Abstract
Several pieces of the puzzle of the natural history of tuberculosis are assembled in this review to illustrate the potential reservoirs and sources of the Mycobacterium tuberculosis complex (MTBC) mycobacteria, their transmission to animals and humans, and their fate in populations, in a co-evolutionary perspective. Millennia-old companions of mammalian and human populations, MTBC are detected in the soil, in which they infect and survive within vegetative amoebae and cysts, except for Mycobacterium canettii. Never detected in the sphere of plants, they are transmissible by transcutaneous, digestive and respiratory routes and cause an infection of the lymphatic system with secondary dissemination in most tissues, in which they determine a specific and non-pathognomonic granulomatous inflammatory reaction; in which MTBC survives in dormant form irrespective of MTBC species and mammalian species; indicating that the current epidemiology in mammalian populations is essentially governed by the probabilities of contact between mammalian species and MTBC species. Individual variabilities in clinical expression of tuberculosis are related to MTBC species, strain and inoculum; host genetic factors; acquired modulations of the inflammatory response; and probably human microbiota. This review of the literature suggests an evolutionary natural history of telluric environmental mycobacteria, satellites of unicellular eukaryotes, transmissible to mammals via the digestive and then respiratory tracts, in which they determine a fatal contagious infection that is primarily lymphatic and a quiescence-mimicking encysted form. This review opens perspectives for microbiological and translational medical research.
Keywords: Microbiota, Mycobacterium, Mycobacterium bovis, Mycobacterium tuberculosis, natural history, sources, transmission, tuberculosis
Introduction
Tuberculosis is one of the leading infectious causes of death worldwide, and it is estimated that approximately one-quarter of the world's population is latently infected with Mycobacterium tuberculosis [1,2]. This fatal infectious disease is caused by 13 species of closely related mycobacteria forming the Mycobacterium tuberculosis complex (MTBC) [3]. Because of their genomic proximity, the exact taxonomic status of these species is debated, and some authors consider the species of the MTBC as ecotypic variants of M. tuberculosis [4]. However, these species or ecotypes have genomic, genetic and phenotypic differences that may explain differences in reservoirs and modes of transmission. Mycobacterium tuberculosis does not cause tuberculosis only in humans, being described in a variety of animals, mainly mammals [5].
The outcome for persons infected with M. tuberculosis is highly variable, ranging from total elimination of the pathogen by the inflammatory and immune system, to long-term asymptomatic transport during so-called dormant tuberculosis, to symptomatic tuberculosis, and finally death [6]. The role of the microbiota in modulating the expression of M. tuberculosis infection is emerging, and the continued transmission of M. tuberculosis is the main factor in maintaining the high incidence of this disease [7].
Mycobacteria of the MTBC
Of the 13 species or ecotypes currently described in MTBC, only nine have been associated with human tuberculosis, including the biliary bacillus Calmette–Guérin (BCG) vaccine [8,9]. MTBCs have 72 highly conserved genomic sequences (>99.9% nucleotide identity) but 73 different phenotypes. It has been suggested that MTBC evolved from a common ancestor via 74 successive DNA deletions/insertions, giving them their differences in pathogenicity [10]. The genomic evolution of these mycobacteria is achieved by a progressive decrease in the coding capacity of their genome, which is exclusively chromosomal, with loss of genomic fragments called deletion regions and invasion of the genome by insertion sequences [11]. Mycobacterium canettii is the closest species to the common ancestor of MTBC, having a genome of 4.48 ± 0.05 Mb [11] coding for unique functions such as the Trans cobalamin gene. Mycobacterium canettii is responsible for non-contagious tuberculosis [12] and has a geographical distribution limited to the Horn of Africa. Microbiologically, M. canettii has a 17-hour doubling time that is one-third shorter than that of M. tuberculosis (25 hours) and produces smooth colonies, unlike M. tuberculosis, which has a rough morphotype [12]. Mycobacterium tuberculosis has a worldwide distribution in six major phylogenetic lineages (lineages 1, 2, 3, 4, 7 and the recently described lineage 8) [13] that are unevenly distributed across the world. L1 and L2 lineages predominate in East and Southeast Asia, while L1 and L3 lineages predominate in the Indian subcontinent, L3 and L4 lineages predominate in Central Asia and Russia, and the L4 lineage predominates in Europe, the Americas, North Africa and the Middle East [14]. It is in sub-Saharan Africa that we find the greatest variety of lineages, because in addition to the L1, L2, L3 and L4 lineages, two new lineages have recently been identified, the L7 lineage in Ethiopia and the L8 lineage in Rwanda and Uganda [[13], [14], [15]]. Mycobacterium africanum has two lineages (L5 and L6) distributed exclusively in West Africa and responsible for tuberculosis that is indistinguishable from that caused by M. tuberculosis [[16], [17], [18]]. The virulence of MTBC is variable and differs within strains of the same species, for example: modern lineages of M. tuberculosis (L2–L4) are more virulent and responsible for most tuberculosis cases in the world [10]. Specifically, the Beijing (L2) strains are the most virulent MTBC for humans with a predisposition to develop further resistance to anti-tuberculosis drugs and have a high capacity for propagation, illustrated by the fact that these strains have been described in cattle and dogs [5,10,19]. This virulence of Beijing strains is correlated with deletions in the pks15/1 gene and in the RD207 region [20]. In contrast, Mycobacterium bovis BCG is the least virulent strain among MTBC, its attenuation follows the loss of the RD1 region in its genome [21].
