Abstract
Background
Preterm infants require high protein intake to achieve adequate growth and development. Although breast milk feeding has many benefits for this population, the protein content is highly variable, and inadequate to support rapid infant growth. This is a 2020 update of a Cochrane Review first published in 1999.
Objectives
To determine whether protein‐supplemented human milk compared with unsupplemented human milk, fed to preterm infants, improves growth, body composition, cardio‐metabolic, and neurodevelopmental outcomes, without significant adverse effects.
Search methods
We used the standard search strategy of Cochrane Neonatal to search Cochrane Central Register of Controlled Trials (CENTRAL 2019, Issue 8) in the Cochrane Library and MEDLINE via PubMed on 23 August 2019. We also searched clinical trials databases and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
Published and unpublished RCTs were eligible if they used random or quasi‐random methods to allocate hospitalised preterm infants who were being fed human milk, to additional protein supplementation or no supplementation.
Data collection and analysis
Two review authors independently abstracted data, assessed risk of bias and the quality of evidence at the outcome level, using GRADE methodology. We performed meta‐analyses, using risk ratio (RR) for dichotomous data, and mean difference (MD) for continuous data, with their respective 95% confidence intervals (CIs). We used a fixed‐effect model and had planned to explore potential causes of heterogeneity via subgroup or sensitivity analyses.
Main results
We included six RCTs, involving 204 preterm infants. The risk of bias for most methodological domains was unclear as there was insufficient detail reported. Low‐quality evidence showed that protein supplementation of human milk may increase in‐hospital rates of growth in weight (MD 3.82 g/kg/day, 95% CI 2.94 to 4.7; five RCTs, 101 infants; I² = 73%), length (MD 0.12 cm/wk, 95% CI 0.07 to 0.17; four RCTs, 68 infants; I² = 89%), and head circumference (MD 0.06 cm/wk, 95% CI 0.01 to 0.12; four RCTs, 68 infants; I² = 84%). Protein supplementation may lead to longer hospital stays (MD 18.5 days, 95% CI 4.39 to 32.61; one RCT, 20 infants; very low‐quality evidence). Very low quality evidence means that the effect of protein supplementation on the risk of feeding intolerance (RR 2.70, 95% CI 0.13 to 58.24; one RCT, 17 infants), or necrotizing enterocolitis (RR 1.11, 95% CI 0.07 to 17.12; one RCT, 76 infants) remains uncertain. No data were available about the effects of protein supplementation on neurodevelopmental outcomes.
Authors' conclusions
Low‐quality evidence showed that protein supplementation of human milk, fed to preterm infants, increased short‐term growth. However, the small sample sizes, low precision, and very low‐quality evidence regarding duration of hospital stay, feeding intolerance, and necrotising enterocolitis precluded any conclusions about these outcomes. There were no data on outcomes after hospital discharge. Our findings may not be generalisable to low‐resource settings, as none of the included studies were conducted in these settings.
Since protein supplementation of human milk is now usually done as a component of multi‐nutrient fortifiers, future studies should compare different amounts of protein in multi‐component fortifiers, and be designed to determine the effects on duration of hospital stay and safety, as well as on long‐term growth, body composition, cardio‐metabolic, and neurodevelopmental outcomes.
Plain language summary
Protein supplementation of human milk for promoting growth in preterm infants
Review question
We reviewed the evidence to see whether the addition of extra protein to human milk, compared with no additional protein, fed to preterm infants, improved growth, body fat, obesity, heart problems, high blood sugar, and brain development, without significant side effects.
Background
Lack of adequate protein intake during the early stages of the preterm infant's life can result in poor growth and development. Preterm infants need more protein than full term babies. Breast milk has numerous benefits for babies born preterm (before 37 weeks), but its protein content is variable, and may not meet the nutritional needs of the rapidly growing preterm infant. Therefore, to meet their higher protein needs, and to promote optimum health and long‐term development, additional protein, in the form of a fortifier, may be added to expressed breast milk for preterm babies.
Study characteristics
We found six randomised trials (trials in which each infant had an equal chance of being chosen to receive either treatment), involving 204 preterm infants. The search is up to date to August 2019.
Key results
Low‐quality evidence showed that the addition of extra protein to breast milk increased short‐term rates of weight gain (five trials), length gain (four trials), and head growth (four trials). Low‐quality evidence from one trial did not show a clear difference in the rate of growth of skin fold thickness (measure of fat under the skin) between the supplemented and unsupplemented groups. Very low‐quality evidence from one trial reported that infants who received additional protein stayed in hospital longer, while very low‐quality evidence from four trials observed higher blood urea nitrogen concentrations (measure of kidney function and protein breakdown) in these infants, compared to those who received no additional protein. Very low‐quality evidence from one trial suggested that adding extra protein to expressed breast milk did not clearly increase the risk of necrotising enterocolitis (inflammation of the intestine) or feeding intolerance, or clearly alter serum albumin concentrations (a measure of blood protein levels). No data were available on the effects of adding extra protein to human milk on long‐term growth, body fat, obesity, high blood sugar, or brain development.
Conclusions
Adding extra protein to human milk for preterm infants may increase short‐term growth. However, its effect on length of hospital stay, feeding intolerance, and necrotizing enterocolitis is uncertain, due to data limitations and very low‐quality evidence. There were no data about effects on later health and development, or effects in low resource settings.
Since protein supplementation of human milk is now usually done as a component of multi‐nutrient fortifiers, future studies should compare different amounts of protein in multi‐component fortifiers, and be designed to determine the effects on length of hospital stay, safety, long‐term growth, body fat, obesity, high blood sugar, and brain development.
Summary of findings
Summary of findings 1. Protein supplementation compared to control for promoting growth in preterm infants.
| Protein supplementation compared to no supplementation for promoting growth in preterm infants | ||||||
| Patient or population: preterm infants Setting: hospital Intervention: protein supplementation Comparison: no protein supplementation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Risk with Protein supplementation | |||||
| Growth: weight (weight gain g/kg/day) | The meand weight gain in the unsupplemented human milk group was 13.3 g/kg/day | Mean weight gain 3.82 g/kg/day higher (2.94 higher to 4.7 higher). | — | 101 (5 RCTs) | ⊕⊕⊝⊝ Low a b | mean difference (MD) 3.82, 95% CI 2.94 to 4.70 |
| Growth: length (cm/week) | The meand length gain in the unsupplemented human milk group was 0.41 cm/week | Mean length gain 0.12 cm/week higher (0.07 higher to 0.17 higher) | — | 68 (4 RCTs) | ⊕⊕⊝⊝ Low a b | MD 0.12, 95% CI 0.07 to 0.17 |
| Growth: head circumference (cm/week) | The meand head circumference gain in the unsupplemented human milk group was 0.68 cm/week | Mean head growth 0.06 cm/week higher (0.01 higher to 0.12 higher) | — | 68 (4 RCTs) | ⊕⊕⊝⊝ Low a b | MD 0.06, 95% CI 0.01 to 0,12 |
| Neurodevelopmental outcomes | see comments | see comments | see comments | see comments | see comments | None of the included studies reported on neurodevelopmental outcomes. |
| Duration of hospital stay (days) | The mean duration of hospital stay in the unsupplemented human milk group was 48.7 days | Mean difference 18.5 days higher (4.39 higher to 32.61 higher) | — | 20 (1 RCT) | ⊕⊝⊝⊝ Very lowa c | MD 18.5, 95% CI 4.39 to 32.61 |
| Feeding intolerance | 0 per 1000e | 0 per 1000 (0 to 0) | RR 2.70 (0.13 to 58.24) | 17 (1 RCT) | ⊕⊝⊝⊝ Very low a c | No events reported in the control group (0/8). One event reported in the fortified group (1/9). |
| Necrotising enterocolitis | 25 per 1000e | 28 per 1000 (2 to 322) | RR 1.11 (0.07 to 18.49) | 76 (1 RCT) | ⊕⊝⊝⊝ Very low a c | One event reported in the control group (1/40). One event reported in the fortified group (1/36). |
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
a Downgraded one level due to risk of bias; most studies rated as unclear due to lack of methodological details
bDowngraded one level due to moderate‐to‐high heterogeneity among the included studies estimating the population mean difference
cDowngraded two levels for imprecision ‐ few events, very wide confidence intervals
dThe base means were calculated as weighted mean, that is, the sum of (the mean from each study multiplied by the weight) divided by a summation of the weights for each study.
eThe assumed base risks were calculated as the total number of events in the control group divided by the total number of participants in the control group.
Background
Description of the condition
Optimum nutrition that meets the special needs of preterm infants remains a challenge. To match intrauterine growth (AAP Committee on Nutrition 1985), preterm infants require higher protein intake than full term infants, to accommodate their higher requirements for protein synthesis (Agostoni 2010; Hay 2010; Underwood 2013). Failing to consume sufficient amounts of protein, especially during the first few weeks, can result in compromised growth and organ development (Embleton 2001; Freitas 2016), particularly of the brain and central nervous system (Agostoni 2010; Claas 2011; Ghods 2011).
Breast milk, fed to the preterm infant, is associated with several benefits, including: reduction in rates of late‐onset sepsis (Schanler 1999), necrotizing enterocolitis (NEC; Sisk 2007), and retinopathy of prematurity (Okamoto 2007). Other benefits include: better feeding tolerance (Boyd 2007), improved neurodevelopmental outcomes (Bertino 2012), lower rates of metabolic syndrome (AAP 2012) and lower low‐density lipoprotein levels in adolescence (Bertino 2012).
Women who give birth preterm initially produce breast milk with higher amounts of protein than are found in full term milk. However, the protein content is inconsistent (Tudehope 2013). It varies between mothers, decreases within a breastfeeding session, and decreases after the first two weeks postnatally, when it is particularly needed to support rapid infant growth (Hay 2009; Su 2014). In addition, mothers of preterm infants face many difficulties that interfere with their establishment and maintenance of milk production. This limitation in breast milk supply may result in a reliance on donor human milk from mothers who gave birth at term, but this contains insufficient protein to support the high protein requirements of the preterm infant (Schanler 2005; Weber 2001).
Further, feeding preterm infants unsupplemented breast milk during neonatal admissions has been associated with inadequate growth (Brooke 1987; Su 2014; Tonkin 2014), which in turn is associated with longer hospital stays, more infections, and adverse short and long‐term developmental outcomes (Ehrenkranz 2006; Ehrenkranz 2010; Lapillonne 2013).
Thus, to meet the higher protein needs of rapidly growing preterm infants, and to promote their optimum health, additional protein in the form of a fortifier may be added to expressed breast milk.
Description of the intervention
Protein fortifiers are usually commercially available, and are produced in liquid or powder forms. They may also contain additional micronutrients and electrolytes, comprise hydrolyzed or intact protein, and can be bovine or human milk‐based. They are mixed with human milk, and fed to the preterm infant once they begin to tolerate enteral feeds (Di Natale 2011; Ziegler 2011).
