Abstract
Background
Gestational diabetes mellitus (GDM) is associated with a range of adverse pregnancy outcomes for mother and infant. The prevention of GDM using lifestyle interventions has proven difficult. The gut microbiome (the composite of bacteria present in the intestines) influences host inflammatory pathways, glucose and lipid metabolism and, in other settings, alteration of the gut microbiome has been shown to impact on these host responses. Probiotics are one way of altering the gut microbiome but little is known about their use in influencing the metabolic environment of pregnancy. This is an update of a review last published in 2014.
Objectives
To systematically assess the effects of probiotic supplements used either alone or in combination with pharmacological and non‐pharmacological interventions on the prevention of GDM.
Search methods
We searched Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (20 March 2020), and reference lists of retrieved studies.
Selection criteria
Randomised and cluster‐randomised trials comparing the use of probiotic supplementation with either placebo or diet for the prevention of the development of GDM. Cluster‐randomised trials were eligible for inclusion but none were identified. Quasi‐randomised and cross‐over design studies were not eligible for inclusion in this review. Studies presented only as abstracts with no subsequent full report of study results were only included if study authors confirmed that data in the abstract came from the final analysis. Otherwise, the abstract was left awaiting classification.
Data collection and analysis
Two review authors independently assessed study eligibility, extracted data and assessed risk of bias of included studies. Data were checked for accuracy.
Main results
In this update, we included seven trials with 1647 participants. Two studies were in overweight and obese women, two in obese women and three did not exclude women based on their weight. All included studies compared probiotics with placebo. The included studies were at low risk of bias overall except for one study that had an unclear risk of bias. We excluded two studies, eight studies were ongoing and three studies are awaiting classification.
Six included studies with 1440 participants evaluated the risk of GDM. It is uncertain if probiotics have any effect on the risk of GDM compared to placebo (mean risk ratio (RR) 0.80, 95% confidence interval (CI) 0.54 to 1.20; 6 studies, 1440 women; low‐certainty evidence). The evidence was low certainty due to substantial heterogeneity and wide CIs that included both appreciable benefit and appreciable harm.
Probiotics increase the risk of pre‐eclampsia compared to placebo (RR 1.85, 95% CI 1.04 to 3.29; 4 studies, 955 women; high‐certainty evidence) and may increase the risk of hypertensive disorders of pregnancy (RR 1.39, 95% CI 0.96 to 2.01, 4 studies, 955 women), although the CIs for hypertensive disorders of pregnancy also indicated probiotics may have no effect.
There were few differences between groups for other primary outcomes. Probiotics make little to no difference in the risk of caesarean section (RR 1.00, 95% CI 0.86 to 1.17; 6 studies, 1520 women; high‐certainty evidence), and probably make little to no difference in maternalweight gain during pregnancy (MD 0.30 kg, 95% CI –0.67 to 1.26; 4 studies, 853 women; moderate‐certainty evidence). Probiotics probably make little to no difference in the incidence of large‐for‐gestational age infants (RR 0.99, 95% CI 0.72 to 1.36; 4 studies, 919 infants; moderate‐certainty evidence) and may make little to no difference in neonatal adiposity (2 studies, 320 infants; data not pooled; low‐certainty evidence). One study reported adiposity as fat mass (MD –0.04 kg, 95% CI –0.12 to 0.04), and one study reported adiposity as percentage fat (MD –0.10%, 95% CI –1.19 to 0.99). We do not know the effect of probiotics on perinatal mortality (RR 0.33, 95% CI 0.01 to 8.02; 3 studies, 709 infants; low‐certainty evidence), a composite measure of neonatal morbidity (RR 0.69, 95% CI 0.36 to 1.35; 2 studies, 623 infants; low‐certainty evidence), or neonatal hypoglycaemia (mean RR 1.15, 95% CI 0.69 to 1.92; 2 studies, 586 infants; low‐certainty evidence). No included studies reported on perineal trauma, postnatal depression, maternal and infant development of diabetes or neurosensory disability.
Authors' conclusions
Low‐certainty evidence from six trials has not clearly identified the effect of probiotics on the risk of GDM. However, high‐certainty evidence suggests there is an increased risk of pre‐eclampsia with probiotic administration. There were no other clear differences between probiotics and placebo among the other primary outcomes. The certainty of evidence for this review's primary outcomes ranged from low to high, with downgrading due to concerns about substantial heterogeneity between studies, wide CIs and low event rates.
Given the risk of harm and little observed benefit, we urge caution in using probiotics during pregnancy.
The apparent effect of probiotics on pre‐eclampsia warrants particular consideration. Eight studies are currently ongoing, and we suggest that these studies take particular care in follow‐up and examination of the effect on pre‐eclampsia and hypertensive disorders of pregnancy. In addition, the underlying potential physiology of the relationship between probiotics and pre‐eclampsia risk should be considered.
Plain language summary
Probiotics to prevent gestational diabetes mellitus
We analysed evidence from randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) investigating probiotic supplements alone or in combination with drug or non‐drug interventions for preventing gestational diabetes mellitus (GDM).
What is the issue?
GDM is a condition where the mother develops high blood sugar levels, usually after 13 weeks of pregnancy. GDM is different from type 2 diabetes in that blood sugar levels are normal before pregnancy, and the levels usually return to normal after pregnancy. GDM is associated with an increased risk of developing type 2 diabetes later in life. Women with GDM are at increased risk of high blood pressure with protein in the urine (pre‐eclampsia) and instrumental delivery or caesarean section. Their infants are more likely to be born large for their gestational age. Probiotics are 'good bacteria' that are usually taken in the form of capsules or drinks to add to the gut bacteria. We are dependent on our gut bacteria to help digest our food, produce certain vitamins, regulate our immune system and keep us healthy by protecting us against disease‐causing bacteria. Probiotics could change a person's metabolism and play a role in the prevention of GDM.
Why is this important?
Women who are overweight or obese, had GDM in a previous pregnancy or have an immediate family member with diabetes are at increased risk of GDM. Current treatment for GDM includes diet with or without medication but does not always prevent the problems associated with GDM. Probiotics could be a simple method for preventing GDM. This review looked at whether there is evidence to show if this is true.
What evidence did we find?
We searched for evidence from randomised controlled trials in March 2020 and identified seven studies with 1647 pregnant women comparing probiotics with inactive placebo (pretend treatment). Two studies were in overweight and obese women, two in obese women and three did not exclude women based on their weight. The overall risk of bias was low except for one study where the risk of bias was unclear.
It is unclear how probiotics affect the risk of developing GDM due to the wide variation in the results of six studies (1440 women, low‐quality evidence). Probiotics increase the risk of developing pre‐eclampsia (4 studies, 955 women; high‐quality evidence). Probiotics make little to no difference to the risk of needing a caesarean section (6 studies, 1520 women; high‐quality evidence), and probably make little to no difference to weight gain during pregnancy (4 studies, 853 women; moderate‐quality evidence) or to the risk of giving birth to a big baby (4 studies, 919 women; moderate‐quality evidence). None of the studies reported information about the risk of perineal trauma (tears during vaginal birth or a surgical incision (episiotomy)), postnatal depression or developing subsequent diabetes.
We do not know if probiotics affect the infant having medical problems after birth because of the variation in results between studies (2 studies, 623 infants; low‐quality evidence). It is also uncertain how probiotics affect infant death (either before birth or as a newborn) (3 studies, 709 infants; low‐certainty evidence), low blood sugar (2 studies, 586 infants; low‐certainty evidence) or body fat (2 studies, 320 infants; low‐certainty evidence). None of the studies reported information about the risk of infants developing diabetes or long‐term conditions that affect brain development.
What does this mean?
Low‐quality evidence from six trials has not clearly identified the effect of probiotics on the risk of GDM. However, high‐quality evidence suggests that probiotics probably increase the risk of pre‐eclampsia. Therefore, there is currently evidence of possible harm with little observed benefit for widespread use of probiotics in pregnancy.
There are eight studies currently ongoing that may help to provide more clarity on the effects of probiotics. It is also important to explore the relationship between probiotics and pre‐eclampsia further.
Summary of findings
Background
Description of the condition
According to the American Diabetes Association (ADA), gestational diabetes mellitus (GDM) is diabetes in pregnancy that is diagnosed during the second or third trimester and was not clearly present prior to pregnancy (ADA 2019). There are multiple sets of diagnostic criteria that are used worldwide, which has caused estimates of prevalence to vary greatly (Buchanan 2012). According to the International Association of Diabetes in Pregnancy Study Group (IADPSG) criteria, approximately 14% of pregnancies worldwide were affected by GDM in 2017 (Cho 2018), and multiple studies have observed that the incidence is rising (Noh 2021; López‐de‐Andrés 2020; Abouzeid 2014; Dabelea 2005).
GDM is associated with a number of maternal and fetal adverse outcomes, and the risk of these outcomes increases with higher fasting plasma glucose levels (HAPO 2008). Women with GDM have higher rates of pre‐eclampsia and need for a caesarean section, and their infants have higher rates of macrosomia, shoulder dystocia, neonatal hypoglycaemia and respiratory distress syndrome (Carr 2011; Dodd 2007; Esakoff 2009; HAPO 2008). In addition, there is an increased risk for metabolic dysfunction for both mother and infant in the long term including diabetes, obesity and metabolic syndrome (Malcolm 2012). Large randomised controlled trials have demonstrated the benefits of treating GDM for preventing many of the associated adverse outcomes (Crowther 2005; Landon 2009), but it is not known if treatment prevents the long‐term adverse effects. In addition, there are substantial costs associated with the treatment of GDM, and cost‐effectiveness has not been clearly demonstrated (Fitria 2019). Therefore, prevention of GDM is favourable.
Prevention efforts have primarily focused on lifestyle interventions such as diet and exercise. The Cochrane Review evaluating the combination of diet and exercise interventions for the prevention of GDM concluded that there is moderate‐quality evidence that this combination of lifestyle interventions can reduce the risk of GDM (Shepherd 2017). However, the Cochrane Reviews evaluating diet and exercise for GDM prevention independently were inconclusive and suggested the need for higher‐quality evidence (Han 2012; Tieu 2017). All these reviews reported that studies involving these interventions were difficult to interpret given heterogeneity and small sample sizes. In addition, there was concern about adherence to these interventions on a population level (Sui 2013).
Due to these concerns, dietary supplements such as probiotics and myo‐inositol are being studied. The Cochrane Review evaluating myo‐inositol use for the prevention of GDM concluded there may be a reduction in GDM risk with its use, but the review authors ultimately suggested the need for further research due to low‐quality evidence (Crawford 2015). One overview of Cochrane Reviews for the prevention of gestational diabetes looked at all these interventions, and they found that no studied intervention resulted in clear benefit or harm, and many of these interventions did not have enough high‐quality evidence to determine an effect (Griffith 2020).
Description of the intervention
According to the World Health Organization, probiotics are defined as "live microorganisms which when administered in adequate amounts confer a health benefit on the host" (FAO/WHO 2006). The health effects provided by probiotics vary depending on the specific species and strain of probiotic used, and, therefore, have been investigated in a wide variety of health conditions. Among the most common probiotics used are members of the genera Lactobacillus, Bifidobacterium and Enterococcus, but products differ greatly in the strains and concentrations used (Syngai 2016; FAO/WHO 2006). Probiotics are available in a variety of food products, such as yoghurt or fermented milks, or as dietary supplements that can be purchased as capsules without a prescription.
How the intervention might work
Studies of the human microbiome have revealed a complex relationship between the microbiome and an individual's overall health and wellbeing. The microbiome is altered by a variety of factors including diet and various health conditions (David 2014), and in turn, the microbiome may influence host metabolism and contribute to the development of obesity and diabetes (Musso 2011). Many studies of the gut microbiome in obese people have revealed an increase in the proportion of bacteria in the Firmicutes phylum and a decrease in bacteria belonging to the Bacteroidetes phylum (John 2016). Similarly, studies in people with type 2 diabetes and glucose intolerance have revealed a reduction in Akkermansia muciniphila and butyrate‐producing bacteria such as Lactobacillus and Bifidobacterium in their microbiome (Brunkwall 2017). These changes in the gut microbiome may be linked to obesity and diabetes through the role bacteria play in host glucose and lipid metabolism (Musso 2011).
Increases in body fat and decreases in insulin sensitivity are normal changes in pregnancy, and these changes appear to be linked to changes in the microbiome as well. Koren 2012 found that the gut microbiome became less diverse as pregnancy progressed, and the microbiome in the third trimester was associated with increased adiposity and insulin insensitivity when transplanted into mice (Koren 2012). In women with GDM, insulin sensitivity is impaired beyond normal levels, leading to hyperglycaemia. Crusell 2018 have looked at the microbiome in women with GDM, and found that the microbiome in GDM differed slightly from that in normal pregnancy and may have resembled that of non‐pregnant women with type 2 diabetes (Crusell 2018).
Given the role the microbiome plays in host glucose and lipid metabolism (Musso 2011), probiotics have been suggested as an intervention for improving glycaemic control in diabetes by helping to restore balance among species of bacteria in the microbiome (Tiderencel 2020). Many randomised controlled trials have examined the use of probiotics in people with type 2 diabetes, and one meta‐analysis of these trials revealed that probiotics were helpful in improving glycaemic control and may have improved glucose metabolism (Tiderencel 2020). GDM is like type 2 diabetes in that there are similar changes in insulin resistance and possibly in the microbiome (Crusell 2018), and, therefore, probiotics may have similar effects for prevention or treatment of GDM. A Cochrane Review evaluating the use of probiotics to treat GDM was published in 2020 (Okesene‐Gafa 2020).
Why it is important to do this review
The incidence of GDM is increasing (Noh 2021; López‐de‐Andrés 2020; Abouzeid 2014; Dabelea 2005), and GDM is associated with significant health implications for both mother and infant (HAPO 2008). Therefore, prevention of GDM is ideal. One study evaluating the use of probiotics in pregnancy suggested that probiotics may reduce the incidence of GDM (Laitinen 2009). Since this initial study, other studies have tried to address this question with mixed results (Asgharian 2020; Callaway 2019; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019; Wickens 2017). Therefore, a systematic review is necessary to synthesise the available evidence for or against the use of probiotics for preventing GDM in pregnancy.
Objectives
To systematically assess the effects of probiotic supplements used either alone or in combination with pharmacological and non‐pharmacological interventions on the prevention of GDM.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised and cluster‐randomised trials; however, we found no cluster‐randomised trials. Quasi‐randomised and cross‐over design studies were not eligible for inclusion in this review. Studies presented only as abstracts with no subsequent full report of study results were only included if we received confirmation from the study authors that the data in the abstract were final. Otherwise, they were listed as awaiting classification.
Types of participants
Studies that included pregnant women not previously diagnosed with diabetes mellitus. Studies of women with GDM in a previous pregnancy but no evidence of diabetes mellitus or GDM in the current pregnancy before entering the trial were eligible for inclusion.
Types of interventions
Probiotic supplementation for prevention of GDM, either alone or in combination with pharmacological (e.g. metformin) or non‐pharmacological (e.g. diet/lifestyle) interventions.
Probiotic supplementation (administered by any method) should have been commenced prior to the diagnosis of GDM and continued for any duration.
Comparison interventions of placebo or diet were eligible.
Trials may have used other interventions in a comparison arm or in combination with the probiotic. These other interventions may have included pharmaceutical probiotic supplements as well as food items supplemented with probiotics.
Types of outcome measures
The outcomes for this review are from the Cochrane Core Outcome Set for GDM prevention.
Primary outcomes
Maternal
Diagnosis of GDM
Hypertensive disorders of pregnancy (including pre‐eclampsia, pregnancy‐induced hypertension and eclampsia)
Caesarean section
Infant
Large‐for‐gestational age
Perinatal mortality (including stillbirth and neonatal mortality)
Mortality or morbidity composite
Secondary outcomes
Maternal
Induction of labour
Perineal trauma
Placental abruption
Postpartum haemorrhage
Postpartum infection
Weight gain during pregnancy
Adherence to the intervention
Behaviour changes associated with the intervention
Relevant biomarker changes associated with the intervention (including adiponectin, free fatty acids, triglycerides, high‐density lipoproteins, low‐density lipoproteins, insulin, etc.)
Sense of wellbeing and quality of life
Views of the intervention
Breastfeeding
Long‐term maternal
Postnatal depression
Postnatal weight retention or return to prepregnancy weight
Body mass index (BMI)
GDM in a subsequent pregnancy
Type 1 diabetes
Type 2 diabetes
Impaired glucose tolerance
Cardiovascular health as defined by trialists (including blood pressure, hypertension, cardiovascular disease and metabolic syndrome)
Infant
Stillbirth
Neonatal mortality
Gestational age at birth
Preterm birth (less than 37 weeks' gestation and less than 32 weeks' gestation)
Apgar score (less than seven at five minutes)
Macrosomia
Small‐for‐gestational age (SGA)
Birthweight and z‐score
Head circumference and z‐score
Length and z‐score
Ponderal index
Adiposity
Shoulder dystocia
Bone fracture
Nerve palsy
Respiratory distress syndrome
Hypoglycaemia as defined by trialists
Hyperbilirubinaemia
Later infant and childhood
Weight and z‐scores
Height and z‐scores
Head circumference and z‐scores
Adiposity (including BMI and skinfold thickness)
Blood pressure
Type 1 diabetes
Type 2 diabetes
Impaired glucose tolerance
Dyslipidaemia or metabolic syndrome
Neurodisability
Educational achievement
Child as an adult
Weight
Height
Adiposity (including BMI and skinfold thickness)
Cardiovascular health as defined by trialists (including blood pressure, hypertension, cardiovascular disease and metabolic syndrome)
Type 1 diabetes
Type 2 diabetes
Impaired glucose tolerance
Dyslipidaemia or metabolic syndrome
Employment, education and social status/achievement
Health service use
Number of hospital or health professional visits (including midwife, obstetrician, physician, dietician and diabetic nurse)
Number of antenatal visits or admissions
Length of antenatal stay
Neonatal intensive care unit admission
Length of postnatal stay (mother)
Length of postnatal stay (baby)
Costs to families associated with the management provided
Costs associated with the intervention
Cost of maternal care
Cost of offspring care
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth's Trials Register by contacting their Information Specialist (20 March 2020).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth's Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings; and the list of journals reviewed via the current awareness service, see the Cochrane Pregnancy and Childbirth's Trials Register (pregnancy.cochrane.org/pregnancy-and-childbirth-groups-trials-register).
Briefly, Cochrane Pregnancy and Childbirth's Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Two people screen search results and review the full text of all relevant trial reports identified through the searching activities. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/) for unpublished, planned and ongoing trial reports (20 March 2020) using the search methods detailed in Appendix 1.
Searching other resources
We searched the reference lists of all retrieved studies.
We applied no language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, see Barrett 2014.
For this update, we used the following methods for assessing the 43 reports that were identified as a result of the updated search.
The following methods section is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors (SJD and MDN) independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted another review author (LC). Since three review authors were also authors on one of the identified studies (Callaway 2019), the two review authors not involved with this study assessed it for inclusion (SJD and SAP).
We created a study flow diagram to map out the number of records identified, included, excluded or awaiting classification (Figure 1).
1.
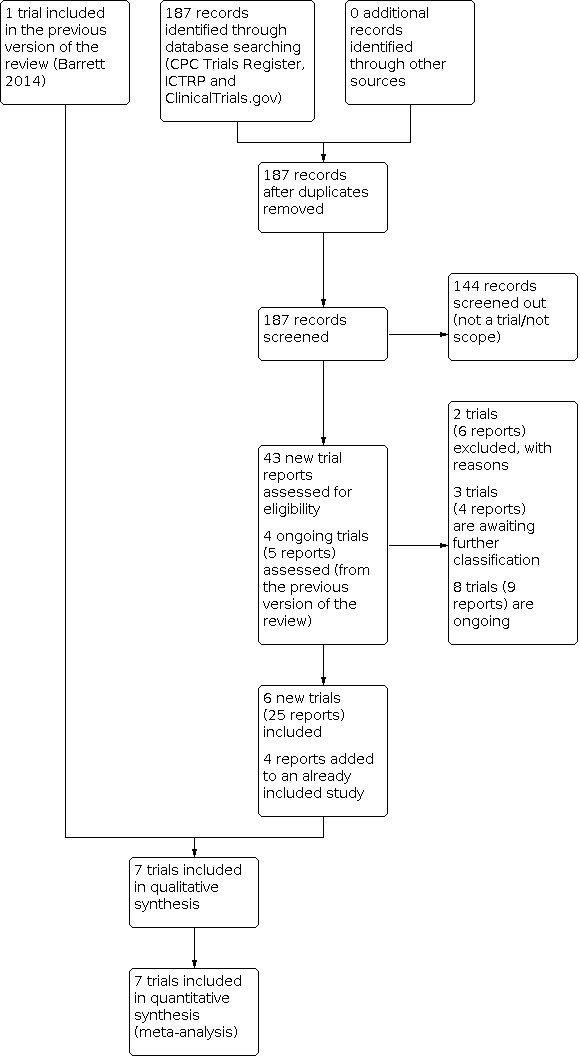
Study flow diagram.
