Abstract
Systemic or brain‐selective hypothermia is a well‐established method for neuroprotection after brain trauma. There is increasing evidence that hypothermia exerts beneficial effects on the brain and may also support regenerative responses after brain damage. Here, we have investigated whether hypothermia influences neurite outgrowth in vitro via modulation of the post‐injury cytokine milieu.
Organotypic brain slices were incubated: deep hypothermia (2 h at 17°C), rewarming (2 h up to 37°C), normothermia (20 h at 37°C). Neurite density and cytokine release (IL 1beta, IL‐6, IL‐10, and TNF‐alpha) were investigated after 24 h. For functional analysis mice deficient in NT‐3/NT‐4 and TNF‐alpha as well as the TNF‐alpha inhibitor etanercept were used. Hypothermia led to a significant increase of neurite outgrowth, which was independent of neurotrophin signaling. In contrast to other cytokines investigated, TNF‐alpha secretion by organotypic brain slices was significantly increased after deep hypothermia. Moreover, hypothermia‐induced neurite extension was abolished after administration of the TNF‐alpha inhibitor and in TNF‐alpha knockout mice. We demonstrate that TNF‐alpha is responsible for inducing neurite outgrowth in the context of deep hypothermia and rewarming. These data suggest that hypothermia not only exerts protective effects in the CNS but may also support neurite outgrowth as a potential mechanism of regeneration.
Keywords: brain slices, hypothermia, TNF‐alpha, neurite outgrowth, traumatic brain injury, regeneration
INTRODUCTION
Traumatic brain injury (TBI) remains a significant cause of death and severe disability worldwide. High morbidity and long‐term problems in performing the activities of daily life are a result of a TBI (34). Systemic or brain‐selective hypothermia has been established as an effective neuroprotective treatment in multiple studies; however, management of unwanted side effects, such as hypotension or hypovolemia, is of key importance (33). Several mechanisms of neuronal protection induced by hypothermia have been proposed, including reduction of the cerebral metabolic rate 9, 21, suppression of epileptic activity and seizures (36) and the reduction of excessive free radical production 27, 34.
Moreover, hypothermia can prevent secondary damage, which is initiated through inflammatory responses following TBI (2). Cytokines and activated microglia extend neuronal injury and sensitize the injured brain to a second insult. We have previously demonstrated that hypothermia suppresses inflammation in stimulated microglial cells via the ERK signaling pathway (38) and downregulates MCP‐1 and interleukin (IL)‐8 release in activated endothelial cells 7, 8.
Finally, there are some indications that hypothermia may not only influence neuronal cell survival but also promote regenerative responses after brain damage (37). Because hypothermia influences the inflammatory response after brain trauma and as inflammatory cytokines play a major role in modulating neurite outgrowth and regeneration 17, 18, we investigated in organotypic brain slice cultures whether hypothermia and rewarming influence neurite outgrowth after injury via modulation of the post‐injury cytokine milieu. We analyzed the secretion of four inflammation‐associated cytokines that have been shown to influence neurite extension. We demonstrated here that tumor necrosis factor (TNF)‐alpha levels were significantly upregulated after hypothermia and rewarming in contrast to IL‐1beta, IL‐6 and IL‐10. In functional assays we provide for the first time evidence that TNF‐alpha is responsible for hypothermia‐induced neurite extension.
These data indicate that TNF‐alpha may play a key role in hypothermia‐associated axon growth as a potential mechanism of hypothermia‐induced regeneration after central nervous system (CNS) trauma.
MATERIALS AND METHODS
Animals and factors
C57BL/6 wild‐type mice, NT‐3 and NT‐4 knockout mice [NT‐3+/+/NT‐4−/− and NT‐3 ± /NT‐4−/−] as well as TNF‐alpha‐deficient mice (1) were used for the experiments on postnatal day two. It was necessary to use NT‐3 ± /NT‐4−/− mice, as those with a complete knockout for both NT‐3 and NT‐4 die within hours after birth. All mice were housed in a conventional animal facility (Center for Anatomy, Charité‐Universitätsmedizin) and the experiments were performed in accordance with German guidelines on the use of laboratory animals.
K252a (Calbiochem, Schwalbach, Germany), which is a potent inhibitor of TrkA, TrkB and TrkC (but not of p75NTR) in nanomolar concentrations and does not block other tyrosine kinases 14, 23, 32, was used in concentrations of 100 nM or 1 µM in dimethyl sulfoxide (DMSO; Calbiochem, Bad Soden, Germany) compared with DMSO alone at the same concentration. Etanercept (Enbrel® Whyeth, Munster, Germany) was used in a concentration of 80 µg/mL to block TNF‐alpha based on an effective in vivo concentration (12).
Acute organotypic slice cultures
Collagen type I from rat tail was dissolved in 0.1 M acetic acid at a final concentration of 2 mg/mL. Organotypic slice cultures were prepared on postnatal day 2 from mouse entorhinal cortex as previously described 20, 39. The collagen cultures were incubated in a humidified atmosphere with 5% CO2 for 24 h according to the time‐temperature protocol.
Time‐temperature protocol
Acute organotypic brain slices were prepared and directly cooled down from 37°C to 17°C. Deep hypothermia (17°C) was maintained for 2 h followed by rewarming up to 37°C for a period of 2 h. After a follow‐up phase of 20 h at 37°C cytokine release and neurite outgrowth were analyzed. Normothermic control slices were incubated at 37°C throughout the experiments.
