Abstract
A persistent cycling cell population in the normal adult human brain is well established. Neural stem cells or neural progenitors have been identified in the subventricular zone and the dentate gyrus subgranular layer (SGL), two areas of persistent neurogenesis. Cycling cells in other human normal brain areas, however, remains to be established. Here, we determined the distribution and identity of these cells in the cortex, the white matter and the hippocampal formation of adult patients with and without chronic temporal lobe epilepsy using immunohistochemistry for the cell cycle markers Ki‐67 (Mib‐1) and minichromosome maintenance protein 2. Rare proliferative neuronal precursors expressing the neuronal antigen neuronal nuclei were restricted to the SGL. In contrast, the oligodendrocyte progenitor cell markers Olig2 and the surface antigen NG2 were expressed by the vast majority of cycling cells scattered throughout the cortex and white matter of both control and epileptic patients. Most of these cycling cells were in early G1 phase, and were significantly more numerous in epileptic than in non‐epileptic patients. These results provide evidence for a persistent gliogenesis in the human cortex and white matter that is enhanced in an epileptic environment.
Keywords: AOPC, cell cycle, epilepsy, human brain, oligodendrocyte
INTRODUCTION
Contrary to a long‐standing dogma, the existence of persistent dividing cells in the normal adult mammalian central nervous system (CNS) is now well established 22, 23, 80.
Rodent cycling cells are distributed throughout the entire CNS, but are particularly concentrated in two regions: the subventricular zone (SVZ) that lies directly adjacent to the ciliated ventricular ependyma and the subgranular layer (SGL) of the hippocampal dentate gyrus. These regions constitute the only well‐established neurogenic areas of the adult rodent CNS, new granule neurons arising from neural progenitors of the SGL 24, 29 and olfactory bulb interneurons arising from neural stem cells of the SVZ 14, 15, 53.
Very low levels of neurogenesis have been described in other adult CNS areas, including the frontal and temporal neocortex 30, 31, the CA1 area of the hippocampus (68) and the spinal cord 82, 83, 87, and the identity of cycling cells found in these areas is still controversial 7, 16, 41, 42, 52. Numerous cycling cells distributed in all regions of the adult rodent CNS express NG2, an integral membrane chondroitin sulfate proteoglycan 10, 25, 35, 48. In the developing CNS, NG2 proteoglycan is expressed by precursor cells of the oligodendrocyte lineage 47, 56. In the mature rodent CNS, the NG2‐immunoreactive cell population remains abundant (5%–8% of all cells), both in the cortex and the white matter 9, 63. First described in 1996, this adult NG2‐positive cells represent a distinct class of macroglial cells in the CNS, presenting two basic morphologies, bipolar and multipolar with fine branching processes forming a lattice‐like network in the cortex and the white matter (55).The ratio of NG2‐positive cells per myelinating oligodendrocytes is approximately of 1:4 in the white matter and of 1:1 in the cortex (9). Their function in the adult CNS is still a matter of debate, but their spatial distribution in close contact to nodes of Ranvier or synapses suggest that they may regulate neuronal electrical activity 5, 59. In addition, their particular physiological properties and ability to bind glutamate could contribute to the glial regulation at the synaptic cleft and/or to the neuron–glia communication 3, 39. NG2‐immunoreactive cells represent 50%–75% of all bromodeoxyuridine‐incorporating cells in the rodent cortex and spinal cord 9, 35, and exhibit a slow turnover rate (9). These cells have been termed adult oligodendrocyte precursor cells (AOPCs) as they co‐express platelet‐derived growth factor receptor‐α (PDGFRα), O4 and can differentiate into oligodendrocyte in vitro (47). Of note, NG2 expression does not extend to mature myelinating oligodendrocytes 9, 10, 55, 56. On the opposite, the transcription factor Olig2 is expressed in both oligodendrocyte progenitors and myelinating oligodendrocytes 49, 50. Interestingly, the co‐expression of Olig2 and NG2 in most non‐endothelial NG2‐positive cells has been demonstrated in the adult murine brain 13, 51 and recently, in the human temporal lobe (67) In the normal adult human CNS, cells expressing markers of rodent oligodendrocytes progenitors have also been observed 27, 75. NG2‐positive cells that appear morphologically identical to their counterparts in rodents have been reported in normal post‐mortem and multiple sclerosis brain parenchyma (6). They are distinct from astrocytes, microglia, lymphocytes or mature proteolipid protein (PLP)‐positive oligodendrocytes and represent an abundant process forming cell population (6).
Evidences for the existence of cycling cells in the human adult CNS have been provided by in vitro 1, 37, 40, 45, 58, 61, 72, 78, and in situ studies on post‐mortem human CNS (17) or intraoperative specimens. Studies using cell cycle markers such as [H3]Thymidine, BrdU, PCNA, Ki‐67 or minichromosome maintenance protein 2 (Mcm2) have identified cycling cells in the human hippocampal formation and SVZ of the lateral ventricles 4, 8, 11, 17, 73, 81. These results, associated with the generation of neurospheres from human brain biopsy samples (72), including subcortical white matter (58) and temporal cortex (78), suggest that cycling cells of the adult human CNS are endowed with stem or progenitor cell properties. However, their number and identity in the human CNS remain largely unknown, despite their therapeutic regenerative potential. A unique and recent quantitative study identified a mitotically active Olig2‐positive cell population in non‐neoplastic adult human temporal epileptic CNS, estimated to one in 5000 cortical or white matter cells (67).
The present study explored the regional distribution and the identity of cycling cells in the adult human neocortex, white matter and hippocampal formation using surgical specimens from epileptic and non‐epileptic patients, and non epileptic autopsy controls. The proliferating cells were determined by immunodetection of the Ki‐67 nuclear protein (Mib‐1), expressed by cells that are in the late G1 through M phases of the cell cycle (26), and of Mcm2 , which is expressed from early G1 up to M phase contrary to Ki‐67 19, 36, 79. We used a large array of antibodies directed against markers of the neuronal, astroglial, oligodendroglial and microglial lineages. We additionally used nestin, reported to be expressed by neural stem and progenitor cells 33, 43, 44, 46, 64.
Using double immunohistochemical procedures, we show that NG2+ cells represent the largest pool of cycling cells in the human adult brain parenchyma, including the SVZ and the SGL. Most of them are in the early G1 phase of the cell cycle, and their numbers increase in the CNS of patients suffering from non‐lesional pharmacoresistant mesial temporal lobe epilepsy.
PATIENTS AND METHODS
Patients
Our study included 10 adult patients (6 women, 4 men) who presented non‐lesional pharmacoresistant mesial temporal lobe epilepsy, treated with a standardized anterior temporal lobectomy including mesial temporal lobe structures (epileptic group). All patients had severe partial epilepsy associated or not with generalized seizures. The median age at first symptoms was 6 years (mean, 9.4; range, 2–20) and the median duration of seizures was 20 years (mean, 15.3; range, 5–32). Their median age at surgery was 25 years (mean, 26.2; range, 22–35). None of the patients underwent pre‐surgical stereotactic electroencephalography.
A first non‐epileptic control group (surgical control group) included 5 adult patients (3 women, 2 men) with a median age of 52 years at surgery (mean, 50; range, 25–66). They were surgically treated for a deep‐seated well‐circumscribed extra‐parenchymal benign lesion (four meningiomas and one schwannoma), without a history of partial or generalized epilepsy. Normal brain samples (cortex and white matter) were issued from brain parenchyma that was removed during surgery to access the tumor site. In addition, a second non‐epileptic and non‐surgical control group (autopsy control group) included 5 patients (2 women, 3 men) with a median age of 52 years at autopsy (mean, 48.6; range, 31–64). None of these patients presented a past history of epilepsy or primitive cerebral tumor. The clinical and pathological features of the patients included in the epileptic and surgical and autopsy control groups are shown in 1, 2, respectively.
Table 1.
Summary of clinical informations of the epileptic patients. Abbreviations: M = male; F = female; PE = partial epilepsy; GE = generalized epilepsy; HS = hippocampal sclerosis.
| Patients | Age at beginning of symptoms (years) | Duration of seizures (years) | Age at surgery (years) | Sex M/F | Symptoms | Histopathology | Post‐surgical interval and fixation time |
|---|---|---|---|---|---|---|---|
| 1 | 3 | 21 | 24 | M | PE | HS (CA2, CA3, CA4) | <10 minutes; 18 h |
| 2 | 19 | 6 | 25 | M | PE + GE | HS (CA1) | <10 minutes; 20 h |
| 3 | 3 | 32 | 35 | F | PE | HS (CA2, CA3) | <10 minutes; 19 h |
| 4 | 20 | 7 | 27 | M | PE | HS (CA1, CA3, CA4) | <10 minutes; 18 h |
| 5 | 3 | 20 | 23 | F | PE | HS (CA1) | <10 minutes; 17 h |
| 6 | 19 | 5 | 24 | F | PE + GE | HS (CA2, CA4) | <10 minutes; 20 h |
| 7 | 6 | 23 | 29 | F | PE | HS (CA2, CA3) | <10 minutes; 17 h |
| 8 | 2 | 20 | 22 | F | PE | HS (CA2, CA4) | <10 minutes; 20 h |
| 9 | 13 | 13 | 26 | M | PE | HS (CA1, CA2, CA3, CA4) | <10 minutes; 17 h |
| 10 | 6 | 21 | 27 | F | PE + GE | HS (CA3) | <10 minutes; 18 h |
Table 2.
