Abstract
Meningiomas are the second most common brain tumors in adults, and meningiomas exhibit a tendency to invade adjacent structures. Compared with high‐grade gliomas, little is known about the molecular changes that potentially underlie the invasive behavior of meningiomas. In this study, we examined the expression and function of the membrane alanyl‐aminopeptidase [mAAP, aminopeptidase N (APN), CD13, EC3.4.11.2] zinc‐dependent ectopeptidase in meningiomas and meningioma cell lines, based on its prior association with tumor invasion in colorectal and renal carcinomas. We found a significant reduction of APNmRNA and protein expression, as well as enzymatic activity, in high‐grade meningiomas. While meningioma tumor cell proliferation was not affected by either pharmacologic APN inhibition or siRNA‐mediated APN silencing, APN pharmacologic and siRNA knockdown significantly reduced meningioma cell invasion in vitro. Next, we employed pathway‐specific cDNA microarray analyses to identify extracellular matrix and adhesion molecules regulated by APN, and found that APN‐siRNA knockdown substantially increased the expression of secreted protein, acidic and rich in cysteine (SPARC)/osteonectin. Finally, we demonstrated that SPARC, which has been previously associated with meningioma invasiveness, was increased in aggressive meningiomas. Collectively, these results suggest that APN expression and enzymatic function is reduced in aggressive meningiomas, and that alterations in the balance between APN and SPARC might favor meningioma invasion.
Keywords: CD13, invasion, meningioma
INTRODUCTION
Meningiomas are among the most frequent tumors of the brain and spinal cord, accounting for 15%–20% of all central nervous system tumors (23). While the vast majority of meningiomas comprises benign World Health Organization (WHO) grade I tumors with good overall survival rates, atypical WHO grade II and anaplastic WHO grade III meningiomas exhibit aggressive clinical behavior and lead to substantially reduced survival rates (23). A limited number of genetic changes associated with meningioma progression have been identified, including losses involving chromosomes 1p, 14q and 6q or chromosome 10 (34). Atypical or anaplastic meningiomas also harbor alterations in the CDKN2A, p14, and CDKN2B tumor suppressor genes (2), insulin‐like growth factor II (IGF‐II) expression (19) and PI3 kinase/Akt pathway activity (17). It is not known which, if any, of these molecular changes confers brain or bone invasiveness.
Invasion of adjacent brain or bone structures is a feature in a fraction of meningiomas of all WHO grades. Several proteins have been proposed as potential markers involved in meningioma invasion. Overexpression of matrix metalloproteinases, such as MMP‐2 and MMP‐9, as well as other extracellular matrix (ECM) proteins, such as tenascin, has been related to increased brain invasiveness in meningiomas 15, 20. In addition, the ECM‐related protein SPARC (also named osteonectin or BM‐40) has been strongly implicated in meningioma invasiveness 26, 30, 35.
Recently, the Zn‐dependent membrane alanyl‐aminopeptidase [mAAP, aminopeptidase N (APN), CD13, EC 3.4.11.2] (also known as APN) has been shown to play a role in tumor biology. The biologic effects of this ectopeptidase were mainly attributed to the regulation of the local balance of peptide hormone and growth factor activation/inactivation [reviewed in Carl‐McGrath et al (5)]; however, APN has also been linked to the control of cell proliferation 4, 27, neoangiogenesis (1) and tumor cell invasion 9, 11, 13, 14. Using a number of different methodological strategies, the invasive potential of tumor cells can be significantly inhibited by downregulation of APN 13, 14, which has been attributed to proteolytic degradation and adhesion to the ECM. In the present study, we show that APN is normally expressed in non‐neoplastic leptomeningeal tissue and benign grade I meningiomas but is decreased in grade III meningiomas. In addition, we show that APN knockdown results in increased meningioma cell motility/invasion. We further demonstrate that the reduction of APN activity results in the induction of SPARC expression in meningiomas. These studies suggest that APN might be partly responsible for the invasive behavior of high‐grade meningiomas.
MATERIALS AND METHODS
Tumor material
Paraffin‐embedded samples from 37 human meningiomas were used for the immunohistochemical studies. The series comprised of 13 WHO grade I meningiomas, 17 atypical meningiomas WHO grade II and 7 anaplastic meningiomas WHO grade III. While all WHO grade I tumors were primary tumors, six atypical meningiomas and six anaplastic meningiomas were recurrent tumors. All tumors analyzed in this study were intracranial meningiomas. Additional tumor material, which has been snap frozen in liquid nitrogen and stored at −80°C, was used for real‐time polymerase chain reaction (PCR) analyses from nine WHO grade I, four atypical WHO grade II and three anaplastic WHO grade III meningiomas, respectively. From these group, four WHO grade I, one WHO grade II and three WHO grade III meningiomas were also studied by Western blotting. All tumors were graded according to the current WHO classification for meningiomas (16).
Cell lines
The cell lines HBL52 (Cell lines service, Heidelberg, Germany) and Ben‐Men‐1 [characterized in Puttmann et al (24)] were both derived from benign meningiomas WHO grade I. The meningioma cell lines F5 and SF3061 [both characterized in Cuevas et al (6)] as well as IOMM‐Lee (31) and KT21MG1 were derived from malignant meningiomas. Cells were maintained in Dulbecco's modified Eagles medium (PAA, Linz, Austria), supplemented with 10% fetal calf serum, L‐glutamine (2 µM) and penicillin (50 IU/mL) at 37°C in 5% CO2.
Immunohistochemistry
Immunohistochemical detection of APN and SPARC in paraffin‐embedded tumor tissue was performed using standard procedures as described previously in detail (17). The CD13/APN antibody was from Novocastra (Newcastle upon Tyne , UK) [clone 38C12, dilution 1:50, pretreatment with 10 mM sodium citrate, pH 6.0 (3 × 10 minutes), 600 W microwave] and has been successfully used in previous studies (28). Liver tissue served as the positive control (Figure 1A, inset). The SPARC (osteonectin) antibody (Haematological Technologies, Inc., Essex Junction, VT, USA) was used as reported in Schittenhelm et al (30). Negative controls comprised omission of the primary antibody and its substitution with an irrelevant mouse monoclonal antibody (data not shown). Immunoexpression of APN and SPARC was graded semiquantitatively as follows: −, no expression; +, weak patchy expression; ++, focal strong expression; and +++, strong expression in more than 50% of tumor tissue.
