[tw] OR "Vitamin G"[tw] OR "Vitamin B2"[tw] OR "Vitamin B 2"[tw] OR Beflavin[tw] OR beflavine[tw] OR flavaxin[tw] OR hyrye[tw] OR lactoflavin[tw] OR lactoflavine[tw] OR ovoflavin[tw] OR pabriflan[tw] OR riboflavin[tw] OR ribovel[tw] OR "83‐88‐5"[tw]) NOT Medline[sb] 7. (Ultraviolet*[tw] OR Ultra‐Violet[tw] OR UV Ray*[tw] OR UV light*[tw] OR "UV‐A"[tw] OR UVA[tw] OR Actinotherap*[tw] OR Actinic Ray*[tw]) NOT Medline[sb] 8. Photosensitiz*[tw] NOT Medline[sb] 9. (Photochemotherap*[tw] OR Photodynamic*[tw]) NOT Medline[sb] 10. (chemical enhancer*[tw] OR iontophor*[tw]) NOT Medline[sb] 11. #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 12. #2 AND #11 13. #1 AND #12
Appendix 5. LILACS search strategy
(keratocon$ OR "conical cornea" OR "corneal ectasia" OR keratectas$ OR MH:C11.204.627$ OR queratocon$ OR ceratocon$) AND (MH:D27.720.470.410.210$ OR (cross link$) OR crosslink$ OR CXL OR MH:D05.750.078.280$ OR MH:D12.776.860.300.250$ OR Collagen OR MH:D27.505.954.122$ OR (Anti Infective$) OR Antiinfective$ OR Antimicrobial$ OR (Anti Microbial$) OR Microbicid$ OR Antiinfecciosos OR "Anti‐Infecciosos" OR MH:D03.438.733.315.650$ OR MH:D03.494.507.650$ OR MH:D08.211.474.650$ OR MH:D23.767.405.650$ OR Riboflavin$ OR "Vitamin G" OR "Vitamin B2" OR "Vitamin B 2" OR Beflavin OR beflavine OR flavaxin OR hyrye OR lactoflavin OR lactoflavine OR ovoflavin OR pabriflan OR riboflavine OR ribovel OR "83‐88‐5" OR MH:E02.774.945$ OR MH:G01.358.500.505.650.891$ OR MH:G01.590.540.891$ OR MH:G01.750.250.650.891$ OR MH:G01.750.750.659$ OR MH:G01.750.770.578.891$ OR MH:G16.500.275.063.725.525.600$ OR MH:G16.500.750.775.525.600$ OR MH:N06.230.300.100.725.525.600$ OR MH:SP4.011.087.698.384.075.166.032$ OR MH:SP4.021.202.133.789$ OR Ultraviolet$ OR (Ultra Violet$) OR (UV Ray$) OR (UV light$) OR "UV‐A" OR UVA OR Actinotherap$ OR (Actinic Ray$) OR MH:D27.505.954.444.600$ OR MH:D27.505.954.600.710$ OR Photosensitiz$ OR Fotosensibilizante$ OR Fotossensibilizante$ OR Photochemotherap$ OR Photodynamic$ OR MH:E02.186.500$ OR MH:E02.319.685$ OR MH:E02.774.722$ OR Fotoquimioterap$ OR mh:"Cornea/DE" OR mh:"Cornea/RE" OR "chemical enhancer" OR "chemical enhancers" OR iontophor$)
Appendix 6. ClinicalTrials.gov search strategy
keratoconus OR "conical cornea" OR "corneal ectasia" OR keratectasia
Appendix 7. WHO ICTRP search strategy
keratoconus OR "conical cornea" OR "corneal ectasia" OR keratectasia
Data and analyses
Comparison 1. Transepithelial corneal collagen crosslinking versus epithelium‐off corneal collagen crosslinking.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mean change in maximum K from baseline or final value | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 at 6 months | 2 | 84 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐2.53, 0.49] |
| 1.1.2 at 12 months or more | 5 | 177 | Mean Difference (IV, Random, 95% CI) | 0.99 [‐0.11, 2.09] |
| 1.2 Proportion of participants whose maximum K decreased by at least 2 diopters | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 Proportion of participants whose maximum K increased by at least 2 diopters | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4 Proportion of participants whose keratoconus remained stable | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5 Mean change in corrected distance visual acuity (logMAR) from baseline or final values | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.5.1 at 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.5.2 at 12 months or more | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.6 Patient questionnaire of subjective visual function parameters (Ocular Surface Disease Index) at 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.7 Adverse outcomes—corneal haze or scarring | 4 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [1.01, 1.14] |
Comparison 2. Transepithelial corneal collagen crosslinking using iontophoresis versus epithelium‐off corneal collagen crosslinking.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Acar 2014.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, randomized controlled trial
Number randomized (total and per group): 13 eyes of 13 participants in total; 6 to transepithelial CXL, and 7 to epithelium‐off Unit of randomization (individual or eye): individual (1 eye per participant was included) Number analyzed (total and per group): 13 eyes of 13 participants in total; 6 in transepithelial CXL, and 7 in epithelium‐off Unit of analysis (individual or eye): individual (1 eye per participant) Exclusions and losses to follow‐up (total and per group): none How were missing data handled?: not applicable Length of follow‐up: 6 months Reported power calculation (Y/N), if yes, sample size and power: not reported |
|
| Participants |
Country: Turkey Setting: Haydarpasa Numune Education and Research Hospital Baseline characteristics 1. Epithelium‐off CXL, n = 7
2. Transepithelial CXL, n = 6
Overall, n = 13
Inclusion criteria: progressive keratoconus defined as an increase in the steepest keratometry of 1.00 D or more in a 1‐year period, >= 0.50 D increase in manifest refraction spherical equivalent, >= 1.00 D increase in manifest cylinder, or need for new contact lens fitting more than once in 2 years Exclusion criteria: a corneal thickness of less than 400 mm, central/paracentral scars in epithelial or stromal layers, history of herpetic keratitis, active ophthalmic infection or inflammation, pregnancy, lactation, and dry eye Baseline equivalence: "no significant difference with respect to age" in the 2 groups; the mean endothelial cell counts, polymegathism, pleomorphism, and central corneal thickness values of the 2 groups were compared and P > 0.05; "confocal microscopy showed no significant differences between the two groups in stromal morphology." |
|
| Interventions | 1 drop of proparacaine HCl 0.5% (Alcain; Alcon Inc., Fort Worth, TX) was instilled 4 times every 5 min, starting 20 min before the intervention. To reduce the risk of UVA exposure, miosis was induced with pilocarpine 1.0% 30 min before the procedure. 1. Transepithelial CXL
2. Epithelium‐off CXL
|
|
| Outcomes |
Primary outcome: in vivo confocal microscopy (the mean endothelial cell counts, morphology of endothelial cells (polymegathism and pleomorphism), and depth of CXL effect in the stroma), and central corneal thickness values Secondary outcomes: none Adverse outcomes:
Measurement time points: examined 1, 4, 7 days after surgery; outcomes were assessed 1 and 6 months after surgery Other issues with outcome assessment (e.g. quality control for outcomes, if any): none |
|
| Notes |
Study period: not reported Publication language: English Trial registration: not found Conflicts of interest: "The authors have no funding or conflicts of interest to disclose." Funding source: "The authors have no funding or conflicts of interest to disclose." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported: "Patients were randomized into two groups: CXL after total epithelial debridement (standard CXL group) and CXL with intact epithelium (transepithelial CXL group)." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment before assignment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The analyses were performed by the same investigator (C.A.U.) blinded to intervention modality." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Neither protocol nor trial registry was available. |
| Other bias | Unclear risk | None identified. |
Al Fayez 2015.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, randomized controlled trial
Number randomized (total and per group): 70 eyes of 70 participants in total; 34 to transepithelial CXL, and 36 to epithelium‐off Unit of randomization (individual or eye): individual (1 eye per participant was included) Number analyzed (total and per group): 70 eyes of 70 participants in total; 34 in transepithelial CXL, and 36 in epithelium‐off Unit of analysis (individual or eye): individual (1 eye per participant) Exclusions and losses to follow‐up (total and per group): not explicitly reported; of 122 eligible participants, only 70 participants (70 eyes) were included in the study; the reasons and methods for not to including 52 participants were not reported How were missing data handled?: not reported Length of follow‐up: "primary outcome measure was change in maximum K (Kmax) results 36 months after treatment. 70 patients in study were followed up for a mean of 40 months" Reported power calculation (Y/N), if yes, sample size and power: not reported |
|
| Participants |
Country: Saudi Arabia Setting: The Eye and Laser Centre and King Abdulaziz University Hospital Baseline characteristics 1. Epithelium‐off CXL, n = 36
2. Transepithelial CXL, n = 34
Overall, n = 70
Inclusion criteria: documented progressive mild and moderate keratoconus (stages I and II on the Amsler–Krumeich scale) with a corneal thickness >= 400 mm, mean K =< 53 D, and a clear cornea with no Vogt striae. (Progression of keratoconus was defined as an increase in the maximum K value or manifest astigmatism >= 1 D within the previous year based on repeated corneal topography.) Exclusion criteria: central corneal scarring, previous ocular surgery, ocular surface pathology or infection, collagen vascular disease, and pregnancy Baseline equivalence: baseline comparable |
|
| Interventions | 1. Transepithelial CXL
2. Epithelium‐off CXL
|
|
| Outcomes |
Primary outcome: maximum K (Kmax) readings Secondary outcomes: comfort on 1‐to‐5 scale, uncorrected logMAR visual acuity, corrected logMAR visual acuity, depth of demarcation line Adverse outcomes:
Measurement time points: 1 week, 1, 3, 6, and 12 months and then every year Other issues with outcome assessment (e.g. quality control for outcomes, if any): numbers of participants whose keratoconus progressed in transepithelial group were different in Results (19) and Abstract (20) |
|
| Notes |
Study period: not reported Publication language: English Trial registration: not found Conflicts of interest: "The authors have no funding or conflicts of interest to disclose" Funding source: "The authors have no funding or conflicts of interest to disclose" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were allocated using a computer‐ generated randomization sequence with randomly variable block sizes of 2 and 4 age and sex‐matched patients, prepared by a biostatistician" |
| Allocation concealment (selection bias) | Low risk | "The allocation was concealed using sequentially numbered sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The surgeon and the patients were aware of the allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Ophthalmic technicians who measured the outcome parameters were masked to the allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers excluded or lost to follow‐up were not explicitly reported. |
| Selective reporting (reporting bias) | High risk | Outcomes of interest (e.g. Kmax, UDVA, CDVA) in the review are reported incompletely (e.g. no standard deviation) so that they could not be incorporated into meta‐analysis. |
| Other bias | Low risk | None identified. |
Al Zubi 2019.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, randomized controlled trial
Number randomized (total and per group): 80 in total; 40 into each group Unit of randomization (individual or eye): individual (1 eye per participant) Number analyzed (total and per group): not explicitly reported Unit of analysis (individual or eye): individual (1 eye per participant) Exclusions and losses to follow‐up (total and per group): not explicitly reported How were missing data handled?: not reported Length of follow‐up: 12 months Reported power calculation (Y/N), if yes, sample size and power: not reported |
|
| Participants |
Country: Jordan Setting: tertiary care Baseline characteristics Transepithelial CXL, n = 40
Epithelium‐off CXL, n = 40
Overall, n = 80
Inclusion criteria: "participants with keratoconus aged 18 years or above with documented progression of keratoconus (greater than 0.5D rise in six months or greater than 1 D rise in steep K/12months), keratometry (between 46 D and 56 D along with the corneal thickness being ≥400 μm) from the thinnest point, and no corneal scarring on presentation were included in this study" Exclusion criteria: none listed Baseline equivalence: it was unclear if participants were comparable at baseline. The authors reported: "While, mean Sim K astigmatism was 6.73 ± 1.98D in group 2 (range 4.3 D to 11.1 D). There was a significant difference between the two groups (P = 0.02)"; however, equivalent data in Table 2 showed a P value of 0.3. |
|
| Interventions | 1. Transepithelial CXL
2. Epithelium‐off CXL
|
|
| Outcomes |
Primary outcomes: CDVA, UDVA, central corneal thickness Secondary outcomes: keratometric astigmatism, flattest keratometry, steepest keratometry Adverse outcomes:
Measurement time points: 3, 6, and 12 months Other issues with outcome assessment (e.g. quality control for outcomes, if any): none |
|
| Notes |
Study period: not reported Publication language: English Trial registration: not found Conflicts of interest: "The authors declare no conflict of interest, financial or otherwise" Funding source: "The authors declare no conflict of interest, financial or otherwise" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was unclear what "odd‐even number allocation method, known as a randomized controlled trial" means: "the keratoconus patients were allocated to one of the two groups in a random fashion in accordance with the odd‐even number allocation method, known as a randomized control trial." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was unclear: "the keratoconus patients were allocated to one of the two groups in a random fashion in accordance with the odd‐even number allocation method, known as a randomized control trial." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers excluded or lost to follow‐up were not explicitly reported. |
| Selective reporting (reporting bias) | High risk | UDVA, which was proposed in the methods, was not reported in the results. |
