Kaposi’s sarcoma-associated herpesvirus (KSHV) is an oncogenic virus and the causative agent of potentially fatal malignancies. Lytic replication of KSHV is an essential part of the viral life cycle, allowing for virus dissemination within the infected host and shedding to infect naive hosts.
KEYWORDS: DNA replication, KSHV, Kaposi's sarcoma-associated herpesvirus, ORF59, histones, processivity factor
ABSTRACT
Kaposi’s sarcoma-associated herpesvirus (KSHV) is a human oncogenic virus and the causative agent of Kaposi’s sarcoma, multicentric Castleman’s disease, and primary effusion lymphoma. During lytic reactivation, there is a temporal cascade of viral gene expression that results in the production of new virions. One of the viral factors that is expressed during reactivation is open reading frame 59 (ORF59), the viral DNA polymerase processivity factor. ORF59 plays an essential role for DNA synthesis and is required for the nuclear localization of the viral DNA polymerase (ORF9) to the origin of lytic replication (oriLyt). In addition to its functions in viral DNA synthesis, ORF59 has been shown to interact with chromatin complexes, including histones and cellular methyltransferases. In this study, a series of KSHV BACmids containing 50-amino acid (aa) deletions within ORF59 were generated to determine the interaction domains between ORF59 and histones, as well as to assess the effects on replication fitness as a result of these interactions. These studies show that in the context of infection, ORF59 51 to 100 and 151 to 200 amino acids (aa) are required for interaction with histones, and ORF59 301 to 396 aa are not required for DNA synthesis. Since full-length ORF59 is known to localize to the nucleus, we performed an immunofluorescent assay (IFA) with the ORF59 deletion mutants and showed that all deletions are localized to the nucleus; this includes the ORF59 deletion without the previously identified nuclear localization signal (NLS). These studies further characterize ORF59 and demonstrate its essential role during lytic replication.
IMPORTANCE Kaposi’s sarcoma-associated herpesvirus (KSHV) is an oncogenic virus and the causative agent of potentially fatal malignancies. Lytic replication of KSHV is an essential part of the viral life cycle, allowing for virus dissemination within the infected host and shedding to infect naive hosts. Viral DNA synthesis is a critical step in the production of new infectious virions. One of the proteins that is vital to this process is open reading frame 59 (ORF59), the viral encoded polymerase processivity factor. Previous work has demonstrated that the function of ORF59 is closely connected to its association with other viral and cellular factors. The studies presented here extend that work to include the interaction between ORF59 and histones. This interaction offers an additional level of regulation of the chromatinized viral genome, ultimately influencing DNA synthesis and transcription dynamics.
INTRODUCTION
Herpesviruses are large double-stranded DNA viruses. There are eight known human herpesviruses. Kaposi’s sarcoma-associated herpesvirus (KSHV), or human herpesvirus-8 (HHV-8), is an oncogenic herpesvirus and the causative agent of Kaposi’s sarcoma (KS), multicentric Castleman’s disease, and primary effusion lymphoma (1, 2). KSHV is in the gammaherpesvirus subfamily; other members include Epstein-Barr virus (EBV), or human herpesvirus-4 (HHV-4), along with the nonhuman primate gammaherpesviruses rhesus macaque rhadinovirus (RRV) and retroperitoneal fibromatosis-associated herpesvirus (RFHV) (3, 4). The distinguishing characteristic of all herpesviruses is the ability to remain within the host after an initial infection by establishing a latent infection, followed by periodic episodes of lytic reactivation and subsequent viral shedding.
During productive lytic replication, there is temporal regulation of viral gene expression. The temporal cascade is divided into three distinct phases, immediate early (IE), early (E), and late (L) (5). The IE phase does not require de novo protein synthesis and produces viral transactivators that are involved in E gene expression. E viral proteins typically participate in viral genome replication. In general, the expression of IE and E genes is not dependent on viral DNA synthesis; however, some E genes may have enhanced accumulation by the onset of viral genome replication. L gene expression occurs after the onset of viral DNA synthesis, and they are generally structural components of virions. Treatment with antiviral compounds that block DNA synthesis, such as acyclic nucleoside analogues or DNA polymerase inhibitors, will consequently prevent L gene expression (6, 7). During viral latency, there are a limited number of viral genes expressed, the main one being latency-associated nuclear antigen (LANA), which tethers the viral genome to the host chromosome to facilitate latent viral DNA persistence (8). In this phase, no infectious virus is produced (9–11).
Herpesvirus DNA synthesis is a tightly regulated process involving cis and trans factors. The cis element within the KSHV genome is termed oriLyt, for lytic origin of replication, and contains specific DNA sequences that act to regulate viral DNA replication. There are two identified lytic origins within the KSHV genome, the leftward oriLyt-L and the rightward oriLyt-R (12). The KSHV origins contain several common features found in other viral origins; these include an AT-rich region that facilitates unwinding of the DNA, transcription factor binding sites, and a promoter region. The K-RTA response element (RRE) is a binding site for the KSHV major transactivator and presumed initiator protein, K-RTA, which is encoded by open reading frame 50 (ORF50) (13, 14). In general, it is thought that herpesvirus initiator proteins, or origin binding proteins (OBP), function to recognize oriLyt and recruit DNA replication proteins and may carry out some enzymatic function to facilitate DNA synthesis (15). This holds true for K-RTA, which binds to the RRE within the origin and can recruit the polymerase processivity factor (ORF59) (13). ORF59, or processivity factor-8 (PF-8), is part of the viral encoded core replication complex, consisting of DNA polymerase (ORF9), single-stranded DNA (ssDNA) binding protein (ORF6), helicase (ORF44), primase (ORF56), and primase-associated factor (ORF40/41). These six core proteins provide the enzymatic machinery to synthesize viral DNA during lytic replication.
Previous studies have characterized the physical and functional properties of ORF59. In immunofluorescent assays (IFA), Vero cells transfected with an ORF59 expression plasmid showed nuclear accumulation of ORF59 protein and also demonstrated that nuclear localization of ORF9, the viral DNA polymerase, is dependent on the presence of ORF59 (16, 17). The proposed nuclear localization sequence (NLS) for ORF59 is residues 369 to 377 near the carboxy terminus (17). ORF59 is phosphorylated by the viral encoded kinase (ORF36) at serines 376, 378, and 379, and mutations of these residues have been reported to decrease virion production (18).
There are studies suggesting that ORF59 has additional functions to support lytic reactivation beyond its role in DNA synthesis. ORF59 is an abundant early transcript, and the viral encoded MTA protein (ORF57) is reported to promote the stability of ORF59 mRNA (19). Proteomic analysis of ORF59 binding partners identified many viral and cellular proteins interacting with ORF59 (20). ORF59 has been shown to block the interaction between DNA-PKcs and Ku, which prevents nonhomologous end joining (NHEJ) repair of double-stranded DNA breaks (21). ORF59 interacts with poly(ADP-ribose) polymerase 1 (PARP-1), which promotes the proteasomal degradation of PARP-1, leading to a decrease in PARP-1-mediated cell cycle control and apoptosis (22). It was also shown that ORF59 interacts with protein arginine methyl transferase 5 (PRMT5), leading to a decrease in the association of PRMT5 and its adaptor protein cooperator of PRMT5 (COPR5). This causes a decrease in symmetric methylation and opens viral chromatin, resulting in an increase in viral gene expression (20). ORF59 interacts with minichromosome maintenance proteins (MCMs), and it is suggested that this interaction disrupts the assembly and normal function of the MCM complex to halt cellular DNA synthesis in order to promote viral replication (23). In addition to protein binding partners, ORF59 has been identified to interact with a viral long noncoding RNA (lncRNA) polyadenylated nuclear transcript (PAN) (24).
Some of the more interesting cellular proteins that ORF59 has been reported to interact with are histones (20). Histones H2B and H4 were found as ORF59 binding partners in a proteomics screen of ORF59 isolated from lytically reactivated cells (20). H2B and H4 are part of the core histone complex and along with their respective dimerization partners, H2A and H3, form a histone octamer (25–28). DNA is wrapped around the core histones to form packaged structural units called nucleosomes, and histone H1, the linker, creates the compactness between the nucleosomes (29–32). Histones, along with the associated chromatin modifications, play a major role in the epigenetic control of latency and lytic reactivation of KSHV (33–36).
Our initial investigation into understanding the interaction between ORF59 and histones began with determining the domain of ORF59 which interacts with histones during lytic infection. For these experiments, we generated an HA-epitope tag of the ORF59 locus in KSHV BAC16 and performed coimmunoprecipitations (co-IPs) between ORF59 and endogenous histones. Characterizing the interaction between ORF59 and histones in a biologically relevant model, such as SLK cells with an inducible K-RTA (iSLK) that carry a KSHV BACmid, which support viral reactivation, is an important step in understanding the dynamic interaction between viral and cellular proteins during an infection. In addition to H2B and H4, which were identified in the ORF59 proteomics screen, we also looked at H2A and H3 because they are part of the core histone complex. Interestingly, the PAN proteomic analysis which identified ORF59 also identified H2A and H1 as PAN binding partners, suggesting that this may represent a larger protein-protein-lncRNA complex (24).
RESULTS
Generation of iSLK BAC16 ORF59-HA deletion mutants and protein expression.
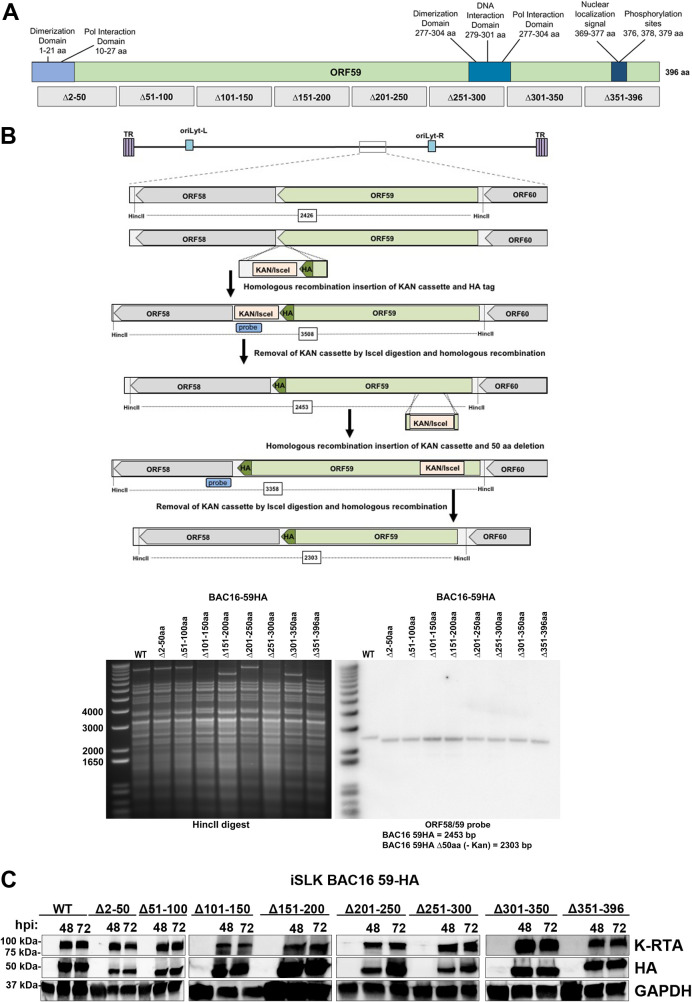
Previous studies focusing on ORF59 protein binding partners indicated that ORF59 interacts with many cellular proteins, including histones (20, 23). To determine the ORF59 amino acid domains that mediate interactions with histones, we generated KSHV BACmids, which contain an in-frame hemagglutinin (HA) tag and 50-aa deletions. Previously identified domains (17, 18, 37–39) and position of the 50-aa deletions are shown in Fig. 1A. KSHV BAC16 ORF59-HA and deletions were constructed using homologous recombination with insertion of a kanamycin I-SceI cassette containing an in-frame HA tag to the carboxyl terminus of ORF59, followed by the seamless removal of the kanamycin cassette. The insertion and removal of the kanamycin cassette were confirmed by Southern blot analysis of wild-type (WT) BAC16, BAC16 ORF59-HA, and ORF59 deletions by DNA digestion with HincII, followed by hybridization with an ORF58/59 probe (Fig. 1B). All BAC recombinants were confirmed by whole-genome DNA sequencing to contain only the specific deletion. No other indels were detected within the viral genome of any of the BAC mutants used in the following experiments. BAC sequences were deposited in the NCBI Sequence Read Archive (SRA), and accession numbers are listed in Materials and Methods. iSLK cells were transfected with either BAC16 ORF59-HA or BAC16 ORF59-HA deletions and selected with 1 μg/ml puromycin, 250 μg/ml G418, and 1,200 μg/ml hygromycin. iSLK BAC16 ORF59-HA and BAC16 ORF59 deletions were reactivated with 0.5 μg/ml doxycycline, 0.5 mM sodium butyrate (NaB), and 20 ng/ml 12-O-tetradecanoyl-phorbol-13-acetate (TPA). To determine if the ORF59 deletion viruses had defects in expression of K-RTA and ORF59, iSLK BAC16 ORF59-HA or ORF59 deletion mutants were reactivated, and protein was harvested at 48 and 72 hours postinduction (hpi). Protein lysates were separated on SDS-PAGE to verify expression of K-RTA, ORF59-HA, and GAPDH using specific antibodies (Fig. 1C). As shown in Fig. 1C, there were no defects in K-RTA and ORF59-HA protein expression in the wild-type (WT) full-length ORF59 or deletion BACmids.
FIG 1.
Generation and expression of KSHV BAC16 with in-frame HA tag of ORF59 and ORF59 deletions. (A) Schematic of ORF59 full-length protein showing previously identified interaction domains and sequential ORF59 50-aa deletion mutants described in this report. (B) KSHV BAC16 ORF59-HA and deletions were constructed using homologous recombination with insertion of the kanamycin I-SceI cassette, which contained an in-frame HA-tag to the carboxy terminus of ORF59, followed by seamless removal of the kanamycin cassette. The insertion and removal of the kanamycin cassette was confirmed by Southern blot analysis using a probe specific to ORF58/59. (C) Expression of ORF59 in iSLK cells upon reactivation. iSLK cells were transfected with either BAC16 ORF59-HA or ORF59 deletions and selected with 1 μg/ml puromycin, 250 μg/ml G418, and 1,200 μg/ml hygromycin. BACmid-containing cells were treated with doxycycline and TPA/NaB as indicated to reactivate virus. Protein was harvested at 48 and 72 hpi along with untreated control.