Figure 1.
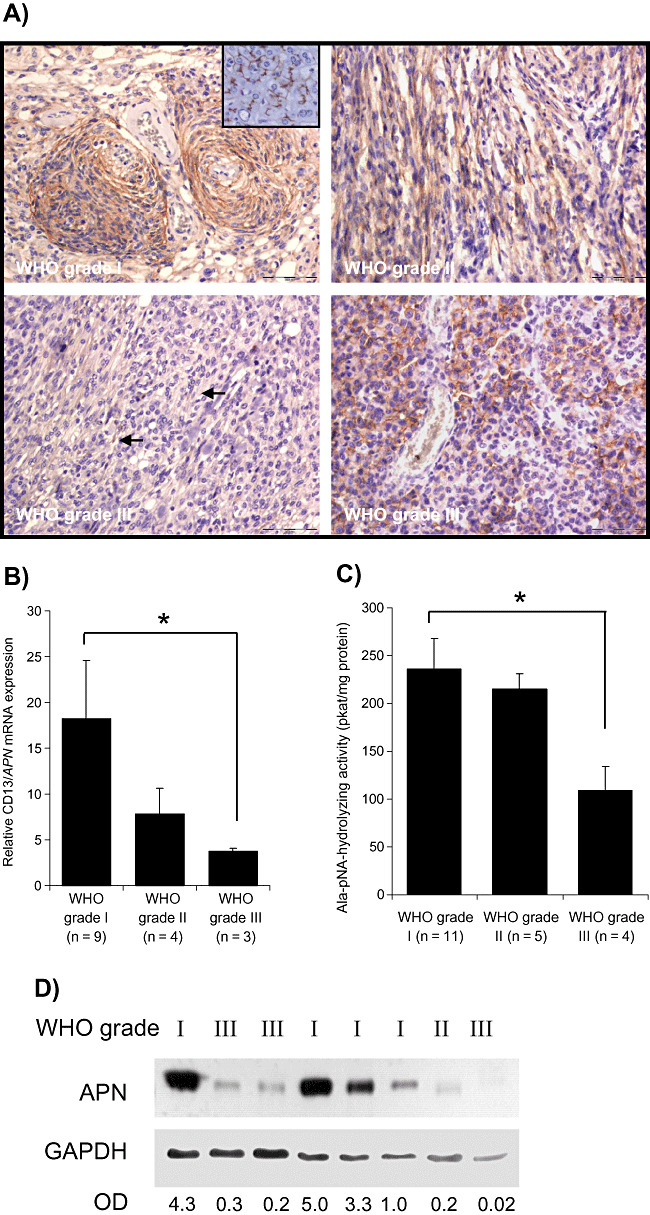
Expression of CD13/aminopeptidase N (APN) in human meningiomas. A. Immunodetection of APN in paraffin‐embedded tumor samples of meningiomas with different grades of malignancy. Strong predominantly membrane‐bound immunoreaction for APN is seen in meningothelial (upper‐left figure) and fibroblastic World Health Organization (WHO) grade I meningiomas. In aggressive WHO grade II (upper‐right figure) or WHO grade III (lower panel) meningiomas, the membrane‐bound APN staining is restricted to a few cells (lower‐left figure, arrows) or cell groups (lower‐right figure). Bars represent 100 µm. Inset shows liver tissue as a positive control. B. Expression of APN mRNA as measured by real‐time polymerase chain reaction (PCR) is decreased in both atypical (grade II) and anaplastic (grade III) meningiomas (*P < 0.05). Values are normalized to α‐tubulin expression; mean ± standard error of the mean (SEM) are shown. C. Enzymatic activity is decreased especially in anaplastic grade III meningiomas (mean ± SEM; *P < 0.05). D. Western blot detection of APN from human meningioma samples confirm reduced APN protein amounts in aggressive grade II or grade III meningiomas. Densitometric values of APN normalized to GAPDH are given below. Abbreviation: OD = optical density.
Real‐time PCR
Total RNA was prepared by using the RNeasy Mini Kit®(Qiagen, Hilden, Germany). Contaminating DNA was removed by Dnase I digestion. cDNA was generated from 1 µg total RNA, and 1/20th of the cDNA mixture was used for quantitative reverse transcription in the iCycler (Bio‐Rad, Munich, Germany). A typical 25 µL reaction mixture contained 12.5 µL 2× Sensi‐Mix (Quantace, London, UK), 0.3 µL of SYBR Green I (Quantace) and 0.5 µmol of the specific primers: APN‐US: 5′‐AgCTCCACACACCgT TCCTg; APN‐DS: gCCCTTggCCATggTgATggTg; SPARC‐US: 5′‐CCT gAT gAg ACA gAg gTg gTg‐3′; and SPARC‐DS: 5′‐TTC TCA TCC AgC TCg CAC AC‐3′. An initial denaturation/activation step (10 minutes, 95°C) was followed by 40 cycles (15 s 95°C, 30 s 58°C, 45 s 72°C). The amounts of mRNA were normalized to α‐tubulin mRNA (human α‐tub‐US, 5′‐CATTTCACCATCTGG GCTGGCTC‐3′; α‐tub‐DS, 5′‐CACCCGTCT TCAGGGCTTCT TGGTTT‐3′).
Determination of APN enzymatic activity
Lysates of human meningiomas or meningioma cells were analyzed colorimetrically in a 96‐well plate by monitoring the cleavage of 2.5 mM H‐Ala‐p‐nitroanilide at 405 nm and 37°C in 50 mM phosphate buffer, pH 7.4 (six wells per lysate). To discriminate between mAAP (mAAP/APN/CD13/EC3.4.11.2) and cytosolic alanyl‐aminopeptidase (cAAP/PSA/EC3.4.11.16), which both exhibit a largely identical substrate specificity, enzymatic activities were determined in the presence of the cAAP‐selective inhibitor PAQ22 (10−5 M) (kindly provided by Dr. Yuichi Hashimoto, Tokyo, Japan) and the nondiscriminating inhibitor phebestin (10−6 M) (SIGMA, Deisenhofen, Germany).
