Abstract
Background
Chronic venous insufficiency (CVI) is a condition in which veins are unable to transport blood unidirectionally towards the heart. CVI usually occurs in the lower limbs. It might result in considerable discomfort, with symptoms such as pain, itchiness and tiredness in the legs. Patients with CVI may also experience swelling and ulcers. Phlebotonics are a class of drugs often used to treat CVI. This is the second update of a review first published in 2005.
Objectives
To assess the efficacy and safety of phlebotonics administered orally or topically for treatment of signs and symptoms of lower extremity CVI.
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, and CINAHL databases and the World Health Organization International Clinical Trials Registry Platform and Clinicaltrials.gov trials register up to 12 November 2019. We searched the reference lists of the articles retrieved by electronic searches for additional citations. We also contacted authors of unpublished studies.
Selection criteria
We included randomised, double‐blind, placebo‐controlled trials (RCTs) assessing the efficacy of phlebotonics (rutosides, hidrosmine, diosmine, calcium dobesilate, chromocarbe, Centella asiatica, disodium flavodate, French maritime pine bark extract, grape seed extract and aminaftone) in patients with CVI at any stage of the disease.
Data collection and analysis
Two review authors independently extracted data and assessed the quality of included RCTs. We estimated the effects of treatment by using risk ratios (RRs), mean differences (MDs) and standardized mean differences (SMDs), according to the outcome assessed. We calculated 95% confidence intervals (CIs) and percentage of heterogeneity (I2). Outcomes of interest were oedema, quality of life (QoL), assessment of CVI and adverse events. We used GRADE criteria to assess the certainty of the evidence.
Main results
We identified three new studies for this update. In total, 69 RCTs of oral phlebotonics were included, but only 56 studies (7690 participants, mean age 50 years) provided quantifiable data for the efficacy analysis. These studies used different phlebotonics (28 on rutosides, 11 on hidrosmine and diosmine, 10 on calcium dobesilate, two on Centella asiatica, two on aminaftone, two on French maritime pine bark extract and one on grape seed extract). No studies evaluating topical phlebotonics, chromocarbe, naftazone or disodium flavodate fulfilled the inclusion criteria.
Moderate‐certainty evidence suggests that phlebotonics probably reduce oedema slightly in the lower legs, compared with placebo (RR 0.70, 95% CI 0.63 to 0.78; 13 studies; 1245 participants); and probably reduce ankle circumference (MD ‐4.27 mm, 95% CI ‐5.61 to ‐2.93 mm; 15 studies; 2010 participants). Moderate‐certainty evidence shows that phlebotonics probably make little or no difference in QoL compared with placebo (SMD ‐0.06, 95% CI ‐0.22 to 0.10; five studies; 1639 participants); and similarly, may have little or no effect on ulcer healing (RR 0.94, 95% CI 0.79 to 1.13; six studies; 461 participants; low‐certainty evidence). Thirty‐seven studies reported on adverse events. Pooled data suggest that phlebotonics probably increase adverse events slightly, compared to placebo (RR 1.14, 95% CI 1.02 to 1.27; 37 studies; 5789 participants; moderate‐certainty evidence). Gastrointestinal disorders were the most frequently reported adverse events. We downgraded our certainty in the evidence from 'high' to 'moderate' because of risk of bias concerns, and further to 'low' because of imprecision.
Authors' conclusions
There is moderate‐certainty evidence that phlebotonics probably reduce oedema slightly, compared to placebo; moderate‐certainty evidence of little or no difference in QoL; and low‐certainty evidence that these drugs do not influence ulcer healing. Moderate‐certainty evidence suggests that phlebotonics are probably associated with a higher risk of adverse events than placebo. Studies included in this systematic review provided only short‐term safety data; therefore, the medium‐ and long‐term safety of phlebotonics could not be estimated. Findings for specific groups of phlebotonics are limited due to small study numbers and heterogeneous results. Additional high‐quality RCTs focusing on clinically important outcomes are needed to improve the evidence base.
Plain language summary
Drugs to improve blood flow for people who have poor blood circulation in the veins of their legs
Background
In chronic venous insufficiency, veins of the lower limbs are unable to transport blood towards the heart. It might be caused by genetic factors, may occur after trauma, or may result from a blood clot. Poor movement of blood up the legs may cause swelling and puffiness, feelings of heaviness, tingling, cramps, pain, varicose veins and changes in skin pigmentation. If severe insufficient blood circulation occurs, ulcers and skin wasting can develop. Drugs such as natural flavonoids extracted from plants and similar synthetic products may improve blood circulation. These drugs are known collectively as venoactive drugs or phlebotonics. This review examined evidence from randomised controlled clinical trials comparing these drugs versus inactive treatment (placebo), generally given over one to three months.
Study characteristics and key results
We identified three new studies for this update. In total, 69 studies met the eligibility criteria for this review. However, we could only use 56 studies (7690 participants; mean age 50 years) in further analysis.
We compared the results and summarised the evidence from the studies. After doing so, we assessed how certain the evidence was. To do this, we considered factors such as the way studies were conducted, study sizes, and consistency of findings across studies. Based on our assessments, we categorised the evidence as potentially being of very low, low, moderate or high certainty.
Moderate‐certainty evidence from 13 studies (involving 1245 people) suggests that phlebotonics probably slightly reduce puffiness (oedema) compared with placebo. Moderate‐certainty evidence suggests that there is little or no difference in quality of life for people taking phlebotonics when compared with placebo. Low‐certainty evidence suggests there is little or no difference in the proportion of healed ulcers with phlebotonics, compared with placebo. Moderate‐certainty evidence from 37 studies (involving 5789 people) suggests that phlebotonics probably produce more side effects, especially gastrointestinal disorders.
Certainty of the evidence
All evidence was of moderate or low certainty. Starting from an initial assumption of high certainty, we downgraded the certainty of evidence by one level for each outcome because of the high risk of bias, primarily due to selective outcome reporting and incomplete outcome data. For the outcome of ulcer healing, we downgraded by an additional level due to statistical imprecision (small number of events). With moderate‐certainty evidence, we are moderately confident in the effect estimates for these outcomes. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. With low‐certainty evidence, our confidence in the effect estimate for that outcome is limited. The true effect may be substantially different from the estimate of the effect.
How‐up‐to date is this review?
The evidence in this Cochrane Review is current to November 2019.
Summary of findings
Summary of findings 1. Do phlebotonics improve signs and symptoms of venous insufficiency when compared with placebo?
| Phlebotonics compared with placebo for venous insufficiency | ||||||
| Patient or population: patients with venous insufficiency Settings: hospital and ambulatory settings Intervention: phlebotonics Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects * | Relative effect (95% CI) | Number of participants (RCTs) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with phlebotonics | |||||
|
Oedema in the lower legs (dichotomous variable) Follow‐up: 1‐6 months |
575 per 1000 | 403 per 1000 (362 to 449) | RR 0.70 (0.63 to 0.78) | 1245 (13 studies) | ⊕⊕⊕⊝ Moderatea | Phebotonics probably slightly reduce oedema in the lower limb compared to placebo |
|
Oedema in the lower legs (ankle circumference, mm) Follow‐up: 1‐12 months |
‐ | Mean ankle circumference in the lower legs in the phlebotonic groups was 4.27 mm lower (5.61 to 2.93 lower) than in the placebo groups | ‐ | 2010 (15 studies) | ⊕⊕⊕⊝ Moderateb | Phlebotonics probably slightly reduce ankle perimeter circumference compared to placebo |
|
Quality of life (CIVIQ and other questionnaires) Follow‐up: mean 2‐12 months |
‐ | The QoL in the phlebotonic groups was 0.06 SMD lower (0.22 lower to 0.1 higher) than in the placebo groups | ‐ | 1639 (5 studies) | ⊕⊕⊕⊝ Moderatec | Phebotonics probably make little or no difference to QoL compared with placebo |
|
Ulcer healing (dichotomous variable) Follow‐up: 1‐12 months |
381 per 1000 | 358 per 1000 (301 to 430) | RR 0.94 (0.79 to 1.13) | 461 (6 studies) | ⊕⊕⊝⊝ Lowd | Phlebotonics may make little or no difference to ulcer healing compared to placebo |
|
Adverse events Follow‐up: 1‐12 months |
158 per 1000 | 180 per 1000 (161 to 200) | RR 1.14 (1.02 to 1.27) | 5789 (37 studies) | ⊕⊕⊕⊝ Moderatee | Phlebotonics probably slightly increase adverse events compared to placebo |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CIVIQ: Chronic Venous Insufficiency International Questionnaire; QoL: quality of life; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aThe certainty of the evidence was downgraded (1 level) to moderate because of overall risk of bias (10 studies had an unclear risk of bias and two had a high risk of bias) bThe certainty of the evidence was downgraded (1 level) to moderate because of overall risk of bias (11 studies had an unclear risk of bias and one had a high risk of bias) cThe certainty of the evidence was downgraded (1 level) to moderate because of overall risk of bias (one study had an unclear risk of bias and two had a high risk of bias) dThe certainty of the evidence was downgraded (2 levels) to low because of overall risk of bias (four studies had an unclear risk of bias and two had a high risk of bias) and imprecision (low number of events) eThe certainty of the evidence was downgraded (1 level) to moderate because of overall risk of bias (28 RCTs had unclear risk of bias and four RCTs had high risk of bias)
Background
Description of the condition
Chronic venous insufficiency (CVI) is a condition in which veins are unable to transport blood unidirectionally toward the heart with flow adapted to tissue drainage needs, temperature regulation and haemodynamic reserve, regardless of their position and activity. CVI first manifests as an increase in venous tension (venous hypertension, or high blood pressure in the veins) with or without reflux (Kurz 1999). Depending on its cause, CVI can be congenital, primary (with undetermined cause) or secondary (post‐thrombotic, post‐traumatic or other). Depending on its pathophysiology, CVI can be related to occlusion (blocked veins), reflux or both. Finally, it might depend on superficial or deep venous systems or on perforator anomalies (Porter 1995).
CVI is an important cause of discomfort and inability to work, and many people find it difficult to live with this condition. Its prevalence has not been clearly determined because available studies regarding this subject are few, and those that are available present limitations. Some studies do not cover the whole pathological spectrum and focus only on varicose veins or ulcers; others do not use standardized definitions of the illness and apply a variety of diagnostic criteria (Nicolaides 2000). As a result, prevalence has been estimated at between 1% and 50% (Evans 1999; Stanhope 1975; Van den Oever 1998). The Framingham Study showed an annual incidence of 2.6% among women and 1.9% among men (Brand 1988). In a recent publication of the Edinburgh Vein Study, annual incidence of CVI was reported as 1% among the general population of the UK (Robertson 2014).
Causes of CVI are unknown, although it has been associated with venous dilation, deformity and valvular venous incompetence. Trophic skin disorders and venous ulcers result from severe varicose illness (Carpentier 2000). Varicose veins have a multi‐factorial origin related to advanced age and certain lifestyles (sedentary life), pregnancy, hereditary factors and obesity. Risk of ulcers may be increased by trauma and previous episodes of deep venous thrombosis (clinical or subclinical) (Scott 1995).
Clinical manifestations of CVI differ according to stage of the illness and can include feelings of heaviness in the extremities, paraesthesia (tingling), cramps, pain, oedema (swellings), varicose veins, skin pigmentation, varicose sores and signs of skin atrophy (wasting). Symptoms are frequently related to extent of disease. Underlying venous disease (superficial, deep or both, with or without obstruction) has a major impact on both manifestations of the disease and response to treatment. Since 1994, criteria develop by the International Consensus Committee on Chronic Venous Disease have been used to define and classify CVI in a standardized fashion (Porter 1995). According to this Consensus, clinical signs (C), aetiology (E), anatomical distribution (A) and physiological conditions (P) ("Clinical‐Etiology‐Anatomy‐Pathophysiology"; CEAP) are used to classify CVI (Porter 1995). A later revision of the CEAP classification established a means of differentiating between chronic venous disorder (referring to all morphology and functional abnormalities of the venous system) and CVI (reserved for more advanced stages of the disease with oedema, skin changes or venous ulcers) (Eklöf 2004). In parallel, a venous clinical severity score (ranging from none (0) to severe (3)) was established to assess pain, varicose veins, venous oedema, skin pigmentation, inflammation, induration, active ulcer (number, duration and size) and use of compression therapy (Vasquez 2010). Recently, a new version of CEAP classification has been published (Lurie 2020), in which Corona phlebectatica was added as a C4c clinical subclass, the modifier “r” introduced for recurrent varicose veins and recurrent venous ulcers and numeric descriptions of the venous segments replaced by their common abbreviations (Lurie 2020).
Description of the intervention
Surgery, sclerotherapy and mechanical compression are generally the preferred treatments for CVI. However, pharmacological treatments or phlebotonics are often used because they are easy to administer, and because compliance with compressive treatments (such as elastic stockings) is often poor.
Phlebotonics represent a heterogeneous group of medications used to treat CVI. Most of these drugs are natural flavonoids extracted from plants. Synthetic products with flavonoid‐like properties are also used to treat venous disorders. In the Anatomical Therapeutic Chemical (ATC) system, phlebotonics are classified as vasoprotective agents (ATC 2015). Within this classification system, active substances are divided into different groups according to the organ or system on which they act and their therapeutic, pharmacological and chemical properties. Phlebotonics are known as venoactive drugs whose mechanism of action is not scientifically well established despite the availability of numerous studies examining their pharmacological and clinical properties. These medications are associated with effects on macrocirculation (e.g. they may improve venous tone) (Tsouderos 1991) and on microcirculatory parameters (e.g. they may decrease capillary hyperpermeability) (Behar 1988).
Why it is important to do this review
Lower limb CVI affects a predominantly adult population and it is a frequent cause for a referral from primary to secondary care (Venous Forum 2011). Although phlebotonics are commercialised in many countries, in others they are not widely available. In some countries, such as Spain, for certain phlebotonics (calcium dobesilate, chromocarbe and naftazone) the CVI indication has been withdrawn, and for several other phlebotonics, such as aminaftone, diosmine, hidrosmine, escin and some rutosides, conditions of use during exacerbations of CVI have been limited to two or three months by the Spanish Ministry of Health (AEM 2002).
Controversy surrounds the clinical relevance of the efficacy and benefit‐risk balance of phlebotonics. Case‐control studies have found that risk of agranulocytosis (reduced numbers of white blood cells, mainly neutrophils) is associated with some phlebotonics (Ibañez 2000; Ibáñez 2005; Kaufman 1991). As efficacy is not well defined and serious harmful effects have been associated with phlebotonics, an evaluation of available evidence is needed.
Objectives
To assess the efficacy and safety of phlebotonics administered orally or topically for treatment of signs and symptoms of lower extremity CVI.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised, double‐blind, controlled trials assessing the efficacy and/or safety of phlebotonics compared with placebo in patients with chronic venous insufficiency (CVI) at any stage of the disease. We did not include studies which were not RCTs or double‐blind. We did not choose specific diagnostic classifications of CVI a priori because most of the studies were carried out before 1994, before the international diagnostic consensus of CVI. Therefore, we included RCTs with different diagnostic criteria. We included studies in which use of compression measures (support tights) was similar across groups.
Types of participants
We included both male and female participants who were 18 years of age and older, suffering from any type of CVI. CVI could be diagnosed according to explicit clinical criteria and/or by objective instruments. Participant background, ethnicity and medical co‐morbidities at the beginning of the study did not influence the decision to include or exclude the study. We excluded studies that included participants with active thrombophlebitis and those including pregnant women.
Types of interventions
We considered the following interventions to treat CVI acceptable for inclusion: treatments including venoactive drugs or phlebotonics, administered orally or topically, at any dosage and independently of the duration of treatment, compared with placebo. We excluded studies that compared phlebotonics among themselves or with any other therapeutic method (i.e. support tights or surgery).
-
Natural products
Flavonoids: rutoside, French maritime pine bark extract (also known as pycnogenol), grape seed extract, diosmine and hidrosmine, disodium flavodate
Saponosides: Centella asiatica
-
Synthetic products
Calcium dobesilate, naftazone, aminaftone, chromocarbe
We excluded escin (horse chestnut seed extract), as it is covered in another Cochrane Review (Pittler 2012).
Pentoxifylline is classified as a peripheral vasodilator, not as a vasoprotective agent (ATC 2015); therefore, we excluded it from this review.
Types of outcome measures
We included studies that assessed any of the following outcome measures.
Primary outcomes
Oedema in the lower limb measured by the dichotomous variable 'oedema' and the continuous variables 'ankle perimeter circumference' and 'volume of the leg'
Specific quality of life (QoL) scales (e.g. Chronic Venous Insufficiency International Questionnaire (CIVIQ))
Secondary outcomes
-
Assessment of CVI: objective signs
Skin manifestations including venous ulcer healing and trophic alterations (e.g. lipodermatosclerosis (hardening of the skin that may cause red/brown pigmentation and is accompanied by wasting of subcutaneous fat), telangiectasia (tiny blood vessels cause threadlike red lines or patterns on the skin), reticular veins (dilated veins that show as a net‐like pattern on the skin), varicose veins (permanently dilated veins)
-
Assessment of CVI: subjective symptoms
Pain in the lower legs
Cramps in the lower legs
Restless legs
Itching in the lower legs
Feeling of heaviness in the lower legs
Swelling in the lower legs
Paraesthesias (abnormal sensations, such as prickling, burning, tingling) in the lower legs
Participant satisfaction
-
Adverse events
Adverse reactions experienced by participants during the trial, as reported by questionnaire or related by participants and specified within the publication
Search methods for identification of studies
Electronic searches
For this update, the Cochrane Vascular Information Specialist conducted systematic searches of the following databases for randomised controlled trials and controlled clinical trials without language, publication year or publication status restrictions:
the Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web searched on 12 November 2019);
the Cochrane Central Register of Controlled Trials (CENTRAL) Cochrane Register of Studies Online (CRSO 2019, issue 10);
MEDLINE (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) (searched from 1 January 2017 to 12 November 2019);
Embase Ovid (searched from 1 January 2017 to 12 November 2019);
CINAHL Ebsco (searched from 1 January 2017 to 12 November 2019); and
AMED Ovid (searched from 1 January 2017 to 12 November 2019).
The Information Specialist modelled search strategies for other databases on the search strategy designed for CENTRAL. Where appropriate, strategies were combined with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6, Lefebvre 2011). Search strategies for major databases are provided in Appendix 1.
The Information Specialist searched the following trials registries on 12 November 2019:
the World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch);
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
For this update, we searched the reference lists of articles retrieved by electronic searches for additional citations.
Data collection and analysis
Selection of studies
For this update, two review authors (RV and DS) independently assessed the eligibility of new studies identified by the searches. A third review author (MMZ) helped to resolve disagreements by discussion.
Data extraction and management
For this update, two review authors (RV and MMZ) independently extracted data from new studies and entered them to a previously tested standardized form. A consensus between reviewers were reached if any data extraction discrepancies occurred. We collected information including characteristics of study participants, characteristics of intervention and control groups and outcome characteristics of every group of participants. For cross‐over studies, we extracted and analyzed only data related to the first period of treatment.
Assessment of risk of bias in included studies
For this update, two review authors (RV and MJMZ) independently assessed the risk of bias of the newly included studies. A consensus between review authors was reached by discussion when there was any disagreement. We specifically assessed the randomisation method (sequence generation and allocation concealment); blinding of participants, caregivers/study researchers and outcome assessors to the intervention; whether outcome data were incomplete; and presence of selection bias. Once this information was gathered, review authors classified each study into one of three levels of risk of bias: low, unclear or high, based on the criteria specified in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
We estimated effects of treatment with phlebotonics by using risk ratios (RRs) for dichotomous variables and mean differences (MDs) or standardized mean differences (SMDs) for continuous variables, along with their corresponding 95% confidence intervals (CIs). We calculated SMDs when studies used different instruments to measure the same variable.
Unit of analysis issues
We took the unit of analysis to be the individual participant. For cross‐over studies, we extracted and analyzed only data related to the first period of treatment.
Dealing with missing data
We analyzed dichotomous variables by applying the intention‐to‐treat (ITT) principle to analyze every individual in the randomly assigned treatment group regardless of whether individuals completed treatment or withdrew prematurely from the study. We included in the ITT analysis only studies that provided data from all randomised participants, or that stated the number of participants lost during follow‐up. We numerically imputed missing values due to withdrawal of participants or loss to follow‐up as therapeutic failures in both comparative groups. For continuous variables, we analyzed data as provided by study authors, either per protocol or as ITT values.
Assessment of heterogeneity
We carried out an analysis to detect the presence of heterogeneity by using the I2 statistic before obtaining global effect estimators. The I2 statistic describes the percentage of total variation across studies that is due to heterogeneity rather than to sampling error (Deeks 2011). When statistical heterogeneity was high (I2 > 75%), we did not pool studies. For levels of I2 less than 50%, we applied a fixed‐effect model; for levels of I2 greater than 50% but less than 75%, we applied a random‐effects model (DerSimonian 1986).
Assessment of reporting biases
We constructed a funnel plot to assess whether the outcome of oedema (dichotomous variable) was subject to publication bias (Figure 1).
1.