Summary of clinical informations of the non‐epileptic control group; surgical control group (1–5); autopsy control group (6–10). Abbreviations: M = male; F = female; RT = right temporal; RPO = right parieto‐occipital.
| Patients | Age at surgery (years) | Sex | Pathology/cause of death | Localization | Post‐surgical interval and fixation time |
|---|---|---|---|---|---|
| 1 | 55 | F | Meningioma | RT | <5 minutes; 18 h |
| 2 | 52 | F | Meningioma | RT | <5 minutes; 15 h |
| 3 | 66 | F | Schwannoma | RT | <5 minutes; 17 h |
| 4 | 52 | M | Meningioma | RPO | <5 minutes; 15 h |
| 5 | 25 | M | Meningioma | RPO | <5 minutes; 23 h |
| 6 | 39 | M | Post‐operative hemorrhage for intramedullary tumor | No lesion | 3 days; 1 month |
| 7 | 61 | M | Sudden death | No lesion | 4 days; 6 months |
| 8 | 31 | F | Cardiogenic choc | No lesion | 1 day; 3 months |
| 9 | 64 | F | Cranial trauma | Cerebral hematoma | 1 day; 3 months |
| 10 | 48 | M | Pulmonary embolus | No lesion | 5 days; 3 months |
Histopathology
All fixed embedded tissues and paraformaldehyde fixed‐frozen tissues were retrieved from the Pathology Archives at the Department of Pathology, at Sainte‐Anne Hospital between July 1993 and July 2005. Samples were fixed in formalin‐zinc (formol 5%; zinc 3 g/L; sodium chloride 8 g/L). The median duration time for formalin‐zinc was 3 months (mean, 3.2; range, 1–6) for post‐mortem brains, and was 16 h (mean, 17.3; range, 15–23) in the epileptic and surgical control groups. According to French bioethical law, after 2004, all patients were informed of the procedure according to the local ethical committee recommendations and gave their informed consent.
In the epileptic group, 10 paraffin‐embedded blocks encompassing the external temporal cortex and 10 blocks encompassing the en bloc hippocampal resection were selected. In addition, frozen tissue from external temporal parenchyma was available for patients 7, 8 and 9.
In the surgical control group, five paraffin‐embedded blocks corresponding to normal cortex and white matter were selected. In addition, frozen tissue from normal brain parenchyma was also available for patient 1.
In the autopsy control group, 10 paraffin‐embedded blocks corresponding to the external temporal parenchyma and the hippocampal formation were selected.
Four‐micron sections from paraffin‐embedded blocks and 8 µm sections from frozen tissue were stained with hemalun‐phloxin and safranin. The slides were reviewed by two pathologists (S. G., P. V.).
In the epileptic group, the external temporal cortex and the hippocampal formation presented neither malformation nor neoplasm on microscopic examination. The external temporal parenchyma displayed classical secondary epileptic lesions such as neuronal loss and gliosis. The hippocampi exhibited: (i) moderate to severe neuronal loss resulting in atrophy of the Ammon's Horn, mainly involving CA3 and CA1, and less frequently CA4 and CA2; (ii) a discrete to moderate granular dispersion; and (iii) a non‐specific astrogliosis. The cerebral parenchyma of the surgical control group as well as the hippocampi of the autopsy control group showed a normal histological aspect.
Single chromogenic immunolabelings
Cell proliferation was evaluated using two antibodies directed against Mcm2 and Ki‐67 (Mib‐1) in all patients. In the epileptic group, both external temporal parenchyma and hippocampal formation were studied. Sections were deparaffinized and subjected to microwave antigen retrieval (Micro MEDT/T Mega, Hacker instruments, Winnsboro, SC, USA) for 30 minutes at 98°C according to manufacturer recommendations. After blocking of non‐specific endogenous peroxidase by H2O2 and non‐specific antibody‐binding sites with the Immunotech kit (Immunotech, Marseille, France), the sections were incubated with one of the following primary antibodies: Mcm2 (1/500, BD Biosciences, San Jose, CA, USA), Ki‐67 (Mib‐1 mouse, 1/75, M7240 Dako, Glostrup, Denmark) and nestin (1/1000, Chemicon, Temecula, CA, USA) for 1 h at ambient temperature. The reaction was revealed using the diaminobenzidine chromogene (kit DAB K3468, Dako, Trappes, France), and the nuclei were counterstained with hemalun. All washes were carried out with phosphate buffered saline. Light microscopic images were digitally captured using a Nikon eclipse E600 microscope (Nikon, Tokyo, Japan) equipped with Nikon DXM 1200 Digital camera. Photomicrographs were assembled for illustrations using the Adobe Photoshop version 7.0.1 software (Adobe, San Jose, CA, USA).
Cell count and statistical analyses
In each section, all non‐endothelial Mcm2 and Mib‐1 immunoreactive cells were counted and topographically reported within the cortex, the white matter or the hippocampal formation. Endothelial cells, easily identified using simple morphological criteria (elongated regular endothelial nuclei organized in longitudinal or circular patterns), were excluded from the counts. For analytical purposes, the hippocampal formation was divided into four distinct zones: (i) the dentate gyrus, including the granule cell layer and the SGL; (ii) the Ammon's horn (hippocampus proper with CA1, CA2, CA3 and CA4); (iii) the subiculum; and (iv) the white matter outside the hippocampal formation. The surface of the analyzed section was calculated using a 2 × 25 magnification, and the LUCIA version 4.71 software (Liberex, Prague, Czech Republic). Mcm2 and Mib‐1 immunoreactive cells were counted with a 40 × 25 magnification on one section of each block. Olig2 immunopositive cells were counted on an adjacent section with a 60 × 25 and 40 × 25 magnification for the white matter and cortex areas, respectively. Results were expressed as number of Mcm2 and Mib‐1‐immunoreactive cells per cm2. Statistical analyses were performed to assess the difference in the number of Mcm2 and Mib‐1 immunoreactive cells per cm2 between epileptic and control groups. Non‐parametric Kruskal–Wallis analysis of variance was used to test significant differences in the different groups. Then, post‐hoc non‐parametric Mann–Whitney rank sum test was also performed to test differences between groups. The level of significance was P < 0.05. Analyses were performed using JMP 5.1 (SAS Institute Inc., Cary, NC, USA).
Double chromogenic immunolabelings
The identification of the cycling cells was performed using double immunolabeling with Mib‐1 as the first primary antibody and with several second primary antibodies directed against markers of various cellular types to identify cells of: (i) the neuronal lineage βIII‐tubulin or Tuj‐1 (20), calretinin (21), microtubule associated protein 2 or MAP2 (38), neuronal nuclei or NeuN (74); (ii) the astroglial lineage vimentin and glial fibrillary associated protein or GFAP (88); (iii) the oligodendroglial lineage Olig2 89, 90, NG2 6, 9, 10 and 2′ 3′‐cyclic nucleotide 3′‐phosphodiesterase or CNPase 65, 76; and (iv) the microglial lineage KP1/CD68 and Ricinus communis agglutinin or RCA (18).
Double immunostainings with Mib‐1/nestin, Mib‐1/vimentin, Mib‐1/GFAP, Mib‐1/RCA, Mib‐1/Kp1, Mib‐1/MAP, Mib‐1/CPNase were performed in each case of the epileptic and non‐epileptic surgical control groups. Double immunostaining with Mib‐1/Tuj‐1 was only performed on hippocampal sections of the epileptic group.
