Abstract
Background
Respiratory morbidity including respiratory distress syndrome (RDS) is a serious complication of preterm birth and the primary cause of early neonatal mortality and disability. Despite early evidence indicating a beneficial effect of antenatal corticosteroids on fetal lung maturation and widespread recommendations to use this treatment in women at risk of preterm delivery, some uncertainty remains about their effectiveness particularly with regard to their use in lower‐resource settings, different gestational ages and high‐risk obstetric groups such as women with hypertension or multiple pregnancies.
This updated review (which supersedes an earlier review Crowley 1996) was first published in 2006 and subsequently updated in 2017.
Objectives
To assess the effects of administering a course of corticosteroids to women prior to anticipated preterm birth (before 37 weeks of pregnancy) on fetal and neonatal morbidity and mortality, maternal mortality and morbidity, and on the child in later life.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (3 September 2020), ClinicalTrials.gov, the databases that contribute to the WHO International Clinical Trials Registry Platform (ICTRP) (3 September 2020), and reference lists of the retrieved studies.
Selection criteria
We considered all randomised controlled comparisons of antenatal corticosteroid administration with placebo, or with no treatment, given to women with a singleton or multiple pregnancy, prior to anticipated preterm delivery (elective, or following rupture of membranes or spontaneous labour), regardless of other co‐morbidity, for inclusion in this review.
Data collection and analysis
We used standard Cochrane Pregnancy and Childbirth methods for data collection and analysis. Two review authors independently assessed trials for inclusion, assessed risk of bias, evaluated trustworthiness based on predefined criteria developed by Cochrane Pregnancy and Childbirth, extracted data and checked them for accuracy, and assessed the certainty of the evidence using the GRADE approach. Primary outcomes included perinatal death, neonatal death, RDS, intraventricular haemorrhage (IVH), birthweight, developmental delay in childhood and maternal death.
Main results
We included 27 studies (11,272 randomised women and 11,925 neonates) from 20 countries. Ten trials (4422 randomised women) took place in lower‐ or middle‐resource settings.
We removed six trials from the analysis that were included in the previous version of the review; this review only includes trials that meet our pre‐defined trustworthiness criteria. In 19 trials the women received a single course of steroids. In the remaining eight trials repeated courses may have been prescribed.
Fifteen trials were judged to be at low risk of bias, two had a high risk of bias in two or more domains and ten trials had a high risk of bias due to lack of blinding (placebo was not used in the control arm.
Overall, the certainty of evidence was moderate to high, but it was downgraded for IVH due to indirectness; for developmental delay due to risk of bias and for maternal adverse outcomes (death, chorioamnionitis and endometritis) due to imprecision.
Neonatal/child outcomes
Antenatal corticosteroids reduce the risk of:
‐ perinatal death (risk ratio (RR) 0.85, 95% confidence interval (CI) 0.77 to 0.93; 9833 infants; 14 studies; high‐certainty evidence; 2.3% fewer, 95% CI 1.1% to 3.6% fewer),
‐ neonatal death (RR 0.78, 95% CI 0.70 to 0.87; 10,609 infants; 22 studies; high‐certainty evidence; 2.6% fewer, 95% CI 1.5% to 3.6% fewer),
‐ respiratory distress syndrome (RR 0.71, 95% CI 0.65 to 0.78; 11,183 infants; studies = 26; high‐certainty evidence; 4.3% fewer, 95% CI 3.2% to 5.2% fewer).
Antenatal corticosteroids probably reduce the risk of IVH (RR 0.58, 95% CI 0.45 to 0.75; 8475 infants; 12 studies; moderate‐certainty evidence; 1.4% fewer, 95% CI 0.8% to1.8% fewer), and probably have little to no effect on birthweight (mean difference (MD) ‐14.02 g, 95% CI ‐33.79 to 5.76; 9551 infants; 19 studies; high‐certainty evidence).
Antenatal corticosteroids probably lead to a reduction in developmental delay in childhood (RR 0.51, 95% CI 0.27 to 0.97; 600 children; 3 studies; moderate‐certainty evidence; 3.8% fewer, 95% CI 0.2% to 5.7% fewer).
Maternal outcomes
Antenatal corticosteroids probably result in little to no difference in maternal death (RR 1.19, 95% CI 0.36 to 3.89; 6244 women; 6 studies; moderate‐certainty evidence; 0.0% fewer, 95% CI 0.1% fewer to 0.5% more), chorioamnionitis (RR 0.86, 95% CI 0.69 to 1.08; 8374 women; 15 studies; moderate‐certainty evidence; 0.5% fewer, 95% CI 1.1% fewer to 0.3% more), and endometritis (RR 1.14, 95% CI 0.82 to 1.58; 6764 women; 10 studies; moderate‐certainty; 0.3% more, 95% CI 0.3% fewer to 1.1% more)
The wide 95% CIs in all of these outcomes include possible benefit and possible harm.
Authors' conclusions
Evidence from this updated review supports the continued use of a single course of antenatal corticosteroids to accelerate fetal lung maturation in women at risk of preterm birth. Treatment with antenatal corticosteroids reduces the risk of perinatal death, neonatal death and RDS and probably reduces the risk of IVH. This evidence is robust, regardless of resource setting (high, middle or low).
Further research should focus on variations in the treatment regimen, effectiveness of the intervention in specific understudied subgroups such as multiple pregnancies and other high‐risk obstetric groups, and the risks and benefits in the very early or very late preterm periods. Additionally, outcomes from existing trials with follow‐up into childhood and adulthood are needed in order to investigate any longer‐term effects of antenatal corticosteroids.
We encourage authors of previous studies to provide further information which may answer any remaining questions about the use of antenatal corticosteroids without the need for further randomised controlled trials. Individual patient data meta‐analyses from published trials are likely to provide answers for most of the remaining clinical uncertainties.
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Adrenal Cortex Hormones; Adrenal Cortex Hormones/administration & dosage; Betamethasone; Betamethasone/administration & dosage; Bias; Cerebral Intraventricular Hemorrhage; Cerebral Intraventricular Hemorrhage/prevention & control; Developmental Disabilities; Developmental Disabilities/epidemiology; Dexamethasone; Dexamethasone/administration & dosage; Fetal Organ Maturity; Fetal Organ Maturity/drug effects; Hydrocortisone; Hydrocortisone/administration & dosage; Lung; Lung/drug effects; Lung/embryology; Maternal Death; Perinatal Death; Premature Birth; Prenatal Care; Prenatal Care/methods; Randomized Controlled Trials as Topic; Respiratory Distress Syndrome, Newborn; Respiratory Distress Syndrome, Newborn/prevention & control
Plain language summary
What are the benefits and risks of giving corticosteroids to pregnant women at risk of premature birth?
Why is this question important?
Babies born prematurely (before 37 weeks of pregnancy) can have trouble breathing if their lungs are not sufficiently developed. Up to half of babies born before 28 weeks, and a third of babies born before 32 weeks, have problems breathing and many babies do not survive. Others may become disabled due to the lack of oxygen they suffer because of the breathing difficulties experienced at birth.
Women who may be at risk of giving birth prematurely can be given corticosteroids to prevent their babies from having trouble breathing once they are born. Corticosteroids are anti‐inflammation medicines that help the baby’s lungs mature before being born. They are usually given to women at risk of early labour, typically as two injections, though they can also be given before planned preterm birth and in some cases a repeat course can be given.
To find out about the benefits and risks of giving corticosteroids to women at risk of giving birth early, we reviewed the evidence from research studies.
How did we identify and evaluate the evidence?
We searched the medical literature for studies that compared the effects of corticosteroids against:
‐ a placebo (dummy) treatment; or
‐ no treatment.
We compared the results and summarised the evidence from all the studies. We rated our confidence in the evidence, based on factors such as study methods and sizes, and the consistency of findings across studies.
What did we find?
We found 27 studies that involved 11,272 women and 11,925 infants. The studies were set in 21 different countries, which included high‐, middle‐ and low‐income countries.
Infant health
Robust evidence shows that corticosteroids:
‐ reduce perinatal deaths (numbers of stillbirths and babies dying in the first 28 days of life);
‐ reduce neonatal deaths (numbers of babies dying in the first 28 days of life);
‐ reduce serious breathing problems in the first hours of life;
‐ have little to no effect on babies’ birth weight.
Corticosteroids probably reduce the risk of:
‐ bleeding inside the brain;
‐ developmental delay in later childhood.
We are only moderately confident about these two findings, either because:
‐ the infants in the studies may not have been representative of all babies born prematurely; or
‐ studies may have been conducted in ways that introduced errors into their results.
Maternal health
The evidence indicates that corticosteroids probably do not affect the risk of:
‐ mothers dying after giving birth;
‐ developing chorioamnionitis (inflammation or infection of the tissues that surround the baby in pregnancy);
‐ developing endometritis (inflammation of the lining of the uterus).
We are only moderately confident about these three findings because they are based on few events. Until we have more evidence from more women, we cannot be certain that there is no difference in risk.
We found little evidence about:
‐ women who were pregnant with multiple babies; women with high blood pressure; or women whose membranes surrounding the baby broke early;
‐ the effects of corticosteroids in babies born prematurely versus very prematurely;
‐ different doses of corticosteroids.
This means that we cannot be certain that the findings in this review apply to all women and babies at risk of premature birth. Nor can we determine which dose of corticosteroids is best.
What does this mean?
Corticosteroids given to women at risk of premature birth improve the chances that, once they are born, their babies will be able to breathe and survive.
The evidence available suggests that corticosteroids are probably not associated with risks for the baby or mother. Further evidence is needed about:
‐ whether corticosteroids work differently for women who expect multiple babies or who have high blood pressure;
‐ whether the benefits and risks of corticosteroids are the same when babies are born very prematurely, or less prematurely;
‐ which dose of corticosteroids works best.
How‐up‐to date is this review?
The evidence in this Cochrane Review is current to September 2020.
A visual summary of some of the results from this review can be found here.
Summary of findings
Summary of findings 1. Neonatal and child outcomes: corticosteroids compared to placebo or no treatment for accelerating fetal lung maturation for women at risk of preterm birth.
| Neonatal and child outcomes: corticosteroids compared to placebo or no treatment for accelerating fetal lung maturation for women at risk of preterm birth | ||||||
| Patient or population: infants born of women at risk of preterm birth Setting: hospitals settings in low‐, middle‐ and high‐resource countries Intervention: corticosteroids Comparison: placebo or no treatment | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| Without Corticosteroids | With Corticosteroids | Difference | ||||
| Perinatal death (composite of fetal death (in utero death) and neonatal death) № of participants: 9833 (14 RCTs) | RR 0.85 (0.77 to 0.93) | Study population | ⊕⊕⊕⊕ HIGH | Corticosteroids reduce perinatal death compared with placebo or no treatment. | ||
| 15.6% | 13.3% (12 to 14.6) | 2.3% fewer (3.6 fewer to 1.1 fewer) | ||||
| Neonatal death (infants born with signs of life who die within the first 28 days) № of participants: 10,609 (22 RCTs) | RR 0.78 (0.70 to 0.87) | Study population | ⊕⊕⊕⊕ HIGH | Corticosteroids reduce neonatal death compared with placebo or no treatment. | ||
| 11.9% | 9.3% (8.3 to 10.3) | 2.6% fewer (3.6 fewer to 1.5 fewer) | ||||
| Respiratory distress syndrome № of participants: 11,183 (26 RCTs) | RR 0.71 (0.65 to 0.78) | Study population | ⊕⊕⊕⊕ HIGH | Corticosteroids reduce respiratory distress syndrome compared with placebo or no treatment. We did not downgrade for risk of bias (two trials) at high risk of bias due to lack of placebo in control) because sensitivity analysis removing those trials made very little difference to the effect estimate. |
||
| 14.8% | 10.5% (9.6 to 11.5) | 4.3% fewer (5.2 fewer to 3.2 fewer) | ||||
| Intraventricular haemorrhage (IVH) № of participants: 8475 (12 RCTs) | RR 0.58 (0.45 to 0.75) | Study population | ⊕⊕⊕⊝ MODERATE 1 | Corticosteroids probably reduce intraventricular haemorrhage compared with placebo or no treatment. We did not downgrade for risk of bias (four trials infants) at high risk of bias due to lack of placebo in control groups) because sensitivity analysis removing those trials made very little difference to the effect estimate. |
||
| 3.3% | 1.9% (1.5 to 2.5) | 1.4% fewer (1.8 fewer to 0.8 fewer) | ||||
| Mean birthweight (g) № of participants: 9551 (19 RCTs) | ‐ | The mean birthweight in the control group ranged from 941 g to 2654 g | ‐ | MD 14.02 lower (33.79 lower to 5.76 higher) | ⊕⊕⊕⊕ HIGH | Corticosteroids result in little to no difference in mean birthweight compared with placebo or no treatment. We did not downgrade for risk of bias (two trials at high risk of bias due to incomplete outcome data) because sensitivity analysis removing those trials made very little difference to the effect estimate. We did not downgrade for imprecision because the confidence interval showed a difference at most on average of 33 g in weight, which is less than 10% of the lightest mean weight in any trial. |
| Developmental delay in childhood
№ of participants: 600 (3 RCTs). Age at follow‐up: 2 to 12 years. |
RR 0.51 (0.27 to 0.97) | 7.7% | 4.0% (2.1 to 7.5) |
3.8% fewer (5.7 fewer to 0.2 fewer) |
⊕⊕⊕⊝ MODERATE 2 | Corticosteroids probably lead to a slight reduction in developmental delay in childhood compared with placebo or no treatment. Additionally, in three studies (778 children) it was uncertain if corticosteroids had any effect on intellectual impairment (RR 0.86, 95% CI 0.44 to 1.69). In two studies (166 children) it was uncertain if corticosteroids had any effect on the risk of visual impairment (RR 0.55, 95% CI 0.24 to 1.23) and in one study (82 children) it was uncertain if corticosteroids have any effect on hearing impairment (RR 0.64, 95% CI 0.04 to 9.87). Another study reported no children with hearing impairment in either group (84 children). |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level for indirectness: in some trials only a subset of infants were screened for IVH; some trials diagnosed IVH at postmortem only.
2 Downgraded one level for risk of bias: unclear randomisation, allocation concealment, incomplete outcome data and selective reporting
Summary of findings 2. Maternal outcomes: corticosteroids compared to placebo or no treatment for accelerating fetal lung maturation for women at risk of preterm birth.
| Maternal outcomes: corticosteroids compared to placebo or no treatment for accelerating fetal lung maturation for women at risk of preterm birth | ||||||
| Patient or population: women at risk of preterm birth Setting: hospitals settings in low‐, middle‐ and high‐resource countries Intervention: corticosteroids Comparison: placebo or no treatment | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| Without Corticosteroids | With Corticosteroids | Difference | ||||
| Maternal death follow up: 90 days № of participants: 6244 (6 RCTs) | RR 1.19 (0.36 to 3.89) | Study population | ⊕⊕⊕⊝ MODERATE 1 | Corticosteroids probably result in little to no difference in maternal death, but the wide 95% CI includes possible benefit and possible harm, compared to placebo or no treatment. Four studies (3174 women) reported zero deaths in either arm. |
||
| 0.2% | 0.2% (0.1 to 0.6) | 0.0% fewer (0.1 fewer to 0.5 more) | ||||
| Chorioamnionitis № of participants: 8374 (15 RCTs) | RR 0.86 (0.69 to 1.08) | Study population | ⊕⊕⊕⊝ MODERATE 2 | Corticosteroids probably make little to no difference to the risk of chorioamnionitis but the wide 95% CI includes possible benefit and possible harm, compared to placebo/no treatment. | ||
| 3.5% | 3.0% (2.4 to 3.8) | 0.5% fewer (1.1 fewer to 0.3 more) | ||||
| Endometritis № of participants: 6764 (10 RCTs) | RR 1.14 (0.82 to 1.58) | Study population | ⊕⊕⊕⊝ MODERATE 1 | Corticosteroids probably make little to no difference to the risk of endometritis but the wide 95% CI includes possible benefit and possible harm, compared to placebo/no treatment. | ||
| 1.8% | 2.1% (1.5 to 2.9) | 0.3% more (0.3 fewer to 1.1 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio, | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level for imprecision: few events and wide 95% CI that includes possible benefit and possible harm
2 Downgraded one level for imprecision: wide 95% CI that includes possible benefit and possible harm
Background
This updated review, which supersedes an earlier review Crowley 1996, was first published in 2006 and subsequently updated in 2017.
Description of the condition
Respiratory distress syndrome (RDS) is a serious complication of preterm birth and the primary cause of early neonatal death and disability (Rodriguez 2002). It affects up to half of babies born before 28 weeks and a third of babies born before 32 weeks. Approximately 42% of extremely low birthweight babies have RDS (Hintz 2007).
Respiratory failure in these infants occurs as a result of surfactant deficiency, poor lung anatomical development and immaturity in other organs. Neonatal survival after preterm birth improves with gestation (Doyle 2001a), reflecting improved maturity of organ systems. However, those who survive early neonatal care are at increased risk of long‐term neurological disability (Doyle 2001b).
Some understanding of fetal lung development may be useful in understanding why RDS occurs and why corticosteroids work. Fetal lung development can be divided into five stages: embryonic, pseudoglandular, canalicular, terminal sac and alveolar. From 28 to 35 weeks' gestation, the alveoli can be counted and with increasing age they become more mature. Lung volume increases four‐fold between 29 weeks and term. Alveolar number shows a curvilinear increase with age but a linear relationship with bodyweight. At birth there are an average of 150 million alveoli (half the expected adult number). The alveoli produce surfactant. The alveolar stage continues for one to two years after birth. In the preterm infant, low alveolar numbers probably contribute to respiratory dysfunction.
The fetal lung also matures biochemically with increasing gestation. Lamellar bodies, which store surfactant, appear at 22 to 24 weeks. Surfactant is a complex mixture of lipids and apoproteins, the main constituents of which are dipalmitoylphosphatidyl choline, phosphatidylglycerol and apoproteins A, B, C and D. Surfactant is needed to maintain stability when breathing out, to prevent collapse of the alveoli. Premature infants have a qualitative and quantitative deficiency of surfactant, which predisposes them to RDS. At the low lung volume associated with expiration, surface tension becomes very high, leading to atelectasis with subsequent intrapulmonary shunting, ventilation perfusion inequalities, and ultimately respiratory failure. Capillary leakage allows inhibitors from plasma to reach alveoli and inactivate any surfactant that may be present. Hypoxia, acidosis and hypothermia (common problems in the very preterm infant) can reduce surfactant synthesis required to replenish surfactant lost from the system. The pulmonary antioxidant system develops in parallel to the surfactant system and deficiency in this also puts the preterm infant at risk of chronic lung disease.
Description of the intervention
While researching the effects of the steroid dexamethasone on premature parturition in fetal sheep in 1969, Liggins found that there was some inflation of the lungs of lambs born at gestations at which the lungs would be expected to be airless (Liggins 1969). Liggins and Howie performed the first randomised controlled trial in humans of betamethasone for the prevention of RDS in 1972 (Liggins 1972a).
Subsequent to the original Liggins study a large number of clinical trials have been performed on the effects of corticosteroids before preterm birth. The first structured review on corticosteroids in preterm birth was published in 1990 (Crowley 1990). This review showed that corticosteroids given prior to preterm birth (as a result of either preterm labour or planned preterm delivery) are effective in preventing RDS and neonatal mortality. Corticosteroid treatment was also associated with a significant reduction in the risk of intraventricular haemorrhage (IVH; bleeding into the brain). Corticosteroids appear to exert major vasoconstrictive effects on fetal cerebral blood flow, protecting the fetus against IVH at rest and when challenged by conditions causing vasodilatation such as hypercapnia (Schwab 2000). Crowley found no effect on necrotising enterocolitis or chronic lung disease from antenatal corticosteroid administration. The influence of the results of the original trial and Crowley's review was the subject of a Wellcome Witness Seminar (Wellcome 2005) held in 2004.
Corticosteroids have become the mainstay of prophylactic treatment in preterm birth, as a result of these findings and subsequent work. However, there have remained a number of outstanding issues regarding the use of antenatal corticosteroids. The original trial by Liggins suggested an increased rate of stillbirth in women with hypertension syndromes (Liggins 1976). There is concern about using corticosteroids in women with premature rupture of membranes due to the possible increased risk of neonatal and maternal infection (Imseis 1996; NIH 1994). The efficacy of this treatment in multiple births has only been addressed retrospectively (Turrentine 1996). From the time of the original Liggins paper, debate has continued around whether the treatment is effective at lower gestations and at differing treatment‐to‐delivery intervals. Debate has also centred around whether treatment is effective at latter gestations, up to and including term delivery (Sotiriadis 2018). These issues will be addressed in this review in subgroup analyses. The effectiveness and safety of repeat doses of corticosteroids for women who remain undelivered, but at increased risk of preterm birth after an initial course of treatment, is addressed in a separate Cochrane Review (Crowther 2015).
Epidemiological evidence and animal work suggests that there may be adverse long‐term consequences of antenatal exposure to corticosteroids (Seckl 2000). Exposure to excess corticosteroids before birth is hypothesised to be a key mechanism underlying the fetal origins of adult disease hypothesis (Barker 1998; Benediktsson 1993). This hypothesis postulates a link between impaired fetal growth, and cardiovascular disease and type 2 diabetes in later life along with their risk factors of impaired glucose tolerance, dyslipidaemia, and hypertension (Barker 1998). A large body of animal experimental work has documented impaired glucose tolerance and increased blood pressure in adult animals after antenatal exposure to corticosteroids (Clark 1998; Dodic 1999; Edwards 2001). Thus, this review has considered blood pressure, glucose intolerance, dyslipidaemia, and hypothalamo‐pituitary‐adrenal axis function in childhood and adulthood.
Experimental animal studies have also shown decreased brain growth in preterm and term infants exposed to single courses of corticosteroid (Huang 1999; Jobe 1998). This review has therefore also addressed long‐term neurodevelopment and other childhood and adult outcomes after antenatal corticosteroid exposure.
How the intervention might work
Liggins (Liggins 1972a) theorised that dexamethasone might have accelerated the appearance of pulmonary surfactant. The hypothesis is that corticosteroids act to trigger the synthesis of ribonucleic acid that codes for particular proteins involved in the biosynthesis of phospholipids or in the breakdown of glycogen. Subsequent work has suggested that, in animal models, corticosteroids mature a number of organ systems (Padbury 1996; Vyas 1997).
Why it is important to do this review
Since the first version of the review was published there is a need for an updated systematic review of the effects of prophylactic corticosteroids for preterm birth, as a result of current interest and due to further published trials. In Roberts 2006 we were able to re‐analyse the Auckland Steroid Study by intention‐to‐treat. In Roberts 2017 we updated the review as the methodology for Cochrane Reviews had changed and we attempted to standardise the review with the Cochrane Review on 'Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes' (Crowther 2015). This current (2020) update has been stimulated by the publication of substantial data from new trials in low‐resource settings becoming available and the need to incorporate this new evidence into the review to provide a more definitive answer to the question of the effectiveness of antenatal corticosteroids.
Objectives
To assess the effects of administering a course of corticosteroids to the mother prior to anticipated preterm birth on fetal and neonatal morbidity and mortality, maternal mortality and morbidity, and on the child in later life.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs) for inclusion in this review, regardless of whether or not they were published in full. Cluster‐randomised as well as individually‐randomised trials were eligible. Quasi‐randomised (e.g. allocation by date of birth or record number) and cross‐over trials were not eligible for inclusion. We excluded trials where non‐randomised cohorts were amalgamated with randomised participants if the results of the randomised participants could not be separated out.
Types of participants
Women, with a singleton or multiple pregnancy, expected to deliver before 37 weeks of pregnancy as a result of either spontaneous preterm labour, preterm prelabour rupture of the membranes or planned preterm delivery, regardless of other co‐morbidity.
Types of interventions
Trials investigating a corticosteroid capable of crossing the placenta (betamethasone, dexamethasone, hydrocortisone, methylprednisolone) compared with placebo or with no treatment.
We excluded trials that tested the effect of corticosteroids along with other co‐interventions. We also excluded trials where all participants received corticosteroids before beginning their allocated treatment of corticosteroids or placebo/no treatment.
Types of outcome measures
We did not use the reporting of particular outcomes as a criterion for eligibility for review. We did not exclude studies from review solely on the grounds of an outcome of interest not being reported. Primary outcomes chosen were those which were thought to be the most clinically valuable in assessing effectiveness and safety of the treatment for the woman and her offspring. Secondary outcomes included possible complications and other measures of effectiveness.
Primary outcomes
For the fetus/neonate.
Perinatal death (composite of fetal death (in utero death) and neonatal death).
Neonatal death (before 28 completed days of life).
Fetal death (in utero death).
Respiratory distress syndrome (RDS).
Moderate/severe RDS.
Chronic lung disease (need for continuous supplemental oxygen at 28 days postnatal age or 36 weeks' postmenstrual age, whichever was later).
Intraventricular haemorrhage (IVH).
Mean birthweight (g).
For the woman.
Maternal death (up to 90 days after giving birth).
Chorioamnionitis (however defined by study authors).
Endometritis (however defined by study authors and including infections).
For the child.
Death.
Neurodevelopmental disability at follow‐up (visual impairment, hearing impairment, intellectual impairment defined as intelligence quotient less than ‐2 standard deviation below population mean, moderate/severe cerebral palsy (however defined by study authors), or developmental delay (defined as developmental quotient less than ‐2 standard deviation below population mean)).
For the child as adult.
Death.
Neurodevelopmental disability at follow‐up (visual impairment, hearing impairment, intellectual impairment defined as intelligence quotient less than ‐2 standard deviation below population mean, moderate/severe cerebral palsy (however defined by study authors), or developmental delay (defined as developmental quotient less than ‐2 standard deviation below population mean)).
Secondary outcomes
For the woman.
Fever after trial entry requiring the use of antibiotics.
Intrapartum fever requiring the use of antibiotics.
Postnatal fever.
Admission to intensive care unit.
Side effects of therapy.
Glucose intolerance (however defined by study authors).
Hypertension (however defined by study authors).
For the fetus/neonate.
Apgar score less than seven at five minutes.
Interval between trial entry and birth.
Mean length at birth (height).
Mean head circumference at birth.
Mean skin fold thickness at birth.
Small‐for‐gestational age (however defined by study authors).
Mean placental weight.
Neonatal blood pressure.
Admission to neonatal intensive care unit (NICU).
Need for inotropic support.
Mean duration of inotropic support (days).
Need for mechanical ventilation/continuous positive airways pressure.
Mean duration of mechanical ventilation/continuous positive airways pressure (days).
Air leak syndrome.
Duration of oxygen supplementation (days).
Surfactant use.
Systemic infection in first 48 hours of life.
Proven infection while in the NICU.
Necrotising enterocolitis.
Hypothalamo‐pituitary‐adrenal (HPA) axis function (however defined by study authors).
For the child.
Mean weight.
Mean head circumference.
Mean height.
Mean skin fold thickness.
Abnormal lung function (however defined by study authors).
Mean blood pressure.
Glucose intolerance (however defined by study authors).
HPA axis function (however defined by study authors).
Dyslipidaemia (however defined by study authors).
Cerebral palsy (however defined by study authors).
Behavioural/learning difficulties (however defined by study authors).
For the child as adult.
Mean weight.
Mean head circumference.
Mean height.
Mean skin fold thickness.
Abnormal lung function (however defined by study authors).
Mean blood pressure.
Glucose intolerance (however defined by study authors).
HPA axis function (however defined by study authors).
Dyslipidaemia (however defined by study authors).
Mean age at puberty.
Bone density (however defined by study authors).
Educational achievement (completion of high school, or however defined by study authors).
For health services.
Mean length of antenatal hospitalisation for women (days).
Mean length of postnatal hospitalisation for women (days).
Mean length of neonatal hospitalisation (days).
Cost of maternal care (in 10s of 1000s of USD).
Cost of neonatal care (in 10s of 1000s of USD).
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (3 September 2020).
The Register is a database containing over 26,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched ClinicalTrials.gov, and the databases that contribute to the WHO International Clinical Trials Registry Platform (ICTRP) (3 September 2020) for unpublished, planned and ongoing trial reports (see Appendix 1 for search terms we used).
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in previous versions of this review, see Roberts 2006; Roberts 2017.
For this update, we used the following methods to assess the new reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors assessed the trials for eligibility and trustworthiness. Trials were not assessed blind, as we knew the author's name, institution and the source of publication. We resolved any disagreement by discussion until we reached consensus.
Screening eligible studies for trustworthiness
All studies meeting our inclusion criteria were evaluated by at least two review authors against predefined criteria to select studies that, based on available information, are deemed to be sufficiently trustworthy to be included in the analysis. The trustworthiness screening tool was developed by Cochrane Pregnancy and Childbirth and contains the following criteria: reasons for rejecting a trial include the following.
Research governance
Was the study prospectively registered (for those studies published after 2010) If not, was there a plausible reason?
When requested, did the trial authors provide/share the protocol and/or ethics approval letter?
Did the trial authors engage in communication with the Cochrane Review authors within the agreed timelines?
Did the trial authors provide IPD data upon request? If not, was there a plausible reason?.
Baseline characteristics
Is the study free from characteristics of the study participants that appear too similar (e.g. distribution of the mean (SD) excessively narrow or excessively wide, as noted by Carlisle 2017)?
Feasibility
Is the study free from characteristics could be implausible? (e.g. large numbers of women with a rare condition (such as severe cholestasis in pregnancy) recruited within 12 months);
In cases with (close to) zero losses to follow‐up, is there a plausible explanation?
Results
Is the study free from results that could be implausible? (e.g. massive risk reduction for main outcomes with small sample size)?
Do the numbers randomised to each group suggest that adequate randomisation methods were used (e.g. is the study free from issues such as unexpectedly even numbers of women ‘randomised’ including a mismatch between the numbers and the methods, if the authors say ‘no blocking was used’ but still end up with equal numbers, or if the authors say they used ‘blocks of 4’ but the final numbers differ by 6)?
Studies assessed as being potentially ‘high risk’ were not included in the review. Where a study was classified as ‘high risk’ we attempted to contact the study authors to address any possible lack of information/concerns. In cases where we could not obtain contact details for the study authors, or where adequate information remained unavailable, the study remained in ‘awaiting classification’ and the reasons and communications with the author (or lack of) were described in detail.
Abstracts
Data from abstracts will only be included if, in addition to the trustworthiness assessment, the study authors have confirmed in writing that the data to be included in the review have come from the final analysis and will not change. If such information is not available/provided, the study will remain in ‘awaiting classification’ (as above).
See Figure 1 for details of how we applied the trustworthiness screening criteria.
1.