| Other bias | Unclear risk | It was unclear if participants were comparable at baseline. The authors reported: "While, mean Sim K astigmatism was 6.73 ± 1.98D in group 2 (range 4.3 D to 11.1 D). There was a significant difference between the two groups (P = 0.02)"; however, equivalent data in Table 2 showed a P value of 0.3. Lack of use of standard outcome measures (authors cite seminal papers that use standard measures) |
Bikbova 2016.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, randomized controlled trial
Number randomized (total and per group): 149 eyes of 119 participants in total; 73 eyes of 62 participants to standard CXL, and 76 eyes of 57 participants to transepithelial CXL Unit of randomization (individual or eye): individual Number analyzed (total and per group): 149 eyes of 119 participants in total; 73 eyes of 62 participants in standard CXL, and 76 eyes of 57 participants in transepithelial CXL Unit of analysis (individual or eye): eye (both eyes of single participant were separately included in the analysis without taking account of non‐independence) Exclusions and losses to follow‐up (total and per group): not explicitly reported How were missing data handled?: not reported Length of follow‐up: 24 months Reported power calculation (Y/N), if yes, sample size and power: not reported |
|
| Participants |
Country: Russia Setting: Ufa Eye Research Institute in Republic of Bashkortostan, Russia Baseline characteristics 1. Epithelium‐off CXL, 62 participants (73 eyes)
2. Transepithelial CXL using iontophoresis, 57 participants (76 eyes)
Overall, 119 participants (149 eyes)
Inclusion criteria: > 18 years of age with documented progression of disease as defined by the following changes over 1 year: an increase in the steepest keratometry value by 1.0 D or more in manifest cylinder, or an increase of 0.5 D or more in manifest spherical equivalent refraction by repeated keratopography ODP‐scans ARK‐1000 Exclusion criteria: pachymetry value of < 400 microns, history of previous ocular infection (e.g. herpes), pregnancy or breastfeeding, corneal scarring Baseline equivalence: baseline comparable |
|
| Interventions | 1. Transepithelial CXL using iontophoresis
2. Epithelium‐off CXL
|
|
| Outcomes |
Primary outcome: mean K (average keratometry in the central 3 mm of cornea) Secondary outcomes: CDVA, depth of demarcation line, anterior corneal astigmatism Adverse outcomes: no endothelial damage was observed; impaired epithelial healing with central haze development was observed in 4 participants at the 6‐month follow‐up in the epithelium‐off CXL group Measurement time points: 1, 3, 6, 12, and 24 months Other issues with outcome assessment (e.g. quality control for outcomes, if any): unit of randomization was the individual, but unit of analysis was the eye. Both eyes of 30 (25.2%) participants were separately included in the analysis, but the analysis did not take into account non‐independence of eyes (unit of analysis error). |
|
| Notes |
Study period: January 2010 to December 2014 Publication language: English Trial registration: NCT02456961 Conflicts of interest: not reported Funding source: State Academy of Science of Republic Bashkortostan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported: "Patients were randomized by unrestricted randomization to either standard CXL or iontophoresis‐assisted transepithelial CXL." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment before assignment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers excluded or lost to follow‐up were not explicitly reported. |
| Selective reporting (reporting bias) | High risk | The authors did not report on Kmax, which is most common criterion followed in CXL; used criterion of "Km" for mean keratometry in central 3‐millimeter zone; no P values regarding change in "Km" after standard CXL (decreased by "2.15 diopters") and after transepithelial CXL (decreased by "0.97 diopter"); did NOT report on % gaining or losing logMAR vision; only report was NO difference in mean change in CDVA between 2 groups at 24 months. |
| Other bias | Unclear risk | Conflicts of interest were not reported. |
Cifariello 2018.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, randomized controlled trial
Number randomized (total and per group): 40 eyes of 32 participants in total; 20 eyes in each group Unit of randomization (individual or eye): individual (both eyes were assigned to the same intervention for participants who were included bilaterally) Number analyzed (total and per group): number analyzed not provided Unit of analysis (individual or eye): eye (both eyes of single participant were separately included in the analysis without taking into account non‐independence) Exclusions and losses to follow‐up (total and per group): not explicitly reported How were missing data handled?: not reported Length of follow‐up: 24 months Reported power calculation (Y/N), if yes, sample size and power: not reported |
|
| Participants |
Country: Italy Setting: university hospital/clinic Baseline characteristics 1. Epithelium‐off CXL, total number of participants unclear
2. Transepithelial CXL, total number of participants unclear
Overall, 32 participants (40 eyes)
Inclusion criteria: patients with evolving keratoconus (defined as clinical and instrumental worsening in prior 6 months); aged between 18 and 40 years, with no evidence of corneal scarring Exclusion criteria: patients with central and paracentral corneal opacities, Vogt’s striae, previous intraocular surgery, history of herpetic keratitis, severe dry eye, concomitant autoimmune diseases Baseline equivalence: no differences in baseline OSDI; statistical comparisons between other preoperative outcomes are not reported; mean age was significantly greater in transepithelial CXL group |
|
| Interventions | 1. Transepithelial CXL
2. Epithelium‐off CXL
|
|
| Outcomes |
Primary outcome: BCVA Secondary outcomes: central and peripheral corneal thickness, K flat, K steep, mean K, fibrotic reaction, corneal alteration of nerves, activated keratocytes, and corneal opacities. Anterior thinning, the presence of inflammatory cells associated with the lenticule, and activation of corneal keratocytes, stromal cell density, OSDI Adverse outcomes:
Measurement time points: day 1, 3, 7, and 15 and then after 1, 6, 12, and 24 months Other issues with outcome assessment (e.g. quality control for outcomes, if any): unit of randomization was the individual, but unit of analysis was the eye. Both eyes of 8 (25%) participants were separately included in the analysis, but the analysis did not take into account non‐independence of eyes (unit of analysis error). It appears that the gender distributions by intervention and overall refer to eyes, not participants. |
|
| Notes |
Study period: June 2014 to June 2015 Publication language: English Trial registration: NCT03598634 (note: the paper incorrectly reports the identification as NCT01350323) Conflicts of interest: "The authors declare that they have no conflicts of interest" Funding source: University of Molise |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not described: "The patients were randomly assigned to one of the two treatment groups (20 eyes were treated with CLX epi‐off, and the other 20 eyes were treated with CLX epi‐on)." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment before assignment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study: "Forty eyes from 32 patients with progressive keratoconus, followed at the University of Molise, Italy, from June 2014 to June 2015, were included in this nonblinded, randomized comparative study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers excluded or lost to follow‐up were not explicitly reported. |
| Selective reporting (reporting bias) | High risk | Baseline data were reported, but the equivalence was not reported (between‐group significance in age, BCVA, and K steep); the results were reported for only one time point (endpoint), although outcomes were measured at multiple time points. |
| Other bias | High risk | Baseline equivalence was not reported except for OSDI, and mean age was significantly greater for transepithelial CXL group than for epithelium‐off CXL group. |
Lombardo 2016.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, randomized controlled trial
Number randomized (total and per group): 34 eyes of 25 participants in total; 22 eyes of 20 participants to T‐ionto CXL, and 12 eyes of 10 participants to standard CXL Unit of randomization (individual or eye): eye (each eye was randomized independently when both eyes of a participant were included in the study) Number analyzed (total and per group): 34 eyes of 25 participants in total; 22 eyes of 20 participants to T‐ionto CXL, and 12 eyes of 10 participants to standard CXL (until 12 months) Unit of analysis (individual or eye): eye (both eyes of single participant were separately included in the analysis without taking account of non‐independence) Exclusions and losses to follow‐up (total and per group): none until 12 months, 2 eyes in T‐ionto CXL group and 1 eye in standard CXL group were lost to follow‐up at 24 months How were missing data handled?: not reported Length of follow‐up: 24 months Reported power calculation (Y/N), if yes, sample size and power: Y, power 81%; sample size 34 cases (allocation ratio 2:1) |
|
| Participants |
Country: Italy Setting: clinical trials center of the Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Fondazione G.B. Bietti (Rome, Italy) Baseline characteristics 1. Epithelium‐off CXL, 10 participants (12 eyes)
2. Transepithelial CXL using iontophoresis, 20 participants (22 eyes)
Overall, 25 participants (34 eyes)
Inclusion criteria: aged between 18 and 46 years; confirmed diagnosis of progressive keratoconus deemed to be progressive if there was an increase of at least 1 D in the Kmax derived by computerized Placido disk corneal topography over the 12 months preceding the operation Exclusion criteria: a minimum corneal thickness less than 400 μm; Kmax steeper than 61 D; any corneal scarring; previous refractive or other corneal or ocular surgery; other ocular disorders (e.g. cataract, glaucoma, herpetic keratitis); pregnant or breastfeeding at the time of enrollment Baseline equivalence: baseline comparable |
|
| Interventions | 1. Transepithelial CXL using iontophoresis
2. Epithelium‐off CXL
2 drops of ofloxacin 0.3% (Monofloxofta; Sooft Italia SpA) were applied in all cases at the end of both treatments. A bandage contact lens was applied only to eyes treated by standard CXL, which remained in place until epithelial closure was confirmed. After surgery, all participants continued ofloxacin 0.3% 5 times daily for 6 days, sodium hyaluronate 0.10% (Ribolisin; Sooft Italia SpA) 6 times daily for 3 months and 3 times daily for up to 6 months, and fluorometholone acetate 0.1% (Fluaton; Bausch & Lomb, Rochester, NY) 2 times daily from days 7 to 21. |
|
| Outcomes |
Primary outcome: keratometry maximum (Kmax) Secondary outcomes: UDVA logMAR units and CDVA logMAR units, obtained using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart at 4 meters contrast sensitivity function (log units) evaluated using the Pelli‐Robson chart manifest refraction (expressed as spherical equivalent; D), endothelial cell density (cells per square millimeter) measured by non‐contact specular microscopy (Perseus; CSO, Florence, Italy), intraocular pressure using a Goldmann applanation tonometer (Haag‐Streit AG, Koeniza, Switzerland) preoperatively and 6 and 12 months postoperatively Adverse outcomes:
Measurement time points: 1, 3, 6, 12, 18, 24 months Other issues with outcome assessment (e.g. quality control for outcomes, if any): "If both eyes of a patient qualified for participation in the study, each eye was randomized independently. Second eyes were treated no earlier than 2 months after the first eyes." The gender distributions for each intervention appear to refer to eyes, not participants. However, it is unclear how "18 men and 3 women" are in the T‐ionto CXL group of "22 eyes of 20 participants," as 21 (18 + 3) does not agree with either the number of eyes or the number of participants. |
|
| Notes |
Study period: recruitment between 31 January 2014 and 30 May 2015 Publication language: English Trial registration: NCT02117999 Conflicts of interest: "No competing interests exist for any author."; "The authors made the following financial disclosure: Supported by the National Framework Program for Research and Innovation PON (grant no. 01_00110), the Italian Ministry of Health, and Fondazione Roma." Funding source: "Supported by the National Framework Program for Research and Innovation PON (grant no. 01_00110), the Italian Ministry of Health, and Fondazione Rome"; "Sooft Italia SpAdFidia Pharma Group provided the medical devices used in this study" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible patients were randomized after enrollment, with an allocation ratio of 2:1, into either the study or control group using a computer‐generated randomization plan with block randomization." |
| Allocation concealment (selection bias) | Low risk | "personnel were protected to foresee the upcoming assignment before randomization. The randomization numbers were secured in the health secretary room. Only after signature of the consent form, the principal investigator was taking the randomization number to assign to the patient" (personal communication) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This is an "unmasked" study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "All data were acquired and analyzed in an unmasked manner." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 eyes in the T‐ionto CXL group and 1 eye in the standard CXL group were lost to follow‐up at 24 months. Intention‐to‐treat analysis was followed. "The Intent‐to‐Treat Population consisted of all participants who were randomized into the trial and performed at least 1 follow‐up visit." (personal communication) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes in the trial registry and outcomes in the methods were reported in the results. |
