Influenza A viruses (IAVs) still pose a major threat to human health worldwide. As a zoonotic virus, IAV can spontaneously overcome species barriers and even reside in new hosts after efficient adaptation.
KEYWORDS: NS1, influenza virus, protein phosphorylation, viral polymerase activity
ABSTRACT
Influenza A virus (IAV) nonstructural protein 1 (NS1) is a protein with multiple functions that are regulated by phosphorylation. Phosphoproteomic screening of H1N1 virus-infected cells revealed that NS1 was phosphorylated at serine 205 in intermediate stages of the viral life cycle. Interestingly, S205 is one of six amino acid changes in NS1 of post-pandemic H1N1 viruses currently circulating in humans compared to the original swine-origin 2009 pandemic (H1N1pdm09) virus, suggesting a role in host adaptation. To identify NS1 functions regulated by S205 phosphorylation, we generated recombinant PR8 H1N1 NS1 mutants with S205G (nonphosphorylatable) or S205N (H1N1pdm09 signature), as well as H1N1pdm09 viruses harboring the reverse mutation NS1 N205S or N205D (phosphomimetic). Replication of PR8 NS1 mutants was attenuated relative to wild-type (WT) virus replication in a porcine cell line. However, PR8 NS1 S205N showed remarkably higher attenuation than PR8 NS1 S205G in a human cell line, highlighting a potential host-independent advantage of phosphorylatable S205, while an asparagine at this position led to a potential host-specific attenuation. Interestingly, PR8 NS1 S205G did not show polymerase activity-enhancing functions, in contrast to the WT, which can be attributed to diminished interaction with cellular restriction factor DDX21. Analysis of the respective kinase mediating S205 phosphorylation indicated an involvement of casein kinase 2 (CK2). CK2 inhibition significantly reduced the replication of WT viruses and decreased NS1–DDX21 interaction, as observed for NS1 S205G. In summary, NS1 S205 is required for efficient NS1–DDX21 binding, resulting in enhanced viral polymerase activity, which is likely to be regulated by transient phosphorylation.
IMPORTANCE Influenza A viruses (IAVs) still pose a major threat to human health worldwide. As a zoonotic virus, IAV can spontaneously overcome species barriers and even reside in new hosts after efficient adaptation. Investigation of the functions of specific adaptational mutations can lead to a deeper understanding of viral replication in specific hosts and can probably help to find new targets for antiviral intervention. In the present study, we analyzed the role of NS1 S205, a phosphorylation site that was reacquired during the circulation of pandemic H1N1pdm09 “swine flu” in the human host. We found that phosphorylation of human H1N1 virus NS1 S205 is mediated by the cellular kinase CK2 and is needed for efficient interaction with human host restriction factor DDX21, mediating NS1-induced enhancement of viral polymerase activity. Therefore, targeting CK2 activity might be an efficient strategy for limiting the replication of IAVs circulating in the human population.
INTRODUCTION
Influenza A virus (IAV) infections still pose a major threat to the human population, with high morbidity and mortality rates, presenting a high economic burden each year. IAV nonstructural protein 1 (NS1) is a multifunctional protein participating mainly in the suppression of antiviral defense mechanisms orchestrated by the cell to fight the invading pathogen. Due to its well-characterized interferon (IFN)-antagonistic functions (1), NS1 is indispensable for efficient viral replication in IFN-competent systems. By the regulation of cell intrinsic innate immune responses, NS1 has also been shown to indirectly promote viral polymerase activity (2, 3). Furthermore, NS1 interacts with different cellular proteins, such as DEAD box RNA helicase DDX21 or RNA-associated protein 55 (RAP55), thereby counteracting DDX21-mediated hijacking of the viral PB1 protein and inhibiting NP targeting to stress granules, respectively (4, 5), Altogether, these functions allow for efficient viral polymerase assembly and increased viral RNA stability, resulting in enhanced viral polymerase activity. Therefore, mutations in NS1, one of the major virulence determinants in IAV infection, contribute to the adaptation to new hosts and thus have been shown to drive viral evolution, besides changes in the viral hemagglutinin and neuraminidase proteins (6, 7). For instance, it has been published that, unlike the original swine-derived 2009 pandemic virus (H1N1pdm09) NS1 protein, NS1 from currently circulating post-pandemic H1N1 viruses in humans has evolved six amino acid mutations (E55K, L90I, I123V, E125D, K131E, and N205S) that restore its ability to bind to cellular cleavage polyadenylation specificity factor 30 (CPSF30), therefore allowing efficient inhibition of host gene expression (8). Interestingly, with these adaptational mutations, NS1 partially converted back to a genotype that had been present in pre-pandemic H1N1 viruses circulating in humans for many years.
Phosphorylation and dephosphorylation are well known to regulate the functions not only of cellular but also of viral proteins. Functional evolution of viral proteins during host adaptation might involve the acquisition of new phosphorylation sites that allow for the fine-tuning of specific functions. Thus, host kinases control phosphorylation dynamics during viral life cycle progression. So far, several phosphorylation sites of IAV NS1 have been identified and functionally analyzed (9–15). Most of these phosphorylation events abrogate the IFN-antagonistic activity of IAV NS1 (S42, T49, T80) by decreasing its RNA-binding affinity (11, 12) or affecting NS1–RIG-I binding (13), while phosphorylation of Y73 and S83 has been shown to be needed for efficient IFN suppression (14). Further phosphorylation events (S48, T215) could not be linked to specific NS1 functions yet, since they did not significantly affect viral replication (10–12). Interestingly, phosphorylation of NS1 at position S205 has been reported in the presence of an NS1 S48A mutant (11) but was not functionally characterized. However, this phosphorylation site is of specific interest, since NS1 S205 was acquired during the circulation of the original swine-derived H1N1pdm09 virus in the human host (8).
Here, we characterized the dynamics of casein kinase 2 (CK2)-mediated NS1 S205 phosphorylation in H1N1 virus infection of human lung epithelial cells, and we provide evidence that this phosphorylation is needed for efficient interaction with cellular DEAD box RNA helicase DDX21, an interferon-induced protein essential for NS1-induced enhancement of viral polymerase activity.
RESULTS
NS1 S205 is dynamically phosphorylated during infection with PR8 H1N1 and is needed for efficient replication.
It has been reported previously that the NS1 protein of currently circulating H1N1pdm09-like viruses in the human population has accumulated six amino acid mutations, including N205S, since the emergence of this virus in 2009 (8). Interestingly, serine had been the predominant amino acid at position 205 in previous human H1N1 viruses until the introduction of H1N1pdm09 (Fig. 1A). NS1 of H1N1pdm09 originates from the classical swine IAV lineage, initially carrying a serine at position 205, circulating in North American pigs (Fig. 1B). In the 2000–2001 winter season, an asparagine at position 205 significantly increased its prevalence among swine H1N1 viruses isolated in Northern temperate regions. Later, in 2009, the H1N1pdm09 reassortant virus that carried an NS1 with N205 crossed the species barriers and jumped into the human population. This virus was reintroduced into the swine population via reverse zoonosis and produced reassortants, while asparagine remained the predominant amino acid at position 205, indicating that swine H1N1 viruses might tolerate both amino acids at position 205. Interestingly, NS1 sequence analysis of other human, swine, and avian IAV isolates revealed that human H3N2 and H2N2 viruses also carry a serine at position 205, whereas in swine H1N2 and H3N2 isolates, the most prevalent amino acid is asparagine. Avian IAVs possess other amino acids at this position, mainly arginine or glycine (Fig. 1C).
FIG 1.
