Abstract
Background
Trigger finger is a common hand condition that occurs when movement of a finger flexor tendon through the first annular (A1) pulley is impaired by degeneration, inflammation, and swelling. This causes pain and restricted movement of the affected finger. Non‐surgical treatment options include activity modification, oral and topical non‐steroidal anti‐inflammatory drugs (NSAIDs), splinting, and local injections with anti‐inflammatory drugs.
Objectives
To review the benefits and harms of non‐steroidal anti‐inflammatory drugs (NSAIDs) versus placebo, glucocorticoids, or different NSAIDs administered by the same route for trigger finger.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL, CNKI (China National Knowledge Infrastructure), ProQuest Dissertations and Theses, www.ClinicalTrials.gov, and the WHO trials portal until 30 September 2020. We applied no language or publication status restrictions.
Selection criteria
We searched for randomised controlled trials (RCTs) and quasi‐randomised trials of adult participants with trigger finger that compared NSAIDs administered topically, orally, or by injection versus placebo, glucocorticoid, or different NSAIDs administered by the same route.
Data collection and analysis
Two or more review authors independently screened the reports, extracted data, and assessed risk of bias and GRADE certainty of evidence. The seven major outcomes were resolution of trigger finger symptoms, persistent moderate or severe symptoms, recurrence of symptoms, total active range of finger motion, residual pain, patient satisfaction, and adverse events. Treatment effects were reported as risk ratios (RRs) and mean differences (MDs) with 95% confidence intervals (CIs).
Main results
Two RCTs conducted in an outpatient hospital setting were included (231 adult participants, mean age 58.6 years, 60% female, 95% to 100% moderate to severe disease). Both studies compared a single injection of a non‐selective NSAID (12.5 mg diclofenac or 15.0 mg ketorolac) given at lower than normal doses with a single injection of a glucocorticoid (triamcinolone 20 mg or 5 mg), with maximum follow‐up duration of 12 weeks or 24 weeks.
In both studies, we detected risk of attrition and performance bias. One study also had risk of selection bias. The effects of treatment were sensitive to assumptions about missing outcomes. All seven outcomes were reported in one study, and five in the other.
NSAID injection may offer little to no benefit over glucocorticoid injection, based on low‐ to very low‐certainty evidence from two trials. Evidence was downgraded for bias and imprecision. There may be little to no difference between groups in resolution of symptoms at 12 to 24 weeks (34% with NSAIDs, 41% with glucocorticoids; absolute effect 7% lower, 95% confidence interval (CI) 16% lower to 5% higher; 2 studies, 231 participants; RR 0.83, 95% CI 0.62 to 1.11; low‐certainty evidence). The rate of persistent moderate to severe symptoms may be higher at 12 to 24 weeks in the NSAIDs group (28%) compared to the glucocorticoid group (14%) (absolute effect 14% higher, 95% CI 2% to 33% higher; 2 studies, 231 participants; RR 2.03, 95% CI 1.19 to 3.46; low‐certainty evidence). We are uncertain whether NSAIDs result in fewer recurrences at 12 to 24 weeks (1%) compared to glucocorticoid (21%) (absolute effect 20% lower, 95% CI 21% to 13% lower; 2 studies, 231 participants; RR 0.07, 95% CI 0.01 to 0.38; very low‐certainty evidence). There may be little to no difference between groups in mean total active motion at 24 weeks (235 degrees with NSAIDs, 240 degrees with glucocorticoid) (absolute effect 5% lower, 95% CI 34.54% lower to 24.54% higher; 1 study, 99 participants; MD ‐5.00, 95% CI ‐34.54 to 24.54; low‐certainty evidence). There may be little to no difference between groups in residual pain at 12 to 24 weeks (20% with NSAIDs, 24% with glucocorticoid) (absolute effect 4% lower, 95% CI 11% lower to 7% higher; 2 studies, 231 participants; RR 0.84, 95% CI 0.54 to 1.31; low‐certainty evidence). There may be little to no difference between groups in participant‐reported treatment success at 24 weeks (64% with NSAIDs, 68% with glucocorticoid) (absolute effect 4% lower, 95% CI 18% lower to 15% higher; 1 study, 121 participants; RR 0.95, 95% CI 0.74 to 1.23; low‐certainty evidence).
We are uncertain whether NSAID injection has an effect on adverse events at 12 to 24 weeks (1% with NSAIDs, 1% with glucocorticoid) (absolute effect 0% difference, 95% CI 2% lower to 3% higher; 2 studies, 231 participants; RR 2.00, 95% CI 0.19 to 21.42; very low‐certainty evidence).
Authors' conclusions
For adults with trigger finger, by 24 weeks' follow‐up, results from two trials show that compared to glucocorticoid injection, NSAID injection offered little to no benefit in the treatment of trigger finger. Specifically, there was no difference in resolution, symptoms, recurrence, total active motion, residual pain, participant‐reported treatment success, or adverse events.
Plain language summary
Which types of medicines work best when injected into the hand to treat a painful swollen finger (trigger finger)?
Key messages
1. A single injection of a medicine called an NSAID (non‐steroidal anti‐inflammatory drug) to treat trigger finger offered little to no benefit 6 months later
2. One injection of an NSAID may not work better than one injection of a steroid to treat the symptoms of trigger finger
3. More people may continue to have moderate to severe symptoms 3 to 6 months after an NSAID injection compared with those who had a steroid injection
4. There were no differences in the numbers of unwanted effects reported after injections of either type of medicine (steroid or non‐steroid)
What is trigger finger?
Trigger finger is a condition that affects one or more of the tendons in your hand. Tendons are tissues that join muscle to bone, to let you move your joints. When a tendon in your hand is swollen and inflamed, bending the affected finger is difficult and painful. Without treatment, the affected finger may become permanently bent, making everyday tasks difficult.
Treating trigger finger
For some people, trigger finger might get better without treatment. If it doesn't, treatments include:
1. rest ‐ avoiding certain activities;
2. physical therapy;
3. strapping the finger to a piece of plastic (splint) to reduce movement;
4. medicines known as NSAIDs taken by mouth or directly through the skin to reduce pain; and
5. steroid medicines injected to reduce swelling.
If none of these treatments works, surgery may be needed to help the tendon move freely again.
Why did we do this Cochrane Review?
Injecting steroid medicines into the hand to treat trigger finger may cause unwanted effects. We wanted to find out if injecting NSAID medicines into the hand could help people with trigger finger and cause fewer unwanted effects than steroids.
What did we do?
We searched for studies that tested NSAID medicines injected to treat trigger finger. We compared results of the studies we found to arrive at an overall estimate of how well an NSAID injection can treat trigger finger.
How up‐to‐date is this review?
We included evidence up to 30 September 2020.
What did we find?
We found 2 studies in 231 adults (average age 59 years; 60% women) who were treated for trigger finger at outpatient clinics in Singapore and Malaysia. One study lasted 3 months; the other lasted 6 months. One study did not report its source of funding; the other study received no commercial funding.
In both studies, the people taking part were given one injection of either:
1. a steroid medicine (called triamcinolone); or
2. an NSAID (diclofenac or ketorolac).
What are the main results of our review?
Compared with a steroid injection, an NSAID injection may have little to no effect on:
1. whether symptoms disappear (evidence from 2 studies in 231 people);
2. how much someone can move the affected finger 3 to 6 months later (1 study in 99 people);
3. how many people still have pain after 3 to 6 months of treatment (2 studies in 231 people); and
4. how many people say their treatment was successful (1 study in 121 people).
On average, for every 100 people treated:
1. symptoms may disappear after 3 months in 34 people who had an NSAID compared to 41 people who had a steroid;
2. 28 people who had an NSAID may still have moderate to severe symptoms after 3 to 6 months compared to 14 people who had a steroid;
3. 20 people who had an NSAID may still feel pain after 3 to 6 months compared to 24 people who had a steroid;
4. 64 people who had an NSAID may say their treatment was successful compared to 68 people who had a steroid.
We are uncertain if an NSAID injection:
1. affects whether symptoms of trigger finger come back after treatment; or
2. causes fewer unwanted effects than a steroid injection (evidence from 2 studies in 231 people).
Limitations of the evidence
Our confidence in the results is limited because they come from two small studies only. These studies used different doses of steroid for injection, which may have affected our comparison of the studies. Further research is likely to change these results or increase our confidence in them..
Summary of findings
Summary of findings 1. NSAID injection compared to glucocorticoid injection for trigger finger.
| NSAID injection compared to glucocorticoid injection for trigger finger | ||||||
| Patient or population: adults (> 18 years of age) with trigger finger Setting: outpatient hand surgery clinic Intervention: NSAID injection at the level of the A1 pulley of the affected finger Comparison: glucocorticoid injection at the level of the A1 pulley of the affected finger | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with glucocorticoid injection | Risk with NSAID injection | |||||
| Resolution at 12 to 24 weeks Quinell Grade (range 0 to 4, resolved disease is grade 0) Follow‐up: range 12 weeks to 24 weeks |
41 per 100 | 34 per 100 (25.4 to 45.5) |
RR 0.83 (0.62 to 1.11) | 231 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | NSAID injection may have little to no effect on resolution of symptoms Absolute difference 7% lower (16% lower to 5% higher),c risk ratio 17% lower (38% lower to 11% higher) |
| Persistent moderate to severe symptoms at 12 to 24 weeks Assessed with Quinnell Grade (range 0 to 4; severe disease Grades 2 to 4) Follow‐up: range 12 weeks to 24 weeks | 14 per 100 | 28 per 100 (16.3 to 47.3) |
RR 2.03 (1.19 to 3.46) | 231 (2 RCTs) | ⊕⊕⊝⊝ LOWa,d | NSAID injection may result in more people with persistent moderate to severe symptoms Absolute difference 14% higher (2% higher to 33% higher), risk ratio 103% higher (19% higher to 246% higher) NNTH 7, 95% CI 3 to 38 |
| Recurrence at 12 to 24 weeks Follow‐up: range 12 weeks to 24 weeks | 21 per 100 | 1 per 100 (0.2 to 7.8) |
RR 0.07 (0.01 to 0.38) | 231 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWe,f | It is uncertain if NSAID injection results in fewer people with recurrence of symptoms Absolute difference 20% lower (21% to 13% lower), risk ratio 93% lower (99% lower to 62% lower) NNTB 6, 95% CI 5 to 8 |
| Total active motion Assessed with goniometer (degrees) Follow‐up: mean 24 weeks | Mean total active motion was 240 degrees | Mean total active motion was 5 degrees lower (34.54 degrees lower to 24.54 degrees higher) |
‐ | 99 (1 RCT) | ⊕⊕⊝⊝ LOWa,g | NSAID injection may have little to no effect on total active motion Absolute difference 5% lower (34.54% lower to 24.54% higher), risk ratio 2.1% lower (14.8% lower to 10.2% higher) |
| Residual pain Assessed with participant‐reported (pain/no pain) measures Follow‐up: range 12 weeks to 24 weeks | 24 per 100 | 20 per 100 (12.9 to 31.4) |
RR 0.84 (0.54 to 1.31) | 231 (2 RCTs) | ⊕⊕⊝⊝ LOWa,g | NSAID injection may have little to no effect on residual pain Absolute difference 4% lower (11% lower to 7% higher), risk ratio 16% lower (46% lower to 31% higher) |
| Participant‐reported treatment success Follow‐up: mean 24 weeks | 68 per 100 | 64 per 100 (50.1 to 83.3) |
RR 0.95 (0.74 to 1.23) | 121 (1 RCT) | ⊕⊕⊝⊝ LOWa,g | NSAID injection may have little to no effect on treatment success Absolute difference 4% lower (18% lower to 15% higher), risk ratio 5% lower (26% lower to 23% higher) |
| Adverse events Follow‐up: range 12 weeks to 24 weeks | 1 per 100 | 1 per 100h (0 to 4.0) | RR 2.00 (0.19 to 21.42i) | 231 (2 RCTs) | ⊕⊕⊝⊝ VERY LOWa,j | It is uncertain if NSAID injection results in fewer adverse events Absolute difference 0% (2% lower to 3% higher), risk ratio 0% (200% lower to 300% higher) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group (steroid injection) and the relative effect of the intervention (and its 95% CI). The exception is the adverse events outcome, for which the absolute effect of the intervention is used (see Footnote 9). CI: confidence interval; MD: mean difference; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; NSAID: non‐steroidal anti‐inflammatory drug; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level for risk of bias (high risk of selection and attrition bias).
bDowngraded by one level for imprecision (95% CI supports both superiority for steroid injections and weak superiority for NSAID injections).
cWith the exception of the adverse events outcome (see Footnote 10),the absolute effect is the simple difference between NSAID and steroid group risks in columns three and two of the table.
dDowngraded by one level for imprecision (95% CI supports both superiority for steroid injection and no important difference between the two interventions).
eDowngraded by two levels for risk of bias (high risk of selection and attrition bias; magnitude and precision of the pooled effect are highly sensitive to inclusion/exclusion of participants not at risk of recurrence; see Analysis 4.1).
fDowngraded by one level for imprecision (95% CI supports substantial or weak superiority for NSAID injections).
gDowngraded by one level for imprecision (95% CI supports superiority for steroid and NSAID injections).
hThe risk in the intervention group was based on the pooled risk ratio because this effect uses data from both studies (Analysis 1.7), unlike the relative effect (see footnote below).
iThis risk ratio estimate is based on only one study (Shakeel 2012), as the risk ratio of the excluded study ‐ Leow 2018 ‐ was not estimable due to zero adverse events in both treatment groups.
jDowngraded by two levels for imprecision (data from only one study ‐ Shakeel 2012 ‐ and very few events).
Background
Description of the condition
Trigger finger is a common condition that occurs when the gliding movement of an inflamed and swollen flexor tendon is obstructed by the narrowed osteofibrous canal of the first annular (A1) pulley, resulting in pain, clicking, catching, and loss of motion of the affected finger (Choudhury 2014). The A1 pulley is a fibrous condensation located at the metacarpophalangeal (MCP) joint. The forearm muscles that flex the fingers to enable grasping transmit force through long cord‐like flexor tendons that cross over the wrist and palm and attach to the fingers. To increase mechanical efficiency, these tendons slide through tunnel‐like tendon sheaths, which anchor the tendons closely to the volar surface of the finger bones (Peters‐Veluthamaningal 2009), in the same way that eyelets on a fishing rod constrain a tense fishing line to follow the curvature of the flexing rod. Trigger finger occurs when the smooth gliding movement of the flexor tendon through the A1 pulley is impaired by swelling of the tendon and the A1 pulley. This results in pain, clicking, catching, and restricted movement of the finger (Choudhury 2014; Makkouk 2008).
Trigger finger is a common hand condition with a prevalence of 2.6% in the general population (Makkouk 2008), more commonly affecting women and people in their fifth and sixth decades (Choudhury 2014). People who have arthritis and diabetes are more susceptible, with prevalence ranging from 5% to 36% (Cagliero 2002; Ray 2011). Trigger finger presents with different levels of severity. The most characteristic complaint is a finger stuck ("triggered") in the flexed position with difficulty returning to full extension (Choudhury 2014; Kolind‐Sørensen 1980; Peters‐Veluthamaningal 2009). It may eventually be released spontaneously with a snapping action (active correction) or with the help of the other hand (passive correction). In more severe cases, the finger may be locked in the bent position due to severe pain and mechanical tightness at the A1 pulley. In milder cases, patients may just experience uneven finger movements with accompanying discomfort and stiffness. Quinnell 1980 proposed a five‐point ordinal scale on which to grade the severity of trigger finger: Grade 0 – normal but possibly some mild crepitus during movement, Grade 1 – no triggering but uneven movement, Grade 2 – triggering but actively correctable, Grade 3 – triggering but passively correctable, Grade 4 – locked finger. Others have used very similar scales, with Grades 0 and 1 indicating no and mild disease, respectively, and Grade 4 indicating a fixed flexion (Eastwood 1992; Green 2005; Patel 1997).
Description of the intervention
The first line of treatment for trigger finger is conservative management, which involves activity modification, administration of topical or oral non‐steroidal anti‐inflammatory drugs (NSAIDs), and splinting (Choudhury 2014; Evans 1988; Patel 1992; Ryzewicz 2006). People with mild symptoms and those who have declined local injections and surgery are offered conservative treatment. Topical NSAIDs such as ketoprofen and oral NSAIDs such as ibuprofen are commonly given to relieve the pain caused by trigger finger. If conservative treatment fails, or if symptoms are severe (Quinell Grade 3 or 4), patients have the option of local glucocorticoid injections or surgery. A Cochrane Review has summarised the efficacy of glucocorticoid injections for trigger finger (Peters‐Veluthamaningal 2009). These injections are given locally at the affected A1 pulley. Variations in injection methods include palmar versus mid‐lateral approach (Jianmongkol 2007), at the A1 pulley versus at the proximal phalanx (Bodor 2009; Pataradool 2011), intra‐sheath versus extra‐sheath (Mardani‐Kivi 2018), and passing through versus not passing through the tendon substance (Akhtar 2006). It is thought that injectable NSAIDs might be an effective alternative to injectable glucocorticoids in situations where glucocorticoids are arguably contraindicated, as in diabetic patients with poor blood sugar control or in patients with infection or poor immunity (Leow 2017).
How the intervention might work
NSAIDs act via the arachidonic pathway, which involves inhibition of the cyclo‐oxygenase (COX) receptors, which in turn inhibits the synthesis of important pro‐inflammatory prostaglandins and blood clotting thromboxanes, resulting in reduced inflammation. NSAIDs are subdivided into two classes: (1) non‐selective NSAIDs, which inhibit both COX‐1 and COX‐2 enzymes, and (2) COX‐2‐selective NSAIDs (Waller 2013). The inflammation, swelling, and pain that impair tendon mobility are counteracted by the anti‐inflammatory and analgesic properties of NSAIDs.
The potent anti‐inflammatory effects of glucocorticoids are mediated by lipocortin‐1 (annexin‐1) synthesis and its downstream effects on phospholipase A2 synthesis and the arachidonic pathway. Glucocorticoids have a larger immunosuppressive effect than NSAIDs, as they work at the hormonal level and suppress the inflammatory process through numerous pathways (Barnes 2009).
Adverse effects of NSAIDs are primarily related to their inhibition of protective prostaglandins and thromboxanes, which increases the risk of adverse effects. Systemic events are expected to be more common with oral NSAIDs because of systemic absorption. These include hepatic injury, gastrointestinal irritation, ulcers, and bleeding, especially with non‐selective NSAIDs (Baigent 2013; Gabriel 1991; Ong 2007; Schneeweiss 2005; Sostres 2010); transient increase in blood pressure, heart attack, and stroke, especially with the thrombogenic COX‐2‐selective NSAIDs (Baigent 2013; Bresalier 2005; Kearney 2006); renal injury and hypertension due to inhibition of blood flow through the kidney (Thomas 2000); dry skin, rash, dermatitis, paraesthesia, pruritis, urticaria, and vesiculobullous rash (Makris 2007); and dizziness, vertigo, and headache (Makris 2007; Rannou 2016). Local events are relevant to topical or injectable NSAIDs but are not expected with an appropriate dosing schedule. Effects occur at the site of application or injection and include bleeding, pain, and allergic reactions.
Adverse effects of glucocorticoids occur at the site of injection and include bleeding, tendon rupture (Fitzgerald 2005; Gyuricza 2009; Wei 2006), skin thinning (Fitzgerald 2005; Gyuricza 2009), and discolouration due to effects on collagen metabolism (Brinks 2010; Friedman 1988). These effects are rare and are reported in very few case studies. Glucocorticoid injections are typically safe. Usually, there is a limit of three injections given at the same site. Systemic events are expected to be very uncommon for topical and local injections of glucocorticoids due to the local route of administration, which minimises systemic absorption. These events would be more common for oral glucocorticoids and may include infection and delayed healing due to immunosuppression (Coutinho 2011), transient hyperglycaemia due to effects on glucose synthesis and transport (Baumgarten 2007; Wang 2006), and allergic reactions.
Why it is important to do this review
Trigger finger is one of the most common hand conditions. Topical and oral NSAIDs are routinely prescribed as first‐line conservative treatment. Clinical experience shows that topical NSAIDs can help to relieve the pain and triggering caused by trigger finger. In people for whom the first line of treatment has failed, or in those with more severe cases, injectable glucocorticoids are an option. Our review summarises available evidence on the effectiveness, safety, and benefits of topical, oral, and injectable NSAIDs, and evaluates their effectiveness as an alternative to glucocorticoids.
Objectives
To review the benefits and harms of non‐steroidal anti‐inflammatory drugs (NSAIDs) versus placebo, glucocorticoids, or different NSAIDs administered by the same route for trigger finger.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) or quasi‐randomised trials were included. Quasi‐random methods of assignment to interventions are systematic methods that are not truly random, such as allocation by date of birth, hospital record number, or alternation. Studies reported as full text or only as an abstract and unpublished data were included. We applied no language restrictions.
Types of participants
Only studies of adults (older than 18 years) with a clinical diagnosis of trigger finger (triggering with or without locking of a finger or pain at the A1 pulley), irrespective of the duration of symptoms, were included. Studies of trigger finger caused by infection were excluded.
Types of interventions
We included all studies using traditional or COX‐2 selective NSAIDs administered topically, orally, or by injection. Comparators included placebo, glucocorticoids, or different NSAIDs delivered by the same route as the intervention.
Types of outcome measures
Follow‐up time points for the outcomes of interest were 4 weeks, 12 weeks, 24 weeks, 38 weeks, and 52 weeks.
Major outcomes
Resolution of trigger finger symptoms (dichotomous: resolved/not resolved): resolution of symptoms was defined as a Quinell Grade (or equivalent) of 0; all other grades were classified as unresolved disease
Persistent moderate to severe trigger finger symptoms (dichotomous: present/absent): moderate to severe symptoms was defined as a Quinell Grade of 2 or higher; Grade 1 was classified as mild, and Grade 0 as resolved
Recurrence of trigger finger symptoms (dichotomous: recurring symptoms/no recurring symptoms): usually measured as the proportion with recurrence (definition of recurrence may vary across trials)
