Abstract
Background
Multiple studies have identified the prognostic relevance of extent of resection in the management of glioma. Different intraoperative technologies have emerged in recent years with unknown comparative efficacy in optimising extent of resection. One previous Cochrane Review provided low‐ to very low‐certainty evidence in single trial analyses and synthesis of results was not possible. The role of intraoperative technology in maximising extent of resection remains uncertain. Due to the multiple complementary technologies available, this research question is amenable to a network meta‐analysis methodological approach.
Objectives
To establish the comparative effectiveness and risk profile of specific intraoperative imaging technologies using a network meta‐analysis and to identify cost analyses and economic evaluations as part of a brief economic commentary.
Search methods
We searched CENTRAL (2020, Issue 5), MEDLINE via Ovid to May week 2 2020, and Embase via Ovid to 2020 week 20. We performed backward searching of all identified studies. We handsearched two journals, Neuro‐oncology and the Journal of Neuro‐oncology from 1990 to 2019 including all conference abstracts. Finally, we contacted recognised experts in neuro‐oncology to identify any additional eligible studies and acquire information on ongoing randomised controlled trials (RCTs).
Selection criteria
RCTs evaluating people of all ages with presumed new or recurrent glial tumours (of any location or histology) from clinical examination and imaging (computed tomography (CT) or magnetic resonance imaging (MRI), or both). Additional imaging modalities (e.g. positron emission tomography, magnetic resonance spectroscopy) were not mandatory. Interventions included fluorescence‐guided surgery, intraoperative ultrasound, neuronavigation (with or without additional image processing, e.g. tractography), and intraoperative MRI.
Data collection and analysis
Two review authors independently assessed the search results for relevance, undertook critical appraisal according to known guidelines, and extracted data using a prespecified pro forma.
Main results
We identified four RCTs, using different intraoperative imaging technologies: intraoperative magnetic resonance imaging (iMRI) (2 trials, with 58 and 14 participants); fluorescence‐guided surgery with 5‐aminolevulinic acid (5‐ALA) (1 trial, 322 participants); and neuronavigation (1 trial, 45 participants). We identified one ongoing trial assessing iMRI with a planned sample size of 304 participants for which results are expected to be published around winter 2020. We identified no published trials for intraoperative ultrasound.
Network meta‐analyses or traditional meta‐analyses were not appropriate due to absence of homogeneous trials across imaging technologies. Of the included trials, there was notable heterogeneity in tumour location and imaging technologies utilised in control arms. There were significant concerns regarding risk of bias in all the included studies.
One trial of iMRI found increased extent of resection (risk ratio (RR) for incomplete resection was 0.13, 95% confidence interval (CI) 0.02 to 0.96; 49 participants; very low‐certainty evidence) and one trial of 5‐ALA (RR for incomplete resection was 0.55, 95% CI 0.42 to 0.71; 270 participants; low‐certainty evidence). The other trial assessing iMRI was stopped early after an unplanned interim analysis including 14 participants; therefore, the trial provided very low‐quality evidence. The trial of neuronavigation provided insufficient data to evaluate the effects on extent of resection.
Reporting of adverse events was incomplete and suggestive of significant reporting bias (very low‐certainty evidence). Overall, the proportion of reported events was low in most trials and, therefore, issues with power to detect differences in outcomes that may or may not have been present. Survival outcomes were not adequately reported, although one trial reported no evidence of improvement in overall survival with 5‐ALA (hazard ratio (HR) 0.82, 95% CI 0.62 to 1.07; 270 participants; low‐certainty evidence). Data for quality of life were only available for one study and there was significant attrition bias (very low‐certainty evidence).
Authors' conclusions
Intraoperative imaging technologies, specifically 5‐ALA and iMRI, may be of benefit in maximising extent of resection in participants with high‐grade glioma. However, this is based on low‐ to very low‐certainty evidence. Therefore, the short‐ and long‐term neurological effects are uncertain. Effects of image‐guided surgery on overall survival, progression‐free survival, and quality of life are unclear. Network and traditional meta‐analyses were not possible due to the identified high risk of bias, heterogeneity, and small trials included in this review. A brief economic commentary found limited economic evidence for the equivocal use of iMRI compared with conventional surgery. In terms of costs, one non‐systematic review of economic studies suggested that, compared with standard surgery, use of image‐guided surgery has an uncertain effect on costs and that 5‐ALA was more costly. Further research, including completion of ongoing trials of ultrasound‐guided surgery, is needed.
Plain language summary
Image‐guided surgery for brain tumours
Background
Surgery has a key role in the management of many types of brain tumour. Removing as much tumour as possible is very important, as in some types of brain tumour this can help people to live longer and to feel better. However, removing a brain tumour may be difficult because the tumour either looks like normal brain tissue or is near brain tissue that is needed for normal functioning. New methods of seeing tumours during surgery (called imaging) have been developed to help surgeons better identify tumour from normal brain tissue.
Questions
Is image‐guided surgery more effective at removing brain tumours than surgery without image guidance?
Is one image‐guidance technology or tool better than another?
Study characteristics
Our search strategy is up to date as of May 2020. We found four trials looking at three different tools to help improve the amount of tumour that is removed. The tumour being evaluated was glioma. Imaging interventions used during surgery included:
– magnetic resonance imaging during surgery to assess the amount of remaining tumour; – fluorescent dye to mark out the tumour (5‐aminolevulinic acid); – imaging before surgery to map out the location of a tumour, which was then used at the time of surgery to guide the surgery (neuronavigation); or – ultrasound imaging during surgery to assess the amount of remaining tumour.
All the studies had compromised methods, which could mean their conclusions were biased. Some studies were funded by the manufacturers of the image guidance technology being evaluated. We intended to use a form of analysis called network meta‐analysis (which can incorporate comparisons of interventions even if they have not been directly compared within trials) to compare each of these interventions and identify which single technology might be best.
Key results
We found low‐ to very low‐certainty evidence that use of image‐guided surgery may result in more of the tumour being removed surgically in some people. The short‐ and long‐term neurological effects are uncertain. We did not have the data to determine whether any of the evaluated technologies affected overall survival, time until disease progression, or quality of life. There was very low‐certainty evidence for neuronavigation across all outcomes in one very small trial, and we identified no trials for ultrasound guidance. We were unable to perform a network meta‐analysis to compare imaging interventions. In terms of costs, compared with standard surgery, use of image‐guided surgery has an uncertain effect on costs and that 5‐aminolevulinic acid was more costly than conventional surgery.
Quality of the evidence
Evidence for intraoperative imaging technology for use in removing brain tumours is sparse and of low to very low certainty. Further research is needed to assess three main questions:
– Is removing more of the tumour better for the patient in the long term? – What are the risks of causing a patient to have worse symptoms by taking out more of the tumour? – How does resection affect a patient's quality of life?
Summary of findings
Background
Description of the condition
Of all primary tumours of the central nervous system, gliomas are the second most common representing 26.0%. The vast majority of gliomas are diffuse astrocytic and oligodendroglial tumours characterised by their diffusely infiltrative behaviour through the brain parenchyma (Louis 2016). This group includes IDH‐mutant (isocitrate dehydrogenase) and wild‐type genetic classifications across histological subtypes of diffuse astrocytoma, anaplastic astrocytoma, oligodendroglioma, anaplastic oligodendroglioma, and glioblastoma. Glioblastoma has a dismal prognosis; median survival in the UK is 6.1 months with a five‐year survival of 3.4% (Brodbelt 2015). There is consensus for low‐grade diffusely infiltrating gliomas and glioblastoma that maximising extent of resection is associated with a more favourable prognosis. Increasingly, intraoperative imaging technologies are being utilised in an effort to maximise the likelihood of gross total resection.
Description of the intervention
There are multiple modalities offering intraoperative imaging technology to assist the neurosurgeon in achieving maximal safe resection of gliomas. Fluorescence‐guided surgery enables tumour tissue to be better visualised to maximise the probability of gross total resection of the enhancing component of gliomas. The most common fluorescent compound, 5‐aminolevulinic acid (5‐ALA), is a precursor of haemoglobin. Administered three hours before induction, the result is an accumulation of fluorescent porphyrins in mitotically active tissue. The fluorescence is visualised with ultraviolet light to identify neoplastic tissue intraoperatively in the surgical field and improve identification of resection margins (Stummer 1998; Stummer 2000). As a medication administration, there is an associated cost per single use. Capital investment required includes a fluorescence filter in any operating theatre microscope. The relative uptake of 5‐ALA is dependent on the tumour characteristics, which limits its utility in accurately differentiating tumour and non‐tumour tissue.
One imaging technology that is currently variably used in the resection of glioma is intraoperative ultrasound (iUS), which relies on the different reflections of ultrasonic wave pulses caused by different tissue types enabling the delineation of neuroanatomical structures including normal‐appearing cortex and brain tumour tissue. The ease in which images can be acquired using a hand‐held device enables continuous assessment by the surgeon as resection proceeds.
Furthermore, this relatively affordable technique can be combined with a third technology, neuronavigation, in order to assist the neurosurgeon in achieving gross total resection. Neuronavigation leverages optical or electromagnetic technology to allow the registration of preoperative imaging on the patient in theatre. Several points are matched between the preoperative scan and the patient in theatre prior to computational registration of the scan on the patient in theatre. Specialised equipment visible to the neuronavigation software can then be used to plan the incision and craniotomy, and the trajectory of targeted biopsies.
The combination of iUS and navigation can overcome one major limitation of neuronavigation, namely brain shift. This occurs when the cranial cavity is entered resulting in a shift of intracranial contents relative to the preoperative scan due to the change in intracranial pressure and removal of cerebrospinal fluid (CSF) and tumour tissue. Such a shift can be adjusted for using iUS with simultaneous registration of three‐dimensional (3D) iUS imaging on preoperative imaging. The software registers the position of the ultrasound probe and maps the 3D neuroanatomical image to allow adjustment of the preoperative imaging reflective of the brain shift that has occurred during the operation.
Finally, intraoperative magnetic resonance imaging (iMRI) involves the availability of either a nearby or portable magnetic resonance imaging (MRI) scanner for use in the operating theatre. MRI as an imaging technique involves creating a strong magnetic field, applying radiofrequency pulses, and analysing effects of this on the tissue of interest. Equivalent strength magnets are available to traditional MRI scanners offering clinically useful resolution to enable a real‐time intraoperative snapshot of the extent of tumour resection. Such a technique theoretically affords the possibility of immediate further resection during the same operative session (Black 1997). Unexpectedly, due to the need for a dedicated MRI scanner in the operating room, iMRI is associated with substantial capital costs both in terms of purchasing the scanner and its installation.
Guidance from the National Institute for Health and Care Excellence (NICE) in the UK was published in July 2018. With respect to intraoperative techniques, the use of 5‐ALA was recommended at initial surgery where the patient has radiologically suspected high‐grade glioma (HGG) and gross total resection of the enhancing component is possible, while other techniques (iMRI, iUS, diffusion tensor imaging, awake craniotomy) could be considered in low‐grade glioma (LGG) and HGG to maximise surgical resection while preserving neurological function (NICE 2018).
How the intervention might work
The purpose of all the above interventions is to maximise safe resection of the tumour which, in the case of LGG and HGG, have been associated with improved overall survival (OS) based on low‐quality evidence (Hart 2019; Jiang 2017). The extent of resection is one of the only modifiable factors demonstrably correlated with OS and, therefore, is an important subject of research. A further benefit of improved detection of tumour and non‐tumour tissue is the minimisation of damage to healthy brain tissue during the operation. In combination, these interventions can be used to maximise resection and improve prognosis and quality of life (QoL) for the patients.
Why it is important to do this review
The technologies described are not used in all cases in all centres and, prior to their introduction, were not subject to the same degree of scrutiny as new medical treatments including phase III studies. The capital costs associated with iMRI are substantial; an ability to compare a single or combination of technologies with alternatives is important to evaluate efficacy and cost‐effectiveness. Given the close relationship between achieving a greater extent of resection and the risk of surgical injury to healthy brain tissue, the associated risks of each technology and its effect on measures of QoL were evaluated.
One review published in 2018 identified four randomised controlled trials (RCTS) of low‐certainty evidence without network meta‐analysis (NMA) (Jenkinson 2018). This review performed an updated search for new evidence published since the publication of Jenkinson 2018, with presentation of previous studies with additional attempts at quantitative analysis to facilitate direct and indirect comparisons of intraoperative imaging technologies used in isolation or in combination.
Objectives
To establish the comparative effectiveness and risk profile of specific intraoperative imaging technologies using a network meta‐analysis and to identify cost analyses and economic evaluations as part of a brief economic commentary.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Participants included in RCTs with presumed new or recurrent glial tumours of any location of histology as identified based on clinical examination and neuroimaging (computed tomography (CT) or MRI, or both). Participant must also have been eligible for randomisation to any intraoperative imaging modality.
Types of interventions
We compared the following interventions with each other or against the standard of care of conventional microsurgery with white light.
iMRI: defined as a portable or fixed scanner with the acquisition of MRI to evaluate extent of resection while the patient remained under anaesthesia.
5‐ALA: fluorescence‐guided surgery defined as the use of a compound to facilitate the intraoperative delineation of tumour and normal brain tissue to assist the surgeon in performing maximal resection of the tumour.
Neuronavigation: image guidance defined as using preoperative imaging to identify intracranial neuroanatomy using optical or electromagnetic technology. Could be integrated with iMRI or iUS (or both) to update imaging to account for brain shift and tumour tissue removed during the treatment.
iUS: either two‐dimensional (2D) or 3D imaging modality defined as the use of an ultrasound probe for the identification of neuroanatomical structures including residual tumour tissue for evaluation of extent of resection.
The interventions above are genuinely competing alternatives and in theory an RCT comparing all the imaging techniques would be possible and participants could be randomly allocated to any of the interventions in isolation or combination. Moreover, combinations of interventions are also possible, particularly the concomitant use of iUS and image guidance to reduce problems associated with brain shift correction of preoperative imaging for brain shift.
Potential effect modifiers include the following.
iMRI: use of portable or fixed scanner, sequences used, use of intravenous contrast agents.
5‐ALA: fluorescence‐guided surgery: exact compound used, time of administration, dose given, microscopic technologies used to detect fluorescence.
Neuronavigation: manufacturer software, optical or electromagnetic technologies.
iUS: 2D or 3D projections.
Furthermore, as this review included both newly diagnosed or recurrent glial tumours in any location, the degree to which studies could have been merged on the network depended on the participants included and the interventions used, with splitting of the network to optimise transitivity when required.
Types of outcome measures
Primary outcomes
Extent of resection: defined as the proportion of tumour tissue removed based on postoperative MRI. Results presented as an absolute volume of resection, percentage resection, and categorical results (gross total resection, subtotal resection, biopsy).
Adverse events (AEs): defined as need for unplanned additional procedures or development of complications including wound haematoma or infection, CSF leak, cerebral oedema, new or worsening focal neurological deficits or seizures, and general medical complications including thromboembolic disease or non‐surgical site infection.
Secondary outcomes
Overall survival (OS): defined as length of time from randomisation to death from any cause.
Progression‐free survival (PFS): defined as length of time from randomisation in RCT to tumour progression based on RANO (Response Assessment in Neuro‐Oncology criteria) consensus of imaging features of the contrast‐enhancing and T2‐weighted‐fluid‐attenuated inversion recovery (T2/FLAIR) non‐enhancing component, new lesions, clinical deterioration not attributable to another cause, death from any cause, or other clear progression of unmeasurable disease (Wen 2010).