PR8 NS1 S205 is phosphorylated in viral replication. (A, B) Amino acid prevalence at NS1 position 205 in all human H1N1 viruses (A) and swine H1N1 isolates (B) from Northern temperate regions from 1930 until 2020 (n, 6 to 5,317 isolates/time frame). (C) Prevalence of a serine at amino acid position 205 in the viral NS1 protein. Human H3N2 and H2N2 isolates, swine H1N2 and H3N2 isolates, and avian isolates of different subtypes isolated from 1902 to 2020 were analyzed (n, 125 to 22,092). (D) Relative phosphorylation of the tryptic peptide of NS1 showing the amount of phosphorylated S205 normalized to total NS1 protein amounts. (E) A549 cells were transfected with WT PR8 NS1 or the S205G substitution mutant. At 24 h p.t., cells were infected with WT PR8 (MOI, 5) for 6 or 8 h. (Left) Phosphoserine-containing proteins were immunoprecipitated (IP) by using pS-specific antibodies, and the precipitation of NS1 was determined by Western blot analysis. Blots are representative of the results of three independent experiments. The phosphorylation levels of NS1 were analyzed by densitometry and normalized to the respective NS1 amounts. (Right) Shown are the levels of phosphorylated NS1 (+ standard deviations) as percentages of the phosphorylation level of NS1 S205 at 6 h p.i. Statistical significance was analyzed by one-way analysis of variance followed by Dunn’s multiple-comparison test.
Noteworthy, phosphoproteomic mass spectrometry (MS) analysis of human lung epithelial A549 cells infected with IAV strain A/PR8/34 and subjected to stable-isotope labeling by amino acids in cell culture (SILAC) revealed a distinct phosphorylation of NS1 at S205 6 h postinfection (p.i.) that seemed to decrease slightly during viral life cycle progression (8 h p.i.) (Fig. 1D). Of note, phosphorylation dynamics were normalized to NS1 protein expression and therefore do not just reflect expression levels in infected cells, since NS1 was readily detectable 4 h p.i., and its expression increased over time. These observations indicate a potential transient function of this phosphorylation event in intermediate stages of the virus life cycle and suggest a potential functional switch of the NS1 protein in the different stages of viral replication mediated by phosphorylation. To verify the phosphorylation of NS1 at the serine at position 205, wild-type (WT) NS1 and a nonphosphorylatable NS1 S205G substitution mutant were overexpressed, and cells were infected with WT PR8 for 6 or 8 h, respectively. Immunoprecipitation (IP) with phosphorylated serine (pS)-specific antibodies and detection with NS1-specific antibodies revealed that NS1 phosphorylation was decreased by approximately 30% in the presence of NS1 S205G (Fig. 1E). These results confirm the phosphorylation of NS1 at the serine at position 205; however, no differences in NS1 phosphorylation levels could be observed between 6 and 8 h p.i.
For deeper analysis of the NS1 functions regulated by phosphorylation, and to investigate whether phosphorylation at S205 contributes to NS1 functional evolution and adaptation to the human host, we generated recombinant PR8 viruses expressing NS1 S205 substitution mutants with either nonphosphorylatable glycine (S205G) or negatively charged residues mimicking constitutive phosphorylation (S205D). Furthermore, we introduced an asparagine (S205N), representing the H1N1pdm09 signature. While the PR8 NS1 S205G and S205N substitution mutants could be rescued, viruses expressing a mimic of constitutive phosphorylation (S205D) were not obtained without the occurrence of additional mutations. In contrast, H1N1pdm09 NS1 N205 substitution mutants allowing for phosphorylation (N205S) or mimicking constitutive phosphorylation (N205D) could be rescued. To analyze whether these substitutions affect viral replication in human lung cells, A549 cells were infected with WT PR8, PR8 NS1 S205G, or PR8 NS1 S205N, respectively. As expected, both substitution mutants were significantly attenuated compared to WT PR8, and PR8 expressing NS1 S205N (the H1N1pdm09 signature) showed the most prominent reduction in viral replication (Fig. 2A). These results indicate that dynamic phosphorylation of NS1 at S205 might be needed for efficient virus replication. To investigate whether this potential dependence of replication on phosphorylation is specific for human cell lines, we also infected a swine lung cell line. Here, both PR8 NS1 substitution mutants were affected to the same extent (Fig. 2B), indicating a host cell line-independent advantage of phosphorylatable S205, while an asparagine at this position led to a potential host-specific attenuation in human cells. As already mentioned, H1N1pdm09 NS1 acquired S205 besides other mutations during continuing circulation in the human host (8). The replication abilities of WT H1N1pdm09 and of H1N1pdm09 NS1 N205S and N205D were explored in human as well as swine lung cell lines. Here, the replication of NS1 substitution mutants was not affected (Fig. 2C and D), highlighting that acquisition of NS1 N205S alone is not sufficient to affect the replication of H1N1pdm09. Since these cell lines might not efficiently reflect the natural situation, the replication of H1N1pdm09 with WT NS1 and substitution mutants was analyzed in primary human bronchial epithelial cells (HBEpCs), potentially allowing for greater phenotypic penetrance. Here, the tendency of increased viral replication in the presence of H1N1pdm09 NS1 N205S was observed, ranging from 2- to 6-fold relative to the WT (Fig. 2E). These results indicate a potential replication advantage in the presence of serine 205 in human cells, as observed previously for PR8, and might present the reason for positive selection of serine at position 205 in H1N1 viruses in the human host.
FIG 2.
A serine at position 205 of the NS1 protein facilitates efficient viral replication. (A to E) Human A549 cells (A, C), porcine WSL-R-HP cells (B, D), or HBEpCs (E) were infected with WT PR8, PR8 NS1 S205G, or PR8 NS1 S205N (A, B) or with WT H1N1pdm09, H1N1pdm09 NS1 N205S, or H1N1pdm09 NS1 N205D (C, D, E) at an MOI of 0.1, and viral replication was analyzed at the indicated time points (A to D) or 24 h p.i. (E). Amounts of viral progeny were analyzed by standard plaque assays and are presented as means ± standard deviations of results from three independent experiments (A) or as results from one experiment representative of two independent experiments (B, C, D). Virus titers obtained after infection of HBEpCs are expressed as mean percentages of the WT H1N1pdm09 NS1 titer ± standard deviations from three independent experiments (E). Statistical significance was analyzed by one-way (E) or two-way (A to D) analysis of variance followed by Tukey’s multiple-comparison test (***, P ≤ 0.001).
A serine at position 205 does not affect NS1 interferon-suppressive functions.
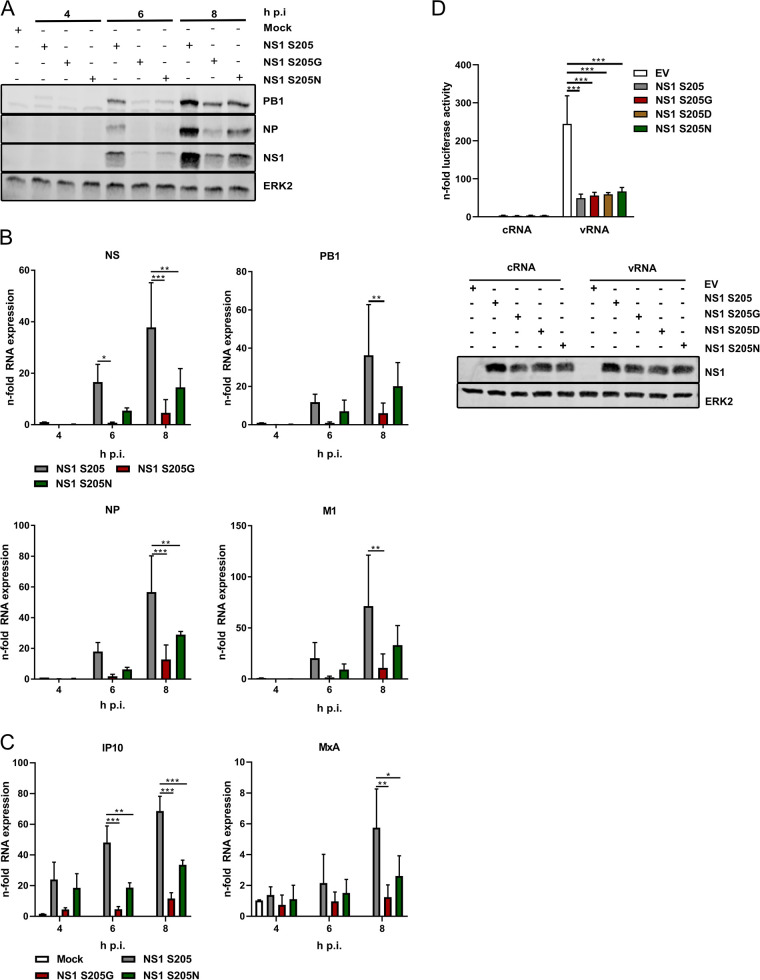
To investigate more deeply the specific role of S205 in NS1 function, viral protein expression was analyzed as an intermediate step in viral replication after infection of A549 cells with WT PR8 or NS1 mutant-expressing viruses. Western blot analysis of viral PB1, NP, and NS1 protein expression in infected A549 cells clearly indicated a dependence of viral protein accumulation on NS1 S205, since both substitution mutants showed lower viral protein expression than the WT, with PR8 NS1 S205G being slightly more affected than PR8 NS1 S205N (Fig. 3A). To analyze whether these NS1 mutants interfere directly with viral protein translation or rather reflect different levels of viral mRNAs produced, quantitative reverse transcription-PCR (qRT-PCR) was performed after infection with PR8 with WT NS1 or substitution mutants. Here, all viral mRNAs analyzed were strongly affected in the presence of S205G compared to the WT, while the S205N mutant showed intermediate mRNA levels (Fig. 3B). These results indicate that the presence of a phosphorylatable serine at position 205 is needed early in viral life cycle progression to ensure efficient viral gene transcription, while the presence of S205N seems to partially replace its function.