Funnel plot of comparison: 1 Phlebotonics vs placebo, outcome: 1.1 Oedema in the lower legs (dichotomous variable).
Data synthesis
We obtained data from the included studies for variables evaluated at the end of treatment. In addition, we obtained data from measures of change when no significant baseline differences were evident between compared groups. We then pooled these together with other similar continuous outcomes.
We split the outcomes of variables measured by ordinal categorical scales into two groups of response. We considered one group as showing success (no signs or symptoms or mild manifestations) and the other as showing failure (moderate, severe or very severe persistence of signs and symptoms).
Subgroup analysis and investigation of heterogeneity
We carried out subgroup analyses in addition to the overall analysis of phlebotonics. These included looking at the effects of the following phlebotonics: rutosides, hidrosmine, diosmine, calcium dobesilate, disodium flavodate, grape seed extract, French maritime pine bark extract, chromocarbe and aminaftone.
Sensitivity analysis
We performed sensitivity analyses to assess the influence on data of assumptions and decisions of review authors during the review process. We re‐analysed data by:
excluding studies that used compression measures;
excluding unpublished studies; and
excluding studies with high or unclear risk of bias in at least one domain.
Summary of findings and assessment of certainty of the evidence
We created one 'Summary of findings' table to present the main findings for 'Phlebotonics compared with placebo for venous insufficiency' using GRADE profiler software (GRADEpro 2008). See Table 1. We used the principles of the GRADE system to assess the certainty of the body of evidence associated with the main outcomes listed below. The GRADE approach appraises the certainty of a body of evidence according to the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Evaluation of the certainty of a body of evidence considers within‐study risk of bias, indirectness of the evidence, inconsistency (heterogeneity in the data), imprecision (precision of effect estimates) and publication bias (Schünemann 2011).
Two review authors (DS and RV) independently assessed the certainty of the body of evidence for the following outcomes.
Oedema in the lower legs (dichotomous variable)
Oedema in the lower legs (circumference mm)
QoL
Ulcer healing
Adverse events
Results
Description of studies
Results of the search
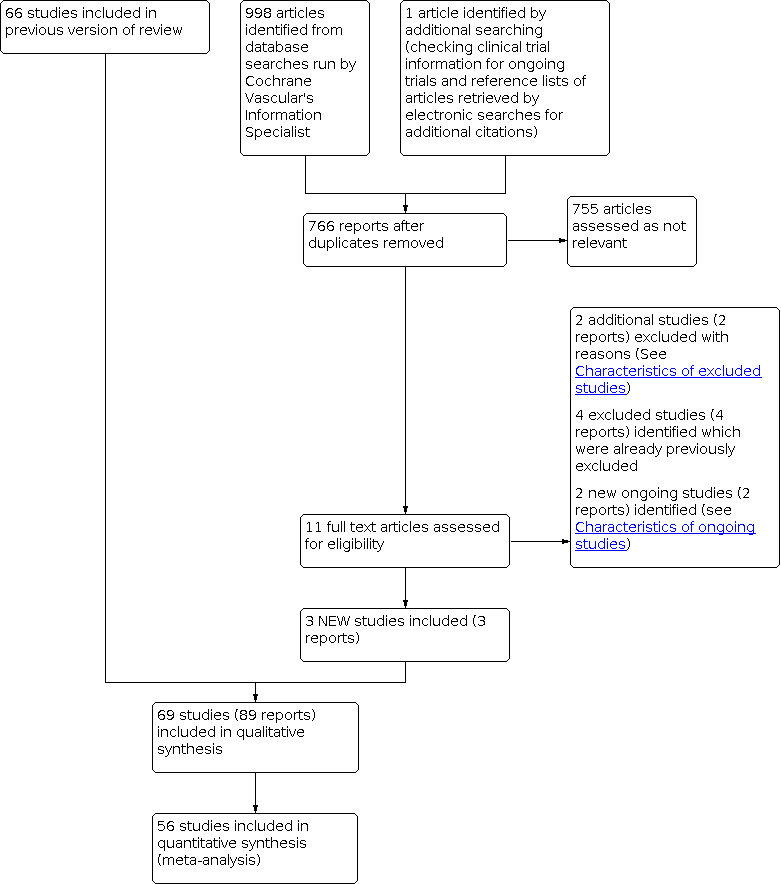
For this update we identified three new included studies (NCT01848210; Rabe 2015; Rabe 2016); two new ongoing studies (Barattini 2019; NCT03833024); and two new studies were excluded (EudraCT2009‐014681‐25; ISRCTN54360155). See Figure 2. Details of all studies are provided in the Characteristics of included studies, Characteristics of ongoing studies and Characteristics of excluded studies tables.
2.
Study flow diagram.
Included studies
For this update, we identified three new included studies (NCT01848210; Rabe 2015; Rabe 2016). In total with those identified in earlier versions, we included 69 studies. See Characteristics of included studies tables.
Most studies were published in English, but four were published in German (Biland 1982; Kiesewetter 1997; Koscielnny 1996; Pedersen 1992), seven in French (Cauwenberge 1978; Chassignolle 1994; Planchon 1990; Thebaut 1985; Vin 1994; Welch 1985; Zucarelli 1987), four in Spanish (Flota‐Cervera 2008; Klüken 1971; Marinello 2002; Serralde 1990), three in Italian (Allegra 1981; Lazzarini 1982; Pecchi 1990), and one in Spanish, French and Dutch (Padrós 1972).
Of the 69 included double‐blind, placebo‐controlled clinical trials, we did not include 13 studies in the efficacy analysis. Of these, 10 studies corresponded to the rutoside group (Bergqvist 1981; Cloarec 1994; Jongste 1986; Mann 1981; Nocker 1990; Prerovsky 1972; Renton 1994; Rose 1970; Rudofsky 1989; Sentou 1984), two corresponded to calcium dobesilate (Padrós 1972; Pecchi 1990) and another corresponded to French bark pine extract (Petrassi 2000).
We excluded these studies from the efficacy analysis for the following reasons.
Only mean data were provided without standard deviations (SDs) or standard errors (SEs) (Sentou 1984).
Medians were provided instead of means (Renton 1994).
Outcomes were reported by graph only (Nocker 1990; Rose 1970; Rudofsky 1989).
First period data were not provided in studies of cross‐over design (Padrós 1972; Prerovsky 1972).
No data were provided for any variable (Bergqvist 1981; Cloarec 1994; Jongste 1986).
Measured changes were reported when significant differences in baseline were noted between compared groups (Mann 1981; Petrassi 2000).
A quasi‐randomisation method was used in which treatments were alternatively allocated depending on participants' order of arrival (Pecchi 1990).
At baseline, a significant imbalance in the ulcer area was evident between groups (1130 mm2 in the rutoside group vs 430 mm2 in the placebo group; P = 0.039) (Mann 1981).
Of the 56 studies with oral phlebotonics included in the efficacy analysis, studied phlebotonics corresponded to 28 studies of rutosides (Balmer 1980; Burnand 1989; Cloarec 1996; Cauwenberge 1972; Cauwenberge 1978; Cesarone 2002; Cornu‐Thenard 1985; Diebschlag 1994; Ihme 1996; Jongste 1986; Jongste 1989; Kiesewetter 1997; Koscielnny 1996; Klüken 1971; Kriner 1985; Languillat 1988; Laurent 1988; MacLennan 1994; NCT01848210; Parrado 1999; Pedersen 1992; Pulvertaft 1983; Schultz‐Ehrenburg 1993; Serralde 1990; Unkauf 1996; Vanscheidt 2002a; Vanscheidt 2002b; Vin 1994), 11 of hidrosmine and diosmine (Chassignolle 1994; Danielsson 2002; Dominguez 1992; Fermoso 1992; Gilly 1994; Guilhou 1997; Planchon 1990; Rabe 2015; Thebaut 1985; Welch 1985; Zucarelli 1987), 10 of calcium dobesilate (Casley‐Smith 1988; DOBESILATO500/2; Flota‐Cervera 2008; Hachen 1982; Labs 2004; Marinello 2002; Martinez‐Zapata 2008; Rabe 2011; Rabe 2016; Widmer 1990), two of Centella asiatica (Allegra 1981; Pointel 1986), two of aminaftone (Belczak 2014; Lazzarini 1982), two of French maritime pine bark extract (Arcangeli 2000; Petrassi 2000) and one of grape seed extract (Thebaut 1985). No studies using topical phlebotonics or chromocarbe or naftazone or disodium flavodate fulfilled the inclusion criteria. Length of treatment and participant follow‐up ranged from 28 days to four months, except for three studies, in which follow‐up lasted six months or more (DOBESILATO500/2; MacLennan 1994; Martinez‐Zapata 2008).
Overall, we included 7690 participants in the meta‐analysis; 83% were female and 17% were male; mean age was 50 years (range 32 to 62 years). The mean number of participants included per clinical trial was 150 (range 20 to 1137). All participants met the respective CVI criteria of every study, although we noted variation between studies in degree of progression to CVI, as well as in diagnostic classification criteria applied. Only 22% of studies reported the diagnostic classification used. Among studies that did report on the diagnostic classification of CVI, the CEAP classification was used most often (Belczak 2014; Danielsson 2002; DOBESILATO500/2; Labs 2004; Marinello 2002; Martinez‐Zapata 2008; Rabe 2011; Rabe 2015; Rabe 2016; Vanscheidt 2002a; Vanscheidt 2002b), followed by Widmer's classification (Casley‐Smith 1988; Cloarec 1996; Koscielnny 1996; Parrado 1999; Unkauf 1996). Wert's was the only other classification used (Kiesewetter 1997).
Differences in severity of disease were observed: some studies were performed with participants at early and symptomatic CVI stages (Cornu‐Thenard 1985; Danielsson 2002; Gilly 1994; Hachen 1982; Thebaut 1985), and others included participants at advanced stages because of long progression of the disease or the presence of venous ulcers (Casley‐Smith 1988; DOBESILATO500/2; Guilhou 1997; Lazzarini 1982; Marinello 2002; Planchon 1990; Schultz‐Ehrenburg 1993; Vanscheidt 2002a). However, most studies included participants at moderate CVI stages with oedema, skin pigmentation, varicose veins and post‐thrombotic syndromes.
Ten studies specified that investigators used additional compression therapy (DOBESILATO500/2; Guilhou 1997; Laurent 1988; Lazzarini 1982; Marinello 2002; Martinez‐Zapata 2008; Planchon 1990; Rabe 2011; Schultz‐Ehrenburg 1993; Zucarelli 1987).
Eleven studies used a visual analogue scale (VAS) to measure subjective variables (Alterkamper 1987; Cesarone 2002; DOBESILATO500/2; Labs 2004; Martinez‐Zapata 2008; Rabe 2011; Rabe 2015; Unkauf 1996; Vanscheidt 2002b; Widmer 1990; Zucarelli 1987). Other studies used ordinal categorical scales with a scoring system from ‐3 to +1 (Hachen 1982), ‐1 to + 1 (Casley‐Smith 1988), 0 to 1 (Ihme 1996), 0 to 2 (Biland 1982; Ihme 1996; Kiesewetter 1997), 0 to 3 (Allegra 1981; Arcangeli 2000; Cloarec 1996; Cornu‐Thenard 1985; Danielsson 2002; Diebschlag 1994; Dominguez 1992; Gilly 1994; Jongste 1989; Languillat 1988; Laurent 1988; Lazzarini 1982; Parrado 1999; Planchon 1990; Pointel 1986; Pulvertaft 1983; Serralde 1990; Thebaut 1985; Tsouderos 1989; Welch 1985), 0 to 4 (Balmer 1980; Chassignolle 1994; Fermoso 1992; Flota‐Cervera 2008), 0 to 5 (NCT01848210; Rabe 2011), 0 to 7 (Labs 2004) or 0 to 9 (Dominguez 1992). Likewise, some of these scales were used to evaluate signs or objective variables such as oedema or trophic disorders. Methods used to measure oedema included metric tape to measure ankle or calf circumference and plethysmographic values (used in most studies) to determine leg volume.
Excluded studies
For this update, we identified two new studies that were excluded (EudraCT2009‐014681‐25; ISRCTN54360155). Four previously excluded studies were also identified by the search (Belczak 2014; Kiesewetter 1997; Prerovsky 1972; NCT01532882), making a total of 104 studies excluded for a variety of reasons (see Characteristics of excluded studies for details). We summarise the exclusion details below.
We excluded 58 studies because the intervention used by researchers was not included in this Cochrane Review (Akbulut 2010; Bacci 2003; Bastide 1976; Batchvarova 1989a; Behar 1993; Bello 1990; Bento 2006; Berson 1978; Bohm 1989; Bolliger 1972; Bosse 1985; Brami 1983; Carstens 1985; Cataldi 2001; Cesarone 2001b; Chiummariello 2009; Cospite 1996; de Parades 1990; Delacroix 1981; Delecluse 1991; Dustmann 1984; Erdlen 1989; Erler 1991; EudraCT2009‐014681‐25; Henriet 1995; Horvath 1985; Janssens 1999a; Kiesewetter 2000; Koltringer 1993; Krähenbühl 1975; Krcílek 1973; Languillat 1988b; Marastoni 1982; Monteil‐Seurin 1993; Morales 1993; NCT02191163; NCT02191254; NCT02191280; Neumann‐Mangoldt 1979; Nill 1970; Ottillinger 2001; Paciaroni 1982; Partsch 1981; Paul 1983; Pauschinger 1987; Pointel 1987b; Pokrovskii 2005; Rabe 2011b; Riccioni 2004; Sanctis 2001; Steiner 1990; Steiner 1992; Topalov 1990; Turio 2000; ISRCTN54360155; Weindorf 1987; Widmer 1972; Zuccarelli 1996).
We excluded 30 studies because researchers assessed no clinical endpoints or reported only outcomes not included in this Cochrane Review (Androulakis 1989; Auteri 1990; Belcaro 1995; Belcaro 2008; Boisseau 1995; Bort 1995; Cesarone 1992; Cesarone 1994; Cesarone 2001; Cesarone 2001c; Cesarone 2002b; Chant 1973; Clemens 1986; Duchene 1988; Forconi 1977; Gonzalez‐Fajardo 1990; Incandela 1995; Incandela 1996; Janssens 1999; Kalus 2004; Kostering 1985; Languillat 1989; Le Dévéhat 1989; Le Dévéhat 1997; Naser‐Hijazi 2004; Neumann 1988; Neumann 1990; Questel 1983; Roztocil 1977; Seydewitz 1992).
We excluded 16 studies because they were not double‐blinded (Belcaro 1989; Blume 1996; Cesarone 2001a; Cesarone 2010; De Anna 1989; De Sanctis 2001; Frausini 1985; Glinski 1999; Granger 1995; Incandela 2001; Incandela 2002; Menyhei 1994; NCT01654016; Petruzzellis 2002; Roztocil 2003; Steru 1988).
Ongoing studies
For this update, we identified two new ongoing studies (Barattini 2019; NCT03833024). This brings the total number of ongoing studies included to four (Barattini 2019; ISRCTN18841175; NCT01532882; NCT03833024). Details of these can be found in the Characteristics of ongoing studies table.
Risk of bias in included studies
Overall, only four studies (Labs 2004; Martinez‐Zapata 2008; Rabe 2011; Vanscheidt 2002a) were at low risk of bias (see Characteristics of included studies, Figure 3 and Figure 4).
3.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
4.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Of the 69 studies included, 19 (28%) submitted details on the randomisation process and were assessed as being at low risk (see Figure 4 and Characteristics of included studies). The remaining studies were all judged to be at an unclear risk of bias.
Only 12 (17%) studies provided an accurate explanation of the allocation concealment process. Two used the sealed envelope method (Danielsson 2002; Pedersen 1992), four used indistinguishable number packaging (Biland 1982; Padrós 1972; Rabe 2011; Rose 1970), one used randomised numbered bottles provided by an external investigator (Belczak 2014), two used allocation concealment by direct phone calls (DOBESILATO500/2; Martinez‐Zapata 2008), and the remaining three studies used computerised random assignment (Jongste 1989; Labs 2004; Vanscheidt 2002a).
Blinding
Of the 69 studies included, 41 (59%) reported that the placebo used was identical to the active treatment; thus participants, study researchers and outcome assessors were blinded to the intervention and these were judged to have a low risk of bias. The other 28 studies did not mention whether placebo had identical characteristics to those of the active drug and so were at an unclear risk of bias (see Figure 4 and Characteristics of included studies).
Incomplete outcome data
Of the 69 studies included, 52 (75%) reported participant withdrawals, and thus were at low risk of bias. The percentage of withdrawn participants ranged from 0% to 42.5% (see Characteristics of included studies). Only eight (12%) studies included in the efficacy analysis stated that investigators carried out an ITT analysis (Dominguez 1992; Guilhou 1997; Ihme 1996; Martinez‐Zapata 2008; Rabe 2011; Rabe 2016; Unkauf 1996; Vanscheidt 2002a). Seven studies had high risk of bias in this domain (Cauwenberge 1978; DOBESILATO500/2; Mann 1981; Rabe 2016; Rose 1970; Sentou 1984; Vanscheidt 2002b): four described an important percentage of losses (42.5% Cauwenberge 1978; 18% Mann 1981; 39% Rose 1970; 34% Vanscheidt 2002b), one interrupted recruitment because financial support was interrupted (DOBESILATO500/2) and one did not specify the number of participants included (Sentou 1984). In the Rabe 2016 study, 14.8% of the randomised participants were lost during follow‐up and major protocol violations were reported for 42.4% of the randomised participants. Ten studies were judged to be at unclear risk of bias because the reasons for dropouts (Cauwenberge 1972), or the number of dropouts, were not provided (Cornu‐Thenard 1985; Kiesewetter 1997; Klüken 1971; Kriner 1985; Lazzarini 1982; Nocker 1990; Padrós 1972; Pedersen 1992), or the standard deviation was lacking in the results (Thebaut 1985).
Selective reporting
Of the 69 studies included, 57 (84%) reported all outcomes specified in the methods section and were judged as being at low risk of reporting bias. We evaluated seven studies as having high risk of selective reporting bias because we noted differences between outcomes reported in the methods and results sections (Cloarec 1994; Jongste 1986; Jongste 1989; Mann 1981; Rabe 2015), and because data before the cross‐over were not reported (Padrós 1972; Rose 1970). One study was interrupted, and results of this study were not published (DOBESILATO500/2). Lazzarini 1982 provided no information about adverse events. Two studies were judged to be at unclear risk of reporting bias because characteristics of participants were not provided ((Allegra 1981) and outcomes were not reported in methods and neither a protocol was published (Klüken 1971).
Figure 1 shows that all studies, except one (Casley‐Smith 1988), are located symmetrically around the effect measure at the top of the pyramid, indicating highly precise results (Cauwenberge 1972; Cornu‐Thenard 1985; Kiesewetter 1997; Klüken 1971; Kriner 1985; Lazzarini 1982; Nocker 1990; Padrós 1972; Pedersen 1992; Thebaut 1985). Apart from one imprecise study favouring phlebotonics, no small or heterogeneous studies provided results favouring placebo or phlebotonics (Casley‐Smith 1988).
Other potential sources of bias
No other potential sources of bias were detected.
Effects of interventions
See: Table 1
See Table 1 for the main comparison. Results of all analyzed outcomes are specified in an additional Table 2. Results of outcomes analyzed by active agent (aminaftone, calcium dobesilate, Centella asiatica, diosmine and hidrosmine, French maritime pine bark extract, grape seed extract and rutosides) are specified in Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; and Table 9, respectively.
1. Results of all outcomes analysed (all phlebotonics).
| Variables | Dichotomous | Continuous |
| Oedema | RR 0.70 (0.63 to 0.78) | ‐ |
| Oedema (mm) | ‐ | MD ‐4.27 (‐5.61 to ‐2.93) |
| Oedema (volume) | ‐ | SMD ‐0.24 (‐0.33 to ‐0.15) |
| Ulcer cured | NS | ‐ |
| Trophic disorders | RR 0.87 (0.81 to 0.95) | ‐ |
| Pain | ‐ | SMD ‐0.35 (‐0.54, ‐0.17) |
| Cramps | RR 0.72 (0.58 to 0.89) | ‐ |
| Restless legs | RR 0.81 (0.72 to 0.91) | ‐ |
| Itching | ‐ | ‐ |
| Heaviness | ‐ | ‐ |
| Swelling | RR 0.63 (0.50 to 0.80) | ‐ |
| Paraesthesia | RR 0.67 (0.50 to 0.88) | NS |
| Quality of life | ‐ | NS |
| Global assessment by the participant | ‐ | ‐ |
| Adverse events | RR 1.14 (1.02 to 1.27) | ‐ |
| Note: No measures of effect are specified when I2 was > 75% for the subgroup | ||
RR: risk ratio MD: mean difference NS: non‐significant RR: risk ratio SMD: standardized mean difference
2. Results by pharmacological group: aminaftone.
| Variables | Dichotomous | Continous |
| Oedema | RR 0.53 (0.28 to 0.99) | SMD ‐0.17 (‐0.61 to 0.28) |
| Ulcer cured | NS | ‐ |
| Trophic disorder | NS | ‐ |
| Pain | RR 0.43 (0.23 to 0.79) | ‐ |
| Cramps | RR 0.56 (0.31 to 0.99) | ‐ |
| Itching | RR 0.53 (0.31 to 0.91) | ‐ |
| Heaviness | RR 0.32 (0.17 to 0.60) | ‐ |
| Quality of live | ‐ | MD ‐10.00 (‐17.01 to ‐2.99) |
| Adverse events | NS | ‐ |
| Note: Only 1 study was analyzed | ||
MD: mean difference NS: non‐significant RR: risk ratio SMD: standardized mean difference
3. Results by pharmacological group: calcium dobesilate.
| Variables | Dichotomous | Continuous |
| Oedema | ‐ | ‐ |
| Oedema (mm) | ‐ | NS |
| Oedema (volume) | ‐ | SMD ‐0.38 (‐0.51 to ‐0.24) |
| Ulcer cured | NS | ‐ |
| Pain | RR 0.53 (0.35 to 0.82) | NS |
| Cramps | RR 0.65 (0.50 to 0.84) | ‐ |
| Restless legs | RR 0.73 (0.59 to 0.91) | NS |
| Itching | ‐ | NS |
| Heaviness | NS | NS |
| Swelling | RR 0.19 (0.08 to 0.41) | NS |
| Paraesthesia | NS | ‐ |
| Quality of life | ‐ | NS |
| Global assessment by the participant | ‐ | SMD ‐0.52 (‐0.71 to ‐0.33) |
| Adverse events | RR 1.22 (1.0 to 1.49) | ‐ |
| Note: No measures of effect are specified when I2 was > 75% for the subgroup | ||
NS: non‐significant RR: risk ratio SMD: standardized mean difference
4. Results by pharmacological group: Centella asiatica.
| Variables | Dichotomous | Continuous |
| Heaviness | NS | ‐ |
| Global assessment by the participant | RR 0.28 (0.14 to 0.57) | ‐ |
| Adverse events | NS | ‐ |
| Note: Only 1 study was analyzed | ||
NS: non‐significant RR: risk ratio
5. Results by pharmacological group: diosmine, hidrosmine.
| Variables | Dichotomous | Continuous |
| Oedema | RR 0.63 (0.46 to 0.86) | ‐ |
| Oedema (mm) | ‐ | MD ‐5.98 (‐7.78 to ‐4.18) |
| Ulcer cured | NS | ‐ |
| Trophic disorder | RR 0.87 (0.81 to 0.94) | ‐ |
| Pain | NS | SMD ‐0.23 (‐0.41 to ‐0.05) |
| Cramps | RR 0.83 (0.70 to 0.98) | SMD ‐0.46 (‐0.78 to ‐0.14) |
| Restless legs | NS | ‐ |
| Itching | NS | ‐ |
| Heaviness | NS | SMD ‐0.69 (‐1.02 to ‐0.36) |
| Swelling | RR 0.70 (0.52 to 0.94) | SMD ‐0.92 (‐1.26 to ‐0.58) |
| Paraesthesia | NS | NS |
| Quality of life | ‐ | NS |
| Global assessment by the participant | ‐ | SMD ‐0.81 (‐1.14 to ‐0.47) |
| Adverse events | NS | ‐ |
| Note: No measures of effect are specified when I2 was > 75% for the subgroup | ||
MD: mean difference NS: non‐significant RR: risk ratio SMD: standardized mean difference
6. Results by pharmacological group: French maritime pine bark extract.
| Variables | Dichotomous | Continuous |
| Pain | RR 0.66 (0.48 to 0.91) | SMD ‐1.39 (‐2.09 to ‐0.69) |
| Heaviness | NS | SMD ‐1.50 (‐2.21 to ‐0.79) |
| Swelling | NS | SMD ‐1.65 (‐2.38 to ‐0.92) |
| Note: Only 1 study was analyzed | ||
NS: non‐significant RR: risk ratio SMD: standardized mean difference
7. Results by pharmacological group: grape seed extract.
| Variables | Dichotomous | Continuous |
| Oedema | NS | ‐ |
| Adverse events | NS | NS |
| Note: Only 1 study was analyzed | ||
NS: non‐significant
8. Results by pharmacological group: rutosides.
| Variables | Dichotomous | Continuous |
| Oedema | RR 0.72 (0.64 to 0.81) | ‐ |
| Oedema (mm) | ‐ | NS |
| Oedema (volume) | ‐ | SMD ‐0.15 (‐0.16 to ‐0.03) |
| Ulcer cured | NS | ‐ |
| Trophic disorder | NS | ‐ |
| Pain | ‐ | SMD ‐0.71 (‐1.23 to ‐0.19) |
| Cramps | RR ‐0.83 (‐1.50 to ‐0.16) | NS |
| Restless legs | NS | ‐ |
| Itching | ‐ | SMD ‐0.58 (‐1.10 to ‐0.06) |
| Heaviness | RR 0.60 (0.48 to 0.74) | ‐ |
| Swelling | RR 0.67 (0.50 to 0.88) | NS |
| Paraesthesias | RR 0.55 (0.37 to 0.83) | NS |
| Global assessment by the participant | ‐ | ‐ |
| Adverse events | RR 1.22 (1.04 to 1.43) | ‐ |
| Note: No measures of effect are specified when I2 was > 75% | ||
NS: non‐significant RR: risk ratio SMD: standardized mean difference
Of the 69 included studies, we excluded 13 studies from the efficacy analysis for the reasons explained under Included studies (Bergqvist 1981; Cloarec 1994; Jongste 1986; Mann 1981; Nocker 1990; Padrós 1972; Pecchi 1990; Petrassi 2000; Prerovsky 1972; Renton 1994; Rose 1970; Rudofsky 1989; Sentou 1984). Belczak 2014 compared three different interventions with placebo. For the analysis, we included only the comparison of aminaftone with placebo because the other two interventions were combinations of different drugs (micronised diosmine and hesperidin; coumarin and troxerutin).
Primary outcomes
Oedema in the lower limb (dichotomous variable)
We included 13 trials in the meta‐analysis: seven corresponding to rutosides (Cauwenberge 1972; Cauwenberge 1978; Cloarec 1996; Ihme 1996; Kriner 1985; MacLennan 1994; Welch 1985), two to calcium dobesilate (Casley‐Smith 1988; Labs 2004), two to hidrosmine and diosmine (Fermoso 1992; Planchon 1990), one to grape seed extract (Thebaut 1985) and one to aminaftone (Lazzarini 1982), with a total of 626 participants in the active treatment group and 619 in the placebo group. The median time to follow‐up was 49 days. Phebotonics probably reduce oedema in the lower limb compared to placebo (RR 0.70, 95% CI 0.63 to 0.78; 13 studies; 1245 participants; moderate‐certainty evidence; Analysis 1.1). The certainty of the evidence was downgraded by one step to moderate because of overall risk of bias (10 studies had an unclear risk of bias and two had a high risk of bias) (Table 1). No differences between the subgroups was detected (test for subgroup differences: P = 0.74).
1.1. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 1: Oedema in the lower legs (dichotomous variable)
Oedema in the lower limb (continuous variables)
Ankle perimeter circumference
We included 15 studies in the meta‐analysis: seven corresponding to rutosides (Cloarec 1996; Cornu‐Thenard 1985; Jongste 1989; MacLennan 1994; Parrado 1999; Vin 1994; Welch 1985), five to calcium dobesilate (Flota‐Cervera 2008; Labs 2004; Martinez‐Zapata 2008; Rabe 2011; Widmer 1990), and three to diosmine (Gilly 1994; Planchon 1990; Tsouderos 1989), with a total of 1001 participants given active treatment and 1009 given placebo. The median time to follow‐up was 60 days. Phlebotonics probably slightly reduce ankle perimeter circumference compared to placebo (MD ‐4.27 mm; 95% CI ‐5.61 to ‐2.93; 15 studies; 2010 participants; moderate‐certainty evidence; Analysis 1.2). The certainty of the evidence was downgraded by one step to moderate because of overall risk of bias (11 studies had an unclear risk of bias and one had a high risk of bias). Differences between the subgroups was detected (test for subgroup differences: P = 0.02) due to a larger effect of diosmin‐hidrosmin.
1.2. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 2: Ankle perimeter circumference (mm)
Volume of the leg
We included 11 studies in the analysis: six corresponding to rutosides (Burnand 1989; Diebschlag 1994; Ihme 1996; Kiesewetter 1997; NCT01848210; Vanscheidt 2002a), four to calcium dobesilate (Casley‐Smith 1988; Rabe 2011;Rabe 2016; Widmer 1990) and one to aminaftone (Belczak 2014), with a total of 686 participants treated with phlebotonics and 706 with placebo. Phlebotonics probably slightly reduce volume of the leg compared to placebo (SMD ‐0.24 mL; 95% CI ‐0.33 to ‐0.15; 11 studies; 2072 participants; moderate‐certainty evidence; Analysis 1.3). The certainty of the evidence was downgraded (1 level) to moderate because of overall risk of bias (seven studies had an unclear risk of bias and one had a high risk of bias). Differences between the subgroups was detected (test for subgroup differences: P = 0.04) due to a larger effect of calcium dobesilate.
1.3. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 3: Volume of the leg (mL)
QoL
Seven studies evaluated QoL (Belczak 2014; Martinez‐Zapata 2008; Rabe 2011; Rabe 2015; Rabe 2016; Vanscheidt 2002a; Vanscheidt 2002b). Vanscheidt 2002a and Vanscheidt 2002b assessed QoL by using a questionnaire (EuroQol Measure of Health‐Related QoL and Freiburg Life Quality Assessment, respectively) and therefore did not provide quantifiable results. Martinez‐Zapata 2008, Rabe 2011, Rabe 2015 and Rabe 2016 evaluated QoL via the Chronic Venous Insufficiency International Questionnaire (CIVIQ). Belczak 2014 used a specific questionnaire for chronic venous disease adapted from Cesarone 2006. Phebotonics probably make little or no difference to QoL compared with placebo (SMD ‐0.06, 95% CI ‐0.22 to 0.10; five studies; 1639 participants; moderate‐certainty evidence; Analysis 1.4). The certainty of the evidence was downgraded by one step to moderate because of overall risk of bias (one study had an unclear risk of bias and two had a high risk of bias). Differences between the subgroups was detected (test for subgroup differences: P = 0.02) due to a larger effect of aminaftone .
1.4. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 4: Quality of life
Secondary outcomes
Assessment of CVI by objective signs: skin manifestations
Ulcer healing (dichotomous variable)
We included six trials in the meta‐analysis: one on aminaftone (Lazzarini 1982), one on calcium dobesilate (DOBESILATO500/2), two on diosmine (Fermoso 1992; Guilhou 1997) and two on rutoside (MacLennan 1994; Schultz‐Ehrenburg 1993), with a total of 230 participants in the active treatment group and 231 in the placebo group. Phlebotonics may make little or no difference to dichotomous variable ulcer cured compared to placebo (RR 0.94; 95% CI 0.79 to 1.13; 6 studies; 461 participants; low‐certainty evidence; Analysis 1.5). The certainty of the evidence was downgraded by two levels to low because of overall risk of bias (four studies had an unclear risk of bias and two had a high risk of bias) and imprecision (low number of total events) (Table 1). No differences between the subgroups was detected (test for subgroup differences: P = 0.21).
1.5. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 5: Ulcer healing
Trophic disorders (dichotomous variable)
We included six studies in the meta‐analysis: four on hidrosmine and diosmine (Fermoso 1992; Gilly 1994; Laurent 1988; Planchon 1990), one on aminaftone (Lazzarini 1982) and one on rutosides (MacLennan 1994), with a total of 355 participants in the phlebotonics group and 350 in the placebo group. Phlebotonics probably slightly improve trophic disorders compared to placebo (RR 0.87, 95% CI 0.81 to 0.95; 6 studies; 705 participants; moderate‐certainty evidence; Analysis 1.6). The certainty of the evidence was downgraded by one level to moderate because of overall risk of bias (five studies had an unclear risk of bias and one had a high risk of bias). No differences between the subgroups was detected (test for subgroup differences: P = 0.80).
1.6. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 6: Trophic disorders (dichotomous variable)
Telangiectasia, reticular veins and varicose veins (dichotomous variable)
Included studies did not report data on improvement in skin signs such as telangiectasia, reticular veins and varicose veins. Only Fermoso 1992 reported results regarding varicose veins. Before treatment, 3/16 (18.8%) participants presented varicose veins in the hidrosmine group and 2/12 participants in the placebo group (16.7%). After treatment, one participant from the hidrosmine group was cured of varicose veins, and no participants from the placebo group were cured.
Assessment of CVI by subjective symptoms
Pain in the lower legs (dichotomous variable)
A total of 21 studies reported on this outcome as a dichotomous variable: 10 on rutosides (Balmer 1980; Cauwenberge 1972; Cauwenberge 1978; Jongste 1989; Klüken 1971; Languillat 1988; Pedersen 1992; Pulvertaft 1983; Vanscheidt 2002a; Welch 1985), five on calcium dobesilate (Casley‐Smith 1988; Flota‐Cervera 2008; Hachen 1982; Rabe 2016; Widmer 1990), four on diosmine and hidrosmine (Biland 1982; Dominguez 1992; Fermoso 1992; Planchon 1990), one on aminaftone (Lazzarini 1982), and one on French maritime pine bark extract (Arcangeli 2000), with a total of 1468 participants treated with phlebotonics and 1130 with placebo (Analysis 1.7). The analysis showed heterogeneity (I2 = 77%); therefore, we did not pool the data.
1.7. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 7: Pain in the lower legs (dichotomous variable)
Pain in the lower legs (continuous variable)
We included 12 studies in the meta‐analysis: five on calcium dobesilate (DOBESILATO500/2; Marinello 2002; Martinez‐Zapata 2008; Rabe 2011; Rabe 2016), three on rutosides (Cloarec 1996; Cornu‐Thenard 1985; Parrado 1999), three on diosmine (Gilly 1994; Planchon 1990; Rabe 2015) and one on French maritime pine bark extract (Arcangeli 2000), with a total of 1110 participants assigned to phlebotonics and 1122 to placebo (Analysis 1.8). Phlebotonics may reduce pain (measured as a continuous variable) in the lower legs compared to placebo (SMD ‐0.35, 95% CI ‐0.54 to ‐0.17; 12 studies; 2232 participants; low‐certainty evidence). The certainty of the evidence was downgraded by two levels to low because of overall risk of bias (seven studies had an unclear risk of bias and three had a high risk of bias) and imprecision. We used a random‐effects model as heterogeneity was detected (I2 = 75%). Differences between the subgroups was detected (test for subgroup differences: P = 0.002) due to differences in results between the subgroup of French maritime pine bark extract compared to the other subgroups.
1.8. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 8: Pain in the lower legs (continuous variable)
Cramps in the lower legs (dichotomous variable)
We included 14 studies in the meta‐analysis: eight on rutosides (Balmer 1980; Cauwenberge 1978; Jongste 1989; Languillat 1988; Pedersen 1992; Pulvertaft 1983; Vin 1994; Welch 1985), three on diosmine and hidrosmine (Biland 1982; Fermoso 1992; Planchon 1990), two on calcium dobesilate (Casley‐Smith 1988; Widmer 1990) and one on aminaftone (Lazzarini 1982), with a total of 1072 participants treated with phlebotonics and 721 with placebo. Phlebotonics probably reduce cramps (measured as a dichotomous variable) compared to placebo (RR 0.72, 95% CI 0.58 to 0.89; 14 studies; 1793 participants; moderate‐certainty evidence; Analysis 1.9) The certainty of the evidence was downgraded by one level to moderate because of overall risk of bias (11 studies had an unclear risk of bias and three had a high risk of bias). We used a random‐effects model as heterogeneity was detected (I2 = 73%). No differences between the subgroups was detected (test for subgroup differences: P = 0.28).
1.9. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 9: Cramps in the lower legs (dichotomous variable)
Cramps in the lower legs (continuous variable)
We included four studies in the meta‐analysis: two on rutosides (Cloarec 1996; Parrado 1999), one on calcium dobesilate (Martinez‐Zapata 2008), and one on diosmine (Gilly 1994), with 363 participants treated with phlebotonics and 366 with placebo (Analysis 1.10). The analysis showed heterogeneity (I2 = 86%); therefore, we did not pool the data.
1.10. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 10: Cramps in the lower legs (continuous variable)
Restless legs (dichotomous variable)
We included seven studies in the meta‐analysis: four on rutosides (Balmer 1980; Cauwenberge 1978; Jongste 1989; Pedersen 1992), two on calcium dobesilate (Casley‐Smith 1988; Widmer 1990), and one on diosmine (Biland 1982). A total of 329 participants were treated with phlebotonics and 323 with placebo (Analysis 1.11). Phebotonics probably slightly reduce restless legs (measured as a dichotomous variable) compared to placebo (RR 0.81, 95% CI 0.72 to 0.91; 7 studies; 652 participants; moderate‐certainty evidence). The certainty of the evidence was downgraded by one level to moderate because of overall risk of bias (five studies had an unclear risk of bias and two had a high risk of bias). No differences between the subgroups was detected (test for subgroup differences: P = 0.41).
1.11. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 11: Restless legs (dichotomous variable)
Itching in the lower legs (dichotomous variable)
We included four studies in the analysis: two on rutoside (Pedersen 1992; Vanscheidt 2002a), one on hidrosmine (Fermoso 1992), and one on aminaftone (Lazzarini 1982). A total of 206 participants were included in the active treatment group and 199 in the placebo group (Analysis 1.12). The analysis showed heterogeneity (I2 = 92%); therefore, we did not pool the data.
1.12. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 12: Itching in the lower legs (dichotomous variable)
Itching in the lower legs (continuous variable)
We included two studies in the analysis: one on calcium dobesilate (Martinez‐Zapata 2008), and one on rutosides (Parrado 1999). A total of 234 participants were treated with phlebotonics and 242 with placebo (Analysis 1.13). The analysis showed heterogeneity (I2 = 82%), and we did not pool the data.
1.13. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 13: Itching in the lower legs (continuous variable)
Feeling of heaviness in the lower legs (dichotomous variable)
We included 19 studies in the analysis: nine on rutosides (Cauwenberge 1972; Cauwenberge 1978; Jongste 1989; Languillat 1988; Pedersen 1992; Pulvertaft 1983; Vanscheidt 2002a; Vin 1994; Welch 1985), four on diosmine and hidrosmine (Dominguez 1992; Fermoso 1992; Planchon 1990; Tsouderos 1989), three on calcium dobesilate (Casley‐Smith 1988; Hachen 1982; Widmer 1990), one on aminaftone (Lazzarini 1982), one on Centella asiatica (Pointel 1986), and one on French maritime pine bark extract (Arcangeli 2000). A total of 1257 participants were included in the active treatment group and 909 in the placebo group (Analysis 1.14). The analysis showed heterogeneity (I2 = 80%), and we did not pool the data.
1.14. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 14: Heaviness in the lower legs (dichotomous variable)
Feeling of heaviness in the lower legs (continuous variable)
We included 10 studies in the analysis: six on rutosides (Alterkamper 1987; Cloarec 1996; Cornu‐Thenard 1985; Diebschlag 1994; Parrado 1999; Unkauf 1996), two on calcium dobesilate (Marinello 2002; Martinez‐Zapata 2008), one on diosmine (Gilly 1994), and one on French maritime pine bark extract (Arcangeli 2000). A total of 557 participants were included in the active treatment group and 557 in the placebo group (Analysis 1.15). The analysis showed heterogeneity (I2 = 91%); therefore, we did not pool the data.
1.15. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 15: Heaviness in the lower legs (continuous variable)
Swelling in the lower legs (dichotomous variable)
We included 14 studies in the analysis: nine on rutosides (Balmer 1980; Cauwenberge 1978; Jongste 1989; Kriner 1985; Languillat 1988; Pedersen 1992; Vanscheidt 2002a; Vin 1994; Welch 1985), two on calcium dobesilate (Casley‐Smith 1988; Hachen 1982), two on diosmine and hidrosmine (Biland 1982; Fermoso 1992), and one on French maritime pine bark extract (Arcangeli 2000), with 544 participants included in the active treatment group and 528 in the placebo group. Phebotonics probably reduce swelling in the lower leg (measured as a dichotomous variable) compared to placebo (RR 0.63, 95% CI 0.50 to 0.80; 14 studies; 1072 participants; moderate‐certainty evidence; Analysis 1.16). The certainty of the evidence was downgraded by one level to moderate because of overall risk of bias (11 studies had an unclear risk of bias and two had a high risk of bias). We used a random‐effects model as heterogeneity was detected (I2 = 69%). Differences between the subgroups was detected (test for subgroup differences: P = 0.007) due to a larger effect of calcium dobesilate.
1.16. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 16: Swelling in the lower legs (dichotomous variable)
Swelling in the lower legs (continuous variable)
We included six studies in the analysis: three on rutosides (Cloarec 1996; Diebschlag 1994; Unkauf 1996), one on diosmine (Gilly 1994), one on calcium dobesilate (Martinez‐Zapata 2008), and one on French maritime pine bark extract (Arcangeli 2000), with 436 participants assigned to active treatment and 435 to placebo (Analysis 1.17). The analysis showed heterogeneity (I2 = 95%), and we did not pool the data.
1.17. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 17: Swelling in the lower legs (continuous variable)
Paraesthesia in the lower legs (dichotomous variable)
We included nine studies in the analysis: four on rutosides (Balmer 1980; Cauwenberge 1978; Pulvertaft 1983; Welch 1985), three on calcium dobesilate (Casley‐Smith 1988; Hachen 1982; Widmer 1990) and two on diosmine and hidrosmine (Fermoso 1992; Planchon 1990), with 896 participants assigned to active treatment and 560 to placebo (Analysis 1.18). Phlebotonics probably reduce paraesthesia in the lower legs (measured as a dichotomous variable) compared to placebo (RR 0.67, 95% CI 0.50 to 0.88; 9 studies; 1456 participants; moderate‐certainty evidence). The certainty of the evidence was downgraded by one level) to moderate because of overall risk of bias (eight studies had an unclear risk of bias and one had a high risk of bias). We used a random‐effects model as heterogeneity was detected (I2 = 72%). No differences between the subgroups was detected (test for subgroup differences: P = 0.32).
1.18. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 18: Paraesthesia in the lower legs (dichotomous variable)
Paraesthesia in the lower legs (continuous variable)
We included two studies in the analysis: one on diosmine (Gilly 1994), and one on rutoside (Cornu‐Thenard 1985), with 97 participants assigned to active treatment and 91 to placebo (Analysis 1.19). It is uncertain whether phlebotonics reduce continuous variable paraesthesia because the certainty of this evidence is very low (SMD ‐0.15, 95% CI ‐0.44 to 0.13; 2 studies; 188 participants). The certainty of the evidence was downgraded by three levels to very low because of risk of bias (one level) and the sample size was small with a high imprecision in the results (two levels). No differences between the subgroups was detected (test for subgroup differences: P = 0.59).
1.19. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 19: Paraesthesia in the lower legs (continuous variable)
Participant satisfaction (dichotomous variable)
We included 16 studies in the analysis: eight on rutosides (Burnand 1989; Cloarec 1996; Jongste 1989; Languillat 1988; Parrado 1999; Pedersen 1992; Pulvertaft 1983; Welch 1985), three on calcium dobesilate (Casley‐Smith 1988; Labs 2004; Rabe 2011), four on diosmine (Biland 1982; Chassignolle 1994; Danielsson 2002; Laurent 1988), and one on Centella asiatica (Allegra 1981), with a total of 1265 participants treated with phlebotonics and 939 with placebo (Analysis 1.20). The analysis showed heterogeneity (I2 = 86%), and we did not pool the data.
1.20. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 20: Participant satisfaction (dichotomous variable)
Participant satisfaction (continuous variable)
We included seven studies in the analysis: four on rutosides (Cesarone 2002; Cloarec 1996; Ihme 1996; Kiesewetter 1997), two on calcium dobesilate (Rabe 2011; Widmer 1990), and one on diosmine (Gilly 1994), with 440 participants treated with phlebotonics and 441 with placebo (Analysis 1.21). The analysis showed heterogeneity (I2 = 85%), and we did not pool the data.
1.21. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 21: Participant satisfaction (continuous variable)
Adverse events
Thirty‐seven studies reported on adverse events. These included 17 trials considering rutosides (Alterkamper 1987; Balmer 1980; Diebschlag 1994; Jongste 1989; Koscielnny 1996; Kriner 1985; Languillat 1988; MacLennan 1994; NCT01848210; Parrado 1999; Serralde 1990; Unkauf 1996; Vanscheidt 2002a; Vanscheidt 2002b; Vin 1994; Welch 1985; Zucarelli 1987), nine on hidrosmine‐diosmine (Biland 1982; Danielsson 2002; Dominguez 1992; Fermoso 1992; Gilly 1994; Guilhou 1997; Laurent 1988; Planchon 1990; Rabe 2015), eight on calcium dobesilate (Flota‐Cervera 2008; Hachen 1982; Labs 2004; Marinello 2002; Martinez‐Zapata 2008; Rabe 2011; Rabe 2016; Widmer 1990), one on aminaftone (Belczak 2014), one on grape seed extract (Thebaut 1985), and one on Centella asiatica (Pointel 1986).
We included in the meta‐analysis a total of 2944 participants treated with phlebotonics and 2845 with placebo. Phlebotonics probably increase adverse events slightly, compared to placebo (RR 1.14, 95% CI 1.02 to 1.27; 37 studies; 5789 participants; moderate‐certainty evidence; Analysis 1.22). The certainty of the evidence was downgraded by one level to moderate because of overall risk of bias (28 RCTs had unclear risk of bias and four RCTs had high risk of bias) (Table 1). No differences between the subgroups was detected (test for subgroup differences: P = 0.36).
1.22. Analysis.

Comparison 1: Phlebotonics versus placebo, Outcome 22: Adverse events
Adverse events analyzed by active agent
Aminaftone
Only one trial reported adverse events (Belczak 2014). One participant presented with headache in the group given Aminaftone, and two in the placebo group dropped out as the result of subjective worsening of leg pain. It is uncertain whether aminaftone reduces adverse events because the certainty of this evidence is very low (RR 0.60, 95% CI 0.06 to 6.32; 79 participants; Analysis 1.22). The certainty of the evidence was downgraded by three levels to very low because Belczak 2014 had unclear risk of bias (one level) and the sample size was very small with a high imprecision in the results (two levels).
Calcium dobesilate
In total, eight trials evaluated adverse events with calcium dobesilate use (Flota‐Cervera 2008; Hachen 1982; Labs 2004; Marinello 2002; Martinez‐Zapata 2008; Rabe 2011; Rabe 2016; Widmer 1990). Nineteen per cent of participants in the calcium dobesilate group (179/932) experienced an adverse event and 15% (133/892) in the placebo group. Calcium dobesilate may make little or no difference to adverse events compared with placebo (RR 1.22, 95% CI 1.00 to 1.49; 8 studies 1824 participants; low‐certainty evidence). The certainty of the evidence was downgraded by 2 levels to low because of overall risk of bias (four RCTs had unclear risk of bias and one RCT had high risk of bias). The most common adverse event was a gastrointestinal event (epigastric discomfort, vomiting). No agranulocytosis or white blood cell disorders were identified. Twenty‐five participants were withdrawn from the calcium dobesilate group and 17 from the placebo group as the result of adverse events.
Centella asiatica
One study reported information on adverse events with Centalla asiatica (Pointel 1986). Thirty‐one per cent of participants in the Centella asiatica group (19/61) suffered from adverse events and 27.3% (9/33) in the placebo group. It is uncertain whether Centella asiatica reduces adverse events because the certainty of this evidence is very‐low (RR 1.14, 95% CI 0.58 to 2.23; 94 participants). The certainty of the evidence was downgraded by three levels to very low because Pointel 1986 had unclear risk of bias (one level) and the sample size was very small with a high imprecision in the results (two levels). Two participants who took Centella asiatica 120 mg withdrew ‐ one because of gastralgia (gastric colic) and the other because of neurological absence (absence of nerve activity). One participant taking placebo discontinued the study because of cyanosis of the extremities (bluish discolouration caused by lack of oxygen in the blood).
Diosmine and hidrosmine
Nine studies reported the number of participants who experienced adverse events (Biland 1982; Danielsson 2002; Dominguez 1992; Fermoso 1992; Gilly 1994; Guilhou 1997; Laurent 1988; Planchon 1990; Rabe 2015). Ninety‐nine adverse events occurred in the hidrosmine and diosmine group (99/720) and 106 (106/709) in the placebo group. Diosmine and hidrosmine may make little or no difference to adverse events compared with placebo (RR 0.93, 95% CI 0.72 to 1.19; 9 studies; 1429 participants; low‐certainty evidence). The certainty of the evidence was downgraded by two levels to low because of overall risk of bias (eight RCTs had unclear risk of bias and one RCT had high risk of bias).
Gastrointestinal disorders were the most reported adverse events (heartburn and nausea): 14 cases were reported in the hidrosmine and diosmine group and 11 in the placebo group.
Thirteen participants withdrew from the hidrosmine group and 12 from the placebo group as the result of adverse events.
Grape seed extract
One study reported information regarding adverse events (Thebaut 1985). Eleven per cent of participants (4/35) receiving active treatment reported adverse effects (three withdrew): two participants had gastralgia, one participant had a headache and one had an allergic reaction. Twenty per cent of participants in the placebo group (8/40) experienced adverse effects (one withdrew); these included constipation, gastralgia, tiredness, dry mouth and discomfort. It is uncertain whether grape seed extract reduces adverse events because of the certainty of this evidence is very low (RR 0.57, 95% CI 0.19 to 1.74; 75 participants). The certainty of the evidence was downgraded by three levels to very low because of Thebaut 1985 had unclear risk of bias (one level) and the sample size was very small with a high imprecision in the results (two levels).
Rutoside
Sixteen trials reported information regarding the number of participants who experienced adverse events (Alterkamper 1987; Balmer 1980; Diebschlag 1994; Jongste 1989; Koscielnny 1996; Kriner 1985; Languillat 1988; MacLennan 1994; Parrado 1999; Serralde 1990; Unkauf 1996; Vanscheidt 2002a; Vanscheidt 2002b; Vin 1994; Welch 1985; Zucarelli 1987).Twenty per cent of participants (233/1160) in the rutoside group suffered from adverse events and 16% (181/1128) in the placebo group. Rutosids may slightly increase adverse events compared to placebo (RR 1.41, 95% CI 1.08 to 1.83; 16 studies; 2288 participants; low‐certainty evidence). The certainty of the evidence was downgraded by two levels to low because of the overall risk of bias (13 RCTs had unclear risk of bias and two had high risk of bias). The most common adverse events were gastrointestinal in nature (constipation, dry mouth, epigastric discomfort, vomiting): 127 in the rutoside group and 81 in the placebo group, followed by headache (23 in the rutoside group, 21 in the placebo group) and tiredness (17 in the rutoside group, nine in the placebo group).
Thirteen participants withdrew from the rutoside group and 22 from the placebo group as the result of adverse events.
Sensitivity analysis
Exclusion of studies using compression measures (elastic stockings)
We carried out sensitivity analysis by re‐analysing the data excluding studies that allowed the use of elastic stockings (Balmer 1980; DOBESILATO500/2; Guilhou 1997; Laurent 1988; MacLennan 1994; Martinez‐Zapata 2008; Rabe 2011; Schultz‐Ehrenburg 1993; Zucarelli 1987). We found that generally, results did not change, except for the following variables.
Phlebotonics may reduce dichotomous variable pain (RR 0.70, 95% CI 0.60 to 0.82; 18 studies; 1818 participants, low‐certainty evidence; Analysis 2.7). The certainty of evidence was downgraded by two levels to low because of overall risk of bias (18 studies had unclear risk of bias and five had high risk of bias). No differences between the subgroups was detected (test for subgroup differences: P = 0.12). In the overall analysis, the results were very heterogeneous, so we did not pool them.
Phlebotonics may reduce dichotomous variable participant satisfaction (RR 0.69, 95% CI 0.53 to 0.90; 12 studies; 1193 participants; low‐certainty evidence; Analysis 2.20). The certainty of evidence was downgraded by two levels to low because of overall risk of bias (10 studies had unclear risk of bias and one had high risk of bias). No differences between the subgroups was detected (test for subgroup differences: P = 0.33). In the overall analysis, the results were very heterogeneous, so we did not pool them.
2.7. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 7: Pain in the lower legs (dichotomous variable)
2.20. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 20: Participant satisfaction (dichotomous variable)
Exclusion of unpublished data
Only one study, which focused on rutosides, was not published (Welch 1985). When we re‐analysed the data while excluding this study, we found results very similar to those of the main analysis for all outcomes.
Exclusion of studies at high or unclear risk of bias
In judging quality levels based on the aforementioned criteria, we identified only four studies with low risk of bias (Labs 2004; Martinez‐Zapata 2008; Rabe 2011; Vanscheidt 2002a). Consequently, limited sensitivity analyses for the included variables were possible.
Results changed only for the following variables:
For the dichotomous variable of oedema, only one study on calcium dobesilate was included with a low risk of bias (Labs 2004). Phlebotonics may make little or no difference in the dichotomous variable oedema compared to placebo (RR 0.99, 95% CI 0.63 to 1.55; 1 study; 260 participants; low‐certainty evidence; Analysis 4.1). The certainty of the evidence was downgraded by two levels to low because Labs 2004 had a small sample size and imprecision.
For the continuous variable of oedema (measure of ankle circumference in mm), three studies on calcium dobesilate were included with a low risk of bias (Labs 2004; Martinez‐Zapata 2008; Rabe 2011). Based on their results, phlebotonics probably make little or no difference in the continuous variable oedema (measure of ankle circumference in mm) compared to placebo (MD ‐2.34 mm, 95% CI ‐8.79 to 4.11; 3 studies; 867 participants; moderate‐certainty evidence; Analysis 4.2). The certainty of the evidence was downgraded by one level for imprecision.
For the dichotomous variable of itching, only one study on rutoside had a low risk of bias (Vanscheidt 2002a). Phlebotonics probably reduce dichotomous variable itching, compared with placebo (RR 0.44, 95% CI 0.32 to 0.62; 231 participants; moderate‐certainty evidence; Analysis 4.7). The certainty of the evidence was downgraded by one level for imprecision.
For the continuous variable of itching, one study on calcium dobesilate was at low risk of bias (Martinez‐Zapata 2008). Phlebotonics probably make little or no difference to the continuous variable itching (MD 4.60 cm, 95% CI ‐5.66 to 14.86; 416 participants; moderate‐certainty evidence; Analysis 4.8). The certainty of the evidence was downgraded by one level by imprecision.
For the dichotomous variable of heaviness, one study on rutoside was at low risk of bias (Vanscheidt 2002a). Phlebotonics probably reduce the dichotomous variable heaviness compared to placebo (RR 0.62, 95% CI 0.47 to 0.82; 231 participants; moderate‐certainty evidence; Analysis 4.9). The certainty of the evidence was downgraded by one level by imprecision.
For the continuous variable of heaviness, one study on calcium dobesilate was at low risk of bias (Martinez‐Zapata 2008). Phlebotonics probably make little or no difference on the continuous variable heaviness compared with placebo (MD ‐2.40 cm, 95% CI ‐7.89 to 3.09; 417 participants; moderate‐certainty evidence; Analysis 4.10). The certainty of the evidence was downgraded by one level for imprecision.
For the continuous variable of swelling, one study on calcium dobesilate was at low risk of bias (Martinez‐Zapata 2008). Phlebotonics probably make little or no difference on the continuous variable swelling, compared to placebo (MD ‐1.30 cm, 95% CI ‐6.72 to 4.12; 417 participants; moderate‐certainty evidence; Analysis 4.12). The certainty of the evidence was downgraded by one level for imprecision.
For the dichotomous variable of participant satisfaction, two studies on calcium dobesilate were at low risk of bias (Labs 2004; Rabe 2011). Phlebotonics probably make little or no difference on the dichotomous variable participant satisfaction compared to placebo (RR 1.04, 95% CI 0.81 to 1.32; 2 studies; 476 participants; moderate‐certainty evidence; Analysis 4.13). The certainty of the evidence was downgraded by one level for imprecision.
For the continuous variable of participant satisfaction, one study on calcium dobesilate had a low risk of bias (Rabe 2011). Phlebotonics probably improve the continuous variable participant satisfaction (MD ‐5.64, 95% CI ‐8.85 to ‐2.43; 223 participants; moderate‐ certainty evidence; Analysis 4.14). The certainty of the evidence was downgraded by one level for imprecision.
For the dichotomous variable of adverse events, four studies were at low risk of bias (Labs 2004; Martinez‐Zapata 2008; Rabe 2011; Vanscheidt 2002a). Phlebotonics probably make little or no difference on the dichotomous variable adverse events compared to placebo (RR 1.59, 95% CI 0.97 to 2.63; four studies; 1257 participants; moderate‐certainty evidence; Analysis 4.15). The certainty of the evidence was downgraded by one level for imprecision (low number of events). No differences between the subgroups was detected (test for subgroup differences: P = 0.70)
4.1. Analysis.

Comparison 4: Sensitivity analysis based on low risk of bias, Outcome 1: Oedema in the lower legs (dichotomous variable)
4.2. Analysis.

Comparison 4: Sensitivity analysis based on low risk of bias, Outcome 2: Ankle perimeter circumference (mm)
4.7. Analysis.

Comparison 4: Sensitivity analysis based on low risk of bias, Outcome 7: Itching in the lower legs (dichotomous variable)
4.8. Analysis.

Comparison 4: Sensitivity analysis based on low risk of bias, Outcome 8: Itching in the lower legs (continuous variable)
4.9. Analysis.

Comparison 4: Sensitivity analysis based on low risk of bias, Outcome 9: Heaviness in the lower legs (dichotomous variable)
4.10. Analysis.

Comparison 4: Sensitivity analysis based on low risk of bias, Outcome 10: Heaviness in the lower legs (continuous variable)
4.12. Analysis.

Comparison 4: Sensitivity analysis based on low risk of bias, Outcome 12: Swelling in the lower legs (continuous variable)
4.13. Analysis.

Comparison 4: Sensitivity analysis based on low risk of bias, Outcome 13: Participant satisfaction (dichotomous variable)
4.14. Analysis.

Comparison 4: Sensitivity analysis based on low risk of bias, Outcome 14: Participant satisfaction (continuous variable)
4.15. Analysis.

