Abstract
Background
The introduction and advancement of enteral feeds for preterm or low birth weight infants is often delayed because of concerns that early full enteral feeding will not be well tolerated or may increase the risk of necrotising enterocolitis. Early full enteral feeding, however, might increase nutrient intake and growth rates; accelerate intestinal physiological, metabolic, and microbiomic postnatal transition; and reduce the risk of complications associated with intravascular devices for fluid administration.
Objectives
To determine how early full enteral feeding, compared with delayed or progressive introduction of enteral feeds, affects growth and adverse events such as necrotising enterocolitis, in preterm or low birth weight infants.
Search methods
We used the standard search strategy of Cochrane Neonatal to search Cochrane Central Register of Controlled Trials; MEDLINE Ovid, Embase Ovid, Maternity & Infant Care Database Ovid, the Cumulative Index to Nursing and Allied Health Literature, and clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials to October 2020.
Selection criteria
Randomised controlled trials that compared early full enteral feeding with delayed or progressive introduction of enteral feeds in preterm or low birth weight infants.
Data collection and analysis
We used the standard methods of Cochrane Neonatal. Two review authors separately assessed trial eligibility, evaluated trial quality, extracted data, and synthesised effect estimates using risk ratios (RR), risk differences, and mean differences (MD) with 95% confidence intervals (CI). We used the GRADE approach to assess the certainty of evidence.
Main results
We included six trials. All were undertaken in the 2010s in neonatal care facilities in India. In total, 526 infants participated. Most were very preterm infants of birth weight between 1000 g and 1500 g. Trials were of good methodological quality, but a potential source of bias was that parents, clinicians, and investigators were not masked. The trials compared early full feeding (60 mL/kg to 80 mL/kg on day one after birth) with minimal enteral feeding (typically 20 mL/kg on day one) supplemented with intravenous fluids. Feed volumes were advanced daily as tolerated by 20 mL/kg to 30 mL/kg body weight to a target steady‐state volume of 150 mL/kg to 180 mL/kg/day. All participating infants were fed preferentially with maternal expressed breast milk, with two trials supplementing insufficient volumes with donor breast milk and four supplementing with preterm formula.
Few data were available to assess growth parameters. One trial (64 participants) reported a slower rate of weight gain (median difference –3.0 g/kg/day), and another (180 participants) reported a faster rate of weight gain in the early full enteral feeding group (MD 1.2 g/kg/day). We did not meta‐analyse these data (very low‐certainty evidence). None of the trials reported rate of head circumference growth. One trial reported that the mean z‐score for weight at hospital discharge was higher in the early full enteral feeding group (MD 0.24, 95% CI 0.06 to 0.42; low‐certainty evidence). Meta‐analyses showed no evidence of an effect on necrotising enterocolitis (RR 0.98, 95% CI 0.38 to 2.54; 6 trials, 522 participants; I² = 51%; very low‐certainty evidence).
Authors' conclusions
Trials provided insufficient data to determine with any certainty how early full enteral feeding, compared with delayed or progressive introduction of enteral feeds, affects growth in preterm or low birth weight infants. We are uncertain whether early full enteral feeding affects the risk of necrotising enterocolitis because of the risk of bias in the trials (due to lack of masking), inconsistency, and imprecision.
Plain language summary
Early full enteral feeds for preterm or low birth weight infants
Review question: do preterm or low birth weight infants (babies born early or small) grow faster and have fewer problems when they receive all their nutrients as milk feeds from shortly after birth (compared with gradually introducing milk feeds while giving fluid or nutrients via an intravenous drip (a slow infused into the bloodstream via a vein))?
Background: 'early full enteral feeding' means that preterm or low birth weight infants receive all their nutrition as milk feeds from shortly after birth, and do not receive any supplemental fluids or nutrition via intravenous drips. Assessing whether this approach is safe and beneficial is particularly relevant to feeding very preterm or very low birth weight infants (born before 32 weeks, or birth weigh less than 1500 g).
Study characteristics: we included six trials, all undertaken in neonatal care units in India during the 2010s. The trials were generally good quality although most were small (involving 526 infants in total). Participants were preterm infants of birth weight 1000 g to 1500 g.
The search is up to date as of July 2020.
Key results: there were insufficient data to show whether infants who received full milk feeds from birth put on weight and grew more quickly than those for whom feeds were introduced gradually during the first week or two after birth. The trials reported no information about the effects early full milk feeds might have on development and growth later in the baby's life. The included trials found no evidence of other potential benefits or harms of early full feeds, including any effects on feeding or bowel problems.
Conclusion: there is not enough evidence to determine whether early full milk feeds benefit preterm or low birth weight infants. New trials would be needed to resolve this uncertainty.
Quality of evidence: we assessed this evidence as being of low or very low quality because the included trials were small with some methodological weaknesses and their findings were inconsistent with each other. This means that further research is very likely to have an important impact on the estimates of effect and our confidence in the findings.
Summary of findings
Summary of findings 1. Early full enteral feeding compared to delayed or progressive feeding for preterm or low birth weight infants.
| Early full compared to delayed/progressive enteral feeding for preterm or low birth weight infants | |||||
| Patient or population: preterm or low birth weight infants Setting: neonatal care facilities (India) Intervention: early full enteral feeding Comparison: delayed/progressive enteral feeding | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with delayed/progressive enteral feeding | Risk difference with early full enteral feeding | ||||
| In hospital rate of weight gain (g/kg/day) until term equivalent | 236 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | — | Meta‐analysis not possible due to different outcome measures. | |
| In hospital rate of head circumference growth (cm/week) until term equivalent – not reported | — | — | — | — | — |
| Growth restriction (z‐score of weight) at hospital discharge | 46 (1 RCT) | ⊕⊕⊝⊝ Lowa,d | — | The mean growth restriction (z‐score of weight) at hospital discharge was –1.09 | Mean 0.24 higher (0.06 higher to 0.42 higher) |
| Necrotising enterocolitis | 522 (6 RCTs) | ⊕⊝⊝⊝ Very lowa,e,f | RR 0.98 (0.38 to 2.54) | Study population | |
| 31 per 1000 | 1 fewer per 1000 (19 fewer to 47 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to risk of surveillance and detection bias owing to lack of masking. bDowngraded one level due to wide range point estimates across the two trials (imprecision). cDowngraded one level due to opposite direction of effect (inconsistency). dDowngraded one level as analysis included data from one small trial (imprecision). eDowngraded one level due to moderate heterogeneity (inconsistency). fDowngraded one level due to wide 95% confidence interval (imprecision).
Background
This Cochrane Review appraised and synthesised data from randomised controlled trials (RCTs) that compared early full enteral feeding with delayed or progressive introduction of enteral feeds (combined with parenteral fluids or nutrition) for preterm or low birth weight (LBW; less than 2500 g) infants.
Description of the condition
Preterm or LBW infants, especially very preterm or very low birth weight (VLBW; less than 1500 g) infants, have few nutrient reserves at birth and are subject to physiological and metabolic stresses that increase their nutrient needs. Recommendations on nutrient requirements for preterm or LBW infants assume that the optimal rate of postnatal growth should be similar to that of uncompromised fetuses of an equivalent gestational age (Agostoni 2010; Tsang 1993). Such levels of nutrient input and growth are rarely achieved and most very preterm or VLBW infants accumulate nutrient deficits during their initial hospital stay (Embleton 2001; Horbar 2015). By the time they are ready to go home, many of these infants are growth restricted compared to their term‐born peers (Clark 2003; Dusick 2003). Growth deficits can persist through childhood and adolescence, and are associated with adverse neurodevelopmental, cognitive, and educational outcomes (Bracewell 2008; Cooke 2003; Doyle 2004; Farooqi 2006; Ford 2000; Hack 1991; Leppänen 2014).
Necrotising enterocolitis
Necrotising enterocolitis, a syndrome of acute intestinal necrosis of unknown aetiology, affects about 1 in 20 very preterm or VLBW infants (Gagliardi 2008; Holman 1997; Moro 2009). Infants who develop necrotising enterocolitis experience more infections, have lower levels of nutrient intake, grow more slowly, and have longer durations of intensive care and hospital stay than gestation‐comparable infants who do not develop necrotising enterocolitis (Bisquera 2002; Guthrie 2003). The associated mortality rate is greater than 20% (Fitzgibbons 2009). Compared with their peers, infants who develop necrotising enterocolitis have a higher incidence of long‐term neurological disability, which may be a consequence of infection and undernutrition during a critical period of brain development (Berrington 2012; Pike 2012; Rees 2007).
In addition to low gestational age at birth, the major risk factor for necrotising enterocolitis is intrauterine growth restriction, especially if it is associated with absent or reversed end diastolic flow velocities in Doppler studies of the fetal aorta or umbilical artery (Dorling 2005; Samuels 2017). Most very preterm or VLBW infants who develop necrotising enterocolitis have received enteral milk feeds (Ramani 2013). Feeding with artificial formula rather than human milk increases the risk of developing necrotising enterocolitis (Quigley 2019; Walsh 2019). However, the available data from RCTs do not provide evidence that delaying the introduction of progressive enteral feeds beyond four days after birth, or advancing feed volumes more slowly than 24 mL/kg/day, affects the risk of necrotising enterocolitis and associated morbidities or mortality in very preterm or VLBW infants (Morgan 2013; Morgan 2014; Oddie 2017).
Early enteral feeding strategies
Evidence exists that early enteral feeding strategies – particularly the timing of introduction and the rate of advancement of milk feeds – affect important outcomes in preterm or LBW infants, including nutrient intake, the risk of necrotising enterocolitis, and growth and development (Embleton 2013). Approaches to early enteral feeding vary by the gestational age and clinical condition of the infant (Hay 2008; Klingenberg 2012). Stable preterm infants born at or more than 32 weeks' gestation, or with a birth weight of 1500 g or greater, are generally treated similarly to well term infants; all fluid and nutrition is supplied enterally from birth, either orally or via an intragastric feeding tube. In contrast, newborn infants who are extremely preterm (born before 28 weeks' gestation), or of extremely low birth weight (ELBW; less than 1000 g) are commonly affected by respiratory distress, have delayed gastric emptying and inefficient intestinal motility, and are at high risk of developing necrotising enterocolitis. These infants tend to be supported with parenteral fluids and nutrition during the first few days after birth. Enteral milk feeds are then introduced in subnutritional volumes (trophic feeds) during the first week after birth, ideally as colostrum or expressed breast milk, and the volume of feeds is increased gradually over the next one to two weeks as the volume of parenteral nutrition is decreased.
There remains substantial variation in practice with regard to early enteral feeding strategies for those preterm infants born at gestations between approximately 28 and 32 weeks (or with birth weights between approximately 1000 g and 1500 g). This variation reflects ongoing uncertainty about whether these infants should be treated in the same way as those infants born at a later gestation (i.e. with full enteral feeds from birth), or more similarly to extremely preterm or ELBW infants (i.e. delayed introduction and gradual advancement of milk feeds while supporting nutritional needs with parenteral nutrition). In many high‐income countries, policy and practice has tended to favour the conservative approach to introducing and advancing enteral feeds for these infants because of concerns that early full enteral feeding might increase the risk of feed intolerance, gastro‐oesophageal reflux and aspiration of stomach contents, hypoglycaemia, and necrotising enterocolitis in very preterm or VLBW infants (de Waard 2018; Klingenberg 2012; Leaf 2013; Maas 2018). However, in low‐ and middle‐income countries with fewer resources for neonatal care, practice is more pragmatic and tends to favour early introduction and advancement of enteral feeds (sometimes facilitated by 'kangaroo' mother care) for stable infants born at 28 weeks' gestation or greater, or with a birth weight of 1000 g or more (Conde‐Agudelo 2016; Sankar 2008).
