Abstract
Background
Intestinal dysbiosis may contribute to the pathogenesis of necrotising enterocolitis (NEC) in very preterm or very low birth weight infants. Dietary supplementation with probiotics to modulate the intestinal microbiome has been proposed as a strategy to reduce the risk of NEC and associated mortality and morbidity.
Objectives
To determine the effect of supplemental probiotics on the risk of NEC and mortality and morbidity in very preterm or very low birth weight infants.
Search methods
We searched Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 2) in the Cochrane Library; MEDLINE Ovid (1946 to 17 Feb 2020), Embase Ovid (1974 to 17 Feb 2020), Maternity & Infant Care Database Ovid (1971 to 17 Feb 2020), the Cumulative Index to Nursing and Allied Health Literature (1982 to 18 Feb 2020). We searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials (RCTs) and quasi‐RCTs.
Selection criteria
We included RCTs and quasi‐RCTs comparing probiotic supplementation with placebo or no probiotics in very preterm or very low birth weight infants.
Data collection and analysis
We used the standard methods of Cochrane Neonatal. Two review authors separately evaluated trial quality, extracted data, and synthesised effect estimates using risk ratio (RR), risk difference (RD), and mean difference. We used the GRADE approach to assess the certainty of evidence for effects on NEC, all‐cause mortality, late‐onset infection, and severe neurodevelopmental impairment.
Main results
We included 56 trials in which 10,812 infants participated. Most trials were small (median sample size 149). Lack of clarity on methods to conceal allocation and mask caregivers or investigators were the main potential sources of bias in about half of the trials. Trials varied by the formulation of the probiotics. The most commonly used preparations contained Bifidobacterium spp.,Lactobacillus spp., Saccharomyces spp., andStreptococcus spp. alone or in combinations.
Meta‐analysis showed that probiotics may reduce the risk of NEC: RR 0.54, 95% CI 0.45 to 0.65 (54 trials, 10,604 infants; I² = 17%); RD ‐0.03, 95% CI ‐0.04 to ‐0.02; number needed to treat for an additional beneficial outcome (NNTB) 33, 95% CI 25 to 50. Evidence was assessed as low certainty because of the limitations in trials design, and the presence of funnel plot asymmetry consistent with publication bias. Sensitivity meta‐analysis of trials at low risk of bias showed a reduced risk of NEC: RR 0.70, 95% CI 0.55 to 0.89 (16 trials, 4597 infants; I² = 25%); RD ‐0.02, 95% CI ‐0.03 to ‐0.01; NNTB 50, 95% CI 33 to 100. Meta‐analyses showed that probiotics probably reduce mortality (RR 0.76, 95% CI 0.65 to 0.89; (51 trials, 10,170 infants; I² = 0%); RD ‐0.02, 95% CI ‐0.02 to ‐0.01; NNTB 50, 95% CI 50 to 100), and late‐onset invasive infection (RR 0.89, 95% CI 0.82 to 0.97; (47 trials, 9762 infants; I² = 19%); RD ‐0.02, 95% CI ‐0.03 to ‐0.01; NNTB 50, 95% CI 33 to 100). Evidence was assessed as moderate certainty for both these outcomes because of the limitations in trials design. Sensitivity meta‐analyses of 16 trials (4597 infants) at low risk of bias did not show an effect on mortality or infection. Meta‐analysis showed that probiotics may have little or no effect on severe neurodevelopmental impairment (RR 1.03, 95% CI 0.84 to 1.26 (five trials, 1518 infants; I² = 0%). The certainty on this evidence is low because of limitations in trials design and serious imprecision of effect estimate. Few data (from seven of the trials) were available for extremely preterm or extremely low birth weight infants. Meta‐analyses did not show effects on NEC, death, or infection (low‐certainty evidence).
Authors' conclusions
Given the low to moderate level of certainty about the effects of probiotic supplements on the risk of NEC and associated morbidity and mortality for very preterm or very low birth weight infants, and particularly for extremely preterm or extremely low birth weight infants, further, large, high‐quality trials are needed to provide evidence of sufficient quality and applicability to inform policy and practice.
Plain language summary
Probiotics for prevention of necrotising enterocolitis in very preterm or very low birthweight infants
Review question Does giving very preterm or very low birth weight infants probiotics prevent necrotising enterocolitis?
Background Very preterm infants (born more than eight weeks' early) and very low birth weight (less than 1.5 kg) are at risk of developing a severe bowel disorder, where a portion of the bowel becomes inflamed, infected, and dies, called necrotising enterocolitis. This condition is associated with death, serious infection, and long‐term disability and developmental problems. One way to help prevent necrotising enterocolitis and associated conditions may be to add probiotics (dietary supplements containing potentially beneficial bacteria or yeasts) to milk feeds.
Study characteristics The search is up to date as of 18 February 2020. We found 56 trials, with, in total, more than 10,000 infant participants. Trials were mostly small, and some had design flaws that might bias their findings.
Key results Combined analyses showed that giving very preterm and very low birth weight infants probiotics may reduce the risk of necrotising enterocolitis, and probably reduces the risk of death and serious infection. There is no evidence of an effect on disability or developmental outcomes. Few trials provided data for extremely preterm infants (born more than 12 weeks' early) and extremely low birth weight (less than 1.0 kg), and these analyses did not show effects on necrotising enterocolitis, death and serious infection.
Certainty of evidence The evidence for an effect on necrotising enterocolitis is "low‐certainty" because of concerns that the effect could have been biased by small trials with unreliable methods.
Summary of findings
Background
The intestinal microbiome may play an important role in the pathogenesis of necrotising enterocolitis (NEC) (Embleton 2017). Probiotics are microorganisms that benefit the host by modulating the intestinal microbiome and promoting mucosal barrier functions and resistance to pathogens. Dietary supplementation with probiotics has been proposed as a strategy to reduce the risk of NEC and associated morbidity and mortality in very preterm or very low birth weight infants (VLBW) infants.
Description of the condition
Necrotising enterocolitis, a syndrome of acute intestinal necrosis of unknown aetiology, affects about 5% of very preterm or VLBW infants (Horbar 2012). The major risk predictors for NEC include being extremely preterm or extremely low birth weight (ELBW), and having evidence of intrauterine growth restriction or absent or reversed end‐diastolic flow velocities in Doppler studies of the foetal aorta or umbilical artery (Samuels 2017). Infants who develop NEC experience more infections, have lower levels of nutrient intake, grow more slowly, and have longer durations of intensive care and hospital stay than gestation‐comparable infants who do not (Battersby 2018; Berrington 2012). The associated mortality rate is about 20%, and infants who develop NEC, especially if associated with bloodstream infections, have a higher risk of neurodevelopmental problems and disability compared with their peers (Hickey 2018; Martin 2010).
The pathogenesis of NEC remains incompletely understood but is thought to involve intestinal dysbiosis, infection and inflammation (Eaton 2017; Mara 2018; Morgan 2011). Emerging evidence supports the theory that the intestinal microbiome affects the risk of developing NEC (Masi 2019; Olm 2019; Stewart 2012; Warner 2016). Most very preterm or VLBW infants who develop NEC have received enteral milk feeds. Feeding with human milk rather than cow's milk formula reduces the risk of NEC (Quigley M 2019). One putative mechanism for this protective effect is that “prebiotic” substances in human milk promote the growth of non‐pathogenic probiotic microorganisms, predominantly lactobacilli and bifidobacteria, that modulate the intestinal microbiome and promote mucosal barrier functions (Embleton 2017; Granger 2020; Walsh 2019). Compared with human milk‐fed term infants, however, very preterm or VLBW infants typically harbour fewer probiotic microorganisms and more potential pathogens such as enterococci and Enterobacteriaceae, which might be due to dysbiotic effects of enteral fasting and antibiotic exposure (Stewart 2017).
Given the putative role of probiotics in maintaining the structure, integrity, and function of the intestinal barrier, the possibility that supplemental probiotics might be effective in preventing NEC is of considerable research interest (Berrington 2019; Patel 2018).
Description of the intervention
The probiotic preparations used most commonly as enteral supplements contain one or more strains of bacteria (typically bifidobacteria or lactobacilli) or the fungus Saccharomyces boulardii (Thomas 2010). Other bacteria with probiotic properties include Bacillus clausii,Enterococcus faecium, and Streptococcus thermophilus. Exogenous probiotics can colonise the mucosal surface of the human gastrointestinal tract (Abdulkadir 2016; Zmora 2018). A range of probiotic supplements, as single‐ or multiple‐strain preparations, are available commercially and have been used to prevent and treat infectious or inflammatory gastrointestinal conditions in adults. Despite biological plausibility and underpinning pre‐clinical studies, however, evidence for benefit remains low certainty for most conditions (Bron 2017; Koretz 2018; Kunk 2019; Lerner 2019; Suez 2019). Furthermore, serious, unexpected adverse events and outcomes have been associated with probiotic supplementation for critically‐ill adults (Besselink 2008; Boyle 2006).
Probiotics for very preterm infants
Policies and practices for the use of probiotic supplements to prevent NEC in very preterm or VLBW infants vary within and between countries (Duffield 2019; Viswanathan 2016). Parents have expressed willingness to consider use of probiotics for their very preterm or VLBW infants if evidence of benefit and safety exists (Sesham 2014). Enteral administration of commercially‐available supplements of lyophilised probiotic microorganisms, usually multi‐species preparations containing lactobacilli or bifidobacteria or both, is established in some settings (Robertson 2020). Routine use outwith trials, however, remains limited because of uncertainty about the optimal constitution of preparations (strains of microorganisms and dosing strategies), quality control and safety issues including contamination of products with potential pathogens, and national licensing processes and regulatory requirements (Berrington 2019; Fleming 2019; Pell 2019; van den Akker 2020; Vermeulen 2020). Although probiotic supplementation in immuno‐competent adults is considered to be safe, exogenous probiotic microorganisms have been reported as causing bacteraemia or fungaemia in very preterm or VLBW infants (Bertelli 2015; Esaiassen 2016; Jenke 2012; Zbinden 2015).
How the intervention might work
Intestinal probiotic microorganisms are thought to exert their beneficial effects via several mechanisms. Probiotics may out‐compete pathogens for nutrients and limit pathogen growth via production of inhibitory organic acids ("post‐biotics") and antimicrobial compounds (Embleton 2017; Patel 2015). Infants supplemented with probiotics harbour fewer potential pathogens in the intestine (Alcon‐Giner 2020). Other putative actions include stimulating differentiation and proliferation of enterocytes, enhancing expression of intestinal digestive enzymes, and improving intestinal mucosal barrier integrity (Bron 2017; Johnson‐Henry 2016; Sanders 2019).
Why it is important to do this review
Necrotising enterocolitis and associated complications, particularly infections, are the commonest causes of mortality and serious morbidity beyond the early neonatal period in very preterm or VLBW infants (Berrington 2012). Since probiotic supplementation might reduce the risk of NEC, appraising and synthesising the trial evidence about the effectiveness and safety of probiotic supplementation could inform practice, policy, and research (Embleton 2016; Quigley E 2019). Currently, international policy statements that exist to guide practice do not make unconditional recommendations for use of any probiotic combination for very preterm or VLBW infants (Marchand 2012; van den Akker 2020).
Objectives
To determine the effect of supplemental probiotics on the risk of necrotising enterocolitis (NEC) and mortality and morbidity in very preterm or very low birth weight (VLBW) infants.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs.
Types of participants
We included very preterm (< 32 weeks' gestation) or extremely low birth weight (VLBW)(< 1500 g) infants (pre‐specified analyses for extremely preterm (< 28 weeks' gestation) or extremely low birth weight (ELBW) (< 1000 g) infants).
Types of interventions
We included enteral administration of any probiotic or probiotic combination for at least one week compared to placebo or no treatment.
We categorised probiotic preparations at the genus level (Bifidobacterium spp.,Lactobacillus spp.,Sacchromyces spp., Streptococcal spp., others, and combinations thereof).
Types of outcome measures
Primary outcomes
-
Necrotising enterocolitis (NEC), confirmed at surgery or autopsy or diagnosed by at least two of the following clinical features (Walsh 1986):
abdominal radiograph showing pneumatosis intestinalis or gas in the portal venous system or free air in the abdomen;
abdominal distension with abdominal radiograph with gaseous distension or frothy appearance of bowel lumen (or both);
blood in stool;
lethargy, hypotonia or apnoea (or combination of these).
All‐cause mortality before discharge from hospital.
Secondary outcomes
Late‐onset invasive infection, as determined by culture of bacteria or fungus from blood or cerebrospinal fluid or from a normally sterile body space (> 48 hours after birth).
Late‐onset infection with the supplemented probiotic microorganism.
Duration of hospitalisation (days).
Neurodevelopmental impairment assessed by a validated test after 12 months' post‐term: neurological evaluations, developmental scores, and classifications of disability, including cerebral palsy and auditory and visual impairment.
Search methods for identification of studies
We used the criteria and standard methods of Cochrane Neonatal.
Electronic searches
We used the standard search strategy of Cochrane Neonatal to search Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 2) in the Cochrane Library; MEDLINE Ovid (1946 to 17 Feb 2020), Embase Ovid (1974 to 17 Feb 2020), Maternity & Infant Care Database Ovid (1971 to 17 Feb 2020), the Cumulative Index to Nursing and Allied Health Literature (1982 to 18 Feb 2020), and clinical trials databases, and conference proceedings (see Appendix 1 for the full search strategies for each database). We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International Trials Registry and Platform, and the ISRCTN Registry).
Searching other resources
We searched the reference lists of any articles selected for inclusion in this review.
Data collection and analysis
We used the standard methods of Cochrane Neonatal.
Selection of studies
One review author (SS) screened titles and abstracts of all records identified by the search and coded records as "order" or "exclude". A second review author (WM) assessed all records coded as "order" and made the final decision about which records were ordered as full‐text articles. SS and SO read the full texts and used a checklist to assess each article's eligibility for inclusion on the basis of pre‐specified inclusion and exclusion criteria. WM checked these decisions.
Data extraction and management
Two review authors (SS and WM or SO) extracted data independently using a data collection form to aid extraction of information on design, methods, participants, interventions, outcomes, and treatment effects from each included study. We discussed disagreements until we reached consensus. If data from the trial reports were insufficient, we contacted trialists for further information.
Assessment of risk of bias in included studies
Two review authors (SS and WM or SO), independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2011) for the following domains.
Sequence generation (selection bias).
allocation concealment (selection bias).
blinding of participants and personnel (performance bias).
blinding of outcome assessment (detection bias).
incomplete outcome data (attrition bias).
We resolved any disagreements by discussion or by a third assessor. See Appendix 2 for a description of risk of bias for each domain.
Measures of treatment effect
We analysed treatment effects in the individual trials using Review Manager 5 (Review Manager 2020), and reported risk ratios (RRs) and risk differences (RDs) for dichotomous data, and mean differences (MDs) for continuous data, with respective 95% confidence intervals (CIs). We determined the number needed to treat for one additional beneficial outcome (NNTB) for analyses with a statistically significant difference in the RD.
Unit of analysis issues
The unit of analysis was the participating infant in individually‐randomised trials. For cluster‐randomised trials, we undertook analyses at the level of the individual while accounting for inter‐cluster correlations in the data using methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). Cross‐over studies were not eligible for inclusion.
Dealing with missing data
We requested additional data from trial investigators when data on important outcomes were missing or were reported unclearly. If unavailable, we planned to undertake sensitivity analyses to assess the potential impact of missing outcome data.
Assessment of heterogeneity
We examined treatment effects in individual trials and heterogeneity between trial results by inspecting the forest plots if more than one trial was included in a meta‐analysis. We calculated the I² statistic for each analysis to quantify inconsistency across studies and to describe the percentage of variability in effect estimates that may be due to heterogeneity rather than to sampling error. If we detected moderate (I² > 50%) or high (I² > 75%) heterogeneity, we planned to explore possible causes (differences in study design, participants, interventions, or outcome assessments).
Assessment of reporting biases
We assessed funnel plot asymmetry visually and with Harbord's modification of Egger's test in meta‐analyses with data from more than nine trials contributing events (Harbord 2006).
Data synthesis
We used a fixed‐effect model for meta‐analysis (as per Cochrane Neonatal recommendations). When moderate or high heterogeneity existed, we planned to examine the potential causes in subgroup (see below) and sensitivity (by methodological quality) analyses.
Subgroup analysis and investigation of heterogeneity
We planned to undertake subgroup analyses by:
genus of probiotics or combinations (Bifidobacterium spp.,Lactobacillus spp.,Sacchromyces spp., Streptococcal spp., others, and combinations thereof);
type of enteral feeding permitted for participating infants (human milk versus formula versus mixed).
Sensitivity analysis
We planned sensitivity analyses to determine how estimates were affected by including only studies at low risk of bias: (i) selection bias (adequate randomisation and allocation concealment), (ii) detection or performance bias (adequate masking of intervention and measurement), (iii) attrition bias (< 20% loss to follow‐up for primary outcome assessment), and (iv) reporting bias (selective reporting).
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence of the following (clinically relevant) outcomes: NEC, all‐cause mortality, late‐onset infection, and severe neurodevelopmental impairment.
Three review authors (WM, MXRR and SO) independently assessed the certainty of the evidence for each of the outcomes above. We considered evidence from RCTs as high certainty but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create two ‘Summary of findings’ tables to report the certainty of the evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence as one of four grades.
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: we are very uncertain about the estimate.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies
Results of the search
See Figure 1.
1.
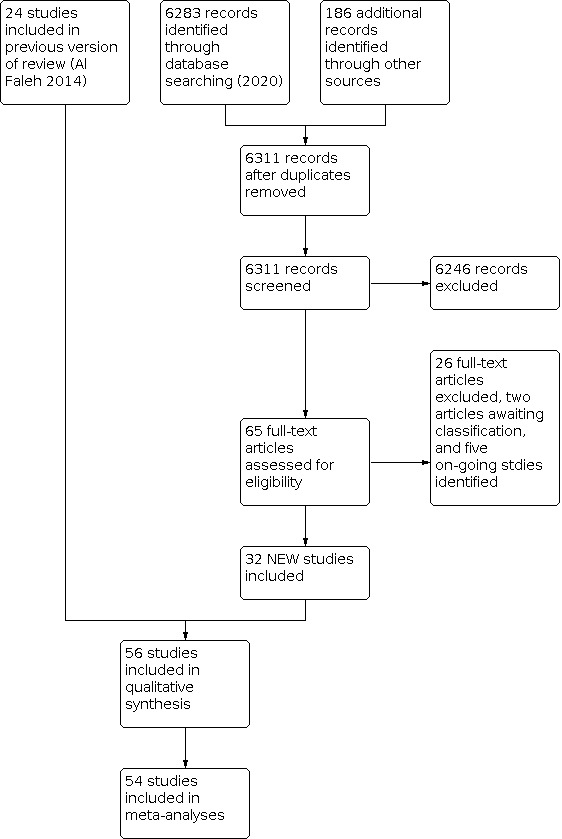
Study flow diagram: review update 2020
Included studies
See: Characteristics of included studies.
We included 56 trials. Most were conducted during the past 20 years (four trials pre‐2000). Geographical spread was wide, though predominantly in Europe (23 trials) and Asia (23 trials). Only one trial took place in sub‐Saharan Africa (Zeber‐Lubecka 2016).
Most trials occurred in single centres. Nine were multicentre (Al‐Hosni 2012; Costeloe 2015; Dani 2002; Dilli 2015; Hays 2015; Jacobs 2013; Lin 2008; Manzoni 2009; Totsu 2014).
In all but one of the trials, individual infants were allocated randomly to intervention or control groups. One trial, based in 19 neonatal units in Japan, used a cluster design, with the unit of randomisation being the neonatal unit (Totsu 2014).
Population
In total, 10,812 infants participated in the 56 included trials. The median number of participants in the trials was 149. Twenty‐one trials enrolled fewer than 100 participants. Twenty trials enrolled between 100 and 199 participants. Twelve trials enrolled between 200 and 499 participants. Three trials enrolled 500 participants or more: Costeloe 2015 (N = 1310); Dani 2002 (N = 585); Jacobs 2013 (N = 1099).
Most trials enrolled only very preterm or VLBW infants, with average birth weight among participants typically 1000 g to 1200 g, and average gestation at birth 28 to 32 weeks'. Eight trials enrolled infants of gestational age up to 34 weeks', or birth weight up to 1800 g (Chandrashekar 2018; Dashti 2014; Fujii 2006; Hernandez‐Enriquez 2016; Mohan 2006; Ren 2010; Strus 2018; Tewari 2015). Because the average gestation at birth was < 32 weeks', or the average birth weight < 1500 g, we included these trials.
Two trials restricted participation to extremely low birth weight (ELBW) infants (Al‐Hosni 2012; Wejryd 2019). Four trials excluded infants who were born with birth weight below the 10th percentile for the reference population ("small‐for‐gestation") (Al‐Hosni 2012; Hays 2015; Indrio 2017; Kitajima 1997). None of the trials specified exclusion of infants who had evidence of absent or reversed end‐diastolic flow velocities detected on antenatal Doppler studies of the foetal aorta or umbilical artery.
In most trials, participating infants were permitted human milk or formula feeding. Seven trials enrolled infants who received human milk only (Roy 2014; Samanta 2009; Shadkam 2015; Shashidhar 2017; Tewari 2015; Van Niekerk 2014; Wejryd 2019), and five trials enrolled only formula‐fed participants (Costalos 2003; Chrzanowska‐Liszewska 2012; Indrio 2017; Reuman 1986; Stratiki 2007).
Interventions and comparisons
The probiotic preparations tested varied. Thirty‐three trials used single‐genus probiotics (most commonly, Bifidobacterium spp. or Lactobacillus spp.), and 23 used multi‐genus combinations (most commonly, Bifidobacterium spp. plus Lactobacillus spp.). These were mostly commercially‐available products supplied by the manufacturer for use in the trial.
Bifidobacterium spp. (14 trials):
B. breve (Costeloe 2015; Fujii 2006; Hikaru 2010; Kitajima 1997; Li 2019; Patole 2014; Wang 2007);
B. lactis (Dilli 2015; Mihatsch 2010; Mohan 2006; Stratiki 2007);
B. bifidum (Totsu 2014);
B. adolescentis (Huang 2009);
B. lactis, or B. longum, or both (three intervention groups) (Hays 2015).
Lactobacillus spp. 13 trials):
L. rhamnosus (Agarwal 2003; Chrzanowska‐Liszewska 2012; Dani 2002; Manzoni 2006; Manzoni 2009; Millar 1993);
L. reuteri (Oncel 2014);
L. acidophilus (Reuman 1986).
Sacchromyces spp. (four trials):
Sacchromyces boulardii (Costalos 2003; Demirel 2013; Serce 2013; Zeber‐Lubecka 2016).
Bacillus spp. (two trials):
Bacillus clausii (Tewari 2015);
Bacillus coagulans* (Sari 2011).
(*Lactobacillus sporogenes in report.)
Bifidobacterium spp. plus Lactobacillus spp. (eight trials):
B. breve and L. casei (Yakult®) (Braga 2011);
B. bifidum, B. longum, B. infantis, L. rhamnosus, L. paracasei , L. casei, L. acidophilus, and L.latis (Cap TS6®) (Chowdhury 2016);
B. bifidum and L. acidophilus (Infloran®) (Lin 2005; Lin 2008; Saengtawesin 2014);
B. longum and L. rhamnosus (Rougé 2009);
B. longum, B. bifidum, B. lactis and L. acidophilus (Roy 2014);
B. longum, B. bifidum, B.infantis and L. acidophilus (Samanta 2009).
Bifidobacterium spp. plus Streptococcus spp. (two trials):
B. infantis, B. lactis and S. thermophilus (Jacobs 2013);
B. infantis, B. bifidum** and S. thermophilus (Bin‐Nun 2005).
(** Lactobacillus bifidus in report)
Bifidobacterium spp. plus Lactobacillus spp. plus Sacchromyces spp. (four trials):
B. infantis, L. rhamnosus, L. casei, L. plantarum, L acidophilus, and S. boulardii (Dutta 2015);
B. bifidum, L acidophilus, and S. boulardii (Hariharan 2016);
B. longum, L.acidophilus,L. rhamnosus, and S. boulardii (Chandrashekar 2018; Shashidhar 2017).
Bifidobacterium spp. plus Lactobacillus spp. plus Streptococcus spp. (five trials):
B longum, B. breve, L. acidophilus, L. rhamnosus, L. bulgaricus, L.casei, and S. thermophilus (Dashti 2014);
B. infantis, L. rhamnosus, L. casei, L. plantarum, L acidophilus, and S. thermophilus (Fernández‐Carrocera 2013);
B. infantis, L acidophilus, and Enterococcus faecium (Kanic 2015);
B. infantis, L. acidophilus, Enterococcus faecium, and Bacillus cereus (Ren 2010);
Bifidobacterium spp. (not specified), L. acidophilus, L. delbrueckii. and S. thermophilus (Rehman 2018).
Most trials initiated probiotic (and placebo if used) administration during the first week after birth, typically with the first enteral feed. The lyophilised probiotics were reconstituted in water or milk, and administered to supply 108 to 109 colony forming units per dose, once or twice daily via a gastric feeding tube. In most trials, the intervention period was at least six weeks, typically until 34 to 36 weeks' postmenstrual age, or until discharge from hospital. Eleven of the trials administered the intervention for a shorter period (from seven to 30 days) (Braga 2011; Costalos 2003; Dutta 2015; Huang 2009; Kitajima 1997; Millar 1993; Mohan 2006; Ren 2010; Reuman 1986; Shadkam 2015; Van Niekerk 2014). One trial continued the intervention until the infant reached 2000 g body weight (Totsu 2014).
Outcomes
Fifty‐four trials reported the number of infants who developed NEC, and 51 trials reported mortality prior to hospital discharge. Forty‐seven trials reported (or provided unpublished data) the number of infants with at least one episode of culture‐confirmed infection. Other in‐hospital outcomes reported included time to establish full enteral feeding, rate of weight gain, and duration of hospital stay (22 trials). Six trials reported neurodevelopmental or cognitive outcomes (Jacobs 2013; Lin 2005; Oncel 2014; Sari 2011; Totsu 2014; Patole 2014). Two trials did not report any of the review outcomes (Agarwal 2003; Li 2019).
Excluded studies
We excluded 26 reports of studies (Characteristics of excluded studies). The most common reasons for exclusion were ineligible population (most participants not very preterm, or VLBW), intervention (prebiotics or synbiotics) and design (not randomised). A further four screened articles were secondary reports for included trials.
Risk of bias in included studies
Methodological quality varied between the included trials (Risk of bias in included studies; Figure 2).
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Twenty‐five of the 56 trials were assessed as being a low risk of selection bias. These employed adequate methods to generate the random sequence, typically computer‐generated, and methods to conceal allocation, typically central or pharmacy allocation, or storage of allocation codes in sealed envelopes (we did not mandate that reports stated that envelopes were "opaque"). Randomisation and allocation concealment methods were not stated in 26 trial reports (unclear risk of bias), and in five "quasi‐randomised" trials, alternate allocation was used (high risk of bias).
Blinding
Twenty‐five trials were assessed as being a low risk of performance bias and detection bias. These were placebo‐controlled (usually maltodextrin), or the report or investigators indicated that preparation of the intervention (mixing the probiotic in milk) was undertaken by staff who were not directly involved in other caregiving duties or outcome assessments (for example, pharmacy staff). In 13 trials, control infants received milk feeds without probiotic supplements, but it was unclear whether staff were aware of the group allocation (unclear risk of bias). Eighteen trials were at high risk of bias due to absence of any masking measures.
Incomplete outcome data
Attrition bias does not appear to be an issue in most trials (outcome data reported for > 80% of randomised cohorts).
Selective reporting
Most reports did not provide access to the trial protocol. It is unlikely, however, that reporting bias was an issue in most trials (low risk of bias) where the review primary and infant‐important outcomes were reported. In trials where the aim was to assess surrogate outcomes such as stool colonisation or intestinal permeability, clinical outcome data were generally available from the investigators.
Effects of interventions
Summary of findings 1. Probiotics compared to control in very preterm or very low birth weight infants.
| Probiotics compared to control in very preterm or very low birth weight infants | ||||||
| Patient or population: very preterm or very low birth weight infants Setting: neonatal care centres globally Intervention: probiotics Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Sensitivity analysis of trials at low risk of bias | |
| Risk with control | Risk with Probiotics | |||||
| Necrotising enterocolitis (before hospital discharge) | Study population | RR 0.54 (0.45 to 0.65) | 10,604 (54 studies) | ⊕⊕⊝⊝ Lowa,b | Sensitivity meta‐analysis of 16 trials (4597 infants) at low risk of bias showed a reduced risk of NEC: RR 0.70, 95% CI 0.55, 0.89 (I² = 25%) | |
| 61 per 1000 | 33 per 1000 (27 to 40) | |||||
| Mortality (all‐cause before hospital discharge) | Study population | RR 0.76 (0.65 to 0.89) | 10,170 (51 studies) | ⊕⊕⊕⊝ Moderatea | Sensitivity meta‐analysis of 16 trials (4597 infants) at low risk of bias did not show an effect: RR 0.86, 95% CI 0.69, 1.07 (I² = 0%) | |
| 65 per 1000 | 49 per 1000 (42 to 58) | |||||
| Invasive infection (before hospital discharge) | Study population | RR 0.89 (0.82 to 0.97) | 9762 (47 studies) | ⊕⊕⊕⊝ Moderatea | Sensitivity meta‐analysis of 16 trials (4597 infants) at low risk of bias did not show an effect: RR 0.90, 95% CI 0.79, 1.02 (I² = 8%) | |
| 173 per 1000 | 154 per 1000 (142 to 168) | |||||
| Severe neurodevelopmental impairment (18 months to 3 years) | Study population | RR 1.03 (0.84 to 1.26) | 1518 (5 studies) | ⊕⊕⊝⊝ Lowa,c | Sensitivity meta‐analysis of two trials (913 infants) at low risk of bias did not show an effect: RR 0.99, 95% CI 0.76, 1.27 (I² = 0%) | |
| 194 per 1000 | 200 per 1000 (163 to 245) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for serious study limitations (high risk of bias due to uncertainty about methods used to generate random sequence, conceal allocation, and mask outcome assessment) in 12 trials
bDowngraded one level for serious publication bias (funnel plot asymmetry and statistical evidence consistent with trial size; trials favouring controls missing)
cDowngraded one level for serious imprecision of effect estimate (95% CI around estimate consistent with substantial harm or benefit)
Summary of findings 2. Probiotics compared to control in extremely preterm or extremely low birth weight infants.
| Probiotics compared to control in extremely preterm or extremely low birth weight infants | |||||
| Patient or population: extremely preterm or extremely low birth weight infants Setting: neonatal care centres globally Intervention: probiotics Comparison: control | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with control (extremely preterm or ELBW) | Risk with Probiotics | ||||
| Necrotising enterocolitis (before hospital discharge) | Study population | RR 0.90 (0.68 to 1.21) | 1712 (8 studies) | ⊕⊕⊝⊝ Low,a,b | |
| 100 per 1000 | 90 per 1000 (68 to 121) | ||||
| Mortality (before hospital discharge) | Study population | RR 0.91 (0.71 to 1.16) | 1661 (6 studies) | ⊕⊕⊝⊝ Low,a,b | |
| 137 per 1000 | 124 per 1000 (97 to 159) | ||||
| Invasive infection (before hospital discharge) | Study population | RR 0.90 (0.76 to 1.06) | 1471 (6 studies) | ⊕⊕⊝⊝ Low,a,b | |
| 282 per 1000 | 254 per 1000 (214 to 299) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level for serious study limitations due to high risk of bias (uncertainty about methods used to generate random sequence, conceal allocation, and mask assessments) in many trials
bDowngraded one level for serious imprecision of effect estimate (95% CI around estimate consistent with substantial harm or benefit)
Comparison 1. Probiotics versus control
Primary outcomes
Necrotising enterocolitis
Meta‐analysis of data from 54 trials (10,604 infants) showed a reduced risk of NEC (Analysis 1.1; Figure 3):
1.1. Analysis.

