CLINICAL HISTORY
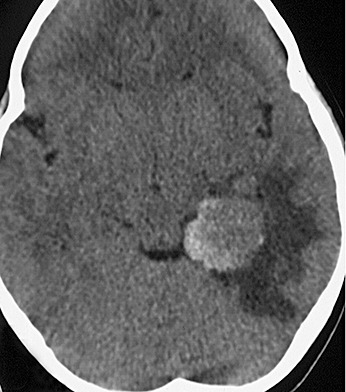
A 6‐year‐old Japanese girl presented with psychomotor seizures including oral automatism with cloudy consciousness, lasting for 2 months. On admission, no neurological deficit was evident except for seizures, and no abnormal manifestations of the skin were observed. Magnetic resonance (MR) images indicated a tumor with peritumoral edema in the left middle cranial fossa, displaying heterogeneous enhancement with intravenous administration of gadolinium. The medial part of the tumor consisted of an irregular‐shaped region, which was hyper‐intense on T1‐weighted images and hypo‐intense on T2‐weighted images (Fig. 1), indicating the presence of certain substances that shorten both T1 and T2 relaxation times, such as melanin, calcium and intracellular methemoglobin (4). This region was highlighted as a high‐density area on computed tomography (Fig. 2). The patient underwent tumor resection through a left occipital craniotomy. The tumor was an extra‐axial mass with focal blackish coloration in its medial portion, and intraoperative frozen sectioning showed a proliferation of pigmented cells among thick collagen fibers.
Figure 1.
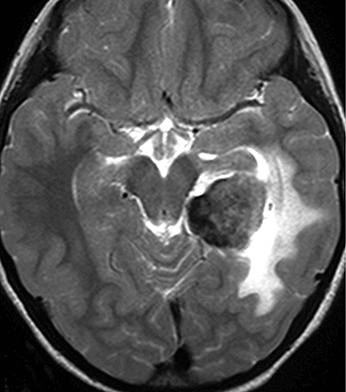
Figure 2.
GROSS AND MICROSCOPIC PATHOLOGY
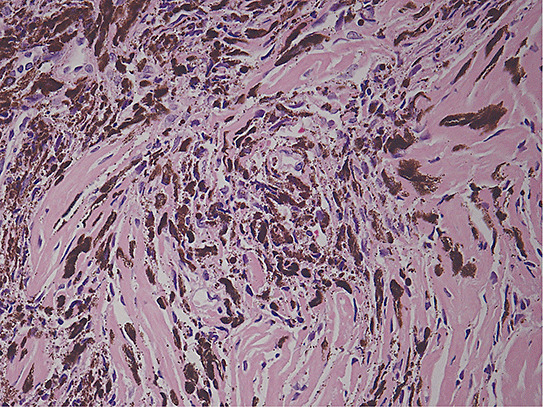
On gross inspection, the specimen was composed of white‐to‐grayish hard tissue, focally showing blackish pigmentation with the highest density at the surface of the tumor. The white‐to‐grayish lesion histologically consisted of a storiform arrangement of spindle‐shaped cells with abundant intercellular collagen fibers and occasional concentric structures (arrow, Fig. 3). There were also clusters of cells with large nuclei and prominent nucleoli (Fig. 4). Invasion of these cells into the surrounding gliotic cerebral tissue was focally noted, which was confirmed by glial fibrillary acidic protein (GFAP)‐immunostaining (asterisks, Fig. 5). While mitotic figures were rarely seen, the MIB‐1 (Ki‐67) staining index was increased up to 5.7% (Fig. 6). On the other hand, the blackish pigmentation at the surface of the tumor was composed of a dense accumulation of pigmented cells in the arachnoid membrane of the existing cerebral tissue, to which the tumor was firmly adhered (upper left: brain tissue, lower right: tumor, Fig. 7). The tumor tissue was highly fibrotic at the site of adhesion, and the pigmented cells infiltrated into the tumor tissue alongside the fibrosis (Fig. 8). Isolated clusters of these pigmented cells were also scattered in the tumor tissue (Fig. 9). These pigmented cells displayed low proliferative activity indicated by the absence of mitotic figures and negative immunoreactivity to Ki‐67.
Figure 3.
