Abstract
Background
Incentive spirometry (IS) is a treatment technique that uses a mechanical device to reduce pulmonary complications during postoperative care. This is an update of a Cochrane review first published in 2007.
Objectives
Update the previously published systematic review to compare the effects of IS for preventing postoperative pulmonary complications in adults undergoing coronary artery bypass graft (CABG).
Search methods
We searched CENTRAL and DARE on The Cochrane Library (Issue 2 of 4 2011), MEDLINE OVID (1948 to May 2011), EMBASE (1980 to Week 20 2011), LILACS (1982 to July 2011) , the Physiotherapy Evidence Database (PEDro) (1980 to July 2011), Allied & Complementary Medicine (AMED) (1985 to May 2011), CINAHL (1982 to May 2011).
Selection criteria
Randomised controlled trials comparing IS with any type of prophylactic physiotherapy for prevention of postoperative pulmonary complications in adults undergoing CABG.
Data collection and analysis
Two reviewers independently evaluated trial quality using the guidelines of the Cochrane Handbook for Systematic Reviews and extracted data from included trials. For continuous outcomes, we used the generic inverse variance method for meta‐analysis and for dichotomous data we used the Peto Odds Ratio.
Main results
This update included 592 participants from seven studies (two new and one that had been excluded in the previous review in 2007. There was no evidence of a difference between groups in the incidence of any pulmonary complications and functional capacity between treatment with IS and treatment with physical therapy, positive pressure breathing techniques (including continuous positive airway pressure (CPAP), bilevel positive airway pressure (BiPAP) and intermittent positive pressure breathing (IPPB), active cycle of breathing techniques (ACBT) or preoperative patient education. Patients treated with IS had worse pulmonary function and arterial oxygenation compared with positive pressure breathing. Based on these studies there was no improvement in the muscle strength between groups who received IS demonstrated by maximal inspiratory pressure and maximal expiratory pressure.
Authors' conclusions
Our update review suggests there is no evidence of benefit from IS in reducing pulmonary complications and in decreasing the negative effects on pulmonary function in patients undergoing CABG. In view of the modest number of patients studied, methodological shortcomings and poor reporting of the included trials, these results should still be interpreted cautiously. An appropriately powered trial of high methodological rigour is needed to determine if there are patients who may derive benefit from IS following CABG.
Plain language summary
The use of incentive spirometry for preventing pulmonary complications in adults people undergoing coronary artery bypass graft surgery
Breathing complications after coronary artery bypass graft (CABG) surgery increases hospital stay and is with associated high healthcare costs. CABG may interfere with the lungs, causing sections of them to collapse which may lead to pneumonia. Re‐inflating areas of the collapsed lung may be done by a device ‐ an incentive spirometer ‐ that reinforces a pattern of breathing which prevents and reverses the process. This device is used alone or in combination with other physiotherapy techniques.
This update included 592 participants from seven studies (two new and one that had been excluded in the previous 2007 review). We found evidence from four small trials that incentive spirometry offers no advantage over standard post‐surgical physical therapy, or preoperative education in preventing breathing complications and pneumonia, improving lung function, or shortening length of hospital stay in patients undergoing CABG. Bigger and better designed trials are needed to determine if there is any role for incentive spirometry.
Background
Description of the condition
The burden of postoperative pulmonary complications following cardiac surgery Despite several advances in the treatment of coronary artery disease, many patients, especially those with multivessel disease and complex anatomies, benefit greatly when subjected to surgical treatment (Mohr 2011). The coronary artery bypass graft (CABG) surgery is the routine procedure for the treatment of patients who present with symptoms of myocardial ischemias (Keenan 2005), and accounts for more resources expended in cardiovascular medicine than any other single procedure (ACC/AHA 1999). Annually, about one million surgeries are carried out in the world (Keenan 2005). The patients receiving CABG present a relatively high risk of developing pulmonary complications, such as atelectasia, pneumonia and pleural effusion. These complications (including mortality) increase the time of hospitalisation and the necessity of financial resources (Pasquina 2003; Ferguson 1999; Lawrence 1995). Transoperatory factors, such as general anaesthesia, pulmonary modifications after extracorporeal circulation, utilization of internal mammary artery as well as postoperative pain, are factors that contribute to the occurrence of pulmonary complications(Groeneveld 2007; Groeneveld 2006; Ferguson 1999; Kips 1997; Mohr 1996; Berrizbetia 1989; Hallbook 1984). Therefore efforts have been made during the last decade to identify patients with the greatest chance of developing complications, and to find techniques to prevent such complications (Ferguson 1999).
Risk factors for postoperative pulmonary complications Manifold variables, whether patient‐related (for example age, constitution, or concomitant pulmonary disease) or care‐related (for example type of surgery, anaesthesia or analgesia), are supposed to have an impact on the efficacy of pulmonary function following surgery (Groeneveld 2007; Groeneveld 2006; Weindler 2001; Ferguson 1999). As the average age of patients undergoing CABG is increasing (due to several factors, including the improvement of therapeutic effectiveness) the effect of age on the incidence of postoperative pulmonary complications is significant (Mortasawi 2004; Hulzebos 2003; Weinstein 1987). Other risk factors generally present in patients with coronary syndromes, including smoking, obesity, physical inactivity, diabetes, raised high‐density lipoprotein levels, systolic hypertension, chronic obstructive pulmonary disease, pain and previus reduction of pulmonary function, also increase the risk of postoperative pulmonary complications (Hulzebos 2003; Daganou 1998; Higgins 1988; Kannel 1980; Kannel 1984; Kannel 1985; Kannel 1986; Kannel 1987; Wilking 1988).
Description of the intervention
Interventions to reduce postoperative pulmonary complications In 1989, in order to guide practice, a consensus conference on perioperative cardiorespiratory physical therapy (modelled on the National Institutes of Health consensus methodology) was held in Canada (CPA 1990). Canadian hospitals with more than 300 beds were surveyed to determine current perioperative cardiorespiratory practice. This survey, with minimal modification to reflect changes in surgical techniques, was repeated in 1997 to determine practice patterns and to document changes since 1990. In the years since the consensus conference, more studies on perioperative physical therapy have been published and surgical techniques have changed (Brooks 2001). Incentive spirometry (IS) is a widely used technique for the prophylaxis and treatment of respiratory complications in postsurgical patients (Cavenaghi 2011; Ferreira 2010; Renault 2009;Renault 2008; Haeffener 2008; Savci 2006; Overend 2001; Wattie 1998; Jenkins 1986; O'Donohue 1985). However, several publications have questioned the effectiveness of IS (Crowe 1977; Dull 1983; Gale 1980; Jenkins 1989; Matte 2000; Oikkonen 1991; Oulton 1981; Stiller 1994; Stock 1984), and the use of the technique is the subject of debate (Restrepo 2011; Freitas 2007; Pasquina 2003; Brooks 2001; Overend 2001).
How the intervention might work
The treatment utilizes an incentive spirometer, a mechanical device developed to reduce pulmonary complications during postoperative care (Chuter 1990). It was developed to imitate natural sighing and yawning, and encourages the patient, through visual and/or audio feedback (Bartlett 1973), to maintain inspiration for a prolonged period, using slow inspiration and deep breaths (AARC 1991; Bartlett 1970; Craven 1974; Meyers 1975; Petz 1979).
Why it is important to do this review
Due to its low cost, incentive spirometers are widely used in hospitals. They are used for treating and preventive purposes regarding pulmonary complications. This device works with visual stimulation to deep inspiration and is largely used by patients in post operatory periods of abdominal and thoracic surgery (Agostini 2008). Despite its low cost, the routine use of IS in CABG will add to the cost of care. Studies evaluating the effectiveness of IS in patients who have had cardiac surgery, however, have been unable to demonstrate the superiority of IS over other techniques (Restrepo 2011; Freitas 2007; Pasquina 2003; Brooks 2001; Overend 2001;Matte 2000; Crowe 1977; Oikkonen 1991;Jenkins 1989; Dull 1983).
Since the last published review, we have included two new outcomes, maximal expiratory pressure (maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP)) and the six minute walk test (6MWT). This is because the peak of postoperative diaphragm dysfunction, with a decrease in its strength, occurs between two to eight hours postoperatively, with a return to pre‐operative values occurring within approximately two weeks. These alterations occur in response to the surgical procedure and can progress to respiratory complications when they modify the initially predicted course for postoperative recovery. The complications are related to the decrease of the contractile capacity of the diaphragm, directly represented by MIP and MEP decrease (Siafakas 1999; Chandler 1984), and the 6MWT. They have now become a common method to determine functional capacity (ATS 2002). For these reasons, it is important to have the best evidence of the beneficial effects of IS after CABG before recommendations for use are applied uniformly. Therefore an updated systematic review evaluating the effectiveness of IS in patients undergoing CABG is necessary.
Objectives
To update the previously published systematic review to compare the effects of IS in preventing postoperative pulmonary complications in adults undergoing CABG. Where available, information is presented on the efficacy of IS in the presence or absence of other treatment or IS associated with other techniques.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
Trials including patients over 18 years of age undergoing CABG, not associated with valve replacements or other procedures.
Types of interventions
Intervention group
IS that allows the patients to accomplish breathing exercises emphasising inspiration with sustained maximal inhalation. The literature search included patients undergoing CABG treated with IS compared with other techniques of physiotherapy treatment for prophylaxis of pulmonary complications. For the purposes of this update, it was necessary to group these variations within broad definitions of the treatment modalities.
Control group
IS was compared with other techniques as described below:
Standard postsurgical physical therapy (PPT)
This included any combination of the following: manual interventions (chest vibration, percussion, chest shaking), forced expiratory with the glottis open (huffing), coughing with sternal support, active exercises of the upper and lower limbs, sitting in a chair, walking, and aerosol therapy.
Continuous positive airway pressure (CPAP) mask therapy
Is a spontaneous ventilatory mode maintaining a supra‐atmospheric pressure in the lung.
Intermittent positive pressure breathing (IPPB)
Is a spontaneous ventilatory mode with of periodic intermittent positive pressure breathing.
Bilevel positive airway pressure (BiPAP or NIV‐2P) mask therapy
Is a barometric ventilatory mode the action of which is determined by the difference between Inspiratory positive airway pressure (IPAP) and expiratory positive airway pressure (EPAP)
The comparisons of IS with positive pressure breathing techniques (CPAP, IPPB, BiPAP or NIV‐2P) plus standard postsurgical physical therapy were also included.
Active cycle of breathing techniques (ACBT)
ACBT consisted of one to two breathing control breaths, three thoracic expansion exercises follows by a three second breath hold at the end of deep inspiration, and forced expiration technique including one to two breathing control breaths combined with one to two huffs.
Types of outcome measures
RCTs reporting any of the following short‐ or long‐term outcomes were eligible for inclusion.
Primary outcomes 1. Atelectasis: radiographic, tomographic or bronchoscopic diagnosis and/or clinical signs with acute respiratory symptoms, for example dyspnoea, cough, abnormal lung sounds. 2. Acute respiratory infection (pneumonia): radiographic diagnosis and/or clinical signs of acute respiratory symptoms for example purulent tracheobronchial secretion, fever (>38°C) or increased circulating leucocytes (>10,000/mm³).
3. Total mortality from respiratory causes: data of the necropsy or clinical inference.
Secondary outcomes 1. Vital capacity (ml)
2. Forced expiratory volume in one second (ml) (FEV1). 2. Arterial Oxygenation (Partial pressure of arterial oxygen per inspired oxygen fraction (PaO2/FiO2).
3. Postoperative days in hospital.
3. Respiratory muscle strength: MIP maximal expiratory pressure MEP.
4. Functional capacity: 6MWT.
5. Perceived quality of life.
6. Economic costs.
Search methods for identification of studies
Eletronic searches
The original searches from December 2004 (Appendix 1) have been updated and were re‐run in August 2009 and May 2011 (Appendix 2).
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library (Issue 2 of 4, 2011, DVD), MEDLINE OVID (1948 to May Week 2 2011), EMBASE OVID (1980 to 2011 Week 20), Allied & Complementary Medicine (AMED) (1985 to May 2011), CINAHL (1982 to May 2011) and Database of Abstracts of Reviews of Effects (DARE) on The Cochrane Library (Issue 2 of 4, 2011, DVD) on 23 May 2011. The searches of LILACS (1982 to July 2011) and the Physiotherapy Evidence Database (PEDro) (1980 to July 2011) have last been run in July 2011.
We used the recommended search strategy for identifying RCTs in MEDLINE for our search in 2004 from the Cochrane Handbook (Clarke 2001). In 2011 we used the updated Cochrane RCT filter ( Lefebvre 2011) for MEDLINE and EMBASE. No language restrictions were applied to the searches.
Searching other resources
Annual meeting abstracts of the American Heart Association, American College of Cardiology, and European Society of Cardiology were also searched from 1996 to July 2011. We searched the references lists of identified studies and other relevant articles and contacted authors of relevant studies to request details of unpublished or ongoing investigations. We used The Cochrane Handbook of Systematic Reviews (Lefebvre 2011) recommended search strategy for identifying RCTs .
Data collection and analysis
Selection of studies
For the update, titles or abstracts of citations retrieved from the literature search were screened for duplicates by the Cochrane Heart Group and lists of potential studies for inclusion in the review were sent to the review authors. All studies that were not randomised controlled trials or that clearly did not fit the inclusion criteria were excluded. Where there was uncertainty as to the nature of the trial or randomisation of participants, a letter was sent to the first named author at the institution stated on the paper. The full text of all other citations were reviewed independently by four review authors to assess eligibility.
Data extraction and management Data were extracted independently by the four authors (ERFSF, BGOS, JRC and ANA). For each included study, all data were extracted and recorded by the review author. Information on the demographics of the study and inclusion and exclusion criteria were detailed. Treatment interventions and durations were listed together with details of any control groups and a full list of outcome measures for each study. This is presented in a tabular format in the Characteristics of included studies table.
Assessment of risk bias in included studies We followed the guidelines of the Cochrane Reviewers' Handbook (Clarke 2003,Higgins 2011). The reviewers (ERFSF, BGOS, JRC and ANA) independently assessed methodological quality of selected studies and assessment of risk bias in included studies, including adequacy of allocation concealment, which was ranked as 'Yes'‐ low risk of bias, 'Unclear' risk of bias and 'No'‐high risk of bias. Any differences of opinion were resolved by discussion and consensus.
All the included studies randomised participants into treatment groups (Dull 1983; Jenkins 1989; Oikkonen 1991; Crowe 1977; Matte 2000; Savci 2006; Romanini 2007). Random number tables were used by one study (Crowe 1977) using computer‐generated random number table, drawing lots by one (Romanini 2007). Despite using computer‐generated random number table in the one study (Crowe 1977), it should be noted that, the numbers in the treated and control groups in this study differ considerably. It would appear that in all of the studies, patients were randomised consecutively after fulfilling any inclusion criteria. This was essential to reduce bias at the allocation stage. Data on inclusion and exclusion criteria are presented in the Characteristics of included studies table. One study (Dull 1983) reported ten patients exclusions after randomisation.
The patients in these studies were not blinded to the types of treatments, therefore all of the studies had a great potential for bias. The measurement of outcome measures was non‐biased, because the assessors were blinded in all studies,
Measures of treatment effect We had hoped to find sufficient trials to carry out a meta‐analysis of all findings using RevMan‐5 software. Owing to limitations in the published material we have conducted a part of the narrative form review and have presented dichotomous outcomes using Peto odds ratios (OR), and 95% confidence intervals (CI). Continuous variables have been expressed as the mean change from baseline to follow up, and the standard deviation difference from baseline to follow up for each comparison group. Where standard deviation differences were not reported allowance was made within patient correlation from baseline to follow up measurement, using the correlation coefficient between the two (see Cochrane Heart Group web site and (Follmann 1992) for details).
Unit of treatment effect
Seven RCTs were included in this update, comparing several different outcomes.
Dealing with missing data
All individual studies would be analysed according to intention to treat analysis.
Data synthesis
When possible, we grouped studies on specific treatment techniques for the purpose of meta‐analysis. This facilitated comparisons between specific IS treatment, as well as comparisons with other techniques.
Subgroup analysis and investigation of heterogeneity
Due to the limited number of trials assessing different treatment options, statistical techniques for looking at heterogeneity of data, publication bias (funnel plot), and subgroup analysis were not applied.
Sensitivity analysis
Due to the limited number of trials we did not undertake sensitivity analysis.
Data analysis
Results were expressed as Peto OR with 95% CI for dichotomous variables. Results for continuous variables were expressed as mean differences (MD) with 95% CI.
Results
Description of studies
Results of the search In this update 366 new references were identified after duplicates were removed, totaling 1148 potentially relevant publications (Figure 1) (Moher 1999). After reading titles and abstracts we excluded 992 papers (generally due to lack of suitability of study design or intervention), and 156 papers were retrieved for further evaluation. Subsequently 137 papers were excluded because they did not meet inclusion criteria. Nineteen studies were eligible for inclusion and detailed assessment, however, subsequently 12 studies were excluded (Vraciu 1977; Iverson 1978; Gale 1980; Oulton 1981; Paul 1981; Stock 1984; Rau 1988; Jenkins 1990; Mahler 1998; Haeffener 2008; Renault 2009; Ferreira 2010), see Characteristics of excluded studies.
1.
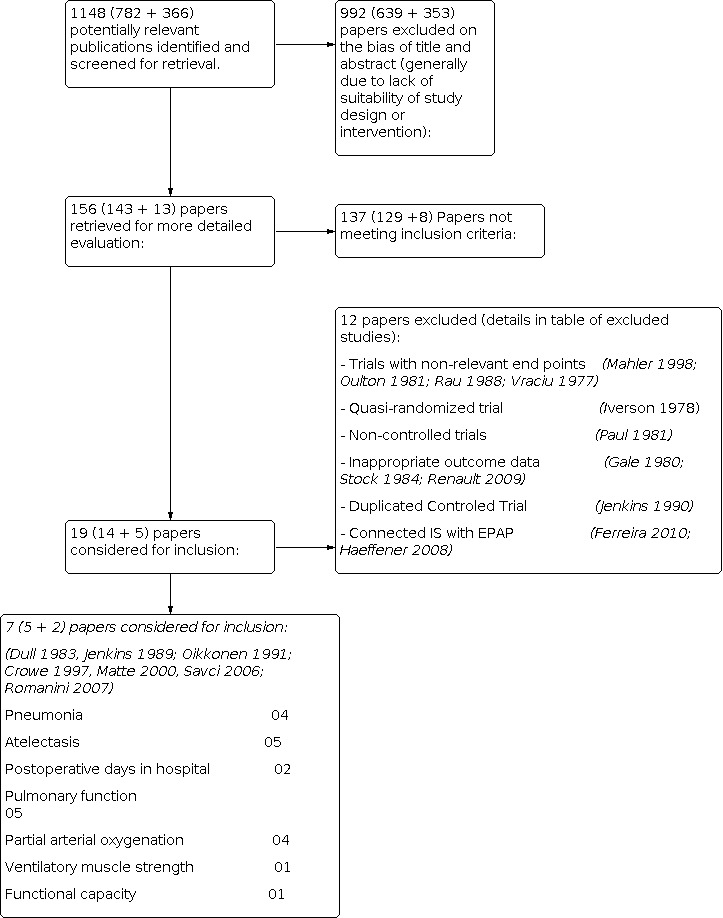
Flow diagram.
We would like to emphasize that one study had been excluded from the previous review because the data had been presented in the form of graphs and illustrations. We have subsequently obtained this data and as such have included it in this review (Dull 1983).
Included studies
Seven RCTs met the inclusion criteria in this update (Dull 1983; Jenkins 1989; Crowe 1977; Matte 2000; Oikkonen 1991; Savci 2006; Romanini 2007) (see Characteristics of included studies), providing a total of 592 patients. Five trials reported data on atelectasis (Jenkins 1989; Oikkonen 1991; Crowe 1977; Matte 2000; Savci 2006), four reported data on pneumonia (Jenkins 1989; Crowe 1977; Matte 2000; Oikkonen 1991), five reported data on pulmonary function (Dull 1983; Jenkins 1989; Oikkonen 1991; Crowe 1977; Matte 2000), four reported data on partial pressure of oxygen and fractional inspired oxygen (PaO2/FiO2) (Jenkins 1989; Matte 2000; Oikkonen 1991; Savci 2006), one reported data on ventilatory muscle strength (MIP and MEP) (Romanini 2007). One study reported data on functional capacity (6MWT) (Savci 2006) and two on postoperative days in hospital (Crowe 1977; Matte 2000). The average group size was 34 patients (range 10‐95 patients), and the range of follow‐up was two to five days.
All trials excluded patients with unstable cardiac status, intubation time longer than 24 hours and patients who failed to co‐operate. Two study only recruited male patients (Jenkins 1989; Savci 2006), two studies excluded subjects with chronic obstructive pulmonary disease (Matte 2000; Oikkonen 1991), one study excluded any subjects over the age of 70 (Oikkonen 1991), one study excluded smokers, a history of cerebrovascular accident, renal dysfunction requiring dialysis, use of immunosuppressive treatments during the 30 day period before surgery, cardiovascular instability or an aneurysm (Savci 2006). Thus, the population included in studies was of low surgical risk because studies excluded participants who took longer to wean off ventilators, pre‐existing lung disease, undergoing emergent CABG surgery, and participants with postoperative cardiac neurological complications.
One study included patients undergoing CABG and valve replacement (Dull 1983), three studies included patients undergoing CABG with the use of internal mammary artery and saphenous vein (Crowe 1977; Jenkins 1989; Oikkonen 1991), and one study included patients undergoing elective CABG with the use of mammary arteries only (Matte 2000; Savci 2006).
The mean age of included patients ranged from 41 to 75 years, 516 patients were male (90.2%), and 76 patients were female (13.3%).
The trials were conducted between 1983 and 2007 in Europe (United Kingdom, Belgium, Finland), North America (Canada and USA), and Brazil.
Interventions
Two hundred and forty eight patients were allocated to IS and 344 patients were allocated to control. In four studies (Dull 1983; Oikkonen 1991;Crowe 1977; Matte 2000) conventional physical therapy (typically considered as a type of generic postsurgical physiotherapy: e.g. early bed mobility, ambulation, basic deep breathing, and coughing exercises) or early mobilization was given to both the intervention and the control groups. Three trials (Crowe 1977; Matte 2000; Romanini 2007) compared IS versus positive airway pressure techniques (CPAP, BiPAP and IPPB) and in two groups (Crowe 1977; Matte 2000) IS was given to both the intervention group and the control group plus physical therapy. Two trials (Jenkins 1989;Romanini 2007) compared IS alone, one study versus conventional physical therapy intervention and versus preoperative education (Jenkins 1989) and other study versus IPPB (Romanini 2007). One trial (Savci 2006) compared the IS plus conventional physical therapy versus active cycle of breathing techniques (ACBT).Table 1
1. Number of patients receiving Incentive Spirometry (IS) and other techniques included studies.
| Study |
Incentive Spirometry (IS) |
Physical Therapy (PT) |
Continuous Positive Airway Pressure (CPAP |
Non invasive ventilation support with bilevel positive airway pressure (BiPAP) |
Intermettente positive pressure breathing (IPPB) |
Active cycle of breathing techniques (ACBT) |
None |
Total patients of study |
| Dull 1983 | 17 | 16 | 16 | 49 | ||||
| Jenkins 1989 | 38 | 35 | 37 | 110 | ||||
| Oikkonen 1991 | 26 | 26 | 52 | |||||
| Crowe 1977 | 90 | 95 | 185 | |||||
| Matte 2000 | 30 | 33 | 33 | 96 | ||||
| Savci 2006 | 30 | 30 | 60 | |||||
| Romanini 2007 | 20 | 20 | 40 | |||||
|
Total patients of techniques |
251 | 146 | 33 | 33 | 46 | 30 | 53 | 592 |
The incentive spirometry intervention ranged from 5 to 10 breaths repeated every two hours or 10 breaths repeated every one to two hours .
Excluded studies
A total of 12 studies were excluded (see Characteristics of excluded studies); four did not have relevant end‐points (Mahler 1998; Oulton 1981; Rau 1988; Vraciu 1977); one was a quasi‐randomised trial (Iverson 1978); one was a non‐controlled trial (Paul 1981); one was a duplicate report (Jenkins 1989); two combined CABG with other interventions and did not present the results for CABG separately (Gale 1980; Stock 1984); one did not present individual results for each group (Renault 2009), and two combined IS connected with EPAP and showed the results in form of graphs or illustrations (Haeffener 2008; Ferreira 2010).
Risk of bias in included studies
Summary with details of the quality assessment are given in the Characteristics of included studies; Figure 2; Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The risk of bias in the included studies were assessed by two review authors. Only one study reported random sequence using a computer‐generated random number table Crowe 1977. No study reported adequate allocation concealment and sequence generation. Two studies (Crowe 1977; Matte 2000) described blinding the observer. Others forms of blinding were not found (participants and professionals). Given the interventions being studied, it would not have been feasible to have blinded the participants or carers to the treatment group. Crowe 1977 had incomplete outcome data. Crowe 1977; Jenkins 1989 had not applied intention to treat analysis. All included studies (Crowe 1977; Dull 1983; Jenkins 1989; Matte 2000; Oikkonen 1991; Romanini 2007; Savci 2006) had adequate follow‐ups.
Effects of interventions
Seven studies involving 592 participants were included in this update of review (Dull 1983; Jenkins 1989; Crowe 1977; Oikkonen 1991; Matte 2000; Savci 2006; Romanini 2007)
Primary outcomes Pulmonary complications
Atelectasis Five trials involving 502 subjects reported this outcome (Jenkins 1989; Crowe 1977; Oikkonen 1991; Matte 2000; Savci 2006), with 213 (42.4%) allocated to IS and 289 (57.6%) to other techniques Table 2. Due to the variability of the comparison we were unable to pool the five trials. There was little evidence of a reduction of atelectasis in any of the comparison groups. In two trials where patients received IS compared with conventional physical therapy, pooled analysis showed no effects between IS and conventional therapy Figure 4, Analysis 1.1.
2. Summary of primary outcomes of included trials.
| Study |
Intervention (IS) Peto Odds Ratio Peto, Fixed, 95% CI |
Control Group Peto Odds Ratio Peto, Fixed, 95% CI |
| Atelectasis | ||
| Jenkins 1989 | 60.5% (23/38) | (Conventional physical therapy) 54.3% (19/35); (Preoperative advice only) 64.9% (24/37) |
| Oikkonen 1991 | 80.7% (21/26) | (IPPB) 61.5% (16/26) |
| Crowe 1977 | 10.1% (9/89) | (Conventional Physical Therapy) 10.5% (10/95) |
| Matte 2000 | 30.0% (9/30) | (CPAP) 15.2% (5/33); (BiPAP) 15.2% (5/33) |
| Savci 2006 | 30.0% (9/30) | (ACBT) 33.3% (10/30) |
| Pneumonia | ||
| Jenkins 1989 | 5.3% (2/38) | (Conventional physical therapy) 11.4% (4/35); (Preoperative advice only) 13.5% (5/37) |
| Oikkonen 1991 | 38.5% (10/26) | (IPPB) 23.1% (6/26) |
| Crowe 1977 | 8.9% (8/90) | (Conventional physical therapy) 10.5% (10/95) |
| Matte 2000 | 3.3% (1/30) | (CPAP) 3.0% (1/33); (BiPAP) 0.0% (0/30) |
4.