Description of the intervention
Early (sometimes termed 'immediate') full enteral feeding means that newborn infants receive all their prescribed nutrition as milk feeds (either human milk or formula) and do not receive any supplemental parenteral fluids or nutrition from birth (Nangia 2018). This approach for feeding preterm infants has been advocated since the earliest days of modern neonatology but has tended to be reserved for clinically stable preterm infants of gestational age at birth of more than approximately 32 weeks (Klingenberg 2012; Smallpeice 1964). In most neonatal care facilities, particularly in high‐income countries, the more common practice is to introduce enteral milk feeds for very preterm or VLBW infants at low volume (trophic feeds or minimal enteral nutrition) and to then advance the feed volume slowly during the next one to two weeks. During this time, infants receive most of their fluids and nutrition parenterally, usually in the form of commercially available solutions containing amino acids, glucose, minerals, vitamins, and fats (Klingenberg 2012).
There are potential disadvantages associated with conservative enteral feeding regimens (Flidel‐Rimon 2004; Flidel‐Rimon 2006). Because gastrointestinal hormone secretion and motility are stimulated by milk feeds, delaying full enteral feeding may diminish the functional adaptation of the gastrointestinal tract and disrupt the patterns of microbial colonisation (Embleton 2017). Intestinal dysmotility and dysbiosis might exacerbate feed intolerance and delay the establishment of enteral feeding independently of parenteral nutrition (Pammi 2017). Prolonging the duration of parenteral nutrition is associated with infectious and metabolic complications that increase mortality and morbidity, prolong hospital stay, and adversely affect growth and development (Embleton 2013). Due to cost and equipment implications, parenteral nutrition is less easily available in low‐ and middle‐income countries. In high‐income settings, earlier achievement of full enteral feeds and an associated reduction of length of hospital stay could be associated with considerable resource savings (Embleton 2014).
How the intervention might work
The aims of early full enteral feeding are to avoid the risks and costs associated with provision of parenteral nutrition and to accelerate gastrointestinal physiological, endocrine and metabolic maturity and so allow infants to attain nutrient intakes to optimise growth and development. Early full enteral feeding in this vulnerable population might reduce the risk of infection associated with intravascular devices used to deliver parenteral nutrition (Flidel‐Rimon 2004). Early provision of breast milk might promote successful expression and lactation and help establish maternal breast milk feeding as the primary source of infant nutrition (Hay 2008; Senterre 2014). However, there is some concern that exclusive enteral nutrition might not be sufficient to maintain normal blood glucose levels during the early metabolic transition phase in very preterm or VLBW infants, particularly in growth‐restricted infants with limited nutrient reserves at birth (Klingenberg 2012). Furthermore, any beneficial effects may be negated if early full enteral feeding increases the risk of feed intolerance or necrotising enterocolitis in very preterm or VLBW infants (Chauhan 2008; Samuels 2017).
Why it is important to do this review
This review focuses on the comparison of early full enteral feeding versus gradual introduction of progressive enteral feeding in combination with parenteral fluids. Other Cochrane Reviews have addressed the questions of whether delaying the introduction of any enteral milk feeding or restricting feed volumes to trophic levels (minimal enteral nutrition) affect the risk of necrotising enterocolitis in very preterm or VLBW infants (Morgan 2013; Morgan 2014). The findings of this review complement these other reviews of early enteral feeding strategies and might inform policy, practice, or research in this field (Chauhan 2008).
Objectives
To determine how early full enteral feeding, compared with delayed or progressive introduction of enteral feeds, affects growth and adverse events such as necrotising enterocolitis, in preterm or low birth weight infants.
Where data were available, we planned subgroup analyses of very preterm or VLBW infants (versus infants born after a longer gestation or with higher birth weight), infants 'small for gestational age' at birth (versus those 'appropriate for gestation'), infants fed with human milk only (versus formula‐fed infants), and trials set in low‐ or middle‐income countries (versus high‐income countries) (see: Subgroup analysis and investigation of heterogeneity).
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs or quasi‐RCTs, including cluster‐RCTs.
Types of participants
We included infants born preterm (less than 37 weeks' gestation) or with LBW (less than 2500 g).
Types of interventions
Intervention
Full enteral feeds from birth without parenteral fluids or nutrition. Early full enteral feeding is defined as sufficient volumes of milk being fed orally or via an enteric feeding tube from soon after birth, without parenteral supplementation at any point.
Comparison
Any other feeding regimen, such as delayed initiation of full milk feeds and gradual advancement of feed volumes while receiving supplemental fluid or nutrients parenterally.
Types of outcome measures
Primary outcomes
In hospital rate of weight gain (g/kg/day) until term equivalent.
In hospital rate of head circumference growth (cm/week) until term equivalent.
Growth restriction: z‐score and proportion of infants who remain below the 10th percentile for the index population distribution of weight at term equivalent.
-
Necrotising enterocolitis, confirmed at surgery or autopsy or diagnosed by at least two of the following clinical features (Kliegman 1987):
abdominal radiograph showing pneumatosis intestinalis or gas in the portal venous system or free air in the abdomen;
abdominal distension with abdominal radiograph with gaseous distension or frothy appearance (or both) of bowel lumen;
blood in stool;
lethargy, hypotonia, or apnoea (or combination of these).
Secondary outcomes
Feed intolerance during the trial intervention period that results in cessation in enteral feeding for more than four hours.
Episodes of hypoglycaemia (investigator defined) requiring treatment with enteral supplement (including milk feed or buccal dextrose gel) or with intravenous fluids (including dextrose solution).
Invasive infection, as determined by culture of bacteria or fungus from blood, cerebrospinal fluid, or from a normally sterile body space.
Duration of birth hospitalisation (days).
All‐cause mortality up to 36 to 44 weeks' postmenstrual age and one‐year post‐term.
Growth parameters assessed beyond infancy: weight, height, or head circumference and proportion of infants who remain below the 10th percentile for the index population's distribution, and measures of body composition (lean/fat mass) and body mass index.
Severe neurodevelopmental disability, assessed beyond infancy until adulthood: non‐ambulant cerebral palsy, developmental quotient more than two standard deviations (SD) below the population mean and blindness (visual acuity less than 6/60) or deafness (any hearing impairment requiring or unimproved by amplification).
Search methods for identification of studies
We used the standard search strategy of Cochrane Neonatal.
Electronic searches
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 10) in the Cochrane Library; MEDLINE Ovid (1946 to October 2020), Embase Ovid (1974 to October 2020), Maternity & Infant Care Database Ovid (1971 to October 2020), and the Cumulative Index to Nursing and Allied Health Literature EBSCO (1982 to October 2020), using terms described in Appendix 1. We limited the search outputs with the relevant search filters for clinical trials as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We applied no language restrictions.
We searched ClinicalTrials.gov and the World Health Organization's International Clinical Trials Registry Platform (www.who.int/ictrp/en/) for completed or ongoing trials.
Searching other resources
We examined the reference lists of studies identified as potentially relevant. We searched the abstracts from annual meetings of the Pediatric Academic Societies (1993 to 2019), the European Society for Paediatric Research (1995 to 2020), the UK Royal College of Paediatrics and Child Health (2000 to 2019), and the Perinatal Society of Australia and New Zealand (2000 to 2019). We considered trials reported only as abstracts to be eligible if sufficient information was available from the report, or from contact with study authors, to fulfil the inclusion criteria (see Dealing with missing data for further details).
Data collection and analysis
We used the standard methods of Cochrane Neonatal.
Selection of studies
Two review authors (JB, BC, or VW) independently screened the titles and abstracts of all studies and assess the full articles of all potentially relevant trials. We excluded studies that did not meet all the inclusion criteria and stated the reasons for exclusion in the Characteristics of excluded studies table. We discussed any disagreements until consensus was achieved.
Data extraction and management
Two review authors (JB, BC, or VW) used a form to independently extract data on design, methodology, participants, interventions, outcomes, and treatment effects from each included study. We discussed any disagreements until we reached a consensus. The data extraction form was based on forms used previously by the author team, with adaptations made to meet the requirements of this review.
Assessment of risk of bias in included studies
Two review authors (JB, BC, or VW) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane 'Risk of bias' tool (Higgins 2017) for the following domains:
sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective reporting (reporting bias);
any other bias.
We resolved any disagreements by discussion. We did not exclude trials on the basis of risk of bias, but planned to conduct sensitivity analyses, if applicable, to explore the consequences of synthesising evidence of variable quality.
See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We analysed the treatment effects in the individual trials and reported the risk ratio (RR) and risk difference (RD) for dichotomous data and the mean difference (MD) for continuous data, with 95% confidence intervals (CI). We planned to report number needed to treat for an additional beneficial outcome (NNTB), or number needed to treat for an additional harmful outcome (NNTH) for analyses with a statistically significant difference in the RD.
Unit of analysis issues
The unit of analysis used was the participating infant in individually randomised trials and the neonatal unit (or subunit) for cluster‐randomised trials.
For cluster‐randomised trials, we planned to undertake analyses at the level of the individual while accounting for clustering in the data using the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017).
Dealing with missing data
Where data were missing without explanation, and could not be derived, we used the following approaches.
Where we had concerns about the extent of missing data or potential bias in missing data, we contacted the original study investigators to request the missing data for primary outcomes only, for studies published within the past 10 years where an email address was easily available from the published papers.
Where possible, we planned to impute missing SD using the coefficient of variation, or calculate this from other available statistics including standard errors, CIs, t values, and P values.
If the data were assumed to be missing at random, we planned to analyse the data without imputing any missing values.
If data could not be assumed to be missing at random, we planned to impute the missing outcomes with replacement values, assuming all to have a poor outcome, and conduct sensitivity analyses to assess any changes in the direction or magnitude of effect resulting from data imputation.
Assessment of heterogeneity
Two review authors (JB, VW, or WM) assessed clinical heterogeneity, and conducted a meta‐analysis when both authors agreed that study participants, interventions, and outcomes were sufficiently similar.
We examined the treatment effects of individual trials and heterogeneity between trial results by inspecting the forest plots. We calculated the I² statistic for each analysis to quantify inconsistency across studies and described the percentage of variability in effect estimates that may have been due to heterogeneity rather than to sampling error. If high levels of heterogeneity were detected (an I² value greater than 75%), we explored the possible sources (e.g. differences in study design, participants, interventions, or completeness of outcome assessments).
Assessment of reporting biases
If there were more than 10 trials in a meta‐analysis, we planned to examine a funnel plot for asymmetry.
Data synthesis
We used the fixed‐effect model in Review Manager 5 for meta‐analyses (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses for the primary outcomes.
Trials in which infants received human milk only (maternal expressed milk or donor human milk) versus those where formula could be given instead of, or as a supplement to, human milk.
Very preterm infants (born at less than 32 weeks' gestation) versus infants born at 32 weeks' gestation or greater.
Very low birth weight infants (less than 1500 g) versus infants with a birth weight of 1500 g or greater.
