Abstract
The demand for expert neuropathologic intraoperative diagnoses often exceeds the available supply and geographic distribution of neuropathology centers. Telepathology has therefore been implemented in recent years to meet this demand. Herein, we draw on our experience with 4 generations of telepathology systems over the past 8 years to discuss the design, initiation and maintenance of an effective telepathology service for neuropathologists, including when to change systems. In addition to workflow efficiency, unique advantages of telepathology include integration into other informatics modalities, quality assurance/quality control (QA/QC) maintenance and the potential for visual data to be readily available to clinicians. Given the improvements in technology and the multiple uses of telepathology, this method for delivering patient care will undoubtedly continue to grow over time.
Keywords: telepathology, neuropathology, intraoperative, consultation
THE NEED FOR TELEPATHOLOGY IN NEUROPATHOLOGY
Two of the great advancements of the 20th century were in the realms of telecommunications and computer science. Innovation in both of these arenas has had broad transformational effects in our society. The delivery and organization of medical care have been fundamentally reshaped by these developments. Within pathology, the broadest effects have been seen in the widespread adoption of new technologies like laboratory information systems (LIS) and digital imaging. These changes have improved workflow efficiency, reduced errors and turnaround time and enhanced communication between pathologists and clinicians. However, these changes have built upon, rather than fundamentally altered, the traditional arrangement of the pathology workspace in which the pathologist delivers services from an office adjacent to a laboratory, situated nearby operating and procedure suites. One reason that more extensive changes have not occurred is that one of the fundamental techniques of pathology—direct microscopic examination of tissue and interpretation of often subtle findings—was simply not feasible by remote examination. Now, the tremendous improvements in high‐resolution imaging, computing power and speed and bandwidth have made it possible to render difficult diagnoses by remote. In some cases it is even preferable to have expert opinions accessible by remote if the only alternative is an on‐site general pathologist who is uncomfortable and untrained in certain subspecialties. This is particularly true in the subspecialty of neuropathology, for although the number of central nervous system (CNS) tumors is low compared with other sites like breast, lung and colon, the demand for expert neuropathologic diagnoses is spread out over a broader geographic range than can be physically met by the existing pool of neuropathologists. Even if there were enough board‐certified neuropathologists for every hospital that does neurosurgery, keeping such specialized physicians on staff is not cost‐effective for many pathology departments and group practices. Furthermore, it would be difficult for neuropathologists in remote sites to maintain diagnostic acuity without handling a sufficient number of cases annually.
If the demand for neuropathologic expertise was strictly limited to postoperative diagnoses on permanent, paraffin‐embedded tissue, such problems could easily be resolved by mailing all pathologic material to large regional centers for processing and in‐depth analysis. Unfortunately, intraoperative consultations need to be done accurately and quickly in “real time.” Adding to the dilemma is the complexity and high‐pressure context of a typical neurosurgical consult, as the consequences of a diagnostic error in the brain or spinal cord are more likely to be serious than elsewhere. For all these reasons, neurosurgeons (and their patients) would prefer that their specimens be analyzed by the best‐trained consultants possible, as fast as possible, while in the OR so they can adjust surgical approaches appropriately.
Utilization of the aforementioned advances in computing power and telecommunications has been of interest in the world of pathology, from the USA to China and everywhere in between 1, 2, 3, 5, 6, 8, 11, 12, 13, 14, 16, 17). This application in pathology, called “telepathology”(18), has been especially practical in neuropathology, particularly in the context of intraoperative consultations. Initial forays into telepathology for neuropathologists were unsuccessful largely because of low image quality, slow rate of image transmission and an inability to directly control the slide on which the tissue was mounted 4, 9, 19). In recent years, improvements in digital imaging, microscopy hardware and network bandwidth have greatly improved the reliability and accuracy of telepathology such that more recent studies have validated this technology in a variety of settings, including neuropathology 7, 9, 10).
