Abstract
Background
Standard treatment for deep vein thrombosis (DVT) aims to reduce immediate complications. Use of thrombolytic clot removal strategies (i.e. thrombolysis (clot dissolving drugs), with or without additional endovascular techniques), could reduce the long‐term complications of post‐thrombotic syndrome (PTS) including pain, swelling, skin discolouration, or venous ulceration in the affected leg. This is the fourth update of a Cochrane Review first published in 2004.
Objectives
To assess the effects of thrombolytic clot removal strategies and anticoagulation compared to anticoagulation alone for the management of people with acute deep vein thrombosis (DVT) of the lower limb.
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, CINAHL and AMED and the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registries to 21 April 2020. We also checked the references of relevant articles to identify additional studies.
Selection criteria
We considered randomised controlled trials (RCTs) examining thrombolysis (with or without adjunctive clot removal strategies) and anticoagulation versus anticoagulation alone for acute DVT.
Data collection and analysis
We used standard methodological procedures as recommended by Cochrane. We assessed the risk of bias in included trials with the Cochrane 'Risk of bias' tool. Certainty of the evidence was evaluated using GRADE. For dichotomous outcomes, we calculated the risk ratio (RR) with the corresponding 95% confidence interval (CI). We pooled data using a fixed‐effect model, unless we identified heterogeneity, in which case we used a random‐effects model. The primary outcomes of interest were clot lysis, bleeding and post thrombotic syndrome.
Main results
Two new studies were added for this update. Therefore, the review now includes a total of 19 RCTs, with 1943 participants. These studies differed with respect to the thrombolytic agent, the doses of the agent and the techniques used to deliver the agent. Systemic, loco‐regional and catheter‐directed thrombolysis (CDT) strategies were all included. For this update, CDT interventions also included those involving pharmacomechanical thrombolysis. Three of the 19 included studies reported one or more domain at high risk of bias. We combined the results as any (all) thrombolysis interventions compared to standard anticoagulation.
Complete clot lysis occurred more frequently in the thrombolysis group at early follow‐up (RR 4.75; 95% CI 1.83 to 12.33; 592 participants; eight studies) and at intermediate follow‐up (RR 2.42; 95% CI 1.42 to 4.12; 654 participants; seven studies; moderate‐certainty evidence). Two studies reported on clot lysis at late follow‐up with no clear benefit from thrombolysis seen at this time point (RR 3.25, 95% CI 0.17 to 62.63; two studies). No differences between strategies (e.g. systemic, loco‐regional and CDT) were detected by subgroup analysis at any of these time points (tests for subgroup differences: P = 0.41, P = 0.37 and P = 0.06 respectively).
Those receiving thrombolysis had increased bleeding complications (6.7% versus 2.2%) (RR 2.45, 95% CI 1.58 to 3.78; 1943 participants, 19 studies; moderate‐certainty evidence). No differences between strategies were detected by subgroup analysis (P = 0.25).
Up to five years after treatment, slightly fewer cases of PTS occurred in those receiving thrombolysis; 50% compared with 53% in the standard anticoagulation (RR 0.78, 95% CI 0.66 to 0.93; 1393 participants, six studies; moderate‐certainty evidence). This was still observed at late follow‐up (beyond five years) in two studies (RR 0.56, 95% CI 0.43 to 0.73; 211 participants; moderate‐certainty evidence).
We used subgroup analysis to investigate if the level of DVT (iliofemoral, femoropopliteal or non‐specified) had an effect on the incidence of PTS. No benefit of thrombolysis was seen for either iliofemoral or femoropopliteal DVT (six studies; test for subgroup differences: P = 0.29). Systemic thrombolysis and CDT had similar levels of effectiveness. Studies of CDT included four trials in femoral and iliofemoral DVT, and results from these are consistent with those from trials of systemic thrombolysis in DVT at other levels of occlusion.
Authors' conclusions
Complete clot lysis occurred more frequently after thrombolysis (with or without additional clot removal strategies) and PTS incidence was slightly reduced. Bleeding complications also increased with thrombolysis, but this risk has decreased over time with the use of stricter exclusion criteria of studies. Evidence suggests that systemic administration of thrombolytics and CDT have similar effectiveness. Using GRADE, we judged the evidence to be of moderate‐certainty, due to many trials having small numbers of participants or events, or both. Future studies are needed to investigate treatment regimes in terms of agent, dose and adjunctive clot removal methods; prioritising patient‐important outcomes, including PTS and quality of life, to aid clinical decision making.
Plain language summary
Thrombolysis for treatment of acute deep vein thrombosis
Background
Deep vein thrombosis (DVT) occurs when a blood clot forms in a leg vein. The clot can break up and move to the lungs, leading to a potentially serious blockage in blood flow (pulmonary embolism or PE). Because of the damage to the leg vein, post‐thrombotic syndrome (PTS) may develop any time over the next couple of years. Symptoms include leg pain, swelling, skin pigmentation and leg ulcers, leading to loss of mobility. Anticoagulants are the standard treatment for DVT or a clot in a leg vein. These medications thin the blood to reduce further clots from forming and prevent PE; yet PTS can still develop. Another way of treating DVT is by thrombolysis. Thrombolysis breaks down the blood clot, and drugs such as streptokinase, urokinase and tissue plasminogen activator are infused into a vein in the arm or foot. In some cases, these drugs may be directly delivered to the site of the clot, using a catheter and X‐ray control. Additional surgical techniques can also be used to help remove the clot. Possible harmful side effects that can happen after both anticoagulation and thrombolysis include bleeding complications, stroke or intracerebral haemorrhage.
To find out whether thrombolytic clot removal strategies and anticoagulation might be better than anticoagulation alone for the management of people with acute DVT of the leg, we reviewed the evidence from research studies.
How did we identify and evaluate the evidence?
First, we searched the medical literature for randomised controlled trials (RCTs), clinical studies where people are randomly put into one of two or more treatment groups. This type of study provides the most robust evidence about the effects of a treatment. We then compared the results, and summarised the evidence from all the studies. Finally, we assessed how certain the evidence was. To do this, we considered factors such as the way studies were conducted, study sizes, and consistency of findings across studies. Based on our assessments, we categorised the evidence as being of very low, low, moderate or high certainty.
What did we find?
We found 19 RCTs that included a total of 1943 people with acute DVT to receive either thrombolysis or anticoagulant treatment. Trials were conducted in Belgium, Canada, Denmark, Egypt, France, Germany, the Netherlands, Norway, South Africa, Sweden, Switzerland, Turkey, the UK and the USA. All trials included men and women ranging in age from 18 to 75 years, with more older adults.
Our review found moderate‐certainty evidence that thrombolysis effectively dissolved the clot so that complete clot breakdown occurred more often with thrombolysis than with standard anticoagulant therapy. Those receiving thrombolysis had more bleeding complications than with standard anticoagulation (6.7% versus 2.2%). Most bleeding episodes occurred in the older studies. Six trials (1393 participants) continued for over six months and found that slightly fewer people developed PTS when treated with thrombolysis; 50% compared with 53% in the standard anticoagulation treatment group. Two trials (211 participants) that continued for over five years showed that fewer people developed PTS when treated with thrombolysis. Use of strict eligibility criteria appears to have improved the safety of this treatment, which is effective delivered directly to the clot by catheter or via the bloodstream from another vein. We did not find any evidence that the position of the clot within the leg made it more or less likely for people to get PTS. Future studies are needed to investigate what clot removal method is most beneficial to patient important outcomes including PTS, bleeding and quality of life.
How up‐to date is this review?
The evidence in this Cochrane Review is current to 21 April 2020.
Summary of findings
Summary of findings 1. Treatment with any thrombolysis strategy for acute deep vein thrombosis.
| Treatment with any thrombolysis clot removal strategy for acute DVT | ||||||
| Patient or population: patients diagnosed with acute DVT Setting: hospital Intervention: thrombolysis1 Comparison: standard anticoagulation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard anticoagulation | Risk with thrombolysis | |||||
|
Complete clot lysis (intermediate, 6 months to under 5 years after treatment) |
Study population | RR 2.42 (1.42 to 4.12) | 654 (7 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | 78 of 244 patients treated with standard anticoagulation had complete clot lysis compared to 198 of 410 in the thrombolysis group | |
| 320 per 1000 | 774 per 1000 (454 to 1000) | |||||
|
Bleeding (early, up to 1 month after treatment) |
Study population | RR 2.45 (1.58 to 3.78) | 1943 (19 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | ||
| 23 per 1000 | 56 per 1000 (36 to 87) |
|||||
|
Post‐thrombotic syndrome (intermediate, 6 months to under 5 years after treatment) |
Study population | RR 0.78 (0.66 to 0.93) | 1393 (6 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | 329 of 622 patients treated with standard anticoagulation developed PTS compared to 383 of 771 treated with thrombolysis. Additional subgroup analysis did not show any differences in PTS incidence between iliofemoral and femoropopliteal DVTs |
|
| 529 per 1000 | 413 per 1000 (349 to 492) | |||||
|
Post‐thrombotic syndrome (late, 5 years follow‐up after treatment) |
Study population | RR 0.56 (0.43 to 0.73) | 211 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | 75 of 107 patients treated with standard anticoagulation developed PTS compared to 41 of 104 treated with thrombolysis | |
| 701 per 1000 | 393 per 1000 (301 to 512) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CDT: catheter‐directed thrombolysis; CI: confidence interval; DVT: deep vein thrombosis; PTS: post‐thrombotic syndrome; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Thrombolysis includes delivery of thrombolytics either systemically, loco‐regionally or by CDT. CDT may include the use of additional endovascular techniques 2Downgraded by one level as the number of participants in the majority of studies included in the analysis is small 3Downgraded by one level as the number of participants and events in the majority of studies included in the analysis is small (only 4/19 studies had over 100 participants)
Background
Description of the condition
Deep vein thrombosis (DVT) is a major health problem, with between 2.5% to 5% of the population affected at some time in their lives (Browse 1999; White 2006). Its main complications are pulmonary embolism (PE) in the short term and post‐thrombotic syndrome (PTS) in the long term. Standard treatment is with anticoagulation (thinning the blood to reduce formation of further clots) and is aimed mainly at the prevention of PE and recurrent DVT (Kearon 2016; NICE guidelines CG144). Despite treatment, over 50% of patients may suffer post‐thrombotic symptoms in the long term, manifested by some degree of pain, swelling, skin pigmentation or venous ulceration of the affected leg (Kahn 2006; Schulman 2006). This usually becomes apparent in the first two years after the thrombotic event (Brandjes 1997; Kahn 2004; Kahn 2008). Most studies report eventual venous ulceration in at least 6% of DVT patients, despite treatment (Johnson 1995; Schulman 2006). The prevalence of venous ulcers in the general population is around 1 in 1000, and between 40% to 50% of patients with venous ulcers have evidence of post‐thrombotic damage (Browse 1999; Kahn 2004). Complications including venous ulcers may result in significant disability and may be difficult to manage in both the community and secondary care. Because complications develop after hospital admission, there is a low level of awareness of these complications amongst the clinicians who dealt with the acute DVT admission.
Description of the intervention
Standard anticoagulation does not actively remove blood clots (Kearon 2012), whereas thrombolytic drugs act to dissolve blood clots by activating plasminogen. This forms an enzyme called plasmin that breaks links between the fibrin molecules, which make up blood clots. The drugs can be administered systemically through a peripheral vein, loco‐regionally via a vein close to the clot or directly via a catheter to the occluding thrombus (Sharafuddin 2003). The latter method, commonly known as catheter‐directed thrombolysis (CDT), more directly targets plasminogen within the clot and is less affected by potential inhibitors in the circulation (Comerota 1993). Thrombolysis may be used alongside a variety of endovascular (catheter‐based) techniques devices to increase drug penetration and speed clot removal. This is known as endovascular or pharmacomechanical catheter‐directed thrombolysis (PCDT) (Vedantham 2009a; Vedantham 2013). These adjunctive techniques may involve mechanical thrombectomy to aid removal of the clot (suction, rotation, rheolysis), and/or balloon angioplasty or stenting, or a combination of these techniques, aiming to preserve venous function (Sharafuddin 2003; Vedantham 2009a).
How the intervention might work
Dissolving the thrombus in the acute phase may reduce the risk of more permanent damage to the structure and function of the vein, in particular venous valvular function, thus lowering the risk of post‐thrombotic complications in the long term. Combining thrombolysis with adjunctive techniques may allow faster and more complete clot removal (Sharafuddin 2003; Vedantham 2009b).
Why it is important to do this review
This systematic review is the fourth update of a previously published Cochrane Review (Armon 2000 (protocol); Watson 2004; Watson 2010; Watson 2014; Watson 2016). Our review draws together previous comparative trials of thrombolysis and anticoagulation to reassess the advantages and disadvantages of thrombolytic therapy in the context of acute lower limb DVT, and to identify areas for future research. Our review now includes evidence from recent trials, including pharmacomechanical thrombectomy, to reflect current clinical practice and to aid decision‐making for professionals and patients.
Objectives
To assess the effects of thrombolytic clot removal strategies and anticoagulation compared to anticoagulation alone for the management of people with acute DVT of the lower limb.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) of thrombolysis (with or without adjunctive clot removal strategies) and anticoagulation, versus anticoagulation alone, for acute lower limb DVT. Any method of randomisation was eligible, and differences in methodological quality were taken into account in the analysis. Trials that were not analysed on an intention‐to‐treat (ITT) basis were included, provided investigators accounted for all randomised participants.
Types of participants
We included trials of adult (aged 18 and over) participants with acute DVT, defined as onset of symptoms within seven days and confirmed by objective testing with, for example, venography or duplex ultrasonography. Trials including participants with chronic or recurrent venous thrombosis were excluded, as were those with participants commencing treatment after a maximum of 21 days from the onset of symptoms. Trials including participants with arm vein thrombosis were included in the update when the majority of cases affected the lower limb.
Types of interventions
We included trials with the use of any thrombolytic agent, the principal ones being streptokinase, urokinase and tissue plasminogen activator (tPA); other agents were included if used for the treatment of acute DVT. All routes of drug lysis administration were considered, as were different dosing regimens of lytic agents. These included systemic and catheter‐directed thrombolysis (CDT) methods. As combinations of clot removal strategies are now frequently used in clinical practice, we also included studies where adjunctive thrombus removal techniques such as thrombectomy, balloon maceration, balloon venoplasty, aspiration, stenting etc, were used in combination with thrombolysis, provided they were compared to standard anticoagulation alone.
Types of outcome measures
Outcome assessments were classified as early (up to one month); intermediate (after six months to five years) or late (more than five years), from the time of intervention (see Included studies). When data were reported between one and six months, we planned to discuss and reassess the definition of our time points as required.
Primary outcomes
Complete clot lysis (defined as achievement of full patency of the affected vein, or complete dissolution of the clot, by objective measures)
Bleeding complications, excluding stroke or intracerebral haemorrhage (defined as bleeding causing treatment to be stopped, requiring transfusion or surgery, or causing chronic or fatal sequelae)
PTS
Secondary outcomes
Any improvement in venous patency (assessed by objective measures such as venography, where pre‐ and post‐comparative data on the degree of restoration of the lumen were available)
Stroke, and in particular haemorrhagic stroke (preferably documented by objective means such as a computerised tomography scan or autopsy)
Venous ulceration rates
Mortality
Recurrent DVT
PE
Venous function (assessed by duplex ultrasound or other objective means such as foot volumetry or ambulatory venous pressure measurements)
Quality of life (QoL)
Cost comparisons
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for RCTs and controlled clinical trials without language, publication year or publication status restrictions:
the Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web searched on 21 April 2020);
the Cochrane Central Register of Controlled Trials (CENTRAL) Cochrane Register of Studies Online (CRSO 2020, Issue 3);
MEDLINE (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) (searched from 1 January 2017 to 21 April 2020);
Embase Ovid (searched from 1 January 2017 to 21 April 2020);
CINAHL Ebsco (searched from 1 January 2017 to 21 April 2020);
AMED Ovid (searched from 1 January 2017 to 21 April 2020).
The Information Specialist modelled search strategies for other databases on the search strategy designed for CENTRAL. Where appropriate, strategies were combined with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying RCTs and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 6 (Lefebvre 2011). Search strategies for major databases are provided in Appendix 1.
The Information Specialist searched the following trials registries on 21 April 2020:
the World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch);
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
The reference lists of articles retrieved by electronic searches were searched for additional citations.
Data collection and analysis
Data were collected from the original papers and authors were contacted for clarification where necessary.
Selection of studies
One review author (CB) screened the titles and abstracts of all articles identified by the search, using Covidence. Non‐relevant articles were removed. Full‐text articles were obtained for the remaining references. Following this, pairs of review authors (from among CB, LW and MA), or one of these review authors and a member of the Cochrane Vascular editorial support team (MS; see Contributions of authors), then independently assessed these articles against the inclusion criteria. Any conflicts were resolved by discussion.
Data extraction and management
Two review authors (CB, LW), or one of these review authors and MS, independently extracted and checked data using pro formas designed by Cochrane Vascular. We contacted authors of ongoing trials to check for available data.
Assessment of risk of bias in included studies
Two review authors (from among CB, LW and MA), or one of these review authors and MS, independently assessed the risk of bias in included studies, following Cochrane Vascular guidelines and using the Cochrane 'Risk of bias' tool (Higgins 2011). Any disagreements were resolved by discussion.
Measures of treatment effect
We performed statistical analyses according to the statistical guidelines for review authors provided by Cochrane Vascular. If appropriate, we calculated a summary statistic for each dichotomous outcome using the risk ratio (RR) and its corresponding 95% confidence interval (CI). We present QoL statistics as reported by the respective studies in terms of means and standard deviations (SD) or standard errors (SE).
Unit of analysis issues
Individual participants were the unit of analysis. If appropriate, the control groups in the multiple arm trials were divided up to avoid double counting in the meta‐analysis.
Dealing with missing data
We conducted ITT analysis where possible. We recalculated any missing statistics from original data, where available. We contacted study authors to request data where it was not possible to identify specific event numbers from the data reported.
Assessment of heterogeneity
Heterogeneity was assessed clinically from descriptions of studies, visually from forest plots and statistically using the Chi2 test. If P < 0.05 for the Chi2 test, we used a random‐effects model; otherwise, we used a fixed‐effect model. We also considered heterogeneity by clinical judgements of differences in participant populations, interventions and outcome assessments.
Assessment of reporting biases
We assessed reporting bias through a review of the identified studies, and considered using funnel plots for outcomes when more than 10 studies with available data were included in a meta‐analysis.
Data synthesis
We pooled studies for meta‐analysis when the interventions, patient groups, outcome measures and timing of outcome assessment were sufficiently similar (determined by consensus). We calculated the pooled RRs and corresponding 95% CIs for dichotomous outcomes. We used a fixed‐effect model unless we identified statistical heterogeneity (as described above), in which case we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We analysed trials together and in subgroups, according to route of administration. We commented on other sources of heterogeneity, such as participant selection, type of DVT, drug or dose, where relevant. Where information was provided, we carried out subgroup analysis by location of DVT (iliofemoral, femoropopliteal or non‐specified).
Sensitivity analysis
We carried out sensitivity analyses for all outcomes where the meta‐analysis included trials judged to have any domain at high risk of bias. To determine if results were robust, we repeated meta‐analyses, excluding these studies.
Summary of findings and assessment of the certainty of the evidence
We created a 'Summary of findings' table using the GRADEpro GDT software (Gradepro GDT 2020). This summarised the evidence comparing thrombolysis to standard anticoagulation for study populations consisting of patients with acute DVT (Table 1). The most important and clinically relevant outcomes (both desirable and undesirable) that were thought to be essential for decision‐making were the outcomes 'complete clot lysis', 'bleeding' and 'post‐thrombotic syndrome'. Assumed control intervention risks were calculated by the mean number of events in the control groups of the selected studies for each outcome. We used the system developed by the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group for grading the certainty of evidence. We judged the certainty of evidence for each outcome to be high, moderate, low or very low, depending on within‐study risk of bias, inconsistency, directness of evidence, imprecision, and publication bias (Atkins 2004).
Results
Description of studies
Results of the search
For this update, two new studies met the criteria for inclusion; both were previously listed as ongoing studies (ATTRACT; CAVA 2020). Seventeen new studies were excluded (Ageno 2016; Ansari 2016; Bulatov 2019; Calik 2015; Deitelzweig 2016; Doyle 1987; Duan 2016; Fan 2015; Jiang 2017; Kim 2017; Kuo 2017; Liu 2013; NCT02414802; NCT02767232; Righini 2016; Song 2019; Yang 2016); two new studies were assessed as ongoing (ChiCTR‐INR‐16009090; NCT02959801); and two new studies were placed in studies awaiting classification (Gong 2018; Su 2017). See Figure 1.
1.
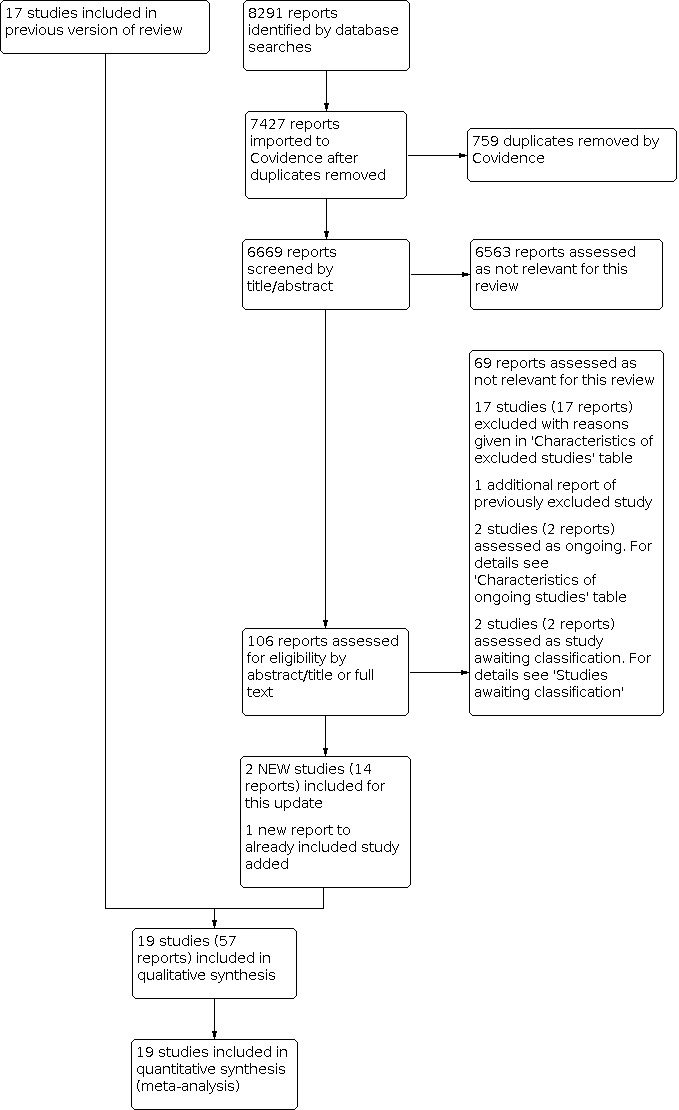
Study flow diagram.
Included studies
In total, we included 19 trials, with 1943 participants (Arnesen 1978; ATTRACT; CAVA 2020; CAVENT; Common 1976; Elliot 1979; Elsharawy 2002; Goldhaber 1990; Goldhaber 1996; Kakkar 1969; Kiil 1981; Marder 1977; Schulman 1986; Schweizer 1998; Schweizer 2000; Tsapogas 1973; Turpie 1990; Ugurlu 2002; Verhaeghe 1989). Studies were carried out from 1969 to 2017.
Participants
Trials were conducted in Belgium, Canada, Denmark, Egypt, France, Germany, the Netherlands, Norway, South Africa, Sweden, Switzerland, Turkey, the UK and the USA. All trials included men and women, with an age range from 18 to 75 years and with a preponderance of older adults. The participants had diverse underlying causes for developing DVT, and varying degrees of level and extent of occlusion. Both Elsharawy 2002 and CAVA 2020 included DVTs affecting femoral and iliofemoral veins; CAVENT included pelvic, femoral and iliofemoral veins; ATTRACT included proximal DVT; other trials included thrombosis affecting different combinations of levels, including popliteal. The only study to include calf vein thrombosis only was Schulman 1986. See Table 2,
1. Level of affected leg veins in included studies.
| Study | Potential levels of leg vein included |
| Arnesen 1978 | proximal to calf |
| ATTRACT | proximal (femoral, common femoral, iliac vein with or without other involved ipsilateral veins) |
| CAVA 2020 | femoral and iliofemoral |
| CAVENT | pelvic, iliofemoral, femoral |
| Common 1976 | not specified |
| Elliot 1979 | proximal |
| Elsharawy 2002 | femoral and iliofemoral |
| Goldhaber 1990 | popliteal or more proximal |
| Goldhaber 1996 | proximal |
| Kakkar 1969 | not specified |
| Kiil 1981 | not specified |
| Marder 1977 | calf up to iliac vein |
| Schulman 1986 | calf vein thrombosis only |
| Schweizer 1998 | not specified |
| Schweizer 2000 | leg or pelvic (popliteal or more proximal) |
| Tsapogas 1973 | not specified |
| Turpie 1990 | proximal |
| Ugurlu 2002 | popliteal up to inferior vena cava |
| Verhaeghe 1989 | popliteal or more proximal |
Participant inclusion criteria
Participant inclusion criteria have become more restrictive over time. In the earliest study (Kakkar 1969), there were only four contra‐indications: surgery within three days, an unhealed wound, peptic ulcer and hypertension. By the time of Schweizer 2000, a more comprehensive list of contra‐indications had been developed including: surgery or head trauma within the previous three months, malignancy, renal and hepatic disorders, and bleeding disorders. In these later studies, the more comprehensive list reduces the proportion of eligible participants.
Interventions
Interventions included systemic, loco‐regional, catheter‐directed and pharmacomechanical thrombolysis. Systemic and loco‐regional techniques differ only in the veins used to deliver an infusion: the arm or foot respectively. Catheter‐directed thrombolysis (CDT) is a more invasive procedure in which a catheter is inserted percutaneously into a vein using imaging to guide to the clot location. The thrombolytic agent is infused through the catheter into the blood clot itself and the position of the catheter is altered according to the progress made in lysing the blood clot. The majority of trials assessed systemic thrombolysis, with streptokinase the most common agent used. The dose used varied: Schulman 1986 used a low‐dose regime of systemic streptokinase, Tsapogas 1973 used loco‐regional streptokinase and Elsharawy 2002 used catheter‐directed streptokinase with frequent radiological assessment, a technique used again in CAVENT. ATTRACT used mechanical therapy in addition to CDT (pharmacomechanical thrombolysis). These adjunctive therapies could include balloon maceration, catheter aspiration, thrombectomy, percutaneous transluminal balloon venoplasty, stent placement, or combination of the above (ATTRACT; Vedantham 2017). CAVA 2020 used ultrasound accelerated CDT to deliver urokinase.
Goldhaber 1990, Turpie 1990 and Verhaeghe 1989 used systemic tPA. While doses of tPA varied, there was no obvious cut‐off for high or low doses. Goldhaber 1996 randomised two regimes of tPA, with and without heparin, compared to heparin alone. The two treatment arms were combined for the purposes of this review. Schweizer 1998 had two treatment arms, loco‐regional tPA and urokinase; and Schweizer 2000 had four treatment arms: systemic streptokinase, systemic urokinase, loco‐regional urokinase and loco‐regional tPA. Kiil 1981 used low‐dose systemic urokinase.
Co‐treatments
Monitoring regimes for heparinisation varied, and length of anticoagulation after the initial phase may be limited to a few months or continued for over a year. In some trials, especially the more recent ones, the use of compression bandages and elevation were reported; and for longer follow‐up, some participants were required to use compression stockings with rigorous ascertainment of compliance with the continued treatment.
Size
Nine studies had fewer than 50 participants (Arnesen 1978; Elsharawy 2002; Goldhaber 1996; Kakkar 1969; Kiil 1981; Marder 1977; Schulman 1986; Tsapogas 1973; Verhaeghe 1989), and four studies had more than 100 participants (ATTRACT; CAVA 2020; CAVENT; Schweizer 2000). Most studies therefore lacked power to detect statistically significant effects. A power calculation was described in four studies (CAVA 2020; CAVENT; Elsharawy 2002; Schweizer 2000). ATTRACT was the largest trial, with 692 participants.
Outcomes
One trial (Verhaeghe 1989) reported results for randomised participants together with non‐randomised participants, but we have used only the data from randomised participants. Some studies reported outcomes using scales that could not be combined (Marder 1977). Removal of the clot was reported using various categorisations. In order to capture as much information as possible, we report in this review both complete clot dissolution or lysis, indicating that the venous patency was 100% restored, and any degree of venographic improvement in patency. Tsapogas 1973 reported partial or complete clearance (75% to 100%), a measure not used in any other study, and others reported partial clearance (50% to 100%). The more recent trials report primarily on PTS and QoL (ATTRACT; CAVA 2020; CAVENT), with CAVENT also reporting cost comparisons.
Length of follow‐up
All trials assessed outcomes in the period immediately after treatment. This was usually at one week, although the range was 36 hours to one month. We collectively grouped these as early outcomes. Intermediate outcomes have been classified as those determined after six months and under five years. No data were reported between this early and intermediate phase (i.e. after one month and before six months). Late outcomes were those reported five years or more after the intervention. PTS was assessed between one and six years. The longest follow‐up (six years) was in the Arnesen 1978 study.
Excluded studies
Seventeen additional trials were excluded for this update (Ageno 2016; Ansari 2016; Bulatov 2019; Calik 2015; Deitelzweig 2016; Doyle 1987; Duan 2016; Fan 2015; Jiang 2017; Kim 2017; Kuo 2017; Liu 2013; NCT02414802; NCT02767232; Righini 2016; Song 2019; Yang 2016). This brings the total number of excluded studies to 40 (Ageno 2016; Ansari 2016; Ansell 1990; Bashir 2014; Bieger 1976; Browse 1968; Bulatov 2019; Cakir 2014; Calik 2015; Deitelzweig 2016; Doyle 1987; Duan 2016; Engelberger 2015; Fan 2015; Jiang 2017; Johansson 1979; Kim 2017; Kuo 2017; Liu 2013; Marini 1991; Markevicius 2004; NCT02414802; NCT02767232; Patra 2014; Persson 1977; Pinto 1997; Righini 2016; Robertson 1967; Santiago 2014; Sas 1985; Schweizer 1996; Silistreli 2004; Song 2019; Sui 2013; Tibbutt 1974; Tibbutt 1977; TORPEDO 2012; Yang 2016; Zhang 2014; Zimmermann 1986). Eight trials did not satisfy the criteria for randomisation (Bashir 2014; Browse 1968; Bulatov 2019; Johansson 1979; Markevicius 2004; Robertson 1967; Santiago 2014; Schweizer 1996). Twenty‐four studies did not include a comparison of thrombolysis versus anticoagulation (Ageno 2016; Ansari 2016; Cakir 2014; Calik 2015; Deitelzweig 2016; Doyle 1987; Duan 2016; Engelberger 2015; Fan 2015; Jiang 2017; Kim 2017; Kuo 2017; Liu 2013; Marini 1991; NCT02414802; Pinto 1997; Righini 2016; Song 2019; Sui 2013; Tibbutt 1974; Tibbutt 1977; Yang 2016; Zhang 2014; Zimmermann 1986); one study was withdrawn due to lack of funding (NCT02767232); DVT was not confirmed objectively in one study (Bieger 1976); and onset of symptoms was beyond 21 days in two studies (Patra 2014; Silistreli 2004). In three cases, insufficient information was obtained despite attempts to contact the authors (Ansell 1990; Persson 1977; Sas 1985). TORPEDO 2012 was excluded as only 33 out 90 participants received thrombolysis. See the Characteristics of excluded studies table for further information.
Ongoing studies
For this update, we identified two new ongoing studies (ChiCTR‐INR‐16009090; NCT02959801). Two previously ongoing studies are now included (ATTRACT; CAVA 2020). We contacted the study investigators of the three ongoing studies, but no data were available (ChiCTR‐INR‐16009090; IRCT201108035625N3; NCT02959801). See Characteristics of ongoing studies for further details.
Studies awaiting classification
For this update, two potential new studies were identified and placed into Studies awaiting classification until a thorough assessment of the full text can be made (Gong 2018; Su 2017).
Risk of bias in included studies
The quality of reporting of most trials was high, see Figure 2 and Figure 3. See the Characteristics of included studies table for detailed information. Minor protocol violations were reported in several studies, and losses to follow‐up were more common in the later phases.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Many studies reported random allocation from a random numbers table or computer‐generated sequence (Arnesen 1978; ATTRACT; CAVA 2020; CAVENT; Elsharawy 2002; Goldhaber 1990; Schulman 1986; Schweizer 1998; Tsapogas 1973; Ugurlu 2002; Verhaeghe 1989), although sometimes this detail was lacking (Common 1976; Elliot 1979; Goldhaber 1996; Kiil 1981; Marder 1977; Schweizer 2000; Turpie 1990; Verhaeghe 1989). Many older studies did not give details on allocation concealment, and this remained a possible risk of bias (Common 1976; Elliot 1979; Elsharawy 2002; Kiil 1981; Marder 1977; Schweizer 1998; Schweizer 2000; Turpie 1990; Ugurlu 2002; Verhaeghe 1989). Studies with good allocation concealment also found significant effects. In some cases, insufficient detail was reported on whether envelopes were sequentially numbered, sealed or opaque (Common 1976; Elliot 1979; Goldhaber 1996; Schulman 1986; Tsapogas 1973).
Blinding
With the exception of Tsapogas 1973, all studies used blinding for the assessment of venograms. Turpie 1990 and Verhaeghe 1989 used identical placebo infusions and therefore were double‐blind. Where participants were not blinded to the treatment group (Arnesen 1978; ATTRACT; CAVA 2020; CAVENT; Common 1976; Elliot 1979; Elsharawy 2002; Goldhaber 1990; Goldhaber 1996; Kakkar 1969; Marder 1977; Schweizer 1998; Schweizer 2000; Tsapogas 1973; Ugurlu 2002), an assessment was made that this introduced a low risk of bias where the assessor was blinded and using objective measures, which was the case in most studies (Arnesen 1978; ATTRACT; CAVA 2020; CAVENT; Common 1976; Elliot 1979; Elsharawy 2002; Goldhaber 1990; Goldhaber 1996; Schulman 1986; Schweizer 1998; Schweizer 2000; Turpie 1990; Ugurlu 2002; Verhaeghe 1989). Blinding participants would be more difficult with more interventional approaches. However, this lack of blinding of participants may have introduced bias in the longer term as participants in receipt of thrombolysis may be more likely to have impressed upon them, or to heed advice given on, the importance of complying with co‐treatments such as compression stockings. For example, compliance was higher in the treatment group in CAVENT. In Kakkar 1969 neither the participants nor outcome assessors were blinded, and this study was therefore judged to have a high risk of bias.
Incomplete outcome data
Most studies did not demonstrate any major differences in follow‐up between the treatment and control groups for the main outcomes, in the early or intermediate follow‐up periods and so were judged to have a low risk of attrition bias. Marder 1977 was assessed as having high risk of bias for this category as it was not possible to separate the data from the three patients who were added non‐randomly after randomisation took place. In ATTRACT, a total of 80 patients missed all PTS assessments. Fifty‐two of these were in the control group (14%), compared to 28 (8%) in the intervention group. Sensitivity analysis carried out by the study authors did not demonstrate a difference in the PTS outcome compared to primary analysis so this was judged to not impact the risk of bias assessment in this domain.
Selective reporting
In some cases subgroups were reported that did not include all trial participants, for example, PTS in those with complete clot lysis, but these were not included in the review. As results including non‐randomised participants were reported in Marder 1977, this was judged to be at high risk of bias. Duplicate reports of studies were identified in the selection process and multiple sources were searched, with no language restriction.
Other potential sources of bias
There were no other specific concerns about bias except for Marder 1977, who added three non‐randomised participants to the study post‐randomisation.
Effects of interventions
See: Table 1
Thrombolysis versus standard anticoagulation
Nineteen studies were included in the meta‐analysis (Arnesen 1978; ATTRACT; CAVA 2020; CAVENT; Common 1976; Elliot 1979; Elsharawy 2002; Goldhaber 1990; Goldhaber 1996; Kakkar 1969; Kiil 1981; Marder 1977; Schulman 1986; Schweizer 1998; Schweizer 2000; Tsapogas 1973; Turpie 1990; Ugurlu 2002; Verhaeghe 1989). Schweizer 2000 had two treatment groups, one systemic thrombolysis and one loco‐regional thrombolysis group. We carried out subgroup analysis to investigate any overall effect of thrombolytics and also to compare the different thrombolysis strategies.
Complete clot lysis
Eight trials with 592 participants reported on the occurrence of early complete clot lysis (up to one month follow‐up) (Common 1976; Elliot 1979; Elsharawy 2002; Goldhaber 1990; Kakkar 1969; Schulman 1986; Schweizer 2000; Ugurlu 2002). In all trials this was more likely in the treatment group, although the extent of the effect varied and the results were statistically heterogeneous. A random‐effects model demonstrated a benefit from thrombolysis treatment (RR 4.75, 95% CI 1.83 to 12.33; P = 0.001; Analysis 1.1). No differences between thrombolysis strategy used were seen with subgroup analysis (test for subgroup differences: P = 0.41).
1.1. Analysis.