Screening eligible studies for scientific integrity/trustworthiness
Two review authors evaluated all studies meeting our inclusion criteria against predefined criteria to select studies that, based on available information, were deemed to be sufficiently trustworthy to be included in the analysis. The criteria are as follows.
Research governance
No prospective trial registration for studies published after 2010 without plausible explanation.
When requested, trial authors refuse to provide/share the protocol or ethics approval letter (or both).
Trial authors refuse to engage in communication with the Cochrane Review authors.
Trial authors refuse to provide individual participant data (IPD) data upon request with no justifiable reason.
Baseline characteristics
Characteristics of the study participants being too similar (distribution of mean (standard deviation (SD)) excessively narrow or excessively wide, as noted by Carlisle 2017.
Feasibility
Implausible numbers (e.g. 500 women with severe cholestasis of pregnancy recruited in 12 months).
(Close to) zero losses to follow‐up without plausible explanation.
Results
Implausible results (e.g. massive risk reduction for main outcomes with small sample size).
Unexpectedly even numbers of women 'randomised' including a mismatch between the numbers and the methods (e.g. if they say no blocking was used but still end up with equal numbers, or they say they used blocks of four but the final numbers differ by six).
Studies assessed as being potentially 'high risk' were not included in the review. Where a study was classified as 'high risk' for one or more of the above criteria, we attempted to contact the study authors to address any possible lack of information/concerns. If adequate information remained unavailable, the study remained in 'awaiting classification' and the reasons and communications with the author (or lack of) described in detail.
The process is described fully in Figure 3.
Abstracts
Data from abstracts were only included if, in addition to the trustworthiness assessment, the study authors confirmed in writing that the data to be included in the review had come from the final analysis and will not change. If such information was not available/provided, the study remained 'awaiting classification' (as above).
Data extraction and management
We designed a form to extract data. For eligible studies, at least two review authors (SJD and MDN for most studies, SJD and SAP for Callaway 2019) extracted data using the agreed form. We resolved discrepancies through discussion, or, if required, through consultation with a third review author. We entered data into Review Manager 5 and checked for accuracy (Review Manager 2014). When information regarding any of the above was unclear, we attempted to contact authors of the original reports to request further details.
Assessment of risk of bias in included studies
Two review authors (SJD and MDN) independently assessed risk of bias for the included studies using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third review author. Different review authors (SJD and SAP) independently assessed risk of bias for Callaway 2019 to limit the effect of conflict of interest.
1. Random sequence generation (checking for possible selection bias)
We described the method used to generate the allocation sequence in sufficient detail to assess whether it produced comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
2. Allocation concealment (checking for possible selection bias)
We described the methods used to conceal allocation to interventions prior to assignment and assessed whether the intervention allocation could have been foreseen in advance of, or during, recruitment or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
3.1. Blinding of participants and personnel (checking for possible performance bias)
We described the methods used to conceal the allocation sequence and determine whether intervention allocation could have been foreseen in advance of, or during, recruitment or changed after assignment. We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
3.2. Blinding of outcome assessment (checking for possible detection bias)
We described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies would be at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias.
4. Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses that we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
5. Selective reporting (checking for reporting bias)
We described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all the study's prespecified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study's prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
6. Other bias (checking for bias due to problems not covered by 1. to 5. above)
We described any important concerns we had about other possible sources of bias.
We assessed whether the included study was free of other problems that could have put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
7. Overall risk of bias
We made explicit judgements about whether the included study was at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to 1. to 6. above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses (see Sensitivity analysis).
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CI).
Continuous data
For continuous data, we used the mean difference (MD) with 95% CIs if outcomes were measured in the same way between trials. We planned to use the standardised mean difference with 95% CIs to combine trials that measured the same outcome, but used different scales.
Unit of analysis issues
Cluster‐randomised trials
We identified no cluster‐randomised trials for inclusion. However, if we identify cluster‐randomised trials in updates of this review, we will include them in the analyses along with individually randomised trials. We will adjust their effect measure using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. Where the cluster‐randomised trial properly accounts for the cluster design, we will extract an estimate of the effect measure directly. Where the cluster‐randomised trial does not properly account for the clustering, we will calculate the effective sample size of the intervention and placebo groups by dividing the sample size by the design effect. The design effect is 1 + (m – 1) × ICC where m is the mean cluster size. We will assess the cluster‐randomised trials and the calculation of the effective sample size will be performed with the assistance of a statistician. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a subgroup analysis to investigate the effects of the randomisation unit.
Studies with more than two intervention groups
In studies with more than two groups, only the two groups that best fit the comparisons used in this review were chosen. When there were more than two groups due to a secondary intervention, the groups without the secondary intervention were chosen if possible to minimise the effect of the secondary intervention on the comparison. If this was not possible, the groups were chosen so that the secondary intervention was the same in both groups. For 2×2 factorial trials, groups were combined where appropriate given the participants were independently randomised to the intervention of interest.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis (i.e. we attempted to include all participants randomised to each group in the analyses, and analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention). The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the I² and Chi² statistics. For random‐effects meta‐analyses we also considered the Tau² statistic. We regarded heterogeneity as substantial if the I² statistic was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Given we included only seven studies, reporting bias analysis was not undertaken. In updates of this review, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager 5 (Review Manager 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect (i.e. where trials examined the same intervention, and the trials' populations and methods were judged sufficiently similar). If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if there was substantial statistical heterogeneity, we used random‐effects meta‐analysis to produce an overall summary, if a mean treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the mean range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the mean treatment effect was not clinically meaningful, we did not combine trials.
If we used random‐effects analyses, we presented the results as the mean treatment effect with 95% CIs, and the estimates of the Tau² and I² statistics.
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses.
History of GDM or family history of type 2 diabetes (yes versus no).
Probiotic dose (more than five billion colony‐forming units (CFU) versus less than five billion CFU).
Probiotic bacterial species (one species versus another species).
Probiotic treatment starting in early pregnancy versus starting at more than 20 weeks' gestation.
Probiotic mode of delivery (capsule versus other).
Probiotic frequency of administration (daily versus other).
In this update of the review, subgroup analysis by history of GDM or family history of type 2 diabetes was not conducted as outcome data were not available in these subgroups. The subgroup analysis by probiotic mode of delivery and frequency of administration were also not conducted since all included studies administered the intervention daily as a capsule. These subgroups will be included in future updates of the review if possible.
Subgroup analysis was restricted to the review's primary outcomes. We assessed subgroup differences by interaction tests available within Review Manager 5 (Review Manager 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² statistic.
Sensitivity analysis
Sensitivity analysis was carried out, where necessary, to explore the influence of diagnostic criteria for GDM. Sensitivity analysis was restricted to the review's primary outcomes.
We planned to conduct a sensitivity analysis to explore the influence of high dropout rates (more than 20%); however, we identified no such studies. This may be possible in updates of this review.
Summary of findings and assessment of the certainty of the evidence
For this update, we used the GRADE approach to assess the certainty of the evidence as outlined in the GRADE Handbook to assess the quality of the body of evidence relating to the following outcomes for the main comparison probiotics versus placebo. If studies comparing probiotics and diet are identified in future updates, we will evaluate this comparison.
Maternal
Diagnosis of GDM
Hypertensive disorders of pregnancy (pre‐eclampsia)
Caesarean section
Perineal trauma
Weight gain during pregnancy
Postnatal depression
Development of subsequent diabetes
Infant
Large‐for‐gestational age
Perinatal mortality
Mortality or morbidity composite
Hypoglycaemia as defined by trialists
Adiposity
Diabetes
Neurodisability
We used GRADEpro GDT to import data from Review Manager 5 (Review Manager 2014) in order to create 'Summary of findings' tables. We produced a summary of the intervention effect and a measure of certainty for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; and Characteristics of ongoing studies tables.
Results of the search
See: Figure 1
We assessed 43 new trial reports from an updated search in March 2020. We also reassessed the four studies (five reports) that were ongoing in the previous version of the review. We included six new trials (25 reports), added four new reports to the previously included study (Laitinen 2009), and excluded two trials (six reports). Three studies (four reports) are awaiting further classification and we added eight studies (nine reports) to the Ongoing studies section.
Screening eligible studies for scientific integrity/trustworthiness
See: Figure 2
2.
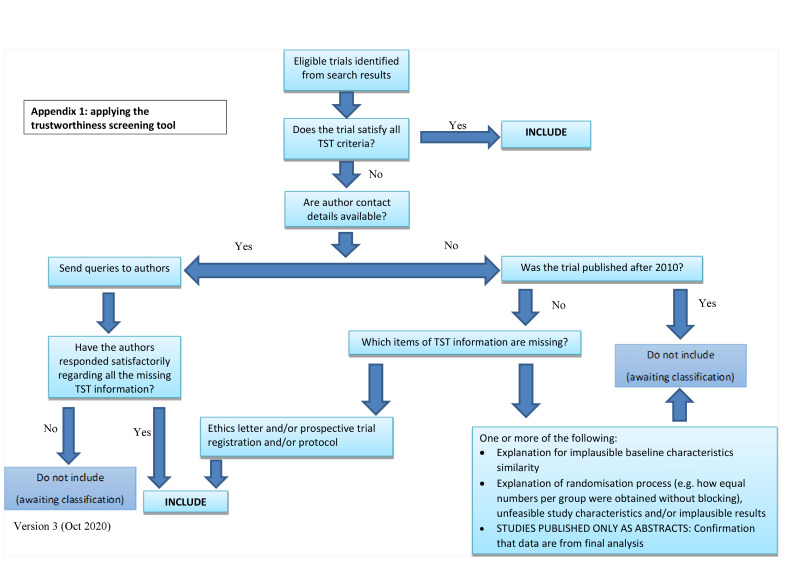
Applying the trustworthiness screening tool
One study is awaiting classification since it was only available in abstract form and confirmation that the presented data came from the final analysis was not received (Charles 2018). Another study is awaiting classification because it was unclear whether the study met our inclusion criteria; we sought clarification from the authors but received no response (Si 2019).
Two studies were at high risk according to the prespecified trustworthiness criteria. One study had almost no losses to follow‐up (Asgharian 2020), and there was insufficient information provided by the study authors for us to make a definitive classification. Therefore, this study remains in studies awaiting classification. The other study had no losses to follow‐up and had limited information regarding their randomisation methods (Jamilian 2016). However, the study authors provided more detail regarding these concerns, and the study was included since it was determined to be at low risk. See Characteristics of included studies; Characteristics of studies awaiting classification for further information.
Included studies
Design
All included studies were parallel randomised controlled trials. Four studies had one intervention arm and one control arm (Callaway 2019; Jamilian 2016; Lindsay 2014; Wickens 2017). Laitinen 2009 had three arms for two interventions. Pellonpera 2019 had four arms for two interventions, and Okesene‐Gafa 2019 was designed as a 2×2 factorial with two interventions.
Sample sizes
The number of women recruited in the included studies ranged from 60 (Jamilian 2016) to 438 women (Pellonpera 2019). The other studies recruited 175 (Lindsay 2014), 230 (Okesene‐Gafa 2019), 256 (Laitinen 2009), 423 (Wickens 2017), and 433 women (Callaway 2019).
Setting
The included studies in this review were conducted in Iran (Jamilian 2016), Australia (Callaway 2019), Finland (Laitinen 2009; Pellonpera 2019), Ireland (Lindsay 2014), and New Zealand (Okesene‐Gafa 2019; Wickens 2017).
Participants
All included studies were conducted in pregnant women with singleton pregnancies without pre‐existing diabetes or other significant health conditions, although two studies included women with a history of atopic disease (Laitinen 2009; Wickens 2017). Two studies were conducted in overweight and obese pregnant women (Callaway 2019; Pellonpera 2019), two in obese pregnant women only (Lindsay 2014; Okesene‐Gafa 2019), and three did not exclude women based on their body mass index (Jamilian 2016; Laitinen 2009; Wickens 2017).
Interventions and comparisons
All seven trials compared probiotics versus placebo. In four trials, women were only randomised to either probiotics or placebo (Callaway 2019; Jamilian 2016; Lindsay 2014; Wickens 2017). Three studies included a second intervention, two of which included a dietary intervention (Laitinen 2009; Okesene‐Gafa 2019), and one included a fish oil capsule (Pellonpera 2019). Laitinen 2009 randomised women to probiotics plus dietary intervention, placebo plus dietary intervention, or placebo plus routine dietary advice. Okesene‐Gafa 2019 first randomised all women to the dietary intervention or routine dietary advice, then randomised all women again to either probiotics or placebo. Pellonpera 2019 randomised women to one of four study arms (probiotics plus fish oil, probiotics plus placebo, placebo plus fish oil or placebo plus placebo). Although two trials included diet as a secondary intervention, the trials did not directly compare probiotics versus diet (Laitinen 2009; Okesene‐Gafa 2019). Therefore, no conclusions could be drawn about this comparison.
Six trials started the intervention prior to 20 weeks' gestation (Callaway 2019; Jamilian 2016; Laitinen 2009; Okesene‐Gafa 2019; Pellonpera 2019; Wickens 2017), and one trial started the intervention at 20 weeks' gestation or later (Lindsay 2014). One study initially started the intervention before 16 weeks' gestation, but it was later changed to before 20 weeks' gestation due to a change in hospital policy (Callaway 2019). Women received the intervention daily in all studies.
All included studies delivered the intervention as a capsule. The dose of probiotic used in the studies varied, with three studies reporting a dose of less than five billion CFUs per species (Callaway 2019; Jamilian 2016; Lindsay 2014), and four studies reporting a dose of greater than five billion CFUs per species (Laitinen 2009; Okesene‐Gafa 2019; Pellonpera 2019; Wickens 2017). However, it is important to note that given decay in probiotics over time from the date of manufacturing, the dose can change. Therefore, studies reported either the minimum or mean dose, so individual participants in each study may have received doses that varied from the reported study dose.
Studies used a variety of different bacterial species and strains, and most used a combination of species for their probiotics. The species were Lactobacillus rhamnosus GG (Callaway 2019; Laitinen 2009; Okesene‐Gafa 2019), Lactobacillus rhamnosus HN001 (Pellonpera 2019; Wickens 2017), Lactobacillus acidophilus LA5 (Jamilian 2016), Lactobacillus casei (Jamilian 2016), Lactobacillus salivarius UCC118 (Lindsay 2014), Bifidobacterium animalis subspecies lactis BB12 (Callaway 2019; Laitinen 2009; Okesene‐Gafa 2019), Bifidobacterium animalis subspecies lactis 420 (Pellonpera 2019), and Bifidobacterium bifidum (Jamilian 2016).
Outcomes
Studies were required to have either the diagnosis of GDM or a marker of glucose metabolism in the third trimester of pregnancy as a reported outcome to be eligible for inclusion. Six studies reported the incidence of GDM (Callaway 2019; Laitinen 2009; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019; Wickens 2017), while one reported laboratory measures of glucose metabolism such as fasting plasma glucose and insulin levels (Jamilian 2016). The studies that reported the incidence of GDM used several different diagnostic criteria, and some studies reported results according to more than one set of diagnostic criteria. Four studies used the IADPSG criteria (Callaway 2019; Okesene‐Gafa 2019; Pellonpera 2019; Wickens 2017; criteria: IADPSG 2010); one study used the Carpenter and Coustan criteria (Lindsay 2014; criteria: Carpenter 1982), one used the modified Fourth International Workshop‐Conference on GDM criteria (Laitinen 2009; criteria: Metzger 1998), one used the local New Zealand criteria (Australasian Diabetes in Pregnancy Society) (Wickens 2017; criteria: Ministry of Health 2014), and one used the local Finnish criteria (Pellonpera 2019; criteria: The Finnish Medical Society Duodecim 2013). The details for each set of diagnostic criteria can be found in Table 3.
1. Diagnostic criteria for GDM.
| Parameter | IADPSG (IADPSG 2010) | Carpenter and Coustan (Carpenter 1982) | Modified Fourth International Workshop‐Conference (Metzger 1998) | New Zealand Guidelines (Ministry of Health 2014) | Finnish Current Care Guidelines (The Finnish Medical Society Duodecim 2013) |
| OGTT (g) | 75 | 100 | 75 | 75 | 75 |
| Fasting (mmol/L) | 5.1 | 5.3 | 4.8 | 5.5 | 5.3 |
| 1 hour (mmol/L) | 10.0 | 10.0 | 10.0 | — | 10.0 |
| 2 hours (mmol/L) | 8.5 | 8.6 | 8.7 | 9.0 | 8.6 |
| 3 hours (mmol/L) | — | 7.8 | — | — | — |
| Elevated values required | 1 | 2 | 1 | 1 | 1 |
IADPSG: International Association of Diabetes and Pregnancy Study Groups; OGTT: oral glucose tolerance test.
At least one study reported the other primary outcomes in this review. Four studies reported hypertensive disorders of pregnancy (Callaway 2019; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019), six studies reported caesarean sections (Callaway 2019; Laitinen 2009; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019; Wickens 2017), four studies reported large‐for‐gestational‐age infants (Callaway 2019; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019), three studies reported perinatal mortality including stillbirth and neonatal mortality (Callaway 2019; Laitinen 2009; Lindsay 2014), and two studies reported a neonatal mortality or morbidity composite measure (Callaway 2019; Okesene‐Gafa 2019).
Further details on the primary and secondary outcomes of each study can be found in the Characteristics of included studies table.
Dates of study
The studies were all conduced between 2002 and 2017, with the following trial dates: April 2002 to November 2005 (Laitinen 2009), March 2012 to March 2013 (Lindsay 2014), commencement in November 2012 with no end date provided (Callaway 2019), December 2012 to November 2014 (Wickens 2017), October 2013 to July 2017 (Pellonpera 2019), March 2015 to July 2015 (Jamilian 2016), and April 2015 to June 2017 (Okesene‐Gafa 2019).
Funding sources
Study authors reported the following sources of funding: National Health and Medical Research Council (Callaway 2019), the Royal Brisbane and Women's Hospital Foundation (Callaway 2019), Vice‐Chancellor for Research, AUMS, Iran (Jamilian 2016), Academy of Finland (Laitinen 2009; Pellonpera 2019), Sigrid‐Juselius Foundation (Laitinen 2009), Juho Vainio Foundation (Laitinen 2009; Pellonpera 2019), Social Insurance Institution of Finland (Laitinen 2009), Raisio (Laitinen 2009), Chr. Hansen A/S (Laitinen 2009; Okesene‐Gafa 2019), Valio Ltd (Laitinen 2009), National Maternity Hospital Medical Fund Ivo Drury Award (Lindsay 2014), Alimentary Health Ltd (Lindsay 2014), Counties Manukau Health (Okesene‐Gafa 2019), Cure Kids Grant (Okesene‐Gafa 2019), Lottery Health Research (Okesene‐Gafa 2019), RANZCOG Two Mercia Barnes Trust (Okesene‐Gafa 2019), Gravida National Centre for Growth and Development (Okesene‐Gafa 2019), University of Auckland Faculty Development Research Fund and Reinvestment Fund (Okesene‐Gafa 2019), Nurture Foundation (Okesene‐Gafa 2019), Heart Foundation of New Zealand (Okesene‐Gafa 2019), Roche Diagnostics International Ltd (Okesene‐Gafa 2019), Turku University Hospital Expert Responsibility Area (Pellonpera 2019), Diabetes Research Foundation (Pellonpera 2019), Business Finland (Pellonpera 2019), the Finnish Medical Foundation (Pellonpera 2019), the University of Turku (Pellonpera 2019), DuPont (Pellonpera 2019), Croda Europe Ltd (Pellonpera 2019), the Health Research Council of New Zealand (Wickens 2017), and Fonterra (Wickens 2017).
Declarations of interest
All included studies stated they had no declarations of interest.
Further details on each study can be found in the Characteristics of included studies table.
Excluded studies
We excluded two studies from this review since the intervention was not started until the third trimester of pregnancy, after GDM would have been diagnosed (Asemi 2013; Taghizadeh 2014). See Characteristics of excluded studies table.
Risk of bias in included studies
Our risk of bias assessment is summarised in Figure 3. The included studies were at low risk of bias in all domains except for Jamilian 2016, which was at unclear risk of selection bias. Three review authors were authors on one of the included studies (HB, MDN and LC) (Callaway 2019). Therefore, the other two review authors (SJD and SAP) assessed risk of bias in Callaway 2019 to minimise any effects from conflicts of interest.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
All included studies used computer‐generated randomisation procedures. Some studies used block randomisation with blocks of four (Pellonpera 2019), six (Laitinen 2009), or random block sizes (Okesene‐Gafa 2019; Wickens 2017). Other studies used simple 1:1 randomisation (Lindsay 2014), or did not state whether blocking methods were used (Callaway 2019; Jamilian 2016). All studies were classified at low risk of bias for random sequence generation.
Allocation concealment
All studies stated that allocation concealment was used. Three studies used sealed, opaque envelopes to conceal the allocation sequence prior to recruitment (Callaway 2019; Laitinen 2009; Lindsay 2014), and three studies assigned participants sequentially using a randomisation sequence generated by a third party and unknown to the study staff responsible for enrolment (Okesene‐Gafa 2019; Pellonpera 2019; Wickens 2017). One study claimed to use allocation concealment but provided no further information, so this study was classified at unclear risk of bias (Jamilian 2016). All other studies were at low risk of bias (Callaway 2019; Laitinen 2009; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019; Wickens 2017).