Forest plot of comparison: 1 Atelectasis, outcome: 1.1 Incentive spirometry versus conventional physical therapy.
1.1. Analysis.

Comparison 1: Atelectasis, Outcome 1: Incentive spirometry versus conventional physical therapy
Acute respiratory infection (Pneumonia) Four trials reported this outcome (Jenkins 1989;Oikkonen 1991; Crowe 1977; Matte 2000), involving 443 patients, with 184 (41.5%) allocated to IS and 259 (58.5%) to other techniques Table 2. Again, due to the variability of the comparison we were unable to pool the four trials. In two trials where patients received IS compared with conventional physical therapy, pooled analysis showed no effects between IS and conventional therapy Figure 5Analysis 2.1
5.

Forest plot of comparison: 2 Pneumonia, outcome: 2.1 Incentive spirometry versus conventional physical therapy.
2.1. Analysis.

Comparison 2: Pneumonia, Outcome 1: Incentive spirometry versus conventional physical therapy
Total mortality from respiratory causes
None of the included studies assessed this outcome.
Secondary outcomes
Vital capacity Six trials reported vital capacity (Oikkonen 1991; Crowe 1977; Jenkins 1989; Matte 2000; Dull 1983; Savci 2006), involving 552 subjects with 231 (41.8%) allocated to IS and 321 (58.2%) to control groups Table 3. In this analysis there was also a great variability of the comparison and we were unable to pool the six trials. However in three trials (Crowe 1977; Dull 1983; Jenkins 1989) where patients received IS compared with conventional physical therapy, pooled analysis showed that there was no favourable effect from IS Analysis 3.1, similar results were found when comparing IS with preoperative physiotherapy advice only Analysis 3.4. People that received treatment with IS had a smaller volume of vital capacity when compared to positive pressure breathing techniques with statistical significance (IS versus CPAP: P=0.01; IS versus BiPAP or NIV‐2P: P=0.0002; IS vs IPPB P< 0.00001) (Matte 2000; Oikkonen 1991) Analysis 3.2; Analysis 3.3; Analysis 3.5. One trial did not report the standard deviation (Crowe 1977) and another trial presented vital capacity as a percentage and can not be analysed (Savci 2006).
3. Summary of secondary outcomes of included trials.
| Study |
Mean difference (IS vs control group) IV, fixed, 95% CI |
| Vital capacity (ml) | |
| Dull 1983 | (Conventional physical therapy) 280.00 [‐16.96, 576.96]; (Preoperative advice only) ‐65.00 [‐521.76; 391.74] |
| Jenkins 1989 | (Conventional physical therapy) 100.00 [‐175.51, 375.51]; (Preoperative advice only) 0.0 [‐277.29, 277.29] |
| Oikkonen 1991 | (IPPB) ‐272.00 [‐350.68, ‐193.32] |
| Crowe 1977 | Standard deviation not available |
| Matte 2000 | (CPAP) ‐338.00 [‐607.33, ‐68.67]; (BiPAP) ‐427.0 [‐655.04, ‐198.96] |
| Savci 2006 | Results presented as percentage |
| Forced expiratory volume in 1 second (ml) | |
| Dull 1983 | (Preoperative advice only) 50.00 [‐278.15, 378.15]; (Conventional physical therapy) 160.00 [‐99.51, 419.51] |
| Jenkins 1989 | (Conventional physical therapy) 0.0 [‐184.16, 184.16]; (Preoperative advice only) 0.0 [‐203.70, 203.70]; |
| Crowe 1977 | (Conventional physical therapy) 55.00 [‐22.84, 132.84] |
| Matte 2000 | (CPAP) ‐183.0 [‐310.09, ‐55.91];(BiPAP) ‐213.0 [‐369.12, ‐56.88] |
| Savci 2006 | Results presented as percentage |
| Arterial oxygenation (PaO2/FiO2) (mmHg) | |
| Jenkins 1989 | (Preoperative advice only) 5.0 [‐13.38, 23.38]; (Conventional physical therapy) ‐5.0 [‐23.68, 13.68] |
| Oikkonen 1991 | (IPPB) ‐35.0 [‐54.57, ‐15.43] |
| Matte 2000 | (CPAP) ‐14.00 [‐35.26, 7.26]; (BiPAP) ‐29.00 [‐53.8, ‐4.20] |
| Savci 2006 | (ACBT) 2.00 [‐39.49, 43.49} |
3.1. Analysis.

Comparison 3: Vital capacity (ml), Outcome 1: Incentive spirometry versus conventional physical therapy
3.4. Analysis.

Comparison 3: Vital capacity (ml), Outcome 4: Incentive spirometry versus preoperative physiotherapy advice only
3.2. Analysis.

Comparison 3: Vital capacity (ml), Outcome 2: Incentive spirometry versus continuous positive airway pressure (CPAP)
3.3. Analysis.

Comparison 3: Vital capacity (ml), Outcome 3: incentive spirometry versus bilevel positive airway pressure (BiPAP or NIV‐2P)
3.5. Analysis.

Comparison 3: Vital capacity (ml), Outcome 5: Incentive spirometry versus Intermittente positive pressure breathing (IPPB)
Forced expiratory volume in one second (FEV1) Five trials involving 500 subjects reported this outcome (Crowe 1977; Jenkins 1989; Matte 2000; Savci 2006; Dull 1983), with 228 (45.6%) allocated to IS and 272 (54.4%) to control groups Table 3. Due to the variability of the comparison we were unable to pool the five trials. There was little evidence of a improvement of VEF1 in any of the comparison groups. In three trials where patients received IS compared with conventional physical therapy (Dull 1983; Jenkins 1989; Crowe 1977) Figure 6Analysis 8.1, and in two trials where patients received IS compared with preoperative advice only (Dull 1983; Jenkins 1989) Analysis 8.2, pooled analysis showed no beneficial effect in favour from IS. People given IS plus physical therapy had a smaller FEV1 compared with positive pressure breathing techniques (Is versus CPAP: P=0.0005 and IS versus BiPAP: P=0.007) (Matte 2000). The FEV1 was presented as percentage in one trial and could not be analysed (Savci 2006).
6.

Forest plot of comparison: 8 Forcede expiratory volume in one second (ml), outcome: 8.1 Incentive spirometry versus conventional physical therapy.
8.1. Analysis.

Comparison 8: Forcede expiratory volume in one second (ml), Outcome 1: Incentive spirometry versus conventional physical therapy
8.2. Analysis.