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 1: Complete clot lysis (early, subgrouped by thrombolysis strategy)
Seven trials with a total of 654 participants reported intermediate clot lysis (after six months) and in all cases this was more likely in the groups treated with thrombolysis (Common 1976; CAVENT; Elliot 1979; Elsharawy 2002; Schulman 1986; Schweizer 1998; Schweizer 2000). There was a benefit seen with thrombolysis treatment, (RR 2.42, 95% CI 1.42 to 4.12; P = 0.001 using a random‐effects model (moderate‐certainty evidence; Analysis 1.2). No differences were seen with subgroup analysis by thrombolysis strategy (test for subgroup differences: P = 0.37). We downgraded the certainty of the evidence from high to moderate due to imprecision.
1.2. Analysis.

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 2: Complete clot lysis (intermediate, subgrouped by thrombolysis strategy)
Two trials with a total of 206 participants reported clot lysis at five years and over (Arnesen 1978; CAVENT). There was no clear benefit of clot lysis with thrombolysis seen at this time point (RR 3.25, 95% CI 0.17 to 62.63; P = 0.44; Analysis 1.3). No differences between thrombolysis strategies used were seen with subgroup analysis (test for subgroup differences: P = 0.06).
1.3. Analysis.

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 3: Complete clot lysis (late, subgrouped by thrombolysis strategy)
CAVA 2020 data on clot lysis are to be published in subsequent papers.
Bleeding
This category excluded cerebral bleeding and minor bleeds, for example, oozing from venepuncture sites and superficial haematomas. All 19 trials reported on the occurrence of bleeding episodes (Arnesen 1978; ATTRACT; CAVA 2020; CAVENT; Common 1976; Elliot 1979; Elsharawy 2002; Goldhaber 1990; Goldhaber 1996; Kakkar 1969; Kiil 1981; Marder 1977; Schulman 1986; Schweizer 1998; Schweizer 2000; Tsapogas 1973; Turpie 1990; Ugurlu 2002; Verhaeghe 1989). Overall, 6.7% (72/1073) of participants in the thrombolysis group experienced a bleeding complication compared to 2.2% (20/870) of participants in the standard anticoagulation group (RR 2.45, 95% CI 1.58 to 3.78; 1943 participants; 19 studies; P < 0.0001; moderate‐certainty evidence; Analysis 1.4). No differences were seen with subgroup analysis by thrombolysis strategy (test for subgroup differences: P = 0.25). We downgraded the certainty of the evidence from high to moderate due to imprecision. We detected no indication of reporting bias (Figure 4).
1.4. Analysis.

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 4: Bleeding (early, subgrouped by thrombolysis strategy)
4.

Funnel plot of comparison: 1 Thrombolysis versus standard anticoagulation, outcome: 1.4 Bleeding (early, subgrouped by thrombolysis strategy).
PTS
Six studies reported clinically assessed PTS at six months to 5 years (intermediate) (ATTRACT; CAVA 2020; CAVENT; Elliot 1979; Schweizer 1998; Schweizer 2000), in a format that could be combined, with a total of 1393 participants. Fewer cases of PTS were reported in those participants receiving thrombolysis compared to those in the control group (RR 0.78, 95% CI 0.66 to 0.93; 1393 participants; six studies; P = 0.006; moderate‐certainty evidence; Analysis 1.5). We downgraded the certainty of the evidence from high to moderate due to imprecision and small number of participants in the majority of the included studies. The incidence in the thrombolysis group compared with the control group was 383/771 (49.6%) versus 329/622 (52.8%, ranging from 35% to 96% in different trials, which may reflect definitions, treatment doses and adjunctive treatments). We detected heterogeneity (P = 0.01), so we used a random‐effects model. No differences were seen with subgroup analysis by thrombolysis strategy (test for subgroup differences: P = 0.22; Analysis 1.5). Two studies provided data for systemic thrombolysis and these showed a reduction in the incidence of PTS (RR 0.54, 95% CI 0.31 to 0.92; 170 participants; P = 0.02). It is of note that both studies used a much higher dose of thrombolytic than those used in other studies (Elliot 1979; Schweizer 2000; see Characteristics of included studies). No clear benefit for CDT was shown by pooling results from the ATTRACT, CAVA 2020 and CAVENT trials (RR 0.89, 95% CI 0.74 to 1.05; 1032 participants; P = 0.17).
1.5. Analysis.

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 5: PTS (intermediate, subgrouped by thrombolysis strategy)
We also carried out subgroup analysis by DVT level (iliofemoral, femoropopliteal or non‐specified). We have included all participants of CAVENT in the iliofemoral group as this population was similar to the ATTRACT iliofemoral group (personal communication with the study authors). In keeping with reports above, overall the thrombolysis group experienced less PTS (RR 0.82, 95% CI 0.71 to 0.94; 1393 participants; six studies, P = 0.006). No differences in PTS incidence between level of DVT were detected between subgroups (test for subgroup differences: P = 0.29; Analysis 1.6).
1.6. Analysis.

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 6: PTS by iliofemoral/fempop (intermediate, subgrouped by location)
Two studies with 211 participants (Arnesen 1978; CAVENT), reported reduced incidence of clinically assessed PTS at over five years (late follow‐up) following thrombolysis (RR 0.56, 95% CI 0.43 to 0.73; 211 participants; two studies; P < 0.0001; moderate‐certainty evidence; Analysis 1.7). We downgraded the certainty of the evidence from high to moderate due to the small number of studies and participants. In the control group, the incidence of PTS was 75/107 (70%) and in the thrombolysis group 41/104 (39%). No differences were seen with subgroup analysis by thrombolysis strategy (test for subgroup differences: P = 0.28).
1.7. Analysis.

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 7: PTS (late, subgrouped by thrombolysis strategy)
Any improvement in venous patency
Nine trials reported on improvements in venous patency defined by a change in occlusion of the affected segment after treatment (Arnesen 1978; Common 1976; Elsharawy 2002; Goldhaber 1990; Goldhaber 1996; Kakkar 1969; Kiil 1981; Turpie 1990; Ugurlu 2002). All of these studies used systemic thrombolysis strategies except for Elsharawy 2002. Out of a total of 421 participants, improvement was more likely in those receiving thrombolysis (RR 2.48; 95% CI 1.35 to 4.57, P = 0.004; Analysis 1.8). Statistical heterogeneity was noted (P < 0.0001), and a random‐effects model was used. The study by Marder 1977, which showed a difference in mean change from venograms, could not be included due to the reporting format used. A greater improvement was noted but for randomised participants this was not reported to be significantly different. Similarly the Verhaeghe 1989 data could not be included in the meta‐analysis. No clear differences were seen with subgroup analysis by thrombolysis strategy (test for subgroup differences: P = 0.05).
1.8. Analysis.

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 8: Any improvement in venous patency (early)
Stroke or intracerebral haemorrhage
Three trials reported the occurrence of stroke or intracerebral haemorrhage (Common 1976; Goldhaber 1990; Marder 1977). All trials described bleeding complications, therefore the absence of mention of any serious neurological complications or cerebral bleeds was taken to indicate that none were detected. Out of a total of 1943 participants three events occurred in the treatment group (3/1943) and none in the control group. The pooled RR was 1.92 (95% CI 0.34 to 10.86; P = 0.46; 19 studies; Analysis 1.9). All studies where stroke occurred were from before 1990.
1.9. Analysis.

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 9: Stroke (early, subgrouped by thrombolysis strategy)
Leg ulceration
Five studies described ulceration of the leg occurring more than six months from trial entry (ATTRACT; CAVENT; Elliot 1979; Schulman 1986; Schweizer 1998). Fifteen events occurred in the treatment group (15/513) and 19/520 in the control group (RR 0.76, 95% CI 0.39 to 1.49; 1033 participants; five studies; P = 0.43; Analysis 1.10). No differences were seen with subgroup analysis by thrombolysis strategy (test for subgroup differences: P = 0.72).
1.10. Analysis.

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 10: Leg ulceration (intermediate, subgrouped by thrombolysis strategy)
Arnesen 1978 reported at a mean of 6.5 years and so fell within the definition of late ulceration. This study involved a small number of participants, with 3/18 control participants experiencing ulceration after six years compared to 0/17 in the thrombolysis participants (RR 0.15, 95% CI 0.01 to 2.72; P = 0.20; Analysis 1.11).
1.11. Analysis.