Infants with a birth weight below the 10th percentile for the reference population ('small for gestation') versus infants with a birth weight at or above the 10th percentile ('appropriate for gestation').
Trials set in low‐ or middle‐income countries versus trials set in high‐income countries (for classification, see datahelpdesk.worldbank.org/knowledgebase/articles/906519#High_income (accessed 18 April 2019)).
Sensitivity analysis
We planned sensitivity analyses to determine if the findings were affected by including only studies of adequate methodology (low risk of bias), defined as those studies with adequate randomisation and allocation concealment, blinding of intervention and measurement, and less than 10% loss to follow‐up assessment of the trial primary outcome.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence of the following primary outcomes: in hospital rate of weight gain (g/kg/day) until term equivalent; in hospital rate of head circumference growth (cm/week) until term equivalent; growth restriction (z score of weight) at hospital discharge; necrotising enterocolitis.
Two review authors (VW and WM) independently assessed the certainty of the evidence for each of the outcomes above. We considered evidence from RCTs as high certainty but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used GRADEpro GDT to create Table 1 to report the certainty of the evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence as one of four grades.
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: we are very uncertain about the estimate.
Results
Description of studies
We included six trials (Bora 2017; Chetry 2014; Jajoo 2017; Nangia 2019; Ramya 2014; Sanghvi 2013). Two reports were available as conference abstracts only (Chetry 2014; Jajoo 2017). One was a published dissertation (Ramya 2014).
Results of the search
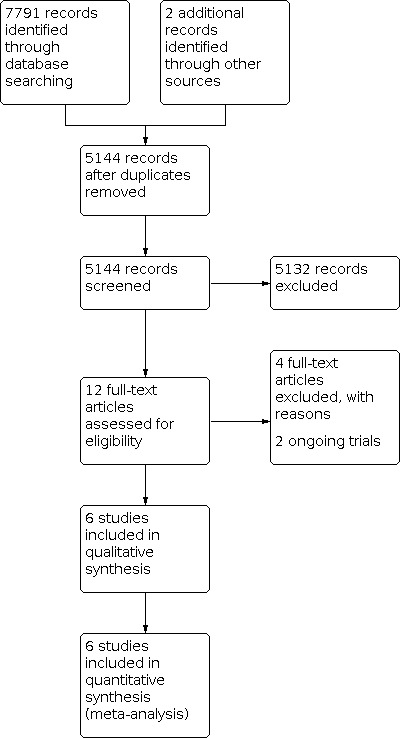
The search identified 7791 records through database searching and two additional records from other sources. After removing duplicates, 5144 records remained. We excluded 5132 irrelevant records and assessed the full text of 12 records. We included six RCTs (Bora 2017; Chetry 2014; Jajoo 2017; Nangia 2019; Ramya 2014; Sanghvi 2013; Characteristics of included studies table). We excluded studies with reasons (Genzel‐Boroviczény 2002; Higgs 1974; Jain 2016; Yu 1979; Characteristics of excluded studies table), and found two ongoing studies (ISRCTN89654042; NCT03708068; Characteristics of ongoing studies table). See Figure 1.
1.
Study flow diagram.
Included studies
All included trials were undertaken during the 2010s by investigators in neonatal care centres in India (see Characteristics of included studies table).
Participants
In total, 526 infants participated (range per trial 60 to 180). Most were medically stable VLBW infants (birth weight 1000 g to 1500 g) of gestational age at birth 28 to 34 weeks. None was extremely preterm or ELBW. Participants included infants born 'small‐for‐gestation' in all trials except Chetry 2014, which excluded infants with birth weight less than 3rd percentile for reference population. All trials excluded infants with congenital anomalies or gastrointestinal or neurological problems from participation. Two trials excluded infants in receipt of respiratory support beyond supplemental oxygen (Jajoo 2017; Nangia 2019). One trial excluded infants who needed mechanical ventilation (Sanghvi 2013).
Interventions
The trials compared early full feeding (60 mL/kg to 80 mL/kg on day one after birth) with minimal enteral feeding (typically 20 mL/kg to 30 mL/kg on day one) supplemented with intravenous fluids (typically 10% dextrose). Feed volumes were advanced daily as tolerated by 20 mL/kg to 30 mL/kg body weight to a target steady‐state volume of 150 mL/kg/day to 180 mL/kg/day.
All participating infants were fed preferentially with maternal expressed breast milk. Two trials used donor breast milk to supplement insufficient volumes of maternal expressed breast milk (Bora 2017; Ramya 2014). The other trials used preterm formula if maternal milk was insufficient. In three trials, human milk was enriched with a multi‐nutrient fortifier when volume of intake exceeded 100 mL/kg/day (Bora 2017; Nangia 2019; Sanghvi 2013).
Outcomes
Outcomes reported most commonly were feed intolerance (reported in various ways but often without accompanying numerical data), days to regain birth weight, duration of birth hospitalisation, necrotising enterocolitis, late‐onset infection, and mortality. None of the trials assessed any outcomes posthospitalisation, including growth and neurodevelopment.
Excluded studies
We excluded four studies after full‐text assessment (Genzel‐Boroviczény 2002; Higgs 1974; Jain 2016; Yu 1979; see Characteristics of excluded studies table).
Ongoing studies
We found two ongoing trials (ISRCTN89654042; NCT03708068; see Characteristics of ongoing studies table).
Risk of bias in included studies
Quality assessments are detailed in the Characteristics of included studies table and summarised in Figure 2.
2.

Risk of bias summary.
Allocation
All trials were at low risk of selection bias as they reported adequate allocation concealment methods (sealed, numbered envelopes).
Blinding
None of the trials masked parents, carers, or clinicians or investigators assessing the trial outcomes (not reported in Sanghvi 2013).
Incomplete outcome data
All trials reported complete outcome assessments of the trial cohorts and were at low risk of attrition bias.
Selective reporting
We were unable to assess reliably whether selective reporting occurred in four of the trials as we identified no trial registrations, protocols, or other indicators of prespecified outcomes; these were at unclear risk of reporting bias (Bora 2017; Chetry 2014; Jajoo 2017; Ramya 2014). The other two trials listed outcomes of interest in their registrations (Nangia 2019; Sanghvi 2013). Both trials reported all prespecified outcomes, and additional, non‐prespecified data as secondary outcomes and were at low risk of reporting bias.
Other potential sources of bias
One trial received no funding and was at low risk of other bias (Sanghvi 2013). The others did not report whether they received trial funding and were at unclear risk of other bias.
Effects of interventions
See: Table 1
Primary outcomes
In hospital rate of weight gain
Two studies reported in hospital rate of weight gain (Chetry 2014 (author correspondence); Nangia 2019).
Chetry 2014 reported a slower rate of weight gain in infants in the early full enteral feeding group (median 15.22 g/kg/day, interquartile range (IQR) 10.9 to 24.6) compared to the incremental feeding group (median 18.23 g/kg/day, IQR 11.36 to 27.3).
Nangia 2019 reported a faster rate of weight gain in the early full enteral feeding group compared with the control group (mean: 6.3 g/kg/day with early full enteral feeding compared to 5.06 g/kg/day with control; SDs not provided).
We did not meta‐analyse these data. We assessed the certainty of the evidence as very low, downgrading for risk of bias, inconsistency, and imprecision.
In hospital rate of head circumference growth
None of the trials reported in hospital rate of head circumference growth.
Growth restriction
1.1. Analysis.

Comparison 1: Early full versus delayed/progressive enteral nutrition, Outcome 1: z‐score for weight at hospital discharge
One study reported z‐score for weight at hospital discharge (Sanghvi 2013).
The definition of growth restriction in the included trial (z‐score at discharge) differed from our prespecified definition (z‐score at term). We made a consensus post hoc decision to include the data based on the investigators' definition.
Analysis showed that infants in the early full enteral feeding group had higher z‐scores that those in the control group (MD 0.24, 95% CI 0.06 to 0.42).
We assessed the certainty of the evidence as low, downgrading for risk of bias and imprecision.
Necrotising enterocolitis
1.2. Analysis.

Comparison 1: Early full versus delayed/progressive enteral nutrition, Outcome 2: Necrotising enterocolitis
Six studies reported necrotising enterocolitis (Bora 2017; Chetry 2014; Jajoo 2017; Nangia 2019; Ramya 2014; Sanghvi 2013). Two trials (which were reported in conference abstracts) did not specify diagnostic criteria for necrotising enterocolitis (Chetry 2014; Jajoo 2017).
There was no evidence of a difference (RR 0.98, 95% CI 0.38 to 2.54; I² = 51%; RD –0.00, 95% CI –0.03 to 0.03; Figure 3).
3.

Forest plot of comparison: 1 Early full versus delayed/progressive enteral nutrition, outcome: 1.2 Necrotising enterocolitis.
We assessed the certainty of the evidence as very low, downgrading for risk of bias, inconsistency (moderate heterogeneity), and imprecision.
Secondary outcomes
Feed intolerance
1.3. Analysis.

Comparison 1: Early full versus delayed/progressive enteral nutrition, Outcome 3: Feed intolerance
Four studies reported feed intolerance (Bora 2017; Chetry 2014; Nangia 2019; Sanghvi 2013). The definitions of feed intolerance used in the included trials did not specify the duration of enteral feed interruption. We made a consensus post hoc decision to include the data based on the investigators' definitions (see Characteristics of included studies table).
There was no evidence of a difference (RR 0.74, 95% CI 0.49 to 1.13; I² = 54%; RD –0.06, 95% CI –0.13 to 0.02; Figure 4).
4.

Forest plot of comparison: 1 Early full versus delayed/progressive enteral nutrition, outcome: 1.3 Feed intolerance.
Episodes of hypoglycaemia
1.4. Analysis.

Comparison 1: Early full versus delayed/progressive enteral nutrition, Outcome 4: Episodes of hypoglycaemia
Two studies reported hypoglycaemia (Nangia 2019; Sanghvi 2013).
There was no evidence of a difference (RR 3.00, 95% CI 0.13 to 70.02; I² not estimable; RD 0.01, 95% CI –0.03 to 0.06; Figure 5).
5.

Forest plot of comparison: 1 Early full versus delayed/progressive enteral nutrition, outcome: 1.4 Episodes of hypoglycaemia.
Invasive infection
1.5. Analysis.

Comparison 1: Early full versus delayed/progressive enteral nutrition, Outcome 5: Invasive infection
Four studies reported invasive infection (Chetry 2014 (author correspondence); Nangia 2019; Ramya 2014; Sanghvi 2013).
There was no evidence of a difference (RR 0.72, 95% CI 0.36 to 1.46; I² = 45%; RD –0.03, 95% CI –0.08 to 0.03; Figure 6).
6.

Forest plot of comparison: 1 Early full versus delayed/progressive enteral nutrition, outcome: 1.5 Invasive infection.
Duration of birth hospitalisation
1.6. Analysis.

Comparison 1: Early full versus delayed/progressive enteral nutrition, Outcome 6: Duration of birth hospitalisation (days)
Five studies reported days of hospitalisation (Bora 2017; Chetry 2014; Jajoo 2017; Nangia 2019; Sanghvi 2013).
Meta‐analysis showed that the early full enteral feeding group had a shorter duration of hospitalisation than infants in the control group (MD –3.07 days, 95% CI –4.13 to –2.02; I² = 90%).