FIG 3.
NS1 S205 substitution mutants show decreased viral protein expression that is independent of the IFN-antagonistic function of NS1. (A, B, C) A549 cells were infected with WT PR8 or substitution mutants at an MOI of 5 and were analyzed 4, 6, and 8 h p.i. for the expression of viral proteins (A) or viral mRNA and cRNA (B) as well as cellular innate immune responses (C). (A) Expression of viral proteins PB1, NP, and NS1 was determined by Western blotting. ERK2 served as a loading control. Blots are representative of the results of three independent experiments. (B, C) The expression of different viral and cellular mRNAs was analyzed by qRT-PCR and is presented as the mean n-fold induction + standard deviation for three independent experiments. Values were normalized to those for WT virus infection at 4 h p.i. (B) or mock-infected controls (C). Statistical analysis was performed by two-way analysis of variance followed by Tukey’s multiple-comparison test (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). (D) Impact of PR8 NS1 S205 substitution on IFN-β promoter activity. Vero cells were transfected with a luciferase reporter construct expressed under the control of the IFN-β promoter in combination with an empty vector (EV), PR8 NS1 S205, or substitution mutants for 24 h. Cells were stimulated with 500 ng of total RNA isolated from infected A549 cells (8 h; MOI, 5; vRNA). Total RNA from uninfected A549 cells was used as a control (cRNA). At 5 h poststimulation, promoter activity was measured by a luciferase assay, and the results are presented as the mean n-fold activity (+ standard deviation) from three independent experiments. Values were normalized to those for the EV cRNA-stimulated controls. Statistical significance was analyzed by two-way analysis of variance followed by Sidak’s multiple-comparison test (***, P ≤ 0.001).
Since the inhibition of type I IFN induction is one of the major functions of NS1 early in infection (1), we wondered whether attenuated virus replication observed in the presence of NS1 S205 substitution mutants might be due to inefficient suppression of the type I IFN cascade. To test this hypothesis, A549 cells were infected with PR8 with WT NS1 or S205 substitution viruses, and the mRNA expression levels of different interferon-stimulated genes (ISGs) were investigated by qRT‐PCR (Fig. 3C). Surprisingly, the most prominent ISG induction was observed in infection with PR8 with WT NS1, indicating that IFN expression and the corresponding ISG expression seem to correlate with the replication abilities of the different viruses. To study the IFN-suppressing activities of NS1 in a replication-independent approach, reporter gene assays were performed. The IFN-β promoter, also known as the IFN-β enhanceosome, comprises three functional transcription factor binding sites for AP‐1, NF‐κB, and IRF‐3; of these, IRF‐3 is the most important site for virus‐specific induction of gene expression downstream of RIG‐I (16). To reveal if IFN-β promoter activity was influenced by the S205G, S205D, or S205N substitution in PR8 NS1, a reporter gene construct expressing a luciferase gene under the control of the IFN-β enhanceosome was transfected in combination with plasmids coding for WT NS1 or the different substitution mutants. After stimulation with viral RNA (vRNA), the major IAV-associated pathogen-associated molecular pattern (PAMP) (17), all NS1 mutants showed the same IFN-antagonistic activity as the WT (Fig. 3D), indicating that amino acid (aa) 205 is not involved in the control of this major NS1 function. Thus, reduced viral gene and protein expression in the presence of NS1 substitution mutants cannot be attributed to changes in the ability of NS1 to suppress the cellular IFN response.
NS1 S205 promotes the NS1-induced enhancement of viral polymerase activity mediated by interaction with cellular DDX21.
Besides its function in the regulation of cell intrinsic innate immune responses, NS1 has been shown to indirectly promote viral polymerase activity by interaction with different cellular proteins (4, 5). To analyze whether NS1 S205 substitution affects NS1-mediated enhancement of polymerase activity, viral ribonucleoprotein (vRNP) reconstitution experiments were performed. Here, all components of the vRNP are cotransfected with a reporter gene construct allowing for polymerase I (pol I)-derived expression of a luciferase vRNA, whose mRNA transcription is mediated by a viral promoter and therefore depends on the activity of the viral polymerase complex (18). As expected, the presence of WT NS1 readily increased viral polymerase activity by roughly 5-fold (Fig. 4A). While the presence of NS1 S205D or NS1 S205N also significantly increased viral polymerase activity, NS1 S205G showed no clear enhancement of viral polymerase function over the activity of vRNPs alone. Importantly, immunofluorescence analyses demonstrated that these results cannot be attributed to a defect in the nuclear localization of NS1 S205G, since the mutant was readily observed in the nuclei of infected cells (Fig. 4B). These results indicate that both NS1 S205 and NS1 S205N can readily induce the enhancement of viral polymerase activity; in the case of NS1 S205, the negative charge provided by phosphorylation potentially facilitates this function as mimicked by NS1 S205D. Interestingly, if this is true, NS1 S205N can compensate for this function by other regulatory means. In order to investigate whether the polymerase activity-enhancing function of H1N1pdm09 NS1 is also affected by the amino acid at position 205, we performed vRNP reconstitution experiments with the H1N1pdm09 RNP. Here, we observed a tendency toward enhanced polymerase function in the presence of the NS1 N205S and N205D substitution mutants, while WT NS1 did not increase viral polymerase activity over that with the vRNP alone (Fig. 4C). These results indicate an advantage in the presence of serine 205 that might be based on the negative charge provided by potential phosphorylation, as reflected by phosphomimetic NS1 S205D. This might be the reason for increased H1N1pdm09 replication in the presence of NS1 N205S (Fig. 2E).
FIG 4.