ORF59 301 to 350 aa are not required for interaction with histones during lytic reactivation of KSHV.
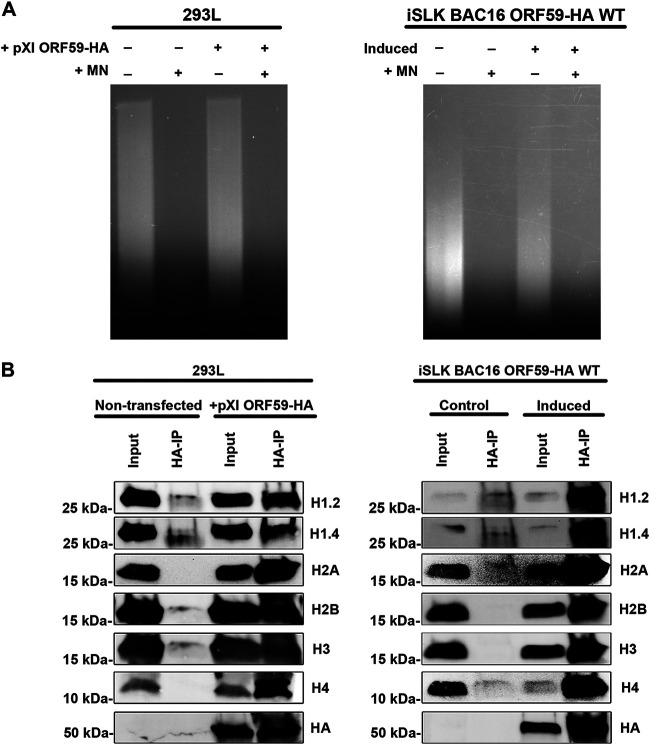
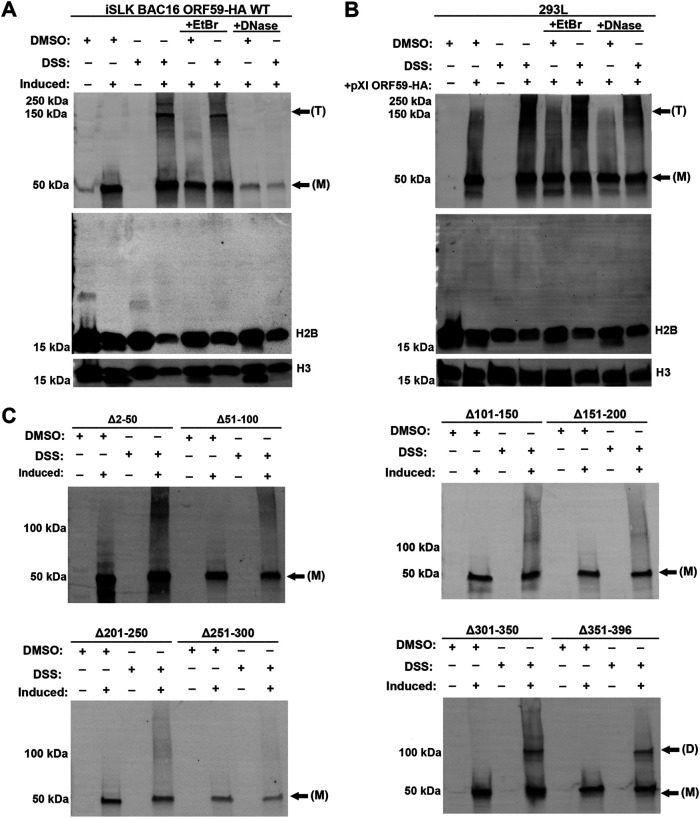
Since both ORF59 and histones are known to interact with DNA, we wanted to confirm that this interaction was not DNA-dependent. 293L cells transfected with pXI ORF59-HA plasmid and iSLK BAC16 ORF59-HA were induced with doxycycline, TPA, and NaB. Following transfection and reactivation, cells were harvested and treated with micrococcal nuclease (MNase) to disrupt the association of proteins to DNA. Lysate was collected and DNA was extracted prior to and following nuclease extraction. DNA was run on a 1% agarose gel (Fig. 2A). Nuclease-treated lysate was used for co-IP. Input protein and immunoprecipitation complexes were identified by Western blot analysis using HA antibody to detect ORF59, followed by detection of cellular histones (Fig. 2B).
FIG 2.
ORF59 301 to 350 aa are not required for interaction with histones during lytic reactivation of KSHV. Co-IP was performed to determine the interaction domain of ORF59 with native histones in the context of infection. Protein lysates were harvested 48 hpi, and protein complexes were isolated using HA-specific antibody and protein G magnetic beads in the presence of EtBr. Immunoprecipitations were analyzed by SDS-PAGE and visualized using histones and HA-specific antibodies.
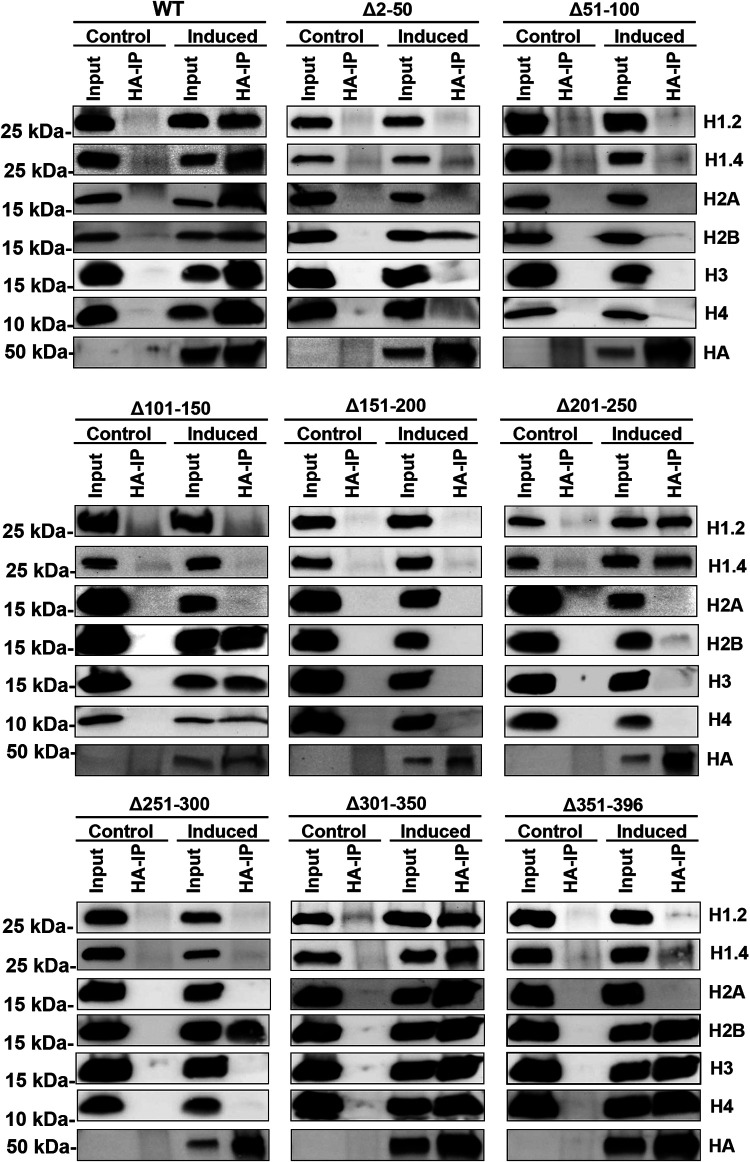
We identified that ORF59 and histones interact independently of the presence of DNA. Next, we wanted to identify the specific amino acid domains of ORF59 required for histone binding during lytic reactivation. iSLK BAC16 ORF59-HA and ORF59 deletion mutants were induced with doxycycline, TPA, and NaB. Following reactivation, protein lysates were treated with ethidium bromide (EtBr) to disrupt the association of proteins to DNA before adding HA-specific antibodies for the co-IP. Input protein and immunoprecipitation complexes were identified by Western blot analysis using HA antibody to detect ORF59, followed by detection of cellular histones with specific antibodies as indicated ((Fig. 3). Interestingly, deletions to 301 to 350 aa at the carboxy terminus of ORF59 do not seem to affect binding to histones. Furthermore, 101 to 150, 251 to 300, and 351 to 396 aa of ORF59 only affect binding to H1 variants and H2A; 201 to 250 and 301 to 350 aa are not required for binding of ORF59 and H1 variants; 51 to 100 and 151 to 250 aa of ORF59 are necessary for binding to all histones. The first 300 aa of ORF59 are necessary for H3 and H4 binding except for 101 to 150 and 301 to 350 aa. Noninduced cells were used as controls. IPs from the noninduced lysate, which did not contain ORF59 or ORF59 deletion proteins, did not precipitate any of the histones, confirming that ORF59 and histones undergo specific protein-protein interactions. In general, deletions to any domain of ORF59, with the exception of 301 to 350 aa, affects the binding of one or more histones.
FIG 3.
ORF59 and histone interactions are DNA-independent. 293L transfected with pXI ORF59-HA and induced iSLK BAC16 ORF59-HA cells were treated with micrococcal nuclease and used for co-IP to determine if ORF59-histone interactions are dependent on the presence of DNA. (A) DNA was collected and extracted prior to and following nuclease treatment. DNA was run on 1% agarose gel. (B) Following treatment, protein complexes were isolated using HA-specific antibody and protein G magnetic beads. Immunoprecipitations were analyzed by SDS-PAGE and visualized using histones and HA-specific antibodies.
ORF59 deletion mutants retain the ability to localize to the nucleus during reactivation.
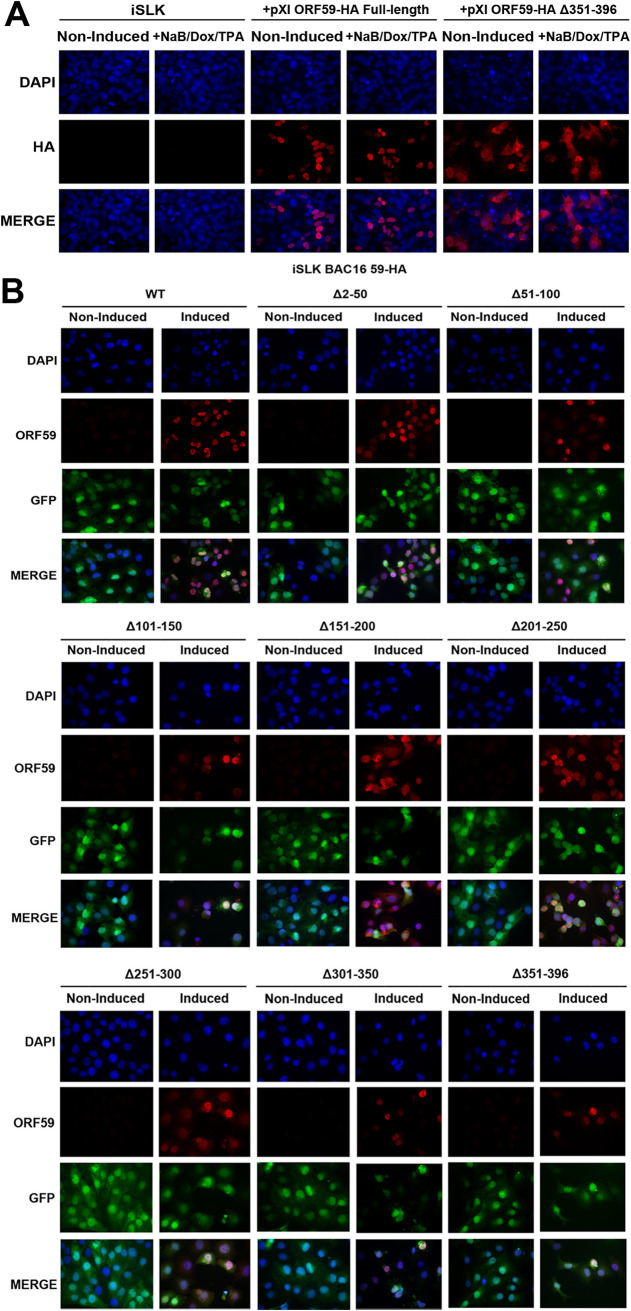
Since the function of ORF59 is dependent on its ability to properly localize to the nucleus, we wanted to determine if the ORF59 deletion mutants had localization defects. Previously published reports using a transient plasmid system showed that the ORF59 NLS is within 369 to 377 aa (17). Using IFA, we assessed the localization of ORF59 by transfecting ORF59 full-length and Δ351-396 plasmids into iSLK cells that did not contain the KSHV BACmid. Since iSLK cells contain an inducible ORF50, we evaluated if the presence of K-RTA or the chemical treatment (Dox, TPA, NaB) would influence the localization of ORF59. Both induced and noninduced iSLK cells were used to visualize ORF59 localization. We observed that the ORF59 Δ351-396 mutant still localizes to the nucleus, in addition to having some protein accumulate in the cytoplasm. Full-length ORF59 was observed to primarily localize to the nucleus (Fig. 4A). We tested the localization of ORF59 deletion mutants in the context of KSHV infection. We observed that the localization of ORF59 in iSLKs containing BAC16 ORF59 and deletion mutants was exclusively nuclear and unaffected by any deletion within ORF59 (Fig. 4B). Our data suggest that even without the reported NLS, ORF59 Δ351-396 still retains the ability to localize to the nucleus. Thus, these data confirm that the ORF59 deletion mutants and any functional defects are not due to aberrant localization. These data also suggest the possibility of a secondary mechanism by which ORF59 can localize to the nucleus beyond the previously described NLS.
FIG 4.
ORF59 deletion mutants retain the ability to localize to the nucleus during reactivation. (A) iSLK cells were transfected with ORF59-HA full-length or Δ351-396 plasmid and induced with doxycycline where indicated. IFA was performed to visualize the localization of ORF59 full-length and ORF59Δ351-396. Cells were visualized at ×40 magnification. (B) IFA was performed to visualize ORF59 localization in noninduced and induced iSLK BAC16 ORF59-HA WT and deletions. Cells were incubated with ORF59 antibody followed by secondary antibody labeled with Alexa Fluor 594. Cells were visualized at ×40 magnification.
