Abstract
Cryptic intracerebral hemorrhage as an etiological factor in fetal hydrocephalus has been postulated but not described at autopsy. Four fetuses with overt hydrocephalus diagnosed by in utero ultrasound examination were examined at autopsy at 19–22 weeks gestation. Although a hemorrhagic etiology was not evident on ultrasound, hemosiderin‐containing macrophages and associated reactive changes were found to obstruct the otherwise well‐formed cerebral aqueduct in all four. Coagulopathy due to thrombocytopenia was implicated in one case. Anomalies involving other parts of the body were identified in two cases, although a direct link to the hydrocephalus was not obvious. The abnormality was isolated in one case. In three cases, possible sites of hemorrhage in the ventricles were identified. This abnormality represents a significant proportion of the fetuses examined for hydrocephalus in our referral center. We discuss the importance of careful autopsy examination in the diagnosis of cryptic intracerebral hemorrhage and the implications for counseling.
Keywords: autopsy, bleeding, cerebral ventricles, fetus, human, hydrocephalus
INTRODUCTION
Hydrocephalus, defined as an abnormal accumulation of cerebrospinal fluid within the head, is usually the result of an obstruction between sites of absorption and production. The causes of fetal hydrocephalus are varied and have prognostic significance 5, 32. In utero hydrocephalus as a consequence of identifiable hemorrhage has been well documented, the majority in the third trimester by readily identifiable hemorrhagic lesions 14, 18, 25, 31, 37. Cryptic intracerebral hemorrhage has been postulated as a cause of fetal hydrocephalus 8, 10, 12; however, by definition, it cannot be diagnosed by current ultrasound examination techniques. Here we report the details of four midgestation fetuses with hydrocephalus caused by minute blood clots in the cerebral aqueduct but no intraventricular hematoma.
METHODS
The four cases were identified over a 15‐year period by the senior author (MRD). Use of the tissue falls within the ethical tissue utilization guidelines of the Department of Pathology. Progressive fetal ventriculomegaly was identified by ultrasonography. Autopsies were performed within 24 h following elective termination of the pregnancies. A detailed chart review was performed in each case. The brain was removed using routine fetal autopsy technique. Weights, measurements and gross appearance were documented, followed by fixation of the brain in 10% neutral buffered formalin. After approximately 2 weeks, the brain was re‐examined. The brainstem was transected at the level of the upper midbrain. As in all cases of fetal hydrocephalus, the midbrain sample was sectioned at multiple levels to ensure adequate inspection of the cerebral aqueduct. The cerebral hemispheres were sectioned in the coronal plane to assess the extent of ventricular dilatation and relevant anatomy. Samples of frontal cerebrum, basal nuclei—including ganglionic eminence, thalamus, temporal lobe, parietal lobe, midbrain, pons and cerebellum—and medulla were dehydrated and embedded in paraffin wax for sectioning (5 µm in thickness). All samples were stained with hematoxylin and eosin for routine histological evaluation. Selected samples were stained with Perls' Prussian blue method to identify hemosiderin within macrophages and Martius scarlet blue to ascertain the presence of fibrin. Selected samples also underwent immunohistochemical staining using the following antibodies: glial fibrillary acidic protein (GFAP) (polyclonal rabbit anti‐GFAP to detect reactive astrocytes; 1:10000 dilution; Dako, Glostrup, Denmark), HLA‐DR (monoclonal mouse antibody to detect reactive microglia; 1:2000 dilution; Dako), CD3 (polyclonal rabbit antibody to detect T lymphocytes; 1:400 dilution; Dako) or CD31 (monoclonal mouse antibody to detect endothelial cells; 1:100 dilution; Dako). Antigen retrieval was performed in a Bull's Eye Decloaking chamber (Biocare Medical, Concord, CA) for 1 minute at 125°C utilizing Dako pH 9 retrieval solution. All antibodies were detected using the Dako Envision system (Dako) and diaminobenzidine precipitation.
Case reports
Case 1
A 36‐year‐old woman (gravida 2 para 1) was investigated because the maternal serum triple test (alpha‐fetoprotein, human chorionic gonadotropin and unconjugated estriol) drawn at 15 weeks gestation was positive; the estimated risk for fetal Down syndrome was 1/299. She was generally healthy with no history of diabetes, although her weight at presentation was 125 kg. She denied tobacco, drug or alcohol use. There was no consanguinity and she had no family history of chromosomal anomalies, spina bifida or anencephaly. She was Rh+. Ultrasonography performed at16 weeks gestational age (by ultrasound criteria) showed hydrocephalus involving both lateral ventricles (16 mm dilation) as well as the third ventricle. The surrounding cortex appeared as a thin rim (Figure 1). The falx cerebri, thalami and face were deemed normal. Fetal motility and fetal heart rate were within normal limits. Amniocentesis performed at the same time revealed normal female karyotype (46,XX). An ultrasound examination repeated at 21 weeks gestational age confirmed the brain findings. Following counseling, the woman decided on a therapeutic abortion. Labor was induced with misoprostol at 22 weeks gestational age.
Figure 1.
