Abstract
Impaired arginine vasopressin (AVP) synthesis and release by the neurohypophyseal system, which includes the neurohypophysis and magnocellular neurons of the paraventricular and supraoptic nuclei, have been postulated in septic shock, but changes in this system have never been assessed in human septic shock, and only partially experimentally. We investigated AVP synthesis and release by the neurohypophyseal system in 9 patients who died from septic shock and 10 controls, and in 20 rats with fecal peritonitis‐induced sepsis and 8 sham‐operation controls. Ten rats died spontaneously from septic shock, and the others were sacrificed. In patients with septic shock, as in rats that died spontaneously following sepsis induction, AVP immunohistochemical expression was decreased in the neurohypophysis and supraoptic magnocellular neurons, whereas it was increased in the paraventricular magnocellular neurons. No significant change was observed in AVP messenger RiboNucleic Acid (mRNA) expression assessed by in situ hybridization in either paraventricular or supraoptic magnocellular cells. This study shows that both in human and experimental septic shock, AVP posttranscriptional synthesis and transport are differently modified in the magnocellular neurons of the supraoptic and paraventricular nuclei. This may account for the inappropriate AVP release in septic shock and suggests that distinct pathogenic mechanisms operate in these nuclei.
Keywords: hypothalamus, neurohypophysis, sepsis, vasopressin
INTRODUCTION
Arginine vasopressin (AVP) released by the neurohypophyseal system is an essential hormone‐controlling body fluid balance and vascular tone. The neurohypophyseal system includes the magnocellular neurons of the hypothalamic supraoptic nucleus (SON) and paraventricular nucleus (PVN) where AVP is synthesized, and the neurohypophysis where AVP is stored before being secreted into the bloodstream (16). The PVN also contains parvocellular neurons that co‐synthesize AVP and corticotropin‐releasing hormone. Both these latter peptides control adrenocorticotropic hormone (ACTH) secretion from the adenohypophysis (16).
Inappropriate circulating AVP levels or so‐called “relative AVP deficiency”(15) has been reported in septic shock, a life‐threatening infection complicated by failure of the cardiovascular system that carries a mortality ranging from 40% to 60% (3). Landry et al first reported that circulating AVP levels were significantly lower in patients with advanced vasodilatory septic shock compared with those in cardiogenic shock, despite comparable values of systolic blood pressure and plasma osmolality (15). Subsequent studies suggested that AVP levels increase in the very early phase of septic shock as a result of release of neurohypophyseal vesicles, and then decline to inappropriately low levels (27). Mechanisms underlying AVP deficiency in the late phase of septic shock remain uncertain 21, 23, 25, although clearance of AVP from plasma appears unaffected (26). A number of studies tend to support a central origin for the AVP deficiency, including depletion of neurohypophyseal vesicles in patients with septic shock (26), neuronal apoptosis within the PVN and SON that is associated with an overexpression of inducible nitric oxide synthase (iNOS) 28, 29, and the observation in septic animals that NO inhibits AVP release (12).
There are only a few morphological studies of AVP synthesis and release in experimental sepsis and, to our knowledge, none in human septic shock. Endotoxin injection, and cecal ligation and puncture have been shown to rapidly increase plasma AVP levels by activating magnocellular neurons of paraventricular 18, 31 and supraoptic nuclei 13, 18. Conversely, it has been experimentally evidenced that relative AVP deficiency is present at 24 h 4, 11. It would be of interest to determine whether this phenomenon persists beyond the 24 h of experimental sepsis, as it is during the post‐acute phase that relative AVP deficiency occurs in septic patients. Experimental fecal peritonitis‐induced prolonged severe sepsis seems a more appropriate model for assessing the pathophysiological mechanisms of long‐lasting sepsis‐induced relative AVP deficiency, and is associated with low circulating vasopressin levels (5). The experimental studies have not concomitantly investigated mRNA and protein expression within the different components of the neurohypophyseal system, leading to an incomplete view of vasopressin synthesis and release. The aim of our study was to investigate changes in AVP content and mRNA expression in the paraventricular and supraoptic nuclei and neurophypophysis of rats that underwent experimental peritonitis, and in our series of patients who died from septic shock 28, 29.
METHODS
In vivo model of fecal peritonitis
Experiments were carried out at the University College London, with Home Office approval in accordance with the Animal Scientific Procedures Act [European Community's Council Directive (24 November 1986; 86/609/EEC)]. Male Wistar rats were instrumented under isoflurane (Abbott, Queenborough, UK) anesthesia maintained via a face mask. Mean weight of the animals was 300 g. Internal jugular venous and carotid arterial catheters (external diameter, 0.96 mm) were inserted and tunneled subcutaneously to the nape of the neck. Catheters were mounted onto a swivel/tether system that enabled unimpaired movement around the cage and free access to food and water after recovery from anesthesia. Both catheters were flushed continuously with 0.15 mL/h heparinized saline (1:1000). Mean arterial pressure was measured (P23XL transducers™, Viggo‐Spectramed, Oxnard, CA, USA) and recorded onto a pre‐calibrated PowerLab system® (ADInstruments, Sydney, NSW, Australia). Sepsis was induced 24 h later by intraperitoneal injection of fecal slurry (0.63 mg/100 g body weight) prepared from the bowel contents of a rat from the same batch. After a further 2 h, intravenous fluid resuscitation [1:1 solution of 6% hetastarch (EloHaes®, Fresenius Kabi, Warrington, UK) and 5% glucose] was commenced at 10 mL/kg/h (this was reduced to 8.75 mL/kg/h between 24 h and 48 h).
Experimental groups
Three groups were constituted in a random manner: sham‐operated controls received no intraperitoneal injection but were otherwise treated identically. Rats that underwent experimental peritonitis were classified as septic if they did not die spontaneously, and as septic with early death (septic ED) if they died spontaneously. In this conscious, fluid‐resuscitated model, blood pressure is maintained by cardiovascular reflexes at approximately 10 mmHg below sham control levels until the preterminal phase, which lasted usually less than 30 minutes. Sham and septic rats were sacrificed, in average, 36 h after injection of slurry. All septic rats with ED died, in average, 24 h after induction of sepsis.