The amino-terminus 2 to 300 aa of ORF59 are necessary for formation of dimers and tetramers.
In continuing an assessment of potential physical and functional defects of the ORF59 deletion mutants, we tested whether ORF59 deletion mutants maintained the ability to form quaternary protein structures during lytic reactivation. To evaluate this, we performed in vitro cross-linking with disuccinimidyl suberate (DSS), a commonly used permeable homobifunctional N-hydroxysuccinimide ester (40, 41). 293L transfected with pXI ORF59-HA plasmid or iSLK BAC16 ORF59-HA WT and deletion mutants were harvested at 24 hours postinduction, resuspended in phosphate-buffered saline (PBS), and treated with EtBr or DNase for 30 min at 4°C or 37°C. The ORF59 monomers and multimers were stabilized by the addition of DSS followed by quenching with Tris-HCl, pH 7.5, and the resulting complexes were resolved by SDS-PAGE. We used an HA-specific antibody and visualized ORF59 monomers (M) of ∼55 kDa, ORF59 homodimers (D) of ∼110 kDa, and homotetramers (T) of ∼220 kDa (Fig. 5A to C). Additionally, we used histone H2B- and H3-specific antibodies to confirm that the detected oligomers were specifically ORF59 and not ORF59-histone complexes. Figure 5A and B show that ORF59 forms higher-order oligomers in the context of infection and transfection. In the case of full-length ORF59 treated with EtBr or DNase, we observed that tetramers were maintained after EtBr treatment but not with DNase treatment. This is most likely due to the cleavage of DNA by DNase, whereas EtBr disrupts DNA structures, suggesting that the observed ORF59 tetramer can maintain a ring-like structure after the intercalation of EtBr. We observed that full-length ORF59 monomers and tetramers were the most abundant protein conformation detected after induction. Conversely, ORF59 deletion mutants from the first amino-terminus 300 aa show the formation of monomers only. Interestingly, homodimers were detected in both Δ301-350 and Δ351-396 aa. These data indicate that the amino terminus mediates ORF59 dimerization and formation of tetramers. Our observations are consistent with previous reports that showed the carboxy terminus of ORF59 is not required for formation of dimers (37). We were not able to detect dimerization in full-length ORF59, although previous studies have reported that ORF59 forms homodimers. The lack of detection of homodimers may be due to the infection model used in the experiments described here, which may have viral factors present that favor the formation or stabilization of the tetramers over the dimers. Deletions to 301 to 396 aa of the carboxy terminus retain ORF59’s ability to form dimers but not tetramers. This suggests that although dimers may be sufficient for DNA synthesis, tetramer formation may play an additional role during replication or at later stages of the viral replication cycle.
FIG 5.
The amino-terminus 2 to 300 aa of ORF59 are necessary for formation of dimers and tetramers. (A to C) (A) iSLK BAC16 ORF59-HA WT and (C) deletions were either noninduced or induced for 24 h, and (B) 293L cells transfected with pXI ORF59-HA were harvested and treated with 500 μM DSS or DMSO vehicle for 30 min, and cross-linking was quenched by addition of 1 mM tris-HCl pH 7.5. Protein complexes were separated by SDS-PAGE gel and analyzed by Western blotting using HA-specific antibody or specific histones H2B and H3. ORF59 (M) monomer, ∼55 kDa, (D) dimer, ∼110 kDa, and (T) tetramer, ∼220 kDa are shown.
ORF59 deletion mutants do not affect expression of IE and E viral transcripts.
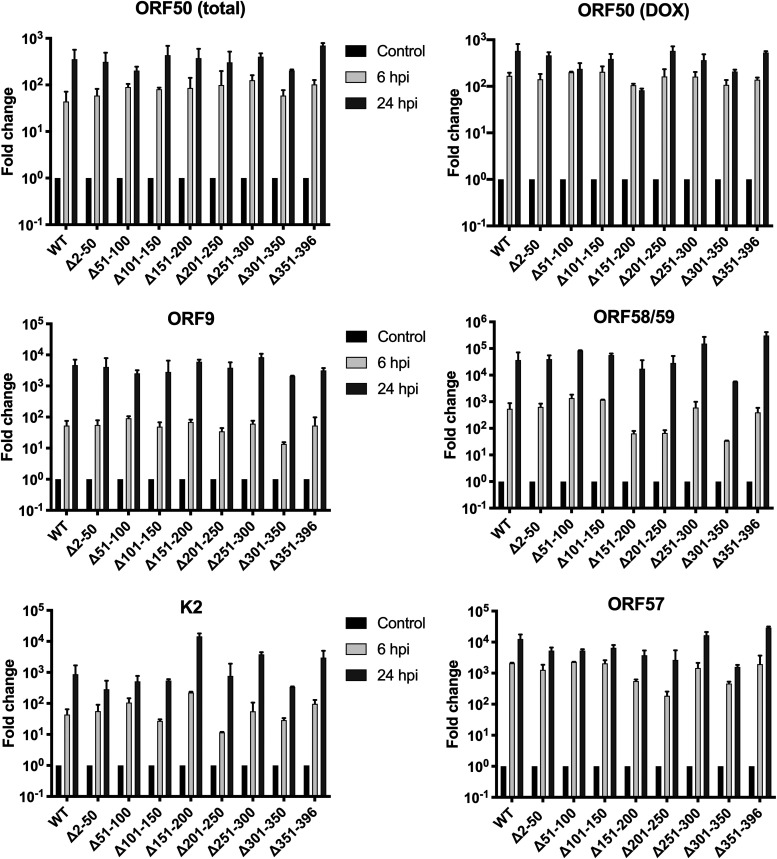
Since the interaction between ORF59 and histones may have implications in the regulation of viral transcription, we were interested in determining if the ORF59 deletion mutants affected the expression of viral transcripts at IE and E points during reactivation. iSLK BAC16 ORF59-HA and ORF59 deletion mutant cell lines were treated with doxycycline, TPA, and NaB. Total RNA was harvested at 6 and 24 hours postinduction (hpi). We measured mRNA accumulation by quantitative PCR (qPCR) using primers/probes specific to ORF50, ORF9, ORF58/59, ORFK2 (vIL-6), and ORF57 transcripts (Fig. 6). For ORF50, we had two sets of primers/probes, one that detected the ORF50 coding region present in both endogenous native ORF50 and doxycycline-induced ORF50 (ORF50 total; Fig. 6). The other set detected the 3′ untranscribed region (UTR) region of the doxycycline-inducible ORF50 transcript from the integrated ORF50 gene in iSLK (ORF50 DOX; Fig. 6). There were no significant differences in the accumulation of IE and E viral mRNA levels measured in the ORF59 deletion mutant cell lines. It is also important to note that although the longer ORF58/ORF59 bicistronic transcript originates from an upstream promoter before the ORF59 start, there is an additional ORF58 promoter within ORF59 that produces a smaller ORF58 transcript, the deletions within 251 to 300 and 301 to 350 disrupts this promoter (42). Because of the bicistronic nature of many transcripts encompassing these loci, there is the potential that the other deletions disrupt motifs that facilitate transcription at this promoter (43).
FIG 6.
ORF59 deletion mutants do not affect expression of IE and E viral transcripts. RNA was harvested and purified from iSLK BAC16 ORF59-HA WT and deletions at 6 and 24 hpi. Purified RNA was DNase treated and used for cDNA reactions, followed by qPCR using primers and probes specific to ORF50, the 3′ UTR region of ORF50 transcript from the dox-inducible promoter of the integrated gene in iSLK, ORF9, ORF58/59, ORFK2 (vIL-6), and ORF57 transcripts. Cycle threshold (CT) values were normalized to cellular control untreated samples. Results represent three independent experiments. Bar graphs represent means ± the standard deviation (SD). Two-way analysis of variance (ANOVA) analysis was performed (P < 0.05).
The carboxy-terminus 301 to 396 aa are not required for viral DNA synthesis.
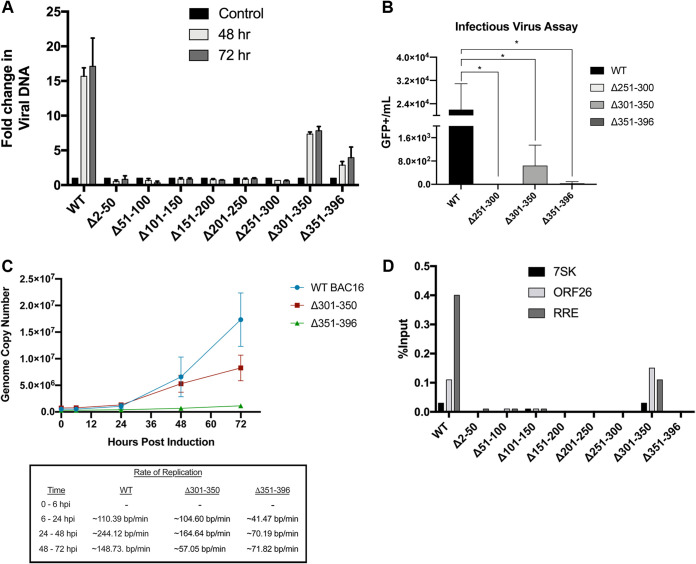
As the ORF59 mutants were not found to affect viral transcripts and protein expression, we measured the ability of the ORF59 deletion mutants to synthesize viral DNA. We performed qPCR on DNA harvested from iSLK BAC16 ORF59-HA WT and ORF59-HA mutants at 48 and 72 hpi and compared the results to DNA harvested from noninduced cells. Using primers/probes specific to ORF26 to detect viral DNA and cellular 7SK as a normalization control, qPCR analysis demonstrates that most of the ORF59 deletion mutants (2 to 300 aa) do not support viral DNA synthesis (Fig. 7A). This was further confirmed by quantifying infectious virus production. WT, Δ251-300, Δ301-350, and Δ351-396 ORF59 mutants were induced for 5 days, and virus was pelleted. Purified virus was used to infect 293L, and green fluorescent protein (GFP) (+) cells were counted 72 dpi and plotted as GFP (+) cells/ml (Fig. 7B). The WT shows ∼2.4 × 104 GFP+/ml, Δ251-300 aa is 0 GFP+/ml, Δ301-350 is ∼800 GFP+/ml, and Δ351-395 aa shows ∼100 GFP+/ml. The 301 to 396 aa deletions at the carboxy terminus show a modest accumulation of viral DNA and infectious virus following reactivation. This indicates that the carboxy terminus is not exclusively required for viral DNA synthesis. We further investigated the effects of ORF59 mutant deletions during viral reactivation by determining the rate of viral DNA replication. The rate of replication of WT, Δ251-300, Δ301-350, and Δ351-396 is reported as base pairs (bp) per minute (min) at indicated times postinfection (Fig. 7C). The number of genomes and rate of replication in the assessed deletions compared to WT are considerably lower. This indicates that mutations to ORF59 at this amino acid domain still retain the ability to synthesize DNA and production of infection virus, albeit at significantly lower levels compared to the WT. We further tested this by performing chromatin immunoprecipitation assays (ChIPs) to determine if ORF59 mutants still retained the ability to bind to DNA after reactivation. ChIPs were performed from iSLK BAC16 ORF59-HA WT and deletion mutants either uninduced or reactivated for 24 hours. We investigated the relative enrichment of ORF59 using primer/probes specific to the K-RTA response element (RRE) of oriLyt and ORF26 for a viral locus outside oriLyt. The detection of ORF59 at the RRE during lytic reactivation has previously been described (13). ORF59 bound chromatin and enrichment were determined by qPCR analysis. In ORF59 mutant cell lines, there was a decrease in enrichment at RRE following reactivation, except for ORF59 Δ301-350 aa. These data suggest that the first amino-terminus 300 aa of ORF59 are important for interacting with viral DNA, and deletions within this region result in disruption of viral replication during lytic reactivation (Fig. 7D). Unexpectedly, the ORF59 Δ351-396 aa did not show an enrichment of ORF59 to the RRE or ORF26, which is surprising considering that this deletion had measurable DNA accumulation (Fig. 7A). One possibility in the discrepancy is that the ORF59 Δ351-396 aa replicates slowly or slightly delayed compared to the WT. This would explain why we are unable to detect ORF59 Δ351-396 aa interacting with viral DNA at 24 hpi but do measure an increase in viral DNA accumulation by 48 and 72 hpi, although at a smaller amount than for the WT. Another possibility is that the interaction between ORF59 Δ351-396 and viral DNA may be below the threshold of detection for the ChIP assay since the amount of accumulated DNA is noticeably lower than that of the WT or the Δ301-350 mutant. Additionally, there may be other functional or heterodimerization domains within the Δ351-396 aa region which contribute to the stability and function of the ORF59 complex.
FIG 7.
The carboxy-terminus 301 to 396 aa are not required for viral DNA synthesis. (A) Total DNA was harvested at 48 and 72 hpi and purified for qPCR using TaqMan primers and probes to determine the relative levels of viral DNA accumulation. CT values were normalized using cellular DNA and compared to control untreated samples. (B) ORF59 mutants produce infectious virus (GFP+/ml) at lower levels than WT infectious virus production of WT and deletions. Infectious virus is reported as described in Materials in Methods. Statistical significance was determined by unpaired t test; *, P < 0.05. (C) Rate of replication of WT and deletions. Induced cells at indicated time points were harvested, and viral genomes were quantified by qPCR. (D) For ORF59 to bind to DNA at the RRE region of oriLyt, 301 to 350 aa are not required. iSLK BAC16 ORF59-HA WT and ORF59 deletion mutants were either uninduced or induced for 24 h and harvested, cross-linked, and used for a ChIP assay to determine the ability of ORF59 and deletions to bind to the RRE region of the origin of viral replication. The first amino-terminus 300 aa of ORF59 do not show a strong association to RRE compared to WT and 301 to 350 aa at the carboxy terminus after lytic reactivation.
DISCUSSION
The initial hypothesis surrounding this investigation was that the interaction between ORF59 and histones may constitute an additional level of viral gene regulation during lytic replication. The dynamic interaction between the viral genome and cellular histones modulates the capacity of the viral DNA template to be utilized for transcription. Chromatinization of the viral genome, resulting in either a heterochromatin state (more condensed and less transcriptionally active) or a euchromatin state (less condensed and more transcriptionally active) ultimately affects the ability of the virus to undergo productive lytic replication or remain latent (33, 36, 44). In addition, recruitment of particular subtypes of histones, especially variants of the H1 linker histone, may facilitate progression through various states of the viral replication cycle (30, 32).