Quality of life (QoL): defined based on validated measures for people with glioma including but not limited to the EORTC QLQ‐C30 and BN20 (European Organisation for Research and Treatment of Cancer Quality of Life assessment specific to brain neoplasms) questionnaires, and FACT‐BrS (Functional Assessment of Cancer Therapy – Brain subscale) (Dirven 2014; Fountain 2016).
Our 'Summary of findings' tables Table 1; Table 2; Table 3 reported the following;
Summary of findings 1. iMRI image‐guided surgery compared to standard surgery for high‐grade glioma.
| iMRI image‐guided surgery compared to standard surgery for high‐grade glioma | ||||||
|
Patient or population: high‐grade glioma Settings: specialist centres Intervention: iMRI image‐guided surgery (based on postoperative MRI) Comparison: standard surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | iMRI image‐guided surgery | |||||
| Extent of resection: incomplete resection | 32a per 100 | 4 per 100 (1 to 31) | RR 0.13 (0.02 to 0.96) | 49 participants (1 study) | ⊕⊝⊝⊝b,c Verylow | Small trial of highly selected participants with potential bias in allocation and performance. 1 other trial reported this outcome but did not contribute towards the analysis. |
| Adverse events | Inadequately and inconsistently reported in the trial | ⊕⊝⊝⊝d Verylow | Adverse events were reported in an inconsistent manner and not according to the manner prespecified in our protocol. | |||
| Overall survival | Not estimable | ⊕⊝⊝⊝d Verylow | Abstract publication only in 2017. 24 (83%) of 29 patients randomly allocated to the iMRI group and 21 (72.4%) of 29 controls were eligible for analysis of overall survival, reported as "iMRI itself did not affect outcome (560 vs. 624 days, p=0.53)". Unable to identify which 8 patients had metastasis (these were excluded from published trial in 2011). | |||
| Progression‐free survival | Not estimable | ⊕⊝⊝⊝d Verylow | Progression‐free survival or time to progression was not adequately reported in the trial. | |||
| Quality of life | Not estimable | ⊕⊝⊝⊝d Verylow | Quality of life was not reported in the trial. | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; iMRI: intraoperative magnetic resonance imaging; MRI: magnetic resonance imaging; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aExpressed in terms of risk of incomplete resection (bad outcome). bSmall trial so quality of the evidence downgraded one level. cHighly selected participants with potential bias in allocation and performance as well as in other 'Risk of bias' domains, thus downgraded two levels. dOutcome was not reported (or inadequately reported for meaningful conclusions to be drawn), therefore giving lowest quality of evidence judgement.
Summary of findings 2. 5‐ALA image‐guided surgery compared to standard surgery for high‐grade glioma.
| 5‐ALA image‐guided surgery compared to standard surgery for high‐grade glioma | ||||||
|
Patient or population: high‐grade glioma Settings: specialist centres Intervention: 5‐ALA image‐guided surgery (based on postoperative MRI) Comparison: standard surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | 5‐ALA image‐guided surgery | |||||
| Extent of resection: incomplete resection | 64a per 100 | 35 per 100 (27 to 45) | RR 0.55 (0.42 to 0.71) | 270 participants (1 study) |
⊕⊕⊝⊝b Low | Highly selected participants with potential bias in allocation and performance. |
| Adverse events | Inadequately and inconsistently reported in the trial | ⊕⊝⊝⊝c Verylow | Adverse events were reported in an inconsistent manner and not according to the manner prespecified in our protocol. | |||
| Overall survival | Not estimable due to reporting of HR and since just a single trial reported on this outcome we did not arbitrarily choose a time to use as a basis to calculate the assumed and corresponding risks as this may be misleading. |
HR 0.82 (0.62 to 1.07) |
270 participants (1 study) |
⊕⊕⊝⊝b Low | The overall quality of this outcome was low in this trial and was downgraded for highly selected participants with potential bias in allocation and performance. | |
| Progression‐free survival | Inadequately reported or not assessed at all in the included trials | ⊕⊝⊝⊝c Verylow | Progression‐free survival or time to progression was not adequately reported in the trial. | |||
| Quality of life | Inadequately reported or not assessed at all in the included trials | ⊕⊝⊝⊝c Verylow | Quality of life was not reported in the trial. | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).f 5‐ALA: 5‐aminolevulinic acid; CI: confidence interval; HR: hazard ratio; MRI: magnetic resonance imaging; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aExpressed in terms of risk of incomplete resection (bad outcome). bHighly selected participants with potential bias in allocation and performance as well as in other 'Risk of bias' domains, thus downgraded by two levels. cOutcome was not reported (or inadequately reported for meaningful conclusions to be drawn), therefore giving lowest quality of evidence judgement.
Summary of findings 3. Neuronavigation image‐guided surgery compared to standard surgery for high‐grade glioma.
| Neuronavigation image‐guided surgery compared to standard surgery for high‐grade glioma | ||||||
|
Patient or population: high‐grade glioma Settings: specialist centres Intervention: neuronavigation image‐guided surgery (based on postoperative MRI) Comparison: standard surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Neuronavigation image‐guided surgery | |||||
| Extent of resection: incomplete resection | Not estimable | Not estimable | Not reported | 45 participants (1 study) | ⊕⊝⊝⊝a,b,c Verylow | Small study of highly selected participants at very high risk of allocation bias. Complete resection was achieved in 3 participants in the control group and 5 participants in the neuronavigation group. However, there was significant attrition, with not all participants completing imaging, and the denominators for these figures were not stated, precluding formal analysis. |
| Adverse events | Inadequately and inconsistently reported in the trial | ⊕⊝⊝⊝c Verylow | Adverse events were reported in an inconsistent manner and not according to the manner prespecified in our protocol. | |||
| Overall survival | Not estimable | ⊕⊝⊝⊝d Verylow | Not reported by trial authors so graded as very low‐quality evidence. | |||
| Progression‐free survival | Not estimable | ⊕⊝⊝⊝c Verylow | Progression‐free survival or time to progression was not reported in the trial. | |||
| Quality of life | Inadequately reported or not assessed at all in the included trials | ⊕⊝⊝⊝d Verylow | Quality of life was reported in the trial but only 19 participants (8 in the neuronavigation arm and 11 in the standard surgery arm) completed questionnaires postoperatively at 3 months, constituting only 64.5% of all eligible participants, and no statistical analysis was presented. | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aSmall trial so quality of the evidence downgraded by one level. b cHighly selected participants with potential bias in allocation and performance as well as in other 'Risk of bias' domains, thus downgraded by two levels. dOutcome was not reported (or inadequately reported for meaningful conclusions to be drawn), therefore giving lowest quality of evidence judgement.
Extent of resection
AEs
OS
PFS
QoL
Decisions on the certainty of the evidence for each outcome followed the most recent recommendations and guidelines (Brignardello‐Petersen 2018; Brignardello‐Petersen 2019a; Brignardello‐Petersen 2019b; Puhan 2014).
Search methods for identification of studies
Non‐English language journals were eligible for inclusion.
Electronic searches
We searched the following databases to 19 May 2020:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 5), in the Cochrane Library;
MEDLINE via Ovid (1946 to May week 2 2020);
Embase via Ovid (1980 to 2020 week 20).
We presented our CENTRAL (Appendix 1), MEDLINE search strategy (Appendix 2), Embase search strategy (Appendix 3). For databases other than MEDLINE, we adapted the search strategies accordingly.
We searched the references of all identified studies for additional eligible studies for the review.
Searching other resources
We undertook a handsearch of the Journal of Neuro‐oncology and Neuro‐oncology from 1990 to 2019 to identify trials that may not have been included in the electronic databases. This search included all conference abstracts published in these journals.
Personal communications
We contacted principal investigators of ongoing relevant trials regarding available data for potential inclusion. For completion of the previous review in 2018, neuro‐oncology experts were contacted to obtain information on current or pending RCTs.
Data collection and analysis
Selection of studies
The selection of studies for the 2018 review are described in Jenkinson 2018.
For the updated search, following automated deduplication of results, two review authors (DGB and MW) independently screened titles and abstracts, and assessed them based on inclusion and exclusion criteria. We utilised the Cochrane author support tool Covidence for title and abstract screening. We undertook full‐text screening of all eligible studies at this stage and further examined them against inclusion and exclusion criteria. We identified disagreements and resolved them through discussion.
Where studies had multiple publications, we collated the reports of the same trial so that each trial, rather than each report, was the unit of interest for the review, and such trials had a single identifier with multiple nested references.
Data extraction and management
Data extraction and management of studies included in the 2018 review are described in Jenkinson 2018.
For any additionally identified studies, two review authors (DMF and MW) independently extracted data into a pre prepared database designed based on an initial pilot of three studies. Where sufficient data were not available from the published paper or additional supplementary material, we contacted authors to request relevant data for completion of the database for each study. We identified differences in the extracted data between review authors and resolved them through discussion. The database included the following fields.
Participant characteristics: age, sex, performance status based on Karnofsky performance score (KPS; Table 4) or World Health Organization score (WHO; Table 5), tumour location, contrast enhancement, tumour histology, tumour mutation status, and methylation status.
Trial characteristics: inclusion and exclusion criteria, randomisation methods and stratification, allocation concealment (if applicable), blinding (of who and when), and statistics. Definitions identified included extent of resection, progression, and AEs.
Interventions: iMRI field strength, imaging sequences, use of contrast, and reporting methods. iUS brand and operator experience, neuronavigation imaging sequences and brand, 5‐ALA dose and timing of administration, use with a microscope.
Outcomes: methods to calculate and measured extent of resection, OS, PFS, and QoL.
Risk of bias in each study.
Duration of follow‐up.
1. Karnofsky performance score.
| Score | Definition |
| 100 | Normal, no complaints, no evidence of disease |
| 90 | Able to carry on normal activity: minor symptoms of disease |
| 80 | Normal activity with effort: some symptoms of disease |
| 70 | Cares for self: unable to carry on normal activity or active work |
| 60 | Requires occasional assistance but is able to care for needs |
| 50 | Requires considerable assistance and frequent medical care |
| 40 | Disabled: requires special care and assistance |
| 30 | Severely disabled: hospitalisation is indicated, death is not imminent |
| 20 | Very sick, hospitalisation is necessary: active treatment is necessary |
| 10 | Moribund, fatal processes are progressing rapidly |
| 0 | Dead |
2. WHO performance score.
| Grade | Definition |
| 0 | Fully active, able to carry on all predisease performance without restriction |
| 1 | Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g. light house work, office work |
| 2 | Ambulatory and capable of all self‐care, but unable to carry out any work activities. Up and about > 50% of waking hours |
| 3 | Capable of only limited self‐care, confined to bed or chair > 50% of waking hours |
| 4 | Completely disabled. Cannot carry out any self‐care. Totally confined to bed or chair |
| 5 | Dead |
WHO: Word Health Organization.
We produced Characteristics of included studies and Characteristics of excluded studies tables.
We extracted data as follows.
For dichotomous outcomes (e.g. extent of resection), we extracted the number of participants in each treatment arm who experienced the outcome of interest and the number of participants assessed at endpoint, in order to estimate a risk ratio (RR) and 95% confidence intervals (CI).
For continuous outcomes (e.g. QoL measures), we extracted the final value and standard deviation of the outcome of interest and the number of participants assessed at endpoint in each treatment arm at the end of follow‐up, in order to estimate the mean difference between treatment arms and its standard error.
For time to event data (OS and PFS), we extracted the log of the hazard ratio (log(HR)) and its standard error from trial reports. If these were not reported, we attempted to estimate the log (HR) and its standard error using the methods of Parmar 1998.
Where possible, all data were extracted relevant to an intention‐to‐treat analysis in which participants are analysed in the groups to which they were assigned.
The time points at which outcomes were collected and reported were noted.
Assessment of risk of bias in included studies
Assessment of risk of bias in studies included in the 2018 review are as described in Jenkinson 2018.
For the NMA, two review authors (DMF and MW) provided independent critical appraisal, with any differences identified and resolved through discussion. We assessed risk of bias in all included RCTs in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). Types of bias considered included selection, performance, detection, attrition and reporting bias. The additional influence of recorded risk of bias on the transitivity assumption in any network meta‐analyses were assessed.
Measures of treatment effect
We measured the level of incoherence where possible (Chapter 11; Higgins 2019), with incoherence factors calculated and statistically tested. We determined treatment effect measurements based on the type of data collected:
continuous data: extent of resection, QoL;
time‐to‐event data: OS, PFS;
dichotomous data: extent of resection.
Unit of analysis issues
We did not anticipate any unit of analysis issues. All network meta‐analyses was planned using Stata (Stata), and this software can deal with issues such as the inclusion of any multi‐arm trials (Chaimani 2017) (i.e. adjust for the correlation between the effect sizes in the NMA).
Dealing with missing data
In the case of missing data required for review outcomes, we contacted study authors as needed. We did not impute missing outcome data.
Assessment of heterogeneity
In the first instance, we decided whether or not included trials were sufficiently clinically and methodologically similar to do a pair‐wise analysis. We then assessed the transitivity assumption based on inclusion criteria and, if deemed sufficiently similar, considered an NMA (Salanti 2014).
If trials appeared similar enough to include, we aimed to further assess transitivity and subsequently heterogeneity between studies by visually inspecting forest plots. Clinical heterogeneity was assessed based on participant characteristics, trial characteristics, and interventions to judge directness. For any pair‐wise analyses in the review, we aimed to report the I2 value and interpret it according to guidelines reported in Section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
We planned to calculate the individual Q statistic for heterogeneity as part of obtaining direct treatment effects in the NMA. We then planned to calculate a Q statistic for inconsistency for global assessment across the NMA based on network estimates for direct and indirect comparisons (Efthimiou 2016). We aimed to report the standard deviation (tau) of the between‐study heterogeneity as outlined in Chapter 11 of Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
Assessment of reporting biases
For the meta‐analysis or NMA, or both, we aimed to construct funnel plots of treatment effect versus precision to investigate the likelihood of publication bias. If these plots suggested that treatment effects may not be sampled from a symmetrical distribution, as assumed by the random‐effects model, we intended to perform additional meta‐analyses using the fixed‐effect model.
Data synthesis
Data synthesis of studies included in the 2018 review are described in Jenkinson 2018.
For the NMA, previously extracted and synthesised data were reviewed and updated if necessary. We planned to generate network plots to demonstrate which direct comparisons the included RCTs had made, with separate network plots for each prespecified outcome as available in the collected data.
For any dichotomous outcomes, we intended to calculate the RR for each study and pooled these.
For continuous outcomes, we intended to pool the mean differences between the treatment arms at the end of follow‐up if all trials measured the outcome on the same scale, otherwise we intended to pool standardised mean differences.
For time‐to‐event data, we intended to pool HRs using the generic inverse variance facility of Review Manager 5 (Review Manager 2014).
We intended to carry out all meta‐analyses in a frequentist framework using Stata using random‐effects models with inverse variance weighting (Stata). We intended to make appropriate decisions about any variability in the interventions and justified all comparisons and, if necessary, split the same interventions in the network if sufficiently different (e.g. measured in a different way).
From each NMA, we intended to report the Surface Under the Cumulative Ranking curve Area (SUCRA) and mean rank statistics to accompany the effect estimates to aid in the interpretation of selecting the most effective imaging technique (Rücker 2015; Salanti 2011). We intended to examine trade‐offs between the different outcomes and interpret all findings in light of risk of bias and GRADE profile for each outcome (GRADE Working Group 2004; Meader 2014).
