Even after decades of intensive research efforts, a safe and efficacious RSV vaccine remains elusive. Expression of heterologous antigens from rVSV vectors has demonstrated several practical and safety advantages over other virus vector systems and live attenuated vaccines.
KEYWORDS: respiratory syncytial virus, cotton rat, vesicular stomatitis virus, fusion protein, G protein
ABSTRACT
Respiratory syncytial virus (RSV) is a major cause of lower respiratory tract (LRT) infections, with increased severity in high-risk human populations, such as infants, the immunocompromised, and the elderly. Although the virus was identified more than 60 years ago, there is still no licensed vaccine available. Over the years, several vaccine delivery strategies have been evaluated. In this study, we developed two recombinant vesicular stomatitis virus (rVSV) vector-based vaccine candidates expressing the RSV-G (attachment) protein (rVSV-G) or F (fusion) protein (rVSV-F). All vectors were evaluated in the cotton rat animal model for their in vivo immunogenicity and protective efficacy against an RSV-A2 virus challenge. Intranasal (i.n.) delivery of rVSV-G and rVSV-F together completely protected the lower respiratory tract (lungs) at doses as low as 103 PFU. In contrast, doses greater than 106 PFU were required to protect the upper respiratory tract (URT) completely. Reimmunization of RSV-immune cotton rats was most effective with rVSV-F. In immunized animals, overall antibody responses were sufficient for protection, whereas CD4 and CD8 T cells were not necessary. A prime-boost immunization regimen increased both protection and neutralizing antibody titers. Overall, mucosally delivered rVSV-vector-based RSV vaccine candidates induce protective immunity and therefore represent a promising immunization regimen against RSV infection.
IMPORTANCE Even after decades of intensive research efforts, a safe and efficacious RSV vaccine remains elusive. Expression of heterologous antigens from rVSV vectors has demonstrated several practical and safety advantages over other virus vector systems and live attenuated vaccines. In this study, we developed safe and efficacious vaccine candidates by expressing the two major immunogenic RSV surface proteins in rVSV vectors and delivering them mucosally in a prime-boost regimen. The main immune parameter responsible for protection was the antibody response. These vaccine candidates induced complete protection of both the upper and lower respiratory tracts.
INTRODUCTION
Worldwide, respiratory syncytial virus (RSV) is the most common viral cause of bronchiolitis and pneumonia in infants and children under 5 years of age leading to hospitalization. Apart from disease caused in children and adults, RSV causes pneumonia in the elderly, chronically immunocompromised individuals, and those suffering from cardiopulmonary illnesses such as cystic fibrosis (1, 2). RSV was associated with hospitalizations 16 times more frequently than influenza virus in children under 1 year of age and required more caregiver time and resource utilization than influenza virus (3). In the postneonatal period, between 1 and 12 months of life, RSV is second only to malaria as a single-agent cause of mortality (4).
The causative agent, human RSV, is an enveloped RNA virus with 15.2-kb negative-sense genome that is nonsegmented, and it belongs to the family Pneumoviridae (5). The RSV genome encodes a total of eight structural proteins. The virion envelope has three glycoproteins, namely, the glycoprotein responsible for attachment (G); the fusion protein (F) for penetration of the cell membrane; and the small hydrophobic protein (SH), which inhibits apoptosis caused by tumor necrosis factor beta (TNF-β) (6). The envelope proteins G and F are the major targets of neutralizing antibodies against RSV, which makes them suitable antigens for vaccine development (7–10).
Although RSV was discovered and characterized in the mid-1950s, there is still no licensed vaccine available for human use. In the 1960s, a formalin-inactivated RSV vaccine (FI-RSV) was developed that led to more severe disease after exposure to wild-type virus (11, 12). Since then, efforts to develop RSV vaccines have continued, resulting in recent promising RSV vaccine strategies that have been evaluated both in animal models and in human clinical trials, such as live attenuated viruses, subunit vaccines (13), DNA vaccines, and viral vector-based vaccines (14). However, many of these attempts have not succeeded in developing a safe and efficacious vaccine candidate due to limitations such as incomplete attenuation, poor immunogenicity, poor antigen expression, or safety issues (1, 15, 16).
Among the available vaccine development strategies, expression of viral target antigens in vaccine vectors represents a promising approach to develop a safe and efficacious RSV vaccine (17). Several such approaches have been reported with RSV proteins expressed from Newcastle disease virus (18), Sendai virus (19), parainfluenza virus (20), vaccinia virus (21, 22), alphavirus (23), adenovirus (24), and vesicular stomatitis virus (rVSV) (17, 25). Among them, the rVSV vector system has been demonstrated to have potential as a vaccine expression platform for many viral diseases, such as HIV (26), influenza virus (27, 28), measles virus (29, 30), and Ebola virus (31), among others. However, so far only the VSV-based Ebola vaccine (rVSV-ZEBOV) has been approved by the FDA (32, 33).
VSV is the prototypic virus of the family Rhabdoviridae and is characterized by a nonsegmented, negative-sense RNA genome with a simple genetic organization encoding five structural proteins. The recombinant VSV vectors have several practical advantages over other viral vector systems. Recombinant VSVs can efficiently incorporate up to 4 kb of foreign DNA into their genome (thus enabling coexpression of multiple heterologous proteins), grow to very high titers in almost all mammalian cell lines (>109 PFU/ml) and do not undergo genetic reassortment or recombination. In the human population, the seroprevalence and pathogenicity of rVSV is very low, and rVSVs are a strong inducer of innate, humoral, and cellular immunity, both systemically and at mucosal sites (34).
In the present study, we used a recombinant VSV vector to express the full-length RSV-G or RSV-F genes as an additional gene unit between the rVSV-G glycoprotein and the large protein (polymerase). To enhance the immunogenicity of the rVSV-G or rVSV-F vectors, we used an intranasal (i.n.) prime-boost vaccination regimen.
RESULTS
Kinetics of expression of recombinant proteins by recombinant VSVs expressing RSV-G and RSV-F proteins.
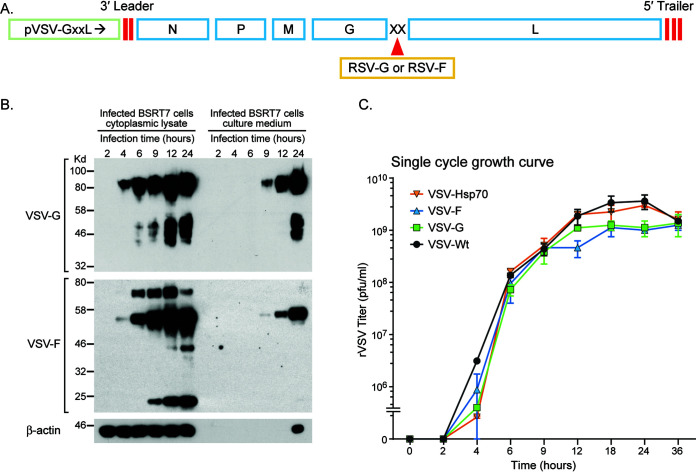
We constructed and rescued VSV expressing the RSV-G or the RSV-F protein in the same manner as that previously described for other VSV recombinants (35, 36), by inserting these transgenes in the VSV G-L gene junction (Fig. 1A). The target genes in the recovered viruses were confirmed by reverse transcription-PCR (RT-PCR) and sequence analysis. The kinetics of RSV-G and RSV-F protein expression by these recombinant VSVs was examined by Western blotting (Fig. 1B). Both proteins were expressed in the infected cells and released into the culture medium. In the cell lysates, the proteins were detectable as early as 4 h postinfection. In the culture medium, RSV-G and RSV-F were detectable at 9 h postinfection. Cumulative aggregation of a protein band lower than 25 kDa was observed only in the rVSV-F-infected cells, possibly due to degradation of the F protein by cellular proteases. In order to assess whether the RSV glycoproteins were incorporated into the VSV envelope, gradient-purified VSV recombinants were tested by enzyme-limited immunosorbent assay (ELISA) with an RSV-specific antiserum with negative results (data not shown). A single-step growth curve analysis in BSRT7 cells was performed to compare the kinetics of released infectious VSV recombinant virus progeny (Fig. 1C). Wild-type VSV (rVSV-Wt) and a control rVSV expressing HSP70 showed steady increase in progeny release, which peaked at 24 h postinfection. The growth of rVSV-G and rVSV-F plateaued at 3- to 4-fold lower levels compared to that of rVSV-wt. Overall, growth kinetics were similar to those of other reported recombinant VSVs (37).
