Abstract
In multiple sclerosis, demyelination occurs beside the white‐matter structures and in the cerebral and cerebellar cortex. We have previously shown that, in the cuprizone model, demyelination is present not only in the corpus callosum but also in the cerebral cortex. Here, we have performed a detailed analysis of the dynamics of de‐ and remyelination in the cerebellar cortex and white matter at nine timepoints in two cerebellar regions. To induce demyelination, C57BL/6 mice were fed with 0.2% cuprizone for 12 weeks followed by a recovery of 8 weeks. Both cortex and white‐matter structures were significantly demyelinated after 12 weeks of cuprizone feeding. Remyelination occurred after withdrawal of cuprizone but was less prominent in the more caudal cerebellar region. Microglia infiltration was prominent in all analyzed cerebellar areas, preceding demyelination by approximately 2–4 weeks, and was delayed in the more caudal cerebellar region. Astrogliosis was also seen but did not reach the extent observed in the cerebrum. In summary, cuprizone feeding provides an excellent model for the investigation of de‐ and remyelination processes in the cerebellar cortex and white matter. Furthermore, demyelination, microglia and astrocyte changes were different in the cerebellum as compared with the cerebrum, indicating region‐dependent pathomechanisms.
Keywords: animal model, C57BL/6 mice, myelin, remyelination
INTRODUCTION
Multiple sclerosis (MS) is an inflammatory disease of the central nervous system (CNS) that leads to demyelination of the white matter and axonal damage (34). However, recent studies have shown that demyelinating lesions also arise in the cerebral cortex and are even more prominent in the cerebellar cortex 8, 16, 17. Cortical demyelination is particularly prominent in primary and secondary progressive MS but is rare in the acute or relapsing form. Demyelination occurs in more than 35% of the total cerebellar cortical area in the progressive forms, and some extreme cases show complete loss of myelin in up to 92% of the cortex (17).
To date, the complex pathomechanisms and functional consequences of demyelination in the cerebral and particularly in the cerebellar cortex are far from being understood. Therefore, animal models have become helpful in exploring the underlying mechanisms. In the cuprizone model, young adult mice are fed the copper chelator cuprizone (bis‐cyclohexanone oxaldihydrazone), which leads to a reproducible demyelination of the white matter within weeks 21, 33. After removal of the toxin, spontaneous remyelination occurs (18). Besides white‐matter demyelination in the corpus callosum, we have recently shown that de‐ and remyelination are also a prominent feature in the cerebral cortex in this model (30).
However, there is a lack of suitable animal models for cerebellar cortical demyelination to date. Here, we analyzed in detail the dynamics of cuprizone‐induced demyelination of the cerebellar cortex and white matter together with microglia activation and changes of astroglia and oligodendrocytes.
MATERIAL AND METHODS
Animals and experiments
C57BL/6 male mice were obtained from Charles River (Sulzfeld, Germany). Animals underwent routine cage maintenance once a week and were microbiologically monitored according to Federation of European Laboratory Animal Science Associations recommendations (28). Food and water were available ad libitum. All research and animal care procedures were approved by the Review Board for the Care of Animal Subjects of the district government (Lower Saxony, Germany) and performed according to international guidelines on the use of laboratory animals.
Demyelination was induced by feeding 8‐week‐old male C57BL/6 mice a diet containing 0.2% cuprizone (bis‐cyclohexanone oxaldihydrazone, Sigma‐Aldrich Inc., St. Louis, MO, USA) mixed into a ground standard rodent chow. For chronic demyelination, the cuprizone diet was maintained for 12 weeks. Thereafter, mice were put on a normal chow for another 8 weeks. At different time points (0, 4, 6, 8, 10, 12, 13, 14 and 20 weeks), mice were perfused with 4% paraformaldehyde (PFA) in phosphate buffer via left cardiac ventricle as previously described (18). A group size of five to six animals was investigated at all time points. The brains were removed, postfixed in 4% PFA and paraffin embedded. For light microscopy, 7‐µm serial paraffin sections were cut and dried at 37°C overnight. Two different regions, one between bregma −5.80 and −6.00 and the other between bregma −6.64 and −6.84 [according to mouse atlas by Paxinos and Franklin (26)], were analyzed.
To explore even longer cuprizone exposure, 8‐week‐old male C57BL/6 mice were fed 0.2% cuprizone for 14 weeks and 16 weeks, respectively, in another experiment.