PRACTICAL CONSIDERATIONS WHEN IMPLEMENTING A TELEPATHOLOGY SYSTEM
Successful implementation of a telepathology system cannot be accomplished in a vacuum; cooperation and input from multiple sources are essential. The first step is partnering with the neurosurgeons that will be using teleneuropathology to ensure that they participate in all relevant decisions. Co‐ownership of the endeavor is essential if the goals of implementation are to be achieved, and this will have the practical and very important effect of reducing the potential for unpleasant interactions in the event of intraoperative system complications. A simple example of this approach is the following: when a vendor representative is showing the neuropathologists how a system works, neurosurgeons should be invited to the demonstration so their input about functionality from a clinical perspective can be obtained. This partnership should include the ability for neurosurgeons to have a role in deciding which system is ultimately purchased. Another practical benefit of this partnership is that neurosurgical departments may also reasonably be asked to share in the cost of the system.
Consideration should be given to the underlying technology on which the telepathology system is based, especially the methods used for image acquisition and viewing. Most products available today are high‐resolution dynamic–robotic (DR) or whole slide imaging (WSI) systems, both of which have proven superior to static imaging systems that show only a series of still shots. Although either DR or WSI modalities are adequate for telepathology, some suggest that WSI is faster than DR (7). Both the DR and WSI modalities enable the neuropathologist to control the slide by remote input, eliminating the need for another pathologist or technician to sit at the remote site and manually operate the microscope according to instructions via telephone. In the case of WSI, the entire slide is scanned at high resolution at once, generating an image around 10 GB that can zoom up and down and be “dragged” around. DR does not create a high‐resolution image of the entire slide, but instead employs a robotic microscope with an attached camera that sends a real‐time image of whatever is on the stage, with the position and magnification controlled by the remote operator (Figure 1A,B). In both cases, the consultant has direct control of the slide and what is studied in detail. Not only does this improve speed, but it also gives the neuropathologist more confidence that the relevant diagnostic areas on a slide have been adequately reviewed and thus reduces the deferral rate. Of particular importance is to ensure that the system implemented has the ability to generate a low‐power “thumbnail” image of the whole slide adjacent to a larger window with the higher magnification view. The system should also have on‐screen control “keys” that are operable by mouse or keyboard. Moreover, the operator should be able to directly click on a portion of the whole slide thumbnail and have the high power view immediately move to those coordinates. In our experience, this latter feature with the thumbnail image is nearly as important as high bandwidth for improving turnaround time.
Figure 1.
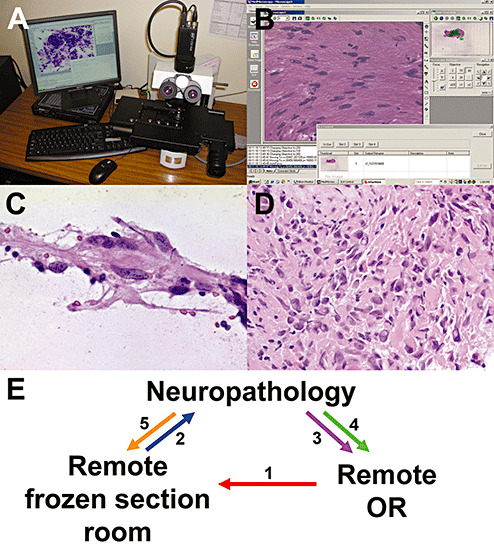
Dynamic–robotic telepathology system. A. The Trestle robotic microscope system has a four‐slide stage, standard microscope objectives and a camera that transmits real‐time images via the Internet to a secure website. The website (B), which can be accessed using any computer worldwide with an Internet connection, has windows displaying a thumbnail whole slide image, high‐magnification image, control “pad” and thumbnail images of any other slide(s) on the stage. An intraoperative smear preparation of a post‐therapy glioblastoma biopsy (C) shows the detail and resolution that this system generates. The diagnosis rendered intraoperatively corresponded to the permanent section of the same glioblastoma with treatment effect (D). E. A telepathology consultation is initiated by the remote neurosurgeon who notifies the remote pathology department (1). The specimen is received and prepared by the remote pathology assistant (PA), who apprises the neuropathologist (2). The neuropathologist immediately contacts the neurosurgeon both for additional clinical information and to provide an update on the biopsy status (3). Once a diagnosis is reached, the neuropathologist communicates this directly to the neurosurgeon (4) and then to the PA (5), who records the diagnosis and any special processing requests (eg, ordering up‐front immunostains, unstained slides, etc.).