FIG 1.
Generation and in vitro characterization of recombinant VSV vectors expressing RSV-G or RSV-F protein. (A) The gene coding for the full-length RSV-G or RSV-F protein was inserted at the junction of VSV glycoprotein and large polymerase genes (G-L junction) in the vector pVSV(+)-GxxL. N, nucleocapsid gene; P, phosphoprotein gene; M, matrix protein gene; G, glycoprotein gene; L, large polymerase gene. (B) To determine the kinetics of protein expression, BSRT7 cells were infected with rVSV-G or rVSV-F at a multiplicity of infection (MOI) of 4. Lysates of the cytoplasmic extracts and infected cell culture medium samples were harvested at the indicated time points postinfection. Equal amounts of total cytoplasmic lysate and culture medium were analyzed by SDS-PAGE, followed by Western blotting using the antibodies described in Materials and Methods. A representative blot of the β-actin as a control protein is also shown. (C) To determine the growth curve of the recombinant VSVs, 90% confluent BSRT7 cells were infected with an MOI of 4, and samples of the culture medium were harvested at the indicated time points. The virus titer was determined by plaque assay on Vero cells. Recombinant wild-type VSV and an unrelated VSV recombinant expressing HSP70 (36) were used as controls. Titers (PFU/ml) represent the averages of the results of two independent experiments.
Mucosal immunization protects the upper and lower respiratory tract of cotton rats.
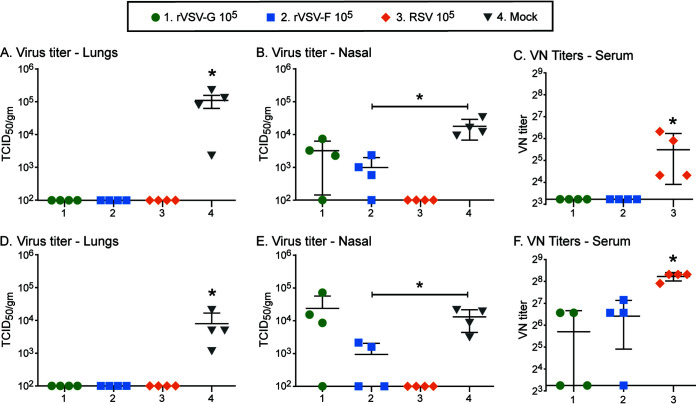
To determine the ability of these recombinant viruses to induce a protective immune response, cotton rats were immunized first subcutaneously with 105 PFU of rVSV-G, rVSV-F, or, as a control, 105 50% tissue culture infectious dose (TCID50) of RSV (Fig. 2A to C). Four weeks later, all animals were infected intranasally with 105 TCID50 of RSV. At 4 days postchallenge, no infectious RSV was detected in the lungs of rVSV-G-, rVSV-F-, or RSV-immunized cotton rats (Fig. 2A). The same was true in nasal samples for RSV-immunized animals. In contrast, the group immunized with rVSV-F was partially protected, whereas animals immunized with rVSV-G were not (Fig. 2B). The incomplete protection of the nose by the VSV recombinants correlated with significantly lower (P < 0.05) serum neutralization titers compared to those of RSV-immunized animals (Fig. 2C). Similarly, RSV-specific mucosal IgG and IgA in both lung and nasal tissue and circulatory IgG antibodies levels in rVSV-G-immunized animals were lower than those in RSV-F- and rVSV-F-immunized animals (data not shown). Following a similar experimental design, cotton rats were immunized with rVSV-G, rVSV-F, or RSV by the intranasal route and infected subsequently with RSV. Intranasal immunization with rVSV-G or rVSV-F resulted in similar results to subcutaneous immunization (complete protection of the lung [Fig. 1D] and partial protection of the nose [Fig. 1E]) and increased induction of RSV-neutralizing antibodies (Fig. 1F). Levels of IgA and IgG in both lung and nasal tissue were comparable in RSV-, rVSV-G-, and rVSV-F-immunized animals (data not shown) and were not tested in subsequent studies.
FIG 2.
One-dose immunization of cotton rats with rVSV-G or rVSV-F. Cotton rats were immunized subcutaneously (A to C) or intranasally (D to F) with 105 PFU of rVSV-G or rVSV-F, or with 105 50% tissue culture infective dose (TCID50) of RSV. Serum samples were collected 28 days postimmunization, and cotton rats were intranasally challenged with 105 TCID50 of RSV. At 4 days postchallenge, animals were euthanized, and lungs and nasal turbinates were harvested. RSV loads in the lungs (A and D) and in nasal homogenates (B and E) were determined. RSV neutralization antibody (VN) titers were determined in serum samples (C and F). Each group consisted of four cotton rats (n = 4), and each dot represents the indicated titer from an individual animal. A statistically significant difference from the naive control group is indicated by * (P < 0.05).
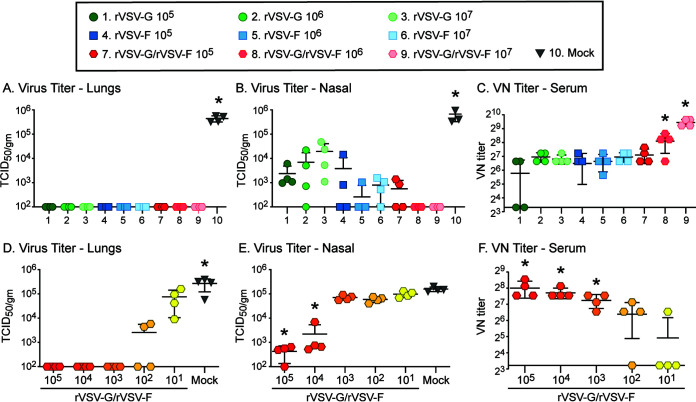
To improve protection after immunization via the intranasal route, increasing doses of the individual VSV recombinants and combinations of rVSV-G and rVSV-F were tested (Fig. 3A to C). Cotton rats were immunized once with a low (105 PFU), medium (106 PFU), or high (107 PFU) dose of either individual rVSV-G or rVSV-F or with combined rVSV-G and rVSV-F (rVSV-G/rVSV-F in equal doses). All doses protected the lungs of immunized animals against RSV challenge and reduced viral titers in the nose (Fig. 3A and B). However, only the medium and high doses (106 or 107 PFU each, groups 8 and 9) of the rVSV-G/rVSV-F combination elicited complete protection of the upper respiratory tract (URT) (Fig. 3B), which correlated with the enhanced titers of neutralizing antibodies (Fig. 3C). These antibody titers were higher than antibody titers after immunization with rVSV-G or rVSV-F (at a dose of 106 PFU or 107 PFU) alone (Fig. 3C). These results demonstrated that rVSV-G or rVSV-F induced dose-dependent protection, but complete protection of the upper respiratory tract was achieved only through a combination of both. To determine the protective capacity of lower doses of rVSV-G/rVSV-F, cotton rats were immunized intranasally with 105, 104, 103, 102, or 101 PFU each of rVSV-G/rVSV-F and challenged after 4 weeks (Fig. 3D to F). The lungs were protected with a rVSV-G/rVSV-F combination dose as low as 103 PFU (Fig. 3D). In the nose, 105 and 104 PFU led to partial protection (Fig. 3E). Virus-neutralizing antibodies were detectable in animals which received as low a dose as102 PFU of rVSV-G/rVSV-F (Fig. 3F). These results demonstrate that protection of the lower respiratory tract (LRT) could be achieved by a dose as low as 103 PFU of the rVSV-G/rVSV-F combination, but a significantly higher dose of 106 or 107 PFU of each was required to induce protective immunity in the upper respiratory tract. To further assess the role of G and F proteins of RSV, we assessed the boosting effect of rVSV-G and rVSV-F in RSV-seropositive animals. Cotton rats were immunized intranasally with RSV and intranasally boosted after 8 weeks with either 107 PFU of rVSV-G or rVSV-F. At 4 weeks postboost, the boost with rVSV-F increased neutralizing antibody titers to 4,400 ± 1,442 (versus RSV-immunized ones at 245 ± 147). A booster immunization with rVSV-G did not lead to an increase in neutralizing antibodies (data not shown).