Total active motion (continuous): defined as the investigator‐reported sum of active range of motion of the three finger joints (metacarpophalangeal, proximal interphalangeal, and distal interphalangeal) as measured by a goniometer in degrees; normal range of motion is as follows: metacarpophalangeal 19 to 71 degrees; proximal interphalangeal 23 to 87 degrees; distal interphalangeal 10 to 64 degrees (Bain 2014)
Residual pain (dichotomous: pain/no pain): defined as participant‐reported residual pain or tenderness at the base of the finger
Severity of pain: reporting of pain as a continuous outcome would be considered if visual analogue or other such pseudo‐continuous scales were used
Participant‐reported treatment success (dichotomous: satisfied/not satisfied): defined as participant‐reported satisfaction with treatment
Adverse effects of treatment (dichotomous: present/absent): defined as the investigator‐reported total number of adverse effects or complications of treatment
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library; MEDLINE (1966 to 2020); Embase (1988 to 2020); the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1937 to 2020); the China National Knowledge Infrastructure (CNKI; 1915 to 2020); and ProQuest Dissertations and Theses (1980 to 2020). A search of www.ClinicalTrials.gov and the World Health Organization (WHO) trials portal was also conducted (www.who.int/ictrp/en/). We applied no restriction on language of publication. The detailed search strategy for MEDLINE (Ovid) is provided in Appendix 1. This search strategy was adapted appropriately for the other electronic databases. The search was conducted on 30 September 2020.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references.
Data collection and analysis
Selection of studies
Two review authors (ML, QZ) independently screened titles and abstracts for possible inclusion. Two review authors (ML, SCT) independently screened the full‐text reports retrieved to confirm eligibility and to document reasons for exclusion. Any disagreement was resolved by consensus. Results at various stages of the selection process were recorded by using the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram (prisma-statement.org/PRISMAStatement/Default.aspx; PRISMA Group).
Data extraction and management
Three review authors (ECSY, ML, QZ) independently extracted the following study data using a standard data collection form. Any disagreement was resolved by consensus.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study settings, withdrawals, and dates of study.
Participants: N, mean age, age range, sex, disease duration, severity of condition, diagnostic criteria, medical history of participants, inclusion criteria, and exclusion criteria.
Interventions: name, dosage, route of administration, and concomitant and excluded medications.
Outcomes: outcomes, treatment group statistics (i.e. number of events, means, standard deviations, and number of participants per treatment group), and time points. If outcomes for time points falling outside the pre‐specified values were reported, we extracted the data corresponding to a time closest to one of the pre‐specified time points to create as far as possible intention‐to‐treat (ITT) samples for analysis
Characteristics of the design of the trial for assessment of risk of bias, including sources of funding and conflicts of interest
Assessment of risk of bias in included studies
Three review authors (ECSY, ML, QZ) independently assessed risk of bias for each study, using the Cochrane ’Risk of bias’ (RoB) tool (Higgins 2017). Two authors of this review are the authors of one of the studies included in this review (Leow 2018). This potential bias was resolved by having Renea Johnston from the Cochrane Musculoskeletal Group and an independent methodologist (ECSY) assess risk of bias, and by involving two methodologists (ECSY, QZ) in all critical steps (Contributions of authors). An independent methodologist (ECSY) assessed risk of bias, and two methodologists (ECSY, QZ) were involved in all critical steps to avoid bias (Contributions of authors).
Any disagreement was resolved by consensus. The following domains were graded as having high, low, or unclear risk of bias (RoB).
Random sequence generation.
Allocation concealment.
Blinding of participants and study personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data.
Selective outcome reporting.
Other potential bias.
Separate RoB judgements were made for performance bias and detection of outcome bias. For performance bias, we considered blinding of participants and study personnel involved in treatment and caregiving. For detection bias, we separately assessed blinding of investigator‐reported outcome measurements (resolution, moderate to severe symptoms, recurrence, total active motion, and adverse events) and blinding of participant‐reported outcome measurements (pain and satisfaction).
Measures of treatment effect
We analysed dichotomous outcomes as risk ratios (RRs) or as Peto odds ratios when intervention effects were small or even zero, and we reported 95% confidence intervals (CIs). We analysed continuous outcomes as mean differences (MDs) or standardised mean differences (SMDs), depending on whether the same scale was used to measure an outcome, and we reported 95% CIs. We entered data presented on a scale with a consistent direction of effect across studies.
When different scales were used to measure the same conceptual outcome (e.g. function), we planned to calculate SMDs, along with corresponding 95% CIs. We would back‐translate SMDs to a typical scale (e.g. 0 to 10 for pain) by multiplying the SMD by a typical among‐person standard deviation (e.g. standard deviation of the control group at baseline from the most representative trial), as per Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2017).
In the Effects of interventions section and in the 'Comments' column of the Characteristics of included studies table, we provided the absolute per cent difference, the relative per cent change from baseline, and the number needed to treat for an additional beneficial outcome (NNTB) (provided only when the effect was clinically significant). For dichotomous outcomes, we calculated the NNTB or the number needed to treat for an additional harmful outcome (NNTH) from the control group event rate and the risk ratio, using the Visual Rx NNT calculator (Cates 2016). We calculated the NNTB or the NNTH for continuous outcomes using the Wells calculator (available at the CMSG Editorial Office; http://musculoskeletal.cochrane.org/).
For dichotomous outcomes, we calculated the absolute risk difference using the risk difference statistic in Review Manager 5 (RevMan 2014), and we expressed the result as a percentage. For continuous outcomes, we calculated the absolute difference as the mean in the intervention group minus the mean in the control group, in original units, expressed as a percentage.
We derived the relative per cent change for dichotomous data as the risk ratio of 1 and expressed it as a percentage. For continuous outcomes, we calculated the relative difference in the change from baseline as the absolute difference divided by the mean of the control group, expressed as a percentage.
Unit of analysis issues
Each finger of a study participant was the unit of analysis; if multiple fingers from individual participants were included (i.e. those participants represent a cluster of units), we employed a variance inflating correction for the correlated outcomes from that study, if not already reported (Higgins 2011). If multiple trial arms were reported in a single trial, we included only the relevant arms. If two comparisons (e.g. drug A versus placebo and drug B versus placebo) were combined in the same meta‐analysis, we halved the control group to avoid double‐counting.
Dealing with missing data
Whenever possible, we contacted the study authors to verify key study characteristics and to obtain missing data.
For dichotomous outcomes, we performed an ITT analysis by using the total number of randomised participants as the denominator for group risks, with participants analysed in their randomised groups. When attrition caused missing outcome data, we presented the ITT analysis scenario, which assumed all study dropouts did not experience the outcome event (middle case 1 scenario) as the default analysis (see Sensitivity analysis). For continuous outcomes, we reported a per‐protocol analysis by using the number of participants who completed follow‐up.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity in terms of participants, interventions, outcomes, and study characteristics to determine if similarity was sufficient for meta‐analysis to be appropriate (see Characteristics of included studies). We quantified statistical heterogeneity by using the I² statistic (Deeks 2017), and we judged inconsistency by using the following guidelines: I² value of 0% to 40% 'might not be important'; 30% to 60% may represent 'moderate' heterogeneity; 50% to 90% may represent 'substantial' heterogeneity; and 75% to 100% represents 'considerable' heterogeneity (Deeks 2017).
Assessment of reporting biases
We checked trial protocols against published reports to assess outcome reporting bias. For studies published after 1 July 2005, we screened the WHO International Clinical Trials Registry Platform for prospectively written trial protocols (www.who.int/ictrp/en/). We reported any commercial funding or serious conflict of interest in the "Other bias" domain. If we identified sufficient studies, and if all other major biases (except publication bias) could be ruled out (Sterne 2017), we examined a funnel plot for asymmetry; otherwise a funnel plot would not be produced.
Data synthesis
We estimated pooled effects only when there was meaningful clinical and methodological similarity; pooling was done by using a random‐effects (RE) model.
Subgroup analysis and investigation of heterogeneity
If data were available, we planned subgroup analyses to explore differences in treatment effects for participants with diabetes mellitus. We found that these patients had poorer treatment outcomes with glucocorticoids and could benefit from NSAIDs (Baumgarten 2007).
Outcomes that we will include in the subgroup analysis include resolution and the severity of trigger finger (e.g. Quinnell grading; Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire) and pain score obtained from a visual analogue scale (VAS).
Sensitivity analysis
When relevant, we assessed the robustness of the meta‐analyses for reviewer decisions in the following areas.
Resolution and recurrence outcomes: inclusion/exclusion of studies at high or unclear risk of selection bias.
Participant‐reported (subjective) outcomes: inclusion/exclusion of studies with inadequate or unclear participant blinding (this was not done, as participant blinding was adequate in all studies).
Method of imputing missing events in the ITT analysis of dichotomous outcomes for participants who were lost to follow‐up (we compared analyses for four ITT data imputation scenarios described in Table 2 and Table 3 for benefit and harm dichotomous outcomes, respectively).
1. ITT case scenarios for 'benefit' dichotomous outcomes (symptom resolution, participant satisfaction).
| ITT scenarios | Dropouts assumed to have the good outcome | |
| NSAID group | Steroid group | |
| Best case (for NSAID) | yes | no |
| Middle case 1 | no | no |
| Middle case 2 | yes | yes |
| Worst case (for NSAID) | no | yes |
ITT: intention‐to‐treat.
NSAID: non‐steroidal anti‐inflammatory drug.
2. ITT case scenarios for 'harm' dichotomous outcomes (moderate to severe symptoms, symptom recurrence, residual pain, adverse events).
| ITT scenarios | Dropouts assumed to have the bad outcome | |
| NSAID group | Steroid group | |
| Best case (for NSAID) | no | yes |
| Middle case 1 | no | no |
| Middle case 2 | yes | yes |
| Worst case (for NSAID) | yes | no |
ITT: intention‐to‐treat.
NSAID: non‐steroidal anti‐inflammatory drug.
Summary of findings and assessment of the certainty of the evidence
We created a 'Summary of findings' (SoF) table for the NSAID injection/glucocorticoid injection comparison using the following outcomes at the final time point.
Resolution of trigger finger.
Moderate to severe symptoms of trigger finger.
Recurrence of trigger finger.
Total active motion of the finger.
Residual pain.
Severity of pain.
Participant‐reported treatment success.
Adverse effects of treatment.
Two review authors (ML, QZ) independently assessed the quality of the evidence. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to studies that contributed data to the meta‐analyses for pre‐specified outcomes, and we reported the quality of evidence as high, moderate, low, or very low. We used GRADEpro software to prepare the SoF tables (GRADEpro GDT 2015). We justified all decisions to downgrade the quality of studies by using footnotes, and we made comments to aid the reader's understanding of the review when necessary. We were aware of distinguishing lack of evidence of effect from lack of effect (Schünemann 2017). We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review. We avoided making recommendations for practice, and our implications for research suggest priorities for future research and outline remaining uncertainties in this area.
Results
Description of studies
See the Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
The latest search results (up till September 2020) are shown in Figure 1. We conducted full‐text screening of 67 records (MEDLINE: 23, Embase: 33, CENTRAL: 5, CINAHL: 6). Overall, we included two studies (Leow 2018; Shakeel 2012), and we excluded 56 studies. We identified no ongoing studies and no studies awaiting classification. Of the excluded studies, we excluded 22 because they examined non‐trigger finger, 27 because they were not RCTs, and seven because they did not include an NSAID treatment arm. We found no records in CNKI nor in ProQuest Dissertations and Theses.
1.
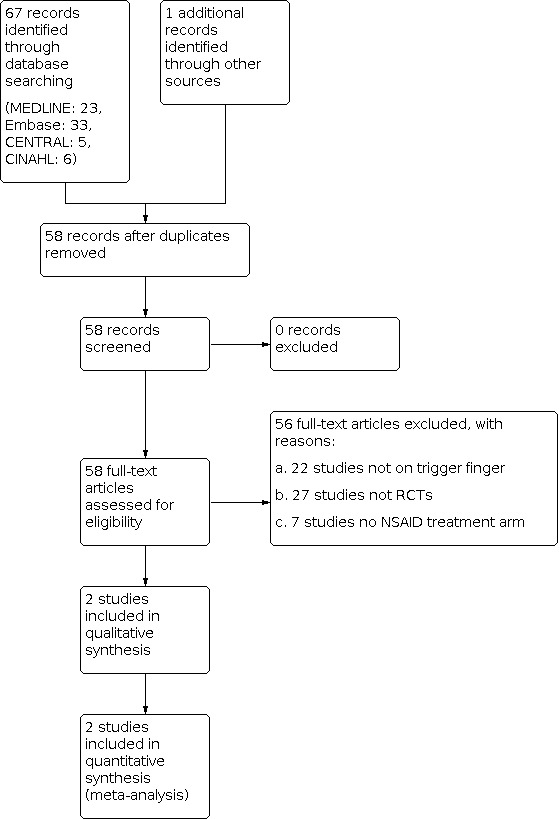
Study flow diagram.
Included studies
We included two RCTs (221 participants; one digit per participant) evaluating the efficacy and safety of an injectable NSAID versus an injectable glucocorticoid (Leow 2018; Shakeel 2012). They included 75 males and 146 females. The mean age of participants in Shakeel 2012 was 60 years, and the mean age of participants in Leow 2018 was 57.5 years.
We found no studies of oral or topical NSAIDs and no studies that compared NSAIDs by any route with placebo or another NSAID.
Participants
Both studies recruited adults without previous injections at the finger site. The study population in Shakeel 2012 had slightly more severe disease ‐ all were Quinell Grade 2 or higher (64% Grade 2, 29% Grade 3, and 7% Grade 4). Leow 2018 included a small proportion with Grade 1 (mild) disease (7.4%), 61.2% with Grade 2, 31.4% with Grade 3, and none with Grade 4 disease. Quinnell 1980 proposed a five‐point ordinal scale to grade the severity of trigger finger: Grade 0 – normal but possibly some mild crepitus during movement, Grade 1 – no triggering but uneven movement, Grade 2 – triggering that is actively correctable, Grade 3 – triggering that is passively correctable, Grade 4 – locked finger. Moderate to severe symptoms were defined as Quinell Grade 2 or higher; Grade 1 was classified as mild; and Grade 0 was resolved. Hand dominance was not recorded in either study.
Interventions
Both studies used single injections of a non‐selective NSAID at lower doses than are typically used in injections. Shakeel 2012 used diclofenac sodium at half the typically used dose (12.5 mg), and Leow 2018 used ketorolac trometamol at one‐quarter (15 mg) the typically used dose in combination with 5 mg 1% lidocaine. Both studies used single injections of triamcinolone acetonide as the control. Shakeel 2012 used 20 mg triamcinolone, and Leow 2018 used 5 mg in combination with 5 mg 1% lidocaine. Shakeel 2012 used an exclusively intrasynovial injection technique; Leow 2018 used combined intrasynovial and extrasynovial injections.
Outcomes
The outcomes measured in Shakeel 2012 were reported at three time points ‐ baseline (time of injection), 3 weeks, and 3 months. Those measured in Leow 2018 were reported at five time points ‐ baseline, 3 weeks, 6 weeks, 12 weeks, and 24 weeks.
Shakeel 2012 used Quinell grades (Table 2), and Leow 2018 used Green's version (Green 2005). Moderate to severe symptoms were defined as Quinell Grade 2 or higher; Grade 1 as mild; and Grade 0 as resolved disease. Both studies defined disease resolution as achieving a grade of zero as assessed by the investigator at the end of follow‐up.
Recurrence of trigger finger symptoms in participants who experienced temporary resolution was reported by Leow 2018; in Shakeel 2012, recurrence was reported but was not explicitly defined.
Pain was measured as a dichotomous outcome in Shakeel 2012 in Leow 2018, pain was measured on a 0 to 10 rating scale and was also reported as a dichotomous outcome (present/absent).
Total active motion and participant satisfaction were reported only by Leow 2018.
Both studies reported adverse events.
Excluded studies
We excluded 56 studies for the following reasons: 22 studies were not about trigger finger (Akdogan 2014; Al‐Homood 2013; Alvarez‐Nemegyei 2004; Andreu 2011; Coleman 2008; Farouk 2010; Gheita 2011; Gigante 2011; Giuliani 2015; Hardy 2013; Heffernan 2001; Lebiedz‐Odrobina 2010; Lintermans 2011; Londono 2012; Reading 2001; Sainsbury 2008; Sheon 1997; Silva 2012; Vega‐Morales 2014; Waimann 2011; Woo 2017; Wysocki 2012); 27 were not RCTs (Akhtar 2005; Bullocks 2009; Choudhury 2014; Crop 2011; Davidson 2011; Egido 2008; Harvard News Letter 2017; Lim 2007; Huisstede 2010; Jacobs 2009; Jacobs 2013; Kamath 2016; Kim 2011; Lim 2017; Ma 2019; Massoud 2018; Mutlu 2017; Nimigan 2006; Panayotopoulos 1992; Pargali 2011; Park 2009; Saldana 2001; Sayilir 2017; Schulman 2013; Soto Quijano 2005; Taras 2010; Wiwanitkit 2012); and seven did not include an NSAID intervention arm (Anderson 1991Chao 2009; Foster 2015; Ilyas 2019; Kosiyatrakul 2018Peters‐Veluthamaningal 2009; Weinheimer 2019).
Risk of bias in included studies
The risk of bias (RoB) assessment is summarised in Figure 2 and in the Characteristics of included studies tables.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We judged Leow 2018 as having low RoB, as the sequence generation was computerised and baseline characteristics were reported and judged as balanced.
Shakeel 2012 used a physical method (drawing marked slips from a container) to randomise treatment. However these researchers did not assess the success of the procedure in balancing baseline characteristics, nor did they report a table of baseline characteristics, so we judged the RoB as unclear.
Allocation concealment
Leow 2018 implemented the allocation through opaque, sequentially numbered envelopes. These envelopes were opened by a research assistant (who was not involved in the treatment or outcome assessment process) after the participant had consented, so we judged the RoB to be low.
In Shakeel 2012, the treatment slips in the container were openly marked with the treatment names "NSAID" or "STEROID", instead of a more secure numerical code (decipherable only by the trial pharmacist). So it would have been possible for a study team member to pre‐draw the slips and create an unblinded random treatment allocation schedule. Even if the slips were drawn in real time and were not pre‐drawn, awareness of the assigned treatment could have influenced the behaviour of the recruiter. As a result, we judged the RoB to be high.
Blinding
Performance bias
Blinding of participants
In both studies, participants were blinded.
Blinding of study personnel
In both studies, study personnel were not blinded.
For both studies, we judged the risk of performance bias as unclear.
Detection bias
Investigator‐reported outcomes
In both studies, these outcomes were measured by blinded assessors, and we judged RoB as low.
Participant‐reported outcomes
In both studies, participants were blinded, and we judged RoB as low.
Incomplete outcome data
In Leow 2018, the attrition rate at 12 weeks (3 months), which is the measurement time point most similar to the latest time point in Shakeel 2012, was 23.7% (NSAID) and 22.6% (glucocorticoid); and at 24 weeks, the attrition rate was 20.3% (NSAID) and 16.1% (glucocorticoid). No reasons were given (except for three participants in the NSAID group who opted for surgery). Study authors performed a per‐protocol analysis that excluded the study dropouts (per personal communication with study author).
In Shakeel 2012, the attrition rate at 3 months was 9.1% in both groups. A per‐protocol analysis (excluding the 10 who did not complete the 3‐month follow‐up) was done.
In both studies, all attrition rates are comparable in magnitude to the outcome event rates, thus making the treatment effects sensitive to assumptions about the outcome status of those who dropped out. We therefore judged RoB as high.
Selective reporting
In both studies, the major outcomes of resolution and moderate to severe symptoms could be determined for all time points mentioned in the Methods section. Even though the results were not reported as point estimates with confidence intervals, we obtained sufficient detail to estimate treatment effects and 95% CIs for all review outcomes measured at the latest follow‐up times. We judged RoB as low.
Other potential sources of bias
Funding for Leow 2018 was received from non‐commercial sources, and the study was judged at low RoB, Shakeel 2012 did not mention study funders, and we judged RoB for this study as unclear.
Effects of interventions
See: Table 1
All review outcomes were reported in at least one study. Outcomes were measured at multiple time points ‐ 3 weeks and 3 months for Shakeel 2012, and 3, 6, 12, and 24 weeks for Leow 2018. Except for Quinnell grades for disease severity, group results for the other outcomes were not reported in full. All investigator‐reported analyses were based on those who completed follow‐up. In both studies, no treatment effect estimates or 95% CIs were reported ‐ only P values. However reported data were sufficient for us to calculate some effect estimates and CIs for the latest follow‐up time point of 24 weeks for Leow 2018 and 3 months (taken as 12 weeks) for Shakeel 2012. We expressed these in a standardised statement, which incorporates a judgement about the clinical importance, direction, and certainty of evidence of the effect (see Data synthesis). We performed sensitivity analyses for various reasons; these are described under the relevant outcome headings.
The only comparison reported was NSAID injection versus glucocorticoid injection (see Table 1).
NSAID injection versus glucocorticoid injection
Major outcomes
Resolution of trigger finger symptoms
Shakeel 2012 measured the severity of trigger finger symptoms using Quinnell grading. However, Leow 2018 used a modified Quinell grading system, which distinguishes between required passive extension (Grade 3a) and inability to actively flex (Grade 3b) for Grade 3 trigger finger, and does not include Grade 4 for a "locked" finger. At baseline, 92.6% (112/121) of participants in Leow 2018 had severe disease (Quinnell Grade 2 or higher) compared to 100% in Shakeel 2012.
Our analysis pooled outcomes measured at 24 weeks in Leow 2018 with those measured at 12 weeks in Shakeel 2012 (Analysis 1.1). For a sensitivity analysis, we pooled only 12‐week outcomes from both studies. The RR of symptom resolution at 12 to 24 weeks was 0.83 in favour of glucocorticoid injection (95% CI 0.62 to 1.11 times higher), and the absolute effect was 7% lower in favour of glucocorticoid injection (95% CI 16% lower to 5% higher); the risk difference was 17% lower (95% CI 38% lower to 11% higher) (Analysis 1.1; Table 1). For adults with trigger finger, NSAID injection may make little to no difference in the chance of symptom resolution at 12 to 24 weeks compared to glucocorticoid injection (2 studies, 231 participants; low‐certainty evidence, downgraded for bias and imprecision).
1.1. Analysis.