Enzyme‐linked immunosorbent assay (ELISA)
Conditioned medium from treated slice cultures was tested for IL‐6, TNF‐alpha, IL‐1beta and IL‐10 using commercially available sandwich ELISAs according to the manufacturer's instructions (BD Bioscience, Heidelberg, Germany). Absorbance was measured at 450 nm in the microplate reader (Multiskan Ascent; Thermo Electron Corporation, Vantaa, Finland).
Immunohistochemistry
The entorhinal cortices (ECs) were washed three times with phosphate‐buffered saline (PBS) and fixed for 20 minutes in 4% paraformaldehyde. The ECs were then incubated for 1 h in PBS with 10% normal goat serum (NGS; Sigma, St. Louis, MO, USA) and 0.2% Triton X‐100 (Sigma) in PBS. The first antibody, Tau‐1 (mouse monoclonal, MAB3420, Millipore, Billerica, MA, USA) or glial fibrillary acidic protein (GFAP; mouse monoclonal, Sigma, G3893), was added (1:500) for 4 h (Tau1) or overnight (GFAP) with 10% NGS in PBS at room temperature (RT). The ECs were then washed three times in PBS (the last time for 20 minutes) and then incubated for 2 h at RT with the secondary antibody goat anti‐mouse (1:1000), AL488 (A11029, Invitrogen, Carlsbad, CA, USA), in PBS with 10% NGS. Immunofluorescence was photodocumented with a fluorescent microscope (BX50; Olympus, Hamburg, Germany) and a CoolSNAPTIMES camera (Photometrics, Tucson, AZ, USA).
Measurement of neurite density
To evaluate neurite density we used a standard protocol for the evaluation of neurite outgrowth as described previously 20, 39 (1, 3), which was improved using image analysis software (Image J, Wayne Rasband, NIH) to quantify neurite density (2, 4). The concave part of the entorhinal cortex explants was photo‐documented using a 10× objective (Olympus IX70). To determine neurite density, image processing was based on the Sobel algorithm, which measures a two‐dimensional spatial gradient emphasizing regions of high spatial density that correspond to neurites. The mean intensity was then calculated in a standardized area parallel to the brain slice edge in a micrograph of every organotypic brain slice.
Figure 1.
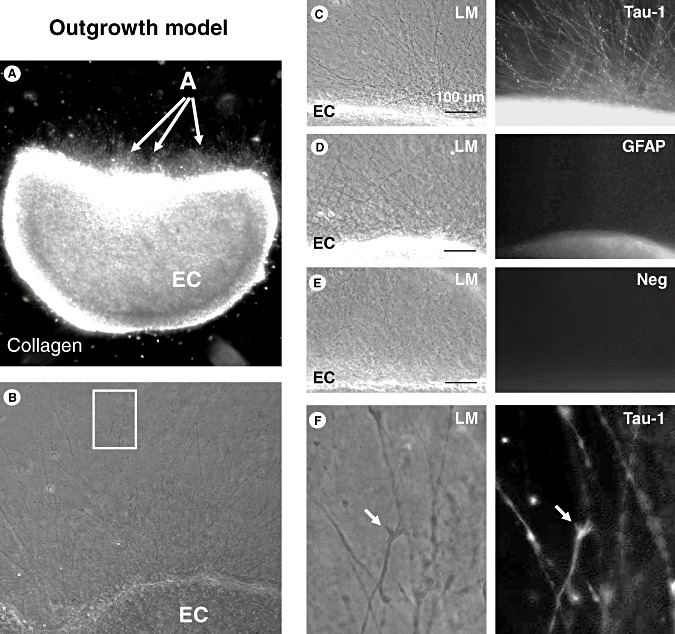
Outgrowth model. A. Outgrowth assay (see Material and Methods section for details); the entorhinal cortex (EC) explant was embedded in a three‐dimensional collagen I gel matrix; the density of the outgrowing neurites (arrows) was photo‐documented at higher magnification and quantified by two blinded investigators. B. Neurite outgrowth in control organotypic brain slices. The white box indicates the area shown at higher magnification in 1F. C. Multiple neurites are visible by light‐microscopy (LM) growing out of the concave part of the EC explant. Immunofluorescence staining using specific antibodies reveals immunreactivity against Tau‐1, which is found on neurites but not on glial cells. D. No immunoreactivity of the extensions is detectable using a specific antibody against glial fibrillary acidic protein (GFAP) as a marker for astrocytes and their extensions. E. No immunoreactivity of the extensions is found using the secondary antibody alone as a negative control. F. Higher magnification of the white box in 1B: Tau‐1‐labeled neurites at light microscopy (LM) show characteristic growth cones that are also immunoreactive for Tau‐1 as a marker for neurites.
Figure 3.