Comparison 8: Forcede expiratory volume in one second (ml), Outcome 2: Incentive spirometry versus preoperative physiotherapy advice only
Arterial Oxygenation (Partial pressure of arterial oxygen per inspired oxygen fraction (PaO2/FiO2) Four trials involving 318 subjects reported this outcome (Jenkins 1989; Oikkonen 1991; Matte 2000; Savci 2006), with 124 (39,0%) allocated to IS and 194 (61.0%) to control groups Table 3. People given IS had smaller PaO2/FiO2 when compared with positive pressure breathing techniques (Matte 2000; Oikkonen 1991) (IS versus BiPAP or NIV‐2P: P=0.02; IS versus IPPB: P=0.0005). However, there was no difference between those receiving IS versus conventional physical therapy or preoperative physiotherapy advice only (Jenkins 1989) or active cycle of breathing techniques (Savci 2006).
Respiratory muscle strength: MIP and MEP.
One study reported these outcomes involving 40 subjects, with 20 (50.0%) allocated to IS and 20 (50.0%) to control group that received IPPB (Romanini 2007) Table 4. The group that received IPPB had better muscle strength assessed by MIP and MEP breathing when compared to the group receiving IS (MIP P=0.04; MEP P=0,07).
4. Summary of secondary outcomes of included trials.
| Study |
Means difference (IS vs control group) IV, fixed, 95% CI |
| Maximum inspiratory pressure (MIP) (cmH2O) | |
| Romanini 2007 | (IPPB) 17.40 [0.61, 34.19] |
| Maximum expiratory pressure (MEP) (cmH2O) | |
| Romanini 2007 | (IPPB) 12.75 [‐0.99, 26.49] |
| Six‐minute walk test (6MWT) (mt) | |
| Savci 2006 | (ACBT)) ‐13.73 [‐59,43, 31.97] |
Functional Capacity ‐ 6MWT
One trial involving 60 subjects used 6MWT as an outcome to assess functional capacity, with 30 (50.0%) allocated to IS and 30 (50.0%) to a control group that received active cycle of breathing techniques demonstrated no difference between treatment groups (Savci 2006) Table 4
Postoperative days in hospital The postoperative days in hospital was reported in two trials (Crowe 1977; Matte 2000), involving 281 subjects, with 120 (42.7%) allocated to IS plus standard postsurgical physical therapy and 161 (57.3%) allocated to standard postsurgical physical therapy. In one study (Crowe 1977) the average number of postoperative days in the IS plus physical therapy group was 9.0 days versus 9.7 days in those given standard postsurgical physical therapy. One study (Matte 2000) evaluated only the stay in the intensive care united and the average number of IS group was 2.2 days versus 2.1 days in those CPAP and versus 2.1 days in those BiPAP or NIV‐2P.
Economic cost
One study mentioned this outcome and demonstrated that the IS cost $7.25. This trial involved 185 subjects, with 90 (48.6%) allocated to IS and 95 (51.4%) to control group that received standard physical therapy (Crowe 1977).
Perceived quality of life
Ferreira et al Ferreira 2010 analysed of life quality, using the SF‐36 and could not find any statistical differences in the majority on the assessed parameters between groups. The only domains which presented significant difference between group concerned the limitations in physical aspects, in which IS and EPAP group presented higher values in comparison to control group.
Discussion
Summary of main results
This update review was set out to determine if there was any advantage of IS (a technique used for many years for preventing pulmonary complication after surgery) over other techniques. Studies comparing IS with other techniques, such as conventional physical therapy, preoperative physiotherapy advice only, CPAP, BiPAP or NIV‐2P, IPPB and ACBT and physiotherapy advice only were included. The results of this update indicate insufficient evidence as to whether IS is effective for preventing postoperative pulmonary complications in adults undergoing CABG when compared with other techniques. Due to the variability of the comparisons we were unable to pool some trials. One of the major drawbacks of the reviewed literature is that the individual studies involved very small numbers of patients and each study considered only a limited number of outcome measures.
Outcomes
There was little evidence of a reduction in atelectasis and pneumonia and better pulmonary function in the subjects undergoing IS when compared with conventional physical therapy (Dull 1983; Jenkins 1989; Crowe 1977). Only one trial (Romanini 2007) indicated that the IS was better in increasing respiratory muscle strength (MIP and MEP) than IPPB. However, other studies indicated that IS did not improve arterial oxygenation (PaO2/FiO2), VC and FEV1 than BiPAP (NIV‐2P) (Oikkonen 1991; Matte 2000) and that IPPB and CPAP were better at improving FEV1 than IS (Matte 2000).
To evaluate functional capacity, the 6MWT was performed in one trial, which concluded that between the groups that underwent IS versus ACBT the functional capacity was well preserved (Savci 2006).
The cost‐effectiveness of IS is of interest and was considered in only one trial (Crowe 1977). This study concluded that, if IS was proven to be as effective as physical therapy, it would be an economic alternative. Stock et al (Stock 1984) reported that IS offers no advantage over coughing and deep breathing, and there is the expense of acquiring a spirometer. Pasquina et al (Pasquina 2003) reported from their systematic review that the average daily cost of labour for each patients was €6 for IS. However Overend et al (Overend 2001) have pointed out that the cost of IS is affected by many factors, including the type of spirometer and the method used (for instance single use versus re‐use following sterilization). If there was no difference in the effectiveness of IS technique over other techniques, the spirometer would add costs that could be neglected in this context.
Perveived quality of life considered in only one study (Ferreira 2010) which indicated that IS may improve physical outcomes but the evidence is weak.
Overall completeness and applicability of evidence
The therapeutic efficacy of IS is still being tested and much discussed in the literature (Pinheiro 2011; Restrepo 2011; Cavenaghi 2011; Ferreira 2010, Renault 2009; Haeffener 2008; Savci 2006; Crowe 1977; Hall 1991; Hall 1996; Kips 1997; Mang 1991; Marini 1984; Oikkonen 1991; Weiner 1997).
This updated systematic review has included data from seven trials investigating the prevention of pulmonary complications using IS after CABG.
The past five published systematic reviews and guidelines ( Restrepo 2011; Brooks 2001; Overend 2001; Pasquina 2003; Thomas 1994) have analysed respiratory physiotherapy for the prevention of pulmonary complications after different operations, but they obtained conflicting results. The benefits of such techniques remained uncertain as data came from different operations and the pooled data came from different end points, such as pulmonary infiltrates, consolidation or atelectasis. Commentators have noted the lack of evidence of efficacy of IS in some studies (Restrepo 2011; Pasquina 2003; Freitas 2007; Celli 1984; Craven 1974; Hall 1996), but others claim that its effectiveness depends on the selection of patients, careful instruction, and supervision of patients during respiratory training (Weindler 2001).
Previous systematic reviews performed by us concluded that there was no evidence of benefit from IS in reducing pulmonary complications and in decreasing the negative effects on pulmonary function in patients undergoing CAGB (Freitas 2007).
Quality of the evidence
Due to the lack of sufficient quality evidence, we still cannot make definitive statements about the effectiveness of IS for the prevention of pulmonary complications after CABG. Most studies were small and did not detect significant differences between groups. In addition, due to the the variability of comparisons it was difficult to pool studies for analysis. In the seven trials included, five different regimens of therapy were tested against IS. This made the pooling of data of dubious value and we did not attempt to do this. The variation in usual practice may be due to the lack of a 'gold standard' method for respiratory physiotherapy (Tramer 1998). The best comparison would be to use a placebo or no intervention along with the total absence of physiotherapy as control (Temple 2000). However this is usually considered unethical. In this review only two trials (Dull 1983; Jenkins 1989) used a virtually no treatment control group. These trials, patients in the control group were seen before the operation by a physiotherapist, who explained the need to move about after surgery and to expectorate excess bronchial secretions, and taught early mobilization with active exercises of the upper and lower limbs, forced expirations with the glottis open (huffing) and coughing with sternal support.
Potencial biases in the review process
This updated review was limited by the low quality of the trials available for inclusion. The methodological descriptions reported inadequate methods of randomisation and concealment of allocation, and there were limitations to blinding.
There were no differences among the three treatment programs in improving lung volumes or preventing postoperative pulmonary complications. This is one of the few studies to include a control group in the research design. Thus the efficacy of IS when compared with the absence of intervention can be based only on these two small trials (Dull 1983; Jenkins 1989).
The IS treatment regimes used in the included studies were different, ranging from five breaths repeated every 2 hours to 10 breaths repeated every hour. Two trials (Crowe 1977; Oikkonen 1991) postulated that insufficient self‐administration of IS might be a possible explanation for the failure of the treatment. This inconsistency suggests uncertainty about the optimal treatment regime for IS.
The high variability in the rate of events was another limitation. For instance, the average incidence of pneumonia was 0‐12% (Crowe 1977; Jenkins 1989; Matte 2000) and atelectasis was 4‐80% (Crowe 1977; Jenkins 1989; Matte 2000; Oikkonen 1991; Savci 2006). Variability may be explained by the absence of a uniform definition of pneumonia and atelectasis in the primary studies and the limited size of trials (only one study included groups of more than 50 patients; Crowe 1977). In small trials, events may happen by random chance (Moore 1998). In the four trials included, the longest observation period was four days, this made it difficult to identify all pulmonary complications. Nosocomial pneumonia, for instance, occurs on average eight days after cardiac surgery (Leal‐Noval 2000).
Additional respiratory physical therapy and/or mobilization and/or analgesia were used as adjunctive treatments in three of the included trials (Crowe 1977; Matte 2000; Oikkonen 1991); however few treatments were described adequately. All these co‐interventions, such as early mobilization (Chulay 1982; Jenkins 1989; Scheidegger 1976) and the intensity and method of postoperative analgesia, may have an impact on pulmonary function (Hedderich 1999). In large RCTs co‐interventions are usually balanced between the groups, but, in small trials bias cannot be excluded.
No trial included in this systematic review evaluated the adverse effects of IS. However, Iverson et al (Iverson 1978) reported that gastrointestinal complaints and nausea were rare in patients using IS, 2% and 0% respectively. This trial was excluded from this systematic review due to low methodological quality and inadequate allocation concealment.
Authors' conclusions
Implications for practice.
For professionals and patients, the results of this review suggest that, in patients undergoing CABG when compared to treatment of physical therapy no evidence of benefit from IS compared with preoperative education or standard postsurgical physical therapy for preventing postoperative pulmonary complications, improving pulmonary function and oxygenation and/or reducing length of hospital stay. There is evidence that IS is better than IPPB in increasing respiratory muscle strength, however this may have some drawbacks compared with other positive airway pressure techniques. Our conclusions are based on a population of low surgical risk (excluded are people who took longer to wean off ventilators, people with pre‐existing lung disease, people undergoing emergent CABG surgery, and people with postoperative cardiac neurological complications). The small number of studies, the modest numbers of patients, the methodological limitations and the adjunctive use of other treatments in these patients means that currently available trials of IS contribute little to making decisions on its use.
Implications for research.
There is room for improvement in the methodological quality of studies aimed at evaluating the efficacy of IS. The methodological areas that can be improved are an adequate sample size, the concealment of allocation following the randomisation procedure, the use of a consensual definition of pulmonary complication and treatment regimes for use of IS.
A large scale RCT to asses the benefits of IS with and without standard postsurgical physical therapy compared with standard post‐surgical physical therapy, and compared with the absence of physical therapy (preoperative education only) in patients undergoing CABG is needed. New RCTs should also follow Consort‐Statement (www.consort‐statement.org) guidelines and that would contribute to clinical decision‐making in dentistry.
What's new
| Date | Event | Description |
|---|---|---|
| 14 May 2018 | Review declared as stable | The review question is considered of low priority for the current portfolio of the Heart Group. We are not aware of new trials and deem it unlikely that a review update would inform clinical practice. |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 3, 2007
| Date | Event | Description |
|---|---|---|
| 31 July 2011 | New citation required but conclusions have not changed | Of 366 additional references found in the update of this review, two new trials were included (Savci 2006; Romanini 2007). One study which had been excluded in the previous review, was included due to additional data being presented (Dull 1983). Results remain unchanged. |
| 31 July 2011 | New search has been performed | Two new outcomes were included: respiratory muscle strength reported by Romanini 2007 and functional capacity by Savci 2006. |
Acknowledgements
Trials Search Co‐ordinator and Feedback Editor of Cochrane Heart Group, for searching databases.
Appendices
Appendix 1. Search strategies 2004
CENTRAL