Blinding
All studies blinded both participants and personnel to probiotic/placebo allocation. While Laitinen 2009 had one group where the probiotic/placebo intervention was not blinded to study staff (placebo plus routine dietary advice), this group was not used for comparison in this review. In addition, in the two studies that used a secondary dietary intervention, participants and personnel were not blinded to the dietary intervention (Laitinen 2009; Okesene‐Gafa 2019). However, the dietary intervention was the same in both the probiotic and placebo groups, and, therefore, the lack of blinding for this intervention did not affect the probiotics versus placebo comparison. Therefore, all included studies were at low risk for bias for both performance and detection bias.
Incomplete outcome data
There was minimal loss to follow‐up at the time of testing for GDM or third trimester measurements of glucose metabolism in all studies. Loss to follow‐up rates ranged from 0% to 13.9% and were similar between groups. All studies were classified as low risk for attrition bias. Attrition rates for each study are shown in the Characteristics of included studies table.
Selective reporting
All studies reported their prespecified or mentioned outcomes. All studies were at low risk for reporting bias.
Other potential sources of bias
The studies had no other sources of bias.
Effects of interventions
Summary of findings 1. Probiotics compared to placebo for preventing gestational diabetes (maternal outcomes).
| Probiotics compared to placebo for preventing gestational diabetes (maternal outcomes) | ||||||
|
Patient or population: preventing gestational diabetes Setting: Australia, Finland, Iran, Ireland, and New Zealand Intervention: probiotics Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Probiotics | |||||
| Diagnosis of gestational diabetes mellitus | Study population | Mean RR 0.80 (0.54 to 1.20) | 1440 (6 RCTs) | ⊕⊕⊝⊝ Lowa,b | — | |
| 191 per 1000 | 153 per 1000 (103 to 229) | |||||
| Hypertensive disorders of pregnancy (pre‐eclampsia) | Study population | RR 1.85 (1.04 to 3.29) | 955 (4 RCTs) | ⊕⊕⊕⊕ High | — | |
| 35 per 1000 | 65 per 1000 (37 to 116) | |||||
| Caesarean section | Study population | RR 1.00 (0.86 to 1.17) | 1520 (6 RCTs) | ⊕⊕⊕⊕ High | — | |
| 285 per 1000 | 285 per 1000 (245 to 333) | |||||
| Perineal trauma | — | — | — | — | — | Not reported |
| Weight gain during pregnancy | The mean weight gain during pregnancy was 9.4–14.8 kg | MD 0.30 kg higher (0.67 lower to 1.26 higher) | — | 853 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | — |
| Postnatal depression | — | — | — | — | — | Not reported |
| Development of subsequent diabetes | — | — | — | — | — | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for serious concerns about inconsistency due to substantial unexplained heterogeneity between studies. bDowngraded one level for serious concerns about imprecision due to a wide CI.
Summary of findings 2. Probiotics compared to placebo for preventing gestational diabetes (infant outcomes).
| Probiotics compared to placebo for preventing gestational diabetes (infant outcomes) | ||||||
|
Patient or population: preventing gestational diabetes Setting: Australia, Finland, Iran, Ireland, and New Zealand Intervention: probiotics Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with probiotics | |||||
| Large‐for‐gestational age | Study population | RR 0.99 (0.72 to 1.36) | 919 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | — | |
| 142 per 1000 | 141 per 1000 (102 to 193) | |||||
| Perinatal mortality (stillbirth and neonatal mortality) | Study population | RR 0.33 (0.01 to 8.02) | 709 (3 RCTs) | ⊕⊕⊝⊝ Lowb | — | |
| 3 per 1000 | 1 per 1000 (0 to 22) | |||||
| Mortality or morbidity composite | Study population | RR 0.69 (0.36 to 1.35) | 623 (2 RCTs) | ⊕⊕⊝⊝ Lowb | — | |
| 61 per 1000 | 42 per 1000 (22 to 83) | |||||
| Hypoglycaemia as defined by trialists | Study population | Mean RR 1.15 (0.69 to 1.92) | 586 (2 RCTs) | ⊕⊕⊝⊝ Lowa,c | — | |
| 135 per 1000 | 155 per 1000 (93 to 259) | |||||
| Adiposity | The included studies showed no appreciable difference in fat mass or % fat between groups. 1 study reported adiposity as fat mass (MD –0.04 kg, 95% CI –0.12 to 0.04) 1 study reported adiposity as % fat (MD –0.10%, 95% CI –1.19 to 0.99) |
— | 320 (2 RCTs data not pooled) | ⊕⊕⊝⊝ Lowd | — | |
| Diabetes | — | — | — | — | — | Not reported |
| Neurodisability | — | — | — | — | — | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for serious concerns about imprecision due to a wide confidence intervals that included both appreciable benefit and harm. bDowngraded two levels for very serious concerns about imprecision due to a very small number of events and a wide confidence intervals that included both appreciable benefit and harm. cDowngraded one level for serious concerns about inconsistency due to unexplained heterogeneity between studies. dDowngraded two levels for very serious concerns about imprecision due to the small sample sizes of the included studies and wide confidence intervals that included both appreciable benefit and harm.
All seven included studies compared probiotics versus placebo. While two studies included a secondary dietary intervention (Laitinen 2009; Okesene‐Gafa 2019), the studies were not conducted in a way that facilitated our second comparison of probiotics versus diet. This comparison will be included in review updates.
Probiotics versus placebo
Four studies had one intervention with the comparison probiotics versus placebo (Callaway 2019; Jamilian 2016; Lindsay 2014; Wickens 2017). In the three studies that had two simultaneous interventions, the study groups used in this review were chosen to balance the effect of the secondary intervention between the probiotic and placebo groups (Laitinen 2009; Okesene‐Gafa 2019; Pellonpera 2019). Laitinen 2009 had three arms, and for this comparison we used two of these arms to isolate the effect of the probiotic intervention (probiotics plus dietary intervention for probiotics, placebo plus dietary intervention for placebo). Okesene‐Gafa 2019 conducted a 2×2 factorial study where all participants were separately randomised to the dietary intervention and probiotics, so we used all groups for this comparison (probiotics with or without dietary intervention for probiotics, placebo with or without dietary intervention for placebo). Pellonpera 2019 was a four‐arm study of two interventions, so we only used the groups without the fish oil intervention for this comparison to isolate the effect of the probiotics (probiotics plus placebo for probiotics, placebo plus placebo for placebo).
Primary outcomes
Maternal
Diagnosis of gestational diabetes mellitus
Six studies reported GDM (Callaway 2019; Laitinen 2009; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019; Wickens 2017). We used a random‐effects model given the substantial heterogeneity present (I² = 64%). It is uncertain if probiotics have any effect on the risk for GDM compared to placebo (average RR 0.80, 95% CI 0.54 to 1.20; 1440 women; I2 = 64%; Tau² = 0.15; Analysis 1.1). Given the substantial heterogeneity and the wide CI including both appreciable benefit and appreciable harm, this evidence was low certainty (Table 1).
1.1. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 1: Gestational diabetes mellitus
The studies that reported GDM used different criteria for the diagnosis. Four studies used IADPSG criteria (Callaway 2019; Okesene‐Gafa 2019; Pellonpera 2019; Wickens 2017), one study used local criteria based on the Fourth International Workshop‐Conference on Gestational Diabetes criteria (Laitinen 2009), and one study used Carpenter and Coustan criteria (Lindsay 2014). Sensitivity analysis was performed based on these criteria, and the results were largely unchanged. There was a reduced risk of GDM when using the Fourth International Workshop‐Conference on Gestational Diabetes criteria, but this is only based on one study and, therefore, should be interpreted with caution.
Subgroup analyses based on whether the reported dose of probiotics was less than five billion CFU (2 studies, 547 participants) or greater than five billion CFU (4 studies, 911 participants) found a difference between the subgroups (Chi² = 6.92, P = 0.009, I² = 85.5%; Analysis 1.2). However, there was still substantial heterogeneity in the subgroup with a dose greater than five billion CFU (Tau² = 0.07, I² = 51%), and the subgroup with a dose less than five billion CFU had only two studies. In addition, given the decay in probiotics over time, this subgroup analysis was conducted based on reported minimum or mean dose and there was no guarantee all participants received the reported dose. Therefore, this subgroup analysis should be interpreted with caution. The subgroup analysis based on bacterial species and duration of treatment revealed no clear differences, although both had subgroups with only one trial (Analysis 1.3; Analysis 1.4).
1.2. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 2: Gestational diabetes mellitus (by dose)
1.3. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 3: Gestational diabetes mellitus (by bacterial species)
1.4. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 4: Gestational diabetes mellitus (by duration of treatment)
Hypertensive disorders of pregnancy (including pre‐eclampsia, pregnancy‐induced hypertension and eclampsia)
Four studies reported hypertensive disorders of pregnancy (Callaway 2019; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019). Probiotics may increase the risk of hypertensive disorders of pregnancy compared to placebo (RR 1.39, 95% CI 0.96 to 2.01; 955 women; I2 = 0%; Analysis 1.5). Subgroup analyses found no differences in the results, although most subgroups only included one or two studies (Analysis 1.6; Analysis 1.7; Analysis 1.8).
1.5. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 5: Hypertensive disorders of pregnancy
1.6. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 6: Hypertensive disorders of pregnancy (by dose)
1.7. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 7: Hypertensive disorders of pregnancy (by bacterial species)
1.8. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 8: Hypertensive disorders of pregnancy (by duration of treatment)
Four studies reported pre‐eclampsia (Callaway 2019; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019). Probiotics increase the risk of pre‐eclampsia compared to placebo (RR 1.85, 95% CI 1.04 to 3.29; 955 women; I2 = 0%; Analysis 1.9; high‐certainty evidence; Table 1).
1.9. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 9: Pre‐eclampsia
Caesarean section
Six studies reported caesarean section (Callaway 2019; Laitinen 2009; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019; Wickens 2017). Probiotics make little to no difference in the rate of caesarean sections compared to placebo (RR 1.00, 95% CI 0.86 to 1.17; 1520 women; I2 = 0%; Analysis 1.10; high‐certainty evidence; Table 1). Subgroup analyses revealed no differences in the results, although most subgroups only included one or two studies (Analysis 1.11; Analysis 1.12; Analysis 1.13).
1.10. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 10: Caesarean section
1.11. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 11: Caesarean section (by dose)
1.12. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 12: Caesarean section (by bacterial species)
1.13. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 13: Caesarean section (by duration of treatment)
Infant
Large‐for‐gestational age
Four studies reported large‐for‐gestational age (Callaway 2019; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019). One study defined large‐for‐gestational age as greater than 90th percentile on customised percentile charts (Okesene‐Gafa 2019), while the other three studies also defined large‐for‐gestational age as greater than 90th percentile but did not specify what charts were used (Callaway 2019; Lindsay 2014; Pellonpera 2019). Probiotics probably make little to no difference in the risk of being large‐for‐gestational age compared to placebo (RR 0.99, 95% CI 0.72 to 1.36; 919 infants; I2 = 0%; Analysis 1.14; moderate‐certainty evidence; Table 2). Subgroup analyses revealed no differences in the results, although most subgroups only included one or two studies (Analysis 1.15; Analysis 1.16; Analysis 1.17).
1.14. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 14: Large‐for‐gestational age
1.15. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 15: Large‐for‐gestational age (by dose)
1.16. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 16: Large‐for‐gestational age (by bacterial species)
1.17. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 17: Large‐for‐gestational age (by duration of treatment)
Perinatal mortality (including stillbirth and neonatal death)
Three studies reported perinatal mortality (Callaway 2019; Laitinen 2009; Lindsay 2014). However, two of these studies had no stillbirths or neonatal deaths in either group (Laitinen 2009; Lindsay 2014), and the other study had only one perinatal death across groups (Callaway 2019). We do not know if probiotics have an effect on perinatal mortality compared to placebo because the wide CI crossed the line of no effect (RR 0.33, 95% CI 0.01 to 8.02; 3 studies, 709 infants; I2 = 0%; Analysis 1.18; low‐certainty evidence; Table 2). This evidence was of low certainty due to the small number of events and very wide CIs. Given the lack of data on this outcome, subgroup analyses would not be meaningful and were not performed.
1.18. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 18: Perinatal mortality (stillbirth and neonatal mortality)
Mortality or morbidity composite
Two studies reported a composite measure of neonatal morbidity (Callaway 2019; Okesene‐Gafa 2019). Callaway 2019 used a composite measure of birth injury including nerve injury, bone fracture and intracranial haemorrhage. Okesene‐Gafa 2019 used a composite measure of morbidity including birth trauma, hypoxic‐ischaemic encephalopathy, sepsis, respiratory distress requiring continuous positive airway pressure and hypoglycaemia requiring intravenous therapy. It is uncertain if probiotics have any effect on neonatal morbidity compared to placebo because the CIs were consistent with appreciable harm and appreciable benefit (RR 0.69, 95% CI 0.36 to 1.35; 623 infants; I2 = 0%; Analysis 1.19; low‐certainty evidence; Table 2). Subgroup analyses were not performed because only two studies reported this outcome.
1.19. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 19: Mortality or morbidity composite
Secondary outcomes
Maternal
Induction of labour
Two studies reported induction of labour (Callaway 2019; Lindsay 2014). Probiotics may make little to no difference in induction of labour rates compared to placebo (RR 1.08, 95% CI 0.85 to 1.39; 544 women; I2 = 23%; Analysis 1.20).
1.20. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 20: Induction of labour
Perineal trauma
No studies reported perineal trauma.
Placental abruption
No studies reported placental abruption.
Postpartum haemorrhage
Two studies reported postpartum haemorrhage (Lindsay 2014; Pellonpera 2019). One study defined postpartum haemorrhage as greater than 1000 mL (Pellonpera 2019), while the other study provided no definition (Lindsay 2014). It is uncertain if probiotics have any effect on the risk of postpartum haemorrhage compared to placebo (RR 1.05, 95% CI 0.60 to 1.85; 324 women; I2 = 0%; Analysis 1.21).
1.21. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 21: Postpartum haemorrhage
Postpartum infection
No studies reported postpartum infection.
Weight gain during pregnancy
Four studies reported weight gain during pregnancy (Callaway 2019; Laitinen 2009; Lindsay 2014; Okesene‐Gafa 2019). Two studies specified the reported weight gain was from baseline to 36 weeks' gestation (Callaway 2019; Okesene‐Gafa 2019), while the other two studies stated "total" weight gain over the course of the pregnancy (Laitinen 2009; Lindsay 2014). There was substantial heterogeneity using a random‐effects model (I² = 40%). Probiotics probably make little to no difference in weight gain during pregnancy compared to placebo (MD 0.30 kg, 95% CI –0.67 to 1.26; 853 women; Analysis 1.22).
1.22. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 22: Weight gain during pregnancy (kg)
Adherence to the intervention
Six studies reported intervention adherence (Jamilian 2016; Laitinen 2009; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019; Wickens 2017). However, it was reported differently by each study, and, therefore, was not amenable to meta‐analysis. Jamilian 2016 reported that all participants received all capsules throughout the intervention (probiotics 30/30 women, placebo 30/30 women). Laitinen 2009 reported that participants reported 99.5% of capsules taken at visit two, 99% at visit three and 95% at visit four; these data were not separated out by group. Lindsay 2014 reported that the number of missed capsules was similar between groups, with 9/63 participants missing three or more capsules in the probiotics group and 12/75 participants missing three or more capsules in the placebo group. Okesene‐Gafa 2019 reported that over 75% of capsules were taken by 87/115 participants, but these data were not separated by group. Pellonpera 2019 reported good compliance by 88.4% of the entire study cohort, and stated that adherence was similar between groups. Wickens 2017 reported median adherence rates with interquartile ranges (IQR), and found no clear difference between groups (probiotics: median 94.9%, IQR 85.7 to 98.8; placebo: median 94.0%, IQR 85.9 to 98.8). Overall, there may be little to no difference in adherence rates between probiotics and placebo.
Behaviour changes associated with the intervention
No studies reported behaviour changes associated with the intervention.
Relevant biomarker changes associated with the intervention (e.g. adiponectin, free fatty acids, triglycerides, high‐density lipoproteins, low‐density lipoproteins, insulin)
All included studies reported at least one relevant biomarker.
Seven studies reported fasting plasma glucose in the third trimester. Given the substantial heterogeneity, we used a random‐effects model (I² = 69%). Probiotics may make little to no difference in fasting plasma glucose levels in the third trimester compared to placebo (MD –0.04 mmol/L, 95% CI –0.12 to 0.05; 1519 women; I2 = 69%; Analysis 1.23).
1.23. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 23: Fasting plasma glucose (mmol/L)
Four studies reported plasma glucose at one hour of a 75 g oral glucose tolerance test (OGTT) (Callaway 2019; Okesene‐Gafa 2019; Pellonpera 2019; Wickens 2017). Probiotics may make little to no difference in one‐hour OGTT results compared to placebo (MD –0.07 mmol/L, 95% CI –0.27 to 0.13; 1110 women; I2 = 0%; Analysis 1.24).
1.24. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 24: 1‐hour oral glucose tolerance test (OGTT) plasma glucose (mmol/L)
Four studies reported plasma glucose at two hours of a 75 g OGTT (Callaway 2019; Okesene‐Gafa 2019; Pellonpera 2019; Wickens 2017). Probiotics may make little to no difference in two‐hour OGTT results compared to placebo (0.02 mmol/L, 95% –0.13 to 0.18; 1186 women; I2 = 0%; Analysis 1.25).
1.25. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 25: 2‐hour OGTT plasma glucose (mmol/L)
Two studies reported triglycerides at the end of the intervention period (Jamilian 2016; Lindsay 2014). Probiotics may slightly reduce triglyceride levels compared to placebo (MD –0.21 mmol/L, 95% CI –0.40 to –0.02; 198 women; I2 = 0%; Analysis 1.26). However, given this is based on two small studies with a wide CI, the certainty of this evidence was low.
1.26. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 26: Triglycerides (mmol/L)
Two studies reported high‐density lipoproteins at the end of the intervention period (Jamilian 2016; Lindsay 2014). Probiotics may make little to no difference in high‐density lipoprotein levels compared to placebo (MD 0.02 mmol/L, 95% CI –0.08 to 0.11; 198 women; I2 = 0%; Analysis 1.27).
1.27. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 27: High‐density lipoprotein (mmol/L)
Two studies reported low‐density lipoproteins at the end of the intervention period (Jamilian 2016; Lindsay 2014). Probiotics may slightly reduce low‐density lipoprotein levels compared to placebo, but the CIs indicates probiotics may make little or no difference (MD –0.22 mmol/L, 95% CI –0.48 to 0.04; 198 women; I2 = 0%; Analysis 1.28).
1.28. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 28: Low‐density lipoprotein (mmol/L)
Two studies reported total cholesterol at the end of the intervention period (Jamilian 2016; Lindsay 2014). Probiotics may slightly reduce total cholesterol levels compared to placebo, but the 95% CI included zero indicating the possibility that probiotics may make little or no difference (MD –0.31 mmol/L, 95% CI –0.62 to –0.00; 198 women; I2 = 0%; Analysis 1.29).
1.29. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 29: Total cholesterol (mmol/L)
Four studies reported insulin levels in the third trimester (Jamilian 2016; Laitinen 2009; Lindsay 2014; Pellonpera 2019). Probiotics may reduce insulin levels slightly compared to placebo (MD –1.95 mU/L, 95% CI –3.01 to –0.88; 538 women; I2 = 0%; Analysis 1.30).
1.30. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 30: Insulin (mU/L)
Sense of wellbeing and quality of life
One study reported sense of wellbeing and quality of life (Okesene‐Gafa 2019). This study reported several different measures of wellbeing and quality of life at 36 weeks' gestation. It is uncertain if probiotics have any effect compared to placebo in Edinburgh Postnatal Depression scores at 36 weeks (MD 0.42, 95% CI –0.89 to 1.73; 164 women; Analysis 1.31.1), Spielberger State‐Trait Anxiety Inventory Short Form scores at 36 weeks (MD –0.94, 95% CI –4.09 to 2.21; 164 women; Analysis 1.31.2), Short‐Form Health Survey scores Mental Component Score (MD 0.31, 95% CI –2.54 to 3.16; 164 women; Analysis 1.31.3) or Physical Component Score (MD 0.87, 95% CI –1.94 to 3.68; 164 women; Analysis 1.31.4).
1.31. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 31: Sense of wellbeing and quality of life
Views of the intervention
One study reported views of the intervention (Lindsay 2014). Specifically, Lindsay 2014 reported if participants thought the "intervention was an inconvenience" (probiotics: 11/56 women, placebo: 12/63 women), "capsules were difficult to swallow" (probiotics: 1/56 women, placebo: 5/64 women), and if they "would consider taking a probiotic in a future pregnancy" (probiotics: 55/55 women, placebo: 60/64 women). Overall, Lindsay 2014 reported no differences in views of the intervention between groups.
