Abstract
Background
Motor imagery (MI) is defined as a mentally rehearsed task in which movement is imagined but is not performed. The approach includes repetitive imagined body movements or rehearsing imagined acts to improve motor performance.
Objectives
To assess the treatment effects of MI for enhancing ability to walk among people following stroke.
Search methods
We searched the Cochrane Stroke Group registry, CENTRAL, MEDLINE, Embase and seven other databases. We also searched trial registries and reference lists. The last searches were conducted on 24 February 2020.
Selection criteria
Randomized controlled trials (RCTs) using MI alone or associated with action observation or physical practice to improve gait in individuals after stroke. The critical outcome was the ability to walk, assessed using either a continuous variable (walking speed) or a dichotomous variable (dependence on personal assistance). Important outcomes included walking endurance, motor function, functional mobility, and adverse events.
Data collection and analysis
Two review authors independently selected the trials according to pre‐defined inclusion criteria, extracted the data, assessed the risk of bias, and applied the GRADE approach to evaluate the certainty of the evidence. The review authors contacted the study authors for clarification and missing data.
Main results
We included 21 studies, involving a total of 762 participants. Participants were in the acute, subacute, or chronic stages of stroke, and had a mean age ranging from 50 to 78 years. All participants presented at least some gait deficit. All studies compared MI training versus other therapies. Most of the included studies used MI associated with physical practice in the experimental groups. The treatment time for the experimental groups ranged from two to eight weeks. There was a high risk of bias for at least one assessed domain in 20 of the 21 included studies.
Regarding our critical outcome, there was very low‐certainty evidence that MI was more beneficial for improving gait (walking speed) compared to other therapies at the end of the treatment (pooled standardized mean difference (SMD) 0.44; 95% confidence interval (CI) 0.06 to 0.81; P = 0.02; six studies; 191 participants; I² = 38%). We did not include the outcome of dependence on personal assistance in the meta‐analysis, because only one study provided information regarding the number of participants that became dependent or independent after interventions.
For our important outcomes, there was very low‐certainty evidence that MI was no more beneficial than other interventions for improving motor function (pooled mean difference (MD) 2.24, 95% CI ‐1.20 to 5.69; P = 0.20; three studies; 130 participants; I² = 87%) and functional mobility at the end of the treatment (pooled SMD 0.55, 95% CI ‐0.45 to 1.56; P = 0.09; four studies; 116 participants; I² = 64.2%). No adverse events were observed in those studies that reported this outcome (seven studies). We were unable to pool data regarding walking endurance and all other outcomes at follow‐up.
Authors' conclusions
We found very low‐certainty evidence regarding the short‐term benefits of MI on walking speed in individuals who have had a stroke, compared to other therapies. Evidence was insufficient to estimate the effect of MI on the dependence on personal assistance and walking endurance. Compared with other therapies, the evidence indicates that MI does not improve motor function and functional mobility after stroke (very low‐certainty evidence). Evidence was also insufficient to estimate the effect of MI on gait, motor function, and functional mobility after stroke compared to placebo or no intervention. Motor Imagery and other therapies used for gait rehabilitation after stroke do not appear to cause significant adverse events.
Keywords: Aged; Female; Humans; Male; Middle Aged; Bias; Gait Disorders, Neurologic; Gait Disorders, Neurologic/etiology; Gait Disorders, Neurologic/rehabilitation; Imagery, Psychotherapy; Imagery, Psychotherapy/methods; Randomized Controlled Trials as Topic; Stroke; Stroke/complications; Stroke Rehabilitation; Stroke Rehabilitation/methods; Walking Speed
Plain language summary
Motor imagery for gait rehabilitation
Review question
Is motor imagery (MI) an effective approach to improve gait (walking ability) in people following stroke?
Background
Post‐stroke gait disability affects independence, mobility, activities of daily living, and participation in community activities. MI is a type of therapy that uses the imagination of movement (without actually moving). It has been recommended in the rehabilitation of people with stroke to promote movement relearning.
Study characteristics
Our most recent search date was 24 February 2020. We included 21 studies, with 762 participants (60% men and 40% women), and a mean age ranging from 50 to 78 years. The participants included in the studies were at different time points after stroke, and the etiology (causes of stroke) was also varied. All participants were able to walk with some difficulty. All included studies compared MI training with another intervention, and physical practice was the most applied therapy in the comparison (control) groups. In the experimental groups, most of the included studies used MI combined with physical practice, and used either kinesthetic (when someone imagines himself or herself) or visual (when the individual observes another person) MI. The treatment time for the experimental groups ranged from two to eight weeks. In general, only three of the included studies conducted a follow‐up assessment after interventions.
Key results
We found very low‐certainty evidence that MI alone or combined with either action observation (a type of imagery in which patients observe movement) or physical practice was superior to other therapies in improving walking speed in a short‐term period. However, there is very low‐certainty evidence that MI was no more beneficial than other therapies for improving motor function and functional mobility at the end of the treatment. There was insufficient evidence to evaluate the effect of MI on independence to perform activities of daily living and walking endurance after stroke, and to evaluate the medium‐ or long‐term effects of MI on all assessed outcomes. Although poorly reported, no adverse events related to MI and other therapies were observed. It is unknown whether the gait of people who have had a stroke could benefit from MI training compared to placebo or no intervention.
Certainty of the evidence
We classified the certainty of the evidence as very low because many studies had methodological concerns and low numbers of participants, and did not follow guidelines for how studies should be reported.
Summary of findings
Summary of findings 1. Summary of findings for the main comparison. Motor imagery compared to other therapies (control) for gait rehabilitation after stroke (only outcomes immediately after intervention).
| Motor imagery compared to other therapies (control) for gait rehabilitation after stroke (only outcomes immediately after intervention) | ||||||
| Patient or population: People performing gait rehabilitation after stroke Setting: Clinical and home environment Intervention: MI Comparison: Other therapies (control) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with MI | |||||
| Walking speed assessed with: 10MWT test, custom systems | ‐ | The mean walking speed in the intervention groups was 0.44 standard mean difference higher (0.06 to 0.81 higher) | ‐ | 191 (6 RCTs) | ⊕⊝⊝⊝ very lowa,b,c | Evidence suggests that MI may increase walking speed |
| Motor function assessed with: FMA‐LE | ‐ | The mean motor function in the intervention groups was 2.24mean difference higher (1.20 lower to 5.69 higher) | ‐ | 130 (3 RCTs) | ⊕⊝⊝⊝ very lowa,b,c,d | MI results show little or no difference in motor function |
| Functional mobility assessed with: RMI, TUGT | ‐ | The mean functional mobility in the intervention groups was 0.55 standard mean difference higher (0.45 lower to 1.56 higher) | ‐ | 116 (4 RCTs) | ⊕⊝⊝⊝ very lowa,b,c,d | MI results show in little or no difference in functional mobility |
| Dependence on personal assistance assessed with: MAS, BI, FAC | See comment | Not estimable | 385 (10 RCTs) | ‐ | Due to the lack of data in the studies regarding this outcome it was not possible to perform the meta‐analysis | |
| Walking endurance assessed with: 6MWT | See comment | ‐ | 30 (1 RCT) | ‐ | Due to not reaching the minimum number of studies (2), it was not possible to perform the meta‐analysis | |
| Adverse events (including pain, falls and all‐cause deaths) | See comment | Not estimable | 252 (7 RCTs) | ‐ | No adverse events were related to the interventions | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 6MWT: 6 Minute Walk Test; 10MWT: 10 Meter Walk Test; BI: Barthel Index; CI: confidence interval; FAC: Functional Ambulation Category; FIM: Functional Independence Measure; FMA‐LE: Fugl‐Meyer Assessment Lower Extremity; MAS: Motor Assessment Scale; MI: motor imagery; RMI: Rivermead Mobility Index; RR: risk ratio; TUGT: Timed Up and Go Test | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded due to several ratings with 'high' or 'unclear' risk of bias for random sequence generation, allocation concealment or blinding of outcome assessment.
bSmall sample size (< 400).
cWide confidence interval.
dModerate or substantial heterogeneity (> 50%).
Background
Description of the condition
According to the World Health Organization (WHO), cardiovascular diseases are the leading cause of death worldwide, accounting for 17.7 million deaths in 2015. Of these, 6.7 million were directly attributed to stroke, making it one of the main non‐communicable causes of death. Stroke can be defined as an acute event caused by a blockage or bleeding that prevents blood from flowing to the brain, often resulting in motor symptoms such as muscle weakness. Stroke represents one of the leading healthcare expenditures and is the second highest cause of disability (WHO 2017). Around 15% to 30% of people with stroke exhibit persistent functional disability, and only 13% of stroke survivors return to work (Chumney 2010; Rayegani 2016).
It is estimated that three months after stroke, 70% of stroke survivors walk at a reduced speed, and 20% remain wheelchair‐bound (Dujovic 2017; Sakuma 2014). The literature reports a direct relationship between motor deficit and function (Jørgensen 1995; Langhorne 2009). Post‐stroke gait disability diminishes independence, mobility, activities of daily living, and participation in community activities (Mikołajewska 2017). Thus, one of the most important goals of post‐stroke rehabilitation is to restore gait pattern and achieve fast walking so that people who have had a stroke can perform their activities of daily living without complications (Chiu 2000; Ji 2015; Whitall 2004). In this respect, evidence indicates that specific high‐intensity repetitive task training improves the process of gait rehabilitation (French 2016; Langhorne 2009; Mehrholz 2017).
Description of the intervention
Exercises involving direct walking practice have been used to improve gait, such as treadmill training (Mehrholz 2017), and overground physical therapy gait training (States 2009), but activities that mimic walking, including imagery/mental practice, have also been used (Barclay 2015). Movement representation techniques, also referred to as mental practice, can be defined as any type of therapy that uses the representation of movement, specifically observation or imagination, or both. These interventions are mirror therapy, action observation, and motor imagery (MI) (Thieme 2016). Mirror therapy is defined as an intervention that uses a mirror to create a reflection of the non‐affected upper or lower limb, and thus provides the individual with normal visual feedback of movement (Ramachandran 1994; Thieme 2016). Action observation refers to the visual perception of a given action performed by others. In the observation, actual performance by another person, or as video or virtual setups, can be used (Thieme 2016). In this review, we will explore the effect of MI.
MI is defined as a mentally rehearsed task in which movement is imagined but is not executed (Kim 2018; Mulder 2007). Because MI is independent of residual motor function, it may provide a substitute for executed movement as a means to activate the motor network (Sharma 2006). This way, the approach includes repetitive imagined body movements or rehearsing imagined acts with the aim to improve motor performance (Carrasco 2016; Li 2017). Motor imagery was initially used to improve athletic performance (Driediger 2006), and has subsequently been suggested for the rehabilitation of people with stroke to promote motor relearning (Driediger 2006; Liu 2004; Moura 2012).
MI for rehabilitation can be conducted in two forms: external or visual, in which people imagine from the standpoint of an external observer (third‐person imagination); and internal or kinesthetic, where people imagine the sensation of their body moving (first‐person imagination) (Carrasco 2016). While the ability to internally represent and to produce actions have common aspects, studies have indicated a dissociation of these processes, which can help to explain why individuals with stroke may be able to generate internal action representations even though they do not have the ability to perform a movement (Johnson 2000; Johnson 2002a). In fact, it is still unclear how well people with stroke are able to perform MI, but it appears that most of these individuals retain their ability for MI (Braun 2017; Johnson 2000; Johnson 2002a). Over the past two decades, whether separately or combined with physical practice (where the movement is executed), MI has demonstrated promising results for rehabilitating gait after stroke (Dickstein 2004; Hwang 2010; Lamontagne 2004). For example, results are promising for increased gait speed (Beyaert 2015; Dickstein 2004).
How the intervention might work
In 1996, Decety suggested that imagining movement activates the same brain areas that are activated when the movements are actually executed. These findings reinforce the idea that if mental stimulation of the action triggers neural activation of relevant motor areas, we can therefore 'exercise' the brain in the absence of a physical movement (Decety 1996).
The neurophysiological basis underlying MI consists of the mirror neuron system, located in the rostral portion of the inferior parietal lobule, pars opercularis of the inferior frontal gyrus and the ventral portion of the premotor cortex. The units that make up this system (mirror neurons) are a class of visuomotor neurons that are activated during execution or observation of movements aimed at an objective (Garrison 2010). During MI, the motor areas involved in the process are the primary motor cortex and several pre‐motor areas, including the supplementary motor area, pre‐supplementary motor area, and ventral and dorsal parts of the premotor cortex (Jeannerod 1995; Kim 2018). These areas are activated during both motor execution and motor imagery tasks; indeed, functional imaging studies have observed activation of brain regions upon motor execution and motor imagery (Johnson 2002b; Lotze 1999; Wang 2016).
A number of hypotheses have been proposed to elucidate the MI functioning mechanism. The first is the mental simulation theory (Munzert 2009), which states that a neural motor network is activated by imagining motor actions (Jeannerod 2001). This activation includes pre‐motor and motor areas and subcortical areas of the brain (Lotze 1999), and basal ganglia (Bonda 1995). In this respect, these subliminal activations improve an individual's learning (Barclay‐Goddard 2011). A second hypothesis proposes that individuals involved in MI rehearse elements of the task, giving them the opportunity to foresee the outcomes of their actions based on previous experience. Therapy participants anticipate possible action trajectories, which they are more likely to use to perform when executing a real movement. As such, individuals develop more efficient ways to approach outcomes (Barclay‐Goddard 2011). Although the exact MI functioning mechanism has not fully been clarified, recent evidence indicates cortical reorganization in people with stroke after treatment with MI, which could result in better gait recovery in this population (Guerra 2017). It is believed that cortical reorganization occurs due to increased primary motor activity, which in turn raises sensorimotor cortex recruitment, resulting in functional improvements (Sun 2013).
Why it is important to do this review
Stroke is considered to be a serious public health issue worldwide, leading to an increasing number of survivors with disabilities (Chumney 2010; Rayegani 2016). Gait recovery is a key aim of post‐stroke rehabilitation, given that it enables survivors to resume most daily activities, reducing the incidence of falls, and other factors that pose a risk to this population. However, stroke survivors may undergo lengthy and challenging treatments, resulting in adoption of passive attitudes to rehabilitation. Motor imagery is an easy, safe, and less tiring technique that increases survivor participation and motivation. Furthermore, MI does not require specific equipment, and is considered to be a low‐cost procedure (Decety 1993; Dickstein 2004; Hosseini 2012). Nevertheless, there is currently insufficient evidence to indicate the best treatment to improve walking after stroke (Barclay 2015).
Recent studies show positive gait rehabilitation results from MI, such as increased lower limb muscle strength and better walking performance in people following stroke (Kumar 2016; Oostra 2015). However, confirming the efficacy of MI in post‐stroke gait requires a thorough investigation of experimental studies on the issue, given that results do not appear to be consistent. Both therapy results and methodological quality of studies need to be assessed, given that treatment protocols vary considerably.
There is a wide variety of intervention protocols that differ in aspects such as frequency of exposure to MI, movements and tasks performed, and duration of therapy (Carrasco 2016). Furthermore, few clinical trials on MI present high methodological quality (Guerra 2017; Winstein 2016). To date, there has been no Cochrane Review exploring the effects of MI on gait among stroke survivors. By conducting a systematic review and meta‐analysis, and assessing the methodological quality of the studies, this review should provide support for evidence‐based clinical decisions. In addition, it will also highlight where further research is needed.
Objectives
To assess the treatment effects of MI for enhancing ability to walk among people following stroke.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomized controlled trials (RCTs), including those available only in summary form. We also included cross‐over trials (using data only from the first phase), provided that allocation of interventions was random. We excluded quasi‐experimental or non‐randomized studies. We included studies regardless of publication date or language.
Types of participants
We included studies in which participants presented with a clinical diagnosis of stroke of any type (including subarachnoid hemorrhage). Eligible participants were at least 18 years of age, of any sex, with any degree of severity of the disease, and at any stage after stroke. We excluded studies in which participants had a mixed etiology of the disease (e.g. acquired brain injury), unless data were available for individuals who only had a stroke.
Types of interventions
We included studies that used MI for gait improvement in people with stroke. We considered the concept of MI as an approach in which the individual imagines the movement, or part of it,without its actual execution. Thus, we selected studies comparing:
MI alone or associated with action observation, physical activity, or functional gait training versus other therapies (including conventional physical therapy);
MI alone or associated with action observation, physical activity or functional gait training versus placebo; and
MI alone or associated with action observation, physical activity or functional gait training versus no therapy.
Types of outcome measures
We extracted the outcomes of interest from the baseline and the evaluation at the end of the intervention period (immediate effects) and follow‐up (medium‐ or long‐term effects). Measures of medium‐term effects were considered as those collected between two weeks to six months after treatment had ended, and measures of long‐term effects if collected more than six months after treatment had ended.
Primary outcomes
The critical outcome was ability to walk, verified using the following continuous and dichotomous variables.
Continuous variable: walking speed, measured by biomechanical analysis or walking tests, or both, considering both preferable/comfortable walking speed and fastest walking speed.
-
Dichotomous variable: dependence on personal assistance. According to Mehrholz and colleagues, dependence was defined "as the inability to walk indoors (with or without a gait aid) without personal assistance or supervision" (Mehrholz 2017). If reported, we used data from functional scales related to walking to define the level of dependence. We considered the following scales and scores (Mehrholz 2017):
Motor Assessment Scale (MAS) (Carr 1985), score of 2 or less for the walking item;
Functional Independence Measure (Hamilton 1994), score of 5 or less for the walking item;
Barthel Index (Collin 1988), score of 3 (independent, but may use any aid) or less for the ambulation item;
Rivermead Mobility Index (Collen 1991), an answer of 'no' to the 'walking inside with an aid if necessary' item; and
Functional Ambulation Category (FAC) (Holden 1984), score of 2 or less.
Secondary outcomes
Walking endurance (distance covered, in meters), measured by Six‐Minute Walk Test or Two‐Minute Walk Test.
Motor function, measured by the Fugl‐Meyer Assessment Scale (Fugl‐Meyer 1975), or Motor Assessment Scale.
Functional mobility (including gait), measured by Rivermead Mobility Index or Timed Up and Go Test (Podsiadlo 1991).
Adverse events (including pain, falls, and all‐cause deaths).
When included studies cited more than one measure for each outcome, we considered the Six‐Minute Walk Test for walking endurance, the Fugl‐Meyer Assessment Scale for motor function, and the Rivermead Mobility Index for functional mobility.
Search methods for identification of studies
See the 'Specialized register' information at the Cochrane Stroke Group's website. We searched for trials in all languages and arranged for translation of relevant articles where necessary.
Electronic searches
We searched the Cochrane Stroke Group trials register (last searched on 3 February 2020) and the following electronic databases.
Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library; Issue 2 of 12, February 2020; last searched 3 February 2020) (Appendix 1);
MEDLINE Ovid (from 1946 to January 31, 2020; last searched 3 February 2020) (Appendix 2);
Embase Ovid (1980 to 2020 Week 05; last searched 3 February 2020) (Appendix 3);
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; from 1982 to present; last searched 3 February 2020) (Appendix 4);
PsycINFO Ovid (from 1806 to January 2020 Week 4; last searched 3 February 2020) (Appendix 5);
AMED Ovid (Allied and Complementary Medicine; from 1985 to January 2020; last searched 3 February 2020) (Appendix 6);
LILACS Bireme (Latin American and Caribbean Health Science Information database; from 1982 to present; last searched 24 February 2020) (Appendix 7);
SPORTDiscus EBSCO (from 1949 to present; last searched 3 February 2020) (Appendix 8);
PEDro (Physiotherapy Evidence Database; www.pedro.org.au/) (24 February 2018) (Appendix 9);
REHABDATA National Rehabilitation Information Center (www.naric.com/?q=en/REHABDATA) (24 February 2018) (Appendix 10).
We developed the MEDLINE search strategy with the help of the Cochrane Stroke Group Information Specialist and we adapted it for the other databases where appropriate. All search strategies deployed were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6, Lefebvre 2011).
We also searched the following trials registries.
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/) (last searched 3 February 2020) (Appendix 11).
World Health Organization (WHO) International Clinical Trials Registry Platform (who.int/ictrp/en/) (last searched 3 February 2020) (Appendix 12).
Stroke Trials Registry (www.strokecenter.org/trials/) (October 15, 2018) (Appendix 13).
Searching other resources
In an effort to identify further published, unpublished and ongoing trials, we did the following:
screened the reference lists of relevant studies to identify further studies for potential inclusion in the review;
used Science Citation Index Cited Reference search for forward tracking of relevant articles;
contacted study authors, researchers and experts in the field to obtain additional information on relevant trials; and
searched for PhD and MSc theses (using ProQuest Thesis database and British Library Ethos database).
Data collection and analysis
Selection of studies
Two review authors (LS and LL) independently screened titles and abstracts of the references obtained from our searching activities and excluded obviously irrelevant reports. We retrieved the full‐text articles for the remaining references. The same two review authors independently screened the full‐text articles to identify studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion, or we consulted a third review author (TR) when required. We gathered multiple reports of the same study so that each study, and not each reference, is the unit of interest in the review. We recorded the selection process and completed a PRISMA flow diagram.
Data extraction and management
Two review authors (LS and LL) independently extracted data from the included studies. When data were lacking or details were unclear, we contacted the study authors for clarification. When there was disagreement regarding data collection, a third review author checked the data (TR). The data collected were:
method used: objectives, study design, instruments used, total duration of the study, form of randomisation, secrecy of the allocation, blindness of the evaluators, institutions or study centers involved, study site, withdrawal and withdrawal of the participants and year of study;
participants: sample size, age, sex, diagnostic criteria, inclusion and exclusion criteria, severity of stroke and stage (acute/subacute and chronic);
intervention: we used the 'Template for intervention description and replication' (TIDieR) checklist and guide to extract data about interventions (Hoffmann 2014); we considered all the 12 points on the TIDierR checklist;
results: critical and important outcomes for each assessment and reassessment; and
notes: funding for experimentation and notable conflicts of interest of the study authors.
Assessment of risk of bias in included studies
Two review authors (LS and LL) independently assessed risk of bias for each study using Cochrane's 'Risk of bias' tool (Higgins 2011). We resolved any disagreements by discussion or by involving another review author (TR). We assessed the risk of bias according to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Any other bias.
We graded any identified biases using table 8.5.a of the Cochrane Handbook for Systematic Reviews of interventions (Higgins 2011). This table provides criteria for analysis and judgement of risk of bias in each of the seven domains. We classified risk of bias in each domain as high, low, or unclear, and we justified each decision and recorded this information in the 'Risk of bias' tables.
The assessment of risk of bias for blinding of participants and personnel depended on the influence that lack of blinding would have. If the participants and personnel were not blinded, and after judging that the outcome measure could be influenced by the knowledge of participants and personnel about which intervention was provided, we assigned a high risk of bias. If we judged that the outcome measure would not be influenced by the knowledge of participants and personnel about the intervention, we assigned a low risk of bias, whether or not the blinding of participants and personnel had happened.
Measures of treatment effect
We measured treatment effects for continuous outcomes using the mean difference (MD) (if at least two studies reported the same outcome measures) or the standardized mean difference (SMD) (when different outcome measures were used). For dichotomous outcomes, we used the risk ratio (RR). We presented the results for each outcome with 95% confidence intervals (CI).
Unit of analysis issues
When we identified cluster‐randomized studies or any non‐parallel designs, we considered their inclusion, following guidance in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We contacted authors of respective studies to request missing information. When we were unable obtained the missing data from study authors and we considered that the missing data might introduce serious bias, we conducted a sensitivity analysis to explore the impact of including such studies in the overall assessment of results. We performed an available case analysis, i.e. we included data for only those participants whose results are known, without assumptions for imputing data. We considered the amount of missing data when determining the risk of bias of each included study.
Assessment of heterogeneity
We visually assessed forest plots, verifying overlap in the confidence intervals of studies (poor overlap may indicate statistical heterogeneity) (Deeks 2011). In addition, we used the I² statistic to measure heterogeneity among trials in each analysis. Values of I² greater than 50% may represent substantial heterogeneity (Deeks 2011).
We explored the reasons for heterogeneity (e.g. setting, participants, interventions, design, and risk of bias). When we found that heterogeneity was caused by one or two studies with peripheral results conflicting with the rest of the studies, we carried out analyses with and without these studies as part of the sensitivity analysis.
Assessment of reporting biases
We planned to examine the presence of publication bias by visual inspection of funnel plot if 10 or more trials were included (Higgins 2011). We attempted to avoid language bias by including trials irrespective of language of publication, and we also provided translation to English when needed. In cases of possible multiple publications from the same trial, we contacted study authors to check whether these publications were duplicates. When we were unable to obtain the necessary information from study authors, we made a judgement based on consideration of criteria such as the recruitment site, trial dates, registry numbers, and whether there were similar or identical patient characteristics in each study. For assessment of selective reporting, when the study protocol or trial registry was available, outcomes in the protocol or trial registry and in the published study were compared. If not, we examined if the outcomes listed in the methods section of a study were reported in the results.
Data synthesis
We analyzed data using Review Manager 5 software (Review Manager 2014), and pooled data for meta‐analysis when we considered studies to be sufficiently similar in terms of participants, interventions, comparisons, and outcomes. We used the random‐effects model for meta‐analysis.
GRADE and 'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes: walking speed, dependence on personal assistance, walking endurance, motor function, functional mobility, and adverse events.
The following comparison is reported in the 'Summary of findings' tables:
Motor imagery (alone or associated with action observation or physical practice) versus other therapies (outcomes immediately after intervention).
We planned to prepare another 'Summary of findings' table for medium‐ and long‐term effects. However, it was not possible due to the lack of follow‐up data.
We reported the number of studies and participants, the relative effect, direction of effect, and the certainty of the evidence (GRADE) for each outcome.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the pre‐specified outcomes (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro GDT software (GRADEpro GDT 2015). We justified all decisions to downgrade the quality of studies using footnotes, and made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We planned to undertake subgroup analyses for all outcomes when feasible to explore the influence of the following:
type of stroke: ischemic or hemorrhagic;
post‐stroke time: acute (less than one month post‐stroke), subacute (between one and six months post‐stroke) and chronic (more than six months after stroke);
length of treatment period or treatment dose;
type of treatment: MI alone or MI associated with action observation or physical practice (physical activity or functional gait training);
walking dependence: independent or dependent of personal assistance (human support or supervision) at the beginning of the study.
Sensitivity analysis
We planned to perform sensitivity analyses for all outcomes when we suspected that missing data introduced important bias, and to assess heterogeneity caused by studies with peripheral results. Furthermore, we carried out the sensitivity analyses by excluding studies from the analysis that were at high risk of bias in one or more of these three domains:
allocation concealment;
blinding of outcome assessment;
random sequence generation.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies
Results of the search
Our searches identified 4769 references. After removal of duplicates, we screened titles and abstracts, and identified 61 potentially eligible references. After reading the full texts of these references, we selected 21 studies for inclusion in the review. The results of the search are summarized in the PRISMA study flow diagram (Figure 1).
1.
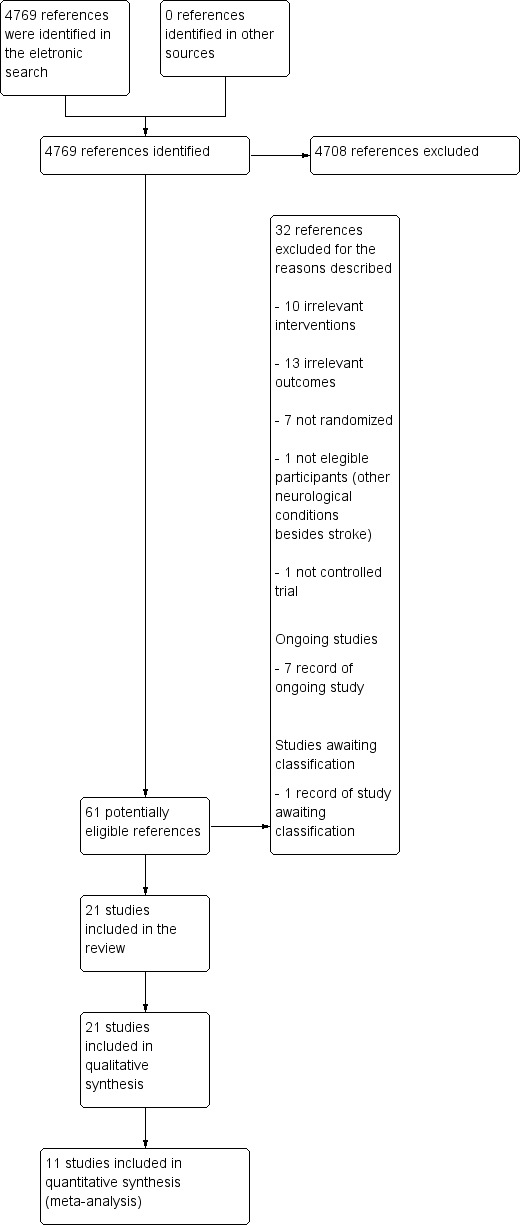
4747Study flow diagram.
Included studies
We included 21 studies in this review (Braun 2012; Cho 2012; Dickstein 2013; Dickstein 2014; Gupta 2017; Kim 2013a; Kumar 2013a; Kumar 2016; Lee 2010; Lee 2011; Lee 2015; Liu 2004; Liu 2009; Oostra 2015; Park 2019; Schuster 2012; Suvadeep 2017; Verma 2011; Yan 2013; Zhang 2013; Zhu 2017). We found one study (an abstract) that referred to the same authors and contained the same data in terms of sample size, interventions, number of participants in each group, outcomes, and results. To avoid duplication, we chose to use the most recent study (Oostra 2015) because data were already fully analyzed, thus providing the most comprehensive results.
Design
We identified 21 RCTs, including one multicenter trial (Braun 2012), two pilot studies (Kumar 2013a; Schuster 2012), and three crossover trials (Dickstein 2013; Dickstein 2014; Zhang 2013).
Sample characteristics
The 21 studies involved a total of 762 participants. The mean age of the participants ranged from 50 years (Oostra 2015), to 78 years (Braun 2012). The sample consisted of 60% men and 40% women. Four studies included participants in the subacute stroke stage (one to six months after stroke) (Gupta 2017; Oostra 2015; Suvadeep 2017; Verma 2011), six in the chronic stroke stage (more than six months after stroke) (Cho 2012; Dickstein 2013; Dickstein 2014; Kim 2013a; Lee 2015; Park 2019), and 11 studies did not report or did not make clear the stroke stage. Fourteen studies specified stroke etiology. Two recruited only participants with ischemic stroke (Liu 2004; Liu 2009) and 12 recruited participants with either ischemic or hemorrhagic stroke (Cho 2012; Dickstein 2013; Dickstein 2014; Kim 2013a; Kumar 2016; Lee 2010; Lee 2015; Oostra 2015; Park 2019; Schuster 2012; Verma 2011; Zhang 2013). Five studies reported the dependence of the participants on personal assistance to walk at study baseline. Three studies reported that participants were either dependent or independent at the beginning of the study (Dickstein 2013; Kumar 2016; Oostra 2015), and three studies reported that participants were considered independent at study entry (Lee 2011; Lee 2015; Verma 2011).
For inclusion and exclusion criteria, see Characteristics of included studies.
Settings
Six studies were carried out in rehabilitation centers (Cho 2012; Kumar 2016; Lee 2011; Oostra 2015; Park 2019; Schuster 2012) and eight studies were conducted in a hospital setting (Dickstein 2013; Lee 2015; Liu 2004; Liu 2009; Verma 2011; Yan 2013; Zhang 2013; Zhu 2017). One study was conducted in a nursing home (Braun 2012), and one was carried out in a community center (Dickstein 2014). In the five other studies, the setting was unclear or not reported.
Interventions
All included studies used MI alone or associated with action observation, physical activity, or functional gait training in the experimental groups. The following interventions and comparisons were used for the trials (see Table 2: General characteristics of included studies).
1. General characteristics of the included studies.
| Included Studies | Experimental Group | Control Group | Frequency and duration | Motor Imagery Protocols |
| Braun 2012 | During therapy, imagery attempts and overt movements are combined: movements are performed to generate sensory information, which are then embedded in the imagery attempts to make them as vivid as possible. The proportions of actual movements and imagery attempts are based on individual preferences. | To compensate for the unguided imagery training, patients in the control group were also encouraged to do 'homework', primarily practicing tasks that they had difficulty with. They were asked to report unguided therapy in logs | The duration of therapy was up to 30 minutes for 6 weeks | Four steps are distinguished: 1) explaining the concept; 2) developing imagery techniques; 3) applying mental practice; and 4) consolidating. The protocol had a conditional and an optional part. To be included in the per protocol analysis (only participants who have received the assigned intervention are taken into account), patients from the experimental branch should have received the conditional parts of the framework: at least 10 sessions of mental practice (step 2) and have practiced outside of supervised therapy time |
| Cho 2012 | MI training was conducted using visual and kinematic imagery separately. In visual imagery, participants imagine normal movement on their non‐paretic side and that their paralytic side moves like their non‐paretic side. Meanwhile, in kinematic imagery, participants imagine sensory information that they can get from their non‐paretic side when they move normally and then imagine that their paralytic side senses the same sensory information and moves like their non‐paretic side | The control group performed only gait training on the treadmill for 30 minutes | 45 minutes for experimental group and 30 minutes for control group, 3 times a week for 6 weeks | Imagery training was applied for 15 minutes, following gait training using a treadmill for 30 minutes. After conducting imagery training, the participants were allowed to relax for 5 minutes. To perform MI training, videos of normal gait movement were shown. During an explanation of normal gait movement by an experienced researcher, participants imagined normal gait movement based on visual materials. Then the researcher asked the participants to explain the movement they were imagining |
| Dickstein 2013 | Participants’ goals were used to select imagined walking tasks for the imagery practice. The imagery scripts were identical for the 3 weekly sessions and changed at the beginning of each week. Both kinesthetic and visual imagery of the walking activities were used during practice | Control treatment consisted of physical therapy for the affected upper extremity. It included 3 types of exercises, each conducted for 3 minutes: 1) a transport‐reach exercise (e.g. spoon to mouth); 2) a bimanual exercise (e.g. folding clothes); and 3) a unimanual manipulation with the involved upper extremity (e.g. placing items in a jar). The functional tasks, chosen according to the participant’s needs, did not involve ambulation | They consisted of 15‐minute sessions conducted 3 times a week for 4 weeks | All sessions were performed while participants sat on a couch with eyes closed. Each session started and ended with 3 minutes of relaxation exercises. Three minutes of imagery practice were conducted for each of 3 imagery environments: the participant’s home, a 'community interior' (public indoor, such as a mall), and a 'community exterior' (public outdoors, such as a street) environment (for a total of 9 minutes) |
| Dickstein 2014 | Both visual and kinesthetic imagery practice of the same motor tasks was applied to both treatments. The tasks were changed once a week. Instructions provided for each session uniformly presented. During visual imagery practice, the participants were encouraged to 'see' themselves performing the requested tasks. Imagery of zooming through a camera was frequently described to assist them in focusing on movement of the target body parts. During the kinesthetic imagery practice, the participants were asked to feel their body parts, focusing on movement of the joint(s) of the affected extremity during the practiced task. Repetitions were introduced, along with reinforcement for the sensations that were associated with the imagery performance | Imagery practice of movements of the affected upper extremity in different home environmental situations | The protocol was applied twice a week for 5 weeks in each community center during the morning hours | 1) Short conversation between the participants and the instructor, with the instructor providing feedback for the participants’ comments on home exercises and feelings 2) Explanation and demonstration of the assignment for the week 3) Relaxation phase (2 to 3 minutes) 4) MI practice (10 minutes) 5) Refocusing on the environment (2 minutes) |
| Gupta 2017 | Participants were asked to close their eyes and imagine they were performing the physically practiced task, similar to one shown in the video; participants were urged to imagine themselves from a first‐person perspective, to feel their trunk, legs, hands and feet to concentrate on their movements. Sequence of the task was verbally explained to the patient for better recalling of sensations in muscles during the movements | Patients in the control group physically performed each of the 5 tasks in a week, 10 times and followed the same routine for successive weeks | Total duration: 3 weeks 4 days per week |
Each patient in the experimental group was shown a video comprising of normal movements of the 5 tasks selected for the week, wherein each task was repeated 3 times. After seeing the video, patients performed each activity physically for 10 repetitions. During the entire exercise schedule, the participant's attention was focused on the position, and movement of their body, on proprioceptive inputs coming from the leg muscles (quadriceps and adductors) and on the tactile sensations of foot contact. Thereafter the patient was asked to narrate the sequence of tasks, rehearsed mentally, by the patient. The same steps were followed for the remaining four tasks. At the end participants were asked to relax |
| Kim 2013a | Consisted of viewing a task video for 20 minutes through a 32‐inch TV installed approximately 2 meters away while sitting in a comfortable armchair, followed by physical training with a therapist for 10 minutes, based on the video. While participants watched the video, they were instructed not to follow the motions of the video or move. Models of videos were normal adult men and women in their 50s, which is similar to the mean age of patients, so as to raise their concentration on understanding the motions | The exercise program included training of the trunk for learning supine to rolling movements, sit to stand, and normal gait pattern, as well as training of the lower extremity, weight shifting, and gait level surface and gait stairs | 30‐minute training session 5 times per week for a period of 4 weeks | The training program consisted of four stages, according to the content and level of difficulty. Participants watched a video of each stage for a period of one week. Stage 1 was composed of pelvic tilting, trunk flexion and extension, and trunk rotation in the sitting position for enhancement of trunk stability and mobility. Stage 2 was composed of sit‐to‐stand and stand‐to‐sit. Stage 3 was composed of a weight shift to the front and back, left and right, and weight shift involved lifting a foot on the block while standing for balance training in the standing position. Stage 4 was composed of a gait level surface and step over obstacle for improvement of gait ability |
| Kumar 2013a | Audio‐based lower extremity tasks for imagery practice. | Patients task‐oriented training for lower extremity. | Experimental group: total intervention time per session was about 60 minutes (45 minutes for physical practice and 15 minutes for mental practice). 5 days a week, for 3 weeks Control group: total intervention time per session was about 45 minutes. 5 days a week, for 3 weeks |
Author did not give details about the MI protocol |
| Kumar 2016 | Mental practice program started with a familiarization period and was followed by training of lower extremity tasks | Task‐specific training program focused on improving the performance and endurance of functional tasks involving the lower extremities such as sit‐to‐stand, reaching in sitting and standing, marching, walking, turning and transfers. Participants were encouraged to perform all the exercises in all of the program sessions to a maximum of 60 minutes with adequate rest periods for 10 to 15 minutes | 45 to 60 minutes per session, conducted 4 times a week for 3 weeks | In the familiarization phase the participants were explained about the basic action thoughts or motor representations of complex movements (e.g. drinking a cup of tea using Structural Dimension Analysis of Motor Memory). To enhance the imagery ability, verbal instructions and explanation of the lower extremity task components which were practiced in physical practice, by means of pre‐recorded audio tape with total duration 15 minutes delivered in participant's own language, before and during the physical practice training. The taped intervention consists of 2 minutes relaxation followed by 12 minutes of cognitive visual images related to the lower extremity task characteristics (e.g. imagine yourself in a warm, relaxing place and you are bending your knee and feel the tightness in your muscles). Participants were then taught to visualize themselves performing the required task and also experience kinesthetic sensations related to the task. This was followed by refocusing of attention to the immediate surroundings and genuine body position |
| Lee 2010 | The visual offerings consisted of 4 courses. Each motion was produced as a moving picture | Functional exercise was applied | (6 weeks, 3 times a week). 30 minutes for imagination training, 1 hour for functional training | Participants were asked to imagine and focus on movements. Participants were then asked to describe the imagined movements |
| Lee 2011 | The provision of visual and auditory information was composed of: watching a video clip of normal gait movement being performed by normal people, and listening to a researcher’s explanation of normal gait movement. MI training was divided between visual imagery and kinematic imagery. In the visual imagery of this study, participants imagined affected leg movement as if it were the unaffected leg after imagining the normal movement of the unaffected side from an external point of view. In the kinematic imagery of this study, participants imagined body moving on the affected side as if it were the unaffected side after imagining the sensory information felt during the movement of the unaffected side | Participants in the control group underwent 30 minutes of treadmill gait training, 3 times a week for 6 weeks | Experimental group: total intervention time per session was about 30 minutes for treadmill gait training and 30 minutes for MI, 3 times a week for 6 weeks Control group: total intervention time per session was about 30 minutes |
Participants in the experimental underwent 30 minutes of treadmill gait training. After that, MI training was composed of imagination of normal gait movement. It was carried out for 15 minutes after provision of visual and auditory information for 15 minutes. The provision of visual and auditory information was composed of: watching a video clip of normal gait movement being performed by normal people, and listening to a researcher’s explanation of normal gait movement |
| Lee 2015 | MI training was performed in the cognitive rehabilitation room at a proper temperature, with no noise, in order to enhance concentration on the MI training. To lower the stress and anxiety of the participants, and relax the body and mind, armchairs with a backrest were used so that participants could comfortably lean on them and close their eyes | The proprioception training program was conducted in 2 phases (phase I and II) Phase I (5 sets for 30 minutes each for 4 weeks): balance pad Phase II (5 sets for 30 minutes each for 4 weeks): balance board |
Total duration: 8 weeks; total time: 30 minutes; 5 days per week Experimental group: Time of MI training applied was 5 minutes and proprioception training program was 25 minutes Control group: the time of proprioception training program applied was 30 minutes |
MI training was divided into mobility imagery and visual imagery. The objective of mobility imagery is to imagine the inner sensory information during actual movements of body from the first‐person view, and the purpose of visual imagery is to imagine one’s own movements of the body from a third‐person view. The mobility imagery training was conducted to encourage the participants to feel the position senses of the ankle, knee, and hip joints, the peripheral muscles, and sole. Participants actively participated in the proprioception training program. In the MI training, therapists asked participants to imagine the contents of the proprioception program for 5 minutes, by directly reading aloud to them while reading. Participants were asked some questions in order to ensure they were adequately performing the imagery training. Proprioception program consisted of 4 sets performed in 25 minutes before the MI training |
| Liu 2004 | In the mental imagery program, participants were trained in the technique of mental imagery to practice specific tasks. Different but related mental imagery skills and the actual performance of tasks were practiced each week to help patients develop competence in using imagery as a learning tool | In the functional retraining program, the demonstration and then practice method was adopted. Participants were required to practice the same tasks following a sequence and training schedule similar to that of the mental imagery program | In both groups, participants received training for a total of 3 weeks with 5 x 1‐hour sessions each week | In the first week, the focus was on analyzing task sequences to facilitate the motor planning and problem identification process using computer‐generated pictures and movies. In the second week, participants identified their own problems for rectification through the use of mental imagery. Picture cards depicting the task sequences were used if participants needed help recalling the steps. In the third week, the focus was on practicing the rectified task performance using mental imagery and actual practice. To further standardize the protocol, a computer program was developed to guide participants to relearn the steps involved in performing each of the 15 tasks. Each step was presented as a picture, with verbal explanations of physical and mental demands of that particular step (to enhance task analysis). Visual aids were also used to help participants' reflection on problems that they encountered when they actually performed the tasks. They watched the video playback to confirm the problems that they identified (to enhance problem identification). Participants were guided to develop strategies to overcome problems |
| Liu 2009 | Participants in the MI group received 1 hour of MI per treatment and those in the functional rehabilitation (FR) group were given conventional occupational therapy | In the FR group, participants were given conventional occupational therapy using demonstration‐and‐practice methods to train them to perform the same 15 daily tasks.= | All treatment protocols were administered 5 times a week for 3 weeks (a total of 15 treatments). Participants in both groups received similar levels of therapist attention during their programs. All participants had 1 hour of physical therapy daily that involved mobilization, strengthening, and walking exercises | 5 tasks with a similar difficulty level were covered each week, progressing from the easiest to the most difficult. The MI intervention involved the participants’ self‐reflection on their abilities and deficits: mentally imagining, then actually performing, the task |
| Oostra 2015 | Participants received a standard rehabilitation program. It consisted of 2 hours physical therapy and 1 hour occupational therapy daily. In addition to standard training, the MI training group received 30‐minute daily mental practice treatment sessions. Each session was individually delivered in a quiet room in the hospital by 2 experienced therapists who were not involved in any other part of the study | The group received the same amount of muscle relaxation therapy over and above the standard rehabilitation training. Muscle relaxation was used to control for therapeutic attention and consisted of relaxation therapy of daily 30‐minute one‐to‐one sessions.The basic principle of this technique is to begin by instructing participants to physically tense particular muscle groups in a given order and then to relax and let go of the muscle contraction. During the same session the participants were asked to concentrate on using diaphragmatic breathing to aid relaxation | All participants received a standard rehabilitation program, consisting of 2 hours physical therapy and 1 hour occupational therapy daily, 5 days per week. In addition to standard training, the experimental group received 30‐minute daily mental practice treatment sessions | Every session started with 2 minutes of relaxation preceding the imaging session. During MI practice participants were seated in a (wheel) chair and instructed to keep their eyes closed. The practice was performed from an internal perspective with both visual (“viewing” themselves performing the task) and kinesthetic mode (“feeling” the experience of performing the task), with emphasis on the latter. During the first week MI training participants were familiarized with the MI technique, whereby the therapist gave visual, auditory, and sensory cueing to each participant, focusing on imaging of environmental situations well known to the participant. During the second week MI training was focused on the individual participant’ gait problems, such as forefoot landing, absence of knee loading response, knee hyperextension in stance, and stiff knee gait. Gait‐specific lower limb movements (hip flexion/extension, knee flexion/extension, ankle flexion/extension) were thus guided by individual gait analysis. In addition, information concerning the participant’s gait problem areas was provided to the MI therapist by the treating rehabilitation therapist. During third and fourth weeks, gait symmetry and velocity were rehearsed using different (MI) walking tasks, focusing on integrating the components practiced previously into the (mental) gait cycle. Participants were asked to pay specific attention to step length and walking speed. Auditory cues were used to guide walking speed. During the last 2 weeks of practice, gait exercises were embedded in activities of daily living. Participants were instructed to “view” and “feel” themselves walking in different situations and environments and on different terrains |
| Park 2019 | The participants in MIT EMG NMES group were asked to comfortably sit on the chair, place their upper limb on the desk, and flex and rotate their elbow about 90. MIT EMG‐NMES consists of 3 phases: relaxation phase, mental imagery phase, and stimulation phase. Each phase proceeded according to the menu presented on the MIT EMG NMES monitor. First, the relaxation phase maintains mental relaxation for 12 seconds. Second, in the mental imagery phase, participants were asked to imagine rigorous sports movements such as tennis stroke, throwing a baseball ball, or spiking a volley ball using their affected upper limb. Finally, in the stimulation phase, once the electric potential generated through mental imagery reaches the set EMG threshold, electrical stimulation is applied to the affected upper limb for 6 seconds, which causes substantial muscle contraction. However, if the electric potential generated by mental imagery did not reach the set threshold, it returned to the relaxation phase without electrical stimulation. The instructions are as follows: “when the relaxation phase lights up on the screen of the device, keep relaxed without imagining the movements. After that, when mental imagery phase lights up on the screen of the device, imagine the intensive movement of the affected upper limb.” | The participants in the EMG NMES group were attached to extensor pollicis brevis and longus using 3 surface electrodes in the same way as the participants in MIT EMG NMES. First, the voluntary wrist extension of the participant was induced, and the threshold was set based on the level of electrical potential according to muscle contraction and the threshold was reset every session. When the electric potential reaches the threshold and electrical stimulation is induced, biphasic pulses with a frequency of 35 Hz and a pulse width of 200 microseconds were applied to the affected upper limb for 6 seconds. Then intensity of stimulation was set to be between 15 and 30mA just as in the MIT EMG‐NMES. If the electrical potential generated by muscle contraction did not reach the threshold, electrical stimulation was automatically applied to the affected upper limb after 20 seconds. | Both groups performed intervention for 30 minutes a day, 5 days a week, for 6 weeks | Consisted of 3 phases: (1) Relaxation phase: participants were asked to maintain mental relaxation for 12 seconds (2) Mental imagery phase: participants were asked to imagine rigorous sports movements such as tennis stroke, throwing a baseball ball, or spiking a volley ball using their affected upper limb (3) Stimulation phase: the electric potential generated through mental imagery reaches the set EMG threshold, electrical stimulation is applied to the affected upper limb for 6 seconds, which causes substantial muscle contraction |
| Schuster 2012 | In total, treatment time was about 45 to 50 minutes. Training consisted of the following aspects: Physical/emotion: imagination of the motor task where it should be performed, without any prior relaxation exercises, in an active and alert state Timing: duration of the motor task should not exceed the real performance duration. Environment: using (personalized) multisensory environmental cues Task/learning/perspective: participants, who preferred the external MI perspective, were asked to switch to the internal perspective after learning and familiarization with the motor task | Besides receiving physiotherapy during a 30‐minute session, participants in the control group listened to a 17‐minute tape (average). The tape started with a short relaxation period (about 3.5 minutes). Afterwards, participants listened to information about stroke: causes, consequences for different body functions and recovery phase, therapy options, prevention of potential complications, self‐help groups and their offers | Total duration per session was about 45 to 50 minutes. A total of 6 therapy sessions during 2 weeks | Complete motor task was divided into its 13 stages. Each stage was imagined 5 times before it was physically practiced once. At the end of each physiotherapy session, participants imagined the complete task 4 times while lying supine on the treatment bench and 4 times while standing against a wall. To control for every imagination trial each of the 8 MI trials were timed with a stopwatch by the participant and by the therapist |
| Suvadeep 2017 | Received 30 minutes of mental imagery, in addition to 30 minutes of conventional therapy which included neurodevelopmental facilitation technique, stretching, and gait training | Received 30 minutes of mirror therapy, in addition to 30 minutes of conventional therapy which included neurodevelopmental facilitation technique, stretching and gait training | Total of 1 hour per day for 5 days per week for 4 weeks | Study author did not give details about the MI protocol |
| Verma 2011 | MI comprised imagining walking abilities and tasks related to a real‐life situation. Participants were familiarized with MI during a pre‐intervention session and educated about the basic imagery principles | Participants in the control group participated in the conventional poststroke lower extremity rehabilitation program based on the Bobath’s neurodevelopmental technique. The control group program was matched for duration, number, and frequency of the sessions with the experimental group program | Experimental group received 15 minutes of MI followed by 25 minutes of TOCCT for a total of 40 minutes, 7 days per week for 2 weeks (14 sessions). Control group program was matched for duration, number, and frequency of the sessions with the experimental group program | The MI program of 15 to 25 minutes was given on an individual basis. Participants were also asked to keep a diary of their MI practice to measure the rehearsal frequency after each treatment session |
| Yan 2013 | Before training, the therapist explained the purpose, method and precautions of the training to the participant, and guided the participant to do the dorsiflexion of the contralateral limbs first, so that they can master the joint activities of the affected side | Conventional rehabilitation therapy + tactiles foot dorsiflexion training, continuous for 6 weeks | Once a day, approximately 20 to 30 minutes, rest on Sunday, for 6 consecutive weeks | Training method: the participant was placed in a quiet room and closed his eyes on the bed, relaxed for 2 to 3 minutes before exercise imaging training, imagined the content as the details of the action of passive foot dorsiflexion in rehabilitation training, and repeatedly felt the amount of ankle joint training. The key action of dorsiflexion of the toes was to relax after approximately 5 to 7 minutes. Rested for 1 to 2 minutes, and then 5 to 7 minutes to imagine exercise. Finally, used 2 minutes to guide the participant back to the treatment room from the imaginary situation, and focused on the body and the surrounding environment to make them feel the body. Changed, listened to the sound of the surrounding environment (such as people's voice, footsteps or noise inside and outside the room), and finally the trainer counted down for 10 seconds, when the time was finished, let the participant open his eyes, rest for a while and then the therapist performed the dorsiflexion training |
| Zhang 2013 | Experimental group received routine training combined with motor imaging therapy in the first stage, and only routine training in the third stage | Control group only conducted routine training in the first stage | The total trial duration was 8 weeks, divided into 3 phases, which were 3 weeks, 2 weeks, and 3 weeks | The therapist does a demonstration, explaining the movements that need to be imagined, explaining the parts of the limbs that need to be moved, and explaining the feeling of movement; participant imagines the movements alone; participant performs the imagination exercises according to the instructions recorded |
| Zhu 2017 | The experimental group was supplemented with electroacupuncture and motor imaging treatment | Routine care, drug treatment, routine rehabilitation treatment and electroacupuncture treatment | Treatment was given once a day, 5 treatments per week. In total, 4 weeks of treatment was performed | MI treatment involves: (1) pre‐training preparation: before the training is performed the participant's level of motor functions is assessed. Cognitive ability and exercise of imagining the action is also evaluated (2) before start of training: adjusting the position of the participant is done. The participant is allowed to see the actual action in a video scene with demonstrations. Therapist stands beside the hemiplegic participant and performs tactile and proprioceptive stimulation. Therapist helps patients complete limb movements and establish a "flow" of the program (3) start of training action: according to the video, the participant follows the orientation to relax the whole body (2 minutes) → visualizes the actual action of the scene of a video of 5 to 10 seconds → then with eyes closed, according to the orientation, it is enough to imagine the action (the therapist remains on the hemiplegic side of the participant and performs tactile and proprioceptive stimulation for 5 to 10 seconds → the participant relaxes for 10 seconds → each operation was repeated 5 times → 20 minutes of the imagination of the movement (4) end of the training course: to focus attention on participant's own body, open their eyes, let the body relax |
MI: motor imagery; MIT‐EMG NMES: motor imagery training and electromyogram‐triggered neuromuscular electrical stimulation; TOCCT: task‐oriented circuit class training
When applying MI, some studies used videos that imitated the execution of specific normal movements and then asked the participants to imagine performing the movement (Cho 2012; Dickstein 2014; Gupta 2017; Kim 2013a; Lee 2011; Liu 2004; Zhu 2017). Nine studies applied MI from previous protocols, and instructions on how the participants should imagine the movements were given at the moment of the intervention (Braun 2012; Dickstein 2013; Lee 2015; Liu 2009; Oostra 2015; Park 2019; Verma 2011; Yan 2013; Zhang 2013). Kumar 2016 used a voice recording to guide participants in imagining the movements.
For MI practice, most of the studies asked the participants to imagine isolated movements related to gait. Cho 2012, Dickstein 2013, Lee 2011, and Oostra 2015 used the full gait for MI practice. Park 2019 asked the participants to imagine rigorous sports movements. Braun 2012, Liu 2009, Zhang 2013, and Zhu 2017 did not specify what kind of imagination was suggested. Most of the studies used both kinesthetic and visual motor imagery. Kim 2013a and Oostra 2015 used only visual imagery, while Liu 2009, Park 2019, Verma 2011, Yan 2013, and Zhang 2013 used only kinesthetic imagery. Kumar 2013a and Suvadeep 2017 did not specify which kind of imagery (if kinesthetic or visual) was used.
Most of the included studies used MI and physical practice in the experimental groups. As established in our review protocol, we understood physical practice as physical activity, functional gait training, or other active physical therapies (including conventional physical therapy). Most of the studies that used physical practice and MI in the experimental groups performed physical practice first, followed by MI; while only Verma 2011 used MI followed by physical practice. Gupta 2017, Kim 2013a, Kumar 2013a, Lee 2011, Liu 2009, and Suvadeep 2017 did not make clear whether MI was applied before or after physical practice. Three studies used only MI in the experimental groups (Dickstein 2013; Dickstein 2014; Liu 2004).
Most of the investigations initiated MI practice by giving instructions. The practice was performed in a calm environment in some of the included studies to reduce the participants' stress. Some studies performed a few minutes of relaxation before starting MI (Cho 2012; Dickstein 2013; Dickstein 2014; Kumar 2016; Oostra 2015; Park 2019; Yan 2013; Zhu 2017). Overall, the studies used protocols and instruments to evaluate the ability to generate motor images, such as the Movement Imagery Questionnaire. No study monitored vital signs or other signals that sought to identify whether the movement was being imagined during MI execution.
Eight studies cited that MI was applied by therapists (in general) (Braun 2012; Kim 2013a; Lee 2015; Liu 2009; Oostra 2015; Verma 2011; Yan 2013; Zhang 2013). Five studies reported that physical therapists applied MI (Dickstein 2013; Dickstein 2014; Gupta 2017; Kumar 2016; Schuster 2012), and Liu 2004 and Park 2019 cited that occupational therapists applied MI. Three studies mentioned that MI was applied by researchers, without further specifications (Cho 2012; Lee 2010; Lee 2011). In all studies, MI was applied personally. No study monitored the participants' adherence to MI treatment.
The time of MI application, in each session, ranged from 30 to 60 minutes. The total treatment dose in the experimental groups ranged from 100 to 1200 minutes over the course of two to eight weeks of therapy. Lee 2011, Lee 2015, Oostra 2015, and Yan 2013 reported a total of more than 1000 minutes of therapy in the experimental groups, while Cho 2012, Dickstein 2013, Dickstein 2014, Kim 2013a, Kumar 2016, Park 2019, Verma 2011, and Zhang 2013 reported less than 1000 minutes of total therapy; the other studies did not define the therapy time per session in the respective experimental groups. In all studies, the treatment frequency ranged from two to six times per week, and only one session per day was performed using MI.
No studies used placebo or no therapy in the control group; all included studies used other therapies to compare the effects of MI. Physical practice was the most often applied therapy in the comparison groups (controls). Only Dickstein 2014 used MI in the comparison group, but for the upper limbs. Suvadeep 2017 used mirror therapy, Oostra 2015 used muscle relaxation, Park 2019 used neuromuscular electrical stimulation, and Zhang 2013 used drug treatment in addition to physical practice in the comparison group. Most studies were composed of two groups, but Kim 2013a, Schuster 2012, and Zhu 2017 had three treatment groups. In the study conducted by Kim 2013a, the control group performed physical practice alone, and two experimental groups performed physical practice associated with action observation or with MI. Schuster 2012 had two experimental groups that performed either MI embedded into physical practice or MI added to physical practice, while the control group performed only physical practice. Zhu 2017 had two experimental groups that received electroacupuncture (either alone or associated with MI) in addition to physical practice, and another group that received only physical practice (control group).
The total treatment dose for the control groups ranged from 12 to 240 minutes, and the therapy lasted two to eight weeks. In all studies, the treatment frequency ranged from two to seven times per week, and only one session per day was performed in the control groups.
Outcomes
As outcomes, fifteen studies measured walking speed, eleven studies assessed dependence on personal assistance, one study measured walking endurance, six studies assessed motor function, and seven studies assessed functional mobility. Most of the studies did not report adverse events.
Our critical outcomes were the ability to walk, measured using the participants' walking speed, and the dependence on personal assistance. For walking speed, the 10‐meter Walk Test (Braun 2012; Cho 2012; Dickstein 2013; Dickstein 2014; Gupta 2017; Kumar 2016; Oostra 2015; Suvadeep 2017), and custom systems (Dickstein 2014; Kim 2013a; Kumar 2013a; Lee 2010; Lee 2011; Lee 2015; Schuster 2012; Verma 2011), were used. The following measures were used to evaluate the dependence on personal assistance: Barthel Index (Braun 2012; Liu 2009; Park 2019; Schuster 2012; Verma 2011; Yan 2013; Zhu 2017); MAS (Suvadeep 2017); and FAC (Kim 2013a; Verma 2011; Zhang 2013).
For our important outcomes, the only measure used to assess walking endurance was the Six‐minute Walk Test (Verma 2011). The Fugl‐Meyer Assessment Scale (items related to lower limbs) was used to evaluate motor function (Cho 2012; Liu 2004; Liu 2009; Oostra 2015; Suvadeep 2017; Yan 2013). For functional mobility, the following measures were used: Timed Up and Go test (Cho 2012; Kim 2013a; Kumar 2013a; Lee 2010; Lee 2015), and the Rivermead Mobility Index (Braun 2012; Verma 2011). Although different outcome measures were used in the included studies, the outcome data were pooled in the meta‐analysis when necessary.
Only Braun 2012, Dickstein 2013, Liu 2004, Liu 2009, Oostra 2015, Schuster 2012, and Verma 2011 reported adverse events, by reporting this directly in the published text or after we requested the information from the study authors. In all of these studies, the study authors reported no adverse events related to the interventions (both control and experimental groups). Three studies assessed falls as an outcome using either the Falls‐Efficacy Scale ‐ Swedish version (Dickstein 2013), or the Activities‐specific Balance Confidence scale (Dickstein 2014; Schuster 2012); however, none of these studies reported whether falls occurred during the trial period. We contacted all the study authors but they were unable to provide this information.
All included studies assessed outcomes immediately at the end of the study, and only three conducted follow‐up. Braun 2012, Dickstein 2014, and Verma 2011 evaluated the medium‐term effects after a follow‐up period of two, four, and 18 weeks post‐treatment, respectively.
As planned before, we intended to conduct separate data analyses for data related to the period immediately after the intervention and follow‐up. For the outcomes reported in advance in our protocol, we could not perform the follow‐up analyses as there were not enough studies to group the data in the meta‐analysis.
Excluded studies
We excluded 32 studies for various reasons (see Characteristics of excluded studies). In addition, one study is awaiting classification (see Characteristics of studies awaiting classification), and seven are ongoing (see Characteristics of ongoing studies).
Risk of bias in included studies
Two review authors independently assessed the methodological quality of the included trials using the ’Risk of bias’ tool. Figure 2 and Figure 3 show the risk of bias summary and the risk of bias graph of the included studies, respectively, showing the review authors' judgments about each risk of bias item.
2.

