Abstract
Purpose
To evaluate the effect of central serous chorioretinopathy (CSCR) on retinal function using dark adaptation in a human subject, and to follow it through resolution of the disease.
Patients
Single patient, 50 years old male patient, with acute CSCR in one eye and resolved old CSCR in the other eye.
Observations
Observational study in patient with CSCR followed through resolution of the subretinal fluid (52 days). Dark adaptation was assessed using the AdaptDx® (Maculogix Inc.) measured by Rod Intercept time (RIT) in minutes. A normal retinal locus of the same eye on the opposite side of the fovea was used as control. Retinal separation (microns) was measured using Spectralis Optical Coherence Tomography (Spectralis®, HRA + OCT, Heidelberg engineering). Change in time to dark adapt, were correlated with retinal separation measured in microns, during the course of CSCR.
The Rod Intercept time was delayed in the area of detached retina compared to the normal region (control) on presentation with retinal separation (RS) of 104 μm. The Rod Intercept time returned to normal as the retinal separation from retinal pigment epithelium decreased and eventually resolved.
Conclusions
This case shows that delay in dark adaptation is proportional to the amount of separation of neurosensory retina from retinal pigment epithelium in CSCR, this may offer a potential of using DA to characterize visual function in CSCR. The association of dark adaptation response with the state of retinal pigment epithelial function and its ability to predict the recurrence of CSCR needs further evaluation.
Keywords: Central serous chorioretinopathy, Dark adaptation
1. Introduction
Central serous chorioretinopathy (CSCR) is a retinal disorder characterized by serous retinal detachment with or without associated retinal pigment epithelial detachment (RPED). In most cases, the Snellen's visual acuity is good in these patients and yet they are very symptomatic. CSCR is self-resolving; however recurrent and chronic episodes can cause decreased vision. In order to evaluate a more sensitive measure of visual function, we measured dark adaptation (DA) function in CSCR and correlated it with the separation of neurosensory retina from the retinal pigment epithelium (RPE). We found that Central Serous Chorioretinopathy leads to delayed dark adaptation, and that the proximity of the RPE and photoreceptor layer is important in dark adaptation.
2. Case report
A 50-year-old male patient, with past history of spontaneously resolved CSCR of the right eye (OD) 5 years back treated elsewhere for symptoms of mild visual distortion that resolved completely with 20/20 vision in a few weeks, presented to the Retina clinic with a three day history of noticing a round dim spot nasal to the center of vision, in his left eye (OS). Best corrected visual acuity (BCVA) was 20/20 OD and 20/25 OS, with pinhole (PH) improving to 20/20. Both eyes had normal intraocular pressure, with full confrontational visual fields and extra ocular movements. Slit lamp biomicroscopy of the anterior segment showed bilateral trace nuclear sclerosis, normal anterior chamber and vitreous. Bilateral fundus examination showed normal disc and vessels. The right macula was notable for pigment mottling with a single subretinal deposit. The left macula showed retinal elevation beginning at fovea and involving about two disc diameter circular area of temporal macula with an orange colored pigment epithelial detachment (PED).