FIG 3.
Dose-dependent protection and humoral immune responses induced by rVSV-G and rVSV-F. (A to C) Cotton rats were immunized intranasally with 105, 106, or 107 PFU of rVSV-G, rVSV-F, or rVSV-G/rVSV-F (each). Serum samples were collected 28 days postimmunization, and cotton rats were intranasally challenged with 105 TCID50 of RSV A2. At 4 days postchallenge, viral titers were statistically significantly different in the immunized versus naive group in the lungs (A) and nasal homogenates (B) (P > 0.05 and lower). Serum samples were tested for virus-neutralizing antibody (C), and groups marked with an asterisk (*) had statistically significantly higher levels (P > 0.05). (D to F) Cotton rats were immunized intranasally with 105, 104, 103, 102, or 101 PFU each of rVSV-G/rVSV-F. Serum samples were collected 28 days postimmunization, and cotton rats were intranasally challenged with 105 TCID50 of RSV A2. At 4 days postchallenge, viral titers were statistically significantly lower (P > 0.05 and lower) in the lungs of immunized animals (D). Viral titers were statistically significantly lower in two groups in the nasal homogenates (E) (P > 0.01). Serum samples were tested for virus-neutralizing antibody (F), and groups marked with an asterisk (*) had statistically significantly higher levels (P > 0.05). Each group consisted of four cotton rats (n = 4), and each dot represents the indicated titer from an individual animal.
CD4+ and CD8+ T-cell-mediated immunity induced by rVSV-G and rVSV-F coimmunization.
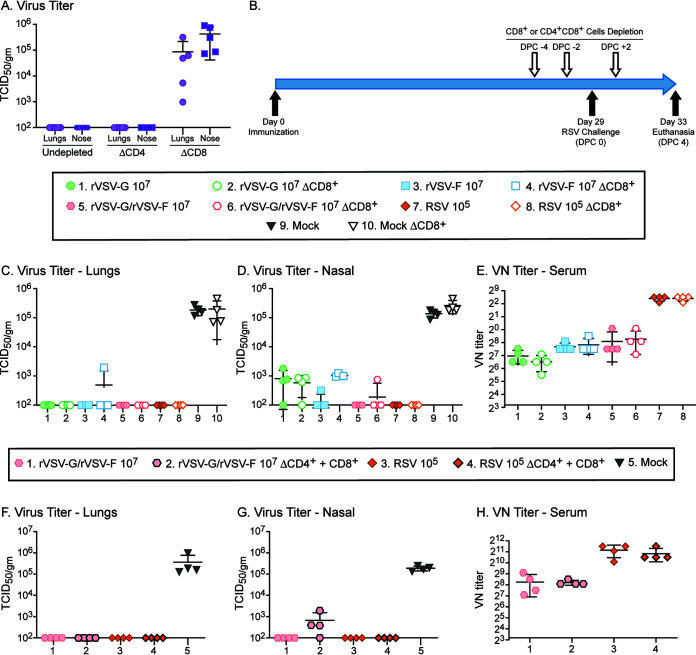
Although neutralizing antibodies clearly correlated with protection against RSV infection, we also assessed the T-cell response after immunization. Since CD4+ T cells play an important role in shaping CD8+ T-cell and B-cell mediated responses, and CD8+ T cells directly contribute to clearing virus infection, we studied the role of CD8+ T cells alone or in interaction with CD4+ T cells. In naive cotton rats, RSV was cleared 7 days after infection, and CD4+ T cell depletion did not affect clearance (Fig. 4A). In contrast, cotton rats depleted of CD8+ T cells still had titers of ∼104.5 TCID50/g tissue in the lungs and nasal tissues (Fig. 4A). To analyze the role of T-cell responses after immunization, we depleted CD8 T cells or both CD8 and CD4 T cells in cotton rats immunized with rVSV-F, rVSV-G, or rVSV-F/rVSV-G. Neither depletion of CD8+ T cells (Fig. 4C) nor combined depletion of CD4+ and CD8+ T cells affected protection of the lower respiratory tract (Fig. 4F). In the upper respiratory tract, protection was reduced in three cotton rats immunized with rVSV-G/rVSV-F after depletion of CD4 and CD8 T cells (Fig. 4G). T-cell depletion did not affect the induction of RSV neutralization (VN) antibodies (Fig. 4E and H). Thus, these results clearly demonstrated that CD8+ T-cell responses are crucial for clearance of virus during primary infection but are not necessary in vaccinated animals.
FIG 4.
Protection and humoral immune responses in rVSV-G/rVSV-F-immunized cotton rats do not rely on T-cell responses. (A) Naive cotton rats overcome RSV infection by day 7 in both lung and nasal tissue. Depletion of CD4 T cells does not change viral clearance whereas depletion of CD8 T cells leads to delayed viral clearance (P > 0.001). (B) Schematic representation of the depletion regimen and animal study design. (C to E) Cotton rats were immunized intranasally with 107 PFU of rVSV-G, rVSV-F, or rVSV-G/rVSV-F, or with 105 TCID50 of RSV and were depleted of CD8+ T cells before RSV challenge 4 weeks later. (F to H) Cotton rats were immunized intranasally with 107 PFU of rVSV-G/rVSV-F or with 105 TCID50 of RSV and were depleted of both CD4 and CD8 T cells before RSV challenge 4 weeks later. Four days later, homogenates of lungs (C and F) and nasal turbinates (D and G) were titrated for RSV. Neutralizing antibodies were measured in serum samples from day 28 after immunization (E and H). Each group consisted of four cotton rats (n = 4), and each dot represents the indicated titer from an individual animal. Dotted line represents the threshold level of detection. All immunized groups were protected in lungs and nasal turbinates (P > 0.001). Serum levels of neutralizing antibodies were higher than those in the other immunized groups (D) (P < 0.05).
Antibodies mediate protection of rVSV-G/rVSV-F-immunized cotton rats.
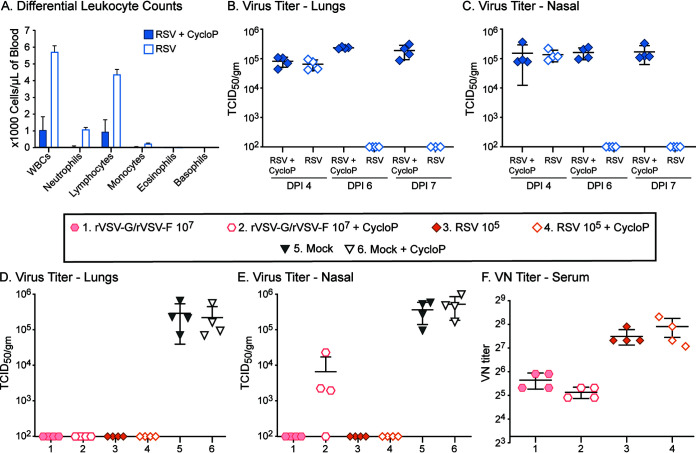
Cellular (innate) immune parameters other than CD4+ and CD8+ T cells might also contribute to the protection against RSV infection. In order to suppress overall cellular immune responses, we treated cotton rats with cyclophosphamide (38, 39). In unimmunized, RSV-infected cotton rats, cyclophosphamide treatment resulted in significantly (P < 0.05) reduced white blood cell counts (on day 7 post RSV infection) (Fig. 5A) and in extended replication of RSV (Fig. 5B and C). In cotton rats immunized with 107 PFU each of rVSV-G/rVSV-F, cyclophosphamide treatment had no effect on the protection of the lungs (Fig. 5D), whereas it had a moderate effect on the protection of nasal passages of rVSV-G/rVSV-F-immunized animals (Fig. 5E). Cyclophosphamide treatment did not influence existing titers of neutralizing antibodies (Fig. 5F). These results also demonstrated that, in cotton rats immunized with either RSV or rVSV-G/rVSV-F, protection was predominantly mediated by antibody responses and that overall cellular immunity played only a minor role in the protection of the upper respiratory tract.
FIG 5.