Protein fortifiers increase the concentrations of protein, and potentially other micronutrients, in expressed breast milk. They are typically administered as a fixed dose per unit volume of breast milk, known as standardized fortification (Di Natale 2013). The amount also can be varied, depending on the measured or estimated protein content of the breast milk, to meet the infant’s needs (targeted fortification).
How the intervention might work
Protein‐fortified human milk is expected to improve postnatal growth and development, in part by providing essential amino acids and energy for tissue growth, and in part by interacting with endocrine systems, such as the insulin‐like growth factor I (IGF‐1) system. IGF‐1 plays an important role in growth, body composition, and cognition of preterm infants (Clemmons 2006; Hansen‐Pupp 2013; Socha 2011). At 30 weeks’ postmenstrual age, there is a reciprocal relationship between IGF‐1 and dietary protein in preterm infants (Hansen‐Pupp 2011). Low protein levels are associated with low IGF‐I concentrations (Yeung 2003), and lower lean mass in childhood (Chiesa 2008; Hellström 2016; Lo 2002). Therefore, the addition of protein to human milk is expected to raise IGF‐1 concentrations, decrease fat mass accretion, and limit the initial growth failure of preterm infants (Kim 2016; Koletzko 2005).
Complications from protein supplementation can occur. For example, fortifiers based on cow's milk (i.e. intact bovine protein) have been associated with the development of allergies in preterm infants from very early contact with heterologous proteins (Srinivasan 2010). In addition, powdered fortifiers are non‐sterile products, and therefore, carry the risk of bacterial contamination, which could predispose the preterm infant to sepsis (D'Netto 2000; Reich 2010). Furthermore, acidified, higher protein fortifiers have been shown to cause feeding intolerance and metabolic imbalances in preterm infants, possibly due to their immature metabolic processes and reduced kidney function (Cibulskis 2015; Thoene 2014). Preterm infants are at increased risk of developing metabolic and renal tubular acidosis (Koletzko 2005; Manz 1997). Thus, fortifiers which have been acidified as a form of sterilisation may have higher acid loads, and result in decreased growth (Kalhoff 1993; Kalhoff 2001). Finally, the addition of liquid fortifiers to human milk may displace the volume of human milk, and cause the infant to receive an inadequate total volume of human milk (Underwood 2013).
Why it is important to do this review
Protein supplementation of human milk would help to increase protein intake in very preterm infants, while retaining the benefits of feeding human milk. However, fortifiers are often expensive, their long‐term benefits, if any, are uncertain, and their use has been associated with some adverse effects (Thoene 2014; Tonkin 2014). It is imperative to determine the benefits and harms of their use in both the short‐ and long‐term.
Objectives
To determine whether protein‐supplemented human milk, compared with unsupplemented human milk, fed to preterm infants, improves growth, body composition, cardio‐metabolic, and neurodevelopmental outcomes, without significant adverse effects.
Methods
Criteria for considering studies for this review
Types of studies
We considered published and unpublished randomised and quasi‐randomised controlled trials for this review.
Types of participants
Preterm infants (less than 37 weeks' gestation) receiving enteral feeding of human milk, within a hospital setting.
Types of interventions
Human milk, with or without additional protein supplementation. Micronutrient supplements were allowed in both groups.
Types of outcome measures
The primary and secondary outcomes for this review were aligned with the outcomes of the Cochrane Review, Multi‐nutrient fortification of human milk for preterm infants (Brown 2016).
Primary outcomes
Growth: weight, length, head circumference, skinfold thickness (WHO 1995), body mass index and measures of body composition (lean, fat mass) and growth restriction (proportion of infants below the 10th percentile for the index population distribution of weight, length, or head circumference). Growth parameters were assessed from birth to hospital discharge, at or after two years’ corrected age, during adolescence, and as adults.
Neurodevelopmental outcomes after 12 months’ post term: neurological evaluations, developmental scores, and classifications of disability, including auditory and visual disability. We defined neurodevelopmental impairment as the presence of one or more of the following: non‐ambulant cerebral palsy, developmental quotient more than two standard deviations below the population mean, blindness (visual acuity less than 6/60), or deafness (any hearing impairment requiring or unimproved by amplification).
Secondary outcomes
Duration of hospital admission
Feeding intolerance that resulted in cessation of or reduction in enteral feeding
Necrotising enterocolitis (NEC)
Blood urea nitrogen (BUN) concentrations
Serum albumin concentrations
Metabolic acidosis, as defined by trialists
Long‐term measures of cardio‐metabolic health, such as insulin resistance, obesity, diabetes, and hypertension
Search methods for identification of studies
Electronic searches
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2019, Issue 8) in the Cochrane Library and MEDLINE via PubMed (2018 to 23 August 2019). We have included the search strategies for each database in Appendix 1. We did not apply language restrictions.
We searched clinical trial registries for ongoing or recently completed trials (ISRCTN Registry). The World Health Organization’s International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en/) and the U.S. National Library of Medicine’s ClinicalTrials.gov (clinicaltrials.gov) were searched via Cochrane CENTRAL.
This search updates the searches conducted for previous versions of the review (Amissah 2018, Kuschel 2000c).
Searching other resources
We also searched the reference lists of any articles selected for inclusion in this review, in order to identify additional relevant articles. We did not search any additional conference proceedings.
Data collection and analysis
We used the criteria and standard methods of Cochrane Neonatal to assess the methodological quality of the included trials.
Two review authors (EA, JB) independently extracted the data, compared data, and resolved differences by discussion, or by consulting with a third review author (JH).
We used the standard methods of Cochrane Neonatal to synthesise the data. We expressed results as relative risk and mean difference.
Selection of studies
For the 2018 update, two review authors (EA and JB) independently screened the titles and abstracts of the records identified by the searches. We resolved conflicts by discussion, or by consulting with a third author (JH). We retrieved the full text of all potentially relevant articles, and linked reports of the same study. Two review authors (EA and JB) independently assessed the full‐text articles for inclusion, using the eligibility criteria. We resolved conflicts by discussion, or by consulting with a third author (JH). We had planned to correspond with investigators to clarify study eligibility and obtain missing results if needed. We used Covidence for the study selection and data collection processes (Covidence).
For the 2020 update, Cochrane Neonatal screened the titles and abstracts identified by the search, as well as potentially relevant full‐text articles, independently and in duplicate in consultation with a review author (JH).
Data extraction and management
We developed a data extraction form prior to data gathering, to enable two review authors to independently extract information from the studies. We extracted data such as source details, study eligibility, study design, participant characteristics, intervention and control details, and outcomes. We resolved conflicts in the data extraction and management process by discussion, or by consulting with a third review author. We then exported the data into Cochrane's review software, Review Manager 5 (Review Manager 2014).
Assessment of risk of bias in included studies
Two review authors (EA and JB) independently assessed the risk of bias (low, high, or unclear) of all included trials, using the Cochrane ‘Risk of bias’ tool, for the following domains (Higgins 2017):
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Any other bias
We resolved conflicts by discussion, or by consulting with a third review author. See Appendix 2 for a more detailed description of risk of bias for each item.
Measures of treatment effect
For dichotomous data, we used the number of events in the control and intervention groups of each study to calculate risk ratios (RRs) with 95% confidence intervals (CIs). For continuous data, we calculated mean differences (MDs) between treatment groups with 95% CIs, where outcomes were measured in the same way. We did not need to use standardised mean differences (SMD) in this update, but they will be used in future updates where outcomes from studies are the same, but different methods have been used to collect the data. We did not calculate numbers needed to treat for an additional beneficial outcome (NNTB) or the numbers needed for an additional harmful outcome (NNTH), due to insufficient data.
Unit of analysis issues
We did not identify any unit of analysis issues. In future updates, if we identify cluster‐randomised trials, we will undertake analysis at the individual level, taking clustering into account, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017).
Dealing with missing data
We noted levels of attrition. We carried out analyses using an intention‐to‐treat basis, where possible, for all of the outcomes. Where possible, we analysed all participants in the treatment group to which they were randomised, regardless of the actual treatment received. We did not contact any of the trial authors. In future updates, if data are missing, we will make an attempt to contact the trial authors. We were unable to conduct sensitivity analyses, and were unable to address the potential impact of missing data on the findings of the review, due to insufficient data.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. This was done by assessing statistical heterogeneity using the Chi² test and the I² statistic. We took an I² measurement greater than 50%, and P < 0.10 in the Chi² test for heterogeneity to indicate moderate‐to‐high heterogeneity. Where we detected moderate‐to‐high heterogeneity, we had planned to explore possible explanations for clinical heterogeneity via subgroup analyses or methodological heterogeneity via sensitivity analyses, or both. We had planned to take clinical and statistical heterogeneity into account when interpreting the results, especially if there was any variation in the direction of effect.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. Some types of reporting bias (e.g. publication bias, multiple publication bias, language bias) reduce the likelihood that all studies eligible for a review will be retrieved. If all eligible studies are not retrieved, the review may be biased. We aimed to conduct a comprehensive search for eligible studies, and were alert for duplication of data. We were unable to formally assess publication bias, as there were insufficient studies for any of the outcomes (10 or more studies required). In future updates, if we find 10 or more studies reporting an outcome, we will assess publication bias by visual inspection of a funnel plot.
Data synthesis
We performed meta‐analyses using Review Manager (Review Manager 2014). We used risk ratio (RR) for dichotomous data, and mean difference (MD) for continuous data, with their respective 95% confidence intervals (CIs). We used a fixed‐effect model to combine data where similar interventions, populations and methods were employed by the trials. We planned to explore potential causes of heterogeneity via sub‐group and sensitivity analyses and assessed the quality of evidence at the outcome level, using GRADE methodology.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook, to assess the quality of evidence for the following (clinically relevant) outcomes: growth, neurodevelopment, duration of hospital admission, feeding intolerance that resulted in cessation or reduction in enteral feeding, and necrotizing enterocolitis (Schünemann 2013).
Two authors (EA and JB) independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality, but downgraded the evidence one level for serious (or two levels for very serious) limitations, based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used GRADEpro GDT software to create a ‘Summary of findings’ table, to summarise results and report the quality of the evidence (GRADEpro GDT).
The GRADE approach leads to an assessment of the quality of a body of evidence at one of four levels:
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We considered whether an overall summary was meaningful by assessing clinical and methodological heterogeneity among trials (see section above on 'Assessment of Heterogeneity'). If we had found moderate‐to‐high heterogeneity, we had planned to perform subgroup and sensitivity analyses.We had planned to carry out the following subgroup analyses to evaluate differences in outcome between: gestational age subgroups (less than 30, versus 30 up to 34, versus 34 up to 37 completed weeks), birth weight subgroups (less than 1 kg versus 1 kg or above), male versus female, and types of protein supplements (bovine versus human, and with versus without micronutrients or minerals). However, there were insufficient data to allow us to conduct any subgroup analyses.
Sensitivity analysis
We had planned to conduct sensitivity analyses by examining only those trials considered to have a low risk of bias for allocation concealment and randomisation. We were unable to do this as all the included studies were judged to be of unclear risk of bias for both allocation concealment and randomisation.
