CSF, caused by CSFV, is a World Organization for Animal Health (OIE) notifiable disease and causes significant financial losses to the pig industry globally. The ESCRT machinery plays an important regulatory role in several members of the genera Flavivirus and Hepacivirus within the family Flaviviridae, such as hepatitis C virus, Japanese encephalitis virus, and dengue virus.
KEYWORDS: classical swine fever virus, ESCRT, entry, replication, Tsg101
ABSTRACT
Classical swine fever (CSF), caused by classical swine fever virus (CSFV), is a highly contagious disease of swine with high morbidity and mortality that negatively affects the pig industry worldwide, in particular in China. Soon after the endocytosis of CSFV, the virus makes full use of the components of host cells to complete its life cycle. The endocytosis sorting complex required for transport (ESCRT) system is a central molecular machine for membrane protein sorting and scission in eukaryotic cells that plays an essential role in many physiological metabolic processes, including invasion and egress of envelope viruses. However, the molecular mechanism that ESCRT uses to regulate the replication of CSFV is unknown. In this study, we demonstrated that the ESCRT-I complex Tsg101 protein participates in clathrin-mediated endocytosis of CSFV and is also involved in CSFV trafficking. Tsg101 assists the virus in entering the host cell through the late endosome (Rab7 and Rab9) and finally reaching the lysosome (Lamp-1). Interestingly, Tsg101 is also involved in the viral replication process by interacting with nonstructural proteins 4B and 5B of CSFV. Finally, confocal microscopy showed that the replication complex of Tsg101 and double-stranded RNA (dsRNA) or NS4B and NS5B protein was close to the endoplasmic reticulum (ER), not the Golgi, in the cytoplasm. Collectively, our finding highlights that Tsg101 regulates the process of CSFV entry and replication, indicating that the ESCRT plays an important role in the life cycle of CSFV. Thus, ESCRT molecules could serve as therapeutic targets to combat CSFV infection.
IMPORTANCE CSF, caused by CSFV, is a World Organization for Animal Health (OIE) notifiable disease and causes significant financial losses to the pig industry globally. The ESCRT machinery plays an important regulatory role in several members of the genera Flavivirus and Hepacivirus within the family Flaviviridae, such as hepatitis C virus, Japanese encephalitis virus, and dengue virus. Previous reports have shown that assembling and budding of these viruses require ESCRT. However, the role of ESCRT in Pestivirus infection remains to be elucidated. We determined the molecular mechanisms of the regulation of CSFV infection by the major subunit Tsg101 of ESCRT-I. Interestingly, Tsg101 plays an essential regulatory role in both clathrin-mediated endocytosis and genome replication of CSFV. Overall, the results of this study provide further insights into the molecular function of ESCRT-I complex protein Tsg101 during CSFV infection, which may serve as a molecular target for pestivirus inhibitors.
INTRODUCTION
Classical swine fever (CSF), caused by classical swine fever virus (CSFV), causes a highly contagious disease of swine with high morbidity and mortality (1, 2). Because of its widespread distribution and significant economic impact on the porcine industry globally, CSF is a notifiable disease according to the World Organization for Animal Health (OIE) (3, 4). China has also classified CSF as a class I animal infectious disease (5). CSFV is an enveloped, positive-stranded RNA virus that belongs to the genus Pestivirus within the family Flaviviridae (6). The virus can grow in pig kidney cells, pig testis cells, and other cell lines, as well as primary bovine and sheep testis cells without producing an apparent cytopathic effect (CPE) (7, 8). After CSFV enters the host cells, it makes full use of the cellular components to complete the viral life cycle (9). In pigs, CSFV infection causes a series of clinical symptoms, including skin and tissue hemorrhages, disseminated intravascular coagulation, and leukopenia (10). Although some progress has been made in understanding CSFV pathogenesis in pigs, the molecular mechanisms of CSFV replication in host cells are still largely unknown.
The endocytic sorting complex required for transport (ESCRT) is an essential molecular machine for membrane protein sorting and scission in eukaryotic cells (11, 12). It has the function of a scission lipid bilayer membrane. It also plays critical roles in many physiological, metabolic processes, such as the formation of poly-vesicles, cytokinesis, plasma membrane repair, nuclear membrane reconstruction, invasion, and egress of enveloped viruses (13, 14). The ESCRT system is composed of more than 20 proteins. According to the complex formed by its components and their functional differences, it can be divided into five complexes, known as ESCRT-0, -I, -II, and -III and vacuolar protein sorting 4 (Vps4), as well as some auxiliary proteins, such as Alix (15).
In eukaryotic cells, ESCRT-I is a heterotetramer, which consists of tumor susceptibility gene 101 (Tsg101 or vps23), vps28, vps37, and mvb12. Tsg101 is expressed in the vacuolar protein sorting (VPS) pathway and plays a central role in the normal physiological function of cells (16). In addition, previous studies showed that Tsg101 regulates the maturation and release of human immunodeficiency virus-1 (HIV-1) (17, 18) and simian immunodeficiency virus (SIV) from infected cells (19). Similarly, the interaction between Tsg101 and VP40 of Marburg virus helped the germination of virus-like particles (VLPs) (20). Recently, ESCRT machinery was found to help replication of Kaposi's sarcoma-associated herpesvirus (KSHV) in host cells. Briefly, soon after KSHV enters the host cells, ESCRT-I (Tsg101) forms a complex with chmp5/6 that transports KSHV from the early endosome (Rab5) to the late endosome (Rab7), followed by entry into the nucleus for replication (21). The deletion of the Tsg101 gene significantly inhibited the transport of the virus from early to late endosomes (21). Thus, we hypothesize that Tsg101 is also involved in entry and replication in the life cycle of CSFV.
Here, we showed that Tsg101 assists the virus in transit from the late endosome (Rab7 and Rab9) to lysosome (Lamp-1), suggesting that Tsg101 plays a role in CSFV trafficking. In addition, Tsg101 regulates CSFV genome replication through its interaction with viral nonstructural proteins 4B (NS4B) and 5B (NS5B). Lastly, confocal microscopy data showed that Tsg101 colocalizes with viral double-stranded RNA (dsRNA), NS4B, and NS5B, which is closely associated with the endoplasmic reticulum (ER), suggesting that Tsg101 is involved in the formation of the replication complex.
RESULTS
Tsg101 is required for CSFV infection.