Comparison 1: NSAID injection vs steroid injection, Outcome 1: Resolution at 12 to 24 weeks
Persistent moderate to severe trigger finger symptoms
This outcome measures the failure of treatment to reduce symptom severity from what was initially a moderate or severe condition to a mild or resolved condition (i.e. persistently moderate or severe symptoms at the end of follow‐up) (Quinnell Grade 2 or higher).
For adults with trigger finger, NSAID injection may increase the chance of persistent moderate to severe symptoms at 12 to 24 weeks compared to glucocorticoid injection (2 studies, 231 participants). The RR of persistent moderate to severe symptoms at 12 to 24 weeks was 2.03 in favour of glucocorticoid injection (95% CI 1.19 to 3.46 times higher) (Analysis 1.2; Table 1). The absolute risk is 14% higher in favour of glucocorticoid injection (95% CI 2% to 33% higher) and the risk difference is 103% higher in favour of glucocorticoid injection (95% CI 19% to 246% higher) (NNTH 7, 95% CI 3 to 38; low‐certainty evidence, downgraded for bias and imprecision).
1.2. Analysis.

Comparison 1: NSAID injection vs steroid injection, Outcome 2: Moderate to severe disease at 12 to 24 weeks
Recurrent trigger finger symptoms
Leow 2018 reported recurrence in the glucocorticoid injection group as a percentage only; exact numbers of events and definitions were obtained by personal communication. Our default analysis counted all randomised participants in the denominator for risk calculations. We also conducted a sensitivity analysis using only participants at risk of recurrence in the denominator of the risk calculations (i.e. those participants who had experienced initial resolution of symptoms during follow‐up).
For adults with trigger finger, it is very uncertain whether NSAID injection decreases the chance of symptom recurrence at 12 to 24 weeks compared to glucocorticoid injection (2 studies, 231 participants). The RR of symptom recurrence at 12 to 24 weeks was 0.07 in favour of NSAID (95% CI 0.01 to 0.38 times higher) (Analysis 1.3Table 1) (NNTB 6, 95% CI 5 to 8; very low‐certainty evidence, downgraded by two levels for bias and by one level for imprecision).
1.3. Analysis.

