Abstract
Background
Despite the health benefits of breastfeeding, initiation and duration rates continue to fall short of international guidelines. Many factors influence a woman's decision to wean; the main reason cited for weaning is associated with lactation complications, such as mastitis.
Mastitis is an inflammation of the breast, with or without infection. It can be viewed as a continuum of disease, from non‐infective inflammation of the breast to infection that may lead to abscess formation.
Objectives
To assess the effectiveness of preventive strategies (for example, breastfeeding education, pharmacological treatments and alternative therapies) on the occurrence or recurrence of non‐infective or infective mastitis in breastfeeding women post‐childbirth.
Search methods
We searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (3 October 2019), and reference lists of retrieved studies.
Selection criteria
We included randomised controlled trials of interventions for preventing mastitis in postpartum breastfeeding women.
Quasi‐randomised controlled trials and trials reported only in abstract form were eligible. We attempted to contact the authors to obtain any unpublished results, wherever possible.
Interventions for preventing mastitis may include: probiotics, specialist breastfeeding advice and holistic approaches.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and assessed the certainty of the evidence using GRADE.
Main results
We included 10 trials (3034 women). Nine trials (2395 women) contributed data. Generally, the trials were at low risk of bias in most domains but some were high risk for blinding, attrition bias, and selective reporting. Selection bias (allocation concealment) was generally unclear. The certainty of evidence was downgraded due to risk of bias and to imprecision (low numbers of women participating in the trials). Conflicts of interest on the part of trial authors, and the involvement of industry funders may also have had an impact on the certainty of the evidence.
Most trials reported our primary outcome of incidence of mastitis but there were almost no data relating to adverse effects, breast pain, duration of breastfeeding, nipple damage, breast abscess or recurrence of mastitis.
Probiotics versus placebo
Probiotics may reduce the risk of mastitis more than placebo (risk ratio (RR) 0.51, 95% confidence interval (CI) 0.35 to 0.75; 2 trials; 399 women; low‐certainty evidence). It is uncertain if probiotics reduce the risk of breast pain or nipple damage because the certainty of evidence is very low. Results for the biggest of these trials (639 women) are currently unavailable due to a contractual agreement between the probiotics supplier and the trialists. Adverse effects were reported in one trial, where no woman in either group experienced any adverse effects.
Antibiotics versus placebo or usual care
The risk of mastitis may be similar between antibiotics and usual care or placebo (RR 0.37, 95% CI 0.10 to 1.34; 3 trials; 429 women; low‐certainty evidence). The risk of mastitis may be similar between antibiotics and fusidic acid ointment (RR 0.22, 95% CI 0.03 to 1.81; 1 trial; 36 women; low‐certainty evidence) or mupirocin ointment (RR 0.44, 95% CI 0.05 to 3.89; 1 trial; 44 women; low‐certainty evidence) but we are uncertain due to the wide CIs. None of the trials reported adverse effects.
Topical treatments versus breastfeeding advice
The risk of mastitis may be similar between fusidic acid ointment and breastfeeding advice (RR 0.77, 95% CI 0.27 to 2.22; 1 trial; 40 women; low‐certainty evidence) and mupirocin ointment and breastfeeding advice (RR 0.39, 95% CI 0.12 to 1.35; 1 trial; 48 women; low‐certainty evidence) but we are uncertain due to the wide CIs.
One trial (42 women) compared topical treatments to each other. The risk of mastitis may be similar between fusidic acid and mupirocin (RR 0.51, 95% CI 0.13 to 2.00; low‐certainty evidence) but we are uncertain due to the wide CIs. Adverse events were not reported.
Specialist breastfeeding education versus usual care
The risk of mastitis (RR 0.93, 95% CI 0.17 to 4.95; 1 trial; 203 women; low‐certainty evidence) and breast pain (RR 0.93, 95% CI 0.36 to 2.37; 1 trial; 203 women; low‐certainty evidence) may be similar but we are uncertain due to the wide CIs. Adverse events were not reported.
Anti‐secretory factor‐inducing cereal versus standard cereal
The risk of mastitis (RR 0.24, 95% CI 0.03 to 1.72; 1 trial; 29 women; low‐certainty evidence) and recurrence of mastitis (RR 0.39, 95% CI 0.03 to 4.57; 1 trial; 7 women; low‐certainty evidence) may be similar but we are uncertain due to the wide CIs. Adverse events were not reported.
Acupoint massage versus routine care
Acupoint massage probably reduces the risk of mastitis compared to routine care (RR 0.38, 95% CI 0.19 to 0.78;1 trial; 400 women; moderate‐certainty evidence) and breast pain (RR 0.13, 95% CI 0.07 to 0.23; 1 trial; 400 women; moderate‐certainty evidence). Adverse events were not reported.
Breast massage and low frequency pulse treatment versus routine care
Breast massage and low frequency pulse treatment may reduce risk of mastitis (RR 0.03, 95% CI 0.00 to 0.21; 1 trial; 300 women; low‐certainty evidence). Adverse events were not reported.
Authors' conclusions
There is some evidence that acupoint massage is probably better than routine care, probiotics may be better than placebo, and breast massage and low frequency pulse treatment may be better than routine care for preventing mastitis. However, it is important to note that we are aware of at least one large trial investigating probiotics whose results have not been made public, therefore, the evidence presented here is incomplete.
The available evidence regarding other interventions, including breastfeeding education, pharmacological treatments and alternative therapies, suggests these may be little better than routine care for preventing mastitis but our conclusions are uncertain due to the low certainty of the evidence.
Future trials should recruit sufficiently large numbers of women in order to detect clinically important differences between interventions and results of future trials should be made publicly available.
Plain language summary
Interventions for the prevention of mastitis following childbirth
We set out to look at the effectiveness of interventions used to prevent breastfeeding women developing inflammation of breast tissue known as mastitis.
What is the issue?
Mastitis is a common complication of breastfeeding. It causes considerable pain and suffering for women and may stop some mothers from breastfeeding their babies for as long as they would like. Several factors contribute to the development of mastitis, such as blocked ducts, the breasts being too full with milk, cracked nipples and the baby being unable to latch on correctly. Mastitis can occur in one or both breasts and be associated with a number of symptoms including breast pain, redness and swelling, and flu‐like symptoms. The symptoms can last from two to three days up to a couple of weeks or more.
Why is this important?
It is important to investigate treatments to prevent mastitis in order to maximise breastfeeding outcomes and duration. Breastfeeding has major health benefits for both babies and their mothers, and healthcare authorities and the World Health Organization recommend that newborn infants should be fed exclusively on breast milk until they are six months of age. We need to ensure mothers, and the doctors and midwives who care for them, know about the best interventions for preventing mastitis in order to help women breastfeed successfully for as long as they want.
What evidence did we find?
We searched for evidence from randomised controlled trials in October 2019 and identified 10 trials (involving 3034 breastfeeding women). Most trials reported how many women were diagnosed with mastitis but there was almost no information about adverse effects, breast pain, duration of breastfeeding, nipple damage, breast abscess or recurrence of mastitis. Some trials were industry funded.
Three trials (1038 women) compared probiotics to placebo. Results for the biggest of these trials (639 women) are currently unavailable because of a contractual agreement between the probiotics supplier and the trialists. Probiotics may reduce the risk of mastitis compared with placebo (low‐certainty evidence). It is uncertain if probiotics reduce the risk of breast pain or nipple damage because the certainty of evidence is very low.
The risk of mastitis may be similar between antibiotics and usual care or placebo (low‐certainty evidence). The risk of mastitis may be similar between antibiotics and fusidic acid ointment, antibiotics and mupirocin ointment, fusidic acid ointment and breastfeeding advice, mupirocin ointment and breastfeeding advice, fusidic acid and mupirocin, a single session of specialist breastfeeding education and routine care, anti‐secretory factor‐inducing cereal and standard cereal, but we are not certain about these results because they come from trials with small numbers of participants and the quality of evidence is low.
Acupoint massage probably reduces the risk of mastitis and breast pain compared with routine care (moderate‐certainty evidence).
Breast massage and low frequency pulse treatment may reduce the risk of mastitis compared with routine care (low‐certainty evidence).
What does this mean?
Acupoint massage probably helps to prevent mastitis and breast pain, probiotics may be better than placebo and breast massage and low frequency pulse treatment may be better than routine care. However, in general, we cannot be sure what the most effective treatments are for preventing mastitis because the certainty of evidence is low due to risk of bias, low numbers of women participating in the trials, and large differences between the treatments which make it difficult to make meaningful comparisons. We are also unsure about the true effectiveness of probiotics because we know of at least one probiotics trial whose results are not publicly available.
Summary of findings
Background
The World Health Organization (WHO) recognises the short‐ and long‐term benefits of breastfeeding and recommends exclusive breastfeeding until six months of age (Kramer 2002; Kramer 2012; World Health Organization 2008). The epidemiologic evidence overwhelmingly supports breastfeeding as being protective of infant, maternal, family and community health (Kramer 2002; Kramer 2012; World Health Organization 2008). The improved nutrition, immunological, psychological, economical and environmental benefits that breastfeeding provides are well documented (Anatolitou 2012; Chezem 2003). Specifically, breast milk protects infants and children against conditions such as gastroenteritis and respiratory infections (Anatolitou 2012; MacDonald 2003); moreover babies who are not breastfed are predisposed to many health complications in later life, including high blood pressure, obesity, non‐insulin dependent diabetes and ischaemic heart disease (Thompson 2005). The short‐ and long‐term benefits of breastfeeding to the mother include the increase of uterine contractions post‐delivery, resulting in a reduction of postpartum bleeding (Chua 1994; Anatolitou 2012). Breastfeeding also enhances a faster return to the pre‐pregnant body weight (Anatolitou 2012; Dewey 1999), as well as possible protection against osteoporosis, ovarian and uterine cancer (Anatolitou 2012; Cummings 1993; Melton 1993; Rosenblatt 1993; Siskind 1997).
Despite the recognised health, emotional, psychosocial and societal benefits of breastfeeding to women and children, breastfeeding rates worldwide are suboptimal, especially among low‐income women. Increasing breastfeeding initiation and duration amongst low‐income women would not only offer improved health benefits to both the mother and infant, but would lessen the economic burden experienced by this group of people within the community (Anatolitou 2012; Guttman 2000; Mitra 2004).
Description of the condition
There are many factors that may influence a woman's decision to cease breastfeeding. However, the main reason cited for stopping breastfeeding is related to complications of lactation (Boakes 2018; Dener 2003). Mastitis is a significant complication and is a common problem in lactating women (Boakes 2018; Dener 2003). This debilitating condition may contribute to weaning in the first three weeks (Boakes 2018; Schwartz 2002) and has been reported as the third most common reason for weaning (Fetherston 1997), with one in four breastfeeding women citing mastitis as the reason they weaned (Michie 2003). However, the incidence of mastitis has been reported to be as high as 33% in breastfeeding women (Jahanfar 2013). Mastitis may also contribute to some women experiencing negative emotions, including distress, depression and anxiety as well as a feeling of helplessness (Amir 2006). The definition of mastitis varies throughout the literature; WHO defines mastitis as "an inflammatory condition of the breast, which may or may not be accompanied by infection" (Amir 2007; Fetherston 1998; World Health Organization 2008). Non‐infective mastitis may result from milk stasis, blocked ducts, engorgement or physical injury to the breast. Infective mastitis may result from cracked or traumatised nipples; interruption in the nipples' integrity provides a route for micro‐organisms to enter the breast (Fetherston 1998). Mastitis can be viewed as a continuum of disease, from non‐infective inflammation of the breast to infection that may lead to abscess formation. Mastitis presents with a plethora of clinical symptoms; it can present unilaterally or bilaterally with breast pain, redness and swelling; and may be associated with flu‐like symptoms (Amir 2007; Jahanfar 2013). The type of mastitis experienced may affect the duration of symptoms, from two to three days to as long as 14 days or more (Thomsen 1984). The prevalence of mastitis varies depending on the definition and the number of weeks postpartum (Kinlay 2001; Potter 2005; Semba 2000). Studies following participants from three to 12 months have reported incidence rates of mastitis of 23.7% to 27.1% (Fetherston 1998; Vogel 1999), while the recurrence of mastitis is between 6.5% and 8.5% (Fetherston 1997; Vogel 1999). However, Boakes conducted a study in 2018 that reported the global prevalence of mastitis ranging from between 1% to 10% in lactating woman (Boakes 2018.
Description of the intervention
Health education and peer support have been identified as interventions that improve the initiation of breastfeeding amongst low‐income populations where breastfeeding initiation rates are typically low (Dyson 2005). However, antenatal breastfeeding education has been explored as an intervention to improve breastfeeding duration rates (Anatolitou 2012; Lumbiganon 2016). The literature also suggests that education and support, along with correct breastfeeding practices such as good positioning and the correct attachment of the baby to the breast, lead to improved breastfeeding exclusivity and duration (Anatolitou 2012; Fetherston 1998; Inch 2006; Potter 2005) and one study has postulated that breastfeeding education may positively impact on the prevention of mastitis (Flores 2002). Breastfeeding education can take many forms, such as in group and/or one‐to‐one sessions, informative literature and telephone and/or online support.
Poor breast attachment and inadequate breast drainage when feeding are issues that have been linked to women developing mastitis (Amir 2014; Bell 1998; Inch 2006). Breastfeeding frequently, alternating the breast that feeds are started from, and the position used to feed the infant, may all help to relieve engorgement. Breast compression or breast massage before latching is an effective way to avoid blocked ducts that may lead to mastitis. Frequent feeding and the use of electric or hand pumps may assist by efficiently emptying the breast, and reduce breast engorgement and milk stasis. Previous work has suggested that if left untreated, these conditions may develop into mastitis (Amir 2014; Foxman 2002). Avoiding the use of ill‐fitting clothes or bras and sleeping on the stomach are among other measures that women can take to reduce pressure on breast tissue. Such pressure can lead to blocked milk ducts or traumatised breast tissue, which is another precursor to mastitis. Taking care of oneself, getting plenty of rest, adequate fluids and a nutritious diet are all seen as preventive treatments to help manage maternal stress and fatigue, which are factors seen to precede mastitis (Spowart 2004; Wambach 2016). Studies by Roberts 1998 have shown that cabbage leaves can be used to help manage engorgement by reducing pain and discomfort. Antibiotics have also been used as a preventive treatment for women that are predisposed to recurrent mastitis (Cusack 2011; Fetherston 1998; Foxman 1994; Jahanfar 2013). However, there is insufficient evidence to confidently conclude that antibiotics therapy is effective in the management of mastitis (Jahanfar 2013).
Other interventions that have been trialed as interventions in the treatment of mastitis are topical ointments to treat painful, infected nipples with a view to preventing the further onset of mastitis (Livingstone 1999), hydrothermically processed cereal with anti‐secretory factor‐inducing properties (Svensson 2004), and acupoint massage (He 2015).
How the intervention might work
Interventions intended to prevent mastitis might work in several ways. Some interventions aim to facilitate milk extraction from the breast, some focus on breastfeeding knowledge and technique, while others are thought to have anti‐inflammatory and anti‐infection effects. The interventions investigated here are underpinned by a range of assumptions:
breastfeeding education; to improve women's understanding of breastfeeding physiology and management, including relaxation, stress and fatigue management, and correct positioning of baby at the breast, thought to reduce the risk of nipple damage as well as facilitate adequate drainage of milk from the breast. Evidence from randomised controlled trials and observational studies shows that counselling and educational interventions delivered at home and in the community help to improve breastfeeding rates (Sinha 2015), therefore, it is possible that these types of interventions could also help to reduce mastitis rates.
acupoint massage and/or breast massage before and during breastfeeding; to facilitate milk extraction from the breast, and to soften breast tissue when draining the breast of milk. A systematic review indicates that massage interventions can help to reduce pain in women with a range of breastfeeding problems (Anderson 2019). Observational study evidence suggests that therapeutic massage can help relieve symptoms in women with engorgement, plugged ducts or mastitis (Witt 2016).