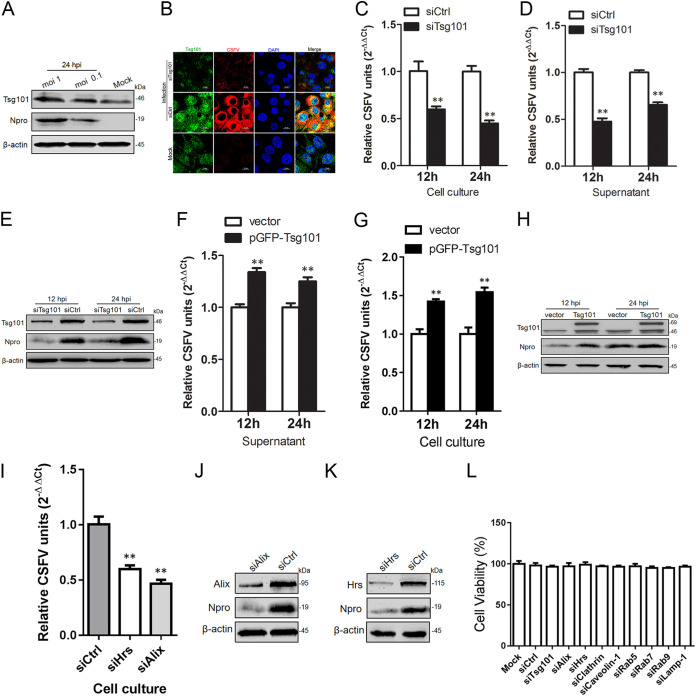
To determine changes in endogenous Tsg101 levels in infected cells, PK-15 cells were infected with CSFV at different multiplicities of infection (MOI), and cell lysates were harvested at 24 h postinfection (hpi). Western blot analyses showed that CSFV infection resulted in an increase of endogenous Tsg101 expression. Specifically, the Tsg101 expression level in cells infected with CSFV at an MOI of 1 was significantly higher than that in cells infected at an MOI of 0.1 (Fig. 1A). Next, to determine whether knockdown of endogenous Tsg101 affects replication of CSFV in host cells, small interfering RNA (siRNA) duplexes targeting Tsg101 were transfected into PK-15 cells and incubated for 24 h after inoculation of CSFV (MOI = 1). As shown in Fig. 1B, the fluorescence intensity of CSFV E2 was significantly reduced in Tsg101 knockdown PK-15 cells compared to that in siCtrl-transfected cells. Subsequently, we evaluated CSFV replication in Tsg101 knockdown cells at various time points after infection. The reverse transcription-quantitative PCR (RT-qPCR) results showed that knockdown of Tsg101 reduced intracellular as well as extracellular viral RNA loads by 40.2% and 55.1% and by 52.4% and 34.5% at 12 and 24 hpi, respectively (Fig. 1C and D). Western blot assay further confirmed that the CSFV Npro expression level had decreased to a greater extent at 12 hpi than at 24 hpi (Fig. 1E).
FIG 1.
Tsg101 is required in CSFV infection. CSFV infection was inhibited when Tsg101 was knocked down. (A) Tsg101 expression was induced significantly upon CSFV infection. Cells were infected with different MOI of CSFV (0.1 and 1) and harvested at 24 hpi for Western blotting using rabbit anti-Tsg101 antibody, rabbit anti-Npro antibody, or mouse anti-β-actin antibody. (B) siTsg101 or siCtrl-transfected cells were infected with CSFV (MOI = 1). At 24 hpi, cells were fixed with 4% PFA and probed with rabbit anti-Tsg101 antibody or mouse anti-E2 antibody (WH303) and then observed by confocal microscopy. Bars = 10 μm. (C and D) siTsg101- or siCtrl-transfected cells were inoculated with CSFV. At 12 and 24 hpi, the cell culture and supernatant, respectively, were harvested for RT-qPCR. Data are means and SD from three independent experiments. **, P < 0.01. (E) The harvested cells described above were subjected to Western blotting using rabbit anti-Tsg101 antibody, rabbit anti-Npro antibody, or mouse anti-β-actin antibody. (F, G, and H) PK-15 cells were transfected with GFP-tagged Tsg101 or vector plasmid and then inoculated with CSFV. At 12 and 24 hpi, the cell culture and supernatant, respectively, were harvested for RT-qPCR and Western blotting as described above. Data are means and SD from three independent experiments. **, P < 0.01. (I, J, and K) siHrs-, siAlix-, or siCtrl-transfected cells were inoculated with CSFV (MOI = 0.1). At 24 hpi, the cell culture was harvested for RT-qPCR and Western blotting described above. Data are means and SD from three independent experiments. **, P < 0.01. (L) Cell viabilities upon transfection with all the siRNA duplexes were assessed by using the Cell Titer 96 AQueous One Solution cell proliferation assay.
In order to determine whether overexpression of Tsg101 affects CSFV infection, PK-15 cells transfected with a wild-type (WT) plasmid expressing Tsg101 were infected with CSFV at an MOI of 0.1, and cell culture and supernatants were harvested at 12 and 24 hpi. RT-qPCR analyses showed that overexpression of Tsg101 resulted in a 33.9% and 25.1% and in 42.3% and 52.6% increases in the intracellular and extracellular viral RNA loads at 12 and 24 hpi, respectively (Fig. 1F and G). Similarly, Western blot assay also showed that PK-15 cells overexpressing Tsg101 had significantly higher CSFV Npro expression levels than control cells (Fig. 1H). To determine whether knockdown of two other important components of ESCRT associated with Tsg101, Hrs and Alix (16, 22, 23), affects replication of CSFV in host cells, small interfering RNA (siRNA) duplexes targeting Hrs or Alix were transfected into PK-15 cells and incubated for 24 h after inoculation of CSFV (MOI = 0.1). The RT-qPCR results showed that knockdown of Hrs and Alix reduced intracellular viral RNA loads by 40.0% and 53.3%, respectively (Fig. 1I). Western blotting assay further confirmed that CSFV Npro expression level was decreased by depletion of Hrs and Alix (Fig. 1J and K). This suggested that some subunit proteins in the ESCRT machine likely acted as the helper of Tsg101 to participate in the viral cycle of CSFV and affected the replication of the virus. Finally, we examined the cytotoxicity of all siRNA duplexes used in this study, and the results showed that all the siRNA primers were not toxic to cells (Fig. 1L). The data together indicated that Tsg101 is necessary for efficient infection of CSFV in host cells.
CSFV entry depends on Tsg101.
To investigate whether Tsg101 is involved in the early stage of CSFV infection, PK-15 cells were infected with CSFV (MOI = 10), adsorbed at 4°C for 1 h, and then incubated for 1 h at 37°C. After fixation, CSFV virions and endogenous Tsg101 were detected with mouse anti-E2 monoclonal antibody (MAb) (WH303) and rabbit anti-Tsg101 antibody, respectively, and observed using confocal microscopy. As shown in Fig. 2A, a clear colocalization of CSFV E2 proteins with endogenous Tsg101 was observed. More interestingly, the colocalization signal increased along with infection time, suggesting accumulation of Tsg101 at the site of CSFV infection. Furthermore, cells were transfected with the pGFP-Tsg101 construct and then infected with CSFV (MOI = 10). At 1 and 2 hpi, intracellular viral genome was quantified by RT-qPCR. The data showed that the viral RNA loads increased by 41.9% and 33.6% at 1 and 2 hpi, respectively, in Tsg101-overexpressing cells (Fig. 2B). Next, we evaluated CSFV entry in Tsg101 knockdown cells. Extracellular viral genome levels obtained from cell culture supernatants of Tsg101 knockdown cells infected with CSFV were measured by RT-qPCR. The results indicated that the viral load was reduced by 43.1% and 62.3% in siTsg101-transfected cells at 1 and 2 hpi, compared with viral load in siCtrl-transfected cells (Fig. 2C). Finally, to determine whether CSFV infection affects the expression of endogenous Tsg101, cell lysates infected with CSFV (MOI = 1) were harvested at different time points and subjected to Western blot analyses. As shown in Fig. 2D, Tsg101 expression significantly increased with CSFV infection in a time-dependent manner. However, there was no significant change in expression levels of the other two ESCRT-related proteins, Alix and Hrs. Last, the grayscale analysis results also correlated with protein expression levels measured by Western blotting (Fig. 2E).