Patient was examined and imaged about every 2 weeks until full recovery (Fig. 1). The near infrared images with the optical coherence tomography (OCT) scans (Spectralis, Heidelberg Engineering. Heidelberg, Germany) were performed to measure height (in microns) of retinal separation (RS). Dark adaptation (DA) was measured periodically from the presentation to resolution of clinical signs (Fig. 2) using the AdaptDx® (Maculogix, Middletown, PA) instrument, with rod-intercept time (RIT) in minutes as the DA parameter. Minimum of 30 minutes of pre-test adaptation to indoor room lighting is done in case of any immediate prior bright light exposure (e.g. Fundus photography or Autofluorescence imaging) to avoid any delaying effect on the test results. Measurements were taken in a dark room using the extended (20 minutes) protocol. Initial photo bleach exposure to a flash of 505-nm for 0.8-ms at an intensity of 1.8 × 104 scot cd/m2, equivalent to 76% bleaching level for rods was used. The flash of light passed through a square aperture sized to bleach a 6° area of the retina centered at 5° from the fovea on either side of the horizontal meridian, with the patient fixating on a light. The test target used was a 2° test stimulus light of 505 nm wavelength beginning 15 seconds after the bleaching flash. Patient was instructed to press a response button when first noticed the stimulus light and then the stimulus light intensity was gradually reduced till stimulus stops being perceived. Threshold was estimated using a 3-down/1-up modified staircase estimate procedure1 and continued at 30 seconds intervals till the recovery of visual sensitivity. The Rod Intercept, measured by the AdaptDx®, is the time in minutes at which the visual sensitivity recovery crosses three log units of recovery after initial bleaching, and is completely rod-mediated characterizing the visual sensitivity recovery rate, or dark adaptation speed. The machine has a central fixation target and stimulus locations at predefined eccentricities along vertical and horizontal meridian. We chose to measure at 5° eccentricity on horizontal meridian because it corresponded with the CSR spot on the retina. We obtained measurements at 5° horizontal from the fovea on either side as 5° location with 6° bleach area, and 2° spot size fell well within the CSCR location; the nasal visual field test corresponded with the area of CSCR located temporal to the fovea, while the temporal visual field test corresponding to the nasal to the fovea retinal location served as the control. DA testing spot was moved by changing the test location in the machine settings. The 5° from fovea superior or inferior location was avoided as control region since the area was very close to the CSCR region border, and considering the 6° bleach area, and 2° size of the test stimulus light, we wanted no overlap between the stimulus light location and the CSCR region in case the area of CSCR expanded after the first measurement. A single DA measurement was performed in the right eye on day 16 in the nasal visual field (region previously affected by CSCR temporal to the fovea) to measure the RIT in the eye with resolved CSCR. The test is designed in such a manner that the flash bleaches a localized predetermined area of retina with patient fixating on a fixation light, so sequential testing at different test spot on opposite side of the fovea would not affect the results. However, to avoid any probable effect, the DA testing in the CSCR and control locations on follow up visits was done by alternating the test sequence of the region tested first.
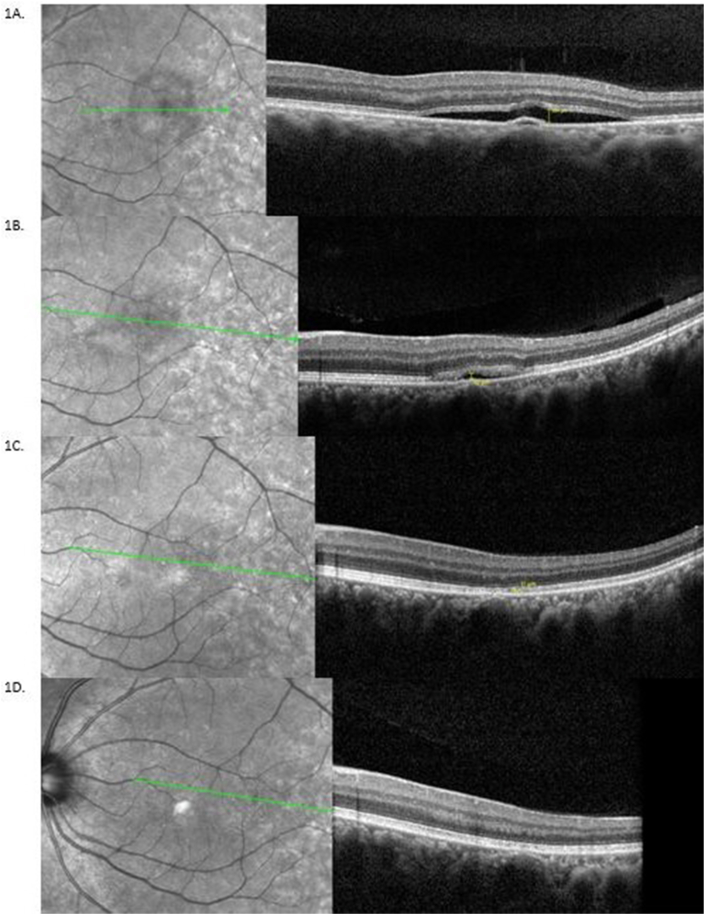
Fig. 1.
