Abstract
This review summarises and discusses recent findings concerning the pathophysiology, clinical presentation, diagnosis, treatment, and outcome of SARS-CoV-2-associated Guillain-Barre syndrome (SC2-GBS). By the end of December 2020, at least 220 patients with SC2-GBS have been published in 95 papers. SC2-GBS is most likely secondary due to an immune reaction against SARS-CoV-2 since the virus has not been found in the CSF of any SC2-GBS patient so far reported. SC2-GBS occurs in each age group and does not differ from non-SC2-GBS regarding clinical presentation and treatment, but the outcome of SC2-GBS is worse compared to non-CS2-GBS patients, and the prevalence/incidence of GBS most likely increased since the outbreak of the pandemic. Early diagnosis of SC2-GBS is warranted to apply appropriate treatment in due time and to improve the overall outcome from the infection.
Keywords: SARS-CoV-2, COVID-19, Guillain-Barre syndrome, Nerve conduction, Immunoglobulins
Introduction
Since the outbreak of the pandemic by the SARS-CoV-2 virus, it became rapidly obvious that the virus not only causes lung disease (COVID-19) but affects other organs as well, particularly the central and peripheral nervous system (PNS, CNS), the kidneys, the intestines, and the heart [1–3]. The most disabling PNS disorder is polyradiculitis (polyradiculoneuritis, Guillain-Barre syndrome (GBS)) [4]. GBS comprises a number of subtypes which include acute, inflammatory, demyelinating neuropathy (AIDP) (classic type), acute, motor, axonal neuropathy (AMAN), acute, motor and sensory, axonal neuropathy (AMSAN), Miller-Fisher syndrome (MFS), polyneuritis cranialis (PNC), the pharyngeal, cervical, and brachial (PCB) variant, and Bickerstaff encephalitis (BFE) [5]. GBS is usually diagnosed according to the Brighton criteria if there is bilateral, progressive, flaccid lower > upper limb paraparesis, if tendon reflexes in weak limbs are diminished, if the disease course is monophasic and if time between onset and nadir ranges from 12 h to 28 days, if cerebrospinal fluid (CSF) investigations reveal a cell count < 50cells/μL, if CSF protein is elevated (dissociation cyto-albuminque (DCA)), and if nerve conduction studies show a demyelinating lesion of motor nerves (AIDP), an axonal lesion of motor nerves (AMAN), or an axonal lesion of motor and sensory nerves (AMSAN) [5]. MFS is diagnosed if there is acute onset ophthalmoplegia, areflexia, ataxia, and DCA. PNC is diagnosed in case of a lesion of a single or multiple cranial nerves and DCA. PCB is diagnosed if there is progressive dysphagia, dysphonia, upper limb weakness, and DCA [5]. BFE is diagnosed if there are pyramidal signs and impaired consciousness in addition to MFS. In the early stages of GBS, upper or lower limb paraplegia with preserved tendon reflexes may occur [5]. There can be even hyperreflexia if the pyramidal tract is involved. All GBS subtypes occur in the setting of a preceding viral or bacterial infection. The type of preceding infection largely determines the subtype and clinical course of GBS. This systematised review summarises and discusses recent findings and future perspectives concerning the pathophysiology, clinical presentation, diagnosis, treatment, and outcome of SARS-CoV-2-associated GBS (SC2-GBS).