Figure 2: Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Of the 21 included studies, nine performed adequate randomization and respected allocation concealment, so we deemed these to be at low risk of bias (Braun 2012; Cho 2012; Gupta 2017; Kim 2013a; Kumar 2016; Oostra 2015; Park 2019; Schuster 2012; Verma 2011). In the studies conducted by Braun 2012, Park 2019, and Schuster 2012, an independent researcher that was not involved in the study was responsible for the allocation of the participants and generated the randomization list from a personalized computer system. Conversely, in the studies of Cho 2012, Gupta 2017, Kim 2013a, Kumar 2016, Oostra 2015, and Verma 2011, randomization was generated from permuted blocks, and the randomization sequence was placed in opaque and sealed envelopes. Zhang 2013 performed randomization according to the hospital admission number, while the other studies did not report how randomization was performed; we classified these as being at high risk or uncertain risk of bias.
Blinding
Blinding refers to the sample participants and the outcome examiners. In our review, due to the nature of the interventions, it was impossible to blind the therapists. Only Cho 2012 reported that both the participants and examiners were blinded, so we categorized this as low risk of bias. Gupta 2017, Kim 2013a, Kumar 2013a, Lee 2010, Lee 2011, Lee 2015, Suvadeep 2017, Yan 2013, Zhang 2013, and Zhu 2017 did not blind the participants and examiners, and we judged them to be at high risk of bias. Braun 2012, Dickstein 2013, Dickstein 2014, Kumar 2016, Liu 2004, Liu 2009, Oostra 2015, Park 2019, Schuster 2012, and Verma 2011 blinded only the participants or the evaluators, or did not clearly explain whether the two domains were blinded; we considered these trials to be at unclear risk of bias.
Incomplete outcome data
We classified Gupta 2017, Kumar 2013a, Lee 2010, Lee 2015, Suvadeep 2017, and Yan 2013 as unclear risk of bias because they did not clearly explain or did not provide information regarding study losses. We considered Cho 2012, Dickstein 2013, Dickstein 2014, Kim 2013a, Kumar 2016, Lee 2011, Liu 2004, Liu 2009, Oostra 2015, Park 2019, Schuster 2012, Verma 2011, Zhang 2013, and Zhu 2017 to be at low risk of bias because there were no sample losses after interventions, or sample losses were adequately justified and balanced between groups.
Selective reporting
We categorized one study as being at high risk of bias because a previous protocol was not presented, and the outcomes were not reported as listed in the study methods (Kumar 2013a). Seventeen studies presented the study registry and/or reported the outcomes, as stated in the methodology (Braun 2012; Cho 2012; Dickstein 2013; Dickstein 2014; Gupta 2017; Kim 2013a; Kumar 2016; Lee 2010; Lee 2011; Lee 2015; Liu 2004; Liu 2009; Oostra 2015; Park 2019; Schuster 2012; Verma 2011; Zhang 2013). Therefore, we considered them to be at low risk of bias.
Other potential sources of bias
We identified no information associated with other potential sources of bias.
Effects of interventions
See: Table 1
See: Table 1.
We were able to use data from 11 studies in meta‐analysis (Braun 2012; Cho 2012; Dickstein 2013; Gupta 2017; Kim 2013a; Kumar 2016; Lee 2011; Lee 2015; Oostra 2015; Verma 2011; Yan 2013). The other studies could not be pooled because some data were not presented. We contacted the study authors but did not obtain these data. At least two studies evaluated our pre‐planned critical outcome (ability to walk) and some of the important outcomes (motor function, functional mobility, and adverse events); walking endurance was evaluated by only one study.
Although we planned to compare the effects of MI (alone or associated with either action observation or physical practice) versus other therapies (including conventional physical therapy), placebo, and no therapies, we found no studies that performed comparisons with placebo or no therapies. Therefore, we performed all analyses comparing MI therapy versus other therapies (as control conditions).
MI therapy versus other therapies (control): effect on ability to walk
1.1 Ability to walk: walking speed
Six studies (191 participants) measured walking speed at the end of the intervention using different measures. The first meta‐analysis included all studies that presented data concerning walking speed, regardless of which unit measure was used. We found very low‐certainty evidence that MI had a greater effect than other therapies on walking speed at the end of the intervention (pooled SMD = 0.44; 95% CI 0.06 to 0.81; P = 0.02; I² = 38%; Analysis 1.1).
1.1. Analysis.