Breastfeeding (e.g. at discharge, six weeks postpartum)
Two studies reported women who were breastfeeding any amount at six months (Laitinen 2009; Wickens 2017). It is uncertain if probiotics have any effect on numbers of women breastfeeding at six months compared to placebo (non‐event RR 1.08, 95% CI 0.77 to 1.50; 552 women; I2 = 0%; Analysis 1.32).
1.32. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 32: Breastfeeding at 6 months
Long‐term maternal
Postnatal depression
No studies reported postnatal depression.
Postnatal weight retention or return to prepregnancy weight
One study reported weight at four to seven days postpartum (Wickens 2017). Probiotics may make little to no difference in postpartum weight compared with placebo (MD –0.10 kg, 95% CI –0.91 to 0.71; 391 women; Analysis 1.33). Laitinen 2009 also reported that weight decreased similarly between groups but provided no data.
1.33. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 33: Postnatal weight retention (kg)
Body mass index
One study reported body mass index at four to seven days postpartum (Wickens 2017), one at one‐year postpartum (Laitinen 2009), and one at four years postpartum (Laitinen 2009). At all time points, there was no clear difference between groups (4 to 7 days: MD –0.10 kg/m², 95% CI –0.38 to 0.18; 391 women; 12 months: MD –0.10 kg/m², 95% CI –0.65 to 0.45; 128 women; 4 years: MD 0.70 kg/m², 95% CI –0.18 to 1.58; 80 women; Analysis 1.34).
1.34. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 34: Body mass index (kg/m2)
Gestational diabetes mellitus in a subsequent pregnancy
No studies reported GDM in a subsequent pregnancy.
Type 1 diabetes
No studies reported type 1 diabetes.
Type 2 diabetes
No studies reported type 2 diabetes.
Impaired glucose tolerance
No studies reported glucose tolerance.
Cardiovascular health as defined by trialists (including blood pressure, hypertension, cardiovascular disease and metabolic syndrome)
No studies reported cardiovascular health.
Infant
Stillbirth
Five studies reported stillbirth (Callaway 2019; Laitinen 2009; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019). Of the 1128 participants, there were six stillbirths. Therefore, we do not know if probiotics reduce stillbirth compared to placebo because there were so few events and the CIs were very wide (RR 0.59, 95% CI 0.14 to 2.46; 1128 women; I2 = 0%; Analysis 1.35).
1.35. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 35: Stillbirth
Neonatal mortality
Three studies reported neonatal mortality (Callaway 2019; Laitinen 2009; Lindsay 2014). However, there were no neonatal mortalities in any study, so the effect of probiotics could not be estimated (Analysis 1.36). Therefore, we do not know if probiotics reduce neonatal mortality compared to placebo.
1.36. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 36: Neonatal mortality
Gestational age at birth
Six studies reported gestational age at birth (Callaway 2019; Laitinen 2009; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019; Wickens 2017). One study reported gestational age at birth as median and IQR, so this study was not included in the meta‐analysis (Wickens 2017). However, Wickens 2017 found no difference between groups (probiotics: 39.7 weeks, IQR 38.7 to 40.7; placebo: 39.6 weeks, IQR 38.7 to 40.4). We included the other five studies in the meta‐analysis (Callaway 2019; Laitinen 2009; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019). Probiotics may make little to no difference in gestational age at birth compared to placebo (MD 0.01 weeks, 95% CI –0.19 to 0.21; 5 studies, 1073 infants; I2 = 26%; Analysis 1.37).
1.37. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 37: Gestational age at birth (weeks)
Preterm birth (less than 37 weeks' gestation and less than 32 weeks' gestation)
Six studies reported preterm birth (Callaway 2019; Laitinen 2009; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019; Wickens 2017). All studies defined preterm birth as prior to 37 weeks' gestation. It is uncertain if probiotics have an effect on the risk of preterm birth compared to placebo (RR 1.32, 95% CI 0.86 to 2.01; 1484 participants; I2 = 0%; Analysis 1.38).
1.38. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 38: Preterm birth
Apgar score (less than seven at five minutes)
Three studies reported Apgar score (Laitinen 2009; Pellonpera 2019; Wickens 2017). However, all studies reported this outcome differently, so the data were not conducive to a meta‐analysis. Laitinen 2009 reported the median and range of five‐minute Apgar scores, which showed no difference between groups (probiotics: median 9, range 6 to 10; placebo: median 9, range 3 to 10). Pellonpera 2019 reported the mean and SD of five‐minute Apgar scores, which showed no difference between groups (probiotics: mean 9.0, SD 0.7; placebo: 9.0, SD 0.8). Finally, Wickens 2017 reported the proportion of infants who had an Apgar score of seven or greater at five minutes and found no difference between groups (probiotics: 200/203; placebo: 198/202). Overall, probiotics may make little to no difference in five‐minute Apgar scores compared to placebo.
Macrosomia
Three studies reported macrosomia defined as a birthweight greater than 4000 g (Callaway 2019; Lindsay 2014; Wickens 2017). Probiotics may make little to no difference in the risk of macrosomia compared to placebo (RR 1.13, 95% CI 0.86 to 1.48; 952 infants; I2 = 20%; Analysis 1.39).
1.39. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 39: Macrosomia
Small‐for‐gestational age
Three studies reported SGA (Callaway 2019; Okesene‐Gafa 2019; Pellonpera 2019). One study defined SGA as less than 10th percentile using customised percentile charts (Okesene‐Gafa 2019), while the other two studies also used less than 10th percentile but did not state which charts they used (Callaway 2019; Pellonpera 2019). Probiotics may reduce the incidence of SGA infants compared to placebo (RR 0.51, 95% CI 0.30 to 0.85; 814 infants; Analysis 1.40).
1.40. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 40: Small‐for‐gestational age
Birthweight and z‐score
Six studies reported birthweight and z‐score (Callaway 2019; Laitinen 2009; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019; Wickens 2017). We used a random‐effects model due to substantial heterogeneity (I² = 42%). It is uncertain if probiotics have an effect on birthweight compared to placebo (MD 26.87 g, 95% CI –49.52 to 103.26; 1524 infants; Analysis 1.41). Two studies also reported birthweight z‐scores (Okesene‐Gafa 2019; Pellonpera 2019), and one study reported birthweight percentiles (Lindsay 2014). Two of these studies reported no difference between groups (Lindsay 2014; Pellonpera 2019), while one study reported a slight increase in birthweight z‐score in the probiotics group compared to placebo (Okesene‐Gafa 2019). We do not know if probiotics affect birthweight z‐score compared to placebo.
1.41. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 41: Birthweight (g)
Head circumference and z‐score
Three studies reported head circumference and z‐score (Laitinen 2009; Okesene‐Gafa 2019; Wickens 2017). Probiotics may make little to no difference in head circumference compared to placebo (MD –0.04 cm, 95% CI –0.27 to 0.18; 789 infants; I2 = 21%; Analysis 1.42). One study reported head circumference z‐scores and also found no difference between groups (Okesene‐Gafa 2019).
1.42. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 42: Head circumference (cm)
Length and z‐score
Three studies reported length and z‐score (Laitinen 2009; Okesene‐Gafa 2019; Wickens 2017). We used a random‐effects model due to substantial heterogeneity (I² = 59%). Probiotics may make little to no difference in length compared to placebo (MD 0.02 cm, 95% CI –0.54 to 0.59; 786 infants; Analysis 1.43). One study reported length z‐scores and also found no difference between groups (Okesene‐Gafa 2019).
1.43. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 43: Length (cm)
Ponderal index
Two studies reported ponderal index (Lindsay 2014; Wickens 2017). Probiotics may make little to no difference in ponderal index compared to placebo (MD 0.25 kg/m3, 95% CI –0.21 to 0.70; 539 infants; I2 = 0%; Analysis 1.44).
1.44. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 44: Ponderal index (kg/m3)
Adiposity
Two studies reported adiposity (Callaway 2019; Okesene‐Gafa 2019). Okesene‐Gafa 2019 reported adiposity as fat mass (MD –0.04 kg, 95% CI –0.12 to 0.04; 110 infants; Analysis 1.45), and Callaway 2019 reported adiposity as percentage fat (MD –0.10%, 95% CI –1.19 to 0.99; 210 infants; Analysis 1.46), which both showed no difference between probiotics and placebo.
1.45. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 45: Adiposity – fat mass (kg)
1.46. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 46: Adiposity – % fat
Shoulder dystocia
No studies reported shoulder dystocia.
Bone fracture
No studies reported bone fracture.
Nerve palsy
No studies reported nerve palsy.
Respiratory distress syndrome
No studies reported respiratory distress syndrome.
Hypoglycaemia as defined by trialists
Two studies reported hypoglycaemia (Callaway 2019; Pellonpera 2019). One study defined hypoglycaemia as a fasting blood sugar level of less than 2.2 mmol/L (Callaway 2019), and one study defined hypoglycaemia as a fasting blood sugar level of less than 2.4 mmol/L (Pellonpera 2019). We used a random‐effects model due to substantial heterogeneity (I² = 37%). Given the heterogeneity and the wide CIs, we do not know if probiotics reduce the risk of hypoglycaemia compared to placebo (mean RR 1.15, 95% CI 0.69 to 1.92; 586 infants; Analysis 1.47).
1.47. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 47: Hypoglycaemia
Hyperbilirubinaemia
Two studies reported hyperbilirubinaemia, both as hyperbilirubinaemia requiring phototherapy (Callaway 2019; Pellonpera 2019). It is uncertain if probiotics have any effect on the risk of hyperbilirubinaemia compared to placebo (RR 0.95, 95% CI 0.66 to 1.38; 593 infants; I2 = 10%; Analysis 1.48).
1.48. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 48: Hyperbilirubinaemia
Later infant and childhood
Weight and z‐scores
One study reported weight gain in grams per month from 0 to 6, 6 to 12 and 12 to 24 months (Laitinen 2009). Another study reported that weights and z‐scores were similar between groups at five months (Okesene‐Gafa 2019). Probiotics may make little to no difference in weight compared to placebo (0 to 6 months: MD –3 g/month, 95% CI –53.07 to 47.07; 6 to 12 months: MD 27 g/month, 95% CI –0.76 to 54.76; 12 to 24 months: MD –19 g/month, 95% CI –42.62 to 4.62; Analysis 1.49).
1.49. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 49: Infant weight gain (g/month)
Height and z‐scores
One study reported growth in centimetres per month from 0 to 6, 6 to 12 and 12 to 24 months (Laitinen 2009). Another study reported that heights and z‐scores were similar between groups at five months (Okesene‐Gafa 2019). Probiotics may make little to no difference in height compared to placebo (0 to 6 months: MD –0.05 cm/month, 95% CI –0.15 to 0.05; 6 to 12 months: MD 0.02 cm/month, 95% CI –0.04 to 0.08; 12 to 24 months: MD 0.01 cm/month, 95% CI –0.04 to 0.06; Analysis 1.50).
1.50. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 50: Infant height (cm/month)
Head circumference and z‐scores
One study reported head circumference with 119 participants at six months of age (Laitinen 2009). Another study reported head circumferences and z‐scores were similar between groups at five months (Okesene‐Gafa 2019). Probiotics may make little to no difference in head circumference compared to placebo (MD 0.30 cm, 95% CI –0.26 to 0.86; Analysis 1.51).
1.51. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 51: Infant head circumference – 6 months (cm)
Adiposity (including body mass index and skinfold thickness)
One study reported that infant body mass indexes and skinfold thicknesses were similar between groups at five months (Okesene‐Gafa 2019). There were no data for meta‐analysis. Probiotics may have no effect on long‐term adiposity in children compared with placebo.
Blood pressure
One study reported mean blood pressures in infants aged six months (Laitinen 2009). Probiotics may make little to no difference in mean blood pressure compared to placebo (MD –1.00 mmHg, 95% CI –4.19 to 2.19; 114 participants; Analysis 1.52).
1.52. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 52: Infant mean blood pressure – 6 months (mmHg)
Type 1 diabetes
No studies reported type 1 diabetes.
Type 2 diabetes
No studies reported type 2 diabetes.
Impaired glucose tolerance
One study reported impaired glucose tolerance (Laitinen 2009). This study used 32–33 split proinsulin as a measure of glucose tolerance at six months, and levels were defined as abnormal if they were above the 85th percentile. We do not know if probiotics affect impair glucose tolerance in children (RR 0.95, 95% CI 0.34 to 2.69; Analysis 1.53).
1.53. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 53: 32–33 split proinsulin > 85th percentile – 6 months
Dyslipidaemia or metabolic syndrome
One study reported dyslipidaemia or metabolic syndrome (Laitinen 2009). Laitinen 2009 stated there were no differences in lipid levels with probiotics in children at one, two and four years of age compared to placebo. There were no data for meta‐analysis.
Neurodisability
No studies reported neurodisability.
Educational achievement
No studies reported educational achievement.
Child as an adult
Weight
No studies reported weight.
Height
No studies reported height.
Adiposity (including body mass index and skinfold thickness)
No studies reported adiposity.
Cardiovascular health as defined by trialists (including blood pressure, hypertension, cardiovascular disease and metabolic syndrome)
No studies reported cardiovascular health.
Type 1 diabetes
No studies reported type 1 diabetes.
Type 2 diabetes
No studies reported type 2 diabetes.
Impaired glucose tolerance
No studies reported impaired glucose tolerance.
Dyslipidaemia or metabolic syndrome
No studies reported dyslipidaemia or metabolic syndrome.
Employment, education and social status/achievement
No studies reported employment, education and social status/achievement.
Health service use
Number of hospital or health professional visits (including midwife, obstetrician, physician, dietician and diabetic nurse)
No studies reported health service use outcomes.
Number of antenatal visits or admissions
No studies reported number of antenatal visits or admissions.
Length of antenatal stay
No studies reported length of antenatal stay.
Neonatal intensive care unit admission
Five studies reported neonatal intensive care unit admission (Callaway 2019; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019; Wickens 2017). All studies reported neonatal intensive care unit admission alone except for Callaway 2019, which reported special care unit admission. Probiotics may make little to no difference in neonatal intensive care unit admissions compared to placebo (RR 0.97, 95% CI 0.75 to 1.26; 1354 infants; I2 = 0%; Analysis 1.54).
1.54. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 54: Neonatal intensive care unit admission
Length of postnatal stay (mother)
No studies reported length of postnatal stay (mother).
Length of postnatal stay (baby)
No studies reported length of postnatal stay (baby).
Costs to families associated with the management provided
No studies reported costs to families associated with the management provided.
Costs associated with the intervention
No studies reported costs associated with the intervention.
Cost of maternal care
No studies reported cost of maternal care.
Cost of offspring care
No studies reported cost of offspring care.
Probiotics versus diet
We found no trials comparing probiotics versus diet.
Discussion
Summary of main results
The aim of this review was to determine the effect of probiotic supplementation during pregnancy on the risk of developing gestational diabetes. Seven trials met our inclusion criteria, and all included studies compared probiotics with placebo.
Six included studies in this review reported the incidence of GDM in 1440 participants. It is uncertain if probiotics have any effect on the risk of GDM compared to placebo because there was substantial heterogeneity between studies and wide CIs that included both appreciable benefit and harm (low‐certainty evidence). Two of these studies reported a reduction in the risk of GDM with probiotics, while the other four studies reported no difference. This heterogeneity was explored through subgroup analysis, and identified no clear causes for the heterogeneity.
Among the other primary outcomes for this review, we found probiotics increase the risk of pre‐eclampsia (high‐certainty evidence) and may increase the risk of hypertensive disorders of pregnancy, although the CIs for hypertensive disorders of pregnancy included the possibility of no effect.
There were few differences between groups for this review's other main outcomes. Probiotics make little to no difference in the risk of caesarean section (high‐certainty evidence), and probably make little to no difference in maternal weight gain during pregnancy (moderate‐certainty evidence). Probiotics probably make little to no difference in the incidence of large‐for‐gestational age infants (moderate‐certainty evidence) and may make little to no difference in neonatal adiposity (low‐certainty evidence, data from two studies not pooled). We do not know the effect of probiotics on perinatal mortality (low‐certainty evidence), a composite measure of neonatal morbidity (low‐certainty evidence) or neonatal hypoglycaemia (low‐certainty evidence) because of serious imprecision in the effect estimates and CIs, which are consistent with possible benefit and possible harm. No included studies reported on perineal trauma, postnatal depression, maternal and infant development of diabetes, and neurosensory disability.
There were few differences between groups for most of the secondary outcomes reported in this review. Among the markers for glucose tolerance, there was no difference for fasting plasma glucose and OGTT results. While there was a reduction in insulin levels with probiotics, this finding was of minimal importance given there were no differences in glucose levels or the diagnosis of GDM. The only other notable finding was that probiotics may reduce the risk of SGA infants compared to placebo. However, SGA was not one of our selected outcomes for inclusion in the 'Summary of findings' tables, therefore, we have not assessed the certainty of the evidence contributing to this outcome. The SGA data were based on relatively few events and it is not certain if the effect estimate is due to chance or a real difference between the intervention and control groups.
Overall completeness and applicability of evidence
This review included seven studies with 1647 participants. Given the incidence of GDM in the study population, interpretation of the results was limited by wide CIs and substantial heterogeneity between studies. We identified eight ongoing or unpublished studies as part of our search, which will add to the body of evidence when published and will hopefully help to overcome some of the present limitations.
The studies that reported the incidence of GDM were conducted in women at high risk of developing GDM due to being overweight or obese, or women with a history of atopic disease of any body mass index. Although this was not formally explored through subgroup analysis, the difference in study populations may explain some of the heterogeneity observed between studies. In particular, the authors would like to note that the two studies conducted in women of any weight with a history of atopic disease were the only two studies to detect a reduction in the risk of GDM with probiotics. Therefore, women with atopic disease may be a population where probiotics are effective. Another possibility is that probiotics are more effective in normal‐weight women compared to the higher‐risk overweight and obese women. Genetic differences or differences in diet between populations may also be responsible, although both Finland and New Zealand have each had one trial that showed benefit and one trial that showed no effect. More data are required before any conclusions can be made, but we plan to explore the effect of probiotics in these populations through subgroup analyses in updates of this review.
While multiple studies reported all our primary outcomes, many of our secondary outcomes were not reported or were reported by only one study. In particular, data were lacking in most of the long‐term outcomes since only one included study had significant long‐term follow‐up. There were also almost no data on health service use apart from neonatal intensive care unit admission. More studies will need to include long‐term follow‐up and data on health service use before any conclusions can be drawn about these outcomes.
Quality of the evidence
Overall, risk of bias among the included studies was low across all domains, apart from one study (Jamilian 2016), which had an unclear risk of bias in relation to allocation concealment. While some studies lacked blinding for secondary interventions, all studies were double blind for the probiotics versus placebo comparison.
We assessed certainty of evidence using GRADE methodology, and this assessment is given in Table 1 and Table 2. Overall, the certainty of evidence ranged from low to high, with downgrading due to concerns around inconsistency and imprecision. Certainty varied widely across the assessed outcomes, with evidence of high certainty for caesarean section, pre‐eclampsia; moderate certainty for weight gain during pregnancy, large‐for‐gestational age and composite neonatal morbidity; and low certainty for GDM, perinatal mortality, neonatal hypoglycaemia and adiposity.
Potential biases in the review process
There are possible sources of bias with any review, and we minimised these sources of bias. We performed the search for studies in this area using the Cochrane Pregnancy and Childbirth Group's Trials Register, which is updated weekly to monthly with information from CENTRAL, MEDLINE, Embase, handsearches from 30 journals and conference proceeding of major conferences, and alerts for a further 44 journals to minimise the risk of missing eligible studies. Two review authors independently assessed studies for inclusion and risk of bias, and resolved any discrepancies through discussion or with the entire author team if necessary.
This review was also uniquely susceptible to bias given three of the review authors (MDN, LC and HB) were also authors on a study that was identified for possible inclusion. For this Cochrane Review, the two review authors who were not involved with the study (SJD and SAP) independently assessed the relevant trial reports for inclusion, determined risk of bias and collected all data to minimise any conflict of interest.
Agreements and disagreements with other studies or reviews
Many reviews have evaluated the impact of probiotics on GDM, either as part of a specific review or a general review on prevention strategies for GDM or the use of probiotics during pregnancy. Since many of the studies included in this review were only published in the past few years, many older reviews on the topic include only one or two studies (Agha‐Jaffar 2016; Barrett 2012; Facchinetti 2014; Gomez Arango 2015; Griffin 2015; Jarde 2018; Lindsay 2013; Plows 2019; Rogozinska 2015; Simmons 2015; van de Vusse 2013). Depending on inclusion criteria and publication date, some combinations of Laitinen 2009, Lindsay 2014, and Wickens 2017, and one study we excluded (Asemi 2013) were included in each review. Overall, these reviews concluded that probiotics may reduce the risk of GDM, but agreed that more data are needed to make firm conclusions.
Two recent systematic reviews have been published that include the newer trials. Masulli 2020 published a systematic review and meta‐analysis including 17 trials evaluating the use of probiotics during pregnancy on various metabolic outcomes in both women with and without GDM. For the diagnosis of GDM outcome, this review included seven studies: the six included in this review (Callaway 2019; Laitinen 2009; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019; Wickens 2017) and one awaiting classification (Asgharian 2020). In agreement with our review, Masulli 2020 reported no benefit with probiotics on the incidence of GDM, although they also demonstrated substantial heterogeneity between studies (RR 0.77, 95% CI 0.51 to 1.16; I2 = 62%).