Comparison 1: NSAID injection vs steroid injection, Outcome 3: Recurrence at 12 to 24 weeks
Total active motion
Only Leow 2018 reported total active range of motion. This is an objective measure of unaided mobility of the three finger joints. A per‐protocol analysis of this continuous outcome is reported.
For adults with trigger finger, NSAID injection may make little to no difference in mean total active motion at 24 weeks compared to glucocorticoid injection. Mean total active range of motion for each group was 235 degrees (NSAID) and 240 degrees (glucocorticoid). The mean difference in total active range of motion at 24 weeks was 5 degrees less in favour of glucocorticoid injection (95% CI 35.54 less to 24.54 more), absolute difference was 5% lower (95% CI 34.54% lower to 24.54% higher), and risk difference was 2.1% lower (95% CI 14.8% lower to 10.2% higher) (Analysis 1.4; Table 1) (1 study, 99 participants; low‐certainty evidence, downgraded for bias and imprecision).
1.4. Analysis.

Comparison 1: NSAID injection vs steroid injection, Outcome 4: Total active motion
Residual pain
Leow 2018 measured residual pain on a 0 to 10 scale, with 0 signifying "no pain". This original analysis was based on comparison of median pain scores. To enable pooling with pain data from Shakeel 2012, a dichotomous outcome was defined (pain/no pain). Compared to Leow 2018, Shakeel 2012 reported considerably fewer residual pain events described as continuous pain at the injection site and classified as a "complication" (adverse event), but they opine that this pain could be unresolved pain from the trigger finger rather than a genuine adverse event. Our default analysis assumes this is residual pain from the presenting disease. But we also performed a sensitivity analysis in which this was not counted and in which suspected under‐reporting of pain was adjusted for by assuming all participants with moderate disease at the end of follow‐up had residual pain (Shakeel 2012).
For adults with trigger finger, NSAID injection may make little to no difference in the chance of residual pain at 12 to 24 weeks compared to glucocorticoid injection. The RR of residual pain at 12 to 24 weeks was 0.84 in favour of NSAID (95% CI 0.54 to 1.31 times higher), absolute difference was 4% lower (95% CI 11% lower to 7% higher), and risk difference was 16% lower (95% CI 46% lower to 31% higher) (2 studies, 231 participants; low‐certainty evidence, downgraded for bias and imprecision) (Analysis 1.5; Table 1).
1.5. Analysis.

Comparison 1: NSAID injection vs steroid injection, Outcome 5: Pain
Participant‐reported treatment success
Only Leow 2018 reported participant satisfaction with treatment.
For adults with trigger finger, NSAID injection may make little to no difference in the chance of participant satisfaction at 24 weeks compared to glucocorticoid injection. The RR of participant‐reported treatment success at 24 weeks was 0.95 in favour of glucocorticoid injection (95% CI 0.74 to 1.23 times higher), absolute difference was 4% lower (95% CI 18% lower to 15% higher), and risk difference was 5% lower (95% CI 26% lower to 23% higher) (1 study, 121 participants; low‐certainty evidence, downgraded for bias and imprecision) (Analysis 1.6; Table 1).
1.6. Analysis.

Comparison 1: NSAID injection vs steroid injection, Outcome 6: Satisfaction
Adverse events
Leow 2018 reported no adverse events in either group. Shakeel 2012 reported "complications", but of the eight reported in the NSAID group, we did not consider six of them to be adverse effects of treatment (five nodular swellings and stiffness at the injection site, one recurrence of triggering). We regarded these as residual symptoms of unresolved disease. Therefore we counted only two adverse events in the NSAID group (both were continuous mild dull aches at the injection site). In the glucocorticoid injection group, ten "complications" were reported, one of which was continuous pain at the injection site and nine recurrences of triggering; we excluded recurrence events and counted only one adverse event in the glucocorticoid injection group. This would be consistent with a systematic review that reported pain at the injection site as a possible adverse reaction following glucocorticoid injection (Brinks 2010).
For adults with trigger finger, It is uncertain if NSAID injection results in fewer adverse events at 12 to 24 weeks compared to glucocorticoid injection. The pooled RR of adverse events at 12 to 24 weeks was not estimable due to zero events in both treatment groups in the Leow 2018 study (Analysis 1.7; Table 1). The estimate from just Shakeel 2012 was RR 2.00 in favour of glucocorticoid injection (95% CI 0.19 to 21.42 times higher) (2 studies, 231 participants; very low‐certainty evidence, downgraded by one level for bias and by two levels for imprecision).
1.7. Analysis.

Comparison 1: NSAID injection vs steroid injection, Outcome 7: Adverse events
Sensitivity analysis
Resolution of trigger finger symptoms
Excluding Shakeel 2012 because of high risk of selection bias leaves just Leow 2018 from which to estimate the effect of treatment on symptom resolution, for an RR of 0.97 compared to the pooled estimate of 0.83, both favouring glucocorticoid injection (Analysis 1.1). Comparison of various ITT scenarios was based on different assumptions about outcome events among participants who were lost to follow‐up (Analysis 2.1). Leow 2018 reported 12 and 10 dropouts in NSAID and glucocorticoid groups, respectively; Shakeel 2012 reported 5 dropouts in each group. Scenario RRs ranged from 0.67 (favouring glucocorticoid) to 1.30 (favouring NSAID). The two middle scenarios had RRs of 0.83 and 0.90 (favouring glucocorticoid injection). Sensitivity to missing data assumptions supports a high risk of attrition bias judgement (Figure 2). Comparing pooling of the 12‐week outcome in Shakeel 2012 with 12‐ or 24‐week outcomes in Leow 2018 revealed favouring of glucocorticoid injection, with a pooled estimate of 0.32 (0.03, 3.03) at 12‐week and 0.83 (0.62, 1.11) at 24‐week follow‐up (Analysis 3.1).
Persistent moderate to severe trigger finger symptoms
Excluding Shakeel 2012 because of high risk of selection bias leaves just Leow 2018 from which to estimate the effects of treatment on persistent symptoms, for an RR of 1.84 compared to the pooled estimate of 2.03, both favouring glucocorticoid injection (Analysis 1.2). When various ITT scenarios were compared (Analysis 2.2), scenario RRs ranged from 1.06 to 3.11 (favouring glucocorticoid injection). The two middle scenarios had RRs of 1.62 and 2.03 (favouring glucocorticoid injection). When pooling of the 12‐week outcome in Shakeel 2012 was compared with pooling of 12‐ or 24‐week outcomes in Leow 2018 (Analysis 3.2), RRs ranged from 2.03 to 3.20 (favouring glucocorticoid injection).
Recurrent trigger finger symptoms
Excluding Shakeel 2012 because of high risk of selection bias leaves just Leow 2018 from which to estimate the effect of treatment on recurrence, for an RR of 0.03 compared to the pooled estimate of 0.07, both favouring NSAID (Analysis 1.3). When various ITT scenarios were compared (Analysis 2.3), scenario RRs ranged from 0.05 to 0.78 (favouring NSAID). The two middle scenarios had RRs of 0.07 and 0.48 (favouring NSAID). When pooling only of participants at risk of symptom recurrence in the denominator of the risk calculation was compared with pooling of all randomised participants (our default analysis) (Analysis 4.1), pooled RRs ranged from 0.07 to 0.13 (favouring NSAID). The larger effect in favour of NSAID seen in the "at risk" analysis is highly imprecise (95% CI ‐0.70 to 0.01), with high heterogeneity (I² = 88%).
Residual pain
When various ITT scenarios were compared (Analysis 2.4), scenario RRs ranged from 0.58 (favouring NSAID) to 2.26 (favouring glucocorticoid injection). The two middle scenarios had RRs of 0.84 and 0.95 (favouring NSAID). When three pain outcome scenarios ‐ including or excluding persistent pain as a bona fide pain outcome and assuming the presence of residual pain among Shakeel 2012 study participants who had Grade 2 severity at the end of follow‐up ‐ were compared (Analysis 5.1), RRs ranged from 0.82 (favouring NSAID) to 1.36 (favouring glucocorticoid).
Participant‐reported treatment success
When various ITT scenarios were compared (Analysis 2.5), scenario RRs ranged from 0.77 (favouring glucocorticoid injection) to 1.25 (favouring NSAID). The two middle scenarios had RRs of 0.95 (favouring glucocorticoid) and 1.01(favouring NSAID).
Adverse events
When various ITT scenarios were compared (Analysis 2.6), pooled scenario RRs ranged from 0.12 (favouring NSAID) to 11.13 (favouring glucocorticoid). The two middle scenarios had RRs of 0.77 (favouring NSAID) and 2.00 (favouring glucocorticoid). Shakeel 2012 opined that the three "continuous pain at injection site" events could be persistent symptoms of the disease rather than true adverse events. A subsequent sensitivity analysis included and excluded these events as adverse events (Analysis 6.1).
Discussion
For this review, we found two studies with 221 participants (aged 40 to 87 years) that compared the effectiveness of non‐steroidal anti‐inflammatory drug (NSAID) and glucocorticoid injections for adults with trigger finger (Leow 2018; Shakeel 2012). Both studies used non‐selective NSAIDs. No studies examined oral, topical, or cyclo‐oxygenase (COX)‐2‐selective NSAIDs or non‐injectable glucocorticoids or placebo.
Summary of main results
Our comparative analyses treated the NSAID injection as the experimental intervention and glucocorticoid as the comparator. Evidence is presented from the perspective of our working hypothesis that injectable NSAIDs might be clinically superior to injectable glucocorticoids.
Evidence on outcomes ranged from low to very low certainty. We found low‐certainty evidence for resolution, persistent moderate to severe symptoms, pain, total active motion, and treatment success reported by participants, and very low‐certainty evidence for recurrence and adverse events.
We considered resolution of symptoms by the end of follow‐up as the most stringent criterion of treatment success, by which absence of symptoms can be considered successful treatment. In this review, low‐certainty evidence suggests that a single NSAID injection may have little to no effect on resolution of symptoms compared with glucocorticoid injection at 12 to 24 weeks. Due to risk of selection and attrition bias and imprecision, we determined the certainty of evidence to be low.
Low‐certainty evidence suggests that a single NSAID injection may result in more people with persistent moderate to severe symptoms compared to a glucocorticoid injection. Data show that NSAID injection may even increase the chance of unresolved symptoms at 12 to 24 weeks. Due to risk of selection and attrition bias and imprecision, we determined the certainty of evidence to be low.
It is uncertain if NSAID injection results in fewer people with recurrence of symptoms due to very low‐certainty evidence; we do not know if there are differences in recurrence rates between groups. The evidence is of very low certainty, downgraded twice for bias and once for imprecision.
Low‐certainty evidence suggests that a single NSAID injection may have little to no effect on total active motion compared with a glucocorticoid injection. The difference of 5 degrees in favour of glucocorticoid is small and is distributed over the range of motion of three joints; we judged this difference to be clinically not significant. Due to risk of selection and attrition bias and imprecision, we determined that the certainty of evidence is low.
Low‐certainty evidence suggests that a single NSAID injection may have little to no effect on residual pain. Concern about the accuracy of reporting of pain in Shakeel 2012 was caused by uncertainty as to whether pain was a presenting symptom or an adverse effect caused by the intervention, or whether pain was under‐reported by participants. Our sensitivity analyses indicate that the default analysis is robust. Due to risk of selection and attrition bias and imprecision, we determined that the certainty of evidence is low.
Low‐certainty evidence suggests that a single NSAID injection may have little to no effect on treatment success. The risk difference of 5% in favour of glucocorticoid is small, and we judged it to be clinically not significant. Due to risk of selection and attrition bias and imprecision, we determined that the certainty of evidence is low.
All reported adverse events were local and were related to the injection procedure. Evidence is of very low certainty, downgraded once for bias and twice for imprecision, and It is uncertain if NSAID injection results in fewer adverse events.
Overall completeness and applicability of evidence
Overall, we found very limited to no randomised controlled trial (RCT) evidence that addresses the objectives of this review. We identified only two eligible studies for the current review (221 participants randomised; 199 study completers) that used lower than normal concentrations of two different non‐selective injectable NSAIDs in comparison with two different doses of triamcinolone glucocorticoid injection (5 and 20 mg). In one study, 5 mg of 1% lidocaine was used as a co‐intervention in both treatment groups. The longest follow‐up times were limited to between 12 and 24 weeks; two follow‐up time points were common to both studies ‐ 3 weeks and 12 weeks. We found small numbers of adverse events reported in the two included studies.
Doses of glucocorticoid (triamcinolone acetonide) given in the two studies were different (Leow 2018; Shakeel 2012). Shakeel 2012 used 20 mg triamcinolone acetonide, and Leow 2018 used 10 mg triamcinolone acetonide. This could have resulted in the difference in treatment efficacy in the glucocorticoid groups. Also, the drug was given neat in Shakeel 2012, but anaesthetic drug (parenteral lidocaine 1%, 10 mg/mL) was added to the glucocorticoid or NSAID in Leow 2018. No study has been conducted to evaluate the efficacy of the drug given neat versus the drug given in combination. However, the literature has recommended mixing anaesthetic drug with glucocorticoid for trigger finger injection (Kale 2017). The type of NSAID used was different in the two studies. Shakeel 2012 used diclofenac sodium, and Leow 2018 used ketorolac trometamol. A previous systematic review on the use of NSAID for osteoarthritis found that dose and type of NSAID used could affect treatment efficacy (da Costa 2017), and a review on low back pain found no differences in efficacy between various NSAIDs (Roelofs PDDM 2008). Thus, it is not conclusively known whether the efficacy of NSAIDs for trigger finger depends on the type of NSAID used.
We found no RCT evidence on the possible effectiveness of a wider range of NSAIDs including COX‐2‐selective ones, administered by oral and topical routes, at higher doses, in combination with anaesthetic drugs (da Costa 2017; Kale 2017; Roelofs PDDM 2008), and we found no evidence of comparisons against a wider range of glucocorticoid, non‐injection routes of delivery or against placebo. Applicability to patients with diabetes is very uncertain. Although about 39% of participants in Shakeel 2012 were diabetic, the reported analysis was not relevant, as it showed how intervention modified the effect of diabetes on symptom severity (see Table 4 of Shakeel 2012), instead of how diabetes modified the effect of intervention on symptom severity.
We suggest that a non‐inferiority analysis perspective may be more relevant when NSAIDs are compared with glucocorticoid treatment, as it is conceivable that the patient and the clinician would be willing to accept some NSAID treatment inferiority in exchange for the benefit of reduced risk of glucocorticoid adverse reactions. If so, this requires an a priori specification of a "non‐inferiority margin", which represents the maximum NSAID treatment inferiority that would be acceptable. However, logic dictates that the non‐inferiority margin should not exceed the inferiority of placebo as assessed in placebo‐controlled trials of glucocorticoid.
An even more crucial consideration is that analysis of just recurring symptoms may not provide a clinically meaningful measure of treatment effectiveness, as it is only one of two possible treatment failure events. The other event that is ignored is failure due to sustained symptom non‐responsiveness (without transient symptom resolution), and this happens to be the dominant failure mode in these two studies (Table 4). Ignoring this other failure event can lead to misleading conclusions about treatment efficacy, as is the case here. A more sensible analysis would combine both types of treatment failure. A sensitivity analysis shows that the treatment effect estimated from only recurrence failures is markedly different in magnitude and direction from that estimated from both types of failure (34% lower risk of failure versus 5% higher risk of failure; Analysis 7.1) and therefore is potentially seriously misleading.
3. Frequency of recurrence and sustained treatment failure events.
| Study | Intervention group | Recurrence failure events | Sustained failure events |
| Leow 2018 | NSAID | 0 | 47 |
| Steroid | 15 | 34 | |
| Total failure | 15 | 81 | |
| Shakeel 2012 | NSAID | 1 | 26 |
| Steroid | 9 | 11 | |
| Total failure | 10 | 37 |
NSAID: non‐steroidal anti‐inflammatory drug.
7.1. Analysis.