Comparison 1: Motor Imagery therapy versus other therapies (control): effect on ability to walk, Outcome 1: Walking speed
Subgroup analysis: type of stroke
We planned to do a subgroup analysis to assess the influence of the type of stroke on walking speed at the end of the intervention. Two studies did not report the type of participant stroke (Gupta 2017; Lee 2011), while four studies reported participants with either ischemic or hemorrhagic stroke (Dickstein 2013; Kumar 2016; Oostra 2015; Verma 2011). However, it was impossible to perform the meta‐analysis for this outcome since these studies did not report disaggregated ischemic and hemorrhagic stroke data.
1.2 Subgroup analysis: post‐stroke time
We analyzed subgroups considering the post‐stroke time (six studies, 191 participants) and pooled the studies in which participants were in the 1) subacute stroke stage, 2) chronic stroke stage, and 3) subacute and chronic stroke stages. We considered the assessment of walking speed performed at the end of the intervention. No significant intergroup difference was found (P = 0.59; I² = 0%; Analysis 1.2).
1.2. Analysis.

Comparison 1: Motor Imagery therapy versus other therapies (control): effect on ability to walk, Outcome 2: Subgroup analysis: post‐stroke time
1.3 Subgroup analysis: treatment dose
We assessed the influence of the treatment dose on walking speed at the end of the intervention (five studies; 161 participants). We analyzed subgroups according to the therapy dose since both control and experimental groups had different therapy times. We compared studies that provided more and less than 1000 minutes of therapy in the experimental groups, and observed no significant intergroup differences (P = 0.31; I² = 1.5%; Analysis 1.3).
1.3. Analysis.

Comparison 1: Motor Imagery therapy versus other therapies (control): effect on ability to walk, Outcome 3: Subgroup analysis: treatment dose
1.4 Subgroup analysis: type of treatment
In the subgroup analysis considering the types of treatment (6 studies, 191 participants), the studies in which MI was used alone were compared with those in which MI was associated with action observation or physical practice. The walking speed at the end of the intervention was considered, and the intergroup analysis revealed no significant difference (P = 0.54; I² = 0%; Analysis 1.4).
1.4. Analysis.

Comparison 1: Motor Imagery therapy versus other therapies (control): effect on ability to walk, Outcome 4: Subgroup analysis: type of treatment
1.5 Subgroup analysis: walking dependence
We analyzed subgroups according to walking dependence to assess the influence on walking speed at the end of the intervention (four studies, 117 participants). We compared studies in which participants were considered 1) dependent and independent on personal assistance, and 2) independent on personal assistance at the beginning of the study. We found no significant intergroup difference (P = 0.44; I² = 0%; Analysis 1.5).
1.5. Analysis.

Comparison 1: Motor Imagery therapy versus other therapies (control): effect on ability to walk, Outcome 5: Subgroup analysis: walking dependence
1.6 Subgroup analysis: forms of application of MI
We analyzed subgroups to assess the influence of the form of application of MI on walking speed at the end of the intervention (six studies; 191 participants). We formed three subgroups: 1) studies that used only visual imagery, 2) studies that used only kinesthetic imagery, and 3) studies that used both visual and kinesthetic imagery. There was no significant intergroup difference (P = 0.23; I² = 31.2% Analysis 1.6).
1.6. Analysis.

Comparison 1: Motor Imagery therapy versus other therapies (control): effect on ability to walk, Outcome 6: Subgroup analysis: forms of application of MI
Ability to walk: walking speed: sensitivity analysis: studies without high risk of bias
We planned to do a sensitivity analysis for the studies that exhibited a high risk of bias in at least one of the following domains: allocation concealment, blinding of outcome assessment, and random sequence generation. We could not perform the analysis because only one study was classified as being at low risk of bias (Kumar 2016).
Ability to walk: walking speed: follow‐up
We planned to do an analysis to verify the follow‐up data concerning walking speed, but it was impossible to do this because only one study performed follow‐up assessment (Verma 2011).
1.7 Ability to walk: dependence on personal assistance
Seven studies used the Barthel Index; three studies used FAC; one study used MAS. Despite the number of studies, we did not do the meta‐analysis because only one study specified the dependence or independence of the participants on personal assistance after the interventions (Verma 2011). Our team contacted the study authors by email to request missing data. However, none of the study authors replied. Verma 2011 indicated that seven participants (46.6%) from the experimental (MI) group and two (13.3%) from the control group exceeded the FAC score (> 2 points); they were thus categorized as independent at the end of the intervention. In Verma 2011, when compared to the control group, statistically significant differences were observed favoring the MI group in both the post‐intervention (P = 0.001) and follow‐up (P = 0.001) assessments.
Motor imagery therapy versus other therapies (control): effect on walking endurance
Only one study measured walking endurance (Verma 2011), and it was thus impossible to conduct a meta‐analysis. This study aimed to investigate the effects of task‐oriented circuit class training with MI on gait abilities of patients with subacute stroke. According to the results, the comparison between the control and experimental groups at the post‐intervention moment was statistically significant (P = 0.005) in favor of the experimental group.
Motor imagery therapy versus other therapies (control): effect on motor function
2.1 Motor function
Six studies compared the immediate effects of MI on motor function at the end of the intervention. All of these studies evaluated motor function using the lower extremity item of the Fugl‐Meyer Scale. However, even after requesting information from the authors, data were not available in three studies. Therefore, for this outcome, only three studies were pooled into meta‐analysis (130 participants). We found very low‐certainty evidence that MI had no greater effect than other therapies on motor function at the end of intervention (pooled MD = 2.24; 95% CI ‐1.20 to 5.69; P = 0.20; I² = 87%; Analysis 2.1).
2.1. Analysis.

Comparison 2: Motor imagery versus other therapies (control): effect on motor function, Outcome 1: Motor function
Subgroup analysis: type of stroke
We planned to do a subgroup analysis to verify the influence of the type of stroke on motor function at the end of the intervention. Only two studies reported the type of stroke of the participants: either ischemic or hemorrhagic stroke (Cho 2012; Oostra 2015). However, it was not possible for us to perform the meta‐analysis because the studies did not report disaggregated ischemic and hemorrhagic stroke data.
2.2 Subgroup analysis: post‐stroke time
Regarding motor function, we analyzed subgroups considering the post‐stroke time (two studies, 70 participants) by pooling studies in which participants were in the subacute or chronic stage of stroke. In the subgroup composed of individuals in the chronic stage, statistical significance was observed for the experimental (MI) group (subgroup 2: MD = 5.50; 95% CI 3.79 to 7.21; P < 0.00001; I² = not applicable; Analysis 2.2). A significant intergroup difference was also found (P = 0.0009; I² = 90.9%; Analysis 2.2).
2.2. Analysis.

Comparison 2: Motor imagery versus other therapies (control): effect on motor function, Outcome 2: Subgroup analysis: post‐stroke time
2.3 Subgroup analysis: treatment dose
We analyzed subgroups to assess the influence of the treatment dose on motor function (three studies, 130 participants). Participants were divided into two subgroups, according to the total therapy time in the experimental groups: one combining studies with more than 1000 minutes, and another with less than 1000 minutes. In the subgroup with less than 1000 minutes of total therapy, statistical significance favoring the experimental (MI) group was observed (subgroup 2: MD = 5.50; 95% CI 3.79 to 7.21; P < 0.00001; I² = not applicable; Analysis 2.3). Intergroup differences were also statistically significant (P = 0.01; I² = 84%; Analysis 2.3).
2.3. Analysis.

Comparison 2: Motor imagery versus other therapies (control): effect on motor function, Outcome 3: Subgroup analysis ‐ treatment dose
Subgroup analyses: type of treatment and walking dependence
We planned the other two subgroup analyses, considering the type of treatment (MI alone or associated with action observation or physical practice) and walking dependence (dependent or independent to walk at the beginning of the study), for the motor function outcome. However, we could not perform these analyses because there were not enough studies to do so. We contacted the study authors to request unreported data about these outcomes, but none of them replied to our request.
2.4 Subgroup analysis: forms of application of MI
In the subgroup analysis considering the influence of the forms of application of MI on motor function (three studies; 130 participants), we formed three subgroups: one combining studies that used only visual imagery, another combining studies that used only kinesthetic imagery, and the third combining studies that used both visual and kinesthetic imagery. In the subgroup that used only kinesthetic imagery as well as in the subgroup that used both forms of MI, statistical significance favoring the experimental (MI) group was found (subgroup 2: MD = 1.90; 95% CI 0.37 to 3.43; P= 0.01; I² = not applicable; Analysis 2.4); (subgroup 3: MD = 5.50; 95% CI 3.79 to 7.21; P < 0.00001; I² = not applicable; Analysis 2.4). Intergroup differences were also statistically significant (P = 0.0004; I² = 87.4% Analysis 2.4).
2.4. Analysis.