Comparison of Infrared fundus image and SDOCT B-scan findings at the initial presentation and serial follow-ups for the left eye: A. Near infrared fundus image and spectral domain Optical Coherence Tomography (SDOCT) B-scan at initial presentation to the clinic. The left eye (OS) shows a well-circumscribed lesion just temporal to fovea corresponding to the serous detachment of the neurosensory retina, with RS measurement of 104μm. B. Infrared fundus images and comparative SDOCT B-scans of the macular area for the left eye at day 16. The macula shows gradually resolving central serous retinopathy (CSCR), with RS of 42μm. B-scans for subsequent follow-up visits were taken at a tilted angle and narrower degree view to ensure capturing the whole extent of CSCR to be able to measure any residual SRF. It was ensured that all the measurements are done on same horizontal raphe C. Infrared fundus images and comparative SDOCT B-scans at day 31 with RS of 11μm. D. The final visit shows a normal appearing macula. SDOCT B-scans of the left eye at day 52 shows resolved fluid and no measurable RS. Retinal separation measurement was performed on the same line scan at maximum fluid height on follow-up visits.
Fig. 2.
Dark adaptation trend for left eye CSCR and Control region and retinal separation height: DA Rod intercept time (RIT) and the separation of retina measurements for the CSCR region starting from initial presentation to day 52. Measurements were taken at 5° horizontal offset from fovea on either side for the left eye. The CSCR region temporal to the fovea, and the normal retina nasal to the fovea for control. In the Right eye RIT was measured on the day 16 in temporal macula 5° from fovea to demonstrate RIT for the area of long resolved CSCR (Represented in the graph by single solid blue block as OD Control). RIT measurements for the nasal field were down-trending, corresponding to resolution of SRF in the CSCR location. By day 52, the retina appears normal without fluid on OCT and the nasal region RIT is similar to the temporal region RIT. The temporal region RITs are consistent with the normal range of RIT described for Adapt Dx9 throughout the serial dark adaptation testing with minimal inter-test variation (average ± std. RIT: 3.7 ± 0.3 minutes). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
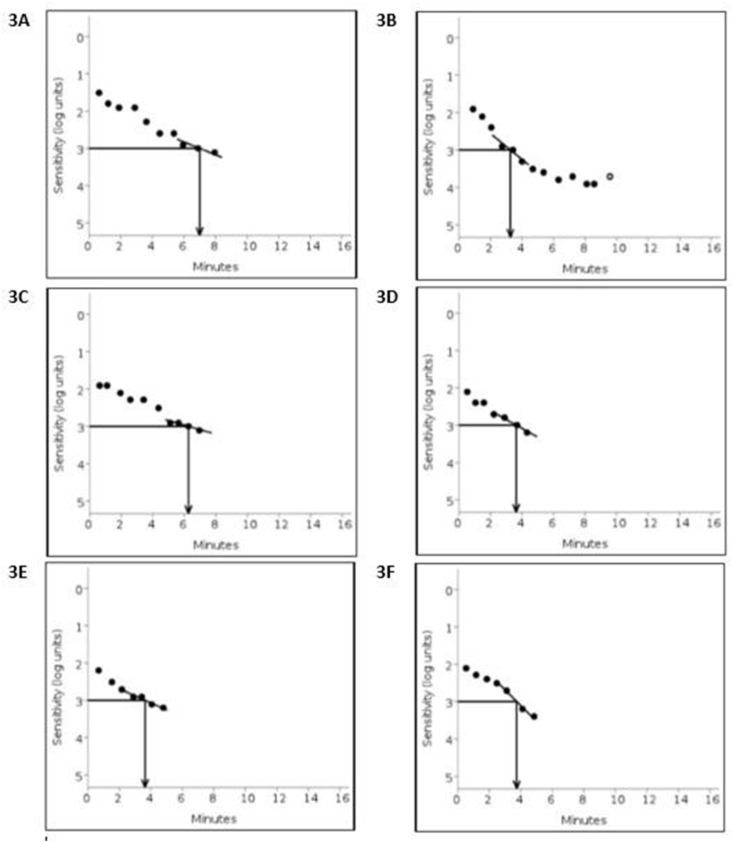
