Abstract
Background
Statins are one of the most prescribed classes of drugs worldwide. Atorvastatin, the most prescribed statin, is currently used to treat conditions such as hypercholesterolaemia and dyslipidaemia. By reducing the level of cholesterol, which is the precursor of the steroidogenesis pathway, atorvastatin may cause a reduction in levels of testosterone and other androgens. Testosterone and other androgens play important roles in biological functions. A potential reduction in androgen levels, caused by atorvastatin might cause negative effects in most settings. In contrast, in the setting of polycystic ovary syndrome (PCOS), reducing excessive levels of androgens with atorvastatin could be beneficial.
Objectives
Primary objective
To quantify the magnitude of the effect of atorvastatin on total testosterone in both males and females, compared to placebo or no treatment.
Secondary objectives
To quantify the magnitude of the effects of atorvastatin on free testosterone, sex hormone binding globin (SHBG), androstenedione, dehydroepiandrosterone sulphate (DHEAS) concentrations, free androgen index (FAI), and withdrawal due to adverse effects (WDAEs) in both males and females, compared to placebo or no treatment.
Search methods
The Cochrane Hypertension Information Specialist searched the following databases for randomized controlled trials (RCTs) up to 9 November 2020: the Cochrane Hypertension Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE; Embase; ;two international trials registries, and the websites of the US Food and Drug Administration, the European Patent Office and the Pfizer pharmaceutical corporation. These searches had no language restrictions. We also contacted authors of relevant articles regarding further published and unpublished work.
Selection criteria
RCTs of daily atorvastatin for at least three weeks, compared with placebo or no treatment, and assessing change in testosterone levels in males or females.
Data collection and analysis
Two review authors independently screened the citations, extracted the data and assessed the risk of bias of the included studies. We used the mean difference (MD) with associated 95% confidence intervals (CI) to report the effect size of continuous outcomes,and the risk ratio (RR) to report effect sizes of the sole dichotomous outcome (WDAEs). We used a fixed‐effect meta‐analytic model to combine effect estimates across studies, and risk ratio to report effect size of the dichotomous outcomes. We used GRADE to assess the certainty of the evidence.
Main results
We included six RCTs involving 265 participants who completed the study and their data was reported. Participants in two of the studies were male with normal lipid profile or mild dyslipidaemia (N = 140); the mean age of participants was 68 years. Participants in four of the studies were female with PCOS (N = 125); the mean age of participants was 32 years. We found no significant difference in testosterone levels in males between atorvastatin and placebo, MD ‐0.20 nmol/L (95% CI ‐0.77 to 0.37). In females, atorvastatin may reduce total testosterone by ‐0.27 nmol/L (95% CI ‐0.50 to ‐0.04), FAI by ‐2.59 nmol/L (95% CI ‐3.62 to ‐1.57), androstenedione by ‐1.37 nmol/L (95% CI ‐2.26 to ‐0.49), and DHEAS by ‐0.63 μmol/l (95% CI ‐1.12 to ‐0.15). Furthermore, compared to placebo, atorvastatin increased SHBG concentrations in females by 3.11 nmol/L (95% CI 0.23 to 5.99). We identified no studies in healthy females (i.e. females with normal testosterone levels) or children (under age 18). Importantly, no study reported on free testosterone levels.
Authors' conclusions
We found no significant difference between atorvastatin and placebo on the levels of total testosterone in males. In females with PCOS, atorvastatin lowered the total testosterone, FAI, androstenedione, and DHEAS. The certainty of evidence ranged from low to very low for both comparisons. More RCTs studying the effect of atorvastatin on testosterone are needed.
Plain language summary
What is the effect of atorvastatin on testosterone and other hormone levels in men and women?
Background
Statins are one of the most prescribed classes of drugs, and atorvastatin is the most used drug in that class. People take statins to lower their blood cholesterol levels and reduce their risk of heart disease. However, statins may also lower levels of testosterone and other androgens. These are hormones that have important functions in male and female health and development.
What did we do?
We wanted to measure the effect of atorvastatin on testosterone and other androgens.We searched for all studies in the medical literature that compared the effect of different doses of atorvastatin to placebo or no treatment in males and females, and also reported on their levels of testosterone and other androgens. We looked for studies in which the treatments people received were decided at random. This type of study usually gives the most reliable evidence about the effects of a treatment. Studies could have participants of any age.
After we identified these studies, we compared the results, and summarized the evidence from all the studies. Finally, we rated our confidence in the evidence ("certainty of the evidence"), based on such factors such as study methods and sizes, and how consistent the findings were across studies.
What did we find?
We found six studies, involving a total of 265 participants. Studies were conducted in China, Finland, Iran, Turkey, the UK and the USA.
Evidence from four studies suggests that atorvastatin may have a potentially beneficial effect in females with polycystic ovary syndrome (PCOS), a set of symptoms that may develop in women with higher than normal androgen levels. In women with PCOS, atorvastatin helped to decrease total testosterone and other androgens.
We found two studies in men, where atorvastatin had no significant effect on total testosterone.
What does this mean?
The certainty of the evidence for all outcomes ranged from low to very low. Our statistical estimates for the effects of atorvastatin on testosterone and other androgens in males and females may be very different from the true effects. More studies are needed to answer this important question.
How up‐to‐date is this evidence?
The evidence from our review is current to November 2020.
Summary of findings
Summary of findings 1. Summary of findings.
|
Population: Male participants Intervention: Atorvastatin Comparison: Placebo or no treatment | |||
| Outcomes | Number of participants (RCTs) | Mean difference of atorvastatin compared to placebo (95% CI) | The certainty of evidence (GRADE) |
| Total testosterone | 140 (2) | ‐0.20 nmol/L (‐0.77 to 0.37) | Very low 1, 2, 3 |
| Acronyms and grades | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: randomized controlled trial | |||
|
GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
1 Unclear risk of selection bias.
2 95% confidence interval around the best effect estimate includes both a reduction and an increase in total testosterone.
3 Evidence was derived from only two studies, which had several limitations.
Summary of findings 2. Summary of findings.
|
Population: Adult female participants with PCOS Intervention: Atorvastatin Comparison: Placebo | |||
| Outcomes | Number of participants (RCTs) | Mean difference of atorvastatin compared to placebo (95% CI) | The certainty of evidence (GRADE) |
| Total testosterone | 125 (4) | ‐0.27 nmol/L (‐0.50 to ‐0.04) | Very low 1, 2,3 |
| FAI | 65 (2) | ‐2.59 (‐3.62 to ‐1.57) | Very low 1, 2, 3 |
| SHBG | 65 (2) | 3.11 nmol/L (0.23 to 5.99) | Low 1, 3 |
| Androstenedione | 125 (4) | ‐1.37 nmol/L (‐2.26 to ‐0.49) | Very low 1, 2, 3 |
| DHEAS | 125 (4) | ‐0.63 μmol/L (‐1.12 to ‐0.15) | Low 1, 3 |
| Acronyms and grades | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; DHEAS: dehydroepiandrosterone sulphate; FAI: free androgen index; RCT: randomized controlled trial; SHBG: sex hormone binding globin | |||
|
GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
1 High risk of bias in source of funding and sponsorship bias in more than one study.
2 Significant heterogeneity.
3 Small sample size.
Background
Androgens are a group of sex steroid hormones that are mainly responsible for the development and maintenance of the male sex organs, and secondary male sex traits. Androgens also impact libido and sexual behavior in both males and females. Circulating androgens include testosterone, dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEAS), androstenedione (A4), androstenediol (A5), and dihydrotestosterone (DHT). Androgens are mainly produced by the testicles, adrenal cortex, and ovaries (Sriram 2010).
Testosterone, C19 H28 O2, is the main male sex hormone. It plays a crucial role in several biological processes, such as regulating the sex drive in both sexes and producing sperm in males. Testosterone is also involved in regulating some other functions in both males and females, e.g. bone mass, muscle mass and strength, fat distribution, and the production of red blood cells (Finkelstein 2013).
Testosterone circulates in the blood in three different forms: as testosterone firmly attached to sex hormone binding globulin (SHBG) (roughly 45% of total testosterone), which is biologically inactive; as free testosterone (approximately 2% to 3% of total testosterone); and as testosterone weakly bound to other proteins, mainly albumin (approximately 50% of total testosterone). Both free testosterone and testosterone attached to albumin are bioavailable for use by tissues (Dunn 1981).
Bioavailable and free testosterone are better measures than total serum testosterone to diagnose abnormal androgen status and their clinical sequelae. However, bioavailable and free testosterone tests are time‐consuming, expensive and not always available. Therefore, determining total testosterone level, which is easy and relatively inexpensive to do, is commonly used to diagnose overt male hypogonadism. However, it is an unreliable marker in patients who have total testosterone levels just below the normal range, or in the low normal range. To overcome this issue and provide a more reliable indicator, some researchers use the SHBG and total testosterone, with a formula to calculate an approximate estimate of bioavailable or free testosterone (Stanworth 2008).
The normal ranges of the serum total testosterone are significantly higher in adult men than in adult women, and vary widely depending on various factors that include the laboratory methods used and the patient's age. There is a significant increase in total testosterone in males after puberty and a reduction of approximately 1% in total testosterone per year in males over thirty years old (Brawer 2004; Feldman 2002; Pagana 2015; refer to Table 3).
1. Total testosterone and free testosterone levels by sex and age.
| Tests of blood: normal findings | ||
| Free testosterone pg/mL | ||
| Age/Tanner stage | Male | Female |
| Postmenopausal | 0.6‐3.8 | |
| 7 months‐9 years (Tanner stage I) | ≤ 3.7 | < 2.2 |
| 10‐13 years (Tanner stage II) | 0.3‐21 | 0.4‐4.5 |
| 14‐15 years (Tanner stage III) | 1.0‐98 | 1.3‐7.5 |
| 16‐17 years (Tanner stage IV) | 35.0‐196 | 1.1‐15.5 |
| 18‐19 years (Tanner stage V) | 41.0‐239 | 0.8‐9.2 |
| Free testosterone % | ||
| Adult male | 1.6‐2.9 | |
| Adult female | 0.1‐0.3 | |
| Total testosterone ng/dL | ||
| Male | Female | |
| 7 months‐9 years (Tanner stage I) | < 30 | < 30 |
| 10‐13 years (Tanner stage II) | < 300 | < 40 |
| 14‐15 years (Tanner stage III) | 170‐540 | < 60 |
| 16‐19 years (Tanner stage IV, V) | 250‐910 | < 70 |
| 20 years and over | 280‐1080 | < 70 |
| Dihydrotestosterone | ||
| Adult male | 240‐650 pg/mL | |
| Adult female | ≤ 300 pg/mL | |
Total testosterone and free testosterone levels by sex and age (Pagana 2015).
Research suggests that low serum testosterone in men is associated with health problems, such as reduction in fertility and decreased libido. Low testosterone in both males and females can increase body fat and the incidence of depression. It can also decrease mass and strength of muscles, decrease body hair, decrease the production of red cells leading to anaemia, and decrease bone density (Demers 2010; Traish 2009).
Description of the condition
Statins are 3‐hydroxy‐3‐methylglutaryl–coenzyme A (HMG‐CoA) reductase inhibitors, a class of drugs mainly prescribed to lower blood cholesterol. They are widely used in adult patients for secondary and primary prevention of cardiovascular events (Stone 2014). They are also used to lower cholesterol in people of all ages with heterozygous familial hypercholesterolaemia and mixed dyslipidaemia (Schaiff 2008). They are considered to be a highly effective class of drugs in reducing low‐density lipoprotein (LDL) cholesterol (Schaiff 2008). Statins are also used to treat women with polycystic ovary syndrome (PCOS) (Sun 2015).
Description of the intervention
Atorvastatin is the most prescribed member of the statin class, which are among the most commonly prescribed medications worldwide (Ioannidis 2014; IQVIA 2017). In Canada, approximately 3 million people aged 20 to 79 (12% of Canada’s population) took a statin medication during the period 2007 to 2011; one in four Canadians are potentially eligible for statin treatment (Hennessy 2016). Studies illustrate that long‐term statin therapy in secondary prevention significantly reduces all‐cause mortality and major adverse cardiovascular events such as myocardial infarction (MI) and stroke. However, it remains controversial whether they reduce mortality in primary prevention patients (Virani 2013; Vrecer 2003).
Atorvastatin is rapidly absorbed after oral administration; reaching peak plasma concentration within one to two hours. Atorvastatin is extensively metabolized by cytochromes P‐450 3A4 and P‐450 3A5 to two active metabolites ortho‐ and para‐hydroxylated derivatives, which contribute to approximately 70% of the inhibitory activity for HMG‐CoA reductase. These two active metabolites also increase the half‐life of inhibitory activity for HMG‐CoA reductase (about 20 to 30 hours). A Cochrane Review shows that atorvastatin 2.5 mg/day to 80 mg/day reduces total blood LDL‐cholesterol by between 27.3% and 52%, respectively (Adams 2015; FDA 2011).
Statins are associated with a number of potential adverse effects. Such adverse effects can be divided into two groups, common adverse effects and serious adverse effects. Common adverse effects include headache, nausea, vomiting, constipation, diarrhoea, rash, insomnia, myalgia, dyspepsia, pain in extremities, upper respiratory infection, arthralgia, elevated creatine kinase (CK), elevated alanine aminotransferase (ALT) or aspartate aminotransferase (AST), cognitive impairment, and gynaecomastia. Serious adverse effects include muscle symptoms, ranging from muscle pain and weakness to rhabdomyolysis, tendon rupture, acute renal failure, hepatotoxicity, pancreatitis, hypersensitivity reaction, anaphylaxis, photosensitivity, toxic epidermal necrolysis, erythema multiforme, Stevens‐Johnson syndrome, thrombocytopaenia, leukopaenia, haemolytic anaemia, diabetes mellitus, and interstitial lung disease (atorvastatin Adverse Reactions ‐ Epocrates Online; Thompson 2016).
The most relevant adverse effect in terms of this review is gynaecomastia. This is listed as a common side effect of statins, which means it occurs in more than 1% of patients. It is a condition clinically defined by an enlargement of male breast tissue, along with other histopathological characteristics including benign proliferation of glandular male breast tissue (Johnson 2009). While the mechanism of action is unclear, statins could lead to gynaecomastia via an increased production of oestrogen, decreased production of androgens, or a combination of both. According to a recent case‐control study based on 6147 cases, the use of statins is associated with an increased risk of gynaecomastia (Skeldon 2018). The occurrence of gynaecomastia among male patients receiving statins most likely reflects an effect of statins to reduce androgen serum levels. This coincides with the hypothesis of our review.
How the intervention might work
The synthesis of steroid hormones occurs within the mitochondria and the endoplasmic reticulum, since the group of required oxidative enzymes exists only within these two intracellular organelles. The transport of free cholesterol from the cytoplasm into the mitochondria is the rate‐limiting step. Within the mitochondria, cholesterol is converted to pregnenolone, the immediate precursor, by cytochrome P450 family 11 subfamily A member 1 (CYP11A1). Organs that contain this enzyme, such as the testicles, adrenal cortex, ovaries, and placenta, are the only ones that participate in the synthesis of steroids. The following steps of steroidogenesis are catalyzed by several enzymes that are categorized into two different groups, the cytochrome P450 (CYP) enzymes (oxidative enzymes) and the hydroxysteroid dehydrogenase (HSD) enzymes (Schiffer 2019).
Atorvastatin inhibits the enzyme that converts HMG‐CoA into a cholesterol precursor called mevalonic acid in hepatocytes. Atorvastatin molecules bind reversibly to the active site, changing the conformation of the enzyme, which leads to preventing the enzyme from acquiring a functional structure. Inhibition of HMG–CoA reductase leads to a decline in the intracellular cholesterol, resulting in the activation of a protease that cleaves the sterol regulatory element binding proteins (SREBPs) from the endoplasmic reticulum. SREBPs, in their turn, are translocated in the nucleus and increase the gene expression for LDL receptors in hepatocytes, which leads to a reduction of the circulating LDL and both intermediate‐density lipoprotein (IDL) and very low‐density lipoprotein (VLDL), precursors for LDL (Stancu 2001).
In terms of its potential effect on testosterone, atorvastatin likely decreases the availability of cholesterol, which is a crucial substrate for testosterone production, leading to a decline in serum testosterone. The reduction of androgen levels can be potentially harmful and an adverse side effect in males and females with normal or below normal testosterone levels. However, women with PCOS (hyperandrogenism) may reap benefits from reduced androgen levels. Distinguishing between these two clinical settings is crucial when the effect of statins on androgen levels is being evaluated.
Why it is important to do this review
Based on the mechanism of action (MOA) of atorvastatin, a decline in the levels of testosterone and other androgens will likely occur in patients taking atorvastatin, as atorvastatin decreases the availability of cholesterol, a crucial precursor for androgen production.
Due to the widely increasing use of atorvastatin and the important role of testosterone in both men and women, concerns have been raised about the effect of atorvastatin on serum testosterone in men and women. A non‐Cochrane systematic review attempted to address the effect of statins on testosterone (Schooling 2013). However, this review has a number of limitations. Firstly, it is out of date (the searches range to the end of 2011). It has a limited search strategy, as the review authors searched only MEDLINE and ISI Web of Science, and restricted their search to publications in the English language. Secondly, the review was limited to adults, thus excluding adolescents and children who are potentially particularly vulnerable to reduced serum testosterone levels during their growing years. Thirdly, no protocol was pre‐published, and there are errors in the data reported by the review. Finally, the systematic review was potentially biased, as it was funded by one of the manufacturers of rosuvastatin, MedImmune, LLC. At present, there is no Cochrane Review that addresses the effect of atorvastatin on serum testosterone levels.
Objectives
To quantify the magnitude of the effects of atorvastatin as compared to placebo or no treatment on total testosterone, free testosterone, SHBG, A4, DHEAS concentrations, FAI, and WDAEs.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) with a minimum duration of three weeks, that compared atorvastatin to placebo or no treatment, and measured one or more of the androgenic outcomes.
Types of participants
Participants of both sexes could be of any age, with normal lipid parameters, or any type of hyperlipidaemia or dyslipidaemia. We included participants with various comorbid conditions: diabetes mellitus, hypertension, metabolic syndrome, chronic renal failure, PCOS, or cardiovascular diseases.
Types of interventions
We included atorvastatin doses that ranged from 2.5 mg to 80 mg, administered daily for at least three weeks. We chose a three‐week time window in order to allow for a steady‐state effect of atorvastatin to occur.
Types of outcome measures
Blood concentrations of different androgens.
Primary outcomes
The primary outcome of this study was the absolute change in total testosterone concentrations. When the absolute change was reported at different time periods, we chose the absolute change reported at the longest time period.
Secondary outcomes
Absolute change in free testosterone
Absolute change in FAI
Absolute change in DHEAS
Absolute change in A4
Absolute change in SHBG
(When the absolute change was reported at different periods for these outcomes, we chose the absolute change at the longest period).
Patient WDAEs
Search methods for identification of studies
Electronic searches
The Cochrane Hypertension Information Specialist searched the following databases without language, publication year or publication status restrictions:
the Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched 9 November 2020);
the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies (CRS‐Web) (searched 9 November 2020);
MEDLINE Ovid (from 1946 onwards), MEDLINE Ovid Epub Ahead of Print, and MEDLINE Ovid In‐Process & Other Non‐Indexed Citations (searched 9 November 2020);
Embase Ovid (from 1974 onwards) (searched 9 November 2020);
ClinicalTrials.gov (www.clinicaltrials.gov) (searched 9 November 2020);
World Health Organization International Clinical Trials Registry Platform (www.who.it.trialsearch) (searched 9 November 2020).
The Information Specialist modeled subject strategies for databases on the search strategy designed for MEDLINE. Where appropriate, these strategies were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomized controlled studies (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.c (Higgins 2011). The MEDLINE search strategy was translated into the other databases using the appropriate controlled vocabulary, as applicable. We present search strategies for major databases in Appendix 1.
Searching other resources
The Cochrane Hypertension Information Specialist searched the Hypertension Specialised Register segment (which includes searches of MEDLINE, Embase, and Epistemonikos for systematic reviews) to retrieve existing reviews relevant to this Cochrane Review, so that we could scan their reference lists for additional trials. The Specialised Register also includes searches for controlled trials of CAB Abstracts & Global Health, CINAHL, ProQuest Dissertations & Theses and Web of Science.
We checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials.
Where necessary, we contacted authors of key papers and abstracts to request additional information about their trials, or clarification and further data if trial reports are unclear.
We also searched websites of the following resources, for additional information.
Pfizer, Inc ( Pfizer is the maker of Lipitor which is the commercial name of atorvastatin). (www.pfizer.ca/en/our_products)
US Food and Drug Administration (www.fda.gov)
European Patent Office (worldwide.espacenet.com)
Data collection and analysis
Selection of studies
Two review authors independently, in duplicate, screened the titles and abstracts of records that were retrieved in the search results. We applied the eligibility criteria and used the Covidence software (Covidence) to code these records for possible inclusion as ‘yes,’ ‘no’ or ‘maybe’. We excluded articles if the citation appeared completely irrelevant, after reading the title and abstract. We resolved disagreements in the coding by discussion to reach consensus. We obtained the full‐text papers for all records coded as ‘yes' and 'maybe' at the title and abstract screening stage. Two review authors independently screened the full‐text papers in duplicate to decide which studies met the inclusion criteria. We resolved disagreements by discussion, with adjudication by a third review author (JMW) in the event of non‐consensus. We provided the reasons for exclusion and bibliographic details for all articles excluded after full‐text review. We identified multiple full‐text papers of the same study, which we linked and treated as a single study. We also checked the list of references in the included studies and the articles that cited the included studies in Google Scholar, to identify relevant articles. We created a PRISMA chart diagram to document and report the flow of records and studies through the systematic review process (Liberati 2009).
Data extraction and management
We used an electronic data extraction form based on the Cochrane Public Health template (Data Extraction and Assessment Template), modified to allow extraction of all data required for this review. Two authors independently performed the data extraction, and followed this by a cross‐check of the data. We described the characteristics of each included study in the 'Characteristics of included studies' table.
Our data extraction form included details on study design, type of masking, PICO (i.e. the study's population, intervention, comparison, and outcome) elements, primary and secondary outcomes, effect estimates, mean differences (MDs), confidence intervals (CIs), standard deviation (SD) for each group and main differences, details of interventions and comparators, duration of interventions, baseline characteristics of the participants, statistical analysis, the follow‐up duration, number of participants lost to follow‐up, and funding sources.
We resolved discrepancies by rechecking the data, as well as through discussion and consensus between the two review authors. When we are not able to reach an agreement, a third review author (JMW) acted as arbiter. When necessary, we contacted the authors of the studies to provide clarification on ambiguous information. All extracted data was entered and double‐checked in RevMan 5 software (RevMan 2014).
Assessment of risk of bias in included studies
We independently assessed the risk of bias of the included studies using the Cochrane 'Risk of bias' tool (version 1) following Chapter 8 of the Cochrane Handbook, for the following domains (Higgins 2011):
sequence generation (selection bias);
allocation sequence concealment (selection bias);
blinding of participants (performance bias);
blinding of outcome assessors (detection bias);
incomplete outcome data (attrition bias);
selective outcome reporting (reporting bias); and
other potential sources of bias (conflict of interest, funding source)
We produced 'Risk of bias' tables as outlined in the Cochrane Handbook, Chapter 8 (Chapter 8). We reported the information obtained in our bias assessment in the 'Risk of bias' tables associated with each included trial.
We included all studies in the meta‐analysis, regardless of our assessment of the risk of bias. We performed a sensitivity analysis, excluding studies with a high risk of bias, to explore their impact on the treatment effect estimate. This risk of bias across studies informed our assessment of the certainty of evidence, using the GRADE framework.
Measures of treatment effect
For continuous outcomes, we calculated the MD by using mean values, corresponding SDs, and treatment arm sizes. Moreover, we analyzed the data using 95% CIs. We summarized dichotomous data (from studies reporting WDAEs) as risk ratios (RRs) with 95% CIs. We used an intention‐to‐treat (ITT) analysis.
Unit of analysis issues
As we included no cross‐over trials, we had no unit of analysis issues for cross‐over trials. For multi‐arm trials, if a study reported more than one intervention arm (with other drugs than atorvastatin), we identified the relevant intervention arm (atorvastatin in any dose) and included that in the review.
Dealing with missing data
We sought missing or unclear data from reports of included studies by contacting the study authors using the contact information provided in the respective articles.
The most common type of value that was not reported was the SD of the change. If a standard error (SE) was given instead of the SD, we used the formula "SD = SE x square root of n" (with 'n' signifying the number of participants) to calculate the SD. We also calculated SD if the 95% CI, P value, or t value were reported in the included studies, according to Chapter 16 of the Cochrane HandbookChapter 16 . If we were not able to obtain the SD from the study authors or calculate from the values mentioned above, we imputed SD using the following hierarchy (listed from highest to lowest):
SD of the end of treatment value;
SD of the baseline treatment value; or
average weighted standard deviation of the change from other trials in the review (Furukawa 2006).
Assessment of heterogeneity
We assessed statistical heterogeneity in results by inspection of a graphical display of the estimated treatment effects from included studies, along with their 95% CIs, and by formal statistical tests of measures of inconsistency (I2 statistic) (Higgins 2002). If the I2 statistic value was greater than 50%, we investigated the reasons behind the heterogeneity by looking for clinical and methodological differences among trials.
Assessment of reporting biases
As fewer than 10 studies contributed to any meta‐analysis, we did not use a funnel plot to detect the extent of risk of reporting bias, based on the symmetry of the plot, as we had planned to do in the protocol. However, we have discussed the other types of reporting bias, for example multiple publication bias, outcome reporting bias, and language bias (Sterne 2011).