administration of topical treatments to painful, infected nipples with the intention of preventing further infection and the onset of mastitis. Purified lanolin may be beneficial in the treatment of sore nipples.The management of sore nipples may reduce the risk of developing mastitis in some women (Spencer 2008).
use of probiotics, whose anti‐inflammatory effects may prevent mastitis. It is thought that supplements containing specific strains of Lactobacilli from human milk may have a protective effect against breast infection in breastfeeding women since the micro‐organisms in the probiotics can travel from the gastrointestinal tract to the mammary glands (Amir 2016). There have been few studies published regarding probiotics in the treatment or prevention of mastitis, and with mixed results, however, healthcare professionals in some parts of the world are already receiving direct marketing of probiotic products despite the paucity of evidence for their effectiveness (Amir 2016).
hydrothermically processed nutritional interventions designed to induce anti‐secretory factor (AF) in human milk, thought to reduce the risk of infection. AF helps to prevent diarrhoea and inflammation of the intestines (Lange 2001) and it is thought that there may be an association between high levels of active AF in plasma and breast milk, and a reduced risk of infection (Gustafsson 2018).
use of prophylactic antibiotics; to prevent the onset of infection and to manage recurrence of mastitis. The use of antibiotics in the early presentation of mastitis is a considered management treatment (Auckland District Health Board 2017) and it is possible that antibiotics could be used as a preventive measure to avoid developing advanced presentation of mastitis.
Why it is important to do this review
Currently, a variety of interventions are used in clinical practice for the prevention of mastitis following childbirth. Uncertainties remain about their effectiveness and their possible impact on breastfeeding. It is important to identify, synthesise and assess the certainty of the existing evidence relating to the effectiveness and safety of interventions to prevent mastitis in order to enable women and clinical decision makers to make better informed decisions. Additionally, new randomised studies have been conducted since the previous version of this review was published in 2012 which need to be incorporated to ensure our findings are up‐to‐date and to help inform international clinical guidelines.
Objectives
To assess the effectiveness of preventive strategies (for example, breastfeeding education, pharmacological treatments and alternative therapies) on the occurrence or recurrence of non‐infective or infective mastitis in breastfeeding women post‐childbirth.
Methods
Criteria for considering studies for this review
Types of studies
Eligible studies were randomised controlled trials (RCTs), quasi‐RCTs and cluster‐randomised trials with the purpose of evaluating one or more interventions to prevent mastitis. Trials reported only in abstract form were also eligible for inclusion.
Types of participants
Postpartum women, either primiparous or multiparous, who are breastfeeding or who intend to breastfeed both exclusively and partially. We included studies where some of the women had had mastitis previously or who had symptoms, such as cracked nipples, but all the studies were in women who did not currently have mastitis.
Types of interventions
Any intervention intended to prevent mastitis versus any other intervention intended to prevent mastitis or versus no intervention (placebo), administered towards the end of pregnancy or in the first few weeks postpartum.
Types of interventions may include:
breastfeeding education, information, and support (including relaxation, stress and fatigue management, correct positioning of baby at the breast);
acupoint and breast massage before and during breastfeeding;
prophylactic antibiotics;
probiotics;
topical ointments;
anti‐secretory factor‐inducing nutritional interventions.
Types of outcome measures
The following primary and secondary outcomes were selected through discussion amongst the author team.
Primary outcomes
Incidence of mastitis within six months postpartum, diagnosed by a combination of women's self‐reported symptoms and clinical examination
Recurrence of mastitis within 12 months postpartum
Secondary outcomes
Breast abscess within six months postpartum
Nipple damage within six months postpartum
Duration of exclusive breastfeeding (where the baby receives no other food or drink, not even water)
Duration of any breastfeeding (where the baby receives breastmilk in addition to any other nutrition)
Breast pain
Breast engorgement
Women's perception of milk supply
Maternal breastfeeding satisfaction (measured by Maternal Breastfeeding Evaluation Scale (Leff 1994))
Maternal breastfeeding confidence (measured by Breastfeeding Self‐efficacy Scale (Dennis 1999))
Cessation of breastfeeding within six months postpartum
Number of women with adverse events
Search methods for identification of studies
The following methods section is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (3 October 2019).
The Register is a database containing over 26,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings; and the list of journals reviewed via the current awareness service; please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (3 October 2019) using the search methods detailed in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
(For details of the search methods used in the previous review, please see Crepinsek 2012.)
Data collection and analysis
For methods used in the previous version of this review, seeCrepinsek 2012.
For this update, the following methods (based on a standard group template) were used for assessing the reports that were identified as a result of the updated search.
Selection of studies
Two review authors (ET and FS) independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors (ET and FS) extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third review author (MC). Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we contacted authors of the original reports to request further details.
Assessment of risk of bias in included studies
Two review authors (ET and FS) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2019). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Time‐to‐event data
We did not identify any data relating to duration of breastfeeding. In future updates, where data are available we will use time‐to‐event analysis and present hazard ratios and 95% confidence intervals.
Unit of analysis issues
Cluster‐randomised trials
No cluster‐randomised trials were identified. In future updates of the review, we will include cluster‐randomised trials in the analyses along with individually randomised trials. We will adjust their sample sizes using the methods described in section 23.1.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials would not be a suitable design for this intervention, therefore, were not eligible for inclusion.
Other unit of analysis issues
The unit of analysis is each woman who is randomised to a treatment group. We analysed trials with more than two arms by treating each pair of arms as a separate comparison.
Dealing with missing data
For included studies, levels of attrition were noted. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis through visual inspection of forest plots and consideration of the I2 statistic.
As strict thresholds for interpretation of I2 are not recommended, we used the guide to interpretation in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity
When I² lay in an area of overlap between two categories (e.g. between 50% and 60%), we considered differences in participants and interventions among the trials contributing data to the analysis (Higgins 2019).
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
In future updates, if there is clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary is treated as the average of the range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials. If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We did not identify substantial heterogeneity. In future updates, we will investigate heterogeneity using subgroup analyses and sensitivity analyses.
In future updates, we will carry out subgroup analysis to investigate if interventions have different effects in women who have previously experienced mastitis after childbirth compared to women who have never had mastitis. We will limit subgroup analysis to the two primary outcomes of incidence of mastitis and recurrence of mastitis.
We will assess subgroup differences in future reviews by interaction tests available within RevMan (RevMan 2014) and we will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We did not identify sufficient numbers of trials to undertake sensitivity analysis but in future updates we plan to carry out sensitivity analyses to explore the effect of risk of bias by excluding trials at high risk of bias from the analysis.
Summary of findings and assessment of the certainty of the evidence
The certainty of the evidence was assessed using the GRADE approach as outlined in the GRADE handbook in order to assess the certainty of the body of evidence relating to the following outcomes.
Incidence of mastitis within six months postpartum.
Recurrence of mastitis within 12 months postpartum.
Breast abscess within six months postpartum.
Nipple damage within six months postpartum.
Duration of any breastfeeding.
Breast pain.
Number of women with adverse events.
The GRADEpro Guideline Development Tool was used to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of certainty for each of the above outcomes were produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
Results of the search
We retrieved 23 trial reports from the updated search and also reassessed (Gensch 2006) which was awaiting classification in the previous version of the review. This trial did not meet the inclusion criteria and was excluded. There are two ongoing studies that will be reviewed at a later date. Five new trials were identified that met the inclusion criteria, giving a total of 10 trials in the review (Figure 1).
1.
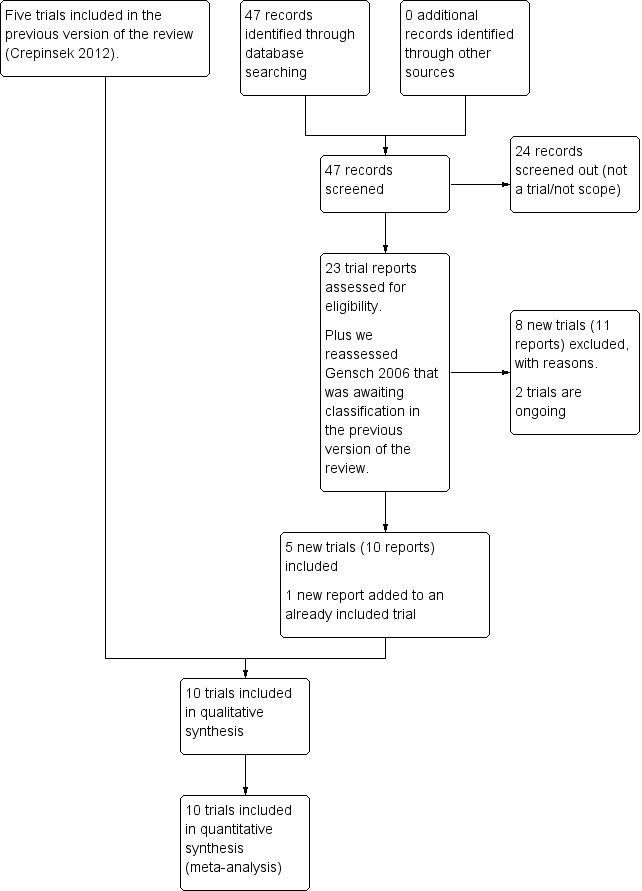
Study flow diagram.
Included studies
We identified 10 trials (3034 women) that met the inclusion criteria (see Characteristics of included studies). The trials were conducted between 1999 and 2018.
Design
All of the included studies were randomised controlled trials (RCTs). One trial had four arms (Livingstone 1999) and the others were all two‐arm trials.
Setting
All of the studies were conducted in middle or high‐income countries. Two trials were conducted in each of the following countries, Australia (Amir 2004; Bond 2018), Spain (Fernandez 2016; Hurtado 2017) and China (Fang 2016; He 2015). The further studies were conducted in Brazil (De Oliveira 2006), Canada (Livingstone 1999), South Africa (Sebitloane 2008) and Sweden (Svensson 2004).
Sample size
The sample size ranged from 10 women (Amir 2004) to 639 women (Bond 2018). The mean number of women randomised in each trial was 304.
Population
The participants in the trials were women with uncomplicated pregnancies and healthy, full‐term infants.
Six trials recruited women who were asymptomatic (Bond 2018; De Oliveira 2006; He 2015; Hurtado 2017; Sebitloane 2008; Svensson 2004)
In two trials, all the women had sore or cracked nipples (Amir 2004; Livingstone 1999). In one trial, all the women had low milk supply (Fang 2016) and in another all the women had a history of mastitis in previous pregnancies (Fernandez 2016).
Interventions
One trial delivered its intervention before the women gave birth (Fernandez 2016). In one trial, the intervention was delivered while the women were in active labour (Sebitloane 2008). In five trials, the interventions began in the immediate postpartum period: before discharge from hospital (De Oliveira 2006; Fang 2016; He 2015); or during the first week postpartum (Hurtado 2017; Svensson 2004). Three trials did not state how long after giving birth they began their interventions (Amir 2004; Bond 2018; Livingstone 1999).
Of the 10 trials that met the pre‐stated inclusion criteria in this review, three trials compared probiotics to placebo (Bond 2018; Fernandez 2016; Hurtado 2017). One trial evaluated breastfeeding education (De Oliveira 2006). One trial compared basic breastfeeding advice in combination with topical treatments ‐ this trial also included an antibiotic arm to the trial (Livingstone 1999). The four arms of the Livingstone 1999 trial were: optimal breastfeeding advice (n = 23); topical 2% mupirocin ointment applied to the nipples (n = 25); topical fusidic acid ointment applied to the nipples (n = 17); and oral antibiotics ‐ cloxacillin/erythromycin (n = 19).
One trial evaluated hydrothermally processed cereals with anti‐secretory factor‐inducing properties. Anti‐secretory factor is a protein found in most human tissue including the placenta and possibly occurring in milk, which has been shown to have possible anti‐infectious and anti‐inflammatory capabilities (Svensson 2004).
Two other trials investigated the use of antibiotics (Amir 2004; Sebitloane 2008). The trial by He 2015 investigated the effects of breast acupoint massage with early breastfeeding and breastfeeding education, while the trial by Fang 2016 investigated breast massage combined with low frequency pulse treatment.
Outcomes
All 10 trials measured the primary outcome, incidence of mastitis, with one trial also reporting mastitis recurrence.
One study reported sore nipples (De Oliveira 2006) and two studies reported breast engorgement (De Oliveira 2006; He 2015).
Breastfeeding outcomes were addressed in two trials. Exclusive breastfeeding was reported in two trials (Fang 2016; He 2015) and any breastfeeding, breastfeeding problems or perceived low milk supply were each addressed in a single trial (Fang 2016).
The mastitis study by Amir 2004 was aborted at 12 months due to poor intervention compliance and lack of eligible participants. The study by Bond 2018 measured the incidence of mastitis up to eight weeks following birth. However, no data were available due to restrictions placed on the authors by the probiotics providers.
De Oliveira 2006 reported the measures of exclusive breastfeeding rates and breastfeeding‐related problems. They also reported the measures of mastitis, sore nipples and engorgement. The study by Fang 2016 reported a hypogalactia degree score, postpartum lactation initiating, milk volume effect and mastitis morbidity. Fernandez 2016 reported the occurrence of mastitis during the first three months after delivery. They also collected data on breast pain from women who had mastitis. Adverse events and side effects related to the ingestion of the probiotic were also reported. He 2015 reported the Initial time of lactation and the amount of lactation, breastfeeding rate after 42 days, breast comfort, swelling, incidence of mastitis after 42 days, and nursing satisfaction.
Hurtado 2017 reported the incidence of clinical mastitis during the first four months of breastfeeding. Mastitis was defined as at least two out of the three breast symptoms (pain, redness, and lump) and at least one of fever or flu‐like symptoms (shivering, hot sweats, or aches). Secondary outcomes ‐ the microbiota of breast milk at the end of the intervention and in mastitis events, monthly questionnaire on evaluation of breast pain, and inflammatory markers in breast milk at the end of intervention and in mastitis events ‐ were also measured.
Livingstone 1999 measured nipple symptoms, breast symptoms and mastitis, while Sebitloane 2008 reported postpartum infections and Svensson 2004 reported the incidence of mastitis.
Sources of funding
Nine trials received state and/or academic institution funding (Amir 2004; Bond 2018; Fang 2016; Fernandez 2016; He 2015; Hurtado 2017; Livingstone 1999; Sebitloane 2008; Svensson 2004).
One trial also received funding from intervention manufacturers (Amir 2004).
One trial did not mention any sources of funding (De Oliveira 2006).
Declarations of interest
One trial declared that the authors had no conflicts of interest but that the intervention and comparator were donated by a private company, which would have no direct influence on the conduct, design or implementation of the trial and that there were no commercial benefits for the trials authors (Bond 2018).
One trial made a declaration of interest because several of its authors were employees of the manufacturer of the probiotic intervention (Hurtado 2017).
Three trials reported that the authors had no conflicts of interest or declarations of interest to declare (Amir 2004; De Oliveira 2006; Fernandez 2016).
Five trials did not mention declarations of interest (Fang 2016; He 2015; Livingstone 1999; Sebitloane 2008; Svensson 2004).
Excluded studies
SeeCharacteristics of excluded studies.
We excluded 41 studies, mostly because the trials in question were not aimed at preventing mastitis, or were not RCTs. Trials were also excluded it they were reporting the treatment of mastitis rather than the prevention of mastitis.
Studies were excluded from the review for the following reasons:
Seven studies were not randomised trials (Blaikeley 1953; Evans 1995; Lawlor‐Smith 1997; Meah 2001; Neifert 1990; Nicholson 1993; Schurz 1978).
Twelve trials were about various aspects of breastfeeding not related to preventing mastitis (Bystrova 2007; Feng 2019; Filteau 1999; Forster 2004; Frank 1987; Gunn 1998; Homer 2001; Kramer 2001; Lumley 2006; Mastromarino 2015; Swift 2003; Waldenstrom 1994).
Six trials were about preventing or treating breast engorgement (McLachlan 1991; NCT03230760; Nikodem 1993; Phillips 1975; Roberts 1995; Roberts 1998).
One trial investigated prevention of subclinical mastitis (Gomo 2003).
One trial investigated antibiotic prophylaxis for caesarean section (Luttkus 1997).
Two trials were about the relationship between HIV and mastitis (ISRCTN98567612; Zadrozny 2017).
Seven trials were about the prevention or treatment of breast or nipple pain or nipple damage (Centuori 1999; Dennis 2012; Gensch 2006; Harvey [date of communication?]; Herd 1986; Maldonado‐Lobon 2015; Nicholson 1985.
Five trials were about the treatment not the prevention of mastitis (Crepinsek 2008, Hager 1996; Kvist 2004; Kvist 2007; Thomsen 1984).
Risk of bias in included studies
Figure 2 and Figure 3 summaries the risk of bias in the included studies.
2.