Comparison 2: Motor imagery versus other therapies (control): effect on motor function, Outcome 4: Subgroup analysis: forms of application of MI
Motor function: sensitivity analysis: studies without high risk of bias
We planned to do a sensitivity analysis for the motor function outcome considering only studies with a low risk of bias in the following domains: allocation concealment, blinding of outcome assessment, and random sequence generation. However, only one study was considered to be at low risk in the above mentioned domains (Cho 2012). We were thus unable to perform the sensitivity analysis.
Motor imagery therapy versus other therapies (control): effect on functional mobility
3.1 Functional mobility
Four studies (116 participants) measured functional mobility at the end of the intervention and used two different measures (Rivermead Mobility Index and Timed Up and Go test). In the studies that used the Timed Up and Go test, values were obtained in 'seconds,' indicating better functional mobility with fewer elapsed seconds.This explains the negative values in the analyses. We found very low‐certainty evidence that MI had no greater effect than other therapies on functional mobility (pooled SMD = 0.55; 95% CI ‐0.45 to 1.56; P = 0.09; I² = 64.2%; Analysis 3.1).
3.1. Analysis.

Comparison 3: Motor imagery versus other therapies (control): effect on functional mobility, Outcome 1: Functional mobility
In the study that evaluated functional mobility using the Rivermead Mobility Index (34 participants), we also found very low‐certainty evidence that the use of MI did not improve functional mobility compared to other therapies at the end of the intervention (pooled SMD = ‐0.34; 95% CI ‐1.02 to 0.34; P = 0.32; I² = not applicable; Analysis 3.1).
In three studies that evaluated this outcome using the Timed Up and Go test (82 participants), we also found very low‐certainty evidence that the use of MI did not improve functional mobility compared to other therapies at the end of the intervention (pooled SMD = 0.88; 95% CI ‐0.38 to 2.14; P = 0.17; I² = 85%; Analysis 3.1).
Subgroup analysis: type of stroke
We planned to do a subgroup analysis considering the influence of the type of stroke on functional mobility at the end of the intervention; however, not enough information was presented in the included studies. We contacted the study authors by email, but received no response.
Subgroup analysis: post‐stroke time
We planned to do a subgroup analysis to assess the influence of the post‐stroke time on functional mobility at the end of the intervention, but only data from the chronic stage of stroke was present in the included studies.
3.2 Subgroup analysis: treatment dose
We assessed the influence of the treatment dose on functional mobility at the end of the intervention (2 studies; 64 participants). Two subgroups were formed according to the total therapy time in the experimental groups: one group receiving more than 1000 minutes of total therapy and another group receiving less than 1000 minutes of total therapy. Statistical significance was observed favoring the experimental (MI) group in the subgroup with less than 1000 minutes of total therapy (subgroup 2: SMD = 2.30; 95% CI 1.31 to 3.28; P < 0.00001; I² = not applicable). Intergroup differences were also statistically significant (P = 0.0005; I² = 91.8%; Analysis 3.2).
3.2. Analysis.

Comparison 3: Motor imagery versus other therapies (control): effect on functional mobility, Outcome 2: Subgroup analysis: treatment dose
Subgroup analyses: type of treatment and walking dependence
The other two subgroup analyses were proposed for functional mobility considering 1) the type of treatment (MI alone or associated with action observation or physical practice), and 2) walking dependence (dependent or independent for walking at the beginning of the study). However, not enough information was reported to conduct the meta‐analysis. We contacted the study authors, but received no reply.
3.3 Functional mobility: sensitivity analysis: studies without high risk of bias
We conducted a sensitivity analysis excluding the studies that presented a high risk of bias in at least one of the following domains: allocation concealment, blinding of outcome assessment, and random sequence generation (two studies; 62 participants). The effect of therapy remained non‐significant (pooled SMD = 0.95; 95% CI ‐1.63 to 3.54; P = 0.47; I² = 95%; Analysis 3.3).
3.3. Analysis.

Comparison 3: Motor imagery versus other therapies (control): effect on functional mobility, Outcome 3: Functional mobility ‐ sensitivity analysis: studies without high risk of bias
Functional mobility: follow‐up
We planned to do an analysis to assess the follow‐up data. It was not possible because only one study performed follow‐up assessment regarding functional mobility (Braun 2012).
3.4 Functional mobility: sensitivity analysis: without peripheral studies
We conducted a sensitivity analysis (three studies; 88 participants) excluding one study that had a sample composed of outpatients. The effect of therapy remained non‐significant (SMD ‐0.00; 95% CI ‐0.42 to 0.42; P = 1.00; I² = 0%; Analysis 3.4).
3.4. Analysis.

Comparison 3: Motor imagery versus other therapies (control): effect on functional mobility, Outcome 4: Functional mobility ‐ sensitivity analysis: without peripheral studies
3.5 Subgroup analysis: forms of application of MI
In the analysis considering the influence of the form of application of MI on functional mobility (three studies; 82 participants), we divided the studies into two subgroups: one group receiving only visual imagery, and the second group receiving both the visual and kinesthetic imageries. No significant intergroup difference was found (P = 0.41; I² = 0%; Analysis 3.5).
3.5. Analysis.