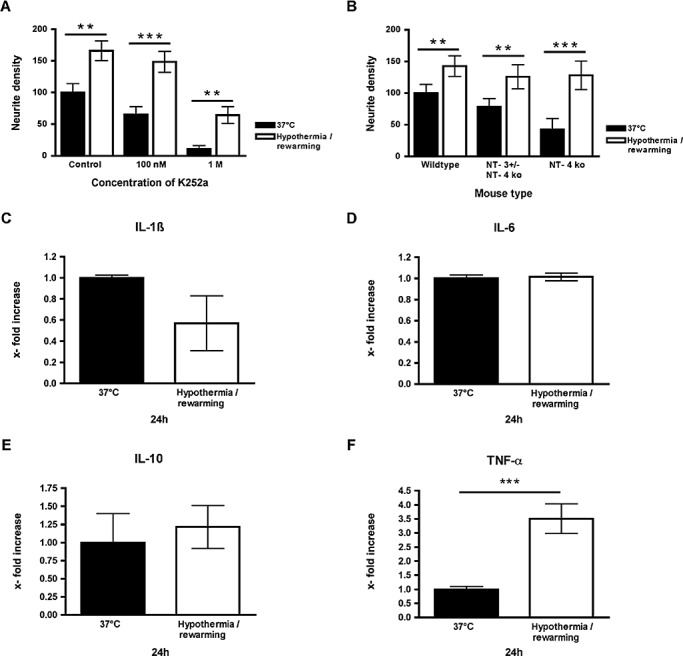
Hypothermia‐induced neurite growth is independent of neurotrophins and deep hypothermia upregulates the secretion of TNF‐alpha by organotypic brain slices. A. Neurite growth is increased by deep hypothermia and rewarming in wildtype mice as well as in NT‐4 or NT‐3 ± /NT‐4 mice. B. Hypothermia‐induced increase in neurite growth remains present after application of the pan‐neurotrophin receptor antagonist K252a, which is a potent inhibitor of TrkA, TrkB and TrkC in nanomolar concentrations compared with the solvent DMSO alone at the same concentration. C–E. The levels of IL‐1beta, IL‐6 and IL‐10 do not significantly change after deep hypothermia and rewarming. F. Deep hypothermia and rewarming significantly upregulate (nearly fourfold) the secretion of TNF‐alpha by organotypic brain slices ***P < 0.001.
Figure 2.
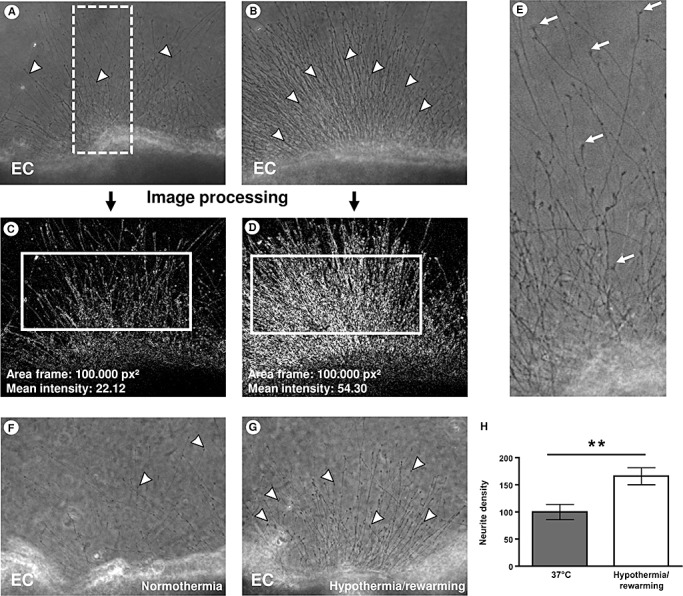
Quantification by image analysis reveals stimulation of neurite outgrowth by hypothermia and rewarming. A, B. Representative photomicrographs of a brain slice with intermediate outgrowth (A) and a brain slice with strong outgrowth (B); the white dotted box in Figure 2A indicates the area shown at higher magnification in 1E. C, D. To quantify the density of the outgrowing neurites (arrowheads) image processing based on the Sobel algorithm was performed to determine the mean intensity in a standardized area parallel to the brain slice edge (indicated as white boxes). E. Higher magnification of the white dotted box in 1A; characteristic growth cones (arrows) are visible at the end of the neurites. F. Control brain slices kept at 37°C during the experiment display average growth of neurites (arrowheads). G. Applying the dynamic time–temperature protocol over 24 h leads to significantly increased neurite density after deep hypothermia and rewarming. H. Analysis of neurite density reveals a significant increase of more than 60% in organotypic brain slice cultures after deep hypothermia. **P < 0.01; arrow heads: single neurites. Abbreviation: EC = entorhinal cortex.
Figure 4.
