Abstract
We have previously demonstrated that the transcription of neuronal repressor element‐1/neuron‐restrictive silencer element (RE1/NRSE)‐regulated genes is reduced in the brain of subjects with Huntington's disease (HD) as a result of increased binding of the repressor element‐1 silencing transcription factor/neuron‐restrictive silencer factor (REST/NRSF) to its RE1/NRSE targets. As specific non‐neuronal REST/NRSF‐regulated genes have been identified in the human genome, we exploited the possibility that the binding of REST/NRSF to its target RE1/NRSE sites may also be altered in the peripheral tissues of HD patients.
Our results show that REST/NRSF occupancy is increased in lymphocytes from HD subjects, thus indicating for the first time that the activity of the RE1/NRSE sites is dysfunctional in vivo. Chromatin immunoprecipitation (ChIP) of the RE1/NRSE sites in lymphocytes may therefore be a reproducible, sensitive and specific means of searching for candidate markers of HD onset and progression.
Keywords: Huntington, Lymphocytes, REST/NRSF
INTRODUCTION
Huntington's disease (HD) is a dominantly inherited and progressive neurodegenerative disorder that is characterized by the loss of neurons in the striatum and cerebral cortex and leads to motor impairment and cognitive decline (15). The disease‐triggering event is an abnormal expansion of a CAG triplet repeat in the 5′ end of the gene encoding for the huntingtin protein (18) and, as a consequence of the elongated polyglutamine (polyQ) stretch, huntingtin loses some of its beneficial activities and becomes toxic to brain neurons (6).
A number of studies have shown that transcriptional dysregulation is a central pathogenic mechanism in HD (8). The expression of many transcripts is altered in the brain of HD patients by mutant huntingtin's ability to affect transcription factor activity, DNA target sequences and chromatin remodeling (8). The affected transcriptional pathways include the repressor element‐1 silencing transcription factor/neuron‐restrictive silencer factor (REST/NRSF), a repressor that controls the expression of neuronal genes by binding to its target sequence repressor element‐1/neuron‐restrictive silencer element (RE1/NRSE). Chromatin immunoprecipitation (ChIP) experiments performed on HD cells, HD mouse brain and post‐mortem brain samples from subjects affected by HD have revealed increased binding of REST/NRSF to its regulatory motif RE1/NRSE, and we have reported that the expression of a number of RE1/NRSE‐regulated neuronal genes, including brain‐derived neurotrophic factor (BDNF), is greatly reduced in HD 20, 21).
In this study, we sought in vivo proof of the aberrant occupancy of RE1/NRSE sites in human HD, concentrating on the RE1/NRSE sites localized in the small number of genes encoding non‐neuronal proteins expressed in human peripheral cells (11). In a preliminary experiments, we found that REST/NRSF occupancy is increased at the RE1/NRSE site within the hepatic transcription factor HNF‐1a locus of livers from HD but not in control mice (21), which suggested searching for RE1/NRSE‐regulated genes actively transcribed in human peripheral cells in order to verify whether REST/NRSF occupancy of these loci reflects the pathological genomic profile observed in the brain. In addition, this might be useful for evaluating various aspects of disease progression and pharmacological treatment.
Using ChIP experiments with fresh lymphocytes from peripheral blood, we found that REST/NRSF occupancy of RE1/NRSE loci in the proximity of the leukocyte immunoglobulin (Ig)‐like receptor, subfamily B, member 4 (LILRB4) and Ig lambda‐like polypeptide 1 precursor (IGLL1) genes was higher in chromatin from HD patients (n = 31) than in controls (n = 17). No alteration was found in pre‐HD gene carriers (n = 5). We also found that increased REST/NRSF occupancy is a specific molecular phenotype caused by the polyQ expansion in huntingtin protein as controls and patients with spinocerebellar ataxia type 1, 2 and 6 (SCA1, SCA2 and SCA6) patients (n = 7) had overlapping REST/NRSF occupancy profiles.
Our results indicate that peripheral lymphocytes from HD patients show increased binding of REST/NRSF to RE1/NRSE loci, thus mirroring a pathological event occurring in the nervous system. Given the precise molecular window in gene transcription assessed by ChIP of the RE1/NRSE sites, our data suggest that ChIP analysis of the RE1/NRSE sites in lymphocytes can be used as a novel, reproducible, sensitive and specific means of assessing the activity of candidate therapeutic agents capable of restoring the transcription of RE1/NRSE‐controlled neuronal genes in the brain.
MATERIAL AND METHODS
Patients
The patients were enrolled, and the molecular, genetic and clinical evaluations were made in collaboration with C. Besta National Neurological Institute (Milan, Italy) in accordance with the 1994 guidelines of the International Huntington's Association and World Federation of Neurology Research Group for HD. All of the eligible participants received verbal and written information about the study, and signed an informed consent form (informed consent was obtained from the relatives of symptomatic HD patients with severe cognitive impairment).
The 60 enrolled subjects included 31 patients with symptomatic HD (grades 1, 2, 3 and 4) and positive genetic tests for the presence of the pathogenic triplet repeat expansion; five presymptomatic HD subjects with a positive family history of HD, but no clinical signs of the disease; seven patients with SCA type 1, 2 and 6; and 17 controls recruited from patients, healthy relatives and spouses. Table 1 shows the subjects' gender, age, disease duration, total function capacity score (TFC), Unified Huntington's Disease Rating Scale (UHDRS) motor score and the length of the CAG repeat on the expanded allele.
Table 1.
Clinical and genetic characteristics of HD, pre‐HD, SCA and healthy patients, expressed as median values (min‐max). Abbreviations: HD = Huntington's disease; SCA = spinocerebellar ataxia; TFC = total function capacity score; UHDRS = Unified Huntington's Disease Rating Scale.
| HD (n = 31) | Pre‐HD (n = 5) | SCA type 1, 2 and 6 (n = 7) | Controls (n = 17) | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Women–men | 11–20 | 2–3 | 5–2 | 10–7 |
| Age (years) | 53.5 (30–81) | 36 (36–47) | 46 (26–69) | 53 (27–71) |
| Disease duration (years) | 6 (1–12) | — | 12 (5–19) | — |
| UHDRS motor score | 24 (1–77) | 0 (0–6) | — | — |
| TFC | 10.5 (1–13) | 13 (13–13) | — | — |
| Disease stages | Stage I = 16 | — | — | — |
| Stage II = 7 | ||||
| Stage III = 7 | ||||
| Stage IV = 1 | ||||
| Genetic characteristics | ||||
| CAG repeats normal | 17 (15–24) | 20 (17–22) | 22 (11–29) | — |
| CAG repeats expanded | 44 (40–52) | 40 (39–43) | 47 (22–53) | — |
Lymphocyte and monocyte isolation and culturing conditions
Blood samples (30 mL) were collected in ethylenediaminetetraacetic acid (EDTA)‐treated tubes (K3 EDTA 15%, Vacutainer® blood collection tubes, Franklin Lakes, NJ, USA), and processed 2 h later under sterile conditions after being diluted with an equal volume of phosphate‐buffered saline (PBS). Lympholyte Mammal (Tebu‐Bio, Yvelines, France) was used for the high‐yield and non‐selective recovery of viable lymphocytes and monocytes as instructed by the manufacturer, and 30 × 106 cells were cultured overnight at 37°C in T‐75 flasks in the presence of Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum to allow the monocytes to adhere. The non‐adherent cells (the lymphocyte population) were collected and centrifuged for 10 minutes at 800 × g.