NS1-mediated enhancement of viral polymerase activity is orchestrated by interaction with cellular DDX21. (A, C) Effects of WT PR8 (A) or H1N1pdm09 (C) NS1 and substitution mutants on viral polymerase activity. Vero cells were transfected with plasmids encoding WT NS1 or different NS1 205 substitution mutants, PB1, PB2, PA, and NP, together with a plasmid that directs the expression of the firefly luciferase reporter RNA minigenome. At 24 h p.t., cells were lysed and firefly luciferase activity was measured. The results are presented as the mean relative polymerase activity (+ standard deviation) for three independent experiments normalized to NS1 expression levels and activity in empty-vector-expressing cells. Confirmation of efficient NS1 overexpression was performed by Western blot analysis. ERK2 served as a loading control for detection. Statistical significance was analyzed by one-way analysis of variance followed by Dunnett’s multiple-comparison test (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). (B) A549 cells were infected at an MOI of 20 with WT PR8 NS1 or the PR8 NS1 S205G or S205N substitution mutant for 8 h. Cells were fixed, and the localization of viral proteins NS1 (green) and NP (red) was analyzed by indirect immunofluorescence. DAPI was used to stain the nuclei. Images are representative of three independent experiments. (D) Analysis of NS1–DDX21 interaction. Vero cells were transfected with Flag-tagged DDX21 in combination with Strep-tagged WT NS1 or S205 substitution mutants. At 24 h p.t., NS1 was pulled down by using Strep-Tactin beads. (Top) Coprecipitation of DDX21 was determined by using Flag-specific antibodies in Western blot analysis. (Bottom) Amounts of coprecipitated DDX21 were analyzed by densitometry of blots from three to six independent experiments and are presented as the level of interaction compared to that with WT NS1 (+ standard deviation). Statistical significance was analyzed by one-way analysis of variance followed by Dunn’s multiple-comparison test (*, P ≤ 0.05). (E) A549 cells were transfected with different DDX21-specific siRNAs or scrambled control siRNAs. At 48 h p.t., cells were infected at an MOI of 0.1 with WT PR8 NS1 or PR8 expressing NS1 S205G or NS1 S205N. (Top) Virus titers were determined 24 h p.i. by standard plaque assays and are presented as the mean virus titer (+ standard deviation) (left) or as the mean percentage, from three independent experiments, of the virus titer for WT infection of control siRNA-transfected cells (right). Statistical significance was analyzed by two-way analysis of variance followed by Tukey’s multiple-comparison test (*, P ≤ 0.05; ***, P ≤ 0.001). (Bottom) Efficient DDX21 knockdown was confirmed by Western blot analysis. ERK1/2 was used as a loading control, and NS1 detection served as a control for infection.
To address the mechanism behind NS1 S205-induced polymerase activity, we analyzed the ability of NS1 to interact with the cellular DEAD box RNA helicase DDX21. It has been reported previously that NS1 hijacks DDX21, thereby inhibiting the DDX21–PB1 interaction that has been shown to interfere with viral polymerase activity (5). Pulldown assays of Strep-tagged WT NS1 and NS1 S205 substitution mutants indicated that the NS1-induced enhancement of viral polymerase activity can be attributed to the interaction with cellular DDX21 (Fig. 4D). While the negatively charged phosphomimetic S205D substitution mutant was able to interact with DDX21 as efficiently as WT NS1 and NS1 S205N, NS1 S205G clearly showed decreased affinity for DDX21 binding, reflecting the results obtained in vRNP reconstitution assays (Fig. 4A). The efficient interaction of NS1 S205N with DDX21 potentially explains why viral polymerase activity was not affected in the presence of this substitution mutant relative to activity in the presence of WT NS1 (Fig. 4A).
In order to investigate whether the replication defect of viruses expressing NS1 S205G can be attributed to decreased binding affinity of NS1 S205G to DDX21, we analyzed the replication efficiencies of PR8 with WT NS1 and PR8 with NS1 S205G in cells in which DDX21 was knocked down by small interfering RNA (siRNA). In contrast to previously published antiviral effects of DDX21 in infection with the IAV strain A/WSN/33 (WSN) (5), downregulation of DDX21 expression by using two different DDX21-specific siRNAs resulted in significant decreases in the replication of WT PR8 correlating with the knockdown efficiencies of the respective siRNAs (Fig. 4E). As expected, the replication of PR8 with NS1 S205G was not affected by siRNA-mediated DDX21 knockdown, verifying that reduced viral replication in the presence of NS1 S205G can be attributed to a decrease in the interaction between DDX21 and NS1, which is needed for efficient NS1-mediated enhancement of viral polymerase activity. However, the replication of PR8 with NS1 S205N also was not affected by the absence of DDX21, although this mutant showed efficient DDX21 interaction (Fig. 4D) and enhancement of polymerase function in reduced experimental systems (Fig. 4A). This observation might be attributed to earlier interference with viral replication in the presence of NS1 S205N, potentially also explaining the decreased viral mRNA expression (Fig. 3B), which is not further affected by decreased viral polymerase activity in the absence of DDX21.
NS1 S205 is phosphorylated by CK2.
The results presented so far demonstrate that the previously detected phosphorylation of NS1 S205 might increase viral polymerase activity, in line with the observed dynamics of S205 phosphorylation, which was first detected at 6 h p.i. and seemed to decrease slightly at 8 h p.i., after the phase at which the promotion of viral transcription/replication is required (∼4 to 6 h p.i.) (Fig. 1D). This dynamic suggests that NS1 S205 phosphorylation occurs in the nucleus. Since nonphosphorylatable NS1 S205G is readily imported into the nucleus (Fig. 4B), phosphorylation at S205 is not needed for efficient nuclear import. Indeed, a search for potential kinases that may phosphorylate NS1 at S205 predicts cellular casein kinase 2 (CK2) (Fig. 5A), a kinase that can be localized in nearly every cellular compartment, including the nucleus (14). In order to investigate whether CK2 might be responsible for the phosphorylation of NS1 S205, a high-affinity ATP-competitive inhibitor of CK2, DMAT (2-dimethylamino-4,5,6,7-tetrabromo-1-H-benzimidazole), was used, and the replication of PR8 with WT NS1 and PR8 with NS1 S205G was analyzed. Here, PR8 expressing WT NS1 showed significantly reduced viral titers, by >1 order of magnitude, when CK2 was inhibited, as observed for PR8 with NS1 S205G (Fig. 5B, left). Strikingly, in the presence of nonphosphorylatable NS1 S205G, replication was only marginally affected by CK2 inhibition, highlighting that NS1 S205 phosphorylation is likely to be mediated by CK2 (Fig. 5B, right). In order to analyze whether CK2 inhibition affects viral replication at the level of viral polymerase activity, the expression of viral RNAs was analyzed. To eliminate potential effects on virus replication induced by changes in NS1 expression levels after infection with WT PR8 or substitution mutants, the different NS1 variants were overexpressed, and cells were subsequently infected with the PR8 ΔNS1 virus (19). Under these conditions, expression levels of different viral genes, such as the NP, M1, and PB1 genes, were significantly lower in the presence of the NS1 S205G or NS1 S205N substitution mutant than in the presence of WT NS1 (Fig. 5C). Furthermore, in the presence of WT NS1, DMAT treatment results in a reduction of viral RNA expression to the same level as that observed for the substitution mutants. Of note, CK2 inhibition also slightly affected viral gene expression after overexpression of the NS1 S205G and S205N substitution mutants. It has been reported previously that CK2 affects IAV replication by phosphorylation of the viral protein PA (20, 21), potentially explaining the slight decreases observed in PR8 NS1 S205G replication and viral gene expression in the presence of the substitution mutants.
FIG 5.