| Other bias | High risk | Medical devices were supplied by industry: "The authors thank Sooft Italia SpA for generously providing the medical devices used in the present work." |
Mastropasqua 2013.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, randomized controlled trial
Number randomized (total and per group): 40 eyes of 35 participants; 20 eyes each group Unit of randomization (individual or eye): eye Number analyzed (total and per group): 40 eyes of 35 participants Unit of analysis (individual or eye): eye Exclusions and losses to follow‐up (total and per group): not explicitly reported How were missing data handled?: not reported Length of follow‐up: 12 months Reported power calculation (Y/N), if yes, sample size and power: not reported |
|
| Participants |
Country: Italy Setting: G. D’Annunzio University of Chieti‐Pescara, Pescara, Italy Baseline characteristics 1. Epithelium‐off CXL
2. Transepithelial CXL
Overall, 35 participants (40 eyes)
Inclusion criteria: progressive keratoconus defined as thickness of the cornea’s thinnest point of at least 400 microns and documented topographic or pachymetric progression of keratoconus during the previous 6 months. Keratoconus progression was confirmed by serial differential corneal topographies and by differential optical pachymetry. Keratoconus progression was defined as a mean central K‐reading change of >= 1.5 D observed in 3 consecutive topographies during the preceding 6 months, or a mean central corneal thickness decrease of >= 5% in 3 consecutive examinations performed in the previous 6 months. Exclusion criteria: not reported Baseline equivalence: not reported |
|
| Interventions | 1. Transepithelial CXL
2. Epithelium‐off CXL
|
|
| Outcomes |
Primary outcome: morphological changes in cornea seen in in vivo confocal microscopy and anterior segment optical coherence topography Secondary outcomes: not reported Adverse outcomes: not reported Measurement time points: 1 week, then 1, 3, 6, and 12 months Other issues with outcome assessment (e.g. quality control for outcomes, if any): none |
|
| Notes |
Study period: not reported Publication language: English Trial registration: not found Conflicts of interest: "The authors have no funding or conflicts of interest to disclose" Funding source: "The authors have no funding or conflicts of interest to disclose" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported: "The eyes examined were randomly assigned to the standard corneal CXL group (20 eyes) or transepithelial corneal CXL group (20 eyes)." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment before assignment was unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers excluded and lost to follow‐up were not explicitly reported. |
| Selective reporting (reporting bias) | Unclear risk | Neither protocol nor trial registry was available. |
| Other bias | Low risk | None identified. |
Nawaz 2015.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, randomized controlled trial
Number randomized (total and per group): 40 eyes of 40 participants; 20 eyes of 20 participants in each group Unit of randomization (individual or eye): individual (1 eye per participant) Number analyzed (total and per group): 40 eyes of 40 participants; 20 eyes of 20 participants in each group Unit of analysis (individual or eye): individual (1 eye per participant) Exclusions and losses to follow‐up (total and per group): not explicitly reported How were missing data handled?: not reported Length of follow‐up: 6 months (12 months for endothelial cell count) Reported power calculation (Y/N), if yes, sample size and power: not reported |
|
| Participants |
Country: India Setting: cornea division at tertiary care hospital Baseline characteristics 1. Epithelium‐off CXL, n = 20
2. Transepithelial CXL, n = 20
Overall, n = 40
Inclusion criteria: patient age over 18 years; documented keratoconus progression (> 1 D increase in steep K/12 months or > 0.5 D in 6 months); no evidence of corneal scarring; keratometry between 47 D and 55 D; corneal thickness at the thinnest point ≥ 400 μm Exclusion criteria: not reported Baseline equivalence: baseline comparable |
|
| Interventions | 1. Transepithelial CXL
2. Epithelium‐off CXL
Topical moxifloxacin 0.3% 4 times a day, prednisolone acetate 1% 4 times a day tapering over 4 weeks starting day 1 in transepithelial CXL group, and after epithelial healing in epithelium‐off group; in both groups, oral non‐steroidal drug 3 times a day for 1 week |
|
| Outcomes |
Primary outcome: CDVA, K mean Secondary outcomes: UDVA, slit‐lamp biomicroscopy, pachymetry, endothelial cell count, Orbscan II Adverse outcomes:
Measurement time points: 1, 3, and 6 months Other issues with outcome assessment (e.g. quality control for outcomes, if any): none |
|
| Notes |
Study period: not reported Publication language: English Trial registration: not found Conflicts of interest: "None declared" Funding source: "Nil" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "These patients were then randomly allocated to either of the two groups according to odd even number method (randomized control trial)." It is unclear what "odd even number method (randomized control trial)" means. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment before assignment was unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers excluded and lost to follow‐up were not explicitly reported. |
| Selective reporting (reporting bias) | High risk | Protocol or trial registry was not available; 1 outcome (UCDVA) in the methods was not reported in the results; only one time point (endpoint) was reported for K steep and K flat. |
| Other bias | Low risk | None identified. |
Razmjoo 2014.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, randomized controlled trial
Number randomized (total and per group): 44 eyes of 22 participants; 22 eyes of 11 participants each Unit of randomization (individual or eye): individual (both eyes of a participant were assigned to the same intervention) Number analyzed (total and per group): 44 eyes of 22 participants; 22 eyes or 11 participants each Unit of analysis (individual or eye): eye; both eyes included in all (22) participants, analyzed separately (unit of analysis error) Exclusions and losses to follow‐up (total and per group): none How were missing data handled?: not applicable Length of follow‐up: 6 months Reported power calculation (Y/N), if yes, sample size and power: not reported |
|
| Participants |
Country: Iran Setting: university hospital Baseline characteristics 1. Epithelium‐off CXL, 11 participants (22 eyes)
2. Transepithelial CXL, 11 participants (22 eyes)
Overall, 22 participants (44 eyes)
Inclusion criteria: age between 16 and 40 years, axial topography consistent with keratoconus, minimum corneal thickness more than 400 μm and a progression of keratoconus in past 12 months (an increase in maximum keratometry (K) of 1.00 D, an increase of refractive astigmatism of 1 D, or increase of refractive error of 0.5 D) Exclusion criteria: individuals with a history of ocular herpes or non‐healing corneal ulcers; current ocular infection; severe preoperative corneal haze or scar; severe ocular surface disease; autoimmune diseases; pregnant Baseline equivalence: baseline comparable |
|
| Interventions | 1. Transepithelial CXL
2. Epithelium‐off CXL
|
|
| Outcomes |
Primary outcome: BCVA Secondary outcomes: cylinder, sphere, intraocular pressure, Kmax, corneal asphericity, corneal thickness at thinnest point, IVA (index of vertical asymmetry), IHD (index of height decentration), ISV (index of surface variance), KI (keratoconus index), CKI (central keratoconus index), IHA (index of height asymmetry), Rmin (minimum radius of curvature), corneal density (as measure of haze) Adverse outcomes: no serious complications were reported Measurement time points: 6 months Other issues with outcome assessment (e.g. quality control for outcomes, if any): all participants were bilaterally included, with both eyes receiving the same intervention; the analysis did not take into account non‐independence of eyes (unit of analysis error) |
|
| Notes |
Study period: July 2012 to December 2012 Publication language: English Trial registration: NCT01809977 Conflicts of interest: "None declared" Funding source: "Nill" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using computer generated random numbers, patients were randomly distributed into two treatment groups, and both eyes were treated with the same method" |
| Allocation concealment (selection bias) | High risk | Personnel could be aware of upcoming assignment: "We enrolled 44 consecutive eyes to the study, the samples allocated to the groups with a pre‐determined table created with random allocation software; the printed list, was not regarded a secret paper but it was only usable for the surgeon performing the procedure. Patients were not aware of which treatment method was utilized on each eye, but the surgeon had to be informed about this." (personal communication) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single masking (outcomes assessor) per ClinicalTrials.gov (NCT01809977) record |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor masked per ClinicalTrials.gov (NCT01809977) record. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in trial registry were reported in published paper. |
| Other bias | Low risk | None identified. |
Rossi 2015.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, randomized controlled trial
Number randomized (total and per group): 20 eyes of 20 participants; 10 eyes of 10 participants each group Unit of randomization (individual or eye): individual (1 eye per participant) Number analyzed (total and per group): 20 eyes of 20 participants; 10 eyes of 10 participants each group Unit of analysis (individual or eye): individual (1 per participant was included) Exclusions and losses to follow‐up (total and per group): none How were missing data handled?: not applicable Length of follow‐up: 12 months Reported power calculation (Y/N), if yes, sample size and power: Y, power of 80% |
|
| Participants |
Country: Italy Setting: Eye Clinic of the Second University of Naples Baseline characteristics 1. Epithelium‐off CXL, n = 10
2. Transepithelial CXL, n = 10
Overall, n = 20
Inclusion criteria: age greater than 18 years and presenting with progressive keratoconus with a documented clinical and instrumental (topographic, pachymetric, or aberrometric) worsening in the previous 6 months of observation Exclusion criteria: any coexisting ocular disease or corneal opacities possibly affecting visual acuity, previous intraocular surgery, history of herpetic keratitis, severe dry eye, and concomitant autoimmune diseases Baseline equivalence: participants in the epi‐off group had better UDVA (P < 0.001), CDVA (P < 0.001), and endothelial cell density (P < 0.05) at baseline |
|
| Interventions | 1. Transepithelial CXL
2. Epithelium‐off CXL
|
|
| Outcomes |
Primary outcome: UDVA, CDVA, slit‐lamp exam, spherical error, spherical equivalent, corneal astigmatism, simulated maximum, minimum, and average keratometry, coma and spherical aberration, central corneal thickness, and endothelial cell density Secondary outcomes: not distinguished Adverse outcomes: no ocular or systemic adverse events were observed. No corneal edema, no haze, and no re‐epithelialization delay were noticed. Measurement time points: 3 and 12 months except for spherical aberration, coma aberration, and root mean sphere Other issues with outcome assessment (e.g. quality control for outcomes, if any): none |
|
| Notes |
Study period: May 2012 to July 2012 Publication language: English Trial registration: not found Conflicts of interest: "The authors report no conflicts of interest in this work" Funding source: "The study had no funding." (personal communication) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to one of the two treatment groups (ten eyes were treated with epi‐off CXL, and the other ten eyes were treated with epi‐on CXL)."; "Randomization was done by a computer‐generated random number list prepared by an investigator with no clinical involvement" (personal communication) |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment before assignment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Patient and the surgeon responsible for the treatment were aware of the allocated arm, technicians and physicians performing the other clinical investigations and data analysts were kept blinded to the allocation." (personal communication) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patient and the surgeon responsible for the treatment were aware of the allocated arm, technicians and physicians performing the other clinical investigations and data analysts were kept blinded to the allocation." (personal communication) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Neither protocol nor clinical registration was available. |
| Other bias | High risk | Participants in the epi‐off group had higher UDVA (P < 0.001), CDVA (P < 0.001), and endothelial cell density (P < 0.05) at baseline. |
Rossi 2018.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, randomized controlled trial (3 arms)
Number randomized (total and per group): 30 eyes of 30 participants; 10 eyes of 10 participants each group Unit of randomization (individual or eye): individual (1 eye per participant) Number analyzed (total and per group): 30 eyes of 30 participants; 10 eyes of 10 participants each group Unit of analysis (individual or eye): individual (1 per participant) Exclusions and losses to follow‐up (total and per group): none (personal communication) How were missing data handled?: not applicable Length of follow‐up: 12 months Reported power calculation (Y/N), if yes, sample size and power: not reported |
|
| Participants |
Country: Italy Setting: University of Campania Baseline characteristics 1. Epithelium‐off CXL, n = 10
2. Transepithelial CXL, n = 10
3. Iontophoresis‐transepithelial CXL, n = 10
Overall, n = 30
