Abstract
Although a large number of amyotrophic lateral sclerosis (ALS) patients have undergone transplantation procedures with olfactory ensheathing cells (OECs) in the Bejing Hospital, to our knowledge, no post‐mortem neuropathologic analyses have been performed. We examined the post‐mortem brain of two Italian patients affected by ALS who underwent cellular transplantation in Beijing with their consent. Our aim was to assess the events following the graft procedure to possibly support the rationale of the treatment strategy. The neuropathologic findings were analyzed on the basis of the limited awareness of the experimental conditions and discussed in relation to the safety, efficacy and long‐term outcome of the transplanted cells. Islands of quiescent, undifferentiated cells within the delivery track persisting for up to 12 months–24 months were found. Prominent glial and inflammatory reaction around the delivery track strongly supports the encasement of the graft. Evidence of axonal regeneration, neuronal differentiation and myelination was not seen. The surgical procedure of implantation was not compatible with a neurotrophic effect. The OEC transplantation did not modify the neuropathology of ALS in the two patients. In conclusion, the present neuropathologic analysis does not support a beneficial effect of fetal OEC implantation into the frontal lobes of ALS patients.
Keywords: amyotrophic lateral sclerosis, neuropathology, olfactory ensheathing cells, transplantation
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by progressive loss of motor neurons leading to progressive paralysis. The cause of the disease is not known, and the prognosis is dismal, as patients usually die within 2 years–5 years after the presentation of symptoms. The condition is incurable: generally acknowledged medical treatments include riluzole, symptomatic drugs, enteral nutrition and noninvasive positive pressure ventilation. Currently, several disease‐modifying treatments for ASL are being studied; these treatments are based on biologically supported rationales and undergo rigorous safety trials, followed by randomized controlled trials to establish the efficacy for a defined group of patients, and use reliable measurements of validated end points.
The isolation and characterization of stem cells and neural precursor cells have suggested novel potential modalities of treating neurodegenerative disorders, including ALS 7, 24, 26.
Since 2001, a neurosurgeon in Beijing, China, has been performing a neural cell transplantation treatment called “olfactory ensheathing cell (OEC) transplantation procedure” in ALS patients, as well as in patients with cerebral palsy, multiple sclerosis, ataxia, stroke and other neurological disorders (17). A slower rate of clinical progression of ALS in the first 4 months post transplantation was reported in a pilot study (16); the ALS score increased after the treatment independently from the OEC transplantation procedure (brain, spinal cord, and brain and spinal cord) (4). However, the articles are in Chinese, and a complete appraisal of the results and modalities is not feasible. Hundreds of ALS patients from all over the world have been treated. As very little scientific and clinical information has been made available to physicians and patients about the experimental procedures employed 4, 16, 17, neurologists have generally not recommended this procedure (5); nevertheless, patients are queuing to be treated in the Chinese hospital 8, 11.
Although a large number of ALS patients have undergone transplantation procedures with OECs in the Chinese hospital (8), to our knowledge, no post‐mortem neuropathologic analysis has been performed to assess the events following the graft procedure and possibly support the rationale of the treatment strategy.
Two Italian patients affected by ALS were diagnosed and followed up in our ALS center until death; they underwent the cellular transplant by the above‐mentioned Chinese team with consent. We examined the post‐mortem brain of the two patients and now report the neuropathologic findings. The pathologic findings were analyzed on the basis of the limited awareness of the experimental conditions and discussed in relation to the safety, efficacy and long‐term outcome of the transplanted cells.
PATIENTS AND METHODS
Patient 1, male, was 64 years old at death. Presentation of the disease was characterized by lower limb weakness, and a diagnosis of definite ALS according to the revised El Escorial criteria (3) was carried out. One year after the diagnosis, the patient travelled to Beijing to undergo the transplantation procedure. The clinical charts produced during his stay in the Chinese hospital reported: “Under local anaesthesia, the olfactory ensheathing cell transplantation procedure was done. 100 µl containing about 2,000,000 cells was injected into the brain of the patient.” Details of the target sites for the injections were not provided. No clinical adverse reaction occurred after the operation. During the following months, the disease progressed, leading to bulbar involvement; the patient died from respiratory insufficiency. The total duration of illness from the first symptoms to death was 25 months.