Applying the trustworthiness screening tool criteria
Data extraction and management
Two review authors extracted the data, checked them for discrepancies and processed them as described in Higgins 2019. We contacted authors of each included trial for further information, if we thought this to be necessary.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the methods as:
low risk of bias (any truly random process, e.g. random number table; computer random‐number generator; tossing a coin, minimisation);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number; quasi‐randomised studies were excluded from the review);
unclear risk of bias (unclear description or no description of randomisation sequence generation).
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determine whether intervention allocation could have been foreseen in advance of, or during, recruitment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study all the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We also provided any information relating to whether the intended blinding was effective. Where blinding was not possible, we assessed whether the lack of blinding was likely to have introduced bias.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel;
low, high or unclear risk of bias for outcome assessors
where low risk of bias was when there was blinding or where we assessed that the outcome or the outcome measurement was not likely to have been influenced by lack of blinding.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We have assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analyses at each stage (compared with the total randomised participants), reasons for attrition/exclusion where reported, and any re‐inclusions in analyses undertaken.
We assessed the methods as:
low risk of bias (e.g. where there were no missing data or where reasons for missing data were balanced across groups);
high risk of bias (e.g. where missing data were likely to be related to outcomes or were not balanced across groups);
unclear risk of bias (e.g. where there was insufficient reporting of attrition or exclusions to permit a judgement to be made).
(5) Selective reporting bias
We described for each included study how we examined the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study's pre‐specified outcomes were reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias
We described for each included study any important concerns we had about other possible sources of bias. For example, was there a potential source of bias related to the specific study design? Was the trial stopped early due to some data‐dependent process? Was there extreme baseline imbalance? Had the study been claimed to be fraudulent?
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of bias;
high risk of bias;
unclear.
Measures of treatment effect
Dichotomous data
We calculated risk ratios (RRs) and 95% confidence intervals (CIs) for dichotomous data.
Continuous data
For continuous data, we used the mean difference (MD) with 95% CI where outcomes were measured using the same instrument. Where different instruments were used we planned to use the standardised mean difference (SMD) with 95% CI with the following interpretations.
SMD 0.8 or greater = large effect
SMD greater than 0.49 and less than 0.8 = medium effect
SMD greater than 0.19 and less than 0.5 = small effect
SMD less than 0.2 = trivial or no effect
Unit of analysis issues
The unit of randomisation was per woman. For maternal outcomes the unit of analysis was per woman. Where possible for multiple pregnancies, the number of babies was used as the denominator for fetal and neonatal outcomes.
In trials with one control arm and more than two intervention arms, we added the intervention arms together for binary outcomes. To avoid double‐counting continuous outcomes we divided the denominator in the control arm by the number of different intervention arms.
Cluster‐randomised trials
We did not identify any cluster‐RCTs. In future updates, if there are cluster‐RCTs that meet our inclusion criteria we will combine them with individually‐randomised trials in accordance with the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We will adjust their standard errors (SEs) using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible) or from a similar trial. If we use ICCs from other sources, we will report this, and conduct sensitivity analyses to investigate the effect of variation in the ICC. We will also acknowledge heterogeneity in the randomisation unit, and perform a subgroup analysis to investigate the effects of the randomisation unit.
Dealing with missing data
In cases where trial data were missing, we first sought information from the original trial investigators. Details of trial authors contacted and the questions asked of them are contained in Characteristics of included studies.
We recognise that some study outcomes may be applicable only to a subset of participants, for instance morbidity outcomes can only occur after live birth. However, if the denominator is not based on the intention‐to‐treat (ITT) principle,i.e. omitting those who died before birth, this could potentially bias the analysis, as the comparison is then not between the randomised groups. Since we cannot be certain that pre‐delivery fetal deaths are unrelated to the intervention, we judged that the more appropriate method would be to use numbers randomised as the denominator for all our outcomes related to the fetus/neonate.
In the absence of appropriate statistical methods in the individual trials to account for missing data, in the following situations we deemed it appropriate to use the denominator reported by the trial rather than the number randomised in order to avoid making potentially misleading assumptions about the outcomes for those women and fetuses with missing data:
where no explanation was given for women who were lost to follow‐up before giving birth and were not included in the analysis of the individual trials;
where women were not included in trial analysis because they were randomised in error, e.g. multiple pregnancy in a singleton pregnancy‐only trial.
Where standard deviations (SDs) were missing from continuous outcome data, we estimated SDs by using the largest SD from the other trials in the same meta‐analysis.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the I² and Chi² statistics and visual inspection of forest plots. We used the following guidance from The Cochrane Handbook of Systematic Reviewsof Interventions to interpret the I² statistic (Higgins 2019):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Where we found substantial heterogeneity we used a random‐effects model to conduct the analysis and attempted to explain possible sources of heterogeneity (Deeks 2011).
Assessment of reporting biases
If there were 10 or more studies in the meta‐analysis we investigated reporting biases (such as publication bias) using funnel plots (Sterne 2011). We assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the RevMan 5.4 software (RevMan 2020). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: that is, where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we have discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we planned not to combine trials.
Subgroup analysis and investigation of heterogeneity
We performed analysis of clinical groups for primary outcomes only (where data were available).
We analysed the following clinical groups:
singleton versus multiple pregnancy;
intact membranes versus ruptured membranes at first dose;
pregnancy‐induced hypertension syndromes;
type of glucocorticoid (betamethasone, dexamethasone, hydrocortisone);
decade of trial (post‐hoc, i.e. not pre‐specified in the protocol);
protocol with weekly repeats (post hoc, i.e. not pre‐specified in the protocol);
gestational age at trial entry (post hoc, i.e. not pre‐specified in the protocol).
All covariates were proposed after deliberation with clinical experts. We planned to explore potential differences in the effect of corticosteroids in distinct clinical populations, such as pregnant women with ruptured membranes or multiple pregnancy, and in different types of trials.
For the main analysis we did not adjust data for multiple pregnancies to take account of non‐independence of outcomes for babies from the same pregnancy. For some outcomes there will be a higher correlation between babies from the same pregnancy than between babies from different pregnancies. The degree of non‐independence of outcomes for babies from multiple pregnancies will vary considerably depending on the outcome and the type of multiple pregnancy. For some outcomes the risk of an adverse event will be highly correlated in babies from the same pregnancy (e.g. preterm birth); while for others the degree of correlation will be lower (e.g. fetal death) but still higher than for babies from different pregnancies. In view of this non‐independence, subgroup analysis examining fetal and neonatal outcomes in singleton versus multiple pregnancies must be interpreted with particular caution.
We found that some trials included in this review had a protocol of weekly repeat doses of corticosteroid if the mother remained undelivered. None of the trials that allowed weekly repeat doses reported outcomes separately for those exposed to repeat doses. We performed a post hoc analysis for primary outcomes of trials where a single course was used versus those where weekly repeat doses were allowed in the protocol to determine if the inclusion of such trials biased our results. Single versus multiple doses of corticosteroids is the subject of another Cochrane Review (Crowther 2015). The analysis in this update will differ from that of the single versus multiple doses review, because the latter review includes only those studies where the women were randomised to either single or multiple doses.
Because the case‐fatality rate for RDS has decreased with improvements in neonatal care, we postulated that the effect of corticosteroids may not be as apparent in more recent trials. This hypothesis was tested in a post‐hoc subgroup analysis with trials grouped by the main decade of recruitment or publication of results.
Many trials did not report outcome data split according to the listed clinical characteristics (covariates). Due to this missing information, the total number of events/participants in subgroup analysis for some outcomes does not match the overall analysis. Wherever possible we have indicated in footnotes on the forest plots where the data are discrepant between the main analysis and the clinical subgroups.
All analyses by the covariates listed above should be considered hypothesis‐generating.
Finally, it should be noted that we did not conduct subgroup analysis where there were too few trials reporting data to conduct meaningful analyses.
Sensitivity analysis
We conducted sensitivity analysis for the primary outcomes based on risk of bias, where we removed studies from the analysis which were judged high risk for random sequence generation, allocation concealment or attrition bias.
We also conducted three post‐hoc sensitivity analyses:
intention‐to‐treat analysis versus available‐case analysis for neonatal/fetus primary outcomes;
intraventricular haemorrhage (IVH): we removed studies from the analysis whose diagnosis of IVH was by postmortem only;
subgroup analyses for different gestational ages at trial entry: we removed studies from the analysis that did not fit into either of the gestational age categories.
Summary of findings and assessment of the certainty of the evidence
We assessed the certainty of evidence using the GRADE approach, as outlined in the GRADE Handbook, for the following outcomes.
For the fetus/neonate
Perinatal death (composite of fetal death (in utero death) and early neonatal death (before seven completed days of life)).
Neonatal death (before 28 completed days of life).
Respiratory distress syndrome (RDS).
Intraventricular haemorrhage (IVH).
Mean birthweight (g).
For the child
Neurodevelopmental disability at follow‐up (visual impairment, hearing impairment, intellectual impairment defined as intelligence quotient less than ‐2 standard deviations below population mean, moderate/severe cerebral palsy (however defined by study authors), or developmental delay (defined as developmental quotient less than ‐2 standard deviation below population mean)).
For the woman
Maternal death (up to 90 days after giving birth.
Chorioamnionitis (however defined by study authors).
Endometritis (however defined by study authors and including infections).
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5 (RevMan 2020) in order to create 'Summary of findings' tables. A summary of the intervention effect and a measure of certainty for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
Results of the search
See Figure 2 for a full description of the study identification process.
2.
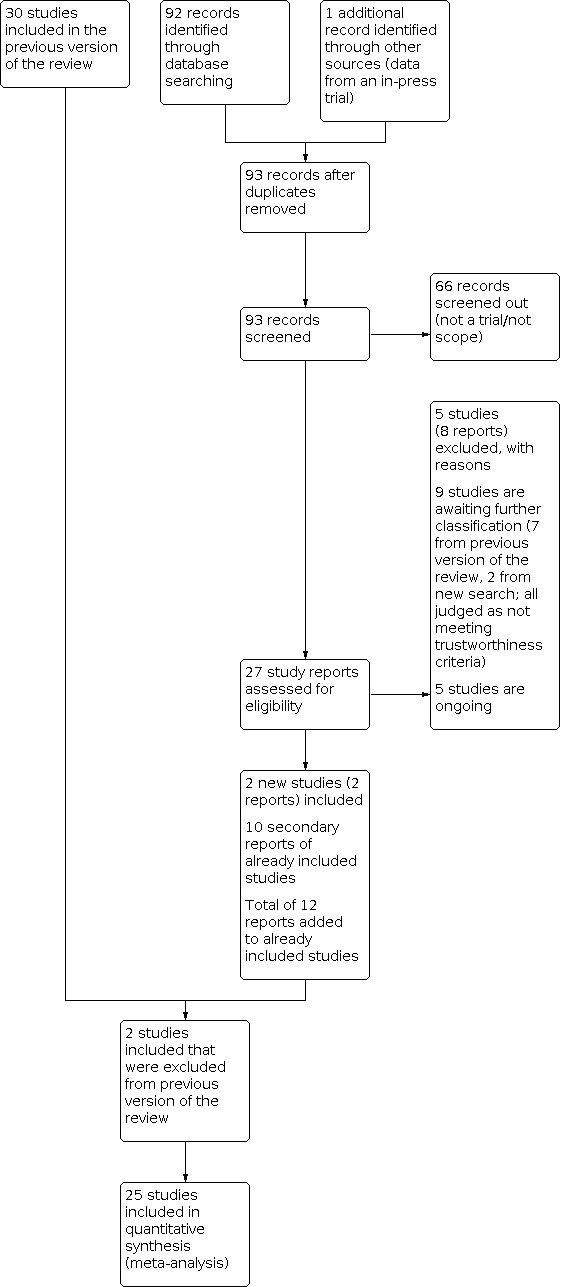
Study flow diagram.
From our assessment of the 27 trial reports that were identified from the search update we found the following:
five ongoing studies;
five studies (eight reports) that did not meet our inclusion criteria;
10 secondary reports of studies already included in the previous version of this review;
four new, completed studies that meet our inclusion criteria.
We also reassessed studies that were excluded from the previous version and included two (Morrison 1978; Schmidt 1984), which had been excluded because they had a large amount of missing data. However, in line with current Cochrane standards we have now included them.
Screening eligible studies for trustworthiness
From the 30 studies included in the previous version of the review and the three eligible studies identified from the update search we judged that nine studies did not meet our criteria for trustworthiness for the following reasons.
Four studies were published only as abstracts and we have not been able to confirm with the trial authors that the data were from the final analyses (Cararach 1991; Carlan 1991; Goodner 1979; Parsons 1988).
Two studies had concerns about randomisation processes where there was no explanation for substantial imbalances between the numbers allocated to each group (Doran 1980; Taeusch 1979).
Three studies published since 2010 demonstrated no evidence of prospective registration (Delibas 2017; Mirzamoradi 2019; Khazardoust 2012).
In all cases we made every effort to contact the authors and either identified no contact details at all or the authors did not respond to our queries ‐ see Studies awaiting classification.
Included studies
Twenty‐seven studies met our inclusion criteria and were assessed as trustworthy (11,272 randomised women and 11,925 neonates). The included studies were conducted over a wide range of gestational ages, including those of extreme prematurity and late prematurity. Obstetric indications for recruitment to trials were premature rupture of membranes, spontaneous preterm labour and planned preterm delivery. Please also refer to the Characteristics of included studies tables for full details.
Two trials were stopped early. One (WHO 2020; 2852 women) was stopped early for infant mortality benefits and strong evidence of a graded dose‐response effect and the other (Shanks 2010; 32 women) was stopped due to problems with recruitment.
Design
All of the studies are randomised controlled trials (RCTs). One four‐arm trial had three intervention groups, which each received a different corticosteroid and the fourth group received placebo (Schmidt 1984). Another four‐arm trial randomised women to expectant management alone, or expectant management plus either betamethasone or ampicillin, or a combination of expectant management plus betamethasone plus ampicillin (Morales 1989). One three‐arm trial (Block 1977) compared two different corticosteroids and a placebo group. One trial (Nelson 1985), had three arms but we did not include one of the arms because it did not meet our inclusion criteria for eligible comparators. The other trials had two arms each.
Setting
The included studies came from a range of healthcare systems and settings. Ten trials (4422 randomised women) took place in lower‐ or middle‐resource settings.
Ten of the studies were conducted in the USA (Block 1977; Collaborative 1981; Garite 1992; Gyamfi‐Bannerman 2016; Lewis 1996; Morales 1989; Nelson 1985; Shanks 2010; Schmidt 1984; Silver 1996), two in Brazil (Amorim 1999; Porto 2011), two in Finland (Kari 1994; Teramo 1980), and one each in India (Ontela 2018), Iran (Mansouri 2010), Colombia (Lopez 1989), South Africa (Dexiprom 1999), Thailand (Attawattanakul 2015), Tunisia (Fekih 2002), Turkey (Balci 2010), the UK (Gamsu 1989), New Zealand (Liggins 1972b), Jordan (Qublan 2001) and the Netherlands (Schutte 1980). One study took place in the USA and Germany (Morrison 1978), and another study took place in Bangladesh, India, Kenya, Nigeria and Pakistan (WHO 2020).
Participants
Multiple pregnancy
The majority of trials recruited only women with singleton pregnancy. Twelve trials (Collaborative 1981, Dexiprom 1999, Fekih 2002, Gamsu 1989, Garite 1992, Kari 1994, Liggins 1972b, Schutte 1980, Schmidt 1984; Silver 1996; Teramo 1980; WHO 2020) recruited women with singleton or multiple pregnancy. Of these, five studies (Collaborative 1981, Gamsu 1989, Liggins 1972b; Silver 1996; WHO 2020) reported some outcome data separately for included women with multiple pregnancy. In two trials recruitment was unclear but we assumed that they only included women with singleton pregnancies since the number of women was the same as the number of infants in each group (Lopez 1989; Morrison 1978).
Membrane status
Several trials specifically excluded women with premature rupture of membranes: Amorim 1999; Attawattanakul 2015; Balci 2010Garite 1992; Shanks 2010. Eight trials reported outcome data for women with premature rupture of membranes (Dexiprom 1999; Lewis 1996; Liggins 1972b; Lopez 1989; Morales 1989; Nelson 1985; Qublan 2001; Schutte 1980). The remaining included trials reported data for a mixed population or the membrane status of included women was unclear. One trial (Liggins 1972b) reported some outcome data separately for women with intact or ruptured membranes.
Gestational age at trial entry
We have attached a table stating the gestational parameters for trials included in the review (Table 3). For the analysis of clinical subgroups for this update, we have compared trials recruiting women at gestational age of less than and including 35 weeks + 0 days with trials recruiting women 34 weeks + 0 days' gestation or greater for the review's primary outcomes. Most trials fall on either side of this division, with the exception of four studies; Block 1977, Collaborative 1981, Liggins 1972b, and Teramo 1980. Data from Liggins 1972b was available for women receiving their first dose at less than 35 weeks + 0 days and from between 35 weeks + 0 days and 37 weeks + 0 days, footnotes detailing this have been added to the appropriate forest plots. The majority of women in the remaining three studies (Block 1977; Collaborative 1981; Teramo 1980) received their first dose prior to 34 weeks + 0 days, therefore we included these studies in the younger gestational age grouping for the analysis (women less than, and including, 35 weeks and 0 days), but undertook a sensitivity analysis with the studies' data removed.
1. Gestational age parameters for included trials.
| Trial | Minimum (weeks+days) |
Maximum (weeks+days) |
| Amorim 1999 | 28+0 | 34+6 |
| Attawattanakul 2015 | 34+0 | 36+6 |
| Balci 2010 | 34+0 | 36+6 |
| Block 1977 | Not reported | 36+6 |
| Collaborative 1981 | 26+0 | 37+0 |
| Dexiprom 1999 | 28+0 | 34+6 |
| Fekih 2002 | 26+0 | 34+6 |
| Gamsu 1989 | Not reported | 34+6 |
| Garite 1992 | 24+0 | 27+6 |
| Gyamfi‐Bannerman 2016 | 34+0 | 36+6 |
| Kari 1994 | 24+0 | 31+6 |
| Lewis 1996 | 24+0 | 34+6 |
| Liggins 1972b | 24+0 | 36+6 |
| Lopez 1989 | 27+0 | 35+0 |
| Mansouri 2010 | 35+0 | 36+6 |
| Morales 1989 | 26+0 | 34+6 |
| Morrison 1978 | Not reported | 34+0 |
| Nelson 1985 | 28+0 | 34+6 |
| Ontela 2018 | 34+0 | 36+6 |
| Porto 2011 | 34+0 | 36+6 |
| Qublan 2001 | 27+0 | 34+6 |
| Schutte 1980 | 26+0 | 32+6 |
| Schmidt 1984 | 26+0 | 32+0 |
| Shanks 2010 | 34+0 | 36+6 |
| Silver 1996 | 24+0 | 29+6 |
| Teramo 1980 | 28+0 | 35+6 |
| WHO 2020 | 26+0 | 33+6 |
Interventions
Type of steroid
The following types of steroids were used.
Dexamethasone (Attawattanakul 2015; Collaborative 1981; Dexiprom 1999; Kari 1994; Ontela 2018; Qublan 2001; Silver 1996; WHO 2020).
Betamethasone (Amorim 1999; Balci 2010; Block 1977; Fekih 2002; Gamsu 1989; Garite 1992; Gyamfi‐Bannerman 2016; Lewis 1996; Liggins 1972b; Lopez 1989; Mansouri 2010; Morales 1989; Nelson 1985; Porto 2011; Schutte 1980; Teramo 1980).
Methylprednisolone (Block 1977; Schmidt 1984).
Hydrocortisone (Morrison 1978; Schmidt 1984).
One study used either betamethasone or dexamethasone in its treatment arm (Shanks 2010).
Comparators
In most trials the control arm received placebo. In the other trials the control arm received no treatment (Attawattanakul 2015; Balci 2010; Lopez 1989; Nelson 1985; Ontela 2018; Shanks 2010), expectant management (Fekih 2002; Lewis 1996; Morales 1989; Qublan 2001), and 6 mg cortisone acetate, which has 1/70th of the corticosteroid potency of the betamethasone administered to the intervention group (Liggins 1972b).
Weekly repeats
Most trials included in this review tested a single course of corticosteroid. Eight studies allowed weekly repeat courses of study medication in their study protocols (Amorim 1999; Fekih 2002; Garite 1992; Lewis 1996; Morales 1989; Qublan 2001; Silver 1996; WHO 2020). We conducted post hoc analysis of primary outcomes comparing studies testing a single course of study medication with studies allowing weekly repeat courses.
Outcomes
The following trials reported data for the outcomes specified in our 'Summary of findings' tables.
Perinatal death: 14 trials (Amorim 1999; Block 1977; Collaborative 1981; Dexiprom 1999; Gamsu 1989; Garite 1992; Gyamfi‐Bannerman 2016; Kari 1994; Liggins 1972b; Ontela 2018; Porto 2011; Qublan 2001; Schutte 1980; WHO 2020).
Neonatal death: 22 trials (Amorim 1999; Block 1977; Collaborative 1981; Dexiprom 1999; Fekih 2002; Gamsu 1989; Garite 1992; Gyamfi‐Bannerman 2016; Kari 1994; Lewis 1996; Liggins 1972b; Lopez 1989; Morales 1989; Morrison 1978; Nelson 1985; Ontela 2018; Porto 2011; Qublan 2001; Schmidt 1984; Schutte 1980; Silver 1996; WHO 2020).
Respiratory distress syndrome (RDS): all trials except one (Shanks 2010).
IVH: 12 trials (Amorim 1999; Fekih 2002; Gamsu 1989; Garite 1992; Gyamfi‐Bannerman 2016; Kari 1994; Lewis 1996; Liggins 1972b; Morales 1989; Qublan 2001; Silver 1996; WHO 2020).
Mean birthweight: 19 trials (Attawattanakul 2015; Balci 2010; Dexiprom 1999; Gamsu 1989; Garite 1992; Gyamfi‐Bannerman 2016; Kari 1994; Lewis 1996; Liggins 1972b; Mansouri 2010; Morales 1989; Morrison 1978; Nelson 1985; Ontela 2018; Porto 2011; Schmidt 1984; Schutte 1980; Silver 1996; WHO 2020).
Neurodevelopmental disability in childhood: five trials (Amorim 1999; Collaborative 1981; Kari 1994; Liggins 1972b; Schutte 1980).
Maternal death: six trials (Amorim 1999; Dexiprom 1999; Gyamfi‐Bannerman 2016; Mansouri 2010; Schutte 1980; WHO 2020).
Chorioamnionitis: 15 trials (Amorim 1999; Attawattanakul 2015; Dexiprom 1999; Fekih 2002; Garite 1992; Gyamfi‐Bannerman 2016; Kari 1994; Lewis 1996; Liggins 1972b; Lopez 1989; Morales 1989; Qublan 2001; Schutte 1980; Silver 1996; WHO 2020).
Endometritis: t10 trials (Amorim 1999; Dexiprom 1999; Garite 1992; Gyamfi‐Bannerman 2016; Lewis 1996; Mansouri 2010; Qublan 2001; Schutte 1980; Silver 1996; WHO 2020).
Five studies (Amorim 1999; Collaborative 1981; Kari 1994; Liggins 1972b; Schutte 1980) reported outcome data related to the infant in childhood.
Only two studies (Liggins 1972b; Schutte 1980) reported outcome data related to the infant in adulthood.
Dates of study
Eight trials were conducted during the 1970s (Block 1977; Collaborative 1981; Gamsu 1989; Liggins 1972b; Morrison 1978; Schmidt 1984; Schutte 1980; Teramo 1980), four during the 1980s (Garite 1992; Lopez 1989; Morales 1989; Nelson 1985), five during the 1990s (Amorim 1999; Dexiprom 1999; Kari 1994; Lewis 1996; Silver 1996), six during the 2000s (Balci 2010; Fekih 2002; Mansouri 2010; Porto 2011; Qublan 2001; Shanks 2010), and four during the 2010s (Attawattanakul 2015; Gyamfi‐Bannerman 2016; Ontela 2018; WHO 2020).
Funding sources
Eleven trials received funding from public, educational or charitable sources (Amorim 1999; Collaborative 1981; Dexiprom 1999; Garite 1992; Gyamfi‐Bannerman 2016; Liggins 1972b; Mansouri 2010; Porto 2011; Schutte 1980; Shanks 2010; WHO 2020).
One trial received funding from commercial sources (Block 1977).
Thirteen trials did not specifically report any information about funding sources (Attawattanakul 2015; Balci 2010; Gamsu 1989; Lewis 1996; Lopez 1989; Morales 1989; Morrison 1978; Nelson 1985; Ontela 2018; Qublan 2001; Schmidt 1984; Silver 1996; Teramo 1980).
Declarations of interest
In five trials the authors declared that they had no competing interests (Attawattanakul 2015; Balci 2010; Gyamfi‐Bannerman 2016; Porto 2011; WHO 2020).
Twenty‐one trials did not mention authors' declarations of interest at all (Amorim 1999; Block 1977; Collaborative 1981; Dexiprom 1999; Gamsu 1989; Garite 1992; Kari 1994; Lewis 1996; Liggins 1972b; Lopez 1989; Morales 1989; Morrison 1978; Nelson 1985; Ontela 2018; Qublan 2001; Schmidt 1984; Schutte 1980; Shanks 2010; Silver 1996; Teramo 1980; Lopez 1989).
In one trial, published in languages other than English, we were unable to obtain enough translated information to know what declarations, if any, were reported (Mansouri 2010).
Excluded studies
We excluded 29 studies. Reasons for exclusion included the following.
The study did not compare a corticosteroid with placebo or no treatment (Abuhamad 1999; Althabe 2015; Dola 1997; Dude 2016; Egerman 1998; Garite 1981; Iams 1985; Magee 1997; Minoui 1998; Mulder 1997; NCT04494529 2020; Rotmensch 1999; Whitt 1976).
The study was not a randomised controlled trial (Asnafei 2004; Grgic 2003; Halac 1990; Liu 2006; Maksic 2008; Morales 1986; Simpson 1985).
Study participants were combined with a non‐randomised cohort and results were not presented separately (Butterfill 1979; Kuhn 1982).
The study was withdrawn without having recruited any participants (NCT02351310 2015).
Several studies compared repeat‐dose corticosteroids and are eligible for inclusion in the Crowther 2015 review (Khandelwal 2012; Koivisto 2007; Kurtzman 2008; McEvoy 2010; Papageorgiou 1979; Romejko‐Wolniewicz 2013).
See the Characteristics of excluded studies table for full details.
Ongoing studies
In addition to one study that was identified in the last version of this review (Roberts 2017), and which does not yet have available results, we identified a further four ongoing studies. All of these trials are investigating the use of corticosteroids in women at the late preterm stage (34 to 36 weeks' gestation), and they aim to recruit a total of 23,500 women, mostly in low‐ and middle‐resource countries.
See Characteristics of ongoing studies for further details.
Risk of bias in included studies
Figure 3 and Figure 4 illustrate the risks of bias which are explained in more detail below.
3.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
4.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Allocation
Sequence generation
We have summarised the methods of randomisation used in the included trials in the Characteristics of included studies table. We judged 15 trials as having low risk of bias for random sequence generation because they used techniques such as computer‐generated or random number‐generated randomisation sequences (Amorim 1999; Balci 2010; Block 1977; Dexiprom 1999; Garite 1992; Gyamfi‐Bannerman 2016; Lewis 1996; Liggins 1972b; Nelson 1985; Ontela 2018; Porto 2011; Qublan 2001; Schmidt 1984; Silver 1996; WHO 2020). The remaining 12 trials did not describe the method of sequence generation in sufficient detail so we judged them as unclear risk of bias (Attawattanakul 2015; Collaborative 1981; Fekih 2002; Gamsu 1989; Kari 1994; Lopez 1989; Mansouri 2010; Morales 1989; Morrison 1978; Schutte 1980; Shanks 2010; Teramo 1980).
Allocation concealment
Twelve trials described adequate allocation concealment procedures and we therefore judged them to be low risk of bias (Amorim 1999; Block 1977; Dexiprom 1999; Gyamfi‐Bannerman 2016; Lewis 1996; Liggins 1972b; Ontela 2018; Porto 2011; Schmidt 1984; Schutte 1980; Silver 1996; WHO 2020). We assessed one trial as having a high risk of bias due to a sealed envelope containing the identity of the contents being attached to each vial "to be opened in emergency only in case of an emergency"; the manuscripts do not state how often these were opened (Collaborative 1981). We judged the remaining trials as unclear risk of bias due to insufficient description of the method of allocation concealment (Attawattanakul 2015; Balci 2010; Fekih 2002; Gamsu 1989; Garite 1992; Kari 1994; Lopez 1989; Mansouri 2010; Morales 1989; Morrison 1978; Nelson 1985; Qublan 2001; Shanks 2010; Teramo 1980).
Blinding
Blinding of participants and personnel
Seventeen of the included trials were placebo controlled and therefore we judged them to be low risk of bias (Amorim 1999; Block 1977; Collaborative 1981; Dexiprom 1999; Gamsu 1989; Garite 1992; Gyamfi‐Bannerman 2016; Kari 1994; Liggins 1972b; Mansouri 2010; Morrison 1978; Porto 2011; Schmidt 1984; Schutte 1980; Silver 1996; Teramo 1980; WHO 2020). The majority of these trials used normal saline, or the vehicle of the corticosteroid preparation, as the placebo. The remaining trials were judged as high risk of bias due to not blinding participants and personnel to the study intervention (Attawattanakul 2015; Balci 2010; Fekih 2002; Lewis 1996; Lopez 1989; Morales 1989; Nelson 1985; Ontela 2018; Qublan 2001; Shanks 2010).
Blinding of outcome assessors
Blinding of outcome assessors was sufficiently reported in 10 trials which we judged as low risk of bias (Amorim 1999; Gyamfi‐Bannerman 2016; Liggins 1972b; Mansouri 2010; Morrison 1978; Porto 2011; Schmidt 1984; Schutte 1980; Silver 1996; WHO 2020). The remaining trials did not describe whether or not outcome assessors were blinded and so were judged to be of unclear risk of bias (Attawattanakul 2015; Balci 2010; Block 1977; Collaborative 1981; Dexiprom 1999; Fekih 2002; Gamsu 1989; Garite 1992; Kari 1994; Lewis 1996; Lopez 1989; Morales 1989; Nelson 1985; Ontela 2018; Qublan 2001; Shanks 2010; Teramo 1980).
Incomplete outcome data
We judged 16 trials as having low risk of attrition bias because they had low, non‐differential attrition and/or the reasons for missing data were not related to the intervention (Amorim 1999; Attawattanakul 2015; Balci 2010; Dexiprom 1999; Gamsu 1989; Garite 1992; Gyamfi‐Bannerman 2016; Kari 1994; Lewis 1996; Lopez 1989; Mansouri 2010; Nelson 1985; Ontela 2018; Qublan 2001; Teramo 1980; WHO 2020). Three trials were assessed as having high risk of bias. Shanks 2010 had over 20% loss to follow‐up and no intention‐to‐treat analysis. Schmidt 1984 and Morrison 1978 did not report the group allocation of the women excluded/lost to follow‐up. We assessed the remaining trials as unclear risk of bias due to lack of information or unknown impact of stated exclusions (Block 1977; Collaborative 1981; Fekih 2002; Liggins 1972b; Morales 1989; Porto 2011; Schutte 1980; Silver 1996).
Selective reporting
We judged two trials to be high risk of bias due to selective reporting. Schmidt 1984 did not provide a protocol and did not report on endometritis despite being specified in methods. Shanks 2010 did not report on hyaline membrane disease despite listing it as an outcome.
One trial (Qublan 2001) was judged to have unclear risk of reporting bias because of discrepancies in numbers reported for one outcome. We contacted the authors for clarification but we did not receive any response.
The remaining trials were judged to have low risk of bias due to selective reporting because they either reported all the outcomes specified in their protocols or prospective trial registrations, or, in the case of trials published before protocols and prospective registration became commonplace, they reported all outcomes in full that were specified in their methods (Amorim 1999; Attawattanakul 2015; Balci 2010; Block 1977; Collaborative 1981; Dexiprom 1999; Fekih 2002; Gamsu 1989; Garite 1992; Gyamfi‐Bannerman 2016; Kari 1994; Lewis 1996; Liggins 1972b; Lopez 1989; Mansouri 2010; Morales 1989; Morrison 1978; Nelson 1985; Ontela 2018; Porto 2011; Schutte 1980; Silver 1996; Teramo 1980; WHO 2020).
Other potential sources of bias
We assessed Shanks 2010 as high risk of other bias because the trial was stopped early due to problems with recruitment. Schmidt 1984 was assessed as high risk of bias as two of the three trial arms were discontinued after 24 months to increase group size in the other trial arm and placebo arm without explanation. We judged one trial as unclear for other potential sources of bias because it is in Persian and we are still awaiting clarification from a translator regarding risk of bias (Mansouri 2010).
We judged the remaining trials as low risk because there was no indication of any other potential sources of bias (Amorim 1999; Attawattanakul 2015; Balci 2010; Block 1977; Collaborative 1981; Dexiprom 1999; Fekih 2002; Gamsu 1989; Garite 1992; Gyamfi‐Bannerman 2016; Kari 1994; Lewis 1996; Liggins 1972b; Lopez 1989; Morales 1989; Morrison 1978; Nelson 1985; Ontela 2018; Porto 2011; Qublan 2001; Schutte 1980; Silver 1996; Teramo 1980; WHO 2020).
Effects of interventions
1. Antenatal corticosteroids versus placebo or no treatment (all included studies)
Primary outcomes
For the fetus or neonate
Perinatal death
Antenatal corticosteroids reduce the risk of perinatal death compared with placebo or no treatment (risk ratio (RR) 0.85, 95% confidence interval (CI) 0.77 to 0.93; 9833 infants; studies = 14; I2 = 28%; high‐certainty evidence; Table 1; Analysis 1.1). With corticosteroids there were 2.3% fewer perinatal deaths than with placebo or treatment (95% CI 1.1% fewer to 3.6% fewer).
1.1. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 1: Perinatal death
The shape of the funnel plot (Figure 5) suggested that some evidence may be missing in areas where results would be statistically non‐significant or in the direction of a poorer outcome with corticosteroids. This may explained by lower methodological quality in smaller studies leading to spuriously inflated effects, or by non‐publication of studies because of the nature of their findings (e.g. statistical significance or direction of effect). However, we did not consider the asymmetry to be pronounced enough to downgrade the certainty of evidence.
5.

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.4 Perinatal deaths
Sensitivity analysis removing one trial at high risk of bias for allocation concealment did not substantially change the effect estimate (RR 0.84, 95% CI 0.76 to 0.92; 9076 infants; studies = 13; I2 = 31%).
For perinatal death all trials reported data using numbers randomised as the denominator therefore there is no difference between our intention‐to‐treat (ITT) analysis and available‐case analysis.
Neonatal death
Antenatal corticosteroids reduce the risk of neonatal death compared with placebo or no treatment (RR 0.78, 95% CI 0.70 to 0.87; 10,609 infants; studies = 22; I2 = 12%; high‐certainty evidence; Table 1; Analysis 1.2).
1.2. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 2: Neonatal death
With corticosteroids there were 2.6% fewer neonatal deaths than with placebo or treatment (95% CI 1.5% fewer to 3.6% fewer). Sensitivity analysis comparing ITT analysis and available‐case analysis had little impact on the effect estimate (available case analysis: RR 0.78, 0.70 to 0.86; 10,189 infants).
Sensitivity analysis removing three trials at high risk of bias for allocation concealment or incomplete outcome data did not substantially change the effect estimate (RR 0.79, 95% CI 0.71 to 0.88; 9954 infants; studies = 19; I2 = 1%).
The shape of the funnel plot (Figure 6) suggested that some evidence may be missing in areas where results would be statistically non‐significant or in the direction of a poorer outcome with corticosteroids. This may explained by lower methodological quality in smaller studies leading to spuriously inflated effects, or by non‐publication of studies because of the nature of their findings (e.g. statistical significance or direction of effect). However, we did not consider the asymmetry to be pronounced enough to downgrade the certainty of evidence.
6.

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.5 Neonatal deaths
Fetal death
Antenatal corticosteroids may have little to no effect on the risk of fetal death (RR 1.01, 95% CI 0.83 to 1.22; 9833 infants; studies = 14; I2 = 0%; Analysis 1.3).
1.3. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 3: Fetal death
The symmetry in the funnel plot did not suggest evidence of bias due to non‐publication of results (Figure 7). Sensitivity analysis removing one trial at high risk of bias for allocation concealment did not substantially change the effect estimate (RR 1.02, 95% CI 0.84 to 1.24; 9076 infants; studies = 13; I2 = 0%).
7.

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.6 Fetal deaths
For fetal death all trials reported data using numbers randomised as the denominator therefore there is no difference between our ITT analysis and available case analysis.
Respiratory distress syndrome (RDS)
Antenatal corticosteroids reduce the risk of RDS compared with placebo or no treatment (RR 0.71, 95% CI 0.65 to 0.78; 11,183 infants; studies = 26; I2 = 48%; high‐certainty evidence; Table 1; Analysis 1.4). With corticosteroids 4.3% fewer infants had RDS than with placebo or treatment (3.2% fewer to 5.2% fewer).
1.4. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 4: Respiratory distress syndrome
Sensitivity analysis comparing ITT analysis and available case analysis (ACA) showed that the two effect estimates are the same (ACA RR 0.71, 95% CI 0.65 to 0.78; participants = 10,321). Sensitivity analysis removing three trials at high risk of bias for allocation concealment and incomplete outcome data did not change the effect estimate (RR 0.71, 95% CI 0.65 to 0.79; 10,203 infants; studies = 23; I2 = 50%).
The moderate heterogeneity in the analysis (I2 = 48%) may be explicable by changes in neonatal care over time. Subgroup analysis based on the decade when the trials took place (Analysis 6.4) suggested that there was a difference in effect on RDS according to the time period of the trial. However, we did not consider heterogeneity to be substantial enough to downgrade the certainty of evidence.
6.4. Analysis.

Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 4: Respiratory distress syndrome ‐ decade of trial
The symmetry in the funnel plot did not suggest evidence of bias due to non‐publication of results (Figure 8).
8.

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.7 Respiratory distress syndrome
Moderate to severe respiratory distress syndrome (RDS)
Fewer infants had moderate to severe RDS in the groups treated with antenatal corticosteroids than in the control groups (RR 0.70, 95% CI 0.59 to 0.83; 4874 infants; studies = 7; I2 = 53%; Analysis 1.5). Sensitivity analysis comparing ITT analysis and available case analysis showed no substantial difference in the effect estimate (ACA RR 0.69, 95% CI 0.59 to 0.82; 4127 infants).
1.5. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 5: Moderate/severe respiratory distress syndrome
Removing one trial at high risk of attrition bias did not change the effect estimate (RR 0.69, 95% CI 0.58 to 0.82; 4031 infants; studies = 6; I2 = 60%).
Chronic lung disease
It is unclear if antenatal corticosteroids have any effect on the risk of chronic lung disease compared with placebo or no treatment (RR 0.86, 95% CI 0.41 to 1.79; 745 infants; studies = 5; I2 = 65%; Analysis 1.6). Sensitivity analysis comparing ITT analysis and available case analysis showed no substantial difference in the effect estimate (ACA RR 0.86, 95% CI 0.42 to 1.79; 695 infants). Because of unexplained statistical heterogeneity we conducted random effects meta‐analysis for this outcome.
1.6. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 6: Chronic lung disease
None of the trials reporting chronic lung disease data were at high risk of bias for random sequence generation, allocation concealment or incomplete outcome data so we did not perform further sensitivity analysis.
Intraventricular haemorrhage (IVH)
Antenatal corticosteroids probably reduce the risk of IIVH compared with placebo or no treatment (RR 0.58, 95% CI 0.45 to 0.75; 8475 infants; studies = 12; I2 = 45%; moderate‐certainty evidence; Table 1; Analysis 1.7). With corticosteroids 1.4% fewer infants had IVH than with placebo or treatment (95% CI 0.8% fewer to 1.8% fewer). Sensitivity analysis comparing ITT analysis and available case analysis showed no substantial difference in the effect estimate (ACA RR 0.57, 95% CI 0.45 to 0.73; 6771 infants).
1.7. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 7: Intraventricular haemorrhage
For illustrative purposes only, the analysis shows which studies reported specifically infants with grade 3‐4 IVH and which studies reported infants with any IVH.
The moderate heterogeneity in the analysis (I2 = 45%) may be partly due to differences in trial protocols, where not all trials routinely screened all infants for IVH. However, we did not consider heterogeneity to be substantial enough for a further downgrade of the certainty of evidence.
Three studies stated which infants were screened for IVH, used ultrasound: liveborn neonates < 1500 g or with signs of neonatal hypoxia (Amorim 1999); neonates < 1500 g by the third day (Morales 1989); liveborn neonates < 34 weeks at birth and liveborn neonates ≥ 34 weeks at birth if indicated (WHO 2020). The other trials reporting IVH diagnosis by ultrasound did not state which infants were screened for IVH.
Three studies reported zero cases of IVH in both arms but they did not report a definition of how IVH was diagnosed or how many infants were screened for IVH (Attawattanakul 2015 194 infants; Mansouri 2010 200 infants; Dexiprom 1999 206 infants).
The symmetry in the funnel plot did not suggest evidence of bias due to non‐publication of results (Figure 9). Sensitivity analysis removing two trials where diagnosis of IVH was by postmortem only (Gamsu 1989; Liggins 1972b) did not substantially change the effect estimate (RR 0.58, 95% CI 0.44 to 0.76; 6989 infants; I2 = 55%).
9.

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.7 Intraventricular haemorrhage.
None of the trials in the analysis of IVH data were at high risk of bias for random sequence generation, allocation concealment or incomplete outcome data so we did not perform further sensitivity analysis.
Mean birthweight
Antenatal corticosteroids result in little to no difference in birthweight (mean difference (MD) ‐14.02, 95% CI ‐33.79 to 5.76; 9551 infants; studies = 19; I2 = 0%; high‐certainty evidence; Table 1; Analysis 1.8).
1.8. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 8: Mean birthweight (g)
The symmetry in the funnel plot did not suggest evidence of bias due to non‐publication of results (Figure 10). Sensitivity analysis removing two trials at high risk of bias for incomplete outcome data did not substantially change the effect estimate (MD ‐12.52, 95% CI ‐32.47 to 7.43; 9328 infants; studies = 19; I2 = 0%).
10.

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.11 Mean birthweight (g)
For the woman
Death
Antenatal corticosteroids probably result in little to no difference in the risk of maternal death but the wide 95% CI includes possible benefit and possible harm (RR 1.19, 95% CI 0.36 to 3.89; 6244 women;studies = 6; I2 = 0%; moderate‐certainty evidence; Table 2; Analysis 1.9). In total six studies reported maternal death but in four of them (3174 women) there were no deaths at all (Dexiprom 1999; Gyamfi‐Bannerman 2016; Mansouri 2010; Schutte 1980). With corticosteroids there are probably 0.0% fewer maternal deaths than with placebo or no treatment (95% CI 0.1% fewer to 0.5% more).
1.9. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 9: Maternal death
There were not enough data to perform sensitivity analysis nor did we produce a funnel plot.
Chorioamnionitis
Antenatal corticosteroids probably result in little to no difference in risk of chorioamnionitis (RR 0.86, 95% CI 0.69 to 1.08; 8374 women; studies = 15; I2 = 0%; moderate‐certainty evidence; Table 2; Analysis 1.10).
1.10. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 10: Chorioamnionitis
With corticosteroids 0.5% fewer women had chorioamnionitis than with placebo or no treatment (95% CI 1.1% fewer to 0.3% more).
The symmetry in the funnel plot did not suggest evidence of bias due to non‐publication of results (Figure 11). None of the trials in the analysis of chorioamnionitis data were at high risk of bias for random sequence generation, allocation concealment or incomplete outcome data so we did not perform sensitivity analysis.
11.

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.2 Chorioamnionitis
Endometritis
Antenatal corticosteroids probably result in little to no difference in the risk of endometritis but wide 95% CI includes possible benefit and possible harm (RR 1.14, 95% CI 0.82 to 1.58; 6764 women; studies = 10; I2 = 20%; moderate‐certainty evidence; Table 2; Analysis 1.11).
1.11. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 11: Endometritis
The symmetry in the funnel plot did not suggest evidence of bias due to non‐publication of results (Figure 12). None of the trials in the analysis of endometritis data were at high risk of bias for random sequence generation, allocation concealment or incomplete outcome data so we did not perform sensitivity analysis.
12.

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.11 Endometritis.
For the child
Death
It is uncertain if antenatal corticosteroids have any effect on the risk of death in childhood (RR 0.68, 95% CI 0.36 to 1.27; 1010 children; studies = 4; I2 = 21%; Analysis 1.12). Removing a trial at high risk of bias for allocation concealment did not substantially change the effect estimate (RR 0.52, 95% CI 0.24 to 1.11; 593 children; studies = 3; I2 = 25%).
1.12. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 12: Death in childhood
Neurodevelopmental disability or developmental delay
Antenatal corticosteroids probably lead to a reduction in developmental delay in childhood (RR 0.51, 95% CI 0.27 to 0.97; 600 children; studies = 3; I2 = 0%; moderate‐certainty evidence; Table 1; Analysis 1.13). Age at follow‐up was between two and 12 years.
1.13. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 13: Neurodevelopmental disability in childhood
We did not identify any data that could be pooled for our composite outcome of neurodevelopment disability but we have presented data that we found relating to the separate aspects neurodevelopment disability. It is uncertain if corticosteroids have any effect on intellectual impairment (RR 0.86, 95% CI 0.44 to 1.69; 778 children; studies = 3; I2 = 0%), visual impairment (RR 0.55, 95% CI 0.24 to 1.23; 166 children; studies = 2; I2 = 0%) or hearing impairment (RR 0.64, 95% CI 0.04 to 9.87; 166 children; studies = 2) (Analysis 1.13).
With so few trials contributing to this outcome, and due to the different ways it is measured, we did not perform sensitivity analysis, nor did we produce a funnel plot.
For the child as adult
Death
It is uncertain if antenatal corticosteroids have any effect on death in adulthood (RR 1.00, 95% CI 0.56 to 1.81; 988 participants; studies = 1; Analysis 1.14).
1.14. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 14: Death into adulthood
Neurodevelopmental disability
It is uncertain if antenatal corticosteroids have any effect on visual impairment in adulthood (RR 0.91, 95% CI 0.53 to 1.55; 192 participants; studies = 1), hearing impairment in adulthood (RR 0.24, 95% CI 0.03 to 2.03; 192 participants; studies = 1) or intellectual impairment in adulthood (RR 0.24, 95% CI 0.01 to 4.95; 273 participants; studies = 2) (Analysis 1.15).
1.15. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 15: Neurodevelopmental disability in adulthood
Secondary outcomes
For the woman
Fever after trial entry requiring the use of antibiotics
It is uncertain if corticosteroids have any effect on a woman's risk of fever requiring antibiotics (RR 0.66, 95% CI 0.36 to 1.21; studies = 3; 363 women; I² = 0%; Analysis 1.16).
1.16. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 16: Fever in women after trial entry requiring the use of antibiotics
Intrapartum fever requiring the use of antibiotics
It is uncertain if corticosteroids have any effect on a woman's risk of intrapartum fever (RR 0.60, 95% CI 0.15 to 2.49; 319 women; studies = 2; I2 = 36%; Analysis 1.17).
1.17. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 17: Intrapartum fever in woman requiring the use of antibiotics
Postnatal fever
Corticosteroids may make little to no difference to a woman's risk of postnatal fever (RR 0.92, 95% CI 0.64 to 1.33; 1323 women; studies = 5; I2 = 0%; Analysis 1.18).
1.18. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 18: Postnatal fever in woman
Admission to intensive care unit
It is uncertain if corticosteroids have any effect on a woman's risk of being admitted to intensive care (RR 0.74, 95% CI 0.26 to 2.05; 319 women; studies = 2; Analysis 1.19).
1.19. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 19: Admission into adult intensive care unit
Side effects of therapy
Seven trials reported no side effects for women in any arm (Attawattanakul 2015; Balci 2010; Morrison 1978; Ontela 2018; Porto 2011; Schutte 1980; Shanks 2010; 1182 women). Two other trials reported a range of different side effects in women: any side effects at first dose (RR 0.69, 95% CI 0.59 to 0.82; 2825 women; studies = 1); dyspnoea (RR 0.33, 95% CI 0.01 to 8.15; 2828 women; studies = 1); gastrointestinal upset (RR 2.99, 95% CI 0.12 to 73.37; 2828 women; studies = 1); hyperglycaemia (RR 0.33, 95% CI 0.01 to 8.15; 2828 women; studies = 1); leucocytosis (RR 0.33, 95% CI 0.01 to 8.15; 2828 women; studies = 1); migraine (RR 1.00, 95% CI 0.06 to 15.93; 2828 women; studies = 1) (Analysis 1.20).
1.20. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 20: Side effects of therapy in women
Glucose intolerance
One small study (Amorim 1999), reported that women in the corticosteroid arm were more likely to have glucose intolerance than in the control arm (RR 2.71, 95% CI 1.14 to 6.46; participants = 123; studies = 1; Analysis 1.21). This study used a treatment regimen that included weekly repeat doses of corticosteroids if the infant remained undelivered.
1.21. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 21: Glucose intolerance
Hypertension
It is uncertain if corticosteroids have any effect on a woman's risk of hypertension (RR 1.03, 95% CI 0.59 to 1.79; 288 women; studies = 2; I2 = 0%) (Analysis 1.22).
1.22. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 22: Hypertension
For the fetus or neonate
Apgar score less than seven at five minutes
Fewer infants exposed to antenatal corticosteroids had an Apgar score less than seven at five minutes of age (RR 0.88, 95% CI 0.78 to 0.98; 5727 infants; studies = 12; I2 = 0%; Analysis 1.23). Sensitivity analysis comparing ITT analysis and available case analysis showed no substantial difference in effect estimate (ACA RR 0.89, 95% CI 0.79 to 0.99; 5243 infants).
1.23. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 23: Apgar < 7 at 5 minutes
Exploration of asymmetry in the funnel plot suggests that the effect estimate may be influenced by smaller trials at higher risk of bias therefore it is possible that the true direction and size of effect are different (Figure 13).
13.

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.30 Apgar < 7 at 5 minutes
Interval between trial entry and birth
Corticosteroids may have little to no effect on the interval between trial entry and birth (MD 0.23 days, 95% CI ‐1.86 to 2.32 days; 1513 infants; studies = 3; I2 = 0%; Analysis 1.24).
1.24. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 24: Mean interval between trial entry and birth (days)
Mean length at birth (height) [cm]
Corticosteroids may have little to no effect on babies' length (height) at birth (MD 0.00 cm, 95% CI ‐0.37 to 0.37 cm; 2766 infants; studies = 1; I2 = 0%; Analysis 1.25).
1.25. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 25: Mean length at birth (cm)
Mean head circumference at birth
Corticosteroids may have little to no effect on babies' head circumference at birth (MD 0.00 cm, 95% CI ‐0.22 to 0.22 cm; 2766 infants; studies = 1; I2 = 0%; Analysis 1.26).
1.26. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 26: Mean head circumference at birth (cm)
Mean skin fold thickness at birth
Not reported.
Small‐for‐gestational age
It is uncertain if antenatal corticosteroids have any effect on incidence of small‐for‐gestational‐age infants (RR 1.11, 95% CI 0.96 to 1.28; 3478 infants; studies = 5; I2 = 0%; Analysis 1.27).
1.27. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 27: Small‐for‐gestational age
Mean placental weight
Not reported.
Neonatal blood pressure
Not reported.
Admission to neonatal intensive care unit (NICU)
Corticosteroids may slightly reduce the risk of being admitted to a NICU (RR 0.96, 95% CI 0.91 to 1.00; 6667 infants; studies = 9; I2 = 34%; Analysis 1.28).
1.28. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 28: Admission to neonatal intensive care unit
Need for inotropic support
Not reported.
Mean duration of inotropic support
Not reported.
Need for mechanical ventilation/continuous positive airways pressure (CPAP)
Treatment with antenatal corticosteroids may lead to less need for ventilation/CPAP (RR 0.75, 95% CI 0.66 to 0.84; 4519 infants; studies = 11; I2 = 4%; Analysis 1.29). The symmetry of the funnel plot did not suggest evidence of bias due to non‐publication of results (Figure 14).
1.29. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 29: Need for mechanical ventilation/CPAP
14.