Comparison 4: Sensitivity analysis based on low risk of bias, Outcome 15: Adverse events
Discussion
Summary of main results
We evaluated the efficacy and safety of phlebotonics in the treatment of CVI. Only analyses of studies using oral phlebotonics were possible because no identified study of topical phlebotonics met the inclusion criteria of this Cochrane Review. This Cochrane Review included 69 RCTs, and analyzed data from 56 trials involving 7690 participants. Studies included in the review generally provided objective measurement of ankle and calf oedema reduction, as well as a subjective assessment of other signs and symptoms of CVI.
According to the ITT analysis, there was moderate‐certainty evidence of a probable beneficial effect on the dichotomous variable oedema. Analyses with continuous variables also showed a probable beneficial effect of phlebotonics on oedema (moderate‐certainty evidence).
However, there was moderate‐certainty evidence of little or no difference in QoL with phlebotonic use compared to placebo; and little or no difference to dichotomous variable ulcer healing (low‐certainty evidence).
Phlebotonic use probably slightly improves trophic disorders compared to placebo (moderate‐certainty evidence). Data on telangiectasia, reticular veins and varicose veins were very limited. Heterogeneity prevented meta‐analysis on dichotomous variable pain but showed phlebotonic use may reduce pain (continuous variable) compared to placebo (low‐certainty evidence). Similarly, a possible benefit was seen in cramps (moderate‐certainty evidence), restless legs (moderate‐certainty evidence); swelling in lower legs (moderate‐certainty evidence); and paraesthesia (dichotomous variable) in the lower legs (moderate‐certainty evidence). However, based on very low‐certainty evidence, it is uncertain whether phlebotonics reduce continuous variable paraesthesia.
Heterogeneity prevented meta‐analysis on the outcomes of itching and feeling of heaviness in the lower legs.
There was moderate‐certainty evidence that the incidence of adverse events was probably slightly increased in the phlebotonics group compared to placebo group. Gastrointestinal disorders were the most frequently reported adverse events among studies that provided this information (rutosides, calcium dobesilate, diosmine‐hidrosmine). Our review did not report agranulocytosis associated with calcium dobesilate, although this adverse effect was described in a previous case‐control study that detected potential risk of agranulocytosis, with an incidence rate of 1.21 cases per 10,000 patient‐years of treatment (Ibañez 2000; Ibáñez 2005). This could be explained by the small number of participants in the included RCTs and the short period of participant follow‐up provided.
The results by type of active drug showed that It is uncertain whether aminaftone reduces dichotomous variables oedema, pain, cramps, itching and heaviness (very low‐certainty evidence). There was low‐certainty evidence that aminaftone may slightly improve the continuous variables oedema (volume) and QoL, and may make little or no difference to adverse events.
Calcium dobesilate may reduce continuous volume of the leg and may slightly improve continuous variable participant satisfaction (low‐certainty evidence); it may reduce dichotomous variable swelling and may slightly reduce the dichotomous variables pain, cramps and restless legs. Meanwhile, based on moderate‐certainty evidence, calcium dobesilate probably makes little or no difference on QoL and dichotomous variables assessment by the participant and adverse events. Furthermore, calcium dobesilate may make little or no differences on the continuous variables ankle perimeter circumference, pain and heaviness; and for the dichotomous variables heaviness and paraesthesia (low‐certainty evidence). Calcium dobesilate does not have an important effect on the continuous variables swelling, cramps and itching (high‐certainty evidence). We do not know if calcium dobesilate improves dichotomous variable oedema and ulcer healing as the certainty of the evidence is very low.
Based on very low‐certainty evidence, it is uncertain whether Centella asiatica compared to placebo reduces dichotomous variable heaviness (Pointel 1986), improves the dichotomous variable participant satisfaction (Allegra 1981) or increases adverse events (Pointel 1986).
Diosmine and hidrosmine, based on a moderate‐certainty evidence, probably slightly reduces ankle perimeter, trophic disorders and cramps; and, probably make little or no differences in dichotomous variable pain, QoL, dichotomous variable participant satisfaction and adverse events, Additionally, based on low‐certainty evidence, they may slightly reduce oedema, cramps, heaviness, swelling and dichotomous variable participant satisfaction. Furthermore, diosmine and hidrosmine, based on low‐certainty evidence, may make little or no difference in paraesthesia, ulcer healing, continuous variable pain and dichotomous variable heaviness. We do not know if these drugs improve itching because the certainty of the evidence is very low.
Based on very low‐certainty evidence, it is uncertain whether French maritime pine bark extract reduces pain, heaviness and swelling. For grape seed extract it is also uncertain if it reduces the dichotomous variable oedema (very low‐certainty evidence).
Rutosides were included in the greatest number of clinical trials. Based on moderate‐certainty evidence, rutosides probably improve oedema, volume of the leg and continuous variable pain; and they probably make little or no difference in ankle perimeter and ulcer healing. Based on low‐certainty evidence, rutosides may slightly reduce heaviness, participant satisfaction, continuous variables cramps and itching, dichotomous variables pain, and paraesthesia. Furthermore, rutosides may make little or no difference in continuous variables swelling and paraesthesia and dichotomous variables trophic disorders, cramps, restless and itching. Rutosides may slightly increase adverse events (low‐certainty evidence).
No evidence was found regarding the efficacy of disodium flavodate, naftazone, chromocarbe or topical phlebotonics.
Sensitivity analyses did not alter the results of this review. Whether elastic stockings were used did not influence pooled results, supporting the view that an appropriate randomisation method results in a homogeneous distribution of the groups under comparison.
Overall completeness and applicability of evidence
Several limitations were identified in the included studies. Only one of five studies specified standard diagnostic criteria for CVI, and different studies applied different criteria. Only eleven studies used the currently accepted CEAP classification (Porter 1995) (Belczak 2014; Danielsson 2002; DOBESILATO500/2; Labs 2004; Marinello 2002; Martinez‐Zapata 2008; Rabe 2011; Rabe 2015; Rabe 2016; Vanscheidt 2002a; Vanscheidt 2002b). Therefore, homogeneity in diagnostic criteria is limited, and potential misclassification bias cannot be ruled out. Furthermore, we were unable to perform subgroup analysis by CVI stage because the severity of CVI was variable.
In most RCTs, the way in which participants were included is heterogeneous, and this may have led to differences in response to treatment. In addition, too few participants were included in the studies with the limitations of imprecision in the results and lack of statistical power to detect a difference between phlebotonics and placebo, when an effect could have occurred (beta error, or type II error). Different instruments were used to measure signs and symptoms, and sometimes results were inconclusive. Only seven RCTs assessed the variable QoL using a standardized questionnaire (Belczak 2014; Martinez‐Zapata 2008; Rabe 2011; Rabe 2015; Rabe 2016;Vanscheidt 2002a; Vanscheidt 2002b), but two studies did not provide quantifiable information (Vanscheidt 2002a; Vanscheidt 2002b). Although some studies favoured phlebotonics, the clinical relevance of these findings remains questionable.
Although infrequent, important signs such as venous ulcers have been poorly evaluated. Only six studies included participants with venous ulcers and when pooled, showed none that yielded a difference in ulcer healing (DOBESILATO500/2; Fermoso 1992; Guilhou 1997; Lazzarini 1982; MacLennan 1994; Schultz‐Ehrenburg 1993).
Only two studies addressing trophic disorders defined this term (MacLennan 1994; Planchon 1990), and four did not (Fermoso 1992; Gilly 1994; Laurent 1988; Lazzarini 1982). However, in two studies, trophic disorders were assessed subjectively as present or absent (Fermoso 1992; MacLennan 1994), or as reported on semi‐quantitative four‐item scales (Gilly 1994; Lazzarini 1982; Planchon 1990). Therefore, although data from the examination of trophic alterations were analyzed, these results should be interpreted with caution.
Most studies provided short‐term results (one to three months). Specificaly, for the primary outcome 'oedema in the lower limb,' the median time of follow‐up was 49 days for oedema measured as a dichotomous outcome and 60 days for oedema measures as a continuous outcome. Given the chronic nature of the disease, more long‐term data on the efficacy and safety of phlebotonics are needed (at least one‐year follow‐up). To achieve homogeneous data collection and to specify evidence on the efficacy of phlebotonics, measurement of signs and symptoms should be standardized. Although we have done a subgroup analysis by drugs, we noted that different doses were involved, and we are unable to comment on which is the optimal dose.
Quality of the evidence
Risk of bias of the included studies is somewhat unclear regarding randomisation and blinding because only a limited number of studies specifically reported details regarding these issues. It is difficult to determine whether this is a result of poor design or publication restrictions. As a result, among the 69 RCTs included in this review, only 39 explained the double‐blinding procedure in detail, 18 provided data on randomisation and 10 explained blinding of the randomisation. Furthermore, seven studies had attrition bias and nine selective reporting with high risk of bias. These issues were not addressed in the remaining included studies, and this adds uncertainty to the evidence. Only four studies were graded as having an overall low risk of bias (Labs 2004; Martinez‐Zapata 2008; Rabe 2011; Vanscheidt 2002a).
In the clinical area of CVI, results lack reliability if the RCT did not include a placebo group because of seasonal exacerbations (spring and summer) that might be self limiting and highly subjective symptoms. Consequently, an adequate control group is needed, and both randomisation and treatment should be appropriately blinded (preferably double‐blinded). For this reason, studies that did not include a control group and single‐blinded studies were excluded from the review. Among studies identified as double‐blinded, those with inappropriate blinding of treatments or randomisation were excluded from the meta‐analyses.
We adopted a conservative approach in our review, which prioritised the ITT analysis in terms of both treatment losses and failures. On the other hand, we used change measures only if conditions of the compared groups at baseline were the same, to avoid bias in the assessment of results related to participants' baseline differences.
We evaluated the certainty of the body of evidence using the GRADE approach (Schünemann 2011), which is based on five considerations including risk of bias (study limitations), directness of the evidence, heterogeneity in the data, precision of effect estimates and additional considerations (including risk of publication bias) to assess the certainty of the body of evidence for a priori selected outcomes (in our review, these included the dichotomous variable of oedema in the lower legs and the continuous variables of oedema in the lower legs, QoL, participants with ulcer healing and participants with adverse events) (Table 1).
With this approach, the overall certainty of evidence is ranked from very low (paraesthesia (continuous variable) to moderate (dichotomous and continuous outcomes of oedema and adverse events; cramps, restless legs, swelling and paraesthesia (dichotomous variable).
Reasons for downgrading the certainty of evidence for the outcome ulcer healing include the presence of selective reporting and incomplete outcome data; for the outcome QoL, we downgraded for incomplete outcome data and imprecision (wide confidence intervals); for the dichotomous variables measurement of oedema we downgraded for incomplete outcome data; and for the continuous variable oedema we downgraded for unclear risk of bias of one trial; for the outcome adverse events we downgraded for incomplete outcome data and indirectness (moderate heterogeneity). See Table 1.
Potential biases in the review process
Any systematic review is influenced by the quality of included studies and reports. In this respect, we classified only four RCTs as having low risk of bias, and we considered most included studies to have moderate risk of potential bias. We excluded RCTs with high risk of bias. Therefore, conclusions about the results of these studies should be interpreted with caution.
The heterogeneity of several analysis variables may be due to the following:
Different diagnosis classification criteria have been applied; therefore, characteristics of the included population in terms of degree of progression of CVI might vary among studies.
No standardisation is involved in measuring variables, given the different scales that have been used, some of which are not validated. Although the same criteria were applied to the data dichotomisation (participants without symptoms/signs or with mild symptoms/sign versus participants with moderate to severe symptoms/signs), these may not be equally relevant, as they result from the application of different scales.
On the other hand, the same subjectivity of collected variables may represent differences among individuals and may influence the variability of results.
In addition, efficacy of evaluated treatments may not be the same because different active principles were used. This explains observed differences among treatments in the subgroup analysis.
All these considerations limit the validity of included clinical trials and the conclusions of this review. The existence of such heterogeneity restricts the importance of its detection in the process of generating hypotheses (i.e. phlebotonics could be effective for treatment of the pain, cramps, heaviness and swelling of CVI).
Only 54% of included studies reported information on adverse events. However, to adequately assess adverse events related to phlebotonics, it is necessary to include observational study designs that were excluded from our review.
Agreements and disagreements with other studies or reviews
Several reviews have tried to evaluate the clinical benefit of phlebotonics. Some of these used poor methods, which did not include information on search strategies and data collection sources, extraction and statistical treatment (diosmine, escin and rutosides (Diehm 1996b); flavonoids, tribenosides, escin and calcium dobesilate (Markwardt 1996); rutosides (Wadworth 1992); flavonoids (Rabe 2013)). Other reviews are more elaborate and were developed systematically (global phlebotonics (Boada 1999); calcium dobesilate (Ciapponi 2004); escin (Pittler 1998); rutosides (Aziz 2015; Poynard 1994); flavonoids (Kakkos 2018)). Five reviews pursued data meta‐analysis (Aziz 2015; Boada 1999; Ciapponi 2004; Kakkos 2018; Poynard 1994).
One review specifically evaluated hydroxyethyl rutosides and the review authors included 15 randomised studies and applied a per‐protocol (PP) analysis. They stated that rutosides were better than control for controlling symptoms of pain, cramps and heaviness (Aziz 2015).
Another review analyzed rutosides and the review authors included 12 randomised, double‐blind, placebo‐controlled studies and applied an ITT analysis. They stated that rutosides were better than placebo for controlling symptoms of pain, cramps, heaviness, swelling and tiredness of affected legs. They did not mention CVI signs (Poynard 1994).
Boada 1999 reviewed all drugs that have been evaluated for CVI through randomised, double‐blind, placebo‐controlled trials without concomitant compression procedures. These included traditional agents such as hidrosmine, diosmine, escin, rutosides and calcium dobesilate, along with other, less usual ones such as extract of Centella asiatica, benzarone, tribenoside, flunarizine, dihydroergotamine mesylate and mucopolysaccharide sulphate. The conclusion of the Boada 1999 review was that phlebotonics might improve leg heaviness in patients with CVI. Review authors presented no conclusive data regarding other signs or symptoms, performed PP rather than ITT analysis and provided no information on individual phlebotonics (Boada 1999).
The review led by Ciapponi 2004 analyzed calcium dobesilate and the review authors included 10 double‐blind, randomised, placebo‐controlled studies and applied a PP analysis. They stated that calcium dobesilate was better than placebo for controlling cramps and discomfort. Subgroup analysis showed greater efficacy in more severe cases of the disease in terms of improving symptoms (pain, heaviness and swelling) and signs (leg volume). Sensitivity analysis based on the ITT analysis did not influence these results (Ciapponi 2004).
Kakkos 2018 evaluated the efficacy of a micronized purified flavonoid fraction (diosmine) in CVD. The systematic review included seven double‐blind, randomised, placebo‐controlled studies. Their results showed a significant improvement for diosmine with respect to signs and symptoms related to CVD, QoL and treatment assessment by the physician. Although our review presents some differences because we pooled studies assessing diosmine and hidrosmine, except for QoL, the general results are in agreement with Kakkos 2018.
With the exception of Aziz 2015 and Kakkos 2018, the above‐cited reviews were published some time ago and have not been updated. Our review provides an update regarding evidence on phlebotonics in general and by drug group.
Authors' conclusions
Implications for practice.
Phlebotonics present limited efficacy for oedema and for some signs and symptoms related to chronic venous insufficiency (CVI).
There is moderate‐certainty evidence that phlebotonics probably slightly reduce oedema compared to placebo; moderate‐certainty evidence of little or no difference in quality of life (QoL); and low‐certainty evidence indicates that these drugs do not influence ulcer healing. Moderate‐certainty evidence shows that phlebotonics are probably associated with higher risk of adverse events than placebo, especially in the subgroup analysis of rutoside group. Studies included in this Cochrane Review provided only short‐term efficacy and safety data; therefore, the middle‐ and long‐term efficacy and safety of phlebotonics could not be estimated. Based on the results of subgroup analysis some phlebotonics were effective for certain symptoms and signs; however, given the limited number of studies and the discordance in their results, these findings are uncertain.
Implications for research.
As a result of the importance of phlebotonics and the limitations of current evidence, high‐quality RCTs are needed to evaluate the efficacy and adverse effects of this group of drugs in an independent and rigorous manner. However, the new studies included in this review have improved methodological aspects and have already considered in a standardized manner the diagnostic classification of participants, measurement of signs and symptoms, larger sample sizes and longer follow‐up, and future trials should continue these recommendations. Additional research regarding QoL and both ulcer healing and trophic disorders is needed, particularly with an accurate definition of the term and the use of objective measurements. More and better assessments of venous ulcers should be made, and QoL surveys specifically validated for CVI should be introduced. Furthermore, currently available data on safety refer to a short administration period; therefore, long‐term observational follow‐up studies are needed to better define the safety profile of each of the phlebotonics and to outline more clearly the risk/benefit ratio.
When the efficacy of phlebotonics is investigated, restriction criteria are recommended to avoid situations that are more likely to result in adverse effects, including long‐term administration, important co‐morbidity, leucopenia, ageing and multiple medications. In addition, researchers involved in these trials should make an explicit statement regarding their conflicts of interest.
What's new
| Date | Event | Description |
|---|---|---|
| 27 May 2020 | New citation required but conclusions have not changed | Searches rerun; three new studies included, two new studies excluded and two new ongoing studies identified. A new author joined the review team. Review text updated according to current Cochrane reporting guidelines. |
| 27 May 2020 | New search has been performed | Searches rerun; three new studies included, two new studies excluded and two new ongoing studies identified. |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 21 August 2015 | New citation required and conclusions have changed | Searches rerun, 6 new studies included, 1 study reclassified as an included study, 115 new studies excluded and 3 new ongoing studies identified. New authors have joined the review team. Risk of bias assessed for all included studies and 'Summary of findings' table added. Review updated according to current Cochrane reporting guidelines |
| 21 August 2015 | New search has been performed | Searches rerun, 6 new studies included, 2 publications added to already included studies and 1 study reclassified as an included study, 115 new studies excluded and 3 new ongoing studies identified |
| 8 July 2008 | Amended | Converted to new review format |
| 14 November 2006 | Amended | Edited update. CDSR citations updated |
Acknowledgements
We would like to thank Marta Roqué for statistical advice provided on the first version of this review. We also want to express our acknowledgement to the Spanish Drug Agency for providing us with several papers used in an in‐house assessment process, and to Dr A Cachá, a member of the Safety Committee Task Force of the Agencia Española del Medicamento, who initially participated in the process of selection of clinical trials. We would like to thank Dr Dolors Capellà for contributions to the previous version of this review. We also acknowledge the Cochrane Vascular Information Specialist for assistance in searching for trials and Cathryn Broderick for her support in the development of the review.
The review authors and the Cochrane Vascular editorial base are grateful to the following peer reviewers for their time and comments: Ralph G DePalma MD, Uniformed Services University of the Health Sciences, USA; Dr Alberto Caggiati, Department of Vascular Surgery, Sapienza University of Rome, Italy; Nji Mbaka Fon, Cameroon
Appendices
Appendix 1. Database search strategies
| Source | Search strategy | Hits retrieved |
| CENTRAL | #1 MESH DESCRIPTOR Venous Insufficiency EXPLODE ALL TREES 533 #2 ((insuffic* or insufic* or CVI or isch* or incompet* or chronic) and (saphenous or vein* or veno*)):TI,AB,KY 6291 #3 (Chronic venous disease):TI,AB,KY 128 #4 CVD:TI,AB,KY 4543 #5 (chronic venous disorder*):TI,AB,KY 28 #6 CEAP:TI,AB,KY 256 #7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 10884 #8 MESH DESCRIPTOR 4‐Aminobenzoic Acid EXPLODE ALL TREES 35 #9 MESH DESCRIPTOR Calcium Dobesilate EXPLODE ALL TREES 51 #10 MESH DESCRIPTOR Centella EXPLODE ALL TREES 17 #11 MESH DESCRIPTOR Coumarins EXPLODE ALL TREES 2108 #12 MESH DESCRIPTOR Diosmin EXPLODE ALL TREES 71 #13 MESH DESCRIPTOR Flavonoids EXPLODE ALL TREES 2521 #14 MESH DESCRIPTOR Hemostatics EXPLODE ALL TREES 5173 #15 MESH DESCRIPTOR Hesperidin EXPLODE ALL TREES 67 #16 MESH DESCRIPTOR Hydroxyethylrutoside EXPLODE ALL TREES 97 #17 MESH DESCRIPTOR Pinus EXPLODE ALL TREES 36 #18 MESH DESCRIPTOR Phytotherapy EXPLODE ALL TREES 3950 #19 MESH DESCRIPTOR Plant Extracts EXPLODE ALL TREES 7693 #20 MESH DESCRIPTOR Rutin EXPLODE ALL TREES 171 #21 MESH DESCRIPTOR Saponins EXPLODE ALL TREES 184 #22 aminaftone*:TI,AB,KY 6 #23 aminaphthone:TI,AB,KY 3 #24 aminaphtone*:TI,AB,KY 8 #25 bioflavonoid*:TI,AB,KY 79 #26 (calcium dobesilate):TI,AB,KY 131 #27 centella:TI,AB,KY 101 #28 chromocarbe*:TI,AB,KY 3 #29 Coumarin*:TI,AB,KY 326 #30 daflon:TI,AB,KY 90 #31 diosmin:TI,AB,KY 147 #32 diosmine:TI,AB,KY 10 #33 diosmiplex:TI,AB,KY 1 #34 (disodium flavodate):TI,AB,KY 3 #35 doxium:TI,AB,KY 42 #36 flavonoids:TI,AB,KY 1083 #37 (french maritime pine):TI,AB,KY 38 #38 (grape seed):TI,AB,KY 184 #39 hesperidin:TI,AB,KY 171 #40 hidrosmin*:TI,AB,KY 7 #41 (horse chestnut):TI,AB,KY 50 #42 hydroxyethylrutoside:TI,AB,KY 98 #43 naftazone*:TI,AB,KY 9 #44 para‐aminobenzoates:TI,AB,KY 37 #45 phlebotonics:TI,AB,KY 6 #46 (plant extract*):TI,AB,KY 4598 #47 pycnogenol*:TI,AB,KY 122 #48 rutin*:TI,AB,KY 219 #49 rutoside*:TI,AB,KY 144 #50 saponin*:TI,AB,KY 255 #51 saponosides:TI,AB,KY 0 #52 troxerutin:TI,AB,KY 72 #53 (vasoprotective agents):TI,AB,KY 0 #54 (venoactive drug*):TI,AB,KY 16 #55 (veno‐active drug*):TI,AB,KY 4 #56 #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 20104 #57 #7 AND #56 559 #58 01/01/2015 TO 12/11/2019:CD 761936 #59 #57 AND #58 182 |
182 |
| Clinicaltrials.gov | Venous Insufficiency OR Chronic venous disease OR chronic venous disorder OR CEAP | 4‐Aminobenzoic Acid OR Calcium Dobesilate OR Centella OR Coumarins OR Diosmin OR Flavonoids OR Hemostatics OR Hesperidin OR Hydroxyethylrutoside OR Pinus OR Phytotherapy OR Plant Extracts OR Rutin OR Saponins | 7 |
| ICTRP Search Portal | 6 | |
| Medline (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) 1946 to present | 1 exp Venous Insufficiency/ 2 ((insuffic* or insufic* or CVI or isch* or incompet* or chronic) and (saphenous or vein* or veno*)).ti,ab. 3 "Chronic venous disease".ti,ab. 4 CVD.ti,ab. 5 " chronic venous disorder*".ti,ab. 6 CEAP.ti,ab. 7 or/1‐6 8 4‐Aminobenzoic Acid/ 9 exp Calcium Dobesilate/ 10 Centella/ 11 exp Coumarins/ 12 Diosmin/ 13 exp Flavonoids/ 14 Hemostatics/ 15 Hesperidin/ 16 Hydroxyethylrutoside/ 17 Phytotherapy/ 18 Pinus/ 19 Plant Extracts/ 20 exp Rutin/ 21 exp Saponins/ 22 aminaftone*.ti,ab. 23 aminaphthone.ti,ab. 24 aminaphtone*.ti,ab. 25 bioflavonoid*.ti,ab. 26 "calcium dobesilate".ti,ab. 27 centella.ti,ab. 28 chromocarbe*.ti,ab. 29 Coumarin*.ti,ab. 30 daflon.ti,ab. 31 diosmin.ti,ab. 32 diosmine.ti,ab. 33 diosmiplex.ti,ab. 34 "disodium flavodate".ti,ab. 35 doxium.ti,ab. 36 flavonoids.ti,ab. 37 "french maritime pine".ti,ab. 38 "grape seed".ti,ab. 39 hesperidin.ti,ab. 40 hidrosmin*.ti,ab. 41 "horse ADJ3 (chestnut or chest‐nut)".ti,ab. 42 "horse chestnut".ti,ab. 43 hydroxyethylrutoside.ti,ab. 44 naftazone*.ti,ab. 45 para‐aminobenzoates.ti,ab. 46 phlebotonics.ti,ab. 47 "plant extract*".ti,ab. 48 pycnogenol*.ti,ab. 49 rutin*.ti,ab. 50 rutoside*.ti,ab. 51 saponin*.ti,ab. 52 saponosides.ti,ab. 53 troxerutin.ti,ab. 54 "vasoprotective agents".ti,ab. 55 "venoactive drug*".ti,ab. 56 "veno‐active drug*".ti,ab. 57 or/8‐56 58 7 and 57 59 randomized controlled trial.pt. 60 controlled clinical trial.pt. 61 randomized.ab. 62 placebo.ab. 63 drug therapy.fs. 64 randomly.ab. 65 trial.ab. 66 groups.ab. 67 or/59‐66 68 exp animals/ not humans.sh. 69 67 not 68 70 58 and 69 |
275 |
| Embase 1974 to present | 1 exp vein insufficiency/ 2 ((insuffic* or insufic* or CVI or isch* or incompet* or chronic) and (saphenous or vein* or veno*)).ti,ab. 3 "Chronic venous disease".ti,ab. 4 CVD.ti,ab. 5 "chronic venous disorder*".ti,ab. 6 CEAP.ti,ab. 7 or/1‐6 8 4 aminobenzoic acid/ 9 exp dobesilate calcium/ 10 Centella/ 11 exp coumarin derivative/ 12 diosmin/ 13 exp flavonoid/ 14 hemostatic agent/ 15 hesperidin/ 16 monoxerutin/ 17 phytotherapy/ 18 pine/ 19 plant extract/ 20 exp rutoside/ 21 exp saponin/ 22 aminaftone*.ti,ab. 23 aminaphthone.ti,ab. 24 aminaphtone*.ti,ab. 25 bioflavonoid*.ti,ab. 26 "calcium dobesilate".ti,ab. 27 centella.ti,ab. 28 chromocarbe*.ti,ab. 29 Coumarin*.ti,ab. 30 daflon.ti,ab. 31 diosmin.ti,ab. 32 diosmine.ti,ab. 33 diosmiplex.ti,ab. 34 "disodium flavodate".ti,ab. 35 doxium.ti,ab. 36 flavonoids.ti,ab. 37 "french maritime pine".ti,ab. 38 "grape seed".ti,ab. 39 hesperidin.ti,ab. 40 hidrosmin*.ti,ab. 41 "horse chestnut".ti,ab. 42 hydroxyethylrutoside.ti,ab. 43 naftazone*.ti,ab. 44 para‐aminobenzoates.ti,ab. 45 phlebotonics.ti,ab. 46 "plant extract*".ti,ab. 47 pycnogenol*.ti,ab. 48 rutin*.ti,ab. 49 rutoside*.ti,ab. 50 saponin*.ti,ab. 51 saponosides.ti,ab. 52 troxerutin.ti,ab. 53 "vasoprotective agents".ti,ab. 54 "venoactive drug*".ti,ab. 55 "veno‐active drug*".ti,ab. 56 or/8‐55 57 7 and 56 58 randomized controlled trial/ 59 controlled clinical trial/ 60 random$.ti,ab. 61 randomization/ 62 intermethod comparison/ 63 placebo.ti,ab. 64 (compare or compared or comparison).ti. 65 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 66 (open adj label).ti,ab. 67 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 68 double blind procedure/ 69 parallel group$1.ti,ab. 70 (crossover or cross over).ti,ab. 71 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 72 (assigned or allocated).ti,ab. 73 (controlled adj7 (study or design or trial)).ti,ab. 74 (volunteer or volunteers).ti,ab. 75 trial.ti. 76 or/58‐75 77 57 and 76 |
443 |
| CINAHL | S64 S48 AND S63 S63 S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 S62 MH "Random Assignment" S61 MH "Triple‐Blind Studies" S60 MH "Double‐Blind Studies" S59 MH "Single‐Blind Studies" S58 MH "Crossover Design" S57 MH "Factorial Design" S56 MH "Placebos" S55 MH "Clinical Trials" S54 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" S53 TX crossover OR "cross‐over" S52 AB placebo* S51 TX random* S50 TX trial* S49 TX "latin square" S48 S7 AND S47 S47 S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 S46 TX veno‐active drug* S45 TX venoactive drug* S44 TX vasoprotective agents S43 TX troxerutin S42 TX saponosides S41 TX saponin* S40 TX rutoside* S39 TX rutin* S38 TX pycnogenol* S37 TX plant extract* S36 TX phlebotonics S35 TX para‐aminobenzoates S34 TX naftazone* S33 TX hydroxyethylrutoside S32 TX horse chestnut S31 TX hidrosmin* S30 TX hesperidin S29 TX grape seed S28 TX french maritime pine S27 TX flavonoids S26 TX doxium S25 TX disodium flavodate S24 TX diosmiplex S23 TX diosmine S22 TX diosmin S21 TX daflon S20 TX Coumarin* S19 TX chromocarbe* S18 TX centella S17 TX calcium dobesilate S16 TX bioflavonoid* S15 TX aminaphtone* S14 TX aminaphthone S13 TX aminaftone* S12 (MH "Rutin") S11 (MH "Plant Extracts+") S10 (MH "Medicine, Herbal+") S9 (MH "Hemostatics+") S8 Flavonoids S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6 S6 TX CEAP S5 TX chronic venous disorder* S4 TX CVD S3 TX Chronic venous disease S2 TX ((insuffic* or insufic* or CVI or isch* or incompet* or chronic) and (saphenous or vein* or veno*)) S1 (MH "Venous Insufficiency+") |
57 |
| AMED (Allied and Complementary Medicine) 1985 to present | 1 exp Venous insufficiency/ 2 ((insuffic* or insufic* or CVI or isch* or incompet* or chronic) and (saphenous or vein* or veno*)).ti,ab. 3 "Chronic venous disease".ti,ab. 4 CVD.ti,ab. 5 "chronic venous disorder*".ti,ab. 6 CEAP.ti,ab. 7 or/1‐6 8 exp CLINICAL TRIALS/ 9 RANDOM ALLOCATION/ 10 DOUBLE BLIND METHOD/ 11 Clinical trial.pt. 12 (clinic* adj trial*).tw. 13 ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw. 14 PLACEBOS/ 15 placebo*.tw. 16 random*.tw. 17 PROSPECTIVE STUDIES/ 18 or/8‐17 19 7 and 18 |
17 |
Appendix 2. Glossary
| Agranulocytosis | also known as agranulosis or granulopenia, is an acute condition involving a severe and dangerous leukopenia (lowered white blood cell count), most commonly of neutrophils, and thus causing a neutropenia in the circulating blood |
| Anatomical Therapeutic Chemical (ATC) Classification System | drug classification system that classifies the active ingredients of drugs according to the organ or system on which they act and their therapeutic, pharmacological, and chemical properties |
| Capillary hyperpermeability | the capacity of a blood vessel wall to allow for the flow of small molecules (drugs, nutrients, water, ions) or even whole cells (lymphocytes on their way to the site of inflammation) in and out of the vessel |
| Chronic venous insufficiency (CVI) | a condition in which veins are unable to transport blood unidirectionally toward the heart, usually occurs in the lower limbs |
| Corona phlebectatica | cutaneous sign of chronic venous insufficiency, characterised by abnormally dilated veins around the ankle |
| Exacerbations | the process of making a problem, bad situation, or negative feeling worse |
| Lipodermatosclerosis | hardening of the skin that may cause red/brown pigmentation and is accompanied by wasting of subcutaneous fat |
| Paraesthesias | abnormal sensations, such as prickling, burning, tingling) in the lower legs |
| Pathophysiology | the disordered physiological processes associated with disease or injury |
| Reticular veins | dilated veins that show as a net‐like pattern on the skin |
| Telangiectasia | condition in which widened venules (tiny blood vessels) cause threadlike red lines or patterns on the skin |
| Thrombosis | formation of a blood clot, known as a thrombus, within a blood vessel. It prevents blood from flowing normally through the circulatory system |
| Varicose veins | permanently dilated veins |
| Vasoprotective drug | medication which acts to alleviate or prevent conditions or diseases which affect the blood vessels |
Data and analyses
Comparison 1. Phlebotonics versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Oedema in the lower legs (dichotomous variable) | 13 | 1245 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.63, 0.78] |
| 1.1.1 Aminaftone | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.28, 0.99] |
| 1.1.2 Calcium dobesilate | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.48, 1.07] |
| 1.1.3 Diosmine, Hidrosmine | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.46, 0.86] |
| 1.1.4 Grape seed extract | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.58, 1.06] |
| 1.1.5 Rutosides | 7 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.64, 0.81] |
| 1.2 Ankle perimeter circumference (mm) | 15 | 2010 | Mean Difference (IV, Fixed, 95% CI) | ‐4.27 [‐5.61, ‐2.93] |
| 1.2.1 Calcium dobesilate | 5 | 1122 | Mean Difference (IV, Fixed, 95% CI) | ‐1.69 [‐4.84, 1.47] |
| 1.2.2 Diosmine, Hidrosmine | 3 | 286 | Mean Difference (IV, Fixed, 95% CI) | ‐5.98 [‐7.78, ‐4.18] |
| 1.2.3 Rutosides | 7 | 602 | Mean Difference (IV, Fixed, 95% CI) | ‐2.45 [‐5.06, 0.15] |
| 1.3 Volume of the leg (mL) | 11 | 2072 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.33, ‐0.15] |
| 1.3.1 Aminaftone | 1 | 79 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.61, 0.28] |
| 1.3.2 Calcium dobesilate | 4 | 826 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.51, ‐0.24] |
| 1.3.3 Rutosides | 6 | 1167 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.26, ‐0.03] |
| 1.4 Quality of life | 5 | 1639 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.22, 0.10] |
| 1.4.1 Aminaftone | 1 | 79 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.10, ‐0.19] |
| 1.4.2 Calcium dobesilate at 3 months of treatment | 3 | 968 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.16, 0.10] |
| 1.4.3 Diosmine, Hidrosmine | 1 | 592 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.12, 0.20] |
| 1.5 Ulcer healing | 6 | 461 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.79, 1.13] |
| 1.5.1 Aminaftone | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.18, 3.18] |
| 1.5.2 Calcium dobesilate | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.69, 1.74] |
| 1.5.3 Diosmine, Hidrosmine | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.69, 1.01] |
| 1.5.4 Rutosides | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.87, 1.86] |
| 1.6 Trophic disorders (dichotomous variable) | 6 | 705 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.95] |
| 1.6.1 Aminaftone | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.41, 1.44] |
| 1.6.2 Diosmine, Hidrosmine | 4 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 1.6.3 Rutosides | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.71, 1.25] |
| 1.7 Pain in the lower legs (dichotomous variable) | 21 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.7.1 Aminaftone | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.23, 0.79] |
| 1.7.2 Calcium dobesilate | 5 | 705 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.35, 0.82] |
| 1.7.3 Diosmine, Hidrosmine | 4 | 271 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.08] |
| 1.7.4 French maritime pine bark extract | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.48, 0.91] |
| 1.7.5 Rutosides | 10 | 1485 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.48, 0.83] |
| 1.8 Pain in the lower legs (continuous variable) | 12 | 2232 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.54, ‐0.17] |
| 1.8.1 Calcium dobesilate | 5 | 1127 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.31, 0.03] |
| 1.8.2 Diosmine, Hidrosmine | 3 | 846 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.41, ‐0.05] |
| 1.8.3 French maritime pine bark extract | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐2.09, ‐0.69] |
| 1.8.4 Rutosides | 3 | 219 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.23, ‐0.19] |
| 1.9 Cramps in the lower legs (dichotomous variable) | 14 | 1793 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.58, 0.89] |
| 1.9.1 Aminaftone | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.31, 0.99] |
| 1.9.2 Calcium dobesilate | 2 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.50, 0.84] |
| 1.9.3 Diosmine, Hidrosmine | 3 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.70, 0.98] |
| 1.9.4 Rutosides | 8 | 1227 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.47, 1.02] |
| 1.10 Cramps in the lower legs (continuous variable) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.10.1 Calcium dobesilate | 1 | 415 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.29, 0.09] |
| 1.10.2 Diosmine, Hidrosmine | 1 | 150 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.78, ‐0.14] |
| 1.10.3 Rutosides | 2 | 164 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.50, ‐0.16] |
| 1.11 Restless legs (dichotomous variable) | 7 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.72, 0.91] |
| 1.11.1 Calcium dobesilate | 2 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.59, 0.91] |
| 1.11.2 Diosmine, Hidrosmine | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.70, 1.15] |
| 1.11.3 Rutosides | 4 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.72, 1.01] |
| 1.12 Itching in the lower legs (dichotomous variable) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.12.1 Aminaftone | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.31, 0.91] |
| 1.12.2 Diosmine, Hidrosmine | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.51, 5.25] |
| 1.12.3 Rutosides | 2 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.21, 2.21] |
| 1.13 Itching in the lower legs (continuous variable) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.13.1 Calcium dobesilate | 1 | 416 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.11, 0.28] |
| 1.13.2 Rutosides | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.10, ‐0.06] |
| 1.14 Heaviness in the lower legs (dichotomous variable) | 19 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.14.1 Aminaftone | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.17, 0.60] |
| 1.14.2 Calcium dobesilate | 3 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.08, 1.42] |
| 1.14.3 Centella asiatica | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.32, 1.19] |
| 1.14.4 Diosmine, Hidrosmine | 4 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.35, 1.05] |
| 1.14.5 French maritime pine bark extract | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.76, 1.07] |
| 1.14.6 Rutosides | 9 | 1420 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.48, 0.74] |
| 1.15 Heaviness in the lower legs (continuous variable) | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.15.1 Calcium dobesilate | 2 | 483 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.23, 0.13] |
| 1.15.2 Diosmine, Hidrosmine | 1 | 150 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.02, ‐0.36] |
| 1.15.3 French maritime pine bark extract | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐2.21, ‐0.79] |
| 1.15.4 Rutosides | 6 | 441 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐1.87, ‐0.36] |
| 1.16 Swelling in the lower legs (dichotomous variable) | 14 | 1072 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.50, 0.80] |
| 1.16.1 Calcium dobesilate | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.08, 0.41] |
| 1.16.2 Diosmine, Hidrosmine | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.52, 0.94] |
| 1.16.3 French maritime pine bark extract | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.64, 1.02] |
| 1.16.4 Rutosides | 9 | 848 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.50, 0.88] |
| 1.17 Swelling in the lower legs (continuous variable) | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.17.1 Calcium dobesilate | 1 | 417 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.24, 0.15] |
| 1.17.2 Diosmine, Hidrosmine | 1 | 150 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.26, ‐0.58] |
| 1.17.3 French maritime pine bark extract | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.65 [‐2.38, ‐0.92] |
| 1.17.4 Rutosides | 3 | 264 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐3.50, 0.04] |
| 1.18 Paraesthesia in the lower legs (dichotomous variable) | 9 | 1456 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.50, 0.88] |
| 1.18.1 Calcium dobesilate | 3 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.51, 1.08] |
| 1.18.2 Diosmine, Hidrosmine | 2 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.62, 1.05] |
| 1.18.3 Rutosides | 4 | 1007 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.37, 0.83] |
| 1.19 Paraesthesia in the lower legs (continuous variable) | 2 | 188 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.44, 0.13] |
| 1.19.1 Diosmine, Hidrosmine | 1 | 150 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.44, 0.21] |
| 1.19.2 Rutosides | 1 | 38 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.96, 0.33] |
| 1.20 Participant satisfaction (dichotomous variable) | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.20.1 Calcium dobesilate | 3 | 506 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.36, 1.46] |
| 1.20.2 Diosmine, Hidrosmine | 4 | 451 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.43, 1.02] |
| 1.20.3 Centella asiatica | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.14, 0.57] |
| 1.20.4 Rutosides | 8 | 1167 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.30, 0.84] |
| 1.21 Participant satisfaction (continuous variable) | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.21.1 Calcium dobesilate | 2 | 448 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.71, ‐0.33] |
| 1.21.2 Diosmine, Hidrosmine | 1 | 150 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.14, ‐0.47] |
| 1.21.3 Rutosides | 4 | 283 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐1.96, ‐0.39] |
| 1.22 Adverse events | 37 | 5789 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.02, 1.27] |
| 1.22.1 Aminaftone | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.06, 6.32] |
| 1.22.2 Calcium dobesilate | 8 | 1824 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.00, 1.49] |
| 1.22.3 Centella asiatica | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.58, 2.23] |
| 1.22.4 Diosmine, Hidrosmine | 9 | 1429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.72, 1.19] |
| 1.22.5 Grape seed extract | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.19, 1.74] |
| 1.22.6 Rutosides | 17 | 2288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.04, 1.43] |
Comparison 2. Sensitivity analysis excluding studies that allowed the use of elastic stockings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Oedema in the lower legs (dichotomous variable) | 12 | 1131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.60, 0.76] |
| 2.1.1 Aminaftone | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.28, 0.99] |
| 2.1.2 Calcium dobesilate | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.48, 1.07] |
| 2.1.3 Diosmine, Hidrosmine | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.46, 0.86] |
| 2.1.4 Grape seed extract | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.58, 1.06] |
| 2.1.5 Rutosides | 6 | 540 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.58, 0.78] |
| 2.2 Ankle perimeter circumference (mm) | 10 | 1212 | Mean Difference (IV, Fixed, 95% CI) | ‐4.59 [‐6.02, ‐3.16] |
| 2.2.1 Calcium dobesilate | 3 | 502 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐4.95, 3.34] |
| 2.2.2 Diosmine, Hidrosmine | 2 | 246 | Mean Difference (IV, Fixed, 95% CI) | ‐5.90 [‐7.72, ‐4.07] |
| 2.2.3 Rutosides | 5 | 464 | Mean Difference (IV, Fixed, 95% CI) | ‐3.28 [‐6.06, ‐0.50] |
| 2.3 Volume of the leg (mL) | 9 | 1153 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.42, ‐0.19] |
| 2.3.1 Aminaftone | 1 | 79 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.61, 0.28] |
| 2.3.2 Calcium dobesilate | 3 | 587 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.49, ‐0.17] |
| 2.3.3 Rutosides | 5 | 487 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.47, ‐0.11] |
| 2.4 Quality of life | 3 | 1022 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.15] |
| 2.4.1 Aminaftone | 1 | 79 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.10, ‐0.19] |
| 2.4.2 Calcium dobesilate | 1 | 351 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.23, 0.18] |
| 2.4.3 Diosmine, Hidrosmine | 1 | 592 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.12, 0.20] |
| 2.5 Ulcer healing | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.27, 3.10] |
| 2.5.1 Aminaftone | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.18, 3.18] |
| 2.5.2 Diosmine, Hidrosmine | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.15, 14.68] |
| 2.6 Trophic disorders (dichotomous variable) | 5 | 601 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.79, 0.94] |
| 2.6.1 Aminaftone | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.41, 1.44] |
| 2.6.2 Diosmine, Hidrosmine | 4 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 2.7 Pain in the lower legs (dichotomous variable) | 18 | 1818 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.60, 0.82] |
| 2.7.1 Aminaftone | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.23, 0.79] |
| 2.7.2 Calcium dobesilate | 5 | 705 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.35, 0.82] |
| 2.7.3 Diosmine, Hidrosmine | 4 | 271 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.08] |
| 2.7.4 Rutosides | 8 | 745 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.61, 0.91] |
| 2.8 Pain in the lower legs (continuous variable) | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.8.1 Calcium dobesilate | 1 | 351 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.42, 0.00] |
| 2.8.2 Diosmine, Hidrosmine | 3 | 846 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.41, ‐0.05] |
| 2.8.3 Rutosides | 2 | 164 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.33, ‐0.59] |
| 2.9 Cramps in the lower legs (dichotomous variable) | 12 | 1603 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.57, 0.91] |
| 2.9.1 Aminaftone | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.31, 0.99] |
| 2.9.2 Calcium dobesilate | 2 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.50, 0.84] |
| 2.9.3 Diosmine, Hidrosmine | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.69, 1.09] |
| 2.9.4 Rutosides | 7 | 1147 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.50, 1.06] |
| 2.10 Cramps in the lower legs (continuous variable) | 3 | 314 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.15, ‐0.24] |
| 2.10.1 Diosmine, Hidrosmine | 1 | 150 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.78, ‐0.14] |
| 2.10.2 Rutosides | 2 | 164 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.50, ‐0.16] |
| 2.11 Restless legs (dichotomous variable) | 6 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.72, 0.91] |
| 2.11.1 Calcium dobesilate | 2 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.59, 0.91] |
| 2.11.2 Diosmine, Hidrosmine | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.70, 1.15] |
| 2.11.3 Rutosides | 3 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.73, 1.01] |
| 2.12 Itching in the lower legs (dichotomous variable) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.12.1 Aminaftone | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.31, 0.91] |
| 2.12.2 Diosmine, Hidrosmine | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.51, 5.25] |
| 2.12.3 Rutosides | 2 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.21, 2.21] |
| 2.13 Itching in the lower legs (continuous variable) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.13.1 Rutosides | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.14 Heaviness in the lower legs (dichotomous variable) | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.14.1 Aminaftone | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.17, 0.60] |
| 2.14.2 Calcium dobesilate | 3 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.08, 1.42] |
| 2.14.3 Centella asiatica | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.32, 1.19] |
| 2.14.4 Diosmine, Hidrosmine | 3 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.29, 1.22] |
| 2.14.5 Rutosides | 8 | 531 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.38, 0.80] |
| 2.15 Heaviness in the lower legs (continuous variable) | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.15.1 Diosmine, Hidrosmine | 1 | 150 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.02, ‐0.36] |
| 2.15.2 Rutosides | 5 | 360 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.27 [‐2.22, ‐0.32] |
| 2.16 Swelling in the lower legs (dichotomous variable) | 12 | 952 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.53, 0.82] |
| 2.16.1 Calcium dobesilate | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.08, 0.41] |
| 2.16.2 Diosmine, Hidrosmine | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.52, 0.94] |
| 2.16.3 Rutosides | 8 | 768 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.58, 0.89] |
| 2.17 Swelling in the lower legs (continuous variable) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.17.1 Diosmine, Hidrosmine | 1 | 150 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.26, ‐0.58] |
| 2.17.2 Rutosides | 3 | 264 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐3.50, 0.04] |
| 2.18 Paraesthesias in the lower legs (dichotomous variable) | 7 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.64, 0.88] |
| 2.18.1 Calcium dobesilate | 3 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.58, 1.01] |
| 2.18.2 Diosmine, Hidrosmine | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.61, 1.06] |
| 2.18.3 Rutosides | 2 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.51, 0.91] |
| 2.19 Paraesthesias in the lower legs (continuous variable) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.19.1 Diosmine, Hidrosmine | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.20 Participant satisfaction (dichotomous variable) | 12 | 1193 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.53, 0.90] |
| 2.20.1 Calcium dobesilate | 3 | 515 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.43, 1.17] |
| 2.20.2 Diosmine, Hidrosmine | 2 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.68, 1.04] |
| 2.20.3 Rutosides | 7 | 507 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.28, 0.98] |
| 2.21 Participant satisfaction (continuous variable) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.21.1 Diosmine, Hidrosmine | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.21.2 Rutosides | 3 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.22 Adverse events | 27 | 3433 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.95, 1.33] |
| 2.22.1 Aminaftone | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.06, 6.32] |
| 2.22.2 Calcium dobesilate | 5 | 935 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.82, 1.53] |
| 2.22.3 Centella asiatica | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.58, 2.23] |
| 2.22.4 Diosmine, Hidrosmine | 7 | 1124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.74, 1.27] |
| 2.22.5 Grape seed extract | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.19, 1.74] |
| 2.22.6 Rutosides | 12 | 1126 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.08, 2.19] |
2.1. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 1: Oedema in the lower legs (dichotomous variable)
2.2. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 2: Ankle perimeter circumference (mm)
2.3. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 3: Volume of the leg (mL)
2.4. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 4: Quality of life
2.5. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 5: Ulcer healing
2.6. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 6: Trophic disorders (dichotomous variable)
2.8. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 8: Pain in the lower legs (continuous variable)
2.9. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 9: Cramps in the lower legs (dichotomous variable)
2.10. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 10: Cramps in the lower legs (continuous variable)
2.11. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 11: Restless legs (dichotomous variable)
2.12. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 12: Itching in the lower legs (dichotomous variable)
2.13. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 13: Itching in the lower legs (continuous variable)
2.14. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 14: Heaviness in the lower legs (dichotomous variable)
2.15. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 15: Heaviness in the lower legs (continuous variable)
2.16. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 16: Swelling in the lower legs (dichotomous variable)
2.17. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 17: Swelling in the lower legs (continuous variable)
2.18. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 18: Paraesthesias in the lower legs (dichotomous variable)
2.19. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 19: Paraesthesias in the lower legs (continuous variable)
2.21. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 21: Participant satisfaction (continuous variable)
2.22. Analysis.