Comparison 3: Motor imagery versus other therapies (control): effect on functional mobility, Outcome 5: Subgroup analysis: forms of application of MI
MI therapy versus other therapies (control): effect on adverse events (pain, fall and all cause deaths)
4.1 Adverse events
Only two studies reported no adverse events (Dickstein 2014; Verma 2011). We contacted the study authors of the other studies by email. Five of the authors replied, informing us that there were no adverse events. Therefore, it was impossible to conduct a meta‐analysis for this outcome.
Discussion
Summary of main results
This review aimed to assess the effects of the treatment with MI on the gait of individuals with stroke. The total number of included studies was 21 and involved 762 participants. The studies compared MI with other therapies, and physical practice was the most applied therapy in the control group. No studies compared MI with placebo or no treatment. Overall, the certainty of the evidence for the outcomes was very low. The main results are presented in the Table 1.
We found very low‐certainty evidence that the use of MI was superior to other therapeutic interventions for improving gait (walking speed) at the end of the treatment. Treatment with MI also improved walking speed regardless of the stage of stroke (subacute or chronic), the type of treatment (either MI alone or combined with action observation or physical practice), and the dependence on personal assistance (dependent or independent at the beginning of the study). However, we observed no difference in the effect of MI on walking speed concerning the treatment dose (less than or more than 1000 minutes of therapy, including MI) and the forms of application of MI (visual imagery, kinesthetic imagery, or both visual and kinesthetic imagery). We have very little confidence in our estimate regarding the effect of MI alone. The only study investigating the effect of MI alone presented a high risk of bias in several domains, concerning the blinding of both the participants and personnel, random sequence generation, and allocation concealment. Therefore, the exact effect of the use of MI alone may be different from our estimate. We could not properly assess the evidence of the effects of MI on dependence for walking because only one study specified whether participants were dependent or independent after the interventions. In this trial, statistically significant differences were observed favoring the MI group at the end of the intervention as well as follow‐up assessments (P = 0.001). We could not assess the effects of MI on walking speed at follow‐up because this outcome was assessed in only one study.
We could not properly assess the effects of MI on walking endurance since only one study reported this outcome. In this trial, a significant difference was observed when comparing the control and experimental groups at the end of the intervention (P = 0.05). We found very low‐certainty evidence that MI was no more beneficial than other therapies on motor function, when assessed using the Fugl‐Meyer Assessment at the end of treatment. We also observed no difference concerning its effects on motor function regardless of the stage of stroke or treatment dose. However, with regard to the forms of MI application, we found a significant difference. We observed high methodological heterogeneity among the studies that reported this outcome, which also presented wide confidence intervals. We could not assess the effects of MI on motor function at follow‐up assessment because the studies only reported data from the post‐intervention assessment.
We found very low‐certainty evidence that there is no beneficial effect of MI, compared to other therapies, on functional mobility, measured using the Timed Up and Go Test or the Rivermead Mobility Index. We also observed no difference concerning its effects on functional mobility at the end of the treatment regardless of the treatment dose and forms of application. We also observed high methodological heterogeneity among the studies that reported this outcome. Despite this, when we removed the studies with peripheral results, the effect remained absent; i.e. both MI and the other therapies proved to have similar effects on functional mobility at the end of the treatment. We could not assess the effects of MI on functional mobility at follow‐up because this assessment was conducted in only one study.
Regarding adverse events, we considered any undesirable episode reported in the studies, including pain, falls, and all‐cause deaths. Most studies did not report whether there were any adverse events, while those that did reported no adverse events related to the interventions. Therefore, it was impossible to group data in the meta‐analysis and assess the certainty of the evidence.
Overall completeness and applicability of evidence
The outcomes analyzed in a systematic review need to be relevant to patients, health professionals, and the general population. Based on this justification, we chose to investigate gait (i.e. ability to walk) and the following important outcomes: motor function, functional mobility, walking endurance, and adverse events from a perspective that encompassed all aspects related to rehabilitation of post‐stroke individuals. Our search identified a significant number of studies that applied MI to improve gait and other functional outcomes related to walking. However, we found relatively few studies that observed the effectiveness of MI using a randomised and controlled design (21 studies); furthermore, the studies presented a small sample size.
Considering that we only included studies comparing MI to other therapies, our results cannot be generalized to include the effects of either placebo or no therapy. Even considering the comparison between MI and other therapies, there are factors producing uncertainty for generalizations. In addition, our results were only related to the short‐term effects.
The population of the included studies was quite heterogeneous (e.g. age, type of stroke, post‐stroke time, and deficit at the beginning of the study).
The majority of the experimental interventions included MI combined with other therapy.
The experimental and control conditions were heterogeneous (e.g. type of training, and especially treatment dose).
Although the application of MI is considered easy and no expensive equipment is required, its application costs were not quantified by the researchers. The results of this review appear to be quite generalizable for inpatient and outpatient settings of high‐income countries.
Quality of the evidence
According to the GRADE criteria, we classified the certainty of the evidence as very low due to the small number of studies included in the review, the wide confidence intervals, the moderate or substantial heterogeneity among studies, and because many studies presented methodological concerns. There was a high risk of bias for at least one assessed domain in 20 of the 21 included studies. Fifteen of the 21 included studies had a high risk of bias for allocation concealment. Nineteen of the 21 included studies had a high risk of bias for blinding participants or personnel. However, a good number of the studies adopted some precautions that may have minimized the presence of other biases, such as sufficient methodological details reported in previously published protocols and presenting results as stated in the methodology. The results of the main meta‐analyses showed a moderate to high inconsistency (moderate, substantial, and considerable heterogeneity).
Potential biases in the review process
The selection process of the studies has been judicious and followed the methodological rigor of Cochrane Reviews. We are confident that our comprehensive search strategy and detailed handsearching have identified all relevant studies. However, it is possible that we did not identify some studies published in the grey literature as well as additional (published or unpublished) trials.
As a potential selection bias, we could not identify the effects of MI on the outcome 'dependence on personal assistance' since the study authors could not provide the required data. For the same reason, some studies were not included in the meta‐analyses (Dickstein 2014; Kumar 2013a; Lee 2010; Liu 2004; Liu 2009; Park 2019; Schuster 2012; Suvadeep 2017; Zhang 2013; Zhu 2017). Another limitation of this review is that most of the studies had methodological shortcomings, such as blinding of outcome assessment, random sequence generation, allocation concealment, incomplete outcome data, and selective reporting. These biases can lead to underestimation or overestimation of the true intervention effect (Higgins 2011).
Agreements and disagreements with other studies or reviews
We found only one systematic review with meta‐analysis of RCTs of MI for improving balance, activities of daily living, and upper and lower limb function (Guerra 2017). This review differed from ours by including outcomes not related to gait. Twelve studies investigated motor performance of the lower limb and/or gait in an overall sample of 343 individuals. Our review appears to have carried out a more recent, broad, and comprehensive search compared to Guerra 2017, and thus we identified a greater number of studies in which MI was used to improve gait after stroke. Guerra 2017 found a significant difference for the outcomes related to gait in favor of MI, specifically related to walking speed (MD = 0.49, 95% CI, 0.09 to 0.89, P = 0.02, I² = 0%), thus corroborating our findings. However, when they conducted a sensitivity analysis by excluding low‐quality studies, no significant differences were found. In line with our conclusions, the review performed by Guerra 2017 suggested that further high‐quality studies, as well as greater standardization of MI interventions, are needed.
Authors' conclusions
Implications for practice.
Overall, compared to other therapies, MI may provide short‐term benefits (very low‐certainty evidence) on gait, when measured using walking speed. Regarding motor function and functional mobility (very low‐certainty evidence), MI was not more beneficial than other therapies. We could not properly estimate the effect of MI on both dependence on personal assistance and walking endurance because only one study reported the values of these outcomes after treatment. It was also impossible to estimate the effect of MI on adverse events since the studies neither reported this outcome nor reported adverse events. So, evidence was insufficient to estimate the effect of MI on dependence on personal assistance, walking endurance, and adverse events.
We only found studies comparing the effects of MI to other therapies, so it was impossible to generalize our results to comparisons between MI and placebo or no treatment. We were only able to analyze data relating to the immediate post‐treatment effects of MI due to the lack of follow‐up data in the included studies. Therefore, it was not possible to reach any conclusion about the potential medium or longer‐term (follow‐up) effects of MI. Overall, the certainty of the evidence in this review was very low due to studies with methodological concerns, small sample sizes, and wide confidence intervals. MI can improve short‐term walking speed when compared to other therapies (action observation and physical practice). However, as we rated the certainty of evidence as very low, our confidence in the estimate of the effect is limited, i.e. the real effect may differ substantially from the estimate of the effect.
Implications for research.
Further RCTs are needed with greater methodological rigor to reduce the risk of bias, and larger samples are needed in order to increase the accuracy of the clinical findings. The RCTs included in this review did not provide sufficient clarity regarding methodology, making it difficult to evaluate the risk of bias and its quality. Moreover, statistical data were not always present in its complete form in the included studies, making it difficult to conduct further analyses that would or would not support the use of the intervention. To minimize the biases of clinical trials, it is suggested to follow the Consolidated Standards of Reporting Trials ‐ CONSORT, which is an international guideline for the writing of clinical trials in the health research area (Moher 2001). In order to provide better descriptions of the interventions, we recommend using the Template for intervention description and replication’ (TIDieR) checklist. This checklist supports complete reporting of descriptions of interventions delivered in clinical studies (Hoffmann 2014).
Some studies evaluated the ability to perform MI by the Movement Imagery Questionnaire, without monitoring the execution of practice. No vital signs, such as heart rate or breathing frequency, were monitored in the studies. If vital signs had been taken into account, we would feel more confident that MI was performed properly. In addition, if there were studies comparing MI to placebo or no intervention, we might have different results, since the control groups in this review only performed physical practice or action observation. Therefore, an overestimation of the effect of the control groups may have occurred. Other important points that could make a difference are the standardization for the minimum application time of MI as well as the presence of follow‐up analyses. These would make us more confident concerning the long‐term effects of MI and clarify whether and under what conditions the therapy produces neural plasticity.
History
Protocol first published: Issue 5, 2018 Review first published: Issue 9, 2020
Acknowledgements
We thank Joshua Cheyne for his support and assistance regarding search strategies. We also thank the Cochrane Stroke Group Editorial team for providing assistance through revision of this review, especially Hazel Fraser, and for their willingness to always help.
Appendices
Appendix 1. CENTRAL search strategy
#1MeSH descriptor: [Cerebrovascular Disorders] this term only #2MeSH descriptor: [Basal Ganglia Cerebrovascular Disease] explode all trees #3MeSH descriptor: [Brain Ischemia] explode all trees #4MeSH descriptor: [Carotid Artery Diseases] explode all trees #5MeSH descriptor: [Intracranial Arterial Diseases] explode all trees #6MeSH descriptor: [Intracranial Embolism and Thrombosis] explode all trees #7MeSH descriptor: [Intracranial Hemorrhages] explode all trees #8MeSH descriptor: [Stroke] explode all trees #9MeSH descriptor: [Brain Infarction] explode all trees #10MeSH descriptor: [Vertebral Artery Dissection] this term only #11((brain* or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) near/3 (isch?emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*)):ti,ab,kw (Word variations have been searched) #12((brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid) near/3 (h?emorrhag* or h?ematoma$ or bleed*)):ti,ab,kw (Word variations have been searched) #13MeSH descriptor: [Hemiplegia] this term only #14MeSH descriptor: [Paresis] explode all trees #15MeSH descriptor: [Gait Disorders, Neurologic] explode all trees #16MeSH descriptor: [Brain Damage, Chronic] this term only #17MeSH descriptor: [Brain Injuries] this term only #18MeSH descriptor: [Brain Concussion] explode all trees #19MeSH descriptor: [Brain Injury, Chronic] this term only #20MeSH descriptor: [Diffuse Axonal Injury] this term only #21MeSH descriptor: [Craniocerebral Trauma] this term only #22MeSH descriptor: [Head Injuries, Closed] explode all trees #23MeSH descriptor: [Brain Abscess] explode all trees #24((brain or head or intracran* or cerebr* or cerebell* or orbit* or brainstem or vertebrobasil*) near/5 (abscess* or injur* or contusion* or hypoxi* or damage* or inflamm* or concussion or trauma* or fractur* or infection* or lesion*)):ti,ab,kw (Word variations have been searched) #25{or #1‐#24} #26MeSH descriptor: [Lower Extremity] explode all trees #27MeSH descriptor: [Foot Joints] explode all trees #28(lower extremit* or leg or legs or ankle* or foot or feet or heel* or toe* or hip or knee or knees or thigh*):ti,ab,kw (Word variations have been searched) #29(walk* or gait* or ambulat* or mobil* or locomot* or balanc* or stride or foot‐drop):ti,ab,kw (Word variations have been searched) #30MeSH descriptor: [Gait] explode all trees #31MeSH descriptor: [Locomotion] this term only #32MeSH descriptor: [Walking] this term only #33{or #26‐#32} #34MeSH descriptor: [Imagination] this term only #35MeSH descriptor: [Imagery (Psychotherapy)] this term only #36MeSH descriptor: [Imitative Behavior] this term only #37MeSH descriptor: [Perception] this term only #38MeSH descriptor: [Illusions] this term only #39MeSH descriptor: [Visual Perception] this term only #40MeSH descriptor: [Psychomotor Performance] explode all trees #41((motor or locomot*) near/3 (imag$ or visual* or ideation)):ti,ab,kw (Word variations have been searched) #42(action near/3 (immitat* or observ* or visuali$ or ideation)):ti,ab,kw (Word variations have been searched) #43((cognitive or covert* or mental) near/3 (practic* or rehears* or represent* or visual* or image*)):ti,ab,kw (Word variations have been searched) #44((visual or mirror*) near/3 (reflection or illusion or feedback or therapy) or visuali?ation):ti,ab,kw (Word variations have been searched)5024 #45{or #34‐#44} #46#25 and #33 and #45
Appendix 2. MEDLINE search strategy
1. cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp cerebral small vessel diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or stroke, lacunar/ or vasospasm, intracranial/ or vertebral artery dissection/ 2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj3 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj3 (h?emorrhag$ or h?ematoma$ or bleed$)).tw. 5. hemiplegia/ or exp paresis/ or exp gait disorders, neurologic/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. exp brain damage, chronic/ or brain injuries/ or exp brain concussion/ or brain injury, chronic/ or diffuse axonal injury/ or craniocerebral trauma/ or exp head injuries, closed/ or exp brain abscess/ 8. ((brain or head or intracran$ or cerebr$ or cerebell$ or orbit$ or brainstem or vertebrobasil$) adj5 (abscess$ or injur$ or contusion$ or hypoxi$ or damage$ or inflamm$ or concussion or trauma$ or fractur$ or infection$ or lesion$)).tw. 9. or/1‐8 10. exp Lower Extremity/ 11. foot joints/ or ankle joint/ 12. (lower extremit$ or leg or legs or ankle$ or foot or feet or heel$ or toe$ or hip or knee or knees or thigh$).tw. 13. (walk$ or gait$ or ambulat$ or mobil$ or locomot$ or balanc$ or stride or foot‐drop).tw. 14. gait/ or locomotion/ or exp walking/ 15. or/10‐14 16. imagination/ or "imagery (psychotherapy)"/ or imitative behavior/ 17. perception/ or illusions/ or visual perception/ 18. exp psychomotor performance/ 19. ((motor or locomot$) adj3 (imag$ or visual$ or ideation)).tw. 20. (action adj3 (immitat$ or observ$ or visuali$ or ideation)).tw. 21. ((cognitive or covert$ or mental) adj3 (practic$ or rehears$ or represent$ or visual$ or image$)).tw. 22. ((visual or mirror$) adj3 (reflection or illusion or feedback or therapy)).tw. 23. or/16‐22 24. randomized controlled trial.pt. 25. controlled clinical trial.pt. 26. randomized.ab. 27. placebo.ab. 28. randomly.ab. 29. trial.ab. 30. groups.ab. 31. or/24‐30 32. 9 and 15 and 23 and 31
Appendix 3. EMBASE search strategy
1. cerebrovascular disease/ or brain disease/ or exp basal ganglion hemorrhage/ or exp brain hemangioma/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or exp cerebral artery disease/ or exp cerebrovascular accident/ or exp cerebrovascular malformation/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or exp vertebrobasilar insufficiency/ 2. stroke patient/ or stroke unit/ 3. (stroke$ or poststroke or post‐stroke or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or SAH).tw. 4. ((brain or cerebell$ or cerebr$ or vertebrobasil$ or hemisphere$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation or basilar artery or vertebral artery) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 5. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemisphere$ or subarachnoid) adj5 (h?emorrhag$ or h$ematoma$ or bleed$)).tw. 6. paralysis/ or exp hemiplegia/ or exp paresis/ 7. (hempar$ or hemipleg$ or paresis or paretic).tw. 8. exp head injury/ or neurologic disease/ or exp brain injury/ or brain abscess/ or brain infection/ or brain tumor/ or brain disease/ or exp brain concussion/ or brain injury/ or brain contusion/ or diffuse axonal injury/ 9. ((brain or head or intracran$ or cerebr$ or cerebell$ or orbit$ or brainstem or vertebrobasil$) adj5 (injur$ or contusion$ or hypoxi$ or damage$ or inflamm$ or concussion or trauma$ or fractur$ or neoplasm$ or lesion$ or tumor$ or tumour$ or cancer$ or infection$)).tw. 10. or/1‐9 11. exp lower limb/ 12. (lower extremit$ or leg or legs or ankle$ or foot or feet or heel$ or toe$ or hip or knee or knees or thigh$).tw. 13. (walk$ or gait$ or ambulat$ or mobil$ or locomot$ or balanc$ or stride or foot‐drop).tw. 14. exp walking/ 15. locomotion/ 16. or/11‐15 17. exp imagery/ 18. imagination/ 19. imitation/ 20. mental function/ 21. perception/ or movement perception/ or exp sensation/ 22. proprioception/ 23. illusion/ 24. psychomotor performance/ or task performance/ 25. ((motor or locomot$) adj3 (imag$ or visual$ or ideation)).tw. 26. (action adj3 (immitat$ or observ$ or visuali$ or ideation)).tw. 27. ((cognitive or covert$ or mental) adj3 (practic$ or rehears$ or represent$ or visual$ or image$)).tw. 28. (((visual or mirror$) adj3 (reflection or illusion or feedback or therapy)) or visuali?ation).tw. 29. or/17‐28 30. Randomized Controlled Trial/ or "randomized controlled trial (topic)"/ 31. Randomization/ 32. Controlled clinical trial/ or "controlled clinical trial (topic)"/ 33. control group/ or controlled study/ 34. clinical trial/ or "clinical trial (topic)"/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ 35. Crossover Procedure/ 36. Double Blind Procedure/ 37. Single Blind Procedure/ or triple blind procedure/ 38. placebo/ or placebo effect/ 39. (random$ or RCT or RCTs).tw. 40. (controlled adj5 (trial$ or stud$)).tw. 41. (clinical$ adj5 trial$).tw. 42. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 43. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 44. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 45. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 46. (cross‐over or cross over or crossover).tw. 47. (placebo$ or sham).tw. 48. trial.ti. 49. (assign$ or allocat$).tw. 50. controls.tw. 51. or/30‐50 52. 10 and 16 and 29 and 51
Appendix 4. CINAHL search strategy
S1(MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR ( (MH "Intracranial Embolism and Thrombosis") ) OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections") OR (MH "Stroke Patients") OR (MH "Stroke Units") S2TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH) S3TI ((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) N5 ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus*)) OR AB ((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) N5 ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus*)) S4TI (( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid ) N5 ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )) OR AB (( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid ) N5 ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )) S5(MH "Hemiplegia") or (MH "Gait Disorders, Neurologic+") S6TI (hemipleg* or hemipar* or paresis or paretic) OR AB (hemipleg* or hemipar* or paresis or paretic) S7(MH "Brain Injuries") OR (MH "Brain Damage, Chronic") OR (MH "Brain Concussion+") OR (MH "Head Injuries") OR (MH "Brain Abscess+") S8TI ( ((brain or head or intracran* or cerebr* or cerebell* or orbit* or brainstem or vertebrobasil*) N5 (abscess* or injur* or contusion* or hypoxi* or damage* or inflamm* or concussion or trauma* or fractur* or infection* or lesion*)) ) OR AB ( ((brain or head or intracran* or cerebr* or cerebell* or orbit* or brainstem or vertebrobasil*) N5 (abscess* or injur* or contusion* or hypoxi* or damage* or inflamm* or concussion or trauma* or fractur* or infection* or lesion*)) ) S9S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 S10(MH "Lower Extremity+") S11(MH "Tarsal Joint+") OR (MH "Toe Joint+") OR (MH "Ankle Joint") OR (MH "Knee Joint+") S12TI ( (lower extremit* or leg or legs or ankle* or foot or feet or heel* or toe* or hip or knee or knees or thigh*) ) OR AB ( (lower extremit* or leg or legs or ankle* or foot or feet or heel* or toe* or hip or knee or knees or thigh*) ) S13TI ( (walk* or gait* or ambulat* or mobil* or locomot* or balanc* or stride or foot‐drop) ) OR AB ( (walk* or gait* or ambulat* or mobil* or locomot* or balanc* or stride or foot‐drop) ) S14(MH "Locomotion+") S15S10 OR S11 OR S12 OR S13 OR S14 S16(MH "Guided Imagery") OR (MH "Imagination") OR (MH "Mirror Therapy") OR (MH "Reflection") S17(MH "Mental Processes") OR (MH "Perception+") S18(MH "Imitative Behavior") S19(MH "Psychomotor Performance+") S20TI ( ((motor or locomot*) N3 (imag* or visual* or ideation)) ) OR AB ( ((motor or locomot*) N3 (imag* or visual* or ideation)) ) S21TI ( (action N3 (immitat* or observ* or visuali* or ideation)) ) OR AB ( (action N3 (immitat* or observ* or visuali* or ideation)) ) S22TI ( ((cognitive or covert* or mental) N3 (practic* or rehears* or represent* or visual* or image*)) ) OR AB ( ((cognitive or covert* or mental) N3 (practic* or rehears* or represent* or visual* or image*)) ) S23TI ( ((visual or mirror*) N3 (reflection or illusion or feedback or therapy)). ) OR AB ( ((visual or mirror*) N3 (reflection or illusion or feedback or therapy)). ) S24S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 S25MH Random Assignment or MH Single‐blind Studies or MH Double‐blind Studies or MH Triple‐blind Studies or MH Crossover design or MH Factorial Design S26TI ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or AB ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or SU ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") S27TI random* or AB random* S28AB "latin square" or TI "latin square" S29TI (crossover or cross‐over) or AB (crossover or cross‐over) or SU (crossover or cross‐over) S30MH Placebos S31AB (singl* or doubl* or trebl* or tripl*) or TI (singl* or doubl* or trebl* or tripl*) S32TI blind* or AB mask* or AB blind* or TI mask* S33S31 and S32 S34TI Placebo* or AB Placebo* or SU Placebo* S35MH Clinical Trials S36TI (Clinical AND Trial) or AB (Clinical AND Trial) or SU (Clinical AND Trial) S37S25 or S26 or S27 or S28 or S29 or S30 or S33 or S34 or S35 or S36 S38S9 AND S15 AND S24 AND S37
Appendix 5. PsycINFO search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or exp cerebral ischemia/ or cerebrovascular accidents/ or subarachnoid hemorrhage/ 2. (stroke$ or post stroke or poststroke or post‐stroke or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj3 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj3 (h?emorrhag$ or h?ematoma$ or bleed$)).tw. 5. traumatic brain injury/ or brain damage/ or brain concussion/ or exp head injuries/ 6. ((brain or head or intracran$ or cerebr$ or cerebell$ or orbit$ or brainstem or vertebrobasil$) adj5 (abscess$ or injur$ or contusion$ or hypoxi$ or damage$ or inflamm$ or concussion or trauma$ or fractur$ or infection$ or lesion$)).tw. 7. hemiparesis/ or hemiplegia/ 8. (hemipleg$ or hemipar$ or paresis or paretic).tw. 9. or/1‐8 10. "leg (anatomy)"/ or ankle/ or "feet (anatomy)"/ or knee/ or thigh/ 11. (lower extremit$ or leg or legs or ankle$ or foot or feet or heel$ or toe$ or hip or knee or knees or thigh$).tw. 12. (walk$ or gait$ or ambulat$ or mobil$ or locomot$ or balanc$ or stride or foot‐drop).tw. 13. gait/ or running/ or walking/ 14. locomotion/ or physical mobility/ 15. or/10‐14 16. exp imagery/ or guided imagery/ or exp imagination/ 17. exp "Imitation (Learning)"/ 18. mirror image/ 19. perception/ or exp extrasensory perception/ or exp "illusions (perception)"/ or object recognition/ or exp spatial perception/ or exp visual perception/ 20. ((motor or locomot$) adj3 (imag$ or visual$ or ideation)).tw. 21. (action adj3 (immitat$ or observ$ or visuali$ or ideation)).tw. 22. ((cognitive or covert$ or mental) adj3 (practic$ or rehears$ or represent$ or visual$ or image$)).tw. 23. (((visual or mirror$) adj3 (reflection or illusion or feedback or therapy)) or visuali?ation).tw. 24. 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 25. clinical trials/ or treatment effectiveness evaluation/ or placebo/ 26. (random$ or RCT or RCTs).tw. 27. (controlled adj5 (trial$ or stud$)).tw. 28. (clinical$ adj5 trial$).tw. 29. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 30. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 31. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 32. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 33. (cross‐over or cross over or crossover).tw. 34. (placebo$ or sham).tw. 35. trial.ti. 36. (assign$ or allocat$).tw. 37. controls.tw. 38. or/25‐37 39. 9 and 15 and 24 and 38
Appendix 6. AMED search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h? ematoma$ or bleed$)).tw. 5. exp brain injuries/ or brain disease/ or brain edema/ or brain neoplasms/ or cerebellar disease/ 6. ((brain or head or intracran$ or cerebr$ or cerebell$) adj5 (injur$ or contusion$ or hypoxi$ or damage$ or inflamm$ or concussion or trauma$ or fractur$ or neoplasm$ or lesion$ or tumor$ or tumour$ or cancer$ or infection$)).tw. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp leg/ 9. ankle joint/ or hip joint/ or knee joint/ or exp tarsal joint/ or exp toe joint/ 10. (lower extremit$ or leg or legs or ankle$ or foot or feet or heel$ or toe$ or hip or knee or knees or thigh$).tw. 11. (walk$ or gait$ or ambulat$ or mobil$ or locomot$ or balanc$ or stride or foot‐drop).tw. 12. movement/ or exp gait/ or exp locomotion/ 13. 8 or 10 or 11 or 12 14. imagery/ 15. exp imagination/ 16. exp perception/ 17. exp proprioception/ 18. ((motor or locomot$) adj3 (imag$ or visual$ or ideation)).tw. 19. (action adj3 (immitat$ or observ$ or visuali$ or ideation)).tw. 20. ((cognitive or covert$ or mental) adj3 (practic$ or rehears$ or represent$ or visual$ or image$)).tw. 21. (((visual or mirror$) adj3 (reflection or illusion or feedback or therapy)) or visuali?ation).tw. 22. 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 23. research design/ 24. clinical trials/ 25. randomized controlled trials/ 26. comparative study/ 27. double blind method/ 28. random allocation/ 29. placebos/ 30. random$.tw. 31. (controlled adj5 (trial$ or stud$)).tw. 32. (clinical$ adj5 trial$).tw. 33. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 34. ((multicenter or multicentre or therapeutic) adj5 (trial$ or stud$)).tw. 35. placebo$.tw. 36. 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 37. 7 and 13 and 22 and 36
Appendix 7. LILACS Bireme search strategy
tw:((tw:(stroke)) OR (tw:(cerebrovascular)) AND (tw:(image*)) OR (tw:(mental practice)) AND (tw:(gait)) OR (tw:(lower limb)) AND (tw:(clinical trial)) OR (tw:(randomized clinical trial)))
Appendix 8. SPORTDiscus search strategy
S1((((DE "STROKE") OR (DE "CEREBROVASCULAR disease")) OR (DE "CAROTID artery")) OR (DE "CEREBRAL embolism & thrombosis" OR DE "CEREBRAL hemorrhage")) S2TI ( (stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva* or SAH) ) OR AB ( (stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva* or SAH) ) S3TI ( ((brain* or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) N3 (isch?emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*)) ) OR AB ( ((brain* or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) N3 (isch?emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*)) ) S4TI ( ((brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid) N3 (h?emorrhag* or h?ematoma* or bleed*)) ) OR AB ( ((brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid) N3 (h?emorrhag* or h?ematoma* or bleed*)) ) S5DE "HEMIPLEGIA" S6TI ( (hemipleg* or hemipar* or paresis or paretic) ) OR AB ( (hemipleg* or hemipar* or paresis or paretic) ) S7DE "BRAIN damage" OR DE "BRAIN diseases" OR DE "BRAIN injuries" S8TI ( ((brain or head or intracran* or cerebr* or cerebell* or orbit* or brainstem or vertebrobasil*) N5 (abscess* or injur* or contusion* or hypoxi* or damage* or inflamm* or concussion or trauma* or fractur* or infection* or lesion*)) ) OR TI ( ((brain or head or intracran* or cerebr* or cerebell* or orbit* or brainstem or vertebrobasil*) N5 (abscess* or injur* or contusion* or hypoxi* or damage* or inflamm* or concussion or trauma* or fractur* or infection* or lesion*)) ) S9S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 S10DE "LEG" OR DE "LEG bones" OR DE "LEG muscles" S11TI ( (lower extremit* or leg or legs or ankle* or foot or feet or heel* or toe* or hip or knee or knees or thigh*) ) OR AB ( (lower extremit* or leg or legs or ankle* or foot or feet or heel* or toe* or hip or knee or knees or thigh*) ) S12TI ( (walk* or gait* or ambulat* or mobil* or locomot* or balanc* or stride or foot‐drop) ) OR AB ( (walk* or gait* or ambulat* or mobil* or locomot* or balanc* or stride or foot‐drop) ) S13(DE "GAIT in humans") OR (DE "LOCOMOTION") S14S10 OR S11 OR S12 OR S13 S15DE "IMAGERY (Psychology)" OR DE "MOTOR imagery (Cognition)" OR DE "VISUALIZATION" S16(DE "PERCEPTUAL‐motor processes") OR (DE "PERCEPTUAL motor learning") S17TI ( ((motor or locomot*) N3 (imag* or visual* or ideation)) ) OR AB ( ((motor or locomot*) N3 (imag* or visual* or ideation)) ) S18TI ( (action N3 (immitat* or observ* or visuali* or ideation)) ) OR AB ( (action N3 (immitat* or observ* or visuali* or ideation)) ) S19TI ( ((cognitive or covert* or mental) N3 (practic* or rehears* or represent* or visual* or image*)) ) OR AB ( ((cognitive or covert* or mental) N3 (practic* or rehears* or represent* or visual* or image*)) ) S20TI ( ((visual or mirror*) N3 (reflection or illusion or feedback or therapy)) ) OR AB ( ((visual or mirror*) N3 (reflection or illusion or feedback or therapy)) ) S21S15 OR S16 OR S17 OR S18 OR S19 OR S20 S22S9 AND S14 AND S21