Comparison 1: Probiotics versus control, Outcome 1: Necrotising enterocolitis
3.

Forest plot of comparison: 1 Probiotics versus control, outcome: 1.1 Necrotising enterocolitis.
Risk ratio (RR) 0.54, 95% confidence interval (CI) 0.45 to 0.65 (I² = 17%);
Risk difference (RD) ‐0.03, 95% CI ‐0.04 to ‐0.02;
NNTB 33; 95% CI 25 to 50.
There was statistically significant evidence of funnel plot asymmetry consistent with trials favouring controls missing from the meta‐analysis (Harbord's modified Egger test for bias ‐0.78, 95% CI ‐1.51 to ‐0.06; P = 0.04) (Figure 4).
4.

Funnel plot of comparison: 1 Probiotics versus control, outcome: 1.1 Necrotising enterocolitis.
We assessed the certainty of evidence as "low" using GRADE approach, downgraded for serious study design limitations and serious risk of publication bias (Table 1).
Mortality
Meta‐analysis of data from 51 trials (10,170 infants) showed a reduced risk of mortality (Analysis 1.2; Figure 5):
1.2. Analysis.

Comparison 1: Probiotics versus control, Outcome 2: Mortality
5.

Forest plot of comparison: 1 Probiotics versus control, outcome: 1.2 Mortality.
RR 0.76, 95% CI 0.65 to 0.89 (I² = 0%);
RD ‐0.02, 95% CI ‐0.02 to ‐0.01;
NNTB 50; 95% CI 50 to 100.
There was some evidence of funnel plot asymmetry (Harbord's modified Egger test for bias ‐0.52, 95% CI ‐1.15 to 0.12, P = 0.11) (Figure 6).
6.

Funnel plot of comparison: 1 Probiotics versus control, outcome: 1.2 Mortality.
We assessed the certainty of evidence as "moderate" using GRADE approach, downgraded for serious study design limitations (risk of bias in included trials) (Table 1).
Secondary outcomes
Invasive infection
Meta‐analysis of data from 47 trials (9762 infants) showed a reduced risk of infection (Analysis 1.3):
1.3. Analysis.

Comparison 1: Probiotics versus control, Outcome 3: Invasive infection
RR 0.89, 95% CI 0.82 to 0.97 (I² = 19%);
RD ‐0.02, 95% CI ‐0.03 to ‐0.01;
NNTB 50; 95% CI 33 to 100.
There was no evidence of funnel plot asymmetry (Harbord's modified Egger test for bias ‐0.07, 95% CI ‐0.86 to 0.73, P = 0.86).
We assessed the certainty of evidence as "moderate" using GRADE approach, downgraded for serious study design limitations (risk of bias in included trials).
Late‐onset infection with the supplemented probiotic microorganism
None of the included studies reported invasive infection caused by the supplemented probiotic microorganisms.
Duration of birth hospitalisation
Meta‐analysis of data from 22 trials (5458 infants) showed a shorter duration of hospitalisation (Analysis 1.4):
1.4. Analysis.

Comparison 1: Probiotics versus control, Outcome 4: Duration of birth hospitalisation (days)
MD ‐1.93 days, 95% CI ‐3.78 to ‐0.08 (I² = 26%).
There was no evidence of funnel plot asymmetry.
Two other trials reported data that could not be meta‐analysed:
Oncel 2014 reported shorter median duration of hospitalisation (38 versus 46 days);
Tewari 2015 reported no difference in duration of hospitalisation.
Neurodevelopmental outcomes
Neurodevelopmental impairment
Five trials reported severe neurodevelopmental impairment (either motor, sensory, or cognitive) in surviving children. Three assessed children using Bayley Scales of Infant Development II (BSID‐II) at 18 to 24 months (Oncel 2014; Sari 2011), or three years (Lin 2005) post‐term. One trial assessed Bayley‐III composite scales, Movement Assessment Battery for Children, and Wechsler Preschool and Primary Scale of Intelligence Full Scale Intelligence Quotient at two to five years (Jacobs 2013). One trial, undertaken in Japan, used the Kyoto Scale of Psychological Development 2001 (similar to the Bayley III scales) and physical examination to assess neurodevelopmental status at 18 months' post‐term (Totsu 2014).
Completeness of neurodevelopmental follow‐up assessment varied (balanced between groups in all trials):
Lin 2005: 90%;
Sari 2011: 84%;
Totsu 2014: 73%;
Oncel 2014: 68%;
Jacobs 2013: 48%.
None of the individual trials, nor a meta‐analysis of data from five trials (1518 infants) showed an effect (Analysis 1.5);
1.5. Analysis.

Comparison 1: Probiotics versus control, Outcome 5: Severe neurodevelopmental impairment
RR 1.03, 95% CI 0.84 to 1.26 (I² = 0%).
We assessed the certainty of evidence as "low" using GRADE approach, downgraded for serious study design limitations (including attrition bias) and for serious imprecision of effect estimate.
Cerebral palsy
None of the individual trials, nor a meta‐analysis of data from five trials (1512 infants) showed an effect (Analysis 1.6):
1.6. Analysis.

Comparison 1: Probiotics versus control, Outcome 6: Cerebral palsy
RR 1.13, 95% CI 0.74 to 1.72 (I² = 18%).
Visual impairment
None of the individual trials, nor a meta‐analysis of data from four trials (1356 infants) showed an effect (Analysis 1.7):
1.7. Analysis.

Comparison 1: Probiotics versus control, Outcome 7: Visual impairment
RR 0.50, 95% CI 0.14 to 1.80 (I² = 0%).
Hearing impairment
None of the individual trials, nor a meta‐analysis of data from four trials (1356 infants) showed an effect (Analysis 1.8):
1.8. Analysis.

Comparison 1: Probiotics versus control, Outcome 8: Hearing impairment
RR 0.46, 95% CI 0.18 to 1.17 (I² = 32%).
Cognitive performance
Patole 2014 assessed 42% of eligible participants aged three to five years using the Mullen's Scale of Early Learning tool. Analysis did not show an effect on the "early learning composite score" (Analysis 1.9):
1.9. Analysis.

Comparison 1: Probiotics versus control, Outcome 9: Continuous early learning composite measure
RR ‐1.00 (95% CI ‐6.38, 4.38).
Probiotics versus control in extremely preterm or ELBW infants
Two trials restricted participation to ELBW infants (Al‐Hosni 2012; Wejryd 2019). Five trials reported subgroup data for extremely preterm or ELBW infants (Costeloe 2015; Jacobs 2013; Oncel 2014; Roy 2014; Tewari 2015; Wang 2007).
Necrotising enterocolitis
Meta‐analysis of data from eight trials (1712 infants) did not show an effect (Analysis 2.1):
2.1. Analysis.

Comparison 2: Probiotics versus control (extremely preterm or ELBW), Outcome 1: Necrotising enterocolitis
RR 0.90, 95% CI 0.68 to 1.21 (I² = 0%).
We assessed the certainty of evidence as "low" using GRADE approach, downgraded one level for study limitations due to high risk of bias and one level for imprecision of effect estimate (Table 2).
Mortality
Meta‐analysis of data from six trials (1661 infants) did not show an effect (Analysis 2.2):
2.2. Analysis.

Comparison 2: Probiotics versus control (extremely preterm or ELBW), Outcome 2: Mortality
RR 0.91, 95% CI 0.71 to 1.16 (I² = 0%)
We assessed the certainty of evidence as "low" using GRADE approach, downgraded one level for serious study limitations due to high risk of bias and one level for serious imprecision of effect estimate (Table 2).
Invasive infection
Meta‐analysis of data from six trials (1471 infants) did not show an effect (Analysis 2.3):
2.3. Analysis.

Comparison 2: Probiotics versus control (extremely preterm or ELBW), Outcome 3: Invasive infection
RR 0.90, 95% CI 0.76 to 1.06 (I² = 0%)
We assessed the certainty of evidence as "low" using GRADE approach, downgraded one level for serious study limitations due to high risk of bias and one level for serious imprecision of effect estimate (Table 2).
Late‐onset infection with the supplemented probiotic microorganism
None of the included studies reported invasive infection caused by the supplemented probiotic microorganisms.
Duration of birth hospitalisation
Analysis of data from one trial (22 infants) did not show an effect:
MD ‐5.40 days, 95% CI ‐14.20 to 3.40)
Neurodevelopmental outcomes
None of the trials reports provided subgroup data for meta‐analysis. Three reports stated that there was not an effect of probiotics on the rate of severe neurodevelopmental impairment in the extremely preterm or ELBW subgroup (Jacobs 2013; Sari 2011; Totsu 2014).
Subgroup comparison by genus of probiotics
Necrotising enterocolitis
There was some evidence of subgroup differences depending on genus of probiotics (Chi² = 11.23, df = 7 (P = 0.13), I² = 37.7%; Analysis 1.1; Figure 3). The largest effect size estimates favoured trials using combinations of:
Lactobacillus spp.
Bifidobacterium spp. plus Lactobacillus spp.
Bifidobacterium spp. plus Streptococcus spp.
Bifidobacterium spp. plus Lactobacillus spp. plus Streptococcus spp.
Mortality
There was no evidence of subgroup differences depending on genus of probiotics (Chi² = 4.40, df = 7 (P = 0.73), I² = 0%; Analysis 1.2; Figure 5).
Invasive infection
There was no evidence of subgroup differences depending on genus of probiotics (Chi² = 2.57, df = 7 (P = 0.92), I² = 0%; Analysis 1.3).
Duration of birth hospitalisation
There was no evidence of subgroup differences depending on genus of probiotics (Chi² = 2.56, df = 6 (P = 0.86), I² = 0%; Analysis 1.4).
Neurodevelopmental outcomes
Neurodevelopmental impairment
There was no evidence of subgroup differences depending on genus of probiotics (Chi² = 1.48, df = 4 (P = 0.83), I² = 0%; Analysis 1.5).
Cerebral palsy
There was no evidence of subgroup differences depending on genus of probiotics (Chi² = 3.66, df = 4 (P = 0.45), I² = 0%; Analysis 1.6).
Visual impairment
There was no evidence of subgroup differences depending on genus of probiotics (Chi² = 1.59, df = 2 (P = 0.45), I² = 0%; Analysis 1.7).
Hearing impairment
There was no evidence of subgroup differences depending on genus of probiotics (Chi² = 3.63, df = 3 (P = 0.30), I² = 17.4%; Analysis 1.8).
Subgroup comparison by type of enteral feed (human milk versus formula versus mixed)
Necrotising enterocolitis
There was no evidence of subgroup differences depending on the type of enteral feed (Chi² = 3.81, df = 2 (P = 0.15), I² = 47.6%; Analysis 3.1).
3.1. Analysis.

Comparison 3: Subgroup analysis by type of feeding, Outcome 1: Necrotising enterocolitis
Mortality
There was no evidence of subgroup differences depending on the type of enteral feed (Chi² = 2.80, df = 2 (P = 0.25), I² = 28.7%; Analysis 3.2).
3.2. Analysis.

Comparison 3: Subgroup analysis by type of feeding, Outcome 2: Mortality
Invasive infection
There was no evidence of subgroup differences depending on the type of enteral feed (Chi² = 3.45, df = 2 (P = 0.18), I² = 42.0%; Analysis 3.3).
3.3. Analysis.

Comparison 3: Subgroup analysis by type of feeding, Outcome 3: Invasive infection
Duration of birth hospitalisation
There was no evidence of subgroup differences depending on the type of enteral feed (Chi² = 1.98, df = 2 (P = 0.37), I² = 0%; Analysis 3.4).
3.4. Analysis.

Comparison 3: Subgroup analysis by type of feeding, Outcome 4: Duration of birth hospitalisation (days)
Neurodevelopmental outcomes
In all trials, participants may have received human milk, or formula, or both.
Sensitivity analyses by risk of bias
Necrotising enterocolitis
There was evidence of subgroup differences depending on risk bias (Chi² = 7.82, df = 2 (P = 0.02), I² = 74.4%). Sensitivity meta‐analysis of 16 trials (4597 infants) at low risk of bias showed a reduced risk of NEC (Analysis 4.1):
4.1. Analysis.

Comparison 4: Sensitivity analyses: Risk of bias, Outcome 1: Necrotising enterocolitis
RR 0.70, 95% CI 0.55, 0.89 (I² = 25%);
RD ‐0.02, 95% CI ‐0.03 to ‐0.01;
NNTB 50; 95% CI 33 to 100.
Mortality
There was no evidence of subgroup differences depending on risk of bias (Chi² = 3.41, df = 2 (P = 0.18), I² = 41.3%). Sensitivity meta‐analysis of 16 trials (4597 infants) at low risk of bias did not show an effect (Analysis 4.2):
4.2. Analysis.