Results
Description of studies
Results of the search
From initial search results of 1990 citations, we identified two additional studies (three publications) for inclusion in this update of the review (Faerk 2001; Greer 1986). For a full description of our selection process, please see our 'Study flow diagram' (Figure 1).
1.
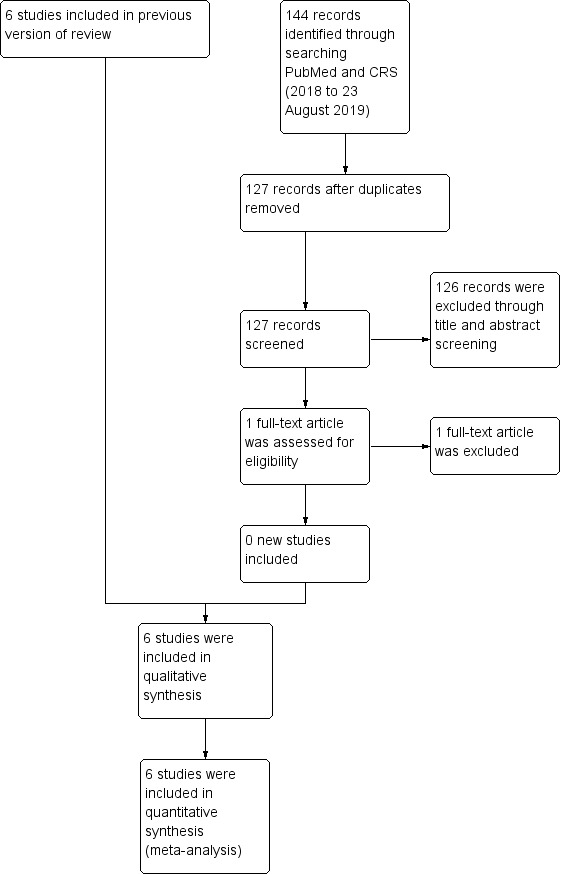
Study flow diagram: review update
Included studies
We included six studies in this review, and extracted data from full‐text publications for all six studies (Boehm 1988a; Faerk 2001; Greer 1986; Polberger 1989; Putet 1987; Rönnholm 1982). All studies were published in English between 1982 and 2001, and included a total of 204 preterm infants who fulfilled our predefined criteria. All the studies were reported to be randomised controlled trials. Four were single centre studies, while two were conducted at two centres each (Faerk 2001; Polberger 1989). Three studies were two‐armed randomised controlled studies (Boehm 1988a; Putet 1987; Rönnholm 1982), Faerk 2001 was three‐armed, and Greer 1986 and Polberger 1989 were four‐armed studies. Sample sizes ranged from 14 (Polberger 1989), to 103 preterm infants (Faerk 2001). Three studies were carried out in Europe (Boehm 1988a; Faerk 2001; Polberger 1989), and one in the USA (Greer 1986), but the locations of the other two were unclear. None of our included studies was conducted in a developing country. We summarised the details of the included studies in the 'Characteristics of included studies' table.
Participants
All the studies examined preterm infants less than 32 gestational weeks, except Boehm 1988a (less than 33 gestational weeks) and Rönnholm 1982 (up to and including 36 gestational weeks). It was not clear what gestational age threshold Putet 1987 studied, but they studied only male infants. Boehm 1988a was the only study that studied the effects of protein supplementation of human milk in standard birth weight groups (very low birth weight (VLBW) and low birth weight (LBW) infants). Greer 1986, Polberger 1989, Putet 1987, and Rönnholm 1982 studied infants with birth weight of less than 1600 g, Faerk 2001 studied infants less than 1200 g and more than 1200 g, and Boehm 1988a studied infants between 1000 g and 1990 g. All studies included infants with no medical problems or major congenital malformations except Faerk 2001, who included infants with medical illnesses, such as bronchopulmonary dysplasia, septicaemia, and intraventricular haemorrhage.
Interventions
Lyophilized human milk protein supplements were used in two studies (Boehm 1988a; Polberger 1989), and bovine casein hydrolysate was used in one study (Putet 1987). One study used bovine whey with mineral supplements (Greer 1986), Faerk 2001 used Eoprotin, and Rönnholm 1982 used human milk protein concentrate. Protein intakes ranged from 0.6 g/kg/day to 4.5 g/kg/day in the intervention groups. All infants in the intervention group of four studies received vitamin and mineral supplements (Faerk 2001; Greer 1986; Polberger 1989; Rönnholm 1982). Four studies used both maternal and donor breast milk (Boehm 1988a; Faerk 2001; Polberger 1989; Rönnholm 1982). Greer 1986 used maternal milk exclusively for infants in both the intervention and control groups, while Putet 1987 did not specify maternal or donor milk. All the studies used standardised rather than targeted fortification.
Comparators
Two studies used unsupplemented human milk alone (Boehm 1988a; Putet 1987), while Faerk 2001, Polberger 1989, and Rönnholm 1982 used human milk supplemented with vitamins and minerals. Greer 1986 used human milk with added vitamin supplements. Faerk 2001 included an unsupplemented arm, in which infants received additional formula if the mother's breast milk was insufficient. Data from this arm of the study were not included in our analysis.
Outcomes
All the studies included at least one of our outcomes of interest. Greer 1986, Polberger 1989, Putet 1987, and Rönnholm 1982 contributed data about the rate of growth, as weight, length, and head circumference. Faerk 2001 contributed weight, length, and head circumference data at term, and Polberger 1989 also provided data on weight at the end of the study. Only one study contributed data on skin fold thickness and duration of hospital stay (Greer 1986), while another contributed data on feeding intolerance and serum albumin concentrations (Polberger 1989). Faerk 2001 provided data on necrotising enterocolitis, and four studies contributed data on blood urea concentrations (Boehm 1988a; Greer 1986; Polberger 1989; Putet 1987). No trial reported data on long‐term growth, body mass index (BMI), body composition, neurodevelopmental, and cardio‐metabolic outcomes.
Excluded studies
We excluded 1 full‐text article from this 2020 update, added to 17 full‐text articles from the 2018 update. In total, fourteen studies (16 publications) used interventions that did not meet our criteria (Abrams 2014; Barrington 2016; Berseth 2012; Bhat 2001; Ditzenberger 2013; Gathwala 2012; Hair 2014; Hayashi 1994; Hill 1997; Kashaki 2018; Modanlou 1986; Moltu 2013; Polberger 1997; Valman 1971), and two studies were not randomised (Bishara 2017; Boehm 1988b). See the 'Characteristics of excluded studies table and Figure 1 for details of exclusions.
Risk of bias in included studies
Overall, we scored most items as unclear risk of bias, as there was insufficient methodological detail to make a judgement. We summarised our evaluations for individual studies in the 'Risk of bias' graph and summary (Figure 2; Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
We judged all six included studies as unclear risk of bias for random sequence generation and allocation concealment as they did not provide sufficient methodological detail to make a judgement (Boehm 1988a; Faerk 2001; Greer 1986; Polberger 1989; Putet 1987; Rönnholm 1982).
Blinding
Of all the studies included, only one had a low risk of performance bias, as they blinded study participants and personnel (Faerk 2001). We judged the remaining five studies as unclear risk of performance bias. We judged detection bias at unclear risk of bias for all six studies.
Incomplete outcome data
We judged two studies to have a low risk of attrition bias (Boehm 1988a; Faerk 2001), but all other studies as unclear risk.
Selective reporting
We judged two studies to have low risk of selective reporting bias (Greer 1986; Putet 1987). We judged Polberger 1989 as high risk of selective reporting bias because the authors did not report head circumference results at all time points, due to an initial finding of a minimal relation to protein intake in their trial. The remaining studies had unclear risk of reporting bias.
Other potential sources of bias
We judged other other potential sources of bias as unclear risk, except for Greer 1986 and Putet 1987, which we judged had low risk of bias, as no extreme baseline imbalances were identified.
Effects of interventions
See: Table 1
1.0 Protein supplementation versus control
1.1 Growth: weight
1.1.1 Weight gain
Five randomised controlled trials, including 101 infants, contributed data (Boehm 1988a; Greer 1986; Polberger 1989; Putet 1987; Rönnholm 1982). Protein supplementation of human milk was associated with more weight gain compared with unsupplemented human milk (mean difference (MD) 3.82 g/kg/day, 95% confidence interval (CI) 2.94 to 4.7; five RCTs, 101 infants; I² = 73%; low‐quality evidence; Analysis 1.1). We downgraded the evidence for risk of bias, as there was insufficient methodological information, and moderate heterogeneity among the studies estimating the population mean difference.
1.1. Analysis.

Comparison 1: Protein supplementation versus no supplementation, Outcome 1: Growth: weight
1.1.2 Weight at term‐equivalent age
Only Faerk 2001 reported the weight at term. There was no evidence of a clear difference between the protein supplemented and the unsupplemented groups (MD 61.0 g, 95% CI –160.23 to 282.23; one RCT, 76 infants; Analysis 1.1).
1.1.3 Weight at the end of the study
Polberger 1989 reported weight at the end of the study, which was when the infant weighed 2200 g or when breastfeeding was initiated. There was no evidence of a clear difference in weight between the protein supplemented and the unsupplemented groups (MD 250.0 g, 95% CI –41.56 to 541.56; one RCT, 14 infants; Analysis 1.1).
1.2 Growth: length
1.2.1 Length gain
Four randomised controlled trials, including 68 infants, contributed data (Greer 1986; Polberger 1989; Putet 1987; Rönnholm 1982). Protein supplementation of human milk was associated with more linear growth compared with unsupplemented human milk (MD 0.12 cm/week, 95% CI 0.07 to 0.17; four RCTs, 68 infants, I² = 89%; low‐quality evidence; Analysis 1.2). The evidence was downgraded for risk of bias, as there was insufficient methodological information, and high heterogeneity among the trials estimating the population mean difference.
1.2. Analysis.

Comparison 1: Protein supplementation versus no supplementation, Outcome 2: Growth: length
1.2.2 Length at term‐equivalent age
Faerk 2001 reported length at term. There was no evidence of a clear difference between the protein supplemented and unsupplemented groups (MD –0.5 cm, 95% CI –1.65 to 0.65; one RCT, 76 infants; Analysis 1.2).
1.3 Growth: head circumference
1.3.1 Head circumference gain
Four randomised controlled trials, including 68 infants, contributed data (Greer 1986; Polberger 1989; Putet 1987; Rönnholm 1982). Protein supplementation of human milk was associated with a greater increase in head circumference compared with unsupplemented human milk (MD 0.06 cm/week, 95% CI 0.01 to 0.12; four RCTs, 68 infants, I² = 84%; low‐quality evidence; Analysis 1.3). We downgraded the evidence for risk of bias, as there was insufficient methodological information and high heterogeneity among the trials estimating the population mean difference.