Cytofluorimetric analysis
Five thousand cells were treated with 1% formaldehyde in PBS and cytofluorimetrically analysed using a Cytomics™ FC 500 Flow Cytometry System (Beckman Coulter, Fullerton, CA, USA) and software for data collection; the acquisition process was stopped after 10.000 total events.
RNA isolation and reverse transcription
Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Genomic DNA was digested with DNA‐free (Applied Biosystems, Foster City, CA, USA) at 37°C for 10 minutes. Total RNA (1 µg) was reverse‐transcribed to single‐stranded cDNA using Superscript III RNaseH reverse transcriptase (Invitrogen) and random primers in a volume of 20 µL as instructed by the manufacturer.
Qualitative polymerase chain reaction (PCR)
PCR was performed in a total volume of 50 µL containing cDNA made from 0.20 µg of RNA, 20 mM Tris‐HCl, pH 8.4, 50 mM KCl, 1.5 mM MgCl2, 0.2 mM dNTPs, 0.4 µM of each primer, and 2 U Taq polymerase (Invitrogen). BDNF IIA, IIB and IIC were amplified by means of amplification cycles consisting of an initial denaturing cycle at 94°C for 5 minutes, followed by 35 cycles of 1 minutes at 94°C, 45 s at 62°C and 45 s at 72°C. LILRB4 was amplified by means of a PCR protocol consisting of an initial denaturing cycle at 94°C for 3 minutes, followed by 35 cycles of 30 s at 95°C, 30 s at 60°C and 30 s at 72°C. For IGGL1 mRNA detection, the PCR protocol consisted of an initial denaturing cycle at 94°C for 3 minutes, followed by 35 cycles of 30 s at 95°C, 30 s at 60°C and 30 s at 72°C. The following primers were used:
-
•
BDNF II: 5′‐GGGCGATAGGAGTCCATTCAGCAC‐3′; and
-
•
BDNF XIII (set up on BDNF coding region): 5′‐CCAAGCC ACCTTGTCCTCGGATG‐3′.
The sizes of the BDNF IIA, IIB and IIC amplification products were, respectively, 483, 400 and 185 bp.
-
•
LILRB4 Fw: 5′‐ GCTGCCGTGAAGAACACACAG ‐3′; and
-
•
LILRB4 Rev: 5′‐ AGTGTCCATCTGTCTGTCCTCT ‐3′.
The size of the amplification product was 207 bp.
-
•
IGLL1 Fw: 5′‐ ACAGCTGCATCGCAGAGCA ‐3′; and
-
•
IGLL1 Rev: 5′‐ TTGGAGCTCCTCAGAGGACG ‐3′.
The size of the amplification product was 142 bp.
The same amount of each cDNA was also independently amplified with specific primers for glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) mRNA. The amplification cycles consisted of an initial denaturing cycle at 94°C for 3 minutes, followed by 35 cycles of 30 s at 95°C, 30 s at 60°C and 30 s at 72°C
The primer sequences for the amplification of GAPDH were:
-
•
GAPDH Fw: 5′‐ AGCTGAACGGGAAGCTCACT ‐3′; and
-
•
GAPDH Rev: 5′‐ AGGTCCACCACTGACACGTTG ‐3′.
The size of the amplified product was 67 bp.
The PCR products were separated by agarose gel electrophoresis.
Real‐time PCR for REST mRNA level
Using an iCycler Thermal Cycler with a Multicolor Real‐time PCR Detection System (Bio‐Rad, Hercules, CA, USA), the PCRs were performed in a total volume of 25 µL containing 5 ng of cDNA, 50 mM KCl, 20 mM Tris‐HCl, pH 8.4, 0.2 mM dNTPs, 25 U/mL iTaq DNA polymerase, 3 mM MgCl2, SYBR Green I, 10 nM fluorescein, stabilisers (iQ SYBR Green Supermix; Bio‐Rad) and 0.2 µM of forward and reverse primers. The amplification cycles consisted of an initial denaturing cycle at 95°C for 3 minutes, followed by 45 cycles of 30 s at 95°C, 30 s at 60°C and 30 s at 72°C. Fluorescence was quantified during the 60°C annealing step, and product formation was confirmed by melting curve analysis (55–94°C). The amounts of the target gene mRNA were normalized to a reference gene (GAPDH) according to Pfaffl (2001). The primer sequences were:
-
•
REST Fw: 5′‐ ACTTTGTCCTTACTCAAGTTCTCAG ‐3′; and
-
•
REST Rev: 5′‐ ATGGCGGGTTACTTCATGTT ‐3′.
The size of the amplified product was 130 bp.
-
•
GAPDH Fw: 5′‐ AGCTGAACGGGAAGCTCACT ‐3′; and
-
•
GAPDH Rev: 5′‐ AGGTCCACCACTGACACGTTG ‐3.
The size of the amplified product was 67 bp.
Chromatin immunoprecipitation
The lymphocytes (70 × 106) were washed three times with PBS and treated with 1% formaldehyde in PBS by rotation for 8 to 10 minutes at 4°C. Fixation was stopped by adding glycine to a final concentration of 125 mM. The cells were washed twice with PBS, and the pellets were suspended in 300 µL of ChIP lysis buffer (10 mM Tris‐HCl, pH 8, 140 mM NaCl, 1 mM EDTA, 1% Triton X‐100, 0.1% sodium deoxycholate supplemented with 1 mM phenylmethylsulfonyl fluoride and the MINI protease inhibitor mixture (Roche, Basel, Switzerland) with 1% SDS (final concentration), and passed through 25–21 ga needles. An additional 2.7 mL of lysis buffer without SDS was added to each sample before sonication. The cell and tissue extract was sonicated five times with 30 s pulses using the microprobe at 40% to 50% output and a 70% duty cycle in glass tubes in order to minimize foaming, and, under these conditions, DNA fragments with an average size of 200–700 bp were obtained.