Inclusion criteria: age >= 18 years; progressive keratoconus with a documented clinical and instrumental (topographic, pachymetric, or aberrometric) worsening in the previous 6 months of observation; thinnest corneal point >= 400 μm in epi‐off CXL and >= 360 μm epi‐on and iontophoresis‐CXL, a clear cornea on slit‐lamp and the absence of scar or severe Vogt striae, which can be considered predictive risk factors for postoperative haze development. The parameters defined to establish keratoconus progression were: worsening of UDVA and/or CDVA of more than 1 Snellen line, an increase in central corneal astigmatism of at least 1.00 D, an increase in the maximum cone apex curvature of at least 1.00 D, a reduction of at least 10 μm or more in the thinnest point. Exclusion criteria: any coexisting ocular disease or corneal opacities possibly affecting visual acuity; previous intraocular surgery; history of herpetic keratitis; severe dry eye; concomitant autoimmune diseases; any lens or retinal disease Baseline equivalence: baseline comparable |
|
| Interventions | 1. Transepithelial CXL
2. Transepithelial CXL using iontophoresis
3. Epithelium‐off CXL
"All patients were discharged with topical tobramycin to apply four times a day for 1 week, dexamethasone phosphate 0.1% four times a day for 2 weeks, then tapering to zero. Orally, amino acids (Aminoftal, SOOFT, Italy) were administered for 2 weeks... . Topical hyaluronic 3 times a day was administered for 3 months. All patients were operated by same surgeon." |
|
| Outcomes |
Primary outcome: examination (spherical error, spherical equivalent), corneal topography (corneal astigmatism, flattest meridian keratometry, steepest meridian keratometry, mean keratometry, apex keratometry, superior‐inferior corneal symmetry index), aberrometry (spherical aberration, coma and root‐mean‐square), central corneal thickness and endothelial cell density. All intra‐ and postoperative adverse events were recorded. Secondary outcomes: not distinguished Adverse outcomes: no ocular or systemic adverse event was observed. No corneal edema, no haze, and no re‐epithelialization delay were noticed. Eye pain was reported in participants in the epi‐off CXL group in the early postoperative correlated with the sudden corneal de‐epithelialization. Measurement time points: 12 months Other issues with outcome assessment (e.g. quality control for outcomes, if any): none |
|
| Notes |
Study period: not reported Publication language: English Trial registration: not found Conflicts of interest: "The authors declare that they have no conflict of interest." Funding source: "The study had no funding." (personal communication) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done by a computer‐generated random number list prepared by an investigator with no clinical involvement" (personal communication) |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment before assignment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Patient and the surgeon responsible for the treatment were aware of the allocated arm, technicians and physicians performing the other clinical investigations and data analysts were kept blinded to the allocation." (personal communication) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patient and the surgeon responsible for the treatment were aware of the allocated arm, technicians and physicians performing the other clinical investigations and data analysts were kept blinded to the allocation." (personal communication) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "No patients were lost in our study. All the participants had the scheduled follow up examinations." (personal communication) |
| Selective reporting (reporting bias) | Unclear risk | Neither protocol nor trial registry was available. |
| Other bias | Low risk | None identified. |
Soeters 2015.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, randomized controlled trial
Number randomized (total and per group): 61 eyes of 61 participants in total; 26 eyes of 26 participants to epi‐off CXL group, and 35 eyes of 35 participants to trans‐CXL group Unit of randomization (individual or eye): individual (1 eye per participant) Number analyzed (total and per group): not explicitly reported Unit of analysis (individual or eye): individual (1 eye per participant) Exclusions and losses to follow‐up (total and per group): 4 (6%) participants in total; 2 participants in each group were lost to follow‐up at the last follow‐up visit. 2 moved abroad; 1 received follow‐up care at another hospital; and 1 was retreated with epi‐off CXL 10 months after the initial trans‐CXL treatment. How were missing data handled?: not reported Length of follow‐up: 12 months Reported power calculation (Y/N), if yes, sample size and power: Y, power 80%, sample size 29 each group |
|
| Participants |
Country: the Netherlands Setting: University Medical Center Utrecht, the Netherlands Baseline characteristics 1. Epithelium‐off CXL, n = 26
2. Transepithelial CXL, n = 35
Overall, n = 61
Inclusion criteria: age > 18 years, a clear central cornea, documented progression as defined by an increase in Kmax, Ksteep, mean keratometry, and/or topographic cylinder value by > 0.5 D over the previous 6 to 12 months Exclusion criteria: minimal pachymetry of less than 400 mm prior to UVA irradiation, pregnancy or breastfeeding, history of previous ocular infection Baseline equivalence: comparable, except a lower spherical equivalent (P = 0.04) and logMAR UDVA (P = 0.03) in the trans‐CXL group |
|
| Interventions | 1. Transepithelial CXL
2. Epithelium‐off CXL
|
|
| Outcomes |
Primary outcome: clinical stabilization of keratoconus 1 year after CXL, defined as a Kmax increase of no more than 1 D over the preoperative Kmax value Secondary outcomes: manifest refraction, UDVA, CDVA, corneal tomography; keratometry, demarcation line, endothelial cell density Adverse outcomes:
Measurement time points: 1, 3, 6, and 12 months Other issues with outcome assessment (e.g. quality control for outcomes, if any): none |
|
| Notes |
Study period: enrollment from 30 May 2011 through 4 September 2013 Publication language: English Trial registration: NCT02349165 Conflicts of interest: "The authors report no conflicts of interest in this work." Funding source: "N. Soeters, R.P.L. Wisse, and D.A. Godefrooij were supported by the Dr F.P. Fischer Stichting (Amersfoort, TheNetherlands). N. Soeters was supported by Stichting Nederlands Oogheelkundig Onderzoek (SNOO, Rotterdam, The Netherlands). The funding organizations had no role in the design or conduct of this research. They provided unrestricted grants." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported. "Patients were randomized using a simple unrestricted randomization procedure to either transepithelial CXL or epi‐off CXL." |
| Allocation concealment (selection bias) | Unclear risk | Authors reported that "sealed envelopes in a box" were used (personal communication), but allocation concealment remains unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study (NCT02349165). "The unequal sample size in this (non‐double‐masked) study can be considered a limitation" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study (NCT02349165). "The unequal sample size in this (non‐double‐masked) study can be considered a limitation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 (6.7%) participants did not complete 1‐year follow‐up (2 lost to follow‐up; 2 protocol deviation). Although intention‐to‐treat analysis was not explicitly reported, the description indicates that outcomes for all participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | All prespecified outcomes were reported in the final report, except the data for demarcation line depth at 6 months. |
| Other bias | High risk | Participants in trans‐CXL group had lower spherical equivalent (P = 0.04) and logMAR UDVA (P = 0.03) at baseline. |
Stojanovic 2014.
| Study characteristics | ||
| Methods |
Study design: intraperson comparative, randomized controlled trial
Number randomized (total and per group): 40 eyes of 20 participants; 20 eyes of 20 participants each group (paired‐eyes design) Unit of randomization (individual or eye): eye (1 eye was randomized to epithelial‐on CXL group, and the fellow eye was assigned to epithelial‐off CXL group) Number analyzed (total and per group): 40 eyes of 20 participants; 20 eyes of 20 participants each group Unit of analysis (individual or eye): eye Exclusions and losses to follow‐up (total and per group): none How were missing data handled?: no missing data Note: endothelial cell count only "available" for 10 participants/20 eyes, with eyes evenly split by design. No imputation was done for this outcome. Length of follow‐up: 12 months Reported power calculation (Y/N), if yes, sample size and power: not reported |
|
| Participants |
Country: Norway Setting: university hospital Baseline characteristics 1. Epithelium‐off CXL, 20 participants (20 eyes)
2. Transepithelial CXL, 20 participants (20 eyes)
Overall, 20 participants (40 eyes)
Inclusion criteria: patients with documented progression of keratoconus during the last 12 months before treatment (increase of astigmatism or myopia by 1.00 D or increase in average simulated keratometry (Sim K) by 1.50 D), minimum corneal thickness of no less than 400 μm at the thinnest point measured by Scheimpflug‐based corneal topo‐/tomography (Precisio, iVIS Technology, Taranto, Italy), Amsler‐Krumeich keratoconus classification stages II to III Exclusion criteria: history of herpes virus keratitis, severe dry eye, concurrent corneal infections, previous ocular surgery, hard contact lens wear ≤ 4 weeks before the baseline examination Baseline equivalence: eye‐level characteristics by intervention were not reported (participant‐level baseline characteristics were identical in studies with a paired‐eyes design, but eye‐level characteristics may not be similar) |
|
| Interventions | 1. Transepithelial CXL
2. Epithelium‐off CXL
|
|
| Outcomes |
Primary outcome: pain, visual acuity and refraction, corneal topography and wavefront aberrations Secondary outcomes: not distinguished Adverse outcomes: no postoperative complications were recorded Measurement time points: 1, 6, and 12 months Other issues with outcome assessment (e.g. quality control for outcomes, if any): participants were included bilaterally, but the analysis did not take into account non‐independence in the intraperson comparative design |
|
| Notes |
Study period: July 2010 to December 2014 Publication language: English Trial registration: NCT01181219 Conflicts of interest: "The authors declare that there is no conflict of interests regarding the publication of this paper" Funding source: "The SynsLaser Surgery AS and the Norwegian Research Council supported this research. No additional external funding was received for this study" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was unclear. "One eye of the patient was randomly chosen to be treated with 'epithelium‐on' CXL and the fellow eye was treated with 'epithelium‐off' CXL. For each patient, the eye with the best CDVA was determined as the 'best eye.' Blocked randomization was used to ensure that each group had an equal number of 'best eyes.'" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment before assignment was not reported. "One eye of the patient was randomly chosen to be treated with 'epithelium‐on' CXL and the fellow eye was treated with 'epithelium‐off' CXL. For each patient, the eye with the best CDVA was determined as the 'best eye.' Blocked randomization was used to ensure that each group had an equal number of 'best eyes.'" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label per clinical trial record (NCT01181219) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label per clinical trial record (NCT01181219) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Specular microscopy was "available" for only 10 participants/20 eyes out of 20 participants/40 eyes. No missing data for all other outcomes, including primary outcomes of BCVA and keratometry. |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the trial registry were reported in the results. |
| Other bias | Unclear risk | Baseline equivalence was unclear because eye‐level characteristics by intervention were not reported (participant‐level baseline characteristics were identical in studies with a paired‐eyes design, but eye‐level characteristics may not be similar). |
BAC: benzylalkonium chloride
BCVA: best‐corrected visual acuity CDVA: corrected distance visual acuity CXL: corneal collagen crosslinking D: diopters
EDTA: ethylenediamine tetraacetic acid HCl: hydrogen chloride
K: keratometry logMAR: logarithm of the minimum angle of resolution
maximum K, or Kmax: maximum keratometry
mean K, or Km: mean keratometry OSDI: ocular surface disease index
SD: standard deviation UDVA, or UCDVA: uncorrected distance visual acuity
UVA: ultraviolet‐A
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akbar 2017 | Not an RCT |
| Bakke 2009 | Not participants of interest |
| Bilgihan 2017 | Not an RCT |
| Bouheraoua 2014 | Not an RCT |
| Buzzonetti 2019 | Not participants of interest |
| Cantemir 2017 | Not an RCT |
| Cassagne 2014 | Preliminary report |
| Eraslan 2017 | Not participants of interest |
| Franch 2015 | Not interventions of interest |
| Godefrooij 2018 | Preliminary report |
| Godefrooij 2019 | Not an RCT |
| Godefrooij 2020 | Not an RCT |
| Henriquez 2017 | Not participants of interest |
| Iqbal 2019 | Not participants of interest |
| IRCT2016112231028N1 | Not the intervention of interest |
| JPRN‐UMIN000009372 | Not an RCT |
| Kopaenko 2018 | Not an RCT |
| Madeira 2019 | Not an RCT |
| Magli 2013 | Not an RCT |
| Mesen 2018 | Not an RCT |
| NCT01868620 | Study was terminated early due to few enrollment |
| NCT03080077 | Not participants of interest |
| Rozema 2013 | Not an RCT |
| Rush 2017 | Not participants of interest |
| Salah 2019 | Not an RCT |
| Serrao 2016 | Preliminary report |
| Spadea 2015 | Not an RCT |
| Spadea 2018 | Not an RCT |
| Touboul 2012 | Not an RCT |
| Vinciguerra 2016 | Not an RCT |
| Vinciguerra 2019 | Not an RCT |
| Yuksel 2015 | Not an RCT |
RCT: randomized controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
ChiCTR1900021768.