Comparison 2: Sensitivity analysis excluding studies that allowed the use of elastic stockings, Outcome 22: Adverse events
Comparison 3. Sensitivity analysis of published studies only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Oedema in the lower legs (dichotomous variable) | 12 | 1088 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.63, 0.78] |
| 3.1.1 Aminaftone | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.28, 0.99] |
| 3.1.2 Calcium dobesilate | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.48, 1.07] |
| 3.1.3 Diosmine, Hidrosmine | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.46, 0.86] |
| 3.1.4 Grape seed extract | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.58, 1.06] |
| 3.1.5 Rutosides | 6 | 497 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.64, 0.81] |
| 3.2 Ankle perimeter circumference (mm) | 13 | 1796 | Mean Difference (IV, Random, 95% CI) | ‐3.61 [‐6.77, ‐0.45] |
| 3.2.1 Calcium dobesilate | 5 | 1122 | Mean Difference (IV, Random, 95% CI) | ‐3.17 [‐8.37, 2.02] |
| 3.2.2 Diosmine, Hidrosmine | 3 | 286 | Mean Difference (IV, Random, 95% CI) | ‐5.98 [‐7.78, ‐4.18] |
| 3.2.3 Rutosides | 5 | 388 | Mean Difference (IV, Random, 95% CI) | ‐2.18 [‐9.79, 5.43] |
| 3.3 Volume of the leg (mL) | 10 | 1392 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.44, ‐0.23] |
| 3.3.1 Aminaftone | 1 | 79 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.61, 0.28] |
| 3.3.2 Calcium dobesilate | 4 | 826 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.51, ‐0.24] |
| 3.3.3 Rutosides | 5 | 487 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.47, ‐0.11] |
| 3.4 Quality of life | 5 | 1639 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.22, 0.10] |
| 3.4.1 Aminaftone | 1 | 79 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.10, ‐0.19] |
| 3.4.2 Calcium dobesilate | 3 | 968 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.16, 0.10] |
| 3.4.3 Diosmine, Hidrosmine | 1 | 592 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.12, 0.20] |
| 3.5 Patients with ulcer (dichotomous variable) | 5 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.78, 1.15] |
| 3.5.1 Aminaftone | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.18, 3.18] |
| 3.5.2 Diosmine, Hidrosmine | 2 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.70, 1.03] |
| 3.5.3 Rutosides | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.84, 1.87] |
| 3.6 Trophic disorders (dichotomous variable) | 6 | 705 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.95] |
| 3.6.1 Aminaftone | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.41, 1.44] |
| 3.6.2 Diosmine, Hidrosmine | 4 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 3.6.3 Rutosides | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.71, 1.25] |
| 3.7 Pain in the lower legs (dichotomous variable) | 19 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.7.1 Aminaftone | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.23, 0.79] |
| 3.7.2 Calcium dobesilate | 5 | 705 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.35, 0.82] |
| 3.7.3 Diosmine, Hidrosmine | 4 | 271 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.08] |
| 3.7.4 French maritime pine bark extract | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.48, 0.91] |
| 3.7.5 Rutosides | 8 | 1318 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.84] |
| 3.8 Pain in the lower legs (continuous variable) | 11 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.8.1 Calcium dobesilate | 4 | 1075 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.34, 0.05] |
| 3.8.2 Diosmine, Hidrosmine | 3 | 846 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.41, ‐0.05] |
| 3.8.3 French maritime pine bark extract | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐2.09, ‐0.69] |
| 3.8.4 Rutosides | 3 | 219 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.23, ‐0.19] |
| 3.9 Cramps in the lower legs (dichotomous variable) | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.9.1 Aminaftone | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.31, 0.99] |
| 3.9.2 Calcium dobesilate | 2 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.50, 0.84] |
| 3.9.3 Diosmine, Hidrosmine | 3 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.70, 0.98] |
| 3.9.4 Rutosides | 6 | 1060 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.45, 1.05] |
| 3.10 Cramps in the lower legs (continuous variable) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.10.1 Calcium dobesilate | 1 | 415 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.29, 0.09] |
| 3.10.2 Diosmine, Hidrosmine | 1 | 150 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.78, ‐0.14] |
| 3.10.3 Rutosides | 2 | 164 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.50, ‐0.16] |
| 3.11 Restless legs (dichotomous variable) | 7 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.72, 0.91] |
| 3.11.1 Calcium dobesilate | 2 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.59, 0.91] |
| 3.11.2 Diosmine, Hidrosmine | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.70, 1.15] |
| 3.11.3 Rutosides | 4 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.72, 1.01] |
| 3.12 Itching in the lower legs (dichotomous variable) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.12.1 Aminaftone | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.31, 0.91] |
| 3.12.2 Diosmine, Hidrosmine | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.51, 5.25] |
| 3.12.3 Rutosides | 2 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.21, 2.21] |
| 3.13 Itching in the lower legs (continuous variable) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.13.1 Calccium dobesilate | 1 | 416 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.11, 0.28] |
| 3.13.2 Rutosides | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.10, ‐0.06] |
| 3.14 Heaviness in the lower legs (dichotomous variable) | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.14.1 Aminaftone | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.17, 0.60] |
| 3.14.2 Calcium dobesilate | 3 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.08, 1.42] |
| 3.14.3 Centella asiatica | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.32, 1.19] |
| 3.14.4 Diosmine, Hidrosmine | 4 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.35, 1.05] |
| 3.14.5 French maritime pine bark extract | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.76, 1.07] |
| 3.14.6 Rutosides | 7 | 1253 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.49, 0.78] |
| 3.15 Heaviness in the lower legs (continuous variable) | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.15.1 Calcium dobesilate | 2 | 483 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.23, 0.13] |
| 3.15.2 Diosmine, Hidrosmine | 1 | 150 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.02, ‐0.36] |
| 3.15.3 French maritime pine bark extract | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐2.21, ‐0.79] |
| 3.15.4 Rutosides | 5 | 360 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.27 [‐2.22, ‐0.32] |
| 3.16 Swelling in the lower legs (dichotomous variable) | 12 | 905 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.49, 0.81] |
| 3.16.1 Calcium dobesilate | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.08, 0.41] |
| 3.16.2 Diosmine, Hidrosmine | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.52, 0.94] |
| 3.16.3 French maritime pine bark extract | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.64, 1.02] |
| 3.16.4 Rutosides | 7 | 681 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.49, 0.91] |
| 3.17 Swelling in the lower legs (continuous variable) | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.17.1 Calcium dobesilate | 1 | 417 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.24, 0.15] |
| 3.17.2 Diosmine, Hidrosmine | 1 | 150 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐1.50, ‐0.80] |
| 3.17.3 French maritime pine bark extract | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.65 [‐2.38, ‐0.92] |
| 3.17.4 Rutosides | 3 | 264 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐3.50, 0.04] |
| 3.18 Paraesthesias in the lower legs (dichotomous variable) | 8 | 1309 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.48, 0.84] |
| 3.18.1 Calcium dobesilate | 3 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.51, 1.08] |
| 3.18.2 Diosmine, Hidrosmine | 2 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.62, 1.05] |
| 3.18.3 Rutosides | 3 | 860 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.35, 0.66] |
| 3.19 Paraesthesias in the lower legs (continuous variable) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.19.1 Diosmine, Hidrosmine | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.20 Participant satisfaction (dichotomous variable) | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.20.1 Calcium dobesilate | 4 | 758 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.61, 1.19] |
| 3.20.2 Centella asiatica | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.14, 0.57] |
| 3.20.3 Diosmine, Hidrosmine | 4 | 451 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.96] |
| 3.20.4 Rutosides | 6 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.26, 0.97] |
| 3.21 Participant satisfaction (continuous variable) | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.21.1 Calcium dobesilate | 2 | 448 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.71, ‐0.33] |
| 3.21.2 Diosmine, Hidrosmine | 1 | 150 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.14, ‐0.47] |
| 3.21.3 Rutosides | 4 | 283 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐1.96, ‐0.39] |
| 3.22 Adverse events | 34 | 4830 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.99, 1.29] |
| 3.22.1 Aminaftone | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.06, 6.32] |
| 3.22.2 Calcium dobesilate | 8 | 1824 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.00, 1.49] |
| 3.22.3 Centella asiatica | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.58, 2.23] |
| 3.22.4 Diosmine, Hidrosmine | 9 | 1429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.72, 1.19] |
| 3.22.5 Grape seed extract | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.19, 1.74] |
| 3.22.6 Rutosides | 14 | 1329 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.02, 1.76] |
3.1. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 1: Oedema in the lower legs (dichotomous variable)
3.2. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 2: Ankle perimeter circumference (mm)
3.3. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 3: Volume of the leg (mL)
3.4. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 4: Quality of life
3.5. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 5: Patients with ulcer (dichotomous variable)
3.6. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 6: Trophic disorders (dichotomous variable)
3.7. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 7: Pain in the lower legs (dichotomous variable)
3.8. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 8: Pain in the lower legs (continuous variable)
3.9. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 9: Cramps in the lower legs (dichotomous variable)
3.10. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 10: Cramps in the lower legs (continuous variable)
3.11. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 11: Restless legs (dichotomous variable)
3.12. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 12: Itching in the lower legs (dichotomous variable)
3.13. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 13: Itching in the lower legs (continuous variable)
3.14. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 14: Heaviness in the lower legs (dichotomous variable)
3.15. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 15: Heaviness in the lower legs (continuous variable)
3.16. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 16: Swelling in the lower legs (dichotomous variable)
3.17. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 17: Swelling in the lower legs (continuous variable)
3.18. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 18: Paraesthesias in the lower legs (dichotomous variable)
3.19. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 19: Paraesthesias in the lower legs (continuous variable)
3.20. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 20: Participant satisfaction (dichotomous variable)
3.21. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 21: Participant satisfaction (continuous variable)
3.22. Analysis.

Comparison 3: Sensitivity analysis of published studies only, Outcome 22: Adverse events
Comparison 4. Sensitivity analysis based on low risk of bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Oedema in the lower legs (dichotomous variable) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1.1 Calcium dobesilate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.2 Ankle perimeter circumference (mm) | 3 | 867 | Mean Difference (IV, Random, 95% CI) | ‐2.34 [‐8.79, 4.11] |
| 4.2.1 Calcium dobesilate | 3 | 867 | Mean Difference (IV, Random, 95% CI) | ‐2.34 [‐8.79, 4.11] |
| 4.3 Quality of life | 2 | 617 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.15, 0.95] |
| 4.3.1 Calcium dobesilate at 3 months of treatment | 2 | 617 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.15, 0.95] |
| 4.4 Pain in the lower legs (dichotomous variable) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.4.1 Rutosides | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.5 Pain in the lower legs (continuous variable) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.5.1 Calcium dobesilate | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.6 Cramps in the lower legs (continuous variable) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.6.1 Calcium dobesilate | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.7 Itching in the lower legs (dichotomous variable) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.7.1 Rutosides | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.8 Itching in the lower legs (continuous variable) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.8.1 Calcium dobesilate | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.9 Heaviness in the lower legs (dichotomous variable) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.9.1 Rutosides | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.10 Heaviness in the lower legs (continuous variable) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.10.1 Calcium dobesilate | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.11 Swelling in the lower legs (dichotomous variable) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.11.1 Rutosides | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.12 Swelling in the lower legs (continuous variable) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.12.1 Calcium dobesilate | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.13 Participant satisfaction (dichotomous variable) | 2 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.81, 1.32] |
| 4.13.1 Calcium dobesilate | 2 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.81, 1.32] |
| 4.14 Participant satisfaction (continuous variable) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.14.1 Calcium dobesilate | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.15 Adverse events | 4 | 1257 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.97, 2.63] |
| 4.15.1 Calcium dobesilate | 3 | 1026 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.76, 3.09] |
| 4.15.2 Rutosides | 1 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.00, 3.34] |
4.3. Analysis.

Comparison 4: Sensitivity analysis based on low risk of bias, Outcome 3: Quality of life
4.4. Analysis.

Comparison 4: Sensitivity analysis based on low risk of bias, Outcome 4: Pain in the lower legs (dichotomous variable)
4.5. Analysis.

Comparison 4: Sensitivity analysis based on low risk of bias, Outcome 5: Pain in the lower legs (continuous variable)
4.6. Analysis.

Comparison 4: Sensitivity analysis based on low risk of bias, Outcome 6: Cramps in the lower legs (continuous variable)
4.11. Analysis.