Comparison 4: Sensitivity analyses: Risk of bias, Outcome 2: Mortality
RR 0.86, 95% CI 0.69, 1.07 (I² = 0%);
RD ‐0.01, 95% CI ‐0.03 to 0.00.
Invasive infection
There was some evidence of subgroup differences depending on risk of bias (Chi² = 4.62, df = 2 (P = 0.10), I² = 56.7%). Sensitivity meta‐analysis of 16 trials (4597 infants) at low risk of bias did not show an effect (Analysis 4.3):
4.3. Analysis.

Comparison 4: Sensitivity analyses: Risk of bias, Outcome 3: Invasive infection
RR 0.90, 95% CI 0.79, 1.02 (I² = 8%);
RD ‐0.02, 95% CI ‐0.04 to 0.00.
Duration of birth hospitalisation
There was no evidence of subgroup differences depending on risk of selection bias (Chi² = 1.30, df = 2 (P = 0.52), I² = 0%). Sensitivity meta‐analysis of six trials (2786 infants) at low risk of bias did not show an effect (Analysis 4.4):
4.4. Analysis.

Comparison 4: Sensitivity analyses: Risk of bias, Outcome 4: Duration of birth hospitalisation (days)
MD ‐2.44 days, 95% CI ‐5.76 to 1.29 (I² = 52%).
Neurodevelopmental outcomes
Neurodevelopmental impairment
There was no evidence of subgroup differences depending on risk of bias (Chi² = 0.30, df = 1 (P = 0.58), I² = 0%). Sensitivity meta‐analysis of two trials (913 infants) at low risk of bias did not show an effect (Analysis 4.5):
4.5. Analysis.

Comparison 4: Sensitivity analyses: Risk of bias, Outcome 5: Severe neurodevelopmental impairment
RR 0.99, 95% CI 0.76, 1.27 (I² = 0%);
RD 0.00, 95% CI ‐0.05 to 0.05.
Cerebral palsy
There was no evidence of subgroup differences depending on risk of bias (Chi² = 0.01, df = 1 (P = 0.92), I² = 0%). Sensitivity meta‐analysis of two trials (913 infants) at low risk of bias did not show an effect (Analysis 4.6):
4.6. Analysis.

Comparison 4: Sensitivity analyses: Risk of bias, Outcome 6: Cerebral palsy
RR 1.14, 95% CI 0.68, 1.92 (I² = 0%);
RD 0.01, 95% CI ‐0.02 to 0.04.
Visual impairment
There was no evidence of subgroup differences depending on risk of performance and detection bias (Chi² = 1.53, df = 1 (P = 0.22), I² = 34.6%). Sensitivity meta‐analysis of two trials (913 infants) at low risk of bias did not show an effect (Analysis 4.7):
4.7. Analysis.

Comparison 4: Sensitivity analyses: Risk of bias, Outcome 7: Visual impairment
RR 2.91, 95% CI 0.12, 71.21 (I² = not applicable);
RD 0.00, 95% CI ‐0.01 to 0.01.
Hearing impairment
There was no evidence of subgroup differences depending on risk of performance and detection bias (Chi² = 1.96, df = 1 (P = 0.16), I² = 48.9%). Sensitivity meta‐analysis of two trials (913 infants) at low risk of bias did not show an effect (Analysis 4.8):
4.8. Analysis.

Comparison 4: Sensitivity analyses: Risk of bias, Outcome 8: Hearing impairment
RR 0.30, 95% CI 0.09, 0.98 (I² = 60%); 0.30 [0.09, 0.98)
RD ‐0.02, 95% CI ‐0.03 to ‐0.00.
Discussion
Summary of main results
Meta‐analyses of data from more than 50 trials, with more than 10,000 participants in total, show that enteral supplementation with probiotics may reduce the risk of NEC, and probably reduces mortality and the risk of late‐onset invasive infection in very preterm or VLBW infants. Sensitivity meta‐analyses of trials at low risk of bias did not show effects on mortality or infection. None of our included studies reported instances of invasive infection caused by the probiotic organisms being tested. Meta‐analyses of data available from five trials do not show an effect on severe neurodevelopmental impairment. According to GRADE assessment, the certainty of the evidence in this review is low to moderate.
Overall completeness and applicability of evidence
Most of the trials were undertaken within the past 20 years in healthcare facilities internationally, but predominantly in Europe and Asia. Few data were available from trials conducted in sub‐Saharan Africa. The findings should be applicable to current care practices for very preterm or VLBW infants including infants 'small for gestation' at birth (only four trials excluded such infants, and none defined evidence of abnormal end‐diastolic flow velocities in fetal Doppler studies as an exclusion criterion). The average event rate for NEC in the control group was 6%, consistent with estimates from prevalence studies in very preterm of VLBW infants in high‐income countries (Battersby 2018). We pre‐specified a comparison including only data for extremely preterm or ELBW infants. Only two small trials, however, restricted participation to this population, and a further five trials reported subgroup data. Meta‐analyses included fewer than 1800 infants, and did not show effects on any of the review outcomes. These estimates are imprecise due to few participants being included in meta‐analyses. The wide confidence intervals around the point estimates do not rule out important benefits or harms in this subpopulation, and are consistent with the effects seen in the meta‐analyses including the entire very preterm or VLBW population.
The review findings are likely to be broadly applicable to infants fed enterally with human milk or formula or both. Formula feeding increases risk of NEC and the risk‐benefit balance of probiotic supplementation could differ between human milk‐ and formula‐fed very preterm or VLBW infants (Quigley M 2019). Pre‐specified subgroup analyses did not show differences in effect sizes between trials that permitted only human milk feeding for participants (seven trials), versus trials in which all infants received only formula (five trials), versus those trials in which infants could be fed with human milk or formula or both (42 trials). The reported data in trials that permitted human milk‐ or formula‐feeding or both were insufficient to analyse subgroups effects at an infant level by type of enteral feeds received.
The main challenge in applying the findings of this review is the heterogeneity of the interventions tested. Subgroup analyses showed some evidence of differences in effect sizes depending on the genus of the probiotics used, with larger effects in trials that used combinations of bifidobacteria and lactobacilli (with or without S. thermophilus). Data from the only two large (> 1000 participants), high‐quality trials support this interpretation (Costeloe 2015; Jacobs 2013). The largest trial of probiotic supplementation yet reported (N = 1310) showed that a single‐strain preparation of Bifidobacterium breve is probably ineffective in reducing NEC (Costeloe 2015). Conversely, the combination of Bifidobacterium infantis, Streptococcus thermophilus and B. lactis used in the other large trial (N = 1099) is probably effective in reducing the risk of NEC (but not mortality or infection) (Jacobs 2013). These findings, although consistent with recent network analyses of different probiotic combinations, should be interpreted cautiously (Bi 2019; Morgan 2020; van den Akker 2018). As indirect comparisons are not randomised, any differences in effect between trials or groups of trials could be due to other factors, including methodological quality, types of participants, setting, and other practices and policies such as feeding protocols and antibiotic stewardship. Effect estimates may be confounded by species and strain level differences that affect how probiotic organisms interact with each other and endogenous microorganisms in the intestine of immature infants (Millar 2012). Consequently, the optimal probiotic composition or combination is unlikely to be determined reliably by analyses of the existing trial data.
Quality of the evidence
We assessed, using GRADE approach, the certainty of evidence as low or moderate for the pre‐specified outcomes (Table 1; Table 2). About half of the trials had methodological quality weaknesses, including in measures used to conceal random allocation and to mask clinicians, parents, and caregivers to the intervention (Figure 2), increasing the risk of bias in outcomes assessment. Knowledge of the intervention group could have affected caregivers' or assessors' subjective perceptions of outcomes, for example, it may have influenced decisions on whether investigate or diagnose NEC or invasive infection.
Most of the included trials were small (median N = 149). The asymmetry evident in the funnel plot for the meta‐analysis of the effect on NEC (and mortality to a lesser extent) was consistent with small‐study bias (Figure 4). One explanation is publication bias ‐ the tendency for articles that report "statistically significant" effects to be submitted and accepted for publication (Gale 2020). Publication bias, as well as other sources of small‐study bias, has become increasingly evident as an important contributor to exaggerated effect size estimates in meta‐analyses of interventions to improve outcomes in very preterm or VLBW infants (Ohlsson 2020; Pammi 2020). Another concern is that in many of the trials that aimed to assess the effect of probiotics on clinical outcomes, it is unclear from most reports how the sample size was defined, and whether trial "stopping rules" existed. If trial investigators were able to monitor accumulating outcome data until an effect on an outcome was detected, this may result a tendency to detect spurious effects that inflate the pooled estimate of effect sizes.
Attrition bias, due to loss of outcome data from randomised participants, was not a concern for the in‐hospital outcomes (NEC, death, infection) assessed in this review. Completeness of long‐term neurodevelopmental outcomes data, however, ranged from 48% to 90% between the trials that reported such assessments. The degree of incomplete "follow up" assessment was balanced across the intervention and control groups in each trial. Although this is reassuring with regard to the impact of attrition bias on effect estimates, some concern remains that the assessed population may not be representative of the entire cohort (Tin 1998). The findings in meta‐analyses that probiotics does not affect neurodevelopmental outcomes are consequently of 'low‐certainty'.
Potential biases in the review process
The main concern with meta‐analysis of the effect on NEC is the possibility that the findings are subject to small‐study biases, including publication bias. There may be a greater availability of data for inclusion in meta‐analyses from trials which reported statistically significant or potentially important effects (Hopewell 2009). We attempted to minimise this threat by searching the proceedings of major international perinatal conferences to identify trial reports that were not published in full form in journals. We cannot be sure that other trials have been undertaken but not reported, and the concern remains that such trials are less likely than published trials to have detected statistically significant or clinically important effects.
We contacted trial investigators for unpublished data (Young 2011). In several cases, authors of "proof of concept" or exploratory trials that aimed primarily to assess whether probiotic administration affected intestinal (stool) colonisation patterns or permeability or immune function were able to provide unpublished clinical outcomes data for inclusion in meta‐analyses.
We did not include any potential risk of bias due to the funding source of the included trials (where reported). In related contexts, such as manufacturers of breast milk substitutes funding infant feeding trials, this conflict is important to note (Cleminson 2015). We did not, however, consider this to be a substantial risk of bias here. Manufacturers of probiotic products supported some of the trials by supplying the intervention at no or low cost (noted in Characteristics of included studies), but we considered that they were unlikely to have a conflict of interest in the trial outcome for this relatively niche indication.
Agreements and disagreements with other studies or reviews
Our findings are broadly consistent with other recent systematic reviews of probiotics for preterm infants (summarised in Jarrett 2019). Our review differs from others in some key respects:
we restricted the population of interest to very preterm and VLBW infants to enhance applicability to those infants at high risk of developing NEC and associated complications;
we included trials that assessed probiotics only, and excluded trials that assessed prebiotics or synbiotics;
we conducted genus‐level subgroup analyses to explore for differences in effect sizes depending upon the probiotic or combination of probiotics assessed;
we included formal statistical evaluation to assess the risk of small‐study bias for the major outcomes;
we pre‐specified sensitivity analyses to determine how trial methodological quality affected effect sizes; and
we included a formal GRADE assessment of the 'certainty' of the evidence at outcomes level to help inform policy, practice, and research (Gephart 2020).
Authors' conclusions
Implications for practice.
Despite the quantity of trial evidence, and the effects shown on necrotising enterocolitis, mortality, and infection, uncertainty remains about how to interpret and apply the trial data of probiotic supplementation for very preterm or VLBW infants. As well as concern that effect size estimates are inflated by biases in the existing trials (including publication bias), the major barrier to implementing the findings is that existing analyses are not able to determine reliably the optimal constitution of probiotics (strains, doses, timing of introduction, duration of use) for routine prophylactic use. A variety of commercially‐available probiotic preparations are in use in a minority of neonatal units internationally, but widespread use appears to be limited by availability and regulatory and licensing issues. Although the data from the included trials are reassuring with regard to safety, probiotic bacteraemia or fungaemia and other adverse effects have been reported in preterm infants (Bertelli 2015; Esaiassen 2016; Jenke 2012; Zbinden 2015). It remains unclear whether different strains or combinations have different safety profiles.
Implications for research.
Given the uncertainty about whether (and which) probiotics affect important outcomes in very preterm or VLBW infants, consideration could be given to further assessment in randomised placebo‐controlled trials. It is essential, firstly, for investigators to determine whether families and clinicians would support a trial of this intervention. Any planned trials should attempt to ensure that caregivers and assessors are masked to the intervention as investigation and diagnosis of important outcomes such as NEC, invasive infection and neurodevelopmental impairment can be subjective. While it may be appropriate to be broadly inclusive of very preterm and VLBW infant participants, trials should ensure sufficient power to assess effects in extremely preterm or ELBW infants, and to explore interactions with the type of enteral feed received.
A key concern in planning any trial is choosing the appropriate intervention to assess. Two options appear favourable. Firstly, a 'confirmatory' trial that uses the probiotic combination (Bifidobacterium infantis, Streptococcus thermophilus and B. lactis) already shown to be likely to reduce the risk of NEC in a large, high‐quality trial in Australasia (Jacobs 2013). Alternatively, investigators may consider a pragmatic choice based on multi‐strain products in established use in their regions (which provides some availability and quality control reassurances with regard to product integrity and safety). Furthermore, investigators could consider whether trials using 'synbiotics' (combinations of probiotics with 'prebiotics' such as human milk oligosaccharides and other milk glycans) are merited alongside trials, or as part of an adaptive design, of probiotics (Underwood 2019).
Unit of randomisation and analysis is another consideration. Although individual infant randomisation is preferred for statistical and analytical reasons, concern exists that cross‐contamination of the trial organisms to infants in the control group will limit the power of the trial to detect an effect (as may have happened in Costeloe 2015). Randomising at the neonatal care centre level (cluster‐RCT) obviates this problem, but inflates the sample size requirement considerably because of inter‐cluster correlation of outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 4 October 2020 | New search has been performed | Inclusion criteria modified to include only very preterm (< 32 weeks' gestation) or very low birth weight infants (< 1500 g) with pre‐specified analyses for extremely preterm (< 28 weeks' gestation) or extremely low birth weight (< 1000 g) infants. The literature was searched in February 2020. Thirty‐two new published trials were identified. |
| 4 October 2020 | New citation required and conclusions have changed | Probiotics may reduce the risk of necrotising enterocolitis, but the certainty of the evidence is "low". |
History
Protocol first published: Issue 4, 2005 Review first published: Issue 1, 2008
| Date | Event | Description |
|---|---|---|
| 1 October 2013 | New citation required but conclusions have not changed | Updated search identified eight new trials for inclusion in this review update. |
| 1 October 2013 | New search has been performed | This updates Al Faleh 2011 |
| 3 November 2010 | New search has been performed | This updates the review "Probiotics for prevention of necrotizing enterocolitis in preterm infants" published in the Cochrane Database of Systematic Reviews (Al Faleh 2008). New authorship: Khalid AlFaleh, Jasim Anabrees, Dirk Bassler, Turki Al‐Kharfi. Updated search identified seven new trials for inclusion in this review update. |
| 3 November 2010 | New citation required and conclusions have changed | With the addition of seven new trials to this update, it brings the total to sixteen eligible trials randomizing 2842 infants. The previous review included nine eligible trials, randomizing 1425 infants. |
| 12 November 2008 | Feedback has been incorporated | Feedback incorporated |
| 22 July 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank all primary investigators who provided additional information about trial methods and outcomes.
We thank Cochrane Neonatal: Colleen Ovelman, Managing Editor, Jane Cracknell, Assistant Managing Editor, Carol Friesen, Information Specialist, and Roger Soll, Co‐coordinating editor, who provided editorial and administrative support; and Kath Wright (CRD, York) for the electronic search strategy and database management.
We thank Yuan Chi for translating two trial reports (Huang 2009; Ren 2010).
We thank Mohan Pammi, Cochrane Neonatal Associate Editor, Robert Boyle and Sarah Hodgkinson (Cochrane Children and Families Network) for peer review.
Khalid AlFaleh, Jasim Anabrees, Dirk Bassler and Turki Al‐Kharfi authored previous iterations of this review (AlFaleh 2005; Al Faleh 2008; Al Faleh 2011; Al Faleh 2014).
Appendices
Appendix 1. Electronic search methodology
Cochrane probiotics search strategies February 2020
Bibliographic databases: Cochrane Central register of Controlled Trials (CENTRAL), CINAHL, Embase, Maternity & Infant Care, MEDLINE
Trial registers: WHO ICTRP & ClinicalTrials.gov
Cochrane Register of Controlled Trials (CENTRAL)
Search date = 18th February 2020; 126 records
#1 MeSH descriptor: [Probiotics] explode all trees
#2 (probiotic*):ti,ab,kw (Word variations have been searched)
#3 MeSH descriptor: [Bifidobacterium] explode all trees
#4 (bifidobacterium*):ti,ab,kw (Word variations have been searched)
#5 MeSH descriptor: [Lactobacillus] explode all trees
#6 (lactobacill*):ti,ab,kw (Word variations have been searched)
#7 MeSH descriptor: [undefined] explode all trees
#8 MeSH descriptor: [Saccharomyces boulardii] this term only
#9 (Saccharomyces):ti,ab,kw (Word variations have been searched)
#10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9
#11 MeSH descriptor: [Prebiotics] explode all trees
#12 (prebiotic*):ti,ab,kw (Word variations have been searched)
#13 MeSH descriptor: [Oligosaccharides] explode all trees
#14 (oligosaccharide*):ti,ab,kw (Word variations have been searched)