Baseline OCT examination showed retinal separation height 104μ and dark adaptation RIT 7.02 min for the CSCR region, and 3.27 min for the control region. (Fig. 1, Fig. 3B). On day 8 the RIT for the CSCR and the control regions were 6.25 min and 3.63 min respectively (Fig. 3C and D). On day 16, a reduction in the RS (42μ) and RIT (5.86 min) were seen (Fig. 1B), control RIT was 4.36 min. Day 31 OCT scans showed mostly resolved CSCR (RS of 11μ, RIT 3.31 min, and control RIT 3.52 min) (Fig. 1C). The central foveal region was not affected by the CSCR, so the Snellen visual acuity was unaffected and pinhole vision was 20/20. On subsequent visits, we focused on measuring only the DA within the area of CSCR. On day 52, follow-up clinical examination demonstrated a visual acuity of 20/20 in both eyes, normal intraocular pressure and stable anterior segment. Fundus examination of OD remained the same as before while OS showed fully absorbed SRF with no RS or PED confirmed by OCT, and RIT of 3.66 minutes; with control RIT of 3.74 minutes (Fig. 1, Fig. 3F).
Fig. 3.
Dark adaptation curves for left eye CSCR and Control region on Initial presentation, Day 8 and Day 52:
3A Initial presentation: dark adaptation RIT 7.02 minutes for the CSCR region, 3B RIT 3.27 minutes for the control region, 3C and 3D: Day 8 the RIT for the CSCR and the control regions 6.25 minutes and 3.63 minutes respectively. 3E Day 52, resolved CSCR confirmed by OCT with RIT of 3.66 minutes with, 3F control region RIT of 3.74 minutes.
3. Discussion
Previous studies by Alpren and Krants,2 Chaung et al.,3 and Van Meel et al.4 have shown delays in visual pigment recovery which happens by regeneration of visual pigment through visual cycle, and reduced photoreceptor sensitivities in patients with CSCR. Chaung et al.3 showed loss of photoreceptor sensitivity was proportional to the degree of retinal separation from RPE by testing multiple patients each one at a different stages of disease evolution. However changing retinal separation from RPE has never been correlated with changing DA, and it has never been shown that DA response in the CSCR region fully recovers with disease remission.2,3 In our patient, delay in DA in the local CSCR region was observed corresponding to the height of RS, both reduced over time becoming similar to the control region (non-CSCR region) after the resolution of CSCR.