Comparison 4: Sensitivity analysis based on low risk of bias, Outcome 11: Swelling in the lower legs (dichotomous variable)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allegra 1981.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: table of random numbers Exclusions post randomisation: none Losses to follow‐up: none |
|
| Participants | Country: Italy Setting: hospital Number: 80 patients Age: not stated Gender: not stated Inclusion criteria: patients with postphlebitic syndrome, oedema of the lower limb, phlebolymphoedema, constitutional venous stasis, varices Exclusion criteria: not stated |
|
| Interventions | Treatment: 2 × 10 mg Centella tablets 3 × per day Control: placebo Duration: 30 days Follow‐up: 30 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The assignment of patients to one of two treatments, labelled as A or B, was made randomly using a special randomization list" Comment: a randomisation list is generally accepted as a fair method of ensuring a random sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information given about methods used for allocation concealment |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Low risk | Comment: no exclusions post randomisation and no losses to follow‐up |
| Selective reporting | Unclear risk | Comment: the number of participants in both groups was described. However, a table with important characteristics was lacking; this could lower the generalisability. Adverse events, tolerability and signs of intolerance were presented |
| Other bias | Low risk | Comment: none detected |
Alterkamper 1987.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 3/50 (6%) |
|
| Participants | Country: France Setting: not stated Number: 50 patients Age: mean 53 ± 9 years Gender: 13 M:37 F Inclusion criteria: symptomatic stage I to II of CVI Exclusion criteria: oedemas requiring compression, post‐thrombotic syndrome, lymphoedema; cardiac, renal or hepatic failure; diuretics; pregnancy; severe disease |
|
| Interventions | Treatment: 1.86 mg ruscus and 75 mg hesperidin. 2 capsules 3 × per day Control: placebo Duration: 28 days Follow‐up: 28 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In a randomized double‐blind study..." Comment: no information given about method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information given about allocation concealment |
| Blinding (patients) | Low risk | Quote: "The Phlebodril and placebo capsules had the same external appearance" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "The Phlebodril and placebo capsules had the same external appearance" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "The Phlebodril and placebo capsules had the same external appearance" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Quote: "Three patients dropped out for reasons unconnected with this study" Comment: number in each group described, and number of participants who dropped out of the study prematurely presented |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Arcangeli 2000.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: none |
|
| Participants | Country: Italy Setting: clinical centre Number: 40 patients Age: mean 57.95 ± 12.78 years pycnogenol group; mean 61.40 ± 10.62 years placebo group Gender: 13 M:27 F Inclusion criteria: symptomatic CVI as a consequence of deep venous thrombosis or idiopathic venous lymphatic deficiency Exclusion criteria: cardiovascular, diuretics, analgesic or anti‐inflammatory drugs |
|
| Interventions | Treatment: French maritime pine bark extract, 100 mg 3 × per day Control: placebo Duration: 69 days Follow‐up: 60 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After the 2‐week run‐in period, the patients were randomly divided into two groups and assigned to a treatment with Pycnogenol, 100 mg × 3/day or a placebo for a period of 2 months" Comment: no method of randomisation stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method of allocation concealment stated |
| Blinding (patients) | Low risk | Quote: "The placebo visually matched the test drug" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "The placebo visually matched the test drug" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "The placebo visually matched the test drug" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: no exclusions post randomisation and no losses to follow‐up described |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Balmer 1980.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: none |
|
| Participants | Country: Switzerland Setting: not stated Number: 40 patients Age: mean 46.2 ± 14.1 years active group; mean 52.3 ± 14.1 years placebo group Gender: 4 M:36 F Inclusion criteria: CVI without venous ulcers Exclusion criteria: varicose ulcers |
|
| Interventions | Treatment: oxirutoside 900 mg per day Control: placebo Duration: 28 days Follow‐up: 28 days Compression therapy was allowed if participants were unwilling to abandon this support |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The trial was double‐blind, randomised, placebo controlled, between patients..." Comment: no information given about method of randomisation used |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information given about method of treatment allocation used |
| Blinding (patients) | Low risk | Quote: "Patients receiving respectively the test drug or identical placebo" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "Patients receiving respectively the test drug or identical placebo" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "Patients receiving respectively the test drug or identical placebo" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: no exclusions post randomisation and no losses to follow‐up |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Belczak 2014.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 9/136 (6.6%) |
|
| Participants | Country: Brazil Setting: Department of Vascular Surgery of Sao Camilo Medical School Number: 136 patients Age: mean 52.8 ± 16.4 years active group; mean 50.6 ± 13.1 years placebo group Gender: 33 M:103 F Inclusion criteria: treatment‐naïve (no history of pharmacological or compression therapy), CVD (CEAP grades 2 to 5) Exclusion criteria: other conditions that might produce lower extremity‐related symptoms |
|
| Interventions | Treatments: micronised diosmine (450 mg) + hesperidin (50 mg), aminaftone (75 mg), coumarin (15 mg), troxerutin (90 mg) Control: placebo Duration: 112 days Follow‐up: 112 days Compression therapy: not used |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Funding: all medications and placebos purchased by the investigators | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly divided into four groups" Comments: no methods of randomisation described |
| Allocation concealment (selection bias) | Low risk | Quote: "All tablets (active and placebo) were randomly divided into numbered bottles by an external investigator..." |
| Blinding (patients) | Low risk | Quote: "All tablets (active and placebo) were randomly divided into numbered bottles by an external investigator, and the contents of each bottle were unmasked only at the time of statistical analysis" |
| Blinding (study researchers) | Low risk | Quote: "All tablets (active and placebo) were randomly divided into numbered bottles by an external investigator, and the contents of each bottle were unmasked only at the time of statistical analysis" |
| Blinding (outcome assessment) | Low risk | Quote: "Assessors were blind to the treatment groups" |
| Incomplete outcome data | Low risk | Comment: very few participants lost to follow‐up |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Bergqvist 1981.
| Study characteristics | ||
| Methods | Study design: randomised, cross‐over, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 6/149 (4%) |
|
| Participants | Country: Sweden Setting: outpatient clinic and local population Number: 149 patients Age: 'adults' Gender: 33 M:116 F Inclusion criteria: symptoms related to varicose veins and CVI Exclusion criteria: not stated |
|
| Interventions | Treatment: oxirutoside 1000 mg intravenous injection followed by 1 tablet of 500 mg per 8 hours Control: placebo Duration: 28 days Follow‐up: 28 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were then randomly allocated to treatment with either HR or identical placebo" Comment: no details of randomisation method provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method of allocation concealment described |
| Blinding (patients) | Low risk | Quote: "The placebo regime was identical" and "... or identical placebo" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "The placebo regime was identical" and "... or identical placebo" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "The placebo regime was identical" and "... or identical placebo" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described. Loss to follow‐up described along with exclusions after randomisation, including reasons |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Biland 1982.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 14/70 (20%) |
|
| Participants | Country: Germany Setting: hospital Number: 70 patients Age: mean 43 ± 13 years diosmine group; mean 39 ± 12.5 years placebo group Gender: 7 M:49 F Inclusion criteria: symptoms related to CVI and oedema Exclusion criteria: phlebitis, venous thromboses, post‐thrombotic syndrome, ulcus cruris, heart insufficiency, recent sclerotherapy or venous stripping, trauma, neuropathy, arthrosis, pregnancy |
|
| Interventions | Treatment: diosmine 450 mg plus hesperidin 50 mg, 2 capsules twice a day Control: placebo Duration: 28 days Follow‐up: 28 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was double‐blind, randomized, placebo with Daflon" Comment: no method of randomisation stated |
| Allocation concealment (selection bias) | Low risk | Quote: "Placebo tablets were given in indistinguishable numbered packaging" Comment: Indistinguishable number packaging ensures a fair method of allocation concealment |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Low risk | Comment: numbers of participants in each group reported, along with participants excluded after randomisation,reasons for exclusion and information on compliance |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Burnand 1989.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: none |
|
| Participants | Country: UK Setting: hospital Number: 49 patients Age: mean 53 years Gender: 18 M:31 F Inclusion criteria: venous reflux by volumetry, with varicose veins and lipodermatosclerosis Exclusion criteria: patients with ankle‐to‐arm arterial Doppler pressure ratio < 1.0 (significant arterial disease) |
|
| Interventions | Treatment: oxerutin (Paroven) 500 mg per 12 hours Control: placebo Duration: 30 days Follow‐up: 30 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A double‐blind controlled trial was undertaken.." and "the two groups of patients were balanced and randomized by trial number so that as far as possible an equal number in each group..." Comment: no details of randomisation method provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods of allocation concealment described |
| Blinding (patients) | Low risk | Quote: "Were given Paroven 500 mg bd or identical placebo" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "Were given Paroven 500 mg bd or identical placebo" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "This code was not broken until the completion of the study" Comment: outcome assessors blinded |
| Incomplete outcome data | Low risk | Comment: neither exclusions post randomisation nor losses to follow‐up described |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Casley‐Smith 1988.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: none |
|
| Participants | Country: Australia Setting: university Number: 60 patients Age: 'adults' Gender: 28 M:32 F Inclusion criteria: 30 normal volunteer participants and 30 patients with CVI grade I to III Widmer (dilated subcutaneous veins, alteration of pigmentation, open or healed crural ulcer) Exclusion criteria: not stated |
|
| Interventions | Treatment: calcium dobesilate 1000 mg per day Control: placebo Duration: 42 days Follow‐up: 42 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A randomized, double‐blind, placebo‐controlled technique was used. Because of carryover effects, a matched‐pair technique was used" Comment: no methods of randomisation stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods of allocation concealment stated |
| Blinding (patients) | Low risk | Quote: "Calcium dobesilate, or an identical placebo, were administered..." Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "Calcium dobesilate, or an identical placebo, were administered..." Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "Calcium dobesilate, or an identical placebo, were administered..." Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: no exclusions post randomisation and no losses to follow‐up described |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Cauwenberge 1972.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 7/44 (16%) |
|
| Participants | Country: Belgium Setting: Liège Number: 44 patients Age: 'adults' Exclusion criteria: not stated Gender: not stated Inclusion criteria: varicose veins and postphlebitic syndrome Exclusion criteria: not stated |
|
| Interventions | Treatment: O‐(beta‐hydroxyethyl)‐rutoside 900 mg per day Control: placebo Duration: 28 days Follow‐up: 28 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Description of 2 clinical trials (CTs): One is a parallel CT, and the other is a cross‐over CT. Only the parallel CT is included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "44 patients were treated randomly and under double‐blind conditions" Comment: no specific methods stated for randomisation of participants |
| Allocation concealment (selection bias) | Unclear risk | Comment: no specific methods stated for allocation concealment |
| Blinding (patients) | Low risk | Quote: "In addition, a placebo identical in appearance to the active drug..." Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "In addition, a placebo identical in appearance to the active drug..." Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "In addition, a placebo identical in appearance to the active drug..." Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Unclear risk | Comment: number in each group described, including drop‐outs and those excluded after randomisation during follow‐up (7/44; 16%); reasons for drop‐out not provided |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Cauwenberge 1978.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: not stated Losses to follow‐up: 51/120 (42.5%) |
|
| Participants | Country: Belgium Setting: Liège Number: 120 patients Age: 'adults' Gender: not stated Inclusion criteria: varicose veins, postphlebitic syndrome Exclusion criteria: symptoms not attributed to CVI |
|
| Interventions | Treatment: O‐(beta‐hydroxyethyl)‐rutoside 1200 mg per day Control: placebo Duration: 90 days Follow‐up: 90 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients are divided into two series according to the degree of symptoms. Within these two series, patients were distributed randomly into two groups, receiving respectively the active ingredient or placebo" Comment: no method of randomisation stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method of allocation concealment stated |
| Blinding (patients) | Low risk | Quote: "We also used a placebo of identical presentation" Comment: Identical placebo ensure double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "We also used a placebo of identical presentation" Comment: Identical placebo ensure double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "We also used a placebo of identical presentation" Comment: Identical placebo ensure double‐blinding |
| Incomplete outcome data | High risk | Comment: number of participants in each group described, but no information given on important characteristics of participants. Number of persons excluded after randomisation was important (51/120; 42.5%). Reasons for exclusion were given |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Cesarone 2002.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: none |
|
| Participants | Country: Italy Setting: hospital Number: 46 patients and 10 healthy individuals Age: 44 to 45 years Gender: percentages/numbers of men and women not specified Inclusion criteria: severe superficial venous incompetence with a normal deep venous system Exclusion criteria: diabetes, peripheral arterial disease |
|
| Interventions | Treatment A: hidroxirutoxide 500 mg tid Treatment B: hidroxirutoxide 1000 mg tid Control (group C): placebo tid Treatment D: hidroxirutoxide 1000 mg/d Duration: 28 days Follow‐up: 28 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no randomisation methods stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods of allocation concealment stated |
| Blinding (patients) | Low risk | Comment: placebo used with the same frequency as in experimental groups |
| Blinding (study researchers) | Low risk | Comment: placebo used with the same frequency as in experimental groups |
| Blinding (outcome assessment) | Low risk | Comment: placebo used with the same frequency as in experimental groups |
| Incomplete outcome data | Low risk | Comment: no exclusions post randomisation and no losses to follow‐up |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Chassignolle 1994.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 4/40 (10%) |
|
| Participants | Country: France Setting: hospital Number: 40 patients Age: 32.0 (1.3) years active group; 35.6 (1.1) years placebo group Gender: female Inclusion criteria: women with functional CVI Exclusion criteria: not stated |
|
| Interventions | Treatment: diosmine 1000 mg per day Control: placebo Duration: 60 days Follow‐up: 60 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to two parallel groups of 20" Comment: no randomisation methods stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods of allocation concealment stated |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described, number of participants who dropped out prematurely stated and reasons for dropping out described |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Cloarec 1994.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: 16/120 (13%) Losses to follow‐up: not stated |
|
| Participants | Country: France Setting: not stated Number: 120 patients Age: mean 50 years Gender: not stated Inclusion criteria: history of CVI for several years Exclusion criteria: not stated |
|
| Interventions | Treatment: O‐(beta‐hydroxyethyl)‐rutoside 2000 mg per day Control: placebo Duration: 56 days Follow‐up: 56 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | This clinical trial is published in abstract format; not possible to extract data showing results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A multicenter double blind randomized clinical trial was designed" Comment: no methods described for randomisation of participants |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information given about methods used for allocation concealment |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Low risk | Comment: only 13% drop‐out rate (16/120) for violation of study protocol reported |
| Selective reporting | High risk | Comment: no protocol identified. In the methods section, subjective symptoms identified that were not reported in the results section (pain, heaviness, swelling, restless leg, cramps, presence of pitting oedema) |
| Other bias | Low risk | Comment: none detected |
Cloarec 1996.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: 5/109 (5%) Losses to follow‐up: none |
|
| Participants | Country: France Setting: outpatient university clinic in a military hospital Number: 109 patients Age: 48 ± 14 years active group; 53.6 ± 13.6 years placebo group Gender: 16 M:88 F Inclusion criteria: CVI (Widmer grade II) and oedema and symptoms Exclusion criteria: elastic stockings, arterial insufficiency, venous ulcers or superficial thrombophlebitis, venous surgery or sclerotherapy in the preceding 6 months, other possible causes of leg oedema, pregnancy, irregular menstrual cycles; therapy with diuretics, steroids, anti‐inflammatories or venous drugs |
|
| Interventions | Treatment: O‐(beta‐hydroxyethyl)‐rutoside 1000 mg per 12 hours Control: placebo Duration: 60 days Follow‐up: 60 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "For this reason, we undertook a randomized, double‐blind, placebo‐controlled trial..." Comment: no methods for randomisation of participants described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods for allocation concealment described |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Low risk | Comment: only 5% drop‐out rate (5/109) for violation of study protocol. Number in each group provided, along with reasons for exclusion after randomisation and information on compliance |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Cornu‐Thenard 1985.
| Study characteristics | ||
| Methods | Study design: randomised, multi‐centre, double‐blind, placebo‐controlled Method of randomisation: random distribution of numbered batches Exclusions post randomisation: not stated Losses to follow‐up: not stated |
|
| Participants | Country: France Setting: not stated Number: 83 patients Age: 20 to 65 years; mean 43.73 ± 11.92 years active group; mean 43.55 ± 11.42 years placebo group Gender: 6 M:77 F Inclusion criteria: symptoms related to CVI Exclusion criteria: severe damage to venous musculature requiring urgent treatment ‐ surgery or sclerosis; surgical operation on venous or deep or superficial vein thrombosis in the past year; sclerosis or heavy support bandages (light support bandages not excluded), major trophic lesions, Raynaud's syndrome, arteritis, lymphoedema, renal or cardiac insufficiency; anti‐migraine treatment, analgesic or anti‐inflammatory treatment, diuretic treatment, low‐sodium diet, treatment for cardiovascular system (except nifedipine) |
|
| Interventions | Treatment: extract Ruscus aculeatus 75 mg plus hesperidin 75 mg plus ascorbic acid 50 mg per day (Cyclo 3) Control: placebo Duration: 60 days Follow‐up: 60 days Light compression therapy allowed |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A double‐blind comparative study against placebo, using two groups treated in parallel, after random distribution of numbered batches of the two treatments to be compared" Comment: seems like a fair method of randomisation was conducted |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information given about methods used for allocation concealment |
| Blinding (patients) | Low risk | Quote: "The two products to be compared were presented in the form of identical capsules for both Cyclo 3 and placebo" Comment: Identical placebo ensures a fair method used for double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "The two products to be compared were presented in the form of identical capsules for both Cyclo 3 and placebo" Comment: Identical placebo ensures a fair method used for double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "The two products to be compared were presented in the form of identical capsules for both Cyclo 3 and placebo" Comment: Identical placebo ensures a fair method used for double‐blinding |
| Incomplete outcome data | Unclear risk | Quote: no information provided about participants who withdrew prematurely from the trial |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Danielsson 2002.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: sealed envelope principle Exclusions post randomisation: none Losses to follow‐up: 4/101 (4%) |
|
| Participants | Country: Sweden Setting: hospital Number: 101 patients Age: 18 to 65 years Gender: 28 M:73 F Inclusion criteria: symptomatic CVI with reflux venous, CEAP II classification Exclusion criteria: diabetes; inflammatory, heart, renal, hepatic or peripheral arterial disease. Treatment with diuretics or anti‐inflammatory drugs (steroids, NSAIDs). Allergic reactions to venoactive drugs |
|
| Interventions | Treatment: micronised purified flavonoid fraction (MPFF) 1000 mg per day Control: placebo Duration: 60 days Follow‐up: 60 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | No description of double‐blind | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "One hundred and one patients with symptomatic CVD were randomly allocated to treatment with either MPFF (51 patients) or placebo..." Comment: no methods described for randomisation of participants |
| Allocation concealment (selection bias) | Low risk | Quote: "After informed consent, patients were randomised in a blinded fashion (sealed envelope principle)" Comment: sealed envelope principle considered a good method to ensure allocation concealment |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described. In addition, information given about numbers of participants who withdrew prematurely (4/101; 4%) |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Diebschlag 1994.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: none |
|
| Participants | Country: Germany Setting: not stated Number: 60 postmenopausal females Age: 'adults' Gender: 60 F Inclusion criteria: stage II CVI (oedema and symptoms) Exclusion criteria: not stated |
|
| Interventions | Treatment: oxerutin 500 mg per day or 1000 mg per day Control: placebo Duration: 84 days Follow‐up period: 112 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study design consisted of a double‐blind placebo controlled, randomized parallel group comparison with three treatment groups" Comment: no methods described for randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods described for allocation concealment |
| Blinding (patients) | Low risk | Quote: "All medications appeared to be identical with respect to volume, colour of the bottle and the smell of solution. The difference in taste could be accepted as this type is of solution was not commercially available and, therefore, unknown and unidentifiable to patients" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "All medications appeared to be identical with respect to volume, colour of the bottle and the smell of solution. The difference in taste could be accepted as this type is of solution was not commercially available and, therefore, unknown and unidentifiable to patients" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "All medications appeared to be identical with respect to volume, colour of the bottle and the smell of solution. The difference in taste could be accepted as this type is of solution was not commercially available and, therefore, unknown and unidentifiable to patients" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: no exclusions post randomisation and no losses to follow‐up |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
DOBESILATO500/2.
| Study characteristics | ||
| Methods | Study design: randomised, multi‐centre, double‐blind, placebo‐controlled Method of randomisation: random list generated by computer Exclusions post randomisation: study interrupted Losses to follow‐up: study interrupted |
|
| Participants | Country: Spain Setting: hospital Number: 69 patients Age: 60.9 (13.9) years placebo; 63.0 (20.5) years calcium dobesilate Gender: 36 M:33 F Inclusion criteria: adult patients with venous ulcer (CEAP 6) that affected epidermis, dermis and/or subcutaneous tissue, with an area superior to 3 cm2, an ankle‐arm index 0.9 or superior and written informed consent of patients Exclusion criteria: diabetes mellitus I or II. Renal failure and dialysis. Vascular surgery needed Impossibility to use compressive measures on the leg. Use of topical antibiotics, silver dressing, growth factors; plasma‐rich platelets, skin graft, pentoxifylline, ultrasound, laser, hyperbaric oxygen, electrical stimulation or vacuum. Pregnancy. Breast feeding. No anti‐contraceptive measures. Allergy or intolerance to phlebotonics. Background of neutropenia or leucopenia. Basal leucocytes < 3.500/mL |
|
| Interventions | Treatment: calcium dobesilate 500 mg 3× per day (capsules) Control: placebo Duration: 180 days Follow‐up period: 365 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Financial support for Laboratories Dr Esteve was withdrawn and the study was interrupted. Register at clinicatrial.gov: NCT00979836 We obtained information from researchers who conducted this unpublished and interrupted clinical trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation to treatment was randomised, centralised and computer stratified in blocks, by ulcer size and centre" Comment: Random sequence ensured by computer‐stratified blocks |
| Allocation concealment (selection bias) | Low risk | Comment: Treatment allocated by researcher phoning the co‐ordinating centre |
| Blinding (patients) | Low risk | Quote: "... to placebo (inactive capsules of identical appearance and weight) twice a day" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "... to placebo (inactive capsules of identical appearance and weight) twice a day" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "... to placebo (inactive capsules of identical appearance and weight) twice a day" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | High risk | Study was interrupted when only 69 of the 230 necessary participants were included |
| Selective reporting | High risk | Study was not published |
| Other bias | Low risk | Comment: none detected |
Dominguez 1992.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: computer‐generated random number table Exclusions post randomisation: none Losses to follow‐up: 7/57 (12%) |
|
| Participants | Country: Spain Setting: hospital Number: 57 patients Age: 20 to 65 years Gender: 5 M:52 F Inclusion criteria: symptomatic CVI and varicose veins and oedema Exclusion criteria: elastic bandages, anti‐inflammatory drugs and diuretics not permitted. Surgical operation, thrombophlebitis, pregnancy, diabetes, cardiopathy, hepatopathy, nephropathy, varicose veins secondary to extrinsic compression and varicose ulcers excluded |
|
| Interventions | Treatment: hidrosmine 600 mg per day Control: placebo Duration: 45 days Follow‐up: 45 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "On entry, patients were assigned to one or other of the two treatment groups according to a computer‐generated random number table" Comment: computer‐generated random number table considered a fair method to ensure good randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods of allocation concealment described |
| Blinding (patients) | Low risk | Quote: "The medications were supplied in identical capsule form" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "The medications were supplied in identical capsule form" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "The medications were supplied in identical capsule form" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group reported, along with information on compliance, drop‐outs (7/57; 12%), reasons for drop‐out and adverse events. ITT analysis conducted |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Fermoso 1992.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 6/34 (18%) |
|
| Participants | Country: Spain Setting: hospital Number: 34 patients Age: mean 53 ± 18 (range 21 to 86) years Gender: 20 M:14 F Inclusion criteria: CVI (varicose veins and/or disturbances of venous circulation by Doppler) Exclusion criteria: not stated |
|
| Interventions | Treatment: hidrosmine 600 mg per day Control: placebo Duration: 28 days Follow‐up: 28 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 34 patients chosen were randomly assigned to two treatment groups" Comment: no methods of randomisation described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods of allocation concealment described |
| Blinding (patients) | Low risk | Quote: "And the other group received placebo capsules indistinguishable from the hidrosmin capsules, according to the double‐blind technique" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "And the other group received placebo capsules indistinguishable from the hidrosmin capsules, according to the double‐blind technique" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "And the other group received placebo capsules indistinguishable from the hidrosmin capsules, according to the double‐blind technique" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described. In addition, number of participants who prematurely withdrew from the study (6/34; 18%) described |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Flota‐Cervera 2008.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: none |
|
| Participants | Country: Mexico Setting: hospital Number: 49 patients (25 in the calcium dobesilate group; 24 in the placebo group) Age: mean 52.20 ± 8.45 years Gender: 5 M:44 F Inclusion criteria: venous oedema Exclusion criteria: not stated |
|
| Interventions | Treatment: calcium dobesilate 1500 mg per day Control: placebo Duration: 49 days Follow‐up: 49 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A single center, prospective, randomized double‐blind, parallel group, placebo‐controlled" Comment: no method of randomisation generation described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A single center, prospective, randomized double‐blind, parallel group, placebo‐controlled" Comment: no method of allocation concealment described |
| Blinding (patients) | Low risk | Comment: placebo capsules identical to calcium dobesilate capsules |
| Blinding (study researchers) | Low risk | Comment: placebo capsules identical to calcium dobesilate capsules |
| Blinding (outcome assessment) | Low risk | Comment: placebo capsules identical to calcium dobesilate capsules |
| Incomplete outcome data | Low risk | Comment: no exclusions post randomisation and no losses to follow‐up |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Gilly 1994.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 10/160 (6%) |
|
| Participants | Country: France Setting: hospital Number: 160 patients Age: 'adults' Gender: 26 M:134 F Inclusion criteria: symptomatic disturbances of the veno‐lymphatic system Exclusion criteria: other or associated vascular diseases; oedema of cardiac, renal or hepatic origin; symptoms or signs of arterial, metabolic, neurological or orthopaedic origin; pregnancy; recent venous surgery; deep or superficial thrombosis in the past 6 months |
|
| Interventions | Treatment: micronised purified flavonoid fraction (MPFF) 1000 mg per day Control: placebo Duration: 42 days Follow‐up: 42 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eighty patients were randomly allocated to the S 5682 group and eighty patients to the placebo group" Comment: no method of randomisation described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method of allocation concealment described |
| Blinding (patients) | Low risk | Quote: "Patients fulfilling the inclusion criteria were randomly allocated to receive one S 5682 tablet twice daily or matching placebo" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "Patients fulfilling the inclusion criteria were randomly allocated to receive one S 5682 tablet twice daily or matching placebo" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "Patients fulfilling the inclusion criteria were randomly allocated to receive one S 5682 tablet twice daily or matching placebo" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described. In addition, adverse events experienced, number of drop‐outs and reasons for drop‐outs described. Methods used for imputing missed data not described. Six per cent of participants lost to follow‐up |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Guilhou 1997.
| Study characteristics | ||
| Methods | Study design: randomised, multi‐centre, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 6/107 (6%) |
|
| Participants | Country: France Setting: hospital Number: 107 patients Age: 'adults' Gender: 30 M:77 F Inclusion criteria: venous ulcers Exclusion criteria: not stated Randomisation of treatment stratified according to ulcer size: < 10 cm or ≥ 10 cm |
|
| Interventions | Treatment: diosmine 450 mg plus hesperidin 50 mg per 12 hours plus compression stockings Control: placebo and standard compression stockings Duration: 60 days Follow‐up: 60 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation of treatment was stratified according to the size of the ulcers" Comment: no method of randomisation described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method of allocation concealment described |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described. ITT analysis conducted. Information provided about participants who withdrew prematurely from the study, along with reasons for premature withdrawal |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Hachen 1982.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 2/50 (4%) |
|
| Participants | Country: Switzerland Setting: hospital Number: 50 females Age: 10 to 45 years Gender: 50 F Inclusion criteria: recent onset of CVI; no venous surgery, presence of symptoms (heaviness, fatigue, etc.) or aggravation during prolonged sitting or standing or during premenstrual periods Exclusion criteria: pregnancy, diabetes, polyneuropathy, osteo‐articular lesions in the legs, arterial peripheral insufficiency, oral contraceptives, poor co‐operation |
|
| Interventions | Treatment: calcium dobesilate 1000 mg per day Control: placebo Duration: 28 days Follow‐up: 28 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Fifty female patients with recent onset of venous insufficiency were randomly allocated to two subgroups receiving either calcium dobesilate or a corresponding placebo" Comment: no method of randomisation of participants described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method of allocation concealment described |
| Blinding (patients) | Low risk | Quote: "Fifty female patients with recent onset of venous insufficiency were randomly allocated to two subgroups receiving either calcium dobesilate or a corresponding placebo" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "Fifty female patients with recent onset of venous insufficiency were randomly allocated to two subgroups receiving either calcium dobesilate or a corresponding placebo" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "Fifty female patients with recent onset of venous insufficiency were randomly allocated to two subgroups receiving either calcium dobesilate or a corresponding placebo" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described. Participants who withdrew prematurely from the trial described, along with reasons for withdrawal. Four per cent of participants lost to follow‐up |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Ihme 1996.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: Rancode computer software Exclusions post randomisation: none Losses to follow‐up: 11/77 (14%) |
|
| Participants | Country: Germany Setting: hospital Number: 77 patients Age: mean 57.3 ± 9.6 years active group; mean 59.8 ± 7.3 years placebo group Gender: 24 M:53 F Inclusion criteria: CVI stages I and II (oedema, symptoms, stem varicosis, post‐thrombotic syndrome, valvular insufficiency of the deep veins) Exclusion criteria: varicosis with surgical indication; active or healed ulcus cruris; acute thrombosis or venous inflammation; oedema due to cardiac or renal insufficiency; treatment with a diuretic, dihydroergotamine or any other drugs for venous therapy; other severe disorder |
|
| Interventions | Treatment: Buckwheat herb tea (rutoside) 270 mg per day Control: placebo Duration: 90 days Follow‐up: 112 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation was carried out by Rancode computer software (IDV Gauting, Germany)" Comment: Randomisation seems like a fair method to ensure a random sequence of participants |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods of allocation concealment described |
| Blinding (patients) | Low risk | Quote: "A blinded taste test with pharmacists demonstrated that the teas were similar in taste and appearance and hard to distinguish" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "A blinded taste test with pharmacists demonstrated that the teas were similar in taste and appearance and hard to distinguish" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "A blinded taste test with pharmacists demonstrated that the teas were similar in taste and appearance and hard to distinguish" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described. Number of drop‐outs and reasons for dropping out of the trial described. ITT analysis conducted |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Jongste 1986.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: none |
|
| Participants | Country: The Netherlands Setting: outpatient Number: 80 patients Age: 20 to 75 years Gender: male and female; breakdown not given Inclusion criteria: unilateral post‐thrombotic syndrome Exclusion criteria: elastic stockings; diuretics; venoactive drugs; open venous ulcers; paralysis of the leg with post‐thrombotic syndrome; arterial disease; oedema of other origin; regular users of anti‐inflammatories, corticosteroids or analgesics |
|
| Interventions | Treatment: O‐(beta‐Hydroxyethyl)‐rutosides 1200 mg per day Control: placebo Duration: 56 days Follow‐up: 56 days |
|
| Outcomes | Primary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The trial was double blind, randomised, placebo controlled between patients" Comment: no methods of randomisation described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods of allocation concealment described. |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Low risk | Quote: number of participants in each group described. No losses reported |
| Selective reporting | High risk | Comment: no published protocol identified. In the methods section, outcomes of “restless legs” and “venous pressure” reported, but in the results/conclusion sections, no data regarding these outcomes reported |
| Other bias | Low risk | Comment: none detected |
Jongste 1989.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: computerised random assignment method used Exclusions post randomisation: 17/101 (17%) Losses to follow‐up: 3 (0.3%) |
|
| Participants | Country: The Netherlands Setting: hospital Number: 101 patients Age: 53 ± 12 years active group; 54 ± 13 years placebo group Gender: 48 M:35 F Inclusion criteria: unilateral post‐thrombotic syndrome > 6 months' duration and history of venography with deep vein thrombosis Exclusion criteria: elastic stockings; veno‐active drugs within 2 weeks of entry into the trial; active venous ulcer; pregnancy; age > 75 years |
|
| Interventions | Treatment: oxirutosides 1200 mg per day Control: placebo Duration: 56 days Follow‐up: 56 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Concealment of placebo not explicit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Upon entering the study, patients were randomly assigned to receive either HR or placebo with the use of a computerized random assignment method" Comment: computerised random assignment method generally accepted as a good method to generate a random sequence of participants |
| Allocation concealment (selection bias) | Low risk | Quote: "A series of coded sealed envelopes for decoding any particular case was supplied to the local hospital pharmacy" Comment: sealed envelopes generally accepted as a good method of allocation concealment |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described, along with number of participants who dropped out and number who experienced adverse events |
| Selective reporting | High risk | Comment: no published protocol identified. In the methods section, outcomes of “restless legs” and “venous pressure” reported, but in the results/conclusion sections, no data regarding these outcomes reported |
| Other bias | Low risk | Comment: none detected |
Kiesewetter 1997.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: not stated Losses to follow‐up: not stated |
|
| Participants | Country: Germany Setting: university Number: 81 patients Age: mean 59 ± 7 years Gender: 26 M:55 F Inclusion criteria: stage I to II of Wert CVI Exclusion criteria: acute thromboses; ulcus cruris; heart insufficiency; recent venous surgery; venoactive drugs |
|
| Interventions | Treatment: 500 mg Buckwheat herb and 30 mg troxerutin. 2 tablets 3 × per day Control: placebo Duration: 84 days Follow‐up: 112 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For randomization of patients, the program was 'Rancode' of the company IDV data analysis and experimental design, Gauting, used" Comment: computerised generation of a random sequence generally accepted as a fair method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information given about methods used for allocation concealment |
| Blinding (patients) | Low risk | Quote: "The same number of identical‐looking placebo tablets consisting of lactose were given" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "The same number of identical‐looking placebo tablets consisting of lactose were given" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "The same number of identical‐looking placebo tablets consisting of lactose were given" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Unclear risk | Comment: number of participants in each group described. No information provided about participants who prematurely dropped out of the study |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Klüken 1971.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: not stated Losses to follow‐up: not stated |
|
| Participants | Country: Germany Setting: hospital Number: 60 patients Age: 'adults' Gender: not stated Inclusion criteria: CVI (varicoses or post‐thrombotic syndrome) Exclusion criteria: not stated |
|
| Interventions | Treatment: troxerutin 75 mg and coumarin 15 mg per day Control: placebo Duration: 21 days Follow‐up: 21 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Double‐blind, randomized, placebo‐controlled. In two parallel groups" Comment: information about methods of randomisation not provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information about methods of allocation concealment provided |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Unclear risk | Comment: number of participants in each group described. No information provided about the number of participants who dropped out of the study prematurely or the number who experienced adverse events |
| Selective reporting | Unclear risk | Comment: no published protocol identified. No outcomes reported in the methods section |
| Other bias | Low risk | Comment: none detected |
Koscielnny 1996.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 6/77 (8%) |
|
| Participants | Country: Germany Setting: university Number: 94 patients selected; 67 randomised Age: 'adults' Gender: not stated Inclusion criteria: CVI stage I to II Widmer Exclusion criteria: not stated |
|
| Interventions | Treatment: Buckwheat herb tea 3 × 1.8 g per day Control: placebo tea Duration: 84 days Follow‐up: 112 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After a placebo period of two weeks, patients were randomly assigned to active treatment or a placebo group" Comment: no information about methods of randomisation provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information about methods of allocation concealment provided |
| Blinding (patients) | Low risk | Quote: "Placebo is with taste and appearance indistinguishable from the treatment" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "Placebo is with taste and appearance indistinguishable from the treatment" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "Placebo is with taste and appearance indistinguishable from the treatment" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in both placebo and treatment groups described, along with the most important participant characteristics, numbers of participants who dropped out prematurely, reasons for drop‐out, influence of drop‐outs and information on compliance |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Kriner 1985.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: not stated Losses to follow‐up: not stated |
|
| Participants | Country: Germany Setting: hospital Number: 50 patients Age: 'adults' Gender: not stated Inclusion criteria: disturbances of venous blood flow, oedema Exclusion criteria: not stated |
|
| Interventions | Treatment: ruscus extract 75 mg and hesperidin 75 mg 2 × 2 capsules per day. rutoside cream once per day Control: placebo Duration: 28 days Follow‐up: 28 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The two groups were balanced and comparable with respect to age, weight, and type and duration of disturbances" Comment: no method of randomisation described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method of allocation concealment described |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Unclear risk | Comment: number in each group described, but important characteristics lacking. In addition, number of participants who dropped out prematurely or were excluded after randomisation not described |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Labs 2004.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: computerised random assignment method Exclusions post randomisation: 7/260 (0.3%), protocol violation Losses to follow‐up: 21/260 (8%) |
|
| Participants | Country: Switzerland Setting: university Number: 260 patients Age: 20 to 70 years Gender: 16 M:201 F Inclusion criteria: CVI class 1 to 4 (CEAP classification), oedema and symptoms Exclusion criteria: CVI class 5 to 6 (CEAP classification); other causes of oedema (cardiac, renal, etc.); hypertension with change in treatment within 6 weeks of study start; obesity; peripheral arterial occlusive disease; venous surgery in the past 12 months or sclerotherapy during the past 6 months; irregular menstrual cycle; elevated transaminases; neutropenia; significant renal insufficiency; gastrointestinal disease; allergy to study medication; pregnant or lactating women; unreliable patient (psychiatric disorders, alcoholism, etc.); compression stockings or bandages; diuretics; venotropic medication; antiphlogistic drugs; corticosteroids; analgesics |
|