The sonicated extracts were centrifuged, and their chromatin yield was evaluated by means of ultraviolet spectrometry. Equal amounts of chromatin were precleared with blocked protein G‐Sepharose and incubated by overnight rotation with 1 µg of primary anti‐REST/NRSF antibodies (Upstate, Millipore, Billerica, MA, USA) and the same amount of non‐specific rabbit IgG as a negative control; as a positive control, 0.5 µg of anti‐histone H3 were used to immunoprecipitate the same chromatin. Protein G‐Sepharose (GE Healthcare, Little Chalfont, UK) was added and followed by 2 h of incubation with rotation. The beads were spun at 10 000× g for 30 s and then washed sequentially with increasing concentrations of salts and non‐ionic detergents: a first wash with a solution of 10 mM Tris‐HCl, pH 8, 500 mM NaCl, 1 mM EDTA, 1% Triton X‐100, 0.1% sodium deoxycholate; and a second wash with 10 mM Tris‐HCl, pH 8, 1 mM EDTA, 250 mM LiCl, 0.5% NP40, 0.5% sodium deoxycholate. Finally, the beads were eluted with 1% SDS in 0.1 mM NaHCO3, and the bound fractions were de‐cross–linked by adding 200 mM NaCl and incubating at 65°C for 6–8 h. The de‐cross–linked samples were treated with RNase and proteinase K, and the DNA was purified using phenol‐chloroform, precipitated with two volumes of absolute ethanol, and then washed twice with 70% ethanol, after which the pellets were resuspended in 50 µL of HPLC water.
Quantitative real‐time PCR (iCycler thermal cycler with Multicolor Real‐time PCR Detection System, Bio‐Rad) with SYBR Green incorporation was used to assess REST/NRSF occupancy, with three independent experiments being performed for each RE1/NRSE‐containing site. The PCRs were performed in a total volume of 20 µL containing equal amounts of input and immunoprecipitated DNA, 50 mM KCl, 20 mM Tris‐HCl, pH 8.4, 0.2 mM dNTPs, 25 U/mL iTaq DNA polymerase, 3 mM MgCl2, SYBR Green I, 10 nM fluorescein, stabilizers (iQ SYBR Green Supermix; Bio‐Rad) and 0.2 µM of forward and reverse primers. Input from the chromatin that had been cross–linked reversed like the analysed samples was used to control PCR effectiveness.
We used primers flanking the RE1/NRSE sites of the following genes:
-
•
LILRB4 Fw: 5′‐ GGCCCAGCTCATACAAATG ‐3′;
-
•
LILRB4 Rev: 5′‐ AGGCTTCCACTGTTTTCTGGT ‐3′;
-
•
IGLL1 Fw: 5′‐ GGAAGCAGCCTTGGACTTTT ‐3′; and
-
•
IGLL1 Rev: 5′‐ GCCCCTGGACTCAAT ‐3′.
In parallel, anti‐REST/NRSF‐immunoprecipitated genomic DNA was also assessed by means of real‐time PCR using primers set on genomic regions distal to any RE1/NRSE sites represented by coding regions of the selected genes (see the primer sequences used for the qualitative PCR experiments). In addition, DNA from the same chromatin immunoprecipitation underwent quantitative real‐time PCR for β‐actin, a gene that is not regulated by REST/NRSF and not proximal to any RE1/NRSEs, using the following primers:
-
•
β‐actin Fw: 5 ‐TGCCTAGGTCACCCACTAATG‐3; and
-
•
β‐actin Rev: 5 ‐GTGGCCCGTGATGAAGGCTA‐3.
The PCR amplification cycle was 95°C for 3 minutes, followed by 45 cycles of 30 s at 95°C, 30 s at 60°C and 30 s at 72°C. Fluorescence was quantified during the 60°C annealing step, and product formation confirmed by melting curve analysis (55–94°C). The immunoprecipitated DNA for the different RE1/NRSE loci was quantified using a standard curve (nanograms of DNA).
The binding of REST/NRSF to RE1/NRSEs was calculated as nanograms of DNA IP REST antibody per nanogram of DNA IP IgG. Similarly, the binding of histone H3 to each of the RE1/NRSEs (the positive control of the ChIP assay) was calculated as nanograms of DNA IP H3 per nanogram of DNA IP IgG.
ChIP scanning assay
This used the ChIP protocol described earlier. Cross‐linked REST/NRSF DNA complexes were precipitated with anti‐REST/NRSF antibody, and quantitative real‐time PCR was performed on the precipitated DNA fragments using three pairs of oligonucleotide primers designed to produce amplicons covering the RE1/NRSE site in the LILRB4 gene (see earlier discussion), and flanking sequences located at about 16.000 bp and 3.000 bp upstream and10.000 bp downstream of the RE1/NRSE; the primers located 16.000 bp upstream of RE1/NRSE overlapped the coding region of LILRB4. The primers were:
-
•
LILRB4cds Fw: 5′‐ GCTGCCGTGAAGAACACACAG ‐3′;
-
•
LILRB4cds Rev: 5′‐ AGTGTCCATCTGTCTGTCCTCT ‐3′;
-
•
LIRB4 3.000 Fw: 5′‐ CATCTGCAAGTACAGTGACAGTG ‐3′;
-
•
LIRB4 3.000 Rev: 5′‐ GCTCCAACTTTCTCAGTCGACTA ‐3′;
-
•
LIRB4 10.000 Fw: 5′‐GCTGTTTACCAGCTAAGAGCTC ‐3′; and
-
•
LIRB4 10.000 Rev: 5′‐CAAGCCATTGAAGCCATTGTCATG ‐3′.
The same procedure was performed to produce amplicons covering the RE1/NRSE site in the IGLL1 locus, and flanking sequences located 28.542 bp upstream and 62.000 bp downstream of the RE1/NRSE; the primers located 28.542 bp upstream of RE1/NRSE overlapped the coding region of IGLL1. The primers were:
-
•
IGLL1cds Fw: 5′‐ ACAGCTGCATCGCAGAGCA ‐3′;
-
•
IGLL1cds Rev: 5′‐ TTGGAGCTCCTCAGAGGACG ‐3′;
-
•
IGLL1 62.000 Fw: 5′‐ CATTCTAGCATAGAGAACACCTGTG ‐3′;
-
•
IGLL1 62.000 Rev: 5′‐ GCATTTACCAATCTAAGTCAAT AGCC ‐3′.