Visual system is able to rapidly adapt to a wide range of light intensities. Dark adaptation refers to the very slow recovery of visual sensitivity in darkness following exposure to intense or prolonged illumination, which bleaches a significant amount of the rhodopsin.5 Dark adaptation visual function has been shown to be delayed in various retinal diseases like age related macular degeneration (AMD). Studies indicate hampered metabolic exchange between the choroid and the photoreceptors in AMD partly by hydrophobic lipid deposition in Bruch's membrane and in the sub RPE space, creating a diffusion barrier.6 This barrier impedes translocation of plasma lipoproteins delivering lipophilic essentials like vitamin A, for rapid outer retinal uptake and distribution.7
The visual cycle requires coordinated activity between the photoreceptors and RPE to regenerate rhodopsin necessary for visual transduction by recycling of its chromophore. Opsin, a G protein–coupled receptor (GPCR) found in photoreceptors, contains an 11-cis-retinal chromophore that undergoes photoisomerization to change the opsin GPCR conformation to all-trans-retinal, which results in a signal transduction cascade hyperpolarizing the photoreceptor cell, and triggering a nerve impulse. After photoisomerization and release from opsin, 11-cis-retinal is reduced to all-trans-retinol and transferred to the adjacent RPE. There, all-trans-retinol is esterified by lecithin–retinol acyltransferase to retinyl ester, then converted to 11-cis-retinol by the isomerohydrolase RPE65, and finally oxidized to 11-cis-retinal before returning to the photoreceptors to combine with opsin to form rhodopsin.8
While many different methods have been used in past to measure dark adaptation recovery, the AdaptDx® device measures the time it takes for eyes to adapt from bright light to darkness, a proprietary parameter called the Rod Intercept (RI). RIT range between 2.5 minutes and 6.5 minutes is considered normal when measured with AdaptDx in patients above 50 years of age. Having a rod intercept > 6.5 minutes is shown to be 90.6% sensitive and 90.5% specific for AMD while a rod intercept ≤ 6.5 minutes is consistent with normal retinal health.9 However, there is no normative data available for CSCR. with this machine. Measuring the rod mediated sensitivity impairment is more relevant in pathology associated with the RPE Bruch's membrane complex and photoreceptor function, as a Muller cell based visual cycle can sustain cone function and account for rapid recovery of cone visual pigment after bleaching for continuous daylight vision, or early recovery of cone mediated dark adaptation independent of RPE-Bruch's membrane.10 The Rod Intercept provides an objective assessment of retinal function that identifies dark adaptation impairment and makes it possible to monitor retinal function. This device has previously shown significant sensitivity and specificity in detecting retinal dysfunction related to age related macular degeneration as stated above.9
The observed delay in RIT in our study could be due to physical separation of RPE and retina leading to disruption of the visual cycle, this hypothesis is supported by other groups: Studies have been published looking at visual function in retinal detachment and CSCR. Alpren and Krants reported delayed cone pigment regeneration kinetics in light by retinal densitometry in Harada's disease patient with serous retinal detachment.2 Van Meel et al. showed association between active leakage measured by fluorescein angiography and slowed regeneration time assessed using foveal densitometry in CSCR, hypothesizing slowed regeneration time by the dilution and removal of retinal by circulating serous fluid.4 Chuang et al.,3 detected rod thresholds by fine matrix perimetry to show loss of sensitivity was directly proportional to the retinal elevation measured by optometer at the fovea,11 measuring levels and regeneration rate of rhodopsin after bleaching by fundus reflectometry.12 Another experimental animal model by Mori et al.13 compared DA recovery within the area of detachment and the surrounding undetached retina immediately after inducing detachment. Dark adaptation was checked by measuring recovery of local ERG and vitreal ERG before and after flashing rabbit retina with light. Although, the study did not show significant difference in time to dark adapt between the detached and attached retina, authors suggested it might be relevant to measure the DA in higher detachments to determine if the recovery becomes abnormal at a threshold height.13
The delayed dark adaptation might also be due to photoreceptor dysfunction which occurs due to the retinal detachment. This was also proposed by Leibrock et al.14 who suggested that recovery of photoreceptor sensitivity is dependent on the regeneration of rhodopsin as well as the decay of photochemical intermediates. Lamb and Pugh15 hypothesize the slowness of recovery results from rate-limited delivery of 11-cis retinal to recombine with opsin, due to either a resistive barrier to delivery or an enzymatic limitation in the processing of 11-cis retinoid. All these processes might be affected during the separation of the retina from the RPE and cause delayed dark adaptation.
The worsening of DA in patients with CSCR could also be due to dysfunction of RPE during the acute phase of the disease. In the canonical visual cycle RPE plays an important function providing the intracellular site and key metabolic enzymes like lecithin retinol acyltransferase and RPE-specific 65 kDa.16 CSCR has been shown to have increased levels of endogenous corticosteroids,17 which may affect electrophysiology of RPE,18 and lead to delays in dark adaptation. Changes in the Bruch's membrane-RPE complex observed in AMD, such as the thickening of Bruch's membrane, and the sub-RPE deposits were shown to hamper the regeneration of visual pigments and the nutrition of photoreceptors,19 which might support importance of RPE in visual pigment regeneration.