| Interventions | Treatment: calcium dobesilate 1500 mg per day Control: placebo Duration: 28 days Follow‐up: 42 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Reasons for withdrawal unknown | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The corresponding boxes were randomized in balanced blocks and were labelled by the sponsor with the study number, the dosage, the batch numbers, with the patient number and with the note 'for clinical trials only'. The randomization was done by BIOMETRIX S. A., CH‐1911 Gland, Switzerland, using appropriate software" Comment: computer‐generated list of random numbers accepted as a good method for generating a random sequence of participants |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation of the study treatment to each patient was done according to the next available consecutive patient number printed on the prescription card and on the label of the box. This number was recorded on each page of the CRF.¨ and ¨Each investigator was provided with a sealed envelope containing the code for each patients randomisation number" Comment: seems like a fair method of allocation concealment |
| Blinding (patients) | Low risk | Quote: "For the double‐blind treatment period, the boxes, labels, and capsules containing Doxium 500 and placebo were identical in appearance for each drug, to ensure patient and investigator blinding" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "For the double‐blind treatment period, the boxes, labels, and capsules containing Doxium 500 and placebo were identical in appearance for each drug, to ensure patient and investigator blinding" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "For the double‐blind treatment period, the boxes, labels, and capsules containing Doxium 500 and placebo were identical in appearance for each drug, to ensure patient and investigator blinding" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described. Adverse events, participant experience, compliance and number of participants who dropped out prematurely reported (29/260 participants) |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Languillat 1988.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: none |
|
| Participants | Country: France Setting: hospital Number: 20 patients Age: 20 and 65 years Gender: 1 M:19 F Inclusion criteria: symptomatic CVI and oedema Exclusion criteria: previous venous sclerosis; surgery or elastic support; trophic disturbances; ulcers or permanent oedema; cardiac, renal, hepatic insufficiency or arterial disease; Raynaud's phenomenon; lymphoedema; pregnancy; venoactive drugs; any significant change in patient lifestyle or work |
|
| Interventions | Treatment: extract Ruscus aculeatus 450 mg plus hesperidin 450 mg plus ascorbic acid 300 mg per 12 hours Control: placebo Duration: 28 days Follow‐up: 42 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This was a double‐blind placebo‐controlled trial with two groups of patients treated in parallel" Comment: no method of randomisation described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method of allocation concealment described |
| Blinding (patients) | Low risk | Quote: "The double‐blind nature of the trial was guaranteed by the strictly identical appearance of treatment units (capsules) as well as their packaging (bottles and bags of treatment kits)" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "The double‐blind nature of the trial was guaranteed by the strictly identical appearance of treatment units (capsules) as well as their packaging (bottles and bags of treatment kits)" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "The double‐blind nature of the trial was guaranteed by the strictly identical appearance of treatment units (capsules) as well as their packaging (bottles and bags of treatment kits)" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described, along with the most important baseline characteristics. No losses reported |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Laurent 1988.
| Study characteristics | ||
| Methods | Study design: 2 randomised, double‐blind, placebo‐controlled studies analysed together Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 5/200 (2.5%) |
|
| Participants | Country: France Setting: hospital Number: 200 patients Age: mean 49 (range 22 to 82) years Gender: 26 M:174 F Inclusion criteria: One study included patients with functional venous insufficiency (presence of symptoms but not signs); n = 83. The other study included patients with chronic organic venous insufficiency (varicose disease, post‐thrombotic syndrome); n = 117 Elastic stockings permitted Exclusion criteria: not exclusively venous symptoms (arterial, neurological or metabolic origin, disorders of static equilibrium); venotropic drugs in the past 3 months; pregnancy; prolonged immobilisation |
|
| Interventions | Treatment: diosmine 450 mg plus hesperidin 50 mg per 12 hours Control: placebo Duration: 60 days Follow‐up: 60 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized trials were conducted versus placebo using appropriate statistical tests determined a priori" Comment: no methods of sequence generation specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods of allocation concealment described |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group provided, along with inclusion and exclusion criteria and characteristics of participants Number of participants who experienced adverse events presented, along with number who dropped out of the study. Losses 2.5% |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Lazzarini 1982.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: not stated Losses to follow‐up: not stated |
|
| Participants | Country: Italy Setting: hospital Number: 100 patients Age: 'adults' Gender: 23 M:74 F Inclusion criteria: stratification for participant groups: varicose legs, ulcer, thrombophlebitis, slight CVI Exclusion criteria: not stated |
|
| Interventions | Treatment: aminaftone 150 mg per day Control: placebo Duration: 60 days Follow‐up: 60 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The trial was conducted in 100 patients, informed consent and randomized into two groups of 50 and 50 and double‐blind treatment, the first with Capillarema and the second with placebo" Comment: method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method of allocation concealment described |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Unclear risk | Comment: number of participants in each group described, but important baseline characteristics lacking. In addition, number of participants who withdrew prematurely not described |
| Selective reporting | High risk | Comment: no information regarding adverse events provided |
| Other bias | Low risk | Comment: none detected |
MacLennan 1994.
| Study characteristics | ||
| Methods | Study design: multicenter, international, parallel, randomised, double‐blind, placebo‐controlled trial Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 16/104 (15%) |
|
| Participants | Country: UK, Germany, Netherland and Belgium Setting: hospital Number: 104 patients Age: ≥ 65 years Gender: 24 M:62 F Inclusion criteria: unilateral or bilateral symptoms and signs of CVI. Compression stockings allowed Exclusion criteria: bed‐bound or with cardiac or renal or hepatic disease or clinically important obesity; arterial insufficiency of the legs |
|
| Interventions | Treatment
Follow‐up: 180 days Participants who wore elastic support stockings had to continue to wear them throughout the study |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was made according to a computer‐generated randomization list in blocks of 10" Comment: computer‐generated randomisation list generally accepted as an appropriate way to generate a random sequence of participants |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method of allocation concealment stated |
| Blinding (patients) | Low risk | Quote: "Patients from each centre in the randomised control group were given placebo capsules identical in appearance" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "Patients from each centre in the randomised control group were given placebo capsules identical in appearance" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "Patients from each centre in the randomised control group were given placebo capsules identical in appearance" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: number of participants described, along with the most important characteristics, number of drop‐outs, adverse events and information on compliance |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Mann 1981.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 5/28 (18%) |
|
| Participants | Country: UK Setting: outpatient Number: 28 patients Age: mean 69 years active treatment; mean 63 years placebo Gender: not stated Inclusion criteria: ≥ 1 venous ulcer Exclusion criteria: not stated |
|
| Interventions | Treatment: hidroxirutoside 1000 mg per day Control: placebo Duration: 90 days Follow‐up: 90 days Concomitant therapy: topical therapy and an "elastoweb" bandage |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided about the method used for randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided about the method used for allocation concealment |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | High risk | Comment: number of participants for each group described, but no information provided about participants lost to follow‐up or dropped out. Data were missing from the analysis and adverse events were not described. Losses were reported as 18% |
| Selective reporting | High risk | Comment: no protocol identified. Differences were noted between methods and results for the following outcomes: tiredness, heaviness, tender legs, distended veins, nights disturbed, daytime cramps |
| Other bias | Low risk | Comment: none detected |
Marinello 2002.
| Study characteristics | ||
| Methods | Study design: randomised, multi‐centre, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 21/123 (17%) |
|
| Participants | Country: Spain Setting: hospital Number: 143 patients Age: mean 52.87 (range 19 to 72) years Gender: 25 M:77 F Inclusion criteria: CVI stage CEAP III, IV and V Exclusion criteria: not stated |
|
| Interventions | Treatment: calcium dobesilate 1000 mg per day or calcium dobesilate 2000 mg per day Control: placebo Duration: 84 days Follow‐up: 84 days Elastic stockings permitted |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In total 143 patients 123 were randomized (41 per treatment group)" Comment: no method of randomisation described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods of allocation concealment described |
| Blinding (patients) | Low risk | Quote: "The placebo had the same characteristics which include active treatment. The oral administration was under the same conditions as the active treatment" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "The placebo had the same characteristics which include active treatment. The oral administration was under the same conditions as the active treatment" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "The placebo had the same characteristics which include active treatment. The oral administration was under the same conditions as the active treatment" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described, along with baseline characteristics. In addition, numbers and information provided about adverse events and participants who withdrew prematurely from the study |
| Selective reporting | Low risk | Comment: no published protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Martinez‐Zapata 2008.
| Study characteristics | ||
| Methods | Study design: randomised, multi‐centre, double‐blind, placebo‐controlled Method of randomisation: computer‐generated random number table Exclusions post randomisation: none Losses to follow‐up: 131/509 (25.7%) |
|
| Participants | Country: Spain Setting: hospital Number: 509 patients Age: mean 53.3 ± 13.3 years treatment group; mean 54.7 ± 14.9 years placebo group Gender: 66 M:443 F Inclusion criteria: adults of either gender with CVD, CEAP clinical grades 1 to 6 and able to complete a QoL questionnaire Exclusion criteria: chronic or acute disease that limited compliance with the protocol, scheduled surgery or sclerotherapy in the coming calendar year, pregnant or lactating women, patients with allergies or known intolerance to the study medication, history of neutropoenia or leucopoenia, baseline serum leucocyte count < 3500/mL |
|
| Interventions | Treatment: 500 mg capsules of oral calcium dobesilate twice a day for 3 months Control: placebo: Inactive capsules of identical appearance and weight Duration: 90 days Follow‐up: 365 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation to treatment was randomised, centralised and computer stratified in blocks of 10 patients, by clinical CEAP classification and centre" Comment: Computer‐stratified blocks ensure a random sequence |
| Allocation concealment (selection bias) | Low risk | Comment: treatment was allocated by researcher phoning the co‐ordinating centre |
| Blinding (patients) | Low risk | Quote: "to placebo (inactive capsules of identical appearance and weight) twice a day" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "to placebo (inactive capsules of identical appearance and weight) twice a day" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "to placebo (inactive capsules of identical appearance and weight) twice a day" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: number in each group was described, and those lost to follow‐up (25.7%) and participants who prematurely withdrew were described. Important characteristics were described, and inclusion and exclusion criteria were reported. ITT analysis was conducted, and imputation technique was described |
| Selective reporting | Low risk | Comment: protocol identified and no differences identified between protocol and article |
| Other bias | Low risk | Comment: none detected |
NCT01848210.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: "by chance, like flipping a coin" Exclusions post randomisation: 166 Losses to follow‐up: 36 |
|
| Participants | Country: Brazil Setting: not specified Number: 829 (411 experimental group and 418 placebo group) included and 711 analysed (383 experimental group and 388 placebo group) Age (mean): 56 (18 to 75 years old) Gender: 83 men and 688 women Inclusion criteria: consent of subject or legal representative. Men or women of any ethnicity, aged between 18 and 75 years, and BMI equal or less than 40. CVI in the reference leg with the clinical classification C3, or C4a or C4b or C5, stable edema. Scoring in "Severity Score of Local Complaints" equal to or higher than 5 total points. Women who are using an effective birth control or who are postmenopausal Exclusion criteria: Deep vein insufficiency or venous obstruction and/or DVT and/or presence of phlebitis in lower limbs during the last 3 months.Surgery at the venous system or sclerotherapy or any treatment for CVI during the last 3 months. History of known or suspected allergy or intolerance to any of the ingredients of the medicinal product under investigation. Serious systemic disease. Hepatitis A, hepatitis B, or C or any liver disease. Use of diuretics. Diabetes insulin‐dependent. History of alcoholism, drug abuse, psychological or emotional problems |
|
| Interventions | Treatment: Coumarin 30 mg, troxerutin 180 mg fixed‐dose combination tablets (Venalot), orally, 3 times daily Control: placebo Duration: 16 weeks Follow‐up: 18 weeks |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Sponsor: Takeda. Results published in clinicaltrials.gov | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants will be randomly assigned (by chance, like flipping a coin) to one of the two treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified. |
| Blinding (patients) | Low risk | Quote: "Placebo (dummy inactive pill) ‐ this is a tablet that looks like the study drug but has no active ingredient. All participants will be asked to take two tablets three times a day throughout the study" |
| Blinding (study researchers) | Low risk | Quote: "Placebo (dummy inactive pill) ‐ this is a tablet that looks like the study drug but has no active ingredient. All participants will be asked to take two tablets three times a day throughout the study" |
| Blinding (outcome assessment) | Low risk | Quote: "Placebo (dummy inactive pill) ‐ this is a tablet that looks like the study drug but has no active ingredient. All participants will be asked to take two tablets three times a day throughout the study" |
| Incomplete outcome data | Low risk | Comment: there was a IIT analysis (patient that taken the treatment at least 28 days) and a PP analysis. The total losses were 166 (20%) |
| Selective reporting | Low risk | Comment: all results are published. |
| Other bias | Low risk | Comment: none detected |
Nocker 1990.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: not stated Losses to follow‐up: not stated |
|
| Participants | Country: Germany Setting: university Number: 30 Age: 55 to 59 years Gender: menopausal females Inclusion criteria: stage II CVI with symptoms Exclusion criteria: venoactive drugs, anti‐inflammatories, corticosteroids or diuretics in the last 8 days before the start of the study; use of compression bandages or elastic stockings |
|
| Interventions | Treatment: oxirutoside 600 mg or 900 mg or 1200 mg or 1500 mg per day Control: placebo Duration: 90 days Follow‐up: 112 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized to one of the five groups, receiving oral solutions of HR in small bottles containing 600, 900, 1200, 1500 mg HR or simply distilled water (controls) with six patients in each group" Comment: no methods described for randomising participants |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods for allocation concealment described |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Unclear risk | Comment: no data given about drop‐outs. Most important characteristics described with inclusion and exclusion criteria |
| Selective reporting | Low risk | Comment: no protocol identified, but no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Padrós 1972.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, cross‐over, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: not stated Losses to follow‐up: not stated |
|
| Participants | Country: Spain Setting: university Number: 30 females Age: 48 to 51 years Gender: female Inclusion criteria: CVI with signs (oedema, venous ectasia) and symptoms (heaviness, paraesthesias) Exclusion criteria: not stated |
|
| Interventions | Treatment: calcium dobesilate 250 mg tablet 3 × per day Control: placebo tablet 3 × per day Duration: 21 days Follow‐up: 28 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no methods of random sequence generation described |
| Allocation concealment (selection bias) | Low risk | Comment: each bottle of treatment was identical and was numbered in a random way |
| Blinding (patients) | Low risk | Comment: each bottle of treatment was identical. Participants did not know the type of treatment administered |
| Blinding (study researchers) | Low risk | Comment: each bottle of treatment was identical. Researcher did not know the type of treatment administered |
| Blinding (outcome assessment) | Low risk | Comment: each bottle of treatment was identical. Assessor did not know the type of treatment administered |
| Incomplete outcome data | Unclear risk | Comment: no information on losses |
| Selective reporting | High risk | Comment: results before cross‐over not reported |
| Other bias | Low risk | Comment: none detected |
Parrado 1999.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: table of random numbers Exclusions post randomisation: none Losses to follow‐up: none |
|
| Participants | Country: Argentina Setting: hospital Number: 60 patients Age: 30 to 70 years Gender: 16 M:44 F Inclusion criteria: CVI, stages I to II of the Widmer classification (pigmentation, oedema, varicoses and symptoms) Exclusion criteria: elastic stockings; urgent surgical treatment or venous surgical treatment or sclerotherapy in previous 6 months; cardiac, renal or hepatic insufficiency; anti‐migraine drugs; analgesics; NSAIDs; diuretics or cardiovascular drugs; pregnant women or women who had given birth during previous 3 months |
|
| Interventions | Treatment: Ruscus aculeatus with hesperidin and vitamin C 300 mg per day Control: placebo Duration: 60 days Follow‐up: 60 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study was double‐blind and patients were randomly allocated to be included in one of two parallel groups by using a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods of allocation concealment described |
| Blinding (patients) | Low risk | Quote: "Bottles, identical in form and presentation contained dobesilate calcium or placebo, according to a randomization code that was opened until the end of experiment" Comments: Identical presentation of intervention and control groups ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "Bottles, identical in form and presentation contained dobesilate calcium or placebo, according to a randomization code that was opened until the end of experiment"; "Neither the patient nor the medical staff did not know the nature of the substance administered, thereby satisfying the conditions of a double‐blind trial" Comments: Identical presentation of intervention and control groups ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "Bottles, identical in form and presentation contained dobesilate calcium or placebo, according to a randomization code that was opened until the end of experiment"; "Neither the patient nor the medical staff did not know the nature of the substance administered, thereby satisfying the conditions of a double‐blind trial" Comments: Identical presentation of intervention and control groups ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: no losses reported |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Pecchi 1990.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: use of alternation by order of arrival of each participant Exclusions post randomisation: none Losses to follow‐up: none |
|
| Participants | Country: Italy Setting: university Number: 40 patients Age: mean 48.2 ± 15.7 years Gender: 4 M:36 F Inclusion criteria: primary CVI and post‐thrombotic syndrome Exclusion criteria: postphlebitic syndrome; severe trophic lesions; no venous oedema; patients taking diuretics, corticosteroids or vasoactive drugs; elastic stockings or bandages |
|
| Interventions | Treatment: calcium dobesilate 1000 mg per day Control: placebo Duration: 30 days Follow‐up: 30 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients admitted to the study were randomly divided into two balanced groups treated respectively with calcium or placebo for one month..." Comment: no method of randomisation described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The allocation to individual patients of either type of treatment was performed according to the access sequence number of the patient" Comment: no method of allocation concealment described |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Low risk | Comment: numbers of participants in both groups described. No losses reported. No baseline characteristics of participants provided |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Pedersen 1992.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: not stated Losses to follow‐up: not stated |
|
| Participants | Country: Denmark Setting: not stated Number: 43 patients Age: 'adults' Gender: 8 M:41 F Inclusion criteria: symptoms of CVI and oedema Exclusion criteria: diuretic drugs; venotonic drugs; pregnant women |
|
| Interventions | Treatment: oxirutoside 900 mg per day Control: placebo Duration: 28 days Follow‐up: 28 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "If patients met the inclusion criteria, they were randomised using envelope method, double‐blind treatment with Venoruton 300 mg × 3 daily or placebo" Comment: method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Quote: "If patients met the inclusion criteria, they were randomised using envelope method, double‐blind treatment with Venoruton 300 mg × 3 daily or placebo" Comment: envelope methods generally accepted as a fair method for allocation concealment |
| Blinding (patients) | Unclear risk | Comment: no methods of blinding described |
| Blinding (study researchers) | Unclear risk | Comment: no methods of blinding described |
| Blinding (outcome assessment) | Unclear risk | Comment: no methods of blinding described |
| Incomplete outcome data | Unclear risk | Comment: number of participants in both groups described, along with the most important characteristics and inclusion and exclusion criteria. Number of participants who withdrew prematurely not described |
| Selective reporting | Low risk | Comment: no protocol identified, but no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Petrassi 2000.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: computer‐elaborated simple randomisation table Exclusions post randomisation: none Losses to follow‐up: none |
|
| Participants | Country: Italy Setting: ambulatory Number: 20 patients Age: 47.7 (3.65) years active group; 36.7 (3.66) placebo group Gender: 3 M:19 F Inclusion criteria: CVI symptoms (heaviness and subcutaneous swelling) and venous pressure > 40 mmHg Exclusion criteria: cardiovascular drugs, diuretic drugs and analgesic or anti‐inflammatory compounds |
|
| Interventions | Treatment: French bark pine extract capsules 100 mg 3 × per day Control: placebo Duration: 60 days Follow‐up: 60 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They were treated with placebo or Pycnogenol 100mg × 3/day for 2 months according to a computer elaborated simple randomization table" Comment: computerised randomisation table generally accepted as a proper way to randomise participants |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method described for allocation concealment |
| Blinding (patients) | Low risk | Quote: "The drugs were prepared in white opaque capsules in order to make the slightly pinkish‐coloured Pycnogenol® indistinct from placebo (lactose)" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "The drugs were prepared in white opaque capsules in order to make the slightly pinkish‐coloured Pycnogenol® indistinct from placebo (lactose)" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "The drugs were prepared in white opaque capsules in order to make the slightly pinkish‐coloured Pycnogenol® indistinct from placebo (lactose)" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: number of participants was described in each group, along with the most important characteristics of participants, including inclusion and exclusion criteria. In addition, information was given about drop‐outs and adverse events |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Planchon 1990.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 6/110 (5%) |
|
| Participants | Country: France Setting: hospital Number: 110 participants Age: mean 50 (range 22 to 79) years Gender: 18 M:92 F Inclusion criteria: symptoms of functional and organic (post‐thrombotic syndrome and varices) CVI Exclusion criteria: venous thrombosis; long‐term immobilisation; hepatic, renal and cardiac oedema; neurological, arterial and metabolic symptoms |
|
| Interventions | Treatment: diosmine 450 mg plus hesperidin 50 mg × 2 capsules per day Control: placebo Duration: 60 days Follow‐up: 60 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The award of the therapeutic group membership made by draw lots was ignored until the complete end of the study by both the clinician and the patients" Comment: drawn seems a method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods of allocation concealment stated |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described, as well as the inclusion and exclusion criteria and the most important characteristics. Numbers of participants who withdrew prematurely were described, including reasons for dropping out, information about compliance and adverse events |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Pointel 1986.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 4 (4%) |
|
| Participants | Country: France Setting: hospital Number: 94 patients Age: mean 49 ± 12 years Gender: 8 M:86 F Inclusion criteria: CVI Exclusion criteria: severe varicose veins requiring an elastic strip, postphlebitic patients, those with unilateral venous insufficiency, those treated with a venoactive drug before the start of the study |
|
| Interventions | Treatment: Centella asiatica (TECA) 120 mg: two 30 mg capsules twice a day vs Centella asiatica (TECA) 60 mg: one 30 mg capsule twice a day Control: placebo Duration: 56 days Follow‐up: 56 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study conducted in four hospitals according to a controlled, randomized, double‐blind (double dummy) study performed on three parallel groups for eight weeks" Comment: no method of randomisation described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods of allocation concealment described |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described, along with inclusion and exclusion criteria and important characteristics for participants. In addition, study author reported the number of adverse events that occurred, the number of participants who withdrew prematurely and reasons for dropping out |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Prerovsky 1972.
| Study characteristics | ||
| Methods | Study design: 2 independent, randomised, double‐blind, cross‐over, placebo‐controlled trials
Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: none |
|
| Participants | Country: Czechoslovakia Setting: research centre Number: 50 patients Age: 'adults' Gender: not stated Inclusion criteria: signs (oedema, pigmentation, post‐thrombotic syndrome) and symptoms of CVI Exclusion criteria: not stated |
|
| Interventions | Treatment: oxirutoside 1200 mg per day Control: placebo Duration: 126 days Follow‐up: 126 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... after the administration of 3 capsules of HR (900 mg) or 3 capsules of placebo in a double blind cross‐over trial in a randomized‐order" Comment: method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method of allocation concealment described |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Low risk | Comment: number of participants described in each group, along with the most important characteristics. However, inclusion and exclusion criteria were, apart from clinical features, not well described. Adverse events and drop‐outs were well described |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Pulvertaft 1983.
| Study characteristics | ||
| Methods | Study design: randomised, multi‐centre, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: not stated Losses to follow‐up: 64/660 (10%) |
|
| Participants | Country: UK Setting: general practice Number: 660 patients Age: 54 years Gender: 220 M:440 F Inclusion criteria: symptomatic CVI Exclusion criteria: not stated |
|
| Interventions | Treatment: oxirutoside 1000 mg per day Control: placebo Duration: 28 days Follow‐up: 28 days Participants who wore elastic support had to continue to wear it throughout the study |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Four patients would receive active treatment with Paroven and one would be randomly and blindly treated with placebo" Comment: no method of randomisation described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method of allocation concealment described |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described, and a table includes the most important characteristics of participants and inclusion and exclusion criteria. In addition, number of participants excluded after randomisation reported |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Rabe 2011.
| Study characteristics | ||
| Methods | Study design: randomised, multi‐centre, double‐blind, placebo‐controlled Method of randomisation: table of random numbers Exclusions post randomisation: 22 (8%) Losses to follow‐up: 32/256 (12.5%) |
|
| Participants | Countries: Germany and Switzerland Setting: not stated Number: 256 patients Age: mean 53.2 ± 11.5 years treatment group; mean 53.5 ± 12.1 years placebo group Gender: 38 M:218 F Inclusion criteria: pitting oedema due to CVI (C3‐C5 according to CEAP classification) and ≥ 1 of the symptoms such as discomfort and pain Exclusion criteria: disease that imitates symptoms of CVI, cardiac insufficiency, ulceration of the lower leg, diabetes mellitus, hypertension, lymphoedema, sclerotherapy during the past 6 months, lipoedema, obesity (BMI > 30 kg/m2), disease of the gastrointestinal tract; female patients who were pregnant, lactating or of childbearing potential and not protected from pregnancy by a sufficiently reliable method; malignant disease |
|
| Interventions | Treatment: calcium dobesilate 1500 mg per day Control: matching placebo Duration: 56 days Follow‐up post treatment: 70 days Elastic stockings permitted |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization with blocks of four was used. The randomization list was produced by an independent person" Comment: Randomisation list ensures a random sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "The study medication was packed in identical boxes marked with a randomization number each newly randomized patient was given the medication with the lowest randomization number available" Comment: Identical boxes with randomisation provision ensure proper allocation concealment |
| Blinding (patients) | Low risk | Quote: ".... or a matching placebo ... The study medication was packed in identical boxes..." Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "The study medication was packed in identical boxes marked with a randomization number each newly randomized patient was given the medication with the lowest randomization number available" ; ".... or a matching placebo ... The study medication was packed in identical boxes..." Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: ".... or a matching placebo ... The study medication was packed in identical boxes..." Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: number in each group described, as were loss to follow‐up and participants who prematurely withdrew. Important characteristics and inclusion and exclusion criteria reported. ITT analysis conducted, but no methods used for imputation of missing values described |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Rabe 2015.
| Study characteristics | ||
| Methods | Study design: randomised, multi‐centre, double‐blind, placebo‐controlled Method of randomisation: not specified Exclusions post randomisation: 48% of patients (no symptoms) Losses to follow‐up: not specified. |
|
| Participants | Countries: Argentina, Austria, Czech Republica, France, Germany, Hungary, Italy, Poland, Portugal, Russia, Slovakia, Spain and Switzerland Setting: ambulatory outpatients Number: 1137 (579 to experimental and 558 to placebo group); 592 (52.1%) patients had CVI with symptoms Age: mean 48.9.2 ± 11.1 years old (symptomatic patients) Gender: 87.3% women (symptomatic patients) Inclusion criteria: ambulatory outpatients, adults, with CEAP C3 or C4A, and at least one venous reflux and vesper leg oedema Exclusion criteria: BMI ≥ 30, dermatoliposclerosis, leg ulcer, idiopathic oedema, lymphoedema, a recent (< 1 year) DVT, dermal infection or inflammation of the leg, recent sclerotherapy or surgical treatment of varicose veins. Treatment with anti‐inflammatories, calcium channel blockers, diuretics, thymoanaleptics, hormones or venous‐active drugs |
|
| Interventions | Treatment: micronized purified flavonoid fraction 1000 mg (2 tablets 500 mg) per day Control: matching placebo Duration: 4 months Follow‐up post treatment: 2 months Elastic stockings: not specified |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The authors did not describe the process of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: The authors did not describe the process of allocation concealment |
| Blinding (patients) | Unclear risk | Comment: The authors did not describe the placebo characteristics (colour and taste). The patient received two tablets of 500 mg of placebo at lunch time as the experimental group that received micronized purified flavonoid fraction |
| Blinding (study researchers) | Unclear risk | Comment:The study used placebo |
| Blinding (outcome assessment) | Unclear risk | Comment: The study used placebo |
| Incomplete outcome data | Low risk | The baseline characteristics were described only for symptomatic patients (52% of the sample size) and were balanced between groups. Although there is not a flowchart about the total patients included, the authors reported a 4.1% of losses in the overall patients and a 3.6% of losses in the symptomatic subgroup |
| Selective reporting | High risk | The main outcome "improvement on leg oedema" was not reported adequately. The authors only referred that there were not significant differences between groups when oedema was assessed using water displacement volumetry. This is a posthoc analysis for only symptomatic patients |
| Other bias | Low risk | Comment: none detected |
Rabe 2016.
| Study characteristics | ||
| Methods | Study design: randomized, double‐blind, placebo‐controlled, multi‐center Phase IV study Method of randomisation: not specified Exclusions post randomisation: the analysis was per ITT but 149 (45.4%) participants presented major protocol violations Losses to follow‐up: 52 (14.8%) participants |
|
| Participants | Countries: Germany, Italy, Poland, Portugal Setting: ambulatory outpatients Number: 351 (177 calcium dobesilate, 177 placebo) Age: mean 54.9 ± 10.7 years Gender: 280 (79.8%) female Inclusion criteria: participants of both sexes, with moderate CVI, as defined by CEAP classification C3 or C4,3 and assessed by clinical evaluation and duplex sonography. Eligible patients presented with a pitting oedema and at least one of the following: discomfort or pain in at least one leg at both the screening and baseline visits. In addition, all patients had to have chronic but stable edema. Exclusion criteria: participants with diseases that mimicked CVI (such as cardiac, hepatic or renal disease or other causes of leg oedema), those with other vascular system disorders (such as cardiac insufficiency, diabetes mellitus, non‐controlled hypertension, recent phlebitis/deep leg vein thrombosis) and those with primary or secondary lymphoedema |
|
| Interventions | Treatment: capsules containing 500 mg of calcium dobesilate (Doxium; batch number 23843) Control: placebo Dose: 3 capsules per day of calcium dobesilate or matching placebo Duration: 12 weeks Follow‐up post treatment: 12 weeks Elastic stockings: not specified |
|
| Outcomes | Primary
Secondary
|
|
| Notes | EudraCT (number 2009‐013391‐44). clinicaltrialsregister.eu/ctr‐search/trial/2009‐013391‐44/IT. Recruitment between 20 April 2010 and 10 November 2011 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "351 were randomized in a 1:1 ratio to treatment with calcium dobesilate or placebo" Comment: There was no information about the generation of the random sequence |
| Allocation concealment (selection bias) | Unclear risk | Quote: "351 were randomized in a 1:1 ratio to treatment with calcium dobesilate or placebo" Comment: There was no information about the allocation concealment |
| Blinding (patients) | Low risk | Quote: "Capsules containing 500 mg of calcium dobesilate (Doxium; batch number 23843) were used. The treatment regimen consisted of three capsules per day of calcium dobesilate or matching placebo" Comment: Placebo were capsules administered at the same posology as Dobesilate |
| Blinding (study researchers) | Low risk | Quote: "Capsules containing 500 mg of calcium dobesilate (Doxium; batch number 23843) were used. The treatment regimen consisted of three capsules per day of calcium dobesilate or matching placebo" Comment: Placebo were capsules administered at the same posology as Dobesilate |
| Blinding (outcome assessment) | Low risk | Quote: "Capsules containing 500 mg of calcium dobesilate (Doxium; batch number 23843) were used. The treatment regimen consisted of three capsules per day of calcium dobesilate or matching placebo" Comment: Placebo were capsules administered at the same posology as Dobesilate |
| Incomplete outcome data | High risk | Comment: 14.8% of the randomised participants were lost during follow‐up and major protocol violations were reported for 42.4% of the randomised participants. |
| Selective reporting | Low risk | Comment: The outcomes specified in the protocol were reported |
| Other bias | Low risk | Comment: none detected |
Renton 1994.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 9/40 (22.5%) |
|
| Participants | Country: UK Setting: ambulatory Number: 40 patients Age: 'adults' Gender: not stated Inclusion criteria: ankle oedema due to mild to moderate venous hypertension Exclusion criteria: peripheral arterial disease, diabetes or normal Doppler ultrasound |
|
| Interventions | Treatment: hidroxirutoside 500 mg × 2 capsules twice a day Control: placebo Duration: 30 days Follow‐up: 30 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After the final examination, the patients were randomised to receive either...." Comment: no method of randomisation described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods of allocation concealment described |
| Blinding (patients) | Low risk | Quote: "The patients were randomised to receive either 2 tablets of 500mg HR twice daily or a placebo of identical appearance" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "The patients were randomised to receive either 2 tablets of 500mg HR twice daily or a placebo of identical appearance" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "The patients were randomised to receive either 2 tablets of 500mg HR twice daily or a placebo of identical appearance" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described, along with information about the most important characteristics and inclusion and exclusion criteria. In addition, study author described the number of participants who experienced adverse events and the number who withdrew prematurely from the study, including reasons for dropping out |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Rose 1970.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, cross‐over, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: not stated Losses to follow‐up: 39% (13/33) |
|
| Participants | Country: UK Setting: hospital Number: 33 patients Age: not stated Gender: not stated Inclusion criteria: CVI associate with varicose disorders or postphlebitic syndrome Exclusion criteria: not stated |
|
| Interventions | Treatment: hidroxirutoside 1200 mg per day Control: placebo Duration: 180 days Follow‐up: 270 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no methods of random sequence generation described |
| Allocation concealment (selection bias) | Low risk | Quote: "The active and the placebo material were numbered in randomised order" Comment: Randomised order prevented knowledge of treatment in advance |
| Blinding (patients) | Low risk | Quote: "The key to the active and placebo capsules was not broken until January of this year, when all the patients had completed treatment for over 6 months" |
| Blinding (study researchers) | Low risk | Quote: "The key to the active and placebo capsules was not broken until January of this year, when all the patients had completed treatment for over 6 months" |
| Blinding (outcome assessment) | Low risk | Quote: "The key to the active and placebo capsules was not broken until January of this year, when all the patients had completed treatment for over 6 months" |
| Incomplete outcome data | High risk | Comment: 39% (13/33) losses; imbalance between groups at the end of follow‐up (17 participants received hidroxirutoside; 8 received placebo) |
| Selective reporting | High risk | Comment: results by symptom before the cross‐over not reported |
| Other bias | Low risk | Comment: none detected |
Rudofsky 1989.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: randomisation stratified by centre Exclusions post randomisation: none Losses to follow‐up: 10/151 (7%) |
|
| Participants | Country: Germany Setting: hospital Number: 151 patients Age: mean 49.7 (range 21 to 73) years Gender: not stated Inclusion criteria: stage I and II CVI, primary varicosis and post‐thrombotic symptoms Exclusion criteria: morning oedema (stage I to II CVI); acute thrombosis; leg ulcer; other peripheral arterial occlusive disorders; heart failure; severe cardiac arrhythmia; severe hypertension; diuretics; dihydroergotamine products; pregnancy |
|
| Interventions | Treatment: ruscus extract plus hesperidinmethylchalcone × 2 capsules 3 times per day for 4 weeks, then 2 capsules twice per day for 8 weeks Control: placebo Duration: 56 days Follow‐up: 56 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Following randomisation, stratified by centre, the patients then received daily 3x2 capsules of identical appearance, containing either active drug or placebo" Comment: no method of randomisation described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method of allocation concealment described |
| Blinding (patients) | Low risk | Quote: "Following randomisation, stratified by centre, the patients then received daily 3×2 capsules of identical appearance, containing either active drug or placebo" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "Following randomisation, stratified by centre, the patients then received daily 3×2 capsules of identical appearance, containing either active drug or placebo" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "Following randomisation, stratified by centre, the patients then received daily 3x2 capsules of identical appearance, containing either active drug or placebo" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described, along with important characteristics and inclusion and exclusion criteria. Number of patients who withdrew prematurely described, but no information on the reasons why participants dropped out |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Schultz‐Ehrenburg 1993.
| Study characteristics | ||
| Methods | Study design: 2 prospective, multi‐centre, randomised, double‐blind, placebo‐controlled trials Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 7/55 (13%) |
|
| Participants | Country: France Setting: outpatient Number: 55 patients Age: 'adults' Gender: not stated Inclusion criteria: unilateral venous leg ulcers and chronic venous insufficiency (deep or superficial) Exclusion criteria: not stated |
|
| Interventions | Treatment
Control: placebo Duration: 84 days Follow‐up: 84 days All participants received pressure bandaging |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Data extraction possible only in trial A | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Two prospective, multicentre, double‐blind, randomized, parallel, placebo‐controlled trial" Comment: no method of randomisation stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method of allocation concealment stated |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described, along with number of losses, but not reasons |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Sentou 1984.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled trial Method of randomisation: not stated Exclusions post randomisation: not stated Losses to follow‐up: 1 participant |
|
| Participants | Country: France Setting: ambulatory Number: not stated Age: 34.6 ± 9.18 years active product; 38.2 ± 12.44 years placebo Gender: female Inclusion criteria: slight or moderate varicose disease Exclusion criteria: surgical indication or trophic disorders, other vasoactive drugs |
|
| Interventions | Treatment: extract Ruscus aculeatus 450 mg plus hesperidin 450 mg plus ascorbic acid 300 mg per day (Cyclo 3: 3 capsules twice per day) Control: placebo Duration: 28 days Follow‐up: 20 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Number of included participants not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The allocation of the subjects to the Cyclo 3 and placebo groups was done at random, in a blind manner, according to the order of admission in the study" Comment: no method of randomisation described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method of allocation concealment described |
| Blinding (patients) | Low risk | Quote: "Cyclo 3® and the placebo had an identical appearance" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "Cyclo 3® and the placebo had an identical appearance" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "Cyclo 3® and the placebo had an identical appearance" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | High risk | Comment: number of included participants not specified. Only 1 participant did not accomplish the study protocol |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Serralde 1990.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled trial Method of randomisation: not stated Exclusions post randomisation: not losses Losses to follow‐up: none |
|
| Participants | Country: Mexico Setting: hospital Number: 52 patients Age: 42.4 ± 11.6 years active treatment; 42.3 ± 8.4 years placebo Gender: 11 M:41 F Inclusion criteria: CVI and oedema Exclusion criteria: venoactive drugs, diuretics, anti‐inflammatories and steroid drugs; elastic stockings or bandages; other causes of oedema; superficial thrombophlebitis; venous ulcer; venous surgery; pregnant women |
|
| Interventions | Treatment: oxirutosides 1000 mg per day Control: placebo Duration: 28 days Follow‐up: 56 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The trial was double‐blind, randomized, placebo‐controlled dose of one tablet of 500 mg twice daily HR or identical placebo" Comment: method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation concealment unclear |
| Blinding (patients) | Low risk | Quote: "The trial was double‐blind, randomized, placebo‐controlled dose of one tablet of 500 mg twice daily HR or identical placebo" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "The trial was double‐blind, randomized, placebo‐controlled dose of one tablet of 500 mg twice daily HR or identical placebo" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "The trial was double‐blind, randomized, placebo‐controlled dose of one tablet of 500 mg twice daily HR or identical placebo" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in both groups described, along with inclusion and exclusion criteria and the most important characteristics. Adverse events presented. No losses |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Thebaut 1985.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled trial Method of randomisation: throwing dice Exclusions post randomisation: not stated Losses to follow‐up: 14/92 (15%) |
|
| Participants | Country: France Setting: ambulatory Number: 92 patients Age: 38.9 ± 10.1 years active treatment; 40.7 ± 11.4 years placebo Gender: 8 M:63 F Inclusion criteria: CVI with leg heaviness or paraesthesias (16 to 65 years old) Exclusion criteria: venoactive drugs, elastic stockings or bandages; deep venous insufficiency by echo Doppler, venous complications, postphlebitic syndrome |