| Methods | Parallel‐group, randomized controlled trial |
| Participants | Inclusion criteria: corneal topography showed progressive aggravation of keratoconus, and the maximum corneal curvature increased by more than 1.00 D within 6 to 12 months. The maximum diopter of cornea > 45.00 D, astigmatism > 1.50 D, and corneal thickness > 400 μm. Exclusion criteria: patients who do not meet the inclusion criteria or who suffer from systemic organic diseases |
| Interventions |
|
| Outcomes |
Primary outcome: corneal topography, corneal biomechanics, corneal endothelium count Secondary outcomes: not reported Length of follow‐up: 3 months |
| Notes | Study name: 'Corneal biomechanical stability after epithelium removal and transepithelial corneal collagen crosslinking for keratoconus' |
D: diopters
Characteristics of ongoing studies [ordered by study ID]
NCT03858036.
| Study name | Corneal collagen cross‐linking (CXL) performed with "Epi‐ON" versus "Epi‐OFF" in eyes with keratoconus and other corneal ectatic disorders |
| Methods | Parallel‐group, randomized controlled trial |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes |
Primary outcome: percentage of eyes that had a greater than 2‐diopter increase in Kmax Secondary outcomes: change in refraction percentage of eyes that had a loss of 2 or more lines in BSCVA; change in uncorrected visual acuity (UCVA); change in thinnest pachymetry Length of follow‐up: 12 months |
| Starting date | First posted 28 February 2019; estimated start date 8 March 2019; estimated primary completion date 31 December 2024 |
| Contact information | Jasmine Ly, OD; jly@liangvision.com |
| Notes |
NCT03990506.
| Study name | Photorefractive Intrastromal Crosslinking (PiXL) for the treatment of progressive keratoconus |
| Methods | Parallel‐group, randomized controlled trial |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes |
Primary outcome: uncorrected visual acuity, keratometry readings, Ocular Discomfort Score Secondary outcomes: refraction, spherical equivalent, corneal endothelial cell density Length of follow‐up: 24 months (planned) |
| Starting date | First posted 19 June 2019; estimated starting date 2 April 2019; estimated completion date 2 June 2021 |
| Contact information | Anders Behndig, MD, PhD; anders.behndig@umu.se |
| Notes |
BSCVA: best‐corrected visual acuity
CXL: corneal collagen crosslinking
HIPAA: Health Insurance Portability and Accountability Act
Kmax: maximum keratometry
UV: ultraviolet
Differences between protocol and review
We added one outcome, 'proportion of participants whose keratoconus remained stable (i.e. no progression)', because there was a paucity of data for the prespecified outcomes, and this outcome was used to examine the success of corneal collagen crosslinking. We modified the data elements in the Data extraction and management section to be appropriate for this review topic. One review author (SMN) was added.
Contributions of authors
Conception and design of study: ICK, KL
Assessing the eligibility of relevant studies: ICK, KL, MR
Critically appraising risk of bias and extracting data: ICK, SMN, MR
Conducting qualitative and quantitative data synthesis: ICK, SMN, BSH
Data entry: ICK, SMN
Drafting the review or commenting on it critically for intellectual content: ICK, SMN, BSH
Final approval of the document to be published: ICK, SMN, KL, BSH, MR
Sources of support
Internal sources
No sources of support supplied
External sources
Cochrane Eyes and Vision US Project, supported by grant 1 U01 EY020522, National Eye Institute, National Institutes of Health, USA
-
National Institute for Health Research (NIHR), UK
Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision (CEV), acknowledged financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
The NIHR also funds the CEV Editorial Base in the UK.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
SMN: no financial interests MR: no financial interests KL: no financial interests BSH: no financial interests ICK: no financial interests
Edited (no change to conclusions)
References
References to studies included in this review
Acar 2014 {published data only}
- Acar BT, Utine CA, Ozturk V, Acar S, Ciftci F. Can the effect of transepithelial corneal collagen cross-linking be improved by increasing the duration of topical riboflavin application? An in vivo confocal microscopy study. Eye Contact Lens 2014;40(4):207-12. [DOI: 10.1097/ICL.0000000000000036] [DOI] [PubMed] [Google Scholar]
Al Fayez 2015 {published data only}
- Al Fayez MF, Alfayez S, Alfayez Y. Transepithelial versus epithelium-off corneal collagen cross-linking for progressive keratoconus: a prospective randomized controlled trial. Cornea 2015;34(Suppl 10):S53-6. [DOI: 10.1097/ICO.0000000000000547] [DOI] [PubMed] [Google Scholar]
Al Zubi 2019 {published data only}
- Al Zubi K, Albakar Y, Nasser R. Transepithelial versus epithelium off crosslinking for treating keratoconus among Jordanians. Open Ophthalmology Journal 2019;13(1):8-14. [DOI: 10.2174/1874364101913010008] [DOI] [Google Scholar]
Bikbova 2016 {published data only}
- Bikbova G, Bikbov M. Standard corneal collagen crosslinking versus transepithelial iontophoresis-assisted corneal crosslinking, 24 months follow-up: randomized control trial. Acta Ophthalmologica 2016;94(7):e600-6. [DOI: 10.1111/aos.13032] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02456961. Standard corneal collagen crosslinking versus transepithelial corneal crosslinking by Iontophoresis of Riboflavin [Comparative study of results of standard corneal collagen crosslinking versus transepithelial corneal crosslinking by Iontophoresis of riboflavin: a randomized control trial]. clinicaltrials.gov/ct2/show/NCT02456961 (first received 29 May 2015).
Cifariello 2018 {published data only}
- Cifariello F, Minicucci M, Di Renzo F, Di Taranto D, Coclite G, Zaccaria S, et al. Epi-off versus epi-on corneal collagen cross-linking in keratoconus patients: a comparative study through 2-year follow-up. Journal of Ophthalmology 2018;2018:Article ID 4947983. [DOI: 10.1155/2018/4947983] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03598634. Epi off versus epi on corneal collagen cross-linking in keratoconus patients [Epi off versus epi on corneal collagen cross-linking in keratoconus patients: a comparative study through 2 years follow-up]. clinicaltrials.gov/ct2/show/NCT03598634 (first received 26 July 2018).
Lombardo 2016 {published and unpublished data}
- Lombardo M, Giannini D, Lombardo G, Serrao S. Randomized controlled trial comparing transepithelial corneal cross-linking using iontophoresis with the Dresden protocol in progressive keratoconus. Ophthalmology 2017;124(6):804-12. [DOI: 10.1016/j.ophtha.2017.01.040] [DOI] [PubMed] [Google Scholar]
- Lombardo M, Serrao S, Lombardo G, Schiano-Lomoriello D. Two-year outcomes of a randomized controlled trial of transepithelial corneal crosslinking with iontophoresis for keratoconus. Journal of Cataract and Refractive Surgery 2019;45(7):992-1000. [DOI: 10.1016/j.jcrs.2019.01.026] [DOI] [PubMed] [Google Scholar]
- Lombardo M, Serrao S, Raffa P, Rosati M, Lombardo G. Novel technique of transepithelial corneal cross-linking using iontophoresis in progressive keratoconus. Journal of Ophthalmology 2016;2016:Article ID 7472542. [DOI: 10.1155/2016/7472542] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02117999. Transepithelial corneal cross-linking using iontophoresis. clinicaltrials.gov/ct2/show/NCT02117999 (first received 21 April 2014).
- Serrao S, Lombardo G, Giannini D, Lombardo M. Corneal topography and aberrometry changes one-year after transepithelial corneal cross-linking using iontophoresis versus standard corneal cross-linking. Investigative Ophthalmology and Visual Science 2017;58(8):ARVO E-abstract 3504.
Mastropasqua 2013 {published data only}
- Mastropasqua L, Nubile M, Lanzini M, Calienno R, Mastropasqua R, Agnifili L, et al. Morphological modification of the cornea after standard and transepithelial corneal cross-linking as imaged by anterior segment optical coherence tomography and laser scanning in vivo confocal microscopy. Cornea 2013;32(6):855-61. [DOI: 10.1097/ICO.0b013e3182844c60] [DOI] [PubMed] [Google Scholar]
Nawaz 2015 {published and unpublished data}
- Nawaz S, Gupta S, Gogia V, Sasikala NK, Panda A. Trans-epithelial versus conventional corneal collagen crosslinking: a randomized trial in keratoconus. Oman Journal of Ophthalmology 2015;8(1):9-13. [DOI: 10.4103/0974-620X.149855] [DOI] [PMC free article] [PubMed] [Google Scholar]
Razmjoo 2014 {published and unpublished data}
- Razmjoo H, Rahimi B, Kharraji M, Koosha N, Peyman A. Corneal haze and visual outcome after collagen crosslinking for keratoconus: a comparison between total epithelium off and partial epithelial removal methods. Advanced Biomedical Research 2014;3:221. [DOI: 10.4103/2277-9175.145677] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossi 2015 {published and unpublished data}
- Rossi S, Orrico A, Santamaria C, Romano V, De Rosa L, Simonelli F, et al. Standard versus trans-epithelial collagen cross-linking in keratoconus patients suitable for standard collagen cross-linking. Clinical Ophthalmology 2015;9:503-9. [DOI: 10.2147/OPTH.S73991] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossi 2018 {published and unpublished data}
- Rossi S, Santamaria C, Boccia R, De Rosa L, D'Alterio FM, Simonelli F, et al. Standard, transepithelial and iontophoresis corneal cross-linking: clinical analysis of three surgical techniques. International Ophthalmology 2018;38(6):2585-92. [DOI: 10.1007/s10792-017-0772-3] [DOI] [PubMed] [Google Scholar]
Soeters 2015 {published and unpublished data}
- Godefrooij DA, El Kandoussi M, Soeters N, Wisse RPL. Higher order optical aberrations and visual acuity in a randomized controlled trial comparing transepithelial versus epithelium-off corneal crosslinking for progressive keratoconus. Clinical Ophthalmology 2017;11:1931-6. [DOI: 10.2147/OPTH.S139358] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02349165. Standard versus transepithelial corneal crosslinking. clinicaltrials.gov/ct2/show/NCT02349165 (first received 28 January 2015).
- Soeters N, Wisse RP, Godefrooij DA, Imhof SM, Tahzib NG. Transepithelial versus epithelium-off corneal cross-linking for the treatment of progressive keratoconus: a randomized controlled trial. American Journal of Ophthalmology 2015;159(5):821-8. [DOI: 10.1016/j.ajo.2015.02.005] [DOI] [PubMed] [Google Scholar]
Stojanovic 2014 {published data only}
- NCT01181219. Transepithelial corneal collagen cross-linking (CXL) in treatment of keratoconus [Behandling av keratoconus med "cornea collagen cross-linking" uten hornhinneepitelfjerning]. clinicaltrials.gov/ct2/show/NCT01181219 (first received 13 August 2010).