Human–environment interfaces: entrance doors and microbiota
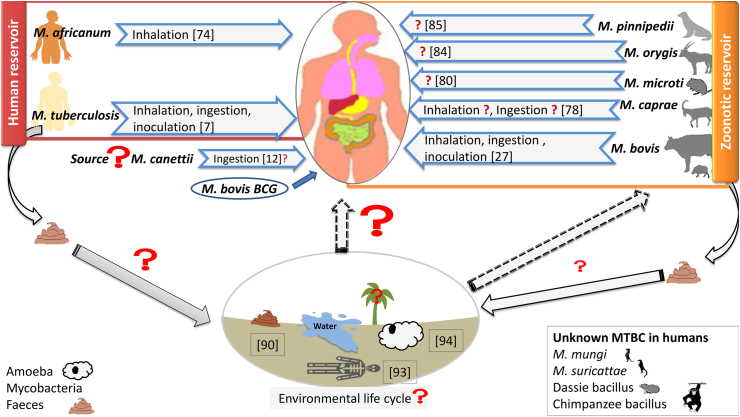
Since the work of Villemin and the introduction of M. tuberculosis culture by Robert Koch, many scientists have tried to determine how tuberculosis is transmitted to humans [7,22]. A large part of the studies on tuberculosis transmission were carried out at the end of the nineteenth/early twentieth century. These studies focused on culture to demonstrate viability, and inoculation of animals to confirm the pathology [7]. Several theories existed at the time, and Calmette and Guérin reported in 1905 the intestinal origin of pulmonary tuberculosis [23]. Other works reported the culture of M. tuberculosis in the environment [7]. It should be considered that, at that time, knowledge of the diversity of mycobacteria (tuberculous and non-tuberculous) was more limited, so that several studies were carried out with environmental mycobacteria, or with MTBC different from M. tuberculosis [7]. An important part of our knowledge about tuberculosis transmission comes from the investigation of cases acquired during care. It has been well recognized since the 1950s that the respiratory tract is the main route of transmission of M. tuberculosis; however, this does not exclude other routes of transmission [7] (Fig. 1).
Fig. 1.
Reservoirs of the Mycobacterium tuberculosis complex and routes of transmission to humans.
Respiratory route
Innovative studies by Riley and Wells in the 1950s and 1960s showed that exposure of guinea pigs to aerosol droplets from patients with pulmonary tuberculosis resulted in substantial tuberculin conversion rates in the guinea pigs [24,25]; and this work has recently been replicated with consistent results [7]. These studies have clarified the basic principles of airborne transmission of pulmonary tuberculosis, which is now widely accepted as the major route of transmission [7,26]. Cases of transmission of M. tuberculosis by aerosolized M. tuberculosis have been reported during autopsy [7] and surgical procedures of incision and irrigation of tuberculous abscesses [7]. Respiratory transmission also includes M. bovis tuberculosis, not only in persons exposed to aerosols from animals infected with M. bovis [27,28], but also by human-to-human transmission of M. bovis [[28], [29], [30]].
In mice, infection with M. tuberculosis aerosols leads to a loss of intestinal microbiota diversity 6 days after infection [31]. These rapid changes in the microbiota are attributed to the host immune response, with all mice showing a recovery of microbial diversity within a few days [31]. The role of the respiratory microbiota in the development of tuberculosis remains uncertain. In addition, the data show that there is a unique microbial diversity between individuals with tuberculosis and healthy controls, but the results remain controversial [32]. Importantly for the diagnosis of tuberculosis by laboratory culture, the protocols include a step of chemical decontamination (sodium hydroxide) of the sputum to remove fast-growing microorganisms to facilitate the growth of mycobacteria [33]. This decontamination may have contributed to the non-observance by culture of certain bacterial communities that could coexist with M. tuberculosis in the lungs and respiratory tract, opening prospects for clinical microbiology work.
Digestive route
Historically, the digestive tract has been the primary route for BCG vaccine administration, illustrating the possibility of systemic passage of MTBC after ingestion [34,35]. This route of administration was abandoned in most countries following the ‘Lübeck Disaster’, and in 1976 Brazil was the last country to abandon this route, because of poor response to skin testing and for economic and operational reasons [36,37]. Currently, the possibility of a digestive gateway for MTBC is neglected despite published evidence, illustrated by the ‘Lübeck Disaster’ in Germany in 1929–1933 [38]. During this episode, oral administration to 251 neonates of BCG vaccine that was accidentally contaminated with M. tuberculosis caused tuberculosis in 228 children, all of whom developed lymph node involvement, while a pulmonary form was reported in 30 children; 71 (28.3%) died of tuberculosis [38]. This accident shed light on the transmissibility of M. tuberculosis through the digestive tract, and in this accident the form of tuberculosis caused by the M. tuberculosis was similar to the forms of tuberculosis caused by M. bovis in humans, most commonly affecting the lymph nodes after entry of the pathogen through the digestive tract [38]. For M. canettii, current clinical and experimental data [12,39] suggest the existence of an unknown environmental reservoir, and digestive transmission via food with local replication in the oropharynx and cervical lymph nodes and increased dissemination in the respiratory and digestive tracts [12]. Digestive transmission of tuberculosis is known particularly for zoonotic tuberculosis caused mainly by M. bovis [27]. Zoonotic tuberculosis is primarily a foodborne disease that follows consumption of unpasteurized milk or milk products [27]. Meat from animals with tuberculosis is not recognized as a vehicle for transmission of M. bovis, at least when cooked, and M. bovis is rarely found in muscle [27]. WHO has estimated that in 2018 zoonotic tuberculosis with M. bovis caused 143 000 new cases and 12 300 deaths [2].
Abdominal tuberculosis represents 12% of extrapulmonary tuberculosis cases and 1%–3% of total tuberculosis cases [40]; 15%–25% of abdominal tuberculosis cases have concomitant pulmonary tuberculosis [41]. Modes of MTBC infection in cases of abdominal tuberculosis include swallowing infected sputum, ingestion of infected food, lymphatic spread from an extra-abdominal focus, and by contiguous spread from urogenital organs [40,42].
Abdominal tuberculosis can be classified into four forms: luminal (ileocaecal), peritoneal, nodal and visceral involving solid intra-abdominal organs [40].
Several series of cases of abdominal tuberculosis have been reported, in some studies this localization is mainly attributed to M. bovis then to M. tuberculosis [[43], [44], [45]].
Alteration of the intestinal microbiota after antibiotic administration has been reported in mouse models to cause susceptibility to tuberculosis. Mice with microbial dysbiosis showed a significant increase in M. tuberculosis in the lungs and spread to the spleen and liver [46]; similar results to those observed at the induction of dysbiosis by Helicobacter hepaticus infection [47].
Tuberculosis of inoculation
Cutaneous inoculation is also a rare route of transmission, reported in some accidental cases among health-care workers [48,49], or in patients following corticosteroid injections or skin trauma [50] and tattooing [51]. This route of inoculation results in primary cutaneous tuberculosis. On the other hand, facial cutaneous tuberculosis, peri-ocular cutaneous tuberculosis, has been described and the authors hypothesized that this unusual presentation could be due to minor trauma followed by inoculation [52]. Annobil et al. described primary tuberculosis of the penis with bilateral hypertrophy of the inguinal lymph nodes in a 4-month-old baby circumcised at 6 weeks of age; possibly related to the barber-operator having moistened the razor with sputum before sharpening it [53]. Also, an anecdotal report of sexual transmission of M. tuberculosis has been described, involving penile cutaneous tuberculosis followed by endometrial tuberculosis in the patient's partner; the two strains of M. tuberculosis were identical by molecular typing [54]. In rare cases, tuberculosis can be contracted after solid organ transplantation from an infected organ donor, and because of immunosuppression, transplant recipients frequently develop extrapulmonary or disseminated TB; however, tuberculosis after solid organ transplantation is most often caused by primary infection or reactivation of a latent infection [55].