Appendix 9. PEDro search strategy
Abstract & Title: stroke gait image*
Subdiscipline: Neurology
Method: Clinical trial
When searching: Mach all search itens (AND)
Appendix 10. REHABDATA search strategy
Current Search: View Articles, including International Research, where Abstract contains: stroke, AND Abstract contains: gait, AND Abstract contains: image*
Current Search: View Articles, including International Research, where Title contains: stroke, AND Title contains: gait, AND Title contains: image*
Current Search: View Articles, including International Research, where Abstract contains: stroke, AND Abstract contains: mental and Abstract contains: practice, OR Abstract contains: motor and Abstract contains: imagery, AND Title contains: trial
Current Search: View Articles, including International Research, where Abstract contains: hemipares*, AND Abstract contains: gait, AND Abstract contains: image*, AND Abstract contains: random*
Appendix 11. ClinicalTrials search strategy
( imagery OR mental practice OR imagination OR action observation OR mirror therapy ) AND ( Brain Infarction OR Intracranial Hemorrhages OR Carotid Artery Diseases OR Brain Ischemia OR Cerebral Hemorrhage OR Cerebrovascular Disorders OR Stroke ) [DISEASE]
Appendix 12. WHO ClinicalTrials search strategy
stroke AND mirror OR stroke AND imagery OR stroke AND action observation cerebrovascular AND mirror OR cerebrovascular AND imagery OR cerebrovascular AND action observation
Appendix 13. Stroke Trials Registry search strategy
stroke AND mirror OR stroke AND imagery OR stroke AND action observation cerebrovascular AND mirror OR cerebrovascular AND imagery OR cerebrovascular AND action observation
Data and analyses
Comparison 1. Motor Imagery therapy versus other therapies (control): effect on ability to walk.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Walking speed | 6 | 191 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.06, 0.81] |
| 1.2 Subgroup analysis: post‐stroke time | 6 | 191 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.06, 0.81] |
| 1.2.1 Subacute | 3 | 104 | Std. Mean Difference (IV, Random, 95% CI) | 0.67 [‐0.08, 1.43] |
| 1.2.2 Chronic | 2 | 47 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.37, 0.78] |
| 1.2.3 Subacute and chronic | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.36, 0.88] |
| 1.3 Subgroup analysis: treatment dose | 5 | 161 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.03, 0.59] |
| 1.3.1 More than 1000 minutes | 2 | 68 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.38, 0.57] |
| 1.3.2 Less than 1000 minutes | 3 | 93 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [0.00, 0.83] |
| 1.4 Subgroup analysis: type of treatment | 6 | 191 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.06, 0.81] |
| 1.4.1 Motor imagery alone | 1 | 23 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.63, 1.01] |
| 1.4.2 Motor imagery associated with action observation or physical practice | 5 | 168 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [0.04, 0.92] |
| 1.5 Subgroup analysis: walking dependence | 4 | 117 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [0.01, 0.74] |
| 1.5.1 Dependent and independent of personal assistance | 2 | 63 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.26, 0.73] |
| 1.5.2 Independent of personal assistance | 2 | 54 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [‐0.06, 1.15] |
| 1.6 Subgroup analysis: forms of application of MI | 6 | 191 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.06, 0.81] |
| 1.6.1 Visual imagery | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.56, 0.62] |
| 1.6.2 Kinesthetic imagery | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.84 [0.08, 1.59] |
| 1.6.3 Both visual and kinesthetic imagery | 4 | 117 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.02, 0.97] |
Comparison 2. Motor imagery versus other therapies (control): effect on motor function.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Motor function | 3 | 130 | Mean Difference (IV, Random, 95% CI) | 2.24 [‐1.20, 5.69] |
| 2.2 Subgroup analysis: post‐stroke time | 2 | 70 | Mean Difference (IV, Random, 95% CI) | 2.08 [‐5.06, 9.22] |
| 2.2.1 Subacute | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐5.75, 2.15] |
| 2.2.2 Chronic | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 5.50 [3.79, 7.21] |
| 2.3 Subgroup analysis ‐ treatment dose | 3 | 130 | Mean Difference (IV, Random, 95% CI) | 2.24 [‐1.20, 5.69] |
| 2.3.1 More than 1000 minutes | 2 | 102 | Mean Difference (IV, Random, 95% CI) | 0.52 [‐2.99, 4.03] |
| 2.3.2 Less than 1000 minutes | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 5.50 [3.79, 7.21] |
| 2.4 Subgroup analysis: forms of application of MI | 3 | 130 | Mean Difference (IV, Random, 95% CI) | 2.24 [‐1.20, 5.69] |
| 2.4.1 Visual imagery | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐5.75, 2.15] |
| 2.4.2 Kinesthetic imagery | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 1.90 [0.37, 3.43] |
| 2.4.3 Both visual and kinesthetic imagery | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 5.50 [3.79, 7.21] |
Comparison 3. Motor imagery versus other therapies (control): effect on functional mobility.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Functional mobility | 4 | 116 | Std. Mean Difference (IV, Random, 95% CI) | 0.55 [‐0.45, 1.56] |
| 3.1.1 Functional mobility ‐ Rivermead mobility index | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.02, 0.34] |
| 3.1.2 Functional mobility ‐ Timed Up and Go test | 3 | 82 | Std. Mean Difference (IV, Random, 95% CI) | 0.88 [‐0.38, 2.14] |
| 3.2 Subgroup analysis: treatment dose | 2 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 1.21 [‐0.85, 3.27] |
| 3.2.1 More than 1000 minutes | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.46, 0.85] |
| 3.2.2 Less than 1000 minutes | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | 2.30 [1.31, 3.28] |
| 3.3 Functional mobility ‐ sensitivity analysis: studies without high risk of bias | 2 | 62 | Std. Mean Difference (IV, Random, 95% CI) | 0.95 [‐1.63, 3.54] |
| 3.4 Functional mobility ‐ sensitivity analysis: without peripheral studies | 3 | 88 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.42, 0.42] |
| 3.5 Subgroup analysis: forms of application of MI | 3 | 82 | Std. Mean Difference (IV, Random, 95% CI) | 0.88 [‐0.38, 2.14] |
| 3.5.1 Visual imagery | 1 | 18 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.68, 1.18] |
| 3.5.2 Both visual and kinesthetic imagery | 2 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 1.21 [‐0.85, 3.27] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Braun 2012.
| Study characteristics | ||
| Methods | Multicenter RCT | |
| Participants | Participants were recruited from a nursing home setting because most older stroke patients in the Netherlands receive rehabilitation at nursing homes, so it was clinically important to study this group Sample size: 36 Inclusion criteria: 1) clinically diagnosed adult stroke patients, between 2 and 10 weeks after stroke onset; 2) sufficient cognitive level and communication skills to engage in mental practice. Clinical judgment of the treating therapist, support from family, and score on the Mini‐Mental State Examination (MMSE preferably > 24) were taken into account Exclusion criteria: 1) patients who had conditions such as rheumatic diseases; 2) patients who had dementia before stroke onset sufficient to cause persistent premorbid disability Mean (SD) age: control group 77.9 (SD 7.4) years; experimental group 77.7 (SD 7.2) years Stroke details: not reported by study authors |
|
| Interventions | Both groups received multi‐professional therapy as usual. Additionally, patients in the experimental group had instruction on mental practice | |
| Outcomes | Outcomes recorded before, after, and at 6 months after treatment Walking speed: 10 Meters Walking Time Dependence on personnel assistance: Barthel Index Functional mobility: Rivermead Mobility Index |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Decentralized randomisation took place by an independent third party blinded to the characteristics of the study participants, based on a computerized (block size 4) randomisation schedule. No stratification took place. The randomisation procedure was the same for all 3 sites" |
| Allocation concealment (selection bias) | Low risk | Quote: “[...] before the envelope was opened to determine their allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “The patients were not blinded to the treatment they received, as they were aware of the treatment content. The rater, however, was blinded for the treatment allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The patients were not blinded to the treatment they received, as they were aware of the treatment content. The rater, however, was blinded for the treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “We probably missed the patients most likely to benefit because patients going home within a few weeks and patients being transferred to a specialized rehabilitation center were not included in the trial. This meant that the patients recruited for this trial were a specific and frail subgroup" |
| Selective reporting (reporting bias) | Low risk | Study authors described what is proposed in the methodology |
| Other bias | Low risk | None detected |
Cho 2012.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 2 research assistants screened volunteers 28 participants: 15 experimental group, 13 control group Inclusion criteria: more than 6 months after stroke onset, no problems with auditory or visual functions, ability to walk > 10 meters independently, not taking any medication, no orthopedic injuries that could influence balance or gait ability, and Mini‐Mental State Examination score > 24 Exclusion criteria: not reported by study authors Mean (SD) age: experimental group: 53.93 (SD 12.60) years; control group: 53.85 (SD 12.44) years Stroke details: not reported by study authors Stroke phase: chronic |
|
| Interventions | Experimental group: imagery training regarding normal gait movement performed in conjunction with gait training may improve gait ability. Imagery training was applied for 15 minutes, following gait training using a treadmill for 30 minutes. After conducting imagery training, the participants were allowed to relax for 5 minutes. To perform motor imagery training, videos of normal gait movement were shown Control group: the control group performed only gait training on the treadmill for 30 minutes |
|
| Outcomes | Outcome recorded before and one day after 6 weeks intervention Walking speed: 10 Meter Walk Test Motor function: Fugl‐Meyer Assessment. Functional mobility: Timed Up and Go Test |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Another research assistant used the tables of random numbers for random allocation of the subjects” |
| Allocation concealment (selection bias) | Low risk | Quote: “Another research assistant used the tables of random numbers for random allocation of the subjects" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Participants, researchers and two research assistants, who helped with the program and the measurements, were unaware of the group assignments" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Participants, researchers and two research assistants, who helped with the program and the measurements, were unaware of the group assignments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram: exclusion = 0; drop‐out = 0 for all outcomes |
| Selective reporting (reporting bias) | Low risk | Quote: ‘there were significant differences between the two groups at follow‐up with respect to all parameters (P < 0.05)” |
| Other bias | Low risk | None detected |
Dickstein 2013.
| Study characteristics | ||
| Methods | Half‐crossover study | |
| Participants | Participants were recruited from the registry of Flieman Geriatric Rehabilitation Hospital in Haifa, Israel. Potential participants were screened, and after the project presentation, consent was obtained in their homes by a physical therapist Inclusion criteria: participants were included if they were community‐dwelling individuals, 60 to 80 years of age, who had sustained a unilateral stroke at least 6 months and no more than 2 years before recruitment. Only people reporting limited indoor and outdoor ambulation after the stroke; Mini Mental State Examination score tested at the home visit was 24 points or higher and who were not receiving physical therapy were included Exclusion criteria: wheelchair use, severe ailments including psychiatric disorders and major depression, and communication deficits Mean (SD) age: 72 (SD 6.9) years Stroke details: all participants: 18 ischemic, 5 hemorrhagic. Assigned to intervention: 9 ischemic, 3 hemorrhagic. Assigned to control: 9 ischemic, 2 hemorrhagic. Severity level of stroke: cortical = 6, subcortical = 11, cortical + subcortical = 1. In 5 participants, the stroke site was not determined Stroke phase: chronic |
|
| Interventions | Experimental group ('integrated imagery practice'): the participants’ goals were used to select the imagined walking tasks for the imagery practice. The imagery scripts were identical for 3 weekly sessions and changed at the beginning of each week. All sessions were performed while the participants sat on a couch with eyes closed. Each session started and ended with 3 minutes of relaxation exercises. Three minutes of imagery practice were conducted for each of 3 imagery environments: the participant’s home, a 'community interior' (public indoor, such as a mall), and a 'community exterior' (public outdoors, such as a street) environment (for a total of 9 minutes). Imagery vividness was enhanced by using environments that were familiar to the participants. Both kinesthetic and visual imagery of the walking activities were used during practice. Motivational imagery was introduced in each session to enhance arousal, stimulate problem‐solving, and provide a sense of satisfaction Control group: control treatment consisted of physical therapy for upper extremity. It included 3 types of exercises, each conducted for 3 minutes: 1) transport‐reach exercise (e.g. spoon to mouth); 2) bimanual exercise (e.g. folding clothes); and 3) unimanual manipulation with the involved upper extremity (e.g. placing items in a jar). Functional tasks, chosen according to the participant’s needs, did not involve ambulation. The tasks were identical for the 3 weekly sessions and changed at the beginning of each week. All participants had motor limitations of paretic upper limb. Control treatment, similar to the experimental treatment, promoted participants’ collaboration |
|
| Outcomes | Outcomes recorded at baseline, post‐intervention, and at 1 month from treatment conclusion Walking speed: 10 Meter Walk Test Pain, falls, and all‐cause deaths: Falls‐Efficacy Scale, Swedish version |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “... randomisation was based on a minimization scheme, which ensured balance in gait speed (with speed of.42m/s dividing subjects into “low‐” and “high‐level” walkers) as well as in age and sex" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | All participants had to be aware of the therapies that were submitted to participate in the study, since it involved physical and cognitive exercise |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Assessments were performed by 2 physical therapists (M.K., A.D.) blinded to group treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missed data balanced between groups |
| Selective reporting (reporting bias) | Low risk | The data of all outcomes were shown |
| Other bias | Low risk | None detected |
Dickstein 2014.
| Study characteristics | ||
| Methods | Full crossover | |
| Participants | Group members met regularly twice a week at each center. After project explanation to each member group, volunteers were recruited to participate in the study Sample size: 16 Inclusion criteria: inclusion criteria were an age range of 30 to 70 years; a time gap of at least 3 months between the stroke and admission to the study; appropriate cognitive ability (Mini Mental State Examination score not lower than 24 points); ability to walk a minimal distance of 10 meters without stopping; absence of any medical condition that would prohibit participation; and absence of any communication problem that would interfere with participation Exclusion criteria: not reported by study authors Mean (SD) age: 63 (SD 7) years Stroke details: stroke territory ‐ anterior circulation = 14, vertebrobasilar = 2 AffectedbBody side: left: 10, right: 6. Type: thromboembolic = 14; hemorrhagic = 2 Stroke phase: chronic |
|
| Interventions | Experimental treatment: motor imagery practice of gait activities Control treatment: motor imagery practice of upper extremity functional movements |
|
| Outcomes | Outcomes were recorded at baseline, post‐intervention, and 5 weeks from treatment conclusion Walking speed: 10 Meter Walk Test, vertical ground reaction forces, measured via the 'Smart Step' system; Tinetti Mobility Test (gait score) Pain, falls, and all‐cause deaths: Activities‐specific Balance Confidence (ABC) scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “The order of assignment to the experimental and the control treatments during the first period was determined by the order of admission to the study" |
| Allocation concealment (selection bias) | High risk | Quote: “The order of assignment to the experimental and the control treatments during the first period was determined by the order of admission to the study” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Two physical therapists served as group instructors in each center, with one instructing the experimental treatment and the other the control treatment. General plans for the exercise regimens in the two centers were established during a workshop that preceded the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Pre‐intervention, post‐intervention, and follow‐up measurements were performed in each center by one evaluator, who was a senior physical therapist that did not participate in the application of the treatments and was blind to the subjects’ treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost data balanced between groups and similar reasons |
| Selective reporting (reporting bias) | Low risk | Differences found between these values at the pre‐ and post‐interventions were not significant for either treatment modality. Likewise, no differences between the effects of the experimental and the control treatments were discerned for any of these tested variables (for all comparisons P > 01) |
| Other bias | Low risk | None detected |
Gupta 2017.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 30 stroke patients were recruited from hospitals in New Delhi, India Sample size: 30 Inclusion criteria: diagnosed as having had a first unilateral cerebral infarction, confirmed by MRI, sub acute phase (1 month to 1 year post stroke), age between 40 and 70 years, both men and women, right and left sides will be included, Mini Mental State Examination score should be more than 24, no severe cognitive impairment, an average score of less than 3 on the Vividness of Movement Imagery Questionnaire, affected upper limb and lower limb tone < 2 on modified Ashworth Scale, ability to walk with or without assistance, independent in performing daily activities, having given their voluntary consent, no orthopedic diseases that would have affected standing balance Exclusion criteria: medically unstable, hemorrhagic lesions, lesions affecting both hemispheres as determined by MRI available in medical records, unilateral neglect, visual and hearing impairment, significant sensory and communication deficits, excessive pain in the affected upper and lower limb as measured by a score of more than or equal to 4 on a 10 point Visual Analogue Scale, musculoskeletal injuries to upper extremity and lower limb, fractures and dislocations, unmanaged seizures, any other neurological disorder, alcohol dependence Mean (SD) age: control group: 58.46 (SD 6.37) years; experimental group: 69.20 (SD 5.69) years Stroke details: not recorded by study authors Strokephase: subacute |
|
| Interventions | Both groups received conventional physical therapy for improving balance and gait Control group: conventional physical therapy Experimental group: conventional physical therapy + MI |
|
| Outcomes | Outcomes recorded pre 1, post 1, post 2 and post 3 (3 weeks) Walking speed: Tinetti Performance Oriented Mobility Assessment (gait tests), 10 Meter Walking Test |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Allocation of subjects into two groups, 15 each, was done according to permuted block randomisation" |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Low risk | All the outcome measures analyzed in the protocol appear in the results |
| Other bias | Low risk | None detected |
Kim 2013a.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Recruitment methods were not reported by the study authors Sample size: 27 Inclusion criteria: 1) having a first‐time ischemic or hemorrhagic stroke; 2) over 6 months since onset; 3) able to walk independently more than 10 meters; 4) more than 24 points on the Mini Mental State Examination; 5) fewer than 36 points on the Vividness Motor Imagery Questionnaire‐2 Exclusion criteria: 1) severe cognitive disabilities, such as unilateral neglect, dementia, and depression; 2) severe aphasia Mean (SD) age: action observation training (n = 9): 55.3 (SD 12.1) years; motor imagery training (n = 9): 54.8 (SD 8.8) years; physical training (n = 9): 59.8 (SD 8.9) years Stroke details: ischemic = 17 (action observation training = 5; motor imagery training = 5; physical training = 7). Hemorrhagic = 10 (action observation training = 4; motor imagery training = 4; physical training = 2) Stroke phase: chronic |
|
| Interventions | Experimental groups: (EG1): physical training + action observation training; (EG2): physical training + motor imagery training Control group: physical training All participants in this study underwent neurodevelopmental therapy for 30 minutes, twice per day, f5 days per week for a period of 4 weeks, according to the schedule of the institution in which they were hospitalized |
|
| Outcomes | Outcomes recorded before and after intervention Walking speed: GaitRite (biomechanical analysis) Dependence on personal assistance: Functional Ambulation Category Functional mobility: Timed Up and Go Test |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “patients were randomly assigned to select a sealed envelope” |
| Allocation concealment (selection bias) | Low risk | Quote: “patients were randomly assigned to select a sealed envelope” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “All participants in this study underwent neurodevelopmental therapy” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “assessment of outcome measures was performed by two physical therapists” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missed data balanced between groups |
| Selective reporting (reporting bias) | Low risk | All expected and pre‐specified outcomes were reported |
| Other bias | Low risk | None detected |
Kumar 2013a.
| Study characteristics | ||
| Methods | Pilot RCT | |
| Participants | Participants recruitment methods were not reported by the study authors Sample size: 26 Inclusion criteria: hemiparetic patients who could walk 10 meters with good imagery ability in KVIQ – 20 ≥ 60 and time‐dependent motor imagery screening test Exclusion criteria: not reported by the study authors Mean (SD) age: not reported by the study authors Stroke details: not reported by the study authors Stroke phase: subacute or chronic |
|
| Interventions | Experimental group (EG) and control group (CG). Bothgroups received physical practice treatment (training for lower extremity for 45 minutes). EG received added 15 minutes of audio‐based lower‐extremity tasks for imagery practice | |
| Outcomes | Outcomes recorded before and after the program (3 weeks of program) Walking speed: Functional Gait Assessment Functional mobility: Timed Up and Go Test |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote:“... were recruited and randomly allocated into physical practice group (n = 13) and physical + mental practice (n = 13)” |
| Allocation concealment (selection bias) | High risk | Quote:“... were recruited and randomly allocated into physical practice group (n = 13) and physical + mental practice (n = 13)” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | High risk | Quote: “Following 3 weeks of training there was a significant difference in FGA and TUG scores in both the groups. Between groups the mean (SD) differences scores of 4.5 (.55) for FGA and 7.3 (.23) for TUGT was statistically significantly (P < 0.05)" |
| Other bias | Low risk | None detected |
Kumar 2016.
| Study characteristics | ||
| Methods | Assessor‐blinded RCT design | |
| Participants | Participants were identified from a retrospective search from inpatient/outpatient registry from April 2012 to June 2013 and were referred for a comprehensive rehabilitation program in Kasturba Medical College and Hospitals, Mangalore, Manipal University, Karnataka, India. Primary investigator (VK, a physical therapist) contacted the potential participants through telephone communication/information letter about the study purpose and interested participants were assessed for eligibility Sample size: 40 Inclusion criteria: 1) unilateral first episode of stroke at least 3 months (ischemic/hemorrhagic) with residual hemiparesis before recruitment, 2) Brunnstorm recovery stage ≥ 5 for lower extremity; 3) Functional Ambulation Category level 2 and above; 4) Mini Mental State Examination score was 24 points or higher; 5) kinesthetic and visual imagery score (KVIQ‐20) only ≥ 60 able to do time‐dependent MI screening test Exclusion criteria: history of CNS diseases, major head injury, neuropsychiatric diseases, cerebellar or brainstem stroke, dizziness or vertigo that limits walking, severe visual defect, peripheral vascular diseases etc. Serious cardiac conditions which required hospitalization in the past 6 months, major musculoskeletal or orthopaedic surgeries in lower extremities and those who participated in MI program related to physical activity within the previous 3 months Mean (SD) age: control group: 51.0 (SD 5.80) years, experimental group: 53.0(SD 6.40) years Stroke details: control group: ischemic 25% to hemorrhagic 75%, experimental group: 40% to 60% Stroke phase: probably subacute and chronic |
|
| Interventions | Experimental group: physical plus mental practice (experimental) group: movement imagery training Control group: physical practice |
|
| Outcomes | Outcomes recorded at baseline, post‐intervention, and at 3 weeks from treatment conclusion Walking speed: 10 Meter Walk Test |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Total 40 participants were randomly assigned to receive either experimental (n = 20) or the control group (n = 20) using block randomization (4 blocks with 10 subjects in each block)" |
| Allocation concealment (selection bias) | Low risk | Quote: “The primary investigator generated the randomization list using computer generated random numbers and allotted each subject intervention assignment which were enclosed in sealed opaque envelopes” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: “A trained physical therapist with five years of experience in stroke rehabilitation was assigned as an independent blinded assessor to administer the outcome measures at two assessment points. Data were collected at baseline and after 3 weeks of intervention period" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no drop‐outs |
| Selective reporting (reporting bias) | Low risk | Quote: “The study results have shown that combined MIT training was found to be more beneficial in comparison to task–specific training alone to improve the paretic muscle strength and gait performance in ambulant stroke subjects" |
| Other bias | Low risk | None detected |
Lee 2010.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Participant recruitment methods were not reported Sample size: 21 Inclusion criteria: participants with Korean Mental State Examination of 21 or more points; who can walk for 10 minutes or more independently; does not take the medication that affects balance; no visual defects and agrees to participate in the study after explaining the purpose Exclusion criteria: not reported by study authors Mean (SD) age: experimental group: 61.45 (SD 4.23) years, control group: 61.70 (SD 3.27) years Stroke details: not reported by study authors Stroke phase: chronic |
|
| Interventions | Imagination training group (experimental group): 1 hour for functional training + 30 minutes for imagination training Functional training group (control group): 1 hour for functional training |
|
| Outcomes | Outcomes recorded before and after treatment Walking speed: GaitRite Functional mobility: Timed Up and Go Test |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “The 21 patients selected were randomly selected as an imaginative training group and functional exercise group, with 11 and 10 patients.” |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded. Personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “Twenty‐one patients who failed to walk for more than 10 minutes and two patients treated in other medical devices were selected and participated in this study.” |
| Selective reporting (reporting bias) | Unclear risk | Not clear |
| Other bias | Low risk | None detected |
Lee 2011.
| Study characteristics | ||
| Methods | RCT | |
| Participants | The participants in this study took part in a rehabilitation program at a community center Sample size: 24 Inclusion criteria: hemiparetic from a single stroke occurring at least 6 months earlier; able to walk 10 meters independently without an assistive device; Mini Mental State Examination scores of 24 or higher; unknown musculoskeletal conditions that would affect the ability to safely walk repeatedly; and absence of serious visual impairment or hearing disorder Exclusion criteria: not reported by study authors Mean (SD) age: experimental group: 60.7 (SD 7.53) years, control group: 61.9 (SD 11.26) years Stroke details: not reported by study authors Stroke phase: not reported by study authors |
|
| Interventions | Both the experimental and control groups received treatment with treadmill gait training. The experimental group received added motor imagery training | |
| Outcomes | Outcomes recorded before and after the program (6 weeks of program) Walking speed: temporal and spatial gait parameters (Gaitrite®) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Subjects were randomly assigned to one of the two groups after initial evaluation using a simple random sampling method for minimizing the selection bias“ |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “Five patients from the experimental group and seven patients from the control group dropped out of the study due to health condition, loss of interesting, refusal to continue and individual circumstances” |
| Selective reporting (reporting bias) | Low risk | All expected and pre‐specified outcomes were reported |
| Other bias | Low risk | None detected |
Lee 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | The participants were patients hospitalized for the treatment of stroke in a hospital located in the Republic of Korea Sample size: 36 Inclusion criteria: more than 6 months since the onset of non‐traumatic and unilateral stroke; score of more than 24 in the Korean version of the Mini Mental State Examination; score of less than 2.26 in the Vividness of Movement Imagery Questions; ability to stand independently for more than 3 minutes; ability to walk farther than 10 meters; no orthopedic diseases that would have affected standing balance Exclusion criteria: not reported by study authors Mean (SD) age: not reported by study authors Stroke details: not reported by study authors Stroke phase: chronic |
|
| Interventions | The experimental group was given MI training for 5 minutes and proprioceptive training (involving exercises with a balance pad and a balance board) for 25 minutes, while the control group was given the same proprioceptive training for 30 minutes | |
| Outcomes | Outcomes recorded before, after and at 4 and 8 weeks after treatment Walking speed: custom systems Functional mobility: Timed Up and Go Test |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “... patients were randomly assigned to either an experimental group of 18 patients or a control group of 18 patients.” |
| Allocation concealment (selection bias) | High risk | Allocation not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Low risk | All the outcome measures analyzed in the protocol appear in the results |
| Other bias | Low risk | None detected |
Liu 2004.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Participants recruitment methods were not recorded by the study authors Sample size: 46 Inclusion criteria: 1) diagnosed as having had a first unilateral cerebral infarction as confirmed by a computed tomography scan, 2) age 60 years or older, 3) independent in performing daily activities before admission, 4) able to communicate effectively, as screened by the Cognistat 19, and 5) having given their voluntary consent Exclusion criteria: not recorded by study authors Mean (SD) age: MI group: 71.0 (SD 6.0) years; functional retraining group: 72.7 (SD 9.4) years Stroke details: all 46 were diagnosed with cerebral infarction in the middle cerebral artery region, with 1‐sided hemiplegia Stroke phase: not recorded by study authors |
|
| Interventions | Functional retraining and MI | |