#15 MeSH descriptor: [Inulin] explode all trees
#16 (inulin*):ti,ab,kw (Word variations have been searched)
#17 ((fructooligosaccharide* or fructo‐oligosaccharide* or FOS or FOSs or galacto‐oligosaccharide* or galactooligosaccharide*)):ti,ab,kw (Word variations have been searched)
#18 MeSH descriptor: [Lactoferrin] explode all trees
#19 (lactoferrin*):ti,ab,kw (Word variations have been searched)
#20 MeSH descriptor: [Lactulose] explode all trees
#21 (lactulose*):ti,ab,kw
#22 #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 or #20 or #21
#23 MeSH descriptor: [Synbiotics] explode all trees
#24 (synbiotic*):ti,ab,kw (Word variations have been searched)
#25 (((probiotic* and prebiotic*) NEAR/4 combin*)):ti,ab,kw (Word variations have been searched)
#26 #23 OR #24 OR #25
#27 #10 AND #22 AND #26
#28 MeSH descriptor: [Infant, Newborn] explode all trees
#29 MeSH descriptor: [Premature Birth] explode all trees
#30 neonat*:ti,ab,kw (Word variations have been searched)
#31 neo‐nat*:ti,ab,kw (Word variations have been searched)
#32 newborn or new born* or newly born*:ti,ab,kw (Word variations have been searched)
#33 preterm or preterms or (pre term) or (pre terms):ti,ab,kw (Word variations have been searched)
#34 preemie* or premie or premies:ti,ab,kw (Word variations have been searched)
#35 prematur* near/3 (birth* or born or deliver*):ti,ab,kw (Word variations have been searched)
#36 low near/3 (birthweight* or birth weight*):ti,ab,kw (Word variations have been searched)
#37 lbw or vlbw or elbw:ti,ab,kw (Word variations have been searched)
#38 infan* or baby or babies:ti,ab,kw (Word variations have been searched)
#39 #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38
#40 #27 AND #39
CINAHL Via EBSCO
27 records; 18th February 2020
S35 S31 AND S34 (27)
S34 S32 OR S33 (616,583)
S33 TX ( (neonat* or neo nat*) ) OR TX ( (newborn* or new born* or newly born*) ) OR TX ( (preterm or preterms or pre term or pre terms) ) OR TX ( (preemie$ or premie or premies) ) OR TX ( (prematur* N3 (birth* or born or deliver*)) ) OR TX ( (low N3 (birthweight* or birth weight*)) ) OR TX ( (lbw or vlbw or elbw) ) OR TX infan* OR TX ( (baby or babies) ) (616,583)
S32 (MH "Infant, Newborn+") (126,178)
S31 S22 AND S30 (107)
S30 S28 not S29 (628,752)
S29 ( MH animals+ OR MH (animal studies) OR TI (animal model*) ) NOT MH (human) (167,644)
S28 S23 OR S24 OR S25 OR S26 OR S27 (657,363)
S27 AB (cluster W3 RCT) (322)
S26 MH placebos OR PT randomized controlled trial OR AB control W5 group OR MH crossover design OR MH comparative studies (401,674)
S25 MH sample size AND AB ( (assigned OR allocated OR control) ) (3,766)
S24 TI ( (randomised OR randomized) ) OR AB random* OR TI trial (337,314)
S23 MH Randomized Controlled Trials OR MH double‐blind studies OR MH single‐blind studies OR MH random assignment OR MH pretest‐posttest design OR MH cluster sample (192,625)
S22 S9 AND S18 AND S21 (240)
S21 S19 OR S20 (366)
S20 TI ( (probiotic* and prebiotic*) N4 combin* ) OR AB ( (probiotic* and prebiotic*) N4 combin* ) (51)
S19 TI Synbiotic* OR AB Synbiotic* (342)
S18 S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 (4,196)
S17 TI Lactoferrin OR AB Lactoferrin (524)
S16 TI fructooligosaccharide* OR AB fructooligosaccharide* OR TI fructo‐oligosaccharide* OR AB fructo‐oligosaccharide* OR TI galactooligosaccharide* OR AB galactooligosaccharide* OR TI galacto‐oligosaccharide* OR AB galacto‐oligosaccharide* (363)
S15 TI Inulin OR AB Inulin (515)
S14 TI lactulose* OR AB lactulose* (481)
S13 TI Oligosaccharides OR AB Oligosaccharides (778)
S12 (MH "Oligosaccharides") (932)
S11 TI Prebiotic* OR AB Prebiotic* (1,270)
S10 (MH "Prebiotics") (1,408)
S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 (10,092)
S8 TI Saccharomyces OR AB Saccharomyces (510)
S7 (MH "Saccharomyces") (47)
S6 TI lactobacillus OR AB lactobacillus (2,281)
S5 (MH "Lactobacillus") OR (MH "Lactobacillus Acidophilus") (2,502)
S4 TI bifidobacterium* OR AB bifidobacterium* (875)
S3 (MH "Bifidobacterium") (946)
S2 TI probiotic* OR AB probiotic* (5,016)
S1 MH "Probiotics" (6,611)
Embase Via OVID
Search date 17th February 2020; 5600 records
Database: Embase <1974 to 2020 February 14>
1 Probiotic Agent/ (34490)
2 probiotic$.ti,ab,kw. (31301)
3 exp bifidobacterium/ (12860)
4 bifidobacterium$.ti,ab,kw. (9740)
5 exp lactobacillus/ (43379)
6 lactobacill$.ti,ab,kw. (38688)
7 Saccharomyces/ or Saccharomyces boulardii/ or Saccharomyces cerevisiae/ (98260)
8 Saccharomyces$.ti,ab,kw. (77090)
9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 (183648)
10 Prebiotic Agent/ (7387)
11 prebiotic$.ti,ab,kw. (9900)
12 exp Oligosaccharide/ (546080)
13 oligosaccharide$.ti,ab,kw. (37361)
14 Galactose oligosaccharide/ (961)
15 (galacto‐oligosaccharide$ or galactooligosaccharide$).ti,ab,kw. (1364)
16 Fructose Oligosaccharide/ (2182)
17 (fructooligosaccharide$ or fructo‐oligosaccharide$ or FOS or FOSs).ti,ab,kw. (35709)
18 Lactulose/ (8835)
19 lactulose$.ti,ab,kw. (5550)
20 Inulin/ (7321)
21 inulin$.ti,ab,kw. (9557)
22 Lactoferrin/ (10431)
23 lactoferrin$.ti,ab,kw. (9054)
24 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 (617217)
25 Synbiotic Agent/ (1624)
26 synbiotic$.ti,ab,kw. (1737)
27 ((probiotic$ and prebiotic$) adj4 combin$).ti,ab,kw. (411)
28 25 or 26 or 27 (2333)
29 9 or 24 or 28 (778900)
30 Newborn/ (516866)
31 Prematurity/ (99389)
32 (neonat$ or neo nat$).ti,ab. (334397)
33 (newborn$ or new born$ or newly born$).ti,ab. (189575)
34 (preterm or preterms or pre term or pre terms).ti,ab. (102056)
35 (preemie$ or premie or premies).ti,ab. (257)
36 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (21105)
37 (low adj3 (birthweight$ or birth weight$)).ti,ab. (42758)
38 (lbw or vlbw or elbw).ti,ab. (11219)
39 infan$.ti,ab. (487240)
40 (baby or babies).ti,ab. (94958)
41 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 (1110575)
42 Randomized controlled trial/ (590055)
43 Controlled clinical study/ (462890)
44 Random$.ti,ab. (1501724)
45 randomization/ (85807)
46 intermethod comparison/ (256520)
47 placebo.ti,ab. (300990)
48 (compare or compared or comparison).ti. (500389)
49 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. (2058845)
50 (open adj label).ti,ab. (76978)
51 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. (228154)
52 double blind procedure/ (169466)
53 parallel group$1.ti,ab. (24938)
54 (crossover or cross over).ti,ab. (103058)
55 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. (322434)
56 (assigned or allocated).ti,ab. (379281)
57 (controlled adj7 (study or design or trial)).ti,ab. (339741)
58 (volunteer or volunteers).ti,ab. (243065)
59 human experiment/ (484405)
60 trial.ti. (291075)
61 or/42‐60 (4900385)
62 (random$ adj sampl$ adj7 ("cross section$" or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.) (7961)
63 Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.) (228646)
64 (((case adj control$) and random$) not randomi?ed controlled).ti,ab. (16824)
65 (Systematic review not (trial or study)).ti. (135640)
66 (nonrandom$ not random$).ti,ab. (15874)
67 "Random field$".ti,ab. (2243)
68 (random cluster adj3 sampl$).ti,ab. (1253)
69 (review.ab. and review.pt.) not trial.ti. (777162)
70 "we searched".ab. and (review.ti. or review.pt.) (30687)
71 "update review".ab. (103)
72 (databases adj4 searched).ab. (33664)
73 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/ (1045069)
74 Animal experiment/ not (human experiment/ or human/) (2213091)
75 or/62‐74 (3395835)
76 61 not 75 (4366247)
77 29 and 41 and 76 (5600)
Maternity & Infant Care Via OVID
Search date 17th February 2020; Records 94
Database: Maternity & Infant Care Database (MIDIRS) <1971 to December 2019>
1 probiotic$.ti,ab,de. (430)
2 bifidobacterium$.ti,ab,de. (153)
3 lactobacill$.ti,ab,de. (306)
4 Saccharomyces$.ti,ab,de. (12)
5 1 or 2 or 3 or 4 (643)
6 prebiotic$.ti,ab,de. (145)
7 oligosaccharide$.ti,ab,de. (139)
8 inulin$.ti,ab,de. (13)
9 (fructooligosaccharide$ or fructo‐oligosaccharide$ or FOS or FOSs).ti,ab,de. (39)
10 (galactooligosaccharide$ or galacto‐oligosaccharide$).ti,ab,de. (35)
11 lactoferrin$.ti,ab,de. (156)
12 lactulose$.ti,ab,de. (27)
13 6 or 7 or 8 or 9 or 10 or 11 or 12 (413)
14 synbiotic$.ti,ab,de. (27)
15 ((probiotic$ and prebiotic$) adj4 combin$).ti,ab,de. (5)
16 14 or 15 (28)
17 5 or 13 or 16 (932)
18 (neonat$ or neo nat$).ti,ab. (46156)
19 (newborn$ or new born$ or newly born$).ti,ab. (20773)
20 (preterm or preterms or pre term or pre terms).ti,ab. (27396)
21 (preemie$ or premie or premies).ti,ab. (56)
22 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (4126)
23 (low adj3 (birthweight$ or birth weight$)).ti,ab. (11086)
24 (lbw or vlbw or elbw).ti,ab. (3170)
25 infan$.ti,ab. (66564)
26 (baby or babies).ti,ab. (29888)
27 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 (123341)
28 17 and 27 (765)
29 limit 28 to randomised controlled trial (94)
MEDLINE Via OVID
Search date 17th February 2020; Records 2054
Database: Ovid MEDLINE(R) ALL <1946 to February 14, 2020>
1 Probiotics/ (16413)
2 probiotic$.ti,ab,kw. (23385)
3 exp bifidobacterium/ (5805)
4 bifidobacterium$.ti,ab,kw. (7563)
5 exp lactobacillus/ (28003)
6 lactobacill$.ti,ab,kw. (34222)
7 Saccharomyces/ or Saccharomyces boulardii/ or Saccharomyces cerevisiae/ (109184)
8 Saccharomyces$.ti,ab,kw. (72585)
9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 (183166)
10 Prebiotics/ (2477)
11 prebiotic$.ti,ab,kw. (8040)
12 Oligosaccharides/ (24163)
13 oligosaccharide$.ti,ab,kw. (33210)
14 (galactooligosaccharides or galacto‐oligosaccharides).ti,ab,kw. (859)
15 (fructooligosaccharide$ or fructo‐oligosaccharide$ or FOS or FOSs).ti,ab,kw. (29851)
16 Lactulose/ (2114)
17 lactulose$.ti,ab,kw. (3524)
18 Inulin/ (6862)
19 inulin$.ti,ab,kw. (8603)
20 Lactoferrin/ (5956)
21 lactoferrin$.ti,ab,kw. (7664)
22 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 (100138)
23 Synbiotics/ (525)
24 synbiotic$.ti,ab,kw. (1327)
25 ((probiotic$ and prebiotic$) adj4 combin$).ti,ab,kw. (313)
26 23 or 24 or 25 (1500)
27 9 or 22 or 26 (276802)
28 exp Infant, Newborn/ (599027)
29 Premature Birth/ (13220)
30 (neonat$ or neo nat$).ti,ab. (258480)
31 (newborn$ or new born$ or newly born$).ti,ab. (163361)
32 (preterm or preterms or pre term or pre terms).ti,ab. (72698)
33 (preemie$ or premie or premies).ti,ab. (166)
34 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (15366)
35 (low adj3 (birthweight$ or birth weight$)).ti,ab. (33943)
36 (lbw or vlbw or elbw).ti,ab. (8192)
37 infan$.ti,ab. (428676)
38 (baby or babies).ti,ab. (68784)
39 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 (1039559)
40 randomized controlled trial.pt. (500729)
41 controlled clinical trial.pt. (93588)
42 randomized.ab. (470135)
43 placebo.ab. (205251)
44 drug therapy.fs. (2181901)
45 randomly.ab. (327315)
46 trial.ab. (494771)
47 groups.ab. (2009585)
48 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 (4636061)
49 exp animals/ not humans.sh. (4674306)
50 48 not 49 (4016966)
51 27 and 39 and 50 (2054)
Appendix 2. 'Risk of bias' tool
Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
Blinding of personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for personnel.
Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
Data and analyses
Comparison 1. Probiotics versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Necrotising enterocolitis | 54 | 10604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.45, 0.65] |
| 1.1.1 Bifidobacterium spp. | 14 | 2988 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.54, 0.96] |
| 1.1.2 Lactobacillus spp. | 12 | 2000 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.28, 0.71] |
| 1.1.3 Sacchromyces spp. | 4 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.44, 1.50] |
| 1.1.4 Bacillus spp. | 2 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.23, 1.61] |
| 1.1.5 Bifidobacterium spp. plus Lactobacillus spp. | 11 | 2041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.23, 0.59] |
| 1.1.6 Bifidobacterium spp. plus Streptococcus spp. | 2 | 1244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.19, 0.68] |
| 1.1.7 Bifidobacterium spp. plus Lactobacillus spp. plus Sacchromyces spp. | 4 | 583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.28, 1.58] |
| 1.1.8 Bifidobacterium spp. plus Lactobacillus spp. plus Streptococcus spp. | 5 | 662 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.22, 0.77] |
| 1.2 Mortality | 51 | 10170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.65, 0.89] |
| 1.2.1 Bifidobacterium spp. | 12 | 2761 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.58, 1.09] |
| 1.2.2 Lactobacillus spp. | 12 | 2000 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.60, 1.37] |
| 1.2.3 Sacchromyces spp. | 3 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.46, 2.70] |
| 1.2.4 Bacillus spp. | 2 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.45, 1.69] |
| 1.2.5 Bifidobacterium spp. plus Lactobacillus spp. | 12 | 2071 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.45, 0.81] |
| 1.2.6 Bifidobacterium spp. plus Streptococcus spp. | 2 | 1244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.52, 1.35] |
| 1.2.7 Bifidobacterium spp. plus Lactobacillus spp. plus Sacchromyces spp. | 4 | 583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.30, 1.49] |
| 1.2.8 Bifidobacterium spp. plus Lactobacillus spp. plus Streptococcus spp. | 4 | 512 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.39, 1.42] |
| 1.3 Invasive infection | 47 | 9762 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.82, 0.97] |
| 1.3.1 Bifidobacterium spp. | 12 | 2736 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.70, 1.02] |
| 1.3.2 Lactobacillus spp. | 11 | 1970 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.76, 1.21] |
| 1.3.3 Sacchromyces spp. | 4 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.58, 1.22] |
| 1.3.4 Bacillus spp. | 2 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.67, 1.51] |
| 1.3.5 Bifidobacterium spp. plus Lactobacillus spp. | 10 | 1913 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.78, 1.08] |
| 1.3.6 Bifidobacterium spp. plus Streptococcus spp. | 2 | 1244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.72, 1.17] |
| 1.3.7 Bifidobacterium spp. plus Lactobacillus spp. plus Sacchromyces spp. | 4 | 583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.53, 1.18] |
| 1.3.8 Bifidobacterium spp. plus Lactobacillus spp. plus Streptococcus spp. | 2 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.63, 1.00] |
| 1.4 Duration of birth hospitalisation (days) | 22 | 5458 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐3.78, ‐0.08] |
| 1.4.1 Bifidobacterium spp. | 4 | 1945 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐6.55, 4.45] |
| 1.4.2 Lactobacillus spp. | 4 | 217 | Mean Difference (IV, Random, 95% CI) | ‐1.95 [‐10.81, 6.90] |
| 1.4.3 Sacchromyces spp. | 2 | 470 | Mean Difference (IV, Random, 95% CI) | ‐2.88 [‐8.06, 2.29] |
| 1.4.4 Bifidobacterium spp. plus Lactobacillus spp. | 7 | 1265 | Mean Difference (IV, Random, 95% CI) | ‐1.74 [‐5.22, 1.73] |
| 1.4.5 Bifidobacterium spp. plus Streptococcus spp. | 1 | 1044 | Mean Difference (IV, Random, 95% CI) | ‐3.00 [‐6.28, 0.28] |
| 1.4.6 Bifidobacterium spp. plus Lactobacillus spp. plus Sacchromyces spp. | 2 | 231 | Mean Difference (IV, Random, 95% CI) | ‐5.65 [‐11.68, 0.38] |
| 1.4.7 Bifidobacterium spp. plus Lactobacillus spp. plus Streptococcus spp. | 2 | 286 | Mean Difference (IV, Random, 95% CI) | 1.69 [‐6.73, 10.11] |
| 1.5 Severe neurodevelopmental impairment | 5 | 1518 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.84, 1.26] |
| 1.5.1 Bifidobacterium spp. | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.34, 1.72] |
| 1.5.2 Lactobacillus spp. | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.69, 1.48] |
| 1.5.3 Bacillus spp. | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.58, 2.07] |
| 1.5.4 Bifidobacterium spp. plus Streptococcus spp. | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.69, 1.36] |
| 1.5.5 Bifidobacterium spp. plus Lactobacillus spp. | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.81, 1.98] |
| 1.6 Cerebral palsy | 5 | 1512 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.74, 1.72] |
| 1.6.1 Bifidobacterium spp. | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.10, 1.36] |
| 1.6.2 Lactobacillus spp. | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.40, 2.08] |
| 1.6.3 Bacillus spp. | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.38, 10.88] |
| 1.6.4 Bifidobacterium spp. plus Streptococcus spp. | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.67, 2.58] |
| 1.6.5 Bifidobacterium spp. plus Lactobacillus spp. | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [0.62, 8.41] |
| 1.7 Visual impairment | 4 | 1356 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.14, 1.80] |
| 1.7.1 Bifidobacterium spp. | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.54] |
| 1.7.2 Lactobacillus spp. | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.7.3 Bifidobacterium spp. plus Streptococcus spp. | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.12, 71.21] |
| 1.7.4 Bifidobacterium spp. plus Lactobacillus spp. | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.02, 1.89] |
| 1.8 Hearing impairment | 4 | 1356 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.18, 1.17] |
| 1.8.1 Bifidobacterium spp. | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.07, 16.10] |
| 1.8.2 Lactobacillus spp. | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.02 [0.12, 73.52] |
| 1.8.3 Bifidobacterium spp. plus Streptococcus spp. | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.04, 0.79] |
| 1.8.4 Bifidobacterium spp. plus Lactobacillus spp. | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.16, 18.64] |
| 1.9 Continuous early learning composite measure | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐6.38, 4.38] |
Comparison 2. Probiotics versus control (extremely preterm or ELBW).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Necrotising enterocolitis | 8 | 1712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.68, 1.21] |
| 2.1.1 Bifidobacterium spp. | 2 | 665 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.70, 1.43] |
| 2.1.2 Lactobacillus spp. | 2 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.36, 1.48] |
| 2.1.3 Bacillus spp. | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.1.4 Bifidobacterium spp. plus Lactobacillus spp. | 2 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.21, 4.79] |
| 2.1.5 Bifidobacterium spp. plus Streptococcus spp. | 1 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.33, 1.60] |
| 2.2 Mortality | 6 | 1661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.71, 1.16] |
| 2.2.1 Bifidobacterium spp. | 1 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.60, 1.61] |
| 2.2.2 Lactobacillus spp. | 2 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.42, 1.42] |
| 2.2.3 Bacillus clausii | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.36, 2.08] |
| 2.2.4 Bifidobacterium spp. plus Lactobacillus spp. | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.18, 3.18] |
| 2.2.5 Bifidobacterium spp. plus Streptococcus spp. | 1 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.65, 1.35] |
| 2.3 Invasive infection | 6 | 1471 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.76, 1.06] |
| 2.3.1 Bifidobacterium spp. | 2 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.73, 1.37] |
| 2.3.2 Lactobacillus spp. | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.67, 1.66] |
| 2.3.3 Bacillus clausii | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.43, 1.47] |
| 2.3.4 Bifidobacterium spp. plus Lactobacillus spp. | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.45, 1.54] |
| 2.3.5 Bifidobacterium spp. plus Streptococcus spp. | 1 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.64, 1.06] |
| 2.4 Duration of birth hospitalisation (days) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐5.40 [‐14.20, 3.40] |
| 2.4.1 Bifidobacterium spp. plus Lactobacillus spp. | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐5.40 [‐14.20, 3.40] |
2.4. Analysis.