FIG 2.
CSFV entry depends on Tsg101. (A) CSFV colocalized with Tsg101. Cells infected with CSFV (MOI = 10) were kept at 4°C for 1 h and then shifted to 37°C. At different intervals, cells were fixed, probed with mouse anti-CSFV E2 monoclonal antibody (WH303) or rabbit anti-Tsg101 antibody, and then observed by confocal microscopy. The nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole). White arrows indicate the colocalized fluorescent signal. Bars = 10 μm. (B) Cells were transfected with pGFP-Tsg101 or vector plasmid and then inoculated with CSFV (MOI = 10) for 1 or 2 h. Viral RNA copies were quantified using RT-qPCR. (C) siTsg101 or siCtrl-transfected cells were inoculated with CSFV (MOI = 10) for 1 or 2 h. Viral RNA copies were quantified using RT-qPCR. Data are means and SD from three independent experiments. **, P < 0.01. (D) Cells were infected with CSFV (MOI = 1) at different times, and lysates were subjected to Western blotting using the indicated antibodies. (E) Western blotting results were analyzed by grayscale analysis with ImageJ software. Data are means and SD from three independent experiments. **, P < 0.01.
Taken together, these results indicated that Tsg101 is required for early infection of CSFV. However, earlier reports showed that Tsg101 was associated with the assembly process of Japanese encephalitis virus (JEV) (24, 25), an important member of the genus Flavivirus used as a virus control for CSFV endocytosis in our previously studied data (26, 27). Here, we also investigated the role of Tsg101 in JEV entry using confocal microscopy and RT-qPCR assay described above. As shown in Fig. 3A, JEV virions did not colocalize with Tsg101 from 15 to 60 min postinfection after entry. In agreement with the immunofluorescence data, the viral RNA genome level of JEV remained unchanged in both Tsg101-overexpressing and Tsg101 knockdown cells infected with JEV (Fig. 3B and C). Obviously, Tsg101 had no effect on JEV entry.
FIG 3.
JEV entry does not depend on Tsg101. (A) JEV does not colocalize with Tsg101. Cells infected with JEV (MOI = 10) were kept at 4°C for 1 h and then shifted to 37°C. At different intervals, cells were fixed and probed with mouse anti-JEV E monoclonal antibody (4B4) or rabbit anti-Tsg101 antibody and then observed by confocal microscopy. The nuclei were stained with DAPI. Bars = 10 μm. (B and C) Cells were transfected with pGFP-Tsg101 or siTsg101 and then inoculated with JEV (MOI = 10) for 1 or 2 h. Viral RNA copies were quantified using RT-qPCR. Data are means and SD from three independent experiments.
Tsg101 delivers CSFV from late endosomes to lysosomes after endocytosis.
In order to study how Tsg101 regulates CSFV endocytosis during the early stage of infection, cells were infected with CSFV (MOI of 10) for 1 h and fixed with 4% paraformaldehyde (PFA). Colocalization of viral E2 protein of the CSFV virion with various endosomal markers (Rab5, -7, -9, -11, and -35) and lysosome was analyzed by immunofluorescence assay. As shown in Fig. 4A, endogenous Tsg101 colocalized with clathrin, Rab7, Rab9, and Lamp-1 but not with caveolin-1, Rab5, and Rab35. There was no evidence of colocalization between Tsg101 and the endosomal markers in mock-infected cells (Fig. 4C). Moreover, Pearson’s correlation analysis indicated that colocalization coefficients for Tsg101 with clathrin, Rab7, Rab9, and Lamp-1 were significantly higher than those of caveolin-1, Rab5, and Rab35, confirming the results obtained with confocal microscopy (Fig. 4B and D). Finally, in order to determine the role of Tsg101 in the inactivation of clathrin, caveolin-1, Rab5, Rab7, and Rab9, we transfected a serious of constructs (wild type [WT] and dominant negative mutant [DN]) overexpressing active or inactive clathrin, caveolin-1, Rab5, Rab7, or Rab9. As expected, we observed that the endogenous Tsg101 colocalized with the WT clathrin, Rab7, or Rab9. However, when the inactive form of clathrin, Rab7, or Rab9 was overexpressed, endogenous Tsg101 did not colocalize with them (Fig. 4E). Moreover, Pearson’s correlation analysis indicated that an obvious colocalization for endogenous Tsg101 with the WT status of clathrin, Rab7, or Rab9, in agreement with the results above (Fig. 4F). Combined with our previous studies (26, 28) that showed the viral trafficking from the endosome to the lysosome and the above fluorescence colocalization signals, these results led us to speculate that CSFV is initially selected in the late endosome (Rab7 and Rab9) and then passed on to ESCRT-I on multivesicular body (MVB) membranes, where Tsg101 protein plays a central role in recognizing the viral particles and activating ESCRT-II and subsequently ESCRT-III complexes, which is in agreement with previous reports (29, 30).
FIG 4.
CSFV recruited Tsg101 to transit from late endosomes to lysosomes after endocytosis. (A and C) Cells infected with CSFV or not (MOI = 10) at 37°C for 1 h were fixed, probed with mouse anti-Tsg101 (red), rabbit anticlathrin, anticaveolin-1, anti-Rab, or anti-Lamp-1 (green), and then observed by confocal microscopy. Bars = 10μm. (B and D) The colocalization analysis was expressed as Pearson’s correlation coefficient, measured for individual cells. Results are means and SD from three independent experiments. **, P < 0.01. (E) Cells were transfected with a series of constructs (wild type [WT] and dominant negative mutant [DN]) overexpressing active or inactive forms of clathrin, caveolin-1, Rab5, Rab7, and Rab9, infected with CSFV (MOI = 10), stained with rabbit anti-Tsg101 antibody (red), and observed by confocal microscopy. Bars = 10μm. (F) The colocalization analysis was expressed as Pearson’s correlation coefficient, measured for individual cells. Results are means and SD from three independent experiments. **, P < 0.01.
Tsg101 is involved in CSFV replication.
In order to study whether Tsg101 is involved in CSFV genome replication, cells were transfected with siRNA specific to Tsg101 and subsequently infected with CSFV at different time points. Cell culture supernatants and cell lysates were collected and analyzed by RT-qPCR and Western blotting, respectively. The knockdown of Tsg101 significantly inhibited the viral RNA and Npro protein levels at various time points, although the inhibitory effect was more pronounced in the early stage of infection (Fig. 5A and B). Furthermore, confocal microscopy showed that the fluorescence intensity of Tsg101 and CSFV dsRNA decreased significantly in the cells transfected with Tsg101-specific siRNAs. In contrast, endogenous Tsg101 and viral dsRNA colocalized in cells transfected with negative-control siRNA, suggesting that Tsg101 may be involved in viral replication (Fig. 5C). In addition, in order to distinguish between input defects and subsequent replication defects, cells were first infected with CSFV and transfected with siRNA duplexes targeting Tsg101. At 18 and 30 h posttransfection, cells were harvested for determining viral replication by RT-qPCR and Western blotting, respectively. The knockdown of Tsg101 significantly inhibited the viral RNA and Npro protein levels at indicated time points (Fig. 5D and E).