1.3. Analysis.

Comparison 1: Protein supplementation versus no supplementation, Outcome 3: Growth: head circumference
1.3.2 Head circumference at term‐equivalent age
Faerk 2001 reported the head circumference at term. There was no evidence of a clear difference between the protein supplemented and the unsupplemented groups (MD 0.3 cm, 95% CI 0.–24 to 0.84; one RCT, 76 infants; Analysis 1.3).
1.4 Growth: skin fold thickness
One trial including 20 children reported data on the rate of growth of skin fold thickness (Greer 1986). Neither the triceps nor the subscapular measurements showed a clear difference between the protein supplemented and unsupplemented groups (triceps MD 0.06 mm/week, 95% CI –0.09 to 0.21; one RCT, 20 infants; subscapular MD 0.0 mm/week, 95% CI –0.17 to 0.17; one RCT, 20 infants; Analysis 1.4).
1.4. Analysis.

Comparison 1: Protein supplementation versus no supplementation, Outcome 4: Growth: skin fold thickness
1.5 Duration of hospital stay
One trial reported on duration of hospital stay (Greer 1986). The protein supplemented group had a longer hospital stay than the unsupplemented group (MD 18.5 days, 95% CI 4.39 to 32.61; one RCT, 20 infants; very low‐quality evidence; Analysis 1.5). We downgraded the evidence for risk of bias, as there was insufficient methodological information to judge the risk of bias, few patients, few events, and very wide confidence intervals.
1.5. Analysis.

Comparison 1: Protein supplementation versus no supplementation, Outcome 5: Duration of hospital stay (days)
1.6 Feeding intolerance
Only Polberger 1989 reported data on feeding intolerance. There was one event reported in the supplemented group and no events reported in the unsupplemented group (risk ratio (RR) 2.70, 95% CI 0.13 to 58.24; one RCT, 17 infants; very low‐quality evidence; Analysis 1.6). We downgraded the evidence for risk of bias, as there was insufficient methodological information to judge the risk of bias, few patients, few events, and very wide confidence intervals.
1.6. Analysis.

Comparison 1: Protein supplementation versus no supplementation, Outcome 6: Feeding intolerance
1.7 Necrotising enterocolitis
One trial reported data on the incidence of necrotising enterocolitis (Faerk 2001). There was one infant in each of the protein supplemented and unsupplemented groups who developed necrotising enterocolitis (RR 1.11, 95% CI 0.07 to 17.12; one RCT, 76 infants; very low‐quality evidence; Analysis 1.7). We downgraded the evidence for risk of bias as there was insufficient methodological information to judge the risk of bias, few patients, few events, and very wide confidence intervals.
1.7. Analysis.

Comparison 1: Protein supplementation versus no supplementation, Outcome 7: Necrotising enterocolitis
1.8 Blood urea nitrogen (BUN)
Four randomised controlled trials contributed data (Boehm 1988a; Greer 1986; Polberger 1989; Putet 1987). BUN concentrations were higher in the protein supplemented group than in the unsupplemented group (MD 0.95 mmol/L, 95% CI 0.81 to 1.09; four RCTs, 81 infants; I² = 56%; Analysis 1.8).
1.8. Analysis.

Comparison 1: Protein supplementation versus no supplementation, Outcome 8: Blood urea (mmol/L)
1.9 Serum albumin concentrations
Only Polberger 1989 reported data on serum albumin concentrations. There was no evidence of a clear difference in serum albumin concentrations between the protein supplemented and unsupplemented groups (MD 2.5 g/L, 95% CI –5.66 to 10.66; one RCT, 11 infants; Analysis 1.9).
1.9. Analysis.

Comparison 1: Protein supplementation versus no supplementation, Outcome 9: Serum albumin (g/L)
No data were reported for the following outcomes: long‐term growth, body mass index (BMI), body composition, neurodevelopmental, and cardio‐metabolic outcomes.
The heterogeneity test indicated moderate‐to‐high variation among studies reporting growth outcomes, but there were insufficient data to allow us to conduct our prespecified subgroup analyses.
Discussion
Summary of main results
The evidence from six randomised controlled trials, involving 204 preterm infants, showed that protein supplementation of human milk may increase in‐hospital rates of weight gain, length gain, and head growth in preterm infants. There was no evidence of a clear difference in the rate of growth of skin fold thickness between the supplemented and unsupplemented groups. Protein supplementation was associated with longer hospital stays in one study, and higher blood urea nitrogen (BUN) concentrations. The evidence did not show that protein supplementation clearly altered the risk of necrotising enterocolitis (NEC) or feeding intolerance, or that it altered serum albumin concentrations. No data were available for the assessment of the effects of protein supplementation on long‐term growth outcomes, body mass index (BMI), body composition, neurodevelopmental, or cardio‐metabolic outcomes. We observed moderate‐to‐high heterogeneity among studies reporting growth outcomes.
Overall completeness and applicability of evidence
The lack of data on long‐term growth, BMI, body composition, neurodevelopmental, and cardio‐metabolic outcomes made it impossible to determine long‐term health and developmental effects of protein supplementation of human milk fed to preterm infants. Few trials reported our secondary outcomes (duration of hospital stay, feeding intolerance, NEC, and serum albumin concentrations), and therefore these outcomes had small sample sizes and very low‐quality evidence supported their estimates of effect, making it difficult to make an evidence‐based statement on these effects. In addition, due to incomplete data reporting, we were unable to identify reasons for the moderate‐to‐high heterogeneity among the sample population. We were also unable to conduct any of our planned subgroup analyses. Finally, the included studies were all performed in developed countries, and so our findings may not be generalisable to preterm infants in less developed countries.
Quality of the evidence
We graded the overall quality of evidence for the primary outcomes as low‐quality, because of lack of reported methodological details and moderate‐to‐high heterogeneity among the studies estimating the population mean difference. Without details such as blinding of study personnel and outcome assessors, it was difficult to adequately judge the risk of bias and quality of evidence, as blinding could have an impact on the assessment of growth parameters, and hence, possibly the estimate of effect size. All secondary outcomes were graded as very low‐quality evidence due to small sample sizes, few events, and small number of studies.
Potential biases in the review process
Due to small numbers of included studies, we were unable to create funnel plots to assess the potential risk of publication or reporting bias. We minimised bias by conducting a systematic search of the literature, and data extraction was undertaken independent by two review authors.
Agreements and disagreements with other studies or reviews
We are not aware of any previous systematic reviews conducted on this topic, except for our previous review, which included four single centre randomised and quasi‐randomised controlled trials published between 1982 and 1989, and involving 90 very low birth weight infants (Kuschel 2000a). The results of this updated systematic review are similar to those of the previous review, including increases in weight gain, length gain, head circumference, and BUN levels in the protein supplemented groups, but no clear effect on albumin concentrations or the risk of NEC. To our knowledge, this review is the first to include body composition and cardio‐metabolic outcomes, for which we found no available data.
Authors' conclusions
Implications for practice.
Protein supplementation of expressed breast milk fed to preterm infants increased short‐term rates of weight gain, length gain, and head growth, without evidence of a clearly increased risk of necrotising enterocolitis or feeding intolerance. Long‐term benefits and harms are unknown. Further, preterm infants fed solely breast milk have other nutritional deficiencies, including energy and minerals, so protein supplementation of human milk is now usually done as a component of multi‐nutrient fortifiers. We conclude that protein fortification of human milk in preterm infants could be considered in settings where the risk of poor postnatal growth is high and multi‐nutrient fortification is not available or feasible.
Implications for research.
Although there was evidence that protein supplementation increased short‐term growth, there were few data, and overall, very low‐quality evidence regarding potential short‐term risks and long‐term benefits and harms. Therefore, future studies should compare different amounts of protein in multi‐component fortifiers, and be designed to determine the effects on duration of hospital stay and safety, as well as on long‐term growth, body composition, cardio‐metabolic, and neurodevelopmental outcomes. These studies should also be conducted in resource‐poor settings.
What's new
| Date | Event | Description |
|---|---|---|
| 26 June 2020 | New search has been performed |
|
| 26 June 2020 | New citation required but conclusions have not changed | A supplementary search was carried out in August 2019. No new included studies were identified (one new excluded study was found).The main conclusions of the original review remained unchanged. |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 3, 1999
| Date | Event | Description |
|---|---|---|
| 2 February 2018 | New search has been performed |
|
| 23 January 2018 | New citation required but conclusions have not changed | The main conclusions of the original review remained unchanged. The updated review identified that protein supplementation led to longer hospital stays compared with no supplementation. |
| 3 November 2008 | Amended | Converted to new review format. |
| 13 December 1999 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Emma Amissah was supported by a doctoral scholarship from the University of Auckland.
Julie Brown was supported by a grant from the Health Research Council of New Zealand.
We acknowledge the support of Caroline A Crowther in preparing this review, and the work of Carl A Kuschel as lead author of the original review (Kuschel 2000c).
We would like to thank Fiona Stewart, Cochrane Children and Families Network, for assistance in screening searches.