Brain sampling was performed in 8 sham, 10 septic and 10 septic ED rats. Whole brains and pituitary glands were removed immediately after animal sacrifice by neck dislocation, or less than 12 h after spontaneous death, and fixed by immersion in 10% formalin. After at least 21 days of formalin fixation, brains were macroscopically examined and cut at the levels of the optic chiasma and brain stem. Each sample was paraffin embedded, and serial sections of 4 µm were then performed using a Leica® (Leica Microsystems SAS, Nanterre, France) microtome until the region of interest was reached. Coronal sections of the SON and PVN, and horizontal sections of the pituitary gland (Figure 1E,G) were then stained with hematoxylin and eosin (H&E) for microscopic examination.
Figure 1.
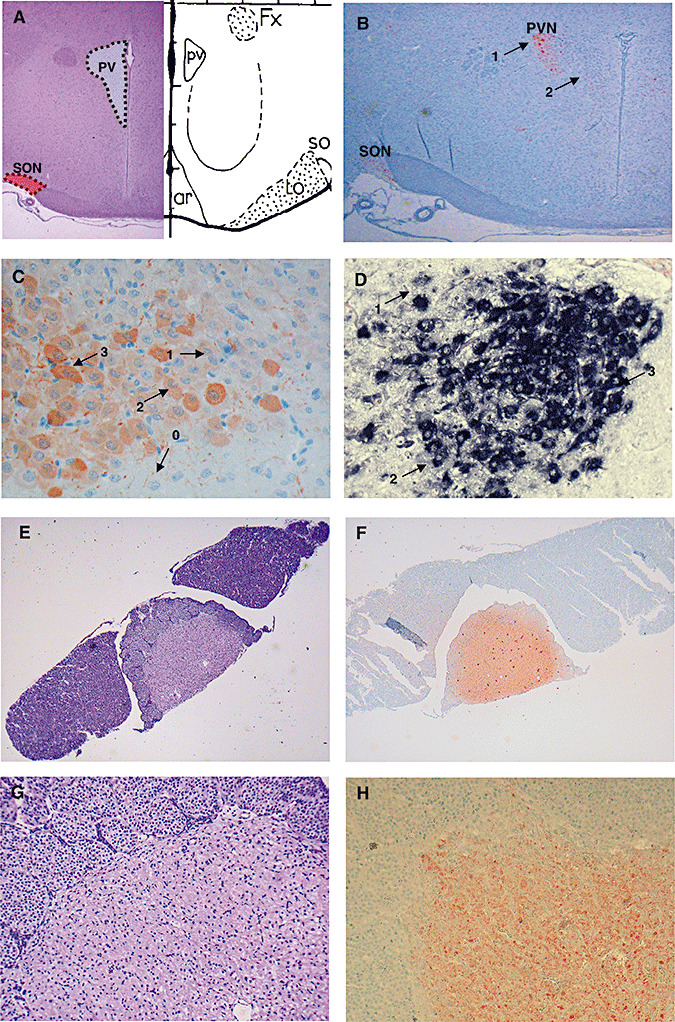
A. Location of supraoptic nucleus (SON, red area) and paraventricular nucleus (PVN, gray area) on a coronal section of control rat brain. B. Arginine vasopressin (AVP) immunohistochemical labeling on a coronal section of control rat brain in SON and PVN (ABC‐peroxidase/Amino‐Ethyl‐Carbazole (AEC) × 10). Arrows 1 and 2 indicate magnocellular and parvocellular neurons, respectively. Magnocellular neurons are larger than parvocellular neurons and are situated in the periphery of the PVN. C. Index of AVP labeling after immunohistochemistry in the PVN (×250). The intensity of AVP immunostaining of each neuron was semiquantitatively scored from 0 to 3. The index was calculated for each nucleus as described in the Methods. D. Index of AVP labeling after in situ hybridization in the PVN (×250). The intensity of AVP in situ hybridization of each neuron was semiquantitatively scored from 0 to 3. E–H. The neurohypophysis is located in the central part of the hypophysis [E hematoxylin and eosin (H&E) × 10; G H&E × 80]. After immunohistochemistry staining for AVP, the whole neurohypophysis shows strong labeling (ABC‐peroxidase/AEC) (F × 10; H × 80).
Patients
Nine non‐survivors from septic shock and 10 non‐septic patients who died from cardiogenic shock or an extracranial cause of sudden death were included (10). None of these patients had severe hyponatremia (<125 mmol/L) or hypernatremia (>150 mmol/L). The clinical data and neuropathological findings in these patients have been previously reported 28, 29. We recorded severity of illness using simplified acute physiology score II (SAPS‐II). Vital signs were recorded continuously, and standard laboratory tests and relevant microbiological data were recorded daily. Duration of shock, cumulative time passed with a hypotension (defined as mean blood pressure less than 60 mmHg) and cumulative time between death and brain sampling were also recorded.
The demographic and clinical data in the patients and controls are summarized in Table 1.
Table 1.