DA response varies with eccentricity of test location20,21 and possibly along meridians.22 However, there is some evidence that for normal vision controls the DA response along horizontal and vertical meridians for the same eccentricities are not significantly different.23 So the key was to select a control location at the same eccentricity as the CSCR spot, but making sure that it does not overlap with the CSCR spot. Therefore in our case, the control spot was chosen as the opposite end at the same eccentricity on the same meridian. Interestingly in our patient, we found delayed DA on the day of presentation, which improved on day 8 in the affected retina even before complete resolution of SRF. The RIT continued to decrease at day 16 and 31 as the RS resolved further (Fig. 2). Also, DA values were within normal range in the OD with prior CSCR, now at 5 years post-resolution. This observation indicates that the physical separation of the neurosensory retina 104 μm away from the RPE might be sufficient to affect the visual cycle and cause localized delays in dark adaptation.
In summary, this case report demonstrates that a patient with CSCR can have delayed DA proportional to the degree of separation, which improves as CSCR resolves. The correlation between the height of a resolving CSCR detachment and delayed recovery of dark adaptation, possibly could be due to Retinal separation, dysfunction of photoreceptors or possibly dysfunction of RPE. This work may have further implications to understanding visual function recovery in other forms of retinal detachment. Even though patients with CSCR are symptomatic, the standard of care visual acuity can be good. Compared to other visual function measures such as visual acuity measured at a fixed central location, which might not be affected by the CSCR, the dark adaptation sensitivity can be precisely measured within the area of diseased retina. Our study for the first time uses FDA approved accessible device AdaptDx to measure DA in these patients. Our case report shows that dark adaptation measured within the localized area affected by the disease may be more sensitive measure of visual function in patients with CSCR and can also be used as an additional way of monitoring recovery of visual function as the disease resolves.
Patient consent
Written consent to publish case details is obtained from the patient.
Funding
No funding or Grant support
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
The following authors have no financial disclosures.
Acknowledgements
None.
This case has not been presented at any conference.
Contributor Information
Archana Nigalye, Email: archana_nigalye@meei.harvard.edu.
Shrinivas Pundlik, Email: shrinivas_pundlik@meei.harvard.edu.
Janice Kim, Email: Janicekim93@gmail.com.
Gang Luo, Email: gang_luo@meei.harvard.edu.
Deeba Husain, Email: deeba_husain@meei.harvard.edu.
References
- 1.Jackson G.R., Edwards J.G. A short-duration dark adaptation protocol for assessment of age-related maculopathy. Journal of Ocular Biology, Diseases, and Informatics. 2008;1:7–11. doi: 10.1007/s12177-008-9002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpern M., Krantz D.H. Visual pigment kinetics in abnormalities of the uvea-retinal epithelium interface in man. Invest Ophthalmol Vis Sci. 1981;20:183–203. [PubMed] [Google Scholar]
- 3.Chuang E.L., Sharp D.M., Fitzke F.W., Kemp C.M., Holden A.L., Bird A.C. Retinal dysfunction in central serous retinopathy. Eye. 1987;1(Pt 1):120–125. doi: 10.1038/eye.1987.18. [DOI] [PubMed] [Google Scholar]
- 4.van Meel G.J., Smith V.C., Pokorny J., van Norren D. Foveal densitometry in central serous choroidopathy. Am J Ophthalmol. 1984;98:359–368. doi: 10.1016/0002-9394(84)90329-5. [DOI] [PubMed] [Google Scholar]