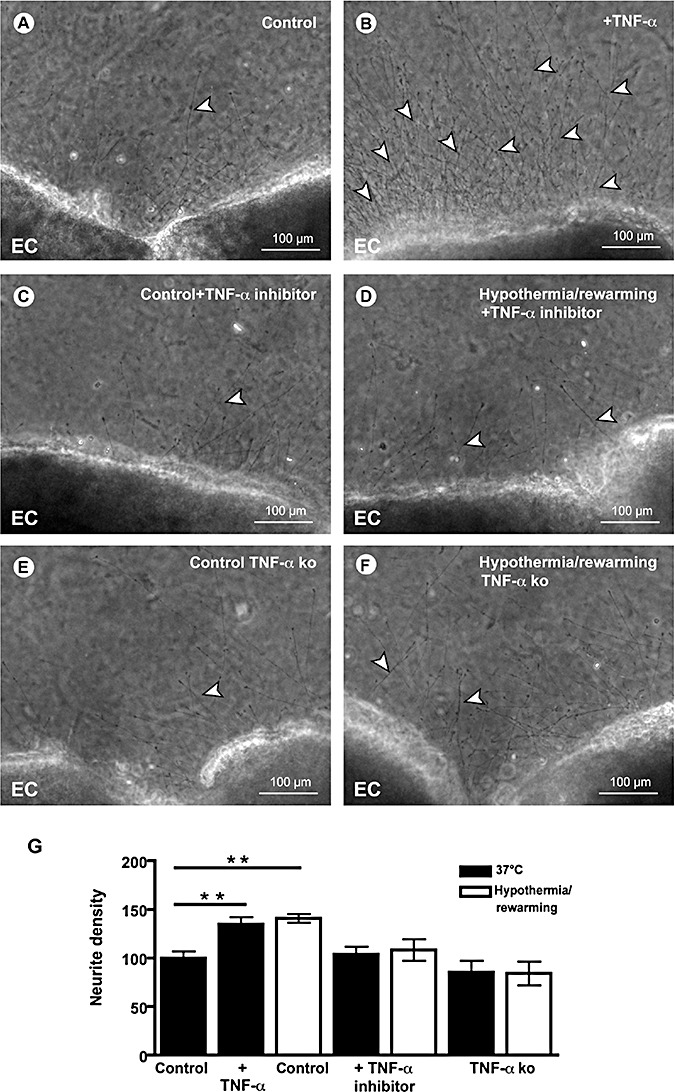
TNF‐alpha is necessary and sufficient for hypothermia‐induced neurite growth. A. Representative photomicrograph of a normothermic organotypic brain slice showing moderate outgrowth of neurites (arrowhead). B. Hypothermia and rewarming induce a substantial increase of the outgrowth of neurites (arrowheads). C. The TNF‐apha inhibitor etanercept does not influence neurite outgrowth of normothermic brain slices compared with controls. D. Etanercept inhibits hypothermia‐induced neurite growth. E. TNF‐alpha absence in brain slices derived from TNF‐alpha‐deficient mice blocks hypothermia‐induced neurite growth. F. TNF‐alpha‐deficient mice fully eliminated the effect of deep hypothermia and rewarming. G. TNF‐alpha significantly increases neurite density similar to hypothermia and rewarming. In the presence of the TNF‐alpha inhibitor etanercept and in the absence of TNF‐alpha in TNF‐alpha‐deficient mice the hypothermia‐induced increase in neurite growth is abolished. **P < 0.01. Abbreviation: EC = entorhinal cortex.
Statistical analysis
The Mann–Whitney U one‐way analysis of variance by ranks was used to determine whether significant differences were present. P values were considered significant when below 0.05.
RESULTS
We investigated the effects of deep hypothermia and rewarming on neurite outgrowth from acute organotypic brain slices using a dynamic time–temperature protocol over 24 h. Organotypic brain slices were embedded in a three‐dimensional collagen matrix (Figure 1A). The concave part of the entorhinal cortex explants was photo‐documented (Figure 1B). To confirm that the observed extensions from the brain slice are neurites, immunofluorescence was performed using a specific antibody against Tau‐1 (Figure 1C). A specific antibody against GFAP as a marker for astrocytes did not mark any extension (Figure 1D) similar to the secondary antibody alone as negative control (Figure 1E). Higher magnification of Tau‐1‐labeled neurites showed characteristic growth cones (Figure 1F, arrows) suggesting that these neurites are axons and not dendrites. To precisely quantify neurite outgrowth we improved a standard protocol 20, 39 by using an image analysis software (Figure 2). The concave part of the entorhinal cortex explants was photo‐documented using a 10× objective. Figure 2A illustrates a brain slice with intermediate outgrowth, and Figure 2B a slice with strong outgrowth; the white box in Figure 2A indicates the area shown at higher magnification in Figure 2E. To quantify the density of the outgrowing neurites (Figure 2A,B; arrowheads) image processing based on the Sobel algorithm was performed (Figure 2C,D). The mean intensity was then calculated in a standardized area parallel to the brain slice edge (indicated as white boxes in Figure 2C,D). At higher magnification characteristic growth cones are visible (Figure 2E, arrows). Compared with control brain slices (Figure 2F) that were kept at 37°C during the experiment, applying the dynamic time‐temperature protocol over 24 h lead to significantly increased neurite density in brain slices after deep hypothermia and rewarming (Figure 2G,H).
As NT‐3 and NT‐4 are the major neurotrophins responsible for neurite growth from organotypic brain slices (Hechler & Hendrix, under review) we investigated brain slices derived from mice either homozygous for NT‐4 deficiency (NT‐4−/−) or with a combined homozygous NT‐4 deficiency and heterozygous NT‐3 deficiency (NT‐3 ± /NT‐4−/−) (a full NT‐3 knockout is lethal). Neurite growth was still increased by hypothermia in brain slices derived from NT‐4 KO mice (Figure 3A). Furthermore, a combination of NT‐4 deficiency and a substantial reduction of NT‐3, in mice homozygous for NT‐4 deficiency and heterozygous for NT‐3 deficiency, did not abolish the growth‐stimulatory effect of hypothermia on neurites (Figure 3A).
To further investigate whether hypothermia‐induced neurite outgrowth is independent of neurotrophin signaling, we applied K252a, which is a potent inhibitor of the neurotrophin receptors TrkA, TrkB and TrkC in nanomolar concentrations 14, 23, 32. The application of 100 nM to the culture medium reduced neurite growth from control slices but did not abolish the significant stimulation of neurite growth by deep hypothermia and rewarming (Figure 3B). A higher concentration of K252a (1 M), which may also block other tyrosine kinases, reduced neurite outgrowth in controls; however, there was still a strong and statistically significant stimulating effect of deep hypothermia and rewarming (Figure 3B).