FIG 5.
Tsg101 is involved in CSFV replication. (A and B) PK-15 cells were transfected with siTsg101 or siCtrl and infected with CSFV for 6, 12, 24, or 48 h. RNA copies and CSFV Npro protein were identified by Western blotting and RT-qPCR, respectively. Data are means and SD from three independent experiments. **, P < 0.01. (C) siTsg101- or siCtrl-transfected cells were inoculated with CSFV for 24 h, fixed with 4% PFA, and probed with mouse anti-dsRNA (red) and rabbit anti-Tsg101 (green). The nuclei were stained with DAPI and observed by confocal microscopy. Bars = 10 μm. (D and E) Cells infected with CSFV for 6 h were transfected with siTsg101 or siCtrl for 18 h and 30 h, respectively. The cell culture was harvested for RT-qPCR and Western blotting. Data are means and SD from three independent experiments. **, P < 0.01. (F) Cells transfected with pFLAG-Core, -NS2, -NS3, -NS4B, -NS5A, -NS5B, or -E2 and stained with rabbit anti-Tsg101 antibody (green) and mouse anti-Flag antibody (red) for confocal microscopy. (G) The colocalization analysis was expressed as Pearson’s correlation coefficient. Results are means and SD from three independent experiments. ***, P < 0.001.
Finally, we transfected PK-15 cells with a series of constructs including the structural and nonstructural proteins of CSFV and observed the cells at 48 h postinfection (hpi) by confocal microscopy. Interestingly, the endogenous Tsg101 protein colocalized with NS4B and NS5B proteins but not with core, E2, NS2, NS3, and NS5A proteins (Fig. 5F). According to Pearson’s correlation analysis, the colocalization coefficients of Tsg101 with NS4B and NS5B were significantly higher than those of other proteins (Fig. 5G). The interaction between Tsg101 and NS4B, or Tsg101 and NS5B, was further investigated by coimmunoprecipitation assay. pGFP-Tsg101 and pFLAG-NS4B or pFLAG-NS5B plasmids were cotransfected into 293T cells. Compared with the vector control, Tsg101 showed interaction with both NS4B and NS5B (Fig. 6A and B), suggesting that Tsg101 is involved in CSFV genome replication. Finally, we investigated the effect of NS4B or NS5B overexpression on the protein level of Tsg101. Western blotting showed that overexpression of neither NS4B nor NS5B affected Tsg101 expression (Fig. 6C).
FIG 6.
The interaction between Tsg101 and NS4B or NS5B. (A and B) HEK293T cells cotransfected with pGFP-Tsg101 or vector and pFLAG-NS4B or -NS5B were prepared for co-IP assay. Proteins were immunoprecipitated with rabbit anti-Tsg101 or mouse anti-Flag antibody. The whole-cell lysates were assayed for expression of Tsg101, FLAG, and GAPDH. (C) Cells were transfected with pFLAG-NS4B, -NS5B, pFLAG-NS4B/-NS5B, pFLAG, pGFP-Tsg101, or pGFP for 48 hpi and harvested for Western blotting using rabbit anti-Tsg101 or mouse anti-Flag antibody, along with β-actin antibody as a loading control.
Tsg101 is associated with the replication complex of CSFV.
To determine whether Tsg101 forms a replication complex with viral NS4B and NS5B proteins, we transfected pFLAG-NS4B and pFLAG-NS5B into PK-15 cells. After 24 h of CSFV infection, cells were stained with antibodies to determine protein colocalization. As shown in Fig. 7A, the endogenous Tsg101 protein colocalized with NS4B, NS5B, and dsRNA, suggesting that Tsg101 may be involved in the formation of a replication complex of CSFV. As a negative control, Tsg101 did not colocalize with NS5A and dsRNA. The results of Pearson’s correlation analysis were consistent with those of colocalization images (Fig. 7B). Subsequently, to study whether the replication complex is associated with the endoplasmic reticulum (ER) or Golgi apparatus, PK-15 cells were transfected with pGFP-Tsg101 and infected with CSFV. At 24 hpi, cells were fixed and observed by confocal microscopy. As shown in Fig. 7C, Tsg101, and dsRNA were both located in the ER but not in the Golgi apparatus, indicating that the replication process occurs in the ER region. Pearson’s correlation analysis also showed there is clear colocalization between Tsg101 and dsRNA, Tsg101 and ER, and ER and dsRNA (Fig. 7D). In contrast, Pearson’s correlation analysis further showed that there is no colocalization between Tsg101 and Golgi or between Golgi and dsRNA (Fig. 7E).
FIG 7.
There is colocalization among Tsg101, dsRNA, and NS4B/5B. (A) Cells were transfected with pFLAG-NS4B, -NS5B or -NS5A, infected with CSFV (MOI = 1) for 24 h, and fixed for probing with rabbit anti-Tsg101 antibody (purple), mouse anti-dsRNA antibody (green), and goat anti-Flag antibody (red). The images were obtained by confocal microscopy. Bars = 10 μm. (B) Results of colocalization analysis among Tsg101, dsRNA, and NS4B, NS5B, or NS5A expressed as Pearson’s correlation coefficient. Results are means and SD from three independent experiments. (C) Cells were transfected with pGFP-Tsg101, infected with CSFV (MOI = 1) for 24 hpi, and then fixed for probing with mouse anti-dsRNA (red) or rabbit anti-ER or Golgi (purple). The images were obtained by confocal microscopy. Bars = 10 μm. Results of colocalization analysis of Tsg101, dsRNA, and ER (D) or Golgi (E) expressed as Pearson’s correlation coefficient. Results are means and SD from three independent experiments.
Finally, we used confocal microscopy to show whether Tsg101 colocalized with NS4B or NS5B in the ER. As shown in Fig. 8A, the endogenous Tsg101 protein with NS4B or with NS5B was located in the ER and not in the Golgi apparatus. Pearson’s correlation analysis also showed colocalization between Tsg101 and NS4B/5B, Tsg101 and ER, and ER and NS4B/5B (Fig. 8B). In contrast, there was no evidence of colocalization with the Golgi apparatus (Fig. 8C). As a negative control, NS5A did not colocalize with Tsg101. More importantly, to prove that Tsg101 was associated with the ER, we used an ER protein extraction kit to extract the ER total proteins. Compared to the mock-infected control, Western blot assay showed that there was a band corresponding to Tsg101 protein in the extract of ER, suggesting that Tsg101 was located in the ER after CSFV infection (Fig. 8D). Taken together, these results show that CSFV utilizes host cell components (Tsg101) to promote viral genome replication.
FIG 8.