Western blotting
Western blot studies were all performed as described in Mawrin et al (17). The following antibodies were used: CD13/APN (Leu‐M7, Becton‐Dickinson (Heidelberg, Germany), 1:200 in phosphate‐buffered saline (PBS)), and SPARC/osteonectin (Santa Cruz, Santa Cruz, CA). Following membrane stripping, equal protein loading was determined using antibodies against glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) or actin as indicated. The resulting images were densitometrically analyzed by using the Gene Profiler software ver3.56 (Scanalytics, Inc.TM, Rockville, MD). Comparison of the different groups was only done on blots processed equally and exposed on the same X‐ray film.
Proliferation assays
Cells were seeded into 96‐well plates at a density of 50 000 cells/well/200 µL cell culture medium (six wells per cell line) and incubated at 37°C and 5% CO2 with the additions indicated in the figure legends. The WM15 antibody was from Acris, Hiddenhausen, Germany. Actinonin was from SIGMA. After 72 h, the cultures were pulsed for additional 6 h with [3H]‐methylthymidine (0.2 µCi per well; Amersham‐Biosciences, Freiburg, Germany), and the incorporated radioactivity was measured by scintillation counting.
APN‐siRNA knockdown
The cells were seeded in six‐well plates at a density of 150 000 cells/well/2 mL cell culture medium. At 60% confluency, the medium was replaced by a fresh one, and the cells were transfected with siRNA. Control siRNA was obtained from Qiagen; predesigned siRNAs for the APN knockdown were from Eurogentec (Seraing, Belgium) (siRNA1: gAA gCU CAA CUA CAC CCU C dTdT, siRNA2: CAA gAA CgC CAA CAg CUC C dTdT). SiLentFect from Bio‐Rad was used for transfection, which was performed according to the manufacturer's instructions. Ten nanomoles of each APN‐siRNA1 and APN‐siRNA2 and 2 µL siLentFect were applied per well. The cells were harvested for RNA preparation or immunoblot analyses after 48 or 72 h, respectively. Control siRNA was used at a concentration of 10 nm.
Meningioma cell invasion assay
The determination of the invasive potential of the malignant meningioma cell line IOMM‐Lee and KT21MG1 was performed by using the BD BioCoat™ Matrigel™ Invasion Chamber from BD Biosciences (Heidelberg, Germany) according to the manufacturer's recommendation. The cells were cultured in 24‐well chambers with and without matrigel at a density of 30 000 cells per 1000 µL per chamber in presence or absence of 10–5 mol/L actinonin. A part of the cells were pretreated with siRNA. Three chambers per cell lines were used, and experiments were done in triplicate. The effective knockdown of the APN gene was controlled by a parallel determination of mRNA expression. After a 24‐h incubation and removal of noninvading cells, the counting of invading cells was evaluated by two independent persons (CW and UL).
Quantitative real‐time PCR array analysis
The pathway‐specific human PCR array related to the screening of ECM and adhesion molecules (PAHS‐013, GE SuperArray, SuperArray Bioscience Corp., Frederick, MD, USA) containing 84 genes important for cell–cell and cell–matrix interactions was used according to the manufacturer's instructions.
Statistical analyses
Differences in the mRNA expression levels, enzymatic activities and cell invasion were determined with the Mann–Whitney test. P < 0.05 was considered statistically significant. All statistical evaluations were performed with the SPSS 13.0 software packageTM (SPSS, Chicago, IL, USA).
RESULTS
APN expression and enzymatic activity is reduced in aggressive meningiomas
Immunohistochemical analysis revealed a reduced immunoexpression of APN especially in anaplastic meningiomas (Table 1, Figure 1A). In some tumors with adjacent normal meningeal tissue, the latter showed usually moderate APN positivity (data not shown). Subgroup analysis of meningiomas showing various types of invasion (Table 2) revealed a reduced or absent APN expression in meningiomas with brain or dural invasion, mostly in the group of anaplastic meningiomas. As suggested in the paper by Rempel et al (26), we also compared APN expression in nonrecurrent and noninvasive tumors with tumors showing invasion and/or being recurrent tumors. We observed that among noninvasive, nonrecurrent tumors (n = 14), seven tumors (50%) showed a complete loss (score: −) or weak focal (score: +) APN expression. In the group with invasion and/or recurrence (n = 23), absent or markedly reduced APN immunoexpression was found in 14 cases (61%). Expression analysis by real‐time PCR (Figure 1B) showed a clear reduction of APN mRNA levels in the grade III meningiomas. Comparable downregulation of APN enzymatic activity was also observed in the grade III meningiomas (Figure 1C). While there was a reduction in APN mRNA expression or APN enzymatic activity in the grade II meningiomas, it did not reach statistical significance. Similarly, Western blot studies revealed that WHO grade I meningiomas contained high amounts of APN protein, while the atypical and anaplastic meningiomas showed reduced APN protein levels (Figure 1D).
Table 1.
Distribution of aminopeptidase N (APN) expression in human meningiomas. Abbreviation: WHO = World Health Organization.
| Immunoexpression score | Meningioma | Atypical meningioma | Anaplastic meningioma |
|---|---|---|---|
| WHO grade I (n = 13) | WHO grade II (n = 17) | WHO grade III (n = 7) | |
| − | 2 (15%) | 6 (35%) | 3 (42%) |
| + | 4 (31%) | 4 (24%) | 2 (29%) |
| ++ | 3 (23%) | 5 (29%) | 2 (29%) |
| +++ | 4 (31%) | 2 (12%) | — |
−, no expression; +, weak patchy expression; ++, strong focal expression; +++, strong expression in more than 50% of tumor tissue.
Table 2.