Demographic and clinical data in 10 patients who died from septic shock and 9 controls.
| N° | Group | Age (years) | Sex | SAPS II at admission | Highest and lowest natremia (mmol/L) | Length of stay (Days) | Duration of hypotension (h)* | Duration of shock (days) |
|---|---|---|---|---|---|---|---|---|
| 1 | Septic shock | 78 | M | 30 | 137–128 | 10 | 8 | 7 |
| 2 | Septic shock | 71 | M | 46 | 137–126 | 13 | 25 | 8 |
| 3 | Septic shock | 71 | F | 91 | 145–139 | 1 | 18 | 1 |
| 4 | Septic shock | 37 | M | 86 | 135–134 | 1 | 7 | 1 |
| 5 | Septic shock | 83 | F | 33 | 131–131 | 34 | 39 | 8 |
| 6 | Septic shock | 51 | F | 94 | 144–132 | 4 | 43 | 4 |
| 7 | Septic shock | 56 | F | 23 | 132–130 | 3 | 10 | 1 |
| 8 | Septic shock | 77 | F | 67 | 141–146 | 2 | 24 | 2 |
| 9 | Septic shock | 71 | M | 102 | 143–128 | 33 | 35 | 18 |
| C1 | Cardiogenic shock | 64 | M | 41 | 146–134 | 17 | 36 | 10 |
| C2 | Cardiogenic shock | 41 | F | 41 | 137–131 | 4 | 2 | 4 |
| C3 | Cardiogenic shock | 67 | M | 85 | 145–145 | 1 | 3 | 1 |
| C4 | Cardiogenic shock | 64 | M | 76 | 142–138 | 1 | 2 | 1 |
| C5 | Cardiogenic shock | 71 | M | 63 | 129–125 | 7 | 5 | 3 |
| C6 | Cardiogenic shock | 65 | M | 20 | 142–131 | 19 | 3 | 5 |
| C7 | Cardiogenic shock | 63 | F | 97 | 137–135 | 2 | 17 | 2 |
| C8 | Sudden death | 20 | M | NA | NA | NA | NA | NA |
| C9 | Sudden death | 56 | M | NA | NA | NA | NA | NA |
| C10 | Sudden death | 35 | M | NA | NA | NA | NA | NA |
Abbreviations—NA: not available Defined as mean arterial pressure less than 60 mm Hg.
Macroscopic examination of the brains was performed after 6 weeks of fixation in 10% formalin (v/v). Samples of the SON and PVN at the level of the anterior and posterior hypothalamus respectively, and pituitary glands were embedded in paraffin. Blocs were cut in 4‐µm serial sections with a Leica® microtome. Sections were stained with H&E.
Immunohistochemistry
Immunohistochemistry was performed on deparaffinized formalin‐fixed sections using an indirect Avidin Biotin Complex (ABC)‐peroxidase method revealed with 3‐3′diaminobenzidine or amino‐ethyl‐carbazole as chromogenes. Heat‐mediated antigen retrieval (90°C, 30 minutes) was performed for hypothalamic samples, but not for pituitary samples. Endogenous peroxidase activity was blocked by incubation in 3% hydrogen peroxide in phosphate‐buffered saline for 10 minutes. Immunohistochemistry was performed using an automated Immunohistochemistry (IHC) slide‐staining system (NexES® IHC Full system, Ventana Medical Systems, Tucson, AZ, USA), using specific primary polyclonal antibodies raised against AVP. For human samples, rabbit polyclonal antisera against AVP (1:500, Peninsula Laboratories, San Carlos, CA, USA) were used. The rat sections were processed with rabbit polyclonal antisera raised against AVP (1:10 000, AB1565®, Chemicon International, Temecula, CA, USA). Sections were incubated for 30 minutes with primary antibodies. Slides were then counterstained with hematoxylin for 4 minutes and mounted in Eukitt® (Electron Microscopy Sciences, Hattfield, PA, USA) for microscopic evaluation. Omission of the primary antibodies and replacement by isotype‐matched irrelevant antibody was used for negative control. Images were acquired using a Leitz Diaplan® microscope (Leitz, Solms, Germany) equipped with a Nikon Coolpix 950® digital camera (Champigny sur Marne, France).
In situ hybridization
In situ hybridization was performed using a 200 bp rat vasopressin cDNA probe (gift from Dr C Rabadan‐Diehl, Bethesda, MD, USA). Sense and antisense probes were synthesized from linearized plasmids using digoxigenin‐Uridine TriPhosphate (UTP) (Roche, France) and Riboprobe® combination system (Promega, Charbonnières, France), as described previously (30). In situ hybridization was performed on 4‐µm paraffin sections using the Ventana Discovery® (Ventana Medical Systems). Briefly, rat tissue hybridization was carried out at 65°C followed by 0.1X standard saline citrate (SSC) washes at 70°C. In situ hybridization of human tissues was performed using digoxigenin‐labeled rat AVP probe at 55°C followed by 0.2X SSC washes at 55°C. Digoxigenin was detected by immunohistochemistry using biotinylated anti‐digoxigenin antibody (Jackson ImmunoResearch, Suffolk, UK) and BlueMap™ kit (Ventana).
Slides were finally mounted with Vectashield® mounting medium (Vector Labs‐AbCys, Paris, France). The labeled cells were observed with an Axioplan 2™ microscope (Zeiss, Göttingen, Germany) equipped with a video camera (Micromax 1 MHz, MicroMax, Roper Scientific, Ottobrun, Germany). Images were acquired with AxioVision™ (Zeiss, Göttingen, Germany) acquisition and analysis software. Sections hybridized with sense probes showed no staining.
Evaluation of immunostaining and in situ hybridization
Immunostaining and in situ hybridization were assessed by two independent observers who were blinded to rat and patient groups (RS, JPB). Counting of magnocellular neurons was performed morphometrically using Image J 1.36b® software (Wayne Rasband, National Institute of Health, Bethesda, MD, USA). For rat analyses, cell counts were performed on one 4 µm‐treated slice containing both the SON and PVN on both sides [at the level −1.4 mm or −1.8 mm from bregma, according to the stereotaxic rat brain atlas of Paxinos and Watson (20)] (Figure 1A,B). For humans, one slice representative of both regions was selected for counting.
The intensity of AVP immunolabeling (immunohistochemistry and in situ hybridization) of each neuron was semiquantitatively scored as 0 (none), 1 (low), 2 (moderate) or 3 (high) in the PVN and SON (Figure 1C,D). To take into account both the amount of positive neurons and the cellular intensity of immunostaining, the following index was then calculated for each nucleus: [1 × number (n) of low intensity cells + 2 × n of moderate intensity cells + 3 × n of high intensity cells] / (total n of neurons on the slice). Therefore, this index ranged from 0 to 3. This index was developed in the hypothalamic nuclei of naive rats and of control patients who did not die from septic shock (Figure 1C,D).