All‐cause mortality
1.7. Analysis.

Comparison 1: Early full versus delayed/progressive enteral nutrition, Outcome 7: All‐cause mortality
Six studies reported mortality (Bora 2017 (author correspondence); Chetry 2014 (author correspondence); Jajoo 2017; Nangia 2019; Ramya 2014; Sanghvi 2013).
There was no evidence of a difference (RR 0.78, 95% CI 0.36 to 1.70; I² = 0%; RD –0.01, 95% CI –0.05 to 0.03).
Long‐term growth
None of the studies reported long‐term growth.
Severe neurodevelopmental disability
None of the studies reported severe neurodevelopmental disability.
Additional post hoc outcome
Days to regain birth weight
1.8. Analysis.

Comparison 1: Early full versus delayed/progressive enteral nutrition, Outcome 8: Days to regain birth weight
Six studies reported days to regain birth weight (Bora 2017; Chetry 2014; Jajoo 2017; Nangia 2019; Ramya 2014; Sanghvi 2013).
Meta‐analysis showed that the early full enteral feeds group regained birth weight earlier than the control group (MD –3.42 days, 95% CI –4.31 to –2.53; I² = 89%).
Subgroup analyses
-
Trials in which infants received human milk only versus those where formula could be given instead of, or as a supplement to, human milk. The test for subgroup differences was statistically significant for:
-
feed intolerance (Chi² = 6.49, df = 1 (P = 0.01), I² = 84.6%); Figure 4):
exclusive human milk: RR 2.04, 95% CI 0.83 to 5.02; I² = not applicable; RD –0.12, 95% CI –0.03 to 0.27;
supplemental formula: RR 0.54, 95% CI 0.33 to 0.88; I² = 0%; RD –0.12, 95% CI –0.21 to ‐0.03
-
duration of hospitalisation (Chi² = 22.73, df = 1 (P < 0.00001), I² = 95.6%;):
exclusive human milk: MD –1.40 days, 95% CI –2.66 to –1.04 days; I² = not applicable;
supplemental formula: MD –7.03 days, 95% CI –8.96 to –5.09 days; I² = 82%;
-
days to regain birth weight (Chi² = 4.25, df = 1 (P = 0.04), I² = 76.5%):
exclusive human milk: MD –1.97, 95% CI –3.67 to –0.26 days; I² = 0%;
Supplemental formula: MD –4.02, 95% CI –4.98 to –3.07 days; I² = 91%.
-
-
Very preterm infants (born at less than 32 weeks' gestation) versus infants born at 32 weeks' gestation or greater.
Subgroup data not available (most trial participants were very preterm infant).
-
Very low birth weight infants (less than 1500 g) versus infants with a birth weight of 1500 g or greater.
All trial participants were very low birth weight infants (1000 g to 1500 g).
-
Infants with a birth weight below the 10th percentile for the reference population ('small for gestation') versus infants with a birth weight at or above the 10th percentile ('appropriate for gestation').
Subgroup data for infants born 'small for gestation' not available.
-
Trials set in low‐ or middle‐income countries versus trials set in high‐income countries.
All the trials were conducted in a middle‐income country (India).
Sensitivity analysis for risk of bias
We were unable to do planned sensitivity analyses to determine if the findings were affected by bias as the trials had a similar risk of bias with low risk for randomisation and allocation concealment, complete or near‐complete follow‐up, but high risk from inadequate masking of intervention and measurement.
Discussion
Summary of main results
Data from six trials, in which 526 VLBW infants participated, provided limited evidence about the effect of early full enteral feeding on growth and development. There are data from two trials on in hospital rates of weight gain, and these have inconsistent results (not suitable to be combined in meta‐analysis). One trial that assessed infant weight at the time of hospital discharge showed that early enteral feeding slightly increased the mean z‐score compared with delayed or progressive advancement of enteral feeds. There are no data on head growth or length at hospital discharge, or for growth parameters following discharge and developmental outcomes assessed beyond infancy.
The growth parameter most commonly reported was the time from birth to regain birth weight. We did not prespecify this outcome, but a post hoc meta‐analysis of data from six trials showed that infants who had early full enteral feeding regained birth weight about three days earlier than infants who had delayed introduction and progressive advancement of feed volumes. Given the paucity of data for other growth parameters, however, the importance of this effect is unclear.
Meta‐analysis of data from six trials found no effect of early full enteral feeding on risk of necrotising enterocolitis. The bounds of the 95% CI for the RD are consistent with either three extra or three fewer cases in every 100 infants who receive early full enteral feeding. We found no evidence of other possible benefits or harms, including insufficient data to determine whether early full enteral feeding is associated with the risk of feed intolerance, invasive infection, duration of birth hospitalisation, mortality, or episodes of hypoglycaemia during the early neonatal period.
Overall completeness and applicability of evidence
Most participants included in the six trials in this review were stable very preterm infants of birth weight between 1000 g and 1500 g. One trial excluded infants who were born 'small for gestation' from participation (Chetry 2014). Two trials excluded infants with evidence of intrauterine growth compromise indicated by the antenatal detection of absent or reversed end‐diastolic flow velocity in the umbilical vessels (Nangia 2019; Ramya 2014). The other trials did not report subgroup data for infants who were 'small for gestation' or growth restricted at birth. It is unclear, therefore, whether the review findings are applicable to infants who have both a high level of nutrient requirement and an elevated risk of developing necrotising enterocolitis (Dorling 2005; Samuels 2017).
In two trials, infants received only human milk (expressed own mother's milk or donor milk) (Bora 2017; Ramya 2014). The other trials used maternal milk preferentially, but used cow's milk formula to supplement any deficits (Chetry 2014; Jajoo 2017; Nangia 2019; Sanghvi 2013). The risk‐benefit balance of early enteral feeding strategies may differ between human milk‐fed and formula‐fed very preterm or VLBW infants (Quigley 2019). Prespecified subgroup analyses, however, found no differences on the risk of necrotising enterocolitis or death between infants who received human milk only versus those where formula could be given instead of, or as a supplement to, human milk.
All the trials included in this review were conducted in neonatal care facilities in India in the 2010s. The review findings may be applicable particularly to neonatal care facilities where staff and equipment costs limit the availability of parenteral fluids or nutrition, as well as to facilities without such resource constraints where early full enteral feeding could complement other infant and family centred care practices (Patel 2018).
Quality of the evidence
The certainty of evidence for the primary outcomes, assessed using GRADE methods, was low or very low, downgraded because of risk of bias in the included trials (lack of masking of investigators, parents, and carers), imprecision of estimates of effect, and inconsistency (heterogeneity) in meta‐analyses (Table 1).
Risk of bias (lack of masking)
In all the included trials, staff and parents knew to which group the participating infant had been allocated (because it is difficult to conceal intravenous access devices and fluids). Lack of masking may have resulted in surveillance and detection biases. It is unclear, however, to what extent lack of masking might have biased effect estimates. It is plausible that clinicians who harboured concerns that early full enteral feeding could contribute to feed intolerance or necrotising enterocolitis, for example, might have undertaken more assessments or tests, or used a lower threshold for subjective criteria, to diagnose these conditions.
Inconsistency (heterogeneity) in meta‐analyses
The meta‐analyses in this review showed moderate to high levels of statistical heterogeneity that were not explained by major differences in trial design or conduct. All the trials were undertaken in secondary care facilities in India during the 2010s. Participants were similar (stable very preterm infants of birth weight between 1000 g and 1500 g). Trials used slightly different rates of advancement of feed volumes, but these were consistent with current practice (20 mL/kg to 30 mL/kg). Trials differed by the modality of intravenous fluids in the control group, with two trials providing parenteral nutrition and four providing 10% dextrose. Given the duration of use of intravenous fluid (up to one week), this may have affected growth outcomes.
The assessed outcomes were similar across the trials. For some outcomes, such as feed intolerance and necrotising enterocolitis, diagnosed using subjective criteria, variation in diagnostic thresholds might be a source of heterogeneity. As these criteria were not explicitly described in all the published reports, we were unable to explore the extent to which variation in outcome definition contributed to inconsistency in estimates of effect.
In prespecified subgroup comparisons of trials in which infants received human milk only versus those where formula could be given as a supplement to human milk, there was no evidence of a differential effect on the risk of necrotising enterocolitis. Small subgroup differences exist for the risk of feed intolerance and time taken to regain birth weight. The subgroup difference in the estimates of effect on the duration of hospitalisation favoured trials in which formula was used as a supplement when maternal milk was insufficient. This might be due to accelerated weight gain in infants receiving preterm formula with higher nutrient density than human milk. Since an attained body weight (ranging from 1300 g to 1600 g across trials) was a key discharge criterion for participants, infants reaching those targets earlier will have shorted durations of hospitalisation.
Imprecision
The estimates of effect were imprecise as indicated by the broad 95% CIs with upper and lower bounds encompassing substantial harm or benefit. This reflects the sample size of the studied population (526 infants in total), the rarity of the key outcomes such as necrotising enterocolitis (3% overall rate in the control groups), and the paucity of outcome data, especially of growth parameters, available for synthesis due to non‐reporting. Ongoing trials of this intervention should recruit sufficient participants to provide data to optimise the information size in future meta‐analyses.
Potential biases in the review process
The main concern is the possibility that findings of this review are subject to publication and other reporting biases. We attempted to minimise this threat by screening the reference lists of included trials and related reviews and searching the proceedings of major international perinatal conferences to identify trial reports that are not (yet) published in full form in academic journals. There were insufficient trials to examine for symmetry of funnel plots as a means of identifying possible publication or small‐study bias.
Agreements and disagreements with other studies or reviews
This review focused on the comparison of early full enteral feeding versus delayed introduction or progressive feed volume advancement. Other Cochrane Reviews have appraised and synthesised the trial evidence for the comparison of different rates of volume advancement and different timing of introduction of progressive enteral feeds. Consistent with the findings of this review, these show that conservative feeding strategies (delaying introduction of enteral feeds until beyond four days after birth, or advancing feed volumes more slowly than by 24 mL/kg daily) do not affect growth parameters or the risk of feed intolerance or necrotising enterocolitis in very preterm infants (Morgan 2014; Oddie 2017).
Authors' conclusions
Implications for practice.
The available trial data do not provide sufficient data to determine how early full enteral feeding, compared with delayed or gradual introduction of enteral feeds (combined with parenteral fluids or nutrition) affects growth and development in preterm or low birth weight infants. While meta‐analyses found no effects on the risk of necrotising enterocolitis, feed intolerance, late onset infection, or death in preterm or low birth weight infants, the certainty of the evidence is low or very low.
Implications for research.
Given the potential for early full enteral feeding to affect important outcomes in preterm or low birth weight infants, this intervention merits further assessment in pragmatic randomised trials powered to detect important effects on growth, as well as potential adverse consequences such as necrotising enterocolitis. One such trial is being conducted in the UK (FEED1) (ISRCTN89654042).
History
Protocol first published: Issue 3, 2020 Review first published: Issue 12, 2020
Acknowledgements
We would like to thank Cochrane Neonatal: Colleen Ovelman, Managing Editor; Jane Cracknell, Assistant Managing Editor; and Roger Soll, Co‐coordinating editor, who provided editorial and administrative support.