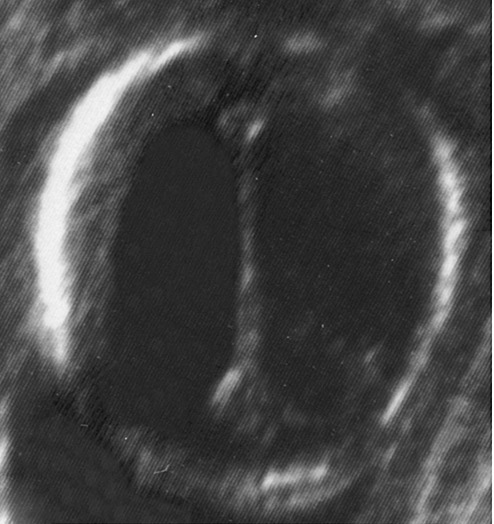
Ultrasound image of the fetal head in the horizontal plane at 21 weeks gestational age showing marked dilatation of the lateral ventricles (Case 1).
At autopsy, there were no abnormalities except for a relatively large head (body weight 397 g and head circumference 20 cm; 35th percentile and 60th percentile, respectively) (28). The brain weighed 80 g (90th percentile). The cerebral hemispheres were enlarged, with shallow calcarine fissures and frontal sulci, consistent with the gestational age. Coronal sections through the cerebral hemispheres demonstrated symmetric enlargement of the lateral ventricles. There was no obvious hemorrhage or discoloration. The corpus callosum was thin. Horizontal sections through the brainstem and cerebellum demonstrated a patent proximal cerebral aqueduct, but no visible aqueduct in the lower midbrain or upper pons. The fourth ventricle was small.
Histopathologic examination showed normal cortical architecture. The ganglionic eminence was intact; it exhibited normal proliferative activity and no evidence of hemorrhage. The proximal aqueduct was wide with blood cells and hemosiderin in the lumen and periaqueductal tissue and focal mineralization (Figure 2a). The caudal aqueduct and rostral fourth ventricle were closed with focal loss of ependyma and substantial collections of hemosiderin‐filled macrophages and cellular debris within the lumen. No vascular malformations were present. Hemosiderin‐containing macrophages were scattered in the leptomeninges. The primary source of hemorrhage could not be identified with certainty.
Figure 2a.
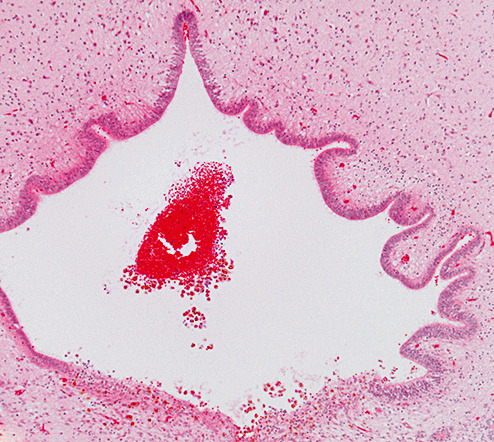
Photomicrograph showing a small blood clot in the proximal aqueduct of Case 1. The posterior aspect (lower part of figure) exhibits hemosiderin‐containing macrophages within the tissue (hematoxylin and eosin stain; magnification 100×).
Subsequent screening for fetal alloimmune thrombocytopenia was negative. During a subsequent pregnancy, detailed ultrasound examination revealed no anomalies, amniocentesis revealed a normal 46,XX karyotype, maternal serum screen showed no increased risk for fetal anomalies and a healthy girl was born.
Case 2
A 20‐year‐old woman (gravida 3 para 1 stillbirth 1) presented for routine ultrasound examination at the fetal assessment unit. Her history was significant for type 1 diabetes mellitus. Several years earlier, she had renal failure due to poor diabetic control, but control had improved during this pregnancy. There was no history of smoking, alcohol or drug use. There was no relevant family history. There was no consanguinity (previous pregnancies were with a different partner). Maternal serum screen did not indicate an increased risk for fetal anomalies. She was Rh+. At 21 weeks gestational age by dates, ultrasound examination showed a small fetus (estimated age 18 weeks) and multiple anomalies including severe oligohydramnios, absence of kidneys and hydrocephalus involving both lateral ventricles, which measured 12 mm in width. Fetal heart rate was within the normal range. There were reduced fetal movements. The placental site was anterior and the fetus was in breech position. Amniocentesis showed a normal male karyotype (46,XY). Following counseling, the woman decided to terminate the pregnancy. Labor was induced at 21 weeks with misoprostol.
The body weight was 269 g and the head circumference 18 cm (15th percentile and 45th percentile, respectively). Autopsy revealed cervical and axillary pterygia, right foot with axial polydactyly, clefted soft palate, hypoplastic mandible, low‐set ears, prominent inner canthal folds, relatively large head (Figure 2b), multiple lumbar hemivertebrae, 11 ribs on the right side, acetabular hypoplasia, renal agenesis with oligohydramnios sequence, hypoplastic lungs and right‐sided aortic arch. One diagnostic consideration was VACTERL (V—Vertebral anomalies, A—Anal atresia, C—Cardiovascular anomalies, T—Tracheoesophageal fistula, E—Esophageal atresia, R—Renal and/or radial anomalies, L—Limb anomalies)‐hydrocephalus association (19). The brain weighed 71 g (90th percentile). The cerebral hemispheres were symmetric and distended with minimal definition of the calcarine sulci and bulging at the infundibulum. Coronal sections demonstrated enlarged lateral ventricles with severe thinning of the cerebrum (3 mm parietal and 5 mm frontal). There was no obvious hemorrhage or discoloration. The third ventricle was only mildly widened, the cerebral aqueduct was very small and the fourth ventricle was small but patent. Macroscopic hemorrhage was not apparent.