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.25 Need for mechanical ventilation/CPAP
Mean duration of mechanical ventilation/continuous positive airways pressure (CPAP)
It is uncertain if corticosteroids have any effect on the duration of mechanical ventilation/CPAP (MD ‐1.91 days, 95% CI ‐4.59 to 0.76 days; 471 infants; studies = 3; I2 = 77%; Analysis 1.30).
1.30. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 30: Mean duration of mechanical ventilation/CPAP (days)
One study (WHO 2020) reported median duration of mechanical ventilation (Analysis 1.31) and median duration of CPAP (Analysis 1.32). There was little difference between the two groups.
1.31. Analysis.
Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 31: Median (IQR) duration of mechanical ventilation (hours)
| Median (IQR) duration of mechanical ventilation (hours) | ||
| Study | Corticosteroids | Placebo |
| WHO 2020 | 18 hours (12‐48) 83 infants |
18 hours (12‐60) 103 infants |
1.32. Analysis.
Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 32: Median (IQR) duration of CPAP (hours)
| Median (IQR) duration of CPAP (hours) | ||
| Study | Corticosteroids | Placebo |
| WHO 2020 | 48 hours (24‐96) 265 infants |
48 hours (24‐84) 337 infants |
Air leak syndrome
It is uncertain if corticosteroids have any effect on the risk of air leak syndrome (RR 0.76, 95% CI 0.32 to 1.80; 2965 infants; studies = 2; I2 = 0%; Analysis 1.33).
1.33. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 33: Air leak syndrome
Duration of oxygen supplementation (days)
In one study, infants receiving corticosteroids required less oxygen supplementation (MD ‐2.86 days, 95% CI ‐5.51 to ‐0.21 days; 73 infants; Analysis 1.34). One study (WHO 2020) reported median duration of oxygen supplementation (Analysis 1.35). The median duration in the corticosteroids group was 36 hours (interquartile range (IQR) 18 to 96; 726 infants)) compared with 48 hours (IQR 12 to 93; 756 infants) in the placebo group.
1.34. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 34: Mean duration of oxygen supplementation (hours)
1.35. Analysis.
Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 35: Median (IQR) duration of oxygen supplementation (hours)
| Median (IQR) duration of oxygen supplementation (hours) | ||
| Study | Corticosteroids | Placebo |
| WHO 2020 | 36 (18‐96) 726 infants |
48 (12‐93) 756 infants |
Surfactant use
Corticosteroids may reduce the need to use surfactant (RR 0.65, 95% CI 0.50 to 0.85; 6104 infants; studies = 6; I2 = 0%; Analysis 1.36).
1.36. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 36: Surfactant use
Systemic infection in first 48 hours of life
Treatment with antenatal corticosteroids may lead to fewer infants having systemic infection in the first 48 hours after birth (RR 0.60, 95% CI 0.41 to 0.88; 1708 infants; studies = 7; I2 = 0%; Analysis 1.37).
1.37. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 37: Systemic infection in the first 48 hours of life
Proven infection while in the neonatal intensive care unit (NICU)
Treatment with antenatal corticosteroids may reduce the risk of infection while in the NICU (RR 0.79, 95% CI 0.64 to 0.98; 5521 infants; studies = 10; I2 = 29%; Analysis 1.38). The symmetry of the funnel plot did not suggest evidence of bias due to non‐publication of results (Figure 15).
1.38. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 38: Proven infection while in the neonatal intensive care unit
15.

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.38 Proven infection while in the neonatal intensive care unit.
Necrotising enterocolitis
Corticosteroids may reduce the risk of necrotising enterocolitis (RR 0.50, 95% CI 0.32 to 0.78; 4702 infants; studies = 10; I2 = 0%; Analysis 1.39). The symmetry of the funnel plot did not suggest evidence of bias due to non‐publication of results (Figure 16).
1.39. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 39: Necrotising enterocolitis
16.

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.39 Necrotising enterocolitis.
Hypothalamo‐pituitary‐adrenal (HPA) axis function
It is uncertain if corticosteroids have any effect on HPA axis function (cortisol MD 3.94 log units, 95% CI ‐3.12 to 11.00 log units; 27 infants; studies = 1; Analysis 1.40).
1.40. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 40: Mean infant HPA axis function (cortisol)
For the child
Mean weight
It is uncertain if corticosteroids have any effect on childhood weight (MD 0.30 kg, 95% CI ‐0.39 to 1.00 kg; 333 children; studies = 2; I2 = 0%; Analysis 1.41) (age at follow‐up was six to 12 years).
1.41. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 41: Mean childhood weight (kg)
Mean head circumference
It is uncertain if corticosteroids have any effect on childhood on head circumference (MD 0.27 cm, 95% CI ‐0.08 to 0.63 cm; 328 children; studies = 2; I2 = 0%; Analysis 1.42) (age at follow‐up was six to 12 years).
1.42. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 42: Mean childhood head circumference (cm)
Mean height
It is uncertain if corticosteroids have any effect on childhood height (MD 1.02 cm, 95% CI ‐0.26 to 2.29 cm; 334 children; studies = 2; I2 = 0%; Analysis 1.43) (age at follow‐up was six to 12 years).
1.43. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 43: Mean childhood height (cm)
Mean skin fold thickness
Not reported.
Abnormal lung function
Not reported.
Mean blood pressure
It is uncertain if corticosteroids have any effect on childhood systolic blood pressure (MD ‐1.60 mmHg, 95% CI ‐4.06 to 0.86 mmHg; 223 children; studies = 1; Analysis 1.44) (age at follow‐up was six to 12 years).
1.44. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 44: Mean childhood systolic blood pressure (mmHg)
Glucose intolerance
Not reported.
Hypothalamo‐pituitary‐adrenal H(PA) axis function
Not reported.
Dyslipidaemia
Not reported.
Cerebral palsy
Antenatal corticosteroids may reduce a child's risk of cerebral palsy but the evidence is uncertain because the confidence interval is wide and includes possible harm (RR 0.60, 95% CI 0.34 to 1.03; 904 children; studies = 5; I2 = 0%; Analysis 1.45) (age at follow‐up was two to 12 years).
1.45. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 45: Cerebral palsy in childhood
Behavioural/learning difficulties
It is uncertain if corticosteroids have any effect on a child's risk of behavioural/learning difficulties (RR 0.86, 95% CI 0.35 to 2.09; participants = 90; studies = 1; Analysis 1.46).
1.46. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 46: Behavioural/learning difficulties in childhood
For the child as adult
Mean weight
It is uncertain if corticosteroids have any effect on weight in adulthood (MD ‐0.83 kg, 95% CI ‐6.41 to 4.76 kg; 538 participants; studies = 2; I2 = 60%; Analysis 1.47).
1.47. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 47: Mean adult weight (kg)
Mean head circumference
It is uncertain if corticosteroids have any effect on head circumference in adulthood (MD 0.03 cm, 95% CI ‐0.33 to 0.38 cm; 537 participants; studies = 2; I2 = 0%; Analysis 1.48).
1.48. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 48: Mean adult head circumference (cm)
Mean height
It is uncertain if corticosteroids have any effect on height in adulthood (MD 0.91 cm, 95% CI ‐0.28 to 2.10 cm; 537 participants; studies = 2; I2 = 0%; Analysis 1.49).
1.49. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 49: Mean adult height (cm)
Mean skin fold thickness
It is uncertain if corticosteroids have any effect on skin fold thickness in adulthood (triceps MD ‐0.02 log units, 95% CI ‐0.11 to 0.07 log units;456 participants; studies = 1; biceps MD ‐0.01 log units, 95% CI ‐0.11 to 0.09 log units; 456 participants; studies = 1; subscapular MD 0.01 log units, 95% CI ‐0.08 to 0.10 log units;441 participants; studies = 1 suprailiac MD ‐0.01 log units, 95% CI ‐0.12 to 0.10 log units; 452 participants; studies = 1; Analysis 1.50).
1.50. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 50: Mean adult skinfold thickness (log values)
Abnormal lung function
It is uncertain if antenatal corticosteroids has any effect on lung function (forced vital capacity) at age 30 years (forced vital capacity MD ‐0.70, 95% CI ‐3.16 to 1.76; 383 participants; studies = 1; Analysis 1.51).
1.51. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 51: Abnormal lung function measured as forced vital capacity (adult)
Mean blood pressure
It is uncertain if corticosteroids have any effect on systolic blood pressure in adulthood (MD ‐0.87 mmHg, 95% CI ‐2.81 to 1.07 mmHg; 545 participants; studies = 2; I2 = 47%; Analysis 1.52).
1.52. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 52: Mean adult systolic blood pressure (mmHg)
Glucose intolerance
Long‐term follow‐up in one study (Liggins 1972b) showed increased insulin release 30 minutes following a fasting 75 g oral glucose tolerance test (MD 0.16 log insulin units, 95% CI 0.04 to 0.28 log insulin units; 412 participants; studies = 1; Analysis 1.53) in 30‐year‐olds who had been exposed to antenatal corticosteroid. Results were inconclusive for fasting glucose concentrations (MD 0.01 mmol/L, 95% CI ‐0.09 to 0.11 mmol/L; 432 participants; studies = 1), or 30 minutes following a 75 g oral glucose tolerance test (MD 0.21 mmol/L, 95% CI ‐0.12 to 0.54 mmol/L; participants = 413; studies = 1). At 120 minutes following a 75 g oral glucose tolerance test, exposure to antenatal corticosteroids was associated with a reduction in glucose concentration (MD ‐0.27 mmol/L; 95% CI ‐0.52 to ‐0.02 mmol/L; 410 participants; studies = 1) (Analysis 1.54). However, the study reported no difference between those exposed to antenatal corticosteroids and those not exposed in the prevalence of diabetes.
1.53. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 53: Mean adult insulin (log values)
1.54. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 54: Mean adult glucose
Hypothalamo‐pituitary‐adrenal (HPA) axis function
It is uncertain if corticosteroids have any effect on HPA axis function in adulthood (cortisol MD 0.06 log units, 95% CI ‐0.02 to 0.14 log units; 444 participants; studies = 1; Analysis 1.55).
1.55. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 55: Mean adult HPA axis function (mean log fasting cortisol)
Dyslipidaemia
Not reported.
Mean age at puberty
It is uncertain if corticosteroids have any effect on mean age at puberty (MD for girls 0 years, 95% CI ‐0.94 to 0.94 years; 38 girls; studies = 1; Analysis 1.56) (data not available for boys).
1.56. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 56: Mean age at puberty (years)
Bone density
One study reported that there was no difference between those exposed to antenatal corticosteroids and those not exposed for bone density at age 30 years in a subset of participants (Liggins 1972b).
Educational achievement
It is uncertain if corticosteroids have any effect on educational achievement defined as attending university or polytechnic education (RR 0.94, 95% CI 0.80 to 1.10; 534 participants; studies = 1; Analysis 1.57).
1.57. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 57: Educational achievement by adulthood (university or polytechnic education)
For health services
Mean length of antenatal hospitalisation for women
It is uncertain if corticosteroids have any effect on length of antenatal hospitalisation (MD ‐0.00 days, 95% CI ‐0.23 to 0.22 days; 412 women; studies = 2; I2 = 0%; Analysis 1.58).
1.58. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 58: Mean length of antenatal hospitalisation (days)
Four other studies reported data relating to overall length of maternal hospital stay (Attawattanakul 2015; Gyamfi‐Bannerman 2016; Mansouri 2010; WHO 2020). In all of the trials there was little to no difference between the groups (Analysis 1.59).
1.59. Analysis.
Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 59: Length of maternal hospital stay
| Length of maternal hospital stay | |||
| Study | Measure | Corticosteroids | Control |
| Attawattanakul 2015 | Overall length of maternal hospital stay (days) (mean (SD)) | 3.57 (0.87) 96 women |
3.58 (0.75) 98 women |
| Gyamfi‐Bannerman 2016 | Overall length of maternal hospital stay (days) (median (IQR)) | 3 (3 to 5) 1427 women |
3 (3 to 5) 1400 women |
| Mansouri 2010 | Number of women requiring a hospital stay of more than three days | 12/100 | 12/100 |
| WHO 2020 | Overall length of maternal hospital stay (days) (median (IQR)) | 8 (4 to 20) 1323 women |
8 (4 to19) 1322 women |
Mean length of postnatal hospitalisation for women
It is uncertain if corticosteroids have any effect on length of postnatal hospitalisation for women (MD 0.00 days, 95% CI ‐1.72 to 1.72 days; 218 women; studies = 1; Analysis 1.60).
1.60. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 60: Mean length of postnatal hospitalisation (days)
Mean length of neonatal hospitalisation
It is uncertain if corticosteroids have any effect on length of neonatal hospitalisation (MD 0.18 days, 95% CI ‐0.51 to 0.87 days; 788 infants; studies = 5; I2 = 0%; Analysis 1.61).
1.61. Analysis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 61: Mean length of neonatal hospitalisation (days)
Two studies (Gyamfi‐Bannerman 2016; WHO 2020) reported median neonatal hospitalisation and found very little difference between the two groups (Analysis 1.62).
1.62. Analysis.
Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 62: Length of neonatal hospitalisation
| Length of neonatal hospitalisation | |||
| Study | Measure | Corticosteroids | Control |
| Gyamfi‐Bannerman 2016 | Overall length of neonatal hospital stay (days) (median (IQR)) | 7 (4 to 12) 1427 infants |
8 (4 to 13) 1400 infants |
| WHO 2020 | Overall length of neonatal hospital stay (days) (median (IQR)) | 8 (3 to17) 1320 infants |
8 (3 to 17) 1301 infants |
Cost of maternal care
not reported
Cost of neonatal care
not reported.
Clinical subgroups
We have analysed the results for prespecified clinical subgroups (covariates) in comparisons 2, 3, 4 and 5, and added further post hoc analyses to explore the possible impact of change in practice over time (comparison 6), protocols with weekly steroid administration (comparison 7), and gestational age at randomisation (comparison 8). Where there was a sufficient number of trials reporting data for meaningful analyses, we have explored the evidence for the review's primary outcomes. These analyses are hypothesis‐generating only and should not be interpreted as conclusive.
2. Antenatal corticosteroids versus placebo or no treatment (singleton and women with multiple pregnancies)
Discrete outcome data for those women delivering multiple pregnancies were available from only five studies (Collaborative 1981; Gamsu 1989; Liggins 1972b; Silver 1996; WHO 2020), with the remainder of the studies including only singleton pregnancies, or reporting data from combined singleton and multiple pregnancies. We have been unable to confirm whether the Mansouri 2010 trial included only singleton pregnancy, but this is suggested by the equal numbers of women and infants reported. We have included data from this study in the singleton subgroup.
For the fetus or neonate
Perinatal death
The test for subgroup differences did not suggest a difference in effect on perinatal death between singleton pregnancies, multiple pregnancies and trials with a combination of singleton and multiple pregnancies (P = 0.77, I2 = 0%, overlapping confidence intervals; Analysis 2.1).
2.1. Analysis.

Comparison 2: Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 1: Perinatal death ‐ single or multiple pregnancy
Neonatal death
The test for subgroup differences did not suggest a difference in effect on neonatal death between singleton pregnancies, multiple pregnancies and trials with a combination of singleton and multiple pregnancies (P = 0.52, I2 = 0%, overlapping confidence intervals; Analysis 2.2).
2.2. Analysis.

Comparison 2: Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 2: Neonatal death ‐ single or multiple pregnancy
Fetal death
The test for subgroup differences did not suggest a difference in effect on fetal death between singleton pregnancies, multiple pregnancies and trials with a combination of singleton and multiple pregnancies (P = 0.42, I2 = 0%, overlapping confidence intervals; Analysis 2.3).
2.3. Analysis.

Comparison 2: Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 3: Fetal death ‐ single or multiple pregnancy
Respiratory distress syndrome (RDS)
The test for subgroup differences did not suggest a difference in effect on RDS in multiple pregnancies compared with singleton pregnancies and trials with a combination of singleton and multiple pregnancies (P = 0.08, I2 = 60%; Analysis 2.4).
2.4. Analysis.

Comparison 2: Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 4: Respiratory distress syndrome ‐ single or multiple pregnancy
Moderate/severe respiratory distress syndrome (RDS)
Insufficient data to perform subgroup analysis.
Chronic lung disease
Insufficient data to perform subgroup analysis.
Intraventricular haemorrhage (IVH)
The test for subgroup differences did not suggest a difference in effect on IVH in multiple pregnancies compared with singleton pregnancies and trials with a combination of singleton and multiple pregnancies (P = 0.56, I2 = 0%; Analysis 2.5).
2.5. Analysis.

Comparison 2: Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 5: Intraventricular haemorrhage ‐ single or multiple pregnancy
Mean birthweight
Insufficient data to perform subgroup analysis.
For the woman
There were insufficient data to perform subgroup analysis for any of the primary outcomes related to the woman.
For the child
There were insufficient data to perform subgroup analysis for any of the primary outcomes related to the child.
For the child as adult
There were insufficient data to perform subgroup analysis for any of the primary outcomes related to the child as adult.
3. Antenatal corticosteroids versus placebo or no treatment (by presence or absence of ruptured membranes at first dose of corticosteroids)
Discrete outcome data from women with intact membranes at the first dose of study medication were available from eight studies (Amorim 1999; Attawattanakul 2015; Block 1977; Collaborative 1981; Garite 1992; Kari 1994; Liggins 1972b; Schmidt 1984), discrete outcome data from women with ruptured membranes at the first dose of study medication were available from ten studies (Block 1977; Dexiprom 1999; Lewis 1996; Liggins 1972b; Lopez 1989; Morales 1989; Nelson 1985; Qublan 2001; Schmidt 1984; Schutte 1980), with the remainder of the studies not reporting rupture of membrane status or reporting combined data from women with intact and ruptured membranes.
Relevant subgroups compared below are: 1. pregnant women with intact membranes, 2. pregnant women with ruptured membranes, and 3. pregnant women for whom membrane status was not reported separately or mixed populations. Analyses with small amounts of data missing are the following: 3.1 Perinatal death (Liggins 1972b); 3.2 Neonatal death (Liggins 1972b); 3.3 Fetal death (Liggins 1972b); 3.4 RDS (Liggins 1972b; Block 1977; Collaborative 1981; Schmidt 1984; Schutte 1980); 3.5 IVH (Liggins 1972b); 3.6 Birthweight (Liggins 1972b); and 3.7 Chorioamnionitis (Liggins 1972b). Overall totals for these outcomes will not match our main analyses in Comparison 1 due to small amounts of missing data where ruptured membrane status was missing for some women.
For the fetus or neonate
Perinatal death
The test for subgroup differences did not suggest a difference in effect on perinatal death between babies born from pregnancies with intact membranes, pregnancies with ruptured membranes and trials with a combination of pregnancies with intact and rupture membranes (P = 0.08, I2 = 60%, overlapping confidence intervals; Analysis 3.1).
3.1. Analysis.

Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 1: Perinatal death ‐ intact or ruptured membranes
Neonatal death
The test for subgroup differences did not suggest a difference in effect on neonatal death between babies born from pregnancies with intact membranes, pregnancies with ruptured membranes and trials with a combination of pregnancies with intact and rupture membranes (P = 0.29, I2 = 20%, overlapping confidence intervals; Analysis 3.2).
3.2. Analysis.

Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 2: Neonatal deaths ‐ intact or ruptured membranes
Fetal death
The test for subgroup differences did not suggest a difference in effect on fetal death between babies born from pregnancies with intact membranes, pregnancies with ruptured membranes and trials with a combination of pregnancies with intact and rupture membranes (P = 0.81, I2 = 0%, overlapping confidence intervals; Analysis 3.3).
3.3. Analysis.

Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 3: Fetal death ‐ intact or ruptured membranes
Respiratory distress syndrome (RDS)
The test for subgroup differences did not suggest a difference in effect on RDS between babies born from pregnancies with intact membranes, pregnancies with ruptured membranes and trials with a combination of pregnancies with intact and ruptured membranes (P = 0.08, I2 = 60%, overlapping confidence intervals; Analysis 3.4).
3.4. Analysis.

Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 4: RDS ‐ intact or ruptured membranes
Moderate/severe respiratory distress syndrome (RDS)
Insufficient data to perform subgroup analysis.
Chronic lung disease
Insufficient data to perform subgroup analysis.
Intraventricular haemorrhage (IVH)
The test for subgroup differences suggested a difference in effect on IVH between babies born from pregnancies with intact membranes, pregnancies with ruptured membranes and trials with a combination of pregnancies with intact and ruptured membranes (P = 0.02, I2 = 76%, overlapping confidence intervals; Analysis 3.5). However, the effect estimates of the intact membranes group and the ruptured membranes group are close to each other, while the difference detected by the statistical test is likely to be caused by the mixed population group. Furthermore, we cannot be certain that the variability in effect estimates is due to genuine subgroup differences rather than chance.
3.5. Analysis.

Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 5: IVH ‐ intact or ruptured membranes
Mean birthweight
The test for subgroup differences suggests there may be a difference in effect on birthweight between babies born from pregnancies with intact membranes, pregnancies with ruptured membranes and trials with a combination of pregnancies with intact and rupture membranes (P = 0.46, I2 = 0%, overlapping confidence intervals; Analysis 3.6).
3.6. Analysis.

Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 6: Birthweight ‐ intact or ruptured membranes
For the woman
Maternal death
Insufficient data to perform subgroup analysis.
Chorioamnionitis
The test for subgroup differences did not suggest a difference in effect on chorioamnionitis between women with intact membranes, women with ruptured membranes and trials with a combination of women with intact and rupture membranes (P = 0.51, I2 = 0%, overlapping confidence intervals; Analysis 3.7).
3.7. Analysis.

Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 7: Chorioamnionitis ‐ intact or ruptured membranes
Endometritis
The test for subgroup differences did not suggest a difference in effect on endometritis between babies born from pregnancies with intact membranes, pregnancies with ruptured membranes and trials with a combination of pregnancies with intact and rupture membranes (P = 0.99, I2 = 0%, overlapping confidence intervals; Analysis 3.8).
3.8. Analysis.

Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 8: Endometritis ‐ intact or ruptured membranes
For the child
There were insufficient data to perform subgroup analysis for any of the primary outcomes related to the child.
For the child as adult
There were insufficient data to perform subgroup analysis for any of the primary outcomes related to the child as adult.
4. Antenatal corticosteroids versus placebo or no treatment (for women with hypertension syndrome)
Meaningful analysis was not possible for several primary outcomes due to the small number of trials reporting results by presence or absence of hypertension syndromes.
For the fetus or neonate
Perinatal death
The test for subgroup differences did not suggest a difference in effect on perinatal death between babies whose mothers had hypertension syndrome, mothers without hypertension syndrome and trials where hypertension was not reported separately (P = 0.99, I2 = 0%, overlapping confidence intervals; Analysis 4.1).
4.1. Analysis.

Comparison 4: Corticosteroids versus placebo or no treatment ‐ hypertension syndrome versus all other trials, Outcome 1: Perinatal deaths ‐ hypertension syndrome vs other trials
Neonatal death
The test for subgroup differences did not suggest a difference in effect on perinatal death between babies whose mothers had hypertension syndrome, mothers without hypertension syndrome and trials where hypertension was not reported separately (P = 0.16, I2 = 46%, overlapping confidence intervals; Analysis 4.2).
4.2. Analysis.

Comparison 4: Corticosteroids versus placebo or no treatment ‐ hypertension syndrome versus all other trials, Outcome 2: Neonatal deaths ‐ hypertension syndrome vs other trials
Fetal death
The test for subgroup differences did not suggest a difference in effect on fetal death between babies whose mothers had hypertension syndrome and babies whose mothers did not have hypertension syndrome or babies in trials where hypertension was not reported separately (P = 0.09, I2 = 59%, overlapping confidence intervals; Analysis 4.3).
4.3. Analysis.

Comparison 4: Corticosteroids versus placebo or no treatment ‐ hypertension syndrome versus all other trials, Outcome 3: Fetal deaths ‐ hypertension syndrome vs other trials
Respiratory distress syndrome (RDS)
The test for subgroup differences suggests there may be a difference in effect on RDS death between babies whose mothers had hypertension syndrome and babies whose mothers did not have hypertension syndrome or babies in trials where hypertension was not reported separately (P = 0.007, I2 = 80%; Analysis 4.4). An examination of the effect estimates and their overlapping confidence intervals suggests that the variability detected by the test for subgroup differences may be related to size of effect but not to direction of effect, whereby the size of effect is larger in the group of women with hypertension than in the other two groups. It should also be noted that the difference in effect size between the trials with women with hypertension and the trials in women without hypertension is small, the cause of the subgroup difference may be due to the group of trials with mixed populations having a difference effect size from the other two groups. Furthermore, we cannot be certain that the variability in effect estimates is due to genuine subgroup differences rather than chance.
4.4. Analysis.

Comparison 4: Corticosteroids versus placebo or no treatment ‐ hypertension syndrome versus all other trials, Outcome 4: Respiratory distress syndrome ‐ hypertension syndrome vs other trials
Moderate/severe respiratory distress syndrome (RDS)
Insufficient data to perform subgroup analysis.
Chronic lung disease
Insufficient data to perform subgroup analysis.
Intraventricular haemorrhage (IVH)
Insufficient data to perform subgroup analysis.
Mean birthweight
Insufficient data to perform subgroup analysis.
For the woman
There were insufficient data to perform subgroup analysis for any of the primary outcomes related to the woman.
For the child
There were insufficient data to perform subgroup analysis for any of the primary outcomes related to the child.
For the child as adult
There were insufficient data to perform subgroup analysis for any of the primary outcomes related to the child as adult.
5. Antenatal corticosteroids versus placebo or no treatment (by type of corticosteroid)
For the fetus or neonate
Perinatal death
The test for subgroup differences did not suggest a difference in effect on perinatal death between different types of corticosteroid (P = 0.62, I2 = 0%, overlapping confidence intervals; Analysis 5.1).
5.1. Analysis.

Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 1: Perinatal death ‐ type of steroid
Neonatal death
The test for subgroup differences did not suggest a difference in effect on neonatal death between different types of corticosteroid (P = 0.62, I2 = 0%, overlapping confidence intervals; Analysis 5.2).
5.2. Analysis.

Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 2: Neonatal death ‐ type of steroid
Fetal death
The test for subgroup differences did not suggest a difference in effect on fetal death between different types of corticosteroid (P = 0.90, I2 = 0%, overlapping confidence intervals; Analysis 5.3).
5.3. Analysis.

Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 3: Fetal death ‐ type of steroid
Respiratory distress syndrome (RDS)
The test for subgroup differences suggests there may be a difference in effect on RDS between different types of corticosteroid (P = 0.04, I2 = 63%, overlapping confidence intervals; Analysis 5.4). The difference seems to be attributable to the groups using dexamethasone and betamethasone. However given the overlapping confidence intervals of the two effect estimates, we cannot be certain that the variability in effect estimates is due to genuine subgroup differences rather than chance.
5.4. Analysis.

Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 4: Respiratory distress syndrome ‐ type of steroid
Moderate/severe respiratory distress syndrome (RDS)
The test for subgroup differences suggests there may be a difference in effect on moderate/severe RDS between different types of corticosteroid (P = 0.03, I2 = 65.8%, overlapping confidence intervals; Analysis 5.5). The difference seems to be attributable to the groups using dexamethasone and betamethasone. However given the overlapping confidence intervals of the two effect estimates, we cannot be certain that the variability in effect estimates is due to genuine subgroup differences rather than chance.
5.5. Analysis.

Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 5: Moderate/severe respiratory distress syndrome ‐ type of steroid
Chronic lung disease
The test for subgroup differences did not suggest a difference in effect on chronic lung disease between different types of corticosteroid (P = 0.17, I2 = 47%, overlapping confidence intervals; Analysis 5.6).
5.6. Analysis.

Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 6: Chronic lung disease ‐ type of steroid
Intraventricular haemorrhage (IVH)
The test for subgroup differences did not suggest a difference in effect on IVH between different types of corticosteroid (P = 0.06, I2 = 71%, overlapping confidence intervals; Analysis 5.7).
5.7. Analysis.

Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 7: IVH ‐ type of steroid
Mean birthweight
The test for subgroup differences did not suggest a difference in effect on mean birthweight between different types of corticosteroid (P = 0.38, I2 = 2%, overlapping confidence intervals; Analysis 5.8).
5.8. Analysis.

Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 8: Birthweight ‐ type of steroid
For the woman
Maternal death
Insufficient data to perform subgroup analysis.
Chorioamnionitis
The test for subgroup differences suggests there may be a difference in effect on chorioamnionitis between different types of corticosteroid (P = 0.02, I2 = 82%; Analysis 5.9). Betamethasone may reduce the risk of chorioamnionitis compared to control treatments, while the effect estimate of dexamethasone compared to control treatments is inconclusive. Taking into consideration the overlapping confidence intervals of the two effect estimates, we cannot be certain that the variability in effect estimates is due to genuine subgroup differences rather than chance.
5.9. Analysis.

Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 9: Chorioamnionitis ‐ type of steroid
Endometritis
The test for subgroup differences did not suggest a difference in effect on endometritis between different types of corticosteroid (P = 0.13, I2 = 57%, overlapping confidence intervals; Analysis 5.10).
5.10. Analysis.

Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 10: Endometritis ‐ type of steroid
For the child
There were insufficient data to perform subgroup analysis for any of the primary outcomes related to the child.
For the child as adult
There were insufficient data to perform subgroup analysis for any of the primary outcomes related to the child as adult.
6. Antenatal corticosteroids versus placebo or no treatment (by decade of trial)
The subgroup tests in RevMan 5 are not ideal to test whether or not there were trends across decades; the test can only indicate if at least one decade differs from another, and not if there is a trend over time. We advise caution when interpreting the findings below, especially regarding survival across decades.
For the fetus or neonate
Perinatal death
The test for subgroup differences suggests there may be a difference in effect on perinatal death according to the decade when the trial took place (P = 0.02, I2 = 65%; Analysis 6.1), whereby there appears to be a difference between trials conducted in the 1970s ‐ 1980s (CIs cross the line of no effect) and later trials (clearly favouring corticosteroids). Reasons for the differences are uncertain and may be due to variation in standard of care across decades or because of trial locations. Furthermore, we cannot be certain that it is due to genuine subgroup differences rather than due to chance.
6.1. Analysis.

Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 1: Perinatal death ‐ decade of trial
Neonatal death
The test for subgroup differences suggests there may be a difference in effect on neonatal death according to the decade when the trial took place (P = 0.03, I2 = 64%; Analysis 6.2), whereby there appears to be a greater effect size in trials conducted in the 2000s compared with 2010s. Reasons for the differences are uncertain and may be due to variation in standard of care across decades or because of trial locations. Furthermore, we cannot be certain that the difference is due to genuine subgroup differences rather than due to chance.
6.2. Analysis.

Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 2: Neonatal death ‐ decade of trial
Fetal death
The test for subgroup differences did not suggest a difference in effect on fetal death according to the decade when the trial took place (P = 0.86, I2 = 0%, overlapping confidence intervals; Analysis 6.3).
6.3. Analysis.

Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 3: Fetal death ‐ decade of trial
Respiratory distress syndrome (RDS)
The test for subgroup differences suggests there may be a difference in effect on RDS according to the decade when the trial took place (P = 0.01, I2 = 69%; Analysis 6.4). In all decades the effect estimates are in the same direction and favour corticosteroid treatment reducing RDS. Their overlapping confidence intervals means that we cannot be certain that the result is due to genuine subgroup differences rather than due to chance.
Moderate/severe respiratory distress syndrome (RDS)
Insufficient data to perform subgroup analysis.
Chronic lung disease
Insufficient data to perform subgroup analysis.
Intraventricular haemorrhage (IVH)
The test for subgroup differences did not suggest a difference in effect on IVH according to the decade when the trial took place (P = 0.10, I2 = 49%, overlapping confidence intervals; Analysis 6.5).
6.5. Analysis.

Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 5: IVH ‐ decade of trial
Mean birthweight
The test for subgroup differences did not suggest a difference in effect on birthweight according to the decade when the trial took place (P = 0.81, I2 = 0%, overlapping confidence intervals; Analysis 6.6).
6.6. Analysis.

Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 6: Birthweight ‐ decade of trial
For the woman
Maternal death
Insufficient data to perform subgroup analysis.
Chorioamnionitis
The test for subgroup differences did not suggest a difference in effect on chorioamnionitis according to the decade when the trial took place (P = 0.07, I2 = 55%, overlapping confidence intervals; Analysis 6.7).
6.7. Analysis.

Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 7: Chorioamnionitis ‐ decade of trial
Endometritis
The test for subgroup differences did not suggest a difference in effect on endometritis according to the decade when the trial took place (P = 0.36, I2 = 7%, overlapping confidence intervals; Analysis 6.8).
6.8. Analysis.

Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 8: Endometritis ‐ decade of trial
For the child
There were insufficient data to perform subgroup analysis for any of the primary outcomes related to the child.
For the child as adult
There were insufficient data to perform subgroup analysis for any of the primary outcomes related to the child as adult.
7. Antenatal corticosteroids versus placebo or no treatment (by presence or absence in protocol of weekly repeat doses of corticosteroid)
Seven of the included studies allowed weekly repeat courses of study medication in their study protocols (Amorim 1999; Garite 1992; Lewis 1996; Morales 1989; Qublan 2001; Silver 1996; WHO 2020).
For the fetus or neonate
Perinatal death
The test for subgroup differences did not suggest a difference in effect on perinatal death between protocols with a single course and those including weekly repeats (P = 0.63, I2 = 0%, overlapping confidence intervals; Analysis 7.1).
7.1. Analysis.

Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 1: Perinatal death ‐ protocol with weekly repeats
Neonatal death
The test for subgroup differences did not suggest a difference in effect on neonatal death between protocols with a single course and those including weekly repeats (P = 0.43, I2 = 0%, overlapping confidence intervals; Analysis 7.2).
7.2. Analysis.

Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 2: Neonatal death ‐ protocol with weekly repeats
Fetal death
The test for subgroup differences did not suggest a difference in effect on fetal death between protocols with a single course and those including weekly repeats (P = 0.68, I2 = 0%, overlapping confidence intervals; Analysis 7.3).
7.3. Analysis.

Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 3: Fetal death ‐ protocol with weekly repeats
Respiratory distress syndrome (RDS)
The test for subgroup differences did not suggest a difference in effect on RDS between protocols with a single course and those including weekly repeats (P = 0.55, I2 = 0%, overlapping confidence intervals; Analysis 7.4).
7.4. Analysis.

Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 4: Respiratory distress syndrome ‐ protocol with weekly repeats
Moderate/severe respiratory distress syndrome (RDS)
The test for subgroup differences did not suggest a difference in effect on moderate/severe RDS between protocols with a single course and those including weekly repeats (P = 0.53, I2 = 0%, overlapping confidence intervals; Analysis 7.5).
7.5. Analysis.

Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 5: Moderate/severe respiratory distress syndrome
Chronic lung disease
Insufficient data to perform subgroup analysis.
Intraventricular haemorrhage (IVH)
The test for subgroup differences did not suggest a difference in effect on IVH between protocols with a single course and those including weekly repeats (P = 0.99, I2 = 0%, overlapping confidence intervals; Analysis 7.6).
7.6. Analysis.

Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 6: IVH ‐ protocol with weekly repeats
Mean birthweight
The test for subgroup differences did not suggest a difference in effect on birthweight between protocols with a single course and those including weekly repeats (P = 0.29, I2 = 12%, overlapping confidence intervals; Analysis 7.7).
7.7. Analysis.

Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 7: Birthweight ‐ protocol with weekly repeats
For the woman
Maternal death
Insufficient data to perform subgroup analysis.
Chorioamnionitis
The test for subgroup differences did not suggest a difference in effect on chorioamnionitis between protocols with a single course and those including weekly repeats (P = 0.68, I2 = 0%, overlapping confidence intervals; Analysis 7.8).
7.8. Analysis.

Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 8: Chorioamnionitis ‐ protocol with weekly repeats
Endometritis
The test for subgroup differences did not suggest a difference in effect on endometritis between protocols with a single course and those including weekly repeats (P = 0.11, I2 = 61%, overlapping confidence intervals; Analysis 7.9).
7.9. Analysis.

Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 9: Endometritis ‐ protocol with weekly repeats
For the child
There were insufficient data to perform subgroup analysis for any of the primary outcomes related to the child.
For the child as adult
There were insufficient data to perform subgroup analysis for any of the primary outcomes related to the child as adult.
8. Gestational age at trial entry (less than or equal to 35 weeks + 0 days; greater than or equal to 34 weeks + 0 days)
We have split studies according to the gestational age at which pregnant women entered trials to receive their first dose of corticosteroids and have considered two, slightly overlapping subgroups: 1) women less than, and including, 35 weeks and 0 days and 2) women greater than, and including, 34 weeks and 0 days. Four studies could be analysed in either group (Block 1977; Collaborative 1981; Liggins 1972b; Teramo 1980). We addressed these issues as follows: data from Liggins 1972b were available for women entering the trial at less than 35 weeks + 0 days and from between 35 weeks + 0 days and 37 weeks + 0 days. The majority of women in the remaining three studies (Block 1977; Collaborative 1981; Teramo 1980) were of less than 34 weeks + 0 days gestation, therefore we included these studies in the younger gestational age grouping for the analysis (women less than and including 35 weeks and 0 days), but we undertook a sensitivity analysis with the studies' data removed.
For the fetus or neonate
Perinatal death
The test for subgroup differences did not suggest a difference in effect on perinatal death between women with pregnancy gestations at less than or equal to 35 weeks and those at 34 weeks or greater (P = 0.13, I2 = 56%, overlapping confidence intervals; Analysis 8.1).
8.1. Analysis.

Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 1: Perinatal death ‐ gestational age at trial entry
Sensitivity analysis removing two studies (Block 1977; Collaborative 1981) did not substantially change the test for subgroup difference (P = 0.12, I2 = 58%).
Neonatal death
The test for subgroup differences did not suggest a difference in effect on neonatal death between women with pregnancy gestations at less than or equal to 35 weeks and those at 34 weeks or greater (P = 0.24, I2 = 28%, overlapping confidence intervals; Analysis 8.2).
8.2. Analysis.

Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 2: Neonatal death ‐ gestational age at trial entry
Sensitivity analysis removing two studies (Block 1977; Collaborative 1981) did not substantially change the test for subgroup difference (P = 0.23, I2 = 32%).
Fetal death
The test for subgroup differences did not suggest a difference in effect on fetal death between women with pregnancy gestations at less than or equal to 35 weeks and those at 34 weeks or greater (P = 0.40, I2 = 0%, overlapping confidence intervals; Analysis 8.3).
8.3. Analysis.

Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 3: Fetal death ‐ gestational age at trial entry
Sensitivity analysis removing two studies (Block 1977; Collaborative 1981) did not change the test for subgroup difference (P = 0.40, I2 = 0%).
Respiratory distress syndrome (RDS)
The test for subgroup differences did not suggest a difference in effect on RDS between women with pregnancy gestations at less than or equal to 35 weeks and those at 34 weeks or greater (P = 0.60, I2 = 0%, overlapping confidence intervals; Analysis 8.4).
8.4. Analysis.

Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 4: Respiratory distress syndrome ‐ gestational age at trial entry
Sensitivity analysis removing three studies (Block 1977; Collaborative 1981; Teramo 1980) did not substantially change the test for subgroup difference (P = 0.58, I2 = 0%).
Moderate/severe respiratory distress syndrome (RDS)
Insufficient data to perform subgroup analysis.
Chronic lung disease
Insufficient data to perform subgroup analysis.
Intraventricular haemorrhage (IVH)
The test for subgroup differences did not suggest a difference in effect on IVH between women with pregnancy gestations at less than or equal to 35 weeks and those at 34 weeks or greater (P = 0.16, I2 = 49%, overlapping confidence intervals; Analysis 8.5).
8.5. Analysis.

Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 5: IVH ‐ gestational age at trial entry
Mean birthweight
The test for subgroup differences did not suggest a difference in effect on birthweight between women with pregnancy gestations at less than or equal to 35 weeks and those at 34 weeks or greater (P = 0.77, I2 = 0%, overlapping confidence intervals; Analysis 8.6).
8.6. Analysis.

Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 6: Birthweight ‐ gestational age at trial entry
The effect estimate in this analysis is slightly different from the main analysis (Analysis 1.8) because the data from one study (Liggins 1972b) have been analysed in six different gestational age groups, whereas in the main analysis we have presented the mean for the overall trial population.
For the woman
Maternal death
Insufficient data to perform subgroup analysis.
Chorioamnionitis
The test for subgroup differences did not suggest a difference in effect on chorioamnionitis between women with pregnancy gestations at less than or equal to 35 weeks and those at 34 weeks or greater (P = 0.11, I2 = 61%, overlapping confidence intervals; Analysis 8.7).
8.7. Analysis.

Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 7: Chorioamnionitis ‐ gestational age at trial entry
Endometritis
Insufficient data to perform subgroup analysis.
For the child
There were insufficient data to perform subgroup analysis for any of the primary outcomes related to the child.
For the child as adult
There were insufficient data to perform subgroup analysis for any of the primary outcomes related to the child as adult.
Discussion
Summary of main results
In this updated review includes 27 studies involving 11,272 women and 11,925 infants. Our results support the conclusion of the previous review (Roberts 2017), that treatment with antenatal corticosteroids reduces perinatal death, neonatal death, respiratory distress syndrome (RDS), and intraventricular haemorrhage (IVH) in preterm infants.
High‐certainty evidence suggests that antenatal corticosteroids reduce the risk of perinatal death, neonatal death and RDS, and have little to no effect on birthweight (high‐certainty evidence; Table 1). Antenatal corticosteroids probably reduce the risk of IVH (moderate‐certainty evidence; Table 1). Antenatal corticosteroids probably reduce the risk of developmental delay in later childhood (moderate‐certainty evidence; Table 1). Further, the evidence suggests that antenatal corticosteroids reduce the risk of moderate and severe RDS. The evidence is uncertain if antenatal corticosteroids have any effect on the risk of death (in childhood or adulthood), or on neurodevelopmental disability in later adulthood.
Moderate‐certainty evidence suggests that antenatal corticosteroids probably result in little to no difference in the risk of maternal death or endometritis but the wide 95% CIs include possible benefit and possible harm (Table 2). Antenatal corticosteroids probably result in little to no difference in the risk of chorioamnionitis (moderate‐certainty evidence; Table 2).
In addition to the benefit of antenatal corticosteroids reducing the risk for a number of our primary outcomes for the fetus or neonate (perinatal death, neonatal death, RDS, moderate and severe RDS, IVH), we found evidence of potential benefit of antenatal corticosteroids reducing the risk for a number of our secondary outcomes for the fetus or neonate: Apgar score < 7 at five minutes, admission to the neonatal intensive care unit (NICU), need for mechanical ventilation/continuous positive airways pressure (CPAP), duration of oxygen supplementation, surfactant use, systemic infection in the first 48 hours of life, proven infection while in the NICU, and necrotising enterocolitis. However, antenatal corticosteroids may have little to no effect on the risk of fetal death. For the remainder of the secondary outcomes for the fetus or neonate, we are uncertain of the overall effect of antenatal corticosteroids.
For the woman, child, and adult we generally found no evidence of harm from antenatal corticosteroids apart from increased glucose tolerance in women and increased insulin resistance in adults; in fact we found some evidence suggesting potentially decreased side effects in women receiving corticosteroids, although for a number of the outcomes investigated there were no or insufficient data.
Overall, the complete body of evidence of antenatal corticosteroids for women at risk of preterm birth strongly favours important clinical benefit for the fetus and neonate, including reducing risk of death and major neonatal morbidity, without evidence of major clinical harm to either the women, the fetus, neonate, child or adult.
Overall completeness and applicability of evidence
We have attempted to identify all available published and unpublished randomised trial data for the use of antenatal corticosteroids for women at risk of preterm birth. Additional data have been obtained and included where possible. We believe that the data are comprehensive and relevant to all women at risk of preterm birth. Comparisons of repeat antenatal corticosteroid regimens, of different antenatal corticosteroids and of the use of antenatal corticosteroids at term before elective birth are described in other Cochrane Reviews (Brownfoot 2013; Crowther 2015; Sotiriadis 2018).
Since the last update of this review (Roberts 2017), we have been able to add substantial evidence from low‐ and middle‐resource settings for most of our primary outcomes. The evidence supplied by these studies is consistent with that of the previous review, and the incorporation of these data into the current review should reassure clinicians who work in these settings that the evidence presented is applicable to the population they serve. This is important, as preterm birth is the leading cause of death in children younger than five years worldwide, with greater burden in low‐ and middle‐resource settings (Chawanpaiboon 2019).
In subgroup analyses we examined the effect of antenatal corticosteroids in women with singleton versus women with multiple pregnancies, in women with intact membranes versus ruptured membranes at first dose, and in women with pregnancy‐induced hypertension syndromes. The test for subgroup differences did not find substantial differences between subgroups to suggest that the applicability of the evidence should be different to one subgroup versus another; in other words the evidence remains applicable to all clinical subgroups examined. However, for a number of the subgroups, the number of studies, and participants within studies, contributing data are limited and thus the subgroup analyses do need to be interpreted with caution.
Whether antenatal corticosteroids are beneficial in the current era of advanced neonatal practice has been questioned on the basis that previous conclusions concerning their benefits drew on data from the 1970s. In this update, we have included 10 studies published since 2000 (Attawattanakul 2015; Balci 2010; Fekih 2002; Gyamfi‐Bannerman 2016; Mansouri 2010; Porto 2011; Ontela 2018; Qublan 2001; Shanks 2010; WHO 2020), as well as analyses for the previous decades. These more recent studies contributed over 60% of the overall data to the review. Overall, the results show consistent benefits of steroid use, without any strong evidence that antenatal corticosteroids are not beneficial in the current era of advanced neonatal practice.
The gestational age range at which antenatal corticosteroids provide benefit has been subject to debate. Four studies enrolled infants between 24 weeks and 0 days and 26 weeks and 0 days, with another three studies not reporting the lower gestational age limit with which they enrolled (Table 3). Ideally, this question should be investigated with individual patient data analysis using a priori agreed gestational age cut‐offs. We examined outcomes based on gestational age divisions of up to, and including, 35 weeks + 0 days and greater than, and including, 34 weeks + 0 days at trial entry. We included data from over 4000 women and infants from trials that enrolled from 34 weeks + 0 days. The test for subgroup differences did not find differences between subgroups for the outcomes of perinatal death, neonatal death, fetal death, RDS, IVH, birthweight or chorioamnionitis. This large body of evidence supports the use of antenatal corticosteroids in women at risk of late preterm birth, although there are currently insufficient data to comment on long‐term effects for the child and adult.
In our subgroup analyses of different corticosteroids we found little evidence of a difference in efficacy between the two types of corticosteroids, apart from a possible difference with less maternal chorioamnionitis occurring with betamethasone. However the confidence intervals overlapped and our analysis is subject to bias as allocation to one type of corticosteroid or the other was not subject to randomisation. Further, our overall results in this subgroup analysis are consistent with another review (Brownfoot 2013; 10 studies; 1089 women and 1161 infants), which compared different corticosteroid regimens and found insufficient evidence to support the use of one corticosteroid over the other.
In our analysis the test for subgroup differences between trials administering a single course of steroids and trial protocols allowing weekly repeats, should the infant remain undelivered, did not suggest a difference in effect. However, this finding should not be interpreted as evidence in support of weekly repeats which is the subject of another Cochrane Review (Crowther 2015)
Quality of the evidence
The evidence described in this review is based on 27 randomised controlled trials comparing antenatal corticosteroids with no antenatal corticosteroids. Overall, the evidence is consistent. There are some limitations in several trials where there was no placebo treatment used in the control group, and therefore the participants and caregivers were not blinded, and there was insufficient information in several trials to enable us to make judgements on the processes of randomisation or allocation concealment. The lack of information is most likely due to the era in which the trials were conducted, when this information was not a requirement for publication. We did not downgrade for risk of bias because our sensitivity analyses did not indicate that the effect estimates would be substantially different with those trials removed from the analysis.
We assessed the evidence for perinatal death, neonatal death, RDS and birthweight as high certainty, which means that future trials are unlikely to change these findings. We assessed the evidence for IVH as moderate certainty because of the complexity involved in diagnosing IVH; earlier studies only reported this outcome at post mortem (consistent with lack of investigative techniques at the time), while latter studies utilised diagnosis by ultrasound, universally (but consistent with clinical practice) not all infants were screened for this outcome and so we cannot be certain that the effect estimate in our analysis is a true reflection of the risk in the whole trial population.
For pregnant women, we assessed the evidence as moderate certainty for three outcomes: maternal death, chorioamnionitis and endometritis. Downgrading in each case was for imprecision due to wide confidence intervals crossing the line of no effect. There were very few data for maternal death.
For the first time, we have considered the identified studies in terms of their trustworthiness in addition to using GRADE and the Cochrane 'Risk of bias' tool. We believe that the steps we have taken to evaluate studies' trustworthiness, and to remove studies whose trustworthiness could not be ascertained, adds to the value of the review and enhances its reliability. Studies were removed from the analysis and put into 'awaiting classification' because of concerns around randomisation processes, lack of evidence of ethics approval and/or prospective trial registration (for studies published after 2010) and, in the case of studies published only as abstracts, lack of confirmation that the available data were from the final analysis. Arguments for including data from studies published only in abstract form centre largely around publication bias in favour of studies with positive results and for studies conducted in environments where English is the primary language. However, subsequent publication of scientific meeting abstracts as full manuscripts is associated with better quality of the studies (Scherer 2018). Furthermore, the inclusion of the four trials with abstract only data available would have added only an additional 179 participants to the RDS analysis, the single primary outcome where all four of these studies contributed data to the previous review (Roberts 2017).
Assessing selective or incomplete reporting of results of randomised controlled trials is notoriously difficult when only published trial reports are available. These concerns led to the call for prospective trial registration (Simes 1986), which was subsequently adopted by the International Committee of Medical Journal Editors in 2004 (De Angelis 2004). In this review, although none of the early trials had been registered the two largest and most recent trials were prospectively registered and reported their pre‐specified outcomes in full.
The need for prospective ethics has been recognised by the Declaration of Helsinki for many decades (The World Medical Association 2013). Our exclusion of three trials published since 2010 due to lack of evidence of ethics approval and/or prospective trial registration appears fair and in keeping with these important principles of high‐quality randomised controlled trial conduct. We have attempted to contact study authors and we acknowledge that further trustworthy evidence may come to light from some studies. We welcome contact from study authors should they wish to engage with us.
Potential biases in the review process
Through our comprehensive search strategy we have attempted to identify all relevant trials regardless of publication status or language. Two or more review authors independently appraised the trials, extracted the data, assessed risk of bias and applied the GRADE method to assess the certainty of evidence. Where data were missing, we have contacted the original trialists and some additional data have been provided that enhances the content of this review.
We are aware of potential bias in our assumption that trials where the number of women is the same is the same as the number of infants included only singleton pregnancies.
A common situation in trials of pregnant women is that some outcomes do not apply to all randomised participants; specifically neonatal mortality and morbidity outcomes can only apply to those fetuses that survive to birth. While the number of 'eligible' participants can be used as a denominator in analysis of neonatal mortality and morbidity outcomes, this approach risks introducing bias as the comparison is not between the randomised groups, particularly if there is a difference in survival to birth between intervention groups. Consistent with this approach we have used the number of fetuses randomised for our primary neonatal mortality and morbidity outcomes (neonatal death, RDS, moderate/severe RDS, chronic lung disease, and IVH). Fourteen studies (9883 infants) provided data for fetal death, with the outcome reported in 399 (6%). While we could have undertaken a sensitivity analysis of our primary neonatal mortality and morbidity outcomes using 'eligible' infants (i.e. removing fetal deaths) as the denominator, this was not deemed necessary as the two groups where very well balanced in terms of fetal deaths (RR 1.01, 95% CI 0.83 to 1.22), and the neonatal mortality and morbidity outcomes analysed had tight confidence levels.
While we applied our trustworthiness screening tool using objectively‐defined criteria we acknowledge that there may be some subjective interpretation in its application. Similarly, there may be an element of subjectivity in the application of 'Risk of bias' assessment and GRADE rating of evidence certainty. To minimise the potential for introducing bias with respect to 'Risk of bias' assessment, GRADE rating and trustworthiness screening we ensured each of these stages was carried out independently by two review authors with agreement reached by consensus through consultation with a third review author where necessary.
Agreements and disagreements with other studies or reviews
Current international recommendations, including those of the World Health Organization, have used earlier versions of this review on which to base their recommendations (WHO 2015).
A systematic review conducted for a bi‐national clinical practice guideline for Australia and New Zealand in 2015 reported on the same maternal and neonatal benefits as the primary outcomes of this systematic review (Antenatal Corticosteroid CPG Panel 2015), and had agreement with respect to the principle findings of this review
As mentioned above, additional Cochrane Reviews consider evidence for repeat antenatal corticosteroid regimens (Crowther 2015), different types of antenatal corticosteroids (Brownfoot 2013), use of antenatal corticosteroids at term before elective birth (Sotiriadis 2018), maternal versus direct fetal administration of corticosteroids (Utama 2018), and strategies for optimising antenatal corticosteroid administration (Rohwer 2020). Ten studies contributing data to the review of repeat antenatal corticosteroids (4733 women and 5700 neonates) found that repeat corticosteroids reduce the risk of neonates experiencing RDS and serious outcomes, but at the expense of lower birthweight (although this was not associated with lower birthweight adjusted for gestational age), without evidence of harm at early childhood follow‐up (Crowther 2015). Four studies contributing data to the review of antenatal corticosteroids at term before elective birth (3856 women and 3893 neonates) found low‐certainty evidence that corticosteroids reduce the risk of RDS, transient tachypnoea, and admission into neonatal special care for the neonate, but not a reduction in need for mechanical ventilation (Sotiriadis 2018). Twelve studies contributing data to the review of different types of antenatal corticosteroids (1557 women and 1661 neonates) found evidence that dexamethasone may reduced the risk of IVH compared to betamethasone, although the authors concluded that more trials are urgently needed (Brownfoot 2013). No studies were identified comparing maternal versus direct fetal routes of corticosteroids (Utama 2018). Three cluster‐randomised controlled trials contributing data to the review of strategies for antenatal corticosteroid administration were unable to be pooled in meta‐analysis. In two trials, promoting the use of antenatal corticosteroids resulted in increased use of antenatal corticosteroids, whereas one trial did not find a difference in the rate of antenatal corticosteroid administration compared to usual care. The authors found in low‐resource settings that a strategy of actively promoting the use of antenatal corticosteroid in women at risk of preterm birth may increase antenatal corticosteroid use in the target population, but may also carry a substantial risk of unnecessary exposure of antenatal corticosteroids in women in whom antenatal corticosteroid is not indicated. At the population level, these effects were probably associated with increased risks of fetal death, perinatal death, neonatal death, and maternal infection (Rohwer 2020). Thus the inclusion of important data from low‐ and medium‐resource settings in the current review provides direct randomised controlled trial evidence that antenatal corticosteroids are effective in women at risk of preterm birth in these settings. However as demonstrated in the Rohwer 2020 review clinicians need to ensure that appropriate women in such settings are targeted.
A number of recent systematic reviews have analysed observational data with respect to the use of antenatal corticosteroids in women at risk of preterm birth before 24 and 25 weeks (Park 2016; 17 studies, 3626 participants; Deshmukh 2017; eight studies, 10,109 participants; Deshmukh 2018 nine studies, 13,443 participants). Authors found moderate‐certainty evidence that antenatal corticosteroids reduced neonatal mortality (Deshmukh 2017; Deshmukh 2018; Park 2016) and IVH (Deshmukh 2017; Deshmukh 2018), which is consistent with our findings from randomised controlled trials.
Authors' conclusions
Implications for practice.
The evidence from this review update supports the use of antenatal corticosteroids in women at risk of preterm birth in low‐, medium‐ and high‐resource settings. Treatment with antenatal corticosteroids reduces the risk of perinatal death, neonatal death, and respiratory distress syndrome (RDS) even in the current era of advanced neonatal care. Antenatal corticosteroids probably also reduce the risk of intraventricular haemorrhage (IVH). and are likely to have little or no effect on birthweight. For women at high risk of preterm birth, antenatal corticosteroids probably have little or no effect on the risk of maternal death, chorioamnionitis or endometritis.
Antenatal corticosteroids can continue to be used in women at high risk of preterm birth. Further information is required regarding the optimal dose‐to‐delivery interval, the optimal corticosteroid, the effects in multiple pregnancy and long‐term effects into adulthood. In situations where there are significant concerns about maternal health, the timing of delivery needs to be considered in terms of the expected risks and benefits to both the mother and infant.
Implications for research.
There is little need for further trials of a single course of antenatal corticosteroids versus placebo in singleton pregnancies in low‐ medium‐ and high‐resource settings. There are few data regarding risks and benefits of antenatal corticosteroids in multiple pregnancies and other high‐risk obstetric groups (e.g. women with diabetes). We encourage authors of previous studies to provide further information, which may answer any remaining questions about the use of antenatal corticosteroids in such pregnancies without the need for further randomised controlled trials. Individual patient data meta‐analysis from published trials is likely to answer some of the evidence gaps. Follow‐up studies into childhood and adulthood, particularly in the late‐preterm‐gestation and repeat‐courses groups are needed.
Feedback
Nachum, September 2002,
Summary
Are there enough data to indicate the efficacy of antenatal steroids in twins?
(Summary of comment received from Zohar Nachum, September 2002)
Reply
Only two small trials report outcome following a multiple pregnancy. Therefore there is currently not enough evidence to support the use of corticosteroids in multiple pregnancy. Nevertheless, in view of the strength of the overall evidence, it would seem sensible to offer a single course of steroids to women with a multiple pregnancy at risk of preterm birth.
(Summary of response from Devender Roberts and Stuart Dalziel, May 2006)
Contributors
Devender Roberts Stuart Dalziel
Preston, August 2002,
Summary
It is unclear whether quasi‐randomised trials should be included. The abstract states they are included, types of studies says they are excluded, and a quasi‐randomised study has been included (Morales 1986).
Also some data appear to be missing from the meta‐analysis. Silver 1995 does not contribute any information to the outcome neonatal death, yet the data are reported in the abstract you reference (7/54 deaths on dexamethasone, 8/42 deaths on placebo).
(Summary of comments received from Carol Preston, August 2002)
Reply
The protocol for the updated review excluded quasi‐randomised studies, and Morales 1986 has therefore been excluded. The data for neonatal deaths in Silver 1995 are now included in the meta‐analysis.
(Summary of response from Devender Roberts and Stuart Dalziel, May 2006)
Contributors
Devender Roberts Stuart Dalziel
Liabsuetrakul, September 2003,
Summary
The results, and reviewer's conclusions, are that administering corticosteroids (24 mg betamethasone, or 24 mg dexamethasone) to women who are expected to give birth at 28‐34 weeks' gestation reduces neonatal morbidity and mortality. However, there is no clarification of how this should be prescribed. Standard regimens are for 48 hours treatment, using either 12 mg betamethasone IM every 24 hours, or 6 mg dexamethasone IM every 12 hours. But data in this review show the maximum benefit for corticosteroids is after 24 hours of treatment.
I have some questions about how to maximise the benefit in clinical practice.
1) For a woman in preterm labor who is being given tocolytic treatment to facilitate steroid administration, how long should tocolytics be continued, 24 hours or 48 hours?
2) Would the benefit of steroids be the same for a modified regimen over 24 hours, for example 8 mg dexamethasone IM every 8 hours for 3 doses, or 12 mg dexamethasone IM every 12 hours? Will this affect adrenal suppression and fetal growth like repeated doses?
3) Do we need a review comparing the benefits and adverse events between different regimens of prophylactic corticosteroids?
(Summary of comments from Tippawan Liabsuetrakul, September 2003)
Reply
These questions have all been addressed by sub‐group analyses in the updated review.
(Summary of response from Devender Roberts and Stuart Dalziel, May 2006)
Contributors
Devender Roberts Stuart Dalziel
Selinger, December 2005,
Summary
Why do the corticosteroids need to be administered by intramuscular injection? Is there any evidence that this is preferable to oral administration?
(Summary of comment from Mark Selinger, December 2005)
Reply
Presumably the original sheep studies were done with parenteral steroids, so perhaps the initial extrapolation to humans was intramuscular use. We are not aware of evidence about the effects of oral administration.
(Summary of response from Devender Roberts and Stuart Dalziel, May 2006)
Contributors
Devender Roberts Stuart Dalziel
Hutchon, May 2006,
Summary
There have been two recent reports(1,2) of 30‐year follow‐up of people recruited whilst in utero to Liggins 1972a. Both used intention‐to‐treat analysis, as does this review. One of these reports (1) stated " that there were similar numbers of neonatal survivors with much the same perinatal morbidity in both treatment and control groups". Clearly this means that Liggins 1972a showed no overall benefit in terms of survival or morbidity, which to me seem the most important end points.
Liggins 1972a forms a major part of this Cochrane review, yet the data from the follow‐up reports differ from those in the review. This new evidence therefore raises questions about the validity of the Cochrane meta‐analysis. There are also discrepancies between this version of the review, and its earlier published versions, for some of the other trials. The version published in Effective care in Pregnancy and Childbirth (3) contained 12 trials reporting the effect of corticosteroids on early neonatal death (0‐7 days). Some of these 12 are in the analysis presented here of corticosteroids versus placebo for the outcome neonatal death (0‐28 days). However, for Liggins 1972a, Block 1977, Gamsu 1989, and Morales 1989 the data remain unchanged between the two reviews. Does this mean there were no deaths from 8‐28 days? We now know this is not true for Liggins 1972a. There is also something peculiar about the randomisation in Schmidt 1984. Between appearing in Effective Care in Pregnancy and Childbirth and inclusion in the Cochrane review 15 women were added to this study, all in the treatment group and with no change in the number of deaths.
I understand an update of the review is in preparation. However, since the early nineties it would have been considered unethical to carry out a randomised trial of steroids versus placebo and so I do not expect any new trials to have become available since the last Cochrane review in 2002.
(Summary of feedback from David Hutchon, May 2006)
References
1. Dalziel SR, Walker NK, Parag V, Mantell C, Rea HH, Rodgers A et al. Cardiovascular risk factors after exposure to antenatal betamethasone: 30‐year follow‐up of a randomised controlled trial. Lancet 2005;365:1856‐62.
2. Dalziel SR, Lim VK, Lambert A, McCarthy D, Parag V, Rodgers A et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in a randomised controlled trial. BMJ 2005;331:665‐8.
3. Table 45.12 In: Chalmers I, Enkin M, Keirse MJNC, eds. Effective care in pregnancy and childbirth. Oxford: Oxford University Press, 1989:754.
Reply
Since Effective Care in Pregnancy and Childbirth appeared, nine randomised controlled trials of antenatal corticosteroids have been published. These trials are now included in the updated Cochrane review. This updated review shows the contribution of each study to the outcome measures, and describes the methodological quality of each included trial.
For Liggins 1972a, the previous Cochrane review (Crowley 1996) included data that were published at that time Hence, data for perinatal death (stillbirth or death in the first week of life) were included. However, the updated Cochrane review includes an intention‐to‐treat analysis of the original data from Liggins 1972a. These data were not available for the previous review (Crowley 1996). This updated review therefore now includes data for neonatal death (death in the first 28 days of life) in Liggins 1972a.
Data reported for Schmidt 1984 included a third arm of women and infants who had been excluded from randomisation. This study is now excluded from the review.
(Suumary of response from Devender Roberts and Stuart Dalziel, May 2006)
Contributors
Devender Roberts Stuart Dalziel
Hutchon, January 2007,
Summary
It is good to see the updated review has incorporated intention to treat analysis for all the trials. In the paragraph entitled "Effects of antenatal corticosteroids for preterm birth" the third sentence referring to the 1990 review by Crowley et al (1) is not strictly correct. "This review showed that corticosteroids ... are effective in preventing respiratory distress syndrome and neonatal mortality." In fact that analysis was for early neonatal deaths (deaths in the first seven days) only. Subsequently the Cochrane review used neonatal deaths (deaths in the first 28 days) and, as I pointed out in my feedback on the last update, data from some of the trials (Liggins 1972a, Block 1977, Gamsu 1989, and Morales 1989) are still the same as the previous data reported as early neonatal deaths. Therefore, to be correct, the above sentence should end "...preventing respiratory distress and early neonatal mortality."
Confusion remains regarding the results of three trials. Differences in the data for neonatal death between this update and the previous version (Table 1) are unexplained. For Block 1977 and Gamsu 1989 the differences are minor, but for Morales 1986 they are larger. These changes merit some comment.
Table 1 Differences in the data for neonatal mortality:
Block 1977 Previous update: Treatment (n/N) = 1/69; Control (n/N) = 5/61 This update: Treatment (n/N) = 1/57; Control (n/N) = 5/53
Gamsu 1989 Previous update: Treatment (n/N) = 14/131; Control (n/N) = 20/137 This update: Treatment (n/N) = 14/130; Control (n/N) = 17/132
Morales 1986 Previous update: Treatment (n/N) = 7/121; Control (n/N) = 13/124 This update: Treatment (n/N) = 7/87; Control (n/N) = 8/78
Finally, data from Liggins 1972a has been adjusted and is now presented as an intention to treat analysis. Precise details about the cause of death are not available. Data for Block 1977, Gamsu 1989 and Morales 1986 are not quite as old as that for Liggins 1972a, nevertheless, it is surprising that secure reanalysis of these studies was available after all these years.
1.Crowley P, Chalmers I, Keirse MJNC. The effects of corticosteroid administration before preterm delivery: an overview of the evidence from controlled trials. British Journal of Obstetrics and Gynaecology 1990; 97:11‐25
(Summary of feedback from David Hutchon, January 2007)
Reply
A reply from the authors will be published as soon as it is available.
Contributors
David Hutchon
Vlassov, 15 March 2008
Summary
The title of the review is misleading; the objectives of the review, as well as the outcomes evaluated, are NOT about fetal lung maturation only.
(Summary of feedback from Vasiliy Vlassov, March 2008)
Reply
The results of the review do include data for outcomes other than fetal lung maturity. For the update, we did not want to significantly alter the title of the review. The intention of the original review was to assess the effect on fetal lung maturation. We felt it would be too radical a change for this first update to have a completely different title. We will consider this comment for future updates.
(Reply from Devender Roberts, June 2008)
Contributors
Devender Roberts
Berghella, 23 January 2013
Summary
This review is one of the best and most comprehensive I have seen. However, I would suggest though adding ‘neonatal hypoglycemia’ as an outcome.
(Comment submitted by Vincenzo Berghella, January 2013)
Reply
Thank you for your comments and for your suggestion regarding neonatal hypoglycemia. In this update we decided not to include this outcome because neonatal hypogylacemia is difficult to define and since we have already included many adverse outcomes for the baby we did not consider this one to be a critical addition to the outcomes.
Contributors
Emma McGoldrick and Fiona Stewart, November 2020.
What's new
| Date | Event | Description |
|---|---|---|
| 8 February 2021 | Amended | Edited the plain language summary to include a link to a visual summary for this updated review. |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 3, 2006
| Date | Event | Description |
|---|---|---|
| 7 January 2021 | Amended | Edited Abstract/Main results to remove superflous 'we' from the text. |
| 19 December 2020 | Feedback has been incorporated | The authors have responded to Feedback 8. |
| 3 September 2020 | New citation required and conclusions have changed | Overall, the conclusions remain the same. However with the addition of additional studies from low‐ and medium‐resource settings the conclusions are more certain for perinatal mortality, and for neonatal mortality and morbidity. This new evidence has been reflected in the conclusions. |
| 3 September 2020 | New search has been performed | Search updated and three recently‐published studies added as well as two studies added that were excluded from the previous version of the review. Six studies included in the previous version did not meet Pregnancy and Childbirth trustworthiness criteria and have not been included in this update. |
| 17 February 2016 | New citation required but conclusions have not changed | Nine new studies added for this update (Attawattanakul 2015; Balci 2010; Goodner 1979a; Gyamfi‐Bannerman 2016; Khazardoust 2012a; Lopez 1989; Mansouri 2010; Porto 2011; Shanks 2010). The review now includes a total of 30 studies. The conclusions remain unchanged. |
| 17 February 2016 | New search has been performed | Search updated. The methods updated and the analyses have been restructured. 'Summary of findings' table has been incorporated. |
| 23 January 2013 | Feedback has been incorporated | Feedback 8 received from Vincenzo Berghella. |
| 30 April 2010 | Amended | Search updated. Fourteen reports added to Studies awaiting classification. |
| 25 June 2008 | Feedback has been incorporated | Feedback from Vasiliy Vlassov added with a reply from the review author. |
| 23 June 2008 | Amended | Converted to new review format. |
| 14 March 2007 | Feedback has been incorporated | Feedback from David Hutchon added. |
| 30 October 2005 | New search has been performed | The review substantially updates the Crowley 2006 review due to new Cochrane guidelines for inclusion and exclusion of studies and the need for the review to be standardised with the repeat courses of prenatal corticosteroids review. Six new trials have been included (Amorim 1999; Dexiprom 1999; Fekih 2002a; Lewis 1996; Nelson 1985; Qublan 2001). Three studies that were included in the previous review have been excluded. The results are now presented as relative risks. Results from recent follow‐up studies have been included. Individual participant data were available from the Liggins and Howie study and these were analysed completely by intention‐to‐treat analysis for the first time. These data contribute nearly a third of the data to the review. This represents an important development. The review also provides new information on corticosteroid use in the presence of rupture of membranes, hypertension syndromes, in multiple pregnancies and according to gestational age at first corticosteroid dose. |
Acknowledgements
P Crowley's first, unstructured review of antenatal corticosteroids was conducted at the suggestion of Professor Dennis Hawkins in 1980. Dr Anne Anderson encouraged her to use it as a basis for an early meta‐analysis in 1981. Her work at the National Perinatal Epidemiology Unit in 1980 to 1981 was funded by the National Maternity Hospital, Dublin at the suggestion of the then Master, Dr Dermot MacDonald. This review was first published in structured form on the Oxford Database of Perinatal Trials in 1989. The preparation and continued updating of the original review would have been impossible without the help of Iain and Jan Chalmers, Marc Keirse, Jini Hetherington, Sonja Henderson and Professor Zarko Alfirevic.
Acknowledgements to Professor James Neilson and Professor Jane Harding for their help with the previous update. Many thanks to Sonja Henderson for sound advice at all times. Acknowledgements also to all the study authors who provided us with additional data.
Thanks to Almira Opardija for translating Grgic 2003. Thanks also to Bita Mesgarpour for translating Mansouri 2010.
For the 2017 update: we acknowledge and thank Tineke Crawford for her contribution to editing the review and updating some of the evidence. We would also like to thank Leanne Jones and Therese Dowswell for their contribution to editing the review.
We acknowledge the contribution of Devener Roberts, Nancy Medley, and Julie Brown for their author contributions to previous versions of this review.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of Cochrane Pregnancy and Childbirth's international panel of consumers and the Group's Statistical Adviser. The authors are grateful to the following peer reviewers for their time and comments: Doris Chou, Maternal and Perinatal Health, Department of Sexual and Reproductive Health and Research, WHO, Geneva; Jim G Thornton, Professor of Obstetrics and Gynaecology, University of Nottingham, UK.
This project was supported by the National Institute for Health Research (NIHR), via Evidence Synthesis Programme funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
S Dalziel's time was supported by Cure Kids New Zealand.
Appendices
Appendix 1. Search methods for ClinicalTrials.gov and the databases that contribute to ICTRP
Each line was run separately
corticosteroid(s) AND pregnancy
corticosteroid(s) AND antenatal
corticosteroid(s) AND prenatal
steroid(s) AND pregnancy
steroid(s) AND antenatal
steroid(s) AND prenatal
dexamethasone AND pregnancy
dexamethasone AND antenatal
dexamethasone AND prenatal
betamethasone AND pregnancy
betamethasone AND antenatal
betamethasone AND prenatal
Data and analyses
Comparison 1. Corticosteroids versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Perinatal death | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| 1.2 Neonatal death | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| 1.3 Fetal death | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.22] |
| 1.4 Respiratory distress syndrome | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| 1.5 Moderate/severe respiratory distress syndrome | 7 | 4874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.59, 0.83] |
| 1.6 Chronic lung disease | 5 | 745 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.41, 1.79] |
| 1.7 Intraventricular haemorrhage | 12 | 8475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| 1.7.1 Any IVH grade | 5 | 720 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.45, 0.84] |
| 1.7.2 IVH Grade 3‐4 | 5 | 6269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.27, 0.88] |
| 1.7.3 IVH diagnosed at postmortem | 2 | 1486 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.34, 1.06] |
| 1.8 Mean birthweight (g) | 19 | 9551 | Mean Difference (IV, Fixed, 95% CI) | ‐14.02 [‐33.79, 5.76] |
| 1.9 Maternal death | 6 | 6244 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.36, 3.89] |
| 1.10 Chorioamnionitis | 15 | 8374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.08] |
| 1.11 Endometritis | 10 | 6764 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.82, 1.58] |
| 1.12 Death in childhood | 4 | 1010 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.36, 1.27] |
| 1.13 Neurodevelopmental disability in childhood | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.13.1 Developmental delay | 3 | 600 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.27, 0.97] |
| 1.13.2 Intellectual impairment | 3 | 778 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.44, 1.69] |
| 1.13.3 Hearing impairment | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.04, 9.87] |
| 1.13.4 Visual impairment | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.24, 1.23] |
| 1.14 Death into adulthood | 1 | 988 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.56, 1.81] |
| 1.15 Neurodevelopmental disability in adulthood | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.15.1 Visual impairment | 1 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.53, 1.55] |
| 1.15.2 Hearing impairment | 1 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 2.03] |
| 1.15.3 Intellectual impairment | 2 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 4.95] |
| 1.16 Fever in women after trial entry requiring the use of antibiotics | 3 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.36, 1.21] |
| 1.17 Intrapartum fever in woman requiring the use of antibiotics | 2 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.15, 2.49] |
| 1.18 Postnatal fever in woman | 5 | 1323 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.64, 1.33] |
| 1.19 Admission into adult intensive care unit | 2 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.26, 2.05] |
| 1.20 Side effects of therapy in women | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.20.1 Any side effects at first dose | 1 | 2825 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.59, 0.82] |
| 1.20.2 Dyspnoea | 1 | 2828 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] |
| 1.20.3 Gastrointestinal upset | 1 | 2828 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.99 [0.12, 73.37] |
| 1.20.4 Hyperglycaemia | 1 | 2828 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] |
| 1.20.5 Leucocytosis | 1 | 2828 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] |
| 1.20.6 Migraine | 1 | 2828 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 15.93] |
| 1.21 Glucose intolerance | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [1.14, 6.46] |
| 1.22 Hypertension | 2 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.59, 1.79] |
| 1.23 Apgar < 7 at 5 minutes | 12 | 5727 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.78, 0.98] |
| 1.24 Mean interval between trial entry and birth (days) | 3 | 1513 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐1.86, 2.32] |
| 1.25 Mean length at birth (cm) | 1 | 2766 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.37, 0.37] |
| 1.26 Mean head circumference at birth (cm) | 1 | 2766 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.22, 0.22] |
| 1.27 Small‐for‐gestational age | 5 | 3478 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.96, 1.28] |
| 1.28 Admission to neonatal intensive care unit | 9 | 6667 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.91, 1.00] |
| 1.29 Need for mechanical ventilation/CPAP | 11 | 4519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.66, 0.84] |
| 1.30 Mean duration of mechanical ventilation/CPAP (days) | 3 | 471 | Mean Difference (IV, Random, 95% CI) | ‐1.91 [‐4.59, 0.76] |
| 1.31 Median (IQR) duration of mechanical ventilation (hours) | 1 | Other data | No numeric data | |
| 1.32 Median (IQR) duration of CPAP (hours) | 1 | Other data | No numeric data | |
| 1.33 Air leak syndrome | 2 | 2965 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.32, 1.80] |
| 1.34 Mean duration of oxygen supplementation (hours) | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐2.86 [‐5.51, ‐0.21] |
| 1.35 Median (IQR) duration of oxygen supplementation (hours) | 1 | Other data | No numeric data | |
| 1.36 Surfactant use | 6 | 6104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.50, 0.85] |
| 1.37 Systemic infection in the first 48 hours of life | 7 | 1708 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.41, 0.88] |
| 1.38 Proven infection while in the neonatal intensive care unit | 10 | 5521 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.64, 0.98] |
| 1.39 Necrotising enterocolitis | 10 | 4702 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.32, 0.78] |
| 1.40 Mean infant HPA axis function (cortisol) | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 3.94 [‐3.12, 11.00] |
| 1.40.1 In babies born < 24 hours after 1st dose | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | 9.00 [‐11.93, 29.93] |
| 1.40.2 In babies born 24‐48 hours after 1st dose | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐8.68, 8.68] |
| 1.40.3 In babies born > 48 hours after 1st dose | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | 13.00 [‐1.90, 27.90] |
| 1.41 Mean childhood weight (kg) | 2 | 333 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.39, 1.00] |
| 1.41.1 Liggins | 1 | 250 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.32, 1.12] |
| 1.41.2 Schutte (females) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐6.55, 1.75] |
| 1.41.3 Schutte (males) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐3.88, 3.68] |
| 1.42 Mean childhood head circumference (cm) | 2 | 328 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.08, 0.63] |
| 1.42.1 Liggins | 1 | 250 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.11, 0.71] |
| 1.42.2 Schutte (females) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.05, 0.85] |
| 1.42.3 Schutte (males) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.51, 1.71] |
| 1.43 Mean childhood height (cm) | 2 | 334 | Mean Difference (IV, Fixed, 95% CI) | 1.02 [‐0.26, 2.29] |
| 1.43.1 Liggins | 1 | 250 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐0.39, 2.39] |
| 1.43.2 Schutte (females) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐3.08, 6.48] |
| 1.43.3 Schutte (males) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐3.79, 4.99] |
| 1.44 Mean childhood systolic blood pressure (mmHg) | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐4.06, 0.86] |
| 1.45 Cerebral palsy in childhood | 5 | 904 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.34, 1.03] |
| 1.46 Behavioural/learning difficulties in childhood | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.35, 2.09] |
| 1.47 Mean adult weight (kg) | 2 | 538 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐6.41, 4.76] |
| 1.47.1 Schutte (females) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐6.00 [‐12.93, 0.93] |
| 1.47.2 Schutte (males) | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐9.91, 7.91] |
| 1.47.3 Liggins | 1 | 458 | Mean Difference (IV, Random, 95% CI) | 2.57 [‐0.72, 5.86] |
| 1.48 Mean adult head circumference (cm) | 2 | 537 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.33, 0.38] |
| 1.48.1 Schutte (females) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐1.03, 1.03] |
| 1.48.2 Schutte (males) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.37, 0.97] |
| 1.48.3 Liggins | 1 | 458 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.34, 0.46] |
| 1.49 Mean adult height (cm) | 2 | 537 | Mean Difference (IV, Fixed, 95% CI) | 0.91 [‐0.28, 2.10] |
| 1.49.1 Schutte (females) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐5.37, 3.37] |
| 1.49.2 Schutte (males) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 3.00 [‐2.30, 8.30] |
| 1.49.3 Liggins (females) | 1 | 234 | Mean Difference (IV, Fixed, 95% CI) | 1.17 [‐0.65, 2.99] |
| 1.49.4 Liggins (males) | 1 | 224 | Mean Difference (IV, Fixed, 95% CI) | 0.75 [‐1.03, 2.53] |
| 1.50 Mean adult skinfold thickness (log values) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.50.1 Triceps | 1 | 456 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.11, 0.07] |