Methods
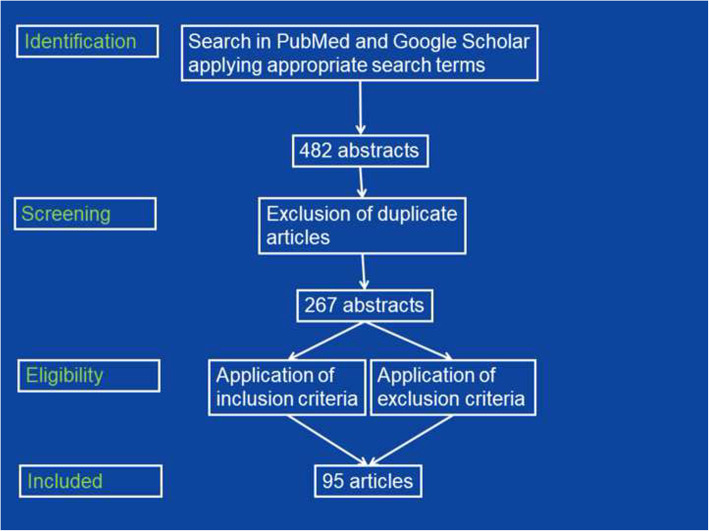
A systematised literature search in the databases PubMed and Google Scholar using the search terms “neuropathy,” “Guillain Barre syndrome,” “polyradiculitis,” “AIDP,” “AMAN,” “AMSAN,” “Miller-Fisher syndrome,” “polyneuritis cranialis,” and “Bickerstaff encephlaitis,” in combination with “SARS-CoV-2,” “COVID-19,” and “coronavirus” was conducted. Additionally, reference lists were checked for further articles meeting the search criteria. Included were only original articles detailing individual patients’ data (age, sex, latency between onset of COVID-19 and SC2-GBS, GBS subtype, results of CSF investigations, treatment, and outcome) and written in English, French, Spanish, Italian, or German, Excluded from data analysis were reviews, abstracts, proceedings, and editorials as well as original studies not specifying individual patients’ data (Fig. 1).
Fig. 1.
Flow chart detailing the search protocol and the results after application of inclusion and exclusion criteria
Main text
By the end of December 2020, at least 220 patients with SC2-GBS have been published in 95 papers (Tables 1 and 2). Age of these patients (reported in n = 215) ranged from 8–94 years (Table 1). Gender (reported in n = 213) was male in 146 and female in 67 (Tables 1 and 2). Onset (reported in n = 165) was identified after/together with/before onset of non-neurological COVID-19 manifestations in 156/3/6 patients (Tables 1 and 2). Latency between onset of COVID-19 and GBS (n = 194) ranged from − 10 to 90 days. One patient remained asymptomatic. The GBS subtype (reported in n = 152) was identified as AIDP (n = 118), AMAN (n = 13), AMSAN (n = 11), MFS (n = 7), PNC (n = 2), the PCB variant (n = 1), and BFE (n = 0). SARS-CoV-2 was not detected in the CSF in any of the patients (Table 1). Therapy of GBS (reported in n = 215) comprised intravenous immunoglobulins (IVIG) (n = 191), plasmapheresis (n = 15), steroids (n = 2), or no therapy (n = 7) (Tables 1 and 2). Forty-one patients required artificial ventilation (Tables 1 and 2). Outcome (reported in n = 168) was assessed as complete recovery (n = 37), partial recovery (n = 119), or death (n = 12) (Tables 1 and 2). No studies about factors determining the outcome of SC2-GBS subtypes were identified.
Table 1.
Patients with SARS-CoV-2 associated polyradiculitis as reported by the end of December 2020
| Age (years) | Sex | Onset | LOO (days) | Subtype | CIC | CM | IVIG | AV | Recovery | Country |
|---|---|---|---|---|---|---|---|---|---|---|
| 61 | f | B | 9 | AIDP | nr | No | Yes | No | Yes | China |
| 65 | m | A | 9 | AMSAN | nd | DM | Yes | No | nr | Iran |
| 54 | m | A | 8 | AIDP | nr | No | Yes | Yes | Yes | USA |
| 70 | f | A | 23 | AIDP | nd | No | Yes | Yes | nr | Italy |
| 66 | f | A | 7 | AIDP | No | nr | Yes | Yes | Yes | Italy |
| 54 | f | A | 21 | AIDP | nd | No | Yes | No | Yes | Germany |