Subgroup analysis and investigation of heterogeneity
Owing to differences in prognosis, we intended to perform subgroup analyses according to tumour type, including:
HGG;
LGG;
primary versus recurrent disease in HGG and primary disease versus disease progression in LGG.
Sensitivity analysis
We planned to perform a sensitivity analysis to investigate how trial quality affected robustness of findings. We also planned a subsequent sensitivity analysis of trials that included objective blinded early postoperative MRI and histology in their assessment of extent of resection.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to assess confidence in estimates of effect (certainty of evidence) associated with specific comparisons, including estimates from direct, indirect, and final NMA (Brignardello‐Petersen 2018; Puhan 2014; Salanti 2014). Our confidence assessments addressed risk of bias (limitations in study design and execution); inconsistency (heterogeneity of estimates of effects across trials); indirectness (differences in population, interventions, or outcomes to the target of the intended meta‐analysis); and imprecision (e.g. 95% CIs are wide and include or are close to null effect). Limitations in these domains could have resulted in a decrease of the certainty of evidence from high to moderate, low, or very low certainty by –1 (serious concern) or –2 (very serious concern). We based indirect evidence on the most dominant loops (i.e. the shortest path between two treatments) and potentially rated it down for intransitivity (differences in study characteristics that may modify treatment effect in the direct comparisons along the path). We intended to obtain the final NMA confidence rating from the higher of the direct and indirect rating excluding imprecision and indented to rate it down for imprecision and incoherence (difference between direct and indirect estimates). We justified all decisions using footnotes and intended to make comments to aid reader's understanding of the review where necessary.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies tables.
Results of the search
The updated literature search to May 2020 identified 425 records: CENTRAL, 117 references, MEDLINE, 87 references; Embase, 221 references. Following preliminary deduplication across the databases, the combined total was 383 references.
We utilised the Cochrane author support tool Covidence for title and abstract screening of the 383 references.
Two review authors (DMF and MW) independently examined the remaining 20 potential studies. We excluded those studies that clearly did not meet the inclusion criteria, and obtained full‐text copies of five potentially relevant references. Subsequently, no additional studies were identified for inclusion (Figure 1; Figure 2).
1.
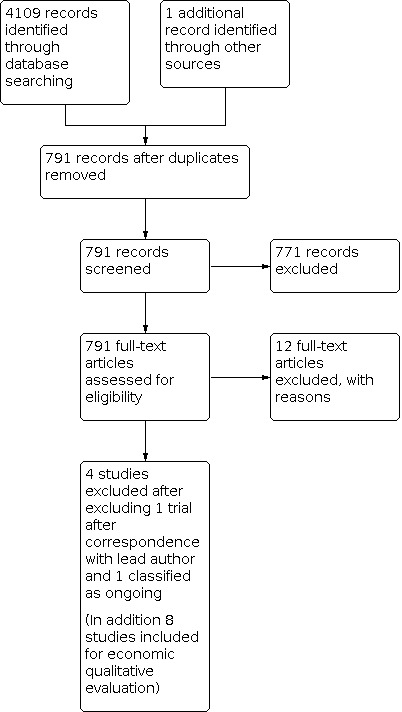
Study flow diagram.
2.
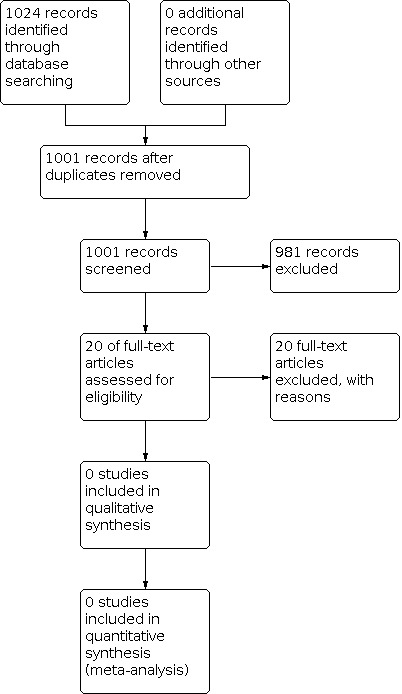
Study flow diagram.
We subsequently re reviewed all ongoing studies identified in the 2018 review (Jenkinson 2018) and updated with additional newly identified ongoing studies (NCT01811121 (RESECT), NCT02150564, NCT03291977 (FLEGME), NCT03531333). One author with an ongoing trial has published several interim analyses (NCT01479686), but the final results remain unpublished. Consequently, this was not included in this version of the review.
Included studies
The four included studies are Kubben 2014; Senft 2011; Stummer 2006 and Willems 2006 and for the purposes of consideration for a NMA are redescribed in detail below and in the Characteristics of included studies table.
In summary, we identified two trials of iMRI (Kubben 2014; Senft 2011), one trial of fluorescence‐guided surgery (Stummer 2006), and one trial of neuronavigation (Willems 2006). We found no eligible studies of ultrasound‐guided surgery.
Intraoperative magnetic resonance imaging
Kubben 2014 recruited 14 participants from multiple centres in Belgium and the Netherlands between 2010 and 2012. Participants had to have a supratentorial brain tumour suspected to be a glioblastoma and an indication for gross total resection. The trial compared surgery with iMRI versus surgery without iMRI (of which either arm could have included neuronavigation). Outcomes were residual tumour volume, complications, QoL (EORTC QLQ‐C30 questionnaire with BN20 brain tumour module, and European Quality of Life‐5 Dimensions (EQ‐5D) questionnaire), and OS. The final results were initially supposed to be an interim analysis, but ultimately the trial was stopped early thereafter. This unplanned interim analysis was not specified a priori, and as a consequence the sample size would not have taken this into account even if the trial had been fully completed. The size of the trial and circumstances around its early completion are reflected in the 'Risk of bias' assessment and GRADE profile (see below).
Senft 2011 recruited 58 participants from a single German neurosurgical centre between 2007 and 2010. Participants had to have a known or suspected glioma that was contrast enhancing and amenable to complete resection. The trial compared surgery with iMRI versus surgery without iMRI (of which either arm could have included neuronavigation). The primary outcome was extent of resection. Secondary outcomes were volume of residual tumour on postoperative MRI, PFS at six months, duration of surgery, and treatment‐related morbidity.
Fluorescence‐guided surgery
Stummer 2006 recruited 322 participants from multiple centres in Germany between 1999 and 2004. Participants had to have a malignant glioma on imaging. The trial compared surgery with 5‐ALA versus surgery without 5‐ALA (of which either arm could have included neuronavigation). Primary outcomes were complete tumour resection on MRI (< 72 hours' postoperation and > 1.5 T) and PFS. Secondary outcomes were residual tumour volume, OS, type and severity of neurological deficits after surgery, and toxic effects.
Neuronavigation
Willems 2006 recruited 45 participants from a single Dutch centre between 1999 and 2002. Participants had to have a solitary intracerebral space‐occupying lesion with (partial) contrast enhancement eligible for surgery with the intention of gross total resection. The trial compared surgery with neuronavigation versus surgery without neuronavigation. Primary outcomes were extent of resection and survival. Secondary outcomes were procedure duration, usefulness of neuronavigation, extent of resection, QoL (EORTC QLQ‐C30 questionnaire with BN20 brain tumour module), and postoperative course (including neurological status and AEs).
Excluded studies
We excluded 32 studies, as follows (see Characteristics of excluded studies).
Five studies were duplicates between searches.
Nine studies were classified as ongoing (see Characteristics of ongoing studies).
Twelve studies were not RCTs (Czyz 2011; Golub 2020; Koc 2008; Stepp 2007; Wu 2003; Wu 2004; Wu 2007; Zhang 2015; Abraham 2018; Abraham 2019; Wadhwa 2019; Waqas 2018).
Three were only presented as abstracts and we were unable to obtain sufficient information even after attempting correspondence with the original trial authors (Chen 2011; Chen 2012; Seddighi 2016).
Three did not directly compare an intraoperative imaging intervention with either another intraoperative imaging intervention or standard surgery (Eljamel 2008; Rohde 2011; Stummer 2017).
All ongoing studies were reviewed, with further written communication in relation to one ongoing RCT performed on 29 June 2020 (NCT01479686). Due to its current status as an active ongoing trial with planned publication of the final report at the time of writing this review, this study is listed as ongoing and will be considered when results are available.
Risk of bias in included studies
Summary data for risk of bias are presented in Figure 3 and Figure 4. A detailed description is provided below and in the Characteristics of included studies table.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation methods
Randomisation methods were described and were satisfactory in all four included trials, for a judgement of low risk of bias (Kubben 2014; Senft 2011; Stummer 2006; Willems 2006).
Allocation concealment
We assessed one trial in which allocation concealment was potentially inadequate (i.e. sealed envelopes) and judged at high risk of bias (Senft 2011), one trial at low risk of bias (Stummer 2006), and the remaining two trials at unclear risk of bias (Kubben 2014; Willems 2006).
Blinding
Three trials performed blinded assessment for extent of resection (Kubben 2014; Senft 2011; Stummer 2006), and one trial for histological assessment (Stummer 2006). Regarding OS, blinding would not affect outcome reporting but could affect subsequent treatment. For QoL, PFS, and AEs, blinding would likely affect the outcomes reported. All trials were not blinded to participants or clinicians.
Incomplete outcome data
One trial accounted for all participants (Kubben 2014). Two trials accounted for all participants, but did not perform an intention‐to‐treat analysis, as those participants that had alternative pathological diagnoses were excluded (Senft 2011; Stummer 2006). In the remaining trial there was evidence of attrition bias for extent of resection (analysis of 32/42 participants) (Willems 2006).
Selective reporting
One trial reported all outcomes and was, therefore, at low risk of reporting bias (Senft 2011). Selective outcome reporting was apparent in three trials: one trial did not report QoL outcomes (Kubben 2014); one trial did not report full outcome data in the form of figures and appropriate statistics for survival, PFS, and AEs for 5‐ALA (Stummer 2006); and one trial did not present full data for survival, QoL, or AEs (Willems 2006). AE data in all studies were particularly poorly reported in terms of total number of events, number of participants with multiple events, and timing of events.
Other potential sources of bias
One of the issues with iMRI is attribution bias. Because surgeons know they can check for residual disease, they do not operate as aggressively as they might if they could not check for residual disease during the operation. So when a scan is done, residual disease is more likely to be detected, removed, and the success of the removal attributed to the iMRI. This is likely to affect outcomes that report a difference between the first intraoperative and final postoperative MRI scans.
Early cessation of trial
All four trials were stopped early based on the results of interim analyses. Kubben 2014 was stopped early based on the results of an interim analysis not specified a priori. Given the low number of participants involved, we excluded this trial from quantitative analysis. Senft 2011 was stopped early based on the results of an interim analysis not specified a priori. Significance values were consequently adjusted (a P < 0.04 was subsequently regarded as significant). Stummer 2006 was stopped early based on the results of a scheduled interim analysis with compensated power calculation. Willems 2006 was stopped early, but no reason was given.
Industry sponsorship
Industry sponsorship was apparent in three trials. Kubben 2014 was financially supported by Medtronic Navigation, but the sponsors "were not involved in writing the protocol, had no access to the data, was not involved in writing the manuscript, and had no veto right for submission." Senft 2011 included authors who had received an honorarium from Medtronic (who manufactured the iMRI machine used in the study), although it was emphasised that the study received no funding from Medtronic. Stummer 2006 was sponsored by medac GmbH (who manufacture Gliolan), which was involved in the study design, quality assurance, and quality control but had no role in the interpretation of data, and the corresponding author had final responsibility for the article (although the author was a paid consultant to both medac GmbH and Zeiss, which manufactures the microscopes used for 5‐ALA). One trial did not state if there were conflicts of interest (Willems 2006).
Effects of interventions
See: Table 1; Table 2; Table 3
Due to considerable heterogeneity across trials and the small number of included trials (four), we did not conduct an NMA or any pair‐wise meta‐analyses. Consequently, we did not conduct any subgroup or sensitivity analyses or investigate publication bias by constructing funnel plots.
Two trials assessed iMRI (Kubben 2014; Senft 2011).
One trial assessed 5‐ALA (Stummer 2006).
One trial assessed neuronavigation (Willems 2006).
Extent of resection
Extent of resection was reported as proportion with incomplete resection in each arm. The RR for the extent of resection in participants with glioma favoured the experimental arms in two of the four trials reporting this outcome, indicating a lower risk of having an incomplete resection with the intervention:
iMRI: Senft 2011 achieved complete tumour resection in 23/24 (96%) participants in the intervention group compared with 17/25 (68%) participants in the control group (RR for incomplete resection 0.13, 95% CI 0.02 to 0.96; very low‐quality evidence). Kubben 2014 reported tumour resection using residual tumour volume and data for complete tumour resections were not available.
5‐ALA: Stummer 2006 performed complete resection in 90/139 (65%) participants in the intervention group versus 47/131 (36%) participants in the control group (RR for incomplete resection 0.55, 95% CI 0.42 to 0.71; low‐quality evidence).
Neuronavigation: Willems 2006 achieved complete resection in three participants in the control group and five participants in the neuronavigation group. However, there was significant attrition, with not all participants having complete imaging, and the denominators for these figures were not stated, precluding meta‐analysis (very low‐quality evidence).
Adverse events
Reporting of AEs was inconsistent between trials and not according to the prespecified manner required in our protocol (Fountain 2020). Specifically, data were not available for participants at risk, participants with multiple events, timing of events, and outcomes of events. Therefore, we adopted a descriptive method using the data available to describe the AEs in each trial.
iMRI: in the trial of Senft 2011, new or aggravated neurological deficits were present in 2/25 (8%) participants in the control group and 3/24 (13%) participants in the iMRI group; intraoperative imaging did not lead to continuation of tumour resection in any of the participants with AEs. Two participants had symptomatic haematomas, which were not attributable to the use of iMRI. In one participant, hemianopia was deliberately accepted due to tumour extension around the temporal horn of the lateral ventricle involving the optic radiation. In the Kubben 2014 trial, one participant in the intervention arm experienced a postoperative haemorrhage.
5‐ALA: AEs were present in 58.7% of the intervention arm versus 57.8% of the control arm in Stummer 2006. Neurological AEs were present in 42.8% of the intervention arm (7.0% grade 3 to 4) and 44.5% of the control arm (5.2% grade 3 to 4). There were significant neurological AEs in 12.4% of the intervention arm versus 11.6% of the control arm. The number of participants with a deterioration in the National Institutes of Health Stroke Scale compared to baseline tended to be higher in the intervention arm at 48 hours (26.2% with 5‐ALA versus 14.5% with control) but not at seven days (20.5% with 5‐ALA versus 10.7% with control), six weeks (17.1% with 5‐ALA versus 11.3% with control), and three months (19.6% with 5‐ALA versus 18.6% with control). No denominators were given for each result, preventing calculation of RRs and CIs.
Neuronavigation: new or worsened neurological deficits were present at three months in 45.5% of participants in the control group and 18.2% of participants in the neuronavigation group in the Willems 2006 trial. During the first three months after surgery, seven participants (31.8%) in the control group and seven (30.4%) in the neuronavigation group experienced a new, non‐neurological AE. In three participants in the neuronavigation group, these events were fatal (pulmonary embolism, cardiac arrest with pulseless electrical activity, and postoperative pulmonary insufficiency). Other AEs included pulmonary or urinary tract infection, surgical removal of an epidural haematoma, surgical cyst drainage, repeated tumour debulking, CSF leakage, postoperative delirium, and insufficiently treated steroid‐induced diabetes.