As described earlier, DNA from the same chromatin immunoprecipitation underwent quantitative real‐time PCR for β‐actin, a gene that is not regulated by REST/NRSF and not proximal to any RE1/NRSEs.
Quantitative PCR was performed in a total volume of 20 µL containing equal amounts of input and immunoprecipitated DNA, 50 mM KCl, 20 mM Tris‐HCl, pH 8.4, 0.2 mM dNTPs, 25 U/ml iTaq DNA polymerase, 3 mM MgCl2, SYBR Green I, 10 nM fluorescein, stabilisers (iQ SYBR Green Supermix; Bio‐Rad) and 0.2 µM of forward and reverse primers, using the following amplification protocol: 95°C for 3 minutes, followed by 45 cycles of 30 s at 95°C, 30 s at 60°C and 30 s at 72°C. Fluorescence was quantified during the 60°C annealing step, and product formation confirmed by melting curve analysis (55–94°C). The immunoprecipitated DNA for the different RE1/NRSE loci was quantified using a standard curve (nanograms of DNA). The binding of REST/NRSF to the RE1/NRSEs was calculated as nanograms of DNA IP REST antibody per nanogram of DNA IP with rabbit IgG.
Statistical analyses
As the REST occupancy and REST mRNA values do not follow a normal distribution, the non‐parametric, two‐tailed Mann–Whitney U‐test was used for the statistical analyses, with a P value of <0.05 being considered significant. Median values were used for the same reason. In each figure, the boundary of the box closest to zero indicates the 25th percentile, the line within the box marks the median or 50th percentile and the boundary of the box farthest from zero indicates the 75th percentile. When 10 or more samples were analyzed, whiskers above and below the box indicate the 90th and 10th percentiles.
Three or more unpaired groups were compared using the non‐parametric Kruskal–Wallis test followed by Dunn's multiple comparison test. The correlations were assessed using Spearman's rank correlation coefficient.
RESULTS
In silico identification of RE1/NRSE sites regulating lymphocyte‐specific genes
We started to search for putative REST/NRSF target genes expressed in the immunosystem by interrogating http://bioinformatics.leeds.ac.uk/RE1db_mkII/, a database containing genomic human and mouse RE1/NRSE sequences that identifies the predictive binding sites of REST/NRSF to RE1/NRSE by means of RE1 position‐specific scoring matrices (PSSMs) and the putative gene regulated by each RE1/NRSE locus (11). Most of the selected REST‐binding sites are functional. Using an RE1 0.91 PSSM cut‐off score and restricting the search to the immuno‐specific RE1/NRSE loci allowed us to identify 13 different RE1/NRSE sites that putatively regulate various genes expressed in the human immune system, of which we selected two that had a high RE1 PSSM cut‐off score and were positioned closest to predicted regulated lymphocyte‐specific genes. The first was an RE1 (ID number hum40753; cut‐off score 0.916) that putatively modulates the leukocyte Ig‐like receptor, subfamily B, member 4 gene (LILRB4), and the second (ID number RE1 hum43244; cut‐off score 0.912) possibly controls the IGLL1 gene.
LILRB4 is a member of the leukocyte Ig‐like receptor (LIR) family, which is found in a gene cluster at chromosomal region 19q13.4. The encoded protein belongs to the subfamily B class of LIR receptors that contains two or four extracellular Ig domains, a transmembrane domain and 2–4 cytoplasmic immunoreceptor tyrosine‐based inhibitory motifs 1, 3). More specifically, LILRB4 is an inhibitory Ig‐like receptor, which transduces a negative signal that inhibits the stimulation of an immune response and then controls inflammatory responses and cytotoxicity in order to help focus the immune response and limit autoreactivity 7, 9).
IGLL1 is a member of the Ig gene superfamily, and the only functional gene of three genes belonging to a lambda‐like gene cluster located in chromosomal region 22q11.2. These genes encode the surrogate light chain subunits of a pre‐B‐cell receptor that is involved in the transduction of signals for cell proliferation, differentiation from the pro‐B to the pre‐B‐cell stage, allelic exclusion at the Ig heavy chain gene locus and promotion of Ig light chain gene rearrangement 12, 14, 17).
Figure 1A shows the position of RE1/NRSE (determined by the RE1/NRSE database and subsequently confirmed by BLAST analyses): the hum40753 RE1/NRSE site is located 15.500 bp 3′ of the LILRB4 gene; and the hum43244 RE1/NRSE site is located 28.542 bp 5′ of the IGLL1 gene.
Figure 1.
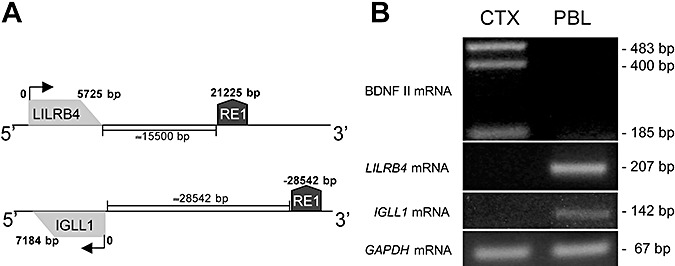
Repressor element‐1 silencing transcription factor/neuron‐restrictive silencer factor (REST/NRSF)‐target genes are expressed in lymphocytes. A. Schematic representation of the positions of the leukocyte immunoglobulin‐like receptor, subfamily B, member 4 (LILRB4) and immunoglobulin lambda‐like polypeptide 1 precursor (IGLL1) REST/NRSF target genes and repressor element‐1/neuron‐restrictive silencer element (RE1/NRSE) on chromosomes 19 and 22. The gray box identifies the gene, and the arrows indicate the transcriptional direction. The RE1/NRSE locus is indicated by the dark grey box. The distance of RE1/NRSE from the genes is calculated by taking the first gene nucleotide (the NC_000019 region from 59865936 to 59871660 for LILRB4 and the NC_000022 region (complement) from 22245312 to 22252495 for IGLL1) as 0. B. Qualitative polymerase chain reaction shows that LILRB4 and IGLL1 are expressed in lymphocytes, but they are not expressed in the cerebral cortex that expresses brain‐derived neurotrophic factor (BDNF) mRNA II, a neuronal isoform of BDNF under the control of REST/NRSF. The presence of three functional donor sites in human BDNF exon II leads to the production of the three BDNF isoforms, BDNF IIA, IIB and IIC. The glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) gene was amplified as a housekeeping gene (data are from one of three independent PCR experiments). Abbreviations: CTX = cortex; PBL = peripheral blood lymphocytes.