In a next step we investigated whether the levels of selected inflammation‐associated cytokines like IL‐1beta, IL‐6, IL‐10 and TNF‐alpha secreted by organotypic brain slices are modulated 24 h after experimental start by deep hypothermia and rewarming. The secretion of IL‐1beta (37°C: 2200 fg/mL ± 110; hypothermia and rewarming: 1200 fg/mL ± 970), IL‐6 (37°C: 166 pg/mL ± 9.19; hypothermia and rewarming: 168 pg/mL ± 9.90) and IL‐10 (37°C: 5700 fg/mL ± 3970; hypothermia and rewarming: 6400 fg/mL ± 3360) by organotypic brain slices was not substantially modulated (Figs 3C–E) in contrast to TNF‐alpha secretion, which was significantly increased nearly fourfold (37°C: 1200 fg/mL ± 230; hypothermia and rewarming: 4300 fg/mL ± 1140) after deep hypothermia and rewarming (Figure 3F).
Based on these finding we further explored whether the TNF‐alpha upregulation plays a causal role in stimulating neurite extension after hypothermic treatment. As a first step, we demonstrated that TNF‐alpha was sufficient to increase neurite extension from brain slices (Figure 4A,B,G). TNF‐alpha increased neurite density by nearly 45%, a similar effect to that of deep hypothermia/rewarming (Figure 4G). Next we used the TNF‐alpha inhibitor etanercept (Figure 4C,D,G), which fully abolished the stimulatory effect of hypothermia and rewarming. Furthermore, the absence of endogenous TNF‐alpha in slices derived from TNF‐alpha‐deficient mice (Figure 4E–G) fully eliminated the effect of deep hypothermia and rewarming.
DISCUSSION
TBI is a major source of death and disability. Estimates run as high as 10 million cases of TBI per year worldwide. Around 40% of the patients are left with permanent neurological disabilities (34). In addition to the emotional effects the financial burden is also enormous.
The neurological outcome after a period of ischemia is established to a substantial degree by mechanisms during the post‐injury period. Therefore ischemia plays a key role in all forms of brain injury and preventing ischemic injury is central to all neuroprotective strategies. One well‐established method for neuroprotection in the context of TBI is the direct application of systemic or brain‐restricted hypothermia to prevent this secondary injury (5).
In a meta‐analysis looking at the effects of hypothermia on clinical neurological outcome in patients with TBI, most investigations showed that cooling can be effective if the treatment is initiated early and continued for long enough and if patients are rewarmed slowly (33). Therefore, in our cell culture model, acute organotypic brain slices were directly cooled down to 17°C and after 2 h the slices were rewarmed over a period of 2 h to 37°C. Deep hypothermia and rewarming hereby led to increased neurite outgrowth.
The multifaceted benefits of hypothermic treatment include a reduced rate of cerebral metabolism and epileptic discharges as well as the production of reactive oxygen species 9, 21, 27, 36. We and others have previously demonstrated that hypothermia also modulates the brain immune system 37, 38, 39, 42.
Based on these findings we addressed the question of whether hypothermia may support not only neuronal cell survival (37) but also regenerative responses after brain damage (38). In contrast to previous studies focused mainly on neuroprotection we investigated whether hypothermia induces neurite growth via neurotrophins and/or inflammation‐associated cytokines—namely IL‐1beta, IL‐6, IL‐10 and TNF‐alpha—that have been shown to modulate neurite outgrowth and regeneration 17, 18. Organotypic brain slice cultures underwent a dynamic time‐temperature protocol of deep hypothermia and rewarming over 24 h resulting in a significant increase of neurite growth.
Organotypic brain slices are a well‐established in vitro model to study, e.g., electrophysiological processes and cell survival as well as neurite plasticity, growth and regeneration (16). As brain slices are acutely cut out of the living brain they are in fact a trauma model because most neurites are dissected, the blood–brain barrier is heavily damaged, many cells die, and astrocytes and immune cells become highly activated 10, 19, 43.
The extensions growing out of the organotypic brain slices show immunoreactivity against Tau‐1, which is primarily found on axons but also on dendrites. In contrast, these extensions do not show imunoreactivity against GFAP, a standard marker for astrocytes and their extensions. Morphological criteria used in single cell cultures (e.g., the presence of an axon hillock, the angle of branches) are not applicable to fasciculated distal ends of neurites exiting from brain slices because the cell bodies are embedded in the slices. The Tau‐1 immunoreactivity and the morphological appearance of the neurites, in particular the presence of multiple growth cones, are good arguments in favor of considering these extensions to be axons.
As NT‐3 and NT‐4 are the major neurotrophins responsible for neurite growth in organotypic brain slices (16), we investigated whether these neurotrophins are also responsible for the hypothermia‐induced increase in neurite growth.
Surprisingly, hypothermia still increases neurite growth even in the absence of NT‐4 in brain slices derived from NT‐4 KO mice. Furthermore, the additional reduction in NT‐3 secretion, in mice homozygous for NT‐4 deficiency and heterozygous for NT‐3 deficiency, does not abolish the growth‐stimulatory effect of hypothermia on neurites.