Data synthesis
We used Review Manager 5 (RevMan 2014) to perform data synthesis, and meta‐analyzed the results of clinically and statistically homogeneous studies. We analyzed data for each group (males, females and overall) separately. We performed meta‐analyses using the Mantel‐Haenszel method for dichotomous outcomes (RR) with 95% CIs, using a fixed‐effect model, and the inverse variance method for continuous outcomes as MD with 95% CIs, using a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We planned on performing subgroup analysis based on the following.
According to various doses
Males versus females
Age ≥ 60 years versus age < 60 years
Children (age < 18 years) versus adult (age ≥ 18 years)
It was not possible to conduct a subgroup analysis based on participant age, as we did not have sufficient data.
Sensitivity analysis
We planned the following sensitivity analyses.
To check if the trials with high risk of bias affected the effect estimate of total testosterone, DHEAS, and A4
To check the difference between the effect estimates of the outcomes derived by using a fixed‐effect model and a random‐effects model
To check the impact of imputed data that we imputed due to missing information on the overall effect size
Summary of findings and assessment of the certainty of the evidence
We used GRADEpro software (GRADEpro GDT 2015) to produce an evidence profile table and used that to generate the 'Summary of Findings' table (Schünemann 2011a; Schünemann 2011b). We considered the following domains in assessing the overall certainty of evidence: limitations in study design and implementation (risk of bias), indirectness of evidence, unexplained heterogeneity or inconsistency of results, imprecision in results and high probability of publication bias. As outlined in the Cochrane Handbook (Schünemann 2011b), we assessed the certainty of the body of evidence for each outcome to be high, moderate, low or very low, and review authors provided comments to support these judgements. RCTs started at a high level of certainty and were downgraded by one level for each domain judged to have some serious concerns (Schünemann 2013).
Results
Description of studies
The summary of the screening process is shown in the PRISMA diagram (Figure 1).
1.
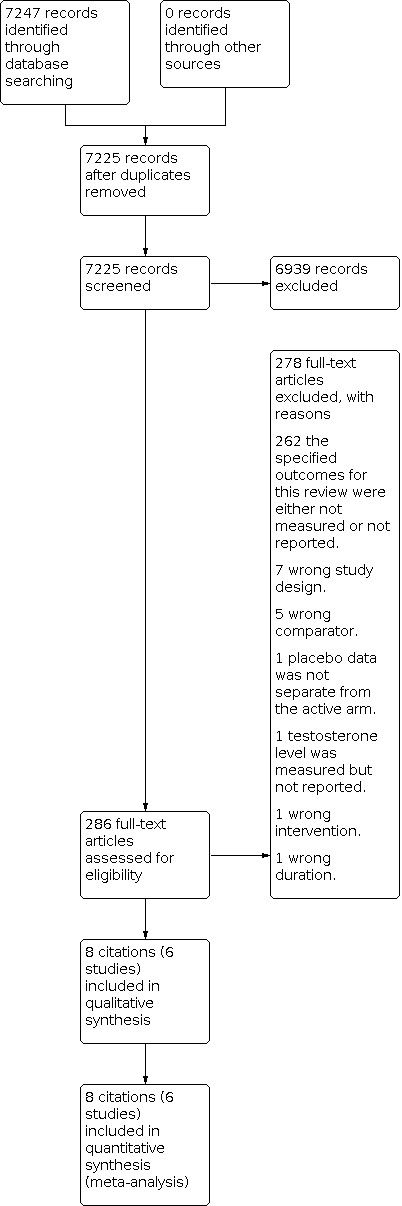
PRISMA diagram for screening studies
Results of the search
Database searching identified 7247 citations. After duplicates were removed, 7225 records remained. After irrelevant citations were removed, 286 records remained. The remaining 286 records were obtained as full‐text articles and assessed for eligibility. Eight citations to six studies (Akbari 2016; Chen 2014; Gokce 2012; Puurunen 2013; Raja‐Khan 2011; Sathyapalan 2009) met the inclusion criteria (Figure 1).
Included studies
We have summarized each included study in the 'Characteristics of included studies'. All six studies were published in English. They included 292 participants who were randomized(154 males and 145 females), but only 265 participants(140 males and 125 females) had their data reported. All of them had a parallel‐group design. Among the six studies included, one was single‐blind, four were double‐blind, and one was open‐label.
Two of the studies included only male participants (N = 140), with a mean age of 68 years. One study (Gokce 2012) included exclusively healthy male participants while the other study (Chen 2014) included male participants with mild dyslipidaemia and osteopaenia. Four of the studies included female participants (N = 125) and the mean age was 32 years. All participants in the four female studies had PCOS. The dose of atorvastatin in the two male studies was 10 mg. The dose of atorvastatin was 20 mg in two of the female studies, and 40 mg in the remaining two studies.
Excluded studies
We excluded 278 articles after reviewing the full‐text articles and recorded the reasons for exclusion. Most articles (262 articles) were excluded because they did not measure and report this review's outcomes of interest. We excluded seven articles because they were not RCTs. In addition, we excluded one study because it measured the testosterone levels but did not report them (see Figure 1 and 'Characteristics of excluded studies').
Risk of bias in included studies
We independently assessed the risk of bias, following the methodology described in Chapter 8 of the Cochrane Handbook (Chapter 8).
We assessed the risk of bias based on seven domains:
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective reporting (reporting bias);
other biases (conflict of interest, industry sponsorship).
We assessed the risk of detection bias and reporting bias in WDAEs separately from their assessment in the other outcomes. We also assessed the risk of attrition bias separately for each of the biomedical outcomes. We classified each domain as being at a low, high or uncertain risk of bias.
In the case of disagreement between review authors, a third review author (JMW) helped to discuss and resolve the disagreement.
The expected direction of risk of bias would be to exaggerate the anti‐androgen effect of atorvastatin as a potential treatment in women with PCOS, and to underestimate the reduction in androgens when it is was studied and measured as a potential adverse effect of atorvastatin treatment in the general patient population (Figure 2; Figure 3).
2.

Risk of bias summary of the included studies
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
We assessed selection bias based on two categories: random sequence generation and allocation concealment.
For random sequence generation, five of the six included studies were classified as having a low risk of bias since they used and described a true randomization method. One study (Akbari 2016) was classified as having an uncertain risk of bias, since the method of randomisation was not described.
For allocation concealment, three included studies (Akbari 2016; Chen 2014; Gokce 2012) were classified as having an uncertain risk of bias because the method of allocation concealment was not reported. The remaining three studies were classified as having a low risk of bias due to the application of appropriate methods, such as central allocation (pharmacy‐controlled randomization and sequentially numbered drug containers) (Puurunen 2013) or allocation by external personnel who were not involved in the trial (Raja‐Khan 2011; Sathyapalan 2009).
Blinding
All six studies were classified as being at low risk of performance and detection bias, as we consider that the individuals measuring laboratory outcomes were not aware of the treatment allocation.
For WDAEs, a lack of blinding could have had an effect. Four studies (Akbari 2016; Puurunen 2013; Raja‐Khan 2011; Sathyapalan 2009) were classified as being at uncertain risk of bias, since the blinding was not described. Two studies (Chen 2014; Gokce 2012) were classified as having high risk of bias, since they were open label and single‐blind, respectively.
Incomplete outcome data
We assessed attrition bias based on six outcomes: total testosterone, free testosterone, free androgen index, A4, SHBG, and DHEAS. For total testosterone, three studies (Akbari 2016; Chen 2014; Sathyapalan 2009) were classified as having low risk of bias given that all participants were included in the final analysis, or missing data were balanced across intervention and control groups, with a similar reason for missing data across groups. Two studies (Gokce 2012; Raja‐Khan 2011) were classified as being at uncertain risk of bias, since 10% of participants did not complete the trial, although the study authors included all participant data in the analysis. One study (Puurunen 2013) was classified as high risk of bias, since 21.1% of participants were not included in the analysis for the atorvastatin group, and 31.6% of participants were not included in the analysis for the placebo group. For A4 and DHEAS, only four studies measured these outcomes. Two studies (Akbari 2016; Sathyapalan 2009) were classified as having a low risk of bias. Puurunen 2013 was classified as high risk of bias, and Raja‐Khan 2011 was assessed to be at uncertain risk of bias. For free testosterone, only one study reported this outcome (Raja‐Khan 2011) and was classified as high risk of bias, since only baseline data was reported. For SHBG and FAI, only two studies measured the outcome: Puurunen 2013 was classified as high risk of bias and Sathyapalan 2009 was classified as low risk of bias.
Selective reporting
We assessed this separately for WDAEs and for all other outcomes. For WDAEs, four studies were classified as having low risk of bias (Akbari 2016; Chen 2014; Gokce 2012; Puurunen 2013) because they recorded and reported the WDAEs in the result section. Two studies (Raja‐Khan 2011; Sathyapalan 2009) were classified as having unclear risk of bias since the WDAEs were not reported separately from withdrawals due to other reasons.
For all other outcomes, three studies (Akbari 2016; Chen 2014; Gokce 2012) were classified as having uncertain risk of bias since no protocol was published and there was insufficient information to evaluate if it was at low or high risk of bias. Two studies (Puurunen 2013; Raja‐Khan 2011) were classified as low risk of bias since all of each study’s prespecified outcomes were reported. One study (Sathyapalan 2009) was classified as having high risk of bias since one reported primary outcome (HS‐CRP) was not prespecified in its trial registration.
Other potential sources of bias
In this domain, the other potential sources of bias assessed included industry sponsorship. Two studies (Chen 2014, Puurunen 2013) were classified as having low risk of bias because they were funded by government grants. Two studies (Akbari 2016; Gokce 2012) were classified as being at uncertain risk of bias since the funding source was not reported. Two studies (Raja‐Khan 2011; Sathyapalan 2009) were classified as high risk of bias because they were funded partially or fully by the Pfizer pharmaceutical company.
Publication bias
We did not pool data from a sufficient number of studies to construct funnel plots for assessing publication bias. However, all studies were published in English. Only six studies from more than 268 placebo‐controlled RCTs identified in our search results reported total testosterone levels, and only two studies reported this outcome in males. There are no studies in healthy females or children (under age 18). This suggests that there are likely RCTs measuring and demonstrating an effect of atorvastatin in reducing testosterone and other androgen levels that were not published, because the results of such RCTs might adversely impact the marketing of atorvastatin.
Effects of interventions
While in the general patient population, the reduction of testosterone levels caused by the administration of lipid‐reducing interventions including atorvastatin is considered an adverse side effect, this reduction is potentially beneficial in trials in which atorvastatin is administrated as a treatment for PCOS. Because the risk of bias is in the opposite direction in these two clinical settings, we presented and pooled these two clinical settings separately.
1. The potential adverse effect of atorvastatin on androgens
Effect of atorvastatin on total testosterone levels (males)
Based on two studies (Chen 2014; Gokce 2012), in 140 male participants, the mean change in total testosterone, using a fixed‐effect model, was ‐0.20 nmol/L (95% CI ‐0.77 to 0.37; P = 0.49). There was no heterogeneity (I2 = 0%) (Figure 4) .
4.

Forest plot of comparison: Atorvastatin versus control (fixed‐effect model), outcome: Total testosterone in males.
Effect of atorvastatin on free testosterone levels (males)
None of the included studies reported data for this outcome.
Effect of atorvastatin on WDAEs in males and females
Based on six studies (Akbari 2016; Chen 2014; Gokce 2012; Puurunen 2013; Raja‐Khan 2011; Sathyapalan 2009) in 292 participants, the RR for WDAEs using the fixed‐effect model was 1.00 (95% CI 0.14 to 7.37; P = 1.00). There was no heterogeneity (I2 = 0%) (Figure 5).
5.

Forest plot of comparison: 5 Atorvastatin versus control (fixed‐effect model), outcome: 5.7 Withdrawal due to adverse effects (WDAEs).
2. The possible beneficial effects of atorvastatin for the treatment of PCOS
Effect of atorvastatin on total testosterone levels
Based on four studies (Akbari 2016; Puurunen 2013; Raja‐Khan 2011; Sathyapalan 2009) in 125 participants, atorvastatin decreased total testosterone levels. The mean decrease, using a fixed‐effect model, was ‐0.27 nmol/L (95% CI ‐0.50 to ‐0.04; P = 0.02). There was substantial heterogeneity (I2 = 84%) (Figure 6).
6.

Effect of atorvastatin on free testosterone levels
None of the included studies reported data for this outcome. Only one study (Raja‐Khan 2011) reported free testosterone levels before the treatment, but did not report it after the treatment.
Effect of atorvastatin on FAI
Only two studies in females (Puurunen 2013; Sathyapalan 2009) reported FAI in 65 participants. Atorvastatin decreased FAI. The mean decrease, using a fixed‐effect model, was ‐2.59 (95% CI ‐3.62 to ‐1.57; P < 0.00001). There was substantial heterogeneity (I2 = 94%) (Analysis 1.7).
1.7. Analysis.

Comparison 1: Atorvastatin versus control (fixed‐effect model), Outcome 7: Free androgen index (FAI)
Effect of atorvastatin on SHBG
Only two studies in females (Puurunen 2013; Sathyapalan 2009) reported SHBG in 65 participants. Atorvastatin increased SHBG. The mean increase, using a fixed‐effect model, was 3.11 nmol/L (95% CI 0.23 to 5.99; P < 0.03). There was no heterogeneity (I2 = 0%) (Analysis 1.3).
1.3. Analysis.

Comparison 1: Atorvastatin versus control (fixed‐effect model), Outcome 3: Sex hormone binding globulin (SHBG)
Effect of atorvastatin on A4
The four studies in females (Akbari 2016; Puurunen 2013; Raja‐Khan 2011; Sathyapalan 2009) reported A4 levels in 125 participants. Atorvastatin decreased A4. The mean decrease, using a fixed‐effect model, was ‐1.37 nmol/L (95% CI ‐2.26 to ‐0.49; P = 0.002). There was substantial heterogeneity (I2 = 68%) (Analysis 1.4).
1.4. Analysis.

Comparison 1: Atorvastatin versus control (fixed‐effect model), Outcome 4: Androstenedione
Effect of atorvastatin on DHEAS
Four studies in females (Akbari 2016; Puurunen 2013; Raja‐Khan 2011; Sathyapalan 2009) reported DHEAS in 125 participants. Atorvastatin decreased DHEAS. The mean decrease, using a fixed‐effect model, was ‐0.63 μmol/L (95% CI ‐1.12 to ‐0.15 P = 0.01). There was no significant heterogeneity (I2 = 9%) (Analysis 1.5).
1.5. Analysis.

Comparison 1: Atorvastatin versus control (fixed‐effect model), Outcome 5: Dehydroepiandrosterone sulfate (DHEAS)
3. Subgroup analysis and exploring heterogeneity
We planned to conduct several subgroup analyses according to dosage and age. Due to the small number of studies, we were not able to conduct subgroup analyses since it is recommended that there should be at least 10 studies in each of the subgroups.
4.Sensitivity analysis
As planned, we conducted a sensitivity analysis to compare the results using the random‐effects model and the fixed‐effect model. While the reduction in total testosterone, FAI, and androstenedione was statistically significant when the fixed‐effect model was used, the reduction in all outcomes mentioned above was not significant when the random‐effects model was applied (Analysis 2.1; Analysis 2.2; Analysis 2.3). We planned to conduct sensitivity analysis on the studies based on their level of risk of bias. Most of the included studies had a similar risk of bias across all the domains, except for industry sponsorship bias and incomplete data for total testosterone. Due to the inadequate number of studies, we were not able to conduct a sensitivity analysis on the included studies based on industry sponsorship.
2.1. Analysis.

Comparison 2: Atorvastatin vs control (random effects model), Outcome 1: Total testosterone in females
2.2. Analysis.

Comparison 2: Atorvastatin vs control (random effects model), Outcome 2: Androstenedione
2.3. Analysis.

Comparison 2: Atorvastatin vs control (random effects model), Outcome 3: Free androgen index (FAI)
Discussion
This review summarizes the effects of atorvastatin on total testosterone, A4, DHEAS, FAI, and SHBG serum levels in males and females. The primary focus of the review was to document the effect of atorvastatin on these outcomes as a potential adverse effect. However, in conducting the review we identified RCTs meeting the inclusion criteria in which patients with PCOS were given atorvastatin with the goal of reducing androgens, as a potential treatment.
For trials studying the possible adverse effect of atorvastatin to decrease androgens, there is a potential bias towards not showing this effect or not reporting RCTs that found this adverse effect. The knowledge that atorvastatin decreased testosterone could result in a negative impact on sales of atorvastatin.
Summary of main results
1. Summary of the results of the possible adverse effect of atorvastatin on androgens
Effect of atorvastatin on total testosterone
In this review, we only found two trials investigating the effects of atorvastatin on androgen levels among males and no studies reporting the effect of atorvastatin on androgens in normal females. This was a surprising and shocking finding, as we anticipated a much larger number of studies looking at the effects of atorvastatin on androgen levels, given the widespread use of atorvastatin. The dearth of studies, the small sample size and the fact that other androgen levels were not reported resulted in the pooled effect of atorvastatin in males showing a statistically non‐significant reduction of total testosterone levels. In addition, the two studies we found used a relatively low dose of atorvastatin, 10 mg/day, when we know that atorvastatin daily doses of 80 mg/day are commonly used. We highly suspect that the companies marketing atorvastatin have successfully suppressed the conduct and/or publication of trials showing the reduction of testosterone and other androgens.
Additionally, it is important to mention that atorvastatin is given as a treatment for children and adolescents with familial hypercholesterolaemia. In this population, where reduction of androgens is more likely to be problematic, we again found no RCTs reporting the effects of atorvastatin on testosterone or other androgens.
Effect of atorvastatin on free testosterone and other androgens
Although free testosterone is a better indicator of the androgen status than total testosterone, no study reported free testosterone levels.
2. Summary of the results of the possible beneficial effects of atorvastatin for the treatment of PCOS
Effect of atorvastatin on total testosterone
For the possible beneficial reduction of total testosterone levels among females with PCOS, the direction of the potential risk of bias is towards a reduction, as the trials are designed to demonstrate that atorvastatin can be used to treat women with PCOS. In this review, we found that atorvastatin decreased total testosterone levels in females by ‐0.27 nmol/l. This finding was based on four studies in females with PCOS.
Effects of atorvastatin on SHBG and FAI
Atorvastatin increased SHBG by 3.11 nmol/l and decreased FAI by 2.59 nmol/L. These results were based on only two studies in females with PCOS. The increase in SHBG levels suggests that more testosterone is bound and that there is less bioavailable free testosterone for tissues. The decrease in FAI corresponds with an increase in SHBG and a decline in total testosterone.
Effect of atorvastatin on DHEAS and androstenedione
Atorvastatin decreased DHEAS concentration in females by 0.63 μmol/L, and androstenedione by 1.37 nmol/L. These results were based on four studies in females with PCOS. These results can be explained by the steroidogenesis pathway; the reduction in DHEAS levels would have led to a reduction in both A4 and testosterone levels. It is important to appreciate that the decline in A4 concentration may be an overestimate, since heterogeneity in the meta‐analysis was substantial and the reduction comes mostly from the Raja‐Khan 2011 study, which was sponsored by the pharmaceutical industry.
Effect of Atorvastatin on WDAEs
In the six trials that reported the impact of atorvastatin on WDAEs, drug therapy had no significant effect on WDAEs compared to placebo or no treatment. However, these were small and short‐term trials and do not represent a good opportunity to determine whether atorvastatin leads to adverse effects.
Overall completeness and applicability of evidence
Surprisingly, there is very little available published data to evaluate the effect of atorvastatin on testosterone and other androgens. Given that we have presented evidence that atorvastatin decreases testosterone and other androgens in women with PCOS, we suspect that atorvastatin also reduces testosterone in other settings. There are likely trials meeting our inclusion criteria, showing this effect of atorvastatin, that have not been published. It is very likely that atorvastatin reduces testosterone as a result of its mechanism of action to reduce cholesterol levels. Further strong evidence that this is occurring is the fact that gynaecomastia is being recognized as an adverse effect of atorvastatin. The two studies in males represented a range in terms of age and health conditions, with a mean age of 68 years. It is possible that atorvastatin does decrease androgen levels more significantly in a younger population .
The participant populations were older males with normal libido (Gokce 2012) and elder men with mild dyslipidaemia (Chen 2014). The overall evidence generated in this review is insufficient to answer the question in the populations studied, and cannot be extrapolated to men and women of all ages, or to adults with sexual dysfunction.
Regarding the studies in females, the participant population was women with PCOS, predominantly in premenopausal age (younger than age 40). Unfortunately, we do not have any trials studying the effect of atorvastatin on females at any age without PCOS. It is, however, likely that atorvastatin does decrease androgen levels in women without PCOS, and that this could have adverse consequences.
There was substantial heterogeneity in the meta‐analysis of the effect of atorvastatin on testosterone in females with PCOS, due to the presence of one study (Sathyapalan 2009). This study was funded by the Pfizer pharmaceutical company. The mean reduction of total testosterone was greater in this study compared to other studies. Thus, we suspect that this reduction may be an overestimate.
There was substantial heterogeneity in the meta‐analysis of the effect of atorvastatin on androstenedione in females, due to the presence of one study (Raja‐Khan 2011). This study was funded by the pharmaceutical industry. The mean reduction of total testosterone was greater in this study, compared to other studies. The unbalanced baseline between the comparison groups in Puurunen 2013 may explain the substantial heterogeneity between the two studies in the FAI meta‐analysis.
Quality of the evidence
We graded the overall certainty of evidence using the GRADE approach, with the GRADEpro GDT software (GRADEpro GDT 2015), and formulated 'Summary of findings' tables, as outlined in the protocol.
We constructed two 'Summary of findings' tables to show the certainty of evidence and a summary of the effects on the outcomes of interest in females (total testosterone, A4, DHEAS, FAI, SHBG, and WDAEs), and total testosterone in males.
In this review, the certainty of evidence ranged from low to very low, which suggests that the estimated effect of atorvastatin may be substantially different from the true effect. Regarding the effect of atorvastatin on total testosterone levels in males, the certainty of evidence was downgraded to very low due to the small size of the studies, wide confidence intervals, and the high risk of bias. Regarding the effect of atorvastatin on total testosterone, A4, and FAI in females, the certainty of evidence was downgraded to very low due to the small size of the studies, significant inconsistency, and the high risk of bias in some domains.
The certainty of evidence for DHEAS and SHBG in studies in females was downgraded to low due to the small sample size and the high risk of bias in some of the studies.
Potential biases in the review process
We employed a robust methodology to assess biases and used comprehensive search strategies to minimize potential for bias by the authors in the selection process of included studies. A few amendments have been implemented on the protocol which were explicitly mentioned and justified.
We have identified several limitations within this review, which are related to the studies that were included. We highly suspect that there are more studies which meet the inclusion criteria and were not published (publication bias), despite our efforts to search the grey literature. Our review's included studies and its data are only applicable to specific population groups, and therefore should not be generalized. The overall sample size of the studies was not sufficiently large to answer our research question. Finally, the reporting of outcomes in some of the included studies tended to be poor in nature. The reporting of the SD of the change in levels was the main problem. In one of the studies in males (Gokce 2012), the authors did not report the SD of the change or the SD of the means, either before or after the intervention. Thus, we imputed the SD of the change from other studies, using the guidance of Furukawa (Furukawa 2006).
In the studies in females, the SD of change was missing, and we had to calculate it from the reported P values. In some studies (Akbari 2016; Puurunen 2013) we imputed the SD of change using the SD of final values. Accordingly, the results should be interpreted with caution. Another major problem related to data reporting was the units of measurements. In one of the included studies (Gokce 2012), the units were reported incorrectly (the testosterone unit was reported as ng/ml instead of ng/dl). Additionally, units were essentially missing, and the author did not report reasons for not including units (Akbari 2016). Furthermore, withdrawal due to adverse effects was not explicitly reported in some of the studies.
Agreements and disagreements with other studies or reviews
One published review (Schooling 2013) examined the effect of atorvastatin on total testosterone. As discussed earlier in this review, Schooling 2013 pooled the effect of all statin agents on testosterone, by assuming that they all had similar effects. We limited the focus of this review only to atorvastatin. Schooling 2013 concluded that statins reduce testosterone in both males and females. However, there are a number of limitations to the Schooling 2013 review, which we have listed in the background.
Another published review (Yang 2019) examined the effect of atorvastatin on DHEAS. Yang pooled the effect of all statin agents on DHEAS and conducted a subgroup that analyzed the effects of atorvastatin and simvastatin separately. According to Yang 2019, atorvastatin significantly reduced DHEAS, compared to simvastatin. The conclusion of Yang 2019, which was based on three of the studies that were included in this review (Puurunen 2013; Raja‐Khan 2011; Sathyapalan 2009), aligns with our review's conclusion.
Statin‐induced gynaecomastia was reported by several studies and case reports as a potential adverse effect among male patients (Jeong 2019; Roberto 2012; Skeldon 2018). Although our review did not find significant reduction in androgens among male patients taking statins, this could be due to publication bias and the limitations of studies included.
This review did not detect a significant difference in WDAEs between atorvastatin and placebo, since only two studies reported WDAEs. A previous review (Adams 2015) with 43 short‐term RCTs reporting WDAEs, also showed no effect of atorvastatin on WDAEs.
Authors' conclusions
Implications for practice.
This review provides the most up‐to‐date clinical evidence on the effects of atorvastatin on testosterone and other androgens in males and females.
The studies in females were limited by trialists to women with polycystic ovary syndrome (PCOS). In adult females with PCOS, very low certainty of evidence shows that atorvastatin (20 mg to 40 mg daily) reduced the following androgens: total testosterone by a mean of ‐0.27 nmol/L, free androgen index (FAI) by ‐2.59, androstenedione by ‐1.37 nmol/L, and dehydroepiandrosterone sulphate (DHEAS) by ‐0.63 µmol /L. Atorvastatin increased sex hormone binding globin (SHBG) by a mean 3.11 nmol/L. The indicated change in the level of androgens illustrates that through lowering cholesterol levels, atorvastatin can lower all the downstream androgens in the steroidogenic pathway. We have judged this evidence to be of low to very low certainty, and it is unknown whether a reduction of androgens by atorvastatin in women with PCOS would have any beneficial clinical effects.