Sections were deparaffinized, dehydrated in alcohol, washed in water then subjected to microwave antigen retrieval at 98°C in a plug citrate (10 mM in H2O, pH 6) for 45 minutes. After blockade of the activity of endogenous peroxidase by H2O2 and the saturation of the non‐specific binding of immunoglobulin with swine normal serum (5% in Tris‐buffered saline Triton), the sections were incubated for 1 h with the antibody Mib‐1 (Ki‐67 rabbit, 18‐0191, Zymed, Fremont, CA, USA) at ambient temperature, then in the Goat Anti‐Rabbit vector (GAR) 1/200 for 30 minutes (Vectastain®, Burlingame, CA, USA). The revelation was performed using the complex alkaline avidin‐biotine‐phosphatase reaction. Nuclear staining was visualized with the kit Rouge Fast Red 0699 (Dako, red color). After several washes with distilled water and Tris Buffered Saline with Tween (TBST), a second blockade of the activity of endogenous peroxidase by H2O2 was carried out for 10 minutes, sections were then incubated for 1 h in one of the following second primary antibodies: anti‐nestin (1/1000, Chemicon), anti‐vimentin (1/400, Dako), anti‐GFAP (1/200, Dako), anti‐Kp1 (1/400, Dako), anti‐RCA (1/10000, Vector, Burlingame, CA, USA), anti‐MAP2 (1/100, Sigma, USA), anti‐ßIII tubulin (Tuj‐1, 1/1250, Sigma, USA) and anti‐CNPase (1/250, Abcam, UK). The revelation was achieved with the peroxidase EnVisionTM polymer K4063 (Dako), and diaminobenzidine as a chromogene (kit DAB K3468, Dako, brown color). Sections were counterstained with hemalun. In addition, in the epileptic group, sections of external temporal cortex and of hippocampal formation containing nestin‐immunoreactive cells (patients 4, 5, 6 and 8) were double immunostained with anti‐nestin/Mib‐1, anti‐nestin/anti‐vimentin, anti‐nestin/anti‐Mcm2 and anti‐nestin/anti‐Olig2, using the methodology described above.
Double immunofluorescence labelings
Double immunofluorescence labelings of Olig2/Ki‐67 were carried out in sections of the external temporal parenchyma of the epileptic group (patients 8 and 10) and of normal parenchyma of the surgical control group (patient 1). In addition, double immunofluorescence labelings of NeuN/Ki‐67 were performed on hippocampal sections of the epileptic group (patients 1 and 9), and NG2/Olig2 was performed in frozen sections of the external temporal parenchyma of the epileptic group (patients 8 and 10) and of normal parenchyma of the surgical control group (patient 1).
Sections were deparaffinized, dehydrated in alcohol, washed in water, prior to being subjected to microwave antigen retrieval as described previously. After the blockade of endogenous peroxidase activities by H2O2 and the saturation of non‐specific antigenic sites by the Immunotech kit for 20 minutes (Immunotech), sections were incubated for 1 h with the primary antibodies Olig2 (1/25, R&D, Minneapolis, MN, USA) or NeuN (1/500, Chemicon) in phosphate buffered saline. Labels were visualized using CY3‐coupled streptavidine (1/3000 in TBST, 40 minutes, ambient temperature, Jackson Labs, Campbell, CA, USA). The second blockade of the antigenic sites was carried out using 5% donkey normal serum in TBST. Sections were then incubated with the second primary mouse antibody Mib‐1 (1/75, M7240 Dako, Denmark). Visualization was achieved using a donkey‐anti‐mouse antibody coupled to Alexa fluor® 488 (1/4000, Molecular Probe, Carlsbad, CA, USA).
Double immunofluorescence labelings of NG2/Mib‐1 and NG2/Olig2 were performed on paraformaldehyde fixed and frozen sections of the external temporal parenchyma (patients 8, 9 and 10 of the epileptic group) and the normal parenchyma (patient 1 of the surgical control group). Frozen sections were incubated in 5% horse normal serum for the first blockade of antigenic sites, followed by an overnight incubation at ambient temperature with anti‐NG2 primary antibody (NG2 1/100, mab 9.2.27, gift from Dr. W. B. Stallcup). Labeling was visualized by a combination of Streptavidine and CY3 fluorochrome (1/3000, Jackson Labs). The second blockade of the antigenic sites was carried out with 5% goat normal serum in TBST concerning Mib‐1 double immunostaining and 5% donkey normal serum for Olig2 double immunostaining. Sections were then incubated in second primary antibody rabbit Mib‐1 (1/100) or goat Olig2 (1/100). Labeling was visualized using GAR (1/200) or donkey antigoat (1/200), directly coupled with Alexa fluor ®488 (1/100, Molecular Probe). Confocal imaging, quantification and photomicrograph production on tissue stained sections were performed using a confocal microscope Leica TCS SP2 (Leica Microsystems, Heerbrugg, Switzerland). Two different lasers were used depending on the fluorochrome. The images were prepared for printing using Adobe Photoshop version 7.0.1 software (Adobe system).
RESULTS
Fixation time affects cycle‐related immunostainings
Comparison of Mib‐1 and Mcm2 immunostainings between the surgical and autopsy control groups showed that no Mib‐1‐positive cells could be detected in the autopsy control group (mean fixation time = 3.2 months). Accordingly, few Mcm2‐positive cells were detected in the cortex and white matter of only two of the five autopsy cases (median number of Mcm2‐positive cells/cm2 = 0.66). On the opposite, the surgical control group (mean fixation time = 18 h), exhibited Mcm2‐positive cells in all cases and in higher number than in the autopsy controls (median number of Mcm2‐positive cells/cm2 = 5.7, Figure 1). These results led us to exclude autopsy cases from further analysis, and focused on surgical control groups. We, nevertheless, present the immunolabelings of the hippocampus in autopsy controls, the only ones available for this region under control conditions.
Figure 1.
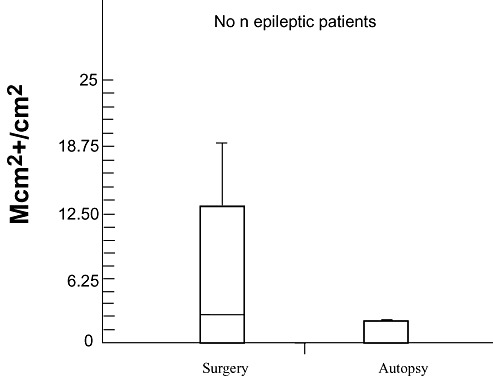
Comparative Mcm‐2 immunodetection in tissues derived from the surgical and autopsy control groups. Fewer Mcm2 labeled cells are detected in the autopsy control group (mean fixation time: 3, 2 months) than in the surgical control group (mean fixation time: 18 h). Abbreviations: Mcm2 = minichromosome maintenance protein 2.
Cycling cells in early G1 phase of the cell cycle are dispersed throughout the cortex and the white matter of control brains
In the surgical control CNS, Mib‐1‐ and Mcm2‐positive cells were found both in the white matter and the cortex, with a preferential location at the zone of junction between the cortex and the white matter (Figure 2). Labeled nuclei were small (8–12 µm in diameter), well‐circumscribed, round to oval, their chromatin condensed in small dots and were devoid of any discernable cytoplasm. In the cortex, none of the pyramidal neurons were labeled. Mib‐1‐ and Mcm2‐positive cells were dispersed (Figure 2A), some of them appearing located close to a neuron (ie, perineuronal satellitosis). In the white matter, some of the positive cells were located close to myelinated fibers, without any vascular tropism (Figure 2C). Some of the Mib‐1 and Mcm2‐positive cells were observed near small capillaries, but were not in close contact with pericytes or endothelial cells.
Figure 2.
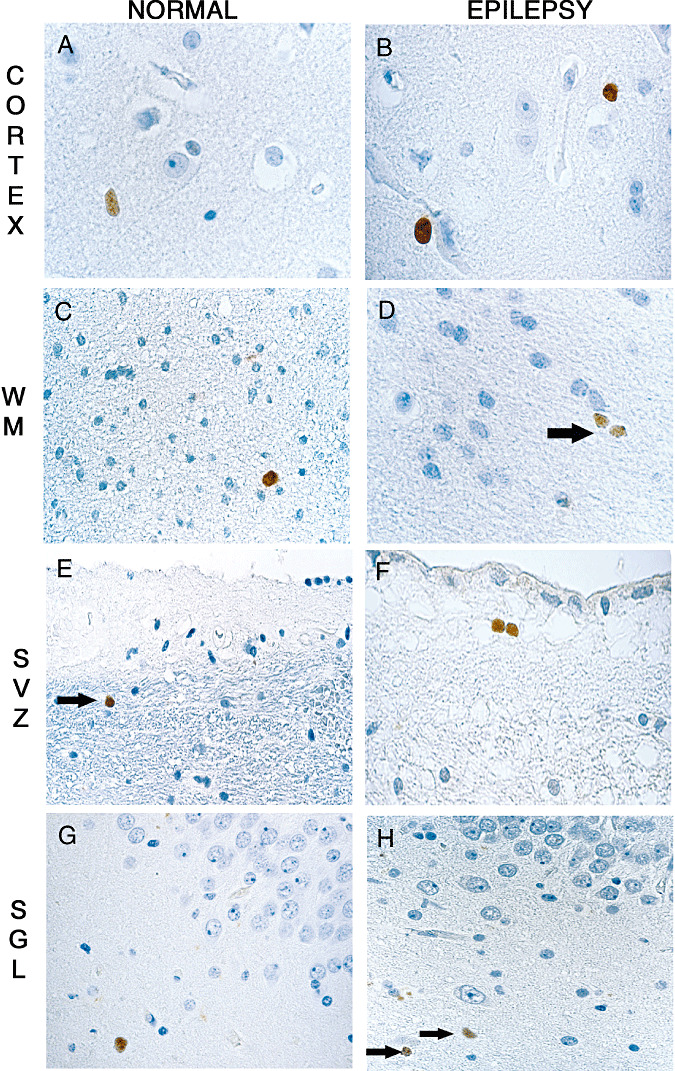
Cycling cells are dispersed in the cortex and the white matter of the control (A,C,E,G) and epileptic brains (B,D,F,H). Mib‐1‐positive nuclei are restricted to round monomorphous regular nuclei, in the cortex (A,B) or in the white matter (C,D, arrow). In the autopsy control group, rare Mcm2‐positive cells are detected in the lateral ventricle SVZ (E, arrow) as well as in the SGL (G). In the SVZ of epileptic patients, pairs of Mcm‐2‐positive nuclei are visualized (F) and some single cells are present in the SGL (H, arrows). Magnification in A,C,D,E,G,H: ×600; B,F: ×1000. Scale bar = 10 µm. Abbreviations: Mcm2 = minichromosome maintenance protein 2; Mib‐1 = Ki‐67; SGL = subgranular layer; SVZ = subventricular zone; WM = white matter.