The third consideration for a telepathology system is the strength of the digital communication line. Older systems often utilized a separate, dedicated integrated services digital network (ISDN) line with a separate control pad and viewscreen, but this was cumbersome and is no longer needed. Virtually, all new DR telepathology systems operate via a secure user‐specific Internet website so that any desktop or laptop computer with a sufficiently high bandwidth connection (10 MBps sufficient, preferably 100 MBps), CPU processing speed (at least 533 MHz, preferably 1 GHz or higher) and RAM (512 MB minimum, over 1 GB optimal) can be used for intraoperative consultations, whether the computer is in the neuropathologist's workspace or even at home. This becomes a particular advantage in the event of a computer “crash,” which can be handled by switching to another computer. The newer DR systems on the market today send images with a size of around 1 megapixel and a resolution down to 0.18 microns/pixel (Figure 1C,D). Processing speed is even more critical in WSI systems, wherein a typical high‐resolution (visual equivalent of 200×) whole slide file is between 2 and 10 GB (15).
Fourth, effective implementation of telepathology for intraoperative use requires technical support not just from the vendor, but from a management team at the site where the system is physically located. Responsibilities of this team should include system installation and ensuring ongoing optimal functionality. They should be available and competent to troubleshoot problems as they arise and determine whether additional vendor support is needed. The neuropathologist should of course be involved in managing any problems, but not as the primary troubleshooter or repair technician. In the rare event of a system failure, the neurosurgeon must immediately be notified. The local on‐call pathologist can then be contacted to physically take over the glass slide analysis. At our institution, an older DR system serves as a backup, and only if both systems fail (which in 2 years has yet to happen) is a local pathologist involved.
Fifth, the pathology staff responsible for physically handling the specimen, either pathologists or pathology assistants (PAs), needs to be well‐trained on how to handle neuropathology specimens. Such education should include how to recognize abnormal brain tissue, make satisfactory smear and touch preparations, and when and how to freeze brain tissue for sectioning. Adequate training of personnel is just as important as image quality. Preferably, this training would come from the consulting neuropathologists, but if physical distance is too great, onsite general pathologists may have to substitute. In our system, a 1 hour session using fresh autopsy brain tissue is sufficient to train a new PA to perform proper touch and smear techniques. Showing them what normal unfixed white and gray matter looks like also improves their ability to recognize abnormal areas in a specimen—for example, to recognize which end of a biopsy core is most likely to produce diagnostic results. Unfortunately, employee turnover requires periodic training of new staff, as well as retraining of existing PAs. In addition to regular feedback during intraoperative consultations, such retraining is necessary about once a year.
Last and most important, full coordination must be established among the operating room, the on‐site pathology staff that physically handles the specimen and neuropathology. This requires a specific workflow with elements involving both the OR and the pathology laboratory (Figure 1E). The neuropathologist must take a leading role in assigning clear‐cut, well‐defined tasks for each participant. At our institution, the neuropathologist is provided with a list of all pending neurosurgical procedures 1 day prior and reviews electronically available patient information on each case, including radiology. During surgery, the neurosurgeon initiates the consult by asking the rotating nurse to call their local pathology gross room and inform them that a specimen is ready for processing. The PA retrieves the specimen from the OR and goes to the frozen section room, where he/she notifies the neuropathologist via text paging. The neuropathologist then calls the frozen section room and discusses with the PA how to best process the specimen. While the specimen is being processed, the neuropathologist opens the telepathology website, calls the OR directly and discusses the case with the neurosurgeon, focusing in particular on relevant clinical information that may not have been available electronically, as well as any information or questions the neurosurgeon would like to convey.