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 11: Leg ulceration (late)
Mortality
Ten trials reported on deaths occurring up to one month after treatment (ATTRACT; Arnesen 1978; Common 1976; Elliot 1979; Elsharawy 2002; Kakkar 1969; Kiil 1981; Marder 1977; Schulman 1986; Schweizer 2000); three trials reported that no deaths occurred in this period (ATTRACT; Elsharawy 2002; Schweizer 2000). A total of five events occurred in the treatment group (5/677) and seven in the control group (7/543), out of a total of 1220 participants. There were relatively few events and no clear difference between the groups (RR 0.76; 95% CI 0.31 to 1.89; P = 0.56; Analysis 1.12). All the recorded events occurred within the systemic thrombolysis subgroup, a test for subgroup differences was not estimable. All trials where deaths occurred at this time point were from before 1987.
1.12. Analysis.

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 12: Mortality (early, subgrouped by thrombolysis strategy)
Four trials with a total of 1144 participants reported mortality occurring up to five years after treatment (ATTRACT; CAVA 2020; Elliot 1979; Schweizer 2000). No deaths were reported in either group in Schweizer 2000; CAVA 2020, ATTRACT and Elliot 1979 reported similar numbers of deaths in each group (RR 0.81, 95% CI 0.39 to 1.69; 1144 participants; four studies; P = 0.58; Analysis 1.13). Most deaths were unrelated to the clot or treatment but rather to underlying conditions. ATTRACT reported one death due to PE in the thrombolysis group. No differences were seen with subgroup analysis by thrombolysis strategy (test for subgroup differences: P = 0.76).
1.13. Analysis.

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 13: Mortality (intermediate, subgrouped by thrombolysis strategy)
Two trials with a total of 230 participants reported mortality after five years follow‐up (Arnesen 1978; CAVENT). Seven deaths occurred in the thrombolysis group (7/111) and 12/119 in the control group with a RR of 0.61 (95% CI 0.25 to 1.50; P = 0.28; Analysis 1.14). No differences were seen with subgroup analysis by thrombolysis strategy (test for subgroup differences: P = 0.17).
1.14. Analysis.

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 14: Mortality (late, subgrouped by thrombolysis strategy)
Recurrent venous thromboembolism (DVT/VTE)
At intermediate time points, one trial reported on recurrent DVT (Arnesen 1978), while ATTRACT, CAVA 2020 and CAVENT reported recurrent VTE. Seventy‐three events (including one fatal PE event in the ATTRACT study) occurred in the treatment group (73/520), compared to 58/547 in the control group. The RR was 1.32, 95% CI 0.96 to 1.83; 1067 participants; four studies; P = 0.09); Analysis 1.15. No differences were seen with subgroup analysis by thrombolysis strategy (test for subgroup differences: P = 0.92).
1.15. Analysis.

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 15: Recurrent DVT (intermediate, subgrouped by thrombolysis strategy)
At five year follow‐up, CAVENT reported 13/87 and 21/89 VTE events in the thrombolysis and anticoagulation groups respectively (RR 0.63, 95% CI 0.34 to 1.18; Analysis 1.16). Thirteen events were in the ipsilateral leg, 10 in the contralateral leg, nine were PE and two were unknown. Six patients with chronic iliac vein occlusions (one in the CDT group and five in the control group), were referred and had endovascular recanalisation with stenting. Although randomised to the treatment group, the CDT patient had not received CDT as planned, due to technical failure (Haig 2016).
1.16. Analysis.

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 16: Recurrent DVT (late, subgrouped by thrombolysis strategy)
PE
Six trials reported the occurrence of a PE in the early phase (Arnesen 1978; Elliot 1979; Elsharawy 2002; Kakkar 1969; Schulman 1986; Schweizer 2000). One study noted the absence of any PE (Schulman 1986). The diagnostic criteria used were variable. With the exception of participants who died from PE (one in the treatment group, two in the control group), transient clinical symptoms often occurred but with no objective diagnostic confirmation described. Where deaths were attributed to PE, postmortem examinations were not mentioned. For this reason, the results should be interpreted with caution. The RR was 1.01 (95% CI 0.33 to 3.05; 443 participants; six studies; P = 0.98; Analysis 1.17). No differences were detected between subgroups (P = 0.43). CAVENT did not measure this outcome at this time point. ATTRACT reported that there were 6/336 recurrent VTE events in the thrombolysis group compared to 4/355 (P = 0.5) within the first 10 days; it is not specified if these were PE or DVT.
1.17. Analysis.

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 17: Pulmonary embolism (early, subgrouped by thrombolysis strategy)
Venous function (intermediate)
Three trials reported on presence of normal venous function (CAVENT; Elsharawy 2002; Schulman 1986). Overall, no clear benefit to venous function with thrombolysis was shown, (RR 2.18; 95% CI 0.86 to 5.54; 255 participants, 3 studies, P = 0.10, Analysis 1.18). Heterogeneity was detected (P = 0.009) so a random‐effects model was used. Subgroup analysis suggests a difference between use of systemic and CDT strategies (P = 0.03) with increased normal venous function being seen in the CDT group (RR 3.18, 95% CI 1.41 to 7.19; 224 participants; two studies; P = 0.005).
1.18. Analysis.