In the hippocampi of the autopsy control group, Mcm2‐labeled cells were observed in two cases (patients 8, 9): in the SVZ around the lateral ventricle (Figure 2E, arrow) or within the SGL (Figure 2G). In the SVZ, no ependymal cells were labeled (Figure 2E). Of note, a pair of Mcm2 immunoreactive cells was observed only in one case (patient 8).
In the cortex and white matter of the surgical control group, the density of Mcm2‐positive cells (median, 13.04 cells/cm2) was higher than that of Mib‐1‐positive cells (median, 4.17 cells/cm2, P = 0.006, Figure 3). Likewise, in the epileptic group, Mcm2‐positive cells densities were higher than Mib‐1‐positive cells densities in the hippocampal formation (Mcm2: median, 60.0 cells/cm2; Mib‐1: median, 30.1 cells/cm2; P = 0.002, Figure 3) and in the external temporal cortex (Mcm2: median, 80.0 cells/cm2; Mib‐1: median, 12.44 cells/cm2; P = 0.001, Figure 3).
Figure 3.
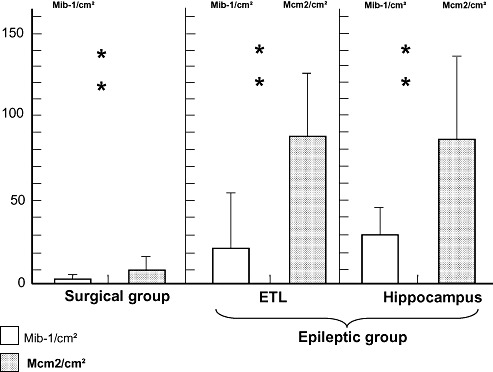
Comparative data show that Mcm2‐positive cells/cm2 are consistently more abundant than Mib‐1‐positive cells/cm2 in the surgical control group (P = 0.006) and in the external temporal lobe (ETL) (P = 0.001) and the hippocampal formation (P = 0.002) of epileptic patients. Abbreviations: Mcm2 = minichromosome maintenance protein 2; Mib‐1 = Ki‐67.
Cycling cells are increased in the external temporal parenchyma of epileptic patients
In the epileptic group, Mib‐1and Mcm2 immunoreactive cells were observed in all patients, in both the external temporal parenchyma and the hippocampal formation. Single or paired Mcm2‐ and Mib‐1‐positive cells exhibited the same morphology and distribution as in the non‐epileptic control group (Figure 2B,D,F,H). In all hippocampal formations, immunopositive Mib‐1or Mcm2 nuclei were frequent in the fimbria and the white matter around the Ammon's horn, but rare in the SGZ and the granule cell layer of the dentate gyrus (Figure 2H, arrows).
The number of Mib‐1and Mcm2 immunopositive cells per cm2 varied according to their location in the external temporal parenchyma and in the hippocampal formation and among patients (Table 3). No statistically significant difference (P = 0.127), was found between the number of Mib‐1‐positive cells per cm2 in the white matter (median, 22.4) as compared with the cortex (median, 6.83) (Figure 4). On the opposite, there was statistically more (P = 0.017) Mib‐1‐positive cells per cm2 in the external temporal parenchyma of the epileptic group (median, 12.4 cells/cm2) than in the surgical control group (median = 4.17 cells/cm2; Figure 5). No statistically significant correlation was found between the numbers of cycling cells per cm2 (Mib‐1 or Mcm2 immunoreactive cells) in the external temporal parenchyma or the hippocampal formation, and the various clinical data, that is, sex, age at the beginning of symptoms, duration of symptoms and age at surgery.
Table 3.
Quantification of Mib‐1‐positive cells/cm2 in the external temporal parenchyma of the epileptic patient group and of the non‐epileptic surgical control group in the cortex (Cx) and the white matter (WM).
| Mib‐1+ cells/cm2 | Mib1+ cells/cm2 Cx | Mib‐1 + cells/cm2 WM | |
|---|---|---|---|
| Epileptic patients | |||
| 1 | 28.45 | 29.33 | 26.92 |
| 2 | 9.1 | 3.18 | 20.9 |
| 3 | 14 | 11.53 | 18.57 |
| 4 | 5.85 | 3.42 | 14.28 |
| 5 | 104.64 | 82.92 | 164 |
| 6 | 10.88 | 5.83 | 19.17 |
| 7 | 9.61 | 5.06 | 24 |
| 8 | 21.28 | 7.84 | 63.26 |
| 9 | 3.77 | 1.7 | 8.18 |
| 10 | 16.75 | 9.03 | 45.23 |
| Non‐epileptic patients | |||
| 1 | 8.33 | 2.78 | 13.33 |
| 2 | 7.14 | 3.57 | 6.67 |
| 3 | 4.17 | 0 | 33.3 |
| 4 | 1.35 | 0 | 9.09 |
| 5 | 0 | 0 | 0 |
Figure 4.

The densities of Mib‐1‐positive cells are not statistically different in the white matter (WM) and the cortex (Cx) of either the surgical control group or the epileptic group. Abbreviations: Mib‐1 = Ki‐67.
Figure 5.
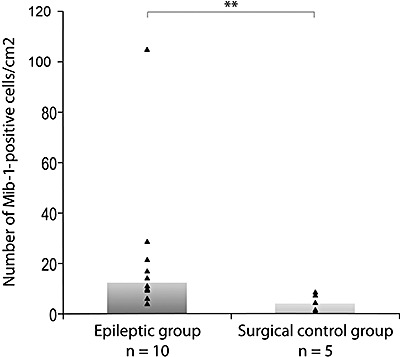
Increased numbers of Mib‐1‐positive cells/cm2 in the external temporal parenchyma of epileptic patients as compared with the surgical control group (P = 0.017).
Cycling cells correspond to Olig2 and NG2‐expressing cells
In order to characterize cycling cells, we performed double immunostainings in both surgical control group (Figure 6) and epileptic patients (Figure 7) for Ki‐67/nestin, Ki‐67/GFAP, Ki‐67/vimentin, Ki‐67/ßIII‐tubulin, Ki‐67/MAP2, Ki‐67/Calretinin and Ki‐67/CNPase. No Mib‐1‐positive cells co‐expressed GFAP (Figure 6A, arrow and Figure 7A,B), vimentin (Figure 7C,D), KP1 (Figure 7E,F), CNPase (Figure 6B, arrow and Figure 7G), MAP2 (Figure 7H), nestin (Figure 7I), calretinin (Figure 7J) or ßIII‐tubulin (not shown). The majority of Mib‐1‐positive cells in the external temporal parenchyma were RCA‐negative except for two cells in one epileptic patient (patient 3, not shown). Exceptional nestin‐immunopositive cells were observed in four patients (patients 4, 5, 6 and 8). They exhibited two distinct morphological features according to their anatomical location. In the white matter, they had a small size (6–8 µm in diameter), and a bipolar morphology with a round to oval nucleus and little discernable cytoplasm. They did not co‐express vimentin (not shown). Nestin‐immunoreactive cells localized in the hilus of the dentate gyrus, and in the CA4 area had a reactive astrocyte‐like morphology, and co‐expressed vimentin, a marker of the astroglial lineage (not shown). Of note, no nestin‐immunoreactive cells co‐expressed Mib‐1, Mcm2 or Olig2.
Figure 6.