|
| Interventions | Treatment: grape seed extract tablets 300 mg every 8 hours Control: placebo Duration: 28 days Follow‐up: 28 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The method chosen was that of a controlled trial conducted a double‐blind placebo‐controlled with throwing dice assigned treatment" Comment: Throwing dice method seems to be a fair method for generating a random sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information about allocation concealment provided |
| Blinding (patients) | Low risk | Quote: "Two parallel groups were thus formed, the E group received 300mg per day of Endolelon three times daily, P group receiving placebo ‐ in all respects identical to the active pills ‐ and using the same frequency" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "Two parallel groups were thus formed, the E group received 300mg per day of Endolelon three times daily, P group receiving placebo ‐ in all respects identical to the active pills ‐ and using the same frequency" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "Two parallel groups were thus formed, the E group received 300mg per day of Endolelon three times daily, P group receiving placebo ‐ in all respects identical to the active pills ‐ and using the same frequency" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Unclear risk | Comment: number of participants in each group described, along with inclusion and exclusion criteria and the most important characteristics. Information about participants who withdrew prematurely described. In addition, standard deviation lacking in the results |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Tsouderos 1989.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled trial Method of randomisation: not stated Exclusions post randomisation: not stated Losses to follow‐up: 4 participants |
|
| Participants | Country: France Setting: hospital Number: 40 patients Age: 'adults' Gender: not stated Inclusion criteria: functional CVI Exclusion criteria: not stated |
|
| Interventions | Treatment: diosmine 450 mg plus hesperidin 50 mg per 12 hours Control: placebo Duration: 60 days Follow‐up: 60 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | This publication describes 3 clinical trials. Only 1 is included here. The others are phase 2 clinical trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All the studies were conducted double blind, according to the methodology of controlled trials" Comment: no method of randomisation described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method of allocation concealment described |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Low risk | Comment: 2 participants lost in each group, but reasons not explained |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Unkauf 1996.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, parallel, placebo‐controlled trial Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 23/133 (17%) |
|
| Participants | Country: Germany Setting: outpatients Number: 133 patients Age: mean 58.9 ± 8.6 years active group; mean 60.6 ± 10.0 years placebo group Gender: 133 F Inclusion criteria: CVI grade II (according to Widmer) Exclusion criteria: premenstrual syndrome oedema; acute phlebitis or thrombosis; cardiac insufficiency or peripheral arterial disease; other venotonic drugs, laxatives, theophylline, diuretics, cardiac glycosides, angiotensin‐converting enzyme or calcium antagonist within preceding 8 days; changes in postmenopausal hormone therapy within preceding 2 months |
|
| Interventions | Treatment: oxerutins 1000 mg per day Control: placebo Duration: 90 days Follow‐up: 90 days All participants received standard compression stockings |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study had a double‐blind, randomised, multi‐centered, paralel‐group design with two treatment groups" Comment: no method of randomisation described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method of allocation concealment described |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described, along with the most important characteristics and inclusion and exclusion criteria. ITT analysis conducted. Information about adverse events, exclusion after randomisation and loss to follow‐up given |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Vanscheidt 2002a.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled trial Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 52/231 (22.5%) |
|
| Participants | Country: Germany Setting: university Number: 231 patients Age: mean 55.1 (range 23 to 78) years Gender: 48 M:183 F Inclusion criteria: stages 3 to 5 of CEAP Exclusion criteria: surgical treatment of CVI; heart insufficiency; arterial occlusive disease; diabetes mellitus; neuropathy; acute thrombosis; lymphoedema; renal insufficiency or impaired liver function; malignant disease; pregnancy or breast feeding; major surgery; drugs with influence on the veins |
|
| Interventions | Treatment: SB‐LOT (15 mg coumarin and 90 mg troxerutin) 2 tablets 3 × per day for 16 weeks Control: placebo Duration: 112 days Follow‐up: 112 days All participants received standard compression stockings during first 4 weeks |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation schedule was generated by the validated PC programme RanCode plus, independently to all study participants. It was based on blocks of 4 patients. All medication was pre‐numbered and distributed to the centres" Comment: computer‐generated table of random numbers ensures a random sequence of participants |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were included in the study by receiving the next consecutive random number. For each patient the study centres were supplied sealed envelopes with the treatment group information" Comment: sealed envelopes and allocation of participants by giving the next consecutive random number ensure fair allocation concealment |
| Blinding (patients) | Low risk | Quote: "Placebo tablets matched the active tablets in taste, smell and appearance" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "Placebo tablets matched the active tablets in taste, smell and appearance" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "Placebo tablets matched the active tablets in taste, smell and appearance" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described, along with the most important characteristics and inclusion and exclusion criteria. In addition, study author stated the number of participants who withdrew from the study prematurely or were excluded after randomisation (22.5%). ITT analysis conducted |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Vanscheidt 2002b.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled trial Method of randomisation: computer‐generated random number table Exclusions post randomisation: not stated Losses to follow‐up: 56/167 (34%) |
|
| Participants | Country: Germany Setting: university Number: 167 patients Age: mean 53.2 ± 13.3 years active group; mean 53 ± 10.9 years placebo group Gender: 166 F Inclusion criteria: stages I and II of Widmer or CEAP 3 to 4 Exclusion criteria: other diseases with oedema, compression therapy for the past 6 months before the study; support stockings; patients more than 30% overweight; any concomitant medication that may interfere with study treatment |
|
| Interventions | Treatment: Ruscus aculeatus 72 to 75 mg per day Control: placebo Duration: 90 days Follow‐up: 90 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was designed as a multi‐center, double‐blind, randomized, placebo‐controlled trial with women suffering from chronic venous insufficiency..." Comment: no method of randomisation described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method of allocation concealment described |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | High risk | Comment: number of participants in each group described, along with important characteristics and inclusion and exclusion criteria. In addition, number of participants who withdrew prematurely described, but percentage was important (34%) and no ITT analysis performed |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Vin 1994.
| Study characteristics | ||
| Methods | Study design: multi‐centre, randomised, double‐blind, placebo‐controlled with a placebo run‐in period Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 4/73 (4%) |
|
| Participants | Country: France Setting: hospital Number: 73 patients Age: mean 55.7 ± 15.8 years active treatment; mean 53.6 ± 16.7 years placebo Gender: 10 M:59 F Inclusion criteria: presence of truncal varicose veins with ostial reflux and subjective symptoms of venous origin Exclusion criteria: occlusive arterial disease; osteoarticular disease; diabetes; acute or chronic inflammatory syndromes; haematological diseases; venoactive drugs; pregnancy; smoking |
|
| Interventions | Treatment: troxerutin 3500 mg per day Control: placebo Duration: 60 days Follow‐up: 60 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was controlled, double‐blind, randomized, multicentre and with a placebo run‐in period" Comment: no method of randomisation stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method of allocation concealment described |
| Blinding (patients) | Low risk | Quote: "They were then randomly allocated to receive either troxerutin, 3500 mg to be taken in the morning for 2 months, or a placebo with identical appearance and taste" Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "They were then randomly allocated to receive either troxerutin, 3500 mg to be taken in the morning for 2 months, or a placebo with identical appearance and taste" Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "They were then randomly allocated to receive either troxerutin, 3500 mg to be taken in the morning for 2 months, or a placebo with identical appearance and taste" Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described, along with the most important characteristics and inclusion and exclusion criteria. In addition, information about participants who withdrew prematurely given, including reasons for dropping out. Adverse events given as well |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Welch 1985.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: not stated Exclusions post randomisation: none Losses to follow‐up: 7/147 (5%) |
|
| Participants | Country: Belgium Setting: hospital Number: 147 patients Age: mean 44.5 ± 14 years active group; mean 43.6 ± 14 years placebo group Gender: 26 M:119 F Inclusion criteria: CVI with oedema and ≥ 1 related symptom Exclusion criteria: elastic stockings or compressive bandages; leg oedema from another origin; arterial insufficiency; superficial thrombophlebitis; varicose eczema or ulcer; diuretics, analgesics, steroids, NSAIDs or other venous drugs; pregnancy |
|
| Interventions | Treatment: O‐(beta‐hydroxyethyl)‐rutoside 1000 mg per day Control: placebo Duration: 28 days Follow‐up: 28 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not given |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation concealment not given |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described, and inclusion and exclusion criteria reported as well for the most important characteristics. Number of participants who dropped out prematurely given, along with numbers of and reasons for adverse events |
| Selective reporting | Low risk | Comment: protocol identified and no differences identified between protocol and article |
| Other bias | Low risk | Comment: none detected |
Widmer 1990.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: randomisation list prepared by statistician Exclusions post randomisation: none Losses to follow‐up: 17/225 (7%) |
|
| Participants | Country: Switzerland Setting: hospital Number: 225 patients Age: 20 to 70 years Gender: 27 M:181 F Inclusion criteria: CVI grade I to II (alterations in pigmentation, with or without subcutaneous veins, oedema and symptoms of the disease) Exclusion criteria: CVI grade III with open or healed varicose ulcer; venous surgery during past 12 months or sclerotherapy during past 6 months; symptomatic peripheral arterial occlusion; renal or cardiac insufficiency; lymphoedema; diabetes; hypertension; overweight; pregnancy; compression therapy or drugs that might interfere with clinical results (diuretics); intolerance to the active drug of the study |
|
| Interventions | Treatment: calcium dobesilate 1500 mg per day Control: placebo Duration: 28 days Follow‐up: 28 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were treated for 28 days with either Doxium or placebo at the dosage of 3 capsules daily, according to a randomization list prepared by the statistician" Comment: randomisation list assumed to be a fair method of assuring a random sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods described for allocation concealment |
| Blinding (patients) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (study researchers) | Unclear risk | Comment: no information given about methods used for blinding |
| Blinding (outcome assessment) | Unclear risk | Comment: no information given about methods used for blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described, including most important characteristics and inclusion and exclusion criteria. In addition, reasons for excluding participants after randomisation given, along with number of participants. Number compliant with medication provided, along with adverse events |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
Zucarelli 1987.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Method of randomisation: throwing dice Exclusions post randomisation: none Losses to follow‐up: 25/149 (16%) |
|
| Participants | Country: France Setting: outpatients Number: 149 patients Age: mean 33 ± 9.4 years active treatment; mean 32 ± 8 years placebo Gender: 149 F Inclusion criteria: CVI stage I (functional symptoms and oedema) Participants allowed to wear elastic support Exclusion criteria: chronic venous with trophic alterations; varices; phlebitis; postphlebitic syndrome; lymphoedema; arteriopathy; pregnancy; other phlebotonics; anti‐inflammatories; diuretics; anti‐platelet or vasculo‐protector treatments |
|
| Interventions | Treatment: coumarin 10.5 mg per day plus troxerutin 1050 mg per day Control: placebo Duration: 90 days Follow‐up: 90 days |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The methodology used was that of a controlled trial against placebo in double‐blind perspective with the drawing of lots to constitute two parallel groups" Comment: Drawing of lots seems like a fair method of generating an adequate sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods of allocation concealment described |
| Blinding (patients) | Low risk | Quote: "Ampules of placebo, which are in all respects comparable to those of the active..." Comment: Identical placebo ensures double‐blinding |
| Blinding (study researchers) | Low risk | Quote: "Ampules of placebo, which are in all respects comparable to those of the active..." Comment: Identical placebo ensures double‐blinding |
| Blinding (outcome assessment) | Low risk | Quote: "Ampules of placebo, which are in all respects comparable to those of the active..." Comment: Identical placebo ensures double‐blinding |
| Incomplete outcome data | Low risk | Comment: number of participants in each group described, along with inclusion and exclusion criteria and the most important characteristics. In addition, tolerance, adverse events and participants who dropped out prematurely described |
| Selective reporting | Low risk | Comment: no protocol identified, and no differences between outcomes reported in the methods section and those reported in the results section |
| Other bias | Low risk | Comment: none detected |
BMI: body mass index CEAP classification (clinical signs (C), aetiology (E), anatomical distribution (A) and physiological conditions (P) of CVI) CIVIQ: Chronic Venous Insufficiency International Questionnaire CT: clinical trial CVD: cardiovascular disease CVI: chronic venous insufficiency DVT: deep vein thrombosis EuroQoL: Descriptive system of health‐related quality of life states FLQA: Freiburg Life Quality Assessment h: hour ITT: intention‐to‐treat LRR: light reflection rheography MPL: most pathological leg NSAIDs: non‐steroidal anti‐inflammatories QoL: quality of life tid: 3 times a day VAS: visual analogue scale WDV: water displacement volumetry
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akbulut 2010 | This study assessed the combination of calcium dobesilate and oxerutin |
| Androulakis 1989 | Principal outcome consists of plethysmographic parameters ‐ a surrogate outcome |
| Auteri 1990 | No clinical endpoints were assessed |
| Bacci 2003 | This study assessed a combination of different active products |
| Bastide 1976 | This study assessed dihydroergotamine, which is not included in our review |
| Batchvarova 1989a | This study assesses a product with escin, which is not included in our review |
| Behar 1993 | This study assesses a product with escin, which is not included in our review |
| Belcaro 1989 | This was a single‐blind study |
| Belcaro 1995 | Outcomes studied were surrogates (laser Doppler and transcutaneous oximetry) |
| Belcaro 2008 | No clinical endpoints were assessed ‐ only microcirculatory parameters |
| Bello 1990 | Calcium dobesilate was combined with a heparinoid |
| Bento 2006 | This study assessed a combination of different active products that contain escin |
| Berson 1978 | Two clinical trials are described. One was a non‐controlled clinical trial, and in the other, the control group was given naftazone |
| Blume 1996 | Inadequate blinding: Initial phase of the trial used 'placebo' that was actually a low concentration of the assessed active drug: coumarin 2 mg and rutoside 100 mg |
| Bohm 1989 | This study assessed the combination of a diuretic and a drug for CVI |
| Boisseau 1995 | Outcomes were not applicable to this review: Biological parameters were measured (erythrocyte aggregation and fibrinolytic activity) |
| Bolliger 1972 | This study assessed the combination of dimethyl sulfoxide and diphenyl butazone with a rutoside |
| Bort 1995 | No clinical endpoints were assessed ‐ only microcirculatory parameters |
| Bosse 1985 | This study compared 2 drugs (Venalot ‐ combination of coumarin and troxerutin ‐ and Benzarone) for CVI |
| Brami 1983 | This study assessed the efficacy of a combination of dyhigroergocriptine mesilate and caffeine for CVI |
| Carstens 1985 | This study assessed the combination of a diuretic and escin (DIU Venostatin) |
| Cataldi 2001 | The drug studied was a combination of several active principles, one of which was rutin |
| Cesarone 1992 | No clinical endpoints were assessed ‐ only microcirculatory parameters |
| Cesarone 1994 | No clinical endpoints were assessed ‐ only microcirculatory parameters |
| Cesarone 2001 | No clinical endpoints were assessed ‐ only microcirculatory parameters |
| Cesarone 2001a | This was a single‐blind study |
| Cesarone 2001b | This study assessed escin in diabetic microangiopathy |
| Cesarone 2001c | The study was about microvascular parameters: PO2, PCO2 and volume parameters. This was a single‐blind study |
| Cesarone 2002b | This study assessed variations in plasma free radicals in participants with CVI |
| Cesarone 2010 | This study was not double‐blinded |
| Chant 1973 | Non‐clinical criteria were given |
| Chiummariello 2009 | The drug evaluated is a combination of different products for CVI. This study was not double‐blinded |
| Clemens 1986 | Only haemodynamic venous parameters were assessed by light reflection rheography |
| Cospite 1996 | This study compared heparan sulphate vs diosmine for CVI |
| De Anna 1989 | This was a single‐blind study |
| Delacroix 1981 | The drug evaluated was escin, which has been excluded from our review |
| Delecluse 1991 | This study compared Diovenor versus a combination of flavonoids |
| de Parades 1990 | This study compared Cyclo 3 Fort vs diosmine plus hesperidin for CVI |
| De Sanctis 2001 | This was a single‐blind study |
| Duchene 1988 | Only haemodynamic venous parameters were assessed by plethysmography |
| Dustmann 1984 | The drug evaluated was escin, which has been excluded from our review |
| Erdlen 1989 | Venostasin contains escin, which has been excluded from our review |
| Erler 1991 | This study assessed escin, which has been excluded from our review |
| EudraCT2009‐014681‐25 | The outcome (reflux) is not included in our review. The comparison are different doses of Ruscus aculeatus (150 mg), hesperidin methyl chalcone (150 mg) and ascorbic acid (100 mg) |
| Forconi 1977 | No clinical endpoints were assessed ‐ only microcirculatory parameters |
| Frausini 1985 | This was a single‐blind study |
| Glinski 1999 | This was an open RCT conducted to examine venous ulcers |
| Gonzalez‐Fajardo 1990 | The outcome assessed was a surrogate (photoplethysmographic evaluation) |
| Granger 1995 | It is not specified that the trial was double‐blind |
| Henriet 1995 | This study compared the efficacy of Diovenor (diosmine) vs a combination of different flavonoids |
| Horvath 1985 | This study assessed the efficacy of dyhidroergotamine, which is not included in our review |
| Incandela 1995 | No clinical endpoints were assessed ‐ only microcirculatory parameters |
| Incandela 1996 | This study looked at the effects of troxerutin on microcirculatory parameters |
| Incandela 2001 | This was a single‐blind study |
| Incandela 2002 | This was a single‐blind study |
| ISRCTN54360155 | Different drugs combinations (acetyl salicylic acid, asiaticoside and acemannan) and there is not a placebo group. |
| Janssens 1999 | No clinical endpoints were assessed ‐ only microcirculatory parameters |
| Janssens 1999a | This study looked at the effects of Ginkor Fort (ginkgo biloba), which is not included in our review |
| Kalus 2004 | No clinical endpoints were assessed ‐ only microcirculatory parameters (cutaneous microcirculation and oxygen supply) |
| Kiesewetter 2000 | This study evaluated red vine leaf extract, an herbal medicine containing several flavonoids that are not included in our review |
| Koltringer 1993 | This study assessed Ginkgo biloba, which is not included in our review |
| Kostering 1985 | This study assessed microcirculatory parameters |
| Krähenbühl 1975 | The bencyclan is a drug with cardiovascular depression effects; it is not included in the review |
| Krcílek 1973 | The drug evaluated was escin, which is not included in our review |
| Languillat 1988b | The drug studied (Veliten) was a combination of rutin, ascorbic acid and alpha‐tocopherol. No clinical endpoints were assessed ‐ only microcirculatory parameters |
| Languillat 1989 | No clinical endpoints were assessed ‐ only microcirculatory parameters |
| Le Dévéhat 1989 | Outcomes were not applicable to this review: microcirculatory and haemorrheological parameters |
| Le Dévéhat 1997 | This study assessed troxerutine for CVI: microcirculatory and haemorrheological parameters |
| Marastoni 1982 | This study assessed dihydroergotamine, which is not included in our review |
| Menyhei 1994 | No placebo group was included |
| Monteil‐Seurin 1993 | This study compared Cyclo 3 Fort vs diosmine |
| Morales 1993 | This RCT assessed escin, which is not included in our review |
| Naser‐Hijazi 2004 | This RCT assessed the combination of coumarin and troxerutin (SB‐LOT) in CVI. The objective of this study was to assess effects of SB‐LOT on blood coagulation |
| NCT01654016 | This is an ongoing single‐blinded (outcome assessor) clinical trial about Daflon |
| NCT02191163 | This study assessed the efficacy of the red‐vine‐leaf extract, which is not included in our review (Antistax) |
| NCT02191254 | This study assessed the efficacy of the red‐vine‐leaf extract, which is not included in our review (Antistax) |
| NCT02191280 | This study assessed the efficacy of the red‐vine‐leaf extract, which is not included in our review (Antistax) |
| Neumann 1988 | No clinical endpoints were assessed ‐ only microcirculatory parameters |
| Neumann 1990 | Only haemodynamic venous parameters were assessed by light reflection rheography and transcutaneous oxygen tension measurement (TcPO2) |
| Neumann‐Mangoldt 1979 | The drug evaluated contained escin and heparin |
| Nill 1970 | This study assessed escin, which is not included in our review |
| Ottillinger 2001 | This study assessed escin, which is not included in our review |
| Paciaroni 1982 | The drug evaluated was escin, which is not included in our review |
| Partsch 1981 | This study assessed oral dyhidroergotamine, which is not included in our review |
| Paul 1983 | The drug evaluated was benzarone, which is not included in our review |
| Pauschinger 1987 | The drug evaluated was escin, which is not included in our review |
| Petruzzellis 2002 | This study included 3 comparative groups (2 of different doses of oxirutoside and 1 of placebo), but treatment concealment was incorrect or was not explained correctly |
| Pointel 1987b | This study assessed vitamin C combined with Ruscus aculeatus and anthocyanosides from Ribes nigrum (helps to maintain the integrity of capillaries) |
| Pokrovskii 2005 | This study assessed Ginkgo biloba, which is not included in our review |
| Questel 1983 | No clinical endpoints were assessed ‐ only microcirculatory parameters |
| Rabe 2011b | This study assessed the efficacy of the red‐vine‐leaf extract, which is not included in our review (Antistax) |
| Riccioni 2004 | This study assessed the efficacy of the combination of troxerutin plus French maritime pine bark |
| Roztocil 1977 | This study assessed microcirculatory parameters (capillary filtration) |
| Roztocil 2003 | This was an RCT that was not blinded |
| Sanctis 2001 | This study assessed escin, which is not included in our review |
| Seydewitz 1992 | Non‐clinical parameters were evaluated in this study |
| Steiner 1990 | This study assessed the drug escin, which is not included in our review |
| Steiner 1992 | This study assessed the drug escin, which is not included in our review |
| Steru 1988 | It is not specified whether this trial was double‐blind |
| Topalov 1990 | This study assessed the efficacy of troxesamol (combination of troxerutin, acetylsalicylic acid and dipyridamole) |
| Turio 2000 | This study assessed the efficacy of a combination of vitamin PP (niacin), vitamin C and phyto‐therapeutic extracts titrated in escin, bromelain and anthocyanosides |
| Weindorf 1987 | This study assessed the efficacy of the combination of Ruscus aculeatus and trimethylhespiridinchalcone |
| Widmer 1972 | The active treatment in this study was phlebolan composed of rutin and several anti‐inflammatory agents such as prednisolone and diphenylbutazone |
| Zuccarelli 1996 | This study assessed GinKor Fort (Ginkgo biloba), which is not included in our review |
CVI: chronic venous insufficiency HR: hidroxy rutoside PO2: pressure of oxygen in blood PCO2: pressure of carbon dioxide in blood RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Barattini 2019.
| Study name | Clinical trial to assess the efficacy of μSmin® Plus (dietary supplement) |
| Methods | Multicentre, randomised, double‐blind, parallel, placebo‐controlled trial |
| Participants | 68 participants adults with CVI between C2‐C4 on the CEAP classification system |
| Interventions | 1 tablet of μSMIN® Plus (corresponding to 450 mg of micronized diosmine) or placebo per day during 8 weeks |
| Outcomes | QoL (CIVIQ‐20 questionnaire), VAS pain scale, CVI symptomatology, and change in the circumference of the affected leg at calf level, investigators and patient global assessment, percentage of subjects who would want to continue with the treatment, treatment compliance and safety |
| Starting date | 24 September 2019 |
| Contact information | Contact: Dionisio Franco Barattini, MD Contact: Dumitru‐Emanuel Dogaru, PM |
| Notes |
ISRCTN18841175.
| Study name | Effects of micronised purified flavonoid fraction on microcirculation in women suffering from CVD |
| Methods | Single‐centre double‐blind randomised placebo‐controlled parallel‐group study |
| Participants | 240 females 18 to 30 years old suffering from primary CVD |
| Interventions | Micronised purified flavonoid fraction 500 mg over 4 menstrual cycles versus placebo |
| Outcomes | Effects on microcirculatory and biological parameters over 4 menstrual cycles |
| Starting date | July 2009 |
| Contact information | Prof Eliete Bouskela. Instituto de Biologia Roberto Alcantara Gomes Dept Ciências Fisiologicasências. Rio de Janeiro. Brazil |
| Notes | Sponsor: Institut de Recherches Internationales Servier (France) |
NCT01532882.
| Study name | Efficacy and safety of diosmine 600 mg versus placebo for painful symptoms in patients with CVD of lower limbs (EDEN) |
| Methods | Multi‐centre controlled randomised double‐blind placebo‐controlled parallel‐group study |
| Participants | 378 patients with painful symptoms of CVD of the lower limbs |
| Interventions | Diosmine 600 mg ‐ DIOVENOR versus placebo (1 tablet per day during 28 days) |
| Outcomes | Primary outcome measure: Change in VAS score for assessment of painful venous symptoms |
| Starting date | January 2012 |
| Contact information | Dr Jean‐Jérôme GUEX, Nice, France |
| Notes | Sponsor: Innotech International |
NCT03833024.
| Study name | The MUFFIN‐PTS Trial |
| Methods | Multicentre, randomised, double‐blind placebo‐controlled trial |
| Participants | 86 participants adults with PTS; Villalta score > 4 with at least two of the following four PTS manifestations (daily heaviness, cramps, pain, and objective oedema) in the leg ipsilateral to a previous objectively diagnosed DVT, or DVT of unknown date but with presence of residual proximal or distal venous obstruction on ultrasound |
| Interventions | Micronized Purified Flavonoid Fraction (MPFF) or placebo for 6 months MPFF 500 mg, bid (morning and evening) for 6 months in addition to their usual treatment (i.e. ECS and/or anticoagulation). |
| Outcomes | Symptoms and signs of PTS and QoL (EQ‐5D‐5L and EQ VAS) measured at 3, 6 and 9 months follow‐up Primary outcome: Change in PTS (6 months). Improvement will be defined as a decrease of at least 30% in the Villalta score or a Villalta score < 5 in the PTS‐affected leg |
| Starting date | 1 December 2019 |
| Contact information | Dr. Susan Kahn, Sir Mortimer B. Davis ‐ Jewish General Hospital, Montreal, Canada |
| Notes |
bid: twice daily CEAP: Clinical‐Etiology‐Anatomy‐Pathophysiology CVD: chronic venous disease CVI: chronic venous insufficiency DVT: deep vein thrombosis ECS: elastic compression stockings mg: milligrams QoL: quality of life PTS: postthrombotic syndrome VAS: visual analogue scale
Differences between protocol and review
2020 version:
We reviewed all previously excluded studies. In keeping with recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), where these meet the definition of 'not relevant,' we removed them and re‐classified them as excluded studies.
2016 version:
In the protocol, we established different assumptions to examine adverse events. In this current review, we simplified the analyses. We calculated the risk of adverse events by considering the number of participants with adverse events reported in the papers as the numerator and the number of participants randomised by group as the denominator.
In the protocol, we considered the Jadad scale (Jadad 1996) and the Cochrane criteria (Clarke 2003) to assess the risk of bias of included RCTs. In this current review, we used only the current Cochrane criteria to assess risk of bias (Higgins 2011).
In the protocol, we considered statistical heterogeneity of P < 0.1 as a reason for not pooling results of the studies. In this current review, we used the I2 statistic and considered I2 > 75% a reason for not pooling the results of RCTs.
In the protocol, we specified to use a random‐effects statistical model in all analyses. In this current review, however, we used this model only when I2 was between 50% and 75%.
In the protocol, we performed a sensitivity analysis by level of quality of studies according to the Cochrane criteria (Clarke 2003). In this current review, we performed a sensitivity analysis that included only studies with low risk of bias according to the Cochrane risk of bias (Higgins 2011).
In the protocol, assessment of publication bias was not specified. In this current review, we constructed a funnel plot to explore publication bias.
In the protocol, the quality of evidence was assessed by the Cochrane criteria. In this current review, we applied GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) (Schünemann 2011) criteria and presented a 'Summary of findings' table (Table 1).
Contributions of authors
MJM: assessed the risk of bias and extracted data for the new studies of this update; responsible for statistical and methodological aspects and for overall compiling of this review; responsible for manuscript development and revision of this review;
RWMV: screened the search of this update; assessed the risk of bias, GRADE and extracted data for the new studies of this update; responsible for manuscript development and revision of this review
DS: screened the search of this update; assessed the risk of bias, GRADE and responsible for manuscript development and revision of this SR
SMU: responsible for manuscript development and revision of this review
ATS: responsible for manuscript development and revision of this review
RMM: provided clinical experience and insight on the protocol and review reports
EV: responsible for manuscript development and revision of this review
XBC: responsible for manuscript development and revision of this review
Sources of support
Internal sources
Iberoamerican Cochrane Centre, Spain
External sources
-
The Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
-
Instituto de Salud Carlos III, Spain
Dr. Mª José Martinez Zapata is funded by a Miguel Servet research contract from the Instituto de Salud Carlos III (CP15/00116).
Declarations of interest
MJM: none known
RWMV: none known
DS: none known
SMU: none known
ATS: none known
RMM: none known
EV: none known
XBC: none known
Dr RM Moreno, Dr X Bonfill Cosp and Dr MJ Martínez‐Zapata were authors of a published double‐blind, placebo‐controlled clinical trial (Martinez‐Zapata 2008) that is included in this review. This study was supported by Laboratorios Dr Esteve, which markets calcium dobesilate (Doxium). Laboratorios Dr Esteve signed a written commitment to fully respect the researchers' independence and to allow dissemination of results, whatever they could be. Furthermore, Dr RM Moreno, Dr X Bonfill Cosp and Dr MJ Martínez‐Zapata were researchers in the included clinical trial DOBESILATO500/2, which was prematurely interrupted because of lack of funding. Dr RM Moreno, Dr X Bonfill Cosp and Dr MJ Martínez‐Zapata have not been involved in study selection, data analysis, ROB and GRADE assessment of these cited clinical trials.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Allegra 1981 {published data only}
- Allegra C Pollari G, Criscuolo A, Bonifacio M, Tabassi D. Centella asiatica extract in venous disorders of the lower limbs. Comparative clinico-instrumental studies with a placebo. Clinica Terapeutica 1981;99(5):507-13. [PubMed] [Google Scholar]
Alterkamper 1987 {published data only}
- Alterkamper H. Efficacy of antivaricotic drugs can be measured objectively. Phlebologie in der Praxis 1987;2(9-10):19-20. [Google Scholar]
Arcangeli 2000 {published data only}
- Arcangeli P. Pycnogenol in chronic venous insufficiency. Fitoterapia 2000;71(3):236-44. [DOI] [PubMed] [Google Scholar]
Balmer 1980 {published data only}
- Balmer A, Limoni C. Clinical, placebo-controlled double-blind study of venoruton in the treatment of chronic venous insufficiency. Importance of the selection of patients. Vasa 1980;9(1):76-82. [PubMed] [Google Scholar]
- Blume J. Therapy of venous oedemas [Tratamento do edema de origem venosa]. Revista Brasileira de Medicina 1994;51(3):283-8. [Google Scholar]
Belczak 2014 {published data only}
- Belczak SQ, Sincos IR, Campos W, Beserra J, Nering G, Aun R. Veno-active drugs for chronic venous disease: a randomized, double-blind, placebo-controlled parallel-design trial. Phlebology 2014;29(7):454-60. [DOI] [PubMed] [Google Scholar]
Bergqvist 1981 {published data only}
- Berqvist D, Hallböök T, Lindblad B, Lindhagen A. A double-blind trial of O-(beta-hydroxyethyl)-rutoside in patients with chronic venous insufficiency. Vasa 1981;10(3):253-60. [PubMed] [Google Scholar]
Biland 1982 {published data only}
- Biland L, Blättler P, Scheibler P, Studer S, Widmer K. Zur therapie sogenannt venosër beinsbeschwerden. Vasa 1982;11(1):53-8. [PubMed] [Google Scholar]
Burnand 1989 {published data only}
- Burnand KG, Powell S, Bishop C, Stacey M, Pulvertaft T. Effect of Paroven on skin oxygenation in patients with varicose veins. Phlebologie 1989;4(1):15-22. [Google Scholar]
Casley‐Smith 1988 {published data only}
- Casley-Smith JR. A double-blind, placebo -controlled, matched-pair trial of the mode of action of 'Doxium' in the treatment of chronic venous insufficiency. In: Davy A and Stemmer R , editors(s). Proceedings of the Union Internationale de Phlebologie - 10th World Congress, Strasbourg, 25-29 September. London and Paris: John Libbey Eurotext Ltd, 1989.
- Casley-Smith JR. A double-blind, placebo-controlled, matched-pair trial of the mode of action of 'Doxium' in the treatment of chronic venous insufficiency. Phlebologie 1989;2:709-11. [Google Scholar]
- Casley-Smith JR. A double-blind trial of calcium dobesilate in chronic venous insufficiency. Angiology 1988;39(10):853-7. [DOI] [PubMed] [Google Scholar]
Cauwenberge 1972 {published data only}
- Van Cauwenberge H. Double-blind study of the efficacy of a soluble rutoside derivative in the treatment of venous disease. Archives of Internal Pharmacodynamics and Therapeutics 1972;196(Suppl 196):122-5. [PubMed] [Google Scholar]
Cauwenberge 1978 {published data only}
- Van Cauwenberge H. Double-blind clinical trial to assess the efficacy of 0-(b-hidroxy-ethyl)-rutosides in the treatment of venous disorders [Etude en double aveugle de l'efficacité de l'0-(b-hidroxyéthyl)-rutosides dans le traitement des affections veineuses]. Mèdicine et Hygiène 1978;35:4175-7. [Google Scholar]
Cesarone 2002 {published data only}
- Cesarone MR, Incandela L, DeSanctis MT, Belcaro G, Griffin M, Ippolito E, et al. Treatment of edema and increased capillary filtration in venous hypertension with HR (Paroven, Venoruton; O-(beta-hydroxyethyl)-rutosides): a clinical, prospective, placebo-controlled, randomized, dose-ranging trial. Journal of Cardiovascular Pharmacology and Therapeutics 2002;7 Suppl 1:S21-4. [DOI] [PubMed] [Google Scholar]
Chassignolle 1994 {published data only}
- Chassignolle JF, Amiel M, Lanfranchi G, Barbe R. Activite therapeutique de daflon 500 mg dans l'insuffisance veineuse fonctionnelle. Journal International de Medicine 1994;Suppl 99:32-5. [Google Scholar]
Cloarec 1994 {published data only}
- Cloarec M, Clement R, Griton P, Guillou GB, Golden G. A double blind three centre trial on efficacy of o-(beta-hydroxyethyl) rutosides in patients with venous insufficiency. International Angiology 1994;13 Suppl 1(2):74. [Google Scholar]
Cloarec 1996 {published data only}
- Cloarec M, Clément R, Griton P. A double-blind clinical trial of hydroxyethylrutosides in the treatment of the symptoms and signs of chronic venous insufficiency. Phlebology 1996;11(2):76-82. [Google Scholar]
Cornu‐Thenard 1985 {published data only}
- Cornu-Thenard A, Dahan B, De Parades B. Study of the action in venous insufficiency of the legs. Pierre Fabre Medicament Laboratoires (Novartis). Report no. DC982GEC51(2), 1985.
Danielsson 2002 {published data only}
- Danielsson G, Jungbeck C, Peterson K, Norgren L. A randomised controlled trial of micronised purified flavonoid fraction versus placebo in patients with chronic venous disease. European Journal of Vascular and Endovascular Surgery 2002;23(1):73-6. [DOI] [PubMed] [Google Scholar]
Diebschlag 1994 {published data only}
- Diebschlag W, Nocker W, Lehmacher W, Rehn D. A clinical comparison of two doses of O-(beta-hydroxyethyl) rutosides (oxerutins) in patients with chronic venous insufficiency. Journal of Pharmaceutical Medicine 1994;4(1):7-14. [Google Scholar]
DOBESILATO500/2 {unpublished data only}
- Fundación Iberoamericana Itaca. Randomized, double-blind multicenter clinical trial comparing the efficacy of calcium dobesilate with placebo in the treatment of ulcer secondaries to chronic venous disease. ClinicalTrials.gov 2009.
Dominguez 1992 {published data only}
- Dominguez C, Brautigham I, González E, González JA, Nazco J, Valiente R, et al. Therapeutic effects of hidrosmin on chronic venous insufficiency of the lower limbs. Current Medical Research and Opinion 1992;12(10):623-30. [DOI] [PubMed] [Google Scholar]
Fermoso 1992 {published data only}
- Fermoso J, Legido AG, Del Pino J, Valiente R. Therapeutic value of hidrosmin in the treatment of venous disorders of the lower limbs. Current Therapeutic Research, Clinical and Experimental 1992;52(1):124-34. [Google Scholar]
Flota‐Cervera 2008 {published and unpublished data}
- Flota-Cervera F, Flota-Ruiz C, Treviño C, Berber A. Randomized, double blind, placebo-controlled clinical trial to evaluate the lymphagogue effect and clinical efficacy of calcium dobesilate in chronic venous disease. Angiology 2008;59(3):352-6. [DOI] [PubMed] [Google Scholar]
- Flota LF. Prospective, randomised, double-blind, placebo controlled, clinical trial that assesses the efficacy of calcium dobesilate in the limphoedema by varicose disease [Estudio clínico prospectivo aleatorizado, doble ciego, con control placebo, para evaluar la eficacia en la resolución del edema de origen lifático, del dobesilato de calcio en pacientes con enfermedad varicosa]. Knoll de Mexico S.A. Laboratorios Dr. Esteve. No de proyec. Knoll-mex-02-99, 003/MEX, 99.
Gilly 1994 {published data only}
- Frileux C, Gilly R. Activité thérapeutic de Daflon 500 mg dans l'insuffisance veineuse chronique des membres inférieurs. Journal Internationale de Medicine 1994;Suppl 99:36-9. [Google Scholar]
- Gilly R, Pillion G, Frileux C. Evaluation of a new venactive micronized flavonoid fraction (S5682) in symptomatic disturbances of the venolymphatic circulation of the lower limb: a double-blind, placebo-controlled study. Phlebology 1994;9(2):67-70. [Google Scholar]
- Thiollet M, Frileux C, Gilly R. Evaluation of a new micronized diosmin in the treatment of chronic venous incompetence: a double-blind, placebo controlled trial. Journal of Vascular Surgery 1992;15(2):447. [Google Scholar]
Guilhou 1997 {published data only}
- Guilhou JJ, Dereure O, Marzin L, Ouvry P, Zuccarelli F, Debure C, et al. Efficacy of Daflon 500 mg in venous leg ulcer healing: a double-blind, randomised, controlled versus placebo trial in 107 patients. Angiology 1997;48(1):77-85. [DOI] [PubMed] [Google Scholar]
Hachen 1982 {published data only}
- Hachen HJ, Lorenz P. Double-blind clinical and plethysmographic study of calcium dobesilate in patients with peripheral microvascular disorders. Angiology 1982;33(7):480-8. [DOI] [PubMed] [Google Scholar]
Ihme 1996 {published data only}
- Ihme N, Kiesewetter H, Jung F, Hoffmann KH, Birk A, Müller A, et al. Leg oedema protection from a buckwheat herb tea in patients with chronic venous insufficiency: a single-centre, randomized, double-blind, placebo-controlled clinical trial. European Journal of Clinical Pharmacology 1996;50(6):443-7. [DOI] [PubMed] [Google Scholar]
Jongste 1986 {published data only}
- De Jongste AB, Ten Cate JW, Huisman MV. The effectiveness of o-(b-hydroxyethyl)-rutosides (HR) in the post-thrombotic syndrome (PTS). Phlebology 1986;85 Suppl 285:837-9. [Google Scholar]
Jongste 1989 {published data only}
- Jongste AB, Jonker JJC, Huisman MV, Cate JW, Azar AJ. A double-blind trial on the short-term efficacy of HR in patients with the post-thrombotic syndrome. Phlebology 1990;5(Suppl 1):21-2. [Google Scholar]
- Jongste AB, Jonker JJC, Huisman MV, den Cate JW, Azar AJ. A double-blind three center clinical trial on the short-term efficacy of O-(beta-hydroxyethyl)-rutosides in patients with post-thrombotic syndrome. Thrombosis and Haemostasis 1989;62(3):826-9. [PubMed] [Google Scholar]
Kiesewetter 1997 {published data only}
- Kiesewetter H, Koscielny J, Grützner K, Müller A, Hoffmann KH, Birk A. Buckwheat herb/troxerutin-combination for the treatment of chronic venous insufficiency. Zeitschrift für Phytotherapie 1997;18(6):341-6. [Google Scholar]
Klüken 1971 {published data only}
- Klüken N. Double-blind clinical trial to assess the therapy with drugs for venous disorders [Estudio clínico doble ciego para tratar de objetivar la terapéutica con venofármacos]. Therapiewoche 1971;21:1. [Google Scholar]
Koscielnny 1996 {published data only}
- Koscielnny J, Radtke H, Hoffmann, Jung F, Müller A, Grützner KI, et al. Fagorutin buckwheat herb tea in chronic venous insufficiency. Zeitschrift für Phytotherapie 1996;17(3):147-59. [Google Scholar]
Kriner 1985 {published data only}
- Kriner E, Braun R, Hirche H, Van Laak HH. Treatment of venous insufficiency. A double-blind trial with Phlebodril. Zeitschrift für Allgemeinmedizin 1985;61(9):309-13. [Google Scholar]
Labs 2004 {published and unpublished data}
- Jaeger K. Efficacy and safety of doxium in chronic venous insufficiency. Double-blind, placebo-controlled multicentre study. International Angiology 2001;20(2 Suppl 1):239. [PubMed] [Google Scholar]
- Jaeger K. Efficacy and safety of doxium in chronic venous insufficiency. OM PHARMA (LAB. ESTEVE). Study number: DX-1994/2.
- Labs KH, Degischer S, Gamba G, Jaeger KA, on behalf of the CVI Study Group. Effectiveness and safety of calcium dobesilate in treating chronic venous insufficiency: randomized, double-blind, placebo-controlled trial. Phebology 2004;19(3):123-9. [Google Scholar]
Languillat 1988 {published data only}
- Languillat N. Trial of Cyclo 3 Fort in venous insufficiency of the lower limbs: Xenon 133 functional investigation of venous circulatory velocity. Pierre Fabre Medicament Laboratories (Novartis). No. Report DC982GEC130(2), 1988.
Laurent 1988 {published data only}
- Laurent R, Gilly R, Frileux C. Clinical evaluation of a venotropic drug in man. Example of Daflon 500 mg. International Angiology 1988;7(2 Suppl):39-43. [PubMed] [Google Scholar]
Lazzarini 1982 {published data only}
- Lazzarini A, Danieli L. Clinical controlled trial of aminaphtone in lower limbs' phlebopathies and phlebopathic ulcers [Sperimentazione clinica controllata del l´aminaftone flebopatie degli arti inferiori e nelle flebopatiche]. Rassegna Internazionale di Clinica e Terapia 1982;62(12):825-44. [Google Scholar]
MacLennan 1994 {published data only}
- Dikland WJ. A clinical trial on the effect of hydroxyethylrutosides on venous refilling time as measured by light reflection rheography. Scripta Phlebologica 1995;3:4-7. [Google Scholar]
- MacLennan WJ, Wilson J, Rattenhuber V, Dikland WJ, Vanerdonckt J, Moriau M. Hydroxyethylrutosides in elderly patients with chronic venous insufficiency. Its efficacy and tolerability. Gerontology 1994;40(1):45-52. [DOI] [PubMed] [Google Scholar]
Mann 1981 {published data only}
- Mann RJ. A double-blind trial of oral O-beta-hydroxyethyl rutosides for stasis leg ulcers. British Journal of Clinical Practice 1981;35:79-81. [PubMed] [Google Scholar]
Marinello 2002 {published and unpublished data}
- Marinello J, et al. Multicentric, randomised, double-blind, placebo controlled, clinical trial that assess the efficacy of calcium dobesilate in the treatment of venous hypertension in patients with chronic venous insufficiency [Ensayo clínico multicéntrico, doble ciego, aleatorizado, controlado con placebo sobre la eficacia de dobesilato de calcio en el tratamiento de la hipertensión venosa en pacientes afectos de insuficiencia venosa crónica en sus extremidades inferiores. Código: ESCLIN-004/99]. Laboratorios Dr. Esteve.
- Marinello J, Videla S. Chronic venous insufficiency of the lower limbs: suitability of transcutaneous blood gas monitoring as an endpoint to evaluate the outcome of pharmacological treatment with calcium dobesilate. Methods and Findings in Experimental and Clinical Pharmacology 2004;26(10):775-80. [DOI] [PubMed] [Google Scholar]
Martinez‐Zapata 2008 {published data only}
- Martinez-Zapata MJ, Moreno RM, Gich I, Urrútia G, Bonfill X, Chronic Venous Insufficiency Study Group. A randomized, double-blind multicentre clinical trial comparing the efficacy of calcium dobesilate with placebo in the treatment of chronic venous disease. European Journal of Vascular and Endovascular Surgery 2008;35(3):358-65. [DOI] [PubMed] [Google Scholar]
NCT01848210 {published data only}
- NCT01848210. Efficacy and safety of coumarin and troxerutin in the symptomatic treatment of chronic venous insufficiency. clinicaltrials.gov/ct2/show/NCT01848210 (first posted 7 May 2013).
Nocker 1990 {published data only}
- Nocker W, Diebschlag W, Lehmacher W. Clinical trials of the dose-related effects of o-(beta-hydroxyethyl)-rutosides in patients with chronic venous insufficiency. Phlebology 1990;5 Suppl 1:23-6. [Google Scholar]
- Nocker W, Diebschlag W, Lehmacher W. Three-month, randomized, double-blind, dose-response study with O-(beta-hydroxyethyl)-rutosides drinking solution. Vasa 1989;18(3):235-8. [PubMed] [Google Scholar]
- Nocker W, Diebschlag W. An investigation of dosage effects with drinking solutions of o-(beta hydroxyethyl)-rutosides. Vasa 1987;16:365-9. [PubMed] [Google Scholar]
Padrós 1972 {published data only}
- Padrós W. Controlled study of the effect of calcium dobesilate on syndromes of venous insufficiency. Medicina Clínica 1972;58(6):515-9. [Google Scholar]
- Padros W. Double blind investigation of clinical effectiveness of calcium dobesilate in the venous insufficiency syndrome (Dutch). Ars Medici Internationaal Tijdschrift voor Praktische Therapie 1977;6(8):777-84. [Google Scholar]
- Padros W. Double blind study of the action of calcium dobesilate on venous insufficiency syndromes. Ars Medici Revue Internationale de Therapie Pratique 1977;32(8):783-90. [Google Scholar]
Parrado 1999 {published data only}
- Parrado F, Buzzi A. A study of the efficacy and tolerability of a preparation containing Ruscus aculeatus in the treatment of chronic venous insufficiency of the lower limbs. Clinical Drug Investigation 1999;18(4):255-61. [Google Scholar]
Pecchi 1990 {published data only}
- Pecchi S, De Franco V, Damiani P, Guerrini M, Di Perri T. Calcium dobesilate in the treatment of primary venous insufficiency of the lower limbs. A controlled clinical study [Il dobesilato di calcio nel trattamento del l´insufficienza venosa primitiva degli arti inferiori]. Clinica Terapeutica 1990;132(6):409-17. [PubMed] [Google Scholar]
Pedersen 1992 {published data only}
- Pedersen FM, Hamberg O, Sorensen MD, Neland K. The effect of O-(beta-hydroxyethyl)-rutoside (Venoruton) on symptomatic venous insufficiency in the lower limbs. Ugeskrift for Laeger 1992;154(38):2561-3. [PubMed] [Google Scholar]