In the neurohypophysis of each rat or patient, stores of AVP were scored with a semiquantitative scale, ranging from 0 to 3, depending on the global immunostaining intensity at the level of the section considered (Figure 1F,H).
Statistical analysis
Variables were expressed as median with interquartile ranges (25th–75th percentile), respectively. Nonparametric analyses were performed using Kruskal–Wallis and and Mann–Whitney test for comparison of quantitative variables between three and two groups, respectively. A P‐value less than 0.05 was deemed significant.
RESULTS
Characterization of the in vivo model
All septic animals with or without ED showed features of illness from about 12 h after injection of fecal slurry, including hunched posture, piloerection, and decreased movement and alertness. At 24 h, there was a significant decrease in mean arterial pressure in surviving septic rats (115 mmHg ± 2 mmHg controls, 106 mmHg ± 4 mmHg septic, P < 0.05). Blood sodium levels were comparable between groups (136 mmol/L ± 1.3 mmol/L septic vs. 138 mmol/L ± 0.8 mmol/L sham controls, P = not significant). In all septic rats with and without ED, laparotomy showed evidence of peritoneal inflammation.
Hypothalamic and neurohypophyseal expression of AVP in rats
The neurohypophyseal expression of AVP varied significantly among groups (Kruskal–Wallis, P = 0.0057) (Figure 2A–C). It was significantly higher in sham [2.75 (2.63–2.75)] than in either septic animals [1.5 (1.25–1.75), Mann–Whitney, P = 0.006] or septic ED animals [1.75 (1.50–1.75), Mann–Whitney, P = 0.006] (Figure 2D). It did not differ between septic and septic ED rats (Mann–Whitney, P = 0.42).
Figure 2.
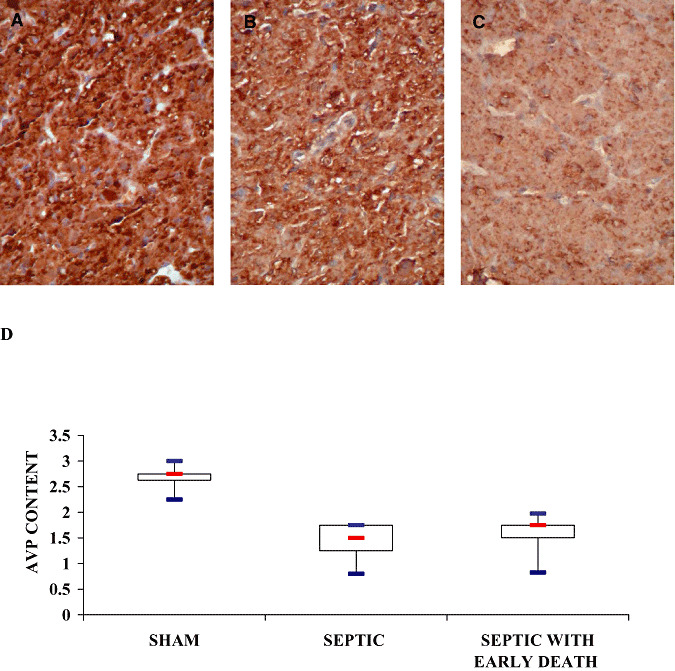
Neurohypophyseal content of vasopressin of sham‐operated (A), septic (B) and septic with early death (septic ED) rats (C). Microphotographs illustrate the decrease in neurohypophyseal content of arginine vasopressin (AVP) in neurohypophysis in line with the severity of sepsis (ABC‐peroxidase/Diaminobenzidine (DAB) × 200). D. Box plots show median (interquartile). In the neurohypophysis, immunostaining intensity varied significantly among groups (Kruskal–Wallis, P = 0.0057) and was significantly higher in sham‐operated than in septic and septic ED rats.
In the SON magnocellular neurons, AVP labeling differed significantly between the three groups (Kruskal–Wallis, P = 0.002). It was significantly higher in sham [1.28 (1.23–1.69)] than in septic animals [1.02 (0.84–1.29), Mann–Whitney, P = 0.04] or septic ED animals [0.47 (0.21–0.94), Mann–Whitney, P = 0.001]. It was also significantly greater in septic than in septic ED rats (Mann–Whitney, P = 0.02) (3, 4). AVP mRNA expression did not differ significantly between groups [sham: 2.43 (2.40–2.61) vs. septic: 2.49 (2.33–2.56) vs. septic ED: 2.69 (2.47–2.72), Kruskal—Wallis, P = 0.37] (3, 4).
Figure 3.
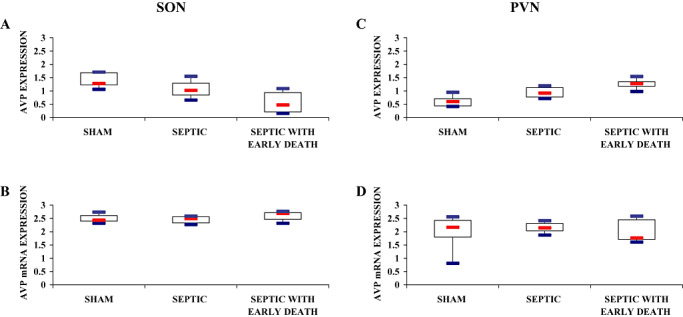
Index of AVP labeling after immunohistochemistry (A,C) and in situ hybridization (B,D) procedures in magnocellular neurons of the supraoptic nucleus (SON; A,B) and paraventricular nucleus (PVN; C,D) of sham‐operated, septic and septic with early death (septic ED) rats. Box plots show median (interquartile). A. In the SON, arginine vasopressin (AVP) protein expression varied significantly among groups and was significantly higher in sham rats than in septic and septic ED rats. C. In the PVN, AVP protein expression varied significantly among groups and was significantly higher in septic ED rats than in sham rats. B., D. In both SON and PVN, vasopressin mRNA expression did not differ statistically between groups.
Figure 4.