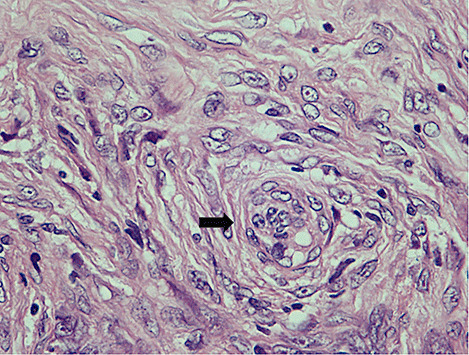
Figure 4.
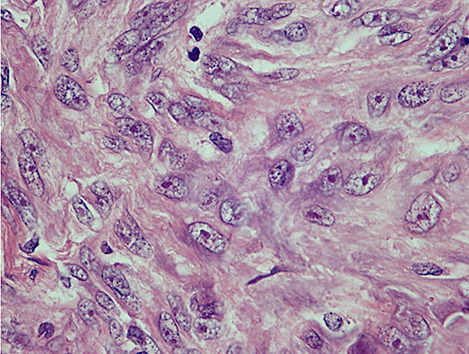
Figure 5.
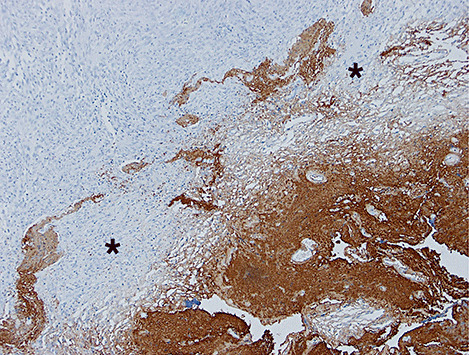
Figure 6.
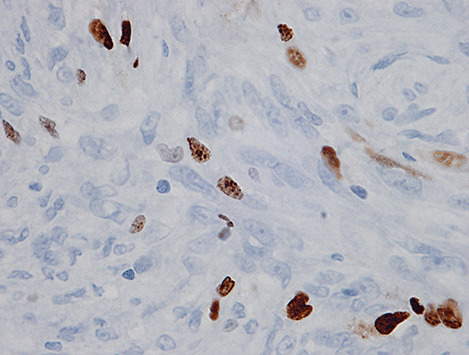
Figure 7.
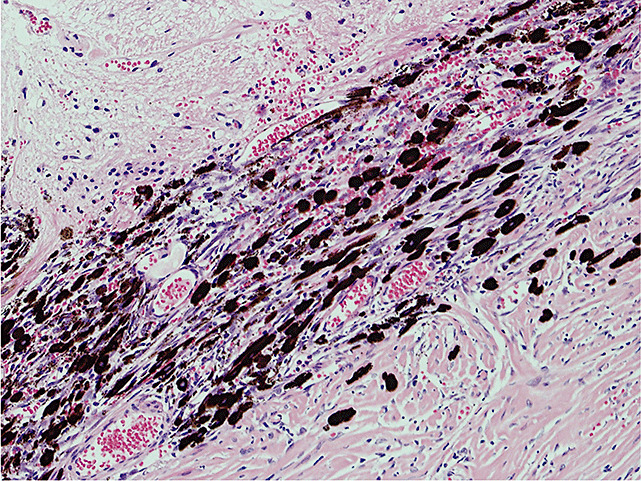
Figure 8.
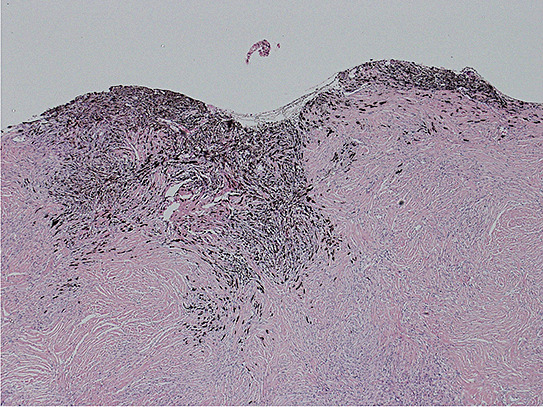
Figure 9.
Further immunohistochemical analyses showed that spindle‐shaped cells focally expressed epithelial membrane antigen (EMA), but not S‐100 protein and HMB‐45 whereas pigmented cells were immunopositive for S‐100 protein, but negative for EMA and HMB‐45.