Protection and humoral immune responses in cyclophosphamide-treated cotton rats immunized with rVSV-G/rVSV-F. (A to C) Cyclophosphamide treatment leads to a severe reduction in the different leukocyte counts (P > 0.001) (A). Naive cotton rats clear RSV infection by day 6, whereas cyclophosphamide-treated cotton rats still have high virus loads in lung (B) and nasal tissue (C) on day 6 and 7 (P > 0.001). (D to F) Cotton rats were immunized intranasally with 107 PFU of rVSV-G/rVSV-F or with 105 TCID50 of RSV and treated with cyclophosphamide or left untreated. Serum samples were collected 28 days postimmunization, and all of the animals were intranasally challenged with 105 TCID50 of RSV. At 4 days postchallenge, RSV loads were determined in lungs (D) and nasal turbinates (E), and neutralizing antibody titers were measured (F). Virus titers were significantly reduced in immunized groups (P > 0.001). Each group consisted of four cotton rats (n = 4), and each dot represents the indicated titer from an individual animal.
Moderately enhanced anamnestic protective response upon prime-boost immunization strategy.
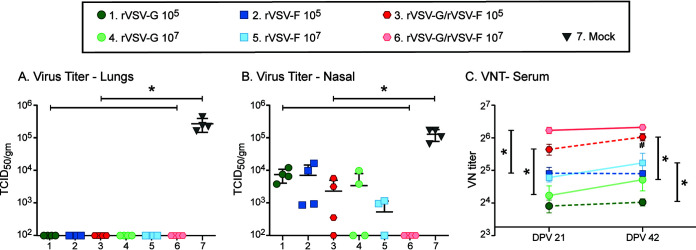
In order to further improve the level of virus-neutralizing antibody titers and protection, we used a prime-boost immunization strategy with a low dose (105 PFU) and a high dose (107 PFU) of rVSV-F, rVSV-G, or rVSV-F/rVSV-G (Fig. 6A to C). Cotton rats were boosted with the same dose 3 weeks after primary vaccination, and the animals were challenged 3 weeks after the second/booster immunization. All groups were protected after RSV challenge in the lungs (Fig. 6A), and immunization with a high dose of rVSV-G/rVSV-F protected the nose. However, the other groups were only partially protected in the nose (Fig. 6B). Immunization with a high dose increased antibody titers after the boost more than immunization with a low dose (Fig. 6C). These results indicated that low-dose prime-boost immunization did not protect the upper respiratory tract, whereas a high dose of rVSV-G/rVSV-F induced protection of the upper respiratory tract, along with moderately enhanced VN antibody levels.
FIG 6.
Protection and humoral immune responses induced by prime-boost immunization regimen of the VSV recombinants. (A to C) Cotton rats were immunized twice intranasally, with a 21-day interval between doses of 105 or 107 PFU of rVSV-G, rVSV-F, or rVSV-G/rVSV-F (each). Serum samples were collected on the day of booster immunization (day 21) and on the day of challenge (day 42), and all the animals were intranasally challenged with 105 TCID50 of RSV. At 4 days postchallenge, all the animals were euthanized, and lungs and nasal turbinates were harvested. Challenge RSV loads were determined in the lungs (A) and nasal homogenates (B), and endpoint RSV neutralization antibody (VN) titers were determined in serum samples (C). Each group consisted of four cotton rats (n = 4), and each dot represents the indicated titer from an individual animal (for line diagrams, each dot represents the mean VN titer of the group). An asterisk (*) represents a statistically significant (P < 0.05) difference between the indicated groups.
DISCUSSION
In the past 2 decades, recombinant VSV has been proven to be an excellent in vivo delivery system. Multiple VSV vector-based biologics are at different stages of preclinical and clinical trials for vaccine administration or cancer therapy (31, 40–43). Notably, a recombinant VSV vaccine vector expressing a glycoprotein of Ebola virus (VSVΔG-ZEBOV-GP) has been approved for human application and shown to be effective (32, 33). In this study, we expressed RSV-G and RSV-F proteins in VSV vectors and evaluated the recombinant viruses for their in vitro growth characteristics and in vivo protective immunogenicity. Similarly to previous studies, insertion of a foreign gene between the G-L junction of VSV resulted in stable vectors, and their in vitro cytopathic effect and growth kinetics resembled that of wild-type VSV (37, 44, 45). In terms of protection, low-dose (105 PFU) immunization with either rVSV-G or rVSV-F protected the lower respiratory tract (LRT), whereas even at a higher dose (107 PFU) immunization did not protect the upper respiratory tract (URT) in all the immunized animals. This is in alignment with several studies which suggest that protection of the LTR is more easily achieved than protection of the URT (46, 47). In contrast, in a previously reported study in cotton rats, low-dose (104 PFU) immunization with a parainfluenza virus 5 (PIV5) vector expressing RSV-G protein elicited partial protection in the lower respiratory tract and complete protection in the upper respiratory tract in the absence of detectable VN antibodies (48). The protection was attributed to nonneutralizing antibodies (48–51). However, when the related recombinant PIV 3 (rPIV3) vector system was used, the RSV-F protein induced complete protection in the LRT and partial protection in the URT (52). In alignment with these findings are our data, which found rVSV-G and rVSV-F administration by the intranasal route to be superior to that by the subcutaneous route. For human immunization, an advantage of the i.n. route would be that it circumvents the neutralizing effects of serum-derived maternal antibodies on the vaccine virus, which can greatly reduce vaccine efficiency in high-risk infants (53, 54). Furthermore, VSV mRNA persists longer (beyond 20 days) after i.n. inoculation than after intramuscular inoculation (4 to 10 days) (55), suggesting that protracted antigen presentation by the i.n. route could contribute in further enhancing ongoing antiviral immune response, especially in inducing a strong T-cell response. For translation of VSV as a biologic into clinical application, not only the efficacy but also the safety of VSV vectors has to be taken into account. The intranasal application of vaccines is not current clinical practice and might raise safety concerns, given that VSV is neurotropic in mice (56), although these findings could not be replicated in nonhuman primates (57, 58). A number of reports have provided data on safety of recombinant VSV in pigs (a natural host [59]), as a vector for oncolytic therapy in dogs (41) and humans (42, 43), and as a vaccine vector (31). Another issue to consider in RSV vaccine development is the question of which group (infants, children, the elderly, adults, pregnant mothers, and the immunocompromised [60]) may be immunized with which vaccine. It is quite possible that each of these groups will receive a different vaccine, thus balancing the need for safety with efficacy of the respective vaccine.
In humans, RSV infection induces predominantly an F-specific antibody response (61), and several F protein-based vaccine candidates are at various stages of development or clinical trials (11, 62, 63). However, in children, the development of G-specific antibodies correlates with a milder disease. In this context, it is interesting to note that rVSV-F, but not rVSV-G, was able to induce high levels of neutralizing antibodies in RSV-immune cotton rats. In our studies, the combination of rVSV-F and rVSV-G was able to induce the highest level of protection in both the LTR and the UTR but did not induce neutralizing antibody titers to levels comparable to those elicited by RSV-A2. This contrasts with a previous study in mice in which intranasal immunization of rVSV expressing spike protein of severe acute respiratory syndrome coronavirus (SARS-CoV) type I induced higher level of VN antibodies than wild-type SARS-CoV (64). In terms of vector development, it may be of interest to express G and F in one VSV vector, as well as to test variants of the G and F protein for putatively better immunity.
In this study, the rVSV vectors were used to answer the question of which parts of the immune system are stimulated by vaccination and are necessary and sufficient to protect the respiratory tract. The depletion of T cells did not abrogate protection, and even suppression of other immune cells through cyclophosphamide was not detrimental. This is remarkable, as cyclophosphamide treatment of naive cotton rats leads to high level of RSV replication for at least 2 weeks (38). In this study, antibodies seemed to be the major protective immune parameter, with a strong correlation with neutralizing antibodies. In patients, the level of protection conferred by anti-RSV neutralizing antibodies titers varies by study, but neutralizing antibody levels are considered to correlate with protection from severe disease (8, 65). However, a few studies have demonstrated that nonneutralizing antibodies may also accomplish protection. This was true in mice that were protected against RSV infection by nonneutralizing antibodies after immunization with VSV vectors expressing modified RSV-G protein (VSVΔG or VSV-Gstem) (17, 25). It has also been suggested that G-specific antibodies may not neutralize in vitro but are able to protect in vivo (17, 50, 66). Similar observations have been reported previously and attributed to the possibility that protection is correlated more with ELISA IgG levels than VN titers (25). Similarly, vaccinia virus expressing RSV-G induced protection with no detectable VN titers, suggesting that direct antibody neutralization is not the sole mechanism of protection (9). Other contributing components to limit the spread of virus in vivo include complement activation and antibody-dependent cellular cytotoxicity (ADCC). In the current study, antibody levels estimated by ELISA were comparable or higher in rVSV-G/rVSV-F groups than in RSV-immunized groups, possibly highlighting the role of other functionalities of antibodies in protection (67).