- 5.Lamb T.D., Pugh E.N., Jr. Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23:307–380. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Curcio C.A., Johnson M., Rudolf M., Huang J.-D. The oil spill in ageing Bruch membrane. Br J Ophthalmol. 2011;95:1638–1645. doi: 10.1136/bjophthalmol-2011-300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owsley C., McGwin G., Clark M.E. Delayed rod-mediated dark adaptation is a functional biomarker for incident early age-related macular degeneration. Ophthalmology. 2016;123:344–351. doi: 10.1016/j.ophtha.2015.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsin A., Betts-Obregon B., Grigsby J. Visual cycle proteins: structure, function, and roles in human retinal disease. J Biol Chem. 2018;293:13016–13021. doi: 10.1074/jbc.AW118.003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson G., Scott I., Kim I., Quillen D., Innaccone A., Edwards J. Diagnostic sensitivity and specificity of dark adaptometry for detection of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55:1427–1431. doi: 10.1167/iovs.13-13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J-s, Nymark S., Frederiksen R. Chromophore supply rate-limits mammalian photoreceptor dark adaptation. J Neurosci. 2014;34:11212–11221. doi: 10.1523/JNEUROSCI.1245-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzke F.W., Holden A.L., Sheen F.H. A Maxwellian-view optometer suitable for electrophysiological and psychophysical research. Vis Res. 1985;25:871–874. doi: 10.1016/0042-6989(85)90196-8. [DOI] [PubMed] [Google Scholar]
- 12.Faulkner D.J., Kemp C.M. Human rhodopsin measurement using a T.V.-based imaging fundus reflectometer. Vis Res. 1984;24:221–231. doi: 10.1016/0042-6989(84)90124-x. [DOI] [PubMed] [Google Scholar]
- 13.Mori T., Pepperberg D.R., Marmor M.F. Dark adaptation in locally detached retina. Invest Ophthalmol Vis Sci. 1990;31:1259–1263. [PubMed] [Google Scholar]
- 14.Leibrock C.S., Reuter T., Lamb T.D. Molecular basis of dark adaptation in rod photoreceptors. Eye. 1998;12(Pt 3b):511–520. doi: 10.1038/eye.1998.139. [DOI] [PubMed] [Google Scholar]
- 15.Lamb T.D., Pugh E.N., Jr. Phototransduction, dark adaptation, and rhodopsin regeneration the proctor lecture. Invest Ophthalmol Vis Sci. 2006;47:5137–5152. doi: 10.1167/iovs.06-0849. [DOI] [PubMed] [Google Scholar]
- 16.Yang G.-Q., Chen T., Tao Y., Zhang Z.-M. Recent advances in the dark adaptation investigations. Int J Ophthalmol. 2015;8:1245–1252. doi: 10.3980/j.issn.2222-3959.2015.06.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garg S.P., Dada T., Talwar D., Biswas N.R. Endogenous cortisol profile in patients with central serous chorioretinopathy. Br J Ophthalmol. 1997;81:962–964. doi: 10.1136/bjo.81.11.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arndt C., Sari A., Ferre M. Electrophysiological effects of corticosteroids on the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2001;42:472–475. [PubMed] [Google Scholar]
- 19.Jackson G.R., Owsley C., Curcio C.A. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res Rev. 2002;1:381–396. doi: 10.1016/s1568-1637(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 20.Flynn O.J., Cukras C.A., Jeffrey B.G. Characterization of rod function phenotypes across a range of age-related macular degeneration severities and subretinal drusenoid deposits. Invest Ophthalmol Vis Sci. 2018;59:2411–2421. doi: 10.1167/iovs.17-22874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Binns A.M., Taylor D.J., Edwards L.A., Crabb D.P. Determining optimal test parameters for assessing dark adaptation in people with intermediate age-related macular degeneration. Invest Ophthalmol Vis Sci. 2018;59:AMD114–AMD121. doi: 10.1167/iovs.18-24211. [DOI] [PubMed] [Google Scholar]
- 22.Proudlock F.A., Khanna A., Gottlob I. Filling-in along horizontal and vertical meridians. Investig Ophthalmol Vis Sci. 2006;47:453–460. doi: 10.1167/iovs.05-0255. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen C.T., Fraser R.G., Tan R. Longitudinal changes in retinotopic rod function in intermediate age-related macular degeneration. Investig Ophthalmol Vis Sci. 2018;59:AMD19–AMD24. doi: 10.1167/iovs.17-23084. [DOI] [PubMed] [Google Scholar]