The studies in males were limited by trialists to men with normal libido. In this specific population, very low‐certainty evidence suggests that using 10 mg of atorvastatin did not have a significant effect on total testosterone levels. It is impossible to know, with the limited data available, whether atorvastatin reduces androgens, but given the mechanism of action, the fact that atorvastatin reduces androgens in women with PCOS and that it can be associated with gynaecomastia, we have a high degree of suspicion that atorvastatin does reduce androgens in most if not all settings. Therefore, healthcare providers should be aware of the potential reduction in androgens by atorvastatin, and inform patients of this potential adverse effect.
Implications for research.
This review provides better understanding of the available evidence on the effects of atorvastatin on androgens and sheds light on the absence of high‐quality evidence in certain patient populations. Based on our findings, we recommend the following:
More randomized controlled trials (RCTs) are needed to evaluate the effects of atorvastatin on androgens in females with PCOS.
More RCTs are needed to evaluate the effects of atorvastatin on androgens in males, using higher doses of atorvastatin.
The review did not find any eligible RCTs that reported the effects of atorvastatin on children, healthy females, or males with abnormal libido. Future RCTs in these populations are needed.
The procedures of randomization and blinding must be described in detail within published reports.
Trials should report the standard deviation (SD) of the change, and the unit of measurement used.
Reporting of withdrawal due to adverse effects should be mandatory for all trials.
Free testosterone should be measured and reported, as it provides a more reliable measure of androgen status.
History
Protocol first published: Issue 12, 2018 Review first published: Issue 1, 2021
Acknowledgements
The review authors would like to acknowledge the help provided by Cochrane Hypertension. We would like to thank Douglas Salzwedel, Information Specialist for Cochrane Hypertension, for designing and conducting the searches, and Ciprian Jauca, Managing Editor for Cochrane Hypertension, for his assistance.
Appendices
Appendix 1. Search strategy
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) <1946 to November 06, 2020> Search Date: 9 November 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 atorvastatin/ (6631) 2 (atorvastatin$ or atorlip or atovarol or cardyl or "ci 981" or ci981 or glustar or lipibec or lipitor or liprimar or liptonorm or lowlipen or sortis or storvas or tahor or torvast or totalip or xarator or "ym 548" or ym548 or zarator).tw,kf. (8996) 3 or/1‐2 (10041) 4 randomized controlled trial.pt. (516561) 5 controlled clinical trial.pt. (93916) 6 randomized.ab. (497951) 7 placebo.ab. (212325) 8 drug therapy.fs. (2249299) 9 randomly.ab. (344379) 10 trial.ab. (526367) 11 groups.ab. (2112842) 12 or/4‐11 (4832805) 13 animals/ not (humans/ and animals/) (4720430) 14 12 not 13 (4197674) 15 3 and 14 (5419) 16 15 and (2013 12$ or 2014$ or 2015$ or 2016$ or 2017$ or 2018$ or 2019$ or 2020$).dt. (1788) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Cochrane Hypertension Specialised Register via Cochrane Register of Studies (CRS‐Web) Search Date: 9 November 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 MESH DESCRIPTOR atorvastatin AND INREGISTER #2 (atorvastatin* OR atorlip OR atovarol OR cardyl OR glustar OR lipibec OR lipitor OR liprimar OR liptonorm OR lowlipen OR sortis OR storvas OR tahor OR torvast OR totalip OR xarator OR zarator) AND INREGISTER #3 #1 OR #2 #4 RCT:DE AND INSEGMENT #5 Review:ODE AND INSEGMENT #6 #4 OR #5 #7 #3 AND #6 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Cochrane Central Register of Controlled Trials (Issue 10, 2020) via Cochrane Register of Studies (CRS‐Web) Search Date: 9 November 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 MESH DESCRIPTOR atorvastatin AND CENTRAL:TARGET #2 (atorvastatin* OR atorlip OR atovarol OR cardyl OR glustar OR lipibec OR lipitor OR liprimar OR liptonorm OR lowlipen OR sortis OR storvas OR tahor OR torvast OR totalip OR xarator OR zarator) AND CENTRAL:TARGET #3 (#1 OR #2) AND CENTRAL:TARGET ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Embase <1974 to 2020 November 06> Search Date: 9 November 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 atorvastatin/ (37934) 2 (atorvastatin$ or atorlip or atovarol or cardyl or "ci 981" or ci981 or glustar or lipibec or lipitor or liprimar or liptonorm or lowlipen or sortis or storvas or tahor or torvast or totalip or xarator or "ym 548" or ym548 or zarator).mp. (39004) 3 or/1‐2 (39004) 4 randomized controlled trial/ (631824) 5 crossover procedure/ (65266) 6 double‐blind procedure/ (178669) 7 (randomi?ed or randomly).tw. (1291636) 8 (crossover$ or cross‐over$).tw. (109824) 9 placebo.ab. (306862) 10 (doubl$ adj blind$).tw. (215272) 11 assign$.ab. (402529) 12 allocat$.ab. (156801) 13 or/4‐12 (1870641) 14 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (6531514) 15 13 not 14 (1633335) 16 3 and 15 (6642) 17 16 and (2013 12$ or 2014$ or 2015$ or 2016$ or 2017$ or 2018$ or 2019$ or 2020$).dc,dd. (2367) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: ClinicalTrials.gov Search Date: 9 November 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Other terms: sex hormone* OR SHBG OR testosterone Study type: Interventional Studies (Clinical Trials) Study Results: All Studies Intervention/treatment: Atorvastatin OR lipitor
Data and analyses
Comparison 1. Atorvastatin versus control (fixed‐effect model).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Total testosterone in males | 2 | 140 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.77, 0.37] |
| 1.2 Total testosterone in females | 4 | 125 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.50, ‐0.04] |
| 1.3 Sex hormone binding globulin (SHBG) | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 3.11 [0.23, 5.99] |
| 1.4 Androstenedione | 4 | 125 | Mean Difference (IV, Fixed, 95% CI) | ‐1.37 [‐2.26, ‐0.49] |
| 1.5 Dehydroepiandrosterone sulfate (DHEAS) | 4 | 125 | Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐1.12, ‐0.15] |
| 1.6 Withdrawal due to adverse effects (WDAEs) | 6 | 292 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.14, 7.37] |
| 1.7 Free androgen index (FAI) | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐2.59 [‐3.62, ‐1.57] |
1.1. Analysis.

Comparison 1: Atorvastatin versus control (fixed‐effect model), Outcome 1: Total testosterone in males
1.2. Analysis.

Comparison 1: Atorvastatin versus control (fixed‐effect model), Outcome 2: Total testosterone in females
1.6. Analysis.

Comparison 1: Atorvastatin versus control (fixed‐effect model), Outcome 6: Withdrawal due to adverse effects (WDAEs)
Comparison 2. Atorvastatin vs control (random effects model).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Total testosterone in females | 4 | 125 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.87, 0.34] |
| 2.2 Androstenedione | 4 | 125 | Mean Difference (IV, Random, 95% CI) | ‐1.56 [‐3.32, 0.20] |
| 2.3 Free androgen index (FAI) | 2 | 65 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐6.30, 2.51] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akbari 2016.
| Study characteristics | ||
| Methods | Study design: Randomized placebo controlled trial Masking: Double‐blind Number of arms: 2 |
|
| Participants |
Inclusion criteria: women with a diagnosis of PCOD based upon existence of 2 of the 3 criteria specified by Rotterdam, BMI > 25.2, LDL > 100, not pregnant, no other reasons for obesity and metabolic and hormone and glands and metabolism issues, no use of metformin and other medicines that create sensitivity to insulin and no use of OCP over the last three months and no consumption of medicines that reduce androgen, not sensitive to atorvastatin, and participants are 18 to 35 years old Exclusion criteria: pregnancy, using metformin or any other medicines that cause sensitivity to insulin and OCP over the last 3 months before the research and androgen reducer (using progesterone for withdrawal or keeping mensuration was ok), LDL < 100, BMI < 25, any hormonal or metabolic disorders not related to PCOS, any sensitivity to atorvastatin exhibited by the rise of liver enzymes more than three times the normal level and severe muscle cramps Baseline Characteristics Placebo
40 mg atorvastatin
|
|
| Interventions |
Intervention characteristics 40 mg/day for 6 weeks Placebo for 6 weeks |
|
| Outcomes | Total testosterone, DHEAS and androstenedione | |
| Statistical analysis and reporting | Sequential nonrandom sampling method was used in both groups. The resulting information was analyzed using SPSS 16. As for qualitative variables, indicators such as frequency, mode, mean, and average were calculated. The frequency of qualitative variables was also measured. Independent t test was used to study qualitative variables, while repeated measures test was used to calculate the changes observed in two groups before and after intervention. Forward linear regression was used to study the confounding effect. | |
| Number of participants lost to follow‐up | None | |
| Source of funding | not reported | |
| Notes | Location: clinic of Firouzgar Hospital, Tehran, Iran Ethical approval: The ethics committee of Iran University of Medical Sciences studied the ethical aspects of this research and approved it under the code 93/D/105/4431. The ethical principles specified in the Treaty of Helsinki were observed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Used block randomization, but the process for selecting the blocks was not specified "People qualified for the research were randomly divided into randomised blocks containing 4 people in each one." |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Objective outcomes like serum total testosterone, DHEAS and androstenedione are not likely to be influences by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Androgen parameters were measured in a laboratory and the outcome measurements were not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): WDAEs | Unclear risk | Blinding method was not described |
| Selective reporting (reporting bias) | Unclear risk | No protocol was found, and there is insufficient information to judge if it is low or high risk of bias |
| Selective reporting (reporting bias) WDAEs | Low risk | WDAE outcome reported |
| Source of funding, sponsorship and conflict of interest | Unclear risk | Source of funding not reported |
| Incomplete outcome data (attrition bias): Total testosterone | Low risk | All participants were included in the efficacy analysis |
| Incomplete outcome data (attrition bias): Androstenedione | Low risk | All participants were included in the efficacy analysis |
| Incomplete outcome data (attrition bias): DHEAS | Low risk | All participants were included in the efficacy analysis |
Chen 2014.
| Study characteristics | ||
| Methods | Study design: Randomized controlled trial Masking: Open label Number of arms: 2 |
|
| Participants |
Inclusion criteria: subjects diagnosed with mild dyslipidaemia and osteopenia before recruitment; and subjects not treated routinely with lipid‐lowering drugs in the last 3 months before recruitment Exclusion criteria: history of medications for diseases affecting bone metabolism; therapy for osteoporosis with diphosphonate and calcitriol within the last 6 months; other medications including diuretics, testosterones, thyroid hormones, glucocorticoids, or immunosuppressants within the last 1 year; relevant diseases: malignant tumours, severe hepatic or renal dysfunction, hyperthyroidism, and hyperparathyroidism. Baseline characteristics Lifestyle guidance
10 mg/day atorvastatin and lifestyle guidance
|
|
| Interventions | 10 mg/day atorvastatin and lifestyle guidance for 12 months lifestyle guidance for 12 months |
|
| Outcomes | Total testosterone (6‐12 months) | |
| Statistical analysis and reporting | Statistical analyses were conducted using SPSS package for Windows, version 17.0. All data were expressed as means SEM. Comparison of baseline characteristics between atorvastatin and control groups was based on independent‐sample t‐test for continuous variables and chi‐square test for categorical variables. In follow‐up, comparison of parameters between groups was performed at 6 and 12 months using independent‐sample t‐test or ANOVA for repeated measurements with treatment group as the independent variables and changing of parameters from baseline as the dependent variables as a function of time. In addition, Mauchly’s test of sphericity was analyzed to evaluate the assumption of ANOVA for repeated measurements to adjust the degrees of freedom, according to Greenhouse–Geisser correction coefficient e, if necessary. Moreover, comparison of parameters within groups at different time points of follow‐up was performed using one‐way ANOVA followed by least significant differences test (LSD test). After obtaining the statistical significance of primary and secondary endpoints, Pearson correlation analysis of changed parameters was undertaken. All analyses were two‐tailed and P value of <0.05 was considered significant. | |
| Number of participants lost to follow‐up | One subject went abroad to visit relatives and three participants were re‐treated due to other reasons | |
| Source of funding | grants from National Nature Science Foundation of China (81370360) | |
| Notes | Location: Ren‐Ji Hospital, Shanghai Jiao‐Tong University Medical School, China Ethical approval: Ethics Committee of Renji Hospital |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Conducted with complete randomization using SPSS package for Windows, version 17.0 |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Objective outcome like serum total testosterone is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Testosterone was measured in a laboratory and the outcome measurement was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): WDAEs | High risk | No blinding of outcome assessment, and the outcome measurement was likely to be influenced by lack of blinding |
| Selective reporting (reporting bias) | Unclear risk | No protocol was found and there is insufficient information to judge if it is low or high risk of bias |
| Selective reporting (reporting bias) WDAEs | Low risk | WDAE outcome reported |
| Source of funding, sponsorship and conflict of interest | Low risk | Government grants from China |
| Incomplete outcome data (attrition bias): Total testosterone | Low risk | (2/32)*100 = 6.25% were not included in the testosterone measurement for the control group (2/32)*100 = 6.25% were not included in the testosterone measurement for the atorvastatin group missing outcome data balanced in numbers across intervention and control groups with similar reason for missing data across groups |
Gokce 2012.
| Study characteristics | ||
| Methods | Study design: Randomized controlled trial Masking: single‐blind Number of arms: 2 |
|
| Participants |
Inclusion criteria: 30–70‐year‐old male patients, normal libido, IIEF< 17, no previous use of PDE5 Is and normal serum testosterone levels 300 ng/dL Exclusion criteria: history of any pelvic surgery, having any kind of medication for ED, having any neurological or mental problem, liver or hepatic insufficiency, use of nitrates, and use of antiandrogens Baseline characteristics Atorvastatin 10 mg/day
No treatment
|
|
| Interventions | 10 mg/day atorvastatin for 3 months no treatment for 3 months |
|
| Outcomes | Total testosterone (3 months) | |
| Statistical analysis and reporting | Sample size estimation was performed by a conventional statistical program by taking into account an effect size of 50% improvement in symptoms, andminimum number of patients needed to reject the null hypothesis was 120. For randomisation, NCSS program was used, and patients were blinded for the treatment. The statistical analysis was performed by SPSS ver. 15.0. (SPSS Inc.,Chicago, Illinois). Data are expressed as numbers and percentages for discrete variables and as means ± SD for continuous variables. The chi‐square analysis or Fisher’s exact test was used to assess the significance of differences between dichotomous variables. Continuous variables were compared by Student’s t‐test or Mann–Whitney U‐test. | |
| Number of participants lost to follow‐up | Atorvastatin group: one lost to follow up and three discontinued; Of the three that discontinued; one was due to nausea and two was due to lack of efficacy No treatment group: two lost to follow up and four discontinued; Of the four that discontinued; one was due to headache and three was due to lack of efficacy |
|
| Source of funding | not reported | |
| Notes | Location: Department of Urology, Ankara University School of Medicine, Adnan Saygun Caddesi, Altındag˘, Ankara, Turkey Department of Cardiology, Ankara University School of Medicine, Adnan Saygun Caddesi, Altındag˘, Ankara, Turkey |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization, NCSS program was used |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Objective outcome like serum total testosterone is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Testosterone was measured in a laboratory and the outcome measurement was not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): WDAEs | High risk | No blinding of outcome assessment, and the outcome measurement was likely to be influenced by lack of blinding |
| Selective reporting (reporting bias) | Unclear risk | No protocol was found and there is insufficient information to judge if it is low or high risk of bias |
| Selective reporting (reporting bias) WDAEs | Low risk | WDAE outcome reported |
| Source of funding, sponsorship and conflict of interest | Unclear risk | No source of funding reported |
| Incomplete outcome data (attrition bias): Total testosterone | Unclear risk | (4/45)*100 = 8.9% were not included in the testosterone measurement for the atorvastatin group (6/45)*100 = 13.3% were not included in the testosterone measurement for the control group |
Puurunen 2013.
| Study characteristics | ||
| Methods | Study design: randomized, double blind, placebo controlled trial Masking: double‐blind Number of arms: 2 |
|
| Participants |
Inclusion criteria: Caucasian women with PCOS diagnosis, not menopausal, and reliable non hormonal contraception. The diagnoses of PCOS had been assigned to all subjects during their earlier visits to the Department of Obstetrics and Gynecology, the women generally being between the ages of 20 and 30 years, and the researchers confirmed the criteria before the present study. Exclusion criteria: T2DM, medication affecting glucose tolerance, lipid metabolism, or steroid synthesis in the preceding 3 months, menopause, regular smoking, abuse of alcohol, previous ovarian drilling, oophorectomy, or hysterectomy, and contraindications regarding the use of atorvastatin. Baseline characteristics Placebo
20 mg/day atorvastatin
|
|
| Interventions | Placebo for 6 months Atorvastatin 20 mg/day for 6 months |
|
| Outcomes | Total testosterone, SHBG, FAI, DHEAS, Androstenedione | |
| Statistical analysis and reporting | "Androgen secretion and glucose metabolism served as primary outcome measures. Secondary outcome measures were CRP concentrations and lipid profile. Sample size (power analysis) was calculated on the basis of the decrease in serum testosterone levels (baseline, [mean SD], 2.96 0.82 nmol/L; at 12 weeks, 1.76 0.70 nmol/L) during 12 weeks simvastatin treatment in women with PCOS in a study carried out by Duleba et al (13). The analysis revealed that each study group needed to have 15 subjects to have 95% power. A dropout rate of 20% was expected; thus, we needed to recruit 36 subjects. All variables with skewed distribution went through logarithmic transformation before statistical analysis. All statistical analyses were conducted using SPSS software (version 15.0 for Windows; SPSS Inc). The limit of statistical significance was set at P.05. To analyze changes in the levels of serum glucose, insulin, hormones, lipids, andCRPand glucose tolerance indexes within the same study group at 0, 3, and 6 months of treatment, repeated‐measures ANOVA was performed for normally distributed variables and Friedman’s test was used for variables with a skewed distribution. Where ANOVA was significant or if the parameters related to glucose metabolism had only 2 time points, the paired‐samples t test was used for normally distributed variables and Wilcoxon’s nonparametric test was used for variables with a skewed distribution. The independent‐samples t test or the Mann‐Whitney U test was used to analyze differences between the study groups. The effect of baseline testosterone was controlled in repeated‐measures ANOVA using baseline testosterone as a covariate." | |
| Number of participants lost to follow‐up | 10 6 in the placebo group and 4 in the atorvastatin group |
|
| Source of funding | The Academy of Finland, the Sigrid Jusélius Foundation, the Finnish Medical Foundation, the National Clinical Graduate School, the Research Foundation of Obstetrics and Gynecology, Oulu University Scholarship Foundation, the North Ostrobothnia Regional fund of the Finnish Cultural Foundation, the Tyyni Tani Foundation of the University of Oulu, and the Finnish‐Norwegian Medical Foundation. | |
| Notes | Location: University of Oulu and Oulu University Hospital, Finland Ethical approval: Ethics Committee of Oulu University Hospital and the Finnish Medicines Agency |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was conducted using a computer‐generated randomisation list in blocks of 6" |
| Allocation concealment (selection bias) | Low risk | Central allocation (pharmacy‐controlled randomisation and sequentially number drug containers) "carried out at the Oulu University Hospital Pharmacy by personnel not involved in the study". "They also repacked the medication in closed envelopes, which were sequentially numbered". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Objective outcome like serum androgens are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Androgens were measured in a laboratory and the outcome measurements were not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): WDAEs | Unclear risk | Blinding method was not described |
| Selective reporting (reporting bias) | Low risk | Study protocol is available (NCT01072097) and all the study's prespecified outcomes were reported |
| Selective reporting (reporting bias) WDAEs | Low risk | WDAEs were reported |
| Source of funding, sponsorship and conflict of interest | Low risk | Government grants "This work was supported by the Academy of Finland, the Sigrid Jusélius Foundation, the Finnish Medical Foundation, the National Clinical Graduate School, the Research Foundation of Obstetrics and Gynecology, Oulu University Scholarship Foundation, the North Ostrobothnia Regional fund of the Finnish Cultural Foundation, the Tyyni Tani Foundation of the University of Oulu, and the Finnish‐Norwegian Medical Foundation". |
| Incomplete outcome data (attrition bias): Total testosterone | High risk | (19‐15)*100/19 = 21.1% were not included in the analysis for the atorvastatin group (19‐13)*100/19 = 31.6% were not included in the analysis for the placebo group |
| Incomplete outcome data (attrition bias): Free androgen index | High risk | (19‐15)*100/19 = 21.1% were not included in the analysis for the atorvastatin group (19‐13)*100/19 = 31.6% were not included in the analysis for the placebo group |
| Incomplete outcome data (attrition bias): Androstenedione | High risk | (19‐15)*100/19 = 21.1% were not included in the analysis for the atorvastatin group (19‐13)*100/19 = 31.6% were not included in the analysis for the placebo group |
| Incomplete outcome data (attrition bias): SHBG | High risk | (19‐15)*100/19 = 21.1% were not included in the analysis for the atorvastatin group (19‐13)*100/19 = 31.6% were not included in the analysis for the placebo group |
| Incomplete outcome data (attrition bias): DHEAS | High risk | (19‐15)*100/19 = 21.1% were not included in the analysis for the atorvastatin group (19‐13)*100/19 = 31.6% were not included in the analysis for the placebo group |
Raja‐Khan 2011.
| Study characteristics | ||
| Methods | Study design: randomized, double‐blind, placebo controlled Masking: double‐blind Number of arms: 2 |
|
| Participants |
Inclusion criteria: Women with PCOS and LDL‐cholesterol > 100 mg/dL were eligible to participate in the study. Exclusion criteria: not reported Baseline characteristics Placebo
40 mg/day atorvastatin
|
|
| Interventions | Placebo for 6 weeks Atorvastatin 40 mg/day for 6 weeks |
|
| Outcomes | Total testosterone, Free testosterone, Androstenedione and DHEAS | |
| Statistical analysis and reporting | Linear mixed‐effects models, extensions of regression that account for within‐subject correlation inherent in pre‐post designs, were fit to continuous outcomes to assess the change from baseline to 6weeks within and between treatment groups. All hypotheses tests were two‐sided. All analyses were performed by intention‐to‐treat using SAS software, version 9.1 (SAS Institute Inc., Cary, NC) | |
| Number of participants lost to follow‐up | 2 | |
| Source of funding | National Institutes of Health (NIH) grant number K12HD055882, GCRC grant M01 RR10732 and construction grant C06 RR016499 to Pennsylvania State University and a research grant from Pfizer | |
| Notes | Location: Department of Medicine, Pennsylvania State University College of Medicine, Hershey, Pennsylvania Ethical approval: The institutional review board of Pennsylvania State University approved the study. Written informed consent was obtained from all participants. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The biostatistician generated a permuted block randomisation scheme" |
| Allocation concealment (selection bias) | Low risk | "The biostatistician generated a permuted block randomisation scheme for the allocation sequence and provided it to the pharmacist" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The atorvastatin and placebo were over encapsulated by the pharmacist so that the participants, research coordinator who administered the intervention, and investigators who assessed the outcomes were blinded to group assignment and objective outcome like serum androgens are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Androgens were measured in a laboratory and the outcome measurements were not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): WDAEs | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | Study protocol is available (NCT00529542) and all the study's prespecified outcomes were reported |
| Selective reporting (reporting bias) WDAEs | High risk | Not reported |
| Source of funding, sponsorship and conflict of interest | High risk | National Institutes of Health (NIH) grant number K12HD055882, GCRC grant M01 RR10732 and construction grant C06 RR016499 to Pennsylvania State University and a research grant from Pfizer N.R‐K. has nothing to disclose. A.R.K. reports ownership of Merck stock. C.S.H. has nothing to disclose. C.M.S. has nothing to disclose. L.M.D. has nothing to disclose. R.S.L. has received speaker honorarium from Merck‐Serono and study support from Solvay |
| Incomplete outcome data (attrition bias): Total testosterone | Unclear risk | (20‐18)*100/20 = 10% did not complete the trial but the authors included all the data of all participants in the analysis |
| Incomplete outcome data (attrition bias): Free testosterone | High risk | Only baseline free testosterone was reported in each group |
| Incomplete outcome data (attrition bias): Androstenedione | Unclear risk | (20‐18)*100/20 = 10% did not complete the trial but the authors included all the data of all participants in the analysis |
| Incomplete outcome data (attrition bias): DHEAS | Unclear risk | (20‐18)*100/20 = 10% did not complete the trial but the authors included all the data of all participants in the analysis |
Sathyapalan 2009.