Active consent for the autopsy was given by the family. The brain was fixed in buffered formalin for 2 weeks and, before cutting, was subjected to a magnetic resonance (MR) study (see below).
Patient 2, male, was 61 years old at death. Symptom presentation was at the respiratory muscles, followed by limb weakness. The diagnosis was definite ALS. The patient was ventilator‐dependent during sleep. He underwent the transplantation procedure twice by the same team that had operated on Patient 1. The first procedure was performed 2 years after presentation of the disease; the second was performed 12 months later. No details of the transplantation procedure were reported in the chart of 2005, while the following description was in the 2006 chart: “Under local anaesthesia, the surgical transplantation of olfactory ensheathing cells procedure was done. 100 µl containing about 2,000,000 cells was injected into the bilateral frontal lobe of the patient's brain.” The patient died 15 months after the second procedure because of the progression of respiratory symptoms. Total disease duration was 4 years.
Active consent for the autopsy was given by the patient's family, complying with the last will of the patient. The brain and spinal cord were fixed in buffered formalin; the MR study was performed before the brain cutting.
Post‐mortem MR study of the brains
The formalin‐fixed brains were placed in a metal‐free plastic container and examined on a 1 Tesla magnetic resonance imaging (MRI) system (General Electric Medical Systems, Milwaukee, WI, USA). The axial, coronal and sagittal series were obtained with the following sequences: spin echo (SE) T1 [repetition time (RT) 500 ms and echo time (ET) 16 ms, number of excitations (Nex) 2, thickness (th) 5 mm, slice gap 0.6]; (SE) proton density‐T2 (RT 2500 ms, ET 30 ms–90 ms, Nex 2, th 5 mm, slice gap 0.6); and three‐dimensional Inversion Recovery‐Fast SPoiled GRadient (IR‐FSPGR) (RT 15 ms, ET 6,7, flip angle 20°, Nex 2, th 2 mm, 94 contiguous 2‐mm axial slices) with multiplanar image reformations (volume rendering).
The brain surfaces were reconstructed and three‐dimensionally rendered (Figure 1A). Using three‐dimensional SPGR data, the surgical pathways were reconstructed and analyzed.
Figure 1.
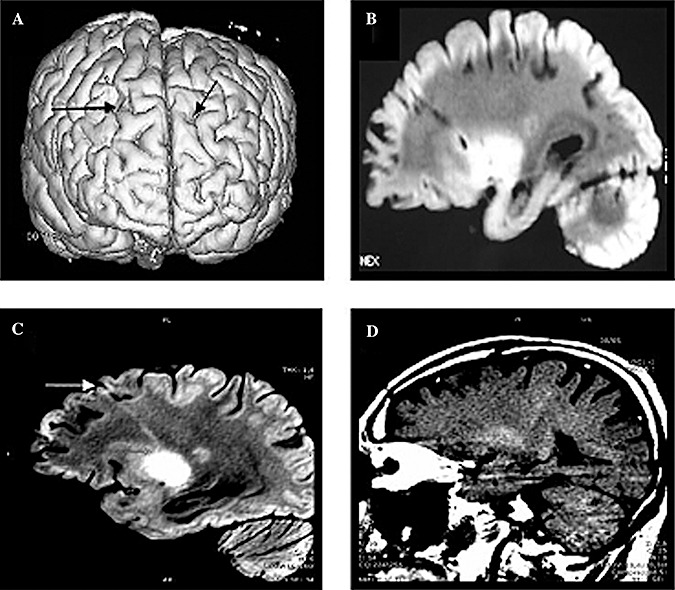
Brain MR images of the patients. Post‐mortem MRI study. A. Cortical surface rendering from the three‐dimensional MRI of Patient 1; the entry holes of the transplantation are visible (arrows). B. T1‐MT‐weighted image of the transplantation track, Patient 1. C. T2‐weighted image of one transplantation track, Patient 2, T2. D. In vivo MR of Patient 1: corticospinal tract hyperintensity on T1‐MT‐weighted image.