Summary of risk of bias assessment of included studies.
3.

Risk of bias judgements
Allocation
Eight of the trials reported adequate sequence generation methods and we judged them at low risk of selection bias for random sequence generation (Amir 2004; Bond 2018; De Oliveira 2006; Fernandez 2016; Hurtado 2017; Livingstone 1999; Sebitloane 2008; Svensson 2004). The remaining two did not provide sufficient information about their randomisation processes so we judged them as having unclear risk of bias (Fang 2016; He 2015).
Four trials also reported adequate methods for concealing allocation so we judged them as having low risk of bias for allocation concealment (Amir 2004; Bond 2018; Livingstone 1999; Sebitloane 2008); the remaining trials were judged to have an unclear risk of bias in regard to allocation concealment because of a lack of information reported in the published papers.
Blinding
Six trials were judged to be at low risk of performance bias because they used blinding for participants and caregivers (Amir 2004; Bond 2018; Fernandez 2016; Hurtado 2017; Sebitloane 2008; Svensson 2004).
Due to the nature of the interventions in three trials (De Oliveira 2006; Fang 2016; He 2015), it was not possible to use blinding for participants or caregivers. It is unclear if lack of blinding could have affected outcomes, therefore these trials were judged as having unclear risk of performance bias.
One trial (Livingstone 1999) explicitly stated that it did not use blinding for participants or caregivers and, therefore, was judged to be at high risk of performance bias.
Six trials were judged to be low risk of detection bias because investigators and outcome assessors were blinded (Bond 2018; De Oliveira 2006; Fernandez 2016; Hurtado 2017; Sebitloane 2008; Svensson 2004).
In four trials, the risk of detection bias was unclear because the authors did not report any details about blinding of outcome assessors (Amir 2004; Fang 2016; He 2015; Livingstone 1999).
Incomplete outcome data
We judged nine studies to have a low risk of attrition bias because they either reported complete data on all participants or had non‐differential attrition (Amir 2004; De Oliveira 2006; Fang 2016; Fernandez 2016; He 2015; Hurtado 2017; Livingstone 1999; Sebitloane 2008; Svensson 2004). There were no results available for the Bond 2018 trial, therefore, the risk of attrition bias was unclear.
Selective reporting
Most trials were judged to be at low risk of reporting bias because they appeared to report all outcomes that were prespecified. However, the results of the Bond 2018 trial were unavailable due to restrictions imposed by the probiotics provider, therefore, we judged this trial to be at high risk of reporting bias. Another trial (Fernandez 2016) was also judged to be at high risk of reporting bias because they did not report outcomes according to the full length of follow‐up that was prespecified in the trial protocol. Another trial was judged to be at high risk of reporting bias because it did not report all outcomes in full (Hurtado 2017).
Other potential sources of bias
We judged eight trials to be at low risk of other bias because there was no suggestion of any other sources of bias (Amir 2004; De Oliveira 2006; Fang 2016; Fernandez 2016; He 2015; Livingstone 1999; Sebitloane 2008; Svensson 2004).
The risk of other bias was unclear in one trial (Bond 2018) because the study authors could not provide a full paper or any results data due to restrictions imposed by the intervention manufacturer; therefore, we did not have sufficient information to judge whether there were any other sources of bias. We judged another trial to be unclear in terms of risk of other bias because we did not have sufficient information to assess whether the authors' paid work for the patent owner of the intervention could have any influence on the results (Hurtado 2017).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9
Summary of findings 1. Probiotics compared to placebo for preventing mastitis after childbirth.
| Probiotics compared to placebo for preventing mastitis after childbirth | ||||||
| Patient or population: postpartum breastfeeding women Setting: obstetric outpatient clinic Intervention: probiotics Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with probiotics | |||||
| Incidence of mastitis within 6 months postpartum | Study population | RR 0.51 (0.35 to 0.75) | 399 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 | ||
| 293 per 1000 | 149 per 1000 (102 to 220) | |||||
| Recurrence of mastitis within 12 months postpartum | Not reported | |||||
| Breast abscess within 6 months postpartum | Not reported | |||||
| Nipple damage within 6 months postpartum | Study population | RR 0.33 (0.11 to 1.01) |
424 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 59 per 1000 | 19 per 1000 (6 to 59) |
|||||
| Duration of any breastfeeding | Not reported | |||||
| Breast pain | Study population | RR 0.81 (0.64 to 1.01) | 335 (2 RCTs) | ⊕⊕⊝⊝ LOW3 4 | ||
| 522 per 1000 | 423 per 1000 (334 to 527) | |||||
| Number of women with adverse events | In one trial no women in either the probiotics group or the placebo group experienced adverse events | 108 (1 RCT) | ⊕⊕⊝⊝ LOW 5 | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded two levels due to risk of bias: unclear allocation concealment, high risk of reporting bias, and missing data
2 Downgraded one level for indirectness: measured as number of women using topical treatment for nipple cracks
3 Downgraded one level due to risk of bias: unclear allocation concealment and high risk of selective reporting
4 Downgraded one level for imprecision: 95% confidence interval is consistent with possible benefit and possible harm
5 Downgraded two levels for imprecision: few participants and no events
Summary of findings 2. Antibiotics compared to usual care or placebo for preventing mastitis after childbirth.
| Antibiotics compared to usual care or placebo for preventing mastitis after childbirth | ||||||
| Patient or population: postpartum breastfeeding women Setting: obstetric outpatient clinic Intervention: antibiotics Comparison: usual care or placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care or placebo | Risk with antibiotics | |||||
| Incidence of mastitis within 6 months postpartum | Study population | RR 0.37 (0.10 to 1.34) | 429 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 | ||
| 37 per 1000 | 14 per 1000 (4 to 49) | |||||
| Recurrence of mastitis within 12 months postpartum | Not reported | |||||
| Breast abscess within 6 months postpartum | Not reported | |||||
| Nipple damage within 6 months postpartum | Not reported | |||||
| Duration of any breastfeeding | Not reported | |||||
| Breast pain | Not reported | |||||
| Number of women with adverse effects | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded two levels due to imprecision: low event rate and wide 95% CIs indicating the true effect may be either appreciable benefit or harm
Summary of findings 3. Antibiotics compared to topical treatments for preventing mastitis after childbirth.
| Antibiotics compared to topical treatments for preventing mastitis after childbirth | ||||||
| Patient or population: postpartum breastfeeding women Setting: obstetric outpatient clinic Intervention: antibiotics Comparison: topical treatments | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with topical treatments | Risk with antibiotics | |||||
| Incidence of mastitis within 6 months postpartum ‐ Antibiotics versus fusidic acid ointment | Study population | RR 0.22 (0.03 to 1.81) | 36 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 235 per 1000 | 52 per 1000 (7 to 426) | |||||
| Incidence of mastitis within 6 months postpartum ‐ Antibiotics versus mupirocin ointment | Study population | RR 0.44 (0.05 to 3.89) | 44 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 120 per 1000 | 53 per 1000 (6 to 467) | |||||
| Recurrence of mastitis within 12 months postpartum | Not reported | |||||
| Breast abscess within 6 months postpartum | Not reported | |||||
| Nipple damage within 6 months postpartum | Not reported | |||||
| Duration of any breastfeeding | Not reported | |||||
| Breast pain | Not reported | |||||
| Number of women with adverse effects | Not reported | |||||
1 Downgraded two levels due to imprecision: single small trial with wide 95% CIs, indicating that the true effect may be either appreciable benefit or harm
Summary of findings 4. Topical treatments compared to breastfeeding advice for preventing mastitis after childbirth.
| Topical treatments compared to breastfeeding advice for preventing mastitis after childbirth | ||||||
| Patient or population: postpartum breastfeeding women Setting: obstetric outpatient clinic Intervention: topical treatments Comparison: breastfeeding advice | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with breastfeeding advice | Risk with topical treatments | |||||
| Incidence of mastitis within 6 months postpartum ‐ Fusidic acid ointment versus breastfeeding advice | Study population | RR 0.77 (0.27 to 2.22) | 40 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 304 per 1000 | 234 per 1000 (82 to 676) | |||||
| Incidence of mastitis within 6 months postpartum ‐ Mupirocin ointment versus breastfeeding advice | Study population | RR 0.39 (0.12 to 1.35) | 48 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 304 per 1000 | 119 per 1000 (37 to 411) | |||||
| Recurrence of mastitis within 12 months postpartum | Not reported | |||||
| Breast abscess within 6 months postpartum | Not reported | |||||
| Nipple damage within 6 months postpartum | Not reported | |||||
| Duration of any breastfeeding | Not reported | |||||
| Breast pain | Not reported | |||||
| Number of women with adverse events | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded two levels due to imprecision: single small trial with wide 95% CIs, indicating that the true effect may be either appreciable benefit or harm
Summary of findings 5. Mupirocin ointment compared to fusidic acid ointment for preventing mastitis after childbirth.
| Mupirocin ointment compared to fusidic acid ointment for preventing mastitis after childbirth | ||||||
| Patient or population: postpartum breastfeeding women Setting: obstetric outpatient clinic Intervention: Mupirocin ointment Comparison: fusidic acid ointment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with fusidic acid ointment | Risk with Mupirocin ointment | |||||
| Incidence of mastitis within 6 months postpartum | Study population | RR 0.51 (0.13 to 2.00) | 42 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 235 per 1000 | 120 per 1000 (31 to 471) | |||||
| Recurrence of mastitis within 12 months postpartum | Not reported | |||||
| Breast abscess within 6 months postpartum | Not reported | |||||
| Nipple damage within 6 months postpartum | Not reported | |||||
| Duration of any breastfeeding | Not reported | |||||
| Breast pain | Not reported | |||||
| Number of women with adverse events | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded two levels due to imprecision: single small trial with wide 95% CIs, indicating that the true effect may be either appreciable benefit or harm
Summary of findings 6. Specialist breastfeeding education compared to usual care for preventing mastitis after childbirth.
| Specialist breastfeeding education compared to usual care for preventing mastitis after childbirth | ||||||
| Patient or population: postpartum breastfeeding women Setting: obstetric outpatient clinic Intervention: specialist breastfeeding education Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with specialist breastfeeding education | |||||
| Incidence of mastitis within 6 months postpartum | Study population | RR 0.93 (0.17 to 4.95) | 203 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | This outcome was measured at 30 days postpartum | |
| 30 per 1000 | 28 per 1000 (5 to 150) | |||||
| Recurrence of mastitis within 12 months postpartum | Not reported | |||||
| Breast abscess within 6 months postpartum | Not reported | |||||
| Nipple damage within 6 months postpartum | Not reported | |||||
| Duration of any breastfeeding | Not reported | |||||
| Breast pain (sore nipples) | Study population | RR 0.93 (0.36 to 2.37) | 203 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 91 per 1000 | 85 per 1000 (33 to 215) | |||||
| Number of women with adverse events | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded two levels due to imprecision: single small trial with wide 95% CIs, indicating that the true effect may be either appreciable benefit or harm
Summary of findings 7. Hydrothermally processed cereal with anti‐secretory factor‐inducing properties versus standard cereal standard cereal for preventing mastitis after childbirth.
| Anti‐secretory factor‐inducing cereal compared to standard cereal for preventing mastitis after childbirth | ||||||
| Patient or population: postpartum breastfeeding women Setting: obstetric outpatient clinic Intervention: anti‐secretory factor‐inducing cereal Comparison: standard cereal | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard cereal | Risk with anti‐secretory factor‐inducing cereal | |||||
| Incidence of mastitis within 6 months postpartum | Study population | RR 0.24 (0.03 to 1.72) | 29 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 353 per 1000 | 85 per 1000 (11 to 607) | |||||
| Recurrence of mastitis within 12 months postpartum | Study population | RR 0.39 (0.03 to 4.57) | 7 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 667 per 1000 | 260 per 1000 (20 to 1000) | |||||
| Breast abscess within 6 months postpartum | Not reported | |||||
| Nipple damage within 6 months postpartum | Not reported | |||||
| Duration of any breastfeeding | Not reported | |||||
| Breast pain | Not reported | |||||
| Number of women with adverse events | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded two levels for imprecision: few participants and wide 95% CIs indicating the true effect may be either appreciable benefit or harm
Summary of findings 8. Acupoint massage compared to routine care for preventing mastitis after childbirth.
| Acupoint massage compared to routine care for preventing mastitis after childbirth | ||||||
|
Patient or population: postpartum breastfeeding women Setting: obstetric outpatient clinic Intervention: acupoint massage Comparison: routine care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with routine care | Risk with acupoint massage | |||||
| Incidence of mastitis within 6 months postpartum) | Study population | RR 0.38 (0.19 to 0.78) | 400 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 130 per 1000 | 49 per 1000 (25 to 101) | |||||
| Recurrence of mastitis within 12 months postpartum | Not reported | |||||
| Breast abscess within 6 months postpartum | Not reported | |||||
| Nipple damage within 6 months postpartum | Not reported | |||||
| Duration of any breastfeeding | Not reported | |||||
| Breast pain | Study population | RR 0.13 (0.07 to 0.23) | 400 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 400 per 1000 | 52 per 1000 (28 to 92) | |||||
| Number of women with adverse events | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to unclear risk of bias across most domains
Summary of findings 9. Breast massage and low frequency pulse treatment compared to routine care for preventing mastitis after childbirth.
| Breast message and low frequency pulse treatment compared to routine care for preventing mastitis after childbirth | ||||||
| Patient or population: postpartum breastfeeding women Setting: obstetric outpatient clinic Intervention: breast message and low frequency pulse treatment Comparison: routine care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with routine care | Risk with breast message and low frequency pulse treatment | |||||
| Incidence of mastitis within 6 months postpartum | Study population | RR 0.03 (0.00 to 0.21) | 300 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 233 per 1000 | 7 per 1000 (0 to 49) | |||||
| Recurrence of mastitis within 12 months postpartum | Not reported | |||||
| Breast abscess within 6 months postpartum | Not reported | |||||
| Nipple damage within 6 months postpartum | Not reported | |||||
| Duration of any breastfeeding | Not reported for either the duration of exclusive or any breastfeeding | |||||
| Breast pain | Not reported | |||||
| Number of women with adverse events | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to unclear risk of selection, performance and detection bias
2 Downgraded one level for imprecision: few events
Comparison one: probiotics versus placebo
Three trials compared probiotics to placebo (Bond 2018; Fernandez 2016; Hurtado 2017). We could not include data from the Bond 2018 trial (639 women) because the probiotics provider would not allow the results to be made public.
Primary outcomes
Incidence of mastitis within six months postpartum
Probiotics may reduce the risk of mastitis more than placebo (risk ratio (RR) 0.51, 95% confidence interval (CI) 0.35 to 0.75; 399 women; studies = 2; I2 = 0%; Analysis 1.1; Table 1; low‐certainty evidence).
1.1. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 1: Incidence of mastitis within 6 months postpartum
Recurrence of mastitis within 12 months postpartum
Not reported.
Secondary outcomes
Breast abscess within six months postpartum
Not reported.
Nipple damage within six months postpartum
One study reported the number of women using topical treatment for nipple cracks. We are uncertain if there is any effect on the risk of nipple damage with probiotics compared with placebo (RR 0.33, 95% CI 0.11 to 1.01; participants = 424; studies = 1; very low‐certainty evidence; Table 1; Analysis 1.2; Hurtado 2017).
1.2. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 2: Nipple damage within 6 months postpartum
Duration of exclusive breastfeeding
Not reported.
Duration of any breastfeeding
Not reported.
Breast pain
It is uncertain if probiotics reduce the risk of breast pain because the certainty of evidence is very low (RR 0.81, 95% CI 0.64 to 1.01; 335 women; studies = 2; I2 = 50%) (Table 1; Analysis 1.3; Fernandez 2016; Hurtado 2017).
1.3. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 3: Breast pain
Since there was some suggestion of statistical heterogeneity in the analysis, we also undertook random‐effects meta‐analysis but the effect estimate did not change substantially (RR 0.86, 95% CI 0.68, 1.09).
Breast engorgement
Not reported.
Women's perception of milk supply
Not reported.
Maternal breastfeeding satisfaction
Not reported.
Maternal breastfeeding confidence
Not reported.
Cessation of breastfeeding within six months postpartum
Not reported.
Number of women with adverse events
One study reported no women in either the probiotics group or the placebo group experienced adverse events (Fernandez 2016).
Comparison two: antibiotics versus usual care or placebo
Two trials compared antibiotics with placebo (Amir 2004 used flucloxacillin; and Sebitloane 2008 used intravenous cefoxitin in HIV‐infected women). Another trial (Livingstone 1999) compared oral cloxacillin/erythromycin with breastfeeding advice alone.
Primary outcomes
Incidence of mastitis within six months postpartum
There may be little or no difference between antibiotics and placebo or breastfeeding advice in terms of risk of mastitis although the CIs were wide and are consistent with both important benefit and harm (RR 0.37, 95% CI 0.10 to 1.34; 3 studies; 429 women; Analysis 3.1; Table 2; low‐certainty evidence).
3.1. Analysis.

Comparison 3: Antibiotics versus placebo or usual care, Outcome 1: Incidence of mastitis within 6 months postpartum
Recurrence of mastitis within 12 months postpartum
Not reported.
Secondary outcomes
Breast abscess within six months postpartum
Not reported.
Nipple damage within six months postpartum
Not reported.
Duration of breastfeeding
Not reported.
Duration of any breastfeeding
Not reported.
Breast pain
Five women in one trial (Livingstone 1999) had "severe sore nipples with deep, radiating, burning breast pain and episodic vasospasms of their nipples unrelated to immediate suckling". However, the trial did not report which intervention groups these women were assigned to.
Breast engorgement
Not reported.
Women's perception of milk supply
Not reported.
Duration of exclusive breastfeeding
Not reported.
Duration of any breastfeeding
Not reported.
Maternal breastfeeding satisfaction
Not reported.
Maternal breastfeeding confidence
Not reported.
Cessation of breastfeeding within six months postpartum
Not reported.
Number of women with adverse events
Not reported.
Comparison three: antibiotics versus topical treatments
One trial (Livingstone 1999) compared oral cloxacillin/erythromycin (19 women) with topical 2% mupirocin ointment (25 women) and with topical fusidic acid (17 women).
Primary outcomes
Incidence of mastitis within six months postpartum
It is uncertain whether antibiotics reduce the risk of mastitis compared to either fusidic acid ointment (RR 0.22, 95% CI 0.03 to 1.81; 36 women; studies = 1) or mupirocin ointment (RR 0.44, 95% CI 0.05 to 3.89; 44 women; studies = 1) because the certainty of evidence is low and the CIs are wide, indicating that the true effect may be either appreciable harm or appreciable benefit (Analysis 2.1; Table 3).
2.1. Analysis.

Comparison 2: Antibiotics versus topical treatments, Outcome 1: Incidence of mastitis within 6 months postpartum
Recurrence of mastitis within 12 months postpartum
Not reported.
Secondary outcomes
Not reported.
Comparison four: topical treatments versus usual care
One trial (Livingstone 1999) compared topical treatments (topical 2% mupirocin (25 women) and topical fusidic acid (17 women) to usual care in the form of optimal breastfeeding advice (23 women).
Primary outcomes
Incidence of mastitis within six months postpartum
It is uncertain whether either fusidic acid ointment (RR 0.77, 95% CI 0.27 to 2.22; 40 women; studies = 1) or mupirocin ointment (RR 0.39, 95% CI 0.12 to 1.35; 48 women; studies = 1) reduce the risk of mastitis more than optimal breastfeeding advice because the certainty of evidence is low and the CIs were wide, indicating that the true effect may be either appreciable harm or appreciable benefit (Table 4; Analysis 4.1).
4.1. Analysis.

Comparison 4: Topical treatments versus breastfeeding advice, Outcome 1: Incidence of mastitis within 6 months postpartum
Secondary outcomes
Not reported.
Comparison five: mupirocin ointment versus fusidic acid ointment
One trial (Livingstone 1999) compared two different topical treatments to each other: 2% mupirocin ointment (25 women) and fusidic acid (17 women).
Primary outcomes
Incidence of mastitis within six months postpartum
It is uncertain if there is any difference in risk of mastitis between fusidic acid ointment and mupirocin ointment because the certainty of evidence is low and the CIs were wide, indicating that the true effect may be either appreciable harm or appreciable benefit (RR 0.51, 95% CI 0.13 to 2.00; 42 women; studies = 1; Table 5; Analysis 5.1).
5.1. Analysis.

Comparison 5: Mupirocin ointment versus fusidic acid ointment, Outcome 1: Incidence of mastitis within 6 months postpartum
Recurrence of mastitis within 12 months postpartum
Not reported.
Secondary outcomes
Not reported.
Comparison six: specialist breastfeeding education versus usual care
One trial (De Oliveira 2006) compared specialist breastfeeding education (74 women) with usual care (137 women).
Primary outcomes
Incidence of mastitis within six months postpartum
It is uncertain if there is a difference in the risk of mastitis comparing specialist breastfeeding education with usual care because the certainty of evidence is low and the CIs were consistent with both appreciable benefit and harm (RR 0.93, 95% CI 0.17 to 4.95; 203 women; studies = 1; Table 6; Analysis 6.1; De Oliveira 2006).
6.1. Analysis.