David Osborn (Cochrane Neonatal Editor) and Thangaraj Abiramalatha (peer reviewer) provided feedback.
We thank Drs Satish Saluja, Mamta Jajoo, and Sushma Nangia for providing unpublished information about their trials (Chetry 2014; Jajoo 2017; Nangia 2019).
Appendices
Appendix 1. Electronic search strategy
CENTRAL via the Cochrane Library
Search date 19 October 2020
ID Search
#1 MeSH descriptor: [Infant, Newborn] explode all trees
#2 MeSH descriptor: [Premature Birth] explode all trees
#3 ((neonat* or neo nat*)):ti,ab,kw OR ((newborn* or new born* or newly born*)):ti,ab,kw OR ((preterm or preterms or pre term or pre terms)):ti,ab,kw OR ((preemie* or premie or premies)):ti,ab,kw OR ((prematur* NEAR/3 (birth* or born or deliver*))):ti,ab,kw (Word variations have been searched)
#4 ((low NEAR/3 (birthweight* or birth weight*))):ti,ab,kw OR ((lbw or vlbw or elbw)):ti,ab,kw OR (infan*):ti,ab,kw OR ((baby or babies)):ti,ab,kw (Word variations have been searched)
#5 #1 OR #2 OR #3 OR #4
#6 MeSH descriptor: [Enteral Nutrition] explode all trees
#7 (((enteral or enteric) NEAR/2 (nutrition or feed*))):ti,ab,kw OR (((oral or sip or tube) NEAR/2 feeding*)):ti,ab,kw OR (((nasogastric or gastrostomy or jejunostomy) NEAR/2 tube*)):ti,ab,kw OR (((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) NEAR/3 enteral feed*)):ti,ab,kw OR (((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) NEAR/3 enteric feed*)):ti,ab,kw (Word variations have been searched)
#8 (((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) NEAR/3 enteral intake*)):ti,ab,kw OR (((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) NEAR/3 enteric intake*)):ti,ab,kw OR (((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) NEAR/3 enteral nutrition)):ti,ab,kw OR (((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) NEAR/3 enteric nutrition)):ti,ab,kw OR (((aggressive* or fast or rapid* or slow* or speed*) NEAR/3 feed*)):ti,ab,kw (Word variations have been searched)
#9 (((aggressive* or fast or rapid* or slow* or speed*) NEAR/3 volume*)):ti,ab,kw OR (trophic feeding*):ti,ab,kw OR (((gut or gastrointestinal) NEAR/2 priming)):ti,ab,kw (Word variations have been searched)
#10 #6 or #7 or #8 or #9
#11 #5 and #10
#12 MeSH descriptor: [Parenteral Nutrition] explode all trees and with qualifier(s): [adverse effects ‐ AE]
#13 MeSH descriptor: [Enterocolitis, Necrotizing] explode all trees and with qualifier(s): [etiology ‐ ET, epidemiology ‐ EP, prevention & control ‐ PC]
#14 MeSH descriptor: [Infections] 1 tree(s) exploded and with qualifier(s): [epidemiology ‐ EP]
#15 (((prevent* or risk*) NEAR/3 necrotising enterocolitis)):ti,ab,kw OR (((prevent* or risk*) NEAR/3 necrotizing enterocolitis)):ti,ab,kw (Word variations have been searched)
#16 #12 or #13 or #14 or #15
#17 #5 and #16
#18 #11 or #17
CINAHL via EBSCO
Search date 19 October 2020
S1 (MH "Infant, Newborn+")
S2 (MH "Childbirth, Premature")
S3 TI ( (neonat* or neo nat*) ) OR AB ( (neonat* or neo nat*) ) OR TI ( (newborn* or new born* or newly born*) ) OR AB ( (newborn* or new born* or newly born*) ) OR TI ( (preterm or preterms or pre term or pre terms) ) OR AB ( (preterm or preterms or pre term or pre terms) )
S4 TI ( (preemie* or premie or premies) ) OR AB ( (preemie* or premie or premies) ) OR TI ( (prematur* N3 (birth* or born or deliver*)) ) OR AB ( (prematur* N3 (birth* or born or deliver*)) ) OR TI ( (low N3 (birthweight* or birth weight*)) ) OR AB ( (low N3 (birthweight* or birth weight*)) )
S5 TI ( (lbw or vlbw or elbw) ) OR AB ( (lbw or vlbw or elbw) ) OR TI infan* OR AB infan* OR TI ( (baby or babies) ) OR AB ( (baby or babies) )
S6 S1 OR S2 OR S3 OR S4 OR S5
S7 (MH "Enteral Nutrition")
S8 TI ( ((enteral or enteric) N2 (nutrition or feed*)) ) OR AB ( ((enteral or enteric) N2 (nutrition or feed*)) ) OR TI ( ((oral or sip or tube) N2 feeding*) ) OR AB ( ((oral or sip or tube) N2 feeding*) ) OR TI ( ((nasogastric or gastrostomy or jejunostomy) N2 tube*) ) OR AB ( ((nasogastric or gastrostomy or jejunostomy) N2 tube*) )
S9 TI ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteral feed*) ) OR AB ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteral feed*) ) OR TI ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteric feed*) ) OR AB ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteric feed*) ) OR TI ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteral intake*) ) OR AB ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteral intake*) )
S10 TI ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteric intake*) ) OR AB ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteric intake*) ) OR TI ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteral nutrition) ) OR AB ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteral nutrition) ) OR TI ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteric nutrition) ) OR AB ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteric nutrition) )
S11 TI ( ((aggressive* or fast or rapid* or slow* or speed*) N3 feed*) ) OR AB ( ((aggressive* or fast or rapid* or slow* or speed*) N3 feed*) ) OR TI ( ((aggressive* or fast or rapid* or slow* or speed*) N3 volume*) ) OR AB ( ((aggressive* or fast or rapid* or slow* or speed*) N3 volume*) ) OR TI trophic feeding OR AB trophic feeding* OR TI ( ((gut or gastrointestinal) N2 priming) ) OR AB ( ((gut or gastrointestinal) N2 priming) )
S12 S7 OR S8 OR S9 OR S10 OR S11
S13 S6 AND S12
S14 (MH "Double‐Blind Studies")
S15 (MH "Single‐Blind Studies")
S16 (MH "Random Assignment")
S17 (MH "Pretest‐Posttest Design")
S18 (MH "Cluster Sample")
S19 TI ( randomized or randomised ) OR AB random* OR TI trial
S20 MH "sample size" AND AB ( (assigned or allocated or control) )
S21 MH placebos
S22 PT randomised controlled trial OR AB control W5 group OR MH "crossover design" OR MH "comparative studies" OR AB cluster W3 RCT
S23 S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22
S24 S13 AND S23
S25 (MH "Parenteral Nutrition/AE")
S26 (MH "Enterocolitis, Necrotizing/CO/ET/EP/PC")
S27 (MH "Infection/EP")
S28 TI ( ((prevent* or risk*) N3 necrotising enterocolitis) ) OR AB ( ((prevent* or risk*) N3 necrotising enterocolitis) ) OR TI ( ((prevent* or risk*) N3 necrotizing enterocolitis) ) OR AB ( ((prevent* or risk*) N3 necrotizing enterocolitis) )
S29 S25 OR S26 OR S27 OR S28
S30 S6 AND S23 AND S29
S31 S24 OR S30
View Results (1,062)
Embase via Ovid
Search date 19 October 2020
Database: Embase <1974 to 2020 Week 42>
1 Newborn/
2 Prematurity/
3 (neonat$ or neo nat$).ti,ab.
4 (newborn$ or new born$ or newly born$).ti,ab.
5 (preterm or preterms or pre term or pre terms).ti,ab.
6 (preemie$ or premie or premies).ti,ab.
7 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab.
8 (low adj3 (birthweight$ or birth weight$)).ti,ab.
9 (lbw or vlbw or elbw).ti,ab.
10 infan$.ti,ab.
11 (baby or babies).ti,ab.
12 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11
13 Enteric Nutrition/
14 ((enteral or enteric) adj2 (nutrition or feed$)).ti,ab.
15 ((oral or sip or tube) adj2 feeding$).ti,ab.
16 ((nasogastric or gastrostomy or jejunostomy) adj2 tube$).ti,ab.
17 ((advanc$ or aggressive$ or delay$ or early or fast or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral feed$).ti,ab.
18 ((advanc$ or aggressive$ or delay$ or early or fast or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric feed$).ti,ab.
19 ((advanc$ or aggressive$ or delay$ or early or fast or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral nutrition).ti,ab.
20 ((advanc$ or aggressive$ or delay$ or early or fast or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric nutrition).ti,ab.
21 ((advanc$ or aggressive$ or delay$ or early or fast or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral intake).ti,ab.
22 ((advanc$ or aggressive$ or delay$ or early or fast or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric intake).ti,ab.
23 ((aggressive$ or fast or rapid$ or slow$ or speed$) adj3 feed$).ti,ab.
24 ((aggressive$ or fast or rapid$ or slow$ or speed$) adj3 volume$).ti,ab.
25 trophic feeding$.ti,ab.
26 ((gut or gastrointestinal) adj2 priming).ti,ab.
27 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26
28 12 and 27
29 Randomized controlled trial/
30 Controlled clinical study/
31 Random$.ti,ab.
32 randomization/
33 intermethod comparison/
34 placebo.ti,ab.
35 (compare or compared or comparison).ti.
36 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab.
37 (open adj label).ti,ab.
38 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
39 double blind procedure/
40 parallel group$1.ti,ab.
41 (crossover or cross over).ti,ab.
42 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
43 (assigned or allocated).ti,ab.
44 (controlled adj7 (study or design or trial)).ti,ab.
45 (volunteer or volunteers).ti,ab.
47 trial.ti.
48 or/29‐47
49 (random$ adj sampl$ adj7 ("cross section$" or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.)
50 Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.)
51 (((case adj control$) and random$) not randomi?ed controlled).ti,ab.
52 (Systematic review not (trial or study)).ti.
53 (nonrandom$ not random$).ti,ab.
54 "Random field$".ti,ab.
55 (random cluster adj3 sampl$).ti,ab.
56 (review.ab. and review.pt.) not trial.ti.
57 "we searched".ab. and (review.ti. or review.pt.)
58 "update review".ab.
59 (databases adj4 searched).ab.
60 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/
61 Animal experiment/ not (human experiment/ or human/)
62 or/49‐61
63 48 not 62
64 28 and 63
65 Parenteral Nutrition/
66 complication/
67 safety/ or patient safety/
68 (adverse$ adj2 (effect$ or event$ or impact$ or outcome$)).ti,ab.
69 (complication$ or risk$ or safe or safely or safer or safety or sequaela or side effect$ or tolerated or toxicities or toxicity).ti,ab. (5056731)
70 65 and (66 or 67 or 68 or 69)
71 Necrotizing Enterocolitis/co, ep, et, pc [Complication, Epidemiology, Etiology, Prevention]
72 ((prevent$ or risk$) adj3 necrotising enterocolitis).ti,ab.
73 ((prevent$ or risk$) adj3 necrotizing enterocolitis).ti,ab.
74 Infection/ep [Epidemiology]
75 70 or 71 or 72 or 73 or 74
76 12 and 63 and 75
77 64 or 76
Maternity and infant Care Via Ovid
Search date 19 October 2020
Database: Maternity & Infant Care Database (MIDIRS) <1971 to August 2020>
1 (neonat$ or neo nat$).ti,ab.