- Stojanovic A, Zhou W, Utheim TP. Corneal collagen cross-linking with and without epithelial removal: a contralateral study with 0.5% hypotonic riboflavin solution. BioMed Research International 2014;2014:Article ID 619398. [DOI: 10.1155/2014/619398] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Akbar 2017 {published data only}
- Akbar B, Intisar-Ul-Haq R, Ishaq M, Fawad A, Arzoo S, Siddique K. Comparison of transepithelial corneal crosslinking with epithelium-off crosslinking (epithelium-off CXL) in adult Pakistani population with progressive keratoconus. Taiwan Journal of Ophthalmology 2017;7(4):185-90. [DOI: 10.4103/tjo.tjo_38_17] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bakke 2009 {published data only}
- Bakke EF, Stojanovic A, Chen X, Drolsum L. Penetration of riboflavin and postoperative pain in corneal collagen crosslinking: excimer laser superficial versus mechanical full-thickness epithelial removal. Journal of Cataract and Refractive Surgery 2009;35(8):1363-6. [DOI: 10.1016/j.jcrs.2009.03.023] [DOI] [PubMed] [Google Scholar]
Bilgihan 2017 {published data only}
- Bilgihan K, Yesilirmak N, Altay Y, Yuvarlak A, Ozdemir HB. Conventional corneal collagen cross-linking versus transepithelial diluted alcohol and iontophoresis-assisted corneal cross-linking in progressive keratoconus. Cornea 2017;36(12):1492-7. [DOI: 10.1097/ICO.0000000000001383] [DOI] [PubMed] [Google Scholar]
Bouheraoua 2014 {published data only}
- Bouheraoua N, Jouve L, El Sanharawi M, Sandali O, Temstet C, Loriaut P, et al. Optical coherence tomography and confocal microscopy following three different protocols of corneal collagen-crosslinking in keratoconus. Investigative Ophthalmology and Visual Science 2014;55(11):7601-9. [DOI: 10.1167/iovs.14-15662] [DOI] [PubMed] [Google Scholar]
Buzzonetti 2019 {published data only}
- Buzzonetti L, Petrocelli G, Valente P, Iarossi G, Ardia R, Petroni S, et al. Iontophoretic transepithelial collagen cross-linking versus epithelium-off collagen cross-linking in pediatric patients: 3-year follow-up. Cornea 2019;38(7):859-63. [DOI: 10.1097/ICO.0000000000001965] [DOI] [PubMed] [Google Scholar]
Cantemir 2017 {published data only}
- Cantemir A, Alexa AI, Galan BG, Anton N, Ciuntu RE, Danielescu C, et al. Iontophoretic collagen cross-linking versus epithelium-off collagen cross-linking for early stage of progressive keratoconus—3 years follow-up study. Acta Ophthalmologica 2017;95(7):e649-55. [DOI: 10.1111/aos.13538] [DOI] [PubMed] [Google Scholar]
Cassagne 2014 {published data only}
- Cassagne M, Delafoy I, Mesplie N, Fournie P, Cochener B, Malecaze F. Transepithelial corneal collagen crosslinking using iontophoresis: preliminary clinical results. Investigative Ophthalmology and Visual Science 2014;55(13):ARVO E-abstract 4216.
Eraslan 2017 {published data only}
- Eraslan M, Toker E, Cerman E, Ozarslan D. Efficacy of epithelium-off and epithelium-on corneal collagen cross-linking in pediatric keratoconus. Eye and Contact Lens 2017;43(3):155-61. [DOI: 10.1097/ICL.0000000000000255] [DOI] [PubMed] [Google Scholar]
Franch 2015 {published data only}
- Franch A, Birattari F, Dal Mas G, Luznik Z, Parekh M, Ferrari S, et al. Evaluation of intrastromal riboflavin concentration in human corneas after three corneal cross-linking imbibition procedures: a pilot study. Journal of Ophthalmology 2015;2015:Article ID 794256. [DOI: 10.1155/2015/794256] [DOI] [PMC free article] [PubMed] [Google Scholar]
Godefrooij 2018 {published data only}
- Godefrooij DA, El Kandoussi M, Soeters N, Wisse RPL. Optical aberrations and their relationship with visual acuity after crosslinking. Acta Ophthalmologica 2018;96:6. [DOI: 10.1111/aos.13736] [DOI] [Google Scholar]
Godefrooij 2019 {published data only}
- Godefrooij DA, Roohe SL, Soeters N, Wisse RP. The independent effect of various crosslinking treatment modalities on treatment effectiveness in keratoconus. Acta Ophthalmologica 2019;97:4-5. [DOI: 10.1111/aos.14061] [DOI] [Google Scholar]
Godefrooij 2020 {published data only}
- Godefrooij DA, Roohé SL, Soeters N, Wisse RP. The independent effect of various cross-linking treatment modalities on treatment effectiveness in keratoconus. Cornea 2020;39(1):63-70. [DOI: 10.1097/ICO.0000000000002168] [DOI] [PubMed] [Google Scholar]
Henriquez 2017 {published data only}
- Henriquez MA, Rodriguez AM, Izquierdo L Jr. Accelerated epi-on versus standard epi-off corneal collagen cross-linking for progressive keratoconus in pediatric patients. Cornea 2017;36(12):1503-8. [DOI: 10.1097/ICO.0000000000001366] [DOI] [PubMed] [Google Scholar]
Iqbal 2019 {published data only}
- Iqbal M, Elmassry A, Saad H, Am Gad A, Ibrahim O, Hamed N, et al. Standard cross-linking protocol versus accelerated and transepithelial cross-linking protocols for treatment of paediatric keratoconus: a 2-year comparative study. Acta Ophthalmologica 2020;98(3):e352-62. [DOI: 10.1111/aos.14275] [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT2016112231028N1 {published data only}
- IRCT2016112231028N1. Comparison of effects and complications of collagen cross-linking surgery with 2 methods of epithelium removal and trans epithelial with using DAYA epithelium disruptor: a randomized-controlled clinical trial. en.irct.ir/trial/24481 (first received 29 March 2017).
JPRN‐UMIN000009372 {published data only}
- JPRN-UMIN000009372. Clinical trial of corneal crosslinking for keratoconus and keratoectasia. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000009372 (first received 21 November 2012).
Kopaenko 2018 {published data only}
- Kopaenko AI, Ivanova NV. Transepithelial corneal collagen crosslinking in patients with progressive keratoconus. Vestnik Oftalmologii 2018;134(2):42-7. [DOI: 10.17116/oftalma2018134242-47] [DOI] [PubMed] [Google Scholar]
Madeira 2019 {published data only}
- Madeira C, Vasques A, Beato J, Godinho G, Torrao L, Falcao M, et al. Transepithelial accelerated versus conventional corneal collagen crosslinking in patients with keratoconus: a comparative study. Clinical Ophthalmology 2019;13:445-52. [DOI: 10.2147/OPTH.S189183] [DOI] [PMC free article] [PubMed] [Google Scholar]
Magli 2013 {published data only}
- Magli A, Forte R, Tortori A, Capasso L, Marsico G, Piozzi E, et al. Epithelium-off corneal collagen cross-linking versus transepithelial cross-linking for pediatric keratoconus. Cornea 2013;32(5):597-601. [DOI: 10.1097/ICO.0b013e31826cf32d] [DOI] [PubMed] [Google Scholar]
Mesen 2018 {published data only}
- Mesen A, Bozkurt B, Kamis U, Okudan S. Correlation of demarcation line depth with medium-term efficacy of different corneal collagen cross-linking protocols in keratoconus. Cornea 2018;37(12):1511-6. [DOI: 10.1097/ICO.0000000000001733] [DOI] [PubMed] [Google Scholar]
NCT01868620 {published data only}
- NCT01868620. Non-inferiority trial of iontophoretic corneal collagen crosslinking (CXL) compared to standard corneal collagen crosslinking in progressive keratoconus. clinicaltrials.gov/show/NCT01868620 (first received 4 June 2013).
NCT03080077 {published data only}
- NCT03080077. Safety and effectiveness of corneal crosslinking (CXL): keratoconus and post-refractive ectasia. clinicaltrials.gov/show/NCT03080077 (first received 15 March 2017).
Rozema 2013 {published data only}
- Rozema JJ, Koppen C, Bral N, Tassignon MJ. Changes in forward and backward light scatter in keratoconus resulting from corneal cross-linking. Asia-Pacific Journal of Ophthalmology 2013;2(1):15-9. [DOI: 10.1097/APO.0b013e3182729df0] [DOI] [PubMed] [Google Scholar]
Rush 2017 {published data only}
- Rush SW, Rush RB. Epithelium-off versus transepithelial corneal collagen crosslinking for progressive corneal ectasia: a randomised and controlled trial. British Journal of Ophthalmology 2017;101(4):503-8. [DOI: 10.1136/bjophthalmol-2016-308914] [DOI] [PubMed] [Google Scholar]
Salah 2019 {published data only}
- Salah Y, Omar K, Sherif A, Azzam S. Study of demarcation line depth in transepithelial versus epithelium-off accelerated cross-linking (AXL) in keratoconus. Journal of Ophthalmology 2019;2019:Article ID 3904565. [DOI: 10.1155/2019/3904565] [DOI] [PMC free article] [PubMed] [Google Scholar]
Serrao 2016 {published data only}
- Serrao S, Lombardo G, Rosati M, Lomoriello DS, Lombardo M. Preliminary results of a randomized controlled trial comparing transepithelial corneal cross-linking with iontophoresis and standard cross-linking in patients with progressive keratoconus. Investigative Ophthalmology and Visual Science 2016;57(12):ARVO E-abstract 2892.
Spadea 2015 {published data only}
- Spadea L, Salvatore S, Paroli MP, Vingolo EM. Recovery of corneal sensitivity after collagen crosslinking with and without epithelial debridement in eyes with keratoconus. Journal of Cataract and Refractive Surgery 2015;41(3):527-32. [DOI: 10.1016/j.jcrs.2014.06.030] [DOI] [PubMed] [Google Scholar]
Spadea 2018 {published data only}
- Spadea L, Di Genova L, Tonti E. Corneal stromal demarcation line after 4 protocols of corneal crosslinking in keratoconus determined with anterior segment optical coherence tomography. Journal of Cataract and Refractive Surgery 2018;44(5):596-602. [DOI: 10.1016/j.jcrs.2018.02.017] [DOI] [PubMed] [Google Scholar]
Touboul 2012 {published data only}
- Touboul D, Efron N, Smadja D, Praud D, Malet F, Colin J. Corneal confocal microscopy following conventional, transepithelial, and accelerated corneal collagen cross-linking procedures for keratoconus. Journal of Refractive Surgery 2012;28(11):769-76. [DOI: 10.3928/1081597X-20121016-01] [DOI] [PubMed] [Google Scholar]
Vinciguerra 2016 {published data only}
- Vinciguerra P, Romano V, Rosetta P, Legrottaglie EF, Piscopo R, Fabiani C, et al. Transepithelial iontophoresis versus standard corneal collagen cross-linking: 1-year results of a prospective clinical study. Journal of Refractive Surgery 2016;32(10):672-8. [DOI: 10.3928/1081597X-20160629-02] [DOI] [PubMed] [Google Scholar]
Vinciguerra 2019 {published data only}
- Vinciguerra P, Rosetta P, Legrottaglie EF, Morenghi E, Mazzotta C, Kaye S, et al. Iontophoresis CXL with and without epithelial debridement versus standard CXL: 2-year clinical results of a prospective clinical study. Journal of Refractive Surgery 2019;35(3):184-90. [DOI: 10.3928/1081597X-20190128-01] [DOI] [PubMed] [Google Scholar]
Yuksel 2015 {published data only}
- Yuksel E, Novruzlu S, Ozmen MC, Bilgihan K. A study comparing standard and transepithelial collagen cross-linking riboflavin solutions: epithelial findings and pain scores. Journal of Ocular Pharmacology and Therapeutics 2015;31(5):296-302. [DOI: 10.1089/jop.2014.0090] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ChiCTR1900021768 {published data only}
- ChiCTR1900021768. Corneal biomechanical stability after epithelium removal and transepithelial corneal collagen crosslinking for keratoconus. www.chictr.org.cn/com/25/showproj.aspx?proj=36716 (first received 8 March 2019).