Figure 2b.
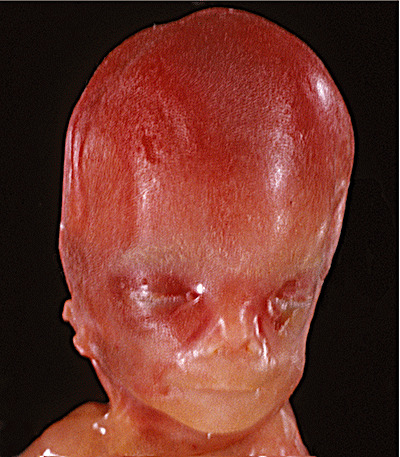
Photograph showing enlarged head of Case 2.
On microscopic examination, the cortical architecture was normal. There were irregularities on the surface of the left ganglionic eminence with focal interruption of the immature ependyma and growth of capillaries onto the ventricle surface; although there was no hemosiderin, this was considered to be scarring at a probable site of hemorrhage. The aqueduct lumen was obstructed by blood and hemosiderin‐containing macrophages (2c, 2d). However, the features of primary aqueduct stenosis/atresia were not present. The ependyma was generally intact, the anterior–posterior diameter was normal and there were no ancillary ependyma‐lined channels in the periaqueductal region.
Figure 2c.
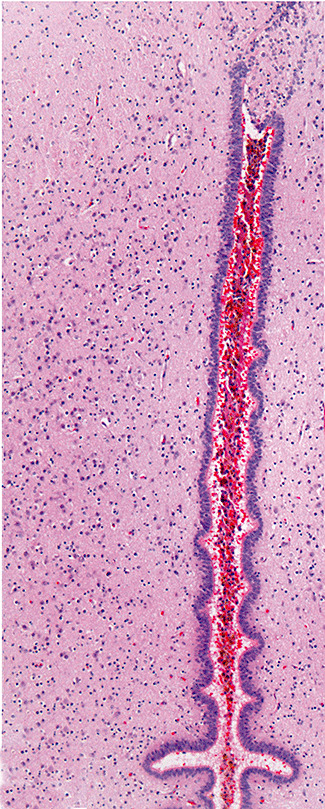
Photomicrograph showing slit‐like cerebral aqueduct occluded by blood (Case 2; hematoxylin and eosin stain; magnification 100×).
Figure 2d.
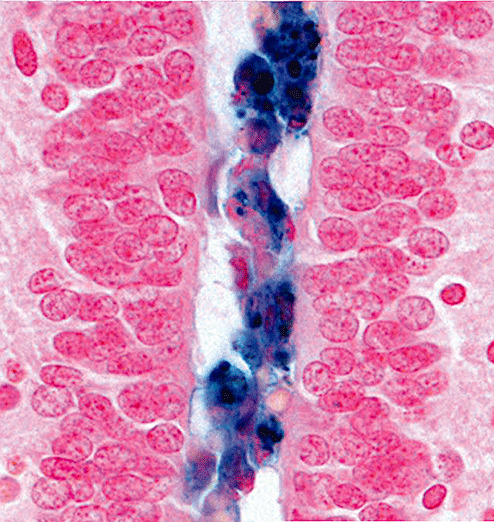
Photomicrograph showing hemosiderin‐containing macrophages in the obstructed lumen (Case 2; Perls' Prussian blue method for iron; magnification 600×).
Clinical follow up was limited. Screening for bleeding diathesis did not occur. During a subsequent hospital admission for diabetic complications, the woman was found to be pregnant. Serum alpha‐fetoprotein level was within normal limits. Ultrasound examination of the fetus revealed tetralogy of Fallot but no other anomalies. The woman developed diabetic ketoacidosis and pancreatitis, following which the fetus died.
Case 3
A 32‐year‐old woman (gravida 3 stillbirth 1 para 1) had a routine ultrasound examination at approximately 18 weeks gestation that showed mild fetal hydrocephalus. There was no significant personal or family medical history and no history of teratogen exposure. A follow‐up fetal assessment at 22 weeks gestation confirmed the presence of severe fetal hydrocephalus, with the right and left lateral ventricles enlarged at 11 mm and 25 mm, respectively. The third ventricle was slightly enlarged. The cerebellum was slightly small. A mass, interpreted as the tongue, protruded from the mouth. Fetal movements were normal. An amniocentesis showed a normal female karyotype (46,XX) and a normal alpha‐fetoprotein level. Following counseling, the woman decided on a therapeutic abortion. Labor was induced with misoprostol at 22 weeks gestation.