Histology and immunohistochemistry
Histology for Luxol‐fast blue periodic acid‐Schiff base (Sigma‐Aldrich, Steinheim, Germany) and immunohistochemistry were performed as previously described (19). Paraffin embedded sections were de‐waxed, rehydrated and microwaved for 5 minutes in 10 mM citrate buffer (pH 6.0). Sections were quenched with H2O2, blocked for 1 h in phosphate buffered saline (PBS) containing 3% normal goat serum, 0.1% Triton X‐100 and then incubated overnight with primary antibody. The following primary antibodies were used: for myelin, proteins proteolipid protein (PLP) (mouse IgG, Serotec, Düsseldorf, Germany, 1:500) and myelin basic protein (MBP) (mouse IgG; Sternberger Monoclonals Inc., Berkeley, CA, USA, 1:1000); for microglia, mac‐3 (rat IgG, BD Pharmingen, Heidelberg, Germany, 1:500); for astrocytes, glial fibrillary acidic protein (GFAP) (mouse IgG, Chemicon, UK, 1:200); and for oligodendrocytes, Nogo‐A (rabbit polyclonal, Chemicon, 1:750). After washing, sections were further incubated with biotinylated antimouse IgG (H + L), antirat IgG (H + L) and antirabbit IgG (H + L) secondary antibodies (Vector Laboratories, Burlingame, UK, 1:500) for 1 h, followed by peroxidase‐coupled avidin‐biotin complex (ABC Kit, Vector Laboratories). Reactivity was visualized with diamino‐3,3′benzidine (DAB, Dako Cytomation, Hamburg, Germany). For cell stainings, slides were counterstained by using Mayer's hemalum solution (Merck, Damstadt, Germany).
Determination of cortical de‐ and remyelination
In order to analyze the extent of de‐ and remyelination in the cerebellar cortex, the sections were immunohistochemically stained for PLP as well as for MBP. The complete cortex of two different regions mentioned above was subsequently analyzed by light microscopy (Leica DMLB, Wetzlar, Germany). Two observers made an assessment of the myelination status of both white matter and cortex of the complete cerebellum in blinded sections from each animal. The distribution of changes was drawn into a sketch of the anatomic region observed, which was subsequently used to generate a distribution map. To assess myelination, three grades of myelin‐loss were defined: grade 1 is light demyelination (shown in blue in the distribution map, see Figure 1, F2); grade 2 is moderate demyelination (shown in red, see Figure 1, F3); and grade 3 means complete loss of myelin (shown in yellow, see Figure 1, F4), while normal myelination was left in white (Figure 1, F1). In addition, camera pictures of five different areas and four different time points of the rostrally located region of the cerebellum were drawn and used as representatives as shown in Figure 1A1–E4.
Figure 1.

Cerebellar de‐ and remyelination demonstrated in images of selected areas, stained for the myelin marker PLP. C57BL/6 mice were fed with cuprizone for 12 weeks and thereafter put on a normal diet. After 6 weeks, there was moderate demyelination in the superior cerebellar peduncle area (E2) but only weak demyelination in the cortex and white matter of the lobes (A2, B2, C2, D2). Severe demyelination was induced after 12 weeks of cuprizone administration and was most prominent in the periphery (A3, B3, C3, D3). At week 20 (after 8 weeks on a normal diet), myelin recovered but did not reach the intensity of the controls. The sketch on top of the figure shows the areas from which the images were taken. F1–4 shows the various degrees of myelination in the cerebellar cortex with normal myelin (F1), mild demyelination (F2, blue in Figure 2), moderate demyelination (F3, red in Figure 2) and severe demyelination (F4, yellow in Figure 2). Scale bars: 100 µm (A1–4, D1–4), 50 µm (B1–4, C1–4), 200 µm (E1–4). PLP = proteolipid protein.
Quantitation of cells in the cerebellar cortex and white matter of the lobes
Quantitation of cells was performed for mature oligodendrocytes (Nogo‐A) (15) as well as for activated microglia (mac‐3). Counting of immunopositive cells with an identified nucleus (counterstaining with hemalum) was performed in both left and right cerebellar lobes, and a mean value is given in the results. Using a magnification of ×40 (Leica DMLB), sections of two different regions of the cerebellum mentioned above were evaluated. In the first more rostrally located region, five different areas were analyzed: lingula cerebelli (LIC), lobulus centralis (LOC), lobus anterior (LA), lobulus simplex (LS) and crus I lobuli ansiformis (C1LA) (see Figure 2A). In the second more caudally located region, four different areas were investigated: comissura cerebelli/nodulus (CC/ND) culmen (CU), lobulus simplex/crus I lobuli ansiformis (LS/C1LA) and crus II lobuli ansiformis/lobulus paramedianus (C2LA/LP) (see Figure 2J). In all analyzed areas, grey and white matters were assessed separately. Values were expressed as number of cells per mm2.
Figure 2.