Petrassi 2000 {published data only}
- Petrassi C, Mastromarino A, Spartera C. Pycnogenol in chronic venous insufficiency. Phytomedicine 2000;7(5):383-8. [DOI] [PubMed] [Google Scholar]
Planchon 1990 {published data only}
- Planchon B. Venous insufficiency and Daflon 500 mg [Insuffisance veineuse et Daflon 500 mg]. Artères et Veines 1990;IX(4):376-80. [Google Scholar]
Pointel 1986 {published data only}
- Pointel JP, Boccalon H, Cloarec M, Le Devehat C, Joubert M. Titrated extract of Centella asiatica (TECA) in the treatment of venous insufficiency of the lower limbs. Angiology 1987;38(1 Pt 1):46-50. [DOI] [PubMed] [Google Scholar]
- Pointel JP. Titrated extract of centella asiatica (TECA) in the treatment of venous insufficiency of the lower limbs. Angiology 1986;37(5):420-1. [DOI] [PubMed] [Google Scholar]
Prerovsky 1972 {published data only}
- Prerovsky I, Roztocil K, Hlavova A, Koleilat Z, Razgova L, Oliva I. The effects of hydroxyethylrutosides after acute and chronic oral administration in patients with venous diseases. A double blind study. Angiologica 1972;9(3-6):408-14. [DOI] [PubMed] [Google Scholar]
Pulvertaft 1983 {published data only}
- Pulvertaft TB. General practice treatment of symptoms of venous insufficiency with oxerutins. Results of a 660 patient multicentre study in the UK. Vasa 1983;12(4):373-6. [PubMed] [Google Scholar]
- Pulvertaft TB. Paroven in the treatment of chronic venous insufficiency. Practitioner 1979;223(1338):838-41. [PubMed] [Google Scholar]
Rabe 2011 {published data only}
- Rabe E, Jaeger KA, Bulitta M, Pannier F. Calcium dobesilate in patients suffering from chronic venous insufficiency: a double-blind, placebo-controlled, clinical trial. Phlebology 2011;26:162–8. [DOI] [PubMed] [Google Scholar]
Rabe 2015 {published data only}
- Rabe E, Agus GB, Roztocil K. Analysis of the effects of micronized purified flavonoid fraction versus placebo on symptoms and quality of life in patients suffering from chronic venous disease: from a prospective randomized trial. International Angiology 2015;34(5):428-36. [PubMed] [Google Scholar]
Rabe 2016 {published data only}
- Rabe E, Ballarini S, Lehr L, Doxium EDX09/01 Study Group. A randomized, double-blind, placebo-controlled, clinical study on the efficacy and safety of calcium dobesilate in the treatment of chronic venous insufficiency. Phlebology 2016;31(4):264-74. [DOI] [PubMed] [Google Scholar]
Renton 1994 {published data only}
- Renton S, Leon M, Belcaro G, Nicolaides AN. The effect of hydroxyethylrutosides on capillary filtration in moderate venous hypertension: a double blind study. International Angiology 1994;13(3):259-62. [PubMed] [Google Scholar]
Rose 1970 {published data only}
- Rose SS. A report on the use of an hydroxyethylrutoside in symptoms due to venous back pressure and allied conditions in the lower limbs. British Journal of Clinical Practice 1970;24(4):161-4. [PubMed] [Google Scholar]
Rudofsky 1989 {published data only}
- Rudofsky G, Diehm C, Grub J, Hartmann M, Schultz-Ehrenburg HU, Bisler H. Ruscus saponines and the flavonoid hesperidinmethylchalcone in the treatment of chronic venous insufficiency. In: Davy A, Stemmer R , editors(s). Proceedings of the Union Internationale de Phlebologie - 10th World Congress, Strasbourg, 25-29 September. London and Paris: John Libbey Eurotext Ltd, 1989:728-30.
- Rudofsky G, Diehm C, Gruss JD, Hartman M, Schultz-Ehrenburg HK, Bisler H. Chronic venous insufficiency: treatment with Ruscus extract and trimethyl hesperidine chalcone. Münchener Medizinische Wochenschrift 1990;132(13):205-10. [Google Scholar]
Schultz‐Ehrenburg 1993 {published data only}
- Schultz-Ehrenburg U, Müller B. Two multicentre clinical trials of two different dosages of O-(beta-hydroxyethyl)-rutosides in the treatment of leg ulcers. Phlebology 1993;8 Suppl 1:29-30. [Google Scholar]
Sentou 1984 {published data only}
- Sentou Y. Double blind study of the activity of Cyclo 3 in man. International Angiology 1984;3:106-9. [Google Scholar]
Serralde 1990 {published data only}
- Serralde CF, Aceves AQ. Clinical trial of the O-(β-hydroxy-ethyl-rutosides) in patients with chronic venous insufficiency [Ensayo clínico de o-(beta-hidroxietil-rutósidos) en pacientes con insuficiencia venosa crónica]. Revista Médica del Hospital General de México 1990;53(2):102-6. [Google Scholar]
Thebaut 1985 {published data only}
- Thebaut JF, Thebaut P, Vin F. Trial of vasculoprotective agent (plant products of the flavan class) in functional manifestations of peripheral venous insufficiency (double-blind trial in 92 cases). Gazette Medicale 1985;92(12):96-100. [Google Scholar]
Tsouderos 1989 {published data only}
- Tsouderos Y. Are the phlebotonic properties shown in clinical pharmacology predictive of a therapeutic benefit in chronic venous insufficiency. Our experience with Daflon 500 mg. International Angiology 1989;8(4):53-9. [PubMed] [Google Scholar]
- Tsouderos Y. Venous tone: are the phlebotonic properties predictive of a therapeutic benefit? A comprehensive view of our experience with Daflon 500 mg. Zeitschrift fur Kardiologie 1991;80 Suppl 7:95-101. [PubMed] [Google Scholar]
Unkauf 1996 {published data only}
- Unkauf M, Rehn D, Klinger J, Motte S, Grobmann K. Investigation of the efficacy of oxerutins compared to placebo in patients with chronic venous insufficiency treated with compression stockings. Arzneimittel-Forschung 1996;46(5):478-82. [PubMed] [Google Scholar]
Vanscheidt 2002a {published data only}
- Vanscheidt W, Rabe E, Naser-Hijazi B, Ramelet AA, Partsch H, Diehm C, et al. The efficacy and safety of a coumarin-/troxerutin-combination (SB-LOT) in patients with chronic venous insufficiency: a double blind placebo-controlled randomised study. Vasa 2002;31(3):185-90. [DOI] [PubMed] [Google Scholar]
Vanscheidt 2002b {published data only}
- Vanscheidt W, Jost V, Wolna P, Lücker A, Muller A, Theurer C, et al. Efficacy and safety of a Butcher's broom preparation (Ruscus aculeatus L. extract) compared to placebo in patients suffering from chronic venous insufficiency. Arzneimittel-Forschung 2002;52(4):243-50. [DOI] [PubMed] [Google Scholar]
Vin 1994 {published data only}
- Vin E, Chabanel A, Taccoen A, Ducros J, Grufaz J, Hutinel B, et al. Action of Veinamitol 3500 mg on clinical and hemorheological data in CVI. International Angiology 1995;14(Suppl 1):99. [Google Scholar]
- Vin F, Chabanel A, Taccoen A, Ducros J, Gruffaz J, Hutinel B, et al. Action de la troxérutine sur les paramètres cliniques, pléthysmographiques et rhéologiques de l'insuffisance veineuse des membres inférieurs. Etude contrôlée contre placebo. Artères et Veines 1992;XI:333-42. [Google Scholar]
- Vin F, Chabanel A, Taccoen A, Ducros J, Gruffaz J, Hutinel B, et al. Double-blind trial of the efficacy of troxerutin in chronic venous insufficiency. Phlebology 1994;9(2):71-6. [Google Scholar]
Welch 1985 {unpublished data only}
- Welch W, Moriau M, Gysel JP. A double-blind, placebo controlled trial of o-(beta-hydroxyethyl)-rutosides in patients with chronic venous insufficiency. Novartis 1985.
Widmer 1990 {published data only}
- Widmer L, Biland L, Barras JP. Doxium 500 in chronic venous insufficiency: a double-blind placebo controlled multicentre study. International Angiology 1990;9(2):105-10. [PubMed] [Google Scholar]
Zucarelli 1987 {published data only}
- Zucarelli F. Clinical efficacy and tolerability of rutin. Double blind, placebo controlled clinical trial. [Efficacité clinique et tolerance de la coumarine rutine. Étude controlée en double aveugle versus placebo]. Gazette Médicale 1987;94(32):80-6. [Google Scholar]
References to studies excluded from this review
Akbulut 2010 {published data only}
- Akbulut B. Calcium dobesilate and oxerutin: effectiveness of combination therapy. Phlebology 2010;25:66-71. [DOI] [PubMed] [Google Scholar]
Androulakis 1989 {published data only}
- Androulakis G, Panoysis PA. Plethysmographic confirmation of the beneficial effect of calcium dobesilate in primary varicose veins. Angiology 1989;40(1):1-4. [DOI] [PubMed] [Google Scholar]
Auteri 1990 {published data only}
- Auteri A, Blardi P, Frigerio C, Lillo L, di Perri T. Pharmacodynamics of troxerutine in patients with chronic venous insufficiency: correlations with plasma drug levels. International Journal of Clinical Pharmacology Research 1990;10(4):235-41. [PubMed] [Google Scholar]
Bacci 2003 {published data only}
- Bacci PA, Allegra C, Botta G, Mancini S. The role of a multifunctional plant complex in phlebolymphology: randomized, placebo-controlled double-blind clinical study. Amercian College of Phlebology. Abstracts from the 17th Annual Congress, August 27 - 31, 2003 — San Diego, California.
Bastide 1976 {published data only}
- Bastide G, Becade P, Goulley Y. Double blind study of Dihydroergotamine Sandoz in venous pathology of lower limbs. Angeiologie 1976;28(5):249-54. [Google Scholar]
Batchvarova 1989a {published data only}
- Batchvarova V. Effet Clinique du Troxevit sur l'Insuffisance Veineuse Chronique. Vol. 2. London & Paris: John Libby Eurotext Ltd, 1989. [Google Scholar]
Behar 1993 {published data only}
- Behar A, Nathan P, Lavieuville M, Allaert FA. Effect of veinotonyl 75 on the capillary permeability test using technetium albumin in cyclic orthostatic edemas. Phlébologie 1993;46(4):721-31. [PubMed] [Google Scholar]
Belcaro 1989 {published data only}
- Belcaro G, Rulo A, Candiani C. Evaluation of the microcirculatory effects of Venorutin in patients with chronic venous hypertension by laser Doppler flowmetry, transcutaneous PO2 and PCO2 measurements, leg volumetry and ambulatory venous pressure measurements. Vasa 1989;18:146-51. [PubMed] [Google Scholar]
- Belcaro G, Rulo A, Candiani C. Evaluation of the microcirculatory effects of Venoruton in patients with chronic venous hypertension by laser-Doppler flowmetry, transcutaneous PO2 and PCO2 measurements, leg volumetry and ambulatory venous pressure measurements. Phlebology 1989;4(1):23-30. [PubMed] [Google Scholar]
Belcaro 1995 {published data only}
- Belcaro G, Rosaria Cesarone M, De Sanctis MT, Incandela L, Laurora G, Fevrier B, et al. Laser doppler and transcutaneous oxymetry: modern investigations to assess drug efficacy in chronic venous insufficiency. International Journal of Microcirculation: Clinical and Experimental 1995;15(Suppl 1):45-9. [DOI] [PubMed] [Google Scholar]
Belcaro 2008 {published data only}
- Belcaro G Cesarone MR, Ledda A, Cacchio M, Ruffini I, Ricci A, et al. O-(beta-hydroxyethyl)-rutosides systemic and local treatment in chronic venous disease and microangiopathy: an independent prospective comparative study. Angiology 2008;59 (Suppl 1):7S-13S. [DOI] [PubMed] [Google Scholar]
Bello 1990 {published data only}
- Bello AA, Meyer K, Garcia AT, Reinaga VV. Calcium dobesilate combined with a heparinoid in the topical treatment of chronic venous insufficiency: a double-blind study. Acta Therapeutica 1990;16(1):79-87. [Google Scholar]
Bento 2006 {published data only}
- Bento C, Brandão DDC, Smith P. A multicentric, IV phase, multidisciplinary, prospective, randomized, double blinded, comparative study to evaluate the castanha-da-índia, rutina, smilax japicanga and polygonum punctatum combination efficacy and tolerability in the treatment of patients suffering from symptomatic venous insufficiency comparing to placebo treatment. Revista Brasileira de Medicina 2006;63(8):422-6. [Google Scholar]
Berson 1978 {published data only}
- Berson I. About a new medicamentous treatment of the varicose syndrome. Schweiz Rundschau Med (PRAXIS) 1978;67:981-3. [PubMed] [Google Scholar]
Blume 1996 {published data only}
- Blume J, Wüstenberg P. Chronic vein insufficiency. Treatment results with benzopyrones during and after compression therapy [Cronisch-venöse Insuffizienz (CVI). Behandlungsergebnisse mit benzopyronen und nach Kompressiontherapie]. Therapiewoche 1996;46(10):540-4. [Google Scholar]
- Blume J. Therapy of venous oedema [Tratamento do edema de origem venosa. Eficácia de um tratamento medicamentoso em combinaçao com o tratamento compresivo]. Revista Brasileira de Medicina 1994;51(3):283-8. [Google Scholar]
Bohm 1989 {published data only}
- Bohm C. Venodiuretics: a new combination of diuretic and edema protective drugs. Medizinische Welt 1989;40(30-31):887-8. [Google Scholar]
Boisseau 1995 {published data only}
- Boisseau MR, Taccoen A, Garreau C, Vergnes C, Roudaut MF, Garreau-Gomez B. Fibrinolysis and hemorheology in chronic venous insufficiency: a double-blind study of troxerutin efficiency. Journal of Cardiovascular Surgery 1995;36(4):369-74. [PubMed] [Google Scholar]
Bolliger 1972 {published data only}
- Bolliger AA. Results of a double blind trial of percutaneously administered rutoside. Angiologica 1972;9:397-400. [PubMed] [Google Scholar]
Bort 1995 {published data only}
- Bort H, Hahn M, Klyscz T, Junger M. The influence of rutosides on increased capillary permeability in chronic venous insufficiency as measured by video capillaroscopy. In: Proceedings of the Union Internationale de Phlebologie - 12th World Congress, London, 3-8 September. 1995.
Bosse 1985 {published data only}
- Bosse K, Drieschner P, Klose L. Comparative studies concerning the effectiveness of therapeutic agents in chronic venous insufficiency. Phlebologie und Proktologie 1985;14:111-4. [Google Scholar]
Brami 1983 {published data only}
- Brami C, Morere MCNK, Megret G, Elbaz C. Double-blind controlled trial against placebo of dihydroergocryptine mesilate plus caffeine in chronic venous insufficiency. Angeiologie 1983;35(8):281-3. [Google Scholar]
Carstens 1985 {published data only}
- Carstens C, Hampel H. Treatment of oedemata in chronic venous insufficiency by means of an additional therapy with DIU Venostatin. Die Medizinische Welt 1985;36:867-70. [Google Scholar]
Cataldi 2001 {published data only}
- Cataldi A, Gasbarro V, Viaggi R, Soverini R, Gresta E, Mascoli F. Effectiveness of the association of alphatocopherol, rutin, melilotus and centella asiatica in the treatment of patients affected by chronic venous insufficiency [Efficacia clinica di un'associazine di alfatocoferoli, rutina, meliloto e centella asiatica nel trattamento di pazienti con insufficienza venosa cronica]. Minerva Cardioangiologica 2001;49(2):159-63. [PubMed] [Google Scholar]
Cesarone 1992 {published data only}
- Cesarone MR, Laurora G, Ricci A, Belcaro G, Pomante P, Candiani C, et al. Acute effects of hydroxyethylrutosides on capillary filtration in normal volunteers, patients with venous hypertension and in patients with diabetic microangiopathy (a dose comparison study). Vasa 1992;21(1):76-80. [PubMed] [Google Scholar]
Cesarone 1994 {published data only}
- Cesarone MR, Laurora G, De Sanctis MT, Incandela L, Grimaldi R, Marelli C, et al. Microcirculatory activity of centella asiatica in venous insufficiency. Minerva Cardioangiologica 1994;42(6):299-304. [PubMed] [Google Scholar]
Cesarone 2001 {published data only}
- Cesarone MR, De Sanctis MT, Incandela L, Belcaro G, Griffin M, Bavera P, et al. Microvascular changes in venous hypertension due to varicose veins after standardized application of Essaven gel--a placebo-controlled, randomized study. Angiology 2001;52 Suppl 3:S11-6. [DOI] [PubMed] [Google Scholar]
Cesarone 2001a {published data only}
- Cesarone MR, Belcaro G, De Sanctis MT, Incandela L, Cacchio M, Bavera P, et al. Effects of the total triterpenic fraction of Centella asiatica in venous hypertensive microangiopathy: a prospective, placebo-controlled, randomized trial. Angiology 2001;52 Suppl 2:S15–8. [PubMed] [Google Scholar]
Cesarone 2001b {published data only}
- Cesarone MR, Incandela L, Belcaro G, Sanctis MT, Ricci A, Griffin M. Two-week topical treatment with Essaven gel in patients with diabetic microangiopathy: a placebo-controlled, randomized study. Angiology 2001;52 Suppl 3:S43-8. [DOI] [PubMed] [Google Scholar]
Cesarone 2001c {published data only}
- Cesarone MR, Belcaro G, Rulo A, Griffin M, Ricci A, Ippolito E, et al. Microcirculatory effects of total triterpenic fraction of Centella asiatica in chronic venous hypertension: measurement by laser Doppler, TcPO2-CO2, and leg volumetry 93. Angiology 2001;52 Suppl 2:S45-8. [PubMed] [Google Scholar]
Cesarone 2002b {published data only}
- Cesarone MR, Incandela L, DeSanctis MT, Belcaro G, Dugall M, Acerbi G. Variations in plasma free radicals in patients with venous hypertension with HR (Paroven, Venoruton; O-(beta-hydroxyethyl)-rutosides): a clinical, prospective, placebo-controlled, randomized trial. Journal of Cardiovascular Pharmacology and Therapeutics 2002;7 Suppl 1:S25-8. [DOI] [PubMed] [Google Scholar]
Cesarone 2010 {published data only}
- Cesarone MR, Belcaro G, Rohdewald P, Pellegrini L, Ledda A, Vinciguerra G, et al. Improvement of signs and symptoms of chronic venous insufficiency and microangiopathy with Pycnogenol: a prospective, controlled study. Phytomedicine 2010;17(11):835-9. [DOI] [PubMed] [Google Scholar]
Chant 1973 {published data only}
- Chant AD. The effect of paroven (HR) of the clearance of sodium-24 from the subcutaneous tissues of the foot in patients with varicose veins. Vasa 1973;2(3):288-91. [PubMed] [Google Scholar]
Chiummariello 2009 {published data only}
- Chiummariello S, De Gado F, Monarca C, Ruggiero M, Carlesimo B, Scuderi N, et al. [Multicentric study on a topical compound with lymph-draining action in the treatment of the phlebostatic ulcer of the inferior limbs]. Il Giornale di chirurgia 2009;30(11-12):497-501. [PubMed] [Google Scholar]
Clemens 1986 {published data only}
- Clemens S, Bisler H, Braun R. Phlebodril: Influences on the venous regurgitation. Phlebologie und Proktologie 1986;15(1):15-9. [Google Scholar]
Cospite 1996 {published data only}
- Cospite M, Milio G. Heparan sulfate vs diosmin: effects on microcirculation in chronic venous insufficiency of the lower extremities. Advances in Therapy 1996;13(3):178-190. [Google Scholar]
De Anna 1989 {published data only}
- De Anna D, Mari F, Intini S, Gasbarro V, Sortini A, Pozza E, et al. Effects of therapy with aminaftone on chronic venous and lymphatic stasis [Effetti della terapia con aminaftone sulla stasi venosa e linfatica cronica]. Minerva Cardioangiologica 1989;37(5):251-4. [PubMed] [Google Scholar]
- De Anna D, Risaliti A, Intini S, Terrosu G, Petri R, Taddeo U, et al. Aminaphtone therapy in venous lymphatic stasis of lower limbs. Phlebologie 1989;2:753-5. [Google Scholar]
- De Anna D, Risaliti A, Intini S, Uzzau A, Terrosu G, Petri R, et al. Aminaphtone therapy in venous and lymphatic stasis of lower limbs. In: Davy A and Stemmer R , editors(s). Proceedings of the Union Internationale de Phlebologie - 10th World Congress, Strasbourg, 25-29-September 1989. Vol. 2. London and Paris: John Libbey Eurotext Ltd, 1989:753-5.
Delacroix 1981 {published data only}
- Delacroix P. Double-blind trial of endotelon (TM) in chronic venous insufficiency. La Revue de Medecine 1981;22(27-28):1793-802. [Google Scholar]
Delecluse 1991 {published data only}
- Delecluse M, Ducros JJ, Egal G, Hamel H, Junk R, Leroux A, et al. [Clinical study of Diovenor 300 mg versus a mixture of flavonoides in 90 % of diosmine in the treatment of symptoms of chronic venous insufficiency in young active females]. Essai Clinique Pragmatique de Diovenor 300 mg Versus Melange de Flavonoides a 90 % de Diosmine Dans le Traitement des Manifestations d'Insuffisance Veineuse Chronique Chez la Femme Active Jeune 1991;10(7):498-503. [Google Scholar]
de Parades 1990 {published data only}
- Parades B, Demarez JP, Cauquil J. Comparative analysis of the therapeutic effects of Cyclo 3 Fort and Diosmin 450 mg in combination with hesperidin 50 mg in venous insufficiency of the legs. Vie Medicale 1990;6:226-32. [Google Scholar]
De Sanctis 2001 {published data only}
- De Sanctis MT, Belcaro G, Incandela L, Cesarone MR, Griffin M, Ippolito E, et al. Treatment of edema and increased capillary filtration in venous hypertension with total triterpenic fraction of Centella asiatica: a clinical, prospective, placebo-controlled, randomized, dose-ranging trial. Angiology 2001;52(Suppl 2):S55-9. [PubMed] [Google Scholar]
Duchene 1988 {published data only}
- Duchene Marullaz P, Amiel M, Barbe R. Evaluation of the clinical pharmacological activity of a phlebotonic agent. Application to the study of Daflon 500 mg. International Angiology 1988;7 Suppl 2:25-32. [PubMed] [Google Scholar]
Dustmann 1984 {published data only}
- Dustmann HO, Godolias G, Seibel K. Foot volume with chronic venous insufficiency while standing: effect of a new treatment. Therapiewoche 1984;34(36):5077-86. [Google Scholar]
Erdlen 1989 {published data only}
- Erdlen F. Clinical efficacy of venostasin. A double blind trial. Medizinische Welt 1989;40(36):994-6. [Google Scholar]
Erler 1991 {published data only}
- Erler M. Horse chestnut seed extract in the therapy of the peripheral venous edema - Clinical therapies in comparison. Medizinische Welt 1991;42(7):593-6. [Google Scholar]
EudraCT2009‐014681‐25 {published data only}
- EudraCT2009-014681-25. Pharmacodynamic and clinical assessment of DC 982 GE (2,4 or 6 capsules per day) in patients with chronic venous disorders: randomised, placebo-controlled, dose effect, double blind, parallel group study. clinicaltrialsregister.eu/ctr-search/search?query=2009-014681-25 (first posted 21 October 2009).
Forconi 1977 {published data only}
- Forconi S, Guerrini M, Di Perri T. Study of the activity of a flavonoid, O-(beta-hydroxyethyl)-rutoside, at high dose levels of venous tone measured by "strain gauge" plethysmography. Vasa 1977;6(3):279-84. [PubMed] [Google Scholar]
Frausini 1985 {published data only}
- Frausini G, Rotatori P, Oliva S. Controlled trial on clinical-dynamic effects of three treatments in chronic venous insufficiency. Giornale Italiano di Angiologica 1985;5(2):147-51. [Google Scholar]
Glinski 1999 {published data only}
- Glinski W, Chodynicka B, Roszkiewicz J, Bogdanowski T, Lecewicz-Torun B, Kaszuba A, et al. Effectiveness of a micronized purified flavonoid fraction (MPFF) in the healing process of lower limb ulcers. An open multicentre study, controlled and randomized. Minerva Cardioangiologica 2001;49(2):107-14. [PubMed] [Google Scholar]
- Glinski W, Chodynicka B, Roszkiewicz J, Bogdanowski T, Lecewicz Torun B, Kaszuba A, et al. The beneficial augmentative effect of micronised purified flavonoid fraction (MPFF) on the healing of leg ulcers: an open, multicentre, controlled, randomised study. Phlebology 1999;14(4):151-7. [Google Scholar]
Gonzalez‐Fajardo 1990 {published data only}
- Gonzalez-Fajardo JA, Rodriguez-Camarero SJ, Marino P, Castro Villamor MA, March Garcia JR, Carpintero Mediavilla L, et al. [Photoplethysmographic evaluation of the effect of a vascular tonic drug]. Angiologia 1990;42(5):167-71. [PubMed] [Google Scholar]
Granger 1995 {published data only}
- Granger C, Laveille C, Vilain C, Jochemsen R. Correlation between haemodynamic parameters of venous tone and clinical symptoms improvement in patients with chronic venous insufficiency. A controlled randomised study of Daflon 200 mg 2 tablets per day versus placebo during two months of treatment. International Angiology 1995;14 Suppl 1:343. [Google Scholar]
Henriet 1995 {published data only}
- Henriet JP. [Functional venous insufficiency: clinical study comparing once a day DIOVENOR 600 mg (600 mg of diosmine d'hemisyntheses) versus two doses per day of a mixture of 500 mg of flavonoides (900 mg of diosmine)]. Phlebologie 1995;48(2):285-90. [Google Scholar]
Horvath 1985 {published data only}
- Horvath W, Tomschi F. [The postthrombotic state and the effect of dihydroergotamine]. Das Postthrombotische Zustandsbild und Seine Beeinflussung Durch Dihyrdroergotamin 1985;14(1):84-9. [PubMed] [Google Scholar]
Incandela 1995 {published data only}
- Incandela L, Cesarone MR, De Sanctis MT, Laurora G, Ricci A, Gerentes I. Evaluation of the microcirculatory effects of veinamitol 3500 mg in chronic venous insufficiency. International Angiology 1995;14 Suppl 1:98-9. [Google Scholar]
Incandela 1996 {published data only}
- Incandela L, De Sanctis MT, Cesarone MR, Laurora G, Belcaro G, Taccoen A, et al. Efficacy of troxerutin in patients with chronic venous insufficiency: a double-blind, placebo-controlled study. Advances in Therapy 1996;13(3):161-6. [Google Scholar]
Incandela 2001 {published data only}
- Incandela L, Belcaro G, De Sanctis MT, Cesarone MR, Griffin M, Ippolito E, et al. Total triterpenic fraction of Centella asiatica in the treatment of venous hypertension: a clinical, prospective, randomized trial using a combined microcirculatory model. Angiology 2001;52 Suppl 2:S61-7. [PubMed] [Google Scholar]
Incandela 2002 {published data only}
- Incandela L, Belcaro G, Renton S, DeSanctis T, Cesarone MR, Bavera P, et al. HR (Paroven, Venoruton; 0-(beta-hydroxyethyl)-rutosides) in venous hypertensive microangiopathy: a prospective, placebo-controlled, randomized trial. Journal of Cardiovascular Pharmacology and Therapeutics 2002;7 Suppl 1:7-10. [DOI] [PubMed] [Google Scholar]
ISRCTN54360155 {published data only}
- ISRCTN54360155. A 3 month clinical trial of herbal medicine combination intended for topical application in patients with leg symptoms complaints due to chronic venous insufficiency: a double-blind randomized controlled trial. www.isrctn.com/ISRCTN54360155 (first posted 5 January 2015).
Janssens 1999 {published data only}
- Janssens D, Michiels C, Guillaume G, Cuisinier B, Louagie Y, Remacle J. Increase in circulating endothelial cells in patients with primary chronic venous insufficiency: protective effect of Ginkor Fort in a randomized double-blind, placebo-controlled clinical trial. Journal of Cardiovascular Pharmacology 1999;33(1):7-11. [DOI] [PubMed] [Google Scholar]
Janssens 1999a {published data only}
- Janssens D, Michiels C, Guillaume G, Cuisinier B, Louagie Y, Remacle J. Increase in circulating endothelial cells in patients with primary chronic venous insufficiency: protective effect of Ginkor Fort in a randomized double-blind, placebo-controlled clinical trial. Journal of Cardiovascular Pharmacology 1999;33(1):7-11. [DOI] [PubMed] [Google Scholar]
Kalus 2004 {published data only}
- Kalus U, Koscielny J, Grigorov A, Schaefer E, Peil H, Kiesewetter H. Improvement of cutaneous microcirculation and oxygen supply in patients with chronic venous insufficiency by orally administered extract of red vine leaves AS 195: a randomised, double-blind, placebo-controlled, crossover study. Drugs in R & D 2004;5(2):63-71. [DOI] [PubMed] [Google Scholar]
- Kalus U, Koscielny J, Grigorov A, Schaefer E, Peil H, Kiesewetter H. Improvement of cutaneous microcirculation and oxygen supply in patients with chronic venous insufficiency by oral therapy with red wine leaf extract AS 195. Vasomed 2004;16(1):20-1. [DOI] [PubMed] [Google Scholar]
Kiesewetter 2000 {published data only}
- Kiesewetter H, Koscielny J, Kalus U, Vix JM, Peil H, Petrini O, et al. Efficacy of orally administered extract of red vine leaf AS 195 (folia vitis viniferae) in chronic venous insufficiency (stages I-II). A randomized, double-blind, placebo-controlled trial. Arzneimittel-Forschung 2000;50(2):109-17. [DOI] [PubMed] [Google Scholar]
Koltringer 1993 {published data only}
- Koltringer P, Langsteger W, Klima G, Reisecker F, Eber O. Hemorheologic effects of ginkgo biloba extract EGb 761. Dose- dependent effect of EGb 761 on microcirculation and viscoelasticity of blood. [German]. Fortschritte der Medizin 1993;111(10):170-2. [PubMed] [Google Scholar]
Kostering 1985 {published data only}
- Kostering H, Bandura B, Merten HA, Wieding JU. The behaviour of blood clotting and its inhibitors under long-term with 5,6-benzo-alpha-pyrone (coumarin). Double-blind study. Arzneimittel-Forschung 1985;35(8):1303-6. [PubMed] [Google Scholar]
Krähenbühl 1975 {published data only}
- Krähenbühl B. Chronic arterial insufficiency of lower limbs: treatment by bencyclan (fludilat). Schweizerische Rundschau fur Medizin Praxis 1975;64(20):632-4. [PubMed] [Google Scholar]
Krcílek 1973 {published data only}
- Krcílek A, Smejkal V. Therapeutic effects of venotonics in clinical pharmacotherapeutical evaluations by double-blind tests. Casopis Lekaru Ceskych 1973;112:930-3. [PubMed] [Google Scholar]
Languillat 1988b {published data only}
- Languillat N, Zucarrelli F. Etude en double aveugle contre placebo de l'activite veinotonique due veliten: evolution de la permeabilite capillare et de lat vitesse de circulation veineuse. Acta Medica Internationale Angiologie 1988;5(83):3-5. [Google Scholar]
Languillat 1989 {published data only}
- Languillat N, Zuccarelli F, Hariton C. Radioisotopic comparative double-blind study of venous capillary permeability and circulation rate after treatment by Veliten (R) in venous insufficiency of inferior limbs. In: Proceedings of the Union Internationale de Phlebologie - 10th World Congress, Strasbourg, 25-29 September. 1989.
Le Dévéhat 1989 {published data only}
- Le Dévéhat C, Vimeux M, Bandoux G. Hemoreological effects of oral troxerutin treatment versus placebo in venous insufficiency of the lower limbs. Clinical Hemorrheology 1989;9(4):543-52. [Google Scholar]
- Le Dévéhat C, Vimeux M, Bondoux G, Khodabandehlou T. Hemorheological effects of oral troxerutin treatment in venous insufficiency of the lower limbs. Phlebologie 1989;2:766-8. [Google Scholar]
- Le Devehat C, Lemoine A. Effet hemorheological de la troxerutine dans l'insuffisance veineuse [Effet hemorheological de la troxerutine dans l'insuffisance veineuse]. In: Negus D, Jantet G, editors(s). Phlebology 1985. Herts, UK: John Libbey and Co Ltd, 1986:850-2. [Google Scholar]
Le Dévéhat 1997 {published data only}
- Le Devehat C, Khodabandehlou T, Vimeux M, Kempf C. Evaluation of haemorheological and microcirculatory disturbances in chronic venous insufficiency: activity of Daflon 500 mg. International Journal of Microcirculation: Clinical and Experimental 1997;17(Suppl 1):27-33. [DOI] [PubMed] [Google Scholar]
Marastoni 1982 {published data only}
- Marastoni F, Crespi B, Montorsi M, De Stefano A. A clinical and instrumental assessment of the effect of dihydroergotamine in lower extremity venous insufficiency. Archivio per le Scienze Mediche 1982;139(2):165-74. [PubMed] [Google Scholar]
Menyhei 1994 {published data only}
- Menyhei G, Acsady G, Hetenyi A, Dubeaux D, Rado G. Chronobiology and clinical activity of daflon 500 mg in chronic venous insufficiency. Phlebology 1994;9 Suppl 1:15-8. [Google Scholar]
Monteil‐Seurin 1993 {published data only}
- Monteil-Seurin J. Lymphatic venous insufficiency: comparative study of Cyclo 3 Fort (TM) versus diosmin. Comptes Rendus de Therapeutique et de Pharmacologie Clinique 1993;11(109):3-7. [Google Scholar]
Morales 1993 {published data only}
- Morales CA, Barros RM. Efficacy and safety on use of dried horse chestnut extract in the treatment of chronic venous insufficiency of the limbs [Eficácia e segurança do extracto seco da semente de castanha-da-India no tratamento da insuficiencia venosa crônica de membros inferiores]. Revista Brasileira de Medicina 1993;50(11):1563-5. [Google Scholar]
Naser‐Hijazi 2004 {published data only}
- Naser-Hijazi B, Gallenkemper G, Rieckemann B, Vanscheidt W. In contrast to its derivatives, coumarin does not influence prothrombin time: results from a randomised placebo-controlled study. Phlebologie 2004;33(1):17-22. [Google Scholar]
- Schmeck-Lindenau HJ, Naser-Hijazi B, Becker EW, Henneicke von HH, Schnitker J. Safety aspects of a coumarin-troxerutin combination regarding liver function in a double-blind placebo-controlled study. International Journal of Clinical Pharmacology and Therapeutics 2003;41(5):193-9. [DOI] [PubMed] [Google Scholar]
NCT01654016 {published data only}
- NCT01654016. Study of antiinflammatory effects of Detralex (Daflon). https://clinicaltrials.gov/ct2/show/NCT01654016 (accessed 9 September 2015).
NCT02191163 {published data only}
- NCT02191163. Efficacy of Antlstax In improving microcirculation of the skin in the leg in patients suffering from chronic venous insufficiency. https://clinicaltrials.gov/ct2/show/NCT02191163 (accessed 9 Sepember 2015).
NCT02191254 {published data only}
- NCT02191254. Efficacy and tolerability of Antlstax in male and female patients sufferlng from chronlc venous insufficiency. https://clinicaltrials.gov/ct2/show/NCT02191254 (accessed 9 September 2015).
NCT02191280 {published data only}
- NCT02191280. Antistax In patients with chronlc venous insufficiency. https://clinicaltrials.gov/ct2/show/NCT02191280 (accessed 9 September 2015).
Neumann 1988 {published data only}
- Neumann HAM, Van Den Broek MTJB. Double blind study of the influence of O-(beta-hydroxyethyl)-rutosides on the TcpO2 and LRR curve in patients with chronic venous insufficiency. International Angiology 1988;7(4):9. [Google Scholar]
Neumann 1990 {published data only}
- Neuman HAM, Van der Broek MJTB. Evaluation of O-(beta-hydroxyethyl)-rutosides in chronic venous insufficiency by means of non-invasive techniques. Phlebology 1990;5 Suppl 1:13-20. [Google Scholar]
Neumann‐Mangoldt 1979 {published data only}
- Neumann-Mangoldt VP. Treatment of venous disorders of the lower extremities with Essaven-capsules, results of a double blind trial. Fortschritte der Medizin 1979;97(45):2117-20. [PubMed] [Google Scholar]
Nill 1970 {published data only}
- Nill HJ, Fischer H, Nill HJ, Fischer H. Comparative investigations concerning the effect of extract of horse chestnut upon the pressure-volume-diagram of patients with venous disorders. [German]. Arztliche Forschung 1970;24(5):141-3. [PubMed] [Google Scholar]
Ottillinger 2001 {published data only}
- Ottillinger B, Greeske K. Rational therapy of chronic venous insufficiency--chances and limits of the therapeutic use of horse-chestnut seeds extract. BMC Cardiovascular Disorders 2001;1(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paciaroni 1982 {published data only}
- Paciaroni E, Marini M. Topical therapy for phlebopathies. Results of a controlled clinical study. Policlinico - Sezione Medica 1982;89:255-64. [ID: 8761] [Google Scholar]
Partsch 1981 {published data only}
- Partsch H. Improvement of venous insufficiency with oral dehydroergotamine [Besserung der venosen insuffizienz durch orales dihydroergotamin]. Die Medizinische Welt 1981;32:1668-71. [PubMed] [Google Scholar]
Paul 1983 {published data only}
- Paul V, Lange A. Benzarone in edema of the legs due to chronic venous insufficiency. Munchener Medizinische Wochenschrift 1983;125(16):343-4. [PubMed] [Google Scholar]
Pauschinger 1987 {published data only}
- Pauschinger P. Clinical and experimental examination of the effect of horse-chestnut extract on the transcapillary filtratrion and the intravasel volume in patients with chronic venous insufficiency [Klinisch experimentelle Untersuchungen zur Wirkung von Rosskastanien-samenextrakt auf die transkapilläre Filtration und das intravasale Volumen an Parienten mit chronisch venöser Insuffizienz]. Phlebol Proktol 1987;16:57-61. [Google Scholar]
Petruzzellis 2002 {published data only}
- Petruzzellis V, Troccoli T, Candiani C, Guarisco R, Lospalluti M, Belcaro G, et al. Oxerutins (Venoruton): efficacy in chronic venous insufficiency - a double-blind, randomized, controlled study. Angiology 2002;53(3):257-63. [DOI] [PubMed] [Google Scholar]
Pointel 1987b {published data only}
- Pointel JP, Got I, Ziegler OI, Fontaine M, Benedetti F, Drouin P, et al. Double-blind controlled study of a combination of vitamin C, Ruscus aculeatus and Ribes nigrum anthocyanosides on capillary filtration in venous insufficiency [Essai controle en double insu d'une association vitamine C, ruscus aculeatus et anthocyanosides de ribes nigrum sur la filtration capillaire dans l'insuffisance veineuse]. Arteres et veines 1987;6(5):395-7. [Google Scholar]
Pokrovskii 2005 {published data only}
- Pokrovskii AV, Sapelkin SV, Galaktionova LA, Fedorov EE. The assessment of medical therapy effectiveness of patients with lower limb chronic venous insufficiency: the results of prospective study with Ginkor Fort. [Russian]. Angiologiia i Sosudistaia Khirurgiia/Angiology and Vascular Surgery 2005;11(3):47-52. [PubMed] [Google Scholar]
Questel 1983 {published data only}
- Questel R, Walrant P. Randomized, placebo-controlled trial of extracts of Ruscus aculeatus and Ribes nigrum plus ascorbic acid in venous insufficiency: Observation of microcirculation by conjunctival capillarography. Gazette Medicale de France 1983;90(6):508-14. [Google Scholar]
Rabe 2011b {published data only}
- Rabe E, Stucker M, Esperester A, Schafer E, Ottillinger B. Efficacy and tolerability of a red-vine-leaf extract in patients suffering from chronic venous insufficiency - results of a double-blind placebo-controlled study. European Journal of Vascular and Endovascular Surgery 2011;41(4):540-7. [DOI] [PubMed] [Google Scholar]
Riccioni 2004 {published data only}
- Riccioni C, Sarcinella R, Izzo A, Palermo G, Liguori M. Effectiveness of Troxerutin in association with Pycnogenol in the pharmacological treatment of venous insufficiency. Minerva Cardioangiologica 2004;52(1):43-8. [PubMed] [Google Scholar]
Roztocil 1977 {published data only}
- Roztocil K, Prerovsky I, Oliva I. The effect of hydroxyethylrutosides on capillary filtration rate in the lower limb of man. European Journal of Clinical Pharmacology 1977;11:435-8. [DOI] [PubMed] [Google Scholar]
Roztocil 2003 {published data only}
- Roztocil K, Stvrtinova V, Strejcek J. Efficacy of a 6-month treatment with Daflon 500 mg in patients with venous leg ulcers associated with chronic venous insufficiency. International Angiology 2003;22(1):24-31. [PubMed] [Google Scholar]
Sanctis 2001 {published data only}
- Sanctis MT, Cesarone MR, Incandela L, Belcaro G, Ricci A, Griffin M. Four-week treatment with Essaven gel in diabetic microangiopathy--a placebo-controlled, randomized study. Angiology 2001;52 Suppl 3:S49-55. [DOI] [PubMed] [Google Scholar]
Seydewitz 1992 {published data only}
- Seydewitz V, Berg D, Staubesand J, Welbers P. Impact of drug treatment on the activity of lysosomal enzymes in the wall of varicose veins [Einflub einer medikamentösen therapie auf die aktivität lysosomaler enzyme in der varikös veränderten Venenwand]. Phlebologie 1992;21(6):288-92. [Google Scholar]
Steiner 1990 {published data only}
- Steiner M. Investigation on the edema-reducing and edema-protective effect of horse chestnut seed extract. Phlebol Proktol 1990;19(5):239-42. [Google Scholar]
Steiner 1992 {published data only}
- Steiner M. The Extent of the Edema Protective Effect of Horse Chestnut Seed Extract. Vol. 2. Paris: John Libbey Eurotext, 1992. [Google Scholar]
Steru 1988 {published data only}
- Steru D, Steru L. Evaluation clinique d'un phlebotrope: application a l'etude du veinamitol [Evaluation clinique d'un phlebotrope: application a l'etude du veinamitol]. Arteres et Veines 1988;7(4):362-4. [Google Scholar]
Topalov 1990 {published data only}
- Topalov Y, Marinov H, Stanchev S. Troxesamol. Clinical application of the preparation in patients with vascular insufficiency of the lower extremities. Medico Biologie Information 1990;4(4):22-5. [Google Scholar]
Turio 2000 {published data only}
- Turio E, Romanelli M, Barachini P. Clinical arid instrumental evaluation of the efficacy of a vasoactive drug containing vitamin PP, vitamin C and phyto-therapeutic extracts titrated in escin, bromelain and anthocyanosides for the treatment of varicose leg ulcers. Giornale Italiano di Dermatologia e Venereologia 2000;135(1):101-5. [Google Scholar]
Weindorf 1987 {published data only}
- Weindorf N, Schultz Ehrenburg U. Controlled study of increasing venous tone in primary varicose veins by oral administration of Ruscus aculeatus and trimethylhespiridinchalcone. Zeitschrift fur Hautkrankheiten 1987;62:28-38. [PubMed] [Google Scholar]
Widmer 1972 {published data only}
- Widmer LK, Glaus L, Raps E. Local treatment of leg disorders and chronic venous insufficiency. Double blind study of 55 patients. Schweizerische Rundschau fur Medizin Praxis 1972;61(42):1300-4. [PubMed] [Google Scholar]
Zuccarelli 1996 {published data only}
- Zuccarelli F. Evaluation of the effectiveness of Ginkor Fort on the symptoms of chronic venous insufficiency. Phlébologie 1996;49(1):105-10. [Google Scholar]
References to ongoing studies
Barattini 2019 {published data only}
- Barattini DF, Dogaru DE. Clinical trial to assess the efficacy of μSmin® Plus. clinicaltrials.gov/ct2/show/NCT04101201 (first posted 24 September 2019).
ISRCTN18841175 {published data only}18841175
- ISRCTN18841175. Effects of micronised purified flavonoic fraction on microcirculation in women suffering from chronic venous disease. www.isrctn.com/ISRCTN18841175 (first posted 20 July 2009).
NCT01532882 {published data only}
- NCT01532882. Efficacy and safety of Diosmin 600mg versus placebo on painful symptomatology in patients with chronic venous disease of lower limbs (EDEN). clinicaltrials.gov/show/NCT01532882 (first posted 15 Febraury 2012).
NCT03833024 {published data only}
- NCT03833024. The MUFFIN-PTS Trial (MUFFIN-PTS). clinicaltrials.gov/ct2/show/NCT03833024 (first posted 6 February 2019).
Additional references
AEM 2002
- Agencia Española del Medicamento (2002). Re-evaluation of the risk-benefit relationship of oral phlebotonic agents [Re-evaluación de la relación beneficio-riesgo de los agentes flebotónicos para administración por via oral]. https://www.aemps.gob.es/informa/notasinformativas/medicamentosusohumano-3/seguridad-1/2002/ni_2002-09_flebotonicos/ (accessed 27 March 2020).
ATC 2015
- Anatomical Therapeutic Chemical (ATC) system classification. http://www.whocc.no/atc_ddd_index/?code=C05 (accessed December 2015).
Aziz 2015
- Aziz Z, Tang WL, Chong NJ, Tho LY. A systematic review of the efficacy and tolerability of hydroxyethylrutosides for improvement of the signs and symptoms of chronic venous insufficiency. Journal of Clinical Pharmacy and Therapeutics 2015;40(2):177-85. [DOI] [PubMed] [Google Scholar]
Behar 1988
- Behar A, Lagrue G, Cohen-Boulakia F, Baillet J. Capillary filtration in idiopathic cyclic edema - effects of Daflon 500 mg. Nuklearmedizin 1988;27(3):105-7. [PubMed] [Google Scholar]
Boada 1999
- Boada JN, Nazco GJ. Therapeutic effect of venotonics in chronic venous insufficiency. A meta-analysis. Clinical Drug Investigation 1999;18(6):413-32. [Google Scholar]
Brand 1988
- Brand FN, Dannenberg AL, Abbott RD, Kannel WB. The epidemiology of varicose veins: the Framingham Study. American Journal of Preventive Medicine 1988;4(2):96-101. [PubMed] [Google Scholar]
Carpentier 2000
- Carpentier PH. Epidemiology and physiopathology of chronic venous leg diseases. Revue du Praticien 2000;50(11):1176-81. [PubMed] [Google Scholar]
Cesarone 2006
- Cesarone MR, Belcaro G, Rohdewald P, Pellegrini L, Ledda A, Vinciguerra G, et al. Comparison of Pycnogenol and Daflon in treating chronic venous insufficiency: a prospective, controlled study. Clinical and Applied Thrombosis/Hemostasis 2006;12(2):205-12. [DOI] [PubMed] [Google Scholar]
Ciapponi 2004
- Ciapponi A, Laffaire E, Roque M. Calcium dobesilate for chronic venous insufficiency: a systematic review. Angiology 2004;55(2):147-54. [DOI] [PubMed] [Google Scholar]
Clarke 2003
- Clarke M, Oxman AD, editors. Cochrane Reviewers’ Handbook [updated March 2003]. Oxford, UK: The Cochrane Library. The Cochrane Collaboration. Update Software; Issue 2, 2003.
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, on behalf of the Cochrane Statistical Methods Group (editors). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane-handbook.org.
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
Diehm 1996b
- Diehm C. The role of oedema protective drugs in the treatment of chronic venous insufficiency: a review of evidence based on placebo-controlled clinical trials with regard to efficacy and tolerance. Phlebology 1996;11(1):23-9. [Google Scholar]
Eklöf 2004
- Eklöf B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, et al, American Venous Forum International Ad Hoc Committee for Revision of the CEAP Classification. Revision of the CEAP classification for chronic venous disorders: consensus statement. Journal of Vascular Surgery 2004;40(6):1248-52. [DOI] [PubMed] [Google Scholar]
Evans 1999
- Evans CJ, Fowkes FG, Ruckley CV, Lee AJ. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. Journal of Epidemiology and Community Health 1999;53(3):149-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- GRADEpro Version 3.2 for Windows [GRADEpro. www.gradepro.org].]. Brozek J, Oxman A, Schünemann H. Ontario, Canada: McMaster University, 2008.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane-handbook.org.
Ibañez 2000
- Ibañez L, Ballarín E, Vidal X, Laporte JR. Agranulocytosis associated with calcium dobesilate. Clinical course and risk estimation with the case-control and the case-population approaches. European Journal of Clinical Pharmacology 2000;56(9-10):763-7. [DOI] [PubMed] [Google Scholar]
Ibáñez 2005
- Ibáñez L, Vidal X, Ballarín E, Laporte JR. Population-based drug-induced agranulocytosis. Archives of Internal Medicine 2005;165(8):869-74. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials 1996;17(1):1-12. [DOI] [PubMed] [Google Scholar]
Kakkos 2018
- Kakkos SK, Nicolaides AN. Efficacy of micronized purified flavonoid fraction (Daflon®) on improving individual symptoms,signs and quality of life in patients with chronic venous disease: a systematic review and meta-analysis of randomized double-blind placebo-controlled trials. Int Angiol 2018;37:143-54. [DOI] [PubMed] [Google Scholar]
Kaufman 1991
- Kaufman DW, Kelly JP, Levy Shapiro S. The Drug Etiology of Agranulocytosis and Aplastic Anemia. New York: Oxford University Press, 1991. [Google Scholar]
Kurz 1999
- Kurz X, Kahn SR, Abenhaim L, Clement D, Norgren L, Baccaglini U, et al. Chronic venous disorders of the leg: epidemiology, outcomes, diagnosis and management. Summary of an evidence-based report of the VEINES task force. Venous Insufficiency Epidemiologic and Economic Studies. International Angiology 1999;18(2):83-102. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6. Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from handbook.cochrane.org 2011.
Lurie 2020
- Lurie F, Passman M, Meisner M, Dalsing M, Masuda E, Welch H, et al. The 2020 update of the CEAP classification system and reporting standards. Journal of Vascular Surgery 2020;8(3):342-352. [DOI] [PubMed] [Google Scholar]
Markwardt 1996
- Markwardt F. Pharmacology of oedema protective drugs. Phlebology 1996;11(1):10-5. [Google Scholar]
Nicolaides 2000
- Nicolaides AN Cardiovascular Disease Educational and Research Trust European Society of Vascular Surgery The International Angiology Scientific Activity Congress Organization International Union of Angiology Union Internationale de Phlebologie at the Abbaye des Vaux de Cernay. Investigation of chronic venous insufficiency: a consensus statement (France, March 5-9, 1997). Circulation 2000;102(20):E126-63. [DOI] [PubMed] [Google Scholar]
Pittler 1998
- Pittler MH, Ernst E. Horse-Chestnut seed extract for chronic venous insufficiency: a criteria-based systematic review. Archives of Dermatology 1998;134(11):1356-60. [DOI] [PubMed] [Google Scholar]
Pittler 2012
- Pittler MH, Ernst E. Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD003230. [DOI: 10.1002/14651858.CD003230.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Porter 1995
- Porter JM, Moneta GL. Reporting standards in venous disease: an update. International Consensus Committee on Chronic Venous Disease. Journal of Vascular Surgery 1995;21(4):635-45. [DOI] [PubMed] [Google Scholar]
Poynard 1994
- Poynard T, Valterio C. Meta-analysis of hydroxyethylrutosides in the treatment of chronic venous insufficiency. Vasa 1994;23(3):244-50. [PubMed] [Google Scholar]
Rabe 2013
- Rabe E, Guex JJ, Morrison N, Ramelet AA, Schuller-Petrovic S, Scuderi A, et al. Treatment of chronic venous disease with flavonoids: recommendations for treatment and further studies. [Review]. Phlebology 2013;28(6):308-19. [DOI] [PubMed] [Google Scholar]
Robertson 2014
- Robertson LA, Evans CJ, Lee AJ, Allan PL, Ruckley CV, Fowkes FG. Incidence and risk factors for venous reflux in the general population: Edinburgh Vein Study. European Journal of Vascular and Endovascular Surgery 2014;48(2):208-14. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and “Summary of findings” tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of interventions Version 5.1.0. London, UK: The Cochrane Collaboration. www.cochrane-handbook.org, 2011. [Google Scholar]
Scott 1995
- Scott TE, LaMorte WW, Gorin DR, Menzoain JO. Risk factors for chronic venous insufficiency: a dual case-control study. Journal of Vascular Surgery 1995;22(5):622-8. [DOI] [PubMed] [Google Scholar]
Stanhope 1975
- Stanhope JM. Varicose veins in a population of New Guinea. International Journal of Epidemiology 1975;4(3):221-5. [DOI] [PubMed] [Google Scholar]
Tsouderos 1991
- Tsouredos Y. Venous tone: are the phlebotonics properties predictive of a therapeutic benefit? A comprehensive view of our experience with Daflon 500 mg. Zeitschrift fur Kardiologie 1991;80 Suppl 7:95-101. [PubMed] [Google Scholar]
Van den Oever 1998
- Van den Oever R, Hepp B, Debbaut B, Simon I. Socio-economic impact of chronic venous insufficiency. An underestimated public health problem. International Angiology 1998;17(3):161-7. [PubMed] [Google Scholar]
Vasquez 2010
- Vasquez MA, Rabe E, McLafferty RB, Shortell CK, Marston WA, Gillespie D, et al. Revision of the venous clinical severity score: venous outcomes consensus statement: special communication of the American Venous Forum Ad Hoc Outcomes Working Group. Journal of Vascular Surgery 2010;52(5):1387-96. [DOI] [PubMed] [Google Scholar]
Venous Forum 2011
- Venous Forum of the Royal Society of Medicine, Berridge D, Bradbury AW, Davies AH, Gohel M, Nyamekye I, et al. Recommendations for the referral and treatment of patients with lower limb chronic venous insufficiency (including varicose veins). Phlebology 2011;26(3):91-3. [DOI] [PubMed] [Google Scholar]
Wadworth 1992
- Wadworth AN, Faulds D. Hydroxyethylrutosides. A review of its pharmacology and therapeutic efficacy in venous insufficiency and related disorders. Drugs 1992;44(6):1013-32. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Martinez 2001
- Martinez MJ, Bonfill X, Moreno RM, Cachà A, Vargas E, Capellà D. Phlebotonics for venous insufficiency. Cochrane Database of Systematic Reviews 2001, Issue 1. Art. No: CD003229. [DOI: 10.1002/14651858.CD003229] [DOI] [PubMed] [Google Scholar]
Martinez‐Zapata 2005
- Martinez-Zapata MJ, Bonfill Cosp X, Moreno RM, Vargas E, Capellà D. Phlebotonics for venous insufficiency. Cochrane Database of Systematic Reviews 2005, Issue 3. Art. No: CD003229. [DOI: 10.1002/14651858.CD003229.pub2] [DOI] [PubMed] [Google Scholar]
Martinez‐Zapata 2016
- Martinez-Zapata MJ, Vernooij RW, Uriona Tuma SM, Stein AT, Moreno RM, Vargas E, Capellà D, Bonfill Cosp X. Phlebotonics for venous insufficiency. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No: CD003229. [DOI: 10.1002/14651858.CD003229.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