It has previously been demonstrated that many RE1/NRSE regulated genes that are silent in non‐neural cells are inaccessible to REST/NRSF 2, 19), and so we evaluated whether LILRB4 and IGLL1 are actively transcribed in cultured human lymphocytes. Figure 1B shows that LILRB4 and IGLL1 mRNAs are present in control lymphocytes but, as expected, not in post‐mortem human cortical tissue. These findings indicate that LILRB4 and IGLL1 are actively transcribed in lymphocytes and, therefore, are potentially accessible to REST/NRSF.
Development of a protocol for assessing REST/NRSF occupancy in human lymphocytes
We then set up an accurate and reproducible method for immunoprecipitating the selected REST/NRSF target sequences in human lymphocytes.
Preparing lymphocytes from fresh blood
The blood samples were processed using Lympholyte Mammal (Tebu‐Bio) a maximum of 2–3 h after they were drawn, and a high‐quality fresh population of lymphocytes and monocytes was obtained. The preparation of lymphocytes from fresh blood is a critical step in this protocol as blood processing after storage at 4°C for longer than 8 h causes a high rate of cell death and red cell contamination that make the samples analytically useless.
ChIP of fresh chromatin from cultured lymphocytes
In a first attempt to perform the REST/NRSF ChIP assay, fresh lymphocytes and monocytes were processed to obtain the chromatin lysates that were then precleared as indicated in the Material and Methods section, and stored at −20°C for practical reasons before being immunoprecipitated with the anti‐REST/NRSF antibody; the REST/NRSF ChIP assay did not work when thawed samples were tested. This clearly indicates that REST/NRSF ChIP must be performed on fresh chromatin samples.
In order to overcome this problem, the lymphocyte/monocyte cultures were set up on the day the blood was collected. In addition to providing fresh cells for the ChIP assay, the culture method allowed us to separate the lymphocytes from monocytes, which adhered to the cell plate. After overnight culture, the suspended lymphocytes were processed to obtain chromatin, which was immediately immunoprecipitated with anti‐REST/NRSF antibodies (see Supporting Information Figure S1 for lymphocytes recovery, counting and plating).
Functional evaluation of REST/NRSF interactions with bona fide RE1/NRSE targets
To verify whether REST/NRSF could bind the LILRB4 RE1/NRSE locus in in vivo human samples, we performed the ChIP assay using two different anti‐REST antibodies: one from UpState and one (Ab 2174) from N. Buckley's lab at King's College, London. Figure 2A shows that αREST/NRSF from UpState and αREST 2174 immunoprecipitate REST/NRSF with the same efficiency. It was also observed that RE1/NRSE occupancy of the LILRB4 locus was greater in HD subjects than in the control subjects. Similar results were obtained when REST/NRSF occupancy was evaluated at the RE1/NRSE proximal to the IGLL1 locus (Supporting Information Figure S2).
Figure 2.
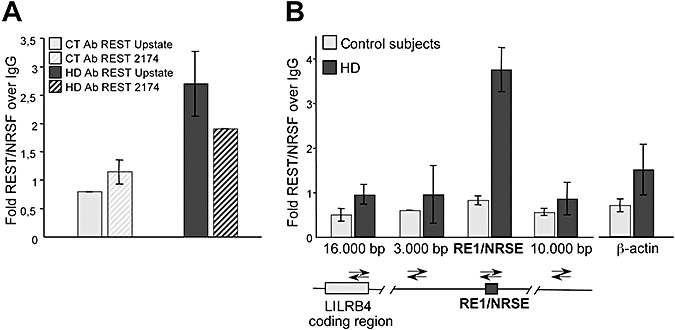
Repressor element‐1 silencing transcription factor/neuron‐restrictive silencer factor (REST/NRSF) chromatin immunoprecipitation (ChIP) in human lymphocytes is specific. A. ChIP at the RE1/NRSE of the leukocyte immunoglobulin‐like receptor, subfamily B, member 4 (LILRB4) locus using two different anti‐REST/NRSF antibodies; the similar results confirm the specificity of the ChIP assay. In addition, repressor element‐1/neuron‐restrictive silencer element (RE1/NRSE) occupancy of the LILRB4 locus was greater in the patients with Huntington's disease (HD) than in the controls (CT). B. ChIP scanning of the LILRB4 locus. Quantitative real‐time polymerase chain reaction was performed on the precipitated DNA fragments using four pairs of oligonucleotide primers designed to produce amplicons covering the RE1/NRSE site in the LILRB4 gene and flanking sequences, located 16.000 bp, on coding region of LILRB4, and 3.000 bp upstream and 10.000 bp downstream of the RE1/NRSE. The scanning ChIP assay shows peak of REST/NRSF occupancy centered at the RE1/NRSE site of the LILRB4 gene. There is no enrichment in distal regions or in the coding region of LILRB4 and the β‐actin gene, which is not regulated by REST/NRSF. Abbreviations: IgG = immunoglobulin G.
ChIP with the H3 antibody was used as a positive control. Histone H3 is a core component of chromatin that is bound to most DNA sequences throughout the genome, including the RE1/NRSE locus. All of the samples used for the analyses showed high levels of H3 occupancy at the RE1/NRSE sites proximal to the selected genes (Supporting Information Figure S3).
ChIP scanning was used to confirm the specificity of REST/NRSF enrichment at the LILRB4 locus. Immunoprecipitated genomic DNA with αREST/NRSF from UpState was assessed by means of real‐time PCR using four pairs of oligonucleotide primers designed to produce amplicons covering the LILRB4 RE1/NRSE site and flanking sequences located 10.000 bp downstream, and 3.000 bp and 16.000 bp upstream of the RE1/NRSE. The assay showed peak REST/NRSF occupancy centered on the RE1/NRSE site of the LILRB4 gene, with no enrichment at distal regions 3.000 bp upstream or 10.000 bp downstream, and no enrichment at a distal 16.000 bp that overlapped with the coding region of LILRB4. ChIP scanning was performed also at the IGLL1 locus (Supporting Information Figure S2).
Finally, no REST/NRSF occupancy was detected at the promoter of the β‐actin gene, which is not regulated by REST/NRSF (Figure 2B), thus further confirming the specificity of the REST/NRSF ChIP assay.