Sketches demonstrating the various degrees of demyelination in both, more rostrally located sections (A–I, between bregma −5.80 and −6.00) and more caudally located sections (J–S, between bregma −6.64 and −6.84). Mice were fed with cuprizone for 12 weeks and thereafter put on a normal diet. The degrees of demyelination were assessed for PLP stainings. White matter demyelination is shown on the left side, while cortical demyelination is shown on the right side of each sketch. Three grades of myelin loss were distinguished: mild demyelination is shown in blue (for detail, see Figure 1, F2 ), moderate demyelination is shown in red (for detail, see Figure 1, F3 ), and severe demyelination is shown in yellow (for detail, see Figure 1, F4 ). The following abbreviations were used to mark the areas: SCP (superior cerebellar peduncle), LIC (lingula cerebelli), LOC (lobulus centralis), LA (lobus anterior), LS (lobulus simplex), C1LA (crus I lobuli ansiformis), CC/ND (comissura cerebelli/nodulus), CU (culmen), LS/C1LA (lobulus simplex/crus I lobuli ansiformis) and C2LA/LP (crus II lobuli ansiformis/lobulus paramedianus). In the more caudally located region, demyelination occurred to a slightly smaller extent by weeks 8 and 10 (D, E) in comparison to the more rostrally located region (M, N). After withdrawal of cuprizone, myelin recovered more slowly in the caudal region (P–S) than in the rostral region (G–I). PLP = proteolipid protein.
Statistical analysis
Statistical analysis was performed by using one‐way analysis of variance (ANOVA) with the factor “time/week” followed by the Fisher‐protected least‐significant difference test for post hoc comparison if appropriate. All data are given as arithmetic means ± standard error of the mean. P values of the different ANOVAs are given in the Results, while group comparisons derived from post hoc analysis are provided in the figures. In the latter case, significant effects are indicated by asterisks (*P < 0.05; **P < 0.01; ***P < 0.001).
RESULTS
Cortical cerebellar demyelination is prominent in the cuprizone model
To determine whether mice show demyelination, PLP‐ and MBP‐stained sections of two different cerebellar regions were investigated. In the rostrally located cerebellar region, weak demyelination was confined to the superior cerebellar peduncle (SCP) and the adjacent area after 4 weeks of cuprizone feeding. After 6 weeks, there was moderate demyelination in the SCP (Figure 1, E2). Only weak demyelination could be found in the cortex and white matter of the lobes (Figure 1, A2, B2, C2, D2). Thereafter, demyelination in both the cortex and white matter of the lobes as well as in the SCP area increased steadily, reaching its peak after week 12 of cuprizone diet. At this time point, the cortex showed severe demyelination most prominent in the periphery (Figure 1, A3, B3, C3, D3). After cuprizone withdrawal, remyelination occurred in all areas, myelin still not reaching the intensity of the controls by week 20 (Figure 1, A4, B4, C4, D4, E4).
To examine whether more severe demyelination can be induced by longer administration of cuprizone, mice were fed cuprizone for 14 weeks as well as for 16 weeks in another set of experiments. However, the degree of demyelination was not changed as compared with week 12 (data not shown).
There was no difference between the analyzed PLP‐ and MBP‐stained sections. Thus, only PLP results are shown in 1, 2. The sensitivity of the luxol‐fast blue (LFB) staining was not sufficient to demonstrate cerebellar cortical myelination, similar to cortical and hippocampal myelination 14, 30.
Demyelination depends on the region of the cerebellum
To further characterize cortical cerebellar demyelination in the cuprizone model, another more caudally located region was investigated. Demyelination occurred here to a slightly lower extent, by weeks 8 and 10, in comparison with the first more rostrally located region (see Figure 2). After changing to normal diet, the myelin recovered in a slower rate in comparison to the first region.
Severe loss of mature oligodendrocytes occurs in response to cuprizone administration
Mature oligodendrocytes were studied by Nogo‐A staining (15). In control animals, the density of Nogo‐A positive oligodendrocytes was lower in the cortex than in the white matter of the lobes. During cuprizone treatment, the amount of Nogo‐A‐positive cells was significantly reduced in all observed areas, both in the more rostrally located region (Figure 3; cortex: LIC P < 0.0001, LOC P < 0.0001, LA P < 0.0001, LS P < 0.0001, C1LA P = 0.0004; white matter: LIC P < 0.0001, LOC P < 0.0001, LA P = 0.004, LS P < 0.0001, C1LA P = 0.0002) and in the more caudally located region (Figure 4; cortex: CC/ND P = 0.0004, CU P < 0.0001, LS/C1LA P < 0.0001, C2LA/LP P = 0.0003; white matter: CC/ND P < 0.0001, CU P < 0.0001, LS/C1LA P < 0.0001, C2LA/LP P < 0.0001). The number of oligodendrocytes changed significantly during the course of cuprizone administration, reaching its lowest density by weeks 4 and 12 while increasing again between weeks 6 and 8 as well as from week 13 on. By week 20, the density of Nogo‐A‐positive cells had fully recovered in the areas of the rostral region (Figure 3), while some areas of the caudal region failed to recover fully (Figure 4).