Microphotographs of arginine vasopressin (AVP) labeling after immunohistochemistry (ABC‐peroxidase/AEC) (A,C,E×250) and in situ hybridization for AVP mRNA (B,D,F×200) in magnocellular neurons of the supraoptic nucleus in sham (A,B), septic (C,D) and two septic with early death (E,F) rats. AVP protein expression decreases in line with the severity of illness, but without change in AVP mRNA expression.
In the PVN magnocellular neurons, AVP immunostaining varied significantly among groups (Kruskal–Wallis, P = 0.005). It was significantly lower in sham [0.60 (0.44–0.71)] than in septic ED rats [1.28 (1.18–1.35), Mann–Whitney, P = 0.009], and also between septic [0.92 (0.77–1.13)] and septic ED rats (Mann–Whitney, P = 0.02) (3, 5). The paraventricular expression of AVP mRNA within the magnocellular neurons did not differ significantly between groups [sham: 2.17 (1.80–2.43) vs. septic: 2.15 (2.04–2.31) vs. septic ED: 1.78 (1.71–2.45), Kruskal–Wallis, P = 0.86] (3, 5).
Figure 5.
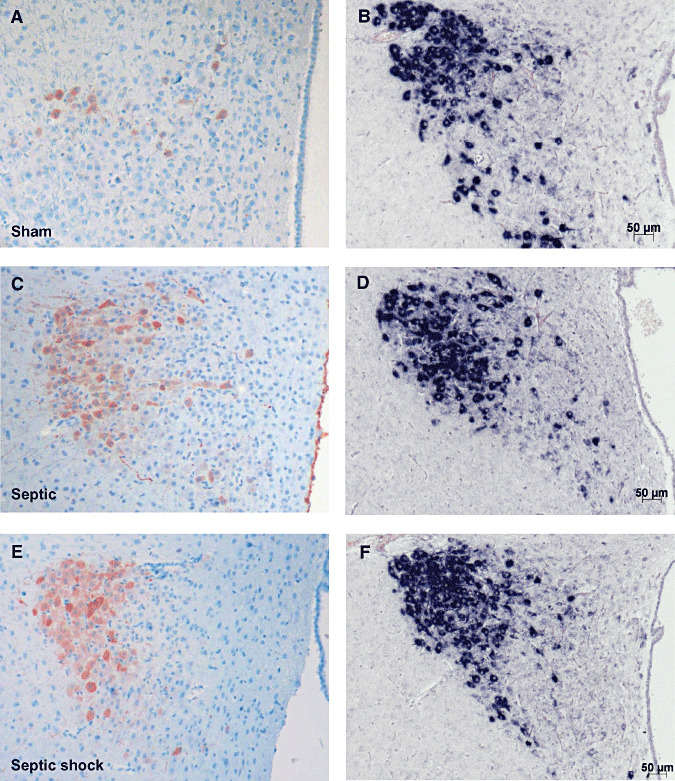
Microphotographs of arginine vasopressin (AVP) labeling after immunohistochemistry (ABC‐peroxidase/AEC) (A,C,E×100) and in situ hybridization for AVP mRNA (B,D,F×100) in magnocellular neurons of paraventricular nucleus in sham (A,B), septic (C,D) and septic with early death (E,F) rats. There is an increase in AVP protein expression in line with the severity of illness, but without change in AVP mRNA expression.
Characterization of patients
The median age of patients who died from septic shock was 71 years (58–76). Their SAPS‐II score was 57 (35–90), the cumulative time of hypotension was 25 h (12–36), and their duration of shock was 6 days (1–8). In patients who died from non‐septic causes, SAPS‐II score was higher [ie, 70 (47–83)], but cumulative time of hypotension and duration of shock were shorter, namely, 3 h (2–5) and 3 days (1–4), respectively. Median highest and lowest blood sodium levels were similar between the two groups [139 mmol/L (136–144) vs. 140 mmol/L (137–142); 131 mmol/L (128–134) vs. 133 mmol/L (131–137)].
Hypothalamic and neurohypophyseal expression of AVP in patients
Neurohypophyseal expression of AVP was significantly lower in septic shock than in non‐septic shock patients [1.50 (0.88–1.63) vs. 2.00 (1.88–2.63), Mann–Whitney, P = 0.05].
The immunohistochemical expression of AVP in the SON was significantly higher in controls than in septic shock [2.69 (2.01–2.71) vs. 1.05 (0.77–1.50), Mann–Whitney, P = 0.02] (6, 7). Supraoptic AVP mRNA expression did not differ significantly between groups [1.87 (1.66–1.98) vs. 1.85 (1.74–2.18), Mann–Whitney, P = 0.99] (6, 7).
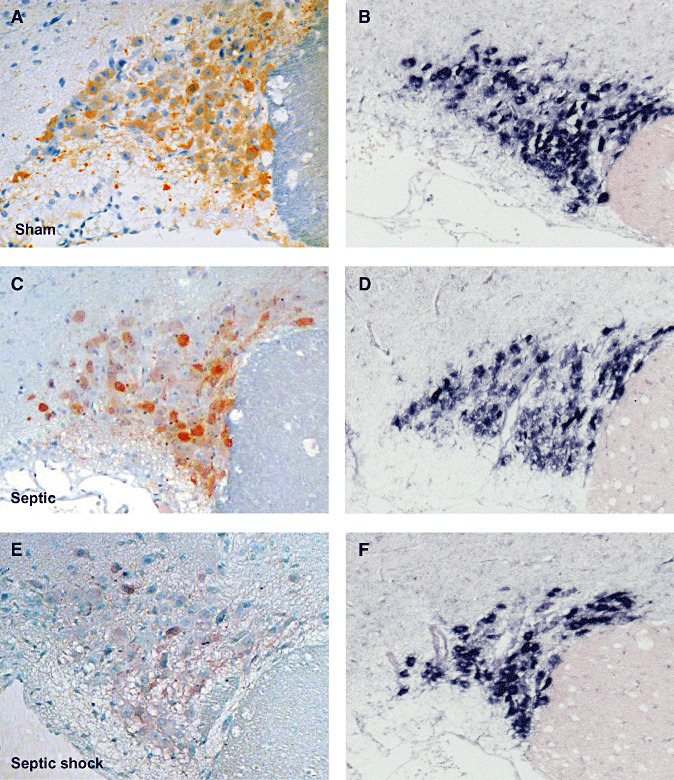
Figure 6.