Comparison 6: Specialist breastfeeding education versus usual care, Outcome 1: Incidence of mastitis within 6 months postpartum
Recurrence of mastitis within 12 months postpartum
Not reported.
Secondary outcomes
Breast abscess within six months postpartum
Not reported.
Nipple damage within six months postpartum
Not reported.
Duration of exclusive breastfeeding
No trials reported duration of exclusive breastfeeding but one trial comparing specialist breastfeeding education with usual care (De Oliveira 2006) reported the rate of exclusive breastfeeding. At seven days' follow‐up, 60/73 in the breastfeeding education group were exclusively breastfeeding, compared to 109/137 in the usual care group (RR 1.03, 95% CI 0.90 to 1.18; 210 women; studies = 1). At 30 days' follow‐up the numbers of women exclusively breastfeeding were 38/71 and 80/132 (RR 0.88, 95% CI 0.68 to 1.14; 203 women; studies = 1) (Analysis 6.4).
6.4. Analysis.

Comparison 6: Specialist breastfeeding education versus usual care, Outcome 4: Exclusive breastfeeding
Duration of any breastfeeding
Not reported.
Breast pain
It is uncertain if there is a difference in the risk of sore nipples comparing specialist breastfeeding education with usual care because the certainty of evidence is low and the CIs were consistent with both appreciable benefit and harm (RR 0.93, 95% CI 0.36 to 2.37; 203 women; studies = 1; (Table 6; Analysis 6.2; De Oliveira 2006; 203 women).
6.2. Analysis.

Comparison 6: Specialist breastfeeding education versus usual care, Outcome 2: Breast pain (sore nipples)
Breast engorgement
One trial (De Oliveira 2006) found little or no difference between breastfeeding education and usual care in the numbers of women with breast engorgement (RR at 30 days' follow‐up 1.04, 95% CI 0.73 to 1.49; 203 women; studies = 1; Analysis 6.3).
6.3. Analysis.

Comparison 6: Specialist breastfeeding education versus usual care, Outcome 3: Breast engorgement
Women's perception of milk supply
Not reported.
Maternal breastfeeding satisfaction
Not reported.
Maternal breastfeeding confidence
Not reported.
Cessation of breastfeeding within six months postpartum
Not reported.
Number of women with adverse events
Not reported.
Comparison seven: hydrothermally processed cereals with anti‐secretory factor‐inducing properties versus standard cereal
One trial investigated cereal intended to induce production of anti‐secretory factor (AF) compared with standard cereal (Svensson 2004).
Primary outcomes
Incidence of mastitis within six months postpartum
In a trial of 29 women comparing consumption of AF‐inducing cereal with standard cereal (Svensson 2004), 1/12 in the intervention group and 6/17 in the standard cereal group had mastitis (RR 0.24, 95% CI 0.03 to 1.72; 29 women; studies = 1; Analysis 7.1). It is uncertain if there is any difference in the risk of mastitis because the certainty of evidence is low and the CIs were consistent with both appreciable harm and benefit (Table 7).
7.1. Analysis.

Comparison 7: Hydrothermally processed cereal with anti‐secretory factor‐inducing properties versus standard cereal, Outcome 1: Incidence of mastitis within 6 months postpartum
Recurrence of mastitis within 12 months postpartum
One trial (Svensson 2004) reported recurrence of mastitis within five weeks. Of the women who had mastitis, there was no recurrence in the AF‐inducing cereal group and recurrence in three of the six women in the standard cereal group (Analysis 7.2). It is uncertain if there is any difference in the risk of recurrence of mastitis because the certainty of evidence is low and the CIs were consistent with both appreciable harm and benefit (Table 7).
7.2. Analysis.

Comparison 7: Hydrothermally processed cereal with anti‐secretory factor‐inducing properties versus standard cereal, Outcome 2: Recurrence of mastitis within 12 months postpartum
Secondary outcomes
None of the secondary outcomes were reported.
Comparison eight: acupoint massage versus routine care
One trial investigated acupoint massage compared with routine care (He 2015).
Primary outcomes
Incidence of mastitis within six months postpartum
Acupoint massage probably reduces the risk of mastitis compared with routine care (RR 0.38, 95% CI 0.19 to 0.78; 400 women; studies = 1; Table 8; moderate‐certainty evidence; Analysis 8.1; He 2015).
8.1. Analysis.

Comparison 8: Acupoint massage versus routine care, Outcome 1: Incidence of mastitis within 6 months postpartum)
Recurrence of mastitis within 12 months postpartum
Not reported.
Secondary outcomes
Breast abscess within six months postpartum
Not reported.
Nipple damage within six months postpartum
Not reported.
Duration of breastfeeding
No trials measured duration of exclusive breastfeeding but, in one trial, the number of women exclusively breastfeeding at 42 days postpartum was 152/200 in the acupoint massage group compared to 80/200 in the usual care group (RR 1.90, 95% CI 1.58 to 2.29; 400 women; studies = 1; Analysis 8.2; He 2015).
8.2. Analysis.

Comparison 8: Acupoint massage versus routine care, Outcome 2: Exclusive breastfeeding (at 42 days postpartum)
Duration of any breastfeeding
Not reported.
Breast pain
Acupoint massage probably reduces the risk of severe breast pain compared to usual care (RR 0.13, 95% CI 0.07 to 0.23; 400 women; studies = 1; He 2015; Analysis 8.3; Table 8; moderate‐certainty evidence).
8.3. Analysis.

Comparison 8: Acupoint massage versus routine care, Outcome 3: Breast pain
Breast engorgement
In one trial, fewer women had breast engorgement in the acupoint massage group compared to the usual care group (RR 0.49, 95% CI 0.37 to 0.65; 400 women; studies = 1; Analysis 8.4; He 2015).
8.4. Analysis.

Comparison 8: Acupoint massage versus routine care, Outcome 4: Breast engorgement
Women's perception of milk supply
In one trial, more women in the acupoint massage group rated their milk supply as 'moderate' or 'a lot' compared with the usual care group (RR 1.26, 95% CI 1.13 to 1.40; 400 women; studies = 1; Analysis 8.5; He 2015).
8.5. Analysis.

Comparison 8: Acupoint massage versus routine care, Outcome 5: Women's perception of milk supply (moderate or better)
Maternal breastfeeding satisfaction
Not reported.
Maternal breastfeeding confidence
Not reported.
Cessation of breastfeeding within six months postpartum
Not reported.
Number of women with adverse events
Not reported.
Comparison nine: breast massage and low frequency pulse treatment versus routine care
One trial investigated breast massage and low frequency pulse treatment compared to routine care (Fang 2016).
Primary outcomes
Incidence of mastitis within six months postpartum
Breast message and low frequency pulse treatment may reduce the risk of mastitis compared with routine care (RR 0.03, 95% CI 0.00 to 0.21; 300 women; studies = 1; Table 9; low‐certainty evidence; Analysis 9.1; Fang 2016).
9.1. Analysis.

Comparison 9: Breast massage and low frequency pulse treatment versus routine care, Outcome 1: Incidence of mastitis within 6 months postpartum
Recurrence of mastitis within 12 months postpartum
Not reported.
Secondary outcomes
Breast abscess within six months postpartum
Not reported.
Nipple damage within six months postpartum
Not reported.
Duration of breastfeeding
No trials measured duration of exclusive breastfeeding but one trial reported more women were breastfeeding exclusively at the end of treatment with breast massage and low frequency pulse treatment compared with routine care group (RR 2.65, 95% CI 1.74 to 4.05; 300 women; studies = 1; Analysis 9.2; Fang 2016).
9.2. Analysis.

Comparison 9: Breast massage and low frequency pulse treatment versus routine care, Outcome 2: Exclusive breastfeeding
Duration of any breastfeeding
No trials measured duration of any breastfeeding but one trial reported more women in the breast message and low frequency pulse treatment were able to breastfeed at end of treatment compared to routine care (RR 1.83, 95% CI 1.57 to 2.12; 300 women; studies = 1; Analysis 9.3; Fang 2016).
9.3. Analysis.

Comparison 9: Breast massage and low frequency pulse treatment versus routine care, Outcome 3: Any breastfeeding
Breast pain
Not reported.
Breast engorgement
Not reported.
Women's perception of milk supply
Women in the breast massage and low frequency pulse treatment group had a perception of greater milk supply than those in the routine care group (MD ‐5.55, 95% CI ‐5.90 to ‐5.20; 300 women; studies = 1; Analysis 9.4; Fang 2016).
9.4. Analysis.

Comparison 9: Breast massage and low frequency pulse treatment versus routine care, Outcome 4: Women's perception of milk supply (0‐14 scale; higher score = less milk supply)
Maternal breastfeeding satisfaction
Not reported.
Maternal breastfeeding confidence
Not reported.
Cessation of breastfeeding within six months postpartum
Fewer women in the breast message and low frequency pulse treatment group stopped breastfeeding than in the routine care group (RR 0.03, 95% CI 0.01 to 0.12; 300 women; studies = 1; Analysis 9.5; Fang 2016). The length of follow‐up was not reported.
9.5. Analysis.

Comparison 9: Breast massage and low frequency pulse treatment versus routine care, Outcome 5: Cessation of breastfeeding (at end of treatment period)
Number of women with adverse events
Not reported.
Discussion
Summary of main results
This review included 10 studies (3034 women), all of which measured incidence of mastitis; one study reported recurrence of mastitis and some studies reported breast pain. We found very little evidence relating to recurrence of mastitis, breast abscess, nipple damage, duration of breastfeeding and adverse events.
Probiotics versus placebo
Three trials compared probiotics with placebo, however we were unable to include the data from the Bond 2018 trial (639 women) because the probiotics provider would not allow the results to be made public. When evaluating the incidence of mastitis within six months postpartum, findings suggest that probiotics may reduce the risk of mastitis more than placebo. Findings suggest that it is uncertain if probiotics reduce the risk of breast pain or nipple damage due to the very low certainty of evidence. The evidence relating to adverse events with probiotics compared with placebo is low‐certainty; only a single trial reported on this outcome and no women were reported to experience any adverse events (Table 1).
Antibiotics versus usual care or placebo
Two trials compared antibiotics with placebo, with one trial using flucloxacillin and the other intravenous cefoxitin. A third trial compared oral cloxacillin/erythromycin with breastfeeding advice alone. Low‐certainty evidence suggests that there may be little to no difference between antibiotics and placebo or breastfeeding advice in terms of risk of mastitis. Five women in one of the trials reported "severe sore nipples with deep, radiating, burning breast pain and episodic vasospasms of their nipples unrelated to immediate suckling". However, the trial did not report to which intervention groups these women were assigned, resulting in a degree of uncertainly as to the true effect of the study intervention (Table 2).
Antibiotics versus topical treatments
Oral cloxacillin/erythromycin were compared with topical 2% mupirocin ointment and topical fusidic acid. Findings from this study indicate that it is uncertain whether antibiotics reduce the risk of mastitis compared to either fusidic acid ointment or mupirocin ointment because the certainty of the evidence is low and the confidence intervals were wide, indicating that the true effect may be either appreciable harm or appreciable benefit (Table 3).
Topical treatments versus usual care
It is uncertain whether either topical fusidic acid ointment or mupirocin ointment reduce the risk of mastitis more than optimal breastfeeding advice. The certainty of evidence is low and the confidence intervals were wide, indicating that the true effect may be either appreciable harm or appreciable benefit (Table 4).
Mupirocin ointment versus fusidic acid ointment
It is uncertain if there is any difference in the risk of mastitis with the use of fusidic acid ointment compared to mupirocin ointment because the certainty of evidence is low and the confidence intervals were wide, indicating that the true effect may be either appreciable harm or appreciable benefit (Table 5).
Specialist breastfeeding education versus usual care
It is uncertain if there is a difference in the risk of mastitis or sore nipples comparing specialist breastfeeding education with usual care because the certainty of evidence is low and the confidence intervals were consistent with both appreciable benefit and harm (Table 6).
Anti‐secretory factor‐inducing cereal versus standard cereal
One trial compared the consumption of AF‐inducing cereal with standard cereal. The difference in the risk of mastitis is uncertain as the certainty of the evidence is low with the confidence intervals consistent with both appreciable harm and benefit. It is uncertain if there is any difference in the risk of recurrence of mastitis because again the certainty of evidence is low and the confidence intervals were consistent with both appreciable harm and benefit (Table 7).
Acupoint massage versus routine care
Acupoint massage probably reduces the risk of mastitis and breast pain compared to routine care. No other important outcomes were reported for this comparison (Table 8).
Breast massage and low frequency pulse treatment versus routine care
Breast massage and low frequency pulse treatment may reduce the risk of mastitis compared with routine care (Table 9). No other important outcomes were reported for this comparison.
Overall completeness and applicability of evidence
The studies we identified involved women who are largely representative of our population of interest and therefore we consider that the evidence presented here is applicable to postpartum women who intend to breastfeed. However, the evidence remains uncertain about the effectiveness of interventions designed to prevent mastitis. Moreover, we are aware that this review does not present the complete evidence relating to probiotics for mastitis prevention because we were unable to obtain data from one of the largest studies we identified (Bond 2018) due to restrictions placed on the trial authors by the probiotics providers.
Two studies were stopped prematurely. One study was abandoned after 12 months due to insufficient recruitment of participants, as some women expressed a reluctance to take antibiotics and other women were overwhelmed with challenges they faced as new mothers (Amir 2004). The authors of this study also recognised in retrospect that a feasibility study would have been valuable prior to doing this trial. Livingstone's study also ceased prematurely, due to ethical concerns about the raised incidence of treatment failure and hence symptoms, amongst the participants that did not receive antibiotics (Livingstone 1999).
One may question whether some of the interventions used were robust enough to prevent mastitis. Svennson's study was found to have flaws regarding the consumption and preparation of the anti‐secretory factor in the cereal (intervention) used (Svensson 2004). The study by De Oliveira and colleagues provided participants with one education session with a lactation consultant; future research is warranted to determine whether the intervention may have proven more effective had there been more than a single session and further follow‐up with the lactation consultant (De Oliveira 2006). This review illustrated problems with complicated interventions requiring many steps or stages, affecting adherence. Fang 2016 (n = 300), Fernandez 2016 (n = 110), Hurtado 2017 (n = 217) had larger participant numbers, however, study design, allocation concealment, and blinding of participants are factors to be considered in the robustness of these studies.
The timing of an intervention and data collection need to be relevant to the participants, the condition measured and the outcomes expected. The study by De Oliveira and colleagues collected data measuring the incidence of mastitis at seven and 30 days within the two groups (De Oliveira 2006). De Oliveira's study may have found different results, had the measures been extended to perhaps three to six months (De Oliveira 2006). Moreover, interventions including education and breastfeeding advice may need to be delivered on an ongoing basis, rather than a single consult.
Withdrawal rates
This review included studies from a variety of countries (Australia, China, Spain, South Africa, Brazil and Sweden). Withdrawal rates were reported in eight of the 10 studies. Two of these studies reported no withdrawals, six studies reported the number of withdrawals, and two studies did not report. When a trial loses a high numbers of participants to follow‐up, this has implications for the completeness of data and evidence presented.
Strategies that can be implemented to improve retention include extended consultation time with participants during recruitment participants to explain and reinforce instruction; designing interventions such as tailoring drug regimens to patient lifestyle; frequent follow‐up when initiating or changing treatment regimens; and the use of reminder calls and alerts to keep participants focused.
Quality of the evidence
Generally, the risk of bias relating to randomisation and attrition was low but many of the studies were inadequately powered and therefore did not give precise estimates of effect. In addition to imprecision due to the small numbers of women participating in the trials, the certainty of evidence has also been downgraded for risk of bias with regard to allocation concealment, blinding, and selective reporting and also due to some evidence of indirectness. The certainty of the evidence ranged from low to very‐low certainty.
We cannot be certain about the evidence we identified relating to probiotics for mastitis prevention because the missing data from the Bond 2018 probiotics trial means that our effect estimates may change substantially should those data be made available and synthesised with the other identified data. The certainty of some of the evidence presented here may be influenced by the study funding sources but since the role of industry funders, or manufacturers who provide interventions for use in trials, remains unclear it is difficult to judge their impact on study results.
Potential biases in the review process
To reduce the risk of bias in the review process as far as possible, we conducted a comprehensive literature search without any restrictions with regard to language, date or publication status. We further reduced the risk of bias by ensuring that two authors independently carried out search result screening, data extraction, 'Risk of bias' assessment and GRADE ratings.
The lead author of this review is also the author of a study that was considered for inclusion in the review. To reduce bias, the lead author had no part in making the final decision about whether it was included or excluded.
However, problems obtaining missing trial data due to restrictions imposed by one of the probiotics manufacturers will inevitably have an impact on the extent to which we can present meaningful conclusions.
Agreements and disagreements with other studies or reviews
The current world literature generally agrees with the need for robust studies in this field. The World Health Organization (WHO 2000) recommends supporting education; prompt attention to any milk stasis and difficulties with feeding; infection control; and management of breast engorgement.
Few other reviews have examined the prevention of mastitis, but are somewhat consistent with the findings of a Cochrane Review of 24 trials (Lumbiganon 2016), which did not find prenatal education to be more effective than usual care in extending the duration of breastfeeding.
Few studies compared acupoint massage, but a Cochrane Review (Mangesi 2016) found acupressure to be less effective than hot and cold compresses in reducing pain from engorgement.
A recent systematic review (Anderson 2019) found that types of breast massage were reported as effective in reducing immediate pain but methods were too inconsistent to be able to draw conclusions. It recommended development of a validated tool for measuring breastfeeding problems. Another review (Pustotina 2016) compared various international guidelines and reviews, concluding that active emptying of the breasts can prevent mastitis.
Whereas Pustotina 2016 concluded that antibiotics were effective in the treatment of mastitis, a Cochrane Review (Jahanfar 2013) found insufficient evidence on the effectiveness of antibiotic therapy for the treatment of lactational mastitis, which is more consistent with our findings.
The use of probiotics is an area of growing interest but the literature has not provided sufficient data to compare with the results of this review.
Authors' conclusions
Implications for practice.
Probiotics may show promise in preventing mastitis but until the data from existing completed trials are available, the certainty of the evidence around probiotics remains low. There is also some evidence that acupoint massage is probably better than routine care. We did not find sufficient evidence to support the use of the other interventions that have been investigated in these trials. With almost no data available on the risk of adverse events, we do not have sufficient evidence to know whether any of these interventions may cause harm.
Implications for research.
This review has identified concerns around the conduct of research on probiotics for preventing mastitis. It is of fundamental importance that data are published from all completed studies in order to comply with ethical obligations to the women who participate in these trials and to ensure that women and clinicians have access to all the available evidence to inform their decisions about treatment.
Future trials should recruit adequate numbers of women and should measure clinically important outcomes, including possible side effects/adverse events. Given the already heterogenous nature of the interventions available for preventing mastitis, further research on the treatments that have been identified here, rather than exploring new treatments, is needed in order to increase the level of certainty of evidence. In this regard, the two ongoing trials that we identified, involving around 700 women, will make a substantial contribution to the evidence base around probiotics for preventing mastitis when their results are reported.
Providing women with evidence‐based robust research findings that support the prevention of mastitis when breastfeeding will help to improve their clinical outcomes and breastfeeding experience. Further research in this area is required to improve the prevention and management of mastitis in breastfeeding women.
What's new
| Date | Event | Description |
|---|---|---|
| 3 October 2019 | New citation required but conclusions have not changed | The conclusions have not changed since the last update. However, it has been rewritten to be more succinct. |
| 3 October 2019 | New search has been performed | Search updated and 5 new trials included in this update. The review now includes a total of 10 trials (3034 women). |
History
Protocol first published: Issue 3, 2008 Review first published: Issue 8, 2010
| Date | Event | Description |
|---|---|---|
| 6 September 2012 | New search has been performed | Search updated. No new trial reports identified. |
| 6 September 2012 | New citation required but conclusions have not changed | Review updated with new search date. |
Acknowledgements
The authors would like to acknowledge Karen New for her assistance and advice with the editorial review of the protocol and Michael Steele who provided ongoing statistical advice.
The authors would also like to acknowledge the support and extensive help that Philippa Middleton provided with the editorial review of both the protocol and original review.
Thanks to Maria Stoyadinova for help with translating Tanchev 2004 and to Millie Anim‐Somuah for help with translating Gurtovoi 1979 and Kulakov 2004, to Anne‐Marie Grant for help with translating Luttkus 1997 and to Aidan Tan for help with Fang 2016 and He 2015.
We would like to acknowledge and thank Dr Neil Smart and Dr Linda Crowe for their previous contribution to this review.
The authors would also like to thank the Cochrane Pregnancy and Childbirth team for their exemplary guidance and support when conducting this review.
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), members of our international panel of consumers and our Group's Statistical Adviser. The authors are grateful to the following peer reviewer for their time and comments: Mahjabeen Khan.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
Appendices
Appendix 1. Search methods for ICTRP and ClinicalTrials.gov
ICTRP
(searched with all synonyms)
mastitis AND breastfeeding
ClinicalTrials.gov
Advanced search
mastitis | Interventional Studies | breastfeeding
Data and analyses
Comparison 1. Probiotics versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Incidence of mastitis within 6 months postpartum | 2 | 399 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.35, 0.75] |
| 1.2 Nipple damage within 6 months postpartum | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.11, 1.01] |
| 1.3 Breast pain | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.64, 1.01] |
| 1.4 Number of women with adverse events | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
1.4. Analysis.