Fate of M. tuberculosis in humans
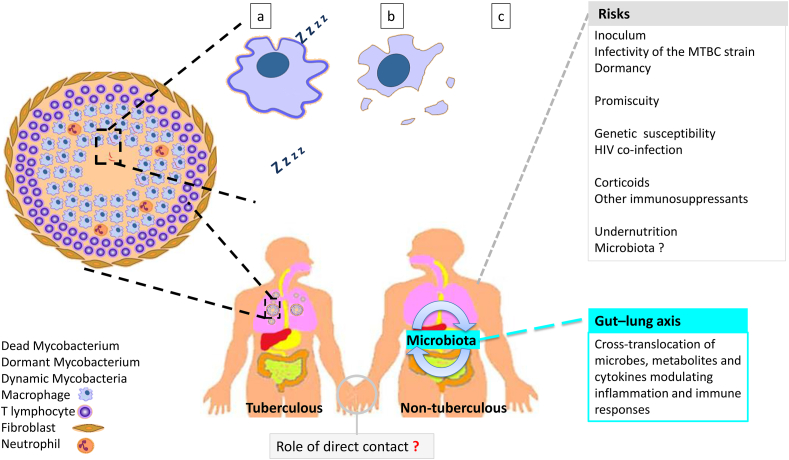
Once inhaled, M. tuberculosis travels from the trachea to the lungs where it is phagocytosed by alveolar macrophages in which it is internalized in the phagosomes and then in the phagolysosomes [56,57]. However, M. tuberculosis can block the acidification and maturation of phagosomes to survive in the host alveolar macrophages [58,59]. Macrophages and other immune cells aggregate to form the granuloma [60], in which mycobacteria are both intracellular within macrophages and extracellular [61]. This granuloma is formed mainly by macrophages infected and uninfected with M. tuberculosis bacilli surrounded by immune cells including granulocytes, dendritic cells, natural killer cells and T and B lymphocytes [60] (Fig. 3). Under these conditions, both innate and adaptive immune defences are involved in the control of M. tuberculosis [62], limiting their replication and leading to a state of equilibrium between the host organism and the pathogen. In the granuloma, M. tuberculosis bacilli are exposed to a variety of stress conditions, in particular hypoxia, which promotes the dormancy of the mycobacteria [62]. This dormant state in M. tuberculosis results in an ability to persist in host tissues without replication for months or even years, without causing tuberculosis disease, and resulting in chronic asymptomatic infection in up to 90% of infected persons—known as latent tuberculosis infection [6,[63], [64], [65]]. On the other hand, 5% of infected persons will develop active tuberculosis [66], whereas others will be competent to eliminate the pathogen. Dormant mycobacteria may reside in old granulomatous lung lesions (in the macrophage and/or caseum), pulmonary lymph nodes [67], or adipose tissue, which is described as a large reservoir housing dormant mycobacteria and preserving them from antimicrobial agents and the host immune system [68]. In 5%–15% of latently infected persons, M. tuberculosis can reactivate, leading to active tuberculosis [68]. Indeed, when host immunity is compromised and environmental conditions around M. tuberculosis become conducive to its reactivation, these bacilli accelerate replication, leading to necrosis of infected macrophages and release of intracellular mycobacteria, which could infect new cells and spread to other tissues [69,70]. In addition to their ability to reactivate, the important role of dormancy in the natural history of tuberculosis lies in the fact that dormant mycobacteria are potentially infectious. Indeed, this has been demonstrated not only experimentally [69,70] but also clinically, where it has been found that dormant mycobacteria can be even more infectious than metabolically active mycobacteria in the expectorant of patients with pulmonary tuberculosis [71].
Fig. 3.
Risks predisposing to the acquisition of tuberculosis and the life cycle of Mycobacterium tuberculosis in the granuloma. (a) Dormant M. tuberculosis bacilli persist in macrophages and caseum for extended periods of time. (b) When environmental conditions become conducive to reactivation, mycobacteria actively replicate leading to disruption of the integrity of the granuloma and spread to other tissues. (c) Under the action of antibiotic treatment and the immune system, initially replicating mycobacteria will be eliminated while a proportion may become dormant and persist.
Tuberculosis in the animate environment: animals
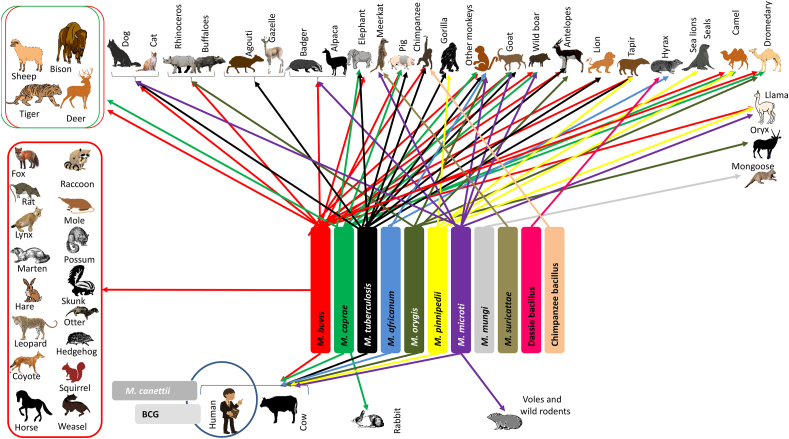
Data from the literature indicate that except for M. bovis BCG, which is a vaccine strain, and M. canettii, all other MTBC (11/13 species) can infect one or more animal species, including humans (Fig. 2). MTBC species are genetically closely related (>99.9% nucleotide identity) and some authors classify them into strains that are adapted to the human host, such as M. tuberculosis and M. africanum, and those that have the potential to spread and transmit in a wide variety of wild and domestic animal hosts [3]. However, it should be noted that the species barrier is not strict and in rare situations M. tuberculosis can infect domestic animals, or wild animals in captivity. More than 15 species of animals can be infected with M. tuberculosis, including parrots [5,72,73]; similarly, M. africanum has been sporadically isolated from African monkeys with active tuberculosis and from cows [18,74]. Five MTBC have already been reported as zoonoses, causing tuberculosis in animals and transmissible to humans, including M. bovis [27,[75], [76], [77]], Mycobacterium caprae [78,79], Mycobacterium microti [[80], [81], [82]], Mycobacterium orygis [83,84] and Mycobacterium pinnipedii [85,86]. However, the main agent of tuberculosis in animals is M. bovis, which can infect a wide range of mammals and has a worldwide distribution in cows [75,87].