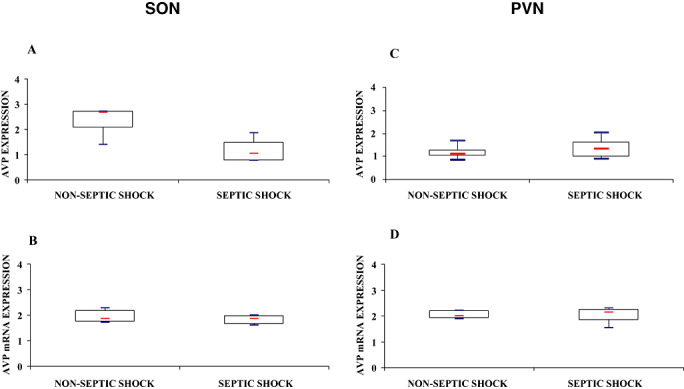
Index of arginine vasopressin (AVP) labeling after immunohistochemistry (A,C) and in situ hybridization for AVP mRNA (B,D) in magnocellular neurons of the supraoptic nucleus (SON; A,B) and paraventricular nucleus (PVN; C,D) of patients who died from septic shock or from non‐septic causes. Box plots show median (interquartile). In septic shock patients, AVP protein expression was significantly lower in the SON and was increased, albeit not significantly, in the PVN. In both nuclei, AVP mRNA did not differ between the two groups.
Figure 7.
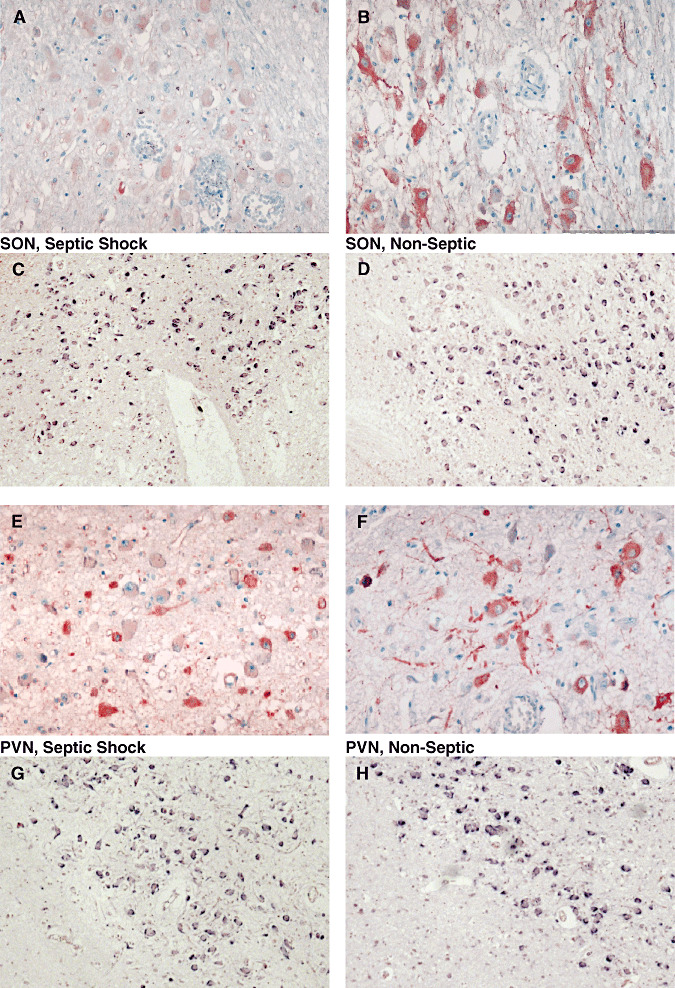
Microphotographs of arginine vasopressin (AVP) labeling after immunohistochemistry (ABC‐peroxidase/AEC) (A,B,E,F×250) and in situ hybridization for AVP mRNA (C,D,G,H×100) in magnocellular neurons of supraoptic nucleus (SON; A–D) and paraventricular nucleus (PVN; E–H) of patients who had died from non‐septic causes (B,D,F,H) and septic shock (A,C,E,G). There is a decrease in AVP protein expression in the SON, and a nonsignificant increase of AVP protein in the PVN in septic shock patients. AVP mRNA expression did not differ between the two groups in either nuclei.
There was no significant difference between septic shock and non‐septic patients for the paraventricular expression of either AVP protein [1.32 (0.96–1.64) vs. 1.10 (0.99–1.28), Mann–Whitney, P = 0.34] or AVP mRNA [2.13 (1.85–2.25) vs. 1.99 (1.90–2.15) Mann–Whitney, P = 0.71] (6, 7).
DISCUSSION
Using immunohistochemistry and in situ hybridization, we have assessed AVP mRNA and protein expression in the different structures of the neurohypophyseal system in 9 patients who died from septic shock and in 20 rats that underwent experimental peritonitis, (from which 10 rats survived and 10 died spontaneously early from septic shock), and compared our findings with those in 10 control patients and 8 sham‐operation control rats. We found, both in rats and in humans, that septic shock is associated with decreased AVP protein expression in the neurohypophysis and SON, and increased AVP protein expression in the PVN. No significant change was observed in AVP mRNA content either in SON or PVN.
These results confirm previous demonstration that neurohypophyseal vesicles containing AVP are depleted during septic shock (27) and suggest impaired AVP posttranscriptional synthesis and transport. It has been recently shown that depletion of neurohypophyseal AVP content is associated with an increase not only in plasma AVP levels, but also in supraoptic and paraventricular AVP content within the 24 h after cecal ligation and puncture in rats (18).These results and our findings are complementary as they account for the early increase and subsequent decrease in plasma AVP level observed in septic animals and patients, respectively. Together, they also support the hypothesis that sepsis‐associated AVP deficiency has a hypothalamic origin.