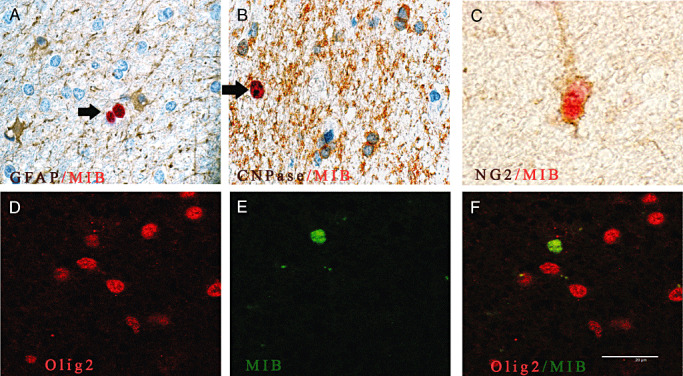
Double immunostainings show that cycling cells co‐express Olig2 or NG2 in the surgical control brain. A,B. No Mib‐1‐positive cells (red, arrow) co‐express GFAP (brown) (A) or CNPase (brown) (B). C. All Mib‐1‐positive cells (red) co‐localized with non‐endothelial NG2‐positive cells (brown). D–F. Confocal microscopy of double immuno‐fluorescence stainings of Olig2 (red, D) and Mib‐1 (green, E) reveal that all Mib‐1‐positive cells co‐express Olig2 (F, overlay of D and E). Magnification in A,B,D,E and F: ×600; C: ×1000. Scale bar = 20 µm. Abbreviations: GFAP = glial fibrillary associated protein; CNPase = 2′ 3′‐cyclic nucleotide 3′‐phosphodiesterase; Mib‐1 = Ki‐67.
Figure 7.
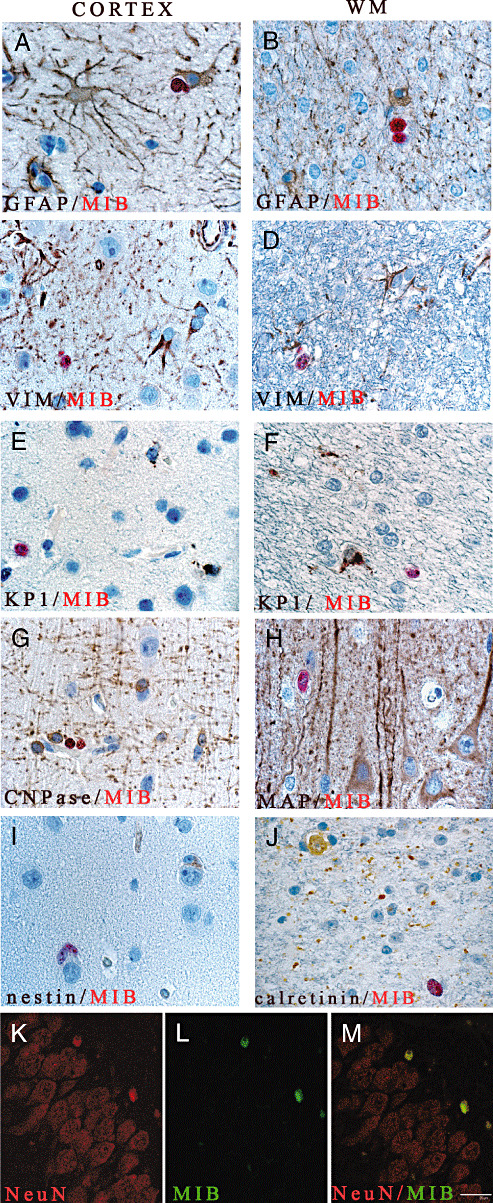
Double immunostainings revealed that cycling cells do not share markers of the astrocytes, microglia, mature oligodendrocytes or neurons, except in the SGL of the epileptic patients. No Mib‐1‐positive cell (red) co‐express GFAP (A,B), vimentin (C,D), KP1 (E,F), CNPase (G), MAP‐2 (H), nestin (I) or calretinin (J). Confocal microscopy of double immuno‐fluorescence stainings of NeuN (red, K) and Mib‐1 (green, L) in cells of the subgranular layer (overlay, M). Magnification = ×400 in A–J, ×600 in K–M. Scale bar = 7 µm. Abbreviations: GFAP = glial fibrillary associated protein; CNPase = 2′ 3′‐cyclic nucleotide 3′‐phosphodiesterase; Mib‐1 = Ki‐67; MAP2 = microtubule associate protein 2; NeuN = neuronal nuclei; WM = white matter.
Except in the SGL of the hippocampus, where a few cells co‐expressing NeuN and Mib‐1 were observed in the SGL in two cases of the epileptic group (patients 2 and 10; Figure 7K–M) in the surgical control and epileptic groups, all Mib‐1‐positive cells co‐expressed the markers NG2 and Olig2 (Figure 6C–F and Figure 8F–L). All NG2 glial cells were Olig2 positive in the white matter (Figure 8B) as in the cortex (Figure 8C), and were easily distinguished from NG2+/Olig2− pericytes (not shown). In the cortex, NG2/Olig2 co‐labeled cells are often seen in a perineuronal satellitosis situation (Figure 8D, arrow). These NG2 cycling cells exceptionally in mitosis (Figure 8E, arrow) exhibited fine cytoplasmic processes (Figure 8F–H). Rare pairs of cycling NG2‐positive cells can also be demonstrated (Figure 8I, arrow).The overall mean of Olig2+/MIB+/cm2 in the cortex was evaluated to 0.031% and 0.04% in the white matter in epileptic patients.
Figure 8.
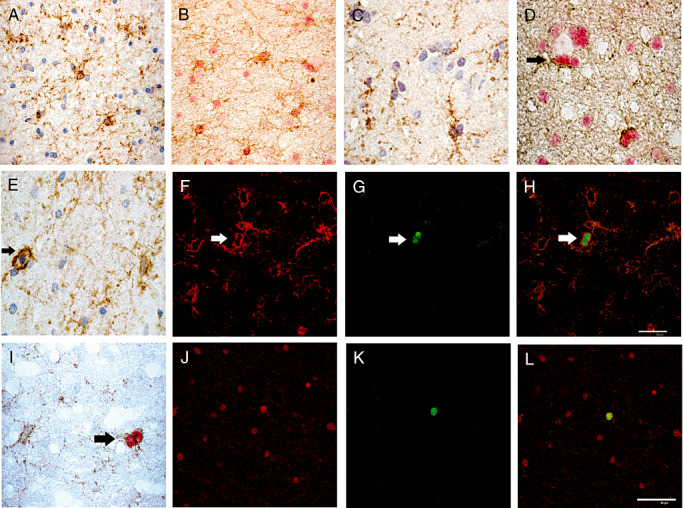
NG2 expressing cells are dispersed in the white matter (A,B,E,I) and the cortex (C,D) in epileptic patients and presented a multipolar appearance. Double immunostainings revealed that all NG2‐positive cells (brown, B,D) co‐expressed Olig2 (red, B,D) in the white matter as well as in the cortex, sometimes in a perineuronal satellitosis location (D, arrow). A unique NG2‐positive cell was in mitosis (brown, E). Double immuno‐fluorescence stainings showed that in epileptic patients cycling cells correspond to Olig2 and NG2‐expressing cells. Confocal microscopy revealed that all Mib‐1‐positive cells (green, G,K) co‐expressed the oligodendrocyte progenitor cells markers NG2 (red, F) or Olig2 (red, J). H,L. overlay respectively of F and G and J and K. Double chromogenic immunostaining of NG2/ Mib‐1 confirm that some paired of fine ramified NG2‐positive cells (brown) co‐expressed Mib‐1 (red) (I). Magnification = ×400 in A–C,E,I; =×600 in D,F–H,J–L, scale bar = 30 µm.
DISCUSSION
Our data show that the “in‐the‐cell‐cycle” cell population distributed throughout the cortex and the white matter of adult human brain exhibit the immunoprofile of oligodendrocyte precursor cells. These cells represent the majority of cycling cells, neuronal precursors being found only in the SGZ of the hippocampus, are for most of them maintained in G1 phase and are more abundant in the chronic epileptic brain parenchyma.
Cycling cells are present in both non‐epileptic and epileptic adult human CNS
The first evidence of dividing cells in the human non‐epileptic adult CNS was directly provided in 1998, using the post‐mortem dentate gyrus of five patients (17). Several in vitro studies indirectly confirmed these data by obtaining neural stem cells from cerebral tissue of the SVZ and hippocampal formation of young adults 37, 44, 64, 78. Recently, two other studies using PCNA (nuclear protein expressed during all phases of the cell cycle) and Mcm2 showed cycling cells in the SGL of the dentate gyrus and in the posterior SVZ of epileptic and non‐epileptic patients (8). Here, we used two complementary markers of cycling cells. One, Ki‐67 labels cells which are in the late G1 phase and beyond. The other, Mcm‐2, labels cells that have entered the cell cycle, including cells in the early G1 phase 19, 36, 79. Mib‐1 detection has been reported to be altered by the fixation time of the tissue (71). Accordingly, we did not observe Mib‐1‐positive cells in the autopsy control group. The paucity of Mcm2‐positive cells in this group, as opposed to surgical groups, indicates that Mcm2 is also strongly affected by long formalin‐fixation times.