Figure 3.
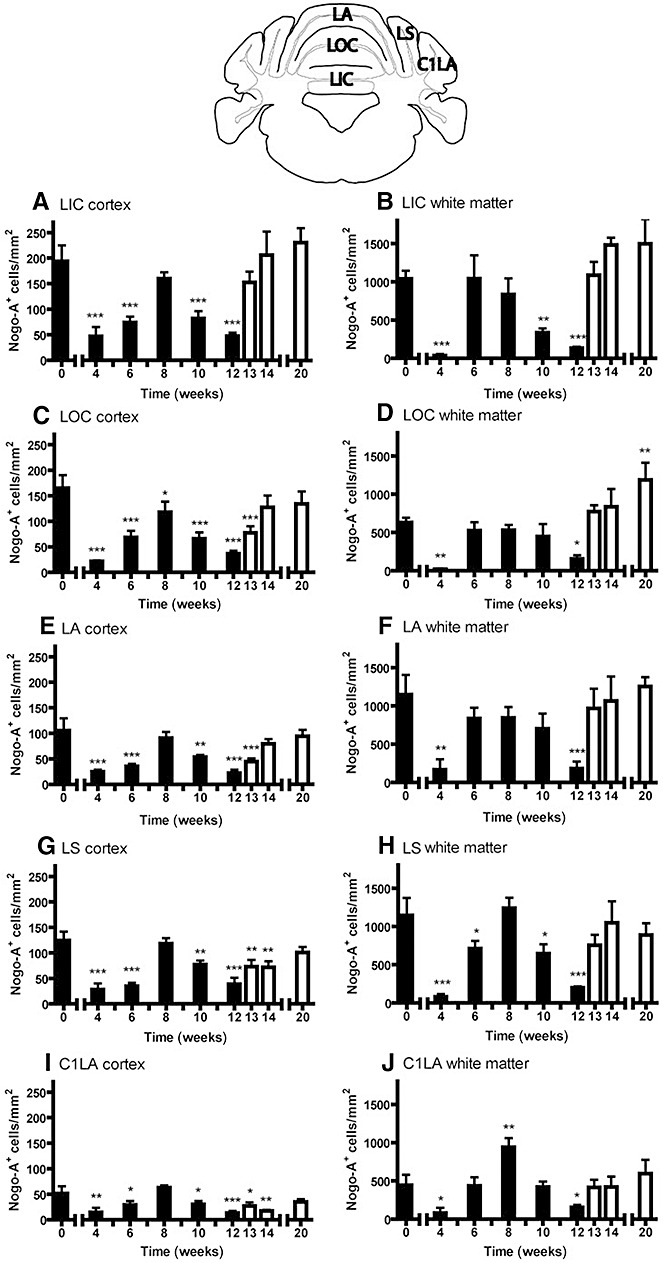
Oligodendrocytes during cuprizone feeding in the more rostral region of the cerebellum. Graphs represent cell quantitation for Nogo‐A in the white matter (B, D, F, H and J) and cortex (A, C, E, G and I) of different lobes. The following areas were analyzed: lingula cerebelli (LIC), lobulus centralis (LOC), lobus anterior (LA), lobulus simplex (LS), crus I lobuli ansiformis (C1LA). C57BL/6 mice were fed with cuprizone for 12 weeks (demyelination, black bars) and were thereafter put on a normal diet (remyelination, white bars). Significant effects vs. controls are indicated by asterisks (*P < 0.05, **P < 0.01 and ***P < 0.001).
Figure 4.

Oligodendrocytes during cuprizone feeding in the more caudally located region of the cerebellum. Graphs represent cell quantitation for Nogo‐A in the white matter (B, D, F and H) and cortex (A, C, E and G) of different lobes. The following areas were analyzed: comissura cerebelli/nodulus (CC/ND), culmen (CU), lobulus simplex/crus I lobuli ansiformis (LS/C1LA), crus II lobuli ansiformis/lobulus paramedianus (C2LA/LP). C57BL/6 mice were fed with cuprizone for 12 weeks (demyelination, black bars) and were thereafter put on a normal diet (remyelination, white bars). Significant effects vs. controls are indicated by asterisks (*P < 0.05, **P < 0.01 and ***P < 0.001).
Microglia response during cuprizone treatment
In order to study activated microglia during cuprizone treatment, we used the marker mac‐3. No activated microglia were found in control animals (see Figure 5). During cuprizone treatment, the amount of activated microglia was significantly increased in all observed areas, both in the more rostrally located region (Figure 6; for all cortical and white‐matter areas P < 0.0001) and in the more caudally located region (Figure 7; cortex: CC/ND P < 0.0001, CU P < 0.0005, LS/C1LA P < 0.0001, C2LA/LP P < 0.002; for all white‐matter areas P < 0.0001). In both regions, the density of mac‐3‐positive cells was higher in the white‐matter structures than in the cortex. In the cortical areas of the rostral region, microglia peaked in average at week 6, while, in the white‐matter structures, microglia reached maximum density by week 8 (Figure 6). Furthermore, region two reached the peak of microglial density in the white matter 2 weeks later than the first region, in average, by week 10, and microglia could still be found there even after discontinuing the cuprizone diet (Figure 7).