(Terms in capitals are exploded MeSH terms, those in lower case text words.) 1 CORONARY ARTERY BYPASS (ME exp) 2 MYOCARDIAL REVASCULARIZATION (ME) 3 CARDIAC SURGICAL PROCEDURES (ME) 4 THORACIC SURGERY (ME) 5 CARDIOPULMONARY BYPASS (ME) 6 (#1 or #2 or #3 or #4 or #5) 7 cabg 8 (coronary near bypass*) 9 (heart near bypass*) 10 (cardiopulmonary near bypass*) 11 (cardiac near surgery) 12 (heart near surgery) 13 (thora* near surgery) 14 (#7 or #8 or #9 or #10 or #11 or #12 or #13) 15 (#6 or #14) 16 SPIROMETRY (ME exp) 17 spiromet* 18 broncospiromet* 19 RESPIRATORY THERAPY (ME) 20 WORK OF BREATHING (ME) 21 bronco spirograph* 22 spirograph* 23 (lung next function) 24 PHYSICAL THERAPY TECHNIQUES (ME) 25 BREATHING EXERCISES (ME) 26 (#16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25) 27 (breath* near exercise*) 28 (breath* near measur*) 29 (incentive near breath*) 30 physiotherap* 31 triflo* 32 spirocare 33 (breath* near device*) 34 (respiratory next therapy) 35 (maxim* near inspira*) 36 coach 37 (#27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36) 38 (#26 or #37) 39 (#15 and #38)
MEDLINE
1 exp Coronary Artery Bypass/ 2 myocardial revascularization/ 3 Cardiac Surgical Procedures/ 4 Thoracic Surgery/ 5 Cardiopulmonary Bypass/ 6 cabg.tw. 7 (coronary adj3 bypass$).tw. 8 (heart adj3 bypass$).tw. 9 (cardiopulmonary adj3 bypass$).tw. 10 cardiac surgery.tw. 11 heart surgery.tw. 12 thoracic surgery.tw. 13 or/1‐12 14 exp Spirometry/ 15 spiromet$.tw. 16 bronchospiromet$.tw. 17 Respiratory Therapy/ 18 Work of Breathing/ 19 bronchospirograph$.tw. 20 spirograph$.tw. 21 lung function.tw. 22 Physical Therapy Techniques/ 23 Breathing Exercises/ 24 (breath$ adj3 exercise$).tw. 25 (breath$ adj3 measur$).tw. 26 (incentive adj3 breath$).tw. 27 physiotherap$.tw. 28 exp Forced Expiratory Flow Rates/ 29 spirocare.tw. 30 triflo.tw. 31 (breath$ adj3 device$).tw. 32 respiratory therap$.tw. 33 (maxim$ adj3 inspira$).tw. 34 coach.tw. 35 or/14‐34 36 13 and 35
EMBASE
1 exp Coronary Artery Bypass/ 2 exp Coronary artery surgery/ 3 Heart Surgery/ 4 Thorax Surgery/ 5 Cardiopulmonary Bypass/ 6 cabg.tw. 7 (coronary adj3 bypass$).tw. 8 (heart adj3 bypass$).tw. 9 (cardiopulmonary adj3 bypass$).tw. 10 cardiac surgery.tw. 11 heart surgery.tw. 12 thoracic surgery.tw. 13 or/1‐12 14 exp Spirometry/ 15 Spirography/ 16 spiromet$.tw. 17 bronchospiromet$.tw. 18 exp Lung function test/ 19 Bronchospirography/ 20 bronchospirograph$.tw. 21 spirograph$.tw. 22 lung function.tw. 23 Physiotherapy/ 24 Breathing Exercise/ 25 (breath$ adj3 exercise$).tw. 26 (breath$ adj3 measur$).tw. 27 (incentive adj3 breath$).tw. 28 physiotherap$.tw. 29 Forced Expiratory Flow/ 30 spirocare.tw. 31 triflo.tw. 32 (breath$ adj3 device$).tw. 33 respiratory therap$.tw. 34 (maxim$ adj3 inspira$).tw. 35 coach.tw. 36 or/14‐35 37 13 and 36 38 controlled study/ 39 clinical trial/ 40 major clinical study 41 random$.tw. 42 randomized controlled trial/ 43 trial.tw. 44 compar$.tw. 45 control$.tw. 46 follow‐up.tw. 47 blind$.tw. 48 double blind procedure/ 49 placebo$.tw. 50 clinical article/ 51 placebo/ 52 doubl$.tw. 53 or/38‐52 54 37 and 53
CINAHL
1 exp Coronary Artery Bypass/ 2 Heart Surgery/ 3 Thorax Surgery/ 4 Cardiopulmonary bypass/ 5 cabg.tw. 6 (coronary adj3 bypass$.tw. 7 (heart adj bypass$).tw. 8 (cardiopulmonary adj3 bypass).tw. 9 cardiac surgery.tw. 10 heart surgery.tw. 11 thoracic surgery.tw. 12 or/1‐11 13 exp spirometry/ 14 spiromet$.tw. 15 bronchospiromet$.tw. 16 exp Respiratory Function tests/ 17 bronchospirograph$.tw. 18 spirograph$.tw. 19 lung function.tw. 20 Physical Therapy/ 21 Breathing Exercises/ 22 (breath$ adj3 exercise$).tw. 23 (breath$ adj3 measur$).tw. 24 (incentive adj3 breath$).tw. 25 physiotherap$.tw. 26 exp respiratory airflow/ 27 spirocare.tw. 28 triflo.tw. 29 (breath$ adj3 device$).tw. 30 respiratory therap$.tw. 31 (maxim$ adj3 inspira$).tw. 32 coach.tw. 33 or/13‐32 34 12 and 33 35 experimental studies/ 36 exp clinical trial/ 37 ((control$ or clinic$ or prospective$) adj5 (trial$ or study or studies)).tw. 38 ((allocate$ or assign$ or divid$) adj5 (condition$ or experiment$ or treatment$ or control$ or group$)).tw. 39 ((singl$ or doubl$) adj (blind$ or mask$).tw. 40 cross?over$.tw. 41 placebo$.tw. 42 exp Clinical research/ 43 comparative studies/ 44 exp evaluation research/ 45 exp "control (research)"/ 46 Random assignment/ 47 exp Prospective studies/ 48 exp Evaluation research/ 49 random$.tw. 50 RCT.tw. 51 (compare$ adj5 (trial$ or study$ or studies)).tw. 52 or/35‐51 54 34 and 52
Appendix 2. Search strategies 2009/2011
CENTRAL and DARE
#1MeSH descriptor Coronary Artery Bypass explode all trees #2MeSH descriptor Myocardial Revascularization explode all trees #3MeSH descriptor Cardiac Surgical Procedures explode all trees #4MeSH descriptor THORACIC SURGERY this term only #5MeSH descriptor CARDIOPULMONARY BYPASS this term only #6(#1 or #2 or #3 or #4 or #5) #7cabg in All Text #8(coronary in All Text near/6 bypass* in All Text) #9(heart in All Text near/6 bypass* in All Text) #10(cardiopulmonary in All Text near/6 bypass* in All Text) #11(cardiac in All Text near/6 surgery in All Text) #12(heart in All Text near/6 surgery in All Text) #13(thora* in All Text near/6 surgery in All Text) #14(#7 or #8 or #9 or #10 or #11 or #12 or #13) #15(#6 or #14) #16MeSH descriptor Spirometry explode all trees #17spiromet* in All Text #18broncospiromet* in All Text #19MeSH descriptor RESPIRATORY THERAPY this term only #20MeSH descriptor WORK OF BREATHING this term only #21broncospirograph* in All Text #22spirograph* in All Text #23lung next function in All Text #24MeSH descriptor Physical Therapy Modalities this term only #25MeSH descriptor BREATHING EXERCISES this term only #26(#16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25) #27(breath* in All Text near/6 exercise* in All Text) #28(breath* in All Text near/6 measur* in All Text) #29(incentive in All Text near/6 breath* in All Text) #30physiotherap* in All Text #31triflo* in All Text #32spirocare in All Text #33(breath* in All Text near/6 device* in All Text) #34respiratory next therapy in All Text #35(maxim* in All Text near/6 inspira* in All Text) #36coach in All Text #37(#27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36) #38(#26 or #37) #39(#15 and #38)
MEDLINE 2009
1 exp Coronary Artery Bypass/ (37737) 2 myocardial revascularization/ (7885) 3 Cardiac Surgical Procedures/ (27271) 4 Thoracic Surgery/ (8782) 5 Cardiopulmonary Bypass/ (16722) 6 cabg.tw. (8782) 7 (coronary adj3 bypass$).tw. (29412) 8 (heart adj3 bypass$).tw. (1448) 9 (cardiopulmonary adj3 bypass$).tw. (19821) 10 cardiac surgery.tw. (18250) 11 heart surgery.tw. (10445) 12 thoracic surgery.tw. (5077) 13 or/1‐12 (112424) 14 exp Spirometry/ (15482) 15 spiromet$.tw. (11079) 16 bronchospiromet$.tw. (208) 17 Respiratory Therapy/ (5158) 18 Work of Breathing/ (1654) 19 bronchospirograph$.tw. (20) 20 spirograph$.tw. (613) 21 lung function.tw. (16247) 22 Physical Therapy Techniques/ (21768) 23 Breathing Exercises/ (2225) 24 (breath$ adj3 exercise$).tw. (1337) 25 (breath$ adj3 measur$).tw. (2763) 26 (incentive adj3 breath$).tw. (27) 27 physiotherap$.tw. (10077) 28 exp Forced Expiratory Flow Rates/ (8155) 29 spirocare.tw. (1) 30 triflo.tw. (13) 31 (breath$ adj3 device$).tw. (275) 32 respiratory therap$.tw. (1335) 33 (maxim$ adj3 inspira$).tw. (1752) 34 coach.tw. (754) 35 or/14‐34 (82156) 36 13 and 35 (838) 37 randomized controlled trial.pt. (278858) 38 controlled clinical trial.pt. (80338) 39 Randomized controlled trials/ (62927) 40 random allocation/ (65783) 41 double blind method/ (103539) 42 single‐blind method/ (13336) 43 or/37‐42 (470716) 44 exp animal/ not humans/ (3440908) 45 43 not 44 (438732) 46 clinical trial.pt. (456939) 47 exp Clinical Trials as Topic/ (220960) 48 (clin$ adj25 trial$).ti,ab. (165102) 49 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ti,ab. (100603) 50 placebos/ (28372) 51 placebo$.ti,ab. (119070) 52 random$.ti,ab. (458323) 53 research design/ (57560) 54 or/46‐53 (993616) 55 54 not 44 (920930) 56 45 or 55 (950392) 57 56 and 36 (189)
MEDLINE 2011
1. exp Coronary Artery Bypass/ 2. Myocardial Revascularization/ 3. Cardiac Surgical Procedures/ 4. Thoracic Surgery/ 5. Cardiopulmonary Bypass/ 6. cabg.tw. 7. (coronary adj3 bypass$).tw. 8. (heart adj3 bypass$).tw. 9. (cardiopulmonary adj3 bypass$).tw. 10. cardiac surgery.tw. 11. heart surgery.tw. 12. thoracic surgery.tw. 13. or/1‐12 14. exp Spirometry/ 15. spiromet$.tw. 16. bronchospiromet$.tw. 17. Respiratory Therapy/ 18. Work of Breathing/ 19. bronchospirograph$.tw. 20. spirograph$.tw. 21. lung function.tw. 22. Physical Therapy Modalities/ 23. Breathing Exercises/ 24. (breath$ adj3 exercise$).tw. 25. (breath$ adj3 measur$).tw. 26. (incentive adj3 breath$).tw. 27. physiotherap$.tw. 28. exp Forced Expiratory Flow Rates/ 29. spirocare.tw. 30. triflo.tw. 31. (breath$ adj3 device$).tw. 32. respiratory therap$.tw. 33. (maxim$ adj3 inspira$).tw. 34. coach.tw. 35. or/14‐34 36. 13 and 35 37. randomized controlled trial.pt. 38. controlled clinical trial.pt. 39. randomized.ab. 40. placebo.ab. 41. drug therapy.fs. 42. randomly.ab. 43. trial.ab. 44. groups.ab. 45. 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 46. exp animals/ not humans.sh. 47. 45 not 46 48. 36 and 47
EMBASE 2009
1 exp Coronary Artery Bypass/ 2 exp Coronary artery surgery/ 3 Heart Surgery/ 4 Thorax Surgery/ 5 Cardiopulmonary Bypass/ 6 cabg.tw. 7 (coronary adj3 bypass$).tw. 8 (heart adj3 bypass$).tw. 9 (cardiopulmonary adj3 bypass$).tw. 10 cardiac surgery.tw. 11 heart surgery.tw. 12 thoracic surgery.tw. 13 or/1‐12 14 exp Spirometry/ 15 Spirography/ 16 spiromet$.tw. 17 bronchospiromet$.tw. 18 exp Lung function test/ 19 Bronchospirography/ 20 bronchospirograph$.tw. 21 spirograph$.tw. 22 lung function.tw. 23 Physiotherapy/ 24 Breathing Exercise/ 25 (breath$ adj3 exercise$).tw. 26 (breath$ adj3 measur$).tw. 27 (incentive adj3 breath$).tw. 28 physiotherap$.tw. 29 Forced Expiratory Flow/ 30 spirocare.tw. 31 triflo.tw. 32 (breath$ adj3 device$).tw. 33 respiratory therap$.tw. 34 (maxim$ adj3 inspira$).tw. 35 coach.tw. 36 or/14‐35 37 13 and 36 38 controlled clinical trial/ 39 random$.tw. 40 randomized controlled trial/ 41 follow‐up.tw. 42 double blind procedure/ 43 placebo$.tw. 44 placebo/ 45 factorial$.ti,ab. 46 (crossover$ or cross‐over$).ti,ab. 47 (double$ adj blind$).ti,ab. 48 (singl$ adj blind$).ti,ab. 49 assign$.ti,ab. 50 allocat$.ti,ab. 51 volunteer$.ti,ab. 52 Crossover Procedure/ 53 Single Blind Procedure/ 54 or/38‐53 55 (exp animals/ or nonhuman/) not human/ 56 54 not 55 57 37 and 56
EMBASE 2011
1. exp coronary artery bypass graft/ 2. exp coronary artery surgery/ 3. heart surgery/ 4. thorax surgery/ 5. cardiopulmonary bypass/ 6. cabg.tw. 7. (coronary adj3 bypass$).tw. 8. (heart adj3 bypass$).tw. 9. (cardiopulmonary adj3 bypass$).tw. 10. cardiac surgery.tw. 11. heart surgery.tw. 12. thoracic surgery.tw. 13. or/1‐12 14. exp spirometry/ 15. spirography/ 16. spiromet$.tw. 17. bronchospiromet$.tw. 18. exp lung function test/ 19. bronchospirography/ 20. bronchospirograph$.tw. 21. spirograph$.tw. 22. lung function.tw. 23. physiotherapy/ 24. breathing exercise/ 25. (breath$ adj3 exercise$).tw. 26. (breath$ adj3 measur$).tw. 27. (incentive adj3 breath$).tw. 28. physiotherap$.tw. 29. forced expiratory flow/ 30. spirocare.tw. 31. triflo.tw. 32. (breath$ adj3 device$).tw. 33. respiratory therap$.tw. 34. (maxim$ adj3 inspira$).tw. 35. coach.tw. 36. or/14‐35 37. 13 and 36 38. random$.tw. 39. factorial$.tw. 40. crossover$.tw. 41. cross over$.tw. 42. cross‐over$.tw. 43. placebo$.tw. 44. (doubl$ adj blind$).tw. 45. (singl$ adj blind$).tw. 46. assign$.tw. 47. allocat$.tw. 48. volunteer$.tw. 49. crossover procedure/ 50. double blind procedure/ 51. randomized controlled trial/ 52. single blind procedure/ 53. 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 54. (animal/ or nonhuman/) not human/ 55. 53 not 54 56. 37 and 55 59. limit 56 to embase
AMED 2009
1 exp Coronary Artery Bypass/ 2 myocardial revascularization/ 3 Heart Surgery/ 4 Thoracic Surgery/ 5 cabg.tw. 6 (coronary adj3 bypass$).tw. 7 (heart adj3 bypass$).tw. 8 (cardiopulmonary adj3 bypass$).tw. 9 cardiac surgery.tw. 10 heart surgery.tw. 11 thoracic surgery.tw. 12 or/1‐11 13 exp Spirometry/ 14 spiromet$.tw. 15 bronchospiromet$.tw. 16 Respiratory Therapy/ 17 bronchospirograph$.tw. 18 spirograph$.tw. 19 lung function.tw. 20 Physiotherapy/ 21 Breathing Exercises/ 22 (breath$ adj3 exercise$).tw. 23 (breath$ adj3 measur$).tw. 24 (incentive adj3 breath$).tw. 25 physiotherap$.tw. 26 exp Forced Expiratory Flow Rates/ 27 spirocare.tw. 28 triflo.tw. 29 (breath$ adj3 device$).tw. 30 respiratory therap$.tw. 31 (maxim$ adj3 inspira$).tw. 32 coach.tw. 33 or/13‐32 34 12 and 33
AMED 2011
1 coronary artery bypass/ 2 myocardial revascularization/ 3 heart surgery/ 4 thoracic surgery/ 5 cabg.tw. 6 (coronary adj3 bypass*).tw. 7 (heart adj3 bypass*).tw. 8 (cardiopulmonary adj3 bypass*).tw. 9 cardiac surgery.tw. 10 heart surgery.tw. 11 thoracic surgery.tw. 12 or/1‐11 13 spirometry/ 14 spiromet*.tw. 15 bronchospiromet*.tw. 16 respiratory therapy/ 17 bronchospirograph*.tw. 18 spirograph*.tw. 19 lung function.tw. 20 Physiotherapy/ 21 breathing exercises/ 22 (breath* adj3 exercise*).tw. 23 (breath* adj3 measur*).tw. 24 (incentive adj3 breath*).tw. 25 physiotherap*.tw. 26 exp forced expiratory flow rates/ 27 spirocare.tw. 28 triflo.tw. 29 (breath* adj3 device*).tw. 30 respiratory therap*.tw. 31 (maxim* adj3 inspira*).tw. 32 coach.tw. 33 or/13‐32 34 12 and 33
CINAHL 2009
( ( (MH "Coronary Artery Bypass+") or cabg or coronary surgery or cardiac surgery or coronary N5 bypass or heart N5 bypass ) ) and ( ( (MH "Spirometry") or spirometr* or bronchospirometr* or spirocare or triflow or coach or spirograph* or (MH "Chest Physical Therapy") or (MH "Rehabilitation, Pulmonary+") or physiotherap* ) )
CINAHL 2011
S10 S3 and S9 S9 S4 or S5 or S6 or S7 or S8 S8 TI (physiotherap*) or AB (physiotherap*) S7 MH "Rehabilitation, Pulmonary+" S6 MH "Chest Physical Therapy" S5 TI (spirometr* or bronchospirometr* or spirocare or triflow or coach or spirograph*) or AB (spirometr* or bronchospirometr* or spirocare or triflow or coach or spirograph*) S4 MH "Spirometry" S3 S1 or S2 S2 TI (cabg or coronary surgery or cardiac surgery or coronary N5 bypass or heart N5 bypass) or AB (cabg or coronary surgery or cardiac surgery or coronary N5 bypass or heart N5 bypass) S1 MH "Coronary Artery Bypass+"
Data and analyses
Comparison 1. Atelectasis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Incentive spirometry versus conventional physical therapy | 2 | 257 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.58, 2.16] |
| 1.2 Incentive spirometry versus continuous positive airway pressure (CPAP) | 1 | 63 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.33 [0.72, 7.58] |
| 1.3 incentive spirometry versus bilevel positive airway pressure (BiPAP or NIV‐2P) | 1 | 63 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.33 [0.72, 7.58] |
| 1.4 Incentive spirometry versus Intermittente positive pressure breathing (IPPB) | 1 | 52 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.51 [0.76, 8.23] |
| 1.5 Incentive spirometry versus active cycle of breathing techniques | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.29, 2.53] |
| 1.6 Incentive spirometry versus preoperative physiotherapy advice only | 1 | 75 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.33, 2.11] |
1.2. Analysis.