The body weight was 625 g (95th percentile). Autopsy findings included orbital hypertelorism, flattened broad nasal ridge, narrow but patent nares and a hairy polyp (epignathus) arising from the hard palate (24). The skull base was entirely normal. The brain weighed 101 g (>95th percentile). The ventricles were severely enlarged (left > right). Development of the calcarine fissure and central sulcus were appropriate for the gestational age. Coronal sections through the cerebral hemispheres demonstrated thinning of the cortical mantle. There was no obvious hemorrhage or discoloration. No patent aqueduct was apparent in the midbrain. The fourth ventricle was small and the cerebellum was normal.
Microscopic examination of the brain showed normal cortical architecture. The corpus callosum was thin. Hemosiderin and a nearby collection of lymphocytes and macrophages were present at the base of the lateral ventricle choroid plexus. This was likely a site of hemorrhage. Inflammation was not found elsewhere in the brain and there was no evidence for an infectious process. The cerebral aqueduct exhibited narrowing in the upper midbrain with occlusion by hemosiderin‐containing macrophages (HLA‐DR immunoreactive). Reactive microglia (HLA‐DR immunoreactive) and hypertrophic astrocytes (GFAP immunoreactive) were present at a few foci along the aqueduct. In the lower midbrain the opposing walls of the aqueduct were fused. Among the ependymal cilia were intact erythrocytes and macrophages (CD68 immunoreactive). Capillaries (CD31 immunoreactive) had grown into the canal (2e, 2f, 2g, 2h). The fourth ventricle was of normal size and the surrounding structures were unremarkable.
Figure 2e.
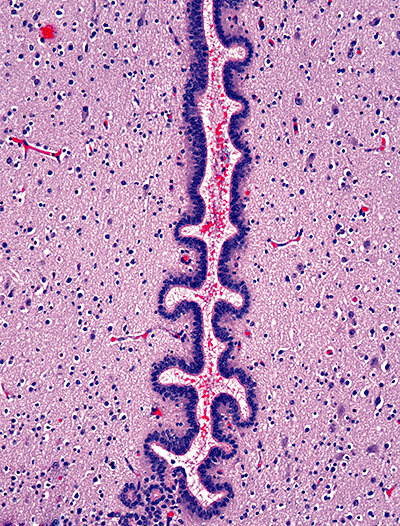
Photomicrograph showing slit‐like cerebral aqueduct occluded by blood (Case 3; hematoxylin and eosin stain; magnification 100×).
Figure 2f.
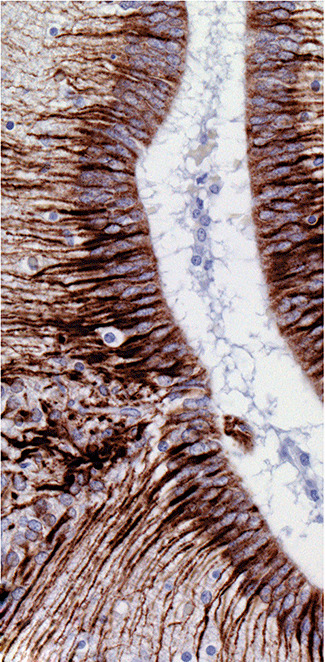
Photomicrograph showing immunoreactivity for glial fibrillary acidic protein around the cerebral aqueduct of Case 3. The basal processes of ependymocytes are prominently labeled and rare immunoreactive cells are present in the occluded lumen (brown diaminobenzidine reaction product and hematoxylin counterstain; magnification 400×).
Figure 2g.
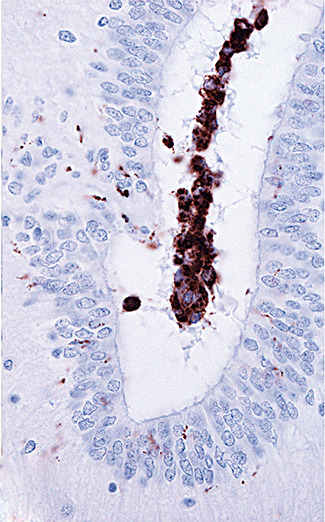
Photomicrograph showing immunoreactivity for CD68 within the cerebral aqueduct of Case 3. The labeled cells are macrophages. Delicate immunoreactive processes of reactive microglia are present in the surrounding tissue (brown diaminobenzidine reaction product and hematoxylin counterstain; magnification 600×).
Figure 2h.
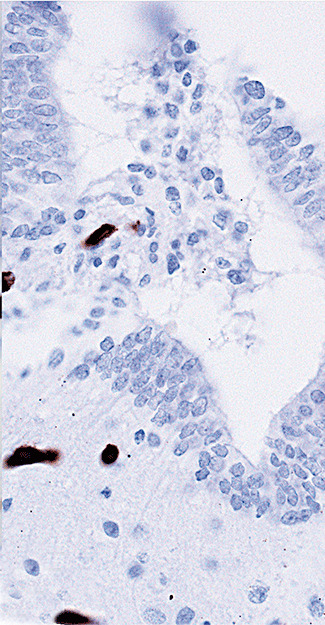
Photomicrograph showing immunoreactivity for CD31 around the cerebral aqueduct of Case 3. The labeled cells are capillary endothelial cells, some of which have grown into the obstructed lumen (brown diaminobenzidine reaction product and hematoxylin counterstain; magnification 600×).