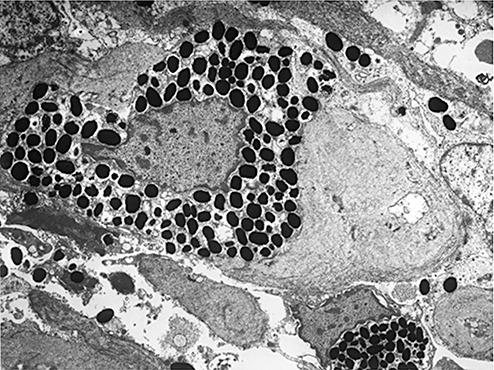
Electron microscopic examination showed that the spindle‐shaped cells had nuclei with cytoplasmic invagination, a fine chromatin pattern and conspicuous nucleoli, and abundant intermediate filaments in their cytoplasm. Interdigitation of the cytoplasmic processes and junctional complexes were also noted at the cell‐to‐cell borders (arrows, Fig. 10). In contrast, pigmented cells contained abundant pigment granules in their cytoplasm (Fig. 11), but displayed no features of spindle‐shaped cells.
Figure 10.
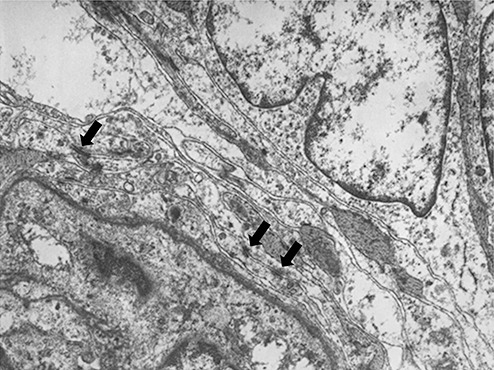
Figure 11.
What is the diagnosis?
DIAGNOSIS
Atypical meningioma associated with reactive hyperplasia and colonization of meningeal melanocytes.
DISCUSSION
The tumor was diagnosed as an atypical meningioma 7, 9. However, we observed accumulation of pigmented cells within the arachnoid and infiltration of these cells into the meningioma, which may be misleading in the diagnosis of melanocytic tumors, including primary melanocytic tumors (melanocytoma, malignant melanoma), melanotic progonoma and intracranial tumors of melanotic variants (melanotic schwannoma). These pigmented cells displayed no mitotic activity and negative immunoreactivity to Ki‐67. No meningioma cells contained melanin pigments. Pigmented cells were immunopositive for S‐100 protein but negative for EMA and HMB‐45, and ultrastructural studies showed that they contained abundant secondary melanosomes (melanin granules), consistent with non‐neoplastic, mature melanocytes (3). Based on these findings we concluded that this marked proliferation and infiltration of melanocytes could be due to reactive hyperplasia and their colonization in the tumor tissue.
“Melanocyte colonization” was originally described in breast carcinomas, and was defined as the presence of dendritic melanocytes in carcinomatous tissue (1). To date, melanocyte colonization has been reported in a variety of tumors, which are essentially non‐pigmented and in most instances of epithelial origin. To the best of our knowledge, only one case of an atypical meningioma with melanocytic colonization has been reported (8). Although there have been several previous reports on “melanotic meningiomas”, these cases are now considered to be meningeal melanocytomas 5, 6, 8 and thus are a different entity from a meningioma with melanocytic colonization. One interesting speculation made on the case of Nestor and colleagues was a racial factor; since the patient was African‐American, the number of melanocytes in the leptomeninges might have been much greater than in general Caucasians (8). However, our case was a Japanese child, indicating that this phenomenon should be taken into consideration regardless of either race or age.
In the central nervous system (CNS), melanocytes are preferentially localized in the leptomeninges especially at the base of the brain (7). Since meningiomas usually have a long‐standing, close association with the leptomeninges during tumor development, the source of the dendritic melanocytes seen in the previously‐reported case of meningioma with melanocytic colonization was speculated to be leptomeningeal in derivation (8). Our case clearly showed unusual expansion of melanocytes in the arachnoid membrane and a continuous flow of melanocytes into the meningioma, indicating that these melanocytes were derived from existing melanocytes normally residing in the leptomeninges.