Comparison 1: Atelectasis, Outcome 2: Incentive spirometry versus continuous positive airway pressure (CPAP)
1.3. Analysis.

Comparison 1: Atelectasis, Outcome 3: incentive spirometry versus bilevel positive airway pressure (BiPAP or NIV‐2P)
1.4. Analysis.

Comparison 1: Atelectasis, Outcome 4: Incentive spirometry versus Intermittente positive pressure breathing (IPPB)
1.5. Analysis.

Comparison 1: Atelectasis, Outcome 5: Incentive spirometry versus active cycle of breathing techniques
1.6. Analysis.

Comparison 1: Atelectasis, Outcome 6: Incentive spirometry versus preoperative physiotherapy advice only
Comparison 2. Pneumonia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Incentive spirometry versus conventional physical therapy | 2 | 258 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.31, 1.64] |
| 2.2 Incentive spirometry versus preoperative physiotherapy advice only | 1 | 75 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.08, 1.79] |
| 2.3 Incentive spirometry versus continuous positive airway pressure (CPAP) | 1 | 63 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.10 [0.07, 18.08] |
| 2.4 incentive spirometry versus bilevel positive airway pressure (BiPAP or NIV‐2P) | 1 | 63 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.17 [0.16, 413.39] |
| 2.5 Incentive spirometry versus Intermittente positive pressure breathing (IPPB) | 1 | 52 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.03 [0.63, 6.52] |
2.2. Analysis.