Relation between invasive meningiomas and aminopeptidase N (APN) expression. Abbreviation: WHO = World Health Organization.
| Type of invasion*/APN immunoexpression | Meningioma | Atypical meningioma | Anaplastic meningioma |
|---|---|---|---|
| WHO grade I (n = 13) | WHO grade II (n = 17) | WHO grade III (n = 7) | |
| Brain invasion | − | 4 (24%) | 5 (71%) |
| APN | −:2 | −:4 | |
| +:1 | ++:1 | ||
| +++:1 | |||
| Dural invasion | 4 (31%) | 4 (24%) | 1 (14%) |
| APN | −:1 | −:2 | + |
| +:2 | +:1 | ||
| ++:1 | ++:1 |
Bone invasion was not observed. Immunoexpression of APN was graded semiquantitatively: −, no expression; +, weak patchy expression; ++, strong focal expression; +++, strong expression in more than 50% of tumor tissue.
APN expression and activity in meningioma cells and its relation to proliferation and invasion
To identify meningioma cell lines with high APN expression for experimental manipulation, we screened six different human meningioma cell lines for APN activity and expression. As shown in Figure 2, APN mRNA (A), protein (B) and enzymatic activity (C) were observed in most of the meningioma cell lines examined. However, high APN levels were noted in two malignant meningioma cell lines (IOMM‐Lee and KT21MG1) and two benign meningioma cell lines (Ben‐Men‐1 and HBL52), while a reduction was evident only for F5 and SF3061. In Figure 2D, we compared cells with high APN levels (IOMM‐Lee) with cells harboring low APN levels (KT21MG1) for the grade of invasiveness in a transwell migration assay, revealing that KT21MG1 cells have lower migration rates than IOMM‐Lee cells.
Figure 2.
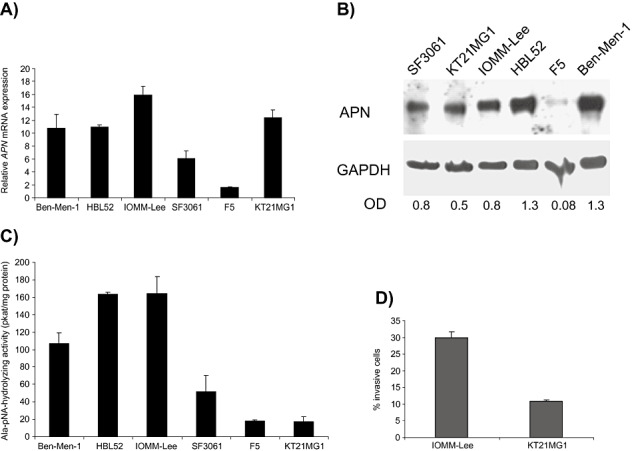
Expression and activity of aminopeptidase N (APN) in meningioma cells. High APN expression can be found on the mRNA level (A), by Western blot analyses (B) and by measuring the enzymatic activity (C), especially in benign meningioma cell lines HBL52 and Ben‐Men‐1, as well as in the malignant meningioma cell line IOMM‐Lee. D. Cells with high APN enzymatic activity (IOMM‐Lee) have higher migration rates than cells with low APN activity (KT21MG1) in a transwell migration assay. Mean ± standard deviation (SD) are shown; densitometric values are given in B. Abbreviations: GAPDH = glyceraldehyde‐3‐phosphate dehydrogenase; OD = optical density.
In order to establish a functional role of APN in meningiomas, we first employed siRNA‐mediated knockdown of APN. As shown in Figure 3A, using two different silencing constructs, we could suppress APN mRNA and protein levels in meningioma cells (IOMM‐Lee cell results shown; comparable results were seen for Ben‐Men‐1 cells). Moreover, APN enzymatic activity was effectively reduced by APN‐siRNA (Figure 3B).
Figure 3.
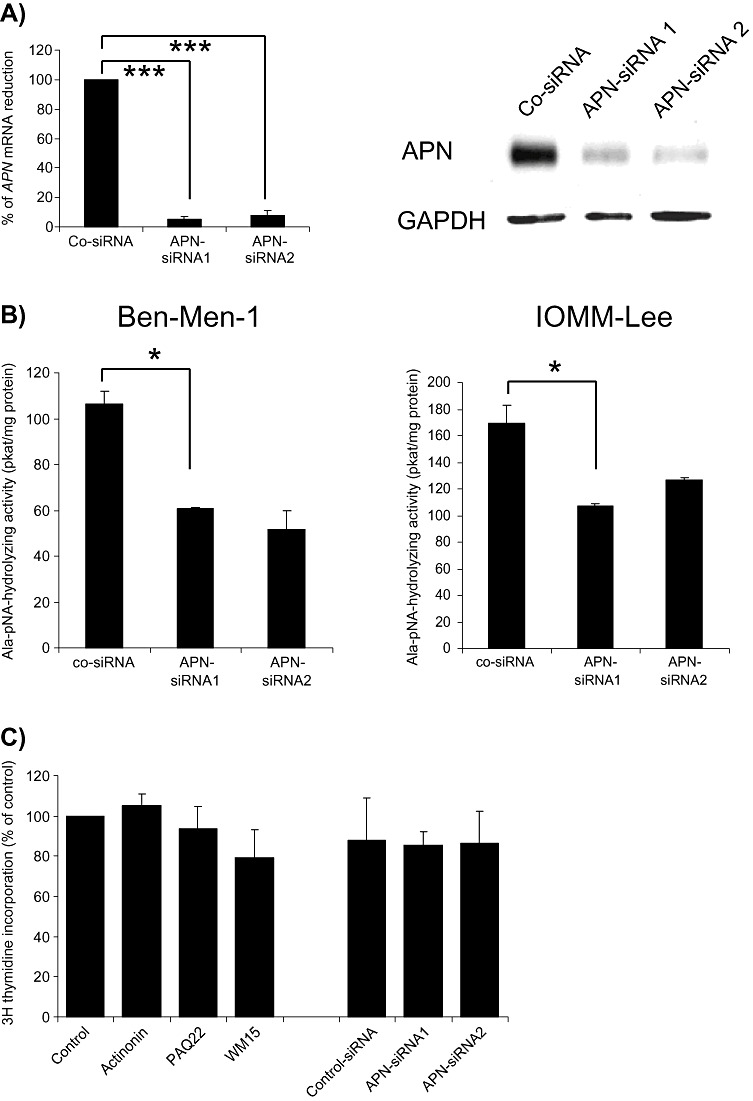
Aminopeptidase N (APN) inhibition in meningioma cells do not affect meningioma cell proliferation. A. Significant inhibition of APN mRNA (left figure; ***P < 0.01) and protein levels (right figure) by two different siRNA species. B. Both Ben‐Men‐1 and IOMM‐Lee cells have reduced APN enzymatic activity following siRNA‐mediated knockdown [mean ± standard error of the mean (SEM); *P < 0.05]. C. No substantial effects on meningioma cell proliferation are seen after inhibition by APN inhibitors (actinonin, PAQ22 or WM15) or by siRNA (mean ± SEM are shown for IOMM‐Lee cells).