When considering the implication for the interaction between ORF59 and histones, one of the goals was to identify the domain of ORF59 that interacts with histones. Although we approached this in a variety of ways, because of the multimeric structure of ORF59 and the interactions between histones (H2A with H2B and H3 with H4) within the octamer, the answer of a specific domain of ORF59 that interacts with histones was difficult to define. However, in the context of infection, we were able to determine that 51 to 100 and 151 to 200 aa of ORF59 are necessary for binding to all histones. Interestingly, it is important to note that we did find differences in the specific domains of ORF59 that interact with each histone, suggesting that the three-dimensional conformation of ORF59 and histone octamer results in the association of unique epitopes in these proteins that confers the interaction. This could be due to the complex multimeric structure of ORF59 and the dynamic interactions of histones within the octamer. Despite investigating the functional role, it remains unclear how the interaction between ORF59 and histones contributes to efficient viral replication. We did gain insight on the ability of ORF59 to localize to the nucleus in the absence of the previously reported NLS and identified that the carboxy terminus is not required for viral DNA synthesis, although it does facilitate more efficient DNA synthesis when present (17). The 2 to 50 and 251 to 300 aa deletions were unable to synthesize viral DNA following reactivation; this is consistent with previous reports that identified ORF9 binding, DNA binding, and dimerization within these regions (17, 37, 45). The internal deletions (Δ51-100, Δ101-150, Δ151-200, and Δ201-250) also were unable to synthesize DNA; we hypothesize that this because it was unable to form a competent tetrameric structure around the replicating DNA, or there could be binding domains within these regions for additional cofactors required for replication which have yet to be identified. The alternative or compounding factor for the observed phenotype is due to a disruption in the ability of ORF59 to interact with K-RTA, which has been shown to be responsible for the recruitment to oriLyt (13). Additionally, we observed that although the 301 to 350 aa of ORF59 retains the ability to bind to histones at levels comparable to the WT, this mutant replicates DNA at lower levels compared to the WT. This interesting phenotype could be due to the observed inability to form tetramers, hindering the ability of viral proteins such as the viral DNA polymerase or K-RTA to properly bind to ORF59 and resulting in the observed defect in viral replication. This domain does not have any reported function but does slightly overlap the polymerase interaction domain (277 to 304) which could account for the deficiency in replication. We determined that ORF59 deletion mutants did not affect the expression of IE and E transcripts. Since most of the ORF59 deletion mutants had a defect in DNA synthesis, late gene expression was not assessed. Any observed defect in late gene expression could be due to the lack of genome replication or due to disfunction in the proposed ORF59 transcription regulation, but because late gene expression is dependent on DNA synthesis, it would be difficult to discriminate between those two possibilities to make a definitive conclusion. A limitation to the interpretation of these results is that there may be undetermined posttranslational expression defects which effect the ability of ORF59 to function normally. In addition, because of a promoter for ORF58 within the internal region of ORF59, we cannot rule out that the observed phenotypes are not due to expression defects in ORF58 or other poly-cistronic transcripts within this region (42, 43).
The structures and functions of processivity factors are relatively conserved across many different types of organisms. Herpesvirus processivity factors have been compared to cellular proliferating cell nuclear antigen (PCNA), acting as a “sliding clamp” and functioning to increase the catalytic activity of DNA polymerase to aid in long-chain DNA synthesis. This interaction has been most well characterized with the EBV processivity factor (BMFR1) and polymerase (BALF5) and herpes simplex virus (HSV) processivity factor (UL42) and polymerase (UL30) (15, 46–50). Comparing the amino acid sequence of KSHV ORF59 to other human herpesvirus processivity factors shows a similarity of 28.5% to BMRF1 (EBV), 21.8% to UL42 (HSV-1), 20.4% to UL44 (human cytomegalovirus [HCMV]), 19.3% to p41 (HHV-6), 17.4% to gene 16 (varicella-zoster virus [VZV]), and 17.3% to U27 (HHV-7) (45). Further information about the homology between the processivity factors can be found when comparing their secondary and oligomeric structures. Interestingly, not all herpesvirus processivity factors form a ring structure similar to what has been reported for PCNA, which forms a head-to-tail trimeric ring (51, 52). HSV-1 UL42 functions as a monomer, and HCMV UL44 forms a head-to-head dimer “C-clamp” (53–61). EBV BMRF1 forms a head-to-head and tail-to-tail tetramer and adopts a ring structure similar to PCNA (62–65). KSHV ORF59 has been reported to form head-to-head dimers forming a C-clamp, but there is also evidence under cross-linking conditions that it may form higher-order tetramers with additional tail-to-tail interactions (37–39). The dimerization domain for ORF59 had previously been mapped to the first 21 residues at the amino terminus and residues 277 to 304 at the carboxy terminus (37). While we observed primarily tetrameric formation of full-length ORF59 in both a plasmid transfected expression of ORF59 in 293L cells and during lytic reactivation of iSLK cells followed by treatment with the cross-linking reagent DSS, it is important to note that previous reports found primarily dimeric ORF59. One of the main differences that may account for the variation includes the use of BS3 as the cross-linking reagent in the previous reports (37).
The rate of nucleotide incorporation for KSHV WT (iSLK BAC16) is slightly lower than what has previously been reported for HSV-1 (66) but consistent with 5-ethynyl-2′-deoxycytidine (EdU) pulse-labeled experiments which showed a longer EdU pulse time needed for KSHV DNA to have visual fluorescence from the nascent EdU-labeled DNA (67). The rates of nucleotide incorporation for ORF59 Δ301-350 and Δ351-396 deletion mutants are considerably lower than that of wild-type virus and correlate with the smaller amounts of DNA accumulation and production of infectious virus. These experiments suggest that the functional efficiency of ORF59 is in part found within 301 to 396 aa.
We identified that the amino-terminus 2 to 300 aa of ORF59 are important for dimer formation, and the 301 to 396 aa at the carboxy terminus still retain the ability to dimerize. Since we determined that mutations to the first 300 aa resulted in a defect in viral synthesis, it was not surprising to observe that these same residues would be important for multimer formation. Although herpesvirus processivity factors retain the basic ability to facilitate efficient DNA synthesis, the structural diversity beyond the conserved DNA binding domain suggests that some may have additional functions, one of those being the interaction between ORF59 and histones. It remains to be seen if the interaction between a herpesvirus processivity factor and cellular histones extends beyond KSHV ORF59 to other members of the herpesvirus family.
MATERIALS AND METHODS
Cell lines.
293L cells were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (Corning). iSLK cells were maintained in DMEM with 10% fetal bovine serum in 1 μg/ml of puromycin and 250 μg/ml of G418. iSLK cells containing the KSHV bacterial artificial chromosome (BAC) were maintained in DMEM with 10% fetal bovine serum, 1 μg/ml of puromycin, 250 μg/ml of G418, and 1,200 μg/ml of hygromycin B. For induction of lytic reactivation in the described cell lines, 1 mM sodium butyrate (NaB) (Sigma-Aldrich), 1 μg/ml of doxycycline (US Biologicals), and 20 ng/ml of phorbol 12-myristate 13-acetate (TPA) (Sigma-Aldrich) were used at the indicated time points. All cell lines were grown and maintained at 37°C in a humidified incubator supplemented with 5% CO2.
Plasmids.
To generate pXI ORF59-HA plasmids and deletion mutant expression plasmids, pXI vector was linearized using EcoRI enzyme for subsequent use in the cloning reaction. gBlocks (IDT) were designed to contain ORF59 with sequential 50-aa deletions followed by an in-frame HA tag (Table 1). ORF59-HA and deletion inserts were cloned into pXI vector and transformed using GeneArt Seamless Cloning and Assembly (Thermo Fisher Scientific) following manufacturer’s instructions.
TABLE 1.
gBlocks for plasmid pXI ORF59 deletion mutantsa
| pXI ORF59-HA plasmid | Sequence |
|---|---|
| WT full-length | gcttcgaattccaccATGCCTGTGGATTTTCACTATGGGGTCAGGGTGGACGTGACCCTCCTGTCTAAAATCAGGAGGGTCAATGAACACATTAAGAGTGCCACTAAAACCGGAGTAGTGCAAGTGCACGGATCGGCTTGCACGCCAACCCTCAGTGTGCTGTCCAGCGTGGGGACAGCTGGCGTTCTGGGGTTAAGAATAAAGAATGCCCTTACGCCCCTGGTGGGACACACGGAAGGCAGTGGAGACGTTAGCTTCAGCTTCAGGAATACGTCCGTCGGTAGCGGCTTCACGCACACGCGTGAGCTATTCGGTGCGAATGTACTCGACGCTGGCATAGCCTTTTATCGAAAGGGGGAGGCATGCGATACGGGGGCCCAACCGCAGTTCGTCAGGACCACCATTTCCTACGGGGACAACCTCACCTCCACCGTGCACAAAAGCGTGGTGGACCAAAAGGGTATCCTACCTTTCCACGATCGGATGGAAGCCGGTGGTAGGACCACCAGGCTTCTCCTCTGTGGCAAAACGGGTGCATTCCTACTTAAGTGGCTAAGGCAGCAAAAAACAAAAGAGGACCAAACGGTGACTGTGTCTGTCAGCGAGACGCTGTCGATCGTGACATTTTCTCTTGGTGGCGTGAGCAAGATCATTGATTTCAAGCCAGAAACCAAACCCGTTTCTGGCTGGGATGGTCTGAAGGGGAAGAAGTCGGTGGATGTGGGCGTGGTGCACACCGACGCCCTGAGCCGGGTTAGCCTGGAGTCCTTAATCGCCGCCCTGAGACTGTGTAAAGTCCCGGGTTGGTTTACCCCCGGGCTGATTTGGCACTCCAACGAAATATTAGAAGTGGAAGGTGTGCCCACTGGCTGCCAATCAGGTGACGTAAAGTTAAGTGTCCTACTTTTAGAGGTAAACCGATCTGTGTCTGCCGAGGGAGGCGAGTCTTCGCAAAAGGTTCCCGATTCTATACCGGACTCCAGGAGGCAGCCGGAATTGGAGTCTCCGGATTCTCCCCCTCTCACACCAGTTGGTCCCTTTGGGCCGCTGGAGGACGCCTCTGAGGACGCTGCCTCTGTCACCAGCTGCCCCCCAGCGGCGCCAACTAAGGACAGCACAAAGAGGCCTCACAAGAGGCGCTCAGACTCGAGCCAGTCCAGGGATCGTGGGAAGGTGCCCAAAACCACATTTAACCCCCTGATTTACCCATACGATGTTCCAGATTACGCTtagggctaagaattctgcagtcgacggta |
| Δ351-396 | gctcaagcttcgaattccaccATGCCTGTGGATTTTCACTATGGGGTCAGGGTGGACGTGACCCTCCTGTCTAAAATCAGGAGGGTCAATGAACACATTAAGAGTGCCACTAAAACCGGAGTAGTGCAAGTGCACGGATCGGCTTGCACGCCAACCCTCAGTGTGCTGTCCAGCGTGGGGACAGCTGGCGTTCTGGGGTTAAGAATAAAGAATGCCCTTACGCCCCTGGTGGGACACACGGAAGGCAGTGGAGACGTTAGCTTCAGCTTCAGGAATACGTCCGTCGGTAGCGGCTTCACGCACACGCGTGAGCTATTCGGTGCGAATGTACTCGACGCTGGCATAGCCTTTTATCGAAAGGGGGAGGCATGCGATACGGGGGCCCAACCGCAGTTCGTCAGGACCACCATTTCCTACGGGGACAACCTCACCTCCACCGTGCACAAAAGCGTGGTGGACCAAAAGGGTATCCTACCTTTCCACGATCGGATGGAAGCCGGTGGTAGGACCACCAGGCTTCTCCTCTGTGGCAAAACGGGTGCATTCCTACTTAAGTGGCTAAGGCAGCAAAAAACAAAAGAGGACCAAACGGTGACTGTGTCTGTCAGCGAGACGCTGTCGATCGTGACATTTTCTCTTGGTGGCGTGAGCAAGATCATTGATTTCAAGCCAGAAACCAAACCCGTTTCTGGCTGGGATGGTCTGAAGGGGAAGAAGTCGGTGGATGTGGGCGTGGTGCACACCGACGCCCTGAGCCGGGTTAGCCTGGAGTCCTTAATCGCCGCCCTGAGACTGTGTAAAGTCCCGGGTTGGTTTACCCCCGGGCTGATTTGGCACTCCAACGAAATATTAGAAGTGGAAGGTGTGCCCACTGGCTGCCAATCAGGTGACGTAAAGTTAAGTGTCCTACTTTTAGAGGTAAACCGATCTGTGTCTGCCGAGGGAGGCGAGTCTTCGCAAAAGGTTCCCGATTCTATACCGGACTCCAGGAGGCAGCCGGAATTGGAGTCTCCGGATTCTCCCCCTCTCACACCAGTTGGTCCCTTTGGGCCGCTGGAGGACGCCTCTGAGGACTACCCATACGATGTTCCAGATTACGCTtagggctaagaattctgcagtcgacggta |
The homologous regions used to target the pXI vector during cloning are underlined. HA tag is indicated by bold font.
Generation of recombinant BACmids with sequential amino acid deletions to ORF59.
The protocol for BAC mutagenesis was followed as previously described (68). Briefly, KSHV BAC16 ORF59 mutants were generated by homologous recombination using gBlocks (IDT) that were designed to contain the kanamycin cassette and I-SceI restriction enzyme site. The cassette was flanked by homologous sequences to ORF59 containing an in-frame HA tag to the carboxyl terminus or targeting 50 aa sequentially throughout the ORF59 region for removal. The gBlocks used are shown in Table 2. Then, 30 ng of gBlocks was electroporated (0.1-cm cuvette, 1.8 kV, 200 Ω, 25 μF) into competent GS1783 Escherichia coli cells harboring BAC16. The Kanr/I-SceI-containing mutants were selected on chloramphenicol and kanamycin plates. Individual bacterial colonies were selected, and correct clones were confirmed by PCR, restriction enzyme digest with HincII, and Southern blot analysis using an ORF58/59-specific probe. The second recombination step for the removal of the Kanr/SceI cassette was performed by treatment with 1% l-arabinose and arabinose-mediated I-SceI enzyme induction. The resultant colonies were analyzed by restriction digest, Southern blot analysis, and the ORF59 mutants containing 50-amino acid deletions were confirmed by sequencing. BAC DNA was purified using a NucleoBond Xtra BAC kit (Clontech) according to the manufacturer’s protocol. Then, 20 to 30 μl of BAC DNA from BAC16 WT DNA and BAC16 ORF59 deletion mutants was transfected into iSLK cells with FuGene HD reagent at a 1:4 ratio according to the manufacturer’s protocol (Promega). The iSLK cells containing the BACmids were selected with 1,200 μg/ml of hygromycin to obtain a pure population of cells.