| 70 | f | A | 3 | AMSAN | No | RA | Yes | No | Partial | Morocco |
| 20 | m | A | 5 | AMAN | nd | No | Yes | No | Yes | India |
| 71 | m | A | 4 | AIDP | No | AHT, AAR, LC | Yes | Yes | Death | Italy |
| 64 | m | A | 11 | AIDP | nd | No | Yes | Yes | nr | France |
| nr | nr | A | 7 | AIDP | No | nr | Yes | No | Partial | Italy |
| nr | nr | A | 10 | AIDP | No | nr | Yes | No | Yes | Italy |
| nr | nr | A | 10 | AMAN | No | nr | Yes | Yes | Partial | Italy |
| nr | nr | A | 5 | AMAN | No | nr | Yes | No | Partial | Italy |
| nr | nr | A | 7 | AMAN | No | nr | Yes, PE | No | nr | Italy |
| 50 | m | A | 3 | MFS, PNC | No | No | Yes | No | Yes | Spain |
| 39 | m | A | 3 | MFS, PNC | No | No | No | No | Yes | Spain |
| 61 | m | A | 10 | MFS | No | No | S | No | Yes | Spain |
| 76 | f | A | 8 | GBS (no NCS) | nd | No | No | nr | Death | Spain |
| ~ 75 | m | B | 10 | AIDP | No | No | Yes | No | Yes | Swiss |
| 43 | m | A | 10 | AIDP | nr | nr | Yes | No | Yes | Spain |
| 64 | m | A | 23 | AIDP | No | nr | Yes | No | Yes | France |
| 72 | m | A | 7 | AIDP | No | AHT, CHD, AL | Yes | Yes | Partial | USA |
| ~ 65 | m | A | 17 | AIDP | No | No | Yes | No | Yes | Italy |
| 67 | f | A | 10 | nr | No | Breast cancer | PE | Yes | Partial | USA |
| 54 | m | A | 14 | AIDP | nd | nr | Yes | No | Partial | USA |
| 43 | m | A | 21 | AIDP | No | nr | Yes | No | Yes | France |
| 71 | f | A | 10 | AIDP | No | nr | Yes | No | Partial | France |
| 36 | m | A | 4 | MFS | nr | nr | Yes | No | Yes | USA |
| 55 | m | A | 20 | AIDP | No | nr | Yes | Yes | Partial | Italy |
| 60 | m | A | 3 | AMSAN | No | nr | Yes | Yes | Partial | Italy |
| 58 | m | AB | 0 | AIDP | No | No | Yes | No | Partial | Canada |
| 52 | f | A | 15 | AIDP | No | nr | Yes | No | Partial | Swiss |
| 63 | f | A | 7 | AIDP | nr | nr | Yes | No | Yes | Swiss |
| 61 | f | A | 22 | AIDP | No | nr | Yes | No | Partial | Swiss |
| 53 | f | B | nr | AIDP | No | No | PE | No | Partial | Turkey |
| 51 | f | A | 14 | MFS | nr | nr | Yes | No | Partial | Spain |
| 56 | f | A | 15 | AIDP | No | nr | Yes | Yes | Partial | Spain |
| 68 | m | A | 14 | AIDP | nr (ASPC) | nr | Yes, PE | Yes | Partial | Austria |
| 55 | f | A | 14 | AIDP | nr | No | Yes | Yes | Partial | Spain |
| 53 | m | A | 24 | AIDP | No | No | Yes | No | Yes | Netherlands |
| 57 | m | A | 6 | AIDP | No | AHT, psoriasis | Yes | Yes | Partial | UK |
| 21 | m | A | 16 | AIDP | nr | AHT, DM | PE | No | Yes | USA |
| 41 | m | A | 10 | AIDP | nr | DM | Yes | No | Partial | Iran |
| 38 | m | A | 16 | AIDP | nr | AHT | PE | No | Yes | Iran |
| 14 | f | A | nr | GBS | nr | No | Yes | No | Yes | Iran |
| 49 | m | A | 14 | AIDP | No | No | Yes | No | Yes | UK |
| 68 | m | A | 5 | AIDP | nr | AHT, HLP | Yes | No | Yes | Italy |
| 11 | m | A | 21 | AIDP | nr | No | Yes | No | Yes | Saudi |
| 15 | m | A | nr | AMAN | No | No | Yes | No | Partial | Brazil |
| 72 | m | A | 18 | AIDP | No | nr | Yes | Yes | Partial | Italy |