In conclusion, recombinant VSV is a suitable vector system to deliver RSV proteins, as the tested VSV recombinants in this study were well tolerated and induced dose-dependent immunity that could be enhanced by mucosal delivery, coadministration of rVSV-G and rVSV-F, and a prime-boost regimen. The outcomes were cumulative enhancement of neutralizing antibody levels and complete protection.
MATERIALS AND METHODS
Recombinant vesicular stomatitis viruses.
VSV-G and VSV-F were generated using pVSV1(+)-GxxL as described previously (35, 36). This VSV vector plasmid (based on the VSV Indiana strain) and support plasmids encoding the VSV nucleoprotein (pN), phosphoprotein (pP), and polymerase (pL) were kindly provided by Sean Whelan and Gail Wertz (68). The RSV-G and RSV-F genes were amplified by high-fidelity RT-PCR from RSV A2-infected cells and cloned into pVSV1(+)-GxxL at the XmaI and XhoI sites (35, 36). All of the transgenes contained the VSV gene start and gene end sequences, and all of the resulting constructs were confirmed by sequencing.
Recovery and purification of recombinant VSVs.
Recovery of the two recombinant VSVs was performed as previously described (35). Briefly, BSRT7 cells were infected for 1 h at a multiplicity of infection (MOI) of 10 with a recombinant vaccinia virus (vTF7-3 [69]) expressing T7 RNA polymerase. The cells were washed with Opti-MEM (Gibco) after removing the vaccinia virus vTF7-3 and cotransfected with pVSV1(+)-GxxL expressing one of the transgenes [pVSV1(+)-G or pVSV1(+)-F] and with support plasmids pN, pP, and pL using Lipofectamine 2000 (Invitrogen). After 96 to 108 h posttransfection, the culture medium was harvested and centrifuged at 1,892 × g for 15 min at 4°C, and the supernatant was filtered through a 0.2-μm pore-size membrane filter (to separate the recovered viruses from vaccinia virus vTF7-3). rVSVs were further passaged and plaque purified on BSRT7 cells. Plaque-derived seed stocks were aliquoted and stored at −80°C. The transgenes were confirmed in the seed stock virus by RT-PCR, and protein expression was confirmed by ELISA and Western blot analysis.
RT-PCR.
Viral RNA from the recovered or seed stock viruses (rVSV-G or rVSV-F) was extracted using the QIAmp viral RNA extraction kit (Qiagen) according to the manufacturer’s recommendations. RNA was reverse transcribed, and the inserted genes were confirmed by the OneStep RT-PCR kit (Qiagen) protocol using two primers complementary to the flanking VSV G gene (5′-CGAGTTGGTATTTATCTTTGC-3′; nucleotide position 4524; 214 nucleotides [nt] upstream of the transgene) and the L gene (5′-GTACGTCATGCGCTCATCG-3′; nucleotide position 4831; 127 nt downstream of the transgene). The resulting PCR products were analyzed by gel electrophoresis using 1% agarose gel.
ELISA for envelope glycoproteins.
Sucrose gradient-purified RSV (106 TCID50) and recombinant VSV (106 TCID50) were added in sodium carbonate-bicarbonate buffer (pH 9.6) to wells of an ELISA polystyrene 96 flat-well plate and incubated overnight at 4°C. Plates were washed with phosphate-buffered saline (PBS)-Tween 20 (0.05%) (PBS-T). To these wells, an RSV-specific goat-horseradish peroxidase (HRP) serum (1:1,000 dilution; ViroStat) was added for 1 h. Plates were washed and developed with 3,3′,5,5′-tetramethylbenzidine (TMB) substrate, and the color development was stopped after 5 min with 2N sulfuric acid. Endpoint optical density values were measured at 450 nm (OD450) and corrected for background.
Western blotting.
BSRT7 cells were infected with rVSV-G and rVSV-F at a multiplicity of infection (MOI) of 4 for 1 h. At the indicated postinfection time points, the cells were lysed in 200 μl of radioimmunoprecipitation assay (RIPA) cell lysis buffer (Sigma) to extract the cytoplasmic proteins. Culture medium was centrifuged first at 1,892 × g for 15 min at 4°C, and the supernatant was subsequently subjected to high-speed centrifugation (18,213 × g for 15 min at 4°C). The pellet was dissolved in 200 μl of RIPA buffer. Cell lysate or purified culture medium (5 μl) was separated by 10% SDS-PAGE in a Mini-Protean 3 electrophoresis cell module (Bio-Rad) and transferred to a nitrocellulose membrane (Bio-Rad) in a XCell IITM blot module (Invitrogen). The blot was probed with specific primary antibodies, followed by species-specific secondary antibodies linked to horseradish peroxidase (HRP), as follows: for the RSV-G protein, rabbit anti-RSV polyclonal IgG (1:1,000; Abcam) and goat anti-rabbit IgG-HRP (Invitrogen); and for F protein, motavizumab, a humanized anti-F monoclonal IgG (1:10,000), followed by rabbit anti-human IgG-HRP (1:20,000; Invitrogen). The blot was developed with SuperSignal West Pico chemiluminescent substrate (Thermo Scientific) and exposed to Biomax MR film (Kodak).
Growth curves.
Confluent cell monolayers of BSRT7 cells were infected at an MOI of 4 with VSV recombinants for 1 h. Aliquots of the culture medium were collected at various time points, and the titer of the virus was determined by plaque assay on Vero cells.
Plaque assay for vesicular stomatitis virus.
For the plaque assay, Vero cells of 90% density in a 6-well plate were incubated at 37°C with 10-fold virus dilutions for 1 h. Subsequently, the virus solution was aspirated, and a 1% ultrapure agarose overlay containing HEPES, penicillin/streptomycin antibiotic, 2× minimal essential medium (MEM), and 10% fetal calf serum (FCS) was applied to each well. Plates were incubated at 32°C and 5% CO2 for 48 h. Cells were fixed with 10% formalin for 1 h, and then the agarose overlay was discarded. Cells were stained with 0.067% crystal violet solution with 10% glutaric dialdehyde, and plaques were counted. To calculate the titer (PFU/ml), the inverse of the dilution of the first well with a number of plaques of greater than or equal to 5 was used and adjusted for the factors of dilution.
Animal experiments.
For immunization experiments, 4- to 6-week-old specific-pathogen-free and RSV-seronegative female cotton rats (Sigmodon hispidus) (CR) were used. Cotton rats were provided water and feed ad libitum. Four cotton rats (n = 4) were allocated for each experimental group. For one-dose immunization studies, the cotton rats were challenged 4 weeks after immunization with the respective vaccine candidate, dose, and route. For two-dose (prime-boost immunization regimen) studies, the cotton rats were immunized a second time 3 weeks after primary immunization (day 21) and challenged 3 weeks after the second immunization (day 42). For subcutaneous immunization, 500 μl of vaccine vector was injected at the flank, and for intranasal immunization, 100 μl of vaccine vector was inoculated intranasally in isoflurane narcosis. For the challenge experiments, 105 TCID50 RSV-A2 strain (in PBS) was inoculated intranasally in a 100-μl volume. At 4 days postchallenge, cotton rats were euthanized through carbon dioxide inhalation. A total of nine animal experiments were conducted in this study. Details of the experimental design of each study are provided in the Results and in respective figure legends. All animal experiments in this study were approved by the Institutional Animal Care and Use Committee of The Ohio State University (Columbus, OH).
RSV titration in respiratory tissue.
Left lung tissue was collected and homogenized in 2 ml of Advanced MEM (Gibco) in a Precellys Evolution tissue homogenizer (Bertin Instruments, France) using ceramic beads. Nasal tissue was homogenized in 3 ml of Advanced MEM using mortar and pestle. Lung and nasal homogenates were titrated using a 50% tissue culture infectious dose (TCID50) assay. Briefly, 10-fold dilutions of the homogenates (100 μl/well) were added to an 80 to 85% confluent monolayer of HEp-2 cells in a 48-well cell culture plate (in 6 replicates) and incubated for 1 h at 37°C. Wells were washed three times with PBS, and MEM/2% FCS was added. After 5 days, the final titer was determined according to the TCID50 method (70). Titers were expressed as TCID50/g of lung or nasal tissue.