| Study characteristics | ||
| Methods | Study design: randomized placebo controlled Masking: double‐blind Number of arms: 2 |
|
| Participants |
Inclusion criteria: female participants with PCOS and biochemical hyperandrogaenemia Subjects had no concurrent illness, were not on any prescription or over‐the‐counter medication that was likely to affect
insulin sensitivity, lipids, or ovarian function including hormonal contraceptives for the preceding 6 months. None of the patients had statin therapy in the past. Subjects were not planning to conceive and were using barrier contraception. Exclusion criteria: Nonclassical 21‐hydroxylase deficiency, hyperprolactinaemia, Cushing’s disease, and androgen‐secreting tumours Baseline characteristics Placebo
40 mg/day atorvastatin
|
|
| Interventions | Placebo for 12 weeks Atorvastatin 40 mg/day for 12 weeks |
|
| Outcomes | Total testosterone, FAI, SHBG | |
| Statistical analysis and reporting | The sample size was based on the study on the known effect of atorvastatin on hsCRP in patients with impaired fasting glucose with the assumption that a similar effect would occur in those patients with PCOS. Powered specifically for CRP, the minimum difference worth detecting/observed difference was 32.7%, estimated within‐group SD was 11.1; therefore, for 90% power and a significance level of 5%, a sample size of 16 per group was calculated. Adjusting for a possible 20% dropout rate meant a total of 40 patients needed to be recruited. Comparisons between both the groups from baseline were carried out using the paired t test for biochemical data and clinical observations. The Wilcoxon signed rank test was applied to biochemical data that violated the assumptions of normality when tested using the Kolmogorov‐Smirnov test. The effect of treatment was evaluated by first calculating the percentage change from baseline for all variables studied and then the percentage change for each variable in each patient group, thus negating the differences in the baseline values of the two groups. Between‐group comparison of percent changes was performed using independent‐samples t test. For all analyses, a two‐tailed P 0.05 was considered to indicate statistical significance. Statistical analysis was performed using SPSS for Windows NT, version 14.0 (SPSS Inc., Chicago, IL). | |
| Number of participants lost to follow‐up | 3 in the atorvastatin group and 2 in the placebo group | |
| Source of funding | Unrestricted grant from Pfizer | |
| Notes | Location: Department of Diabetes and Endocrinology , University of Hull, Hull HU6 7RX, United Kingdom;
Department of Clinical Biochemistry , Hull Royal Infirmary, Hull HU3 2JZ, United Kingdom; and Department of
Obstetric Ultrasound, Hull and East Yorkshire Women’s and Children’s Hospital, Hull HU3 2PZ, United Kingdom Ethical approval: All patients gave informed consent. The study was approved by the Hull and East Riding Local Research Ethics committee |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list |
| Allocation concealment (selection bias) | Low risk | Labelling was done by personnel not involved in the trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind objective outcome like serum androgens are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Androgens were measured in a laboratory and the outcome measurements were not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias): WDAEs | Unclear risk | Blinding method was not described |
| Selective reporting (reporting bias) | High risk | One reported primary outcome (hs‐CRP) was not prespecified in the ISRCTN24474824 registry |
| Selective reporting (reporting bias) WDAEs | High risk | Atorvastatin 1 out of 20 for non‐compliance Placebo 2 out of 20 for non‐compliance non‐compliance may be due to WDAEs |
| Source of funding, sponsorship and conflict of interest | High risk | Unrestricted grant from Pfizer sponsors had no input into study design, its execution, or interpretation of the findings All of the authors have got nothing else to disclose but in 2017 they claimed that the research did not receive any specific grant from any funding agency in the public, commercial or not‐for‐profit sector |
| Incomplete outcome data (attrition bias): Total testosterone | Low risk | Atorvastatin group: (20‐19)*100/20 = 5% were not included in the analysis Placebo group: (20‐18)*100/20 = 10% were not included in the analysis Both groups: (40‐37)*100/40 = 7.5% were not included in the analysis |
| Incomplete outcome data (attrition bias): Free androgen index | Low risk | Atorvastatin group: (20‐19)*100/20 = 5% were not included in the analysis Placebo group: (20‐18)*100/20 = 10% were not included in the analysis Both groups: (40‐37)*100/40 = 7.5% were not included in the analysis |
| Incomplete outcome data (attrition bias): Androstenedione | Low risk | Atorvastatin group: (20‐19)*100/20 = 5% were not included in the analysis Placebo group: (20‐18)*100/20 = 10% were not included in the analysis Both groups: (40‐37)*100/40 = 7.5% were not included in the analysis |
| Incomplete outcome data (attrition bias): SHBG | Low risk | Atorvastatin group: (20‐19)*100/20 = 5% were not included in the analysis Placebo group: (20‐18)*100/20 = 10% were not included in the analysis Both groups: (40‐37)*100/40 = 7.5% were not included in the analysis |
| Incomplete outcome data (attrition bias): DHEAS | Low risk | Atorvastatin group: (20‐19)*100/20 = 5% were not included in the analysis Placebo group: (20‐18)*100/20 = 10% were not included in the analysis Both groups: (40‐37)*100/40 = 7.5% were not included in the analysis |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Almroth 2009 | Outcomes specified for this review were not reported or measured |
| Amarenco 1999 | Outcomes specified for this review were not reported or measured |
| Amarenco 2006 | Outcomes specified for this review were not reported or measured |
| Amarenco 2007a | Outcomes specified for this review were not reported or measured |
| Amarenco 2007b | Outcomes specified for this review were not reported or measured |
| Amarenco 2009a | Outcomes specified for this review were not reported or measured |
| Amarenco 2009b | Outcomes specified for this review were not reported or measured |
| Amarenco 2009c | Outcomes specified for this review were not reported or measured |
| Amarenco 2010 | Outcomes specified for this review were not reported or measured |
| Amarenco 2014 | Outcomes specified for this review were not reported or measured |
| Amudha 2008 | Outcomes specified for this review were not reported or measured |
| Anonymous 1998 | Outcomes specified for this review were not reported or measured |
| Bakker‐Arkema 1996 | Outcomes specified for this review were not reported or measured |
| Baspinar 2016 | Wrong study design; Not randomized |
| Berthold 2004 | Outcomes specified for this review were not reported or measured |
| Bleda 2012 | Outcomes specified for this review were not reported or measured |
| Bloomfield 2009 | Outcomes specified for this review were not reported or measured |
| Braamskamp 2015 | Wrong study design (non‐randomized) |
| Cai 2014 | Wrong study design (systematic review) |
| Chan 2002a | Outcomes specified for this review were not reported or measured |
| Chan 2002b | Outcomes specified for this review were not reported or measured |
| Chan 2002c | Outcomes specified for this review were not reported or measured |
| Chan 2006 | Outcomes specified for this review were not reported or measured |
| Chapman 2011 | Outcomes specified for this review were not reported or measured |
| Clough 2009 | Outcomes specified for this review were not reported or measured |
| Cohn 2009 | Outcomes specified for this review were not reported or measured |
| Colhoun 2004 | Outcomes specified for this review were not reported or measured |
| Crisostomo 2008 | Outcomes specified for this review were not reported or measured |
| Cubeddu 2006 | Outcomes specified for this review were not reported or measured |
| Cui 2006 | Outcomes specified for this review were not reported or measured |
| Dallinga‐Thie 2006a | Outcomes specified for this review were not reported or measured |
| Dallinga‐Thie 2006b | Outcomes specified for this review were not reported or measured |
| Davidson 2002 | Outcomes specified for this review were not reported or measured |
| Deanfield 2010 | Outcomes specified for this review were not reported or measured |
| Diabetes 2001 | Outcomes specified for this review were not reported or measured |
| Diepeveen 2005 | Outcomes specified for this review were not reported or measured |
| Dogra 2007 | Outcomes specified for this review were not reported or measured |
| Economides 2004 | Outcomes specified for this review were not reported or measured |
| Elkawy 2014 | Testosterone level was measured but not reported |
| EUCTR2004‐000139‐27‐SE | Outcomes specified for this review were not reported or measured |
| Faludi 2004 | Outcomes specified for this review were not reported or measured |
| Ferrier 2002a | Outcomes specified for this review were not reported or measured |
| Ferrier 2002b | Outcomes specified for this review were not reported or measured |
| Fogari 2004 | Outcomes specified for this review were not reported or measured |
| Fogari 2006 | Outcomes specified for this review were not reported or measured |
| Freed 2002 | Outcomes specified for this review were not reported or measured |
| Ge 2008 | Outcomes specified for this review were not reported or measured |
| Gentile 2000 | Outcomes specified for this review were not reported or measured |
| GlaxoSmithKline 2000 | Outcomes specified for this review were not reported or measured |
| Glinkina 2008 | Outcomes specified for this review were not reported or measured |
| Goldstein 2008 | Outcomes specified for this review were not reported or measured |
| Goldstein 2009 | Outcomes specified for this review were not reported or measured |
| Grimm 2010 | Outcomes specified for this review were not reported or measured |
| Guo 2012 | Outcomes specified for this review were not reported or measured |
| Hernandez 2010 | Outcomes specified for this review were not reported or measured |
| Holman 2009 | Outcomes specified for this review were not reported or measured |
| Holmberg 2005 | Outcomes specified for this review were not reported or measured |
| Horwich 2011 | Outcomes specified for this review were not reported or measured |
| Hunninghake 2001 | Outcomes specified for this review were not reported or measured |
| Huptas 2006 | Outcomes specified for this review were not reported or measured |
| Iavenko 2014 | Outcomes specified for this review were not reported or measured |
| Inukai 2011 | Outcomes specified for this review were not reported or measured |
| IRCT201208299626N1 | Wrong comparator |
| Ishola 2017 | Wrong study design (animal study) |
| Issa 2012 | Outcomes specified for this review were not reported or measured |
| Jayaram 2007 | Outcomes specified for this review were not reported or measured |
| J‐CLAS 1997 | Outcomes specified for this review were not reported or measured |
| Joy 2008 | Outcomes specified for this review were not reported or measured |
| JPRN‐UMIN000001114 | Outcomes specified for this review were not reported or measured |
| Kadoglou 2011 | Outcomes specified for this review were not reported or measured |
| Kajimoto 2009 | Outcomes specified for this review were not reported or measured |
| Kanadasi 2006 | Outcomes specified for this review were not reported or measured |
| Kappelle 2009 | Outcomes specified for this review were not reported or measured |
| Kappelle 2010 | Outcomes specified for this review were not reported or measured |
| Karam 2008 | Outcomes specified for this review were not reported or measured |
| Kishi 2009 | Outcomes specified for this review were not reported or measured |
| Kitas 2019 | Outcomes specified for this review were not reported or measured |
| Knopp 2006 | Outcomes specified for this review were not reported or measured |
| Koh 2005 | Outcomes specified for this review were not reported or measured |
| Koh 2010 | Outcomes specified for this review were not reported or measured |
| Kom 2007 | Outcomes specified for this review were not reported or measured |
| Konduracka 2008 | Outcomes specified for this review were not reported or measured |
| Krysiak 2010 | Outcomes specified for this review were not reported or measured |
| Krysiak 2014 | Wrong comparator; absence of controls |
| Krysiak 2016 | Wrong study design (non‐randomized) |
| Kubler 2003 | Outcomes specified for this review were not reported or measured |
| Kuznetsova 2010 | wrong duration (14 days) |
| Lamon‐Fava 2007 | Outcomes specified for this review were not reported or measured |
| Lavallee 2009 | Outcomes specified for this review were not reported or measured |
| Lawrence 2004 | Outcomes specified for this review were not reported or measured |
| Lewandowski 2008 | Outcomes specified for this review were not reported or measured |
| Li 2006 | Outcomes specified for this review were not reported or measured |
| Li 2010 | Outcomes specified for this review were not reported or measured |
| Lins 2004 | Outcomes specified for this review were not reported or measured |
| Liu 2009 | Outcomes specified for this review were not reported or measured |
| Loughrey 2013 | Outcomes specified for this review were not reported or measured |
| Macchia 2012 | Outcomes specified for this review were not reported or measured |
| Macin 2005 | Outcomes specified for this review were not reported or measured |
| Magen 2004 | Outcomes specified for this review were not reported or measured |
| Maggioni 2018 | Outcomes specified for this review were not reported or measured |
| Mal'gina 2007 | Outcomes specified for this review were not reported or measured |
| Malekzadeh 2010 | Outcomes specified for this review were not reported or measured |
| Manuel 2003 | Outcomes specified for this review were not reported or measured |
| Marais 1997 | Outcomes specified for this review were not reported or measured |
| Marchesi 2000 | Outcomes specified for this review were not reported or measured |
| Martin 2011 | Outcomes specified for this review were not reported or measured |
| Martin‐Ventura 2005 | Outcomes specified for this review were not reported or measured |
| McCrindle 2003 | Outcomes specified for this review were not reported or measured |
| Mehta 2007 | Outcomes specified for this review were not reported or measured |
| Meng 2009 | Outcomes specified for this review were not reported or measured |
| Messerli 2006 | Outcomes specified for this review were not reported or measured |
| Mills 2007 | Outcomes specified for this review were not reported or measured |
| Mishra 2005 | Outcomes specified for this review were not reported or measured |
| Moga 2005 | Outcomes specified for this review were not reported or measured |
| Mohler 2003 | Outcomes specified for this review were not reported or measured |
| Moini 2012 | Outcomes specified for this review were not reported or measured |
| Monteiro 2008 | Outcomes specified for this review were not reported or measured |
| Muscari 2001 | Outcomes specified for this review were not reported or measured |
| Nakamura 1997 | Outcomes specified for this review were not reported or measured |
| Nakamura 2007 | Outcomes specified for this review were not reported or measured |
| Naoumova 1997 | Outcomes specified for this review were not reported or measured |
| Naoumova 1999 | Outcomes specified for this review were not reported or measured |
| Naoumova 2003 | Outcomes specified for this review were not reported or measured |
| Nawawi 2003 | Outcomes specified for this review were not reported or measured |
| Nawrocki 1995 | Outcomes specified for this review were not reported or measured |
| NCT00433823 | Wrong comparator; no placebo; |
| NCT00522158 | Wrong comparator; no placebo |
| NCT00603590 | Outcomes specified for this review were not reported or measured |
| NCT01555632 | Wrong setting; Study was withdrawn before participants were enrolled |
| NCT02497638 | Wrong setting; Not started yet |
| NCT03784703 | Outcomes specified for this review were not reported or measured |
| NCT04101136 | Outcomes specified for this review were not reported or measured |
| Negi 2011 | Outcomes specified for this review were not reported or measured |
| Neil 2010 | Outcomes specified for this review were not reported or measured |
| Neutel 2009 | Outcomes specified for this review were not reported or measured |
| Nicholls 2011 | Outcomes specified for this review were not reported or measured |
| Nikitina 2009 | Outcomes specified for this review were not reported or measured |
| Nissen 2007 | Outcomes specified for this review were not reported or measured |
| O'Neill 2001 | Outcomes specified for this review were not reported or measured |
| Okazaki 2004 | Outcomes specified for this review were not reported or measured |
| Okopien 2004 | Outcomes specified for this review were not reported or measured |
| Okopien 2005 | Outcomes specified for this review were not reported or measured |
| Olivotti 2002 | Outcomes specified for this review were not reported or measured |
| Olsson 2001 | Outcomes specified for this review were not reported or measured |
| Olsson 2005 | Outcomes specified for this review were not reported or measured |
| Olsson 2007 | Outcomes specified for this review were not reported or measured |
| Ooi 2012 | Outcomes specified for this review were not reported or measured |
| Oranje 2001 | Outcomes specified for this review were not reported or measured |
| Ormiston 2004 | Placebo data not reported separate from the active treatment data |
| Orr 2009 | Outcomes specified for this review were not reported or measured |
| Oshima 2008 | Outcomes specified for this review were not reported or measured |
| Ozkiris 2007 | Outcomes specified for this review were not reported or measured |
| Packard 2007 | Outcomes specified for this review were not reported or measured |
| Paiva 2003 | Outcomes specified for this review were not reported or measured |
| Paiva 2005 | Outcomes specified for this review were not reported or measured |
| Panahi 2011 | Outcomes specified for this review were not reported or measured |
| Paolisso 2000 | Outcomes specified for this review were not reported or measured |
| Papathanasiou 2008 | Outcomes specified for this review were not reported or measured |
| Parini 2008 | Outcomes specified for this review were not reported or measured |
| Parker 2011 | Outcomes specified for this review were not reported or measured |
| Parker 2013 | Outcomes specified for this review were not reported or measured |
| Pedro‐Botet 2001 | Outcomes specified for this review were not reported or measured |
| Peng 2018 | Outcomes specified for this review were not reported or measured |
| Petri 2011 | Outcomes specified for this review were not reported or measured |
| Pfiser 2003 | Outcomes specified for this review were not reported or measured |
| Pfizer 2003a | Outcomes specified for this review were not reported or measured |
| Pfizer 2003b | Outcomes specified for this review were not reported or measured |
| Pfizer 2003c | Outcomes specified for this review were not reported or measured |
| Pfizer 2004a | Outcomes specified for this review were not reported or measured |
| Pfizer 2004b | Outcomes specified for this review were not reported or measured |
| Pfizer 2004c | Outcomes specified for this review were not reported or measured |
| Pfizer 2005 | Outcomes specified for this review were not reported or measured |
| Pfizer 2006a | Outcomes specified for this review were not reported or measured |
| Pfizer 2006b | Outcomes specified for this review were not reported or measured |
| Pfizer 2007 | Outcomes specified for this review were not reported or measured |
| Pfizer 2009 | Outcomes specified for this review were not reported or measured |
| Pfizer 2010 | Outcomes specified for this review were not reported or measured |
| Piperi 2004 | Outcomes specified for this review were not reported or measured |
| Plazak 2011 | Outcomes specified for this review were not reported or measured |
| Pons‐Rejraji 2014 | Wrong comparator; No control |
| Pontrelli 2002 | Outcomes specified for this review were not reported or measured |
| Preston 2007 | Outcomes specified for this review were not reported or measured |
| Raal 2000 | Outcomes specified for this review were not reported or measured |
| Raal 2003 | Wrong study design (3‐SEQUENCE CROSSOVER) |
| Raison 2002 | Outcomes specified for this review were not reported or measured |
| Reiter 2005 | Outcomes specified for this review were not reported or measured |
| Renders 2001 | Outcomes specified for this review were not reported or measured |
| Riahi 2006 | Outcomes specified for this review were not reported or measured |
| Rosenson 2009 | Outcomes specified for this review were not reported or measured |
| Sadik 2010 | Outcomes specified for this review were not reported or measured |
| Samentzas 2015 | Wrong study design |
| Samy 2011 | Outcomes specified for this review were not reported or measured |
| Sardo 2002 | Outcomes specified for this review were not reported or measured |
| Sardo 2005 | Outcomes specified for this review were not reported or measured |
| Sasmazel 2010 | Outcomes specified for this review were not reported or measured |
| Sathyapalan 2019 | Outcomes specified for this review were not reported or measured |
| Schaefer 2002 | Outcomes specified for this review were not reported or measured |
| Schaefer 2004 | Outcomes specified for this review were not reported or measured |
| Schaefer 2005 | Outcomes specified for this review were not reported or measured |
| Schanberg 2012 | Outcomes specified for this review were not reported or measured |
| Schneider 2004 | Outcomes specified for this review were not reported or measured |
| Schrott 1998 | Outcomes specified for this review were not reported or measured |
| Sdringola 2008 | Outcomes specified for this review were not reported or measured |
| See 2003 | Outcomes specified for this review were not reported or measured |
| Sever 2003 | Outcomes specified for this review were not reported or measured |
| Sever 2005 | Outcomes specified for this review were not reported or measured |
| Sever 2010 | Outcomes specified for this review were not reported or measured |
| Sharma 2001 | Outcomes specified for this review were not reported or measured |
| Sillesen 2007a | Outcomes specified for this review were not reported or measured |
| Sillesen 2007b | Outcomes specified for this review were not reported or measured |
| Sillesen 2008 | Outcomes specified for this review were not reported or measured |
| Singh 2008 | Outcomes specified for this review were not reported or measured |
| Sinski 2009 | Outcomes specified for this review were not reported or measured |
| Soedamah‐Muthu 2003 | Outcomes specified for this review were not reported or measured |
| Soedamah‐Muthu 2015 | Outcomes specified for this review were not reported or measured |
| Sola 2006 | Outcomes specified for this review were not reported or measured |
| Solem 2006 | Outcomes specified for this review were not reported or measured |
| Sparks 2005 | Outcomes specified for this review were not reported or measured |
| Sposito 2003 | Outcomes specified for this review were not reported or measured |
| Stalenhoef 2005 | Outcomes specified for this review were not reported or measured |
| Stefanadi 2009 | Outcomes specified for this review were not reported or measured |
| Stein 2012 | Outcomes specified for this review were not reported or measured |
| Strey 2005 | Outcomes specified for this review were not reported or measured |
| Strey 2006 | Outcomes specified for this review were not reported or measured |
| Suleiman 2012 | Outcomes specified for this review were not reported or measured |
| Szramka 2007 | Outcomes specified for this review were not reported or measured |
| Tan 2002 | Outcomes specified for this review were not reported or measured |
| Tanaka 2001 | Outcomes specified for this review were not reported or measured |
| Tanaka 2009 | Outcomes specified for this review were not reported or measured |
| Taneva 2006 | Outcomes specified for this review were not reported or measured |
| Tannous 1999 | Outcomes specified for this review were not reported or measured |
| Tanriverdi 2005 | Outcomes specified for this review were not reported or measured |
| Tantikosoom 2005 | Outcomes specified for this review were not reported or measured |
| Tehrani 2010 | Outcomes specified for this review were not reported or measured |
| Teshima 2009 | Outcomes specified for this review were not reported or measured |
| Tousoulis 2005a | Outcomes specified for this review were not reported or measured |
| Tousoulis 2005b | Outcomes specified for this review were not reported or measured |
| Tousoulis 2005c | Outcomes specified for this review were not reported or measured |
| Tousoulis 2006a | Outcomes specified for this review were not reported or measured |
| Tousoulis 2006b | Outcomes specified for this review were not reported or measured |
| Tousoulis 2007 | Outcomes specified for this review were not reported or measured |
| Tousoulis 2010 | Outcomes specified for this review were not reported or measured |
| Tousoulis 2011 | Outcomes specified for this review were not reported or measured |
| Tremblay 2011 | Outcomes specified for this review were not reported or measured |
| Tsai 2008 | Outcomes specified for this review were not reported or measured |
| Urso 2005 | Outcomes specified for this review were not reported or measured |
| van de Ree 2003 | Outcomes specified for this review were not reported or measured |
| Van De Ree 2003 | Outcomes specified for this review were not reported or measured |
| van der Harst 2005 | Outcomes specified for this review were not reported or measured |
| van Doorn 2006 | Outcomes specified for this review were not reported or measured |
| van Hoek 2009 | Outcomes specified for this review were not reported or measured |
| Van J 2002 | Outcomes specified for this review were not reported or measured |
| Vansant 2001 | Outcomes specified for this review were not reported or measured |
| van Venrooij 2002 | Outcomes specified for this review were not reported or measured |
| van Venrooij 2003 | Outcomes specified for this review were not reported or measured |
| Vernaglione 2004 | Outcomes specified for this review were not reported or measured |
| von Eynatten 2009 | Outcomes specified for this review were not reported or measured |
| Vrtovec 2005 | Outcomes specified for this review were not reported or measured |
| Wang 2001 | Outcomes specified for this review were not reported or measured |
| Wanner 2005 | Outcomes specified for this review were not reported or measured |
| Wassmann 2004 | Outcomes specified for this review were not reported or measured |
| Waters 2000 | Outcomes specified for this review were not reported or measured |
| Watts 2003a | Outcomes specified for this review were not reported or measured |
| Watts 2003b | Outcomes specified for this review were not reported or measured |
| Williams 2009 | Outcomes specified for this review were not reported or measured |
| Wissing 2006 | Outcomes specified for this review were not reported or measured |
| Wojnicz 2006 | Outcomes specified for this review were not reported or measured |
| Wu 2007 | Outcomes specified for this review were not reported or measured |
| Xia 2009 | Outcomes specified for this review were not reported or measured |
| Xie 2010 | Outcomes specified for this review were not reported or measured |
| Yamada 2007 | Outcomes specified for this review were not reported or measured |
| Yamada 2009 | Outcomes specified for this review were not reported or measured |
| Yokoyama 2005 | Outcomes specified for this review were not reported or measured |
| Young 2008 | Outcomes specified for this review were not reported or measured |
| Zeng 2012 | Outcomes specified for this review were not reported or measured |
| Zhang 2009a | Outcomes specified for this review were not reported or measured |
| Zhang 2009b | Outcomes specified for this review were not reported or measured |
| Zheng 2019 | Outcomes specified for this review were not reported or measured |
Differences between protocol and review
According to the published protocol, we intended to analyze only four secondary outcomes: free testosterone levels, FAI, SHBG and WDAEs. Because of the clinical importance of DHEAS and androstenedione in conditions such as POCS, we decided to add them as secondary outcomes in the review.
Contributions of authors
James M Wright (JMW) formulated the idea, developed the basis of the protocol, and contributed to data analysis, interpretation of the final result, and editing of the final draft of the review.
Muhammad Ismail Shawish (MIS) and Bahador Bagheri (BB) drafted the protocol, with help from JMW.
MIS, BB and SPA independently assessed studies for inclusion or exclusion and assessed the risk of bias of all included studies.
MIS extracted data, checked data entry, conducted data analysis, interpreted study results, and drafted the final review.
Vijaya Musini (VM) contributed to data analysis, interpretation of the final result, and editing of the final draft of the review.
All review authors reviewed and approved the final version.
Sources of support
Internal sources
-
Department of Anesthesiology, Pharmacology & Therapeutics, University of BC, Canada
infrastructure
External sources
-
British Columbia Ministry of Health, Canada
Therapeutics Initiative grant
Declarations of interest
Muhammad Ismail Shawish: nothing to declare.
Bahador Bagheri: nothing to declare.
Vijaya Musini: nothing to declare.
Stephen Adams: nothing to declare.
James Wright: nothing to declare.