Case 4
A 28‐year‐old woman (gravida 3 para 2) underwent ultrasound imaging at approximately 17 weeks gestation. Ventriculomegaly with cortical thinning was identified. Intracardiac fetal blood sampling revealed severe fetal thrombocytopenia. Fetal karyotype was normal male (46,XY). Past obstetric history included one liveborn child with congenital hydrocephalus, who died within 1 h of birth (no autopsy record was available). Another child had severe thrombocytopenia as an infant, but suffered no long‐term sequelae (unfortunately, the available records do not specify the sex of the other children). Once counseled, the woman opted for a therapeutic termination of pregnancy. Labor was induced with prostaglandins at 19 weeks gestation and a 122 g male fetus (<5th percentile) was delivered.
External examination showed extensive petechial hemorrhage involving the entire body surface. Apart from a tall appearing head (13 cm diameter; <5th percentile) and retrognathia, no other developmental abnormalities were present. The surfaces of all internal organs showed petechial hemorrhages. On sectioning of the brain (weight not recorded), petechiae could be identified along the walls of the lateral ventricles. There was no intraventricular hematoma. Microscopically, sites of hemorrhage (up to 600 µm diameter) were found near the ventricular surface of the upper midbrain and thalamus (Figure 2i), as well as in the lateral cerebellum. The narrowest part of the cerebral aqueduct was obstructed by blood debris and macrophages (Figure 2j). In addition, hemosiderin‐containing macrophages were present in the fourth ventricle choroid plexus and in the leptomeninges near the fourth ventricle outlets.
Figure 2i.
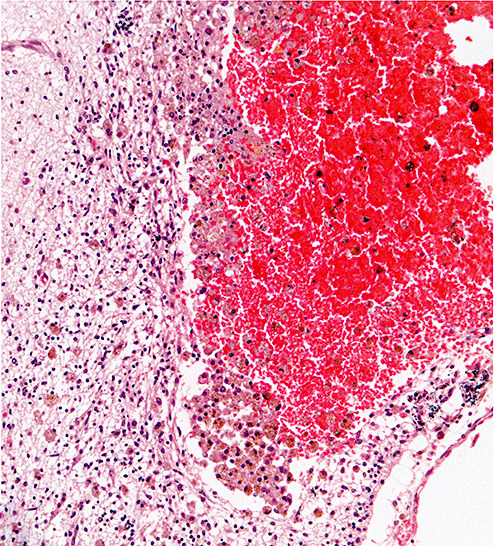
Photomicrograph showing hemorrhagic site in thalamus of Case 4. The small hematoma exhibits hemosiderin‐containing macrophages within the surrounding tissue (hematoxylin and eosin stain; magnification 200×).
Figure 2j.
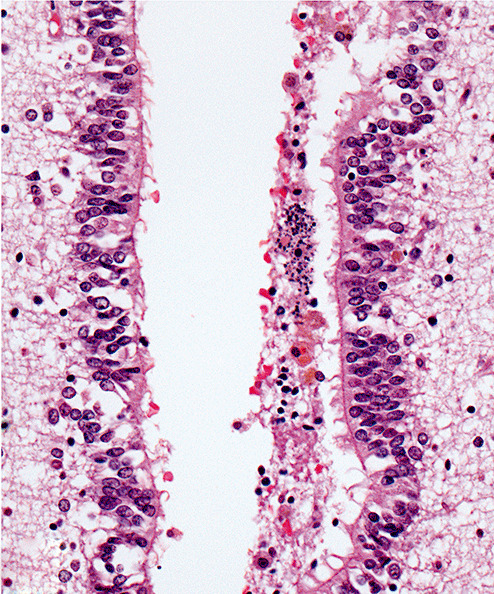
Photomicrograph showing cerebral aqueduct of Case 4 occluded by blood breakdown products including hemosiderin‐containing macrophages. Granular calcification is apparent. (hematoxylin and eosin stain; magnification 600×).
DISCUSSION
Fetal/congenital hydrocephalus is a heterogeneous condition with an incidence of 4–10/10 000 pregnancies 5, 13, 16, 39. Although one of the most important predictors of outcome is etiology, the factors involved in the development of fetal hydrocephalus are varied and not necessarily easy to identify during the early intrauterine period. Size of the ventricles, maturity of the fetus when hydrocephalus develops and associated malformations all contribute to the long‐term prognosis. In general, outcomes are superior if mild to moderate hydrocephalus develops later in pregnancy when no complicating brain or chromosomal anomalies are present 11, 22, 32.
These four cases are interesting because midgestation fetal hydrocephalus was caused by tiny blood clots in the cerebral aqueduct in the absence of overt intraventricular blood. Rapidly progressive ventricular enlargement can disrupt blood vessels in the ventricle walls, particularly at the lateral angles of the frontal horns and less often in the floor of the third ventricle (6). Such secondary lesions were not identified in these cases. In Cases 2 and 3, subtle changes suggested choroid plexus and ganglionic eminence as sources of blood, and in Case 4, the source was obvious. In all cases, the clots were located at sites of aqueductal obstruction, leading to the conclusion that they are the cause and not the result of the hydrocephalus. The hemosiderin‐filled macrophages in the cerebral aqueduct must be distinguished from the macrophages that appear on the ventricle wall in response to fetal hydrocephalus 9, 40. Finally, a significant proportion of premature infants who die soon after birth are found to have acute hemorrhagic lesions in the germinal matrix (7). This type of hematoma was not identified in any of these cases, and furthermore, the aqueductal clots were all subacute with evidence of organization in some. Hemorrhagic obstruction of the aqueduct has been documented in a postnatal case with overt intraventricular bleeding (17).