2 (newborn$ or new born$ or newly born$).ti,ab.
3 (preterm or preterms or pre term or pre terms).ti,ab.
4 (preemie$ or premie or premies).ti,ab.
5 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab.
6 (low adj3 (birthweight$ or birth weight$)).ti,ab.
7 (lbw or vlbw or elbw).ti,ab.
8 infan$.ti,ab.
9 (baby or babies).ti,ab.
10 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9
11 (Infant ‐ premature or Infant ‐ very low birth weight or Infant ‐ newborn).de.
12 10 or 11
13 Enteral nutrition.de.
14 ((enteral or enteric) adj2 (nutrition or feed$)).ti,ab.
15 ((oral or sip or tube) adj2 feeding$).ti,ab.
16 ((nasogastric or gastrostomy or jejunostomy) adj2 tube$).ti,ab.
17 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral feed$).ti,ab.
18 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric feed$).ti,ab.
19 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral intake$).ti,ab.
20 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric intake$).ti,ab.
21 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral nutrition).ti,ab.
22 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric nutrition).ti,ab.
23 ((aggressive$ or fast or rapid$ or slow$ or speed$) adj3 feed$).ti,ab.
24 ((aggressive$ or fast or rapid$ or slow$ or speed$) adj3 volume$).ti,ab.
25 trophic feeding$.ti,ab.
26 ((gut or gastrointestinal) adj2 priming).ti,ab.
27 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26
28 12 and 27
29 limit 28 to randomised controlled trial
30 Parenteral nutrition.de.
31 Enterocolitis ‐ necrotizing.de.
32 (adverse$ adj2 (effect$ or event$ or impact$ or outcome$)).ti,ab.
33 (complication$ or risk$ or safe or safely or safer or safety or sequaela or side effect$ or tolerated or toxicities or toxicity).ti,ab.
34 Complications.de.
35 safety.de.
36 (30 or 31) and (32 or 33 or 34 or 35)
37 ((prevent$ or risk$) adj3 necrotising enterocolitis).ti,ab.
38 ((prevent$ or risk$) adj3 necrotizing enterocolitis).ti,ab.
39 36 or 37 or 38
40 limit 39 to randomised controlled trial
41 29 or 40
MEDLINE via Ovid
Search date 19 October 2020
Database: Ovid MEDLINE(R) ALL <1946 to October 16, 2020>
1 exp Infant, Newborn/
2 Premature Birth/
3 (neonat$ or neo nat$).ti,ab.
4 (newborn$ or new born$ or newly born$).ti,ab.
5 (preterm or preterms or pre term or pre terms).ti,ab.
6 (preemie$ or premie or premies).ti,ab.
7 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab.
8 (low adj3 (birthweight$ or birth weight$)).ti,ab.
9 (lbw or vlbw or elbw).ti,ab.
10 infan$.ti,ab.
11 (baby or babies).ti,ab.
12 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11
13 Enteral Nutrition/
14 ((enteral or enteric) adj2 (nutrition or feed$)).ti,ab.
15 ((oral or sip or tube) adj2 feeding$).ti,ab.
16 ((nasogastric or gastrostomy or jejunostomy) adj2 tube$).ti,ab.
17 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral feed$).ti,ab.
18 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric feed$).ti,ab.
19 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral intake$).ti,ab.
20 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric intake$).ti,ab.
21 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral nutrition).ti,ab.
22 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric nutrition).ti,ab.
23 ((aggressive$ or fast or rapid$ or slow$ or speed$) adj3 feed$).ti,ab.
24 ((aggressive$ or fast or rapid$ or slow$ or speed$) adj3 volume$).ti,ab.
25 trophic feeding$.ti,ab.
26 ((gut or gastrointestinal) adj2 priming).ti,ab.
27 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26
28 12 and 27
29 Parenteral Nutrition/ae [Adverse Effects]
30 Enterocolitis, Necrotizing/ep, et, pc [Epidemiology, Etiology, Prevention & Control]
31 ((prevent$ or risk$) adj3 necrotising enterocolitis).ti,ab.
32 ((prevent$ or risk$) adj3 necrotizing enterocolitis).ti,ab.
33 Infection/ep [Epidemiology]
34 29 or 30 or 31 or 32 or 33
35 12 and 34
36 28 or 35
37 randomized controlled trial.pt.
38 controlled clinical trial.pt.
39 randomized.ab.
40 placebo.ab.
41 drug therapy.fs.
42 randomly.ab.
43 trial.ab.
44 groups.ab.
45 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44
46 exp animals/ not humans.sh.
47 45 not 46
48 36 and 47
Trials Registers
ClinicalTrials.gov
Search one – Enteral feeding restricted to interventional studies about children aged birth to 17
Search two – Enterocolitis, necrotizing restricted to infants, interventional studies
WHO ICTRP
Search one – enteral nutrition
Search two – necrotizing enterocolitis
Appendix 2. 'Risk of bias' tool
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk, or unclear risk for participants; and
low risk, high risk, or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we reincluded missing data in the analyses. We categorised the methods as:
low risk (less than 20% missing data);
high risk (20% or greater missing data); or
unclear risk.
6. Selective reporting bias. Were reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it was clear that all the study's prespecified outcomes and all expected outcomes of interest to the review were reported);
high risk (where not all the study's prespecified outcomes were reported; one or more reported primary outcomes were not prespecified outcomes of interest and were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data dependent process). We assessed whether each study was free of other problems that could have put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Early full versus delayed/progressive enteral nutrition.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 z‐score for weight at hospital discharge | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [0.06, 0.42] |
| 1.2 Necrotising enterocolitis | 6 | 522 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.38, 2.54] |
| 1.2.1 Exclusive human milk | 2 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.08 [0.47, 35.26] |
| 1.2.2 Supplemental formula | 4 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.16, 1.82] |
| 1.3 Feed intolerance | 4 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.49, 1.13] |
| 1.3.1 Exclusive human milk | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.83, 5.02] |
| 1.3.2 Supplemental formula | 3 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.33, 0.88] |
| 1.4 Episodes of hypoglycaemia | 2 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 70.02] |
| 1.5 Invasive infection | 4 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.36, 1.46] |
| 1.5.1 Exclusive human milk | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.56, 7.58] |
| 1.5.2 Supplemental formula | 3 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.18, 1.09] |
| 1.6 Duration of birth hospitalisation (days) | 5 | 436 | Mean Difference (IV, Fixed, 95% CI) | ‐3.07 [‐4.13, ‐2.02] |
| 1.6.1 Exclusive human milk | 1 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐2.66, ‐0.14] |
| 1.6.2 Supplemental formula | 4 | 333 | Mean Difference (IV, Fixed, 95% CI) | ‐7.03 [‐8.96, ‐5.09] |
| 1.7 All‐cause mortality | 6 | 522 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.36, 1.70] |
| 1.7.1 Exclusive human milk | 2 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.29, 3.59] |
| 1.7.2 Supplemental formula | 4 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.24, 1.80] |
| 1.8 Days to regain birth weight | 6 | 505 | Mean Difference (IV, Fixed, 95% CI) | ‐3.53 [‐4.37, ‐2.70] |
| 1.8.1 Exclusive human milk | 2 | 168 | Mean Difference (IV, Fixed, 95% CI) | ‐1.97 [‐3.67, ‐0.26] |
| 1.8.2 Supplemental formula | 4 | 337 | Mean Difference (IV, Fixed, 95% CI) | ‐4.02 [‐4.98, ‐3.07] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bora 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 107 "stable" VLBW infants (1000–1500 g birth weight), irrespective of gestational age at birth Setting: Department of Paediatrics, Assam Medical College, Assam, India |
|
| Interventions | Intervention (n = 52): full enteral feeds (80 mL/kg/day) with expressed breast milk or donor breast milk Control (n = 55): minimal enteral feeds (20 mL/kg/day) supplemented with intravenous 10% dextrose Feed volumes increased by 20 mL/kg/day as tolerated |
|
| Outcomes |
|
|
| Notes | Human milk was enriched with a multi‐nutrient fortifier when infants reached an intake volume of volume of 100 mL/kg/day. The intervention group contained more infants born to mothers with pregnancy induced hypertension (51%) than the control group (31%). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Near‐complete (103/107 participants). |
| Selective reporting (reporting bias) | Unclear risk | No protocol to compare. All outcomes in methods section reported. |
| Other bias | Unclear risk | No funding source reported. |
Chetry 2014.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 64 "stable" VLBW infants (1000–1500 g birth weight), of gestational age 30–34 weeks at birth, and > 3rd percentile for weight Setting: Sir Ganga Ram Hospital, New Delhi, India |
|
| Interventions | Intervention (n = 31): full enteral feeds (80 mL/kg/day) with expressed breast milk if available or preterm formula Control (n = 33): minimal enteral feeds (20 mL/kg/day) supplemented with parenteral nutrition Feed volumes increased by 20 mL/kg/day subject as tolerated |
|
| Outcomes |
|
|
| Notes | Further information via author communication (satishsaluja@gmail.com) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete. |
| Selective reporting (reporting bias) | Unclear risk | Abstract and author correspondence only. No protocol or methods to compare outcomes reported. |
| Other bias | Unclear risk | No definition given for necrotising enterocolitis. Funding source not reported. |
Jajoo 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 60 "stable" VLBW (1000–1499 g birth weight), of gestational age 29–33 weeks at birth Setting: Department of Paediatrics, Chacha Nehru Bal Chikitsalaya, New Delhi, India |
|
| Interventions | Intervention (n = 31): full enteral feeds (80 mL/kg/day) with expressed maternal milk or formula Control (n = 29): minimal enteral feeds (30 mL/kg/day) supplemented with intravenous 10% dextrose Feed volumes increased by 20 mL/kg/day as tolerated |
|
| Outcomes |
|
|
| Notes | Further information via author communication (mamtajajoo123@gmail.com) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely to have been masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unlikely to have been masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. No protocol available to compare. Additional to primary and secondary outcome in methods section, they reported duration of hospital stay and mortality. |
| Other bias | Unclear risk | Funding source not reported. |
Nangia 2019.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 180 "stable" VLBW infants (1000–1499 g birth weight), of gestational age 28–34 weeks at birth Setting: Department of Neonatology, Lady Hardinge Medical College, New Delhi, India |
|
| Interventions | Intervention (n = 91): full enteral feeds (80 mL/kg/day) with expressed breast milk or preterm formula Control (n = 89): minimal enteral feeds (20 mL/kg/day) supplemented with intravenous 10% dextrose Feed volumes advanced by 20 mL/kg/day for 2 days, then 30 mL/kg/day |
|
| Outcomes |
|
|
| Notes | Human milk was enriched with a multi‐nutrient fortifier when infants reached an intake volume of volume of 100 mL/kg/day. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete. |
| Selective reporting (reporting bias) | Low risk | All outcomes on trial registry reported. Additional data not specified as outcomes in trial registry also reported as secondary outcomes (necrotising enterocolitis, days to regain birth weight, discharge weight, hyperglycaemia, hypoglycaemia, patent ductus arteriosus, apnoea, duration of antibiotics, duration of intravenous fluids, intraventricular haemorrhage, shock). |
| Other bias | Unclear risk | Funding source not reported. |
Ramya 2014.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 69 stable VLBW infants (1000–1500 g birth weight) Setting: Madras Medical College, Chennai, Tamil Nadu, India |
|
| Interventions | Intervention (n = 34): full enteral feeds (60 mL/kg/day) with expressed maternal milk or donor breast milk Control (n = 35): minimal enteral feeds (20–40 mL/kg/day) supplemented with parenteral nutrition Feed volumes advanced by 20 mL/kg/day as tolerated |
|
| Outcomes |
|
|
| Notes | Nutrient fortifiers not used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete. |
| Selective reporting (reporting bias) | Unclear risk | No protocol. |
| Other bias | Unclear risk | Study for dissertation submitted to University for DM (Neonatology). No funding source reported. |
Sanghvi 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 46 VLBW infants (1200–1500 g birth weight), irrespective of gestational age at birth Setting: Prince Aly Khan Hospital, Mumbai, India |
|
| Interventions | Intervention (n = 23): full enteral feeds (80 mL/kg/day) with expressed maternal milk or preterm formula Control (n = 23): minimal enteral feeds (20 mL/kg/day) supplemented with parenteral nutrition Feed volumes increased by 20 mL/kg/day as tolerated |
|
| Outcomes |
|
|
| Notes | Human milk was enriched with a multi‐nutrient fortifier when infants reached an intake volume of volume of 100 mL/kg/day. Fewer infants in the intervention group than the control group received respiratory support via a continuous positive airway pressure device. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated (variable block size randomisation, generated by statistician who was not part of the study). |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not masked – carers could not be blinded to treatment allocation because of nature of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete. |
| Selective reporting (reporting bias) | Low risk | Retrospectively registered (2012). All outcomes reported in trial registry reported. Reported additional outcomes of feeding intolerance, weight at discharge, z‐score of weight at discharge, length and head circumference at discharge. |
| Other bias | Low risk | Received no monetary support. |
n: number of participants; SD: standard deviation; VLBW: very low birth weight.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Genzel‐Boroviczény 2002 | Non‐randomised observational study comparing apolipoprotein A1 and high‐density lipoprotein distributions in 3 groups of infants (infants with parenteral nutrition receiving early feedings, preterm infants and term infants receiving enteral nutrition). |
| Higgs 1974 | Did not meet inclusion criteria for intervention and comparator. Intervention of total parenteral nutrition was compared to a milk feeding regimen supplemented with intravenous dextrose. |
| Jain 2016 | Did not meet inclusion criteria for intervention and comparator. This study compared the speed of increasing milk feeds supplemented by intravenous dextrose/parenteral nutrition. |
| Yu 1979 | Did not meet inclusion criteria for intervention and comparator. Intervention of total parenteral nutrition was compared to a milk feeding regimen supplemented with intravenous dextrose. |
Characteristics of ongoing studies [ordered by study ID]
ISRCTN89654042.