The fecal peritonitis insult administered to the animals reflects a clinically realistic scenario. This model has been well validated 5, 6, with the septic animals presenting with clinical and biologic features of organ dysfunction and a 3‐day mortality rate of 40%–50%. This model (14) and others (5) have previously reported inappropriately low plasma AVP levels. These animals do not become markedly hypotensive until the preterminal stages, in part because of ample fluid resuscitation, and in part because of preserved cardiovascular reflexes and sympathetic activation in this conscious model. Compared with sham‐operated animals, blood pressure in the septic animals is hyporesponsive to exogenous catecholamines (14), and also falls markedly on anesthesia or administration of ganglion‐blocking agents (L. Barrett, unpublished data).
The similarities in impairment of AVP synthesis and transport observed in both septic rats and septic patients make this impairment unlikely to result only from agonic or post‐mortem phenomenon. These similarities also strengthen the relevance of our experimental model and selection of patients. Although patients who died from septic and non‐septic shock are not matched in terms of duration of shock and hypotension, this discrepancy does not undermine our hypothesis of sepsis‐associated dysfunction of the neurohypophyseal system. Indeed, a more prolonged hemodynamic stimulation should have rather resulted in higher expression of AVP in the septic patients. It also has to be noted that sodium levels, a major modulator of AVP synthesis from magnocellular cells, were comparable between septic patients and rats, and controls.
The differences in AVP protein and mRNA expression in the SON and the PVN are puzzling. In the SON, the decrease in AVP protein and unchanged AVP mRNA expression imply impaired AVP synthesis. In the PVN, the increase in AVP protein and unchanged AVP mRNA expression suggest impaired AVP transport, as AVP‐containing vesicles are depleted in the neurohypophysis. It seems likely that other factors may operate. In addition to osmotic and baroreflex regulation, the vasopressinergic system is also regulated by cytokines, prostaglandin, Gamma Amino Butyric Acid (GABA), angiotensin II and opioids 7, 14, 17, 27. NO is clearly involved as sepsis increases iNOS expression within the PVN and SON 11, 12, 28, and experimental inhibition of iNOS increases AVP secretion 11, 12. Interestingly, preliminary results obtained in 10 septic or septic rats with ED indicated that expression of iNOS was higher in SON than in PVN (1.3 ± 0.5 vs. 0.6 ± 0.5, P = 0.01, Wilcoxon signed‐rank test), in keeping with the discrepancy in AVP synthesis between these two nuclei. Noradrenergic projections from the nucleus tractus solitarii and ventrolateral medulla, the main brain centers controlling the baroreflex, are powerful modulators of AVP secretion by magnocellular neurons (16). Interestingly, septic shock is often associated with impaired baroreflex sensitivity and neuronal apoptosis in brain stem autonomic nuclei 2, 10, 19, 24, 28, and a recent study tent to show that blocking NO production alters neuronal activation in brain stem autonomic nuclei of rats in the late phase of polymicrobial sepsis (8). Thus, increased iNOS‐related production of NO and impaired baroreflex regulation may account for the relative AVP deficiency seen in septic shock. However, ACTH secretion is enhanced by iNOS‐related NO 1, 22, and is controlled by neural input from brain stem autonomic nuclei (9) and by AVP released by PVN neurons. Thus, differential expression (protein and mRNA) in the SON and PVN may reflect different mechanisms of regulation. In particular, the preservation of adrenal function through ACTH release from the PVN may be more crucial than simply maintaining AVP release in life‐threatening conditions such as septic shock. Further studies are needed to support these hypotheses.
ACKNOWLEDGMENTS
The authors wish to thank Patrice Castagnet for the valuable technical help.
Source of support: this study was funded by a grant from the Fondation Garches.
REFERENCES
- 1. Akasaka S, Nomura M, Nishii H, Fujimoto N, Ueta Y, Tsutsui M et al (2006) The hypothalamo‐pituitary axis responses to lipopolysaccharide‐induced endotoxemia in mice lacking inducible nitric oxide synthase. Brain Res 1089:1–9. [DOI] [PubMed] [Google Scholar]
- 2. Annane D, Trabold F, Sharshar T, Jarrin I, Blanc AS, Raphael JC, Gajdos P (1999) Inappropriate sympathetic activation at onset of septic shock: a spectral analysis approach. Am J Respir Crit Care Med 160:458–465. [DOI] [PubMed] [Google Scholar]
- 3. Annane D, Bellissant E, Cavaillon JM (2005) Septic shock. Lancet 365:63–78. [DOI] [PubMed] [Google Scholar]
- 4. Athayde LA, Oliveira‐Pelegrin GR, Nomizo A, Faccioli LH, Rocha MJ (2009) Blocking central leukotrienes synthesis affects vasopressin release during sepsis. Neuroscience 160:829–836. Epub 2009 March 2011. [DOI] [PubMed] [Google Scholar]
- 5. Barrett LK, Orie NN, Taylor V, Stidwill RP, Clapp LH, Singer M (2007) Differential effects of vasopressin and norepinephrine on vascular reactivity in a long‐term rodent model of sepsis. Crit Care Med 35:2337–2343. [DOI] [PubMed] [Google Scholar]