New
References
References to studies included in this review
Akbari 2016 {published data only}
- Akbari M, Almasi A, Naderi Z, Kouhpayezadeh J, Pourali R, Hossinzadeh Z. The effect of atorvastatin on the ovarian arterial blood flow and serum androgen level in PCOS patient. Biomedical and Pharmacology Journal 2016;9(3):1041-1048. [DOI: 10.13005/bpj/1046] [DOI] [Google Scholar]
Chen 2014 {published data only}
- Chen ZG, Cai HJ, Jin X, Lu JH, Wang J, Fang NY. Effects of atorvastatin on bone mineral density (BMD) and bone metabolism in elderly males with osteopenia and mild dyslipidemia: A 1-year randomized trial. Archives of Gerontology & Geriatrics 2014;59(3):515-21. [DOI: 10.1016/j.archger.2014.07.006] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gokce 2012 {published data only}
- Gokce MI, Gulpinar O, Ozturk E, Gulec S, Yaman O. Effect of atorvastatin on erectile functions in comparison with regular tadalafil use. A prospective single-blind study. International Urology & Nephrology 2012;44(3):683-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Puurunen 2013 {published data only}
- Puurunen J, Piltonen T, Puukka K, Ruokonen A, Savolainen MJ, Bloigu R, et al. Statin therapy worsens insulin sensitivity in women with polycystic ovary syndrome (PCOS): a prospective, randomized, double-blind, placebo-controlled study. Journal of Clinical Endocrinology & Metabolism 2013;98(12):4798-807. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Raja‐Khan 2011 {published data only}
- *.Raja-Khan N, Kunselman AR, Hogeman CS, Stetter CM, Demers LM, Legro RS. Effects of atorvastatin on vascular function, inflammation, and androgens in women with polycystic ovary syndrome: a double-blind, randomized, placebo-controlled trial. Fertility & Sterility 2011;95(5):1849-52. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sathyapalan 2009 {published data only}
- Sathyapalan T, Coady AM, Kilpatrick ES, Atkin SL. The effect of atorvastatin on pancreatic beta cell requirement in women with polycystic ovary syndrome. Endocrine Connections 2017;6(8):811-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Sathyapalan T, Kilpatrick ES, Coady AM, Atkin SL. The effect of atorvastatin in patients with polycystic ovary syndrome: a randomized double-blind placebo-controlled study. Journal of Clinical Endocrinology & Metabolism 2009;94(1):103-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sathyapalan T, Smith KA, Coady AM, Kilpatrick ES, Atkin SL. Atorvastatin therapy decreases androstenedione and dehydroepiandrosterone sulphate concentrations in patients with polycystic ovary syndrome: randomized controlled study. Annals of Clinical Biochemistry 2012;49(Pt 1):80-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Almroth 2009 {published data only}
- Almroth H, Hoglund N, Boman K, Englund A, Jensen S, Kjellman B, et al. Atorvastatin and persistent atrial fibrillation following cardioversion: a randomized placebo-controlled multicentre study. European Heart Journal 2009;30(7):827-33. [DOI] [PubMed] [Google Scholar]
Amarenco 1999 {published data only}
- Amarenco P, Bogousslavsky J, Callahan A, Goldstein L, Hennerici M, Sillesen H, et al. Effect of atorvastatin compared with placebo on cerebrovascular end points in patients with previous stroke or transient ischemic attack - The SPARCL Study. Cerebrovascular Diseases 1999;9(Suppl 1):108. [MEDLINE: ] [Google Scholar]
Amarenco 2006 {published data only}
- Amarenco P, Bogousslavsky J, Callahan Iii A, Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transient ischemic attack. New England Journal of Medicine 2006;355(6):549-59. [DOI] [PubMed] [Google Scholar]
Amarenco 2007a {published data only}
- Amarenco P, Goldstein LB, Szarek M, Sillesen H, Rudolph AE, Callahan A, et al. Effects of intense low-density lipoprotein cholesterol reduction in patients with stroke or transient ischemic attack: the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke 2007;38(12):3198-204. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Amarenco 2007b {published data only}
- Amarenco P, Lavallee PC, Mazighi M, Labreuche J. The role of statins in the prevention of stroke. Archives of Medical Science 2007;3(4 SUPPL. A):S109-S114. [EMBASE: 2008090028] [Google Scholar]
Amarenco 2009a {published data only}
- Amarenco P, Goldstein LB, Callahan 3rd A, Sillesen H, Hennerici MG, O'Neill BJ, et al. Baseline blood pressure, low- and high-density lipoproteins, and triglycerides and the risk of vascular events in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Atherosclerosis 2009;204(2):515-20. [DOI] [PubMed] [Google Scholar]
Amarenco 2009b {published data only}
- Amarenco P, Benavente O, Goldstein LB, Callahan A, Sillesen H, Hennerici MG, et al. Results of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial by stroke subtypes. Stroke 2009;40(4):1405-1409. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Amarenco 2009c {published data only}
- Amarenco P, Goldstein LB, Messig M, O'Neill BJ, Callahan A, Sillesen H, et al. Relative and cumulative effects of lipid and blood pressure control in the stroke prevention by aggressive reduction in cholesterol levels trial. Neurology EMT - stroke prevention blood pressure regulation neurology risk patient density ischemic heart disease transient ischemic attack hazard ratio cholesterol lipid high density lipoprotein low density lipoprotein triacylglycerol placebo atorvastat 2009;73(4):331. [Google Scholar]
Amarenco 2010 {published data only}
- Amarenco P, Goldstein LB, Sillesen H, Benavente O, Zweifler RM, Callahan A, et al. Coronary heart disease risk in patients with stroke or transient ischemic attack and no known coronary heart disease: findings from the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke; a Journal of Cerebral Circulation 2010;41(3):426-30. [DOI] [PubMed] [Google Scholar]
Amarenco 2014 {published data only}
- Amarenco P, Callahan A 3rd, Campese VM, Goldstein LB, Hennerici MG, Messig M et al. Effect of high-dose atorvastatin on renal function in subjects with stroke or transient ischemic attack in the SPARCL trial. Stroke 2014;45(10):2974-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Amudha 2008 {published data only}
- Amudha K, Choy AM, Mustafa MR, Lang CC. Short-term effect of atorvastatin on endothelial function in healthy offspring of parents with type 2 diabetes mellitus. Cardiovascular Therapeutics 2008;26(4):253-61. [DOI] [PubMed] [Google Scholar]
Anonymous 1998 {published data only}
- Anonymous. Lipid lowering - New study with atorvastatin [Lipidsenker - Neue studie mit atorvastatin]. Deutsche Apotheker Zeitung 1998;138(29):30-1. [EMBASE: 1998252217] [Google Scholar]
Bakker‐Arkema 1996 {published data only}
- Bakker-Arkema RG, Davidson MH, Goldstein RJ, Davignon J, Isaacsohn JL, Weiss SR, et al. Efficacy and safety of a new HMG-CoA reductase inhibitor, atorvastatin, in patients with hypertriglyceridemia. JAMA 1996;275(2):128-33. [PubMed] [Google Scholar]
Baspinar 2016 {published data only}
- Baspinar O, Bayram F, Korkmaz S, Aksu M, Kocer D, Dizdar OS, et al. The effects of statin treatment on adrenal and sexual function and nitric oxide levels in hypercholesterolemic male patients treated with a statin. Journal of Clinical Lipidology 2016;10(6):1452-61. [DOI] [PubMed] [Google Scholar]
Berthold 2004 {published data only}
- Berthold HK, Unverdorben S, Zittermann A, Degenhardt R, Baumeister B, Unverdorben M, et al. Age-dependent effects of atorvastatin on biochemical bone turnover markers: a randomized controlled trial in postmenopausal women. Osteoporosis International 2004;15(6):459-67. [DOI] [PubMed] [Google Scholar]
Bleda 2012 {published data only}
- Bleda S, De Haro J, Varela C, Esparza L, Rodriguez J, Acin F. Improving total-cholesterol/HDL-cholesterol ratio results in an endothelial dysfunction recovery in peripheral artery disease patients. Cholesterol 2012;2012:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bloomfield 2009 {published data only}
- Bloomfield D, Carlson GL, Sapre A, Tribble D, McKenney JM, Littlejohn TW, et al. Efficacy and safety of the cholesteryl ester transfer protein inhibitor anacetrapib as monotherapy and coadministered with atorvastatin in dyslipidemic patients. American Heart Journal 2009;157(2):352-60. [DOI] [PubMed] [Google Scholar]
Braamskamp 2015 {published data only}
- Braamskamp MJ, Kusters DM, Wiegman A, Avis HJ, Wijburg FA, Kastelein JJ et al. Gonadal steroids, gonadotropins and DHEAS in young adults with familial hypercholesterolemia who had initiated statin therapy in childhood. Atherosclerosis 2015;241(2):427-32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cai 2014 {published data only}
- Cai X, Tian Y, Wu T, Cao CX, Bu SY, Wang KJ. The role of statins in erectile dysfunction: a systematic review and meta-analysis. Asian Journal of Andrology 2014;16(3):461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2002a {published data only}
- Chan DC, Watts GF, Mori TA, Barrett PH, Beilin LJ, Redgrave TG. Factorial study of the effects of atorvastatin and fish oil on dyslipidaemia in visceral obesity. European Journal of Clinical Investigation 2002;32(6):429-36. [DOI] [PubMed] [Google Scholar]
Chan 2002b {published data only}
- Chan DC, Watts GF, Barrett PHR, Martins IJ, James AP, Mamo JCL, et al. Effect of atorvastatin on chylomicron remnant metabolism in visceral obesity: a study employing a new stable isotope breath test. Journal of Lipid Research 2002;43(5):706-12. [PubMed] [Google Scholar]
Chan 2002c {published data only}
- Chan DC, Watts GF, Barrett PH, Beilin LJ, Redgrave TG, Mori TA. Regulatory effects of HMG CoA reductase inhibitor and fish oils on apolipoprotein B-100 kinetics in insulin-resistant obese male subjects with dyslipidemia. Diabetes 2002;51(8):2377-86. [DOI] [PubMed] [Google Scholar]
Chan 2006 {published data only}
- Chan DC, Watts GF, Nguyen MN, Barrett PH. Factorial study of the effect of n-3 fatty acid supplementation and atorvastatin on the kinetics of HDL apolipoproteins A-I and A-II in men with abdominal obesity. American Journal of Clinical Nutrition 2006;84(1):37-43. [DOI] [PubMed] [Google Scholar]
Chapman 2011 {published data only}
- Chapman N, Chang CL, Caulfield M, Dahlof B, Feder G, Sever PS, et al. Ethnic variations in lipid-lowering in response to a statin (EVIREST): a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT). Ethnicity & Disease 2011;21(2):150-7. [PubMed] [Google Scholar]
Clough 2009 {published data only}
- Clough GF, Turzyniecka M, Walter L, Krentz AJ, Wild SH, Chipperfield AJ, et al. Muscle microvascular dysfunction in central obesity is related to muscle insulin insensitivity but is not reversed by high-dose statin treatment. Diabetes 2009;58(5):1185-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohn 2009 {published data only}
- Cohn JN, Wilson DJ, Neutel J, Houston M, Weinberger MH, Grimm Jr R, et al. Coadministered amlodipine and atorvastatin produces early improvements in arterial wall compliance in hypertensive patients with dyslipidemia. American Journal of Hypertension 2009;22(2):137-44. [DOI] [PubMed] [Google Scholar]
Colhoun 2004 {published data only}
- Colhoun HM, Betteridge DJ, Durrington PN, Persell SD. Cholesterol lowering with atorvastatin for the primary prevention of cardiovascular disease in diabetic adults. Journal of Clinical Outcomes Management 2004;11(11):682-5. [Google Scholar]
Crisostomo 2008 {published data only}
- Crisostomo LM, Souza CA, Mendes CM, Coimbra SR, Favarato D, Luz PL. Vascular and metabolic response to statin in the mildly hypertensive hypercholesterolemic elderly. Clinics (Sao Paulo, Brazil) 2008;63(5):589-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cubeddu 2006 {published data only}
- Cubeddu LX, Cubeddu RJ, Heimowitz T, Restrepo B, Lamas GA, Weinberg GB. Comparative lipid-lowering effects of policosanol and atorvastatin: a randomized, parallel, double-blind, placebo-controlled trial. American Heart Journal 2006;152(5):982.e1-5. [DOI] [PubMed] [Google Scholar]
Cui 2006 {published data only}
- Cui B, Jiang H. [Effect of atorvastatin on endothelium-dependent vasodilatation function in patients with essential hypertension complicated by hyperlipidemia]. Wuhan Daxue Xuebao 2006;27(6):786-9. [Google Scholar]
Dallinga‐Thie 2006a {published data only}
- Dallinga-Thie GM, Tol A, Hattori H, Rensen PC, Sijbrands EJ. Plasma phospholipid transfer protein activity is decreased in type 2 diabetes during treatment with atorvastatin: a role for apolipoprotein E? Diabetes 2006;55(5):1491-6. [DOI] [PubMed] [Google Scholar]
Dallinga‐Thie 2006b {published data only}
- Dallinga-Thie GM, Tol A, Hattori H, Vark-van der Zee LC, Jansen H, Sijbrands EJ, et al. Plasma apolipoprotein A5 and triglycerides in type 2 diabetes. Diabetologia 2006;49(7):1505-11. [DOI] [PubMed] [Google Scholar]
Davidson 2002 {published data only}
- Davidson M, Ma P, Stein EA, Gotto Jr AM, Raza A, Chitra R, et al. Comparison of effects on low-density lipoprotein cholesterol and high-density lipoprotein cholesterol with rosuvastatin versus atorvastatin in patients with type IIa or IIb hypercholesterolemia. American Journal of Cardiology 2002;89(3 PG - 268-275):268-75. [DOI] [PubMed] [Google Scholar]
Deanfield 2010 {published data only}
- Deanfield JE, Sellier P, Thaulow E, Bultas J, Yunis C, Shi H, et al. Potent anti-ischaemic effects of statins in chronic stable angina: incremental benefit beyond lipid lowering? European Heart Journal 2010;31(21):2650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diabetes 2001 {published data only}
- Diabetes Atorvastin Lipid Intervention Study Group. The effect of aggressive versus standard lipid lowering by atorvastatin on diabetic dyslipidemia: the DALI study: a double-blind, randomized, placebo-controlled trial in patients with type 2 diabetes and diabetic dyslipidemia. Diabetes Care 2001;24(8):1335-41. [DOI] [PubMed] [Google Scholar]
Diepeveen 2005 {published data only}
- Diepeveen SH, Verhoeven GW, Van Der Palen J, Dikkeschei LD, Van Tits LJ, Kolsters G, et al. Effects of atorvastatin and vitamin E on lipoproteins and oxidative stress in dialysis patients: a randomised-controlled trial. Journal of Internal Medicine 2005;257(5):438-45. [DOI] [PubMed] [Google Scholar]
Dogra 2007 {published data only}
- Dogra G, Irish A, Chan D, Watts G. A randomized trial of the effect of statin and fibrate therapy on arterial function in CKD. American Journal of Kidney Diseases 2007;49(6):776-85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Economides 2004 {published data only}
- Economides PA, Caselli A, Tiani E, Khaodhiar L, Horton ES, Veves A. The effects of atorvastatin on endothelial function in diabetic patients and subjects at risk for type 2 diabetes. Journal of Clinical Endocrinology and Metabolism 2004;89(2):740-7. [DOI] [PubMed] [Google Scholar]
Elkawy 2014 {published data only}
- Elkawy KA, El-Sisi A, Hegazy S, Salem K. Comparing the effects of atorvastatin and vitamin E on endothelial function of sildenafil non-responders. FASEB Journal 2014;28(1 SUPPL 1):no pagination. [Google Scholar]
EUCTR2004‐000139‐27‐SE {published data only}
- EUCTR2004-000139-27-SE. A Phase 3, Double-Blind, Placebo-Controlled, Randomized, Parallel Group, Multicenter Study of the Efficacy, Safety, and Tolerability of Fixed Combination Torcetrapib/Atorvastatin Administered Orally, Once Daily for 6 Months, Compared to Atorvastatin Alone or Placebo, in Subjects With Mixed Dyslipidemia (Frederickson Types IIa and IIb). - N/A. http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2004-000139-27-SE 2004.
Faludi 2004 {published data only}
- Faludi AA, Aldrighi JM, Bertolami MC, Saleh MH, Silva RA, Nakamura Y, et al. Progesterone abolishes estrogen and/or atorvastatin endothelium dependent vasodilatory effects. Atherosclerosis 2004;177(1):89-96. [DOI] [PubMed] [Google Scholar]
Ferrier 2002a {published data only}
- Ferrier KE, Muhlmann MH, Baguet JP, Cameron JD, Jennings GL, Dart AM, et al. Intensive cholesterol reduction lowers blood pressure and large artery stiffness in isolated systolic hypertension. Journal of the American College of Cardiology 2002;39(6):1020-5. [DOI] [PubMed] [Google Scholar]
Ferrier 2002b {published data only}
- Ferrier KE, Muhlmann MH, Baguet JP, Cameron JD, Jennings GL, Dart AM, et al. Cholesterol reduction and artery compliance in hypertension. Cardiology Review 2002;19(14):14-6. [DOI] [PubMed] [Google Scholar]
Fogari 2004 {published data only}
- Fogari R, Derosa G, Lazzari P, Zoppi A, Fogari E, Rinaldi A, et al. Effect of amlodipine-atorvastatin combination on fibrinolysis in hypertensive hypercholesterolemic patients with insulin resistance. American Journal of Hypertension 2004;17(9):823-7. [DOI] [PubMed] [Google Scholar]
Fogari 2006 {published data only}
- Fogari R, Preti P, Zoppi A, Lazzari P, Corradi L, Fogari E, et al. Effects of amlodipine-atorvastatin combination on inflammation markers and insulin sensitivity in normocholesterolemic obese hypertensive patients. European Journal of Clinical Pharmacology 2006;62(10):817-22. [DOI] [PubMed] [Google Scholar]
Freed 2002 {published data only}
- Freed MI, Ratner R, Marcovina SM, Kreider MM, Biswas N, Cohen BR, et al. Effects of rosiglitazone alone and in combination with atorvastatin on the metabolic abnormalities in type 2 diabetes mellitus. American Journal of Cardiology 2002;90(9):947-52. [DOI] [PubMed] [Google Scholar]
Ge 2008 {published data only}
- Ge CJ, Lu SZ, Chen YD, Wu XF, Hu SJ, Ji Y. Synergistic effect of amlodipine and atorvastatin on blood pressure, left ventricular remodeling, and C-reactive protein in hypertensive patients with primary hypercholesterolemia. Heart and Vessels 2008;23(2):91-5. [DOI] [PubMed] [Google Scholar]
Gentile 2000 {published data only}
- Gentile S, Turco S, Guarino G, Sasso CF, Amodio M, Magliano P, et al. Comparative efficacy study of atorvastatin vs simvastatin, pravastatin, lovastatin and placebo in type 2 diabetic patients with hypercholesterolaemia. Diabetes, Obesity & Metabolism 2000;2(6):355-62. [DOI] [PubMed] [Google Scholar]
GlaxoSmithKline 2000 {published data only}
- GlaxoSmithKline. A 16-Week Randomized, Double-Blind, Parallel-Group Study to Evaluate the Safety and Efficacy of Hypolipidemic Therapy with an HMGCoA Reductase Inhibitor (Atorvastatin) in Type 2 Diabetes Mellitus Patients Treated with Rosiglitazone (BRL 49653C) 4mg bd. Protocol No. 49653/108 2000.
Glinkina 2008 {published data only}
- Glinkina IV, Zilov AV, Mel'nichenko GA, Roitman AP, Il'in AV. Comparison of the effects of carbohydrate metabolism compensation and atorvastatin treatment on lipid metabolism and c-reactive protein in type 2 diabetes mellitus. Terapevticheskii Arkhiv 2008;80(12):17-22. [PubMed] [Google Scholar]
Goldstein 2008 {published data only}
- Goldstein LB, Amarenco P, Lamonte M, Gilbert S, Messig M, Callahan A, et al. Relative effects of statin therapy on stroke and cardiovascular events in men and women: secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Study. Stroke 2008;39(9):2444-8. [DOI] [PubMed] [Google Scholar]
Goldstein 2009 {published data only}
- Goldstein LB, Amarenco P, Zivin J, Messig M, Altafullah I, Callahan A, et al. Statin treatment and stroke outcome in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke; a Journal of Cerebral Circulation 2009;40(11):3526-31. [DOI] [PubMed] [Google Scholar]
Grimm 2010 {published data only}
- Grimm R, Malik M, Yunis C, Sutradhar S, Kursun A, TOGETHER Investigators. Simultaneous treatment to attain blood pressure and lipid goals and reduced CV risk burden using amlodipine/atorvastatin single-pill therapy in treated hypertensive participants in a randomized controlled trial. Vascular Health and Risk Management 2010;6:261-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guo 2012 {published data only}
- Guo S, Wang R, Yang Z, Li K, Wang Q. Effects of atorvastatin on serum lipids, serum inflammation and plaque morphology in patients with stable atherosclerotic plaques. Experimental and Therapeutic Medicine 2012;4(6):1069-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernandez 2010 {published data only}
- Hernandez C, Francisco G, Ciudin A, Chacon P, Montoro B, Llaverias G, et al. Effect of atorvastatin on lipoprotein (a) and interleukin-10: a randomized placebo-controlled trial. Diabetes & Metabolism 2010;37(2):124-30. [DOI] [PubMed] [Google Scholar]
Holman 2009 {published data only}
- Holman RR, Paul S, Farmer A, Tucker L, Stratton IM, Neil HA, et al. Atorvastatin in Factorial with Omega-3 EE90 Risk Reduction in Diabetes (AFORRD): a randomised controlled trial. Diabetologia 2009;52(1):50-9. [DOI] [PubMed] [Google Scholar]
Holmberg 2005 {published data only}
- Holmberg B, Brannstrom M, Bucht B, Crougneau V, Dimeny E, Ekspong A, et al. Safety and efficacy of atorvastatin in patients with severe renal dysfunction. Scandinavian Journal of Urology and Nephrology 2005;39(6):503-10. [DOI] [PubMed] [Google Scholar]
Horwich 2011 {published data only}
- Horwich TB, Middlekauff HR, Maclellan WR, Fonarow GC. Statins do not significantly affect muscle sympathetic nerve activity in humans with nonischemic heart failure: a double-blind placebo-controlled trial. Journal of Cardiac Failure 2011;17(11):879-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunninghake 2001 {published data only}
- Hunninghake D, Insull Jr W, Toth P, Davidson D, Donovan J M, Burke S K. Coadministration of colesevelam hydrochloride with atorvastatin lowers LDL cholesterol additively. Atherosclerosis 2001;158(2):407-16. [DOI] [PubMed] [Google Scholar]
Huptas 2006 {published data only}
- Huptas S, Geiss H C, Otto C, Parhofer K G. Effect of atorvastatin (10 mg/day) on glucose metabolism in patients with the metabolic syndrome. American Journal of Cardiology 2006;98(1):66-9. [DOI] [PubMed] [Google Scholar]
Iavenko 2014 {published data only}
- Iakovenko EI, Evdakimova AA, Toguzova ZA, Sharvadze GG, Mamedov MN. Dose-dependent effect of atorvastatin on erectile function and androgen status in men with high cardiovascular risk. Kardiologiia 2014;54(2):37-42. [DOI] [PubMed] [Google Scholar]
Inukai 2011 {published data only}
- Inukai K, Iuchi T, Sumita T, Ito D, Ikebukuro K, Imai K, et al. [Combination therapy with angiotensin receptor blocker (ARB) plus atorvastatin reduced proteinuria in type 2 diabetic patients with diabetic nephropathy]. Therapeutic Research 2011;32(7):947-53. [EMBASE: 2011473820] [Google Scholar]
IRCT201208299626N1 {published data only}
- IRCT201208299626N1. The effect of atorvastatin on biochemical and hemostatic profile of patients with polycystic ovary syndrome. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201208299626N1 2012.
Ishola 2017 {published data only}
- Ishola IO, Tijani HK, Dosumu OO, Anunobi CC, Oshodi TO. Atorvastatin attenuates testosterone-induced benign prostatic hyperplasia in rats: role of peroxisome proliferator-activated receptor-gamma and cyclo-oxygenase-2. Fundamental & Clinical Pharmacology 2017;31(6):652-62. [DOI] [PubMed] [Google Scholar]
Issa 2012 {published data only}
- Issa MH, Cerda A, Genvigir FD, Cavalli SA, Bertolami MC, Faludi AA, et al. Atorvastatin and hormone therapy effects on APOE mRNA expression in hypercholesterolemic postmenopausal women. Journal of Steroid Biochemistry and Molecular Biology 2012;128(3-5):139-44. [DOI] [PubMed] [Google Scholar]
Jayaram 2007 {published data only}
- Jayaram S, Prasad HB, Sovani VB, Langade DG, Mane PR. Randomised study to compare the efficacy and safety of isapgol plus atorvastatin versus atorvastatin alone in subjects with hypercholesterolaemia. Journal of the Indian Medical Association 2007;105(3):142-145,150. [PubMed] [Google Scholar]
J‐CLAS 1997 {published data only}
- Anonymous 1997. Efficacy of atorvastatin in primary hypercholesterolemia. Japan Cholesterol Lowering Atorvastatin Study (J-CLAS) Group. American Journal of Cardiology 1997;79(9):1248-52. [PubMed] [Google Scholar]
Joy 2008 {published data only}
- Joy MS, Dornbrook-Lavender KA, Chin H, Hogan SL, Denu-Ciocca C. Effects of atorvastatin on Lp(a) and lipoprotein profiles in hemodialysis patients. Annals of Pharmacotherapy 2008;42(1):9-15. [DOI] [PubMed] [Google Scholar]
JPRN‐UMIN000001114 {published data only}
- JPRN-UMIN000001114. Effects of lipid lowering by atorvastatin on carotid atherosclerotic plaque (ACAP) study: a randomized trial for analysis of qualitative change in carotid atherosclerrotic plaque with IB echo and Black Blood MRI. http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000001114 2008.