Neuropathology
On the basis of the MR images, the positions of the needle tracks were detected, and the brains were cut accordingly. The microscopic features of the cerebral cortex and subcortical white matter crossed by the tracks were analyzed; the tracks were either longitudinally or transversally sectioned. In addition to routine histological methods (Luxol fast blue, periodic acid Shiff (PAS) and Bodian), antibodies (Table 1) were used for the immunohistochemical demonstration of antigens relevant to glial and/or neuronal differentiation, neurotrophins, proliferation, myelin protein, immune and inflammatory response, mesodermal reaction, OEC immunophenotype as reported elsewhere (15) and the hallmark inclusions of ALS. Immunohistochemical reactions were performed after a standard microwave antigen retrieval protocol using citrate buffer, pH 6, prior to incubation of the sections in the primary antibodies; citrate buffer, pH 9, was used before MIB1 and SMI31+32 antibodies. As a subsequent step, anti‐mouse immunoglobulin ImmPRESS™ reagent kit (Vector Laboratories, Burlingame, CA, USA) and anti‐rabbit Envision™ System‐labeled polymer‐HRP (DakoCytomation, Glostrup, Denmark) were used. The labeling was visualized using a Strep‐ABC Vectastain® kit (Vector Laboratories) and peroxidase substrate kit Vector® VIP (Vector Laboratories).
Table 1.
Immunohistochemistry. Panel of antibodies.
| Antibody | Antigen Retrieval | Specificity and aim | |
|---|---|---|---|
| GFAP | Mouse Dako 1:100 | no | OEC phenotype1,15,18,24 |
| Nestin | Mouse Chemicon 1:200 | yes | |
| Fibronectin | Rabbit Sigma 1:200 | yes | |
| NGFR p75 | Mouse Santa Cruz 1:100 | yes | |
| NGF | Rabbit Novus Biologicals 1:100 | yes | Neurotrophines |
| NT‐4 | Rabbit Novus Biologicals 1:200 | yes | |
| CD45 (LCA) | Mouse Dako 1:100 | yes | Inflammation |
| CD20cy | Mouse Dako 1:150 | yes | |
| CD3 | Rabbit Dako 1:50 | yes | |
| CD68 | Mouse Dako 1:100 | yes | Macrophages, microglia |
| HLA‐DR (CR3/43) | Mouse Dako 1:200 | yes | |
| Ki‐67 (MIB‐1) | Mouse Dako 1:50 | yes | Proliferating cells |
| MBP | Rabbit Dako 1:400 | yes | Myelin basic protein |
| Synaptophisin | Mouse Dako 1:40 | yes | Neuronal differentiation |
| SMI31+SMI32 | Mouse Covance 1:1000 | yes | |
| Ubiquitin | Rabbit Dako 1:200 | no | Inclusion bodies of ALS |
| TDP‐43 | Rabbit Proteintech Group 1:200 | yes | |
| Vimentin | Mouse Neomarkers 1:100 | yes | Fibroblasts, glia, blood vessels |
Abbreviations: GFAP = glial fibrillary acidic protein; NGFR p75 = nerve growth factor receptor protein 75; NGF = nerve growth factor; NT‐4 = neurotrophin 4; LCA = leucocyte common antigen; MBP = myelin basic protein; TDP‐43 = TAR DNA binding protein 43.
Fluorescence in situ hybridization using an X chromosome centromeric probe (Vysis Inc., Downers Grove, IL, USA) was performed as described (22) on the nervous tissue around the needle track. The finding of cells with two nuclear signals would be direct evidence of the presence of heterologous cells within the brain.
With the aim of distinguishing the specific changes induced by transplantation from those generated solely by the tissue reaction to the needle wound itself, we comparatively analyzed the neuropathology of the white matter surrounding a ventricular catheter positioned for reducing the intracranial hypertension in a brain tumor patient.
RESULTS
Imaging of post‐mortem brains
MR images showed bilateral asymmetrical linear signals progressing from the prefrontal cortex to the basal ganglia region, ending in proximity to either the caudate or the putamen (Figure 1A–C). Two linear signals in Patient 1 and four in Patient 2 were observable. These were hyperintense in T2, hypointense in T1‐MT and seemingly corresponded to the tracks of the needle. The linear images were not in the area of the corticospinal tract, as shown by comparing a brain MRI of Patient 1, performed 1 year before death, in which the characteristic neuroimaging finding of a hyperintense signal along the corticospinal tract on T1‐MT (Figure 1D), T2‐ and proton density‐weighted and fluid attenuated inversion recovery (FLAIR) sequences, was evident. No signal corresponding to the corticospinal tract was visible in the post‐mortem MR images.