As mice with a full knockout of NT‐3 are not viable, we cannot determine with these experiments whether the remaining NT‐3 still plays a role in hypothermia‐induced neurite extension. To address the question of whether hypothermia‐induced neurite outgrowth is independent of neurotrophin signaling, we applied K252a, which is a potent inhibitor of the neurotrophin receptors TrkA, TrkB and TrkC in nanomolar concentrations 14, 23, 32. Surprisingly, neurotrophin receptor inhibition by K252a application did not abolish hypothermia‐induced increase in neurite extension. These data indicate that the increase of neurite extension by hypothermia is independent of neurotrophin signaling.
There is increasing evidence in the literature and from our own laboratory 17, 18 that inflammation‐associated cytokines play a key role in the stimulation of axonal regeneration. Therefore, we further investigated whether hypothermia influences neurite outgrowth in vitro via modulation of the post‐injury cytokine milieu. The analysis of the levels of IL‐1beta, IL‐6, IL‐10 and TNF‐alpha, which are known to influence neurite extension, revealed that only TNF‐alpha secretion is significantly increased (nearly four fold) after deep hypothermia and rewarming of organotypic brain slices.
TNF‐alpha is a pleiotropic cytokine that induces neuroprotective and neurotoxic effects after CNS injury 25, 26, 29. It is upregulated within 1 h after CNS injuries in neurons, astrocytes, macrophages and microglia. TNF‐alpha exerts pro‐ or anti‐apoptotic effects on CNS neurons depending on cell type and assay 4, 6, 30. Similarly, TNF‐alpha has differential effects on neurite extension 17, 18. Previously, we and others have demonstrated in organotypic dorsal root ganglia cultures that TNF‐alpha reduces neurite outgrowth in the peripheral nervous system 15, 24. In organotypic mesencephalic brain slices TNF‐alpha has been shown to support glia‐dependent neurite growth (28). In the present study we have demonstrated that TNF‐alpha increases neurite outgrowth in brain slices by nearly 45%, thus, at a level comparable with the stimulatory effects of hypothermia.
The use of the TNF‐alpha inhibitor etanercept as well as the absence of endogenous TNF‐alpha in slices derived from TNF‐alpha‐deficient mice fully abolished the effect of deep hypothermia and rewarming. Interestingly, etanercept did not substantially influence cell survival (data not shown), suggesting that hypothermia‐induced neurite extension is mediated by TNF‐alpha whereas hypothermia‐induced cell survival is an independently regulated process.
There are good indications that TNF‐alpha may have protective and maybe even proregenerative functions in the damaged CNS in vivo. In two models of hippocampal excitotoxic and ischemic injury, mice deficient in both TNF receptors were found to be more susceptible 3, 11. Preconditioning with TNF‐alpha appears to be neuroprotective in ischemic cerebral injury 13, 31. TNF receptor‐deficient mice also show exacerbated brain damage after traumatic injury (41). Finally, TNF‐alpha appears to facilitate the regeneration of the injured adult rabbit optic nerve.
In line with this discussion, it has been shown that chilling and rewarming of murine brain slices lead to a substantial proliferation of dendritic spines of mature hippocampal neurons (22). Similar structural changes have been reported for dendrites of CA3 pyramidal cells in ground squirrels after arousal from hibernation (35), suggesting a physiological role of neuronal plasticity after hypothermia and rewarming. The role of TNF‐alpha in these processes is not known.
However, as TNF‐alpha is a pleiotropic cytokine exerting neuroprotective as well as neurotoxic effects after CNS injury (40), it will be important to analyze whether the TNF‐alpha‐induced neurite growth shown in the present study is truly beneficial (i.e., pro‐regenerative) or whether it may even have detrimental effects such as contributing to posttraumatic epileptogenesis.
The data presented here clearly show that TNF‐alpha is necessary and sufficient to increase neurite outgrowth in the context of deep hypothermia and rewarming. This study provides for the first time evidence that hypothermia not only exerts protective effects in the CNS but also supports neurite outgrowth via TNF‐alpha upregulation as a possible mechanism of regeneration.
ACKNOWLEDGMENTS
The authors wish to thank Doreen Lüdecke for excellent technical assistance and Anne Gale for editing the manuscript. NT‐3 and NT‐4 knockout mice [NT‐3+/+/NT‐4−/− and NT‐3 ± /NT‐4−/−] were a kind gift from G. Lewin, Max‐Delbrück Center for Molecular Medicine, Berlin‐Buch, Germany, respectively. This study was supported in part by grants from the European union (EFRE) to KS and SH and by Hasselt University to SH (BOF09NI07). FB is a member of the Marie‐Curie network CORTEX, DH was a member of GRK1258.