Kadoglou 2011 {published data only}
- Kadoglou NPE, Vrabas IS, Kapelouzou A, Lampropoulos S, Sailer N, Kostakis A, et al. Impact of atorvastatin on serum vaspin levels in hypercholesterolemic patients with moderate cardiovascular risk. Regulatory Peptides 2011;170(1-3):57-61. [DOI] [PubMed] [Google Scholar]
Kajimoto 2009 {published data only}
- Kajimoto K, Miyauchi K, Kasai T, Shimada K, Kojima Y, Shimada A, et al. Short-term 20-mg atorvastatin therapy reduces key inflammatory factors including c-Jun N-terminal kinase and dendritic cells and matrix metalloproteinase expression in human abdominal aortic aneurysmal wall. Atherosclerosis 2009;206(2):505-11. [DOI] [PubMed] [Google Scholar]
Kanadasi 2006 {published data only}
- Kanadasi M, Cayli M, Demirtas M, Inal T, Demir M, Koc M, et al. The effect of early statin treatment on inflammation and cardiac events in acute coronary syndrome patients with low-density lipoprotein cholesterol. Heart and Vessels 2006;21(5):291-7. [DOI] [PubMed] [Google Scholar]
Kappelle 2009 {published data only}
- Kappelle P, Zwang L, Huisman MV, Banga JD, Sluiter WJ, Dallinga-Thie GM, et al. Atorvastatin affects low density lipoprotein and non-high density lipoprotein cholesterol relations with apolipoprotein B in type 2 diabetes mellitus: Modification by triglycerides and cholesteryl ester transfer protein. Expert Opinion on Therapeutic Targets 2009;13(7):743-51. [DOI] [PubMed] [Google Scholar]
Kappelle 2010 {published data only}
- Kappelle PJ, Dallinga-Thie GM, Dullaart RP, Diabetes Atorvastatin Lipid Intervention (DALI) study group. Atorvastatin treatment lowers fasting remnant-like particle cholesterol and LDL subfraction cholesterol without affecting LDL size in type 2 diabetes mellitus: Relevance for non-HDL cholesterol and apolipoprotein B guideline targets. Biochimica et Biophysica Acta 2010;1801(1):89-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Karam 2008 {published data only}
- Karam JG, Loney-Hutchinson L, McFarlane SI. High-dose atorvastatin after stroke or transient ischemic attack: the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. Journal of the Cardiometabolic Syndrome 2008;3(1):68-9. [DOI] [PubMed] [Google Scholar]
Kishi 2009 {published data only}
- Kishi T, Yamada A, Okamatsu S, Sunagawa K. Atorvastatin might improve ventricular electrostability and decelerate the deterioration of renal function in patients with heart failure and diabetes mellitus. Journal of Cardiology 2009;53(3):341-8. [DOI] [PubMed] [Google Scholar]
Kitas 2019 {published data only}
- Kitas GD, Nightingale P, Armitage J, Sattar N, Belch JJF, Symmons DPM, TRACE RA Consortium. Trial of atorvastatin for the primary prevention of cardiovascular events in patients with rheumatoid arthritis (TRACE RA): a multicenter, randomized, placebo controlled trial. Arthritis & Rheumatology 2019;71(9):1437-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knopp 2006 {published data only}
- Knopp RH, d'Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care 2006;29(7):1478-85. [DOI] [PubMed] [Google Scholar]
Koh 2005 {published data only}
- Koh KK, Quon MJ, Han SH, Chung WJ, Ahn JY, Seo YH, et al. Additive beneficial effects of fenofibrate combined with atorvastatin in the treatment of combined hyperlipidemia. Journal of the American College of Cardiology 2005;45(10):1649-53. [DOI] [PubMed] [Google Scholar]
Koh 2010 {published data only}
- Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Shin EK, et al. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. Journal of the American College of Cardiology 2010;55(12):1209-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kom 2007 {published data only}
- Kom G D, Schwedhelm E, Maas R, Schneider L, Benndorf R, Boger R H. Impact of atorvastatin treatment on platelet-activating factor acetylhydrolase and 15-F(2trans)-isoprostane in hypercholesterolaemic patients. British Journal of Clinical Pharmacology 2007;63(6):672-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Konduracka 2008 {published data only}
- Konduracka E, Galicka-Latala D, Cieslik G, Rostoff P, Fedak D, Sieradzki J, et al. Effect of atorvastatin on endothelial function and inflammation in long-duration type 1 diabetic patients without coronary heart disease and arterial hypertension. Diabetes, Obesity & Metabolism 2008;10(9):719-25. [DOI] [PubMed] [Google Scholar]
Krysiak 2010 {published data only}
- Krysiak R, Gdula-Dymek A, Bachowski R, Okopien B. Pleiotropic effects of atorvastatin and fenofibrate in metabolic syndrome and different types of pre-diabetes. Diabetes Care 2010;33(10):2266-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krysiak 2014 {published data only}
- Krysiak R, Okopien B. The effect of atorvastatin and atorvastatin-ezetimibe combination therapy on androgen production in hyperandrogenic women with elevated cholesterol levels. Experimental and Clinical Endocrinology & Diabetes 2014;123(2):75-9. [DOI] [PubMed] [Google Scholar]
Krysiak 2016 {published data only}
- Krysiak R, Gilowski W, Okopien B. The effect of testosterone on cardiometabolic risk factors in atorvastatin-treated men with late-onset hypogonadism. Pharmacological Reports: PR 2016;68(1):196-200. [DOI] [PubMed] [Google Scholar]
Kubler 2003 {published data only}
- Kubler W, Ssever P, Dahlof B, Poulter NR, Wedel H, Beevers G, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cariac Outcomes Trial - Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Zeitschrift fur Kardiologie 2003;92(7):613. [EMBASE: 2003300606] [Google Scholar]
Kuznetsova 2010 {published data only}
- Kuznetsova MA, Vaulin NA, Masenko VP, Gratsianskii NA. Non-ST-elevation acute coronary syndrome. Comparison of effects of atorvastatin and rosuvastatin on blood levels of lipids and markers of inflammation. Kardiologiia 2010;50(2):21-5. [PubMed] [Google Scholar]
Lamon‐Fava 2007 {published data only}
- Lamon-Fava S, Diffenderfer MR, Barrett PH, Buchsbaum A, Matthan NR, Lichtenstein AH, et al. Effects of different doses of atorvastatin on human apolipoprotein B-100, B-48, and A-I metabolism. Journal of Lipid Research 2007;48(8):1746-53. [DOI] [PubMed] [Google Scholar]
Lavallee 2009 {published data only}
- Lavallee PC, Labreuche J, Gongora-Rivera F, Jaramillo A, Brenner D, Klein IF, et al. Placebo-controlled trial of high-dose atorvastatin in patients with severe cerebral small vessel disease. Stroke 2009;40(5):1721-8. [DOI] [PubMed] [Google Scholar]
Lawrence 2004 {published data only}
- Lawrence JM, Reid J, Taylor GJ, Stirling C, Reckless JP. The effect of high dose atorvastatin therapy on lipids and lipoprotein subfractions in overweight patients with type 2 diabetes. Atherosclerosis 2004;174(1):141-9. [DOI] [PubMed] [Google Scholar]
Lewandowski 2008 {published data only}
- Lewandowski M, Kornacewicz-Jach Z, Millo B, Zielonka J, Czechowska M, Kaliszczak R, et al. The influence of low dose atorvastatin on inflammatory marker levels in patients with acute coronary syndrome and its potential clinical value. Cardiology Journal 2008;15(4):357-64. [PubMed] [Google Scholar]
Li 2006 {published data only}
- Li XP, Duan J, Zhao SP, Tan MY, Xu ZM, Zhang DQ. [Efficacy and safety of extended-release niacin alone or with atorvastatin for lipid profile modification]. Zhonghua Yi Xue Za Zhi 2006;86(34):2399-403. [PubMed] [Google Scholar]
Li 2010 {published data only}
- Li WL, Ke W, Deng XR. Influence of atorvastatin on ischemic adverse events following cerebral middle artery stent implantation: A 12-month follow-up in 24 cases. [Chinese]. Journal of Clinical Rehabilitative Tissue Engineering Research 2010;14(4):736-9. [Google Scholar]
Lins 2004 {published data only}
- Lins RL, Matthys KE, Billiouw JM, Dratwa M, Dupont P, Lameire NH, et al. Lipid and apoprotein changes during atorvastatin up-titration in hemodialysis patients with hypercholesterolemia: a placebo-controlled study. Clinical Nephrology 2004;62(4):287-94. [DOI] [PubMed] [Google Scholar]
Liu 2009 {published data only}
- Liu M, Wang F, Wang Y, Jin R. Atorvastatin improves endothelial function and cardiac performance in patients with dilated cardiomyopathy: the role of inflammation. Cardiovascular Drugs & Therapy 2009;23(5):369-76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Loughrey 2013 {published data only}
- Loughrey BV, McGinty A, Young IS, McCance DR, Powell LA. Increased circulating CC chemokine levels in the metabolic syndrome are reduced by low-dose atorvastatin treatment: evidence from a randomized controlled trial. Clinical Endocrinology 2013;79(6):800-6. [DOI] [PubMed] [Google Scholar]
Macchia 2012 {published data only}
- Macchia A, Laffaye N, Comignani PD, Cornejo PE, Igarzabal C, Scazziota AS, et al. Statins but not aspirin reduce thrombotic risk assessed by thrombin generation in diabetic patients without cardiovascular events: the RATIONAL trial. PloS one 2012;7(3):e32894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macin 2005 {published data only}
- Macin SM, Perna ER, Farias EF, Franciosi V, Cialzeta JR, Brizuela M, et al. Atorvastatin has an important acute anti-inflammatory effect in patients with acute coronary syndrome: results of a randomized, double-blind, placebo-controlled study. American Heart Journal 2005;149(3):451-7. [DOI] [PubMed] [Google Scholar]
Magen 2004 {published data only}
- Magen E, Viskoper R, Mishal J, Priluk R, Berezovsky A, Laszt A, et al. Resistant arterial hypertension and hyperlipidemia: atorvastatin, not vitamin C, for blood pressure control. Israel Medical Association Journal 2004;6(12):742-6. [PubMed] [Google Scholar]
Maggioni 2018 {published data only}
- Maggioni AP, Filardi PP. The REVEAL study [Lo studio REVEAL]. Giornale Italiano di Cardiologia 2018;19(2):71-6. [DOI] [PubMed] [Google Scholar]
Mal'gina 2007 {published data only}
- Mal'gina MP, Ignatyeva OI, Moroshkina NV, Skorobogatova YV, Nedoshivin AO, Berkovich OA. Effectiveness, safety, and endothelial function effects of atorvastatin lipid-lowering therapy in coronary heart disease patients undergoing percutaneous coronary intervention. Cardiovascular Therapy and Prevention 2007;6(3):50-5. [Google Scholar]
Malekzadeh 2010 {published data only}
- Malekzadeh F, Marshall T, Pourshams A, Gharravi M, Aslani A, Nateghi A, et al. A pilot double-blind randomised placebo-controlled trial of the effects of fixed-dose combination therapy ('polypill') on cardiovascular risk factors. International Journal of Clinical Practice 2010;64(9):1220-7. [DOI] [PubMed] [Google Scholar]
Manuel 2003 {published data only}
- Manuel Y Keenoy B, Van Campenhout C, Vertommen J, De Leeuw I. Effects of Atorvastatin on LDL sub-fractions and peroxidation in type 1 diabetic patients: a randomised double-blind placebo-controlled study. Diabetes/Metabolism Research Reviews 2003;19(6):478-86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marais 1997 {published data only}
- Marais AD, Naoumova RP, Firth JC, Penny C, Neuwirth CK, Thompson GR. Decreased production of low density lipoprotein by atorvastatin after apheresis in homozygous familial hypercholesterolemia. Journal of Lipid Research 1997;38(10):2071-8. [PubMed] [Google Scholar]
Marchesi 2000 {published data only}
- Marchesi S, Lupattelli G, Siepi D, Schillaci G, Vaudo G, Roscini AR, et al. Short-term atorvastatin treatment improves endothelial function in hypercholesterolemic women. Journal of Cardiovascular Pharmacology 2000;36(5):617-21. [DOI] [PubMed] [Google Scholar]
Martin 2011 {published data only}
- Martin S, Herder C, Schloot N C, Koenig W, Heise T, Heinemann L, et al. Residual beta cell function in newly diagnosed type 1 diabetes after treatment with atorvastatin: the Randomized DIATOR Trial. PloS one 2011;6(3):e17554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin‐Ventura 2005 {published data only}
- Martin-Ventura JL, Blanco-Colio LM, Gomez-Hernandez A, Munoz-Garcia B, Vega M, Serrano J, et al. Intensive treatment with atorvastatin reduces inflammation in mononuclear cells and human atherosclerotic lesions in one month. Stroke 2005;36(8):1796-800. [DOI] [PubMed] [Google Scholar]
McCrindle 2003 {published data only}
- McCrindle BW, Ose L, Marais AD. Efficacy and safety of atorvastatin in children and adolescents with familial hypercholesterolemia or severe hyperlipidemia: a multicenter, randomized, placebo-controlled trial. Journal of Pediatrics 2003;143(1):74-80. [DOI] [PubMed] [Google Scholar]
Mehta 2007 {published data only}
- Mehta A, Shah U, Parikh K, Chag M, Baxi H, Chandarana A, et al. Effect of pioglitazone and its combination with statins in coronary artery disease patients with hyperinsulinemia. Canadian Journal of Physiology & Pharmacology 2007;85(6):628-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meng 2009 {published data only}
- Meng X, Qie L, Wang Y, Zhong M, Li L. Assessment of arterial stiffness affected by atorvastatin in coronary artery disease using pulse wave velocity. Clinical & Investigative Medicine. Medecine Clinique et Experimentale 2009;32(6):E238. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Messerli 2006 {published data only}
- Messerli FH, Bakris GL, Ferrera D, Houston MC, Petrella RJ, Flack JM, et al for the AVALON Investigators. Efficacy and safety of coadministered amlodipine and atorvastatin in patients with hypertension and dyslipidemia: results of the AVALON trial. Journal of Clinical Hypertension 2006;8(8):571-81; quiz 582-3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mills 2007 {published data only}
- Mills IW, Crossland A, Patel A, Ramonas H. Atorvastatin treatment for men with lower urinary tract symptoms and benign prostatic enlargement. European Urology 2007;52(2):503-9. [DOI] [PubMed] [Google Scholar]
Mishra 2005 {published data only}
- Mishra M, Durrington P, Mackness M, Siddals K W, Kaushal K, Davies R, et al. The effect of atorvastatin on serum lipoproteins in acromegaly. Clinical Endocrinology 2005;62(6):650-5. [DOI] [PubMed] [Google Scholar]
Moga 2005 {published data only}
- Moga M. The influence of atorvastatin therapy on myocardial ischemia in patients with stable angina pectoris. [Polish] [Wplyw leczenia atorwastatyna na stopien niedokrwienia miesnia sercowego u pacjentow ze stabilna dlawica piersiowa]. Folia Cardiologica 2005;12(7):471-80. [Google Scholar]
Mohler 2003 {published data only}
- Mohler ER, Hiatt WR, Creager MA. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation 2003;108(12):1481-6. [DOI] [PubMed] [Google Scholar]
Moini 2012 {published data only}
- Moini A, Azimi G, Farivar A. Evaluation of atorvastatin for the treatment of patients with asthma: A double-blind randomized clinical trial. Allergy, Asthma & Immunology Research 2012;4(5):290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Monteiro 2008 {published data only}
- Monteiro CMC, Oliveira L, Izar MCO, Santos AO, Povoa RMS, Fischer SM, et al. Early effects of lipid lowering treatment in subjects with metabolic syndrome and acute coronary syndromes. International Journal of Atherosclerosis 2008;3(2):93-9. [Google Scholar]
Muscari 2001 {published data only}
- Muscari A, Bastagi L, Poggiopollini G, Tomassetti V, Massarelli G, Boni P, et al. Short term effect of atorvastatin and vitamin E on serum levels of C3, a sensitive marker of the risk of myocardial infarction in men. Cardiovascular Drugs & Therapy 2001;15(5):453-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nakamura 1997 {published data only}
- Nakamura H, Ohashi Y, Maruhama Y, Ninomiya K, Toyota T, Oikawa S, et al. Efficacy of atorvastatin in primary hypercholesterolemia. American Journal of Cardiology 1997;79(9):1248-52. [PubMed] [Google Scholar]
Nakamura 2007 {published data only}
- Nakamura T, Kodama Y, Takano H, Umetani K, Fujioka D, Saito Y, et al. Increase in circulating levels of adiponectin after treatment with statin and fibrate in patients with coronary artery disease and hyperlipidemia. Atherosclerosis 2007;193(2):449-51. [DOI] [PubMed] [Google Scholar]
Naoumova 1997 {published data only}
- Naoumova RP, Dunn S, Rallidis L, Abu-Muhana O, Neuwirth C, Rendell N B, et al. Prolonged inhibition of cholesterol synthesis explains the efficacy of atorvastatin. Journal of Lipid Research 1997;38(7):1496-500. [PubMed] [Google Scholar]
Naoumova 1999 {published data only}
- Naoumova RP, O'Neill FH, Dunn S, Neuwirth CKY, Taylor GW, Axelson M, et al. Effect of inhibiting HMG-CoA reductase on 7alpha-hydroxy-4-cholesten-3-one, a marker of bile acid synthesis: Contrasting findings in patients with and without prior up-regulation of the latter pathway. European Journal of Clinical Investigation 1999;29(5):404-12. [DOI] [PubMed] [Google Scholar]
Naoumova 2003 {published data only}
- Naoumova RP, Patel DD, O'Neill FH, Thompson GR, Knight BL. Treatment with atorvastatin alters the ratio of interleukin-12/interleukin-10 gene expression [corrected]. European Journal of Clinical Investigation 2003;33(1):88-91. [DOI] [PubMed] [Google Scholar]
Nawawi 2003 {published data only}
- Nawawi H, Osman NS, Yusoff K, Khalid BA. Reduction in serum levels of adhesion molecules, interleukin-6 and C-reactive protein following short-term low-dose atorvastatin treatment in patients with non-familial hypercholesterolemia. Hormone & Metabolic Research 2003;35(8):479-85. [DOI] [PubMed] [Google Scholar]
Nawrocki 1995 {published data only}
- Nawrocki JW, Weiss SR, Davidson MH, Sprecher DL, Schwartz SL, Lupien PJ, et al. Reduction of LDL cholesterol by 25% to 60% in patients with primary hypercholesterolemia by atorvastatin, a new HMG-CoA reductase inhibitor. Arterosclerosis, Thrombosis & Vascular Biology 1995;15(5):678-82. [DOI] [PubMed] [Google Scholar]
NCT00433823 {published data only}
- NCT00433823. A Study Evaluating the Effects of Lipid Lowering Treatment on Steroid Synthesis. https://clinicaltrials.gov/show/nct00433823.
NCT00522158 {published data only}
- NCT00522158. Effects of Achieving Very Low LDL-Cholesterol After Treatment With Statins on Steroidogenesis and Cognition. https://clinicaltrials.gov/show/nct00522158.
NCT00603590 {published data only}
- NCT00603590. Phase II Study of Heart Polypill Safety and Efficacy in Primary Prevention of Cardiovascular Disease. Https://clinicaltrials.gov/show/nct00603590 2008;(PG -).
NCT01555632 {published data only}
- NCT01555632. Atorvastatin Calcium in Preventing Metabolic Syndrome in Patients With Prostate Cancer Receiving Long-Term Androgen-Deprivation Therapy. https://clinicaltrials.gov/show/nct01555632.
NCT02497638 {published data only}
- NCT02497638. LIpitor and biGuanide to Androgen Delay Trial. https://clinicaltrials.gov/show/nct02497638.
NCT03784703 {published data only}
- NCT03784703. Type 2 Diabetic Patients Maintained on Statin Therapy. Https://clinicaltrials.gov/show/nct03784703 2018;(PG -).
NCT04101136 {published data only}
- NCT04101136. Effect of Atorvastatin on Subclinical Atherosclerosis. https://clinicaltrials.gov/show/NCT04101136 2019;(PG -).
Negi 2011 {published data only}
- Negi S, Shukrullah I, Veledar E, Bloom HL, Jones DP, Dudley SC. Statin therapy for the prevention of atrial fibrillation trial (SToP AF trial). Journal of Cardiovascular Electrophysiology 2011;22(4):414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neil 2010 {published data only}
- Neil HA, Ceglarek U, Thiery J, Paul S, Farmer A, Holman RR. Impact of atorvastatin and omega-3 ethyl esters 90 on plasma plant sterol concentrations and cholesterol synthesis in type 2 diabetes: a randomised placebo controlled factorial trial. Atherosclerosis 2010;213(2):512-7. [DOI] [PubMed] [Google Scholar]
Neutel 2009 {published data only}
- Neutel JM, Bestermann WH, Dyess EM, Graff A, Kursun A, Sutradhar S, et al. The use of a single-pill calcium channel blocker/statin combination in the management of hypertension and dyslipidemia: a randomized, placebo-controlled, multicenter study. Journal of Clinical Hypertension 2009;11(1):22-30. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nicholls 2011 {published data only}
- Nicholls SJ, Brewer HB, Kastelein JJ, Krueger KA, Wang MD, Shao M, et al. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol: a randomized controlled trial. JAMA 2011;306(19):2099-109. [DOI] [PubMed] [Google Scholar]
Nikitina 2009 {published data only}
- Nikitina NM, Rebrov AP. The use of atorvastatin in patients with rheumatoid arthritis with hyperlipidemia. Kardiologiia 2009;49(9):21-6. [PubMed] [Google Scholar]
Nissen 2007 {published data only}
- Nissen SE, Nicholls SJ, Wolski K, Howey DC, McErlean E, Wang MD, et al. Effects of a potent and selective PPAR-alpha agonist in patients with atherogenic dyslipidemia or hypercholesterolemia: two randomized controlled trials. JAMA 2007;297(12):1362-73. [DOI] [PubMed] [Google Scholar]
O'Neill 2001 {published data only}
- O'Neill FH, Patel DD, Knight BL, Neuwirth CK, Bourbon M, Soutar AK, et al. Determinants of variable response to statin treatment in patients with refractory familial hypercholesterolemia. Arteriosclerosis, Thrombosis, & Vascular Biology 2001;21(5):832-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Okazaki 2004 {published data only}
- Okazaki S, Yokoyama T, Miyauchi K, Shimada K, Kurata T, Sato H, et al. Early statin treatment in patients with acute coronary syndrome: demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: the ESTABLISH Study. Circulation 2004;110(9):1061-8. [DOI] [PubMed] [Google Scholar]
Okopien 2004 {published data only}
- Okopien B, Krysiak R, Herman ZS. Effect of monthly atorvastatin treatment on hemostasis. International Journal of Clinical Pharmacology & Therapeutics 2004;42(11):589-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Okopien 2005 {published data only}
- Okopien B, Krysiak R, Haberka M, Herman ZS. Effect of monthly atorvastatin and fenofibrate treatment on monocyte chemoattractant protein-1 release in patients with primary mixed dyslipidemia. Journal of Cardiovascular Pharmacology 2005;45(4):314-20. [DOI] [PubMed] [Google Scholar]
Olivotti 2002 {published data only}
- Olivotti L, Ghigliotti G, Spallarossa P, Leslie S, Rossettin P, Barsotti A, et al. High doses of atorvastatin do not affect activity of prothrombinase in patients with acute coronary syndromes. Blood Coagulation & Fibrinolysis 2002;13(4):315-22. [DOI] [PubMed] [Google Scholar]
Olsson 2001 {published data only}
- Olsson AG, Pears J, McKellar J, Mizan J, Raza A. Effect of rosuvastatin on low-density lipoprotein cholesterol in patients with hypercholesterolemia. American Journal of Cardiology 2001;88(5):504-8. [DOI] [PubMed] [Google Scholar]
Olsson 2005 {published data only}
- Olsson AG, Schwartz GG, Szarek M, Sasiela WJ, Ezekowitz MD, Ganz P, et al. High-density lipoprotein, but not low-density lipoprotein cholesterol levels influence short-term prognosis after acute coronary syndrome: results from the MIRACL trial. European Heart Journal 2005;26(9):890-6. [DOI] [PubMed] [Google Scholar]
Olsson 2007 {published data only}
- Olsson AG, Schwartz GG, Szarek M, Luo D, Jamieson MJ. Effects of high-dose atorvastatin in patients > or =65 years of age with acute coronary syndrome (from the myocardial ischemia reduction with aggressive cholesterol lowering [MIRACL] study). American Journal of Cardiology 2007;99(5):632-5. [DOI] [PubMed] [Google Scholar]
Ooi 2012 {published data only}
- Ooi EMM, Ng TWK, Watts GF, Chan DC, Barrett PHR. Effect of fenofibrate and atorvastatin on VLDL apoE metabolism in men with the metabolic syndrome. Journal of Lipid Research 2012;53(11):2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oranje 2001 {published data only}
- Oranje WA, Sels JP, Rondas-Colbers GJ, Lemmens PJ, Wolffenbuttel BH. Effect of atorvastatin on LDL oxidation and antioxidants in normocholesterolemic type 2 diabetic patients. Clinica Chimica Acta 2001;311(2):91-4. [DOI] [PubMed] [Google Scholar]
Ormiston 2004 {published data only}
- Ormiston T, Wolkowitz OM, Reus VI, Johnson R, Manfredi F. Hormonal changes with cholesterol reduction: a double-blind pilot study. Journal of Clinical Pharmacy & Therapeutics 2004;29(1):71-3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Orr 2009 {published data only}
- Orr JS, Dengo AL, Rivero JM, Davy KP. Arterial destiffening with atorvastatin in overweight and obese middle-aged and older adults. Hypertension 2009;54(4):763-8. [DOI] [PubMed] [Google Scholar]
Oshima 2008 {published data only}
- Oshima E. LDL-cholesterol lowering therapy by atorvastatin have an impact on the carotid intima-media thickness in the hypercholesterolemia patient with type 2 diabetes. Therapeutic Research 2008;29(11):1933-9. [Google Scholar]
Ozkiris 2007 {published data only}
- Ozkiris A, Erkilic K, Koc A, Mistik S. Effect of atorvastatin on ocular blood flow velocities in patients with diabetic retinopathy. British Journal of Ophthalmology 2007;91(1):69-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Packard 2007 {published data only}
- Packard RR, Schlegel S, Senouf D, Burger F, Sigaud P, Perneger T, et al. Atorvastatin treatment and vaccination efficacy. Journal of Clinical Pharmacology 2007;47(8):1022-7. [DOI] [PubMed] [Google Scholar]
Paiva 2003 {published data only}
- Paiva H, Laasko J, Lehtimaki T, Isomustajarvi M, Ruokonene I, Laaksonen R. Effect of high-dose statin treatment on plasma concentrations of endogenous nitric oxide synthase inhibitors. Journal of Cardiovascular Pharmacology 2003;41(2):219-22. [DOI] [PubMed] [Google Scholar]
Paiva 2005 {published data only}
- Paiva H, Thelen KM, Van Coster R, Smet J, De Paepe B, Mattila KM, et al. High-dose statins and skeletal muscle metabolism in humans: a randomized, controlled trial. Clinical Pharmacology & Therapeutics 2005;78(1):60-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Panahi 2011 {published data only}
- Panahi Y, Pishgoo B, Beiraghdar F, Araghi Z M, Sahebkar A, Abolhasani E. Results of a randomized,open-label,clinical trial investigating the effects of supplementation with Heracleumpersicum extract as an adjunctive therapy for dyslipidemia. Thescientificworldjournal 2011;11:592-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paolisso 2000 {published data only}
- Paolisso G, Barbagallo M, Petrella G, Ragno E, Barbieri M, Giordano M, et al. Effects of simvastatin and atorvastatin administration on insulin resistance and respiratory quotient in aged dyslipidemic non-insulin dependent diabetic patients. Atherosclerosis 2000;150(1):121-7. [DOI] [PubMed] [Google Scholar]
Papathanasiou 2008 {published data only}
- Papathanasiou AI, Lourida ES, Tsironis LD, Goudevenos JA, Tselepis AD. Short- and long-term elevation of autoantibody titers against oxidized LDL in patients with acute coronary syndromes. Role of the lipoprotein-associated phospholipase A2 and the effect of atorvastatin treatment. Atherosclerosis 2008;196(1):289-97. [DOI] [PubMed] [Google Scholar]
Parini 2008 {published data only}
- Parini P, Gustafsson U, Davis MA, Larsson L, Einarsson C, Wilson M, et al. Cholesterol synthesis inhibition elicits an integrated molecular response in human livers including decreased ACAT2. Arteriosclerosis, Thrombosis, & Vascular Biology 2008;28(6):1200-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parker 2011 {published data only}
- Parker BA, Capizzi JA, Augeri AL, Grimaldi AS, Michael White C, Thompson PD. Atorvastatin increases exercise leg blood flow in healthy adults. Atherosclerosis 2011;219(2):768-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parker 2013 {published data only}
- Parker BA, Capizzi JA, Grimaldi AS, Clarkson PM, Cole SM, Keadle J, et al. Effect of statins on skeletal muscle function. Circulation 2013;127(1):96-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pedro‐Botet 2001 {published data only}
- Pedro-Botet J, Schaefer EJ, Bakker-Arkema RG, Black DM, Stein EM, Corella D, et al. Apolipoprotein E genotype affects plasma lipid response to atorvastatin in a gender specific manner. Atherosclerosis 2001;158(1):183-93. [DOI] [PubMed] [Google Scholar]
Peng 2018 {published data only}
- Peng Y, Ou BQ, Li HH, Liang FL. Atorvastatin and folic acid improve cardiac function and inhibit ventricular remodeling in chronic heart failure patients with hyperhomocysteinemia. Journal of the Hong Kong College of Cardiology 2018;26(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petri 2011 {published data only}
- Petri MA, Kiani AN, Post W, Christopher-Stine L, Magder LS. Lupus Atherosclerosis Prevention Study (LAPS). Annals of the Rheumatic Diseases 2011;70(5):760-5. [DOI] [PubMed] [Google Scholar]
Pfiser 2003 {published data only}
- Pfiser. The atorvastatin efficacy and safety study in diabetic patients to determine starting dose for effectively reducing lipids. (The ADSL study). Protocol No. A2581080 2003.