REST/NRSF occupancy of RE1/NRSE targets is higher in HD lymphocytes
We designed a pilot study to assess REST/NRSF occupancy of the LILRB4 and IGLL1 RE1/NRSE sites in lymphocytes taken from controls and HD subjects. These ChIP analyses were performed on chromatin extracted from cultured lymphocytes taken from 31 HD patients, five presymptomatic gene carriers, 17 controls and seven patients with SCA1‐2‐6. In all cases, the patients were examined in the clinic where their blood was drawn and immediately processed.
Figure 3A shows REST/NRSF occupancy at the LILRB4 locus in each of the analysed samples. The endogenous binding of REST/NRSF to the LILRB4 locus was greater in the chromatin samples from the HD subjects than in the samples taken from controls. As most of the REST/NRSF occupancy values in the control and HD groups did not fit a normal distribution curve, the non‐parametric Mann–Whitney U‐test was used to determine the statistical significance of REST/NRSF occupancy between the two cohorts, and it revealed that occupancy was significantly increased in the HD group (P = 0.0003, two‐tailed test); no increase in REST/NRSF occupancy was found in the small group of five presymptomatic gene carriers (P > 0.05, Kruskal–Wallis test). In parallel with the HD subjects, the seven SCA1‐2‐6 patients were tested and the values of REST/NRSF occupancy at the RE1/NRSE site proximal to the LILRB4 locus were similar to those detected in control subjects (P > 0.05, Kruskal–Wallis test). These data suggest that increased REST/NRSF occupancy in HD lymphocytes is a specific molecular phenotype determined by the polyQ expansion in huntingtin protein. Figure 3B shows the median values, which better represent the average value of not normally distributed data and also reduce the skewing caused by outliers.
Figure 3.
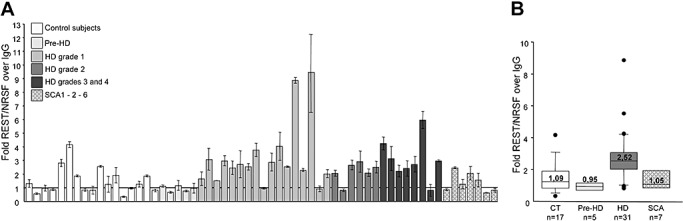
Repressor element‐1 silencing transcription factor/neuron‐restrictive silencer factor (REST/NRSF) occupancy of the repressor element‐1/neuron‐restrictive silencer element (RE1/NRSE) locus of the leukocyte immunoglobulin‐like receptor, subfamily B, member 4 (LILRB4) gene. A. REST/NRSF occupancy of the RE1/NRSE locus of the LILRB4 gene in control (CT), pre‐Huntington's disease (HD), HD and spinocerebellar ataxia type 1, 2, and 6 (SCA type 1, 2 and 6) subjects. Each column represents the average value ± standard deviation of three independent quantitative polymerase chain reaction experiments using genomic DNA immunoprecipitated with anti‐REST/NRSF antibody. B. Median REST/NRSF occupancy in the CT and HD groups. REST/NRSF occupancy was significantly greater in the HD patients than in the CT (two‐tailed Mann–Whitney test: P = 0.0003), and in the pre‐HD (Kruskal–Wallis test: P < 0.01) and SCA subjects (Kruskal–Wallis test: P < 0.05), with no statistical difference between the CT and pre‐HD (Kruskal–Wallis test: P > 0.05) or spinocerebellar ataxia (SCA) samples (Kruskal–Wallis test: P > 0.05). The boundary of the box closest to zero indicates the 25th percentile, the line within the box marks the median and the boundary of the box farthest from zero indicates the 75th percentile. When 10 or more samples were analyzed, whiskers above and below the box indicate the 90th and 10th percentiles. Outliers are indicated as black circles. Abbreviations: IgG = immunoglobulin G.
To confirm the presence of an altered REST/NRSF genomic profile in HD lymphocytes, we analyzed the association between REST/NRSF and the RE1/NRSE locus proximal to the IGLL1 gene. Figure 4A shows the REST/NRSF occupancy of the RE1/NRSE IGLL1 locus in controls (n = 10) and presymptomatic (n = 5), HD (n = 13) and SCA1‐2‐6 patients (n = 6). In line with the ChIP LILRB4 locus data, REST/NRSF showed a closer association with the RE1/NRSE of the IGLL1 gene in HD lymphocytes (Mann–Whitney U‐test P = 0.0002, two‐tailed). There was no difference in REST/NRSF occupancy between the controls and the presymptomatic gene carriers (P > 0.05, Kruskal–Wallis test) or SCA1‐2 patients (P > 0.05, Kruskal–Wallis test).
Figure 4.
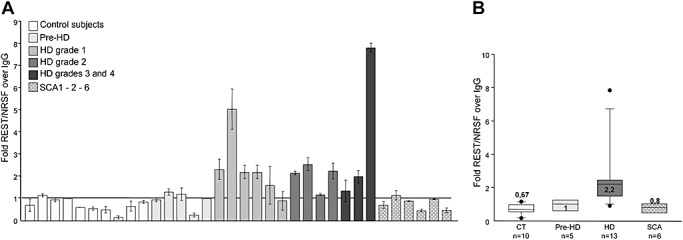
Repressor element‐1 silencing transcription factor/neuron‐restrictive silencer factor (REST/NRSF) occupancy at the repressor element‐1/neuron‐restrictive silencer element (RE1/NRSE) of the immunoglobulin lambda‐like polypeptide 1 precursor (IGLL1) gene. A. REST/NRSF occupancy of the RE1/NRSE of the IGLL1 gene. Each column represents the average value ± standard deviation of three independent quantitative polymerase chain reaction experiments. B. Median REST/NRSF occupancy levels in the control (CT) and Huntington's disease (HD) groups. RE1/NRSE occupancy was significantly greater in the HD patients than in the CT (two‐tailed Mann–Whitney test: P = 0.0002), and in the pre‐HD (Kruskal–Wallis test: P < 0.01) and spinocerebellar ataxia (SCA) subjects (Kruskal–Wallis test: P < 0.05), with no statistical difference between the CT and the pre‐HD (Kruskal–Wallis test: P > 0.05) or SCA subjects (Kruskal–Wallis test: P > 0.01). The boundary of the box closest to zero indicates the 25th percentile, the line within the box marks the median and the boundary of the box farthest from zero indicates the 75th percentile. When 10 or more samples were analyzed, whiskers above and below the box indicate the 90th and 10th percentiles. Outliers are indicated as black circles.