| 1.50.2 Biceps | 1 | 456 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.11, 0.09] |
| 1.50.3 Subscapular | 1 | 441 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.08, 0.10] |
| 1.50.4 Suprailiac | 1 | 452 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.12, 0.10] |
| 1.51 Abnormal lung function measured as forced vital capacity (adult) | 1 | 383 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐3.16, 1.76] |
| 1.52 Mean adult systolic blood pressure (mmHg) | 2 | 545 | Mean Difference (IV, Fixed, 95% CI) | ‐0.87 [‐2.81, 1.07] |
| 1.52.1 Schutte (females) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐4.00 [‐9.12, 1.12] |
| 1.52.2 Schutte (males) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐3.00 [‐7.17, 1.17] |
| 1.52.3 Liggins | 1 | 455 | Mean Difference (IV, Fixed, 95% CI) | 0.55 [‐1.88, 2.98] |
| 1.53 Mean adult insulin (log values) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.53.1 Fasting | 1 | 435 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.03, 0.19] |
| 1.53.2 30 minutes fasting following a 75 g oral glucose tolerance test | 1 | 412 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [0.04, 0.28] |
| 1.53.3 120 minutes following a 75 g oral glucose tolerance test | 1 | 428 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.27, 0.07] |
| 1.54 Mean adult glucose | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.54.1 Fasting | 1 | 432 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.09, 0.11] |
| 1.54.2 30 minutes fasting following a 75 g oral glucose tolerance test | 1 | 413 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.12, 0.54] |
| 1.54.3 120 minutes following a 75 g oral glucose tolerance test | 1 | 410 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.52, ‐0.02] |
| 1.55 Mean adult HPA axis function (mean log fasting cortisol) | 1 | 444 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.02, 0.14] |
| 1.56 Mean age at puberty (years) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.56.1 Schutte (females) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.94, 0.94] |
| 1.57 Educational achievement by adulthood (university or polytechnic education) | 1 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.10] |
| 1.58 Mean length of antenatal hospitalisation (days) | 2 | 412 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.23, 0.22] |
| 1.59 Length of maternal hospital stay | 4 | Other data | No numeric data | |
| 1.60 Mean length of postnatal hospitalisation (days) | 1 | 218 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐1.72, 1.72] |
| 1.61 Mean length of neonatal hospitalisation (days) | 5 | 788 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.51, 0.87] |
| 1.62 Length of neonatal hospitalisation | 2 | Other data | No numeric data |
Comparison 2. Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Perinatal death ‐ single or multiple pregnancy | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| 2.1.1 In babies born from singleton pregnancies | 7 | 5492 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.99] |
| 2.1.2 In babies born from multiple pregnancies | 2 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.41, 1.22] |
| 2.1.3 Mixed population | 7 | 4089 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.77, 0.96] |
| 2.2 Neonatal death ‐ single or multiple pregnancy | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| 2.2.1 In babies born from singleton pregnancies | 13 | 8453 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.71, 0.91] |
| 2.2.2 In babies born from multiple pregnancies | 3 | 813 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.57, 1.02] |
| 2.2.3 Mixed population | 9 | 1343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.50, 0.90] |
| 2.3 Fetal death ‐ single or multiple pregnancy | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.21] |
| 2.3.1 In babies born from singleton pregnancies | 7 | 5492 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.76, 1.46] |
| 2.3.2 In babies born from multiple pregnancies | 2 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.20, 1.40] |
| 2.3.3 Mixed population | 7 | 4089 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.80, 1.29] |
| 2.4 Respiratory distress syndrome ‐ single or multiple pregnancy | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| 2.4.1 In babies born from singleton pregnancies | 17 | 6731 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.57, 0.74] |
| 2.4.2 In babies born from multiple pregnancies | 4 | 323 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.20] |
| 2.4.3 Mixed population | 9 | 4129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.68, 0.92] |
| 2.5 Intraventricular haemorrhage ‐ single or multiple pregnancy | 12 | 8475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| 2.5.1 In babies born from singleton pregnancies | 6 | 4494 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.35, 0.75] |
| 2.5.2 In babies born from multiple pregnancies | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.08, 2.26] |
| 2.5.3 Mixed population | 6 | 3831 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.48, 0.94] |
Comparison 3. Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Perinatal death ‐ intact or ruptured membranes | 14 | 9804 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| 3.1.1 In babies born from pregnancies with intact membranes at 1st dose | 4 | 1332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.71, 1.10] |
| 3.1.2 In babies born from pregnancies with ruptured membranes at 1st dose | 3 | 688 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.47, 0.83] |
| 3.1.3 Not reported or mixed population | 8 | 7784 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.78, 0.97] |
| 3.2 Neonatal deaths ‐ intact or ruptured membranes | 22 | 10580 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| 3.2.1 In babies born from pregnancies with intact membranes at 1st dose | 4 | 1332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.60, 1.05] |
| 3.2.2 In babies born from pregnancies with ruptured membranes at 1st dose | 7 | 1014 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.46, 0.84] |
| 3.2.3 Not reported or mixed population | 12 | 8234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.71, 0.91] |
| 3.3 Fetal death ‐ intact or ruptured membranes | 14 | 9804 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.22] |
| 3.3.1 In babies born from pregnancies with intact membranes at 1st dose | 4 | 1332 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.73, 1.64] |
| 3.3.2 In babies born from pregnancies with ruptured membranes at 1st dose | 3 | 688 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.46, 1.61] |
| 3.3.3 Not reported or mixed population | 8 | 7784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.80, 1.26] |
| 3.4 RDS ‐ intact or ruptured membranes | 26 | 11079 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.64, 0.78] |
| 3.4.1 In babies born from pregnancies with intact membranes at 1st dose | 8 | 1924 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.50, 0.71] |
| 3.4.2 In babies born from pregnancies with ruptured membranes at 1st dose | 10 | 1202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.60, 0.87] |
| 3.4.3 Not reported or mixed population | 13 | 7953 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.67, 0.88] |
| 3.5 IVH ‐ intact or ruptured membranes | 12 | 8446 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| 3.5.1 In babies born from pregnancies with intact membranes at 1st dose | 4 | 1332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.28, 0.66] |
| 3.5.2 In babies born from pregnancies with ruptured membranes at 1st dose | 4 | 722 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.28, 0.79] |
| 3.5.3 Not reported or mixed population | 5 | 6392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.64, 1.38] |
| 3.6 Birthweight ‐ intact or ruptured membranes | 19 | 9522 | Mean Difference (IV, Fixed, 95% CI) | ‐14.86 [‐34.59, 4.87] |
| 3.6.1 In babies born from pregnancies with intact membranes at 1st dose | 4 | 1301 | Mean Difference (IV, Fixed, 95% CI) | ‐30.27 [‐100.43, 39.89] |
| 3.6.2 In babies born from pregnancies with ruptured membranes at 1st dose | 5 | 835 | Mean Difference (IV, Fixed, 95% CI) | ‐49.72 [‐113.91, 14.46] |
| 3.6.3 Not reported or mixed population | 11 | 7386 | Mean Difference (IV, Fixed, 95% CI) | ‐9.40 [‐31.10, 12.30] |
| 3.7 Chorioamnionitis ‐ intact or ruptured membranes | 15 | 8345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.70, 1.09] |
| 3.7.1 In women with intact membranes at 1st dose | 4 | 1243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.50, 1.40] |
| 3.7.2 In women with ruptured membranes at 1st dose | 7 | 1129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.72, 1.48] |
| 3.7.3 Not reported or mixed population | 5 | 5973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.54, 1.09] |
| 3.8 Endometritis ‐ intact or ruptured membranes | 10 | 6764 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.82, 1.58] |
| 3.8.1 In women with intact membranes at 1st dose | 2 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.61, 2.00] |
| 3.8.2 In women with ruptured membranes at 1st dose | 4 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.55, 2.25] |
| 3.8.3 Not reported or mixed population | 5 | 5998 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.73, 1.87] |
Comparison 4. Corticosteroids versus placebo or no treatment ‐ hypertension syndrome versus all other trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Perinatal deaths ‐ hypertension syndrome vs other trials | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| 4.1.1 Hypertension syndrome | 2 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.57, 1.20] |
| 4.1.2 No hypertension syndrome or hypertension syndromes excluded | 1 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.67, 1.10] |
| 4.1.3 Hypertension not reported separately | 12 | 8397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.76, 0.94] |
| 4.2 Neonatal deaths ‐ hypertension syndrome vs other trials | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| 4.2.1 Hypertension syndrome | 2 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.28, 0.83] |
| 4.2.2 No hypertension syndrome or hypertension syndromes excluded | 1 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.25] |
| 4.2.3 Hypertension not reported separately | 20 | 9173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.88] |
| 4.3 Fetal deaths ‐ hypertension syndrome vs other trials | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.22] |
| 4.3.1 Women with hypertension syndrome | 3 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.91, 3.28] |
| 4.3.2 No hypertension syndrome or hypertension syndromes excluded | 2 | 1373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.49, 1.12] |
| 4.3.3 Hypertension not reported separately | 11 | 8129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.83, 1.30] |
| 4.4 Respiratory distress syndrome ‐ hypertension syndrome vs other trials | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| 4.4.1 Hypertension syndrome | 5 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.34, 0.69] |
| 4.4.2 No hypertension syndrome or hypertension syndromes excluded | 7 | 2511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.48, 0.74] |
| 4.4.3 Hypertension not reported separately | 19 | 8254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
Comparison 5. Corticosteroids versus placebo or no treatment ‐ type of steroid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Perinatal death ‐ type of steroid | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| 5.1.1 Dexamethasone | 6 | 4673 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.95] |
| 5.1.2 Betamethasone | 8 | 5092 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.99] |
| 5.1.3 Methylprednisolone | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.43, 5.43] |
| 5.2 Neonatal death ‐ type of steroid | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| 5.2.1 Dexamethasone | 7 | 4769 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.71, 0.91] |
| 5.2.2 Betamethasone | 14 | 5593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.59, 0.89] |
| 5.2.3 Hydrocortisone | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.20, 1.47] |
| 5.2.4 Methylprednisolone | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.42, 3.12] |
| 5.3 Fetal death ‐ type of steroid | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.83, 1.21] |
| 5.3.1 Dexamethasone | 6 | 4673 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.78, 1.25] |
| 5.3.2 Betamethasone | 8 | 5092 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.74, 1.42] |
| 5.3.3 Methylprednisolone | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.08, 47.36] |
| 5.4 Respiratory distress syndrome ‐ type of steroid | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.65, 0.78] |
| 5.4.1 Dexamethasone | 8 | 4963 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.70, 0.92] |
| 5.4.2 Betamethasone | 17 | 5973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.55, 0.72] |
| 5.4.3 Hydrocortisone | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.36, 1.28] |
| 5.4.4 Methylprednisolone | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.56, 2.27] |
| 5.5 Moderate/severe respiratory distress syndrome ‐ type of steroid | 7 | 4874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.59, 0.83] |
| 5.5.1 Dexamethasone | 2 | 3166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.67, 1.03] |
| 5.5.2 Betamethasone | 5 | 1655 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.37, 0.67] |
| 5.5.3 Hydrocortisone | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.32, 6.63] |
| 5.5.4 Methylprednisolone | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.26, 5.31] |
| 5.6 Chronic lung disease ‐ type of steroid | 5 | 745 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.41, 1.79] |
| 5.6.1 Dexamethasone | 2 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [0.41, 9.16] |
| 5.6.2 Betamethasone | 3 | 460 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.21, 1.42] |
| 5.7 IVH ‐ type of steroid | 12 | 8475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| 5.7.1 Dexamethasone | 4 | 3494 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.54, 1.13] |
| 5.7.2 Betamethasone | 8 | 4981 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.34, 0.68] |
| 5.8 Birthweight ‐ type of steroid | 19 | 9551 | Mean Difference (IV, Fixed, 95% CI) | ‐14.02 [‐33.79, 5.76] |
| 5.8.1 Dexamethasone | 6 | 3972 | Mean Difference (IV, Fixed, 95% CI) | 3.84 [‐31.09, 38.76] |
| 5.8.2 Betamethasone | 12 | 5401 | Mean Difference (IV, Fixed, 95% CI) | ‐20.40 [‐44.61, 3.81] |
| 5.8.3 Hydrocortisone | 2 | 151 | Mean Difference (IV, Fixed, 95% CI) | ‐146.68 [‐371.30, 77.93] |
| 5.8.4 Methylprednisolone | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐121.00 [‐430.59, 188.59] |
| 5.9 Chorioamnionitis ‐ type of steroid | 15 | 8374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.08] |
| 5.9.1 Dexamethasone | 6 | 3621 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.84, 1.71] |
| 5.9.2 Betamethasone | 9 | 4753 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.51, 0.93] |
| 5.10 Endometritis ‐ type of steroid | 10 | 6764 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.82, 1.58] |
| 5.10.1 Dexamethasone | 4 | 3270 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.92, 2.90] |
| 5.10.2 Betamethasone | 6 | 3494 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.63, 1.42] |
Comparison 6. Corticosteroids versus placebo or no treatment ‐ decade of trial.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Perinatal death ‐ decade of trial | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| 6.1.1 Trials conducted in 1970s | 5 | 2520 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74, 1.06] |
| 6.1.2 Trials conducted in 1980s | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.59, 2.21] |
| 6.1.3 Trials conducted in 1990s | 3 | 615 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.46, 0.97] |
| 6.1.4 Trials conducted in 2000s | 2 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.31, 0.70] |
| 6.1.5 Trials conducted in 2010s | 3 | 6207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.79, 0.99] |
| 6.2 Neonatal death ‐ decade of trial | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| 6.2.1 Trials conducted in 1970s | 7 | 2743 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.67, 1.04] |
| 6.2.2 Trials conducted in 1980s | 4 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.55, 1.49] |
| 6.2.3 Trials conducted in 1990s | 5 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.40, 0.90] |
| 6.2.4 Trials conducted in 2000s | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.31, 0.66] |
| 6.2.5 Trials conducted in 2010s | 4 | 6482 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.72, 0.96] |
| 6.3 Fetal death ‐ decade of trial | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.22] |
| 6.3.1 Trials conducted in 1970s | 5 | 2520 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.69, 1.32] |
| 6.3.2 Trials conducted in 1980s | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.42 [0.37, 31.41] |
| 6.3.3 Trials conducted in 1990s | 3 | 615 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.49, 2.36] |
| 6.3.4 Trials conducted in 2000s | 2 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.19, 4.50] |
| 6.3.5 Trials conducted in 2010s | 3 | 6207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.30] |
| 6.4 Respiratory distress syndrome ‐ decade of trial | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| 6.4.1 Trials conducted in 1970s | 8 | 2823 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.54, 0.78] |
| 6.4.2 Trials conducted in 1980s | 4 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.55, 0.88] |
| 6.4.3 Trials conducted in 1990s | 5 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.65, 0.92] |
| 6.4.4 Trials conducted in 2000s | 5 | 845 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.26, 0.59] |
| 6.4.5 Trials conducted in 2010s | 4 | 6401 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 6.5 IVH ‐ decade of trial | 12 | 8475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| 6.5.1 Trials conducted in 1970s | 1 | 1218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.33, 1.12] |
| 6.5.2 Trials conducted in 1980s | 3 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.26, 0.81] |
| 6.5.3 Trials conducted in 1990s | 4 | 580 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.44, 0.90] |
| 6.5.4 Trials conducted in 2000s | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.15, 0.73] |
| 6.5.5 Trials conducted in 2010s | 2 | 5897 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [0.69, 8.31] |
| 6.6 Birthweight ‐ decade of trial | 19 | 9551 | Mean Difference (IV, Fixed, 95% CI) | ‐14.02 [‐33.79, 5.76] |
| 6.6.1 Trials conducted in 1970s | 5 | 1822 | Mean Difference (IV, Fixed, 95% CI) | ‐41.39 [‐110.05, 27.26] |
| 6.6.2 Trials conducted in 1980s | 3 | 280 | Mean Difference (IV, Fixed, 95% CI) | ‐19.60 [‐108.55, 69.35] |
| 6.6.3 Trials conducted in 1990s | 4 | 569 | Mean Difference (IV, Fixed, 95% CI) | ‐33.13 [‐102.39, 36.13] |
| 6.6.4 Trials conducted in 2000s | 3 | 573 | Mean Difference (IV, Fixed, 95% CI) | ‐20.77 [‐61.95, 20.41] |
| 6.6.5 Trials conducted in 2010s | 4 | 6307 | Mean Difference (IV, Fixed, 95% CI) | ‐3.82 [‐30.36, 22.72] |
| 6.7 Chorioamnionitis ‐ decade of trial | 15 | 8374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.08] |
| 6.7.1 Trials conducted in 1970s | 2 | 1237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.46, 1.17] |
| 6.7.2 Trials conducted in 1980s | 3 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.25, 1.01] |
| 6.7.3 Trials conducted in 1990s | 5 | 731 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.85, 1.89] |
| 6.7.4 Trials conducted in 2000s | 2 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.59, 6.95] |
| 6.7.5 Trials conducted in 2010s | 3 | 5873 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.48, 1.11] |
| 6.8 Endometritis ‐ decade of trial | 10 | 6764 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.82, 1.58] |
| 6.8.1 Trials conducted in 1970s | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.07, 15.86] |
| 6.8.2 Trials conducted in 1980s | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.88, 6.06] |
| 6.8.3 Trials conducted in 1990s | 4 | 574 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.53, 1.44] |
| 6.8.4 Trials conducted in 2000s | 4 | 6018 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.75, 2.03] |
Comparison 7. Corticosteroids versus placebo or no treatment ‐ weekly repeats.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Perinatal death ‐ protocol with weekly repeats | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| 7.1.1 Single course only | 10 | 6329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74, 1.04] |
| 7.1.2 Courses including weekly repeats | 4 | 3504 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.75, 0.93] |
| 7.2 Neonatal death ‐ protocol with weekly repeats | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| 7.2.1 Single course only | 14 | 6636 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.68, 1.02] |
| 7.2.2 Courses including weekly repeats | 8 | 3973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.67, 0.86] |
| 7.3 Fetal death ‐ protocol with weekly repeats | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.22] |
| 7.3.1 Single course only | 10 | 6329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.70, 1.31] |
| 7.3.2 Courses including weekly repeats | 4 | 3504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.82, 1.31] |
| 7.4 Respiratory distress syndrome ‐ protocol with weekly repeats | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| 7.4.1 Single course only | 18 | 7210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.64, 0.83] |
| 7.4.2 Courses including weekly repeats | 8 | 3973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.60, 0.79] |
| 7.5 Moderate/severe respiratory distress syndrome | 7 | 4874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.59, 0.83] |
| 7.5.1 Single course only | 3 | 1359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.47, 0.88] |
| 7.5.2 Courses including weekly repeats | 4 | 3515 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.59, 0.89] |
| 7.6 IVH ‐ protocol with weekly repeats | 12 | 8475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| 7.6.1 Single course only | 4 | 4502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.37, 0.91] |
| 7.6.2 Courses including weekly repeats | 8 | 3973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.43, 0.79] |
| 7.7 Birthweight ‐ protocol with weekly repeats | 19 | 9551 | Mean Difference (IV, Fixed, 95% CI) | ‐14.02 [‐33.79, 5.76] |
| 7.7.1 Single course only | 14 | 6165 | Mean Difference (IV, Fixed, 95% CI) | ‐20.90 [‐44.39, 2.60] |
| 7.7.2 Courses including weekly repeats | 5 | 3386 | Mean Difference (IV, Fixed, 95% CI) | 2.72 [‐33.92, 39.36] |
| 7.8 Chorioamnionitis ‐ protocol with weekly repeats | 15 | 8374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.08] |
| 7.8.1 Single courses only | 7 | 4659 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.61, 1.11] |
| 7.8.2 Courses including weekly repeats | 8 | 3715 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.65, 1.28] |
| 7.9 Endometritis ‐ protocol with weekly repeats | 10 | 6764 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.82, 1.58] |
| 7.9.1 Single courses only | 4 | 3332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.49, 1.39] |
| 7.9.2 Courses including weekly repeats | 6 | 3432 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.94, 2.19] |
Comparison 8. Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Perinatal death ‐ gestational age at trial entry | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.77, 0.92] |
| 8.1.1 Less than or equal to 35 weeks + 0 days | 11 | 6185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.76, 0.91] |
| 8.1.2 Greater than or equal to 34 weeks + 0 days | 4 | 3648 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.68, 4.28] |
| 8.2 Neonatal death ‐ gestational age at trial entry | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.86] |
| 8.2.1 Less than or equal to 35 weeks + 0 days | 19 | 6961 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.69, 0.86] |
| 8.2.2 Greater than or equal to 34 weeks + 0 days | 4 | 3648 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.49, 4.61] |
| 8.3 Fetal death ‐ gestational age at trial entry | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.83, 1.20] |
| 8.3.1 Less than or equal to 35 weeks + 0 days | 11 | 6185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.81, 1.19] |
| 8.3.2 Greater than or equal to 34 weeks + 0 days | 4 | 3648 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.42, 8.82] |
| 8.4 Respiratory distress syndrome ‐ gestational age at trial entry | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| 8.4.1 Less than or equal to 35 weeks + 0 days | 20 | 7041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.63, 0.78] |
| 8.4.2 Greater than or equal to 34 weeks + 0 days | 7 | 4142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.95] |
| 8.5 IVH ‐ gestational age at trial entry | 12 | 8475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.74] |
| 8.5.1 Less than or equal to 35 weeks + 0 days | 11 | 5412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.44, 0.72] |
| 8.5.2 Greater than or equal to 34 weeks + 0 days | 2 | 3063 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.91 [0.24, 102.09] |
| 8.6 Birthweight ‐ gestational age at trial entry | 19 | 9551 | Mean Difference (IV, Fixed, 95% CI) | ‐13.36 [‐32.99, 6.26] |
| 8.6.1 Less than or equal to 35 weeks + 0 days | 13 | 5412 | Mean Difference (IV, Fixed, 95% CI) | ‐9.78 [‐40.81, 21.24] |
| 8.6.2 Greater than or equal to 34 weeks + 0 days | 7 | 4139 | Mean Difference (IV, Fixed, 95% CI) | ‐15.75 [‐41.09, 9.58] |
| 8.7 Chorioamnionitis ‐ gestational age at trial entry | 15 | 8374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.68, 1.07] |
| 8.7.1 Less than or equal to 35 weeks + 0 days | 13 | 5132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.73, 1.20] |
| 8.7.2 Greater than or equal to 34 weeks + 0 days | 3 | 3242 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.34, 0.99] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amorim 1999.
| Study characteristics | ||
| Methods | Type of study: RCT Method of treatment allocation: computer‐generated randomisation sequence with randomisation code kept by the chief pharmacist. The pharmacy provided coded drug boxes. Stratification: none stated Placebo: yes, same volume of similar appearing vehicle Sample size calculation: yes Intention‐to‐treat analyses: no Losses to follow‐up: yes, 2 (1%) women in the placebo group dropped out after randomisation | |
| Participants | Location: Instituto Materno‐Infantil de Pernambuco, Recife, state of Pernambuco, Brazil Eligibility criteria: women with severe pre‐eclampsia, singleton pregnancy with a live fetus and gestational age between 26‐34 weeks. Likely minimal interval of 24 hours between drug administration and delivery. Lung immaturity was confirmed by the foam test in fetuses of 30‐34 weeks. Gestational age range: 26‐34 weeks Exclusion criteria: indication for immediate delivery, diabetes, PROM, maternal disease, congenital malformations, perinatal haemolytic disease, Group B streptococcal infection Total recruited: 220 women and infants. 110 women and infants in each arm | |
| Interventions | 12 mg betamethasone IM, repeated after 24 hours and weekly thereafter if delivery had not occurred. Control group received identical placebo. Delivery was at 34 weeks or in the presence of maternal or fetal compromise in both groups. | |
| Outcomes | Maternal outcomes (death, chorioamnionitis, maternal infection, fever after trial entry requiring antibiotics, intrapartum fever requiring antibiotics, postnatal fever, admission to ICU, glucose intolerance, hypertension), fetal/neonatal outcomes (fetal death, neonatal death, RDS, chronic lung disease, IVH, birthweight, Apgar score < 7, interval between trial entry and delivery, small‐for‐gestational age, admission to NICU, need for mechanical ventilation/CPAP, duration of oxygen supplementation, surfactant use, systemic infection in the first 48 hours of life, proven infection while in the NICU, necrotising enterocolitis), childhood outcomes (death, developmental delay, cerebral palsy) and health service outcomes reported (length of antenatal hospitalisation for women, length of postnatal hospitalisation for women, length of neonatal hospitalisation) | |
| Notes | Further information obtained from the study authors, including substantial unpublished data Dates of the study: April 1997‐June 1998 Funding sources: "supported by Instituto Materno‐Infantil de Pernambuco, Brazil" Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated randomisation sequence." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation code kept by the chief pharmacist." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessors was not described, but it is likely as the authors state, "the data analysis was carried out without knowledge of which of the treatments corresponded to corticosteroid and which to placebo". The code was fully broken only after the analysis was completed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two women (1%) in the placebo group voluntarily dropped out after randomisation. |
| Selective reporting (reporting bias) | Low risk | Study protocol was not available, but study appears to report on all pre‐specified outcomes |
| Other bias | Low risk | The groups were comparable at baseline. The study appears free of other sources of bias. |
Attawattanakul 2015.
| Study characteristics | ||
| Methods | Type of study: open‐label RCT
Method of treatment allocation: method of randomisation not stated. Block randomisation used Stratification: none stated Placebo: no, comparison was no treatment Sample size calculation: "Sample size was calculated to have type one error of 5 per cent and 80 per cent power to detect a reduction of 50 per cent in rate of respiratory distress. Rate of respiratory distress in late preterm infant was assumed to be 28.9 percent based on Wang ML, et al. Accordingly, the number of study population was at least 95 pregnant women in each group." Intention‐to‐treat analyses: yes Losses to follow‐up: no |
|
| Participants | Location: Chonburi Hospital, Thailand
Eligibility criteria: all pregnant women with singleton pregnancy admitted in labour (defined as "regular uterine contraction at least 4 times in 20 minutes or 8 times in 60 minutes and cervical dilatation more than 1 cm and cervical effacement at least 80 percent") with a gestational age of 34 weeks + 0 days to 36 weeks + 6 days Gestational age range: 34 weeks + 0 days to 36 weeks + 6 days Exclusion criteria: "Participants who had history of corticosteroid administration in current pregnancy, history of dexamethasone allergy, systemic infection, multifetal pregnancy, complicated pregnancy including overt diabetes mellitus, gestational diabetes mellitus (GDM), pregnancy induced hypertension (PIH), placenta previa and abruptio placentae, positive or unknown sexual transmitted disease serology, PROM, evidence of fetal amniotic membrane leakage confirmed by two of the following test; pooling, nitrazine test, fern test or cough test, known fetal intrauterine restriction, oligohydramnios, non‐reassuring fetal heart rate tracing, fetal death, fetal anomaly, suspicious of chorioamnionitis (fetal tachycardia >160/min, maternal fever > 37.8°C, uterine tenderness, foul smelling amniotic fluid), cervical dilatation more than 7 cm, were excluded from our study." Total recruited: 194 women and infants; 96 women and infants in the treatment arm and 98 women and infants in the control arm. |
|
| Interventions | The treatment group received 6 mg dexamethasone IM, up to 4 doses 12 hours apart. The control group received no treatment. |
|
| Outcomes | Maternal outcomes (chorioamnionitis, side effects of therapy in women) Fetal/neonatal outcomes (RDS, IVH, birthweight, necrotising enterocolitis, systemic infection in the first 48 hours of life, need for mechanical ventilation/CPAP, Apgar score < 7, admission to NICU) |
|
| Notes | Labour augmentation performed if needed even if women had not received full course of steroids. 6 (6%) women in the intervention group received a full course of steroids; most women (75/96 (78%)) in the intervention arm received just 1 dose of dexamethasone. Data for 'maternal local or systemic adverse reactions to treatment' have been included in the review under our outcome of maternal side effects. Data from the trial are available for the following outcomes: low birthweight (not defined); hypoglycaemia in infant; need for respiratory support in infant (6/96 treatment and 14/98 control; (these data are in addition to the need for 'positive pressure ventilation' included in the review outcome 'need for mechanical ventilation'); and maternal length of stay (not separated into intrapartum and postpartum) Contact details: 3803 Qiss Bldg. A2 5,6Fl. Room 501‐2,601‐2 Rama IV Rd., Phra Khanong, Khlong Toei, Bangkok ‐ 10110 Email: info@takaraivfbkk.com Information from trialist (received July 2020): "The protocol was developed and approved by our IRB committee prior to the enrolment. This trial prospectively register at Chonburi hospital but do not have online registration number." Dates of the study: March 2013‐March 2014 Funding sources: not stated Declarations of interest: authors declare no competing interests |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Method reported as block randomisation only |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label, participants would have been aware of allocation. Delivery nurse not blinded but all other hospital staff delivering care were blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The data were retrieved from chart review and hospital staff were blinded apart from delivery room nurses. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 women in the dexamethasone delivered after 1 week and were included in ITT analysis |
| Selective reporting (reporting bias) | Low risk | Relevant outcome data reported |
| Other bias | Low risk | The groups were comparable at baseline. |
Balci 2010.
| Study characteristics | ||
| Methods | Type of study: RCT Method of treatment allocation: computer‐generated random number table, sequential sealed envelopes, not stated if opaque Stratification: none stated Placebo: no, comparison was no treatment Sample size calculation: not stated Intention‐to‐treat analyses: yes Losses to follow‐up: 30 infants with fetal distress, meconium‐stained liquor and who delivered within less than 24 hours were excluded from the study (14 in control group, 16 in steroid group) | |
| Participants | Location: Dept of Obstetrics and Gynecology, Hospital of Meram, Faculty of Medicine, Selcuk University, Konya, Turkey
Eligibility criteria: 34‐36 weeks' gestation based on LMP. If unsure dates, fetal biometric measurements of 33‐36 weeks on abdominal ultrasonography (done on admission). The mother had had at least 2 contractions lasting more than 30 seconds in 10 min on cardiotocography, and cervical dilatation > 3 cm with 80% effacement. Patients who delivered at least 24 hours after betamethasone administration were included in this study. Gestational age range: 34 + 0‐36 + 0 weeks. Exclusion criteria: obstetric complications (severe IUGR, pre‐eclampsia, placental abruption, placenta praevia), multiple pregnancies, those who had already received antenatal corticosteroid therapy, PROM, or suspicion of chorioamnionitis, fetal anomaly, fetal distress, severe systemic disease (heart disease, hyperthyroidism, hypothyroidism, renal disease, diabetes mellitus) Total recruited: 100 (50 women and babies in each group) |
|
| Interventions | The treatment group received a single dose of 12 mg betamethasone IM. The control group received no treatment. Women who delivered at least 24 hours after betamethasone administration were included in the study. |
|
| Outcomes | Apgar score at 1 and 5 minutes, need for resuscitation, development of RDS | |
| Notes | Email: drobalci@yahoo.com or drobalci@hotmail.com Dates of the study: January 2007 to May 2009 Funding sources: not reported Declarations of interest: correspondence from author (Sept 2020) "There is no conflict of interest in terms of funding source for any or any of the authors of the our published article" Correspondence from author (August 2020) confirmed baseline data similarity was due to chance and that there was no prospective trial registration. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Generated by a computer" |
| Allocation concealment (selection bias) | Unclear risk | Quote:"Sequential sealed envelopes" not stated if opaque or not |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to comparison group receiving no treatment and treatment group receiving corticosteroids, blinding of participants and personnel would not have been possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors is not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | No protocol but all outcomes specified in methods are reported in full. |
| Other bias | Low risk | No indication of any other sources of bias. |
Block 1977.
| Study characteristics | ||
| Methods | Type of study: RCT Method of treatment allocation: computer‐generated randomisation sequence. Coded drug boxes were provided. Stratification: none stated Placebo: yes, normal saline Sample size calculation: no Intention‐to‐treat analyses: no | |
| Participants | Location: Department of Gynecology and Obstetrics at the University of Oklahoma College of Medicine, Oklahoma City, Oklahoma, USA Eligibility criteria: women with preterm labour and PROM Gestational age range: not stated Exclusion criteria: not stated Total recruited: 167 women randomised (14 delivered elsewhere and were lost to follow‐up). Data are available on 169 infants (60 infants in the betamethasone arm, 41 infants in the methylprednisolone arm, and 54 infants in the control arm). | |
| Interventions | Group A: 12 mg betamethasone IM repeated after 24 hours if delivery had not occurred
Group B: 125 mg methylprednisolone IM repeated after 24 hours if delivery had not occurred GRoup C: Control group received 1 mL normal saline IM repeated after 24 hours if delivery had not occurred. If there was evidence of progressive cervical dilatation an alcohol infusion was given in order to attempt to delay delivery for at least 48 hours. In women with PROM delivery was induced if serial white blood cell counts or temperatures became elevated regardless of time elapsed since drug administration. |
|
| Outcomes | Fetal/neonatal outcomes reported (fetal death, neonatal death, RDS, need for mechanical ventilation/CPAP) | |
| Notes | Further information was requested from the study authors but there was no reply. Dates of the study: not stated in manuscript, the study is coded as 1970s for the review Funding sources: quote: "supported in part by a grant from Schering Corporation, Kenilworth, New Jersey, USA; and The Upjohn Company, Kalamazoo, Michigan, USA" Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"Computer generated randomisation sequence." |
| Allocation concealment (selection bias) | Low risk | Quote:"Consecutively numbered boxes containing randomly selected study drug or placebo." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clinicians were never aware of the contents of the coded box. Placeob was saline so it is likely that participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 14 (10%) women delivered elsewhere and were lost to follow‐up. 6 (4%) women were excluded from analyses as they failed to complete the protocol (1 in the betamethasone group, 2 in the methylprednisolone group, and 3 in the control group). |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes. |
| Other bias | Low risk | Nothing to indicate any other sources of bias. |
Collaborative 1981.
| Study characteristics | ||
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Coded drug boxes with sequentially‐numbered vials containing study drug were used. Sealed envelope containing the identity of the contents of was attached to each vial quote:"to be opened in emergency only in case of an emergency". The manuscripts do not state how often these were opened. Stratification: yes, within each hospital Placebo: yes, identical appearance Sample size calculation: yes Intention‐to‐treat analyses: no Losses to follow‐up: yes, 2 (0%) infants in the control arm were lost to RDS follow‐up as neonates and 240 (37%) children were lost to follow‐up at age 3 (124 in the treatment arm and 116 in the control arm) | |
| Participants | Location: 5 university hospitals in the USA Eligibility criteria: women at high risk of preterm delivery. L/S ratio < 2.0 in cases of uncertain gestation, hyperthyroidism, hypertension, placental insufficiency, drug addiction, methadone use or gestational age > 34 weeks Gestational age range: 26‐37 weeks Exclusion criteria: > 5 cm of cervical dilatation, anticipated delivery < 24 hours or > 7 days, intrauterine infection, previous glucocorticoid treatment, history of peptic ulcer disease, active tuberculosis, viral keratitis, severe fetal Rhesus sensitisation, infant unlikely to be available for follow‐up Total recruited: 696 women and 757 infants; 349 women and 378 infants in the treatment arm and 347 women and 379 infants in the control arm | |
| Interventions | 4 doses of 5 mg dexamethasone phosphate IM 12 hours apart Control group received placebo | |
| Outcomes | Maternal outcomes (postnatal fever), fetal/neonatal outcomes (fetal death, neonatal death, RDS, birthweight, interval between trial entry and delivery, systemic infection in the first 48 hours of life, proven infection while in the NICU, necrotising enterocolitis), childhood outcomes (death, lung function, developmental delay, intellectual impairment, cerebral palsy) and health service outcomes were reported (length of neonatal hospitalisation) | |
| Notes | Further information was requested from the authors but there was no reply Funding sources: Division of Lung Diseases of the National Heart, Lung and Blood Institute (National Institutes of Health, USA). "Merck, Sharpe and Dohme provided the drug and placebo preparations used in this study. This acknowledgement of appreciation is in no way an endorsement of a particular product." Study dates: March 1977‐March 1980 Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | High risk | Sealed envelope containing the identity of the contents of was attached to each vial quote:"to be opened in emergency only in case of an emergency". The manuscripts do not state how often these were opened. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote:"Both placebo and steroid were dispensed as 10 ml clear, colourless solutions which differed only in that one contained the steroid". It is likely that participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 (0.27%) infants in the control arm were lost to RDS follow‐up as neonates. At age 3, 240 (37%) children were lost to follow‐up (124 in the treatment arm and 116 in the control arm), or had died (47 in the treatment arm and 46 in the control arm). |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Dexiprom 1999.
| Study characteristics | ||
| Methods | Type of study: RCT Method of treatment allocation: computer‐generated randomisation. Sequentially‐numbered drug boxes were used. Stratification: yes, by hospital Placebo: yes, normal saline Sample size calculation: yes Intention‐to‐treat analyses: no Losses to follow‐up: yes, 7 (3%) women and infants were excluded from analysis (3 women did not have PROM, 2 women were < 26 weeks at randomisation, 1 woman received off‐protocol corticosteroid, a neonatal bed was not available in 1 case) | |
| Participants | Location: 6 hospitals in South Africa Eligibility criteria: women with PROM between 28‐34 weeks or with an estimated fetal weights of 1000 g to 2000 g if the gestational age was unknown Gestational age range: 28‐34 weeks Exclusion criteria: cervical dilatation > 4 cm, evidence of infection, evidence of antepartum haemorrhage, < 19 years old Total recruited: 204 women and 208 infants; 102 women and 105 infants in the treatment arm and 102 women and 103 infants in the control arm | |
| Interventions | 2 doses of 12 mg dexamethasone IM 24 hours apart
Control group received placebo All women also received ampicillin, metronidazole and hexoprenaline if contractions present in < 24 hours |
|
| Outcomes | Maternal outcomes (maternal death, chorioamnionitis, endometritis, postnatal fever), fetal/neonatal outcomes reported (fetal death, neonatal death, RDS, IVH, birthweight, need for mechanical ventilation/CPAP, systemic infection in the first 48 hours of life, necrotising enterocolitis) | |
| Notes | Study authors supplied additional data Emailed study authors July 2020 to clarify ethic approval; reply received that all sites had ethics approval from the University in each case. Study dates: not stated in the manuscripts, the study is coded as 1990s for the review Funding sources: quote: "the authors acknowledge the Medical Research Council for funding this study and Donmed Pharmaceuticals for supplying the dexamethasone and saline vials." Declarations of interest: not reported Study was discontinued before target sample size was reached due to increasing body of evidence of the use of corticosteroids in women with PPROM being advantageous to the infants, and it was felt unnecessary to conduct further trials of antenatal corticosteroids in women with PPROM |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation. Sequentially‐numbered drug boxes were used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants likely as identical looking placebo was used. Blinding of study personnel was not described, other than "double blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 (3%) women and infants were excluded from analysis (3 women did not have PROM, 2 women were < 26 weeks at randomisation, 1 woman received off‐protocol corticosteroid, a neonatal bed was not available in 1 case) |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Fekih 2002.
| Study characteristics | ||
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Stratification: none stated Placebo: no Sample size calculation: no Intention‐to‐treat analyses: no Losses to follow‐up: yes, number of post‐randomisation exclusions not stated | |
| Participants | Location: CHU Farhat Hached, Sousse, Tunisia Eligibility criteria: women in preterm labour Gestational age range: 26‐34 weeks Exclusion criteria: gestational diabetes, > 4 cm cervical dilatation, fetal abnormalities, contraindication to corticosteroids, delivery elsewhere or after 34 weeks (post‐randomisation exclusions) Total recruited: 118 women and 131 infants; 59 women and 63 infants in the treatment arm and 59 women and 68 infants in the control arm | |
| Interventions | Abstract and full report state slightly different protocols for the intervention arm. The abstract stated that 24 mg betamethasone was given as two 12 mg IM doses at 24 hours apart. The full text states that this regimen was repeated weekly. Women had two doses of 12 mg given 24 hours apart, and this regimen was repeated weekly. Control group received expectant management | |
| Outcomes | Maternal outcomes (chorioamnionitis, postnatal fever) and fetal/neonatal outcomes reported (neonatal death, RDS, IVH) | |