We next sought to determine whether APN downregulation might affect tumor cell proliferation. For these experiments, we treated meningioma cells with either APN‐siRNA, the APN inhibitors actinonin or PAQ22, or an anti‐APN antibody (WM15). As shown for IOMM‐Lee cells (Figure 3C), none of these inhibitory approaches suppressed tumor cell proliferation (comparable data were obtained for all meningioma cell lines). These data suggest that APN does not regulate meningioma tumor cell proliferation in vitro.
As APN has also been implicated in the regulation of cell motility/invasion, we analyzed the motility/invasion of IOMM‐Lee cells in a transwell migration assay following APN inhibition. In these experiments, the number of invasive meningioma cells was significantly inhibited both by APN‐siRNA (Figure 4A) and by actinonin (Figure 4B).
Figure 4.
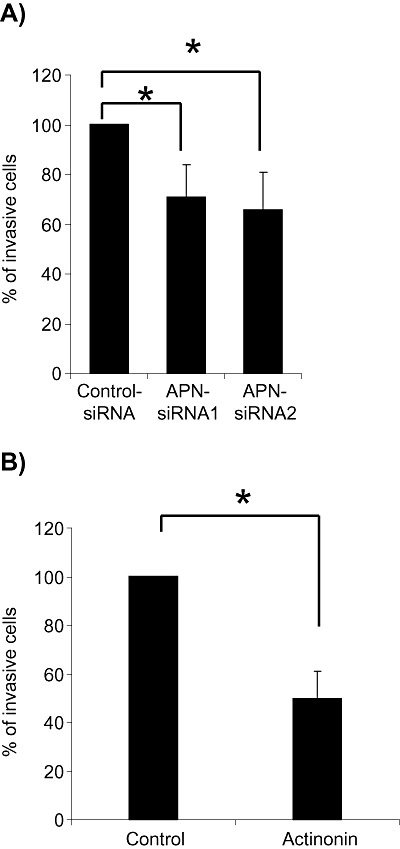
Invasiveness of IOMM‐Lee meningioma cells is significantly inhibited by aminopeptidase N (APN)‐siRNA knockdown (A) or APN inhibition by actinonin (B) [mean ± standard error of the mean (SEM);*P < 0.05].
APN regulates SPARC expression in meningiomas
The above experiments suggest that APN function in meningiomas is likely related to tumor cell migration, rather than proliferation. To identify possible candidate genes regulated by APN in meningioma cells, we compared mRNA expression levels of APN‐siRNA‐treated IOMM‐Lee cells with appropriate controls using a pathway‐specific real‐time PCR array containing 84 genes implicated in cell–cell and cell–matrix interactions. These analyses revealed only two genes with significant changes in the expression levels: ADAMTS38 and SPARC. ADAMTS38 was downregulated 10‐fold in APN‐siRNA‐treated IOMM‐Lee cells, whereas SPARC was increased by 3.73‐fold.
Previous papers had reported that SPARC immunoexpression is increased in aggressive and invasive meningiomas (26), prompting us to analyze the relation between APN and SPARC in meningiomas in more detail. As shown in Figure 5A, SPARC mRNA expression was increased following APN‐siRNA treatment. Figure 5B shows SPARC mRNA levels in all six cell lines, revealing an inverse relation to the APN mRNA amounts (Figure 2A). Western blot analysis of APN‐siRNA‐treated cells also shows an increase of SPARC with decreasing APN (Figure 5C). However, the analysis of SPARC mRNA expression following actinonin treatment did not show conclusive results; while SPARC levels in Ben‐Men‐1, IOMM‐Lee and HBL52 cells were nearly unchanged, in KT21MG1, we observed an increase, but in SF3061 and F5 cells, we observed a decrease in SPARC mRNA levels. Next, we employed real‐time PCR to examine SPARC mRNA expression in fresh surgical specimens of meningiomas of different malignancy grades. We found that SPARC mRNA expression was significantly increased in grade III meningiomas relative to grade I meningiomas (Figure 5D). Lastly, we compared the expression of APN and SPARC in five selected grade I and five selected grade III meningiomas with known APN expression pattern, and found an inverse relation between SPARC and APN expression, with high APN expression but low SPARC levels in low‐grade meningiomas, and high SPARC expression in aggressive meningiomas characterized by low APN expression (Figure 5E, Table 3). This relation was also confirmed by analyzing the mRNA expression levels of SPARC and APN in meningiomas (Figure 5F). Reprobing of the APN blot from Figure 1D revealed that in two out of three anaplastic meningiomas, a strong SPARC signal was detected, while grade I meningiomas with high APN had low SPARC expression (Figure 5G). Taken together, these data suggest that in a fraction of meningiomas, there is an inverse relation between APN and SPARC expression.
Figure 5.