Spearman's correlation analyses showed that REST/NRSF occupancy in HD patients did not correlate with the UHDRS motor score (RE1/NRSE in LILRB4: Spearman's ρ (Sρ) −0.2160; P = 0.24; RE1/NRSE in IGLL1: Sρ−0.03444; P = 0.91), TFC (RE1/NRSE in LILRB4: Sρ−0.056; P = 0.76; RE1/NRSE in IGLL1: Sρ = 0.3223; P =0.28), age (RE1/NRSE in LILRB4: Sρ = 0.1254; P = 0.5014; RE1/NRSE in IGLL1: Sρ = 0.1956; P = 0.52) or disease duration (RE1/NRSE in LILRB4: Sρ = 0.2791; P = 0.13; RE1/NRSE in IGLL1: Sρ = 0.2808; P = 0.35). Finally, there was no correlation between REST/NRSF occupancy and the length of the CAG repeat on the expanded allele, possibly because of the very similar CAG repeat length among the HD patients. Twenty‐nine of the 31 HD patients were being treated with two or more drugs that mainly included selective reuptake inhibitors (selective serotonin re‐uptake inhibitors, selective serotonin–norepinephrine re‐uptake inhibitors), neuroleptics and benzodiazepines (see Supporting Information Table S1), but because of the small number of untreated HD patients, we could not look for any effect of drug treatment on REST/NRSF occupancy.
REST/NRSF mRNA levels in control and HD patients
To demonstrate that the increased binding of REST/NRSF to RE1/NRSE in HD lymphocytes did not depend on increased levels of REST/NRSF, we performed a real‐time PCR analysis to evaluate REST/NRSF mRNA levels on total RNA extracted from the cultured lymphocytes of 11 HD and 6 control subjects. No differences were found in the level of REST/NRSF mRNA normalized to GAPDH mRNA content (Figure 5). We therefore concluded that the increased occupancy of RE1/NRSE by REST/NRSF in human HD lymphocytes is caused by the increased binding of REST/NRSF to the RE1/NRSE sites 19, 20).
Figure 5.
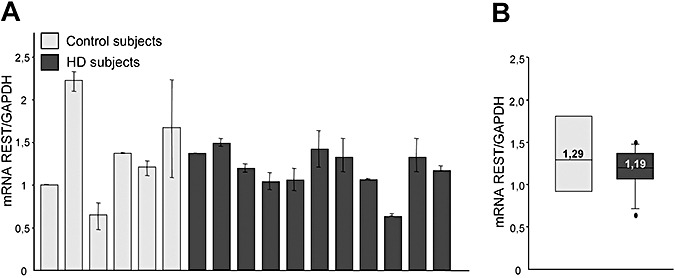
Levels of repressor element‐1 silencing transcription factor/neuron‐restrictive silencer factor (REST/NRSF) mRNA in lymphocytes from subjects with Huntington disease (HD) and control subjects. Total levels of REST/NRSF mRNA were analyzed by means of quantitative polymerase chain reaction (PCR) and normalized to the level of glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) mRNA. A. REST/NRSF mRNA levels were similar in the controls and HD subjects. B. Median REST/NRSF mRNA levels. The boundary of the box closest to zero indicates the 25th percentile, the line within the box marks the median and the boundary of the box farthest from zero indicates the 75th percentile. When 10 or more samples were analyzed, whiskers above and below the box indicate the 90th and 10th percentiles. Outliers are indicated as black circles. The values are the averages ± standard deviation of three independent PCR experiments.
DISCUSSION
Transcription factor occupancy profiling has previously been applied to cell and mouse models of HD and human brain tissues 10, 21) but not to the blood of HD patients. We therefore used ChIP to provide the first functional evidence that the binding of the REST/NRSF repressor to its target RE1/NRSE sites can be monitored in fresh human lymphocytes.
The application of REST/NRSF ChIP to a small cohort of HD and control subjects demonstrated that mutant huntingtin increases REST/NRSF occupancy at RE1/NRSE sites in lymphocytes from HD patients in comparison with controls, thus clearly indicating that the genome of HD lymphocytes has similar pathological hallmarks to those previously characterized in HD brain 20, 21).
Although tested in a small number of cases, the increased REST/NRSF occupancy was not found in the circulating cells of presymptomatic individuals carrying the CAG mutation, which suggests that increased REST/NRSF binding in blood may occur at the same time as the appearance of disease symptoms. Interestingly, REST/NRSF occupancy was similar in the controls and the SCA1‐2‐6 patients, and so we suggest that increased REST/NRSF occupancy at selected RE1/NRSE sites may be a specific molecular phenotype strictly related to the presence of the elongated polyQ expansion in huntingtin.
We did not find any correlations with disease severity, the length of the polyQ expansion or other disease parameters because of the small number of analyzed patients. However, we do provide evidence that chromatin from human lymphocytes represents a valuable tool when seeking peripheral transcriptional dysfunctions, and that DNA target sequences on genomic DNA may be reliable peripheral markers of HD. We also found that two major conditions need to be considered when approaching ChIP studies of blood: to use fresh blood and immunoprecipitate the target sites on fresh chromatin. The time between blood drawing, storage and processing greatly affects the quality and specificity of the ChIP assay, as well as the yield and quality of RNA and protein, as suggested by our other studies of human blood (Tarditi et al, unpub. obs.). Coordination between clinical sites and laboratories is imperative for the immediate processing of blood samples for ChIP studies.
As mutant huntingtin affects widely expressed transcription factors, transcriptional dysregulation in HD has recently been the subject of various analytical approaches aimed at identifying potential biomarkers. Although the early results indicated a battery of mRNA as potential markers of HD progression in blood (4), subsequent studies based on the same approach have not confirmed these data (16), indicating that transcriptomic analyses may be a highly variable and risky means of tracking molecular changes in human samples when seeking correlations with HD onset and progression. These findings therefore suggest that other biomarker identification strategies monitoring transcriptional dysfunction are particularly important. Our data indicating that the immunoprecipitation of selected transcription factor target sites is reproducible, sensitive and specific suggest that ChIP may be more advantageous than strategies based on detecting blood RNA. The specificity of the targeted genomic sequence may significantly reduce multifactorial variability in the event controlling the production and stability of specific mRNA or protein.
We concluded that ChIP of the RE1/NRSE sites is a suitable indicator of HD peripheral dysfunction at specific transcription factor binding sites. However, we are conscious that it is premature to propose the REST/NRSF ChIP as a biomarker because the analyses should be extended to a larger population of control and HD subjects.
Supporting information
Figure S1. A schematic representation of the procedure to isolate fresh lymphocytes to be used in the REST ChIP assay.
Figure S2. The graph shows the positive control routinely performed in the assay ie, histone H3 at the RE1/NRSE loci calculated as ng DNA IP H3/ng DNA IP IgG. Similar controls were performed on chromatin lysates from all the subjects included in this study.