For resident melanocytes to colonize into surrounding tissues and tumors, Azzopardi and Eusebi alluded to the necessity of melanocyte hyperplasia before they migrate towards the tumor tissue (1). A dense accumulation of non‐proliferative, mature melanocytes in our case indicated that reactive hyperplasia of melanocytes relevant to their colonization could occur in the tumors of the CNS. In the practice of clinical pathology, it is necessary to differentiate tumors with melanocytic features from tumors of melanocytic origin such as malignant melanoma, because of the difference in their biological behavior, aggressiveness and clinical outcome. Therefore, it is important for surgical pathologists to recognize this phenomenon and differentiate a meningioma with reactive hyperplasia of melanocytes from primary pigmented tumors of the meninges (melanocytoma and malignant melanomas) (2), a metastatic malignant melanoma to a meningioma (10), and other intracranial tumors of melanotic variants, especially in frozen or small biopsy samples.
ABSTRACT
A 6‐year‐old Japanese girl presented with psychomotor seizures. Magnetic resonance (MR) images disclosed a mass lesion in the left middle cranial fossa with an internal irregular‐shaped area, which was hyperintense on T1‐weighted images and hypointense on T2‐weighted images. Gross total resection of the tumor was performed through left occipital craniotomy. The tumor was white‐to‐grayish hard tissue, focally showing blackish pigmentation. Histological, immunohistochemical and electron‐microscopical analyses revealed that white‐to‐grayish hard tissue corresponded to an atypical meningioma, and the blackish pigmentation of the tumor was composed of non‐neoplastic, reactive hyperplasia and colonization of meningeal melanocytes in the meningioma tissue. A meningioma with reactive hyperplasia and colonization of meningeal melanocytes is unusual, but it is clinically important to differentiate this entity from other melanocytic tumors.
REFERENCES
- 1. Azzopardi JG, Eusebi V (1977) Melanocyte colonization and pigmentation of breast carcinoma. Histopathology 1:21–30. [DOI] [PubMed] [Google Scholar]
- 2. Burger PC, Scheithauer BW, Vogel FS (2002) Intracranial Meninges (Melanocytoma, Malignant Melanoma). In: Surgical Pathology Of The Nervous System And Its Coverings, 4th edition, Chapter 2, pp 73–76, 85–87, Churchill Livingstone: New York, NY. [Google Scholar]
- 3. Carlson JA, Ross JS, Slominski A, Linette G, Mysliborski J, Hill J, Mihm M Jr. (2005) Molecular diagnostics in melanoma. J Am Acad Dermatol 52:743–775. [DOI] [PubMed] [Google Scholar]
- 4. Jackson EF, Ginsberg LE, Schomer DF, Leeds NE (1997) A review of MRI pulse sequences and techniques in neuroimaging. Surg Neurol 47:185–199. [DOI] [PubMed] [Google Scholar]
- 5. Lach B, Russell N, Benoit B, Atack D (1988) Cellular blue nevus (“melanocytoma”) of the spinal meninges: electron microscopic and immunohistochemical features. Neurosurgery 22:773–780. [DOI] [PubMed] [Google Scholar]
- 6. Limas C, Tio FO (1972) Meningeal melanocytoma (“melanotic meningioma”). Its melanocytic origin as revealed by electron microscopy. Cancer 30:1286–1294. [DOI] [PubMed] [Google Scholar]
- 7. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds.) (2007) WHO Classification of Tumours of the Central Nervous System, 4th edition, International Agency for Research on Cancer: Lyon. [Google Scholar]
- 8. Nestor SL, Perry A, Kurtkaya O, Abell‐Aleff P, Rosemblat AM, Burger PC, Scheithauer BW (2003) Melanocytic colonization of a meningothelial meningioma: histopathological and ultrastructural findings with immunohistochemical and genetic correlation: case report. Neurosurgery 53:211–214. [DOI] [PubMed] [Google Scholar]
- 9. Perry A, Scheithauer BW, Stafford SL, Lohse CM, Wollan PC (1999) “Malignancy” in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer 85:2046–2056. [DOI] [PubMed] [Google Scholar]
- 10. Wong A, Koszyca B, Blumbergs PC, Sandhu N, Halcrow S (1999) Malignant melanoma metastatic to a meningioma. Pathology 31:162–165. [DOI] [PubMed] [Google Scholar]