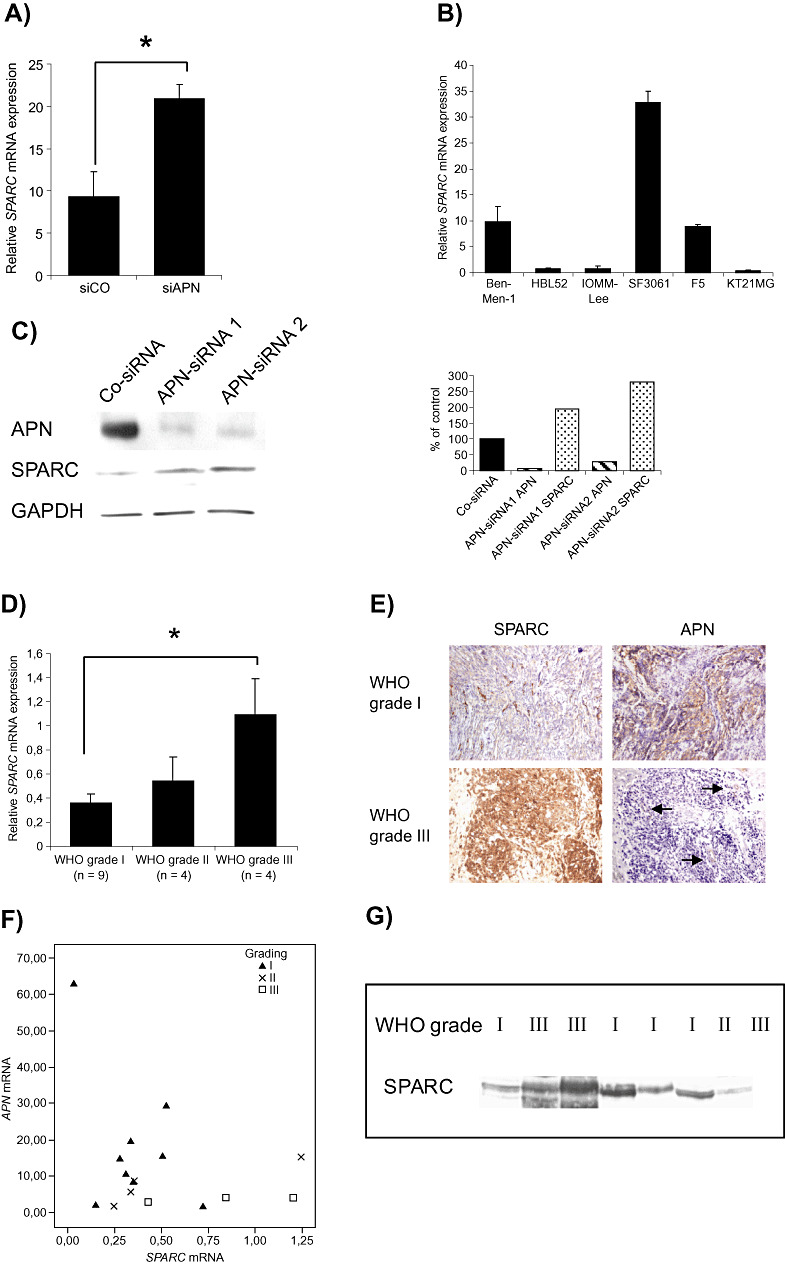
Aminopeptidase N (APN) expression in meningiomas is inversely related to SPARC expression. A. siRNA knockdown in IOMM‐Lee meningioma cells is associated with increased SPARC mRNA expression [mean ± standard error of the mean (SEM); *P < 0.05]. B. SPARC mRNA levels in six meningioma cell lines. C. APN‐siRNA‐mediated reduction of APN protein level is associated with increased SPARC protein (right figure shows normalized densitometric analyses for band intensities). D. Measuring of SPARC mRNA in human meningiomas reveals significantly increased levels in aggressive meningiomas, confirming previous immunohistochemical findings (26) (mean ± SEM; *P < 0.05). E. Association between SPARC and APN immunoexpression in grade I and grade III human meningiomas. An inverse relation is present with high SPARC but low APN expression in grade III meningiomas and vice versa in grade I meningioma. Serial sections of the same tumor region are shown. Arrows indicate retained APN staining in blood vessels in an otherwise APN‐negative meningioma. F. Scatter plot depicting the correlation between APN and SPARC mRNA expression in 16 meningiomas of different World Health Organization (WHO) grade. G. Reprobing of the APN blot (Figure 1D) with an anti‐SPARC antibody shows an inverse relation between APN and SPARC expression in the majority of tumors.
Table 3.
Relation between aminopeptidase N (APN) and SPARC immunoexpression in meningiomas. Abbreviation: WHO = World Health Organization.
| Case number | WHO grade | APN expression | SPARC expression |
|---|---|---|---|
| 1 | I | +++ | + |
| 2 | I | +++ | + |
| 3 | I | ++ | + |
| 4 | I | +++ | ++ |
| 5 | I | +++ | + |
| 6 | III | − | ++ |
| 7 | III | − | +++ |
| 8 | III | − | ++ |
| 9 | III | + | +++ |
| 10 | III | + | +++ |
−, no expression; +, weak patchy expression; ++, strong focal expression; +++, strong expression in more than 50% of tumor tissue.
DISCUSSION
The present study shows that the APN (CD13/APN) is highly expressed and functionally active in benign human meningiomas, but APN expression is reduced in a fraction of atypical and anaplastic meningiomas. Furthermore, while APN activity does not regulate tumor cell proliferation, the loss of APN is involved in meningioma cell invasion and ECM degradation. We established that SPARC, which is known to be increased in aggressive meningiomas, is at least partly regulated by APN levels in human meningiomas.
CD13/APN belongs to the increasing family of ectopeptidases, which have been implicated in tumor cell migration, metastatic behavior and neoangiogenesis [reviewed in Carl‐McGrath et al (5)]. APN regulation of neoangiogenesis has reached great attention, because proper blood vessel formation has been shown to be critically dependent on APN function 1, 22. Apn‐null mice are characterized by normal development but showed deficits in oxygen‐related retinal neovascularization (25). However, given the multifunctional characteristics of APN, it is also likely that APN has additional functions beyond the control of angiogenesis.
Conflicting data have been reported concerning CD13/APN expression and function in tumors. Colon, lung and breast cancer contain high levels of CD13/APN 7, 18 and CD13/APN expression correlated with increased malignant behavior 10, 11. In renal cancer, CD13/APN levels are reduced (12), and human ovarian cancer xenografts overexpressing CD13/APN are characterized by reduced growth (32). To date, no investigation has addressed the role of CD13/APN in meningiomas.