Appendices
Appendix 1. Search methods 2020 update
MEDLINE via PubMed
(((((((((Dietary Protein[MeSH]) OR protein[tiab])) OR (((hydrolysate[tiab] OR hydrolys*[tiab] OR hydrolyz*[tiab])) NOT formula*))) AND ((((Milk, Human[MeSH]) OR breastmilk*[tiab])) OR (((human[tiab] OR breast[tiab] OR expressed[tiab] OR mother*[tiab] OR maternal[tiab] OR donor*[tiab])) AND milk*[tiab])))) AND ((((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))))))
Cochrane Central Register of Controlled Trials
| 1 | MESH DESCRIPTOR Milk, Human EXPLODE ALL AND CENTRAL:TARGET |
| 2 | ((human OR breast OR expressed) NEAR2 milk*):TI,AB,KW AND CENTRAL:TARGET |
| 3 | ((mother* or maternal or donor*) NEAR2 milk*): TI,AB,KW AND CENTRAL:TARGET |
| 4 | #1 OR #2 OR #3 |
| 5 | (infan* or newborn or neonat* or premature or preterm or very low birth weight or low birth weight or VLBW or LBW) AND CENTRAL:TARGET |
| 6 | MESH DESCRIPTOR Dietary Proteins EXPLODE ALL AND CENTRAL:TARGET |
| 7 | ((protein or hydrolysate or hydrolys* or hydrolyz*) NOT formula*):TI,AB,KW AND CENTRAL:TARGET |
| 8 | #6 OR #7 |
| 9 | #4 AND #5 AND #8 |
Appendix 2. Risk of bias tool
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorized the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table, computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth, hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorized the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation, consecutively numbered sealed opaque envelopes);
high risk (open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth); or
unclear risk
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
low risk, high risk, or unclear risk for participants; and
low risk, high risk, or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorized the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data, including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups, or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear risk
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Appendix 3. Previous search methods
MEDLINE via PubMed
(((((((((Dietary Protein[MeSH]) OR protein[tiab])) OR (((hydrolysate[tiab] OR hydrolys*[tiab] OR hydrolyz*[tiab])) NOT formula*))) AND ((((Milk, Human[MeSH]) OR breastmilk*[tiab])) OR (((human[tiab] OR breast[tiab] OR expressed[tiab] OR mother*[tiab] OR maternal[tiab] OR donor*[tiab])) AND milk*[tiab])))) AND ((((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))))))
Cochrane Central Register of Controlled Trials
| 1 | MESH DESCRIPTOR Milk, Human EXPLODE ALL AND CENTRAL:TARGET |
| 2 | ((human OR breast OR expressed) NEAR2 milk*):TI,AB,KW AND CENTRAL:TARGET |
| 3 | ((mother* or maternal or donor*) NEAR2 milk*): TI,AB,KW AND CENTRAL:TARGET |
| 4 | #1 OR #2 OR #3 |
| 5 | (infan* or newborn or neonat* or premature or preterm or very low birth weight or low birth weight or VLBW or LBW) AND CENTRAL:TARGET |
| 6 | MESH DESCRIPTOR Dietary Proteins EXPLODE ALL AND CENTRAL:TARGET |
| 7 | ((protein or hydrolysate or hydrolys* or hydrolyz*) NOT formula*):TI,AB,KW AND CENTRAL:TARGET |
| 8 | #6 OR #7 |
| 9 | #4 AND #5 AND #8 |
CINAHL
| S1 | MH "Dietary Proteins+" OR ( ( TI ( protein OR hydrolysate OR hydrolys* OR hydrolyz* ) ) NOT formula* ) OR ( ( AB ( protein OR hydrolysate OR hydrolys* OR hydrolyz* ) ) NOT formula* ) |
| S2 | MH "Milk, Human+" OR TI ( ((human or breast or expressed) N2 milk*) ) OR AB ( ((human or breast or expressed) N2 milk*) ) OR TI ( ((mother* or maternal or donor*) N2 milk*) ) OR AB ( ((mother* or maternal or donor*) N2 milk*) ) OR TI "breastmilk" OR AB "breastmilk" |
| S3 | ( (infan* OR newborn OR neonat* OR premature OR low birth weight OR VLBW OR LBW) ) AND ( (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) ) |
| S4 | S1 AND S2 AND S3 |
Embase
| 1 | exp protein intake/ |
| 2 | ((protein or hydrolysate or hydrolys* or hydrolyz*) not formula$).ti,ab. |
| 3 | 1 or 2 |
| 4 | exp breast milk/ |
| 5 | ((human or breast or expressed) adj2 milk$).ti,ab. |
| 6 | ((mother$ or maternal or donor$) adj2 milk$).ti,ab. |
| 7 | 4 or 5 or 6 |
| 8 | (infan* or newborn or neonat* or premature or very low birth weight or low birth weight or VLBW or LBW).mp. |
| 9 | exp infant/ |
| 10 | 8 or 9 |
| 11 | (human not animal).mp. |
| 12 | (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial).mp. |
| 13 | 10 and 11 and 12 |
| 14 | 3 and 7 and 13 |
Data and analyses
Comparison 1. Protein supplementation versus no supplementation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Growth: weight | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 Weight gain (g/kg/day) | 5 | 101 | Mean Difference (IV, Fixed, 95% CI) | 3.82 [2.94, 4.70] |
| 1.1.2 Weight at term‐equivalent age (grams) | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 61.00 [‐160.23, 282.23] |
| 1.1.3 Weight at end of study (grams) | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 250.00 [‐41.56, 541.56] |
| 1.2 Growth: length | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Length gain (cm/week) | 4 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [0.07, 0.17] |
| 1.2.2 Length at term‐equivalent age (cm) | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐1.65, 0.65] |
| 1.3 Growth: head circumference | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 Head growth (cm/week) | 4 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [0.01, 0.12] |
| 1.3.2 Head circumference at term‐equivalent age (cm) | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.24, 0.84] |
| 1.4 Growth: skin fold thickness | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.4.1 Skinfold thickness (mm/week): triceps | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.4.2 Skinfold thickness (mm/week): subscapular | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.5 Duration of hospital stay (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.6 Feeding intolerance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7 Necrotising enterocolitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.8 Blood urea (mmol/L) | 4 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.95 [0.81, 1.09] |
| 1.9 Serum albumin (g/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Boehm 1988a.
| Study characteristics | ||
| Methods | Parallel randomised controlled trial, single centre | |
| Participants | Inclusion criteria: infants without major clinical problems, VLBW or LBW infants. Exclusion criteria: not specified Setting: neonatal unit of the Karl Marx University Children’s Hospital in Leipzig, Germany Timing: not specified |
|
| Interventions | Lyophilised human milk protein (6 g/100mL) supplementation of maternal or donor preterm human milk (17 infants) versus unsupplemented human milk (16 infants) Not stated when intervention ceased |
|
| Outcomes | Primary outcomes: outcomes not specified into primary and secondary, but protein intake on 8th and 21st days of age, weight gain, daily serum urea, and alpha‐amino‐nitrogen concentrations taken 60 minutes after a feed were measured Secondary outcomes: not specified |
|
| Notes | Conflicts of interest: no details Source of funding: no details Both the VLBW and LBW arms of the study were included in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants randomised appear to have been analysed |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear by comparison of the methods and results sections that all the results of pre‐specified outcomes were stated. |
| Other bias | Unclear risk | No other sources of bias noted. |
Faerk 2001.
| Study characteristics | ||
| Methods | Parallel randomised controlled trial, multiple centres | |
| Participants | Inclusion criteria: preterm infants with gestational age of < 32 weeks and no major congenital malformations Exclusion criteria: not stated Setting: NICU at Rigshospitalet and Hvidovre Hospital in Copenhagen. Some infants were discharged to local neonatal units at Glostrup and Hilleroed Hospitals, but continued in the study. Timing: not stated |
|
| Interventions | Human milk (mother + donor) supplemented with protein, calcium, and phosphate (Eoprotin, 0.4 g protein, 35 mg Ca and 17 mg P/100 mL; N = 51) versus human milk + phosphate (10 mg P/100 mL; N = 52) All infants received 800 IU of vitamin D daily. The intervention ceased at the end of the 36th gestational week or when infants were fully breast fed, whichever came first. |
|
| Outcomes | Primary: total body mineral content at term by DEXA scan Secondary: body weight, crown‐heel length, head circumference, body composition, and bone area at term. |
|
| Notes | Conflicts of interest: not stated Source of funding: not stated This study had three arms ‐ unsupplemented human milk or formula versus human milk supplemented with phosphate versus human milk supplemented with protein, calcium and phosphate. Infants in the unsupplemented arm were fed only their own mother's milk. However, if the mother's breast milk was insufficient, her infant was fed formula as well. Data on the unsupplemented human milk or formula arm were not included in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of how the random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | The randomisation was done in an independent centre in Copenhagen (a human milk bank). They used sealed envelopes which gave some assurance. However, it was not stated if the envelopes were opaque. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “The study was kept blinded for the PI, the parents, all the attending physicians, and at Hvidovre Hospital, Rigshospitalet, all the attending staff”. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 217 infants fulfilled the inclusion criteria, parents of 52 infants refused to participate. 165 were randomised, 15 withdrew from the fortifier group due to unacceptable or no DEXA scans and other reasons, while 12 withdrew from the unfortified group also for unacceptable or no DEXA scans and other reasons. Other reasons included: abdominal discomfort in three cases, non‐acceptance of blinding principle in one case, on request of the attending physician in one case, and in two cases no reasons were stated. The remaining 11 withdrawals were from the formula group, which is not part of this review. |
| Selective reporting (reporting bias) | Low risk | The study protocol was available and all of the study’s prespecified (primary and secondary) outcomes were reported in the prespecified way. |
| Other bias | Unclear risk | No demographic tables were provided to make the assessment. The sample included sick preterm infants |
Greer 1986.
| Study characteristics | ||
| Methods | Parallel randomised controlled trial, single centre | |
| Participants | Inclusion criteria: "all infants with birth weight < 1600 grams, gestational age < 32 weeks who were admitted to the neonatal intensive care unit at Madison General Hospital were potentially eligible for enrolment into the study". Included infants received less than 3 weeks of mechanical ventilation and were receiving full enteral feedings by 21 days of age. No infants had chronic lung disease (supplemental oxygen ≥ 28 days) or received furosemide therapy. Exclusion criteria: major congenital anomalies, chronic intrauterine infection, significant gastrointestinal disease (hepatitis, cholestatic jaundice, malabsorption), seizure disorders requiring anticonvulsant therapy, or if there was more than a 2‐week discrepancy between the two determinations of gestational age (history of last menstrual period and Ballard examination for infants > 27 gestational weeks or fetal ultrasound dating for those ≤ 27 weeks' gestational age) Setting: neonatal units at Madison General hospital, Wisconsin Timing: 28 February 1983 to 1 April 1985 |
|
| Interventions | Exclusively mother's own milk supplemented with 0.85 gm/dL bovine whey, 90 mg/dL calcium, and 45 mg/dL phosphorus (N = 10) versus mother's own milk with no fortification (N = 10) All infants in these groups, except those fed formula, received a standard daily multivitamin supplement containing 400 IU of vitamin D. Not clear when the intervention ceased. |
|
| Outcomes | Primary outcomes: outcomes were not specified as primary or secondary, but short‐term growth parameters assessed were weight, length, head circumference, and skin fold thickness. Bone mineral content, bone width, serum calcium, phosphorus, creatinine, blood urea nitrogen, total protein, methionine, taurine and cysteine concentrations, alkaline phosphatase, urinary calcium, phosphate, inorganic sulphate, and creatinine concentrations, Breast milk composition and length of stay were also assessed. | |
| Notes | Conflicts of interest: no details Source of funding: Ross Laboratories, Columbus, Ohio This study had four arms ‐ unsupplemented human milk versus human milk supplemented with protein, calcium, and phosphorus, and Similac Special Care (special formula) versus standard formula. Data from the formula arms of this study were not used in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors stated that the 38 infants who completed the study represented "22% (38/176) of the total number of admitted infants who met the criteria for birth weight and gestational age". However, no flow chart or details were provided concerning attrition. |
| Selective reporting (reporting bias) | Low risk | There was no protocol available, but all pre‐specified outcomes in the methods section were reported. |
| Other bias | Low risk | No baseline imbalances identified and no other flaws of study design noted |
Polberger 1989.