The other recently published systematic review on the topic was a network meta‐analysis evaluating a variety of interventions to prevent GDM specifically in overweight and obese pregnant women (Chatzakis 2019). In total, the review included 23 studies, five of which evaluated the use of probiotics. Four of these studies were included in our review (Callaway 2019; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019), while the other study is awaiting classification (Asgharian 2020). Overall, Chatzakis 2019 found no interventions to be superior to placebo. Their direct comparison of probiotics and placebo revealed no clear differences between groups in agreement with our review (RR 1.02, 95% CI 0.78 to 1.32). However, there was no substantial heterogeneity between the studies as seen in our review, which is likely reflective of the fact that Chatzakis 2019 limited their review to overweight and obese women. The effect of body mass index was not evaluated through subgroup analysis in our review, but further supports our observation that probiotics may not be effective for preventing GDM in overweight and obese women.
Authors' conclusions
Implications for practice.
Probiotics may increase, decrease or make little to no difference in the risk of gestational diabetes mellitus (GDM), although the current evidence is of low certainty due to concerns regarding imprecision and inconsistency. While analysis revealed a small reduction in insulin levels with probiotics, this is unlikely to be clinically meaningful. Given the substantial heterogeneity observed between studies in the risk of GDM, there may be certain populations in which probiotics are effective, but there is currently insufficient evidence to identify these populations.
High‐certainty evidence suggests that probiotics probably increase the risk of pre‐eclampsia and could increase hypertensive disorders of pregnancy but the 95% confidence intervals for hypertensive disorders of pregnancy includes the possibility of no effect. While further research is needed to explore the underlying potential physiology of this relationship, given the potential risk of harm and little observed benefit, we urge caution in using probiotics during pregnancy at this time.
Implications for research.
This review identified high‐certainty evidence that probiotics increase the risk of pre‐eclampsia, so great care needs to be taken in any future study of probiotics in pregnancy. Safety needs to be carefully monitored, and women in these studies need to be made aware of this outcome when informed consent is obtained. In the eight studies that are currently ongoing, particular care should be taken in participant follow‐up and analysis of the effect of probiotics on pre‐eclampsia. Further research is needed to elucidate the underlying potential physiology of the relationship between probiotics and pre‐eclampsia. The effect of probiotics on risk of small‐for‐gestational age infants should be explored further.
More data are required to fully determine the effect of probiotics on the risk for GDM. While future studies should be conducted with caution, the eight ongoing studies will hopefully explore sources of the substantial heterogeneity in the current data. In the studies where women were recruited for a personal or family history of atopic disease (Laitinen 2009; Wickens 2017), there appears to be a differential impact on GDM diagnosis compared to studies where women were recruited specifically for being at high risk for GDM (Callaway 2019; Lindsay 2014; Okesene‐Gafa 2019; Pellonpera 2019). If probiotics are deemed to be safe to use in pregnancy, this difference should be explored further.
What's new
| Date | Event | Description |
|---|---|---|
| 20 March 2020 | New citation required and conclusions have changed | In the previous version of the review, only 1 study was included that showed a decrease in the risk of gestational diabetes mellitus with probiotics. For this updated review, we identified 6 new studies that were eligible for inclusion that brought the total participant count from 256 to 1647. The meta‐analysis performed on this much larger body of evidence revealed no clear difference in the risk of gestational diabetes with probiotics compared to placebo. |
| 20 March 2020 | New search has been performed | Search updated and 6 new studies found to be eligible for inclusion. |
History
Protocol first published: Issue 7, 2012 Review first published: Issue 2, 2014
Acknowledgements
As part of the prepublication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser. The authors are grateful to the following peer reviewers for their time and comments: Dr Mohammad Othman, Assistant Professor of Obstetrics and Gynaecology, Faculty of Medicine, Albaha University, Saudi Arabia; Dr Amita Ray, Professor and Head of Department, Department of Obstetrics and Gynaecology, IQ City Medical College, Durgapur, India.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
We thank Louise Conwell for her contribution to previous versions of this review.
Appendices
Appendix 1. Search methods for ICTRP and ClinicalTrials.gov
ICTRP
(each line was run separately)
probiotics AND pregnancy
probiotics AND pregnant
ClinicalTrials.gov
Advanced search
Interventional Studies | Pregnancy | probiotics
Data and analyses
Comparison 1. Probiotics versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Gestational diabetes mellitus | 6 | 1440 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.54, 1.20] |
| 1.2 Gestational diabetes mellitus (by dose) | 6 | 1440 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.54, 1.20] |
| 1.2.1 < 5 billion CFU | 2 | 547 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.94, 2.30] |
| 1.2.2 > 5 billion CFU | 4 | 893 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.46, 0.98] |
| 1.3 Gestational diabetes mellitus (by bacterial species) | 6 | 1440 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.54, 1.20] |
| 1.3.1 Lactobacillusrhamnosus + Bifidobacteriumanimalis | 4 | 931 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.50, 1.37] |
| 1.3.2 Lactobacillus rhamnosus | 1 | 373 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.32, 1.08] |
| 1.3.3 Lactobacillus salivarius | 1 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.25, 5.70] |
| 1.4 Gestational diabetes mellitus (by duration of treatment) | 6 | 1440 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.54, 1.20] |
| 1.4.1 Started early pregnancy | 5 | 1304 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.51, 1.20] |
| 1.4.2 Started ≥ 20 weeks' gestation | 1 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.25, 5.70] |
| 1.5 Hypertensive disorders of pregnancy | 4 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.96, 2.01] |
| 1.6 Hypertensive disorders of pregnancy (by dose) | 4 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.96, 2.01] |
| 1.6.1 < 5 billion CFU | 2 | 545 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.87, 2.12] |
| 1.6.2 > 5 billion CFU | 2 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.77, 2.81] |
| 1.7 Hypertensive disorders of pregnancy (by bacterial species) | 4 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.96, 2.01] |
| 1.7.1 Lactobacillusrhamnosus + Bifidobacteriumanimalis | 3 | 819 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.92, 1.98] |
| 1.7.2 Lactobacillus salivarius | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.49, 7.99] |
| 1.8 Hypertensive disorders of pregnancy (by duration of treatment) | 4 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.96, 2.01] |
| 1.8.1 Started early pregnancy | 3 | 819 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.92, 1.98] |
| 1.8.2 Started ≥ 20 weeks' gestation | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.49, 7.99] |
| 1.9 Pre‐eclampsia | 4 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.04, 3.29] |
| 1.10 Caesarean section | 6 | 1520 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.86, 1.17] |
| 1.11 Caesarean section (by dose) | 6 | 1520 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.86, 1.17] |
| 1.11.1 < 5 billion CFU | 2 | 547 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.73, 1.14] |
| 1.11.2 > 5 billion CFU | 4 | 973 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.87, 1.36] |
| 1.12 Caesarean section (by bacterial species) | 6 | 1520 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.86, 1.17] |
| 1.12.1 Lactobacillusrhamnosus + Bifidobacteriumanimalis | 4 | 977 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.81, 1.19] |
| 1.12.2 Lactobacillus rhamnosus | 1 | 407 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.79, 1.51] |
| 1.12.3 Lactobacillus salivarius | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.59, 1.55] |
| 1.13 Caesarean section (by duration of treatment) | 6 | 1520 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.86, 1.17] |
| 1.13.1 Started early pregnancy | 5 | 1384 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.86, 1.19] |
| 1.13.2 Started ≥ 20 weeks' gestation | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.59, 1.55] |
| 1.14 Large‐for‐gestational age | 4 | 919 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.72, 1.36] |
| 1.15 Large‐for‐gestational age (by dose) | 4 | 919 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.72, 1.36] |
| 1.15.1 < 5 billion CFU | 2 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.72, 1.62] |
| 1.15.2 > 5 billion CFU | 2 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.53, 1.46] |
| 1.16 Large‐for‐gestational age (by bacterial species) | 4 | 919 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.72, 1.36] |
| 1.16.1 Lactobacillusrhamnosus + Bifidobacteriumanimalis | 3 | 783 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.71, 1.38] |
| 1.16.2 Lactobacillus salivarius | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.36, 2.89] |
| 1.17 Large‐for‐gestational age (by duration of treatment) | 4 | 919 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.72, 1.36] |
| 1.17.1 Started early pregnancy | 3 | 783 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.71, 1.38] |
| 1.17.2 Started ≥ 20 weeks' gestation | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.36, 2.89] |
| 1.18 Perinatal mortality (stillbirth and neonatal mortality) | 3 | 709 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.01, 8.02] |
| 1.19 Mortality or morbidity composite | 2 | 623 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.36, 1.35] |
| 1.20 Induction of labour | 2 | 544 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.85, 1.39] |
| 1.21 Postpartum haemorrhage | 2 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.60, 1.85] |
| 1.22 Weight gain during pregnancy (kg) | 4 | 853 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.67, 1.26] |
| 1.23 Fasting plasma glucose (mmol/L) | 7 | 1519 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.12, 0.05] |
| 1.24 1‐hour oral glucose tolerance test (OGTT) plasma glucose (mmol/L) | 4 | 1110 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.27, 0.13] |
| 1.25 2‐hour OGTT plasma glucose (mmol/L) | 4 | 1186 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.13, 0.18] |
| 1.26 Triglycerides (mmol/L) | 2 | 198 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.40, ‐0.02] |
| 1.27 High‐density lipoprotein (mmol/L) | 2 | 198 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.08, 0.11] |
| 1.28 Low‐density lipoprotein (mmol/L) | 2 | 198 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.48, 0.04] |
| 1.29 Total cholesterol (mmol/L) | 2 | 198 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.62, ‐0.00] |
| 1.30 Insulin (mU/L) | 4 | 538 | Mean Difference (IV, Fixed, 95% CI) | ‐1.95 [‐3.01, ‐0.88] |
| 1.31 Sense of wellbeing and quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.31.1 Edinburgh Postnatal Depression Score – 36 weeks | 1 | 164 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [‐0.89, 1.73] |
| 1.31.2 Spielberger State‐Trait Anxiety Inventory Short Form Score – 36 weeks | 1 | 164 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐4.09, 2.21] |
| 1.31.3 12‐Item Short‐Form Health Survey – Mental Component Score, 36 weeks | 1 | 164 | Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐2.54, 3.16] |
| 1.31.4 12‐Item Short‐Form Health Survey – Physical Component Score, 36 weeks | 1 | 164 | Mean Difference (IV, Fixed, 95% CI) | 0.87 [‐1.94, 3.68] |
| 1.32 Breastfeeding at 6 months | 2 | 552 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.77, 1.50] |
| 1.33 Postnatal weight retention (kg) | 1 | 391 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.91, 0.71] |
| 1.34 Body mass index (kg/m2) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.34.1 4–7 days postpartum | 1 | 391 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.38, 0.18] |
| 1.34.2 12 months postpartum | 1 | 128 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.65, 0.45] |
| 1.34.3 4 years postpartum | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.18, 1.58] |
| 1.35 Stillbirth | 5 | 1128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.14, 2.46] |
| 1.36 Neonatal mortality | 3 | 709 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.37 Gestational age at birth (weeks) | 5 | 1073 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.19, 0.21] |
| 1.38 Preterm birth | 6 | 1484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.86, 2.01] |
| 1.39 Macrosomia | 3 | 952 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.86, 1.48] |
| 1.40 Small‐for‐gestational age | 3 | 814 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.30, 0.85] |
| 1.41 Birthweight (g) | 6 | 1524 | Mean Difference (IV, Random, 95% CI) | 26.87 [‐49.52, 103.26] |
| 1.42 Head circumference (cm) | 3 | 789 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.27, 0.18] |
| 1.43 Length (cm) | 3 | 786 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.54, 0.59] |
| 1.44 Ponderal index (kg/m3) | 2 | 539 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.21, 0.70] |
| 1.45 Adiposity – fat mass (kg) | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.12, 0.04] |
| 1.46 Adiposity – % fat | 1 | 210 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.19, 0.99] |
| 1.47 Hypoglycaemia | 2 | 586 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.69, 1.92] |
| 1.48 Hyperbilirubinaemia | 2 | 593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.66, 1.38] |
| 1.49 Infant weight gain (g/month) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.49.1 at 0–6 months | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | ‐3.00 [‐53.07, 47.07] |
| 1.49.2 at 6–12 months | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | 27.00 [‐0.76, 54.76] |
| 1.49.3 at 12–24 months | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐19.00 [‐42.62, 4.62] |
| 1.50 Infant height (cm/month) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.50.1 at 0–6 months | 1 | 156 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.15, 0.05] |
| 1.50.2 at 6–12 months | 1 | 156 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.04, 0.08] |
| 1.50.3 at 12–24 months | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.04, 0.06] |
| 1.51 Infant head circumference – 6 months (cm) | 1 | 119 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.26, 0.86] |
| 1.52 Infant mean blood pressure – 6 months (mmHg) | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐4.19, 2.19] |
| 1.53 32–33 split proinsulin > 85th percentile – 6 months | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.34, 2.69] |
| 1.54 Neonatal intensive care unit admission | 5 | 1354 | Risk Ratio (IV, Fixed, 95% CI) | 0.97 [0.75, 1.26] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Callaway 2019.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled trial Blinding: double blind Location: Royal Brisbane & Women's Hospital, Redcliffe Hospital, and Mater Mothers Hospital in Brisbane, Australia |
|
| Participants | Inclusion criteria: < 16 weeks' gestation (changed to < 20 weeks' gestation during the study), singleton pregnancy, BMI > 25 kg/m2, > 18 years of age, able to read and understand English, and ability to provide informed consent Exclusion criteria: > 16 weeks' gestation (changed to > 20 weeks' gestation during the study), multiple pregnancy, known pre‐existing diabetes, impaired fasting glucose or impaired glucose tolerance, GDM prior to recruitment, taking medications likely to influence glucose metabolism, medical conditions associated with altered glucose metabolism, known major fetal abnormality noted on 12‐week ultrasound examination and known ingestion of probiotics |
|
| Interventions | Probiotic (n = 219): capsule containing Lactobacillus rhamnosus GG and Bifidobacterium lactis BB12 at 109 CFU taken daily from enrolment until birth Placebo (n = 214): capsule containing microcrystalline cellulose and dextrose anhydrate taken daily from enrolment until birth |
|
| Outcomes | Primary: diagnosis of GDM Secondary: gestational weight gain, pre‐eclampsia, induction of labour, caesarean delivery, change in prevalence of L rhamnosus and B lactis in gut microbiome, change in lipids and inflammatory profile, change in dietary indices and physical activity levels between baseline and 28 weeks; gestation, neonatal body composition, preterm delivery, shoulder dystocia, hypoglycaemia, neonatal treatment with supplementary fluids/feeds, nerve palsy, admission to NICU, jaundice requiring phototherapy, bone fracture, perinatal death, visit attendance, adherence to probiotic/placebo regimen, birthweight and congenital anomaly |
|
| Notes | Sources of funding: National Health and Medical Research Council grant APP1028575, Royal Brisbane and Women’s Hospital Foundation Study dates: commenced November 2012 Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation conducted using computer‐generated random number codes stratified by centre and BMI category. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes used for randomisation, and all medical staff, research assistants, nursing staff and participants were blinded to the randomised allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All medical staff, research assistants, nursing staff and participants were blinded to the randomised allocation. Placebo and probiotic supplements were identically packaged. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All study staff and participants were blinded to the randomised allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was minimal and similar in both groups (10/214 participants in the placebo group, 12/219 participants in the probiotic group). |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported. |
| Other bias | Low risk | No other sources of bias identified. |
Jamilian 2016.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled trial Blinding: double blind Location: Arak University of Medical Sciences, Arak, Iran |
|
| Participants | Inclusion criteria: first half of pregnancy (≤ 20 weeks' gestation), aged 18–37 years Exclusion criteria: recognised cause of recurrent miscarriages or a structural uterine abnormality distorting the cavity, history of rheumatoid arthritis, thyroid, parathyroid, or adrenal diseases, hepatic or renal failure |
|
| Interventions | Probiotic (n = 30): capsule containing Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum at 2 × 109 taken once per day for 12 weeks Placebo (n = 30): capsule containing starch taken once per day for 12 weeks |
|
| Outcomes | Primary: insulin levels after intervention Secondary: fasting blood sugar, glutathione, HDL‐cholesterol, hs‐CRP, LDL, malondialdehyde, nitric oxide, total antioxidant capacity, total cholesterol, triglycerides, and VLDL after the intervention period. |
|
| Notes | Sources of funding: grant from Vice‐Chancellor for Research, AUMS, Iran Study dates: March 2015 to July 2015 Declarations of interest: none declared This study was initially deemed high risk for scientific integrity/trustworthiness given no participants were lost to follow‐up over the course of the study and the numbers were equal in both groups with no mention of what type of randomisation method was used. Clarification from the study authors was sought on 11 May 2020 and 28 May 2020. The authors confirmed that all participants completed the study given their methods for ensuring patient follow‐up and high levels of adherence to specialist care in the study population. The authors also confirmed that randomisation was conducted using computer‐generated random numbers to select equal groups of 30 participants. We deemed the authors responses sufficient to change the study classification to low risk. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed using computer‐generated numbers. |
| Allocation concealment (selection bias) | Unclear risk | While the authors stated allocation was concealed, no details were provided as to how allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The group assignments were concealed from the researchers and participants until the final analyses were completed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Group assignments were not revealed to the researchers until after the final analyses were completed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost to follow‐up during the study. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the trial registry were reported. |
| Other bias | Low risk | No other sources of bias identified. |
Laitinen 2009.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial Blinding: double blind for probiotics/placebo, single blind for dietary intervention Location: Turku University Hospital, Turku, Finland |
|
| Participants | Inclusion criteria: < 17 weeks' gestation Exclusion criteria: metabolic or chronic diseases such as diabetes apart from atopic eczema, allergic rhinitis or asthma |
|
| Interventions | Probiotic + dietary intervention (n = 85): capsule containing Lactobacillus rhamnosus GG, ATCC 53 103 and Bifidobacterium lactis BB12 at dose of 1010 CFU taken daily from early pregnancy until the end of exclusive breastfeeding + intensive dietary counselling aiming to conform to currently recommended pregnancy diet Placebo + dietary intervention (n = 86): capsule containing microcrystalline cellulose and dextrose anhydrate taken daily from early pregnancy until the end of exclusive breastfeeding + intensive dietary counselling aiming to conform to currently recommended pregnancy diet Placebo + routine diet (n = 85): capsule containing microcrystalline cellulose and dextrose anhydrate taken daily from early pregnancy until the end of exclusive breastfeeding |
|
| Outcomes | Primary: maternal glucose metabolism as measured by plasma glucose, blood HbA1c, serum insulin and HOMA and QUICKI indices at baseline, third trimester of pregnancy, 1, 6 and 12 months postpartum. | |
| Notes | Sources of funding: Academy of Finland, Sigrid‐Juselius Foundation, Juho Vainio Foundation, Social Insurance Institution of Finland, Raisio, Chr. Hansen, Valio Ltd. Study dates: April 2002 to November 2005 Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation of 6 women. The use of only 1 block size could make it possible to guess the randomisation of the dietary intervention of the last participant of each block. However, since the probiotic/placebo randomisation was double blind and we only included the 2 groups who received the dietary intervention, the selection bias risk for probiotics vs placebo is still considered low. |
| Allocation concealment (selection bias) | Low risk | Randomisation list generated by a non‐investigator statistician, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo/probiotic allocation was blind to both participants and personnel, dietary therapy was not blinded to personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All personnel who handled or analysed blood samples were blind to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal loss to follow‐up by assessment of glucose tolerance. Total loss to follow‐up was 18.75% by 1‐year postpartum. |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes they intended to report. |
| Other bias | Low risk | No other biases detected. |
Lindsay 2014.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled trial Blinding: double blind for probiotics/placebo Location: National Maternity Hospital, Dublin, Ireland |
|
| Participants | Inclusion criteria: < 20 weeks' gestation, BMI 30–39.9 kg/m2 at first pregnancy visit, singleton pregnancy, and aged > 18 years Exclusion criteria: history of gestational or non‐gestational diabetes (type 1 or 2), presence of fetal anomaly, multiple pregnancy, and inability to give full informed consent |
|
| Interventions | Probiotic (n = 83): capsule containing 109 CFU of Lactobacillus salivarius UCC118 taken once daily from 24 to 28 weeks' gestation Placebo (n = 92): placebo capsule taken once daily from 24 to 28 weeks' gestation |
|
| Outcomes | Primary: change in maternal fasting glucose Secondary: incidence of gestational diabetes and impaired glucose tolerance, neonatal anthropometric measures, metabolic variables, gestational weight gain, pre‐eclampsia, delivery complications, cord blood metabolic variables, fetal growth at 34 weeks' gestation, 5‐minute Apgar score and NICU admission. |
|
| Notes | Sources of funding: National Maternity Hospital Medical Fund with support from an Ivo Drury Award, Alimentary Health Ltd Study dates: March 2012 to March 2013 Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation conducted by an independent researcher using a computer‐generated, simple randomisation process in a 1:1 ratio. |
| Allocation concealment (selection bias) | Low risk | Randomisation sequence was concealed in sequentially numbered, sealed, opaque envelopes that were not opened until after enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All capsules were identical in appearance, and all participants and researchers were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All researchers were blind to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the participants who ever received capsules, only 1 participant from each group stopped taking the capsules (1/64 in the probiotic group, 1/76 in the placebo group). No participants were lost to follow‐up, and intention‐to‐treat analyses were performed. |
| Selective reporting (reporting bias) | Low risk | Results for all prespecified outcomes were reported. |
| Other bias | Low risk | No other bias identified. |
Okesene‐Gafa 2019.