Comparison 2: Pneumonia, Outcome 2: Incentive spirometry versus preoperative physiotherapy advice only
2.3. Analysis.

Comparison 2: Pneumonia, Outcome 3: Incentive spirometry versus continuous positive airway pressure (CPAP)
2.4. Analysis.

Comparison 2: Pneumonia, Outcome 4: incentive spirometry versus bilevel positive airway pressure (BiPAP or NIV‐2P)
2.5. Analysis.

Comparison 2: Pneumonia, Outcome 5: Incentive spirometry versus Intermittente positive pressure breathing (IPPB)
Comparison 3. Vital capacity (ml).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Incentive spirometry versus conventional physical therapy | 3 | 291 | Mean Difference (IV, Fixed, 95% CI) | 183.26 [‐18.71, 385.24] |
| 3.2 Incentive spirometry versus continuous positive airway pressure (CPAP) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐338.00 [‐607.33, ‐68.67] |
| 3.3 incentive spirometry versus bilevel positive airway pressure (BiPAP or NIV‐2P) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐427.00 [‐655.04, ‐198.96] |
| 3.4 Incentive spirometry versus preoperative physiotherapy advice only | 2 | 106 | Mean Difference (IV, Fixed, 95% CI) | ‐17.34 [‐253.25, 218.57] |
| 3.5 Incentive spirometry versus Intermittente positive pressure breathing (IPPB) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐272.00 [‐350.68, ‐193.32] |
Comparison 4. Arterial oxygenation (PaO2/FiO2).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Incentive spirometry versus preoperative physiotherapy advice only | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 5.00 [‐13.38, 23.38] |
| 4.2 Incentive spirometry versus conventional physical therapy | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐5.00 [‐23.68, 13.68] |
| 4.3 Incentive spirometry versus continuous positive airway pressure (CPAP) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐14.00 [‐35.26, 7.26] |
| 4.4 incentive spirometry versus bilevel positive airway pressure (BiPAP or NIV‐2P) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐29.00 [‐53.80, ‐4.20] |
| 4.5 Incentive spirometry versus Intermittente positive pressure breathing (IPPB) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐35.00 [‐54.57, ‐15.43] |
| 4.6 Incentive spirometry versus active cycle of breathing techniques | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [‐39.49, 43.49] |
4.1. Analysis.

Comparison 4: Arterial oxygenation (PaO2/FiO2), Outcome 1: Incentive spirometry versus preoperative physiotherapy advice only
4.2. Analysis.

Comparison 4: Arterial oxygenation (PaO2/FiO2), Outcome 2: Incentive spirometry versus conventional physical therapy
4.3. Analysis.

Comparison 4: Arterial oxygenation (PaO2/FiO2), Outcome 3: Incentive spirometry versus continuous positive airway pressure (CPAP)
4.4. Analysis.

Comparison 4: Arterial oxygenation (PaO2/FiO2), Outcome 4: incentive spirometry versus bilevel positive airway pressure (BiPAP or NIV‐2P)
4.5. Analysis.

Comparison 4: Arterial oxygenation (PaO2/FiO2), Outcome 5: Incentive spirometry versus Intermittente positive pressure breathing (IPPB)
4.6. Analysis.

Comparison 4: Arterial oxygenation (PaO2/FiO2), Outcome 6: Incentive spirometry versus active cycle of breathing techniques
Comparison 5. Maximum inspiratory pressure (MIP).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Incentive spirometry versus Intermittente positive pressure breathing (IPPB) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 17.40 [0.61, 34.19] |
5.1. Analysis.

Comparison 5: Maximum inspiratory pressure (MIP), Outcome 1: Incentive spirometry versus Intermittente positive pressure breathing (IPPB)
Comparison 6. maximum expiratory pressure (MEP).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Incentive spirometry versus Intermittente positive pressure breathing (IPPB) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 12.75 [‐0.99, 26.49] |
6.1. Analysis.

Comparison 6: maximum expiratory pressure (MEP), Outcome 1: Incentive spirometry versus Intermittente positive pressure breathing (IPPB)
Comparison 7. Six‐minute walk test (6MWT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Incentive spirometry versus active cycle of breathing techniques | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐13.73 [‐59.43, 31.97] |
7.1. Analysis.

Comparison 7: Six‐minute walk test (6MWT), Outcome 1: Incentive spirometry versus active cycle of breathing techniques
Comparison 8. Forcede expiratory volume in one second (ml).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Incentive spirometry versus conventional physical therapy | 3 | 291 | Mean Difference (IV, Fixed, 95% CI) | 54.70 [‐14.41, 123.81] |
| 8.2 Incentive spirometry versus preoperative physiotherapy advice only | 2 | 108 | Mean Difference (IV, Fixed, 95% CI) | 13.91 [‐159.16, 186.98] |
| 8.3 Incentive spirometry versus continuous positive airway pressure (CPAP) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐183.00 [‐310.09, ‐55.91] |
| 8.4 incentive spirometry versus bilevel positive airway pressure (BiPAP or NIV‐2P) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐213.00 [‐369.12, ‐56.88] |
8.3. Analysis.

Comparison 8: Forcede expiratory volume in one second (ml), Outcome 3: Incentive spirometry versus continuous positive airway pressure (CPAP)
8.4. Analysis.