Fig. 2.
Species distribution of Mycobacterium tuberculosis complex in mammals; based on references [8,23,26,28,29,32,33,37,38,85].
Other MTBC species identified in animals have never been reported in humans, including Mycobacterium mungi, Mycobacterium suricattae, Dassie bacillus and Chimpanzee bacillus [8,9,86,88]. These data suggest that there is no specificity between MTBC species and infected mammalian species, and that the current distribution of MTBC species among mammals results from the probability of contact.
Tuberculosis in the inanimate environment
Current data in the literature depict a scenario in which MTBC were initially environmental mycobacteria that evolved from unicellular eukaryotic (amoeba) opportunistic pathogens to mammalian opportunistic pathogens and then to contagious pathogens in humans (Fig. 1). Some studies have shown the persistence of M. tuberculosis and M. bovis in soil experimentally inoculated in the laboratory under controlled temperature and humidity conditions for a period of 12 months [89]. This experimental observation was followed by field observations in Tehran, Iran, where 1% of the soil samples and 10% of the water samples were found to have grown M. tuberculosis, which was re-cultivated 9 months after sampling; and whose genotypes determined by spoligotyping corresponded in part to those of tuberculosis patients diagnosed in Tehran [90]. Recent work has reviewed all the experimental and field observation data to confirm the possibility of prolonged storage of MTBC in soil [7]. Patients with pulmonary tuberculosis pass M. tuberculosis in the stool, which is an alternative to sputum for the diagnosis of tuberculosis by culture [91] and by molecular biology [92]. Individuals infected with M. tuberculosis could contaminate the environment [90]. In addition, there has been a reported case of transmission of M. tuberculosis to an embalmer from the corpse of a patient who died of pulmonary tuberculosis [93], illustrating that the corpses of tuberculosis patients could be a source of soil infection [5].
Some experimental observations indicate the survival of MTBC within the vegetative forms of free amoebae of the genus Acanthamoeba [94]. It has been shown experimentally that the five free amoebae Acanthamoeba polyphaga, Acanthamoeba castellanii, Acanthamoeba lenticulata, Vermamoeba vermiformis and Dictyostellium discoideum can be infected by M. bovis; it is encysted by each of these five amoebae; it persists for at least 60 days within the cysts; and that experimental inhalation of vegetative amoebae and cysts infected with M. bovis causes pulmonary tuberculosis in BALB/c mice [95]. This intra-amoebic life was the occasion for genetic exchanges between the host amoeba and the mycobacteria [96]. Interestingly, only M. canettii is not cyst-positive at the time of amoeba cyst formation, unlike M. tuberculosis, which can probably survive for extended periods of time within the cyst, as it has been shown that Acanthamoeba amoeba cysts can survive for 50 decades [97]. The mechanism of M. canettii's early exit is not known, even though this MTBC codes for an active cellulase that could cleave the cellulose wall of the developing cyst [94]. Also, the modality of survival of MTBC inside the amoeba cyst is unknown even though they could be dormant mycobacteria [98]. All these observations suggest the possibility of an environmental cycle independent of the usual hosts, the possibility of which is not documented. However, the role of soil as a source of contamination of certain mammals does not seem unreasonable (Fig. 1).
Conclusions and perspectives
This review of the literature from different geographical and thematic sources describe scenarios of reservoirs, sources, modes of transmission and fates of MTBC in animal and human populations that are broader than those usually reported. Indeed, tuberculosis can be understood as an infection by telluric bacteria initially pathogenic to unicellular eukaryotes, which progressively acquired the capacity to infect pluricellular eukaryotes and then to be directly transmissible from host to host conferring the contagious character currently observed in human populations. The observation of pulmonary tuberculosis alone obscures the knowledge of these possible scenarios, diminishing vigilance on current and past alternative modalities for prehistoric populations.
Funding sources
This work was funded by the IHU Méditerranée Infection (Marseille, France) and by the French Government under the Investissements d’avenir (Investments for the Future) programme managed by the Agence Nationale de la Recherche (ANR, fr: National Agency for Research) (reference: Méditerranée Infection 10-IAHU- 03).
Conflict of interest
None declared.