Neuropathology
The entry holes of the needle were variably located between the first and the second frontal gyrus, in proximity to 9, 10 and 46 Brodmann areas (Figure 1A). By adequate serial sectioning, the course of the injection tract was reached and followed from the entry in the frontal cortex to the end. Microscopically, no core delivery site of cells was identified at the terminal part of the delivery track; the end of track was found in brain areas different for each track, as expected, given the lack of stereotactic control for the grafting procedure (Figure 2A,B).
Figure 2.
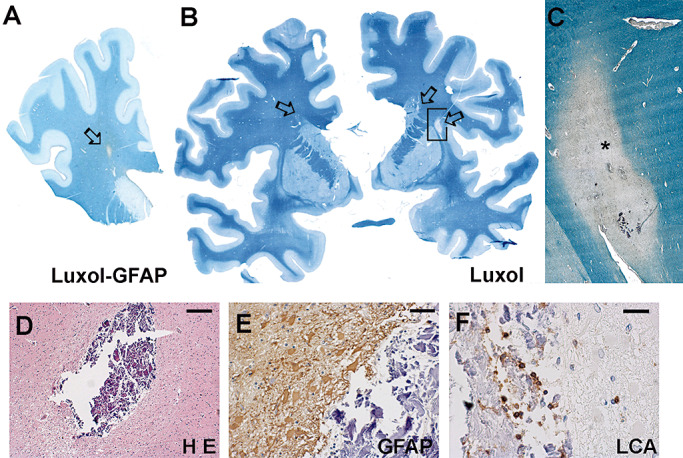
Patient 1: in the paraffin section, the track is visible by lack of myelin and a GFAP‐positive rim (A). Patient 2: three tracks are seen (arrows) (B). (C) is a higher magnification of (B). The track in the cerebral cortex (D) surrounded by gliosis (E) and inflammatory cells (F). The * corresponds to the track. Scale bars: 200 µm (D); 50 µm (E,F). Abbreviations: H E = Hematoxylin‐Eosin; LCA = Leucocyte commom antigen.
Immediately below the entry site in the cortex, necrotic calcified material was found (Figure 2D). It was surrounded by a prominent astroglial reaction with numerous hypertrophic astrocytes (Figure 2E) and an inflammatory reaction characterized by mononuclear cells and foamy macrophages (Figure 2F). Perivascular cuffs of lymphocytes were also found in the cerebral cortex 1 mm–2 mm far from the needle track.
The delivery pathway in its course through the subcortical white matter hosted islands of apparently distinct cells confined within the track and sharply delimited from the surrounding white matter (Figure 3A,B). The immunoreactivity of the islands demonstrated that they did not represent a mesenchymal scar. The nuclei of the cells within the track appeared different from those of the cells in the surrounding white matter (Figure 3C,D); no mitosis was observed, and no cell expressed the proliferation marker Ki‐67. Rare glial fibrillary acidic protein (GFAP)‐ or nestin‐positive cytoplasms (Figure 3E) were observed within the islands, whereas no immunoreactivity for myelin basic protein (Figure 3F), neurofilament (Figure 3G) and nerve growth factor receptor (NGFR) p75 was observed within the islands. Expression of neurotrophins was not evidenced; fibronectin antibody immunostained the vessel walls (Figure 3H).
Figure 3.