The identification of aqueductal obstruction by blood raises counseling issues. In Case 1, this appears to be an isolated phenomenon, probably without significant risk of inheritability. In Case 4, there was a known coagulopathy with overt hemorrhagic consequences throughout the body. In Case 3, the association of epignathus and hydrocephalus appears to be entirely coincidental; no similar cases could be found in the literature. As such, the risk of recurrence is probably low. Case 2 is more problematic as it is obviously syndromic. One possible underlying cause is diabetic embryopathy (29). In most cases of VACTERL with hydrocephalus, the hydrocephalus is caused by aqueduct stenosis or atresia 19, 27. The senior author has examined the brains of three infants with VACTERL with hydrocephalus; in all three, the aqueduct channel was either tiny or absent and replaced by clusters of ependymal cells (unpublished findings). The case described in this report is entirely different; here, the aqueduct dimensions were normal but the lumen was occluded by blood clot. Because hemorrhage was the cause of aqueduct obstruction in this case, the VACTERL association and the hydrocephalus should be considered coincidental. VACTERL has been reported in conjunction with Fanconi anemia (4); in rare cases, thrombocytopenia due to Fanconi anemia can present in the neonatal period (34). Another possible explanation for bleeding is the poorly controlled maternal diabetes, which can cause impaired fetal blood clotting (35). Other maternal factors, such as vasoactive and anticoagulant drugs, thrombocytopenia and other coagulopathies, pre‐eclampsia, infections and even minor abdominal trauma are recognized risk factors for fetal intracranial hemorrhage 20, 21, 37, 38.
Cryptic intraventricular hemorrhage is perhaps an underrecognized pathogenesis for fetal hydrocephalus. Review of the records of the Department of Pathology at the Health Sciences Centre in Manitoba, Canada showed that ∼2.7 cases per year are submitted following fetal terminations for hydrocephalus. During the period these cases were observed, other causes of fetal hydrocephalus leading to termination of pregnancy included myelomeningocele with aqueductal stenosis/atresia (7 cases), isolated aqueductal stenosis/atresia (2 cases), venous angioma compressing the aqueduct (1 case), complex posterior fossa malformation (2 cases), unilateral hydrocephalus due to abnormality of foramen of Monro (2 cases), aqueduct scarring with calcification possibly due to viral infection (2 cases), lissencephaly (2 cases), isolated large cerebellar hemorrhage (1 case), maternal warfarin associated with fetal brain hemorrhage (1 case), congenital teratoma (1 case) and indeterminate cause associated with chromosomal anomaly (2 cases); in the remainder, the cause was not identifiable with certainty because of autolytic changes. Thus, in our population, obstruction of the cerebral aqueduct by tiny blood clots contributes to fetal hydrocephalus in a conservative estimate of >5% of cases. It is not clear why this has not been reported previously. In our catchment area (population ∼1.2 million), ∼8 fetal hydrocephalus cases per year are diagnosed by ultrasonography (39). The precise pathology was not described in that study, and the fate of those not terminated is beyond the scope of this manuscript.
Currently accepted screening tests, including maternal serum assays and ultrasound technology, cannot diagnose either cryptic intracerebral hemorrhage or the subsequent aqueduct stenosis 5, 39. In this situation, magnetic resonance imaging (MRI) might be an important adjuvant to ultrasound examination 3, 15, 23, 30, due to its superior soft tissue contrast and its sensitivity to iron‐containing molecules such as hemosiderin (1). However, the fast sequence T2‐weighted spin echo images often obtained in fetuses likely would not detect small hemosiderin aggregates. Gradient echo or fast low‐angle shot T1‐weighted imaging would be more likely to detect this type of abnormality 26, 36, although we could not find any reports of fetal MRI with abnormalities suggestive of hemosiderin in the aqueduct. Current postnatal surgical management of fetal hydrocephalus has a less than favorable outcome 11, 33. There is renewed interest for intrauterine shunting, at least in an experimental setting 2, 41. Hydrocephalus due to aqueductal occlusion by blood without major destructive process might have a relatively good prognosis if identified and treated early.
The four cases described here illustrate that cryptic intracerebral hemorrhage may be an etiological factor in the pathogenesis of early onset fetal hydrocephalus. Comprehensive autopsy with detailed microscopic examination of the brain is necessary to document the aqueductal abnormalities and possible sites of hemorrhage, which may not be apparent macroscopically. Although focal hemorrhage is most likely sporadic, it demands investigation of the blood coagulation system. Counseling with respect to the likelihood of recurrence will differ if fetal hydrocephalus is secondary to a minor hemorrhagic event rather than a complex malformative process.
CONFLICTS OF INTEREST
None.