TABLE 2.
gBlocks for BAC16 ORF59 deletion mutantsa
| ORF59-HA | Sequence |
|---|---|
| Δ2-50 | GCTTTCCTGTGATTTCTCTGTGCGTCTACGGCCGCAATCATGAGCGTGGGGACAGCTGGCGTTCGATTTATTCAACAAAGCCACGTTGTGTCTCAAAATCTCTGATGTTACATTGCACAAGATAAAAATATATCATCATGAACAATAAAACTGTCTGCTTACATAAACAGTAATACAAGGGGTGTTATGAGCCATATTCAACGGGAAACGTCTTGCTCGAGGCCGCGATTAAATTCCAACATGGATGCTGATTTATATGGGTATAAATGGGCTCGCGATAATGTCGGGCAATCAGGTGCGACAATCTATCGATTGTATGGGAAGCCCGATGCGCCAGAGTTGTTTCTGAAACATGGCAAAGGTAGCGTTGCCAATGATGTTACAGATGAGATGGTCAGACTAAACTGGCTGACGGAATTTATGCCTCTTCCGACCATCAAGCATTTTATCCGTACTCCTGATGATGCATGGTTACTCACCACTGCGATCCCCGGGAAAACAGCATTCCAGGTATTAGAAGAATATCCTGATTCAGGTGAAAATATTGTTGATGCGCTGGCAGTGTTCCTGCGCCGGTTGCATTCGATTCCTGTTTGTAATTGTCCTTTTAACAGCGATCGCGTATTTCGTCTCGCTCAGGCGCAATCACGAATGAATAACGGTTTGGTTGATGCGAGTGATTTTGATGACGAGCGTAATGGCTGGCCTGTTGAACAAGTCTGGAAAGAAATGCATAAGCTTTTGCCATTCTCACCGGATTCAGTCGTCACTCATGGTGATTTCTCACTTGATAACCTTATTTTTGACGAGGGGAAATTAATAGGTTGTATTGATGTTGGACGAGTCGGAATCGCAGACCGATACCAGGATCTTGCCATCCTATGGAACTGCCTCGGTGAGTTTTCTCCTTCATTACAGAAACGGCTTTTTCAAAAATATGGTATTGATAATCCTGATATGAATAAATTGCAGTTTCATTTGATGCTCGATGAGTTTTTCTAATCAGAATTGGTTAATTGGTTGTAACACTGGCATTACCCTGTTATCCCTAGATCGATGTACGGGCCAGATATACGCGGCGTCTACGGCCGCAATCATGAGCGTGGGGACAGCTGGCGTTCTGGGGTTAAGAATAAAGAAT |
| Δ51-100 | CAAGTGCACGGATCGGCTTGCACGCCAACCCTCAGTGTGCTGTCCGCGAATGTACTCGACGCTGGCCGATTTATTCAACAAAGCCACGTTGTGTCTCAAAATCTCTGATGTTACATTGCACAAGATAAAAATATATCATCATGAACAATAAAACTGTCTGCTTACATAAACAGTAATACAAGGGGTGTTATGAGCCATATTCAACGGGAAACGTCTTGCTCGAGGCCGCGATTAAATTCCAACATGGATGCTGATTTATATGGGTATAAATGGGCTCGCGATAATGTCGGGCAATCAGGTGCGACAATCTATCGATTGTATGGGAAGCCCGATGCGCCAGAGTTGTTTCTGAAACATGGCAAAGGTAGCGTTGCCAATGATGTTACAGATGAGATGGTCAGACTAAACTGGCTGACGGAATTTATGCCTCTTCCGACCATCAAGCATTTTATCCGTACTCCTGATGATGCATGGTTACTCACCACTGCGATCCCCGGGAAAACAGCATTCCAGGTATTAGAAGAATATCCTGATTCAGGTGAAAATATTGTTGATGCGCTGGCAGTGTTCCTGCGCCGGTTGCATTCGATTCCTGTTTGTAATTGTCCTTTTAACAGCGATCGCGTATTTCGTCTCGCTCAGGCGCAATCACGAATGAATAACGGTTTGGTTGATGCGAGTGATTTTGATGACGAGCGTAATGGCTGGCCTGTTGAACAAGTCTGGAAAGAAATGCATAAGCTTTTGCCATTCTCACCGGATTCAGTCGTCACTCATGGTGATTTCTCACTTGATAACCTTATTTTTGACGAGGGGAAATTAATAGGTTGTATTGATGTTGGACGAGTCGGAATCGCAGACCGATACCAGGATCTTGCCATCCTATGGAACTGCCTCGGTGAGTTTTCTCCTTCATTACAGAAACGGCTTTTTCAAAAATATGGTATTGATAATCCTGATATGAATAAATTGCAGTTTCATTTGATGCTCGATGAGTTTTTCTAATCAGAATTGGTTAATTGGTTGTAACACTGGCATTACCCTGTTATCCCTAGATCGATGTACGGGCCAGATATACGCGCCAACCCTCAGTGTGCTGTCCGCGAATGTACTCGACGCTGGCATAGCCTTTTATCGAAAGGGGGAG |
| Δ101-150 | ACGTCCGTCGGTAGCGGCTTCACGCACACGCGTGAGCTATTCGGTCTACCTTTCCACGATCGGATGCGATTTATTCAACAAAGCCACGTTGTGTCTCAAAATCTCTGATGTTACATTGCACAAGATAAAAATATATCATCATGAACAATAAAACTGTCTGCTTACATAAACAGTAATACAAGGGGTGTTATGAGCCATATTCAACGGGAAACGTCTTGCTCGAGGCCGCGATTAAATTCCAACATGGATGCTGATTTATATGGGTATAAATGGGCTCGCGATAATGTCGGGCAATCAGGTGCGACAATCTATCGATTGTATGGGAAGCCCGATGCGCCAGAGTTGTTTCTGAAACATGGCAAAGGTAGCGTTGCCAATGATGTTACAGATGAGATGGTCAGACTAAACTGGCTGACGGAATTTATGCCTCTTCCGACCATCAAGCATTTTATCCGTACTCCTGATGATGCATGGTTACTCACCACTGCGATCCCCGGGAAAACAGCATTCCAGGTATTAGAAGAATATCCTGATTCAGGTGAAAATATTGTTGATGCGCTGGCAGTGTTCCTGCGCCGGTTGCATTCGATTCCTGTTTGTAATTGTCCTTTTAACAGCGATCGCGTATTTCGTCTCGCTCAGGCGCAATCACGAATGAATAACGGTTTGGTTGATGCGAGTGATTTTGATGACGAGCGTAATGGCTGGCCTGTTGAACAAGTCTGGAAAGAAATGCATAAGCTTTTGCCATTCTCACCGGATTCAGTCGTCACTCATGGTGATTTCTCACTTGATAACCTTATTTTTGACGAGGGGAAATTAATAGGTTGTATTGATGTTGGACGAGTCGGAATCGCAGACCGATACCAGGATCTTGCCATCCTATGGAACTGCCTCGGTGAGTTTTCTCCTTCATTACAGAAACGGCTTTTTCAAAAATATGGTATTGATAATCCTGATATGAATAAATTGCAGTTTCATTTGATGCTCGATGAGTTTTTCTAATCAGAATTGGTTAATTGGTTGTAACACTGGCATTACCCTGTTATCCCTAGATCGATGTACGGGCCAGATATACGCGCACACGCGTGAGCTATTCGGTCTACCTTTCCACGATCGGATGGAAGCCGGTGGTAGGACCACCAGG |
| Δ151-200 | CTCACCTCCACCGTGCACAAAAGCGTGGTGGACCAAAAGGGTATCATCGTGACATTTTCTCTTGGTCGATTTATTCAACAAAGCCACGTTGTGTCTCAAAATCTCTGATGTTACATTGCACAAGATAAAAATATATCATCATGAACAATAAAACTGTCTGCTTACATAAACAGTAATACAAGGGGTGTTATGAGCCATATTCAACGGGAAACGTCTTGCTCGAGGCCGCGATTAAATTCCAACATGGATGCTGATTTATATGGGTATAAATGGGCTCGCGATAATGTCGGGCAATCAGGTGCGACAATCTATCGATTGTATGGGAAGCCCGATGCGCCAGAGTTGTTTCTGAAACATGGCAAAGGTAGCGTTGCCAATGATGTTACAGATGAGATGGTCAGACTAAACTGGCTGACGGAATTTATGCCTCTTCCGACCATCAAGCATTTTATCCGTACTCCTGATGATGCATGGTTACTCACCACTGCGATCCCCGGGAAAACAGCATTCCAGGTATTAGAAGAATATCCTGATTCAGGTGAAAATATTGTTGATGCGCTGGCAGTGTTCCTGCGCCGGTTGCATTCGATTCCTGTTTGTAATTGTCCTTTTAACAGCGATCGCGTATTTCGTCTCGCTCAGGCGCAATCACGAATGAATAACGGTTTGGTTGATGCGAGTGATTTTGATGACGAGCGTAATGGCTGGCCTGTTGAACAAGTCTGGAAAGAAATGCATAAGCTTTTGCCATTCTCACCGGATTCAGTCGTCACTCATGGTGATTTCTCACTTGATAACCTTATTTTTGACGAGGGGAAATTAATAGGTTGTATTGATGTTGGACGAGTCGGAATCGCAGACCGATACCAGGATCTTGCCATCCTATGGAACTGCCTCGGTGAGTTTTCTCCTTCATTACAGAAACGGCTTTTTCAAAAATATGGTATTGATAATCCTGATATGAATAAATTGCAGTTTCATTTGATGCTCGATGAGTTTTTCTAATCAGAATTGGTTAATTGGTTGTAACACTGGCATTACCCTGTTATCCCTAGATCGATGTACGGGCCAGATATACGCGGTGGTGGACCAAAAGGGTATCATCGTGACATTTTCTCTTGGTGGCGTGAGCAAGATCATTGATTTC |
| Δ201-250 | GAGGACCAAACGGTGACTGTGTCTGTCAGCGAGACGCTGTCGTCCTTAATCGCCGCCCTGAGACGATTTATTCAACAAAGCCACGTTGTGTCTCAAAATCTCTGATGTTACATTGCACAAGATAAAAATATATCATCATGAACAATAAAACTGTCTGCTTACATAAACAGTAATACAAGGGGTGTTATGAGCCATATTCAACGGGAAACGTCTTGCTCGAGGCCGCGATTAAATTCCAACATGGATGCTGATTTATATGGGTATAAATGGGCTCGCGATAATGTCGGGCAATCAGGTGCGACAATCTATCGATTGTATGGGAAGCCCGATGCGCCAGAGTTGTTTCTGAAACATGGCAAAGGTAGCGTTGCCAATGATGTTACAGATGAGATGGTCAGACTAAACTGGCTGACGGAATTTATGCCTCTTCCGACCATCAAGCATTTTATCCGTACTCCTGATGATGCATGGTTACTCACCACTGCGATCCCCGGGAAAACAGCATTCCAGGTATTAGAAGAATATCCTGATTCAGGTGAAAATATTGTTGATGCGCTGGCAGTGTTCCTGCGCCGGTTGCATTCGATTCCTGTTTGTAATTGTCCTTTTAACAGCGATCGCGTATTTCGTCTCGCTCAGGCGCAATCACGAATGAATAACGGTTTGGTTGATGCGAGTGATTTTGATGACGAGCGTAATGGCTGGCCTGTTGAACAAGTCTGGAAAGAAATGCATAAGCTTTTGCCATTCTCACCGGATTCAGTCGTCACTCATGGTGATTTCTCACTTGATAACCTTATTTTTGACGAGGGGAAATTAATAGGTTGTATTGATGTTGGACGAGTCGGAATCGCAGACCGATACCAGGATCTTGCCATCCTATGGAACTGCCTCGGTGAGTTTTCTCCTTCATTACAGAAACGGCTTTTTCAAAAATATGGTATTGATAATCCTGATATGAATAAATTGCAGTTTCATTTGATGCTCGATGAGTTTTTCTAATCAGAATTGGTTAATTGGTTGTAACACTGGCATTACCCTGTTATCCCTAGATCGATGTACGGGCCAGATATACGCGTCTGTCAGCGAGACGCTGTCGTCCTTAATCGCCGCCCTGAGACTGTGTAAAGTCCCGGGTTGG |
| Δ251-300 | GGCGTGGTGCACACCGACGCCCTGAGCCGGGTTAGCCTGGAGAACCGATCTGTGTCTGCCGAGCGATTTATTCAACAAAGCCACGTTGTGTCTCAAAATCTCTGATGTTACATTGCACAAGATAAAAATATATCATCATGAACAATAAAACTGTCTGCTTACATAAACAGTAATACAAGGGGTGTTATGAGCCATATTCAACGGGAAACGTCTTGCTCGAGGCCGCGATTAAATTCCAACATGGATGCTGATTTATATGGGTATAAATGGGCTCGCGATAATGTCGGGCAATCAGGTGCGACAATCTATCGATTGTATGGGAAGCCCGATGCGCCAGAGTTGTTTCTGAAACATGGCAAAGGTAGCGTTGCCAATGATGTTACAGATGAGATGGTCAGACTAAACTGGCTGACGGAATTTATGCCTCTTCCGACCATCAAGCATTTTATCCGTACTCCTGATGATGCATGGTTACTCACCACTGCGATCCCCGGGAAAACAGCATTCCAGGTATTAGAAGAATATCCTGATTCAGGTGAAAATATTGTTGATGCGCTGGCAGTGTTCCTGCGCCGGTTGCATTCGATTCCTGTTTGTAATTGTCCTTTTAACAGCGATCGCGTATTTCGTCTCGCTCAGGCGCAATCACGAATGAATAACGGTTTGGTTGATGCGAGTGATTTTGATGACGAGCGTAATGGCTGGCCTGTTGAACAAGTCTGGAAAGAAATGCATAAGCTTTTGCCATTCTCACCGGATTCAGTCGTCACTCATGGTGATTTCTCACTTGATAACCTTATTTTTGACGAGGGGAAATTAATAGGTTGTATTGATGTTGGACGAGTCGGAATCGCAGACCGATACCAGGATCTTGCCATCCTATGGAACTGCCTCGGTGAGTTTTCTCCTTCATTACAGAAACGGCTTTTTCAAAAATATGGTATTGATAATCCTGATATGAATAAATTGCAGTTTCATTTGATGCTCGATGAGTTTTTCTAATCAGAATTGGTTAATTGGTTGTAACACTGGCATTACCCTGTTATCCCTAGATCGATGTACGGGCCAGATATACGCGCTGAGCCGGGTTAGCCTGGAGAACCGATCTGTGTCTGCCGAGGGAGGCGAGTCTTCGCAAAAG |
| Δ301-350 | CAATCAGGTGACGTAAAGTTAAGTGTCCTACTTTTAGAGGTAGACGCTGCCTCTGTCACCAGCCGATTTATTCAACAAAGCCACGTTGTGTCTCAAAATCTCTGATGTTACATTGCACAAGATAAAAATATATCATCATGAACAATAAAACTGTCTGCTTACATAAACAGTAATACAAGGGGTGTTATGAGCCATATTCAACGGGAAACGTCTTGCTCGAGGCCGCGATTAAATTCCAACATGGATGCTGATTTATATGGGTATAAATGGGCTCGCGATAATGTCGGGCAATCAGGTGCGACAATCTATCGATTGTATGGGAAGCCCGATGCGCCAGAGTTGTTTCTGAAACATGGCAAAGGTAGCGTTGCCAATGATGTTACAGATGAGATGGTCAGACTAAACTGGCTGACGGAATTTATGCCTCTTCCGACCATCAAGCATTTTATCCGTACTCCTGATGATGCATGGTTACTCACCACTGCGATCCCCGGGAAAACAGCATTCCAGGTATTAGAAGAATATCCTGATTCAGGTGAAAATATTGTTGATGCGCTGGCAGTGTTCCTGCGCCGGTTGCATTCGATTCCTGTTTGTAATTGTCCTTTTAACAGCGATCGCGTATTTCGTCTCGCTCAGGCGCAATCACGAATGAATAACGGTTTGGTTGATGCGAGTGATTTTGATGACGAGCGTAATGGCTGGCCTGTTGAACAAGTCTGGAAAGAAATGCATAAGCTTTTGCCATTCTCACCGGATTCAGTCGTCACTCATGGTGATTTCTCACTTGATAACCTTATTTTTGACGAGGGGAAATTAATAGGTTGTATTGATGTTGGACGAGTCGGAATCGCAGACCGATACCAGGATCTTGCCATCCTATGGAACTGCCTCGGTGAGTTTTCTCCTTCATTACAGAAACGGCTTTTTCAAAAATATGGTATTGATAATCCTGATATGAATAAATTGCAGTTTCATTTGATGCTCGATGAGTTTTTCTAATCAGAATTGGTTAATTGGTTGTAACACTGGCATTACCCTGTTATCCCTAGATCGATGTACGGGCCAGATATACGCGAGTGTCCTACTTTTAGAGGTAGACGCTGCCTCTGTCACCAGCTGCCCCCCAGCGGCGCCAACTAAGGACAGCACAAAGAGGCCTCACAAGAGGCGCTCAGACTCGAGCCAGTCCAGGGATCGTGGGAAGGTGCCCAAAACCACATTTAACCCCCTGATT |