Comparison 2: Probiotics versus control (extremely preterm or ELBW), Outcome 4: Duration of birth hospitalisation (days)
Comparison 3. Subgroup analysis by type of feeding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Necrotising enterocolitis | 54 | 10604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.45, 0.65] |
| 3.1.1 Human milk only | 8 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.16, 0.57] |
| 3.1.2 Mixed‐ human milk or formula or both | 42 | 9364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.48, 0.70] |
| 3.1.3 Formula only | 4 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.16, 1.18] |
| 3.2 Mortality | 51 | 10271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.65, 0.89] |
| 3.2.1 Human milk only | 8 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.41, 1.00] |
| 3.2.2 Mixed‐ human milk or formula or both | 40 | 9118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.67, 0.94] |
| 3.2.3 Formula only | 3 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.04, 1.21] |
| 3.3 Invasive infection | 47 | 9762 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.82, 0.97] |
| 3.3.1 Human milk only | 8 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.59, 0.96] |
| 3.3.2 Mixed‐ human milk or formula or both | 36 | 8552 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.83, 1.00] |
| 3.3.3 Formula only | 3 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.11, 1.49] |
| 3.4 Duration of birth hospitalisation (days) | 22 | 5458 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐3.78, ‐0.08] |
| 3.4.1 Human milk only | 4 | 366 | Mean Difference (IV, Random, 95% CI) | ‐3.95 [‐7.70, ‐0.21] |
| 3.4.2 Mixed‐ human milk or formualor both | 16 | 5002 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐2.84, 0.85] |
| 3.4.3 Formula only | 2 | 90 | Mean Difference (IV, Random, 95% CI) | 1.50 [‐26.33, 29.32] |
Comparison 4. Sensitivity analyses: Risk of bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Necrotising enterocolitis | 54 | 10800 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.46, 0.65] |
| 4.1.1 LOW | 16 | 4597 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.55, 0.89] |
| 4.1.2 UNCLEAR | 20 | 3905 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.31, 0.59] |
| 4.1.3 HIGH | 18 | 2298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.26, 0.63] |
| 4.2 Mortality | 51 | 10170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.65, 0.89] |
| 4.2.1 LOW | 16 | 4597 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.07] |
| 4.2.2 UNCLEAR | 19 | 3818 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.54, 0.94] |
| 4.2.3 HIGH | 16 | 1755 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.38, 0.85] |
| 4.3 Invasive infection | 47 | 9762 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.82, 0.97] |
| 4.3.1 LOW | 16 | 4597 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.79, 1.02] |
| 4.3.2 UNCLEAR | 18 | 3700 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.84, 1.10] |
| 4.3.3 HIGH | 13 | 1465 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.59, 0.90] |
| 4.4 Duration of birth hospitalisation (days) | 22 | 5458 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐3.78, ‐0.08] |
| 4.4.1 LOW | 6 | 2786 | Mean Difference (IV, Random, 95% CI) | ‐2.24 [‐5.76, 1.29] |
| 4.4.2 UNCLEAR | 8 | 1675 | Mean Difference (IV, Random, 95% CI) | ‐0.99 [‐4.07, 2.10] |
| 4.4.3 HIGH | 8 | 997 | Mean Difference (IV, Random, 95% CI) | ‐3.92 [‐7.91, 0.07] |
| 4.5 Severe neurodevelopmental impairment | 5 | 1518 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.84, 1.26] |
| 4.5.1 LOW | 2 | 913 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.76, 1.27] |
| 4.5.2 UNCLEAR | 3 | 605 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.79, 1.54] |
| 4.6 Cerebral palsy | 5 | 1512 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.74, 1.72] |
| 4.6.1 LOW | 2 | 913 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.68, 1.92] |
| 4.6.2 UNCLEAR | 3 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.52, 2.28] |
| 4.7 Visual impairment | 4 | 1356 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.14, 1.80] |
| 4.7.1 LOW | 2 | 913 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.12, 71.21] |
| 4.7.2 UNCLEAR | 2 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.06, 1.49] |
| 4.8 Hearing impairment | 4 | 1356 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.18, 1.17] |
| 4.8.1 LOW | 2 | 913 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.09, 0.98] |
| 4.8.2 UNCLEAR | 2 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.23, 8.29] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agarwal 2003.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 39 VLBW infants | |
| Interventions | Probiotics (N = 24): Lactobacillus rhamnosus GG once daily with human milk or formula for 21 days or discharge from hospital Control (N = 15): unsupplemented milk feeds |
|
| Outcomes |
(NEC, death, infection not reported) |
|
| Notes | India (1999 to 2000) Funding: UK National Institute for Health (Fogarty Grant TW‐00601) and Conagra Foods Inc., USA (supplied intervention) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Unlikely |
| Selective reporting (reporting bias) | Unclear risk | No clinical outcomes reported |
Al‐Hosni 2012.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 101 ELBW infants (appropriate for gestational age) | |
| Interventions | Probiotic (N = 50): Lactobacillus rhamnosus GG (LGG) and Bifidobacterium infantis added to the 1st milk feed and continued once daily until discharge or until 34 weeks' postmenstrual age Control (N = 51): unsupplemented milk feeds | |
| Outcomes |
|
|
| Notes | USA (2009 to 2011) Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unsupplemented milk feeds‐ not placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Bin‐Nun 2005.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 145 VLBW infants | |
| Interventions | Probiotics (N = 72): "Lactobacillus bifidus" (likely Bifidobactrium bifidum), Streptococcus thermophilus, and B. infantis added to expressed breast milk or formula enteral feeds daily until 36 weeks' postmenstrual age Control (N = 73): unsupplemented milk feeds | |
| Outcomes |
|
|
| Notes | Israel (2001 to 2004) Funding: Solgar, Wyeth (manufacturer of intervention) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unsupplemented milk feeds‐ not placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Unclear risk | Data published in an abstract form on two previous occasions at the Society of Pediatrics Research (SPR 2003, 2005) with different inclusion criteria and clinical outcomes |
Braga 2011.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 231 VLBW infants (birth weight 750 g to 1500 g) | |
| Interventions | Probiotics (N = 119): Lactobacillus casei and Bifidobacterium breve (Yakult ‐ LB) in human milk once daily until day 30 or hospital discharge Control (N = 112): unsupplemented milk feeds | |
| Outcomes |
|
|
| Notes | Brazil (2007 to 2008)
Funding: public/state. External Study Committee terminated trial early (quote:) "for a clear benefit" after enrolment of 231 infants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sealed envelope with group allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unsupplemented milk feeds‐ not placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Chandrashekar 2018.
| Study characteristics | ||
| Methods | Quasi‐RCT | |
| Participants | 145 preterm infants of gestation < 34 weeks' (most participants were very preterm or VLBW) | |
| Interventions | Probitics (N = 72): Lactobacillus acidophilus, L. rhamnosus, Bifidobacterium longum, and Saccharomyces boulardii with human milk or formula feeds until discharge from hospital Control (N = 73): unsupplemented milk feeds (no placebo) |
|
| Outcomes |
|
|
| Notes | India (2014 to 2015) Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Simple random sampling method" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Near‐complete (5 participants withdrawn pre‐analysis) |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Chowdhury 2016.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 119 VLBW Infants (28 to 33 weeks' gestation) | |
| Interventions | Probiotics (N = 60): (quote:) "Cap TS6" containing Lactobacillus rhamnosus GG, L. paracasei , L. casei, L. acidophilus, Lactococcus latis, Bifidobacterium bifidum, B. longum, B. infantis) in human milk once daily until discharge Control (N = 59): unsupplemented milk feeds |
|
| Outcomes |
|
|
| Notes | Bangladesh (2012 to 2015) Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | First allocation by lottery, and subsequent by alternate allocation |
| Allocation concealment (selection bias) | High risk | Unconcealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Chrzanowska‐Liszewska 2012.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 47 very preterm infants (birth weight > 1000 g) | |
| Interventions | Probiotics (N = 21): Lactobacillus rhamnosus GG, added to formula, once daily until day 42 Control (N = 26): maltodextrin placebo added to formula |
|
| Outcomes |
|
|
| Notes | Poland (2008 to 2009) Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Coded capsules containing probiotics or placebo |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Costalos 2003.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 87 formula‐fed infants of gestational age at birth 28 to 32 weeks. | |
| Interventions | Probiotics (N = 51): Saccharomyces boulardii added to formula every 12 hours during the 1st week of life when enteral feed are tolerated for 30 days Control (N = 36): maltodextrin placebo | |
| Outcomes |
|
|
| Notes | Greece (period of study: not specified) Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Cards with allocation in sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Near‐complete (5 infants with incomplete data were not included in analyses) |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Costeloe 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 1310 infants born before 31 weeks' gestation | |
| Interventions | Probiotics (N = 650): Bifidobacterium breve BBG‐001 once daily until 36 weeks’ postmenstrual age or discharge from hospital Control (N = 660): corn starch placebo |
|
| Outcomes |
|
|
| Notes | UK (24 neonatal units; 2010 to 2013) Funding: by UK National Institute for Health Research Health Technology Assessment programme (ISRCTN 05511098) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Web‐based |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | No |
Dani 2002.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 585 VLBW infants (or < 33 weeks' gestation at birth) | |
| Interventions | Probiotics (N = 295): Lactobacillus rhamnosus GG added to milk (human or formula) feeds once daily until hospital discharge Control (N = 290): maltodextrin placebo | |
| Outcomes |
|
|
| Notes | Italy (12 centres; study period not specified) Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Sealed envelope containing allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked (placebo‐controlled) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Dashti 2014.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 136 preterm infants of birth weight 700 g to 1800 g (most participants very preterm or VLBW) | |
| Interventions | Probiotics (N = 69): Lactobacillus acidophilus, L. rhamnosus, L. bulgaricus, L. casei, Streptococcus thermophilus, Bifidobacterium longum, B. breve added to milk feeds once daily until hospital discharge Control (N = 67): placebo powder (not described) | |
| Outcomes |
|
|
| Notes | Iran (2010 to 2011) Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked (placebo‐controlled) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Demirel 2013.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 271 VLBW infants (gestational age ≤ 32 weeks at birth) | |
| Interventions | Probiotics (N = 135): Saccharomyces boulardii added to human milk or formula once a day, starting with the 1st feed, until hospital discharge Control (N = 136): unsupplemented milk | |
| Outcomes |
|
|
| Notes | Turkey (2011) Funding: not stated ClinicalTrials.gov Identifier: NCT01315821 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Allocations sealed in opaque, sequentially‐numbered envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Dilli 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 200 very preterm or VLBW infants | |
| Interventions | Probiotics (N = 100): Bifidobacterium lactis added to human milk or formula once daily for 8 weeks (or hospital discharge) Control (N= 100): maltodextrin powder placebo |
|
| Outcomes |
|
|
| Notes | Turkey (5 centres: 2011 to 2014) Funding: not stated NB. This was a 4‐arm RCT‐ 2 other groups were prebiotic (N = 100) and synbiotic (n + 100) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked (placebo‐controlled) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Dutta 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 149 infants (27 to 33 weeks' gestation at birth) | |
| Interventions | Probiotics (N = 114): Lactobacillus acidophilus, L. rhamnosus, Bifidobacterium longum, and Saccharomyces boulardii (3 groups: (quote:) "low‐dose" (109) for 21 days or quote:) "high‐dose" (1010) 2 times daily with human milk or formula feeds for 14 or 21 days Control (N = 35): maltodextrin placebo for 21 days |
|
| Outcomes |
|
|
| Notes | India (study period not stated) Funding: Aristo Pharmaceuticals Pvt Ltd, Madhya Pradesh, India provided the sachets of probiotics and placebo free of cost |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Fernández‐Carrocera 2013.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 150 VLBW infants | |
| Interventions | Probiotics (N = 75): Lactobacillus rhamnosus, L. casei, L. plantarum, L acidophilus, Bifidobacteruim infantis, and Streptococcus thermophilus added to human milk or formula (duration intervention not stated) Control (N = 75): unsupplemented milk feeds | |
| Outcomes |
|
|
| Notes | Mexico (2007 to 2010) Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Staff unable to predict allocation by number |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unsupplemented milk feeds‐ not placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Fujii 2006.
| Study characteristics | ||
| Methods | Quasi‐RCT | |
| Participants | 19 preterm infants (most very preterm or VLBW) | |
| Interventions | Probiotics group (N = 11): Bifidobacterium breve 2 times daily with human milk or formula feeds until hospital discharge Control (N = 8): unsupplemented milk feeds | |
| Outcomes |
|
|
| Notes | Japan (2000 to 2002) Published: 2004 Funding: Morinaja Milk industry and Meiji Dairies (manufactured intervention) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Hariharan 2016.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 196 very preterm infants with birth weight < 1250 g | |
| Interventions | Probiotics (N = 93): Lactobacillus acidophilus, Bifidobacterium bifidum, Saccharomyces boulardii 2 times daily in milk feeds for 6 weeks Control (N = 103): unsupplemented feeds |
|
| Outcomes |
|
|
| Notes | India (study period not stated) Funding: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Hays 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 199 very preterm infants (gestation at birth 25 to 31 weeks), and birth weight 700 g to 1600 g that was appropriate for gestational age | |
| Interventions | Probiotics (3 groups: N = 145): Bifidobacterium lactis, or B. longum, or both once daily in sterile water for 4 to 6 weeks (depending on gestation at birth) Control (N = 52): maltodextrin placebo |
|
| Outcomes |
|
|
| Notes | France (three centres: 2007 to 2010) Funding: Nestle France (Marne‐la‐Vallee, France) and Nestec (Vevey, Switzerland) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, sealed, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked (placebo‐controlled) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Hernandez‐Enriquez 2016.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 44 preterm infants < 34 weeks' gestation or ≤ 1550 g birth weight (most infants very preterm or VLBW) | |
| Interventions | Intervention (N = 24): Lactobacillus reuteri once daily for 1st 10 days after birth Control (N = 20): placebo (sterile water) |
|
| Outcomes |
|
|
| Notes | Mexico (2012 to 2013) Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Simple randomisation sequence" |
| Allocation concealment (selection bias) | Low risk | Seaed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete |
| Selective reporting (reporting bias) | Unclear risk | Unlikely |
Hikaru 2010.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 208 VLBW infants | |
| Interventions | Probiotics (N = 108): Bifidobacterium breve in human milk or formula once daily until discharge from the intensive care unit Control (N = 100): unsupplemented milk feeds |
|
| Outcomes |
(NEC not reported) |
|
| Notes | Japan (2001 to 2013) Funding: Morinaga Milk Industry Co. Ltd. (supplied Bifidobacterium breve preparation) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Huang 2009.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 183 VLBW infants who survived 7 days after birth and began enteral feeding | |
| Interventions | Probiotics (N = 95): Bifidobacterium adolescentis twice daily with milk feeds daily for 7 days Control (N = 88): unsupplemented milk feeds |
|
| Outcomes | NEC (unclear whether death or infection assessed) | |
| Notes | China (single centre, study dates not stated) Translation from Chinese courtesy of Yuan Chi |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to assess |
| Selective reporting (reporting bias) | Unclear risk | Mortality and infection not reported |
Indrio 2017.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 60 preterm infants of gestational age 28 to 32 weeks' at birth | |
| Interventions | Probiotics (N = 30): Lactobacillus reuteri DSM 17938 suspended in sunflower and medium‐chain triglyceride oils, given once daily until day 30 Control (N = 30): identical oils without probiotics |
|
| Outcomes |
|
|
| Notes | Italy (2011 to 2012) Funding: University of Bari, Italy ClinicalTrials.gov no. NCT00985816 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked (placebo‐controlled) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Unclear risk | Unlikely |
Jacobs 2013.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 1099 very preterm VLBW infants | |
| Interventions | Probiotics (N = 548): Bifidobacterium infantis, Streptococcus thermophilus and B. lactis once daily in human milk or formula until discharge from hospital or term corrected age. Control (N = 551): maltodextrin powder placebo |
|
| Outcomes |
|
|
| Notes | Australasia (10 centres; 2007 to 2011) Funding: National Health and Research Medical Council, Australia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked (placebo‐controlled) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete for in hospital outcomes (Neurodevelopmental assessment = 48%) |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Kanic 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 80 VLBW infants | |
| Interventions | Probiotics (N = 40): Lactobacillus acidophilus, Enterococcus faecium, Bifidobacterium infantis 2 times daily with milk feeds until discharge from hospital Control (N = 40): unsupplemented milk feeds |
|
| Outcomes |
|
|
| Notes | Slovenia (2008 to 2011) Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate allocation (quote: "quasi‐randomised") |
| Allocation concealment (selection bias) | High risk | Unconcealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Kitajima 1997.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 91 VLBW infants | |
| Interventions | Probiotics (N = 45): Bifidobacterium breve in distilled water once daily for 28 days Control (N = 46): distilled water | |
| Outcomes |
(NEC, death, infection‐ data courtesy of investigators) |
|
| Notes | Japan (1990 to 1991) Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Near‐complete (4 participants not included in analyses) |
| Selective reporting (reporting bias) | Low risk | Data |
Li 2019.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 30 VLBW infants | |
| Interventions | Probiotics (N = 16): Lactobacillus plantarum, Bifidobacterium longum, B. bifidum once daily with milk feeds until 36 weeks' postmenstrual age. Control (N = 14): 5% glucose solution |
|
| Outcomes |
(NEC, death, infection not reported (author contacted in May 2020)) |
|
| Notes | China (2014 to 2015) Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Concealed by the principal investigator according to sequential numbers" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked (intervention and control solutions identical) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | > 50% outcome data unreported |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine |
Lin 2005.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 367 VLBW infants | |
| Interventions | Probiotics (N = 180): Lactobacillus acidophilus and Bifidobacterium. infantis (Infloran®) 2 times daily with human milk until discharge from hospital Control (N = 187): unsupplemented milk feeds (no placebo) | |
| Outcomes |
|
|
| Notes | Taiwan (1999 to 2003) Funding: Research Department of China Medical University Hospital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | Low risk | Opaque, sequentially numbered, sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unsupplemented milk feeds‐ not placebo‐controlled (investigators aware of allocation) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Near‐complete (90% for neurodevelopmental assessments) |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Lin 2008.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 434 VLBW infants | |
| Interventions | Probiotics (N = 217): Bifidobacterium bifidum and Lactobacillus acidophilus, added to human milk or formula 2 times daily for 6 weeks Control (N = 217): unsupplemented milk feeds |
|
| Outcomes |
|
|
| Notes | Taiwan (7 centres: 2005 to 2007)
Funding: National Science Council of Taiwan ClinicalTrials.gov Identifier: NCT00540033 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Allocated centrally |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unsupplemented milk feeds‐ not placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Manzoni 2006.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 80 VLBW infants | |
| Interventions | Probiotics (N = 39): Lactoacillus casei subspecies rhamnosus with human milk until 6 weeks or hospital discharge Control (N = 41): unsupplemented milk feeds | |
| Outcomes |
|
|
| Notes | Italy (2004 to 2005) Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Manzoni 2009.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 485 VLBW infants | |
| Interventions | Probiotics (N = 238): Lactoacillus casei subspecies rhamnosus with human milk or formula until 4 (VLBW) or 6 (ELBW) weeks plus bovine lactoferrin (100 mg/day) Control (N = 247): bovine lactoferrin alone (All doses including placebo were diluted in prepared milk so as to maintain masking) | |
| Outcomes |
|
|
| Notes | Italy (11 centres: 2007 to 2008) Funding: Dicofarm SpA (manufacturer of intervention) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Pharmacy allocation (remote) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Unclear risk | Data for invasive infection in complete cohort not reported in primary publication (available to derive from later publications) |
Mihatsch 2010.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 180 VLBW infants (< 30 weeks' gestation) | |
| Interventions | Probiotics (N = 91): Bifidobacterium lactis BB12 mixed with powdered fortifier in human milk or formula once daily for 6 weeks Control (N = 89): powdered fortifier placebo | |
| Outcomes |
|
|
| Notes | Germany (2000 to 2003 ) Funding: Nestlé AG, Frankfurt, Germany |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked (placebo‐controlled) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Millar 1993.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 20 infants < 33 weeks' gestation (most participants very preterm or VLBW) | |
| Interventions | Probiotics (N = 10): Lactobacillus rhamnosus GG mixed with human milk or formula 2 times daily for 14 days, starting with 1st feed Control (N = 10): unsupplemented milk feeds | |
| Outcomes |
(NEC, death (courtesy of investigators)) |
|
| Notes | UK (1991 to 1992) Funding: Wessex Medical Trust | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unsupplemented milk feeds‐ not placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Mohan 2006.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 69 preterm infants (most participants were very preterm or VLBW) | |
| Interventions | Probiotics (N = 37): Bifidobacterium lactis in milk feeds from 1st day after birth for 21 days Control (N = 32): unsupplemented milk feeds |
|
| Outcomes |
(NEC, death, infection (courtesy of investigators)) |
|