Comparison 7: Sensitivity analysis (treatment failure), Outcome 1: Types of treatment failure (unresolved symptoms)
Quality of the evidence
We assessed the quality of evidence for each outcome at the individual study level (risk of bias) and at the pooled data level (certainty of evidence).
Of the eight risk of bias (RoB) domains, Leow 2018 showed high risk of bias due to incompleteness of outcome data and unclear risk of bias for performance bias regarding blinding of participants and study personnel; the remaining six domains were at low risk of bias. Shakeel 2012 showed high risk of bias for allocation concealment and incompleteness of outcome data and unclear risk of bias for random sequence generation, blinding of participants and study personnel (performance bias), and other biases (non‐reporting of funding); the remaining three domains were at low risk of bias.
We found low‐certainty evidence for five (resolution, moderate to severe symptoms, total active motion, residual pain, and participant satisfaction) of the seven outcomes. We downgraded the evidence for risk of bias and imprecision. We found very low‐certainty evidence for symptom recurrence, as the risk of bias was judged very serious and the estimate imprecise. We found very low‐certainty evidence for adverse events, downgraded twice for imprecision, as data were derived from only one study and very few events were reported.
We are unable to comment on the clinical significance of a difference of 5 degrees lost in total active motion. This may be due to slight variability during measurements, and participants may not have any deficits in function.
Potential biases in the review process
Two authors of this review are the authors of one of the studies included in this review (Leow 2018). We resolved this potential bias by having Renea Johnston from the Cochrane Musculoskeletal Group and independent methodologist ECSY assess the risk of bias, and by involving two methodologists (ECSY, QZ) in all critical steps (Contributions of authors).
Agreements and disagreements with other studies or reviews
No other published review has considered NSAIDs for trigger finger. No prospective studies have examined the efficacy of topical or oral NSAIDs and glucocorticoids. We summarise the findings of a Cochrane Review synthesising the evidence for glucocorticoid injection for trigger finger (Peters‐Veluthamaningal 2009). These review authors found only two pseudo‐randomised studies (63 participants), both of which reported the dichotomous outcome "treatment success" (resolved, asymptomatic, or sufficiently improved to justify no further treatment), with maximum follow‐up of 1 month and 4 months. Corticosteroid injection with lidocaine was more effective than lidocaine alone for treatment success at 4 weeks (risk ratio 3.15, 95% confidence interval (CI) 1.34 to 7.40). The number needed to treat for an additional beneficial outcome was 3. No adverse events or side effects were reported. The findings from this review cannot be compared to the results of our review due to differences in interventions. We do not have another published review on NSAIDs for trigger finger against which to compare the findings of the present review.
Authors' conclusions
Implications for practice.
Low‐ to very low‐certainty evidence suggests that NSAID injection may offer little to no benefit over glucocorticoid injection for adults with trigger finger at 24 weeks' follow‐up. A single NSAID injection may have little to no effect on the number of people with symptom resolution, the number of people with residual pain, the number reporting treatment success, and the mean total active motion. NSAID injection may result in more people with moderate to severe symptoms. It is uncertain if NSAID injection results in fewer people with recurrence of symptoms, or fewer adverse events, due to the small number of events reported. There may be patient‐specific reasons for avoiding glucocorticoid injections, such as concerns about blood sugar control and anxieties about steroid‐associated adverse effects, for which an injectable NSAID may be considered; however the certainty of evidence is low.
Implications for research.
Although topical, oral, and injectable NSAIDs have been used in clinical practice for trigger finger, no studies have evaluated their efficacy against placebo.
Given that the choice of using an NSAID rather than a glucocorticoid injection is likely to be motivated by the reduced risk of adverse effects, and because it is unlikely that NSAIDs are more potent ant‐inflammatory agents than steroids, it would not be unreasonable for future RCTs to adopt a non‐inferiority rather than a superiority hypothesis perspective. Methods used by clinicians and patients to elicit the appropriate non‐inferiority margin will be crucial and should be clearly described.
History
Protocol first published: Issue 9, 2017 Review first published: Issue 4, 2021
Acknowledgements
The methods section is based on the standard Cochrane Musculoskeletal Group Protocol template. The review authors would like to thank Ms Anne Lyddiatt (consumer reviewer) and Dr. Teemu V Karjalainen, Monash Department of Clinical Epidemiology, Cabrini Institute and Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia for peer reviewing this review.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
1. exp Trigger Finger Disorder/
2. (trigger adj (finger$ or thumb$ or digit$)).tw.
3. (snapping adj (finger$ or thumb$ or digit$)). tw.
4. (locking adj (finger$ or thumb$ or digit$)).tw.
5. or/1‐4
6. exp Anti‐Inflammatory Agents, Non‐Steroidal/
7. nsaid$.tw.
8. nonsteroidal anti‐inflammator$.tw.
9. non‐steroidal anti‐inflammator$.tw.
10. exp diclofenac/ or (diclofenac$ or fenoprofen$ or flurbiprofen$).tw.
11. exp indometacin/ or indometacin$.tw.
12. exp ketoprofen/ or (ketoprofen$ or ketorolac$ or piroxicam$ or bromfenac$ or oxyphenbutazone$ or suprofen$).tw.
13. exp ibuprofen/ or ibuprofen$.tw.
14. exp naproxen/ or naproxen$.tw.
15. exp celecoxib/ or (celecoxib$ or etoricoxib$).tw.
16. or/6‐15
17. 5 and 16
18. randomized controlled trial.pt.
19. controlled clinical trial.pt.
20. randomized.ab.
21. placebo.ab.
22. drug therapy.fs.
23. randomly.ab.
24. trial.ab.
25. groups.ab.
26. or/18‐25
27. exp animals/ not humans.sh.
28. 26 not 27
29. 17 and 28
Appendix 2. Unpublished data (pain score)
Pain score between NSAID and steroid group patients
| Pain score | Median (IQR) | P value | |
| NSAID | Steroid | ||
| Baseline | 5 (2 to 7) | 5 (3.2 to 7) | 0.5111 |
| 3 weeks | 4 (2 to 5) | 0 (0 to 1) | < 0.0001 |
| 6 weeks | 3 (0.8 to 4.2) | 0 (0 to 0) | < 0.0001 |
| 12 weeks | 2 (0 to 4) | 0 (0 to 0) | < 0.0001 |
| 24 weeks | 0 (0 to 2) | 1 (0 to 3) | 0.3724 |
IQR: interquartile ratio.
NSAID: non‐steroidal anti‐inflammatory drug.
Appendix 3. Unpublished data (total active motion)
Total active motion (TAM) between NSAID and steroid group patients
| TAM | Median (IQR) | P value | |
| NSAID | Steroid | ||
| Baseline | 220 (160 to 250) | 205 (127.5 to 237.5) | 0.1961 |
| 3 weeks | 215 (140 to 250) | 235 (160 to 260) | 0.2109 |
| 6 weeks | 230 (152.5 to 247.5) | 247.5 (157.5 to 265) | 0.1203 |
| 12 weeks | 225 (180 to 260) | 240 (182.5 to 260) | 0.5550 |
| 24 weeks | 235 (165 to 260) | 240 (148.8 to 256.2) | 0.9078 |
IQR: interquartile ratio.
NSAID: non‐steroidal anti‐inflammatory drug.
Appendix 4. CINAHL (EBSCOhost) search strategy
1. TX ( trigger N9 (finger# or thumb# or digit#) ) OR TX ( snapping N9 (finger# or thumb# or digit#) ) OR TX ( locking N9 (finger# or thumb# or digit#) )
2. TX nsaid# OR TX nonsteroidal anti‐inflammator# OR TX non‐steroidal anti‐inflammator# OR TX ( diclofenac# or fenoprofen# or flurbiprofen# or indometacin# or ketoprofen# or ketorolac# or piroxicam# or bromfenac# or oxyphenbutazone# or suprofen# or ibuprofen# or naproxen# or celecoxib# or etoricoxib# )
3. S1 AND S2
Appendix 5. Embase (Ovid) search strategy
1. exp Trigger Finger Disorder/
2. (trigger adj (finger$ or thumb$ or digit$)).tw.
3. (snapping adj (finger$ or thumb$ or digit$)). tw.
4. (locking adj (finger$ or thumb$ or digit$)).tw.
5. or/1‐4
6. exp Anti‐Inflammatory Agents, Non‐Steroidal/
7. nsaid$.tw.
8. nonsteroidal anti‐inflammator$.tw.
9. non‐steroidal anti‐inflammator$.tw.
10. exp diclofenac/ or (diclofenac$ or fenoprofen$ or flurbiprofen$).tw.
11. exp indometacin/ or indometacin$.tw.
12. exp ketoprofen/ or (ketoprofen$ or ketorolac$ or piroxicam$ or bromfenac$ or oxyphenbutazone$ or suprofen$).tw.
13. exp ibuprofen/ or ibuprofen$.tw.
14. exp naproxen/ or naproxen$.tw.
15. exp celecoxib/ or (celecoxib$ or etoricoxib$).tw.
16. or/6‐15
17. 5 and 16
18. crossover‐procedure/ or double‐blind procedure/ or randomized controlled trial/ or single‐blind procedure/ or (random* or factorial* or crossover* or cross over* or placebo* or (doubl* adj blind*) or (singl* adj blind*) or assign* or allocat* or volunteer*).tw.
19. 17 and 18
Appendix 6. CENTRAL search strategy
1. [mh "Trigger Finger Disorder"]
2. trigger near (finger* or thumb* or digit*)
3. snapping near (finger* or thumb* or digit*)
4. locking near (finger* or thumb* or digit*)
5. #1 or #2 or #3 or #4
6. [mh "Anti‐Inflammatory Agents, Non‐Steroidal"]
7. nsaid*
8. nonsteroidal anti‐inflammator*
9. non‐steroidal anti‐inflammator*
10. [mh diclofenac] or diclofenac* or fenoprofen* or flurbiprofen*
11. [mh indometacin] or indometacin*
12. [mh ketoprofen] or ketoprofen* or ketorolac* or piroxicam* or bromfenac* or oxyphenbutazone* or suprofen*
13. [mh ibuprofen] or ibuprofen*
14. [mh naproxen] or naproxen*
15. [mh celecoxib] or celecoxib* or etoricoxib*
16. #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15
17. #5 and #16
Data and analyses
Comparison 1. NSAID injection vs steroid injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Resolution at 12 to 24 weeks | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.62, 1.11] |
| 1.2 Moderate to severe disease at 12 to 24 weeks | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.19, 3.46] |
| 1.3 Recurrence at 12 to 24 weeks | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.01, 0.38] |
| 1.4 Total active motion | 1 | 99 | Mean Difference (IV, Random, 95% CI) | ‐5.00 [‐34.54, 24.54] |
| 1.5 Pain | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.54, 1.31] |
| 1.6 Satisfaction | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.74, 1.23] |
| 1.7 Adverse events | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.19, 21.42] |
Comparison 2. Sensitivity analysis (ITT scenarios).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Resolution at 12 to 24 weeks (ITT scenarios) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1.1 Best case | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.62, 2.73] |
| 2.1.2 Middle case 1 | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.62, 1.11] |
| 2.1.3 Middle case 2 | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.69, 1.18] |
| 2.1.4 Worst case | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.51, 0.87] |
| 2.1.5 Per‐protocol | 2 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.10] |
| 2.2 Moderate to severe disease at 12 to 24 weeks (ITT scenarios) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.2.1 Best case | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.70, 1.60] |
| 2.2.2 Middle case 1 | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.19, 3.46] |
| 2.2.3 Middle case 2 | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [1.14, 2.31] |
| 2.2.4 Worst case | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 3.11 [1.91, 5.07] |
| 2.2.5 Per‐protocol | 2 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [1.25, 3.52] |
| 2.3 Recurrence at 12 to 24 weeks (ITT scenarios) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.3.1 Best case | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.24] |
| 2.3.2 Middle case 1 | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.01, 0.38] |
| 2.3.3 Middle case 2 | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.29, 0.78] |
| 2.3.4 Worst case | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.45, 1.35] |
| 2.3.5 Per‐protocol | 2 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.01, 0.39] |
| 2.4 Pain (ITT scenarios) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.4.1 Best case | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.39, 0.85] |
| 2.4.2 Middle case 1 | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.54, 1.31] |
| 2.4.3 Middle case 2 | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.71, 1.28] |
| 2.4.4 Worst case | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [0.43, 11.86] |
| 2.4.5 Per‐protocol | 2 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.59, 1.32] |
| 2.5 Satisfaction (ITT scenarios) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.5.1 Best case | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.02, 1.53] |
| 2.5.2 Middle case 1 | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.74, 1.23] |
| 2.5.3 Middle case 2 | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.87, 1.18] |
| 2.5.4 Worst case | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.62, 0.96] |
| 2.5.5 Per‐protocol | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.21] |
| 2.6 Adverse events (ITT scenarios) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.6.1 Best case | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 1.90] |
| 2.6.2 Middle case 1 | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.19, 21.42] |
| 2.6.3 Middle case 2 | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.45, 1.32] |
| 2.6.4 Worst case | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 11.13 [2.11, 58.60] |
| 2.6.5 Per‐protocol | 2 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.19, 21.36] |
2.1. Analysis.