Comparison 1: Probiotics versus placebo, Outcome 4: Number of women with adverse events
Comparison 2. Antibiotics versus topical treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Incidence of mastitis within 6 months postpartum | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 Antibiotics versus fusidic acid ointment | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.03, 1.81] |
| 2.1.2 Antibiotics versus mupirocin ointment | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.05, 3.89] |
Comparison 3. Antibiotics versus placebo or usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Incidence of mastitis within 6 months postpartum | 3 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.10, 1.34] |
Comparison 4. Topical treatments versus breastfeeding advice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Incidence of mastitis within 6 months postpartum | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1.1 Fusidic acid ointment versus breastfeeding advice | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.27, 2.22] |
| 4.1.2 Mupirocin ointment versus breastfeeding advice | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.12, 1.35] |
Comparison 5. Mupirocin ointment versus fusidic acid ointment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Incidence of mastitis within 6 months postpartum | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.13, 2.00] |
Comparison 6. Specialist breastfeeding education versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Incidence of mastitis within 6 months postpartum | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1.1 At hospital discharge | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 6.1.2 At 7 days | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.75 [0.35, 40.70] |
| 6.1.3 At 30 days | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.17, 4.95] |
| 6.2 Breast pain (sore nipples) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.2.1 At hospital discharge | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.72, 1.36] |
| 6.2.2 At 7 days | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.66, 1.22] |
| 6.2.3 At 30 days | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.36, 2.37] |
| 6.3 Breast engorgement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.3.1 At hospital discharge | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.03, 14.87] |
| 6.3.2 At 7 days | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.71, 1.53] |
| 6.3.3 At 30 days | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.73, 1.49] |
| 6.4 Exclusive breastfeeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.4.1 At 7 days | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.18] |
| 6.4.2 At 30 days | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.68, 1.14] |
Comparison 7. Hydrothermally processed cereal with anti‐secretory factor‐inducing properties versus standard cereal.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Incidence of mastitis within 6 months postpartum | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 1.72] |
| 7.2 Recurrence of mastitis within 12 months postpartum | 1 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.03, 4.57] |
Comparison 8. Acupoint massage versus routine care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Incidence of mastitis within 6 months postpartum) | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.19, 0.78] |
| 8.2 Exclusive breastfeeding (at 42 days postpartum) | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.58, 2.29] |
| 8.3 Breast pain | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.07, 0.23] |
| 8.4 Breast engorgement | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.37, 0.65] |
| 8.5 Women's perception of milk supply (moderate or better) | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.13, 1.40] |
Comparison 9. Breast massage and low frequency pulse treatment versus routine care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Incidence of mastitis within 6 months postpartum | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.21] |
| 9.2 Exclusive breastfeeding | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [1.74, 4.05] |
| 9.3 Any breastfeeding | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.57, 2.12] |
| 9.4 Women's perception of milk supply (0‐14 scale; higher score = less milk supply) | 1 | 300 | Mean Difference (IV, Fixed, 95% CI) | ‐5.55 [‐5.90, ‐5.20] |
| 9.5 Cessation of breastfeeding (at end of treatment period) | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.01, 0.12] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amir 2004.
| Study characteristics | ||
| Methods | Design: randomised controlled trial Setting: hospitals in Melbourne, Australia |
|
| Participants | Number of participants: (N = 10) breastfeeding postpartum women with cracked nipples colonised with Staphylococcus aureus Inclusion criteria: lactating women with Staphylococcus aureus‐colonised nipples wishing to breastfeed Exclusion criteria: cracked nipples that were not colonised with Staphylococcus aureus |
|
| Interventions | Number of participants: (N = 10) Intervention: prophylactic antibiotics (flucloxacillin capsules taken for 7 days); (N = 5) Control: placebo (capsules with glucose powder taken for 7 days); (N = 5) Women with a positive nipple culture for Staphylococcus aureus had a follow‐up visit at 1 week. Women with negative nipple cultures had telephone follow‐up at 1 week. All participants had a final telephone interview at 6 weeks. |
|
| Outcomes | Mastitis study aborted at 12 months due to poor intervention compliance and lack of eligible participants Primary outcome: incidence of mastitis, defined as "at least two breast symptoms (pain, redness, lump) and at least one of fever or 'flu‐like' symptoms" Secondary outcome: nipple damage, defined as "mild 1 or 2 mm wide; moderate 3–9 mm wide; severe: greater than 10 mm wide and/or yellow colour visible in crack. In addition to a clinical assessment, a more permanent record of nipple damage was created using digital photography. It was planned for the photographs to be reviewed independently by three lactation consultants, in order to allow a thorough assessment of nipple damage and changes over time, rather than relying on the clinical assessment alone." |
|
| Notes | After 12 months, only 10 of the planned total of 133 women had been randomised to the trial and so the trial was stopped early. The author for this trial was contacted to clarify risks of bias (July 2020). Dates of study: recruitment was carried out between 2001 and 2002. The study was completed in 2004. Funding sources: 1 author received a National Health and Medical Research Council Medical Public Health PhD Scholarship, a grant from the Medical Research Foundation for Women and Babies and postgraduate support grants from the Faculty of Health Sciences, La Trobe University. CSL Ltd donated the flucloxacillin capsules and empty placebo capsules. Declarations of interest: "None declared" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The pharmacist used a random numbers table to label the capsules (placebo or active); sequence was stratified by hospitals in blocks of 10. "Randomisation was conducted by the Director of Pharmacy at the Royal Women's Hospital according to a random numbers table, stratified according to hospital, in blocks of ten". |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intervention and placebo capsules were identical. "The flucloxacillin and identical placebo capsules were put into bottles and labelled with a study number (e.g. RWH 001, RWH 002, MHW 001, MHW 002, etc.) by the pharmacy department of the Royal Women's Hospital". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The researcher was not involved in the randomisation process and was unaware of the treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2/5 women in the placebo group dropped out of the study as they did not wish to take medications. Analysis was intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | This is a report of a 'failed' RCT that was stopped because of a range of problems, including slow recruitment. The authors reported in full the number of women who developed mastitis and which group they were allocated to. Mastitis incidence was the primary outcome of the study and the authors have reported transparently why they were not able to measure any other outcomes. |
| Other bias | Low risk | Trial stopped early but there was nothing to indicate any other sources of bias. |
Bond 2018.
| Study characteristics | ||
| Methods | Design: randomised controlled trial Setting: Australia |
|
| Participants | Number of participants: (N = 639) Number of dropouts (per group if available): (N = 19) Inclusion criteria: women ≥ 18 years of age who have delivered a singleton baby at 37 weeks’ gestation or later; not currently taking commercial probiotics containing Lactobacillus fermentum; own a smartphone; with intention at the time of consent to breastfeed their baby for at least 2 months following birth Exclusion criteria: women with a history of Raynaud syndrome will not be eligible to participate in the trial. Any delivery/breast complication rendering the infant unable to breastfeed will be excluded. Women unable to speak/understand English will not be consented. |
|
| Interventions | Intervention A: (N = 311) probiotics containing Lactobacillus fermentum CECT5716 (1 × 1010 CFU/mL): 1 sachet daily, preferably at the same time each day for a period of 8 weeks following the birth of her baby. The contents of the sachet should be mixed with water, juice or milk, stirred and consumed immediately. Control B: (N = 309) as per intervention group but sachets do not contain Lactobacillus fermentum Duration of treatment: 8 weeks Duration of follow‐up: 2, 6 and 12 months postpartum |
|
| Outcomes | Incidence of mastitis up to 8 weeks following birth as measured by 1) clinical diagnosis of mastitis OR 2) at least 2 of the following breast symptoms: pain,redness/inflammation, lump/swelling AND at least 1 of the following systemic symptoms: flu‐like symptoms (body aches, headaches and chills) or fever ≥ 38°C. Breastfeeding duration (total/partial), recurrence of mastitis, development of breast abscess, cracked nipples, use of antibiotics, overall maternal health and well‐being, breastfeeding support, number of doctor’s visits for probable mastitis, overall doctor’s visits, adverse effects of treatment, incidence of primary mastitis between 2 and 6 months postpartum, maternal lifestyle factors which may affect breastfeeding outcomes, acceptability and compliance of the trial product Secondary infant outcomes: growth (height and weight) and well‐being in the first year of life (measured at 2, 6 and 12 months) as assessed via self‐report of health conditions including infections (gastrointestinal, respiratory), doctor’s visits, admission to hospital, allergic reactions and/or use of antibiotics |
|
| Notes | Dates of study: April 2015 to December 2016 Funding sources: "Funding was provided by the The Ramsay Research and Teaching Fund of Royal North Shore Hospital and The Kolling Institute of Medical Research. NN was supported by an Australian NHMRC Career Development Fellowship (#APP1067066). In‐kind support was provided by Intersect Australia Ltd for eResearch support and development of APProve‐Lite. The funders have no role in the design and conduct of the study”. Declarations of interest: “The probiotic and placebo sachets will be donated for the trial by Puremedic Pty Ltd, who will have no direct influence on the conduct, design or implementation of the trial. No trial material will bear the company name or logo. There are no commercial benefits to the researchers as a result of this trial. The authors have no conflicts of interest to report”. Correspondence from lead author diana.bond@sydney.edu.au: no data are available due to restrictions placed on the authors by the probiotics providers (date of last correspondence with trial author: January 2020. Author contacted again July 2020 to ask for any further update; response received from Diana Bond to say unfortunately the situation has not changed and they are still not able to publish the results). Retrospective trial registration |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation schedule will be prepared and centrally administered by a researcher not involved in patient care. A computer random number generator will be used to prepare the randomisation schedule in blocks of 4 and 6, and stratified by the incidence of previous mastitis." |
| Allocation concealment (selection bias) | Low risk | "Participants using the mobile phone application system (APProve‐Lite) will be randomised via a central password‐protected web‐based application developed by the APProve clinical trial unit. Concealment for participants using the ‘standard’ approach (not the APProve‐Lite system) will be via opaque, sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The randomisation sequence will be concealed until all data has been collected. The participant and researcher will be blinded as to treatment allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The researcher will be blinded as to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No results available |
| Selective reporting (reporting bias) | High risk | Results are unavailable due to restrictions imposed by the probiotics provider. |
| Other bias | Unclear risk | Insufficient information available |
De Oliveira 2006.
| Study characteristics | ||
| Methods | Design: randomised controlled trial. Setting: Porto Alegre, Brazil (women were recruited from June to November 2003) |
|
| Participants | Number of participants: (N = 211) breastfeeding mother‐infant pairs. Inclusion criteria: healthy non‐twin newborns with birthweight ≥ 2500 g Exclusion criteria: mother‐infant pairs unable to stay together due to a health concern in either the mother or the infant |
|
| Interventions | Intervention: breastfeeding education session (30 minutes) with a lactation consultant and an experienced breastfeeding nurse in hospital (N = 74) Control: usual care (N = 137) All women received a follow‐up home visit at day 7 and day 30. |
|
| Outcomes | Measures of exclusive breastfeeding rates and breastfeeding‐related problems Measure of mastitis, sore nipples and engorgement | |
| Notes | No contact details available for the trial authors Dates of study: June to November 2003 Funding sources: not stated Declarations of interest: "No reported competing interests" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned using 2 different coloured balls from a bag, 1 colour for the intervention, 1 colour for the control |
| Allocation concealment (selection bias) | Unclear risk | 2 different coloured balls from a bag, 1 colour for the intervention, 1 colour for the control |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind women or caregivers; self‐reported outcomes could be affected |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The researchers responsible for the breastfeeding evaluations did not participate in the intervention and were blinded to the group to which the mother‐infant pairs had been assigned." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between 5% and 9.9%. The original number of participants in the experimental group and the control group was 74 and 137, respectively. At the time of data analysis, there had been a loss of participants in both groups, 3 participants in the experimental group leaving 71 women and 5 women in the control group leaving 132 women. |
| Selective reporting (reporting bias) | Low risk | Protocol publication was not common practice at the time. No evidence of selective reporting |
| Other bias | Low risk | No apparent evidence of other bias |
Fang 2016.
| Study characteristics | ||
| Methods | Design: randomised controlled trial Setting: China |
|
| Participants | Number of participants: (N = 300) Number of dropouts (per group if available): not reported Inclusion criteria: postpartum women with low milk supply, age 21 – 45, singleton, not pre/post‐term, no obstetric conditions Exclusion criteria: not reported |
|
| Interventions | Intervention A: (N = 150) breast massage combined with low frequency pulse treatment. Massage from base to nipple, until breast is no longer swollen/distended, and is soft, and the milk has smooth flow. Trapped milk is manually expressed. In a seated position, massage clockwise for 5 minutes, 2‐3 times daily over the swollen areas. Express any trapped milk 2‐3 times a day. Manual expression of milk/trapped pus is done repeatedly with increasing pressure, using the blocked ducts, 2‐3 times a day. Gentle massage of the acupoints for 5 minutes until the area feels aching/sore 2‐3 times daily. Low frequency pulse treatment is performed using SOKO 900I machine, with mother in a supine position. Electrodes are placed on the breast and on the back. Alternating current is applied titrated up slowly till maternal tolerance. 60 minutes, twice daily Control B: (N = 150) routine care. No further details reported Duration of treatment: not reported Duration of follow‐up: not reported |
|
| Outcomes | Hypogalactia degree score, postpartum lactation initiating, milk volume effect, mastitis morbidity | |
| Notes | Dates of study: January 2013 to August 2015 Funding sources: Guangzhou District Huizhou city Science Plan Foundation (2015Y249) Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. Given the intervention is a massage, participants are unlikely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 300 participants had their data reported. |
| Selective reporting (reporting bias) | Low risk | No protocol to compare. All outcomes described in methods were reported. |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Fernandez 2016.
| Study characteristics | ||
| Methods | Design: randomised controlled trial Setting: Spain |
|
| Participants | Number of participants: (N = 110) Number of dropouts (per group if available): A 0/55, B 2/55 Inclusion criteria: women aged 25 to 35 with normal pregnancy, healthy status, and a history of lactational mastitis after at least 1 previous pregnancy Exclusion criteria: women ingesting probiotic supplements or receiving antibiotic treatment in the previous 30 days, any kind of health problems related to pregnancy, symptomatic vaginal infections, allergy to cow's milk protein, intolerance to lactose |
|