Comparison 1: Thrombolysis versus standard anticoagulation, Outcome 18: Venous function (intermediate, subgrouped by thrombolysis strategy)
QoL
Only ATTRACT, CAVA 2020 and CAVENT measured QoL. All three studies used the Venous Insufficiency Epidemological and Economic Study Quality of Life (VEINES‐QOL) measure, which includes both an overall score and a symptom score. In addition, CAVENT and CAVA 2020 used the generic instrument EQ‐5D; and ATTRACT and CAVA 2020 used the Medical Outcomes Study 36‐Item Short Form Health Survey (SF‐36). This includes both a physical component score (PCS) and mental component score (MCS). CAVA 2020 also used the Pain Disability Index (PDI), which reports limitations in daily activities due to pain (scored from 0 to 10, 0 no limitations, 10 fully disabled). VEINES‐QoL, EQ‐5D and SF‐36 scores range from 0 to 100, higher scores indicating better QoL or health perception. A difference of 3 to 4 points is considered clinically meaningful.
After 12 months, CAVA 2020 reported that there were no differences between the CDT and standard treatment group for any health‐related and disease‐specific QoL assessment (mean (SD) VEINES‐Sym score for CDT was 50.1 (11.1) compared to 49.7 (9) in the standard treatment group; mean (SD) SF36 general scores were 65.6 (17.8) in the CDT group compared to 64.9 (22.8) in the standard treatment group; mean (SD) EQ5D score for the CDT group was 85.7 (15.0) compared to 82.3 (21.0) in the standard treatment group; and using the PDI, the mean (SD) CDT group score was 8.7 (12.4) compared to 13.1 (16.3) in the standard treatment group.
After 24 months CAVENT reported there were no differences in QoL between the additional CDT and standard treatment arms; mean difference for the EQ‐5D index was 0.04 (95% CI ‐0.10 to 0.17), for the VEINES‐QOL score 0.2 (95% CI ‐2.8 to 3.0) and for the VEINES‐Sym score 0.5 (95% CI ‐2.4 to 3.4; P > 0.37). After five years, CAVENT reported no difference in mean generic QoL scores, disease specific QoL scores, or symptom severity score between the groups (Enden 2012; Enden 2013a).
Independent of treatment arms, patients with PTS had poorer outcomes than patients without PTS after 24 months; mean difference for EQ‐5D was 0.09 (95% CI 0.03 to 0.15), for VEINES‐QOL score 8.6 (95% CI 5.9 to 11.2) and for VEINES‐Sym score 9.8 (95% CI 7.3 to 12.3; P < 0.001). After five years the EQ‐5D, VEINES‐QOL and VEINES‐Sym scores for patients with PTS were lower than for those without PTS (Enden 2012; Enden 2013a).
After 24 months, ATTRACT reported no difference between the groups with the SF‐36 form (mean (SE) 11.18 (0.91) in the thrombolysis group compared to 10.06 (0.97) in the control group (P = 0.37)); with the VEINES‐QOL score (mean (SE) 27.67 (1.71) in the thrombolysis group compared to 23.47 (1.83) in the control group (P = 0.37)); or the VEINES‐Sym score (mean (SE) 20.58 (1.70) in the thrombolysis group compared to 17.31 (1.81) in the control group (P = 0.17)). A report of a secondary analysis of the ATTRACT study by Kahn 2018 reported VEINES‐QOL scores were better in the thrombolysis group compared to the control group at 30 days (mean (SE) 64.9 (1.4) versus 60.3 (1.4); P = 0.018) and six months (77.0 (1.4) versus 73.1 (1.4); P = 0.044), respectively. This improvement was also detected in the iliofemoral subgroup but not in the femoropopliteal subgroup (Kahn 2018).
Cost comparisons
Only CAVENT has reported on this outcome (Enden 2013b). Additional CDT accumulated 32.31 quality‐adjusted life years (QALYs) compared with 31.68 QALYs after standard treatment. The lifetime cost of CDT was USD 64,709 compared to USD 51,866 with standard treatment. The incremental cost‐effectiveness ratio was USD 20,429 per QALY gained, and the study authors concluded that the probability that CDT was cost‐effective was 82% at a willingness to pay threshold of USD 50,000 per QALY gained (Enden 2013b). CDT may have additional costs compared to systemic administration.
Sensitivity analyses
We carried out sensitivity analyses for all outcomes where the meta‐analysis included trials judged to have any domain at high risk of bias. To determine if results were robust, meta‐analyses were repeated excluding the following studies: Kakkar 1969; Marder 1977; Tsapogas 1973. Forest plots and summary figures were visually assessed and for all outcomes the results remained consistent.
Discussion
Summary of main results
Complete clot lysis was more likely following thrombolysis at both early (RR 4.75, 95% CI 1.83 to 12.33), and intermediate time points (RR 2.42, 95% CI 1.42 to 4.12; moderate‐certainty evidence). The use of objective classification of the degree of lysis would assist, in the future, with quantifying this outcome and the patency of the veins. This benefit is off set by the increased incidence of major bleeding (RR 2.45, 95% CI 1.58 to 3.78; moderate‐certainty evidence). The rationale for the use of thrombolysis for DVT is to prevent long‐term complications related to poor venus function including PTS and ulceration. In this meta‐analysis involving 19 studies, 53% of control participants at intermediate time points and 70% at late follow‐up experienced PTS, which is in line with other estimates. Pooling all types of thrombolysis, the results showed a slight reduction in the risk of PTS with use of thrombolysis at the intermediate time point (RR 0.78, 95% CI 0.66 to 0.93; moderate‐certainty evidence); and at late follow‐up (RR 0.56, 95% CI 0.43 to 0.73; moderate‐certainty evidence). The clinical importance of this reduction is difficult to interpret. The overall benefit of thrombolysis is reduced compared to the previous version of our review (Watson 2016), due to the inclusion of one and two year data from the CAVA 2020 trial and a large multi‐centre trial (ATTRACT), which, in contrast to CAVENT, reported no benefit on incidence of PTS following thrombolysis. Differences between ATTRACT, CAVA 2020 and CAVENT include size (692 participants in ATTRACT versus 209 participants in CAVENT), and a greater use of mechanical adjunctive therapies, versus the longer thrombolytic infusion times in ATTRACT and CAVA 2020 compared to CAVENT. CAVENT primarily recruited patients with iliofemoral DVT, as did CAVA 2020; while in ATTRACT, 43% of participants had femoropopliteal DVT, a population less likely to develop PTS (Kahn 2008). However, subgroup analysis by the ATTRACT study authors did not indicate a difference in PTS incidence between these two levels of DVT. For this update, we carried out subgroup analysis to investigate any effect on PTS incidence by DVT level. This failed to demonstrate any clear effect between iliofemoral, femoropopliteal or non‐specified level of DVT. The authors of ATTRACT highlighted an increased number of participants in the control group who did not attend PTS assessments, suggesting this may have lead to an underestimation of treatment effect. Sensitivity analysis carried out by them did not support this possibility. The ATTRACT authors reported a decrease in the severity of PTS in the pharmacomechanical group compared to the anticoagulant group (RR 0.73 95% CI 0.54 to 0.98; P = 0.04). Subsequent publications from the ATTRACT study reporting on stratified analysis highlight that patients with iliofemoral DVT experienced less severe PTS and improved venous disease‐specific QoL (Comerota 2019). The CAVA 2020 trial reported a higher number of recurrent thrombotic events in the CDT group as a result of in‐stent‐thrombosis. Recurrent thrombosis is one of the main risk factors for PTS and may partly explain why the results did not favour CDT in this iliofemoral population, as had been expected (Prandoni 2004).
This updated meta‐analysis indicates that thrombolysis improved venous patency, with the majority of studies reporting on this outcome using systemic delivery routes (RR 2.48, 95% CI 1.35 to 4.57). The risk of inducing unwanted bleeding with thrombolytics has been the most important factor limiting its use for patients with DVT. Most bleeding episodes and deaths occurred in the earlier studies. Bleeding episodes (excluding stroke) causing interruption of therapy, interventions such as transfusion, or chronic sequelae (a condition following as a consequence of a disease) occurred more often with thrombolysis than with standard anticoagulation. There is no strong evidence that one particular route of administration or agent was excessively hazardous in this respect, although it is notable that no bleeding occurred in the Elsharawy 2002 study. This may have been due to strict exclusion criteria and the close radiological monitoring and dose titration depending upon clot lysis. A high proportion of patients with DVT are, however, unsuitable for thrombolytic treatment because of extensive contra‐indications. Three intracerebral bleeds occurred in these trials (Common 1976; Goldhaber 1990; Marder 1977). Adoption of current contra‐indications may have prevented these events in more recent trials. A stroke occurred in a participant with polycythaemia rubra vera who received streptokinase (Common 1976), an intracranial bleed in a participant with controlled hypertension treated with tPA (Goldhaber 1990), and a fatal intracranial haemorrhage in a patient with a remote history of cerebrovascular accident (Marder 1977). Two of the early deaths in the treatment groups may also have been prevented with the use of current contra‐indications to thrombolysis: a participant with metastatic carcinoma (Common 1976), and a participant with recent surgery (Kakkar 1969).
Four trials with a total of 1144 participants reported on mortality occurring up to five years after treatment (ATTRACT; CAVA 2020; Elliot 1979; Schweizer 2000). No deaths were reported in either group in Schweizer 2000; ATTRACT; CAVA 2020 and Elliot 1979 reported similar numbers of deaths in each group. One trial (Schweizer 2000), reported the absence of further PE episodes at one year, and ATTRACT reported one death due to PE in the thrombolysis group. Results relating to PE were inconclusive due to uncertainty surrounding diagnosis. Two PE were reported in the CAVA 2020 standard anticoagulation group compared to none in the CDT group. There was no clear evidence of any differences between the groups in ulceration beyond six months, or recurrent VTE or DVT. While overall no benefit was seen in the thrombolysis group on venous function, increased venous function was suggested in the CDT group, though this should be interpreted with caution due to the limited number of participants.
CAVENT examined both QoL and cost‐effectiveness. ATTRACT and CAVA 2020 also reported QoL. For QoL, there was no clear difference between CDT and standard treatment although PTS was associated with a lower QoL (ATTRACT). The incremental cost‐effectiveness ratio was USD 20,429 per QALY gained (Enden 2013b). This incremental cost‐effectiveness ratio for CDT is within the range for approval by bodies making recommendation for implementation (Dakin 2014; NICE PMG9).
Overall completeness and applicability of evidence
The evidence presented is highly relevant to determining the effect of thrombolysis for DVT. The effectiveness of newer catheter‐directed methods appears to be similar to that of systemic administration. Evidence suggests effectiveness at levels not limited to iliofemoral. As there is a degree of consistency in the results of trials over time, as well as in different settings, it is likely that the findings have external validity. Further evidence is desirable to confirm the effect of newer methods, and the factors predicting more successful outcomes. For this update, we have been able to include data from ATTRACT and CAVA 2020, where clinicians used a combination of invasive procedures, which reflects newer available strategies to remove clots. With respect to standard treatment with anticoagulation, selected patients with extensive DVT may benefit from systemic thrombolysis or by endovascular interventions such as catheter‐directed and percutaneous mechanical thrombectomy if this were considered safe. This is consistent with the current 'Recommendations and link to evidence' from National Institute for Clinical Excellence (NICE) guidelines (NICE guidelines CG144). There are implications for inpatient treatment, where anticoagulation for DVT is now delivered in outpatient settings, and for the resourcing of more invasive procedures.
No comparisons between thrombolysis and subcutaneous low molecular weight heparin, administered at home, for DVT were identified.
There were not enough data in this review to make any definitive comparison between the different agents or doses of thrombolytics used. Strepokinase and urokinase are more common in older studies, with tPA typically used more recently. Streptokinase appears to have been most widely studied but treatment doses varied widely, as did doses of other thrombolytics and control anticoagulant regimes. ATTRACT mentions the option of using the newer non‐vitamin K oral anticoagulant drugs (rivaroxaban) but it is not known what percentage of participants received this. CAVA 2020 reported use of acenocoumarol, phenprocoumon, DOAC and LMWH. We do not have enough information to draw any conclusions over the question of whether use of the newer anticoagulants has improved outcomes in the standard treatment groups.
Quality of the evidence
This evidence is based on 19 trials involving 1943 participants from a range of countries and settings. The key limitation of the studies is the paucity of long‐term follow‐up. The methodological quality of the studies was mostly high, and the results were consistent across a range of settings and patient groups. Using GRADE assessment, the body of evidence relating to complete clot lysis (intermediate), bleeding (early) and PTS (intermediate and late) was judged to be of moderate certainty, downgraded due to many trials having low numbers of participants or events, or both (See Table 1). There were obvious differences between the inclusion criteria and the conduct of studies completed over 40 years ago compared to more recent studies. However, the results across studies were consistent and we have reasonable confidence in the results.
Potential biases in the review process
It is likely that all relevant studies were identified and included. Relevant data were requested or obtained from study authors, although for older studies this was less likely to be successful. Efforts were made to reduce bias in the review process by ensuring double independent data extraction and quality assessment of studies.
Agreements and disagreements with other studies or reviews
The evidence presented here is consistent with findings of other reviews, which have included a broader range of evidence than RCTs. A review of the literature by Patterson 2010 concluded that in carefully selected patients, CDT offered benefits in treatment, although further trial evidence was needed. Vedantham 2010 indicated benefits in CDT for people with extensive acute iliofemoral DVT, low expected bleeding risk and good functional status, although Comerota 2008 also emphasised a need for further research. A meta‐analysis by Du 2015 included both randomised and non‐randomised studies and had similar findings. Systemic thrombolysis is not current practice, although this review suggests that it has similar effectiveness to CDT, possibly due to its higher dosing regimes (Characteristics of included studies). More recent reviews, which also include data from ATTRACT, report that although a benefit from CDT/PMT (in terms of PTS incidence or severity) may be seen in selected patients (iliofemoral DVT), it is unclear if this benefit outweighs the increased bleeding risk and costs (ten Cate‐Hoek 2018; Chiasakul 2018; Poston 2018).
Authors' conclusions
Implications for practice.
Complete clot lysis occurred more frequently after thrombolysis (with or without additional clot removal strategies) and the proportion of patients with chronic disabling leg symptoms from post‐thrombotic syndrome (PTS) was slightly reduced up to five years from treatment. There was an increased risk of bleeding after thrombolysis, but this risk has decreased over time with the use of stricter exclusion criteria of studies. Results from systemic thrombolysis and catheter‐directed thrombolysis (CDT) appear similar. Using GRADE methods, we judged the evidence to be of moderate‐certainty, due to many trials having small numbers of participants.
Implications for research.
Future trials need to be large enough to detect significant clinical outcomes and ideally last two to five years to estimate the long‐term effect of thrombolysis. CDT differs significantly, as a technique, from systemic thrombolysis and further investigation is needed using this method, particularly in the long term. It may worth be re‐visiting whether systemic thrombolysis can be used safely in the modern era with careful patient selection and with the newer anticoagulants now available. There are also resource implications to introducing systemic or CDT in selected patients, due to the need for availability of skilled staff and interventional resources. Access to such treatment where outpatient management of deep vein thrombosis (DVT) is undertaken may require service changes. These factors will require appropriate consideration in health economic studies that assess costs and cost‐effectiveness.
Use of thrombolysis in combination with interventional methods of clot removal may offer benefit to specific groups of patients, but information on these populations is still limited, as is information comparing specific interventions and the resulting pathophysiological effects. This is an important area for study and future trials should focus on DVT patient subsets, including predicting which patients are at most risk of developing severe PTS. Newer agents that cause less systemic bleeding may hold promise for this condition.
It may be useful to differentiate the effects of PTS and thrombolysis on younger and older patients, the specific level of the clot, and differing times from the initial event, for example 14 days or 21 days or sooner from symptom onset. The measurement and quantification of lysis, resulting patency of the vein and assessment of PTS is an area for further study. Priority should be given to patient important outcomes such as PTS, bleeding and quality of life. Secondary analysis from recent studies highlights the importance of new studies being powered to detect differences in the severity of PTS and the subsequent impact of this on quality of life (QoL). Exclusions, such as malignancy, warrant further study as these may become less significant in certain circumstances with safer methods of treatment. Further research is also needed on cost and QoL issues.
What's new
| Date | Event | Description |
|---|---|---|
| 24 July 2020 | New citation required but conclusions have not changed | Search updated. Two new included studies and two new ongoing studies identified. Seventeen new studies excluded. Conclusions not changed. |
| 24 July 2020 | New search has been performed | Search updated. Two new included studies and two new ongoing studies identified. Seventeen new studies excluded. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 25 February 2016 | New citation required but conclusions have not changed | Search updated. No new included studies. Seven new studies excluded. Two new ongoing studies added. New data from previously included study added. Text amended to reflect current Cochrane policy. 'Summary of findings' table added. |
| 25 February 2016 | New search has been performed | Search updated. No new included studies. New data from previously included study added. Seven new studies excluded. Two new ongoing studies added. |
| 6 June 2013 | New citation required but conclusions have not changed | New search carried out. New author joined the review team. One new study included, four previously excluded studies now included. One new study excluded. Risk of bias assessed for all included studies and text updated. No change to conclusions. |
| 6 June 2013 | New search has been performed | One new study included, four previously excluded studies now included. One new study excluded. |
| 11 November 2009 | Amended | Some graph labels changed and minor edits made to the text. |
| 3 November 2008 | Amended | Converted to new review format. |
| 12 November 2007 | New search has been performed | Four additional excluded studies added. Dates of searches updated. Plain Lanugage Summary provided by the Cochrane Consumer Network added and edited by author. Minor copy edits throughout text. Analyses graphs copy edited for uniformity in presentation. Technical edits performed to clarify outcome statistics. Conclusions remain unchanged. |
Acknowledgements
We would like to thank Dr Jonathon Michaels, who was involved with formulating the original protocol.
The review authors, and the Cochrane Vascular Editorial base wish to thank the peer referees for their comments: Scott M Stevens MD FRCP, Co‐Director Thrombosis Clinic & Thrombosis Research Group, Intermountain Medical Center, UT, USA; Avi Leader MD, Specialist in Internal Medicine and Hematology, Rabin Medical Center, Petah Tikva, Israel; Dr Ronald LG Flumignan, Sao Paulo, Brazil; Ahmed HS Ibrahim, Egypt.
Appendices
Appendix 1. Database searches
| Source | Search strategy | Hits retrieved |
| CENTRAL via CRSO | #1 MESH DESCRIPTOR Thrombosis 1312 #2 MESH DESCRIPTOR Thromboembolism 955 #3 MESH DESCRIPTOR Venous Thromboembolism 955 #4 MESH DESCRIPTOR Venous Thrombosis EXPLODE ALL TREES 2111 #5 (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol*):TI,AB,KY 21669 #6 MESH DESCRIPTOR Pulmonary Embolism EXPLODE ALL TREES 784 #7 (PE or DVT or VTE):TI,AB,KY 5820 #8 (((vein* or ven*) near thromb*)):TI,AB,KY 7563 #9 (blood near3 clot*):TI,AB,KY 3621 #10 (pulmonary near3 clot*):TI,AB,KY 6 #11 (lung near3 clot*):TI,AB,KY 5 #12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 28188 #13 MESH DESCRIPTOR Thrombolytic Therapy EXPLODE ALL TREES 1582 #14 MESH DESCRIPTOR Fibrinolytic Agents EXPLODE ALL TREES 11397 #15 MESH DESCRIPTOR Fibrinolysis EXPLODE ALL TREES 964 #16 MESH DESCRIPTOR Plasminogen Activators EXPLODE ALL TREES 2302 #17 (plasminogen near2 activator* ):TI,AB,KY 3945 #18 (tPA or t‐PA or rtPA or rt‐PA):TI,AB,KY 2385 #19 (thromboly* or fibrinoly* or antithrombotic or antithrombic):TI,AB,KY 9637 #20 (recanalis* or recanaliz*):TI,AB,KY 1209 #21 ((((clot* or thrombus) near3 (lyse or lysis or dissolve* or dissolution)))):TI,AB,KY 1143 #22 urokinase:TI,AB,KY 849 #23 alteplase:TI,AB,KY 887 #24 reteplase:TI,AB,KY 113 #25 tenecteplase:TI,AB,KY 184 #26 saruplase:TI,AB,KY 33 #27 anistreplase:TI,AB,KY 156 #28 monteplase:TI,AB,KY 14 #29 streptokinase:TI,AB,KY 1309 #30 staphylokinase:TI,AB,KY 18 #31 (avelizin or awelysin):TI,AB,KY 0 #32 (celiase or distreptase or Kabikinase or kabivitrum):TI,AB,KY 12 #33 (Streptase or streptodecase or apsac or Abbokinase or renokinase ):TI,AB,KY 111 #34 (Actilyse or Activase or Eminase or Retavase or Rapilysin or desmopletase or u‐pa or alfimeprase):TI,AB,KY 92 #35 streptodornase:TI,AB,KY 50 #36 (pro?urokinase or rpro?uk ):TI,AB,KY 46 #37 (lumbrokinase or duteplase or lanoteplase or pamiteplase):TI,AB,KY 45 #38 #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 20438 #39 #12 AND #38 6346 #40 01/01/2016 TO 09/04/2018:CD 229232 #41 #39 AND #40 1272 |
10.4.18 ‐ 1272 18.3.19 – 915 21.4.20 ‐ 1769 |
| Clinicaltrials.gov | Venous Thrombosis OR Pulmonary Embolism OR Thromboembolism | Thrombolytic Therapy OR Fibrinolytic Agents OR Fibrinolysis OR Plasminogen Activators | Start date on or after 01/01/2016 | 10.4.18 ‐ 59 18.3.19 – 22 21.4.20 ‐ 31 |
| ICTRP Search Portal | Venous Thrombosis OR Pulmonary Embolism OR Thromboembolism | Thrombolytic Therapy OR Fibrinolytic Agents OR Fibrinolysis OR Plasminogen Activators | Start date on or after 01/01/2016 | 10.4.18 ‐ 1 18.3.19 – 2 21.4.20 ‐ not available |
| MEDLINE | 1 exp Venous Thrombosis/ 51052 2 exp Pulmonary Embolism/ 35949 3 (PE or DVT or VTE).ti,ab. 45773 4 ((vein* or ven*) adj thromb*).ti,ab. 60287 5 (pulmonary adj3 clot*).ti,ab. 185 6 (lung adj3 clot*).ti,ab. 46 7 or/1‐6 138870 8 exp Thrombolytic Therapy/ 22161 9 exp Fibrinolytic Agents/ 160669 10 exp FIBRINOLYSIS/ 20608 11 exp Plasminogen Activators/ 38080 12 (plasminogen adj2 activator*).ti,ab. 34984 13 (tPA or t‐PA or rtPA or rt‐PA).ti,ab. 28773 14 (thromboly* or fibrinoly* or antithrombotic or antithrombic).ti,ab. 76361 15 (recanalis* or recanaliz*).ti,ab. 11766 16 ((clot* or thrombus) adj3 (lyse or lysis or dissolve* or dissolution)).ti,ab. 3584 17 urokinase.ti,ab. 14299 18 alteplase.ti,ab. 1618 19 reteplase.ti,ab. 336 20 tenecteplase.ti,ab. 428 21 saruplase.ti,ab. 56 22 anistreplase.ti,ab. 178 23 monteplase.ti,ab. 29 24 streptokinase.ti,ab. 6869 25 staphylokinase.ti,ab. 407 26 (avelizin or awelysin).ti,ab. 19 27 (celiase or distreptase or Kabikinase or kabivitrum).ti,ab. 83 28 (Streptase or streptodecase or apsac or Abbokinase or renokinase).ti,ab. 357 29 (Actilyse or Activase or Eminase or Retavase or Rapilysin or desmopletase or u‐pa or alfimeprase).ti,ab. 2043 30 streptodornase.ti,ab. 541 31 (pro?urokinase or rpro?uk).ti,ab. 182 32 (lumbrokinase or duteplase or lanoteplase or pamiteplase).ti,ab. 124 33 or/8‐32 254354 34 7 and 33 24396 35 randomized controlled trial.pt. 457131 36 controlled clinical trial.pt. 92290 37 randomized.ab. 407065 38 placebo.ab. 187639 39 drug therapy.fs. 2005209 40 randomly.ab. 287700 41 trial.ab. 422799 42 groups.ab. 1779227 43 or/35‐42 4171773 44 exp animals/ not humans.sh. 4440009 45 43 not 44 3605151 46 34 and 45 12548 47 (2017* or 2018*).ed. 1177446 48 46 and 47 569 |
10.4.18 ‐ 569 18.3.19 ‐ 612 21.4.20 ‐ 644 |
| EMBASE | 1 exp vein thrombosis/ 116494 2 exp lung embolism/ 82827 3 (PE or DVT or VTE).ti,ab. 73611 4 ((vein* or ven*) adj thromb*).ti,ab. 89856 5 (pulmonary adj3 clot*).ti,ab. 286 6 (lung adj3 clot*).ti,ab. 74 7 or/1‐6 238518 8 exp Thrombolytic Therapy/ 21923 9 exp Fibrinolytic Agents/ 124797 10 exp FIBRINOLYSIS/ 70117 11 exp Plasminogen Activators/ 76700 12 (plasminogen adj2 activator*).ti,ab. 43374 13 (tPA or t‐PA or rtPA or rt‐PA).ti,ab. 38466 14 (thromboly* or fibrinoly* or antithrombotic or antithrombic).ti,ab. 105914 15 (recanalis* or recanaliz*).ti,ab. 18768 16 ((clot* or thrombus) adj3 (lyse or lysis or dissolve* or dissolution)).ti,ab. 5034 17 urokinase.ti,ab. 17500 18 alteplase.ti,ab. 2878 19 reteplase.ti,ab. 462 20 tenecteplase.ti,ab. 694 21 saruplase.ti,ab. 76 22 anistreplase.ti,ab. 207 23 monteplase.ti,ab. 50 24 streptokinase.ti,ab. 8122 25 staphylokinase.ti,ab. 486 26 (avelizin or awelysin).ti,ab. 14 27 (celiase or distreptase or Kabikinase or kabivitrum).ti,ab. 86 28 (Streptase or streptodecase or apsac or Abbokinase or renokinase).ti,ab. 406 29 (Actilyse or Activase or Eminase or Retavase or Rapilysin or desmopletase or u‐pa or alfimeprase).ti,ab. 2315 30 streptodornase.ti,ab. 488 31 (pro?urokinase or rpro?uk).ti,ab. 229 32 (lumbrokinase or duteplase or lanoteplase or pamiteplase).ti,ab. 179 33 or/8‐32 241003 34 7 and 33 30842 35 randomized controlled trial/ 497383 36 controlled clinical trial/ 459782 37 random$.ti,ab. 1290605 38 randomization/ 77656 39 intermethod comparison/ 232951 40 placebo.ti,ab. 270387 41 (compare or compared or comparison).ti. 464955 42 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1722305 43 (open adj label).ti,ab. 63394 44 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 206964 45 double blind procedure/ 148646 46 parallel group$1.ti,ab. 21541 47 (crossover or cross over).ti,ab. 91947 48 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 278864 49 (assigned or allocated).ti,ab. 327251 50 (controlled adj7 (study or design or trial)).ti,ab. 290679 51 (volunteer or volunteers).ti,ab. 222490 52 trial.ti. 247135 53 or/35‐52 3982033 54 34 and 53 5459 55 (2017* or 2018*).dc. 2298109 56 54 and 55 646 |
