CLINICAL DETAILS
A 72‐year‐old man presented abruptly with a grand mal seizure while he was dancing. He was admitted to our hospital and physical examination detected mild left upper limb paresis. His medical history included idiopathic hypertension and non insulin‐dependent diabetes, both under specific drug therapy. Cranial magnetic resonance imaging (MRI) revealed a 4 × 4 × 3‐cm extraaxial lesion, isointense on T1‐weighted images and hyperintense on T2‐weighted images, with heterogenous enhancement. It was located in the posterior right frontal convexity with poorly defined boundaries and remarkable perilesional edema (Fig. 1). The patient then underwent total microsurgical resection of the hypervascular intracranial mass. During operation, the tumor was found to be a mass partially encapsulated with dural attachment. The surgical appearance was felt to be atypical for meningioma. The dissection showed a prominent and arterialized venous drainage and no evident cleavage plane in some areas. A small piece of tumor was submitted for frozen section (Fig 2).
Figure 1.
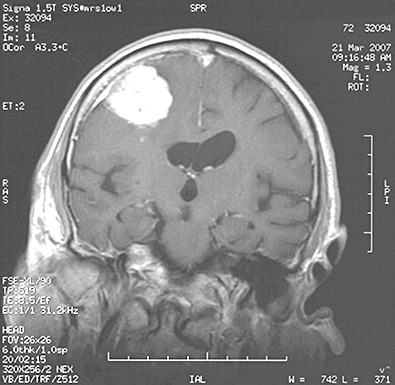
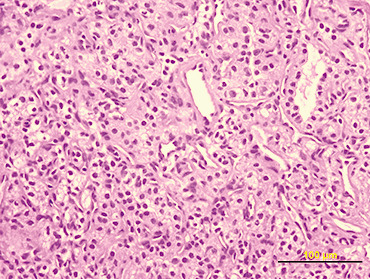
Figure 2.
PATHOLOGICAL EXAMINATION
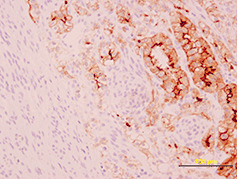
On macroscopic examination, the tumor was irregularly spherical (17 gm weight and 4 × 4 × 3 cm mass). Under a rough gray surface, an extensive solid and soft yellowish area emerged. Serial sections showed that this area occupied two‐third of the tumor. Histological examination showed a large central soft area that consisted of polyhedral cells with clear cytoplasm and mild atypia, arranged in alveolar and glandular patterns with scarce fibrovascular stroma (Fig 2). The peripheral zone was composed of uniform sheets of polygonal and spindle cells (2, 3). Psammoma bodies were also identified in the tumor (Fig. 3). Immunohistochemical staining for cytokeratin 8 (Fig. 4) and CD 10 (Fig. 5) showed positivity only in the clear cell component.
Figure 3.
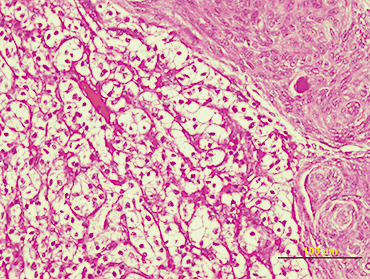
Figure 4.
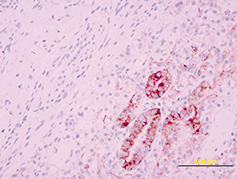
Figure 5.
DIAGNOSIS
Metastatic clear‐cell type of renal carcinoma in a meningothelial meningioma.
He had an uneventful post‐operative period. The postoperative evaluation was completed with a body CT‐scan, which revealed a mass‐lesion in the right kidney and two nodules in the right lung. He underwent whole brain radiotherapy, right radical nephrectomy and adjuvant chemotherapy. The patient was neurological symptom‐free on follow‐up after 3 months.
DISCUSSION
Tumor to tumor metastasis is a rare pathological event. Meningiomas have been found to be the most common intracranial tumor to host a metastatic neoplasia. Among these, breast followed by lung carcinoma were the most common primary sites (1). Several theories and biological factors (metabolic, hemodynamic, hormonal, immunological and molecular) have been advanced concerning the genesis of this unusual event (2). Radiologic techniques cannot reliably exclude the presence of metastatic tumor within a meningioma, nor can they establish a definitive diagnosis between meningioma and dural metastasis (10). Both possibilities should be considered in those patients presenting with a solitary intracranial lesion suggestive of meningioma whether or not they harbor an extracranial primary tumor at the same time.