| Outcomes | Outcomes recorded before and after 3 weeks of treatment Motor function: Fugl‐Meyer Assessment Scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Each patient was then randomly assigned by means of drawing lots to either the mental imagery group or the functional retraining group" |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “All clinical assessments were conducted by 2 occupational therapists who were blind to the study. Both of them received training in the administration of all the clinical instruments used in the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Three patients dropped out during the first week of the program: 1 from the mental imagery group and the other 2 from the functional retraining group. They were all readmitted to an acute hospital: 2 because of a second stroke and 1 because of renal failure" |
| Selective reporting (reporting bias) | Low risk | The statistical difference was presented for all outcomes |
| Other bias | Low risk | None detected |
Liu 2009.
| Study characteristics | ||
| Methods | Single‐blind, RCT | |
| Participants | Participants recruitment methods were not recorded by the study authors Sample size: 34 Inclusion criteria: patients were included if they had experienced a first acute stroke; sustained unilateral cerebral infarction within the middle carotid artery system; aged over 60 years; independent in their daily activities before the stroke; able to communicate effectively and were cognitively intact when assessed using a validated neurocognitive functioning test (Cognistat, Northern California Neurobehavioral Group, CA, USA) Exclusion criteria: not reported by study authors Mean (SD) age: conventional occupational therapy group: 68.1 (SD 10.5) years; MI group: 70.4 (SD 9.8) years Stroke details: all cases are ischemic Stroke phase: unspecified |
|
| Interventions | Experimental group: participants in the MI group received 1 hour of MI per treatment. The MI intervention involved the patients’ self‐reflection on their abilities and deficits: mentally imagining, then actually performing, the task. Average time spent on MI and in actual practice was 30 minutes each Control group: conventional occupational therapy: participants were given conventional occupational therapy using demonstration‐and‐practice methods to train them to perform the same 15 daily tasks All participants had 1 hour of physical therapy daily that involved mobilization, strengthening, and walking exercises. All treatment protocols were administered 5 times a week for 3 weeks (a total of 15 treatments). All patients were trained to relearn 15 daily tasks. Five tasks with a similar level of difficulty were covered each week, progressing from the easiest to the most difficult |
|
| Outcomes | Outcomes recorded before and after intervention Dependence on personal assistance: Barthel Index Motor function: Fugl‐Meyer Assessment Scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomized by drawing lots for either the MI or FR programs” |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “the assessors were blinded to the nature of the intervention” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for the lack of data not related to the result |
| Selective reporting (reporting bias) | Low risk | Study authors presented what they proposed in the methodology |
| Other bias | Low risk | None detected |
Oostra 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | All patients sustained stroke between August 2009 and June 2013. Patients were recruited via the University Hospital and from hospitals in East and West Flanders to the Rehabilitation Centre, University Hospital of Ghent MI training: 21, muscle relaxation: 23 Sample size: 44 Inclusion criteria: 1) had experienced a first‐ever stroke less than 1 year before entering the study; 2) able to walk 10 meters with minimal assistance (Functional Ambulation Category ≥ 3); 3) able to pass the Time Dependent Motor Imagery screening test; 4) between 16 and 70 years old; and 5) did not have psychiatric symptoms or any other neurological disease Exclusion criteria: not reported by study authors Mean (SD) age: MI training group: 50.3 (SD 12.8) years; muscle relaxation group: 53.7 (SD 12.0) years Stroke details: MI training group: 13 ischaemic/8 hemorrhagic; muscle relaxation group: 15 ischemic/8 hemorrhagic Stroke phase: subacute |
|
| Interventions | Experimental group: MI training: practice was performed from an internal perspective with both a visual (“viewing” themselves performing the task) and kinesthetic mode (“feeling” the experience of performing the task), with emphasis on the latter Control group: muscle relaxation: this group, on the other hand, received the same amount of muscle relaxation therapy over and above the standard rehabilitation training All patients in both groups received a standard rehabilitation program, consisting of 2 hours physical therapy and 1 hour occupational therapy daily, 5 days per week |
|
| Outcomes | Outcomes recorded at baseline and after 6 weeks of intervention Walking speed: 10 Meter Walk Test. Motor function: Lower‐extremity Fugl‐Meyer Assessment Scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “a process of blinded random number allocation” |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | All the participants had to be aware of the therapies to participate in the study, since it involved physical and cognitive exercise |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “the physician responsible for assessment of patients throughout the study remained blinded to the patients’ group allocation for the full duration of the trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “None of the participants dropped out during the study" |
| Selective reporting (reporting bias) | Low risk | Quote: “The 10‐m walk scores and lower extremity Fugl‐Meyer assessment (LE‐FMA) scores improved significantly in both groups after treatment (P < 0.001 for both values). We also found a significant group interaction effect for the 10‐m walk test (F(1,43) = 4.5, P < 0.05), revealing a significantly reduced walking duration in the MIT group compared with the MR group. There was no significant interaction between session and group for the LE‐FMA score". |
| Other bias | Low risk | None detected |
Park 2019.
| Study characteristics | ||
| Methods | Assessor‐blind RCT | |
| Participants | 79 people were recruited from the local rehabilitation hospital in Korea. Among all participants, 68 participants were finally selected as study participants. Inclusion and exclusion criteria were derived from a previous study. Inclusion criteria: 1) participants with a first‐time cerebral infarction or cerebral hemorrhage which had been ascertained by computer tomography or magnetic resonance imaging for at least 6 months, 2) participants able to have an active wrist extension at least 10, 3) Modified Ashworth Scale grade on the muscles affecting on the wrist and fingers of affected upper limb 2, 4) intact general cognitive function as determined by the Korean version of Mini Mental Examination score 24, and 5) abnormal movement imagery ability as confirmed by the Vividness of Movement Imagery Questionnaire average score 2.26 Exclusion criteria: 1) participants with artificial cardiac pacemaker, 2) Medical Research Council grade on the affected upper limb is 0, 3) affected upper limb pain determined Visual Analogue Scale 5, and 4) participants with skin lesions on the electrodes |
|
| Interventions | Experimental group: the participants in MIT EMG‐NMES group were asked to comfortably sit on the chair, place their upper limb on the desk, and flex and rotate their elbow about 90. MIT EMG‐NMES consists of 3 phases: relaxation phase, mental imagery phase, and stimulation phase. Each phase proceeded according to the menu presented on the monitor of MIT EMG‐NMES Control group: the participants in EMG‐NMES group were attached to extensor pollicis brevis and longus using 3 surface electrodes in the same way as the participants in MIT EMG‐NMES group All the sessions were conducted by an occupational therapist with 6 years of clinical experience. All participants performed 30‐minute sessions per day 5 days per week for 6 weeks |
|
| Outcomes | Upper limb function: Action Research Arm Test and Fugl–Meyer Assessment Activities of daily living: Korean version of Modified Barthel Index |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was designed accordance with CONSORT guidelines and computer‐generated by one occupational therapist who was not involved in participant recruitment" |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All assessors were blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All losses were reported by the study author |
| Selective reporting (reporting bias) | Low risk | All expected and pre‐specified outcomes were reported |
| Other bias | Low risk | None detected |
Schuster 2012.
| Study characteristics | ||
| Methods | Pilot RCT | |
| Participants | Patients were recruited from the rehabilitation centre database. Sample size: 39. Inclusion criteria: first ischemic or hemorrhagic stroke at least 3 months before, able to stand with or without a cane for at least 30 seconds on a normal hard floor, able to walk 20 meters with or without a cane or an orthosis, older than 18 years, score at least 20 on the Mini Mental State Examination, given written informed consent Exclusion criteria: joint replacements (knee, hip, shoulder), motor task limiting pain in the upper or lower body evaluated with the 11‐point Visual Analogue Scale, limited range of motion in the hip, knee, ankle joints or toes, bodyweight exceeding 90 kilograms, or had a comprised mental capacity to give written informed consent Mean (SD) age: embedded motor imagery training (EG1) = 65.8 (SD 10.2) years, added motor imagery training (EG2) = 59.7 (SD 13.0) years, control group = 64.4 (SD 6.8) years Stroke details: in total 29 patients with an ischemic and 10 patients with a hemorrhagic stroke participated in the study Stroke phase: probably subacute and chronic |
|
| Interventions | Experimental group: embedded motor imagery training (EG1) and added motor imagery training (EG2) Control group: besides receiving physiotherapy during a 30‐minute session, participants in the control group listened to a 17‐minute tape (average). The total intervention time per session was about 45 to 50 minutes. The rationale for this was to provide control group participants the same therapeutic attention as applied in EG1 and EG2 All 3 study groups performed the motor task ‘Going down, laying on the floor, and getting up again’ 10 times: during the 4 measurement events and in each of the 6 physiotherapy sessions. After reaching the stage supine lying on a mat on the floor, patients rested for a short while, typically less than 10 seconds, before getting up again in the reversed stage order All patients received 6 physiotherapy sessions over a 2‐week intervention period |
|
| Outcomes | Outcomes recorded before, after and at 2 weeks after treatment Dependence on personal assistance: Barthel index Pain, falls, and all‐cause deaths: Activities Specific Balance Confidence Scale (fear of falling) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “An independent researcher, who did not work in our institution, produced a computer‐generated randomization list (MATLAB 2007b, Mathworks Inc., USA) and sent it to the pharmacist in our institution" |
| Allocation concealment (selection bias) | Low risk | Quote: “The pharmacist created sealed envelopes including group allocation, each for one patient”; “Both (researcher, pharmacist) were not involved in the current study" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “the project leader requested the sealed envelope respective to the patient number from the pharmacist and gave it to the patient after finalization of T0. If possible, patients unsealed the envelope themselves" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Two blinded examiners performed all necessary assessments twice at baseline (BL), before intervention (T0), after intervention (T1), and after a two‐week follow‐up (FU) period" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 patient excluded from analysis ‐ reason: patient only received 2 of 6 intervention sessions Losses to follow‐up = 3 |
| Selective reporting (reporting bias) | Low risk | The study author presented that which was proposed in the methodology |
| Other bias | Low risk | None detected |
Suvadeep 2017.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Methods of recruitment were not reported by the study authors Sample size: 30 Inclusion criteria: patients with first episode of unilateral stroke, confined to the territory of middle cerebral artery, with hemiparesis, 3 to 12 months post stroke, men and women aged 50 to 65 years, Brunnstrom recovery stage 2 and above, with no severe cognitive deficit i.e. Mini Mental State Examination Score > 24.9, ability to walk with supervision and/or aids > 10 meters, able to understand and follow simple verbal instructions Exclusion criteria: patients with unilateral neglect, apraxia, impaired vision or aphasia, any diagnosed case of psychiatric disorder, any diagnosed case of neurological, musculoskeletal, cardiopulmonary disorder Mean (SD) age: not reported by study authors Stroke details: not reported by study authors Stroke phase: subacute |
|
| Interventions | Experimental group: MI group received 30 minutes of MI therapy in addition to 30 minutes of conventional therapy which included neurodevelopmental facilitation technique, stretching and gait training Control group: the mirror group received 30 minutes of MI therapy in addition to 30 minutes of conventional therapy which included neurodevelopmental facilitation technique, stretching and gait training |
|
| Outcomes | Outcomes recorded at baseline, post‐intervention at 1 month from treatment conclusion Walking speed: 10 Meter Walk Test Motor function: Fugl‐Meyer Assessment Lower‐ Extremity Scale Score Dependence on personal assistance: Motor Assessment Scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “They were randomly assigned to either the Group‐A i.e. Mirror Group (N=15) or the Group B i.e. Mental Imagery (N = 15).” |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Neither exclusions nor lost of data were reported |
| Selective reporting (reporting bias) | Low risk | Study authors presented what they had proposed in the methodology |
| Other bias | Low risk | None detected |
Verma 2011.
| Study characteristics | ||
| Methods | Randomized, controlled, assessor‐blinded trial | |
| Participants | Potential participants were identified from an inpatient neurology ward. The investigator (a neuro physician) assessed the participants to determine their eligibility for the study Sample size: 30 Inclusion criteria: 1) first episode of unilateral stroke with hemiparesis during the last month, 2) Functional Ambulation Classification level II and above, 3) ability to understand instructions (Hindi Mental State Examination > 24), 4) ambulatory before stroke, 5) ability to cope with the intensive training program, 6) ability for mental imaging (Movement Imagery Questionnaire ‐ revised second version ≥ 25), and 7) National Institutes of Health Stroke Scale score less than 14 Exclusion criteria: 1) history of any other neurological pathology such as Parkinson disease and epilepsy, 2) conditions affecting balance, 3) neglect, 4) dementia, 5) impaired vision, 6) impaired conscious level, 7) concomitant medical illness, 8) musculoskeletal conditions affecting lower limbs, 9) cardiovascular instability (resting systolic blood pressure > 200 mm Hg and resting diastolic blood pressure > 100 mm Hg), and 10) serious cardiac conditions (hospitalization for heart disease within 3 months, active angina, serious cardiac arrhythmias, hypertrophic cardiomyopathy, severe aortic stenosis) Mean (SD) age: control group: 55.07 (SD 6.80) years, experimental group: 53.27 (SD 8.53) years Stroke details: control group: 12 ischemic/3 hemorrhagic; experimental group: 11 ischemic/4 hemorrhagic Stroke phase: subacute |
|
| Interventions | Experimental group: task‐oriented circuit class training with MI. The participants were familiarized with MI during a pre‐intervention session and educated about the basic imagery principles. MI program of 15 to 25 minutes was given on an individual basis. Participants were also asked to keep a diary of their MI practice to measure the rehearsal frequency after each treatment session. The program included different workstations and was intended to improve the meaningful tasks related to walking competency, such as balance control, stair walking, turning, transfers, and speed walking Control group: Bobath’s neurodevelopmental technique. Participants in the control group participated in the conventional post‐stroke lower extremity rehabilitation program based on the Bobath neurodevelopmental technique. The control group program was matched for duration, number, and frequency of the sessions with the experimental group program |
|
| Outcomes | Outcomes recorded at baseline, post‐intervention and at 6 weeks from treatment conclusion Walking speed: 10 Meter Walk Test Dependence on personal assistance: Barthel Index and Functional Ambulatory Category Walking endurance: 6 Minute Walk Test Functional mobility: Rivermead Visual Gait Assessment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “the patients were randomly assigned to either the experimental group (n = 15) or the control group (n = 15) using computer‐generated random numbers” |
| Allocation concealment (selection bias) | Low risk | Quote: “The intervention assignments were enclosed in sealed envelopes, which were opaque and sequentially numbered. A resident physician at the study site conducted the random‐number program. However, the resident physician was blinded to the research protocol and was not involved in the trial" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The subjects were blinded for intervention of interest" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missed data balanced between groups |
| Selective reporting (reporting bias) | Low risk | Quote: “Statistically significant differences were observed in the changes between the groups at post and follow‐up assessment for FAC, RVGA, cadence, Speed‐C, and 6MWT (F: P = .001–.049; U: P = .001). There was a significant difference of 1 median score between the groups both for FAC across the assessments. Further analysis was done using the Kaplan–Meier curve (survival analysis) for FAC level 5 as an event of interest and day of achievement (day 42 as the last day of observation). Seven (46.6%) subjects in the experimental group reached the FAC level 5 (by day 31), although only 2 (13.3%) subjects in the control group could reach the level (by day 39) (Mantel‐Cox: P < .036)” |
| Other bias | Low risk | None detected |
Yan 2013.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Patients admitted at the Department of Rehabilitation Medicine from January 2012 to October 2012 were selected Sample size: 60 Inclusion criteria: first onset of stroke, in line with the diagnostic criteria established by the 4th National Cerebrovascular Disease Conference, diagnosis of cerebral infarction or cerebral hemorrhage by head CT or MRI, Brunnstrom staging 2 to 3, patients after medical and surgical symptomatic treatment, vital signs stable, clear consciousness, no obvious cognitive impairment, no sensory aphasia. All cases ranged from 14 days to 3 months without bone and joint, muscle disease and heart, liver and kidney lesions Exclusion criteria: not reported by the study authors Mean (SD) age: joint training group: 53.6 (SD 11.5) years; passive training group: 50.5 (SD 12.8) years Stroke details: not reported by the study authors Stroke phase: not reported by the study authors |
|
| Interventions | Passive training group and joint training group: both groups received conventional rehabilitation therapy. Passive training group patients were given tactiles foot dorsiflexion training, at the same time, joint training group patients were given imagined foot dorsiflexion training and tactiles foot dorsiflexion training, continuous for 6 weeks | |
| Outcomes | Outcomes recorded before and after treatment Dependence on personal assistance: Barthel Index Motor function: Fugl‐Meyer Assessment ‐ Lower Extremity |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Stroke patients with lower limb hemiplegia were randomly divided into passive training group and joint training group”. |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no exclusions or loss of participants or data |
| Selective reporting (reporting bias) | Low risk | Statistical difference was presented for all outcomes |
| Other bias | Low risk | None detected |
Zhang 2013.
| Study characteristics | ||
| Methods | Cross‐over experimental study | |
| Participants | Participants with first stroke patients admitted to the Rehabilitation Department of Ruijin Hospital Branch of Shanghai Inclusion criteria: 1) meets the diagnostic criteria formulated by the Fourth National Conference on Cerebrovascular Diseases in 1995 (and confirmed by CT and/or MRI examination of the brain; 2) first onset, and the course of disease is < 6 months, unilateral paralysis, neurological symptoms are stable; 3) Kinesthetic and Visual Imagery Questionnaire > 25 points, which can complete the evaluation and treatment of the entire treatment cycle; 4) disease diagnosis is clear, vital signs are stable, and disease symptoms are no longer progression over 48 hours; 5) lower limb hemiplegia (lower limb muscle strength 33) Exclusion criteria: 1) severe pain or stoma in the lower limb; 2) cognitive dysfunction (simple intelligence points, points 2586 or more, need to be able to walk, guide, Mini Mental State Examination) or unqualified, sensory aphasia; 3) accompanied by difficulty in understanding, dementia, severe 1.5 heart, liver, renal insufficiency and mental illness |
|
| Interventions | Experimental group: Group A received routine training combined with MI therapy in the first stage, and only routine training in the third stage Control group: Group B only conducted routine training in the first stage Both groups underwent neurological drug treatment and routine rehabilitation training, including bed posture correction, upper limb function training, sitting, standing balance function training, standing, sitting training, physical factor treatment, walking training and daily life activity training, etc |
|
| Outcomes | 1) Fugl‐Meyer Motor Function Scale ‐ Lower Limb 2) Tineti Gait Assessment Scale (ability to walk) 3) Functional Ambulation Category |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “Thirty‐six patients were odd‐numbered according to the order of admission, and even numbers were divided into groups A and B, with 18 in each group”. |
| Allocation concealment (selection bias) | High risk | Quote: “Thirty‐six patients were odd‐numbered according to the order of admission, and even numbers were divided into groups A and B, with 18 in each group”. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “A total of 38 patients met the inclusion criteria, of which 1 refused motor imaging therapy, 1 was lost to follow‐up in the trial, and 36 patients were finally included in the statistics". |
| Selective reporting (reporting bias) | Low risk | All expected and pre‐specified outcomes were reported |
| Other bias | Low risk | None detected |
Zhu 2017.
| Study characteristics | ||
| Methods | RCT | |
| Participants | All 90 patients were from the Acupuncture and Rehabilitation Department of Zhongda Hospital affiliated to Southeast University, from October 2015 to September 2016, and the inpatients of the Department of Neurology of Nanjing Brain Hospital affiliated to Nanjing Medical University Sample size: 87 Inclusion criteria: patients with 'cerebral infarction' according to the 'Diagnostic Points for Various Cerebrovascular Diseases' adopted by the Fourth National Conference of Cerebral Vascular Diseases of the Chinese Medical Association, and confirmed by CT or MRI Exclusion criteria: 1) transient ischemic attack, lacunar infarction without hemiplegic sequelae; 2) relapses, multiple and large area cerebral infarction; 3) patients treated with thrombolytic therapy; 4) with Temporal Slope Syndrome (Pusher Synthesis), 5) patients with unilateral neglect; patients with muscular disorders bone and joint disease, or severe primary disease of the heart, lung, liver, kidney, hematopoietic system and endocrine system, as well as patients with psychosis and cancer; 6) patients with bilateral paralysis and complete paralysis Mean (SD) age: comprehensive group: 66 (SD 10) years; rehabilitation group: 63 (SD 9) years; electroacupuncture group: 67 (SD 11) years Stroke details: not reported by the study authors Stroke phase: not reported by the study authors |
|
| Interventions | Rehabilitation group: patients in the rehabilitation group were treated with regular care, medication and rehabilitation training for 20 minutes each time Electroacupuncture group; patients in the electroacupuncture group were treated mainly with electroacupuncture. An electroacupuncture device was connected for 30 minutes after rehabilitation training Comprehensive group: patients in the comprehensive group were treated with electroacupuncture as the electroacupuncture group and MI therapy. MI therapy was performed 30 minutes after electroacupuncture treatment and lasted 20 minutes Patients in all 3 groups received routine care and medication for cerebral infarction as well as regular rehabilitation (rehabilitation training, 20 minutes each time) The treatment was given once a day, 5 treatments per week, and in total 4‐week treatment was performed |
|
| Outcomes | Outcomes recorded before and after treatment Dependence on personal assistance: Barthel index |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Ninety patients with hemiplegic cerebral infarction were randomly divided into a rehabilitation group, an EA group and a comprehensive group, 30 patients in each one" |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Three cases did not finish the trial and finally 87 cases were included into analysis, including 30 cases in the rehabilitation group, 29 cases in the EA group and 28 cases in the comprehensive group" |
| Selective reporting (reporting bias) | Low risk | The statistical difference was presented for all outcomes |
| Other bias | Low risk | None detected |
CT: computed tomography; EMG NMES: electromyogram‐triggered neuromuscular electrical stimulation; MI: motor imagery; MIT‐EMG NMES: motor imagery training and electromyogram‐triggered neuromuscular electrical stimulation; MRI: magnetic resonance imaging; RCT: randomized controlled trial; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bae 2015 | Not an RCT |
| Bang 2013 | The intervention was not MI |
| Bovend'Eerdt 2010 | Some participants had other neurological conditions and did not present isolated stroke data |
| Choi 2013 | Not an RCT |
| Dunsky 2008 | Not an RCT |
| Ghanjal 2014 | The intervention was not MI |
| Guttman 2012 | Not an RCT |
| Hatwar 2019 | Not an RCT |
| Hwang 2010 | Not an RCT |
| Ietswaart 2011 | The effects were observed in upper limb |
| Ji 2015 | The intervention was not MI |
| Kim 2011 | There was no control group |
| Kim 2012 | The intervention was not MI |
| Kim 2013b | Did not assess outcomes of interest |
| Kumar 2013b | Did not assess outcomes of interest |
| Lee 2016 | The intervention was not MI |
| Malouin 2004 | Did not assess outcomes of interest |
| Malouin 2009 | Did not assess outcomes of interest |
| Mihara 2012 | Did not assess outcomes of interest |
| Mohan 2013 | The intervention was not MI |
| Page 2001 | The effects were observed in upper limb |
| Page 2005 | Did not assess outcomes of interest |
| Page 2007 | The effects were observed in upper limb |
| Page 2009 | The effects were observed in upper limb |
| Park 2013 | The intervention was not MI |
| Park 2015 | The intervention was not MI |
| Pheung‐phrarattanatrai 2015 | Not an RCT |
| Saito 2013 | Did not assess outcomes of interest |
| Schuster 2009 | Did not assess outcomes of interest |
| Sun 2011 | Not an RCT |
| Sütbeyaz 2007 | The intervention was not MI |
| Tyson 2015 | The intervention was not MI |
MI: motor imagery; RCT: randomized controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
Zhang 2014.
| Methods | Cross‐control design |
| Participants | A total of 40 hospitalized patients with hemiplegia after stroke who met the inclusion criteria were selected and divided into the group A (n = 20) and group B (n = 20) |
| Interventions | The experiment was divided into phase I (week 1 to 3), phase II (week 4 to 5), and phase III (week 6 to 8). For group A, patients were treated with routine rehabilitation training combined with Tai‐Ji exercise MI therapy at phase I and routine training at phase III. For group B, patients were treated with routine rehabilitation training at phase I and routine training combined with Tai‐Ji exercise MI therapy at the phase III. Phase II was the washout period and patients were not treated with routine rehabilitation training or MI therapy during phase II |
| Outcomes | The walk function of patients was evaluated by the lower extremity part of the Fugl‐Meyer Motor Assessment, Functional Ambulation Category, and Tinetti Gait Assessment before the experiment and 3, 5, and 8 weeks after the intervention |
| Notes |
MI: motor imagery
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1800019581.
| Study name | Effects of motor imagery training on lower limb motor function of patients with chronic stroke |
| Methods | Not reported |
| Participants | Patients after stroke |
| Interventions | Not reported |
| Outcomes | Not reported |
| Starting date | January 2017 |
| Contact information | yxj3913@163.com |
| Notes |
ChiCTR‐IOR‐16008137.
| Study name | Graded motor imagery based on mirror neuron on rehabilitative training for stroke patients: a BOLD‐fMRI study |
| Methods | Inclusion criteria
Exclusion criteria
|
| Participants | 30 patients after stroke |
| Interventions | Experimental group: routine rehabilitative training + graded MI training Control group: routine rehabilitative training |
| Outcomes |
|
| Starting date | June 2014 |
| Contact information | tuwenzhan@163.com |
| Notes |
ISRCTN33487341.
| Study name | Mental practice‐based rehabilitation training aimed at improving arm function and performance of daily activities in stroke: a randomized clinical trial |
| Methods | A multi‐centre, single‐blinded, placebo‐controlled randomized trial |
| Participants | 160 patients after stroke |
| Interventions | Intervention: mental practice training: training program 3 times a day (10 to 15 minutes) during 10 weeks in additional to therapy as usual. The training is guided by CD‐Rom. Different training tasks are available depending on the functional level of the patient. Patients can practice at home, in the hospital, or in a rehabilitation centre. An occupational therapist will coach during the program Control group: patients will be instructed to practice additional bimanual upper extremity techniques based on conservative neurodevelopmental principles. Training intensity is 3 times a day during 10 weeks |
| Outcomes | Upper extremity functioning assessed on activity level:
Upper extremity functioning assessed on impairment and participation level:
Both critical and important outcome measures will be assessed at baseline, after 10 weeks and 6 and 12 months |
| Starting date | January 2008 |
| Contact information | j.verbunt@srl.nl |
| Notes |
NCT01993563.
| Study name | Graded motor imagery for patients within a year after stroke |
| Methods | Interventional (clinical trial) |
| Participants | Patients after stroke |
| Interventions | Experimental group: graded MI program includes three steps: implicit MI (IMI); explicit MI (EMI); mirror box therapy (MT) IMI included a training based on hand laterality discrimination tasks. During these tasks 60 pictures of right and left hands are projected randomly on a 15" screen. Patients are asked to choose whether the images seen are right or left and therefore to click respectively the right or the left button on a mouse EMI training consists of imagining a movement without actual performing it. It will be introduced during IMI's last 2 sessions and gradually enhanced increasing the complexity of motor skills to be imagined. The therapist shows or explains in detail the movements the patient has to mentally rehearse MT treatments will start with simply watching the unaffected hand in the mirror and increased toward functional movement. When possible, gentle movement with the affected hand will be encouraged behind the reflecting part of the mirror Control group: patients will undergo to a standard treatment, that is thought to be the best option for that specific patient. In this hospital, treatment options include motor training, functional training, occupational therapy, bilateral arm training or motor treatment using virtual reality devices |
| Outcomes |
|
| Starting date | September 2014 |
| Contact information | andrea.turolla@ospedalesancamillo.net/ |
| Notes |
NCT03436810.
| Study name | Effect of structured progressive task‐oriented circuit class training with motor imagery on gait in stroke |
| Methods | RCT |
| Participants | 40 patients with stroke from the departments of physical medicine and rehabilitation, North Okkalapa General Hospital, East General Hospital and National Rehabilitation Hospital, Yangon, Myanmar will participate in this study Inclusion criteria: first stroke and paresis on unilateral side of the body, aged 18 to 75 years, post‐stroke duration 3 to 12 months, middle cerebral artery involvement, ability to walk at least 10 meters with or without using assistance, Functional Ambulation Category ≥ 3, Mini Mental State Examination ≥ 24, National Institutes of Health Stroke Scale (NIHSS) < 14, MI ability by the Kinesthetic and Visual Imagery Questionnaire (KVIQ‐10) ≥ 3 Exclusion criteria: unstable cardiopulmonary problems, other neurological conditions such as Parkinson's disease, Alzheimer's disease, or epilepsy, orthopedic and rheumatologic disorders with weight bearing pain, unable to communicate or unable to follow commands, serious cardiac conditions, patients with unilateral spatial neglect, patients with ataxic movement |