RSV specific neutralization assay.
Serum was separated from blood collected in BD Microtainer blood collection tubes by centrifugation for 2 min at 18,213 × g.
For determination of neutralizing antibodies, serial 2-fold dilutions of serum samples in Advanced MEM (Gibco) were prepared in a 96-well tissue culture flat-bottomed plate with a starting dilution of 1:10 (50 μl/well). Subsequently, 50 TCID50/well of RSV in Advanced MEM (Gibco) was added in equal volume to each well and incubated for 1 h at 37°C. RSV-A2-hyperimmune serum and RSV-seronegative serum samples served as positive and negative controls, and titer of the virus used for the neutralization assay was redetermined. After virus incubation with serum, HEp-2 cells (5,000 cells/well in 100 μl of MEM containing 5% FBS) were added, and the plate was incubated at 37°C and 5% CO2 for 4 days. The endpoint neutralization titer was determined as the reciprocal of the highest dilution at which 100% inhibition/neutralization of RSV-induced cytoplasmic effect (CPE) was observed. Sera without neutralizing ability at a 1:10 dilution were considered negative.
T-cell depletion and cyclophosphamide treatment.
Cotton rats were inoculated intraperitoneally on days −4, −2, and 2 after infection with 0.5 mg of a cotton rat CD8 alpha-specific monoclonal antibody alone or in combination with 0.5 mg of a cotton rat CD4 monoclonal antibody (18). Mouse IgG2a α-CRCD8 and mouse IgG1 α-CRCD4 was purchased from Virion Systems, Inc. (Maryland, USA) and purified via the montage antibody purification protocol (MilliporeSigma, Massachusetts, USA).
For depletion of leukocytes, cotton rats were inoculated intraperitoneally on days −4, −2, 0, 2, and 4 after infection with 150 mg/kg of cyclophosphamide (Sigma) (38).
Statistical analysis.
The data were expressed as the mean ± standard deviation (SD) of the mean. Statistical analysis was performed by one-way analysis of variance followed by Tukey’s multiple-comparison post hoc test for the majority of the data analysis, and a P value of less than 0.05 (P < 0.05) was considered a statistically significant difference. The unpaired Student’s t test was applied for comparison of depleted or undepleted groups (Fig. 4) and to compare the virus antibody-neutralizing titers or RSV-specific IgG levels between days 21 and 42 after primary vaccination in prime-boost immunization studies (Fig. 6).
REFERENCES
- 1.Falsey AR, Walsh EE. 1996. Safety and immunogenicity of a respiratory syncytial virus subunit vaccine (PFP-2) in ambulatory adults over age 60. Vaccine 14:1214–1218. doi: 10.1016/s0264-410x(96)00030-8. [DOI] [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng PY, Steiner C, Abedi GR, Anderson LJ, Brammer L, Shay DK. 2012. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infect Dis 54:1427–1436. doi: 10.1093/cid/cis211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afonso CL, Amarasinghe GK, Bányai K, Bào Y, Basler CF, Bavari S, Bejerman N, Blasdell KR, Briand F-X, Briese T, Bukreyev A, Calisher CH, Chandran K, Chéng J, Clawson AN, Collins PL, Dietzgen RG, Dolnik O, Domier LL, Dürrwald R, Dye JM, Easton AJ, Ebihara H, Farkas SL, Freitas-Astúa J, Formenty P, Fouchier RAM, Fù Y, Ghedin E, Goodin MM, Hewson R, Horie M, Hyndman TH, Jiāng D, Kitajima EW, Kobinger GP, Kondo H, Kurath G, Lamb RA, Lenardon S, Leroy EM, Li C-X, Lin X-D, Liú L, Longdon B, Marton S, Maisner A, Mühlberger E, Netesov SV, Nowotny N, et al. 2016. Taxonomy of the order Mononegavirales: update 2016. Arch Virol 161:2351–2360. doi: 10.1007/s00705-016-2880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuentes S, Tran KC, Luthra P, Teng MN, He B. 2007. Function of the respiratory syncytial virus small hydrophobic protein. J Virol 81:8361–8366. doi: 10.1128/JVI.02717-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jt M, Van Kirk JE, Wright PF, Chanock RM. 1971. Experimental respiratory syncytial virus infection of adults: possible mechanisms of resistance to infection and illness. J Immunol 107:123–130. [PubMed] [Google Scholar]
- 8.Walsh EE, Falsey AR. 2004. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis 190:373–378. doi: 10.1086/421524. [DOI] [PubMed] [Google Scholar]
- 9.Stott EJ, Ball LA, Young KK, Furze J, Wertz GW. 1986. Human respiratory syncytial virus glycoprotein G expressed from a recombinant vaccinia virus vector protects mice against live-virus challenge. J Virol 60:607–613. doi: 10.1128/JVI.60.2.607-613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wertz GW, Stott EJ, Young KK, Anderson K, Ball LA. 1987. Expression of the fusion protein of human respiratory syncytial virus from recombinant vaccinia virus vectors and protection of vaccinated mice. J Virol 61:293–301. doi: 10.1128/JVI.61.2.293-301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karron RA, Buchholz UJ, Collins PL. 2013. Live-attenuated respiratory syncytial virus vaccines. Curr Top Microbiol Immunol 372:259–284. doi: 10.1007/978-3-642-38919-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. 1969. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 13.Madhi SA, Polack FP, Piedra PA, Munoz FM, Trenholme AA, Simoes EAF, Swamy GK, Agrawal S, Ahmed K, August A, Baqui AH, Calvert A, Chen J, Cho I, Cotton MF, Cutland CL, Englund JA, Fix A, Gonik B, Hammitt L, Heath PT, de Jesus JN, Jones CE, Khalil A, Kimberlin DW, Libster R, Llapur CJ, Lucero M, Perez Marc G, Marshall HS, Masenya MS, Martinon-Torres F, Meece JK, Nolan TM, Osman A, Perrett KP, Plested JS, Richmond PC, Snape MD, Shakib JH, Shinde V, Stoney T, Thomas DN, Tita AT, Varner MW, Vatish M, Vrbicky K, Wen J, Zaman K, Zar HJ, Glenn GM, Fries LF, Prepare Study Group. 2020. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med 383:426–439. doi: 10.1056/NEJMoa1908380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer G, Deplanche M, Schelcher F. 2008. Human and bovine respiratory syncytial virus vaccine research and development. Comp Immunol Microbiol Infect Dis 31:191–225. doi: 10.1016/j.cimid.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Belshe RB, Anderson EL, Walsh EE. 1993. Immunogenicity of purified F glycoprotein of respiratory syncytial virus: clinical and immune responses to subsequent natural infection in children. J Infect Dis 168:1024–1029. doi: 10.1093/infdis/168.4.1024. [DOI] [PubMed] [Google Scholar]
- 16.Dudas RA, Karron RA. 1998. Respiratory syncytial virus vaccines. Clin Microbiol Rev 11:430–439. doi: 10.1128/CMR.11.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson JE, McNeil LK, Megati S, Witko SE, Roopchand VS, Obregon JH, Illenberger DM, Kotash CS, Nowak RM, Braunstein E, Yurgelonis I, Jansen KU, Kalyan NK, Sidhu MK. 2013. Non-propagating, recombinant vesicular stomatitis virus vectors encoding respiratory syncytial virus proteins generate potent humoral and cellular immunity against RSV and are protective in mice. Immunol Lett 150:134–144. doi: 10.1016/j.imlet.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Sobrido L, Gitiban N, Fernandez-Sesma A, Cros J, Mertz SE, Jewell NA, Hammond S, Flano E, Durbin RK, Garcia-Sastre A, Durbin JE. 2006. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector. J Virol 80:1130–1139. doi: 10.1128/JVI.80.3.1130-1139.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takimoto T, Hurwitz JL, Zhan X, Krishnamurthy S, Prouser C, Brown B, Coleclough C, Boyd K, Scroggs RA, Portner A, Slobod KS. 2005. Recombinant Sendai virus as a novel vaccine candidate for respiratory syncytial virus. Viral Immunol 18:255–266. doi: 10.1089/vim.2005.18.255. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt AC, McAuliffe JM, Murphy BR, Collins PL. 2001. Recombinant bovine/human parainfluenza virus type 3 (B/HPIV3) expressing the respiratory syncytial virus (RSV) G and F proteins can be used to achieve simultaneous mucosal immunization against RSV and HPIV3. J Virol 75:4594–4603. doi: 10.1128/JVI.75.10.4594-4603.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olszewska W, Suezer Y, Sutter G, Openshaw PJ. 2004. Protective and disease-enhancing immune responses induced by recombinant modified vaccinia Ankara (MVA) expressing respiratory syncytial virus proteins. Vaccine 23:215–221. doi: 10.1016/j.vaccine.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Crowe JE, Jr, Collins PL, London WT, Chanock RM, Murphy BR. 1993. A comparison in chimpanzees of the immunogenicity and efficacy of live attenuated respiratory syncytial virus (RSV) temperature-sensitive mutant vaccines and vaccinia virus recombinants that express the surface glycoproteins of RSV. Vaccine 11:1395–1404. doi: 10.1016/0264-410X(93)90168-W. [DOI] [PubMed] [Google Scholar]