Comparison 2: Sensitivity analysis (ITT scenarios), Outcome 1: Resolution at 12 to 24 weeks (ITT scenarios)
2.2. Analysis.

Comparison 2: Sensitivity analysis (ITT scenarios), Outcome 2: Moderate to severe disease at 12 to 24 weeks (ITT scenarios)
2.3. Analysis.

Comparison 2: Sensitivity analysis (ITT scenarios), Outcome 3: Recurrence at 12 to 24 weeks (ITT scenarios)
2.4. Analysis.

Comparison 2: Sensitivity analysis (ITT scenarios), Outcome 4: Pain (ITT scenarios)
2.5. Analysis.

Comparison 2: Sensitivity analysis (ITT scenarios), Outcome 5: Satisfaction (ITT scenarios)
2.6. Analysis.

Comparison 2: Sensitivity analysis (ITT scenarios), Outcome 6: Adverse events (ITT scenarios)
Comparison 3. Sensitivity analysis (12/24‐week follow‐up).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Resolution | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1.1 Longest follow‐up time | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.62, 1.11] |
| 3.1.2 Up to 12‐week follow‐up | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.03, 3.03] |
| 3.2 Moderate to severe disease | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.2.1 Longest follow‐up time | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.19, 3.46] |
| 3.2.2 Up to 12‐week follow‐up | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 3.20 [1.71, 5.98] |
3.1. Analysis.

Comparison 3: Sensitivity analysis (12/24‐week follow‐up), Outcome 1: Resolution
3.2. Analysis.

Comparison 3: Sensitivity analysis (12/24‐week follow‐up), Outcome 2: Moderate to severe disease
Comparison 4. Sensitivity analysis for the recurrence outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Recurrence (scenarios) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1.1 Only those at risk of recurrence | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.02, 0.63] |
| 4.1.2 All randomised | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.01, 0.38] |
4.1. Analysis.

Comparison 4: Sensitivity analysis for the recurrence outcome, Outcome 1: Recurrence (scenarios)
Comparison 5. Sensitivity analysis for the pain outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Pain (scenarios) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1.1 Include continuous pain | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.54, 1.31] |
| 5.1.2 Exclude continuous pain | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.52, 1.28] |
| 5.1.3 Painful Grade 2 disease | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.41, 4.52] |
5.1. Analysis.

Comparison 5: Sensitivity analysis for the pain outcome, Outcome 1: Pain (scenarios)
Comparison 6. Sensitivity analysis for the adverse events outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Adverse events (scenarios) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1.1 Continuous pain is not an AE | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 6.1.2 Continuous pain is an AE | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.19, 21.42] |
6.1. Analysis.