References
- 1.Cohen A., Mathiasen V.D., Schön T., Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Resp J. 2019;54 doi: 10.1183/13993003.00655-2019. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . World Health Organization (WHO); Geneva (Switzerland): 2019. Global tuberculosis report 2019. [Google Scholar]
- 3.Brites D., Loiseau C., Menardo F., Borrell S., Boniotti M.B., Warren R. A new phylogenetic framework for the animal-adapted Mycobacterium tuberculosis Complex. Front Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riojas M.A., McGough K.J., Rider-Riojas C.J., Rastogi N., Hazbón M.H. Phylogenomic analysis of the species of the Mycobacterium tuberculosis complex demonstrates that Mycobacterium africanum, Mycobacterium bovis, Mycobacterium caprae, Mycobacterium microti and Mycobacterium pinnipedii are later heterotypic synonyms of Mycobacterium tuberculosis. Int J Syst Evol Microbiol. 2018;68:324–332. doi: 10.1099/ijsem.0.002507. [DOI] [PubMed] [Google Scholar]
- 5.Ghodbane R., Drancourt M. Non-human sources of Mycobacterium tuberculosis. Tuberculosis. 2013;93:589–595. doi: 10.1016/j.tube.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Bhavanam S., Rayat G.R., Keelan M., Kunimoto D., Drews S.J. Understanding the pathophysiology of the human TB lung granuloma using in vitro granuloma models. Future Microbiol. 2016;11:1073–1089. doi: 10.2217/fmb-2016-0005. [DOI] [PubMed] [Google Scholar]
- 7.Martinez L., Verma R., Croda J., Horsburgh C.R., Walter K.S., Degner N. Detection, survival and infectious potential of Mycobacterium tuberculosis in the environment: a review of the evidence and epidemiological implications. Eur Resp J. 2019;53 doi: 10.1183/13993003.02302-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coscolla M., Lewin A., Metzger S., Maetz-Rennsing K., Calvignac-Spencer S., Nitsche A. Novel Mycobacterium tuberculosis complex isolate from a wild chimpanzee. Emerg Infect Dis. 2013;19:969–976. doi: 10.3201/eid1906.121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parsons S.D.C., Drewe J.A., Gey van Pittius N.C., Warren R.M., van Helden P.D. Novel cause of tuberculosis in meerkats, South Africa. Emerg Infect Dis. 2013;19:2004–2007. doi: 10.3201/eid1912.130268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia X., Yang L., Dong M., Chen S., Lv L., Cao D. The bioinformatics analysis of comparative genomics of Mycobacterium tuberculosis Complex (MTBC) provides insight into dissimilarities between intraspecific groups differing in host association, virulence, and epitope diversity. Front Cell Infect Microbiol. 2017;7 doi: 10.3389/fcimb.2017.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Supply P., Marceau M., Mangenot S., Roche D., Rouanet C., Khanna V. Genomic analysis of smooth tubercle bacilli provides insights into ancestry and pathoadaptation of Mycobacterium tuberculosis. Nat Genet. 2013;45:172–179. doi: 10.1038/ng.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aboubaker Osman D., Bouzid F., Canaan S., Drancourt M. Smooth tubercle bacilli: neglected opportunistic tropical pathogens. Front Public Health. 2015;3:283. doi: 10.3389/fpubh.2015.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ngabonziza, Semuto Jean Claude. A sister lineage of the Mycobacterium tuberculosis complex discovered in the African Great Lakes region. bioRxiv. 2020 doi: 10.1101/2020.01.20.912998. 01.20.912998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brites D., Gagneux S. The nature and evolution of genomic diversity in the Mycobacterium tuberculosis complex. In: Gagneux S., editor. Strain variation in the Mycobacterium tuberculosis complex: its role in biology, epidemiology and control. Springer International Publishing; Cham: 2017. pp. 1–26. [DOI] [PubMed] [Google Scholar]
- 15.Comas I., Coscolla M., Luo T., Borrell S., Holt K.E., Kato-Maeda M. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat Genet. 2013;45:1176–1182. doi: 10.1038/ng.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Jong B.C., Hill P.C., Brookes R.H., Gagneux S., Jeffries D.J., Otu J.K. Mycobacterium africanum elicits an attenuated T cell response to early secreted antigenic target, 6 kDa, in patients with tuberculosis and their household contacts. J Infect Dis. 2006;193:1279–1286. doi: 10.1086/502977. [DOI] [PubMed] [Google Scholar]
- 17.Sharma A., Bloss E., Heilig C.M., Click E.S. Tuberculosis caused by Mycobacterium africanum, United States, 2004–2013. Emerging Infect Dis. 2016;22:396–403. doi: 10.3201/eid2203.151505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeboah-Manu D., de Jong B.C., Gehre F. The biology and epidemiology of Mycobacterium africanum. Adv Exp Med Biol. 2017;1019:117–133. doi: 10.1007/978-3-319-64371-7_6. [DOI] [PubMed] [Google Scholar]
- 19.Parsons S.D.C., Gous T.A., Warren R.M., van Helden P.D. Pulmonary Mycobacterium tuberculosis (Beijing strain) infection in a stray dog. J South Afr Vet Assoc. 2008;79:95–98. doi: 10.4102/jsava.v79i2.252. [DOI] [PubMed] [Google Scholar]
- 20.Lam J.T., Ho P.L., Weng X.H., Zhang W.H., Chen S., Yam W.C. Rv2820c of Beijing/W strains enhances Mycobacterium tuberculosis survival in human macrophages. Mycobact Dis. 2011;1:1–4. [Google Scholar]
- 21.Lewis K.N., Liao R., Guinn K.M., Hickey M.J., Smith S., Behr M.A. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille calmette–guérin attenuation. J Infect Dis. 2003;187:117–123. doi: 10.1086/345862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cambau E., Drancourt M. Steps towards the discovery of Mycobacterium tuberculosis by Robert Koch, 1882. Clin Microbiol Infect. 2014;20:196–201. doi: 10.1111/1469-0691.12555. [DOI] [PubMed] [Google Scholar]
- 23.Calmette A., Guérin C. Origine intestinale de la tuberculose pulmonaire. Ann Inst Pasteur. 1905;19:601–618. [Google Scholar]
- 24.Sultan L., Nyka W., Mills C., O’Grady F., Wells W., Riley R.L. Tuberculosis disseminators. Am Rev Respir Dis. 1960;82:358–369. doi: 10.1164/arrd.1960.82.3.358. [DOI] [PubMed] [Google Scholar]