Figure 5.

Demonstration of microglia infiltration at 6 weeks of cuprizone diet in comparison to controls. The cerebellar sections were immunohistochemically stained for the microglial marker mac‐3 and were then counterstained with Mayer's hemalum solution. At week 6 of cuprizone diet, mac‐3 positive cells could be seen in both the cortex and white matter (A2, B2), whereas no activated microglia could be detected in control animals (A1, B1). The sketch on top of the figure shows the areas from which the images were taken. Scale bars: 100 µm (A1, A2), 50 µm (B1, B2).
Figure 6.

Activated microglia during cuprizone feeding in the more rostrally located region of the cerebellum. Graphs represent cell quantitation for mac‐3 in the white matter (B, D, F, H and J) and cortex (A, C, E, G and I) of different lobes. The following lobes were analyzed: lingula cerebelli (LIC), lobulus centralis (LOC), lobus anterior (LA), lobulus simplex (LS), crus I lobuli ansiformis (C1LA). C57BL/6 mice were fed with cuprizone for 12 weeks (demyelination, black bars) and were thereafter put on a normal diet (remyelination, white bars). Significant effects vs. controls are indicated by asterisks (*P < 0.05, **P < 0.01 and ***P < 0.001).
Figure 7.

Activated microglia during cuprizone feeding in the more caudally located region of the cerebellum. Graphs represent cell quantitation for mac‐3 in the white matter (B, D, F and H) and cortex (A, C, E and G) of different lobes. The following areas were analyzed: comissura cerebelli/nodulus (CC/ND), culmen (CU), lobulus simplex/crus I lobuli ansiformis (LS/C1LA), crus II lobuli ansiformis/lobulus paramedianus (C2LA/LP). C57BL/6 mice were fed with cuprizone for 12 weeks (demyelination, black bars) and were thereafter put on a normal diet (remyelination, white bars). Significant effects vs. controls are indicated by asterisks (*P < 0.05, **P < 0.01 and ***P < 0.001).
Astrocytosis during cuprizone treatment
Before cuprizone treatment, only few astrocytes could be found in the area of the rostral region, mainly located periventricularly and around the SCP area. Very few GFAP‐positive cells could be found in the lobes of the cerebellum (Figure 8, A1, B1). After 4 weeks of cuprizone‐induced demyelination, the number of astrocytes increased periventricularly, while astrocytes in the cortex and white matter of the lobes were first seen after 6 weeks of cuprizone treatment (Figure 8, A2, B2). Astrocytosis reached its peak both in cortex and white matter by week 8 (Figure 8, A3, B3), quickly declining afterward. A weak astrocytosis in the area around the ventricle could still be observed at week 20. A similar development of astrocytosis in the course of cuprizone administration could also be observed in the more caudally located second region of the cerebellum (data not shown).
Figure 8.

Astrocytosis during cuprizone treatment was shown by using immunohistochemical staining for GFAP. In untreated control animals, GFAP‐positive cells were absent or only sporadically seen in the lobes of the cerebellum (A1, B1) and in the cerebral cortex (C1). The number of GFAP‐positive astrocytes increased after 6 weeks of cuprizone‐induced demyelination (A2, B2), reaching its maximum by week 8. The extent of astrocytosis was more prominent in the cerebral cortex (C2, C3). The sketches on top of the figure show the areas from which the images were taken. Scale bars: 50 µm (A1–3), 100 µm (B1–3, C1–3). GFAP = glial fibrillary acidic protein.
The extent of astrocytosis in the cerebellum, although being a prominent feature of cuprizone administration, seems to be smaller than the astrocytosis observed in the cerebrum. Differences in astrocytosis between cerebellum and cerebrum are demonstrated in Figure 8.
DISCUSSION
The present study shows that feeding the copper chelator cuprizone to young adult C57BL/6 mice reliably causes severe demyelination of the cerebellar cortex and the white matter of the lobes. Although it has long been recognized that myelin damage can occur in the superior cerebellar peduncles (3), the extent of cerebellar cortical demyelination has not been investigated yet.