Comparison 6: Sensitivity analysis for the adverse events outcome, Outcome 1: Adverse events (scenarios)
Comparison 7. Sensitivity analysis (treatment failure).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Types of treatment failure (unresolved symptoms) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1.1 Recurrence failure (only those at risk) | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.02, 0.63] |
| 7.1.2 All cases of unresolved symptom failure | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.82, 1.48] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Leow 2018.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: single‐blinded (patients were blinded) Controlled: corticosteroid (triamcinolone) Centre: single‐centre Arms: 2 arms Setting: hand surgery outpatient clinic in Singapore Time period: January 2014 and June 2016 Sample size calculation: to detect a difference in 1 severity grade at significance level of 0.05, power of 0.8, and standard deviation of 0.6, 90 patients are required (45 per group). An anticipated dropout rate of 25% was considered; therefore, 121 patients were recruited into the study Analysis: per‐protocol |
|
| Participants | Inclusion criteria: adults (21 years and older), 1 trigger digit requiring injection treatment Exclusion criteria: ketorolac or triamcinolone allergy, pregnant or breastfeeding, previous triamcinolone injection to the same digit Baseline characteristics
NSAID group baseline
Steroid group baseline
|
|
| Interventions |
NSAID group (N = 59): single injection 0.5 mL parenteral ketorolac trometamol (30 mg/mL) + 0.5 mL parenteral lidocaine 1% (10 mg/mL) Control group (N = 62): single injection 0.5 mL parenteral triamcinolone acetonide (10 mg/mL) + 0.5 mL parenteral lidocaine 1% (10 mg/mL) Method of injection: by aseptic technique, a combined intrasynovial and extrasynovial injection was given at the level of the A1 pulley with a 25‐gauge needle and a 3‐mL syringe. The injection was given by physicians involved in the study |
|
| Outcomes | Time points: baseline, 3 weeks, 6 weeks, 12 weeks, 24 weeks
|
|
| Notes |
Source: Funding Section (p 6). Funding was obtained from the Biomechanics Laboratory Programme grant and the Surgery Academic Clinical Programme seed grant and the Singapore Ministry of Health National Medical Research Council, Centre Grant Unpublished data were provided by the study author (median and interquartile range for pain (Appendix 2); range of motion (Appendix 3)) Trial registration: Health Sciences Authority (HSE Ref: HPRG/CTB 78:10/13‐073) Adverse events: no adverse events occurred in NSAID nor control groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Source: Methods section (p 2): "computerized randomisation was used ...". Table 1 (p 3) of baseline characteristics Comment: randomisation was conducted by an assistant who was not involved in the trial (personal communication). Following randomisation, baseline characteristics and symptoms were found to be generally similar in both groups |
| Allocation concealment (selection bias) | Low risk |
Source: Methods section (p 2): "... and sealed envelopes containing the treatment groups were opened after the patient had consented to participate in the study" Comment: envelopes were opaque and were sequentially numbered; they were opened by the research assistant |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Source: Methods section (p 2): "this was a single blinded study and patients were not informed of the drugs they were given" Comment: The drugs were not repackaged, and although there was a slight difference in drug colour, patients were unlikely to be able to identify the drug even though they could see the syringe. Success of blinding was not measured Personnel injecting the drugs were not blinded |
| Blinding of outcome assessment Investigator‐reported outcomes | Low risk |
Source: personal communication with study author Comment: investigator assessing outcomes (resolution, severe disease, total active motion, adverse events) was blinded |
| Blinding of outcome assessment Participant‐reported outcomes | Low risk |
Source: Methods section (p 2): "this was a single blinded study and patients were not informed of the drugs they were given" Comment: as participants were blinded, this means that assessment of all participant‐reported outcomes (pain and satisfaction) was also blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Source: Figure 1 (p 3) Comment: loss to follow‐up at 24 weeks was 20.3% (12/59) for the NSAID group and 16.1% (10/62) for the steroid group. Reasons were not mentioned except for 3 in the NSAID group who opted for surgery |
| Selective reporting (reporting bias) | Low risk | Comment: no published trial protocol was available, but all review outcomes that were measured could be derived from what was reported or from was obtained by contacting the study authors |
| Other bias | Low risk | none apparent |
Shakeel 2012.
| Study characteristics | ||
| Methods | Allocation: randomised Blinding: double‐blinded (patient and assessor) Controlled: corticosteroid (triamcinolone acetonide) Centre: single‐centre Arms: 2 arms Setting: orthopaedic outpatient clinic in Malaysia Time period: not mentioned Sample size calculation: not performed Analysis: per‐protocol |
|
| Participants | Inclusion criteria: clinically diagnosed patients with trigger digits of at least grade 2 by Quinnell, no previous treatment of the trigger digit (e.g. splinting, injection, therapy) Exclusion criteria: unconsented patients, patients with prior treatment of the trigger digit (e.g. splinting, injection, therapy), patients younger than16 years of age, patients with trigger digits due to rheumatoid arthritis, patients with infection at the site of injection Baseline characteristics
NSAID group baseline
Steroid group baseline
|
|
| Interventions |
NSAID group (N = 55): 12.5 mg diclofenac sodium Control group (N = 55): 20 mg triamcinolone acetonide Method of injection: a single injection was administered at the level of the A1 pulley of the affected finger, at the mouth of the pulley, with a 3‐mL syringe and a 25‐gauge needle at an angle of 45 degrees directed distally to the plane of the palm. As the finger was flexed and extended, the needle was withdrawn slowly to a point at which the needle no longer moved but a grating sensation was felt by the investigator as the pulley touched the needle tip |
|
| Outcomes | Time points: baseline, 3 weeks, 3 months
|
|
| Notes | Trial registration: not reported Adverse event: 1 in the steroid group (pain at the injection site) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Source: Materials and Methods section (p 1320): "treatment was determined by drawing from a container with equal numbers of slips marked “STEROID” or “NSAID”" Comment: randomisation was done through a physical process rather than a software algorithm; hence it is unclear whether allocation was truly random. Baseline comparability was not mentioned, and no baseline characteristics table was provided |
| Allocation concealment (selection bias) | High risk |
Source: Materials and Methods section (p 1320): "treatment was determined by drawing from a container with equal numbers of slips marked “STEROID” or “NSAID”" Comment: since the result of each draw is not concealed by a treatment code number, the trial recruiter would be aware of treatment allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Source: Materials and Methods section (p 1320): "this research was conducted as a prospective, randomised, double‐blinded controlled study" Comment: no further details were given, but participant blinding was probably effective due to equal injection volumes (0.5 mL) and the fact that both were potentially active agents (i.e. not placebo). The investigator injecting the drugs was not blinded |
| Blinding of outcome assessment Investigator‐reported outcomes | Low risk | Source: Materials and Methods section (p 1320): "after the injection, the patients were followed up and assessed by blinded assessors 3 weeks and 3 months after injection" |
| Blinding of outcome assessment Participant‐reported outcomes | Low risk | Comment: participants were probably blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Source: Materials and Methods section (p 1320): "however, 10 patients (5 patients from each group) were lost to follow‐up" Comment: loss to follow‐up at 3 months was 9.1% (5/55) in both NSAID and steroid groups. Reasons were not mentioned |
| Selective reporting (reporting bias) | Low risk | Comment: no published trial protocol was available, but all review outcomes that were measured could be derived from what was reported |
| Other bias | Unclear risk | Insufficient information to assess this risk. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akdogan 2014 | Not trigger finger |
| Akhtar 2005 | Not an RCT |
| Al‐Homood 2013 | Not trigger finger |
| Alvarez‐Nemegyei 2004 | Not trigger finger |
| Anderson 1991 | No NSAID arm |
| Andreu 2011 | Not trigger finger |
| Bullocks 2009 | Not an RCT |
| Chao 2009 | No NSAID arm |
| Choudhury 2014 | Not an RCT |
| Coleman 2008 | Not trigger finger |
| Crop 2011 | Not an RCT |
| Davidson 2011 | Not an RCT |
| Egido 2008 | Not an RCT |
| Farouk 2010 | Not trigger finger |
| Foster 2015 | No NSAID arm |
| Gheita 2011 | Not trigger finger |
| Gigante 2011 | Not trigger finger |
| Giuliani 2015 | Not trigger finger |
| Hardy 2013 | Not trigger finger |
| Harvard News Letter 2017 | Not an RCT |
| Heffernan 2001 | Not trigger finger |
| Huisstede 2010 | Not an RCT |
| Ilyas 2019 | No NSAID arm |
| Jacobs 2009 | Not an RCT |
| Jacobs 2013 | Not an RCT |
| Kamath 2016 | Not an RCT |
| Kim 2011 | Not an RCT |
| Kosiyatrakul 2018 | No NSAID arm |
| Lebiedz‐Odrobina 2010 | Not trigger finger |
| Lim 2007 | Not an RCT |
| Lim 2017 | Not an RCT |
| Lintermans 2011 | Not trigger finger |
| Londono 2012 | Not trigger finger |
| Ma 2019 | Not an RCT |
| Massoud 2018 | Not an RCT |
| Mutlu 2017 | Not an RCT |
| Nimigan 2006 | Not an RCT |
| Panayotopoulos 1992 | Not an RCT |
| Pargali 2011 | Not an RCT |
| Park 2009 | Not an RCT |
| Peters‐Veluthamaningal 2009 | No NSAID arm |
| Reading 2001 | Not trigger finger |
| Sainsbury 2008 | Not trigger finger |
| Saldana 2001 | Not an RCT |
| Sayilir 2017 | Not an RCT |
| Schulman 2013 | Not an RCT |
| Sheon 1997 | Not trigger finger |
| Silva 2012 | Not trigger finger |
| Soto Quijano 2005 | Not an RCT |
| Stepan 2018 | Not an RCT |
| Taras 2010 | Not an RCT |
| Vega‐Morales 2014 | Not trigger finger |
| Waimann 2011 | Not trigger finger |
| Weinheimer 2019 | No NSAID arm |
| Wiwanitkit 2012 | Not an RCT |
| Woo 2017 | Not trigger finger |
| Wysocki 2012 | Not trigger finger |
NSAID: non‐steroidal anti‐inflammatory drug.
RCT: randomised controlled trial.
Differences between protocol and review
The severity outcome was renamed "persistent moderate to severe symptoms". Shakeel 2012 reported pain only as dichotomous, so to enable pooling, we reported pain as a dichotomous outcome.
Contributions of authors
Mabel Leow: drafted the review protocol, screened trial reports, reviewed full‐text trial reports, extracted data, assessed RoB, assessed CoE, edited the review.
Qishi Zheng: screened trial reports, extracted data, performed statistical analysis, assessed CoE, edited the review.
Edwin SY Chan: revised the protocol, assessed RoB, extracted data, performed the sensitivity analyses, checked analyses, assessed CoE, edited the review.
Luming Shi: reviewed the protocol, edited the review.
Shian Chao Tay: provided clinical expertise, reviewed the protocol, screened trial reports, reviewed full‐text trial reports, edited the review.
Sources of support
Internal sources
-
The study is funded by the Biomechanics Laboratory Programme Grant and the Surgery Academic Clinical Programme seed grant (Reference no: GRSG13NSL). Both grants are funded by the SingHealth Duke‐NUS Academic Medical Centre, facilitated by the Joint Office of Academic Medicine (JOAM). This is an initiative of Surgery Academic Clinical Programme (SURG ACP), hosted at the Singapore General Hospital (SGH), Singapore
Manpower support for Mabel Leow in 2016
External sources
National Medical Research Council Block grant funding to Cochrane Singapore, Singapore
Declarations of interest
Mabel Qi He Leow: author of included trial.
Qishi Zheng: none known.
Luming Shi: none known.
Shian Chao Tay: author of included trial.
Edwin SY Chan: received funding from a National Medical Research Council Block grant; funding was not provided for this work in particular.
The authors of included studies did not appraise their own studies for inclusion or risk of bias. This was done by Renea Johnston, from Cochrane Musculoskeletal. The trial authors were not responsible for data extraction; this was done by Qishi Zheng.
New
References
References to studies included in this review
Leow 2018 {published and unpublished data}
Shakeel 2012 {published data only}
- Shakeel H, Ahmad TS. Steroid injection versus NSAID injection for trigger finger: a comparative study of early outcomes. Journal of Hand Surgery American Volume 2012;37(7):1319-23. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akdogan 2014 {published data only}
- Akdogan A, Eroglu A. Comparison of the effect of lidocaine adding dexketoprofen and paracetamol in intravenous regional anesthesia. BioMed Research International 2014;2014 (no pagination)(938108):1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Akhtar 2005 {published data only}
- Akhtar S, Bradley MJ, Quinton DN, Burke FD. Management and referral for trigger finger/thumb. British Medical Journal 2005;331(7507):30-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Homood 2013 {published data only}
- Al-Homood IA. Rheumatic conditions in patients with diabetes mellitus. Clinical Rheumatology 2013;32(5):527-33. [DOI] [PubMed] [Google Scholar]
Alvarez‐Nemegyei 2004 {published data only}
- Alvarez-Nemegyei J, Canoso JJ. Evidence-based soft tissue rheumatology: epicondylitis and hand stenosing, tendinopathy. Journal of Clinical Rheumatology 2004;10(1):33-40. [DOI] [PubMed] [Google Scholar]
Anderson 1991 {published data only}
- Anderson B, Kaye S. Treatment of flexor tenosynovitis of the hand ('trigger finger') with corticosteroids. A prospective study of the response to local injection. Archives of Internal Medicine 1991;151(1):153-6. [PubMed] [Google Scholar]
Andreu 2011 {published data only}
- Andreu JL, Oton T, Silva-Fernandez L, Sanz J. Hand pain other than carpal tunnel syndrome (CTS): the role of occupational factors. Best Practice and Research: Clinical Rheumatology 2011;25(1):31-42. [DOI] [PubMed] [Google Scholar]
Bullocks 2009 {published data only}
- Bullocks JM, Downey CR, Gibler DPG, Netscher DT. Crystal deposition disease masquerading as proliferative tenosynovitis and its associated sequelae. Annals of Plastic Surgery 2009;62(2):128-33. [DOI] [PubMed] [Google Scholar]
Chao 2009 {published data only}
- Chao M, Wu S, Yan T. The effect of miniscalpel-needle versus steroid injection for trigger thumb release. Journal of Hand Surgery European Volume 2009;34(4):522-5. [DOI] [PubMed] [Google Scholar]
Choudhury 2014 {published data only}
- Choudhury MM, Tay SC. Prospective study on the management of trigger finger. Hand Surgery 2014;19(3):393-7. [DOI] [PubMed] [Google Scholar]
Coleman 2008 {published data only}
- Coleman RE, Bolten WW, Lansdown M, Dale S, Jackisch C, Merkel D, et al. Aromatase inhibitor-induced arthralgia: clinical experience and treatment recommendations. Cancer Treatment Reviews 2008;34(3):275-82. [DOI] [PubMed] [Google Scholar]
Crop 2011 {published data only}
- Crop JA, Bunt CW. Doctor, my thumb hurts. Journal of Family Practice 2011;60(6):329-32. [PubMed] [Google Scholar]
Davidson 2011 {published data only}
- Davidson J, Jayaraman S. Guided interventions in musculoskeletal ultrasound: what's the evidence? Clinical Radiology 2011;66(2):140-52. [DOI] [PubMed] [Google Scholar]
Egido 2008 {published data only}
- Egido V, Fuentes A, Buisan L. [Systemic mastocytosis and anesthesia: a case report]. Revista Espanola de Anestesiologia y Reanimacion 2008;55(1):61-2. [DOI] [PubMed] [Google Scholar]
Farouk 2010 {published data only}
- Farouk S, Aly A. Quality of lidocaine analgesia with and without midazolam for intravenous regional anesthesia. Journal of Anesthesia 2010;24(6):864-8. [DOI] [PubMed] [Google Scholar]
Foster 2015 {published data only}
- Foster ZJ, Voss TT, Hatch J, Frimodig A. Corticosteroid injections for common musculoskeletal conditions. American Family Physician 2015;92(8):694-9. [PubMed] [Google Scholar]
Gheita 2011 {published data only}
- Gheita T, Fawzy S, Rizk A, Hussein H. Impaired bone formation and osteoporosis in postmenopausal elderly onset rheumatoid arthritis patients. Egyptian Rheumatologist 2011;33(3):155-62. [Google Scholar]
Gigante 2011 {published data only}
- Gigante A, Callegari L. The role of intra-articular hyaluronan (Sinovial) in the treatment of osteoarthritis. Rheumatology International 2011;31(4):427-44. [DOI] [PubMed] [Google Scholar]
Giuliani 2015 {published data only}
- Giuliani E, Bianchi A, Marcuzzi A, Landi A, Barbieri A. Ibuprofen timing for hand surgery in ambulatory care. Acta Ortopedica Brasileira 2015;23(4):188-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hardy 2013 {published data only}
- Hardy G. Reports from the American Diabetes Association's 73rd scientific sessions, 21-25 June 2013, Chicago, Illinois. Cardiovascular Journal of Africa 2013;24(7):291-2. [Google Scholar]
Harvard News Letter 2017 {published data only}
- Best ways to cope with hand pain. Harvard Health Letter 2017;42(9):4-4.
Heffernan 2001 {published data only}
- Heffernan AM. Transcutaneous spinal electroanalgesia: its effects in healthy volunteers, acute and chronic pain patients. Doctoral dissertation, Medicine. Ann Arbor: University of Leicester; 2001;U161884:294.
Huisstede 2010 {published data only}
- Huisstede BM, Middelkoop M, Randsdorp MS, Glerum S, Koes BW. Effectiveness of Interventions of specific complaints of the arm, neck, and/or shoulder: 3 - Musculoskeletal disorders of the hand: an update. Archives of Physical Medicine and Rehabilitation 2010;91(2):298-314. [DOI] [PubMed] [Google Scholar]
Ilyas 2019 {published data only}
- Ilyas Asif M, Miller Andrew J, Graham Jack G, Matzon Jonas L. A prospective, randomized, double-blinded trial comparing acetaminophen, ibuprofen, and oxycodone for pain management after hand surgery. Orthopedics 2019;42(2):110-5. [DOI] [PubMed] [Google Scholar]
Jacobs 2009 {published data only}
- Jacobs JW. How to perform local soft-tissue glucocorticoid injections. Best Practice and Research: Clinical Rheumatology 2009;23(2):193-219. [DOI] [PubMed] [Google Scholar]
Jacobs 2013 {published data only}