REFERENCES
- 1. Abe K, Yarovinsky FO, Murakami T, Shakhov AN, Tumanov AV, Ito D et al (2003) Distinct contributions of TNF and LT cytokines to the development of dendritic cells in vitro and their recruitment in vivo . Blood 101:1477–1483. [DOI] [PubMed] [Google Scholar]
- 2. Atkins CM, Oliva AA Jr, Alonso OF, Chen S, Bramlett HM, Hu BR, Dietrich WD (2007) Hypothermia treatment potentiates ERK1/2 activation after traumatic brain injury. Eur J Neurosci 26:810–819. [DOI] [PubMed] [Google Scholar]
- 3. Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK et al (1996) Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med 2:788–794. [DOI] [PubMed] [Google Scholar]
- 4. Cheng B, Christakos S, Mattson MP (1994) Tumor necrosis factors protect neurons against metabolic‐excitotoxic insults and promote maintenance of calcium homeostasis. Neuron 12:139–153. [DOI] [PubMed] [Google Scholar]
- 5. Christian E, Zada G, Sung G, Giannotta SL (2008) A review of selective hypothermia in the management of traumatic brain injury. Neurosurg Focus 25:E9. [DOI] [PubMed] [Google Scholar]
- 6. Clarke P, Meintzer SM, Spalding AC, Johnson GL, Tyler KL (2001) Caspase 8‐dependent sensitization of cancer cells to TRAIL‐induced apoptosis following reovirus‐infection. Oncogene 20:6910–6919. [DOI] [PubMed] [Google Scholar]
- 7. Diestel A, Roessler J, Berger F, Schmitt KR (2008) Hypothermia downregulates inflammation but enhances IL‐6 secretion by stimulated endothelial cells. Cryobiology 57:216–222. [DOI] [PubMed] [Google Scholar]
- 8. Diestel A, Roessler J, Pohl‐Schickinger A, Koster A, Drescher C, Berger F, Schmitt KR (2009) Specific p38 inhibition in stimulated endothelial cells: a possible new anti‐inflammatory strategy after hypothermia and rewarming. Vascul Pharmacol 51:246–252. [DOI] [PubMed] [Google Scholar]
- 9. Ehrlich MP, McCullough JN, Zhang N, Weisz DJ, Juvonen T, Bodian CA, Griepp RB (2002) Effect of hypothermia on cerebral blood flow and metabolism in the pig. Ann Thorac Surg 73:191–197. [DOI] [PubMed] [Google Scholar]
- 10. Eyupoglu IY, Savaskan NE, Brauer AU, Nitsch R, Heimrich B (2003) Identification of neuronal cell death in a model of degeneration in the hippocampus. Brain Res Brain Res Protoc 11:1–8. [DOI] [PubMed] [Google Scholar]
- 11. Gary DS, Bruce‐Keller AJ, Kindy MS, Mattson MP (1998) Ischemic and excitotoxic brain injury is enhanced in mice lacking the p55 tumor necrosis factor receptor. J Cereb Blood Flow Metab 18:1283–1287. [DOI] [PubMed] [Google Scholar]
- 12. Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muia C, Bramanti P, Cuzzocrea S (2006) Immunomodulatory effects of etanercept in an experimental model of spinal cord injury. J Pharmacol Exp Ther 316:1006–1016. [DOI] [PubMed] [Google Scholar]
- 13. Ginis I, Jaiswal R, Klimanis D, Liu J, Greenspon J, Hallenbeck JM (2002) TNF‐alpha‐induced tolerance to ischemic injury involves differential control of NF‐kappaB transactivation: the role of NF‐kappaB association with p300 adaptor. J Cereb Blood Flow Metab 22:142–152. [DOI] [PubMed] [Google Scholar]
- 14. Goldberg DJ, Wu DY (1996) Tyrosine phosphorylation and protrusive structures of the growth cone. Perspect Dev Neurobiol 4:183–192. [PubMed] [Google Scholar]
- 15. Golz G, Uhlmann L, Ludecke D, Markgraf N, Nitsch R, Hendrix S (2006) The cytokine/neurotrophin axis in peripheral axon outgrowth. Eur J Neurosci 24:2721–2730. [DOI] [PubMed] [Google Scholar]
- 16. Hechler D, Nitsch R, Hendrix S (2006) Green‐fluorescent‐protein‐expressing mice as models for the study of axonal growth and regeneration in vitro . Brain Res Rev 52:160–169. [DOI] [PubMed] [Google Scholar]
- 17. Hendrix S, Nitsch R (2007) The role of T helper cells in neuroprotection and regeneration. J Neuroimmunol 184:100–112. [DOI] [PubMed] [Google Scholar]
- 18. Hendrix S, Peters EM (2007) Neuronal plasticity and neuroregeneration in the skin—the role of inflammation. J Neuroimmunol 184:113–126. [DOI] [PubMed] [Google Scholar]
- 19. Heppner FL, Skutella T, Hailer NP, Haas D, Nitsch R (1998) Activated microglial cells migrate towards sites of excitotoxic neuronal injury inside organotypic hippocampal slice cultures. Eur J Neurosci 10:3284–3290. [DOI] [PubMed] [Google Scholar]
- 20. Holtje M, Djalali S, Hofmann F, Munster‐Wandowski A, Hendrix S, Boato F et al (2009) A 29‐amino acid fragment of Clostridium botulinum C3 protein enhances neuronal outgrowth, connectivity, and reinnervation. Faseb J 23:1115–1126. [DOI] [PubMed] [Google Scholar]
- 21. Jiang JY, Xu W, Yang PF, Gao GY, Gao YG, Liang YM et al (2006) Marked protection by selective cerebral profound hypothermia after complete cerebral ischemia in primates. J Neurotrauma 23:1847–1856. [DOI] [PubMed] [Google Scholar]
- 22. Kirov SA, Petrak LJ, Fiala JC, Harris KM (2004) Dendritic spines disappear with chilling but proliferate excessively upon rewarming of mature hippocampus. Neuroscience 127:69–80. [DOI] [PubMed] [Google Scholar]