Our results obtained in surgical specimens ascertain the presence of cycling cells (ie, Mib‐1‐ and Mcm2‐positive cells), predominantly in the white matter of the external temporal parenchyma of the adult CNS in both epileptic and non‐epileptic patients. These data are in accordance with recent findings (67) evaluating the number of Ki‐67‐positive cells at 0.046% in the white matter of human temporal lobe in epileptic patients, compared with 0.034% in the cortex. In good agreement with our findings, a great individual regional variability of the repartition of Mib‐1‐positive cells, without any correlation with sub‐localization, patient age, gender and underlying pathology, was also reported. In all our cases, the density of Mcm2‐positive cells was higher than that of Ki‐67‐positive cells, suggesting that a significant proportion of cycling cells in the human CNS (Mcm2‐positive and Ki‐67‐negative cells) are either slowly dividing cells/extended G1 phase or are blocked in various G1 check points. Because the duration of G1 phase is extremely variable, a substantial fraction of cells may differ markedly in Ki‐67 and Mcm2 expression patterns. We also observed that Ki‐67 was more fixation‐dependent than Mcm2 and in agreement with others (70); we recommend to use Mcm2 labeling to assess the cell cycle, on histological archival material. Interestingly, in the rat adult intact spinal cord, a long cell cycle duration for NG2 expressing cells has been documented, estimated of 5–7 days (35).
Anterior temporal lobectomy including mesial temporal lobe structures is an effective treatment of non‐lesional pharmacoresistant mesial temporal epilepsy (85).
In contrast to rodent CNS, we observed only rare cycling cells in the SGL of the dentate gyrus of the epileptic patients. The paucity of these cells may result from their lower proliferation rate in humans than in rodent epileptic dentate gyrus. Alternatively, chronic epilepsy may not only affect neuronal survival, as illustrated by the severe neuronal loss, but also the viability of cycling cells.
Enhanced numbers of cycling cells have been previously reported in the SGL of chronic epileptic patients 4, 8, 11. Our results show that enhanced number of cycling cells in chronic epileptic patients occurs also in the external temporal parenchyma.
Most cycling cells express NG2 in the adult CNS
Adult rodent CNS contain not only neural progenitors in the SVZ and the SGL but also glial progenitor cells (ie, self‐renewing precursors giving birth to astrocytes and oligodendrocytes) that have been identified throughout the neuraxis, from the cortex to the spinal cord 10, 35, 62. In humans, previous in situ studies focused either on neurogenesis or on the identification of cycling cells but disregarded cycling glial progenitors 4, 8, 11. Moreover, several studies have reported the identification of cycling cells lacking the expression of markers of astrocytes, neurons, endothelial cells and neural progenitor cells 4, 8, 11. Very recently, Rhee et al identified a mitotically active Olig2 and NG2 cell population in human temporal lobe, estimated to be 1/2500 to 5000 in subcortical white matter of chronic epileptic patients (67). Thus, no comparative data between the spatial distribution and the relative proportion of neurogenesis and gliogenesis in adult human CNS are available.
Here, we show that almost all Mib‐1‐positive cells co‐express Olig2 or NG2, including progenitors in the hippocampal formation. These co‐labeled cells harbor a bipolar or multipolar appearance, with delicate NG2‐positive processes identical to those described in the adult rodent brain 9, 10, 54, 55. Cycling cells expressing NG2 represent only a fraction of the total NG2‐positive population were observed.
Several rodent studies have shown that NG2 immunoreactive cells are mitotically active, with a slow turnover rate evaluated to 5–7 days (35) and represent the main pool of proliferative progenitor cells (70%) in the normal rodent CNS 25, 48, 75. Retroviral lineage studies in rodents suggest that a fraction of NG2 cycling cells give rise to oligodendrocytes (48). Recently, Dimou et al confirmed, using a permanent reporter gene expression in the Olig2 locus, that virtually all labeled BrdU were Olig2+ and NG2+, outside of the neurogenic niches of the adult rodent brain. They also demonstrated that NG2+ cells gave rise to new mature myelinating oligodendrocytes in the undamaged adult white matter, whereas progeny of these cells in the cortex remained NG2+(13). Our results are in accordance with these data and raise the possibility that NG2 cycling cells present in the human brain correspond also to AOPCs.
No data are actually available concerning PDGFRα or O4 expression in this specific cycling AOPC cell population. Scolding et al described rare PDGFRα expressing cells (in order of 1%) in normal post‐mortem white matter, without any data concerning their turnover rate (71)
Numbers of NG2‐positive cells are altered in response to neurological disease. In experimental models of demyelinating diseases and in multiple sclerosis, bipolar NG2‐positive cells increase in the immediate peripheral area of the demyelinated lesion 6, 57, 66, 86. These observations suggest that AOPCs are able to react in response to a local signal and might represent a source of new oligodendrocytes for remyelination (12). The present study confirmed that rarer cycling cells expressing neuronal markers can be observed in the SGL of chronic epileptic patients than in adult primates or adult rodent SGL, estimated daily to 1/2000 granule cells (22).
Recent studies of gliogenesis in rodents suggest that these cells could also be mobilized in response to epilepsy 60, 84. Electroconvulsive seizures have been shown to counterbalance corticosterone‐induced inhibition of gliogenesis in the hippocampus (81). Similarly, Parent et al report that prolonged seizures increase gliogenesis within the SVZ and attract new glial cells in the injured hippocampal areas (58). The consequences of these ectopic glial cells are actually unknown but understanding how newly generated glia influence the function of the injured hippocampal formation may provide novel insights into epileptogenic mechanisms. The signals leading to oligodendroglial progenitor proliferation in such pathological conditions remain to be identified. Several growth factors such as basic fibroblast growth factor and vascular endothelial factor are up‐regulated in response to seizures 32, 34, 69. NG2 is able to bind with a high affinity to basic fibroblast growth factor (bFGF) and PDGF‐AA (28) that are critical mitogens for oligodendrocyte progenitors (2). Progenitors submitted in vitro to NG2 blocking antibodies that fail to proliferate normally in response to growth factors (55). Additionally, oligodendrocytes progenitor cells culture‐derived from NG2 knockout mice, do not respond to bFGF and PDGF‐AA, and progress along the oligodendroglial lineage, contrary to wild‐type progenitors that maintain an immature phenotype (77). These results suggest a role for NG2 in the proliferation responsiveness of AOPCs to bFGF induced by seizures. Interestingly, cycling NG2 cells from the substantia nigra of adult rats capable of giving birth to new glial cells in situ, but also to new neurons in vitro and in adult hippocampi has been identified (47). Determining whether the cycling NG2 cell population we identified in the adult human brain is endowed with the potential to give birth to macroglial and/or neurons, at least in vitro should open novel insights for cell replacement strategies.