Cellular casein kinase 2 is required for efficient replication by catalyzing NS1 S205 phosphorylation, which is needed for DDX21 interaction. (A) Schematic representation of the CK2 recognition site in comparison to the sequence containing PR8 NS1 S205. (B) A549 cells were pretreated with the CK2-specific inhibitor DMAT (5 μM) or DMSO for 1 h. Cells were infected at an MOI of 0.1 with WT PR8 NS1 or PR8 expressing NS1 S205G and were treated with DMSO or DMAT (5 μM) for 24 h. Virus-containing supernatants were harvested, and viral titers were determined by standard plaque assays and are presented as means (+ standard deviations) from three independent experiments (left) or as the percentage of reduction in viral replication in DMAT-treated cells (right). The level of viral replication in DMSO-treated cells was arbitrarily set to 100%. Statistical significance was determined by using two-way analysis of variance followed by Sidak’s multiple-comparison test (*, P ≤ 0.05; **, P ≤ 0.01). (C) A549 cells were transfected with NS1 S205 or substitution mutants for 24 h. Subsequently, cells were preincubated with DMSO or DMAT (5 μM) for 1 h and were then infected with PR8 ΔNS1 (MOI = 1) in the presence of DMSO or DMAT. Expression levels of NP, M1, and PB1 mRNAs and cRNAs were analyzed 4 and 8 h p.i. by qRT-PCR and are presented as mean n-fold induction (+ standard deviation) from one experiment representative of two independent experiments. Values were normalized to those for cells overexpressing NS1 S205 at 4 h p.i. Statistical significance was analyzed by two-way analysis of variance followed by Tukey’s multiple-comparison test (**, P ≤ 0.01; ***, P ≤ 0.001). (D) Effects of CK2 inhibition on the NS1–DDX21 interaction. HEK293 cells were transfected with Flag-tagged DDX21 in combination with Strep-tagged WT NS1 or substitution mutants. At 18 h p.t., cells were treated with the CK2 inhibitor DMAT (5 μM) or DMSO for 6 h, and NS1 was pulled down by using Strep-Tactin Sepharose beads. Coprecipitation of DDX21 was determined by using Flag-specific antibodies, and efficient Strep-NS1 pulldown was confirmed by using NS1-specific antibodies in Western blot analysis. Blots are representative of the results of two independent experiments.
Next, we investigated whether CK2 inhibition results in decreased NS1–DDX21 binding due to the absence of S205 phosphorylation. As expected, CK2 inhibition reduced the amounts of DDX21 coprecipitated with WT NS1 to the same level as that observed with the NS1 S205G substitution mutant (Fig. 5D). These data confirm that CK2 is a candidate kinase for NS1 phosphorylation at S205, which orchestrates the binding of NS1 to DDX21, resulting in an enhancement of viral polymerase activity.
DISCUSSION
In the present study, we analyzed the function of a newly identified phosphorylation site in the influenza A virus protein NS1. Recently, the serine at position 205 has been reported to be involved in the continuous adaptation of H1N1pdm09 virus to the human host. Interestingly, pre-pandemic human H1N1 viruses also showed a high prevalence of serine 205, indicating a prominent function of this phosphorylatable amino acid in IAV replication in the human host. Here, we provide evidence of NS1 S205 phosphorylation in PR8 infection of human lung epithelial cells that is most likely mediated by the cellular kinase CK2. This phosphorylation facilitates efficient NS1–DDX21 interaction, which is indispensable for NS1-mediated enhancement of viral polymerase activity.
Remarkably, NS1 of the swine-origin H1N1pdm09 virus seems to tolerate either serine or asparagine at position 205 without affecting virus replication in cell lines of human or swine origin. This observation is in line with the amino acid prevalence at position 205 observed in NS1 of H1N1 viruses of swine origin isolated in Northern temperate regions, where the amino acids serine and asparagine were detected in recent decades with changing prevalences. These results suggest that the genetic background of the virus determines the effect of substitutions introduced at position 205 (Table 1). However, infection of primary human bronchial epithelial cells with WT H1N1pdm09 and substitution mutants also indicated a replication advantage in the presence of NS1 N205S as observed for PR8, although to a lower extent. Interestingly, while the replication of PR8 expressing NS1 S205G showed the same attenuation as that of a virus expressing NS1 S205N in a cell line of swine origin, the substitution of serine for asparagine led to a more pronounced reduction in replication in a human cell line. These results indicate that a phosphorylatable serine at position 205 in the genetic background of human H1N1 viruses might be, in general, advantageous for viral replication in cell lines of both species, while the replacement of serine with asparagine leads to an attenuation especially in the human cell line, perhaps partially explaining the discrete selection pressure for the adaptational mutation of H1N1pdm09 NS1 N205S. The reasons for the highly attenuated phenotype observed in PR8 NS1 S205N infection still remain elusive. So far, effects on the NS1-mediated suppression of the IFN system or its enhancement of viral polymerase activity can be excluded; however, the detailed mechanism of attenuation needs further investigation.
TABLE 1.
Summary of research findings with different NS1 variants in the two virus genetic backgroundsa
This table summarizes the effects of NS1 S205 or N205 on different steps in the viral replication cycle in the genetic background of the two H1N1 viruses PR8 and H1N1pdm09.
In this study, we provide evidence that in the presence of NS1 S205, phosphorylation is needed for efficient PR8 NS1-mediated enhancement of viral polymerase activity. The NS1 phosphorylation dynamic as determined by mass spectrometry pointed toward its phosphorylation in the nucleus, and kinase prediction, in turn, identified CK2 as a kinase potentially involved in NS1 phosphorylation at S205. CK2 has already been reported previously to affect viral replication by phosphorylation of the viral protein PA, which is required for efficient viral polymerase activity (20, 21). Consequently, we observed a slight titer reduction in the presence of the CK2 inhibitor DMAT in PR8 NS1 S205G infection also, as well as decreased viral RNA expression in the presence of both NS1 S205 substitution mutants, which might most likely be attributed to missing PA phosphorylation. While, according to our data, CK2 is a most likely candidate, it might not be the only kinase mediating the effects observed. Besides reducing replication, inhibition of CK2 abrogated DDX21–NS1 interaction to the same extent as S205G substitution, highlighting the requirement of PR8 NS1 S205 phosphorylation for efficient DDX21 binding. Surprisingly, the DDX21 interaction site on NS1 identified previously (5) differs from the region of NS1 exhibiting the phosphosite. The serine at position 205 is located in the disordered C-terminal tail that could not be crystallized yet. It is conceivable that this flexible tail hinders the DDX21–NS1 interaction by masking the interaction site mapped to the N-terminal RNA-binding domain comprising R37/38, K41, and R44 in the 3-dimensional structure of NS1 that might be exposed due to S205 phosphorylation mediated by CK2. This “open” conformation seems to be provided not only by the phosphorylation of serine 205 but also by the presence of asparagine 205, which allows for DDX21 interaction and enhances polymerase activity just as phosphorylated S205 does.
Surprisingly, in contrast to the findings of a previous study, we observed reduced viral replication in the absence of cellular DDX21. DDX21 has been reported to interact with IAV NS1, which hijacks DDX21, preventing its interaction with IAV PB1. Binding of DDX21 to PB1 has been reported to hinder efficient viral polymerase complex assembly, thus reducing viral polymerase activity (5). In the aforementioned study, the replication of an avian influenza virus, as well as the replication of human WSN, was analyzed. In that study, DDX21 came into focus due to its coprecipitation with CPSF30–NS1 complexes. However, PR8 as well as swine-origin H1N1pdm09 NS1 proteins are known for their inability to interact with CPSF30 (22, 23). Therefore, we cannot exclude the possibility that reasons for different effects of DDX21–NS1 interaction on virus replication might underlie the inability of PR8 NS1 to interact with CPSF30. Furthermore, sequence variations within the viral PB1 protein allowing for or abrogating DDX21 interaction might also be responsible for the discrepancies between the two studies.
The present study underlines the importance of understanding the function of adaptational mutations in order to gain deeper knowledge of viral replication mechanisms and the functional evolution of viral proteins. Molecular analysis of reacquired or newly emerging modification sites can help to identify new targets for antiviral intervention.
MATERIALS AND METHODS
Cell lines.
Most cell lines used in this study were originally purchased from the ATCC and have been passaged in the laboratory. At regular intervals, cells are checked for their identity by single nucleotide polymorphism (SNP) profiling (Multiplexion). Swine lung WSL-R-HP cells were obtained from the Collection of Cell Lines in Veterinary Medicine of the Friedrich-Loeffler-Institute (Federal Research Institute for Animal Health, Greifswald–Insel Riems, Germany), while primary human bronchial epithelial cells (HBEpCs) were purchased from PromoCell and cultivated in Airway Epithelial Cell growth medium (PromoCell). Human embryonic kidney 293 (HEK293) cells, human alveolar lung epithelial cells (A549), WSL-R-HP, and green monkey epithelial cells (Vero) were grown in Dulbecco’s modified Eagle medium (DMEM). Madin-Darby canine kidney epithelial (MDCKII) cells were grown in minimum essential medium (MEM). All media were supplemented with 10% fetal bovine serum (FBS), and cell lines were incubated at 37°C under a 5% CO2 atmosphere.