Cerebellar cortical demyelination could first be observed from week 6 onward and reached its maximum by week 12. However, our demyelination findings differ from previous observations made in the cerebrum. As previously described, severe demyelination could be seen in the cerebral cortex already after 6 weeks of cuprizone administration (30). The apparent delay of demyelination in the cerebellar cortex suggests different pathomechanisms. After withdrawal of cuprizone from the diet, remyelination occurred. Yet, it did not reach the degree of myelination observed in control animals. Interestingly, remyelination progressed more slowly in the more caudally located cerebellar region than in the more rostral region. Anatomic variations of cuprizone‐induced demyelination were already described for the hippocampus (14) and the corpus callosum, where mostly the caudal part was demyelinated, but the rostral part did not (31). Also, in the human demyelinating disease MS, the extent of demyelination varies between different CNS regions, and demyelination of the grey matter is most extensive in the cerebellum and spinal cord (8). The pathology of grey‐matter lesions was found to be different from that of white‐matter lesions, cortical lesions showing macrophage and lymphocyte infiltration in a smaller extent (5). The observed variations in demyelination may be explained by different region‐specific microglia function. It has been shown that microglia exhibit region‐dependent (cerebellum, hippocampus, cerebral cortex, spinal cord) differences in morphology, expression of surface immunoregulatory proteins, migratory response to adenosine triphosphate (ATP) and phagocytose capacity 6, 7.
Consistent with previous findings on grey‐matter demyelinating lesions both in the cortex (30) and the hippocampus 10, 14, 25, LFB staining was not sensitive enough to reveal demyelination in the cortex of the cerebellum. Thus, in order to investigate cortical myelin lesions, immunohistochemical staining for myelin proteins needs to be performed.
Similar to previous reports in the cerebrum (20), there was a loss of mature oligodendrocytes in the cerebellum in response to cuprizone treatment. In untreated animals, the density of Nogo‐A‐positive oligodendrocytes was lower in the cortex than in the white matter of the lobes by a factor of approximately 10. During the course of cuprizone administration, the number of oligodendrocytes decreased, reaching its lowest density by weeks 4 and 12 while increasing again between weeks 6 and 8 as well as from week 13 onward. The increase of oligodendrocytes between weeks 6 and 8 may be explained by an attempt to remyelinate with subsequent secondary damage. As reported for the corpus callosum, remyelination begins despite continued cuprizone feeding 18, 21, and, when cuprizone is further on fed, demyelination occurs again. Eight weeks after withdrawal of cuprizone, mature oligodendrocytes had fully recovered in the areas of the rostral region. However, in some areas of the caudal region, oligodendrocytes failed to recover fully, which might be the cause for the impaired remyelination.
Along with demyelination, microgliosis occurred in all cerebellar areas, with a lower density in the cerebellar cortex by a factor of approximately 10 than in the white matter of the lobes. These findings of white‐matter microgliosis are in accordance with previous findings on microglia infiltration in cuprizone‐induced demyelination of the cerebral areas 22, 29. The peak of activated microglia was observed in the cerebellar areas about 2–4 weeks before maximal demyelination. After withdrawal of the toxin, microglia numbers quickly decreased in all areas of the more rostrally located region while persisting in all areas of the white matter in the more caudally located region. This persistent microglia activation might be a reason why remyelination progressed more slowly in the second more caudally located cerebellar region. Microglia play an important role in response to myelin damage through their capacity to migrate, proliferate and phagocytose myelin debris and their expression of cytokines and trophic factors (24). In experimental autoimmune encephalomyelitis (EAE), persistent microglial activation has been associated with neuropathology of the cortex (27). However, in recent years, it has been shown that microglia have not only neurodegenerative but also neuroprotective functions by promoting remyelination. In lysolecithin‐induced demyelination, depletion of microglia/macrophages impaired remyelination (12). Also, reduction of microglia/macrophages corresponded with delayed recruitment of oligodendrocyte progenitor cells (13).
Astrogliosis also occurred in cerebellar structures upon cuprizone treatment. Again, these observations are in accordance with astrogliosis demonstrated in the corpus callosum and cortex in the cuprizone model 9, 30. Interestingly, the cerebellum exhibited a much lower density of astrogliosis than the cerebrum. The role of this astrogliosis is not yet clarified, and it is unknown if this represents a deleterious or reparative mechanism. It can only be speculated that the low grade of astrogliosis and the delayed course of demyelination of the cerebellar structures might be related.
In recent years, both cerebral and cerebellar cortical lesions have been recognized as an important feature in the disease course of patients with MS, especially in the primary and secondary progressive forms 4, 17. Remyelination regularly occurs in these lesions (1), and promoting remyelination could be a promising approach to develop new therapeutic strategies. Still, the pathogenesis of cortical demyelinating lesions and of the subsequent remyelination is not well understood. Therefore, animal models are a useful tool for further investigations. In EAE models, demyelinating cortical lesions can be induced in selected animals. In rats, cerebral cortical demyelination could be induced only in certain rat strains; yet, even here, no demyelination was observed in the cerebellar cortex. Nevertheless, all analyzed rat strains developed extensive white‐matter demyelination (32). However, animal models have advantages and disadvantages. In contrast to demyelinating lesions induced by focal injections of proinflammatory mediators (23), in the cuprizone model, no breakdown of the blood–brain barrier occurs 2, 11. This offers the opportunity to investigate the pathomechanisms of de‐ and remyelination uninfluenced by the periphery. Furthermore, the cuprizone model offers consistent anatomically reproducible and well‐detectable de‐ and remyelination processes (21).