ACKNOWLEDGMENTS
We thank Sharon Allen and Susan Janeczko for technical processing of the tissues. We thank Dr. M. Bunge for helpful discussions of the imaging. Dr. Del Bigio holds the Canada Research Chair in Developmental Neuropathology.
REFERENCES
- 1. Brass SD, Chen NK, Mulkern RV, Bakshi R (2006) Magnetic resonance imaging of iron deposition in neurological disorders. Top Magn Reson Imaging 17:31–40. [DOI] [PubMed] [Google Scholar]
- 2. Bruner JP, Davis G, Tulipan N (2006) Intrauterine shunt for obstructive hydrocephalus—still not ready. Fetal Diagn Ther 21:532–539. [DOI] [PubMed] [Google Scholar]
- 3. Canapicchi R, Cioni G, Strigini FA, Abbruzzese A, Bartalena L, Lencioni G (1998) Prenatal diagnosis of periventricular hemorrhage by fetal brain magnetic resonance imaging. Childs Nerv Syst 14:689–692. [DOI] [PubMed] [Google Scholar]
- 4. Cox PM, Gibson RA, Morgan N, Brueton LA (1997) VACTERL with hydrocephalus in twins due to Fanconi anemia (FA): mutation in the FAC gene. Am J Med Genet 68:86–90. [PubMed] [Google Scholar]
- 5. Davis GH (2003) Fetal hydrocephalus. Clin Perinatol 30:531–539. [DOI] [PubMed] [Google Scholar]
- 6. Del Bigio MR (2004) Cellular damage and prevention in childhood hydrocephalus. Brain Pathol 14:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Del Bigio MR (2004) Hemorrhagic lesions. In: Developmental Neuropathology, Golden JA, Harding BN (eds), pp. 150–155. International Society of Neuropathology Press: Basel, Switzerland. [Google Scholar]
- 8. Fukui K, Morioka T, Nishio S, Mihara F, Nakayama H, Tsukimori K, Fukui M (2001) Fetal germinal matrix and intraventricular haemorrhage diagnosed by MRI. Neuroradiology 43:68–72. [DOI] [PubMed] [Google Scholar]
- 9. Fukumizu M, Takashima S, Becker LE (1996) Glial reaction in periventricular areas of the brainstem in fetal and neonatal posthemorrhagic hydrocephalus and congenital hydrocephalus. Brain Dev 18:40–45. [DOI] [PubMed] [Google Scholar]
- 10. Fusch C, Ozdoba C, Kuhn P, Durig P, Remonda L, Muller C et al (1997) Perinatal ultrasonography and magnetic resonance imaging findings in congenital hydrocephalus associated with fetal intraventricular hemorrhage. Am J Obstet Gynecol 177:512–518. [DOI] [PubMed] [Google Scholar]
- 11. Futagi Y, Suzuki Y, Toribe Y, Morimoto K (2002) Neurodevelopmental outcome in children with fetal hydrocephalus. Pediatr Neurol 27:111–116. [DOI] [PubMed] [Google Scholar]
- 12. Ghi T, Simonazzi G, Perolo A, Savelli L, Sandri F, Bernardi B et al (2003) Outcome of antenatally diagnosed intracranial hemorrhage: case series and review of the literature. Ultrasound Obstet Gynecol 22:121–130. [DOI] [PubMed] [Google Scholar]
- 13. Gucciardi E, Pietrusiak MA, Reynolds DL, Rouleau J (2002) Incidence of neural tube defects in Ontario, 1986–1999. CMAJ 167:237–240. [PMC free article] [PubMed] [Google Scholar]
- 14. Gunn TR, Mora JD, Becroft DM (1988) Congenital hydrocephalus secondary to prenatal intracranial haemorrhage. Aust N Z J Obstet Gynaecol 28:197–200. [DOI] [PubMed] [Google Scholar]
- 15. Hashimoto I, Tada K, Nakatsuka M, Nakata T, Inoue N, Takata M et al (1999) Fetal hydrocephalus secondary to intraventricular hemorrhage diagnosed by ultrasonography and in utero fast magnetic resonance imaging. A case report. Fetal Diagn Ther 14:248–253. [DOI] [PubMed] [Google Scholar]
- 16. Hay S (1971) Incidence of selected congenital malformations in Iowa. Am J Epidemiol 94:572–584. [DOI] [PubMed] [Google Scholar]
- 17. Hill A, Rozdilsky B (1984) Congenital hydrocephalus secondary to intra‐uterine germinal matrix/intraventricular haemorrhage. Dev Med Child Neurol 26:524–527. [DOI] [PubMed] [Google Scholar]
- 18. Huang YF, Chen WC, Tseng JJ, Ho ES, Chou MM (2006) Fetal intracranial hemorrhage (fetal stroke): report of four antenatally diagnosed cases and review of the literature. Taiwan J Obstet Gynecol 45:135–141. [DOI] [PubMed] [Google Scholar]
- 19. Iafolla AK, McConkie‐Rosell A, Chen YT (1991) VATER and hydrocephalus: distinct syndrome? Am J Med Genet 38:46–51. [DOI] [PubMed] [Google Scholar]