10.4.18 ‐ 646 18.3.19 – 655 21.4.20 ‐ 639 |
| CINAHL | S56 S54 AND S55 60 S55 EM 2017 OR EM 2018 304,727 S54 S40 AND S53 1,040 S53 S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 337,202 S52 (MH "Random Assignment") 37,695 S51 (MH "Single‐Blind Studies") or (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") 32,564 S50 MH "Crossover Design" 11,081 S49 MH "Factorial Design" 912 S48 MH "Placebos" 8,341 S47 MH "Clinical Trials" 93,020 S46 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" 4,372 S45 TX crossover OR "cross‐over" 14,364 S44 AB placebo* 27,917 S43 TX random* 215,775 S42 TX trial* 246,753 S41 TX "latin square" 141 S40 S13 AND S39 3,932 S39 S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 14,556 S38 TX lumbrokinase or duteplase or lanoteplase or pamiteplase 14 S37 TX pro?urokinase or rpro?uk 0 S36 TX streptodornase 6 S35 TX Actilyse or Activase or Eminase or Retavase or Rapilysin or desmopletase or u‐pa or alfimeprase 55 S34 TX Streptase or streptodecase or apsac or Abbokinase or renokinase 25 S33 TX celiase or distreptase or Kabikinase or kabivitrum 2 S32 TX avelizin or awelysin 0 S31 TX staphylokinase 7 S30 TX streptokinase 420 S29 TX monteplase 2 S28 TX anistreplase 21 S27 TX saruplase 1 S26 TX tenecteplase 94 S25 TX reteplase 71 S24 TX alteplase 448 S23 TX urokinase 590 S22 TX (clot* or thrombus) n3 (lyse or lysis or dissolve* or dissolution) 238 S21 TX recanalis* or recanaliz* 1,104 S20 TX thromboly* or fibrinoly* or antithrombotic or antithrombic 11,686 S19 TX tPA or t‐PA or rtPA or rt‐PA 1,683 S18 TX plasminogen n2 activator* 4,608 S17 (MH "Plasminogen Activators") 367 S16 (MH "Fibrinolysis") 586 S15 (MH "Fibrinolytic Agents") 4,280 S14 (MH "Thrombolytic Therapy") 4,433 S13 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 44,352 S12 TX lung n3 clot* 21 S11 TX pulmonary n3 clot* 29 S10 TX blood n3 clot* 894 S9 TX (vein* or ven*) n thromb* 121 S8 TX PE or DVT or VTE 10,884 S7 (MH "Pulmonary Embolism") 4,686 S6 TX Pulmonary Embolism 6,325 S5 TX (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol*): 35,783 S4 (MH "Venous Thrombosis+") 6,345 S3 (MH "Venous Thromboembolism") 3,043 S2 (MH "Thromboembolism") 3,211 S1 (MH "Thrombosis") 4,598 |
10.4.18 ‐ 60 18.3.19 – 119 21.4.20 ‐ 192 |
| AMED | 1 exp Thrombolytic Therapy/ 0 2 exp Fibrinolytic Agents/ 7 3 exp FIBRINOLYSIS/ 16 4 exp Plasminogen Activators/ 0 5 (plasminogen adj2 activator*).ti,ab. 45 6 (tPA or t‐PA or rtPA or rt‐PA).ti,ab. 155 7 (thromboly* or fibrinoly* or antithrombotic or antithrombic).ti,ab. 168 8 (recanalis* or recanaliz*).ti,ab. 17 9 ((clot* or thrombus) adj3 (lyse or lysis or dissolve* or dissolution)).ti,ab. 5 10 urokinase.ti,ab. 10 11 alteplase.ti,ab. 2 12 reteplase.ti,ab. 0 13 tenecteplase.ti,ab. 0 14 saruplase.ti,ab. 0 15 anistreplase.ti,ab. 0 16 monteplase.ti,ab. 0 17 streptokinase.ti,ab. 1 18 staphylokinase.ti,ab. 1 19 (avelizin or awelysin).ti,ab. 0 20 (celiase or distreptase or Kabikinase or kabivitrum).ti,ab. 0 21 (Streptase or streptodecase or apsac or Abbokinase or renokinase).ti,ab. 0 22 (Actilyse or Activase or Eminase or Retavase or Rapilysin or desmopletase or u‐pa or alfimeprase).ti,ab. 3 23 streptodornase.ti,ab. 0 24 (pro?urokinase or rpro?uk).ti,ab. 1 25 (lumbrokinase or duteplase or lanoteplase or pamiteplase).ti,ab. 4 26 or/1‐25 353 27 exp Thrombosis/ 302 28 exp Pulmonary embolism/ 53 29 (PE or DVT or VTE).ti,ab. 243 30 ((vein* or ven*) adj thromb*).ti,ab. 308 31 or/27‐30 589 32 26 and 31 39 33 ("2017" or "2018").yr. 240 34 32 and 33 1 |
10.4.18 ‐ 1 18.3.19 – 0 21.4.20 ‐ 1 |
Appendix 2. Glossary
| Term | Meaning |
| Adjunctive | An additional therapy |
| Anti‐coagulation | Drugs which prevent blood clotting, thin the blood |
| Catheter‐directed thrombolysis (CDT) | Technique using catheters to direct treatment into the blood clot |
| Deep vein thrombosis (DVT) | Blood clot in a deep vein, usually leg |
| Endovascular techniques | Minimally invasive techniques |
| Femoralpopliteal | Clot located in the segment between the femoral vein and popliteal vein |
| Heterogeneity | Differences between study design and participants |
| Iliofemoral | Clot located in the segment between the iliac vein and the femoral vein |
| Loco‐regional | Drug delivery restricted to near the clot |
| Pharmacomechanical | Combination of drug and mechanical treatments |
| Post thrombotic syndrome (PTS) | Complication seen after DVT |
| Pulmonary embolism (PE) | Blood clot in the lung |
| Systemic | Drug delivery is not to a specific part but through whole body |
| Thrombolytic | Drugs which dissolve blood clots |
| Thrombosis | Formation of a blood clot |
| Venous ulceration | Chronic wound that is caused by problems with blood flow in the leg |
Data and analyses
Comparison 1. Thrombolysis versus standard anticoagulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Complete clot lysis (early, subgrouped by thrombolysis strategy) | 8 | 592 | Risk Ratio (M‐H, Random, 95% CI) | 4.75 [1.83, 12.33] |
| 1.1.1 Systemic | 7 | 432 | Risk Ratio (M‐H, Random, 95% CI) | 3.65 [1.40, 9.56] |
| 1.1.2 Loco‐regional | 1 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 10.55 [0.66, 168.79] |
| 1.1.3 CDT | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 21.79 [1.38, 343.26] |
| 1.2 Complete clot lysis (intermediate, subgrouped by thrombolysis strategy) | 7 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 2.42 [1.42, 4.12] |
| 1.2.1 Systemic | 4 | 239 | Risk Ratio (M‐H, Random, 95% CI) | 3.80 [1.46, 9.93] |
| 1.2.2 Loco‐regional | 2 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [1.03, 2.97] |
| 1.2.3 CDT | 2 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 2.52 [0.52, 12.17] |
| 1.3 Complete clot lysis (late, subgrouped by thrombolysis strategy) | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 3.25 [0.17, 62.63] |
| 1.3.1 Systemic | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 16.76 [1.03, 272.11] |
| 1.3.2 Loco‐regional | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.3.3 CDT | 1 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.94, 1.33] |
| 1.4 Bleeding (early, subgrouped by thrombolysis strategy) | 19 | 1943 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [1.58, 3.78] |
| 1.4.1 Systemic | 14 | 685 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.24, 3.19] |
| 1.4.2 Loco‐regional | 2 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.07 [0.41, 23.05] |
| 1.4.3 CDT | 4 | 1067 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.30 [1.67, 31.98] |
| 1.5 PTS (intermediate, subgrouped by thrombolysis strategy) | 6 | 1393 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.66, 0.93] |
| 1.5.1 Systemic | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.31, 0.92] |
| 1.5.2 Loco‐regional | 2 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.73, 1.07] |
| 1.5.3 CDT | 3 | 1032 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.05] |
| 1.6 PTS by iliofemoral/fempop (intermediate, subgrouped by location) | 6 | 1393 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.71, 0.94] |
| 1.6.1 Iliofemoral DVT | 4 | 777 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.01] |
| 1.6.2 Femoropopliteal DVT | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.76, 1.27] |
| 1.6.3 Unspecified DVT | 2 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.69, 0.92] |
| 1.7 PTS (late, subgrouped by thrombolysis strategy) | 2 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.43, 0.73] |
| 1.7.1 Systemic | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.14, 0.88] |
| 1.7.2 loco‐regional | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.7.3 CDT | 1 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.45, 0.79] |
| 1.8 Any improvement in venous patency (early) | 9 | 421 | Risk Ratio (M‐H, Random, 95% CI) | 2.48 [1.35, 4.57] |
| 1.8.1 Systemic | 8 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [1.28, 3.70] |
| 1.8.2 CDT | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 35.05 [2.28, 539.63] |
| 1.9 Stroke (early, subgrouped by thrombolysis strategy) | 19 | 1943 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.34, 10.86] |
| 1.9.1 Systemic | 14 | 685 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.34, 10.86] |
| 1.9.2 Loco‐regional | 2 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.9.3 CDT | 4 | 1067 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.10 Leg ulceration (intermediate, subgrouped by thrombolysis strategy) | 5 | 1033 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.39, 1.49] |
| 1.10.1 Systemic | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.53] |
| 1.10.2 Loco‐regional | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.17, 13.60] |
| 1.10.3 CDT | 2 | 880 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.36, 1.54] |
| 1.11 Leg ulceration (late) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.12 Mortality (early, subgrouped by thrombolysis strategy) | 10 | 1220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.31, 1.89] |
| 1.12.1 Systemic | 8 | 369 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.31, 1.89] |
| 1.12.2 Loco‐regional | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.12.3 CDT | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.13 Mortality (intermediate, subgrouped by thrombolysis strategy) | 4 | 1144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.39, 1.69] |
| 1.13.1 Systemic | 2 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.27, 3.43] |
| 1.13.2 Loco‐regional | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.13.3 CDT | 2 | 843 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.31, 1.86] |
| 1.14 Mortality (late, subgrouped by thrombolysis strategy) | 2 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.25, 1.50] |
| 1.14.1 Systemic | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.34, 5.24] |
| 1.14.2 CDT | 1 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.10, 1.30] |
| 1.15 Recurrent DVT (intermediate, subgrouped by thrombolysis strategy) | 4 | 1067 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.96, 1.83] |
| 1.15.1 Systemic | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.37, 5.40] |
| 1.15.2 Loco‐regional | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.15.3 CDT | 3 | 1032 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.94, 1.84] |
| 1.16 Recurrent DVT (late, subgrouped by thrombolysis strategy) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.16.1 Systemic | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.16.2 CDT | 1 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.34, 1.18] |
| 1.17 Pulmonary embolism (early, subgrouped by thrombolysis strategy) | 6 | 433 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.33, 3.05] |
| 1.17.1 Systemic | 5 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.36, 4.10] |
| 1.17.2 Loco‐regional | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.17.3 CDT | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.26] |
| 1.18 Venous function (intermediate, subgrouped by thrombolysis strategy) | 3 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [0.86, 5.54] |
| 1.18.1 Systemic | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.59, 1.83] |
| 1.18.2 Loco‐regional | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.18.3 CDT | 2 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 3.18 [1.41, 7.19] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arnesen 1978.
| Study characteristics | ||
| Methods | Allocation: random Single blind Exclusions after randomisation: 1 Loss to follow‐up: nil |
|
| Participants | Country: Norway Participants: 43 Age: < 70 years Sex: Male and female Inclusion criteria: inpatients with venographically confirmed DVT extending proximally beyond the calf < 5 days duration Exclusion criteria: bleeding dysfunction; surgery within 7 days; GI/GU bleeding; stroke; diastolic BP > 120 mmHg; hypertensive retinopathy grade 3 ‐ 4; renal/hepatic insufficiency; pregnancy; malignancy; age > 70 |
|
| Interventions | Treatment: streptokinase 250,000 U loading IV, then 100,000 IU/hour IV 72 ‐ 96 hours Control: heparin 15,000 IU IV bolus, 30,000 IU infusion IV 72 ‐ 90 hours Co‐treatment: hydrocortisone 100 mg IV, then prednisolone 10 mg three times daily during streptokinase infusion. Warfarin begun after streptokinase along with heparin until warfarin effective In control group, warfarin begun after 72 ‐ 90 hours with continuation of heparin until warfarin effective |
|
| Outcomes | 21 days: mortality; PE; major bleeding; clot lysis 6 years: mortality; recurrent DVT; post‐thrombotic syndrome; leg ulceration |
|
| Funding | Not reported | |
| Declaration of interests | Not reported | |
| Notes | 40 randomised, 1 excluded as diagnosis of DVT in error 3 patients included who were not randomised, 2 streptokinase, 1 control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...performed by our statistician on the basis of random numbers" |
| Allocation concealment (selection bias) | Low risk | " ...allocation to the treatment groups was performed by using sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible due to intervention but judged low risk as outcome assessment well described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The radiologic evaluation was done without knowledge of the treatment given" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None |
ATTRACT.
| Study characteristics | ||
| Methods | Multi‐centre, RCT to determine whether PMT prevents PTS in patients with proximal DVT Blinding: single Exclusions post randomisation: 1 Loss to follow‐up: 62 in PCT group, 86 in control group |
|
| Participants | Country: 56 clinical centres in the United States Participants: 692 (337 PCT, 355 control) Age (range): 53 (42 ‐ 62) Sex: male and female Inclusion criteria: symptomatic proximal DVT involving the femoral, common femoral, or iliac vein (with or without other involved ipsilateral veins) Exclusion criteria: younger than 16 or older than 75 years of age, were pregnant, had had symptoms for more than 14 days, were at high bleeding risk, had active cancer, had established PTS or had had ipsilateral DVT in the previous 2 years |
|
| Interventions | Patients in both groups received initial and long‐term anticoagulant therapy and were provided ECS at the 10‐day follow‐up visit and every 6 months Treatment: "rt‐PA (alteplase (Activase, Genentech) at a dose of <35 mg) was delivered into the thrombus by one of three methods. If the popliteal vein was occluded or the inferior vena cava was involved, physicians were required to use “infusion‐first” therapy, which started with rt‐PA infusion through a multi‐sidehole catheter of the physician's choice for no longer than 30 hours. For the remaining patients, physicians were required to first attempt single‐session thrombus removal with rapid delivery of rt‐PA through the AngioJet Rheolytic Thrombectomy System (Boston Scien‐tific) or the Trellis Peripheral Infusion System (Covidien) and then to infuse rt‐PA for no longer than 24 hours if residual thrombus was present. After the initial delivery of rt‐PA, physicians could use balloon maceration, catheter aspiration, thrombectomy with the use of the AngioJet or Trellis system, percutaneous transluminal balloon venoplasty, stent placement (iliac or common femoral vein), or a combination of procedures to clear residual thrombus and treat obstructive lesions. Stenting was encouraged for lesions that were causing 50% or greater narrowing of the diameter of the vein, robust collateral filling, or a mean pressure gradient of more than 2 mm Hg. Treatment was discontinued when there was at least 90% thrombus removal with restoration of flow or when there was a serious complication.The INR was required to be 1.6 or lower at the start of PMT. During the procedure, patients received twice‐daily sc injections of LMWH in therapeutic doses or UFH infusions (with the dose reduced to 6 to 12 U per kg of body weight per hour (maximum, 1000 U per hour) during rt‐PA infusions). Additional UFH boluses (up to 50 units per kg) were given during the procedure at the physician’s discretion" Control: initial and long‐term anticoagulant therapy and ECS |
|
| Outcomes | Primary: development and severity of PTS (defined as a Villalta score of 5 or higher or an ulcer in the leg with the index DVT, at any time between the 6‐month follow‐up visit and the 24‐month follow‐up visit. Patients were also counted as having PTS if they underwent an unplanned endovascular procedure to treat severe venous symptoms) Secondary: Health‐related quality of life Treatment failures that are not PTS Presenting DVT symptoms Degree of resolution of thrombus with PCDT Bleeding Symptomatic PE Symptomatic recurrent DVT Death Trial outcomes were assessed at 10 and 30 days and 6, 12, 18, and 24 months after randomisation |
|
| Funding | The trial drug and additional funding were provided by Genentech. Compression stockings were donated by BSN Medical | |
| Declaration of interests | "These companies played no role in the design or conduct of the trial or in the analysis or reporting of the data" | |
| Notes | ClinicalTrials.gov number: NCT00790335 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization sequence, with varying block sizes, was computer‐generated by an independent statistician" |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly assigned in a 1:1 ratio to the pharmacomechanical‐thrombolysis group or the control group (no procedural intervention) with the use of a Web‐based central randomization system that ensured concealment of the treatment assignments" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible due to intervention but judged low risk as outcome assessment well described and use of more than one measurement scores |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The clinical personnel who performed assessments of efficacy outcomes and the adjudicators of safety and efficacy outcomes were unaware of the treatment assignments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors reported detailed descriptions, intention to treat and per protocol outcomes. 80 patients missed all PTS assessments and 52 of these were in the control group (14%), compared to 28 (8%) in the intervention group. Sensitivity analysis carried out by the study authors did not demonstrate a difference in the PTS outcome compared to primary analysis so this was not judged to impact the risk of bias assessment in this domain |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Within 7 days after randomisation, 5 patients who had been assigned to the control group underwent pharmacomechanical thrombolysis, and 11 patients who had been assigned to the pharmacomechanical thrombolysis group did not undergo the procedure. These patients were clearly reported and excluded from the per‐protocol analysis |
CAVA 2020.
| Study characteristics | ||
| Methods | Multi‐centre RCT Blinding: single Exclusions post randomisation: 32; 14 intervention group (8 withdrew and 6 screen failures), 18 control group (4 screen failures and 14 withdrew) Loss to follow up: 16 CDT group, 14 control group Analysis: modified intention to treat, and per‐protocol analysis | |
| Participants | Country: The Netherlands
Setting: 15 hospitals Participants: 184 (91 intervention; 93 control) Age (range): 52 (18 ‐ 85) Sex: male (77), female (75) Inclusion criteria: objectively documented first‐time iliofemoral DVT (i.e. complete or partial thrombosis of the common femoral vein or more cranial vein segments) with acute symptoms for no longer than 14 days, a life expectancy of more than 6 months, and no previous thrombus in the affected limb Exclusion criteria: pre‐existent signs of venous insufficiency (CEAP classification C3 or higher); history of gastrointestinal bleeding, cerebrovascular accident, or CNS disease within 1 year; severe hypertension (systolic blood pressure > 180 mmHg or diastolic blood pressure > 100 mmHg); active malignancy (metastatic, progressive, or treated within the previous 6 months); increased alanine transaminase levels (more than three times the upper limit of normal [34 international units (IU)/L for women and 45 IU/L for men]); renal failure (estimated glomerular filtration rate < 30 mL/min); major surgery within 6 weeks; pregnancy; or impaired mobility |
|
| Interventions | Patients in both groups received initial and long‐term anticoagulation therapy according to international guidelines. Custom‐fitted knee‐high elastic compression stockings (30 to 40 mmHg pressure) initiated within 24 h after DVT diagnosis with replacement every 6 months were prescribed to all patients. Patients were instructed to use compression stockings during waking hours of every day for a minimum of 24 months after the DVT. Intervention group (77): started no later than 21 days after the onset of symptoms, performed using urokinase (Medacinase, Lamepro, Netherlands) in combination with the Ekos Endowavesystem (EKOS Corporation, Bothell, WA, USA); total bolus dose of 250 000 IU urokinase in 10 mL NaCl was administered directly after placement of the thrombolysis catheter followed by a total of 100 000 IU/h through continuous infusion during the intervention. Simultaneously, a therapeutic dose of heparin (a total of 1000 IU/h) was administered through the sheath to prevent new thrombus formation. During thrombolysis (maximum duration of 96 h) the patient was confined to bed. During the intervention, standard anticoagulation treatment would be stopped and patients would receive therapeutic doses of LMWH to prevent further thrombosis. When the intervention was stopped, patients would be restarted on their regular anticoagulant drugs 1 h after removal of the sheath. Coagulation status was assessed every 6 h to inform decisions on dose adjustment, dose interruption, or treatment termination Control group (75): initial and long‐term anticoagulation therapy according to international guidelines,with vitamin K antagonists (acenocoumarol or phenprocoumon), direct oral anticoagulants (rivaroxaban, apixaban, and dabigatran), or LMWH |
|
| Outcomes | Primary: PTS at 12 months; major bleeding Secondary outcomes: recurrent VTE; PE; in‐stent thrombosis; death; health‐related QoL CAVA 2020 reports that data on clot lysis are to be published in subsequent papers |
|
| Funding | Funded by The Netherlands Organisation for Health Research and Development (ZonMw), Maastricht University, Medical Centre, BTG‐Interventional Medicine. | |
| Declaration of interests | Study authors had no competing interests and funders had "no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication" | |
| Notes | May be underpowered for some outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A web‐based randomisation programme (TENALEA, ALEA version release 2.2) was used with a random variable block size (2–12), and randomisation was stratified for participating centre and age in three strata (18–50 years, 51–70 years, and 71–85 years)." |
| Allocation concealment (selection bias) | Low risk | "The allocated treatment was communicated to the patient by the central study coordinator performing the randomisation" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients received standard treatment for deep‐vein thrombosis at their local hospital and were asked not to disclose their allocation during visits with their treating physician or (local) study personnel. Treating physicians were informed of the patient’s participation in the study, but not on the treatment allocation." Not possible due to intervention but judged low risk as outcome assessment well described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The coordinating researcher at Maastricht University Medical Centre responsible for collecting, maintaining, and analysing the data was masked to assignment." Comment: single blind, outcome assessor blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The primary outcome analysis was a modified intention‐ to‐treat analysis including all patients who were randomly assigned, except those who did not pass screening and patients who immediately withdrew consent before start of allocated treatment" Comment: relevant data reported for modified ITT analysis |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported or explained as planned to be reported in future manuscripts |
| Other bias | Unclear risk | No evidence of other bias |
CAVENT.
| Study characteristics | ||
| Methods | Multicentre, open label, randomised controlled trial of the efficacy and safety of additional catheter‐directed thrombolysis (CDT) with alteplase Three years duration (January 2006 to January 2009) Ethical approval obtained |
|
| Participants | Country: Recruited from 20 centres, 8 hospital trusts in Norway Total randomised: 189 Age: 18 to 75 years Sex: Male and female Inclusion criteria: objectively verified (diagnostic imaging) first time DVT in the upper thigh, common iliac vein, or combined iliofemoral segment, symptom duration up to 21 days Exclusion criteria: Anticoagulant treatment before trial entry (> 7 days previous), contraindications to thrombolytic treatment, indications for thrombolytic treatment, severe anaemia, thrombocytopenia, severe renal failure, sever hypertension, pregnancy or thrombosis within 7 days postpartum, less than 14 days postsurgery or post‐trauma, history of subarachnoid or intracerebral bleeding, disease with life expectancy less than 24 months, drug misuse or mental disease that could interfere with treatment and follow‐up, former ipsilateral proximal DVT, malignant disease needing chemotherapy, any thrombolytic treatment within 7 days before trial inclusion |
|
| Interventions | Treatment with CDT (number randomised 90) Anticoagulation with subcutaneous LMWH (dalteparin or enoxaparin) for at least 5 days, discontinued for at least 8 hours before CDTreintroduced with warfarin 1 hour after procedure. Infusion catheter covering thrombosed segments introduced under ultrasound. 20 mg alteplase diluted 500 mL 0.9% NaCl given at 0.01 mg/kg per hr for a maximum 96 hrs. Maximum dose 20 mg/24 hrs. Unfractionated heparin given simultaneously as a continuous iv infusion, dose adjusted to keep activated partial thromboplastin time at 1.2 to 1.7 times higher than the upper normal limit. No additional antiplatelet treatment given. Use of adjunctive angioplasty and stents to establish flow and obtain less than 50% residual stenosis left to the discretion of the operator. Advised to wear knee high elastic compression stockings (class II) daily for 24 months Control (number randomised 99) Anticoagulation with subcutaneous LMWH (dalteparin or enoxaparin) and warfarin for at least 5 days, followed by warfarin alone to target intensity INR 2 to 3. Advised to wear knee high elastic compression stockings (class II) daily for 24 months |
|
| Outcomes | PTS at 6 and 24 months, and 5 years measured using Villalta score and classified as PTS if score 5 or over, or if venous ulcer present