Once the slide is scanned, the neuropathologist analyzes it, saves digital images of diagnostic fields to a secure server, arrives at an interpretation and calls the OR back to inform the neurosurgeon of the diagnosis. The neuropathologist then calls the PA back and tells them what to write on the requisition form and how to handle any excess tissue. The case is completed when the neuropathologist records the consultation in a logbook, including the time from the initial text page to the diagnosis plus any problems that were encountered. The pathology lab at the remote site sends the specimen in fixative to the neuropathologist's center, where it is grossed and histologically processed like any other case. In the rare event of telepathology system failure, the neuropathologist calls the PA and tells him/her to notify their local on‐call pathologist, who then directly analyzes the tissue as they would any other intraoperative consult. The specific workflow will necessarily vary to reflect the needs of each institution. While detailed and well documented, this workflow also needs to be flexible and adapted as necessary to the practical realities of communication and cooperation between clinical services. If this is done, over time a fluid workflow with robust communication will develop around the technology of whatever system is used.
As we have shown, all these factors must be addressed before an effective telepathology system can be established. In our experience, a year is the minimal time required between the decision to initiate telepathology and its actual implementation. During this time, face‐to‐face meetings among neurosurgery, neuropathology and the remote site pathology staff are most helpful in facilitating a smooth transition.
MAINTAINING AN ESTABLISHED TELEPATHOLOGY SYSTEM
It is possible to develop a workflow and standard operating procedures to support telepathology in most neuropathology laboratory environments, and it is remarkable how quickly telepathology becomes routine. However, a larger issue is the development of skills and comfort among users of the system. An essential aim is to help neuropathologists embrace the technology, in particular, those with extensive experience using traditional intraoperative consultation methods. Making all users a part of the decision‐making process from the start encourages this transition. Most of the newer systems available today, either DR or WSI, are quite user‐friendly and are designed to mimic the “feel” of traditional microscopy with familiar tools like the computer mouse. Critical to ensuring that users accept telepathology is to build the system with adequate image resolution, bandwidth and computing power so that it mimics conventional microscopy as closely as possible. The neuropathologist should also be easily able to control image brightness and focal depth to suit individual needs. While telepathology is now feasible, it is not yet as fast as traditional microscopy; intraoperative consultations will take more time. Everyone, from the neuropathologist to the neurosurgeon, needs to understand this and realize that it is still more cost‐effective and efficient than trying to supply in‐house neuropathologic expertise at every site where neurosurgeries are performed.
Another matter is how to handle the likely increase in volume over time. In our institution, the number of intraoperative consultations done by telepathology increased from 13 in 2002 to 160 in 2006 (9), and in 2007, over 170 cases were diagnosed by telepathology. Likewise, the proportion of all intraoperative consults handled by telepathology grew from 13% in 2002 to 32% in 2007 and has remained at about a third of all cases in 2008 (Figure 2). Not only does this increase in volume come from the primary off‐site center, but as the neuropathology group gains a reputation for expertise with this tool, additional sites may wish to enlist the group's services. For example, in our situation at University of Pittsburgh Medical Center (UPMC) Presbyterian Hospital, the primary off‐site center is UPMC Shadyside Hospital, separated by 18 city blocks. However, Children's Hospital of Pittsburgh is relocating from its current site (attached to Presbyterian) to a neighborhood even farther away than Shadyside, and their neurosurgeons want neuropathologists available via telepathology. Out‐of‐state neurosurgery group practices have also expressed interest in establishing a telepathology service with us. Expanding the service provides more revenue for the neuropathology division but naturally increases workload. The main point to remember is that the telepathology service will likely grow once implemented, underscoring the effectiveness of current telepathology systems. Thus, be prepared to handle an increase in demand.