- 25.Riley R.L., Mills C.C., Nyka W., Weinstock N., Storey P.B., Sultan L.U. Aerial dissemination of pulmonary tuberculosis. A two-year study of contagion in a tuberculosis ward. Am J Hyg. 1959;70:185–196. doi: 10.1093/oxfordjournals.aje.a117542. [DOI] [PubMed] [Google Scholar]
- 26.Yates T.A., Khan P.Y., Knight G.M., Taylor J.G., McHugh T.D., Lipman M. The transmission of Mycobacterium tuberculosis in high burden settings. Lancet Infect Dis. 2016;16:227–238. doi: 10.1016/S1473-3099(15)00499-5. [DOI] [PubMed] [Google Scholar]
- 27.Macedo Couto R., Ranzani O.T., Waldman E.A. Zoonotic Tuberculosis in humans: control, surveillance, and the one health approach. Epidemiol Rev. 2019;41:130–144. doi: 10.1093/epirev/mxz002. [DOI] [PubMed] [Google Scholar]
- 28.Olea-Popelka F., Muwonge A., Perera A., Dean A.S., Mumford E., Erlacher-Vindel E. Zoonotic tuberculosis in human beings caused by Mycobacterium bovis—a call for action. Lancet Infect Dis. 2017;17:e21–e25. doi: 10.1016/S1473-3099(16)30139-6. [DOI] [PubMed] [Google Scholar]
- 29.Sunder S., Lanotte P., Godreuil S., Martin C., Boschiroli M.L., Besnier J.M. Human-to-human transmission of tuberculosis caused by Mycobacterium bovis in immunocompetent patients. J Clin Microbiol. 2009;47:1249–1251. doi: 10.1128/JCM.02042-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans J.T., Smith E.G., Banerjee A., Smith R.M., Dale J., Innes J.A. Cluster of human tuberculosis caused by Mycobacterium bovis: evidence for person-to-person transmission in the UK. Lancet. 2007;369:1270–1276. doi: 10.1016/S0140-6736(07)60598-4. [DOI] [PubMed] [Google Scholar]
- 31.Winglee K., Eloe-Fadrosh E., Gupta S., Guo H., Fraser C., Bishai W. Aerosol Mycobacterium tuberculosis infection causes rapid loss of diversity in gut microbiota. PLOS ONE. 2014;9 doi: 10.1371/journal.pone.0097048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eshetie S., van Soolingen D. The respiratory microbiota: new insights into pulmonary tuberculosis. BMC Infect Dis. 2019;19:92. doi: 10.1186/s12879-019-3712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asmar S., Drancourt M. Rapid culture-based diagnosis of pulmonary tuberculosis in developed and developing countries. Front Microbiol. 2015;6 doi: 10.3389/fmicb.2015.01184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Negre L., Goyal R.K., B. C. G Vaccination in practice. Ind Med Gaz. 1938;73:566–572. [PMC free article] [PubMed] [Google Scholar]
- 35.Calmette A., Weill-Halle B., Saenz A., Costil L. Demonstration experimentale du passage des bacille-vaccins BCG a travers la muquese de l’intestin chez l’enfant et chez le singe. Bull Acad Med. 1933;110:203–206. [Google Scholar]
- 36.Mortatti R.C., Maia L.C., Fonseca L.S. Absorption of Mycobacterium bovis BCG administered by the oral route. Vaccine. 1987;5:109–114. doi: 10.1016/0264-410x(87)90056-9. [DOI] [PubMed] [Google Scholar]
- 37.Benévolo-de-Andrade T.C., Monteiro-Maia R., Cosgrove C., Castello-Branco L.R.R. BCG Moreau Rio de Janeiro: an oral vaccine against tuberculosis—review. Memórias Do Instituto Oswaldo Cruz. 2005;100:459–465. doi: 10.1590/s0074-02762005000500002. [DOI] [PubMed] [Google Scholar]
- 38.Fox G.J., Orlova M., Schurr E. Tuberculosis in newborns: the lessons of the “Lübeck Disaster” (1929–1933) PLOS Pathogens. 2016;12 doi: 10.1371/journal.ppat.1005271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bouzid F., Brégeon F., Lepidi H., Donoghue H.D., Minnikin D.E., Drancourt M. Ready experimental translocation of Mycobacterium canettii yields pulmonary tuberculosis. Infect Immun. 2017;85 doi: 10.1128/IAI.00507-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho J.-K., Choi Y.M., Lee S.S., Park H.K., Cha R.R., Kim W.S. Clinical features and outcomes of abdominal tuberculosis in southeastern Korea: 12 years of experience. BMC Infect Dis. 2018;18:699. doi: 10.1186/s12879-018-3635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Debi U., Ravisankar V., Prasad K.K., Sinha S.K., Sharma A.K. Abdominal tuberculosis of the gastrointestinal tract: revisited. World J Gastroenterol. 2014;20:14831–14840. doi: 10.3748/wjg.v20.i40.14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abu-Zidan F.M., Sheek-Hussein M. Diagnosis of abdominal tuberculosis: lessons learned over 30 years: pectoral assay. World J Emerg Surg. 2019;14:33. doi: 10.1186/s13017-019-0252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veeragandham R.S., Lynch F.P., Canty T.G., Collins D.L., Dankner W.M. Abdominal tuberculosis in children: review of 26 cases. J Pediatr Surg. 1996;31:170–176. doi: 10.1016/s0022-3468(96)90342-5. [DOI] [PubMed] [Google Scholar]
- 44.Probert C.S., Jayanthi V., Wicks A.C., Carr-Locke D.L., Garner P., Mayberry J.F. Epidemiological study of abdominal tuberculosis among Indian migrants and the indigenous population of Leicester, 1972–1989. Gut. 1992;33:1085–1088. doi: 10.1136/gut.33.8.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torres-Gonzalez P., Cervera-Hernandez M.E., Martinez-Gamboa A., Garcia-Garcia L., Cruz-Hervert L.P., Bobadilla-del Valle M. Human tuberculosis caused by Mycobacterium bovis: a retrospective comparison with Mycobacterium tuberculosis in a Mexican tertiary care centre, 2000–2015. BMC Infect Dis. 2016;16:657. doi: 10.1186/s12879-016-2001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan N., Vidyarthi A., Nadeem S., Negi S., Nair G., Agrewala J.N. Alteration in the gut microbiota provokes susceptibility to tuberculosis. Front Immunol. 2016;7 doi: 10.3389/fimmu.2016.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Majlessi L., Sayes F., Bureau J.-F., Pawlik A., Michel V., Jouvion G. Colonization with Helicobacter is concomitant with modified gut microbiota and drastic failure of the immune control of Mycobacterium tuberculosis. Mucosal Immunol. 2017;10:1178–1189. doi: 10.1038/mi.2016.140. [DOI] [PubMed] [Google Scholar]
- 48.Kramer F., Sasse S.A., Simms J.C., Leedom J.M. Primary cutaneous tuberculosis after a needlestick injury from a patient with AIDS and undiagnosed tuberculosis. Ann Intern Med. 1993;119:594–595. doi: 10.7326/0003-4819-119-7_part_1-199310010-00007. [DOI] [PubMed] [Google Scholar]