| 72 | m | A | 30 | AIDP | No | nr | Yes | Yes | Partial | Italy |
| 49 | f | A | 14 | AIDP | No | nr | Yes | No | Partial | Italy |
| 94 | m | A | 33 | AIDP | nr | nr | S | No | Partial | Italy |
| 76 | m | A | 22 | AIDP | No | nr | Yes | Yes | Partial | Italy |
| 64 | m | A | nr | GBS? | nr | DM | Yes | Yes | Yes | Japan |
| 77 | m | A | nr | AIDP | nr | AHT, HLP | Yes | No | Yes | Spain |
| 58 | f | A | 6 | AIDP | No | nr | PE | No | Yes | USA |
| 56 | f | A | 7 | AIDP | No | AHT, thyroxin ↓ | nr | nr | Partial | Germany |
| 61 | f | A | 14 | AMAN | No | AHT, HLP | PE | No | Yes | USA |
| 75 | m | A | nr | nr | No | spinal trauma | Yes | No | Yes | USA |
| 37 | nr | A | 10 | nr | nr | nr | nr | nr | nr | Belgium |
| 60 | f | A | 22 | nr | nr | Migraine | Yes | No | Partial | USA |
| ∅57 | 33 m | nr | 0–37 | nr | nr | nr | Yes, n = 46 | nr | Death, n = 1 | UK, n = 47 |
| PE, n = 1 | nr, n = 46 | |||||||||
| 51 | m | A | 12 | AIDP | No | nr | Yes | Yes | Partial | Germany |
| 34 | m | A | 4 | PNC | nr | Strabism | Yes | No | Partial | USA |
| 71 | f | A | Days | PNC | nr | AHT | No | No | Partial | USA |
| 65 | m | A | 3 | AIDP | nr | No | Yes | No | Yes | Germany |
| 74 | f | A | nr | AIDP | No | Lymphoma | Yes | No | Yes | Spain |
| 49 | m | A | 14 | MFS | No | Crohn’s disease | Yes | Yes | partial | USA |
| 65 | f | A | nr | AIDP | nr | Fibromyalgia | Yes | Yes | Death | Italy |
| 12 | m | A | 7 | nr | nr | No | Yes | Yes | Death | Tanzania |
| 88 | f | A | 2 | AMSAN | nr | nr | PE | Yes | Partial | Iran |
| 47 | m | A | 7 | AMSAN | nr | nr | PE | Yes | Death | Iran |
| 58 | m | A | 9 | AMSAN | nr | nr | Yes, PE | Yes | Death | Iran |
| 54 | m | A | 3 | nr | nr | GBS, DN | Yes | No | Yes | USA |
| 57 | m | A | nr | AMAN | nr | nr | Yes | No | nr | Italy |
| 37 | m | A | 14 | AIDP | nr | nr | Yes | Yes | Partial | Iran |
| 41 | m | A | 10 | AIDP | No | nr | Yes | No | Yes | Guinea |
| 76 | m | A | 7 | AIDP | No | Cardiomyopathy | Yes | No | Partial | France |
| ∅59.2 | 22 m | A | 16–35 | AIDP, n = 23 | nr | Several | n = 25 | n = 5 | Partial | UK, n = 30 |
| AMAN, n = 2 | PE, n = 2 | |||||||||
| 44 | m | A | nr | nr | nr | AHT, asthma | Yes | No | Yes | USA |
| 54 | f | A | 20 | AMAN | nr | Asthma | No | No | Partial | Japan |
| 55 | f | A | 11 | AMSAN | nr | Lung disease | Yes | Yes | Death | Iran |
| 8 | m | B | nr | AIDP | No | No | Yes | Yes | Partial | USA |
| 65 | m | A | 14 | AIDP | nr | nr | Yes | No | Partial | Iran |
| 70 | f | A | 90 | nr | nr | RSD | Yes | No | Yes | USA |
| 55 | f | A | 10 | AMAN | nr | DM, AHT | Yes | No | Partial | India |
| 72 | m | A | 6 | AIDP | nr | AHT | Yes | Yes | Death | India |
| 55 | m | A | 7 | AMSAN | nr | DM, AHT, RI | Yes | No | Partial | India |
| 49 | m | A | 10 | AIDP | nr | DM, AHT | Yes | No | Partial | India |
| 53 | m | A | nr | nr | nr | nr | Yes | No | Partial | Italy |
| 36 | m | A | 18 | AIDP | nr | AHT, NTX | Yes | Yes | Partial | USA |