References to ongoing studies
NCT03858036 {published data only}
- NCT03858036. Corneal collagen cross-linking (CXL) performed with "Epi-ON" versus "Epi-OFF" in eyes with keratoconus and other corneal ectatic disorders [Comparison of corneal collagen cross-linking (CXL) performed with "Epi-ON" versus "Epi-OFF" in eyes with keratoconus and other corneal ectatic disorders]. clinicaltrials.gov/ct2/show/NCT03858036 (first received 28 February 2019).
NCT03990506 {published data only}
- NCT03990506. Photorefractive intrastromal crosslinking (PiXL) for the treatment of progressive keratoconus [Comparison of epi-off and epi-on photorefractive intrastromal crosslinking (PiXL) for progressive keratoconus]. clinicaltrials.gov/ct2/show/NCT03990506 (first received 19 June 2019).
Additional references
Abelson 1980
- Abelson MB, Collin HB, Gillette TE, Dohlman CH. Recurrent keratoconus after keratoplasty. American Journal of Ophthalmology 1980;90(5):672-6. [DOI] [PubMed] [Google Scholar]
Akhtar 2008
- Akhtar S, Bron AJ, Salvi SM, Hawkesworth NR, Tuft SJ, Meek KM. Ultrastructural analysis of collagen fibrils and proteoglycans in keratoconus. Acta Ophthalmologica 2008;86(7):764-72. [DOI] [PubMed] [Google Scholar]
Alio 2018
- Alio JL, Vega-Estrada A, Sanz P, Osman AA, Kamal AM, Mamoon A, et al. Corneal morphologic characteristics in patients with Down syndrome. JAMA Ophthalmology 2018;136(9):971-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Asri 2011
- Asri D, Touboul D, Fournié P, Malet F, Garra C, Gallois A, et al. Corneal collagen crosslinking in progressive keratoconus: multicenter results from the French national reference center for keratoconus. Journal of Cataract and Refractive Surgery 2011;37(12):2137-43. [DOI] [PubMed] [Google Scholar]
Buzzonetti 2015
- Buzzonetti L, Petrocelli G, Valente P, Iarossi G, Ardia R, Petroni S. Iontophoretic transepithelial corneal cross-linking to halt keratoconus in pediatric cases: 15-month follow-up. Cornea 2015;34(5):512-5. [DOI] [PubMed] [Google Scholar]
Caporossi 2010
- Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. American Journal of Ophthalmology 2010;149(4):585-93. [DOI] [PubMed] [Google Scholar]
Caporossi 2012
- Caporossi A, Mazzotta C, Baiocchi S, Caporossi T, Paradiso AL. Transepithelial corneal collagen crosslinking for keratoconus: qualitative investigation by in vivo HRT II confocal analysis. European Journal of Ophthalmology 2012;22(Suppl 7):S81-8. [DOI] [PubMed] [Google Scholar]
Caporossi 2013
- Caporossi A, Mazzotta C, Paradiso AL, Baiocchi S, Marigliani D, Caporossi T. Transepithelial corneal collagen crosslinking for progressive keratoconus: 24-month clinical results. Journal of Cataract and Refractive Surgery 2013;39(8):1157-63. [DOI] [PubMed] [Google Scholar]
Cavas‐Martínez 2016
- Cavas-Martínez F, De la Cruz Sánchez E, Nieto Martínez J, Fernández Cañavate FJ, Fernández-Pacheco DG. Corneal topography in keratoconus: state of the art. Eye and Vision 2016;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Çerman 2015
- Çerman E, Toker E, Ozarslan Ozcan D. Transepithelial versus epithelium-off crosslinking in adults with progressive keratoconus. Journal of Cataract and Refractive Surgery 2015;41(7):1416-25. [DOI] [PubMed] [Google Scholar]
Chan 2007
- Chan CC, Sharma M, Boxer BS. Effect of inferior segment Intacs with and without C3R on keratoconus. Journal of Cataract and Refractive Surgery 2007;33(1):75-80. [DOI] [PubMed] [Google Scholar]
Clearfield 2017
- Clearfield E, Hawkins BS, Kuo IC. Conjunctival autograft versus amniotic membrane transplantation for treatment of pterygium: findings from a Cochrane Systematic Review. American Journal of Ophthalmology 2017;182:8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 15 October 2019. Available at covidence.org.
Cozma 2005
- Cozma I, Atherley C, James NJ. Influence of ethnic origin on the incidence of keratoconus and associated atopic disease in Asian and white patients. Eye 2005;19(8):924-5. [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JPT, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2/.
Doors 2009
- Doors M, Tahzib NG, Eggink FA, Berendschot TT, Webers CA, Nuijts RM. Use of anterior segment optical coherence tomography to study corneal changes after collagen cross-linking. American Journal of Ophthalmology 2009;148(6):844-51. [DOI] [PubMed] [Google Scholar]
Elder 1994
- Elder MJ. Leber congenital amaurosis and its association with keratoconus and keratoglobus. Journal of Pediatric Ophthalmology and Strabismus 1994;31(1):38-40. [DOI] [PubMed] [Google Scholar]
ETDRS 1985
- Early Treatment Diabetic Retinopathy Study (ETDRS) research group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Archives of Ophthalmology 1985;103(12):1796-806. [PubMed] [Google Scholar]
Filippello 2012
- Filippello M, Stagni E, O’Brart D. Transepithelial corneal collagen crosslinking: bilateral study. Journal of Cataract and Refractive Surgery 2012;38(2):283-91. [DOI] [PubMed] [Google Scholar]
Georgiou 2004
- Georgiou T, Funnell CL, Cassels-Brown A, O’Conor R. Influence of ethnic origin on the incidence of keratoconus and associated atopic disease in Asians and white patients. Eye 2004;18(4):379-83. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130-6. [PMC free article] [PubMed] [Google Scholar]
Godefrooij 2016
- Godefrooij DA, Gans R, Imhof SM, Wisse RPL. Nationwide reduction in the number of corneal transplantations for keratoconus following the implementation of cross-linking. Acta Ophthalmologica 2016;94(7):675-8. [DOI] [PubMed] [Google Scholar]
Godefrooij 2017a
- Godefrooij DA, Wit GA, Uiterwaal CS, Imhof SM, Wisse RP. Age-specific incidence and prevalence of keratoconus: a nationwide registration study. American Journal of Ophthalmology 2017;175:169-72. [DOI] [PubMed] [Google Scholar]
Godefrooij 2017b
- Godefrooij DA, Mangen MJ, Chan E, O'Brart DPS, Imhof SM, Wit GA, et al. Cost-effectiveness analysis of corneal collagen crosslinking for progressive keratoconus. Ophthalmology 2017;124(10):1485-95. [DOI] [PubMed] [Google Scholar]
Godel 1978
- Godel V, Blumenthal M, Iaina A. Congenital Leber amaurosis, keratoconus, and mental retardation in familial juvenile nephronophtisis. Journal of Pediatric Ophthalmology and Strabismus 1978;15(2):89-91. [DOI] [PubMed] [Google Scholar]
Gordon 2006
- Gordon MO, Steger-May K, Szczotka-Flynn L, Riley C, Joslin CE, Weissman BA, et al. Baseline factors predictive of incident penetrating keratoplasty in keratoconus. American Journal of Ophthalmology 2006;142(6):923-30. [DOI] [PubMed] [Google Scholar]
Greenstein 2010
- Greenstein SA, Fry KL, Bhatt J, Hersh PS. Natural history of corneal haze after collagen crosslinking for keratoconus and corneal ectasia: Scheimpflug and biomicroscopic analysis. Journal of Cataract and Refractive Surgery 2010;36(12):2105-14. [DOI] [PubMed] [Google Scholar]
Greenstein 2013
- Greenstein SA, Hersh PS. Characteristics influencing outcomes of corneal collagen crosslinking for keratoconus and ectasia: implications for patient selection. Journal of Cataract and Refractive Surgery 2013;39(8):1133-40. [DOI] [PubMed] [Google Scholar]
Haberman 2018
- Haberman ID, Lang PZ, Broncano AF, Kim SW, Hafezi F, Randleman JB. Epithelial remodeling after corneal crosslinking using higher fluence and accelerated treatment time. Journal of Cataract and Refractive Surgery 2018;44(3):306-12. [DOI] [PubMed] [Google Scholar]
Hayes 2008
- Hayes S, O’Brart DP, Lamdin LS, Doutch J, Samaras K, Marshall J, et al. Effect of complete epithelial debridement before riboflavin-ultraviolet-A corneal collagen cross-linking therapy. Journal of Cataract and Refractive Surgery 2008;34(4):657-61. [DOI] [PubMed] [Google Scholar]
Hersh 2011
- Hersh PS, Greenstein SA, Fry KL. Corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. Journal of Cataract and Refractive Surgery 2011;37(1):149-60. [DOI] [PubMed] [Google Scholar]
Hersh 2017
- Hersh PS, Stulting RD, Muller D, Durrie DS, Rajpal RK, United States Crosslinking Study Group. United States Multicenter Clinical trial of corneal collagen crosslinking for keratoconus treatment. Ophthalmology 2017;124(9):1259-70. [DOI] [PubMed] [Google Scholar]
Hersh 2018
- Hersh PS, Lai MJ, Gelles JD, Lesniak SP. Transepithelial corneal crosslinking for keratoconus. Journal of Cataract and Refractive Surgery 2018;44(3):313-22. [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2/.