- 23. Koizumi H, Morita M, Mikami S, Shibayama E, Uchikoshi T (1998) Immunohistochemical analysis of TrkA neurotrophin receptor expression in human non‐neuronal carcinomas. Pathol Int 48:93–101. [DOI] [PubMed] [Google Scholar]
- 24. Larsson K, Rydevik B, Olmarker K (2005) Disc related cytokines inhibit axonal outgrowth from dorsal root ganglion cells in vitro . Spine (Phila Pa 1976) 30:621–624. [DOI] [PubMed] [Google Scholar]
- 25. Lotocki G, Alonso OF, Dietrich WD, Keane RW (2004) Tumor necrosis factor receptor 1 and its signaling intermediates are recruited to lipid rafts in the traumatized brain. J Neurosci 24:11010–11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lotocki G, De Rivero Vaccari JP, Perez ER, Alonso OF, Curbelo K, Keane RW, Dietrich WD (2006) Therapeutic hypothermia modulates TNFR1 signaling in the traumatized brain via early transient activation of the JNK pathway and suppression of XIAP cleavage. Eur J Neurosci 24:2283–2290. [DOI] [PubMed] [Google Scholar]
- 27. Maier CM, Sun GH, Cheng D, Yenari MA, Chan PH, Steinberg GK (2002) Effects of mild hypothermia on superoxide anion production, superoxide dismutase expression, and activity following transient focal cerebral ischemia. Neurobiol Dis 11:28–42. [DOI] [PubMed] [Google Scholar]
- 28. Marschinke F, Stromberg I (2008) Dual effects of TNFalpha on nerve fiber formation from ventral mesencephalic organotypic tissue cultures. Brain Res 1215:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin‐Villalba A, Hahne M, Kleber S, Vogel J, Falk W, Schenkel J, Krammer PH (2001) Therapeutic neutralization of CD95‐ligand and TNF attenuates brain damage in stroke. Cell Death Differ 8:679–686. [DOI] [PubMed] [Google Scholar]
- 30. McGuire TR, Bociek GR, Pavletic SZ, Hock L, Lynch J, Schneider J et al (2001) Organ dysfunction following stem cell transplantation: relationship to plasma cytokine concentrations. Bone Marrow Transplant 28:889–893. [DOI] [PubMed] [Google Scholar]
- 31. Nawashiro H, Tasaki K, Ruetzler CA, Hallenbeck JM (1997) TNF‐alpha pretreatment induces protective effects against focal cerebral ischemia in mice. J Cereb Blood Flow Metab 17:483–490. [DOI] [PubMed] [Google Scholar]
- 32. Ohmichi M, Pang L, Ribon V, Gazit A, Levitzki A, Saltiel AR (1993) The tyrosine kinase inhibitor tyrphostin blocks the cellular actions of nerve growth factor. Biochemistry 32:4650–4658. [DOI] [PubMed] [Google Scholar]
- 33. Polderman KH (2008) Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet 371:1955–1969. [DOI] [PubMed] [Google Scholar]
- 34. Polderman KH (2009) Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 37(7 Suppl.):S186–S202. [DOI] [PubMed] [Google Scholar]
- 35. Popov VI, Bocharova LS, Bragin AG (1992) Repeated changes of dendritic morphology in the hippocampus of ground squirrels in the course of hibernation. Neuroscience 48:45–51. [DOI] [PubMed] [Google Scholar]
- 36. Schmitt FC, Buchheim K, Meierkord H, Holtkamp M (2006) Anticonvulsant properties of hypothermia in experimental status epilepticus. Neurobiol Dis 23:689–696. [DOI] [PubMed] [Google Scholar]
- 37. Schmitt KR, Kern C, Berger F, Ullrich O, Hendrix S, Abdul‐Khaliq H (2006) Methylprednisolone attenuates hypothermia‐ and rewarming‐induced cytotoxicity and IL‐6 release in isolated primary astrocytes, neurons and BV‐2 microglia cells. Neurosci Lett 404:309–314. [DOI] [PubMed] [Google Scholar]
- 38. Schmitt KR, Diestel A, Lehnardt S, Schwartlander R, Lange PE, Berger F et al (2007) Hypothermia suppresses inflammation via ERK signaling pathway in stimulated microglial cells. J Neuroimmunol 189:7–16. [DOI] [PubMed] [Google Scholar]
- 39. Schmitt KR, Kern C, Lange PE, Berger F, Abdul‐Khaliq H, Hendrix S (2007) S100B modulates IL‐6 release and cytotoxicity from hypothermic brain cells and inhibits hypothermia‐induced axonal outgrowth. Neurosci Res 59:68–73. [DOI] [PubMed] [Google Scholar]
- 40. Sriram K, O'Callaghan JP (2007) Divergent roles for tumor necrosis factor‐alpha in the brain. J Neuroimmune Pharmacol 2:140–153. [DOI] [PubMed] [Google Scholar]
- 41. Sullivan PG, Bruce‐Keller AJ, Rabchevsky AG, Christakos S, Clair DK, Mattson MP, Scheff SW (1999) Exacerbation of damage and altered NF‐kappaB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J Neurosci 19:6248–6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Y, Chambers KC (2002) Cooling lesions of the lateral parabrachial nucleus during LiCl activation block acquisition of conditioned taste avoidance in male rats. Brain Res 934:7–22. [DOI] [PubMed] [Google Scholar]
- 43. Wolf SA, Fisher J, Bechmann I, Steiner B, Kwidzinski E, Nitsch R (2002) Neuroprotection by T‐cells depends on their subtype and activation state. J Neuroimmunol 133:72–80. [DOI] [PubMed] [Google Scholar]