- 49.Huang D., Yin H. Primary inoculation tuberculosis after an accidental scalpel injury. Infection. 2013;41:841–844. doi: 10.1007/s15010-013-0442-y. [DOI] [PubMed] [Google Scholar]
- 50.De Jong J.W., van Altena R. Non-respiratory tuberculosis with Mycobacterium tuberculosis after penetrating lesions of the skin: five case histories [Case Study] 2000;4:1184–1187. [PubMed] [Google Scholar]
- 51.Horney D.A., Gaither J.M., Lauer R., Norins A.L., Mathur P.N. Cutaneous inoculation tuberculosis secondary to “jailhouse tattooing. Arch Dermatol. 1985;121:648–650. [PubMed] [Google Scholar]
- 52.Chowdhury M.M., Varma C., Howell S., Holt P.J., Statham B.N. Facial cutaneous tuberculosis: an unusual presentation. Clin Exp Dermatol. 2000;25:48–50. doi: 10.1046/j.1365-2230.2000.00572.x. [DOI] [PubMed] [Google Scholar]
- 53.Annobil S.H., Al-Hilfi A., Kazi T. Primary tuberculosis of the penis in an infant. Tubercle. 1990;71:229–230. doi: 10.1016/0041-3879(90)90083-k. [DOI] [PubMed] [Google Scholar]
- 54.Angus B.J., Yates M., Conlon C., Byren I. Cutaneous tuberculosis of the penis and sexual transmission of tuberculosis confirmed by molecular typing. Clin Infect Dis. 2001;33:E132–E134. doi: 10.1086/324360. [DOI] [PubMed] [Google Scholar]
- 55.Abad C.L.R., Razonable R.R. Mycobacterium tuberculosis after solid organ transplantation: a review of more than 2000 cases. Clin Transplant. 2018;32:e13259. doi: 10.1111/ctr.13259. [DOI] [PubMed] [Google Scholar]
- 56.Pieters J. Mycobacterium tuberculosis and the macrophage: maintaining a balance. Cell Host Microbe. 2008;3:399–407. doi: 10.1016/j.chom.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 57.Cambier C.J., Takaki K.K., Larson R.P., Hernandez R.E., Tobin D.M., Urdahl K.B. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature. 2014;505:218–222. doi: 10.1038/nature12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ehrt S., Schnappinger D. Mycobacterial survival strategies in the phagosome: defence against host stresses. Cell Microbiol. 2009;11:1170–1178. doi: 10.1111/j.1462-5822.2009.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Houben D., Demangel C., Ingen J van, Perez J., Baldeón L., Abdallah A.M. ESX-1-mediated translocation to the cytosol controls virulence of mycobacteria. Cell Microbiol. 2012;14:1287–1298. doi: 10.1111/j.1462-5822.2012.01799.x. [DOI] [PubMed] [Google Scholar]
- 60.Pagán A.J., Ramakrishnan L. The formation and function of granulomas. Annu Rev Immunol. 2018;36:639–665. doi: 10.1146/annurev-immunol-032712-100022. [DOI] [PubMed] [Google Scholar]
- 61.Getahun H., Matteelli A., Chaisson R.E., Raviglione M. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015;372:2127–2135. doi: 10.1056/NEJMra1405427. [DOI] [PubMed] [Google Scholar]
- 62.Dutta N.K., Karakousis P.C. Latent Tuberculosis infection: myths, models, and molecular mechanisms. Microbiol Mol Biol Rev. 2014;78:343–371. doi: 10.1128/MMBR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wayne L.G. Dormancy of Mycobacterium tuberculosis and latency of disease. Eur J Clin Microbiol Infect Dis. 1994;13:908–914. doi: 10.1007/BF02111491. [DOI] [PubMed] [Google Scholar]
- 64.Cardona P.-J., Ruiz-Manzano J. On the nature of Mycobacterium tuberculosis-latent bacilli. Eur Respir J. 2004;24:1044–1051. doi: 10.1183/09031936.04.00072604. [DOI] [PubMed] [Google Scholar]
- 65.Rittershaus E.S.C., Baek S.-H., Sassetti C.M. The normalcy of dormancy: common themes in microbial quiescence. Cell Host Microbe. 2013;13:643–651. doi: 10.1016/j.chom.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koul A., Arnoult E., Lounis N., Guillemont J., Andries K. The challenge of new drug discovery for tuberculosis. Nature. 2011;469:483–490. doi: 10.1038/nature09657. [DOI] [PubMed] [Google Scholar]
- 67.Parrish N.M., Dick J.D., Bishai W.R. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 1998;6:107–112. doi: 10.1016/s0966-842x(98)01216-5. [DOI] [PubMed] [Google Scholar]
- 68.Neyrolles O., Hernández-Pando R., Pietri-Rouxel F., Fornès P., Tailleux L., Payán J.A.B. Is adipose tissue a place for Mycobacterium tuberculosis persistence? PLOS ONE. 2006;1:e43. doi: 10.1371/journal.pone.0000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woolhiser L., Tamayo M.H., Wang B., Gruppo V., Belisle J.T., Lenaerts A.J. In vivo adaptation of the Wayne model of latent tuberculosis. Infect Immun. 2007;75:2621–2625. doi: 10.1128/IAI.00918-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dietrich J., Roy S., Rosenkrands I., Lindenstrøm T., Filskov J., Rasmussen E.M. Differential influence of nutrient-starved Mycobacterium tuberculosis on adaptive immunity results in progressive tuberculosis disease and pathology. Infect Immun. 2015;83:4731–4739. doi: 10.1128/IAI.01055-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Datta S., Sherman J.M., Tovar M.A., Bravard M.A., Valencia T., Montoya R. Sputum microscopy with fluorescein diacetate predicts tuberculosis infectiousness. J Infect Dis. 2017;216:514–524. doi: 10.1093/infdis/jix229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mukherjee F., Bahekar V.S., Prasad A., Rana S.K., Kanani A., Sharma G.K. Isolation of Mycobacterium tuberculosis from Antelope cervicapra and Gazelle bennettii in India and confirmation by molecular tests. Eur J Wildl Res. 2015;61:783–787. [Google Scholar]
- 73.Adeogun A., Omobowale O., Owuamanam C., Alaka O., Taiwo V., van Soolingen D. Mycobacterium tuberculosis and Dual M. tuberculosis/M. bovis infection as the cause of tuberculosis in a gorilla and a lioness, respectively, in Ibadan Zoo, Nigeria. Case Rep Vet Med. 2016;2016 doi: 10.1155/2016/8568237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Jong B.C., Antonio M., Gagneux S. Mycobacterium africanum—review of an important cause of human tuberculosis in West Africa. PLoS Negl Trop Dis. 2010;4 doi: 10.1371/journal.pntd.0000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Palmer M.V. Mycobacterium bovis: characteristics of wildlife reservoir hosts. Transbound Emerg Dis. 2013;60:1–13. doi: 10.1111/tbed.12115. [DOI] [PubMed] [Google Scholar]