Pfizer 2003a {published data only}
- Pfizer Inc. A Multi-National, Prospective Randomized Double-Blind, Multi-Center, Placebo-Controlled Study To Evaluate Efficacy And Safety Of A Fixed Combination Therapy Of Amlodipine And Atorvastatin In The Treatment Of Concurrent Hypertension And Hyperlipidemia - Th. Protocol No. A3841003 2003.
Pfizer 2003b {published data only}
- Pfizer Inc. Double-blind, placebo-controlled, dose ranging trial to evaluate the efficacy of atorvastatin on bone mineral density and markers for bone turnover in postmenopausal women with dyslipidemia and at risk for osteoporosis. Protocol No. A2581049 2003.
Pfizer 2003c {published data only}
- Pfizer Inc. A Multicenter, Randomized, Double-Blind, Placebo-Controlled And Open-Label Evaluation Of The Safety And Efficacy Of Dual Therapy With Atorvastatin Plus Amlodipine When Compared To Either Therapy Alone In The Treatment Of Patients With Simultaneous Hyperlipidemia And Hypertension. (The Avalon Study). Protocol no. A3841001 2003.
Pfizer 2004a {published data only}
- Pfizer Inc. A 4-year, double-blind, randomized, placebo-controlled, study of atorvastatin as prevention of CHD endpoints in patients with (Type II) non-insulin dependent diabetes mellitus. (ASPEN). Protocol No. 0981-071 2004.
Pfizer 2004b {published data only}
- Pfizer Inc. Impact Of Atorvastatin On The Distribution, Composition And Metabolism Of LDL (Low Density Lipoprotein) And HDL (High Density Lipoprotein) Subfractions: A Double-Blind Placebo-Controlled Phase IV Study With Patients Suffering From Combined Hyperlipidemia And Diabetes - Atorvastatin And LDL Profile In NIDDM (ALPIN Study). https://www.clinicaltrials.gov/ct2/show/NCT00640549 2004.
Pfizer 2004c {published data only}
- Pfizer Inc. A multicenter, double blind, randomized, placebo controlled parallel group study to assess the efficacy and safety of atorvastatin in the treatment of men with lower urinary tract symptoms and urinary outflow obstruction associated with benign prostatic enlargement. Protocol No. A2581125 2004.
Pfizer 2005 {published data only}
- Pfizer Inc. Phase 2, multi-center, double-blind, placebo-controlled, randomized, parallel group, dose response study of the safety and efficacy of CP-529,414 combination with open-label atorvastatin once daily (QD) for 12 weeks in subjects with hypercholesterolemia and without overt cardiovascular disease. Pfizer 2005.
Pfizer 2006a {published data only}
- Pfizer Inc. A multi-center, randomized, open-label study to evaluate efficacy and safety of dual therapy with atorvastatin plus amlodipine when compared to amlodipine therapy alone in the treatment of subjects with concurrent hyperlipidemia and hypertension. Protocol No. A3841026 2006.
Pfizer 2006b {published data only}
- Pfizer Inc. A phase 3, double-blind, placebo-controlled, randomized, parallel group, multicenter study of the efficacy, safety and tolerability of fixed combination torcetrapib/atorvastatin administered orally, once daily for 6 months, compared to atorvastatin alone or placebo, in subjects with mixed dyslipidemia (Frederickson types IIa and IIb). Protocol No. A5091018 2006.
Pfizer 2007 {published data only}
- Pfizer Inc. Carotid Atorvastatin Study in Hyperlipidemic Post-MEnopausal Women: a Randomised Evaluation of Atorvastatin Versus Placebo (CASHMERE). Protocol A2581051. Http://pdf.clinicalstudyresults.org/documents/company-study_2902_0.pdf 2007:1-11.
Pfizer 2009 {published data only}
- Pfizer Inc. Atorvastatin and cardiovascular complications in Type II diabetes dialysis patients. The German Diabetes Dialysis Study: 4D Study. Determination of Cardiovascular endpoints in NIDDM dialysis patients. Protocol No. CT-981-423-239 2009.
Pfizer 2010 {published data only}
- Pfizer Inc. A double-blind, placebo-controlled study of atorvastatin as prevention of CHD in high risk patients with non-insulin-dependent diabetes mellitus(CARDS). Protocol No. 0981-430-102 2010.
Piperi 2004 {published data only}
- Piperi C, Kalofoutis C, Lagogianni I, Troupis G, Kalofoutis A. Comparison of raloxifene and atorvastatin effects on serum lipids composition of healthy post-menopausal women. Molecular & Cellular Biochemistry 2004;261(1):71-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Plazak 2011 {published data only}
- Plazak W, Gryga K, Dziedzic H, Tomkiewicz-Pajak L, Konieczynska M, Podolec P, et al. Influence of atorvastatin on coronary calcifications and myocardial perfusion defects in systemic lupus erythematosus patients: a prospective, randomized, double-masked, placebo-controlled study. Arthritis Research & Therapy 2011;13(4):R117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pons‐Rejraji 2014 {published data only}
- Pons-Rejraji H, Brugnon F, Sion B, Maqdasy S, Gouby G, Pereira B, et al. Evaluation of atorvastatin efficacy and toxicity on spermatozoa, accessory glands and gonadal hormones of healthy men: A pilot prospective clinical trial. Reproductive Biology & Endocrinology 2014;12(1):12. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pontrelli 2002 {published data only}
- Pontrelli L, Parris W, Adeli K, Cheung R C. Atorvastatin treatment beneficially alters the lipoprotein profile and increases low-density lipoprotein particle diameter in patients with combined dyslipidemia and impaired fasting glucose/type 2 diabetes. Metabolism: Clinical & Experimental 2002;51(3):334-42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Preston 2007 {published data only}
- Preston RA, Harvey P, Herfert O, Dykstra G, Jukema J W, Sun F, et al. A randomized, placebo-controlled trial to evaluate the efficacy, safety, and pharmacodynamic interaction of coadministered amlodipine and atorvastatin in 1660 patients with concomitant hypertension and dyslipidemia: the respond trial. Journal of Clinical Pharmacology 2007;47(12):1555-69. [DOI] [PubMed] [Google Scholar]
Raal 2000 {published data only}
- Raal FJ, Pappu AS, Illingworth DR, Pilcher GJ, Marais AD, Firth JC, et al. Inhibition of cholesterol synthesis by atorvastatin in homozygous familial hypercholesterolaemia. Atherosclerosis 2000;150(2):421-8. [DOI] [PubMed] [Google Scholar]
Raal 2003 {published data only}
- Raal FJ, Marais AD, Klepack E, Lovalvo J, McLain R, Heinonen T. Avasimibe, an ACAT inhibitor, enhances the lipid lowering effect of atorvastatin in subjects with homozygous familial hypercholesterolemia. Atherosclerosis 2003;171(2):273-9. [DOI] [PubMed] [Google Scholar]
Raison 2002 {published data only}
- Raison J, Rudnichi A, Safar ME. Effects of atorvastatin on aortic pulse wave velocity in patients with hypertension and hypercholesterolaemia: a preliminary study. Journal of Human Hypertension 2002;16(10):705-10. [DOI] [PubMed] [Google Scholar]
Reiter 2005 {published data only}
- Reiter M, Wirth S, Pourazim A, Baghestanian M, Minar E, Bucek RA. Statin therapy has no significant effect on skin tissue cholesterol: results from a prospective randomized trial. Clinical Chemistry 2005;51(1):252-4. [DOI] [PubMed] [Google Scholar]
Renders 2001 {published data only}
- Renders L, Mayer-Kadner I, Koch C, Scharffe S, Burkhardt K, Veelken R, et al. Efficacy and drug interactions of the new HMG-CoA reductase inhibitors cerivastatin and atorvastatin in CsA-treated renal transplant recipients. Nephrology, Dialysis, Transplantation 2001;16(1):141-6. [DOI] [PubMed] [Google Scholar]
Riahi 2006 {published data only}
- Riahi S, Schmidt EB, Amanavicius N, Karmisholt J, Jensen HS, Christoffersen RP, et al. The effect of atorvastatin on heart rate variability and lipoproteins in patients treated with coronary bypass surgery. International Journal of Cardiology 2006;111(3):436-41. [DOI] [PubMed] [Google Scholar]
Rosenson 2009 {published data only}
- Rosenson RS, Otvos JD, Hsia J. Effects of rosuvastatin and atorvastatin on LDL and HDL particle concentrations in patients with metabolic syndrome: a randomized, double-blind, controlled study. Diabetes Care 2009;32(6):1087-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sadik 2010 {published data only}
- Sadik HY, Moore TL, Vail A, Murray A, Anderson M, Blann A, et al. Lack of effect of 8 weeks atorvastatin on microvascular endothelial function in patients with systemic sclerosis. Rheumatology 2010;49(5):990-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Samentzas 2015 {published data only}
- Samentzas AA, Sionis G, Papadimitriou D, Papasaikas D, Blazakis G, Paschalis A, et al. The effect of statins in erectile dysfunction. European Journal of Heart Failure 2015;17(Supplement 1):243. [Google Scholar]
Samy 2011 {published data only}
- Samy W, Hassanian MA. Paraoxonase-1 activity, malondialdehyde and glutathione peroxidase in non-alcoholic fatty liver disease and the effect of atorvastatin. Arab Journal of Gastroenterology 2011;12(2):80-5. [DOI] [PubMed] [Google Scholar]
Sardo 2002 {published data only}
- Sardo MA, Castaldo M, Cinquegrani M, Bonaiuto M, Maesano A, Versace A, et al. Effects of atorvastatin treatment on sICAM-1 and plasma nitric oxide levels in hypercholesterolemic subjects. Clinical & Applied Thrombosis/Hemostasis 2002;8(3):257-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sardo 2005 {published data only}
- Sardo MA, Campo S, Bonaiuto M, Bonaiuto A, Saitta C, Trimarchi G, et al. Antioxidant effect of atorvastatin is independent of PON1 gene T(-107)C, Q192R and L55M polymorphisms in hypercholesterolaemic patients. Current Medical Research & Opinion 2005;21(5):777-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sasmazel 2010 {published data only}
- Sasmazel A, Baysal A, Fedekar A, Buyukbayrak F, Bugra O, Erdem H, et al. The effect of statin therapy on stimulation of endothelium-derived nitric oxide before and after coronary artery bypass surgery. Heart Surgery Forum 2010;13(4):E243-6. [DOI] [PubMed] [Google Scholar]
Sathyapalan 2019 {published data only}
- Sathyapalan T, Hobkirk JP, Javed Z, Carroll S, Coady AM, Pemberton P, et al. The effect of atorvastatin (and subsequent metformin) on adipose tissue acylation-stimulatory-protein concentration and inflammatory biomarkers in overweight/obese women with polycystic ovary syndrome. Frontiers in Endocrinology 2019;10(JUN):Article Number: 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schaefer 2002 {published data only}
- Schaefer EJ, McNamara JR, Tayler T, Daly JA, Gleason JA, Seman LJ, et al. Effects of atorvastatin on fasting and postprandial lipoprotein subclasses in coronary heart disease patients versus control subjects. American Journal of Cardiology 2002;90(7):689-96. [DOI] [PubMed] [Google Scholar]
Schaefer 2004 {published data only}
- Schaefer EJ, McNamara JR, Tayler T, Daly JA, Gleason JL, Seman LJ, et al. Comparisons of effects of statins (atorvastatin, fluvastatin, lovastatin, pravastatin, and simvastatin) on fasting and postprandial lipoproteins in patients with coronary heart disease versus control subjects. American Journal of Cardiology 2004;93(1):31-9. [DOI] [PubMed] [Google Scholar]
Schaefer 2005 {published data only}
- Schaefer EJ, McNamara JR, Asztalos BF, Tayler T, Daly JA, Gleason JL, et al. Effects of atorvastatin versus other statins on fasting and postprandial C-reactive protein and lipoprotein-associated phospholipase A2 in patients with coronary heart disease versus control subjects. American Journal of Cardiology 2005;95(9):1025-32. [DOI] [PubMed] [Google Scholar]
Schanberg 2012 {published data only}
- Schanberg LE, Sandborg C, Barnhart HX, Ardoin SP, Yow E, Evans GW, et al. Use of atorvastatin in systemic lupus erythematosus in children and adolescents. Arthritis & Rheumatism 2012;64(1):285-96. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schneider 2004 {published data only}
- Schneider JG, Eynatten M, Parhofer KG, Volkmer JE, Schiekofer S, Hamann A, et al. Atorvastatin improves diabetic dyslipidemia and increases lipoprotein lipase activity in vivo. Atherosclerosis 2004;175(2):325-31. [DOI] [PubMed] [Google Scholar]
Schrott 1998 {published data only}
- Schrott H, Fereshetian AG, Knopp RH, Bays H, Jones PH, Littlejohn III TW, et al. A multicenter, placebo-controlled, dose-ranging study of atorvastatin. Journal of Cardiovascular Pharmacology & Therapeutics 1998;3(2):119-24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sdringola 2008 {published data only}
- Sdringola S, Gould KL, Zamarka LG, McLain R, Garner J. A 6 month randomized, double blind, placebo controlled, multi-center trial of high dose atorvastatin on myocardial perfusion abnormalities by positron emission tomography in coronary artery disease. American Heart Journal 2008;155(2):245-53. [DOI] [PubMed] [Google Scholar]
See 2003 {published data only}
- See VY, DeNofrio D, Goldberg L, Chang G, Sasseen B, Kolansky DM, et al. Effect of atorvastatin on postcardiac transplant increase in low-density lipoprotein cholesterol reduces development of intimal hyperplasia and progression of endothelial dysfunction. American Journal of Cardiology 2003;92(1):11-5. [DOI] [PubMed] [Google Scholar]
Sever 2003 {published data only}
- Sever PS, Dahlof B, Poulter N R, Wedel H, Beevers G, Caulfield M, et al for the ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trials-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361(9364):1149-58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sever 2005 {published data only}
- Sever PS, Poulter NR, Dahlof B, Wedel H, Anglo-Scandinavian Cardiac Outcomes Trial Investigators. Different time course for prevention of coronary and stroke events by atorvastatin in the Anglo-Scandinavian Cardiac Outcomes Trial - Lipid Lowering Arm (ASCOT-LLA). American Journal of Cardiology 2005;96(5A):39F-44F. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sever 2010 {published data only}
- Sever PS, Poulter NR, Dahlof B, Wedel H, ASCOT Investigators. Antihypertensive therapy and the benefits of atorvastatin in the Anglo-Scandinavian Cardiac Outcomes Trial: lipid-lowering arm extension. Journal of Hypertension 2010;27(5):947-54. [DOI] [PubMed] [Google Scholar]
Sharma 2001 {published data only}
- Sharma AK. SPARCL Study. A double-blind, randomised, placebo-controlled study of Atorvastatin as prevention of cerebrovascular events in patients with a previous transient ischaemic attack or stroke. National Research Register. Issue 1 2001.