The replication complex of CSFV, including Tsg101, NS4B/5B, and dsRNA, was associated with the ER. (A) Cells transfected with pFLAG-NS4B, -NS5B or -NS5A, infected with CSFV (MOI = 1) for 24 h, fixed for probing with mouse anti-Tsg101 antibody (green), goat anti-Flag antibody (red), rabbit anticalnexin antibody (purple), or rabbit anti-GM130 antibody (purple), and then observed by confocal microscopy. Bars = 10 μm. The colocalization coefficients of Tsg101, NS4B, NS5B or NS5A, and ER (B) and Golgi (C) were expressed as Pearson’s correlation coefficient. Results are means and SD from three independent experiments. (D) Cells infected with CSFV (MOI = 1) were subjected to extraction with an ER protein extraction kit at 24 hpi. The extracts were subjected to Western blotting and stained with rabbit anti-Tsg101 antibody or rabbit anti-VPS28 antibody, along with rabbit anticalnexin antibody as a loading control.
DISCUSSION
After CSFV enters the host cells, a series of reactions occur with intracellular components that regulate different stages of the viral life cycle, including entry, trafficking, replication, assembly, and release (31, 32). Our previous studies have found that CSFV enters PK-15 cells through clathrin-mediated endocytosis or 3D4/21 cells through caveolin-mediated endocytosis (26–28). After endocytosis, CSFV transits through early endosomes (Rab5) to late (Rab7) or recycling (Rab11) endosomes and finally to lysosomes (Lamp-1) to stimulate membrane fusion. It is well known that ESCRT has a regulatory function during endocytosis and intracellular transport of virions. However, the events by which ESCRT machinery is recruited to participate in CSFV endocytosis during the process of CSFV trafficking from endosomes to lysosomes are unknown. Moreover, it is worth determining the key subunits of ESCRT, which play a role in regulating CSFV infection. Therefore, these molecular mechanisms have been further elucidated in detail.
Tsg101 in the ESCRT-I complex functions mainly as vacuolar sorting machinery and helps in segregating cargos into typical small vesicles that finally bud into MVBs (33). Several studies have shown the association of ESCRT complex proteins with traditional clathrin-mediated endocytosis (30, 34) and micropinocytosis (35), suggesting that ESCRT machinery can be recruited to virus endocytosis. In addition, Tsg101 was shown to play important roles during viral assembly and egress (36, 37). The study focused on the role of Tsg101 in the late stage of the assembly process at the site of budding at the plasma membrane. Other recent studies also showed that the nucleoprotein NP of the Marburg virus interacts with Tsg101 for efficient budding of virus. Similarly, influenza virus HA (33) and Nipah virus C protein have been shown to interact with Tsg101 for the efficient release of live virus (38).
Budding and entry of viruses are two separate events and require interactions of several host cellular proteins with their viral counterparts. By combining our previous studies that showed the viral trafficking from the endosome to the lysosome and the data in this study, we created a schematic model that depicts a novel role of Tsg101 protein in CSFV entry and replication (Fig. 9). First, the expression level of Tsg101 increased significantly in infected cells, especially in the early stages of the viral life cycle. Upon this point, we speculated that Tsg101 was recruited to the cell membrane for virion transmission. Confocal microscopy showed that endogenous Tsg101 colocalized with clathrin, Rab7, Rab9, and Lamp-1 but not with caveolin-1, Rab5, and Rab35, indicating that Tsg101 is involved in CSFV entry via the clathrin-mediated pathway and then transit from the late endosomes (Rab7 and Rab9) to lysosomes (Lamp-1) for membrane fusion before uncoating. These data demonstrated that Tsg101 plays a role in the trafficking of CSFV after it enters the cell. Previous reports showed that Tsg101 distinctly regulated efficient entry of Autographa california multiple nucleopolyhedrovirus (AcMNPV) (39, 40) and KSHV (21). In accordance with our expectation, JEV could not recruit Tsg101 to promote virus endocytosis as a negative control. However, some previous findings had shown that Tsg101 interacted with viral NS3 proteins (24) and was recruited to the site of viral particle formation (25). Therefore, Tsg101 plays different roles among members of the family Flaviviridae, such as JEV and CSFV. Strikingly, Luyet and colleagues showed that Tsg101 played a direct role in nucleocapsid release within multivesicular endosomes to the cytoplasm, presumably by controlling the back-fusion process (39). In the light of these findings, it would be interesting to evaluate whether Tsg101 plays a role in the process of membrane fusion and uncoating of CSFV.
FIG 9.
Schematic model depicting CSFV life cycle and the role of Tsg101 protein in PK-15 cells. (Step 1) CSFV entry into PK-15 cells begins with the clathrin proteins; virus is then transported to the late endosomes. (Step 2) In the late endosomes, Tsg101 interacts with Rab7 and Rab9 and assists the transport of CSFV to the lysosomes. (Step 3) In the lysosomes, the interaction of Tsg101 with Lamp-1 leads to uncoating and transportation to the endoplasmic reticulum area. (Step 4) Simultaneously, Tsg101 interacts with nonstructural proteins NS4B and NS5B of CSFV and forms a replication complex in the endoplasmic reticulum for the replication of viral genome. Finally, the replication complex is degraded and transported to the Golgi apparatus.
Importantly, our study showed that Tsg101 also participated in CSFV RNA genome replication. Confocal microscopy and immunoprecipitation showed that Tsg101 interacts with CSFV NS4B and NS5B. Moreover, Tsg101 colocalized with dsRNA and NS4B/5B in the ER but not the Golgi. It is well known that CSFV NS4B/5B is the main component of the virus replication complex (41, 42). Therefore, we speculated that the formation of the replication complex induced by Tsg101 is located in the ER region for the synthesis of viral nucleic acids. In agreement with our data, it was previously shown that the recruitment of Tsg101 (Vps23p) is needed for the precise assembly of the replicase complex of plus-stranded RNA viruses, which might help the virus evade recognition by the host defense surveillance system and/or prevent viral RNA destruction by the gene silencing machinery (43, 44). Thus, we assumed that the replication complex of CSFV consisting of Tsg101, dsRNA, and viral proteins performs a similar function. In addition, our study has shown that the expression of Tsg101 significantly increased after CSFV entry and replication. Based on the interaction of NS4B and NS5B with Tsg101, we initially suspected that NS4B and NS5B overexpression would result in an increased protein level of Tsg101. Unexpectedly, overexpression of neither NS4B nor NS5B upregulated the protein level of Tsg101. The data together strengthen the conclusion that Tsg101 regulates the formation of the replication complex to enhance CSFV replication. However, identification of cellular factors and/or viral proteins that mediate upregulation of Tsg101 expression warrants further investigation.
Overall, the data presented in this study provide further insight into the role of Tsg101 in CSFV infection, as follows: (i) the ESCRT-I complex protein Tsg101 plays an important role in CSFV virion trafficking and is essential for clathrin-mediated entry of virus; (ii) Tsg101 is important for the transport of CSFV virion-containing late endosomes to lysosomes to achieve a productive infection; (iii) Tsg101 interacts substantially with NS4B/5B and dsRNA, followed by the formation of replication complex associated with the ER but not the Golgi, during CSFV genome replication. The highlights of our comprehensive study suggest that Tsg101 can serve as a potential target to control CSFV infection.
MATERIALS AND METHODS
Virus, cells, and plasmids.