- 76.Broughan J.M., Downs S.H., Crawshaw T.R., Upton P.A., Brewer J., Clifton-Hadley R.S. Mycobacterium bovis infections in domesticated non-bovine mammalian species. Part 1: review of epidemiology and laboratory submissions in Great Britain 2004–2010. Vet J. 2013;198:339–345. doi: 10.1016/j.tvjl.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 77.Michelet L., De Cruz K., Hénault S., Tambosco J., Richomme C., Réveillaud É. Mycobacterium bovis infection of red fox, France. Emerging Infect Dis. 2018;24:1150–1153. doi: 10.3201/eid2406.180094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Prodinger W.M., Indra A., Koksalan O.K., Kilicaslan Z., Richter E. Mycobacterium caprae infection in humans. Expert Rev Anti Infect Ther. 2014;12:1501–1513. doi: 10.1586/14787210.2014.974560. [DOI] [PubMed] [Google Scholar]
- 79.Krajewska M., Zabost A., Welz M., Lipiec M., Orłowska B., Anusz K. Transmission of Mycobacterium caprae in a herd of European bison in the Bieszczady mountains, southern Poland. Eur J Wildl Res. 2015;61:429–433. [Google Scholar]
- 80.Panteix G., Gutierrez M.C., Boschiroli M.L., Rouviere M., Plaidy A., Pressac D. Pulmonary tuberculosis due to Mycobacterium microti: a study of six recent cases in France. J Med Microbiol. 2010;59:984–989. doi: 10.1099/jmm.0.019372-0. [DOI] [PubMed] [Google Scholar]
- 81.Michelet L., Cruz K de, Zanella G., Aaziz R., Bulach T., Karoui C. Infection with Mycobacterium microti in animals in France. J Clin Microbiol. 2015;53:981–985. doi: 10.1128/JCM.02713-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zanolari P., Robert N., Lyashchenko K.P., Pfyffer G.E., Greenwald R., Esfandiari J. Tuberculosis caused by Mycobacterium microti in South American camelids. J Vet Intern Med. 2009;23:1266–1272. doi: 10.1111/j.1939-1676.2009.0377.x. [DOI] [PubMed] [Google Scholar]
- 83.Dawson K.L., Bell A., Kawakami R.P., Coley K., Yates G., Collins D.M. Transmission of Mycobacterium orygis (M. tuberculosis Complex Species) from a tuberculosis patient to a dairy cow in New Zealand. J Clin Microbiol. 2012;50:3136–3138. doi: 10.1128/JCM.01652-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marcos L.A., Spitzer E.D., Mahapatra R., Ma Y., Halse T.A., Shea J. Mycobacterium orygis lymphadenitis in New York, USA. Emerging Infect Dis. 2017;23:1749–1751. doi: 10.3201/eid2310.170490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zmak L., Obrovac M., Jankovic Makek M., Perko G., Trkanjec J.T. 2019. From Peruvian mummies to living humans: first case of pulmonary tuberculosis caused by Mycobacterium pinnipedii. [DOI] [PubMed] [Google Scholar]
- 86.Rodriguez-Campos S., Smith N.H., Boniotti M.B., Aranaz A. Overview and phylogeny of Mycobacterium tuberculosis complex organisms: implications for diagnostics and legislation of bovine tuberculosis. Res Vet Sci. 2014;97 doi: 10.1016/j.rvsc.2014.02.009. S5–19. [DOI] [PubMed] [Google Scholar]
- 87.Michel A.L., Müller B., van Helden P.D. Mycobacterium bovis at the animal–human interface: a problem, or not? Vet Microbiol. 2010;140:371–381. doi: 10.1016/j.vetmic.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 88.Alexander K.A., Laver P.N., Michel A.L., Williams M., van Helden P.D., Warren R.M. Novel Mycobacterium tuberculosis complex pathogen, M. mungi. Emerg Infect Dis. 2010;16:1296–1299. doi: 10.3201/eid1608.100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ghodbane R., Medie F.M., Lepidi H., Nappez C., Drancourt M. Long-term survival of tuberculosis complex mycobacteria in soil. Microbiology. 2014;160:496–501. doi: 10.1099/mic.0.073379-0. [DOI] [PubMed] [Google Scholar]
- 90.Velayati A.A., Farnia P., Mozafari M., Malekshahian D., Farahbod A.M., Seif S. Identification and genotyping of Mycobacterium tuberculosis isolated from water and soil samples of a metropolitan city. Chest. 2015;147:1094–1102. doi: 10.1378/chest.14-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Asmar S., Chatellier S., Mirande C., Belkum A van, Canard I., Raoult D. A novel solid medium for culturing Mycobacterium tuberculosis isolates from clinical specimens. J Clin Microbiol. 2015;53:2566–2569. doi: 10.1128/JCM.01149-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.El Khéchine A., Henry M., Raoult D., Drancourt M. Detection of Mycobacterium tuberculosis complex organisms in the stools of patients with pulmonary tuberculosis. Microbiology. 2009;155:2384–2389. doi: 10.1099/mic.0.026484-0. [DOI] [PubMed] [Google Scholar]
- 93.Sterling T.R., Pope D.S., Bishai W.R., Harrington S., Gershon R.R., Chaisson R.E. Transmission of Mycobacterium tuberculosis from a cadaver to an embalmer. N Engl J Med. 2000;342:246–248. doi: 10.1056/NEJM200001273420404. [DOI] [PubMed] [Google Scholar]
- 94.Mba Medie F., Ben Salah I., Henrissat B., Raoult D., Drancourt M. Mycobacterium tuberculosis complex mycobacteria as amoeba-resistant organisms. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sanchez-Hidalgo A., Obregón-Henao A., Wheat W.H., Jackson M., Gonzalez-Juarrero M. Mycobacterium bovis hosted by free-living-amoebae permits their long-term persistence survival outside of host mammalian cells and remain capable of transmitting disease to mice. Environ Microbiol. 2017;19:4010–4021. doi: 10.1111/1462-2920.13810. [DOI] [PubMed] [Google Scholar]
- 96.Lamrabet O., Merhej V., Pontarotti P., Raoult D., Drancourt M. The genealogic tree of mycobacteria reveals a long-standing sympatric life into free-living protozoa. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Appelt S., Armougom F., Le Bailly M., Robert C., Drancourt M. Polyphasic analysis of a Middle Ages coprolite microbiota, Belgium. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salah I.B., Ghigo E., Drancourt M. Free-living amoebae, a training field for macrophage resistance of mycobacteria. Clin Microbiol Infect. 2009;15:894–905. doi: 10.1111/j.1469-0691.2009.03011.x. [DOI] [PubMed] [Google Scholar]