| Interventions | Intervention A: (N = 55) probiotics: daily ingestion of 50 g freeze‐dried powder in capsules, 9 log10 colony‐forming units of L. salivarius PS2 from c.30 weeks of pregnancy until delivery Control B: (N = 53) placebo: excipient (powdered milk), 100 mg/once a day from c.30 weeks of pregnancy until birth Duration of treatment: approximately 10 weeks Duration of follow‐up: 3 months after delivery |
|
| Outcomes | Occurrence of mastitis during first 3 months after delivery. Breast pain scores collected from women who had mastitis. Adverse events and side effects related to the ingestion of the probiotic. | |
| Notes | Dates of study: not reported Funding sources: “This work was supported by AGL2013‐41980‐P project from the Ministerio de Economía y Competitividad (Spain)”. Declarations of interest: “All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed”. Contacted author July 2020 to inquire about dates of study, allocation concealment and reporting of outcomes; awaiting reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “computer‐generated allocation sequence” |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and caregivers blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differential attrition. All withdrawals stated as not having any relation to the study |
| Selective reporting (reporting bias) | High risk | Trial registration stated primary outcome would be measured until 6 months postpartum but these data were not reported. |
| Other bias | Low risk | Nothing to indicate any other source of bias |
He 2015.
| Study characteristics | ||
| Methods | Design: randomised controlled trial Setting: China |
|
| Participants | Number of participants: (N = 400) primipara Number of dropouts (per group if available): N = 0 Inclusion criteria: primipara, age 23‐30, healthy, without obstetric conditions or complications, no breast development issues or breast disease, no neonatal respiratory distress or congenital malformation, no treatment contraindications, no psychiatric conditions, consenting to study Exclusion criteria: low mood/abnormal condition, previous pregnancies, age < 23 or > 30, has breast issues, has neonatal issues (severe respiratory distress or congenital malformation) |
|
| Interventions | Intervention A: (N = 200) breast acupoint massage. 2 hours after natural vaginal birth or after return to ward post‐caesarean section, a nurse delivered the following treatment:
Control B: (N = 200) routine care. As per the intervention group, except the acupoint massage Duration of treatment: not reported Duration of follow‐up: 42 days postpartum |
|
| Outcomes | Initial time of lactation, amount of lactation, breastfeeding rate after 42 days, breast comfort, swelling, incidence of mastitis after 42 days, nursing satisfaction | |
| Notes | Dates of study: June to December 2014 Funding sources: Wenzhou City Science Department Support Foundation (Y20140445) Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned, and unlikely as the personnel needs to perform the acupoint massage |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method not described. For subjective outcomes, women may have been their own outcome assessors and are unlikely to have been blinded but this was not described in the trial report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 200 per group, all had results reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol to compare against. All stated outcomes in the methods were reported. |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Hurtado 2017.
| Study characteristics | ||
| Methods | Design: randomised controlled trial Setting: Spain |
|
| Participants | Number of participants: (N = 625) Number of dropouts (per group if available): A 164, B 170 Inclusion criteria: healthy women between 18 and 45 years with development of normal pregnancy, childbirth took place 1– 6 days before recruitment, birth between 37 and 42 weeks of gestation, women who had received preventive antibiotic treatment between 48 hours before and 48 hours after childbirth (1 dose was sufficient for inclusion regardless of the type of antibiotic), and women with firm intention to breastfeed their children for at least 16 weeks Exclusion criteria: mammary pathologies or children’s pathologies that hinder or preclude breastfeeding and low expectation of adherence to the study protocol |
|
| Interventions | Intervention A: (N = 303) probiotics: 1 capsule/day containing L. fermentum 3 x 109 CFU Control B: (N = 322) placebo: 1 placebo capsule/day containing maltodextrin Duration of treatment: 16 weeks Duration of follow‐up: 16 weeks postpartum |
|
| Outcomes | Incidence of clinical mastitis during the first 4 months of breastfeeding. Mastitis defined as at least 2 out of the 3 breast symptoms (pain, redness, and lump) and at least 1 of fever or flu‐like symptoms (shivering, hot sweats, or aches) Secondary outcomes: microbiota of breast milk at the end of intervention and in mastitis events, monthly questionnaire on evaluation of breast pain, and inflammatory markers in breast milk at the end of intervention and in mastitis events | |
| Notes | Dates of study: August 2013 to July 2015 Funding sources: “This study was financed by the Andalusian Government and co‐financed by the European Regional Development Fund under the Andalusia’s 2007–2013 Global Innovation‐Technology‐Enterprise Grant”. Declarations of interest: “JAM‐L, MPD‐R. MO, JF, OB, CR, ADV, and AS are workers of Biosearch Life, owner of the patent of Lactobacillus fermentum CECT5716". Contacted author 16th July 2020 to query the numbers of women withdrawing from the trial because it appeared that some women with mastitis were not included in the mastitis incidence analysis; email address of correspondence author was no longer active and we could not find contact details for the other authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “randomization generated by a computer program” |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and caregivers blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators and outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition in both groups, but not differential (164/303 and 170/322), mainly due to “voluntary resignation” (56/303 and 54/322) and other reasons related to breastfeeding difficulties (70/303 and 76/322 women decided to stop breastfeeding due to perception of insufficient milk or mastitis, 5/303 and 7/322 due to gastrointestinal problems in infants or maternal rash) Some women appeared to have discontinued the intervention due to developing mastitis; these women were not included in the four‐month analysis. Analysis was per‐protocol. |
| Selective reporting (reporting bias) | High risk | Not all outcomes were reported in full. |
| Other bias | Unclear risk | Some of the authors work for the patent owner of the intervention (JAM‐L, MPD‐R, MO, JF, OB, CR, ADV, and AS). It was not clear if this had any influence on the results. |
Livingstone 1999.
| Study characteristics | ||
| Methods | Design: prospective, randomised clinical trial. This study trial led basic breastfeeding advice with a combination of antibiotics and topical ointments. Setting: Canada, Vancouver Breastfeeding Center |
|
| Participants | Number of participants: (N = 84) Inclusion criteria: (N = 84). postpartum breastfeeding women with sore or cracked nipples. Mothers attending breastfeeding clinic for breastfeeding problems, cracked/sore nipples, positive Staphyloccocus aureus results Exclusion criteria: mothers with local or system spread of infection such as cellulitis, ascending lactiferous duct infection or mastitis |
|
| Interventions | Interventions: 4 intervention groups: 1. Optimal breastfeeding technique (basic breastfeeding advice) (N = 23) 2. Topical 2% mupirocin ointment to nipples, (N = 25) 3. Topical fusidic acid ointment to nipples, (N = 17) 4. Oral antibiotics ‐ cloxacillin/erythromycin, (N = 19) |
|
| Outcomes | Measured nipple symptoms, breast symptoms and mastitis | |
| Notes | 100% compliance ‐ highly‐motivated breastfeeding women Intention‐to‐treat not used This study was stopped prematurely ‐ women who did not receive antibiotics perceived to have a higher rate of mastitis Authors contacted July 2020 to clarify study dates and declarations of interest; email address no longer active Dates of study: not stated Funding sources: funding in part was supported by the Department of Family Practice, Vancouver General Hospital. Berkowitz associates provided statistical consultation. Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 100 tags were alternatively labelled A, B, C, D and placed in an envelope. |
| Allocation concealment (selection bias) | Low risk | Each case randomly assigned by drawing a tag from the envelope |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "This was an open study and outcome measures could be subjected to bias". Lack of blinding could influence women's and caregivers' perception of symptoms. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specifically reported if outcome assessors were blinded (i.e. we did not know if people recording women's and caregivers' perception of symptoms were blinded) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses reported |
| Selective reporting (reporting bias) | Low risk | Protocol publication was not common practice at the time but there was nothing to indicate selective reporting. |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Sebitloane 2008.
| Study characteristics | ||
| Methods | Design: randomised controlled trial. This study investigated antibiotics versus placebo. Setting: King Edward VIII and Addington Hospital in Durban, South Africa |
|
| Participants | Number of participants: (N = 615) HIV infected women > 18 years, ≥ 36 weeks' gestation with anticipated vaginal delivery at King Edward VIII and Addington Hospital in Durban, South Africa between February 2003 and May 2005 Inclusion criteria: "HIV‐infected women 18 years old for whom vaginal delivery was anticipated" Exclusion criteria: "women who were HIV uninfected, women who did not wish to know their HIV status, women with obstetric conditions that necessitated a planned cesarean delivery, and women with a known allergy to penicillin. After enrolment and randomization, women were further excluded if they had an emergency cesarean delivery." Women who delivered at the hospitals in the study and were eligible for randomisation (N = 675); of these N = 60 had a planned caesarean section and were excluded. Number of participants (N = 305): women who were randomised and received cefoxitin Number in control group (N = 310) were randomly assigned the placebo. Following this, a further (N = 92) women from the intervention group and (N = 99) from the placebo group were excluded because they had an emergency caesarean delivery. |
|
| Interventions | Group 1 (n = 213) 2 g dose of cefoxitin intravenously over 20 minutes during active labour Group 2 (n = 212) water placebo administered over the same period of time |
|
| Outcomes | Postpartum infections Follow‐up evaluation: of the 213 women assigned randomly to the cefoxitin group, 182 (85%) returned for the follow‐up evaluation at 1 week and 184 (86%) returned at 2 weeks. Of the 212 women assigned the placebo, 180 (85%) returned for the follow‐up at 1 week and 178 (84%) returned at the 2 week for follow‐up. |
|
| Notes | Clinicians blinded to intervention. Women were excluded if they had an emergency caesarean delivery after randomisation. The randomised groups were comparable with regards to age, parity, gestational age at delivery and most baseline haematology. Contacted author July 2020 to ask for details about missing data, why women were excluded from the analysis; awaiting reply Dates of study: the study was conducted between February 2003 and May 2005. Funding sources: supported by grant RES 112‐02 from Secure the Future‐HIV Research Institute (Bristol‐Myers Squibb, New York, NY). Berkowitz associates provided statistical consultation. Declarations of interest: not reported. Correspondence with author outlined that, the loss to follow‐up was not by design. Loss to follow‐up was as a result of patients who did not return and were not contactable. Some patients move back to rural/out of town communities after delivery. Mastitis and other sources of infection (e.g. UTI) were sought and recorded as such if found. This study does have a protocol which was reviewed by their local ethics review committee. A study number was assigned, which the author will make available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated by statistician. Syringes labelled D001‐D686; participants were given the drug during labour according to the next available number. |
| Allocation concealment (selection bias) | Low risk | The statistician generated a computer‐based allocation of each study numbered 1‐686 into either group 1 or 2 which represented either cefoxitin or placebo. Only the pharmacist was aware of the drug code for the duration of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and clinicians blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators blinded ("Only the pharmacist was aware of the drug code for the entire duration of the study.") |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data > 20%. The original number of participants in the study was 716, of which 675 delivered within the study premises. 60 of these women were not randomised. Finally 615 women were randomised, with 305 women in the experimental group and 310 in the control group. The 1‐week follow‐up resulted in 182 participants in the experimental group and 180 in the control group. At the 2‐week follow‐up there were 184 in the experimental group and 178 in the control group. No reasons given for loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Protocol publication was not common practice at the time but there was nothing to indicate evidence of selective reporting. |
| Other bias | Low risk | No apparent evidence of other bias |
Svensson 2004.
| Study characteristics | ||
| Methods | Design: randomised controlled trial. This study trial led the use of anti‐secretory factor in cereal to prevent mastitis. Setting: Sweden |
|
| Participants | Number of participants: (N = 40) postpartum breastfeeding women that had normal deliveries and have healthy full‐term infants, were randomly divided into 2 groups. Participants were breastfeeding or intended to breastfeed. All mothers were Swedish or raised in Sweden. No clear indication of inclusion or exclusion criteria were reported. |
|
| Interventions | Number of participants: (N = 40) Intervention group: (N = 20) received hydrothermally processed cereals (HPC) with specific AF‐inducing properties. The cereals of the HPC were treated in a process similar to malting. The content of sugars and amino acids in the cereals at the end of the hydrothermic process has previously been described. After processing, the cereals were dried to 10% moisture. Control group: (N = 20) similar cereal without AF‐inducing properties Participants requested to eat 50 g of cereal every day for a period of 5 weeks. The active material, as well as the placebo food, was available in the form of cereals produced by BioDoc AB, Stockholm, Sweden. Duration of follow‐up 5 weeks |
|
| Outcomes | Incidence of mastitis between groups | |
| Notes | No difference between the groups, regarding background, obstetric data, age, education, parity, type of anaesthesia used during the delivery, child sex and birth rate. Loss of participants to follow‐up > 20% Dates of study: data were collected April–August 2002. Funding sources: the authors acknowledged financial support from AS‐Factor AB and the Swedish State under the LUA agreement (grant no. I33913). Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned (sealed envelopes that were opened consecutively) to 1 of 2 groups |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes that were opened consecutively |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Mothers and researchers were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Differential attrition. The original number of participants in the study was 40. 11 of these participants dropped out in the first 2 weeks. 7 mothers in the experimental group and 3 in the control group. 1 mother was excluded because of incorrect compliance with the intervention. The final number for the experimental group was 12 mothers and 17 for the control group. One of the mothers in the control group failed to provide a milk sample at the end of the study. Final data analysis was on 12 mothers from the experimental group and 17 from the control group. |
| Selective reporting (reporting bias) | Low risk | Protocol publication was not common practice at the time but there was nothing to indicate evidence of selective reporting. |
| Other bias | Low risk | No apparent evidence of other bias |
AF: anti‐secretory factor CFU: colony forming unit HPC: hydrothermically processed cereal UTI: urinary tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12607000438459008 | This trial is about treating the early signs of mastitis, not focused on prevention. |
| Blaikeley 1953 | This study is not an RCT. |
| Bystrova 2007 | This is not a trial of mastitis prevention. It is an RCT on the effect of different postnatal ward practices on lactation performance. |
| Centuori 1999 | This is not a trial of mastitis prevention. It is an RCT on treating sore nipples. |
| Crepinsek 2008 | This trial focuses on treating mastitis, not on prevention. |
| Dennis 2012 | This trial is about nipple pain, not about mastitis prevention. |
| Evans 1995 | This study is not an RCT. |
| Feng 2019 | This trial is not about preventing mastitis. |
| Filteau 1999 | This is not a trial of mastitis prevention. It is an RCT of postpartum maternal vitamin A supplementation. |
| Forster 2004 | This is an RCT of strategies to increase breastfeeding initiation and duration. |
| Frank 1987 | This is not a trial of mastitis prevention. It is an RCT of discharge packs and counselling to increase breastfeeding duration. |
| Gensch 2006 | This trial is about treating nipple pain, not about preventing mastitis. |
| Gomo 2003 | This is a micronutrient RCT looking at preventing 'subclinical' mastitis. |
| Gunn 1998 | This trial did not evaluate interventions for preventing mastitis ‐ it is a trial comparing early postnatal check up with a GP (at 1 week) with the usual 6‐week check‐up. |
| Hager 1996 | Treatment of mastitis, not prevention |
| Harvey [date of communication?] | This is an RCT/quasi‐RCT for preventing sore nipples. |