| Study characteristics | ||
| Methods | Study design: parallel 2×2 factorial randomised controlled trial Blinding: double blind for probiotic intervention, no blinding for dietary intervention Location: Counties Manukau Health region, South Auckland, New Zealand |
|
| Participants | Inclusion criteria: singleton pregnancy, BMI ≥ 30 kg/m2, 12–17.6 weeks' gestation and able to provide informed consent Exclusion criteria: pre‐existing diabetes or HbA1c ≥ 50 mmol/mol at booking in, taking probiotic supplements, known congenital abnormality, medications or medical conditions that alter glucose metabolism, multiple pregnancy, bariatric surgery and severe hyperemesis |
|
| Interventions | First randomisation: dietary intervention Dietary intervention (n = 116): multifaceted intervention including encounters with nutrition advisor, behaviour change techniques, physical activity advice and motivational texting Routine diet (n = 114): routine dietary advice including a pamphlet about diet, healthy weight gain and physical activity in pregnancy Second randomisation: probiotic intervention Probiotic (n = 115): capsule containing Lactobacillus rhamnosus GG and Bifidobacterium lactis BB12 at a dose of 7 × 109 CFU taken daily from enrolment to delivery Placebo (n = 115): capsule containing microcrystalline cellulose and dextrose anhydrate taken daily from enrolment to delivery |
|
| Outcomes | Primary: proportion of women with excessive gestational weight gain and infant birthweight Secondary: maternal pregnancy glucose metabolism, changes in diet quality and dietary patterns, functional health and well‐being, depression and anxiety scores, maternal adiposity postpartum, gestational diabetes, pregnancy‐induced hypertension, mode of birth, blood lipid concentrations, maternal feedback about study participation, neonatal anthropometry, gestational age at birth, LGA, small‐for‐gestational age, NICU admission, neonatal composite morbidity, breastfeeding, infant anthropometry, infant feeding, infant nutritional intake, attendance at study visits, adherence to probiotic/placebo regimen and cost effectiveness of the intervention |
|
| Notes | Sources of funding: Counties Manukau Health, Cure Kids Grant 2556, Lottery Health Research 353084, RANZCOG Two Mercia Barnes Trust, Gravida National Centre for Growth and Development, University of Auckland Faculty Development Research Fund and Reinvestment Fund, Nurture Foundation, Heart Foundation of New Zealand, Roche Diagnostics International Ltd, Chr. Hansen A/S Study dates: April 2015 to June 2017 Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation conducted using a web‐based protocol (randomize.net) using random block sizes, stratified by clinical site and BMI category. |
| Allocation concealment (selection bias) | Low risk | The research midwife responsible for enrolment did not have access to the probiotic/placebo allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The capsules were identically packaged and were labelled by a third party using a pre‐allocated random list. Only the project manager had access to the probiotic/placebo allocation, and all participants and other staff were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The project manager was the only study staff member with access to the probiotic/placebo allocation, and the protocol stated that the primary outcomes were not subject to bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were minimal losses to follow‐up, and losses were similar between groups (7/115 participants in the probiotics group, 6/115 participants in the placebo group). |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported. |
| Other bias | Low risk | No other sources of bias identified. |
Pellonpera 2019.
| Study characteristics | ||
| Methods | Study design: parallel 4‐arm randomised controlled trial of 2 interventions Blinding: double blind for both interventions Location: Turku University Hospital/University of Turku, Finland |
|
| Participants | Inclusion criteria: self‐reported prepregnancy BMI ≥ 25 kg/m2, < 18 weeks' gestation, and absence of chronic disease (except for asthma and allergies) Exclusion criteria: diabetes before pregnancy (including HbA1c ≥ 6.5% or fasting glucose ≥ 7.0 mmol/L at randomisation), multifetal pregnancy, chronic diseases impacting metabolic or gastrointestinal health, refusal to terminate the intake of other probiotic or fish oil supplements, diagnosis or history of coagulopathy, and use of anticoagulants |
|
| Interventions | Intervention 1: probiotics Probiotic: capsules containing Lactobacillus rhamnosus HN001 and Bifidobacterium animalis ssp lactis 420 at dose of 1010 CFU taken daily from enrolment until 6 months postpartum Placebo: capsules containing microcrystalline cellulose taken once daily from enrolment until 6 months postpartum Intervention 2: fish oil Fish oil: capsules containing 2.4 g n‐3 fatty acids (79% docosahexaenoic acid, 9.4% eicosapentaenoic acid) taken daily from enrolment until 6 months postpartum Placebo: capsules containing 2.4 g medium‐chain fatty acids (54.6% capric acid C8, 40.3% caprylic acid C10) taken daily from enrolment until 6 months postpartum Study arms: Arm 1: probiotics + fish oil (n = 109) Arm 2: probiotics + placebo (n = 110) Arm 3: placebo + fish oil (n = 109) Arm 4: placebo + placebo (n = 110) |
|
| Outcomes | Primary: prevalence of GDM and fasting glucose levels Secondary: change in insulin and HOMA values, need for medication in GDM management, gestational hypertensive disorders, mode of delivery, postpartum haemorrhage, birthweight and macrosomia |
|
| Notes | Sources of funding: Academy of Finland, state research funding for university‐level health research of the Turku University Hospital Expert Responsibility Area, the Diabetes Research Foundation, the Juho Vainio Foundation, Business Finland, the Finish Medical Foundation, the University of Turku, DuPont, and Croda Europe Ltd. Study dates: October 2013 to July 2017 Declarations of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed by a statistician not involved in the study in permutated blocks of 4 and stratified by parity and history of GDM. |
| Allocation concealment (selection bias) | Low risk | Participants were assigned from the randomisation list in order of recruitment, and the staff responsible for enrolment were blinded to the intervention. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All staff responsible for enrolment, study visits and outcome assessment and all participants were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All staff responsible for outcome assessment were blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were minimal losses to follow‐up, and losses were similar between groups (18/109 participants in the probiotics + fish oil group, 11/110 participants in the probiotics + placebo group, 13/109 participants in the placebo + fish oil group, and 19/110 participants in the placebo + placebo group for the primary outcome of GDM). |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | No other sources of bias identified. |
Wickens 2017.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial Blinding: double blind for probiotics/placebo Location: Wellington and Auckland, New Zealand |
|
| Participants | Inclusion criteria: 14–16 weeks' gestation, English speaking, intend to breastfeed, and personal history or child's biological father's history of asthma, eczema or allergic rhinitis treated by a doctor or pharmacist Exclusion criteria: aged < 16 years, did not intend to stay in either of the study centres for the 18 months following enrolment, serious immunological disorder that suppresses immune function or taking immunosuppressant drugs, known cardiac valve disease for which antibiotic prophylaxis was required when undergoing dental procedures, history of transplant or HIV, long‐term continuous antibiotic therapy, IVF pregnancy, pre‐enrolment scan showing major fetal abnormality, using or intend to use probiotic drinks or supplements themselves or in their child, participation in another RCT, severe allergy to cow's milk, previously participated in the study with an older child, deemed unsuitable for participation due to medical reason, or pre‐existing type 1 or 2 diabetes (only for OGTT and GDM outcomes) |
|
| Interventions | Probiotic (n = 212): capsules containing Lactobacillus rhamnosus HN001 at 6 × 109 CFU taken once daily from 16 weeks' gestation until 6 months after birth or until no longer breastfeeding Placebo (n = 211): capsules containing corn‐derived maltodextrin taken once daily until 6 months after birth or no longer breastfeeding |
|
| Outcomes | Primary: infant eczema and atopic sensitisation at age 12 months Secondary: GDM (OGTT 75 g using ADIPS criteria), bacterial vaginosis, group B strep colonisation, and maternal postpartum depression and anxiety. |
|
| Notes | Sources of funding: Health Research Council of New Zealand, Fonterra Study dates: December 2012 to November 2014 Declarations of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation stratified by study centre and performed in blocks of random lengths according to a computer‐generated random list. |
| Allocation concealment (selection bias) | Low risk | Research staff assigned women to consecutive study numbers, and the randomisation list was managed by an external group (Fonterra Co‐operative Group Ltd) who concealed the list from all study staff and participants. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The randomisation sequence was concealed from all study staff and participants. Placebo capsules had the same look and smell as the probiotic capsules, and both were provided in opaque bottles by an external company (Alaron Products Ltd). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Randomisation sequence concealed from all study staff. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up minimal and similar between groups (5/212 participants in the probiotic group and 4/211 participants in the placebo group at gestational diabetes testing). |
| Selective reporting (reporting bias) | Low risk | All predetermined gestational diabetes outcomes were reported. Subgroup analysis was conducted that was not prespecified, but this was clearly stated. |
| Other bias | Low risk | No other sources of bias identified. |
ADIPS: Australasian Diabetes in Pregnancy Society; BMI: body mass index; CFU: colony‐forming units; GDM: gestational diabetes mellitus; HbA1c: haemoglobin A1c; HDL: high‐density lipoprotein; HOMA: Homeostatic Model Assessment; hs‐CRP: high‐sensitivity C‐reactive protein; IUFD: intrauterine fetal demise; IVF: in vitro fertilisation; LDL: low‐density lipoprotein; LGA: large‐for‐gestational age; n: number of participants; NICU: neonatal intensive care unit; OGTT: oral glucose tolerance test; QUICKI: quantitative insulin sensitivity check index; RCT: randomised controlled trial; VLDL: very low‐density lipoprotein.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Asemi 2013 | Probiotics were started in the third trimester, after gestational diabetes would have been diagnosed. In addition, diagnosis of gestational diabetes was not a study outcome. |
| Taghizadeh 2014 | Women did not start the intervention until 27 weeks' gestation, which was too late to prevent gestational diabetes. In addition, diagnosis of gestational diabetes was not a study outcome. |
Characteristics of studies awaiting classification [ordered by study ID]
Asgharian 2020.
| Methods | Study design: parallel randomised controlled trial Blinding: double blind Location: 5 public health centres northwest of Tabriz, Iran |
| Participants | Inclusion criteria: 20–22 weeks' gestation, pre‐ or early‐pregnancy BMI ≥ 25 kg/m2, aged ≥ 18 years, fasting plasma glucose < 92 mg/dL Exclusion criteria: multiple pregnancy, history of GDM, taking any medication likely to influence glucose metabolism (metformin, corticosteroids, immunosuppressants, etc.), medical conditions associated with altered glucose metabolism (Cushing's syndrome, hepatic cirrhosis), regular consumption of probiotics for any reason, smoking, regular use of alcohol or illegal drugs, any antibiotic intake during current pregnancy, illiteracy/low literacy, established major fetal anomaly |
| Interventions | Probiotic (n = 65): yoghurt containing 5 × 108 CFU Lactobacillus acidophilus La5 and Bifidobacterium lactis BB12 with starter bacteria (Streptococcus thermophilus and Lactobacillus delbrueckii subspecies bulgaricus at 107 CFU/g) with 100 g taken daily from 24 weeks' gestation until delivery Placebo (n = 65): yoghurt containing only starter bacteria (Streptococcus thermophilus and Lactobacillus delbrueckii subspecies bulgaricus at 107 CFU/g) with 100 g taken daily from 24 weeks' gestation until delivery |
| Outcomes | Primary: fasting plasma glucose and 1‐and 2‐hour plasma glucose after 75 g OGTT. Secondary: GDM, weight gain over pregnancy, pre‐eclampsia, preterm delivery, delivery mode, satisfaction with the yoghurts, total serum bilirubin (3–5 days after birth), infant weight, infant length, infant head circumference, macrosomia, LGA, neonatal jaundice, jaundice treatments and neonatal death (within 30 days after birth). |
| Notes | Sources of funding: Research Vice‐Chancellor of Tabriz University of Medical Sciences Study dates: April 2016 to September 2017 Declarations of interest: none declared Only 1 participant in each group did not complete the study due to intrauterine fetal death. Since there were almost 0 losses to follow‐up, this study was considered high risk according to our scientific integrity/trustworthiness criteria. We contacted study authors for clarification on 11 May 2020, and the study authors provided information about how participants were followed up, but did not confirm that all other participants completed the study. We determined this was insufficient information to make a final risk assessment. Further information was requested on 20 May 2020 and 11 June 2020, but we received no response. |
Charles 2018.
| Methods | Study design: parallel, randomised controlled trial Blinding: double blind Location: Barts Health NHS Trust and Homerton University Hospital, London, UK |
| Participants | Inclusion criteria: aged ≥ 16 years, 9–14 weeks' gestation Exclusion criteria: lack of informed consent |
| Interventions | Probiotic (n unknown): capsule containing Lactobacillus rhamnosus GR‐1 and Lactobacillus reuteri RC‐1 at 2.5 × 109 CFU and excipients given daily from early pregnancy until delivery Placebo (n unknown): capsule containing excipients only given daily from early pregnancy until delivery |
| Outcomes | Primary: vaginal flora during pregnancy, recruitment of eligible women, and intervention adherence Secondary: reasons for participation and adherence, core outcomes from studies on preterm birth prevention |
| Notes | Sources of funding: Queen Mary University of London, UK Study dates: May 2016 to June 2017 Declarations of interest: not reported Study was only published in abstract form at the time of publication. We attempted to contact the study authors on 11 May 2020 and 28 May 2020 to confirm if the presented data came from the final analysis, but we did not receive a response. |
Si 2019.
| Methods | Study design: parallel, randomised controlled trial Blinding: double blind Location: The Second Hospital of Jilin University, Changchun, Jilin, China |
| Participants | Inclusion criteria: first antenatal visit before 12 weeks' gestation, singleton pregnancy, meeting diagnostic criteria for GDM, and fasting blood glucose > 5.1 mmol/L, 2‐hour OGTT result > 8.5 mmol/L, or both Exclusion criteria: hypertension, kidney disease, cardiovascular disease, medications that may interfere with sugar or lipid metabolism, taking antioxidants, placenta previa, threatened abortion, artificial infertility and history of previous adverse pregnancy |
| Interventions | Probiotic (n = 113): 5 g black garlic fermented by Lactobacillus bulgaricus daily for 40 weeks Placebo (n = 113): 5 g black garlic without fermentation daily for 40 weeks |
| Outcomes | Primary: proportion of participants who were screened for GDM with an OGTT within 4 weeks after the study start date Secondary: gestational age at delivery, weight gain during pregnancy, presence of induced labour, caesarean section, pre‐eclampsia, stillbirth, neonatal death, low‐birthweight infants, macrosomia, preterm infants, respiratory distress syndrome, hyperbilirubinaemia and neonatal intensive care unit admission |
| Notes | Sources of funding: Projects of Science and Technology Development Plan of Jilin Province (grant number 20160101054JC) Study dates: September 2015 to June 2016 Declarations of interest: none declared It was unclear whether this study met our inclusion criteria as we were unable to determine whether participants were diagnosed with gestational diabetes before or after enrolment in the study. We sought clarification from the study authors on 22 April 2020 and 6 May 2020, but received no response. |
BMI: body mass index; CFU: colony‐forming unit; GDM: gestational diabetes mellitus; LGA: large‐for‐gestational age; n: number of participants; OGTT: oral glucose tolerance test.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12620000359932.
| Study name | Probiotics in the prevention of gestational diabetes mellitus in women at increased risk: a prospective randomised controlled trial |
| Methods | Study design: parallel randomised controlled trial Blinding: double blind Location: North West Regional Hospital, Burnie, Tasmania, Australia |
| Participants | Inclusion criteria: aged 18–40 years, > 10 weeks' gestation, singleton pregnancy, increased risk of gestational diabetes as per the Australasian Diabetes in Pregnancy Society criteria (previous GDM, family history of diabetes (first‐degree relative with diabetes or a sister with GDM), BMI > 35 kg/m2, previous macrosomia (baby birthweight > 4500 g or > 90th centile), or polycystic ovarian syndrome) Exclusion criteria: unable to read and understand English, unable to provide informed consent, aged < 18 years, pregnancy > 16 weeks' gestation at recruitment, known pre‐existing diabetes, impaired fasting glucose or impaired glucose tolerance, GDM prior to recruitment as diagnosed by early pregnancy glucose testing, medications likely to influence glucose metabolism (e.g. metformin, glucocorticoids, immunosuppressants), medical conditions with altered glucose metabolism (e.g. Cushing's syndrome, hepatic cirrhosis), major fetal anomaly on ultrasound, current ingestion of probiotics via capsules or sachets and antibiotic use during the study period |
| Interventions | Probiotic: capsule taken once daily from enrolment until results are received from their glucose tolerance test (26–28 weeks' gestation) containing a combination of strains Lactobacillus rhamnosus and Bifidobacterium animalis ssp lactis with or without Bifidobacterium breve and Bifidobacterium longum Placebo: capsule taken once daily from enrolment until the results are received from their glucose tolerance test (26–28 weeks' gestation) containing maize‐derived maltodextrin |
| Outcomes | Primary: incidence of gestational diabetes Secondary: change in weight from booking‐in appointment (approximately 12 weeks' gestation) until routine antenatal care appointment at 26–28 weeks' gestation |
| Starting date | 1 April 2020 |
| Contact information | Anushika Samarage, North West Regional Hospital, anushika.samarage@ths.tas.gov.au |
| Notes | Sources of funding: North West Regional Hospital Declarations of interest: not reported |
Godfrey 2017.
| Study name | Nutritional intervention preconception and during pregnancy to maintain healthy glucose metabolism and offspring health (NiPPeR) |
| Methods | Study design: parallel randomised controlled trial Blinding: double blind Location: research and hospital facilities in Auckland (University of Auckland, Auckland, Waitemata and Counties Manukau District Health Boards and Clinics, New Zealand), Singapore (National University Hospital and National University Health System Investigational Medicine Unit), and Southampton (National Institute for Health Research Wellcome Trust Southampton Clinical Research Facility and Princess Anne Hospital, University Hospital Southampton, UK) |
| Participants | Inclusion criteria: aged 18–38 years; living in Southampton, Singapore or Auckland; planning to have maternity care in Southampton and Auckland if in Southampton or Auckland; willing to deliver at the National University Hospital if in Singapore; planning to conceive within 6 months (but conception up to 12 months after phenotyping will be included); Chinese, Malay, Indian or mixed Chinese/Malay/Indian ethnicity if in Singapore and able to provide written informed consent Exclusion criteria: pregnant or lactating at recruitment; assisted fertility apart from clomifene or letrozole alone; pre‐existing type 1 or 2 diabetes; oral or implanted contraception currently or in the last month or with an intrauterine contraceptive device in situ; metformin or systemic steroids currently or in the last month; anticonvulsant medication currently or in the last month; treatment for HIV, hepatitis B or hepatitis C currently or in the last month; and known serious food allergy |
| Interventions | Probiotic: nutritional drink containing myo‐inositol, vitamin D, riboflavin, vitamin B6, vitamin B12 and zinc with standard folic acid, iodine, calcium, beta‐carotene and iron with probiotics containing Lactobacillus rhamnosus NCC 4007 and Bifidobacterium animalis species lactis NCC 2818 taken twice daily starting before conception Placebo: control drink containing standard amounts of folic acid, beta‐carotene, iron, calcium and iodine taken twice daily starting before conception |
| Outcomes | Primary: maternal glucose metabolism at 28 weeks' gestation Secondary: maintenance of a healthy pregnancy, reduction in maternal micronutrient insufficiency, alteration in gut microbiota, alteration in maternal metabolomic and epigenetic biomarkers, enhancement of breast milk micronutrient content, altered immunological factors, epigenetic and metabolomic profiles, and maintenance of healthy lactogenesis, neonatal adiposity, birthweight and size for gestational age, reduced adiposity gain during infancy, reduction in cord blood C‐peptide, promotion of offspring wellbeing and healthy cardiometabolic risk factors, alteration in offspring metabolomic and epigenetic biomarkers and alteration in offspring gut microbiota |
| Starting date | 3 August 2015 |
| Contact information | Keith M Godfrey, NIHR Southampton Biomedical Research Centre and MRC Lifecourse Epidemiology Unit, UK, kmg@mrc.soton.ac.uk |
| Notes | Sources of funding: UK Medical Research Council (as part of an MRC award to the MRC Lifecourse Epidemiology Unit), the Singapore government (as part of the Growth, Development, and Metabolism Programme of the Singapore Institute for Clinical Sciences), the New Zealand government (as part of the Gravida, Centre of Research Excellence: Growth and Development) and Nestec SA Declarations of interest: KMG has received reimbursement for speaking at conferences sponsored by companies selling nutritional products. KMG, WSC, CYS, SYC, SJB and GCB are part of an academic consortium that has received research funding from Abbott Nutrition, Nestle and Danone. GCB is member of the Scientific Advisory Board and of the Asia‐Pacific grant panel of BASF. LE, TMS, ISZ, KM and SKT are employees of Nestec SA working at the Nestle Research Centre. |
Halkjaer 2016.