Figure 2.
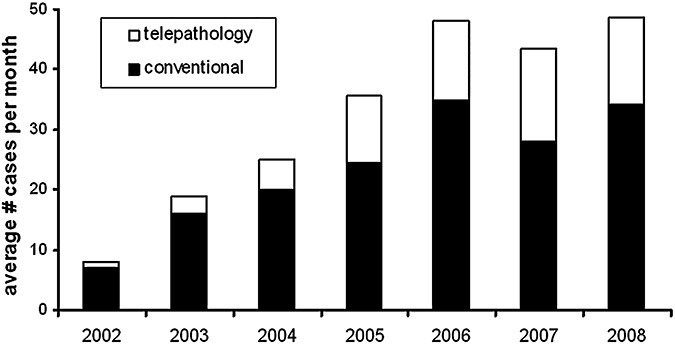
Growth of conventional and intraoperative consultations over time. All intraoperative consultations from January 2002 to October 2008 were tracked. Black bars represent the average number of monthly consultations done by conventional in‐house microscopy. White bars represent the average number of monthly consultations done by telepathology.
CHANGING TELEPATHOLOGY SYSTEMS
As Moore's law (the rule that economical computing speed, memory capacity and image resolution double every 2 years) makes clear, any system used for telepathology will undergo rapid evolution. Commercially available telepathology systems consistently increase in speed, image resolution and the number of new features. It is thus important to decide when the benefits from all those improvements outweigh the cost of a new system and the additional work required to install, modify workflow and retrain users. At UPMC, we are on our fourth generation of telepathology systems; a brief discussion of each will illustrate our own experience in determining when upgrades are appropriate.
Our first generation was a nonrobotic video‐conference system that sent low‐resolution real‐time microscope video images (national television system committee (NTSC), 307 kilopixels) selected by a remote site pathologist through a dedicated ISDN connection that sent information at the slow rate of 384 Kbps to a television screen. It was an easy decision to discard that operation as it never generated any true intraoperative consultations, had frequent technical breakdowns and required one pathologist at each end plus one IT technician at each end.
The second generation was a hybrid static/dynamic nonrobotic Nikon DN100 (Nikon, Melville, NY, USA) microscope that utilized a standard Web browser and broadcast high‐resolution images. The accuracy of that system ended up being comparable to subsequent generations of telepathology systems (Figure 3) but still needed a remote site pathologist to operate, thus reducing cost‐effectiveness as two pathologists were doing the work of one. That and the lack of a whole slide thumbnail view made switching to a third generation high‐resolution robotic system desirable.
Figure 3.
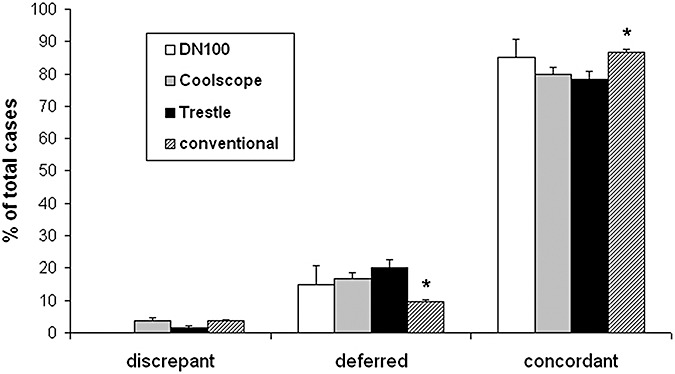
Accuracy of intraoperative diagnoses using second (DN100, 40 cases), third (Coolscope, 361 cases) and fourth generation (Trestle, 263 cases) telepathology systems is close to conventional microscopy (1986 cases). Neuropathology cases with intraoperative consultations were collected from January 2002 to October 2008. Each intraoperative diagnosis was compared with the final diagnosis while blinded to modality. Criteria for intraoperative diagnosis grading was similar to that described previously (9). There is a higher deferral rate and slightly lower overall concordance via telepathology, but discrepancy rates are similar. Thus, when an on‐site neuropathologist is not feasible, telepathology provides an alternative that may be preferable to an untrained general surgical pathologist. *P < 0.001 for conventional vs. both Coolscope and Trestle.