- 6. Brealey D, Karyampudi S, Jacques TS, Novelli M, Stidwill R, Taylor V et al (2004) Mitochondrial dysfunction in a long‐term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol 286:R491–R497. Epub 2003 November 2006. [DOI] [PubMed] [Google Scholar]
- 7. Brown CH, Russell JA, Leng G (2000) Opioid modulation of magnocellular neurosecretory cell activity. Neurosci Res 36:97–120. [DOI] [PubMed] [Google Scholar]
- 8. Bruhn FH, Correa PB, Oliveira‐Pelegrin GR, Rocha MJ (2009) Blocking systemic nitric oxide production alters neuronal activation in brain structures involved in cardiovascular regulation during polymicrobial sepsis. Neurosci Lett 453:141–146. Epub 2009 February 2021. [DOI] [PubMed] [Google Scholar]
- 9. Carrasco GA, Van de Kar LD (2003) Neuroendocrine pharmacology of stress. Eur J Pharmacol 463:235–272. [DOI] [PubMed] [Google Scholar]
- 10. Chan SH, Wu KL, Wang LL, Chan JY (2005) Nitric oxide‐ and superoxide‐dependent mitochondrial signaling in endotoxin‐induced apoptosis in the rostral ventrolateral medulla of rats. Free Radic Biol Med 39:603–618. [DOI] [PubMed] [Google Scholar]
- 11. Correa PB, Pancoto JA, De Oliveira‐Pelegrin GR, Carnio EC, Rocha MJ (2007) Participation of iNOS‐derived NO in hypothalamic activation and vasopressin release during polymicrobial sepsis. J Neuroimmunol 183:17–25. Epub 2006 December 2014. [DOI] [PubMed] [Google Scholar]
- 12. Giusti‐Paiva A, De Castro M, Antunes‐Rodrigues J, Carnio EC (2002) Inducible nitric oxide synthase pathway in the central nervous system and vasopressin release during experimental septic shock. Crit Care Med 30:1306–1310. [DOI] [PubMed] [Google Scholar]
- 13. Grinevich V, Ma XM, Jirikowski G, Verbalis J, Aguilera G (2003) Lipopolysaccharide endotoxin potentiates the effect of osmotic stimulation on vasopressin synthesis and secretion in the rat hypothalamus. J Neuroendocrinol 15:141–149. [DOI] [PubMed] [Google Scholar]
- 14. Holmes CL, Patel BM, Russell JA, Walley KR (2001) Physiology of vasopressin relevant to management of septic shock. Chest 120:989–1002. [DOI] [PubMed] [Google Scholar]
- 15. Landry DW, Levin HR, Gallant EM, Ashton RC Jr, Seo S, D'Alessandro D et al (1997) Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation 95:1122–1125. [DOI] [PubMed] [Google Scholar]
- 16. Leng G, Brown CH, Russell JA (1999) Physiological pathways regulating the activity of magnocellular neurosecretory cells. Prog Neurobiol 57:625–655. [DOI] [PubMed] [Google Scholar]
- 17. Leng G, Brown CH, Bull PM, Brown D, Scullion S, Currie J et al (2001) Responses of magnocellular neurons to osmotic stimulation involves coactivation of excitatory and inhibitory input: an experimental and theoretical analysis. J Neurosci 21:6967–6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oliveira‐Pelegrin GR, Ravanelli MI, Branco LG, Rocha MJ (2009) Thermoregulation and vasopressin secretion during polymicrobial sepsis. Neuroimmunomodulation 16:45–53. Epub 2008 December 2015. [DOI] [PubMed] [Google Scholar]
- 19. Pancoto JA, Correa PB, Oliveira‐Pelegrin GR, Rocha MJ (2008) Autonomic dysfunction in experimental sepsis induced by cecal ligation and puncture. Auton Neurosci 138:57–63. Epub 2007 December 2003. [DOI] [PubMed] [Google Scholar]
- 20. Paxinos G, Watson C (1997) The Rat Brain in Stereotaxic Coordinates. Academic Press: New York. [Google Scholar]
- 21. Reid IA (1997) Role of vasopressin deficiency in the vasodilation of septic shock. Circulation 95:1108–1110. [DOI] [PubMed] [Google Scholar]
- 22. Rivier C (1998) Role of nitric oxide and carbon monoxide in modulating the ACTH response to immune and nonimmune signals. Neuroimmunomodulation 5:203–213. [DOI] [PubMed] [Google Scholar]
- 23. Russell JA (2007) Vasopressin in septic shock. Crit Care Med 35:S609–S615. [DOI] [PubMed] [Google Scholar]
- 24. Sayk F, Vietheer A, Schaaf B, Wellhoener P, Weitz G, Lehnert H, Dodt C (2008) Endotoxemia causes central downregulation of sympathetic vasomotor tone in healthy humans. Am J Physiol Regul Integr Comp Physiol 295:R891–R898. Epub 2008 July 2016. [DOI] [PubMed] [Google Scholar]
- 25. Sharshar T, Annane D (2008) Endocrine effects of vasopressin in critically ill patients. Best Pract Res Clin Anaesthesiol 22:265–273. [DOI] [PubMed] [Google Scholar]
- 26. Sharshar T, Carlier R, Blanchard A, Feydy A, Gray F, Paillard M et al (2002) Depletion of neurohypophyseal content of vasopressin in septic shock. Crit Care Med 30:497–500. [DOI] [PubMed] [Google Scholar]
- 27. Sharshar T, Blanchard A, Paillard M, Raphael JC, Gajdos P, Annane D (2003) Circulating vasopressin levels in septic shock. Crit Care Med 31:1752–1758. [DOI] [PubMed] [Google Scholar]
- 28. Sharshar T, Gray F, Lorin de la Grandmaison G, Hopkinson NS, Ross E, Dorandeu A et al (2003) Apoptosis of neurons in cardiovascular autonomic centres triggered by inducible nitric oxide synthase after death from septic shock. Lancet 362:1799–1805. [DOI] [PubMed] [Google Scholar]
- 29. Sharshar T, Annane D, De La Grandmaison GL, Brouland JP, Hopkinson NS, Gray F (2004) The neuropathology of septic shock. Brain Pathol 14:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wilkinson DG, Nieto MA (1993) Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol 225:361–373. [DOI] [PubMed] [Google Scholar]
- 31. Xia Y, Krukoff TL (2003) Differential neuronal activation in the hypothalamic paraventricular nucleus and autonomic/neuroendocrine responses to I.C.V. endotoxin. Neuroscience 121:219–231. [DOI] [PubMed] [Google Scholar]