Kamaev 2012
- Kamaev P, Friedman MD, Sherr E, Muller D. Photochemical kinetics of corneal cross-linking with riboflavin. Investigative Ophthalmology & Visual Science 2012 Apr 30;53(4):2360-7. [DOI] [PubMed] [Google Scholar]
Kanellopoulos 2009
- Kanellopoulos AJ. Collagen cross-linking in early keratoconus with riboflavin in a femtosecond laser-created pocket: initial clinical results. Journal of Refractive Surgery 2009 Nov;25(11):1034-7. [DOI] [PubMed] [Google Scholar]
Kennedy 1986
- Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. American Journal of Ophthalmology 1986;101(3):267-73. [DOI] [PubMed] [Google Scholar]
Khairy 2014
- Khairy HA, Marey HM, Ellakwa AF. Epithelium-on corneal crosslinking treatment of progressive keratoconus: a prospective, consecutive study. Clinical Ophthalmology 2014;8:819-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knox Cartwright 2011
- Knox Cartwright NE, Tyrer JR, Marshall J. Age-related differences in the elasticity of the human cornea. Investigative Ophthalmology and Visual Science 2011;52(7):4324-9. [DOI] [PubMed] [Google Scholar]
Kobashi 2018
- Kobashi H, Rong SS, Ciolino JB. Transepithelial versus epithelium-off corneal crosslinking for corneal ectasia. Journal of Cataract and Refractive Surgery 2018;44(12):1507-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kocak 2014
- Kocak I, Aydin A, Kaya F, Koc H. Comparison of transepithelial corneal collagen crosslinking with epithelium-off crosslinking in progressive keratoconus. Journal Francais d'Ophtalmologie 2014;37(5):371-6. [DOI] [PubMed] [Google Scholar]
Koppen 2012
- Koppen C, Wouters K, Mathysen D, Rozema J, Tassignon MJ. Refractive and topographic results of benzalkonium chloride-assisted transepithelial crosslinking. Journal of Cataract and Refractive Surgery 2012;38(6):1000-5. [DOI] [PubMed] [Google Scholar]
Kuo 1997
- Kuo IC, Seitz B, LaBree L, McDonnell P. Can zinc prevent apoptosis of anterior keratocytes after superficial keratectomy? Cornea 1997;16(5):550-5. [PubMed] [Google Scholar]
Kymionis 2014
- Kymionis GD, Grentzelos MA, Kankariya VP, Liakopoulos DA, Portaliou DM, Tsoulnaras KI, et al. Safety of high-intensity corneal collagen crosslinking. Journal of Cataract and Refractive Surgery 2014;40(8):1337-40. [DOI] [PubMed] [Google Scholar]
Kymionis 2017
- Kymionis GD, Kontadakis GA, Hashemi KK. Accelerated versus conventional corneal crosslinking for refractive instability: an update. Current Opinion in Ophthalmology 2017;28(4):343-7. [DOI] [PubMed] [Google Scholar]
Leccisotti 2010
- Leccisotti A, Islam T. Transepithelial corneal collagen cross-linking in keratoconus. Journal of Refractive Surgery 2010;26(12):942-8. [DOI] [PubMed] [Google Scholar]
Leung 2017
- Leung VC, Pechlivanoglou P, Chew HF, Hatch W. Corneal collagen cross-linking in the management of keratoconus in Canada: a cost-effectiveness analysis. Ophthalmology 2017;124(8):1108-19. [DOI] [PubMed] [Google Scholar]
Li 2017
- Li W, Wang B. Efficacy and safety of transepithelial corneal collagen crosslinking surgery versus standard corneal collagen crosslinking surgery for keratoconus: a meta-analysis of randomized controlled trials. BMC Ophthalmology 2017;17(1):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazzotta 2007
- Mazzotta C, Balestrazzi A, Baiocchi S, Traversi C, Caporossi A. Stromal haze after combined riboflavin-UVA corneal collagen cross-linking in keratoconus: in vivo confocal microscopic evaluation. Clinical and Experimental Ophthalmology 2007;35(6):580-2. [DOI] [PubMed] [Google Scholar]
Mazzotta 2014
- Mazzotta C, Traversi C, Paradiso AL, Latronico ME, Rechichi M. Pulsed light accelerated crosslinking versus continuous light accelerated crosslinking: one-year results. Journal of Ophthalmology 2014;2014:604731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Medeiros 2016
- Medeiros CS, Giacomin NT, Bueno RL, Ghanem RC, Moraes HV Jr, Santhiago MR. Accelerated corneal collagen crosslinking: technique, efficacy, safety, and applications. Journal of Cataract and Refractive Surgery 2016;42(12):1826-35. [DOI] [PubMed] [Google Scholar]
Meek 2005
- Meek KM, Tuft SJ, Huang Y, Gill PS, Hayes S, Newton RH, et al. Changes in collagen orientation and distribution in keratoconus corneas. Investigative Ophthalmology and Visual Science 2005;46(6):1948-56. [DOI] [PubMed] [Google Scholar]
NICE 2019
- National Institute for Health and Care Excellence. Photochemical corneal collagen cross‑linkage using riboflavin and ultraviolet A for keratoconus and keratectasia. www.nice.org.uk/guidance/ipg466 (accessed 15 October 2019).
O'Brart 2013
- O'Brart DP, Kwong TQ, Patel P, McDonald RJ, O'Brart NA. Long-term follow-up of riboflavin/ultraviolet A (370 nm) corneal collagen cross-linking to halt the progression of keratoconus. British Journal of Ophthalmology 2013;97(4):433-7. [DOI] [PubMed] [Google Scholar]
O'Brart 2015
- O'Brart DP, Patel P, Lascaratos G, Wagh VK, Tam C, Lee J, et al. Corneal cross-linking to halt the progression of keratoconus and corneal ectasia: seven-year follow-up. American Journal of Ophthalmology 2015;160(6):1154-63. [DOI] [PubMed] [Google Scholar]
Pearson 2000
- Pearson AR, Soneji B, Sarvananthan N, Sandford-Smith JH. Does ethnic origin influence the incidence or severity of keratoconus? Eye 2000;14(Pt 4):625-8. [DOI] [PubMed] [Google Scholar]
Podskochy 2004
- Podskochy A. Protective role of corneal epithelium against ultraviolet radiation damage. Acta Ophthalmologica 2004;82(6):714-7. [DOI] [PubMed] [Google Scholar]
Poli 2015
- Poli M, Lefevre A, Auxenfans C, Burillon C. Corneal collagen cross-linking for the treatment of progressive corneal ectasia: 6-year prospective outcome in a French population. American Journal of Ophthalmology 2015;160(4):654-62. [DOI] [PubMed] [Google Scholar]
Price 2016
- Price MO, Price DA, Bucci FA Jr, Durrie DS, Bond WI, Price FW Jr. Three-year longitudinal survey comparing visual satisfaction with LASIK and contact lenses. Ophthalmology 2016;123(8):1659-66. [DOI] [PubMed] [Google Scholar]
Price 2018a
- Price MO, Feng MT, Price FW Jr. Patient satisfaction with epithelium-off corneal crosslinking. Journal of Cataract and Refractive Surgery 2018;44(3):323-8. [DOI] [PubMed] [Google Scholar]
Price 2018b
- Price MO, Fairchild K, Feng MT, Price FW Jr. Prospective randomized trial of corneal cross-linking riboflavin dosing frequencies for treatment of keratoconus and corneal ectasia. Ophthalmology 2018;125(4):505-11. [DOI] [PubMed] [Google Scholar]
Rabinowitz 1990
- Rabinowitz YS, Garbus J, McDonnell PJ. Computer-assisted corneal topography in family members of patients with keratoconus. Archives of Ophthalmology 1990;108(3):365-71. [DOI] [PubMed] [Google Scholar]
Rabinowitz 1998
- Rabinowitz YS. Keratoconus. Survey of Ophthalmology 1998;42(4):297-319. [DOI] [PubMed] [Google Scholar]
Rados 1948
- Rados A. Conical cornea and mongolism. Archives of Ophthalmology 1948;40(4):454-78. [DOI] [PubMed] [Google Scholar]
Raiskup 2015
- Raiskup F, Theuring A, Pillunat LE, Spoerl E. Corneal collagen crosslinking with riboflavin and ultraviolet-A light in progressive keratoconus: ten-year results. Journal of Cataract and Refractive Surgery 2015;41(1):41-6. [DOI] [PubMed] [Google Scholar]
Raiskup‐Wolf 2008
- Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen cross-linking with riboflavin and ultraviolet-A light in keratoconus: long-term results. Journal of Cataract and Refractive Surgery 2008;34(3):796-801. [DOI] [PubMed] [Google Scholar]
Rebenitsch 2011
- Rebenitsch RL, Kymes SM, Walline JJ, Gordon MO. The lifetime economic burden of keratoconus: a decision analysis using a Markov model. American Journal of Ophthalmology 2011;151(5):768-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rosenblat 2016
- Rosenblat E, Hersh PS. Intraoperative corneal thickness change and clinical outcomes after corneal collagen crosslinking: standard crosslinking versus hypotonic riboflavin. Journal of Cataract & Refractive Surgery 2016;42(4):596-605. [DOI] [PubMed] [Google Scholar]
Sandvik 2015
- Sandvik GF, Thorsrud A, Råen M, Østern AE, Sæthre M, Drolsum L. Does corneal collagen cross-linking reduce the need for keratoplasties in patients with keratoconus? Cornea 2015;34(9):991-5. [DOI] [PubMed] [Google Scholar]
Seiler 2006
- Seiler T, Hafezi F. Corneal cross-linking induced stromal demarcation line. Cornea 2006;25(9):1057-9. [DOI] [PubMed] [Google Scholar]
Shalchi 2015
- Shalchi Z, Wang X, Nanavaty MA. Safety and efficacy of epithelium removal and transepithelial corneal collagen crosslinking for keratoconus. Eye 2015;29(1):15-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinhab 2014
- Sinhab MM. Five Steps to Start Your Refractive Surgery: a Case-Based Systematic Approach. 1st edition. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd, 2014. [Google Scholar]
Spadea 2012
- Spadea L, Mencucci R. Transepithelial corneal collagen cross-linking in ultrathin keratoconic corneas. Clinical Ophthalmology 2012;6:1785-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spoerl 1998
- Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Experimental Eye Research 1998;66(1):97-103. [DOI] [PubMed] [Google Scholar]
Spoerl 1999
- Spoerl E, Seiler T. Techniques for stiffening the cornea. Journal of Refractive Surgery 1999;15(6):711-3. [DOI] [PubMed] [Google Scholar]
Spoerl 2004a
- Spoerl E, Wollensak G, Seiler T. Increased resistance of crosslinked cornea against enzymatic digestion. Current Eye Research 2004;29(1):35-40. [DOI] [PubMed] [Google Scholar]
Spoerl 2004b
- Spoerl E, Wollensak G, Dittert DD, Seiler T. Thermomechanical behaviour of collagen cross-linked porcine cornea. Ophthalmologica 2004;218(2):136-40. [DOI] [PubMed] [Google Scholar]
Stojanovic 2012
- Stojanovic A, Chen X, Jin N, Zhang T, Stojanovic F, Raeder S, et al. Safety and efficacy of epithelium-on corneal collagen cross-linking using a multifactorial approach to achieve proper stromal riboflavin saturation. Journal of Ophthalmology 2012;2012:Article ID 498435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toker 2017
- Toker E, Çerman E, Özcan DÖ, Seferoğlu ÖB. Efficacy of different accelerated corneal crosslinking protocols for progressive keratoconus. Journal of Cataract and Refractive Surgery 2017;43(8):1089-99. [DOI] [PubMed] [Google Scholar]
Tuft 1994
- Tuft SJ, Moodaley LC, Gregory WM, Davison CR, Buckley RJ. Prognostic factors for the progression of keratoconus. Ophthalmology 1994;101(3):439-47. [DOI] [PubMed] [Google Scholar]
Wen 2018
- Wen D, Song B, Li Q, Tu R, Huang Y, Wang Q, et al. Comparison of epithelium-off versus transepithelial corneal collagen cross-linking for keratoconus: a systematic review and meta-analysis. Cornea 2018;37(8):1018-24. [DOI] [PubMed] [Google Scholar]
Wittig‐Silva 2008
- Wittig-Silva C, Whiting M, Lamoureux E, Lindsay RG, Sullivan LJ, Snibson GR. A randomized controlled trial of corneal collagen cross-linking in progressive keratoconus: preliminary results. Journal of Refractive Surgery 2008;24(7):S720-5. [DOI] [PubMed] [Google Scholar]
Wittig‐Silva 2014
- Wittig-Silva C, Chan E, Islam FM, Wu T, Whiting M, Snibson GR. A randomized, controlled trial of corneal collagen cross-linking in progressive keratoconus: three-year results. Ophthalmology 2014;121(4):812-21. [DOI] [PubMed] [Google Scholar]
Wollensak 2003a
- Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen cross-linking for the treatment of keratoconus. American Journal of Ophthalmology 2003;135(5):620-7. [DOI] [PubMed] [Google Scholar]
Wollensak 2003b
- Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. Journal of Cataract and Refractive Surgery 2003;29(9):1780-5. [DOI] [PubMed] [Google Scholar]
Wollensak 2007
- Wollensak G, Iomdina E, Dittert DD, Herbst H. Wound healing in the rabbit cornea after corneal cross-linking with riboflavin and UVA. Cornea 2007;26(5):600-5. [DOI] [PubMed] [Google Scholar]
Wollensak 2009
- Wollensak G, Iomdina E. Biomechanical and histological changes after corneal crosslinking with and without epithelial debridement. Journal of Cataract and Refractive Surgery 2009;35(3):540-6. [DOI] [PubMed] [Google Scholar]
Zadnik 1998
- Zadnik K, Barr JT, Edrington TB, Everett DF, Jameson M, McMahon TT, et al. Baseline findings in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) study. Investigative Ophthalmology and Visual Science 1998;39(13):2537-46. [PubMed] [Google Scholar]
References to other published versions of this review
Kuo 2020
- Kuo IC, Hawkins BS, Ren M, Lindsley KB. Transepithelial versus epithelium-off corneal crosslinking for progressive keratoconus. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD013512. [DOI: 10.1002/14651858.CD013512] [DOI] [PMC free article] [PubMed] [Google Scholar]