Classic swine fever virus (CSFV), Shimen strain (GenBank accession number AF092448), and the highly virulent Japanese encephalitis virus (JEV), NJ2008 strain (GenBank accession number GQ918133), were used in this study as described previously (26, 27). Porcine kidney cells (PK-15) were maintained in Dulbecco’s modified essential medium (DMEM; GIBCO) supplemented with 10% fetal bovine serum (FBS) (GIBCO, Invitrogen), 0.2% NaHCO3, 100 μg/ml streptomycin, and 100 IU/ml penicillin (GIBCO, Invitrogen) at 37°C with 5% CO2. The pFLAG-E2, pFLAG-Core, pFLAG-NS3, pFLAG-NS4B, pFLAG-NS5A, and pFLAG-NS5B plasmids were generated by cloning corresponding CSFV genes into the p3×FLAG-CMV-7.1 vector (45). The pGFP-Tsg101 plasmid was constructed in the laboratory following standard molecular cloning procedures. The authenticity of each construct was confirmed by DNA sequencing.
Plasmids and siRNA transfections.
PK-15 cells were grown to 70% confluence on coverslip dishes and transfected with the appropriate plasmid (2.5 μg) using Lipofectamine 3000 (Invitrogen) according to the manufacturer’s instructions. At 6 h posttransfection (hpt), the transfection mixture was replaced with DMEM containing 2% FBS, and cells were incubated for an additional 48 h. For RNA knockdown, PK-15 cells were transfected with 100 nM siRNA using Lipofectamine 3000 according to the manufacturer’s instructions. The siRNA duplexes used in the study are Tsg101 (ab125011) and the negative-control siRNA (sc-37007; Santa Cruz). At 48 hpt, cells were infected with CSFV or JEV, and virus replication was evaluated by RT-qPCR (MOI = 0.1) or confocal fluorescence microscopy (MOI = 1) at 24 hpi.
Confocal fluorescence microscopy.
PK-15 cells grown on dishes were infected with CSFV at an MOI of 10 at 4°C for 1 h, rinsed, and shifted to 37°C. After incubation, the monolayers were fixed with 4% PFA in phosphate-buffered saline (PBS) and permeabilized with 0.1% Triton X-100. For visualization of CSFV or JEV and Tsg101, cells were stained with mouse anti-CSFV E2 antibody (WH303), mouse anti-JEV E antibody (4B4), or rabbit anti-Tsg101 (ab125011). To visualize the colocalization of Tsg101 and clathrin, caveolin-1, Rab proteins, or Lamp-1, cells were stained with mouse anti-Tsg101 antibody (4A10; GeneTex) and rabbit anti-Rab antibody. To visualize the CSFV structural and nonstructural proteins, cells were stained with rabbit anti-Tsg101 antibody (ab125011) and mouse anti-Flag antibody (F1804; Sigma). For visualization of dsRNA and Tsg101, cells were stained with goat anti-Flag antibody (ab1257), rabbit anti-Tsg101 antibody, and mouse double-stranded-RNA (dsRNA) monoclonal antibody J2 (Scicons). For visualization of Tsg101 and ER or Golgi, cells were stained with goat anti-Flag antibody and mouse anti-Tsg101 antibody; the endoplasmic reticulum (ER) was stained with rabbit anticalnexin antibody (ab13505), and the Golgi apparatus was stained with rabbit anti-GM130 antibody (ab52649) (46). The colocalization coefficients were calculated using professional quantitative colocalization analysis software (Nikon A1) included with the Nikon A1 confocal microscope and expressed as Pearson’s correlation coefficient.
Coimmunoprecipitation and Western blotting.
For coimmunoprecipitation (co-IP), PK-15 cells were cotransfected with pGFP-Tsg101 and pFLAG plasmids (pFLAG, pFLAG-NS4B, and pFLAG-NS5B). At 48 hpt, cells were lysed in NP-40 lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM NaF, 1 mM Na3O4; pH 7.4) for 30 min at 4°C. Lysates were cleared by centrifugation at 1,000 × g for 10 min at 4°C. A 25% aliquot of the supernatant (whole-cell lysate) was removed from all samples for later use. The remaining 75% of the lysate was incubated with 0.5 μg of the appropriate control IgG and 20 μl of a protein A/G Plus–agarose slurry (sc-2003; Santa Cruz) for 4 h at 4°C with rotation. Agarose beads were removed by centrifugation at 1,000 × g for 5 min at 4°C. The lysates were then treated with 5 μg of mouse anti-Flag antibody (F1804; Sigma) or rabbit anti-Tsg101 antibody (ab125011) and incubated with rotation for 4 to 6 h at 4°C. Protein A/G Plus–agarose slurry was added to each sample and incubated for 2 h more under the same conditions. The agarose beads were collected by centrifugation, washed with NP-40 lysis buffer at least three times, and then resuspended in 2× SDS loading buffer for SDS-PAGE and Western blotting.
Immunoprecipitates and whole-cell lysates were probed with mouse anti-Flag MAb, rabbit anti-Tsg101 MAb, or mouse anti-β-actin (sc47778; Santa Cruz). The cell samples were washed three times with ice-cold PBS and lysed in cold lysis buffer (1% Triton X-100, 1 mM PMSF in PBS) for 30 min. Lysates were clarified by centrifugation at 12,000 × g for 10 min. Proteins in the lysates were separated by SDS-PAGE, transferred to nitrocellulose membranes, and then probed with the indicated antibodies. β-Actin was used as a loading control. In order to determine levels of indicated proteins, the corresponding protein/actin quantity was used to calculate the grayscale values using ImageJ 7.0 software.
ER protein extraction.
Isolation of the ER proteins was performed with a commercial kit (BB-31454; BestBio). Cells were harvested after centrifugation at 500 × g for 3 min and then washed with cold PBS three times. The cell precipitate was fully homogenized after adding 500 μl solution A for 10 min at 4°C, and the lysate was collected. After centrifugation at 1,000 × g for 5 min at 4°C, cell supernatant was collected and then centrifuged at 11,000 × g for 10 min at 4°C. Subsequently, the supernatant was transferred to a fresh tube and centrifuged at 100,000 × g for 45 min at 4°C. The precipitation was collected, added to 400 μl solution B, and then centrifuged at 100,000 × g for another 45 min at 4°C. Finally, the precipitate was resuspended in solution C and contained the ER proteins for Western blotting (47, 48).
Cell viability assay.
PK-15 cells were seeded in a 96-well plate (104 cells/well) and treated with different amounts of siRNA for 24 h. The cytotoxic effect of the siRNA on PK-15 cells was evaluated by an MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay. The reagent’s fluorescence was measured with a fluorescence microplate reader after 4 h of incubation at 37°C. No cytotoxicity was observed in cells treated with the indicated concentrations of the siRNA duplexes.
Statistical analysis.
All data are presented as means ± standard deviations (SD). Student's t test was used to compare the data from pairs of treated and untreated groups. Statistical significance is indicated by asterisks in the figures. All statistical analyses and calculations were performed using Prism 6 (GraphPad Software, Inc., La Jolla, CA).
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (31872471 and 31930109).