REFERENCES
- 1. Arsenijevic Y, Villemure JG, Brunet JF, Bloch JJ, Deglon N, Kostic C et al (2001) Isolation of multipotent neural precursors residing in the cortex of the adult human brain. Exp Neurol 170:48–62. [DOI] [PubMed] [Google Scholar]
- 2. Baron W, Metz B, Bansal R, Hoekstra D, De Vries H (2000) PDGF and FGF‐2 signaling in oligodendrocyte progenitor cells: regulation of proliferation and differentiation by multiple intracellular signaling pathways. Mol Cell Neurosci 15:314–329. [DOI] [PubMed] [Google Scholar]
- 3. Bergles DE, Tzingounis AV, Jahr CE (2002) Comparison of coupled and uncoupled currents during glutamate uptake by GLT‐1 transporters. J Neurosci 22:10153–10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blumcke I, Schewe JC, Normann S, Brustle O, Schramm J, Elger CE, Wiestler OD (2001) Increase of nestin‐immunoreactive neural precursor cells in the dentate gyrus of pediatric patients with early‐onset temporal lobe epilepsy. Hippocampus 11:311– 321. [DOI] [PubMed] [Google Scholar]
- 5. Butt AM, Duncan A, Hornby MF, Kirvell SL, Hunter A, Levine JM, Berry M (1999) Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia 26:84–91. [PubMed] [Google Scholar]
- 6. Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD (2000) NG2‐positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci 20:6404–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen J, Magavi SS, Macklis JD (2004) Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proc Natl Acad Sci USA 101:16357–16362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crespel A, Rigau V, Coubes P, Rousset MC, De Bock F, Okano H et al (2005) Increased number of neural progenitors in human temporal lobe epilepsy. Neurobiol Dis 19:436–450. [DOI] [PubMed] [Google Scholar]
- 9. Dawson MR, Levine JM, Reynolds R (2000) NG2‐expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res 61:471–479. [DOI] [PubMed] [Google Scholar]
- 10. Dawson MR, Polito A, Levine JM, Reynolds R (2003) NG2‐expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci 24:476–488. [DOI] [PubMed] [Google Scholar]
- 11. Del Bigio MR (1999) Proliferative status of cells in adult human dentate gyrus. Microsc Res Tech 45:353–358. [DOI] [PubMed] [Google Scholar]
- 12. Di Bello IC, Dawson MR, Levine JM, Reynolds R (1999) Generation of oligodendroglial progenitors in acute inflammatory demyelinating lesions of the rat brain stem is associated with demyelination rather than inflammation. J Neurocytol 28:365–381. [DOI] [PubMed] [Google Scholar]
- 13. Dimou L, Simon C, Kirchhoff F, Takebayashi H, Gotz M (2008) Progeny of Olig2‐expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci 28:10434–10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doetsch F (2003) The glial identity of neural stem cells. Nat Neurosci 6:1127–1134. [DOI] [PubMed] [Google Scholar]
- 15. Doetsch F, Caille I, Lim DA, Garcia‐Verdugo JM, Alvarez‐Buylla A (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97:703–716. [DOI] [PubMed] [Google Scholar]
- 16. Ehninger D, Kempermann G (2003) Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cereb Cortex 13:845–851. [DOI] [PubMed] [Google Scholar]
- 17. Eriksson PS, Perfilieva E, Bjork‐Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4:1313–1317. [DOI] [PubMed] [Google Scholar]
- 18. Esiri MM, Morris CS (1991) Immunocytochemical study of macrophages and microglial cells and extracellular matrix components in human CNS disease. 2. Non‐neoplastic diseases. J Neurol Sci 101:59–72. [DOI] [PubMed] [Google Scholar]
- 19. Eward KL, Obermann EC, Shreeram S, Loddo M, Fanshawe T, Williams C et al (2004) DNA replication licensing in somatic and germ cells. J Cell Sci 117:5875–5886. [DOI] [PubMed] [Google Scholar]
- 20. Fanarraga ML, Avila J, Zabala JC (1999) Expression of unphosphorylated class III beta‐tubulin isotype in neuroepithelial cells demonstrates neuroblast commitment and differentiation. Eur J Neurosci 11:517–527. [PubMed] [Google Scholar]
- 21. Fonseca M, Soriano E (1995) Calretinin‐immunoreactive neurons in the normal human temporal cortex and in Alzheimer's disease. Brain Res 691:83–91. [DOI] [PubMed] [Google Scholar]
- 22. Gage FH (2000) Mammalian neural stem cells. Science 287:1433–1438. [DOI] [PubMed] [Google Scholar]
- 23. Gage FH (2002) Neurogenesis in the adult brain. J Neurosci 22:612–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J (1998) Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol 36:249–266. [DOI] [PubMed] [Google Scholar]
- 25. Gensert JM, Goldman JE (2001) Heterogeneity of cycling glial progenitors in the adult mammalian cortex and white matter. J Neurobiol 48:75–86. [PubMed] [Google Scholar]
- 26. Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H (1984) Cell cycle analysis of a cell proliferation‐associated human nuclear antigen defined by the monoclonal antibody Ki‐67. J Immunol 133:1710–1715. [PubMed] [Google Scholar]
- 27. Gogate N, Verma L, Zhou JM, Milward E, Rusten R, O'Connor M et al (1994) Plasticity in the adult human oligodendrocyte lineage. J Neurosci 14:4571–4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goretzki L, Burg MA, Grako KA, Stallcup WB (1999) High‐affinity binding of basic fibroblast growth factor and platelet‐derived growth factor‐AA to the core protein of the NG2 proteoglycan. J Biol Chem 274:16831–16837. [DOI] [PubMed] [Google Scholar]
- 29. Gould E, Tanapat P (1997) Lesion‐induced proliferation of neuronal progenitors in the dentate gyrus of the adult rat. Neuroscience 80:427–436. [DOI] [PubMed] [Google Scholar]
- 30. Gould E, Reeves AJ, Graziano MS, Gross CG (1999) Neurogenesis in the neocortex of adult primates. Science 286:548–552. [DOI] [PubMed] [Google Scholar]
- 31. Gould E, Vail N, Wagers M, Gross CG (2001) Adult‐generated hippocampal and neocortical neurons in macaques have a transient existence. Proc Natl Acad Sci USA 98:10910–10917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grinspan JB, Stern JL, Franceschini B, Pleasure D (1993) Trophic effects of basic fibroblast growth factor (bFGF) on differentiated oligodendroglia: a mechanism for regeneration of the oligodendroglial lineage. J Neurosci Res 36:672–680. [DOI] [PubMed] [Google Scholar]
- 33. Gu H, Wang S, Messam CA, Yao Z (2002) Distribution of nestin immunoreactivity in the normal adult human forebrain. Brain Res 943:174–180. [DOI] [PubMed] [Google Scholar]
- 34. Hagihara H, Hara M, Tsunekawa K, Nakagawa Y, Sawada M, Nakano K (2005) Tonic‐clonic seizures induce division of neuronal progenitor cells with concomitant changes in expression of neurotrophic factors in the brain of pilocarpine‐treated mice. Brain Res Mol Brain Res 139:258–266. [DOI] [PubMed] [Google Scholar]
- 35. Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J et al (2000) Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci 20:2218–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ishimi Y, Komamura Y, You Z, Kimura H (1998) Biochemical function of mouse minichromosome maintenance 2 protein. J Biol Chem 273:8369–8375. [DOI] [PubMed] [Google Scholar]
- 37. Johansson CB, Svensson M, Wallstedt L, Janson AM, Frisen J (1999) Neural stem cells in the adult human brain. Exp Cell Res 253:733–736. [DOI] [PubMed] [Google Scholar]
- 38. Johnson GV, Jope RS (1992) The role of microtubule‐associated protein 2 (MAP‐2) in neuronal growth, plasticity, and degeneration. J Neurosci Res 33:505–512. [DOI] [PubMed] [Google Scholar]
- 39. Karadottir R, Hamilton NB, Bakiri Y, Attwell D (2008) Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci 11:450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kirschenbaum B, Nedergaard M, Preuss A, Barami K, Fraser RA, Goldman SA (1994) In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain. Cereb Cortex 4:576–589. [DOI] [PubMed] [Google Scholar]
- 41. Koketsu D, Mikami A, Miyamoto Y, Hisatsune T (2003) Nonrenewal of neurons in the cerebral neocortex of adult macaque monkeys. J Neurosci 23:937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kornack DR, Rakic P (2001) The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc Natl Acad Sci USA 98:4752–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G (2003) Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol 467:455–463. [DOI] [PubMed] [Google Scholar]
- 44. Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B, Thomas LB et al (1999) Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol 156:333–344. [DOI] [PubMed] [Google Scholar]
- 45. Laywell ED, Kukekov VG, Steindler DA (1999) Multipotent neurospheres can be derived from forebrain subependymal zone and spinal cord of adult mice after protracted postmortem intervals. Exp Neurol 156:430–433. [DOI] [PubMed] [Google Scholar]
- 46. Lendahl U, Zimmerman LB, McKay RD (1990) CNS stem cells express a new class of intermediate filament protein. Cell 60:585–595. [DOI] [PubMed] [Google Scholar]