Overall survival
iMRI: Senft 2011 did not assess OS in the initial publication, although an abstract was published in 2017 included 24/29 (83%) of patients allocated to iMRI group and 21/29 (72.4%) of controls and reported that "iMRI itself did not affect outcome (560 versus 624 days, P = 0.53)". Kubben 2014 did not report OS in the prespecified manner for inclusion.
5‐ALA: in Stummer 2006, there was no difference in OS between the intervention and control arms (HR 0.82, 95% CI 0.62 to 1.07). Median survival was also reported and this was 15.2 months (95% CI 12.9 to 17.5) in the intervention arm versus 13.5 months (95% CI 12.0 to 14.7) in the control arm.
Neuronavigation: Willems 2006 reported the HR to be 1.6, however, no CIs were available or able to be calculated. The median survival time was reported to be nine months in the control arm and 5.6 months in the intervention arm.
Progression‐free survival or time to progression
iMRI: In Senft 2011, HRs or their respective CIs were not available and could not be calculated. The median PFS in the intervention arm was 226 days (95% CI 0.0 to 454) versus 154 days (95% CI 60 to 248) in the control arm. Kubben 2014 did not assess these outcomes.
5‐ALA: HRs and their respective CIs were not available and could not be calculated in Stummer 2006. Median PFS was 5.1 months (95% CI 3.4 to 6.0) in the intervention arm versus 3.6 months (95% CI 3.2 to 4.4 months) in the control arm.
Neuronavigation: Willems 2006 did not assess time to progression or PFS.
Quality of life
iMRI: Senft 2011 and Kubben 2014 did not report data for QoL.
5‐ALA: Stummer 2006 did not assess QoL.
Neuronavigation: In Willems 2006, QoL questionnaires at three months' postoperatively were completed by 19 participants (eight in the neuronavigation arm and 11 in the standard surgery arm), constituting 64.5% of all eligible participants. The questionnaire included the EORTC QLQ‐C30 with 30 general questions, with additionally the 20‐question brain tumour module (BN20). Out of 26 outcome measures that were presented, the direction of change differed in seven (all in the BN20 group): four were in favour of the neuronavigation group and three were in favour of standard surgery. No statistical analysis was presented.
We considered this evidence to be of low to very low quality for all reported outcomes (Table 1; Table 2; Table 3).
For all effects of interventions, meta‐analysis and NMA were not performed. Although all unit of analyses were appropriate, the included studies were excessively heterogeneous clinically for pair‐wise comparison, and there was an insufficient number of studies across interventions with low sample sizes with variations in the image guidance tools used in the control arms (predominantly utilisation of neuronavigation).
Brief economic commentary
To supplement the main systematic review of effects, we sought to identify cost analyses and economic evaluations that compared the interventions with each other or between different variants of the same intervention. A search of MEDLINE and Embase identified seven such studies (Abraham 2019; Eljamel 2016; Esteves 2015a; Hall 2003; Kowalik 2000; Makary 2011; Schulder 2003; Slof 2015) (one study was reported in two papers – Hall 2003; Kowalik 2000). Of the seven studies, four studies compared iMRI to conventional surgery (Abraham 2019; Kowalik 2000; Makary 2011; Schulder 2003); two compared 5‐ALA with white light surgery (Esteves 2015a; Slof 2015); and one compared conventional, 5‐ALA, fluorescein, ultrasound, and iMRI surgery (Eljamel 2016). Three studies were conducted in the USA (Kowalik 2000; Makary 2011; Schulder 2003), one in Portugal (Esteves 2015a), and one in Spain (Slof 2015), and for two it was unclear (but was probably the USA) (Abraham 2019; Eljamel 2016). Four studies were based on non‐randomised retrospective comparative cohorts (Kowalik 2000; Makary 2011; Schulder 2003; Slof 2015); one was based on a review and pair‐wise meta‐analyses (Eljamel 2016), and one used data from a trial and retrospective cohorts (Esteves 2015a). One study parameterised their microsimulation model with data from prospective and retrospective cohorts studies and a randomised trial (Abraham 2019). Costs estimates were derived from existing cost analysis and cost‐effectiveness analysis (including Eljamel 2016). Utility data were derived from a previous health technology assessment and preference elicitation studies, all utility data were derived using the standard gamble method. The studies based on single cohort studies all involved fewer than 100 participants, except for Slof 2015, which included 254 participants who received 5‐ALA and 120 who received white light surgery. All the studies except two (Abraham 2019; Esteves 2015a;), which integrated data using a Markov model and a microsimulation model respectively, were based on comparisons of individual patient level data. In terms of costs, what costs were included and over what time horizon varied markedly. Two studies considered costs over the patient lifetime (Abraham 2019; Esteves 2015a), and one only considered the drug cost (Slof 2015). The other studies considered costs incurred in hospital for the index surgery. Costs were reported in USD in five studies (Abraham 2019; Esteves 2015a; Kowalik 2000; Makary 2011; Schulder 2003), but the price year was stated only in two studies (Abraham 2019; Makary 2011). The other two studies reported costs in EUR, and the price year was 2012 in one study (Esteves 2015a), and not stated in the other (Slof 2015). Two studies were cost analyses only (Kowalik 2000; Schulder 2003). Effects were resection rates (Eljamel 2016), resection‐free years (Makary 2011), quality‐adjusted life years (QALY) (Abraham 2019; Eljamel 2016; Esteves 2015a; Slof 2015), PFS (Esteves 2015a), and life years (Esteves 2015a).
For the comparison of iMRI with conventional surgery, two studies reported a potential cost saving driven by reductions in length of stay (Kowalik 2000; Schulder 2003), and third study reported lower mean costs that were not statistically significant (Makary 2011). The one cost‐effectiveness analysis (Makary 2011) reported a lower complication rate for iMRI versus cMRI in people presenting for their initial tumour resection as well as a longer interval to repeat resection (20.1 months versus 6.7 months; P = 0.02); further results suggested iMRI was more cost‐effective in terms of cost per resection‐free years. Another study reported that iMRI was the most costly of conventional, 5‐ALA, fluorescein, and ultrasound‐assisted surgery (Eljamel 2016). iMRI was the least cost‐effective, but the results could not be replicated from the data presented in the study. Estimates of cost‐effectiveness (and cost over a longer follow‐up) need to considered due to the very limited evidence for iMRI where there is a benefit shown in terms of extent of resection but no evidence in the review of clinical effectiveness on OS. The one cost utility analysis was based on a microsimulation model and simulated cost and outcomes for a hypothetical cohort of 100,000 participants in each arm, the authors did not discuss the rationale for this sample size (Abraham 2019). The authors found that iMRI yielded an incremental benefit of 0.18 QALYs at an incremental cost of USD 13,447, which resulting in an incremental cost‐effectiveness ratio of USD 76,442 per QALY. The authors concluded that there was a 99.5% chance of cost‐effectiveness at a willingness‐to‐pay threshold of USD 100,000 per QALY based on probabilistic sensitivity analysis. The authors did not discuss the reasons for selecting a USD 100,000 per QALY cost‐effectiveness threshold.
For the comparison of 5‐ALA and standard surgery, 5‐ALA was on average more costly in both studies, but results in more quality adjusted life years over the patient lifetime or over the time to progression of disease (Esteves 2015a; Slof 2015). In both cases, the study authors concluded that the extra costs were worth the extra QALYs and that these conclusions were consistent over all sensitivity analyses conducted. These findings of extra effectiveness in the economic studies need to be considered in context of the findings of the review of the best available clinical effectiveness data summarised above.
We did not subject the identified cost analyses and economic evaluations to critical appraisal and we did not attempt to draw any firm or general conclusions regarding the relative costs or efficiency of the iMRI strategies compared. The evidence seems to state there is additional benefit in the additional intraoperative imaging strategies but the value of this benefit seems to vary. The evidence needs to be considered in the context of the decision makers' local context and with different national acceptability thresholds for cost‐effectiveness.
Discussion
Summary of main results
We identified four RCTs in total; two RCTs for iMRI (Kubben 2014; Senft 2011), one for surgery with 5‐ALA (Stummer 2006), and one assessing neuronavigation using preoperative MRI (Willems 2006). Formal NMA standard pair‐wise meta‐analyses were not possible due to the different comparisons and variability in the control arm population between trials. Therefore, we were limited to performing a narrative synthesis of the included trials.
Two trials demonstrated a benefit for intraoperative imaging technology in terms of extent of resection (the primary outcome) (iMRI: Senft 2011; 5‐ALA: Stummer 2006). OS data were available for 5‐ALA and iMRI; there was no evidence that 5‐ALA or iMRI improved OS. Two trials provided data for PFS, and were not available in the format specified (HRs and their variance). Nevertheless, there was a suggestion that 5‐ALA increased PFS compared with standard surgery. One trial reported QoL data, and there was significant attrition and reporting bias. AE reporting varied considerably between trials but in general was poorly performed. With 5‐ALA, it appeared that neurological deterioration was more common after fluorescence‐guided surgery. The studies that reported this effect noted that it occurred mainly among people with fixed deficits and early after surgery, but there was subsequently a trend towards recovery (Stummer 2006). Other AEs appeared to be rare and similar in frequency between study arms.
We did not subject the three identified cost analyses and economic evaluations to critical appraisal and we did not attempt to draw any firm or general conclusions regarding the relative costs or efficiency of the iMRI strategies compared. The evidence seems to state there is additional benefit in the additional intraoperative imaging strategies but the value of this benefit seems to vary. The evidence needs to be considered in the context of the decision makers local context and with different national acceptability thresholds for cost‐effectiveness.
Overall completeness and applicability of evidence
The overall evidence base is incomplete across comparisons and outcomes reported. We only identified four trials and all were small with the exception of one (Stummer 2006), which included 322 participants. The other three included a total of 117 participants, which did not take into account considerable attrition for most outcomes. Furthermore, all the identified trials included highly selected participants in specialised centres, and the applicability of these findings to a more general population needs to be carefully considered. Participants included in the trials tended to be generally young and of good performance status. In addition, most trials also clearly specified the types of tumours that were to be included, and would not have randomised those patients with eloquent tumours or where a complete resection was not feasible. Potentially those enrolled in one of the iMRI trials (Senft 2011) were likely to have more resectable or less eloquent tumours than those in the 5‐ALA trial (Stummer 2006), given the far higher resection rates in both arms of the iMRI study (96% iMRI and 68% control versus 65% 5‐ALA and 36% control).
The majority of included trials only enrolled participants with probable HGG. We identified no RCTs for ultrasound‐guided surgery, which may reflect the less widespread application of this particular technology. There are theoretical advantages to this technology, such as relative affordability, repeatability, and possibly better sensitivity in low‐grade tumours than the other included intraoperative imaging modalities. Nevertheless, it currently does not have the same evidence base as other intraoperative imaging modalities to recommend its use in routine clinical practice.
Quality of the evidence
The certainty of the evidence across all outcomes ranged from low to very low. Therefore, further evidence will certainly have an impact on the results of this review and additional studies in this area are vital. It is clearly feasible to perform RCTs for new surgical interventions, and it appears now to have become standard practice to perform an RCT for assessing new intraoperative imaging technologies. The openness of major centres to enrolling participants in RCTs to provide clear outcome data is a major step forward in neuro‐oncology. Some aspects of the included trials were at low risk of bias, such as randomisation methods and blinded, objective reporting for extent of resection. However, the overall the risk of bias was high, and there were consistent concerns with stopping trials early and the role of industry involvement (Table 1; Table 2; Table 3).
Extent of resection was the primary outcome for all the included trials. This has the advantage of being the outcome most directly influenced by intraoperative imaging. However, there is still no evidence from RCTs that resection (either total or less than total) improves outcomes for HGG over biopsy alone (Hart 2019). Subgroup analyses, particularly for the 5‐ALA trial (Stummer 2006), have shown that those participants that have a complete resection of all contrast‐enhancing tumour survive longer than those with residual tumour (Pichlmeier 2008). Studies of chemotherapy have also found that those without residual tumour survive longer (Stupp 2005). While this is not direct evidence in favour of complete resection, but rather a post hoc non‐randomised subgroup analysis, it is becoming increasingly apparent that a complete tumour resection is desirable, particularly when it can be achieved safely. Precisely how much a complete resection contributes towards the overall outcome is unclear. New methods of imaging (e.g. amino acid positron emission tomography) have found that tumours frequently extend out from the contrast‐enhancing margin on MRI (Miwa 2004). However, validation of this approach has yet to be established, and the need for a cyclotron makes widespread application and testing a challenge in the UK; therefore, MRI for assessing residual tumour remains the current standard of care.
After extent of resection, studies tended to focus on PFS rather than OS. There are certain advantages to this in that possibly fewer participants are required and the results may be available sooner. Additionally, it may provide a more direct assessment of the effect of the primary intervention that is not confounded by subsequent therapy. However, it can be argued that OS should remain the main outcome of interest. First, survival is so short in HGG that the practical benefits of assessing PFS are less relevant. Second, assessment of PFS can be more subjective, and is critically dependent on the timing and interpretation of imaging, which can often be complicated (Wen 2010).
Quality control for surgical neuro‐oncology trials is an important area (Chang 2007). Standardisation of reporting is required to allow clear comparisons between trials in meta‐analyses. Detailed reporting is required for tumour location with regard to eloquent brain; operative technique used; postoperative imaging protocol; assessment of extent of resection; and recording of AEs (including total numbers of events, total number of participants at risk, number of participants with multiple events; severity, timing, and outcome of events, i.e. resolution or persistence of neurological deficits).
Potential biases in the review process
We took multiple steps in the original published and updated review process to minimise bias, including double independent literature sift and data extraction, not pooling results due to heterogeneity, and using strict inclusion criteria. Overall, these steps acted to minimise bias and restrict the review to the best available evidence.
Notably, the majority of trials identified through the search strategy were not RCTs. It could be argued that excluding this volume of data biases our review and that it would be more appropriate to consider a Cochrane Review of non‐randomised studies (NRS). In particular in the completion of a meta‐analysis and NMA, there have recently been peer‐reviewed publications on this topic (see Agreements and disagreements with other studies or reviews).
However, the issue of selection bias is critical, particularly in surgical trials. Participants enrolled in an NRS are likely to have a better prognosis than a control population, and it is impossible to accurately account for this bias without using randomisation. Therefore, it would be unclear what benefit intraoperative imaging had on the overall outcome. Meta‐analysis of RCTs remains the most reliable way of assessing the benefits of specific intraoperative imaging modalities. However, NRS may also have a role, particularly regarding technology development and reporting of AEs.
This review included two specific groups of technologies, those that used imaging obtained intraoperatively and those that used imaging obtained preoperatively for use in an intraoperative manner. We felt that both methods were suitable for comparison, as the goals are similar: namely, to achieve maximal safe resection via the application of surgical technology. A major concern with preoperative imaging is intraoperative brain shift, whereby anatomical localisation is affected by events that occur during surgery (e.g. anaesthesia, brain retraction, tumour resection, dural opening, and CSF drainage). Imaging obtained intraoperatively can theoretically account for brain shift and allow more accurate navigation than imaging obtained preoperatively. In this review, we found that a single trial did not demonstrate an effect for intraoperative imaging utilising preoperatively acquired data (Willems 2006).