| Study characteristics | ||
| Methods | Parallel randomised controlled trial, in two neonatal units | |
| Participants | Inclusion criteria: birth weight < 1500 grams, appropriate‐for‐gestational‐age, tolerance of complete enteral feeding (170 mL/kg/day), no obvious disease or major malformations, no oxygen therapy, informed parental consent, and acceptance of a blind trial Exclusion criteria: not stated Setting: two neonatal units of the University of Lund in Malmo and Lund Timing: not stated |
|
| Interventions | 1.0 grams lyophilised human milk protein per 100 mL unpasteurised human milk (maternal or term banked donor; N = 7) versus unsupplemented milk (N = 7)
Intervention ceased when the infant reached approximately 2200 g or was breast fed. All infants were supplemented with additional vitamins, calcium lactate (30 mg/kg/day) and sodium phosphate (20 mg/kg/day). From 4 weeks, 2 mg/kg/day elemental iron was given to all infants. |
|
| Outcomes | The outcomes were not specified as primary or secondary, but the following were assessed: short‐term growth parameters (weight, crown‐heel length, occipitofrontal head circumference), intake of protein, fat, carbohydrates, energy, and electrolytes (sodium, potassium, calcium). | |
| Notes | Conflicts of interest: no details Source of Funding: supported in part by the Swedish Medical Research Council, Grant No. B85‐ I'IX‐06259, and Stiftelsen Saniarite This study had four arms: unsupplemented versus supplemented with protein versus supplemented with fat versus supplemented with fat and protein. The analyses of the fat and combined fat and protein arms are discussed in other reviews on multi‐component and fat supplementation respectively (Brown 2016; Kuschel 2000b). Of the 34 infants enrolled in the study: 6 were withdrawn following randomisation for apnoea (N = 2), intolerance to accepting to the fixed volume (N = 3) and need for intravenous therapy (N = 1). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | The study used closed envelopes without specifying if they were opaque or not. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study was stated to be double blinded, but who was blinded was not specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided as to who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The missing data i.e. 6 infants, < 20%, were excluded for the following reasons: 2 had apnoea, 3 developed feeding intolerance, and 1 needed IV therapy. However, the authors did not report whether there were any differences between infants excluded and included in the study. |
| Selective reporting (reporting bias) | High risk | The authors stated “head circumference showed so little relation to protein intake that it was not included in further statistical evaluation (Table 3)”. This could suggest possible selective reporting and analytical bias, as not all outcomes were reported. |
| Other bias | Unclear risk | The authors stated "there was a difference in sex distribution between the groups, but later analyses confirmed that this difference had no implications on the results". However, no further details were provided. |
Putet 1987.
| Study characteristics | ||
| Methods | Parallel randomised controlled trial, single centre | |
| Participants | Inclusion criteria: VLBW boys of appropriate weight for gestational age and no infant had medical problems at the time of the study Exclusion criteria: not stated Setting: not stated Timing: not stated |
|
| Interventions | Cow's milk casein hydrolysate (1 g per 100 mL) added to pooled human milk (8 infants) versus unsupplemented pooled human milk (8 infants). Duration of intervention was not clear. | |
| Outcomes | Primary: not specified as primary and secondary outcomes, but short‐term growth parameters were assessed, including weight, length, head circumference. Total serum protein, BUN, acid‐base status, free amino acids, nitrogen retention, bicarbonate level, oxygen consumption, and energy expenditures were also assessed. | |
| Notes | Conflicts of interest: no details Source of funding: supported by a grant from University Claude Bernard (Lyon) and by an INSERM Contract (PRC 123.0228) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details |
| Selective reporting (reporting bias) | Low risk | The protocol was not available, but it appeared that all pre‐specified outcomes were measured and reported. |
| Other bias | Low risk | No other apparent sources of bias related to the study design noted. No extreme baseline imbalances observed. |
Rönnholm 1982.
| Study characteristics | ||
| Methods | Parallel randomised controlled trial, single centre | |
| Participants | Inclusion criteria: infants with birth weight less than 1500 grams were enrolled at 2 days of age, if free of major illness and major malformation Exclusion criteria: infants were excluded after randomisation if they developed major disease, died, or were lost to follow up Setting: not stated Timing: not stated Source of funding: supported by a grant from University Claude Bernard (Lyon) and by an INSERM Contract (PRC 123.0228). |
|
| Interventions | 0.8 g human milk protein per 100 mL human milk (pasteurised maternal or term donor milk) (N = 10) versus unsupplemented human milk (N = 8).Target fluid volume 200 mL/kg/day. All infants received supplemental iron and vitamins. Control infants received calcium supplements (10 mg/kg/day). It is not clear when the intervention was ceased. | |
| Outcomes | Not specified into primary and secondary, but short‐term growth parameters, such as weight, length, head circumference were measured. Serum protein concentrations, BUN, acid‐base balance, and plasma concentrations of tyrosine and phenylalanine were measured at two weeks of age. | |
| Notes | Conflicts of interest: no details Source of funding: grants from the Foundation for Pediatric Research in Finland Data were extracted from the 1982 publication (Rönnholm 1982). In the 1986 report (Ronholm 1986), half the infants in each group received supplementation with MCT oil, 1.0 g/100 mL and it was not possible to extract data for protein supplementation alone versus unsupplemented milk alone. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of the 23 preterm infants included in the study, 5 (21.7%) were excluded from the study because they subsequently developed major disease, died, or were lost to follow‐up. The authors did not report whether there were any differences between infants excluded and included in the study. |
| Selective reporting (reporting bias) | Unclear risk | Comparing the methods to the results section, it appeared all outcomes measured were reported, except for the acid‐base status, which did not appear to be prespecified. |
| Other bias | Unclear risk | No baseline characteristics were provided to assess for baseline imbalances. |
Abbreviations
VLBW: very low birth weight
LBW: low birth weight
DEXA: dual‐energy X‐ray absorptiometry
NICU: neonatal intensive care unit
PI: principal investigator
BUN: blood urea nitrogen
MCT: medium chain triglyceride
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abrams 2014 | Comparison of human milk‐based versus cow’s milk‐based protein supplementation |
| Barrington 2016 | Comparison of human milk or formula versus milk with 100 mg/day of bovine lactoferrin powder |
| Berseth 2012 | Unable to locate the full article |
| Bhat 2001 | Supplementation was with a multi‐nutrient fortifier |
| Bishara 2017 | Not a randomised trial |
| Boehm 1988b | No evidence of randomisation |
| Ditzenberger 2013 | Composition of the fortifier was not clear, infants received formula when mothers milk was insufficient, and a Microlipid was added to increase caloric density. |
| Gathwala 2012 | Supplementation was with a multi‐nutrient fortifier. |
| Hair 2014 | The control group received supplementation. |
| Hayashi 1994 | The fortifier contained extra carbohydrate. |
| Hill 1997 | Studies in the abstract of podium presentations were not related to our review. |
| Kashaki 2018 | Both groups received breast milk fortifier. |
| Modanlou 1986 | Supplementation was with a multi‐nutrient fortifier. |
| Moltu 2013 | The control group received supplementation. |
| Polberger 1997 | No unsupplemented arm |
| Valman 1971 | The intervention was either expressed human milk and breast milk substitute or evaporated milk formula. |
Differences between protocol and review
For the 2018 update:
The original protocol was published in 1997, and was the basis of the last version of this review, written in 1999. We aligned the review outcomes in the 2018 update with those of the Cochrane Review, Multi‐nutrient fortification of human milk for preterm infants (Brown 2016). We added body mass index and measures of body composition as part of growth parameters of the primary outcome. We also included new secondary outcome measures: long‐term measures of cardio‐metabolic health (such as insulin resistance, obesity, diabetes, and hypertension) We also added the 'Summary of findings' table and GRADE recommendations, which were not included in the original protocol. We expanded the search terms to find relevant studies for this update; therefore, we ran the search without any date limits to ensure that no eligible studies were missed.
For the 2020 update:
As of July 2019, Cochrane Neonatal no longer searches Embase for its reviews. RCTs and controlled clinical trials (CCTs) from Embase are added to the Cochrane Central Register of Controlled Trials (CENTRAL) via a robust process (see How CENTRAL is created). Cochrane Neonatal has validated their searches to ensure that relevant Embase records are found while searching CENTRAL.
Also starting in July 2019, Cochrane Neonatal no longer searches for RCTs and CCTs on the following platforms: ClinicalTrials.gov or from The World Health Organization’s International Clinical Trials Registry Platform (ICTRP), as records from both platforms are added to CENTRAL on a monthly basis (see How CENTRAL is created). Comprehensive search strategies are executed in CENTRAL to retrieve relevant records. The ISRCTN (at www.isrctn.com/, formerly Controlled‐trials.com), is searched separately.
We did not search CINAHL for this update. The 2018 search methods are listed in Appendix 3.
Contributions of authors
For the 2018 update:
Emma Amissah searched for published, unpublished, and ongoing studies, assessed study eligibility, performed data extraction and ’Risk of bias’ assessment of included studies, analysed data, interpreted results of the analysis, and updated the review. She wrote all drafts and addressed comments from co‐authors.
Julie Brown assessed study eligibility, performed data extraction and ’Risk of bias’ assessment of included studies, assisted in the interpretation of analyses, and provided comments on drafts.
Jane Harding answered queries on trial eligibility, assisted in the interpretation of analyses, and provided comments on all drafts of the review.
All authors read and approved the final version of the review.
For the 2020 update:
Only the search was updated, with no new trials found (only one new excluded trial). The search was screened by Cochrane Neonatal in consultation with Jane Harding. All previous author contributions remain the same, as the text of the review remains largely unchanged.
Sources of support
Internal sources
Liggins Institute, The University of Auckland, Auckland, New Zealand
External sources
We acknowledge the support from the Australian and New Zealand Pregnancy and Childbirth Satellite at the Liggins Institute, University of Auckland, New Zealand
-
National Institute for Health Research, UK
Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research (NIHR) Cochrane Programme Grant (16/114/03). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR or the UK Department of Health.
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
The Gerber Foundation, USA
Editorial support for this review, as part of a suite of preterm nutrition reviews, has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private, 501(c)(3) foundation not related to Gerber Products Company in any way.
Declarations of interest
EA receives a scholarship in the form of a stipend from the University of Auckland as a PhD student.
JB is currently employed by a medical writing company. The preparation of this review took place prior to this employment and her current work is not related to the topic of this review.
JH has partial salary support from research grants from the Health Research Council of New Zealand. The Council has no role in the production of this review. An undirected research grant is pending from Biomed Ltd, Auckland, New Zealand. This company makes dextrose gel.
The methods section of this review is based on a standard template used by Cochrane Neonatal.