| 57 | m | A | 17 | AIDP | nr | nr | Yes | No | Partial | Italy |
| ∅53 | 11 m | A | 0.5–28 | AIDP | No, n = 4 | nr | Yes, n = 15 | nr | Partial, | Italy, n = 17 |
| PE, n = 2 | Death, n = 1 | |||||||||
| 54 | f | AB | 0 | nr | No | AHT | Yes | No | Partial | Spain |
| 58 | f | A | 14 | nr | nr | Disc prolapse | Yes | No | Partial | USA |
| 65 | m | A | nr | AIDP | nr | nr | Yes | No | Partial | Italy |
| 73 | m | AB | 0 | AIDP | No | nr | Yes | No | Partial | Italy |
| 55 | m | A | 20 | AIDP/MFS | No | nr | Yes | No | Partial | Italy |
| 46 | f | A | 3 | AIDP | No | nr | Yes | No | Partial | Italy |
| 60 | m | A | 20 | AMSAN | No | nr | Yes | No | Partial | Italy |
| 63 | f | A | 15 | AMSAN | nr | nr | Yes | No | Partial | Italy |
| ~ 35 | m | A | nr | AMAN | No | nr | Yes | No | Partial | UK |
| 49 | m | A | 11 | PCB | No | AHT, seminoma | No | No | Partial | Italy |
| 54 | m | A | 4 | AIDP | nr | AHT, obesity | Yes | Yes | Partial | Spain |
| 54 | nr | nr | nr | nr | No | AHT, HLP | Yes | Yes | Yes | Spain |
| 72 | f | A | 8 | AIDP | No | nr | Yes | Yes | Partial | Italy |
| 48 | m | A | 18 | AIDP | nr | DM | PE | No | Partial | USA |
| 46 | m | A | 18 | AIDP | nr | nr | No | No | Partial | Iran |
| 65 | m | A | 10 | AIDP | nr | nr | Yes | No | Partial | Iran |
| 66 | f | B | No symptom | AIDP | nr | nr | Yes | No | Partial | Italy |
| 66 | f | A | 30 | AIDP | nr | DM, AHT, arthritis | Yes | No | Partial | Iran |
| 55 | f | A | 31 | AMSAN | nr | COPD | Yes | Yes | Death | Iran |
| 14 | f | A | nr | nr | nr | No | Yes | No | Yes | Iran |
| 38 | m | A | 16 | AIDP | nr | No | PE | No | Partial | Iran |
| 20-63 | 7 m | nr | nr | AIDP | nr | nr | Yes | No | Partial | UK, n = 7 |
| 65 | m | A | 5 | AIDP | nr | DM, AHT | Yes | Yes | Death | Sudan |
| 43 | m | A | 10 | AIDP | nr | nr | Yes | No | Partial | Spain |
| 63 | m | A | 1 | MFS | nr | nr | No | No | Partial | UK |
| 61 | m | A | nr | MFS | No | nr | Yes | No | Yes | Germany |
| 58 | m | B | nr | AIDP | nr | nr | Yes | Yes | Partial | UK |
| 70 | f | A | 15 | AMAN | nr | AHTt, obesity | Yes, PE | No | Partial | Italy |
A, onset of GBS after onset of non-neurological manifestations; AAR Aortic aneurysm repair; AHT Arterial hypertension; AL Alcoholism, ASPC Antibodies for SARS-CoV-2 positive in CSF; AV Artificial ventilation; B, onset of GBS before onset of non-neurological manifestations; CHD Coronary heart disease; CIC CoV2 in CSF; CM Comorbidities; DM Diabetes; f Female; HLP Hyperlipidaemia; LC Lung cancer; LOO Latency between onset of GBS and COVID-19 respectively vice versa; m Male; nd Not done; nr Not reported; NCS Nerve conduction study; NTX Renal transplantation; pc Personal communication; PCB Pharyngeal, cervical, brachial variant of GBS; PE Plasma exchange; PNC Polyneuritis cranialis; RA Rheumatoid arthritis; RI Renal insufficiency; RSD Reflex sympathetic dystrophy; S Steroids
Table 2.