Another technique that is commonly used in neuro‐oncology surgery is awake craniotomy. This is often perceived as a technology to make surgery safer by allowing intraoperative mapping of eloquent brain. It is not typically regarded as a technique to maximise extent of resection and was, therefore, not included in this review.
We did not subject these studies to critical appraisal, and we did not attempt to draw any firm or general conclusions regarding the relative costs or efficiency of the interventions being compared. For the comparison of iMRI surgery with conventional surgery, it is clear that the available economic evidence is, at best, equivocal. For the comparison of 5‐ALA with white light surgery, the available economic evidence indicates that, from an economic perspective, use of 5‐ALA could be a promising strategy but effectiveness data used in the economic studies were not consistent with the findings of the review of effectiveness.
Agreements and disagreements with other studies or reviews
Aforementioned interim analyses of an ongoing trial of iMRI is broadly in agreement with the findings of this review (NCT01479686). This reported outcomes on 202 participants with follow‐up data for 177 patients. Complete resection was achieved in 86% of the iMRI arm versus 45% in the control arm (P < 0.0001). Patients in the iMRI arm with eloquent HGGs had significantly longer PFS and OS compared to the control group. There were no AEs of iMRI reported.
Golub and colleagues have recently published a NMA of 5‐ALA and iMRI in HGG surgery (Golub 2020). The NMA included 11 randomised and NRS, including two RCTs from this review (Senft 2011; Stummer 2006), although notably Senft 2011 was classed as a retrospective study. This NMA also included the data published from the interim results of the RCT not included in this review (NCT01479686). The NMA performed revealed that both iMRI and 5‐ALA were superior to neuronavigation in achieving gross total resection, with a smaller number of studies additionally demonstrating superior PFS and OS. There was no evidence of superiority between iMRI and 5‐ALA demonstrated (Golub 2020).
Furthermore, Coburger and Wirtz performed a systematic review of fluorescence‐guided surgery by 5‐ALA and iMRI in HGG (Coburger 2019). Given the broader inclusion criteria of randomised and NRS without a control group, a total of 22 studies were included in the review including two RCTs from this review (Senft 2011; Stummer 2006). The review concluded that both iMRI and 5‐ALA were superior to neuronavigation in achieving gross total resection of non‐eloquent lesions, while additionally not increasing the rate of permanent neurological deficit or reduction in QoL (Coburger 2019).
Authors' conclusions
Implications for practice.
Intraoperative imaging technologies, specifically intraoperative magnetic resonance imaging (iMRI) and 5‐aminolevulinic acid (5‐ALA), may be of benefit in maximising extent of resection in participants with high‐grade glioma. However, this is based on low‐ to very low‐quality evidence, and is, therefore, very uncertain. The short‐ and long‐term neurological effects remain uncertain.
The purpose of these technologies is to make surgical resection safer and more effective. Patient selection, patient‐specific information, and informed consent are all essential to ensure that these technologies are used appropriately in the pathway of care. Standardisation of patient management using evidence‐based clinical practice will ensure consistent surgical standards of care wherever a patient is treated.
Patient selection must be emphasised. All the trials included predominantly young participants of good performance status and with a well‐defined tumour in a non‐eloquent region that was amenable to safe complete resection.
Due to the absence of sufficient evidence of high quality, it is not possible to draw any firm conclusions regarding the superiority of one intraoperative imaging technology from another. The utility of each technology should be considered on a case‐by‐case basis with involvement of the patient as early as possible until more robust evidence is identified.
Implications for research.
The current studies provide a very limited knowledge base upon which to consider implementing such technologies and the quality of the evidence is this review is low or very low‐certainty for all outcomes involving all comparisons. Important questions remain about benefit in terms of overall survival, progression‐free survival, and the risk of adverse events. Future trials could be done with a similar design to those already performed but with simple improvements to the trial methodology and outcome reporting.
A direct comparison between individual intraoperative imaging technologies could be of benefit to compare their relative merits and in particular help to provide cost‐effectiveness data. The most logical comparison would be between iMRI and 5‐ALA, while ultrasound and advanced imaging neuronavigation (e.g. tractography of functional imaging based) have theoretical advantages but currently have not been the subject of a completed and published randomised controlled trial (RCT). However, units with access to all technologies are likely to be rare, and patients who are suitable for either procedure are likely to be very highly selected, although experience‐based RCTs are a possible way around this. Nevertheless, there are ongoing RCTs comparing different forms of image‐guided surgery, and these can hopefully be incorporated into an update of this review once they are completed (see Characteristics of ongoing studies table, in particular NCT01479686). A network meta‐analysis may then be possible, allowing indirect comparisons of each technology, and a formal economic analysis could allow financial factors to be facilitated into the equation.
Evidence regarding extent of resection and the means with which to achieve this is becoming stronger, but this still needs to be balanced with making surgery safer. Awake craniotomy is probably the main means of enabling a maximal safe resection, particularly with tumours in eloquent areas. A comparison of tractography or functional magnetic resonance imaging‐guided surgery versus awake craniotomy is potentially a relevant question for resection of tumours in eloquent areas.
History
Protocol first published: Issue 5, 2020 Review first published: Issue 12, 2020
Acknowledgements
We thank the Gynaecological, Neuro‐oncology and Orphan Cancers (GNOC) Information Specialist, Jo Platt, for preparing the search strategy; the Managing Editors, Gail Quinn and Clare Jess, for their advice and editorial support; and Assistant Managing Editor, Tracey Harrison, for administrative support. We also thank Robin Grant, Co‐ordinating Editor for GNOC for clinical support. We would also like to acknowledge Tess Lawrie and Luke Vale who contributed to earlier versions of this review.
This project is supported by the National Institute for Health Research (NIHR), via Cochrane Programme Grant funding (NIHR 16/144) to the GNOC. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the National Health Service, or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1. MeSH descriptor: [Central Nervous System Neoplasms] explode all trees #2. ((central nervous system or CNS or brain* or cerebral* or intracerebral or intra‐cerebral or intracranial or intra‐cranial or spine or spinal or astrocytic or oligodendroglial or ependymal) near/5 (cancer* or tumor* or tumour* or malignan* or neoplas* or carcinoma* or metastat*)) #3. MeSH descriptor: [Neoplasms, Neuroepithelial] explode all trees #4. ((cranial or paraspinal or meninges or haematopoietic system or germ cell or germ‐cell or sellar or glioneural or neuroectodermal or embryonal or neuroepithelial or pineal or choroid plexus or teratoid or rhabdoid) near/5 (tumor* or tumour*)) #5. MeSH descriptor: [Glioma] explode all trees #6. glioma* or glial* or astrocytoma* or xanthoastrocytoma* or glioblastoma* or gliosarcoma* or oligodendrogli* or oligoastrocyt* or ependym* or subependym* or astroblastoma* or ganglioglioma* or gangliocytoma* or neurocytoma* or liponeurocytoma* or pineocytoma* or pineoblastoma* or medulloblastoma* or neuroblastoma* or ganglioneuroblastoma*or medulloepithelioma* or GBM* #7. #1 or #2 or #3 or #4 or #5 or #6 #8. MeSH descriptor: [Magnetic Resonance Imaging] explode all trees #9. intra operative magnetic resonance imag* or intra‐operative magnetic resonance imag* or intra operative MRI or intra‐operative MRI or iMRI or ioMRI or IOMRI or IoMRI or MRI or MRi or NMRI or NMRi or magnetic resonance imag* or tractography #10. MeSH descriptor: [Ultrasonography] explode all trees #11. (2D or 3D) near/5 (ultras* or US) #12. ((intra‐operative or intraoperative) near/5 (ultras* or US or IOUS or imag* or navigat* or technolog* or modalit* or eval* or monitor*)) #13 volumetric reconstruction or Sonowand or SonoWand #14. MeSH descriptor: [Neuronavigation] this term only #15. MeSH descriptor: [Surgery, Computer‐Assisted] this term only #16. navigat* or neuronavigat* or neuro‐navigat* or image guid* #17. Brainlab or Stealth #18. MeSH descriptor: [Monitoring, Intraoperative] explode all trees #19. MeSH descriptor: [Fluorescence] this term only #20. MeSH descriptor: [Aminolevulinic Acid] this term only #21. fluorescen* or immunofluorescen* #22. aminolevulinic acid or 5‐aminolevulinic acid #23. ALA or 5‐ALA or Gliolan #24. #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 #25. #7 and #24
Appendix 2. MEDLINE search strategy
Intervention search strategy
1. exp Central Nervous System Neoplasms/ 2. ((central nervous system or CNS or brain* or cerebral* or intracerebral or intra‐cerebral or intracranial or intra‐cranial or spine or spinal or astrocytic or oligodendroglial or ependymal) adj5 (cancer* or tumor* or tumour* or malignan* or neoplas* or carcinoma* or metastat*)).mp. 3. exp neoplasms, neuroepithelial/ 4. ((cranial or paraspinal or meninges or haematopoietic system or germ cell or germ‐cell or sellar or glioneural or neuroectodermal or embryonal or neuroepithelial or pineal or choroid plexus or teratoid or rhabdoid) adj5 (tumor* or tumour*)).mp. 5. exp Glioma/ 6. (glioma* or glial* or astrocytoma* or xanthoastrocytoma* or glioblastoma* or gliosarcoma* or oligodendrogli* or oligoastrocyt* or ependym* or subependym* or astroblastoma* or ganglioglioma* or gangliocytoma* or neurocytoma* or liponeurocytoma* or pineocytoma* or pineoblastoma* or medulloblastoma* or neuroblastoma* or ganglioneuroblastoma*or medulloepithelioma* or GBM*).mp. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp Magnetic Resonance Imaging/ 9. (intra operative magnetic resonance imag* or intra‐operative magnetic resonance imag* or intra operative MRI or intra‐operative MRI or iMRI or ioMRI or IOMRI or IoMRI or MRI or MRi or NMRI or NMRi or magnetic resonance imag* or tractography).mp. 10. exp Ultrasonography/ 11. ((2D or 3D) adj5 (ultras* or US)).mp. 12. ((intra‐operative or intraoperative) adj5 (ultras* or US or IOUS or imag* or navigat* or technolog* or modalit* or eval* or monitor*)).mp. 13. (volumetric reconstruction or Sonowand or SonoWand).mp. 14. Neuronavigation/ 15. Surgery, Computer‐Assisted/ 16. (navigat* or neuronavigat* or neuro‐navigat* or image guid*).mp. 17. (Brainlab or Stealth).mp. 18. exp Monitoring, Intraoperative/ 19. Fluorescence/ 20. Aminolevulinic Acid/ 21. (fluorescen* or immunofluorescen*).mp. 22. (aminolevulinic acid or 5‐aminolevulinic acid).mp. 23. (ALA or 5‐ALA or Gliolan).mp. 24. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 25. 7 and 24 26. randomized controlled trial.pt. 27. controlled clinical trial.pt. 28. randomized.ab. 29. placebo.ab. 30. clinical trials as topic.sh. 31. randomly.ab. 32. trial.ti. 33. 26 or 27 or 28 or 29 or 30 or 31 or 32 34. (animals not (humans and animals)).sh. 35. 33 not 34 36. 25 and 35
Key mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier ab = abstract sh = subject heading ti = title pt = publication type
Economic search strategy
1. exp Central Nervous System Neoplasms/ 2. ((central nervous system or CNS or brain* or cerebral* or intracerebral or intra‐cerebral or intracranial or intra‐cranial or spine or spinal or astrocytic or oligodendroglial or ependymal) adj5 (cancer* or tumor* or tumour* or malignan* or neoplas* or carcinoma* or metastat*)).mp. 3. exp neoplasms, neuroepithelial/ 4. ((cranial or paraspinal or meninges or haematopoietic system or germ cell or germ‐cell or sellar or glioneural or neuroectodermal or embryonal or neuroepithelial or pineal or choroid plexus or teratoid or rhabdoid) adj5 (tumor* or tumour*)).mp. 5. exp Glioma/ 6. (glioma* or glial* or astrocytoma* or xanthoastrocytoma* or glioblastoma* or gliosarcoma* or oligodendrogli* or oligoastrocyt* or ependym* or subependym* or astroblastoma* or ganglioglioma* or gangliocytoma* or neurocytoma* or liponeurocytoma* or pineocytoma* or pineoblastoma* or medulloblastoma* or neuroblastoma* or ganglioneuroblastoma*or medulloepithelioma* or GBM*).mp. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp Magnetic Resonance Imaging/ 9. (intra operative magnetic resonance imag* or intra‐operative magnetic resonance imag* or intra operative MRI or intra‐operative MRI or iMRI or ioMRI or IOMRI or IoMRI or MRI or MRi or NMRI or NMRi or magnetic resonance imag* or tractography).mp. 10. exp Ultrasonography/ 11. ((2D or 3D) adj5 (ultras* or US)).mp. 12. ((intra‐operative or intraoperative) adj5 (ultras* or US or IOUS or imag* or navigat* or technolog* or modalit* or eval* or monitor*)).mp. 13. (volumetric reconstruction or Sonowand or SonoWand).mp. 14. Neuronavigation/ 15. Surgery, Computer‐Assisted/ 16. (navigat* or neuronavigat* or neuro‐navigat* or image guid*).mp. 17. (Brainlab or Stealth).mp. 18. exp Monitoring, Intraoperative/ 19. Fluorescence/ 20. Aminolevulinic Acid/ 21. (fluorescen* or immunofluorescen*).mp. 22. (aminolevulinic acid or 5‐aminolevulinic acid).mp. 23. (ALA or 5‐ALA or Gliolan).mp. 24. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 25. 7 and 24 26. economics/ 27. exp "costs and cost analysis"/ 28. economics, dental/ 29. exp "economics, hospital"/ 30. economics, medical/ 31. economics, nursing/ 32. economics, pharmaceutical/ 33. (economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. 34. (expenditure$ not energy).ti,ab. 35. (value adj1 money).ti,ab. 36. budget$.ti,ab. 37. 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 38. ((energy or oxygen) adj cost).ti,ab. 39. (metabolic adj cost).ti,ab. 40. ((energy or oxygen) adj expenditure).ti,ab. 41. 38 or 39 or 40 42. 37 not 41 43. letter.pt. 44. editorial.pt. 45. historical article.pt. 46. 43 or 44 or 45 47. 42 not 46 48. Animals/ 49. Humans/ 50. 48 not (48 and 49) 51. 47 not 50 52. 25 and 51
Key mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier ab=abstract sh=subject heading ti=title pt=publication type
Appendix 3. Embase search strategy
Intervention search strategy
1. exp central nervous system tumor/ 2. ((central nervous system or CNS or brain* or cerebral* or intracerebral or intra‐cerebral or intracranial or intra‐cranial or spine or spinal or astrocytic or oligodendroglial or ependymal) adj5 (cancer* or tumor* or tumour* or malignan* or neoplas* or carcinoma* or metastat*)).mp. 3. exp neuroepithelioma/ 4. ((cranial or paraspinal or meninges or haematopoietic system or germ cell or germ‐cell or sellar or glioneural or neuroectodermal or embryonal or neuroepithelial or pineal or choroid plexus or teratoid or rhabdoid) adj5 (tumor* or tumour*)).mp. 5. exp glioma/ 6. (glioma* or glial* or astrocytoma* or xanthoastrocytoma* or glioblastoma* or gliosarcoma* or oligodendrogli* or oligoastrocyt* or ependym* or subependym* or astroblastoma* or ganglioglioma* or gangliocytoma* or neurocytoma* or liponeurocytoma* or pineocytoma* or pineoblastoma* or medulloblastoma* or neuroblastoma* or ganglioneuroblastoma*or medulloepithelioma* or GBM*).mp. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp nuclear magnetic resonance imaging/ 9. (intra operative magnetic resonance imag* or intra‐operative magnetic resonance imag* or intra operative MRI or intra‐operative MRI or iMRI or ioMRI or IOMRI or IoMRI or MRI or MRi or NMRI or NMRi or magnetic resonance imag* or tractography).mp. 10. exp echography/ 11. ((2D or 3D) adj5 (ultras* or US)).mp. 12. ((intra‐operative or intraoperative) adj5 (ultras* or US or IOUS or imag* or navigat* or technolog* or modalit* or eval* or monitor*)).mp. 13. (volumetric reconstruction or Sonowand or SonoWand).mp. 14. neuronavigation/ 15. computer assisted surgery/ 16. (navigat* or neuronavigat* or neuro‐navigat* or image guid*).mp. 17. (Brainlab or Stealth).mp. 18. exp intraoperative monitoring/ 19. fluorescence/ 20. aminolevulinic acid/ 21. (fluorescen* or immunofluorescen*).mp. 22. (aminolevulinic acid or 5‐aminolevulinic acid).mp. 23. (ALA or 5‐ALA or Gliolan).mp. 24. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 25. 7 and 24 26. crossover procedure/ 27. double‐blind procedure/ 28. randomized controlled trial/ 29. single‐blind procedure/ 30. random*.mp. 31. factorial*.mp. 32. (crossover* or cross over* or cross‐over*).mp. 33. placebo*.mp. 34. (double* adj blind*).mp. 35. (singl* adj blind*).mp. 36. assign*.mp. 37. allocat*.mp. 38. volunteer*.mp. 39. 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 40. 25 and 39 41. (exp animal/ or nonhuman/ or exp animal experiment/) not human/ 42. 40 not 41