- Jacobs JWG, Michels-Van Amelsfort JMR. How to perform local soft-tissue glucocorticoid injections? Best Practice and Research: Clinical Rheumatology 2013;27(2):171-94. [DOI] [PubMed] [Google Scholar]
Kamath 2016 {published data only}
- Kamath SU, Kamath SS. Musculoskeletal manifestations in diabetes mellitus. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2016;7(2):1548-52. [Google Scholar]
Kim 2011 {published data only}
- Kim JY, Sung JH, Seo JG. Sesamoid arthritis mimicking trigger thumb. Orthopedics 2011;34(3):228. [DOI] [PubMed] [Google Scholar]
Kosiyatrakul 2018 {published data only}
- Kosiyatrakul A, Loketkrawee W, Luenam S. Different dosages of triamcinolone acetonide injection for the treatment of trigger finger and thumb: a randomized controlled trial. Journal of Hand Surgery Asian Pacific Volume 2018;23(2):163-9. [DOI: 10.1142/S24244835518500157] [DOI] [PubMed] [Google Scholar]
Lebiedz‐Odrobina 2010 {published data only}
- Lebiedz-Odrobina D, Kay J. Rheumatic manifestations of diabetes mellitus. Rheumatic Disease Clinics of North America 2010;36(4):681-99. [DOI] [PubMed] [Google Scholar]
Lim 2007 {published data only}
- Lim MH, Lim KK, Rasheed MZ, Narayanan S, Beng-Hoi Tan A. Outcome of open trigger digit release. Journal of Hand Surgery European Volume 2007;32(4):457-9. [DOI] [PubMed] [Google Scholar]
Lim 2017 {published data only}
- Lim SH, Ng JY, Kang L. Three-dimensional printing of a microneedle array on personalized curved surfaces for dual-pronged treatment of trigger finger. Biofabrication 2017 Jan 10;9(1):015010. [DOI: 10.1088/1758-5090/9/1/015010] [DOI] [PubMed] [Google Scholar]
Lintermans 2011 {published data only}
- Lintermans A, Neven P. Pharmacology of arthralgia with estrogen deprivation. Steroids 2011;76(8):781-5. [DOI] [PubMed] [Google Scholar]
Londono 2012 {published data only}
- Londono J, Santos AM, Santos PI, Cubidez MF, Guzman C, Valle-Onate R. Therapeutic efficacy and safety of methotrexate + leflunomide in Colombian patients with active rheumatoid arthritis refractory to conventional treatment. [Portuguese]. Revista Brasileira de Reumatologia 2012;52(6):837-45. [PubMed] [Google Scholar]
Ma 2019 {published data only}
- Ma S, Wang C, Li J, Zhang Z, Yu Y, Lv F. Efficacy of corticosteroid injection for treatment of trigger finger: a meta-analysis of randomized controlled trials. Journal of Investigative Surgery 2019;32(5):433-41. [DOI: 10.1080/08941939.2018.1424970] [DOI] [PubMed] [Google Scholar]
Massoud 2018 {published data only}
- Massoud AEAA, Fouaad AA, Abdelkareem MM, El Baqary AMA. Evaluation of the accuracy of trigger finger injection using ultrasound. Egyptian Journal of Hospital Medicine 2018;73(11):7988-96. [Google Scholar]
Mutlu 2017 {published data only}
- Mutlu T, Cicek H, Tuhanioglu U, Seyfettinoglu F, Ogur HU. A comparison of 4 different patient groups in the treatment of trigger finger: is treatment according to grade important? Journal of Clinical and Analytical Medicine 2017;8:97-101. [Google Scholar]
Nimigan 2006 {published data only}
- Nimigan AS, Rosenblatt Y, Gan BS. Trigger fingers: a review. Critical Reviews in Physical and Rehabilitation Medicine 2006;18(4):303-16. [Google Scholar]
Panayotopoulos 1992 {published data only}
- Panayotopoulos E, Fortis AP, Armoni A, Dimakopoulos P, Lambiris E. Trigger digit: the needle or the knife? Journal of Hand Surgery British Volume 1992;17(2):239-40. [DOI] [PubMed] [Google Scholar]
Pargali 2011 {published data only}
- Pargali N, Habibzadeh F. Bilateral trigger finger in a 5-year-old child: case report. Journal of Plastic, Reconstructive and Aesthetic Surgery 2011;64(11):e283-4. [DOI] [PubMed] [Google Scholar]
Park 2009 {published data only}
- Park J, Dumanian GA. Shower emboli and digital necrosis after a single corticosteroid injection for trigger thumb: case report. Journal of Hand Surgery 2009;34(2):313-6. [DOI] [PubMed] [Google Scholar]
Peters‐Veluthamaningal 2009 {published data only}
- Peters-Veluthamaningal C, Van der Windt DA, Winters JC, Meyboom-de Jong B. Corticosteroid injection for trigger finger in adults. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD005617. [DOI: 10.1002/14651858.CD005617.pub2] [DOI] [PubMed] [Google Scholar]
Reading 2001 {published data only}
- Reading AD. Femoral component fixation in total hip arthroplasty: a comparison of normal and reduced viscosity cements employing modern cementation techniques. Doctoral dissertation, Medicine. Ann Arbor: University of Leicester (United Kingdom); 2001;U140132:411.
Sainsbury 2008 {published data only}
- Sainsbury DCG, Hidvegi N, Blair JW. Intra-tendinous gout in a repaired flexor digitorum profundus. Journal of Hand Surgery European Volume 2008;33(4):528-9. [DOI] [PubMed] [Google Scholar]
Saldana 2001 {published data only}
- Saldana MJ. Trigger digits: diagnosis and treatment. Journal of the American Academy of Orthopaedic Surgeons 2001;9(4):246-52. [DOI] [PubMed] [Google Scholar]
Sayilir 2017 {published data only}
- Sayilir S. Trigger finger mimicking sesamoid bone: a cause of diagnostic delay. Turk Osteoporoz Dergisi 2017;23(1):44. [Google Scholar]
Schulman 2013 {published data only}
- Schulman R, Levchenko A, Lombardo SR, Walkoski SA, Moroz A. Trigger finger in a male with diabetes successfully treated with acupuncture and osteopathic manipulative treatment. Medical Acupuncture 2013;25(1):74-7. [Google Scholar]
Sheon 1997 {published data only}
- Sheon RP. Repetitive strain injury: 2. Diagnostic and treatment tips on six common problems. Postgraduate Medicine 1997;102(4):72-85. [DOI] [PubMed] [Google Scholar]
Silva 2012 {published data only}
- Silva MBG, Skare TL. Musculoskeletal disorders in diabetes mellitus. Revista Brasileira de Reumatologia 2012;52(4):601-5. [DOI] [PubMed] [Google Scholar]
Soto Quijano 2005 {published data only}
- Soto-Quijano DA, Rivera-Tavarez CE. Work-related musculoskeletal disorders of the upper extremity. Critical Reviews in Physical and Rehabilitation Medicine 2005;17(1):65-82. [Google Scholar]
Stepan 2018 {published data only}
- Stepan JG, London DA, Osei DA, Boyer MI, Dardas AZ, Calfee RP. Perioperative celecoxib and postoperative opioid use in hand surgery: a prospective cohort study. J Hand Surg Am 2018;43(4):346-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taras 2010 {published data only}
- Taras JS, Valentine BJ. Tenosynovitis. Current Orthopaedic Practice 2010;21(6):609-14. [Google Scholar]
Vega‐Morales 2014 {published data only}
- Vega-Morales D, Barron-Almazan M, Arana-Guajardo A. Comorbidities in a Mexican mestizo cohort with established rheumatoid arthritis. Annals of the Rheumatic Diseases 2014;73(3):e14. [DOI] [PubMed] [Google Scholar]
Waimann 2011 {published data only}
- Waimann CA, Lu H, Suarez Almazor ME. Rheumatic manifestations of primary and metastatic bone tumors and paraneoplastic bone disease. Rheumatic Disease Clinics of North America 2011;37(4):527-49. [DOI] [PubMed] [Google Scholar]
Weinheimer 2019 {published data only}
- Weinheimer K, Michelotti B, Silver J, Taylor K, Payatakes A. A prospective, randomized, double-blinded controlled trial comparing ibuprofen and acetaminophen versus hydrocodone and acetaminophen for soft tissue hand procedures. Journal of Hand Surgery American Volume 2019;44(5):387-93. [DOI] [PubMed] [Google Scholar]
Wiwanitkit 2012 {published data only}
- Wiwanitkit S, Wiwanitkit V. Trigger digits and diabetes mellitus. North American Journal of Medical Sciences 2012;4(3):117-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woo 2017 {published data only}
- Woo SH, Lee YK, Kim JM, Cheon HJ, Chung WHJ. Hand and wrist injuries in golfers and their treatment. Hand Clinics 2017;33(1):81-96. [DOI] [PubMed] [Google Scholar]
Wysocki 2012 {published data only}
- Wysocki RW, Biswas D, Bayne CO. Injection therapy in the management of musculoskeletal injuries: hand and wrist. Operative Techniques in Sports Medicine 2012;20(2):132-41. [Google Scholar]
Additional references
Akhtar 2006
- Akhtar S, Burke FD. Study to outline the efficacy and illustrate techniques for steroid injection for trigger finger and thumb. Postgraduate Medical Journal 2006;82(973):763-6. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baigent 2013
- Baigent C, Bhala N, Emberson J, Merhi A, Abramson S, Arber N, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs. Lancet 2013;382(9894):769-79. [DOI: 10.1016/S0140-6736(13)60900-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bain 2014
- Bain GI, Polites N, Higgs BG, Heptinstall JR, McGrath MA. The functional range of motion of the finger joints. Journal of Hand Surgery European Volume 2014;40:406-11. [DOI: ] [DOI] [PubMed] [Google Scholar]
Barnes 2009
- Barnes PJ. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. British Journal of Pharmacology 2006;148(3):245-54. [DOI: 10.1038/sj.bjp.0706736] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baumgarten 2007
- Baumgarten KM, David G, Martin IB. Corticosteroid injection in diabetic patients with trigger finger. Journal of Bone and Joint Surgery 2007;89(12):2604-11. [DOI: ] [DOI] [PubMed] [Google Scholar]
Bodor 2009
- Bodor M, Flossman T. Ultrasound-guided first annular pulley injection for trigger finger. Journal of Ultrasound in Medicine 2009;28(6):737-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bresalier 2005
- Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. New England Journal of Medicine 2005;352(11):1092-102. [DOI: 10.1056/NEJMoa050493] [DOI] [PubMed] [Google Scholar]
Brinks 2010
- Brinks A, Koes BW, Volkers AC, Verhaar JA, Bierma-Zeinstra SM. Adverse effects of extra-articular corticosteroid injections: a systematic review. BMC Musculoskeletal Disorders 2010;11:206. [DOI: 10.1186/1471-2474-11-206] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cagliero 2002
- Cagliero E, Apruzzese W, Perlmutter GS. Skeletal disorders of the hand and shoulder in patients with diabetes mellitus. Americal Journal of Medicine 2002;112(6):487-90. [DOI: ] [DOI] [PubMed] [Google Scholar]
Coutinho 2011
- Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Molecular and Cellular Endocrinology 2011;335(1):2-13. [DOI: 10.1016/j.mce.2010.04.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
da Costa 2017
- da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Juni P, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. The Lancet 2017;390(10090):e21-e33. [DOI: ] [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9. Analysing data and undertaking meta-analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Eastwood 1992
- Eastwood DM, Gupta KJ, Johnson DP. Percutaneous release of the trigger finger: an office procedure. Journal of Hand Surgery American Volume 1992;17:114-7. [DOI] [PubMed] [Google Scholar]
Evans 1988
- Evans RB, Hunter JM, Burkhalter WE. Conservative management of the trigger finger: a new approach. Journal of Hand Therapy 1988;1(2):59-68. [DOI: ] [Google Scholar]
Fitzgerald 2005
- Fitzgerald BT, Hofmeister EP, Thompson MA. Delayed flexor digitorum superficialis and profundus ruptures in a trigger finger after a steroid injection: a case report. Journal of Hand Surgery American Volume 2005;30(3):479-82. [DOI: 10.1016/j.jhsa.2004.10.011] [DOI] [PubMed] [Google Scholar]
Friedman 1988
- Friedman SJ, Butler DF, Pittelkow MR. Perilesional linear atrophy and hypopigmentation after intralesional corticosteroid therapy. Report of two cases and a review of the literature. Journal of the American Academy of Dermatology 1988;19(3):537-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gabriel 1991
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs: a meta-analysis. Annals of Internal Medicine 1991;115(10):787-96. [DOI: 10.7326/0003-4819-115-10-787] [DOI] [PubMed] [Google Scholar]
Green 2005
- Green D, Hotchkiss R, Pederson W, Wolfe S. Green's Operative Hand Surgery, 5th edition. London: Churchill Livingstone, 2005. [Google Scholar]
Gyuricza 2009
- Gyuricza C, Umoh E, Wolfe SW. Multiple pulley rupture following corticosteroid injection for trigger digit: case report. Journal of Hand Surgery American Volume 2009;34(8):1444-8. [DOI: 10.1016/j.jhsa.2009.04.037] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16. Special topics in statistics. [Cochrane Handbook for Systematic Reviews of Interventions ]. In: Higgins JPT, Green S, editors(s). The Cochrane Collaboration, 2011. Version 5.1.0 (updated March 2011) edition. Cochrane, 2011. [Google Scholar]
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8. Assessing risk of bias in included studies. [Cochrane Handbook for Systematic Reviews of Interventions]. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editors(s). Available from www.training.cochrane.org/handbook. Version 5.2.0 (updated June 2017) edition. Cochrane, 2017. [Google Scholar]
Jianmongkol 2007
- Jianmongkol S, Kosuwon W, Thammaroj T. Intra-tendon sheath injection for trigger finger: the randomized controlled trial. Hand Surgery 2007;12(2):79-82. [DOI: ] [DOI] [PubMed] [Google Scholar]
Kale 2017
- Kale S. Trigger finger treatment and management. Medscape: Orthopaedic Surgery 24 Oct 2017. [https://emedicine.medscape.com/article/1244693-treatment]
Kearney 2006
- Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 2006;332(7553):1302-8. [DOI: 10.1136/bmj.332.7553.1302] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kolind‐Sørensen 1980
- Kolind-Sørensen V. Treatment of trigger fingers. Acta Orthopaedica Scandinavica 1970;41(1):428-32. [DOI: 10.3109/17453677008991530] [DOI] [PubMed] [Google Scholar]
Makkouk 2008
- Makkouk AH, Oetgen ME, Swigart CR, Dodds SD. Trigger finger: etiology, evaluation and treatment. Current Reviews in Musculoskeletal Medicine 2008;1(2):92-6. [DOI: 10.1007/s12178-009-9012-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Makris 2007
- Makris UE, Kohler MJ, Fraenkel L. Adverse effects (AEs) of topical NSAIDs in older adults with osteoarthritis (OA): a systematic review of the literature. Journal of Rheumatology 2010;37(6):1236-43. [DOI: 10.3899/jrheum.090935] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mardani‐Kivi 2018
- Mardani-Kivi M, Karimi-Mobarakeh M, Babaei JA, Keyhani S, Saheb-Ekhtiari K, Hashemi-Motlagh K. Intra-sheath versus extra-sheath ultrasound guided corticosteroid injection for trigger finger: a triple blinded randomized clinical trial. The Physician and Sportsmedicine 2018;46(1):93-7. [DOI: 10.1080/00913847.2018.1400897] [DOI] [PubMed] [Google Scholar]
Ong 2007
- Ong CK, Lirk P, Tan CH, Seymour RA. An evidenced-base update on nonsteroidal anti-inflammatory drugs. Clinical Medicine and Research 2007;5(1):19-34. [DOI: 10.3121/cmr.2007.698] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pataradool 2011
- Pataradool K, Buranapuntaruk T. Proximal phalanx injection for trigger finger: randomized controlled trial. Hand Surgery 2011;16(3):313-317. [DOI: 10.1142/S0218810411005606] [DOI] [PubMed] [Google Scholar]
Patel 1992
- Patel MR, Bassini L. Trigger fingers and thumb: when to splint, inject, or operate. Journal of Hand Surgery 1992;17(1):110-3. [DOI: 10.1016/0363-5023(92)90124-8] [DOI] [PubMed] [Google Scholar]
Patel 1997
- Patel MR, Moradia VJ. Percutaneous release of trigger digit with and without cortisone injection. Journal of Hand Surgery American Volume 1997;22:150-5. [DOI] [PubMed] [Google Scholar]
Peters‐Veluthamaningal 2009
- Peters-Veluthamaningal C, van der Windt DAWM, Winters JC, Meyboom-de Jong B. Corticosteroid injection for trigger finger in adults. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD005617. [DOI: 10.1002/14651858.CD005617.pub2] [DOI] [PubMed] [Google Scholar]
PRISMA Group
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI: 10.1136/bmj.b2535] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quinnell 1980
- Quinnell RC. Conservative management of trigger finger. Practitioner 1980;224(1340):187-90. [DOI: ] [PubMed] [Google Scholar]
Rannou 2016
- Rannou F, Pelletier J, Martel-Pelletier J. Efficacy and safety of topical NSAIDs in the management of osteoarthritis: evidence from real-life setting trials and surveys. Seminars in Arthritis and Rheumatism 2016;45(4):S18-S21. [DOI: ] [DOI] [PubMed] [Google Scholar]
Ray 2011
- Ray S, Datta AK, Sinhamahapatra P, Ray I, Mukhopadhyay P, Dasgupta S. Prevalence of rheumatic conditions in patients with diabetes mellitus in a tertiary care hospital. Journal of the Indian Medical Association 2011;109(2):74-8. [PMID: ] [PubMed] [Google Scholar]
Roelofs PDDM 2008
- Roelofs PDDM, Deyo RA, Koes BW, Scholten RJPM, Tulder MW. Non-steroidal anti-inflammatory drugs for low-back pain. Cochrane Database of Systematic Reviews 23 January 2008, Issue 4. Art. No: CD000396. [DOI: 10.1002/14651858.CD000396.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryzewicz 2006
- Ryzewicz M, Wolk JM. Trigger digits: principles, management, and complications. Journal of Hand Surgery 2006;31(1):135-46. [DOI: 10.1016/j.jhsa.2005.10.013] [DOI] [PubMed] [Google Scholar]
Schneeweiss 2005
- Schneeweiss S, Glynn RJ, Avorn J, Solomon DH. A Medicare database review found that physician preferences increasingly outweighed patient characteristics as determinants of first-time prescriptions for COX-2 inhibitors. Journal of Clinical Epidemiology 2005;58(1):98-102. [DOI: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12. Interpreting results and drawing conclusions. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Sostres 2010
- Sostres C, Gargallo CJ, Arroyo MT, Lanas A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Gastroenterology 2010;24(2):121–32. [DOI: ] [DOI] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JAC, Egger M, Moher D, Boutron I. Chapter 10. Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available at www.training.cochrane.org/handbook.
Thomas 2000
- Thomas MC. Diuretics, ACE inhibitors and NSAIDs - the triple whammy. Medical Journal of Australia 2000;172(4):184-5. [DOI] [PubMed] [Google Scholar]
Waller 2013
- Waller DFG, Sampson T. Medical Pharmacology and Therapeutics. 4th edition. China: Elsevier Health Sciences, 2013. [Google Scholar]
Wang 2006
- Wang AA, Hutchinson D. The effect of corticosteroid injection for trigger finger on blood glucose level in diabetic patients. Journal of Hand Surgery 2006;31(6):979-81. [DOI: 10.1016/j.jhsa.2006.03.022] [DOI] [PubMed] [Google Scholar]
Wei 2006
- Wei AS, Callaci JJ, Juknelis D, Marra G, Tonino P, Freedman KB, et al. The effect of corticosteroid on collagen expression in injured rotator cuff tendon. Joutnal of Bone and Joint Surgery 2006;88(6):1331-8. [DOI: 10.2106/JBJS.E.00806] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Leow 2017
- Leow MQH, Zheng Q, Shi L, Tay SC, Chan ESY. Non‐steroidal anti‐inflammatory drugs (NSAIDS) for trigger finger.. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD012789. [DOI: 10.1002/14651858.CD012789] [DOI] [PMC free article] [PubMed] [Google Scholar]