REFERENCES
- 1.Moennig V. 2015. The control of classical swine fever in wild boar. Front Microbiol 6:1211. doi: 10.3389/fmicb.2015.01211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou B. 2019. Classical swine fever in China—an update minireview. Front Vet Sci 6:187. doi: 10.3389/fvets.2019.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Postel A, Austermann-Busch S, Petrov A, Moennig V, Becher P. 2018. Epidemiology, diagnosis and control of classical swine fever: Recent developments and future challenges. Transbound Emerg Dis 65(Suppl 1):248–261. doi: 10.1111/tbed.12676. [DOI] [PubMed] [Google Scholar]
- 4.Blome S, Moß C, Reimann I, König P, Beer M. 2017. Classical swine fever vaccines—state-of-the-art. Vet Microbiol 206:10–20. doi: 10.1016/j.vetmic.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Gong W, Li J, Wang Z, Sun J, Mi S, Lu Z, Cao J, Dou Z, Sun Y, Wang P, Yuan K, Zhang L, Zhou X, He S, Tu C. 2019. Virulence evaluation of classical swine fever virus subgenotype 2.1 and 2.2 isolates circulating in China. Vet Microbiol 232:114–120. doi: 10.1016/j.vetmic.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Meyers G, Thiel HJ, Rumenapf T. 1996. Classical swine fever virus: recovery of infectious viruses from cDNA constructs and generation of recombinant cytopathogenic defective interfering particles. J Virol 70:1588–1595. doi: 10.1128/JVI.70.3.1588-1595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paton DJ, McGoldrick A, Greiser-Wilke I, Parchariyanon S, Song JY, Liou PP, Stadejek T, Lowings JP, Bjorklund H, Belak S. 2000. Genetic typing of classical swine fever virus. Vet Microbiol 73:137–157. doi: 10.1016/s0378-1135(00)00141-3. [DOI] [PubMed] [Google Scholar]
- 8.Tautz N, Tews BA, Meyers G. 2015. The molecular biology of pestiviruses. Adv Virus Res 93:47–160. doi: 10.1016/bs.aivir.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Ji W, Guo Z, Ding NZ, He CQ. 2015. Studying classical swine fever virus: making the best of a bad virus. Virus Res 197:35–47. doi: 10.1016/j.virusres.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Gong W, Li J, Wang Z, Sun J, Mi S, Xu J, Cao J, Hou Y, Wang D, Huo X, Sun Y, Wang P, Yuan K, Gao Y, Zhou X, He S, Tu C. 2019. Commercial E2 subunit vaccine provides full protection to pigs against lethal challenge with 4 strains of classical swine fever virus genotype 2. Vet Microbiol 237:108403. doi: 10.1016/j.vetmic.2019.108403. [DOI] [PubMed] [Google Scholar]
- 11.Wegner CS, Rodahl LM, Stenmark H. 2011. ESCRT proteins and cell signalling. Traffic 12:1291–1297. doi: 10.1111/j.1600-0854.2011.01210.x. [DOI] [PubMed] [Google Scholar]
- 12.Vietri M, Radulovic M, Stenmark H. 2020. The many functions of ESCRTs. Nat Rev Mol Cell Biol 21:25–42. doi: 10.1038/s41580-019-0177-4. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt O, Teis D. 2012. The ESCRT machinery. Curr Biol 22:R116–120. doi: 10.1016/j.cub.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horner DS, Pasini ME, Beltrame M, Mastrodonato V, Morelli E, Vaccari T. 2018. ESCRT genes and regulation of developmental signaling. Semin Cell Dev Biol 74:29–39. doi: 10.1016/j.semcdb.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 15.Christ L, Raiborg C, Wenzel EM, Campsteijn C, Stenmark H. 2017. Cellular functions and molecular mechanisms of the ESCRT membrane-scission machinery. Trends Biochem Sci 42:42–56. doi: 10.1016/j.tibs.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Lu Q, Hope LW, Brasch M, Reinhard C, Cohen SN. 2003. TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation. Proc Natl Acad Sci U S A 100:7626–7631. doi: 10.1073/pnas.0932599100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrus JE, von Schwedler UK, Pornillos OW, Morham SG, Zavitz KH, Wang HE, Wettstein DA, Stray KM, Cote M, Rich RL, Myszka DG, Sundquist WI. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107:55–65. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- 18.Goff A, Ehrlich LS, Cohen SN, Carter CA. 2003. Tsg101 control of human immunodeficiency virus type 1 Gag trafficking and release. J Virol 77:9173–9182. doi: 10.1128/jvi.77.17.9173-9182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhai Q, Landesman MB, Robinson H, Sundquist WI, Hill CP. 2011. Identification and structural characterization of the ALIX-binding late domains of simian immunodeficiency virus SIVmac239 and SIVagmTan-1. J Virol 85:632–637. doi: 10.1128/JVI.01683-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makino A, Yamayoshi S, Shinya K, Noda T, Kawaoka Y. 2011. Identification of amino acids in Marburg virus VP40 that are important for virus-like particle budding. J Infect Dis 204(Suppl 3):S871–S877. doi: 10.1093/infdis/jir309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar B, Dutta D, Iqbal J, Ansari MA, Roy A, Chikoti L, Pisano G, Veettil MV, Chandran B. 2016. ESCRT-I protein Tsg101 plays a role in the post-macropinocytic trafficking and infection of endothelial cells by Kaposi's sarcoma-associated herpesvirus. PLoS Pathog 12:e1005960. doi: 10.1371/journal.ppat.1005960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veettil MV, Kumar B, Ansari MA, Dutta D, Iqbal J, Gjyshi O, Bottero V, Chandran B. 2016. ESCRT-0 component Hrs promotes macropinocytosis of Kaposi's sarcoma-associated herpesvirus in human dermal microvascular endothelial cells. J Virol 90:3860–3872. doi: 10.1128/JVI.02704-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christ L, Wenzel EM, Liestol K, Raiborg C, Campsteijn C, Stenmark H. 2016. ALIX and ESCRT-I/II function as parallel ESCRT-III recruiters in cytokinetic abscission. J Cell Biol 212:499–513. doi: 10.1083/jcb.201507009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiou CT, Hu CA, Chen PH, Liao CL, Lin YL, Wang JJ. 2003. Association of Japanese encephalitis virus NS3 protein with microtubules and tumour susceptibility gene 101 (TSG101) protein. J Gen Virol 84:2795–2805. doi: 10.1099/vir.0.19201-0. [DOI] [PubMed] [Google Scholar]
- 25.Tabata K, Arimoto M, Arakawa M, Nara A, Saito K, Omori H, Arai A, Ishikawa T, Konishi E, Suzuki R, Matsuura Y, Morita E. 2016. Unique requirement for ESCRT factors in flavivirus particle formation on the endoplasmic reticulum. Cell Rep 16:2339–2347. doi: 10.1016/j.celrep.2016.07.068. [DOI] [PubMed] [Google Scholar]