Key mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier ab=abstract sh=subject heading ti=title pt=publication type
Economic search strategy
1. exp central nervous system tumor/ 2. ((central nervous system or CNS or brain* or cerebral* or intracerebral or intra‐cerebral or intracranial or intra‐cranial or spine or spinal or astrocytic or oligodendroglial or ependymal) adj5 (cancer* or tumor* or tumour* or malignan* or neoplas* or carcinoma* or metastat*)).mp. 3. exp neuroepithelioma/ 4. ((cranial or paraspinal or meninges or haematopoietic system or germ cell or germ‐cell or sellar or glioneural or neuroectodermal or embryonal or neuroepithelial or pineal or choroid plexus or teratoid or rhabdoid) adj5 (tumor* or tumour*)).mp. 5. exp glioma/ 6. (glioma* or glial* or astrocytoma* or xanthoastrocytoma* or glioblastoma* or gliosarcoma* or oligodendrogli* or oligoastrocyt* or ependym* or subependym* or astroblastoma* or ganglioglioma* or gangliocytoma* or neurocytoma* or liponeurocytoma* or pineocytoma* or pineoblastoma* or medulloblastoma* or neuroblastoma* or ganglioneuroblastoma*or medulloepithelioma* or GBM*).mp. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp nuclear magnetic resonance imaging/ 9. (intra operative magnetic resonance imag* or intra‐operative magnetic resonance imag* or intra operative MRI or intra‐operative MRI or iMRI or ioMRI or IOMRI or IoMRI or MRI or MRi or NMRI or NMRi or magnetic resonance imag* or tractography).mp. 10. exp echography/ 11. ((2D or 3D) adj5 (ultras* or US)).mp. 12. ((intra‐operative or intraoperative) adj5 (ultras* or US or IOUS or imag* or navigat* or technolog* or modalit* or eval* or monitor*)).mp. 13. (volumetric reconstruction or Sonowand or SonoWand).mp. 14. neuronavigation/ 15. computer assisted surgery/ 16. (navigat* or neuronavigat* or neuro‐navigat* or image guid*).mp. 17. (Brainlab or Stealth).mp. 18. exp intraoperative monitoring/ 19. fluorescence/ 20. aminolevulinic acid/ 21. (fluorescen* or immunofluorescen*).mp. 22. (aminolevulinic acid or 5‐aminolevulinic acid).mp. 23. (ALA or 5‐ALA or Gliolan).mp. 24. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 25. 7 and 24 26. Health Economics/ 27. exp Economic Evaluation/ 28. exp Health Care Cost/ 29. pharmacoeconomics/ 30. 26 or 27 or 28 or 29 31. (econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. 32. (expenditure$ not energy).ti,ab. 33. (value adj2 money).ti,ab. 34. budget$.ti,ab. 35. 31 or 32 or 33 or 34 36. 30 or 35 37. letter.pt. 38. editorial.pt. 39. note.pt. 40. 37 or 38 or 39 41. 36 not 40 42. (metabolic adj cost).ti,ab. 43. ((energy or oxygen) adj cost).ti,ab. 44. ((energy or oxygen) adj expenditure).ti,ab. 45. 42 or 43 or 44 46. 41 not 45 47. 25 and 46 48. (exp animal/ or nonhuman/ or exp animal experiment/) not human/ 49. 47 not 48
Key mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier ab=abstract sh=subject heading ti=title pt=publication type
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kubben 2014.
| Study characteristics | ||
| Methods | Randomised controlled trial. Randomisation: performed by the first author using specific software for randomisation in clinical trials. No randomisation blocks used. Sample size: to reduce the chance for type I errors (false positive), used an alpha of 0.05. To reduce the chance for type II errors (false negative), used a beta of 0.2 leading to a power of 0.8. Considered a 10% additional resection of the preoperative tumour volume as the minimal clinically relevant difference, with an estimated standard deviation of approximately 12%. This led to 23 patients in each treatment group. To compensate for loss to follow‐up, intention was to include a total of 54 patients. Blinding: neurosurgeon could not be blinded for the procedure. No intention to blind the physicians on the ward or the patients. Volumetric assessment of pre‐ and postoperative tumour volume performed by a single blinded researcher. |
|
| Participants | Inclusion criteria: supratentorial brain tumour suspected to be glioblastoma on contrast‐enhanced diagnostic MRI, indication for gross total resection of the tumour, aged ≥ 18 years, WHO Performance Scale ≥ 2, ASA class ≥ 3, adequate knowledge of the Dutch or French language, and informed consent. Exclusion criteria: recurrent brain tumour, multiple brain tumour localisations, earlier skull radiotherapy, earlier chemotherapy for glioblastoma, chronic kidney disease or other renal function disorder, and a known magnetic resonance‐contrast allergy. |
|
| Interventions | Intervention: low field iMRI (Medtronic PoleStar N20 0.15 Tesla moveable magnet and the StarShield tent) Control: neuronavigation‐guided tumour resection |
|
| Outcomes | RTV; complications; quality of life (EORTC QLQ‐C30 questionnaire with QLQ‐BN20 brain tumour module, and EQ‐5D questionnaire); overall survival | |
| Notes | Sponsored by Medtronic. Quote: "This study is part of the PhD thesis of the first author, and has been financially supported by Medtronic Navigation. Medtronic Navigation was not involved in writing the protocol, had no access to the data, was not involved in writing the manuscript, and had no veto right for submission." Definitions: RTV percentage was used as the primary endpoint to assess extent of tumour resection. Pre‐ and postoperative tumour volume was calculated by segmenting the hyperintense area on contrast‐enhanced T1 MRI (including enclosed central necrosis) and subtracting the hyperintense area on native T1 MRI to compensate for blood in the resection cavity. Measurements were performed using OsiriX software (Pixmeo SARL, Bernex, Switzerland) on Mac OS X using a Wacom Bamboo pen mouse for contour drawing. Postoperative tumour volume was divided by preoperative tumour volume to calculate the fraction of RTV. Multiplying the fraction with 100% provided the RTV. In formula: RTV = (postoperative contrast enhancement/preoperative contrast enhancement) × 100% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed by the first author using TEN‐ALEA software for randomisation in clinical trials. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The neurosurgeon could not be blinded for the procedure. We did not intend to blind the physicians on the ward, nor the patients. Volumetric assessment of pre‐ and postoperative tumor volume was performed by a single blinded researcher." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The neurosurgeon could not be blinded for the procedure. We did not intend to blind the physicians on the ward, nor the patients. Volumetric assessment of pre‐ and postoperative tumor volume was performed by a single blinded researcher." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for and included in the analysis. |
| Selective reporting (reporting bias) | High risk | Quality of life data not reported. Quote: "After consultation of a health‐technology assessment expert we decided to refrain from any further statistical analyses due to the small sample size." |
| Other bias | High risk |
|
Senft 2011.
| Study characteristics | ||
| Methods | Randomised controlled trial. Randomisation: blocks of 4 on a 1‐to‐1 ratio using BiAS for Windows 9.01 by an assistant who had no clinical involvement in the trial. Sample size: sample size calculation was done to detect a difference of 25% between groups for the primary endpoint with a power of 80%. Blinding: investigators who assessed eligibility of participants and scheduled surgeries were masked to treatment group assignment by use of a sealed envelope design. Surgeons and participants were not masked to the treatment group assignment, but the neuroradiologist who analysed MRI data was masked. |
|
| Participants | Inclusion criteria: adults aged ≥ 18 years with known or suspected gliomas showing distinct contrast enhancement on T1‐weighted MRI amenable to radiologically complete resection. Exclusion criteria: presence of cardiopulmonary or hepatorenal comorbidities; tumours that crossed the midline or were located in the basal ganglia, cerebellum, brain stem, or otherwise in close proximity to eloquent brain structures prohibiting or questioning complete resectability; contraindications to MRI examination (e.g. pacemaker); and inability to give consent due to neuropsychological deficits or a language barrier. |
|
| Interventions | Intervention: mobile intraoperative ultralow field (0.15 Tesla) MRI system (PoleStar N‐20, Odin Medical Technologies, Yokneam, Israel and Medtronic, Louisville, CO, USA). Control: conventional microneurosurgical resection including Cavitron Ultrasonic Aspirator (CUSA) and neuronavigation. Use of intraoperative ultrasound or fluorescence‐guided surgery with 5‐aminolevulinic acid was not allowed in either group. |
|
| Outcomes | Primary: extent of resection. Secondary: volume of residual tumour on postoperative MRI and PFS at 6 months; duration of surgery; and treatment‐related morbidity. |
|
| Notes | Definitions: All participants underwent high‐field MRI at 1.5 T or 3.0 T with and without contrast agent within 7 days before surgery and within 72 hours after surgery. 1 masked, independent, and experienced neuroradiologist (AB) assessed MRIs to establish the extent of resection and undertake volumetric analyses of the tumours and tumour residues. Residual tumour defined as detectable contrast enhancement on T1‐weighted imaging with a volume > 0.175 cm3 on postoperative MRI as done previously. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation of participants in blocks of 4 on a 1‐to‐1 ratio using BiAS for Windows 9.01 by an assistant who had no clinical involvement in the trial. |
| Allocation concealment (selection bias) | High risk | Sealed envelope design. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Surgeons and participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only the neuroradiologist analysing the MRI data was blinded, which is important for assessing extent of resection. Assessors of clinical outcomes were not masked, which would have affected PFS and treatment‐related morbidity. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 49/58 participants analysed (4 excluded in each arm due to diagnosis of a metastasis, and 1 in the iMRI arm withdrew consent). |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported (extent of resection, RTV, PFS, and treatment‐related morbidity). |
| Other bias | High risk |
|
Stummer 2006.
| Study characteristics | ||
| Methods | Randomised controlled trial. Randomisation: dynamic allocation algorithm at a separate research unit, in which participants were allocated to minimise the imbalance between treatment groups. No permuted block randomisation applied. Treatment allocation was communicated to local investigators first by telephone and additionally by fax. Sample size: initial power calculations estimated 350 participants were required for an 80% power, but to allow premature study termination an interim analysis was scheduled after 270 participants whereby a 20\5 difference in PFS could be identified with a power of 80%. |
|
| Participants | Inclusion criteria: aged 18–72 years with suspected (as assessed by study surgeon) newly diagnosed and untreated malignant glioma. Tumours were to have a distinct ring‐like pattern of contrast enhancement with thick irregular walls on MRI and a core area of reduced signal suggestive of tumour necrosis. Exclusion criteria: tumours in the midline, basal ganglia, cerebellum, or brain stem; > 1 contrast‐enhancing lesion; substantial, non‐contrast‐enhancing tumour with areas suggesting low‐grade glioma with malignant transformation; medical reasons precluding MRI; inability to give consent; tumour location that did not enable complete resection; KPS ≤ 60; renal or liver insufficiency; and history of previous systemic malignancy. |
|
| Interventions | Intervention: 5‐aminolevulinic acid (20 mg/kg bodyweight; medac, Wedel, Germany) in freshly prepared solutions orally 3 hours (range 2–4) preoperatively. Solutions were prepared by dissolving the contents of a vial (1.5 g) in 50 mL of drinking water. Surgery was done using a modified neurosurgical microscope (OPMI Neuro/NC4 system with fluorescence kit, Carl Zeiss Surgical GmbH, Oberkochen, Germany), which enabled switching from conventional white xenon illumination to violet–blue excitation light. Control: conventional microsurgery with white light. No placebo. For participants assigned white light, the tumour was resected using conventional illumination. |
|
| Outcomes | Primary endpoints: complete tumour resection on MRI (< 72 hours postoperation and > 1.5 T) and PFS. Secondary endpoints: RTV, overall survival, type and severity of neurological deficits after surgery, and toxic effects. Follow‐up at 6 weeks then 3 months and subsequently at 3 monthly intervals until 18 months. |
|
| Notes | Residual tumour was defined as contrast enhancement with a volume > 0.175 cm3. Progression was defined as the occurrence of a new tumour lesion with a volume > 0.175 cm3, or an increase in RTV > 25%. PFS defined radiologically in the initial trial and by combined measures in the follow‐up paper (radiological criteria as above plus any new tumour or neurological worsening as defined by an NIHSS score increase > 1). Adverse events classified according to the US National Cancer Institute common toxicity criteria (version 1.0). The NIHSS was used to measure postoperative deficits at 2 and 7 days after surgery, radiological progression at 6 weeks, then at 3, 6, 9, 12, 15, and 18 months' postsurgery. Intercentre consistency not presented. The manufacturer of 5‐aminolevulinic acid (medac GmbH) was involved in the trial, and authors received assistance from the sponsor. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Performed independently with a dynamic allocation algorithm. |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was communicated by telephone and fax. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of surgeons, participants, or those involved with treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Neuropathology and neuroradiological assessments were blinded, which is important for assessing extent of resection. Clinical outcome assessment was not blinded, which would have affected PFS and adverse events. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13 participants were excluded for major violations of MRI inclusion criteria. 34 participants were excluded for histological criteria. In total, out of 322 randomly assigned participants, 270 were analysed intention to treat and 251 per protocol. |
| Selective reporting (reporting bias) | High risk | Full outcome data were not presented for survival, PFS, and adverse events (particularly in the earlier article and less so in the follow‐up paper). For example, Kaplan‐Meier plots with hazard ratio, 95% confidence interval, and log‐rank analyses for the full cohort were not presented for survival, PFS (no hazard ratio with 95% confidence interval), or time to deteriorate in the NIHSS (subgroup only of those with complete resection). Timing and severity of all adverse events were not fully documented (e.g. no data on wound infections or related complications and medical complications such as pulmonary thromboembolism). |
| Other bias | High risk |
|
Willems 2006.
| Study characteristics | ||
| Methods | Randomised controlled trial. Randomisation: stratified by age (< 45 or ≥ 45 years) and KPS (≤ 70 or > 70), and they were evenly randomised to SS (without neuronavigation) or SN (with neuronavigation) using a computer‐generated list with allocation codes in random order, balanced for each stratum using blocks of 4. Sample size: based on the results of a power analysis (details not specified in the paper), the authors planned to include 182 participants in the study, but the trial was stopped at 45 participants after an early pilot analysis. There was no blinding. |
|
| Participants | Inclusion criteria: solitary intracerebral space‐occupying lesion with (partial) contrast enhancement eligible for surgical debulking with the intention of gross total resection. Exclusion criteria: previous neurosurgical treatment or any other known primary tumour elsewhere in the body. |
|
| Interventions | Intervention: neuronavigation was performed with bone fiducial markers. Preoperative magnetic resonance images were obtained using a 0.5‐T system with contrast‐enhanced T1 weighted images. Volumetric measurements were performed to assess total lesion volume. Functional grading was recorded according to the MD Anderson scheme (Sawaya 1998). Planning involved localisation using fiducial markers, trajectory planning, and segmentation of the tumour boundary. Tools included an infrared pointer or mechanically tracked operating microscope. | |
| Outcomes | Primary outcome: extent of resection and survival. Other outcomes: procedure duration, usefulness of neuronavigation, extent of resection, quality of life, and postoperative course (including neurological status and adverse events). |
|
| Notes | There were 3 early deaths in the navigation arm from systemic causes, which, with the low numbers in each arm, skewed the results. Interim analysis/abbreviated study. Definitions: Postoperative magnetic resonance images were obtained within 72 hours and subject to volumetric analysis. Clinical assessment was performed postoperatively within 3 days, 1 week, 6 weeks, and 3 months to assess adverse events and neurological status (using KPS and Barthel Index scores). Quality of life questionnaires (EORTC QLQ‐C30 questionnaire with BN20 brain tumour module, and EQ‐5D questionnaire) were filled out preoperatively and approximately 3 months after surgery. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to SS (without neuronavigation) or SN (with neuronavigation) using a computer‐generated list with allocation codes in random order, balanced for each stratum using blocks of 4. However, groups were not evenly distributed at baseline, with more eloquently located tumours in the standard surgery arm and histology with more metastasis in the navigation arm (although the latter was a variable that could not have been determined preoperatively). |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 participant was excluded due to an alternative diagnosis (meningioma). Postoperative imaging was only assessed in 34/45 participants for tumour volume and 40/45 for contrast‐enhancing volume. Data for quality of life at 3 months were only reported on 64.5% of the total eligible population. |
| Selective reporting (reporting bias) | High risk | All outcome measures were reported to a degree. However, full data with suitable presentation and analysis were not available for survival (no Kaplan‐Meier plots), quality of life (no statistical analysis), and adverse events (no presentation of numbers of events). |
| Other bias | High risk | Trial was significantly underpowered and was terminated prematurely. Out of 280 potentially eligible participants, only 46 participants were included, with a planned target of 182. |
ASA: American Society of Anesthesiologists; EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer quality of life assessment; EQ‐5D: European Quality of Life‐5 Dimensions; KPS: Karnofsky performance score; iMRI: intraoperative magnetic resonance imaging; MRI: magnetic resonance imaging; NIHSS: National Institutes of Health Stroke Scale; PFS: progression‐free survival; RTV: residual tumour volume; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abraham 2018 | Not a randomised controlled trial |
| Abraham 2019 | Not a randomised controlled trial |
| Chen 2011 | Only abstract available (report is ofpublished conference proceedings). Contacted authors but no reply. Insufficient information available to fully assess trial for either qualitative or quantitative inclusion |
| Eljamel 2008 | The addition ofrepetitive photodynamic therapy essentially precludes analysis ofthis trial as a test ofintraoperative imaging alone |
| Golub 2020 | |
| Rohde 2011 | This trial assessed specificity and sensitivity ofintraoperative 3D ultrasound as diagnostic test rather than treatment option |
| Seddighi 2016 | Only abstract available (report is of published conference proceedings). Contacted authors but no reply. Insufficient information available to fully assess trial for either qualitative or quantitative inclusion |
| Stepp 2007 | Further report of Stummer 2006 trial; only new data are on spectroscopy and photodynamic therapy |
| Stummer 2017 | A randomised controlled trial on the diagnostic effects of different doses of 5‐aminolevulinic acid (clinical, spec‐trophotometric, pathological) |
| Wadhwa 2019 | Not a randomised controlled trial |
| Waqas 2018 | Not a randomised controlled trial |
| Wu 2003 | Author stated that this was not a randomised controlled trial |
| Wu 2004 | Author stated that this was not a randomised controlled trial |
| Wu 2007 | Author stated that this was not a randomised controlled trial |
| Zhang 2015 | Not a randomised controlled trial; “patient selection was based on economic status and the availability of iMRI" |
| Chen 2012 | Only abstract available (report is ofpublished conference proceedings). Contacted authors but no reply. Insufficient information available to fully assess trial for either qualitative or quantitative inclusion |
| Czyz 2011 | Not a randomised controlled trial |
| Koc 2008 | Prospective study; participants were not randomised |
Characteristics of ongoing studies [ordered by study ID]
NCT00752323.
| Study name | Imaging procedure using ALA in finding residual tumor in grade IV malignant astrocytoma |
| Methods | "Randomised" – possibly diagnostic only trial design |
| Participants | Newly diagnosed and recurrent grade IV glioma |
| Interventions | 2 doses of 5‐ALA |
| Outcomes |
|
| Starting date | August 2008 |
| Contact information | Andrew Sloan |
| Notes | Case Medical Center, Cleveland, OH, USA |
NCT00977327.
| Study name | Comparison of neuronavigational systems for resection‐control of brain tumors |
| Methods | Randomised trial |
| Participants | Neuroradiological evidence of a brain lesion |
| Interventions | iMRI (PoleStar N‐20) vs iUS (SonoWand) |
| Outcomes |
|
| Starting date | 2009 |
| Contact information | Andrew Kanner |
| Notes | Tel Aviv, Israel |
NCT01479686.
| Study name | iMRI guided resection in cerebral glioma surgery |
| Methods | Randomised controlled trial |
| Participants | All adults aged 18–70 years with newly diagnosed supratentorial lesion involving the frontal, temporal, parietal, occipital, insular lobe, or a combination that is an untreated suspected malignant cerebral glioma (WHO grade II–IV) with ; with preoperative assessment of attainable radiologically gross total tumour resection |
| Interventions | iMRI vs conventional neuronavigation |
| Outcomes | Primary: EOR Secondary: progression‐free survival, overall survival, and surgery‐related morbidity |
| Starting date | September 2011 |
| Contact information | Jinsong Wu |
| Notes | Interim analysis – awaiting full results. Definitions: GTR was defined as the complete disappearance of all enhancing lesions (T1‐weighted) for HGG and the complete disappearance of all non‐enhancing (T2‐weighted fluid‐attenuated inversion recovery) lesions for LGG. The EORs were assessed quantitatively in volumetric analyses and stratified as follows: GTR, 100% resection; subtotal resection, 90% resection; partial resection, 70% resection; and biopsy. |
NCT01502280 (BALANCE).
| Study name | Fluorescence‐guided surgery for low‐ and high‐grade gliomas |
| Methods | Randomised. Single‐blind |
| Participants | Newly diagnosed glioma (HGG and LGG) |
| Interventions | 5‐ALA (Gliolan) vs placebo (ascorbic acid) |
| Outcomes | Volume of residual disease; overall survival; and 6‐month progression‐free survival |
| Starting date | November 2010 |
| Contact information | Nader Sanai (principal investigator), Norissa Honea (overall contact) |
| Notes | Barrow, Phoenix, AZ, USA |
NCT01798771 (IMAGER).
| Study name | Intraoperative MRI and 5‐ALA guidance to improve the extent of resection in brain tumor surgery (IMAGER) |
| Methods | Randomised |
| Participants | Newly diagnosed supratentorial intra‐axial brain tumour suspicious for malignant glioma. Deemed resectable |
| Interventions | iMRI + 5‐ALA vs 5‐ALA alone |
| Outcomes | EOR (according to postoperative MRI within 72 hours); volumetric EOR; progression‐free survival; quality of life; National Institutes of Health Stroke Scale |
| Starting date | February 2013 |
| Contact information | Christian Senft |
| Notes | Johann Wolfgang Goethe University Hospitals, Germany |
NCT01811121 (RESECT).
| Study name | Medico‐economic evaluation of surgery guided by fluorescence for the optimization of resection of glioblastomas (RESECT) |
| Methods | Randomised |
| Participants | All competent adults greater than 18 years of age with an intracerebral supratentorial hemispheric, newly‐diagnosed and previously untreated space occupying lesion with MRI characteristics suggestive of glioblastoma in a location amenable to complete resection |
| Interventions | 5‐ALA vs placebo (ascorbic acid) |
| Outcomes | EOR (according to postoperative MRI within 48 hours); volumetric EOR; progression‐free survival; quality of life; procedure duration |
| Starting date | February 2013 |
| Contact information | Jacques Guyotat |
| Notes | Hospices Civils de Lyon, France |
NCT02150564.
| Study name | 3D Ultra Sound for Resection of Brain Tumors (Sono RCT) |
| Methods | Randomised |
| Participants | All radiologically‐suspected, previously untreated, supratentorial malignant gliomas being considered for debulking surgery in adults aged above 18 with a resectable lesion undergoing surgical therapy |
| Interventions | iUS with neuronavigation vs. neuronavigation only |
| Outcomes | GTR rate; EOR (according to postoperative MRI within 72 hours); accuracy of US; further resection prompted; survival |
| Starting date | October 2016 |
| Contact information | Aliasgar V Moiyadi |
| Notes | Tata Memorial Hospital, India |
NCT03291977 (FLEGME).
| Study name | Interest of fluorescein in fluorescence‐guided resection of gliomas (FLEGME) |
| Methods | Randomized |
| Participants | All adults aged between 18 and 79 with a parenchymal lesion typical for a glioblastoma where gross total removal is possible |
| Interventions | Fluorescéine Sodique Faure vs white‐light surgery |
| Outcomes | GTR rate; EOR (according to postoperative MRI within 48 hours); volumetric EOR; occurrence of new neurological deficits, occurrence of anaphylactic events |
| Starting date | September 2017 |
| Contact information | Pierre‐Jean Le Reste |
| Notes | Rennes University Hospital, France |
NCT03531333.
| Study name | Intraoperative Ultrasound Guided Glioma Surgery; a Randomised, Controlled Trial. (US‐GLIOMA) |
| Methods | Randomized |
| Participants | Newly diagnosed, untreated, contrast‐enhancing presumed high‐grade glioma with intention to perform gross total resection |
| Interventions | iUS guided vs. standard surgery |
| Outcomes | GTR rate; EOR (according to postoperative MRI); neurological outcome; quality of life; surgery associated neurological deficits; survival |
| Starting date | May 2018 |
| Contact information | A.J.P.E. VIncent |
| Notes | Erasmus MC, Rotterdam, Zuid‐Holland, Netherlands |
5‐ALA: 5‐aminolevulinic acid; EOR: extent of resection; GTR: gross total resection; HGG: high‐grade glioma; iMRI: intraoperative magnetic resonance imaging; LGG: low‐grade glioma; MRI: magnetic resonance imaging; WHO: World Health Organization.
Differences between protocol and review
We included a brief economic commentary (BEC) as provided in the 2018 review and updated in the 2020 review, but not specified in the 2020 protocol for the network meta‐analysis (Fountain 2020).
Contributions of authors
DMF, AB and DGB drafted the protocol. DGB, MGH, CW and MDJ wrote sections in the 2018 review included in this review. DMF, AB and MW included additional search results and details relating to the network meta‐analysis. All authors approved the final version of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
NIHR 16/144 Cochrane Programme Grant Scheme, UK
Declarations of interest
DMF: funded with an NIHR Academic Clinical Fellowship and recipient of a Cancer Research UK Predoctoral Bursary AB: None known DGB: None known MW: None known MGH: None known HB: None known AK: None known CW: None known MDJ: None known
New
References
References to studies included in this review
Kubben 2014 {published data only (unpublished sought but not used)}
- Kubben PL, Scholtes F, Schijns OE, Ter Laak-Poort MP, Teernstra OP, Kessels AG, et al. Intraoperative magnetic resonance imaging versus standard neuronavigation for the neurosurgical treatment of glioblastoma: a randomized controlled trial. Surgical Neurology International 2014;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Senft 2011 {published data only (unpublished sought but not used)}
- Gessler F, Baumgarten B, Harter P, Baehr O, Franz K, Forster M, Bink A, Seifert V, Senft C. SURG-08. RESECTION OF CONTRAST ENHANCING TISSUE PROLONGS OVERALL SURVIVAL IN GLIOMAS – SECONDARY ENDPOINT ANALYSIS OF A RANDOMIZED CONTROLLED TRIAL ON INTRAOPERATIVE MRI USE. Neuro-Oncology November 2017;19(suppl_6):vi237. [Google Scholar]
- Senft C, Bink A, Franz K, Gasser T, Seifert V. Intra-operative MRI-guided vs. conventional microsurgical brain tumor resection - results of a prospective randomized trial. Journal of Neurosurgery 2011;115:Abstract 602. [Google Scholar]
- Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncology 2011;12(11):997-1003. [DOI] [PubMed] [Google Scholar]
- Senft C, Bink A, Heckelmann M, Gasser T, Seifert V. Glioma extent of resection and ultra-low-field iMRI: interim analysis of a prospective randomized controlled trial. Acta Neurochirurgica Supplementum 2011;109:49-53. [DOI] [PubMed] [Google Scholar]
Stummer 2006 {published data only}
- Pichelmeier U, Bink A, Schackert G, Stummer W, ALA Glioma Study Group. Resection and survival in glioblastoma multiforme: an RTOG recursive partitioning analysis of ALA study patients. Neuro-oncology 2008;10(6):1025-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Roulen HJ, ALA Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncology 2006;7(5):392-401. [DOI] [PubMed] [Google Scholar]
- Stummer W, Reulen HJ, Meinel T, Pichelmeier U, Schumacher W, Tonn JC, et al, ALA Glioma Study Group. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 2008;62:564-76. [DOI] [PubMed] [Google Scholar]
- Stummer W, Tonn JC, Mehdorn HM, Nestler U, Franz K, Goetz C, et al, ALA-Glioma Study group. Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. Journal of Neurosurgery 2011;114(3):613-23. [DOI] [PubMed] [Google Scholar]
Willems 2006 {published data only}
- Willems PW, Taphoorn MJ, Burger H, Berkelbach van der Sprenkel JW, Tulleken CA. Effectiveness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: a randomized controlled trial. Journal of Neurosurgery 2006;104(3):360-8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abraham 2018 {published data only}
- Abraham P, Sarkar R, Brandel M, Wali A, Rennert R, Ramos CL, et al. Cost-effectiveness of intraoperative MRI in the treatment of high-grade gliomas. Neuro-oncology 2018;20(Suppl 6):vi146. [Google Scholar]
Abraham 2019 {published data only}
- Abraham P, Sarkar R, Brandel MG, Wali AR, Rennert RC, Lopez Ramos C, et al. Cost-effectiveness of intraoperative MRI for treatment of high-grade gliomas. Radiology 2019;291(3):689-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2011 {published data only}
- Chen X, Meng X, Zhang J, Wang F, Zhao Y, Xu BN. Low- grade insular glioma resection with 1.5T intra-operative MRI: preliminary results of a prospective randomized trial. Neuro-oncology 2011;13(iii):57. [Google Scholar]
Chen 2012 {published data only (unpublished sought but not used)}
- Chen X, Meng X, Zhang J, Li F, Li J, Xu BN. Low- grade insular glioma resection with 1.5t intra-operative MRI: preliminary results of a prospective randomized trial. Journal of Neurosurgery 2012;117(2):A406–7. [Google Scholar]
Czyz 2011 {published data only}
- Czyz M, Tabakow P, Lechowicz-Glogowska B, Jarmundowicz W. Prospective study on the efficacy of low-field intraoperative magnetic resonance imaging in neurosurgical operations. Neurologia i Neurochirurgia Polska 2011;45(3):226-34. [DOI] [PubMed] [Google Scholar]
Eljamel 2008 {published data only}
- Eljamel MS, Goodman C, Moseley H. ALA and Photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: a single centre phase III randomised controlled trial. Lasers in Medical Science 2008;23(4):361-7. [DOI] [PubMed] [Google Scholar]
Golub 2020 {published data only}
- Golub D, Hyde J, Dogra S, Nicholson J, Kirkwood KA, Gohel P, et al. Intraoperative MRI versus 5-ALA in high-grade glioma resection: a network meta-analysis. Journal of Neurosurgery 2020 Feb;21:1-15. [DOI] [PubMed] [Google Scholar]
Koc 2008 {published data only}
- Koc K. Fluorescein sodium-guided surgery in glioblastoma multiforme: a prospective evaluation. British Journal of Neurosurgery 2008;22(1):99-103. [DOI] [PubMed] [Google Scholar]
Rohde 2011 {published data only}
- Rohde V, Coenen VA. Intraoperative 3-dimensional ultrasound for resection control during brain tumour removal: preliminary results of a prospective randomized study. Acta Neuropathologica 2011;109(Supplementum):187-90. [DOI] [PubMed] [Google Scholar]
Seddighi 2016 {published data only (unpublished sought but not used)}
- Seddighi A, Seddighi AS, Nikouei A, Mohseni G. Image guided surgery using neuronavigation system in resection of cerebral gliomas involving eloquent cortical areas in pediatric population. Neuro-oncology 2016;18(iii):128. [Google Scholar]
Stepp 2007 {published data only}
- Stepp H, Beck T, Pongratz T, Meinel T, Kreth FW, Tonn JCh, et al. ALA and malignant glioma: fluorescence- guided resection and photodynamic treatment. Journal of Environmental Pathology, Toxicology and Oncology 2007;26(2):157-64. [DOI] [PubMed] [Google Scholar]
Stummer 2017 {published data only}
- Stummer W, Stepp H, Wiestler OD, Pichlmeier U. Randomized, prospective double-blinded study comparing 3 different doses of 5-aminolevulinic acid for fluorescence-guided resections of malignant gliomas. Neurosurgery 2017;81(2):230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wadhwa 2019 {published data only}
- Wadhwa S, Malhotra M, Walia B, Kumar A, Aggarwal B. Role of Intraoperative MRI (iMRI) in neurosurgery, potential imaging tool for maximizing gross total resection (GTR) rate of CNS tumours. Annals of Oncology 2019;30(Suppl 5):v156-7. [Google Scholar]
Waqas 2018 {published data only}
- Waqas M, Shamim MS. Sodium fluorescein guided resection of malignant glioma. Journal of the Pakistan Medical Association 2018;68(6):968-70. [PubMed] [Google Scholar]
Wu 2003 {published data only (unpublished sought but not used)}
- Wu JS, Zhou LF, Hong XN, Mao Y, Du GH. Role of diffusion tensor imaging in neuronavigation surgery of brain tumors involving pyramidal tracts. Zhonghua Wai Ke za Zhi [Chinese Journal of Surgery] 2003;41(9):662-6. [PubMed] [Google Scholar]
Wu 2004 {published data only (unpublished sought but not used)}
- Wu JS, Zhou LF, Gao GJ, Mao Y, Du GH. Integrating functional magnetic resonance imaging in neuronavigation surgery of brain tumors involving motor corte. Chinese Medical Journal 2004;84(8):632-6. [PubMed] [Google Scholar]
Wu 2007 {published data only}
- Wu JS, Mao Y, Zhou LF, Tang WJ, Hu J, Song YY, et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery 2007;61(5):935-9. [DOI] [PubMed] [Google Scholar]
Zhang 2015 {published data only}
- Zhang J, Chen X, Zhao Y, Wang F, Li F, Xu B. Impact of intraoperative magnetic resonance imaging and functional neuronavigation on surgical outcome in patients with gliomas involving language areas. Neurosurgical Review 2015;38(2):319-30. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00752323 {published data only}
- Imaging procedure using ALA in finding residual tumor in grade IV malignant astrocytoma. Ongoing study. August 2008. Contact author for more information.
NCT00977327 {published data only}
- Comparison of neuronavigational systems for resection-control of brain tumors. Ongoing study. 2009. Contact author for more information.
NCT01479686 {published data only (unpublished sought but not used)}
- Gong X, Yao CJ, Yuan SW, Zhuang DX, Qiu TM, Lu JF, et al. 3.0T iMRI-guided resection of eloquent high-grade gliomas: preliminary results of a randomised controlled trial. Lancet 2015;386:S11. [Google Scholar]
- Wu JS, Gong X, Song YY, Zhuang DX, Yao CJ, Qiu TM, et al. 3.0-T intraoperative magnetic resonance imaging-guided resection in cerebral glioma surgery: interim analysis of a prospective, randomized, triple-blind, parallel-controlled trial. Neurosurgery 2014;61(Suppl 1):145-54. [DOI] [PubMed] [Google Scholar]
- Wu JS, Gong X, Song YY, Zhuang DX, Yao CJ, Qiu TM, et al. 3.0T iMRI guided resection in cerebral glioma surgery: interim analysis of a prospective, randomized, triple-blind, parallel- controlled trial. Clinical Neurosurgery 2013;60:167. [DOI] [PubMed] [Google Scholar]
NCT01502280 (BALANCE) {published data only}
- Fluorescence-guided surgery for low- and high-grade gliomas. Ongoing study. November 2010. Contact author for more information.
NCT01798771 (IMAGER) {published data only}
- Intraoperative MRI and 5-ALA guidance to improve the extent of resection in brain tumor surgery (IMAGER). Ongoing study. February 2013. Contact author for more information.
NCT01811121 (RESECT) {published data only}
- Medico-economic evaluation of surgery guided by fluorescence for the optimization of resection of glioblastomas (RESECT). Ongoing study. February 2013. Contact author for more information.
NCT02150564 {published and unpublished data}
- 3D Ultra Sound for Resection of Brain Tumors (Sono RCT). Ongoing study. October 2016. Contact author for more information.
NCT03291977 (FLEGME) {published data only}
- Interest of fluorescein in fluorescence-guided resection of gliomas (FLEGME). Ongoing study. September 2017. Contact author for more information.
NCT03531333 {published data only}
- Intraoperative Ultrasound Guided Glioma Surgery; a Randomised, Controlled Trial. (US-GLIOMA). Ongoing study. May 2018. Contact author for more information.
Additional references
Black 1997
- Black PM, Moriarty T, Alexander E, Stieg P, Woodard EJ, Gleason PL, et al. Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery 1997;41(4):831-45. [DOI] [PubMed] [Google Scholar]
Brignardello‐Petersen 2018
- Brignardello-Petersen R, Bonner A, Alexander PE, Siemieniuk RA, Furukawa TA, Rochwerg B, et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. Journal of Clinical Epidemiology 2018;93:36-44. [DOI] [PubMed] [Google Scholar]
Brignardello‐Petersen 2019a
- Brignardello-Petersen R, Mustafa RA, Siemieniuk RA, Murad MH, Agoritsas T, Izcovich A, et al, GRADE Working Group. GRADE approach to rate the certainty from a network meta-analysis: addressing incoherence. Journal of Clinical Epidemiology 2019;108:77-85. [DOI] [PubMed] [Google Scholar]
Brignardello‐Petersen 2019b
- Brignardello-Petersen R, Murad MH, Walter SD, McLeod S, Carrasco-Labra A, Rochwerg B, et al, GRADE Working Group. GRADE approach to rate the certainty from a network meta-analysis: avoiding spurious judgments of imprecision in sparse networks. Journal of Clinical Epidemiology 2019;105:60-7. [DOI] [PubMed] [Google Scholar]
Brodbelt 2015
- Brodbelt A, Greenberg D, Winters T, Williams M, Vernon S, Collins VP. Glioblastoma in England: 2007–2011. European Journal of Cancer 2015;51(4):533-42. [DOI] [PubMed] [Google Scholar]
Chaimani 2017
- Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. Journal of Clinical Epidemiology 2017;83:65-74. [DOI] [PubMed] [Google Scholar]
Chang 2007
- Chang S, Vogelbaum M, Lang FF, Haines S, Kunwar S, Chiocca EA, et al. GNOSIS: guidelines for neuro-oncology: standards for investigational studies – reporting of surgically based therapeutic clinical trials. Journal of Neuro-oncology 2007;82(2):211-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Coburger 2019
- Coburger J, Wirtz CR. Fluorescence guided surgery by 5-ALA and intraoperative MRI in high grade glioma: a systematic review. Journal of Neuro-oncology 2019;141:533-46. [DOI] [PubMed] [Google Scholar]
Dirven 2014
- Dirven L, Aaronson NK, Heimans JJ, Taphoorn MJ. Health-related quality of life in high-grade glioma patients. Chinese Journal of Cancer 2014;33(1):40-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Efthimiou 2016
- Efthimiou O, Debray TP, Valkenhoef G, Trelle S, Panayidou K, Moons KG, et al. GetReal in network meta-analysis: a review of the methodology. Research Synthesis Methods 2016;7(3):236-63. [DOI] [PubMed] [Google Scholar]
Eljamel 2016
- Eljamel MS, Mahboob SO. The effectiveness and cost-effectiveness of intraoperative imaging in high-grade glioma resection; a comparative review of intraoperative ALA, fluorescein, ultrasound and MRI. Photodiagnosis and Photodynamic Therapy 2016;16:35-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Esteves 2015a
- Esteves S, Alves M, Castel-Branco M, Stummer W. A pilot cost-effectiveness analysis of treatments in newly diagnosed high-grade gliomas: the example of 5-aminolevulinic acid compared with white-light surgery. Neurosurgery 2015;76(5):552-62; discussion 562. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fountain 2016
- Fountain DM, Allen D, Joannides AJ, Nandi D, Santarius T, Chari A. Reporting of patient-reported health-related quality of life in adults with diffuse low-grade glioma: a systematic review. Neuro-oncology 2016;18(11):1475-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE Working Group 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2003
- Hall WA, Kowalik K, Liu H, Truwit CL, Kucharezyk J. Costs and benefits of intraoperative MR-guided brain tumor resection. Acta Neurochirurgica. Supplement 2003;85:137-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hart 2019
- Hart MG, Grant GR, Solyom EF, Grant R. Biopsy versus resection for high-grade glioma. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD002034. [DOI: 10.1002/14651858.CD002034.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Jenkinson 2018
- Jenkinson MD, Barone DG, Bryant A, Vale L, Bulbeck H, Lawrie TA, et al. Intraoperative imaging technology to maximise extent of resection for glioma. Cochrane Database of Systematic Reviews 2018, Issue 1. Art. No: CD012788. [DOI: 10.1002/14651858.CD012788.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiang 2017
- Jiang B, Chaichana K, Veeravagu A, Chang SD, Black KL, Patil CG. Biopsy versus resection for the management of low-grade gliomas. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD009319. [DOI: 10.1002/14651858.CD009319.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kowalik 2000
- Kowalik K, Truwit C, Hall W, Kucharczyk J. Initial assessment of costs and benefits of MRI-guided brain tumor resection. European Radiology 2000;10 Suppl 3:S366-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Louis 2016
- Louis DN, Perry A, Reifenberger G, Von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathologica 2016;131(6):803-20. [DOI] [PubMed] [Google Scholar]
Makary 2011
- Makary M, Chiocca EA, Erminy N, Antor M, Bergese SD, Abdel-Rasoul M, et al. Clinical and economic outcomes of low-field intraoperative MRI-guided tumor resection neurosurgery. Journal of Magnetic Resonance Imaging 2011;34(5):1022-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miwa 2004
- Miwa K, Shinoda J, Yano H, Okumura A, Iwama T, Nakashima T, et al. Discrepancy between lesion distributions on methionine PET and MR images in patients with glioblastoma multiforme: insight from a PET and MR fusion image study. Journal of Neurology, Neurosurgery, and Psychiatry 2004;75(10):1457-62. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2018
- National Institute for Health and Care Excellence. Brain tumours (primary) and brain metastases in adults (NG99). www.nice.org.uk/guidance/ng99 (accessed prior to 6 October 2020).
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Pichlmeier 2008
- Pichlmeier U, Bink A, Schackert G, Stummer W. Resection and survival in glioblastoma multiforme: an RTOG recursive partitioning analysis of ALA study patients. Neuro-oncology 2008;10(6):1025-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al, GRADE Working Group. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;24(349):g5630. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rücker 2015
- Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Medical Research Methodology 2015;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163-71. [DOI] [PubMed] [Google Scholar]
Salanti 2014
- Salanti G, Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PloS One 2014;9(7):e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sawaya 1998
- Sawaya R, Hammoud M, Schoppa D, Hess KR, Wu SZ, Shi WM, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 1998;42:1044-56. [DOI] [PubMed] [Google Scholar]
Schulder 2003
- Schulder M, Carmel PW. Intraoperative magnetic resonance imaging: impact on brain tumor surgery. Cancer Control 2003;10(2):115-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Slof 2015
- Slof J, DÃÂez Valle R, Galván J. Cost-effectiveness of 5-aminolevulinic acid-induced fluorescence in malignant glioma surgery. Neurologia (Barcelona, Spain) 2015;30(3):163-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stata [Computer program]
- Stata. Version 15. College Station, TX, USA: StataCorp, 2017. Available at www.stata.com.
Stummer 1998
- Stummer W, Stocker S, Wagner S, Stepp H, Fritsch C, Goetz C, et al. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid- induced porphyrin fluorescence. Neurosurgery 1998;42(3):518-26. [DOI] [PubMed] [Google Scholar]
Stummer 2000
- Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. Journal of Neurosurgery 2000;93(6):1003-13. [DOI] [PubMed] [Google Scholar]
Stupp 2005
- Stupp R, Mason WP, den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England Journal of Medicine 2005;352(10):987-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wen 2010
- Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. Journal of Clinical Oncology 2010;28(11):1963-72. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Fountain 2020
- Fountain DM, Jenkinson MD, Bryant A, Vale L, Bulbeck H, Hart MG, et al. Intraoperative imaging technology to maximise extent of resection for glioma: a network meta-analysis. Cochrane Database of Systematic Reviews 2020, Issue 5. Art. No: CD013630. [DOI: 10.1002/14651858.CD013630] [DOI] [PMC free article] [PubMed] [Google Scholar]