| Notes | Article in French, abstract in English. Article translated by review authors (La Tunisie Medicale, 2002, Vol 80; No. 5: 260‐265). Further information was requested from the study authors but there was no reply Study dates: January 1998‐June 1999 Funding sources: not stated Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is unlikely as placebo was not used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of post‐randomisation exclusions not stated |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Gamsu 1989.
| Study characteristics | ||
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Stratification: yes, by hospital Placebo: yes, vehicle of betamethasone preparation Sample size calculation: no Intention‐to‐treat analyses: yes Losses to follow‐up: no | |
| Participants | Location: 11 hospitals in the UK Eligibility criteria: women with spontaneous or planned preterm delivery Gestational age range: < 34 weeks Exclusion criteria: contraindication to corticosteroids, contraindications to postponing delivery, diabetes, suspected intrauterine infection Total recruited: 251 women and 268 infants; 126 women and 131 infants in the treatment arm and 125 women and 137 infants in the control arm | |
| Interventions | 6 doses of 4 mg betamethasone phosphate IM 8 hours apart
Control group received 6 doses of placebo All women with spontaneous labour received IV salbutamol |
|
| Outcomes | Fetal/neonatal outcomes reported (fetal death, neonatal death, RDS, IVH, birthweight, systemic infection in the first 48 hours of life) | |
| Notes | Study dates: mid 1975‐February 1978 Funding sources: not reported. Quote: "the compilers would like to acknowledge...Glaxo (Group Research Ltd) for the generous provision of computing facilities and for the supply of betamethasone and placebo and their distribution" Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is likely that participants were blinded as placebo was used. Blinding of study personnel was not described other than "double‐blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Garite 1992.
| Study characteristics | ||
| Methods | Type of study: RCT Method of treatment allocation: random‐number table generated randomisation sequence by pharmacy. The pharmacy provided consecutive sealed envelopes. Stratification: none stated Placebo: yes, normal saline Sample size calculation: no Intention‐to‐treat analyses: no Losses to follow‐up: yes, 5 (7%) women delivered elsewhere and were lost to follow‐up (4 in treatment arm and 1 in control arm) | |
| Participants | Location: Long Beach Memorial Women's Hospital, California, USA Eligibility criteria: women likely to deliver between 24 hours and 7 days with spontaneous preterm labour or planned preterm delivery Gestational age range: 24‐27 + 6 weeks Exclusion criteria: PROM, clinical or laboratory evidence of infection, contraindication to or previously given corticosteroids, diabetes Total recruited: 76 women and 82 infants; 37 women and 40 infants in the treatment arm and 39 women and 42 infants in the control arm | |
| Interventions | 2 doses of 6 mg betamethasone acetate and 6 mg betamethasone phosphate IM 24 hours apart, repeated weekly if still < 28 weeks and thought likely to deliver within the next week Control group received 2 doses of placebo. Women undelivered after 28 weeks and 1 week post their last dose of study medication were allowed glucocorticoids at the discretion of their physicians. | |
| Outcomes | Maternal outcomes (chorioamnionitis, endometritis), fetal/neonatal outcomes reported (fetal death, neonatal death, RDS, chronic lung disease, IVH, birthweight, Apgar < 7, need for mechanical ventilation/CPAP, duration of mechanical ventilation/CPAP, proven neonatal infection while in NICU) | |
| Notes | It is not stated how many women received corticosteroids off protocol. Study dates: December 1984‐May 1990 Funding sources: quote: "supported by a grant from the Long Beach Memorial Foundation" Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table generated randomisation sequence by pharmacy |
| Allocation concealment (selection bias) | Unclear risk | The pharmacy provided consecutive sealed envelopes, not stated if envelopes were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is likely that participants were blinded as placebo was used. Blinding of study personnel was not described other than "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 (7%) women delivered elsewhere and were lost to follow‐up (4 in treatment arm and 1 in control arm). |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Gyamfi‐Bannerman 2016.
| Study characteristics | ||
| Methods | Type of study: double‐blind, RCT
Method of treatment allocation: simple urn method of randomisation Stratification: yes, according to clinical site and gestational age (34‐35 weeks and 36 weeks) Placebo: yes, matching placebo Sample size calculation: yes Intention‐to‐treat analyses: yes Losses to follow‐up: yes, 4 (0.11%) lost to follow‐up; 2 in each treatment group |
|
| Participants | Location: 17 university‐based clinics in the USA. All centres affiliated with the Maternal–Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Eligibility criteria: women with singleton pregnancy 34 weeks + 0 d‐36 weeks + 5 d gestation at "high probability" of preterm delivery. Quote: "High probability was defined as either preterm labor with intact membranes and at least 3 cm dilation or 75% cervical effacement, or spontaneous rupture of the membranes. If neither of these criteria applied, a high probability was defined as expected preterm delivery for any other indication either through induction or cesarean section between 24 hours and 7 days after the planned randomisation, as determined by the obstetrical provider." Gestational age range: 34 weeks + 0 days‐36 weeks + 5 days Exclusion criteria: expected delivery < 12 hours for any reason, already received antenatal corticosteroids in current pregnancy, chorioamnionitis, 8 cm or more cervical dilation, non‐reassuring fetal status requiring immediate delivery, no gestational age dating by ultrasound before 32 weeks for women with known date for last menstrual period, women without ultrasound dating before 24 weeks' gestation with unknown date of last menstrual period Total recruited: 2831 women and 2831 infants; 1429 women and 1429 infants in the treatment arm and 1402 women and 1402 infants in the control arm | |
| Interventions | Treatment group: (n = 1429 randomised) 2 IM injections of 12 mg betamethasone (equal parts betamethasone sodium phosphate and betamethasone acetate) administered 24 hors apart Control group received matching placebo "For those enrolled because of an indication for preterm delivery, labor inductions were expected to start by 36 weeks 5 d, and cesarean deliveries were to be scheduled by 36 weeks 6 days and not before 24 hours after randomization." Control: (n = 1402 randomised) placebo IM injections as above Follow up: to 28 d for oxygen dependency outcome |
|
| Outcomes | Maternal outcome (maternal death, chorioamnionitis, side effects of therapy in women), fetal/neonatal outcomes (perinatal death, fetal death, neonatal death, RDS, IVH, birthweight, necrotising enterocolitis, proven infection while in NICU, need for mechanical ventilation/CPAP, surfactant use, air leak syndrome, Apgar score < 7, small for gestation age, admission to NICU) We asked study authors to clarify the mechanical ventilation/CPAP data presented in Table 2 of the publication; we are unsure if outcome categories are exclusive or not. We have not included data from this trial in the meta‐analysis for 1.25 due to these concerns; data will be included at the next update if confirmed by study authors. Data from trial are available for following non‐review outcomes: maternal serious adverse events, infant serious adverse events, hypoglycaemia in infant. Length of stay (maternal and infant) reported as median with IQR only. Randomisation to delivery interval reported as median with IQR only |
|
| Notes | Supplementary appendix published online with data tables and additional information on trial methods relevant to risk of bias. Contact author confirmed no maternal deaths and blinding of researchers abstracting data from maternal and neonatal charts (24.2.2016 by email) ClinicalTrials.gov number, NCT01222247. Ruptured membranes occurred in 22.1% intervention and 21.7% controls
"A total of 860 of 1429 women (60.2%) in the betamethasone group and 826 of 1402 (58.9%) in the placebo group received the prespecified two doses of study medication. Of the 1145 women who did not receive a second dose, 1083 (94.6%) delivered before 24 hours; 6 women did not receive any of the assigned study medication. (In the placebo group, 3 women who consented to participate in the trial subsequently declined the injection, 1 woman delivered after randomization but before the first dose, and 1 received open label betamethasone. In the betamethasone group, 1 woman was in active labor with complete cervical dilation at the time of randomization)" Study dates: October 2010‐February 2015 Funding sources: National Heart, Lung, and Blood Institute, USA; Eunice Kennedy Shriver National Institute of Child Health and Human Development, USA; National Center for Advancing Translational Sciences, National Institutes of Health, USA Declarations of interest: "No potential conflict of interest relevant to this article was reported." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Independent data‐coordinating centre with the use of the simple urn method, with stratification according to clinical site and gestational age category (34 to 35 weeks vs. 36 weeks)" |
| Allocation concealment (selection bias) | Low risk | Remote centre performed randomisation and packaged intervention and placebo |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical treatment and placebo packs prepared remotely. Women and staff blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trained research staff extracted data from maternal and neonatal staff; authors confirmed by email that these researchers were blinded. Charts of babies admitted to special care were reviewed by blinded staff for respiratory outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two women in each group lost to follow‐up. Data available for 2827 neonates |
| Selective reporting (reporting bias) | Low risk | Supplementary outcome data published online with paper |
| Other bias | Low risk | Few baseline imbalances apart from mean maternal age (28.6 versus 27.8 years) and Hispanic ethnic background (28.3 versus. 32%) |
Kari 1994.
| Study characteristics | ||
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Stratification: yes, according to gestational age (24 to 27.9 weeks and 28 to 31.9 weeks) at each hospital Placebo: yes, normal saline Sample size calculation: yes Intention‐to‐treat analyses: yes Losses to follow‐up: yes, 10 (11%) children in the follow‐up study at age 2 (2 in the treatment arm and 8 in the control arm) | |
| Participants | Location: 5 hospitals in Finland Eligibility criteria: women with preterm labour or threatened preterm delivery due to pre‐eclampsia Gestational age range: 24 to 31.9 weeks Exclusion criteria: rupture of membranes, chorioamnionitis, congenital abnormalities, proven lung maturity, insulin‐treated diabetes, previously treated with corticosteroids Total recruited: 157 women and 189 infants; 77 women and 95 infants in the treatment arm and 80 women and 94 infants in the control arm | |
| Interventions | 4 doses of 6 mg dexamethasone sodium phosphate IM 12 hours apart Control group received 4 doses of placebo. Rescue treatment with exogenous human surfactant was given to infants born 24‐33 weeks, who at 2 to 24 hours of age required mechanical ventilation with > 40% oxygen for RDS. | |
| Outcomes | Maternal outcome (chorioamnionitis), fetal/neonatal outcomes (fetal death, neonatal death, RDS, chronic lung disease, IVH, birthweight, surfactant use, necrotising enterocolitis, small‐for‐gestational age) and childhood outcomes reported (death, neurodevelopmental delay) | |
| Notes | Efficacy analysis restricted to 91 infants in treatment arm and 88 infants in control arm. 3 infants excluded for protocol violations (1 mother with twins in placebo arm was given corticosteroid, 1 infant in the treatment arm developed RDS but was not given surfactant as it was not available) and 6 infants were excluded because of congenital malformations (2 treatment, 4 placebo) Study dates: April 1989‐October 1991 Funding: Foundation for Pediatric Research, Finland; Orange County Infant Care Specialists, Finland; The Orion Corporation Research Foundation, Finland; Instrumentarium Corporation Research Foundation, Finland; Arvo and Lea Ylppo Foundation, Finland; Rinnekoti Foundation, Finland; and Organon Company, Oss, The Netherlands Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated.Quote: "Randomisation in each participating hospital was performed in blocks of 10" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The investigators and those who provided care were unaware of the treatment allocation". It is likely that participants were blinded as "ampoules containing betamethasone and placebo were identical". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 (11%) children in the follow‐up study at age 2 (2 in the treatment arm and 8 in the control arm). 1 female placebo‐treated infant born at 27 weeks' gestation died 3 months after the expected date of delivery, 4 infants were lost due to parental refusal, 2 were living overseas, and 3 were in other regions of the country. Efficacy analysis restricted to 91 infants in treatment arm and 88 infants in control arm. 3 infants excluded for protocol violations (1 mother with twins in placebo arm was given corticosteroid, 1 infant in the treatment arm developed RDS but was not given surfactant as it was not available) and 6 infants were excluded because of congenital malformations (2 treatment, 4 placebo); low attrition and not differential. |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Lewis 1996.
| Study characteristics | ||
| Methods | Type of study: RCT Method of treatment allocation: random‐number table generated randomisation sequence by clinical research nurse uninvolved in clinical care. Sequentially‐numbered sealed opaque envelopes used. Stratification: none stated Placebo: no Sample size calculation: no Intention‐to‐treat analyses: no Losses to follow‐up: yes, 2 (2%) women left hospital after randomisation and were lost to follow‐up (1 woman in each arm) | |
| Participants | Location: Louisiana State University Medical Center, Shreveport, Louisiana, USA Eligibility criteria: women with singleton pregnancies with PROM. Women were randomised 12 to 24 hours after receiving IV ampicillin‐sulbactam Gestational age range: 24‐34 weeks Exclusion criteria: evidence of infection, vaginal examination, cerclage, allergic to penicillin, contraindication to expectant management, lung maturity confirmed by L/S ratio if 32 weeks or more Total recruited: 79 women and infants; 39 women and infants in the treatment arm and 40 women and infants in the control arm | |
| Interventions | The treatment group received 12 mg IM betamethasone repeated at 24 hours and weekly if the women had not delivered. The control group received expectant management. | |
| Outcomes | Maternal outcomes (chorioamnionitis, endometritis), fetal/neonatal outcomes (neonatal death, RDS, IVH, birthweight, Apgar < 7, interval between trial entry and delivery, admission to NICU, surfactant use, proven neonatal infection while in NICU, necrotising enterocolitis) and health service outcome reported (length of neonatal hospitalisation) | |
| Notes | Emailed authors July 2020 to enquire about the dates the study took place; awaiting reply. Timeframe: not stated in manuscript, the study is coded as 1990s for the review Funding sources: not stated Declarations of interest not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clinical research nurse uninvolved in clinical care generated randomisation sequence by using random‐number table, with a random permuted block size of 10 |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered sealed opaque envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comparison was "no treatment" so blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 (2%) women left hospital against medical advice after randomisation and were lost to follow‐up (1 women in each arm) |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other sources of bias |
Liggins 1972b.
| Study characteristics | ||
| Methods | Type of study: RCT Method of treatment allocation: random‐number table generated randomisation sequence by chief pharmacist. Pharmacy provided coded drug ampoules containing treatment or placebo Stratification: no Placebo: yes, of identical appearance Sample‐size calculation: no Intention‐to‐treat analyses: yes Losses to follow‐up: yes, 54 (18%) children in the follow‐up study at ages 4‐6 years (31 in the treatment arm and 23 in the control arm) and 412 (44%) adults in the follow‐up study at age 30 years (219 in the treatment arm and 193 in the control arm) |
|
| Participants | Location: National Women's Hospital, Auckland, New Zealand Eligibility criteria: women with threatened or planned preterm delivery Gestational age range: 24‐36 weeks Exclusion criteria: imminent delivery, contraindication to corticosteroids Total recruited: 1142 women and 1218 infants; 560 women and 601 infants in the treatment arm and 582 women and 617 infants in the control arm |
|
| Interventions | The treatment group 2 doses of 6 mg betamethasone phosphate and 6 mg betamethasone acetate IM 24 hours apart. After the first 717 women had enrolled, the treatment intervention was doubled to 2 doses of 12 mg betamethasone phosphate and 12 mg betamethasone acetate IM 24 hours apart. The control group received 6 mg cortisone acetate, which has 1/70th of the corticosteroid potency of the betamethasone. | |
| Outcomes | Maternal outcome (chorioamnionitis), fetal/neonatal outcomes (fetal death, neonatal death, RDS, cerebroventricular haemorrhage, mean birthweight, Apgar score < 7, mean interval between trial entry and delivery, proven infection while in NICU), childhood outcomes (death, mean weight, mean height, mean head circumference, mean lung function, mean blood pressure, intellectual impairment, cerebral palsy) and adulthood outcomes were reported (death, mean weight, mean height, mean head circumference, mean skin fold thickness, mean blood pressure, glucose impairment, HPA axis function, mean cholesterol, educational achievement, visual impairment, hearing impairment, intellectual impairment) | |
| Notes | Review includes new intention‐to‐treat analysis of the complete study and additional data due to the study authors providing individual participant study records Study dates: December 1969 and February 1974 Funding sources: Health Research Council of New Zealand, Auckland, New Zealand; Auckland Medical Research Foundation, Auckland, New Zealand; and New Zealand Lottery Grants Board, Wellington, New Zealand Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table generated randomisation sequence by chief pharmacist |
| Allocation concealment (selection bias) | Low risk | Pharmacy provided coded drug ampoules containing treatment or placebo |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of study personnel was not described. It is likely that participants were blinded as placebo was of identical appearance to the corticosteroid. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | For the diagnosis of RDS, clinical records and chest radiographs were assessed separately and independently, by 1 of the study authors, and by a radiologist. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Incomplete outcome data for 54 (18%) children in the follow‐up study at ages 4‐6 (31 in the treatment arm and 23 in the control arm) and 412 (44%) adults in the follow‐up study at age 30 (219 in the treatment arm and 193 in the control arm) |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other sources of bias. |
Lopez 1989.
| Study characteristics | ||
| Methods | Type of study: RCT
Method of treatment allocation: not described Stratification: not stated Placebo: no. Sample size calculation: not stated Intention‐to‐treat analyses: not stated however, all those randomised were analysed Losses to follow‐up: nil |
|
| Participants | Location: Department of Obstetrics and Gynecology, Faculty of Medicine, National Univeristy of Colombia
Eligibility criteria: PROM (confirmed using speculoscopy and ultrasound), no signs of infection, not in labour at time of hospitalisation
Gestational age range: 27‐35 weeks' gestation
Exclusion criteria: not stated
Total recruited: 20 control group, 20 study group 40 women, 40 infants |
|
| Interventions | The treatment group received 2 doses of 12 mg betamethasone IM, 12 hours apart. The control group received no treatment. |
|
| Outcomes | Neonatal mortality, RDS, Apgar score < 7 at 5 minutes, systemic infection in first 48 hours | |
| Notes | Original article in Spanish, translated into English Study dates: August 1983‐December 1985 Funding: not stated Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated other than quote: "patients were classified randomly into groups" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comparison is "no treatment" so blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other sources of bias exist. |
Mansouri 2010.
| Study characteristics | ||
| Methods | Type of study: double‐blind RCT
Method of treatment allocation: not described Stratification: not stated Placebo: yes, placebo‐controlled Sample size calculation: not stated Intention‐to‐treat analyses: yes Losses to follow‐up: no Double‐blind, randomised controlled trial in Kurdistan University of Medical Sciences, Sanandaj, Iran |
|
| Participants | Location: Kurdistan University of Medical Sciences, Sanandaj, Iran Eligibility criteria: women at high risk of preterm labour, not described Gestational age range: 35‐36 weeks Exclusion criteria: not stated in our translation Total recruited: 200 women and 200 infants; 100 women and 100 infants in the treatment arm and 100 women and 100 infants in the control arm | |
| Interventions | The treatment group received 2 doses of 12 mg betamethasone, IM. The control group received a placebo of normal saline. |
|
| Outcomes | Maternal outcome (maternal death, maternal infections), fetal/neonatal outcomes reported (RDS, birthweight, necrotising enterocolitis, systemic infection in the first 48 hours of life, need for mechanical ventilation/CPAP, Apgar < 7 at 5 minutes, admission to NICU) | |
| Notes | Original article in Persian; we have obtained a truncated translation for this update. Our translator was unable to translate the definition of respiratory distress syndrome but said that the outcome was based on defined symptoms and confirmed by a paediatrician. Additonal outcome data for this trial are: Maternal length of stay > 3 days (equal numbers in treatment arms) is reported narratively above: mean birthweight and SD in kg has been analysed as g. Data for the trial outcome of 'need for respiratory support' has been included in the review analysis 1.26 'need for mechanical ventilation'. We have been unable to confirm whether the trial included only singleton pregnancy, but this is suggested by the equal numbers of women and infants reported. We have included data from this trial in the singleton subgroup. We had no information about membrane status from our translation, and so this trial has been included in the 'not reported or mixed population subgroup.' Maternal length of stay > 3 days (equal numbers in both arms) is reported narratively. We emailed study investigators for clarification and additional information with no reply (2/2016). Study dates: "during 2007" stated Funding: Kurdistan University of medical sciences Declarations of interest: not included in English translation, unclear if reported in original language |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Generation of sequence not stated, but block method specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial described as double‐blind. Placebo‐controlled trial, and researchers and women were blind to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Neonatal outcomes extracted by blinded paediatrician. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reported for all women randomised |
| Selective reporting (reporting bias) | Low risk | Relevant outcome data reported |
| Other bias | Unclear risk | We have obtained a basic translation, but future correspondence with authors may clarify some of the risk of bias domains above. |
Morales 1989.
| Study characteristics | ||
| Methods | Type of study: four‐arm randomised controlled trial Method of treatment allocation: method of randomisation not stated. Sealed envelopes were used. Stratification: none stated Placebo: no Sample size calculation: no Intention‐to‐treat analyses: no Losses to follow‐up: no | |
| Participants | Location: 3 hospitals in Florida, USA Eligibility criteria: women with singleton pregnancies with PROM Gestational age range: 26 and 34 weeks Exclusion criteria: PROM < 12 hours before onset of labour, uterine tenderness, foul smelling lochia, fetal tachycardia, allergy to penicillin, congenital abnormalities, L/S ratio 2 or more, unable to obtain an L/S ratio, Dubowitz‐assigned gestational age different from obstetric assessment by 3 weeks (post‐randomisation exclusion) Total recruited: 165 women and infants; 87 women and infants in the treatment arm and 78 women and infants in the control arm | |
| Interventions | 4 treatment arms. Group 1, expectant management. Group 2, expectant management plus 2 doses of 12 mg betamethasone IM 24 hours apart, repeated weekly if the women remained undelivered. Group 3, expectant management plus 2 g ampicillin IV every 6 hours until cervical cultures were negative. Group 4, combination of group 2 and 3 management. We combined Groups 2 and 4 in the treatment arm for the review, and groups 1 and 3 in the control arm for the review. |
|
| Outcomes | Maternal outcome (chorioamnionitis), fetal/neonatal outcomes reported (neonatal death, RDS, chronic lung disease, IVH, birthweight, proven neonatal infection while in NICU, necrotising enterocolitis, duration of mechanical ventilation/CPAP) | |
| Notes | Further information requested from study authors but there was no reply. No information was available on post‐randomisation exclusions Study dates: January 1986‐March 1988 Funding sources: not stated Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | "Sealed envelopes" were used. Not further described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | As comparison was expectant management, blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No losses to follow‐up noted. No information was available on post‐randomisation exclusions as per exclusion criteria |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other sources of bias exist |
Morrison 1978.
| Study characteristics | ||
| Methods | Parallel group, two‐arm RCT City of Memphis Hospitals, Tennessee, USA Fifth General Hospital, Stuttgart, Germany Stratification: no Placebo: yes Sample size calculation: no Intention‐to‐treat analyses: no |
|
| Participants | Inclusion criteria: women in premature labour or had fetomaternal disorders requiring early delivery Exclusion criteria: women with medical or operative disease that interdicted steroid therapy, women receiving immunosuppressive therapy, allergies to corticosteroids, currently taking corticosteroids, women with mature lecithin/sphingomyelin ratios thought to be in premature labour. Women in active labour or with fetomaternal complications were not entered in the study if delivery was anticipated within 24 hours. Gestational age: less than 34 weeks 196 women randomised: unclear how many randomised to each group. Reported numbers for analysis are 67 women, 67 infants in intervention group and 59 women, 59 infants in control group |
|
| Interventions | Experimental: hydrocortisone 100 mg per mL. Five ml administered every 12 hours over a 48‐hour period. Control: placebo (lactose and water 100 mg permL. Five mL administered every 12 hours over a 48‐hour period. Participants followed up for six months after delivery. Some followed up for three years but no useable data for those time points |
|
| Outcomes | RDS Neonatal death Birthweight Side effects of therapy |
|
| Notes | Study dates: July 1972 to December 1975 Study authors’ declarations of interest: not reported Funding sources: not reported. Authors reported participating in a trial sponsored by the National Heart, Lung, and Blood institute, unclear if this refers to the current trial or to a different one, and unclear if the National Heart, Lung, and Blood institute has a funding role. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “randomized fashion” ‐ not further details reported |
| Allocation concealment (selection bias) | Unclear risk | Unclear if allocation concealment was attempted: quote: “After entry to the study, each patient was assigned an identification number by the research technician. The number was recorded in a log book in the perinatal laboratory. A multidose vial bearing the identification number contained 25 ml of the test drug or placebo). These numbered vials were prepared in the Medicinal Chemistry Department and used in both hospitals” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Numbered vials prepared in the Medicinal Chemistry Dept and used in both hospitals…were clear and colourless and of the same relative viscosity …after delivery the vial was returned to the research technician so that the remaining liquid could be measured to validate the amount of drug given”. “the master code was retained by a cooperating member of the Dept of Biochemistry…to prevent unblinding” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The master code for the drug allocation was retained by a cooperating member of the Department of Biochemistry, who did not have access to the L/S ratios, clinical data or follow‐up results. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 60/196 were excluded after delivery because of evidence of hypoxia, obstetric factors affecting the neonate, or because delivery did not occur within 7 days. A further 10 women did not return for follow‐up visits and were excluded from the analysis. The group allocation of these 70 women is not reported. |
| Selective reporting (reporting bias) | Low risk | No protocol available (would not be common practice at the time of conducting this trial). Outcomes reported as expected. |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Nelson 1985.
| Study characteristics | ||
| Methods | Type of study: RCT Method of treatment allocation: random‐number table generated randomisation sequence with consecutive sealed envelopes used. Stratification: none stated Placebo: no Sample size calculation: no Intention‐to‐treat analyses: yes Losses to follow‐up: no | |
| Participants | Location: Wake Forest University Medical Center, North Carolina, USA Eligibility criteria: women with PROM Gestational age range: 28 and 34 weeks Exclusion criteria: fetal distress, active labour, cervical dilatation > 3 cm, sensitivity to tocolytics, PROM > 24 hours, existing infection Total recruited: 44 women and infants; 22 women and infants in each arm | |
| Interventions | 3 treatment arms. Group 1: 2 doses of 6 mg or 12 mg betamethasone IM 12 hours apart, delivery 24 to 48 hours after PROM and after 24 hours of corticosteroid therapy. Group 2 received similar treatment to group 1 except that no steroids were administered. Delivery 24 to 48 hours after PROM. Group 3, expectant management (no tocolytics or steroids). We did not include Group 3 in the review. |
|
| Outcomes | Fetal/neonatal outcomes (neonatal death, RDS, proven neonatal infection while in NICU) and health service outcome reported (length of neonatal hospitalisation) | |
| Notes | Authors provided further information Study dates: not stated in manuscript, the study is coded as 1980s for the review Funding sources: not stated Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Consecutive, sealed envelopes were used, not stated if opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was not possible due to the nature of the comparison. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up or exclusions |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other sources of bias exist. |
Ontela 2018.
| Study characteristics | ||
| Methods | Two‐arm parallel RCT Setting: Obstetrics and Gynaecology Department in collaboration with the Department of Neonatology at the Jawaharlal Nehru Institute of Postgraduate Medical Education and Research, India |
|
| Participants | Inclusion criteria: women admitted to the labor room with a period of gestation between 34 and 36 + 6 weeks with a singleton pregnancy with risk of preterm delivery either spontaneously or requiring termination for maternal (or) fetal indication Exclusion criteria: those who were far advanced in labour (5 cm or more dilated) or had received the previous course of steroids or had chorioamnionitis at admission, women with multiple gestations, gestational diabetes and diabetes mellitus, major congenital malformations in the fetus and those undergoing a pre‐labour scheduled cesarean section Gestational age: 34 weeks to 36 weeks + 6 days Number randomised: 310 women (155 to each group) Number analysed: 309 women, 309 infants (1 stillbirth was not included in analysis) |
|
| Interventions | Experimental: dexamethasone 6 mg administered intramuscularly 12 hourly, four doses in total. Control: no treatment All babies followed up until discharge. |
|
| Outcomes | Composite respiratory morbidities (transient tachypnoea; respiratory distress syndrome) Quote: “Other complications such as hypoglycemia, hypothermia, poor feeding, sepsis and neonatal mortality were also noted. We followed up all the babies until discharge from the hospital.” |
|
| Notes | Study authors’ declarations of interest: not reported Funding sources: not reported Study dates: main trial report states February 2015 to June 2016, 2018 conference abstract states October 2014 to June 2016 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:“computer‐generated random numbers” |
| Allocation concealment (selection bias) | Low risk | Quote:“sequentially numbered, sealed, opaque envelopes, from an independent data coordinating party” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants (control received no treatment or placebo) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote:“There was no loss to follow‐up after randomization. One case in the study group was excluded from analysis because it was stillborn.” |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in trial registry record and in methods are all reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Porto 2011.
| Study characteristics | ||
| Methods | Type of study: RCT
Method of treatment allocation: sealed cardboard boxes numbered according to random number table generated by a statistician not involved in the study Stratification: not stated Placebo: yes, identical to corticosteroid in appearance, volume and colour Sample size calculation: yes Intention‐to‐treat analyses: yes Losses to follow‐up: 43 (13%) women (19 in corticosteroid group and 24 in placebo group) were discharged from hospital still pregnant and were considered post‐randomisation loss to follow‐up. 2 (1%) women were excluded from the placebo group as they were found to be ineligible after randomisation (multiple pregnancy, and term pregnancy). Two infant stillbirths were also excluded. |
|
| Participants | Location: Instituto de Medicina Integral Professor Fernando Figueira, Recife, Pernambuco, Brazil Eligibility criteria: 34‐36 + 6 weeks' gestation at risk of imminent premature delivery (either spontaneously or if early delivery was recommended as a result of problems with mother or fetus) Gestational age range: 34‐36 + 6 weeks' gestation Exclusion criteria: multiple pregnancy, major congenital malformations, haemorrhage symptoms with active bleeding, clinical evidence of chorioamnionitis, previous use of antenatal corticosteroids, need for immediate resolution of pregnancy for maternal or fetal reasons Total recruited: 320 women and infants; 163 women and infants in the treatment arm and 157 women and infants in the control arm | |
| Interventions | The treatment group received 2 doses of 12 mg IM betamethasone 24 hours apart. The control group received IM saline as placebo. |
|
| Outcomes | Maternal outcomes (side effects of therapy in women) and fetal/neonatal outcomes (fetal deaths, neonatal deaths, RDS, birthweight, proven infection while in NICU, need for mechanical ventilation/CPAP, mean duration of mechanical ventilation/CPAP, surfactant use, small for gestational age, admission to NICU) | |
| Notes | For infant outcomes, we have used the denominator stated in the published report excluding women who left the trial pregnant. An intention‐to‐treat analysis should have included these women, so for 'Summary of findings' outcomes we carried out a sensitivity analysis to determine if the denominator used made a difference to the overall pooled effect estimate; it did not (data not shown) Attempted to contact trial authors in July 2020 to clarify ethics approval but email address is no longer in use. Study dates: April 2008‐June 2010 Funding sources: quote: "This study was supported by the Instituto de Medicina Integral Prof Fernando Figueira‐IMIP (www.imip.org.br), a private, not for profit healthcare organisation based in Recife, Pernambuco, Brazil, where the study was carried out. The institute did not interfere with study design or analysis and the funding covered all study expenses, including purchase of the drug and placebo." Declarations of interest: All authors declare: "no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table was prepared by a statistician not involved in the study, using random allocation software |
| Allocation concealment (selection bias) | Low risk | The hospital pharmacy prepared sealed cardboard boxes numbered according to the random number table, and containing either betamethasone or placebo, identical in appearance, volume and colour. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators, physicians caring for the women, the women themselves and the statistician were all blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators, physicians caring for the women, the women themselves and the statistician were all blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 43 (13%) women (19 in steroid group and 24 in placebo group) were discharged from hospital still pregnant and were considered post‐randomisation losses to follow‐up. 2 (1%) women were excluded from the placebo group as they were found to be ineligible after randomisation (multiple pregnancy, and term pregnancy). |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes appear to have been reported. |
| Other bias | Low risk | Nothing to indicate any other |
Qublan 2001.
| Study characteristics | ||
| Methods | Type of study: RCT Method of treatment allocation: random‐number table generated randomisation sequence Allocation concealment unclear. Stratification: none stated Placebo: no Sample size calculation: no Intention‐to‐treat analyses: yes Losses to follow‐up: no | |
| Participants | Location: 2 military hospitals in Jordan Eligibility criteria: women with singleton pregnancies and PROM Gestational age range: 27‐34 weeks Exclusion criteria: lethal congenital anomaly, fetal death, infection, expected delivery within 12 hours Total recruited: 139 women and infants; 72 women and infants in the treatment arm and 67 women and infants in the control arm | |
| Interventions | The treatment group received 4 doses of 6 mg dexamethasone IM 12 hours apart, repeated if women had not delivered after 1 week. The control group received expectant management. | |
| Outcomes | Maternal outcomes (chorioamnionitis, endometritis), fetal/neonatal outcomes (fetal death, neonatal death, RDS, IVH, proven neonatal infection while in NICU, necrotising enterocolitis, Apgar < 7) and health service outcome reported (length of neonatal hospitalisation) | |
| Notes | Study authors contacted for further information but no reply. Discrepancy in number of infants with necrotising enterocolitis in manuscript Study dates: January 1997‐February 1999 Funding: not stated Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table generated randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was not possible due to the nature of the comparison. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up or exclusions stated |
| Selective reporting (reporting bias) | Unclear risk | Discrepancy in number of infants with necrotising enterocolitis in manuscript |
| Other bias | Low risk | No indication of any other source of bias |
Schmidt 1984.
| Study characteristics | ||
| Methods | Parallel, four‐arm RCT. California, USA |
|
| Participants | Inclusion criteria: preterm labour or having premature rupture of the membranes (or both), gestational age 26 weeks to 32 weeks or estimated fetal weight of 750 g to 1750 g, cervical examination had to be > 5 cm Exclusion criteria: women known to have a disease that might significantly affected by glucocorticosteroids or tocolytic agents or both. If bacteria were seen on Gram stain of amniotic fluid, the woman was excluded. Gestational age: 26 weeks to 32 weeks, or estimated fetal weight of 750 to 1750 g. Number randomised:144 women (149 infants). Numbers randomised to each group are not reported. Numbers analysed: hydrocortisone 15 infants, 15 women; methylprednisolone 17 infants, 16 women; betamethasone 34 infants, 32 women; control 31 infants, 29 women. |
|
| Interventions | Experimental 1: hydrocortisone 250 mg. Two IM doses, given 24 hours apart. Experimental 2: methylprednisolone 125 mg. Two IM doses, given 24 hours apart. Experimental 3: betamethasone 12 mg. Two IM doses, given 24 hours apart. Control: placebo (saline). Two IM doses, given 24 hours apart. |
|
| Outcomes | RDS Neonatal death Birth weight |
|
| Notes | Study dates: July 1977 to April 1980 Study authors’ declarations of interest: not reported Funding sources: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “the sequence of drugs had been determined by means of a table of random numbers” |
| Allocation concealment (selection bias) | Low risk | Quote:“consecutively numbered opaque bags” “the bags were prepared by the project nurses who then sealed them and kept a record of their use” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote:“the record book was not available to those providing patient care or who were evaluating the data” “each dosage consisted of a 2 ml volume prepared by a nurse on the oncology ward, who had no contact with obstetric patients” “the syringe barrel was covered with an opaque label so the care‐givers could not determine visually which drug was being administered.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors seem to be care‐givers, who are blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 52/144 women and their infants excluded from analysis (28 because of birth weights > 2500 g, 13 due to incomplete maternal or baby information, 6 due to procedural errors, 3 due to mature L/S ratio, 2 stillbirths). The group allocation of these women is not reported. |
| Selective reporting (reporting bias) | High risk | No protocol, most outcomes reported in full but no data reported for endometritis although it is specified in the methods. |
| Other bias | High risk | Quote:“hydrocortisone and methylprednisolone groups discontinued at 24 months to increase group size in remaining two regimens” |
Schutte 1980.
| Study characteristics | ||
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Coded drug ampoules were provided. Randomisation code was only known to pharmacist. Stratification: none stated Placebo: yes, normal saline Sample size calculation: no Intention‐to‐treat analyses: no Losses to follow‐up: yes, 12 (12%) children in the follow‐up study at ages 10‐12 years (4 in the treatment arm and 8 in the control arm) and 21 (21%) adults in the follow‐up study at age 20 years (10 in the treatment arm and 11 in the control arm) | |
| Participants | Location: Department of Obstetrics and Gynaecology and Department of Neonatology, Wilhelmina Gasthuis, University of Amsterdam, Amsterdam, the Netherlands. Eligibility criteria: women with preterm labour in whom it was possible to delay delivery by at least 12 hours Gestational age range: 26‐32 weeks. Exclusion criteria: no contraindications to the use of corticosteroids or orciprenaline (insulin‐treated diabetes, hyperthyroidism, infection, severe hypertension, cardiac disease, marked fetal growth retardation or fetal distress) Total recruited: 104 women and 122 infants; 53 women and 64 infants in the treatment arm and 51 women and 58 infants in the control arm | |
| Interventions | The treatment group received 8 mg betamethasone phosphate and 6 mg betamethasone acetate IM repeated after 24 hours.
The control group received an identical placebo. All women received orciprenaline infusion and bed‐rest until 32 weeks. |
|
| Outcomes | Maternal outcomes (death, chorioamnionitis, maternal infections, fever after trial entry requiring antibiotics, intrapartum fever requiring antibiotics, postnatal fever, admission to ICU, side effects of therapy), fetal/neonatal outcomes (fetal death, neonatal death, RDS, IVH, birthweight, Apgar score < 7), childhood outcomes (weight, height, head circumference, lung function, visual impairment, hearing impairment, intellectual impairment, cerebral palsy, behavioural/learning difficulties) and adulthood outcomes were reported (weight, height, head circumference, blood pressure, intellectual impairment, age at puberty) | |
| Notes | Initial study report included a third arm of women (n = 133) and infants (n = 164) who had been excluded from randomisation because they were: 1. already in labour (n = 80) and could not be prolonged for at least 12 hours or were already 33 weeks' gestation, or; 2. (n = 53) contra‐indicated for corticosteroids, or; 3. wrongly excluded (n = 5). These women and infants are not included in the review. Two perinatal deaths in the corticosteroid treatment arm were excluded for: 1. intrauterine fetal death due to solutio placentae, and 2. death due to prolapsed umbilical cord. These deaths have been included in the analyses. Infections in infants are listed in Table 6 of the Schutte 1979 original report. There are deaths associated with these infections, and it is not clear when these infections or deaths occurred, or if they have been included in the reported numbers for neonatal or perinatal deaths. Study dates: April 1974‐April 1977 Funding sources: Dutch Foundation for Research on Prevention (Praeventiefonds Project 28‐1145), the Netherlands Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Low risk | Coded drug ampoules prepared by pharmacist |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial described as double blind, with pharmacist preparing identical treatment and control ampoules |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Staff were blinded to treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two perinatal deaths in the corticosteroids group were excluded. Data for infant infections specify additional deaths, and it is unclear whether or not these deaths are counted in the overall total for perinatal deaths. The inclusion of these deaths will not change the overall conclusions of meta‐analysis in favour of corticosteroid use. We are unclear as to the impact of exclusions on results, especially for the outcome of perinatal deaths. |