Viruses.
The recombinant system of the A/Puerto Rico/8/34 virus was a kind gift from E. Hoffmann (St. Jude Children’s Research Hospital, Memphis, TN, USA) (GenBank accession numbers CY084013.1, CY038901.1, CY084019.1, CY047401.1, CY105898.1, CY105896.1, CY105905.1, and CY038907.1). The A/Puerto Rico/8/34 ΔNS1 virus (19) and the recombinant system of the A/Hamburg/04/2009 virus were obtained from T. Wolff (Robert Koch Institute, Berlin, Germany) (GenBank accession numbers GQ166207, GQ166209, GQ166211, GQ166213, GQ166215, GQ166217, GQ166219, and GQ166221).
Reagents and plasmids.
Cells were preincubated with the CK2-specific inhibitor DMAT (dimethyl sulfoxide [DMSO] soluble; Calbiochem) at 5 μM for 1 h at 37°C as indicated in the legend to Fig. 5.
The DDX21-expressing vector pcDNA5-Flag-DDX21 was a kind gift from M. Bohnsack (Institute for Molecular Biology, University Medical Center [UMG], Georg-August-University, Göttingen, Germany). The luciferase reporter construct pTATA-IFNβ-luc was a kind gift from J. Hiscott (Istituto Pasteur Italia Cenci Bolognetti Foundation, Rome, Italy) and contains the whole IFN-β promoter upstream of the luciferase gene. pHW72‐Luc was obtained from R. Webster (St. Jude Children's Research Hospital, Memphis, TN). pcDNA3-Strep-NS1 plasmids were generated by linker ligation. Specifically, double-stranded oligonucleotides containing the Strep-tag sequence (forward [fw], 5′-GATCATGTGGAGCCACCCGCAGTTCGAAAAAGGAGGAATG-3′; reverse [rev], 5′-GATCCATTCCTCCTTTTTCGAACTGCGGGTGGCTCCACAT-3′) were digested with the BamHI and EcoRI restriction enzymes and were cloned into the pcDNA3-NS1 plasmid.
NS1 sequence analysis.
The sequences of the NS1 proteins of human A/H1N1, A/H3N2, and A/H2N2 or swine A/H1N1, A/H3N2, and A/H1N2 virus strains (Northern temperate regions), as well as those of all avian IAVs isolated from 1902 until 2020, were aligned and analyzed for amino acid prevalence at position 205 (n, 6 to 5,317 sequences/time frame). Sequences were obtained from the Influenza Virus Resource at the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/genomes/FLU/Database/nph-select.cgi?go=database) and were analyzed by using the MegAlign Pro (DNAStar) server.
Virus infection.
Vero, WSL-R-HP, and A549 cells, as well as HBEpCs, were infected with wild-type or mutant PR8 or H1N1pdm09 viruses in phosphate-buffered saline (PBS) (1% penicillin-streptomycin, 0.2% [vol/vol] bovine serum albumin, 0.01% MgCl2, 0.01% CaCl2) at multiplicities of infection (MOI) of 0.1 to 5, as indicated, for 30 min at 37°C. The virus inoculum was removed, and monolayers were washed with PBS. DMEM or MEM supplemented with 1% penicillin-streptomycin, 0.2% (vol/vol) bovine serum albumin, 0.01% MgCl2, 0.01% CaCl2, and 0.15 μg l-(tosylamido-2-phenyl) ethyl chloromethyl ketone (TPCK)-treated trypsin was added, and plates were incubated at 37°C. Supernatants or cell lysates were harvested at the time points indicated in the figures. For immunofluorescence, synchronization of infection was performed at 4°C for 30 min. Afterwards, the virus inoculum was removed, and cells were washed with PBS. Subsequently, PBS for infection was added to allow the attached viruses to enter the cells for 30 min at 37°C.
Generation of recombinant viruses.
PR8 NS1 S205G, S205D, and S205N, as well as H1N1pdm09 NS1 N205S and N205D, were generated by using a set of eight plasmids based on the bidirectional pHW2000 plasmid reverse genetics system (24). Vectors carrying the amino acid changes from serine (AGC) to glycine (GGC), aspartic acid (GAT), or asparagine (AAC), as well as from asparagine (AAC) to serine (AGC) or aspartic acid (GAT), in position 205 were generated by site-directed mutagenesis. Primer sequences for the following NS1 variants are given in parentheses: PR8 NS1 S205G (fw, 5′-AGATTCGCTTGGAGAGGCAGTAATGAGAATG-3′; rev, 5′-CATTCTCATTACTGCCTCTCCAAGCGAATCT-3′), PR8 NS1 S205D (fw, 5′-GATTCGCTTGGAGAGATAGTAATGAGAATGGG-3′; rev, 5′-CCCATTCTCATTACTATCTCTCCAAGCGAATC-3′), PR8 NS1 S205N (fw, 5′-GATTCGCTTGGAGAAATAGTAATGAGAATGGG-3′; rev, 5′-CCCATTCTCATTACTATTTCTCCAAGCGAATC-3′), H1N1pdm09 NS1 N205S (fw, 5′-GATTCGCTTGGAGAAGCTGTGATGAGAATGG-3′; rev, 5′-CCATTCTCATCACAGCTTCTCCAAGCGAATC-3′), and H1N1pdm09 NS1 N205D (fw, 5′-AGATTCGCTTGGAGAGACTGTGATGAGAATG-3′; rev, 5′-CATTCTCATCACAGTCTCTCCAAGCGAATCT-3′). Of note, substitution of PR8 NS1 S205G for S205 does not change the amino acid sequence of the nuclear export protein (NEP), while the introduction of S205N leads to a change from alanine (A) to threonine (T) at position 48; however, this change is also naturally selected for in H1N1pdm09. The opposite substitution is introduced into the NEP for H1N1pdm09 N205S, where threonine (T) is changed to alanine (A), while mutation of NS1 N205 to aspartic acid does not change the amino acid sequence of the NEP. Confluent cocultures of HEK293 and MDCKII cells were transfected with 1 μg DNA of each plasmid encoding the eight viral segments by using Opti-MEM and Lipofectamine 2000. The medium was removed 16 h posttransfection (p.t.), and fresh DMEM supplemented with 1% penicillin-streptomycin, 0.2% (vol/vol) bovine serum albumin, 0.01% MgCl2, 0.01% CaCl2, and 0.15 μg TPCK-treated trypsin was added. Supernatants were centrifuged for 10 min at 4,000 rpm (4°C) and were titrated by standard plaque assays. Viruses were plaque purified and further propagated on MDCKII cells.
Virus titration by standard plaque assay.
MDCKII cells were grown in 6-well plates until full confluence. Serial 10-fold dilutions of virus-containing supernatants were used to infect MDCKII cells, and the cells were incubated for 30 min at 37°C. The virus inoculum was removed and replaced by plaque medium (14.2% [vol/vol] 10× MEM, 0.3% [vol/vol] NaHCO3, 0.014% [vol/vol] DEAE-dextran, 1.4% [vol/vol] 100× penicillin-streptomycin, 0.3% [vol/vol] BSA, 0.9% [vol/vol] agar, 0.01% [vol/vol] MgCl2, 0.01% [vol/vol] CaCl2, 0.15 μg TPCK-trypsin). Cells were incubated upside down for ∼72 h at 37°C.
Western blot analysis.