- 23.Elliott MB, Chen T, Terio NB, Chong SY, Abdullah R, Luckay A, Egan MA, Boutilier LA, Melville K, Lerch RA, Long D, Eldridge JH, Parks CL, Udem SA, Hancock GE. 2007. Alphavirus replicon particles encoding the fusion or attachment glycoproteins of respiratory syncytial virus elicit protective immune responses in BALB/c mice and functional serum antibodies in rhesus macaques. Vaccine 25:7132–7144. doi: 10.1016/j.vaccine.2007.07.065. [DOI] [PubMed] [Google Scholar]
- 24.Hsu KH, Lubeck MD, Davis AR, Bhat RA, Selling BH, Bhat BM, Mizutani S, Murphy BR, Collins PL, Chanock RM. 1992. Immunogenicity of recombinant adenovirus-respiratory syncytial virus vaccines with adenovirus types 4, 5, and 7 vectors in dogs and a chimpanzee. J Infect Dis 166:769–775. doi: 10.1093/infdis/166.4.769. [DOI] [PubMed] [Google Scholar]
- 25.Kahn JS, Roberts A, Weibel C, Buonocore L, Rose JK. 2001. Replication-competent or attenuated, nonpropagating vesicular stomatitis viruses expressing respiratory syncytial virus (RSV) antigens protect mice against RSV challenge. J Virol 75:11079–11087. doi: 10.1128/JVI.75.22.11079-11087.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnell MJ, Johnson JE, Buonocore L, Rose JK. 1997. Construction of a novel virus that targets HIV-1-infected cells and controls HIV-1 infection. Cell 90:849–857. doi: 10.1016/s0092-8674(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 27.Roberts A, Buonocore L, Price R, Forman J, Rose JK. 1999. Attenuated vesicular stomatitis viruses as vaccine vectors. J Virol 73:3723–3732. doi: 10.1128/JVI.73.5.3723-3732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts A, Kretzschmar E, Perkins AS, Forman J, Price R, Buonocore L, Kawaoka Y, Rose JK. 1998. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J Virol 72:4704–4711. doi: 10.1128/JVI.72.6.4704-4711.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlereth B, Buonocore L, Tietz A, Meulen VT, Rose JK, Niewiesk S. 2003. Successful mucosal immunization of cotton rats in the presence of measles virus-specific antibodies depends on degree of attenuation of vaccine vector and virus dose. J Gen Virol 84:2145–2151. doi: 10.1099/vir.0.19050-0. [DOI] [PubMed] [Google Scholar]
- 30.Schlereth B, Rose JK, Buonocore L, ter Meulen V, Niewiesk S. 2000. Successful vaccine-induced seroconversion by single-dose immunization in the presence of measles virus-specific maternal antibodies. J Virol 74:4652–4657. doi: 10.1128/jvi.74.10.4652-4657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suder E, Furuyama W, Feldmann H, Marzi A, de Wit E. 2018. The vesicular stomatitis virus-based Ebola virus vaccine: from concept to clinical trials. Hum Vaccin Immunother 14:2107–2113. doi: 10.1080/21645515.2018.1473698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke EC, Bradfute SB. 2017. Advances in Ebola virus vaccination. Lancet Infect Dis 17:787–788. doi: 10.1016/S1473-3099(17)30320-1. [DOI] [PubMed] [Google Scholar]
- 33.Heppner DG, Kemp TL, Martin BK, Ramsey WJ, Nichols R, Dasen EJ, Link CJ, Das R, Xu ZJ, Sheldon EA, Nowak TA, Monath TP, Heppner DG, Kemp TL, Martin BK, Ramsey WJ, Nichols R, Dasen EJ, Fusco J, Crowell J, Link C, Creager J, Monath TP, Das R, Xu ZJ, Klein R, Nowak T, Gerstenberger E, Bliss R, Sheldon EA, Feldman RA, Essink BJ, Smith WB, Chu L, Seger WM, Saleh J, Borders JL, Adams M, team Vs. 2017. Safety and immunogenicity of the rVSVG-ZEBOV-GP Ebola virus vaccine candidate in healthy adults: a phase 1b randomised, multicentre, double-blind, placebo-controlled, dose-response study. Lancet Infect Dis 17:854–866. doi: 10.1016/S1473-3099(17)30313-4. [DOI] [PubMed] [Google Scholar]
- 34.Geisbert TW, Feldmann H. 2011. Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg virus infections. J Infect Dis 204 Suppl 3:S1075–81. doi: 10.1093/infdis/jir349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma Y, Li J. 2011. Vesicular stomatitis virus as a vector to deliver virus-like particles of human norovirus: a new vaccine candidate against an important noncultivable virus. J Virol 85:2942–2952. doi: 10.1128/JVI.02332-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma Y, Duan Y, Wei Y, Liang X, Niewiesk S, Oglesbee M, Li J. 2014. Heat shock protein 70 enhances mucosal immunity against human norovirus when coexpressed from a vesicular stomatitis virus vector. J Virol 88:5122–5137. doi: 10.1128/JVI.00019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garbutt M, Liebscher R, Wahl-Jensen V, Jones S, Moller P, Wagner R, Volchkov V, Klenk HD, Feldmann H, Stroher U. 2004. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J Virol 78:5458–5465. doi: 10.1128/jvi.78.10.5458-5465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson RA, Prince GA, Suffin SC, Horswood RL, Chanock RM. 1982. Respiratory syncytial virus infection in cyclophosphamide-treated cotton rats. Infect Immun 37:369–373. doi: 10.1128/IAI.37.1.369-373.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMurry ST, Lochmiller RL, Vestey MR, Qualls CW, Jr, Elangbam CS. 1991. Acute effects of benzene and cyclophosphamide exposure on cellular and humoral immunity of cotton rats, Sigmodon hispidus. Bull Environ Contam Toxicol 46:937–945. doi: 10.1007/BF01689741. [DOI] [PubMed] [Google Scholar]
- 40.Majid AM, Barber GN. 2006. Recombinant vesicular stomatitis virus (VSV) and other strategies in HCV vaccine designs and immunotherapy. In Tan SL (ed), Hepatitis C viruses: genomes and molecular biology. Horizon Bioscience, Norfolk, UK. [PubMed] [Google Scholar]
- 41.Naik S, Galyon GD, Jenks NJ, Steele MB, Miller AC, Allstadt SD, Suksanpaisan L, Peng KW, Federspiel MJ, Russell SJ, LeBlanc AK. 2018. Comparative oncology evaluation of intravenous recombinant oncolytic vesicular stomatitis virus therapy in spontaneous canine cancer. Mol Cancer Ther 17:316–326. doi: 10.1158/1535-7163.MCT-17-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merchan JR, Patel M, Cripe TP, Old MO, Strauss JF, Thomassen A, Diaz RM, Peng KW, Russell SJ, Russell L, Reckner M, Wiegert E, Bexon AS, Powell SF. 2020. Relationship of infusion duration to safety, efficacy, and pharmacodynamics (PD): second part of a phase I-II study using VSV-IFNβ-NIS (VV1) oncolytic virus in patients with refractory solid tumors. Jco 38:3090–3090. doi: 10.1200/JCO.2020.38.15_suppl.3090. [DOI] [Google Scholar]
- 43.Bakkum-Gamez J, Block MS, Packiriswamy N, Brunton BA, Deepak U, Mitchell JM, Suksanpaisan L, Atherton P, Dueck A, Russell SJ, Lacy MQ, Peng K-W. 2018. Abstract CT072: First in human (FIH) dose escalation studies of intravenous administration of VSV-IFNβ-NIS (Voyager-V1™) in stage IV or recurrent endometrial cancer. Cancer Res 78:CT072. doi: 10.1158/1538-7445.AM2018-CT072. [DOI] [Google Scholar]