| Δ351-396 | ACACCAGTTGGTCCCTTTGGGCCGCTGGAGGACGCCTCTGAGCACCATGTGCCGCCTGGACAGCGATTTATTCAACAAAGCCACGTTGTGTCTCAAAATCTCTGATGTTACATTGCACAAGATAAAAATATATCATCATGAACAATAAAACTGTCTGCTTACATAAACAGTAATACAAGGGGTGTTATGAGCCATATTCAACGGGAAACGTCTTGCTCGAGGCCGCGATTAAATTCCAACATGGATGCTGATTTATATGGGTATAAATGGGCTCGCGATAATGTCGGGCAATCAGGTGCGACAATCTATCGATTGTATGGGAAGCCCGATGCGCCAGAGTTGTTTCTGAAACATGGCAAAGGTAGCGTTGCCAATGATGTTACAGATGAGATGGTCAGACTAAACTGGCTGACGGAATTTATGCCTCTTCCGACCATCAAGCATTTTATCCGTACTCCTGATGATGCATGGTTACTCACCACTGCGATCCCCGGGAAAACAGCATTCCAGGTATTAGAAGAATATCCTGATTCAGGTGAAAATATTGTTGATGCGCTGGCAGTGTTCCTGCGCCGGTTGCATTCGATTCCTGTTTGTAATTGTCCTTTTAACAGCGATCGCGTATTTCGTCTCGCTCAGGCGCAATCACGAATGAATAACGGTTTGGTTGATGCGAGTGATTTTGATGACGAGCGTAATGGCTGGCCTGTTGAACAAGTCTGGAAAGAAATGCATAAGCTTTTGCCATTCTCACCGGATTCAGTCGTCACTCATGGTGATTTCTCACTTGATAACCTTATTTTTGACGAGGGGAAATTAATAGGTTGTATTGATGTTGGACGAGTCGGAATCGCAGACCGATACCAGGATCTTGCCATCCTATGGAACTGCCTCGGTGAGTTTTCTCCTTCATTACAGAAACGGCTTTTTCAAAAATATGGTATTGATAATCCTGATATGAATAAATTGCAGTTTCATTTGATGCTCGATGAGTTTTTCTAATCAGAATTGGTTAATTGGTTGTAACACTGGCATTACCCTGTTATCCCTAGATCGATGTACGGGCCAGATATACGCGCCGCTGGAGGACGCCTCTGAGTACCCATACGATGTTCCAGATTACGCTCACCATGTGCCGCCTGGACAGTGAGCGCGCTCTGTCGCTCTTCAG |
| C-terminus in-frame HA tag | AACCACATTTAACCCCCTGATTTACCCATACGATGTTCCAGATTACGCTTGACACCATGTGCCGCCTGGACACGATTTATTCAACAAAGCCACGTTGTGTCTCAAAATCTCTGATGTTACATTGCACAAGATAAAAATATATCATCATGAACAATAAAACTGTCTGCTTACATAAACAGTAATACAAGGGGTGTTATGAGCCATATTCAACGGGAAACGTCTTGCTCGAGGCCGCGATTAAATTCCAACATGGATGCTGATTTATATGGGTATAAATGGGCTCGCGATAATGTCGGGCAATCAGGTGCGACAATCTATCGATTGTATGGGAAGCCCGATGCGCCAGAGTTGTTTCTGAAACATGGCAAAGGTAGCGTTGCCAATGATGTTACAGATGAGATGGTCAGACTAAACTGGCTGACGGAATTTATGCCTCTTCCGACCATCAAGCATTTTATCCGTACTCCTGATGATGCATGGTTACTCACCACTGCGATCCCCGGGAAAACAGCATTCCAGGTATTAGAAGAATATCCTGATTCAGGTGAAAATATTGTTGATGCGCTGGCAGTGTTCCTGCGCCGGTTGCATTCGATTCCTGTTTGTAATTGTCCTTTTAACAGCGATCGCGTATTTCGTCTCGCTCAGGCGCAATCACGAATGAATAACGGTTTGGTTGATGCGAGTGATTTTGATGACGAGCGTAATGGCTGGCCTGTTGAACAAGTCTGGAAAGAAATGCATAAGCTTTTGCCATTCTCACCGGATTCAGTCGTCACTCATGGTGATTTCTCACTTGATAACCTTATTTTTGACGAGGGGAAATTAATAGGTTGTATTGATGTTGGACGAGTCGGAATCGCAGACCGATACCAGGATCTTGCCATCCTATGGAACTGCCTCGGTGAGTTTTCTCCTTCATTACAGAAACGGCTTTTTCAAAAATATGGTATTGATAATCCTGATATGAATAAATTGCAGTTTCATTTGATGCTCGATGAGTTTTTCTAATCAGAATTGGTTAATTGGTTGTAACACTGGCATTACCCTGTTATCCCTAGATCGATGTACGGGCCAGATATACGCGCGATGTTCCAGATTACGCTTGACACCATGTGCCGCCTGGACAGTGAGCGCGCTCTGTC |
The homologous regions used to target specific viral sequences and subsequent generation of deletions within the ORF are underlined. The remaining sequence is the Kan cassette.
Sequencing.
WT BAC16, BAC16 ORF59-HA, and ORF59-HA deletion mutants were sequenced at the UNR genomic center using an Illumina NextSeq instrument. Fasta files were aligned to the reference genome using CLC Genomics Workbench (Qiagen). All sequences have been deposited and are accessible in the NCBI Sequence Read Archive (SRA).
Coimmunoprecipitation (co-IP) and Western blotting.
Where indicated, 293Ls were transfected with 10 μg of pXI ORF59-HA plasmid using TransIT LT1 (Mirrus). iSLK BAC16 ORF59-HA WT and ORF59 deletion mutants were induced for either 24 or 48 hours with 0.5 mM NaB, 0.5 μg/ml of doxycycline, and 10 ng/ml of TPA. For nuclease experiments, cells were harvested by scraping cells with PBS. Cells were sonicated using 2 to 3, 10-second pulses with a probe disruptor (Misonix 200; Fisher). Then, 25 μl of lysate was collected as the nontreated control. Cells were then treated with 5 μl micrococcal nuclease along with reaction buffer and bovine serum albumin (BSA) at 100 μg/ml for 15 min at 37°C following the manufacturer’s instructions (New England BioLabs). Following digestion, 25 μl was collected as treated samples. Total DNA was collected from both nontreated and treated samples by phenol-chloroform extraction and then chloroform extraction. DNA was precipitated with 100% ethanol and resuspended in 1× Tris-EDTA (TE). The collected DNA was analyzed on 1% agarose gel. The remaining lysate was used for co-IP. For ORF59-histone co-IP, iSLK BAC16 WT and ORF59 deletions mutant protein extracts were prepared using 500 μl of Pierce IP lysis buffer and protease inhibitors (Sigma-Aldrich P8340). Lysates were sonicated using 2 to 3, 10-second pulses with a probe disruptor (Misonix 200; Fisher) and incubated with 8 μl of ethidium bromide for 30 min on ice to disrupt DNA structures. Lysates were centrifuged at 13,000 rpm for 10 min at 4°C to remove cellular debris. Supernatant was precleared with mouse IgG-agarose conjugate (Santa Cruz Biotechnology) at 4°C for 1 hour, and 5% of the lysate was saved as input. Protein G magnetic beads (Life Technologies) were preblocked with 1 ml of IP lysis and 1% BSA for 30 min at room temperature and washed twice with IP lysis. Then, 50 μl of the preblocked magnetic protein G beads (Life Technology) was added to the remaining lysate along with antihemagglutinin (anti-HA) rabbit antibody (Sigma-Aldrich) at a 1:400 dilution. This mixture was rotated at 4°C overnight. The beads were washed three times with 1 ml of IP lysis buffer, each time with rotation for 5 min at room temperature. After the final wash, the beads were resuspended in 100 μl of Laemmli sample buffer (Bio-Rad) with beta-mercaptoethanol and boiled for 5 min. Then, 50 μl of protein was separated through an SDS-PAGE gel and subsequently transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). Blots were blocked for 10 min with Odyssey blocking buffer in Tris-buffered saline TBS (LI-COR) and then incubated with the following specific primary antibodies: mouse anti-HA at 1:1,000 (catalog no. H3663 and H9658), rabbit anti-HA at 1:2,000 (catalog no. H6908), and rabbit anti-Flag at 1:2,000 (catalog no. F7424); all were obtained from Sigma-Aldrich. Rabbit anti-RTA at 1:5,000 (provided by Don Ganem, UCSF), 1:100 mouse anti-ORF59 hybridoma supernatant (GenScript), and 1:250 mouse anti-ORF59 (provided by Bala Chandran, University of South Florida). Rabbit anti-H1.2 (1:2,000) (catalog no. 17677), rabbit anti-H2A (1:2,000) (catalog no. 18255), mouse anti-H2B (1:1,000) (catalog no. 52484), rabbit anti-H3 (1:5,000) (catalog no. 1791), and rabbit anti-GAPDH (1:2500) (catalog no. 128915) were all obtained from abcam. Rabbit H1.4 (1:2,000) (catalog no. CD4J50) was purchased from cell signaling. Rabbit anti-H4 (1:1,000) (catalog no. 128915) was purchased from Diagenode. All primary antibodies were incubated at 4°C overnight, followed by washing with Tris-buffered saline with Tween 20 (TBST) (10 mM Tris-Hcl, pH 7.5, 150 mM NaCl, 0.05% Tween 20) buffer, and incubation with either IR-Dye 680 and 800 anti-mouse or anti-rabbit (LI-COR) secondary antibody was conjugated for 30 min, and proteins were detected using a ChemiDoc MP imaging system (Bio-Rad).
Real-time qPCR.
For quantitating viral DNA synthesis, iSLK cells were infected with either WT or ORF59 deletions at 48 and 72 hpi, and uninduced controls were used. Total genomic DNA was harvested by adding DNA extraction buffer (2% SDS, 100 mM Tris-HCl, pH 8.0, 10 mM EDTA, and 50 mg/ml of proteinase K). Cell lysates were incubated at 65°C for 1 to 2 hours, followed by phenol-chloroform extraction and then chloroform extraction. DNA was precipitated with 100% ethanol and resuspend in 1× TE. DNA was analyzed by qPCR with SsoAdvanced Universal Probes Supermix (Bio-Rad) according to the manufacturer’s instructions. KSHV-specific primers were used to quantitate viral DNA synthesis and were normalized to 7SK. Each qPCR was performed in triplicate. Selected KSHV transcript primers/probes are shown in Table 3.
TABLE 3.