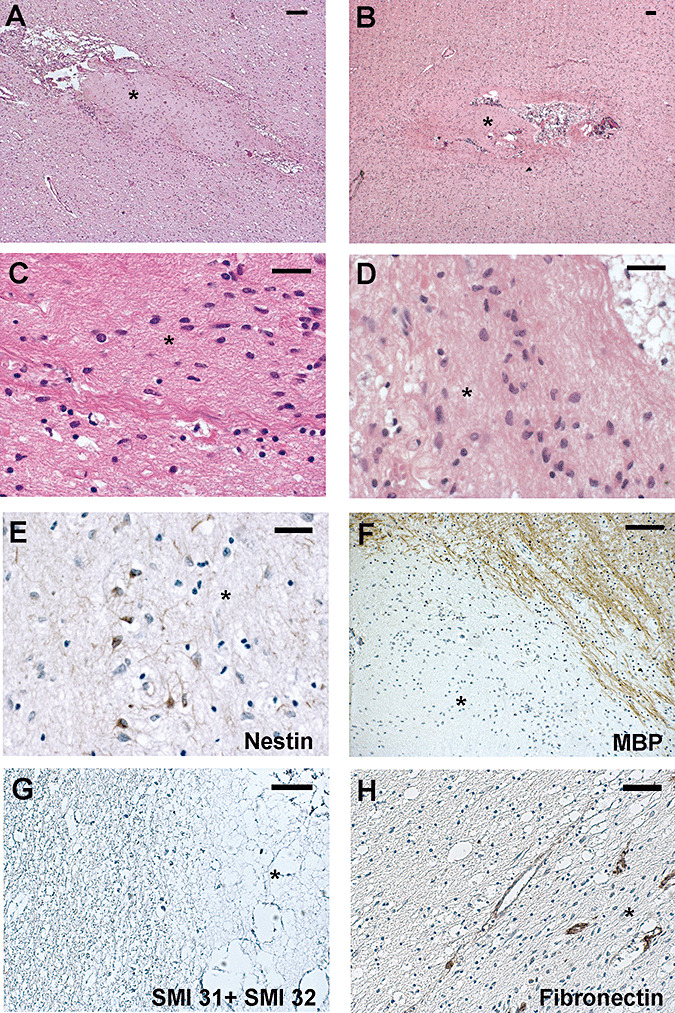
The track in the white matter contains islands distinct from the surrounding tissue (A,B). The cells within the islands (C,D). Sparse nestin‐positive cells (E). The immunostaining of myelin basic protein (F) and neurofilaments (G) stops at the edge of the island. Fibronectin antibody stains the vessel walls. The * marks the island. Scale bar: 100 µm (A,B,F,H); 50 µm (G); 20 µm (C,D,E). Abbreviation: MBP = myelin basic protein.
The sharp border of the islands was evident in slides stained for myelin (Luxol fast blue B) (Figure 2C). Prominent astrogliosis characterized by giant GFAP‐positive reactive astrocytes surrounded the packed cells (Figure 4B).
Figure 4.
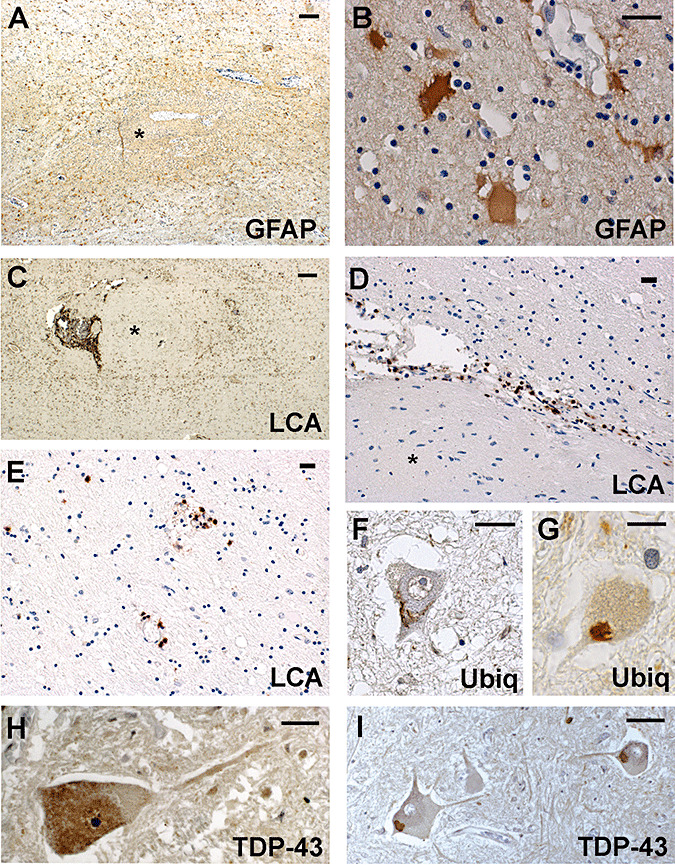
The reaction of the white matter around the islands: a prominent gliosis (A) characterized by giant reactive astrocytes (B); a prominent inflammatory reaction (C) at the edge (D) and distant from the island (E). Spinal motor neurons with ubiquitinated inclusions (F,G), diffuse TDP‐43‐positive cytoplasm (H) and skeins (I). The * marks the island. Scale bars: 200 µm (A,C); 20 µm (B,D–I). Abbreviations: GFAP = glial fibrillary acidic protein; LCA = leucocyte common antigen; Ubiq = ubiquitin.
An inflammatory reaction consisting of perivascular cuffs of lymphocytes and microglia was found within and around the islands (Figure 4C) and in the surrounding white matter, both close to and far from the needle track (Figure 4D,E). No multinucleated giant cells were found.
The brain and spinal cord were also analyzed for well‐known neuropathologic hallmarks of ALS. The motor neurons of the XII cranial nerve in both patients as well as the motor neurons of anterior horns in Patient 2 were reduced in number; typical ubiquitin‐ and TAR DNA binding protein 43 (TDP‐43)‐positive intracytoplasmic skeins and diffuse cytoplasmic TDP‐43 immunostaining (14) were present in the motor neurons (Figure 4F–I). The lateral columns of the spinal cord and the bulbar pyramids showed a partial loss of myelin staining and a moderate microglial reaction.
The overall findings were similar in the two patients. The in situ hybridization gave negative results: no cell showed two nuclear signals.
In comparison, the glial and inflammatory reaction surrounding the site of the ventricular catheter in the brain tumor patient was much less prominent; giant reactive astrocytes were not observed.
DISCUSSION
The use of fetal neural cells or neural precursor cells is a new promising therapy for relentlessly progressive neurodegenerative disorders. Strategies and prospects for their use in ALS have been recently discussed 6, 19. Stem cells are supposed to replace lost or dysfunctional neurons, provided their functional integration into the damaged neural circuitry takes place.
Concerning replacement and integration, promising results come from experiments in animal models of motor neuron impairment 9, 13; nevertheless, the complexity of ALS pathology makes this therapeutic avenue less promising in human patients. Correct differentiation into neurons in non‐neurogenic areas of adult central nervous system (CNS), correct placement of grafted cells in the proper CNS regions, and correct pathfinding and connectivity 6, 19 are the major obstacles to replacement therapy of ALS.
As an alternate to replacement, stem cells or neural precursor cells can have neurotrophic potential improving the functional deficits of diseased neurons. A stem cell‐based neurotrophic therapy is potentially a more promising approach for preventing degeneration or restoring function to damaged motor neurons of ALS patients, provided the transplanted cells migrate and survive into the focal areas.
The mechanisms of possible neurotrophic action of OECs were recently reviewed: OECs promote axon regeneration and functional recovery indirectly by augmenting the endogenous capacity of host Schwann cells to invade the damaged spinal cord and by providing a permissive microenvironment for successful axonal regeneration (2). OECs from rodents express either the low‐affinity neurotrophin receptor p75 or fibronectin in variable proportion depending on culture conditions (1); after implantation into the adult rat brain, p75 immunoreactivity is preserved (24). In vitro cultured human OECs express GFAP (18). The characterization of OECs used by the Chinese neurosurgeon was indirectly reported by distinct authors (15): cultured cells were positive for human nestin and for GFAP. The immunophenotype of grafted human OECs is unknown.
On these bases, the present neuropathologic study of the two ALS patients who underwent the “OEC transplantation” was aimed at assessing the survival and differentiation of transplanted cells, presence of neurotrophins, changes induced in the nervous tissue and modifications of the hallmark pathologic changes of ALS in the brain and spinal cord.
The changes in the needle track area are not attributable to the mere tissue reaction to the needle wound. Our findings suggest persistence of quiescent cells within the delivery track for up to 12 months–24 months, mostly in the white matter part of the track. Properties of OECs, such as the expression of nestin and GFAP, were recognized in sparse cells; the nature of the other numerous cells encased in the needle tracks remains an open question. They do not show the immunophenotype of a scar tissue; therefore, it is possible that they represent quiescent, undifferentiated survivors of the transplanted OECs that neither express neurotrophins nor produce myelin and axons.