Figure S3. A. ChIP at the RE1/NRSE of the IGLL1 locus using two different anti REST/NRSF antibodies. B. ChIP scanning was used to confirm the specificity of REST/NRSF enrichment at the IGLL1 locus.
Table S1. Pharmacological treatments administered to the patients included in this study.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
ACKNOWLEDGMENTS
We are very grateful to Alberto Clivio of the Department of Preclinical Science, Faculty of Medicine, University of Milan, for his generous hospitality and collaboration. This research was funded by Fondazione Cariplo (Italy), NeuroNE (European Union's 6th Framework Programme) and STEM‐HD (European Union's 6th Framework Programme) to E. Cattaneo, who is a member and coordinator of the Huntingtin Function Team, Huntington's Disease Society of America Coalition for the Cure.
REFERENCES
- 1. Arm JP, Nwankwo C, Austen KF (1997) Molecular identification of a novel family of human Ig superfamily members that possess immunoreceptor tyrosine‐based inhibition motifs and homology to the mouse gp49b1 inhibitory receptor. J Immunol 159:2342–2349. [PubMed] [Google Scholar]
- 2. Belyaev ND, Wood IC, Bruce AW, Street M, Trinh JB, Buckley NJ (2004) Distinct RE‐1 silencing transcription factor‐containing complexes interact with different target genes. J Biol Chem 279:556–561. [DOI] [PubMed] [Google Scholar]
- 3. Borges L, Hsu M‐L, Fanger N, Kubin M, Cosman D (1997) A family of human lymphoid and myeloid Ig‐like receptors, some of which bind to MHC class I molecules. J Immunol 159:5192–5196. [PubMed] [Google Scholar]
- 4. Borovecki F, Lovrecic L, Zhou J, Jeong H, Then F, Rosas HD et al (2005) Genome‐wide expression profiling of human blood reveals biomarkers for Huntington's disease. Proc Natl Acad Sci USA 102:11023–11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruce AW, Donaldson IJ, Wood IC, Yerbury SA, Sadowski MI, Chapman M et al (2004) Genome‐wide analysis of repressor element 1 silencing transcription factor/neuron‐restrictive silencing factor (REST/NRSF) target genes. Proc Natl Acad Sci USA 101:10458–10463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cattaneo E, Zuccato C, Tartari M (2005) Normal huntingtin function: an alternative approach to Huntington's disease. Nat Rev Neurosci 6:919–930. [DOI] [PubMed] [Google Scholar]
- 7. Cella M, Dohring C, Samaridis J, Dessing M, Brockhaus M, Lanzavecchia A, Colonna M (1997) A novel inhibitory receptor (ILT3) expressed on monocytes, macrophages, and dendritic cells involved in antigen processing. J Exp Med 185:1743–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cha JH (2007) Transcriptional signatures in Huntington's disease. Prog Neurobiol 83:228–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, Piazza F et al (2002) Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol 3:237–243. [DOI] [PubMed] [Google Scholar]
- 10. Chen‐Plotkin AS, Sadri‐Vakili G, Yohrling GJ, Braveman MW, Benn CL, Glajch KE et al (2006) Decreased association of the transcription factor Sp1 with genes downregulated in Huntington's disease. Neurobiol Dis 22:233–241. [DOI] [PubMed] [Google Scholar]
- 11. Johnson R, Gamblin RJ, Ooi L, Bruce AW, Donaldson IJ, Westhead DR et al (2006) Identification of the REST regulon reveals extensive transposable element‐mediated binding site duplication. Nucleic Acids Res 34:3862–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mattei MG, Fumoux F, Roeckel N, Fougereau M, Schiff C (1991) The human pre‐B‐specific lambda‐like cluster is located in the 22q11.2–22q12.3 region, distal to the IgC lambda locus. Genomics 9:544–546. [DOI] [PubMed] [Google Scholar]
- 13. Pfaffl MW (2001) A new mathematical model from qualification in real time RT‐PCR. Nucleic Acids Res 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pillai S, Baltimore D (1988) The omega and iota surrogate immunoglobulin light chains. Curr Top Microbiol Immunol 137:136–139. [DOI] [PubMed] [Google Scholar]
- 15. Reiner A, Albin RL, Anderson KD, D'Amato CJ, Penney JB, Young AB(1998) Differential loss of striatal projection neurons in Huntington disease. Proc Natl Acad Sci 85:5733–5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Runne H, Kuhn A, Wild EJ, Pratyaksha W, Kristiansen M, Isaacs JD et al (2007) Analysis of potential transcriptomic biomarkers for Huntington's disease in peripheral blood. Proc Natl Acad Sci USA 104:14424–14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schiff C, Milili M, Fougereau M. (1989) Isolation of early immunoglobulin lambda‐like gene transcripts in human fetal liver. Eur J Immunol 19:1873–1878. [DOI] [PubMed] [Google Scholar]
- 18. The Huntington's Disease Collaborative Research Group (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72:971–983. [DOI] [PubMed] [Google Scholar]
- 19. Wood IC, Belyaev ND, Bruce AW, Jones C, Mistry M, Roopra A, Buckley NJ (2003) Interaction of the repressor element 1‐silencing transcription factor (REST) with target genes. J Mol Biol 334:863–874. [DOI] [PubMed] [Google Scholar]
- 20. Zuccato C, Tartari M, Crotti A, Goffredo D, Valenza M, Conti L et al (2003) Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE‐controlled neuronal genes. Nat Genet 35:76–83. [DOI] [PubMed] [Google Scholar]
- 21. Zuccato C, Belyaev N, Conforti P, Ooi L, Tartari M, Papadimou E et al (2007) Widespread disruption of repressor element‐1 silencing transcription factor/neuron‐restrictive silencer factor occupancy at its target genes in Huntington's disease. J Neurosci 27:6972–6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. A schematic representation of the procedure to isolate fresh lymphocytes to be used in the REST ChIP assay.
Figure S2. The graph shows the positive control routinely performed in the assay ie, histone H3 at the RE1/NRSE loci calculated as ng DNA IP H3/ng DNA IP IgG. Similar controls were performed on chromatin lysates from all the subjects included in this study.
Figure S3. A. ChIP at the RE1/NRSE of the IGLL1 locus using two different anti REST/NRSF antibodies. B. ChIP scanning was used to confirm the specificity of REST/NRSF enrichment at the IGLL1 locus.
Table S1. Pharmacological treatments administered to the patients included in this study.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Supporting info item