Iliofemoral patency, graded daily during thrombolysis, 6 months and 24 months and 5 years Bleeding complications defined as major if clinically overt, or haemoglobin decrease of 2 g per decilitre or more, transfusion of 2 or more units of red cells or whole blood, retroperitoneal or intracranial, occurred in a critical organ or contributed to death Clinically relevant/non‐major bleeding: epistaxis requiring intervention, large visible haematoma on skin, spontaneous macroscopic haematuria Venous function: at 6 months and 24 months, doppler ultrasound using pneumatic cuff with patient standing, standardised compression unit, venous incompetence with reflux valve closure time > 0.5 seconds Functionally significant venous obstruction was indicated by a decline in the plethysmographic curve measured by air plethysmography (APG) (Macrola, Norway). Iliofemoral patency was defined as regained when flow in the pelvic and femoral vein and complete compressibility of the femoral vein was assessed by ultrasound; and no functional venous obstruction was indicated by APG Recurrent VTE; verified with routine imaging at local trial site Mortality at 24 months and 5 years Health related quality of life: EQ‐5D measuring mobility, self care, activity, pain and anxiety at 6 month, 24 months and 5 years VEINES QoL/Sym specific to lower limb problems, measures symptoms, limitation, psychological impact over 4 weeks and change over a year, carried out at 6 months, 24 months and 5 years. VEINES‐QOL assesses QoL and VEINES‐Sym measures symptom severity only Cost‐effectiveness: Markov model, examining PTS, bleeding from CDT and post DVT states, costs in US$, third party payer and lifetime horizon. One way and probabilistic sensitivity analysis in hypothetical cohort age 50. Discounted costs and utilities 3% annually. Long term cumulative incidence after 8 years 30% PTS, 88% severe PTS. QALY, costs, incremental cost‐effectiveness ratio |
|
| Funding | "The study was financially supported by grants from the Research Council of Norway (running costs, grant 175465/V50), the South‐Eastern Norway Health Authority (fellowship to TE), the University of Oslo (fellowship to TE), and Oslo University Hospital Ullevål." | |
| Declaration of interests | "We declare that we have no conflicts of interest." | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...multi‐centre, open label, randomised controlled trial..". Random sequence generated with the website www.randomization.com |
| Allocation concealment (selection bias) | Low risk | "...sealed opaque, numbered envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants not possible due to the nature of the interventions, judged not to effect outcome as these very well defined |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors had "no knowledge of patient history or treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Well described. "Missing outcome data because of withdrawal of consent or death from cancer or other causes not related to CDT or anticoagulation were assumed to be missing independently of treatment and not included in the analyses" |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Other bias unlikely although we note that compliance with compression stockings is slightly higher in intervention group: 63% versus 52% |
Common 1976.
| Study characteristics | ||
| Methods | Allocation: random Single blind Exclusions after randomisation: nil Losses to follow‐up: 23 at 7 months |
|
| Participants | Country: USA Participants: 50 Age: > 18 years Sex: Male and female Inclusion criteria: venographically confirmed DVT duration < 14 days Exclusion criteria: pregnancy; surgery or childbirth < 10 days; bleeding dysfunction; peptic ulcer; recent streptococcal infection; active TB; carotid bruit; stroke < 6 months; diastolic BP > 100 mmHg; atrial fibrillation; hypertensive retinopathy grade 3/4; hepatic/renal biopsy aortography < 14 days |
|
| Interventions | Treatment: hydrocortisone 100 mg IV then streptokinase IV 250,000 U over 30 minutes, then 100,000 U/hour titrated for 72 hours. Followed by IV heparin titrated over 7 days Control: IV heparin 150 U/kg loading dose then titrated for 10 days Co‐treatment: warfarin given from day 6 ‐ 7 |
|
| Outcomes | 3 ‐ 10 days: clot lysis; bleeding; stroke; mortality 7 months: clot lysis |
|
| Funding | Supported in part by US Public Health Service GRANT HL‐05828; the General Reseach Centers Program of the Division of Research Resources, National Institutes of Health; and by the Hoechst Pharmaceutical Company | |
| Declaration of interests | Not reported | |
| Notes | Did not specify whether arm vein thrombosis included or not. Strepokinase supplied by Hoechst Pharmaceutical Company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomized" but no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not described but judged as low risk of bias as outcome assessment blinding described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "..two radiologists who were unaware of the patient's treatment were evaluated the venograms..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None |
Elliot 1979.
| Study characteristics | ||
| Methods | A prospective, controlled, randomised, comparative study to compare conventional full dose heparin and streptokinase (Kabikinase) | |
| Participants | Country: South Africa Total randomised: 51 (strep 26, hep 25) Sex: Male (17) and female (34) Mean age hep group: 51 years; strep group: 48 years Inclusion criteria: proximal vein thrombosis diagnosed by bilateral ascending phlebograph and less than 8 days clinical history of DVT Exclusion criteria: any surgery within 7 days or neurosurgical within 2 months, pregnancy, menstruation, haemorrhagic diatheses, diastolic blood pressure of 110 mmHg, suspected or know bleeding lesions, cerebrovascular accident within 6 months, recent streptococcal infection, previous streptokinase therapy within 6 months, liver or renal disease 2 patients in strep group had axillary vein thrombosis |
|
| Interventions | Treatment: 100 mg of hydrocortisone 15 mins prior to first streptokinase dose and repeated 6 hourly for duration of strep treatment. Strepokinase (Kabikinase) loading dose of 600,000 U given by infusion over a period of 30 mins. Then 100,000 U hourly for 3 days by infusion pump. Then heparin for 4 days dose adjusted to maintain Lee‐White clotting time to at least 2.5 ‐ 3 normal Control: At diagnosis 10,000 U of heparin given by iv injection. Then 10,000 U iv 6 hourly using constant infusion pump. Dose adjusted to maintain Lee‐White clotting time to at least 2.5 ‐ 3 normal Treatment continued for 7 days 30 mg warfarin given as a loading dose to both groups 36 hours before heparin therapy terminated, warfarin continued for 8 weeks, dose adjusted to maintain pro‐thrombin index 40 ‐ 60 per cent All participants bed rest for duration, foot of bed raised by 60 cm, elastic support provided |
|
| Outcomes | Mortality, complete lysis, bleeding, PE, valve function, PTS symptoms 6‐33 months (mean 19 months) | |
| Funding | "Financial assistance from South African Medical Research Council, University of Cape Town Staff Research Fund, The Neltie Atkinson Trust and Pharmacal Ethicals is gratefully received" | |
| Declaration of interests | Not reported | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No details given but judged low risk as outcome assessment well described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "..all radiographs were assessed on a blind basis" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None |
Elsharawy 2002.
| Study characteristics | ||
| Methods | Allocation: random Single blind Exclusions after randomisation ‐ nil Losses to follow‐up ‐ nil |
|
| Participants | Country: Egypt Participants: 35 Age: < 70 years Sex: Male and female Inclusion criteria: iliofemoral venous thrombosis confirmed by duplex or venography duration < 10 days; life expectancy > 6 months Exclusion criteria: surgery < 14 days; previous CVA/CNS disease; GI bleed < 1 year; BP > 180/100; pregnancy etc.; other contraindications to thrombolysis not explicitly described |
|
| Interventions | Treatment: catheter‐directed thrombolysis with streptokinase using popliteal approach. Pulse spray given then vein assessed using contrast every 15 minutes. In 1 hour 1 million U given. Followed by low dose infusion 100,000 U/hour, assessed every 12 hours. Stopped when complete lysis achieved, no progress in 12 hours or complication occurred. Followed by anticoagulation Control: heparin IV bolus 5000 U, then adjusted continuous infusion. Warfarin begun the same evening Co treatment: none described |
|
| Outcomes | 1 week: clot lysis; bleeding; mortality; PE 6 months: clot lysis; venous function |
|
| Funding | Not reported | |
| Declaration of interests | Not reported | |
| Notes | Catheter‐directed thrombolysis, as distinct from systemic or loco‐regional | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer designated cards assigning patients to either groups" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible due to intervention but judged low risk as outcome assessment well described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | " ..panel unaware of the sequencing of the studies or if images were obtained at baseline, 24 ‐ 48 hours after randomisation or before discharge" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete data available |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes reported |
| Other bias | Low risk | None |
Goldhaber 1990.
| Study characteristics | ||
| Methods | Allocation: random Single blind Exclusions after randomisation: nil Losses to follow‐up: nil |
|
| Participants | Country: USA Participants: 64 patients, 65 randomisations Age: 18 to 75 years Sex: Male and female Inclusion criteria: venographically documented DVT, in popliteal or more proximal veins < 14 days duration Exclusion criteria: major bleeding; bleeding dysfunction; stroke; head trauma < 3 months; GI/GU bleed < 4 weeks; trauma/surgery < 14 days; renal/hepatic dysfunction; therapeutic warfarin; lactation/pregnancy; low platelet count; contraindication to contrast agent |
|
| Interventions | Treatment (2 groups):
tPA alone 0.05 mg/kg/hour IV over 24 hours, then heparin 100U/kg bolus, then 1000 U/hour, adjusted tPA as above plus heparin concomitantly as above Control: heparin alone 100 U/kg bolus, then 1000 U/hour Co‐treatment: warfarin begun in all groups on second day Heparin adjusted in all groups |
|
| Outcomes | 36 hours: clot lysis; bleeding | |
| Funding | Not reported | |
| Declaration of interests | Not reported | |
| Notes | 2 patients were not treated according to randomisation, one receiving tPA, one receiving heparin 5 of 65 venograms not analysed. 1 patient with recurrent DVT was re‐entered ‐ 64 patients 65 randomisations |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned to (groups) by opening the appropriate consecutively numbered sealed envelope according to a 2:2:1 allocation scheme. Seperate treatment assignments were generated block random number sequences" |
| Allocation concealment (selection bias) | Unclear risk | Open label trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Both patients and investigators knew which drug regimen was being utilized" but judged low risk as outcome assessment well described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Images compared and assessed by a vascular imaging panel that was blinded to randomization assignment and unaware of whether images were obtained at baseline, 24 to 48 hours after randomization or before discharge" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None |
Goldhaber 1996.
| Study characteristics | ||
| Methods | Randomised controlled trial to assess efficacy and safety of rUK compared to heparin alone September 1992 to April 1994 361 screened, total randomised: 17 Allocation on 1:1 basis on morning of treatment Open labelled study Written informed consent |
|
| Participants | Country: USA
Participants: 17
Symptoms of DVT < 14 days
Age: > 18 years Sex: Male and female Inclusion criteria: DVT diagnosed by ultrasonography or venography for proximal lower extremity (popliteal,femoral, iliac veins with or without calf vein thrombosis) or MRI for upper extremity (brachial, axillary, subclavian, internal jugular veins) Exclusion criteria: stroke, intracranial disease or trauma, major chronic bleeding, major GI bleeding within one year, major urological bleeding 1 month, trauma or major surgery at non‐compressible site within 14 days, hypertension > 180/110 mmHg, haematocrit < 25% or platelet count < 100,000/mm3, pregnancy, nursing mothers, occult blood in stool, gross haematuria |
|
| Interventions | Recombinant urokinase group: 3 bolus infusions of 250,000 U in 5 mins via peripheral vein followed by continuous infusion of 750,000 U over 25 mins and 8 hours after initial dose. Final dose 24 hours after initial dose. Heparin administered 12 hours after first rUK dose for 12 hours until final rUK dose. Three hours after final rUK hep resumed to maintain activated PPT time of 60 to 80 seconds. Warfarin started the same evening to maintain INR of 2 to 3 Heparin group: initial bolus of 5000 to 10,000 U if they were not already receiving IV hep, then continuous infusion adjusted to maintain activated PPT time of 60 to 80 seconds. First dose of warfarin given within 24 hours of randomisation, target INR was 2 to 3 |
|
| Outcomes | Clot lysis, venous flow, blood count and bleeding complications, fibrinogen levels | |
| Funding | "Supported, in part, by a grant from Abbott Laboratories. Dr. Goldhaber receives support from the National Heart, Lung and Blood Institute Academic Award in Systemic and Vascular Medicine (HL 02663)." | |
| Declaration of interests | ‐ | |
| Notes | 1 patient in each group had upper extremity DVT UK group had longer duration of symptoms (6 days versus 3 days) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not described |
| Allocation concealment (selection bias) | Unclear risk | Open label |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No details given but judged low risk as outcome assessment well described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "...images compared and assessed by vascular panel blinded to randomization assignment and time point of image" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None |
Kakkar 1969.
| Study characteristics | ||
| Methods | Allocation: random Single blind Exclusions after randomisation: 2 Losses to follow‐up: nil |
|
| Participants | Country: UK Participants: 30 Age: 18 to 77 years Sex: Male and female Inclusion criteria: venographically confirmed DVT of leg duration < 4 days Exclusion criteria: surgery < 3 days; unhealed wound; peptic ulcer; diastolic BP > 100 mmHg |
|
| Interventions | Treatment: (2 groups) streptokinase 500,000 U IV over 30 minutes, 900,000 U every 6 hours for 5 days or (Arwin) 80 U in 6 hours, then 80 units in 15 minutes, then 40 ‐ 80 U every 6 hours for 5 days Control: heparin 10,000 U over 5 minutes, then 10,000 to 15,000 U every 6 hours for 5 days Co‐treatment: oral anticoagulation commenced at end of infusions. Bed rest, leg elevation, bandages to all groups |
|
| Outcomes | 1 month: mortality; PE; clot lysis; bleeding 6 to 12 months: clot lysis after partial lysis |
|
| Funding | Not reported | |
| Declaration of interests | Not reported | |
| Notes | 1 excluded as died of PE in heparin group. 1 excluded due to bleeding in streptokinase group Included 7 patients with tibial vein thrombosis only (4 heparin, 2 streptokinase, 1 Arwin) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Description not clear |
| Allocation concealment (selection bias) | Unclear risk | Description not clear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None |
Kiil 1981.
| Study characteristics | ||
| Methods | Allocation: random Double blind Exclusions after randomisation: 1 Losses to follow‐up: nil |
|
| Participants | Country: Denmark Participants: 20 Age: 17 to 79 years Sex: Male and female Inclusion criteria: venographically confirmed DVT duration < 72 hours Exclusion criteria: not described |
|
| Interventions | Treatment: urokinase 200,000 U IV over 24 hours. After 18 hours, heparin loading dose of 15,000 units then 40,000 U/day for 5 days Control: heparin 40,000 U/day IV for 6 days Co‐treatment: not described |
|
| Outcomes | 6 days: clot lysis; bleeding 2 weeks: mortality |
|
| Funding | Not reported | |
| Declaration of interests | Not reported | |
| Notes | 1 excluded from heparin group due to bleeding. Low dose urokinase. Did not specify whether calf vein thrombosis was included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "....randomly separated" but no further details given |
| Allocation concealment (selection bias) | Unclear risk | "...allocation of the patients ... was performed by one of the participants" no further details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "....mixture of liquids to be infused was performed by one of the participants" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ".....clinical evaluation and interpretation of phlebograms were preformed in a double‐blind fashion" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions explained |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None |
Marder 1977.
| Study characteristics | ||
| Methods | Randomised controlled trial, single blind, "..to provide evidence that lytic agents are more effective than heparin in dissolving venous thrombi" Declaration of Helsinki, written and verbal explanation of procedures and risks of study, written and informed consent |
|
| Participants | Country: USA
Participants: 24 randomised; 12 heparin and 12 strep (plus 3 non‐randomised) Age over 18 years mean age in hep 50.2 and strep 54.7 years Male and females with venographically proved peripheral DVT Mean symptom duration in heparin group was 6.2 days and 8.5 days for the strep group Patients were included in study if "no evidence of hemorrhagic tendency, active gastrointestinal or genitourinary bleeding, severe system hypertension, atrial fibrillation, pregnancy, 10 days post partum, surgery, hepatic or renal biopsy, translumbar aortography. Four patients in strep group had tumours, three had obstructed venous return in veins which contained thrombus. Two patients (one each heparin and strep), had thrombosis of upper extremity" |
|
| Interventions | All patients iv bolus injection of 100 mg hydrocortisone prior to start of strep or hep Treatment: strep was administered as a priming dose of 250,000 U in 20 minute, followed by a maintenance infusion of 100,000 U/hour for 72 hours Control: heparin was administered as an initial iv dose of 150 U/kg of body weight over 5 minutes followed by a 72 hour infusion at a rate which prolonged the PTT to 60 to 100 seconds After 72 hours of treatment both groups received continuous or intermittent iv heparin according to guidelines. A maintenance dose of warfarin (coumadin) was administered on day seven and heparin was discontinued when the prothrombin time was prolonged to 1.5 to 2.5 times the control value. Warfarin was continued for three months or longer at physicians discretion |
|
| Outcomes | Venography (pre‐treatment and five days post treatment), haemostasis, complications | |
| Funding | Supported by Grants 14217 and 5759‐07 of the National Heart and Lung Institute and Grant 5 MO 1 RR 349 of the General Clinical Research Centers Branch, National Institutes of Health, Bethesda, Md., and by Hoechst‐Roussel Pharmaceuticals, Inc., Somerville, NJ | |
| Declaration of interests | Not reported | |
| Notes | Three patients were added in a non‐randomised fashion to the streptokinase group. Mean age 56 years and symptom duration 8.7 days. These patients were added as three patients from the randomised group did not have follow‐up venograms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "..after entry patients were randomly allocated to either the heparin or the streptokinase group..." but it is not clear by which method this was done |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No attempt to blind described but this judged low risk as outcome assessment blinded and clearly described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | For assessment of venography "films were interpreted independently (by two authors)...without knowing the drug administered or whether the study was before or after treatment". For bleeding no clear definition for grading or assessment are given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Although possible to separate the non‐randomised data for venography, it is not possible to do so for bleeding outcomes |
| Selective reporting (reporting bias) | High risk | Not possible to determine which results from randomised patients for all outcomes |
| Other bias | High risk | Three non‐randomised patients added to study post‐randomisation |
Schulman 1986.
| Study characteristics | ||
| Methods | Allocation: random Single blind Exclusions after randomisation: 2 Losses to follow‐up: nil |
|
| Participants | Country: Sweden Participants: 38 Age: 26 to 74 years Sex: Male and female Inclusion criteria: venographically confirmed calf vein thrombosis duration < 7 days Exclusion criteria: previous thrombosis same leg; contraindication to thrombolysis |
|
| Interventions | Treatment: streptokinase 50,000 IU IV over 15 minutes then 100,000 IU over 12 hours for up to 7 days, titrated. Given with 5000 IU heparin IV over 12 hours. Warfarin begun after streptokinase ended Control: heparin 5000 IU IV bolus then 30,000 IU per day, titrated for 7 days. Warfarin begun simultaneously Co‐treatment: paracetamol, hydrocortisone or moduretic if necessary. 24 hours bed rest. Warfarin given for 5 to 6 months. Leg elevation. Elastic bandages. Elastic stockings where swelling or venous insufficiency detected at discharge or follow‐up |
|
| Outcomes | 1 week: bleeding; clot lysis (venographic score); mortality; stroke; PE 1 month: clot lysis 1 year: clot lysis Up to 5 years: post‐thrombotic syndrome; foot volumetry |
|
| Funding | This work was supported by grants from the Karolinska Institute | |
| Declaration of interests | Not reported | |
| Notes | Low dose streptokinase. 2 patients excluded after randomisation, as they had previous thromboses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised, prospective study" but no further details given |
| Allocation concealment (selection bias) | Low risk | "Allocated using sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible due to the nature of the interventions but judged low risk as outcome assessment well described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "..venograms were evaluated blindly in retrospect by one and the same radiologist" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None |
Schweizer 1998.
| Study characteristics | ||
| Methods | Allocation: random Single blind Exclusions after randomisation: 2 Losses to follow‐up: 1 |
|
| Participants | Country: Germany Participants: 69 Age: 22 to 58 years Sex: Male and female Inclusion criteria: venographically confirmed DVT of leg duration < 7 days Exclusion criteria: PE; calf vein thrombosis; recurrent DVT; GI/GU bleed; inflammatory bowel disease; acute pancreatitis; surgery within 4 weeks; IM injection within 10 days; hypertensive retinopathy grade 3 or 4; intracerebral disease; cerebral surgery or trauma within 3 months; malignancy not in remission; diabetic retinopathy stage 3 or 4; renal or hepatic failure; bleeding dysfunction; pregnancy, lactation, delivery within 20 days |
|
| Interventions | Treatment: (2 groups) tPA 20 mg IV into pedal vein over 4 hours each day for 7 days. Heparin IV given concomitantly, with adjustment Urokinase 100,000 IU/hr IV into pedal vein continuously for 7 days. Heparin IV for 7 days. Plasminogen monitored Warfarin from day 7 to 12 months Control: heparin IV, adjusted for 7 days Co‐treatment: bed rest and compression treatment. Warfarin from day 7‐ 12 months in treatment groups. Warfarin begun immediately, for 12 months in control group. Compression for 12 months for all patients |
|
| Outcomes | 7 days: bleeding; clot lysis (no results for control group) 1 year: post‐thrombotic syndrome |
|
| Funding | Not reported | |
| Declaration of interests | Not reported | |
| Notes | Loco‐regional thrombolysis. 2 patients excluded due to bleeding, 1 tPA, 1 urokinase. 1 lost to follow‐up from control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...designed by a biometrician who was not involved in the study" |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not described but judged unlikely to influence outcome assessment as well described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "...evaluated by an independent radiologist who was unaware of the treatment the patients had received" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None |
Schweizer 2000.
| Study characteristics | ||
| Methods | Allocation: random Single blind Exclusions after randomisation: nil Losses to follow‐up: 12 |
|
| Participants | Country: Germany Participants: 250 Age: mean 40 years Sex: Male and female Inclusion criteria: thrombosis of popliteal or more proximal veins confirmed by venogram at more than one level duration < 9 days Exclusion criteria: no PE; recurrent DVT; calf vein thrombosis only; GI/GU bleeding; inflammatory bowel disease < 12 months; acute pancreatitis; surgery or head trauma < 3 months; IM injection < 10 days; hypertension; diabetic retinopathy stage 3 ‐ 4; malignancy; renal or hepatic failure; bleeding dysfunction; pregnancy, lactation, delivery within 20 days |
|
| Interventions | Treatment: (4 groups) local tPA 20 mg/day, over 4 hours via pedal vein for 4 to 7 days. IV heparin given simultaneously at 1000 IU/hour, adjusted Local urokinase 100,000 IU/day infused continuously. Fibrinogen and plasminogen monitored. Heparin IV given concomitantly Systemic streptokinase 3,000,000 U/day over 6 hours in conjunction with heparin for up to 7 days. Premedication: hydrocortisone 100 mg, ranitidine 50 mg, clemastine 2 mg Systemic urokinase 5,000,000 IU/day over 4 hours for up to 7 days. IV heparin given concomitantly Control: heparin IV, adjusted Co‐treatment: bedrest, compression bandages, warfarin and compression treatment continued for 12 months |
|
| Outcomes | 7 days: PE; major bleeding; mortality; clot lysis 1 year: clot lysis, PTS |
|
| Funding | Not reported | |
| Declaration of interests | Not reported | |
| Notes | 4 losses to follow‐up in systemic urokinase, systemic streptokinase and control groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned" no further details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not described but judged low as outcome assessment well described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "..one dedicated radiologist, blinded to the patient' treatment regimens, evaluated the venograms, while another assessed the sonographic data" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None |
Tsapogas 1973.
| Study characteristics | ||
| Methods | Allocation: random Not blind Exclusions after randomisation: nil Losses to follow‐up: nil |
|
| Participants | Country: USA Participants: 34 Age: mean 57 years Sex: Male and female Inclusion criteria: DVT confirmed by venogram duration < 5 days Exclusion criteria: diastolic BP > 120 mmHg; peptic ulceration; bleeding dysfunction; allergic condition; surgery < 7 days; recent streptococcal infection; streptokinase given < 6 months |
|
| Interventions | Treatment: titrated dose of streptokinase IV into ankle vein 100 mg hydrocortisone IV prior to therapy and daily for 5 days. Streptokinase 100,000 U/hr maintained and adjusted up to 72 hours. IV heparin for 1 week 6 to 12 hours after streptokinase Control: heparin IV into affected limb, 7000 U bolus then 1500 U/hr adjusted. Continued for 7 days after 48 hours of treatment Co‐treatment: bed rest, elevation of leg. Warfarin 2 days before end of therapy, continued for 4 weeks |
|
| Outcomes | 7 days: clot lysis | |
| Funding | Not reported | |
| Declaration of interests | Not reported | |
| Notes | Loco‐regional administration of streptokinase and heparin; calf vein thrombosis included, number not specified, equal in both groups. Streptokinase (Kabikinase, Sweden) was provided by AB Kabi, Stockholm, through Cutter Laboratories, Inc., Berkeley, California | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Based on a list of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | "Arranged by using sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None |
Turpie 1990.
| Study characteristics | ||
| Methods | Allocation: random Double blind Exclusions after randomisation: nil Losses to follow‐up: 37 |
|
| Participants | Country: Canada Participants: 83 Age: < 75 years Sex: not described Inclusion criteria: venographically confirmed proximal DVT of lower limb duration < 7 days Exclusion criteria: bleeding dysfunction; active bleeding; peptic ulcer; stroke or intracranial process < 2 months; surgery, trauma, childbirth, biopsy, vessel puncture < 7 days |
|
| Interventions | Treatment: IV heparin 5000 U bolus then 30,000 U/24 hours, adjusted for 7 ‐ 10 days
Phase 1: two chain tPA 0.5 mg/kg IV over 4 hours
Phase 2: one chain tPA 0.5 mg/kg IV over 8 hours and repeated in 24 hours Control: identical placebo to tPA depending on phase, plus heparin as above Co‐treatment: warfarin commenced for 3 months |
|
| Outcomes | 24 ‐ 48 hours: clot lysis; bleeding 3 years: post‐thrombotic syndrome |
|
| Funding | This study was supported by a grant (MA 9872) from the Medical Research Council of Canada | |
| Declaration of interests | Not reported | |
| Notes | 22 died, 15 "not available" for intermediate to late follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated" no further details |
| Allocation concealment (selection bias) | Unclear risk | Not described clearly |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Identical appearing placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Venograms interpreted by an independent panel without knowledge of the clinical findings or the treatment group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All reported |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | None |
Ugurlu 2002.
| Study characteristics | ||
| Methods | Prospective study to compare efficacy and safety of low dose, slow infusion thrombolysis Randomised |
|
| Participants | Country: Turkey Age: 18 to 70 years Number: 97, 50 low dose strep, 47 hep June 1995 to May 1999 Sex: Male and female Informed consent Baseline characteristics similar Inclusion criteria: DVT confirmed with high resolution colour duplex Exclusion criteria: history of stroke, intracranial haemorrhage, major GI, urological ir genital haemorrhage, major trauma or surgery within 20 days, hypertension, known bleeding diathesis, post partum, nursing or pregnant women |
|
| Interventions | Strepokinase group: Methylprednisone 250 mg IV with IV antihistaminic prior to 250,000 U given in 30 mins via forearm vein, then infusion of 100,000 U/hour. Infusion stopped when a dose of 1,500,000 U. Then heparin according to prothrombin and partial thromboplastin times and duplex study done. Urokinase administered in 2 patients who had severe allergic reaction to strep ‐ bolus of 100,000 U then infusion of 100,000 U per hour for a total dose of either 1,500,000 or 3,000,000 U Heparin group: bolus of 5000 U, then infusion of 1‐1500 U/hr. Dose adjusted according to the activated partial thromboplastin time Both groups: bed rest and elevation, coumadin started 48 hours later according to prothrombin times, INR of 2 ‐ 3 |
|
| Outcomes | Venous flow, clinical assessment, haemorrhagic complications, allergic reaction | |
| Funding | Not reported | |
| Declaration of interests | Not reported | |
| Notes | Recurrent DVT included (30% each group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised number table" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible but judged low risk as outcome assessment well described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "...initial and post‐treatment duplex studies preformed by same radiologist unaware of groups.." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None |
Verhaeghe 1989.
| Study characteristics | ||
| Methods | Allocation: random Double blind Exclusions after randomisation: nil Losses to follow‐up: nil |
|
| Participants | Country: France, Belgium, Switzerland Participants: 21 (in randomised phase only) Age: 22 to 74 years Sex: Male and female Inclusion criteria: hospitalised patients with DVT of popliteal or more proximal veins of the lower leg, confirmed by venography duration < 10 days Exclusion criteria: pregnancy; major surgery < 72 hours; stroke < 6 months; head trauma < 1 month; diastolic BP > 120 mmHg; renal/hepatic disease; peptic ulcer; bleeding dysfunction; contraindication to heparin |
|
| Interventions | Treatment: (2 groups) IV tPA 100 mg on day 1, 50 mg tPA on day 2. 10% of dose given as bolus IV tPA 50 mg on day 1, repeated on day 2. 10% of dose given as bolus Control: identical placebo infusion as above Co‐treatment: heparin 5000 U IV bolus then continuous infusion of 1000 U per hour for up to 72 hours |
|
| Outcomes | 72 hours: clot lysis; bleeding | |
| Funding | Not reported | |
| Declaration of interests | Not reported | |
| Notes | Included initial open label phase in some results (11 additional patients) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allotted" not described further |
| Allocation concealment (selection bias) | Unclear risk | Not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Two radiologists interpreted all films without knowing the drug administered or whether the venography was before or after trial treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "No protocol violations" |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None |
APG: air plethysmography BP: blood pressure CDT: catheter‐directed thrombolysis CNS: central nervous system CVA: cerebrovascular accident DVT: deep vein thrombosis ECS: elastic compression stocking GI: gastrointestinal GU: genitourinary hep: heparin IM: intramuscular INR: international normalized ratio IU: international unit IV: intravenous LMWH: low molecular weight heparin PE: pulmonary embolism RCT: randomised controlled trial rt‐PA: recombinant tissue plasminogen activator strep: streptokinase sc: subcutaneous TB: tuberculosis PCDT: percutaneous catheter directed thrombolysis PMT: pharmacomechanical thrombolysis PTS: post thrombotic syndrome QoL: quality of life tPA: tissue plasminogen activator U: unit UFH: unfractionated heparin VEINES‐QOL: Venous Insufficiency Epidemiological and Economic Study Quality of Life VTE: venous thromboembolism
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ageno 2016 | Rivaroxaban versus standard anticoagulation |
| Ansari 2016 | Compared CDT versus ultrasound accelerated CDT |
| Ansell 1990 | Insufficient information despite contacting author |
| Bashir 2014 | Not randomised |
| Bieger 1976 | DVT not confirmed objectively |
| Browse 1968 | Not randomised |
| Bulatov 2019 | Not randomised |
| Cakir 2014 | Thrombectomy not thrombolysis |
| Calik 2015 | Thrombectomy not thrombolysis |
| Deitelzweig 2016 | Rivaroxaban versus anticoagulant |
| Doyle 1987 | Subcutaneous heparin versus iv heparin |
| Duan 2016 | Study investigated different CDT approaches |
| Engelberger 2015 | CDT versus CDT |
| Fan 2015 | Balloon thrombectomy versus thrombolysis |
| Jiang 2017 | CDT versus CDT plus stent |
| Johansson 1979 | Not truly randomised |
| Kim 2017 | Pharmacomechanical thrombectomy versus catheter directed aspiration thrombectomy |
| Kuo 2017 | CDT versus pharmacomechanical thrombectomy |
| Liu 2013 | Compared different doses of urokinase during CDT |
| Marini 1991 | Both groups received thrombolysis |
| Markevicius 2004 | Not truly randomised |
| NCT02414802 | CDT versus CDT plus thrombectomy |
| NCT02767232 | Study withdrawn due to not receiving National Institute of Health funding. Age inclusion criteria 6 ‐ 21 years, so planned to include children |
| Patra 2014 | Included patients with DVT 0 ‐ 8 weeks, not clear if randomised, CDT in addition to thrombectomy |
| Persson 1977 | Insufficient information, unable to contact author |
| Pinto 1997 | No thrombolytic |
| Righini 2016 | LMWH versus placebo in low risk calf VTE |
| Robertson 1967 | Not truly randomised |
| Santiago 2014 | Prospective observational clinical study in children only |
| Sas 1985 | Insufficient information, unable to contact author |
| Schweizer 1996 | Control group not randomised |
| Silistreli 2004 | Included patients with symptoms for more than 21 days |
| Song 2019 | CDT versus CDT |
| Sui 2013 | Compares thrombolytics, not CDT versus anticoagulant |
| Tibbutt 1974 | Ancrod used as control |
| Tibbutt 1977 | All patients received streptokinase |
| TORPEDO 2012 | Only 33 out of 90 patients received thrombolysis |
| Yang 2016 | CDT versus systemic thrombolysis |
| Zhang 2014 | CDT versus CDT plus angioplasty |
| Zimmermann 1986 | Both groups received thrombolysis |
CDT: catheter‐directed thrombolysis DVT: deep vein thrombosis iv: intravenous VTE: venous thromboembolism
Characteristics of studies awaiting classification [ordered by study ID]
Gong 2018.
| Methods | To compare the safety and clinical efficacy of rt‐PA and UK in CDT for the treatment of subacute iliofemoral DVT |
| Participants | Subacute DVT patients (116) |
| Interventions | CDT with either rt‐PA or UK, or simple anticoagulation treatment |
| Outcomes | Thrombolysis duration, rt‐PA or UK dosages, thrombolytic rate and clinical efficacy rate |
| Notes | Full text requested |
Su 2017.
| Methods | "One hundred and thirty‐nine patients with deep venous thrombosis of early lower extremities ....were selected and randomly divided into the AC group or CDT and AC group" |
| Participants | Patients with DVT of early lower extremities |
| Interventions | AC and AC combined with CDT |
| Outcomes | Thrombolytic effects, adverse reactions, PTS and quality of life |
| Notes | Full text requested |
AC: anticoagulation CDT: catheter directed thrombolysis DVT: deep vein thrombosis PTS: post‐thrombotic syndrome rt‐PA: recombinant human tissue plasminogen activator UK: urokinase
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐INR‐16009090.
| Study name | Combined pharmacomechanical thrombectomy and CDT for acute lower extremity DVT: a multicenter prospective control study |
| Methods | Unclear |
| Participants | First‐time acute IFDVT or first‐time acute femoropopliteal venous thrombosis; duration of disease = 14 days; aged between 18 to 70 years |
| Interventions | Group A: angioJet+CDT; Group B: CDT; Group C: systemic thrombolysis |
| Outcomes | Thrombolysis rate;Thrombolysis time;Vascular patency rate |
| Starting date | Registered 25 August 2016 |
| Contact information | Xiaoqiang Li; The Second Affiliated Hospital of Soochow University, China |
| Notes | chictr.org.cn/showprojen.aspx?proj=15097 (accessed 24 July 2020) |
IRCT201108035625N3.
| Study name | Traditional medical treatment versus interventional approach in acute iliofemoral vein thrombosis |
| Methods | Single centre randomised controlled clinical trial comparing the effect of conventional therapy (heparin followed by warfarin) with interventional therapy (thrombolysis with or without angioplasty and stenting) on venous patency in patients admitted with acute iliofemoral DVT to Tehran Heart Center emergency department |
| Participants | Patients with acute extensive iliofemoral venous thrombosis |
| Interventions | Intervention: lytic therapy will be achieved by placing a catheter in the contralateral femoral vein, the right internal jugular vein, or the ipsilateral popliteal vein for direct intra‐clot infusion. Streptokinase will be given as a loading dose of 250,000 units followed by infusion of 100,000 units per hour for 24 to 48 hours. Heparin will be administered concomitantly with the lytic therapy and continued until therapeutic anticoagulation with warfarin will be accomplished. After lytic therapy, further intervention (PTA/stenting) will be performed if there is an underlying venous stenosis of 50% or more. Stent placement will be done with appropriate selected stents (self‐expanding stainless steel wall stents). All stented patients will be given warfarin indefinitely (INR 2 – 3). Lysis will be considered complete if there is less than 5% residual thrombus Control: conventional treatment will consist of intravenous heparin followed by warfarin. All patients will be treated with limb elevation and moist heat during their initial admission and maintained on prescription gradient compression stockings |
| Outcomes | Venous patency and symptom changes |
| Starting date | August 2011 |
| Contact information | Dr Yaser Jenab Tehran Heart Center |
| Notes | irct.ir/searchresult.php?keyword=&id=5625&number=3&prt=2274&total=10&m=1 (accessed 29/02/2016) |
NCT02959801.
| Study name | Outcome of Percutaneous Mechanical Thrombectomy to Treat Acute Deep Venous Thrombosis |
| Methods | The purpose of this study was to compare the efficacy of percutaneous mechanical thrombectomy (PMT) followed by standard anticoagulant therapy, with anticoagulation therapy alone, for the treatment of acute proximal lower extremity deep vein thrombosis |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | PMT uses a number of catheter‐based mechanical devices to deliver the thrombolytic agent as well as to produce some combination of thrombus fragmentation, distribution of thrombolytic drugs throughout the thrombus, and/or thrombus aspiration |
| Outcomes | Primary: post‐thrombotic syndrome (one year) Secondary: complication (one year) death, bleeding, pulmonary embolism, recurrence |
| Starting date | January 2016 |
| Contact information | Dr Junlai Zhao Beijing Tsinghua Chang Gung Hospital Beijing, China, 102218 |
| Notes |
CDT: catheter‐directed thrombolysis DVT: deep vein thrombosis IFDVT: ileofemoral deep vein thrombosis INR: international normalised ratio PMT: percutaneous mechanical thrombectomy PTA: percutaneous transluminal angioplasty PTS: post‐thrombotic syndrome
Differences between protocol and review
After consideration, the review authors decided to increase the inclusion period of acute symptoms of DVT from 14 to 21 days as this is more commonly used in recent studies. Trials previously excluded due to this were reassessed and included.
In the initial published version, the quality of the trials was investigated using the methods of Jadad (Jadad 1996) and Schulz (Schultz 1995). In keeping with updated Cochrane Collaboration requirements, methodological quality has now been assessed using the Cochrane risk of bias tool (Higgins 2011).
For the 2016 update, we changed the time point definitions to differentiate late outcomes after five years as two studies (Arnesen 1978; CAVENT) now reported results within this period. Due to this Arnesen 1978 data was re‐categorised from intermediate to late.
For the 2020 update, the review title was amended from 'Thrombolysis for acute DVT' to 'Thrombolytic strategies versus standard anticoagulation for acute deep vein thrombosis of the lower limb'. This was to reflect current clinical practice where thrombolysis is frequently carried out in combination with additional strategies to aid removal of the clot, not typically as a stand alone treatment. We added the term 'adult' to Types of participants to clarify only studies involving adult participants would be considered for inclusion. Outcomes were reordered to simplify and reflect those of most clinical relevance. To do this the previous primary outcomes of 'improvement in venous patency', 'stroke', 'venous ulceration rates' and 'mortality' were moved to secondary outcomes. Data were checked to ensure that event numbers for PTS included those patients reported as having ulcers, as two older studies reported these separately. Where necessary, PTS data were corrected to include ulcer events as this was considered clinically appropriate. Checks revealed PTS data for one previously included study (Schweizer 2000), and these data were added. We presented subgroup analysis for this update by delivery method to allow comparison between the routes. In the previous version these results were presented separately. We carried out additional subgroup analysis by level of DVT as it was possible to report this data from the ATTRACT study separately.
Contributions of authors
CB: assessed reference list, extracted data, updated review text LW: assessed reference list, extracted data, updated review text MPA: assessed reference list, updated review text, resolved differences where required
Contributions of editorial support
Marlene Stewart (MS; Managing Editor): co‐ordinated the editorial process; edited the review and assisted with full‐text article screening and data extraction.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
CB is a member of Cochrane Vascular's editorial base staff. Where appropriate, editorial tasks were carried out by other group members. LW has declared that she received travel and accomodation fees from the European Society of Angiology for speaking at the 2012 meeting on this topic. LW is an editor for Cochrane Vascular but had no editor role for this review. MPA: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Arnesen 1978 {published data only}
- Arnesen H, Heilo A, Jakobsen E, Ly B, Skaga E. A prospective study of streptokinase and heparin in the treatment of deep vein thrombosis. Acta Medica Scandinavica 1978;203(6):457-63. [DOI] [PubMed] [Google Scholar]
- Arnesen H, Hoiseth A, Ly B. Streptokinase of heparin in the treatment of deep vein thrombosis. Follow-up results of a prospective study. Acta Medica Scandinavica 1982;211(1-2):65-8. [PubMed] [Google Scholar]
- Arnesen H. The late results of treatment with streptokinase or heparin in patients with acute deep vein thrombosis. Thrombosis and Haemostasis 1983;50(1):329-Abstract No. 1039. [Google Scholar]
ATTRACT {published data only}
- Comerota AJ, Kearon C, Gu CS, Julian JA, Goldhaber SZ, Kahn SR, et al. Endovascular thrombus removal for acute iliofemoral deep vein thrombosis: analysis from a stratified multicenter randomized trial. Circulation 2019;139(9):1162-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comerota AJ. The ATTRACT trial: rationale for early intervention for iliofemoral DVT. Perspectives in Vascular Surgery and Endovascular Therapy 2009;21(4):221-4. [DOI] [PubMed] [Google Scholar]
- Kahn SR, Julian JA, Kearon C, Gu CS, Cohen DJ, Magnuson EA, et al. Health-related quality of life after pharmacomechanical catheter-directed thrombolysis for proximal deep vein thrombosis. Research and Practice in Thrombosis and Haemostasis 2018;2 (Suppl 1):213-14. [Google Scholar]
- Kahn SR, Julian JA, Kearon C, Gu CS, Cohen DJ, Magnuson EA, et al. Quality of life after pharmacomechanical catheter-directed thrombolysis for proximal deep venous thrombosis. Journal of Vascular Surgery: Venous and Lymphatic Disorders 2020;8(1):8-23.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearon C, Gu CS, Julian JA, Goldhaber SZ, Comerota AJ, Gornik HL, et al. Pharmacomechanical catheter-directed thrombolysis in acute femoral-popliteal deep vein thrombosis: analysis from a stratified randomized trial. Thrombosis and Haemostasis 2019;119(4):633-44. [DOI] [PubMed] [Google Scholar]
- Magnuson EA, Chinnakondepalli K, Vilain K, Kearon C, Julian JA, Kahn SR, et al. Cost-effectiveness of pharmacomechanical catheter-directed thrombolysis versus standard anticoagulation in patients with proximal deep vein thrombosis: results from the ATTRACT trial. Circulation 2019;12(10):e005659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00790335. Acute venous thrombosis: thrombus removal with adjunctive catheter-directed thrombolysis. clinicaltrials.gov/show/NCT00790335 (first posted 13 November 2008).
- Vedantham S, Goldhaber SZ, Julian JA, Kahn SR, Jaff MR, Cohen DJ, et al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. New England Journal of Medicine 2017;377:2240-52. [DOI: 10.1056/NEJMoa1615066] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedantham S, Goldhaber SZ, Kahn SR, Julian J, Magnuson E, Jaff MR, et al. Rationale and design of the ATTRACT Study: a multicenter randomized trial to evaluate pharmacomechanical catheter-directed thrombolysis for the prevention of postthrombotic syndrome in patients with proximal deep vein thrombosis. American Heart Journal 2013;165(4):523-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg I, Vedantham S, Salter A, Hadley G, l-Hammadi N, Kearon C, et al. Relationships between the use of pharmacomechanical catheter-directed thrombolysis, sonographic findings, and clinical outcomes in patients with acute proximal DVT: results from the ATTRACT multicenter randomized trial. Vascular Medicine (United Kingdom) 2019;24(5):442-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
CAVA 2020 {published data only}
- DUTCH CAVA-trial. Ultrasound accelerated catheter-directed thrombolysis for primary iliofemoral deep vein thrombosis (IFDVT) compared to non-invasive conventional anticoagulant therapy alone: a Dutch randomized controlled multicenter clinical trial. clinicaltrials.gov/ct2/show/NCT00970619 (first posted 2 September 2009).
- EUCTR2014-005145-51. A randomised controlled multicenter trial comparing ultrasound-accelerated catheter-directed thrombolysis, combined with standard anticoagulant therapy, with standard anticoagulant therapy alone, for acute primary iliofemoral deep vein thrombosis. clinicaltrialsregister.eu/ctr-search/trial/2014-005145-51/DE (first posted 18 August 2015).
- Notten P, Ten Cate-Hoek AJ, Arnoldussen C, Strijkers RHW, Smet A, Tick LW, et al. Ultrasound-accelerated catheter-directed thrombolysis versus anticoagulation for the prevention of post-thrombotic syndrome (CAVA): a single-blind, multicentre, randomised trial. The Lancet. Haematology 2020;7(1):e40-e49. [DOI] [PubMed] [Google Scholar]
- Strijkers RHW, Ten Cate-Hoek AJ, Prins MH, Ten CH, Wittens CHA. Design of the catheter directed thrombolysis and anticoagulation therapy vs. anticoagulation alone; the Dutch cava-study; multicenter randomized controlled trial. Journal of Thrombosis and Haemostasis 2011;9:190-1. [Google Scholar]
CAVENT {published data only}
- Enden T, Haig Y, Holme G. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012;379(9810):31-8. [DOI] [PubMed] [Google Scholar]
- Enden T, Klow NE, Sandvik L, Slagsvold CE, Ghanima W, Hafsahl G, et al. Catheter-directed thrombolysis vs. anticoagulant therapy alone in deep vein thrombosis: results of an open randomized, controlled trial reporting on short-term patency. Journal of Thrombosis and Haemostasis 2009;7(8):1268-75. [DOI] [PubMed] [Google Scholar]
- Enden T, Sandvik L, Klow NE, Hafsahl G, Holme PA, Holmen LO, et al. Catheter-directed Venous Thrombolysis in acute iliofemoral vein thrombosis-the CaVenT Study: rationale and design of a multicenter, randomized, controlled, clinical trial (NCT00251771). American Heart Journal 2007;154(5):808-14. [DOI] [PubMed] [Google Scholar]
- Enden TR, Haig Y, Kløw NE, Slagsvold CE, Sandvik L, Ghanima W, et al. Improved functional outcome after additional catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis: results of a randomized controlled clinical trial (the CaVenT study). Blood 2011;118(21):LBA-1. [Google Scholar]
- Enden TR, Resch S, White C, Wik HS, Klow NE, Sandset PM. Cost-effectiveness of additional catheter-directed thrombolysis for deep vein thrombosis. Journal of Thrombosis and Haemostasis 2013;11:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enden TR, Resch S, White C, Wik HS, Klow NE, Sandset PM. Health-related quality of life after catheter-directed thrombolysis for deep vein thrombosis: secondary outcomes of the randomised, non-blinded, parallel-group CaVenT study. BMJ Open 2013;3(8):e002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enden TR, Slagsvold CE, Klow NE, Sandset PM. Additional catheter-directed venous thrombolysis in iliofemoral deep vein thrombosis; short-term results from the cavent study, a multicenter, randomized controlled trial. Journal of Thrombosis and Haemostasis 2009;7 Suppl 2:Abstract no: AS-MO-007.
- Enden TR, Slagsvold CE, Klow NE, Sandset PM. Adjunctive catheter-directed venous thrombolysis in iliofemoral deep vein thrombosis; short-term results from the CaVenT study, a multicenter randomized controlled trial [Abstract No. 989]. Blood 2008;112(11):365. [Google Scholar]
- Enden TR, Wik HS, Kvam AK, Haig Y, Klow NE, Sandset PM. Health-related quality of life after catheter-directed thrombolysis for deep vein thrombosis; from the CaVenT study. Journal of Thrombosis and Haemostasis 2013;11:29-30. [Google Scholar]
- EUCTR2005-004486-42. Catheter-directed venous thrombolysis (CaVenT) in acute iliofemoral vein thrombosis - an open randomized, controlled, clinical trial - CaVenT. clinicaltrialsregister.eu/ctr-search/trial/2005-004486-42/NO (first posted 4 November 2005).
- Haig Y, Enden T, Grøtta O, Kløw NE, Slagsvold CE, Ghanima W, et al. Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematology 2016;3(2):e64-e71. [DOI] [PubMed] [Google Scholar]
- Haig Y, Enden T, Slagsvold CE, Sandvik L, Sandset P, Kløw NE. Residual rates of reflux and obstruction and their correlation to post-thrombotic syndrome in a randomized study on catheter-directed thrombolysis for deep vein thrombosis. Journal of Vascular Surgery and Venous Lymphatic Disorders 2014;2(2):123-30. [DOI] [PubMed] [Google Scholar]
- Haig Y, Enden T, Slagsvold CE, Sandvik L, Sandset PM, Kløw NE. Determinants of early and long-term efficacy of catheter-directed thrombolysis in proximal deep vein thrombosis. Journal of Vascular and Interventional Radiology 2013;24:17-24. [DOI] [PubMed] [Google Scholar]
- Haig Y, Enden T, Slagsvold CE, Sandvik L, Sandset PM, Klow NE, et al. Five-year outcome after catheter-directed thrombolysis for upper femoral and/or iliac vein thrombosis: results of a randomized controlled trial (the CaVenT Study). In: Cardiovascular and Interventional Radiology. Vol. 38. 2016:S193.
- Haig Y, Enden T. Additional catheter-directed thrombolysis for high proximal deep vein thrombosis; 5 year results of a randomized controlled trial (the CaVenT Study). In: Journal of Thrombosis and Haemostasis. Vol. 13. 2015:49.
- NCT00251771. CaVenT: Catheter-directed venous thrombolysis in acute iliofemoral vein thrombosis--the CaVenT study: rationale and design of a multicenter, randomized, controlled, clinical trial (NCT00251771). clinicaltrials.gov/ct/show/NCT00251771?order=1 (first posted 10 November 2005). [DOI] [PubMed]
- Sandset PM. Catheter-directed thrombolysis reduced the postthrombotic syndrome in acute iliofemoral DVT. Annals of Internal Medicine 2012;156(12):31-8. [DOI] [PubMed] [Google Scholar]
Common 1976 {published data only}
- Common HH , Seaman AJ, Rosch J, Porter JM, Dotter CT. Deep vein thrombosis treated with streptokinase or heparin. Follow-up of a randomized study. Angiology 1976;27(11):645-54. [DOI] [PubMed] [Google Scholar]
- Porter JM, Seaman AJ, Common HH , Rosch J, Eidemiller LR, Calhoun AD. Comparison of heparin and streptokinase in the treatment of venous thrombosis. American Surgeon 1975;41(9):511-9. [PubMed] [Google Scholar]
- Rosch J, Dotter CT, Seaman AJ, Porter JM, Common HH . Healing of deep venous thrombosis: venographic findings in a randomized study comparing streptokinase and heparin. American Journal of Roentgenology 1976;127(4):553-8. [DOI] [PubMed] [Google Scholar]
- Seaman AJ, Common HH , Rosch J, Dotter CT, Porter JM, Lindell TD, et al. Deep vein thrombosis treated with streptokinase or heparin. A randomized study. Angiology 1976;27(10):549-56. [DOI] [PubMed] [Google Scholar]
Elliot 1979 {published data only}
- Elliott MS, Immelman EJ, Jeffery P, Benatar SR, Funston MR, Smith JA, et al. A comparative randomized trial of heparin versus streptokinase in the treatment of acute proximal venous thrombosis: an interim report of a prospective trial. British Journal of Surgery 1979;66(12):838-43. [DOI] [PubMed] [Google Scholar]
Elsharawy 2002 {published data only}
- Elsharawy M, Elzayat E. Early results of thrombolysis vs anticoagulation in iliofemoral venous thrombosis. A randomised clinical trial. European Journal of Vascular and Endovascular Surgery 2002;24(3):209-14. [DOI] [PubMed] [Google Scholar]
Goldhaber 1990 {published data only}
- Goldhaber SZ, Meyerovitz MF, Green D, Vogelzang RL, Citrin P, Heit J, et al. Randomized controlled trial of tissue plasminogen activator in proximal deep venous thrombosis. American Journal of Medicine 1990;88(3):235-40. [DOI] [PubMed] [Google Scholar]
- Green D, Goldhaber S, Meyerovitz MF, Citrin P, Vogelzang R, Heit J, et al. A multicenter trial of recombinant human tissue-type plasminogen activator (Activase) in proximal deep vein thrombosis. Thrombosis and Haemostasis 1989;62(1):406-Abstract No 1289. [Google Scholar]
Goldhaber 1996 {published data only}
- Goldhaber SZ, Hirsch DR, MacDougall RC, Polak JF, Creager MA. Bolus recombinant urokinase versus heparin in deep venous thrombosis: a randomized controlled trial. American Heart Journal 1996;132(2 Pt 1):314-8. [DOI] [PubMed] [Google Scholar]
Kakkar 1969 {published data only}
- Kakkar VV, Flanc C, Howe CT, O'Shea M, Flute PT. Treatment of deep vein thrombosis. A trial of heparin, streptokinase and arvin. British Medical Journal 1969;1(647):806-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar VV, Flanc C, O'Shea M, Flute P, Howe CT, Clarke MB. Treatment of deep-vein thrombosis - a random trial. British Journal of Surgery 1968;55(11):858. [PubMed] [Google Scholar]
- Kakkar VV, Howe CT, Laws JW, Flanc C. Late results of treatment of deep vein thrombosis. British Medical Journal 1969;1(5647):810-1. [PMC free article] [PubMed] [Google Scholar]
- Kakkar VV. Results of streptokinase therapy in deep venous thrombosis. Postgraduate Medical Journal 1973;Suppl:60-4. [Google Scholar]
Kiil 1981 {published data only}
- Kiil J, Carvalho A, Sakso P, Nielsen HO. Urokinase or heparin in the management of patients with deep vein thrombosis? Acta Chirurgica Scandinavica 1981;147(7):529-32. [PubMed] [Google Scholar]
Marder 1977 {published data only}
- Marder VJ, Soulen RL, Atichartakarn V, Budzynski AZ, Parulekar S, Kim JR, et al. Quantitative venographic assessment of deep vein thrombosis in the evaluation of streptokinase and heparin therapy. Journal of Laboratory and Clinical Medicine 1977;89(5):1018-29. [PubMed] [Google Scholar]
Schulman 1986 {published data only}
- Schulman S, Granqvist S, Juhlin-Dannfelt A, Lockner D. Long-term sequelae of calf vein thrombosis treated with heparin or low-dose streptokinase. Acta Medica Scandinavica 1986;219(4):349-57. [DOI] [PubMed] [Google Scholar]
Schweizer 1998 {published data only}
- Schweizer J, Elix H, Altmann E, Hellner G, Forkmann L. Comparative results of thrombolysis treatment with rt-PA and urokinase: a pilot study. VASA 1998;27(3):167-71. [PubMed] [Google Scholar]
Schweizer 2000 {published data only}
- Schweizer J, Kirch W, Koch R, Elix H, Hellner G, Forkmann L, et al. Short- and long-term results after thrombolytic treatment of deep venous thrombosis. Journal of the American College of Cardiology 2000;36(4):1336-43. [DOI] [PubMed] [Google Scholar]
Tsapogas 1973 {published data only}
- Tsapogas MJ, Peabody RA, Wu KT, Karmody AM, Devaraj KT, Eckert C. Controlled study of thrombolytic therapy in deep vein thrombosis. Surgery 1973;74(6):973-84. [PubMed] [Google Scholar]
Turpie 1990 {published data only}
- Hirsh J. Thrombolytic therapy for venous thrombosis and pulmonary embolism. Thrombosis and Haemostasis 1989;62(1):547-Abstract No 1739. [Google Scholar]
- Turpie AG, Levine MN, Hirsh J, Ginsberg JS, Cruickshank M, Jay R, et al. Tissue plasminogen activator (rt-PA) vs heparin in deep vein thrombosis. Results of a randomized trial. Chest 1990;97(4 Suppl):172S-5S. [PubMed] [Google Scholar]
Ugurlu 2002 {published data only}
- Ugurlu B, Kazaz H, Oto O, Hazan E, Sariosmanolu N. Low dose systemic thrombolytic therapy for treatment of deep venous thrombosis. Journal of Cardiovascular Surgery 2002;43(6):881-5. [PubMed] [Google Scholar]
Verhaeghe 1989 {published data only}
- Verhaeghe R, Besse P, Bounameaux H, Marbet GA. Multicenter pilot study of the efficacy and safety of systematic rt-PA administration in the treatment of deep vein thrombosis of the lower extremities and/or pelvis. Thrombosis Research 1989;55(1):5-11. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ageno 2016 {published data only}
- Ageno W, Mantovani LG, Haas S, Kreutz R, Monje D, Schneider J, et al. Safety and effectiveness of oral rivaroxaban versus standard anticoagulation for the treatment of symptomatic deep-vein thrombosis (XALIA): an international, prospective, non-interventional study. The Lancet Haematology 2016;3(1):e12-21. [DOI] [PubMed] [Google Scholar]
Ansari 2016 {published data only}
- Ansari M, Garcia D. Comparison of cathter guided thrombolysis and ultrasound accelerated thrombolysis for the treatment of acute ileo-femoral DVT. Journal of the American College of Cardiology 2016;68(18 Supplement 1):B322-3. [Google Scholar]
Ansell 1990 {published data only}
- Ansell JE, Repice NI, Klassen VA. Treatment of deep venous thrombosis (DVT) with a 5-day low-dose infusion of tissue plasminogen activator (rt-PA). Arteriosclerosis 1990;10:937A. [Google Scholar]
Bashir 2014 {published data only}
- Bashir R. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation vs anticoagulation alone to treat lower-extremity proximal deep vein thrombosis. JAMA Internal Medicine 2014;174(9):1494-501. [DOI] [PubMed] [Google Scholar]
Bieger 1976 {published data only}
- Bieger R, Boekhout-Mussert RJ, Hohmann F, Loeliger EA. Is streptokinase useful in the treatment of deep venous thrombosis? Acta Medica Scandinavica 1976;199(1-2):81-8. [DOI] [PubMed] [Google Scholar]
Browse 1968 {published data only}
- Browse NL, Thomas ML, Pim HP. Streptokinase and deep vein thrombosis. British Medical Journal 1968;3(5620):717-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bulatov 2019 {published data only}
- Bulatov V, Ilyukhin EA, Fomin KN, Galchenko MI, Rosukhovskii DA, Porembskaya OY, et al. Immediate and early results of catheter-directed thrombolysis for lower extremity deep vein thrombosis: a pilot study. Flebologiya 2019;13(3):211-19. [Google Scholar]
Cakir 2014 {published data only}
- Cakir V, Gulcu A, Akay E, Capar AE, Gencpinar T, Kucuk B, et al. Use of percutaneous aspiration thrombectomy vs. anticoagulation therapy to treat acute iliofemoral venous thrombosis: 1-year follow-up results of a randomised, clinical trial. Cardiovascular and Interventional Radiology 2014;37(4):969-76. [DOI] [PubMed] [Google Scholar]
Calik 2015 {published data only}
- Calik ES, Dag O, Kaygin MA, Onk OA, Erkut B. Pharmacomechanical thrombectomy for acute symptomatic lower extremity deep venous thrombosis. Heart Surgery Forum 2015;18(4):E178-83. [DOI] [PubMed] [Google Scholar]
Deitelzweig 2016 {published data only}
- Deitelzweig S, Laliberte F, Crivera C, Germain G, Bookhart BK, Olson WH, et al. Hospitalizations and other health care resource utilization among patients with deep vein thrombosis treated with rivaroxaban versus low-molecular-weight heparin and warfarin in the outpatient setting. Clinical Therapeutics 2016;38(8):1803-16.e3. [DOI] [PubMed] [Google Scholar]
Doyle 1987 {published data only}
- Doyle DJ, Turpie AG, Hirsh J, Best C, Kinch D, Levine MN, et al. Adjusted subcutaneous heparin or continuous intravenous heparin in patients with acute deep vein thrombosis. A randomized trial. Annals of Internal Medicine 1987;107(4):441-5. [DOI] [PubMed] [Google Scholar]
Duan 2016 {published data only}
- Duan PF, Ni CF. Randomized study of different approaches for catheter-directed thrombolysis for lower-extremity acute deep venous thrombosis. Journal of the Formosan Medical Association 2016;115(8):652-7. [DOI] [PubMed] [Google Scholar]
Engelberger 2015 {published data only}
- Engelberger RP, Spirk D, Willenberg T, Alatri A, Do DD, Baumgartner I, et al. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis. Circulation: Cardiovascular Interventions 2015;8:e002027. [DOI] [PubMed] [Google Scholar]
- Engelberger RP, Stuck A, Spirk D, Willenberg T, Haine A, Periard D, et al. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis: 1 year follow up data of a randomized-controlled trial. Journal of Thrombosis and Haemostasis 2017;15(7):1351-60. [DOI] [PubMed] [Google Scholar]
Fan 2015 {published data only}
- Fan G, Li B, Dong L, Wang ML, Wang JH. Modified balloon thrombectomy technique for acute lower extremity deep vein thrombosis. Chinese Journal of Interventional Imaging and Therapy 2015;12(8):468-71. [Google Scholar]
Jiang 2017 {published data only}
- Jiang K, Li XQ, Sang HF, Qian AM, Rong JJ, Li CL. Mid-term outcome of endovascular treatment for acute lower extremity deep venous thrombosis. Phlebology 2017;32(3):200-6. [DOI] [PubMed] [Google Scholar]
Johansson 1979 {published data only}
- Johansson L, Nylander G, Hedner U, Nilsson IM. Comparison of streptokinase with heparin: late results in the treatment of deep venous thrombosis. Acta Medica Scandinavica 1979;206(1-2):93-8. [DOI] [PubMed] [Google Scholar]
Kim 2017 {published data only}
- Kim JY. Pharmacomechanical thrombectomy (PMT) with angiojet solent omni compared with catheter directed aspiration thrombectomy (CDAT) for treatment of acute deep vein thrombosis (DVT). Journal of the American College of Cardiology 2017;69(16 (Suppl 1)):S71. [Google Scholar]
Kuo 2017 {published data only}
- Kuo TT, Huang CY, Hsu CP, Lee CY. Catheter-directed thrombolysis and pharmacomechanical thrombectomy improve midterm outcome in acute iliofemoral deep vein thrombosis. Journal of the Chinese Medical Association 2017;80(2):72-9. [DOI] [PubMed] [Google Scholar]
Liu 2013 {published data only}
- Liu GP, Ge MY, Li L, Ren YW, Kong DM, Zhang M, et al. Analysis on dose and effect of urokinase in patients with lower limb deep vein thrombosis treated with catheter-directed thrombolysis. Chinese Journal of Interventional Imaging and Therapy 2013;10(1):11-4. [Google Scholar]
Marini 1991 {published data only}
- Marini M, Tovar E, Batlle J, Rodriquez E, Beraza A, Cachaza FF, et al. Local venous thrombolysis in eighty-two cases: New treatment approaches. Thrombosis and Haemostasis 1991;65(6):1132-Abstract No 1573. [Google Scholar]
Markevicius 2004 {published data only}
- Markevicius N, Apanavicius G, Scerbinskas S. Comparison between long-term results of catheter-directed thrombolysis and anticoagulation in the treatment of acute iliofemoral deep vein thrombosis. Phlebology 2004;19(3):148-9. [Google Scholar]
NCT02414802 {published data only}
- NCT02414802. Study of a novel thrombectomy device to treat acute iliofemoral deep venous thrombosis. clinicaltrials.gov/ct2/show/NCT02414802 (first posted 13 April 2015).
NCT02767232 {published data only}
- NCT02767232. Pediatric high-risk deep venous thrombosis lytic outcomes trial (PHLO). clinicaltrials.gov/ct2/show/NCT02767232 (first posted 10 May 2016).
Patra 2014 {published data only}
- Patra S. Catheter directed thrombolysis along with mechanical thromboaspiration versus anticoagulation alone in the management of lower limb deep venous thrombosis - a comparative study. Journal of American College Cardiology 2014;64(16 Suppl 1):C203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Persson 1977 {published data only}
- Persson AV, Foti CE, Pierce LE, Sower ND. Role of streptokinase in treatment of venous thrombosis. Thrombosis et Diathesis Haemorrhagica 1977;38(1):298. [Google Scholar]
Pinto 1997 {published data only}
- Pinto A, Corrao S, Galati D, Arnone S, Licata A, Parrinello G, et al. Sulodexide versus calcium heparin in the medium-term treatment of deep vein thrombosis of the lower limbs. Angiology 1997;48(9):805-11. [DOI] [PubMed] [Google Scholar]
Righini 2016 {published data only}
- Righini M, Galanaud JP, Guenneguez H, Brisot D, Diard A, Faisse P, et al. Anticoagulant therapy for symptomatic calf deep vein thrombosis (CACTUS): a randomised, double-blind, placebo-controlled trial. The Lancet Haematology 2016;3(12):e556-62. [DOI] [PubMed] [Google Scholar]
Robertson 1967 {published data only}
- Robertson BR, Nilsson IM, Nylander G, Olow B. Effect of streptokinase and heparin on patients with deep venous thrombosis. A coded examination. Acta Chirurgica Scandinavica 1967;133(3):205-15. [PubMed] [Google Scholar]
- Robertson BR, Nilsson IM, Nylander G. Thrombolytic effect of streptokinase as evaluated by phlebography of deep venous thrombi of the leg. Acta Chirurgica Scandinavica 1970;136(3):173-80. [PubMed] [Google Scholar]
- Robertson BR, Nilsson IM, Nylander G. Value of streptokinase and heparin in treatment of acute deep venous thrombosis. A coded investigation. Acta Chirurgica Scandinavica 1968;134(3):203-8. [PubMed] [Google Scholar]
Santiago 2014 {published data only}
- Santiago MJ, Lopez-Herce J, Del Castillo DB. Thrombolytic therapy using a low dose of tissue plasminogen activator in children. Catheterization and Cardiovascular Interventions 2014;83(2):339-40. [DOI] [PubMed] [Google Scholar]
Sas 1985 {published data only}
- Sas G, Peto I. Late results of streptokinase/heparin treatment in deep venous thrombosis. Thrombosis and Haemostasis 1985;54(1):174-Abstract No 01030. [Google Scholar]
Schweizer 1996 {published data only}
- Schweizer J, Spranger CH, Henkel A, Nirade A, Kaulen R, Altmann E. Long-term outcome after venous thrombolysis with rt-PA and urokinase [Langzeitergebnisse nach venoser thrombolyse mit rt-PA und urokinase]. Phlebologie 1996;25(5):173-6. [Google Scholar]
Silistreli 2004 {published data only}
- Silistreli E, Bekis R, Serbest O, Arslan G, Ulker O, Catalyurek H, et al. Platelet scintigraphy results of heparin versus streptokinase treatment in acute deep vein thrombosis. Scandinavian Cardiovascular Journal 2004;38(6):380-2. [DOI] [PubMed] [Google Scholar]
Song 2019 {published data only}
- Song J, He X, Zhao B, Shi W, Gu J. Application of AngioJet thrombus aspiration device for treatment of acute iliofemoral vein thrombosis. International Journal of Clinical and Experimental Medicine 2019;12(6):7517-26. [Google Scholar]
Sui 2013 {published data only}
- Sui SG, Wang SL, Sun P, Xiao Y, Shi HF. Catheter-directed thrombolytic therapy with use of reteplase and urokinase for the treatment of acute deep venous thrombosis of lower extremity: an observation of clinical results. Journal of Interventional Radiology 2013;22(1):57-60. [Google Scholar]
Tibbutt 1974 {published data only}
- Tibbutt DA, Williams EW, Walker MW, Chesterman CN, Holt JM, Sharp AA. Controlled trial of ancrod and streptokinase in the treatment of deep vein thrombosis of lower limb. British Journal of Haematology 1974;27(3):407-14. [DOI] [PubMed] [Google Scholar]
Tibbutt 1977 {published data only}
- Tibbutt DA, Chesterman CN, Williams EW, Faulkner T, Sharp AA. Controlled trial of the sequential use of streptokinase and ancrod in the treatment of deep vein thrombosis of lower limb. Thrombosis and Haemostasis 1977;37(2):222-32. [PubMed] [Google Scholar]
TORPEDO 2012 {published data only}
- Sharifi M, Bay C, Mehdipour M, Sharifi J. Thrombus obliteration by rapid percutaneous endovenous intervention in deep venous occlusion (TORPEDO) trial: midterm results. Journal of Endovascular Therapy 2012;2:273-80. [DOI] [PubMed] [Google Scholar]
- Sharifi M, Mehdipour M, Bay C, Smith G, Sharifi J. Endovenous therapy for deep venous thrombosis: the TORPEDO trial. Catheterization and Cardiovascular Interventions 2010;76(3):316-25. [DOI] [PubMed] [Google Scholar]
Yang 2016 {published data only}
- Yang B, Xu XD, Gao P, Yu JX, Li Y, Zhu AD, et al. Catheter-directed thrombolysis via small saphenous veins for treating acute deep venous thrombosis. Medical Science Monitor 2016;22:2972-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2014 {published data only}
- Zhang X, Ren Q, Jiang X, Sun J, Gong J, Tang B, et al. A prospective randomized trial of catheter-directed thrombolysis with additional balloon dilatation for iliofemoral deep venous thrombosis: a single-center experience. Cardiovascular and Interventional Radiology 2014;37(4):958-68. [DOI] [PubMed] [Google Scholar]
Zimmermann 1986 {published data only}
- Zimmermann R, Epping J, Rasche H, Krzywanek HJ, Breddin K, Rudolph T, et al. Urokinase and streptokinase treatment of deep venous thrombosis. Results of a randomized study. Haemostasis 1986;16 Suppl 5:9. [Google Scholar]
References to studies awaiting assessment
Gong 2018 {published data only}
- Gong M, Gu J, Chen G, He X, Lou W, Chen L, et al. Interventional treatment by catheter-directed thrombolysis for subacute iliofemoral venous thrombosis: comparison of instant efficacy between recombinant human tissue plasminogen activator and urokinase. Chinese Journal of Radiology 2018;52(1):51-7. [Google Scholar]
Su 2017 {published data only}
- Su SF, Tian YF, Chen LB, Yan B. Comparison of therapeutic efficacy of anticoagulation and its combination with catheter-directed thrombolysis for deep venous thrombosis of lower extremities. Zhongguo shi yan xue ye xue za zhi 2017;25(5):1509-13. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR‐INR‐16009090 {published data only}
- ChiCTR-INR-16009090. Combined pharmacomechanical thrombectomy and catheter-directed thrombolysis for acute lower extremity deep venous thrombosis: a multicenter prospective control study. chictr.org.cn/showprojen.aspx?proj=15097 (first posted 25 August 2016).
IRCT201108035625N3 {published data only}
- IRCT201108035625N3. Comparing the effect of conventional therapy (heparin followed by warfarin) with interventional therapy (thrombolysis with or without angioplasty and stenting) on venous patency in patients who admitted with acute iliofemoral DVT in Tehran Heart Center Emergency Department. en.irct.ir/trial/6154 (first received 13 August 2011).
NCT02959801 {published data only}
- NCT02959801. Outcome of percutaneous mechanical thrombectomy to treat acute deep venous thrombosis. clinicaltrials.gov/show/NCT02959801 (first posted 9 November 2016).
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. British Medical Journal 2004;328(7454):1490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brandjes 1997
- Brandjes DP, Buller HR, Heijboer H, Huisman MV, Rijk M, Jagt H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet 1997;349(9054):759-62. [DOI] [PubMed] [Google Scholar]
Browse 1999
- Browse NL, Burnand KG, Lea Thomas M. Deep vein thrombosis: pathology, diagnosis and treatment. In: Browse NL, Burnand KG, Irvine AT, Wilson NM, editors(s). Diseases of the veins. 2nd edition. London: Edward Arnold, 1999:443-74. [Google Scholar]
Chiasakul 2018
- Chiasakul T, Cuker A. The case for catheter-directed thrombolysis in selected patients with acute proximal deep vein thrombosis. Blood Advances 2018;2(14):1799-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Comerota 1993
- Comerota AJ, Aldridge SC. Thrombolytic therapy for deep venous thrombosis: a clinical review. Canadian Journal of Surgery 1993;36(4):359-64. [PubMed] [Google Scholar]
Comerota 2008
- Comerota AJ, Gravatt MH. Current status of thrombolysis for acute deep vein thrombosis. Phlebolymphology 2008;15(3):85-93. [Google Scholar]
Comerota 2019
- Comerota AJ, Kearon C, Gu CS, Julian JA, Goldhaber SZ, Kahn S, et al. Endovascular thrombus removal for acute iliofemoral deep vein thrombosis: analysis from a stratified multicenter randomised trial. Circulation 2019;139(9):1162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version (accessed 23 July 2018). Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Dakin 2014
- Dakin H, Devlin H, Parkin D, Rice N. NICE's cost-effectiveness threshold. herc.ox.ac.uk/research/nhs-uk-healthcare-system/studies/nice2019s-cost-effectiveness-threshold (accessed 20 September 2016).
Du 2015
- Du GC, Zhang MC, Zhao JC. Catheter-directed thrombolysis plus anticoagulation versus anticoagulation alone in the treatment of proximal deep vein thrombosis: a meta-analysis. Vasa European Journal of Vascular Medicine 2015;44(3):195-22. [DOI] [PubMed] [Google Scholar]
Enden 2012
- Enden T, Haig Y, Holme G. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012;379(9810):31-8. [DOI] [PubMed] [Google Scholar]
Enden 2013a
- Enden T, Wik HS, Kvam AK, Haig Y, Kløw NE, Sandset PM. Health-related quality of life after catheter-directed thrombolysis for deep vein thrombosis: Secondary outcomes of the randomised, non-blinded, parallel-group CaVenT study. BMJ Open 2013;3(8):e002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Enden 2013b
- Enden TR, Resch S, White C, Wik HS, Klow NE, Sandset PM. Cost-effectiveness of additional catheter-directed thrombolysis for deep vein thrombosis. Journal of Thrombosis and Haemostasis 2013;11(6):1032-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gradepro GDT 2020 [Computer program]
- GRADEpro GDT: GRADEpro Guideline Development Tool. McMaster University (developed by Evidence Prime), 2020. Available from gradepro.org.
Haig 2016
- Haig Y, Enden T, Grøtta O, Kløw NE, Slagsvold CE, Ghanima W, et al. Post-thrombotic syndrome after catheter-directed thrombolysis for deep Vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematology 2016;3(2):e64-e71. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials 1996;17(1):1-12. [DOI] [PubMed] [Google Scholar]
Johnson 1995
- Johnson BF, Manzo RA, Bergelin RO, Strandness DE Jr. Relationship between changes in the deep venous system and the development of the postthrombotic syndrome after an acute episode of lower limb deep vein thrombosis: a one-to six-year follow-up. Journal of Vascular Surgery 1995;21(2):307-12. [DOI] [PubMed] [Google Scholar]
Kahn 2004
- Kahn SR, Ginsberg JS. Relationship between deep venous thrombosis and the postthrombotic syndrome. Archives of Internal Medicine 2004;164:17–26. [DOI] [PubMed] [Google Scholar]
Kahn 2006
- Kahn SR. The post-thrombotic syndrome: progress and pitfalls. British Journal of Haematology 2006;134:357-65. [DOI: 10.1111/j.1365-2141.2006.06200] [DOI] [PubMed] [Google Scholar]
Kahn 2008
- Kahn SR, Shrier I, Julian JA, Ducruet T, Arsenault L, Miron M, et al. Determinants and time course of the post thrombotic syndrome after acute deep venous thrombosis. Annals of Internal Medicine 2008;149:698-707. [DOI: 10.7326/0003-4819-149-10-200811180-00004] [DOI] [PubMed] [Google Scholar]
Kahn 2018
- Kahn SR, Julian JA, Kearon C, Gu CS, Cohen DJ, Magnuson EA, et al. Health-related quality of life after pharmacomechanical catheter-directed thrombolysis for proximal deep vein thrombosis. Research and Practice in Thrombosis and Haemostasis 2018;2 (Suppl 1):213-4. [Google Scholar]
Kearon 2012
- Kearon C, Comerota AJ. Antithrombotic therapy for VTE disease. Antithrombotic therapy and prevention of thrombosis, 9th ed; American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141(2):e419S-94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kearon 2016
- Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest 2016;149(2):315-52. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
NICE guidelines CG144
- National institute for Health and Care Excellence. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. nice.org.uk/guidance/cg144/evidence (accessed 15 September 2016).
NICE PMG9
- NICE Appraisal Committee. Guide to the methods of technology appraisal (PMG9). nice.org.uk/process/pmg9/chapter/the-reference-case (accessed 20 September 2016).
Patterson 2010
- Patterson BO, Hinchcliffe R, Loftus IM, Thompson MM, Holt PJE. DVT: a new era in anticoagulant therapy. Arteriosclerosis, Thrombosis, and Vascular Biology 2010;30:669-74. [DOI] [PubMed] [Google Scholar]
Poston 2018
- Poston JN, Garcia DA. The case against catheter-directed thrombolysis in patients with proximal deep vein thrombosis. Blood Advances 2018;2(14):1803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prandoni 2004
- Prandoni P, Lensing AWA, Prins MH, Frulla M, Marchiori A, Bernardi E, et al. Below‐knee elastic compression stockings to prevent the post‐thrombotic syndrome. A randomized, controlled trial. Annals of Internal Medicine 2004;141:249–56. [DOI] [PubMed] [Google Scholar]
Schulman 2006
- Schulman S, Lindmarker P, Holmström M, Lärfars G, Carlsson A, Nicol P, et al. Post-thrombotic syndrome, recurrence, and death 10 years after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. Journal of Thrombosis and Haemostasis 2006;4(4):734-42. [DOI] [PubMed] [Google Scholar]
Schultz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12. [DOI] [PubMed] [Google Scholar]
Sharafuddin 2003
- Sharafuddin MJ, Sun S, Hoballah JJ, Youness FM, Sharp WJ, Roh BS. Endovascular management of venous thrombotic and occlusive diseases of the lower extremities. Journal of Vascular and Interventional Radiology 2003;14:405–23. [DOI] [PubMed] [Google Scholar]
ten Cate‐Hoek 2018
- ten Cate-Hoek AJ. Prevention and treatment of the post-thrombotic syndrome. Research and Practice in thrombosis and Haemostasis 2018;2:209-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vedantham 2009a
- Vedantham S, Grassi CJ, Ferral H, Patel NH, Thorpe PE, Antonacci VP, et al. Reporting standards for endovascular treatment of lower extremity deep vein thrombosis. Journal of Vascular and Interventional Radiology 2009;20(7 Suppl):S391-408. [DOI] [PubMed] [Google Scholar]
Vedantham 2009b
- Vedantham S, Thorpe PE, Cardella JF, Grassi CJ, Patel NH, Ferral H, et al. Quality improvement guidelines for the treatment of lower extremity deep vein thrombosis with use of endovascular thrombus removal. Journal of Vascular and Interventional Radiology 2009;20(7 Suppl):S227–39. [DOI] [PubMed] [Google Scholar]
Vedantham 2010
- Vedantham S. Catheter directed thrombolysis for deep vein thrombosis. Current Opinion Haematology 2010;17(5):464-8. [DOI] [PubMed] [Google Scholar]
Vedantham 2013
- Vedantham S, Goldhaber SZ, Kahn SR, Julian J, Magnuson E, Jaff MR, et al. Rationale and design of the ATTRACT Study: A multicenter randomized trial to evaluate pharmacomechanical catheter-directed thrombolysis for the prevention of postthrombotic syndrome in patients with proximal deep vein thrombosis. American Heart Journal 2013;165(4):523-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vedantham 2017
- Vedantham S, Goldhaber SZ, Julian JA, Kahn SR, Jaff MR, Cohen DJ, et al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. New England Journal of Medicine 2017;377:2240-52. [DOI: 10.1056/NEJMoa1615066] [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2006
- White RH. The epidemiology of venous thromboembolism. Circulation 2003;107(23):14-8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Armon 2000
- Armon MP, Michaels JA. Thrombolysis for acute deep vein thrombosis. Cochrane Database of Systematic Reviews 2000, Issue 2. Art. No: CD002783. [DOI: 10.1002/14651858.CD002783] [DOI] [PubMed] [Google Scholar]
Watson 2004
- Watson L, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database of Systematic Reviews 2004, Issue 3. Art. No: CD002783. [DOI: 10.1002/14651858.CD002783.pub2] [DOI] [PubMed] [Google Scholar]
Watson 2010
- Watson L, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD002783. [DOI: 10.1002/14651858.CD002783.pub2] [DOI] [PubMed] [Google Scholar]
Watson 2014
- Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database of Systematic Reviews 2014, Issue 1. Art. No: CD002783. [DOI: 10.1002/14651858.CD002783.pub3] [DOI] [PubMed] [Google Scholar]
Watson 2016
- Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No: CD002783. [DOI: 10.1002/14651858.CD002783.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]