As shown previously (9), the third generation Coolscope TM (Nikon, Melville, NY, USA) robotic microscope was equivalent to conventional microscopy in overall concordance after several years of use. However, telepathology still trended toward a higher deferral rate for most diagnostic categories. Image resolution was adequate (1.2 megapixel image at 0.5 microns/pixel), but focus could not be rapidly fine tuned, and only one slide could be loaded into the machine at a time, slowing turnaround time when multiple slides needed analysis. The slide had to be thoroughly wiped clean of excess mounting medium or the internal components of the tray would be fouled. Image transmission was between 3.75 and 7.5 frames/s (lower on higher magnification), and only 2 objectives (5× and 20×) were available, with a 2× internal magnifying lens producing the equivalent of 10× and 40×.
Our fourth generation system Trestle (Trestle Holdings, Newport Beach, CA, USA) has addressed these problems (Figure 1A–D). In particular, its ability to automatically hold and scan multiple slides, generate high‐resolution digital images (0.18 microns/pixel) and transmit them between 10 and 30 frames/s (lower at higher magnification) and the option to store them in a virtual microscopy database prompted our institution to purchase several microscopes and link them via a digital pathology imaging group. Thus, one system serves both clinical diagnostics and education. The objectives and slide tray are external, making simple repairs and cleanup easier, plus the slides do not need to be completely free of excess mounting medium. At the present time, no plans are in the works to upgrade any further as our clinical and educational needs are being met satisfactorily.
Overall, these telepathology systems have produced a higher rate of deferrals than by conventional microscopy, but the rate of discrepancies is the same or lower and the overall concordance between the intraoperative and final diagnoses (ie, accuracy) is still quite high (Figure 3). Common tumors encountered in neuropathology, like gliomas, pituitary adenoma, meningiomas and metastatic carcinomas, have low rates of diagnostic discrepancies and deferrals in telepathology, similar to conventional microscopy. Likewise, rarer tumors (eg, pineocytomas or germinomas) and diagnostically challenging neoplasms like lymphomas have lower accuracy rates in both telepathology and conventional microscopy. Compared with conventional microscopy, it is more difficult to confidently diagnose tissue as being non‐neoplastic, for example, in a demyelinating lesion or an infarct. Even in these situations, though, the surgeon can usually be given enough descriptive information about the biopsy to help guide the rest of the procedure. Nevertheless, if considering implementation of a telepathology system, expect that the overall accuracy will be slightly lower than if an on‐site neuropathologist was available, because deferrals are more likely. However, this is still preferable to having an untrained general surgical pathologist handle difficult neuropathology consultations.
Each upgrade has, naturally, come with increased cost. In particular, the fourth generation system was five times as expensive as the third generation system. Without the ability to use this new system for additional educational and research purposes, the expense of the fourth generation would likely have been prohibitive. Thus, each institution that uses or is considering telepathology must consider cost, and if the budget is limited, something equivalent to our third generation might be optimal.
INTERFACING OF TELEPATHOLOGY WITH OTHER FACETS OF PATHOLOGY
The digital nature of telepathology output inherently lends itself to integration with other aspects of daily practice in pathology. As described above, during the intraoperative consultation, diagnostic fields are captured digitally and saved to a secure server. Later, when the case has been accessioned and assigned a unique specimen number in our electronic LIS, the images are imported into the case file as part of the image gallery associated with that case. Additional images of radiology, permanent sections, immunohistochemistry and fluorescence in situ hybridization studies are also incorporated into the image gallery. These images thus become part of the patient's permanent electronic record and can be retrieved at any time by those with username/password access to the LIS.