| Interventions | Experimental group: receives training programs of MI for 25 minutes and task‐oriented circuit class training for 65 minutes. Overall duration of program session will be 90 minutes. Training for 3 times a week over duration of 4 weeks Control group: receives programs of health education for 25 minutes and task‐oriented circuit class training for 65 minutes. Overall duration will be 90 minutes. They will be trained 3 times a week over a duration of 4 weeks |
| Outcomes |
|
| Starting date | February 2018 |
| Contact information | Nilar Aung: nilaraun@gmail.com |
| Notes | Date accessed: November 2018 |
NCT04086004.
| Study name | Dual task balance training with additional motor imagery practice in stroke |
| Methods | RCT |
| Participants | 34 participants after stroke |
| Interventions | Group I: experimental MI: this group will receive dual task balance training for 30 minutes/day with additional mental imagery for 10 minutes/day, 3 days/week, for a period of 8 weeks Group II: control dual task training: this group will receive dual task balance training for 40 minutes for 3 days/ week for 8 weeks |
| Outcomes |
|
| Starting date | February 2020 |
| Contact information | imran.amjad@riphah.edu.pk |
| Notes |
NCT04215679.
| Study name | Effect of motor imagery with virtual reality in patients with stroke |
| Methods | RCT |
| Participants | 36 participants after stroke |
| Interventions | Group 1: 3‐dimensional immersive virtual reality (IVR) application In this group, individuals will be included in a game program that will last for 3 days a week for a total of 6 weeks and 45 minutes a day. Individuals will use the IVR to rehabilitate functions that are frequently used in daily life through task‐oriented games. The IVR device will be placed on the head of the individual by closing the eyes of the individual and the Leap Motion device will be used to enable individuals to see their own hands in a virtual reality environment. In order to ensure the safety of individuals, practices shall be carried out with the individual sitting in the chair and leaning against the back. A total of 3 different games will be used for upper extremity function, each game will be 15 minutes and the total session time will be 45 minutes Group 2: MI MI will be performed with the eyes closed. In addition, for the safety of the individual, the individual will sit comfortably in a chair in a quiet environment and sit back. In the MI group, individuals will be shown videos of the 3 games for 2 times in the IVR group and will be asked to imagine that they perform the same functions in the IVR games. The motor imagery will be 3 days a week for a total of 6 weeks and 45 minutes per day (including rest periods) Group 3: conventional physiotherapy Individuals in this group will be randomly recruited from hospitalized stroke volunteers. Since these individuals receive routine rehabilitation 5 days a week, they will be evaluated at the beginning and end of 18 sessions over a total period of 6 weeks. Conventional physiotherapy will include normal joint movements, muscle strengthening exercises, balance and mobility exercises, and exercises to improve daily life activity |
| Outcomes |
|
| Starting date | December 2020 |
| Contact information | avciseb@hotmail.com |
| Notes |
MI: motor imagery; MRI: magnetic resonance imaging; RCT: randomized controlled trial
Differences between protocol and review
It was impossible to perform the meta‐analyses for the outcome 'dependence on personal assistance' due to the lack of information in the included studies. It was also impossible to conduct the meta‐analyses for 'walking endurance' due to an insufficient number of studies. It was also impossible to perform the meta‐analysis for adverse events because the studies neither reported this outcome nor reported the adverse events. We could not conduct the follow‐up analyses because we did not have enough quantitative studies to include, or the included studies did not perform this assessment.
It was impossible to carry out subgroup analyses regarding the type of stroke in all outcomes because not all included studies provided this information or there was a lack of available data.
Other analyses mentioned in the protocol could not be performed for the same above‐mentioned reasons; however, we sought to complete the analyses of the results in their entirety. In addition, we performed a subgroup analysis considering the form of application of MI (visual imagery, kinesthetic imagery, or both the visual and kinesthetic imageries) for all outcomes (walking speed, motor function, and functional mobility), which was not stated in our original protocol.
Contributions of authors
Stephano Silva: conducted the review, assessed the quality of the evidence, performed statistical analyses, interpreted the results, and was in charge of writing the review.
Lorenna RDM Borges: helped in methodological planning and in the statistical analysis.
Lorenna Santiago: study selection, data extraction and assessment of risk of bias.
Larissa Lucena: study selection, data extraction and assessment of risk of bias.
Ana Raquel Rodrigues Lindquist: helped in methodological planning.
Tatiana Ribeiro: was the reviewing judge, assessed evidence quality, helped interpret the results, guided in statistical analysis and corrected the review.
All authors approved the protocol and the final review.
Sources of support
Internal sources
Department of Physical Therapy, Federal University of Rio Grande do Norte, Brazil
External sources
-
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil
This work was supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior ‐ Brazil (CAPES) [Financial code 001].
Declarations of interest
Stephano Silva: none known.
Lorenna RDM Borges: none known.
Lorenna Santiago: none known.
Larissa Lucena: none known.
Ana Raquel Rodrigues Lindquist: none known.
Tatiana Ribeiro: none known.
New
References
References to studies included in this review
Braun 2012 {published data only}
- Braun S, Beurskens A, Kleynen M, Oudelaar B, Schols J, Wade D. A multicenter randomized controlled trial to compare subacute ‘Treatment as Usual’ with and without mental practice among persons with stroke in Dutch nursing homes. Journal of the American Medical Directors Association 2012;13:1-7. [DOI] [PubMed] [Google Scholar]
Cho 2012 {published data only}
- Cho H-Y, Lee G-C. Effects of motor imagery training on balance and gait abilities in post-stroke patients: a randomized controlled trial. Clinical Rehabilitation 2012;27:675-80. [DOI] [PubMed] [Google Scholar]
Dickstein 2013 {published data only}
- Dickstein R, Deutsch J, Yoeli Y, Kafr M, Falash F, Dunsky A, et al. Effects of integrated motor imagery practice on gait of individuals with chronic stroke: a half- crossover randomized study. Archives of Physical Medicine and Rehabilitation 2013;94:2119-25. [DOI] [PubMed] [Google Scholar]
Dickstein 2014 {published data only}
- Dickstein R, Levy S, Shefi S, Holtzman S, Peleg S, Vatine J-j. Motor imagery group practice for gait rehabilitation in individuals with post-stroke hemiparesis: A pilot study. NeuroRehabilitation 2014;34(2):267-276. [DOI] [PubMed] [Google Scholar]
Gupta 2017 {published data only}
- Gupta A. Motor imagery in gait and balance rehabilitation for post stroke hemiparesis. Global Journal of Research Analysis 2017;6:7-11. [Google Scholar]
Kim 2013a {published data only}
- Kim J-H, Lee B-H. Action observation training for functional activities after stroke: a pilot randomized controlled trial. NeuroRehabilitation 2013;33:565-74. [DOI] [PubMed] [Google Scholar]
Kumar 2013a {published data only}
- Kumar V, Chakrapani M, Shennoy U, Suresh B. Effects of mental practice on functional mobility in ambulant stroke subjects: a pilot randomized clinical trial. Cerebrovascular Diseases 2013;36:94. [Google Scholar]
Kumar 2016 {published data only}
- Kumar V, Chakrapani M, Kedambadi R. Motor imagery training on muscle strength and gait performance in ambulant stroke subjects: a randomized clinical trial. Journal of Clinical and Diagnostic Research 2016;10:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2010 {published data only}
- Lee W, Lee C, Chang S. Effectiveness of imagery training of functional training on the balance and gait in stroke patients. Coaching Skills Development Center 2010;12:201-11. [Google Scholar]
Lee 2011 {published data only}
- Lee G, Song C, Lee Y, Cho H, Lee S. Effects of motor imagery training on gait ability of patients with chronic stroke. Journal of Physical Therapy Science 2011;23:197–200. [Google Scholar]
Lee 2015 {published data only}
- Lee H, Kim H, Ahn M. Effects of proprioception training with exercise imagery on balance ability of stroke patients. Journal of Physical Therapy Science 2015;27:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2004 {published data only}
- Liu K, Chan C, Lee T, Hui-Chan C. Mental imagery for promoting relearning for people after stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2004;85:1403-8. [DOI] [PubMed] [Google Scholar]
Liu 2009 {published data only}
- Liu KP. Use of mental imagery to improve task generalisation after a stroke. Hong Kong Medical Journal 2009;15(3 Suppl 4):37-41. [PubMed] [Google Scholar]
Oostra 2015 {published data only}
- Oostra K, Oomen A, Vanderstraeten G, Vingerhoets G. Influence of motor imagery training on gait rehabilitation in sub-acute stroke: a randomized controlled trial. Journal of Rehabilitation Medicine 2015;47:204-9. [DOI] [PubMed] [Google Scholar]
Park 2019 {published data only}
- Park J-H. Effects of mental imagery training combined electromyogram-triggered neuromuscular electrical stimulation on upper limb function and activities of daily living in patients with chronic stroke: a randomized controlled trial. Disability and Rehabilitation 2019 4 Apr [Epub ahead of print]. [DOI: 10.1080/09638288.2019.1577502] [DOI] [PubMed]
Schuster 2012 {published data only}
- Schuster C, Butler J, Andrews B, Kischka U, Ettlin T. Comparison of embedded and added motor imagery training in patients after stroke: results of a randomised controlled pilot trial. Trials 2012;13:1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suvadeep 2017 {published data only}
- Suvadeep D, Charu C, Mehta DD, Mehndiratta MM. Comparison between mirror therapy and mental imagery in improving ankle motor recovery in sub acute stroke patients. Indian Journal of Physiotherapy and Occupational Therapy 2017;11:169-73. [Google Scholar]
Verma 2011 {published data only}
- Verma R, Arya K, Garg R, Singh T. Task-oriented circuit class training program with motor imagery for gait rehabilitation in poststroke patients: a randomized controlled trial. Topics in Stroke Rehabilitation 2011;18:620-32. [DOI] [PubMed] [Google Scholar]
Yan 2013 {published data only}
- Yan L, Mei F, Ping L. Influence of motor imagery therapy combined with passive foot dorsiflexion training on lower limb motor function rehabilitation in stroke patients. Chinese Nursing Research 2013;27:970-2. [Google Scholar]
Zhang 2013 {published data only}
- Zhang H-Y, Pazi L-Y, Zhang Y-Q, Zha L-S, Xu Y, Yuan X-l, et al. Effect of motor imagery therapy on walking ability in patients with stroke and hemiplegia. Journal of Shanghai (Medical Science) 2013;33:1225-30. [Google Scholar]
Zhu 2017 {published data only}
- Zhu F, Gao J, Gao R, He Y, Liu L, Ai B. Clinical efficacy of electroacupuncture combined with motor imagery therapy on hemiplegic cerebral infarction. Zhongguo Zhen Jiu 2017;37:927-31. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bae 2015 {published data only}
- Bae Y-H, Ko Y-J, Ha H-G, Ahn S-Y, Lee W-H, Lee S-M. An efficacy study on improving balance and gait in subacute stroke patients by balance training with additional motor imagery: a pilot study. Journal Physical Therapy Science 2015;27:3245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bang 2013 {published data only}
- Bang D, Shin W, Kim S, Choi J. The effects of action observational training on walking ability in chronic stroke patients: a double-blind randomized controlled trial. Clinical Rehabilitation 2013;27:1118-25. [DOI] [PubMed] [Google Scholar]
Bovend'Eerdt 2010 {published data only}
- Bovend'Eerdt T, Dawes H, Sackley C, Izadi H, Wade D. An integrated motor imagery program to improve functional task performance in neurorehabilitation: a single-blind randomized controlled trial.. Archives of Physical Medicine and Rehabilitation 2010;91:939-46. [DOI] [PubMed] [Google Scholar]
Choi 2013 {published data only}
- Choi B-R, Hwang S-J, Lee H-W, Kang S-Y, Jeon H-S. Group locomotor imagery training-combined knowledge of performance in community-dwelling individuals with chronic stroke: a pilot study. Physical Therapy Korea 2013;20:74-80. [Google Scholar]
Dunsky 2008 {published data only}
- Dunsky A, Dickstein R, Marcovitz E, Levy S, Deutsch J. Home-based motor imagery training for gait rehabilitation of people with chronic poststroke hemiparesis. Archives of Physical Medicine and Rehabilitation 2008;89:1580-8. [DOI] [PubMed] [Google Scholar]
Ghanjal 2014 {published data only}
- Ghanjal A, Torkaman G, Ghabaee M, Ebrahimi E, Motaqhey M. Effect of action observation and imitation on improving the functional activities indices in hemiplegic patients based on mirror neurons theory. Journal of Mazandaran University of Medical Sciences 2014;24:136-50. [Google Scholar]
Guttman 2012 {published data only}
- Guttman A, Burstin A, Brown R, Bril S, Dickstein R. Motor imagery practice for improving sit to stand and reaching to grasp in individuals with poststroke hemiparesis. Topics in Stroke Rehabilitation 2012;19:306-19. [DOI] [PubMed] [Google Scholar]
Hatwar 2019 {published data only}
- Hatwar N, Suchetha P, Kumar D. Combined effectiveness of mirror therapy and motor imagery on gait in stroke patients. International Journal of Current Research and Review 2019;11:5-10. [Google Scholar]
Hwang 2010 {published data only}
- Hwang S, Jeon H-S, Yi C-H, Kwon O-Y, Cho S-H, You S-H. Locomotor imagery training improves gait performance in people with chronic hemiparetic stroke: a controlled clinical trial. Clinical Rehabilitation 2010;24:514-22. [DOI] [PubMed] [Google Scholar]
Ietswaart 2011 {published data only}
- Ietswaart M, Johnston M, Dijkerman H, Joice S, Scott C, MacWalter R, et al. Mental practice with motor imagery in stroke recovery: randomized controlled trial of efficacy. Brain 2011;134:1373-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ji 2015 {published data only}
- Ji S-G, Kim M-K. The effects of mirror therapy on the gait of subacute stroke patients: a randomized controlled trial. Clinical Rehabilitation 2015;29(4):348-54. [DOI] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim J-S, Oh D-W, Kim S-Y, Choi J-D. Visual and kinesthetic locomotor imagery training integrated with auditory step rhythm for walking performance of patients with chronic stroke. Clinical Rehabilitation 2011;25:134-45. [DOI] [PubMed] [Google Scholar]
Kim 2012 {published data only}
- Kim J-S, Kim K. Clinical feasibility of action observation based on mirror neuron system on walking performance in post stroke patients. Journal of Physical Therapy Science 2012;24:597-9. [Google Scholar]
Kim 2013b {published data only}
- Kim J-H, Chung E-J, Lee B-H. A study of analysis of the brain wave with respected to action observation and motor imagery: a pilot randomized controlled trial. Journal of Physical Therapy Science 2013;25:779-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 2013b {published data only}
- Kumar V, Chakrapani M, Shennoy U. Effects of mental practice on functional mobility and quality of life in ambulant stroke subjects–at pilot randomized controlled trial. International Journal of Scientific Research 2013;2:434-7. [Google Scholar]
Lee 2016 {published data only}
- Lee D, Lee G, Jeong J. Mirror therapy with neuromuscular electrical stimulation for improving motor function of stroke survivors: a pilot randomized clinical study. Technology and Health Care 2016;24:503-11. [DOI] [PubMed] [Google Scholar]
Malouin 2004 {published data only}
- Malouin F, Belleville S, Richards C, Desrosiers J, Doyon J. Working memory and mental practice outcomes after stroke. Archives of Physical Medicine and Rehabilitation 2004;85:177-83. [DOI] [PubMed] [Google Scholar]
Malouin 2009 {published data only}
- Malouin F, Richards C, Durand A, Doyon J. Added value of mental practice combined with a small amount of physical practice on the relearning of rising and sitting post-stroke: a pilot study. Journal of Physical Therapy Science 2009;33:195-202. [DOI] [PubMed] [Google Scholar]
Mihara 2012 {published data only}
- Mihara M, Miyai I, Hattori N, Hatakenaka M, Yagura H, Kawano T, et al. Neurofeedback using real-time near-infrared spectroscopy enhances motor imagery related cortical activation. PLoS One 2012;7:322-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohan 2013 {published data only}
- Mohan U, Babu K, Kumar V, Suresh V, Misri K, Chakrapani M. Effectiveness of mirror therapy on lower extremity motor recovery, balance and mobility in patients with acute stroke: a randomized sham-controlled pilot trial. Annals of Indian Academy of Neurology 2013;16:634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2001 {published data only}
- Page J, Levine P, Sisto S, Johnston V. A randomized efficacy and feasibility study of imagery in acute stroke. Clinical Rehabilitation 2001;15:233-40. [DOI] [PubMed] [Google Scholar]
Page 2005 {published data only}
- Page J, Levine P, Leonard A. Effects of mental practice on affected limb use and function in chronic stroke. Archives of Physical Medicine and Rehabilitation 2005;86:399-402. [DOI] [PubMed] [Google Scholar]
Page 2007 {published data only}
- Page S, Levine P, Leonard A. Mental practice in chronic stroke: results of a randomized, placebo-controlled trial. Stroke 2007;38:1293-7. [DOI] [PubMed] [Google Scholar]
Page 2009 {published data only}
- Page S, Levine P, Khoury J. Modified constraint-induced therapy combined with mental practice: thinking through better motor outcomes. Stroke 2009;40:551-4. [DOI] [PubMed] [Google Scholar]
Park 2013 {published data only}
- Park C, Kang K. The effects of additional action observational training for functional electrical stimulation treatment on weight bearing, stability and gait velocity of hemiplegic patients. Journal of Physical Therapy Science 2013;25:1173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2015 {published data only}
- Park E, Hwangbo G. The effects of action observation gait training on the static balance and walking ability of stroke patients. Journal of Physical Therapy Science 2015;27:341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pheung‐phrarattanatrai 2015 {published data only}
- Pheung-phrarattanatrai A, Bovonsunthonchai S, Heingkaew V, Prayoonwiwat N, Chotik-anuchit S. Improvement of gait symmetry in patients with stroke by motor imagery. Journal of the Medical Association of Thailand 2015;98:113-8. [PubMed] [Google Scholar]
Saito 2013 {published data only}
- Saito M, Asaka T, Fukushima J. Effects of motor imagery combined with repetitive task practice on sitting balance of hemiplegic patients. Journal of Physical Therapy Science 2013;25:183-8. [Google Scholar]
Schuster 2009 {published data only}
- Schuster C, Butler J, Andrews B, Kischka U, Ettlin T. Comparison of embedded and added motor imagery training in patients after stroke: study protocol of a randomised controlled pilot trial using a mixed methods approach. Trials 2009;10:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2011 {published data only}
- Sun H, Xiang Y, Yang M. Neurological rehabilitation of stroke patients via motor imaginary-based brain-computer interface technology. Neural Regeneration Research 2011;6:2198-202. [Google Scholar]
Sütbeyaz 2007 {published data only}
- Sütbeyaz S, Yavuzer G, Sezer N, Koseoglu BF. Mirror therapy enhances lower-extremity motor recovery and motor functioning after stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2007;88:555-9. [DOI] [PubMed] [Google Scholar]
Tyson 2015 {published data only}
- Tyson S, Wilkinson J, Thomas N, Selles R, McCabe C, Tyrrell P, et al. Phase II pragmatic randomized controlled trial of patient-led therapies (mirror therapy and lower-limb exercises) during inpatient stroke rehabilitation. Neurorehabilitation and Neural Repair 2015;29:818-26. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Zhang 2014 {published data only}
References to ongoing studies
ChiCTR1800019581 {published data only}
- ChiCTR1800019581. Effects of motor imagery training on lower limb motor function of patients with chronic stroke. http://www.medresman.org.cn/pub/cn/proj/projectshow.aspx?proj=4475 (first received 19 November 2018).
ChiCTR‐IOR‐16008137 {published data only}
- ChiCTR-IOR-16008137. Graded motor imagery based on mirror neuron on rehabilitative training for stroke patients: a BOLD-fMRI study. http://www.chictr.org.cn/showproj.aspx?proj=13608 (first received 9 April 2016).
ISRCTN33487341 {published data only}
- ISRCTN33487341. Mental practice-based rehabilitation training to improve arm function and daily activity performance in stroke patients: a randomized clinical trial. http://www.isrctn.com/ISRCTN33487341 (first received 7 December 2007). [DOI: 10.1186/1471-2377-8-7] [DOI] [PMC free article] [PubMed]
NCT01993563 {published data only}
- NCT01993563. Graded motor imagery for patients within a year after stroke. https://clinicaltrials.gov/ct2/show/NCT01993563 (first received 25 November 2013).
NCT03436810 {published data only}
- NCT03436810. Effect of structured progressive task-oriented circuit class training with motor imagery on gait in stroke. https://clinicaltrials.gov/ct2/show/study/NCT03436810 (first received 19 February 2018).
NCT04086004 {published data only}
- NCT04086004. Dual task balance training with additional motor imagery practice in stroke. https://clinicaltrials.gov/ct2/show/NCT04086004 (first received 11 September 2019).
NCT04215679 {published data only}
- NCT04215679. Effect of motor imagery with virtual reality in patients with stroke. https://www.clinicaltrials.gov/ct2/show/NCT04215679 (first received 2 January 2020).
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barclay 2015
- Barclay RE, Stevenson TJ, Poluha W, Ripat J, Nett C, Srikesavan CS. Interventions for improving community ambulation in individuals with stroke. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD010200. [DOI: 10.1002/14651858.CD010200] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barclay‐Goddard 2011
- Barclay-Goddard RE, Stevenson TJ, Poluha W, Thalman L. Mental practice for treating upper extremity deficits in individuals with hemiparesis after stroke. Cochrane Database of Systematic Reviews 2011, Issue 5. Art. No: CD005950. [DOI: 10.1002/14651858.CD005950.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beyaert 2015
Bonda 1995
- Bonda E, Petride M, Frey S, Evans A. Neural correlates of mental transformations of the body-in-space. Proceedings of the National Academy of Sciences of the United States of America 1995;92(24):11180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Braun 2017
- Braun N, Kranczioch C, Liepert J, Dettmers C, Zich C, Büsching I, et al. Motor imagery impairment in postacute stroke patients. Neural Plasticity 2017. [DOI: 10.1155/2017/4653256] [DOI] [PMC free article] [PubMed]
Carr 1985
- Carr JH, Shepherd RB, Nordholm L, Lynne D. Investigation of a new motor assessment scale for stroke patients. Physical Therapy 1985;65(2):175-80. [DOI] [PubMed] [Google Scholar]
Carrasco 2016
- Carrasco GD, Cantalapiedra AJ. Effectiveness of motor imagery or mental practice in functional recovery after stroke: a systematic review. Neurologia 2016;31(1):43-52. [DOI] [PubMed] [Google Scholar]
Chiu 2000
- Chiu HC, Chern JY, Shi HY, Chen SH, Chang JK. Physical functioning and health-related quality of life: before and after total hip replacement. Kaohsiung Journal of Medical Sciences. 2000;16(6):285-92. [PubMed] [Google Scholar]
Chumney 2010
- Chumney D, Nollinger K, Shesko K, Skop K, Spencer M, Newton RA. Ability of Functional Independence Measure to accurately predict functional outcome of stroke-specific population: systematic review. Journal of Rehabilitation Research and Development 2010;47(1):17-29. [DOI] [PubMed] [Google Scholar]
Collen 1991
- Collen FM, Wade DT, Robb GF, Bradshaw CM. The Rivermead Mobility Index: a further development of the Rivermead Motor Assessment. International Disability Studies 1991;13(2):50-4. [DOI] [PubMed] [Google Scholar]
Collin 1988
- Collin C, Wade DR, Davies S, Horne V. Barthel ADL Index: a reliability study. International Disability Studies 1988;10(2):61-3. [DOI] [PubMed] [Google Scholar]
Decety 1993
- Decety J. Should motor imagery be used in physiotherapy? Recent advances in cognitive neurosciences. Physiotherapy Theory and Practice 1993;9(4):193-203. [Google Scholar]
Decety 1996
- Decety J. The neurophysiological basis of motor imagery. Behavioural Brain Research 1996;77(1-2):45-52. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dickstein 2004
- Dickstein R, Dunsky A, Marcovitz E. Motor imagery for gait rehabilitation in post-stroke hemiparesis. Physical Therapy 2004;84(12):1167-77. [PubMed] [Google Scholar]
Driediger 2006
- Driediger M, Hall C, Callow N. Imagery use by injured athletes: a qualitative analysis. Journal of Sports Sciences 2006;24(3):261-71. [DOI] [PubMed] [Google Scholar]
Dujovic 2017
- Dujovic SD, Malesevi J, Malesevi N, Vidakovic AS, Bijelic G, Kellere T, et al. Novel multi-pad functional electrical stimulation in stroke patients: a single-blind randomized study. NeuroRehabilitation 2017;41(4):791-800. [DOI] [PubMed] [Google Scholar]
French 2016
- French B, Thomas LH, Couple J, McMahon NE, Connell L, Harrison J, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No: CD006073. [DOI: 10.1002/14651858.CD006073.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fugl‐Meyer 1975
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. A method for evaluation of physical performance. Scandanavian Journal of Rehabilitation Medicine 1975;7:13-31. [PubMed] [Google Scholar]
Garrison 2010
- Garrison KA, Winstein CJ, Aziz-Zadeh L. The mirror neuron system: a neural substrate for methods in stroke rehabilitation. Neurorehabilitation and Neural Repair 2010;24:404-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADEpro GDT. Version accessed prior to 23 April 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Guerra 2017
- Guerra ZF, Lucchetti ALG, Lucchetti G. Motor imagery training after stroke: a systematic review and meta-analysis of randomized controlled trials. Journal of Neurologic Physical Therapy 2017;41(4):205-14. [DOI] [PubMed] [Google Scholar]
Hamilton 1994
- Hamilton BB, Laughlin JA, Fiedler RC, Granger CV. Interrater reliability of the 7-level functional independence measure (FIM). Scandinavian Journal of Rehabilitation Medicine 1994;26(3):115-9. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane-handbook.org.
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI: 10.1136/bmj.g1687] [DOI] [PubMed] [Google Scholar]
Holden 1984
- Holden MK, Gill KM, Magliozzi MR, Nathan J, Peihl-Baker L. Clinical gait assessment in the neurologically impaired: reliability and meaningfulness. Physical Therapy 1984;64(1):35-40. [DOI] [PubMed] [Google Scholar]
Hosseini 2012
- Hosseini SA, Fallahpour M, Sayadi M, Gharib M, Haghgoo H. The impact of mental practice on stroke patients' postural balance. Journal of the Neurological Sciences 2012;322(1-2):263-7. [DOI] [PubMed] [Google Scholar]
Jeannerod 1995
- Jeannerod M, Decety J. Mental motor imagery: a window into the representational stages of action. Current Opinion in Neurobiology 1995;5(6):727-32. [DOI] [PubMed] [Google Scholar]
Jeannerod 2001
- Jeannerod M. Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage 2001;14(1 Pt 2):S103-9. [DOI] [PubMed] [Google Scholar]
Johnson 2000
- Johnson SH. Imagining the impossible: intact motor representations in hemiplegics. NeuroReport 2000;11(4):729-32. [DOI] [PubMed] [Google Scholar]
Johnson 2002a
- Johnson SH, Sprehn G, Saykin AJ. Intact motor imagery in chronic upper limb hemiplegics: evidence for activity-independent action representations. Journal of Cognitive Neuroscience 2002;14(6):841–52. [DOI] [PubMed] [Google Scholar]
Johnson 2002b
- Johnson SH, Rotte M, Grafton ST, Hinrichs H, Gazzaniga MS, Heinze HJ. Selective activation of a parietofrontal circuit during implicitly imagined prehension. Neuroimage 2002;17(4):1693-704. [DOI] [PubMed] [Google Scholar]
Jørgensen 1995
- Jørgensen H, Nakayama H, Ho R, Olsen T. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Archives of Physical Medicine and Rehabilitation 1995;76(1):27-32. [DOI] [PubMed] [Google Scholar]
Kim 2018
- Kim YK, Park E, Lee A, Im CH, Kim YH. Changes in network connectivity during motor imagery and execution. PloS One 2018;13(1):1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamontagne 2004
- Lamontagne A, Fung J. Faster is better: implications for speed-intensive gait training after stroke. Stroke 2004;35(11):2543-8. [DOI] [PubMed] [Google Scholar]
Langhorne 2009
- Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurology 2009;8(8):741-54. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Li 2017
- Li RQ, Li ZM, Tan JY, Chen GL, Lin WY. Effects of motor imagery on walking function and balance in patients after stroke: a quantitative synthesis of randomized controlled trials. Complementary Therapies in Clinical Practice 2017 May 26 [Epub ahead of print]. [DOI: 10.1016/j.ctcp.2017.05.009] [DOI] [PubMed]
Lotze 1999
- Lotze M, Montoya P, Erb M, Hülsmann E, Flor H, Klose U, et al. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI study. Journal of Cognitive Neuroscience 1999;11(5):491-501. [DOI] [PubMed] [Google Scholar]
Mehrholz 2017
- Mehrholz J, Pohl M, Elsner B. Treadmill training and body weight support for walking after stroke. Cochrane Database of Systematic Reviews 2017, Issue 8. Art. No: CD002840. [DOI: 10.1002/14651858.CD002840.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mikołajewska 2017
- Mikołajewska E. Bobath and traditional approaches in post-stroke gait rehabilitation in adults. Biomedical Human Kinetics 2017;9(1):27-33. [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357(9263):1191-4. [PubMed] [Google Scholar]
Moura 2012
- Moura DMS. Intervention Proposal to Assist the Motor and Cognitive Rehabilitation of Patients with Stroke [Proposta de Intervenção para Auxiliar a Reabilitação Motora e Cognitiva de Pacientes com Acidente Vascular Cerebral] [Masters thesis]. Natal (Brazil): Federal University of Rio Grande do Norte, 2012. [Google Scholar]
Mulder 2007
- Mulder TH. Motor imagery and action observation: cognitive tools for rehabilitation. Journal of Neural Transmission 2007;114(10):1265-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munzert 2009
- Munzert J, Lorey B, Zentgraf K. Cognitive motor processes: the role of motor imagery in the study of motor representations. Brain Research Reviews 2009;60(2):306-26. [DOI] [PubMed] [Google Scholar]
Podsiadlo 1991
- Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. Journal of the American Geriatric Society 1991;39(2):142-8. [DOI] [PubMed] [Google Scholar]
Ramachandran 1994
- Ramachandran VS. Phantom limbs, neglect syndromes, repressed memories, and Freudian psychology. International Review of Neurobiology 1994;37:291-333. [DOI] [PubMed] [Google Scholar]
Rayegani 2016
- Rayegani SM, Raeissadat SA, Alikhani E, Bayat M, Bahrami MH, Karimzadeh A. Evaluation of complete functional status of patients with stroke by Functional Independence Measure scale on admission, discharge, and six months poststroke. Iranian Journal of Neurology 2016;15(4):202-8. [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sakuma 2014
- Sakuma K, Ohata K, Izumi K, Shiotsuka Y, Yasui T, Ibuki S, et al. Relation between abnormal synergy and gait in patients after stroke. Journal of Neuroengineering and Rehabilitation 2014;25:111-41. [DOI: 10.1186/1743-0003-11-141] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2006
- Sharma N, Pomeroy VM, Baron J-C. Motor imagery: a backdoor to the motor system after stroke? Stroke 2006;37:1941–52. [DOI] [PubMed] [Google Scholar]
States 2009
- States RA, Pappas E, Salem Y. Overground physical therapy gait training for chronic stroke patients with mobility deficits. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD006075. [DOI: 10.1002/14651858.CD006075.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2013
- Sun L, Yin D, Zhu Y, Fan M, Zang L, Wu Y, et al. Cortical reorganization after motor imagery training in chronic stroke patients with severe motor impairment: a longitudinal fMRI study. Neuroradiology 2013;55(7):913-25. [DOI] [PubMed] [Google Scholar]
Thieme 2016
- Thieme H, Morkisch N, Rietz C, Dohle C, Borgetto B. Techniques for treatment of limb pain: a systematic review and meta-analysis. Journal of Pain 2016;17(2):167-80. [DOI] [PubMed] [Google Scholar]
Wang 2016
- Wang L, Zhang J, Zhang Y, Yan R, Liu H, Qiu M. Conditional Granger causality analysis of effective connectivity during motor imagery and motor execution in stroke patients. BioMed Research International 2016 April 20 [Epub ahead of print]. [DOI: 10.1155/2016/3870863] [DOI] [PMC free article] [PubMed]
Whitall 2004
- Whitall J. Stroke rehabilitation research: time to answer more specific questions? Neurorehabilitation and Neural Repair 2004;18(1):3-8. [DOI] [PubMed] [Google Scholar]
WHO 2017
- World Health Organization. Cardiovascular diseases fact sheet. www.who.int/mediacentre/factsheets/fs317/en/ (accessed 12 March 2017).
Winstein 2016
- Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016;47(6):e98-169. [DOI] [PubMed] [Google Scholar]