Summary of findings in 220 patients with SC2-GBS
| Number of papers retrieved: 95 | |
| Number of SC2-GBS: 220 | |
| Number of patients with SC2-GBS subtypes: AIDP (n = 118), AMAN (n = 18), AMSAN (n = 11), MFS (n = 7), PNC (n = 2), PCB (n = 1), BSE (n = 0) | |
| Age range of patients: 8 to 94 years | |
| Gender: male (n = 146), female (67) | |
| Onset: after COVID-19 (n = 156), together with COVID-19 (n = 3), before COVID-19 (n = 6) | |
| Latency between onset of COVID-19 and GBS: − 10 to + 90 days | |
| Therapy: IVIG (n = 191), plasmapheresis (n = 15), steroids (n = 2), MV (n = 41) | |
| Outcome: CR (n = 37), PR (n = 119), death (n = 12) |
CR Complete recovery; MV Mechanical ventilation; PR Partial recovery
Discussion
This systematic review shows that SC2-GBS is not due to a direct attack of the virus but rather due to an immunological reaction to the virus. It also shows that the number of reports about SC2-GBS is increasing and that the outcome is worse compared to non-SC2-GBS [6].
Though the number of cases with SC2-GBS is increasing suggesting that the overall prevalence of GBS has increased since the outbreak of the pandemic, there are conflicting results concerning this matter. In a UK study of 47 SC2-GBS patients, the prevalence of GBS did not increase between March 2020 and May 2020 as compared to the years 2016–2019 [6]. On the contrary, a retrospective, multi-centre study from northern Italy of 34 SC2-GBS patients showed that the estimated incidence of GBS in March 2020 and April 2020 increased from 0.93/100000/year in 2019 to 2.43/100000/year in 2020 [7]. There are several reasons why SC2-GBS may be missed and why the prevalence of GBS in fact increased since onset of the pandemic. First, SC2-GBS may go undetected due to misinterpretation as increased weakness or sensory disturbances of a pre-existing neuropathy. Second, SC2-GBS may be misinterpreted as critical ill neuropathy. Third, work-up for neuropathy may be incomplete due to mild manifestations or due to occurrence during ICU stay.
Before diagnosing SC2-GBS, it is crucial to exclude various differential diagnoses. These include previously existing neuropathy, critical ill myopathy, critical ill neuropathy, toxic neuropathy, or neuropathy or myopathy due to side effects of applied drugs. Lopinavir and tocilizumab have been reported to cause neuropathy [8, 9]. There are also reports indicating that chloroquine may induce neuropathy [10].
If GBS develops during immobilisation for artificial ventilation, diagnosing SC2-GBS becomes challenging [7]. In patients under artificial ventilation for COVID-19, SC2-GBS should be considered if clinical neurologic exam suggests neuropathy and if patients cannot be weaned from the respirator. In this case, nerve conduction studies and investigations of the CSF should be initiated. Diagnosing SC2-GBS is crucial as appropriate treatment may improve the overall outcome of COVID-19 patients [11].
In some cases, SC2-GBS develops before classical clinical manifestations of the infection [12] being explained by subclinical infection with the virus prior to onset of GBS or the incubation time of SARS-CoV-2, which is up to 14 days [7].
Though there are no prediction models for the outcome or the need of artificial ventilation in SC2-GBS patients available, there are indications that the outcome is poor if there are complications from hypercoagulability (stroke, pulmonary embolism) and if there are superinfections or sepsis.
Most of the studies included in this review did not specify if respiratory failure in SC2-GBS patients resulted from brainstem encephalitis, BFE, involvement of the respiratory muscles in GBS, from pneumonia ending up as acute, respiratory distress syndrome (ARDS), from pulmonary embolism, heart failure, or from mixtures of these conditions. Specifying the cause of respiratory failure however is crucial as treatment and outcome may differ significantly among these conditions.
Conclusions
SC2-GBS is most likely secondary to an immune reaction against SARS-CoV-2 since the virus has not been found in CSF of any SC2-GBS patient reported. SC2-GBS occurs at any age. SC2-GBS does not differ from non-SC2-GBS regarding clinical presentation and treatment, but the outcome of SC2-GBS is worse compared to non-CS2-GBS patients. The prevalence/incidence of GBS most likely increased since the outbreak of the pandemic. Since there are no studies about the optimal treatment of SC2-GBS subtypes available, they should be treated empirically in the same way as non-SC2-GBS subtypes. Early diagnosis of SC2-GBS is warranted because if appropriate treatment is applied in due time, the overall outcome from the infection may improve.