| Notes | Germany (2003 to 2005) Funding: Nestlé, Konolfingen, Switzerland | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Central allocation (web‐based) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unsupplemented milk feeds‐ not placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Oncel 2014.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 424 VLBW infants (and gestational age ≤ 32 weeks' at birth) | |
| Interventions | Probiotics (N = 213) Lactobacillus reuteri DSM 17938 once daily with milk feeds until discharge from hospital Placebo (N = 211): placebo containing only oil base |
|
| Outcomes |
|
|
| Notes | Turkey (2012 to 2013) Funding: not stated ClinicalTrials.gov Identifier: NCT01531179 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Opaque, sequentially numbered sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Near‐complete (8 participants withdrawn by family) for in hospital outcomes (Neurodevelopmental assessment = 68%) |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Oshiro 2019.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 35 VLBW infants | |
| Interventions | Probiotics (N = 17): Bifidobacterium breve BBG‐01 in human milk feeds once daily during the hospital stay Control (N = 18): placebo |
|
| Outcomes |
|
|
| Notes | Japan (2015 to 2017) Funding: Yakult Honsha Company, Japan (manufacturer of intervention) Additional data via personal communication: Dr Yuichiro Yamashiro UMIN Registration No. UMIN000005412 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked (probiotic added to milk by dieticians who were not involved in the care of the infant) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Masked |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Patole 2014.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 159 VLBW infants (< 33 weeks' gestation at birth) | |
| Interventions | Probiotics (N = 79): Bifidobacterium breve M‐16V in milk feeds once daily until term equivalent Control (N = 80): maltodextrin placebo |
|
| Outcomes | Probiotic colonisation of stool NEC, death, infection, blood culture‐positive sepsis by B. breve M‐16V (neurodevelopmental outcomes‐ Agrawal 2020) |
|
| Notes | Australia (2009 to 2012) Funding: Morinaga Milk Industry Company, Japan supplied the product free for the trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed, coded envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked (placebo‐controlled) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Near‐complete (6 infants withdrawn) |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Rehman 2018.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 146 VLBW preterm infants (gestational age at birth > 26 weeks') | |
| Interventions | Probiotics (N = 70): Bifidobacterium spp (not specified), Lactobacilli acidophilhis, Streptococcus thermophilus, L. delbrueckii with human milk or formula 2 times daily until hospital discharge Control (N = 70): unsupplemented milk feeds |
|
| Outcomes |
|
|
| Notes | Pakistan (2014) Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Unclear risk | Infection not reported |
Ren 2010.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 150 preterm infants (most participants were very preterm) | |
| Interventions | Probiotics (N = 79): Bifidobacterium infantis, Lactobacillus acidophilus, Bacillus cereus, and Enterococcus faecalis in milk feeds twice daily from day 7 after birth for 7 days (route translated as "oral or nasal"‐ presumed to refer to oro‐gastric or naso‐gastric tube) Control (N = 80): unsupplemented milk feeds |
|
| Outcomes | NEC | |
| Notes | China (single centre, 2006‐2008) Translation from Chinese courtesy of Yuan Chi |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Drawing lots" |
| Allocation concealment (selection bias) | Unclear risk | Safeguards unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to assess |
| Selective reporting (reporting bias) | Unclear risk | Mortality and infection not reported |
Reuman 1986.
| Study characteristics | ||
| Methods | Quasi‐RCT | |
| Participants | 30 very preterm infants (birth weight < 2000 g) | |
| Interventions | Probiotics (N = 15): Lactobacillus acidophilus in formula daily for 28 days Control (N = 15): unsupplemented formula feeds |
|
| Outcomes |
|
|
| Notes | US (early 1980s) Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random number charts and the last digit of patient's chart number, then alternate allocation of next participant |
| Allocation concealment (selection bias) | High risk | Unconcealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Unclear risk | Infection not reported |
Rougé 2009.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 94 very preterm or VLBW infants | |
| Interventions | Probiotics (N = 45): Lactobacillus rhamnosus GG and Bifidobacterium longum with human milk or formula once daily until discharge from hospital Control (N = 49): maltodextrin placebo |
|
| Outcomes |
|
|
| Notes | France (2005 to 2007) Funding: Programme Hospitalier de Recherche Clinique of the French Ministry of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Centrally allocated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked (placebo‐controlled) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Roy 2014.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 112 preterm VLBW infants | |
| Interventions | Probiotics (N = 56): Lactobacillus acidophilus, Bifidobacterium longum, B. bifidum, B. lactis 2 times daily with human milk for 6 weeks or until discharged from hospital Control (N = 56): sterile water as (quote:) "placebo" |
|
| Outcomes |
|
|
| Notes | India (2012 to 2013) Funding: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Centrally allocated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Sadowska‐Krawczenko 2012.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 55 very preterm or VLBW infants | |
| Interventions | Probiotics (N = 30): Lactobacillus rhamnosus 2 times daily in 2 mL of 5% dextrose until discharge from hospital Control (N = 25): maltodextrin placebo |
|
| Outcomes |
|
|
| Notes | Poland (2008 to 2009) Funding: Biomed Lublin, Poland supplied the intervention |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked (placebo‐controlled) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Saengtawesin 2014.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 60 VLBW infants with gestational age ≤ 34 weeks' at birth | |
| Interventions | Probiotics (N = 31): Lactobacillus acidophilus and Bifidobacterium bifidum (Infloran®) once daily with human milk or formula until 6 weeks or hospital discharge Control (N = 29): unsupplemented milk feeds | |
| Outcomes |
|
|
| Notes | Thailand (2012 to 2013) Funding: Queen Sirikit National Institute of Child Health, Perinatal Society of Thailand and DKSH (Thailand) Limited |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Samanta 2009.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 186 very preterm or VLBW infants | |
| Interventions | Probiotics (N = 91): Bifidobacteria infantis, B. bifidum, B. longum and Lactobacillus acidophilus with human milk 2 times daily until hospital discharge Control (N = 95): unsupplemented human milk feeds |
|
| Outcomes |
|
|
| Notes | India (2007 to 2008) Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Sari 2011.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 221 VLBW infants (gestational age < 33 weeks' at birth) | |
| Interventions | Probiotics (N = 110): Lactobacillus sporogenes in human milk or formula once daily until discharge from hospital Control (N = 111): unsupplemented milk feeds | |
| Outcomes |
|
|
| Notes | Turkey (2008 to 2009) Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Opaque, sequentially numbered, sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Caregivers masked, investigators not masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete for in hospital outcomes (Neurodevelopmental assessment = 84%) |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Serce 2013.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 208 very preterm or VLBW infants | |
| Interventions | Probiotics (N = 104): Saccharomyces boulardii in human milk or formula once daily until discharge from hospital Control (N = 104): unsupplemented milk feeds | |
| Outcomes |
|
|
| Notes | Turkey (2010 to 2011) Funding: Biocodex supplied the intervention |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Opaque, sequentially‐numbered, sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unsupplemented milk feeds‐ not placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Shadkam 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 60 preterm infants born between 28 to 34 weeks' gestation and birth weight 1000 g to 1800 g (most participants were very preterm or VLBW) | |
| Interventions | Probiotics (N = 30): Lactobacillus reuteri DSM 17938 2 times daily with human milk until full enteral feeding was reached (about 2 weeks) Control (N =30): unsupplemented milk feeds |
|
| Outcomes |
|
|
| Notes | Iran (2012 to 2013) Funding: Shahid Sadughi University, Iran |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States that random allocation software was used |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unsupplemented milk feeds‐ not placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Shashidhar 2017.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 104 VLBW infants | |
| Interventions | Probiotics (N = 52): Lactobacillus acidophilus, L. rhamnosus, Bifidobacterium longum and Saccharomyces boulardii (Darolac) once daily in human milk until discharge from hospital Control (N = 52): unsupplemented milk feeds |
|
| Outcomes |
|
|
| Notes | India (2012 to 2013) Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, opaque. sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unsupplemented milk feeds‐ not placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Near‐complete (3 infants in each group withdrawn) |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Stratiki 2007.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 77 preterm infants with gestation at birth > 26 weeks' (most participants were very preterm or VLBW) | |
| Interventions | Probiotics (N = 41): Bifidobacterium lactis supplemented formula for 30 days Control (N = 36): unsupplemented formula feeds |
|
| Outcomes |
|
|
| Notes | Greece (2004 to 2005) Funding: Nestlé, Vevey provide the B. lactis supplemented formula | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers generator |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unsupplemented milk feeds‐ not placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Near‐complete (3 infants not included in analyses) |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Strus 2018.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 181 preterm infants ≤ 34 weeks' gestation and birth weight 750 g to 1800 g (most participants were very preterm or VLBW) | |
| Interventions | Probiotics (N = 90): Lactobacillus rhamnosus KL53A and Bifidobacterium breve PB04 in milk feeds for 6 weeks or until hospital discharge Control (N = 91): maltodextrin placebo |
|
| Outcomes |
|
|
| Notes | Poland (2012 to 2013) Funding: IBSS BIOMED S.A., Krakow, Poland ClinicalTrials.gov no. NCT02073214 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Centrally allocated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked (placebo‐controlled) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Tewari 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 244 preterm infants < 34 weeks' gestation at birth (most participants were very preterm or VLBW) | |
| Interventions | Probiotics (N = 121): Bacillus clausii 3 times daily with human milk for 6 weeks, or until discharge or death or occurrence of late‐onset invasive infection Control (N= 123): sterile water placebo (probiotic and the placebo were identical in appearance) |
|
| Outcomes | NEC, death, infection, duration of hospital stay | |
| Notes | India (2012 to 14) Funding: Enterogermina, Sanofi‐Aventis, Italy supplied intervention |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Web‐based |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Totsu 2014.
| Study characteristics | ||
| Methods | Cluster‐RCT | |
| Participants | 283 VLBW infants in 19 neonatal centres | |
| Interventions | Probiotics (N = 10 centres; 153 infants*): Bifidobacterium bifidum with human milk or formula feeds 2 times daily until infant reached 2000 g body weight Control (N = 9 centres; 130 infants*): maltodextrin placebo *Inter‐cluster correlation correction of data for inclusion in meta‐analyses achieved by dividing numerators and denominator by the design effect (1.2779): Probiotics: adjusted N = 120 for in hospital outcomes; N = 80 for neurodevelopmental assessment outcomes Control: adjusted N = 102 for in hospital outcomes; N = 82 for neurodevelopmental assessment outcomes |
|
| Outcomes |
|
|
| Notes | Japan (19 centres: 2010 to 2011) Funding: Meiji, Tokyo, Japan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated (stratified by (quote: "patient volume" of centre) |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked (placebo‐controlled) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete for in hospital outcomes (Neurodevelopmental assessment = 73%) |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Van Niekerk 2014.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 184 VLBW infants (< 1250 g) | |
| Interventions | Probiotics (N = 91): Lactobacillus rhamnosus GG and Bifidobacterium infantis daily with human milk feeds for 4 weeks Control (N = 93): MCT oil placebo in milk feeds |
|
| Outcomes |
|
|
| Notes | South Africa (2011 to 2012) Funding: National Research Foundation, Nestle Nutrition Institute Africa, Medical Research Council and the Faculty of Medicine and Health Sciences, Stellenbosch University ClinicalTrials.gov no. NCT01868737 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent statistician‐generated |
| Allocation concealment (selection bias) | Low risk | Pharmacy allocation (stratified by maternal HIV status) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked (placebo‐controlled) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Wang 2007.
| Study characteristics | ||
| Methods | Quasi‐RCT | |
| Participants | 44 VLBW infants | |
| Interventions | Probiotics (N = 22): Bifidobacterium breve in milk feeds 2 times daily until hospital discharge Control (N = 33): unsupplemented milk feeds |
|
| Outcomes |
(NEC (courtesy of investigators)) |
|
| Notes | Japan (2001 to 2004) Funding: intervention provided by Morinaga Milk Industry, Kanagawa, Japan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate allocation |
| Allocation concealment (selection bias) | High risk | Unconcealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely (did not aim to assess clinical outcomes) |
Wejryd 2019.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 141 ELBW infants (of gestation born < 28 weeks') | |
| Interventions | Probiotics (N = 72): Lactobacillus reuteri DSM 17938 once daily with human milk until 36 weeks' postmenstrual age Control (N = 69): maltodextrin placebo |
|
| Outcomes |
|
|
| Notes | Sweden (10 centres: 2012 to 2015) Funding: Swedish Research Council, the Swedish Society for Medical Research, the Swedish Society of Medicine, the Research Council for the South‐East Sweden, ALF Grants, Region Ostergotland, the Ekhaga Foundation, and BioGaia AB ClinicalTrials.gov no. NCT01603368 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Centrally coded by sequential study number |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked (placebo‐controlled) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete |
| Selective reporting (reporting bias) | Low risk | Unlikely |
Zeber‐Lubecka 2016.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 55 preterm infant < 33 weeks' gestation (most participants were very preterm or VLBW) | |
| Interventions | Probiotics (N = 28): Saccharomyces boulardii once daily with human milk or formula feeds for six weeks Control (N = 27): maltodextrin placebo |
|
| Outcomes |
(NEC, death, infection‐ no events courtesy investigators) |
|
| Notes | Poland (study period not stated) Funding: The National Science Centre, Poland |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described (quote: "randomly divided") |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked (placebo‐controlled) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data from each group (10 from probiotics and 6 from placebo) – not accounted for |
| Selective reporting (reporting bias) | Low risk | Unlikely (primary aim to study intestinal microbiome) |
BBG‐01: Bifidobacterium breve;BSID: the Bayley Scales of Infant Development; ELBW: extremely low birth weight; g: gram(s); HIV: human immunodeficiency virus; LGG:Lactobacillus rhamnosus GG; MCT: medium chain triglycerides; MDI: Mental Developmental Index; NEC: necrotising enterocolitis; PDI: Psychomotor Development Index; RCT: randomised controlled trial; SD: standard deviation; VLBW: very low birth weight.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arora 2017 | Most participants not very preterm or VLBW. |
| Awad 2010 | Most participants not very preterm or VLBW. |
| Chi 2019 | Not an RCT. |
| Dasopoulou 2015 | RCT of prebiotics. |
| Deng 2010 | Most participants not very preterm or VLBW. |
| Denkel 2016 | Not an RCT. |
| Di 2010 | Most participants not very preterm or VLBW. |
| Dongol‐Singh 2017 | Most participants not very preterm or VLBW. |
| Hua 2014 | Most participants not very preterm or VLBW. |
| Hussain 2016 | Most participants not very preterm or VLBW. |
| Kaban 2019 | Most participants not very preterm or VLBW. |
| Ke 2008 | Most participants not very preterm or VLBW. |
| Koksal 2015 | RCT of synbiotics |
| Moles 2015 | A pilot study with including 5 infants. |
| Partty 2013 | Most participants not very preterm or VLBW. |
| Qiao 2017 | Most participants not very preterm or VLBW. |
| Rojas 2012 | Most participants not very preterm or VLBW. |
| Romeo 2011 | Most participants not very preterm or VLBW. |
| Shujie 2011 | Most participants not very preterm or VLBW. |
| Sinha 2015 | Most participants not very preterm or VLBW. |
| Thanhaeuser 2014 | Not an RCT. |
| Uhlemann 1999 | Most participants not very preterm or VLBW. |
| Underwood 2014 | RCT of prebiotics |
| Xu 2016 | Most participants not very preterm or VLBW. |
| Zhou 2012 | Most participants not very preterm or VLBW. |
| Zhuang 2007 | Most participants not very preterm or VLBW. |
RCT: randomised controlled trial; VLBW: very low birth weight
Characteristics of studies awaiting classification [ordered by study ID]
Coleta 2013.
| Methods | Randomised controlled trial |
| Participants | 60 preterm infants |
| Interventions | Probiotics (N = 31): human milk with Lactobacillus reuteri Control (N = 21): human milk alone |
| Outcomes | Efficacy of probiotics on digestive tolerance to enteral feeding |
| Notes | Romania (study period not stated) Unlikley to have been reported fully (unable to contact investigators) |
Punnahitananda 2006.
| Methods | RCT |
| Participants | VLBW infants |
| Interventions | Lactobacillus acidophilus and Bifidobacterium infantis |
| Outcomes | Late‐onset infection, NEC, feeding tolerance, time to full enteral feeding |
| Notes | Data presented at 14th Congress of the Federation of Asia Oceania Perinatal Societies, 2006, Bangkok,Thailand (report not available) |
NEC: necrotising enterocolitis; RCT: randomised controlled trial; VLBW: very low birth weight
Characteristics of ongoing studies [ordered by study ID]
Marisen 2019.
| Study name | Efficacy of Bifidobacterium longum, B. infantis and Lactobacillus acidophilus probiotics to prevent gut dysbiosis in preterm infants of 28‐ 32 weeks' gestation: a randomised, placebo‐controlled, double‐blind, multicentre trial: the PRIMAL Clinical Study protocol |
| Methods | RCT |
| Participants | Preterm infants (28 to 32 weeks') |
| Interventions | Bifidobacterium longum, B. infantis, and Lactobacillus acidophilus |
| Outcomes | Stool colonisation |
| Starting date | 2020 |
| Contact information | Christoph Hartel, Department of Paediatrics, University of Lübeck, Germany |
| Notes | Trial registration number: DRKS00013197 |
NCT00977912.
| Study name | Necrotizing enterocolitis (Nec) and B. Lactis in premature babies |
| Methods | RCT |
| Participants | VLBW infants |
| Interventions | B. lactis for 6 weeks |
| Outcomes | NEC, antibiotic administration, stool microbiology |
| Starting date | November 2009 |
| Contact information | Dr Peter Cooper, University of Witwatetersrand & Charlotte Maxek Johannestburg Academic Hospital, Zambia |
| Notes | (Quote:) "Terminated" in 2013 ‐ unlikely to have been completed (not reported) |
NCT01181791.
| Study name | Effects of Lactobacillus reuteri in premature infants (reuteri) |
| Methods | RCT |
| Participants | VLBW infant |
| Interventions | Lactobacillus reuteri during hospitalisation |
| Outcomes | Time to reach full enteral feeds, stool colonisation and Intestinal immunological response |
| Starting date | 2010 |
| Contact information | Teresa del Moral, University of Miami |
| Notes | Chile (Quote:) "Terminated" because of slow recruitment‐ unlikely to have been reported |
NCT01375309.
| Study name | Bifidobacterium supplementation for very low birth weight infants (Bifido(RCT)) |
| Methods | RCT |
| Participants | VLBW infants |
| Interventions | Bifidobacterium bifidum (duration not clear) |
| Outcomes | Time to full enteral feeding, weight gain, NEC |
| Starting date | 2011 |
| Contact information | Satoshi Kusuda, Professor of Neonatology, Tokyo Women's Medical University |
| Notes | (Quote:) "Completed" 2012 ‐ unlikely to have been reported |
NCT04541771.
| Study name | The role of Lactobacillus reuteri in preventing necrotizing enterocolitis (NEC) in pre‐term infants (NEC) |
| Methods | RCT |
| Participants | Preterm infants (28 to 34 weeks') |
| Interventions | Lactobacillus reuteri until 35 weeks' of gestation or discharged from hospital |
| Outcomes | NEC, infection |
| Starting date | 2020 |
| Contact information | Dr Summera Tabasum, The Children Complex & The Institute of Child Health, Multan |
| Notes | ClinicalTrials.gov identifier: NCT04541771 |
NEC: necrotising enterocolitis; RCT: randomised controlled trial; VLBW: very low birth weight
Differences between protocol and review
In the 2020 update:
new authors updated this review;
we restricted the population of interest to very preterm and VLBW infants in order to enhance applicability to those infants at high risk of developing NEC and associated complications;
we added the methodology and plan for 'Summary of findings' tables and GRADE recommendations, which were not included in the original protocol (AlFaleh 2005), or in previous publications of the review (Al Faleh 2008; Al Faleh 2011; Al Faleh 2014);
we updated the search strategy; and
we updated the "Risk of Bias" assessments.
Contributions of authors
SS and SO screened and appraised reports identified in the updated search, and extracted and analysed data.
NM undertook analyses for small‐study bias.
WM and MXRR arbitrated inclusion and data extraction disagreements, assessed the certainty of the evidence (GRADE), and drafted the review.
All authors contributed to the final manuscript.
Sources of support
Internal sources
-
Centre for Reviews and Dissemination, University of York, UK
Host department
-
Department of Clinical Epidemiology and Biostatistics. Faculty of Medicine. Pontificia Universidad Javeriana, Colombia
Host department
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
National Institute of Health Research (NIHR), UK
This report describes independent research funded by a UK NIHR Cochrane Incentive Award. The views expressed in this publication are those of the review authors and are not necessarily those of the NHS, the NIHR, or the UK Department of Health
Declarations of interest
SS is funded by the UK National Institute of Health Research (NIHR) for the review.
NM: the UK NIHR pays a grant to NM's institution.
MXRR has no interest to declare.
SO: the UK NIHR pays a grant to SO's institution. (SR‐PG 13/89/12).
WM: the UK NIHR pays a grant to WM's institution. WM is co‐coordinating editor of Cochrane Neonatal.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Agarwal 2003 {published data only}
- Agarwal R, Sharma N, Chaudhry R, Deorari A, Paul VK, Gewolb IH, et al. Effects of oral Lactobacillus GG on enteric microflora in low-birth-weight neonates. Journal of Pediatric Gastroenterology and Nutrition 2003;36(3):397-402. [DOI: 10.1097/00005176-200303000-00019] [PMID: ] [DOI] [PubMed] [Google Scholar]
Al‐Hosni 2012 {published data only}
- Al-Hosni M, Duenas M, Hawk M, Stewart LA, Borghese RA, Cahoon M, et al. Probiotics-supplemented feeding in extremely low-birth-weight infants. Journal of Perinatology 2012;32(4):253–9. [DOI: 10.1038/jp.2011.51] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Havranek T, Al-Hosni M, Armbrecht E. Probiotics supplementation increases intestinal blood flow velocity in extremely low birth weight preterm infants10.1038/jp.2012.37. Journal of Perinatology 2013;33(1):40-4. [DOI: 10.1038/jp.2012.37] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bin‐Nun 2005 {published data only}
- Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. Journal of Pediatrics 2005;147(2):192-6. [DOI: 10.1016/j.jpeds.2005.03.054] [PMID: ] [DOI] [PubMed] [Google Scholar]
Braga 2011 {published data only}
- Braga TD, da Silva GA, Lira PI, Carvalho Lima M. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. American Journal of Clinical Nutrition 2011;93(1):81–6. [DOI: 10.3945/ajcn.2010.29799] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chandrashekar 2018 {published data only}
- Chandrashekar GS, Shettigar S, Varghese TC. Role of probiotics in prevention of necrotizing enterocolitis in preterm neonates. Indian Journal of Child Health 2018;5(2):112-5. [DOI: 10.32677/IJCH.2018.v05.i02.010] [DOI] [Google Scholar]
Chowdhury 2016 {published data only}
- Chowdhury T, Ali MM, Hossain MM, Singh J, Yousuf AM, Yasmin F, et al. Efficacy of probiotics versus placebo in the prevention of necrotizing enterocolitis in preterm very low birth weight infants: a double-blind randomized controlled trial. Journal of the College of Physicians and Surgeons Pakistan 2016;26(9):770-4. [PMID: ] [PubMed] [Google Scholar]
Chrzanowska‐Liszewska 2012 {published data only}
- Chrzanowska-Liszewska D, Seliga-Siwecka J, Kornacka MK. The effect of Lactobacillus rhamnosus GG supplemented enteral feeding on the microbiotic flora of preterm infants-double blinded randomized control trial. Early Human Development 2012;88(1):57–60. [DOI: 10.1016/j.earlhumdev.2011.07.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Costalos 2003 {published data only}
- Costalos C, Skouteri V, Gounaris A, Sevastiadou S, Triandafilidou A, Ekonomidou C, et al. Enteral feeding of premature infants with Saccharomyces boulardii. Early Human Development 2003;74(2):89-96. [DOI: 10.1016/s0378-3782(03)00090-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Costeloe 2015 {published data only}
- Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MR. Bifidobacterium breve BBG-001 in very preterm infants:a randomised controlled phase 3 trial. Lancet 2015;15:01027-2. [DOI: 10.1016/S0140-6736(15)01027-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dani 2002 {published data only}
- Dani C, Biadaioli R, Bertini G, Martelli E, Rubaltelli FF. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biology of the Neonate 2002;82(2):103-8. [DOI: 10.1159/000063096] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dashti 2014 {published data only}
- Dashti AS, Afjey SA, Basiry A, Shirvani F, Seifi K, Taheri ZM. Prophylactic probiotics for prevention of necrotizing enterocolitis (NEC) in low birth weight neonates. Archives of Pediatric Infectious Diseases 2014;1(4):174-9. [DOI: 10.5812/pedinfect.11603] [DOI] [Google Scholar]
Demirel 2013 {published data only}
- Demirel G, Erdeve O, Celik IH, Dilmen U. Saccharomyces boulardii for prevention of necrotizing enterocolitis in preterm infants: a randomized, controlled study. Acta Paediatrica 2013;102(12):e560-5. [DOI: 10.1111/apa.12416] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dilli 2015 {published data only}
- Dilli D, Aydin B, Fettah ND, Ozyazıcı E, Beken S, Zenciroglu A, et al. The propre-save study: effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis on very low birth weight infants. Journal of Pediatrics 2015;166:545-51. [DOI: 10.1016/j.jpeds.2014.12.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dutta 2015 {published data only}
- Dutta S, Ray P, Narang A. Comparison of stool colonization in premature infants by three dose regimes of a probiotic combination: a randomized controlled trial. American Journal of Perinatology 2015;32:733–40. [DOI: 10.1055/s-0034-1395473] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fernández‐Carrocera 2013 {published data only}
- Fernández-Carrocera LA, Solis-Herrera A, Cabanillas-Ayón M, Gallardo-Sarmiento RB, García-Pérez CS, Montaño-Rodríguez R, et al. Double-blind, randomised clinical assay to evaluate the efficacy of probiotics in preterm newborns weighing less than 1500 g in the prevention of necrotising enterocolitis. Archives of Diseases in Childhood. Fetal and Neonatal Edition 2013;98(1):F5-9. [DOI: 10.1136/archdischild-2011-300435] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fujii 2006 {published data only}
- Fujii T, Ohtsuka Y, Lee T, Kudo T, Shoji H, Sato H, et al. Bifidobacterium breve enhances transforming growth factor beta1 signaling by regulating Smad7 expression in preterm infants. Journal of Pediatric Gastroenterology and Nutrition 2006;43(1):83-8. [DOI: 10.1097/01.mpg.0000228100.04702.f8] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Li Y, Shimizu T, Hosaka A, Kaneko N, Ohtsuka Y, Yamashiro Y. Effects of bifidobacterium breve supplementation on intestinal flora of low birth weight infants. Pediatrics International 2004;46(5):509-15. [DOI: 10.1111/j.1442-200x.2004.01953.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hariharan 2016 {published data only}
- Hariharan D, Balasubramanian L, Kannappan V, Veluswami G. Probiotic supplementation in VLBW preterm infants improves feeding tolerance and reduces risk of gram negative sepsis. In: Journal of Pediatric Gastroenterology and Nutrition. Vol. 62. 49th Annual Meeting of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition, 2016:655.
Hays 2015 {published data only}
- Hays S, Jacquot A, Gauthier H, Kempf C, Beissel A, Pidoux O, et al. Probiotics and growth in preterm infants: a randomized controlled trial. Clinical Nutrition 2015;35(4):802-11. [DOI: 10.1016/j.clnu.2015.06.006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hernandez‐Enriquez 2016 {published data only}
- Hernandez-Enriquez NP, Rosas-Sumano AB, Monzoy-Ventre MA, Galicia-Flores L. Lactobacillus reuteri DSM 17938 in preventing necrotizing enterocolitis in preterm newborns. Pilot study of efficacy and safety [Lactobacillus reuteri DSM 17938 en la prevención de enterocolitis necrosante en recién nacidos prematuros. Estudio piloto de eficacia y seguridad]. Revista Mexicana de Pediatría 2016;83(2):37-43. [Google Scholar]
Hikaru 2010 {published data only}
- Hikaru U, Koichi S, Yayoi S, Hiromichi S, Hiroaki S, Yoshikazu O. Bifidobacteria prevents preterm infants from developing infection and sepsis. International Journal of Probiotics and Prebiotics 2010;5(1):33-6. [Google Scholar]
Huang 2009 {published data only}
- Huang B, Yang H, Huang X. Probiotics supplementation for prevention of necrotizing enterocolitis in very low-birth-weight neonates: a randomized, controlled trial. Journal of Guangdong Medical College 2009;27:37-9. [Google Scholar]
Indrio 2017 {published data only}
- Indrio F, Riezzo G, Tafuri S, Ficarella M, Carlucci B, Bisceglia M, et al. Probiotic supplementation in preterm: feeding intolerance and hospital cost. Nutrients 2017;9(9):965. [DOI: 10.3390/nu9090965] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobs 2013 {published data only}
- Jacobs SE, Tobin JM, Opie GF, Donath S, Tabrizi SN, Pirotta M, et al. Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics 2013;132(6):1055-62. [DOI: 10.1542/peds.2013-1339] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Plummer EL, Bulach DM, Murray GL, Jacobs SE, Tabrizi SN, Garland SM, ProPrems Study Group. Gut microbiota of preterm infants supplemented with probiotics: sub-study of the ProPrems trial. BMC Microbiology 2018;18(1):184. [DOI: 10.1186/s12866-018-1326-1] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanic 2015 {published data only}
- Kanic Z, Turk DM, Burja S, Kanic V, Dinevski D. Influence of a combination of probiotics on bacterial infections in very low birthweight newborns. Wiener Klinische Wochenschrift 2015;127:S210-5. [DOI: 10.1007/s00508-015-0845-0] [DOI: ] [DOI] [PubMed] [Google Scholar]
Kitajima 1997 {published data only}
- Kitajima H, Sumida Y, Tanaka R, Yuki N, Takayama H, Fujimura M. Early administration of Bifidobacterium breve to preterm infants: randomised controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 1997;76(2):F101-7. [DOI: 10.1136/fn.76.2.f101] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2019 {published data only}
- Li YF, Zhu CR, Gong XL, Li HL, Xiong LK, Wang KJ, et al. Beneficial effects of probiotic treatment on gut microbiota in very low birth weight infants. Gastroenterology Research and Practice 2019;3682836:eCollection 2019. [DOI: 10.1155/2019/3682836] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2005 {published data only}
- Chou IC, Kuo HT, Chang JS, Wu SF, Chiu HY, Su BH, et al. Lack of effects of oral probiotics on growth and neurodevelopmental outcomes in preterm very low birth weight infants. Journal of Pediatrics 2010;156(3):393-6. [DOI: 10.1016/j.jpeds.2009.09.051] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, Yeh TF, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2005;115(1):1-4. [DOI: 10.1542/peds.2004-1463] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lin 2008 {published data only}
- Lin HC, Hsu CH, Chen HL, Chung MY, Hsu JF, Lien RI, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics 2008;122(4):693–700. [DOI: 10.1542/peds.2007-3007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Manzoni 2006 {published data only}
- Manzoni P, Mostert M, Leonessa ML, Priolo C, Farina D, Monetti C, et al. Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species in preterm neonates: a randomized study. Clinical Infectious Diseases 2006;42(12):1735-42. [DOI: 10.1086/504324] [PMID: ] [DOI] [PubMed] [Google Scholar]
Manzoni 2009 {published data only}
- Manzoni P, Meyer M, Stolfi I, Rinaldi M, Cattani S, Pugni L, et al. Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very-low-birth-weight neonates: a randomized clinical trial. Early Human Development 2014;90(Suppl 1):S60-5. [DOI: 10.1016/S0378-3782(14)70020-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Manzoni P, Rinaldi M, Cattani S, Pugni L, Romeo MG, Messner H, et al, Italian Task Force for the Study and Prevention of Neonatal Fungal Infections, Italian Society of Neonatology. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA 2009;302(13):1421-8. [DOI: 10.1001/jama.2009.1403] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Manzoni P, Sánchez RG, Meyer M, Stolfi I, Pugni L, Messner H, et al, Italian Task Force for the Study, and Prevention of Neonatal Fungal Infections and the Italian Society of Neonatology. Exposure to gastric acid inhibitors increases the risk of infection in preterm very low birth weight infants but concomitant administration of lactoferrin counteracts this effect. Journal of Pediatrics 2018;193:62-7.e1. [DOI: 10.1016/j.jpeds.2017.09.080] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mihatsch 2010 {published data only}
- Mihatsch WA, Vossbeck S, Eikmanns B, Hoegel J, Pohlandt F. Effect of Bifidobacterium lactis on the incidence of nosocomial infections in very-low-birth-weight infants: a randomized controlled trial. Neonatology 2010;98(2):156–63. [DOI: 10.1159/000280291] [PMID: ] [DOI] [PubMed] [Google Scholar]
Millar 1993 {published data only}
- Millar MR, Bacon C, Smith SL, Walker V, Hall MA. Enteral feeding of premature infants with Lactobacillus GG. Archives of Disease in Childhood 1993;69(5 Spec No):483-7. [DOI: 10.1136/adc.69.5_spec_no.483] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansbridge EM, Walker V, Hall MA, Smith SL, Millar MR, Bacon C, et al. Effects of feeding premature infants with Lactobacillus GG on gut fermentation. Archives of Disease in Childhood 1993;69(5 Spec No):488-92. [DOI: 10.1136/adc.69.5_spec_no.488] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohan 2006 {published and unpublished data}
- Mohan R, Koebnick C, Schildt J, Schmidt S, Mueller M, Possner M, et al. Effects of Bifidobacterium lactis Bb12 supplementation on intestinal microbiota of preterm infants: a double-blind, placebo-controlled, randomized study. Journal of Clinical Microbiology 2006;44(11):4025–31. [DOI: 10.1128/JCM.00767-06] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oncel 2014 {published data only}
- Akar M, Eras Z, Oncel MY, Arayici S, Guzoglu N, Canpolat FE, et al. Impact of oral probiotics on neurodevelopmental outcomes in preterm infants. Journal of Maternal-Fetal & Neonatal Medicine 2017;30(4):411-5. [DOI: 10.1080/14767058.2016.1174683] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Oncel MY, Sari FN, Arayici S, Guzoglu N, Erdeve O, Uras N, et al. Lactobacillus reuteri for the prevention of necrotising enterocolitis in very low birth weight infants: a randomised controlled trial. Archives of Disease in Childhood Fetal & Neonatal Edition 2014;99(2):F110–5. [DOI: 10.1136/archdischild-2013-304745] [PMID: ] [DOI] [PubMed] [Google Scholar]
Oshiro 2019 {published data only}
- Oshiro T, Nagata S, Wang C, Takahashi T, Tsuji H, Asahara T, et al. Bifidobacterium supplementation of colostrum and breast milk enhances weight gain and metabolic responses associated with microbiota establishment in very-preterm infants. Biomedicine Hub 2019;4(3):1-10. [DOI: 10.1159/000502935] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patole 2014 {published data only}
- Agrawal S, Pestell CF, Granich J, Rao S, Nathan E, Wray JA, et al. Difficulties in developmental follow-up of preterm neonates in a randomised-controlled trial of Bifidobacterium breve M16-V—Experience from Western Australia. Early Human Development 2020;151:105165. [DOI: 10.1016/j.earlhumdev.2020.105165] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Patole S, Keil AD, Chang A, Nathan E, Doherty D, Simmer K, et al. Effect of Bifidobacterium breve M-16V supplementation on fecal bifidobacteria in preterm neonates - a randomised double blind placebo controlled trial. PLOS One 2014;9(3):e89511. [DOI: 10.1371/journal.pone.008951] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rehman 2018 {published data only}
- Rehman SU, Iqbal A, Ali W. Role of probiotics in reducing frequency of necrotizing enterocolitis in preterm neonates. Pakistan Pediatric Journal 2018;42(3):171-6. [Google Scholar]
Ren 2010 {published data only}
- Ren B. Preventive effect of Bifidobacterium tetravaccine tablets in premature infants with necrotizing enterocolitis. Journal of Pediatric Pharmacy 2010;16(2):24-5. [Google Scholar]
Reuman 1986 {published data only}
- Reuman PD, Duckworth DH, Smith KL, Kagan R, Bucciarelli RL, Ayoub EM. Lack of effect of Lactobacillus on gastrointestinal bacterial colonization in premature infants. Pediatric Infectious Disease 1986;5(6):663-8. [DOI: 10.1097/00006454-198611000-00013] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rougé 2009 {published data only}
- Rougé C, Piloquet H, Butel MJ, Berger B, Rochat F, Ferraris L, et al. Oral supplementation with probiotics in very low-birth-weight preterm infants: a randomized,double-blind, placebo-controlled trial. American Journal of Clinical Nutrition 2009;89(6):1828-35. [DOI: 10.3945/ajcn.2008.26919] [PMID: ] [DOI] [PubMed] [Google Scholar]
Roy 2014 {published data only}
- Roy A, Chaudhuri J, Sarkar D, Ghosh P, Chakraborty S. Role of enteric supplementation of probiotics on late-onset sepsis by Candida species in preterm low birth weight neonates: a randomized, double blind, placebo-controlled trial. North American Journal of Medical Sciences 2014;6:50-7. [DOI: 10.4103/1947-2714.125870] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sadowska‐Krawczenko 2012 {published data only}
- Sadowska-Krawczenko IK, Polak P, Wietlicka-Piszcz A, Szajewska H. Lactobacilllus rhamnosus ATC A07FA for preventing necrotizing enterocolitis in very-low-birth-weight preterm infants: a randomized controlled trial (preliminary results) [Ocena skutecznoci Lactobacillus rhamnosus ATC A07FAw zapobieganiu martwiczego zapalenia jelit wczeniaków z bardzoma urodzeniow mas ciaa: badanie z randomizacj (wstpnewyniki)]. Polish Journal of Pediatrics 2012;87(2):139-45. [Google Scholar]
Saengtawesin 2014 {published data only}
- Saengtawesin V, Tangpolkaiwalsak R, Kanjanapattankul W. Effect of oral probiotics supplementation in the prevention of necrotizing enterocolitis among very low birth weight preterm infants. Journal of the Medical Association of Thailand 2014;97(Suppl. 6):S20-5. [PMID: 25391168] [PubMed] [Google Scholar]
Samanta 2009 {published data only}
- Samanta M, Sarkar M, Ghosh P, Ghosh JK, Sinha MK, Chatterjee S. Prophylactic probiotics for prevention of necrotizing enterocolitis in very low birth weight newborns. Journal of Tropical Pediatrics 2009;55(2):128-31. [DOI: 10.1093/tropej/fmn091] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sari 2011 {published data only}
- Sari FN, Dizdar EA, Oguz S, Erdeve O, Uras N, Dilmen U. Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: a randomized, controlled trial. European Journal of Clinical Nutrition 2011;65(4):434-9. [DOI: 10.1038/ejcn.2010.278] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Sari FN, Eras Z, Dizdar EA, Erdeve O, Oguz SS, Uras N, et al. Do oral probiotics affect growth and neurodevelopmental outcomes in very low-birth-weight preterm infants? American Journal of Perinatology 2012;29(8):579-86. [DOI: 10.1055/s-0032-1311981] [DOI: 10.1055/s-0032-1311981] [PMID: ] [DOI] [PubMed] [Google Scholar]
Serce 2013 {published data only}
- Serce O, Benzer D, Gursoy T, Karatekin G, Ovali F. Efficacy of saccharomyces boulardii on necrotizing enterocolitis or sepsis in very low birth weight infants: a randomised controlled trial. Early Human Development 2013;89(12):1033–6. [DOI: 10.1016/j.earlhumdev.2013.08.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Serce O, Gursoy T, Ovali F, Karatekin G. Effects of Saccharomyces boulardii on neonatal hyperbilirubinemia: a randomized controlled trial. American Journal of Perinatology 2015;30(2):137-42. [DOI: 10.1055/s-0034-1376390] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shadkam 2015 {published data only}
- Shadkam MN, Jalalizadeh F, Nasiriani K. Effects of probiotic lactobacillus reuteri (DSM 17938) on the incidence of necrotizing enterocolitis in very low birth weight premature infants. Iranian Journal of Neonatology 2015;6(4):15-20. [DOI: 10.22038/IJN.2015.6143] [DOI] [Google Scholar]
Shashidhar 2017 {published data only}
- Shashidhar A, Suman Rao PN, Nesargi S, Bhat S, Chandrakala BS. Probiotics for promoting feed tolerance in very low birth weight neonates - a randomized controlled trial. Indian Pediatrics 2017;54(5):363-7. [DOI: 10.1007/s13312-017-1106-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stratiki 2007 {published data only}
- Stratiki Z, Costalos C, Sevastiadou S, Kastanidou O, Skouroliakou M, Giakoumatou A, et al. The effect of a bifidobacteria supplemented bovine milk on intestinal permeability of preterm infants. Early Human Development 2007;83(9):575–9. [DOI: 10.1016/j.earlhumdev.2006.12.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Strus 2018 {published data only}
- Strus M, Helwich E, Lauterbach R, Rzepecka-Węglarz B, Nowicka K, Wilińska M, et al. Effects of oral probiotic supplementation on gut Lactobacillus and Bifidobacterium populations and the clinical status of low-birth-weight preterm neonates: a multicenter randomized, double-blind,placebo-controlled trial. Infection and Drug Resistance 2018;11:1557-71. [DOI: 10.2147/IDR.S166348] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tewari 2015 {published data only}
- Tewari VV, Dubey SK, Gupta G. Bacillus clausii for prevention of late-onset sepsis in preterm infants: a randomized controlled trial. Journal of Tropical Pediatrics 2015;61:377–85. [DOI: 10.1093/tropej/fmv050] [PMID: ] [DOI] [PubMed] [Google Scholar]
Totsu 2014 {published data only}
- Totsu S, Terahara M, Kusuda S. Probiotics and the development of very low birthweight infants: follow-up study of a randomised trial. BMJ Paediatrics Open 2018;2(1):e000256. [DOI: 10.1136/bmjpo-2018-000256] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totsu S, Yamasaki C, Terahara M, Uchiyama A, Kusuda S, Probiotics Study Group in Japan. Bifidobacterium and enteral feeding in preterm infants: cluster-randomized trial. Pediatrics International 2014;56(5):714-9. [DOI: 10.1111/ped.12330] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Niekerk 2014 {published data only}
- Van Niekerk E, Kirsten GF, Nel DG, Blaauw R. Probiotics, feeding tolerance, and growth: a comparison between HIV-exposed and unexposed very low birth weight infants. Nutrition 2014;30(6):645-53. [DOI: 10.1016/j.nut.2013.10.024] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wang 2007 {published data only}
- Wang C, Shoji H, Sato H, Nagata S, Ohtsuka Y, Shimizu T, et al. Effects of oral administration of bifidobacterium breve on fecal lactic acid and short-chain fatty acids in low birth weight infants. Journal of Pediatric Gastroenterology and Nutrition 2007;44(2):252-7. [DOI: 10.1097/01.mpg.0000252184.89922.5f] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wejryd 2019 {published data only}
- Wejryd E, Marchini G, Frimmel V, Jonsson B, Abrahamsson T. Probiotics promoted head growth in extremely low birthweight infants in a double-blind placebo-controlled trial. Acta Paediatrica 2019;108(1):62-9. [DOI: 10.1111/apa.14497] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zeber‐Lubecka 2016 {published data only}
- Zeber-Lubecka N, Kulecka M, Ambrozkiewicz F, Paziewska A, Lechowicz M, Konopka E, et al. Effect of Saccharomyces boulardii and mode of delivery on the early development of the gut microbial community in preterm infants. PLOS One 2016;11(2):e0150306. [DOI: 10.1371/journal.pone.0150306] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Arora 2017 {published data only}
- Arora S, Khurana MS, Saini R. To study the role of probiotics in the prevention of necrotizing enterocolitis in preterm neonates. International Journal of Contempary Pediatrics 2017;4(5):6. [DOI: 10.18203/2349-3291.ijcp20173787] [DOI] [Google Scholar]
Awad 2010 {published data only}
- Awad H, Mokhtar H, Imam SS, Gad GI, Hafez H, Aboushady N. Comparison between killed and living probiotic usage versus placebo for the prevention of necrotizing enterocolitis and sepsis in neonates. Pakistan Journal of Biological Sciences 2010;13(6):253-62. [DOI: 10.3923/pjbs.2010.253.262] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chi 2019 {published data only}
- Chi C, Xue Y, Liu R, Wang Y, Lv N, Zeng H, et al. Effects of a formula with a probiotic Bifidobacterium lactis supplement on the gut microbiota of low birth weight infants. European Journal of Nutrition 2019;59(4):1493–503. [DOI: 10.1007/s00394-019-02006-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dasopoulou 2015 {published data only}
- Dasopoulou M, Briana DD, Boutsikou T, Karakasidou E, Roma E, Costalos C, et al. Motilin and gastrin secretion and lipid profile in preterm neonates following prebiotics supplementation: a double-blind randomized controlled study. Journal of Parenteral and Enteral Nutrition 2015;39(3):359-68. [DOI: 10.1177/0148607113510182] [PMID: ] [DOI] [PubMed] [Google Scholar]
Deng 2010 {published data only}
- Deng J, Chen K. Early minimal feeding combined with probiotics to prevent necrotizing enterocolitis in preterm infant. Chinese Journal of Modern Drug Application 2010;4(6):13-4. [Google Scholar]
Denkel 2016 {published data only}
- Denkel LA, Schwab F, Garten L, Geffers C, Gastmeier P, Piening B. Protective effect of dual-strain probiotics in preterm infants: a multi-center time series analysis. PLOS One 2016;11(6):e0158136. [DOI: 10.1371/journal.pone.0158136] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Di 2010 {published data only}
- Di M, Li X. Effects of Bifidobacterium supplementation for prevention of necrotizing enterocolitis in preterm infants: a randomized, controlled trial. Zhong Guo She Qu Yi Shi 2010;231:69. [Google Scholar]
Dongol‐Singh 2017 {published data only}
- Dongol Singh S, Klobassa DS, Resch B, Urlesberger B, Shrestha RP. Placebo controlled introduction of prophylactic supplementation of probiotics to decrease the incidence of necrotizing enterocolitis at Dhulikhel Hospital in Nepal. Kathmandu University Medical Journal 2017;15(60):319-23. [PMID: ] [PubMed] [Google Scholar]
Hua 2014 {published data only}
- Hua X-T, Tang J, Mu D-Z. Effect of oral administration of probiotics on intestinal colonization with drug resistant bacteria in preterm infants [口服益生菌对早产儿肠道耐药菌定植的影响]. Chinese Journal of Contempoary Pediatrics 2014;16(6):606-9. [PMID: ] [PubMed] [Google Scholar]
Hussain 2016 {published data only}
- Hussain M, Jabeen S, Subhani RU. Role of probiotics in prevention of necrotizing enterocolitis in preterm low birth weight neonates. Pakistan Journal of Medicine and Health Sciences 2016;10:455-9. [Google Scholar]
Kaban 2019 {published data only}
- Kaban RK, Hegar B, Rohsiswatmo R, Handryastuti S, Amelia N, Muktiarti D, et al. Lactobacillus reuteri DSM 17938 improves feeding intolerance in preterm infants. Pediatric Gastroenterology, Hepatology & Nutrition 2019;22(6):545-53. [DOI: 10.5223/pghn.2019.22.6.545] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ke 2008 {published data only}