Core editorial and administrative support for the 2020 update of this review has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private foundation, independent from the Gerber Products Company. The grantor has no input on the content of the review or the editorial process.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Boehm 1988a {published data only}
- Boehm G, Müller DM, Beyreiss K, Räihä NC. Evidence of functional immaturity of the ornithine-urea cycle in very-low-birthweight infants. Biology of the Neonate 1988;54(3):121-5. [DOI: 10.1159/000242842] [PMID: ] [DOI] [PubMed] [Google Scholar]
Faerk 2001 {published data only}
- Faerk J, Petersen S, Peitersen B, Michaelsen KF. Diet, growth, and bone mineralization in premature infants. Advances in Experimental Medicine and Biology 2001;501:479-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Faerk J, Petersen S, Peitersen B, Michaelsen KF. Diet and bone mineral content at term in premature infants. Pediatric Research 2000;47(1):148-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
Greer 1986 {published data only}
- Greer FR, McCormick A, Loker J. Increased urinary excretion of inorganic sulfate in premature infants fed bovine milk protein. Journal of Pediatrics 1986;109(4):692-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Greer FR, McCormick A. Improved bone mineralization and growth in premature infants fed fortified own mother’s milk. Journal of Pediatrics 1988;112(6):961-9; Erratum in: Journal of Pediatrics 1988; 113(6):1118. [PMID: ] [DOI] [PubMed] [Google Scholar]
Polberger 1989 {published and unpublished data}
- Polberger SK, Axelsson IA, Räihä NC. Growth of very low birth weight infants on varying amounts of human milk protein. Pediatric Research 1989;25(4):414-9. [DOI: 10.1203/00006450-198904000-00022] [PMID: 2726319 ] [DOI] [PubMed] [Google Scholar]
- Polberger SK, Axelsson IE, Räihä NC. Amino acid concentrations in plasma and urine in very low birth weight infants fed protein-unenriched or human milk protein-enriched human milk. Pediatrics 1990;86(6):909-15. [PMID: ] [PubMed] [Google Scholar]
- Polberger SK, Fex GA, Axelsson IE, Räihä NC. Eleven plasma proteins as indicators of protein nutritional status in very low birth weight infants. Pediatrics 1990;86(6):916-21. [PMID: ] [PubMed] [Google Scholar]
Putet 1987 {published and unpublished data}
- Putet G, Rigo J, Salle B, Senterre J. Supplementation of pooled human milk with casein hydrolysate: energy and nitrogen balance and weight gain composition in very low birth weight infants. Pediatric Research 1987;21(5):458-61. [DOI: 10.1203/00006450-198705000-00007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rönnholm 1982 {published data only}
- Rönnholm KA, Perheentupa J, Siimes MA. Supplementation with human milk protein improves growth of small premature infants fed human milk. Pediatrics 1986;77(5):649-53. [PMID: ] [PubMed] [Google Scholar]
- Rönnholm KA, Siimes MA. Haemoglobin concentration depends on protein intake in small preterm infants fed human milk. Archives of Disease in Childhood 1985;60(2):99-104. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnholm KA, Simell O, Siimes MA. Human milk protein and medium-chain triglyceride oil supplementation of human milk: plasma amino acids in very low-birth-weight infants. Pediatrics 1984;74(5):792-9. [PMID: ] [PubMed] [Google Scholar]
- Rönnholm KA, Sipila O, Siimes MA. Human milk protein supplementation for the prevention of hypoproteinemia without metabolic imbalance in breast milk-fed, very low-birth-weight infants. Journal of Pediatrics 1982;101(2):243-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abrams 2014 {published data only}
- Abrams SA, Schanler RJ, Lee M, Rechtman DJ. Greater mortality and morbidity in extremely preterm infants fed a diet containing cow milk protein products. Breastfeeding Medicine 2014;9(6):281-5. [DOI: 10.1089/bfm.2014.0024] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barrington 2016 {published data only}
- Barrington KJ, Assaad MA, Janvier A. The Lacuna Trial: a double-blind randomized controlled pilot trial of lactoferrin supplementation in the very preterm infant. Journal of Perinatology 2016;36(8):666-9. [DOI: 10.1038/jp.2016.24] [PMID: ] [DOI] [PubMed] [Google Scholar]
Berseth 2012 {published data only}
- Berseth C, Harris C, Mitmesser S. Weight gain in very premature human milk fed infants Is significantly related to protein and non-protein intake. In: Pediatric Academic Societies Annual Meeting; 2012 April 28 - May 1; Boston MA, United States. 2012.
Bhat 2001 {published data only}
- Bhat BA, Gupta B. Effects of human milk fortification on morbidity factors in very low birth weight infants. Annals of Saudi Medicine 2001;21(5-6):292-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Bhat BA, Gupta B. Effects of human milk fortification on morbidity factors in very low birth weight infants. Annals of Saudi Medicine 2003;23(1-2):28-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bishara 2017 {published data only}
- Bishara R, Ng D, Asbury M, Ng E, Unger S, Gibbins S, et al. Nutrient intake and growth of infants enrolled in the donor milk for improved neurodevelopmental outcomes (GTA-DoMINO) trial: comparison to clinical targets. FASEB Journal 2017;31(1 Suppl 1):lb295. [Google Scholar]
Boehm 1988b {published data only}
- Boehm G, Senger H, Muller D, Beyreiss K, Raiha NC. Metabolic differences between AGA- and SGA-infants of very low birthweight. II. Relationship to protein intake. Acta Paediatrica Scandinavica Sep 1988;77(5):642-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ditzenberger 2013 {published data only}
- Ditzenberger GR, Wallen LD, Phelan L, Escoe S, Collins SD. Supplemental protein and postnatal growth of very low birth weight infants: a randomized trial. Journal of Neonatal-perinatal Medicine 2013;6(4):285-94. [DOI: 10.3233/NPM-1371213] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gathwala 2012 {published data only}
- Gathwala G, Shaw CK, Shaw P, Batra R. Effect of fortification of breast milk on the growth of preterm neonates. Eastern Journal of Medicine 2012;17(1):30-5. [Google Scholar]
Hair 2014 {published data only}
- Hair AB, Blanco CL, Moreira AG, Hawthorne KM, Lee ML, Rechtman DJ, et al. Randomized trial of human milk cream as a supplement to standard fortification of an exclusive human milk-based diet in infants 750-1250 g birth weight. Journal of Pediatrics 2014;165(5):915-20. [DOI: 10.1016/j.jpeds.2014.07.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hayashi 1994 {published data only}
- Hayashi T, Takeuchi T, Itabashi K, Okuyama K. Nutrient balance, metabolic response, and bone growth in VLBW infants fed fortified human milk. Early Human Development 1994;39(1):27-36. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hill 1997 {published data only}
- Hill R. Abstracts of podium presentations. Infants and children... proceedings of the Communicating Nursing Research Conference and WIN Assembly held May 1-3, 1997 in Portland, Oregon. Communicating Nursing Research 1997;30:153-6. [Google Scholar]
Kashaki 2018 {published data only}
- Kashaki M, Mazouri A, Bordhar A, Saboute M, Behnamfar Z, Talebi A. Effect of protein supplementation on the growth of infants weighing less than 1,000 grams hospitalized on the neonatal intensive care unit of Akbar Abadi Hospital in Tehran, Iran (2015-2016). Iranian Journal of Neonatology 2018;9(3):49-56. [DOI: 10.22038/ijn.2019.27558.1368] [DOI] [Google Scholar]
Modanlou 1986 {published data only}
- Modanlou HD, Lim MO, Hansen JW, Sickles V. Growth, biochemical status, and mineral metabolism in very-low-birth-weight infants receiving fortified preterm human milk. Journal of Pediatric Gastroenterology and Nutrition 1986;5(5):762-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Moltu 2013 {published data only}
- Moltu SJ, Strommen K, Blakstad EW, Almaas AN, Westerberg AC, Braekke K, et al. Enhanced feeding in very-low-birth-weight infants may cause electrolyte disturbances and septicemia--a randomized, controlled trial. Clinical Nutrition (Edinburgh, Scotland) 2013;32(2):207-12. [DOI: 10.1016/j.clnu.2012.09.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Polberger 1997 {published data only}
- Polberger S, Raiha NC, Juvonen P, Moro G, Minoli I. A new human milk fortifier (HMF). A comparison of growth and metabolic indices with human milk protein (HMP) fortification in preterm infants. Pediatric Research 1997;41(4 Pt 2):238A. [Google Scholar]
Valman 1971 {published data only}
- Valman HB, Brown RJ, Palmer T, Oberholzer VG, Levin B. Protein intake and plasma amino-acids of infants of low birth weight. BMJ 1971;4(5790):789-91. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
AAP 2012
- Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2012;129(3):e827-41. [DOI: 10.1542/peds.2011-3552] [PMID: ] [DOI] [PubMed] [Google Scholar]
AAP Committee on Nutrition 1985
- American Academy of Pediatrics Committee on Nutrition. Nutritional needs of low-birth-weight infants. Pediatrics 1985;75(5):976-86. [PMID: ] [PubMed] [Google Scholar]
Agostoni 2010
- Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, et al, ESPGHAN Committee on Nutrition. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2010;50(1):85-91. [DOI: 10.1097/MPG.0b013e3181adaee0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bertino 2012
- Bertino E, Nicola PD, Giuliani F, Peila C, Cester E, Vassia C, et al. Benefits of human milk in preterm infant feeding. Journal of Pediatric and Neonatal Individualized Medicine (JPNIM) 2012;1(1):19-24. [Google Scholar]
Boyd 2007
- Boyd CA, Quigley MA, Brocklehurst P. Donor breast milk versus infant formula for preterm infants: systematic review and meta-analysis. Archives of Disease in Childhood. Fetal and Neonatal Edition 2007;92(3):F169-75. [DOI: 10.1136/adc.2005.089490] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brooke 1987
- Brooke OG, Onubogu O, Heath R, Carter ND. Human milk and preterm formula compared for effects on growth and metabolism. Archives of Disease in Childhood 1987;62(9):917-23. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2016
- Brown JV, Embleton ND, Harding JE, McGuire W. Multi-nutrient fortification of human milk for preterm infants. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD000343. [DOI: 10.1002/14651858.CD000343.pub3] [DOI] [PubMed] [Google Scholar]
Chiesa 2008
- Chiesa C, Osborn JF, Haass C, Natale F, Spinelli M, Scapillati E, et al. Ghrelin, leptin, IGF-1, IGFBP-3, and insulin concentrations at birth: is there a relationship with fetal growth and neonatal anthropometry? Clinical Chemistry 2008;54(3):550-8. [DOI: 10.1373/clinchem.2007.095299] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cibulskis 2015
- Cibulskis CC, Armbrecht ES. Association of metabolic acidosis with bovine milk-based human milk fortifiers. Journal of Perinatology 2015;35(2):115-9. [DOI: 10.1038/jp.2014.143] [PMID: ] [DOI] [PubMed] [Google Scholar]
Claas 2011
- Claas MJ, Vries LS, Koopman C, Uniken VM, Eijsermans MJ, Bruinse HW, et al. Postnatal growth of preterm born children ≤ 750 g at birth. Early Human Development 2011;87(7):495-507. [DOI: 10.1016/j.earlhumdev.2011.04.009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Clemmons 2006
- Clemmons DR. Clinical utility of measurements of insulin-like growth factor 1. Nature Clinical Practice. Endocrinology & Metabolism 2006;2(8):436-46. [DOI: 10.1038/ncpendmet0244] [PMID: ] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Version accessed 1 September 2017. Melbourne, Australia: Veritas Health Innovation, 2015. Available at www.covidence.org.