Acknowledgements
None
Abbreviations
- AIDP
Acute, inflammatory, demyelinating polyneuropathy
- AMAN
Acute, motor axonal neuropathy
- AMSAN
Acute, motor and sensory, axonal neuropathy
- ARDS
Acute, respiratory distress syndrome
- BFE
Bickerstaff encephalitis
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- GBS
Guillain-Barre syndrome
- IVIG
Intravenous immunoglobulins
- MFS
Miller-Fisher syndrome
- PCB
Pharyngeal, cervical, and brachial variant
- PNC
Polyneuritis cranialis
- PNS
Peripheral nervous system
- SC2-GBS
SARS-CoV-2-associated GBS
Authors’ contributions
JF: design, literature search, discussion, first draft, critical comments, FS: literature search, discussion, critical comments, final approval.
All authors have read and approved the manuscript and ensured that this is the case.
Funding
None received
Availability of data and materials
Not applicable
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Munjal M, Das S, Chatterjee N, Setra AE, Govil D. Systemic involvement of novel coronavirus (COVID-19): a review of literature. Indian J Crit Care Med. 2020;24(7):565–569. doi: 10.5005/jp-journals-10071-23498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.da Rosa MR, Francelino Silva Junior LC, Santos Santana FM, Farias de Oliveira T, Campos Alcântara R, Monteiro Arnozo G, et al. Clinical manifestations of COVID-19 in the general population: systematic review. Wien Klin Wochenschr. 2020:1–6. (in press) [DOI] [PMC free article] [PubMed]
- 3.Baig AM, Sanders EC. Potential neuroinvasive pathways of SARS-CoV-2: deciphering the spectrum of neurological deficit seen in coronavirus disease-2019 (COVID-19) J Med Virol. 2020;92(10):1845–1857. doi: 10.1002/jmv.26105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paliwal VK, Garg RK, Gupta A, Tejan N. Neuromuscular presentations in patients with COVID-19. Neurol Sci. 2020;41(11):3039–3056. doi: 10.1007/s10072-020-04708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen TP, Taylor RS. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2021. Guillain Barre syndrome. 2020 Nov 19. [Google Scholar]
- 6.Keddie S, Pakpoor J, Mousele C, Pipis M, Machado PM, Foster M, et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. Brain. 2020:awaa433. (in press) [DOI] [PMC free article] [PubMed]
- 7.Filosto M, Cotti Piccinelli S, Gazzina S, Foresti C, Frigeni B, Servalli MC, et al. Guillain-Barré syndrome and COVID-19: an observational multicentre study from two Italian hotspot regions. J Neurol Neurosurg Psychiatry. 2020; jnnp-2020-324837. [DOI] [PubMed]
- 8.Khanlou H, Valdes-Sueiras M, Farthing C. Peripheral neuropathy induced by lopinavir-saquinavir-ritonavir combination therapy in an HIV-infected patient. J Int Assoc Physicians AIDS Care (Chic) 2007;6:155. doi: 10.1177/1545109707302756. [DOI] [PubMed] [Google Scholar]
- 9.Sugiura F, Kojima T, Oguchi T, Urata S, Yuzawa Y, Sakakibara A, Hayashi H, Nishimoto N, Ishiguro N. A case of peripheral neuropathy and skin ulcer in a patient with rheumatoid arthritis after a single infusion of tocilizumab. Mod Rheumatol. 2009;19(2):199–203. doi: 10.3109/s10165-008-0132-2. [DOI] [PubMed] [Google Scholar]
- 10.Becerra-Cuñat JL, Coll-Cantí J, Gelpí-Mantius E, Ferrer-Avellí X, Lozano-Sánchez M, Millán-Torné M, Ojanguren I, Ariza A, Olivé A. Chloroquine-induced myopathy and neuropathy: progressive tetraparesis with areflexia that simulates a polyradiculoneuropathy. Two case reports. Rev Neurol. 2003;36(6):523–526. [PubMed] [Google Scholar]
- 11.Dubey D, Kapotic M, Freeman M, Sawhney A, Rojas JC, Warnack W, Vernino S. Factors contributing to delay in diagnosis of Guillain-Barré syndrome and impact on clinical outcome. Muscle Nerve. 2016;53(3):384–387. doi: 10.1002/mus.24772. [DOI] [PubMed] [Google Scholar]
- 12.Finsterer J, Scorza FA, Fiorini AC. SARS-CoV-2-associated guillain-barre syndrome in 62 patients. Eur J Neurol. 2020;28(1):e10–e12. doi: 10.1111/ene.14544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable