Abstract
Background
Antisocial personality disorder (AsPD) is associated with rule‐breaking, criminality, substance use, unemployment, relationship difficulties, and premature death. Certain types of medication (drugs) may help people with AsPD. This review updates a previous Cochrane review, published in 2010.
Objectives
To assess the benefits and adverse effects of pharmacological interventions for adults with AsPD.
Search methods
We searched CENTRAL, MEDLINE, Embase, 13 other databases and two trials registers up to 5 September 2019. We also checked reference lists and contacted study authors to identify studies.
Selection criteria
Randomised controlled trials in which adults (age 18 years and over) with a diagnosis of AsPD or dissocial personality disorder were allocated to a pharmacological intervention or placebo control condition.
Data collection and analysis
Four authors independently selected studies and extracted data. We assessed risk of bias and created 'Summary of findings tables' and assessed the certainty of the evidence using the GRADE framework. The primary outcomes were: aggression; reconviction; global state/global functioning; social functioning; and adverse events.
Main results
We included 11 studies (three new to this update), involving 416 participants with AsPD. Most studies (10/11) were conducted in North America. Seven studies were conducted exclusively in an outpatient setting, one in an inpatient setting, and one in prison; two studies used multiple settings. The average age of participants ranged from 28.6 years to 45.1 years (overall mean age 39.6 years). Participants were predominantly (90%) male. Study duration ranged from 6 to 24 weeks, with no follow‐up period. Data were available from only four studies involving 274 participants with AsPD. All the available data came from unreplicated, single reports, and did not allow independent statistical analysis to be conducted. Many review findings were limited to descriptive summaries based on analyses carried out and reported by the trial investigators.
No study set out to recruit participants on the basis of having AsPD; many participants presented primarily with substance abuse problems. The studies reported on four primary outcomes and six secondary outcomes. Primary outcomes were aggression (six studies) global/state functioning (three studies), social functioning (one study), and adverse events (seven studies). Secondary outcomes were leaving the study early (eight studies), substance misuse (five studies), employment status (one study), impulsivity (one study), anger (three studies), and mental state (three studies). No study reported data on the primary outcome of reconviction or the secondary outcomes of quality of life, engagement with services, satisfaction with treatment, housing/accommodation status, economic outcomes or prison/service outcomes.
Eleven different drugs were compared with placebo, but data for AsPD participants were only available for five comparisons. Three classes of drug were represented: antiepileptic; antidepressant; and dopamine agonist (anti‐Parkinsonian) drugs. We considered selection bias to be unclear in 8/11 studies, attrition bias to be high in 7/11 studies, and performance bias to be low in 7/11 studies. Using GRADE, we rated the certainty of evidence for each outcome in this review as very low, meaning that we have very little confidence in the effect estimates reported.
Phenytoin (antiepileptic) versus placebo
One study (60 participants) reported very low‐certainty evidence that phenytoin (300 mg/day), compared to placebo, may reduce the mean frequency of aggressive acts per week (phenytoin mean = 0.33, no standard deviation (SD) reported; placebo mean = 0.51, no SD reported) in male prisoners with aggression (skewed data) at endpoint (six weeks). The same study (60 participants) reported no evidence of difference between phenytoin and placebo in the number of participants reporting the adverse event of nausea during week one (odds ratio (OR) 1.00, 95% confidence interval (CI) 0.06 to 16.76; very low‐certainty evidence). The study authors also reported that no important side effects were detectable via blood cell counts or liver enzyme tests (very low‐certainty evidence).
The study did not measure reconviction, global/state functioning or social functioning.
Desipramine (antidepressant) versus placebo
One study (29 participants) reported no evidence of a difference between desipramine (250 to 300 mg/day) and placebo on mean social functioning scores (desipramine = 0.19; placebo = 0.21), assessed with the family‐social domain of the Addiction Severity Index (scores range from zero to one, with higher values indicating worse social functioning), at endpoint (12 weeks) (very low‐certainty evidence).
Neither of the studies included in this comparison measured the other primary outcomes: aggression; reconviction; global/state functioning; or adverse events.
Nortriptyline (antidepressant) versus placebo
One study (20 participants) reported no evidence of a difference between nortriptyline (25 to 75 mg/day) and placebo on mean global state/functioning scores (nortriptyline = 0.3; placebo = 0.7), assessed with the Symptom Check List‐90 (SCL‐90) Global Severity Index (GSI; mean of subscale scores, ranging from zero to four, with higher scores indicating greater severity of symptoms), at endpoint (six months) in men with alcohol dependency (very low‐certainty evidence).
The study measured side effects but did not report data on adverse events for the AsPD subgroup.
The study did not measure aggression, reconviction or social functioning.
Bromocriptine (dopamine agonist) versus placebo
One study (18 participants) reported no evidence of difference between bromocriptine (15 mg/day) and placebo on mean global state/functioning scores (bromocriptine = 0.4; placebo = 0.7), measured with the GSI of the SCL‐90 at endpoint (six months) (very low‐certainty evidence).
The study did not provide data on adverse effects, but reported that 12 patients randomised to the bromocriptine group experienced severe side effects, five of whom dropped out of the study in the first two days due to nausea and severe flu‐like symptoms (very low‐certainty evidence).
The study did not measure aggression, reconviction and social functioning.
Amantadine (dopamine agonist) versus placebo
The study in this comparison did not measure any of the primary outcomes.
Authors' conclusions
The evidence summarised in this review is insufficient to draw any conclusion about the use of pharmacological interventions in the treatment of antisocial personality disorder. The evidence comes from single, unreplicated studies of mostly older medications. The studies also have methodological issues that severely limit the confidence we can draw from their results. Future studies should recruit participants on the basis of having AsPD, and use relevant outcome measures, including reconviction.
Plain language summary
The use of medication to treat people with antisocial personality disorder
Background
People with antisocial personality disorder (AsPD) may behave in a way that is harmful to themselves or others, and is against the law. They can be dishonest and act aggressively without thinking. Many misuse drugs and alcohol. Certain types of medication (drugs) may help people with AsPD. This review updates one published in 2010.
Review question
What are the beneficial and harmful effects of medication on aggression, reconviction (reoffending), and people's ability to function in society?
Study characteristics
We searched for relevant studies up to 5 September 2019 and found 11 randomised controlled trials (RCT); a type of study in which people were allocated at random (by chance alone) to have either a medication (drug) or a placebo (dummy tablet).
The studies included 416 AsPD participants, mostly male (90%), with an average age of 39.6 years. Most studies (10/11) were carried out in North America in outpatient clinics (seven studies). Two studies were conducted in mixed settings and one apiece in an inpatient hospital or prison. Studies lasted between six and 24 weeks, and had no follow‐up period. Data were only available from four of the 11 included studies for 274 participants with AsPD.
Some studies reported on important outcomes in AsPD: aggression (six studies), global state/functioning (three studies), social functioning (one study) and adverse effects (seven studies). Some reported on other outcomes: leaving the study early (eight studies), substance misuse (five studies), employment status (one study), impulsivity (one study), anger (three studies), and mental state (three studies). No study reported data on reconviction, quality of life, engagement with services, satisfaction with treatment, housing/accommodation status, economic or prison/service outcomes.
No study set out to recruit participants on the basis of having AsPD. Many participants presented primarily with substance abuse problems. The studies used methods that increased the risk of data being biased or untrue (e.g. not reporting all of their outcomes) and that did not allow independent statistics to be calculated for this review.
The studies assessed 11 medications but comparison data for AsPD participants were available for only three different types of medication and placebo: antiepileptics (drugs to treat epilepsy); antidepressants (drugs to treat depression); and dopamine agonists (drugs to treat Parkinson's disease).
Main results
Phenytoin (antiepileptic) versus placebo
One study (60 participants) found very low‐certainty evidence that, compared to placebo, phenytoin may reduce the average frequency of aggressive acts per week in aggressive male prisoners with AsPD at six weeks. The number of participants reporting sickness during week one did not differ across groups, and no side effects were detectable by blood tests. We are very uncertain about these findings.
Desipramine (antidepressant) versus placebo
One study (29 participants) found no evidence of a difference in social functioning scores at 12 weeks, between a drug used to treat depression (desipramine) and placebo. We are very uncertain about these findings.
Nortriptyline (antidepressant) versus placebo
One study (20 participants) found no evidence of a difference in global state/functioning scores in men with alcohol dependency at six months, between a different antidepressant (nortriptyline) and placebo. We are very uncertain about these findings.
Bromocriptine (dopamine agonist) versus placebo
One study (18 participants) found no evidence of a difference in global state/functioning scores at six months, between a drug used to treat Parkinson's disease (bromocriptine) and placebo. Twelve participants randomised to the bromocriptine group experienced side effects, five of whom dropped out due to sickness and flu‐like symptoms in the first two days. We are very uncertain about these findings.
Amantadine (dopamine agonist) versus placebo
None of the included studies assessed the effectiveness of another treatment for Parkinson's disease (amantadine) for any of the primary outcomes.
Conclusions
The certainty of the evidence is very low, meaning that we are not confident in the findings. There is not enough evidence to determine whether or not medication is a helpful treatment for people with AsPD.
Further research is required to clarify which medications, if any, are effective for treating the main features of AsPD. Future studies should recruit participants on the basis of having AsPD, and include reconviction as an outcome measure.
Summary of findings
Background
Description of the condition
Antisocial personality disorder (AsPD) is one of the 10 specific personality disorder categories in the current edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5). The DSM‐5 defines personality disorder as "an enduring pattern of inner experience and behaviour that deviates markedly from the expectations of the individual's culture, is pervasive and inflexible, has an onset in adolescence or early adulthood, is stable over time, and leads to distress or impairment" (p 645). The general criteria for personality disorder according to DSM‐5 are given in Table 7.
1. DSM‐5 general criteria for personality disorder.
| Criteria | Description (taken from DSM‐5, p 646‐7) |
| A. | An enduring pattern of inner experience and behavior that deviates markedly from the expectations of the individual’s culture. This pattern is manifested in two (or more) of the following areas:
|
| B. | The enduring pattern is inflexible and pervasive across a broad range of personal and social situations. |
| C. | The enduring pattern leads to clinically significant distress or impairment in social, occupational, or other important areas of functioning. |
| D. | The pattern is stable and of long duration, and its onset can be traced back at least to adolescence or early adulthood. |
| E. | The enduring pattern is not better explained as a manifestation or consequence of another mental disorder. |
| F. | The enduring pattern is not attributable to the physiological effects of a substance (e.g. a drug of abuse, a medication) or a another medical condition (e.g. head trauma). |
AsPD is described in the DSM‐5 as “a pattern of disregard for, and violation of, the rights of others” (p 645). According to the DSM‐5, in order to be diagnosed with AsPD (301.7), a person must fulfil both the general criteria for personality disorder outlined above and also the specific criteria for AsPD (criteria A, B, C and D, as shown in Table 8). DSM‐5 also states, in reference to the traits of AsPD, that “this pattern has also been referred to as psychopathy, sociopathy or dyssocial personality disorder” (p 659). There continues, however, to be debate about the status of psychopathy compared to AsPD (for example, see Ogloff 2006), how it is measured and the degree to which it is subject to change, which is beyond the scope of this review.
2. DSM‐5 diagnostic criteria for antisocial personality disorder ‐ (301.7).
| Criteria | Description (taken from DSM‐5, p 659) |
| A. | A pervasive pattern of disregard for and violation of the rights of others, occurring since age 15 years, as indicated by three (or more) of the following: 1. Failure to conform to social norms with respect to lawful behaviors, as indicated by repeatedly performing acts that are grounds for arrest. 2. Deceitfulness, as indicated by repeated lying, use of aliases, or conning others for personal profit or pleasure. 3. Impulsivity or failure to plan ahead. 4. Irritability and aggressiveness, as indicated by repeated physical fights or assaults. 5. Reckless disregard for safety of self or others. 6. Consistent irresponsibility, as indicated by repeated failure to sustain consistent work behavior or honor financial obligations. 7. Lack of remorse, as indicated by being indifferent to or rationalizing having hurt, mistreated, or stolen from another. |
| B. | The individual is at least 18 years. |
| C. | There is evidence of conduct disorder with onset before age of 15 years. |
| D. | The occurrence of antisocial behavior is not exclusively during the course of schizophrenia or bipolar disorder. |
The International Classification of Diseases ‐ 10th Edition (ICD‐10) classifies this condition as dissocial personality disorder (F60.2). AsPD and dissocial personality disorder are often used interchangeably by clinicians and they describe a very similar presentation. While there is considerable overlap between these two diagnostic systems, they differ in two respects. First, the DSM‐5 requires that those meeting the diagnostic criteria also show evidence of conduct disorder with onset before the age of 15 years, whereas there is no such requirement using ICD‐10 criteria when making the diagnosis of dissocial personality disorder. However, a study comparing participants meeting the full criteria for AsPD (which the DSM‐5 has retained) with those who otherwise fulfilled criteria for AsPD, but who did not demonstrate evidence of childhood conduct disorder, did not find any clinically important differences (Perdikouri 2007). Second, dissocial personality disorder focuses more on interpersonal deficits (for example, incapacity to experience guilt, a very low tolerance of frustration, proneness to blame others) and less on antisocial behaviour. Table 9 shows the ICD‐10 diagnostic criteria to diagnose dissocial personality disorder (F60.2).
3. ICD‐10 diagnostic criteria for dissocial personality disorder ‐ (F60.2).
| Description (taken fromICD‐10) |
| Personality disorder, usually coming to attention because of gross disparity between behaviour and the prevailing social norms, and characterized by: a. callous unconcern for the feelings of others; b. gross and persistent attitude of irresponsibility and disregard for social norms, rules and obligations; c. incapacity to maintain enduring relationships, though having no difficulty in establishing them; d. very low tolerance to frustration and a low threshold for discharge of aggression, including violence; e. incapacity to experience guilt or to profit from experience, particularly punishment; and f. marked proneness to blame others, or to offer plausible rationalizations for the behaviour that has brought the patient into conflict with society. There may also be persistent irritability as an associated feature. Conduct disorder during childhood and adolescents, though not invariably present, may further support the diagnosis. |
It is acknowledged that the classification and diagnosis of personality disorder is an area of controversy and complexity with ongoing debate about the usefulness of multiple categories of personality disorder versus a dimensional approach (Tyrer 2015, Skodol 2018). Others feel the very label of personality disorder to be pejorative and unhelpful (Johnstone 2018). Indeed, a major paradigm shift in the conceptualisation of personality disorder has been suggested in the latest iteration of the International Classification of Diseases (ICD‐11). The proposed ICD‐11 model takes a dimensional approach and is made up of three components: a general severity rating, five maladaptive personality trait domains, and a borderline pattern qualifier (Oltmanns 2019). The proposed classification changes to personality disorder, however, are outside the scope of this review, which is focused on interventions for AsPD, as defined in the current, predominant classification systems of DSM‐5 and ICD‐10.
Most studies report the prevalence of AsPD to be between 2% and 3% in the general population (Moran 1999; Coid 2006; NICE 2010). A systematic review and meta‐analysis of the prevalence of personality disorders in the general adult population in Western countries found a prevalence rate for AsPD of 3% (Volkert 2018). Prevalence rates are considerably higher in men compared with women (Dolan 2009; NICE 2015) and a 3:1 ratio of men to women has been described (Compton 2005). It has also been suggested that there are sex differences in how this condition may present, with women with AsPD being less likely than men with AsPD to present with violent antisocial behaviour (Alegria 2013). AsPD (and other personality disorder diagnoses) may be less likely to be diagnosed in non‐white populations (McGilloway 2010).
As would be expected, AsPD is especially common in prison settings. In the UK prison population, the prevalence of people with AsPD has been identified as 63% in male remand prisoners, 49% in male sentenced prisoners and 31% in female prisoners (Singleton 1998). A systematic review of mental disorders in prisoners examined 62 studies from 12 countries and reported the prevalence of AsPD in male prisoners to be 47%, with prisoners approximately 10 times more likely to have AsPD than the general population (Fazel 2002).
The condition is associated with a wide range of disturbance, including greatly increased rates of criminality, substance use, unemployment, homelessness and relationship difficulties (Martens 2000), as well as negative long‐term outcome. Many adults with AsPD are imprisoned at some point in their life. Although follow‐up studies have demonstrated some improvement over the longer term, particularly in rates of re‐offending (Weissman 1993; Grilo 1998; Martens 2000), men with AsPD who reduce their offending behaviour over time may nonetheless continue to have major problems in their interpersonal relationships (Paris 2003). Black 1996 found that men with AsPD who were younger than 40 years of age had a strikingly high rate of premature death, and obtained a value of 33 for the standardized mortality rate (the age‐adjusted ratio of observed deaths to expected deaths), meaning that they were 33 times more likely to die than males of the same age without this condition. This increased mortality was linked not only to an increased rate of suicide but also to reckless behaviours such as drug misuse and aggression. A 27‐year follow‐up study also found AsPD to be a strong predictor of all cause mortality (Krasnova 2019). Black 2015 noted that earlier age of onset has been linked to poorer long‐term outcomes, although marriage, employment, early incarceration and degree of socialization may act as moderating factors. Follow‐up studies in forensic psychiatric settings suggest a similarly concerning picture. For example, Davies 2007 reported that 20 years after discharge from a medium‐secure unit almost half of the patients were reconvicted, with reconviction rates higher in those with personality disorder compared to those with mental illness (such as schizophrenia and bipolar affective disorder). Similarly, Coid 2015 examined reconviction after discharge from seven medium‐secure units in England and Wales and found that patients with personality disorder were more than two and a half times more likely than those with schizophrenia/schizoaffective disorder to violently offend after discharge.
Significant comorbidity exists between AsPD and many other mental health conditions; mood and anxiety disorders are common (Goodwin 2003; Black 2010; Galbraith 2014). The presence of personality disorder co‐occurring with another mental health condition may have a negative impact on the outcome of the latter (Newton‐Howes 2006; Skodol 2005). There is a particularly strong association between AsPD and substance use disorders (Robins 1998). Compared to those without AsPD, those with AsPD are 15 times more likely to meet the criteria for drug dependence, and seven times more likely to meet the criteria for alcohol dependence (Trull 2010). Guy 2018 reported that 77% of people with AsPD met the lifetime criteria for alcohol use disorder.
Description of the intervention
It has been argued that adults with personality disorders may respond to pharmacological interventions that target both their state (temporary/transient) and trait (stable/enduring) symptoms, highlighting the need to evaluate drug treatments that target the cognitive‐perceptual, affective, impulsive‐behavioural and anxious‐fearful domains of personality disorder (Soloff 1998). Several authors have reviewed the evidence relating to treatment of personality disorders with antidepressants, benzodiazepines, anticonvulsants, psychostimulants, antipsychotics and mood stabilisers (Stein 1992; Dolan 1993; Warren 2001; Duggan 2008; Lieb 2010).
Stein 1992 concluded that small doses of neuroleptics may afford some benefits for people with well‐defined borderline and schizotypal personality disorders. Dolan 1993 argued that carbamazepine had been shown to improve overactivity, aggression and impulse control in psychopathic and antisocial personality disorders. They also concluded that lithium maintenance treatment may be of benefit to explosive and impulsive individuals, holding some hope for those with AsPD. Warren 2001 concluded that selective serotonin reuptake inhibitor (SSRI) antidepressants may improve personality disorder symptoms and anger. They noted, however, that the evidence for pharmacological intervention was very poor, since the studies included in their review suffered serious methodological limitations, including small sample sizes, highly selected participants, high dropout rates, short duration or lack of long‐term follow‐up. Similar limitations were noted by Duggan 2008, although their review favoured the use of anticonvulsants to reduce aggression, and anti‐psychotics to reduce cognitive perceptual and mental state disturbance.
Overall, these reviews found the evidence base for pharmacological interventions for AsPD to be weak, or lacking, since the bulk of the studies reviewed had been restricted to individuals with borderline personality disorder (BPD). Therefore, it is important to consider all relevant studies, without restriction on the pharmaceutical agents, and to consider pharmacological interventions where drugs are given not only as monotherapy but also as an adjunctive intervention.
How the intervention might work
Several arguments have been put forward to justify pharmacological treatment for personality disorders (Tyrer 2004), and there is a growing body of evidence that personality disorders are associated with neurochemical abnormalities, whether inherited or arising out of physical or psychological trauma (Coccaro 1998; Skodol 2002). One justification for the use of pharmacotherapy is that it has the potential to modulate neurotransmitter function and so may be able to correct imbalances in the central nervous system of people with personality disorder to a more normal neurochemical state (Markovitz 2004).
The main neurotransmitter system which may be implicated in AsPD is the serotonergic system (Coccaro 1996; Deakin 2003). For example, impulsive and aggressive features of the disorder have been linked to serotonergic system deficits (Sugden 2006). The serotonergic system has been found to be less responsive to pharmacological challenges (i.e. a study where drugs are given to increase serotonin levels) in people with AsPD than those in healthy individuals (Moss 1990). Brain activations following such challenges are reduced in AsPD participants, as demonstrated in functional imaging studies (Völlm 2010). The biological factors contributing to both antisocial behaviour and criminality may also include the under‐arousal of the autonomic system (Dinn 2000; Raine 2000). Raised testosterone levels have been implicated in a range of antisocial behaviours, psychosocial problems, psychoactive substance misuse and violence (Mazur 1998), but no argument has been put forward to justify the use of antiandrogens in AsPD.
In an alternative approach, Soloff 1998 suggests that the likely impact of drugs on the primary symptoms in personality disorder can broadly be predicted from drug effects when used in other disorders such as anxiety, depression or psychosis. On this basis, medication is matched to the primary symptom group, so that antipsychotic medication would be the preferred drug treatment for cognitive‐perceptual symptoms, and mood stabilisers and SSRIs would be indicated for impulsive‐behavioural dyscontrol.
In practice, there are reports of behavioural dyscontrol improving in response to lithium (Links 1990) and anticonvulsants such as carbamazepine (Cowdry 1988), sodium valproate (Stein 1995) and divalproex sodium (Wilcox 1995) in non‐AsPD samples. There are also a number of reports on the use of SSRIs to reduce aggressive and impulsive behaviour (Bond 2005).
Why it is important to do this review
AsPD is an important condition that has a considerable impact on individuals, families and society more widely (Black 1999; NICE 2010). Even by the most conservative estimate, AsPD appears to have the same prevalence in men as schizophrenia, the condition that receives the greatest attention from mental health professionals. Furthermore, AsPD is associated with significant costs (Sampson 2013), arising from emotional and physical damage to victims and damage to property, as well as service utilisation (for instance, in terms of health services, use of police time, and involvement from the criminal justice system and prison services). Related costs include increased use of healthcare facilities, lost employment opportunities, family disruption, gambling, and problems related to alcohol and substance misuse (Myers 1998; Home Office 1999). In one study, lifetime public costs for a group of adults with a history of conduct disorder were found to be 10 times those for a similar group without the disorder (Scott 2001). Around 50% of adolescents with conduct disorder will go onto develop adult AsPD (Scott 2001).
Despite this, there is currently a dearth of evidence on how best to treat people diagnosed with AsPD, and the few reviews that have been carried out to date have been inconclusive. Dolan 1993 reviewed the use of numerous drug groups amongst people with AsPD and psychopathic disorder, but identified only a small number of studies, and noted that these were limited by poor methodology and lack of long‐term follow‐up. They found the evidence base for pharmacological treatments for AsPD to be poor, a conclusion endorsed by the Reed Committee (Reed 1994). They recommended that the UK Department of Health and the Home Office should encourage further research into this area, with added attention to female and ethnic minority groups (Reed 1994). A further review failed to uncover a more credible evidence base (Warren 2001). Increased interest in developing and evaluating pharmacological treatments for personality disorders in recent years has provided the impetus to conduct the first Cochrane Review, which systematically reviewed the literature on the use of pharmacological interventions for AsPD up to 2010 (Khalifa 2010). New evidence has emerged since then, suggesting that an updated systematic review is now timely.
Objectives
To assess the benefits and adverse effects of pharmacological interventions for adults with AsPD.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), in which participants have been randomly allocated to an experimental group and a placebo control group. We included all relevant RCTs, including cross‐over trials, with or without blinding, published in any language.
Types of participants
We included studies involving adult (18 years or over) men or women with a diagnosis of AsPD or dissocial personality disorder defined by the DSM (DSM‐IV; DSM‐IV‐TR; DSM‐5) and ICD‐10 diagnostic classification systems. We excluded studies of people with major functional mental illnesses (i.e. schizophrenia, schizoaffective disorder or bipolar disorder), organic brain disease, and intellectual disability. The decision to exclude persons with these conditions is based on the rationale that the presence of such disorders (and the possible confounding effects of any associated management or treatment) might obscure whatever other psychopathology (including personality disorder) might be present. However, we included studies of people diagnosed AspD who also had other comorbid personality disorders or other mental health problems. We placed no restrictions on setting and included studies with participants living in the community as well as those incarcerated in prison or detained in hospital settings. We included studies with subsamples of patients with AsPD provided that the data for this group were available separately. We also included studies where participants with a AsPD diagnosis comprised at least 75% of the sample. Lastly, we required studies where participants with antisocial or dissocial personality disorder formed a small subgroup to have randomised at least five people with AsPD.
Types of interventions
People with personality disorders may respond to pharmacological interventions that target both their state and trait symptoms. Although it has been argued that drug treatments that target the cognitive‐perceptual, affective, impulsive‐behavioural and anxious‐fearful domains of personality disorder need to be evaluated (Soloff 1998), we carried out the review without any a priori assumptions about the effectiveness of certain drugs in a specific domain.
We included studies of any drug(s) with psychotropic properties, including those falling within the following classes of pharmacological interventions (as defined by the British National Formulary 2018):
hypnotics, anxiolytics and barbiturates;
antipsychotic drugs (including depot injections);
antidepressant drugs; tricyclic and related, monoamine‐oxidase inhibitors, SSRIs and related, and other antidepressant drugs;
central nervous system stimulants;
antiepileptics, mood stabilising agents/antimanic drugs;
drugs used in essential tremor, chorea, tics and related disorders;
drugs used in substance dependence;
dopaminergic drugs used in Parkinsonism; and
others.
We included studies evaluating a combination of drug interventions. We included studies in which the drug being evaluated was given as an adjunct to another drug, but only where the comparison was between the adjunct and a placebo adjunct. Studies in which the comparison was between one drug and another drug or between a pharmacological and a psychological intervention are reported separately. We considered cross‐over trials for inclusion in the review only where the trial was evaluating interventions with a temporary effect in the treatment of stable conditions, and where long‐term follow‐up was not required (Higgins 2011a). A summary of the inclusion and exclusion criteria of the review is provided in Table 10.
4. Summary of the review inclusion and exclusion criteria.
| Inclusion criteria | ||||
| Studies | Participants | Interventions | Primary outcomesa | Secondary outcomesa |
|
|
|
|
|
| Exclusion criteria | ||||
| Studies | Participants | Interventions | Primary outcomes | Secondary outcomes |
| ‐ |
|
|
‐ | ‐ |
aStudies reporting on at least one primary or secondary outcome were considered for inclusion. bThese studies are reported separately.
We included studies where the active drug treatment was compared to an inert 'placebo' (dummy pill/capsule/liquid) containing an inactive substance such as starch, sugar or saline.
Types of outcome measures
The primary and secondary outcomes are listed below in terms of single constructs. Given the relatively stable nature of traits of AsPD (by definition) we chose outcomes that could be subject to change and that were potentially measurable by a variety of means (including self‐report and observation).
Some traits, such as risk‐taking, are difficult to measure directly. Given the large negative impact of aggression and reconviction, we thought these particularly important. Such outcomes could represent a final common pathway encompassing a variety of traits, including failure to confirm to social norms, deceitfulness, impulsivity, recklessness, irresponsibility and lack of remorse. These outcomes are also measurable by self‐report, psychometrics, observed behaviour, informant information and official records. We were also mindful of the issues described in DSM‐5 (p 659): “Because deceit and manipulation are central features of antisocial personality disorder, it may be especially helpful to integrate information acquired from systematic clinical assessments with information collected from collateral sources." We anticipated that the studies included in this review would have used a range of outcome measures (for example, aggression could have been measured by a self‐report instrument or by an external observer). We provide examples of potential measures of each outcome. However, we also accepted other, similar ways of recording each outcome.
Whilst acknowledging that the nature of the disorder can lead to difficulty in long‐term follow‐up of individuals with AsPD, we reported relevant outcomes without restriction on period of follow‐up. We aimed to divide outcomes into immediate (within six months), short‐term (> 6 months to 24 months), medium‐term (> 24 months to five years) and long‐term (beyond five years) follow‐up, if there were sufficient studies to warrant this.
Primary outcomes
Aggression (trait aggression or state/dynamic/current aggression; reduction in aggressive behaviour or aggressive feelings; continuous or dichotomous outcome), measured through improvement in scores on the Aggression Questionnaire (Buss 1992), the Modified Overt Aggression Scale (Malone 1994), or a similar, validated instrument; or as number of observed incidents.
Recidivism (continuous, dichotomous or time‐to‐event outcome depending on how these data were reported), measured as reconviction in terms of the overall reconviction rate or numbers reconvicted for the sample (continuous data), time to reconviction/reoffending (time‐to‐event data), recidivism yes or no (dichotomous). Non‐convicted offences identified by self‐report or incident reporting, etc. and reported in the same way.
Global state/functioning (continuous outcome), measured through improvement on the Global Assessment of Functioning numeric scale (DSM‐IV‐TR).
Social functioning (continuous or dichotomous outcome), measured through improvement in scores on the Social Adjustment Scale (Weissman 1976), the Social Functioning Questionnaire (Tyrer 2005), or a similar, validated instrument; or a proxy measure of social functioning (e.g. decreased level of support required/time taken to achieve leave from hospital).
Adverse events (dichotomous outcome), measured as incidence of overall adverse events and of the three most common adverse events.
Secondary outcomes
Quality of life (self‐reported improvement in overall quality of life; continuous outcome), measured through improvement in scores on the European Quality Of Life instrument (EuroQoL Group 1990), or a similar, validated instrument.
Engagement with services (health‐seeking engagement with services; continuous outcome), measured though improvement in scores on the Service Engagement Scale (Tait 2002), or a similar, validated instrument.
Satisfaction with treatment (continuous outcome), measured through improvement in scores on the Client Satisfaction Questionnaire (Attkisson 1982), or a similar, validated instrument.
Leaving the study early (continuous or dichotomous outcome), measured as proportion of participants discontinuing treatment.
Substance misuse (continuous or dichotomous outcome), measured as improvement on the Substance Use Rating Scale, patient version (Duke 1994), or a similar, validated instrument.
Employment status (continuous outcome), measured as number of days in employment over the assessment period.
Housing/accommodation status (continuous outcome), measured as number of days living in independent housing/accommodation over the assessment period.
Economic outcomes (any economic outcome such as cost‐effectiveness; continuous outcome), measured using cost‐benefit ratios or incremental cost‐effectiveness ratios (ICERs).
Impulsivity (state or trait; self‐reported improvement in impulsivity; continuous outcome), measured through reduction in scores on the Barratt Impulsivity Scale (Patton 1995), or a similar, validated instrument.
Anger (self‐reported improvement in anger expression and control; continuous outcome), measured through reduction in scores on the State‐Trait Anger Expression Inventory‐2 (Spielberger 1999), or a similar, validated instrument.
Mental state (continuous outcome): general mental state, such as ratings of general mental health symptoms, measured by the Brief Psychiatric Rating Scale (BPRS; Overall 1962) or Symptom‐Check List‐90 (SCL‐90; Derogatis 1973); or specific symptoms, such as dissociative experiences measured by Dissociative Experiences Scale (DES; Carlson 1993), mood/anxiety measured by the Hospital Anxiety and Depression Scale (HADS; Zigmond 1983) or the Beck Anxiety and Depression Scale (BADS; Beck 1988); or global mental health, measured by Clinical Outcomes in Routine Evaluation–Outcome Measure (CORE‐OM; Barkham 2001).
Prison/service outcomes (continuous outcome) recording: treatment of people in the community; duration of treatment programme; or changes in services provided by through care/probation teams.
Other outcomes measured in the included studies that did not fall into one of the above categories (continuous or dichotomous outcomes dependent upon how the outcomes were reported).
Search methods for identification of studies
The searches for the previous version of this review were designed to find studies for a suite of reviews on a range of personality disorders. For this update, we revised the population section of the strategy by including only the search terms relevant to antisocial personality disorder. We also made changes to the databases we searched (see Differences between protocol and review).
Electronic searches
We searched the following electronic databases and trial registers (to update the search conducted for Khalifa 2010) on 3 October 2016, 31 October 2017, 3 and 4 October 2018 and 5 September 2019.
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 9), in the Cochrane Library (searched 5 September 2019), which includes the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register.
MEDLINE Ovid (1946 to August Week 5 2019).
MEDLINE In‐Process & Other Non‐Indexed Citations Ovid (searched 5 September 2019).
MEDLINE Epub Ahead of Print Ovid (searched 5 September 2019).
Embase OVID (1974 to 2019 Week 37).
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1937 to 5 September 2019).
PsycINFO OVID (1967 to September Week 1 2019).
Science Citation Index Web of Science (1970 to 5 September 2019).
Social Sciences Citation Index Web of Science (1970 to 5 September 2019).
Conference Proceedings Citation Index ‐ Science Web of Science (1990 to 5 September 2019).
Conference Proceedings Citation Index ‐ Social Science & Humanities Web of Science (1990 to 5 September 2019).
Sociological Abstracts Proquest (1952 to 5 September 2019).
Criminal Justice Abstracts EBSCOhost (1910 to 5 September 2019).
Cochrane Database of Systematic Reviews (2019, Issue 9), part of the Cochrane Library (searched 5 September 2019).
Database of Abstracts of Reviews of Effects (2015, Issue 2. Final Issue), part of the Cochrane Library (searched 5 September 2019).
ClinicalTrials.gov (www.clinicaltrials.gov/ct2/home; searched 5 September 2019).
WHO (World Health Organization) ICTRP (International Clinical Trials Registry Platform; apps.who.int/trialsearch/AdvSearch.aspx; searched 5 September 2019).
WorldCat (limited to theses; www.worldcat.org; searched 5 September 2019).
Detailed search strategies for this update are reported in Appendix 1. The searches were designed to find records for two separate reviews of interventions for AsPD or dissocial personality disorder: a) psychological interventions and b) pharmacological interventions. For this review, we selected only those studies that were relevant to pharmacological interventions. Search strategies used up to September 2009 for the previous version of the review are reported in Khalifa 2010.
Searching other resources
We searched the reference lists of included and excluded studies for additional trials. We also examined bibliographies of systematic reviews identified in the search to identify relevant studies. We contacted the authors of relevant studies to enquire about other sources of information, and contacted the first author or corresponding author of each included study for information regarding unpublished data.
Data collection and analysis
In the following sections, we report only the methods that we were able to use in this review. We direct the reader to our protocol, Khalifa 2009, and Table 11, for information on additional methods that we intend to use in future updates of this review, should data permit.
5. Additional methods for future updates.
| Issue | Method |
| Types of outcome measures | We may reconsider the primary and secondary outcomes in future reviews, to include pre‐clinical markers such as 'facial emotional recognition' or additional features of AsPD as listed in DSM‐5, ICD‐10, or future iterations of these guidelines e.g. ICD‐11. |
| Unit of analysis issues |
Cluster‐randomised trials Where trials use clustered randomisation, study investigators may present their results after appropriately adjusting for clustering effects (robust standard errors or hierarchical linear models). Where it is unclear whether this was done, we will contact the study investigators for further information. If appropriate adjustments were not used, we will request individual participant data and re‐analyse using multilevel models which control for clustering. Following this, we will carry out meta‐analysis in Review Manager 5 (RevMan5; Review Manager 2014), using the generic inverse method (Higgins 2011a). If appropriate adjustments were not used, we will follow the method described by Donner 2001, imputing an intra‐cluster correlation coefficient and adjusting for sample size. If there is insufficient information to adjust for clustering, we will enter outcome data into RevMan5 using the individual as the unit of analysis, and then use sensitivity analysis used to assess the potential biasing effects of inadequately adjusted clustered trials. |
|
Cross‐over trials Should we be able to conduct a meta‐analysis combining the results of cross‐over trials, we will use the inverse variance methods recommended by Elbourne (Elbourne 2002), data permitting. When conducting a meta‐analysis combining the results of cross‐over trials. | |
| Dealing with missing data |
Missing dichotomous data We will report missing data and dropouts for each included study and report the number of participants who are included in the final analysis as a proportion of all participants in each study. We will provide reasons for the missing data in the narrative summary where these are available. |
|
Missing standard deviations The standard deviations of the outcome measures should be reported for each group in each trial. If these are not given, we will calculate these, where possible, from standard errors, confidence intervals, t‐values, F values or P values using the method described in the Cochrane Handbook for Systematic Reviews of Interventions, section 7.7.3.3 (Higgins 2011a). If these data are not available, we will impute standard deviations using relevant data (for example, standard deviations or correlation coefficients) from other, similar studies (Follman 1992) but only if, after seeking statistical advice, to do so is deemed practical and appropriate. Given that trials in this area are often conducted with small samples, any imputations (and the assumptions behind them) are likely to have an important impact. We will therefore follow, where possible, the method suggested by Higgins 2008 for weighting studies with imputed data. | |
|
Loss to follow up We will report separately all data from studies where more than 50% of participants in any group were lost to follow‐up, and will exclude these from any meta‐analyses. The impact of including studies with high attrition rates (25 to 50%) will be subjected to sensitivity analysis. If inclusion of data from this group results in a substantive change in the estimate of effect of the primary outcomes, we will not add data from these studies to trials with less attrition, but will present them separately. We will assess the extent to which the results of the review could be altered by the missing data by conducting a sensitivity analysis based on consideration of 'best‐case' and 'worst‐case' scenarios (Gamble 2005). Here, the 'best‐case' scenario is that where all participants with missing outcomes in the experimental condition had good outcomes, and all those with missing outcomes in the control condition had poor outcomes; the 'worst‐case' scenario is the converse (Higgins 2011a, section 16.2.2).For example, in studies with less than 50% dropout rate, we will consider people leaving early to have had the negative outcome, except for adverse effects such as death. | |
| Assessment of heterogeneity | We will assess the extent of between‐trial differences and the consistency of results of any meta‐analysis in three ways: first, by visual inspection of the forest plots; second, by performing the Chi2 test of heterogeneity (where a significance level less than 0.10 will be interpreted as evidence of heterogeneity); and third, by examining the I2statistic (Higgins 2011a; section 9.5.2). The I2statistic describes approximately the proportion of variation in point estimates due to heterogeneity rather than sampling error. We will consider I2values less than 30% as indicating low heterogeneity, values in the range 31% to 69% as indicating moderate heterogeneity, and values greater than 70% as indicating high heterogeneity. We will attempt to identify any significant determinants of heterogeneity categorised at moderate or high. |
| Assessment of reporting biases | We will draw funnel plots (effect size versus standard error) to assess publication bias, if we find sufficient studies. Asymmetry of the plots may indicate publication bias, although they may also represent a true relationship between trial size and effect size. If such a relationship is identified, we will further examine the clinical diversity of the studies as a possible explanation (Egger 1997; Jakobsen 2014; Lieb 2016). If insufficient data is available to employ statistical techniques, we will look at descriptive methods (such as time elapsed between the study and publication) to assess potential reporting bias. |
| Data synthesis | We will conduct meta‐analyses to combine comparable outcome measures across studies. In carrying out meta‐analysis, the weight to be given to each study is the inverse of the variance, so that the more precise estimates (from larger studies with more events) are given more weight. Where studies provide both endpoint and change data for continuous outcomes, we will perform a meta‐analysis that combines both types of data using the methods described by Da Costa 2013. We will undertake a quantitative synthesis of the data using both fixed and random effects models. Random‐effects models will be used because studies may include somewhat different treatments or populations. Outcome measures will be grouped by length of follow‐up. In addition, the weighted average of the results of all the available studies will be used to provide an estimate of the effect of antiepileptic drugs for aggression and impulsiveness. Where appropriate and if a sufficient number of studies are found, we will use regression techniques to investigate the effects of differences in the study characteristics on the estimate of the treatment effects. Statistical advice will be sought before attempting meta‐regression. If meta‐regression is performed, this will be executed using a random effects model. We will consider pooling outcomes reported at different time points where this does not obscure the clinical significance of the outcome being assessed. To address the issue of multiplicity, future reviews should consider the following:
|
| Subgroup analysis and investigation of heterogeneity | We will undertake a subgroup analysis to examine the effect on primary outcomes of:
|
| Sensitivity analysis | We will undertake sensitivity analyses to investigate the robustness of the overall findings in relation to certain study characteristics. A priori sensitivity analyses are planned, data permitting, for:
|
Selection of studies
Working independently, two review authors read the titles and abstracts generated by the searches and discarded those that were clearly irrelevant. . They next obtained the full‐text reports of those deemed potentially relevant or which more information was needed to determine relevance, and assessed them against the inclusion criteria (Criteria for considering studies for this review). The reviewers resolved uncertainties concerning the appropriateness of studies for inclusion in the review through consultation with a third review author who had not been involved in the initial screening.
For studies reported in a language other than English, we initially examined the English version of the title and abstract, where provided, to decide whether or not the study met the inclusion criteria. We obtained a translation of any non‐English language abstract or full paper where this was necessary for a decision to be made; two of the review authors undertook translations for three records.
We present our selection process in a PRISMA diagram (Moher 2009).
Data extraction and management
Four review authors extracted data independently for all studies using a data extraction form which had previously been piloted (see Appendix 2). Where data were not available in the published trial reports, we contacted the study authors and asked them to supply the missing information. Two review authors entered the data into Review Manager 5 (Review Manager 2014); a third review author checked the data entry into RevMan for accuracy. Study data was finalised through comparison and consensus of two independent extractions with any disagreements resolved by consultation with a third author; less than 5% of papers required such discussion.
Assessment of risk of bias in included studies
For each included study, two reviewers independently completed Cochrane’s tool for assessing risk of bias (Higgins 2011a; Higgins 2011b), resolving any disagreements through consultation with a third reviewer. We assessed the papers against the following areas of possible bias:
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants (performance bias);
blinding of personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective outcome reporting; and
other bias, including allegiance bias and treatment adherence.
For each domain, we assigned ratings of 'high,' 'low' or 'unclear' risk of bias, where we considered the risk of bias to be high, low or uncertain/unknown, respectively.
Overall risk of bias
We assessed the overall risk of bias within studies using the method recommended by Higgins 2011b. We assessed a study at low risk of bias overall if we rated it at low risk of bias on all key domains; at unclear risk of bias overall where we assessed the study at unclear risk of bias on one or more key domains; and at high risk of bias overall where we rated the study at high risk of bias on one or more key domains. If a single domain was rated at high risk but other domains were unclear, we rated the study at high risk of bias overall. We used the results of this assessment to inform our GRADE judgements (see section on 'Summary of findings' below).
Measures of treatment effect
Dichotomous data
For dichotomous (binary) data, we used the odds ratio (OR) with 95% confidence intervals (CI), to summarise results within each study. We chose the OR because it has statistical advantages relating to its sampling distribution and its suitability for modelling, and because it is a relative measure and therefore can be used to combine studies.
Continuous data
For continuous data, such as the measurement of impulsiveness and aggression on a scale, we compared the mean score for each outcome as determined by a standardized tool between the two groups to give a mean difference (MD), and presented these with 95% CI. We used the mean difference (MD) where more than one study reported the same outcome measures. We used the standardized mean difference (SMD) where studies reported different outcome measures of the same construct.
We reported continuous data that were skewed in a separate table, and did not calculate treatment effect sizes in order to minimise the risk of applying parametric statistics to data that depart significantly from a normal distribution. However, if the trial investigators provided results of their own statistical analysis on such data, we reported their results descriptively within the section on Effects of interventions. We define skewness as occurring when, for a scale or measure with positive values and a minimum value of zero, the mean was less than twice the standard deviation (Altman 1996).
Time‐to‐event data
For time‐to‐event data, we used the hazard ratio (HR) with 95% CI. Reconviction (dichotomous or time‐to‐event outcome dependent upon how the outcome was reported), was measured as the overall reconviction rate for the sample or as an analysis of time to reconviction (please see Differences between protocol and review).
Other
Where possible, we made comparisons at specific, clinically relevant follow‐up periods: immediate (within six months), short‐term (> 6 months to 24 months), medium‐term (> 24 months to five years) and long‐term (beyond five years) follow‐up (please see Differences between protocol and review).
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials. For information on how we will handle these issues should they arise in future updates of this review, please see our protocol, Khalifa 2009, and Table 11.
Cross‐over trials
We identified two cross‐over trials but only one provided cross‐over data for one comparison. Where data presented from a cross‐over trial were restricted (and more information was not available from the original investigators), we presented data within the first phase only, up to the point of cross‐over.
Multi‐arm trials
We identified three multi‐arm trials. We included all eligible outcome measures for all trial arms in this review. We used pair‐wise comparisons of individual trial arms against placebo to avoid a potential unit‐of‐analysis error from this approach.
Dealing with missing data
We attempted to contact the original investigators to request any missing or incomplete outcome data and information on whether or not the data could be assumed to be ‘missing at random.' If these data were made available to us, we included the data in the review. If data were not forthcoming, we attempted to contact at least one of the co‐investigators. We permitted a reasonable length of time (at least 12 weeks) for the investigator(s) to supply the missing data before we proceeded with the analysis. We considered 12 weeks to be sufficient, as most contacts provided a response within a few days.
We used intention‐to‐treat analysis for studies with data missing from participants who dropped out of trials before completion. We assumed missing data were random if no explanation was received from study authors, and reported missing data information in the 'incomplete outcome data' section of the 'Risk of Bias' tables. See Table 11 for information about future updates of this review.
Assessment of heterogeneity
We assessed the clinical heterogeneity of trials in relation to the medication type, clinical setting, and the population from which AsPD participants were drawn. We assessed the methodological heterogeneity in relation to the trial design (e.g. parallel/cross‐over). Please see Table 11 for information about assessment of heterogeneity in future updates of this review where pooling may be required.
Assessment of reporting biases
Due to insufficient data we were unable to conduct our preplanned funnel plots (Khalifa 2009; Table 11) to assess reporting bias. Please see Table 11 for information about future updates of this review.
Data synthesis
Due to insufficient data we were unable to conduct meta‐analyses, and therefore provide a narrative summary of the data. Although we considered multiplicity (the concern that performing multiple comparisons increases the risk of falsely rejecting the null hypothesis) this was not an issue in this review as the available data did not allow the making of multiple comparisons. We have outlined how we will address multiplicity and other issues in future reviews in Table 11.
Subgroup analysis and investigation of heterogeneity
Due to insufficient data we were unable to conduct any of our preplanned subgroup analyses (see Khalifa 2009). See Table 11 for information about future updates of this review.
Sensitivity analysis
Due to insufficient data we were unable to conduct any of our preplanned sensitivity analyses (see Khalifa 2009). See Table 11 for information about future updates of this review.
Summary of findings and assessment of the certainty of the evidence
Following the guidelines set out in Schünemann 2013, we used GRADEpro GDT software (GRADEpro GDT) to prepare a 'Summary of findings' table for the following comparisons.
Phenytoin (antiepileptic) versus placebo
Desipramine (antidepressant) versus placebo
Nortriptyline (antidepressant) versus placebo
Bromocriptine (dopamine agonist) versus placebo
Amantadine (dopamine agonist) versus placebo
We included all primary outcomes (aggression, reconviction, global/state functioning, social functioning and adverse events), for immediate, short, medium and long‐term time points, in the 'Summary of findings' tables, presenting pooled data where possible, and single study data narratively.
Two review authors independently assessed the certainty of evidence for all primary outcomes with available data using the GRADE approach (Schünemann 2013), which takes into account the risk of bias, level of inconsistency, indirectness, imprecision and publication bias. We rated the certainty of the evidence for each outcome as being high, moderate, low or very low, and where relevant, provided reasons for downgrading the certainty of the evidence in the footnotes of the tables. We resolved any disagreements by discussion, or in consultation with a third review author. Less than 5% of studies required this discussion.
Results
Description of studies
Results of the search
For the original version of this review (Khalifa 2010), we searched from the inception of each database to September 2009. These searches identified in excess of 16,398 records, 26 of which appeared to merit closer inspection. From these, we identified eight studies (from 11 reports) that met the eligibility criteria.
We ran the searches for this update from September 2009 to September 2019. and found a total of 35,562 records. Once duplicate records were removed, we were left with 24,801 unique records, which we screened by title and abstract. We excluded 24,656 irrelevant records, and retrieved the full texts of the remaining 145 records for closer inspection. From these, we identified three new studies (from four reports) that fully met the inclusion criteria. We calculated the inter‐rater agreement for the selection of studies by the review authors, and obtained a kappa of 0.69; the strength of this agreement is classified as good by Altman 1996 (< 0.20 = poor; 0.21 to 0.40 = fair, 0.41 to 0.60 = moderate; 0.61 to 0.80 = good, and 0.81 to 1.00 = very good).
In total, this review now has 11 included studies (from 15 reports) and 29 excluded studies (from 33 reports). Three studies are awaiting classification, and one study is ongoing. Figure 1 shows the flow of studies for this updated review, as recommended by Stovold 2014.
1.
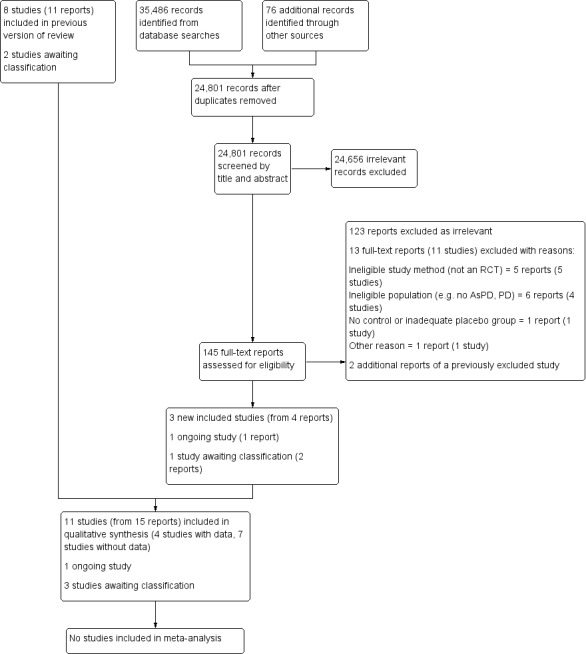
Study flow diagram showing the results of the updated literature search (5 September 2019)
Included studies
We identified 11 studies (416 AsPD participants) that fully met the inclusion criteria (Arndt 1994; Leal 1994; Powell 1995; Barratt 1997; Stanford 2001; Hollander 2003; Stanford 2005; Ralevski 2007; Coccaro 2009; Gowin 2012; Konstenius 2014). All participants with AsPD in the included studies were diagnosed under DSM criteria. Data from those participants with AsPD were available for only four of the 11 studies (Arndt 1994; Leal 1994; Powell 1995; Barratt 1997). The data from the remaining seven studies were not available for the following reasons. The AsPD data reported by Ralevski 2007 were not split by allocation condition, and no data on the AsPD subgroup were available for Stanford 2001, Hollander 2003, Stanford 2005, Coccaro 2009, Gowin 2012 and Konstenius 2014 at the time this review was prepared. We made four requests for further information and received one response from the authors of the Coccaro 2009 study (see 'Notes' section in Characteristics of included studies for details). The 11 included studies involved a total of 15 comparisons of a drug versus placebo. There were some important differences between the studies. We summarise these differences and the main study characteristics below. Further details are provided in the Characteristics of included studies tables.
Design
Nine of the 11 included studies were parallel RCTs (Arndt 1994; Leal 1994; Powell 1995; Hollander 2003; Stanford 2005; Ralevski 2007; Coccaro 2009; Gowin 2012; Konstenius 2014) and two were cross‐over designs (Barratt 1997; Stanford 2001). Of the nine parallel RCTs, five were two‐condition comparisons of a drug against a placebo (Arndt 1994; Hollander 2003; Coccaro 2009; Gowin 2012; Konstenius 2014), two were three‐condition comparisons of two drugs against a placebo (Leal 1994; Powell 1995), one was a four‐condition comparison involving three drugs against a placebo (Stanford 2005), and one was a four‐condition comparison of two drugs against placebo, both separately and in combination with each other (Ralevski 2007). Both studies with cross‐over designs were of a single drug against a placebo (Barratt 1997; Stanford 2001) and both recruited participants with recurrent aggression.
Sample sizes
There was some variation in sample size among studies. Ten studies unambiguously reported the number of randomised participants with AsPD (387 AsPD participants randomised); the sample size for these studies ranged from 6 (Gowin 2012) to 150 (Barratt 1997) (mean = 38.7, median = 18.0). The number with AsPD randomised in the eleventh study was not reported, although 29 AsPD participants completed to the study endpoint (Arndt 1994).
However, sample size data were available to us for only four trials (which include Arndt 1994). In these four studies, 274 participants with AsPD were randomised, with the sample size ranging from 19 (Leal 1994) to 150 (Barratt 1997) (mean = 68.5, median = 52.5; calculation based on an assumption that 50% of the Arndt 1994 sample was AsPD). The proportion of participants completing was reported unambiguously in only three of the four studies: 84% for Barratt 1997 in a prison sample; 57.9% for Leal 1994 in an inpatient sample; and 44.6% for Powell 1995 where participants were in an outpatient setting at the study endpoint.
It is important to note that none of the 11 studies set out to recruit participants on the basis of having a diagnosis of AsPD. In 10 studies, participants with AsPD formed a subgroup that accounted for between 4% and 59% of the trial's sample. However, in one study (Barratt 1997), participants were recruited on the basis of recurrent aggression and subsequent assessment revealed that 100% met the criteria for AsPD.
Setting
All but one of the studies were carried out in North America; Konstenius 2014 was conducted in Sweden. Six were single‐centre trials (Arndt 1994; Leal 1994; Stanford 2001; Stanford 2005; Coccaro 2009; Gowin 2012). Four were multi‐centre trials: Powell 1995 with two sites; Ralevski 2007 and Konstenius 2014 with three sites; Hollander 2003 with 19 sites. One study, Barratt 1997, did not report the number of sites. The trials took place in a number of very different settings. Seven studies were conducted exclusively in an outpatient setting (Arndt 1994; Stanford 2001; Hollander 2003; Stanford 2005; Ralevski 2007; Coccaro 2009; Gowin 2012) and one in an inpatient setting (Leal 1994). One study involved participants who were inpatients at baseline but moved to outpatient status during the course of treatment (Powell 1995). One study was set in prison (Barratt 1997), and one had participants who were prisoners at baseline but moved to outpatient status during the course of treatment (Konstenius 2014).
The duration of the trials ranged between six (Gowin 2012) and 24 weeks (Konstenius 2014; Powell 1995) (mean = 14.2 weeks, median = 13.0 weeks).
Participants
Participants were restricted to males in six studies (Arndt 1994; Powell 1995; Barratt 1997; Stanford 2001; Stanford 2005; Konstenius 2014). The remaining studies had a mix of male and female participants. All studies randomised more men than women. The overall mix was 90% men compared to 10% women. All 11 studies involved adult participants with the mean age per study ranging between 28.6 (Gowin 2012) and 45.1 years (Stanford 2001) (mean = 39.6 years). Five studies focused on participants with substance misuse difficulties. For these, inclusion criteria included cocaine dependency (Arndt 1994), cocaine and opioid dependency (Leal 1994), alcohol dependency (Powell 1995; Ralevski 2007) and amphetamine dependence (Konstenius 2014). The remaining six studies recruited participants on the basis of having displayed recurrent aggression, which was defined as impulsive aggression in four studies (Stanford 2001; Hollander 2003; Stanford 2005; Gowin 2012), as impulsive or premeditated aggression in one study (Barratt 1997), and as intermittent explosive disorder in one study (Coccaro 2009).
The precise definition of AsPD and the method by which it was assessed varied between the studies. Seven studies used DSM‐IV criteria: Hollander 2003; Ralevski 2007; Coccaro 2009; Gowin 2012; and Konstenius 2014 made assessments using the Structured Clinical Interview‐II (SCID‐II), while Stanford 2001 and Stanford 2005 stated "assessed by a licensed clinical psychologist" (further details not reported). Three studies used DSM‐III‐R criteria: Barratt 1997 and Powell 1995 made assessments using the Psychiatric Diagnostic Interview‐Revised (PDI‐R); and Leal 1994 made assessments using the SCID‐II. One study, Arndt 1994, used DSM‐III criteria and made assessments using the NIMH (National Institutes of Mental Health) Diagnostic Interview Schedule (DIS).
Ethnicity of participants was not always reported. For the six studies where it was, 56.7% of randomised participants were described as either "white" or "Caucasian" (Leal 1994; Powell 1995; Barratt 1997; Hollander 2003; Ralevski 2007; Coccaro 2009).
Interventions
Eleven drugs were compared to placebo in the 11 included studies. These were categorised as follows.
Antiepileptics: carbamazepine (one study: Stanford 2005); phenytoin (three studies: Barratt 1997; Stanford 2001; Stanford 2005); tiagabine (one study: Gowin 2012); valproate (one study: Stanford 2005), and divalproex (one study: Hollander 2003)
Antidepressants: desipramine (two studies: Arndt 1994; Leal 1994); fluoxetine (one study: Coccaro 2009); and nortriptyline (one study: Powell 1995)
Central nervous system (CNS) stimulant: methylphenidate (one study: Konstenius 2014)
Dopamine agonists: amantadine (one study: Leal 1994); and bromocriptine (one study: Powell 1995)
Opioid antagonists: naltrexone (one study: Ralevski 2007)
In each case, the route of administration was oral (by tablets, capsules or liquid). Studies varied in the way they reported the dose administered to the treatment group: a fixed daily dose (mg/day), or an adjusted dose in an attempt to achieve a target blood serum concentration (ng/ml or mg/ml). Details are provided in the Characteristics of included studies tables but can be summarised as follows.
Antiepileptics
One study involved carbamazepine (Stanford 2005: 450 mg/day for men with aggression, but with no data available for the AsPD subgroup)
Three studies involved phenytoin (Barratt 1997: 300 mg/day for prisoners with aggression; and Stanford 2001 and Stanford 2005: 300 mg/day for outpatient men with aggression, but with no data available for the AsPD subgroup that made up 43% and 59% of the total sample respectively)
One study involved tiagabine (Gowin 2012: 4 to 12 mg/day for community‐living adults on probation or parole, but with no data available for the AsPD subgroup that made up 50% of the total sample)
Two studies involved either valproate, full name sodium valproate (Stanford 2005: 750 mg/day for men with aggression, but with no data available for the AsPD subgroup that made up 59% of the sample) or divalproex, full name divalproex sodium (Hollander 2003: maximum 30 mg/kg/day for outpatients with aggression, but with no data available for the AsPD subgroup that made up 9% of the total population). Divalproex sodium is an equimolar compound of sodium valproate and valproic acid; because these two drugs are regarded as equivalent in efficacy and have similar side effect profiles, we consider them together in this review.
Antidepressants
Two studies involved desipramine (Arndt 1994: 250 to 300 mg/day for men with cocaine dependency; Leal 1994: 150 mg/day for adults with opioid and cocaine dependency)
One study involved fluoxetine (Coccaro 2009: 20 to 60 mg/day for adults with intermittent explosive disorder, but with no data available for the AsPD subgroup that made up 12% of the total sample)
One study involved nortriptyline (Powell 1995: 25 to 75 mg/day for men with alcohol dependency)
Central nervous system stimulant
One study involved methylphenidate (Konstenius 2014: 18 to 180 mg/day for male prisoners with co‐diagnoses of attention deficit hyperactivity disorder (ADHD) and amphetamine dependence, but with no data available for the AsPD subgroup that made up 52% of the total sample)
Dopamine agnostics
One study involved amantadine (Leal 1994: 300 mg/day for adults with opioid and cocaine dependency)
One study involved bromocriptine (Powell 1995: 15 mg/day for men with alcohol dependency)
Opioid antagonists
One study involved naltrexone (Ralevski 2007: 50 mg/day for adults with alcohol dependency)
The duration of the interventions ranged between six (Gowin 2012) and 24 weeks (Konstenius 2014; Powell 1995) (mean = 12.2 weeks, median = 12.0 weeks). None of the 11 studies followed up participants beyond the end of the intervention period.
All studies had measures in place that would allow for assessment of compliance with the medication regime. Blood tests were used by 10/11 studies (Arndt 1994; Leal 1994; Powell 1995; Barratt 1997; Stanford 2001; Hollander 2003; Stanford 2005; Ralevski 2007; Coccaro 2009; Konstenius 2014) and one study, Gowin 2012, used the timing of opening of the medication bottle and looked at breath alcohol and urine analysis.
Outcomes
Primary outcomes
Studies varied in terms of choice of primary outcomes considered in this review.
Aggression
Six studies assessed aggression as an outcome: Barratt 1997 using a modification to the Overt Aggression Scale (OAS; a weighted behavioural assessment of aggressive behaviour categories (verbal aggression, physical aggression against objects/self/others; higher scores = more severe aggression)); Hollander 2003 and Coccaro 2009 using the OAS‐Modified (OAS‐M; a weighted clinician‐rated semi‐structured interview; scores range from zero to 100 where higher scores = more severe aggression), but with no data available for the subgroup with AsPD; Stanford 2001 and Stanford 2005 using the OAS, but neither with any data available for the subgroup with AsPD; Gowin 2012 using the Point Subtraction Aggression Paradigm (PSAP; a behavioural measure of aggression in response to provocation), Buss‐Perry Aggression Questionnaire (BPAQ; 29 items rated on five‐point Likert scale ranging from one (extremely uncharacteristic) to five (extremely characteristic)), Lifetime History of Aggression Questionnaire (LHA; 11‐item, semi‐structured interview assessing three components of aggression history (aggression, social consequences or antisocial behaviour, and self‐directed aggression; total score = sum of total number of occurrences since 13 years old on five‐point scale (zero = no events, two = one, two or three events, three = four to nine events, four = 10 or more events), and Retrospective Overt Aggression Scale (ROAS; a weighted clinician‐rated semi‐structured interview, with scores ranging from zero to 100; higher scores = more severe aggression), but with no data available for the subgroup with AsPD.
Reconviction
No studies reported on reconviction.
Global/state functioning
Four studies assessed global state/functioning as an outcome: Powell 1995 using the Global Assessment Scale (GAS; a clinician‐rated assessment with a range of zero to 100; higher scores indicate better functioning) and the General Severity Index subscale (GSI; average score of all 90 items of the Symptom Check List‐90 (SCL‐90; 90 items rated on five‐point scale of distress ranging from zero (none) to four (extreme)); and Hollander 2003, Coccaro 2009 and Konstenius 2014 using the Clinical Global Impression (CGI) scale (three items, across three domains; two domains (severity of illness, global improvement) rated by clinicians on seven‐point scale ranging from one (normal, not at all ill) to seven (among the most extremely ill patients); third domain (efficacy index) also rated by clinicians and ranges from zero (marked improvement and no side‐effects) to four (unchanged or worse and side‐effects outweigh the therapeutic effects)), but had no data available for the subgroup with AsPD.
Social functioning
Only one study, Arndt 1994, considered the outcome of social functioning using the family‐social domain of the Addiction Severity Index (ASI; 10‐point interview assessment of lifetime and current problem severity, from zero to one (no real problem, treatment not indicated) to eight to nine (extreme problem, treatment absolutely necessary)) in seven problem areas affected by substance use disorder; ASI also provides composite scores (range from zero = no problems, to one = severe problems) for each domain based on client responses to items measuring behaviour in the 30 days prior to interview).
Adverse events
Seven studies reported on adverse events: Barratt 1997 using blood cell counts and liver function tests; and Powell 1995, Hollander 2003, Ralevski 2007, Coccaro 2009, Gowin 2012 and Konstenius 2014 using self‐reported side effects, but had no data available for the subgroup with AsPD.
Secondary outcomes
Studies varied in terms of their choice of secondary outcomes.
Leaving the study early
Eight studies reported on this outcome. Leal 1994 and Powell 1995 reported on the proportion of participants discontinuing treatment. Hollander 2003, Stanford 2005, Ralevski 2007, Coccaro 2009, Gowin 2012 and Konstenius 2014 reported similarly, but had no data available for the subgroup with AsPD.
Substance misuse
Three studies reported on substance misuse: drugs. Leal 1994 reported dollars spent on cocaine per week and cocaine abstinence measured as percentage of cocaine‐free urine samples. Arndt 1994 reported cocaine‐positive urinalysis results, drug domain of the ASI, days opiate use, days cocaine use, and cocaine craving scores. Konstenius 2014 reported urinalysis and scores on ASI.
Two studies reported on the outcome of substance misuse: alcohol. Powell 1995 reported number of drinking days in the last 30 days; alcohol craving scores (using a visual analogue scale); self‐report of longest period of total abstinence during the six‐month study; abstinence from drinking at endpoint; severity of alcohol misuse as measured with the Alcohol Severity Scale (ASS; 32‐item questionnaire, scores range from zero to 32, higher scores indicate severe alcohol problems); alcohol dependence measured with the Severity of Alcohol Dependence Questionnaire (SADQ; 20 items across five subscales rated on four‐point scale ranging from almost never (zero) to nearly always (three); total scores range from 0 to 60 with higher scores indicating severe alcohol dependence); and both patient and clinical ratings of drinking behaviour. Ralevski 2007 used the Timeline Follow‐Back Interview (number of drinking days from 30 to 360 days from interview) and alcohol craving measured with the Obsessive Compulsive Drinking Scale (14 items across two subscales: obsessive subscale (six items); as well as the compulsive subscale (eight items); rated on a five‐point Likert scale ranging from zero (least) to four (most); total score ranges from zero to 56). No allocation group data were available in Ralevski 2007 for the subgroup with AsPD.
Employment status
Arndt 1994 was the only study to consider employment status. This study used the employment domain of the ASI, days worked in the last 30 days, and employment income.
Impulsivity
Gowin 2012 was the only study to report on impulsivity. This study used the Eysenck Impulsivity Venturesomeness Questionnaire (EIVQ; 54 items assessing three subscales (impulsiveness, venturesomeness, and empathy); answers marked as 'yes' or 'no') and the Barratt Impulsiveness Scale‐2 (BIS‐II; 30 items scored on a four‐point Likert scale ranging from one (rarely or never) to four (almost always/always); higher scores indicate greater impulsivity), but had no data available for the AsPD subsample.
Anger
Three studies reported on anger. Barratt 1997 and Stanford 2001 used the Anger‐Hostility subscale (12 items, scores range = 0 to 48; higher scores indicate greater anger/hostility) of the Profile of Moods Scale (POMS; 65 adjectives rated on five‐point scale, ranging from not at all (zero) to extremely (four)). Gowin 2012 used the State‐Trait Anger Expression Inventory (STAXI; (44 items rated on a four‐point Likert scale from one (almost never) to four (almost always), total scores range from 0 to 132). None of the three studies had any data available for the AsPD subgroup.
Mental state
Three studies reported on mental state. Two studies reported on depression. Powell 1995 used the depression subscale (13 items, mean score of all 13 items) of the SCL‐90 and the Beck Depression Inventory (BDI; 21 items rated on four‐point scale ranging from 0 to three (anchors vary across items); total score ranges from 0 to 63 with higher scores indicating more depressive symptoms). Arndt 1994 used the BDI. One study, Powell 1995, reported on anxiety using the anxiety subscale (10 items, mean score of all 10 items) of the SCL‐90 and the Beck Anxiety Index (BAI; 21 items rated on four‐point scale ranging from zero (not at all) to three (severely ‐ it bothered me a lot); total scores range from zero to 63 with higher scores indicating higher levels of anxiety). Another study, Konstenius 2014, reported on psychiatric symptoms using the Outcomes Questionnaire‐45 (OQ‐45; 45 items rated on five‐point Likert scale (ranging from zero (never) to four (almost always); total score ranges from 0 to 180, the higher the score the more disturbed the patient), symptoms of ADHD using Conners’ Adult ADHD Self‐Rating scale (CAARS:SV; 18‐item screening version, with items scored zero (not at all, never) to three (very much, very frequently)) and cravings using the Craving for Amphetamine Scale (seven‐point scale).
No study reported on quality of life, engagement with services, satisfaction with treatment, housing/accommodation status, economic or prison/service outcomes.
Other relevant outcomes
One study, Arndt 1994, reported on the outcome of illegal activity, using days of illegal activity in the last 30 days, illegal income, and the Illegal domain on the ASI. One study, Gowin 2012, reported on cognitive functioning using the Shipley Institute of Living Scale (SILS; 60 items across two subscales: vocabulary (40 multiple‐choice items) and verbal reasoning (20 items)).
Study funding sources
The 11 included studies were funded by a variety of sources, including research councils, charities, commercial organisations and government departments. Five studies were funded by grants from a single organisation (Arndt 1994; Leal 1994; Powell 1995; Hollander 2003; Gowin 2012), and six studies received financial support from two or more organisations (Barratt 1997; Stanford 2001; Stanford 2005; Ralevski 2007; Coccaro 2009; Konstenius 2014). Six studies were funded by grants from major research councils such as the National Institute on Alcohol Abuse and Alcoholism (USA) (Powell 1995); the National Institute on Drug Abuse (USA) (Arndt 1994; Leal 1994; Gowin 2012); the National Institute of Mental Health (USA) (Coccaro 2009) and the Swedish Research Council (Konstenius 2014). Four studies received some of their funding from charitable organisations (Barratt 1997; Stanford 2001; Stanford 2005; Ralevski 2007) and two were funded by commercial laboratories (Hollander 2003; Coccaro 2009). We provide full details of study funding as a note in each of the Characteristics of included studies tables.
Excluded studies
We excluded 138 full‐text reports from the updated searches. Of these, 123 reports were clearly irrelevant and are not reported in any more detail. The remaining 11 studies (from 13 reports) initially appeared to meet the inclusion criteria, but on closer inspection did not. We also identified two additional reports for a previously excluded study, and changed the study ID to Kool 2003. The reasons for excluding these studies, together with 18 studies (18 reports) from the original review are reported in the Characteristics of excluded studies tables, following guidance in Chapter 4 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2019).
In summary, we excluded 29 studies for the following reasons. Five studies were not RCTs (Lane 2009; Todorovic 2012; Surekha 2013; Alcorn 2015; Patrizi 2019). Six studies did not have an adequate control or placebo comparison group (Mattes 1990; Battaglia 1999; Aberg‐Wisted 2000; Joyce 2003; Kool 2003; Jariani 2010). Fifteen studies had an ineligible population; in two studies AsPD was an exclusion criteria (Shea 1990; Fournier 2008); six studies did not assess AsPD (Allen 1976; Ekselius 1998; Dunlop 2011; George 2011; Johnson 2013; Kampman 2013); three studies had no participants with AsPD (Alpert 1990; Noyes 1991; Agosti 2002); and four studies did not diagnose personality disorder (Black 1994; Mattes 2005; Nickel 2005; Mattes 2008). Three studies were excluded for other reasons: in two studies there were too few randomised participants with AsPD (see Selection of studies section; Patience 1995; Coccaro 1997); and one study was excluded as it did not assess any of the primary or secondary outcomes of this review (Timmermann 2017).
Studies awaiting classification
We identified three studies in which the sample comprised a mixture of personality disorders where it remains unclear whether the investigators had included a subgroup of participants with a diagnosis of AsPD (Verkes 1998; Hellerstein 2000; Charney 2015). We sought clarification from the trial investigators but no further information was available at the time this review was prepared. We summarise the three studies below. Further details are provided in the Characteristics of studies awaiting classification tables.
Charney 2015 compared citalopram plus treatment as usual (TAU) (individual and group psychotherapy) with placebo plus TAU in adult outpatients with alcohol abuse or dependence. Personality disorder was assessed at 12 weeks and 24% of the sample met criteria for DSM‐IV cluster B personality disorder.
Hellerstein 2000 compared sertraline, imipramine and placebo in outpatients with early‐onset dysthymia. This study may have recruited a subgroup with AsPD, since 48 participants had DSM‐III‐R cluster B personality disorder.
Verkes 1998 compared paroxetine with placebo in outpatients with repeated suicidal attempts but without major depression. This study may have recruited a subgroup with AsPD, since at least one cluster B personality disorder was present in 74 out of 91 participants.
Ongoing studies
We identified one ongoing study (EudraCT2010‐018740‐13), investigating the short‐ and long‐term effects of oxytocin on empathy and social behaviour in autistic and antisocial male adults.
The study aims to recruit 78 male participants with AsPD or autism spectrum disorder, aged 18 to 30 years, with an intelligence quotient of 80 or higher. Participants with AsPD will be required to have a previous DSM‐IV diagnosis of early onset conduct disorder, a clinical score on the SCID‐II and score of 30 or more on the Psychopathy Check List‐Revised (PCL‐R), to ensure the inclusion of only highly callous, remorseless individuals in the AsPD group. The four‐week intervention trial has two conditions: oxytocin (syntocinon, intranasal administration, twice per day) and placebo nasal spray (intranasal administration, twice per day). Outcomes include social functioning, assessed by the Social Responsiveness Scale (SRS‐A; to be completed by an adult informant who knows the participant in naturalistic social settings) and by the Symptom Check List‐90 (SCL‐90; completed by the participant). See Characteristics of ongoing studies tables for further detail.
Risk of bias in included studies
Reporting varied considerably among the included studies. The methodological quality of the included studies was poor overall. We attempted to contact the study investigators wherever the available trial reports provided insufficient information for decisions to be made about the likely risk of bias. We were successful in regard to one study (Arndt 1994). We summarise below the risk of bias for the 11 included studies. Studies with data that could be extracted for the antisocial or dissocial PD subgroup (n = 4) are summarised separately from those for which data were unavailable (n = 7). This allows the reader to make a separate judgement about possible bias associated with the quantitative data from which conclusions are drawn in this review. Full details of our assessment of the risk of bias for each included study are provided in the 'Risk of bias' tables beneath the Characteristics of included studies tables. Graphical summaries of the risk of bias in each included study are presented in Figure 2 and Figure 3.
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.

Methodological quality summary: review authors' judgements on the methodological quality of included studies.
Allocation
With data (four studies)
We judged the potential for risk of bias from the method of random sequence generation as unclear in all four studies because the investigators reported that participants had been allocated at random, but provided no further information on how this had been achieved (Arndt 1994; Leal 1994; Powell 1995; Barratt 1997).
We also classified concealment of the allocation sequence unclear in each of these studies, again because the available information was insufficient to allow a judgment to be made.
Without data (seven studies)
We considered the generation of random allocation sequence to be adequate in three studies where allocation was performed using a table of random numbers (Stanford 2001; Stanford 2005) or a computer‐based program (Konstenius 2014), and therefore rated the potential for risk of bias as low for these studies. For three studies, Hollander 2003, Ralevski 2007 and Coccaro 2009, we assessed the risk of bias for random sequence allocation as being unclear because the available information was insufficient to allow a judgment to be made. We judged one study, Gowin 2012, to be at high risk of bias for random sequence allocation because no information was given on the method of randomization and 12 participants were randomised exactly to control (n = 6) versus experimental (n = 6) conditions, with each group having one female and three AsPD participants.
For risk of bias due to allocation concealment, we rated two studies at low risk of bias due to stated methodology (Stanford 2005; Konstenius 2014); four studies at unclear risk (due to insufficient information to allow a judgement to be made) (Stanford 2001; Hollander 2003; Ralevski 2007; Coccaro 2009); and one study at high risk of bias due to the precisely balanced randomization outcome reported above (Gowin 2012) .
Blinding
With data (four studies)
We considered blinding of participants to be adequate in only one study (Powell 1995), and rated the risk of bias as low. We rated the risk of bias as unclear in the other three studies because the information available was insufficient to allow a judgment to be made (Arndt 1994; Leal 1994; Barratt 1997).
For two studies, we judged the adequacy of blinding of personnel as adequate (Arndt 1992; Powell 1995). For two studies, we judged the adequacy of personnel blinding as unclear, because insufficient information was provided (Leal 1994; Barratt 1997).
We judged the adequacy of blinding of outcome assessors as adequate for only one study, Arndt 1994, and unclear for the other three studies, again because of insufficient information.
Without data (seven studies)
We considered blinding of participants to be adequate in four studies (Stanford 2001; Hollander 2003; Stanford 2005; Gowin 2012), but unclear in three studies, because the information available was insufficient to allow a judgment to be made (Ralevski 2007; Coccaro 2009; Konstenius 2014).
We considered the blinding of personnel to be adequate in five studies (Stanford 2001; Hollander 2003; Stanford 2005; Coccaro 2009; Gowin 2012), but unclear in two studies, because the information available was insufficient to allow a judgment to be made (Ralevski 2007; Konstenius 2014).
We judged blinding of outcome assessors as adequate in two studies (Stanford 2001; Coccaro 2009), but unclear for the other five studies, because of insufficient information.
Incomplete outcome data
With data (four studies)
We rated three studies at high risk of attrition bias as they did not adequately address the handling of incomplete outcome data (Arndt 1994; Leal 1994; Powell 1995). We rated one study as having an unclear risk of attrition bias (Barratt 1997). The overall proportion of participants completing was reported unambiguously in only three studies: 84% for Barratt 1997 in a prison sample; 57.9% for Leal 1994 in an inpatient sample; and 44.6% for Powell 1995 in which participants were outpatients at study endpoint. In reporting their data, Arndt 1994, Barratt 1997 and Powell 1995 provided analysis only for those participants classed by the investigators as "completers."
Without data (seven studies)
We judged one of the seven studies to have adequately addressed any incomplete outcome data; Coccaro 2009. We judged two studies at unclear risk of attrition bias; Hollander 2003 because there was insufficient information to judge whether reasons for missing data were balanced across conditions, and Ralevski 2007 because the trial investigators provided no information on either the numbers randomised to each condition or on the extent of the missing data for each condition. We rated four studies at high risk of incomplete outcome data (Stanford 2001; Stanford 2005; Gowin 2012; Konstenius 2014).
Selective reporting
With data (four studies)
We judged that all four studies appeared to have reported on all the measures they set out to use, and at all time scales, as far as could be discerned from the published reports. However, without access to the original protocols, we rated the risk of bias as being unclear for all four studies.
Without data (seven studies)
We considered six studies to be at unclear risk of selective reporting. We rated one study (Ralevski 2007) at unclear risk of bias because a companion paper by Petrakis 2005 indicated that adverse events were measured weekly via the Hopkins Symptom Checklist, although these were not reported in Ralevski 2007. We rated five studies as unclear risk of bias as there was no original study protocol available (Stanford 2001; Hollander 2003; Stanford 2005; Coccaro 2009; Gowin 2012). We judged one study, Konstenius 2014, to be at high risk of reporting bias because the six secondary outcomes that were stated in the protocol were not reported in the study paper.
Other potential sources of bias
With data (four studies)
We judged two studies to be free of other potential sources of bias (Arndt 1994; Powell 1995). We rated one study, Barratt 1997, at unclear risk of other bias because of the possibility of bias in the selection by the investigators of two subgroups for analysis. We rated Leal 1994 at high risk of other bias because of the possibility of false negative results arising from urinalysis carried out twice weekly when the usual detectability window for cocaine is six to eight hours. It was also unclear in Leal 1994 whether participants continued to receive contingency management during the trial and, if so, whether this was similar across the conditions. Since the latter involves monetary incentives in return for a clean urine sample, differences in percentages of cocaine‐free urine samples may be related to such incentives rather than the effects of medications.
Without data (seven studies)
We judged two studies, Ralevski 2007 and Konstenius 2014, to be free of other potential sources of bias. We rated five studies to be at high risk of other sources of bias. Stanford 2001 and Stanford 2005, were rated at high risk of other bias because in both studies the investigators declared their research to be sponsored by the Dreyfus Health Foundation. This foundation was established to study and disseminate information, and to sponsor collaborative, clinical and basic health research, on the 'benefits' of phenytoin. Coccaro 2009 was rated at high risk of other bias as the study was financed, in part, by a grant from Eli Lilly Research Laboratories (Eli Lilly also provided the study drug and placebo). Gowin 2012 was rated at high risk of other bias as a vested interest was identified between the study authors and the developer of the primary outcome assessment tool (PSAP). Hollander 2003 was rated to be at high risk of other bias as the study authors declared multiple links to the funding source, Abbott Laboratories, and other pharmacy companies.
Effects of interventions
See: Table 1; Table 3; Table 4; Table 5; Table 6
Summary of findings 1. Phenytoin (antiepileptic) versus placebo.
| Phenytoin (antiepileptics) versus placebo | ||||||
| Patient or population: adults with antisocial personality disorder Setting: prisons; multiple sites; USA Intervention: phenytoin (oral, 300 mg/day (am: 200 mg; pm: 100 mg) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) |
Number of participants (studies) |
Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with phenytoin | |||||
|
Aggression Measured by: frequency of aggressive acts Follow‐up: end of 6‐week treatment course |
The mean frequency of aggressive acts per week was lower in the phenytoin group (mean = 0.33; SD = not reported) than in the placebo group (mean = 0.51; SD = not reported) | ‐ | 60 (1 RCT) |
⊕⊝⊝⊝ Very lowa | Narrative results only. Skewed summary data and completer analysis by the trial investigators (see Table 2). | |
| Reconviction | ‐ | ‐ | ‐ | ‐ | ‐ | No data available |
| Global state/functioning | ‐ | ‐ | ‐ | ‐ | ‐ | No data available |
| Social functioning | ‐ | ‐ | ‐ | ‐ | ‐ | No data available |
|
Adverse events Measured by: number of participants reporting nausea during week 1 Follow‐up: week 1 |
Study population | OR 1.00 (0.06 to 16.76) | 60 (1 RCT) |
⊕⊝⊝⊝ Very lowa | ‐ | |
| 33 per 1000 | 33 per 1000 (0 fewer; from 31 fewer to 333 more) | |||||
| The study authors reported that no side effects were detectable via blood cell counts or liver enzyme tests | ‐ | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; SD: Standard deviation | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aEvidence downgraded three levels overall. We downgraded two levels for limitations in the design/implementation suggest the findings are at high risk of bias (from 'sequence generation' bias, 'allocation concealment' bias, 'blinding of participants' bias, 'blinding of personnel' bias and 'blinding of outcome assessors' 'incomplete outcome data' bias and 'other' bias), and one level for imprecision due to optimal information size criterion not being met (downgraded one level).
Summary of findings 2. Desipramine (antidepressant) versus placebo.
| Desipramine (antidepressant) versus placebo | ||||||
|
Patient or population: adults with antisocial personality disorder Setting: inpatient; USA Intervention: desipramine (oral, 250 to 300 mg/day) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) |
Number of participants (studies) |
Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with desipramine | |||||
| Aggression | ‐ | ‐ | ‐ | ‐ | ‐ | No data available |
| Reconviction | ‐ | ‐ | ‐ | ‐ | ‐ | No data available |
| Global state/functioning | ‐ | ‐ | ‐ | ‐ | ‐ | No data available |
|
Social functioning Measured by: the Family‐Social domain of ASI (scores range from 0 to 1, with higher values indicating worse social functioning) Follow‐up: end of 12‐week treatment |
There was no difference in ASI mean scores between participants taking desipramine (0.19) and those taking placebo (0.21) | ‐ | 29 (1 RCT) |
⊕⊝⊝⊝ Very lowa | ‐ | |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | No data available |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ANCOVA: Analysis of covariance;ASI: Addiction Severity Index;CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aEvidence downgraded three levels. We downgraded one level for limitations in the design/implementation, suggesting possible risk of attrition bias; one level due to imprecision due to optimal information size criterion not being met; and one level due to indirectness, as outcome is measured by a questionnaire.
Summary of findings 3. Nortriptyline (antidepressant) versus placebo.
| Nortriptyline (antidepressant) versus placebo | ||||||
|
Patient or population: men with alcohol dependency Setting: inpatient and later outpatient; USA Intervention: nortriptyline (oral, 25 to 75 mg/day) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) |
Number of participants (studies) |
Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with nortriptyline | |||||
| Aggression | ‐ | ‐ | ‐ | ‐ | ‐ | No data available |
| Reconviction | ‐ | ‐ | ‐ | ‐ | ‐ | No data available |
|
Global state/functioning Measured by: Global Assessment of Functioning Scale (scores range from 0‐100, with higher scores indicating better functioning) and the General Severity Index (GSI) subscale of the Symptom Check List‐90 (GSI), which uses mean of subscale scores (range from 0‐4, with higher scores indicating greater severity of symptoms) Follow‐up: end of treatment (6 months) |
There was no difference in GSI mean scores between participants taking nortriptyline (0.3) and those taking placebo (0.7) | ‐ | 20 (1 RCT) |
⊕⊝⊝⊝ Very lowa | ‐ | |
| Social functioning | ‐ | ‐ | ‐ | ‐ | ‐ | No data available |
| Adverse events | ‐ | ‐ | ‐ | 20 1 (RCT) |
‐ | No data available. One study measured side effects, however it did not report data on adverse events for the ASPD subgroup in either condition |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AsPD: Antisocial personality disorder; CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aEvidence downgraded three levels. We downgraded one level due to limitations in the design/implementation, suggesting possible risk of bias (attrition bias, selection bias, allocation bias and blinding of outcome assessor bias), and two levels due to very serious imprecision due to optimal information size criterion not being met and non‐reporting of outcome data.
Summary of findings 4. Bromocriptine (dopamine agonist) versus placebo.
| Bromocriptine (dopamine agonist) versus placebo | ||||||
|
Patient or population: adults with antisocial personality disorder Setting: inpatient and outpatient; USA Intervention: bromocriptine (oral, 15 mg/day) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) |
Number of participants (studies) |
Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with bromocriptine | |||||
| Aggression | ‐ | ‐ | ‐ | ‐ | ‐ | No data available |
| Reconviction | ‐ | ‐ | ‐ | ‐ | ‐ | No data available |
|
Global state/functioning Measured by: General Severity Index (GSI) subscale of the Symptom Check List‐90 (GSI), which uses mean subscale scores (range from 0‐4, with higher scores indicating greater severity of symptoms) Follow‐up: 6 months |
There was no difference in GSI mean scores between participants taking bromocriptine (0.3) and those taking placebo (0.7) | ‐ | 18 (1 RCT) |
⊕⊝⊝⊝ Very lowa | ‐ | |
| Social functioning | ‐ | ‐ | ‐ | ‐ | ‐ | No data available |
|
Adverse events Measured by: self‐reported medication side effects Follow‐up: 2 days |
12 patients in the bromocriptine group experienced severe side effects. Of these, 5 dropped out of study in first 2 days due to severe nausea and flu‐like symptoms | ‐ | 18 (1 RCT) |
⊕⊝⊝⊝ Very lowa | ‐ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; SD: Standard deviation | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aEvidence downgraded three levels. We downgraded one level for limitations in the design/implementation, suggesting a possible risk of bias (attrition bias, selection bias, allocation bias and blinding of outcome assessor bias), and two levels for very serious imprecision due to the optimal information size criterion not being met and non‐reporting of outcome data.
Summary of findings 5. Amantadine (dopamine agonist) versus placebo.
| Amantadine (dopamine agonist) versus placebo | ||||||
|
Patient or population: adults with antisocial personality disorder Setting: inpatient; USA Intervention: amantadine (oral, 300 mg/day) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) |
Number of participants (studies)a |
Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with amantadine | |||||
| Aggression | ‐ | ‐ | ‐ | ‐ | ‐ | No data available |
| Reconviction | ‐ | ‐ | ‐ | ‐ | ‐ | No data available |
| Global state/functioning | ‐ | ‐ | ‐ | ‐ | ‐ | No data available |
| Social functioning | ‐ | ‐ | ‐ | ‐ | ‐ | No data available |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | No data available |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aOne study (with 12 participants) included in this comparison. No data were available for any of the primary outcomes; however, data were available for the secondary outcomes.
1. Phenytoin (antiepileptic) versus placebo
One study, Barratt 1997, provided data for this comparison (incarcerated men with aggression; dose 300 mg/day; n = 126, with analysis of 60) and is summarised below. (See also Table 1).
Primary outcomes
Aggression
Barratt 1997 (60 participants) reported skewed summary data (see Table 2) for the overall sample as well as the "impulsive aggression" and "non‐impulsive aggression" subgroups. Overall, these data indicate a difference between conditions at endpoint (six weeks) for mean frequency of aggressive acts (P < 0.001) and for mean intensity of aggressive acts (P < 0.01), favouring phenytoin in both cases. For the impulsive aggression subgroup, these data indicate a difference between conditions at endpoint (six weeks) for mean frequency of aggressive acts (phenytoin mean = 0.33, placebo mean = 0.51; very low‐certainty evidence; P < 0.01) and for mean intensity of aggressive acts (P < 0.01), favouring phenytoin in both cases. For the non‐impulsive aggression subgroup, the data indicate no difference between conditions at endpoint (six weeks) for either mean frequency or mean intensity of aggressive acts (analysis of variance (ANOVA), Greenhouse‐Geisser adjusted, completer analysis by the trial investigators).
6. Comparison 1. Phenytoin (300 mg/day) versus placebo: aggression (skewed data).
| Study | Outcome | Experimental group | Control group | Statistic | Comments | ||||
| n | Mean | SD | n | Mean | SD | ||||
| Overall | |||||||||
| Barratt 1997 | Frequency of aggressive acts per week at 6 weeks | 60 | 0.33 | No data | 60 | 0.51 | No data | F1, 58 = 9.64 (repeated measure ANOVA, Greenhouse‐Geisser adjusted; P < 0.001) | Favours phenytoin |
| Barratt 1997 | Intensity of aggressive acts per week at 6 weeks | 60 | 2.61 | No data | 60 | 3.96 | No data | F1, 58 = 8.23 (repeated measure ANOVA, Greenhouse‐Geisser adjusted; P < 0.01) | Favours phenytoin |
| Impulsive aggression subgroup | |||||||||
| Barratt 1997 | Frequency of aggressive acts per week at 6 weeks | 30 | 0.20 | 0.19 | 30 | 0.52 | 0.46 | Subgroup effect (impulsive vs non‐impulsive): F1, 58 = 9.21 (repeated measure ANOVA, Greenhouse‐Geisser adjusted; P < 0.01) | Favours phenytoin |
| Subgroup by drug‐placebo effect: F1, 58 = 9.50 (repeated measure ANOVA, Greenhouse‐Geisser adjusted; P < 0.01) | |||||||||
| Barratt 1997 | Intensity of aggressive acts per week at 6 weeks | 30 | 2.11 | 1.20 | 30 | 4.16 | 1.92 | Subgroup effect (impulsive vs non‐impulsive): F1, 58 = 4.78 (repeated measure ANOVA, Greenhouse‐Geisser adjusted; P < 0.05) | Favours phenytoin |
| Subgroup by drug‐placebo effect: F1, 58 = 9.74 (repeated measure ANOVA, Greenhouse‐Geisser adjusted; P < 0.01) | |||||||||
| Non‐impulsive aggression subgroup | |||||||||
| Barratt 1997 | Frequency of aggressive acts per week at 6 weeks | 30 | 0.42 | 0.24 | 30 | 0.51 | 0.48 | Subgroup effect (impulsive vs non‐impulsive): F1, 58 = 9.21 (repeated measure ANOVA, Greenhouse‐Geisser adjusted; P < 0.01) | Favours neither condition |
| Subgroup by drug‐placebo effect: F1, 58 = 9.50 (repeated measure ANOVA, Greenhouse‐Geisser adjusted; P < 0.01) | |||||||||
| Barratt 1997 | Intensity of aggressive acts per week at 6 weeks | 30 | 3.40 | 1.29 | 30 | 3.76 | 1.59 | Subgroup effect (impulsive vs non‐impulsive) F1, 58 = 4.78 (repeated measure ANOVA, Greenhouse‐Geisser adjusted; P < 0.05) | Favours neither condition |
| Subgroup by drug‐placebo effect: F1, 58 = 9.74 (repeated measure ANOVA, Greenhouse‐Geisser adjusted; P < 0.01) | |||||||||
| ANOVA: Analysis of variance; SD: standard deviation. | |||||||||
Adverse events
Barratt 1997 provided data indicating no evidence of a difference between phenytoin and placebo conditions for the presence of nausea (OR 1.00, 95% CI 0.06 to 16.76; P = 1.00; 1 study, 60 participants; very low‐certainty evidence; Analysis 1.1). Barratt 1997 also reported that there were no side‐effects detectable via blood cell counts or liver enzyme tests, but did not provide these data.
1.1. Analysis.

Comparison 1: Phenytoin (antiepileptic) versus placebo, Outcome 1: Adverse events: nausea
The study did not measure the other primary outcomes: reconviction; global/state functioning; or social functioning.
Secondary outcomes: anger
Barratt 1997 (60 participants) reported skewed summary data indicating no difference between conditions in scores on the anger‐hostility subscale of the Profile of Moods Scale at endpoint (six weeks) for both impulsive and non‐impulsive aggression subgroups (Table 12; ANOVA, Greenhouse‐Geisser adjusted; completer analysis by the trial investigators).
7. Comparison 1. Phenytoin (300 mg/day) versus placebo: anger‐hostility (skewed data).
| Study | Outcome (instrument) | Experimental group | Control group | Statistic | Comments | ||||
| n | Mean | SD | n | Mean | SD | ||||
| Impulsive aggression subgroup | |||||||||
| Barratt 1997 | Anger and hostility (POMS anger‐hostility subscale at 6 weeks) | 30 | 20.4 | No data | 30 | 22.3 | No data | Scores not reduced from baseline (ANOVA, Greenhouse‐Geisser adjusted; no further details given) | Favours neither condition |
| Non‐impulsive aggression subgroup | |||||||||
| Barratt 1997 | Anger and hostility (POMS anger‐hostility subscale at 6 weeks) | 30 | 11.2 | No data | 30 | 12.5 | No data | Scores not reduced from baseline (ANOVA, Greenhouse‐Geisser adjusted; no further details given) | Favours neither condition |
| ANOVA: Analysis of variance; POMS: Profile of Mood States; SD: standard deviation. | |||||||||
The study did not measure the other secondary outcomes: quality of life; engagement with services; satisfaction with treatment; leaving the study early; substance misuse; employment status; housing/accomodation status; economic outcomes; impulsivity; mental state or prison/service outcomes.
2. Desipramine (antidepressant) versus placebo
We included two studies in this comparison: Leal 1994 (methadone‐maintained inpatient adults with opioid and cocaine dependency; dose 150 mg/day; n = 11) and Arndt 1994 (methadone‐maintained outpatient men with cocaine dependency; dose 250 to 300 mg/day; n = 29 with AsPD completers). See Table 3.
Primary outcomes: social functioning
Arndt 1994 (29 participants) reported data indicating no clear evidence of a difference between desipramine and placebo on scores on the family‐social domain of the Addiction Severity Index (ASI). Means at the end of 12‐week treatment intervention were 0.19 for the desipramine group and 0.21 for the placebo group (very low‐certainty evidence). Trial investigators carried out a between‐group analysis of co‐variance (ANCOVA) using baseline values (30 days before start of treatment) (P > 0.05).
Neither of the studies in this comparison measured the other primary outcomes: aggression; reconviction; global/state functioning; or adverse events.
Secondary outcomes
Leaving the study early
Leal 1994 reported data indicating no difference between desipramine and placebo in the number of participants leaving the study early (OR 1.20, 95% CI 0.07 to 19.63; P = 0.90; Analysis 2.1).
2.1. Analysis.

Comparison 2: Desipramine (antidepressant) versus placebo, Outcome 1: Leaving the study early
Substance misuse: drugs
For the outcome of dollars (USD) spent on cocaine per week, Leal 1994 reported skewed summary data for desipramine and placebo conditions at week one (desipramine mean = USD 184, (standard deviation (SD) = 177), placebo mean = USD 70 (SD = 29)), week six (desipramine mean = USD 98 (SD = 101), placebo mean = USD 32 (SD = 33)), and week 12 (desipramine mean = USD 76 (SD = 69), placebo mean = USD (SD = 64)). The trial investigators reported no statistical analysis that compared desipramine and placebo groups.
For the outcome of cocaine abstinence, Leal 1994 reported the percentage of (twice‐weekly) urinalyses that were cocaine‐free for first two weeks (desipramine = 21%, placebo = 0%), for weeks five and six (desipramine = 18%, placebo = 25%), and for the last two weeks of the study (desipramine = 20%, placebo = 0%). Trial investigators reported no statistical analysis that compared desipramine and placebo groups on this outcome.
Arndt 1994 reported no difference between conditions on mean percentage of cocaine‐positive urinalysis (performed twice weekly throughout the study on random schedule and evaluated for cocaine metabolite (benzoylecgonine)) results across all 12 weeks of the study (desipramine = 78%, placebo = 77%).
Arndt 1994 reported data indicating no difference between desipramine and placebo conditions on drug domain scores, days of opiate use, and days of cocaine use from the ASI, and on cocaine craving scores from the Cocaine Craving Scale and Quantitative Cocaine Inventory (Table 13; between‐groups ANCOVA using baseline value as covariate; analysis by trial investigators).
8. Comparison 5. Desipramine versus placebo: substance misuse.
| Study | Outcomea |
Experimental group (n = 17 completed) |
Control group (n = 12 completed) |
Statisticc |
| Arndt 1994 | ASI (drug factor scores)b | 0.259 | 0.23 | Favours neither condition (P > 0.05) |
| Arndt 1994 | Days of opiate use | 2 | 2 | Favours neither condition (P > 0.05) |
| Arndt 1994 | Days of cocaine use | 9 | 8 | Favours neither condition (P > 0.05) |
| Arndt 1994 | Cocaine craving scores | 8 | 7 | Favours neither condition (P > 0.05) |
| ANCOVA: Analysis of covariance; ASI: Addiction Severity Index | ||||
a12‐week end of treatment means bASI factor scores range from 0 to 1 with larger values indicating greater problem severity cBetween‐groups ANCOVA using baseline value as covariate; baseline values reflect the 30 days before start of treatment; analysis carried out by trial investigators
Employment status
Arndt 1994 reported data indicating no difference between desipramine and placebo conditions on employment domain scores and days worked in the last 30 days from the ASI (Table 14; between‐groups ANCOVA using baseline value as covariate; analysis by trial investigators). They did, however, report results of a similar analysis suggesting a greater employment income in the placebo group compared with the desipramine group (P < 0.05, Table 14).
9. Comparison 5. Desipramine versus placebo: employment status.
| Study | Outcomea |
Experimental group (n = 17 completed) |
Control group (n = 12 completed) |
Statisticc |
| Arndt 1994 | ASI (employment factor)b | 0.606 | 0.495 | Favours neither condition (P = 0.08) |
| Arndt 1994 | Days worked in past 30 days | 9 | 10 | Favours neither condition (P > 0.05) |
| Arndt 1994 | Employment income | US $479 | US $1049 | Favours control (P < 0.05) |
| ANCOVA: Analysis of covariance; ASI: Addiction Severity Index | ||||
a12‐week end of treatment means bASI factor scores range from 0 to 1 with larger values indicating greater problem severity cBetween‐groups ANCOVA using baseline value as covariate; baseline values reflect the 30 days before start of treatment; analysis carried out by trial investigators
Mental state: depression
Arndt 1994 reported data indicating no difference between the desipramine (mean = 7, n = 17) and placebo (mean = 8, n = 12) groups on depression scores assessed with the BDI (between‐groups ANCOVA using baseline value as covariate; P > 0.05, no effect size measure or F value provided, analysis by trial investigators).
Other outcomes: illegal activity
Arndt 1994 reported data indicating no difference between desipramine and placebo conditions on illegal domain scores, days of illegal activity in the last 30 days and illegal income from the ASI (Table 15; between‐groups ANCOVA using baseline value as covariate; analysis by trial investigators).
10. Comparison 5. Desipramine versus placebo: illegal activity.
| Study | Outcomea |
Experimental group (n = 17 completed) |
Control group (n = 12 completed) |
Statisticc |
| Arndt 1994 | ASI (legal factor scores)b | 0.150 | 0.062 | Favours neither condition (P > 0.05) |
| Arndt 1994 | Days of illegal activity | 4 | 2 | Favours neither condition (P > 0.05) |
| Arndt 1994 | Illegal income | US $251 | US $176 | Favours neither condition (P > 0.05) |
| ANCOVA: Analysis of covariance; ASI: Addiction Severity Index | ||||
a12‐week end of treatment means bASI factor scores range from 0 to 1 with larger values indicating greater problem severity cBetween‐groups ANCOVA using baseline value as covariate; baseline values reflect the 30 days before start of treatment; analysis carried out by trial investigators
Neither of the studies included in this comparison measured the following outcomes: quality of life; engagement with services; satisfaction with treatment; housing/accommodation status; economic outcomes; impulsivity; anger; mental state; or prison/service outcomes.
3. Nortriptyline (antidepressant) versus placebo
We included one study in this comparison: Powell 1995 (inpatient, and later outpatient, men with alcohol dependency; dose 25 to 75 mg/day; n = 20 completers). See Table 4.
Primary outcomes
Global state/functioning
Powell 1995 (20 participants) reported data indicating no difference between nortriptyline and placebo for global functioning measured with the GSI subscale of the SCL‐90 (nortriptyline mean = 0.3, placebo mean = 0.7; very low‐certainty evidence) and the GAF (Table 16; 3‐way ANOVA; comorbidity x treatment x time; analysis by trial investigators).
11. Comparison 6. Nortriptyline versus placebo: global functioning.
| Study | Outcome (instrument) | Experimental groupa (n = 11) | Control groupa (n = 9) | Statisticb | ||
| Mean scores at baseline | Mean scores at endpoint | Means scores at baseline | Mean scores at endpoint | |||
| Powell 1995 | Global functioning (GAS‐high)c | 59.3 | 77.1 | 57.9 | 75.7 | Favours neither condition |
| Powell 1995 | Global functioning (GAS‐low)c | 37.6 | 51.7 | 34.8 | 43.0 | Favours neither condition |
| Powell 1995 | Global functioning (SCL‐90 GSI subscale)d | 1.0 | 0.3 | 0.8 | 0.7 | Favours neither condition |
| GAS: Global Assessment Scale;GSI: Global Severity Index; SCL‐90: Symptom Check List‐90 | ||||||
aStandard deviations not reported bTrend‐over‐time analyses based on measurements at baseline and six months, with additional measurements at 2, 4, and 6 weeks, and then at 2, 3, 4, and 5 months; analyses conducted by trial investigators cHigh scores indicate better functioning dHigh scores indicate greater severity
Adverse events
Powell 1995 measured medication side effects but did not report data on adverse events in the nortriptyline and placebo conditions for their AsPD subgroup.
The study did not measure the other primary outcomes: aggression; reconviction; or social functioning.
Secondary outcomes
Leaving the study early
Powell 1995 did not provide data on leaving the study early for nortriptyline and placebo conditions for their AsPD subgroup. They reported however, that “the dropout rates for the comorbidity and medication subgroups ranged from 52.1% to 55.4%, and were not significantly different."
Substance misuse: alcohol
Powell 1995 reported graphical data indicating a difference for nortriptyline versus placebo on mean number of drinking days (nortriptyline mean = 9.5, placebo mean = 42.2; no SD provided), favouring nortriptyline (two‐way ANOVA; comorbidity x medication: F (4, 89) = 2.60; P < 0.05; Tukey post‐hoc tests for each comorbidity subgroup indicated a medication effect for AsPD with nortriptyline subgroup only; P < 0.05; completer analysis by trial investigators).
Powell 1995 reported graphical data indicating no difference between nortriptyline (n = 11) and placebo (n = 9) conditions on mean alcohol craving scores measured on a visual analogue scale (nortriptyline mean = 5.3; placebo mean = 9.5; no SD provided); three‐way ANOVA; comorbidity x medication x time; no main effects or interactions; completer analysis by trial investigators.
Powell 1995 reported an analysis indicating no difference between any medication (nortriptyline or bromocriptine) and placebo conditions on participants' self‐report of longest period of total abstinence during the six‐month study (two‐way ANOVA; comorbidity x medication: F (2, 90) = 3.02; completer analysis by trial investigators). The study authors did not provide data on the difference between nortriptyline and placebo alone.
Powell 1995 additionally reported that seven of the eleven (64%) completers in the nortriptyline group were abstinent from drinking at six months compared to one out of nine (11%) of completers in the placebo group.
Powell 1995 reported data indicating no difference between nortriptyline and placebo conditions on severity of alcohol misuse on Alcohol Severity Scale scores, on patient's rating of drinking, and on clinical rating of drinking (Table 17). They did, however, find a greater improvement over time for nortriptyline compared to placebo on alcohol dependence measured with the SAD‐Q (Table 17; 3‐way ANOVA; comorbidity x treatment x time; P < 0.01; analysis by trial investigators).
12. Comparison 6. Nortriptyline versus placebo: severity of alcohol misuse.
| Study | Outcomea (instrument) | Experimental groupb (n = 11) | Control groupb (n = 9) | Statisticc | ||
| Mean scores at baseline | Mean scores at endpoint | Mean scores at baseline | Mean scores at endpoint | |||
| Powell 1995 | Alcohol severity (ASS) | 20.9 | 4.6 | 20.7 | 14.4 | Favours neither condition |
| Powell 1995 | Patient rating of drinking | 4.6 | 1.8 | 4.7 | 3.6 | Favours neither condition |
| Powell 1995 | Clinical rating of drinking | 5.9 | 2.4 | 6.7 | 4.6 | Favours neither condition |
| Powell 1995 | Alcohol dependence (SAD‐Q) | 27.3 | 4.6 | 20.8 | 15.7 | Favours nortriptyline 3‐way ANOVA; post‐hoc test showed greater improvement over time (P < 0.01) for nortriptyline compared to placebo |
| ANOVA: Analysis of variance; ASS: Alcohol Severity Scale;SAD‐Q: Severity of Alcohol Dependence Questionnaire | ||||||
aHigh scores indicate greater severity bStandard deviations not reported cTrend‐over‐time analyses based on measurements at baseline and six months, with additional measurements at 2, 4, and 6 weeks, and then at 2, 3, 4, and 5 months; analyses conducted by trial investigators
Mental state
Powell 1995 reported data indicating no difference between nortriptyline and placebo conditions on depressive symptoms measured with the depression subscale of the SCL‐90 and the BDI (Table 18; 3‐way ANOVA; comorbidity x treatment x time; analysis by trial investigators).
13. Comparison 6. Nortriptyline versus placebo: depression and anxiety.
| Study |
Outcomea (instrument) |
Experimental groupb (n = 11) | Control groupb (n = 9) | Statisticc | ||
| Mean scores at baseline | Mean scores at endpoint | Mean scores at baseline | Mean scores at endpoint | |||
| Powell 1995 | Depression (BDI) | 11.2 | 3.1 | 5.7 | 5.4 | Favours neither condition |
| Powell 1995 | Depression (SCL‐90 depression subscale) | 1.3 | 0.4 | 0.8 | 0.7 | Favours neither condition |
| Powell 1995 | Anxiety (BAI) | 9.4 | 4.3 | 4.3 | 9.1 | Favours nortriptyline 3‐way ANOVA; post‐hoc test showed greater improvement over time (P < 0.05) for nortriptyline compared to placebo |
| Powell 1995 | Anxiety (SCL‐90 anxiety subscale) | 0.8 | 0.2 | 0.7 | 0.6 | Favours neither condition |
| BAI: Beck Anxiety Inventory; BDI: Beck Depression Inventory; SCL‐90: Symptom Check List‐90 | ||||||
aHigh scores indicate greater severity bStandard deviations not reported cTrend‐over‐time analyses based on measurements at baseline and six months, with additional measurements at 2, 4, and 6 weeks, and then at 2, 3, 4, and 5 months; analyses conducted by trial investigators
Powell 1995 reported data indicating a difference between nortriptyline and placebo conditions, favouring nortriptyline on anxiety symptoms measured using the BAI (P < 0.05), but no difference between the groups on the anxiety subscale of the SCL‐90 (Table 18; 3‐way ANOVA; comorbidity x treatment x time; analysis by trial investigators).
This study did not measure the other secondary outcomes: quality of life; engagement with services; satisfaction with treatment; employment status; housing/accommodation status; economic outcomes; impulsivity; anger; or prison/service outcomes.
4. Bromocriptine (dopamine agonist) versus placebo
We included one study in this comparison: Powell 1995 (inpatient, and later outpatient, men with alcohol dependency; dose 15 mg/day; n = 18 completers; data provided graphically and no SD provided). See Table 5 for this comparison.
Primary outcomes
Global state/functioning
Powell 1995 (18 participants) reported data indicating no difference at six month follow up between bromocriptine and placebo conditions for global functioning measured with the GSI subscale of the SCL‐90 (mean = 0.4 versus 0.7, respectively; very low‐certainty evidence) and the GAS (Table 19. 3‐way ANOVA; comorbidity x treatment x time; analysis by trial investigators).
14. Comparison 9. Bromocriptine versus placebo: global functioning.
| Study | Outcome (instrument) | Experimental groupa (n = 9) | Control groupa (n = 9) | Statisticb | ||
| Mean scores at baseline | Mean scores at endpoint | Mean scores at baseline | Mean scores at endpoint | |||
| Powell 1995 | Global functioning (GAS‐high)c | 55.8 | 76.0 | 57.9 | 75.7 | Favours neither condition |
| Powell 1995 | Global functioning (GAS‐low)c | 36.0 | 47.4 | 34.8 | 43.0 | Favours neither condition |
| Powell 1995 | Global functioning (SCL‐90 GSI subscale)d | 0.8 | 0.4 | 0.8 | 0.7 | Favours neither condition |
| GAS: Global Assessment Scale; GSI: Global Severity Index; SCL‐90: Symptom Check List‐90 | ||||||
aStandard deviations not reported bTrend‐over‐time analyses based on measurements at baseline and six months, with additional measurements at 2, 4, and 6 weeks, and then at 2, 3, 4, and 5 months; analyses conducted by trial investigators cHigh scores indicate better functioning dHigh scores indicate greater severity
Adverse events
Powell 1995 (18 participants) did not provide data on adverse events in the bromocriptine and placebo conditions for their AsPD subgroup. However, they reported that “...12 experienced severe side effects (five of these, all taking bromocriptine, dropped out in the first 2 days because of severe nausea and flu‐like symptoms)" (very low‐certainty evidence).
The study did not measure the other primary outcomes: aggression; reconviction; or social functioning.
Secondary outcomes
Leaving the study early
Powell 1995 did not provide data on leaving the study early for bromocriptine and placebo conditions for their AsPD subgroup. However, they reported that “the dropout rates for the comorbidity and medication subgroups ranged from 52.1% to 55.4%, and were not significantly different."
Substance misuse: alcohol
Powell 1995 reported graphical data indicating no difference between bromocriptine versus placebo on mean number of drinking days (19 versus 42.2, respectively; two‐way ANOVA; comorbidity x medication: F (4, 89) = 2.60; P < 0.05; however, Tukey post‐hoc tests did not provide evidence of an effect for AsPD with bromocriptine subgroup; completer analysis by trial investigators).
Powell 1995 reported graphical data indicating no difference between bromocriptine versus placebo on mean alcohol craving scores measured on a visual analogue scale (6.3 versus 9.5, respectively; three‐way ANOVA; comorbidity x medication x time; no main effects or interactions; completer analysis by trial investigators).
Powell 1995 reported an analysis indicating no difference between medication (bromocriptine or nortriptyline) and placebo conditions on participants' self‐report of longest period of total abstinence during the six‐month study (two‐way ANOVA; comorbidity x medication: F (2, 90) = 3.02; completer analysis by trial investigators). The study authors did not provide data on the difference between bromocriptine and placebo alone.
Powell 1995 additionally reported that three out of nine (33%) completers in the bromocriptine group were abstinent from drinking at six months compared to one out of nine (11%) of completers in the placebo group.
Powell 1995 reported data indicating no difference between bromocriptine and placebo conditions on severity of alcohol misuse measured with the Alcohol Severity Scale, both patient and clinical ratings of drinking, and alcohol dependence on the SAD‐Q (Table 20; 3‐way ANOVA; comorbidity x treatment x time; analysis by trial investigators).
15. Comparison 9. Bromocriptine versus placebo: severity of alcohol misuse.
| Study | Outcomea (instrument) | Experimental groupb (n = 9) | Control groupb (n = 9) | |||
| Mean scores at baseline | Mean scores at endpoint | Mean scores at baseline | Mean scores at endpoint | Statisticc | ||
| Powell 1995 | Alcohol severity (ASS) | 22.8 | 10.8 | 20.7 | 14.4 | Favours neither condition |
| Powell 1995 | Patient rating of drinking | 4.8 | 2.9 | 4.7 | 3.6 | Favours neither condition |
| Powell 1995 | Clinical rating of drinking | 6.6 | 3.6 | 6.7 | 4.6 | Favours neither condition |
| Powell 1995 | Alcohol dependence (SADQ scores) | 28.6 | 17.3 | 20.8 | 15.7 | Favours neither condition |
| ASS: Alcohol Severity Scale; SADQ: Severity of Alcohol Dependence Questionnaire | ||||||
aHigh scores indicate greater severity bStandard deviations not reported cTrend‐over‐time analyses based on measurements at baseline and six months, with additional measurements at 2, 4, and 6 weeks, and then at 2, 3, 4, and 5 months; analyses conducted by trial investigators
Mental state
Powell 1995 additionally reported data indicating no difference between bromocriptine and placebo conditions on depressive symptoms measured with the depression subscale of the SCL‐90 and the BDI (Table 21; 3‐way ANOVA; comorbidity x treatment x time; analysis by trial investigators).
16. Comparison 9. Bromocriptine versus placebo: depression and anxiety.
| Study | Outcomea (instrument) | Experimental groupb (n = 9) | Control groupb (n = 9) | Statisticc | ||
| Mean scores at baseline | Mean scores at endpoint | Mean scores at baseline | Mean scores at endpoint | |||
| Powell 1995 | Depression (BDI) | 12.7 | 3.3 | 5.7 | 5.4 | Favours neither condition |
| Powell 1995 | Depression (SCL‐90 depression subscale) | 0.9 | 0.4 | 0.8 | 0.7 | Favours neither condition |
| Powell 1995 | Anxiety (BAI) | 6.8 | 1.9 | 4.3 | 9.1 | Favours bromocriptine 3‐way ANOVA; post‐hoc test showed greater improvement over time (P < 0.05) for bromocriptine compared to placebo |
| Powell 1995 | Anxiety (SCL‐90 anxiety subscale) | 0.7 | 0.6 | 0.7 | 0.6 | Favours neither condition |
| BAI: Beck Anxiety Inventory; BDI: Beck Depression Inventory; SCL‐90: Symptom Check List‐90 | ||||||
aHgh scores indicate greater severity bStandard deviations not reported cTrend‐over‐time analyses based on measurements at baseline and six months, with additional measurements at 2, 4, and 6 weeks, and then at 2, 3, 4, and 5 months; analyses conducted by trial investigators
Powell 1995 additionally reported data indicating a difference between bromocriptine and placebo conditions favouring bromocriptine on anxiety symptoms measured using the BAI (Table 21; 3‐way ANOVA; comorbidity x treatment x time; P < 0.05; analysis by trial investigators), but no difference between the groups using the anxiety subscale of the SCL‐90 (Table 21).
This study did not measure the other secondary outcomes: quality of life; engagement with services; satisfaction with treatment; employment status; housing/accommodation status; economic outcomes; impulsivity; anger; mental state; or prison/service outcomes.
5. Amantadine (dopamine agonist) versus placebo
One study was included in this comparison: Leal 1994 (methadone‐maintained inpatient adults with opioid and cocaine dependency; dose 300 mg/day; n = 12). See Table 6.
Primary outcomes
The study did not measure any of the primary outcomes: aggression; reconviction; global/state functioning; social functioning; or adverse events.
Secondary outcomes
Leaving the study early
Leal 1994 reported data indicating little or no difference between amantadine and placebo conditions for the outcome of "leaving the study early" (OR 5.00, 95% CI 0.34 to 72.77, P = 0.24; 1 study, 12 participants; Analysis 3.1).
3.1. Analysis.

Comparison 3: Amantadine (dopamine agonist) versus placebo, Outcome 1: Leaving the study early
Substance misuse: drugs
For the outcome of "dollars spent on cocaine per week," Leal 1994 reported skewed summary data for amantadine and placebo conditions at week one (amantadine mean = 162 (SD = 138), placebo mean = 70 (SD = 29)), week six (amantadine mean = 115 (SD = 127), placebo mean = 32 (SD = 33)), and week 12 (amantadine mean = 70 (SD = 63), placebo mean= 59 (64)).
For the outcome of "cocaine abstinence," Leal 1994 reported the percentage of (twice‐weekly) urinalyses that were cocaine‐free for first the two weeks (amantadine = 6%, placebo = 0%), for weeks five and six (amantadine = 6%, placebo = 25%), and for the last two weeks of the study (amantadine = 0%, placebo = 0%).
This study did not measure the other secondary outcomes: quality of life; engagement with services; satisfaction with treatment; employment status; housing/accommodation status; economic outcomes; impulsivity; anger; and mental state.
Discussion
Summary of main results
This review was relatively broad in its focus since it sought evidence on the effectiveness of any pharmacological intervention in the treatment of AsPD. We found considerable differences between the studies in terms of participants, size of sample, intervention, and choice of outcome measures. We identified 11 studies (416 AsPD participants) that fully met the inclusion criteria for this review (Arndt 1994; Leal 1994; Powell 1995; Barratt 1997; Stanford 2001; Hollander 2003; Stanford 2005; Ralevski 2007; Coccaro 2009; Gowin 2012; Konstenius 2014). Included studies reported on all but one of the primary outcomes of this review (i.e. aggression (six studies); global state/functioning (three studies); social functioning (one study); and adverse effects (seven studies)); no study reported on reconviction. Based on analyses conducted by the trial investigators, we found only one drug (phenytoin) which, when compared to placebo, was superior in terms of improvement in at least one primary outcome in at least one study; and two drugs (nortriptyline, bromocriptine) which, when compared to placebo, were superior in terms of improvement in at least one secondary outcome in at least one study.
Much of the quantitative data available from the studies included in this review were either inadequately summarised by the trial investigators or else met our criteria for skewed data as described in the section on 'Measures of treatment effect.' Therefore, in the absence of raw data from the trial investigators, we presented most of the quantitative data in Additional tables and reported statistics on comparisons between conditions calculated by the trial investigators, rather than performing our own analyses. Where data were skewed, we did not carry out any synthesis of primary or secondary outcome data via meta‐analysis because either (a) data for an outcome were available from only one study, or (b) we wanted to minimise the risk of applying parametric statistics to skewed data, which, by definition, are not normally distributed. Therefore, the summaries that follow below are essentially descriptive, and relate to our primary outcomes only.
Phenytoin (antiepileptic) versus placebo
The evidence is very uncertain about the effect of a 300 mg/day dose of phenytoin, compared to placebo, on the mean frequency of aggressive acts at endpoint (six weeks) in one study of 60 male prisoners with aggression (Barratt 1997).
The same study, Barratt 1997, reported no evidence of difference between 300 mg/day of phenytoin and placebo for the adverse event of nausea during week one. The study authors also reported that no important side effects were detectable via blood cell counts or liver enzyme tests. However, the evidence is very uncertain.
Desipramine (antidepressant) versus placebo
One study of 29 methadone‐maintained outpatient men with cocaine dependency, Arndt 1994, reported no evidence of a difference between a 250 to 300 mg/day dose of desipramine and placebo on social functioning scores, measured by the family‐social domain of the Addiction Severity Index at endpoint (12 weeks). However, the evidence is very uncertain.
Nortriptyline (antidepressant) versus placebo
The evidence is very uncertain about the effect of a dose of 25 to 75 mg/day of nortriptyline, compared to placebo, on global functioning scores, measured by the Global Severity Index (GSI) subscale of the Symptom Check List‐90 (SCL‐90) at endpoint (six months), in one study of 20 inpatient/outpatient men with alcohol dependency (Powell 1995).
Bromocriptine (dopamine agonist) versus placebo
One study of 18 inpatient/outpatient men with alcohol dependency, Powell 1995, reported no evidence of a difference between a 15 mg/day dose of bromocriptine and placebo on global functioning scores, measured by the GSI subscale of the SCL‐90 at endpoint (six months). However, the evidence is very uncertain.
The study did not provide data on adverse effects but reported that 12 patients in the bromocriptine group experienced severe side effects, and five of these participants dropped out in the first two days due to nausea and severe flu‐like symptoms. Again, the evidence is very uncertain.
Amantadine (dopamine agonist) versus placebo
None of the included studies in this comparison measured any of the primary outcomes.
Only Barratt 1997 reported a change in a specific antisocial behaviour (phenytoin on aggression). No study provided a comprehensive analysis of adverse effects. The finding by Barratt 1997 that phenytoin reduced acts of impulsive but not premeditated aggression, compared to placebo, is in line with evidence from the wider literature on aggression (Huband 2010) which suggests that different forms of aggression are underpinned by different mechanisms. The differences between impulsive or reactive aggression and premeditated or instrumental aggression have been well documented (e.g. Blair 2001). We suggest, therefore, that studies evaluating the effectiveness of interventions for aggression ‐ both generally and in the context of AsPD ‐ should use outcome measures that enable distinctions to be made according to the evidence‐based typologies of aggression.
As described in the introduction, AsPD is a prevalent condition, which is associated with considerable personal and societal adverse consequences. It also has major negative economic consequences, as it is associated with poor occupational productivity and increased criminal justice costs. Consequently, one might expect that identifying the interventions that might reduce this impact would be a research priority. Unfortunately, the conclusion of this review is similar to many that preceded it, in that there is little, good quality evidence as to what might (or might not) be effective for this condition.The first point to make is how few studies there were to consider. It is also worth noting here that none of the 11 studies set out to recruit participants on the basis of having a diagnosis of AsPD. The second concerns the design and methodological quality of the 11 included studies. While the underlying personality structure of AsPD comprises different traits, such as impulsivity, lack of remorse and irritability, it is persistent rule‐breaking that is its most common behavioural manifestation. Although focusing on behaviour rather than on the underlying personality structure has been frowned upon by some commentators (e.g. Livesley 2007), we argue that persistent rule‐breaking is akin to a final common pathway manifestation of the underlying personality structure. If one accepts this argument, it is disappointing that none of the included studies had reconviction as their primary outcome, and only one, Arndt 1994, reported on illegal activity. Furthermore, four of the 11 included studies were trials to reduce substance misuse. As many within the sample of substance misusers also satisfied criteria for AsPD, there was an opportunity to report on these separately. Hence, strictly speaking, these were not interventions for AsPD; rather, they were interventions to reduce substance misuse in a sample, some of whom also satisfied criteria for AsPD. While these studies had some limitations, there is some evidence that nortriptyline may reduce some aspects of alcohol misuse in this population, and that both nortriptyline and bromocriptine may reduce anxiety symptoms in individuals with AsPD and alcohol dependency. The remaining four studies focused on aggressive behaviour, although data on AsPD participants were available for only one of these, which makes it difficult to draw any robust conclusion.
In the light of the important adverse cost consequences of the condition, it was also disappointing that none of the studies considered the economic impact of their intervention or conducted longer follow‐ups. It is also important to note that most included studies were older trials from the mid‐1990s, with no testing of more recently developed medications.
Overall completeness and applicability of evidence
The evidence obtained from the included studies is relevant to the review question, but is incomplete for the following reasons.
Although we compared 11 different pharmacological interventions, none of the studies evaluated the primary outcome of reconviction.
The reporting of adverse events was low given that medications were being compared; only one study had data for AsPD participants out of the seven studies (7/11) that reported adverse events.
No study reported on the secondary outcomes of quality of life, engagement with services, satisfaction with treatment, housing/accommodation status or economic outcomes.
The majority of studies did not focus primarily on the treatment of AsPD, and only one study recruited a sample in which all participants had this diagnosis.
Five studies focused on participants with substance misuse difficulties. Although drug/alcohol misuse is often relevant to people with AsPD, having a substance misuse problem is not part of the diagnostic criteria for AsPD.
All studies with usable data were trials of older medications, such as the antiepileptic drug phenytoin and tricyclic antidepressants such as nortriptyline, which are no longer widely used and which have been largely superseded by newer drugs with more favourable side‐effect profiles.
The review relies on data from only four of the 11 included studies, despite attempts to contact the trial investigators for information on the AsPD subgroups.
The study samples were heterogeneous, encompassing, for example, both prisoners and outpatients. In addition, AsPD was diagnosed under three similar but not identical rubrics (DSM‐III, DSM‐III‐R, and DSM‐IV).
The data available were generally insufficient to allow any independent statistical analysis.
All the available data were derived from unreplicated single reports.
There was inconsistency in the way primary and secondary outcomes were measured and reported.
Quality of the evidence
The 11 studies that met the criteria for inclusion in this review involved a total of 416 participants with AsPD. Of these 11, only four studies involving 274 participants with AsPD provided usable data. All of the included studies were RCTs. However, as Guyatt 2011 acknowledges, even RCTs can be limited by problems such as failure to conceal allocation, failure to blind, loss to follow‐up, failure to use the intention‐to‐treat principle, stopping early for apparent benefit, and selective reporting of outcomes. Such issues increase the risk of bias, which, in turn, can overestimate the benefits and underestimate the harms identified (Moher 1998; Moher 2010). We used the GRADE approach to assess the certainty of the reported evidence (Schünemann 2013), and considered the risk of bias, inconsistency, indirectness and imprecision of the evidence. The certainty of evidence from all included studies with data for AsPD participants was assessed separately for individual outcomes. In every case, the evidence was downgraded due to a combination of issues with risk of bias (high or possible risk of bias), indirectness (due to use of self‐reported questionnaire), or imprecision (due to small sample size/optimal information size criteria not being met or non‐reporting of outcome data). The largest risk of bias in the included studies came from incomplete outcome data (attrition bias) and 'other' bias. We rated the certainty of the evidence for all primary outcomes as very low (i.e. we have very little confidence in the effect estimate, and the true effect is likely to be substantially difference from the estimate of effect).
Although Guyatt 2011 suggests that a single, very large, rigorously planned and conducted multi‐centre RCT may provide high‐certainty evidence, others suggest that there should be at least two independent, well‐conducted RCTs or single‐case experiments for a treatment to be considered effective (Chambless 1998). The majority of studies included in this review reported small sample sizes, and it was not possible to pool data given the heterogeneity of the interventions and participants. In light of this, we consider that the body of evidence summarised in this review is insufficient to allow any conclusion to be drawn about the use of pharmacological interventions in the treatment of AsPD.
A further limitation with the certainty of the evidence arises from an acknowledgement that personality disorder, in general, is a complex condition and clinical outcomes are best measured across multiple domains (see Khalifa 2010 ). A broad approach to outcome evaluation in personality disorder has been recognised by international experts in the field (e.g. Crawford 2007).
Potential biases in the review process
We acknowledge that a small number of decisions taken during the review process may have introduced 'selective reporting bias' to the review. First, the decision to include studies with two treatment conditions where the trial investigators randomised ‘at least five people with AsPD’ may have resulted in the exclusion of a small number of studies. In this case, we considered that the potential for bias was minimal, as any excluded studies with very small numbers were usually not RCTs. Second, the 12‐week cut‐off period for receiving missing data from study authors could have resulted in relevant data being omitted from the review. In this way, it could be interpreted that we selectively reported the missing data and that the review is open to reporting bias. However, this is not the case, as no missing data were excluded. Third, we decided to include only studies where at least 75% of participants were diagnosed with AsPD. Although this appeared clinically and scientifically appropriate, this decision may have introduced reporting bias to the review.
Agreements and disagreements with other studies or reviews
An earlier review on the pharmacotherapy of personality disorders found no RCTs and only one case report on treatment of AsPD with risperidone (Markovitz 2004). A more recent review (Ripoll 2011), which summarised the evidence base for pharmacotherapy in people with personality disorder, including those with ASPD, found some evidence in favour of lithium for reducing "serious rule infractions," phenytoin for reducing impulsive (not premeditated) aggression, and oxazepam for reducing hostility. The review used evidence from studies involving incarcerated individuals likely to have been antisocial based on past histories of violence and criminality, and included four studies (Sheard 1971; Sheard 1976; Lion 1979; Barratt 1991), which were excluded from the current review because there was no indication that AsPD was represented in the sample.
The most recent and wide‐ranging relevant review with which to compare our findings is that carried out in the development of the NICE clinical guideline on AsPD (NICE 2010; updated in 2013). In reporting their systematic review, the NICE guideline authors observed that "the state of current practice in relation to the use of pharmacological interventions to treat antisocial personality disorder is unclear, but it is likely that pharmacological interventions are used in this population to treat symptoms rather than as an intervention for the disorder" (NICE 2010). They also noted three difficulties that arise when attempting to assess the effectiveness of drug interventions within this client group: lack of clarity as to whether the medication is being used to target AsPD or a comorbid mental illness; the possibility that comorbid substance misuse may diminish response rates; and the likelihood that multiple neurotransmitter systems are involved making drug selection difficult. In recognition of this, they chose to consider not only interventions that targeted AsPD itself, but also those that targeted the symptoms or behaviours associated with the diagnosis (such as anger, impulsivity and aggression), as well as interventions specifically for offenders regardless of diagnosis. Thus, the review described by NICE 2010 is much broader than our current review, which focuses solely on studies of participants with a diagnosis of AsPD.
Although this review and the NICE 2015 review identified the same five studies targeting treatment of AsPD and treatment of comorbid disorder in people with AsPD (i.e. Leal 1994; Powell 1995; Barratt 1997; Hollander 2003; Stanford 2005), there were several differences.
This review identified three additional studies which were not included in the NICE review (Arndt 1994; Stanford 2001; Ralevski 2007); although only one of these, Arndt 1994, had data available for the AsPD subgroup.
NICE 2010 considered three additional studies that were excluded from this review because there was no indication that AsPD was represented in the sample: Mattes 2005 on oxcarbazepine versus placebo in outpatients with impulsive aggression; Mattes 2008 on levetiracetam versus placebo in outpatients with impulsive aggression; and Nickel 2005 on topiramate versus placebo in male outpatients with aggression.
NICE 2010 considered two further trials looking specifically at offenders (Gottschalk 1973; Sheard 1976). These studies were not eligible for inclusion in this review because the participants had no formal diagnosis of AsPD.
NICE 2010 found "no consistent evidence, including that from uncontrolled studies, that supported the use of any pharmacological intervention to treat antisocial personality disorder, or to treat the behaviour and symptoms that underline the specific diagnostic criteria for antisocial personality disorder." They also found "no evidence on the cost‐effectiveness of pharmacological interventions for AsPD with or without substance misuse" (NICE 2010).
The overall recommendations from NICE 2010 were that (a) "pharmacological interventions should not be routinely used for the treatment of AsPD or associated behaviours of aggression, anger and impulsivity," and (b) "pharmacological interventions for comorbid mental disorders, in particular depression and anxiety, should be in line with recommendations in the relevant NICE guideline" (NICE 2010).
This review similarly concludes that good quality evidence favouring any pharmacological intervention for AsPD is virtually non‐existent.
Authors' conclusions
Implications for practice.
This review concludes that there is insufficient evidence to support or refute the effectiveness of any pharmacological intervention for AsPD. In the absence of good quality trial data, the use of pharmacological interventions to treat people with AsPD in clinical practice remains a matter for the clinician, who will wish to weigh the limited evidence of effectiveness against any risk of possible harm. It should ideally be based on consultation with the patient, their family and carers (subject to their consent) and the multi‐disciplinary team involved in the individual's care.
Implications for research.
As the evidence in this review came from single studies, the pharmacological interventions reported here require replication to confirm apparent efficacy or lack of effect (Jakobsen 2014). Given the very few studies that could be considered in this review, there is clearly an imperative to conduct well‐designed trials using pharmacological approaches. Such trials should recruit sufficient numbers of people on the basis of having the disorder and use outcomes measures that are of particular relevance to AsPD. They should focus also on recently marketed drugs that have largely replaced older medications that are no longer widely used (for example, nortriptyline and phenytoin).
In addition, we are concerned to note that the four trials whose data could be used in this review were all published more than a decade ago, so interest in trials for pharmacological interventions for this group appears to be diminishing rather than increasing. We speculate that one of the reasons for this reluctance by the industry to develop treatments for this group is a fear of litigation were something to go wrong. Whatever the reason, given the poor evidence base, we recognise that these initial trials need to be comparisons of active treatment against placebo rather than 'head‐to‐head' investigations of one active medication against another. A major problem in carrying out trials involving AsPD participants is that this is a challenging group to retain in treatment, as people with AsPD are often treatment‐rejecting rather than treatment‐seeking (NICE 2010). However, this caveat does not apply to those in prison, where there is a large number of individuals incarcerated with AsPD. If this were the population chosen, then reconviction on release ought to be the outcome, as reconviction is a relatively common outcome in many with AsPD; approximately two thirds of those released from prison reoffend within two years (Home Office 1999; ONS 2004). We suggest, therefore, that reconviction is chosen as a primary outcome in such a trial, preferably in conjunction with an economic evaluation. If there was a consensus on a single outcome measured across studies, then it would be possible to make cross‐study comparisons, a task that is difficult to perform at present because of the wide range of outcomes and outcome measures that are used.
What's new
| Date | Event | Description |
|---|---|---|
| 29 June 2020 | New citation required but conclusions have not changed | The addition of 3 new studies has not changed the conclusions of the review. |
| 5 September 2019 | New search has been performed | The review was updated following a new search on 29 September 2016, and top‐up searches on 31 October 2017, 3 October 2018 and 5 September 2019. |
History
Protocol first published: Issue 1, 2009 Review first published: Issue 8, 2010
Notes
None.
Acknowledgements
We gratefully acknowledge: Margaret Anderson (Cochrane Developmental, Psychosocial and Learning Problems) for running the electronic searches and Joanne Duffield (Cochrane Developmental, Psychosocial and Learning Problems) for her ongoing advice and support during the preparation of this review. We would like to acknowledge Nottinghamshire Healthcare NHS Foundation Trust for supporting this work. We would like to thank the previous authors of this review (Jutta M Stoffers‐Winterling, Nick Huband, Michael Ferriter, Klaus Lieb and Conor Duggan) and all of the primary study authors who responded to our requests and provided further information. Finally, we would like to thank the following reviewers for their comments on an earlier version of this update: Gemma L Clayton, Department of Population Health Sciences, Bristol Medical School, University of Bristol, UK; and Odie Gieder, Community Engagement Advisory Network, Vancouver, Canada.
Appendices
Appendix 1. Search strategies
CENTRAL, in the Cochrane Library
Searched 2009 to 3 October 2016 (1371 records) Searched 31 October 2017 (66 records) Searched 3 October 2018 (359 records) Searched 5 September 2019 (296 records)
#1[mh "Antisocial Personality Disorder"] #2[mh ^"personality disorder"] #3(asocial* or antisocial* or anti next social* or dissocial* or dis next social* or dyssocial* or dys next social*) #4(self next defeating or masochistic) #5multi next impulsiv* #6((moral* or amoral or "a‐moral") near/5 (character* or personalit*)) #7[mh ^"Multiple Personality Disorder"] #8[mh Narcissism] #9narciss* #10(sociopath* or socio next path*) #11(psychopath or psychopaths or psychopathic*) #12(psycho next path or psycho next paths or psycho next pathic*) #13[mh sadism] #14sadis* #15(self next defeating or masochist*) #16[mh "Disruptive, Impulse Control, and Conduct Disorders"] #17[mh Aggression] #18[mh "Impulsive behavior"] #19((aggress* or deceitful* or impulsiv* or irritab* or reckless*) near/5 (person* or disorder*)) #20"Cluster B" #21"F60.2" #22"301.7" #23{or #1‐#22} Publication Year from 2009 to 2016, in Trials #24{or #1‐#22} Publication Year from 2016 to 2017, in Trials #25{or #1‐#22} Publication Year from 2017 to 2018, in Trials #26{or #1‐#22} Publication Year from 2018 to 2019, in Trials
MEDLINE Ovid
Searched 2009 to 29 September 2016 (3988 records) Searched 31 October 2017 (635 records) Searched 3 October 2018 (614 records) Searched 5 September 2019 (525 records)
1 Antisocial Personality Disorder/ 2 personality disorders/ 3 (asocial$ or antisocial$ or anti‐social$ or dissocial$ or dis‐social$ or dyssocial$ or dys‐social$).tw,kf. 4 (self‐defeating or masochistic).tw,kf. 5 multi‐impulsiv$.tw,kf. 6 ((moral$ or amoral or "a‐moral") adj5 (character$ or personalit$)).tw,kf. 7 Multiple Personality Disorder/ 8 Narcissism/ 9 narciss$.tw,kf. 10 (sociopath$ or socio‐path$).tw,kf. 11 (psychopath$2 or psycho‐path$2).tw,kf. 12 sadism/ 13 sadis$.tw,kf. 14 (self‐defeating or masochist$).tw,kf. 15 "Disruptive, Impulse Control, and Conduct Disorders"/ 16 Aggression/ 17 Impulsive behavior/ 18 ((aggress$ or deceitful$ or impulsiv$ or irritab$ or reckless$) adj5 (person$ or disorder$)).tw,kf. 19 Cluster B.tw,kf. 20 "F60.2".tw,kf. 21 "301.7".tw,kf. 22 or/1‐21 23 randomized controlled trial.pt. 24 controlled clinical trial.pt. 25 randomi#ed.ab. 26 placebo$.ab. 27 drug therapy.fs. 28 randomly.ab. 29 trial.ab. 30 groups.ab. 31 or/23‐30 32 exp animals/ not humans.sh. 33 31 not 32 34 22 and 33 35 limit 34 to yr="2009 ‐Current" 36 limit 34 to ed=20160901‐20171019 37 limit 34 to ed=20171020‐20180920 38 limit 34 to ed=20180921‐20190829
MEDLINE In‐Process & Other Non‐Indexed Citations Ovid
Searched 30 September 2016 (840 records) Searched 31 October 2017 (471 records) Searched 3 October 2018 (474 records) Searched 5 September 2019 (514 records)
1 (asocial$ or antisocial$ or anti‐social$ or dissocial$ or dis‐social$ or dyssocial$ or dys‐social$).tw,kf. 2 (self‐defeating or masochistic).tw,kf. 3 multi‐impulsiv$.tw,kf. 4 ((moral$ or amoral or "a‐moral") adj5 (character$ or personalit$)).tw,kf. 5 narciss$.tw,kf. 6 (sociopath$ or socio‐path$).tw,kf. 7 (psychopath$2 or psycho‐path$2).tw,kf. 8 sadis$.tw,kf. 9 (self‐defeating or masochist$).tw,kf. 10 Cluster B.tw,kf. 11 "F60.2".tw,kf. 12 "301.7".tw,kf. 13 ((aggress$ or deceitful$ or impulsiv$ or irritab$ or reckless$) adj5 (disorder$ or person$)).tw,kf. 14 or/1‐13 15 (random$ or trial$ or control$ or group$ or placebo$ or blind$ or prospectiv$ or longitudinal$ or meta‐analys$ or systematic review$).tw,kf. 16 14 and 15
MEDLINE Epub Ahead of Print Ovid
Searched 30 September 2016 (381 records) Searched 31 October 2017 (175 records) Searched 3 October 2018 (171 records) Searched 5 September 2019 (187 records)
1 (asocial$ or antisocial$ or anti‐social$ or dissocial$ or dis‐social$ or dyssocial$ or dys‐social$).tw,kf. 2 (self‐defeating or masochistic).tw,kf. 3 multi‐impulsiv$.tw,kf. 4 ((moral$ or amoral or "a‐moral") adj5 (character$ or personalit$)).tw,kf. 5 narciss$.tw,kf. 6 (sociopath$ or socio‐path$).tw,kf. 7 (psychopath$2 or psycho‐path$2).tw,kf. 8 sadis$.tw,kf. 9 (self‐defeating or masochist$).tw,kf. 10 Cluster B.tw,kf. 11 "F60.2".tw,kf. 12 "301.7".tw,kf. 13 ((aggress$ or deceitful$ or impulsiv$ or irritab$ or reckless$) adj5 (disorder$ or person$)).tw,kf. 14 or/1‐13 15 (random$ or trial$ or control$ or group$ or placebo$ or blind$ or prospectiv$ or longitudinal$ or meta‐analys$ or systematic review$).tw,kf. 16 14 and 15
Embase Ovid
Searched 2009 to 30 September 2016 (3060 records) Searched 2016 to November 2017 (219 records) Searched 2017 to 3 October 2018 (344 records) Searched 2018 to 5 September 2019 (382 records)
1 Antisocial Personality Disorder/ 2 *Personality disorder/ 3 ((asocial$ or antisocial$ or anti‐social$ or dissocial$ or dis‐social$ or dyssocial$ or dys‐social$) adj5 (person$ or disorder$)).tw,kw. 4 (self‐defeating or masochistic).tw,kw. 5 ((moral$ or amoral or "a‐moral") adj5 (character$ or personalit$)).tw,kw. 6 multiple personality/ 7 narcissism/ 8 narciss$.tw,kw. 9 (sociopath$ or socio‐path$).tw,kw. 10 psychopathy/ 11 (psychopath$2 or psycho‐path$2).tw,kw. 12 sadism/ 13 sadis$.tw,kw. 14 masochism/ 15 (self‐defeating or masochist$).tw,kw. 16 impulse control disorder/ 17 *impulsiveness/ 18 *Aggression/ 19 ((aggress$ or deceitful$ or impulsiv$ or irritab$ or reckless$) adj5 (person$ or disorder$)).tw,kw. 20 Cluster B.tw,kw. 21 "F60.2".tw,kw. 22 "301.7".tw,kw. 23 or/1‐22 24 Randomized controlled trial/ 25 controlled clinical trial/ 26 Single blind procedure/ 27 Double blind procedure/ 28 triple blind procedure/ 29 Crossover procedure/ 30 (crossover or cross‐over).tw. 31 ((singl$ or doubl$ or tripl$ or trebl$) adj1 (blind$ or mask$)).tw. 32 Placebo/ 33 placebo.tw. 34 prospective.tw. 35 factorial$.tw. 36 random$.tw. 37 assign$.ab. 38 allocat$.tw. 39 volunteer$.ab. 40 or/24‐39 41 23 and 40 42 limit 41 to yr="2009 ‐Current" 43 remove duplicates from 42 44 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 45 human/ or normal human/ or human cell/ 46 44 and 45 47 44 not 46 48 43 not 47
CINAHL Plus EBSCOhost
Searched 2009 to 3 October 2016 (2426 records) Searched 2016 to 2 November 2017 (199 records) Searched 2017 to3 October 2018 (714 records) Searched 2018 to 5 September 2019 (422 records)
S1(MH "Antisocial Personality Disorder") S2(MH "Personality Disorders") S3(asocial* or antisocial* or anti‐social* or dissocial* or dis‐social* or dyssocial* or dys‐social*) S4multi‐impulsiv* S5((moral* or amoral or "a‐moral") N5 (character* or personalit*)) S6(MH "Multiple‐Personality Disorder") S7(MH "Narcissism") S8narciss* S9(sociopath* or socio‐path*) S10(psychopath or psychopaths or psychopathic or psycho‐path*) S11sadis* S12(MH "Disruptive Behavior") S13(MH "Aggression") S14MH social behavior disorders S15(MH "Deception") S16((aggress* or deceitful* or impulsiv* or irritab* or reckless*) N5 (person* or disorder*)) S17"Cluster B" S18"F60.2" S19"301.7" S20S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 S21(MH "Clinical Trials+") S22MH random assignment S23(MH "Meta Analysis") S24(MH "Crossover Design") S25(MH "Quantitative Studies") S26PT randomized controlled trial S27PT Clinical trial S28(clinical trial*) or (control* N2 trial*) S29("follow‐up study" or "follow‐up research") S30(prospectiv* study or prospectiv* research) S31(evaluat* N2 study or evaluat* N2 research) S32(MH "Program Evaluation") S33(MH "Treatment Outcomes") S34TI(single N2 mask* or single N2 blind*) OR AB(single N2 mask* or single N2 blind*) S35TI((doubl* N2 mask*) or (doubl* N2 blind*)) OR AB((doubl* N2 mask*) or (doubl* N2 blind*)) S36TI ((tripl* N2 mask*) or (tripl* N2 blind*)) or ((trebl* N2 mask*) or (trebl* N2 blind*)) OR AB((tripl* N2 mask*) or (tripl* N2 blind*)) or ((trebl* N2 mask*) or (trebl* N2 blind*) S37random* S38S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 S39S20 AND S38
PsycINFO OVID
Searched 2009 to 30 September 2016 (6366 records) Searched 2016 to 2 November 2017.(1072 records) Searched 2017 to 3 October 2018 (704 records) Searched 2018 to 5 September 2019 (684 records)
1 Antisocial Personality Disorder/ 2 *Personality Disorders/ 3 ((asocial$ or antisocial$ or anti‐social$ or dissocial$ or dis‐social$ or dyssocial$ or dys‐social$) adj5 (person$ or disorder$)).tw,id. 4 (self‐defeating or masochistic).tw,id. 5 ((moral$ or amoral or "a‐moral") adj5 (character$ or personalit$)).tw,id. 6 Dissociative Identity Disorder/ 7 NARCISSISM/ 8 narciss$.tw,id. 9 (sociopath$ or socio‐path$).tw,id. 10 psychopathy/ 11 (psychopath$2 or psycho‐path$2).tw,id. 12 Sadism/ 13 sadis$.tw,id. 14 MASOCHISM/ ) 15 Self‐Defeating Behavior/ 16 (self‐defeating or masochist$).tw,id. 17 exp Impulse Control Disorders/ 18 Impulsiveness/ 19 Aggressiveness/ 20 *Aggressive behavior/ 21 ((aggress$ or deceitful$ or impulsiv$ or irritab$ or reckless$) adj5 (person$ or disorder$)).tw,id. 22 Cluster B.tw,id. 23 "F60.2".tw,id. 24 "301.7".tw,id. 25 or/1‐24 26 clinical trials/ 27 longitudinal studies/ 28 exp program evaluation/ 29 exp Treatment Effectiveness Evaluation/ 30 random$.tw. 31 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).tw. 32 (crossover$ or "cross over$").tw. 33 trial$.tw. 34 group$.ab. 35 treatment effectiveness evaluation/ 36 ((singl$ or doubl$ or tripl$ or trebl$) adj1 (blind$ or mask$)).tw. 37 prospective.tw. 38 factorial$.tw. 39 (assign$ or allocat$).ab. 40 control.ab. 41 placebo.ab. 42 (crossover or cross‐over).tw. 43 or/26‐42 44 25 and 43
Science Citation Index Web of Science
Searched 2019 to 3 October 2016 (1233 records) Searched 2016 to 2 November 2017 (198 records) Searched 2017 to 3 October 2018 (181 records) Searched 2018 to 5 September 2019 (170 records)
#14 #13 AND #12 DocType=All document types; Language=All languages; #13 TS=(random* or trial* or control* or group* or placebo* or blind* or prospectiv* or longitudinal* or meta‐analys* or systematic review*) DocType=All document types; Language=All languages; #12 #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #2 OR #1 DocType=All document types; Language=All languages; #11 TS="301.7" DocType=All document types; Language=All languages; #10 TS="F60.2" DocType=All document types; Language=All languages; #9 TS=("Cluster B" and (person* or trait* or character*)) DocType=All document types; Language=All languages; #8 TS=sadis* DocType=All document types; Language=All languages; #7 TS=(psychopath or psychopaths or psychopathic ) DocType=All document types; Language=All languages; #6 TS=(sociopath* or socio‐path*) DocType=All document types; Language=All languages; #5 TS=narciss* DocType=All document types; Language=All languages; #4 TS=((moral* or amoral or "a‐moral") near/5 (character* or personalit*)) DocType=All document types; Language=All languages; #3 TS=multi‐impulsiv* DocType=All document types; Language=All languages; #2 TS=(self‐defeating or masochistic) DocType=All document types; Language=All languages; #1 TS= ((asocial* or antisocial* or anti‐social* or dissocial* or dis‐social* or dyssocial* or dys‐social*) NEAR/5 (person* )) DocType=All document types; Language=All languages;
Social Science Citation Index Web of Science
Searched 2019 to 3 October 2016 (2119 records) Searched 2016 to 2 November 2017 (386 records) Searched 2017 to 3 October 2018 (378 records) Searched 2018 to 5 September 2019 (363 records)
#14 #13 AND #12 DocType=All document types; Language=All languages; #13 TS=(random* or trial* or control* or group* or placebo* or blind* or prospectiv* or longitudinal* or meta‐analys* or systematic review*) DocType=All document types; Language=All languages; #12 #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #2 OR #1 DocType=All document types; Language=All languages; #11 TS="301.7" DocType=All document types; Language=All languages; #10 TS="F60.2" DocType=All document types; Language=All languages; #9 TS=("Cluster B" and (person* or trait* or character*)) DocType=All document types; Language=All languages; #8 TS=sadis* DocType=All document types; Language=All languages; #7 TS=(psychopath or psychopaths or psychopathic ) DocType=All document types; Language=All languages; #6 TS=(sociopath* or socio‐path*) DocType=All document types; Language=All languages; #5 TS=narciss* DocType=All document types; Language=All languages; #4 TS=((moral* or amoral or "a‐moral") near/5 (character* or personalit*)) DocType=All document types; Language=All languages; #3 TS=multi‐impulsiv* DocType=All document types; Language=All languages; #2 TS=(self‐defeating or masochistic) DocType=All document types; Language=All languages; #1 TS= ((asocial* or antisocial* or anti‐social* or dissocial* or dis‐social* or dyssocial* or dys‐social*) NEAR/5 (person* )) DocType=All document types; Language=All languages;
Conference Proceedings Citation Indexes ‐ Science, ‐ Social Science & Humanities Web of Science
Searched 2019 to 3 October 2016 (19 records) Searched 2016 to 2 November 2017 (17 records) Searched 2017 to 3 October 2018 (18 records) Searched 2018 to 5 September 2019 (18 records)
#14 #13 AND #12 DocType=All document types; Language=All languages; #13 TS=(random* or trial* or control* or group* or placebo* or blind* or prospectiv* or longitudinal* or meta‐analys* or systematic review*) DocType=All document types; Language=All languages; #12 #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #2 OR #1 DocType=All document types; Language=All languages; #11 TS="301.7" DocType=All document types; Language=All languages; #10 TS="F60.2" DocType=All document types; Language=All languages; #9 TS=("Cluster B" and (person* or trait* or character*)) DocType=All document types; Language=All languages; #8 TS=sadis* DocType=All document types; Language=All languages; #7 TS=(psychopath or psychopaths or psychopathic ) DocType=All document types; Language=All languages; #6 TS=(sociopath* or socio‐path*) DocType=All document types; Language=All languages; #5 TS=narciss* DocType=All document types; Language=All languages; #4 TS=((moral* or amoral or "a‐moral") near/5 (character* or personalit*)) DocType=All document types; Language=All languages; #3 TS=multi‐impulsiv* DocType=All document types; Language=All languages; #2 TS=(self‐defeating or masochistic) DocType=All document types; Language=All languages; #1 TS= ((asocial* or antisocial* or anti‐social* or dissocial* or dis‐social* or dyssocial* or dys‐social*) NEAR/5 (person* )) DocType=All document types; Language=All languages;
Sociological Abstracts Proquest
Searched 2009 to 3 October 2016 (878 records) Searched 2016 to 2 November 2017 (87 records) Searched 2017 to 3 October 2018(89 records) Searched 2018 to 5 September 2019. (86 records)
(SU.EXACT("Personality Disorders") OR SU.EXACT("Sociopathic Personality") OR TI,AB(asocial* or antisocial* or anti‐social* or dissocial* or dis‐social* or dyssocial* or dys‐social*) OR TI,AB(self‐defeating or masochistic*) OR TI,AB( narciss* or sociopath* or socio‐path* or psychopath* or sadis*) OR TI,AB((aggress* or deceitful* or impulsiv* or irritab* or reckless*) NEAR/5 (person* or disorder*)) OR TI,AB("Cluster B" or "F60.2" or "301.7")) AND (SU.EXACT("Random Samples") OR SU.EXACT("Effectiveness") OR SU.EXACT("Intervention") OR SU.EXACT("Treatment Outcomes") OR SU.EXACT("Evaluation Research") OR SU.EXACT("Program Evaluation") OR SU.EXACT("Comparative Analysis") OR TI,AB(random* OR trial* OR control* OR placebo OR intervention* OR treat* OR evaluat* ))
Criminal Justice Abstracts EBSCOhost
Searched 2009 to 3 October 2016 (1104 records) Searched 2016 to 2 November 2017. Deduplicated with previous records (144 records) Searched 2017 to 3 October 2018 (164 records) Searched 2018 to 5 September 2019 (123 records)
S10 S6 AND S9 S9 S7 OR S8 S8 TI(random* OR control* OR placebo OR intervention* OR treat* OR therap* ) OR AB(random* OR control* OR placebo OR intervention* OR treat* OR therap*) S7 (ZU "randomized controlled trials") or (ZU "randomized controlled trials ‐‐ research") S6 S1 OR S2 OR S3 OR S4 OR S5 S5 ("Cluster B" or "F60.2" or "301.7") S4 (narciss* or sociopath* or "socio‐path*" or psychopath* or sadis*) N5 (person* or disorder*) S3 (self‐defeating or masochistic*) S2 antisocial or anti‐social or dissocial OR "dis‐social" OR dys‐social OR dyssocial S1 (ZU "antisocial personality disorders")
Cochrane Database of Systematic Reviews, part of the Cochrane Library
Searched 3 October 2016 (9 records) Searched 31 October 2017 (1 record) Searched 3 October 2018 (8 records) Searched 5 September 2019 (0 records)
#1[mh "Antisocial Personality Disorder"] #2((asocial* or antisocial* or anti next social* or dissocial* or dis next social* or dyssocial* or dys next social*) next/5 (person* or disorder*)):ti,ab,kw #3(self next defeating or masochistic):ti,ab,kw #4multi next impulsiv*:ti,ab,kw #5((moral* or amoral or "a‐moral") near/5 (character* or personalit*)):ti,ab,kw #6[mh ^"Multiple Personality Disorder"] #7[mh Narcissism] #8narciss*:ti,ab,kw #9(sociopath* or socio next path*):ti,ab,kw #10(psychopath or psychopaths or psychopathic*):ti,ab,kw #11(psycho next path or psycho next paths or psycho next pathic*):ti,ab,kw #12[mh sadism] #13sadis*:ti,ab,kw #14(self next defeating or masochist*):ti,ab,kw #15"Cluster B":ti,ab,kw #16"F60.2":ti,ab,kw #17"301.7":ti,ab,kw #18{or #1‐#17} #19[mh "Disruptive, Impulse Control, and Conduct Disorders"] #20[mh Aggression] #21[mh "Impulsive behavior"] #22((aggress* or conduct* or deceitful* or disruptiv* or impulsiv* or irritab* or reckless*) next/5 (person* or disorder*)):ti,ab,kw in Cochrane Reviews (Reviews and Protocols) #23{or #19‐#22} #24[mh ^"personality disorders"] #25(personalit* near/3 disorder*):ti,ab,kw #26#24 or #25 #27#23 and #26 #28#18 or #27 Publication Year from 2009 to 2016, in Cochrane Reviews (Reviews and Protocols) and Other Reviews #29#18 or #27 Publication Year from 2016 to 2017, in Cochrane Reviews (Reviews and Protocols) #30#18 or #27 Publication Year from 2017 to 2018, in Cochrane Reviews (Reviews and Protocols) #31#18 or #27 Publication Year from 2018 to 2019, in Cochrane Reviews (Reviews and Protocols)
Database of Abstracts of Reviews of Effects, part of the Cochrane Library
Searched 2009 to 2016 (5 records). Final issue. No new content added after this issue.
#1[mh "Antisocial Personality Disorder"] #2((asocial* or antisocial* or anti next social* or dissocial* or dis next social* or dyssocial* or dys next social*) next/5 (person* or disorder*)):ti,ab,kw #3(self next defeating or masochistic):ti,ab,kw #4multi next impulsiv*:ti,ab,kw #5((moral* or amoral or "a‐moral") near/5 (character* or personalit*)):ti,ab,kw #6[mh ^"Multiple Personality Disorder"] #7[mh Narcissism] #8narciss*:ti,ab,kw #9(sociopath* or socio next path*):ti,ab,kw #10(psychopath or psychopaths or psychopathic*):ti,ab,kw #11(psycho next path or psycho next paths or psycho next pathic*):ti,ab,kw #12[mh sadism] #13sadis*:ti,ab,kw #14(self next defeating or masochist*):ti,ab,kw #15"Cluster B":ti,ab,kw #16"F60.2":ti,ab,kw #17"301.7":ti,ab,kw #18{or #1‐#17} #19[mh "Disruptive, Impulse Control, and Conduct Disorders"] #20[mh Aggression] #21[mh "Impulsive behavior"] #22((aggress* or conduct* or deceitful* or disruptiv* or impulsiv* or irritab* or reckless*) next/5 (person* or disorder*)):ti,ab,kw in Cochrane Reviews (Reviews and Protocols) #23{or #19‐#22} #24[mh ^"personality disorders"] #25(personalit* near/3 disorder*):ti,ab,kw #26#24 or #25 #27#23 and #26 #28#18 or #27 Publication Year from 2009 to 2016, in Other Reviews
ClinicalTrials.gov (www.clinicaltrials.gov/ct2/home)
Searched 3 October 2016 (14 records) Searched 3 November 2017 for trials registered between 1 October 2016 and 3 November 2017 (1 record) Searched 4 October 2018 for trials registered between 3 November 2017 and 4 October 2018(3 records) Searched 5 September 2019 for trials registered between 4 October 2018 and 5 September 2019 (3 records)
antisocial personality disorder | Interventional Studies
WHO ICTRP (apps.who.int/trialsearch/AdvSearch.aspx)
Searched all years 3 October 2016 (41 records) Searched 3 November 2017 for trials registered between 1 October 2016 and 3 November 2017 (5 records) Searched 4 October 2018 for trials registered between 3 November 2017 and 4 October 2018 (10 records) Searched 5 September 2019 for trials registered between 4 October 2018 and 5 September 2019 (3 records)
antisocial personality OR antisocial AND disorder OR antisocial AND behaviour
WorldCat (theses only; www.worldcat.org)
Searched 3 October 2016 (6 records) Searched 2016 to 31 October 2017 (3 records) Searched 2017 to 4 October 2018 (1 record) Searched 2018 to 5 September 2019 (3 records)
KW: ("ANTISOCIAL PERSONALITY DISORDER" OR "ANTI‐SOCIAL PERSONALITY DISORDER ") AND KW:(TREAT* OR RANDOM* OR THERAP* OR INTERVENTION*)
Appendix 2. Data extraction sheet
Pharmacological interventions for antisocial personality disorder
Source
| Corresponding number on journal article | Trial ID (e.g. Plizska 2000) |
| Trial registry with ID (search www.clinicaltrials.gov from 2008 and apps.who.int/trialsearch/Default.aspx from 2004) | |
| Full citation | |
| Form filled by (date, name) | |
| Author contact information | |
| Other publications on same study | |
| Publication type | |
| Country of origin |
Eligibility
| Confirm eligibility: yes/no/awaiting |
| At least 5 or more ASPD participants: yes/no |
| ASPD: Antisocial personality disorder |
Correspondence
| Correspondence required: yes/no |
Method
| Corresponding number on journal article | How randomized (individual/cluster)? | Number of participants receiving: Intervention = Control = |
| Location (e.g. hospital, out clinic) | ||
| Summary (method) | A X‐week trial with X arms: |
| Methods | Allocation: Blinding: Duration of trial: Duration of participation: Setting: Phases: Intended follow‐up period: Validated instruments used: Unvalidated instruments used: |
| Participants | Number of participants screened: |
|
Control group Method of recruitment of participants: Number of participants included: (male, female) Number of participants followed up: Number of withdrawals: (reason) Diagnosis of ASPD: DSM/ICD Means of assessment: Age: mean years (range) IQ: Medication naive: % Ethnicity: Pre‐existing substance misuse: specify if drugs/alcohol Other comorbid diagnoses: Comedication: | |
|
Experimental group Method of recruitment of participants: Number of participants included: (male, female) Number of participants followed up: Number of withdrawals: (reason) Diagnosis of ASPD: DSM/ICD Means of assessment: Age: mean years (range) IQ: Medication naive: % Ethnicity: Pre‐existing substance misuse: Other comorbid diagnoses: Comedication: | |
| Inclusion criteria met | |
| Exclusion criteria met | |
| Interventions |
Experimental group Medication name: Medication type: (e.g. neuroleptic/antipsychotic) No. randomised to group: Mean medication dosage: Mode of delivery: Administration schedule: Duration: days/weeks/months Level of therapeutic dose (is treatment dose >/< than this?): Washout before study initiation: hours before testing¨ Titration period: duration Adherence to treatment regime: |
|
Control/comparison group Comparison name: Medication type (if applicable): No. randomised to group: Mean medication dosage: Mode of delivery: Administration schedule: Duration: days/weeks/months Washout before study initiation: hours before testing¨ Titration period: duration Adherence to treatment regime: | |
| Outcomes (if possible, identify if outcomes are immediate (within 6 months), short term (> 6 months to 24 months), medium term (> 24 months to 5 years) and long term (beyond 5 years)) |
Primary
|
Secondary
| |
| Statistical results (reported means, standard deviation, standard errors, confidence intervals, F values or P values and range) for key variables: |
| Notes | Sample size calculation: |
| Power: under/adequately powered | |
| Ethics approval: | |
| Comments from study authors | |
| Limitations of study | |
| Strengths of study | |
| Key conclusion of study authors | |
| Supplemental information regarding data received through personal email correspondence with the authors in month/year. |
| Any additional comments you would like to make about this study: |
Risk of bias
| Item | Quote to support decision | Risk of bias (high, unclear, low) |
| Random sequence generation (selection bias)/generation of allocation sequence | ||
| Allocation concealment (selection bias) | ||
| Blinding of personnel to intervention received | ||
| Blinding of participants to intervention received | ||
| Blinding of participants and personnel (performance bias) | ||
| Blinding of outcome assessment (detection bias) | ||
| Incomplete outcome data (ITT, imputation method) (attrition bias) | ||
| Selective outcome reporting (according to protocol?) | ||
| Vested interest (funding or author affiliations, or both) | ||
| Publication bias | ||
| Language bias | ||
| Other sources of bias | ||
| ITT: Intention to treat | ||
Data and analyses
Comparison 1. Phenytoin (antiepileptic) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Adverse events: nausea | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Desipramine (antidepressant) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Leaving the study early | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Amantadine (dopamine agonist) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Leaving the study early | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by year]
Arndt 1994.
| Study characteristics | ||
| Methods | Design: placebo‐controlled parallel trial | |
| Participants |
Participants: methadone‐maintained male outpatients with cocaine dependency (AsPD subgroup) Sex: male only Age: whole sample mean = 40.5 years (range = 29 to 59 years) Unit of allocation: individual participant Number randomised: 79 (number with AsPD not reported) (see note 1) Number completing: 29 with AsPD (desipramine = 17, control = 12) (see note 1) Setting: outpatient; single site; Philadelphia, USA Inclusion criteria: cocaine dependence lasting at least 3 months (DSM‐III; National Institute Mental Health Diagnostic Interview Schedule); aged 20 to 60 years; cocaine‐positive urine over 1 month prior to being contacted for participation Exclusion criteria: medical condition contraindicating desipramine use; cocaine misuse disorder lasting less than 3 months Ethnicity: whole sample = 90% black American Baseline characteristics: whole sample = male service veterans (100%); on methadone maintenance for at least 1 month (100%); average methadone dose 45 mg/day (range = 15‐85 mg/day); reported using cocaine intravenously (83%); reported 'free basing' (15%); reported intranasal use (11%); employed (79%); educated to high school degree level (53%); some college education (29%); married (35%); never married (35%); separated or divorced (30%) |
|
| Interventions | Two conditions:
All participants received standard clinical services including weekly drug counselling, social work services as needed, employment counselling, referral psychiatric and medical care Duration of intervention: 12 weeks Duration of trial: 12 weeks Length of follow‐up: participants were not followed up beyond the study period Dose adjustment: desipramine 50 mg/day initially increased by 50 mg every 2 to 4 days as tolerated to a target dose of 250 to 300 mg/day; mean blood levels 185 mg/ml (range 85 to 270 mg/ml) |
|
| Outcomes |
Primary outcomes Social functioning: days family/social problems in past 30 days (Addiction Severity Index; ASI) Secondary outcomes Substance misuse: urinalysis; days opiate use in past 30 days via the ASI; days cocaine use in past 30 days (ASI); days depressant use in past 30 days (ASI); cocaine craving score (see note 2) Employment status: days worked in past 30 days (ASI); employment income (ASI) Other outcomes Depression (Beck Depression Inventory; BDI); days medical problems in past 30 days (ASI); days illegal activity in past 30 days (ASI); illegal income (ASI); days psychological problems past 30 days (ASI) Timing of outcome assessments Weekly urinalysis; biweekly blood assay; measures such as ASI at baseline, 4,8 and 12 weeks. |
|
| Notes |
Study funding: National Institute on Drug Abuse (USA) Declaration of interests: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information given. Clarification about method of sequence generation has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information given. Clarification about method of sequence generation has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Blinding (performance bias and detection bias) of participants | Unclear risk | Comment: Investigators described the study as "double‐blind" and report that an independent researcher "gave directions for changing the dose given to the patients receiving placebo so that the double‐blind condition was maintained" (Arndt 1992, p.889, column 2). No further information given. |
| Blinding (performance bias and detection bias) of personnel | Low risk | Comment: Investigators reported that "study physicians were blind to the blood level results [which were] provided to an independent research physician who recommended increases or decreases to achieve the desirable blood level" (Arndt 1994, p.152, column 2), and also that the BDI outcome measure was "administered by trained research technicians who were experimentally blind and independent of the treatment program" (Arndt 1992, p.889, column 1). Review authors judged that appropriate care was taken to ensure blinding of study personnel, and that it was unlikely that this blinding could have been broken. |
| Blinding (performance bias and detection bias) of outcome assessors | Low risk | Comment: Investigators reported that "study physicians were blind to the blood level results [which were] provided to an independent research physician who recommended increases or decreases to achieve the desirable blood level" (Arndt 1994, p.152, column 2), and also that the BDI outcome measure was "administered by trained research technicians who were experimentally blind and independent of the treatment program" (Arndt 1992, p.889, column 1). Review authors judged that appropriate care was taken to ensure blinding of outcome assessors, and that it was unlikely that this blinding could have been broken. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Comment: Incomplete outcome data arise from participants not completing the study and applies to all measured outcomes. Investigators did not provide numbers of non‐completers for the AsPD subgroup, but supplied the following data for the whole sample. For the desipramine group, 17 out of 53 (32%) discontinued because of side effects (4), non‐compliance with the protocol (4), hospitalisation unrelated to desipramine treatment (3), legal violations (3), or for other reasons (3). For the control group, 3 out of 26 (12%) discontinued (reasons not given). Numbers of missing data are thus not balanced between experimental conditions for the whole sample. Clarification has been requested from the trial investigators but no further information was available at the time this review was prepared. In this review, data from the 29 participants with AsPD who completed the study were included in the analysis (desipramine condition, n = 17; control condition, n = 12). |
| Selective reporting (reporting bias) | Unclear risk | Comment: The study protocol is not available; however, the published report included all expected outcomes, including those that were pre‐specified in the methods. |
| Other bias | Low risk | Comment: The study appeared to have no other obvious sources of bias. |
Ralevski 2007.
| Study characteristics | ||
| Methods | Design: placebo‐controlled parallel trial | |
| Participants |
Participants: treatment‐seeking adults with alcohol dependency and a current Axis I disorder (subgroup with AsPD) Sex: AsPD subgroup = 93 male, 2 female Age: AsPD subgroup mean = 44.2 (SD = 6.6) years Unit of allocation: individual participant Number randomised: AsPD subgroup = 95 (numbers allocated to treatment and control conditions not reported; see note 1) Number completing: unclear Setting: outpatient; 3 sites; USA (New England) Inclusion criteria: whole sample (see note 1) = treatment‐seeking; alcohol dependency with at least one other current Axis I disorder (DSM‐IV; SCID‐I); alcohol use within the past 30 days; stable dose of psychiatric medication for at least 2 weeks if on medication. Additionally for AsPD subgroup = presence of AsPD diagnosis (DSM‐IV; SCID‐II) Exclusion criteria: unstable psychotic symptoms; serious current psychiatric symptoms such as suicidal or homicidal ideation; current opiate dependence; contraindication to the use of naltrexone and disulfiram, including liver function tests greater than 3 times the normal Ethnicity: AsPD subgroup = 72.6% White (n = 69); 14.7% Black (n = 14); 7.4% Hispanic (n = 7); 4.2% native American (n = 4); 1% other (n = 1) Baseline characteristics: AsPD subgroup = all veterans; mean duration of alcohol use = 25.3 (SD = 8.9) years; mean number of drinking days in last 30 days = 14.3 (SD = 12.3) days; mean total number of drinks in last 30 days = 326.5 (SD =338.7); mean number of heavy drinking days in last 30 days = 13.4 (SD = 12.1); mean baseline on Alcohol Dependence Scale (ADS) score = 23.5 (SD = 7.8); any psychiatric medication (82.3%, n = 79); antidepressants (58.5%, n = 55); anxiolytics (6.3%, n = 6); mood stabilizers (40.6%, n = 39); antipsychotics (22.9%, n = 22); taking more than one medication (43.7%, n = 42) |
|
| Interventions | Two conditions (see note 2):
All participants received weekly clinical management or compliance enhancement therapy focused on discussing negative consequences of drinking, relapse prevention, compliance monitoring and psychoeducation plus treatment as usual (rehabilitation with aftercare and supported housing options) Duration of intervention: 12 weeks Duration of trial: 12 weeks (no washout period) Length of follow‐up: participants were not followed up beyond the end of the intervention period Dose adjustment: no information |
|
| Outcomes |
Primary outcomes Adverse events: Hopkins Symptom Checklist (self‐report) (described in Petrakis 2005 (p.1130, col 1) but no details reported for AsPD subgroup) Secondary outcomes Substance misuse: Alcohol use (Timeline Follow‐Back Interview (TLFB)); Alcohol craving (Obsessive Compulsive Drinking Scale (OCDS)) Leaving the study early: treatment retention Timing of outcome assessments TLFB and OCDS assessed weekly for 12 weeks |
|
| Notes |
Study funding: Veterans Affairs Merit Grant and the VA New England VISN I Mental Illness Research and Clinical Center (USA) Declaration of interests: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No information provided. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information provided on how allocation sequence was concealed. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Blinding (performance bias and detection bias) of participants | Unclear risk | Comment: Trial investigators reported that naltrexone was given in a double‐blinded fashion, but provided no further information. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Blinding (performance bias and detection bias) of personnel | Unclear risk | Comment: Trial investigators reported that naltrexone was given in a double‐blinded fashion, but provided no further information. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | Comment: Trial investigators reported that naltrexone was given in a double‐blinded fashion, but provided no further information. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Incomplete outcome data arise from participants not completing the study and applies to all measured outcomes. Trial investigators provided no information on the numbers randomised to each condition, nor on the extent of missing data. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Selective reporting (reporting bias) | Unclear risk | Comment: A companion paper (Petrakis 2005) indicated that adverse events were measured weekly via the Hopkins Symptom Checklist, but these are not reported here or in that paper. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Other bias | Low risk | Comment: The study appeared free from other sources of potential bias, although the trial investigators acknowledge the possibility of bias arising from the confounding effects of Axis I disorders and their inability to test whether improvement in personality disorder symptoms were related to medication treatment. |
Barratt 1997.
| Study characteristics | ||
| Methods | Design: placebo‐controlled, cross‐over trial | |
| Participants |
Participants: male prisoners with recurrent aggressive behaviour Sex: male only Age: adults; age not reported Unit of allocation: individual participant Number randomised: 150 Number completing: 126; results reported for 60 (30 with primarily impulsive aggression and 30 with primarily premeditated aggression; remaining 66 had committed mixed types of aggression and were not included) (see note 1) Setting: prisons; multiple sites; USA (Texas) Inclusion criteria: history of at least 3 documented aggressive acts within prison over a 3‐month period prior to commencing the study. Aggressive acts classified as impulsive and non‐impulsive based on interview and prison reports (see note 2) Exclusion criteria: verbal and performance IQ of less than 80; DSM‐III‐R axis I disorder, as measured by Psychiatric Diagnostic Interview‐revised (PDI‐R); neurological or other serious medical disorder; taking medication Ethnicity: not reported here, but reported in the baseline study paper (Barratt 1997a); African‐American (53%), Hispanic (24%); white (24%) Baseline characteristics: DSM‐III‐R AsPD (100%); history of aggressive behaviour prior to incarceration (98%); participants had mean of 6.2 incidents of aggression recorded against them while in prison; lifetime substance misuse diagnosis (55%). Other baseline measures, including event‐related potentials (ERP), Profile of Mood States (POMS), neuropsychology measures and personality traits are reported in the baseline study paper (Barratt 1997a), although investigators noted there was only a 90% overlap between the 2 studies |
|
| Interventions | Two conditions:
Duration of intervention: 6 weeks for each condition with 1‐week washout between the 2 phases Duration of trial: 13 weeks (cross‐over trial; two phases; 1‐week washout period between phases) Length of follow‐up: participants were not followed up beyond the end of the intervention period Dose adjustment: no information given |
|
| Outcomes |
Primary outcomes Aggression: frequency of aggressive acts; intensity of aggressive acts (modified Overt Aggression Scale) Adverse events: nausea; blood cell counts; liver function tests Secondary outcomes Anger: Profile of Mood States (POMS) anger‐hostility subscale Other outcomes P300 peak amplitude and latency (electroencephalogram, ERP oddball task); phenytoin blood levels (in relation to frequency and intensity of aggressive acts); other subscales of Profile of Mood States (tension‐anxiety, vigour, depression‐dejection, fatigue inertia, confusion bewilderment) Timing of outcome assessments Baseline, then every two weeks |
|
| Notes |
Study funding: Health Foundation; Rogosin Institute; New York Hospital‐Cornell Medical Center (USA) Declaration of interests: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Investigators reported that participants “were randomly assigned to an initial drug/placebo condition” (p 3), suggesting that the order of treatments was randomised in this cross‐over trial. No further details reported. Insufficient information provided to allow judgment to be made. Clarification about method of sequence generation has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details reported. Insufficient information provided to allow judgment to be made. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Blinding (performance bias and detection bias) of participants | Unclear risk | Comment: Investigators describe the study as "double‐blind". No further details reported. Insufficient information provided to allow judgment to be made, although bloods were taken during placebo treatment, possibly to maintain blinding. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Blinding (performance bias and detection bias) of personnel | Unclear risk | Comment: Investigators describe the study as "double‐blind". No further details reported. Insufficient information provided to allow judgment to be made. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | Comment: Investigators describe the study as "double‐blind". No further details reported. Insufficient information provided to allow judgment to be made. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Insufficient reporting of attrition to permit judgement of ‘high’ or ‘low’. Incomplete outcome data arise from participants not completing the study and applies to all measured outcomes. It appears that 24 did not complete study, and a subgroup of 66 (with 'mixed' type of aggression) were excluded by investigators. Clarification has been requested from the trial investigators but no further information was available at the time this review was prepared. In this review, data from the subgroup of 60 completers were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: The study protocol is not available but it seems clear that the published report includes all expected outcomes from the methods. |
| Other bias | Unclear risk | Comment: There was a one‐week placebo washout period between phases in this cross‐over trial and the trial investigators reported no significant cross‐over effects for the aggression measures for the combined groups suggesting the study was not biased by carryover effects. An important source of bias would be the criminogenic programmes delivered in prison if such programmes were delivered in different proportions to each randomised condition, although no information is provided on this. It is also not clear whether participants were engaged in any of these during the study period. It is not clear what effects the exclusion of ‘mixed aggression group’ would have on the results. Of the 150 participants randomised, results were reported for 60 of 126 completers only (30 committing primarily impulsive and 30 committing primarily premeditated aggression; remaining 66 had committed mixed types of aggression and were not included). Thus there is the possibility of bias arising through excluding the ‘mixed aggression’ group, although it is unclear what effect this would have on the results. |
Stanford 2001.
| Study characteristics | ||
| Methods | Design: placebo‐controlled cross‐over trial | |
| Participants |
Participants: men with DSM‐IV personality disorder and impulsive aggressive behaviour (subgroup with AsPD; see note 1) Sex: male only Age: whole sample, including non‐AsPD participants mean = 45.1 (SD = 6.8) years Unit of allocation: individual participant Number randomised: 46 in whole sample, 10 with AsPD (see note 1) Number completing: not reported Setting: outpatient; single site; USA (New Orleans) Inclusion criteria: over past 6 months, several discrete participant‐identified episodes of failure to resist aggressive impulses resulting in serious assaultive acts or destruction of property; degree of aggressiveness expressed during the episodes was grossly out of proportion to any precipitating psychosocial stressor; at least two such episodes during the month prior to entering the study; score of 8 or higher on the Irritability sub scale of the Buss‐Durkee Hostility Inventory (BDHI); must have identified an individual willing to document any impulsive‐aggressive outbursts that occurred during the study Exclusion criteria: female (due to potential teratogenic effects of phenytoin); verbal IQ < 80; diagnosis of a DSM‐IV‐TR axis I psychiatric disorder; present use of medication; medical/neurological problems (including seizures); liver enzymes not within normal limits Ethnicity: not reported Baseline characteristics: mean verbal IQ = 105.8 (SD = 10.7); mean = 14.3 (SD = 2.4) years education; DSM‐IV personality disorder diagnoses for phase 1 completers: obsessive‐compulsive personality disorder (n = 12), AsPD (n = 10), narcissistic personality disorder (n = 1) |
|
| Interventions | Two conditions:
Duration of intervention: 6 weeks Duration of trial: 16 weeks (cross‐over trial; two phases, 2‐week placebo baseline period, and 2‐week placebo washout period between phases) Length of follow‐up: participants were not followed up beyond the end of the intervention period Dose adjustment: no details reported |
|
| Outcomes |
Primary outcomes Aggression (observer‐reported): Overt Aggression Scale (OAS) scores Secondary outcomes Anger‐hostility: Profile of Mood States (POMS) anger‐hostility subscale scores Other outcomes Psychophysiological recordings (including evoked potentials) Timing of outcome assessments POMS assessed at baseline and then every two weeks up to week 16. OAS reported as required within the 16 weeks (i.e. for every impulsive aggressive incident) |
|
| Notes |
Study funding: Dreyfus Health Foundation, The Rogosin Institute, New York Hospital‐Cornell Medical Center (USA) Declaration of interests: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Investigators report that “subjects were randomly assigned” (p.195, col. 2) suggesting that the order of treatments was randomised in this cross‐over trial. Further details obtained from trial investigators (2009 email from M Stanford to J Dennis clarifying trial methods; unreferenced) indicated that sequence generation was achieved by use of computer generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information to allow a judgement to be made. Clarification has been requested from the trial investigators but no further information was available at the time this review was prepared. |
| Blinding (performance bias and detection bias) of participants | Low risk | Comment: Investigators described the study as “double‐blind”. Response from trial investigators suggests that appropriate care was taken to ensure blinding of participants. |
| Blinding (performance bias and detection bias) of personnel | Low risk | Comment: Investigators described the study as “double‐blind”. Response from trial investigators suggests that appropriate care was taken to ensure blinding of personnel. |
| Blinding (performance bias and detection bias) of outcome assessors | Low risk | Comment: Investigators described the study as “double‐blind”. Response from trial investigators suggests that appropriate care was taken to ensure blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Incomplete outcome data arise from participants not completing the study and applies to all measured outcomes. Overall, 17 of 46 were non‐completers and a further 6 were excluded giving a 50% missing data rate. Review authors judged risk of bias to be high pending data from the AsPD subgroup becoming available. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Study protocol is not available but it seems clear that the published report includes all expected outcomes. |
| Other bias | High risk | Comment: The investigators declared their research sponsored by the Dreyfus Health Foundation, which is focused on phenytoin and was established “to study, collect, and disseminate information and sponsor collaborative, clinical, and basic health research on its benefits”. The authors have insufficient information to assess whether this constitutes a risk of bias. The trial investigators reported a two‐week placebo washout period between phases in this cross‐over trial which will have reduced the possibility of carryover effects and the study appeared to be free of other sources of bias. |
Leal 1994.
| Study characteristics | ||
| Methods | Design: placebo‐controlled parallel trial | |
| Participants |
Participants: methadone‐maintained inpatients meeting DSM‐III‐R criteria for opioid and cocaine dependence (non‐depressed AsPD subgroup) Sex: 11 male, 8 female (for the AsPD subgroup) Age: AsPD subgroup mean = 33.0 (SD = 4.5) years Unit of allocation: individual participant Number randomised: 19 (desipramine = 7, amantadine = 8, control = 4) (see note 1) Number completing: 11 (desipramine = 5, amantadine = 3, control = 3) (see note 1) Setting: inpatient; single site; USA (Yale) Inclusion criteria: AsPD diagnosis without depression (DSM‐III‐R; SCID‐II); opioid and cocaine dependency (DSM‐III‐R) Exclusion criteria: concurrent DSM‐III‐R depression; zidovudine treatment for AIDS; medical contra‐indications, including asthma, renal dysfunction, high blood pressure and diabetes; current alcoholism; refusal to use adequate birth control if female Ethnicity: AsPD subgroup = 68% White (n = 13) Baseline characteristics: AsPD subgroup (see note 1) = married (74%, n = 14); mean methadone dose 57 (SD 11) mg/day; mean time on methadone 4.5 (SD 2.7) months; mean time using heroin 12.0 (SD 6.2) years; mean time using cocaine 7.5 (SD 6.1) years; mean expenditure on cocaine 1141 (SD 1379) US Dollars/month; lifetime diagnosis alcohol misuse disorder (58%), mean time intoxicated 1.7 (SD 3.6) days/month; mean Addiction Severity Index factor scores: psychiatric, 4.3 (SD 2.4); medical, 3.3 (SD 2.0); job, 5.9 (SD 2.7); alcohol, 3.5 (SD 2.5); drug, 8.2 (SD 0.7); family, 5.6 (SD 2.1) |
|
| Interventions | Three conditions:
Duration of intervention: 12 weeks Duration of trial: 12 weeks Length of follow‐up: participants were not followed up beyond the end of the intervention period Dose adjustment: no information given |
|
| Outcomes |
Primary outcomes None Secondary outcomes Leaving the study early: treatment retention in the first and the last 6 weeks of treatment Substance misuse: urinalysis to detect cocaine‐free urine samples (on‐site enzyme‐multiplied immunoassay (EMIT) system); total US Dollars/week spent on cocaine (self report) Other outcomes Depression (Beck Depression Inventory) Timing of outcome assessments Biweekly urine toxicology; blood assay at week 4; weekly BDI and self reported spend on drugs |
|
| Notes |
Study funding: National Institute on Drug Abuse (USA) Declaration of interests: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Investigators described a " . . . randomised, double‐blind trial. . ." (p.32, col 2). No further details reported. Insufficient information to permit judgement on adequacy of sequence generation. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Investigators described a " . . . randomised, double‐blind trial. . ." (p.32, col 2). No further details reported. Insufficient information to permit judgement on adequacy of allocation concealment. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Blinding (performance bias and detection bias) of participants | Unclear risk | Comment: Investigators described the trial as " double‐blind " (p.32, col 2). No further details reported. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Blinding (performance bias and detection bias) of personnel | Unclear risk | Comment: Investigators described the trial as " double‐blind " (p.32, col 2). No further details reported. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | Investigators describe the trial as " double‐blind " (p.32, col 2). No further details reported. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Incomplete outcome data arise from participants not completing the study and applies to all measured outcomes. The investigators reported that “fifteen patients left the study for medication non‐compliance, four for incarceration, and one for medical reasons” (p.32, col 2), but these figures apply to the whole sample including non‐AsPD participants. For the AsPD subgroup, 5/8 (63%) were missing from the amantadine group, 2/7 (29%) were missing from the desipramine group and 1/4 (25%) missing from the control group ‐ all for reasons that are unclear and no breakdown by experimental group was provided. Clarification has been requested from the trial investigators but no further information was available at the time this review was prepared. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Study protocol is not available but it seems clear that the published report includes all expected outcomes, including those that were pre‐specified. |
| Other bias | High risk |
Comment: Two potential sources of bias were identified. First, it is not clear whether patients continued to receive general substance misuse counselling and behavioural contingency management during this trial, and if so whether this was similar for both treatment and control conditions. This is important since the latter involves monetary incentives in return for a clean urine sample. Differences in percentages of cocaine‐free urine samples may be related to that rather than the effects of medications. Second, urinalysis was carried out twice weekly, but the detectability window for cocaine is 6‐8 hours (Wolff 1999) which increases the possibility of false negative results. It is noteworthy that four participants meeting criteria for AsPD plus dysthymia were included in the non‐ASP group and so their results are not included; investigators justify this because "the diagnosis of depression has been reported to favourably affect the treatment outcome of patients with antisocial personality disorder" (page 32, col 1). |
Stanford 2005.
| Study characteristics | ||
| Methods | Design: placebo‐controlled parallel trial | |
| Participants |
Participants: men with recurrent impulsive aggressive behaviour (subgroup with AsPD; see note 1) Sex: male only Age: phenytoin mean = 28.7 years; carbamazepine mean = 34.9 years; valproate mean = 33.6 years; placebo mean = 34.8 years (all figures for whole sample, including non‐AsPD participants) Unit of allocation: individual participant Number randomised: 38 in whole sample, 17 with AsPD (see note 1; breakdown by treatment condition not supplied) Number completing: not known Setting: outpatient; single site; USA (vicinity of New Orleans) Inclusion criteria: over past 6 months, several discrete episodes of failure to resist aggressive impulses resulting in serious assaultive acts or destruction of property; degree of aggressiveness expressed during the episodes was grossly out of proportion to any precipitating psychosocial stressor; at least 2 such episodes during the month prior to entering the study; score of 8 or higher on the Irritability subscale of the Buss‐Durkee Hostility Inventory (BDHI); must have identified an individual willing to document any impulsive‐aggressive outbursts that occurred during the study Exclusion criteria: female (due to potential teratogenic effects of phenytoin); verbal IQ < 80; current bipolar disorder; current thought disorder; present use of psychoactive medication; history of medical/neurological problems (including seizures); non‐native English speaker; liver enzymes not within normal limits Ethnicity: not reported Baseline characteristics: for 29 completers overall = at least one Axis I diagnosis (n = 12); major depression (n = 5); alcohol misuse (n = 7); substance misuse (n = 4); at least one Axis II diagnosis (n = 24); AsPD (n = 17); borderline personality disorder (n = 3) |
|
| Interventions | Four conditions:
Duration of intervention: 6 weeks Duration of trial: 8 weeks (treatment preceded by 2‐week placebo‐baseline period) Length of follow‐up: participants were not followed up beyond the end of the intervention period Dose adjustment: not reported. Serum blood levels measured after sixth week of administration |
|
| Outcomes |
Primary outcomes Aggression (observer‐reported): Overt Aggression Scale (OAS) scores, averaged over 4 2‐week periods (placebo‐baseline, 0‐2 weeks, 2‐4 weeks, 4‐6 weeks) Secondary outcomes Leaving the study early: proportion of participants discontinuing treatment Other outcomes None reported Timing of outcome assessments Blood assay at week 6 |
|
| Notes |
Study funding: Dreyfus Health Foundation, The Rogosin Institute, New York Hospital‐Cornell Medical Center (USA) Declaration of interests: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Sequence generation achieved using a random numbers table. Randomisation was therefore true and unlikely to introduce bias. |
| Allocation concealment (selection bias) | Low risk | Comment: Investigators state “anticonvulsants and placebo were administered in identical, unmarked capsules obtained from a local pharmacy” (p.74, col 1). The lead author [MS] “was responsible for the random assignment and the maintenance/administrations of all study medication. He was not involved in participant assessment subsequent to the placebo‐baseline” (p.73, col 2). |
| Blinding (performance bias and detection bias) of participants | Low risk | Comment: Investigators state “anticonvulsants and placebo were administered in identical, unmarked capsules obtained from a local pharmacy” (p.74, col 1). Appropriate care appears to have been taken to ensure blinding of participants. Unlikely that this blinding could have been broken and therefore unlikely that this lead to the introduction of bias. |
| Blinding (performance bias and detection bias) of personnel | Low risk | Comment: Appropriate care appears to have been taken to ensure blinding of personnel. Unlikely that this blinding could have been broken. |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | Comment: Insufficient information to allow a judgement to be made. Clarification has been requested from the trial investigators but no further information was available at the time this review was prepared. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Incomplete outcome data arise from participants not completing the study and applies to all measured outcomes. Overall, 14 of 43 (33%) were non‐completers. This is a large proportion of participants dropping out, review authors therefore judged risk of bias to be high, pending data from the AsPD subgroup becoming available. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Study protocol is not available but it seems clear that the published report includes all expected outcomes, including those that were pre‐specified. |
| Other bias | High risk | Comment: Investigators report that this study was sponsored by the Dreyfus Health Foundation which is focused on phenytoin and, according to its website, was established “to study, collect, and disseminate information and sponsor collaboration, clinical, and basic health research into its [phenytoin’s] benefits”. This raises the potential for bias in a study such as this which compares phenytoin with other anticonvulsants as well as against placebo. |
Powell 1995.
| Study characteristics | ||
| Methods | Design: placebo‐controlled parallel trial | |
| Participants |
Participants: men with alcohol dependence and comorbid psychiatric disorders (subgroup with AsPD) Sex: male only Age: not reported for AsPD subgroup; for the whole sample, mean 41.3 (SD = 9.2) years Unit of allocation: individual participant Number randomised: 65 with AsPD (out of 216; see note 1) Number completing: 29 with AsPD (out of 99; see note 1) Setting: initially inpatient, then outpatient; two sites; USA (Kansas and Topeka) Inclusion criteria: alcohol dependence (DSM‐III‐R; measured using the PDI‐R) Exclusion criteria: medical condition contraindicating use of tricyclic antidepressants or bromocriptine; receiving psychotropic medication (see note 2), living more than 150 miles from treatment site Ethnicity: for the whole sample: white (63%); black (32%); native American (2%); other (3%) Baseline characteristics: for the whole sample: all veterans; college degree (4%); some college or vocational training (39%); high school education or equivalent (47%); less than high school education (10%); married (21%); separated (15%); divorced/widowed (43%); never married (20%); employed full or part time in the month before admission (50%); retired (4%); student (1%); unemployed (45%) |
|
| Interventions | Three conditions:
In analysis, the 2 placebo groups were combined such that total completed = 9 (see note 3) Duration of intervention: 6 months Duration of trial: 6 months Length of follow‐up: participants were not followed up beyond the end of the intervention period Dose adjustment: nortriptyline dose adjusted to therapeutic levels (50 to 150 ng/ml plasma) with corresponding increase in capsules made for placebo patients; bromocriptine initially 2.5 mg 3 times daily, but increased to 5 mg 3 times daily from month 4 to month 6; placebo as matching capsules with number adjusted to match a participant in the active treatment group Other notes: participants reimbursed 15 US Dollars for each clinic visit; payment was not made until the participant either dropped out or completed the study |
|
| Outcomes |
Primary outcomes Global state/functioning: Global Assessment Scale (GAS); global severity index sub scale of the Symptom Check List‐90 (SCL‐90) Adverse effects: medication side effects via participant self‐report Secondary outcomes Substance misuse: severity of alcoholism (Alcohol Severity Scale (ASS), self report); alcohol craving (visual analogue scale); alcohol dependence (Severity of Alcohol Dependence Questionnaire (SADQ)) Other outcomes depression (Beck Depression Inventory; SCL‐90 depression sub scale); anxiety (Beck Anxiety Inventory (BAI); SCL‐90 anxiety sub scale); problem behaviours (Problem Behavior Checklist) Timing of outcome assessments Monthly blood levels; GAS, SCL‐90, ASS, SADQ, BDI at baseline and 6‐month follow‐up |
|
| Notes |
Study funding: National Institute of Alcohol Abuse and Alcoholism (USA) Declaration of interests: The study authors state "We thank Sandoz Pharmaceuticals Corp. for supplying us with Parlodel (bromocriptine mesylate) and Pamelor (nortriptyline HCI*)." (quote, p 468) *HCl = hydrochloride |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: A two‐stage randomization process was used; patients within each of the subgroups (alcoholism alone, alcoholism + mood/anxiety disorders, alcoholism + AsPD) "were first randomly assigned to the bromocriptine or nortriptyline arm of the study. Patients within each drug arm were then randomised to receive either active drug or placebo" (p.463, col 1). No further information given. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Allocation concealment (selection bias) | Unclear risk | Comment: A two‐stage randomization process was used (see above). "Patients within each drug arm were then randomised to receive either active drug or placebo" (p.463, col 1). No further information given. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Blinding (performance bias and detection bias) of participants | Low risk | Comment: Investigators reported that "both active medications and placebo were prepared as identical‐appearing capsules. . . . when the number of pills was increased for a given patient, a corresponding increase in pills was made for a placebo patient" (p.463, col 1). Review authors judged that appropriate care was taken to ensure blinding of participants, and that it was unlikely that this blinding could have been broken. |
| Blinding (performance bias and detection bias) of personnel | Low risk | Comment: Investigators reported that "at each visit, a physician blinded to the patients' treatment assignment obtained blood samples and systematically recorded pill counts, medication side effects and other medical information" (p.463, col 2). Review authors judged that appropriate care was taken to ensure blinding of trial personnel, and that it was unlikely that this blinding could have been broken. |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | Comment: Investigators reported that "a research assistant then recorded number of drinking days and patient rating of alcohol craving since the last follow‐up visit" (p.463, col 2), but there was no indication that this research assistant was blinded, other than the statement that the study was "double‐blind". Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Comment: Incomplete outcome data arise from participants not completing the study and applies to all measured outcomes. The investigators reported significant dropout rates for the comorbidity and medication subgroups which ranged from 52.1% to 55.4%, and note that there were 99 participants (overall) who completed the study and 117 (overall) who did not (p.464, col 1). No details on missing data were provided for the AsPD subgroup. Clarification has been requested from the trial investigators but no further information was available at the time this review was prepared. In this review, data from the 29 participants with AsPD who completed the study were included in the analysis (bromocriptine condition, n = 9; nortriptyline condition, n = 11; control condition, n = 9). |
| Selective reporting (reporting bias) | Unclear risk | Comment: Study protocol is not available but it seems clear that the published report includes all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: The study appeared to be free of other sources of bias. |
Hollander 2003.
| Study characteristics | ||
| Methods | Design: placebo‐controlled parallel trial | |
| Participants |
Participants: adults with impulsive aggression (subgroup with AsPD; see note 1) Sex: mixed (72.5% men in whole sample, including non‐AsPD participants) Age: whole sample, including non‐AsPD participants, mean = 40.3 years (range = 19 to 67 years) Unit of allocation: individual participant Number randomised: 233 in whole sample; 9 with AsPD (see note 1; breakdown by treatment condition not reported) Number completing: not reported Setting: outpatient; 19 sites; USA Inclusion criteria: aged 18 to 65 years; diagnosis of cluster B personality disorder (DSM‐IV; Stuctured Clinical Interview for DSM‐IV (SCID‐II)) or intermittent explosive disorder (IED), or post‐traumatic stress disorder (PTSD); average of two episodes of physical or verbal aggressive outbursts per week for at least a month prior to screening, causing marked distress or impairment in occupational or interpersonal function where the aggressive behaviour was judged to be neither premeditated nor committed to achieve a tangible objective; minimum score of 15 on OAS at first screening visit and at either the second screening visit or at randomization; if receiving psychotherapy, have a stable psychotherapy schedule for at least 3 months prior to screening and maintained throughout the study Exclusion criteria: lifetime bipolar I disorder; bipolar II disorder with hypomania in the last year or a baseline Mania Syndrome Scale Score >= 12; major depressive disorder > 15 on Hamilton Depression Rating scale (HAM‐D); history of schizophrenia or other psychotic disorder; symptoms of dementia; serious homicidal or suicidal ideation; impulsive aggression resulting from previous head trauma or other medical condition; pregnant or lactating females; clinically abnormal laboratory data; unstable medical condition; any underlying condition that would confound the interpretation of study results; concurrent use of psychotropic medication, with exception of selective serotonin reuptake inhibitors (SSRI), tricyclic antidepressants and stimulants if taken at a stable dose for at least 2 months prior to screening and continued at same dose throughout the study; participants specifically prohibited from use of benzodiazepines, mood stabilisers, anticonvulsants, monoamine oxidase inhibitors (MAOI) and antipsychotic agents (see note 2) Ethnicity: 195 Caucasian, 26 black, 12 other (whole sample, including non‐AsPD participants) Baseline characteristics: (for whole sample, including non‐AsPD) at least one psychiatric hospitalisation (n = 36); history of alcohol misuse/dependence (n = 75); history of drug misuse/dependence (n = 38); history of incarceration (n = 52) |
|
| Interventions | Two conditions:
Duration of intervention: 12 weeks Duration of trial: 15 weeks (treatment preceded by screening period not exceeding 14 days and followed by 1 week tapering period) Length of follow‐up: participants were not followed up beyond the end of the intervention period Dose adjustment: initiated at 500 mg/day, and increased by 250 mg every 3 to 7 days during first 3 weeks of treatment, based on individual clinical response and tolerance. Maximum dose 30 mg/kg/day |
|
| Outcomes |
Primary outcomes Aggression (self‐reported): Overt Aggression Scale‐Modified (OAS‐M) scores Global state/functioning: Clinical Global Impression (CGI) scores Adverse events: assessment by attending physician Secondary outcomes Leaving the study early: proportion of participants discontinuing treatment Other outcomes None Timing of outcome assessments Blood assay at weeks 3, 6 and 12; OAS‐M at baseline then weekly (with telephone visits at weeks 5 and 7); CGI baseline and once a week, excluding weeks 5 and 7 |
|
| Notes |
Study funding: Abbott Laboratories, Abbott Park (Illinois, USA) Declaration of interests: The study authors report "Dr Hollander has received research grants from Abbott Laboratories, Bristol Myers Squibb, Eli Lilly and Company, Pfizer Laboratories, Solvay, and Wyeth‐Ayerst. He has served as a consultant to and member of the Speakers Bureau of Abbott Laboratories, Solvay, and Wyeth‐Ayerst. Dr Swann has received grant support from Abbott Laboratories, Glaxo SmithKline, UCB Pharma, Bristol Myers Squibb, Eli Lilly, and Shire Laboratories. He has served as a consultant for Abbott Laboratories, Pfizer Laboratories, Shire Laboratories, UCB Pharma, Glaxo SmithKline, Novartis, Eli Lilly, and Bristol Myers Squibb. He has served on Speakers’ Bureaus for Abbott Laboratories, Janssen Pharmaceuticals, Novartis, Glaxo SmithKline, and Pfizer Laboratories. Dr Coccaro is a consultant to Abbott Laboratories and to Eli Lilly Pharmaceuticals. He is a member of the Speakers Bureau for Abbott Laboratories, Eli Lilly Pharmaceuticals, Glaxo SmithKline, and Forest Pharmaceuticals and has received research grants from Abbott Laboratories and Eli Lilly Pharmaceuticals. Dr McElroy is a consultant to Abbott Laboratories and is a member of the company’s Speakers Bureau and Divalproex Advisory Board. She has also received research grants from Abbott Laboratories, and Eli Lilly and Company. Dr Nemeroff reports the following: Grants/Research: Abbott Laboratories; AstraZeneca; Bristol‐Myers‐Squibb; Forest Laboratories; Janssen Pharmaceutica; Eli Lilly; GlaxoSmithKline; NARSAD; NIMH; Organon; Pfizer Pharmaceuticals; Pharmacia‐Upjohn; Stanley Foundation/NAMI; and Wyeth‐Ayerst. Consultant: Abbott Laboratories; Acadia Pharmaceuticals; AstraZeneca; Bristol‐Myers‐Squibb; Cephalon Pharmaceuticals; Corcept; Cypress Biosciences; Forest Laboratories; GlaxoSmithKline; Janssen Pharmaceutica; Eli Lilly; Merck; Mindsense; Neurocrine Biosciences; Novartis; Organon; Otsuka; Pharmacia‐Upjohn; Sanofi; Somerset; Vela Pharmaceuticals; and Wyeth‐Ayerst. Speakers Bureau: Abbott Laboratories; AstraZeneca; Bristol‐ Myers‐Squibb; Eli Lilly; Forest Laboratories; GlaxoSmithKline; Janssen Pharmaceutica; Organon; Pfizer Pharmaceuticals; and Wyeth‐Ayerst. Stockholder: Corcept and applies only to Dr. Nemeroff Drs Tracy, Wozniak, and Sommerville are employees of Abbott Laboratories." (quote, p 1195‐6) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Investigators reported "patients were randomised in equal numbers, within each of the three diagnostic groups, to receive either divalproex sodium delayed‐release tablets. . . or matching placebo" (col 1, page 1188). No further details given. Insufficient information to permit judgement on adequacy of sequence generation. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information to permit judgement on adequacy of allocation concealment. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared. |
| Blinding (performance bias and detection bias) of participants | Low risk | Comment: Investigators describe study throughout as "double‐blind" and that participants received a "matching placebo". Review authors judged that blinding of participants was adequate and that it was unlikely that this blinding could have been broken. |
| Blinding (performance bias and detection bias) of personnel | Low risk | Comment: Investigators reported "An unblinded person from the central laboratory reported serum valproate levels. . . to the investigators, so that the dose of the study drug could be adjusted appropriately. In order to preserve the study blind, sham valproate levels were reported for selected placebo patients" (p.1188, col 1). Review authors judged that blinding of personnel was adequate and that it was unlikely that this blinding could have been broken. |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | Comment: Insufficient information to permit judgement on adequacy of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Incomplete outcome data arise from participants not completing the study and applies to all measured outcomes. Overall, 54/124 (44%) of the treatment group and 47/122 (39%) of the control group discontinued prematurely, with reasons for non‐completion approximately balanced between conditions. Review authors unable to make a judgement unless data from the (small) AsPD subgroup (n = 9) become available. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Study protocol is not available but it seems clear that the published report included all expected outcomes, including those that were pre‐specified. |
| Other bias | High risk | Comment: The investigators note that the mean final valproate serum level was 64.2 μg/ml, which is well below possible therapeutic range (80‐120 μg/ml) based on previous studies. The study authors declare multiple links to the funding source (Abbott Laboratories) and other pharmacy companies. |
Coccaro 2009.
| Study characteristics | ||
| Methods | Design: combination data from two parallel randomised placebo‐control studies | |
| Participants |
Participants: adults with intermittent explosive disorder (see notes 1 and 2) Sex: (see note 3) whole sample = 77 male, 23 female; intervention = 49 male, 16 female; control = 28 male, 7 female Age: (see note 3) whole sample mean = 36.8 years (SD = 8.7); intervention mean = 37.7 years (SD = 8.9); control mean = 35.5 years (SD = 8.1) Unit of allocation: individual; 2:1 ratio allocation to intervention or control Number randomised: (see note 3) whole sample = 100; intervention = 6, control = 35) (see note 1) Number completing: (see note 3) whole sample at week 12 = 35 (54%) participants in experimental group and 20 (57%) in control group retained in study Setting: community outpatient Inclusion criteria: males and females with lifetime histories of problematic impulsive aggressive behaviour; meet DSM‐IV criteria for personality disorder and have defined histories of impulsive aggressive behaviour Exclusion criteria: lifetime history of mania or hypomania, schizophrenia, or delusional disorder; current major depression; currently dependent on alcohol or other drugs of abuse Ethnicity: ‘race’ reported for whole sample (see note 2); White (n = 85), African American (n = 12), Other (n = 3) Baseline characteristics: (whole sample) (see notes 2 and 4) GAF function score mean = 56.2 (SD = 6.7); LHA aggression score mean = 18.0 (SD = 5.2); OAS‐M aggression score (raw score) mean = 47.5 (SD = 76.1); OAS‐M irritability score (raw score) mean = 6.1 (SD = 1.3); HAM‐D‐21 score (raw score) mean = 4.9 (SD = 3.5); current history of mood disorder n = 27 (27%); current history of anxiety disorder n = 17 (17%); lifetime history of mood disorder n = 58 (58%); lifetime history of anxiety disorder n = 25 (25%); lifetime history of alcoholism n = 35 (35%); lifetime history of drug dependence n = 27 (27%). Axis II diagnoses: borderline n = 20 (20%); narcissistic n = 15 (15%); AsPD n = 12 (12%); histrionic n = 4 (4%); obsessive‐compulsive n = 26 (26%); avoidant n = 5 (5%); dependent n = 0 (0%); paranoid n = 24 (24%); schizoid n = 2 (2%); schizotypal n = 1 (1%) |
|
| Interventions | Two conditions:
Both groups had 2‐week placebo lead in period before the 12‐week intervention/control condition commenced. Duration of intervention: 12 weeks Duration of trial: 14 weeks: 2 week placebo lead in phase + 12 week treatment phase Length of follow‐up: none. Trial ran between July 1990 and July 1999 Dose adjustment: Trial investigators report “For the first 4 weeks of the double‐blind treatment phase, the fluoxetine dose was set at 20 mg p.o. q.d. At the end of week 4 (or later), fluoxetine (or placebo) could be raised to 40 mg (2 placebo capsules) if the patient’s average OAS‐M aggression score for the previous 2 weeks had not decreased to < 25% of the patient’s average OAS‐M aggression score during the placebo lead‐in phase. Fluoxetine could be increased to a maximum of 60 mg q.d. (3 placebo capsules) again after week 8 if the average OAS‐M aggression score for the previous 2 weeks still had not dropped to < 25% of the average OAS‐M aggression score at randomization.” (p 656, col.1) |
|
| Outcomes |
Primary outcomes All measured at two‐week intervals (1–2, 3–4, 5–6, 7–8, 9–10 and 11–12 weeks) Aggression: Overt Aggression Scale ‐ Modiied (OAS‐M) aggression scores; OAS‐M irritability scores Global state/functioning: global response to treatment measured by Clinical Global Impressions‐Improvement scale (CGI‐I) Adverse events: any adverse event; specific adverse events (e.g. sexual dysfunction, sleep disturbance, nausea/vomiting, jitteriness/restlessness, appetite disturbance, diarrhoea, rash, dry mouth, indigestion, fatigue, headache) Secondary outcomes Leaving the study early: retention in study Other outcomes: Differences between responders and non‐responders treated with fluoxetine Timing of outcome assessments Blood assay at week 4, 8 and 12; OAS‐M and CGI‐I weekly |
|
| Notes |
Study funding: National Institute of Mental Health and Eli Lilly Research Laboratories Declaration of interests: The study authors report that "Eli Lilly also provided the study drug and placebo. Fluoxetine plasma level assessments were performed under the supervision of Thomas B. Cooper, M.A., Analytic Psychopharmacology Laboratory, Nathan S. Kline Institute for Psychiatric Research, Orangeburg, N.Y. Mr. Cooper reports no financial affiliations or other relationships relevant to the subject of this article. Dr. Coccaro has been a consultant to Azevan. Dr. Kavoussi is an employee of GlaxoSmithKline. Dr. Lee reports no additional financial or other relationship relevant to the subject of this article." (quote, p 653) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not reported in 2009 paper |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not reported in 2009 paper |
| Blinding (performance bias and detection bias) of participants | Unclear risk | Comment: Fluoxetine‐treated subjects were assigned fewer study capsules than placebo‐treated subjects at each of the 2 dosage‐decision points; review authors are unclear if this may impact on blinding of participants. |
| Blinding (performance bias and detection bias) of personnel | Low risk | Comment: All assessments were made blind to study assignment. OAS‐M scores were determined by a trained behavioral assessor; all other assessments were performed by the research psychiatrist (R.J.K.). |
| Blinding (performance bias and detection bias) of outcome assessors | Low risk | Comment: All assessments were made blind to study assignment. OAS‐M scores were determined by a trained behavioral assessor; all other assessments were performed by the research psychiatrist (R.J.K.). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Endpoint (i.e., last observation carried forward) for all subjects and completer analyses were also performed. Intention to treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No protocol available. Study is an amalgamation of two sets of subjects studied by the study team at the same study site and study authors report that changes were made to entry criteria, based on interim analysis. All outcome measures stated in the methods are reported. |
| Other bias | High risk |
Comment: Vested interest: the project was supported in part by National Institute of Mental Health grants RO1MH47495 and KO2MH00951 (Dr. Coccaro) and by a grant from the Eli Lilly Research Laboratories. Eli Lilly also provided the study drug and placebo. Fluoxetine plasma level assessments were performed under the supervision of Thomas B. Cooper, M.A., Analytic Psychopharmacology Laboratory, Nathan S. Kline Institute for Psychiatric Research, Orangeburg, N.Y. Mr. Cooper reports no financial affiliations or other relationships relevant to the subject of this article. Dr. Coccaro has been a consultant to Azevan. Dr. Kavoussi is an employee of GlaxoSmithKline. |
Gowin 2012.
| Study characteristics | ||
| Methods | Design: placebo‐controlled parallel trial | |
| Participants |
Participants: community living adults on probation or parole, in Houston area, USA Sex: (see note 1) whole sample (n = 12) = 10 male (83.3%), 2 female (16.7%); intervention (n = 6) = 5 male (83.3%), 1 female (16.7%); control (n = 6) = 5 male (83.3%), 1 female (16.7%) Age: (see note 1) intervention mean = 25.17 years (SD = 3.82), control mean = 32.00 years (SD = 5.02) Unit of allocation: individual Number randomised: 15 (see note 1); intervention = 6, control = 6. Three participants removed for positive urinalysis tests for prohibited substances (group membership not provided) Number completing: 12 (see note 1); intervention = 6 (100%), control = 100%) Setting: community clinic Inclusion criteria: free of illicit and prescription drugs during study period; participants on parole or probation; authors state that these participants were sought “because of high incidence of antisocial and aggressive behaviour associated with this population” (quote, p 983, column 2) Exclusion criteria: medical conditions (e.g. HIV, seizures, cardiovascular disease); pregnancy; any current or past psychiatric illness and axis I disorders (except past substance abuse/dependence) Ethnicity: (whole sample; see note 1) intervention = African‐American (n = 6, 100%), control = African‐American (n = 5, 83.33%) and Hispanic (n = 1, 16.67%) Baseline characteristics: Intervention group: high school education (n = 6, 100%); conduct disorder present (n = 3, 50%); ASPD present (n = 3, 50%); smoker (yes) (n = 4, 66.67%); number of cigarettes/day (mean = 4.33, SD = 4.59); on parole (n = 3, 50%); on probation (n = 2, 33.33%); Shipley Wechsler Adult Intelligence Scale (WAIS) (mean 105.33, SD = 12.66), Shipley WAIS‐R (mean 94.67, SD = 14.38). Control group; high school education (n = 5, 83.33%); conduct disorder present (n = 4, 66.67%); ASPD present (n = 3, 50%); smoker (yes) (n = 5, 83.33%); number of cigarettes/day (mean = 4.67, SD = 3.44); on parole (n = 1, 16.67%); on probation (n = 2, 33.33%); Shipley WAIS (mean 105.83, SD = 5.11); Shipley WAIS‐R (mean 96.50, SD = 6.25) |
|
| Interventions | Two conditions:
Duration of intervention: 6 weeks Duration of trial: 6 weeks Length of follow‐up: none Dose adjustment: increasing dose of tiagabine; 4 mg tiagabine (week 3); 8 mg tiagabine (week 4); 12 mg tiagabine (week 5) |
|
| Outcomes |
Primary outcomes Aggression: Point Subtraction Aggression Paradigm (PSAP); Buss‐Perry Aggression Questionnaire (BPAQ); Lifetime History of Aggression Questionnaire (LHA); Retrospective Overt Aggression Scale (ROAS) Adverse events: medication side effects Secondary outcomes Leaving the study early; 3 randomised participants removed for positive urinalysis tests for prohibited substances (details of group membership not provided) Impulsivity; Eysenck Impulsivity Venturesomeness Questionnaire (EIVQ; Barratt Impulsiveness Scale (BIS‐II) Anger:State‐Trait Anger Expression Inventory (STAXI) Other outcomes Cognitive assessment; Shipley Institute of Living Scale (SILS) Timing of outcome assessments PSAP and cognitive assessment 2‐3 days per week; questionnaires at week 5 |
|
| Notes |
Study funding: National Institute on Drug Abuse (USA) Declaration of interests: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: No information given on method of randomization, however 12 subjects randomised exactly to 6 control vs. 6 experimental, with each group having x1 female and 3 ASPD participants. This suggests that a truly random process was not used meaning that bias may have been introduced. |
| Allocation concealment (selection bias) | High risk | Comment: No information given on method of randomization, however 12 subjects randomised exactly to 6 control vs. 6 experimental, with each group having x1 female and 3 ASPD participants. This suggests that a truly random process was not used meaning that bias may have been introduced. |
| Blinding (performance bias and detection bias) of participants | Low risk | Comment: Placebo and tiagabine capsules were manufactured to look the same. There was however no assessment of whether participants were aware of which group they were in at the end of the study. |
| Blinding (performance bias and detection bias) of personnel | Low risk | Comment: Research assistants conducting drug administration and administering medication event monitoring system (MEMS) bottles were blind to allocation. |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | Comment: Use of computerized task may have reduced opportunity for bias from assessors however no information provided for potential impact on questionnaire‐based outcomes, or data analysis. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Did not use intention to treat (ITT). Appears that 3 subjects left study early due to current substance use but no further information or group membership information given. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No protocol published prior to trial. |
| Other bias | High risk | Comment: Funded by National Institutes of Health grants, but apparent closeness of the authors of this paper and the developer of the PSAP who is acknowledged in the paper for “consultation, mentoring, and expertise of Don R Cherek, PhD without whom these experiments would not be possible” (p.989, col.1) |
Konstenius 2014.
| Study characteristics | ||
| Methods | Design: placebo‐controlled parallel trial | |
| Participants |
Participants: Swedish prisoners about to be released to the community with co‐diagnoses of attention deficit hyperactivity disorder (ADHD) and amphetamine dependence Sex: whole sample = 100% male (see note 1) Age: (see note 1) intervention mean = 41 years (SD = 7.5), control mean = 42 years (SD = 11.7) Unit of Allocation: block randomization (block size = 2); intervention to control ratio of 1:1 Number randomised: whole sample = 54 (see note 1); intervention = 27, control = 27 Number completing: whole sample = 10 (see note 1); intervention = 8, control = 2 Setting: medium security prison and outpatient/community clinic in Sweden Inclusion criteria: male; aged 18‐65 years; meet DSM‐IV criteria for attention deficit hyperactivity disorder (ADHD); meet DSM‐IV criteria for amphetamine dependence prior to current incarceration; used amphetamine a minimum of 12 occasions during last 12 weeks prior to incarceration; consenting to participate Exclusion criteria: meet DSM‐IV diagnosis of substance dependence (except nicotine) currently or in 12 months prior to current incarceration; major psychiatric disorder (schizophrenia, severe depression); current use of antipsychotic medication; current use of benzodiazepine; traces of following substances in urine: amphetamine, benzodiazepine, cannabis, cocaine, dextropropoxyphene [opioid], opiates; serious somatic disease (e.g. hyperthyroidism, moderate/severe hypertension); known hypersensitivity to methylphenidate Ethnicity: not stated Baseline characteristics: Intervention group Demographic data: married /cohabitant (n = 8, 31%); homeless (n = 11, 41%); born in Sweden (n = 24, 93%); diagnosed with hepatitis (n = 20, 77%); years of education (mean = 9.6 years, SD = 2.2); estimated IQ (mean = 90, SD = 9.9) Substance use measures: age of onset in substance use (mean = 13.0 years, SD = 1.8); age of onset amphetamine use (mean = 18.2 years, SD = 4.5); number of participants using amphetamine by injection (n = 24, 89%); age of onset of use by injection (mean = 20.5 years, SD = 6.2); life‐time years of amphetamine use (mean = 20.6 years, SD = 10.2) Additional DSM‐IV diagnosis: number of participants with any Axis I diagnosis (n = 21, 96%); number of axis I diagnoses (mean = 1.4, SD = 0.7); number of participants with any axis II diagnosis (n = 19, 70%); number of axis II diagnoses (mean = 1.4, SD = 1.8); number of participants with antisocial personality disorder (n = 17, 63%); participant attempted suicide in life‐time (n = 4, 15%); psychiatric symptoms measured by OQ45 score (mean = 111.5, SD = 3.7) ADHD measures: inattentive subtype (n = 4, 15%); hyperactive subtype (n = 3, 11%); combined subtype (n = 20, 74%) Criminality measures: age at first prison sentence (mean = 28.7 years, SD = 8.7); number of prison sentences (mean = 10.5, SD = 7.3); total length of prison sentences (mean = 67.7 months, SD = 79.4); length of current prison sentence (mean = 5.30 years, SD = 3.76) Control group Demographic data: married /cohabitant (n = 8, 31%); homeless (n = 10, 37%); born in Sweden (n = 23, 93%): diagnosed with hepatitis (n = 20, 77%); years of education (mean = 9.6 years, SD = 1.9); estimated IQ (mean = 94, SD = 12.0) Substance use measures: age of onset in substance use (mean = 12.2 years, SD = 2.2); age of onset amphetamine use (mean 19.3 years, SD = 7.2); number of participants using amphetamine by injection (n = 25, 93%); age of onset use by injection (mean 20.8 years, SD = 5.4); life‐time years of amphetamine use (mean 18.3 years, SD = 12.7). Additional DSM‐IV diagnosis: number of participants with any axis I diagnosis (n = 16, 76%); number of axis I diagnoses (mean = 1.5, SD = 0.8); number of participants with any axis II diagnosis (n = 15, 56%); number of axis II diagnoses (mean = 1.6, SD = 2.2); number of participants with antisocial personality disorder (n = 11, 41%); participant attempted suicide in life‐time (n = 9, 35%); psychiatric symptoms measured by OQ45 score (mean = 114.8, SD = 3.6) ADHD measures: inattentive subtype (n = 3, 11%); hyperactive subtype (n = 5, 19%); combined subtype (n = 19, 70%) Criminality measures: age at first prison sentence (mean = 27.4 years, SD = 9.6); number of prison sentences (mean = 12.3, SD = 8.8), total length of prison sentences (mean = 62.0 months, SD = 55.5); length of current prison sentence (mean = 6.89 years, SD = 6.07) |
|
| Interventions | Two conditions:
Duration of intervention: 24 weeks Duration of trial: 24 weeks Length of follow‐up: participants were not followed up beyond the study period Dose adjustment: initial dose 18 mg methylphenidate, titrated up to a maximum dose of 180 mg/day (36 mg increase every 3 days) |
|
| Outcomes |
Primary outcomes Global state/functioning; Clinical Global Impression (CGI) scale Adverse events; headache; abdominal discomfort; sleep problems; loss of appetite; depressed mood; increased blood pressure; sweating; fatigue; anxiety; dry mouth; craving; chest pain; muscular pain; restlessness; procrastination; dizziness; skin problems; hears voices; palpitations; tics; agitation; lower self‐esteem; suicidal ideation Secondary outcomes Leaving the study early; proportion of participants discontinuing treatment Substance misuse; urinalysis and scores on Addiction Severity Index (ASI) Mental state; psychiatric symptoms measured by Outcomes Questionnaire 45 (OQ‐45); Conners’ adult ADHD self‐rating scale (CAARS:SV); Craving for Amphetamine Scale (CAS) Other outcomes None reported Timing of outcome assessments ADHD symptoms weekly for first 6 weeks, then every 4 weeks. CGI at baseline than weeks 12 and 24. Weekly assessment for drug urinalysis, CAS, adverse events, blood pressure, pulse and weight. Blood and liver function at weeks 4, 8, 12, 16 and 22. |
|
| Notes |
Study funding: Swedish National Board of Health and Welfare, Swedish Research Council and Stockholm County Council Declaration of interests: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: The trial authors report “randomization list generated by an independent pharmacist using the computer‐based program DESIGN”...“randomized into two parallel groups…with block size of 2” |
| Allocation concealment (selection bias) | Low risk | Comment: The trial authors report "Block randomization was used because of the length of the trial and the nature of the medication effect, and was unknown to the principle investigator and the study staff". (p.441, col.2) |
| Blinding (performance bias and detection bias) of participants | Unclear risk | Comment: The trial authors describes study as a ”double‐blind” trial but no further information given on how this was done. |
| Blinding (performance bias and detection bias) of personnel | Unclear risk | Comment: The trial authors report "Block randomization was used because of the length of the trial and the nature of the medication effect, and was unknown to the principle investigator and the study staff". (p.441, col.2) |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | Comment: The trial authors describes study as a ”double‐blind” trial but no further information given on how this was done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Used ITT for primary analysis but for repeated measures also used last observation carried forward (LOCF) |
| Selective reporting (reporting bias) | High risk |
Comment: The following secondary outcomes are listed in the trial registry protocol but are not reported in the paper: 1. Relapse to crime (readmission to prison or other legal action for criminal offence, self‐reported criminality) 2. Reduction in psychiatric symptoms, assessed using Outcome Questionnaire 45 (other than reporting “no significant changes in other psychiatric symptoms” 3. Plasma concentration of methylphenidate 4. Reduction of problems in attention assessed by Connors' Continuous Performance Test (CPT) 5. Self‐reported drug use, assessed using the Addiction Severity Index (ASI) and time‐line follow‐back 6. Interpersonal Problems (Inventory of Interpersonal problems (IIP)) |
| Other bias | Low risk |
Comment: Authors declared no conflicts of interests, authors give their affiliation as universities, with no evidence of pharmaceutical company funding. Protocol states source of funding: ”Addiction Centre Stockholm (Beroendecentrum Stockholm) (Sweden), National Psychiatric Services Coordination Taskgroup (Nationell Psykiatri Samordning) (Sweden)” |
ADHD = attention deficit hyperactivity disorder; ADS = Alcohol Dependence Scale; ASI = Addiction Severity Index; AsPD = antisocial personality disorder; ASS = Alcohol Severity Scale; BAI = Beck Anxiety Inventory; BDHI = Buss‐Durkee Hostility Inventory; BDI = Beck Depression Inventory; BIS‐II = Barratt Impulsiveness Scale; BPAQ = Buss‐Perry Aggression Questionnaire; CAARS:SV = Conners’ adult ADHD self‐rating scale; CAS = Craving for Amphetamine Scale; CGI = Clinical Global Impression; CGI‐I = Clinical Global Impression‐Improvement scale; CPT = Connors' Continuous Performance Test; DSM‐III = Diagnostic and Statistical Manual of Mental Disorders, Third Edition; DSM‐III‐R = Diagnostic and Statistical Manual of Mental Disorders, Third Edition–Revised; DSM‐IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; DSM‐IV TR = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition 4–Text Revision; EIVQ = Eysenck Impulsivity Venturesomeness Questionnaire; EMIT = enzyme‐multiplied immunoassay; ERP = event‐related potentials; GAF = Global Assessment of Functioning; GAS = Global Assessment Scale; HAM‐D = Hamilton Depression Rating scale; HCl = hydrochloride; HIV = human immunodeficiency virus: IED = intermittent explosive disorder; IIP = Inventory of Interpersonal problems; IQ = intelligence quotient; ITT = intention to treat; LHA = Lifetime History of Aggression Questionnaire; LOCF = last observation carried forward; MANOVA = multivariate analysis of variance; MAOI = monoamine oxidase inhibitor; MEMS = medication event monitoring system; OAS = Overt Aggression Scale; OAS‐M = Overt Aggression Scale–Modified; OCDS = Obsessive Compulsive Drinking Scale; OQ‐45 = Outcomes Questionnaire‐45; OROS = osmotic release oral system; p.o. = per os (by mouth); POMS = Profile Of Mood States; PSAP = Point Subtraction Aggression Paradigm; PTSD = post‐traumatic stress disorder; q.d. = quaque die (every day); ROAS = Retrospective Overt Aggression Scale; SADQ = Severity of Alcohol Dependence Questionnaire; SCID‐II = Structured Clinical Interview for DSM‐IV; SCL‐90 = Symptom Check List‐90; SD = standard deviation; SILS = Shipley Institute of Living Scale; SSRI = selective serotonin reuptake inhibitor; STAXI = State‐Trait Anger Expression Inventory; TLFB = Timeline Follow‐Back Interview; USA = United States of America.
Characteristics of excluded studies [ordered by year]
| Study | Reason for exclusion |
|---|---|
| Alcorn 2015 | 'Counterbalanced dose' trial of self‐administered intranasal oxytocin on aggressive responding in men with AsPD. Excluded because participants were not randomised and there was no control group |
| Allen 1976 | Cross‐over trial in which 41 "sociopathic" prisoners received a random sequence of four active substances (amphetamine, caffeine, imipramine and chlorpromazine) and one inactive placebo. Excluded because participants were not assessed for possible diagnosis of AsPD |
| Alpert 1990 | RCT of nadolol versus placebo for violent psychiatric patients. Excluded because nadolol is not a drug with known psychotropic properties, because none of the participants had a diagnosis of AsPD, and because most had a major functional mental illness (i.e. schizophrenic disorder, schizoaffective disorder or bipolar disorder) |
| Mattes 1990 | RCT comparing carbamazepine versus propranolol for temper outbursts. A subgroup of participants (n = 8) had AsPD. Excluded because of lack of a placebo control condition |
| Shea 1990 | RCT in which 250 participants with a primary diagnosis of major depressive disorder were randomised to four conditions, of which one was imipramine plus clinical management and one was placebo plus clinical management. AsPD was an exclusion criterion |
| Noyes 1991 | RCT comparing alprazolam, diazepam and placebo in patients with panic disorder. Investigators examined the effect of co‐morbid personality disorder traits on treatment outcome, but report only mean Personality Disorder Questionnaire (PDQ) trait scores. Excluded because no indication that any participants with a diagnosis of AsPD were randomised |
| Black 1994 | RCT comparing fluvoxamine, cognitive therapy and placebo for participants with panic disorder. Excluded because no specific personality disorder diagnoses were reported and there was no indication that any subgroup had AsPD |
| Patience 1995 | RCT in which 113 participants meeting DSM‐III criteria for major depression were randomly assigned to 1 of 4 conditions of which 1 was amitriptyline and 1 was routine primary care. Excluded because only 8 participants were diagnosed with AsPD, which is too few to allow calculation of mean and SD when randomised to 4 treatment conditions |
| Coccaro 1997 | RCT comparing fluoxetine with placebo in adult outpatients with personality disorder and a history of impulsive aggression and irritability. Excluded because only 4 participants with AsPD were diagnosed, which is too few to allow calculation of mean and SD when randomised to 2 treatment conditions |
| Ekselius 1998 | RCT of sertraline versus citalopram in depressed patients in primary care. Excluded because no assessment of AsPD was made, and because there was no placebo control condition |
| Battaglia 1999 | RCT of depot fluphenazine ('low dose' versus 'ultra low dose') for multiple suicide attempters in the emergency department. Cluster B personality disorder was represented in the sample, but unclear whether any participants had an AsPD diagnosis. Excluded because of lack of a placebo control group |
| Aberg‐Wisted 2000 | RCT of sertraline versus paroxetine for outpatients with major depression. Investigators examined effects of comorbid axis II disorder by personality disorder cluster. No indication that any subgroup of participants had AsPD. Excluded because of lack of a placebo control group |
| Agosti 2002 | RCT of fluoxetine versus imipramine versus placebo in outpatient with major depression. Excluded because no participants were reported with a diagnosis of AsPD |
| Joyce 2003 | RCT comparing fluoxetine with nortriptyline in patients with major depression. Differential drug response was compared in three groups; with BPD, with other personality disorder and with no personality disorder. 6 participants had AsPD. Excluded because of lack of a placebo control group |
| Kool 2003 | RCT comparing psychodynamic supportive therapy plus pharmacotherapy with pharmacotherapy alone for depressive disorder in adult patients (article in Dutch). Excluded because there was no placebo control condition and only 3 participants had an AsPD diagnosis |
| Mattes 2005 | RCT comparing oxcarbazepine with placebo in outpatients with impulsive aggression. Excluded because no diagnosis of personality disorder was made |
| Nickel 2005 | RCT comparing topiramate with placebo in male outpatients with aggression. Excluded because no diagnosis of personality disorder was made |
| Fournier 2008 | RCT comparing paroxetine, placebo and cognitive therapy in outpatients with depressive disorder. Excluded because presence of AsPD was an exclusion criterion for the trial |
| Mattes 2008 | RCT comparing levetiracetam with placebo in outpatients with impulsive aggression. Excluded because no diagnosis of personality disorder was made |
| Lane 2009 | Within‐participants controlled trial of topiramate for individuals with histories of substance abuse and antisocial behavior. 5 participants had an AsPD diagnosis. Excluded because participants were not randomised and there was no standard placebo control condition. |
| Jariani 2010 | Randomised superiority trial of olanzapine and sertraline on personality disorder in patients with methadone maintenance therapy. All participants had a diagnosis of borderline personality disorder. Excluded as there was no AsPD reported and there was no placebo control condition |
| Dunlop 2011 | The paper reports a secondary analysis of superiority trial of sertraline combined with tri‐iodothyronine (T3) or placebo on psychopathic traits in patients with major depressive disorder (Garlow 2007). Excluded because AsPD diagnosis was not assessed and because of a lack of a placebo control group |
| George 2011 | Randomised placebo‐controlled trial of fluoxetine treatment for alcoholic perpetrators of domestic violence. Excluded because no assessment of AsPD was made |
| Todorovic 2012 | Superiority trial of sertraline plus carbamazepine, valproate or lamotrigine for patients diagnosed with personality disorder and comorbid major depression. Excluded as the study was not randomised and there was no placebo control group |
| Kampman 2013 | Randomised placebo‐controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence in men and women receiving individual CBT relapse prevention therapy. Excluded because no participants were reported with a diagnosis of AsPD |
| Surekha 2013 | Clinical trial of lithium in reducing aggression and impulsivity in patients with a diagnosis of AsPD. Excluded because participants were not randomised and there was no placebo control group |
| Johnson 2013 | Double‐blind RCT comparing topiramate + cognitive behavioural therapy (CBT) with placebo + CBT in cocaine‐dependent adults. Excluded because no diagnosis of personality disorder was made |
| Timmermann 2017 | Randomised, placebo‐controlled, cross‐over trial of the neuropeptide oxytocin for individuals with AsPD (n = 22) and healthy controls (n = 29). Excluded as this is a pre‐clinical neuropsychiatric study looking at improvement in facial emotion recognition and also does not address any of the primary or secondary outcomes in this review. |
| Patrizi 2019 | Retrospective, cohort study of inhaled loxapine (antipsychotic medication) for inpatients with personality disorder who present with psychiatric agitation. Excluded as the study is not an RCT |
AsPD = antisocial personality disorder; BPD = borderline personality disorder; CBT = cognitive behavioural therapy; DSM‐III = Diagnostic and Statistical manual of Mental disorders, Third Edition; RCT = randomised controlled trial; SD = standard deviation.
Characteristics of studies awaiting classification [ordered by year]
Verkes 1998.
| Methods | Design: placebo‐controlled parallel trial |
| Participants |
Participants: outpatients with repeated suicidal attempts but without major depression Sex: 37 male (17 paroxetine group, 20 placebo group); 54 female (29 paroxetine group, 25 placebo group) (data not extractable for any AsPD subgroup; see note 1) Age: mean for paroxetine group = 34.1 (SD = 11.6), mean for control group = 37.1 (SD = 13.0) years (data not extractable for any AsPD subgroup; see note 1) Unit of allocation: individual participant Number randomised: 91 (paroxetine = 46, placebo = 44; see note 1) Number completing: at 8 weeks: paroxetine = 28, placebo = 30; at 52 weeks: paroxetine = 11, placebo = 8 (see note 1) Setting: outpatient; 2 sites; Netherlands (Rotterdam, Leiden) Inclusion criteria: at least one previous suicide attempt; aged 18 years or older Exclusion criteria: major affective disorder; psychotic disorder; currently taking antidepressant or antipsychotic medication; organic mental disorder; dependency on alcohol or substances; using prohibited medication; serious physical disease; unable to co‐operate Ethnicity: not reported Baseline characteristics: Intervention (paroxetine) group History of previous suicide attempts: major repeaters (≧ 5 previous attempts) n = 16 (35%), minor repeater (1‐4 attempts) n = 30 (65%); history of deliberate self‐harm: n = 8 (17%); history of alcohol abuse: n = 19 (41%); mean number of DSM‐III‐R cluster A criteria met = 10.0 (SD = 4.2), mean number of DSM‐III‐R cluster B criteria met = 14.9 (SD = 4.4), mean number of DSM‐III‐R cluster C criteria met = 14.3 (SD = 6.1); mean baseline score on BDI = 28.5 (SD = 11.9); mean baseline score on BHS = 13.4 (SD = 4.1); mean baseline score on STAXI = 22.0 (SD = 9.5). Control (placebo) group History of previous suicide attempts, major repeaters (≧ 5 previous attempts) n = 12 (27%), minor repeater (1‐4 attempts) n = 33 (73%); history of deliberate self‐harm: n = 7 (16%); history of alcohol abuse: n = 21 (47%); mean number of DSM‐III‐R cluster A criteria met = 10.5 (SD = 4.7), mean number of DSM‐III‐R cluster B criteria met = 14.5 (SD = 5.4), mean number of DSM‐III‐R cluster C criteria met = 12.8 (SD = 5.2); mean baseline score on BDI = 28.1 (SD = 11.9); mean baseline score on BHS = 13.7 (SD = 4.1); mean baseline score on STAXI = 20.8 (SD = 10.2). |
| Interventions | Two conditions:
In addition to medication, supportive psychotherapy offered weekly to fortnightly to all participants. Duration of intervention: up to 52 weeks Duration of trial: up to 52 weeks Length of follow‐up: participants were not followed up beyond the end of the intervention period Dose adjustment: initial placebo washout period of 2 weeks; then 20 mg/day paroxetine for one week followed by a fixed dose of 40 mg/day for up to 52 weeks (or matching placebo) |
| Outcomes |
Primary outcomes None reported Secondary outcomes Leaving the study early: Anger: STAXI scores Other outcomes Subsequent suicide attempts; depression (Beck Depression Inventory; self report); hopelessness (Beck Hopelessness Scale, self‐report) |
| Notes |
|
Hellerstein 2000.
| Methods | Design: placebo‐controlled parallel trial |
| Participants |
Participants: outpatients with early‐onset dysthymia Sex: 266 female, 144 male (data not extractable for any AsPD subgroup; see note 1) Age: mean 42.0 (SD = 9.0) years (data not extractable for any AsPD subgroup; see note 1) Unit of allocation: individual participant. Number randomised: 410 (sertraline = 134, imipramine = 136, control = 140; see note 1) Number completing: completion rates: sertraline = 84%, imipramine = 67%, placebo = 76% (data not extractable for any AsPD subgroup; see note 1) Setting: outpatient; multi‐centre (17 sites), North America Inclusion criteria: early‐onset dysthymia (DSM‐III‐R) of at least 5 years' duration; score of 12 or higher on 29‐item Hamilton Depression Rating Scale (SAD version) at end of 1‐week single‐blind placebo washout period Exclusion criteria: major depression; pregnancy or lactation; history of drug or alcohol dependency/misuse within preceding 6 months; serious risk of suicide; current primary diagnosis of panic disorder or generalised anxiety disorder; lifetime diagnosis of bipolar disorder, obsessive compulsive disorder, or any psychotic disorder; failure to respond in two or more prior antidepressant trials; previous adequate trial of imipramine or sertraline treatment Ethnicity: Caucasian (95%, n = 390) (data not extractable for any AsPD subgroup; see note 1) |
| Interventions | Three conditions:
Duration of intervention: 10 weeks Duration of trial: 10 weeks Length of follow‐up: participants were not followed up beyond the end of the intervention period Dose adjustment: sertraline initially 50 mg/day and titrated after weeks 4, 6 and 7 to a maximum of 200 mg/day; imipramine initially 50 mg/day and titrated weekly to a maximum of 300 mg/day; all participants received 4 identical capsules containing either placebo, 50 mg sertraline, or 50 or 100 mg imipramine |
| Outcomes |
Primary outcomes Social functioning: Social Adjustment Scale scores Secondary outcomes Leaving the study early: Other outcomes Changes in personality dimensions (Tridimensional Personality Questionnaire) |
| Notes |
|
Charney 2015.
| Methods | Design: placebo‐controlled parallel trial |
| Participants |
Participants: outpatients with alcohol abuse or dependence Sex: 80 female, 185 male Age: 18‐65 (placebo mean age = 44.7 years; citalopram mean age = 46.0 years) Unit of allocation: individual participant Number randomised: 265 (citalopram = 138, placebo = 127) Number completing:141 (citalopram = 72, placebo = 69) Setting: outpatient: specialist addiction unit in Canada Inclusion criteria: DSM‐IV diagnosis of alcohol abuse or dependence Exclusion criteria: having a second substance use disorder (other than nicotine dependence); psychotic or organic brain disorder; taking any psychiatric medications including SSRI; requiring inpatient detoxification or psychiatric admission; pregnant or breastfeeding; history of serious adverse reactions or intolerance to SSRIs Ethnicity: 92% Caucasian |
| Interventions | Two conditions (citalopram + TAU and placebo + TAU)
Duration of intervention: 12 weeks Duration of trial: 12 weeks Length of follow‐up: none Dose adjustment: citalopram started at 20 mg per day for first week; from week 2 to week 12 citalopram administered at 40 mg per day; same number of capsules provided to the two groups |
| Outcomes |
Primary outcomes None reported Secondary outcomes Leaving the study early: n withdrawn for medical reasons; n discontinued intervention Substance misuse: Addiciton Severity Index (ASI); urine samples for toxicology analysis (cloned enzyme donor immunoassay); number of days alcohol intake; number of drinks per drinking day; Can$ spent on alcohol; max number of days abstinent; abstinent at 12 weeks Impulsivity: Barratt Impulsiveness Scale (BIS) Mental State: Hamilton Rating Scale for Depression (HAM‐D); Clinical Global Impression Scale (CGI); Axis‐1 disorders using SCID‐I; Personality disorders using SCID‐II; Beck Depression Inventory (BDI); Beck Anxiety Inventory (BAI); Symptom checklist (SCL‐90) Other outcomes None reported |
| Notes | Email correspondence with author (KG) on 17 January 2017 (see Charney 2015) who confirmed no AsPD data available: "No, not possible to do this breakdown for now. We did the analyses by clusters, since there were too few of each PD to analyse separately. Sorry about this..." (quote) |
ASI = Addiction Severity Index; AsPD = antisocial personality disorder; BAI = Beck Anxiety Inventory; BDI = Beck Depression Inventory; BIS = Barratt Impulsiveness Scale; CGI = Clinical Global Impression; DSM‐III‐R = Diagnostic and Statistical Manual of Mental Disorders, Third Edition–Revised; HAM‐D = Hamilton Depression Rating scale; RCT: randomised controlled trial; SCID‐I = Structured Clinical Interview for DSM‐IV Axis 1 disorders; SCID‐II = Structured Clinical Interview for DSM‐IV; SCL‐90 = Symptom Check List‐90; SD = standard deviation; SSRI = selective serotonin reuptake inhibitor; TAU = treatment as usual.
Characteristics of ongoing studies [ordered by year]
EudraCT2010‐018740‐13.
| Study name |
Short title: Oxytocin effects in autistic and antisocial male adults Full title: Short‐ and long‐term effects of oxytocin on empathy and social behaviour in autistic and antisocial male adults |
| Methods | Design: single site, cross‐over, double‐blind, RCT |
| Participants |
Participants: aim to recruit 78 participants with antisocial personality disorder/autism spectrum disorder Sex: males only Age: adults aged 18‐30 years Inclusion criteria: IQ of 80 or higher; all participants must have normal or corrected to normal vision Additional inclusion criteria for participants with anti‐social personality disorder:
Exclusion criteria: participants may not have a nasal congestion due to cold or allergies; all participants must be free of psychotropic medication or neuroleptics and stimulant medication; participants may not have a history of alcohol or drug dependence. For the empathy experiment, all participants are required to abstain from stimulants, XTC, soft drugs and alcohol for about 20 hours, from caffeine for about 4 hours and from cigarette smoking and taking food for 2 hours before testing. This will, in the first place, rely on informed consent. The use of cocaine, amphetamine, methamphetamine, cannabis and XTC will be controlled for by saliva samples taken before starting the experiment. Blood serum controls will be carried out on the use of methylphenidate and alcohol. Participants who did not abstain for the time of the experiment will be excluded from further participation or data analysis. |
| Interventions | Two conditions:
Duration of intervention: 4 weeks Duration of trial: not stated Length of follow‐up: none stated Dose adjustment: none stated |
| Outcomes |
Primary outcomes Social functioning: assessed by (1) the Social Responsiveness Scale (the SRS‐A), to be completed by an adult informant who knows the participant in naturalistic social settings; and (2) by a symptom checklist, to be completed by the participant himself (i.e. the SCL 90 that has been used for treatment studies in forensic settings before) Secondary outcomes None stated Other outcomes None stated |
| Starting date | Favourable ethics opinion given on 25 August 2011 |
| Contact information | University Medical Center Groningen, Netherlands |
| Notes | www.clinicaltrialsregister.eu/ctr-search/trial/2010-018740-13/NL#A |
AsPD = antisocial personality disorder; IQ = intelligence Quotient; PCL‐R = Psychopathy Checklist‐Revised; RCT = randomised controlled trial; SCID‐II = Structured Clinical Interview for DSM‐IV; SRS‐A = Social Responsiveness Scale; XTC = ecstasy or 3,4‐Methylenedioxymethamphetamine.
Differences between protocol and review
This review differs from the original protocol (Khalifa 2009) and previous review (Khalifa 2010) in the following ways.
Authorship
Following the publication of the protocol (Khalifa 2009), Michael Ferriter joined the team who produced the original version of this review (Khalifa 2010).
For this update, the following review authors stepped down: Conor Duggan, Jutta Stoffers, Nick Huband, Michael Ferriter and Klaus Lieb. They were replaced by Simon Gibbon, Natalie H‐Y Cheung and Lucy McCarthy.
Types of participants
For this update, we added an additional restriction to this section to apply to studies in which participants with AsPD formed a small subgroup.
This required that studies with two treatment conditions should have randomised at least five people with AsPD to be included in the review. The rationale was that variance and standard deviation could not be calculated in samples of two or less, and so a two‐condition study randomising less than five (relevant) participants would have at least one arm for which standard deviation could not be calculated (Newman 1939).
We included in this update, studies where AsPD group or subgroup data were not available, but where at least 75% of participants had a diagnosis of AsPD. We chose a threshold of 75% as this appeared pragmatic and reflects that the overwhelming majority of participants have AsPD.
Types of outcome measures
-
For this update, we:
modified the criteria for the outcome of 'social functioning' to also include proxy measures of social functioning in order to reflect clinically relevant changes (e.g. decreased level of support required/time taken to achieve leave from hospital);
modified the outcome of 'substance misuse,' so that a reader would find it easier to differentiate drug misuse outcomes from alcohol misuse outcomes(specifically, we replaced it with two separate categories: substance misuse: drugs and substance misuse: alcohol);
added two additional secondary outcomes 'Mental state' and 'Prison and service outcomes' to collect data on outcomes relevant to participants' general mental health symptoms (i.e. specific symptoms such as dissociative experiences, mood/anxiety, or global mental health) and use of prison/probation services (e.g. treatment of people in the community, duration of treatment programme, changes in services provided by through care/probation teams), respectively;
reported other outcomes measured in the included studies that did not fall into one of the above categories (continuous or dichotomous outcomes dependent upon how the outcomes were reported); and
took the decision to exclude any study that did not report any of our primary or secondary outcomes, as any additional outcomes would be considered to be clinically irrelevant, trivial or potentially confusing, and the review is already looking at a large number of clinically‐relevant outcomes (five primary outcomes and 12 secondary outcomes).
We acknowledge that there was an oversight in the original protocol regarding the possible use of dichotomous or time‐to‐event data for certain outcomes (e.g. reconviction, leaving the study early and adverse events); these outcomes are more likely to be dichotomous (or time‐to‐event), rather than continuous, data.
Search methods for identification of studies
In the previous version of the review (Khalifa 2010), we added the National Criminal Justice Reference Service Abstracts Database, to capture relevant studies in the justice and drug‐related literature.
-
For this update, we:
revised the list of electronic databases, either because we no longer had access (ASSIA, BIOSIS, Dissertation Abstracts which we replaced with WorldCat, National Criminal Justice Reference Service Abstracts which we replaced with Criminal Justice Abstracts), or because previous searches were unproductive (OpenSIGLE (now OpenGrey) COPAC (which has since been replaced by Library Hub Discover) and Zetoc);
added two daily updated segments of MEDLINE, which were unavailable last time (MEDLINE Epub Ahead of Print and MEDLINE In‐Process & Other Non‐Indexed Citations);
used the Cochrane Database of Systematic Reviews and DARE to identify other relevant systematic reviews in order to search their reference lists;
did not search the specialized register of the Cochrane Schizophenia group because people with comorbid major functional mental illnesses (including schizophrenia) were excluded from this review; and
search trials registers using WHO ICTRP as metaRegister of Controlled Trials (ISRCTN) was no longer available.
Data collection and analysis
This update omits six analyses specified in the original protocol because of insufficient data. (See Table 11).
-
In this update, we added the following new methods, which we may use in future updates of this review (see Table 11).
We may reconsider the primary and secondary outcomes in future reviews, to include pre‐clinical markers such as 'facial emotional recognition' or additional features of AsPD as listed in DSM‐5, ICD‐10, or future iterations of these guidelines e.g. ICD‐11
If insufficient data are available to employ statistical techniques, we will look at descriptive methods (such as time elapsed between the study and publication), in order to assess potential reporting bias.
We have decided to summarise both endpoint and change data (or both) for continuous outcomes in future updates of this review, and have specified that we will perform a meta‐analysis that combines both types of data using the methods described by Da Costa 2013, since both types may be included together in meta‐analysis when using the MD (Higgins 2011a).
We will consider pooling outcomes reported at different time points where this does not obscure the clinical significance of the outcome being assessed.
We explained how we would manage issues of multiplicity should they arise in future updates of the review, as this was missing from the protocol.
Summary of findings and assessment of the certainty of the evidence
In keeping with current recommendations, we included a new section on 'Summary of findings and assessment of the certainty of the evidence' in this update, in which we explain how we assessed the certainty of the evidence for clinically relevant outcomes and summarised these in a 'Summary of findings' table.
Contributions of authors
Najat Khalifa selected studies for inclusion, extracted data, assessed risk of bias, rated the certainty of the evidence, acted as an arbiter (where necessary), provided a clinical perspective, and wrote and revised the final report. Dr Khalifa is the guarantor of this review.
Simon Gibbon selected studies for inclusion, extracted data, assessed risk of bias, rated the certainty of the evidence, acted as an arbiter (where necessary), and wrote and revised the final report.
Natalie Cheung selected studies, extracted data, assessed risk of bias, rated the certainty of the evidence, and contacted the authors of papers for additional information.
Birgit Völlm obtained and reviewed a report of a study published in the German language and provided resources for the review.
Lucy McCarthy coordinated the review, selected studies for inclusion, extracted data, assessed risk of bias, rated the certainty of the evidence, interpreted the data, entered the data into Review Manager 5 (Review Manager 2014), wrote to authors of papers for additional information, and wrote and revised the final report.
Sources of support
Internal sources
-
Nottinghamshire Healthcare NHS Foundation Trust, UK
Provided financial support for the time of LM, SG, NC and NK to facilitate the review.
External sources
None, Other
Declarations of interest
Najat R Khalifa ‐ none known.
Simon Gibbon ‐ none known.
Birgit A Völlm ‐ none known.
Natalie H‐Y Cheung ‐ none known.
Lucy McCarthy ‐ none known.
Disclaimer: The results of a Cochrane Review can be interpreted differently depending on people's perspectives and circumstances. please consider the conclusions presented carefully. They are the opinions of review authors, and are not necessarily those of the NHS or the Department of Health.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Arndt 1994 {published data only}
- 2010 review team. Cochrane enquiry [personal communication]. Email to: Arndt study team 2009.
- Arndt IO, Dorozynski L, Woody GE, McLellan AT, O'Brien CP. Desipramine treatment of cocaine dependence in methadone-maintained patients. Archives of General Psychiatry 1992;49(11):888-93. [DOI: 10.1001/archpsyc.1992.01820110052008] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Arndt IO, McClellan AT, Dorozynsky L, Woody GE, O'Brien CP. Desipramine treatment for cocaine dependence: role of antisocial personality disorder. Journal of Nervous and Mental Disease 1994;182(3):151-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Barratt 1997 {published data only}
- Barratt ES, Stanford MS, Felthous AR, Kent TA. The effects of phenytoin on impulsive and premeditated aggression: a controlled study. Journal of Clinical Psychopharmacology 1997;17(5):341-9. [PMID: ] [DOI] [PubMed]
- Barratt ES, Stanford MS, Kent TA, Felthous A. Neuropsychological and cognitive psychophysiological substrates of impulsive aggression. Biological Psychiatry 1997;41(10):1045-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Coccaro 2009 {published data only}
- Coccaro E. Cochrane review enquiry and request [personal communication]. Email to: L McCarthy 15 September 2017.
- Coccaro EF, Lee RJ, Kavoussi RJ. A double-blind, randomized, placebo-controlled trial of fluoxetine in patients with intermittent explosive disorder. Journal of Clinical Psychiatry 2009;70(5):653-62. [DOI: 10.4088/JCP.08m04150] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gowin 2012 {published data only}
- Gowin JL, Green CE, Alcorn JL, Swann AC, Moeller FG, Lane SD. Chronic tiagabine administration and aggressive responding in individuals with a history of substance abuse and antisocial behavior. Journal of Psychopharmacology 2012;26(7):982-93. [DOI: 10.1177/0269881111408962] [PMC4777893] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hollander 2003 {published data only}
- Hollander E, Tracy KA, Swann AC, Coccaro EF, McElroy SL, Wozniak P, et al. Divalproex in the treatment of impulsive aggression: efficacy in cluster B personality disorders. Neuropsychopharmacology 2003;28(6):1186-97. [DOI: 10.1038/sj.npp.1300153] [PMID: ] [DOI] [PubMed] [Google Scholar]
Konstenius 2014 {published data only}
- Cheung N. Methylphenidate for attention deficit hyperactivity disorder and drug relapse in criminal offenders with substance dependence [personal communication]. Email to: Konstenius M 1 Februrary 2017. [DOI] [PMC free article] [PubMed]
- Franck J, Konstenius M, Jayaram-Lindström N, Philips B, Guterstam J. ADHD in drug addiction: a RCT on the feasibility of methylphenidate treatment in criminal amphetamine users. International Journal of Neuropsychopharmacology 2012;15(Suppl 1):S41. [DOI: 10.1017/S1461145712000508] [jS-36-004j] [DOI] [Google Scholar]
- Konstenius M, Jayaram-Lindström N, Guterstam J, Beck O, Philips B, Franck J. Methylphenidate for attention deficit hyperactivity disorder and drug relapse in criminal offenders with substance dependence: a 24-week randomized placebo-controlled trial. Addiction 2014;109(3):440-9. [DOI: 10.1111/add.12369] [PMC4226329] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leal 1994 {published data only}
- Leal J, Ziedonis D, Kosten T. Antisocial personality disorder as a prognostic factor for pharmacotherapy of cocaine dependence. Drug and Alcohol Dependence 1994;35(1):31-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Powell 1995 {published data only}
- Powell BJ, Campbell JL, Landon JF, Liskow BI, Thomas HM, Nickel EJ, et al. A double-blind, placebo-controlled study of nortriptyline and bromocriptine in male alcoholics subtyped by comorbid psychiatric disorders. Alcoholism, Clinical and Experimental Research 1995;19(2):462-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ralevski 2007 {published data only}
- Petrakis IL, Poling J, Levinson C, Nich C, Carroll K, Rounsaville B. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biological Psychiatry 2005;57(10):1128-37. [DOI: 10.1016/j.biopsych.2005.02.016] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ralevski E, Ball S, Nich C, Limoncelli D, Petrakis I. The impact of personality disorders on alcohol-use outcomes in a pharmacotherapy trial for alcohol dependence and comorbid Axis I disorders. American Journal on Addictions 2007;16(6):443-9. [DOI: 10.1080/10550490701643336] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stanford 2001 {published data only}
- Stanford MS, Houston RJ, Mathias CW, Greve KW, Villemarette-Pittman NR, Adams D. A double-blind placebo-controlled crossover study of phenytoin in individuals with impulsive aggression. Psychiatry Research 2001;103(2-3):193-203. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stanford 2005 {published data only}
- Stanford MS, Helfritz LE, Conklin SM, Villemarette-Pittman NR, Greve KW, Adams D, et al. A comparison of anticonvulsants in the treatment of impulsive aggression. Experimental and Clinical Psychopharmacology 2005;13(1):72-7. [DOI: 10.1037/1064-1297.13.1.72] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aberg‐Wisted 2000 {published data only}
- Aberg-Wistedt A, Agren H, Ekselius L, Bengtsson F, Akerblad AC. Sertraline versus paroxetine in major depression: clinical outcome after six months of continuous therapy. Journal of Clinical Psychopharmacology 2000;20(6):645-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Agosti 2002 {published data only}
- Agosti V, McGrath PJ. Comparison of the effects of fluoxetine, imipramine and placebo on personality in atypical depression. Journal of Affective Disorders 2002;71(1-3):113-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Alcorn 2015 {published data only}
- Alcorn JL 3rd, Rathnayaka N, Swann AC, Moeller FG, Lane SD. Effects of intranasal oxytocin on aggressive responding in antisocial personality disorder. Psychological Record 2015;65(4):691-703. [DOI: 10.1007/s40732-015-0139-y] [PMC4807973] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 1976 {published data only}
- Allen HE, Dinitz S, Foster TW, Goldman H, Lindner LA. Sociopathy: an experiment in internal environmental control. American Behavioral Scientist 1976;20(2):215-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
Alpert 1990 {published data only}
- Alpert M, Allan ER, Citrome L, Laury G, Sison C, Sudilovsky A. A double-blind, placebo-controlled study of adjunctive nadolol in the management of violent psychiatric patients. Psychopharmacology Bulletin 1990;26(3):367-71. [PMID: ] [PubMed] [Google Scholar]
Battaglia 1999 {published data only}
- Battaglia J, Wolff TK, Wagner-Johnson DS, Rush AJ, Carmody TJ, Basco MR. Structured diagnostic assessment and depot fluphenazine treatment of multiple suicide attempters in the emergency department. International Clinical Psychopharmacology 1999;14(6):361-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Black 1994 {published data only}
- Black DW, Wesner RB, Gabal J, Bowers W, Monahan P. Predictors of short-term treatment response in 66 patients with panic disorder. Journal of Affective Disorders 1994;30(4):233-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Coccaro 1997 {published data only}
- Coccaro EF, Kavoussi RJ. Fluoxetine and impulsive aggressive behaviour in personality-disordered subjects. Archives of General Psychiatry 1997;54(12):1081-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dunlop 2011 {published data only}
- Dunlop BW, DeFife JA, Marx L, Garlow SJ, Nemeroff CB, Lilienfeld SO. The effects of sertraline on psychopathic traits. International Clinical Psychopharmacology 2011;26(6):329-37. [DOI: 10.1097/YIC.0b013e32834b80df] [PMC3202964] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlow SJ, Dunlop BW, Neyman K, Ninan PT, Nemeroff CB. The addition of tri-iodothyronine (T3) to sertraline does not enhance antidepressant response in patients with major depressive disorder. In: Annual Meeting of the American College of Neuropsychopharmacology; 2007 Dec; Boca Raton (FL). 2007.
Ekselius 1998 {published data only}
- Ekselius L, Von Knorring L. Personality disorder comorbidity with major depression and response to treatment with sertraline or citalopram. International Clinical Psychopharmacology 1998;13(5):205-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fournier 2008 {published data only}
- Fournier JC, DeRubeis RJ, Shelton RC, Gallop R, Amsterdam JD, Hollon SD. Antidepressant medications v. cognitive therapy in people with depression with or without personality disorder. British Journal of Psychiatry 2008;192(2):124-9. [DOI: 10.1192/bjp.bp.107.037234] [PMC2682552] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
George 2011 {published data only}
- George DT, Phillips MJ, Lifshitz M, Lionetti TA, Spero DE, Ghassemzedeh N, et al. Fluoxetine treatment of alcoholic perpetrators of domestic violence: a 12-week, double-blind, randomized, placebo-controlled intervention study. Journal of Clinical Psychiatry 2011;72(1):60-5. [DOI: 10.4088/JCP.09m05256gry] [PMC3026856] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jariani 2010 {published data only}
- Jariani M, Saaki M, Nazari H, Birjandi M. The effect of olanzapine and sertraline on personality disorder in patients with methadone maintenance therapy. Psychiatria Danubina 2010;22(4):544-7. [PMID: ] [PubMed] [Google Scholar]
Johnson 2013 {published data only}
- Blevins D, Wang XQ, Sharma S, Ait-Daoud N. Impulsiveness as a predictor of topiramate response for cocaine use disorder. American Journal on Addictions 2019;28(2):71-6. [DOI: 10.1111/ajad.12858] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Wang XQ, Penberthy JK, Javors MA, Seneviratne C, et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry 2013;70(12):1338-46. [DOI: 10.1001/jamapsychiatry.2013.2295] [PMID: ] [DOI] [PubMed] [Google Scholar]
Joyce 2003 {published data only}
- Joyce PR, Mulder RT, Luty SE, Mackenzie JM, Sullivan PF, Cloninger RC. Borderline personality disorder in major depression: symptomatology, temperament, character, differential drug response, and 6-month outcome. Comprehensive Psychiatry 2003;44(1):35-43. [DOI: 10.1053/comp.2003.50001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kampman 2013 {published data only}
- Kampman KM, Pettinati HM, Lynch KG, Spratt K, Wierzbicki MR, O’Brien CP. A double-blind, placebo-controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug and Alcohol Dependence 2013;133(1):94-9. [DOI: 10.1016/j.drugalcdep.2013.05.026] [PMC3786029] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kool 2003 {published data only}
- Kool S, Dekker J, Duijsens IJ, De Jonghe F, Puite B. Changes in personality pathology after pharmacotherapy and combined therapy for depressed patients. Journal of Personality Disorders 2003b;17(1):60-72. [DOI: 10.1521/pedi.17.1.60.24058 ] [DOI] [PubMed] [Google Scholar]
- Kool S, Dekker J, Duijsens IJ, De Jonghe F, Puite B. Efficacy of combined therapy and pharmacotherapy for depressed patients with or without personality disorders. Harvard Review of Psychiatry 2003a;11(3):133-41. [DOI: 10.1080/10673220303950] [DOI] [PubMed] [Google Scholar]
- Kool S, Schoevers R, Duijsens IJ, Peen J, Van AAlst G, De Jonghe F, et al. Treatment of depressive disorder and comorbid personality pathology: combined therapy versus pharmacotherapy [Behandeling van de depressieve stoornis en comorbide persoonlijkheidspathologie: gecombineerde therapie versus farmacotherapie]. Tijdschrift voor Psychiatrie 2007;49(6):361-72. [www.tijdschriftvoorpsychiatrie.nl/assets/articles/articles_1635pdf.pdf] [PMID: ] [PubMed] [Google Scholar]
Lane 2009 {published data only}
- Lane SD, Gowin JL, Green CE, Steinberg JL, Moeller FG, Cherek DR. Acute topiramate differentially affects human aggressive responding at low vs. moderate doses in subjects with histories of substance abuse and antisocial behavior. Pharmacology, Biochemistry and Behavior 2009;92(2):357-62. [ PMC2745401] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mattes 1990 {published data only}
- Mattes JA. Comparative effectiveness of carbamazepine and propranolol for rage outbursts. Journal of Neuropsychiatry and Clinical Neurosciences 1990;2(2):159-64. [DOI: 10.1176/jnp.2.2.159] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mattes 2005 {published data only}
- Mattes JA. Oxcarbazepine in patients with impulsive aggression: a double-blind, placebo-controlled trial. Journal of Clinical Psychopharmacology 2005;25(6):575-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mattes 2008 {published data only}
- Mattes JA. Levetiracetam in patients with impulsive aggression: a double-blind, placebo-controlled trial. Journal of Clinical Psychiatry 2008;69(2):310-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nickel 2005 {published data only}
- Nickel MK, Nickel C, Kaplan P, Lahmann C, Mühlbacher M, Tritt K, et al. Treatment of aggression with topiramate in male borderline patients: a double-blind, placebo-controlled study. Biological Psychiatry 2005;57(5):495-9. [DOI: 10.1016/j.biopsych.2004.11.044] [PMID: ] [DOI] [PubMed] [Google Scholar]
Noyes 1991 {published data only}
- Noyes R Jr, Reich JH, Suelzer M, Christiansen J. Personality traits associated with panic disorder: change associated with treatment. Comprehensive Psychiatry 1991;32(4):283-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Patience 1995 {published data only}
- Patience DA, McGuire RJ, Scott Al, Freeman CP. The Edinburgh Primary Care Depression Study: personality disorder and outcome. British Journal of Psychiatry 1995;167(3):324-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Patrizi 2019 {published data only}
- Patrizi B, Navarro-Haro MV, Gasol M. Inhaled loxapine for agitation in patients with personality disorder: an initial approach. European Neuropsychopharmacology 2019;29(1):122-6. [DOI: 10.1016/j.euroneuro.2018.10.009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shea 1990 {published data only}
- Shea MT, Pilkonis PA, Beckham E, Collins JF, Elkin I, Sotsky SM, et al. Personality disorders and treatment outcome in the NIMH Treatment of Depression Collaborative Research Program. American Journal of Psychiatry 1990;147(6):711-8. [DOI: 10.1176/ajp.147.6.711] [PMID: ] [DOI] [PubMed] [Google Scholar]
Surekha 2013 {published data only}
- Surekha P, Govil S. Efficacy of lithium as anti-aggression agent in patients with antisocial personality disorder. In: 65th Annual National Conference of the Indian Psychiatric Society; 2013 Jan 10-13; Bangalore (IN). Vol. 55. 2013:S71-2. [Abstract # F.14.9] [www.indianjpsychiatry.org/showBackIssue.asp?issn=0019-5545;year=2013;volume=55;issue=5;month=January;supp=Y]
Timmermann 2017 {published data only}
- Timmermann M, Jeung H, Schmitt R, Boll S, Freitag CM, Bertsch K, et al. Oxytocin improves facial emotion recognition in young adults with antisocial personality disorder. Psychoneuroendocrinology 2017;85:158-64. [DOI: 10.1016/j.psyneuen.2017.07.483] [PMID: ] [DOI] [PubMed] [Google Scholar]
Todorovic 2012 {published data only}
- Todorovic M, Perunicic I, Rakovic I. Mood stabilisers in the treatment of personality disorders and comorbid depression. European Neuropsychopharmacology 2012;22(Suppl 2):S231. [DOI: 10.1016/S0924-977X(12)70342-5] [Abstract # P.2.a.013] [DOI] [Google Scholar]
References to studies awaiting assessment
Charney 2015 {published data only}
- Charney DA, Heath LM, Zikos E, Palacios-Boix J, Gill KJ. Poorer drinking outcomes with citalopram treatment for alcohol dependence: a randomized, double-blind, placebo-controlled trial. Alcoholism: Clinical and Experimental Research 2015;39(9):1756-65. [DOI: 10.1111/acer.12802] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gill K. Poorer drinking outcomes with citalopram treatment for alcohol dependence: a randomized, double-blind, placebo-controlled trial [personal communication]. Email to: N Cheung 17 January 2017. [DOI] [PubMed]
Hellerstein 2000 {published data only}
- 2010 review team. Cochrane enquiry [personal communication]. Email to: D Hellerstein 2010.
- Hellerstein DJ, Kocsis JH, Chapman D, Stewart JW, Harrison W. Double-blind comparison of sertraline, imipramine, and placebo in the treatment of dysthymia: effects on personality. American Journal of Psychiatry 2000;157(9):1436-44. [DOI: 10.1176/appi.ajp.157.9.1436] [PMID: ] [DOI] [PubMed] [Google Scholar]
Verkes 1998 {published data only}
- 2010 review team. Cochrane enquiry [personal communication]. Email to: R Verkes 2010.
- Verkes RJ, Van der Mast RC, Hengeveld MW, Tuyl JP, Zwinderman AH, Van Kempen GM. Reduction by paroxetine of suicidal behavior in patients with repeated suicide attempts but not major depression. American Journal of Psychiatry 1998;155(4):543-7. [DOI: 10.1176/ajp.155.4.543] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
EudraCT2010‐018740‐13 {published data only}
- EudraCT2010-018740-13. Oxytocin effects in autistic and antisocial male adults [Short- and long-term effects of oxytocin on empathy and social behaviour in autistic and antisocial male adults]. www.clinicaltrialsregister.eu/ctr-search/trial/2010-018740-13/NL#A (first received 8 April 2010).
Additional references
Alegria 2013
- Alegria AA, Blanco C, Petry NM, Skodol AE, Liu S-M, Grant B, et al. Sex differences in antisocial personality disorder: results from the National Epidemiological Survey on Alcohol and Related Conditions. Personality Disorders 2013;4(3):214–22. [DOI: 10.1037/a0031681] [PMC3767421] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [PMC2352469] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arndt 1992
- Arndt IO, Dorozynski L, Woody GE, McLellan AT, O'Brien CP. Desipramine treatment of cocaine dependence in methadone-maintained patients. Archives of General Psychiatry 1992;49(11):888-93. [DOI: 10.1001/archpsyc.1992.01820110052008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Attkisson 1982
- Attkisson CC, Zwick R. The Client Satisfaction Questionnaire. Psychometric properties and correlations with service utilization and psychotherapy outcome. Evaluation and Program Planning 1982;5(3):233-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Barkham 2001
- Barkham M, Margison F, Leach C, Lucock M, Mellor-Clark J, Evans C, et al. Service profiling and outcomes benchmarking using the CORE-OM: toward practice-based evidence in the psychological therapies. Journal of Consulting and Clinical Psychology 2001;69(2):184-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Barratt 1991
- Barratt ES, Kent TA, Bryant SG, Felthous AR. A controlled trial of phenytoin in impulsive aggression. Journal of Clinical Psychopharmacology 1991;11(6):388–9. [DOI: 10.1097/00004714-199112000-00017] [PMID: ] [DOI] [PubMed] [Google Scholar]
Barratt 1997a
- Barratt ES, Stanford MS, Kent TA, Felthous A. Neuropsychological and cognitive psychophysiological substrates of impulsive aggression. Biological Psychiatry 1997;41(10):1045-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Beck 1988
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology 1988;56(6):893-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Black 1996
- Black DW, Baumgard CH, Bell SE, Kao C. Death rates in 71 men with antisocial personality disorder: a comparison with general population mortality. Psychosomatics 1996;37(2):131-6. [DOI: 10.1016/S0033-3182(96)71579-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Black 1999
- Black DW, Larson CL. Bad Boys, Bad Men: Confronting Antisocial Personality Disorder. Oxford: Oxford University Press, 1999. [Google Scholar]
Black 2010
- Black DW, Gunter T, Loveless P, Allen J, Sieleni B. Antisocial personality disorder in incarcerated offenders: psychiatric comorbidity and quality of life. Annals of Clinical Psychiatry 2010;22(2):113-20. [PMID: ] [PubMed] [Google Scholar]
Black 2015
- Black DW. The natural history of antisocial personality disorder. Canadian Journal of Psychiatry 2015;60(7):309–14. [DOI: 10.1177/070674371506000703] [PMC4500180] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blair 2001
- Blair RJ. Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. Journal of Neurology, Neurosurgery and Psychiatry 2001;71(6):727-31. [PMC1737625] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bond 2005
- Bond AJ. Antidepressant treatments and human aggression. European Journal of Pharmacology 2005;526(1-3):218-25. [DOI: 10.1016/j.ejphar.2005.09.067] [DOI] [PubMed] [Google Scholar]
British National Formulary 2018
- Joint Formulary Committee. British National Formulary. 75th edition. London (UK): Pharmaceutical Press, 2018. [Google Scholar]
Buss 1992
- Buss AH, Perry M. The Aggression Questionnaire. Journal of Personality and Social Psychology 1992;63(3):452-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Carlson 1993
- Carlson EB, Putnam FW. An update on the Dissociative Experiences Scale. Dissociation: Progress in the Dissociative Disorders 1993;6(1):16-27. [psycnet.apa.org/record/1994-27927-001] [Google Scholar]
Chambless 1998
- Chambless DL, Hollon SD. Defining empirically supported therapies. Journal of Consulting and Clinical Psychology 1998;66(1):7-18. [DOI: 10.1037/0022-006X.66.1.7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Coccaro 1996
- Coccaro EF, Kavoussi RJ, Sheline YI, Lish JD, Csernansky JG. Impulsive aggression in personality disorder correlates with tritiated paroxetine binding in the platelet. Archives of General Psychiatry 1996;53(6):531-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Coccaro 1998
- Coccaro EF, Kavoussi RJ, Hauger RL, Cooper TB, Ferris CF. Cerebrospinal fluid vasopressin levels: correlates with aggression and serotonin function in personality-disordered subjects. Archives of General Psychiatry 1998;55(8):708-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Coid 2006
- Coid J, Yang M, Tyrer P, Roberts A, Ullrich S. Prevalence and correlates of personality disorder in Great Britain. British Journal of Psychiatry 2006;188:423-31. [DOI: 10.1192/bjp.188.5.423] [PMID: ] [DOI] [PubMed] [Google Scholar]
Coid 2015
- Coid JW, Yang M, Ullrich S, Hickey N, Kahtan N, Freestone M. Psychiatric diagnosis and differential risks of offending following discharge. International Journal of Law and Psychiatry 2015;38:68-74. [DOI: 10.1016/j.ijlp.2015.01.009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Compton 2005
- Compton WM, Conway KP, Stinson FS, Colliver JD, Grant BF. Prevalence, correlates, and comorbidity of DSM-IV antisocial personality syndromes and alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry 2005;66(6):677-85. [DOI: 10.4088/JCP.v66n0602] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cowdry 1988
- Cowdry RW, Gardner DL. Pharmacotherapy of borderline personality disorder: alprazolam, carbamazepine, trifluoperazine and tranylcypromine. Archives of General Psychiatry 1988;45(2):111-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Crawford 2007
- Crawford M, Rutter D, Price K, Weaver T, Josson M, Tyrer P, et al. Learning the lessons: a multi-method evaluation of dedicated community-based services for people with personality disorder. www.netscc.ac.uk/hsdr/files/project/SDO_FR_08-1404-083_V01.pdf (accessed 1st November 2017).
Da Costa 2013
- Da Costa BR, Nüesch E, Rutjes AW, Johnston BC, Reichenbach S, Trelle S, et al. Combining follow-up and change data is valid in meta-analyses of continuous outcomes: meta-epidemiological study. Journal of Clinical Epidemiology 2013;66(8):847-55. [DOI: 10.1016/j.jclinepi.2013.03.009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Davies 2007
- Davies S, Clarke M, Hollin C, Duggan C. Long-term outcomes after discharge from medium secure care: a cause for concern. British Journal of Psychiatry 2007;191:70-4. [DOI: 10.1192/bjp.bp.106.029215] [PMID: ] [DOI] [PubMed] [Google Scholar]
Deakin 2003
- Deakin JF. Depression and antisocial personality disorder: two contrasting disorders of 5HT function. Journal of Neural Transmission 2003;64(Suppl):79-93. [DOI: 10.1007/978-3-7091-6020-6_5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Derogatis 1973
- Derrogatis LR, Lipman RS, Covi I. SCL-90: an outpatient psychiatric rating scale--preliminary report. Psychopharmacology Bulletin 1973;9(1):13-28. [PMID: ] [PubMed] [Google Scholar]
Dinn 2000
- Dinn WM, Harris CL. Neurocognitive function in antisocial personality disorder. Psychiatry Research 2000;97(2-3):173-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dolan 1993
- Dolan B, Coid J. Psychopathic and Anti-Social Personality Disorders: Treatment and Research Issues. London (UK): Gaskell, 1993. [Google Scholar]
Dolan 2009
- Dolan Mairead, Völlm Birgit. Antisocial personality disorder and psychopathy in women: A literature review on the reliability and validity of assessment instruments. Women and Criminality 2009;32(1):2-9. [DOI] [PubMed] [Google Scholar]
Donner 2001
- Donner A, Piaggio G, Villar J. Statistical methods for the meta-analysis of cluster randomization trials. Statistical Methods in Medical Research 2001;10(5):325-38. [DOI: 10.1177/096228020101000502] [PMID: ] [DOI] [PubMed] [Google Scholar]
DSM‐5
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th edition. Washington (DC): American Psychiatric Association, 2013. [Google Scholar]
DSM‐IV
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th edition. Washington (DC): American Psychiatric Association, 1994. [Google Scholar]
DSM‐IV‐TR
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). 4th edition. Washington (DC): American Psychiatric Association, 2000. [Google Scholar]
Duggan 2008
- Duggan C, Huband N, Smailagic N, Ferriter M, Adams C. The use of pharmacological treatments for people with personality disorder: a systematic review of randomized controlled trials. Personality and Mental Health 2008;2(3):119-70. [DOI: 10.1002/pmh.41] [DOI] [Google Scholar]
Duke 1994
- Duke PJ, Pantelis C, Barnes TR. South Westminster Schizophrenia Survey. Alcohol use and its relationship to symptoms, tardive dyskinesia and illness onset. British Journal of Psychiatry 1994;164(5):630-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey-Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [PMC2127453] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
EuroQoL Group 1990
- EuroQoL Group. EuroQoL: a new facility for the measurement of health-related quality of life. Health Policy 1990;16(3):199-208. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fazel 2002
- Fazel S, Danesh J. Serious mental disorder in 23000 prisoners: a systematic review of 62 surveys. Lancet 2002;359(9306):545-50. [DOI: 10.1016/S0140-6736(02)07740-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Follman 1992
- Follmann D, Elliot P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Galbraith 2014
- Galbraith T, Heimberg RG, Wang S, Schneier FR, Blanco C. Comorbidity of social anxiety disorder and antisocial personality disorder in the National Epidemiological Survey on Alcohol and Related Conditions (NESARC). Journal of Anxiety Disorders 2014;28(1):57–66. [DOI: 10.1016/j.janxdis.2013.11.009] [PMC3951602] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gamble 2005
- Gamble C, Hollis S. Uncertainty method improved on best-worst case analysis in a binary meta-analysis. Journal of Clinical Epidemiology 2005;58(6):579-88. [10.1016/j.jclinepi.2004.09.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
Garlow 2007
- Garlow SJ, Dunlop BW, Neyman K, Ninan PT, Nemeroff CB. The addition of tri-iodothyronine (T3) to sertraline does not enhance antidepressant response in patients with major depressive disorder. In: 46th Annual Meeting of the American College of Neuropsychopharmacology; 2007 Dec 9-13; Boca Raton (FL). 2007.
Goodwin 2003
- Goodwin RD, Hamilton SP. Lifetime comorbidity of antisocial personality disorder and anxiety disorders among adults in the community. Psychiatry Research 2003;117(2):159-66. [DOI: 10.1016/S0165-1781(02)00320-7] [DOI] [PubMed] [Google Scholar]
Gottschalk 1973
- Gottschalk LA, Covi L, Uliana R, Bates DE. Effects of diphenylhydantoin on anxiety and hostility in institutionalized prisoners. Comprehensive Psychiatry 1973;14(6):503-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 1 November 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Grilo 1998
- Grilo CM, McGlashan TH, Oldham JM. Course and stability of personality disorders. Journal of Practical Psychiatry and Behavioural Health 1998;4(2):61-75. [DOI: 10.1097/00131746-199803000-00001] [DOI] [Google Scholar]
Guy 2018
- Guy N, Newton-Howes G, Ford H, Williman J, Foulds J. The prevalence of comorbid alcohol use disorder in the presence of personality disorder: systematic review and explanatory modelling. Personality and Mental Health 2018;12(3):216-28. [DOI: 10.1002/pmh.1415] [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence--study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407-15. [DOI: 10.1016/j.jclinepi.2010.07.017] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, White IR, Wood AM. Imputation methods for missing outcome data in meta-analysis of clinical trials. Clinical Trials 2008;5(3):225-39. [DOI: 10.1177/1740774508091600] [PMC2602608] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0. www.training.cochrane.org/handbook Updated July 2019. [WEBSITE: www.training.cochrane.org/handbook]
Home Office 1999
- Home Office, Department of Health. Managing Dangerous People with Severe Personality Disorder: Proposals for Policy Development; July 1999. webarchive.nationalarchives.gov.uk/+/http:/www.homeoffice.gov.uk/documents/cons-1999-personality-disorder?view=Binary 1999.
Huband 2010
- Huband N, Ferriter M, Nathan R, Jones H. Antiepileptics for aggression and associated impulsivity. Cochrane Database of Systematic Reviews 2010, Issue 2. Art. No: CD003499. [DOI: 10.1002/14651858.CD003499.pub3] [PMC4163499] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ICD‐10
- World Health Organization (WHO). The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva (CH): World Health Organization, 1992. [Google Scholar]
ICD‐11
- World Health Organization. ICD-11: International Classification of Diseases for Mortality and Morbidity Statistics 11th Revision. www.who.int/classifications/icd/en (accessed 3 July 2020).
Jakobsen 2014
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Medical Research Methodology 2014;14:120. [DOI: 10.1186/1471-2288-14-120] [PMC4251848] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnstone 2018
- Johnstone L, Boyle M, Cromby J, Dillon J, Harper D, Kinderman P, et al. The Power Threat Meaning Framework: Towards the identification of patterns in emotional distress, unusual experiences and troubled or troubling behaviour, as an alternative to functional psychiatric diagnosis. Leicester (UK): British Psychological Society, 2018. [Google Scholar]
Krasnova 2019
- Krasnova A, Eaton WW, Samuels JF. Antisocial personality and risks of cause-specific mortality: results from the Epidemiologic Catchment Area study with 27 years of follow-up. Social Psychiatry and Psychiatric Epidemiology 2019;54(5):617-25. [DOI: 10.1007/s00127-018-1628-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019.. Available from www.training.cochrane.org/handbook.
Lieb 2010
- Lieb K, Völlm B, Rücker G, Timmer A, Stoffers JM. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. British Journal of Psychiatry 2010;196(1):4-12. [DOI: 10.1192/bjp.bp.108.062984] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lieb 2016
- Lieb K, Von der Osten-Sacken J, Stoffers-Winterling J, Reiss N, Barth J. Conflicts of interest and spin in reviews of psychological therapies: a systematic review. BMJ Open 2016;6(4):e010606. [DOI: 10.1136/bmjopen-2015-010606] [DOI] [PMC free article] [PubMed] [Google Scholar]
Links 1990
- Links PS, Steiner M, Boiago I, Irwin D. Lithium therapy for borderline patients: preliminary findings. Journal of Personality Disorders 1990;4(2):173-81. [DOI: 10.1521/pedi.1990.4.2.173] [psycnet.apa.org/record/1990-31662-001] [DOI] [Google Scholar]
Lion 1979
- Lion JR. Benzodiazepines in the treatment of aggressive patients. Journal of Clinical Psychiatry 1979;40(2):70–1. [PMID: ] [PubMed] [Google Scholar]
Livesley 2007
- Livesley WJ. A framework for integrating dimensional and categorical classifications of personality disorder. Journal of Personality Disorders 2007;21(2):199-224. [DOI: 10.1521/pedi.2007.21.2.199] [PMID: ] [DOI] [PubMed] [Google Scholar]
Malone 1994
- Malone RP, Luebbert J, Pena-Ariet M, Biesecker K, Delaney MA. The Overt Aggression Scale in the study of lithium in aggressive conduct disorder. Psychopharmacology Bulletin 1994;30(2):215-8. [PMID: ] [PubMed] [Google Scholar]
Markovitz 2004
- Markovitz PJ. Recent trends in the pharmacotherapy of personality disorders. Journal of Personality Disorders 2004;18(1):90-101. [PMID: ] [DOI] [PubMed] [Google Scholar]
Martens 2000
- Martens WHJ. Antisocial and psychopathic personality disorders: causes, course, and remission - a review article. International Journal of Offender Therapy and Comparative Criminology 2000;44(4):406-30. [DOI: 10.1177/0306624X00444002] [psycnet.apa.org/record/2001-00934-001] [DOI] [Google Scholar]
Mazur 1998
- Mazur A, Booth A. Testosterone and dominance in men. Behavioral and Brain Sciences 1998;21(3):353-97. [PMID: ] [PubMed] [Google Scholar]
McGilloway 2010
- McGilloway A, Hall RE, Lee T, Bhui KS. A systematic review of personality disorder, race and ethnicity: prevalence, aetiology and treatment. BMC Psychiatry 2010;10:33. [DOI: 10.1186/1471-244X-10-33] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 1998
- Moher D. CONSORT: an evolving tool to help improve the quality of reports of randomized controlled trials. Consolidated Standards of Reporting Trials. JAMA 1998;279(18):1489-91. [DOI: 10.1001/jama.279.18.1489] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [PMC2707599] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2010
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. [DOI: 10.1136/bmj.c869] [PMC2844943] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moran 1999
- Moran P. The epidemiology of antisocial personality disorder. Social Psychiatry and Psychiatric Epidemiology 1999;34(5):231-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Moss 1990
- Moss HB, Yao JK, Panzak GL. Serotonergic responsivity and behavioral dimensions in antisocial personality disorder with substance abuse. Biological Psychiatry 1990;28(4):325-38. [PMID: ] [DOI] [PubMed] [Google Scholar]
Myers 1998
- Myers MG, Stewart DG, Brown SA. Progression from conduct disorder to antisocial personality disorder following treatment for adolescent substance abuse. American Journal of Psychiatry 1998;155(4):479-85. [DOI: 10.1176/ajp.155.4.479] [PMID: ] [DOI] [PubMed] [Google Scholar]
Newman 1939
- Newman D. The distribution of range in samples from a normal population, expressed in terms of an independent estimate of standard deviation. Biometrika 1939;31:20-30. [DOI: 10.1093/biomet/31.1-2.20] [psycnet.apa.org/record/1940-00666-001] [DOI] [Google Scholar]
Newton‐Howes 2006
- Newton-Howes G, Tyrer P, Johnson T. Personality disorder and the outcome of depression: meta-analysis of published studies. British Journal of Psychiatry 2006;188:13-20. [DOI: 10.1192/bjp.188.1.13] [PMID: ] [DOI] [PubMed] [Google Scholar]
NICE 2010
- National Institute for Health and Care Excellence. Antisocial personality disorder: prevention and management. www.nice.org.uk/guidance/cg77/evidence/full-guideline-242104429 (accessed 29 April 2019). [PubMed]
NICE 2015
- National Institute for Health and Care Excellence. Personality disorders: borderline and antisocial; 11 June 2015. www.nice.org.uk/guidance/qs88 2015. [ISBN: 978-1-4731-1247-6]
Ogloff 2006
- Ogloff JRP. Psychopathy/antisocial personality disorder conundrum. Australian & New Zealand Journal of Psychiatry 2006;40(6-7):519-28. [DOI: 10.1080/j.1440-1614.2006.01834.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Oltmanns 2019
- Oltmanns JR, Widiger TA. Evaluating the assessment of the ICD-11 personality disorder diagnostic system. Psychological Assessment 2019;31(5):674-84. [DOI: 10.1037/pas0000693] [PMC6488396] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ONS 2004
- Office for National Statistics. Social Trends 34. London (UK): Office of National Statistics, 2004. [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799-812. [DOI: 10.2466/pr0.1962.10.3.799] [psycnet.apa.org/record/1963-05043-001] [DOI] [Google Scholar]
Paris 2003
- Paris J. Personality Disorders Over Time: Precursors, Course, and Outcome. Arlington (VA): American Psychiatric Association, 2003. [DOI] [PubMed] [Google Scholar]
Patton 1995
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology 1995;51(6):768-74. [PMID: ] [DOI] [PubMed] [Google Scholar]
Perdikouri 2007
- Perdikouri M, Rathbone G, Huband N, Duggan C. A comparison of adults with antisocial personality traits with and without childhood conduct disorder. Annals of Clinical Psychiatry 2007;19(1):17-23. [DOI: 10.1080/10401230601163501] [PMID: ] [DOI] [PubMed] [Google Scholar]
Petrakis 2005
- Petrakis IL, Poling J, Levinson C, Nich C, Carroll K, Rounsaville B. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biological Psychiatry 2005;57(10):1128-37. [DOI: 10.1016/j.biopsych.2005.02.016] [PMID: ] [DOI] [PubMed] [Google Scholar]
Raine 2000
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Archives of General Psychiatry 2000;57(2):119-27. [PMID: ] [DOI] [PubMed] [Google Scholar]
Reed 1994
- Department of Health, Home Office. Report of the Department of Health and Home Office Working Group on Psychopathic Disorder (Chairman, Dr John Reed, CB). London (UK): Department of Health; Home Office, 1994. [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ripoll 2011
- Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. International Journal of Neuropsychopharmacology 2011;14(9):1257–88. [DOI: 10.1017/S1461145711000071] [PMID: ] [DOI] [PubMed] [Google Scholar]
Robins 1998
- Robins LN. The intimate connection between antisocial personality and substance abuse. Social Psychiatry and Psychiatric Epidemiology 1998;33(8):393-9. [DOI: 10.1007/s001270050071] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sampson 2013
- Sampson CJ, James M, Huband N, Geelan S, McMurran M. Cost implications of treatment non‐completion in a forensic personality disorder service. Criminal Behaviour and Mental Health 2013;23(5):321-35. [DOI: 10.1002/cbm.1866] [PMC3920639] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Scott 2001
- Scott S, Knapp M, Henderson J, Maughan B. Financial cost of social exclusion: follow up study of antisocial children into adulthood. BMJ 2001;323(7306):191-4. [DOI: 10.1136/bmj.323.7306.191] [PMC35269] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheard 1971
- Sheard MH. Effect of lithium on human aggression. Nature 1971;230(5289):113–4. [DOI: 10.1038/230113a0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sheard 1976
- Sheard MH, Marini JL, Bridges CI, Wagner E. The effect of lithium on impulsive aggressive behavior in man. American Journal of Psychiatry 1976;133(12):1409-13. [DOI: 10.117/ajp.133.12.1409] [PMID: ] [DOI] [PubMed] [Google Scholar]
Singleton 1998
- Singleton N, Melzer H, Gatward R. Psychiatric Morbidity Among Prisoners in England and Wales. London (UK): HM Stationery Office, 1998. [Google Scholar]
Skodol 2002
- Skodol AE, Siever LJ, Livesley WJ, Gunderson JG, Pfohl B, Widiger TA. The borderline diagnosis II: biology, genetics and clinical course. Biological Psychiatry 2002;51(12):951-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Skodol 2005
- Skodol AE, Oldham JM, Bender DS, Dyck IR, Stout RL, Morey LC, et al. Dimensional representations of DSM-IV personality disorders: relationships to functional impairment. American Journal of Psychiatry 2005;162(10):1919-25. [DOI: 10.1176/appi.ajp.162.10.1919] [PMID: ] [DOI] [PubMed] [Google Scholar]
Skodol 2018
- Skodol Andrew E. Can Personality Disorders Be Redefined in Personality Trait Terms? American Journal of Psychiatry 2018;175(7):590-592. [DOI] [PubMed] [Google Scholar]
Soloff 1998
- Soloff PH. Algorithms for pharmacological treatment of personality dimensions: symptom-specific treatments for cognitive-perceptual, affective, and impulsive-behavioral dysregulation. Bulletin of the Menninger Clinic 1998;62(2):195-214. [PMID: ] [PubMed] [Google Scholar]
Spielberger 1999
- Spielberger CD. STAXI-2: State-Trait Anger Expression Inventory-2: Professional Manual. Odessa (FL): Psychological Assessment Resources, 1999. [Google Scholar]
Stein 1992
- Stein GS. Drug treatment of the personality disorders. British Journal of Psychiatry 1992;161(2):167-84. [DOI: 10.1192/bjp.161.2.167] [DOI] [PubMed] [Google Scholar]
Stein 1995
- Stein DJ, Simeon D, Frenkel M, Islan MN, Hollander E. An open trial of valproate in borderline personality disorder. Journal of Clinical Psychiatry 1995;56(11):506-10. [PMID: ] [PubMed] [Google Scholar]
Stovold 2014
- Stovold E, Beecher D, Foxlee R, Noel-Storr A. Study flow diagrams in Cochrane systematic review updates: an adapted PRISMA flow diagram. Systematic Reviews 2014;3:54. [DOI: 10.1186/2046-4053-3-54] [PMC4046496] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sugden 2006
- Sugden SG, Kile SJ, Hendren RL. Neurodevelopmental pathways to aggression: a model to understand and target treatment in youth. Journal of Neuropsychiatry and Clinical Neurosciences 2006;18(3):302-17. [DOI: 10.1176/jnp.2006.18.3.302] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tait 2002
- Tait L, Birchwood M, Trower P. A new scale (SES) to measure engagement with community mental health services. Journal of Mental Health 2002;11(2):191-8. [DOI: 10.1080/09638230020023570-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Trull 2010
- Trull TJ, Jahng S, Tomko RL, Wood PK, Sher KJ. Revised NESARC personality disorder diagnoses: gender, prevalence, and comorbidity with substance dependence disorders. Journal of Personality Disorders 2010;24(4):412-26. [DOI: 10.1521/pedi.2010.24.4.412] [PMC3771514] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tyrer 2004
- Tyrer P, Bateman AW. Drug treatment for personality disorders. Advances in Psychiatric Treatment 2004;10(5):389-98. [DOI: 10.1192/apt.10.5.389] [DOI] [Google Scholar]
Tyrer 2005
- Tyrer P, Nur U, Crawford M, Karlsen S, McLean C, Rao B, et al. The Social Functioning Questionniare: a rapid and robust measure of perceived functioning. International Journal of Social Psychiatry 2005;51(3):265-75. [PMID: ] [PubMed] [Google Scholar]
Tyrer 2015
- Tyrer P, Reed GM, Crawford MJ. Classification, assessment, prevalence, and effect of personality disorder. The Lancet 2015;385(9969):717-26. [DOI: 10.1016/S0140-6736(14)61995-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Volkert 2018
- Volkert J, Gablonski TC, Rabung S. Prevalence of personality disorders in the general adult population in Western countries: systematic review and meta-analysis. British Journal of Psychiatry 2018;213(6):709-15. [DOI: 10.1192/bjp.2018.202] [PMID: ] [DOI] [PubMed] [Google Scholar]
Völlm 2010
- Völlm B, Richardson P, McKie S, Reniers R, Elliott R, Anderson IM, et al. Neuronal correlates and serotonergic modulation of behavioural inhibition and reward in healthy and antisocial individuals. Journal of Psychiatric Research 2010;44(3):123-31. [DOI: 10.1016/j.jpsychires.2009.07.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Warren 2001
- Warren F, Preedy-Fayers K, McGauley G, Pickering A, Norton K, Geddes JR, et al. Review of Treatments for Severe Personality Disorder. London (UK): Home Office, 2001. [Google Scholar]
Weissman 1976
- Weissman MM, Bothwell S. Assessment of social adjustment by patient self-report. Archives of General Psychiatry 1976;33(9):1111-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Weissman 1993
- Weissman MM. The epidemiology of personality disorders: a 1990 update. Journal of Personality Disorders 1993;Suppl 1:44-62. [psycnet.apa.org/record/1993-33722-001] [Google Scholar]
Wilcox 1995
- Wilcox JA. Divalproex sodium as a treatment for borderline personality disorder. Annals of Clinical Psychiatry 1995;7(1):33-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wolff 1999
- Wolff K, Farrell M, Marsden J, Monteiro MG, Ali R, Welch S, et al. A review of biological indicators of illicit drug use: practical considerations and clinical usefulness. Addiction 1999;94(9):1279-98. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67(6):361-70. [DOI: 10.1111/j.1600-0447.1983.tb09716.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Khalifa 2009
- Khalifa N, Duggan C, Stoffers JM, Huband N, Völlm BA, Lieb K. Pharmacological interventions for antisocial personality disorder. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD007667. [DOI: 10.1002/14651858.CD007667] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khalifa 2010
- Khalifa N, Duggan C, Stoffers J, Huband N, Völlm BA, Ferriter M, et al. Pharmacological interventions for antisocial personality disorder. Cochrane Database of Systematic Reviews 2010, Issue 8. Art. No: CD007667. [DOI: 10.1002/14651858.CD007667.pub2] [PMC4160654] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]