The prominent glial reaction observed around the track mirrors the increased proliferation of reactive astrocytes induced by OECs in vitro (21) and suggests that the human brain is not inclined to integrate the exogenous cells. The encasement of OECs within the graft and their negligible migratory properties have also been reported in injured nervous tissue of rats 1, 20, 23 and in the substantia nigra of an animal model of Parkinson's disease 10, 12. We are aware that tracking individual cells grafted into the brain of the two patients was not possible under the present conditions, the cells being unmarked. However, the overall neuropathologic aspect strongly supports the encasement of the grafted cells.
The role of the immune response in graft outcome is of utmost importance. The inflammatory signs observed in the two brains suggest that the implanted cells elicit a lifelong reaction; as they are derived from fetal CNS, their immunogenic properties are low, and inflammation is not very prominent but still persistent. Consistently, the two patients had no clinical adverse reaction after the procedure, even if they were not immunosuppressed after the transplantation. As a matter of fact, 6.74% of patients experience various perioperative complications after the grafting procedure, as reported by the Chinese team (17).
The neurotrophic effect of stem cells requires that grafted cells are correctly directed to the proper cerebral regions. No contact between needle tracks and corticospinal tracts was revealed by post‐mortem MR imaging of the two brains. In addition, the neuropathologic features of ALS were not changed after the transplantation procedure. Moreover, the course of illness and disease duration of the two patients were within the normal ranges of ALS patients.
In conclusion, the long‐term fate of OECs transplanted into the human brain corresponds to the persistence of quiescent, undifferentiated cells within the needle track, encased by a prominent glial reaction; evidence of axonal regeneration, neuronal differentiation, myelination and neurotrophin expression was not seen. The surgical procedure of implantation is not permissive of a neurotrophic effect, and OEC transplantation did not modify the neuropathology of ALS.
The use of unproven and new therapeutic measures in the treatment of patients affected by diseases without any effective therapy is accepted by the World Medical Association (WMA) Declaration of Helsinki (25). Transplantation of OECs into the brain of ALS patients is one example of a new therapeutic measure. However, the WMA statement does not exempt new treatments from relying on current consensual scientific experience, data and procedures, or from verifying the effects of the treatment, not only on a clinical but also on a pathological basis. Even given the methodological limitations of our study, the results do not support that the transplantation of fetal OECs into the frontal lobe can have any beneficial effect in ALS patients.
ACKNOWLEDGMENTS
The study was supported by Compagnia di San Paolo, Turin, Italy, Grant 5040.2004.1424. The authors wish to express their gratitude to the patients for the donation of brain tissues used in this study.
REFERENCES
- 1. Andrews MR, Stelzner DJ (2007) Evaluation of olfactory ensheathing and schwann cells after implantation into a dorsal injury of adult rat spinal cord. J Neurotrauma 24:1773–1792. [DOI] [PubMed] [Google Scholar]
- 2. Boyd JG, Doucette R, Kawaja MD (2005) Defining the role of olfactory ensheathing cells in facilitating axon remyelination following damage to the spinal cord. FASEB J 19:694–703. [DOI] [PubMed] [Google Scholar]
- 3. Brooks BR, Miller RG, Swash M, Munsat TL (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299. [DOI] [PubMed] [Google Scholar]
- 4. Chen L, Huang H, Zhang J, Zhang F, Liu Y, Xi H et al (2007) Short‐term outcome of olfactory ensheathing cell transplantation for treatment of amyotrophic lateral sclerosis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 21:961–966. [PubMed] [Google Scholar]
- 5. Chew S, Khandji AG, Montes J, Mitsumoto H, Gordon PH (2007) Olfactory ensheathing glia injection in Beijing: misleading patients with ALS. Amyotroph Lateral Scler 8:314–316. [DOI] [PubMed] [Google Scholar]
- 6. Christou YA, Moore HD, Shaw PJ, Monk PN (2007) Embryonic stem cells and prospects for their use in regenerative medicine approaches to motor neurone disease. Neuropathol Appl Neurobiol 33:485–498. [DOI] [PubMed] [Google Scholar]