Brain metastases in renal cell carcinoma are not rare events, although it is very rare from a renal cell carcinoma staged T1‐2 without systemic metastases. Both metastases in advanced staging cases and isolated metastasis discovered some years after nephrectomy have been reported (7). Only a few cases of renal cell carcinoma metastasizing to an intracranial meningioma have been reported 3, 5, 6, 8, 9. In those cases, a primary renal tumor was known before the neurosurgical procedure. Our case is the first reported metastatic renal cell carcinoma within a meningothelial meningioma without previous knowledge of the existence of the primary malignant tumor.
Therefore, this case could be considered unique: tumor‐to tumor metastasis as the clinical onset of an extracranial neoplasia. Due to the overlapping in clinical presentation and despite advances in imaging, pathological examination is still required for its correct diagnosis. In such cases it is imperative to exclude the occurrence of collision between tumors. In order to assess a true metastasis in the tumor, criteria suggested by Campbell et al could be used (4).
ABSTRACT
Metastasis of an extracranial malignant neoplasm to a meningioma is a rare event. We report a case of a 72‐year‐old man who presented abruptly with a grand mal seizure. Neuroradiological examination revealed an extraaxial lesion located in the posterior right frontal convexity with poorly defined boundaries. Histological and immunohistochemical examination showed that the lesion was a meningothelial meningioma harboring metastatic renal cell carcinoma. The MRI could be indicative but not specific of this type of lesion. Some cases of intracranial meningiomas containing metastatic carcinomas have been published, but to our knowledge only five cases of renal cell carcinoma metastasizing to a meningioma have been reported. Possible explanations for this type of “tumor in tumor” lesion are reviewed.
REFERENCES
- 1. Altinoz MA, Santaguida C, Guiot MC, Del Maestro RF (2005) Spinal hemangioblastoma containing metastatic renal cell carcinoma in von Hippel‐Lindau disease. Case report and review of the literature. J Neurosurg Spine 2005 3:495–500. [DOI] [PubMed] [Google Scholar]
- 2. Benedetto N, Perrini P, Scollato A, Buccoliero AM, Di Lorenzo N (2007) Intracranial meningioma containing metastatic colon carcinoma. Acta Neurochir 149:799–803. [DOI] [PubMed] [Google Scholar]
- 3. Breadmore R, House R, Gonzales M (1994) Metastasis of renal cell carcinoma to a meningioma. Australas Radiol 38:141–3. [DOI] [PubMed] [Google Scholar]
- 4. Campbell LV, Gilbert E, Chamberlein CR Jr, Watne AL (1968) Metastasis of cancer to cancer. Cancer 22:635–643. [DOI] [PubMed] [Google Scholar]
- 5. Han HS, Kim EY, Han JY, Kim YB, Hwang TS, Chu YC (2000) Metastatic renal cell carcinoma in a meningioma: a case report. J Korean Med Sci 15:593–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kimiwada T, Motohashi O, Kumabe T, Watanabe M, Tominaga T (2004) Lipomatous meningioma of the brain harboring metastatic renal‐cell carcinoma: a case report. Brain Tumor Pathol 21:47–52. [DOI] [PubMed] [Google Scholar]
- 7. Sadatomo T, Yuki K, Migita K, Taniguchi E, Kodama Y, Kurisu K (2005) Solitary brain metastasis from renal cell carcinoma carcinoma 15 years after nephrectomy: case report. Neurol Med Chir 45:423–7. [DOI] [PubMed] [Google Scholar]
- 8. Storberg DH (1957) Metastases of carcinoma to meningioma. J Neurosurg 14:337–343. [DOI] [PubMed] [Google Scholar]
- 9. Stortbecker TP (1951) Metastatic hypernephroma of the brain from a neurosurgical point of view. J Neurosurg 8:185–197. [DOI] [PubMed] [Google Scholar]
- 10. Tagle P, Villanueva P, Torrealba G, Huete I (2002) Intracraneal metastasis or meningioma? A uncommon clinical diagnostic dilemma. Surg Neurol 58:241–5. [DOI] [PubMed] [Google Scholar]