Comparison 8: Forcede expiratory volume in one second (ml), Outcome 4: incentive spirometry versus bilevel positive airway pressure (BiPAP or NIV‐2P)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Crowe 1977.
| Study characteristics | ||
| Methods | Randomisation using a computer‐generated random number table. | |
| Participants | 185 patients with chronic airflow limitation (153 men) following CABG. | |
| Interventions | Group 1 (n=95) were randomly assigned to postoperative physical therapy only. Group 2 (n = 90) were randomly assigned to postoperative physical therapy plus incentive spirometry. The incentive spirometry device was volume oriented. | |
| Outcomes | Primary outcome measure: atelectasis, marked collapse or consolidation (estimated by chest X‐ray). Secondary outcome measures included: estimation of lung infection, oxygen saturation, and number of postoperative days in hospital. | |
| Notes | Subjects receiving mammary artery conduits were equally distributed between the two treatment groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Quote: "..."subjects were assigned randomly to one of two treatment protocols using a computer‐generated random number table. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding (performance bias and detection bias) | Low risk | Only the evaluator was blind, no information about the blinding of patient and the therapist. Quote: "...These measurements were read and categorized by a single observer, who was blind to the treatment allocation of the patient." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In some outcomes (e.g. Incidence of atelectasis, an evidence of pleural effusion, oxygen saturation) a smaller number of patients were evaluated. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Intention to treat analysis | High risk | 199 patients eligible to entry criteria, but only 185 were analysed. |
| Follow‐up | Low risk | Patients assessed in the preoperatively and postoperatively at first, second, and third days. |
Dull 1983.
| Study characteristics | ||
| Methods | Method of randomisation unclear. | |
| Participants | 49 consecutive patients scheduled for cardiopulmonary bypass surgery, specifically CABG or valve replacement. | |
| Interventions | Four hours after extubation, patients were randomly assigned to three groups. Group 1 (n =16) received early mobilization twice a day (ankle circumduction, range of motion to all extremities, three maximal coughs and encouragement and assistance to turn from side to side, sit up, or stand up); group 2 (n=16) received early mobilization plus maximal inspiratory breathing exercises (10 repetitions of maximal inhalation from residual volume) four times a day; group 3 (n=17) received early mobilization plus incentive spirometry (10 repetitions of maximal inhalations from residual volume with an incentive spirometer) four times a day. | |
| Outcomes | Slow vital capacity, forced vital capacity; forced expiratory volume in one second, percentage of the forced vital capacity exhaled within the first second and forced slow between 200 and 1200 ml of forced vital capacity. | |
| Notes | The spirocare incentive breathing exercises were used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Quote: "... patients were randomly assigned to one of three exercise programs." |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding (performance bias and detection bias) | High risk | No information about the blinding of patients, therapist end evaluator. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No drop‐outs reported. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Intention to treat analysis | Low risk | 49 patients eligible to entry criteria, 10 were eventually withdrawn from the study. However 49 subjects were analysed. |
| Follow‐up | Low risk | Patients assessed four hours after extubation, and postoperative at first and second and third days. |
Jenkins 1989.
| Study characteristics | ||
| Methods | Stratified randomisation for age. | |
| Participants | 110 consecutive men undergoing CABG. | |
| Interventions | All patients were seen before surgery by a physiotherapist, who explained to need to move about after surgery and to expectorate excess bronchial secretions. Group 1 (n = 35) received usual postoperative physical therapy: three to five consecutive deep breaths were interspersed between a period of quite breathing, in the sitting or half lying position. Group 2 (n = 38) were taught to use an incentive spirometer (Triflo II, Sherwood Medical Industries) in the sitting or half lying position. Three to five consecutive breaths with the spirometer were interposed between period of quiet breathing. Group 3 (n = 37) received the preoperative physiotherapy advice only. | |
| Outcomes | Vital capacity, functional residual capacity, and forced expiratory volume in 1 second (FEV1), at the bedside on the afternoon of the second postoperative day and at the same time each successive day. | |
| Notes | Only white men were considered for inclusion in the study. Patients who had previously had cardiac surgery and those unable to walk the length of the ward (64 metres) for reasons other than angina were excluded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "... Stratified randomisation to balance for age and forced expiratory ratio (FER) ..." |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) | High risk | No information about the blinding of patients, therapist end evaluator. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No drop‐outs reported. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Intention to treat analysis | High risk | 141 patients were considered for the study, however 110 patients were available for analysis |
| Follow‐up | Low risk | Quote: "...After surgery (which was performed via a median sternotomy) all patients were seen by a physiotherapist at least twice on days one and two and at least once daily on days three to five." |
Matte 2000.
| Study characteristics | ||
| Methods | Method of randomisation unclear. | |
| Participants | 96 patients undergoing elective CABG with the use of mammary arteries. | |
| Interventions | After extubation, patients were randomly assigned to three groups. Group 1 (n = 30) received routine chest physiotherapy (RCP: coughing exercises, aerosol therapy, mobilizations) plus incentive spirometry (Coach‐volume) 20/2 h. Group 2 (n = 33) received RCP plus continuous positive airway pressure (CPAP), 5cm H2O, 1h/3h. Group 3 (n = 33) received RCP plus non invasive ventilation with two levels of pressure (NIV‐2P), with expiratory positive airway pressure (EPAP) set at 5 cm H2O and peak inspiratory positive airway pressure (IPAP) at 12 cm H2O 1h/3h. | |
| Outcomes | Vital capacity and forced expiratory volume in 1 second were measured on the first postoperative day before the start of the study and on the second postoperative day (24 h after the start of the study). Chest X‐rays obtained preoperatively and on the first and second postoperative day were analysed for atelectasis. Other measurements: arterial and mixed venous oxygen content, blood gases. | |
| Notes | Patients with chronic obstructive pulmonary disease were excluded. Blood was drawn from the radial and the pulmonary artery for gas analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...After extubation, patients were randomly assigned to 3 groups." |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) | Low risk | The evaluators were blind, but no information about the blinding of patient and the therapist |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No drop‐outs reported. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Intention to treat analysis | Low risk | Ninety‐six patients undergoing elective CABG were included, six patients were excluded for non conformity with the study protocol, however 96 subjects were analysed. |
| Follow‐up | Low risk | The patients were assessed in the preoperative, and postoperative at first and second days. |
Oikkonen 1991.
| Study characteristics | ||
| Methods | Method of randomisation unclear. | |
| Participants | 52 patients (22 men) following CABG. | |
| Interventions | Group 1 (n = 26) received incentive spirometry plus conventional postoperative physical therapy. The incentive spirometer (DHD Coach, DHD Medical Products, USA) was equipped with a volume‐oriented goal indication and the aimed at inhalation exceeded 3 was repeated at least five times per session. Group 2 (n = 26) received intermittent positive pressure breathing (IPPB) (Bennett PR2, Puritan Bennett, USA) plus conventional postoperative physical therapy. The peak airway pressure was adjusted, ranging from 10 to 15 cm H2O at each session. | |
| Outcomes | Vital capacity (expiratory), slow vital capacity, peak expiratory flow. Chest X‐rays were analysed for atelectasis, pulmonary infiltration and pleural effusion. Arterial blood gas values were taken. | |
| Notes | Patients with chronic obstructive pulmonary disease were excluded. Saphenous vein aorta coronary graft, and usually LIMA‐LAD grafts were constructed under hypothermic bypass. Cold cardioplegia and local pericardial cooling were used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... The patients were randomly allocates to receive IPPB or IS in addition to CPT." |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) | High risk | No information about the blinding of patients, therapist end evaluator. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No drop‐outs reported. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Intention to treat analysis | Low risk | 52 consecutive patients scheduled for CABG were select for the study, 9 were excluded (age older than 70 years, weight exceeding the ideal weight by more than 20 percent, history chronic obstructive pulmonary disease (COPD), thoracic anomalies, etc.), however 52 were analysed. |
| Follow‐up | Low risk | The patients were assessed in the preoperative, and postoperative at one to seven days. |
Romanini 2007.
| Study characteristics | ||
| Methods | Randomized by drawing lots, prior to the surgery. | |
| Participants | 40 patients submitted to myocardial revascularization surgery. | |
| Interventions | The patients in the IPPB group (n=20 ‐ 40% women and 60% men) were submitted to intermittent positive pressure breathing (IPPB) with a rubber facial mask for ten minutes, followed by a 5‐minute interval and a new application for 10 minutes. The IS group (n=20 ‐ 20% women and 80% men) was submitted for the same time and interval. |
|
| Outcomes | The following postoperative parameters were assessed: oxygen saturation (SpO2) in ambient air measured by a pulse oxymeter manufactured by Nonin®, model 9500, current volume (CV = MV (minute volume)/RF (respiratory frequency), measured in a Whigth ventilometer manufactured by Respirameter®, model Ferraris‐Mark 8, maximum inspiratory pressure (Ipmax or MIP) and maximum expiratory pressure (Epmax orMEP), which was measured in a Gerar® equipment. These data were collected 24, 48 and 72 hours after surgery. | |
| Notes | The volume‐oriented incentive spirometry, using a Voldyne model 5000 TM, (Sherwood Medical, USA) and Müller Reanimator (MR) manufactured by Engesp® was used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...The study was prospective and the patients were randomised by drawing lots, prior to the surgery, and divided into 2 groups: IPPB: 20 patients; Incentive Spirometry: 20 patients. (It was not necessary to change patients from the groups due to clinical criteria). |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) | High risk | No information about the blinding of patients, therapist end evaluator. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No drop‐outs reported. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Intention to treat analysis | Unclear risk | Insufficient information |
| Follow‐up | Low risk | The patients were assessed in the preoperative, and postoperative at first and second days. |
Savci 2006.
| Study characteristics | ||
| Methods | Method of randomisation unclear | |
| Participants | Sixty male patients (41‐75 years) with CABG. | |
| Interventions | All patients received basic post‐operative respiratory physiotherapy including breathing exercises, instruction in huffing and coughing techniques, mobilization, and active exercises of upper limbs and thorax.The patients were instructed to sit out of bed and stand up on the first post‐operative day (2‐3 times). They walked 30 m in the intensive care unit in the morning and 80 m on the ward in the afternoon on the first post‐operative. On the third post‐operative day, they walked freely in the corridor. Active cycle breathing techniques (ACBT) consisted of 1‐2 breathing control breaths, three thoracic expansion exercises followed by a 3 second breath hold at the end of deep inspiration, and the forced expiration techniques including 1‐2 breathing control breaths combined with 1‐2 huffs. Manual techniques were not included in ACBT. Siting or high sitting positions were used during the treatment. IS was applied as three deep breaths followed by a 3‐second breath hold at the end of the deep inspirations. Afterward, 1‐2 huffing was performed with 1‐2 breathing control. On the first and the second post‐operative days, treatment was applied twice a day, 1 minutes per session. From the third day following surgery, it was applied once a day for 15 minutes. | |
| Outcomes | Vital capacity (VC), forced vital capacity (FVC), forced expiratory volume in one second (FEV1), peak expiratory flow rate (PEF), functional capacity (six‐minute walk test (6MWT)), pain intensity, partial oxygen pressure (PaO2), partial arterial carbon dioxide pressure (PaCO2), oxygen saturation (SaO2), bicarbonate level (HCO3), and arterial pH. | |
| Notes | All patients had left internal mammaria artery graft | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... Eligible patients were randomly allocated to received either active cycle of breathing techniques or incentive spirometer." |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) | High risk | No information about the blinding of patients, therapist end evaluator. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No drop‐outs reported. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Intention to treat analysis | Unclear risk | Insufficient information. |
| Follow‐up | Unclear risk | The patients were assessed in the preoperative, and postoperative at one to five days. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ferreira 2010 | Combined IS connected with EPAP |
| Gale 1980 | Combined CABG with other interventions, and did not separate the results for CABG |
| Haeffener 2008 | Combined IS connected with EPAP and showed the results in form of graphs or illustrations. |
| Iverson 1978 | Paper did not meet inclusion criteria. Allocation concealment inadequate. |
| Jenkins 1990 | Duplicated trial |
| Mahler 1998 | Trial with non‐relevant end‐points. |
| Oulton 1981 | Trial with non‐relevant end‐points. |
| Paul 1981 | Paper did not meet inclusion criteria. Trial not controlled. |
| Rau 1988 | Trial with non‐relevant end‐points. |
| Renault 2009 | Reported inconsistent data. |
| Stock 1984 | Combined CABG with other interventions, and did not separate of the results for CABG. |
| Vraciu 1977 | Trial with non‐relevant end‐points. |
CABG = coronary artery bypass graft
Contributions of authors
Eliane Regina Ferreira Sernache de Freitas (ERFSF) and Alvaro Nagib Atallah (ANA) proposed the protocol and guarantor of review. Jefferson Rosa Cardoso (JRC), Bernardo Garcia de Oliveira Soares (BGOS) and ERFSF assessed the titles and abstracts identified by the electronic search. ERFSF, JRC and ANA undertook the assessment of risk of bias. ERFSF and JRC extracted the information from the RCT. All authors corrected the last draft.
Sources of support
Internal sources
Federal University of São Paulo, Brazil
University of Northern Paraná (UNOPAR), Brazil
External sources
No sources of support supplied
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Crowe 1977 {published data only}
- Crowe JM, Brandlay CA. The effectiveness of incentive spirometry with physical therapy for high-risk patients after coronary artery bypass surgery. Physical Therapy 1977;77(3):260-8. [DOI] [PubMed] [Google Scholar]
Dull 1983 {published data only}
- Dull JL, Dull WL. Are maximal inspiratory breathing exercises or incentive spirometry better than early mobilization after cardiopulmonary bypass? Physical Therapy 1983;63(5):655-659. [DOI] [PubMed] [Google Scholar]
Jenkins 1989 {published data only}
- Jenkins SC, Soutar SA, Loukota JM, Johnson LC, Moxham J. Physiotherapy after coronary artery surgery: are breathing exercises necessary? Thorax 1989;44(8):634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matte 2000 {published data only}
- Matte P, Jacquet L, Van Dyck V, Goenen M. Effects of conventional physiotherapy, continuous positive airway pressure and non-invasive ventilatory support with bilevel positive airway pressure after coronary artery bypass grafting. Acta Anaesthesiologica Scandinavica 2000;44(1):75-81. [DOI] [PubMed] [Google Scholar]
Oikkonen 1991 {published data only}
- Oikkonen M, Karjalainen K, Kahara V, Kuosa R, Schavikin L. Comparison of incentive spirometry and intermittent positive pressure breathing after coronary artery bypass graft. Chest 1991;99(1):60-5. [DOI] [PubMed] [Google Scholar]
Romanini 2007 {published data only}
- Romanini W, Muller AP, Carvalho KAT, Olandoski M, Faria-Neto JR, Mendes FL, et al. The effects of intermittent positive pressure and incentive spirometry in the postoperative of myocardial revascularizarion [Os efeitos da pressão positiva intermitente e do incentivador respiratório no pós-operatório de revascularização miocárdica]. Arq Bras Cardiol 2007;89(2):105-110. [DOI] [PubMed] [Google Scholar]
Savci 2006 {published data only}
- Savci S Sakinç S, Ince DI, Arikan H, Zehr C, Buran Y, Kuralay E. Active cycle of breathing techniques and incentive spirometer in coronary artery bypass graft surgery. Fizyoterapi Rehabilitasyon 2006;17(2):61-69. [Google Scholar]