| Study name | Effect of probiotics (Vivomixx) on weight, microbiota and glucose tolerance in obese pregnant women and their newborn |
| Methods | Study design: parallel randomised controlled trial Blinding: double bind Location: Copenhagen University Hospital Hvidovre, Copenhagen, Denmark |
| Participants | Inclusion criteria: aged > 18 years, prepregnancy BMI 30–34.9 kg/m2, nulliparous, singleton pregnancy, ability to read and speak Danish, and normal ultrasound scan of the fetus at gestational age 12–14 weeks Exclusion criteria: pregnancy > 20 weeks' gestation at recruitment, pregestational diabetes or other serious diseases, multiple pregnancy, previous bariatric surgery, ingestion of probiotics for > 1 month before inclusion or ingestion of probiotics other than the study probiotics and alcohol or drug abuse |
| Interventions | Probiotic: Vivomixx capsule containing Streptococcus thermophilus DSM 24731, Bifidobacterium breve DSM 24732, Bifidobacterium infantis DSM 24737, Lactobacillus acidophilus DSM 24735, Lactobacillus plantarum DSM 24730, Lactobacillus paracasei DSM 24733, and Lactobacillus delbrueckii subspecies bulgaricus DSM 24734 taken twice daily from 14–20 weeks' gestation to delivery Placebo: capsule containing microcrystalline cellulose, magnesium stearate and silicon dioxide taken twice daily from 14–20 weeks' gestation until delivery |
| Outcomes | Primary: gestational weight gain and change in maternal fasting glucose from 14–20 weeks' to 27–30 weeks' gestation Secondary: changes in microbiota and inflammatory markers in mother and child, changes in vaginal microbiological profile and frequency of urinary tract infections, changes in concentrations of lipids and inflammatory markers, incidence of GDM, pre‐eclampsia, and gestational hypertension, change in mode of delivery, gestational age at birth, macrosomia, large‐ and small‐for‐gestational age, diet, physical activity levels, breastfeeding, birthweight and z‐score, Apgar scores, umbilical cord pH, neonatal intensive care unit admission, and child's weight gain and body composition at 9 months |
| Starting date | March 2015 |
| Contact information | Andreas Munk Petersen, Copenhagen University Hospital Hvidovre and University of Copenhagen, Denmark, andreas.munk.petersen@regionh.dk |
| Notes | Sources of funding: grants from Jeppe Juhls og hustru Ovita Juhls Mindelegat, Else og Mogens Wedell‐Wedellborgs Fond, Aase og Ejnar Danielsens Fond, Knud og Edith Eriksens Mindefond, Toyota‐Fonden Denmark, Next Gen Pharma India Pvt. Ltd., and Faculty of Health and Medical Sciences University of Copenhagen Declarations of interest: probiotics, placebo capsules and a 6‐month salary for SIH donated by Next Gen Pharma India Pvt. Ltd. |
IRCT20161025030502N2.
| Study name | Effect of oral probiotic lactofem on metabolic parameters in overweight pregnant women referred to prenatal clinics of the Shiraz hospitals in 2016 |
| Methods | Study design: parallel randomised controlled trial Blinding: double blind Location: not stated |
| Participants | Inclusion criteria: aged 18–35 years, willing to participate in research, prepregnancy BMI 25–30 kg/m2, 20–24 weeks' gestation, no medical comorbidities (including diabetes; hypertension; liver disease; kidney, adrenal or thyroid conditions; hypercholesterolaemia or bleeding), no medication use affecting glucose, fat metabolism, or blood pressure, normal diet and non‐smoker Exclusion criteria: prior use of probiotics, allergies to medication or placebo, the occurrence of acute bleeding and pre‐eclampsia |
| Interventions | Probiotic: capsule taken every 12 hours from 20 to 36 weeks' gestation Placebo: capsule taken every 12 hours from 20 to 36 weeks' gestation |
| Outcomes | Primary: fasting and 2‐hour post breakfast blood glucose levels, plasma lipids and blood pressure |
| Starting date | 21 November 2016 |
| Contact information | Sara Azima, Shiraz University of Medical Sciences, Iran |
| Notes | Sources of funding: Shiraz University of Medical Sciences Declarations of interest: not reported |
IRCT20180509039595N1.
| Study name | The effect of probiotic capsule on prevention of gestational diabetes in high‐risk pre‐diabetic pregnant women |
| Methods | Study design: parallel randomised controlled trial Blinding: double blind Location: not stated |
| Participants | Inclusion criteria: aged 16–49 years, willing to participate, literate, minimum score 2/20 on flowchart to assess GDM risk factors, have a telephone number, prediabetic, 14–16 weeks' gestation, normal screening tests for fetal abnormalities, single pregnancy and fasting blood glucose < 92 mg/dL and < 120 mg/dL 2 hours after food Exclusion criteria: allergy to cow's milk, severe mental problems, stressful incidence in the last 3 months, smoking, intention to terminate pregnancy, immune disorders, medical conditions, pregnancy with IVF and taking antibiotics continuously over the past 3 months |
| Interventions | Probiotic: capsule daily and routine care Placebo: capsule daily and routine care |
| Outcomes | Primary: frequency of abnormal OGTT after 70 days of intervention |
| Starting date | 22 May 2018 |
| Contact information | Mahdieh Ebrahimzadeh, Mashhad University of Medical Sciences, Iran |
| Notes | Sources of funding: Mashhad University of Medical Sciences Declarations of interest: not reported |
NCT01436448.
| Study name | Probiotics (Lactobacillus rhamnosus) in reducing glucose intolerance during and after pregnancy (GRIP) |
| Methods | Study design: randomised controlled trial Blinding: double blind for probiotics/placebo Location: Karachi, Pakistan |
| Participants | Inclusion criteria: high‐risk pregnancy (defined as ≥ 1 of maternal age ≥ 35 years, family history of diabetes among a first‐degree relative or BMI > 23 kg/m2), attending antenatal clinic at 12–14 weeks' gestation, singleton pregnancy and planning delivery at the study hospital Exclusion criteria: history of GDM; known diabetes mellitus; known chronic diseases; medication such as corticosteroids, azathioprine or antiepileptic drugs; known polycystic ovarian syndrome and not a resident of Karachi |
| Interventions | Probiotic: capsule containing Lactobacillus rhamnosus at 1010 CFU taken daily until delivery. Placebo: capsule containing microcrystalline cellulose taken daily until delivery |
| Outcomes | Primary: glucose tolerance by OGTT using ADA guidelines between 24–28 weeks' gestation and at 6–8 weeks' postpartum. Secondary: feasibility, intervention compliance, maternal safety and fetal/neonatal safety |
| Starting date | October 2011 |
| Contact information | Principal Investigator: Bilal Ahmed, MSc, Aga Khan University |
| Notes | Sources of funding: not stated Declarations of interest: not reported |
NCT03240419.
| Study name | Probiotic supplementation in obese pregnant women. a feasibility study |
| Methods | Study design: parallel randomised controlled trial Blinding: double blind Location: Little Rock, Arkansas, US |
| Participants | Inclusion criteria: BMI ≥ 30 kg/m2, aged ≥ 18 years, singleton pregnancy, < 12 weeks' gestation, consuming < 1 serving of yoghurt with live cultures or cultured milk per week, and conceived without assisted fertility treatments Exclusion criteria: women with pre‐existing medical conditions (e.g. diabetes, hypertension, thyroid disorders, heart disease or immune disorders); immunosuppressed women; women taking medications during pregnancy known to affect fetal growth; women using recreational drugs, tobacco or alcohol during pregnancy; milk intolerance/allergy and consuming probiotic supplements |
| Interventions | Probiotic: capsule containing Bifidobacterium BB‐12 and Lactobacillus rhamnosus LGG with minimum of 6.5 × 109 CFU per capsule taken once daily from recruitment until delivery Placebo: capsule containing microcrystalline cellulose, maltodextrin, silicon dioxide, and magnesium stearate taken once daily from recruitment until delivery |
| Outcomes | Primary: change in acceptance of intervention throughout pregnancy and change in compliance with intervention throughout pregnancy |
| Starting date | 23 August 2017 |
| Contact information | Eva C Diaz Fuentes, University of Arkansas, US, ecdiazfuentes@uams.edu |
| Notes | Sources of funding: Arkansas Children's Hospital Research Institute Declarations of interest: not reported |
NCT04009889.
| Study name | Double blind, randomised, controlled trial on impact of oral probiotic blend (Lactobacillus rhamnosus GG, L. crispatus LBV88, L. rhamnosus LBV96, L. jensenii LBV116 and L. gasseri LBV150) on pregnancy outcome |
| Methods | Study design: parallel randomised controlled trial Blinding: double blind Location: Clinical Research Center Kiel GmbH, Kiel, Schleswig‐Holstein, Germany |
| Participants | Inclusion criteria: pregnant women aged > 18 years, < 14 weeks' gestation, willing to consume the study product, willing to abstain from probiotic food and supplements containing probiotics, and able to provide written informed consent Exclusion criteria: enrolment in another clinical study or having finished another clinical study within the last 4 weeks before inclusion; diabetes mellitus; acute metabolic disorder interfering with glucose metabolism; known cancer < 5 years ago; any ano‐rectal infection; disease or surgery in the medical history or current that may impact microbiota; anus praeter; hypersensitivity allergy or reaction to any component of the test product; any disease or condition that might significantly compromise the hematopoietic, renal, endocrine, pulmonary, hepatic, gastrointestinal, cardiovascular, immunological, central nervous, dermatological or any other body system; history of active hepatitis B or C; history of HIV; regular medical treatments including non‐prescription medications that may impact study aims; major cognitive or psychiatric disorders; present drug abuse or alcoholism; reformed alcoholic and legal incapacity |
| Interventions | Probiotic: capsule containing a probiotic blend of 5 different Lactobacilli (L rhamnosus GG, L. crispatus LBV88, L rhamnosus LBV96, L jensenii LBV116 and L gasseri LBV150) taken from before 14 weeks' gestation until delivery Placebo: capsule containing microcrystalline cellulose, magnesium stearate and silicon dioxide from before 14 weeks' gestation until delivery |
| Outcomes | Primary: HOMA‐IR values in weeks 24–28 and weeks 36–40 |
| Starting date | 27 March 2018 |
| Contact information | Christiane Laue, Clinical Research Center Kiel GmbH, Germany, c.laue@crc-kiel.de |
| Notes | Funding sources: i‐Health, Inc. Declarations of interest: not reported |
ADA: American Diabetes Association; BMI: body mass index; CFU: colony‐forming unit; GDM: gestational diabetes mellitus; HOMA‐IR: Homeostatic Model Assessment of Insulin Resistance; IVF: in vitro fertilisation; OGTT: oral glucose tolerance test.
Differences between protocol and review
In this review update, we added in an additional search of ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/). We have also updated our outcomes to be in line with the Cochrane Pregnancy and Childbirth GDM Standardised Outcome Set, and we have incorporated 'Summary of findings' tables using GRADE to evaluate the evidence. Finally, we split our comparison into probiotics versus placebo and probiotics versus diet in place of probiotics versus all other interventions.
We also assessed all previously included eligible studies using Cochrane Pregnancy and Childbirth's trustworthiness screening tool.
Contributions of authors
HB and MDN developed the protocol. LC edited and commented on the protocol. HB and MDN wrote the review, assessed the citations and studies found for inclusion, risk of bias and data analysis. LC assisted with data interpretation, and edited and commented on the review.
Contributions for 2019/20 update
SJD developed the updated protocol.
HB, MDN and LC edited and commented on the updated protocol.
SJD and MDN assessed studies for inclusion and risk of bias and collected data for most studies.
SJD and SAP assessed Callaway 2019 for inclusion and risk of bias and collected data to limit conflict of interest.
SJD wrote the updated review.
MDN, HB, LC and SAP assisted with data interpretation, and edited and commented on the review.
Sources of support
Internal sources
-
The University of Queensland, Australia
salary
-
Royal Brisbane and Women's Hospital, Australia
salary
-
University of Melbourne, Australia
salary
-
Mater Health, Australia
salary
External sources
-
Mater Foundation, Australia
The Mater Foundation provided support for Helen L Barrett's work on this review.
-
NHMRC Early Career Research Fellowship, Australia
Helen L Barrett is the recipient of an NHMRC Early Career Research Fellowship (APP1120070), which provided support for her work on this review.
Declarations of interest
SJD: none.
HB: recieved a grant from the NHMRC, Australia (a competitive government research grant) to undertake a randomised control trial of probiotics for the prevention of gestational diabetes mellitus and I was an associate investigator this trial (Callaway 2019). In this review, HB was not involved in any decisions relating to this trial: assessment of the trial for inclusion, assessment of risk of bias and data extraction were carried out by individuals who were not directly involved in the trial. SJD and SAP carried out these tasks. Chr. Hansen A/S have donated the probiotics and matching placebo to the SPRING study (Callaway 2019) that was conducted by authors Barrett, Dekker Nitert and Callaway. While the authors are grateful for this donation from Chr.Hansen A/S, the conduct of the study, analysis and publication of the results is entirely independent of Chr. Hansen A/S.
SAP: none.
LC: was chief investigator in a trial examining the use of probiotics for preventing gestational diabetes mellitus (Callaway 2019), which was funded from a grant from the NHMRC, Australia (a competitive government research grant). In this review, LC was not involved in any decisions relating to this trial: assessment of the trial for inclusion, assessment of risk of bias and data extraction were carried out by individuals who were not directly involved in the trial. SJD and SAP carried out these tasks. Chr. Hansen A/S have donated the probiotics and matching placebo to the SPRING study (Callaway 2019) that was conducted by authors Barrett, Dekker Nitert and Callaway. While the authors are grateful for this donation from Chr.Hansen A/S, the conduct of the study, analysis and publication of the results is entirely independent of Chr. Hansen A/S.
MDN: was scientific lead in a trial examining the use of probiotics for preventing gestational diabetes mellitus (Callaway 2019), which was funded from a grant from the NHMRC, Australia (a competitive government research grant). In this review, MDN was not involved in any decisions relating to this trial: assessment of the trial for inclusion, assessment of risk of bias and data extraction were carried out by individuals who were not directly involved in the trial. SJD and SAP carried out these tasks. Chr. Hansen A/S have donated the probiotics and matching placebo to the SPRING study (Callaway 2019) that was conducted by authors Barrett, Dekker Nitert and Callaway. While the authors are grateful for this donation from Chr.Hansen A/S, the conduct of the study, analysis and publication of the results is entirely independent of Chr. Hansen A/S.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Callaway 2019 {published data only}ACTRN12611001208998
- Callaway L. Randomized placebo controlled trial of probiotics in overweight and obese women to assess the prevention of gestational diabetes. www.anzctr.org.au/trial_view.aspx?ID=347738 (accessed 6 July 2012).
- Callaway LK, McIntyre HD, Barrett HL, Foxcroft K, Tremellen A, Lingwood BE, et al. Probiotics for the prevention of gestational diabetes mellitus in overweight and obese women: findings from the SPRING double-blind randomized controlled trial. Diabetes Care 2019;42(3):364-71. [CENTRAL: CN-01915884] [EMBASE: 2001611803] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Dekker Nitert M, Barrett HL, Foxcroft K, Tremellen A, Wilkinson S, Lingwood B, et al. SPRING: an RCT study of probiotics in the prevention of gestational diabetes mellitus in overweight and obese women. BMC Pregnancy and Childbirth 2013;13:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jamilian 2016 {published data only}IRCT201503035623N38
- IRCT201503035623N38. Effect of probiotic in the prevention of spontaneously abortion. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201503035623N38 2015. [CENTRAL: CN-01833874]
- Jamilian M, Bahmani F, Vahedpoor Z, Salmani A, Tajabadi-Ebrahimi M, Jafari P, et al. Effects of probiotic supplementation on metabolic status in pregnant women: a randomized, double-blind, placebo-controlled trial. Archives of Iranian Medicine 2016;19(10):687-92. [CENTRAL: CN-01307046] [PMID: ] [PubMed] [Google Scholar]
Laitinen 2009 {published data only}
- Aaltonen J, Ojala T, Laitinen K, Piirainen TJ, Poussa TA, Isolauri E. Evidence of infant blood pressure programming by maternal nutrition during pregnancy: a prospective randomized controlled intervention study. Journal of Pediatrics 2008;152:79-84. [DOI] [PubMed] [Google Scholar]
- Aaltonen J, Ojala T, Laitinen K, Poussa T, Ozanne S, Isolauri E. Impact of maternal diet during pregnancy and breastfeeding on infant metabolic programming: a prospective randomized controlled study. European Journal of Clinical Nutrition 2011;65(1):10-9. [DOI] [PubMed] [Google Scholar]
- Hoppu U, Isolauri E, Koskinen P, Laitinen K. Diet and blood lipids in 1-4 year-old children. Nutrition Metabolism & Cardiovascular Diseases 2013;23(10):980-6. [DOI] [PubMed] [Google Scholar]
- Ilmonen J, Isolauri E, Poussa T, Laitinen K. Impact of dietary counselling and probiotic intervention on maternal anthropometric measurements during and after pregnancy: a randomized placebo-controlled trial. Clinical Nutrition (Edinburgh, Scotland) 2011;30(2):156-64. [CENTRAL: CN-00786714] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Jaakkola J, Isolauri E, Poussa T, Laitinen K. Benefits of repeated individual dietary counselling in long-term weight control in women after delivery. Maternal & Child Nutrition 2015;11(4):1041-8. [CENTRAL: CN-01253756] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen K, Poussa T, Isolauri E. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. British Journal of Nutrition 2009;101(11):1679-87. [DOI] [PubMed] [Google Scholar]
- Luoto R, Laitinen K, Nermes M, Isolauri E. Impact of maternal probiotic-supplemented dietary counseling during pregnancy on colostrum adiponectin concentration: a prospective, randomized, placebo-controlled study. Early Human Development 2012;88(6):339-44. [DOI] [PubMed] [Google Scholar]
- Luoto R, Laitinen K, Nermes M, Isolauri E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. British Journal of Nutrition 2010;103(12):1792-9. [DOI] [PubMed] [Google Scholar]
- Piirainen T, Isolauri E, Lagstrom H, Laitinen K. Impact of dietary counselling on nutrient intake during pregnancy: a prospective cohort study. British Journal of Nutrition 2006;96(6):1095-104. [DOI] [PubMed] [Google Scholar]
- Vahamiko S, Isolauri E, Laitinen K. Weight status and dietary intake determine serum leptin concentrations in pregnant and lactating women and their infants. British Journal of Nutrition 2013;110(6):1098-106. [DOI] [PubMed] [Google Scholar]
Lindsay 2014 {published data only}ISRCTN97241163
- Lindsay K, Kennelly M, Smith T, Maguire O, Shanahan F, Brennan L, et al. Probiotics in obese pregnancy to reduce maternal fasting glucose: a randomised controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(Suppl 1):A156. [DOI] [PubMed] [Google Scholar]
- Lindsay K, Maguire O, Smith T, Brennan L, McAuliffe F. A randomized controlled trial of probiotics to reduce maternal glycaemia in obese pregnancy. American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S342. [Google Scholar]
- Lindsay KL, Brennan L, McAuliffe FM. Acceptability of and compliance with a probiotic capsule intervention in pregnancy. International Journal of Gynecology and Obstetrics 2014;125(3):279-80. [DOI] [PubMed] [Google Scholar]
- Lindsay KL, Brennan L, McAuliffe FM. Probiotics in obese pregnancy to reduce maternal fasting glucose: a randomized controlled trial. The Power of Programming 2014: International Conference on Developmental Origins of Adiposity and Long-term Health; 2014 Mar 13-15; Munich, Germany.
- Lindsay KL, Kennelly M, Culliton M, Smith T, Maguire OC, Shanahan F, et al. Probiotics in obese pregnancy do not reduce maternal fasting glucose: a double-blind, placebo-controlled, randomized trial. American Journal of Clinical Nutrition 2014;99:1432-9. [DOI] [PubMed] [Google Scholar]
- McAuliffe F. A randomised control trial of probiotics in pregnancy to reduce maternal glucose in obese and gestational diabetic women. www.controlled-trials.com/ISRCTN97241163 (accessed 6 July 2012).