Primers pairs used for qPCR
| Gene | Primer 1 | Primer 2 | Probe |
|---|---|---|---|
| 7SK | 5′-TGA CTA CCC TAC GTT CTC CTA C-3′ | 5′-GTC AAG GGT ATA CGA GTA GCT G-3′ | 5′-/56-FAM/CCC TGC TAG/ZEN/AAC CTC CAA ACA AGC T/3IABkFQ/-3′ |
| PPIA | 5′-CAA GAC TGA GAT GCA CAA GTG-3′ | 5′-GTG GCG GAT TTG ATC ATT TGG-3′ | 5′-/56-FAM/AAT TCA CGC/ZEN/AGA AGG AAC CAG ACA GT/3IABkFQ/-3′ |
| ORF9 | 5′-AGG TGG GAA ACT ACT GTG TTA TT-3′ | 5′-TGG CCA GCT TGG CTA TTT-3′ | /56-FAM/TGC TAC GGT/ZEN/TTC AGA CCC ATG TTG AG/3IABkFQ/ |
| ORF26 | 5′-ATG GCA CTC GAC AAG AGT ATA G-3′ | 5′-GGC AGT ACG CTC CCT ATT T-3′ | 5′-/56-FAM/AGA CTC TTC/ZEN/GCT GAT GAA CTG GCC/3IABkFQ/-3′ |
| ORF50 | 5′-CTG GTA CAG TCC TTG CAG AAT A-3′ | 5′-CAG AGT CTA TTC GCC CTG TTA G-3′ | 5′-/56-FAM/CCC TGA AAC/ZEN/ATG GGA TGT CGG GTC/3IABkFQ/-3′ |
| DOX ORF50 3′ UTR | 5′-TCT GGA GCA TGC GCT TTA G-3′ | 5′-CTA CCG GTG GAT GTG GAA TG-3′ | 5′-/56-FAM/CTA CAC AAG/ZEN/TGG CCT CTG GCC T/3IABkFQ/-3′ |
| ORF57 | 5′-GAT GGG AAA CCG CTT AGT AGA G-3′ | 5′-GCC TGG GAT AGT TAG GAC AAA G-3′ | 5-‘/56-FAM/TAA CCT TCT/ZEN/TGG CGA GGT CAA GCT/3IABkFQ/-3′ |
| ORF58/59 | 5′-GTG GCT AGC GGA TAA GGT AAC-3′ | 5′-CAG TGT TTG CTG CTG TAG TTT G-3′ | 5′-/56-FAM/CCG GTA TGA/ZEN/AGG GCA CAC GAG AAA/3IABkFQ/-3′ |
| ORFK2 | 5′-TCC CTG AAG CCT CCC TAA TA-3′ | 5′-GAA GAC CTT AGG ATG GGA CAT AC-3′ | 5′-/56-FAM/TTT GGG TGG/ZEN/ACT GTA GTG CGT CTT/3IABkFQ/-3′ |
| RRE OriLyt | 5′-CTG GGT GGT TTC GGT AGA TG-3′ | 5′-TCA AAT GGG CGT AAC CGT AG-3′ | 5′-/56-FAM/AAC ATG GGT/ZEN/GGC TAA CGC CTA CAT/3IABkFQ/-3′ |
For quantitating expression of viral transcripts, iSLK cells infected with WT or ORF59 deletion mutants were induced and harvested at 6 and 24 hpi. Uninduced cells were used as the control. Total RNA was isolated using a PureLink RNA minikit (Invitrogen). DNA was removed from extracted RNA using a Turbo DNA-free kit (Invitrogen). cDNA was generated using an iScript cDNA synthesis kit (Bio-Rad) and amplified on a T100 thermal cycler (Bio-Rad). qPCR was performed in triplicate using the KSHV-specific primers in Table 3 normalized to cellular cyclophilin (PPIA).
Immunofluorescence assay (IFA).
The iSLKs were grown on coverslips and transfected with either ORF59-HA full-length (2 μg) or ORF59-HA Δ351-396 plasmid (5 μg) using TransIT LT1 (Mirrus). Six hours posttransfection, cells were induced with 1 μg/ml of doxycycline for ∼18 hours. Cells were washed once with PBS, fixed in 4% paraformaldehyde for 15 min, washed with 1 ml of 1% BSA, and permeabilized with 0.5% Triton X-100 for 20 min. Cells were washed twice with 1% BSA and blocked with 3% goat serum for 30 min. Then, the cells were incubated with specific primary antibodies for 2 hours and then washed three times with 1% BSA. Alexa Fluor-conjugated secondary antibody (Invitrogen) was added for 30 min. Cells were washed three times with 1% BSA. Cells were mounted onto glass slides using ProLong Gold Antifade reagent with DAPI (Life Technologies) and visualized using a fluorescence microscope (Carl Ziess, Inc.). For infection experiments, iSLK BAC16 ORF59-HA WT and ORF59-HA deletion mutants were grown over coverslips and induced for 24 hours. IFA was performed as previously described.
Chemical protein cross-linking.
All cross-linking experiments were performed in freshly made solution of disuccinimidyl suberate (DSS) (Thermo Scientific; catalog no. A39267) dissolved in DMSO as a 50-mM stock solution. 293L transfected with 10 μg of pXI ORF59-HA, iSLK BAC16 ORF59-HA full-length and deletions were plated at 5 × 106 cells and induced with 0.5 mM sodium butyrate, 0.5 μg/ml of doxycycline, and 10 ng/ml of TPA for 24 hours. Cells were washed once with 1× Hanks balanced salt solution (HBSS), trypsinized, and suspended in PBS. Cells were spun down for 10 min at 1,200 rpm at room temperature, and the pellet was washed twice with phosphate-buffered saline (PBS). The cell pellet was resuspended to a final volume of 0.2 ml. Where indicated, samples were treated with 8 μl EtBr or DNase using a Turbo DNA-free kit (Invitrogen) for 30 min at 4°C or 37°C, respectively. Samples were treated with DSS at a 1:100 dilution to a final concentration of 500 μM for 30 min rotating at room temperature. The cross-linking reaction was stopped by quenching with 200 mM Tris-HCl, pH 7.5, to a final concentration of 1 mM and incubated for 15 min at room temperature. Finally, an equal amount of 2 × SDS loading buffer was added to the samples, and protein complexes were resolved on SDS-PAGE. HA-specific antibody was used to detect ORF59 monomers and higher-order oligomers. In addition, H1.2-, H2B-, and H3-specific antibodies were used.
Infectious virus assay.
The iSLK BAC16 WT and ORF59 deletions were induced for 5 days. Cells were harvested and freeze-thawed three times. Debris was spun down three times for 10 min at 4,000 rpm. Virus was pelleted by centrifugation at 28,000 rpm for 90 min. Pellet was resuspended in 0.5 ml of medium. 293L cells were infected at different serial dilutions, and GFP foci were counted at 72 hpi. GFP (+) cells/ml were plotted.
Quantification of viral rate of replication.
A total of 250,000 cells of iSLK BAC16 WT and ORF59 deletions were plated onto 3.5-cm dishes and induced with NaB/Dox and TPA for 0, 6, 24, and 72 hpi. Total genomic DNA was harvested by adding DNA extraction buffer (2% SDS, 100 mM Tris-HCl, pH 8.0, 10 mM EDTA, and 50 mg/ml of proteinase K). Cell lysates were incubated at 65°C for 1 to 2 hours, followed by phenol-chloroform extraction and then chloroform extraction. DNA was precipitated with 100% ethanol and resuspend in 1× TE. DNA was analyzed by qPCR with SsoAdvanced Universal Probes Supermix (Bio-Rad) according to manufacturer’s instructions. KSHV-specific primers were used to quantitate viral DNA synthesis and were normalized to 7SK. Each qPCR was performed in triplicate. Selected KSHV transcript primers/probes are shown in Table 3. Standard curves were generated using KSHV BAC16 DNA. The rate of viral DNA replication was calculated as base pairs synthesized per minute using the following formula as previously reported:
Chromatin immunoprecipitation (ChIP).
iDeal ChIP-seq kit for histones (Diagenode, Inc.) assay was performed following manufacturer’s instructions. Briefly, approximately 5 million cells were cross-linked by addition of 1% formaldehyde for 15 min. The reaction was quenched by the addition of glycine. Cells were washed twice with 1 ml of PBS and resuspended in 4 ml of iL1 buffer and incubated at for 4°C for 10 min followed by centrifugation at 500 × g for 5 min at 4°C. The cell pellets were resuspended in 4 ml of iL2 buffer and incubated for 4°C for 10 min followed by centrifugation at 500 × g for 5 min at 4°C. Pellet was resuspended in 0.5 ml of iS1 shearing buffer supplemented with protease inhibitors, and DNA was sonicated using disruptor Misonix 200 (Fisher) to an average fragment length of 200 to 300 bp. Cell debris was removed by centrifugation at 16,000 × g for 10 min at 4°C, and 5 μl of the total volume was saved as input. The remaining fraction was used for chromatin capture using HA-specific antibody to ORF59 and rotated overnight at 4°C. DNA-protein complexes were washed once with iW1 for 5 min rotating at 4°C and repeated with iW2, iW3, and iW4. Samples were eluted with iE1 and iE2 and reverse cross-linked overnight at 65°C. DNA was then precipitated using IPure beads v2 and eluted with 25 μl of buffer C. Purified DNA was used as the template for the qPCR assay using primers specific to 7SK, ORF26, and the RRE region of oriLyt (Table 3).
Data availability.
All sequences for the viruses used and generated in this study have been deposited in NCBI’s Sequence Read Archive (SRA) and are accessible through the following accession numbers: SRX7856828 (KSHV WT BAC16), SRX7856829 (KSHV BAC16 ORF59HA), SRX7856830 (KSHV BAC16 ORF59HA Δ2-50), SRX7856821 (KSHV BAC16 ORF59HA Δ51-100), SRX7856822 (KSHV BAC16 ORF59HA Δ101-150), SRX7856823 (KSHV BAC16 ORF59HA Δ151-200), SRX7856824 (KSHV BAC16 ORF59HA Δ201-250), SRX7856825 (KSHV BAC16 ORF59HA Δ251-300), SRX7856826 (KSHV BAC16 ORF59HA Δ301-350), SRX7856827 (KSHV BAC16 ORF59HA Δ351-396).
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (NIH) NIAID (R01 AI123011 to C.C.R.), the University of Nevada, Reno (UNR) IDeA Network of Biomedical Research Excellence (INBRE) (P20 GM103440 to I.V.G. and C.C.R.), and UNR startup funds to C.C.R.
We declare that we have no conflicts of interest with the contents of this article.