| Study name | Fluids exclusively enterally from day one in premature infants |
| Methods | Randomised controlled trial |
| Participants | Preterm infants born at 30–32 completed weeks' gestation |
| Interventions | Intervention: full milk feeds (minimum of 60 mL/kg each day) from day 1 Control: maximum of 30 mL/kg from day 1 along with intravenous fluids/nutrition as per usual care |
| Outcomes | Primary outcome: duration of birth hospitalisation |
| Starting date | 2019 |
| Contact information | Garry Meakin, University of Nottingham, UK |
| Notes | Sample size: 2080 |
NCT03708068.
| Study name | Early exclusive enteral nutrition in early preterm infants |
| Methods | Randomised controlled trial |
| Participants | Preterm infants born at 30–33 completed weeks' gestation, and of birth weight > 1000 g |
| Interventions | Intervention: early full enteral feeds: feeds commence at ≥ 80% of reference daily fluid intake from day 1 Control: enteral feeds commence at 15–30 mL/kg on day 1, then advanced by 15–30 mL/kg per day on second day onwards until infant reaches full enteral feeds |
| Outcomes | Primary outcome: time to achieve full enteral feeds defined as 140 mL/kg/day sustained for ≥ 3 days |
| Starting date | 2019 |
| Contact information | Belal Alshaikh, University of Calgary, Canada |
| Notes | Sample size: 68 |
Differences between protocol and review
In the protocol (Walsh 2020), we stated a primary outcome of growth measured by "in hospital rate of weight gain (g/kg/day) until term equivalent". Only two studies reported this outcome, with Chetry 2014 reporting the result as the median and Nangia 2019 only reporting the mean with no standard deviations. The outcome of weight gain is an important outcome when comparing preterm feeding. All studies reported days to regain birth weight, and we decided to include this outcome in post hoc analyses.
The definition of growth restriction in Sanghvi 2013 (z‐score at discharge) differed from our prespecified definition (z‐score at term). We made a consensus post hoc decision to include the data based on the investigators' definition (Analysis 1.1).
The definitions of feed intolerance used in the included trials did not specify the duration of enteral feed interruption (Bora 2017; Nangia 2019; Sanghvi 2013). We made a consensus post hoc decision to include the data based on the investigators' definitions (Analysis 1.3).
Contributions of authors
VW, JB, BC, and WM screened and appraised reports identified in the search, extracted and analysed data from included studies and drafted the review.
SO and WM arbitrated inclusion and data extraction disagreements.
All authors drafted the final review.
Sources of support
Internal sources
Centre for Reviews and Dissemination, University of York, UK
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
National Institute for Health Research (NIHR), UK
This report is independent research funded by a UK NIHR Cochrane Programme Grant (16/114/03). The views expressed in this publication are those of the review authors and are not necessarily those of the National Health Service, the NIHR, or the UK Department of Health.
-
The Gerber Foundation, USA
Editorial support for this review, as part of a suite of preterm nutrition reviews, has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private, 501(c)(3) foundation not related to Gerber Products Company in any way.
Declarations of interest
VW: none.
JB: none.
BC: none.
SO and WM are the co‐investigators for the "Fluids exclusively enteral from day one in premature infants" trial (ISRCTN89654042).
WM is Cochrane Neonatal co‐coordinating editor.
Core editorial and administrative support for this review has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private foundation, independent from the Gerber Products Company. The grantor has no input on the content of the review or the editorial process (see Sources of support).
New
References
References to studies included in this review
Bora 2017 {published data only}
- Bora R, Murthy NB. In resource limited areas complete enteral feed in stable very low birth weight infants (1000–1500 g) started within 24 h of life can improve nutritional outcome. Journal of Maternal-Fetal & Neonatal Medicine 2017;30(21):2572-7. [DOI: 10.1080/14767058.2016.1256992] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chetry 2014 {published data only}
- Chetry S, Kler N, Saluja S, Garg P, Soni A, Thakur A. A randomised controlled trial comparing initiation of total enteral feeds on 1st day of life with standard feeding regimen in stable very low birth weight infants born between > 30-34 weeks gestation and 1000-1500 gms. In: Pediatric Academic Societies Annual Meeting; 2014 May 3–6; Vancouver (BC). 2014:2390;442.
Jajoo 2017 {published data only}
- Jajoo M, Arora N, Dabas V, Talukdar B, Kumari S. Early total versus gradual advancement of early nutrition in healthy preterm very low birth weight neonates in a tertiary care nursery: a randomised controlled trial. In: Pediatric Academic Societies Annual Meeting; 2017 May 6–9; San Francisco (CA). 2017:3800;28.
Nangia 2019 {published data only}
- Nangia S, Vadivel V, Thukral A, Saili A. Early total enteral feeding versus conventional enteral feeding in stable very-low-birth-weight infants: a randomised controlled trial. Neonatology 2019;115(3):256-62. [DOI: 10.1159/000496015] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ramya 2014 {published data only}
- Ramya S. Does Full Enteral Feeding from Day One of Life Influence Weight Gain in Haemodynamically Stable Babies Weighing Between 1000–1500 Grams as Against Standard Feeding? An Open Label Randomised Controlled Trial [Dissertation]. Chennai (India): Tamilnadu Dr MGR Medical University, 2014. [Google Scholar]
Sanghvi 2013 {published data only}
- Sanghvi KP, Joshi P, Nabi F, Kabra N. Feasibility of exclusive enteral feeds from birth in VLBW infants >1200 g – an RCT. Acta Paediatrica (Oslo, Norway : 1992) 2013;102(7):e299-304. [DOI: 10.1111/apa.12254] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Genzel‐Boroviczény 2002 {published data only}
- Genzel-Boroviczény O, Göbel Y, Koletzko B. Effect of early enteral feeding on apolipoprotein A1 levels and high-density lipoprotein heterogeneity in preterm infants. Annals of Nutrition and Metabolism 2002;46(3-4):121-7. [DOI: 10.1159/000063080] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgs 1974 {published data only}
- Higgs SC, Malan AF, De Heese HV. A comparison of oral feeding and total parenteral nutrition in infants of very low birthweight. South African Medical Journal 1974;48(52):2169-73. [PMID: ] [PubMed] [Google Scholar]
Jain 2016 {published data only}
- Jain S, Mukhopadhyay K, Jain V, Kumar P. Slow versus rapid enteral feed in preterm neonates with antenatal absent end diastolic flow. Journal of Maternal-fetal & Neonatal Medicine 2016;29(17):2828-33. [DOI: 10.3109/14767058.2015.1105954] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yu 1979 {published data only}
- Yu VY, James B, Hendry P, MacMahon RA. Total parenteral nutrition in very low birthweight infants: a controlled trial. Archives of Disease in Childhood 1979;54(9):653-61. [DOI: 10.1136/adc.54.9.653] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN89654042 {published data only (unpublished sought but not used)}
- ISRCTN89654042. Fluids exclusively enterally from day one in premature infants. isrctn.com/ISRCTN89654042 (first received 10 September 2019).
NCT03708068 {published data only (unpublished sought but not used)}
- NCT03708068. Early exclusive enteral nutrition in early preterm infants. clinicaltrials.gov/ct2/show/NCT03708068 (first received 16 October 2018).