One key use of this archive is at our weekly QA/QC meeting, where cases are presented simply by opening up each case‐specific gallery and showing one image at a time. This allows all neuropathology division members to see exactly what the on‐service neuropathologist saw at the time of the consultation, compare the intraoperative images with corresponding paraffin‐embedded images and to discuss the diagnosis as a group. Thus, everyone benefits from each other's experiences. When telepathology was still relatively new to our division, all telepathology cases were addressed at the QA/QC meeting. Now, as faculty members have grown comfortable and skilled with the technique, only interesting or difficult cases are presented. We also use such images to test trainees on their ability to diagnose both classic and challenging CNS lesions via telepathology, thereby ensuring that all rotating residents are exposed to this technology.
FUTURE DIRECTIONS
Besides the obvious utility of continued improvements in resolution, scanning and transmission speeds and image processing time, other developments in telepathology would be desirable. For instance, one key problem with most current setups is that there is no mechanism for transmitting quality images of the gross specimen. It would be most helpful if the PA could place a small biopsy (which is typical for most neuropathology specimens) under a dedicated gross camera that linked directly with the telepathology website. This would allow the neuropathologist to look at the gross specimen, identify which portions are likely diagnostic and instruct the PA to select those targeted areas for processing.
Another improvement would establish OR access to the images being seen in real time by the consulting neuropathologist. This would allow the neurosurgeon to see on a flat screen TV exactly what the neuropathologist is seeing without having to break scrub, leave the OR and go to the frozen room. It would have the additional benefit of reassuring the neurosurgeon that the biopsy is being processed and studied, reducing the urge to place follow‐up phone calls to the neuropathologist. Similar systems currently exist where an OR has both video and audio input and output capabilities, thus linking the pathologist to the operative field, while the neurosurgeon can see microscopic images of frozen sections in real time. It would therefore not be terribly challenging to develop a comparable setup when dealing with telepathology consultations.
A third key advancement will be the integration of intraoperative digital pathology images in other bioinformatics venues. For example, digital images from an intraoperative preparation could be directly linked to corresponding snap‐frozen excess tissue in a tumor bank database. Anyone withdrawing tissue from that bank would thus have access to the archived images, thus verifying that the tissue withdrawn is indeed of the appropriate pathology. In addition to storage in the electronic pathology LIS, such images could also be linked to electronic radiology, neurosurgery and neuro‐oncology information systems for easy access by other physicians involved in the patient's care.
SUMMARY
Telepathology is an innovative use of modern technology to provide accurate, cost‐effective consultations in the intraoperative setting. It is particularly useful in neuropathology because of the geographic distribution of neurosurgical cases, relative infrequency of such cases and scarcity of neuropathologists. Successful implementation of telepathology requires careful consideration and advance planning, most especially in training remote‐site pathology staff and establishing a rapid flow of communication between all involved parties. The digital nature of this method lends itself well to integration with QA/QC activities and other bioinformatics resources. This technology will likely become part of the routine practice for many neuropathologists, and someday may even completely supplant the traditional in‐person analysis that has been a staple of neuropathology practice since the days of Harvey Cushing a century ago.
Affiliation: CH was supported by a Callie Rohr/American Brain Tumor Association Fellowship.
The authors know of no potential financial conflict of interest in the publication of this study.