Infected cells were washed twice with PBS and lysed in radioimmunoprecipitation assay (RIPA) buffer (25 mM Tris-HCl [pH 8], 137 mM NaCl, 10% glycerol, 0.1% SDS, 0.5% sodium deoxycholate, 1% NP-40, 2 mM EDTA [pH 8]) supplemented with protease inhibitor cocktail. Lysates were cleared by centrifugation at 20,000 × g for 15 min at 4°C. Relative protein concentrations were measured by a Bradford protein assay, and 5× Laemmli buffer was added. Equal amounts of total protein were separated by SDS-PAGE and transferred to nitrocellulose membranes. Five percent BSA diluted in TBS-T buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.2% Triton X-100) was used for blocking. Primary and secondary antibodies were diluted in TBS-T buffer supplemented with BSA (5% [wt/vol]), added to the membranes, and incubated for 1 h. Antisera directed against influenza A virus NS1 (catalog no. GTX125990), PB1 (GTX125923), and NP (GTX125989) were purchased from GeneTex. Flag (F3165) and DDX21 (ab126968) antibodies were obtained from Sigma and Abcam, respectively, and ERK2 (SC-1649) antibodies were from Santa Cruz Biotechnology. Documentation using fluorescence was performed with the Li-Cor Odyssey Fc imaging system, and semiquantification of protein expression was performed using Image Studio software.
siRNA-mediated gene silencing.
Human DDX21 siRNAs (siRNA 1, 5′-CCAGCGCUCCUUGAUCAACUCAAAU-3′; siRNA 2, 5′-AGGCCAGAAGCGGAGUUUCAGUAAA-3′) (12), as well as a control siRNA (5′-UUCUCCGAACGUGUCACGU-3′), were synthesized by Eurofins Genomics GmbH. Transfection of A549 cells was performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocols.
qRT-PCR.
Isolation of total RNA from cell monolayers was performed using the RNeasy Plus minikit (Qiagen) or the Monarch Total RNA Miniprep kit (New England Biolabs [NEB]) according to the manufacturer’s protocols. One microgram of RNA was used to synthesize cDNA with the RevertAid reverse transcriptase with 0.5 μg of oligo(dT). Quantitative RT-PCRs (qRT-PCRs) were performed using Brilliant III SYBR green QPCR master mix according to the manufacturer’s instructions. Primer sequences are as follows: for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-GCAAATTCCATGGCACCGT-3′ (fw) and 5′-GCCCCACTTGATTTGGAGG-3′ (rev); for MxA, 5′-GTTTCCGAAGTGGACATCGCA-3′ (fw) and 5′-GAAGGGCAACTCCTGACAGT-3′ (rev); for IP10, 5′-GTTTCCGAAGTGGACATCGCA-3′ (fw) and 5′-AGACATCTCTTCTCACCCTTC-3′ (rev); for NP, 5′-CGATCGTGCCTTCCTTTG-3′ (fw) and 5′-AGTAGAAACAAGGGTATTTTTCTTTAATTGTCG-3′ (rev); for PB1, 5′-CATACAGAAGACCAGTCGGGAT-3′ (fw) and 5′-GTCTGAGCTCTTCAATGGTGGA-3′ (rev); for NS, 5′-GAGGACTTGAATGGAATGATAACA-3′ (fw) and 5′-GTCTCAATTCTTCAATCAATCAACCATC-3′ (rev); for M1, 5′-TGCAAAAACATCTTCCAAGTCTCTG-3′ (fw) and 5′-AGATGAGTCTTCTAACCGAGGTCG-3′ (rev). The threshold cycle (CT) values obtained were normalized to GAPDH expression levels and quantified by using the ΔΔCT method (25).
Indirect immunofluorescence.
A549 cells were seeded in 24-well plates onto coverslips, infected, and fixed at the time points indicated in the figures with 4% paraformaldehyde in PBS. After 15 min, cells were washed twice with PBS. Permeabilization was performed with 0.1% Triton X-100 for 15 min with gentle shaking and subsequent washing (twice) with PBS. Blocking was performed with 3% BSA for 1 h at room temperature. Coverslips with diluted mouse anti-NP (Bio-Rad) and rabbit anti-NS1 (GeneTex) primary antibodies were incubated for 2 h at room temperature. Alexa Fluor 488- and Alexa Fluor 568-conjugated secondary antibodies were used for visualization. DAPI (4′,6-diamidino-2-phenylindole) was added in the last washing step for 10 min, and cells on coverslips were mounted on slides using fluorescence mounting medium. Slides were analyzed using an LSM 800 confocal microscope, and pictures were prepared with Fiji/ImageJ software, version 1.51n.
Reporter gene assays.
Transfection of Vero cells with the pTATA-IFNβ-luc luciferase reporter plasmid (0.3 μg) in combination with the different NS1 expression plasmids (1 μg) was performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. At 24 h p.t., cells were transfected with 500 ng total RNA isolated from infected cells (vRNA) or mock-infected cells (control RNA) (MOI, 5; 8 h). Luciferase assays were carried out 8 h poststimulation as described previously (26). Relative light units were normalized to protein concentrations determined by the Bradford assay.
Viral minigenome assays were performed by using the luciferase reporter gene plasmid pHW72‐luc, which is driven by a pol I transcription unit containing the noncoding region of the IAV M gene. Vero cells were transfected with pHW72‐luc (0.5 μg), pHW2000‐PB2, pHW2000‐PB1, pHW2000‐PA, or pHW2000‐NP (all from IAV PR8 or H1N1pdm09; 0.25 μg each; 0.4 μg NP) in combination with the different NS1 expression plasmids (1 μg), and viral polymerase activity was measured 24 h p.t. as described above.
Coimmunoprecipitation and pulldown.
Infected A549 cells overexpressing NS1 S205 or substitution mutants were lysed with RIPA buffer (see above) containing protease and phosphatase inhibitors. After clearance by centrifugation, serine-phosphorylated proteins were immunoprecipitated by using antibodies directed against phosphoserine (ab9332; Abcam) and protein G agarose beads (Roche) overnight (o/n) at 4°C. Vero cells overexpressing DDX21-Flag in combination with different NS1-Strep mutants were lysed with Triton lysis buffer (TLB) (20 mM Tris [pH 7.4], 137 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM EDTA, 50 mM glycerol 2-phosphate, 20 mM sodium pyrophosphate [pH 8.3]) containing protease and phosphatase inhibitors. Lysates were cleared by centrifugation, and supernatants were incubated with Strep-Tactin Sepharose (IBA Lifesciences) for 4 h at 4°C. Complexes were washed three times with the respective lysis buffer and resolved by SDS-PAGE with subsequent electrotransfer to nitrocellulose membranes.
Mass spectrometry.
For the screening of influenza A virus NS1 phosphorylation, the stable-isotope labeling by amino acids in cell culture (SILAC) method was used. Human A549 cells stably labeled with either “light” lysine (12C6, 14N2) and arginine (12C6, 14N4), “medium” lysine (13C6, 14N2) and arginine (13C6, 14N2), or “heavy” lysine (13C6, 15N2) and arginine (13C6, 15N4) were infected with PR8 (MOI, 5) for 0, 2, 4, 6, or 8 h. Thereby “medium”‐labeled and “heavy”‐labeled cells were infected for 2 or 6 h and 4 or 8 h, respectively, while “light”‐labeled cells were always used as noninfected controls (0 h). Lysates from cells infected for 0, 2, and 4 h were mixed equally and used as the first sample, while equally mixed lysates from cells infected for 0, 6, and 8 h were used as the second sample. After tryptic digestion, samples were subjected to proteome analysis and a separate phosphoproteome analysis by cation-exchange chromatography with or without TiO2‐based phosphopeptide enrichment chromatography, respectively, followed by liquid chromatography-tandem mass spectrometry (LC–MS-MS) analysis on a Proxeon Easy‐nLC instrument coupled to an LTQ‐Orbitrap XL mass spectrometer to discriminate between regulation at the P-site and the protein level. Data processing, including kinase motif scanning, was performed by using Mascot and MaxQuant software (version 1.0.14.3) as described previously (27, 28). The phosphorylation intensity of NS1-derived peptides was quantified over time (medium/heavy ratio) in relation to the amount of total NS1 protein expressed.
Quantification and statistical analysis.