Okesene‐Gafa 2019 {published and unpublished data}ACTRN12615000400561
- ACTRN12615000400561. A four-armed randomised controlled demonstration trial of a multifaceted dietary intervention and probiotic capsules in obese pregnant women in the Counties Manukau Health region. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12615000400561 (first received 10 April 2015). [CENTRAL: CN-02089252]
- Dawe JP, McCowan LM, Wilson J, Okesene-Gafa KA, Serlachius AS. Probiotics and maternal mental health: a randomised controlled trial among pregnant women with obesity. Scientific Reports 2020;10(1):1291. [CENTRAL: CN-02082808] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinlay C, Okesene-Gafa K, Taylor R, Wall C, Rush E, McCowan M, et al. Dietary intervention and/or probiotic capsules in obese pregnant women and infant growth and feeding at 5 months: healthy mums and babies (HUMBA) trial. Journal of Paediatrics and Child Health 2019;55:35. [CENTRAL: CN-01940481] [EMBASE: 627192706] [Google Scholar]
- Okesene-Gafa K, Li M, Taylor RS, Thompson JM, Crowther CA, McKinlay CJ, et al. A randomised controlled demonstration trial of multifaceted nutritional intervention and or probiotics: the healthy mums and babies (HUMBA) trial. BMC Pregnancy and Childbirth 2016;16(1):373. [CENTRAL: CN-01426401] [EMBASE: 613355126] [PMCID: PMC5123375] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okesene-Gafa K, Li M, Taylor RS, Thompson JM, Crowther CA, McKinlay CJ, et al. Correction: a randomised controlled demonstration trial of multifaceted nutritional intervention and or probiotics: the healthy mums and babies (HUMBA) trial. BMC Pregnancy and Childbirth 2018;18(1):130. [CENTRAL: CN-01607638] [EMBASE: 621978171] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okesene-Gafa K, McKinlay CJ, Li M, Taylor RS, Wall CR, Rush EC, et al. Dietary intervention and/or probiotic capsules in obese pregnant women-effect on gestational weight gain and infant birthweight: healthy mums and babies (HUMBA) trial. Journal of Paediatrics and Child Health 2018;54(Suppl 1):4-5. [CENTRAL: CN-01572063] [EMBASE: 621532607] [Google Scholar]
- Okesene-Gafa KA, Li M, McKinlay CJ, Taylor RS, Rush EC, Wall CR, et al. Effect of antenatal dietary interventions in maternal obesity on pregnancy weight-gain and birthweight: Healthy Mums and Babies (HUMBA) randomized trial. American Journal of Obstetrics and Gynecology 2019;221(2):152.e1-e13. [CENTRAL: CN-01917291] [EMBASE: 626863937] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pellonpera 2019 {published data only}
- Laitinen K. Nutrition and pregnancy intervention study. clinicaltrials.gov/ct2/show/NCT01922791 (first received 14 August 2013).
- Pellonpera O, Mokkala K, Houttu N, Vahlberg T, Koivuniemi E, Tertti K, et al. Efficacy of fish oil and/or probiotic intervention on the incidence of gestational diabetes mellitus in an at-risk group of overweight and obese women: a randomized, placebo-controlled, double-blind clinical trial. Diabetes Care 2019;42(6):1009-17. [CENTRAL: CN-01963280] [EMBASE: 2002045489] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wickens 2017 {published data only}ACTRN12612000196842
- Barthow C, Wickens K, Stanley T, Mitchell EA, Maude R, Abels P, et al. The Probiotics in Pregnancy Study (PiP study): rationale and design of a double-blind randomised controlled trial to improve maternal health during pregnancy and prevent infant eczema and allergy. BMC Pregnancy and Childbirth 2016;16(1):133. [CENTRAL: CN-01425048] [EMBASE: 20160469757] [PMCID: PMC4891898] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude R, Barthow C, Wickens K, Murphy R, Abels P, Stone P, et al. Can early pregnancy probiotic supplementation reduce the rate of gestational diabetes? In: 31st International Confederation of Midwives Triennial Congress. Midwives – Making a Difference in the World; 2017 Jun 18-22; Toronto, Canada. 2017:P1.089. [CENTRAL: CN-02082804]
- Wickens K, Barthow C, Mitchell EA, Stanley TV, Purdie G, Rowden J, et al. Maternal supplementation alone with lactobacillus rhamnosus HN001 during pregnancy and breastfeeding does not reduce infant eczema. Pediatric Allergy and Immunology 2018;29(3):296-302. [CENTRAL: CN-01608243] [EMBASE: 621550781] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Wickens K. A randomized placebo controlled trial of the effects of the probiotic Lactobacillus rhamnosus HN001 taken from the 1st trimester of pregnancy till 6 months post partum, if breastfeeding, on the development of eczema and atopic sensitization in infants by age 12 months. www.anzctr.org.au/trial_view.aspx?ID=362049 (first received 7 February 2012).
- Wickens KL, Barthow CA, Murphy R, Abels PR, Maude RM, Stone PR, et al. Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: a randomised controlled trial. British Journal of Nutrition 2017;117(6):804-13. [CENTRAL: CN-01381612] [EMBASE: 615245454] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Asemi 2013 {published data only}
- Asemi Z, Esmaillzadeh A. Effect of daily consumption of probiotic yoghurt on serum levels of calcium, iron and liver enzymes in pregnant women. International Journal of Preventive Medicine 2013;4(8):949-55. [CENTRAL: CN-00916065] [EMBASE: 369812874] [PMC free article] [PubMed] [Google Scholar]
- Asemi Z, Jazayeri S, Najafi M, Samimi M, Mofid V, Shidfar F, et al. Effects of daily consumption of probiotic yoghurt on inflammatory factors in pregnant women: a randomized controlled trial. Pakistan Journal of Biological Sciences : PJBS 2011;14(8):476-82. [CENTRAL: CN-00798130] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Asemi Z, Samimi M, Tabasi Z, Talebian P, Azarbad Z, Hydarzadeh Z, et al. Effect of daily consumption of probiotic yoghurt on lipid profiles in pregnant women: a randomized controlled clinical trial. Journal of Maternal-fetal & Neonatal Medicine 2012;25(9):1552-6. [CENTRAL: CN-00971455] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Asemi Z, Samimi M, Tabassi Z, Naghibi M, Rahimi A, Khorammian H, et al. Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: a randomized controlled trial. European Journal of Clinical Nutrition 2013;67(1):71-4. [DOI] [PubMed] [Google Scholar]
- IRCT138811282394N3. Comparison of effects of consumption of probiotic and conventional yogurt on oxidative stress and serum TNF-α and CRP in pregnant women and anthropometric indices of neonates. www.irct.ir/trial/2075 (first received 6 June 2011). [CENTRAL: CN-01804915]
Taghizadeh 2014 {published data only}
- Taghizadeh M, Asemi Z. Effects of synbiotic food consumption on glycemic status and serum hs-CRP in pregnant women: a randomized controlled clinical trial. Hormones 2014;13(3):398-406. [CENTRAL: CN-00999375] [EMBASE: 2014487933] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Asgharian 2020 {published data only}IRCT201604013706N31
- Asgharian H, Homayouni-Rad A, Mirghafourvand M, Mohammad-Alizadeh-Charandabi S. Effect of probiotic yoghurt on plasma glucose in overweight and obese pregnant women: a randomized controlled clinical trial. European Journal of Nutrition 2020;59(1):205-15. [CENTRAL: CN-01936694] [EMBASE: 627693578] [PMID: ] [DOI] [PubMed] [Google Scholar]
- IRCT201604013706N31. Effect of probiotic yoghurt on level of blood glucose in obese and overweight pregnant women. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201604013706N31 2016. [CENTRAL: CN-01856226]
Charles 2018 {published data only}
- Charles D, Drymoussi Z, Thangaratinam S. The effect of probiotic supplementation on gestational diabetes mellitus and metabolic outcomes: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2018;125:10. [CENTRAL: CN-01713894] [EMBASE: 625506633] [Google Scholar]
Si 2019 {published data only}
- Si L, Lin R, Jia Y, Jian W, Yu Q, Wang M, et al. Lactobacillus bulgaricus improves antioxidant capacity of black garlic in the prevention of gestational diabetes mellitus: a randomized control trial. Bioscience Reports 2019;39:BSR20182254. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12620000359932 {published data only}
- ACTRN12620000359932. Probiotics in the prevention of gestational diabetes mellitus in women at increased risk: a prospective randomised controlled trial. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12620000359932 (first received 28 January 2020).
Godfrey 2017 {published data only}
- Godfrey KM, Cutfield W, Chan S-Y, Baker PN, Chong Y-S, Aris IBM, et al. Nutritional intervention preconception and during pregnancy to maintain healthy glucose metabolism and offspring health ("NiPPeR"): study protocol for a randomised controlled trial. Trials 2017;18:131. [CENTRAL: CN-01454636] [EMBASE: 614884778] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Halkjaer 2016 {published data only}
- Halkjaer SI, Petersen AM, Nilas L, Cortes D, Carlsen EM, Halldorsson TI, et al. Effects of probiotics (Vivomixx) in obese pregnant women and their newborn: study protocol for a randomized controlled trial. Trials 2016;17(1):491. [CENTRAL: CN-01447521] [PMCID: PMC5057415] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02508844. Effect of probiotics (Vivomixx) on weight, microbiota and glucose tolerance in obese pregnant women and their newborn. clinicaltrials.gov/show/NCT02508844 (first received 27 July 2015). [CENTRAL: CN-02032725]
IRCT20161025030502N2 {published data only}IRCT20161025030502N2
- IRCT20161025030502N2. Effect of oral probiotic lactofem on metabolic parameters in overweight pregnant women referred to prenatal clinics of the shiraz hospitals in 2016. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20161025030502N2 2016. [CENTRAL: CN-01896627]
IRCT20180509039595N1 {published data only}
- IRCT20180509039595N1. The effect of probiotic capsule on prevention of gestational diabetes. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20180509039595N1 2018. [CENTRAL: CN-01907075]
NCT01436448 {published data only}
- NCT01436448. Effects of probiotics (Lactobacillus rhamnosus) in reducing glucose intolerance during and after pregnancy: a double blind randomized controlled trial in antenatal clinic of Karachi-Pakistan (GRIP). clinicaltrials.gov/show/NCT01436448 (first received 19 September 2011).
NCT03240419 {published data only}
- NCT03240419. Prenatal probiotic intervention. clinicaltrials.gov/show/NCT03240419 (first received 7 August 2017). [CENTRAL: CN-02089253]
NCT04009889 {published data only}
- NCT04009889. Impact of oral probiotic blend on pregnancy outcome. clinicaltrials.gov/show/NCT04009889 (first received 5 July 2019). [CENTRAL: CN-01953073]
Additional references
Abouzeid 2014
- Abouzeid M, Versace VL, Janus ED, Davey MA, Philpot B, Oats J, et al. A population-based observational study of diabetes during pregnancy in Victoria, Australia, 1999–2008. BMJ Open 2014;4(11):e005394. [DOI] [PMC free article] [PubMed] [Google Scholar]
ADA 2019
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes – 2019. Diabetes Care 2019;42(Suppl 1):S13-S28. [DOI] [PubMed] [Google Scholar]
Agha‐Jaffar 2016
- Agha-Jaffar R, Oliver N, Johnston D, Robinson S. Gestational diabetes mellitus: does an effective prevention strategy exist? Nature Reviews. Endocrinology 2016;12(9):533-46. [DOI] [PubMed] [Google Scholar]
Barrett 2012
- Barrett HL, Callaway LK, Nitert MD. Probiotics: a potential role in the prevention of gestational diabetes. Acta Diabetologia 2012;49(Suppl 1):S1-S13. [DOI] [PubMed] [Google Scholar]
Brunkwall 2017
- Brunkwall L, Orho-Melander M. The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: from current human evidence to future possibilities. Diabetologia 2017;60(6):943-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchanan 2012
- Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nature Reviews. Endocrinology 2012;8(11):639-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carlisle 2017
- Carlisle JB. Data fabrication and other reasons for non-random sampling in 5087 randomised, controlled trials in anaesthetic and general medical journals. Anaesthesia 2017;72:944-52. [DOI] [PubMed] [Google Scholar]
Carpenter 1982
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. American Journal of Obstetrics and Gynecology 1982;144(7):768-73. [DOI] [PubMed] [Google Scholar]
Carr 2011
- Carr DB, Newton KM, Utzschneider KM, Faulenbach MV, Kahn SE, Easterling TR, et al. Gestational diabetes or lesser degrees of glucose intolerance and risk of preeclampsia. Hypertension in Pregnancy 2011;30(2):153-63. [DOI] [PubMed] [Google Scholar]
Chatzakis 2019
- Chatzakis C, Goulis DG, Mareti E, Eleftheriades M, Zavlanos A, Dinas K, et al. Prevention of gestational diabetes mellitus in overweight or obese pregnant women: a network meta-analysis. Diabetes Research and Clinical Practice 2019;158:107924. [DOI] [PubMed] [Google Scholar]
Cho 2018
- Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Research and Clinical Practice 2018;138:271-81. [DOI] [PubMed] [Google Scholar]
Crawford 2015
- Crawford TJ, Crowther CA, Alsweiler J, Brown J. Antenatal dietary supplementation with myo-inositol in women during pregnancy for preventing gestational diabetes. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD011507. [DOI: 10.1002/14651858.CD011507.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crowther 2005
- Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine 2005;352(24):2477-86. [DOI] [PubMed] [Google Scholar]
Crusell 2018
- Crusell MK, Hansen TH, Nielsen T, Allin KH, Ruhlemann MC, Damm P, et al. Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 2018;6(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dabelea 2005
- Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS, et al. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care 2005;28(3):579-84. [DOI] [PubMed] [Google Scholar]
David 2014
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505(7484):559-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dodd 2007
- Dodd JM, Crowther CA, Antoniou G, Baghurst P, Robinson JS. Screening for gestational diabetes: the effect of varying blood glucose definitions in the prediction of adverse maternal and infant health outcomes. Australian & New Zealand Journal of Obstetrics & Gynaecology 2007;47(4):307-12. [DOI] [PubMed] [Google Scholar]
Esakoff 2009
- Esakoff TF, Cheng YW, Sparks TN, Caughey AB. The association between birthweight 4000 g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. American Journal of Obstetrics and Gynecology 2009;200(6):672 e1-4. [DOI] [PubMed] [Google Scholar]
Facchinetti 2014
- Facchinetti F, Dante G, Petrella E, Neri I. Dietary interventions, lifestyle changes, and dietary supplements in preventing gestational diabetes mellitus: a literature review. Obstetrical & Gynecological Survey 2014;69(11):669-80. [DOI] [PubMed] [Google Scholar]
FAO/WHO 2006
- Food and Agriculture Organization of the United Nations, World Health Organization. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. http://www.fao.org/3/a0512e/a0512e.pdf 2006;accessed January 2021.
Fitria 2019
- Fitria N, Asselt AD, Postma MJ. Cost-effectiveness of controlling gestational diabetes mellitus: a systematic review. European Journal of Health Economics 2019;20(3):407-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gomez Arango 2015
- Gomez Arango LF, Barrett HL, Callaway LK, Nitert MD. Probiotics and pregnancy. Current Diabetes Reports 2015;15(1):567. [DOI] [PubMed] [Google Scholar]
GRADE Handbook
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available at gdt.guidelinedevelopment.org/app/handbook/handbook.html.
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed January 2021. Available at gradepro.org.
Griffin 2015
- Griffin C. Probiotics in obstetrics and gynaecology. Australian & New Zealand Journal of Obstetrics & Gynaecology 2015;55(3):201-9. [DOI] [PubMed] [Google Scholar]
Griffith 2020
- Griffith R J, Alsweiler J, Moore AE, Brown S, Middleton P, Shepherd E, et al. Interventions to prevent women from developing gestational diabetes mellitus: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2020, Issue 6. Art. No: CD012394. [DOI: 10.1002/14651858.CD012394.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2012
- Han S, Middleton P, Crowther CA. Exercise for pregnant women for preventing gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2012, Issue 7. Art. No: CD009021. [DOI: 10.1002/14651858.CD009021.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
HAPO 2008
- The HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine 2008;358:1991-2002. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
IADPSG 2010
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33(3):676-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jarde 2018
- Jarde A, Lewis-Mikhael AM, Moayyedi P, Stearns JC, Collins SM, Beyene J, et al. Pregnancy outcomes in women taking probiotics or prebiotics: a systematic review and meta-analysis. BMC Pregnancy Childbirth 2018;18(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
John 2016
- John GK, Mullin GE. The gut microbiome and obesity. Current Oncology Reports 2016;18(7):45. [DOI] [PubMed] [Google Scholar]
Koren 2012
- Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Backhed HK, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012;150(3):470-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Landon 2009
- Landon MB, Spong CY, Thom EC, Marshall W, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. New England Journal of Medicine 2009;361:1339-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindsay 2013
- Lindsay KL, Walsh CA, Brennan L, McAuliffe FM. Probiotics in pregnancy and maternal outcomes: a systematic review. Journal of Maternal Fetal and Neonatal Medicine 2013;26(8):772-8. [DOI] [PubMed] [Google Scholar]
López‐de‐Andrés 2020
- López-de-Andrés A, Perez-Farinos N, Hernández-Barrera V, Palomar-Gallego MA, Carabantes-Alarcón D, Zamorano-León JJ, Miguel-Diez J, Jimenez-Garcia R. A Population-Based Study of Diabetes During Pregnancy in Spain (2009-2015): Trends in Incidence, Obstetric Interventions, and Pregnancy Outcomes.. Journal of Clinical Medicine 2020;9(2):582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malcolm 2012
- Malcolm J. Through the looking glass: gestational diabetes as a predictor of maternal and offspring long-term health. Diabetes/Metabolism Research and Reviews 2012;28(4):307-11. [DOI] [PubMed] [Google Scholar]
Masulli 2020
- Masulli M, Vitacolonna E, Fraticelli F, Della Pepa G, Mannucci E, Monami M. Effects of probiotic supplementation during pregnancy on metabolic outcomes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Research and Clinical Practice 2020;162:108111. [DOI] [PubMed] [Google Scholar]
Metzger 1998
- Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care 1998;21 Suppl 2:B161-7. [PubMed] [Google Scholar]
Ministry of Health 2014
- Ministry of Health. Screening, Diagnosis and Management of Gestational Diabetes in New Zealand: a Clinical Practice Guideline. Wellington (NZ): Ministry of Health, 2014. [Google Scholar]
Musso 2011
- Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annual Review of Medicine 2011;62:361-80. [DOI] [PubMed] [Google Scholar]
Noh 2021
- Noh Y, Choe S-A, Shin J-Y. Trends and associated maternal characteristics of antidiabetic medication use among pregnant women in South Korea. Scientific Reports 2021;11:Article number: 4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okesene‐Gafa 2020
- Okesene-Gafa KA, Moore AE, Jordan V, McCowan L, Crowther CA. Probiotic treatment for women with gestational diabetes to improve maternal and infant health and well-being. Cochrane Database of Systematic Reviews 2020, Issue 6. Art. No: CD012970. [DOI: 10.1002/14651858.CD012970.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Plows 2019
- Plows JF, Reynolds CM, Vickers MH, Baker PN, Stanley JL. Nutritional supplementation for the prevention and/or treatment of gestational diabetes mellitus. Current Diabetes Reports 2019;19(9):73. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rogozinska 2015
- Rogozinska E, Chamillard M, Hitman GA, Khan KS, Thangaratinam S. Nutritional manipulation for the primary prevention of gestational diabetes mellitus: a meta-analysis of randomised studies. PLoS One 2015;10(2):e0115526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shepherd 2017
- Shepherd E, Gomersall JC, Tieu J, Han S, Crowther CA, Middleton P. Combined diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD010443. [DOI: 10.1002/14651858.CD010443.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simmons 2015
- Simmons D. Prevention of gestational diabetes mellitus: where are we now? Diabetes, Obesity & Metabolism 2015;17(9):824-34. [DOI] [PubMed] [Google Scholar]
Sui 2013
- Sui Z, Turnbull DA, Dodd JM. Overweight and obese women's perceptions about making healthy change during pregnancy: a mixed method study. Maternal and Child Health Journal 2013;17(10):1879-87. [DOI] [PubMed] [Google Scholar]
Syngai 2016
- Syngai GG, Gopi R, Bharali R, Dey S, Lakshmanan GM, Ahmed G. Probiotics - the versatile functional food ingredients. Journal of Food Science and Technology 2016;53(2):921-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
The Finnish Medical Society Duodecim 2013
- Duodecim of the Finnish Medical Association, the Medical Council of the Finnish Diabetes Association and the Finnish Gynecological Association. Gestational Diabetes: Current Care Guidelines. Helsinki (Finland): The Finnish Medical Society Duodecim, 2013. [Google Scholar]
Tiderencel 2020
- Tiderencel KA, Hutcheon DA, Ziegler J. Probiotics for the treatment of type 2 diabetes: a review of randomized controlled trials. Diabetes/Metabolism Research and Reviews 2020;36(1):e3213. [DOI] [PubMed] [Google Scholar]
Tieu 2017
- Tieu J, Shepherd E, Middleton P, Crowther CA. Dietary advice interventions in pregnancy for preventing gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No: CD006674. [DOI: 10.1002/14651858.CD006674.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
van de Vusse 2013
- de Vusse L, Hanson L, Safdar N. Perinatal outcomes of prenatal probiotic and prebiotic administration: an integrative review. Journal of Perinatal & Neonatal Nursing 2013;27(4):288-301. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Barrett 2014
- Barrett HL, Dekker Nitert M, Conwell LS, Callaway LK. Probiotics for preventing gestational diabetes. Cochrane Database of Systematic Reviews 2014, Issue 2. Art. No: CD009951. [DOI: 10.1002/14651858.CD009951.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