- 26.Shi BJ, Liu CC, Zhou J, Wang SQ, Gao ZC, Zhang XM, Zhou B, Chen PY. 2016. Entry of classical swine fever virus into PK-15 cells via a pH-, dynamin-, and cholesterol-dependent, clathrin-mediated endocytic pathway that requires Rab5 and Rab7. J Virol 90:9194–9208. doi: 10.1128/JVI.00688-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu CC, Zhang YN, Li ZY, Hou JX, Zhou J, Kan L, Zhou B, Chen PY. 2017. Rab5 and Rab11 are required for clathrin-dependent endocytosis of Japanese encephalitis virus in BHK-21 cells. J Virol 91:e01113-17. doi: 10.1128/JVI.01113-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang YN, Liu YY, Xiao FC, Liu CC, Liang XD, Chen J, Zhou J, Baloch AS, Kan L, Zhou B, Qiu HJ. 2018. Rab5, Rab7, and Rab11 are required for caveola-dependent endocytosis of classical swine fever virus in porcine alveolar macrophages. J Virol 92:e00797-18. doi: 10.1128/JVI.00797-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasqual G, Rojek JM, Masin M, Chatton JY, Kunz S. 2011. Old world arenaviruses enter the host cell via the multivesicular body and depend on the endosomal sorting complex required for transport. PLoS Pathog 7:e1002232. doi: 10.1371/journal.ppat.1002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shtanko O, Nikitina RA, Altuntas CZ, Chepurnov AA, Davey RA. 2014. Crimean-Congo hemorrhagic fever virus entry into host cells occurs through the multivesicular body and requires ESCRT regulators. PLoS Pathog 10:e1004390. doi: 10.1371/journal.ppat.1004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ning P, Gao L, Zhou Y, Hu C, Lin Z, Gong C, Guo K, Zhang X. 2016. Caveolin-1-mediated endocytic pathway is involved in classical swine fever virus Shimen infection of porcine alveolar macrophages. Vet Microbiol 195:81–86. doi: 10.1016/j.vetmic.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 32.Zheng G, Li LF, Zhang Y, Qu L, Wang W, Li M, Yu S, Zhou M, Luo Y, Sun Y, Munir M, Li S, Qiu HJ. 2020. MERTK is a host factor that promotes classical swine fever virus entry and antagonizes innate immune response in PK-15 cells. Emerg Microbes Infect 9:571–581. doi: 10.1080/22221751.2020.1738278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanyal S, Ashour J, Maruyama T, Altenburg AF, Cragnolini JJ, Bilate A, Avalos AM, Kundrat L, Garcia-Sastre A, Ploegh HL. 2013. Type I interferon imposes a TSG101/ISG15 checkpoint at the Golgi for glycoprotein trafficking during influenza virus infection. Cell Host Microbe 14:510–521. doi: 10.1016/j.chom.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuh AL, Audhya A. 2014. The ESCRT machinery: from the plasma membrane to endosomes and back again. Crit Rev Biochem Mol Biol 49:242–261. doi: 10.3109/10409238.2014.881777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar B, Roy A, Veettil MV, Chandran B. 2018. Insight into the roles of E3 ubiquitin ligase c-Cbl, ESCRT machinery, and host cell signaling in Kaposi's sarcoma-associated herpesvirus entry and trafficking. J Virol 92:e01376-17. doi: 10.1128/JVI.01376-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strickland M, Ehrlich LS, Watanabe S, Khan M, Strub MP, Luan CH, Powell MD, Leis J, Tjandra N, Carter CA. 2017. Tsg101 chaperone function revealed by HIV-1 assembly inhibitors. Nat Commun 8:1391. doi: 10.1038/s41467-017-01426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kovalev N, de Castro Martin IF, Pogany J, Barajas D, Pathak K, Risco C, Nagy PD. 2016. Role of viral RNA and co-opted cellular ESCRT-I and ESCRT-III factors in formation of tombusvirus spherules harboring the tombusvirus replicase. J Virol 90:3611–3626. doi: 10.1128/JVI.02775-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park A, Yun T, Vigant F, Pernet O, Won ST, Dawes BE, Bartkowski W, Freiberg AN, Lee B. 2016. Nipah virus C protein recruits Tsg101 to promote the efficient release of virus in an ESCRT-dependent pathway. PLoS Pathog 12:e1005659. doi: 10.1371/journal.ppat.1005659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luyet PP, Falguieres T, Pons V, Pattnaik AK, Gruenberg J. 2008. The ESCRT-I subunit TSG101 controls endosome-to-cytosol release of viral RNA. Traffic 9:2279–2290. doi: 10.1111/j.1600-0854.2008.00820.x. [DOI] [PubMed] [Google Scholar]
- 40.Yue Q, Yu Q, Yang Q, Xu Y, Guo Y, Blissard GW, Li Z. 2017. Distinct roles of cellular ESCRT-I and ESCRT-III proteins in efficient entry and egress of budded virions of Autographa californica multiple nucleopolyhedrovirus. J Virol 92:e01636-17. doi: 10.1128/JVI.01636-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamp B, Riedel C, Roman-Sosa G, Heimann M, Jacobi S, Becher P, Thiel HJ, Rumenapf T. 2011. Biosynthesis of classical swine fever virus nonstructural proteins. J Virol 85:3607–3620. doi: 10.1128/JVI.02206-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lv H, Dong W, Cao Z, Lin J, Ouyang Y, Guo K, Li C, Zhang Y. 2018. Classical swine fever virus non-structural protein 4B binds tank-binding kinase 1. J Biosci 43:947–957. doi: 10.1007/s12038-018-9802-1. [DOI] [PubMed] [Google Scholar]
- 43.Panavas T, Serviene E, Brasher J, Nagy PD. 2005. Yeast genome-wide screen reveals dissimilar sets of host genes affecting replication of RNA viruses. Proc Natl Acad Sci U S A 102:7326–7331. doi: 10.1073/pnas.0502604102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barajas D, Jiang Y, Nagy PD. 2009. A unique role for the host ESCRT proteins in replication of Tomato bushy stunt virus. PLoS Pathog 5:e1000705. doi: 10.1371/journal.ppat.1000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou J, Chen J, Zhang XM, Gao ZC, Liu CC, Zhang YN, Hou JX, Li ZY, Kan L, Li WL, Zhou B. 2018. Porcine Mx1 protein inhibits classical swine fever virus replication by targeting nonstructural protein NS5B. J Virol 92:e02147-17. doi: 10.1128/JVI.02147-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang XD, Zhang YN, Liu CC, Chen J, Chen XN, Sattar Baloch A, Zhou B. 2019. U18666A inhibits classical swine fever virus replication through interference with intracellular cholesterol trafficking. Vet Microbiol 238:108436. doi: 10.1016/j.vetmic.2019.108436. [DOI] [PubMed] [Google Scholar]
- 47.Jiang X, Liao XH, Huang LL, Sun H, Liu Q, Zhang L. 2019. Overexpression of augmenter of liver regeneration (ALR) mitigates the effect of H2O2-induced endoplasmic reticulum stress in renal tubule epithelial cells. Apoptosis 24:278–289. doi: 10.1007/s10495-019-01517-z. [DOI] [PubMed] [Google Scholar]
- 48.Li M, Wang J, Fu L, Lu Y, Xu J, Zhou L, Zhu H, Fang L, Feng Z, Xie T, Zhou X. 2020. Network pharmacology-based prediction and verification of Qingluo Tongbi formula to reduce liver toxicity of Tripterygium wilfordii via UGT2B7 in endoplasmic reticulum. Med Sci Monit 26:e920376. doi: 10.12659/MSM.920376. [DOI] [PMC free article] [PubMed] [Google Scholar]