- Ke D, Su Z, Li L. Effects of Bifido supplement for prevention of necrotizing enterocolitis in preterm infants: a randomized controlled trial. Chinese Pediatric Emergency Medicine 2008;12:69-71. [Google Scholar]
Koksal 2015 {published data only}
- Köksal N, Varal İ, Özkan H, Bagcı O, Doğan P. Effect of probiotic support on feeding intolerance and mortality at preterm infants. In: Journal of Perinatal Medicine. Vol. 43. 2015:P-0612.
Moles 2015 {published data only}
- Moles L, Escribano E, De Andres J, Montes MT, Rodriguez JM, Jimenez E, et al. Administration of Bifidobacterium breve PS12929 and Lactobacillus salivarius PS12934, two strains isolated from human milk, to very low and extremely low birth weight preterm infants: A pilot study. Journal of Immunology Research 2015;2015:538171. [DOI: 10.1155/2015/538171] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Partty 2013 {published data only}
- Partty A, Luoto R, Kalliomaki M, Salminen S, Isolauri E. Effects of early prebiotic and probiotic supplementation on development of gut microbiota and fussing and crying in preterm infants: a randomized, double-blind, placebo-controlled trial. Journal of Pediatrics 2013;163:1272-7. [DOI: 10.1016/j.jpeds.2013.05.035] [PMID: ] [DOI] [PubMed] [Google Scholar]
Qiao 2017 {published data only}
- Qiao LX, Zhu WY, Zhang HY, Wang H. Effect of early administration of probiotics on gut microflora and feeding in pre-term infants: arandomized controlled trial. Journal of Maternal-Fetal & Neonatal Medicine 2017;30(1):13-6. [DOI: 10.3109/14767058.2016.1163674] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rojas 2012 {published data only}
- Rojas MA, Lozano JM, Rojas MX, Rodriguez VA, Rondon MA, Bastidas JA, et al. Prophylactic probiotics to prevent death and nosocomial infection in preterm infants. Pediatrics 2012 Nov;130(5):e1113-20. [DOI: 10.1542/peds.2011-3584] [PMID: ] [DOI] [PubMed] [Google Scholar]
Romeo 2011 {published data only}
- Romeo MG, Romeo DM, Trovato L, Oliveri S, Palermo F, Cota F, et al. Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: incidence of late-onset sepsis and neurological outcome. Journal of Perinatology 2011;31(1):63–9. [DOI: 10.1038/jp.2010.57] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shujie 2011 {published data only}
- Shujie Y, Haiying Y, Bin G, Shu X, Xianglan D, Jiang W. The clinical application value of endangered preterm infants given earlier amounts of micro feedings and adding probiotics. Journal of Pediatric Pharmacy 2011;17:21-4. [Google Scholar]
Sinha 2015 {published data only}
- Sinha A, Gupta SS, Chellani H, Maliye C, Kumari V, Arya S, et al. Role of probiotics VSL#3 in prevention of suspected sepsis in low birthweight infants in India: a randomised controlled trial. BMJ Open 2015;5(7):e006564. [DOI: 10.1136/bmjopen-2014-006564] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thanhaeuser 2014 {published data only}
- Thanhaeuser M, Repa A, Weber M, Endress D, Kreissl A, Binder C, et al. Probiotics (infloran) for NEC prevention: Influence of enteral nutrition. In: Archives of Disease in Childhood. 5th Congress of the European Academy of Paediatric Societies, EAPS 2014, Barcelona, Spain. Vol. 99. 2014:A176-7.
Uhlemann 1999 {published data only}
- Uhlemann M, Heine W, Mohr C, Plath C, Pap S. Effects of oral administration of bifidobacteria on intestinal microflora in premature and newborn infants newborn infants. Zeitschrift fur Geburtshilfe und Neonatologie 1999;203(5):213-7. [PMID: ] [PubMed] [Google Scholar]
Underwood 2014 {published data only}
- Underwood MA, Kalanetra KM, Bokulich NA, Mirmiran M, Barile D, Tancredi DJ, et al. Prebiotic oligosaccharides in premature infants. Journal of Pediatric Gastroenterology and Nutrition 2014;58(3):352-60. [DOI: 10.1097/MPG.0000000000000211] [PMID: ] [DOI] [PubMed] [Google Scholar]
Xu 2016 {published data only}
- Xu L, Wang Y, Wang Y, Fu J, Sun M, Mao Z et al. A double-blinded randomized trial on growth and feeding tolerance with Saccharomyces boulardii CNCMI-745 in formula-fed preterm infants. Jornal de Pediatria 2016;92(3):296-301. [DOI: 10.1016/j.jped.2015.08.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhou 2012 {published data only}
- Zhou N. The observation of effect of probiotics in the prevention of neonatal necrotizing enterocolitis. Chinese Journal of Ethnomedicine and Ethnopharmacy 2012;21:81. [Google Scholar]
Zhuang 2007 {published data only}
- Zhuang X-Y, Li X-Y, Gao X-X, Su L-D. Relative factors of neonatal necrotizing enterocolitis and preventive effect of microeco-preparation. Journal of Applied Clinical Pediatrics 2006;22:1392-3. [Google Scholar]
References to studies awaiting assessment
Coleta 2013 {published data only}
- Coleta E, Gheonea M, Sarbu M. Oral supplementation with probiotics in premature infants-a randomised clinical trial. In: Intensive Care Medicine. 24th Annual Meeting of the European Society of Paediatric and Neonatal Intensive Care edition. Vol. 39. Rotterdam, Netherlands, 2013:S113.
Punnahitananda 2006 {unpublished data only}ISRCTN39142169
- Punnahitananda S, Thaithumyanon P, Soongsawang K. Nosocomial infection and necrotizing enterocolitis in preterm neonates treated with Lactobacillus acidophilus and Bifidobacterium infantis in a neonatal intensive care unit: a randomized controlled study. In: 14th Congress of the Federation of Asia Oceania Perinatal Societies. Bangkok, Thailand, 2006.
References to ongoing studies
Marisen 2019 {published data only}
- Marisen J, Hais A, Meyer C, Van Rossum T, Bunte LM, Frommhold D, et al. Efficacy of Bifidobacterium longum, B. infantis and Lactobacillus acidophilus probiotics to prevent gut dysbiosis in preterm infants of 28+0-32+6 weeks of gestation: a randomised, placebo-controlled, double-blind, multicentre trial: the PRIMAL Clinical Study protocol. BMJ Open 2019;9(11):e032617. [DOI: 10.1136/bmjopen-2019-032617] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00977912 {unpublished data only}
- NCT00977912. Necrotizing enterocolitis (Nec) and B. Lactis in premature babies [Prevention of NEC in preterm Infants with B. lactis]. clinicaltrials.gov/ct2/show/NCT00977912 (first received 16 September 2009).
NCT01181791 {unpublished data only}
- NCT01181791. Effects of Lactobacillus reuteri in premature infants (reuteri) [Pilot study to evaluate the effects of Lactobacillus reuteri in preterm newborns]. clinicaltrials.gov/ct2/show/NCT01181791 (first received 13 August 2010).
NCT01375309 {unpublished data only}
- NCT01375309. Bifidobacterium supplementation for very low birth weight infants (Bifido(RCT)) [Effect of bifidobacterium bifidum supplementation on morbidity of very low birth weight infants]. clinicaltrials.gov/ct2/show/NCT01375309 (first received 17 June 2011).
NCT04541771 {published data only}
- NCT04541771. The role of Lactobacillus reuteri in preventing necrotizing enterocolitis (NEC) in pre-term infants (NEC) [The role of Lactobacillus reuteri (L. reuteri) in preventing necrotizing enterocolitis (NEC) in pre-term infants less than 34 weeks of gestation]. clinicaltrials.gov/ct2/show/NCT04541771 (first received 9 September 2020).
Additional references
Abdulkadir 2016
- Abdulkadir B, Nelson A, Skeath T, Marrs EC, Perry JD, Cummings SP, et al. Routine use of probiotics in preterm infants: longitudinal impact on the microbiome and metabolome. Neonatology 2016;109(4):239-47. [DOI: 10.1159/000442936] [PMID: ] [DOI] [PubMed] [Google Scholar]
Alcon‐Giner 2020
- Alcon-Giner C, Dalby MJ, Caim S, , Ketskemety J, Shaw A, Sim K, et al. Microbiota supplementation with Bifidobacterium and Lactobacillus modifies the preterm infant gut microbiota and metabolome: an observational study. Cell Reports Medicine 2020;1(5):100077. [DOI: 10.1016/j.xcrm.2020.100077] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Battersby 2018
- Battersby C, Santhalingam T, Costeloe K, Modi N. Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Archives of Disease in Childhood Fetal and Neonatal Edition 2018;103(2):F182-9. [DOI: 10.1136/archdischild-2017-313880] [PMID: ] [DOI] [PubMed] [Google Scholar]
Berrington 2012
- Berrington JE, Hearn RI, Bythell M, Wright C, Embleton ND. Deaths in preterm infants: changing pathology over 2 decades. Journal of Pediatrics 2012;160(1):49-53. [DOI: 10.1016/j.jpeds.2011.06.046] [PMID: ] [DOI] [PubMed] [Google Scholar]
Berrington 2019
- Berrington JE, Zalewski S. The future of probiotics in the preterm infant. Early Human Deveopment 2019;135:75-81. [DOI: 10.1016/j.earlhumdev.2019.05.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bertelli 2015
- Bertelli C, Pillonel T, Torregrossa A, Prod'hom G, Fischer CJ, Greub G, et al. Bifidobacterium longum bacteremia in preterm infants receiving probiotics. Clinical Infectious Diseases 2015;60(6):924-7. [DOI: 10.1093/cid/ciu946] [PMID: ] [DOI] [PubMed] [Google Scholar]
Besselink 2008
- Besselink MG, Santvoort HC, Buskens E, Boermeester MA, Goor H, Timmerman HM, Dutch Acute Pancreatitis Study Group. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008;371(9613):651-9. [DOI] [PubMed] [Google Scholar]
Bi 2019
- Bi LW, Yan BL, Yang QY, Li MM, Cui HL. Which is the best probiotic treatment strategy to prevent the necrotizing enterocolitis in premature infants: a network meta-analysis revealing the efficacy and safety. Medicine 2019;98(41):e17521. [DOI: 10.1097/MD.0000000000017521] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyle 2006
- Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? American Journal of Clinical Nutrition 2006;83(6):1446-7. [DOI] [PubMed] [Google Scholar]
Bron 2017
- Bron PA, Kleerebezem M, Brummer R, Cani PD, Mercenier A, MacDonald TT, et al. Can probiotics modulate human disease by impacting intestinal barrier function? British Journal of Nutrition 2017;117(1):93-107. [DOI: 10.1017/S0007114516004037] [PMID: 28102115] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cleminson 2015
- Cleminson J, Oddie S, Renfrew MJ, McGuire W. Being baby friendly: evidence-based breastfeeding support. Archives of Disease in Childhood- Fetal and Neonatal Edition 2015;100(2):F173-8. [DOI] [PubMed] [Google Scholar]
Duffield 2019
- Duffield SD, Clarke P. Current use of probiotics to prevent necrotising enterocolitis. Archives of Disease in Childhood Fetal and Neonatal Edition 2019;104(2):F228. [DOI: 10.1136/archdischild-2018-316199] [PMID: ] [DOI] [PubMed] [Google Scholar]
Eaton 2017
- Eaton S, Rees CM, Hall NJ. Current research on the epidemiology, pathogenesis, and management of necrotizing enterocolitis. Neonatology 2017;111(4):423-30. [DOI: 10.1159/000458462] [PMID: ] [DOI] [PubMed] [Google Scholar]
Embleton 2016
- Embleton ND, Zalewski S, Berrington JE. Probiotics for prevention of necrotizing enterocolitis and sepsis in preterm infants. Current Opinion in Infectious Diseases 2016;29(3):256-61. [DOI: 10.1097/QCO.0000000000000269] [PMID: 27023404] [DOI] [PubMed] [Google Scholar]
Embleton 2017
- Embleton ND, Berrington JE, Dorling J, Ewer AK, Juszczak E, Kirby JA, et al. Mechanisms affecting the gut of preterm infants in enteral feeding trials. Frontiers in Nutrition 2017;4:14. [DOI: 10.3389/fnut.2017.00014] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Esaiassen 2016
- Esaiassen E, Cavanagh P, Hjerde E, Simonsen GS, Stoen R, Klingenberg C. Bifidobacterium longum subspecies infantis bacteremia in 3 extremely preterm infants receiving probiotics. Emerging Infectious Diseases 2016;22(9):1664-6. [DOI: 10.3201/eid2209.160033] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleming 2019
- Fleming PF, Berrington JE, Jacobs SE. Addressing safety concerns of probiotic use in preterm babies. Early Human Development 2019;135:72-4. [DOI: 10.1016/j.earlhumdev.2019.05.016] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gale 2020
- Gale C, McGuire W, Juszczak E. Randomised controlled trials for informing perinatal care. Neonatology 2020;117(1):8-14. [DOI: 10.1159/000499881] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gephart 2020
- Gephart SM, Underwood MA, Rosito S, Kim JH, Caplan M. Grading the evidence to identify strategies to modify risk for necrotizing enterocolitis. Pediatric Research 2020;88(Suppl 1):41-7. [DOI: 10.1038/s41390-020-1079-z] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 8 May 2018. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Granger 2020
- Granger CL, Embleton ND, Palmer JM, Lamb CA, Berrington JE, Stewart CJ. Maternal breast milk, infant gut microbiome, and the impact on preterm infant health. Acta Paediatrica 2020;00:1-8. [DOI: 10.1111/apa.15534] [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443-57. [DOI: 10.1002/sim.2380] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hickey 2018
- Hickey M, Georgieff M, Ramel S. Neurodevelopmental outcomes following necrotizing enterocolitis. Seminars in Fetal and Neonatal Medicine 2018;23(6):426-32. [DOI: 10.1016/j.siny.2018.08.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA: on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Hopewell 2009
- Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: MR000006. [DOI: 10.1002/14651858.MR000006.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Horbar 2012
- Horbar JH, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics 2012;129(6):1019-26. [DOI: 10.1542/peds.2011-3028] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jarrett 2019
- Jarrett P, Meczner A, Costeloe K, Fleming P. Historical aspects of probiotic use to prevent necrotising enterocolitis in preterm babies. Early Human Development 2019;135:51-7. [DOI: 10.1016/j.earlhumdev.2019.05.015] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jenke 2012
- Jenke A, Ruf EM, Hoppe T, Heldmann M, Wirth S. Bifidobacterium septicaemia in an extremely low-birthweight infant under probiotic therapy. Archives of Disease in Childhood-Fetal and Neonatal Edition 2012;97(3):F217-8. [DOI: 10.1136/archdischild-2011-300838] [PMID: ] [DOI] [PubMed] [Google Scholar]
Johnson‐Henry 2016
- Johnson-Henry KC, Abrahamsson TR, Wu RY, Sherman PM. Probiotics, prebiotics, and synbiotics for the prevention of necrotizing enterocolitis. Advances in Nutrition 2016;7(5):928-37. [DOI: 10.3945/an.116.012237] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koretz 2018
- Koretz RL. Probiotics in gastroenterology: How pro Is the evidence in adults? American Journal of Gastroenterology 2018;113(8):1125-36. [DOI: 10.1038/s41395-018-0138-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kunk 2019
- Kunk D. Probiotics: elixir or empty promise. Lancet Gastroenterology & Hepatology 2019;4(2):81. [DOI: 10.1016/S2468-1253(18)30415-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lerner 2019
- Lerner A, Shoenfeld Y, Matthias T. Probiotics: if it does not help it does not do any harm. Really? Microorganisms 2019;7(4):104. [10.3390/microorganisms7040104] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mara 2018
- Mara MA, Good M, Weitkamp JH. Innate and adaptive immunity in necrotizing enterocolitis. In: Seminars in Fetal and Neonatal Medicine. Vol. 23. Elsevier, 2018:394-9. [DOI: 10.1016/j.siny.2018.08.002] [DOI] [PMC free article] [PubMed]
Marchand 2012
- Marchand V, Canadian Paediatric Society, Nutrition and Gastroenterology Committee. Using probiotics in the paediatric population. Paediatrics and Child Health 2012;17(10):575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2010
- Martin CR, Dammann O, Allred EN, Patel S, O'Shea TM, Kuban KC, et al. Neurodevelopment of extremely preterm infants who had necrotizing enterocolitis with or without late bacteremia. Journal of Pediatrics 2010;157(5):751-6. [DOI: 10.1016/j.jpeds.2010.05.042] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Masi 2019
- Masi AC, Stewart CJ. The role of the preterm intestinal microbiome in sepsis and necrotising enterocolitis. Early Human Development 2019;138:104854. [DOI: 10.1016/j.earlhumdev.2019.104854] [PMID: ] [DOI] [PubMed] [Google Scholar]
Millar 2012
- Millar M, Wilks M, Fleming P, Costeloe K. Should the use of probiotics in the preterm be routine? Archives of Disease in Childhood - Fetal and Neonatal Edition 2012;97(1):F70. [DOI: 10.1136/adc.2009.178939] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morgan 2011
- Morgan JA, Young L, McGuire W. Pathogenesis and prevention of necrotizing enterocolitis. Current Opinion in Infectious Diseases 2011;24(3):183-9. [DOI: 10.1097/QCO.0b013e328345d5b5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morgan 2020
- Morgan RL, Preidis GA, Kashyap PC, Weizman AV, Sadeghirad B, McMaster Probiotic, Prebiotic, and Synbiotic Work Group. Probiotics reduce mortality and morbidity in preterm, low-birth-weight infants: a systematic review and network meta-analysis of randomized trials. Gastroenterology 2020;159(2):467-80. [DOI: 10.1053/j.gastro.2020.05.096] [PMID: 32592699] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ohlsson 2020
- Ohlsson A, Lacy JB. Intravenous immunoglobulin for suspected or proven infection in neonates. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD001239. [DOI: 10.1002/14651858.CD001239.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olm 2019
- Olm MR, Bhattacharya N, Crits-Christoph A, Firek BA, Baker R, Song YS, et al. Necrotizing enterocolitis is preceded by increased gut bacterial replication, Klebsiella, and fimbriae-encoding bacteria. Science Advances 2019;5(12):eaax5727. [DOI: 10.1126/sciadv.aax5727] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pammi 2020
- Pammi M, Suresh G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database of Systematic Reviews 2020, Issue 3. Art. No: CD007137. [DOI: 10.1002/14651858.CD007137.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2015
- Patel RM, Denning PW. Intestinal microbiota and its relationship with necrotising enterocolitis. Pediatric Research 2015;78(3):232-8. [DOI: 10.1038/pr.2015.97] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2018
- Patel RM, Underwood MA. Probiotics and necrotizing enterocolitis. Seminars in Pediatric Surgery 2018;27(1):39-46. [DOI: 10.1053/j.sempedsurg.2017.11.008] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pell 2019
- Pell LG, Loutet MG, Roth DE, Sherman PM. Arguments against routine administration of probiotics for NEC prevention. Current Opinion in Pediatrics 2019;31(2):195-201. [DOI: 10.1097/MOP.0000000000000730] [PMID: 30624281] [DOI] [PubMed] [Google Scholar]
Quigley E 2019
- Quigley EM. Prebiotics and probiotics in digestive health. Clinical Gastroenterology and Hepatology 2019;17(2):333-44. [DOI: 10.1016/j.cgh.2018.09.028] [PMID: ] [DOI] [PubMed] [Google Scholar]
Quigley M 2019
- Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2019, Issue 7. Art. No: CD002971. [DOI: 10.1002/14651858.CD002971.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Robertson 2020
- Robertson C, Savva GM, Clapuci R, Jones J, Maimouni H, Brown E, et al. Incidence of necrotising enterocolitis before and after introducing routine prophylactic Lactobacillus and Bifidobacterium probiotics.. Archives of Disease in Childhood-Fetal and Neonatal Edition 2020;105(4):380-6. [DOI: 10.1136/archdischild-2019-317346] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Samuels 2017
- Samuels N, de Graaf RA, Jonge RC, Reiss IKM, Vermeulen MJ. Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC Pediatrics 2017;17(1):105. [DOI: 10.1186/s12887-017-0847-3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanders 2019
- Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nature Reviews Gastroenterology & Hepatology 2019;16(10):605-16. [DOI: 10.1038/s41575-019-0173-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sesham 2014
- Sesham R, Oddie S, Embleton ND, Clarke P. Probiotics for preterm neonates: parents' perspectives and present prevalence. Archives of Disease in Childhood-Fetal and Neonatal Edition 2014;99(4):F345. [DOI: 10.1136/archdischild-2014-306344] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stewart 2012
- Stewart CJ, Marrs EC, Magorrian S, Nelson A, Lanyon C, Perry JD, et al. The preterm gut microbiota: changes associated with necrotizing enterocolitis and infection. Acta Paediatrica 2012;101(11):1121-7. [DOI: 10.1111/j.1651-2227.2012.02801.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stewart 2017
- Stewart CJ, Embleton ND, Marrs EC, Smith DP, Fofanova T, Nelson A, et al. Longitudinal development of the gut microbiome and metabolome in preterm neonates with late onset sepsis and healthy controls. Microbiome 2017;5(1):75. [DOI: 10.1186/s40168-017-0295-1] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suez 2019
- Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nature Medicine 2019;25(5):716-29. [DOI: 10.1038/s41591-019-0439-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thomas 2010
- Thomas DW, Greer FR, American Academy of Pediatrics Committee on Nutrition, American Academy of Pediatrics Section on Gastroenterology, Hepatology, and Nutrition. Probiotics and prebiotics in pediatrics. Pediatrics 2010;126(6):1217-31. [DOI: 10.1542/peds.2010-2548] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tin 1998
- Tin W, Fritz S, Wariyar U, Hey E. Outcome of very preterm birth: children reviewed with ease at 2 years differ from those followed up with difficulty. Archives of Disease in Childhood- Fetal and Neonatal Edition 1998;79(2):F83-7. [DOI: 10.1136/fn.79.2.f83] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Underwood 2019
- Underwood MA. Probiotics and human milk oligosaccharides in premature infants. Neoreviews 2019;20(1):e1-1. [DOI: 10.1542/neo.20-1-e1] [PMID: ] [DOI] [PubMed] [Google Scholar]
van den Akker 2018
- den Akker CH, Goudoever JB, Szajewska H, Embleton ND, Hojsak I, Reid D, et al, ESPGHAN Working Group for Probiotics, Prebiotics & Committee on Nutrition. Probiotics for preterm infants: a strain-specific systematic review and network meta-analysis. Journal of Pediatric Gastroenterology and Nutrition 2018;67(1):103-22. [DOI: 10.1097/MPG.0000000000001897] [PMID: ] [DOI] [PubMed] [Google Scholar]
van den Akker 2020
- den Akker CH, Goudoever JB, Shamir R, Domellof M, Embleton ND, Hojsak I, et al. Probiotics and preterm infants: a position paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for Probiotics and Prebiotics. Journal of Pediatric Gastroenterology and Nutrition 2020;70(5):664-80. [DOI: 10.1097/MPG.0000000000002655] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vermeulen 2020
- Vermeulen MJ, Luijendijk A, Toledo L, Kaam AH, Reiss IK. Quality of probiotic products for preterm infants: contamination and missing strains. Acta Paediatrica 2020;109(2):276-9. [DOI: 10.1111/apa.14976] [PMID: ] [DOI] [PubMed] [Google Scholar]
Viswanathan 2016
- Viswanathan S, Lau C, Akbari H, Hoyen C, Walsh MC. Survey and evidence based review of probiotics used in very low birth weight preterm infants within the United States. Journal of Perinatology 2016;36(12):1106-11. [DOI: 10.1038/jp.2016.144] [PMID: ] [DOI] [PubMed] [Google Scholar]
Walsh 1986
- Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatric Clinics of North America 1986;33(1):179-201. [DOI: 10.1016/s0031-3955(16)34975-6] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2019
- Walsh V, McGuire W. Immunonutrition for Preterm Infants. Neonatology 2019;115(4):398-405. [DOI: 10.1159/000497332] [PMID: ] [DOI] [PubMed] [Google Scholar]
Warner 2016
- Warner BB, Deych E, Zhou Y, Hall-Moore C, Weinstock GM, Sodergren E, et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet 2016;387(10031):1928-36. [DOI: 10.1016/S0140-6736(16)00081-7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2011
- Young T, Hopewell S. Methods for obtaining unpublished data. Cochrane Database of Systematic Reviews 2011, Issue 11. Art. No: MR000027. [DOI: 10.1002/14651858.MR000027.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zbinden 2015
- Zbinden A, Zbinden R, Berger C, Arlettaz R. Case series of Bifidobacterium longum bacteremia in three preterm infants on probiotic therapy. Neonatology 2015;107(1):56-9. [DOI: 10.1159/000367985] [PMID: 25402825] [DOI] [PubMed] [Google Scholar]
Zmora 2018
- Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 2018;174(6):1388-405. [DOI: 10.1016/j.cell.2018.08.041] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Al Faleh 2008
- Alfaleh K, Bassler D. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD005496. [DOI: 10.1002/14651858.CD005496.pub2] [DOI] [PubMed] [Google Scholar]
Al Faleh 2011
- Alfaleh K, Anabrees J, Bassler D, Al-Kharfi T. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database of Systematic Reviews 2011, Issue 3. Art. No: CD005496. [DOI: 10.1002/14651858.CD005496.pub3] [DOI] [PubMed] [Google Scholar]
Al Faleh 2014
- AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database of Systematic Reviews 2014, Issue 4. Art. No: CD005496. [DOI: 10.1002/14651858.CD005496.pub4] [DOI] [PubMed] [Google Scholar]
AlFaleh 2005
- AlFaleh KM, Bassler D. Probiotics for prevention of mortality and morbidity in preterm infants. Cochrane Database of Systematic Reviews 2005, Issue 4. Art. No: CD005496. [DOI: 10.1002/14651858.CD005496] [DOI] [PubMed] [Google Scholar]