Additional references
Agostoni 2010
- Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, et al, ESPGHAN Committee on Nutrition. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2010;50(1):85-91. [DOI: 10.1097/MPG.0b013e3181adaee0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Berrington 2012
- Berrington JE, Hearn RI, Bythell M, Wright C, Embleton ND. Deaths in preterm infants: changing pathology over 2 decades. Journal of Pedodontics 2012;160(1):49-53.e1. [DOI: 10.1016/j.jpeds.2011.06.046] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bisquera 2002
- Bisquera JA, Cooper TR, Berseth CL. Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birth weight infants. Pediatrics 2002;109(3):423-8. [DOI: 10.1542/peds.109.3.423] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bracewell 2008
- Bracewell MA, Hennessy EM, Wolke D, Marlow N. The EPICure study: growth and blood pressure at 6 years of age following extremely preterm birth. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(2):F108-14. [DOI: 10.1136/adc.2007.118596] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chauhan 2008
- Chauhan M, Henderson G, McGuire W. Enteral feeding for very low birth weight infants: reducing the risk of necrotising enterocolitis. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(2):F162-6. [DOI: 10.1136/adc.2007.115824] [PMID: ] [DOI] [PubMed] [Google Scholar]
Clark 2003
- Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics 2003;111(5 Pt 1):986-90. [DOI: 10.1542/peds.111.5.986] [PMID: ] [DOI] [PubMed] [Google Scholar]
Conde‐Agudelo 2016
- Conde-Agudelo A, Diaz-Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database of Systematic Reviews 2016, Issue 8. Art. No: CD002771. [DOI: 10.1002/14651858.CD002771.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooke 2003
- Cooke RW, Foulder-Hughes L. Growth impairment in the very preterm and cognitive and motor performance at 7 years. Archives of Disease in Childhood 2003;88(6):482-7. [DOI: 10.1136/adc.88.6.482] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Waard 2018
- Waard M, Li Y, Zhu Y, Ayede AI, Berrington J, Bloomfield FH, et al. Time to full enteral feeding for very low-birth-weight infants varies markedly among hospitals worldwide but may not be associated with incidence of necrotizing enterocolitis: the NEOMUNE-NeoNutriNet cohort study. Journal of Parenteral and Enteral Nutrition 2018;43:658-67. [DOI: 10.1002/jpen.1466] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dorling 2005
- Dorling J, Kempley S, Leaf A. Feeding growth restricted preterm infants with abnormal antenatal Doppler results. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90(5):F359-63. [DOI: 10.1136/adc.2004.060350] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doyle 2004
- Doyle LW, Faber B, Callanan C, Ford GW, Davis NM. Extremely low birth weight and body size in early adulthood. Archives of Disease in Childhood 2004;89(4):347-50. [DOI: 10.1136/adc.2002.025924] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dusick 2003
- Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA. Growth failure in the preterm infant: can we catch up? Seminars in Perinatology 2003;27(4):302-10. [DOI: 10.1016/s0146-0005(03)00044-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Embleton 2001
- Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics 2001;107(2):270-3. [DOI: 10.1542/peds.107.2.270] [PMID: ] [DOI] [PubMed] [Google Scholar]
Embleton 2013
- Embleton ND. Early nutrition and later outcomes in preterm infants. World Review of Nutrition and Dietetics 2013;106:26-32. [DOI: 10.1159/000342553] [PMID: ] [DOI] [PubMed] [Google Scholar]
Embleton 2014
- Embleton ND, Simmer K. Practice of parenteral nutrition in VLBW and ELBW infants. World Review of Nutrition and Dietetics 2014;110:177-89. [DOI: 10.1159/000358466] [PMID: ] [DOI] [PubMed] [Google Scholar]
Embleton 2017
- Embleton ND, Berrington JE, Dorling J, Ewer AK, Juszczak E, Kirby JA, et al. Mechanisms affecting the gut of preterm infants in enteral feeding trials. Frontiers in Nutrition 2017;4:14. [DOI: 10.3389/fnut.2017.00014] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Farooqi 2006
- Farooqi A, Hägglöf B, Sedin G, Gothefors L, Serenius F. Growth in 10- to 12-year-old children born at 23 to 25 weeks' gestation in the 1990s: a Swedish national prospective follow-up study. Pediatrics 2006;118(5):e1452-65. [DOI: 10.1542/peds.2006-1069] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fitzgibbons 2009
- Fitzgibbons SC, Ching Y, Yu D, Carpenter J, Kenny M, Weldon C, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. Journal of Pediatric Surgery 2009;44(6):1072-5. [DOI: 10.1016/j.jpedsurg.2009.02.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
Flidel‐Rimon 2004
- Flidel-Rimon O, Friedman S, Lev E, Juster-Reicher A, Amitay M, Shinwell ES. Early enteral feeding and nosocomial sepsis in very low birthweight infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2004;89(4):F289-92. [DOI: 10.1136/adc.2002.021923] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flidel‐Rimon 2006
- Flidel-Rimon O, Branski D, Shinwell ES. The fear of necrotizing enterocolitis versus achieving optimal growth in preterm infants – an opinion. Acta Paediatrica 2006;95(11):1341-4. [DOI: 10.1080/08035250600719713] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ford 2000
- Ford GW, Doyle LW, Davis NM, Callanan C. Very low birth weight and growth into adolescence. Archives of Pediatrics and Adolescent Medicine 2000;154(8):778-84. [DOI: 10.1001/archpedi.154.8.778] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gagliardi 2008
- Gagliardi L, Bellu R, Cardilli V, De Curtis M. Necrotising enterocolitis in very low birth weight infants in Italy: incidence and non-nutritional risk factors. Journal of Pediatric Gastroenterology and Nutrition 2008;47(2):206-10. [DOI: 10.1097/MPG.0b013e318174e855] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 7 August 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Guthrie 2003
- Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. Journal of Perinatology 2003;23(4):278-85. [DOI: 10.1038/sj.jp.7210892] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hack 1991
- Hack M, Breslau N, Weissman B, Aram D, Klein N, Borawski E. Effect of very low birth weight and subnormal head size on cognitive abilities at school age. New England Journal of Medicine 1991;325(4):231-7. [DOI: 10.1056/NEJM199107253250403] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hay 2008
- Hay WW Jr. Strategies for feeding the preterm infant. Neonatology 2008;94(4):245-54. [DOI: 10.1159/000151643] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook.
Holman 1997
- Holman RC, Stoll BJ, Clarke MJ, Glass RI. The epidemiology of necrotizing enterocolitis infant mortality in the United States. American Journal of Public Health 1997;87(12):2026-31. [DOI: 10.2105/ajph.87.12.2026] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Horbar 2015
- Horbar JD, Ehrenkranz RA, Badger GJ, Edwards EM, Morrow KA, Soll RF, et al. Weight growth velocity and postnatal growth failure in infants 501 to 1500 grams: 2000–2013. Pediatrics 2015;136(1):e84-92. [DOI: 10.1542/peds.2015-0129] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kliegman 1987
- Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Current Problems in Pediatrics 1987;17(4):213-88. [DOI: 10.1016/0045-9380(87)90031-4] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klingenberg 2012
- Klingenberg C, Embleton ND, Jacobs SE, O'Connell LA, Kuschel CA. Enteral feeding practices in very preterm infants: an international survey. Archives of Disease in Childhood. Fetal and Neonatal Edition 2012;97(1):F56-61. [DOI: 10.1136/adc.2010.204123 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Leaf 2013
- Leaf A. Introducing enteral feeds in the high-risk preterm infant. Seminars in Fetal & Neonatal Medicine 2013;18(3):150-4. [DOI: 10.1016/j.siny.2013.03.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Leppänen 2014
- Leppänen M, Lapinleimu H, Lind A, Matomäki J, Lehtonen L, Haataja L, et al, PIPARI Study Group. Antenatal and postnatal growth and 5-year cognitive outcome in very preterm infants. Pediatrics 2014;133(1):63-70. [DOI: 10.1542/peds.2013-1187] [PMID: ] [DOI] [PubMed] [Google Scholar]
Maas 2018
- Maas C, Franz AR, Krogh S, Arand J, Poets CF. Growth and morbidity of extremely preterm infants after early full enteral nutrition. Archives of Disease in Childhood. Fetal and Neonatal Edition 2018;103(1):F79-81. [DOI: 10.1136/archdischild-2017-312917] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morgan 2013
- Morgan J, Bombell S, McGuire W. Early trophic feeding versus enteral fasting for very preterm or very low birth weight infants. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No: CD000504. [DOI: 10.1002/14651858.CD000504.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgan 2014
- Morgan J, Young L, McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No: CD001970. [DOI: 10.1002/14651858.CD001970.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moro 2009
- Moro M, Pérez-Rodriguez J, Figueras-Aloy J, Fernández C, Doménech E, Jiménez R, et al. Predischarge morbidities in extremely and very low-birth-weight infants in Spanish neonatal units. American Journal of Perinatology 2009;26(5):335-43. [DOI: 10.1055/s-0028-1110083] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nangia 2018
- Nangia S, Bishnoi A, Goel A, Mandal P, Tiwari S, Saili A. Early total enteral feeding in stable very low birth weight Infants: a before and after study. Journal of Tropical Pediatrics 2018;64(1):24-30. [DOI: 10.1093/tropej/fmx023] [PMID: ] [DOI] [PubMed] [Google Scholar]
Oddie 2017
- Oddie SJ, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database of Systematic Reviews 2017, Issue 8. Art. No: CD001241. [DOI: 10.1002/14651858.CD001241.pub7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pammi 2017
- Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 2017;5(1):31. [DOI: 10.1186/s40168-017-0248-8] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2018
- Patel N, Ballantyne A, Bowker G, Weightman J, Weightman S. Family Integrated Care: changing the culture in the neonatal unit. Archives of Disease in Childhood 2018;103(5):415-9. [DOI: 10.1136/archdischild-2017-313282] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pike 2012
- Pike K, Brocklehurst P, Jones D, Kenyon S, Salt A, Taylor D, et al. Outcomes at 7 years for babies who developed neonatal necrotising enterocolitis: the ORACLE Children Study. Archives of Disease in Childhood. Fetal and Neonatal Edition 2012;97(5):F318-22. [DOI: 10.1136/fetalneonatal-2011-300244] [PMID: ] [DOI] [PubMed] [Google Scholar]
Quigley 2019
- Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2019, Issue 7. Art. No: CD002971. [DOI: 10.1002/14651858.CD002971.pub5] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramani 2013
- Ramani M, Ambalavanan N. Feeding practices and necrotizing enterocolitis. Clinics in Perinatology 2013;40(1):1-10. [DOI: 10.1016/j.clp.2012.12.001] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rees 2007
- Rees CM, Pierro A, Eaton S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Archives of Disease in Childhood. Fetal and Neonatal Edition 2007;92(3):F193-8. [DOI: 10.1136/adc.2006.099929] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Samuels 2017
- Samuels N, de Graaf RA, Jonge RC, Reiss IK, Vermeulen MJ. Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC Pediatrics 2017;17(1):105. [DOI: 10.1186/s12887-017-0847-3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sankar 2008
- Sankar MJ, Agarwal R, Mishra S, Deorari AK, Paul VK. Feeding of low birth weight infants. Indian Journal of Pediatrics 2008;75(5):459-69. [DOI: 10.1007/s12098-008-0073-y] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Senterre 2014
- Senterre T. Practice of enteral nutrition in very low birth weight and extremely low birth weight infants. World Review of Nutrition and Dietetics 2014;110:201-14. [DOI: 10.1159/000358468] [PMID: ] [DOI] [PubMed] [Google Scholar]
Smallpeice 1964
- Smallpeice V, Davies PA. Immediate feeding of premature infants with undiluted breast-milk. Lancet 1964;2(7374):1349-52. [DOI: 10.1016/s0140-6736(64)91152-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tsang 1993
- Tsang RC, Lucas A, Uauy R, Zlotkin S. Nutritional Needs of the Newborn Infant: Scientific Basis and Practical Guidelines. Pawling (NY): Caduceus Medical Publishers, 1993. [Google Scholar]
Walsh 2019
- Walsh V, McGuire W. Immunonutrition for preterm infants. Neonatology 2019;115(4):398-405. [DOI: 10.1159/000497332] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Walsh 2020
- Walsh V, Brown JV, Copperthwaite BR, Oddie SJ, McGuire W. Early full enteral feeding for preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2020, Issue 3. Art. No: CD013542. [DOI: 10.1002/14651858.CD013542] [DOI] [PMC free article] [PubMed] [Google Scholar]