In conclusion, the present work demonstrates that cuprizone administration offers an excellent model to study de‐ and remyelination processes in the cortex of the cerebellum. Cerebellar demyelination, as well as microglia and astrocyte responses, was different compared with the cerebral areas, indicating region‐dependent different pathomechanisms.
ACKNOWLEDGMENTS
This work has been supported by Marie Curie Actions; Grant number: MST‐CT‐2005 021014, and Georg‐Christoph‐Lichtenberg Fellowship by the State of Lower Saxony.
REFERENCES
- 1. Albert M, Antel J, Bruck W, Stadelmann C (2007) Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol 2:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bakker DA, Ludwin SK (1987) Blood‐brain barrier permeability during Cuprizone‐induced demyelination. Implications for the pathogenesis of immune‐mediated demyelinating diseases. J Neurol Sci 2:125–137. [DOI] [PubMed] [Google Scholar]
- 3. Blakemore WF (1973) Demyelination of the superior cerebellar peduncle in the mouse induced by cuprizone. J Neurol Sci 1:63–72. [DOI] [PubMed] [Google Scholar]
- 4. Bo L, Vedeler CA, Nyland HI, Trapp BD, Mork SJ (2003) Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol 7:723–732. [DOI] [PubMed] [Google Scholar]
- 5. Bo L, Geurts JJ, Mork SJ, Van Der Valk P (2006) Grey matter pathology in multiple sclerosis. Acta Neurol Scand Suppl 183:48–50. [DOI] [PubMed] [Google Scholar]
- 6. Haas AH, Boddeke HW, Brouwer N, Biber K (2007) Optimized isolation enables ex vivo analysis of microglia from various central nervous system regions. Glia 13:1374–1384. [DOI] [PubMed] [Google Scholar]
- 7. Haas AH, Boddeke HW, Biber K (2008) Region‐specific expression of immunoregulatory proteins on microglia in the healthy CNS. Glia 8:888–894. [DOI] [PubMed] [Google Scholar]
- 8. Gilmore CP, Donaldson I, Bo L, Owens T, Lowe JS, Evangelou N (2009) Regional variations in the extent and pattern of grey matter demyelination in multiple sclerosis: a comparison between the cerebral cortex, cerebellar cortex, deep grey matter nuclei and the spinal cord. J Neurol Neurosurg Psychiatry 2:182–187. [DOI] [PubMed] [Google Scholar]
- 9. Hiremath MM, Saito Y, Knapp GW, Ting JP, Suzuki K, Matsushima GK (1998) Microglial/macrophage accumulation during cuprizone‐induced demyelination in C57BL/6 mice. J Neuroimmunol 1–2:38–49. [DOI] [PubMed] [Google Scholar]
- 10. Hoffmann K, Lindner M, Groticke I, Stangel M, Loscher W (2008) Epileptic seizures and hippocampal damage after cuprizone‐induced demyelination in C57BL/6 mice. Exp Neurol 2:308–321. [DOI] [PubMed] [Google Scholar]
- 11. Kondo A, Nakano T, Suzuki K (1987) Blood‐brain barrier permeability to horseradish peroxidase in twitcher and cuprizone‐intoxicated mice. Brain Res 1:186–190. [DOI] [PubMed] [Google Scholar]
- 12. Kotter MR, Setzu A, Sim FJ, Van Rooijen N, Franklin RJ (2001) Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin‐induced demyelination. Glia 3:204–212. [DOI] [PubMed] [Google Scholar]
- 13. Kotter MR, Zhao C, Van Rooijen N, Franklin RJ (2005) Macrophage‐depletion induced impairment of experimental CNS remyelination is associated with a reduced oligodendrocyte progenitor cell response and altered growth factor expression. Neurobiol Dis 1:166–175. [DOI] [PubMed] [Google Scholar]
- 14. Koutsoudaki PN, Skripuletz T, Gudi V, Moharregh‐Khiabani D, Hildebrandt H, Trebst C, Stangel M (2009) Demyelination of the hippocampus is prominent in the cuprizone model. Neurosci Lett 1:83–88. [DOI] [PubMed] [Google Scholar]
- 15. Kuhlmann T, Remington L, Maruschak B, Owens T, Bruck W (2007) Nogo‐A is a reliable oligodendroglial marker in adult human and mouse CNS and in demyelinated lesions. J Neuropathol Exp Neurol 3:238–246. [DOI] [PubMed] [Google Scholar]
- 16. Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M et al (2005) Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 11:2705–2712. [DOI] [PubMed] [Google Scholar]