| Selective reporting (reporting bias) | Low risk | Primary outcome of the trial was RDS; this and other important outcomes are reported |
| Other bias | Low risk | Nothing to indicate any other sources of bias. |
Shanks 2010.
| Study characteristics | ||
| Methods | Type of study: RCT
Method of treatment allocation: not stated other than quote:"randomly assigned" Stratification: not stated Placebo: no Sample size calculation: yes Intention‐to‐treat analyses: no Losses to follow‐up: 7 (22%) women (3 in the study group and 4 in the control group) delivered within 7 days of their initial testing for fetal lung maturity and were excluded from the analysis |
|
| Participants | Location: Barnes‐Jewish Hospital, St Louis, Missouri, USA Eligibility criteria: singleton gestation, between 34 + 0 and 36 + 6 weeks' gestation, immature TDx‐FLM‐II test (< 45 mg/g) (this test measures surfactant to albumin ratio) after clinically indicated amniocentesis to test for fetal lung maturity. Gestational age range: 34 + 0 ‐36 + 6 weeks' gestation Exclusion criteria: multiple gestations, ruptured membranes, uncertain gestational ages, previous steroid treatment in current pregnancy, delivery before completing the steroid course, those unwilling or unable to comply with study protocol Total recruited: 32 women and infants; 13 women and infants in the treatment arm and 19 women and infants in the control arm | |
| Interventions | The treatment group received either 2 doses of betamethasone 12 mg IM 24 hours apart, or 4 doses of dexamethasone 6 mg IM 12 hours apart. The control group received no treatment. |
|
| Outcomes | Maternal outcomes (side effects of therapy in women) and fetal/neonatal outcomes (need for mechanical ventilation/CPAP, admission to NICU) | |
| Notes | This study was stopped early due to difficulties in participant recruitment. Study dates: May 2003‐May 2008 Funding sources: supported in part by a Clinical and Translational Science Award, and by a grant from the National Centre for Research Resources, a component of the National Institute of Health and NIH Roadmap for Medical Research Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"Randomly assigned" not further described |
| Allocation concealment (selection bias) | Unclear risk | Quote:"Sealed envelopes" not further described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control group received no treatment so blinding of participants and study personnel would not have been possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention is made of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7 (22%) women (3 in the study group and 4 in the control group) delivered within 7 days of their initial testing for fetal lung maturity and were excluded from the analysis. No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Hyaline membrane disease is listed as an outcome, but not reported |
| Other bias | High risk | This study was stopped early due to difficulties in patient recruitment |
Silver 1996.
| Study characteristics | ||
| Methods | Type of study: RCT Method of treatment allocation: computer‐generated randomisation sequence used Pharmacy provided identical syringes labelled with the woman's study number. Stratification: none stated Placebo: yes, normal saline Sample size calculation: yes Intention‐to‐treat analyses: no Losses to follow‐up: 124 women initially recruited, of whom 49 (40%) remained undelivered after 29 weeks and were not included in the review | |
| Participants | Location: Northwestern University Medical School, Chicago, Illinois, USA Eligibility criteria: women at risk of delivery between 24‐29 weeks Gestational age range: 24‐29 weeks Exclusion criteria: infection, maternal or fetal indications for urgent delivery Total recruited: 75 women and 96 infants; 39 women and 54 infants in the treatment arm and 36 women and 42 infants in the control arm | |
| Interventions | The treatment group received 4 doses of 5 mg dexamethasone IM 12 hours apart, repeated weekly if the women remained undelivered.
The control group received placebo. All infants born < 30 weeks received prophylactic surfactant at birth. |
|
| Outcomes | Maternal outcomes (chorioamnionitis, endometritis) and fetal/neonatal outcomes reported (neonatal death, RDS, chronic lung disease, IVH, small‐for‐gestational age, birthweight, necrotising enterocolitis) | |
| Notes | Those women undelivered after 29 weeks were eligible for corticosteroid outside the study protocol. These women and their infants are not included in the review as it was not possible to separate out control women who subsequently received corticosteroids Study dates: April 1990‐June 1994 Funding sources: not stated Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence used |
| Allocation concealment (selection bias) | Low risk | Pharmacy provided identical syringes labelled with the woman's study number. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote:"Clinical personnel and the patient were effectively blinded to study group assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The severity of RDS, and diagnosis of IVH were quote:"confirmed independently by chart reviews conducted by 1 of the authors blinded to study group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 49 (40%) of the 124 women initially recruited, remained undelivered after 29 weeks and were not included in the review. |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other sources of bias. |
Teramo 1980.
| Study characteristics | ||
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Coded drug boxes used. Stratification: none stated Placebo: yes, normal saline Sample size calculation: no Intention‐to‐treat analyses: yes Losses to follow‐up: no | |
| Participants | Location: University of Helsinki, Finland Eligibility criteria: women with preterm labour and cervical dilatation < 4 cm without progression of labour upon initial observation of up to 12 hours Gestational age range: 28 ‐35 weeks Exclusion criteria: pre‐eclampsia, diabetes Total recruited: 74 women and 80 infants; 36 women and 38 infants in the treatment arm and 38 women and 42 infants in the control arm | |
| Interventions | The treatment group received 2 doses of 12 mg betamethasone IM 24 hours apart. The control group received placebo. | |
| Outcomes | Fetal/neonatal outcomes reported (RDS, HPA axis function) | |
| Notes | Study dates: not stated in manuscript, the study is coded as 1980s for the review Funding sources: not stated Declarations of interest: not reported The study was discontinued early because the overall incidence of RDS was too low for any meaningful conclusions concerning the efficacy of prevention. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Coded drug boxes were used but it is not clear how they were coded, e.g. if they were sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is likely that participants were blinded due to the use of a placebo quote:"similar in appearance" to the corticosteroid. Blinding of study personnel was not described other than quote: "ampoules were administered to the patients using the double‐blind principle". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up or exclusions stated |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other risk of bias |
WHO 2020.
| Study characteristics | ||
| Methods | Type of study: multicountry, multicentre, individually‐randomised, parallel‐group, double‐blind, placebo‐controlled trial Method of treatment allocation: 1:1 Stratification: site‐stratified individual randomisation with balanced permuted blocks of size 10 were used Placebo: yes Sample size calculation: yes |
|
| Participants | Location: Bangladesh, India, Kenya, Nigeria and Pakistan Inclusion criteria: pregnant women (with confirmed live fetuses) who were at risk of preterm birth between 26 weeks 0 days and 33 weeks 6 days; birth planned or expected in the next 48 hours (following preterm prelabour rupture of membranes, spontaneous labor, or provider‐initiated preterm birth). Exclusion criteria: clinical signs of severe infection; major congenital fetal anomalies; concurrent or recent (within the past two weeks) use of systemic glucocorticoids; participation in another trial; or contraindication to glucocorticoids Total recruited: 2852 women (3070 infants) randomised (A 1429 women, 1544 infants; B 1423 women, 1526 infants) Gestational age range: between 26 weeks 0 days and 33 weeks 6 days |
|
| Interventions | Group A: 6 mg dexamethasone administered every 12 hours, to a maximum of four doses, or until hospital discharge or birth Group B: placebo administered every 12 hours, to a maximum of four doses, or until hospital discharge or birth Women were eligible for a repeat course if they had not given birth after seven completed days but still met inclusion criteria. The repeat course was identical to the first course, and the same as the initial allocation |
|
| Outcomes |
For the neonate:
For the woman:
|
|
| Notes | Study dates: December 2017 through November 2019 Funding sources: Quote:“This trial was primarily funded by the Bill and Melinda Gates Foundation (Grant OPP1136821). Additional support was provided by UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Sexual and Reproductive Health and Research; and Department of Maternal, Newborn, Child, Adolescent Health, and Ageing, of the World Health Organization, Geneva, Switzerland.” Declarations of interest: Quote:“The authors declare that they have no competing interests.” Trial stopped early: the Data Safety Monitoring Monitoring board "recommended the trial be stopped for infant mortality benefits, and strong evidence of a graded dose‐response effect, in the context of existing evidence of benefits of antenatal glucocorticoids." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:“The computer‐generated randomization sequence was prepared centrally at WHO” |
| Allocation concealment (selection bias) | Low risk | Quote:“All sites received serially numbered identical treatment packs containing 4mg/mL ampules of dexamethasone or placebo for two full courses” "The assignment schedule was stored at WHO. Once eligibility was confirmed and consent obtained, trained study staff randomized a woman by taking the next numbered treatment pack from the dispenser (which was designed to ensure packs were taken sequentially" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote:“Trial participants, care providers, and investigators were not aware of group assignments.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:“Trial participants, care providers, and investigators were not aware of group assignments.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition. Quote:“Over 99% of randomized women and infants completed follow‐up” |
| Selective reporting (reporting bias) | Low risk | All outcomes that were pre‐specified in the protocol are reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
CPAP: continuous positive airways pressure GDM: gestational diabetes mellitus HPA: hypothalamic‐pituitary‐adrenal ICU: intensive care unit IM: intramuscular ITT: intention‐to‐treat IQR: interquartile range IUGR: Iintrauterine growth restriction IV: intravenous IVH: intraventricular haemorrhage LMP: last menstrual period NICU: neonatal intensive care unit PIH: pregnancy induced hypertension PROM: premature rupture of membranes PPROM: prolonged premature rupture of membranes RCT: randomised controlled trial RDS: respiratory distress syndrome SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abuhamad 1999 | This abstract compares TRH + betamethasone with betamethasone + placebo. |
| Althabe 2015 | This is a trial of strategies to optimise use of corticosteroids. |
| Asnafei 2004 | This study is quasi‐experimental. |
| Butterfill 1979 | Randomised participants are combined with a non‐randomised cohort and cannot be analysed separately. |
| Dola 1997 | This abstract compares TRH + betamethasone with betamethasone + placebo. |
| Dude 2016 | Not an eligible comparison |
| Egerman 1998 | This trial compares oral vs IM dexamethasone in the prevention of RDS. It does not meet our entry criteria for inclusion of studies for the review. |
| Garite 1981 | This trial compares a policy of corticosteroid therapy followed by elective delivery with a policy of withholding corticosteroids and awaiting delivery so the independent effect of the 2 co‐interventions cannot be evaluated separately. |
| Grgic 2003 | Not a randomised trial. Outcomes for women who received steroids were compared with those that did not. Information obtained from translation sheet. Original article in Bosnian. |
| Halac 1990 | Not a randomised trial. Women were allocated to placebo if they were expected to deliver within 24 hours and to betamethasone if labour was not expected within 24 hours. |
| Iams 1985 | Corticosteroid therapy (hydrocortisone) and co‐intervention of elective delivery was compared to expectant management in PROM. The independent effect of the 2 co‐interventions cannot be evaluated separately. |
| Khandelwal 2012 | Compared different doses of corticosteroid: 12‐hourly vs 24‐hourly. The study includes a repeat dose of corticosteroids and is eligible for inclusion in a different review, 'Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes' Crowther 2015. |
| Koivisto 2007 | The study includes a repeat dose of corticosteroids and is eligible for inclusion in a different review, 'Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes' Crowther 2015. |
| Kuhn 1982 | Randomised participants are combined with a non‐randomised cohort and cannot be analysed separately. |
| Kurtzman 2008 | The study includes a repeat dose of corticosteroids and is eligible for inclusion in a different review, 'Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes' Crowther 2015. All women received corticosteroids before the beginning of the trial. |
| Liu 2006 | Quasi‐randomised study that allocated women according to the in‐patient sequence. Compared the effect of dexamethasone combined with vitamin K, dexamethasone alone and no dexamethasone or vitamin K on periventricular/intraventricular haemorrhage. |
| Magee 1997 | This study compares the effects of betamethasone vs dexamethasone on antenatal fetal heart rate. |
| Maksic 2008 | This study appears to be an observational study of 163 premature infants, 80 of whom were exposed to antenatal corticosteroids, and 83 of whom were not. |
| McEvoy 2010 | This trial compares repeat dose corticosteroids and is eligible for inclusion a different review, 'Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes' Crowther 2015. |
| Minoui 1998 | This study compares the effects of betamethasone vs dexamethasone on antenatal fetal heart rate. |
| Morales 1986 | Quasi‐randomised using medical record number. |
| Mulder 1997 | This study compares the effects of betamethasone vs dexamethasone on antenatal fetal heart rate. |
| NCT02351310 2015 | Trial status: quote: "Withdrawn (Company decided not to pursue this study.)" Seems to have recruited no participants. |
| NCT04494529 2020 | Ineligible comparator |
| Papageorgiou 1979 | Ineligible intervention: weekly repeats of betamethasone. |
| Romejko‐Wolniewicz 2013 | This is a head‐to‐head trial of 2 different regimens and is eligible for the Cochrane Review entitled 'Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth' Brownfoot 2013. |
| Rotmensch 1999 | This study compares the effects of betamethasone vs dexamethasone on antenatal fetal heart rate. |
| Simpson 1985 | Quasi‐randomised study. Randomised participants are combined with a non‐randomised cohort and cannot be analysed separately. |
| Whitt 1976 | This trial compares IM betamethasone with IV cortisol. It does not meet our entry criteria for inclusion of studies for the review. |
IM: intramuscular IV: intravenous PROM: premature rupture of membranes RDS: respiratory distress syndrome TRH: thyrotropin‐releasing hormone vs: versus
Characteristics of studies awaiting classification [ordered by study ID]
Cararach 1991.
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Stratification: none stated Placebo: no Sample size calculation: no Intention‐to‐treat analyses: yes Losses to follow‐up: no Funding: FIS; Perinatal Section of SEGO |
| Participants | Location: Hospital Clinic, University of Barcelona, Spain Timeframe: 1987‐1990 Eligibility criteria: women with PROM Gestational age range: 28‐30 weeks Exclusion criteria: none stated Total recruited: 18 women and infants; 12 women and infants in the treatment arm and 6 women and infants in the control arm |
| Interventions | Type and dose of corticosteroid used in the treatment group is not stated Control group received expectant management |
| Outcomes | Fetal/neonatal outcome reported (RDS) |
| Notes | Abstract only: not included in 2020 update of review because there are no contact details available for the trialists and no way to confirm that the data presented in the abstract are the final data. |
Carlan 1991.
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Stratification: none stated Placebo: no Sample size calculation: no Intention‐to‐treat analyses: no Losses to follow‐up: yes, 2 (8%) infants with documented pulmonary maturity and 5 (17%) women with subsequent sealed membranes were not analysed |
| Participants | Location: University of South Florida Medical School, Tampa, Florida, USA Eligibility criteria: women with PROM Gestational age range: 24‐34 weeks Exclusion criteria: not stated Total recruited: the number randomised to each group is not stated. Data are available on 24 women and infants; 13 women and infants in the treatment arm and 11 women and infants in the control arm |
| Interventions | 12 mg betamethasone IM repeated after 24 hours and weekly thereafter until delivery or 34 weeks. Control group received expectant management. |
| Outcomes | Maternal outcome (chorioamnionitis), fetal/neonatal outcomes (RDS, birthweight, days of mechanical ventilation/CPAP) and health service outcomes reported (days in NICU, neonatal days in hospital, neonatal hospital cost). However due to lack of SD data only chorioamnionitis and RDS data were included in the review. |
| Notes | This study included a third arm (12 mg betamethasone IM 24‐hourly for 2 doses and 400 mcg methylprednisolone IV 8‐hourly for 6 doses, repeated weekly until delivery or 34 weeks. The data for the review report the betamethasone and control arms only. Further information was requested from the study authors but there was no reply. Study dates: not stated in manuscript, the study is coded as 1990s for the review Funding sources: not stated Declarations of interest: not stated Abstract only: not included in 2020 update of review because there are no contact details available for the trialists and no way to confirm that the data presented in the abstract are the final data. |
Delibas 2017.
| Methods | Two arm, parallel RCT Setting: Obstetrics and Gynecology Department at the Ataturk University School of Medicine, Turkey |
| Participants | Inclusion criteria: 28–34th gestational week, systolic blood pressure of 140 mmHg, to 160 mmHg, diastolic blood pressure of 90 mmHg, to 100 mmHg, proteinuria 300 mg and < 5 g in 24‐hour urine or ≥1 proteinuria in spot urine test, and diagnosis of mild preeclampsia Exclusion criteria: karyotypic suspicion of anatomic defects, intrauterine growth retardation, or ruptured membranes, and those under treatment with tocolytic agents, magnesium sulphate, or benzodiazepines that may affect BPP and Doppler parameters Number randomised: 40 (20 to each group) |
| Interventions | Experimental: betamethasone 12 mg, two doses, 24 hours apart. Control: placebo (saline). Same volume as intervention group. Followed up until 72 hours after drug administration |
| Outcomes | Fetal biophysical profile score Doppler measurements of arteries |
| Notes | Study dates: May 2009 and September 2010 Declarations of interest: "All authors declare that they have no conflict of interest. The authors alone are responsible for the content and writing of this article" Funding sources: not reported Not included in 2020 update: contacted authors in September and October 2020 to query prospective trial registration. |
Doran 1980.
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Coded drug boxes were provided. Randomisation code was kept on file at the Pharmacy Department of Toronto General Hospital. Stratification: yes, by gestational age into 2 subgroups; 24‐32 weeks and 33‐34 weeks Placebo: yes, vehicle of steroid preparation consisting of 0.2 mg benzalkonium chloride and 0.1 mg disodium edentate per mL Sample size calculation: no Intention‐to‐treat analyses: yes Losses to follow‐up: no Funding: |
| Participants | Location: 6 teaching hospitals in Toronto, Canada Eligibility criteria: women with PROM, spontaneous preterm labour or planned preterm delivery Gestational age range: 24 and 34 weeks. Exclusion criteria: women with pre‐eclampsia or in whom steroids were contraindicated on medical grounds. Total recruited: 137 women and 144 infants; 75 women and 81 infants in the treatment arm and 62 women and 63 infants in the control arm |
| Interventions | 4 doses of 3 mg betamethasone acetate and 3 mg betamethasone sodium phosphate IM 12 hours apart Control group received 4 doses of identical placebo |
| Outcomes | Fetal/neonatal outcomes were reported (fetal death, neonatal death, RDS, IVH, birthweight, days of mechanical ventilation) |
| Notes | Study dates: January 1975‐June 1978 Funding sources: The Hospital for Sick Children Foundation, Canada; Schering Corporation, Canada; Ontario Ministry of Health Provincial Research Grant PR 279, Canada Not included in 2020 update: no contact details available, unable to get details of randomisation process to explain why 75 were allocated to intervention and 62 to placebo. |
Goodner 1979.
| Methods | Type of study: RCT (abstract)
Method of treatment allocation: not described Stratification: not described Placebo: yes, saline Sample size calculation: not stated Intention‐to‐treat analyses: not stated Losses to follow‐up: not stated |
| Participants | Location: Temple University Hospital, Philadelphia, Pennsylvania, USA Eligibility criteria: any pregnant woman expected to deliver prior to 34 weeks' gestation between July 1976 and July 1978 at Department of Obs & Gyne at Temple University Hospital Gestational age range: prior to 34 weeks Exclusion criteria: not stated Total recruited: 45 placebo, 47 steroids |
| Interventions | Treatment group received an IM injection of betamethasone. The control group received an IM injection of saline as placebo. |
| Outcomes | Neonatal mortality, RDS |
| Notes | Study dates: July 1976‐July 1978 Funding sources: not stated Declarations of interest: not stated Abstract only: not included in 2020 update of review because there are no contact details available for the trialists and no way to confirm that the data presented in the abstract are the final data. |
Khazardoust 2012.
| Methods | Type of study: double‐blind RCT
Method of treatment allocation: computer generated Stratification: none stated Placebo: yes, placebo‐controlled Sample size calculation: not described Intention‐to‐treat analyses: no, 5 (13%) of participants in the intervention arm were excluded from analysis post randomisation Losses to follow‐up: yes, as above |
| Participants | Location: obstetric emergency department of Vali‐e‐Asr,Hospital, Tehran, Iran Eligibility criteria: patients at risk of preterm labor as determined by routine ultrasound examination in the first trimester Gestational age range: 34‐37 weeks Exclusion criteria: only primigravid women with signs of preterm labour were eligible, including quote: "palpable uterine contractions every 5‐8 minutes and Bishop score of 4 and higher associated with cervical dilatation of more than 1 cm and at least 50% of effacement." quote: "Women with systemic diseases, maternal hypertension before or during pregnancy, uterine tenderness, chorioamnionitis signs, symptomatic vaginal infection, rupture of membranes, current use of antibiotics, induced pregnancy, and history of smoking were excluded." Total recruited: 80 women and 80 infants; 40 women and 40 infants in the treatment arm and 40 women and 40 infants in the control arm |
| Interventions | The treatment group received 2 doses of 12 mg betamethasone IM. The control group received placebo of saline as per regimen above. |
| Outcomes | No outcomes available for the review |
| Notes | Data are provided on endocervical cytokine levels in women who delivered within and after 1 week but no outcome data available for the review are presented Study dates: June 2006 to July 2010 Funding sources: quote: "The study was supported by Tehran University of Medial Sciences. The assays were performed At Shahed University of Medical Sciences which we would like to thanks the staff and co‐operation of that center in this study." Declarations of interest: not reported Not included in 2020 update: no protocol or prospective registration |
Mirzamoradi 2019.
| Methods | Two‐arm, parallel RCT Setting: Shahid Beheshti University of Medical Sciences, Iran |
| Participants | Inclusion criteria: Women with a single pregnancy at 34–36 weeks and 6 days of gestation, with a high probability of late preterm delivery Exclusion criteria: those with dilatation of 4 cm or more, known congenital malformations, antenatal administration of glucocorticoids before the 34th week, fetal death, major fetal anomalies or non survivable fetus, administration of systemic corticosteroid due to other indications, gestational diabetes, prepregnancy diabetes mellitus, maternal contraindication for betamethasone, chorioamnionitis, unwillingness to participate in the study or/and participation in other intervention study Number randomised: 240 (120 per group) Number analysed: 240 women |
| Interventions | Experimental: 12 mg of betamethasone in two doses with an interval of 24 hours Control: no treatment |
| Outcomes | Primary outcome: composite endpoint describing the need for respiratory support by 72 hours of age consisting of one or more of the following continuous positive airway pressure (CPAP) or high flow nasal cannula (HFNC) for at least two consecutive hours, respiratory distress syndrome or need for mechanical ventilation Birthweight First minute Agpar score Fifth minute Agpar score Need for resuscitation Need for oxygen for more than one hour Need for CPAP or continuous high‐flow nasal canula oxygen therapy Need for continuous high oxygen with FiO2 more than 30% Mechanical ventilation Extracorporeal membrane oxygenation RDS Transient tachypnoea of the newborn Apnoea Bronchopulmonary dysplasia Pneumonia Need for surfactant Umbilical cord blood pH Admission to NICU Duration of NICU admission Duration of hospitalisation in neonatal ward after NICU |
| Notes | Study dates: January 2017 to July 2017 Declarations of interest: quote: "No potential conflict of interest was reported by the authors" Funding sources: not reported Not included in 2020 update: contacted authors in September and October 2020 to query prospective trial registration; awaiting reply. |
Parsons 1988.
| Methods | Type of study: RCT Method of treatment allocation: method of randomisation not stated. Stratification: none stated Placebo: no Sample size calculation: no Intention‐to‐treat analyses: yes Losses to follow‐up: no |
| Participants | Location: University of Illinois, Chicago, USA Eligibility criteria: women with PROM and < 4 cm of cervical dilatation Gestational age range: 25‐32 weeks Exclusion criteria: infection, fetal distress, fetal anomalies, contraindication to tocolysis Total recruited: 45 women and infants; 23 women and infants in the treatment arm and 22 women and infants in the control arm |
| Interventions | The treatment group received 2 doses of 12 mg betamethasone IM 12 hours apart repeated weekly until 32 weeks. The control group received expectant management. |
| Outcomes | Fetal/neonatal outcomes reported (fetal death, neonatal death, RDS, systemic infection in the first 48 hours of life, proven neonatal infection while in NICU) |
| Notes | Study dates: not stated in manuscript, the study is coded as 1980s for the review Funding: not stated Declarations of interest: not stated Abstract only: not included in 2020 update of review because there are no contact details available for the trialists and no way to confirm that the data presented in the abstract are the final data. |
Taeusch 1979.
| Methods | Type of study: RCT
Method of treatment allocation: method of randomisation not stated. Coded drug boxes used Stratification: yes, by gestational age at entry Placebo: yes, normal saline Sample size calculation: yes Intention‐to‐treat analyses: no Losses to follow‐up: yes, data not available for maternal outcomes on 4 women (2 in each treatment arm) |
| Participants | Location: 2 hospitals in Boston, USA Eligibility criteria: women with preterm labour, PROM or with cervical dilatation < 5 cm at 33 weeks or less and women with an L/S ratio < 2 if > 33 weeks or who had a previous infant with RDS Gestational age range: not stated Exclusion criteria: indication for immediate delivery, obstetrician objection, pre‐eclampsia, previously received corticosteroids Total recruited: 122 women and 127 infants recruited |
| Interventions | The treatment group received 6 doses of 4 mg dexamethasone phosphate IM 8 hours apart. The control group received placebo. |
| Outcomes | Maternal outcomes (endometritis, fever after trial entry requiring antibiotics) and fetal/neonatal outcomes reported (fetal death, neonatal death, RDS, chronic lung disease, IVH, proven neonatal infection while in NICU) |
| Notes | Study authors contacted for further information but there was no reply Study dates: January 1975‐March 1977 Funding sources: not stated Not included in 2020 update: no contact details available, unable to get details of randomisation process to explain imbalance between groups (56 infants in intervention group and 71 in placebo group. |
BPP: biophysical profile CPAP: continuous positive airways pressure IM: intramuscular IV: intravenous IVH: intraventricular haemorrhage NICU: neonatal intensive care unit PROM: premature rupture of membranes RCT: randomised controlled trial RDS: respiratory distress syndrome SD: standard deviation
Characteristics of ongoing studies [ordered by study ID]
ACTRN12617001494325 2017.
| Study name | The WHO ACTION‐II (Antenatal CorticosTeroids for Improving Outcomes in preterm Newborns) Trial |
| Methods | Randomised controlled trial Setting: hospitals in Bangladesh, India, Kenya, Nigeria, Pakistan Participants, care givers, outcome assessors and data analysts will all be blinded |
| Participants | Inclusion criteria
Exclusion criteria
Target sample size: 22,589 women |
| Interventions | Experimental group: a single course of 6 mg dexamethasone by intramuscular injection, administered every 12 hours, to a total of four (4) doses (time points 0 hours, 12 hours, 24 hours and 36 hours). If the full regimen is completed, the woman would have received a total of 24 mg in divided doses. No repeat course(s) will be used. Control group: Identical placebo (normal saline), administered according to the same regimen as for dexamethasone, with one injection every 12 hours to a total of four doses. Follow‐up of enrolled women and newborns to 28 days postpartum/postnatal |
| Outcomes | Neonatal death (death of a liveborn within 28 completed days of life) Stillbirth or neonatal death (any death of a fetus (post enrolment), or death of a live birth within 28 completed days of life among all enrolled participants. Possible maternal bacterial infection (occurrence of maternal fever, or clinically suspected or confirmed infection, for which therapeutic antibiotics were used) Stillbirth Early neonatal death Neonatal sepsis Severe intraventricular haemorrhage (sIVH) Neonatal hypoglycaemia Apgar score <7 at 5 minutes Maternal death Maternal fever (greater than or equal to 38.0 C ) Chorioamnionitis Postpartum endometritis Wound infection Non‐obstetric infection Major neonatal resuscitation at birth Timing of breast milk feeding initiation Timing to full enteral feeding Use of oxygen therapy Continuous positive airway pressure (CPAP) ventilation Mechanical ventilation Use of therapeutic antibiotics for 5 or more days Surfactant treatment Length of newborn's hospital stay after birth Admission of newborn to a special newborn care unit Newborn readmission for healthcare at facility Maternal therapeutic antibiotic use Any use of antibiotics in an enrolled participant (maternal) while in facility (prophylactic or therapeutic) Length of total maternal hospitalisation for birth Maternal readmission for healthcare at facility Any maternal referral to another facility Compliance with study allocation Total number of treatment or placebo doses received Time from initiation of first dose until birth Gestational age at birth |
| Starting date | Registered October 2017 (prospectively registered) Last update: October 2010 |
| Contact information | Dr A. Metin Gulmezoglu gulmezoglum@who.int |
| Notes | Funding sources: Bill and Melinda Gates Foundation |
Hong 2019.
| Study name | Effects of antenatal corticosteroids in twin neonates with late preterm birth (ACTWIN [Antenatal Corticosteroids in TWIN late preterm neonates] trial): study protocol for a randomized controlled trial |
| Methods | Multicentre, randomised, double‐blind placebo‐controlled trial. Participant and care provider will be blinded. Setting: obstetric departments of two hospitals in South Korea, Seoul National University Hospital and Cheil General Hospital and Women’s Healthcare Centre |
| Participants | Estimated enrolment: 808 participants Inclusion Criteria
Exclusion Criteria
|
| Interventions | Intervention women will receive intramuscular betamethasone 12 mg (3 mL) twice in a 24‐hour interval. Comparator: women will receive 3 mL of normal saline twice in a 24‐hour interval. |
| Outcomes | Primary outcomes
Secondary outcomes
Other Outcome Measures Necrotising enterocolitis Birth weight 1‐minute, 5‐minute Apgar score Hypoglycaemia up to 28 days after birth Hyperbilirubinaemia up to 28 days after birth Feeding difficulty up to 28 days after birth Neonatal infectious morbidity up to 28 days after birth Seizures/encephalopathy up to 28 days after birth Hospital day of NICU admission up to 28 days after birth |
| Starting date | May 2018 Estimated completion date: December 2022 |
| Contact information | Seung Mi Lee smleemd@hanmail.net |
| Notes |
IRCT2014102919037N2 2014.
| Study name | Evaluation of the effect of betamethasone onnNeonatal outcomes in pregnancies |
| Methods | Randomised, double‐blind, parallel group Setting: Kosar Obstetric and Gynecology Hospita, Qazvin, Iran |
| Participants | Target sample size: 140 women Inclusion criteria: pregnant women aged between 18 to 39 years who are candidate for delivery in 34‐36th week of gestational age Exclusion criteria: delivery after 37th week of gestational age; diabetes; fetal anomalies; delivery before receiving the two doses of the drug; multiple pregnancy |
| Interventions | Group 1: betamethasone. Intramuscularly injection of 6 mL of betamethasone solution (4 mg/mL) (divided into two 3 mL injection with a 12‐hour interval) Group 2: placebo. Intramuscularly injection of 6 mL of normal saline solution (9 mg/mL) (divided into two 3 mL injection with a 12‐hour interval) |
| Outcomes | Respiratory distress syndrome Agpar Need for newborn admission |
| Starting date | Registered October 2014 ("registered while recruiting") |
| Contact information | Mahdieh Yousef‐Zanjani yusefi.mahdieh@yahoo.com Dr. Maryam Jafari dr.jafari1981@gmail.com |
| Notes |
NCT01206946 2010.
| Study name | Effect of antenatal steroids for women at risk of late preterm delivery on neonatal respiratory morbidity |
| Methods | Randomised parallel assignment Setting: various hospitals in Lebanon Blinding: participants, care provider, outcome assessor and investigator |
| Participants | Estimated recruitment: 700 participants Inclusion criteria: women between 34 0/7‐ 36 6/7 weeks of gestation, at high risk of preterm birth Exclusion criteria
|
| Interventions | Group 1: betamethasone: a single course of betamethasone (two doses of 12 mg/dose given at 24‐hourly intervals) Group 2: saline: two doses of 2 mL of normal saline given at 24‐hourly intervals |
| Outcomes | Primary outcome
Respiratory Distress Syndrome during first three days of life Secondary outcomes
|
| Starting date | Study start date: September 2010 Estimated completion date: September 2013 Last update was June 2011 (Status: recruiting) |
| Contact information | Principal Investigator: Khalid Yunis, MD American University of Beirut Medical Center |
| Notes |
NCT03446937 2018.
| Study name | Effect of antenatal corticosteroids on neonatal morbidity |
| Methods | Randomised parallel assignment three‐arm trial Blinding: participant, care provider, investigator Setting: Ahmadu Bello University Teaching Hospital Shika‐Zaria, Nigeria |
| Participants | 100 participants Inclusion criteria
Exclusion criteria
|
| Interventions | Group 1: dexamethasone sodium phosphate injection 12 mg X doses to be given 12 hours apart Group 2: betamethasone sodium phosphate injection12 mg X 2 doses given 12 hours apart Group 3: placebo. two doses of intramuscular injection of water for injection given 12 hours apart |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | Start date: December 2017 Completion date: May 2019 |
| Contact information | Anisah Yahya, MBBS, Ahmadu Bello University, Nigeria moc.oohay@yhasina |
| Notes |
ACS: antenatal corticosteroids NICU: neonatal intensive care unit
Differences between protocol and review
The methods have been updated to current standard methods text for the Cochrane Pregnancy and Childbirth Group.
The following subgroups were not pre‐specified in the protocol:
decade of trial;
gestational age at trial entry;
protocol with weekly repeats.
In the 2016 update, comparison one was re‐structured to include only the main analysis, with all clinical groups moved to subsequent comparisons. We have also deleted subgroups from previous versions of the review related to post‐randomisation variables (gestational age to delivery and ruptured membranes at specific time points). A 'Summary of findings' table has been incorporated in this update (2016).
We clarified the primary outcome of deaths (fetal/neonatal) to perinatal deaths. Neonatal deaths and fetal deaths are still presented separately as primary outcomes.
We renamed outcomes of mean length for children and adults as mean height.
In response to referee feedback we changed the name of the primary outcome 'puerperal sepsis' to 'endometritis (including infections)'. Most trials (7/10) in this analysis specifically reported endometritis.
2020 update
We used pre‐defined criteria to assess the trustworthiness of studies that otherwise meet the review's inclusion criteria. We put any studies that were assessed as untrustworthy into 'awaiting classification' and did not include them in the review.
To ensure a consistent approach in our analysis we applied the intention‐to‐treat principle for all outcomes related to the neonate/fetus, and we expanded our methods in this regard in the 'Dealing with missing data' section.
We amended our methods for assessing heterogeneity using up‐to‐date Cochrane methods.
We checked and re‐analysed the data relating to the intraventricular haemorrhage(IVH) to take into different methods of diagnosing IVH.
For the child and the child as adult, we removed the secondary outcomes relating to visual impairment, hearing impairment, developmental delay and intellectual impairment since these are all included in the primary outcome of neurodevelopmental disability.
We added two outcomes to the 'Summary of findings' table ‐ neonatal death and neurodevelopmental disability ‐ because we believe these outcomes are important in presenting a more complete summary of the evidence.
Contributions of authors
For this update the contributions of each author are listed below.
Fiona Stewart assessed all trials for trustworthiness, extracted and entered data, assessed risk of bias, assessed certainty of evidence, drafted 'Summary of findings' tables, drafted text of the review. Emma McGoldrick assessed all trials for trustworthiness, extracted data, assessed risk of bias, re‐analysed intraventricular haemorrhage (IVH) data. Roses Parker, for a proportion of trials, extracted data, assessed risk of bias and assessed for trustworthiness. She also assessed certainty of evidence and drafted 'Summary of findings' tables. Stuart Dalziel reviewed all aspects of review and contributed to text.
Sources of support
Internal sources
University of Auckland, New Zealand
The University of Liverpool, UK
Liverpool Women's NHS Foundation Trust, UK
External sources
Harris‐Wellbeing of Women Preterm Birth Centre, UK
Cure Kids, New Zealand
Declarations of interest
Fiona Stewart: none known
Emma McGoldrick: none known
Roses Parker: none known
Stuart Dalziel reports receiving research funding, not associated with the review topic, from Cure Kids, Health Research Council, and Starship Foundation, New Zealand. Stuart Dalziel is employed by The University of Auckland and Auckland District Health Board. He is also on the board of the Advanced Paediatric Life Support New Zealand.
These authors contributed equally to this work
These authors contributed equally to this work
Edited (no change to conclusions)
References
References to studies included in this review
Amorim 1999 {published and unpublished data}
- Amorim MM, Santos LC, Faundes A. Corticosteroid therapy for prevention of respiratory distress syndrome in severe preeclampsia. American Journal of Obstetrics and Gynecology 1999;180(5):1283-8. [DOI] [PubMed] [Google Scholar]
Attawattanakul 2015 {published data only}
- Attawattanakul N, Tansupswatdikul P. Effects of antenatal dexamethasone on respiratory distress in late preterm infant: a randomized controlled trial. Thai Journal of Obstetrics and Gynaecology 2015;23:25-33. [Google Scholar]
Balci 2010 {published data only}
- Balci O, Ozdemir S, Mahmoud AS, Acar A, Colakoglu MC. The effect of antenatal steroids on fetal lung maturation between the 34th and 36th week of pregnancy. Gynecologic and Obstetric Investigation 2010;70(2):95-9. [DOI] [PubMed] [Google Scholar]
Block 1977 {published data only}
- Block MF, Kling OR, Crosby WM. Antenatal glucocorticoid therapy for the prevention of respiratory distress syndrome in the premature infant. Obstetrics & Gynecology 1977;50:186-90. [PubMed] [Google Scholar]
Collaborative 1981 {published data only}
- Bauer CR, Morrison JC, Poole WK, Korones SB, Boehm JJ, Rigatto H, et al. A decreased incidence of necrotizing enterocolitis after prenatal glucocorticoid therapy. Pediatrics 1984;73:682-8. [PubMed] [Google Scholar]
- Burkett G, Bauer CR, Morrison JC, Curet LB. Effect of prenatal dexamethasone administration on the prevention of respiratory distress syndrome in twin pregnancies. Journal of Perinatology 1986;6:304-8. [Google Scholar]
- Collaborative Group on Antenatal Steroid Therapy. Amniotic fluid phospholipids after maternal administration of dexamethasone. American Journal of Obstetrics and Gynecology 1983;145:484-90. [DOI] [PubMed] [Google Scholar]
- Collaborative Group on Antenatal Steroid Therapy. Effect of antenatal dexamethasone administration in the infant: long term follow-up. Journal of Pediatrics 1984;105:259-67. [DOI] [PubMed] [Google Scholar]
- *.Collaborative Group on Antenatal Steroid Therapy. Effect of antenatal dexamethasone administration on the prevention of respiratory distress syndrome. American Journal of Obstetrics and Gynecology 1981;141:276-87. [PubMed] [Google Scholar]
- Curet LB, Rao AV, Zachman RD, Morrison J, Burkett G, Poole K, et al. Maternal smoking and respiratory distress syndrome. American Journal of Obstetrics and Gynecology 1983;147:446-50. [DOI] [PubMed] [Google Scholar]
- Haning RV, Curet LB, Poole K, Boehnlein LM, Kuzma DL, Meier SM. Effects of fetal sex and dexamethasone on preterm maternal serum concentrations of human chorionic gonadotropin, progesterone, estrone, estradiol, and estriol. American Journal of Obstetrics and Gynecology 1989;161:1549-53. [DOI] [PubMed] [Google Scholar]
- Wiebicke W, Poynter A, Chernick V. Normal lung growth following antenatal dexamethasone treatment for respiratory distress syndrome. Pediatric Pulmonology 1988;5:27-30. [DOI] [PubMed] [Google Scholar]
- Zachman RD, Bauer CR, Boehm J, Korones SB, Rigatto H, Rao AV. Effect of antenatal dexamethasone on neonatal leukocyte count. Journal of Perinatology 1988;8:111-3. [PubMed] [Google Scholar]
- Zachman RD. The NIH multicenter study and miscellaneous clinical trials of antenatal corticosteroid administration. In: Farrell PM, editors(s). Lung Development: Biological and Clinical Perspectives. Vol. II. London & New York: Academic Press, 1982:275-96. [Google Scholar]
Dexiprom 1999 {published and unpublished data}
- Pattinson RC, Funk M, Makin JD, Ficki H. The effect of dexamethasone on the immune system of women with preterm premature rupture of membranes: a randomised controlled trial. In: 15th Conference on Priorities in Perinatal Care in Southern Africa; 1996 March 5-8; Goudini Spa, South Africa. 1996.
- *.Pattinson RC, Makin JD, Funk M, Delport SD, Macdonald AP, Norman K. The use of dexamethasone in women with preterm premature rupture of membranes: a multicentre double blind, placebo controlled randomised trial. South African Medical Journal 1999;89(8):865-70. [PubMed] [Google Scholar]
- Pattinson RC. A meta-analysis of the use of corticosteroids in pregnancies complicated by preterm premature rupture of membranes. South African Medical Journal 1999;89(8):870-3. [PubMed] [Google Scholar]
- The DEXIPROM Study Group. The use of dexamethasone in women with preterm premature rupture of membranes: a multicentre placebo controlled randomised controlled trial. In: 16th Conference on Priorities in Perinatal Care; 1997; South Africa. 1997:32-4.
Fekih 2002 {published data only}
- Fekih M, Chaieb A, Sboui H, Denguezli W, Hidar S, Khairi H. Value of prenatal corticotherapy in the prevention of hyaline membrane disease in premature infants. Randomized prospective study [Apport de la corticotherapie antenatale dans la prevention de la maladie des membranes hyalines chez le premature. Etude prospective randomisee]. Tunisie Medicale 2002;80(5):260-5. [PubMed] [Google Scholar]
Gamsu 1989 {published data only}
- Donnai P. UK multicentre trial of betamethasone for the prevention of respiratory distress syndrome. In: Proceedings of the 6th European Congress of Perinatal Medicine; 1978 Aug 29-Sept 1; Vienna, Austria. 1978:Abstract no: 81.
- *.Gamsu HR, Mullinger BM, Donnai P, Dash CH. Antenatal administration of betamethasone to prevent respiratory distress syndrome in preterm infants: report of a UK multicentre trial. British Journal of Obstetrics and Gynaecology 1989;96:401-10. [DOI] [PubMed] [Google Scholar]
Garite 1992 {published data only}
- *.Garite TJ, Rumney PJ, Briggs GG, Harding JA, Nageotte MP, Towers CV, et al. A randomized placebo-controlled trial of betamethasone for the prevention of respiratory distress syndrome at 24-28 weeks gestation. American Journal of Obstetrics and Gynecology 1992;166:646-51. [DOI] [PubMed] [Google Scholar]
- Garite TJ, Rumney PJ, Briggs GG. A randomized, placebo-controlled trial of betamethasone for the prevention of respiratory distress syndrome at 24-28 weeks gestation. Surgery, Gynecology and Obstetrics 1993;176:37. [DOI] [PubMed] [Google Scholar]