| Herd 1986 | This is an RCT for treating nipple trauma. |
| Homer 2001 | This is a continuity of care RCT. |
| ISRCTN98567612 | This trial is about the effects of HIV treatment on mastitis, not about mastitis prevention. |
| Kramer 2001 | This is an RCT of breastfeeding promotion. |
| Kvist 2004 | This is a treatment trial. |
| Kvist 2007 | This is a treatment trial. |
| Lawlor‐Smith 1997 | This is not an RCT. |
| Lumley 2006 | This is an RCT of resources, information and support for postpartum women. |
| Luttkus 1997 | This is an RCT of antibiotic prophylaxis for caesarean section. |
| Maldonado‐Lobon 2015 | This is a trial about treating women with breast pain that is not associated with mastitis. |
| Mastromarino 2015 | This trial is not about preventing mastitis. |
| McLachlan 1991 | This is an RCT of ultrasound treatment for breast engorgement. |
| Meah 2001 | This is not an RCT. It is a letter re Kramer 2001. |
| NCT03230760 | This trial is about treating breast engorgement. |
| Neifert 1990 | This is not an RCT. |
| Nicholson 1985 | This is an RCT of treating cracked nipples. |
| Nicholson 1993 | This is not an RCT. |
| Nikodem 1993 | This is an RCT for preventing breast engorgement. |
| Phillips 1975 | This is an RCT for preventing breast engorgement. |
| Roberts 1995 | This is an RCT for treating breast engorgement. |
| Roberts 1998 | This is an RCT for treating breast engorgement. |
| Schurz 1978 | This is a quasi‐randomised trial (women were allocated by the first letter of their surname). |
| Swift 2003 | This is an RCT of lactation suppression (breast binding). |
| Thomsen 1984 | Treatment of mastitis, not prevention |
| Waldenstrom 1994 | This trial did not evaluate interventions for preventing mastitis ‐ it is an RCT comparing birth centre care versus usual obstetric care. |
| Zadrozny 2017 | Ineligible intervention: this trial investigated the relationship between HIV treatment and incidence of mastitis. |
GP: general practitioner HIV: human immunodeficiency virus RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT04032899.
| Study name | Multicenter, randomised, double‐blind, controlled parallel nutritional intervention study to evaluate the effect of consumption during pregnancy and the lactation period of Lactobacillus fermentum CECT5716 on the incidence of mastitis |
| Methods | Quadruple‐blind RCT Setting: Spain |
| Participants | Target size: 480 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: 1 capsule per day with L. fermentum CECT5716 3x109 CFU mixed with maltodextrins from week 28‐32 of gestation up to 16 weeks after delivery Control group (placebo): 1 capsule per day with maltodextrins from week 28‐32 of gestation up to 16 weeks after delivery |
| Outcomes | Incidence of mastitis, microbiota of breast milk, recurrence of mastitis, breast pain questionnaire, cessation of breastfeeding, percentage of infants who receive exclusive breastfeeding, immunoglobulins in breast milk, minerals in breast milk, baby faeces microbiota, incidence of caesareans and incidence of antibiotic use during delivery, baby's anthropometric measures, data about the intestinal health of the baby, data about sleep parameters of the baby All outcomes to be measured at 4 months |
| Starting date | April 15, 2019 |
| Contact information | Principal Investigator: Nicolás Mendoza, MD, PhD |
| Notes |
NL4243.
| Study name | A randomised, double‐blind, placebo‐controlled intervention study to assess the preventive effect of new probiotic strain on lactational mastitis |
| Methods | Double‐blind RCT Setting: the Netherlands |
| Participants | Target size: 300 participants Inclusion criteria: ‐ Healthy pregnant, adults (> 18 years of age) ‐ Before/during the 35th week of pregnancy ‐ Intending to breastfeed her infant ‐ Written informed consent Exclusion criteria: ‐ Pre‐gravid body mass index (BMI) < 18 or > 30 ‐ Use of probiotic supplements during the third trimester of current pregnancy ‐ Enhanced chance of premature delivery (before 37 weeks of gestation) ‐ Current or previous illnesses which could interfere with the study, like other mammary pathologies (e.g. abscesses, Raynaud's syndrome, breast cancer) ‐ Short bowel syndrome ‐ Impaired intestinal epithelial barrier (e.g. diarrhoeal illness, intestinal inflammation) ‐ Serious underlying disease predisposing to infection (e.g. HIV, auto‐immune diabetes, multiple organ failure, malignancy, severe burns, severe acute pancreatitis) ‐ Heart failure and cardiac medical history (e.g. artificial heart valve, medical history of infectious endocarditis, rheumatic fever and cardiac malformation) ‐ History of aggressive immunosuppressive therapy (e.g. radiotherapy, cancer chemotherapy) ‐ Traumatic injury of the gastrointestinal tract ‐ Surgery, including dental surgery, within 1 month prior to inclusion (V1) ‐ Investigator's uncertainty about the willingness/ability of the subject to comply with protocol requirements ‐ Participation in any other clinical trial within 2 weeks prior to entry into the study |
| Interventions | Intervention group: probiotic supplement Control group: placebo supplement |
| Outcomes | Rate of mastitis, count of recurrent episodes of mastitis, rate of breastfeeding withdrawal (complete, partial discontinuation) |
| Starting date | 1st April 2014 |
| Contact information | Mieke Roelofs Email: mieke.roelofs@danone.com |
| Notes | Correspondence from trialist 15th November 2019: "We are currently working on the manuscript of the study, so unfortunately I am not allowed to share any data yet". |
BMI: body mass index CFU: colony‐forming units HIV: human immunodeficiency virus RCT: randomised controlled trial
Differences between protocol and review
We have added in a search of ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP).
We have added secondary outcomes, which are deemed to be of clinical importance, related to breast abscess, nipple damage, breast pain, engorgement, milk supply and adverse events.
We have assessed the certainty of evidence using GRADE and we have presented 'Summary of Findings' tables.
Contributions of authors
Maree Crepinsek is the primary author as well as the contact author. The conception, design, and co‐ordination of the review have been done by Maree Crepinsek. Maree has also provided a clinical perspective for the review, as well as originally writing the review in Review Manager. Fiona Stewart and Emily Taylor independently reviewed all articles found in the search for this review. The team of authors decided on the inclusion or exclusion criteria, types of interventions and outcome measures.
Fiona Stewart and Emily Taylor independently extracted the data from the selected articles for analysis. Keryl Michener has provided support as a librarian, ensuring all search strategies and additional searching was conducted as well as providing ongoing support during the review process. All authors have been involved in editing the drafts of this review prior to submission.
Sources of support
Internal sources
PHCRED Faculty of Health Science and Medicine Bond University, Queensland, Australia
External sources
Herston Health Science Library, University of Queensland, Brisbane, Australia
Declarations of interest
Maree Crepinsek was the primary investigator on a randomised controlled trial that was considered for inclusion in this review. The trial, titled 'Self‐management versus usual care for the treatment of mastitis following childbirth: a randomised controlled trial' (Crepinsek 2008), commenced in January 2008 and was halted soon afterwards. The study was assessed by two other authors of this review and it was excluded because it was about the treatment of mastitis, not the prevention of mastitis.
Emily Taylor: none known.
Keryl Michener: none known.
Fiona Stewart: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Amir 2004 {published data only}
- Amir LH, Lumley J, Garland SM. A failed RCT to determine if antibiotics prevent mastitis: cracked nipples colonized with staphylococcus aureus: a randomised treatment trial [ISRCTN65289389]. BMC Pregnancy and Childbirth 2004;4(19):1-6. [DOI: 10.1186/1471-2393-4-19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN65289389. Cracked nipples colonized with Staphylococcus aureus: a randomised treatment trial. www.isrctn.com/ISRCTN65289389 (first received 9 December 2002).
Bond 2018 {published and unpublished data}
- ACTRN12615000923561. Evaluation of the probiotic lactobacillus fermentum CECT5716 for the prevention of mastitis in breastfeeding women: a randomised controlled trial. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12615000923561 (first received 4 September 2015). [DOI] [PMC free article] [PubMed]
- Bond DM, Morris JM, Gordon A, Phipps H, Kelly C, Shand A, et al. Evaluation of the probiotic lactobacillus fermentum CECT5716 for the prevention of mastitis in breastfeeding women: the APProve randomised controlled trial. Journal of Paediatrics and Child Health 2018;54(Suppl 1):9-10. [Google Scholar]
- Bond DM, Morris JM, Nassar N. Study protocol: evaluation of the probiotic lactobacillus fermentum CECT5716 for the prevention of mastitis in breastfeeding women: a randomised controlled trial. BMC Pregnancy and Childbirth 2017;17:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Oliveira 2006 {published data only}
- De Oliveira LD, Giugliani ERJ, Do Espirito Santo LC, Franca MC, Weigert EML, Kohler C, et al. Effect of intervention to improve breastfeeding technique on the frequency of exclusive breastfeeding and lactation-related problems. Journal of Human Lactation 2006;22(3):315-21. [DOI: 10.1177/08903344062900221] [DOI] [PubMed] [Google Scholar]
Fang 2016 {published data only}
- Fang JL. Impact of methods breast massage combined with low frequency pulse treatment on puerperium breastfeeding and prevention mastitis. China Modern Medicine [Zhong Guo Dang Dai Yi Yao Za Zhi] 2016;23(11):63-5. [Google Scholar]
Fernandez 2016 {published data only}
- Fernandez L, Cardenas N, Arroyo R, Manzano S, Jimenez E, Martin V, et al. Prevention of infectious mastitis by oral administration of lactobacillus salivarius PS2 during late pregnancy. Clinical Infectious Diseases 2016;62(5):568-73. [DOI] [PubMed] [Google Scholar]
- Fernandez L, Cardenas N, Arroyo R, Manzano S, Jimenez E, Martyn V, et al. Prevention of infectious mastitis by oral administration of lactobacillus salivarius PS2 during late pregnancy. Breastfeeding Medicine 2016;11(2):A-49, Abstract no: P-66. [DOI] [PubMed] [Google Scholar]
- NCT01505361. Oral administration of a probiotic strain to pregnant women: effects on the prevention of lactational mastitis and on the eradication of GBS colonization. clinicaltrials.gov/ct2/show/NCT01505361 (first received 6 January 2012).
He 2015 {published data only}
- He L, Zhu XM, Sun GM, Zhu XQ. Clinical study of breast acupoint massages effect for dredging breast. Liaoning Journal of Traditional Chinese Medicine [Liaoning Zhong Yi Za Zhi] 2015;42(10):1895-7. [Google Scholar]
Hurtado 2017 {published data only}
- Hurtado JA, Maldonado-Lobon JA, Diaz-Ropero MP, Olivares M, Fonolla J, Flores-Rojas K, et al. Oral administration to nursing women of lactobacillus fermentum CECT5716 prevents lactational mastitis development: a randomized controlled trial. Breastfeeding Medicine 2017;12(4):202-9. [Google Scholar]
- NCT02203877. Evaluation of lactobacillus fermentum CECT5716 on the incidence of mastitis. clinicaltrials.gov/ct2/show/NCT02203877 (first received 30 July 2014).
Livingstone 1999 {published data only}
- Livingstone V, Stringer LJ. The treatment of staphylococcus aureus infected sore nipples: a randomised comparative study. Journal of Human Lactation 1999;15(3):241-6. [DOI: 10.1177/089033449901500315] [DOI] [PubMed] [Google Scholar]
Sebitloane 2008 {published data only}
- Sebitloane HM, Moodley J, Esterhuizen TM. Prophylactic antibiotics for the prevention of postpartum infectious morbidity in women infected with human immunodeficiency virus: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2008;198(2):189.e1-189.e6. [DOI] [PubMed] [Google Scholar]
Svensson 2004 {published data only}
- Svensson K, Lange S, Lonnroth I, Widstrom AM, Hanson LA. Induction of anti-secretory factor in human milk may prevent mastitis. Acta Paediatrica 2004;93(9):1228-31. [ISSN 0803-5253] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12607000438459008 {published data only}
- ACTRN12607000438459. Self-management versus usual care for the treatment of mastitis following childbirth: a randomised control trial. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=82235&isReview=true (first received 29 August 2007).
Blaikeley 1953 {published data only}
- Blaikeley J, Clarke S, MacKeith R, Ogden KM. Breast-feeding: factors affecting success. Journal of Obstetrics and Gynaecology of the British Empire 1953;60:657-69. [DOI] [PubMed] [Google Scholar]
Bystrova 2007 {published data only}
- Bystrova K, Widstrom AM, Matthiesen AS, Ransjo-Arvidson AB, Welles-Nystrom B, Vorontsov I, et al. Early lactation performance in primiparous and multiparous women in relation to different maternity home practices. A randomised trial in St. Petersburg. International Breastfeeding Journal 2007;2(9):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Centuori 1999 {published data only}
- Centuori S, Burmaz T, Ronfani L, Fragiacomo M, Quintero S, Pavan C, et al. Nipple care, sore nipples, and breastfeeding: a randomised trial. Journal Human Lactation 1999;15:125-30. [DOI] [PubMed] [Google Scholar]
Crepinsek 2008 {published data only}
- Crepinsek AM. Self-management versus usual care for the treatment of mastitis following childbirth: a randomised control trial. Australian New Zealand Clinical Trials Register (www.anzctr.org.au) Accessed 19 February 2008. [Google Scholar]
Dennis 2012 {published data only}
- Dennis CL, Schottle N, Hodnett E, McQueen K. An all-purpose nipple ointment versus lanolin in treating painful damaged nipples in breastfeeding women: a randomized controlled trial. Breastfeeding Medicine 2012;7(6):473-9. [DOI] [PubMed] [Google Scholar]
Evans 1995 {published data only}
- Evans K, Evans R, Simmer K. Effect of the method of breast feeding on breast engorgement, mastitis and infantile colic. Acta Paediatrica 1995;84(8):849-52. [DOI] [PubMed] [Google Scholar]
Feng 2019 {published data only}
- ChiCTR-IOC-17012598. A prospective randomised controlled trial of modified inverted nipple correction with breastfeeding function preserved. www.chictr.org.cn/com/25/showprojen.aspx?proj=18594 (first received 6 September 2017).
- Feng R, Li W, Yu B, Zhou Y. A modified inverted nipple correction technique that preserves breastfeeding. Aesthetic Surgery Journal 2019;39(6):NP165–75. [DOI] [PubMed] [Google Scholar]
Filteau 1999 {published data only}
- Filteau SM, Lietz G, Mulokozi G, Bilotta S, Henry CJ, Tomkins AM. Milk cytokines and subclinical breast inflammation in Tanzanian women: effects of dietary red palm oil or sunflower oil supplementation. Immunology 1999;97:595-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forster 2004 {published data only}
- Forster D, McLachlan H, Lumley J, Beanland C, Waldenstrom U, Amir L. Two mid-pregnancy interventions to increase the initiation and duration of breastfeeding: a randomised controlled trial. Birth 2004;31(3):176-82. [DOI] [PubMed] [Google Scholar]
- Forster D, McLachlan H, Lumley J, Beanland C, Waldenstrom U, Harris H, et al. ABFAB. Attachment to the breast and family attitudes to breastfeeding. The effect of breastfeeding education in the middle of pregnancy on the initiation and duration of breastfeeding: a randomised controlled trial. BMC Pregnancy and Childbirth 2003;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster DA, McLachlan HL, Lumley J, Beanland CJ, Waldenstrom U, Short RV, et al. ABFAB: attachment to the breast and family attitudes towards breastfeeding. The effect of breastfeeding education in the middle of pregnancy on the duration of breastfeeding: a randomised controlled trial. In: Perinatal Society of Australia and New Zealand 7th Annual Congress; 2003 March 9-12; Tasmania, Australia. 2003:A70. [DOI] [PMC free article] [PubMed]
Frank 1987 {published data only}
- Frank DA, Wirtz SJ, Sorenson JR, Heeren T. Commercial discharge packs and breast-feeding counselling: effects on infant-feeding practices in a randomised trial. Pediatrics 1987;80(6):845-54. [PubMed] [Google Scholar]
Gensch 2006 {published data only}
- Gensch M, Wockel A, Abou-Dakn M. Effects of lanolin on nipple pain of breastfeeding mothers. Geburtshilfe und Frauenheilkunde 2006;67:43. [Google Scholar]
Gomo 2003 {published data only}
- Gomo E, Filteau SM, Tomkins AM, Ndhlovu P, Michaelsen KF, Friis H. Subclinical mastitis among HIV-infected and uninfected Zimbabwean women participating in a multi micronutrient supplementation trial. Transactions of the Royal Society of Tropical Medicine and Hygiene 2003;97(2):212-6. [DOI] [PubMed] [Google Scholar]
Gunn 1998 {published and unpublished data}
- Gunn J, Lumley J, Chondros P, Young D. Does an early postnatal check-up improve maternal health: results from a randomised trial in Australian general practice. BJOG: an international journal of obstetrics and gynaecology 1998;105(9):991-7. [DOI] [PubMed] [Google Scholar]
Hager 1996 {published data only}
- Hager WD, Barton JR. Treatment of sporadic acute puerperal mastitis. Infectious Diseases in Obstetrics and Gynecology 1996;4(2):97-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harvey [date of communication?] {published data only}
- Controlled trial of chlorhexidine in aerosol spray for the prevention of sore nipples. Personal communication 1988.
Herd 1986 {published data only}
- Herd B, Feeney JG. Two aerosol sprays in nipple trauma. Practitioner 1986;230:31-8. [PubMed] [Google Scholar]
Homer 2001 {published data only}
- Homer CSE, Davis GK, Brodie PM, Sheehan A, Barclay LM, Wills J, et al. Collaboration in maternity care: a randomised controlled trial comparing community-based continuity of care with standard hospital care. BJOG: an international journal of obstetrics and gynaecology 2001;108(1):16-22. [DOI] [PubMed] [Google Scholar]
ISRCTN98567612 {published data only}ISRCTN98567612
- ISRCTN98567612. Effect of the probiotic lactobacillus reuteri on breast inflammation and lactational mastitis. www.isrctn.com/ISRCTN98567612 (first received 30 March 2016).
Kramer 2001 {published data only}
- Kramer MS, Chalmers B, Hodnett ED, Sevkovskaya Z, Dzikovich I, Shapiro S, et al. PRomotion Of Breastfeeding Intervention Trial (PROBIT): a randomised trial in the Republic of Belarus. JAMA 2001;285(4):413-20. [DOI] [PubMed] [Google Scholar]