One major finding of the present study is the demonstration that APN is functionally active in human meningiomas. Meningiomas constitute a major fraction of intracranial tumors, and meningioma progression and/or invasion of adjacent structures poses a significant clinical challenge. While some important genetic alterations associated with meningioma development have been uncovered, the functional basis of meningioma invasion remains less well defined. High‐grade meningiomas show increased levels of collagenases such as MMP‐2 and MMP‐9 20, 21. APN itself has been shown to mediate invasion in different tumors by the initiation of collagen type IV degradation (29). However, although we found CD13/APN expression in invasive meningioma tumor cell clusters in some tumors, our data clearly demonstrated that CD13/APN expression levels and enzymatic activity is generally reduced in aggressive grade III meningiomas.
One of the functions of APN relates to the deregulation of the local balance of peptide and growth factor activation/inactivation. APN may act in this way to cleave regulatory peptides, such as bradykinin or somatostatin [reviewed in Carl‐McGrath et al (5)]. In osteosarcoma cells, APN directly regulates cell attachment and metastasis (14), whereas in other tumor cell lines, such as gastric carcinoma (4) or gliomas (unpublished data), APN inhibition is clearly associated with reduced tumor cell proliferation. Our data also suggest that proliferation control in meningiomas is not regulated by APN, but that its primary activity is the control of cell motility/invasion.
To identify putative invasion factors regulated by APN expression in meningiomas, we employed a cDNA PCR screen using pathway‐specific gene arrays. Using a siRNA approach with highly effective downregulation of APN in IOMM‐Lee malignant meningioma cells, we found two genes with significant changes in the expression levels following APN knockdown. One was the disintegrin and metalloprotease ADAMTS8, which has recently been described to be downregulated in brain tumors (8). This study also included five meningiomas, with ADAMTS8 downregulation found in one tumor. However, detailed studies regarding ADAMTS8 in meningiomas have not been published. ADAMTS8 is preferentially associated with antiangiogenic features (33) and the association between CD13 and ADAMTS8 expression, and increased vascularity or expression of angiogenic factors such as vascular endothelial growth factor (VEGF) in meningiomas should be clarified in further studies.
SPARC has been associated with proliferation, cellular adhesion and angiogenesis (3). Along these lines, Rempel et al reported that SPARC immunoexpression was significantly increased in invasive meningiomas irrespective of the WHO grade (26). Here, we show that SPARC mRNA levels are significantly increased in aggressive meningiomas. We had previously shown that SPARC expression is present at the brain‐tumor border in meningiomas but did not find a correlation between SPARC and collagen type IV expression (30). Our finding of an inverse relation between APN and SPARC expression in human meningiomas suggests that APN regulation of SPARC expression might contribute to the invasive properties of these common brain tumors.
ACKNOWLEDGMENTS
We thank Ines Helmecke, Desiree Weber, Doris Trzeczak, Ines Schellhase and Katja Mook for excellent technical work. We also thank Dr. Sandra Rempel (Detroit) and Dr. Elmar Kirches (Magdeburg) for helpful discussions. We appreciate the support from the Vincent Buono Fund (to DHG) and by a grant (to CM) from the Deutsche Forschungsgemeinschaft SFB604 (A1).
REFERENCES
- 1. Bhagwat SV, Lahdenranta J, Giordano R, Arap W, Pasqualini R, Shapiro LH (2001) CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood 97:652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bostrom J, Meyer‐Puttlitz B, Wolter M, Blaschke B, Weber RG, Lichter P et al (2001) Alterations of the tumor suppressor genes CDKN2A (p16(INK4a)), p14(ARF), CDKN2B (p15(INK4b)), and CDKN2C (p18(INK4c)) in atypical and anaplastic meningiomas. Am J Pathol 159:661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bradshaw AD, Sage EH. (2001) SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest 107:1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carl‐McGrath S, Lendeckel U, Ebert M, Wolter AB, Roessner A, Rocken C (2004) The ectopeptidases CD10, CD13, CD26, and CD143 are upregulated in gastric cancer. Int J Oncol 25:1223–1232. [PubMed] [Google Scholar]
- 5. Carl‐McGrath S, Lendeckel U, Ebert M, Rocken C (2006) Ectopeptidases in tumour biology: a review. Histol Histopathol 21:1339–1353. [DOI] [PubMed] [Google Scholar]
- 6. Cuevas IC, Slocum AL, Jun P, Costello JF, Bollen AW, Riggins GJ et al (2005) Meningioma transcript profiles reveal deregulated Notch signaling pathway. Cancer Res 65:5070–5075. [DOI] [PubMed] [Google Scholar]
- 7. Dixon J, Kaklamanis L, Turley H, Hickson ID, Leek RD, Harris AL, Gatter KC (1994) Expression of aminopeptidase‐n (CD 13) in normal tissues and malignant neoplasms of epithelial and lymphoid origin. J Clin Pathol 47:43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dunn JR, Reed JE, Du Plessis DG, Shaw EJ, Reeves P, Gee AL et al (2006) Expression of ADAMTS‐8, a secreted protease with antiangiogenic properties, is downregulated in brain tumours. Br J Cancer 94:1186–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fujii H, Nakajima M, Saiki I, Yoneda J, Azuma I, Tsuruo T (1995) Human melanoma invasion and metastasis enhancement by high expression of aminopeptidase N/CD13. Clin Exp Metastasis 13:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hashida H, Takabayashi A, Kanai M, Adachi M, Kondo K, Kohno N et al (2002) Aminopeptidase N is involved in cell motility and angiogenesis: its clinical significance in human colon cancer. Gastroenterology 122:376–386. [DOI] [PubMed] [Google Scholar]