- 20. Kaplan C (2002) Alloimmune thrombocytopenia of the fetus and the newborn. Blood Rev 16:69–72. [DOI] [PubMed] [Google Scholar]
- 21. Kim MW, Choi HM (2006) Fetal hydrocephalus in a pregnancy complicated by idiopathic thrombocytopenic purpura. J Ultrasound Med 25:777–780. [DOI] [PubMed] [Google Scholar]
- 22. Kirkinen P, Serlo W, Jouppila P, Ryynanen M, Martikainen A (1996) Long‐term outcome of fetal hydrocephaly. J Child Neurol 11:189–192. [DOI] [PubMed] [Google Scholar]
- 23. Kirkinen P, Partanen K, Ryynanen M, Orden MR (1997) Fetal intracranial hemorrhage. Imaging by ultrasound and magnetic resonance imaging. J Reprod Med 42:467–472. [PubMed] [Google Scholar]
- 24. Kumar B, Sharma SB (2008) Neonatal oral tumors: congenital epulis and epignathus. J Pediatr Surg 43:e9–e11. [DOI] [PubMed] [Google Scholar]
- 25. Leidig E, Dannecker G, Pfeiffer KH, Salinas R, Peiffer J (1988) Intrauterine development of posthaemorrhagic hydrocephalus. Eur J Pediatr 147:26–29. [DOI] [PubMed] [Google Scholar]
- 26. Levine D, Barnes PD, Robertson RR, Wong G, Mehta TS (2003) Fast MR imaging of fetal central nervous system abnormalities. Radiology 229:51–61. [DOI] [PubMed] [Google Scholar]
- 27. Lomas FE, Dahlstrom JE, Ford JH (1998) VACTERL with hydrocephalus: family with X‐linked VACTERL‐H. Am J Med Genet 76:74–78. [DOI] [PubMed] [Google Scholar]
- 28. Maroun LL, Graem N (2005) Autopsy standards of body parameters and fresh organ weights in nonmacerated and macerated human fetuses. Pediatr Dev Pathol 8:204–217. [DOI] [PubMed] [Google Scholar]
- 29. McLeod L, Ray JG (2002) Prevention and detection of diabetic embryopathy. Community Genet 5:33–39. [DOI] [PubMed] [Google Scholar]
- 30. Messing‐Junger AM, Rohrig A, Stressig R, Schaper J, Turowski B, Blondin D (2009) Fetal MRI of the central nervous system: clinical relevance. Childs Nerv Syst 25:165–171. [DOI] [PubMed] [Google Scholar]
- 31. Morioka T, Hashiguchi K, Nagata S, Miyagi Y, Mihara F, Hikino S et al (2006) Fetal germinal matrix and intraventricular hemorrhage. Pediatr Neurosurg 42:354–361. [DOI] [PubMed] [Google Scholar]
- 32. Oi S (2003) Diagnosis, outcome, and management of fetal abnormalities: fetal hydrocephalus. Childs Nerv Syst 19:508–516. [DOI] [PubMed] [Google Scholar]
- 33. Oi S, Honda Y, Hidaka M, Sato O, Matsumoto S (1998) Intrauterine high‐resolution magnetic resonance imaging in fetal hydrocephalus and prenatal estimation of postnatal outcomes with “perspective classification”. J Neurosurg 88:685–694. [DOI] [PubMed] [Google Scholar]
- 34. Roberts I, Stanworth S, Murray NA (2008) Thrombocytopenia in the neonate. Blood Rev 22:173–186. [DOI] [PubMed] [Google Scholar]
- 35. Salvesen DR, Brudenell MJ, Nicolaides KH (1992) Fetal polycythemia and thrombocytopenia in pregnancies complicated by maternal diabetes mellitus. Am J Obstet Gynecol 166:1287–1293. [DOI] [PubMed] [Google Scholar]
- 36. Shakudo M, Inoue Y, Mochizuki K, Manabe T, Mura K, Matsu R et al (2001) Fast MR imaging and ultrafast MR imaging of fetal central nervous system abnormalities. Osaka City Med J 47:127–135. [PubMed] [Google Scholar]
- 37. Sherer DM, Anyaegbunam A, Onyeije C (1998) Antepartum fetal intracranial hemorrhage, predisposing factors and prenatal sonography: a review. Am J Perinatol 15:431–441. [DOI] [PubMed] [Google Scholar]
- 38. Strigini FA, Cioni G, Canapicchi R, Nardini V, Capriello P, Carmignani A (2001) Fetal intracranial hemorrhage: is minor maternal trauma a possible pathogenetic factor? Ultrasound Obstet Gynecol 18:335–342. [DOI] [PubMed] [Google Scholar]
- 39. Szajkowski TP, Chodirker BN, Macdonald KM, Evans JA (2006) Maternal serum alpha‐fetoprotein levels in fetal hydrocephalus: a retrospective population based study. BMC Pregnancy Childbirth 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ulfig N, Bohl J, Neudorfer F, Rezaie P (2004) Brain macrophages and microglia in human fetal hydrocephalus. Brain Dev 26:307–315. [DOI] [PubMed] [Google Scholar]
- 41. Von Koch CS, Gupta N, Sutton LN, Sun PP (2003) In utero surgery for hydrocephalus. Childs Nerv Syst 19:574–586. [DOI] [PubMed] [Google Scholar]