- 17. Kutzelnigg A, Faber‐Rod JC, Bauer J, Lucchinetti CF, Sorensen PS, Laursen H et al (2007) Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol 1:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lindner M, Heine S, Haastert K, Garde N, Fokuhl J, Linsmeier F et al (2008) Sequential myelin protein expression during remyelination reveals fast and efficient repair after central nervous system demyelination. Neuropathol Appl Neurobiol 1:105–114. [DOI] [PubMed] [Google Scholar]
- 19. Lindner M, Trebst C, Heine S, Skripuletz T, Koutsoudaki PN, Stangel M (2008) The chemokine receptor CXCR2 is differentially regulated on glial cells in vivo but is not required for successful remyelination after cuprizone‐induced demyelination. Glia 10:1104–1113. [DOI] [PubMed] [Google Scholar]
- 20. Mason JL, Toews A, Hostettler JD, Morell P, Suzuki K, Goldman JE, Matsushima GK (2004) Oligodendrocytes and progenitors become progressively depleted within chronically demyelinated lesions. Am J Pathol 5:1673–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsushima GK, Morell P (2001) The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol 1:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McMahon EJ, Suzuki K, Matsushima GK (2002) Peripheral macrophage recruitment in cuprizone‐induced CNS demyelination despite an intact blood‐brain barrier. J Neuroimmunol 1–2:32–45. [DOI] [PubMed] [Google Scholar]
- 23. Merkler D, Ernsting T, Kerschensteiner M, Bruck W, Stadelmann C (2006) A new focal EAE model of cortical demyelination: multiple sclerosis‐like lesions with rapid resolution of inflammation and extensive remyelination. Brain 8:1972–1983. [DOI] [PubMed] [Google Scholar]
- 24. Neumann H, Kotter MR, Franklin RJ (2009) Debris clearance by microglia: an essential link between degeneration and regeneration. Brain 2:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Norkute A, Hieble A, Braun A, Johann S, Clarner T, Baumgartner W et al (2009) Cuprizone treatment induces demyelination and astrocytosis in the mouse hippocampus. J Neurosci Res 6: 1343–1355. [DOI] [PubMed] [Google Scholar]
- 26. Paxinos G, Frankin KBJ (2001) The Mouse Brain in Stereotaxic Coordinates. Academic Press: New York. [Google Scholar]
- 27. Rasmussen S, Wang Y, Kivisakk P, Bronson RT, Meyer M, Imitola J, Khoury SJ (2007) Persistent activation of microglia is associated with neuronal dysfunction of callosal projecting pathways and multiple sclerosis‐like lesions in relapsing—remitting experimental autoimmune encephalomyelitis. Brain 11:2816–2829. [DOI] [PubMed] [Google Scholar]
- 28. Rehbinder C, Baneux P, Forbes D, Van Herck H, Nicklas W, Rugaya Z, Winkler G (1996) FELASA recommendations for the health monitoring of mouse, rat, hamster, gerbil, guinea pig and rabbit experimental units. Report of the Federation of European Laboratory Animal Science Associations (FELASA) Working Group on Animal Health accepted by the FELASA Board of Management, November 1995. Lab Anim 3:193–208. [DOI] [PubMed] [Google Scholar]
- 29. Remington LT, Babcock AA, Zehntner SP, Owens T (2007) Microglial recruitment, activation, and proliferation in response to primary demyelination. Am J Pathol 5:1713–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Skripuletz T, Lindner M, Kotsiari A, Garde N, Fokuhl J, Linsmeier F et al (2008) Cortical demyelination is prominent in the murine cuprizone model and is strain‐dependent. Am J Pathol 4: 1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stidworthy MF, Genoud S, Suter U, Mantei N, Franklin RJ (2003) Quantifying the early stages of remyelination following cuprizone‐induced demyelination. Brain Pathol 3: 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Storch MK, Bauer J, Linington C, Olsson T, Weissert R, Lassmann H (2006) Cortical demyelination can be modeled in specific rat models of autoimmune encephalomyelitis and is major histocompatability complex (MHC) haplotype‐related. J Neuropathol Exp Neurol 12:1137–1142. [DOI] [PubMed] [Google Scholar]
- 33. Torkildsen O, Brunborg LA, Myhr KM, Bo L (2008) The cuprizone model for demyelination. Acta Neurol Scand Suppl 188:72–76. [DOI] [PubMed] [Google Scholar]
- 34. Trapp BD, Nave KA (2008) Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci 31:247–269. [DOI] [PubMed] [Google Scholar]