- 47. Levine JM, Stincone F, Lee YS (1993) Development and differentiation of glial precursor cells in the rat cerebellum. Glia 7:307–321. [DOI] [PubMed] [Google Scholar]
- 48. Levison SW, Young GM, Goldman JE (1999) Cycling cells in the adult rat neocortex preferentially generate oligodendroglia. J Neurosci Res 57:435–446. [PubMed] [Google Scholar]
- 49. Ligon KL, Alberta JA, Kho AT, Weiss J, Kwaan MR, Nutt CL et al (2004) The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J Neuropathol Exp Neurol 63:499–509. [DOI] [PubMed] [Google Scholar]
- 50. Ligon KL, Fancy SP, Franklin RJ, Rowitch DH (2006) Olig gene function in CNS development and disease. Glia 54:1–10. [DOI] [PubMed] [Google Scholar]
- 51. Ligon KL, Kesari S, Kitada M, Sun T, Arnett HA, Alberta JA et al (2006) Development of NG2 neural progenitor cells requires Olig gene function. Proc Natl Acad Sci USA 103:7853–7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Magavi SS, Leavitt BR, Macklis JD (2000) Induction of neurogenesis in the neocortex of adult mice. Nature 405:951–955. [DOI] [PubMed] [Google Scholar]
- 53. Morshead CM (2004) Adult neural stem cells: attempting to solve the identity crisis. Dev Neurosci 26:93–100. [DOI] [PubMed] [Google Scholar]
- 54. Munakata S, Hendricks JB (1993) Effect of fixation time and microwave oven heating time on retrieval of the Ki‐67 antigen from paraffin‐embedded tissue. J Histochem Cytochem 41:1241–1246. [DOI] [PubMed] [Google Scholar]
- 55. Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB (1996) Interaction between NG2 proteoglycan and PDGF alpha‐receptor on O2A progenitor cells is required for optimal response to PDGF. J Neurosci Res 43:315–330. [DOI] [PubMed] [Google Scholar]
- 56. Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB (1996) Co‐localization of NG2 proteoglycan and PDGF alpha‐receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res 43:299–314. [DOI] [PubMed] [Google Scholar]
- 57. Nishiyama A, Chang A, Trapp BD (1999) NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp Neurol 58:1113–1124. [DOI] [PubMed] [Google Scholar]
- 58. Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G 2nd, Jiang L et al (2003) Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med 9:439–447. [DOI] [PubMed] [Google Scholar]
- 59. Ong WY, Levine JM (1999) A light and electron microscopic study of NG2 chondroitin sulfate proteoglycan‐positive oligodendrocyte precursor cells in the normal and kainate‐lesioned rat hippocampus. Neuroscience 92:83–95. [DOI] [PubMed] [Google Scholar]
- 60. Parent JM, Von Dem Bussche N, Lowenstein DH (2006) Prolonged seizures recruit caudal subventricular zone glial progenitors into the injured hippocampus. Hippocampus 16:321–328. [DOI] [PubMed] [Google Scholar]
- 61. Pincus DW, Keyoung HM, Harrison‐Restelli C, Goodman RR, Fraser RA, Edgar M et al (1998) Fibroblast growth factor‐2/brain‐derived neurotrophic factor‐associated maturation of new neurons generated from adult human subependymal cells. Ann Neurol 43:576–585. [DOI] [PubMed] [Google Scholar]
- 62. Pringle NP, Mudhar HS, Collarini EJ, Richardson WD (1992) PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha‐receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development 115:535–551. [DOI] [PubMed] [Google Scholar]
- 63. Reynolds R, Hardy R (1997) Oligodendroglial progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex in vivo. J Neurosci Res 47:455–470. [DOI] [PubMed] [Google Scholar]
- 64. Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255:1707–1710. [DOI] [PubMed] [Google Scholar]
- 65. Reynolds R, Carey EM, Herschkowitz N (1989) Immunohistochemical localization of myelin basic protein and 2′,3′‐cyclic nucleotide 3′‐phosphohydrolase in flattened membrane expansions produced by cultured oligodendrocytes. Neuroscience 28:181–188. [DOI] [PubMed] [Google Scholar]
- 66. Reynolds R, Cenci di Bello I, Dawson M, Levine J (2001) The response of adult oligodendrocyte progenitors to demyelination in EAE. Prog Brain Res 132:165–174. [DOI] [PubMed] [Google Scholar]
- 67. Rhee W, Ray S, Yokoo H, Hoane ME, Lee CC, Mikheev AM et al (2008) Quantitative analysis of mitotic Olig2 cells in adult human brain and gliomas: implications for glioma histogenesis and biology. Glia [DOI] [PMC free article] [PubMed]
- 68. Rietze R, Poulin P, Weiss S (2000) Mitotically active cells that generate neurons and astrocytes are present in multiple regions of the adult mouse hippocampus. J Comp Neurol 424:397–408. [PubMed] [Google Scholar]
- 69. Riva MA, Donati E, Tascedda F, Zolli M, Racagni G (1994) Short‐ and long‐term induction of basic fibroblast growth factor gene expression in rat central nervous system following kainate injection. Neuroscience 59:55–65. [DOI] [PubMed] [Google Scholar]
- 70. Rodins K, Cheale M, Coleman N, Fox SB (2002) Minichromosome maintenance protein 2 expression in normal kidney and renal cell carcinomas: relationship to tumor dormancy and potential clinical utility. Clin Cancer Res 8:1075–1081. [PubMed] [Google Scholar]
- 71. Rowlands DC, Brown HE, Barber PC, Jones EL (1991) The effect of tissue fixation on immunostaining for proliferating cell nuclear antigen with the monoclonal antibody PC10. J Pathol 165:356–357. [DOI] [PubMed] [Google Scholar]
- 72. Roy NS, Wang S, Jiang L, Kang J, Benraiss A, Harrison‐Restelli C et al (2000) In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat Med 6:271–277. [DOI] [PubMed] [Google Scholar]
- 73. Sanai N, Tramontin AD, Quinones‐Hinojosa A, Barbaro NM, Gupta N, Kunwar S et al (2004) Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 427:740–744. [DOI] [PubMed] [Google Scholar]
- 74. Sarnat HB, Nochlin D, Born DE (1998) Neuronal nuclear antigen (NeuN): a marker of neuronal maturation in early human fetal nervous system. Brain Dev 20:88–94. [DOI] [PubMed] [Google Scholar]
- 75. Scolding N, Franklin R, Stevens S, Heldin CH, Compston A, Newcombe J (1998) Oligodendrocyte progenitors are present in the normal adult human CNS and in the lesions of multiple sclerosis. Brain 121:2221–2228. [DOI] [PubMed] [Google Scholar]
- 76. Sprinkle TJ (1989) 2′,3′‐cyclic nucleotide 3′‐phosphodiesterase, an oligodendrocyte‐Schwann cell and myelin‐associated enzyme of the nervous system. Crit Rev Neurobiol 4:235–301. [PubMed] [Google Scholar]
- 77. Stallcup WB (2002) The NG2 proteoglycan: past insights and future prospects. J Neurocytol 31:423–435. [DOI] [PubMed] [Google Scholar]
- 78. Suslov ON, Kukekov VG, Ignatova TN, Steindler DA (2002) Neural stem cell heterogeneity demonstrated by molecular phenotyping of clonal neurospheres. Proc Natl Acad Sci USA 99:14506–14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tachibana KE, Gonzalez MA, Coleman N (2005) Cell‐cycle‐dependent regulation of DNA replication and its relevance to cancer pathology. J Pathol 205:123–129. [DOI] [PubMed] [Google Scholar]
- 80. Temple S (2001) The development of neural stem cells. Nature 414:112–117. [DOI] [PubMed] [Google Scholar]
- 81. Thom M, Martinian L, Williams G, Stoeber K, Sisodiya SM (2005) Cell proliferation and granule cell dispersion in human hippocampal sclerosis. J Neuropathol Exp Neurol 64:194–201. [DOI] [PubMed] [Google Scholar]
- 82. Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA (1996) Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci 16:7599–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Weiss S, Reynolds BA, Vescovi AL, Morshead C, Craig CG, Van Der Kooy D (1996) Is there a neural stem cell in the mammalian forebrain? Trends Neurosci 19:387–393. [DOI] [PubMed] [Google Scholar]
- 84. Wennstrom M, Hellsten J, Ekstrand J, Lindgren H, Tingstrom A (2006) Corticosterone‐induced inhibition of gliogenesis in rat hippocampus is counteracted by electroconvulsive seizures. Biol Psychiatry 59:178–186. [DOI] [PubMed] [Google Scholar]
- 85. Wiebe S, Blume WT, Girvin JP, Eliasziw M (2001) A randomized, controlled trial of surgery for temporal‐lobe epilepsy. N Engl J Med 345:311–318. [DOI] [PubMed] [Google Scholar]
- 86. Wolswijk G (2002) Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain 125:338–349. [DOI] [PubMed] [Google Scholar]
- 87. Yamamoto S, Nagao M, Sugimori M, Kosako H, Nakatomi H, Yamamoto N et al (2001) Transcription factor expression and Notch‐dependent regulation of neural progenitors in the adult rat spinal cord. J Neurosci 21:9814–9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yang HY, Lieska N, Shao D, Kriho V, Pappas GD (1994) Proteins of the intermediate filament cytoskeleton as markers for astrocytes and human astrocytomas. Mol Chem Neuropathol 21:155–176. [DOI] [PubMed] [Google Scholar]
- 89. Yokoo H, Nobusawa S, Takebayashi H, Ikenaka K, Isoda K, Kamiya M et al (2004) Anti‐human Olig2 antibody as a useful immunohistochemical marker of normal oligodendrocytes and gliomas. Am J Pathol 164:1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhou Q, Choi G, Anderson DJ (2001) The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron 31:791–807. [DOI] [PubMed] [Google Scholar]