- 7. Conti L, Reitano E, Cattaneo E (2006) Neural stem cell systems: diversities and properties after transplantation in animal models of diseases. Brain Pathol 16:143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cyranoski D (2005) Fetal‐cell therapy: paper chase. Nature 437:810–811. [DOI] [PubMed] [Google Scholar]
- 9. Deshpande DM, Kim YS, Martinez T, Carmen J, Dike S, Shats I et al (2006) Recovery from paralysis in adult rats using embryonic stem cells. Ann Neurol 60:32–44. [DOI] [PubMed] [Google Scholar]
- 10. Dewar D, Bentley D, Barnett SC (2007) Implantation of pure cultured olfactory ensheathing cells in an animal model of parkinsonism. Acta Neurochir (Wien) 149:407–414. [DOI] [PubMed] [Google Scholar]
- 11. Dobkin BH, Curt A, Guest J (2006) Cellular transplants in China: observational study from the largest human experiment in chronic spinal cord injury. Neurorehabil Neural Repair 20:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R et al (2001) Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med 344:710–719. [DOI] [PubMed] [Google Scholar]
- 13. Gao J, Coggeshall RE, Tarasenko YI, Wu P (2005) Human neural stem cell‐derived cholinergic neurons innervate muscle in motoneuron deficient adult rats. Neuroscience 131:257–262. [DOI] [PubMed] [Google Scholar]
- 14. Giordana MT, Piccinini M, Grifoni S, De Marco G, Vercellino M, Magistrello M et al (2009) TDP‐43 redistribution is an early event in sporadic amyotrophic lateral sclerosis. Brain Pathol [E‐pub ahead of print; DOI: 10.1111/j.1750‐3639‐2009.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guest J, Herrera LP, Qian T (2006) Rapid recovery of segmental neurological function in a tetraplegic patient following transplantation of fetal olfactory bulb‐derived cells. Spinal Cord 44:135–142. [DOI] [PubMed] [Google Scholar]
- 16. Huang H, Chen L, Xi H, Wang H, Zhang J, Zhang F, Liu Y (2008) Fetal olfactory ensheathing cells transplantation in amyotrophic lateral sclerosis patients: a controlled pilot study. Clin Transplant 22:710–718. [DOI] [PubMed] [Google Scholar]
- 17. Huang H, Chen L, Xi H, Wang Q, Zhang J, Liu Y, Zhang F (2009) Olfactory ensheathing cell transplantation for central nervous system diseases in 1255 patients. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 23:14–20. [PubMed] [Google Scholar]
- 18. Kato T, Honmou O, Uede T, Hashi K, Kocsis JD (2000) Transplantation of human olfactory ensheathing cells elicits remyelination of demyelinated rat spinal cord. Glia 30:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lepore AC, Maragakis NJ (2007) Targeted stem cell transplantation strategies in ALS. Neurochem Int 50:966–975. [DOI] [PubMed] [Google Scholar]
- 20. Lu P, Yang H, Culbertson M, Graham L, Roskams AJ, Tuszynski MH (2006) Olfactory ensheathing cells do not exhibit unique migratory or axonal growth‐promoting properties after spinal cord injury. J Neurosci 26:11120–11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Toole DA, West AK, Chuah MI (2007) Effect of olfactory ensheathing cells on reactive astrocytes in vitro. Cell Mol Life Sci 64:1303–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qian J, Bostwick DG, Takahashi S, Borell TJ, Herath JF, Lieber MM, Jenkins RB (1995) Chromosomal anomalies in prostatic intraepithelial neoplasia and carcinoma detected by fluorescence in situ hybridization. Cancer Res 55:5408–5414. [PubMed] [Google Scholar]
- 23. Smale KA, Doucette R, Kawaja MD (1996) Implantation of olfactory ensheathing cells in the adult rat brain following fimbria‐fornix transection. Exp Neurol 137:225–233. [DOI] [PubMed] [Google Scholar]
- 24. Suzuki M, Svendsen CN (2008) Combining growth factor and stem cell therapy for amyotrophic lateral sclerosis. Trends Neurosci 31:192–198. [DOI] [PubMed] [Google Scholar]
- 25. World Medical Association (2008) Declaration of Helsinki‐Ethical Principles for Medical Research Involving Human Subjects. Available at: http://www.wma.net/en/30publications/10policies/b3/index.html (accessed 2009). [PubMed]
- 26. Yamamoto M, Tanaka F, Tatsumi H, Sobue G (2008) A strategy for developing effective amyotropic lateral sclerosis pharmacotherapy: from clinical trials to novel pharmacotherapeutic strategies. Expert Opin Pharmacother 9:1845–1857. [DOI] [PubMed] [Google Scholar]