D'Netto 2000
- D'Netto MA, Herson VC, Hussain N, Ricci A, Brown RT, Hyams JS, et al. Allergic gastroenteropathy in preterm infants. Journal of Pediatrics 2000;137(4):480-6. [DOI: 10.1067/mpd.2000.108563] [PMID: ] [DOI] [PubMed] [Google Scholar]
Di Natale 2011
- Di Natale C, Coclite E, Di Ventura L, Di Fabio S. Fortification of maternal milk for preterm infants. Journal of Maternal-fetal & Neonatal Medicine 2011;24(Suppl 1):41-3. [DOI: 10.3109/14767058.2011.607569] [PMID: ] [DOI] [PubMed] [Google Scholar]
Di Natale 2013
- Di Natale C, Di Fabio S. Fortification of maternal milk. Journal of Pediatric and Neonatal Individualized Medicine (JPNIM) 2013;2(2):e020224. [Google Scholar]
Ehrenkranz 2006
- Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006;117(4):1253-61. [DOI: 10.1542/peds.2005-1368] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ehrenkranz 2010
- Ehrenkranz RA. Early nutritional support and outcomes in ELBW infants. Early Human Development 2010;86(Suppl 1):21-5. [DOI: 10.1016/j.earlhumdev.2010.01.014] [PMID: ] [DOI] [PubMed] [Google Scholar]
Embleton 2001
- Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics 2001;107(2):270-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Freitas 2016
- Freitas BA, Priore SE, Lima LM, Franceschini SC. Extrauterine growth restriction: universal problem among premature infants [Restrição de crescimento extrauterino: problema universal entre os prematuros]. Revista de Nutrição, 2016;29(1):53-64. [Google Scholar]
Ghods 2011
- Ghods E, Kreissl A, Brandstetter S, Fuiko R, Widhalm K. Head circumference catch-up growth among preterm very low birth weight infants: effect on neurodevelopmental outcome. Journal of Perinatal Medicine 2011;39(5):579-86. [DOI: 10.1515/JPM.2011.049] [PMID: 21740330 ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University GRADEpro GDT. Version accessed 1 September 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014. Available at gradepro.org.
Hansen‐Pupp 2011
- Hansen-Pupp I, Löfqvist C, Polberger S, Niklasson A, Fellman V, Hellström A, et al. Influence of insulin-like growth factor I and nutrition during phases of postnatal growth in very preterm infants. Pediatric Research 2011;69(5 Pt 1):448-53. [DOI: 10.1203/PDR.0b013e3182115000] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hansen‐Pupp 2013
- Hansen-Pupp I, Hövel H, Löfqvist C, Hellström-Westas L, Fellman V, Hüppi P, et al. Circulatory insulin-like growth factor-I and brain volumes in relation to neurodevelopmental outcome in very preterm infants. Pediatric Research 2013;74(5):564-9. [DOI: 10.1038/pr.2013.135] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hay 2009
- Hay WW. Optimizing protein intake in preterm infants. Journal of Perinatology 2009;29(7):465-6. [DOI: 10.1038/jp.2009.53] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hay 2010
- Hay WW, Thureen P. Protein for preterm infants: how much is needed? How much is enough? How much is too much? Pediatrics and Neonatology 2010;51(4):198-207. [DOI: 10.1016/S1875-9572(10)60039-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hellström 2016
- Hellström A, Ley D, Hansen-Pupp I, Hallberg B, Löfqvist C, Marter L, et al. Insulin-like growth factor 1 has multisystem effects on foetal and preterm infant development. Acta Paediatrica 2016;105(6):576-86. [DOI: 10.1111/apa.13350] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from https://training.cochrane.org/handbook.
Kalhoff 1993
- Kalhoff H, Manz F, Diekmann L, Kunz C, Stock GJ, Weisser F. Decreased growth rate of low-birth-weight infants with prolonged maximum renal acid stimulation. Acta Paediatrica 1993;82(6-7):522-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kalhoff 2001
- Kalhoff H, Manz F. Nutrition, acid-base status and growth in early childhood. European Journal of Nutrition 2001;40(5):221-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kim 2016
- Kim MJ. Enteral nutrition for optimal growth in preterm infants. Korean Journal of Pediatrics 2016;59(12):466-70. [DOI: 10.3345/kjp.2016.59.12.466] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koletzko 2005
- Koletzko B, Goulet O, Hunt J, Krohn K, Shamir R, Parenteral Nutrition Guidelines Working Group, European Society for Clinical Nutrition and Metabolism, European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), European Society of Paediatric Research (ESPR). 1. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), Supported by the European Society of Paediatric Research (ESPR). Journal of Pediatric Gastroenterology and Nutrition 2005;41(Suppl 2):S1-87. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kuschel 2000a
- Kuschel CA, Harding JE. Protein supplementation of human milk for promoting growth in preterm infants. Cochrane Database of Systematic Reviews 2000, Issue 2. Art. No: CD000433. [DOI: 10.1002/14651858.CD000433] [DOI] [PubMed] [Google Scholar]
Kuschel 2000b
- Kuschel CA, Harding JE, Kumaran VS. Fat supplementation of human milk for promoting growth in preterm infants. Cochrane Database of Systematic Reviews 2000, Issue 2. Art. No: CD000341. [DOI: 10.1002/14651858.CD000341] [DOI] [PubMed] [Google Scholar]
Lapillonne 2013
- Lapillonne A, Griffin IJ. Feeding preterm infants today for later metabolic and cardiovascular outcomes. Journal of Pediatrics 2013;162(3 Suppl):S7-16. [DOI: 10.1016/j.jpeds.2012.11.048] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lo 2002
- Lo HC, Tsao LY, Hsu WY, Chen HN, Yu WK, Chi CY. Relation of cord serum levels of growth hormone, insulin-like growth factors, insulin-like growth factor binding proteins, leptin, and interleukin-6 with birth weight, birth length, and head circumference in term and preterm neonates. Nutrition 2002;18(7-8):604-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Manz 1997
- Manz F, Kalhoff H, Remer T. Renal acid excretion in early infancy. Pediatric Nephrology (Berlin, Germany) 1997;11(2):231-43. [DOI: ] [DOI] [PubMed] [Google Scholar]
Okamoto 2007
- Okamoto T, Shirai M, Kokubo M, Takahashi S, Kajino M, Takase M, et al. Human milk reduces the risk of retinal detachment in extremely low-birthweight infants. Pediatrics International 2007;49(6):894-7. [DOI: 10.1111/j.1442-200X.2007.02483.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Reich 2010
- Reich F, König R, Wiese W, Klein G. Prevalence of Cronobacter spp. in a powdered infant formula processing environment. International Journal of Food Microbiology 2010;140(2-3):214-7. [DOI: 10.1016/j.ijfoodmicro.2010.03.031] [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ronholm 1986
- Rönnholm KA, Perheentupa J, Siimes MA. Supplementation with human milk protein improves growth of small premature infants fed human milk. Pediatrics 1986;77(5):649-53. [PMID: ] [PubMed] [Google Scholar]
Schanler 1999
- Schanler RJ, Shulman RJ, Lau C. Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics 1999;103(6 Pt 1):1150-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schanler 2005
- Schanler RJ, Lau C, Hurst NM, Smith EO. Randomized trial of donor human milk versus preterm formula as substitutes for mothers' own milk in the feeding of extremely premature infants. Pediatrics 2005;116(2):400-6. [DOI: 10.1542/peds.2004-1974] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sisk 2007
- Sisk PM, Lovelady CA, Dillard RG, Gruber KJ, O'Shea TM. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. Journal of Perinatology 2007;27(7):428-33. [DOI: 10.1038/sj.jp.7211758] [PMID: ] [DOI] [PubMed] [Google Scholar]
Socha 2011
- Socha P, Grote V, Gruszfeld D, Janas R, Demmelmair H, Closa-Monasterolo R, et al. Milk protein intake, the metabolic-endocrine response, and growth in infancy: data from a randomized clinical trial. American Journal of Clinical Nutrition 2011;94(6 Suppl):1776S-84S. [DOI: 10.3945/ajcn.110.000596] [PMID: ] [DOI] [PubMed] [Google Scholar]
Srinivasan 2010
- Srinivasan P, Brandler M, D'Souza A, Millman P, Moreau H. Allergic enterocolitis presenting as recurrent necrotizing enterocolitis in preterm neonates. Journal of Perinatology 2010;30(6):431-3. [DOI: 10.1038/jp.2009.153] [PMID: ] [DOI] [PubMed] [Google Scholar]
Su 2014
- Su BH. Optimizing nutrition in preterm infants. Pediatrics and Neonatology 2014;55(1):5-13. [DOI: 10.1016/j.pedneo.2013.07.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thoene 2014
- Thoene M, Hanson C, Lyden E, Dugick L, Ruybal L, Anderson-Berry A. Comparison of the effect of two human milk fortifiers on clinical outcomes in premature infants. Nutrients 2014;6(1):261-75. [DOI: 10.3390/nu6010261] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tonkin 2014
- Tonkin EL, Collins CT, Miller J. Protein intake and growth in preterm infants: a systematic review. Global Pediatric Health 2014;1:2333794X14554698. [DOI: 10.1177/2333794X14554698] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tudehope 2013
- Tudehope DI. Human milk and the nutritional needs of preterm infants. Journal of Pediatrics 2013;162(3 Suppl):S17-25. [DOI: 10.1016/j.jpeds.2012.11.049] [PMID: ] [DOI] [PubMed] [Google Scholar]
Underwood 2013
- Underwood MA. Human milk for the premature infant. Pediatric Clinics of North America 2013;60(1):189-207. [DOI: 10.1016/j.pcl.2012.09.008] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weber 2001
- Weber A, Loui A, Jochum F, Bührer C, Obladen M. Breast milk from mothers of very low birthweight infants: variability in fat and protein content. Acta Paediatrica 2001;90(7):772-5. [PMID: ] [PubMed] [Google Scholar]
WHO 1995
- World Health Organization. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. who.int/nutrition/publications/growth_physical_status/en/ (accessed 2 February 2018).
Yeung 2003
- Yeung MY, Smyth JP. Nutritionally regulated hormonal factors in prolonged postnatal growth retardation and its associated adverse neurodevelopmental outcome in extreme prematurity. Biology of the Neonate 2003;84(1):1-23. [DOI: 10.1159/000071438] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ziegler 2011
- Ziegler EE. Meeting the nutritional needs of the low-birth-weight infant. Annals of Nutrition & Metabolism 2011;58(Suppl 1):8-18. [DOI: 10.1159/000323381] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Amissah 2018
- Amissah EA, Brown J, Harding JE. Protein supplementation of human milk for promoting growth in preterm infants. Cochrane Database of Systematic Reviews 2018, Issue Issue 6. Art. No.: CD000433. Art. No: CD000433. [DOI: 10.1002/14651858.CD000433.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuschel 2000c
- Kuschel CA, Harding JE. Protein supplementation of human milk for promoting growth in preterm infants. Cochrane Database of Systematic Reviews 2000, Issue 2. Art. No: CD000433. [DOI: 10.1002/14651858.CD000433] [DOI] [PubMed] [Google Scholar]