Kvist 2004 {published data only}
- Kvist LJ, Wilde Larsson B, Hall-Lord ML, Rydhstroem H. Effects of acupuncture and care interventions on the outcome of inflammatory symptoms of the breast in lactating women. International Nursing Review 2004;51(1):56-64. [DOI] [PubMed] [Google Scholar]
Kvist 2007 {published data only}
- Kvist LJ, Hall-Lord ML, Rydhstroem H, Larsson BW. A randomised-controlled trial in Sweden of acupuncture and care interventions for the relief of inflammatory symptoms of the breast during lactation. Midwifery 2007;23(2):184-95. [DOI] [PubMed] [Google Scholar]
Lawlor‐Smith 1997 {published data only}
- Lawlor-Smith C, McIntyre E, Bruce J. Effective breastfeeding support in general practice. Australian Family Physician 1997;26(5):573-80. [PubMed] [Google Scholar]
Lumley 2006 {published data only}
- Lumley J, Watson L, Small R. PRISM (Program of Resources, Information and Support for Mothers): a community-randomised trial to reduce depression and improve women's physical health six months after birth. BMC Public Health 2006;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luttkus 1997 {published data only}
- Luttkus A, Fiebelkorn J, Dudenhausen W. Antibiotic prophylaxis in cases of emergency caesarean section. Geburtshilfe und Frauenheilkunde 1997;57(9):510-4. [Google Scholar]
Maldonado‐Lobon 2015 {published data only}
- Maldonado-Lobon JA, Diaz-Lopez MA, Carputo R, Duarte P, Diaz-Ropero MP, Valero AD, et al. Lactobacillus fermentum CECT 5716 reduces staphylococcus load in the breastmilk of lactating mothers suffering breast pain: a randomized controlled trial. Breastfeeding Medicine 2015;10(9):425-32. [DOI] [PubMed] [Google Scholar]
- NCT02093338. Study of intervention nutritional, multicenter, randomized, blind, parallel group to evaluate the efficacy of lactobacillus fermentum CECT5716 to reduce the load bacterial in potential pathogens in milk of mothers with breast pain. clinicaltrials.gov/ct2/show/NCT02093338 (first received 21 March 2014).
Mastromarino 2015 {published data only}
- Mastromarino P, Capobianco D, Miccheli A, Pratico G, Campagna G, Laforgia N, et al. Administration of a multistrain probiotic product (VSL#3) to women in the perinatal period differentially affects breast milk beneficial microbiota in relation to mode of delivery. Pharmacological Research 2015;95-96:63-70. [DOI] [PubMed] [Google Scholar]
- NCT01367470. Effects on cytokines, immunoglobulins, antibodies, sphingomyelinase and PAF hydrolysis capacity in the maternal milk after probiotic VSL#3 administration in the last four weeks of gestation and first month of lactation. clinicaltrials.gov/ct2/show/NCT01367470 (first received 7 June 2011).
McLachlan 1991 {published data only}
- McLachlan Z, Milne EJ, Lumley J, Walker BL. Ultrasound treatment for breast engorgement: a randomised double blind trial. Australian Journal of Physiotherapy 1991;37(1):23-8. [DOI] [PubMed] [Google Scholar]
Meah 2001 {published data only}
- Meah S. A breastfeeding intervention increased breast feeding and reduced GI tract infections and atopic eczema. Evidence-Based Nursing 2001;4(4):106. [Google Scholar]
NCT03230760 {published data only}
- NCT03230760. Acupuncture for breast engorgement during lactation: a pilot study. clinicaltrials.gov/ct2/show/NCT03230760 (first received 26 July 2017).
Neifert 1990 {published data only}
- Neifert M, DeMarzo S, Seacat J, Young D, Leff M, Orleans M. The influence of breast surgery, breast appearance, and pregnancy-induced breast changes on lactation sufficiency as measured by infant weight gain. Birth 1990;17(1):31-8. [DOI] [PubMed] [Google Scholar]
Nicholson 1985 {published data only}
- Nicholson W. Cracked nipples in breastfeeding mothers - a randomised trial of three methods of management. Nursing Mothers Association of Australia Newsletter 1985;21:7-10.
Nicholson 1993 {published data only}
- Nicholson WL. The use of nipple shields by breastfeeding women. Australian College of Midwives Incorporated Journal 1993;6(2):18-24. [DOI] [PubMed] [Google Scholar]
Nikodem 1993 {published data only}
- Nikodem VC, Danziger D, Gebka N, Gulmezoglu AM, Hofmeyr GJ. Do cabbage leaves prevent breast engorgement? A randomised controlled study. Birth 1993;20(2):61-4. [DOI] [PubMed] [Google Scholar]
Phillips 1975 {published data only}
- Phillips WP. Prevention of postpartum breast engorgement: double-blind comparison of chlorotrianisene 72 mg and placebo. Journal of the Arkansas Medical Society 1975;72(4):163-7. [PubMed] [Google Scholar]
Roberts 1995 {published data only}
- Roberts KL. A comparison of chilled cabbage leaves and chilled gel packs in reducing breast engorgement. Journal of Human Lactation 1995;11(1):17-20. [DOI] [PubMed] [Google Scholar]
Roberts 1998 {published data only}
- Roberts KL, Reiter M, Schuster D. Effects of cabbage leaf extract on breast engorgement. Journal of Human Lactation 1998;14(3):231-6. [DOI] [PubMed] [Google Scholar]
Schurz 1978 {published data only}
- Schurz AR, Kobermann M. Comparison of nipple care in the puerperium with powder and ointment. Geburtshilfe und Frauenheilkunde 1978;38:573-6. [PubMed] [Google Scholar]
Swift 2003 {published data only}
- Swift K, Janke J. Breast binding... is it all that it's wrapped up to be? Journal of Obstetric, Gynecologic, and Neonatal Nursing 2003;32(3):332-9. [DOI] [PubMed] [Google Scholar]
Thomsen 1984 {published data only}
- Thomsen AC, Espersen T, Maigaard S. Course and treatment of milk stasis, noninfectious inflammation of the breast, and infectious mastitis in nursing women. American Journal of Obstetrics and Gynecology 1984;149:492-5. [DOI] [PubMed] [Google Scholar]
Waldenstrom 1994 {published data only}
- Waldenstrom U, Nilsson CA. No effect of birth centre care on either duration or experience of breast feeding, but more complications: findings from a randomised controlled trial. Midwifery 1994;10:8-17. [DOI] [PubMed] [Google Scholar]
Zadrozny 2017 {published data only}
- Zadrozny S, Westreich D, Hudgens MG, Chasela C, Jamieson DJ, Martinson F, et al. Effect of postnatal HIV treatment on clinical mastitis and breast inflammation in HIV-infected breast-feeding women. Paediatric and Perinatal Epidemiology 2017;31(2):134-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT04032899 {published data only}
- NCT04032899. Multicenter, randomized, double-blind, controlled parallel nutritional intervention study to evaluate the effect of consumption during pregnancy and the lactation period of lactobacillus fermentum CECT5716 on the incidence of mastitis. www.clinicaltrials.gov/ct2/show/NCT04032899 (first received 25 July 2019).
NL4243 {published data only}
- NL4243. A randomized, double-blind, placebo-controlled intervention study to assess the preventive effect of new probiotic strain on lactational mastitis. www.trialregister.nl/trial/4243 (first received 7 January 2014).
Additional references
Amir 2007
- Amir L, Forster D, Lumley J, McLachlan H. A descriptive study of mastitis in Australian breastfeeding women: incidence and determinants. BMC Public Health 2007;7(62):no pagination. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amir 2014
- Amir L. ABM Clinical Protocol #4: Mastitis. Breastfeeding Medicine 2014;9:239-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amir 2016
- Amir L, Griffin L, Cullinane M, Garland S. Probiotics and mastitis: evidence-based marketing? International Breasfeeding Journal 2016;11:Article 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anatolitou 2012
- Anatolitou F. Human milk benefits and breastfeeding. Journal of Pediatric and Neonatal Individualized Medicine 2012;1:11-18. [Google Scholar]
Anderson 2019
- Anderson L, Kynoch K, Kildea S, Lee N. Effectiveness of breast massage for the treatment of women with breastfeeding problems: a systematic review. JBI Database of Systematic Reviews and Implementation Reports 2019;17(8):1668-94. [DOI: ] [DOI] [PubMed] [Google Scholar]
Auckland District Health Board 2017
- Auckland District Health Board. Guideline: Mastitis Prevention and Treatment. nationalwomenshealth.adhb.govt.nz/assets/Womens-health/Documents/Policies-and-guidelines/Mastitis-Prevention-and-Treatment-.pdf (accessed prior to 12 September 2020).
Bell 1998
- Bell KK, Rawlings NL. Promoting breast-feeding by managing common lactation problems. Nurse Practitioner 1998;23(6):102-4, 106, 109-10. [PubMed] [Google Scholar]
Boakes 2018
- Boakes E, Woods A, Johnson N, Kadoglou N. Breast Infections: a review of diagnosis and management practices. European Journal of Breast Health 2018;14:136-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chezem 2003
- Chezem J, Friesen C, Boettcher J. Breastfeeding knowledge, breastfeeding confidence, and infant feeding plans: effects on actual feeding practices. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2003;32(1):40-7. [DOI] [PubMed] [Google Scholar]
Chua 1994
- Chua S, Arulkumaran S, Lim I, Selemat N, Ratnam SS. Influence of breastfeeding and nipple stimulation on postpartum uterine activity. British Journal of Obstetrics and Gynaecology 1994;101:804-5. [DOI] [PubMed] [Google Scholar]
Cummings 1993
- Cummings R, Klineberg R. Breastfeeding and other reproductive factors and the risk of hip fractures in elderly women. International Journal of Epidemiology 1993;22:684-91. [DOI] [PubMed] [Google Scholar]
Cusack 2011
- Cusack, L Brennan M. Lactational mastitis and breast abscess: diagnosis and management in general practice. Australian Family Physician 2011;40(12):976-9. [PubMed] [Google Scholar]
Dener 2003
- Dener C, Inan A. Breast abscesses in lactating women. World Journal of Surgery 2003;27:130-3. [DOI] [PubMed] [Google Scholar]
Dennis 1999
- Dennis CL, Faux S. Development and psychometric testing of the breastfeeding self-efficacy scale. Research in Nursing and Health 1999;22:399–409. [DOI] [PubMed] [Google Scholar]
Dewey 1999
- Dewey KG. Maternal weight loss patterns during prolonged lactation. Australian Journal of Clinical Nutrition 1999;58:162-6. [DOI] [PubMed] [Google Scholar]
Dyson 2005
- Dyson L, McCormick FM, Renfrew MJ. Interventions for promoting the initiation of breastfeeding. Cochrane Database of Systematic Reviews 2005, Issue 2. Art. No: CD001688. [DOI: 10.1002/14651858.CD001688.pub2] [DOI] [PubMed] [Google Scholar]
Fetherston 1997
- Fetherston C. Characteristics of lactation mastitis in a Western Australian cohort. Breastfeeding Review 1997;5(2):5-11. [0729-2759 (Print)] [PubMed] [Google Scholar]
Fetherston 1998
- Fetherston C. Risk factors for lactation mastitis. Journal of Human Lactation 1998;14(2):101-9. [DOI] [PubMed] [Google Scholar]
Flores 2002
- Flores M, Filteau S. Effect of lactation counselling on subclinical mastitis among Bangladeshi women. Annals of Tropical Paediatrics 2002;22(1):85-8. [0272-4936 (Print)] [DOI] [PubMed] [Google Scholar]
Foxman 1994
- Foxman B, Schwartz K, Looman SJ. Breastfeeding practices and lactation mastitis. Social Science & Medicine 1994;38(5):755-61. [0277-9536 (Print)] [DOI] [PubMed] [Google Scholar]
Foxman 2002
- Foxman B, D'Arcy H, Gillespie B, Bobo JK, Schwartz K. Lactation mastitis: occurrence and medical management among 946 breastfeeding women in the United States. American Journal of Epidemiology 2002;155(2):103-14. [0002-9262 (Print)] [DOI] [PubMed] [Google Scholar]
Gurtovoi 1979
- Gurtovoi BL. Principles of treatment of postpartum mastitis. Akusherstvo i Ginekologiia 1979;11:40-4. [PubMed] [Google Scholar]
Gustafsson 2018
- Gustafsson A, Granström E, Stecksén-Blicks C, West CE, Silfverdal SA. The antisecretory factor in plasma and breast milk in breastfeeding mothers — a prospective cohort study in Sweden. Nutrients 2018;10:1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guttman 2000
- Guttman N, Zimmerman DR. Low-income mothers' views on breastfeeding. Social Science & Medicine 2000;50:1457-73. [DOI] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 [updated July 2019]. Cochrane, 2019. Available from handbook.cochrane.org.
Inch 2006
- Inch S. Breastfeeding problems: prevention and management. Community Practitioner 2006;79(5):165-7. [1462-2815 (Print)] [PubMed] [Google Scholar]
Jahanfar 2013
- Jahanfar S, Ng CJ, Teng CL. Antibiotics for mastitis in breastfeeding women. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD005458. [DOI: 10.1002/14651858.CD005458.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinlay 2001
- Kinlay JR, O'Connell DL, Kinlay S. Risk factors for mastitis in breastfeeding women: results of a prospective cohort study. Australian and New Zealand Journal of Public Health 2001;25(2):115-20. [1326-0200 (Print)] [DOI] [PubMed] [Google Scholar]
Kramer 2002
- Kramer MS, Kakuma R. Optimal duration of breastfeeding. Cochrane Database of Systematic Reviews 2002, Issue 1. Art. No: CD003517. [DOI: 10.1002/14651858.CD003517] [DOI] [PubMed] [Google Scholar]
Kramer 2012
- Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database of Systematic Reviews 2012, Issue 8. Art. No: CD003517. [DOI: 10.1002/14651858.CD003517.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kulakov 2004
- Kulakov AA, Shkoda SM, Astasvov EI, Protasenko PYA, Petrov VI. Lactation mastitis: problems and perspectives. Khiruugiia 2004;6:36-8. [PubMed] [Google Scholar]
Lange 2001
- Lange S, Lönnroth I. The antisecretory factor: synthesis, anatomical and cellular distribution, and biological action in experimental and clinical studies. International Review of Cytology 2001;210:39-75. [DOI] [PubMed] [Google Scholar]
Leff 1994
- Leff EW, Jefferis SC, Gagne MP. The development of the maternal breastfeeding evaluation scale. Journal of Human Lactation 1994;10(2):105–11. [DOI] [PubMed] [Google Scholar]
Lumbiganon 2016
- Lumbiganon P, Martis R, Laopaiboon M, Festin MR, Ho JJ, Hakimi M. Antenatal breastfeeding education for increasing breastfeeding duration. Cochrane Database of Systematic Reviews 2016, Issue 12. Art. No: CD006425. [DOI: 10.1002/14651858.CD006425.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
MacDonald 2003
- MacDonald A. Is breast best? Is early solid feeding harmful? Journal of the Royal Society of Health 2003;123(3):169-74. [0264-0325 (Print)] [DOI] [PubMed] [Google Scholar]
Mangesi 2016
- Mangesi L, Zakarija‐Grkovic I. Treatments for breast engorgement during lactation. Cochrane Database of Systematic Reviews 2016, Issue 6. Art. No: CD006946. [DOI: 10.1002/14651858.CD006946.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Melton 1993
- Melton LJ, Bryant SC, Wahner HW, O'Fallon WM, Malkasian GD. Influence of breastfeeding and other reproductive factors on bone mass later in life. Osteoporosis International 1993;3:76-83. [DOI] [PubMed] [Google Scholar]
Michie 2003
- Michie C, Lockie F, Lynn W. The challenge of mastitis. Archives of Disease in Childhood 2003;88(9):818-21. [1468-2044 (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitra 2004
- Mitra AK, Khoury AJ, Hinton AW, Carothers C. Predictors of breastfeeding intention among low-income women. Maternal and Child Health Journal 2004;8(2):65-70. [DOI] [PubMed] [Google Scholar]
Potter 2005
- Potter B. A multi-method approach to measuring mastitis incidence. Community Practitioner 2005;78:169-73. [PubMed] [Google Scholar]
Pustotina 2016
- Pustotina O. Management of mastitis and breast engorgement in breastfeeding women. Journal of Maternal-Fetal & Neonatal Medicine 2016;29(19):3121-5. [PMID: ] [PMID: doi: 10.3109/14767058.2015.1114092] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenblatt 1993
- Rosenblatt K, Thomas D. Lactation and the risk of epithelial ovarian cancer. International Journal of Epidemiology 1993;22:192-7. [DOI] [PubMed] [Google Scholar]
Schwartz 2002
- Schwartz K, D'Arcy HJS, Gillespie B, Bobo J, Longeway M, Foxman B. Factors associated with weaning in the first 3 months postpartum. Journal of Family Practice 2002;51(5):439-44. [00943509] [PubMed] [Google Scholar]
Semba 2000
- Semba RD. Mastitis and transmission of human immunodeficiency virus through breast milk. Annals of the New York Academy of Sciences 2000;918:156-62. [0077-8923 (Print)] [DOI] [PubMed] [Google Scholar]
Sinha 2015
- Sinha B, Chowdhury R, Jeeva Sankar M, Martines J, Taneja S, Mazumder S, et al. Interventions to improve breastfeeding outcomes: a systematic review and meta-analysis. Acta Pædiatrica 2015;104:114-35. [DOI] [PubMed] [Google Scholar]
Siskind 1997
- Siskind V, Green A, Bain C, Purdie D. Breastfeeding, menopause, and epithelial ovarian cancer. Epidemiology 1997;8:188-91. [DOI] [PubMed] [Google Scholar]
Spencer 2008
- Spencer JP. Management of mastitis in breastfeeding women. American Family Physician 2008;78(6):727-31. [PubMed] [Google Scholar]
Spowart 2004
- Spowart K, Heinig J, Ishii K. Dealing with mastitis. Journal of Human Lactation 2004;20(2):238-9. [DOI] [PubMed] [Google Scholar]
Tanchev 2004
- Tanchev S, Vulkova S, Georgieva V, Gesheva IU, Tsvetkov M. Lansinoh in the treatment of sore nipples in breastfeeding women. Akusherstvo i Ginekologiia 2004;43 Suppl 3:27-30. [PubMed] [Google Scholar]
Thompson 2005
- Thompson J. Breastfeeding: benefits and implications. Part two. Community Practitioner 2005;78(6):218-9. [1462-2815 (Print)] [PubMed] [Google Scholar]
Vogel 1999
- Vogel A, Hutchison BL, Mitchell EA. Mastitis in the first year postpartum. Birth 1999;26(4):218-25. [0730-7659 (Print)] [DOI] [PubMed] [Google Scholar]
Wambach 2016
- Wambach K, Riordan J. Breastfeeding and Human Lactation. 5th (enhanced) edition. Jones & Bartlett, 2016. [Google Scholar]
WHO 2000
- World Health Organization. Mastitis Causes and Management. Geneva: World Health Organisation, 2000. [DOI: ] [Google Scholar]
Witt 2016
- Witt AM, Bolman M, Kredit S, Vanic A. Therapeutic breast massage in lactation for the management of engorgement, plugged ducts and mastitis. Journal of Human Lactation 2016;32:123-31. [DOI] [PubMed] [Google Scholar]
World Health Organization 2008
- WHO, UNICEF, USAID, AED, UCDAVIS, IFPRI. Indicators for assessing infant and young child feeding practices. Part 1. Definitions. Conclusions of a consensus meeting held November 6-8 2007 in Washington DC, USA. www.who.int/maternal_child_adolescent/documents/9789241596664/en/ (accessed prior to 12 September 2020). [ISBN: 978 92 4 159666 4]
References to other published versions of this review
Crepinsek 2012
- Crepinsek MA, Crowe L, Michener K, Smart NA. Interventions for preventing mastitis after childbirth. Cochrane Database of Systematic Reviews 2012, Issue 10. Art. No: CD007239. [DOI: 10.1002/14651858.CD007239.pub3] [DOI] [PubMed] [Google Scholar]