REFERENCES
- 1.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 2.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. 1995. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 3.Desrosiers RC, Sasseville VG, Czajak SC, Zhang X, Mansfield KG, Kaur A, Johnson RP, Lackner AA, Jung JU. 1997. A herpesvirus of rhesus monkeys related to the human Kaposi’s sarcoma-associated herpesvirus. J Virol 71:9764–9769. doi: 10.1128/JVI.71.12.9764-9769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosch ML, Harper E, Schmidt A, Strand KB, Thormahlen S, Thouless ME, Wang Y. 1999. Activation in vivo of retroperitoneal fibromatosis-associated herpesvirus, a simian homologue of human herpesvirus-8. J Gen Virol 80:467–475. doi: 10.1099/0022-1317-80-2-467. [DOI] [PubMed] [Google Scholar]
- 5.Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K. 2007. Human herpesviruses: biology, therapy, and immunoprophylaxis, Cambridge University Press, Cambridge, UK. [PubMed] [Google Scholar]
- 6.Cannon JS, Hamzeh F, Moore S, Nicholas J, Ambinder RF. 1999. Human herpesvirus 8-encoded thymidine kinase and phosphotransferase homologues confer sensitivity to ganciclovir. J Virol 73:4786–4793. doi: 10.1128/JVI.73.6.4786-4793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kedes DH, Ganem D. 1997. Sensitivity of Kaposi’s sarcoma-associated herpesvirus replication to antiviral drugs. Implications for potential therapy. J Clin Invest 99:2082–2086. doi: 10.1172/JCI119380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballestas ME, Kaye KM. 2001. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds TR DNA. J Virol 75:3250–3258. doi: 10.1128/JVI.75.7.3250-3258.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kedes DH, Lagunoff M, Renne R, Ganem D. 1997. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi’s sarcoma-associated herpesvirus. J Clin Invest 100:2606–2610. doi: 10.1172/JCI119804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballestas ME, Chatis PA, Kaye KM. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641–644. doi: 10.1126/science.284.5414.641. [DOI] [PubMed] [Google Scholar]
- 11.Komatsu T, Barbera AJ, Ballestas ME, Kaye KM. 2001. The Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen. Viral Immunol 14:311–317. doi: 10.1089/08828240152716565. [DOI] [PubMed] [Google Scholar]
- 12.AuCoin DP, Colletti KS, Xu Y, Cei SA, Pari GS. 2002. Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) contains two functional lytic origins of DNA replication. J Virol 76:7890–7896. doi: 10.1128/jvi.76.15.7890-7896.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossetto CC, Susilarini NK, Pari GS. 2011. Interaction of Kaposi’s sarcoma-associated herpesvirus ORF59 with oriLyt is dependent on binding with K-Rta. J Virol 85:3833–3841. doi: 10.1128/JVI.02361-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.AuCoin DP, Colletti KS, Cei SA, Papousková I, Tarrant M, Pari GS. 2004. Amplification of the Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 lytic origin of DNA replication is dependent upon a cis-acting AT-rich region and an ORF50 response element and the trans-acting factors ORF50 (K-Rta) and K8 (K-bZIP). Virology 318:542–555. doi: 10.1016/j.virol.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Weller SK, Coen DM. 2012. Herpes simplex viruses: mechanisms of DNA replication. Cold Spring Harb Perspect Biol 4:a013011. doi: 10.1101/cshperspect.a013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu FY, Ahn JH, Alcendor DJ, Jang WJ, Xiao J, Hayward SD, Hayward GS. 2001. Origin-independent assembly of Kaposi’s sarcoma-associated herpesvirus DNA replication compartments in transient cotransfection assays and association with the ORF-K8 protein and cellular PML. J Virol 75:1487–1506. doi: 10.1128/JVI.75.3.1487-1506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Ciustea M, Ricciardi RP. 2005. Processivity factor of KSHV contains a nuclear localization signal and binding domains for transporting viral DNA polymerase into the nucleus. Virology 340:183–191. doi: 10.1016/j.virol.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 18.McDowell ME, Purushothaman P, Rossetto CC, Pari GS, Verma SC. 2013. Phosphorylation of Kaposi’s sarcoma-associated herpesvirus processivity factor ORF59 by a viral kinase modulates its ability to associate with RTA and oriLyt. J Virol 87:8038–8052. doi: 10.1128/JVI.03460-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massimelli MJ, Majerciak V, Kang JG, Liewehr DJ, Steinberg SM, Zheng ZM. 2015. Multiple regions of Kaposi’s sarcoma-associated herpesvirus ORF59 RNA are required for its expression mediated by viral ORF57 and cellular RBM15. Viruses 7:496–510. doi: 10.3390/v7020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strahan RC, McDowell-Sargent M, Uppal T, Purushothaman P, Verma SC. 2017. KSHV encoded ORF59 modulates histone arginine methylation of the viral genome to promote viral reactivation. PLoS Pathog 13:e1006482. doi: 10.1371/journal.ppat.1006482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao Y, Chen J, Liao Q, Wu Y, Peng C, Chen X. 2013. Lytic infection of Kaposi’s sarcoma-associated herpesvirus induces DNA double-strand breaks and impairs non-homologous end joining. J Gen Virol 94:1870–1875. doi: 10.1099/vir.0.053033-0. [DOI] [PubMed] [Google Scholar]
- 22.Chung WC, Park JH, Kang HR, Song MJ. 2015. Downregulation of poly(ADP-Ribose) polymerase 1 by a viral processivity factor facilitates lytic replication of gammaherpesvirus. J Virol 89:9676–9682. doi: 10.1128/JVI.00559-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strahan R, Dabral P, Dingman K, Stadler C, Hiura K, Verma SC. 2018. Kaposi’s sarcoma-associated herpesvirus deregulates host cellular replication during lytic reactivation by disrupting the MCM complex through ORF59. J Virol 92:e00739-18. doi: 10.1128/JVI.00739-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossetto CC, Pari GS. 2011. Kaposi’s sarcoma-associated herpesvirus noncoding polyadenylated nuclear RNA interacts with virus- and host cell-encoded proteins and suppresses expression of genes involved in immune modulation. J Virol 85:13290–13297. doi: 10.1128/JVI.05886-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kornberg RD. 1974. Chromatin structure: a repeating unit of histones and DNA. Science 184:868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- 26.Oudet P, Gross-Bellard M, Chambon P. 1975. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell 4:281–300. doi: 10.1016/0092-8674(75)90149-x. [DOI] [PubMed] [Google Scholar]
- 27.Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. 1997. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 28.Armeev GA, Gribkova AK, Pospelova I, Komarova GA, Shaytan AK. 2019. Linking chromatin composition and structural dynamics at the nucleosome level. Curr Opin Struct Biol 56:46–55. doi: 10.1016/j.sbi.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Thoma F, Koller T, Klug A. 1979. Involvement of histone H1 in the organization of the nucleosome and of the salt-dependent superstructures of chromatin. J Cell Biol 83:403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Misteli T, Gunjan A, Hock R, Bustin M, Brown DT. 2000. Dynamic binding of histone H1 to chromatin in living cells. Nature 408:877–881. doi: 10.1038/35048610. [DOI] [PubMed] [Google Scholar]
- 31.Catez F, Ueda T, Bustin M. 2006. Determinants of histone H1 mobility and chromatin binding in living cells. Nat Struct Mol Biol 13:305–310. doi: 10.1038/nsmb1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hergeth SP, Schneider R. 2015. The H1 linker histones: multifunctional proteins beyond the nucleosomal core particle. EMBO Rep 16:1439–1453. doi: 10.15252/embr.201540749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hopcraft SE, Pattenden SG, James LI, Frye S, Dittmer DP, Damania B. 2018. Chromatin remodeling controls Kaposi’s sarcoma-associated herpesvirus reactivation from latency. PLoS Pathog 14:e1007267. doi: 10.1371/journal.ppat.1007267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toth Z, Brulois K, Jung JU. 2013. The chromatin landscape of Kaposi’s sarcoma-associated herpesvirus. Viruses 5:1346–1373. doi: 10.3390/v5051346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mercier A, Arias C, Madrid AS, Holdorf MM, Ganem D. 2014. Site-specific association with host and viral chromatin by Kaposi’s sarcoma-associated herpesvirus LANA and its reversal during lytic reactivation. J Virol 88:6762–6777. doi: 10.1128/JVI.00268-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu F, Zhou J, Wiedmer A, Madden K, Yuan Y, Lieberman PM. 2003. Chromatin remodeling of the Kaposi’s sarcoma-associated herpesvirus ORF50 promoter correlates with reactivation from latency. J Virol 77:11425–11435. doi: 10.1128/jvi.77.21.11425-11435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X, Lin K, Ricciardi RP. 2004. Human Kaposi’s sarcoma herpesvirus processivity factor-8 functions as a dimer in DNA synthesis. J Biol Chem 279:28375–28386. doi: 10.1074/jbc.M400032200. [DOI] [PubMed] [Google Scholar]
- 38.Zhou X, Liao Q, Ricciardi RP, Peng C, Chen X. 2010. Kaposi’s sarcoma-associated herpesvirus processivity factor-8 dimerizes in cytoplasm before being translocated to nucleus. Biochem Biophys Res Commun 397:520–525. doi: 10.1016/j.bbrc.2010.05.147. [DOI] [PubMed] [Google Scholar]
- 39.Baltz JL, Filman DJ, Ciustea M, Silverman JE, Lautenschlager CL, Coen DM, Ricciardi RP, Hogle JM. 2009. The crystal structure of PF-8, the DNA polymerase accessory subunit from Kaposi’s sarcoma-associated herpesvirus. J Virol 83:12215–12228. doi: 10.1128/JVI.01158-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tellinghuisen TL, Kuhn RJ. 2000. Nucleic acid-dependent cross-linking of the nucleocapsid protein of Sindbis virus. J Virol 74:4302–4309. doi: 10.1128/jvi.74.9.4302-4309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selzer L, Su Z, Pintilie GD, Chiu W, Kirkegaard K. 2020. Full-length three-dimensional structure of the influenza A virus M1 protein and its organization into a matrix layer. PLoS Biol 18:e3000827. doi: 10.1371/journal.pbio.3000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Majerciak V, Yamanegi K, Zheng ZM. 2006. Gene structure and expression of Kaposi’s sarcoma-associated herpesvirus ORF56, ORF57, ORF58, and ORF59. J Virol 80:11968–11981. doi: 10.1128/JVI.01394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majerciak V, Ni T, Yang W, Meng B, Zhu J, Zheng ZM. 2013. A viral genome landscape of RNA polyadenylation from KSHV latent to lytic infection. PLoS Pathog 9:e1003749. doi: 10.1371/journal.ppat.1003749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uppal T, Jha HC, Verma SC, Robertson ES. 2015. Chromatinization of the KSHV genome during the KSHV life cycle. Cancers (Basel) 7:112–142. doi: 10.3390/cancers7010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin K, Dai CY, Ricciardi RP. 1998. Cloning and functional analysis of Kaposi’s sarcoma-associated herpesvirus DNA polymerase and its processivity factor. J Virol 72:6228–6232. doi: 10.1128/JVI.72.7.6228-6232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiehl A, Dorsky DI. 1995. Bipartite DNA-binding region of the Epstein-Barr virus BMRF1 product essential for DNA polymerase accessory function. J Virol 69:1669–1677. doi: 10.1128/JVI.69.3.1669-1677.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li JS, Zhou BS, Dutschman GE, Grill SP, Tan RS, Cheng YC. 1987. Association of Epstein-Barr virus early antigen diffuse component and virus-specified DNA polymerase activity. J Virol 61:2947–2949. doi: 10.1128/JVI.61.9.2947-2949.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsurumi T, Daikoku T, Nishiyama Y. 1994. Further characterization of the interaction between the Epstein-Barr virus DNA polymerase catalytic subunit and its accessory subunit with regard to the 3′-to-5′' exonucleolytic activity and stability of initiation complex at primer terminus. J Virol 68:3354–3363. doi: 10.1128/JVI.68.5.3354-3363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gottlieb J, Challberg MD. 1994. Interaction of herpes simplex virus type 1 DNA polymerase and the UL42 accessory protein with a model primer template. J Virol 68:4937–4945. doi: 10.1128/JVI.68.8.4937-4945.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaudhuri M, Parris DS. 2002. Evidence against a simple tethering model for enhancement of herpes simplex virus DNA polymerase processivity by accessory protein UL42. J Virol 76:10270–10281. doi: 10.1128/jvi.76.20.10270-10281.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gulbis JM, Kelman Z, Hurwitz J, O’Donnell M, Kuriyan J. 1996. Structure of the C-terminal region of p21(WAF1/CIP1) complexed with human PCNA. Cell 87:297–306. doi: 10.1016/S0092-8674(00)81347-1. [DOI] [PubMed] [Google Scholar]
- 52.Krishna TS, Kong XP, Gary S, Burgers PM, Kuriyan J. 1994. Crystal structure of the eukaryotic DNA polymerase processivity factor PCNA. Cell 79:1233–1243. doi: 10.1016/0092-8674(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 53.Gallo ML, Jackwood DH, Murphy M, Marsden HS, Parris DS. 1988. Purification of the herpes simplex virus type 1 65-kilodalton DNA-binding protein: properties of the protein and evidence of its association with the virus-encoded DNA polymerase. J Virol 62:2874–2883. doi: 10.1128/JVI.62.8.2874-2883.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gottlieb J, Marcy AI, Coen DM, Challberg MD. 1990. The herpes simplex virus type 1 UL42 gene product: a subunit of DNA polymerase that functions to increase processivity. J Virol 64:5976–5987. doi: 10.1128/JVI.64.12.5976-5987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamatake RK, Bifano M, Tenney DJ, Hurlburt WW, Cordingley MG. 1993. The herpes simplex virus type 1 DNA polymerase accessory protein, UL42, contains a functional protease-resistant domain. J Gen Virol 74:2181–2189. doi: 10.1099/0022-1317-74-10-2181. [DOI] [PubMed] [Google Scholar]
- 56.Zuccola HJ, Filman DJ, Coen DM, Hogle JM. 2000. The crystal structure of an unusual processivity factor, herpes simplex virus UL42, bound to the C terminus of its cognate polymerase. Mol Cell 5:267–278. doi: 10.1016/S1097-2765(00)80422-0. [DOI] [PubMed] [Google Scholar]
- 57.Randell JC, Coen DM. 2004. The herpes simplex virus processivity factor, UL42, binds DNA as a monomer. J Mol Biol 335:409–413. doi: 10.1016/j.jmb.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 58.Appleton BA, Loregian A, Filman DJ, Coen DM, Hogle JM. 2004. The cytomegalovirus DNA polymerase subunit UL44 forms a C clamp-shaped dimer. Mol Cell 15:233–244. doi: 10.1016/j.molcel.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 59.Komazin-Meredith G, Mirchev R, Golan DE, van Oijen AM, Coen DM. 2008. Hopping of a processivity factor on DNA revealed by single-molecule assays of diffusion. Proc Natl Acad Sci U S A 105:10721–10726. doi: 10.1073/pnas.0802676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Appleton BA, Brooks J, Loregian A, Filman DJ, Coen DM, Hogle JM. 2006. Crystal structure of the cytomegalovirus DNA polymerase subunit UL44 in complex with the C terminus from the catalytic subunit. Differences in structure and function relative to unliganded UL44. J Biol Chem 281:5224–5232. doi: 10.1074/jbc.M506900200. [DOI] [PubMed] [Google Scholar]
- 61.Komazin-Meredith G, Petrella RJ, Santos WL, Filman DJ, Hogle JM, Verdine GL, Karplus M, Coen DM. 2008. The human cytomegalovirus UL44 C clamp wraps around DNA. Structure 16:1214–1225. doi: 10.1016/j.str.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Makhov AM, Subramanian D, Holley-Guthrie E, Kenney SC, Griffith JD. 2004. The Epstein-Barr virus polymerase accessory factor BMRF1 adopts a ring-shaped structure as visualized by electron microscopy. J Biol Chem 279:40358–40361. doi: 10.1074/jbc.M408733200. [DOI] [PubMed] [Google Scholar]
- 63.Murayama K, Nakayama S, Kato-Murayama M, Akasaka R, Ohbayashi N, Kamewari-Hayami Y, Terada T, Shirouzu M, Tsurumi T, Yokoyama S. 2009. Crystal structure of Epstein-Barr virus DNA polymerase processivity factor BMRF1. J Biol Chem 284:35896–35905. doi: 10.1074/jbc.M109.051581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakayama S, Murata T, Yasui Y, Murayama K, Isomura H, Kanda T, Tsurumi T. 2010. Tetrameric ring formation of Epstein-Barr virus polymerase processivity factor is crucial for viral replication. J Virol 84:12589–12598. doi: 10.1128/JVI.01394-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loregian A, Sinigalia E, Mercorelli B, Palù G, Coen DM. 2007. Binding parameters and thermodynamics of the interaction of the human cytomegalovirus DNA polymerase accessory protein, UL44, with DNA: implications for the processivity mechanism. Nucleic Acids Res 35:4779–4791. doi: 10.1093/nar/gkm506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dembowski JA, DeLuca NA. 2015. Selective recruitment of nuclear factors to productively replicating herpes simplex virus genomes. PLoS Pathog 11:e1004939. doi: 10.1371/journal.ppat.1004939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manska S, Octaviano R, Rossetto CC. 2020. 5-Ethynyl-2′-deoxycytidine and 5-ethynyl-2′-deoxyuridine are differentially incorporated in cells infected with HSV-1, HCMV, and KSHV viruses. J Biol Chem 295:5871–5890. doi: 10.1074/jbc.RA119.012378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tischer BK, Smith GA, Osterrieder N. 2010. En passant mutagenesis: a two step markerless red recombination system. Methods Mol Biol 634:421–430. doi: 10.1007/978-1-60761-652-8_30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All sequences for the viruses used and generated in this study have been deposited in NCBI’s Sequence Read Archive (SRA) and are accessible through the following accession numbers: SRX7856828 (KSHV WT BAC16), SRX7856829 (KSHV BAC16 ORF59HA), SRX7856830 (KSHV BAC16 ORF59HA Δ2-50), SRX7856821 (KSHV BAC16 ORF59HA Δ51-100), SRX7856822 (KSHV BAC16 ORF59HA Δ101-150), SRX7856823 (KSHV BAC16 ORF59HA Δ151-200), SRX7856824 (KSHV BAC16 ORF59HA Δ201-250), SRX7856825 (KSHV BAC16 ORF59HA Δ251-300), SRX7856826 (KSHV BAC16 ORF59HA Δ301-350), SRX7856827 (KSHV BAC16 ORF59HA Δ351-396).