- 11. Ishii K, Usui S, Sugimura Y, Yoshida S, Hioki T, Tatematsu M et al (2001) Aminopeptidase N regulated by zinc in human prostate participates in tumor cell invasion. Int J Cancer 92:49–54. [PubMed] [Google Scholar]
- 12. Ishii K, Usui S, Yamamoto H, Sugimura Y, Tatematsu M, Hirano K (2001) Decreases of metallothionein and aminopeptidase N in renal cancer tissues. J Biochem (Tokyo) 129:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kido A, Krueger S, Haeckel C, Roessner A (1999) Possible contribution of aminopeptidase N (APN/CD13) to invasive potential enhanced by interleukin‐6 and soluble interleukin‐6 receptor in human osteosarcoma cell lines. Clin Exp Metastasis 17:857–863. [DOI] [PubMed] [Google Scholar]
- 14. Kido A, Krueger S, Haeckel C, Roessner A (2003) Inhibitory effect of antisense aminopeptidase N (APN/CD13) cDNA transfection on the invasive potential of osteosarcoma cells. Clin Exp Metastasis 20:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kilic T, Bayri Y, Ozduman K, Acar M, Diren S, Kurtkaya O et al (2002) Tenascin in meningioma: expression is correlated with anaplasia, vascular endothelial growth factor expression, and peritumoral edema but not with tumor border shape. Neurosurgery 51:183–192. [DOI] [PubMed] [Google Scholar]
- 16. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (2007) WHO Classification of Tumours of the Central Nervous System, 4th edn. IARC Press: Lyon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mawrin C, Sasse T, Kirches E, Kropf S, Schneider T, Grimm C et al (2005) Different activation of mitogen‐activated protein kinase and Akt signaling is associated with aggressive phenotype of human meningiomas. Clin Cancer Res 11:4074–4082. [DOI] [PubMed] [Google Scholar]
- 18. Mechtersheimer G, Moller P (1990) Expression of aminopeptidase N (CD13) in mesenchymal tumors. Am J Pathol 137:1215–1222. [PMC free article] [PubMed] [Google Scholar]
- 19. Nordqvist AC, Peyrard M, Pettersson H, Mathiesen T, Collins VP, Dumanski JP, Schalling M (1997) A high ratio of insulin‐like growth factor II/insulin‐like growth factor binding protein 2 messenger RNA as a marker for anaplasia in meningiomas. Cancer Res 57:2611–2614. [PubMed] [Google Scholar]
- 20. Nordqvist AC, Smurawa H, Mathiesen T (2001) Expression of matrix metalloproteinases 2 and 9 in meningiomas associated with different degrees of brain invasiveness and edema. J Neurosurg 95:839–844. [DOI] [PubMed] [Google Scholar]
- 21. Okada M, Miyake K, Matsumoto Y, Kawai N, Kunishio K, Nagao S (2004) Matrix metalloproteinase‐2 and matrix metalloproteinase‐9 expressions correlate with the recurrence of intracranial meningiomas. J Neurooncol 66:29–37. [DOI] [PubMed] [Google Scholar]
- 22. Pasqualini R, Koivunen E, Kain R, Lahdenranta J, Sakamoto M, Stryhn A et al (2000) Aminopeptidase N is a receptor for tumor‐homing peptides and a target for inhibiting angiogenesis. Cancer Res 60:722–727. [PMC free article] [PubMed] [Google Scholar]
- 23. Perry A, Gutmann DH, Reifenberger G (2004) Molecular pathogenesis of meningiomas. J Neurooncol 70:183–202. [DOI] [PubMed] [Google Scholar]
- 24. Puttmann S, Senner V, Braune S, Hillmann B, Exeler R, Rickert CH, Paulus W (2005) Establishment of a benign meningioma cell line by hTERT‐mediated immortalization. Lab Invest 85:1163–1171. [DOI] [PubMed] [Google Scholar]
- 25. Rangel R, Sun Y, Guzman‐Rojas L, Ozawa MG, Sun J, Giordano RJ et al (2007) Impaired angiogenesis in aminopeptidase N‐null mice. Proc Natl Acad Sci USA 104:4588–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rempel SA, Ge S, Gutierrez JA. (1999) SPARC a potential diagnostic marker of invasive meningiomas. Clin Cancer Res 5:237–241. [PubMed] [Google Scholar]
- 27. Rocken C, Carl‐McGrath S, Grantzdorffer I, Mantke R, Roessner A, Lendeckel U (2004) Ectopeptidases are differentially expressed in hepatocellular carcinomas. Int J Oncol 24:487–495. [PubMed] [Google Scholar]
- 28. Rocken C, Licht J, Roessner A, Carl‐McGrath S (2005) Canalicular immunostaining of aminopeptidase N (CD13) as a diagnostic marker for hepatocellular carcinoma. J Clin Pathol 58:1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saiki I, Fujii H, Yoneda J, Abe F, Nakajima M, Tsuruo T, Azuma I (1993) Role of aminopeptidase N (CD13) in tumor‐cell invasion and extracellular matrix degradation. Int J Cancer 54:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schittenhelm J, Mittelbronn M, Roser F, Tatagiba M, Mawrin C, Bornemann A (2006) Patterns of SPARC expression and basement membrane intactness at the tumour‐brain border of invasive meningiomas. Neuropathol Appl Neurobiol 32:525–531. [DOI] [PubMed] [Google Scholar]
- 31. Surace EI, Lusis E, Murakami Y, Scheithauer BW, Perry A, Gutmann DH (2004) Loss of tumor suppressor in lung cancer‐1 (TSLC1) expression in meningioma correlates with increased malignancy grade and reduced patient survival. J Neuropathol Exp Neurol 63:1015–1027. [DOI] [PubMed] [Google Scholar]
- 32. Van Hensbergen Y, Broxterman HJ, Rana S, Van Diest PJ, Duyndam MC, Hoekman K et al (2004) Reduced growth, increased vascular area, and reduced response to cisplatin in CD13‐overexpressing human ovarian cancer xenografts. Clin Cancer Res 10:1180–1191. [DOI] [PubMed] [Google Scholar]
- 33. Vazquez F, Hastings G, Ortega MA, Lane TF, Oikemus S, Lombardo M, Iruela‐Arispe ML (1999) METH‐1, a human ortholog of ADAMTS‐1, and METH‐2 are members of a new family of proteins with angio‐inhibitory activity. J Biol Chem 274:23349–23357. [DOI] [PubMed] [Google Scholar]
- 34. Weber RG, Bostrom J, Wolter M, Baudis M, Collins VP, Reifenberger G, Lichter P (1997) Analysis of genomic alterations in benign, atypical, and anaplastic meningiomas: toward a genetic model of meningioma progression. Proc Natl Acad Sci USA 94:14719–14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zeltner L, Schittenhelm J, Mittelbronn M, Roser F, Tatagiba M, Mawrin C et al (2007) The astrocytic response towards invasive meningiomas. Neuropathol Appl Neurobiol 33:163–168. [DOI] [PubMed] [Google Scholar]