References to studies excluded from this review
Ferreira 2010 {published data only}
- Ferreira GM, Haeffner MP, Barreto SSM, Dall' Ago. Incentive spirometry with expiratory positive airway pressure brings benefits after myocardial revascularization. Arquivos Brasileiros de Cardiologia 2010;94(2):230-235. [DOI] [PubMed] [Google Scholar]
Gale 1980 {published data only}
- Gale GD, Sanders DE. Incentive spirometry: its value after cardiac surgery. Canadian Anaesthetists' Society Journal 1980;27(5):475-80. [DOI] [PubMed] [Google Scholar]
Haeffener 2008 {published data only}
- Haeffener MP, Ferreira GM, Barreto SSM, Arena R, DallÁgo P. Incentive spirometry with expiratory positive airway pressure reduces pulmonary complications, improves pulmonary function and 6-minute walk distance in patients undergoing coronary artery bypass graft surgery. American Heart Journal 2008;156(5):900.e2-900.e7. [DOI: ] [DOI] [PubMed] [Google Scholar]
Iverson 1978 {published data only}
- Iverson LIG, Ecker RR, Fox HE, May IA. A comparative study of IPPB, the incentive spirometer, and blow bottles: the prevention of atelectasis following cardiac surgery. Annals of Thoracic Surgery 1978;25(3):197-200. [DOI] [PubMed] [Google Scholar]
Jenkins 1990 {published data only}
- Jenkins SC, Soutar SA, Loukota JM, Johnson LC, Moxham J. A comparison of breathing exercises, incentive spirometry and mobilisation after coronary artery. Physiotherapy Theory and Practice 1990;6(3):117-126. [Google Scholar]
Mahler 1998 {published data only}
- Mahler HI, Kulik JA. Effects of preparatory videotapes on self-efficacy beliefs and recovery from coronary bypass surgery. Annals of Behavioral Medicine 1998;20(1):39-46. [DOI] [PubMed] [Google Scholar]
Oulton 1981 {published data only}
- Oulton JL, Hobbs GM, Hicken P. Incentive breathing devices and chest physiotherapy: a controlled therapy. Canadian Journal of Surgery 1981;24(6):638-40. [PubMed] [Google Scholar]
Paul 1981 {published data only}
- Paul WL, Dows JB. Postoperative atelectasis. Intermittent positive pressure breathing, incentive spirometry and face-mask positive and-expiratory pressure. Archives of Surgery 1981;116(7):861-3. [DOI] [PubMed] [Google Scholar]
Rau 1988 {published data only}
- Rau JL Jr, Thomas L, Haynes RL. The effect of method of administering incentive spirometry on postoperative pulmonary complications in coronary artery bypass patients. Respiratory Care 1988;33(9):771-8. [Google Scholar]
Renault 2009 {unpublished data only}
- Renault JA, Costa-Val R, Rosseti MB, Houri Neto M. Comparison between deep breathing exercises and incentive spirometry after CABG surgery. Revista Brasileira de Cirurgia Cardiovascular 2009;24(2):165-172. [DOI] [PubMed] [Google Scholar]
Stock 1984 {published data only}
- Stock MC, Dows JB, Cooper RB, Lebenson IM, Cleveland J, Weaver DE, et al. Comparison of continuous positive airway pressure, incentive spirometry, and conservative therapy after cardiac operations. Critical Care Medicine 1984;12(11):969-72. [DOI] [PubMed] [Google Scholar]
Vraciu 1977 {published data only}
- Vraciu JK, Vraciu RA. Effectiveness of breathing exercises in preventing pulmonary complications following open heart surgery. Physical Therapy 1977;57(12):1367-71. [DOI] [PubMed] [Google Scholar]
Additional references
AARC 1991
- Hilling L, Bakow E, Fink J, Kelly C, Sobush D, Southorn PA. AARC (American Association for Respiratory Care) clinical practice guideline. Incentive spirometry. Respiratory Care 1991;36(12):1402-5. [PubMed] [Google Scholar]
ACC/AHA 1999
- Eagle KA, Guyton RA, Davidiff R, Ewy GA, Fonger J, Gardner TJ, et al. ACC/AHA Guidelines for Coronary Artery Bypass Graft Surgery: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1991 Guidelines for Coronary Artery Bypass Graft Surgery). Journal of the American College of Cardiology 1999;34(4):1262-347. [DOI] [PubMed] [Google Scholar]
Agostini 2008
- Agostini P, Calvert R, Subramanian H, Naidu B. Is incentive spirometry effective following thoracic surgery? Interactive Cardiovascular and Thoracic Surgery 2008;7(2):297-300. [DOI: 10.1510/icvts.2007.171025] [DOI] [PubMed] [Google Scholar]
ATS 2002
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories American Thoracic Society. ATS statement: guidelines for the six-minute walk test. Am j Respir Crit Care Med 2002;166:111-117. [DOI] [PubMed] [Google Scholar]
Bartlett 1970
- Bartlett RH, Krop P, Hanson EL, Moore FD. Physiology of yawning and its application to postoperative care. Surgical Forum 1970;21:223-4. [PubMed] [Google Scholar]
Bartlett 1973
- Bartlett RH, Gazzaniga AB, Geraghty TR. Respiratory maneuvers to prevent postoperative pulmonary complications. A critical review. JAMA 1973;224:1017-21. [PubMed] [Google Scholar]
Berrizbetia 1989
- Berrizbeitia LD, Tessler S, Jacobowitz IJ, Kaplan P, Budzilowicz L, Cunningham JN. Effect of sternotomy and coronary bypass surgery on postoperative pulmonary mechanics. Comparison of internal mammary and saphenous vein bypass grafts. Chest 1989;96(4):873-876. [DOI: 10.1378/chest.96.4.873] [DOI] [PubMed] [Google Scholar]
Brooks 2001
- Brooks D, Crowe J, Kelsey CJ, Lacy JB, Parsons J, Solway S. A clinical practice guideline on peri-operative cardiorespiratory physical therapy. Physiotherapy Canada 2001;53(1):9-25. [Google Scholar]
Cavenaghi 2011
- Cavenaghi S, Ferreira LL, Marino LH, Lamari NM. Respiratory physiotherapy in the pre and postoperative myocardial revascularization surgery. Revista Brasileira de Cirurgia Cardiovascular 2011;26(3):455-461. [DOI: 10.5935/1678-9741.20110022] [DOI] [PubMed] [Google Scholar]
Celli 1984
- Celli BR, Rodriguez KS, Snider GL. A controlled trial of intermittent positive pressure breathing, incentive spirometry, and deep breathing exercises in preventing pulmonary complications after abdominal surgery. American Review of Respiratory Disease 1984;130(1):12-5. [DOI] [PubMed] [Google Scholar]
Chandler 1984
- Chandler KW, Rozas CJ, Kory RC, Goldman AL. Bilateral diaphragmatic paralysis complicating local cardiac hypothermia during open heart surgery. Am J Med 1984;77(2):243-249. [DOI] [PubMed] [Google Scholar]
Chulay 1982
- Chulay M, Brown J, Summer W. Effect of postoperative immobilization after coronary artery bypass surgery. Critical Care Medicine 1982;10(3):176-9. [DOI] [PubMed] [Google Scholar]
Chuter 1990
- Chuter TM, Weissman C, Mathews DM, Starker PM. Diaphragmatic breathing maneuvers and movement of the diaphragm after cholecystectomy. Chest 1990;97(5):1110-4. [DOI] [PubMed] [Google Scholar]
Clarke 2001
- Clarke M, Oxman AD. Cochrane Database of Systematic Reviews. Oxford: Update Software, 2001. [Google Scholar]
Clarke 2003
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook 4.1.6 [updated January 2003 ]. Oxford: Update Software, 2003. [Google Scholar]
CPA 1990
- Canadian Physiotherapy Association (CPA). Final Consensus Statement. Contact 1990 (April).
Craven 1974
- Craven JL, Evans GA, Davenport PJ, Williams RHP. The evaluation of incentive spirometry in the management of postoperative pulmonary complications. British Journal of Surgery 1974;61(10):793-7. [DOI] [PubMed] [Google Scholar]
Daganou 1998
- Daganou M, Dimopoulou I, Michalopoulos N, Papadopoulos K, Karakatsani A, Geroulanos S, et al. Respiratory complications after coronary artery bypass surgery with unilateral or bilateral internal mammary artery grafting. Chest 1998;113(5):1285-1289. [DOI: 10.1378/chest.113.5.1285] [DOI] [PubMed] [Google Scholar]
Ferguson 1999
- Ferguson MK. Preoperative assessment of pulmonary risk. Chest 1999;115(5 Suppl):58S-63S. [DOI] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliot P, Suh I, Cuter J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769-73. [DOI] [PubMed] [Google Scholar]
Freitas 2007
- Freitas ERFS, Soares BGO, CArdoso JR, Atallh AN. Incentive spirometry for preventing pulmonary complications after coronary artery bypass graft. Cochrane Database of Systematic Reviews 2007, Issue 3. Art. No: CD004466. [DOI: 10.1002/14651858.CD004466.pub2] [DOI] [PubMed] [Google Scholar]
Groeneveld 2006
- Groeneveld AB, Verheij J, van den Berg FG, Wisselink W, Rauwerda JA. Increased pulmonary capillary permeability and extravascular lung water after major vascular surgery: effect on radiography and ventilatory variables. European journal of anaesthesiology 2006;23(1):36-41. [DOI] [PubMed] [Google Scholar]
Groeneveld 2007
- Groeneveld AB, Jansen EK, Verheij J. Mechanisms of pulmonary dysfunction after on-pump and off-pump cardiac surgery: a prospective cohort study. Journal of cardiothoracic surgery 2007;2(11):1-7. [DOI: 10.1186/1749-8090-2-11] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 1991
- Hall JL, Tarala R, Harris J, Tapper J, Christiansen K. Incentive spirometry versus routine chest physiotherapy for prevention of pulmonary complications after abdominal surgery. Lancet 1991;337(8747):953-6. [DOI] [PubMed] [Google Scholar]
Hall 1996
- Hall JC, Tarala RA, Tapper J, Hall JL. Prevention of respiratory complications after abdominal surgery: A randomised clinical trial. BMJ 1996;312(7024):148-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hallbook 1984
- Hallbook T, Lindblad B, Lindroth B, Wolff T. Prophylaxis against pulmonary complications in patients undergoing gall-bladder surgery. A comparison between early mobilization, physiotherapy with and without bronchodilatation. Annales Chirurgiae et Gynaecologiae 1984;73(2):55-8. [PubMed] [Google Scholar]
Hedderich 1999
- Hedderich R, Ness TJ. Analgesia for trauma and burns. Critical Care Clinics 1999;15(1):167-84. [DOI] [PubMed] [Google Scholar]
Higgins 1988
- Higgins M, Kannel WB, Garrison R, Pinski J, Stokes J 3rd. Hazards of obesity. The Framingham experience. Acta Medica Scandinavica - Supplementum 1988;723:23-36. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Wiley-Blackwell 2011.
Hulzebos 2003
- Hulzebos EH, Van Meeteren NL, De Bie RA, Dagnelie PC, Helders PJ. Prediction of postoperative pulmonary complications on the basis of preoperative risk factors in patients who had undergone coronary artery bypass graft surgery. Physical therapy 2011;113(5):994-1002. [DOI: 10.1213/ANE.0b013e31822c94a8] [DOI] [PubMed] [Google Scholar]
Jenkins 1986
- Jenkins SC, Soutar SA. A survey into the use of incentive spirometry following coronary artery by-pass graft surgery. Physiotherapy 1986;72(10):492-3. [Google Scholar]
Kannel 1980
- Kannel WB, Dawber JR, Mc Gee DL. Perspectives on systolic hypertension. The Framingham study. Circulation 1980;61(6):1179-82. [DOI] [PubMed] [Google Scholar]
Kannel 1984
- Kannel WB, McGee DL, Castelli WP. Latest perspectives on cigarette smoking and cardiovascular disease: The Framingham study. Journal of Cardiac Rehabilitation 1984;4(7):267-77. [Google Scholar]
Kannel 1985
- Kannel WB, Wilson PWF, Blair SN. Epidemiologic assessment of the role of physical activity and fitness in development of cardiovascular disease. American Heart Journal 1985;109(4):876-85. [DOI] [PubMed] [Google Scholar]
Kannel 1986
- Kannel WB. Hypertension: Relationship with other risk factors. Drugs 1986;31(Suppl 1):1-11. [DOI] [PubMed] [Google Scholar]
Kannel 1987
- Kannel WB, Sytkowski PA. Atherosclerosis risk factors. Pharmacology & Therapeutics 1987;32(3):207-35. [DOI] [PubMed] [Google Scholar]
Keenan 2005
- Keenan TD, Abu-Omar Y, Taggart DP. Bypassing the pump: changing practices in coronary artery surgery.. Chest 2005;128(1):363-369. [DOI: 10.1378/chest.128.1.363] [DOI] [PubMed] [Google Scholar]
Kips 1997
- Kips JC. Preoperative pulmonary evaluation. Acta Clinica Belgica 1997;52(5):301-5. [DOI] [PubMed] [Google Scholar]
Lawrence 1995
- Lawrence VA, Hilsenbeck SG, Mulrow CD, Dhanbla R, Sapp J, Page CP. Incidence and hospital stay for cardiac and pulmonary complications after abdominal surgery. Journal of General Internal Medicine 1995;10(12):671-8. [DOI] [PubMed] [Google Scholar]
Leal‐Noval 2000
- Leal-Noval SR, Marquez-Vacaro JA, Garcia-Curiel A, Camacho-Larana P, Rincon-Ferrari MD, Ordonez-Fernandez A, et al. Nosocomial pneumonia in patients undergoing heart surgery. Critical Care Medicine 2000;28(4):935-40. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies.. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org..
Mang 1991
- Mang H, Kacmarek RM. Prevention of pulmonary complications after abdominal surgery. Lancet 1991;228(8762):312-3. [DOI] [PubMed] [Google Scholar]
Marini 1984
- Marini JJ. Postoperative atelectasis: pathophysiology, clinical importance, and principles of management. Respiratory Care 1984;29(5):516-28. [Google Scholar]
Meyers 1975
- Meyers JR, Lembeck L, O'Kane H, Baue AE. Changes in functional residual capacity of the lung after operation. Archives of Surgery 1975;110(5):567-83. [DOI] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Lancet 1999;354(9193):1896-900. [DOI] [PubMed] [Google Scholar]
Mohr 1996
- Mohr DN, Lavender RC. Preoperative pulmonary evaluation. Identifying patients at increased risk for complications. Postgraduate Medicine 1996;100(5):241-56. [DOI] [PubMed] [Google Scholar]
Mohr 2011
- Mohr FW, Rastan AJ, Serruys PW, Kappetein AP, Holmes DR, Pomar JL, et al. Complex coronary anatomy in coronary artery bypass graft surgery: impact of complex coronary anatomy in modern bypass surgery? Lessons learned from the SYNTAX trial after two years.. The Journal of thoracic and cardiovascular surgery 2011;141(1):130-140. [DOI: 10.1016/j.jtcvs.2010.07.094] [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramer MR, Collins SL, McQuay HJ. Size is everything - large amounts of information are need to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209-16. [DOI] [PubMed] [Google Scholar]
Mortasawi 2004
- Mortasawi A, Arnrich B, Walter J, Frerichs I, Rosendahl U, Ennker J. Impact of age on the results of coronary artery bypass grafting. Asian Cardiovascular & Thoracic Annals 2004;12(4):324-9. [DOI] [PubMed] [Google Scholar]
O'Donohue 1985
- O' Donohue WJ Jr. National survey of the usage of lung expansion modalities for the prevention and treatment of post-operative atelectasis following abdominal and thoracic surgery. Chest 1985;87(1):76-80. [DOI] [PubMed] [Google Scholar]
Overend 2001
- Overend TJ, Anderson CM, Lucy SD, Bhatia C, Jonsson B, Timmermans C. The effect of incentive spirometry on postoperative pulmonary complications: a systematic review. Chest 2001;120(3):971-8. [DOI] [PubMed] [Google Scholar]
Pasquina 2003
- Pasquina P, Tramers MR, Walder B. Prophylactic respiratory physiotherapy after cardiac surgery: systematic review. BMJ 2003;327(7428):1379-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petz 1979
- Petz TJ. Physiologic effect of IPPB, blow bottles and motivates spirometry. Current Reviews in Respiratory Therapy 1979;1:107-11. [Google Scholar]
Pinheiro 2011
- Pinheiro AC, Novais MCM, Gomes Neto M, Rodrigues MVH, Rodrigues Junior ES, Aras-Junior R, et al. Estimation of lung vital capacity before and after coronary artery bypass grafting surgery: a comparison of incentive spirometer and ventilometry. Journal of Cardiothoracic Surgery 2011;6(70):1-5. [DOI: 10.1186/1749-8090-6-70] [DOI] [PMC free article] [PubMed] [Google Scholar]
Renault 2008
- Renault JA, Costa-Val Ricardo, Rosseti MB. Respiratory physiotherapy in the pulmonary dysfunction after cardiac surgery [Fisioterapia respiratoria na disfunção pulmonar pós-cirurgia cardiaca]. Revista Brasileira de Cirurgia Cardiovascular 2008;23(4):562-569. [DOI] [PubMed] [Google Scholar]
Restrepo 2011
- Restrepo RD, Wettstein R, Wittnebel L, Tracy M. Incentive spirometry: 2011. Respiratory Care 2011;56(10):1600-1604. [PMID: 22008401] [DOI] [PubMed] [Google Scholar]
Scheidegger 1976
- Scheidegger D, Bentz L, Piolino C, Pusterla C, Gigon JP. Influence of early mobilisation of pulmonary function in surgical patients. European Journal of Intensive Care Medicine 1976;2(1):35-40. [DOI] [PubMed] [Google Scholar]
Siafakas 1999
- Siafakas NM, Mitrouska I, Bouros D, Georgopoulous D. Surgery and the respiratory muscle. Thorax 1999;54(5):458-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stiller 1994
- Stiller K, Montarello J, Wallace M, Daff M, Grant R, Jenkins S, et al. Efficacy of breathing and coughing exercises in the prevention of pulmonary complications after coronary artery surgery. Chest 1994;105(3):741-7. [DOI] [PubMed] [Google Scholar]
Temple 2000
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatment. Part 1: Ethical and scientific issues. Annals of Internal Medicine 2000;133(6):455-63. [DOI] [PubMed] [Google Scholar]
Thomas 1994
- Thomas JA, McIntosh JM. Are incentive spirometry, intermittent positive pressure breathing, and deep breathing exercises effective in the prevention of postoperative pulmonary complications after upper abdominal surgery? A systematic overview and meta-analysis. Physical Therapy 1994;74(1):3-10. [DOI] [PubMed] [Google Scholar]
Tramer 1998
- Tramer MR, Reynolds DJ, Moore RA, McQuay HJ. When placebo controlled trials are essential and equivalence trials are inadequate. BMJ 1998;317(7162):875-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wattie 1998
- Wattie J. Incentive spirometry following coronary artery bypass surgery. Physiotherapy 1998;84(10):508-14. [Google Scholar]
Weindler 2001
- Weindler J, Kiefer RT. The efficacy of postoperative incentive spirometry is influenced by the device-specific imposed work of breathing. Chest 2001;119(6):1858-64. [DOI] [PubMed] [Google Scholar]
Weiner 1997
- Weiner P, Man A, Weiner M, Rabner M, Waizman J, Magadle R, et al. The effect of incentive spirometry and inspiratory muscle training on pulmonary function after lung resection. Journal of Thoracic & Cardiovascular Surgery 1997;113(3):552-7. [DOI] [PubMed] [Google Scholar]
Weinstein 1987
- Weinstein MC, Coxson PG, Williams LW, Pass TM, Stason WB, Goldman L. Forecasting coronary heart disease incidence, mortality, and cost: the Coronary Heart Disease Policy Model. American Journal of Public Health 1987;77(11):1417-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilking 1988
- Wilking S, Belanger A, Kannel WB, D'Agostino RB, Steel K. Determinants of isolated systolic hypertension. JAMA 1988;260(23):3451-55. [PubMed] [Google Scholar]