Statistical significance was analyzed, and graphs were generated, by using GraphPad Prism, version 6.01. The particular tests used and the sample sizes can be found in the respective figure legends. Band intensities in Western blots were analyzed by using Image Studio, version 5.2, and final editing of immunofluorescence pictures was performed by using Fiji/ImageJ, version 1.51n.
ACKNOWLEDGMENTS
We thank Danielle Brandes, Michael Wojak, Michael Sulk, Saskia Hinse, Marcel E. Friedrich, and Felicia Hwa for excellent technical assistance.
This work was supported by the German Research Foundation (DFG) (grants Lu477/23-1, RTG 2220 project number 281125614 A1 and A2, CRU342 P4 and P6 as well as BO5122/1-1) and the German Academic Exchange Service (DAAD). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Nogales A, Martinez-Sobrido L, Topham DJ, DeDiego ML. 2018. Modulation of innate immune responses by the influenza A NS1 and PA-X proteins. Viruses 10:708. doi: 10.3390/v10120708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marc D. 2014. Influenza virus non-structural protein NS1: interferon antagonism and beyond. J Gen Virol 95:2594–2611. doi: 10.1099/vir.0.069542-0. [DOI] [PubMed] [Google Scholar]
- 3.Hale BG, Randall RE, Ortin J, Jackson D. 2008. The multifunctional NS1 protein of influenza A viruses. J Gen Virol 89:2359–2376. doi: 10.1099/vir.0.2008/004606-0. [DOI] [PubMed] [Google Scholar]
- 4.Mok BW, Song W, Wang P, Tai H, Chen Y, Zheng M, Wen X, Lau SY, Wu WL, Matsumoto K, Yuen KY, Chen H. 2012. The NS1 protein of influenza A virus interacts with cellular processing bodies and stress granules through RNA-associated protein 55 (RAP55) during virus infection. J Virol 86:12695–12707. doi: 10.1128/JVI.00647-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen G, Liu CH, Zhou L, Krug RM. 2014. Cellular DDX21 RNA helicase inhibits influenza A virus replication but is counteracted by the viral NS1 protein. Cell Host Microbe 15:484–493. doi: 10.1016/j.chom.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe T, Imai M, Kawaoka Y. 2017. NS1 is the fluid for “flu-transmission.” Proc Natl Acad Sci U S A 114:11012–11014. doi: 10.1073/pnas.1715239114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanin M, Wong S-S, Barman S, Kaewborisuth C, Vogel P, Rubrum A, Darnell D, Marinova-Petkova A, Krauss S, Webby RJ, Webster RG. 2017. Molecular basis of mammalian transmissibility of avian H1N1 influenza viruses and their pandemic potential. Proc Natl Acad Sci U S A 114:11217–11222. doi: 10.1073/pnas.1713974114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark AM, Nogales A, Martinez-Sobrido L, Topham DJ, DeDiego ML. 2017. Functional evolution of influenza virus NS1 protein in currently circulating human 2009 pandemic H1N1 viruses. J Virol 91:e00721-17. doi: 10.1128/JVI.00721-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutchinson EC, Denham EM, Thomas B, Trudgian DC, Hester SS, Ridlova G, York A, Turrell L, Fodor E. 2012. Mapping the phosphoproteome of influenza A and B viruses by mass spectrometry. PLoS Pathog 8:e1002993. doi: 10.1371/journal.ppat.1002993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hale BG, Knebel A, Botting CH, Galloway CS, Precious BL, Jackson D, Elliott RM, Randall RE. 2009. CDK/ERK-mediated phosphorylation of the human influenza A virus NS1 protein at threonine-215. Virology 383:6–11. doi: 10.1016/j.virol.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Hsiang TY, Zhou L, Krug RM. 2012. Roles of the phosphorylation of specific serines and threonines in the NS1 protein of human influenza A viruses. J Virol 86:10370–10376. doi: 10.1128/JVI.00732-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kathum OA, Schrader T, Anhlan D, Nordhoff C, Liedmann S, Pande A, Mellmann A, Ehrhardt C, Wixler V, Ludwig S. 2016. Phosphorylation of influenza A virus NS1 protein at threonine 49 suppresses its interferon antagonistic activity. Cell Microbiol 18:784–791. doi: 10.1111/cmi.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng W, Cao S, Chen C, Li J, Zhang S, Jiang J, Niu Y, Fan W, Li Y, Bi Y, Gao GF, Sun L, Liu W. 2017. Threonine 80 phosphorylation of non-structural protein 1 regulates the replication of influenza A virus by reducing the binding affinity with RIG-I. Cell Microbiol 19:e12643. doi: 10.1111/cmi.12643. [DOI] [PubMed] [Google Scholar]
- 14.Cheng J, Tao J, Li B, Shi Y, Liu H. 2019. The tyrosine 73 and serine 83 dephosphorylation of H1N1 swine influenza virus NS1 protein attenuates virus replication and induces high levels of beta interferon. Virol J 16:152. doi: 10.1186/s12985-019-1255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng J, Zhang C, Tao J, Li B, Shi Y, Liu H. 2018. Effects of the S42 residue of the H1N1 swine influenza virus NS1 protein on interferon responses and virus replication. Virol J 15:57. doi: 10.1186/s12985-018-0971-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randall RE, Goodbourn S. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol 89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 17.Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, Reis e Sousa C. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 18.Lutz A, Dyall J, Olivo PD, Pekosz A. 2005. Virus-inducible reporter genes as a tool for detecting and quantifying influenza A virus replication. J Virol Methods 126:13–20. doi: 10.1016/j.jviromet.2005.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García-Sastre A, Egorov A, Matassov D, Brandt S, Levy DE, Durbin JE, Palese P, Muster T. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324–330. doi: 10.1006/viro.1998.9508. [DOI] [PubMed] [Google Scholar]
- 20.Perales B, Sanz-Ezquerro JJ, Gastaminza P, Ortega J, Santaren JF, Ortin J, Nieto A. 2000. The replication activity of influenza virus polymerase is linked to the capacity of the PA subunit to induce proteolysis. J Virol 74:1307–1312. doi: 10.1128/jvi.74.3.1307-1312.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanz-Ezquerro JJ, Fernández Santarén J, Sierra T, Aragón T, Ortega J, Ortín J, Smith GL, Nieto A. 1998. The PA influenza virus polymerase subunit is a phosphorylated protein. J Gen Virol 79:471–478. doi: 10.1099/0022-1317-79-3-471. [DOI] [PubMed] [Google Scholar]
- 22.Kochs G, Garcia-Sastre A, Martinez-Sobrido L. 2007. Multiple anti-interferon actions of the influenza A virus NS1 protein. J Virol 81:7011–7021. doi: 10.1128/JVI.02581-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hale BG, Steel J, Medina RA, Manicassamy B, Ye J, Hickman D, Hai R, Schmolke M, Lowen AC, Perez DR, Garcia-Sastre A. 2010. Inefficient control of host gene expression by the 2009 pandemic H1N1 influenza A virus NS1 protein. J Virol 84:6909–6922. doi: 10.1128/JVI.00081-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. 2000. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A 97:6108–6113. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Borgeling Y, Schmolke M, Viemann D, Nordhoff C, Roth J, Ludwig S. 2014. Inhibition of p38 mitogen-activated protein kinase impairs influenza virus-induced primary and secondary host gene responses and protects mice from lethal H5N1 infection. J Biol Chem 289:13–27. doi: 10.1074/jbc.M113.469239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpy A, Patel A, Tay YD, Hagan IM, Macek B. 2015. Nic1 inactivation enables stable isotope labeling with 13C615N4-arginine in Schizosaccharomyces pombe. Mol Cell Proteomics 14:243–250. doi: 10.1074/mcp.O114.045302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macek J, Ptacek P, Klima J. 2009. Determination of tolterodine and its 5-hydroxymethyl metabolite in human plasma by hydrophilic interaction liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 877:968–974. doi: 10.1016/j.jchromb.2009.02.036. [DOI] [PubMed] [Google Scholar]