REFERENCES
- 1. Ayad E, Sicurello F (2008) Telepathology in emerging countries pilot project between Italy and Egypt. Diagn Pathol 3(Suppl.1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Banach L, Stepien A, Schneider J, Wichrzycka‐Lancaster E (2008) Dynamic active telepathology over National Health Laboratory service network, South Africa: feasibility study using Nikon Coolscope. Diagn Pathol 3(Suppl.1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baruah MK (2005) The practice of telepathology in India. J Postgrad Med 51:316–318. [PubMed] [Google Scholar]
- 4. Becker JRL, Specht CS, Jones R, Rueda‐Pedraza ME, O'Leary TJ (1993) Use of remote video microscopy (telepathology) as an adjunct to neurosurgical frozen section consultation. Human Pathol 24:909–911. [DOI] [PubMed] [Google Scholar]
- 5. Crimmins D, Crooks D, Pickles A, Morris K (2005) Use of telepathology to provide rapid diagnosis of neurosurgical specimens. Neurochirurgie 51:84–88. [DOI] [PubMed] [Google Scholar]
- 6. Dunn BE, Choi H, Almagro UA, Recla DL (2001) Combined robotic and nonrobotic telepathology as an integral service component of a geographically dispersed laboratory network. Human Pathol 32:1300–1303. [DOI] [PubMed] [Google Scholar]
- 7. Evans AJ, Chetty R, Clarke BA, Croul S, Kiehl R, Perez‐Ordonez B, Asa SL (2008) Comparison of whole‐slide imaging to robotic microscopy for primary frozen section diagnosis by telepathology. In: United States and Canadian Academy of Pathology Annual Meeting, United States and Canadian Academy of Pathology, Denver, CO.
- 8. Furness P (2007) A randomized controlled trial of the diagnostic accuracy of internet‐based telepathology compared with conventional microscopy. Histopathology 50:266–273. [DOI] [PubMed] [Google Scholar]
- 9. Horbinski C, Fine JL, Medina‐Flores R, Yagi Y, Wiley CA (2007) Telepathology for intraoperative neuropathologic consultations at an academic medical center: a 5‐year report. J Neuropathol Exp Neurol 66:750–759. [DOI] [PubMed] [Google Scholar]
- 10. Hutarew G, Schlicker HU, Idriceanu C, Strasser F, Dietze O (2006) Four years experience with teleneuropathology. J Telemed Telecare 12:387–391. [DOI] [PubMed] [Google Scholar]
- 11. Kayser K (2000) Telepathology in Europe. Anal Cell Pathol 21:95–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li X, Gong E, McNutt MA, Liu J, Li F, Li T et al (2008) Assessment of diagnostic accuracy and feasibility of dynamic telepathology in China. Hum Pathol 39:236–242. [DOI] [PubMed] [Google Scholar]
- 13. Liang WY, Hsu CY, Lai CR, Ho DM, Chiang IJ (2008) Low‐cost telepathology system for intraoperative frozen‐section consultation: our experience and review of the literature. Hum Pathol 39:56–62. [DOI] [PubMed] [Google Scholar]
- 14. Maher L, Craig A, Menezes G (2007) A national survey of telemedicine in the Republic of Ireland. J Telemed Telecare 13:348–351. [DOI] [PubMed] [Google Scholar]
- 15. Montalto MC (2008) Pathology RE‐imagined: the history of digital radiology and the future of anatomic pathology. Arch Pathol Lab Med 132:764–765. [DOI] [PubMed] [Google Scholar]
- 16. Tsuchihashi Y, Takamatsu T, Hashimoto Y, Takashima T, Nakano K, Fujita S (2008) Use of virtual slide system for quick frozen intra‐operative telepathology diagnosis in Kyoto, Japan. Diagn Pathol 3(Suppl.1):S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vari S, Cserneky M, Kadar A, Szende B (2005) Development of present and future of telepathology in Hungary. Pathol Oncol Res 11:174–177. [DOI] [PubMed] [Google Scholar]
- 18. Weinstein RS, Bloom KJ, Rozek LS (1987) Telepathology and the networking of pathology diagnostic services. Arch Pathol Lab Med 111:646–652. [PubMed] [Google Scholar]
- 19. Weinstein RS, Descour MR, Liang C, Bhattacharyya AK, Graham AR, Davis JR et al (2001) Telepathology overview: from concept to implementation [see comment. Human Pathol 32:1283–1299. [DOI] [PubMed] [Google Scholar]