Gyamfi‐Bannerman 2016 {published data only}
- Battarbee Ashley N. 41: fetal metabolic biomarkers after late preterm betamethasone and their association with neonatal hypoglycemia. American Journal of Obstetrics and Gynecology 2020;222(1):S35-S36. [CENTRAL: CN-02074433] [EMBASE: 2004455290] [Google Scholar]
- Bicocca MJ, Chen H-Y, Blackwell S, Sibai B, Mfmu N. 457: does prepregnancy weight or maternal BMI at betamethasone administration impact late preterm respiratory morbidity? American Journal of Obstetrics and Gynecology 2019;220(1):S306-S307. [CENTRAL: CN-01758135] [EMBASE: 2001405365] [DOI] [PubMed] [Google Scholar]
- Carpenter J, Jablonski K, Koncinsky J, Varner M, Joss-Moore L. Antenatal steroids do not affect splicing or DNA methylation of the glucocorticoid receptor gene in cord blood T-cells. Reproductive Sciences (Thousand Oaks, Calif.) 2017;24(Suppl 1):258A. [CENTRAL: CN-01363475] [EMBASE: 615322896] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty MS, Chen H-Y, Chauhan SP, Sibai BM. 464: time interval from betamethasone to delivery among women at risk for late preterm delivery. American Journal of Obstetrics and Gynecology 2019;220(1):S310-1. [CENTRAL: CN-01710941] [EMBASE: 2001405276] [Google Scholar]
- *.Gyamfi-Bannerman C, Thom EA, Blackwell SC, Tita AT, Reddy UM, Saade GR, et al. Antenatal betamethasone for women at risk for late preterm delivery. New England Journal of Medicine 2016;374(14):1311-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyamfi-Bannerman C, Zupancic JA, Sandoval G, Grobman WA, Blackwell SC, Tita AT, et al. Cost-effectiveness of antenatal corticosteroid therapy vs no therapy in women at risk of late preterm delivery: a secondary analysis of a randomized clinical trial. JAMA Pediatrics 2019;173(5):462-8. [CENTRAL: CN-01916423] [EMBASE: 626717642] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyamfi-Bannerman C. Antenatal late preterm steroids (ALPS): a randomized trial to reduce neonatal respiratory morbidity. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S2. [Google Scholar]
- Gyamfi-Bannerman C. Respiratory morbidity after betamethasone (BMZ) treatment in infants of women at risk for late preterm delivery (LPD). American Journal of Obstetrics and Gynecology 2018;218(1 Suppl 1):S353. [CENTRAL: CN-01450832] [EMBASE: 620309716] [Google Scholar]
- Gyamfi-Bannerman C. Respiratory morbidity in pregnancies at risk for late preterm delivery in relation to cord blood concentrations of betamethasone and cortisol. Reproductive Sciences (thousand Oaks, Calif.) 2016;23(Suppl 1):274A. [EMBASE: 72226697] [Google Scholar]
- NCT01222247. Antenatal late preterm steroids (ALPS): a randomized placebo-controlled trial. clinicaltrials.gov/ct2/show/NCT01222247 (Date first received: 14 October 2010).
- Purisch S, Handal-Orefice R, Ananth CV, Gyamfi-Bannerman C. Effect of BMI on neonatal respiratory outcomes in women at risk for late preterm birth. American Journal of Obstetrics and Gynecology. Conference: 38th Annual Meeting of the Society for Maternal-fetal Medicine: the Pregnancy Meeting. United States 2018;218(1 Suppl 1):S393-S394. [CENTRAL: CN-01450813] [EMBASE: 620309949] [Google Scholar]
- Viteri OA, Doty MS, Alrais MA, Sibai BM. 471: intended administration of antenatal late preterm steroids: is a single dose enough? American Journal of Obstetrics and Gynecology 2019;220(1):S315. [CENTRAL: CN-01710606] [EMBASE: 2001405752] [Google Scholar]
- Werner EF, Romano ME, Rouse DJ, Sandoval G, Gyamfi-Bannerman C, Blackwell SC, et al. Association of gestational diabetes mellitus with neonatal respiratory morbidity. Obstetrics and Gynecology 2019;133(2):349-53. [CENTRAL: CN-01788495] [EMBASE: 626098249] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kari 1994 {published data only}
- Eronen M, Kari A, Pesonen E, Hallman M. The effect of antenatal dexamethasone administration on the fetal and neonatal ductus arteriosus: a randomised double-blind study. American Journal of Diseases of Children 1993;147:187-92. [DOI] [PubMed] [Google Scholar]
- Kari MA, Akino T, Hallman M. Prenatal dexamethasone (DEX) treatment before preterm delivery and rescue therapy of exogenous surfactant- surfactant components and surface activity in airway specimens (AS). In: Proceedings of the 14th European Congress of Perinatal Medicine; 1994 June 5-8; Helsinki, Finland. 1994:Abstract no: 486.
- *.Kari MA, Hallman M, Eronen M, Teramo K, Virtanen M, Koivisto M, et al. Prenatal dexamethasone treatment in conjunction with rescue therapy of human surfactant: a randomised placebo-controlled multicenter study. Pediatrics 1994;93:730-6. [PubMed] [Google Scholar]
- Salokorpi T, Sajaniemi N, Hallback H, Kari A, Rita H, Wendt L. Randomized study of the effect of antenatal dexamethasone on growth and development of premature children at the corrected age of 2 years. Acta Paediatrica 1997;86:294-8. [DOI] [PubMed] [Google Scholar]
Lewis 1996 {published data only}
- Lewis D, Brody K, Edwards M, Brouillette RM, Burlison S, London SN. Preterm premature ruptured membranes: a randomized trial of steroids after treatment with antibiotics. Obstetrics & Gynecology 1996;88(5):801-5. [DOI] [PubMed] [Google Scholar]
Liggins 1972b {published and unpublished data}
- Dalziel SR, Fenwick S, Cundy T, Parag V, Beck TJ, Rodgers A, et al. Peak bone mass after exposure to antenatal betamethasone and prematurity: follow-up of a randomized controlled trial. Journal of Bone & Mineral Research 2006;21(8):1175-86. [DOI] [PubMed] [Google Scholar]
- Dalziel SR, Liang A, Parag V, Rodgers A, Harding JE. Blood pressure at 6 years of age after prenatal exposure to betamethasone: follow-up results of a randomized, controlled trial. Pediatrics 2004;114:e373-e377. [DOI] [PubMed] [Google Scholar]
- Dalziel SR, Lim VK, Lambert A, McCarthy D, Parag V, Rodgers A, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ 2005;331:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel SR, Lim VK, Lambert A, McCarthy D, Parag V, Rodgers A, et al. Psychological functioning and health-related quality of life in adulthood after preterm birth. Developmental Medicine and Child Neurology 2007;49(8):597-602. [DOI] [PubMed] [Google Scholar]
- Dalziel SR, Parag V, Harding JE. Blood pressure at 6 years of age following exposure to antenatal betamethasone. In: 7th Annual Congress of the Perinatal Society of Australia and New Zealand; 2003 March 9-12; Tasmania, Australia. 2003:P13.
- Dalziel SR, Rea HH, Walker NK, Parag V, Mantell C, Rodgers A, et al. Long term effects of antenatal betamethasone on lung function: 30 year follow up of a randomised controlled trial. Thorax 2006;61(8):678-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel SR, Walker NK, Parag V, Mantell C, Rea HH, Rodgers A, et al. Cardiovascular risk factors after exposure to antenatal betamethasone: 30-year follow-up of a randomised controlled trial. Lancet 2005;365:1856-62. [DOI] [PubMed] [Google Scholar]
- Dalziel SR, Walker NK, Parag V, Mantell C, Rea HH, Rodgers A, et al. Long-term effects of antenatal exposure to betamethasone: thirty year follow-up of a randomised controlled trial [abstract]. Pediatric Research 2004;55 Suppl:101. [Google Scholar]
- Dalziel SR, Walker NR, Parag V, Mantell C, Rea HH, Rodgers A, et al. Long-term effects of antenatal exposure to betamethasone: thirty year follow-up of a randomized controlled trial. In: Pediatric Academic Societies Annual Meeting; 2004 May 1-4; San Francisco, USA. 2004.
- Harding JE, Pang J, Knight DB, Liggins GC. Do antenatal corticosteroids help in the setting of preterm rupture of membranes? American Journal of Obstetrics and Gynecology 2001;184:131-9. [DOI] [PubMed] [Google Scholar]
- Howie RN, Liggins GC. Clinical trial of antepartum betamethasone therapy for prevention of respiratory distress in pre-term infants. In: Anderson AB, Beard RW, Brudenell JM, Dunn PM, editors(s). Pre-term Labour. London: RCOG, 1977:281-9. [Google Scholar]
- Howie RN, Liggins GC. Prevention of respiratory distress syndrome in premature infants by antepartum glucocorticoid treatment. In: Villee CA, Villee DB, Zuckerman J, editors(s). Respiratory Distress Syndrome. London & New York: Academic Press, 1973:369-80. [Google Scholar]
- Howie RN, Liggins GC. The New Zealand study of antepartum glucocorticoid treatment. In: Farrell PM, editors(s). Lung Development: Biological and Clinical Perspectives, II. Academic Press: London & New York, 1982:255-65. [Google Scholar]
- Howie RN. Pharmacological acceleration of lung maturation. In: Villee CA, Villee DB, Zuckerman J, editors(s). Respiratory Distress Syndrome. London & New York: Academic Press, 1986:385-96. [Google Scholar]
- *.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972;50:515-25. [PubMed] [Google Scholar]
- Liggins GC, Howie RN. Prevention of respiratory distress syndrome by antepartum corticosteroid therapy. In: Proceedings of Sir Joseph Barcroft Centenary Symposium, Fetal and Neonatal Physiology; 1972; UK. Cambridge University Press: UK, 1973:613-7.
- Liggins GC, Howie RN. Prevention of respiratory distress syndrome by maternal steroid therapy. In: Gluck L, editors(s). Modern Perinatal Medicine. Chicago: Yearbook Publishers, 1974:415-24. [Google Scholar]
- Liggins GC. Prenatal glucocorticoid treatment: prevention of respiratory distress syndrome. In: Moore TD , editors(s). Lung maturation and the prevention of hyaline membrane disease, report of 70th Ross Conference on Paediatric Research. Ross Labs, 1976:97-103.
- MacArthur B, Howie RN, DeZoete A, Elkins J, Liang AY. Long term follow up of children exposed to betamethasone in utero. In: Tejani N, editors(s). Obstetrical Events and Developmental Sequelae. CRC Press, 1989:81-9. [Google Scholar]
- MacArthur B, Howie RN, DeZoete A, Elkins J. Cognitive and psychosocial development of 4-year-old children whose mothers were treated antenatally with betamethasone. Pediatrics 1981;68:638-43. [PubMed] [Google Scholar]
- MacArthur B, Howie RN, DeZoete A, Elkins J. School progress and cognitive development of 6-year-old children whose mothers were treated antenatally with betamethasone. Pediatrics 1982;70:99-105. [PubMed] [Google Scholar]
Lopez 1989 {published data only}
- Lopez AL, Rojas RL, Rodriguez MV, Sanchez AJ. Use of corticoids in preterm pregnancy with premature rupture of membranes [Uso de los corticoides en embarazo pretermino con ruptura prematura de membranas]. Revista Colombiana de Obstetricia y Ginecologia 1989;40:147-51. [Google Scholar]
Mansouri 2010 {published data only}
- IRCT138901193666N1. Effect of antenatal betamethasone on prevention of respiratory distress syndrome among neonates with gestational age of 35-36 weeks. www.irct.ir (Date first received: 17 May 2010).
- *.Mansouri M, Seyedolshohadaei F, Company F, Setare S, Mazhari S. Effect of antenatal betamethasone on prevention of respiratory distress syndrome among neonates with gestational age of 35-36 weeks. Journal of Gorgan University of Medical Sciences 2010;12(3):18-23. [Google Scholar]
Morales 1989 {published data only}
- Morales WJ, Angel JL, O'Brien WF, Knuppel RA. Use of ampicillin and corticosteroids in premature rupture of membranes: a randomized study. Obstetrics & Gynecology 1989;73:721-6. [PubMed] [Google Scholar]
Morrison 1978 {published data only}
- Morrison JC, Schneider JM, Whybrew WD, Bucovaz ET. Effect of corticosteroids and fetomaternal disorders on the L:S ratio. Obstetrics & Gynecology 1980;56:583-90. [PubMed] [Google Scholar]
- Morrison JC, Schneider JM, Whybrew WD, Bucovaz ET. Effect of corticosteroids and fetomaternal disorders on the L:S ratio. Surgery, Gynecology and Obstetrics 1981;153:464. [PubMed] [Google Scholar]
- *.Morrison JC, Whybrew WD, Bucovaz ET, Scheiner JM. Injection of corticosteroids into mother to prevent neonatal respiratory distress syndrome. American Journal of Obstetrics and Gynecology 1978;131:358-66. [DOI] [PubMed] [Google Scholar]
Nelson 1985 {published data only}
- Nelson LH, Meis PJ, Hatjis CG, Ernest JM, Dillard R, Schey HM. Premature rupture of membranes: a prospective randomized evaluation of steroids, latent phase and expectant management. Obstetrics & Gynecology 1985;66:55-8. [PubMed] [Google Scholar]
Ontela 2018 {published data only}
- CTRI/2016/12/007570. Effct of antenatal corticosteroids on respiratory morbidity in late preterm newborns - a randomized controlled trial. http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2016/12/007570 2016. [CENTRAL: CN-01807588]
- Dorairajan G, Ontela V, Vishnu B, Chinnakali P. Effect of antenatal steroids on respiratory morbidity of late preterm new-borns: a randomized controlled trial. https://epostersonline.com/rcog2018/node/58?view=true (accessed 4 September 2020). [DOI] [PubMed]
- Dorairajan G, Ontella V, Bhat V, Chinnakali P. Effect of antenatal dexamethasone on respiratory morbidity of late preterm newborns: a randomized controlled trial. BJOG: an international journal of obstetrics and gynaecology 2018;125(Suppl 1):67-8. [CENTRAL: CN-01571886] [EMBASE: 621569896] [Google Scholar]
- *.Ontela V, Dorairajan G, Bhat VB, Chinnakali P. Effect of antenatal steroids on respiratory morbidity of late preterm newborns: a randomized controlled trial. Journal of Tropical Pediatrics 2018;64(6):531-8. [CENTRAL: CN-01991657] [EMBASE: 626300500] [PMID: ] [DOI] [PubMed] [Google Scholar]
Porto 2011 {published data only}
- Porto AM, Coutinho IC, Correia JB, Amorim MM. Effectiveness of antenatal corticosteroids in reducing respiratory disorders in late preterm infants: randomised clinical trial. BMJ 2011;342:d1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qublan 2001 {published data only}
- Qublan H, Malkawi H, Hiasat M, Hindawi IM, Al-Taani MI, Abu-Khait SA, et al. The effect of antenatal corticosteroid therapy on pregnancies complicated by premature rupture of membranes. Clinical & Experimental Obstetrics & Gynecology 2001;28(3):183-6. [PubMed] [Google Scholar]
Schmidt 1984 {published data only}
- Schmidt PL, Sims ME, Strassner HT, Paul RH, Mueller E, McCart D. Effect of antepartum glucocorticoid administration upon neonatal respiratory distress syndrome and perinatal infection. American Journal of Obstetrics and Gynecology 1984;148:178-86. [DOI] [PubMed] [Google Scholar]
Schutte 1980 {published data only}
- Dessens AB, Haas HS, Koppe JG. Twenty year follow up of antenatal corticosteroid treatment. Pediatrics 2000;105(6):1325. [DOI] [PubMed] [Google Scholar]
- Dessens AB, Smolders-de Haas H, Koppe JG. Twenty year follow up in antenatally corticosteroid-treated subjects. Prenatal and Neonatal Medicine 1998;3 Suppl 1:32. [Google Scholar]
- Schmand B, Neuvel J, Smolder-de Haas H, Hoeks J, Treffers PE, Koppe JG. Psychological development of children who were treated antenatally with corticosteroids to prevent respiratory distress syndrome. Pediatrics 1990;86:58-64. [PubMed] [Google Scholar]
- Schutte MF, Koppe JG, Treffers PE, Breur W. The influence of 'treatment' in premature delivery on incidence of RDS. In: Proceedings of the 6th European Congress of Perinatal Medicine; 1978 Aug 29-Sept 1; Vienna, Austria. 1978:Abstract no: 80.
- Schutte MF, Treffers PE, Koppe JG, Breur W, Filedt Kok JC. The clinical use of corticosteroids for the acceleration of fetal lung maturity [Klinische toepassing van corticosteroiden ter bevordering van de foetale long-rijpheid]. Nederlands Tijdschrift voor Geneeskunde 1979;123(11):420-7. [PubMed] [Google Scholar]
- *.Schutte MF, Treffers PE, Koppe JG, Breur W. The influence of betamethasone and orciprenaline on the incidence of respiratory distress syndrome in the newborn after preterm labour. British Journal of Obstetrics and Gynaecology 1980;87:127-31. [DOI] [PubMed] [Google Scholar]
- Smolders-de Haas H, Neuvel J, Schmand B, Treffers PE, Koppe JG, Hoeks J. Physical development and medical history of children who were treated antenatally with corticosteroids to prevent respiratory distress syndrome: a 10- to 12- year follow up. Pediatrics 1990;86(1):65-70. [PubMed] [Google Scholar]
Shanks 2010 {published data only}
- Shanks A, Gross G, Shim T, Allsworth J, Moga C, Sadovsky Y, et al. Antenatal steroids for enhancement of fetal lung maturity after 34 weeks: lung maturity and antenatal steroids (LUMAS) study. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Shanks A, Gross G, Shim T, Allsworth J, Sadovsky Y, Bildirici I. Administration of steroids after 34 weeks of gestation enhances fetal lung maturity profiles. American Journal of Obstetrics and Gynecology 2010;203(1):47.e1-47.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silver 1996 {published data only}
- Silver RK, Vyskocil CR, Solomon SL, Farrell EE, MacGregor SN, Neerhof MG. Randomized trial of antenatal dexamethasone in surfactant-treated infants delivered prior to 30 weeks of gestation. American Journal of Obstetrics and Gynecology 1995;172:254. [DOI] [PubMed] [Google Scholar]
- *.Silver RK, Vyskocil CR, Solomon SL, Ragin A, Neerhof MG, Farrell EE. Randomized trial of antenatal dexamethasone in surfactant-treated infants delivered prior to 30 weeks of gestation. Obstetrics & Gynecology 1996;87:683-91. [DOI] [PubMed] [Google Scholar]
Teramo 1980 {published data only}
- Teramo K, Hallman M, Raivio KO. Maternal glucocorticoid in unplanned premature labor. Pediatric Research 1980;14:326-9. [DOI] [PubMed] [Google Scholar]
WHO 2020 {published and unpublished data}
- ACTRN12617000476336. The WHO ACTION-I (Antenatal CorticosTeroids for Improving Outcomes in preterm Newborns) Trial: a multi-country, multi-centre, two-arm, parallel, double-blind, placebo-controlled, randomized trial of antenatal corticosteroids for women at risk of imminent birth in the early preterm period in hospitals in low-resource countries to improve newborn outcomes. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12617000476336 (first received 2017) 2017. [CENTRAL: CN-01812305] [DOI] [PMC free article] [PubMed]
- *.WHO ACTION Trials Collaborators. Antenatal dexamethasone for early preterm birth in low-resource countries. New England Journal of Medicine 2020;October 23:DOI: 10.1056/NEJMoa2022398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO ACTION Trials Collaborators. The World Health Organization ACTION-I (Antenatal CorTicosteroids for Improving Outcomes in preterm Newborns) Trial: a multi-country, multi-centre, two-arm, parallel, double-blind, placebo-controlled, individually randomized trial of antenatal corticosteroids for women at risk of imminent birth in the early preterm period in hospitals in low-resource countries. Trials 2019;20(1):507. [CENTRAL: CN-02010986] [EMBASE: 628924308] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abuhamad 1999 {published data only}
- Abuhamad A, Green G, Heyl P, Veciana M. The combined use of corticosteroids and thyrotropin releasing hormone in pregnancies with preterm rupture of membranes: a randomised double blind controlled trial. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S96. [Google Scholar]
Althabe 2015 {published data only}
- Althabe F, Belizan JM, Mazzoni A, Berrueta M, Hemingway-Foday J, Koso-Thomas M, et al. Antenatal corticosteroids trial in preterm births to increase neonatal survival in developing countries: study protocol. Reproductive Health 2012;9(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Althabe F, Belizan JM, McClure EM, Hemingway-Foday J, Berrueta M, Mazzoni A, et al. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster-randomised trial. Lancet 2015;385(9968):629-39. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RL, Thorsten VR, Althabe F, Saleem S, Garces A, Carlo WA, et al. The global network antenatal corticosteroids trial: impact on stillbirth. Reproductive Health 2016;13(1):68. [CENTRAL: CN-01368862] [EMBASE: 610555843] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01084096. Trial of the use of antenatal corticosteroids in developing countries. https://clinicaltrials.gov/ct2/show/NCT01084096 (first received 2010).
Asnafei 2004 {published data only}
- Asnafei N, Pourreza R, Miri SM. Pregnancy outcome in premature delivery of between 34-37 weeks and the effects of corticosteroid on it. Journal of the Gorgan University of Medical Sciences 2004;6(2):57-60. [Google Scholar]
Butterfill 1979 {published data only}
- Butterfill AM, Harvey DR. Follow-up study of babies exposed to betamethasone before birth. Archives of Disease in Childhood 1979;54:725. [Google Scholar]
Dola 1997 {published data only}
- Dola C, Nageotte M, Rumney P, Towers C, Asrat T, Freeman R, et al. The effect of antenatal treatment with betamethasone and thyrotropin releasing hormone in patients with preterm premature rupture of membranes. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S49. [Google Scholar]
Dude 2016 {published data only}
- Dude C, Dude A, Gilner J, Swamy G, Grotegut C. Predicting preterm delivery: using the MFMU BEARS trial data to optimize corticosteroid use in women at risk for preterm delivery. Reproductive Sciences (Thousand Oaks, Calif.) 2016;23(Suppl 1):188A-9A. [CENTRAL: CN-02011932] [Google Scholar]
Egerman 1998 {published data only}
- Egerman RS, Mercer B, Doss JL, Sibai BM. A randomized controlled trial of oral and intramuscular dexamethasone in the prevention of neonatal respiratory distress syndrome. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S19. [DOI] [PubMed] [Google Scholar]
- *.Egerman RS, Mercer BM, Doss JL, Sibai BM. A randomized, controlled trial of oral and intramuscular dexamethasone in the prevention of neonatal respiratory distress syndrome. American Journal of Obstetrics and Gynecology 1998;179(5):1120-3. [DOI] [PubMed] [Google Scholar]
- Egerman RS, Pierce WF 4th, Andersen RN, Umstot ES, Carr TL, Sibai BM. A comparison of the bioavailability of oral and intramuscular dexamethasone in women in late pregnancy. Obstetrics & Gynecology 1997;89(2):276-80. [DOI] [PubMed] [Google Scholar]
- Egerman RS, Walker RA, Doss JL, Mercer B, Sibai BM, Andersen RN. A comparison between oral and intramuscular dexamethasone in suppressing unconjugated estriol levels during the third trimester. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S182. [DOI] [PubMed] [Google Scholar]
- Egerman RS, Walker RA, Mercer BM, Doss JL, Sibai BM, Andersen RA. Comparison between oral and intramuscular dexamethasone in suppressing unconjugated estriol levels during the third trimester. American Journal of Obstetrics and Gynecology 1998;179(5):1234-6. [DOI] [PubMed] [Google Scholar]
Garite 1981 {published data only}
- Garite TJ, Freeman RK, Linzey EM, Braly PS, Dorchester WL. Prospective randomized study of corticosteroids in the management of premature rupture of the membranes and the premature gestation. American Journal of Obstetrics and Gynecology 1981;141:508-15. [DOI] [PubMed] [Google Scholar]
Grgic 2003 {published data only}
- Grgic G, Fatusic Z, Bogdanovic G. Stimulation of fetal lung maturation with dexamethasone in unexpected premature labor. Medicinski Arhiv 2003;57(5-6):291-4. [PubMed] [Google Scholar]
Halac 1990 {published data only}
- Halac E, Halac J, Begue EF, Casanas JM, Idiveri DR, Petit JF, et al. Prenatal and postnatal corticosteroid therapy to prevent neonatal necrotizing enterocolitis: a controlled trial. Journal of Pediatrics 1990;117:132-8. [DOI] [PubMed] [Google Scholar]
Iams 1985 {published data only}
- Iams JD, Talbert ML, Barrows H, Sachs L. Management of preterm prematurely ruptured membranes: a prospective randomized comparison of observation vs use of steroids and timed delivery. American Journal of Obstetrics and Gynecology 1985;151:32-8. [DOI] [PubMed] [Google Scholar]
Khandelwal 2012 {published data only}
- Khandelwal M, Chang E, Hansen C, Hunter K, Milcarek B. Betamethasone dosing interval -12 or 24 hours apart? American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S10-S11. [DOI] [PubMed] [Google Scholar]
- *.Khandelwal M, Chang E, Hansen C, Hunter K, Milcarek B. Betamethasone dosing interval: 12 or 24 hours apart? A randomized, noninferiority open trial. American Journal of Obstetrics and Gynecology 2012;206(3):201.e1-11. [DOI] [PubMed] [Google Scholar]
Koivisto 2007 {published data only}
- Koivisto M, Peltoniemi OM, Saarela T, Tammela O, Jouppila P, Hallman M. Blood glucose level in preterm infants after antenatal exposure to glucocorticoid. Acta Paediatrica 2007;96(5):664-8. [DOI] [PubMed] [Google Scholar]
Kuhn 1982 {published data only}
- Kuhn RJP, Speirs AL, Pepperell RJ, Eggers TR, Doyle LW, Hutchinson A. Betamethasone, albuterol and threatened premature delivery. Obstetrics & Gynecology 1982;60:403-8. [PubMed] [Google Scholar]
Kurtzman 2008 {published data only}
- Kurtzman J, Garite T, Clark R, Maurel K, The OCRN. Impact of a 'rescue course' of antenatal corticosteroids (ACS): a multi-center randomized placebo controlled trial. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S2. [DOI] [PubMed] [Google Scholar]
Liu 2006 {published data only}
- Liu J, Wang Q, Zhao JH, Chen YH, Qin GL. The combined antenatal corticosteroids and vitamin K therapy for preventing periventricular-intraventricular hemorrhage in premature newborns less than 35 weeks gestation. Journal of Tropical Pediatrics 2006;52(5):355-9. [DOI] [PubMed] [Google Scholar]
Magee 1997 {published data only}
- Magee LA, Dawes GS, Moulden M, Redman CW. A randomised controlled comparison of betamethasone with dexamethasone: effects on the antenatal fetal heart rate. British Journal of Obstetrics and Gynaecology 1997;104(11):1233-8. [DOI] [PubMed] [Google Scholar]
Maksic 2008 {published data only}
- Maksic H, Hadzagic-Catibusic F, Heljic S, Dizdarevic J. The effects of antenatal corticosteroid treatment on IVH-PVH of premature infants. Bosnian Journal of Basic Medical Sciences 2008;8(1):58-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
McEvoy 2010 {published data only}
- McEvoy C, Schilling D, Clay N, Spitale P, Durand M. Neurodevelopmental outcome and growth in infants randomized to a single rescue course of antenatal steroids. In: Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30-May 3; Denver, Colorado, USA. 2011:3829.270.
- *.McEvoy C, Schilling D, Peters D, Tillotson C, Spitale P, Wallen L, et al. Respiratory compliance in preterm infants after a single rescue course of antenatal steroids: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2010;202(6):544.e1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy C, Schilling D, Segel S, Spitale P, Wallen L, Bowling S, et al. Improved respiratory compliance in preterm infants after a single rescue course of antenatal steroids: a randomized trial. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy C, Schilling D, Spitale P, Gravett M, Durand M. Pulmonary function and respiratory outcomes at 12-24 months in preterm infants randomized to a single rescue course of antenatal steroids. In: Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1-4; Vancouver, Canada. 2010.
- McEvoy C, Schilling D, Spitale P, Wallen L, Segel S, Bowling S, et al. Improved respiratory compliance after a single rescue course of antenatal steroids: a randomized controlled trial. In: Pediatric Academic Societies Annual Meeting; 2007 May 5-8; Toronto, Canada. 2007.
- McEvoy C, Schilling D, Spitale P, Wallen L, Segel S, Bowling S, et al. Improved respiratory compliance after a single rescue course of antenatal steroids: a randomized controlled trial. In: Pediatric Academic Societies Annual Meeting; 2008 May 2-6; Honolulu, Hawaii. 2008.
- McEvoy C, Schilling D, Spitale P, Wallen P, Segel S, Bowling S, et al. Growth and respiratory outcomes after a single rescue course of antenatal steroids: a randomized trial. In: Pediatric Academic Societies Annual Meeting; 2009 May 2-5; Baltimore, USA. 2009.
- McEvoy CT, Schilling D, Segal S, Spitale P, Wallen L, Bowling S, et al. Improved respiratory compliance in preterm infants <34 weeks after a single rescue course of antenatal steroids. American Journal of Respiratory and Critical Care Medicine 2009;179:A4127 [Poster #423]. [Google Scholar]
Minoui 1998 {published data only}
- *.Minoui S, Ville Y, Senat M, Multon O, Fernandez H, Frydman R. Effect of dexamethasone and betamethasone on fetal heart rate variability in preterm labour: a randomized study. British Journal of Obstetrics and Gynaecology 1998;105:749-55. [DOI] [PubMed] [Google Scholar]
- Minoui S, Ville Y, Senat MV, Multon O, Fernandez H, Frydman R. Effect of dexamethasone and betamethasone on fetal heart rate variability in preterm labor a randomized study. Prenatal and Neonatal Medicine 1996;1 Suppl 1:156. [DOI] [PubMed] [Google Scholar]
Morales 1986 {published data only}
- Morales WJ, Diebel D, Lazar AJ, Zadrozny D. The effect of antenatal dexamethasone administration on the prevention of respiratory distress syndrome in preterm gestations with premature rupture of membranes. American Journal of Obstetrics and Gynecology 1986;154:591-5. [DOI] [PubMed] [Google Scholar]
Mulder 1997 {published data only}
- Mulder EJ, Derks JB, Visser GH. Antenatal corticosteroid therapy and fetal behaviour: a randomised evaluation of betamethasone and dexamethasone. British Journal of Obstetrics and Gynaecology 1997;104(11):1239-47. [DOI] [PubMed] [Google Scholar]
NCT02351310 2015 {published data only}
- NCT02351310. Effectiveness of ACS in extreme preemies [Effects of antenatal corticosteroids in patients with early (22 - 23w6d) threatened preterm birth]. Https://clinicaltrials.gov/show/NCT02351310 (first received 2015 Jan 27). [CENTRAL: CN-01551840]
NCT04494529 2020 {published data only}
- NCT04494529. Single Dose Antenatal Corticosteroids (SNACS) for women at risk of preterm birth [Single Dose Antenatal Corticosteroids (SNACS) pilot randomized control trial for women at risk of preterm birth]. https://clinicaltrials.gov/show/NCT04494529 (first received 2020 Jul 31). [CENTRAL: CN-02145746]
Papageorgiou 1979 {published data only}
- Papageorgiou AN, Desgranges MF, Masson M, Colle E, Shatz R, Gelfand MM. The antenatal use of betamethasone in the prevention of respiratory distress syndrome: a controlled blind study. Pediatrics 1979;63:73-9. [PubMed] [Google Scholar]
Romejko‐Wolniewicz 2013 {published data only}
- Romejko-Wolniewicz E, Oleszczuk L, Zareba-Szczudlik J, Czajkowski K. Dosage regimen of antenatal steroids prior to preterm delivery and effects on maternal and neonatal outcomes. Journal of Maternal-Fetal and Neonatal Medicine 2013;26(3):237-41. [DOI] [PubMed] [Google Scholar]
Rotmensch 1999 {published data only}
- Rotmensch S, Liberati M, Vishne T, Celentano C, Ben-Rafael Z, Bellati U. The effects of betamethasone versus dexamethasone on computer-analysed fetal heart rate characteristics: a prospective randomized trial. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S185. [Google Scholar]
- *.Rotmensch S, Liberati M, Vishne TH, Celentano C, Ben-Rafael Z, Bellati U. The effect of betamethasone and dexamethasone on the fetal heart rate patterns and biophysical activities. A prospective randomized trial. Acta Obstetricia et Gynecologica Scandinavica 1999;78(6):493-500. [PubMed] [Google Scholar]
Simpson 1985 {published data only}
- Simpson G, Harbert G. Use of beta-methasone in management of preterm gestation with premature rupture of membranes. Obstetrics & Gynecology 1985;66:168-75. [PubMed] [Google Scholar]
Whitt 1976 {published data only}
- Whitt GG, Buster JE, Killam AP, Scragg WH. A comparison of two glucocorticoid regimens for acceleration of fetal lung maturation in premature labor. American Journal of Obstetrics and Gynecology 1976;124:479-82. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Cararach 1991 {published data only}
- Botet F, Cararach V, Sentis J. Premature rupture of membranes in early pregnancy. Neonatal prognosis. Journal of Perinatal Medicine 1994;22:45-52. [DOI] [PubMed] [Google Scholar]
- *.Cararach V, Botet F, Sentis J, Carmona F. A multicenter, prospective randomized study in premature rupture of membranes (PROM). Maternal and perinatal complications. International Journal of Gynecology and Obstetrics 1991;36 Suppl:267. [Google Scholar]
- Cararach V, Sentis J, Botet F, De Los Rios L. A multicenter, prospective randomized study in premature rupture of membranes (PROM). Respiratory and infectious complications in the newborn. In: Proceedings of the 12th European Congress of Perinatal Medicine; 1990; Lyon, France. 1990:216.
Carlan 1991 {published data only}
- Carlan SJ, Parsons M, O'Brien WF, Krammer J. Pharmacologic pulmonary maturation in preterm premature rupture of membranes. American Journal of Obstetrics and Gynecology 1991;164:371. [Google Scholar]
Delibas 2017 {published data only}
- Delibas IB, Ingec M, Yapca OE. Does antenatal betamethasone have negative effects on fetal activities and hemodynamics in cases of preeclampsia without severe features? A prospective, placebo-controlled, randomized study. Journal of Maternal-fetal & Neonatal Medicine 2016 Nov;30(22):2671-8. [CENTRAL: CN-01602216] [PMID: ] [DOI] [PubMed] [Google Scholar]
Doran 1980 {published data only}
- Doran TA, Swyer P, MacMurray B, Mahon W, Enhorning G, Bernstein A, et al. Results of a double blind controlled study on the use of betamethasone in the prevention of respiratory distress syndrome. American Journal of Obstetrics and Gynecology 1980;136:313-20. [DOI] [PubMed] [Google Scholar]
Goodner 1979 {published data only}
- Goodner DM. Antenatal steroids in the treatment of respiratory distress syndrome. In: 9th World Congress of Gynecology and Obstetrics; 1979 October 26-31; Tokyo, Japan. 1979:362.
Khazardoust 2012 {published data only}
- Hantoushzadeh S, Javadian P, Salmanian B, Ghazanfari T, Kermani A, Abbasalizadeh F, et al. Betamethasone effects on the endocervical inflammatory cytokines in preterm labor: a randomized clinical trial. International Immunopharmacology 2011;11(8):1116-9. [DOI] [PubMed] [Google Scholar]
- *.Khazardoust S, Javadian P, Salmanian B, Zandevakil F, Abbasalizadeh F, Alimohamadi S, et al. A clinical randomized trial on endocervical inflammatory cytokines and Betamethasone in prime-gravid pregnant women at risk of preterm labor. Iranian Journal of Immunology 2012;9(3):199-207. [PubMed] [Google Scholar]
Mirzamoradi 2019 {published data only}
- IRCT20120918010876N3. Assessment of efficacy of antenatal betamethasone on neonatal respiratory outcome of late preterm delivery (34-37 weeks) [Assessment of efficacy of antenatal betamethasone on neonatal respiratory outcome of late preterm delivery (34-37 weeks) in singleton pregnancies]. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20120918010876N3 2017. [CENTRAL: CN-01898197]
- *.Mirzamoradi M, Hasani NF, Jamali R, Heidar Z, Bakhtiyari M. Evaluation of the effect of antenatal betamethasone on neonatal respiratory morbidities in late preterm deliveries (34-37 weeks). Journal of Maternal-fetal & Neonatal Medicine 2019;33(15):2533-40. [CENTRAL: CN-01787845] [EMBASE: 625854285] [PMID: ] [DOI] [PubMed] [Google Scholar]
Parsons 1988 {published data only}
- *.Parsons MT, Sobel D, Cummiskey K, Constantine L, Roitman J. Steroid, antibiotic and tocolytic vs no steroid, antibiotic and tocolytic management in patients with preterm PROM at 25-32 weeks. In: Proceedings of the 8th Annual Meeting of the Society of Perinatal Obstetricians; 1988 Feb 3-6; Las Vegas, Nevada. 1988:44.
- Sobel D, Parsons M, Roitman J, McAlpine L, Cumminsky K. Antenatal antibiotics in PROM prevents congenital bacterial infection. Pediatric Research 1988;23:476A. [Google Scholar]
Taeusch 1979 {published data only}
- Taeusch HW Jr, Frigoletto F, Kitzmiller J, Avery ME, Hehre A, Fromm B, et al. Risk of respiratory distress syndrome after prenatal dexamethasone treatment. Pediatrics 1979;63:64-72. [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12617001494325 2017 {published data only}
- ACTRN12617001494325. The WHO ACTION-II (Antenatal CorticosTeroids for Improving Outcomes in preterm Newborns) Trial: a multi-country, multi-centre, two-arm, parallel, double-blind, placebo-controlled, randomized trial of antenatal corticosteroids for women at risk of imminent birth in the late preterm period in hospitals in low-resource countries to improve newborn outcomes. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12617001494325 2017. [CENTRAL: CN-01884536] [DOI] [PMC free article] [PubMed]
Hong 2019 {published data only}
- *.Hong S, Lee SM, Kwak DW, Lee J, Kim SY, Oh JW, et al. Effects of antenatal corticosteroids in twin neonates with late preterm birth (ACTWIN [Antenatal Corticosteroids in TWIN late preterm neonates] trial): study protocol for a randomized controlled trial. BMC Pregnancy and Childbirth 2019;19:1. [CENTRAL: CN-01939009] [EMBASE: 627072824] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03547791. Effects of ACS in twin with LPB: study Protocol for a RCT. https://clinicaltrials.gov/show/NCT03547791 (first received 2018 Jun 6). [CENTRAL: CN-02044889]
IRCT2014102919037N2 2014 {published data only}
- IRCT2014102919037N2. Evaluation of the effect of betamethasone on neonatal outcomes in pregnancies [Evaluation of the effect of betamethasone on neonatal outcomes in pregnancies in late preterm pregnancies]. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2014102919037N2 2014. [CENTRAL: CN-01877791]
NCT01206946 2010 {published data only}
- NCT01206946. Efficacy of antenatal steroids in Reducing respiratory morbidities in late preterm infants [Efficacy of antenatal steroids for women at risk of late preterm delivery on neonatal respiratory morbidity]. https://clinicaltrials.gov/show/NCT01206946 (first received 2010 Sep 21). [CENTRAL: CN-01501010]
NCT03446937 2018 {published data only}
- NCT03446937. Effect of antenatal corticosteroids on neonatal morbidity [Effect of antenatal corticosteroids on neonatal morbidity]. Https://clinicaltrials.gov/show/NCT03446937 (first received 2018 Feb 5). [CENTRAL: CN-01483469]
Additional references
Antenatal Corticosteroid CPG Panel 2015
- Antenatal Corticosteroids Clinical Practice Guidelines Panel. Antenatal corticosteroids given to women prior to birth to improve fetal, infant, child and adult health: Clinical Practice Guidelines. Liggins Institute, The University of Auckland, Auckland, New Zealand 2015.
Barker 1998
- Barker DJP. Mothers, Babies and Health in Later Life. 2nd edition. London: Churchill Livingstone, 1998. [Google Scholar]
Benediktsson 1993
- Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet 1993;341(8841):339-41. [DOI] [PubMed] [Google Scholar]
Brownfoot 2013
- Brownfoot FC, Gagliardi DI, Bain E, Middleton P, Crowther CA. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD006764. [DOI: 10.1002/14651858.CD006764.pub3] [DOI] [PubMed] [Google Scholar]
Carlisle 2017
- Carlisle JB. Data fabrication and other reasons for non-random sampling in 5087 randomised, controlled trial in anaesthetic and general medical journals. Anaesthesia 2017;72:944-52. [DOI] [PubMed] [Google Scholar]
Chawanpaiboon 2019
- Chawanpaiboon S, Vogel JP, Moller A, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Global Health 2019;7:e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 1998
- Clark PM. Programming of the hypothalamo-pituitary-adrenal axis and the fetal origins of adult disease hypothesis. European Journal of Pediatrics 1998;157(1 Suppl):S7-S10. [DOI] [PubMed] [Google Scholar]
Crowley 1990
- Crowley P, Chalmers I, Keirse MJ. The effects of corticosteroid administration before preterm delivery: an overview of the evidence from controlled trials. British Journal of Obstetrics and Gynaecology 1990;97:11-25. [DOI] [PubMed] [Google Scholar]
Crowther 2015
- Crowther CA, McKinlay CJD, Middleton P, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD003935. [DOI: 10.1002/14651858.CD003935.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Angelis 2004
- De Angelis C, Drazen JM, Frizelle FA, et al. Clinical Trial Registration: A Statement from the International Committee of Medical Journal Editors. New England Journal of Medicine 2004;351:1250-1. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Deshmukh 2017
- Deshmukh M, Patole S. Antenatal corticosteroids for neonates born before 25 Weeks-A systematic review and meta-analysis. PLOS One 2017;12(9):e0176090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deshmukh 2018
- Deshmukh M, Patole S. Antenatal corticosteroids in impending preterm deliveries before 25 weeks' gestation. Archives of Disease in Childhood. Fetal and Neonatal Edition 2018;103(2):F173-F176. [DOI] [PubMed] [Google Scholar]
Dodic 1999
- Dodic M, Wintour EM, Whitworth JA, Coghlan JP. Effect of steroid hormones on blood pressure. Clinical & Experimental Pharmacology & Physiology 1999;26(7):550-2. [DOI] [PubMed] [Google Scholar]
Doyle 2001a
- Doyle LW. Victorian Infant Collaborative Study Group. Outcome at 5 years of age of children 23 to 27 weeks' gestation: refining the prognosis. Pediatrics 2001;108(1):134-41. [DOI] [PubMed] [Google Scholar]
Doyle 2001b
- Doyle LW, Casalaz D. Victorian Infant Collaborative Study Group. Outcome at 14 years of extremely low birthweight infants: a regional study. Archives of Diseases in Childhood: Fetal and Neonatal Edition 2001;85(3):F159-F164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2001
- Edwards LJ, Coulter CL, Symonds ME, McMillen IC. Prenatal undernutrition, glucocorticoids and the programming of adult hypertension. Clinical & Experimental Pharmacology & Physiology 2001;28(11):938-41. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
Hintz 2007
- Hintz SR, Van Meurs KP, Perritt R, Poole WK, Das A, Stevenson DK, et al. Neurodevelopmental outcomes of premature infants with severe respiratory failure enrolled in a randomized controlled trial of inhaled nitric oxide. Journal of Pediatrics 2007;151:e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 1999
- Huang WL, Beazley LD, Quinlivan JA, Evans SF, Nenham JP, Dunlop SA. Effect of corticosteroids on brain growth in fetal sheep. Obstetrics & Gynecology 1999;94(2):213-8. [DOI] [PubMed] [Google Scholar]
Imseis 1996
- Imseis HM, Iams JD. Glucocorticoid use in patients with preterm premature rupture of fetal membranes. Seminars in Perinatology 1996;20(5):439-50. [DOI] [PubMed] [Google Scholar]
Jobe 1998
- Jobe AH, Wada N, Berry LM, Ikegami M, Ervin MG. Single and repetitive maternal glucocorticoid exposures reduce fetal growth in sheep. American Journal of Obstetrics and Gynecology 1998;178(5):880-5. [DOI] [PubMed] [Google Scholar]
Liggins 1969
- Liggins GC. Premature delivery of foetal lambs infused with corticosteroids. Journal of Endocrinology 1969;45:515-23. [DOI] [PubMed] [Google Scholar]
Liggins 1972a
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972;50(4):515-25. [PubMed] [Google Scholar]
Liggins 1976
- Liggins GC. Prenatal glucocorticoid treatment: prevention of respiratory distress syndrome. In: Moore TD , editors(s). Lung maturation and the prevention of hyaline membrane disease, Report of the Seventieth Ross Conference on Pediatric Research, Columbus, Ohio. 1976:97-103.
NIH 1994
- National Institutes of Health (NIH) Consensus Development Conference Statement. Effect of corticosteroids for fetal maturation on perinatal outcomes. American Journal of Obstetrics and Gynecology 1994;173:246-52. [Google Scholar]
Padbury 1996
- Padbury JF, Ervin MG, Polk DH. Extrapulmonary effects of antenatally administered steroids. Journal of Pediatrics 1996;128(2):167-72. [DOI] [PubMed] [Google Scholar]
Park 2016
- Park CK, Isayama T, McDonald SD. Antenatal corticosteroid therapy before 24 weeks of gestation: a systematic review and meta-analysis. Obstetrics and Gynecology 2016;127(4):715-25. [DOI] [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. The Cochrane Collaboration, 2014.
Rodriguez 2002
- Rodriguez RJ, Martin RJ, Fanaroff AA. Respiratory distress syndrome and its management. In: Fanaroff AA, Martin RJ, editors(s). Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant. St. Louis: Mosby, 2002:1001-10. [Google Scholar]
Rohwer 2020
- Rohwer AC, Oladapo OT, Hofmeyr GJ. Strategies for optimising antenatal corticosteroid administration for women with anticipated preterm birth. Cochrane Database of Systematic Reviews 2020, Issue 5. Art. No: CD013633. [DOI: 10.1002/14651858.CD013633] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scherer 2018
- Scherer RW, Meerpohl JJ, Pfeifer N, Schmucker C, Schwarzer G, Elm E. Full publication of results initially presented in abstracts. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: MR000005. [DOI: 10.1002/14651858.MR000005.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwab 2000
- Schwab M, Roedel M, Anwar MA, Muler T, Schubert H, Buchwalder LF, et al. Effects of betamethasone administration to the fetal sheep in late gestation on fetal cerebral blood flow. Journal of Physiology 2000;528(3):619-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seckl 2000
- Seckl JR, Cleasby M, Nyirenda MJ. Glucocorticoids, 11beta-hydroxysteroid dehydrogenase, and fetal programming. Kidney International 2000;57(4):1412-7. [DOI] [PubMed] [Google Scholar]
Simes 1986
- Simes RJ. Publication bias: the case for an international registry of clinical trials. Journal of Clinical Oncology 1986;4:1529-41. [DOI] [PubMed] [Google Scholar]
Sotiriadis 2018
- Sotiriadis A, Makrydimas G, Papatheodorou S, Ioannidis JP, McGoldrick E. Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD006614. [DOI: 10.1002/14651858.CD006614.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
The World Medical Association 2013
- The World Medical Association. Declaration of Helsinki. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ 2013;Version 8.
Turrentine 1996
- Turrentine MA, Wilson PD, Wilkins IA. A retrospective analysis of the effect of antenatal steroid on the incidence of respiratory distress syndrome in preterm twin pregnancies. American Journal of Perinatology 1996;13(6):351-4. [DOI] [PubMed] [Google Scholar]
Utama 2018
- Utama DP, Crowther. Transplacental versus direct fetal corticosteroid treatment for accelerating fetal lung maturation where there is a risk of preterm birth. Cochrane Database of Systematic Reviews 2018, Issue 6. Art. No: CD008981. [DOI: 10.1002/14651858.CD008981.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vyas 1997
- Vyas J, Kotecha S. Effects of antenatal and postnatal corticosteroids on the preterm lung. Archives of Disease in Childhood: Fetal and Neonatal Edition 1997;77(2):F147-F150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wellcome 2005
- Reynolds LA, Tansey EM. Prenatal corticosteroids for reducing morbidity and mortality after preterm birth. The transcript of a Witness Seminar; The Wellcome Trust Centre for History of Medicine at UCL, London 2005;25.
WHO 2015
- WHO. WHO recommendations on interventions to improve preterm birth outcomes: evidence base. https://www.who.int/reproductivehealth/publications/maternal_perinatal_health/preterm-birth-guideline/en/ 2015. [PubMed]
References to other published versions of this review
Crowley 1996
- Crowley P. Prophylactic corticosteroids for preterm birth. Cochrane Database of Systematic Reviews 1996, Issue 1. Art. No: CD000065. [DOI: 10.1002/14651858.CD000065] [DOI] [PubMed] [Google Scholar]
Crowley 2003
- Crowley P, Roberts D, Dalziel SR, Shaw BNJ. Antenatal corticosteroids to accelerate fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2003, Issue 4. Art. No: CD004454. [DOI: 10.1002/14651858.CD004454] [DOI] [Google Scholar]
Crowley 2006
- Crowley P. Prophylactic corticosteroids for preterm birth. Cochrane Database of Systematic Reviews 2006, Issue 3. Art. No: CD000065. [DOI: 10.1002/14651858.CD000065.pub2] [DOI] [PubMed] [Google Scholar]
Roberts 2006
- Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2006, Issue 3. Art. No: CD004454. [DOI: 10.1002/14651858.CD004454.pub2] [DOI] [PubMed] [Google Scholar]
Roberts 2017
- Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2017, Issue 3. Art. No: CD004454. [DOI: 10.1002/14651858.CD004454.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