- 44.Quinones-Kochs MI, Schnell MJ, Buonocore L, Rose JK. 2001. Mechanisms of loss of foreign gene expression in recombinant vesicular stomatitis viruses. Virology 287:427–435. doi: 10.1006/viro.2001.1058. [DOI] [PubMed] [Google Scholar]
- 45.Schnell MJ, Buonocore L, Whitt MA, Rose JK. 1996. The minimal conserved transcription stop-start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J Virol 70:2318–2323. doi: 10.1128/JVI.70.4.2318-2323.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prince GA, Horswood RL, Chanock RM. 1985. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J Virol 55:517–520. doi: 10.1128/JVI.55.3.517-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson SA, Ottolini MG, Darnell ME, Porter DD, Prince GA. 1996. Unilateral nasal infection of cotton rats with respiratory syncytial virus allows assessment of local and systemic immunity. J Gen Virol 77:101–108. doi: 10.1099/0022-1317-77-1-101. [DOI] [PubMed] [Google Scholar]
- 48.Wang D, Phan S, DiStefano DJ, Citron MP, Callahan CL, Indrawati L, Dubey SA, Heidecker GJ, Govindarajan D, Liang X, He B, Espeseth AS. 2017. A single-dose recombinant parainfluenza virus 5-vectored vaccine expressing respiratory syncytial virus (RSV) F or G protein protected cotton rats and african green monkeys from RSV challenge. J Virol 91. doi: 10.1128/JVI.00066-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyoglu-Barnum S, Todd SO, Chirkova T, Barnum TR, Gaston KA, Haynes LM, Tripp RA, Moore ML, Anderson LJ. 2015. An anti-G protein monoclonal antibody treats RSV disease more effectively than an anti-F monoclonal antibody in BALB/c mice. Virology 483:117–125. doi: 10.1016/j.virol.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miao C, Radu GU, Caidi H, Tripp RA, Anderson LJ, Haynes LM. 2009. Treatment with respiratory syncytial virus G glycoprotein monoclonal antibody or F(ab′)2 components mediates reduced pulmonary inflammation in mice. J Gen Virol 90:1119–1123. doi: 10.1099/vir.0.009308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Radu GU, Caidi H, Miao C, Tripp RA, Anderson LJ, Haynes LM. 2010. Prophylactic treatment with a G glycoprotein monoclonal antibody reduces pulmonary inflammation in respiratory syncytial virus (RSV)-challenged naive and formalin-inactivated RSV-immunized BALB/c mice. J Virol 84:9632–9636. doi: 10.1128/JVI.00451-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang B, Surman S, Amaro-Carambot E, Kabatova B, Mackow N, Lingemann M, Yang L, McLellan JS, Graham BS, Kwong PD, Schaap-Nutt A, Collins PL, Munir S. 2015. Enhanced neutralizing antibody response induced by respiratory syncytial virus prefusion F protein expressed by a vaccine candidate. J Virol 89:9499–9510. doi: 10.1128/JVI.01373-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy BR, Graham BS, Prince GA, Walsh EE, Chanock RM, Karzon DT, Wright PF. 1986. Serum and nasal-wash immunoglobulin G and A antibody response of infants and children to respiratory syncytial virus F and G glycoproteins following primary infection. J Clin Microbiol 23:1009–1014. doi: 10.1128/JCM.23.6.1009-1014.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murphy BR, Olmsted RA, Collins PL, Chanock RM, Prince GA. 1988. Passive transfer of respiratory syncytial virus (RSV) antiserum suppresses the immune response to the RSV fusion (F) and large (G) glycoproteins expressed by recombinant vaccinia viruses. J Virol 62:3907–3910. doi: 10.1128/JVI.62.10.3907-3910.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turner DL, Cauley LS, Khanna KM, Lefrancois L. 2007. Persistent antigen presentation after acute vesicular stomatitis virus infection. J Virol 81:2039–2046. doi: 10.1128/JVI.02167-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hastie E, Cataldi M, Marriott I, Grdzelishvili VZ. 2013. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res 176:16–32. doi: 10.1016/j.virusres.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clarke DK, Nasar F, Chong S, Johnson JE, Coleman JW, Lee M, Witko SE, Kotash CS, Abdullah R, Megati S, Luckay A, Nowak B, Lackner A, Price RE, Little P, Kalyan N, Randolf V, Javadian A, Zamb TJ, Parks CL, Egan MA, Eldridge J, Hendry M, Udem SA. 2014. Neurovirulence and immunogenicity of attenuated recombinant vesicular stomatitis viruses in nonhuman primates. J Virol 88:6690–6701. doi: 10.1128/JVI.03441-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson JE, Nasar F, Coleman JW, Price RE, Javadian A, Draper K, Lee M, Reilly PA, Clarke DK, Hendry RM, Udem SA. 2007. Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non-human primates. Virology 360:36–49. doi: 10.1016/j.virol.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Velazquez-Salinas L, Naik S, Pauszek SJ, Peng KW, Russell SJ, Rodriguez LL. 2017. Oncolytic recombinant vesicular stomatitis virus (VSV) is nonpathogenic and nontransmissible in pigs, a natural host of VSV. Hum Gene Ther Clin Dev 28:108–115. doi: 10.1089/humc.2017.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blanco JCG, Boukhvalova MS, Morrison TG, Vogel SN. 2018. A multifaceted approach to RSV vaccination. Hum Vaccin Immunother 14:1734–1745. doi: 10.1080/21645515.2018.1472183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Capella C, Chaiwatpongsakorn S, Gorrell E, Risch ZA, Ye F, Mertz SE, Johnson SM, Moore-Clingenpeel M, Ramilo O, Mejias A, Peeples ME. 2017. Prefusion F, postfusion F, G antibodies, and disease severity in infants and young children with acute respiratory syncytial virus infection. J Infect Dis 216:1398–1406. doi: 10.1093/infdis/jix489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Collins PL, Melero JA. 2011. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res 162:80–99. doi: 10.1016/j.virusres.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loomis RJ, Johnson PR. 2013. Gene-based vaccine approaches for respiratory syncytial virus. Curr Top Microbiol Immunol 372:307–324. doi: 10.1007/978-3-642-38919-1_15. [DOI] [PubMed] [Google Scholar]
- 64.Kapadia SU, Rose JK, Lamirande E, Vogel L, Subbarao K, Roberts A. 2005. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology 340:174–182. doi: 10.1016/j.virol.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee FE, Walsh EE, Falsey AR, Betts RF, Treanor JJ. 2004. Experimental infection of humans with A2 respiratory syncytial virus. Antiviral Res 63:191–196. doi: 10.1016/j.antiviral.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 66.Haynes LM, Caidi H, Radu GU, Miao C, Harcourt JL, Tripp RA, Anderson LJ. 2009. Therapeutic monoclonal antibody treatment targeting respiratory syncytial virus (RSV) G protein mediates viral clearance and reduces the pathogenesis of RSV infection in BALB/c mice. J Infect Dis 200:439–447. doi: 10.1086/600108. [DOI] [PubMed] [Google Scholar]
- 67.Lefrancois L. 1984. Protection against lethal viral infection by neutralizing and nonneutralizing monoclonal antibodies: distinct mechanisms of action in vivo. J Virol 51:208–214. doi: 10.1128/JVI.51.1.208-214.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whelan SP, Ball LA, Barr JN, Wertz GT. 1995. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc Natl Acad Sci U S A 92:8388–8392. doi: 10.1073/pnas.92.18.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fuerst TR, Niles EG, Studier FW, Moss B. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A 83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reed LJ, Muench H. 1938. A simple method of estimating fifty per cent endpoints. American J Hygiene 27:493–497. doi: 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]