Sillesen 2007a {published data only}
- Sillesen H, Amarenco P, Szarek M, Callahan A, Goldstein LB, Hennerici M, et al. Atorvastatin treatment in patients with carotid stenosis is associated with a marked reduction in the risk of stroke, cardiac events and endarterectomy: a substudy of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke; a journal of cerebral circulation 2007;38(2):457. [CENTRAL: CN-00603188] [Google Scholar]
Sillesen 2007b {published data only}
- Sillesen H, Fernandes JG, Berwanger O, Szarek M, Amarenco P, Callahan A, et al. Atorvastatin treatment in patients with carotid stenosis is associated with a marked reduction in the risk of stroke, cardiovascular events and carotid revascularisation procedures. A substudy of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Trial. Cerebrovascular diseases (basel, switzerland) 2007;23(Suppl 2):55. [CENTRAL: CN-00603199] [Google Scholar]
Sillesen 2008 {published data only}
- Sillesen H, Amarenco P, Hennerici M G, Callahan A, Goldstein L B, Zivin J, et al for the Stroke Prevention by Aggressive Reduction in Cholesterol Levels Investigators. Atorvastatin reduces the risk of cardiovascular events in patients with carotid atherosclerosis: a secondary analysis of the stroke prevention by aggressive reduction in cholesterol levels (SPARCL) trial. Stroke 2008;39(12):3297-302. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Singh 2008 {published data only}
- Singh U, Devaraj S, Jialal I, Siegel D. Comparison effect of atorvastatin (10 versus 80 mg) on biomarkers of inflammation and oxidative stress in subjects with metabolic syndrome. American Journal of Cardiology 2008;102(3):321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinski 2009 {published data only}
- Sinski M, Lewandowski J, Ciarka A, Bidiuk J, Abramczyk P, Dobosiewicz A, et al. Atorvastatin reduces sympathetic activity and increases baroreceptor reflex sensitivity in patients with hypercholesterolaemia and systemic arterial hypertension. Kardiologia Polska 2009;67(6):613-20. [PubMed] [Google Scholar]
Soedamah‐Muthu 2003 {published data only}
- Soedamah-Muthu SS, Colhoun HM, Thomason MJ, Betteridge DJ, Durrington PN, Hitman GA, et al. The effect of atorvastatin on serum lipids, lipoproteins and NMR spectroscopy defined lipoprotein subclasses in type 2 diabetic patients with ischaemic heart disease. Atherosclerosis 2003;167(2):243-55. [DOI] [PubMed] [Google Scholar]
Soedamah‐Muthu 2015 {published data only}
- Soedamah-Muthu SS, Livingstone SJ, Charlton-Menys V, Betteridge DJ, Hitman GA, Neil HA, et al. Effect of atorvastatin on C-reactive protein and benefits for cardiovascular disease in patients with type 2 diabetes: analyses from the Collaborative Atorvastatin Diabetes Trial DBP - 20150422. Diabetologia 2015;58(7):1494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sola 2006 {published data only}
- Sola S, Mir MQ, Lerakis S, Tandon N, Khan BV. Atorvastatin improves left ventricular systolic function and serum markers of inflammation in nonischemic heart failure. Journal of the American College of Cardiology 2006;47(2):332-7. [DOI] [PubMed] [Google Scholar]
Solem 2006 {published data only}
- Solem J, Levin M, Karlsson T, Grip L, Albertsson P, Wiklund O. Composition of coronary plaques obtained by directional atherectomy in stable angina: its relation to serum lipids and statin treatment. Journal of Internal Medicine 2006;259(3):267-75. [DOI] [PubMed] [Google Scholar]
Sparks 2005 {published data only}
- Sparks DL, Sabbagh MN, Connor DJ, Lopez J, Launer LJ, Petanceska S, et al. Atorvastatin therapy lowers circulating cholesterol but not free radical activity in advance of identifiable clinical benefit in the treatment of mild-to-moderate AD. Current Alzheimer Research 2005;2(3):343-53. [DOI] [PubMed] [Google Scholar]
Sposito 2003 {published data only}
- Sposito AC, Santos RD, Amancio RF, Ramires JA, Chapman MJ, Maranhao RC. Atorvastatin enhances the plasma clearance of chylomicron-like emulsions in subjects with atherogenic dyslipidemia: relevance to the in vivo metabolism of triglyceride-rich lipoproteins. Atherosclerosis 2003;166(2):311-21. [DOI] [PubMed] [Google Scholar]
Stalenhoef 2005 {published data only}
- Stalenhoef AF, Ballantyne CM, Sarti C, Murin J, Tonstad S, Rose H, et al. A comparative study with rosuvastatin in subjects with metabolic syndrome: results of the COMETS study. European Heart Journal 2005;26(24):2664-72. [DOI] [PubMed] [Google Scholar]
Stefanadi 2009 {published data only}
- Stefanadi E, Tousoulis D, Antoniades C, Katsi V, Bosinakou E, Vavuranakis E, et al. Early initiation of low-dose atorvastatin treatment after an acute ST-elevated myocardial infarction, decreases inflammatory process and prevents endothelial injury and activation. International Journal of Cardiology 2009;133(2):266-8. [DOI] [PubMed] [Google Scholar]
Stein 2012 {published data only}
- Stein EA, Mellis S, Yancopoulos GD, Stahl N, Logan D, Smith WB, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. New England Journal of Medicine 2012;366(12):1108-18. [DOI] [PubMed] [Google Scholar]
Strey 2005 {published data only}
- Strey CH, Young JM, Molyneux SL, George PM, Florkowski CM, Scott RS, et al. Endothelium-ameliorating effects of statin therapy and coenzyme Q10 reductions in chronic heart failure. Atherosclerosis 2005;179(1):201-6. [DOI] [PubMed] [Google Scholar]
Strey 2006 {published data only}
- Strey CH, Young JM, Lainchbury JH, Frampton CM, Nicholls MG, Richards AM, et al. Short-term statin treatment improves endothelial function and neurohormonal imbalance in normocholesterolaemic patients with non-ischaemic heart failure. Heart 2006;92(11):1603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suleiman 2012 {published data only}
- Suleiman M, Koestler C, Lerman A, Lopez-Jimenez F, Herges R, Hodge D, et al. Atorvastatin for prevention of atrial fibrillation recurrence following pulmonary vein isolation: a double-blind, placebo-controlled, randomized trial. Heart Rhythm 2012;9(2):172-8. [DOI] [PubMed] [Google Scholar]
Szramka 2007 {published data only}
- Szramka M, Harriss L, Ninnio D, Windebank E, Brack J, Skiba M, et al. The effect of rapid lipid lowering with atorvastatin on autonomic parameters in patients with coronary artery disease. International Journal of Cardiology 2007;117(2):287-91. [DOI] [PubMed] [Google Scholar]
Tan 2002 {published data only}
- Tan KC, Chow WS, Tam SC, Ai VH, Lam CH, Lam KS. Atorvastatin lowers C-reactive protein and improves endothelium-dependent vasodilation in type 2 diabetes mellitus. Journal of Clinical Endocrinology & Metabolism 2002;87(2):563-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tanaka 2001 {published data only}
- Tanaka A, Yamada N, Saito Y, Kawakami M, Ohashi Y, Akanuma Y. A double-blind trial on the effects of atorvastatin on glycemic control in Japanese diabetic patients with hypercholesterolemia. Clinica Chimica Acta 2001;312(1-2):41-7. [DOI] [PubMed] [Google Scholar]
Tanaka 2009 {published data only}
- Tanaka M. Effect of atorvastatin on lipid profile and blood glucose level in hypercholesterolemic type 2 diabetic patients. Therapeutic Research 2009;30(10):1631-7. [Google Scholar]
Taneva 2006 {published data only}
- Taneva E, Borucki K, Wiens L, Makarova R, Schmidt-Lucke C, Luley C, et al. Early effects on endothelial function of atorvastatin 40 mg twice daily and its withdrawal. American Journal of Cardiology 2006;97(7):1002-6. [DOI] [PubMed] [Google Scholar]
Tannous 1999 {published data only}
- Tannous M, Cheung R, Vignini A, Mutus B. Atorvastatin increases ecNOS levels in human platelets of hyperlipidemic subjects. Thrombosis & Haemostasis 1999;82(5):1390-4. [MEDLINE: ] [PubMed] [Google Scholar]
Tanriverdi 2005 {published data only}
- Tanriverdi HA, Barut A, Sarikaya S. Statins have additive effects to vertebral bone mineral density in combination with risedronate in hypercholesterolemic postmenopausal women. European Journal of Obstetrics, Gynecology, & Reproductive Biology 2005;120(1):63-8. [DOI] [PubMed] [Google Scholar]
Tantikosoom 2005 {published data only}
- Tantikosoom W, Thinkhamrop B, Kiatchusakul S, Jarernsiripornkul N, Srinakarin J, Ojongpian S. Randomized trial of atorvastatin in improving endothelial function in diabetics without prior coronary disease and having average cholesterol level. Chotmaihet Thangphaet [Journal of the Medical Association of Thailand] 2005;88(3):399-406. [PubMed] [Google Scholar]
Tehrani 2010 {published data only}
- Tehrani S, Mobarrez F, Antovic A, Santesson P, Lins PE, Adamson U, et al. Atorvastatin has antithrombotic effects in patients with type 1 diabetes and dyslipidemia. Thrombosis Research 2010;126(3):e225-31. [DOI] [PubMed] [Google Scholar]
Teshima 2009 {published data only}
- Teshima Y, Yufu K, Akioka H, Iwao T, Anan F, Nakagawa M, et al. Early atorvastatin therapy improves cardiac function in patients with acute myocardial infarction. Journal of Cardiology 2009;53(1):58-64. [DOI] [PubMed] [Google Scholar]
Tousoulis 2005a {published data only}
- Tousoulis D, Antoniades C, Bosinakou E, Kotsopoulou M, Pitsavos C, Vlachopoulos C, et al. Effects of atorvastatin on reactive hyperemia and inflammatory process in patients with congestive heart failure. Atherosclerosis 2005;178(2):359-63. [DOI] [PubMed] [Google Scholar]
Tousoulis 2005b {published data only}
- Tousoulis D, Antoniades C, Bosinakou E, Kotsopoulou M, Tsioufis C, Tentolouris C, et al. Effects of atorvastatin on reactive hyperaemia and the thrombosis-fibrinolysis system in patients with heart failure. Heart 2005;91(1):27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tousoulis 2005c {published data only}
- Tousoulis D, Antoniades C, Vassiliadou C, Toutouza M, Pitsavos C, Tentolouris C, et al. Effects of combined administration of low dose atorvastatin and vitamin E on inflammatory markers and endothelial function in patients with heart failure. European Journal of Heart Failure 2005;7(7):1126-32. [DOI] [PubMed] [Google Scholar]
Tousoulis 2006a {published data only}
- Tousoulis D, Antoniades C, Katsi V, Bosinakou E, Kotsopoulou M, Tsioufis C, et al. The impact of early administration of low-dose atorvastatin treatment on inflammatory process, in patients with unstable angina and low cholesterol level. International Journal of Cardiology 2006;109(1):48-52. [DOI] [PubMed] [Google Scholar]
Tousoulis 2006b {published data only}
- Tousoulis D, Bosinakou E, Kotsopoulou M, Antoniades C, Katsi V, Stefanadis C. Effects of early administration of atorvastatin treatment on thrombotic process in normocholesterolemic patients with unstable angina. International Journal of Cardiology 2006;106(3):333-7. [DOI] [PubMed] [Google Scholar]
Tousoulis 2007 {published data only}
- Tousoulis D, Antoniades C, Vasiliadou C, Kourtellaris P, Koniari K, Marinou K, et al. Effects of atorvastatin and vitamin C on forearm hyperaemic blood flow, asymmentrical dimethylarginine levels and the inflammatory process in patients with type 2 diabetes mellitus. Heart 2007;93(2):244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tousoulis 2010 {published data only}
- Tousoulis D, Koniari K, Antoniades C, Miliou A, Noutsou M, Nikolopoulou A, et al. Impact of 6 weeks of treatment with low-dose metformin and atorvastatin on glucose-induced changes of endothelial function in adults with newly diagnosed type 2 diabetes mellitus: A single-blind study. Clinical Therapeutics 2010;32(10):1720-8. [DOI] [PubMed] [Google Scholar]
Tousoulis 2011 {published data only}
- Tousoulis D, Koniari K, Antoniades C, Papageorgiou N, Miliou A, Noutsou M, et al. Combined effects of atorvastatin and metformin on glucose-induced variations of inflammatory process in patients with diabetes mellitus. International Journal of Cardiology 2011;149(1):46-9. [DOI] [PubMed] [Google Scholar]
Tremblay 2011 {published data only}
- Tremblay AJ, Lamarche B, Lemelin V, Hoos L, Benjannet S, Seidah NG, et al. Atorvastatin increases intestinal expression of NPC1L1 in hyperlipidemic men. Journal of Lipid Research 2011;52(3):558-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsai 2008 {published data only}
- Tsai CT, Lai LP, Hwang JJ, Wang YC, Chiang FT, Lin JL. Atorvastatin prevents atrial fibrillation in patients with bradyarrhythmias and implantation of an atrial-based or dual-chamber pacemaker: a prospective randomized trial. American Heart Journal 2008;156(1):65-70. [DOI] [PubMed] [Google Scholar]
Urso 2005 {published data only}
- Urso ML, Clarkson PM, Hittel D, Hoffman EP, Thompson PD. Changes in ubiquitin proteasome pathway gene expression in skeletal muscle with exercise and statins. Arteriosclerosis, Thrombosis & Vascular Biology 2005;25(12):2560-6. [DOI] [PubMed] [Google Scholar]
van de Ree 2003 {published data only}
- de Ree MA, Huisman MV, Princen HM, Meinders AE, Kluft C, DALI-Study Group. Strong decrease of high sensitivity C-reactive protein with high-dose atorvastatin in patients with type 2 diabetes mellitus. Atherosclerosis 2003;166(1):129-35. [DOI] [PubMed] [Google Scholar]
Van De Ree 2003 {published data only}
- Van De Ree MA, De Maat MP, Kluft C, Meinders AE, Princen HM, Huisman MV, DALI Study Group. Decrease of hemostatic cardiovascular risk factors by aggressive vs. conventional atorvastatin treatment in patients with Type 2 diabetes mellitus. Journal of Thrombosis & Haemostasis 2003;1(8):1753-7. [DOI] [PubMed] [Google Scholar]
van der Harst 2005 {published data only}
- Harst P, Wagenaar LJ, Buikema H, Voors AA, Plokker HW, Morshuis WJ, et al. Effect of intensive versus moderate lipid lowering on endothelial function and vascular responsiveness to angiotensin II in stable coronary artery disease. American Journal of Cardiology 2005;96(10):1361-4. [DOI] [PubMed] [Google Scholar]
van Doorn 2006 {published data only}
- Doorn MB, Espirito Santo SM, Meijer P, Kamerling I, Schoemaker RC, Dirsch V, et al. Effect of garlic powder on C-reactive protein and plasma lipids in overweight and smoking subjects. American Journal of Clinical Nutrition 2006;84(6):1324-9. [DOI] [PubMed] [Google Scholar]
van Hoek 2009 {published data only}
- Hoek M, Tol A, Vark-van der Zee LC, Jansen H, Kastelein JJ, Sijbrands EJ, et al. Role of plasma adiponectin on the HDL-cholesterol raising effect of atorvastatin in patients with type 2 diabetes. Current Medical Research & Opinion 2009;25(1):93-101. [DOI] [PubMed] [Google Scholar]
Van J 2002 {published data only}
- Van JT, Pan J, Wasty T, Chan E, Wu X, Charles MA. Comparison of extended-release niacin and atorvastatin mono-therapies and combination treatment of the atherogenic lipid profile in diabetes mellitus. American Journal of Cardiology 2002;89(11):1306-8. [DOI] [PubMed] [Google Scholar]
Vansant 2001 {published data only}
- Vansant G, Mertens A, Muls E. The effect of atorvastatin on postprandial lipidaemia in overweight or obese women homozygous for apo E3. Acta Cardiologica 2001;56(3):149-54. [DOI] [PubMed] [Google Scholar]
van Venrooij 2002 {published data only}
- Venrooij FV, de Ree MA, Bots ML, Stolk RP, Huisman MV, Banga JD, DALI Study Group. Aggressive lipid lowering does not improve endothelial function in type 2 diabetes: the Diabetes Atorvastatin Lipid Intervention (DALI) Study: a randomized, double-blind, placebo-controlled trial. Diabetes Care 2002;25(7):1211-6. [DOI] [PubMed] [Google Scholar]
van Venrooij 2003 {published data only}
- Venrooij FV, Stolk RP, Banga JD, Sijmonsma TP, Tol A, Erkelens DW, et al for the DALI Study Group. Common cholesteryl ester transfer protein gene polymorphisms and the effect of atorvastatin therapy in type 2 diabetes. Diabetes Care 2003;26(4):1216-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vernaglione 2004 {published data only}
- Vernaglione L, Cristofano C, Muscogiuri P, Chimienti S. Does atorvastatin influence serum C-reactive protein levels in patients on long-term hemodialysis? American Journal of Kidney Diseases 2004;43(3):471-8. [DOI] [PubMed] [Google Scholar]
von Eynatten 2009 {published data only}
- Eynatten M, Liu D, Bluemm A, Schuster T, Baumann M, Lutz J, et al. Changes in adiponectin multimer distribution in response to atorvastatin treatment in patients with type 2 diabetes. Clinical Endocrinology 2009;71(1):27-32. [DOI] [PubMed] [Google Scholar]
Vrtovec 2005 {published data only}
- Vrtovec B, Okrajsek R, Golicnik A, Ferjan M, Starc V, Radovancevic B. Atorvastatin therapy increases heart rate variability, decreases QT variability, and shortens QTc interval duration in patients with advanced chronic heart failure. Journal of Cardiac Failure 2005;11(9):684-90. [DOI] [PubMed] [Google Scholar]
Wang 2001 {published data only}
- Wang KY, Ting CT. A randomized, double-blind, placebo-controlled, 8-week study to evaluate the efficacy and safety of once daily atorvastatin (10 mg) in patients with elevated LDL-cholesterol. Japanese Heart Journal 2001;42(6):725-38. [DOI] [PubMed] [Google Scholar]
Wanner 2005 {published data only}
- Wanner C, Krane V, Marz W, Olschewski M, Mann J F, Ruf G, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. New England Journal of Medicine 2005;353(3):238-48. [DOI] [PubMed] [Google Scholar]
Wassmann 2004 {published data only}
- Wassmann S, Ribaudo N, Faul A, Laufs U, Bohm M, Nickenig G. Effect of atorvastatin 80 mg on endothelial cell function (forearm blood flow) in patients with pretreatment serum low-density lipoprotein cholesterol levels <130 mg/dl. American Journal of Cardiology 2004;93(1):84-8. [DOI] [PubMed] [Google Scholar]
Waters 2000 {published data only}
- Waters D. Comparison of aggressive lipid lowering with Atorvastatin vs. Revascularization Treatments (AVERT) and conventional care for the reduction of ischemic events in patients with stable coronary artery disease. Cardiovascular Reviews & Reports 2000;21(1):26-31. [Google Scholar]
Watts 2003a {published data only}
- Watts GF, Barrett PH, Ji J, Serone AP, Chan DC, Croft K D, et al. Differential regulation of lipoprotein kinetics by atorvastatin and fenofibrate in subjects with the metabolic syndrome. Diabetes 2003;52(3):803-11. [DOI] [PubMed] [Google Scholar]
Watts 2003b {published data only}
- Watts GF, Chan DC, Barrett PH, O'Neill FH, Thompson GR. Effect of a statin on hepatic apolipoprotein B-100 secretion and plasma campesterol levels in the metabolic syndrome. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 2003;27(7):862-5. [DOI] [PubMed] [Google Scholar]
Williams 2009 {published data only}
- Williams B, Lacy PS, Cruickshank JK, Collier D, Hughes AD, Stanton A, et al for the CAFE and ASCOT Investigators. Impact of statin therapy on central aortic pressures and hemodynamics: principal results of the Conduit Artery Function Evaluation-Lipid-Lowering Arm (CAFE-LLA) Study. Circulation 2009;119(1):53-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wissing 2006 {published data only}
- Wissing KM, Unger P, Ghisdal L, Broeders N, Berkenboom G, Carpentier Y, et al. Effect of atorvastatin therapy and conversion to tacrolimus on hypercholesterolemia and endothelial dysfunction after renal transplantation. Transplantation 2006;82(6):771-8. [DOI] [PubMed] [Google Scholar]
Wojnicz 2006 {published data only}
- Wojnicz R, Wilczek K, Nowalany-Kozielska E, Szygula-Jurkiewicz B, Nowak J, Polonski L et al. Usefulness of atorvastatin in patients with heart failure due to inflammatory dilated cardiomyopathy and elevated cholesterol levels. American Journal of Cardiology 2006;97(6):899-904. [DOI] [PubMed] [Google Scholar]
Wu 2007 {published data only}
- Wu J, Wu G, Wang MP, Liu DL, Hu ZJ, Xu GY. [Effect of atorvastatin on carotid intima-medial of thickness of primary hypertension patients of Han nationality in China]. [Chinese]. Zhonghua Yi Xue Za Zhi 2007;87(31):2215-7. [PubMed] [Google Scholar]
Xia 2009 {published data only}
- Xia J, Zhou WZ, Liu XH. [Effects of low-dose atorvastatin in combination with aspirin on carotid atherosclerotic plaques and blood biochemical parameters in patients with cerebral infarction]. Chinese Journal of Cerebrovascular Diseases 2009;6(4):189-93. [Google Scholar]
Xie 2010 {published data only}
- Xie RQ, Cui W, Liu F, Yang C, Pei WN, Lu JC. Statin therapy shortens QTc, QTcd, and improves cardiac function in patients with chronic heart failure. International Journal of Cardiology 2010;140(2):255-7. [DOI] [PubMed] [Google Scholar]
Yamada 2007 {published data only}
- Yamada T, Node K, Mine T, Morita T, Kioka H, Tsukamoto Y, et al. Long-term effect of atorvastatin on neurohumoral activation and cardiac function in patients with chronic heart failure: a prospective randomized controlled study. American Heart Journal 2007;153(6):1055.e1-8. [DOI] [PubMed] [Google Scholar]
Yamada 2009 {published data only}
- Yamada K, Yoshimura S, Kawasaki M, Enomoto Y, Asano T, Minatoguchi S, et al. Effects of atorvastatin on carotid atherosclerotic plaques: a randomized trial for quantitative tissue characterization of carotid atherosclerotic plaques with integrated backscatter ultrasound. Cerebrovascular Diseases 2009;28(4):417-24. [DOI] [PubMed] [Google Scholar]
Yokoyama 2005 {published data only}
- Yokoyama M, Komiyama N, Courtney BK, Nakayama T, Namikawa S, Kuriyama N, et al. Plasma low-density lipoprotein reduction and structural effects on coronary atherosclerotic plaques by atorvastatin as clinically assessed with intravascular ultrasound radio-frequency signal analysis: a randomized prospective study. American Heart Journal 2005;150(2):287. [DOI] [PubMed] [Google Scholar]
Young 2008 {published data only}
- Young JM, Strey CH, George PM, Florkowski CM, Sies CW, Frampton CM, et al. Effect of atorvastatin on plasma levels of asymmetric dimethylarginine in patients with non-ischaemic heart failure. European Journal of Heart Failure 2008;10(5):463-6. [DOI] [PubMed] [Google Scholar]
Zeng 2012 {published data only}
- Zeng WJ, Xiong CM, Zhao L, Shan GL, Liu ZH, Xue F, et al for the Atorvastatin in Pulmonary Arterial Hypertension (APATH) Study Group. Atorvastatin in pulmonary arterial hypertension (Apath) Study. European Respiratory Journal 2012;40(1):67-74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhang 2009a {published data only}
- Zhang L. Effect of atorvastatin combined with Tongxinluo capsule on intra-media thickness of common carotid artery 91 patients with primary hypertension. China Tropical Medicine 2009;9:1314-5. [Google Scholar]
Zhang 2009b {published data only}
- Zhang J, Li J. Effects of different doses of atorvastatin on lipid levels and nervous function in patients with acute cerebral infarction. [Chinese]. Chinese Journal of Contemporary Neurology and Neurosurgery 2009;9(1):46-9. [Google Scholar]
Zheng 2019 {published data only}
- Zheng Y, Sun Z, Ding H. Effects of atorvastatin on growth differentiation factor 15, brain natriuretic peptide, cardiac troponin I and adiponectin in patients with acute myocardial infarction. Acta Medica Mediterranea 2019;35(5):2381-5. [Google Scholar]
Additional references
Adams 2015
- Adams SP, Tsang M, Wright JM. Atorvastatin for lowering lipids. Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No: CD008226. [DOI: 10.1002/14651858.CD008226.pub3] [DOI] [Google Scholar]
atorvastatin Adverse Reactions ‐ Epocrates Online
- atorvastatin Adverse Reactions - Epocrates Online. https://online.epocrates.com/drugs/87805/atorvastatin/Adverse-Reactions.
Brawer 2004
- Brawer MK. Testosterone replacement in men with andropause: an overview. Reviews in Urology 2004;6(Suppl 6):S9-S15. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Chapter 16
- Julian PT Higgins and Jonathan J Deeks. Chapter 16: Special topics in statistics.In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Chapter 8
- Julian PT Higgins, Douglas G Altman and Jonathan AC Sterne, editor(s). Chapter 8: Assessing risk of bias in included studies. In Julian PT Higgins, Douglas G Altman and Jonathan AC Sterne, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Covidence [Computer program]
- Covidence systematic review software. Veritas Health InnovationCovidence. Melbourne, Australia: Veritas Health Innovation, 2018. Available at www.covidence.org.
Data Extraction and Assessment Template
- Data Extraction and Assessment Template. https://training.cochrane.org/sites/training.cochrane.org/files/public/uploads/resources/downloadable_resources/English/Collecting%20data%20-%20form%20for%20RCTs%20only.doc 2011.
Demers 2010
- Demers LM. Androgen deficiency in women; role of accurate testosterone measurements. Maturitas 2010;67(1):39-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dunn 1981
- Dunn JF, NIisula BC, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. The Journal of Clinical Endocrinology & Metabolism 1981;53(1):58-68. [PMID: ] [DOI] [PubMed] [Google Scholar]
FDA 2011
- US Food and Drug Administration, Center for Drug Evaluation and Research. Atorvastatin calcium tablets for oral administration (approval package). www.accessdata.fda.gov/drugsatfda_docs/anda/2011/076477Orig1s000.pdf 2011.
Feldman 2002
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. The Journal of Clinical Endocrinology & Metabolism 2002;87(2):589-98. [PMID: ] [DOI] [PubMed] [Google Scholar]
Finkelstein 2013
- Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, Jones BF, Barry CV, Wulczyn KE, Thomas BJ, Leder BZ. Gonadal steroids and body composition, strength, and sexual function in men. New England Journal of Medicine 2013;369(11):1011-22. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Hennessy 2016
- Hennessy DA, Tanuseputro P, Tuna M, Bennett C, Perez R, Shields M, Ko DT, Tu J, Manuel DG. Population health impact of statin treatment in Canada. Health Reports 2016;27(1):20-8. [PMID: ] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ioannidis 2014
- Ioannidis JP. More than a billion people taking statins? Potential implications of the new cardiovascular guidelines. JAMA 2014;311(5):463. [PMID: ] [DOI] [PubMed] [Google Scholar]
IQVIA 2017
- IQVIA Institute for Human Data Science. Medicines use and spending in the U.S. A Review of 2016 and Outlook to 2021. www.iqvia.com/institute/reports/medicines-use-and-spending-in-the-us-a-review-of-2016 2017.
Jeong 2019
- Jeong Joon Hoon, Kim Yun Seong, Lee Sang Kwon. A case report of gynecomastia due to rosuvastatin. Korean Journal of Family Practice 2019;9(5):471-4. [Google Scholar]
Johnson 2009
- Johnson Ruth E, Murad M Hassan. Gynecomastia: Pathophysiology, Evaluation, and Management. Mayo Clinic Proceedings 2009;84(11):1010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati Alessandro, Altman Douglas G, Tetzlaff Jennifer, Mulrow Cynthia, Gøtzsche Peter C, Ioannidis John PA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed.) 2009;339. [DOI] [PMC free article] [PubMed]
Pagana 2015
- Pagana KD, Pagana TJ, Pagana TN. Mosby's Diagnostic and Laboratory Test Reference. 12th edition. Elsevier, 2015. [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberto 2012
- Roberto Giuseppe, Biagi Chiara, Montanaro Nicola, Koci Ariola, Moretti Ugo, Motola Domenico. Statin-associated gynecomastia: Evidence coming from the Italian spontaneous ADR reporting database and literature. European Journal of Clinical Pharmacology 2012;68(6):1007-11. [DOI] [PubMed] [Google Scholar]
Schaiff 2008
- Schaiff RA, Moe RM, Krichbaum DW. An overview of cholesterol management. American Health & Drug Benefits 2008;1(9):39-48. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Schiffer 2019
- Schiffer Lina, Barnard Lise, Baranowski Elizabeth S, Gilligan Lorna C, Taylor Angela E, Arlt Wiebke, et al. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: A comprehensive review. The Journal of steroid biochemistry and molecular biology 2019;194:105439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schooling 2013
- Schooling CM, Au Yeung SL, Freeman G, Cowling BJ. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Medicine 2013;11(1):57. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH, editor(s). Chapter 11: Presenting results and ’Summary of findings’ tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Available from guidelinedevelopment.org/handbook 2013.
Skeldon 2018
- Skeldon SC, Carleton B, Brophy JM, Sodhi M, Etminan M. Statin medications and the risk of gynecomastia. Clinical Endocrinology 2018;89(4):470-473. [DOI: 10.1111/cen.13794] [DOI] [PubMed] [Google Scholar]
Sriram 2010
- Sriram Dharmarajan, Yogeeswari Perumal. Medicinal Chemistry. Delhi: Pearson, 2010. [Google Scholar]
Stancu 2001
- Stancu C, Sima A. Statins: mechanism of action and effects. Journal of Cellular and Molecular Medicine 2001;5(4):378-87. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanworth 2008
- Stanworth RD, Jones TH. Testosterone for the aging male; current evidence and recommended practice. Clinical Interventions in Aging 2008;3(1):25-44. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Stone 2014
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA Guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Journal of the American College of Cardiology 2014;63(25 Pt B):2889-934. [DOI: 10.1016/j.jacc.2013.11.002] [DOI] [PubMed] [Google Scholar]
Sun 2015
- Sun J, Yuan Y, Cai R, Sun H, Zhou Y, Wang P, Huang R, Xia W, Wang S. An investigation into the therapeutic effects of statins with metformin on polycystic ovary syndrome: a meta-analysis of randomised controlled trials. BMJ Open 2015;5(3):e007280. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 2016
- Thompson PD, Panza G, Zaleski A, Taylor B. Statin-associated side effects. Journal of the American College of Cardiology 2016;67(20):2395-410. [PMID: ] [DOI] [PubMed] [Google Scholar]
Traish 2009
- Traish Abdulmaged M, Guay Andre, Feeley Robert, Saad Farid. The dark side of testosterone deficiency: I. Metabolic syndrome and erectile dysfunction. Journal of andrology 2009;30(1):10-22. [DOI] [PubMed] [Google Scholar]
Virani 2013
- Virani Salim S. Statins in the primary and secondary prevention of cardiovascular disease in women: Facts and myths. Texas Heart Institute Journal 2013///;40(3):288-289. [PMC free article] [PubMed] [Google Scholar]
Vrecer 2003
- Vrecer M, Turk S, Drinovec J, Mrhar A. Use of statins in primary and secondary prevention of coronary heart disease and ischemic stroke. Meta-analysis of randomized trials. International Journal of Clinical Pharmacology and Therapeutics 2003;41(12):567-77. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yang 2019
- Yang S, Gu YY, Jing F, Yu CX, Guan QB. The Effect of Statins on Levels of Dehydroepiandrosterone (DHEA) in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Medical Science Monitor 2019;25:590-597. [DOI: 10.12659/MSM.914128] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Shawish 2018
- Shawish MI, Bagheri B, Musini VM, Adams SP, Wright JM. Effect of atorvastatin on testosterone levels. Cochrane Database of Systematic Reviews 2018, Issue 12. Art. No: CD013211. [DOI: 10.1002/14651858.CD013211] [DOI] [PMC free article] [PubMed] [Google Scholar]


